The effective tax rate on the Group’s earnings is affected by the tax rates applicable in the UK and the US. Changes in tax laws or in their application with respect to matters, such as transfer pricing, that relate to the proportion of the Group’s earnings, which may currently be taxed at more favorable rates, could increase the Group’s effective tax rate and adversely affect its net earnings.
ACAMBIS MAY NOT BE ABLE TO COMPLETE ITS TRANSITION FROM AN R&D-BASED ENTITY INTO AN INTEGRATED BIOPHARMACEUTICAL CONCERN
Acambis continues to implement changes to complete its transition from a group predominantly focused on R&D to one with integrated biopharmaceutical capabilities. These changes are not yet complete and there is a risk that they will not be successful, which will create substantial near-term costs and impede Acambis’ ability to perform effectively in the future.
Acambis is a biopharmaceutical company operating in the infectious disease arena, with a focus on developing new vaccines. It is headquartered in Cambridge, UK. The majority of its operations are based in the US, with R&D in Cambridge, MA, manufacturing in Canton, MA, a sales and marketing operation in Miami, FL and a fill and finish facility in Rockville, MD that Acambis leased in May 2005.
Acambis aims to become a fully integrated, profitable biopharmaceutical company, targeting infectious diseases with vaccines and other biological products, and generating predictable and sustainable revenues through both organic growth and acquisitions. To deliver that goal, it is focused on making the most of the opportunities for its smallpox franchise, building a high-value pipeline, building core capabilities to maximize the value of the pipeline and increasing its recurring revenue streams.
Acambis was established in 1992 in the UK as Peptide Therapeutics Limited and floated on the London Stock Exchange in November 1995. It listed its shares in the form of ADRs on NASDAQ in February 2001.
In May 1999, it acquired a US-based vaccine research company, OraVax, Inc. and, in December 2000, was renamed Acambis plc. Around that time, it refocused its operations on the development of vaccines and subsequently sold its drug discovery business, Mimetrix, to Medivir AB, a Swedish biotechnology company.
Much of its cash has come from government contracts for its new, investigational smallpox vaccine, which is sold to the US and other governments under an FDA Investigational New Drug (IND) application.
Acambis started work on developing a new smallpox vaccine in 2000, having been awarded a contract by the US Government, and was awarded a major stockpiling contract in November 2001. Acambis has now supplied the US with 182.5 million doses of its investigational ACAM2000 vaccine (see further information in Item 10C (a)). It has also supplied the vaccine to 14 other governments around the world as an investigational product. The ACAM2000 program was awarded fast-track status in December 2004. In April 2006, Acambis completed submission of the Biologics License Application to the US FDA to seek licensure of ACAM2000.
In partnership with Baxter, Acambis is developing an attenuated smallpox vaccine, MVA3000, which is a Modified Vaccinia Ankara-based investigational smallpox vaccine. To date, Acambis has been awarded two National Institute of Allergy and Infectious Diseases (NIAID) contracts relating to the development and manufacture of MVA3000. It is currently bidding for a US Government stockpiling contract.
Acambis aims to maximize and retain the value of its products by manufacturing and selling the vaccines it develops, wherever feasible. It has established a bulk manufacturing/purification capability in-house and acquired a lyophilisation and fill/finish facility in May 2005. In addition, the Group added a sales, marketing and distribution capability in 2003 through the acquisition of Berna Products Corporation (BPC), based in Miami, FL, which sells Vivotif®, a licensed oral typhoid vaccine, in North America.
CAPITAL EXPENDITURE IN RECENT YEARS
During 2005, Acambis made additions of short leasehold land and buildings of £3.6m (2004 - £1.5m, 2003 - £2.9m), acquired £0.9m of laboratory and manufacturing equipment (2004 - £0.9m, 2003 - £1.9m) and £0.7m of office equipment (2004 - £0.9m, 2003 - £1.0m). Additions in 2005 and 2004 were principally in the US. Additions in 2003 were primarily related to the reactivation of a manufacturing facility in the US. In the period from December 31, 2005 to the end of April 2006, the Group has made additions of £nil for laboratory and manufacturing equipment and £0.3m for computer equipment. These additions were in the US. All additions in 2003, 2004 and 2005 were financed using cash, and related predominantly to the cost of redeveloping and expanding areas of the US R&D facility. Expenditure incurred during 2005 primarily relates to acquiring the fill/finish facility in Rockville MD, in May 2005.
THE VACCINE INDUSTRY
There are an estimated 200 companies operating in the vaccine arena, selling or developing around 600 products. The five major players – sanofi pasteur (SP), GlaxoSmithKline (GSK), Merck, Wyeth and Novartis – generated around 80% of the $8.5bn worldwide sales in 2004. Other sales were shared between mid-size companies such as Acambis, Baxter Vaccines, Crucell, Intercell and Solvay.
Typically, these mid-size companies, like Acambis, employ between 250 and 1,000 employees, are often investing in developing their infrastructures, including manufacturing and marketing, and tend to have varied geographical coverage. Companies such as these may sell some products and have late-stage development programs but may not be inclined to out-license products except to gain experience or coverage that they lack.
The need for long-term investment in R&D, considerable manufacturing capacity and capability, and the ever-increasing regulatory burden have established high barriers to entry and encouraged industry consolidation to bring the acquirer access to products, expertise or expanded geographical reach. Several of the leading pharmaceutical companies are also investing in their vaccine divisions, including through acquisition. Recent examples include GSK’s acquisition of ID Biomedical, Novartis’ acquisition of Chiron and Crucell’s acquisition of Berna Biotech.
Page 11 of 122
Although the majority of sales today are for pediatric vaccines, elective vaccination of adolescents, adults and the elderly is gaining profile. Target markets include vaccines for diseases associated with pregnancy, sexual contact, drug use and hospital-acquired infections, as well as the more well-known travel/military vaccination markets, biodefense vaccines and the high-profile influenza market.
The infectious disease arena and, in particular, infectious disease vaccines offer major opportunities for companies such as Acambis. Through factors such as an increased emphasis on preventative medicine in Western countries, emergence of new diseases, continued growth in travel to endemic regions and concerns about the threat of viruses and bacteria being used as biological weapons, vaccines are now recognized internationally as a critical part of health management strategies. Vaccines, which are Acambis’ focus, continue to represent the fastest-growing infectious disease sector.
ACAMBIS’ PRODUCTS
The majority of Acambis’ revenues today come from government contracts relating to its investigational smallpox vaccines, ACAM2000 and MVA3000, with the US Government being its principal customer. In addition, it sells a typhoid vaccine, Vivotif, in North America. Acambis plans to drive future revenues through sales of other products that it is currently developing and by seeking to acquire additional products that are licensed or close to licensure. In addition to its smallpox vaccines, it has three other proprietary vaccines in development. ChimeriVax-JE, a vaccine against the Japanese encephalitis virus, is undergoing Phase 3 clinical trials in the US and Australia and will undergo pediatric trials in India. ChimeriVax-West Nile, a vaccine against the West Nile virus, entered Phase 2 testing in December 2005. C. difficile, a vaccine against the hospital-acquired Clostridium difficile bacterium, is currently undergoing Phase 1 trials and is expected to enter Phase 2 trials later in 2006. In addition, Acambis has developed ChimeriVax-Dengue, a vaccine against dengue fever, and out-licensed this program to SP. The vaccine is undergoing Phase 2 trials. In 2005, Acambis established a program to develop a ‘universal’ influenza vaccine, using technology acquired from a US biotechnology company, Apovia Inc (Apovia), and collaboration with a Belgian research institute. It aims to undertake initial trials of a vaccine candidate in 2007.
ACAMBIS’ OPERATIONS
Acambis’ headquarters are based in Cambridge, UK where, in addition to head office functions, it has a clinical and regulatory team, business development and sales and marketing departments. Acambis’ principal research and development operation is located in Cambridge, MA. Acambis’ bulk/purification manufacturing facility is in Canton, MA, together with associated functions such as quality control and quality assurance. Acambis’ fill/finish facility, which is in the process of being fitted out, is based in Rockville, MD. Its sales, marketing and distribution infrastructure is based in Miami, FL and it also has an operation in Toronto, Canada.
ACAMBIS’ MARKETS
The majority of Acambis’ revenues today come from government contracts relating to its investigational smallpox vaccines, ACAM2000 and MVA3000, with the US Government being its principal customer. In addition, it sells a typhoid vaccine, Vivotif, in North America.
Of the investigational vaccines currently being in clinical development, the nearest to market is ChimeriVax-JE. The target markets for this vaccine are people living in endemic regions and travelers and military personnel visiting those regions. Acambis has partnered with Bharat, which will support commercialization of ChimeriVax-JE in India. The primary market for a West Nile vaccine is expected to be the US and the focus for C. difficile is likely to be the US, Canada and Europe.
THE REGULATORY ENVIRONMENT
The process to gain a license to sell a vaccine takes a long time and requires a significant amount of investment. An application for licensure of a vaccine requires a vast amount of data from the results of clinical and pre-clinical testing and about the manufacturing process, and the standards expected of an approvable product are ever increasing.
Page 12 of 122
The regulatory authority that has, to date, overseen much of Acambis’ work is the FDA. However, licensure of Acambis’ vaccines will often also be sought from other regulatory authorities, such as the EMEA and the Australian Therapeutics Goods Administration (TGA).
STRATEGY
As part of its long-term strategy, Acambis has four key drivers:
| - | Maximize the smallpox franchise |
| - | Build a billion dollar pipeline |
| - | Fully integrate from concept to commercialization |
| - | Increase recurring revenue streams. |
MAXIMISE THE SMALLPOX FRANCHISE
Acambis has supplied more doses of smallpox vaccine to more governments than any other company. By capitalizing on its strengths, Acambis aims to make the most of the opportunities available to it – ACAM2000 warm-base manufacturing for the US, stockpiling ACAM2000 for other governments and delivering MVA3000 to the US Strategic National Stockpile – in order to generate funds to invest in its pipeline.
BUILD A BILLION DOLLAR PIPELINE
Acambis’ pipeline is its principal asset for creating shareholder value. By delivering on what it has today – short- and medium-term projects with a mix of commercial opportunities – it aims to establish a sound base for its portfolio. By adding, over time, other products that target sizeable commercial opportunities, it aims to build a high-value portfolio.
FULLY INTEGRATE FROM CONCEPT TO COMMERCIALISATION
Acambis wants to generate as much value as possible from its pipeline. In time, it wants to develop, manufacture and sell its own products wherever possible. For now, profits from sales of Vivotif are already contributing to its pipeline investment, its manufacturing assets are helping it to control costs and timelines, and it complements its strengths where necessary through partnerships with other companies.
INCREASE RECURRING REVENUE STREAMS
The cost of developing new products is significant, as is investing in assets that can generate greater value in the long term, such as manufacturing. Acambis’ aim is to capitalize on all sources of funding available to it to supplement shareholder investment. In addition to bidding for smallpox contracts, it also aims to increase the size and diversity of its recurring revenue streams, particularly through using its sales and distribution infrastructure.
BASIS OF PREPARATION OF FINANCIAL STATEMENTS
The financial statements contained within this Form 20-F have been prepared in accordance with IFRS as adopted by the European Union (‘IFRS’). A reconciliation to US GAAP is set out in note 29 in the financial statements. The principal differences between IFRS and US GAAP accounting arise on revenue recognition timing differences, accounting for share options, deferred tax and the treatment of goodwill. These differences are described within note 29 within the financial statements.
TURNOVER
The Group’s turnover comprises product sales, license fees, contract research fees and milestone payments.
The Directors are of the opinion that the Group has only one class of business.
Page 13 of 122
The following table presents revenue and certain asset and capital expenditure information regarding the Group’s geographic segments.
| | | | Europe | | | | North America |
| | 2005
£m | | 2004
£m | | 2005
£m | | 2004
£m |
Revenue: | | | | | | | | |
Sales to external customers | | 1.8 | | 8.5 | | 39.1 | | 77.0 |
Other segment information: | | | | | | | | |
Total assets | | 79.3 | | 104.0 | | 54.1 | | 59.9 |
Capital additions: | | | | | | | | |
Tangible fixed assets | | — | | — | | 5.2 | | 3.1 |
Intangible assets | | — | | — | | 0.6 | | 0.2 |
In 2005, sales to Europe represented 4.4% and sales to North America represented 95.6% of total sales.
EMPLOYEES
At December 31, 2005, Group headcount had increased to 285 (2004 – 270).
ADR SPLIT
In February 2004, Acambis undertook a change in the ratio of its NASDAQ-listed ADRs, which has had the effect of bringing the price of its ADR more in line with the prices of peer group companies.
Since listing on NASDAQ in February 2001 until December 2003, Acambis’ ADR price had risen from approximately $18 to around $60. To ensure continued accessibility for both institutional and private investors in the US, Acambis took the decision to change the ADR ratio from one ADR for 10 ordinary shares to one ADR for two ordinary shares. All ADR holders on the register as at February 20, 2004 were issued on February 23, 2004 with four additional ADRs for each one held.
C | Organizational structure |
SUBSIDIARY UNDERTAKINGS at December 31, 2005 | | | | | | | |
Company name | | Main business | | Country of incorporation | | Parent company | | % owned | |
Acambis Research Limited | | Corporate administration and sales | | England and Wales | | Acambis plc | | 100% | |
Acambis Inc. | | R&D, sales and manufacturing | | US | | Acambis plc | | 100% | |
Berna Products Corporation | | Sales, marketing and distribution | | US | | Acambis Inc. | | 100% | |
Smallpox Biosecurity Limited | | Marketing | | England and Wales | | Acambis plc | | 100% | |
These subsidiaries are all consolidated into the Group accounts.
D | Property, plant and equipment |
DESCRIPTION OF PROPERTY
The following table summarizes the premises that Acambis currently leases:
Location | | Use | | Approximate
area | | Lease dated | | Lease
term | |
Peterhouse Technology Park, Cambridge, UK | | R&D/office | | 30,000 sq ft | | Dec 1998 | | 25 years | |
Cambridge, MA, USA | | R&D/office | | 53,000 sq ft | | Jan 1996 | | 15 years | |
Cambridge, MA, USA | | Office | | 6,000 sq ft | | Oct 2005 | | 5½ years | |
Canton MA, USA | | Manufacturing | | 47,000 sq ft | | Dec 2001 | | 5 years | |
Page 14 of 122
Location | | Use | | Approximate
area | | Lease dated | | Lease term |
Canton MA, USA | | Office/warehousing | | 27,000 sq ft | | Apr 2002 | | 5 years |
Coral Gables FL, USA | | Office | | 11,000 sq ft | | Jun 2006 | | 3 years |
Toronto, Canada | | Office | | 3,000 sq ft | | Jun 2005 | | 3 years |
Rockville MD. USA | | Fill & Finish Facility | | 58,000 sq ft | | May 2005 | | 12 years |
In March 2000, the Group entered into a sub-lease with Medivir UK Limited (Medivir) with respect to a part of the facility at Peterhouse Technology Park in the UK. In December 2003, this sub-lease was amended, such that 45% of the facility was rented to Medivir until September 2004 with an option to extend until November 2004. Medivir terminated the sub-lease in September 2004. During 2005, Medivir contributed £nil (2004 - £0.2m) in operating lease rentals relating to land and buildings.
The Group believes its properties to be adequately maintained and suitable for their intended use.
Item 5 | Operating and financial review and prospects |
The information in this ‘Operating and financial review and prospects’ section should be read in conjunction with the financial statements and the Notes thereto and Item 3: Key Information, D: Risk Factors.
OVERVIEW OF 2005
INTRODUCTION
Acambis’ most notable achievement in 2005 was the progression of each of its proprietary programs into the next stage of development, including ChimeriVax-JE, which is now undergoing pivotal Phase 3 trials in Australia and the US. It also expanded the pipeline with the addition of an exciting influenza vaccine program by acquiring an ongoing program and starting a research collaboration.
Its capabilities were increased through the acquisition of a fill/finish capability in the US, which has given Acambis the opportunity not only to bring in-house an increasingly scarce resource but also to complete its manufacturing supply chain.
In addition to building its pipeline and capabilities, Acambis has an ongoing aim to exploit its competitive strengths in the smallpox arena to gain as much value as possible from its franchise of products: ACAM2000, MVA3000 and C-VIG. It made good progress with its existing MVA3000 contract, including delivering 500,000 doses to the US Government, and, in January 2006, started submission of a US license application for ACAM2000, which was completed in April 2006.
Acambis is now in a litigation process relating to MVA as a result of complaints filed against it by BN in the US in August 2005. A further suit was filed in Austria in February 2006. BN alleges that Acambis has used its trade secrets in the development of Acambis’ MVA3000 vaccine and that it is infringing BN’s patents. Acambis strongly believes these allegations are without foundation and it is vigorously defending its position.
Acambis’ financial performance during 2005 was in line with management expectations. The guidance it gave at the beginning of the year was for £40m of predictable revenues and the actual performance was £40.9m.
Page 15 of 122
The fact that almost 60% of this revenue was recognized in the fourth quarter of the year highlights one of the principal challenges of predicting and relying on biodefense contract revenues and Acambis continues to pursue opportunities to build more recurring revenues. In this area, 2005 was a particularly good year for sales of Vivotif as Acambis was able to capitalize on availability issues for the competitor typhoid vaccine to improve revenues and market share.
SMALLPOX FRANCHISE UPDATE
ACAM2000
Following a pre-Biologics License Application (BLA) meeting with the FDA in November 2005, Acambis started submission of its BLA for ACAM2000 in January 2006 and completed it in April 2006. This is the culmination of over five years of work to provide the US Government with a next-generation, licensed smallpox vaccine. Acambis submitted the BLA on a ‘rolling’ basis under the fast-track status awarded to the program in December 2004. The submission included safety, tolerability and immunogenicity data obtained from clinical trials of ACAM2000 conducted in more than 3,800 subjects. Given ACAM2000’s fast-track status, Acambis expects to receive the FDA’s decision on its application before the end of the year.
Acambis is currently in negotiations with the US Government about a contract for Acambis to provide warm-base manufacturing for ACAM2000 on a long-term basis. This is intended to maintain its facilities in a state of production readiness and, if necessary, to provide the US with ongoing surge capacity in smallpox vaccine production. In September, Acambis reported that the CDC had indicated that it would be proceeding with a warm-base manufacturing contract during US Government Fiscal Year 2006, which runs from October 1, 2005 through September 30, 2006. Acambis is on track to achieve that timeline.
Management remains confident that there are further opportunities to sell ACAM2000 to other governments. Whilst Acambis did not achieve any significant sales during 2005, discussions with various countries indicate that some may be awaiting the outcome of the US product license application process before proceeding with their procurement decisions.
MVA3000
During 2005, Acambis made excellent progress on its existing contract with the US Government agency, the NIAID, including delivering 500,000 doses of its MVA vaccine, MVA3000, in December.
It also initiated a Phase 2 safety and immunogenicity trial, enrolment for which is now complete.
Together with its co-development partner Baxter, Acambis submitted a bid for a US Government stockpiling contract in October 2005. This was in response to a Request for Proposals (RFP) issued by the Department of Health and Human Services. The RFP is for the manufacture of up to 20 million doses of MVA attenuated smallpox vaccine and advanced clinical testing up to and including obtaining a product license. It also includes options for the purchase of up to 60 million additional doses of MVA and warm-base manufacturing over the longer term. The 500,000 doses Acambis delivered in December were produced at the scale required for this stockpiling process.
Management believes that Acambis’ strong track record with the US Government, its partnership with Baxter and its demonstrated ability to manufacture and deliver large quantities of both MVA3000 and ACAM2000 put Acambis in a strong competitive position.
MVA litigation
Acambis is continuing vigorously to oppose any and all legal actions filed by BN with regard to MVA.
Page 16 of 122
In February 2006, BN filed a suit against Acambis in Austria. This follows complaints lodged with the International Trade Commission (ITC) and District Court of Delaware in August 2005. The complaint filed with the Commercial Court in Vienna alleges infringement of a European patent awarded to Bavarian Nordic in December 2005. The European patent appears to claim technology similar to BN’s US patents, which are in dispute in the US litigation. Acambis has filed an opposition to the European patent.
A hearing was held at the International Trade Commission in May 2006. In advance of the hearing, Motions for Summary Determinations were filed, which had the effect of narrowing the scope of the ITC hearing such that its entire focus was the patent dispute, which Acambis considers to be the principal question in the litigation. The judge’s Initial Determination on the case is expected in the third quarter of 2006, after which his decision will be reviewed by a panel of ITC Commissioners who are required to rule on the decision by the end of November.
C-VIG
During the course of 2005, Acambis helped Cangene Corporation (Cangene) to win its first major vaccinia immune globulin (VIG) contract outside the US. Cangene was awarded a contract of C$17m (c£8.5m) in September to supply doses of its C-VIG product to the UK Government. As sales agent to Cangene, Acambis receives a royalty on the sales achieved under the contract. C-VIG was licensed by the US FDA in 2005.
RESEARCH AND DEVELOPMENT UPDATE
ChimeriVax-JE
Acambis’ ChimeriVax-JE vaccine against the mosquito-borne Japanese encephalitis (JE) virus is now undergoing pivotal Phase 3 testing. The two clinical trials, which are being conducted in multiple centers in Australia and the US, are testing the safety and efficacy of a single-dose regimen of ChimeriVax-JE in more than 2,800 subjects. Recruitment for the trials was completed in April 2006.
Preparations are underway to start a Phase 2 pediatric trial in India, where children are the primary target population for a JE vaccine. The pediatric data will supplement those generated in Acambis’ ongoing Phase 3 trials and previous Phase 1 and 2 studies to support license applications for both the endemic regions and the travel market. It is targeting submissions of license applications in both India and Australia in 2007.
There is a large unmet public health need for a single-dose, convenient and affordable vaccine against JE, which could make it simpler, faster, easier and cheaper for healthcare providers to administer vaccines, particularly in regions where achieving compliance to multi-dose regimens can be difficult. An epidemic in northern India in 2005 resulted in 6,340 cases and more than 1,200 deaths, mostly of children.
India is one of Acambis’ primary markets for ChimeriVax-JE and to support commercialization of the vaccine in the region it established a collaboration with one of India’s leading biotechnology companies, Bharat Biotech at the end of 2005. Under the agreement, Bharat Biotech will undertake end-stage fill/finish processing of ChimeriVax-JE at its facilities in India and, once the product is approved, will market and distribute the vaccine in India and neighboring countries. Acambis is currently pursuing the necessary import and export requirements with a view to completing technology transfer to Bharat Biotech in time to use material produced by Bharat Biotech in planned Phase 3 trials in India. It is also pursuing partnerships to target other endemic countries and the travelers’ market.
ChimeriVax-West Nile
Acambis is continuing to lead the field in developing a human vaccine against the mosquito-borne West Nile virus, which is endemic in the US. Having become the first to complete a Phase 1 trial, it was also the first to enter Phase 2 clinical testing.
Page 17 of 122
It initiated a Phase 2 trial in December 2005 to test ChimeriVax-West Nile in more than 200 subjects in the US. The aim of the randomized, double-blind, placebo-controlled trial is to evaluate the safety, tolerability and immunogenicity of ChimeriVax-West Nile in healthy adults and elderly subjects. Having tested different dose levels in young adults, the optimal dose will be taken forward for testing in subjects aged 50 and above. This age group is likely to be the initial target population for a West Nile vaccine as they are at most risk of severe disease following infection. Recruitment for the healthy adults’ portion of the trial is now complete.
In its Phase 1 safety and immunogenicity trial, results from which were published in April 2005, of the subjects who received a single dose of ChimeriVax-West Nile, 96% in the high-dose group and 100% in the low-dose group developed high titres of West Nile-neutralizing antibodies 28 days after vaccination.
Intervet, which is a leading manufacturer of veterinary vaccines, is aiming to launch its West Nile veterinary vaccine in the US during the 2006 season. The West Nile virus is a particular problem for horses. Intervet’s vaccine was developed from the ChimeriVax technology licensed from Acambis and Acambis will receive royalties from sales of the Intervet product.
C. difficile
In February 2006, Acambis announced results from the first of two Phase 1 trials of its vaccine against C. difficile, a leading cause of hospital-acquired infections. In the 50-subject placebo-controlled trial in healthy adults, antibody responses were seen in all 37 subjects who received our vaccine. No subjects experienced unexpected or serious vaccine-related adverse events.
Enrolment has been completed in a second Phase 1 trial that is designed to explore the safety, tolerability and immunogenicity of its vaccine in healthy elderly subjects at different dose levels. This is the first trial of the vaccine in one of the key target populations for the product. Acambis aims to complete Phase 1 testing in the second half of the year and then to begin Phase 2 trials.
ChimeriVax-Dengue
Following completion in the first quarter of 2005 of a Phase 1 trial of a tetravalent formulation of the ChimeriVax-Dengue vaccine, the lead responsibility for further clinical testing and development passed during 2005 to (SP), to whom Acambis has licensed worldwide rights. Results from the trial showed seroconversion to all four dengue virus serotypes. SP has progressed the vaccine into Phase 2 clinical trials.
Influenza
In August 2005, Acambis announced that it had initiated a program to develop a universal influenza vaccine. The aim of the program is to develop a vaccine that can target all strains of influenza, removing the need for annual reformulations and annual vaccinations.
To achieve this, Acambis acquired a technology previously being developed by Apovia, and established a research collaboration with the Flanders Interuniversity Institute for Biotechnology (VIB), a Belgian research institute. A major component of the new candidate(s) is M2e, the extracellular domain of the ion channel protein M2, which is specific to influenza ‘A’. Being highly conserved, M2e is intended to elicit protective immune responses against all strains of influenza ‘A’.
While Acambis’ ultimate goal is to develop a vaccine that is universally effective against all ‘A’ and ‘B’ strains of the influenza virus, which would be required for complete protection against seasonal influenza, the ‘A’ strain candidate it is developing could be suitable as a vaccine against pandemic influenza. All previous pandemics have been caused by ‘A’ strains of the virus. With a vaccine that targets all ‘A’ strains, governments would be able to stockpile vaccine doses for use in the event of a pandemic instead of waiting for the appropriate strain to be identified before vaccine manufacture can be undertaken.
Page 18 of 122
Pre-clinical development of its pandemic vaccine candidate is ongoing and it aims to enter clinical trials in early 2007. Acambis’ longer term program is currently at the research stage.
VIVOTIF®
Vivotif, the oral typhoid vaccine Acambis sells in the US, had a strong year in 2005, with sales volumes 81% up over 2004. This was primarily as a result of Acambis’ ability to capitalize on the competitor product’s lack of availability for part of the year.
ARILVAXTM
Acambis has US sales and marketing rights to ARILVAX, a yellow fever vaccine that is owned and manufactured by Chiron. In February 2004, Acambis withdrew its BLA application for ARILVAX following initial submission in December 2003. Acambis is in ongoing discussions with Chiron and its now parent company Novartis AG, to resolve a way forward for the ARILVAX program.
IMPORTANT INFORMATION REGARDING THE FINANCIAL POSITION OF ACAMBIS
The review of trading results set out below should be read in conjunction with the risk factors noted in Item 3, on the understanding that the following uncertainties exist in the Group’s business:
| • | Acambis has not yet completed the full clinical development and subsequent registration of any product candidate. |
| • | Acambis has a major contract with the US Government relating to manufacture of a smallpox vaccine and continuing the process of taking that investigational product through to licensure. The costs associated with this program and revenue recognition from it will have a material impact on Acambis’ financial results until the product is approved. |
| • | Acambis conducts a substantial part of its business outside the UK and is, therefore, subject to fluctuations in the exchange rate with other currencies, particularly the US dollar. |
| • | Acambis has two manufacturing facilities, both located in the US. The first in Canton, MA comprises bulk/purification capacity and the second in Rockville, MD comprises lyophilisation and fill/finish operations currently sufficient for clinical trial scale material. The Rockville facility is currently being re-commissioned. The loss of either facility may have a material adverse impact on Acambis’ financial results. |
The following four major risks have been identified as those most pertinent to Acambis in 2006.
RELIANCE ON THE US GOVERNMENT AND THE SMALLPOX VACCINE PRODUCES IRREGULAR REVENUE STREAMS AND MAY IMPAIR FUTURE PERFORMANCE
Acambis is reliant on the smallpox franchise from the US Government for the vast majority of its revenue. Orders for additional smallpox product can come at unpredictable intervals or may not be placed at all, resulting in a potential lack of recurring revenue. There is no guarantee that Acambis will be awarded future contracts, or that demand for the Group’s smallpox franchise will continue or that it will continue to contribute to Acambis’ revenue stream.
FUTURE ACAM2000 REVENUES COULD BE JEOPARDIZED IF ACAMBIS DOES NOT RECEIVE A WARM-BASE MANURACTURING CONTRACT OR IF THE FDA DOES NOT APPROVE ACAM2000
The award of a warm-base manufacturing contract for ACAM2000 vaccine production has been delayed. The risks are that this is further delayed, is of less commercial value than anticipated or does not materialize. If the contract is awarded it may not be as profitable as anticipated or it may not be able to deliver on it in accordance with its obligation. The contract may not be sufficient to fully utilize its manufacturing and fill and finish facilities. There is no guarantee that Acambis will be awarded future contracts; that demand for the warm-base manufacturing will continue or that it will continue to contribute to Acambis’ revenue stream.
Page 19 of 122
If the US Food and Drug Administration (FDA) subsequently refuses to license ACAM2000, future revenues of this product will be materially reduced, harming Acambis’ results of operations.
OPERATIONS
There is a risk that the Company’s fill and finish plant in Rockville, MD will not be ready in time to meet the demands of the warm base contract in 2007. Additionally Acambis still has no experience in taking a product through licensure to sales. Finally the Group’s commercial arrangements with Bharat Biotech are unproven. There is a risk that the partnership will fail to deliver.
ACAMBIS IS DEPENDENT ON THE SUCCESS OF ITS PIPELINE
The Acambis product development pipeline continues to be a major driver of medium-and long-term value. There is a risk that there might be no or insufficient reward in marketing these products, that one or more of them fails in development or clinical trials, that development delays threaten first-to-market advantage or that increasing costs negatively impact potential returns. In addition products may fail or be withdrawn at any time, before or after licensure.
RESULTS OF OPERATIONS
The information contained below covers the results prepared under IFRS for the year ended December 31, 2005 compared to the prior year ended December 31, 2004, which have been restated to IFRS.
TRADING RESULTS
The information below covers the results for the year ended December 31, 2005 compared to the prior year ended December 31, 2004.
Revenue for the year was £40.9m (2004 - £85.5m). The main sources of revenue during 2005 were the two contracts with the NIAID for MVA3000, the fixed-price 155 million-dose ACAM2000 contract with the CDC and product sales of Vivotif. In 2004, revenues also included sales of 27.5 million doses of the ACAM2000 vaccine to the CDC.
Cost of sales in 2005 decreased to £27.6m (2004 - £35.0m). These costs are in line with revenues generated in the year. The gross profit margin for the year decreased to 32.5% (2004 – 59.1%). This reflects the change in the mix of revenues recorded in the two years. During 2004, the gross margin was positively impacted by the reassessment and reduction of costs under the ACAM2000 155 million-dose contract following the decision to close out the two Phase 3 clinical trials early.
Expenditure on R&D increased in the year to £34.1m (2004 - £29.3m) as a result of the successful progression of projects into later stages of development, most notably the initiation of Phase 3 trials for ChimeriVax-JE during the second half of 2005. The Group continues to expense certain of the costs relating to its manufacturing facility to R&D in line with utilization of the facility for process development and manufacturing work for our R&D programs. During 2005, Acambis also started to incur operational costs for the fill/finish facility.
Sales and marketing costs in the year were £2.6m (2004 - £2.8m). Administrative costs were £7.7m (2004 - £5.5m) and include costs incurred and a provision accrued, together totaling around £3m, in relation to the MVA litigation. In 2004, administrative costs included two non-cash exceptional items of £2.6m. During 2004, the Group also recorded £10.2m of exceptional other operating income relating to the settlement with Baxter in respect of the termination of a contract manufacturing agreement.
Interest receivable in the year was £4.0m (2004 - £4.8m). The reduction was as a result of lower cash levels during 2005 compared with 2004. Interest payable was £1.0m (2004 - £0.9m), which primarily comprised interest payable on the lease-financing facility that was put in place for the reactivation of the manufacturing plant in Canton, MA.
Pre-tax loss for 2005 was £27.7m (2004 – pre-tax profit of £27.0m). The change compared with 2004 is primarily a result of higher revenue, increased gross margin and exceptional income recorded during 2004 and increased R&D costs in 2005. This was in line with management’s expectations.
Page 20 of 122
In 2005, the Group recorded a tax credit of £0.7m (2004 – charge of £7.3m). The effective tax rate for 2005 was 6.1% (2004 – 27.0%). The lower effective tax rate in 2005 was principally a result of being in a loss-making position during the period leading to the refund of certain taxes paid in previous profitable periods and movements in deferred tax liabilities.
Investing activities
During 2005, Acambis spent £1.7m (2004 - £0.8m) on the final payments for the BPC acquisition which increased in 2005, in part, as a result of achieving higher sales of Vivotif.
Capital expenditure in 2005 was £3.7m (2004 - £3.4m). Expenditure during the year related to the costs to redevelop and expand areas of the US R&D facility, as well as the acquisition of assets for the Rockville fill/finish facility, which was leased in May 2005.
Balance sheet highlights
i) Cash/debtors
Cash, cash equivalents and liquid investments of the Group at December 31, 2005 amounted to £68.0m (December 31, 2004 - £101.8m). The reduction in cash during the year is a result of increased investment in the R&D pipeline, together with the capital investments in the US R&D facility and the leasing of the Rockville fill/finish facility.
During the year, trade and other receivables increased to £20.6m (December 31, 2004 - £13.7m), principally as a result of an amount owing at the end of 2005 relating to the shipment of 500,000 doses of MVA3000 (debtor value of £10.1m) vaccine to the NIAID under the MVA3000 contract. This debt has been settled since the year-end.
ii) Inventory
Inventory held at December 31, 2005 amounted to £3.6m (December 31, 2004 - £6.0m). The balance principally represents work-in-progress and finished goods in relation to the ACAM2000 and Vivotif vaccines. The reduction seen in the year is partly a result of a provision made against ACAM2000 inventory during the third quarter of 2005.
iii) Current liabilities: amounts falling due in one year
At December 31, 2005, current liabilities were £46.8m (December 31, 2004 - £47.6m). A proportion of this balance relates to accruals and deferred income arising under the ACAM2000 155 million-dose contract with the CDC. At December 31, 2005, deferred income relating to this contract was £2.0m (December 31, 2004 - £16.5m). The deferred revenue balance will unwind during 2006 as the BLA submission process concludes. Trade and other payables were £16.1m at December 31, 2005 (December 31, 2004 - £8.3m). The increase at the end of 2005 was principally attributable to the trade creditor to Baxter for the production of 500,000 doses of MVA3000. This creditor has been settled since the year-end.
iii) Short-term borrowings and financial liabilities
The combined balance of the US dollar-denominated financing facilities was £12.8m at December 31, 2005 (December 31, 2004 - £13.0m). The balance on the lease-financing facility was £7.1m at December 31, 2005 (December 31, 2004 - £9.4m). The balance on the overdraft facility at December 31, 2005 was £4.0m (December 31, 2004 - £3.6m), the increase being attributable to exchange rate movements in the period. The remaining balance of £1.7m at December 31, 2005 (December 31, 2004 - £nil) relates to the discounted value of the future payments for the Rockville fill/finish facility leased earlier in 2005, payable between 2006 and 2017.
Page 21 of 122
CRITICAL ACCOUNTING POLICIES
The preparation of the Group’s financial statements requires it to make estimates and judgments that affect the reported amount of net assets at the date of the financial statements and the reported amounts of revenues and expenses during the period.
Critical accounting policies are those that have a significant impact on the Group’s results and require the most difficult, subjective or complex judgments by management. For a full description of the Group’s accounting policies please refer to note 1 within the Group’s financial statements. Acambis’ critical accounting policies include the following:
REVENUE
Group revenue comprises the value of sales from products and income (excluding VAT and taxes, trade discounts and intra-group transactions) derived from contract research fees and license fees receivable from third parties in the normal course of business. Revenue from product sales is recognized when the risks and rewards of ownership have been transferred to the customer. Where the Group is required to undertake R&D activities any associated revenue is deferred and recognized over the period over which the services are performed. Contract research fees are recognized in the accounting period in which the related work is carried out. Milestones receivable are recognized when they fall contractually due.
Profit is recognized on long-term contracts when the final outcome can be assessed with reasonable certainty by including turnover and related costs within the income statement as contract activity progresses. Turnover is recognized according to the extent of performance under the contract. In determining the degree of contractual performance, reference is made to the costs incurred in relation to the total estimated expected costs.
The ACAM2000 smallpox vaccine contract with the CDC, awarded to Acambis in November 2001, is a fixed-fee arrangement requiring the delivery of products as well as a concurrent R&D program. As the two transactions are linked in such a way that the commercial effect cannot be understood without reference to the series of transactions as a whole, this arrangement has been treated as a single long-term contract, whose elements have not been accounted for separately as required under IAS18.
Turnover and profits are recognized according to the extent of performance under the contract, as described above. Manufacturing costs in respect of this contract are deemed to be incurred when the risks and rewards of ownership have been transferred, as described above; R&D costs are recognized as incurred.
The complexity of the estimation process and issues related to the assumptions, risks and uncertainties inherent with the application of the revenue recognition policies, including the calculation of percentage-to-completion, affect the amounts reported in the financial statements. If the business conditions were different, or if different assumptions were used in the application of this accounting policy, it is likely that materially different amounts would be reported in the financial statements.
SHARE-BASED PAYMENT TRANSACTIONS
Employees (including Directors) of the Group may receive some remuneration in the form of share-based payment transactions, whereby employees render services in exchange for shares or rights over shares (equity-settled transactions).
The cost of equity-settled transactions with employees is measured by reference to the fair value at the date at which they are granted. Fair value is determined in conjunction with an external valuer, using a binomial option pricing model for the Savings-Related Share Option Scheme (SAYE Scheme) and the Employee Share Purchase Plan (ESPP). The fair value of awards made under the 1996 Acambis Share Option Scheme (the 1996 Plan), the 1999 Acambis Share Option Plan (the 1999 Plan) and the Long Term Incentive Plan (LTIP) is measured using a binomial option pricing model adjusted to reflect the Total Shareholder Return (TSR) market based performance condition.
Page 22 of 128
For all options and awards with a TSR market based performance condition the pricing model used follows similar principles to the Monte Carlo approach to value the award and takes into account the fact that TSR vesting and share price performance are not independent.
The cost of equity-settled transactions is recognized, together with a corresponding increase in equity, over the period in which the performance conditions are fulfilled, ending on the date on which the relevant employees become fully entitled to the award (vesting date).
The cumulative expense recognized for equity-settled transactions at each reporting date until the vesting date reflects the extent to which the vesting period has expired and the number of awards that, in the opinion of the Directors, will ultimately vest. The cost is allocated to R&D costs, sales and marketing costs and administration costs on the basis of headcount.
No expense is recognized for awards that do not ultimately vest, except for awards where vesting is conditional upon a market condition. These are treated as vesting, irrespective of whether or not the market condition is satisfied, provided that all other performance conditions are satisfied.
In a profitable year, the dilutive effect of outstanding options is reflected as additional share dilution in the computation of earnings per share.
The Group has an employee share incentive plan and an employee share trust for the granting of non-transferable options to executives and senior employees. Shares in the Group held by the employee share trust are treated as treasury shares and presented in the balance sheet as a deduction from equity.
The Group has taken advantage of the transitional provisions of IFRS2 ‘Share based payments’ in respect of equity settled awards and has applied IFRS2 only to equity-settled awards granted after November, 7 2002 that had not vested on December 31, 2004.
The complexity of the estimation process and issues related to the assumptions, risks and uncertainties inherent with the application of the share-based payment transactions policies, including the calculation of the binomial option pricing model, affect the amounts reported in the financial statements. If different assumptions were used in the application of this accounting policy, it is likely that materially different amounts would be reported in the financial statements.
TAXATION
Deferred income tax is provided, using the liability method, on all temporary differences at the balance sheet date between the tax bases of assets and liabilities and their carrying amounts for financial reporting purposes.
Deferred income tax assets and liabilities are recognized for all deductible temporary differences, carry-forward of unused tax losses, to the extent that it is probable that taxable profit will be available against which the deductible temporary differences, carry-forward of unused tax losses can be utilized:
| • | Except where the deferred income tax asset or liability relating to the deductible temporary difference arises from the initial recognition of an asset or liability in a transaction that is not a business combination and, at the time of the transaction, affects neither the accounting profit nor taxable profit or loss; and |
| • | In respect of deductible temporary differences associated with investments in subsidiaries, associates and interests in joint ventures, deferred tax assets or liabilities are only recognized to the extent that it is probable that the temporary differences will reverse in the foreseeable future and taxable profit will be available against which the temporary difference can be utilized. |
In the UK and the US, the Group is entitled to a tax deduction for the amount treated as compensation on exercise of certain employee share options under each jurisdiction’s tax rules. As explained under ‘Share-based payment transactions’ above, a compensation expense is recorded in the Group’s income statement over the period from the grant date to the vesting date of the relevant options.
Page 23 of 122
As there is a temporary difference between the accounting and tax bases, a deferred tax asset is recorded. The deferred tax asset arising is calculated by comparing the estimated amount of tax deduction to be obtained in the future (based on the Company’s share price at the balance sheet date) with the cumulative amount of the compensation expense recorded in the income statement. If the amount of estimated future tax deduction exceeds the cumulative amount of the remuneration expense at the statutory tax rate, the excess is recorded directly in equity, against the profit and loss reserve.
No compensation charge is recorded in respect of options granted before 7 November 2002 or in respect of those options which have been exercised or have lapsed before December 31, 2004. Nevertheless, tax deductions have arisen and will continue to arise on these options. The tax effects arising in relation to these options are recorded directly in equity, against the profit and loss reserve.
The carrying amount of deferred income tax assets is reviewed at each balance sheet date and reduced to the extent that it is no longer probable that sufficient taxable profit will be available to allow all or part of the deferred income tax asset to be utilized.
Deferred income tax assets and liabilities are measured at the tax rates that apply to the period when the asset is realized or the liability is settled, based on tax rates (and tax laws) that have been enacted or substantively enacted at the balance sheet date. Income tax relating to items recognized directly in equity is recognized in equity and not in the income statement.
The complexity of the estimation process and issues related to the assumptions, risks and uncertainties inherent with the application of the taxation policies, including the calculation of deferred income tax assets or liabilities, affect the amounts reported in the financial statements. If the business conditions were different, or if different assumptions were used in the application of this accounting policy, it is likely that materially different amounts would be reported in the financial statements.
GOODWILL
Goodwill on acquisition is initially measured at cost, being the excess of the cost of the business combination over the acquirer’s interest in the net fair value of the identifiable assets, liabilities and contingent liabilities. The fair value of the consideration is determined by applying appropriate discounts to contingent and deferred consideration, to the level where the Group considers those liabilities will be payable. Where the consideration for the acquisition of a business includes non-interest bearing cash payments due after more than one year, the liability is recorded at its present value, after applying a discount rate that approximates to that which a lender would typically require for a similar transaction, and taking into account the risk/likelihood of the payment being made.
Where revisions are made to the expected amounts of contingent consideration payable as a result of changes to estimates, such changes are accounted for at the date of the change in estimate. Following initial recognition, goodwill is not amortized but is measured at cost less any accumulated impairment losses. Goodwill is reviewed for impairment annually or more frequently if events or changes in circumstances indicate that the carrying value may be impaired.
The complexity of the estimation process and issues related to the assumptions, risks and uncertainties inherent with the application of the goodwill policies, including the calculation of net present value of future cash flows, affect the amounts reported in the financial statements. If different assumptions were used in the application of this accounting policy, it is likely that materially different amounts would be reported in the financial statements.
PROVISIONS
Provisions are recognized when the Group has a present obligation (legal or constructive) as a result of a past event, it is probable that costs will be required to be incurred to settle the obligation and a reliable estimate can be made of the amount of the obligation. Legal fees associated with litigation arising as a result of circumstances in existence at the balance sheet date are provided for based on management’s best estimate of costs to be incurred.
The complexity of the estimation process and issues related to the assumptions, risks and uncertainties inherent with the application of the provisions policies, including the assessment of the legal outcome and the costs to be incurred, affect the amounts reported in the financial statements. If different assumptions were used in the application of this accounting policy, it is likely that materially different amounts would be reported in the financial statements.
Page 24 of 122
INVENTORY PROVISIONS
The Group assesses at each reporting date whether there is an indication that inventory may be impaired, or if the estimate of the net realisable value of the inventory is lower than the carrying value. Inventory is valued at the lower of its cost and its net realisable value, and any write down is recognised in the income statement.
The complexity of the estimation process and issues related to the assumptions, risks and uncertainties inherent with the application of the inventory provisions policies, including the assessment of net realizable value, affect the amounts reported in the financial statements. If the business conditions were different, or if different assumptions were used in the application of this accounting policy, it is likely that materially different amounts would be reported in the financial statements.
B | Liquidity and capital resources |
The Group had aggregate cash and liquid resources of £68.0m at December 31, 2005 (2004 – £101.8m), a decrease of £33.8m since the start of the year (2004 –£23.4m). During 2005, Acambis received £0.2m (2004 – £1.9m) primarily being attributable to the issue of new shares to satisfy share option exercises. Cash outflow from operating activities during the year was £29.3m (2004 – £20.7m). During 2005, Acambis made additions of short leasehold land and buildings of £3.6m (2004 – £1.5m), acquired £0.9m of laboratory and manufacturing equipment (2004 – £0.9m) and £0.7m of office equipment (2004 – £0.7m).
From incorporation through to December 31, 2005, Acambis has financed its operations primarily from equity issuances totaling £96.3m and sales of product totaling £396.9m. At December 31, 2005, Acambis held investments in Acambis ordinary shares through Acambis’ Employee Share Ownership Trust (market value at December 31, 2005 of £0.8m, 2004 – £0.2m).
At December 31, 2005, the balance on the ARILVAX overdraft facility (as explained in note 18 of the financial statements) was £4.0m ($7m) (2004 – £3.6m). At December 31, 2005, the Group did not have any undrawn borrowing facilities in respect of this overdraft facility (2004 – £nil). Given certain circumstances, this facility, may be repayable within one year. In December 2001, the Group secured lease financing for up to $40m (approximately £21m) with Baxter in respect of its Canton, MA, US manufacturing facility. At December 31, 2005, the Group had an outstanding liability of £7.1m under this financing facility (2004 – £9.9m). At December 31, 2005, the Group had $27.9m (approximately £16.3m) undrawn borrowing facilities in respect of this financing facility (2004 – £8.2m). The repayment terms of this facility are described in note 18 within the financial statements.
In May 2005 the Group acquired a leasehold of a fill/finish facility for c. £1.8m ($3m) upfront and a further c. £2.6m ($4.5m) in equal installments between 2006 and 2017. The balance relating to the discounted value of future payments is £1.7m at December 31, 2005 (2004 - £nil).
At the end of the year, capital commitments contracted but not provided for were £0.1m (2004 – £0.2m, 2003 – £0.2m).
Acambis’ future capital requirements will depend on many factors, including, but not limited to, the expenditure required to maintain the manufacturing facility, the progress of R&D programs, pre-clinical and clinical testing, the time and costs involved in obtaining regulatory approvals, the cost of filing, prosecuting and enforcing any patent claims and other intellectual property rights, competing technological and market developments, changes in its existing research relationships, ability to establish collaborative arrangements, receipt of any license fees and royalties, the acquisition of additional facilities and capital expenditure.
Acambis believes that there will be sufficient cash to fund its operations through 2006. Changes in R&D plans or other events affecting its operations, however, may result in accelerated or unexpected expenditures. If additional funds are raised by issuing equity securities, dilution to existing shareholders may result and future investors may be granted rights superior to those of existing shareholders.
Page 25 of 122
C | Research and development, patents and licenses, etc. |
BUILD A HIGH VALUE PIPELINE
Acambis’ pipeline is the principal asset for creating shareholder value. By delivering on what the Group has today – short- and medium-term projects with a range of commercial opportunities – the Group is establishing a sound base for its portfolio. By adding, over time, other products that offer significant commercial potential, Acambis can turn today’s base into a high value pipeline.
FULLY INTEGRATE FROM CONCEPT TO COMMERCIALISATION
Acambis wants to generate as much value as possible from its pipeline. In time, the Group will develop, manufacture and sell its own products wherever it can create value by doing so. For now, profits from sales of Vivotif® are already contributing to the pipeline investment and the manufacturing assets are helping us to control costs and timelines. Acambis complements its strengths where necessary through partnerships with other companies.
See also Item 10C for material contracts, and Item 7 for information on related party transactions. Contractual obligations under lease commitments are outlined in note 26 within the financial statements.
FIVE-YEAR HISTORICAL REVENUES
Five years ago, Acambis’ revenues came from external R&D funding. The US Government smallpox vaccine contract the Group won in 2001 drove revenues for the next three years. The goal now is to supplement further government contracts with recurrent revenues from product sales.
During 2005 Acambis continued to record sales of ACAM2000 smallpox vaccine to the US CDC under the 155-million dose contract. Revenue was also recorded in respect of the two MVA contracts with the NIAID.
The smallpox vaccine opportunity, initiated with the ACAM2000 155-million dose contract, highlighted to Acambis the potential for expansion by playing to existing areas of expertise. Following the US Government contract, Acambis has established a smallpox vaccine franchise that aims not only to maximize sales of ACAM2000 but also to exploit the potential of two related products, VIG and MVA.
Acambis also sells Vivotif® through its North American sales and distribution infrastructure.
E | Off balance sheet arrangements |
The Group leases its property and certain equipment under non-cancelable operating agreements, which expire at various dates until 2023. The future amounts payable under current lease commitments at December 31, 2005 were as follows:
Page 26 of 122
| | Total £m |
2006 | | 2.4 |
2007 | | 2.3 |
2008 | | 2.3 |
2009 | | 3.3 |
2010 | | 1.9 |
Thereafter | | 8.2 |
Total | | 20.4 |
F | Tabular disclosure of contractual obligations |
The following are the Group’s contractual obligations:
Payments due by period | | Total | | Less than
1 year | | 1-3 years | | 3-5 years | | More than
5 years |
2005 | | £m | | £m | | £m | | £m | | £m |
Short-term borrowings | | 4.0 | | 4.0 | | - | | - | | - |
Obligations under finance leases | | 7.1 | | 7.1 | | - | | - | | - |
Other financial liabilities | | 1.7 | | 0.1 | | 0.2 | | 0.2 | | 1.2 |
Derivative financial liabilities | | - | | - | | - | | - | | - |
Operating leases – land and buildings | | 20.1 | | 2.3 | | 4.5 | | 5.2 | | 8.1 |
Operating leases – plant and machinery | | 0.3 | | 0.1 | | 0.2 | | - | | - |
Purchase obligations | | 0.1 | | 0.1 | | - | | - | | - |
Total | | 33.3 | | 13.7 | | 4.9 | | 5.4 | | 9.3 |
Payments due by period | | Total | | Less than
1 year | | 1-3 years | | 3-5 years | | More than
5 years |
2004 | | £m | | £m | | £m | | £m | | £m |
Short-term borrowings | | 3.6 | | 3.6 | | - | | - | | - |
Obligations under finance leases | | 9.4 | | 3.1 | | 6.3 | | - | | - |
Other financial liabilities | | - | | - | | - | | - | | - |
Derivative financial liabilities | | 0.1 | | 0.1 | | - | | - | | - |
Operating leases – land and buildings | | 12.5 | | 1.7 | | 1.8 | | 1.4 | | 7.6 |
Operating leases – plant and machinery | | 0.2 | | 0.1 | | 0.1 | | - | | - |
Purchase obligations | | 0.2 | | 0.2 | | - | | - | | - |
Total | | 26.0 | | 8.8 | | 8.2 | | 1.4 | | 7.6 |
SHORT-TERM BORROWINGS
Under the terms of the agreement between Acambis and Evans Vaccines Limited (a subsidiary of Chiron Corporation, which has been acquired by Novartis AG) given certain conditions the obligation under the bank overdraft facility of £4.0m at December 31, 2005 (2004 – £3.6m) for part of the costs incurred on the ARILVAX project may be repayable within one year. The facility is underwritten by Chiron. Chiron has granted to Acambis 100% of the marketing rights to ARILVAX in the US, whilst retaining an option to buy back 50% of the profits from the US sales in return for refunding to Acambis the costs that Acambis has incurred on the ARILVAX program. The overdraft facility was fully utilized at December 31, 2005 (2004 – fully utilized) and was renewed in January 2006 for a further year.
During the year, an exchange loss of £0.4m (2004 – gain of £0.3m) was recorded in the income statement, resulting from the revaluation of this US dollar-denominated facility.
Page 27 of 122
OBLIGATIONS UNDER FINANCE LEASES
In December 2001, the Group committed to a finance lease of up to $40m (c£21m), repayable within five years, relating to the purchase and sale-and-leaseback of capital assets within the manufacturing plant. This was arranged through Baxter and was approved by shareholders in December 2001. In 2001, the Group drew down $18.6m (£14.0m) and has made no further draw-downs from the facility. The repayment schedule for the lease financing required that interest only was repaid in 2003 and capital and interest are repayable over 2004 to 2006. The Group had an option to repurchase all of the facility’s assets in December 2003, and on each anniversary thereafter, for the capital balance outstanding at that time, plus any accrued but unpaid interest due at the time, and a make-whole payment (discounted to present value) equal to the projected future interest stream payable to the end of the lease term. At December 31, 2005 the balance outstanding on the facility was £7.1m (2004-£9.4m).
OTHER FINANCIAL LIABILITIES
In May 2005, the Group acquired a leasehold of a fill/finish facility for c. £1.8m ($3m) upfront and a further c. £2.6m ($4.5m) in equal installments between 2006 and 2017. The balance relating to the discounted value of future payments is £1.7m at December 31, 2005 (2004 – £nil).
OPERATING LEASES
In 2005 the majority of the operating lease obligation total of £20.1m relates to the facility at Peterhouse Technology Park in the UK which has a total obligation of £10.0m at December 31, 2005 (2004 - £10.6m).
In March 2000, the Group entered into a sub-lease with Medivir UK Limited (Medivir) in respect of 50% of the facility at Peterhouse Technology Park in the UK. In December 2003, this sub-lease was amended, with only 45% of the facility being rented to Medivir from that point. Medivir terminated the sub-lease in September 2004. During 2005, Medivir contributed £nil (£0.2m – 2004) in operating lease rentals relating to land and buildings.
In May 2005 the Group entered into an operating lease on the Rockville fill and finish facility giving rise to an additional obligation of £3.5m at December 31, 2005 (2004 - £nil).
In October 2005 the Group extended the operating lease on the R&D/office facility at Cambridge in the US resulting in an obligation of £5.6m at December 31, 2005 (2004 - £1.0m).
PURCHASE OBLIGATIONS
At the end of the year, the Group had capital commitments contracted but not provided for in the financial statements.
Under the safe harbor provisions of the US Private Securities Litigation Reform Act of 1995, the Company cautions investors that any forward-looking statements or projections made in this document are subject to risks and uncertainties that may cause actual results to differ materially from those projected. These forward-looking statements are based on estimates and assumptions made by the management of Acambis and are believed to be reasonable, though are inherently uncertain and difficult to predict. Actual results or experience could differ materially from the forward-looking statements. Factors that may affect the Group’s operations are discussed in the operating and financial review, risk factors and the corporate governance statement sections contained within this 20-F.
Item 6 | Directors, senior management and employees |
A | Directors and senior management |
| | Alan Smith, 61, a member of the Chartered Institute of Public Finance and Accountancy, joined the Board of Acambis on November 3, 1995 as a Non-executive Director and was appointed Non-executive Chairman on May 20, 1999. The Board considers Alan to be an independent Non-executive Director. |
Page 28 of 122
| | He is Chairman of the Nominations Committee. He was Group Managing Director of Anglian Water plc until December 1997 and is currently Chairman of Avlar Bioventures Limited and Medical Devices Innovations Limited, and a Non-executive Director of CeNeS Pharmaceuticals Plc. Mr. Smith is participating in the continuing Professional Development program of the Chartered Institute of Public Finance and Accountancy. |
2 | GORDON CAMERON OBE, CHIEF EXECUTIVE OFFICER (CEO) |
| | Gordon Cameron, 40, was appointed CEO on February 23, 2004. He was originally appointed to the Board on March 1, 1997 as CFO (formerly Finance Director), having joined Acambis in 1996 from the corporate finance department at N M Rothschild where he had advised Acambis on its listing on the LSE. From March 31, 2001 until his appointment as CEO, Gordon was additionally President of Acambis’ US division, Acambis Inc. In 2004, he was appointed an Officer of the Order of the British Empire for services to the British biotechnology industry in the US. |
| | Gordon was instrumental in Acambis winning and executing on the major smallpox vaccine supply and R&D contracts with the US Government. He combines considerable financial experience with the extensive industry knowledge he has developed during more than nine years with Acambis. |
3 | DAVID LAWRENCE, CHIEF FINANCIAL OFFICER (CFO) |
| | David Lawrence, 43, was appointed to the Board on July 8, 2004 (with an official start date of August 31, 2004) from Chiron Vaccines, where he was Vice President of Finance. In his role at Chiron Vaccines, David was responsible for all aspects of finance and accounting, and also for strategic planning and business development. In particular, he played a lead role in Chiron’s acquisition of PowderJect Pharmaceuticals plc and the subsequent disposal of various non-core assets/businesses. Prior to Chiron, the majority of David’s career had been spent with GSK, which he joined in 1988. |
| | David has considerable industry knowledge and strong financial and management skills that, coupled with the experience he has gained through playing an active role in the rapid growth of Chiron, will be invaluable in the management of Acambis’ continued growth. His responsibilities at Acambis include overseeing the finance function and corporate development. |
4 | THOMAS MONATH, NON-EXECUTIVE DIRECTOR |
| | Tom Monath, 65, a qualified medical doctor, joined the Group in 1992 and was appointed to the Board as Chief Scientific Officer (CSO) on March 12, 2002. Tom stood down from his role as CSO on June 23, 2006, and at that point took up the position of Non-executive Director. The Board does not therefore consider Tom to be an independent Non-executive Director. Tom will resign from this position on September 1, 2006. |
| | Prior to joining Acambis, he worked as Colonel and Chief of the Virology Division of the US Army Medical Research Institute of Infectious Disease. Previously, during almost 20 years as Director of the CDC’s Division of Vector-Borne Infectious Diseases, he was instrumental in building the division into a key centre for research into arthropod-borne viruses such as yellow fever. |
| | Tom is responsible for the direction of Acambis’ programs to develop vaccines against infectious diseases such as smallpox, JE, West Nile and C.difficile, and led the development of Acambis’ proprietary ChimeriVax technology. He served as a member of the US National Vaccine Advisory Committee. During his career, he has published more than 300 scientific papers and six books, including a seminal work on flaviviruses. Among other external positions, he is an Adjunct Professor at Harvard School of Public Health, and President of the American Society of Tropical Medicine and Hygiene. Tom attends conferences and publishes papers and chapters with respect to maintaining his professional medical accreditation. |
5 | RANDAL CHASE, NON-EXECUTIVE DIRECTOR |
| | Randal Chase, 56, was appointed to the Board of Acambis as a Non-executive Director on October 1, 2004. The Board considers Randal to be an independent Non-executive Director. Most recently, he was President of Shire Biologics, until its sale to ID Biomedical in 2004. Previously in his career, Randal was President of North American Vaccine and President of Aventis Pasteur Canada. He has a PhD in biochemistry from the University of British Columbia. |
Page 29 of 122
| | Randal is currently Chairman of Medicago Inc, which has applied for listing on the Toronto Stock Exchange Venture Exchange, a Director of ConjuChem Inc., which is listed on the Toronto Stock Exchange and of Bioject Medical Technologies Inc. which is listed on NASDAQ. He is an Executive Director and Chairman of Molecular Templates, Inc., which is privately held. |
6 | ALAN DALBY, NON-EXECUTIVE DIRECTOR |
| | Alan Dalby, 69, became a Non-executive Director of Acambis on May 1, 1998. He is the senior independent Non-executive Director and Chairman of the Remuneration Committee. The Board considers Alan to be an independent Non-executive Director. Alan was previously an Executive Director of SmithKline, a predecessor company to GSK, and retired from the role of Chairman of Reckitt Benckiser plc in 2001. He is a Director of Alteon, Inc., a US-based biotechnology company. |
7 | PETER FELLNER, NON EXECUTIVE DIRECTOR |
| | Peter Fellner, 62, was appointed to the Board of Acambis as a Non-executive Director on February 7, 2006. The Board considers him to be an independent Non-executive Director. Peter was Celltech Group plc’s Chief Executive Officer from 1990 to 2003 and Chairman from 2003 until its acquisition by the Belgian biopharmaceutical company, UCB SA, in 2004. Before Celltech, Peter was Chief Executive Officer of Roche UK from 1986 to 1990, having previously been Director of Roche UK Research Centre from 1984 to 1986. He is Chairman of Vernalis plc and Astex Therapeutics Limited, a director of UCB SA, and a Non-executive Director of QinetiQ Group plc, Evotec AG and Bespak plc. |
He is also a director of Oxford University’s technology transfer group, Isis Innovation Limited, and a Member of the UK’s Medical Research Council.
8 | ROSS GRAHAM, NON-EXECUTIVE DIRECTOR |
| | Ross Graham, 58, was appointed to the Board of Acambis as a Non-executive Director on March 25, 2004. The Board considers him to be an independent Non-executive Director. He is Chairman of the Audit Committee, and has been identified by the Board as having recent and relevant financial experience. Ross was most recently Corporate Development Director of Misys plc, which he joined as Finance Director in 1987 at the time of its flotation, and was appointed Corporate Development Director in 1998 with Board responsibility for corporate transactions and management of strategic alliances. He stepped down from Misys’ Board of Directors at the end of 2003 after more than 16 years. Prior to his career at Misys, Ross was a Partner with Arthur Young, a predecessor firm to Ernst & Young, where he qualified as a Chartered Accountant. He is also a Non-executive Director of Wolfson Microelectronics plc, Psion plc and Patientline plc, and Non-executive Chairman of Vecta Software Corporation Ltd. Ross attends numerous courses and lectures on audit, financial, remuneration and non-executive related matters. |
9 | ELIZABETH BROWN, COMPANY SECRETARY |
| | Elizabeth Brown, 34, was appointed Company Secretary on July 1, 2002. Elizabeth is a certified accountant and joined Acambis in 1996. In her core role as Vice President of Financial Management, Elizabeth is responsible for financial performance measurement, budgeting and long-term financial planning. In addition, Elizabeth has, in the last few years, overseen the development of Acambis’ risk management systems. |
| | The directors who served during 2005 were: |
Executive: Gordon Cameron, Dr Thomas Monath and David Lawrence.
Non-executive: Alan Smith, Alan Dalby, Michael Lytton (resigned April 11, 2006), Ross Graham, Randal Chase.
Peter Fellner was appointed as a Non-executive Director on February 6, 2006.
| | The usual business address of all of the Directors is the registered office of the company, except Dr Thomas Monath whose usual business address is that of Acambis Inc, at 38 Sidney Street, Cambridge, MA in the US. |
| | In accordance with the Company’s Articles of Association, Alan Smith and Alan Dalby retired by rotation at the 2006 Annual General Meeting (AGM) and were re-elected. In addition Dr Peter Fellner, who was appointed since the 2005 AGM, was re-elected at the 2006 AGM. |
Page 30 of 122
In accordance with the Directors’ Remuneration Report Regulations 2002, a resolution to approve the Remuneration Committee’s Report was put to shareholders and approved at the 2006 AGM on June 23, 2006.
COMPONENTS OF EXECUTIVE DIRECTORS’ REMUNERATION
Set out below are the Remuneration Committee’s compensation guidelines.
BASIC SALARY AND BENEFITS
In determining the basic salary of each Director, the Committee takes into account, and intends to take into account in respect of future financial years, the individual’s responsibilities, and pay levels are set in the light of independent assessment of market practices. Basic salaries for Executive Directors are reviewed annually and compared to salary levels in a group of comparably sized biotechnology companies. The Committee also takes into consideration percentage increases for all employees when reviewing salary increases. For US-based Executive Directors, salary levels in companies of a similar size to Acambis Inc. are also reviewed for comparative purposes. Salary reviews take account of all responsibility changes. Benefits offered to all Executive Directors comprise private healthcare, life assurance, permanent health insurance, private telephone and the use of Group assets. Mr Lawrence receives a car allowance as well as a benefit relating to travel up to the point of his relocation.
ANNUAL BONUS
Bonuses are non-pensionable and based on a percentage of basic salary. In 2005, the maximum annual bonus was 75% of basic salary. The maximum 75% bonus level can only be achieved for significantly outperforming budgeted targets.
Following adoption of the new share-based incentive schemes at the 2006 AGM, (see section E Share Ownership below) the annual maximum bonus percentage was increased from 75% to 125% of salary, but with 40% of any bonus to be deferred for three years in the form of Acambis shares. The deferral will be compulsory with no matching award, and will be lost on resignation or dismissal during the deferral period.
Bonuses are paid at the discretion of the Committee in recognition of the Directors’ contributions to the success of the Group. Objectives are set that are considered to be both challenging and realistic. The performance metrics on which bonus payments are assessed are a mix of short-term financial, product development and business development targets. For 2005, the bonuses awarded to Executive Directors were determined following an evaluation of Group performance to agreed objectives. Following termination, any bonuses paid are at the discretion of the Committee.
LONG TERM INCENTIVES
All Executive Directors are eligible to participate in the Company’s share option schemes.
The performance-linked share option schemes consist of an HM Revenue and Customs-approved executive scheme and unapproved executive schemes. These schemes are described in more detail in Part E, Share Ownership.
PENSION SCHEME
In the UK, the Group operates a self-administered, defined contribution, HM Revenue and customs-approved pension scheme for the Executive Directors. The Group contributes 18% of basic salary into this scheme on behalf of each Executive Director. No other benefits are pensionable. In the US, the Group offers a 401k Savings and Retirement Plan for all employees, including Executive Directors based in the US. Participants may contribute up to 15% of their annual compensation into the plan.
Page 31 of 122
The Group can make discretionary matching contributions, up to a maximum of 3% of basic salary. Pension costs for each Director are shown under the Directors’ Remuneration section.
During 2005, the Group reviewed the impact of any changes arising from the UK pensions legislation which came into force in April 2006. As the Group operates defined contribution arrangements, no significant changes were effected, and the overall cost to the Group did not increase as a result of this legislative change.
NON-EXECUTIVE DIRECTORS’ FEES AND TERMS
The Non-executive Directors’ fees are determined, and it is intended shall be determined in future financial years, by the Board on the basis of independent advice on current levels in similar businesses. Fees are reviewed periodically. Non-executive Directors are not eligible and do not participate in pensions, incentives, bonuses or any similar payments other than out-of-pocket travel and accommodation costs in connection with the performance of their duties. Non-executive Directors’ fees comprise a basic fee plus an additional fee for chairing a committee. Consideration is given to the time commitment required of Non-executive Directors when setting their fees. Non-executive Directors’ fees are not dependent on specific meeting attendance or linked to the number of hours of time spent on Group matters. Whilst there is no set time commitment specified in Non-executive Directors’ service contracts, it is expected that they attend all relevant meetings. Non-executive Directors are entitled to their fees during any notice period.
The Board believes that it is in the Group’s best interest for Non-executive Directors to serve a minimum three-year term before retiring by rotation. Typically, Acambis expects them to serve two three-year terms, although they may be invited to continue in office for a further period.
DIRECTORS’ REMUNERATION
The total remuneration of the Directors for the year ended December 31, 2005 (shown below) comprised salaries, benefits, bonuses, pension contributions and Non-executive Director fees. During the year, no Directors waived emoluments (2004 – £nil).
The remuneration received by each Director who served during the year was as follows:
Directors | | Basic
salary/fees
£’000 | | Benefits9
£’000 | | Bonus
£’000 | | Total
2005
£’000 | | Total
2004
£’000 | | Pension
2005
£’000 | | Pension
2004
£’000 |
Executive: | | | | | | | | | | | | | | |
Gordon Cameron1 | | 344 | | 43 | | 103 | | 490 | | 423 | | 63 | | 57 |
David Lawrence2 | | 187 | | 24 | | 56 | | 267 | | 94 | | 34 | | 11 |
Nicolas Higgins3 | | – | | – | | – | | – | | 474 | | – | | 33 |
Dr Thomas Monath4, 10 | | 179 | | 8 | | 51 | | 238 | | 212 | | 4 | | 3 |
Total | | 710 | | 75 | | 210 | | 995 | | 1,203 | | 101 | | 104 |
Non-executive: | | | | | | | | | | | | | | |
Alan Smith | | 70 | | – | | – | | 70 | | 70 | | – | | – |
Dr Randal Chase5 | | 33 | | – | | – | | 33 | | 8 | | – | | – |
Alan Dalby | | 37 | | – | | – | | 37 | | 37 | | – | | – |
Michael Lytton6 | | 34 | | – | | – | | 34 | | 34 | | – | | – |
Ross Graham7 | | 37 | | – | | – | | 37 | | 29 | | – | | – |
Victor Schmitt8 | | – | | – | | – | | – | | – | | – | | – |
Total | | 211 | | – | | – | | 211 | | 178 | | – | | – |
Total | | 921 | | 75 | | 210 | | 1,206 | | 1,381 | | 101 | | 104 |
Page 32 of 122
NOTES
1 | In 2005, Mr Cameron received a benefit valued at £15,000 (2004 – £22,000) in relation to the provision by the Group of accommodation and travel whilst he was located in the US. Total remuneration in 2005 includes $352,000 which Mr Cameron received in dollars, translated at an average exchange rate of £1: $1.821. (2004 – $511,000 translated at an average rate of £1: $1.832) as a result of him residing in the US for part of the year. Mr Cameron was appointed Chief Executive Officer in February 2004, and his remuneration during 2004 therefore represents a part year as Chief Financial Officer and a part year as Chief Executive Officer. |
2 | Remuneration paid to Mr Lawrence includes a benefit valued at £10,000 (2004 – £8,000) in relation to provision by the Group of travel costs and accommodation. Remuneration for 2004 relates to the period from August 31, 2004 being his employment start date. |
3 | Mr Higgins resigned from the Board on December 31, 2004. Amounts in 2004 represent remuneration paid up until the date of his resignation plus a payment of £240,000 (gross) as compensation for loss of office. |
4 | All of Dr Monath’s remuneration was paid in dollars and has been translated at an average exchange rate of £1: $1.821. |
5 | Dr Chase was appointed to the Board on October 1, 2004. Amounts in 2004 represent fees from the date of his appointment. |
6 | Mr Lytton resigned from the Board on April 11, 2006. |
7 | Mr Graham was appointed to the Board on March 25, 2004. Amounts in 2004 represent fees from the date of his appointment. |
8 | Mr Schmitt resigned from the Board on January 21, 2004. Under the terms of his appointment he did not receive fees. |
9 | Benefits offered to all Executive Directors comprise private healthcare, life assurance, permanent health insurance and private telephone. In addition, all Executive Directors, with the exception of Dr Monath, receive a car allowance. |
10 | Dr Monath resigned as Chief Scientific Officer and became a Non-executive Director on June 23, 2006. |
The following statement describes the main principles of corporate governance and how they have been applied by Acambis.
COMPLIANCE WITH THE COMBINED CODE
The Combined Code (the code) was republished in July 2003 and restated in July 2005 by the Financial Reporting Council and incorporated the previous code (as published in 1998 by the Hampnel Committee) and related guidance that had been issued since that date: the Turnbull Guidance on Internal Control; the Smith Guidance On Audit Committees; and various items of good practice guidance from the Higgs Report. The code has been applicable for reporting years beginning on or after 1 November 2003 and, therefore, was adopted by Acambis from the 2004 financial year. The overriding principle of the code is that companies must comply with it or explain why they have not.
The following section highlights the areas where Acambis previously did not comply with the code and notes the progress made to address those areas:
CODE PROVISION | PROGRESS MADE SINCE PUBLICATION OF THE 2004 FORM 20-F |
B REMUNERATION B.2.1
A statement on whether remuneration consultants have any other connection with the Company should be available on the Acambis website. | A disclosure is made within the Corporate Governance Statement, ‘Remuneration Committee’ section. A statement has been available on the Acambis website since early 2005. |
C ACCOUNTABILITY AND AUDIT
C.3.4 Arrangements should be in place for the reporting and management of concerns raised by staff about possible financial or other improprieties. | In November 2004, the Audit Committee approved a whistleblowing policy. The procedure was developed during 2005 and rolled out to the Group in early 2006. |
STATEMENT OF APPLYING THE PRINCIPLES OF GOOD GOVERNANCE
Acambis has applied the Principles of Good Governance set out in Section 1 of the Combined Code by complying with the Code of Best Practice, with the exception of those points reported above.
Page 33 of 122
Further explanation of how the principles have been applied is set out below and, in relation to Directors’ remuneration, in the ‘Components of Executive Directors Remuneration’ above.
THE BIA CODE OF BEST PRACTICE (BIA CODE)
Acambis, as a member of the BioIndustry Association (BIA), has also complied with the principles in the BIA Code and maintains and develops procedures to support compliance with its specific provisions. The BIA Code was introduced in 1999, is obligatory for all BIA members, and includes principles and provisions relating to corporate governance matters, access to external advice, confidentiality, dealings in a company’s shares and standards of public announcements. It is intended to operate by reference to the particular circumstances of bioscience companies in support of the Combined Code.
THE BOARD AND COMMITTEES
BOARD OF DIRECTORS
At December 31, 2005 the Board comprised the Chairman, three Executive Directors and four independent Non-executive Directors. It meets, in person, at least six times a year, with additional meetings as required. The Chairman also meets with just the Non-executive Directors, without the Executive Directors being present and the Non-executive Directors meet without the Chairman being present. The Board has identified Alan Dalby as the Senior Independent Director. During 2005, the Board met eight times. It oversees and approves Acambis’ business and commercial strategy major transactions, financial statements and operating and capital expenditure budgets, and monitors progress. The information provided to the Board includes strategic and operational reviews, management accounting summaries and specific reports that provide details in respect of the ongoing running of the business. The Executive Directors are fully involved with the management of the Group’s strategic direction. A formal schedule of matters reserved for the Board exists and is available on the Company’s website.
The Board is appraised of views of the investment community via biannual independent perception audits and weekly updates on analyst publications. All Directors have access to professional advice and training at the Company’s cost and to the services of the Company Secretary in the furtherance of their duties. The Board ensures that all newly appointed Directors receive a formal induction including, but not limited to, latest analyst reports, shareholder perception reports, Board and committee minutes, meetings with senior management and internal corporate literature.
The Board delegates the day-to-day responsibility of managing the Group to a number of committees, details of which are set out below. Written terms of reference exist for the Audit, Remuneration and Nominations Committees. These were available during the year, and are now published on the Company’s website.
AUDIT COMMITTEE
The Audit Committee currently consists of Ross Graham and Randal Chase, who are both independent Non-executive Directors, and is chaired by Ross Graham. It examines and reviews, on behalf of the Board, internal financial controls, financial and accounting policies and practices, the form and content of financial reports and statements, compliance with corporate governance best practice and the appointment and work of the external auditors. In 2005 the Company appointed Ernst & Young LLP to review the tax strategy for the Group. The Audit Committee reviews non-audit services provided by the external auditors on an ongoing basis to ensure that auditor objectivity and independence is safeguarded. In advance of any non-audit service engagements, the Audit Committee reviews whether objectivity and independence may be impaired and where appropriate, engages alternative external accountants. The Audit Committee reviews the type of service and fee level in this respect. The policy to ensure that the external auditors do not provide prohibited services was formalized in early 2005. The Audit Committee reports to the Board on these matters. The external auditors, PwC, have provided the Company written assurances under International Standard on Auditing (UK and Ireland) 260 ‘Communication of audit matters to those charged with governance’ and Independence Standards Board Standard No. 1, ‘Independence Discussions with Audit Committees’, that they are independent accountants with respect to the Company, within the meaning of UK and SEC regulatory and professional requirements, and that the objectivity of the audit engagement partner and the audit staff is not impaired.
Page 34 of 122
The CEO, the CFO and the external auditors may be in attendance at meetings. The Audit Committee meets, as a minimum, four times a year and at least once during the year without any Executive Directors present. During 2005, the Audit Committee met six times.
REMUNERATION COMMITTEE
The Remuneration Committee is made up of all of the independent Non-executive Directors and is chaired by Alan Dalby. It determines, on behalf of the Board, the Group’s policy for executive remuneration and the individual remuneration packages for the Executive Directors. The CEO may be in attendance at meetings, except when his own remuneration is being considered. The Committee met twice in 2005, and has access to professional advice in the furtherance of its duties. During 2005, the Remuneration Committee continued to work with Towers Perrin and also appointed The Hay Group to provide a Group-wide review of remuneration policy and strategy.
NOMINATIONS COMMITTEE
The Nominations Committee comprises the Chairman and all of the independent Non-executive Directors and is chaired by Alan Smith. It has responsibility for proposing to the Board any new appointments of both Executive and Non-executive Directors. The Chairman would not chair the Nominations Committee if it were dealing with the appointment of the successor to the Chairman. The Nominations Committee did not meet during 2005, and no appointments were made to the Board during that year.
OPERATIONAL MANAGEMENT
The Board delegates operational management to an Executive Committee made up of the Executive Directors, the Senior Vice President, Clinical Operations and Regulatory Affairs and the Senior Vice President, Operations. It is chaired by the CEO, meets on a monthly basis and makes recommendations to the Board.
DIRECTORS’ SERVICE CONTRACTS
Details of the service contracts of those who served as Directors during the year are:
Director | Contract
date | Notice period |
Executive: | | |
Gordon Cameron | Mar 1, 97 | 12 months |
David Lawrence | Jul 8, 04 | 12 months |
Dr Thomas Monath1,4 | Mar 12, 02 | 12 months |
Non-executive: | | |
Alan Smith2,4 | Jan 1, 98 | 3 months |
Dr Randal Chase | Oct 1, 04 | 3 months |
Alan Dalby2,4 | Mar 25, 98 | 3 months |
Ross Graham | Mar 25, 04 | 3 months |
Michael Lytton3 | Mar 12, 01 | 3 months |
NOTES
1. | Dr Monath resigned as Chief Scientific Officer and became a Non-executive Director on June 23, 2006. |
2 | Mr Smith and Mr Dalby faced re-election as Directors of the Company at the 2006 AGM, being Directors who are retiring by rotation in accordance with the Articles of Association of the Company. The resolutions were passed by shareholders on June 23, 2006. |
3. | Mr Lytton resigned from the Board on April 11, 2006. |
4. | The service contracts for these Directors were reviewed and updated in March 2005 to bring them in line with best practice. |
All Executive Directors have rolling contracts with 12-month notice periods, in line with current best practice. On early termination of contract, an Executive Director would be entitled to basic salary and benefits for the notice period.
The Remuneration Committee believes that, in the event of early termination of an Executive Director’s contract, it is appropriate to examine the specific circumstances of each case. Where appropriate, the Committee may agree to a phased payment of compensation over a fixed term.
Page 35 of 122
During this term, if the Executive Director were to find a new position the principle of mitigation would apply and payments would cease. The Committee does, however, reserve the right to make a payment in lieu of any period of notice.
The Board believes that it is in the Company’s best interest for Executive Directors to serve a minimum three-year term before retiring by rotation. Under the terms of their contracts, Non-executive Directors do not take any part of their fees in the form of Acambis shares.
EXTERNAL APPOINTMENTS
The Remuneration Committee recognizes that Executive Directors may be invited to take up other non-executive directorships or public service appointments and that these can broaden the experience and knowledge of the Director, from which the Group would benefit. Accordingly, subject to Board approval, they may accept non-executive appointments, as long as these are not likely to lead to a conflict of interest. They are also allowed to retain any fees paid under such appointments. During 2005, no Executive Directors held any non-executive directorships.
The average monthly number of employees during the year (including Executive Directors) was:
| UK
Number | US
Number | 2005
Number | 2004
Number |
| | | | |
Research and development | 8 | 93 | 101 | 118 |
Sales and marketing | 3 | 19 | 22 | 19 |
Manufacturing | - | 90 | 90 | 87 |
Administration | 19 | 43 | 62 | 65 |
| 30 | 245 | 275 | 289 |
At December 31, 2005, the Group had 285 employees (2004 – 270) and the Company had three employees, all of whom were Directors (2004 – four).
LONG-TERM SHARE INCENTIVE ARRANGEMENTS
The Committee principally seeks to incentivise Executive Directors by offering participation in share-based long-term incentive schemes.
Executive Directors currently participate in grants of share options under the Acambis 1995 savings-related share option scheme, the Acambis 1996 Approved Share Option scheme, the Acambis 1999 Share Option Plan and in grants of performance shares under the Acambis Share Incentive Plan. These plans and the performance conditions that apply to awards under these plans are described in more detail below.
During 2005 Executive Directors were eligible to receive an annual grant of options of up to one times basic salary per annum (granted in two half-yearly tranches) and an annual grant of performance shares of up to one times basic salary per annum.
At the beginning of 2004, the Committee reviewed the performance conditions applicable to share options and determined that there would be no retesting of performance conditions for options granted from 2004 onwards. In the 2004 Report on Form 20-F, the Committee acknowledged that the performance conditions applicable to its long-term incentives were the same for share options and performance shares. At the 2006 AGM certain changes to its long-term incentives offered, were adopted, which addressed this point.
Page 36 of 122
The Committee acknowledges the importance of updating shareholders on the current performance of grants made to Executive Directors for share options and performance shares against pre-set conditions. This information is provided in the Directors’ remuneration section of this report.
Following the approval granted at the 2003 AGM to revise the dilution limits of issued ordinary share capital of the Company from time to time to 5% over a five-year period, to date the Company has, on average, remained within the 1% per annum limit agreed. A recommendation was passed at the 2006 AGM to amend this limit to allow for a 4.5% dilution over any three-year period whilst remaining within an overall 10% dilution limit over a 10-year period.
All Executive Directors are eligible to participate in the Company’s share-based incentive schemes.
The performance-linked share option schemes consist of an HM Revenue and Customs-approved executive scheme and unapproved executive schemes.
At the 2006 AGM the following new share-based incentive schemes were approved;
| • | the Acambis 2006 Approved Share Option Plan providing for the grant of options which qualify for favorable UK tax treatment (the Approved Plan) |
| • | the Acambis 2006 Unapproved Share Option Plan providing for the grant of share options and Stock Settled Stock Appreciation Rights (defined below) (SSSARs) to employees (the Unapproved Plan) and |
| • | the Acambis 2006 Deferred Bonus Plan providing for part of an executive’s bonus to be taken in the form of shares, the entitlement to which will be deferred (the Deferred Bonus Plan). |
It is intended that no more awards will be made under the Acambis 1999 Unapproved Share Option Scheme, or the Acambis Long Term Incentive Share Plan (LTIP). Awards under the LTIP will be replaced by enhanced share option grants and the Deferred Bonus Plan. The Remuneration Committee believes that these changes place a greater emphasis on absolute growth in shareholder value whilst also encouraging retention of key executives.
The ability to grant SSSARs has been introduced as a way of enabling the Company to make better use of new issue shares by reducing the number of shares required to provide the same net-of-tax gain to employees as an equivalent share option. As employees will not be obliged to sell any of the shares obtained on the exercise of an SSSAR to fund an immediate tax liability, the grant of SSSARs is also intended to aid the retention of shares. No SSSARs will be granted under the Approved Plan since, in normal circumstances, there is no UK tax liability on the exercise of options under the Approved Plan.
Grants made under the Approved Plan and the Unapproved Plan in 2006 will be at the discretion of the Committee and their exercise will be subject to performance conditions relating to the performance of Acambis’ total shareholder return (TSR) compared with a comparator group of other companies within the industry.
These companies are:
AGI Therapeutics plc | | Innovata plc |
Allergy Therapeutics plc | | NeuTec Pharma PLC |
Alizyme plc | | Oxford BioMedica plc |
Antisoma plc | | Prostrakan Group plc |
ARK Therapeutics Group PLC | | Protherics PLC |
Axis-Shield plc | | Sinclair Pharma plc |
Cambridge Antibody Technology Group PLC | | SkyePharma PLC |
Dechra Pharmaceuticals PLC | | Vectura Group PLC |
Goldshield Group PLC | | Vernalis Group plc |
GW Pharmaceuticals plc | | XTL Biopharmaceuticals Ltd |
Page 37 of 122
These companies are all the biotechnology companies listed on the London Stock Exchange (LSE) and Alternative Investment Market (AIM) with a market capitalization greater than £60m but excluding Alliance UniChem Plc, AstraZeneca PLC, GSK plc and Shire Pharmaceuticals Group plc. The Committee has chosen this group as being the most appropriate for Acambis. As before, during 2005 the Committee did consider including selected US biotechnology companies within the TSR comparator group and again concluded that they did not consider this appropriate given the Group is primarily compared to other UK-based biotechnology companies. This will continue to be reviewed in future years.
Performance-related options or SSSARs would vest in full only if Acambis were ranked in the top quartile of the comparator group. If Acambis were ranked below the median level, options or SSSARs would lapse. At a median ranking, 30% of the options or SSSARs would vest, whilst a ranking between the median and top quartile would vest on a straight-line basis between 30% and 100%.
In addition, the exercise of any options or SSSARs will be conditional on the Remuneration Committee being satisfied that there has been an overall improvement in the Company’s underlying performance over the relevant period. There will be no retesting of performance conditions.
The introduction of the Deferred Bonus Plan is intended as an aid to retention of key senior executives. For Executive Directors, 40% of any annual bonus earned with respect to the relevant performance criteria will be awarded in the form of Acambis shares, which will vest after a three-year period. Awards under the Deferred Bonus Plan will not be performance linked but will not vest if the executive is dismissed or leaves voluntarily during the three-year vesting period.
For the purposes of the TSR calculation, the Company’s TSR will be averaged over the three months preceding the commencement of the period and the three months preceding a measurement date to ensure that results are not influenced by short-term volatility. TSR calculations are performed by an independent party.
EXECUTIVE DIRECTORS’ SHARE OWNERSHIP GUIDELINES
The Committee encourages Executive Directors to build and maintain substantial interests in Acambis shares, thereby aligning their interests with other shareholders. During 2005, all Executive Directors increased their shareholdings in the Company. Until April 2006, the Committee had agreed not to introduce formal share ownership guidelines. In April 2006, the Remuneration Committee agreed to formalize share ownership guidelines for Executive Directors. The new policy encourages Directors to build and maintain a shareholding of 100% of salary, recognizing that fluctuations in share price will cause the actual percentage to vary. It is envisaged that this shareholding will be built up over time through share purchases and through retaining a portion of net gains under the Company’s long-term incentive plans.
DIRECTORS’ INTERESTS IN SHARES
The Directors who served during the year had the following beneficial interests in the shares of the Company:
| | Number of ordinary
10p shares held
at Dec 31, 05 | Number of ordinary
10p shares held
at Dec 31, 04 |
Gordon Cameron1 | 283,442 | 278,442 |
Dr Randal Chase | 10,000 | – |
Alan Dalby | 5,000 | 5,000 |
Ross Graham | 6,128 | 6,128 |
David Lawrence2 | 800 | – |
Michael Lytton4 | 21,789 | 18,022 |
Dr Thomas Monath3 | 70,842 | 60,842 |
Alan Smith | 1,800 | 1,800 |
Page 38 of 122
| NOTES |
1 | 40,885 of the shares owned by Mr Cameron are held in trust on his behalf by the Trustees of the Acambis Employees’ Trust (2004 – 35,885 shares). |
2 | Mr Lawrence holds 800 shares on behalf of certain family members (connected persons). |
3 | 10,000 of the shares owned by Dr Monath are held in trust on his behalf by the Trustees of the Acambis Employees’ Trust (2004 – Nil). Dr Monath resigned as Chief Scientific Officer and became a Non-executive Director on June 23, 2006. |
4 | Mr Lytton resigned from the Board on April 11, 2006. |
Individually, each of the Directors beneficially owns less than 1% of the total issued share capital. As at June 26, 2006, and at December 31, 2005 the Directors had no interests in shares of any other Group company.
On March 24, 2006, Mr Lawrence purchased 7,500 shares for himself and on behalf of certain family members and Dr Fellner (appointed to the Board on February 6, 2006) purchased 14,000 shares. Except for these purchases, there have been no changes in the interests of the current Directors in the share capital of the Company since December 31, 2005.
The Executive Directors also have an interest as potential beneficiaries in the 84,972 ordinary shares held at June 26, 2006 by the Trustees of the Acambis Employees’ Trust.
DIRECTORS’ INTERESTS IN SHARE OPTIONS AND PERFORMANCE CONDITIONS
The Directors who held office at December 31, 2005 hold options to acquire ordinary shares of the Company under the Acambis 1996 Approved Share Option Scheme (1996 Scheme), the Acambis 1995 Savings-Related Share Option Scheme (SAYE Scheme) and the Acambis 1999 Share Option Plan (1999 Plan) as follows:
Director | | Scheme | | | Jan 1, 2005 | | Granted | | Dec 31, 2005 | | Exercise
price | | Earliest
date of
exercise | | Expiry
date | | % performance
conditions
met at
Dec 31, 056 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Gordon Cameron | | 1996 | 1 | | 17,685 | | – | | 17,685 | | £1.70 | | Dec 20, 99 | | Dec 20, 06 | | 100% |
| | 1999 | 2 | | 13,911 | | – | | 13,911 | | £3.33 | | Dec 31, 04 | | Dec 31, 11 | | 100% |
| | 1999 | 2 | | 30,545 | | – | | 30,545 | | £3.04 | | Apr 26, 05 | | Apr 26, 12 | | 53% |
| | 1999 | 2 | | 39,116 | | – | | 39,116 | | £2.33 | | Sep 26, 05 | | Sep 26, 12 | | 61% |
| | 1999 | 3 | | 27,469 | | – | | 27,469 | | £3.23 | | May 14, 06 | | May 14, 13 | | Nil |
| | 1999 | 3 | | 32,561 | | – | | 32,561 | | £2.76 | | Dec 19, 06 | | Dec 19, 13 | | Nil |
| | 1999 | 4 | | 43,350 | | – | | 43,350 | | £3.46 | | Mar 24, 07 | | Mar 12, 14 | | Nil |
| | 1999 | 4 | | 60,440 | | – | | 60,440 | | £2.73 | | Oct 12, 07 | | Oct 12, 14 | | Nil |
| | 1999 | 4 | | – | | 78,538 | | 78,538 | | £2.19 | | May 31, 08 | | May 31, 15 | | Nil |
| | 1999 | 4 | | – | | 68,525 | | 68,525 | | £2.51 | | Sep 12, 08 | | Sep 12, 15 | | Nil |
| | SAYE | 5 | | 5,250 | | – | | 5,250 | | £1.80 | | Dec 1, 05 | | Jun 1, 06 | | N/A |
| | SAYE | 5 | | – | | 4,651 | | 4,651 | | £2.01 | | Dec 1, 08 | | May 31, 09 | | N/A |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total | | | | | 270,327 | | 151,714 | | 422,041 | | | | | | | | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
David Lawrence | | 1996 | 4 | | 10,989 | | – | | 10,989 | | £2.73 | | Oct 12, 07 | | Oct 12, 14 | | Nil |
| | 1999 | 4 | | 117,216 | | – | | 117,216 | | £2.73 | | Oct 12, 07 | | Oct 12, 14 | | Nil |
| | 1999 | 4 | | – | | 42,808 | | 42,808 | | £2.19 | | May 31, 08 | | May 31, 15 | | Nil |
| | 1999 | 4 | | – | | 37,350 | | 37,350 | | £2.51 | | Sep 12, 08 | | Sep 12, 15 | | Nil |
| | SAYE | 5 | | – | | 4,651 | | 4,651 | | £2.01 | | Dec 1, 08 | | May 31, 09 | | N/A |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total | | | | | 128,205 | | 84,809 | | 213,014 | | | | | | | | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Dr Thomas | | 1999 | 2 | | 30,403 | | – | | 30,403 | | £3.04 | | Apr 26, 05 | | Apr 26, 12 | | 53% |
Monath7 | | 1999 | 2 | | 38,575 | | – | | 38,575 | | £2.33 | | Sep 26, 05 | | Sep 26, 12 | | 61% |
| | 1999 | 3 | | 26,993 | | – | | 26,993 | | £3.23 | | May 14, 06 | | May 14, 13 | | Nil |
| | 1999 | 3 | | 30,752 | | – | | 30,752 | | £2.76 | | Dec 19, 06 | | Dec 19, 13 | | Nil |
| | 1999 | 4 | | 23,470 | | – | | 23,470 | | £3.46 | | Mar 24, 07 | | Mar 24, 14 | | Nil |
| | 1999 | 4 | | 31,834 | | – | | 31,834 | | £2.73 | | Oct 12, 07 | | Oct 12, 14 | | Nil |
| | 1999 | 4 | | – | | 40,709 | | 40,709 | | £2.19 | | May 31, 08 | | May 31, 15 | | Nil |
| | 1999 | 4 | | – | | 35,995 | | 35,995 | | £2.51 | | Sep 12, 08 | | Sep 12, 15 | | Nil |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total | | | | | 182,027 | | 76,704 | | 258,731 | | | | | | | | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Page 39 of 122
NOTES
1 | The performance condition for those options granted under the 1996 Scheme until the end of 2000 is that either: |
a) | the percentage growth in the Company’s share price over the three years from the date of grant must exceed the percentage growth in the total return for the FTSE All-Share index over that three-year period; or |
b) | that the average percentage share price movements of the Company over each of the three years beginning on a date not earlier than the grant date and ending on the date of exercise must exceed the average movements in the FTSE All-Share Index over each of those three years. |
2 | The performance condition for those options granted until under the 1999 Plan compares the Company’s TSR to the TSR of a chosen group of pharmaceutical and biotechnology companies over a three-year period. A median ranking must be achieved before any part of the option may be exercised (50% of the option) and an upper quartile ranking must be achieved for the option to vest in full. This condition if not initially achieved in full can be further measured over a four- or five-year period measured from the same fixed-base point. |
3 | The performance condition for those options granted under the 1999 Plan is the same as that outlined in note 2 above, except that only 30% of the option may be exercised if the Company achieves a median ranking. Performance can only be re-measured once over a four-year period and there is also a requirement before the option can be exercised for the Committee to be satisfied with the Company’s underlying financial performance over the performance period. |
4 | The performance condition for these options granted under the 1996 Scheme and the 1999 Plan is the same as that outlined in note 3, except that the performance is measured only once, at the end of the three-year period. |
5 | No performance conditions apply to SAYE options. |
6 | Data in this column is intended to illustrate the percentage of awards which would have vested at December 31, 2005 based on the performance conditions applying to those grants. Should the awards have vested at December 31, 2005, a time apportionment factor would also apply based on the period of time from the date of award to December 31, 2005, where the full three years to vest had not been reached. These data are unaudited |
7 | Dr Monath resigned as Chief Scientific Officer and became a Non-executive Director on June 23, 2006. |
All of the above options were granted for nil consideration and are held over 10p ordinary shares in the Company. The market value of the options at the time of grant are as detailed in the ‘Exercise price’ column. The market price of shares at December 31, 2005 was 207.0p and the range during the year was 203.5p to 283.0p per share.
Further information on each of the Company’s share option schemes, including the number of options outstanding, exercise prices and exercise periods, is set out in note 25 to the financial statements.
LONG-TERM SHARE INCENTIVE PLAN
Awards have been made to Executive Directors of the Company under the LTIP1 as follows:
| | | | | | | | | | | | | | | | % performance |
| | | | | | | | | | | | Value | | Award | | Vesting | | conditions
met at
Dec 31, |
Directors | | Jan 1, 2005 | | Awarded | | Vested | | Lapsed | | Dec 31, 2005 | | Vested £ | | date | | date | | 20059 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Gordon | | 59,366 | 2,3 | – | | (20,600 | ) | (38,766 | ) | – | | 45,320 | | Apr 22, 02 | | Apr 22, 05 | | N/A |
Cameron | | 54,939 | 2 | – | | – | | – | | 54,939 | | – | | May 14, 03 | | May 14, 06 | | Nil |
| | 86,704 | 4 | – | | – | | – | | 86,704 | | – | | Mar 24, 04 | | Mar 24, 07 | | Nil |
| | 8,971 | 5 | – | | – | | – | | 8,971 | | – | | Oct 05, 04 | | Oct 05, 06 | | 100% |
| | – | | 1,250 | 6 | – | | – | | 1,250 | | – | | May 27, 05 | | May 27, 07 | | 100% |
| | – | | 157,077 | 4,7 | – | | – | | 157,077 | | – | | May 31, 05 | | May 31, 08 | | Nil |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total | | 209,980 | | 158,327 | | (20,600 | ) | (38,766 | ) | 308,941 | | 45,320 | | | | | | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
David Lawrence | | – | | 85,616 | 4,7 | – | | – | | 85,616 | | – | | May 31, 05 | | May 31, 08 | | Nil |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total | | | | 85,616 | | – | | – | | 85,616 | | | | | | | | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Dr | | 59,090 | 2,3 | – | | (20,504 | ) | (38,586 | ) | – | | 45,109 | | Apr 22, 02 | | Apr 22, 05 | | N/A |
Thomas | | 53,987 | 2 | – | | – | | – | | 53,987 | | – | | May 14, 03 | | May 14, 06 | | Nil |
Monath10 | | 46,943 | 4 | – | | – | | – | | 46,943 | | – | | Mar 24, 04 | | Mar 24, 07 | | Nil |
| | – | | 2,500 | 8 | – | | – | | 2,500 | | – | | May 27, 05 | | May 27, 07 | | 100% |
| | – | | 81,418 | 4,7 | – | | – | | 81,418 | | – | | May 31, 05 | | May 31, 08 | | Nil |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total | | 160,020 | | 83,918 | | (20,504 | ) | (38,586 | ) | 184,848 | | 45,109 | | | | | | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Page 40 of 122
NOTES
1 | The exercise price for all awards made under the LTIP is £1.00 in total for the exercise of any number of shares comprised in an award. All LTIP awards are held over ordinary 10p shares in the Company. |
2 | The performance condition for these awards compares the Company’s TSR to the TSR of a chosen group of pharmaceutical and biotechnology companies over a three-year period. A median ranking must be achieved before any part of the award may vest (30% of the award) and an upper quartile ranking must be achieved for the award to vest in full. After three years, vested plan shares may be left in the Trust and participants can then receive a grant of a further one matching share for each four plan shares so deposited. The matching shares will vest provided the participant remains employed and does not withdraw those plan shares for a further two years. The matching award component was not offered after 2003. |
3 | These awards were made on April 22, 2002, at which time the share price was 321.0p per share. On April 22, 2005, these awards vested at which time the share price was 220.0p per share. Following the measurement of the performance condition, 34.7% of the options vested, and the balance lapsed. These awards were exercised on May 27, 2005. |
4 | The performance condition for these awards compares the Company’s TSR to the TSR of a chosen group of pharmaceutical and biotechnology companies over a three-year period. A median ranking must be achieved before any part of the award may vest (30% of the award) and an upper quartile ranking must be achieved for the award to vest in full. |
5 | Following the exercise of an LTIP award on 5 October, 2004, Mr Cameron elected to leave 35,885 of those plan shares with the Trust. Under the rules of the Plan, Mr Cameron is entitled to receive an additional 8,971 shares, one matching share for each four plan shares so deposited, so long as he retains those shares in the Trust for a period of two years from date of award. |
6 | Following the exercise of an LTIP award on May 27, 2005, Mr Cameron elected to leave 5,000 of those plan shares with the Trust. Under the rules of the Plan, Mr Cameron is entitled to receive an additional 1,250 shares, one matching share for each four plan shares so deposited, so long as he retains those shares in the Trust for a period of two years from date of award. |
7 | These awards were made on 31 May 2005, at which time the share price was 217.75p per share. |
8 | Following the exercise of an LTIP award on May 27, 2005, Dr Monath elected to leave 10,000 of those plan shares with the Trust. Under the rules of the Plan, Dr Monath is entitled to receive an additional 2,500 shares, one matching share for each four plan shares so deposited, so long as he retains those shares in the Trust for a period of two years from date of award. |
9 | Data in this column is intended to illustrate the percentage of the awards which would have vested at December 31 2005 based on the performance conditions applying to those grants. Should the awards have vested at December 31 2005, a time apportionment factor would also apply based on the period of time from the date of award to December 31, 2005, where the full three years to vest had not been reached. These data are unaudited. |
10 | Dr Monath resigned as Chief Scientific Officer and became a Non-executive Director on June 23, 2006 |
GAINS MADE BY DIRECTORS ON SHARE OPTIONS AND LTIPS
The table below shows gains made by individual Directors from the exercise of share options and LTIPs. The gains are calculated as at the exercise date, although the shares may have been retained.
| | 2005 | | 2004 | |
| | £000 | | £000 | |
Gordon Cameron | | 45 | | 740 | |
Nicolas Higgins (resigned December 31, 2004) | | – | | 574 | |
Dr Thomas Monath (resigned as CSO on June 23, 2006) | | 45 | | 497 | |
Total gains on share options and LTIPs | | 90 | | 1,811 | |
ACAMBIS’ TSR PERFORMANCE
Acambis’ TSR performance is shown against a comparator group of all pharmaceutical and biotechnology
companies listed on LSE and AIM with a market capitalization greater than £60m but excluding Alliance
UniChem Plc, AstraZeneca PLC, GSK plc and Shire Pharmaceuticals Group plc. This index has been chosen as Acambis is a constituent of this sector. These companies are:
Page 41 of 122
AGI Therapeutics plc | | Innovata plc |
Allergy Therapeutics plc | | NeuTec Pharma PLC |
Alizyme plc | | Oxford BioMedica plc |
Antisoma plc | | Prostrakan Group plc |
Ardana plc | | Protherics PLC |
ARK Therapeutics Group PLC | | Sinclair Pharma plc |
Axis-Shield plc | | SkyePharma PLC |
Cambridge Antibody Technology Group PLC | | Vectura Group PLC |
Dechra Pharmaceuticals PLC | | Vernalis Group plc |
Goldshield Group PLC | | XTL Biopharmaceuticals Ltd |
GW Pharmaceuticals plc | | |
The following table details the five-year rebased TSR performance of Acambis and its chosen index.
| | | | Pharmaceuticals | |
| | Acambis | | & Biotech Index | |
December 31, 2000 | | 100 | % | 100 | % |
December 31, 2001 | | 339 | % | 55 | % |
December 31, 2002 | | 268 | % | 27 | % |
December 31, 2003 | | 295 | % | 41 | % |
December 31, 2004 | | 243 | % | 41 | % |
December 31, 2005 | | 200 | % | 44 | % |
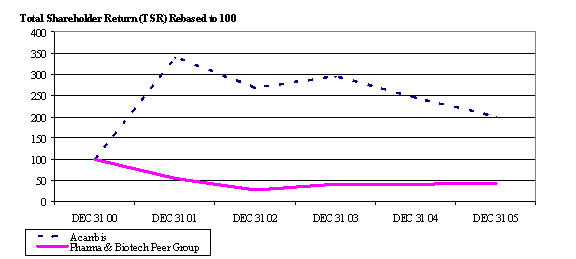
This graph illustrates the TSR performance (share price growth plus dividends paid) of Acambis compared to a ‘broad equity market index’ over the past five years, as required by legislation. Acambis’ TSR performance is shown against a peer group of pharmaceutical and biotechnology companies, comprising LSE- and AIM-listed companies with a market capitalization of over £60m, excluding Alliance UniChem Plc, AstraZeneca plc, GSK plc and Shire Pharmaceuticals Group plc. This index has been chosen as the most appropriate form of ‘broad equity market index’ against which the Company’s performance should be graphed, as Acambis is a constituent of this sector.
SHARE OPTION SCHEMES
The Group operates several share option schemes. Options outstanding under the various schemes are as follows:
Page 42 of 122
Scheme | | Jan 1, 04 ’000 | | Granted ’000 | | Exercised ’000 | | Lapsed ’000 | | Dec 31, 04 ’000 | |
19951 | | 5 | | – | | (5 | ) | – | | – | |
19962 | | 318 | | 70 | | (113 | ) | (42 | ) | 233 | |
19993 | | 3,925 | | 864 | | (1,277 | ) | (339 | ) | 3,173 | |
Scheme | | Jan 1, 04 ’000 | | Granted ’000 | | Exercised ’000 | | Lapsed ’000 | | Dec 31, 04 ’000 | |
SAYE4 | | 192 | | 24 | | (105 | ) | (6 | ) | 105 | |
ESPP5 | | 79 | | 20 | | – | | (14 | ) | 85 | |
1990 US6 | | 167 | | – | | (46 | ) | – | | 121 | |
1995 US7 | | 190 | | – | | (35 | ) | – | | 155 | |
Total | | 4,876 | | 978 | | (1,581 | ) | (401 | ) | 3,872 | |
| | | | | | | | | | | |
Scheme | | ’000 | | ’000 | | ’000 | | ’000 | | ’000 | |
19962 | | 233 | | 36 | | (10 | ) | (59 | ) | 200 | |
19993 | | 3,173 | | 806 | | (104 | ) | (342 | ) | 3,533 | |
SAYE4 | | 105 | | 38 | | (12 | ) | (50 | ) | 81 | |
ESPP5 | | 85 | | 50 | | – | | (60 | ) | 75 | |
1990 US6 | | 121 | | – | | – | | (107 | ) | 14 | |
1995 US7 | | 155 | | – | | – | | (28 | ) | 127 | |
Total | | 3,872 | | 930 | | (126 | ) | (646 | ) | 4,030 | |
NOTES
1 | The Acambis 1995 Unapproved Share Option Scheme |
2 | The Acambis 1996 Approved Share Option Scheme |
3 | The Acambis 1999 Share Option Plan |
4 | The 1995 Savings-Related Share Option Scheme |
5 | The Employee Stock Purchase Plan set up in 2003 for US-based employees. This plan is similar to the UK Save-As-You-Earn scheme |
6 | The Acambis 1990 Stock Incentive Plan |
7 | The Acambis 1995 Stock Incentive Plan |
Year of grant | | Weighted average
exercise price | | Period exercisable
in normal
circumstances | | Number
outstanding | |
1996 | | | $26.02 | | 1999–2006 | | 30,547 | |
1996 | | | £1.70 | | 1999–2006 | | 17,685 | |
1997 | | | $4.89 | | 2000–2007 | | 105,443 | |
1999 | | | $1.68 | | 2002–2009 | | 5,090 | |
1999 | | | £0.36 | | 2002–2009 | | 85,434 | |
2000 | | | £0.92 | | 2003–2006 | | 250,000 | |
2000 | | | £0.96 | | 2003–2010 | | 3,600 | |
2001 | | | £1.25 | | 2004–2006 | | 208,000 | |
2001 | | | £3.33 | | 2004–2006 | | 19,520 | |
2001 | | | £1.38 | | 2004–2011 | | 258,201 | |
2002 | | | £2.46 | | 2005–2006 | | 237,152 | |
2002 | | | £1.80 | | 2005–2006 | | 16,065 | |
2002 | | | £2.62 | | 2005–2012 | | 363,419 | |
2003 | | | £3.26 | | 2006–2007 | | 265,716 | |
2003 | | | £2.74 | | 2006–2007 | | 8,346 | |
2003 | | | £3.00 | | 2006–2013 | | 359,265 | |
2004 | | | £2.65 | | 2006 | | 24,798 | |
2004 | | | £2.81 | | 2007–2008 | | 377,340 | |
2004 | | | £2.36 | | 2007–2008 | | 17,896 | |
2004 | | | £2.91 | | 2007–2014 | | 462,949 | |
2005 | | | £1.87 | | 2007 | | 50,652 | |
2005 | | | £2.46 | | 2008–2009 | | 344,440 | |
2005 | | | £2.01 | | 2008–2009 | | 38,414 | |
2005 | | | £2.34 | | 2008–2015 | | 479,895 | |
Total | | | | | | | 4,029,867 | |
Page 44 of 122
Whilst they have no present intention of utilizing such authority, at the AGM held on June 23, 2006 the shareholders approved a resolution to allot shares up to an aggregate nominal value of £3,264,670 (32,646,700 ordinary shares of 10p each), being the un-issued ordinary shares of the Company at April 21, 2006.
The Group operates an HM Revenue and Customs-approved Save-As-You-Earn scheme in the UK and an Employee Share Purchase Plan scheme in the US.
Item 7 | Major shareholders and related party transactions |
The shareholdings in the table set out below represent the shareholdings amounting to 3% or more of the ordinary share capital of the Company that had been notified to the Company in accordance with sections 198 to 208 of the Companies Act 1985, at the time of publication of the 2004 Annual Report and Form 20-F and this 2005 Form 20-F.
The figures in the columns entitled ‘2004 Annual Report on Form 20-F’ do not necessarily represent the current shareholdings or percentages held by the respective shareholders.
| | As at June 6, 2006 | | 2004 Annual Report
on Form 20-F | |
| | Number of
shares held | | Percentage | | Number of
shares held | | Percentage | |
INVESCO Perpetual UK Investment Series1 | | 20,410,000 | | 19.01 | % | 19,341,000 | | 18.03 | % |
F&C Asset Management | | 10,646,451 | | 9.92 | % | 10,646,451 | | 9.93 | % |
Legal & General Investment Management Ltd | | 6,423,031 | | 5.98 | % | 6,467,972 | | 6.03 | % |
Goldman Sachs Group, Inc | | 5,455,883 | | 5.08 | % | 3,260,918 | | 3.04 | % |
Phylon Fund Limited | | 3,922,000 | | 3.65 | % | – | | – | |
HBOS plc | | 3,260,033 | | 3.04 | % | – | | – | |
Morley Fund Management Limited | | – | | – | | 6,356,645 | | 5.93 | % |
| NOTES |
1 | The Amvescap Group, which includes Invesco Perpetual UK Investment Series is Acambis’ single largest shareholder, owning 30,198,065 shares (28.13%). |
As far as is known to the Directors, the Company is not directly or indirectly owned or controlled by another corporation or by any other government and the only shareholder directly or indirectly owning more than 10% of the Company is shown in the above table. All shareholders have the same voting rights.
ANALYSIS OF SHARE REGISTER AT JUNE 6, 2006 | | | | Percentage | |
Shareholding | | Number
of holders | | Percentage of
total holders | | Number
of shares | | of issued
share capital | |
1-1,000 | | 1,336 | | 57.61 | % | 665,600 | | 0.62 | |
1,001-5,000 | | 619 | | 26.69 | % | 1,400,906 | | 1.30 | |
5,001-100,000 | | 261 | | 11.26 | % | 5,226,868 | | 4.87 | |
100,001-500,000 | | 64 | | 2.76 | % | 14,782,005 | | 13.77 | |
500,001-1,000,000 | | 19 | | 0.82 | % | 14,071,524 | | 13.10 | |
1,000,001 and over | | 20 | | 0.86 | % | 71,227,324 | | 66.34 | |
| | 2,319 | | 100.00 | % | 107,374,227 | | 100.0 | |
US record holders, including American Depository Receipt (ADR) holders, held approximately 8.5% of the issued share capital of ordinary 10p shares.
Page 45 of 122
B | Related party transactions |
The Group has a 50% interest in the Pasteur Merieux-OraVax joint venture (the Joint Venture) (see note 21 to the financial statements. For the year ended December 31, 2005, the Group has included turnover of £nil (2004 – £0.1m) in respect of costs incurred in performing services for the Joint Venture and a loss of £nil (2004 – £0.1m) within its Group financial statements. At December 31, 2005, the amounts the Group owed to the Joint Venture amounted to £0.4m (2004 – £0.3m). Amounts owed by the Joint Venture to the Group at December 31, 2005 were £0.3m (2004 – £0.3m).
Item 8 | Financial information |
| | |
| A | Consolidated statements and other financial information |
See the financial statements for financial information.
There is no difference between IFRS as adopted by the European Union and IFRS on the Group’s results.
The Group has taken advantage of the accommodation granted by the SEC for foreign private issuers for their first year of reporting under IFRS to file two years rather than three years of statements of income, changes in shareholders’ equity and cash flows prepared in accordance with IFRS, with appropriate related disclosure.
DIVIDEND POLICY
Acambis has never paid any cash dividends on its shares and does not anticipate paying cash dividends for the foreseeable future.
| Item 9 | The offer and listing |
| | |
| A | The offer and listing details |
COMPARATIVE MARKET PRICE INFORMATION
Acambis shares are traded on the London Stock Exchange under the symbol ‘ACM’ and on the US NASDAQ National Market in the form of ADRs under the symbol ‘ACAM’.
The following tables set out the high and low closing mid-market prices for Acambis’ shares and close prices for ADRs:
| | | | Shares | | | | ADRs | |
| | High | | Low | | High | | Low | |
Calendar year | | Pence per ordinary share | | Dollars per ADR | |
| | | | | | | | | |
2001 | | 353.0 | | 103.5 | | 10.22 | | 3.33 | |
2002 | | 379.0 | | 181.0 | | 11.06 | | 5.67 | |
2003 | | 396.0 | | 207.5 | | 12.85 | | 6.40 | |
2004 First quarter | | 371.0 | | 300.0 | | 14.41 | | 11.04 | |
Second quarter | | 364.0 | | 300.0 | | 13.63 | | 10.55 | |
Third quarter | | 352.5 | | 292.3 | | 13.30 | | 10.48 | |
Fourth quarter | | 300.0 | | 244.3 | | 10.70 | | 9.46 | |
2005 First quarter | | 283.0 | | 237.8 | | 10.70 | | 9.03 | |
Second quarter | | 240.8 | | 212.0 | | 9.22 | | 7.93 | |
Third quarter | | 262.5 | | 220.0 | | 9.88 | | 7.98 | |
Fourth quarter | | 240.0 | | 203.5 | | 8.66 | | 7.15 | |
Monthly high and low prices (for the last full six months) are as follows: | | | | | | | | | |
December 2005 | | 214.3 | | 205.8 | | 7.74 | | 7.15 | |
January 2006 | | 205.5 | | 195.0 | | 7.26 | | 6.80 | |
February 2006 | | 229.3 | | 197.5 | | 7.94 | | 6.81 | |
March 2006 | | 215.3 | | 194.8 | | 7.75 | | 6.74 | |
April 2006 | | 192.8 | | 182.0 | | 6.93 | | 6.30 | |
May 2006 | | 184.5 | | 156.0 | | 6.93 | | 5.91 | |
Page 46 of 122
As of June 6, 2006, the mid-market price of an Acambis share was 159.5p and the close price of an Acambis ADR was $6.00. The number of outstanding ordinary shares of 10p each at that date was 107,374,227.
On February 23, 2004, Acambis announced a change in the ratio of its ADR, which has had the effect of bringing the price of its ADR more in line with the price of peer group companies. Since listing on NASDAQ in February 2001, Acambis’ ADR price has risen from approximately $18 to around $60. To ensure continued accessibility for both institutional and private investors in the US, Acambis took the decision to change the ADR ratio from one ADR for 10 ordinary shares to one ADR for two ordinary shares. All ADR holders on the register as at February 20, 2004 were issued on February 23, 2004 with four additional ADRs for each one held. The high and low data for ADRs shown in the table above reflect the current ratio.
NATURE OF TRADING MARKET
Acambis’ ordinary shares are traded on the London Stock Exchange under the symbol ‘ACM’ and on the NASDAQ National Market in the form of ADRs under the symbol ‘ACAM’.
D | Not applicable |
E | Not applicable |
F | Not applicable |
Item 10 | Additional information |
A | Not applicable |
B | Memorandum and Articles of Association |
Memorandum of Association and Articles of Association
The following is a summary of the rights under the Company’s Memorandum of Association and Articles of Association relating to voting, dividends, transfers and rights upon liquidation, which attach to the Acambis shares.
Memorandum
The Memorandum of Association of the Company provides that the Company’s principal objects are, inter alia, to act as or carry on the business of a holding company and to carry on activities ancillary thereto. The objects of the Company are set out in full in clause 4 of its Memorandum of Association.
Page 47 of 122
Articles of Association
The Articles of Association contain, inter alia, provisions to the following effect.
The issued and un-issued ordinary shares of 10 pence each in the capital of the Company are eligible for settlement in CREST (a paperless settlement system used by the London Stock Exchange) as contemplated by the Uncertificated Securities Regulations 1995 and effective under the CREST Regulations. Existing Ordinary Shares can therefore be held in registered (certificated or uncertificated) form.
Any shareholder may effect the transfer of some or all his certificated shares by an instrument of transfer in writing in the usual form or in any other form approved by the directors or, in the case of uncertificated shares, in accordance with the CREST Regulations.
The share transfer form must be signed by or on behalf of the transferor and, in the case of a partly paid share, also on behalf of the transferee. The transferor will continue to be treated as a shareholder until the name of the transferee is entered in the register of members for the relevant share or shares.
The directors may, without giving any reason, refuse to register any transfer of shares which are:
| (i) | Not fully paid provided that, if any of these shares have been admitted to the Official List of the London Stock Exchange, this does not stop dealings in the shares from taking place on an open and proper basis; |
| (ii) | In respect of more than one class of share; and |
| (iii) | For certificated shares, whether or not fully paid, in favor of more than four transferees or renouncees. |
The Board may also refuse to register a transfer of uncertificated shares in accordance with the CREST Regulations.
If the directors decide not to register a share transfer, they must no later than two months after the transfer or the relevant operator-instruction was received, in each case by Acambis, send notice of the refusal to the transferee.
Subject to any special rights or restrictions as to voting which are given to any shares (as to which there are none at present), on a show of hands every shareholder who is present in person has one vote, and on a poll every shareholder present in person or by proxy has one vote for every share for which he is the holder. In the case of joint holders, the vote of the person whose name stands first in the register of members is accepted to the exclusion of any vote tendered by any other joint holder. Unless the directors decide otherwise, a shareholder may not vote at any general or class meeting or exercise any other right in relation to meetings while any amount of money relating to his shares remains outstanding.
Subject to the Companies Act 1985, the Company may, by ordinary resolution, declare dividends in accordance with the respective rights of the shareholders, but no dividend shall exceed the amount recommended by the board. Subject to the Companies Act 1985, the directors may pay such interim dividends as appear to them to be justified by the financial position of the Company on shares of any class. If the share capital is divided into different classes, the directors may pay interim dividends on shares which confer deferred or non-preferred rights with regards to dividend as well as on shares which confer preferential rights with regard to dividend, but no interim dividend shall be paid on shares carrying deferred or non-preferred rights if, at the time of payment, any preferential dividend is in arrears. The directors may also pay fixed dividends on any class of share carrying a fixed dividend on the dates prescribed for the payment of such dividends. Except as otherwise provided by the rights attached to the shares, all dividends shall be divided and paid proportionately to the amounts paid up on the shares on which the dividend is paid during any period in respect of which the dividend is paid.
Except as otherwise provided by the Articles of Association or the rights attached to any shares, a dividend or any other money payable in respect of a share can be paid in whatever currency the directors decide.
Page 48 of 122
Directors may deduct any amount relating to shares which remains outstanding from any dividend or other money payable to the shareholder on or in respect of any share held by him.
The Company may stop sending dividend payments through the post, or cease using any other method of payment (including payment through CREST) if, for two consecutive dividends, the dividend payments have been returned undelivered or remain un-cashed during the period for which they are valid or the payments by any other method have failed and, in the case of any one dividend, reasonable enquiries have failed to establish any new address or account of the registered shareholder.
Any dividend which remains unclaimed for 12 years from the date when it was declared or became due for payment, shall be forfeited and go back to the Company.
The Board may, if authorized by the Company at any annual general meeting, offer ordinary shareholders the right to elect to receive ordinary shares instead of some or all of their cash (a scrip dividend).
Upon the recommendation of the directors, the Company may by ordinary resolution direct that a dividend be satisfied wholly or partly by the distribution of specific assets (in particular, paid up shares or debentures of any other company). Where any difficulty arises with regard to the distribution, the directors may resolve it as they think fit and in particular (but without limitation) may authorize any person to sell and transfer any fraction (or ignore fractions) and fix the value for distribution of any assets, and may determine that cash shall be paid to any member upon the basis of that value in order to adjust the rights of members, and may vest any assets in trustees.
(d) Distribution of assets on a winding up |
If the Company is wound up the liquidator may, with the sanction of an extraordinary resolution divide among the shareholders (the division among shareholders to be decided upon by the liquidator) in kind the whole or any part of the assets of Acambis whether the assets consist of property of one or different kinds and at such property value as the liquidator deems fair. With the like authority, the liquidator may vest the whole or any part of the assets in trustees upon such trusts for the benefit of the shareholders as he may determine, but no shareholder shall be compelled to accept any assets upon which there is a liability.
(e) Alternative of share capital |
The Company may, by ordinary resolution, increase its capital, consolidate (or consolidate and then divide) all or any of its share capital into shares of a larger nominal amount than its existing shares and cancel any shares which, at the date of the passing of the ordinary resolution have not been taken, or agreed to be taken and reduce the amount of the Company’s share capital by the amount of the cancelled shares. Subject to the Companies Act 1985, the Company may also divide some or all of its existing shares into shares of a smaller nominal value by ordinary resolution and provide that as between the holders of the divided shares different rights and restrictions apply.
The directors have the power to deal with any fractions of shares resulting from a consolidation, including selling any shares representing fractions for the best price reasonably obtainable and distributing the net proceeds of sale among shareholders in proportion to their fractional entitlements.
The Company may by special resolution reduce its share capital, any capital redemption reserve, any share premium account or any other un-distributable reserve in any way. The Company may, subject to the Companies Act 1985, the rules of the UK Listing Authority (if applicable) and to any special rights previously given to holders of existing shares, purchase any of its own shares (including redeemable shares).
If recommended by the directors, the Company can by ordinary resolution capitalize any sum which is part of the Company’s reserves or which the Company is holding as net profits.
Page 49 of 122
(f) Restrictions on shareholders |
If any shareholder or any other person who the Company has reasonable cause to believe has an interest in the Company’s shares has been duly served with a statutory notice and has not, within 14 days, provided details of those who have an interest and the extent of their interest in that particular shareholding (the ‘Default Shares’), the shareholder shall not be entitled in respect of the Default Shares to attend or vote either personally or by proxy at a general meeting of the Company or a meeting of the holders of any class of shares or to exercise any other right in relation to general meetings of Acambis or meeting of the holders of any class of shares.
Where the Default Shares represent 0.25 percent or more (in nominal value or number) of the issued shares of a class, then the Company shall be entitled to withhold any dividend (or part thereof), any right to receive shares instead of a dividend or other money which would otherwise be payable in respect of the Default Shares. Where the Default Shares represent 0.25 percent or more (in nominal value or number) of the issued shares of a class, then no transfer of the Default Shares shall be registered unless the shareholder is not himself in default as regards supplying the information required and provides evidence, to the satisfaction of the directors, that no person in default as regards supplying such information is interested in any of the shares which are the subject of the transfer; or registration is required by the Uncertificated Securities Regulations 2001.
Subject to the Companies Act 1985 and to special rights previously given to holders of existing shares, Acambis may issue shares with any rights or restrictions attached to them. Rights or restrictions can be decided upon by either an ordinary resolution or as long as there is no conflict with any resolution, by the directors.
Subject to the Companies Act 1985, the rights attached to any class of shares may be changed or abrogated, with the written approval of shareholders holding at least three quarters in nominal value of the issued shares of that class or with the sanction of an extraordinary resolution passed at a separate meeting of the holders of the relevant class of shares.
The provisions of the Articles of Association relating to general meetings will apply to any such separate class meeting except that:
| (i) | the necessary quorum is two shareholders in person or by proxy who own at least one third in nominal value of the issued shares of the class; |
| (ii) | if at an adjourned meeting, a quorum as defined is not present, one person who holds shares of the class, or his proxy, will be a quorum; and |
| (iii) | any shareholder who is present in person or by proxy can demand a poll at which every shareholder who is present in person or by proxy is entitled to one vote for every share he has of the class (but this is subject to any special rights or restrictions which are attached to any class of shares). |
| | (i) | Number |
| | | Subject to the passing of an ordinary resolution changing the provisions in the Articles of Association, the number of directors (other than alternate directors) shall not be subject to a maximum but shall not be less than two. |
| | (ii) | Age |
| | | No person will be appointed a director or be required to stop being a director because he has reached a particular age. Notice of a meeting at which a resolution will be proposed to re-appoint or appoint a director who is age 70 or more must state the fact that the relevant director is aged 70 or more. |
| | (iii) | Appointment |
| | | Directors may be appointed by ordinary resolution or by the board of Acambis and a director need not be a Shareholder. |
| | | A director appointed by the board of Acambis holds office only until the next following annual general meeting and is not taken into account in determining the directors who are to retire by rotation at that meeting. |
Page 50 of 122
| | (iv) | Removal |
| | | In addition to any power to remove directors under the Companies Act 1985, the Company may pass an ordinary resolution to remove a director from office even though his time in office has not ended and may (subject to the Articles of Association) elect a person to replace a director who has been removed in this way by passing an ordinary resolution. |
| | (v) | Retirement by rotation |
| | | At every annual general meeting, one third of the directors will retire by rotation and be eligible for re-election. If one-third is not a whole number, the number of directors to retire is the number nearest to but not greater than one third. The directors to retire will be, firstly, those who wish to retire and not offer themselves for re-election, secondly, those who have been directors longest since they were last elected. If there are directors who were last elected on the same date, and they cannot agree who is to retire, they must draw lots to decide. In addition, any director who would not otherwise be required to retire by rotation must retire by rotation at the third annual general meeting since his last appointment or re-appointment. |
| | (vi) | Remuneration |
| | | The total fees paid to all of the directors must not exceed £300,000 per annum or such other higher amount as may from time to time be decided by ordinary resolution. The fees shall be distinct from salary, remuneration due in accordance with employment or executive office. Each director may be paid reasonable expenses incurred in attending and returning from board meetings, committee meetings, and general meetings or otherwise properly and reasonably incurred in connection with Acambis business or in the performance of his duties as a director. A director who resides abroad or otherwise performs services which in the opinion of the directors is outside the scope of ordinary duties of the director, may be paid extra remuneration by way of salary, commission or otherwise as the directors may determine. |
| | (vii) | Pensions and gratuities for directors |
| | | The board of Acambis may provide pensions or other benefits to any director or former director of the Company, or any relation or dependent of such person. |
| | | Permitted interests—Subject to the provisions of the Companies Act 1985 and provided that a director discloses the nature and extent of his interest to the board, a director can do any one or more of the following: |
| • | be a party to, or otherwise interested in any existing or proposed contract, transaction or arrangement with or involving the Company or another company in which the Company has some interest; |
| • | hold any other position (other than auditor) in the Company as well as being a director; and, |
| • | hold any position within any other Company in which the Company has an interest |
Any director who is so interested does not have to account to the Company for any benefit he receives as a result of any of the above.
| (viii) | When Director may count in quorum or vote |
A Director can not vote or be counted in the quorum in relation to a resolution in respect of any contract or arrangement or any other proposal whatsoever in which he has an interest which (together with any interest of any person connected with him) to his knowledge is a material interest, otherwise than by virtue of his interests in shares or debentures or other securities of or otherwise in or through the Company unless, the resolution concerns:
| • | the giving of any guarantee, security or indemnity to him in respect of money lent or obligations incurred by him or by any other person at the request of, or for the benefit of, the Company or any of its subsidiary undertakings |
| • | the giving of any guarantee, security or indemnity to a third party in respect of a debt or obligation of the Company or any of its subsidiary undertakings for which he himself has assumed responsibility in whole or in part and whether alone or jointly with others under a guarantee or indemnity or by the giving of security; |
| • | any matter relating to an offer of shares, debentures or other securities of or by the Company or any of its subsidiary undertakings in which offer the Director is or may be entitled to participate as a holder of securities or in the underwriting or sub-underwriting of which the Director is to participate; |
Page 51 of 122
| • | any contract, transaction, arrangement or proposal to which the Company is or is to be a party relating to another company, including any subsidiary of the Company, in which he and any persons connected with him do not to his knowledge (directly or indirectly) hold an interest in shares whether as an officer or shareholder, creditor or otherwise representing one per cent. or more of any class of the equity share capital, or the voting rights, in that company; |
| • | any contract, transaction, arrangement or proposal concerning insurance, which the Company proposes to maintain or purchase for the benefit of Directors or for the benefit of persons including Directors. |
The directors may exercise powers to make provision for the benefit of employees or former employees of the Company or any of its subsidiaries in connection with the cessation or transfer of the whole or part of the business of the Company or that subsidiary.
| • | The directors may exercise all the Company’s powers to borrow money; to pledge or grant any security over all or any of the Company’s undertaking, property and assets (present and future) and uncalled capital, to issue debentures and other securities; and to give security for any debt, liability or obligation of the Company or of any third party. |
| • | The Directors will restrict the borrowings of the Company and exercise all voting and other rights, or powers of control exercisable by the Company in relation to its subsidiary undertakings so far as to secure (as regards subsidiary undertakings to the extent possible) that the aggregate principal amount (including any premium payable on final repayment) outstanding of all moneys borrowed by the Company and its subsidiary undertakings (excluding amounts borrowed by any member of the Group from any other member of the Group) shall not at any time, save with the previous sanction of an ordinary resolution of the Company, exceed a sum equal to five times the adjusted capital and reserves (as detailed in the Company’s Articles of Association). |
(j) Shareholders’ Meeting |
Subject to the provisions of the Companies Act 1985, the annual general meeting is held once in every calendar year. The board of Acambis may call extraordinary general meetings at any time. At general meetings of Acambis two members present in person and who are entitled to vote is a quorum. Notice periods and the majorities required for the passing of resolutions are those provided for under the Companies Act 1985.
(k) Untraced shareholders |
The Company may sell in such manner and for such price as the Directors think fit any share held by a member if:
| (i) | for a period of six years before the giving of notices referred to in (iii) below no check, order or warrant for amounts payable in respect of the share, has been cashed by the member or person entitled by death or bankruptcy or otherwise by operation of law to the share and, so far as the Directors are aware, no communication in respect of the share has been received by the Company from such member or person and no such amount has been claimed by the person entitled to it; |
| (ii) | during that period at least two dividends (whether interim or final) in respect of the share have become payable; |
| (iii) | the Company has, after the expiration of that period, advertised in both a national daily newspaper published in the UK and in a newspaper circulating in the area of the last known address to which checks, orders or warrants were sent and by notice to the Quotations Department of the London Stock Exchange if shares of the class concerned are listed or dealt in on that exchange, given notice of its intention to sell such share; and |
| (iv) | the Company has not during the further period of three months after the date of the advertisements referred to in (iii) above or of the last of the two advertisements to be published if they are published on different dates and prior to the sale of the share received any communication in respect of the share from the member or person concerned. |
Page 52 of 122
To sell any shares in this way, the directors may appoint anyone to transfer the shares. This transfer will be just as effective as if it had been signed by the holder, or by a person who is entitled to the shares by law. The person to whom the shares are transferred will not be bound to concern himself as to what is done with the purchase moneys nor will his ownership be affected even if the sale is irregular or invalid in any way.
The net proceeds of sale will belong to the Company which will be indebted to the former member. The Company must record the name of the shareholder, of (if known) the person who would have been entitled to the shares by law, as a creditor for the money in its accounts. The Company will not be a trustee of the money and will not be liable to pay interest on it. The Company can use the money, and any money earned by using the money, for its business or in any other way that the directors decide, but the money cannot be invested in the Company’s shares or in the shares of any holding company of the Company.
Quorum Requirement Exemption
In 2000, the Company obtained an exemption from the quorum standard for NASDAQ-listed companies set forth in NASDAQ Rule 4350(f). This standard requires that the quorum for meetings of shareholders be no less than 33 1/3% of the outstanding shares. NASDAQ granted an exemption on the grounds that this standard is contrary to generally accepted business practices in the United Kingdom.
Pursuant to this exemption, the Company’s quorum standards, as set forth in the Company’s Articles of Association and summarized in paragraph (i) above, follow the relevant quorum standards applicable to companies in the United Kingdom. These standards provide for a quorum in the event that at least two persons entitled to vote upon the business to be transacted are present in person or by proxy at a shareholder meeting.
(a) US CDC agreements
In prior years, the Group was awarded two distinct contracts (ACAM1000 and ACAM2000) by the CDC to develop and manufacture smallpox vaccines for the purposes of countering the threat of bioterrorism.
ACAM1000 CONTRACT
This contract was awarded in September 2000. It required research and development of a new smallpox vaccine, manufacture of a 40 million-dose stockpile and maintenance of that stockpile for 20 years. In 2003, the US Government decided to consolidate the activities ongoing under this contract and that awarded to Acambis in November 2001 (see ‘ACAM2000 contract’, below). As a result, activities under the ACAM1000 contract were wound down and final settlement of the contract was successfully negotiated in February 2006.
During 2005 the Group recorded turnover in relation to this contract of £0.2m (2004 – £1.4m) for R&D costs incurred by the Group and funded by the CDC.
ACAM2000 CONTRACT
In November 2001, Acambis was awarded a second contract by the CDC to develop, manufacture and deliver 155 million doses of investigational ACAM2000 vaccine. The initial value of this contract, which carries a fixed price, was $428m (approximately £234m). This contract was divided into two principal components: funding to take the vaccine through clinical trials to FDA licensure; and manufacture of the vaccine. The funding for the R&D element was received over 2002 and 2003, whereas the funding for the manufacture was received on delivery of the vaccine, which started during 2002 and was completed in 2004. As a result of the US Government’s decision to consolidate activities under Acambis’ ACAM1000 and ACAM2000 contracts, Acambis received an order for an additional 27.5 million doses of ACAM2000 under the terms of the ACAM2000 contract in 2003 and completed delivery of those doses in 2004.
Page 53 of 122
A small amount of work on this contract is continuing in 2006, principally relating to submission of the product license application to the FDA.
Turnover of £12.8m has been recorded in 2005 in relation to this contract (2004 – £66.5m).
(b) US NIAID contracts
In February 2003, Acambis was awarded a $9.2m contract by NIAID, part of the NIH, to develop a new MVA vaccine, deliver several thousand doses of the vaccine to NIAID and conduct a Phase I clinical trial in healthy adults. The contract is structured on a ‘cost plus fixed fee’ basis. For this contract, Acambis partnered with Baxter Healthcare Corporation, its strategic partner, such that Baxter manufactured the doses of vaccine at its manufacturing site. Acambis acted as the prime contractor and Baxter as sub-contractor, leveraging each other’s strengths and capabilities.
In September 2004, Acambis was one of two companies to win a second US Government contract for the development and manufacture of investigational MVA vaccine from the NIAID. As with the first contract, Acambis is co-developing its MVA vaccine candidate with Baxter. Under the principal part of this contract, worth approximately $76m, Acambis has delivered 500,000 doses of vaccine and is carrying out clinical trials.
Turnover of £17.2m was recorded under these contracts in 2005 (2004 – £1.9m).
(c) Baxter agreements
ACAM2000 SUB-CONTRACT
In November 2001, Acambis entered into a sub-contract with Baxter for the purchase of crude bulk smallpox vaccine and the subsequent assembly of various components into multi-dose kits of smallpox vaccine.
CANTON LONG-TERM LEASE FINANCE FACILITY
In December 2001, Acambis entered into a long-term lease-finance facility with Baxter in respect of Acambis’ manufacturing plant. Further details of this facility are described within ‘Financial liabilities’ in note 18 to the financial statements..
ACAM2000 DISTRIBUTION AGREEMENT
In December 2002, Acambis entered into a distribution, manufacturing and license agreement with Baxter, giving Baxter exclusive distribution rights for ACAM2000 smallpox vaccine in all countries of the world, excluding the US and the UK. In 2005, turnover of £nil (2004 – £9.3m) was recorded under this agreement.
MVA SUB-CONTRACT
As noted above in (b), Baxter is a manufacturing sub-contractor with respect to the MVA contract with the NIAID.
(d) Evans Vaccines agreement
In September 1999, the Group entered into an agreement with Medeva Pharma Limited to obtain regulatory approval in the US for ARILVAXTM yellow fever vaccine. Medeva Pharma Limited assigned this agreement to Evans Vaccines Limited, a wholly-owned subsidiary of Chiron Vaccines, in October 2000. The Group funded 100% of the costs of the clinical trials and regulatory submission. As described in note 18 to the financial statements, the costs are being partly financed through an overdraft facility up to a maximum of $7m, being underwritten by Chiron. Chiron has granted to Acambis 100% of the marketing rights to ARILVAXTM in the US, whilst still retaining an option to buy back 50% of the profits from the US sales in return for refunding to the Group the costs the Group has incurred on the ARILVAXTM program. No turnover was recorded in 2005 (2004 – £nil) under this agreement.
Page 54 of 122
In February 2004, Acambis withdrew its BLA application for ARILVAX, and Acambis is in ongoing discussions with Chiron and its now parent company Novartis AG, to resolve a way forward for the ARILVAX program.
(e) Cangene agreement
Since March 2003 Acambis has had an agreement with Cangene Corporation (Cangene) to market Cangene’s VIG product in markets outside North America and Israel. Turnover of £0.4m was recorded in 2005 (2004 – £nil) under this agreement. Baxter is a marketing sub-agent with respect to this agreement.
(f) Berna Biotech supply agreement
In August 2003, Acambis acquired BPC. BPC has exclusive North American sales and distribution rights, up to 2010, to Vivotif®, an oral typhoid vaccine manufactured by Berna Biotech AG. Licensed in over 50 countries around the world and the only orally administered typhoid vaccine currently available, Vivotif® has been registered and sold in the US since 1990 and Canada since 1994. BPC employs 16 people, with operations in Miami and Toronto, from where it promotes and distributes Vivotif® to customers throughout North America.
The Group paid amounts of £1.9m in 2005 in relation to this contract (2004 - £1.7m).
(g) Fill/finish facility capability
In May 2005, Acambis added a lyophilisation and fill/finish capability based in Rockville, MD, through a leasing agreement, from BioReliance Corporation. Acambis made an upfront payment of $3.0m in May 2005 with an additional $4.5m payable over a period of 12 years, until 2017.
The facility was designed to produce liquid and lyophilized material at a scale sufficient for clinical trials. Acambis plans to establish GMP-compliant fill and finish operations at a commercial scale suitable for some of the vaccines in its development pipeline.
EXCHANGE CONTROLS AND OTHER LIMITATIONS AFFECTING SECURITY HOLDERS
There are no UK Government laws, decrees or regulations restricting the import or export of capital or affecting the remittance of dividends or other payments to holders of the Company’s ordinary shares who are non-residents of the UK. There are no limitations relating only to non-residents of the UK under English law or the Company’s Memorandum and Articles of Association on the right to be a holder of, and to vote in respect of, the Company’s ordinary shares.
EXCHANGE RATE INFORMATION
References in this Form 20-F to ‘US dollars’, ‘$’, or ‘¢’ are to the currency of the United States. References to pounds ‘sterling’, ‘pounds’, ‘£’, ‘pence’’ or ‘p’ are to the currency of the United Kingdom. There are 100 pence to each pound. Solely for your convenience, this report contains translations of certain pounds sterling amounts into US dollars at specified rates. These translations should not be taken as assurances that the pound sterling amounts actually represent such US dollar amounts or could be converted into US dollars at the rate indicated or at any other rate.
The table below sets forth, for the periods and dates indicated the exchange rate for the US dollar against the pound based on the noon buying rate, expressed in dollars per pound sterling. The period average is based on the average of the noon buying rates on the last day of each month during the period.
Page 55 of 122
| | Period Average | | Period End | | High | | Low | |
Year ended December 31, | | | | | | | | | |
2001 | | 1.4400 | | 1.4555 | | 1.5047 | | 1.3726 | |
2002 | | 1.5035 | | 1.6095 | | 1.6095 | | 1.4092 | |
2003 | | 1.6453 | | 1.7905 | | 1.7905 | | 1.5497 | |
2004 | | 1.8355 | | 1.9199 | | 1.9492 | | 1.7584 | |
2005 | | 1.8120 | | 1.7168 | | 1.9268 | | 1.7150 | |
Monthly high and low rates (for the last full six months) are: | | | | | | | | | |
December 2005 | | | | | | 1.7723 | | 1.7150 | |
January 2006 | | | | | | 1.7728 | | 1.7168 | |
February 2006 | | | | | | 1.7728 | | 1.7406 | |
March 2006 | | | | | | 1.7534 | | 1.7260 | |
April 2006 | | | | | | 1.7822 | | 1.7346 | |
May 2006 | | | | | | 1.8906 | | 1.8176 | |
As of June 6, 2006, the spot exchange rate for pounds sterling was $1.8858.
UK TAX CONSEQUENCES OF OWNING ACAMBIS SHARES
TAXATION OF DIVIDENDS
There is no withholding tax on dividends paid by a UK company. Under the provisions of the income tax convention which came into force between the UK and the US in relation to dividends paid after May 1, 2003, payments in respect of tax credits are no longer made to US holders on dividends paid by UK companies.
UK TAXATION OF CAPITAL GAINS
A US holder who is not resident, or ordinarily resident, for tax purposes in the UK will not be liable for UK tax on capital gains on the disposal of Acambis shares unless the US holder carries on a trade, profession or vocation in the UK through a branch or agency and the Acambis shares are or have been used by, held by, or acquired for use by or for the purposes of such trade, profession, vocation, branch or agent. In certain circumstances, however, a person who has been resident in the UK and again becomes resident after a period of non-residence may be taxed on gains realized during the period of non-residence.
UK INHERITANCE TAX ON ESTATES AND GIFTS
The estate and gift tax convention in force between the US and the UK provides that the UK tax to which the convention applies is capital transfer tax and that it will also apply to identical or substantially similar taxes which are imposed subsequently. Capital transfer tax in the UK has been replaced by inheritance tax. It is understood that, in practice, the US tax authorities and the UK HM Revenue and Customs apply the convention on the basis that inheritance tax has replaced capital transfer tax as the tax to which the convention now applies, although the convention has not been amended to that effect.
On the basis of that practice, Acambis shares held in the US by an individual who is domiciled for the purposes of the estate and gift tax convention in the US and is not for the purposes of the convention a national of the UK, will not be subject to inheritance tax on the individual’s death or on a transfer of the Acambis shares during the individual’s lifetime. However, special rules apply in the case of Acambis shares held in trust or as part of the business property of a permanent establishment in the UK or related to the fixed base in the UK of a person providing independent personal services.
UK STAMP DUTY AND STAMP DUTY RESERVE TAX
Any transfer of Acambis shares will result in a stamp duty liability at the rate of 0.5% (rounded to the nearest £5) of the consideration (which liability is generally payable by the purchaser of the Acambis shares).
Page 56 of 122
There is no charge to ad valorem stamp duty on gifts. On a transfer of Acambis shares from a nominee to the beneficial owner, if the nominee has at all times held the Acambis shares on behalf of the transferee, under which no beneficial interest passes and which is neither a sale, nor arises under or following a contract of sale, nor is in contemplation of sale, a fixed £5 stamp duty will be payable.
Stamp duty reserve tax, generally at a rate of 0.5% on the consideration, is currently payable on any agreement to transfer ordinary shares or any interest therein unless:
• | an instrument transferring the shares is duly executed and stamped within the appropriate time limits; and |
• | stamp duty, generally at a rate of 0.5% (rounded to the nearest £5) of consideration (or part thereof), is paid. |
Increased UK stamp duty and stamp duty reserve tax charges will apply if the Acambis shares are issued or transferred to a custodian for a clearing system or to a depositary who issues depositary receipts in respect of such shares. These are generally at the rate of 1.5% of the consideration paid or the market value of the Acambis shares, depending on the circumstances.
The above summary is not intended to constitute a complete analysis of all of the UK tax consequences of the ownership or disposition of Acambis shares. This discussion is included for general information purposes only and may not apply to a particular shareholder in light of such shareholder’s particular circumstances. Shareholders are urged to consult their own tax advisers as to the particular tax consequences to them of the ownership or disposition of Acambis shares, including the application of state, local and other foreign tax laws.
US TAX CONSEQUENCES OF OWNING ACAMBIS SHARES
The following summary sets out the principal US federal tax consequences of the purchase, ownership and disposition of the Company’s shares or ADRs in respect of such shares by a ‘US Holder’ (as defined below) and is not intended to be a complete analysis or listing of all the possible tax consequences of such purchase, ownership or disposition. As used herein a ‘US Holder’ means a beneficial owner of Acambis’ shares or ADRs that is: a citizen or resident of the US; a corporation (or other entity taxable as a corporation for US federal income tax purposes) created or organized in or under the laws of the US, or any political subdivision thereof; an estate whose income is includible in gross income for US federal income tax purposes regardless of its source; or a trust, if a court within the US is able to exercise primary supervision over the administration of the trust and one or more US persons have the authority to control all substantial decisions of the trust, or if the trust has in effect a valid election to be treated as a US person.
This summary deals only with shares and ADRs held as capital assets and does not address any special tax consequences that may be applicable to US Holders who are subject to special treatment under the current income tax convention between the US and the UK which came into effect on March 31, 2003 (the ‘Treaty’), or the US Internal Revenue Code of 1986, as amended, such as dealers in securities or foreign currency, traders who elect mark-to-market accounting, financial institutions or financial services entities, insurance companies, persons subject to the alternative minimum tax, tax-exempt entities or private foundations, persons that hold the shares or ADRs as part of a straddle, hedge, conversion or constructive sale transaction or other integrated financial transaction, persons whose functional currency is other than the US dollar, certain expatriates or former long-term residents of the US, persons who alone, or together with one or more associated persons, control or controlled (directly, indirectly or constructively) 10% or more of the voting shares of the Company; persons who acquire shares or ADRs as compensation for services; or a US Holder who is resident or ordinarily resident for tax purposes in the UK, a US corporation which is resident in the UK by reason of being managed and controlled in the UK, or a US Holder who, or a US corporation which, has a permanent establishment in the UK.
In addition, the following summary assumes that US Holders are residents of the US for purposes of the Treaty and are entitled to the benefits of the Treaty and does not consider the tax consequences to person that elect to extend the application of the former income tax treaty between the US and the UK. Prospective investors are advised to consult their tax advisers with respect to the tax consequences of the purchase, ownership and disposition of shares or ADRs, including specifically the consequences under state and local tax laws.
Page 57 of 122
The statements regarding US and UK tax laws set out below are based on US federal and UK tax laws and UK Inland Revenue practice in force on the date of this Form 20-F and are subject to change after that date, potentially with retroactive effect. This summary does not address the tax consequences to partnerships, other pass-through entities or persons who hold shares or ADRs through a partnership or other pass-through entity. In addition, this discussion does not address any aspect of state, local or non-US tax laws or the possible application of US federal gift or estate tax.
US Holders of ADRs will be treated as the owners of the underlying shares for purposes of the double taxation conventions relating to income and estate and gift taxes between the US and the UK and for the purposes of the US Internal Revenue Code of 1986, as amended (the ‘Code’).
TAXATION OF DIVIDENDS
Subject to the discussion below under ‘Tax Consequences of PFIC Status’, any dividend paid by the Company will generally be included in the gross income of a US Holder as dividend income for US federal income tax purposes to the extent made from the Company’s current or accumulated earnings and profits, as determined under US federal income tax principles. Distributions in excess of such current and accumulated earnings and profits will be applied against and will reduce the US Holder’s tax basis in the shares or ADRs and to the extent in excess of such tax basis will be treated as a gain from the sale or exchange of the shares or ADRs. The amount of any dividend paid in pounds sterling will equal the US dollar value of the pounds sterling received calculated by reference to the exchange rate in effect on the day that the dividend is received by the US Holder, in the case of shares, or by the Depositary (or its Custodian), in the case of ADRs, regardless of whether the dividend payment is converted into US dollars. Foreign currency exchange gain or loss, if any, realized on a subsequent sale or other disposition of pounds generally will be treated as US source ordinary income or loss to the US Holder.
Dividends paid by the Company to an individual US Holder which constitute ‘qualified dividend income’ will be subject to tax at a reduced rate of tax of 15%. For this purpose, qualified dividend income includes dividends from foreign corporations paid prior to January 1, 2011 if (a) the shares of such corporation with respect to which such dividend is paid are readily tradable on an established securities market in the US, including NASDAQ, or (b) such corporation is eligible for the benefits of the Treaty. The Company believes that it is eligible for the benefits of the Treaty. Dividends, however, will not qualify for the reduced rate if the Company is treated for the tax year in which dividends are paid (or in the prior year) as a ‘passive foreign investment company’ for US federal income tax purposes. While the Company does not believe it is a passive foreign investment company, see the discussion below at ‘Tax Consequences of PFIC Status’. Accordingly, dividend distributions with respect to the Company’s shares or ADRs should be treated as qualified dividend income eligible for the reduced 15% US federal income tax rate. However an individual US Holder will not be entitled to the reduced rate unless the holder meets certain holding period requirements. Any days during which such a US Holder has diminished its risk of loss on the shares or ADRs are not counted towards meeting such requirement. In addition, an individual US holder will not be entitled to the reduced rate on dividends to the extent such US Holder is under an obligation to make related payments on substantially similar or related property; or such US Holder elects to treat the dividend income as ‘investment income’ pursuant to Section 163(d)(4) of the Code.
For the purpose of calculating foreign tax credit limitations, dividends paid by the Company will be treated as income from sources outside the US. The limitation on foreign taxes eligible for credit is calculated separately with respect to specific classes of income. For this purpose, dividends paid by the Company will, under current law, be ‘passive income’ or, in the case of certain US Holders, ‘financial services income.’ Recently enacted legislation will modify the foreign tax credit limitation by reducing the number of classes of foreign source income to two for taxable years beginning after December 31, 2006. Under that change in law, dividends paid by the Company will generally constitute ‘passive category income’ but could, in the case of certain US Holders, constitute ‘general category income.’ Special rules apply to individuals whose foreign source income during the taxable year consists entirely of ‘qualified passive income’ and whose creditable foreign taxes paid or accrued during the taxable year do not exceed US$300 (US$600 in the case of a joint return).
Page 58 of 122
In particular circumstances, a US Holder that (i) has held the Company shares or ADRs for less than a specified minimum period during which it is not protected from risk of loss, (ii) is obligated to make payments related to the dividends, or (iii) holds the Company shares or ADRs in arrangements in which the US Holder’s expected economic profit, after non-US taxes, is insubstantial will not be allowed a foreign tax credit for foreign taxes imposed on dividends paid on the shares or ADRs. Significant restrictions apply for foreign tax credit limitation purposes with respect to receipts of ‘qualified dividend income’ as described in the preceding paragraph.
TAXATION OF CAPITAL GAINS
Subject to the discussion below at ‘Tax Consequences of PFIC Status’, upon a sale, exchange or other disposition of shares or ADRs, a US Holder will recognize gain or loss for US federal income tax purposes in an amount equal to the difference between the US dollar value of the amount realized and the US Holder’s tax basis (determined in US dollars) in such shares or ADRs. Generally, such gain or loss will be a capital gain or loss and will be a long-term capital gain or loss if the US Holder’s holding period for such shares or ADRs exceeds one year. Any such gain or loss generally will be income or loss from sources within the US for foreign tax credit limitation purposes. Long-term capital gains of a non-corporate US Holder are generally subject to a maximum tax rate of 15%. The deductibility of a capital loss recognized on the sale or exchange of shares or ADRs is subject to limitations.
If the shares or ADRs are publicly traded, a disposition of such shares or ADRs will be considered to occur on the ‘trade date’, regardless of the US Holder’s method of accounting. A US Holder that uses the cash method of accounting calculates the US dollar value of the proceeds received on the sale on the date that the sale settles. However, a US Holder that uses the accrual method of accounting is required to calculate the value of the proceeds of the sale on the ‘trade date’ and, therefore, may realize a foreign currency gain or loss, unless such US Holder has elected to use the settlement date to determine its proceeds of sale for purposes of calculating such foreign currency gain or loss. In addition, a US Holder that receives foreign currency upon the sale or exchange of the shares or ADRs and converts the foreign currency into US dollars subsequent to receipt will have a foreign exchange gain or loss based on any appreciation or depreciation in the value of the foreign currency against the US dollar. A foreign exchange gain or loss will generally be US source ordinary income or loss.
TAX CONSEQUENCES OF PASSIVE FOREIGN INVESTMENT COMPANY (PFIC) STATUS
The Company will be a PFIC, if 75% or more of its gross income in a taxable year, including its pro rata share of the gross income of any company, US or foreign, in which the Company is considered to own 25% or more of the shares by value, is passive income. Alternatively, the Company will be a PFIC if 50% or more of its gross assets in a taxable year, averaged over the year and generally determined based on fair market value and including its pro rata share of the assets of any company, US or foreign, in which the Company is considered to own 25% or more of the shares by value, are held for the production of, or produce, passive income. Because the PFIC determination is made annually on the basis of the Company’s income and assets, including goodwill, we cannot assure you that we will not be a PFIC in the current or subsequent taxable years. As a result of its cash position, the Company may be a classified as a PFIC under the asset test in the event that the price of the ordinary shares declines substantially. Acambis will monitor its status and will, promptly following the end of any taxable year for which it is determined it to be a PFIC, notify US Holders of such status.
If the Company were a PFIC, and a US Holder did not make an election to treat it as a ‘qualified electing fund’ or a ‘mark to market’ election (each as described below):
• | excess distributions to a US Holder would be taxed in a special way. ‘Excess distributions’ are amounts received by a US Holder with respect to shares in any taxable year that exceed 125% of the average distributions received by such US Holder in the shorter of either the three previous years or such US Holder’s holding period before the present taxable year. Excess distributions must be allocated rateably to each day that a US Holder has held shares or ADRs. A US Holder must include amounts allocated to the current taxable year and to years prior to becoming a PFIC in its gross income as ordinary income for the current taxable year. A US Holder must pay tax on amounts allocated to each other taxable year at the highest rate in effect for that year on ordinary income and the tax is subject to an interest charge at the rate applicable to deficiencies for income tax; and |
• | the entire amount of gain realized by a US Holder upon the sale or other disposition of shares or ADRs also will be treated as an excess distribution and will be subject to taxation and an interest charge as described above. |
Page 59 of 122
The special PFIC rules described above will not apply to a US Holder if the US Holder makes an election to treat the Company as a qualified electing fund (QEF) in the first taxable year in which the US Holder owns shares or ADRs and if the Company complies with certain reporting requirements. Instead, a shareholder of a qualified electing fund is required for each taxable year to include in income a pro rata share of the ordinary earnings of the qualified electing fund as ordinary income and a pro rata share of the net capital gain of the qualified electing fund as long-term capital gain. In certain circumstances a separate election may be available to defer payment of taxes, which deferral is subject to an interest charge.
The QEF election is made on a shareholder-by-shareholder basis and can be revoked only with the consent of the IRS. A shareholder makes a QEF election by attaching a completed IRS Form 8621, using the information provided in the PFIC annual information statement, to such shareholder’s timely filed US federal income tax return. Even if a QEF election is not made, a shareholder in a PFIC who is a US person must file a completed IRS Form 8621 with such shareholder’s US federal income tax return every year. The Company will make available to US Holders upon request the annual information statement to make a QEF election and report inclusions there under.
US Holders may also be able to avoid the interest charge described above by making a mark to market election. Such election is available to the extent that the shares or ADRs held are ‘regularly traded’ on certain US stock exchanges (including NASDAQ), or on a foreign stock exchange that meets the following requirements:
• | the foreign exchange is regulated or supervised by a governmental authority of the country in which the exchange is located; |
• | the foreign exchange has trading volume, listing, financial disclosure, and other requirements designed to prevent fraudulent and manipulative acts and practices, remove impediments to, and perfect the mechanism of, a free and open market, and to protect investors; |
• | the laws of the country in which the exchange is located and the rules of the exchange ensure that these requirements are actually enforced; and |
• | the rules of the exchange effectively promote active trading of listed stocks. |
For this purpose, shares or ADRs will be considered regularly traded if, among other requirements, they are traded (other than in de minimis quantities) on at least 15 days during each calendar quarter. A US Holder who makes a mark-to-market election will not be subject to the PFIC rules described above. Instead, subject to certain limitations, such US Holder would essentially be required to take into account the difference, if any, between the fair market value and the adjusted tax basis of its shares or ADRs at the end of a taxable year as ordinary income (or, subject to certain limitations, ordinary loss), in calculating its income for such year. In addition, gains from an actual sale or other disposition of shares or ADRs will be treated as ordinary income, and any losses will be treated as ordinary losses to the extent of any “mark to market” gains for prior years that have not previously been reversed by losses.
The Company believes that it is not currently a PFIC and it does not anticipate becoming a PFIC. This belief is based in part on the Company’s market capitalization and the rules applicable to valuing the assets of publicly traded companies. The tests for determining PFIC status are applied annually and it is difficult to make accurate predictions of future income and assets, which are relevant to this determination. A decline in market capitalization or a significant increase in the amount of royalty income the Company receive could cause the Company to become a PFIC. Accordingly, the Company cannot assure you that it will not be treated as a PFIC in future years.
US Holders who hold shares or ADRs during a period when the Company is a PFIC will be subject to the foregoing rules, even if the Company ceases to be a PFIC. US Holders are urged to consult their tax advisors about the PFIC rules, including the advisability of choosing to make QEF or ‘retroactive QEF’ election and the availability of the mark-to-market election.
Page 60 of 122
US INFORMATION REPORTING AND US BACKUP WITHHOLDING TAX
Dividends paid on shares or ADRs may be subject to US information reporting requirements and backup withholding tax (currently 28%). In addition, the payment of the proceeds of a sale, exchange or redemption of shares or ADRs to a US Holder or non-US holder in the US, or through US or US-related persons, may be subject to US information reporting requirements and backup withholding tax (currently 28%).
US Holders can avoid the imposition of backup withholding tax by reporting their taxpayer identification number to their broker or paying agent on US Internal Revenue Service Form W-9. Non-US holders can avoid the imposition of backup withholding tax by providing a duly completed US Internal Revenue Service Form W-8BEN, W-8ECI or W-8IMY, as appropriate, to their broker or paying agent. Backup withholding is not an additional tax. Any amounts withheld under the backup withholding rules from a payment to a holder will be allowed as a refund or a credit against such holder’s US federal income tax liability, provided that the required returns are filed with US Internal Revenue Service on a timely basis.
Certain documents referred to in this Form 20-F are available for inspection at the registered office of the Company.
Item 11 | Quantitative and qualitative disclosures about market risk |
FINANCIAL INSTRUMENTS
The Group’s financial instruments comprise primarily cash and liquid resources, a finance lease facility amounts owing on the fill/finish facility, an overdraft facility, foreign currency contracts, short- and long-term debtors receivable under the Canton settlement and various items, such as trade debtors and trade creditors, which arise directly from its operations. The main purpose of these financial instruments is to provide working capital for the Group’s operations.
The main risks arising from the Group’s activities and involving the use of financial instruments are foreign currency risk, interest rate risk and liquidity risk. The Board reviews and agrees the Group’s objectives and policies for managing each of these risks. Details of the Group’s objectives and policies, both during the year and since the year end, are set out below, along with numerical disclosures for each category of financial instrument. Except where indicated, these disclosures are indicative of the situation throughout the year.
FOREIGN CURRENCY RISK
The Group has subsidiaries that operate and trade in the US, with revenues, expenses and financing denominated principally in US dollars. Through these overseas operations, the Group is subject to foreign exchange risk, including the risk of fluctuations in the Group’s net investment in, and reported profits from, foreign subsidiaries when translated into sterling. In addition, the UK trading subsidiary enters into contracts in a variety of foreign currencies.
The Group had overall surplus cash funds throughout the year, but had to determine in which currency to hold cash available for working capital and surplus funds. This was done with reference to anticipated future expenditure patterns and relative returns on funds held in different currencies. The Group’s current policy is to hold surplus funds in sterling over the long term, which currently achieves a higher interest rate return, whilst mitigating the risk of fluctuations in the Group’s net assets, when reported in sterling.
Page 61 of 122
From time to time, the Group makes use of forward contracts in order to reduce uncertainty over the sterling value of anticipated US dollar receipts, thereby reducing uncertainty over the level of the Group’s results when reported in sterling. Typically in 2005 the Group took out forward contracts for known significant foreign currency transactions only. There were no forward contracts outstanding at the year end.
During the year, the Group also used dual currency deposits for both euro and US dollar deposits, allowing an enhanced interest rate to be earned, which may, at maturity, be converted into sterling or dollars at the banks’ discretion, at a rate previously agreed. The Group had no dual currency deposits outstanding at the year end.
Where Group companies have monetary assets and liabilities denominated in currencies other than their functional currency, these balances are translated into that subsidiary’s functional currency. With the exception of gains and losses on those inter-company balances that are considered to be ‘as permanent as equity’ and recorded in reserves, foreign exchange gains and losses arising are recorded immediately in the income statement. These amounts include sterling-denominated cash balances held in the US, US dollar- and euro-denominated balances held by the Company, and a US dollar-denominated overdraft facility held by a UK subsidiary. In addition, the Group has other current assets and liabilities denominated in foreign currencies, which the Board does not consider to be significant.
INTEREST RATE RISK
The Group finances its operations predominantly through cash and liquid resources generated through operating activities, from the issuance of equity shares, through finance leases and through an overdraft facility. It is the Group’s policy to invest surplus cash on deposit, or in money market funds managed by professional money managers.
The performance of the investments is reviewed by management on a regular basis to ensure that competitive rates of return are being achieved, subject to the Board’s requirement relating to the accessibility of funds and standing of financial institutions used. The Board reviews regularly the financing facilities available to the Group to ensure competitive rates of interest are being obtained.
During the year, the Group invested in a cash deposit which accrues interest dependent on the sterling LIBOR (the London Interbank Offered Rate). This deposit of £10.0m was outstanding at the year-end and was valued at £10.0m (2004 – deposit £5.8m, valued at £5.7m).
The following table sets out the carrying value by maturity, for each financial instrument that is exposed to interest rate risk.
| | Within one year | | One–two years | | Total | |
2005 | | £m | | £m | | £m | |
Floating rate: | | | | | | | |
Cash | | 11.0 | | – | | 11.0 | |
| | | | | | | |
Fixed rate: | | | | | | | |
Short-term deposits | | 38.2 | | – | | 38.2 | |
Liquid investments | | 8.8 | | 10.0 | | 18.8 | |
Obligations under finance leases | | (7.1 | ) | – | | (7.1 | ) |
Page 62 of 122
| | Within one year | | One–two years | | Total | |
2004 | | £m | | £m | | £m | |
Floating rate: | | | | | | | |
Cash | | 30.2 | | – | | 30.2 | |
| | | | | | | |
Fixed rate: | | | | | | | |
Cash | | 0.7 | | – | | 0.7 | |
Short-term deposits | | 50.1 | | – | | 50.1 | |
Liquid investments | | 20.8 | | – | | 20.8 | |
Obligations under finance leases | | (3.1 | ) | (6.3 | ) | (9.4 | ) |
LIQUIDITY RISK
The Board monitors the level of cash and liquid resources on a regular basis, and management on a daily basis, to ensure that the Group has sufficient liquid funds to enable it to meet its commitments as they fall due. This is achieved through the production and review of cash forecasts, including sensitivity analyses. Approximately 60% of the Group’s cash and liquid resources are managed on a discretionary basis by a third party within strict parameters that have been set by the Board. The remainder is invested in managed funds or invested in bank deposits within the parameters set by the Board. These parameters include the requirement that the institutions used must have a minimum rating of Aa2 long-term or P-1 short-term, and a maximum investment with any one counter-party of £20m.
FAIR VALUES OF FINANCIAL ASSETS AND FINANCIAL LIABILITIES
There is no material difference between the book values and fair values of the Group’s financial assets and liabilities as at December 31, 2005. Fair values have been calculated by discounting cash flows at prevailing interest rates.
| | 2005 | | 2004 | |
| | £m | | £m | |
Assets: | | | | | |
Foreign currency contracts | | 0.1 | | – | |
Liabilities: | | | | | |
Foreign currency contracts | | – | | (0.1 | ) |
In accordance with IAS39, the Group has reviewed all contracts for embedded derivatives that are required to be separately accounted for if they do not meet certain requirements set out in the standard. This derivative is fair valued based on discounted future cash flows with gains and losses passing through the income statement as hedge accounting is not available.
The Group has an embedded derivative deposit which accrues interest dependent on UK LIBOR (the London Interbank Offered Rate).
During the year, the Group also used dual currency deposits for both euro and US dollar deposits, allowing an enhanced interest rate to be earned, which may, at maturity, be converted into sterling or dollars at the banks’ discretion, at a rate previously agreed. The Group had no dual currency deposits outstanding at the year-end (2004 – none).
From time to time, the Group makes use of forward contracts in order to reduce uncertainty over the sterling value of anticipated US dollar receipts, thereby reducing uncertainty over the level of the Group’s results when reported in sterling. Typically, in 2005 the Group took out forward contracts for known significant foreign currency only. The Group had no forward contracts outstanding at the year-end (2004 – a forward contract to sell dollars and buy sterling outstanding at the year-end).
Page 63 of 122
Item 15 | Controls and procedures |
A | Disclosure controls and procedures |
The Board has applied principle C.2 of the Combined Code by establishing a process for identifying, evaluating and managing the significant risks the Group faces. This process has been in place since the start of 2000 and is in accordance with Internal Control: Guidance for Directors on the Combined Code published in September 1999. The Board is responsible for the Group’s overall system of controls and procedures and for reviewing its effectiveness. Such a system manages rather than eliminates the risk of failure to achieve business objectives.
The Board regularly reviews the risks to which the business is exposed and the controls in place to mitigate those risks. It delegates the operational management of the business risk process to the Executive Directors, including the Chief Executive Officer and the Chief Financial Officer, who in turn have put in place a specific working group comprising senior management from different areas of the business to carry out reviews on a periodic basis. From 2005, the Operations Committee, which comprises senior operational managers, reports to the Executive Committee, and which has oversight of the day-to-day operational activities of Acambis, has been responsible for managing the risk reviews.
In conjunction with the reviews described above, the Chief Executive Officer and Chief Financial Officer evaluated the effectiveness of the design and operation of the Company’s disclosure controls and procedures as of December 31, 2005. Based upon that evaluation, the Chief Executive Officer and Chief Financial Officer concluded that the design and operation of these disclosure controls and procedures were effective as of December 31, 2005.
B | Management’s annual report on internal control over financial reporting |
In compliance with provision C.2.1 of the Combined Code, the Board reviews the effectiveness of the Group’s system of internal control. The Board’s monitoring covers all material controls, including financial, operational and compliance controls and risk management. It is based, principally, on reviewing reports from management to consider whether significant risks are identified, evaluated, managed and controlled and whether any significant weaknesses are promptly remedied or indicate a need for more extensive monitoring. The Board has also performed a specific assessment for the purpose of this Annual Report on form 20-F considering all significant aspects of internal control arising during the year. The Audit Committee assists the Board in discharging its review responsibilities.
The Combined Code was republished in July 2003 and restated in July 2005 by the Financial Reporting Council and incorporated the previous code (as published in 1998 by the Hampel Committee) and related guidance that had been issued since that date: the Turnbull Guidance on Internal Control; the Smith Guidance On Audit Committees; and various items of good practice guidance from the Higgs Report. The code has been applicable for reporting years beginning on or after November 1, 2003 and, therefore, was adopted by Acambis from its 2004 financial year, which began on January 1, 2004. The overriding principle of the Combined Code is that companies must comply with it or explain why they have not. The following section highlights the areas where Acambis previously did not comply with the code and notes the progress made to address those areas:
Page 64 of 122
CODE PROVISION | | PROGRESS MADE SINCE PUBLICATION OF THE 2004 FORM 20-F | |
B REMUNERATION
B.2.1 A statement on whether remuneration consultants have any other connection with the Company should be available on the Acambis website. | | A disclosure is made in the Corporate Governance Statement in the ‘Remuneration Committee’ section. A statement has been available on the Acambis website since early 2005. | |
C ACCOUNTABILITY AND AUDIT
C.3.4 Arrangements should be in place for the reporting and management of concerns raised by staff about possible financial or other improprieties. | | In November 2004, the Audit Committee approved a whistleblowing policy. The procedure was developed during 2005 and rolled out to the Group in early 2006. | |
D | Changes in internal control over financial reporting |
During the period covered by this Annual Report on Form 20-F, there were no changes in the Group’s internal control over financial reporting identified in connection with management’s evaluation of such control that materially affected, or are reasonably likely to materially affect, the Group’s internal control over financial reporting.
Item 16A | Audit committee financial expert |
Ross Graham was appointed to the Board of Acambis as a Non-executive Director on March 25, 2004 and was also appointed at that time as Chairman of the Audit Committee. Mr Graham is considered to be an independent Non-executive Director. He meets the requirements of an ‘audit committee financial expert’, having been a qualified Chartered Accountant for over 30 years, having served as Chief Financial Officer of Misys plc, a company quoted on the LSE, until 1998, and having served as Audit Committee Chairman on the Board of Wolfson Microelectronics plc since September 2003.
The Group has complied with the provision of the Code of Best Practice set out in Section 1 of the UK Combined Code throughout 2005, and applied the Principles of Good Governance as set out within the Combined Code. Additionally, the Group applied a formal code of conduct and ethics for senior financial officers, which was adopted in July, 2005. This code met the requirements of the Sarbanes-Oxley Act 2002 and the requirements of the NASDAQ National Market.
Item 16C | Principal accountant fees and services |
During the year the Group obtained the following services from the Group’s auditors:
| | 2005 | | 2004 | |
| | £m | | £m | |
Audit fees | | | 0.2 | | | 0.1 | |
Audit related fees | | | 0.1 | | | 0.1 | |
Tax fees | | | 0.2 | | | 0.3 | |
All other fees | | | - | | | - | |
| | | 0.5 | | | 0.5 | |
Page 65 of 122
The Audit Committee follows an Audit and Non-Audit Services Pre-Approval practice, which applies to the Group’s primary auditors and any other firm serving as an auditor to any entities in the Group. The Audit Committee has delegated the pre-approval of non-audit services to be performed by the primary auditors to the Audit Committee Chairman, and, where appropriate the Audit Committee Chairman refers back to the full Audit Committee for approval. The policy requires all audit engagements to be approved by the Audit Committee Chairman or by the full Audit Committee. It prohibits Group entities from engaging the auditors in activities prohibited by the SEC. The practice permits the auditors to be engaged for other services provided the engagement meets the criteria of pre-approved activities and is notified to the Audit Committee.
Item 17 | Financial statements |
DIRECTORS’ RESPONSIBILITIES
Company law requires the Directors to prepare financial statements for each financial year that give a true and fair view of the state of affairs of the Company and the Group and of the profit or loss of the Group for that period.
FINANCIAL STATEMENTS, INCLUDING ADOPTION OF GOING CONCERN BASIS
After making enquiries, the Directors have a reasonable expectation that the Company and the Group have adequate resources to continue in operational existence for the foreseeable future. For this reason, they continue to adopt the going concern basis in preparing the financial statements.
In preparing the financial statements, the Directors are required to:
• | select suitable accounting policies and then apply them consistently; |
• | make judgments and estimates that are reasonable and prudent; |
• | state whether applicable accounting standards have been followed, subject to any material departure disclosed and explained in the financial statements; and |
• | prepare the financial statements on the going concern basis unless it is inappropriate to presume that the Group will continue in business. |
The Directors are responsible for keeping proper accounting records that disclose with reasonable accuracy at any time the financial position of the Company and the Group and enable them to ensure that the financial statements comply with the Companies Act 1985. They are also responsible for safeguarding the assets of the Company and the Group and, hence, for taking reasonable steps for the prevention and detection of fraud and other irregularities.
The Directors are responsible for the maintenance and integrity of the Group’s website. Uncertainty regarding legal requirements is compounded, as information published on the Internet is accessible in many countries with different legal requirements relating to the preparation and dissemination of financial statements.
Page 66 of 122
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
TO THE BOARD OF DIRECTORS AND THE SHAREHOLDERS OF ACAMBIS PLC
In our opinion, the accompanying consolidated balance sheets, and the related consolidated income statements, statements of changes in shareholders’ equity and consolidated cash flow statements present fairly, in all material respects, the financial position of Acambis plc and its subsidiaries at 31 December 2005 and 2004, and the results of their operations and their cash flows for each of the two years in the period ended 31 December 2005, in conformity with International Financial Reporting Standards as adopted by the European Union. These financial statements are the responsibility of the Company’s management; our responsibility is to express an opinion on these financial statements based on our audits. We conducted our audits of these statements in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
International Financial Reporting Standards as adopted by the European Union vary in certain significant respects from accounting principles generally accepted in the United States of America. Information relating to the nature and effect of such differences is presented in Note 29 to the consolidated financial statements.
PricewaterhouseCoopers LLP
Cambridge, England
June 26, 2006
Page 67 of 122
CONSOLIDATED INCOME STATEMENT FOR THE YEARS ENDED DECEMBER 31
| | | | 2005 | | 2004 | |
| | Notes | | £m | | £m | |
| | | | | | | |
Revenue | | 2 | | 40.9 | | 85.5 | |
Cost of sales | | | | (27.6 | ) | (35.0 | ) |
Gross profit | | | | 13.3 | | 50.5 | |
Research and development costs | | | | (34.1 | ) | (29.3 | ) |
Sales and marketing costs | | | | (2.6 | ) | (2.8 | ) |
Administration costs (including costs relating to Canton plant impairment and restructuring costs) | | 3 | | (7.7 | ) | (5.5 | ) |
Other operating income – settlement of Canton agreement | | 3 | | – | | 10.2 | |
Other operating income – fair value of shares received for grant of license | | 3 | | 0.4 | | – | |
Operating (loss)/profit | | 4 | | (30.7 | ) | 23.1 | |
Finance income | | 3 | | 4.0 | | 4.8 | |
Finance costs | | 3 | | (1.0 | ) | (0.9 | ) |
(Loss)/profit on ordinary activities before taxation | | | | (27.7 | ) | 27.0 | |
Taxation – UK | | 5 | | (1.7 | ) | (3.7 | ) |
Taxation – overseas | | 5 | | 2.4 | | (3.6 | ) |
(Loss)/profit on ordinary activities after taxation attributable to shareholders | | | | (27.0 | ) | 19.7 | |
Basic (loss)/earnings per ordinary share (in pence) | | 6 | | (25.2 | ) p | 18.5 | p |
Diluted (loss)/earnings per ordinary share (in pence) | | 6 | | (25.2 | ) p | 18.1 | p |
A statement of changes in equity is given in note 24.
The accompanying notes are an integral part of this consolidated income statement.
All amounts in 2005 and 2004 arise from continuing operations.
CONSOLIDATED STATEMENT OF RECOGNIZED INCOME AND EXPENSES FOR THE YEARS ENDED DECEMBER 31
| | 2005 | | 2004 | |
| | £m | | £m | |
| | | | | |
Retained (loss)/profit for the year | | (27.0 | ) | 19.7 | |
Gain/(loss) on foreign currency exchange | | 1.6 | | (2.5 | ) |
Revaluation of available-for-sale investment (net of deferred tax) | | 0.1 | | – | |
Total (expense)/income recognized for the year | | (25.3 | ) | 17.2 | |
Page 68 of 122
CONSOLIDATED BALANCE SHEET AT DECEMBER 31
| | | | 2005 | | 2004 | |
| | Notes | | £m | | £m | |
Assets | | | | | | | | | |
Non-current assets | | | | | | | | | |
Goodwill | | 8 | | | 14.9 | | | 15.4 | |
Other intangible assets | | 9 | | | 4.2 | | | 4.1 | |
Property, plant and equipment | | 10 | | | 19.8 | | | 18.5 | |
Deferred tax asset | | 5 | | | 0.3 | | | – | |
Financial assets: available-for-sale investment | | 12 | | | 0.6 | | | – | |
Other non-current assets | | 13 | | | – | | | 2.5 | |
| | | | | 39.8 | | | 40.5 | |
Current assets | | | | | | | | | |
Inventory | | 14 | | | 3.6 | | | 6.0 | |
Current tax assets | | | | | 1.3 | | | 1.9 | |
Trade and other receivables | | 15 | | | 20.6 | | | 13.7 | |
Financial assets: derivative financial instruments | | 16 | | | 0.1 | | | – | |
Liquid investments | | 16 | | | 18.8 | | | 20.8 | |
Cash and cash equivalents | | 17 | | | 49.2 | | | 81.0 | |
| | | | | 93.6 | | | 123.4 | |
Liabilities | | | | | | | | | |
Current liabilities | | | | | | | | | |
Financial liabilities: | | | | | | | | | |
– short-term borrowings | | 18 | | | (4.0 | ) | | (3.6 | ) |
– short-term financial liabilities | | 18 | | | (7.2 | ) | | (3.1 | ) |
– derivative financial instruments | | 16 | | | – | | | (0.1 | ) |
Trade and other payables | | 19 | | | (16.1 | ) | | (8.3 | ) |
Accruals and deferred income | | | | | (14.1 | ) | | (27.9 | ) |
Income tax payable | | | | | (3.1 | ) | | (4.6 | ) |
Provisions | | 20 | | | (2.3 | ) | | – | |
| | | | | (46.8 | ) | | (47.6 | ) |
Net current assets | | | | | 46.8 | | | 75.8 | |
| | | | | | | | | |
Non-current liabilities | | | | | | | | | |
Investment in Joint Venture | | 21 | | | (0.3 | ) | | (0.3 | ) |
Long-term financial liabilities | | 18 | | | (1.6 | ) | | (6.3 | ) |
Other non-current liabilities | | 22 | | | – | | | (0.5 | ) |
Deferred tax liabilities | | 5 | | | (1.7 | ) | | (1.7 | ) |
| | | | | (3.6 | ) | | (8.8 | ) |
Net assets | | | | | 83.0 | | | 107.5 | |
| | | | | | | | | |
Shareholders’ equity | | | | | | | | | |
Share capital | | 23 | | | 10.7 | | | 10.7 | |
Share premium | | 24 | | | 98.0 | | | 97.8 | |
Other reserves | | 24 | | | (0.9 | ) | | (2.5 | ) |
Retained earnings | | 24 | | | (24.8 | ) | | 1.5 | |
Total shareholders’ equity | | | | | 83.0 | | | 107.5 | |
Page 69 of 122
COMPANY BALANCE SHEET AT DECEMBER 31
| | Notes | | 2005
£m | | 2004
£m | |
Assets | | | | | | | |
Non-current assets | | | | | | | |
Investments in subsidiaries | | 11 | | 15.9 | | 15.5 | |
Amounts owed by subsidiary undertakings | | | | 29.2 | | 26.1 | |
Other non-current assets | | 13 | | – | | 0.6 | |
| | | | 45.1 | | 42.2 | |
Current assets | | | | | | | |
Trade and other receivables | | 15 | | 2.5 | | 1.2 | |
Amounts owed by subsidiary undertakings | | | | 17.6 | | – | |
Financial assets: derivative financial instruments | | 16 | | 0.1 | | – | |
Liquid investments | | 16 | | 18.8 | | 17.8 | |
Cash and cash equivalents | | 17 | | 42.5 | | 70.3 | |
| | | | 81.5 | | 89.3 | |
Liabilities | | | | | | | |
Current liabilities | | | | | | | |
Trade and other payables | | 19 | | – | | (0.1 | ) |
Amounts owed to subsidiary undertakings | | | | – | | (16.0 | ) |
Accruals and deferred income | | | | (1.1 | ) | (0.7 | ) |
Financial liabilities: derivative financial instruments | | 16 | | – | | (0.1 | ) |
Income tax payable | | | | (2.1 | ) | (1.1 | ) |
| | | | (3.2 | ) | (18.0 | ) |
Net current assets | | | | 78.3 | | 71.3 | |
| | | | | | | | |
Net assets | | | | 123.4 | | 113.5 | |
| | | | | | | |
Shareholders’ equity | | | | | | | |
Share capital | | 23 | | 10.7 | | 10.7 | |
Share premium | | 24 | | 97.8 | | 97.6 | |
Retained earnings | | 24 | | 14.9 | | 5.2 | |
Total shareholders’ equity | | | | 123.4 | | 113.5 | |
Page 70 of 122
CONSOLIDATED CASH FLOW STATEMENT FOR THE YEARS ENDED DECEMBER 31
| | Notes | | 2005
£m | | 2004
£m | |
Operating activities | | | | | | | |
(Loss)/profit on ordinary activities before tax | | | | (27.7 | ) | 27.0 | |
Depreciation and amortization | | | | 5.3 | | 6.3 | |
Increase in working capital | | | | (2.8 | ) | (51.1 | ) |
Other non-cash movements | | | | (0.7 | ) | 2.6 | |
Net finance costs | | | | (3.0 | ) | (3.9 | ) |
Taxes paid | | | | (0.4 | ) | (1.6 | ) |
| | | | | | | |
Cash flows from operating activities | | | | (29.3 | ) | (20.7 | ) |
Investing activities | | | | | | | |
Purchase of business operations | | | | (1.7 | ) | (0.8 | ) |
Disposal of investments | | | | – | | 0.7 | |
Purchase of intangibles | | | | (0.4 | ) | – | |
Purchase of property, plant and equipment | | | | (3.7 | ) | (3.4 | ) |
| | | | | | | |
Cash flows used in investing activities | | | | (5.8 | ) | (3.5 | ) |
Financing activities | | | | | | | |
Interest element of finance lease payments | | | | (0.6 | ) | (0.7 | ) |
Interest paid | | | | (0.2 | ) | (0.1 | ) |
Interest received | | | | 3.8 | | 4.4 | |
Proceeds from issues of shares | | | | 0.2 | | 1.9 | |
Purchase of own shares | | 24 | | (0.2 | ) | – | |
Capital element of finance lease payments | | | | (3.3 | ) | (2.5 | ) |
Purchase of liquid investments | | | | (34.8 | ) | (62.6 | ) |
Sale of liquid investments | | | | 36.8 | | 59.6 | |
Cash flows from financing activities | | | | 1.7 | | – | |
| | | | | | | |
Decrease in cash and cash equivalents | | | | (33.4 | ) | (24.2 | ) |
Net foreign exchange difference | | | | 1.6 | | (2.2 | ) |
Cash and cash equivalents at January 1, | | 17 | | 81.0 | | 107.4 | |
| | | | | | | |
Cash and cash equivalents at December 31, | | 17 | | 49.2 | | 81.0 | |
COMPANY CASH FLOW STATEMENT FOR THE YEARS ENDED DECEMBER 31
| | Notes | | 2005
£m | | 2004
£m | |
Operating activities | | | | | | | |
Profit on ordinary activities before tax | | | | 6.9 | | 6.7 | |
Increase in working capital | | | | (33.0 | ) | (1.2 | ) |
Other non-cash movements | | | | 3.6 | | 3.7 | |
Net finance costs | | | | (6.3 | ) | (4.8 | ) |
Taxes paid | | | | (1.7 | ) | (0.5 | ) |
Cash flows(used in)/from operating activities | | | | (30.5 | ) | 3.9 | |
Page 71 of 122
COMPANY CASH FLOW STATEMENT FOR THE YEARS ENDED DECEMBER 31 (cont’d)
Financing activities | | | | | | | |
Interest received | | | | 5.8 | | 4.5 | |
Proceeds from issues of shares | | | | 0.2 | | 1.9 | |
Purchase of own shares | | 24 | | (0.2 | ) | – | |
Purchase of liquid investments | | | | (34.8 | ) | (33.8 | ) |
Sale of liquid investments | | | | 33.8 | | 27.0 | |
Cash flows from/(used in) financing activities | | | | 4.8 | | (0.4 | ) |
(Decrease)/increase in cash and cash equivalents | | | | (25.7 | ) | 3.5 | |
| | | | | | | | |
Net foreign exchange difference | | | | (2.1 | ) | (1.1 | ) |
Cash and cash equivalents at January 1, | | 17 | | 70.3 | | 67.9 | |
| | | | | | | |
Cash and cash equivalents at December 31, | | 17 | | 42.5 | | 70.3 | |
The accompanying notes are an integral part of this Company cash flow statement.
Notes to the Group financial statements
1 ACCOUNTING POLICIES
BASIS OF PREPARATION
The consolidated financial statements of Acambis plc have been prepared in accordance with IFRS and International Financial Reporting Interpretations Committee interpretations that have been adopted for use in the European Union and with those parts of the Companies Act 1985 applicable to companies reporting under IFRS. The consolidated financial statements have been prepared on a historical cost basis as modified by the revaluation of available-for-sale investments, except for derivative financial instruments which have been measured at fair value. The consolidated financial statements are presented in pounds sterling and all values are rounded to one decimal point of the nearest million (£m) except where otherwise indicated.
The preparation of financial statements in conformity with generally accepted accounting principles requires the use of estimates and assumptions that affect the reported amounts of assets and liabilities at the date of the financial statements and of revenues and expenses during the reporting period. Although these estimates are based on Management’s best knowledge of the amount, event or action, actual results may ultimately differ from those estimates.
CHANGE IN ACCOUNTING POLICY
The consolidated financial statements of Acambis plc have been prepared, for the first time, in accordance with IFRS. The effect of adoption of IFRS is described in note 28 of these financial statements. A summary of the more important Group accounting policies is set out below.
These accounting policies have been consistently applied in the preparation of these financial statements.
TRANSITIONAL PROVISIONS
The rules for first-time adopters of IFRS are set out in IFRS1 ‘First time adoption of IFRS’, which allows certain transitional provisions. Acambis has applied the exemption granted by IFRS1 to goodwill acquired before August 2003. The value of goodwill relating to the acquisition of Acambis Inc. in 1999 is frozen as at January 1, 2004, whilst that relating to the acquisition of BPC in August 2003 has been restated in accordance with IFRS3 ‘Business combinations’.
BASIS OF CONSOLIDATION
The Group financial statements include and consolidate the financial statements of Acambis plc and each of its subsidiary undertakings. Acquisitions made by the Group are accounted for under the acquisition method of accounting and the Group financial statements include the results of such subsidiaries from the relevant date of acquisition. Intra-Group transactions and profits are eliminated fully on consolidation.
Page 72 of 122
REVENUE
Group revenue comprises the value of sales from products and income (excluding VAT and taxes, trade discounts and intra-Group transactions) derived from contract research fees and license fees receivable from third parties in the normal course of business. Revenue from product sales is recognized when the risks and rewards of ownership have been transferred to the customer. Where the Group is required to undertake R&D activities and the fee is creditable against services provided by the Group, that revenue is deferred and recognized over the period over which the services are performed. Contract research fees are recognized in the accounting period in which the related work is carried out. Milestones receivable are recognized when they fall contractually due.
Profit is recognized on long-term contracts when the final outcome can be assessed with reasonable certainty by including turnover and related costs within the income statement as contract activity progresses. Turnover is recognized according to the extent of performance under the contract. In determining the degree of contractual performance, reference is made to the costs incurred in relation to the total estimated expected costs.
The smallpox vaccine contract with the CDC, awarded to Acambis in November 2001, is a fixed-fee arrangement requiring the delivery of products as well as a concurrent R&D program. As the two transactions are linked in such a way that the commercial effect cannot be understood without reference to the series of transactions as a whole, this arrangement has been treated as a single long-term contract, whose elements have not been accounted for separately as required under IAS18.
Turnover and profits are recognized according to the extent of performance under the contract, as described above. Manufacturing costs in respect of this contract are deemed to be incurred when the risks and rewards of ownership have been transferred, as described above; R&D costs are recognized as incurred.
COST OF SALES
The Group has classified manufacturing costs and costs that are directly attributable to funded research and vaccine manufacture as cost of sales.
RESEARCH AND DEVELOPMENT COSTS
Research costs are expensed as incurred. Internally generated expenditure arising from development (or from the development phase of an internal project) is capitalized if, and only if, it satisfies all of six specified criteria in IAS38 ‘Intangible assets’. It is management’s opinion that it is not possible to satisfy the requirement to demonstrate the technical feasibility of a project, and that it will generate probable future economic benefits, until final submission for regulatory approval has been obtained.
SHARE-BASED PAYMENT TRANSACTIONS
Employees (including Directors) of the Group may receive some remuneration in the form of share-based payment transactions, whereby employees render services in exchange for shares or rights over shares (equity-settled transactions).
The cost of equity-settled transactions with employees is measured by reference to the fair value at the date at which they are granted. Fair value is determined in conjunction with an external valuer, using a binomial option pricing model for the SAYE Scheme and the ESPP. The fair value of awards made under the 1996 Acambis Share Option Scheme (the 1996 Plan), the 1999 Acambis Share Option Plan (the 1999 Plan) and the LTIP is measured using a binomial option pricing model adjusted to reflect the TSR market-based performance condition. For all options and awards with a TSR market-based performance condition the pricing model used follows similar principles to the Monte Carlo approach to value the award and takes into account the fact that TSR vesting and share price performance are not independent.
The cost of equity-settled transactions is recognized, together with a corresponding increase in equity, over the year in which the performance conditions are fulfilled, ending on the date on which the relevant employees become fully entitled to the award (vesting date). The cumulative expense recognized for equity-settled transactions at each reporting date until the vesting date reflects the extent to which the vesting period has expired and the number of awards that, in the opinion of the Directors, will ultimately vest. The cost is allocated to R&D costs, sales and marketing costs and administration costs on the basis of headcount.
Page 73 of 122
No expense is recognized for awards that do not ultimately vest, except for awards where vesting is conditional upon a market condition. These are treated as vesting, irrespective of whether or not the market condition is satisfied, provided that all other performance conditions are satisfied.
The dilutive effect of outstanding options is reflected as additional share dilution in the computation of earnings per share. The Group has an employee share incentive plan and an employee share trust for the granting of non-transferable options to executives and senior employees. Shares in the Group held by the employee share trust are treated as treasury shares and presented in the balance sheet as a deduction from equity.
The Group has taken advantage of the transitional provisions of IFRS2 ‘Share based payments’ in respect of equity-settled awards and has applied IFRS2 only to equity-settled awards granted after 7 November 2002 that had not vested on December 31, 2004.
In the Company accounts, the granting of options to employees of subsidiaries is deemed a capital contribution.
TAXATION
The tax expense represents the sum of the tax currently payable and deferred tax, including UK corporation tax and foreign tax.
The tax currently payable is based on taxable profit for the year. Taxable profit differs from net profit as reported in the income statement because it excludes items of income or expense that are taxable or deductible in other years and it further excludes items that are never taxable or deductible. The Group’s liability for current tax is calculated using tax rates that have been enacted or substantively enacted by the balance sheet date.
Deferred income tax is provided, using the liability method, on all temporary differences at the balance sheet date between the tax bases of assets and liabilities and their carrying amounts for financial reporting purposes.
Deferred income tax assets and liabilities are recognized for all deductible temporary differences, carry-forward of unused tax losses, to the extent that it is probable that taxable profit will be available against which the deductible temporary differences, carry-forward of unused tax losses can be utilized:
• | except where the deferred income tax asset or liability relating to the deductible temporary difference arises from the initial recognition of an asset or liability in a transaction that is not a business combination and, at the time of the transaction, affects neither the accounting profit nor taxable profit or loss; and |
• | in respect of deductible temporary differences associated with investments in subsidiaries, associates and interests in joint ventures, deferred tax assets or liabilities are only recognized to the extent that it is probable that the temporary differences will reverse in the foreseeable future and taxable profit will be available against which the temporary difference can be utilized. |
In the UK and the US, the Group is entitled to a tax deduction for the amount treated as compensation on exercise of certain employee share options under each jurisdiction’s tax rules. As explained under ‘Share-based payment transactions’ above, a compensation expense is recorded in the Group’s income statement over the period from the grant date to the vesting date of the relevant options. As there is a temporary difference between the accounting and tax bases, a deferred tax asset is recorded. The deferred tax asset arising is calculated by comparing the estimated amount of tax deduction to be obtained in the future (based on the Company’s share price at the balance sheet date) with the cumulative amount of the compensation expense recorded in the income statement. If the amount of estimated future tax deduction exceeds the cumulative amount of the remuneration expense at the statutory tax rate, the excess is recorded directly in equity, against the profit and loss reserve.
No compensation charge is recorded in respect of options granted before 7 November 2002 or in respect of those options which have been exercised or have lapsed before December 31, 2004. Nevertheless, tax deductions have arisen and will continue to arise on these options.
Page 74 of 122
The tax effects arising in relation to these options are recorded directly in equity, against the profit and loss reserve.
The carrying amount of deferred income tax assets is reviewed at each balance sheet date and reduced to the extent that it is no longer probable that sufficient taxable profit will be available to allow all or part of the deferred income tax asset to be utilized.
Deferred income tax assets and liabilities are measured at the tax rates that apply to the period when the asset is realized or the liability is settled, based on tax rates (and tax laws) that have been enacted or substantively enacted at the balance sheet date. Income tax relating to items recognized directly in equity is recognized in equity and not in the income statement.
GOODWILL
Goodwill on acquisition is initially measured at cost, being the excess of the cost of the business combination over the acquirer’s interest in the net fair value of the identifiable assets, liabilities and contingent liabilities. The fair value of the consideration is determined by applying appropriate discounts to contingent and deferred consideration, to the level where the Group considers those liabilities will be payable. Where the consideration for the acquisition of a business includes non-interest bearing cash payments due after more than one year, the liability is recorded at its present value, after applying a discount rate that approximates to that which a lender would typically require for a similar transaction, and taking into account the risk/likelihood of the payment being made.
Where revisions are made to the expected amounts of contingent consideration payable as a result of changes to estimates, such changes are accounted for at the date of the change in estimate. Following initial recognition, goodwill is not amortized but is measured at cost less any accumulated impairment losses. Goodwill is reviewed for impairment annually or more frequently if events or changes in circumstances indicate that the carrying value may be impaired.
INTANGIBLE ASSETS
Separately identifiable intangible assets acquired are capitalized at cost and those acquired from a business acquisition are capitalized at fair value as at the date of acquisition. Following initial recognition, the cost model is applied. The useful lives of these intangible assets are assessed to be either finite or indefinite. Where amortization is charged on assets with finite lives, this expense is taken to the income statement. In the case of assets acquired relating to BPC this is through the ‘Cost of sales’ line item.
Intangible assets are tested for impairment when a trigger event occurs. Useful lives are also examined on an annual basis and adjustments, where applicable, are made on a prospective basis. Useful lives are as follows: Distribution contract – 88 months Software assets – 3 years R&D technology – Variable, depending on technology
PROPERTY, PLANT AND EQUIPMENT
Property, plant and equipment is stated at cost less accumulated depreciation and any impairment in value. Land is not depreciated. Depreciation is calculated on a straight-line basis over the estimated useful life of the asset as follows:
Freehold buildings | 39 years |
Leasehold buildings | 15 years or term of lease if shorter |
Laboratory and manufacturing equipment | 4 to 7 years |
Office equipment | 3 to 5 years |
The carrying values of property, plant and equipment are reviewed for impairment when events or changes in circumstances indicate the carrying value may not be recoverable. If any such indication exists and where the carrying values exceed the estimated recoverable amount, the assets or cash-generating units are written down to their recoverable amount. The recoverable amount of property, plant and equipment is the greater of net selling price and value in use. In assessing value in use, the estimated future cash flows are discounted to their present value using a pre-tax discount rate that reflects current market assessments of the time value of money and the risks specific to the asset.
Page 75 of 122
For an asset that does not generate largely independent cash inflows, the recoverable amount is determined for the cash-generating unit to which the asset belongs. Impairment losses are recognized in the income statement.
An item of property, plant and equipment is de-recognized upon disposal or when no future economic benefits are expected to arise from the continued use of the asset. Any gain or loss arising on de-recognition of the asset (calculated as the difference between the net disposal proceeds and the carrying amount of the item) is included in the income statement in the year the item is derecognized.
The Group does not capitalize interest charges on loans to fund the purchase of tangible fixed assets.
INVESTMENTS
Investments in subsidiaries are shown at cost less any provision for impairment. Available-for-sale investments are recorded at fair value. Unrealized holding gains and any temporary unrealized holding losses after the initial recognition are reflected through reserves, net of related taxes. Impairment losses and realized gains and losses are reported in the income statement.
RECOVERABLE AMOUNT OF NON-CURRENT ASSETS
At each reporting date, the Group assesses whether there is any indication that an asset may be impaired. Where an indicator of impairment exists, the Group makes a formal estimate of recoverable amount. Where the carrying amount of an asset exceeds its recoverable amount the asset is considered impaired and is written down to its recoverable amount. Recoverable amount is the higher of an asset’s or cash-generating unit’s fair value less costs to sell and its value in use and is determined for an individual asset, unless the asset does not generate cash inflows that are largely independent of those from other assets or groups of assets.
INVENTORIES, EXCLUDING LONG-TERM CONTRACTS
Inventories are valued at the lower of cost and net realizable value.
Costs incurred in bringing each product to its present location and condition are accounted for as follows:
Raw materials | purchase cost on a first-in, first-out basis |
Finished goods and work in progress | cost of direct materials and labor and a proportion of manufacturing overheads based on normal operating capacity but excluding borrowing costs. |
Net realizable value is the estimated selling price in the ordinary course of business, less estimated costs of completion and the estimated costs necessary to make the sale.
The Group assesses at each reporting date whether there is an indication that inventory may be impaired, or if the estimate of the net realisable value of the inventory is lower than the carrying value. Inventory is valued at the lower of its cost and its net realisable value, and any write down is recognised in the income statement.
FINANCIAL INSTRUMENTS
From time to time, the Group uses derivative financial instruments in the form of sterling and foreign currency contracts to hedge its risks associated with foreign currency fluctuations and those in the form of yield-enhancing deposits to maximize interest rates. Such derivative financial instruments are stated at fair value with movements in fair value recorded in the income statement. The fair value of forward exchange contracts is calculated by reference to current forward exchange rates for contracts with similar maturity profiles.
The Group makes certain deposits in foreign currencies for fixed terms (dual currency deposits) which, at the option of the bank, mature in that foreign currency or are converted to another currency at a pre-agreed exchange rate. The Group considers that such arrangements contain an embedded derivative element, which is separated from the host contract and accounted for as a derivative financial instrument under IAS39 ‘Recognition and measurement of financial instruments’. This is initially stated in the balance sheet at cost. After initial recognition, it is measured at fair value with movements in fair value recorded in the income statement.
Page 76 of 122
A gain or loss arising from a change in the fair value of a financial asset or financial liability classified as at fair value through the profit or loss is recognized in the income statements.
CASH AND CASH EQUIVALENTS
Cash and cash equivalents comprise cash at bank and in hand and short-term deposits with an original maturity of three months or less.
BORROWING COSTS
Borrowing costs are recognized as an expense when incurred.
ACCOUNTING FOR DERIVATIVE FINANCIAL INSTRUMENTS AND HEDGING ACTIVITIES
Derivatives are initially accounted and measured at fair value on the date a derivative contract is entered into and subsequently measured at fair value. The gain or loss on re-measurement is taken to the income statement except where the derivative is a designated cash-flow hedging instrument. The accounting treatment of derivatives classified as hedges depends on their designation, which occurs on the date on which a commitment to the derivative contract is made.
The Group designates derivatives as:
• | A hedge of the fair value of an asset or liability (fair value hedge); |
• | A hedge of the income/cost of a highly probable forecasted transaction or commitment (cash flow hedge); or |
• | A hedge of a net investment in a foreign entity. |
In order to qualify for hedge accounting, the Group is required to document in advance the relationship between the item being hedged and the hedging instrument. The Group is also required to document and demonstrate an assessment of the relationship between the hedged item and the hedging instrument, which shows that the hedge will be highly effective on an ongoing basis.
This effectiveness testing is re-performed at each period end to ensure that the hedge remains highly effective. Gains or losses on fair value hedges that are regarded as highly effective are recorded in the income statement with the gain or loss on the hedged item attributable to the hedged risk.
Gains or losses on cash flow hedges that are regarded as highly effective are recognized in equity. Where the forecast transaction results in a financial asset or liability only gains or losses previously recognized in equity are reclassified to profit or loss in the same period as the asset or liability affects profit or loss. Where the forecasted transaction or commitment results in a non-financial asset or liability, any gains or losses previously deferred in equity are included in the cost of the related asset or liability. If the forecasted transaction or commitment results in future income or expenditure, gains or losses deferred in equity are transferred to the income statement in the same period as the underlying income or expenditure. The ineffective portions of the gain or loss on the hedging instrument are recognized in profit or loss. For the portion of hedges deemed ineffective or transactions that do not qualify for hedge accounting under IAS39, any change in assets or liabilities is recognized immediately in the income statement. Where a hedge no longer meets the effectiveness criteria, any gains or losses deferred in equity are only transferred to the income statement when the committed or forecasted transaction is recognized in the income statement. However, where an entity applied cash flow hedge accounting for a forecasted or committed transaction that is no longer expected to occur, then the cumulative gain or loss that has been recorded in equity is transferred to the income statement. When a hedging instrument expires or is sold, any cumulative gain or loss existing in equity at the time remains in equity and is recognized when the forecast transaction is ultimately recognized in the income statement.
LEASES
Finance leases, which transfer to the Group the risks and benefits incidental to ownership of the leased item, are capitalized at the inception of the lease at the fair value of the leased property or, if lower, at the present value of the minimum lease payments. Lease payments are apportioned between the finance charges and reduction of the lease liability so as to achieve a constant rate of interest on the remaining balance of the liability. Finance charges are charged directly against income.
Where the Group enters into transactions which meet the criteria for a sale and finance leaseback, the difference between the sale price of the asset and its previous carrying value is deferred and amortized over the lease term.
Page 77 of 122
Capitalized leased assets are depreciated over the shorter of the estimated useful life of the asset or the lease term. Leases where the lessor retains the risks and benefits of ownership of the asset are classified as operating leases.
Operating lease payments are recognized as an expense in the income statement on a straight-line basis over the lease term.
PROVISIONS
Provisions are recognized when the Group has a present obligation (legal or constructive) as a result of a past event, it is probable that costs will be required to be incurred to settle the obligation and a reliable estimate can be made of the amount of the obligation. Legal fees associated with litigation arising as a result of circumstances in existence at the balance sheet date are provided for based on management’s best estimate of costs to be incurred.
FOREIGN CURRENCY AND TRANSLATION
Transactions denominated in foreign currencies are recorded in the functional currency of the Group entity at actual exchange rates as at the date of the transaction. Monetary assets and liabilities denominated in foreign currencies are translated at the rates ruling at the balance sheet date. All differences are taken to the income statement except where financing of a foreign subsidiary through long-term loans and deferred trading balances is intended to be as permanent as equity, such loans and inter-Company balances are treated as part of the net investment and, as such, any exchange differences arising are dealt with as adjustments to reserves.
Assets and liabilities of overseas subsidiary and joint venture undertakings are translated into sterling at rates of exchange ruling at the balance sheet date. The results and cash flows of overseas subsidiary and joint venture undertakings are translated into sterling using average rates of exchange. Exchange adjustments arising when the opening net assets and the profits for the year retained by overseas subsidiary and joint venture undertakings are translated into sterling are taken directly to equity. On disposal of a foreign entity, accumulated exchange differences are recognized in the income statement as a component of the gain or loss on disposal.
Goodwill and fair value adjustments arising on the acquisition of a foreign entity are treated as assets and liabilities of the acquiring company and are recorded at the exchange rate at the date of the transaction. The Group has taken advantage of the provisions under IFRS1, and does not have to apply this to acquisitions made before August 2003.
ESOP TRUST
The Company recognizes the assets and liabilities of the ESOP trust in its own accounts and shares held by the trust are recorded at cost as a deduction in arriving at shareholders’ funds until such time as the shares vest unconditionally to employees. The trust is a separately administered trust, funded by loans from the Company, whose assets comprise shares in the Company.
FUTURE PRONOUNCEMENTS
At the date of approval of these financial statements the following standards and interpretations which have not been applied in these financial statements were in issue but not yet effective:
• | an amendment to IAS21 ‘The effects of changes in foreign exchange rates’ in respect of an entity’s investment in foreign operations; |
• | an amendment to IAS1 ‘Presentation of financial statements’ requiring new disclosures about entities’ management of their capital resources; |
• | amendments to IAS39 and IFRS4 ‘Insurance contracts’ which clarify whether financial guarantees fall within the scope of IAS39 or IFRS4 and stipulate the measurement method to be applied to such guarantees; |
• | an amendment to IAS39 to permit hedge accounting for certain forecast intra-Group transactions; and |
• | a new accounting standard, IFRS7 ‘Financial instruments: Disclosures’. This standard replaces IAS30 ‘Disclosures in the financial statements of banks and similar institutions’ and the disclosure requirements in IAS32 ‘Financial instruments: disclosure and presentation’ and locates in one place all disclosures relating to financial instruments. The new requirements incorporate many of IAS32’s disclosures as well as additional qualitative and quantitative disclosures on the risks arising from financial instruments. |
Page 78 of 122
The Directors believe the adoption of these standards and interpretations in the future periods will have no material impact on the financial statements when they come into effect for periods after January 1, 2006.
SEGMENTAL INFORMATION
The Group’s primary reporting format is business segments and its secondary format is geographic segments. At December 2005, the Group is organized on a worldwide basis in one business segment of vaccines, and into two geographical areas of Europe and North America. Transfer prices between segments are set on an arm’s length basis in a manner similar to transactions with third parties. The Group’s geographical segments are determined by location of operations.
GEOGRAPHICAL SEGMENT
The following table presents revenue and certain asset and capital expenditure information regarding the Group’s geographic segments.
| | | | Europe | | | | North America | | | | Total
Group |
| | 2005
£m | | 2004
£m | | 2005
£m | | 2004
£m | | 2005
£m | | 2004
£m |
Revenue: | | | | | | | | | | | | |
Sales to external customers | | 1.8 | | 8.5 | | 39.1 | | 77.0 | | 40.9 | | 85.5 |
Other segment information: | | | | | | | | | | | | |
Total assets | | 79.3 | | 104.0 | | 54.1 | | 59.9 | | 133.4 | | 163.9 |
Total assets | | 79.3 | | 104.0 | | 54.1 | | 59.9 | | 133.4 | | 163.9 |
| | | | | | | | | | | | |
Capital additions: | | | | | | | | | | | | |
Tangible fixed assets | | – | | – | | 5.2 | | 3.1 | | 5.2 | | 3.1 |
Intangible assets | | – | | – | | 0.6 | | 0.2 | | 0.6 | | 0.2 |
The Company’s business is to invest in its subsidiaries and, therefore, it operates as a single segment.
i) OTHER INCOME
In May 2005, the Group sold information and rights of a previous R&D project in exchange for shares, valued at £0.4m at the time. The shares are held on the balance sheet as a financial asset (see note 12). In May 2004, the Group reached a c. £10.6m ($19m) agreement with Baxter to terminate the Canton manufacturing agreement under which Baxter was to place manufacturing orders at Acambis’ Canton facility. The first £5.1m ($9m) was received in May 2004 and the second installment of £2.6m ($5m) was received in January 2005. The third and final installment of £2.9m ($5m) was received in January 2006. The Group discounted future cash receipts and, as a result, recorded other operating income of £10.2m in 2004. In 2005 £0.2m (2004 – £0.2m) was recorded within finance income (see (iii) below), reflecting the staged payment nature of the agreement.
ii) ADMINISTRATION COSTS
| | 2005
£m | | 2004
£m |
Administration costs | | 7.7 | | 2.9 |
Canton plant impairment | | – | | 1.9 |
Restructuring costs | | – | | 0.7 |
Total administration costs | | 7.7 | | 5.5 |
Page 79 of 122
iii) FINANCE INCOME
| | 2005
£m | | 2004
£m |
Unwinding of discounts in relation to deferred debtors | | 0.2 | | 0.2 |
Interest receivable | | 3.8 | | 4.6 |
Total finance income | | 4.0 | | 4.8 |
iv) FINANCE COSTS
| | 2005
£m | | 2004
£m |
On bank overdrafts | | 0.2 | | 0.1 |
Interest element of finance leases | | 0.6 | | 0.7 |
Unwinding of discounts in relation to contingent and deferred consideration | | 0.2 | | 0.1 |
Total finance costs | | 1.0 | | 0.9 |
v) STAFF COSTS
| | 2005
£m | | 2004
£m |
Wages and salaries | | 14.4 | | 14.5 |
Social security costs | | 1.1 | | 1.4 |
Other pension and 401k costs | | 0.4 | | 0.4 |
Cost of share-based payments | | 0.8 | | 0.7 |
Total employee benefits | | 16.7 | | 17.0 |
During 2004, a third-party company to which the Group provided administrative services paid a share of the Group’s administrative costs, including £0.2m for staff costs. This arrangement ceased in 2004 and these costs are included in the comparative figures shown above only.
The average monthly number of employees during the year (including Executive Directors) was:
| | UK
Number | | US
Number | | 2005
Number | | 2004
Number |
Research and development | | 8 | | 93 | | 101 | | 118 |
Sales and marketing | | 3 | | 19 | | 22 | | 19 |
Manufacturing | | – | | 90 | | 90 | | 87 |
Administration | | 19 | | 43 | | 62 | | 65 |
| | 30 | | 245 | | 275 | | 289 |
At December 31, 2005, the Group had 285 employees (2004 – 270) and the Company had three employees, all of whom were Directors (2004 – four). The staff costs for the Company are shown in the remuneration report.
Page 80 of 122
The following items are included in operating (loss)/profit:
| 2005
£m | | 2004
£m |
Depreciation of fixed assets: | | | |
– owned | 3.1 | | 2.7 |
– held under finance leases | 1.9 | | 1.9 |
Cost of share-based payments (note 25) | 0.8 | | 0.7 |
Amounts paid to the Group’s Auditors (see below) | 0.5 | | 0.5 |
Operating lease charges for plant and equipment | 0.1 | | 0.1 |
Operating lease charges for property | 2.2 | | 1.8 |
Loss on disposal of fixed assets | 0.1 | | 0.1 |
Repairs and maintenance costs for property, plant and equipment | 0.5 | | 0.4 |
Exchange (loss)/gain on foreign currency borrowings | (0.4 | ) | 0.3 |
Cost of inventories recognized as expenses | 3.0 | | 17.9 |
Amortization of intangibles in cost of sales | 0.7 | | 0.7 |
Amortization of intangibles in operating expenses | 0.2 | | 0.1 |
During the year the Group obtained the following services from the Group’s Auditors:
| 2005
£m | | 2004
£m |
Audit services: | | | |
– statutory audit | 0.2 | | 0.1 |
– related regulatory reporting | 0.1 | | 0.1 |
Tax services: | | | |
– compliance services | 0.1 | | 0.1 |
– advisory services | 0.1 | | 0.2 |
| 0.5 | | 0.5 |
The Company incurred £0.2m (2004 – £0.2m) of costs with the Group’s Auditors.
Tax is charged on profits made in the country where each Group company is based. Major components of income tax expense for the year are as follows:
| | 2005 | | 2004 | |
Analysis of (credit)/charge in the consolidated income statement | | £m | | £m | |
Current income tax | | (0.3 | ) | 4.2 | |
Deferred taxation | | (0.4 | ) | 3.1 | |
Income tax (benefit)/expense in the consolidated income statement | | (0.7 | ) | 7.3 | |
| | | | | |
Tax on items charged to equity | | | | | |
Current income tax credit on employee share schemes | | – | | (1.2 | ) |
Deferred tax on revaluation of available-for-sale investment | | 0.1 | | – | |
Income tax expense/(benefit) reported in equity | | 0.1 | | (1.2 | ) |
Page 81 of 122
A reconciliation of income tax expense applicable to accounting profit before tax at the statutory income tax rate to total taxation for the Group is as follows:
| | 2005
£m | | 2004
£m | |
(Loss)/profit before tax | | (27.7 | ) | 27.0 | |
| | | | | |
At the standard rate of corporation tax in the UK of 30% (2004 – 30%) | | (8.3 | ) | 8.1 | |
Effects of: | | | | | |
Utilization of tax losses | | (2.9 | ) | (3.6 | ) |
Losses carried forward | | 13.7 | | – | |
Expenses not deductible for tax purposes | | 0.2 | | (1.1 | ) |
Adjustments in respect of foreign tax rates | | (3.5 | ) | 0.2 | |
Other timing differences | | (0.5 | ) | 3.7 | |
Adjustments to tax in respect of prior period | | 0.6 | | – | |
Total taxation | | (0.7 | ) | 7.3 | |
Movements in the deferred tax account are as follows:
| | Deferred tax
asset | | Deferred tax
liability | |
| | 2005
£m | | 2004
£m | | 2005
£m | | 2004
£m | |
At January 1, | | — | | 2.1 | | (1.7 | ) | (1.8 | ) |
Accelerated capital allowances | | 0.3 | | — | | — | | (2.7 | ) |
Short-term timing differences | | — | | — | | — | | 2.6 | |
Exchange differences | | — | | — | | 0.2 | | 0.2 | |
Available-for-sale investment | | — | | — | | (0.2 | ) | — | |
Tax losses | | — | | (2.1 | ) | — | | — | |
At December 31, | | 0.3 | | — | | (1.7 | ) | (1.7 | ) |
The Company has no deferred tax balances.
No deferred tax is recognized on the un-remitted earnings of overseas subsidiaries and joint ventures. The Directors have determined that, as earnings are continually reinvested by the Group, undistributed earnings of the subsidiaries and joint ventures will not be distributed in the foreseeable future.
Deferred tax assets and liabilities are only offset where there is a legally enforceable right of offset and there is an intention to settle the balances net. No balances have been offset in the current or previous years.
UNRECOGNIZED DEFERRED TAX ASSETS/(LIABILITIES)
| 2005
£m | | 2004
£m |
Tax losses | 7.9 | | 0.6 |
R&D tax credit | 0.7 | | – |
Short-term timing differences | (0.6 | ) | – |
Other | 0.4 | | – |
At December 31, | 8.4 | | 0.6 |
Deferred tax assets have not been recognized in respect of tax losses because there is uncertainty in the probability that they will be recoverable in the foreseeable future.
6 | EARNINGS PER ORDINARY SHARE (BASIC AND FULLY DILUTED) |
Basic EPS is calculated by dividing the earnings attributable to ordinary shareholders by the weighted average number of ordinary shares in issue during the year, excluding those held in the employee share trust (see note 24), which are treated as cancelled until the shares vest unconditionally with the employees.
For fully diluted EPS, the weighted average number of ordinary shares in issue is adjusted to assume conversion of dilutive potential ordinary shares.
Page 82 of 122
The Group’s potentially dilutive securities consist of share options and performance shares.
For basic and diluted EPS, the weighted average numbers of shares used in the calculations are set out below:
| | 2005 | | 2004 |
| Earnings
£m | Weighted
average
number
of shares | Earnings
£m | Weighted
average
number
of shares |
Basic EPS | | | | |
(Loss)/earnings attributable to ordinary shareholders | (27.0) | 107,211,367 | 19.7 | 106,300,080 |
Effect of dilutive securities: | | | | |
Options | – | – | – | 2,349,309 |
Adjusted (loss)/earnings | (27.0) | 107,211,367 | 19.7 | 108,649,389 |
| 2005 | 2004 |
| Per share
amount
pence | Per share
amount
pence |
Basic EPS | | |
(Loss)/earnings attributable to ordinary shareholders | (25.2) | 18.5 |
Effect of dilutive securities: | | |
Options | – | (0.4) |
Diluted EPS | (25.2) | 18.1 |
7 | PARENT COMPANY RESULTS FOR THE YEAR | |
| | | | |
As permitted by Section 230 of the Companies Act 1985, a separate income statement for the Company is not presented. The Company’s profit for the year was £9.1m (2004 – £3.2m).
Cost | £m | |
At January 1, 2005 | 21.0 | |
Adjustment to contingent consideration | (0.8 | ) |
Exchange movement | 0.3 | |
At December 31, 2005 | 20.5 | |
Amortisation at January 1, and December 31, 2005 | 5.6 | |
Net book value at December 31, 2005 | 14.9 | |
Net book value at December 31, 2004 | 15.4 | |
Goodwill arose when Acambis Inc. was acquired in 1999 and when BPC was acquired in August 2003.
In 2003, the Group acquired BPC for $6.5m (£4.0m) cash, $2.0m (c. £1.1m) of deferred consideration and $3.2m (c. £1.8m) of contingent consideration. During 2005, deferred consideration of $1.6m (£0.9m) (2004 – $0.6m (£0.3m)) and contingent consideration of $1.5m (£0.8m) (2004 – $0.9m (£0.5m)) was paid.
During 2005, the conditions for the payment of the remainder of the contingent consideration were not met and $1.3m (£0.7m) (2004 – £nil) was deducted from the purchase price.
IMPAIRMENT TESTING OF GOODWILL
Goodwill acquired through business combinations has been allocated to the business as a whole. Acambis operates as a global business and does not have cash-generating units at a level lower than the Group as a whole.
Page 83 of 122
During the year, the goodwill has been tested for impairment in accordance with IAS36 ‘Impairment of assets’. The recoverable value, which is the higher of the Group’s net selling price and its value in use, has been calculated based on the market capitalization of the Group. No impairment charges were made.
| | Distribution contract
£m | | Software
assets
£m | | R&D
technology £m | | Total
£m | |
Cost | | | | | | | | | |
At January 1, 2005 | | 4.7 | | 0.6 | | – | | 5.3 | |
Additions | | – | | 0.2 | | 0.4 | | 0.6 | |
Exchange movement | | 0.5 | | – | | – | | 0.5 | |
At December 31, 2005 | | 5.2 | | 0.8 | | 0.4 | | 6.4 | |
Amortization | | | | | | | | | |
At January 1, 2005 | | 0.9 | | 0.3 | | – | | 1.2 | |
Charge for year | | 0.7 | | 0.2 | | – | | 0.9 | |
Exchange movement | | 0.1 | | – | | – | | 0.1 | |
At December 31, 2005 | | 1.7 | | 0.5 | | – | | 2.2 | |
Net book value at December 31, 2005 | | 3.5 | | 0.3 | | 0.4 | | 4.2 | |
At January 1, 2004 | | 5.0 | | 0.4 | | – | | 5.4 | |
Additions | | – | | 0.2 | | – | | 0.2 | |
Exchange movement | | (0.3 | ) | – | | – | | (0.3 | ) |
At December 31, 2004 | | 4.7 | | 0.6 | | – | | 5.3 | |
Amortization | | | | | | | | | |
At January 1, 2004 | | 0.2 | | 0.2 | | – | | 0.4 | |
Charge for year | | 0.7 | | 0.1 | | – | | 0.8 | |
At December 31, 2004 | | 0.9 | | 0.3 | | – | | 1.2 | |
Net book value at December 31, 2004 | | 3.8 | | 0.3 | | v | | 4.1 | |
10 | PROPERTY, PLANT AND EQUIPMENT |
| | Freehold
land and
buildings | | Short
leasehold
improvements | | Manufacturing
and laboratory
equipment | | Office
equipment | | Total | |
| | £m | | £m | | £m | | £m | | £m | |
Cost | | | | | | | | | | | | | | | | |
January 1, 2005 | | | 0.6 | | | 20.4 | | | 6.8 | | | 2.6 | | | 30.4 | |
Additions | | | – | | | 3.6 | | | 0.9 | | | 0.7 | | | 5.2 | |
Disposals | | | – | | | – | | | – | | | (0.3 | ) | | (0.3 | ) |
Exchange movement | | | – | | | 2.5 | | | 1.3 | | | 0.4 | | | 4.2 | |
At December 31, 2005 | | | 0.6 | | | 26.5 | | | 9.0 | | | 3.4 | | | 39.5 | |
Depreciation | | | | | | | | | | | | | | | | |
At January 1, 2005 | | | – | | | 8.6 | | | 2.0 | | | 1.3 | | | 11.9 | |
Charge for year | | | – | | | 3.1 | | | 1.2 | | | 0.7 | | | 5.0 | |
Impairment | | | – | | | 0.9 | | | – | | | – | | | 0.9 | |
Disposals | | | – | | | – | | | – | | | (0.2 | ) | | (0.2 | ) |
Exchange movement | | | – | | | 1.1 | | | 0.7 | | | 0.3 | | | 2.1 | |
At December 31, 2005 | | | – | | | 13.7 | | | 3.9 | | | 2.1 | | | 19.7 | |
Net book value | | | | | | | | | | | | | | | | |
At December 31, 2005 | | | 0.6 | | | 12.8 | | | 5.1 | | | 1.3 | | | 19.8 | |
| | | | | | | | | | | | | | | | |
Net book value of assets held under finance leases included above: | | | | | | | | | | | | | | | | |
At January 1, 2005 | | | – | | | 4.8 | | | 0.8 | | | – | | | 5.6 | |
At December 31, 2005 | | | – | | | 3.5 | | | 0.7 | | | – | | | 4.2 | |
Page 84 of 122
| | Freehold
land and
buildings | | Short
leasehold
improvements | | Manufacturing
and laboratory
equipment | | Office
equipment | | Total | |
| | £m | | £m | | £m | | £m | | £m | |
Cost | | | | | | | | | | | | | | | | |
January 1, 2004 | | | 0.6 | | | 20.3 | | | 8.4 | | | 2.1 | | | 31.4 | |
Additions | | | – | | | 1.5 | | | 0.9 | | | 0.7 | | | 3.1 | |
Disposals | | | – | | | (0.2 | ) | | (1.8 | ) | | – | | | (2.0 | ) |
Exchange movement | | | – | | | (1.2 | ) | | (0.7 | ) | | (0.2 | ) | | (2.1 | ) |
At December 31, 2004 | | | 0.6 | | | 20.4 | | | 6.8 | | | 2.6 | | | 30.4 | |
Depreciation | | | | | | | | | | | | | | | | |
At January 1, 2004 | | | – | | | 5.2 | | | 2.0 | | | 0.7 | | | 7.9 | |
Charge for year | | | – | | | 2.5 | | | 1.4 | | | 0.7 | | | 4.6 | |
Impairment | | | – | | | 1.8 | | | 0.1 | | | – | | | 1.9 | |
Disposals | | | – | | | (0.3 | ) | | (1.2 | ) | | – | | | (1.5 | ) |
Exchange movement | | | – | | | (0.6 | ) | | (0.3 | ) | | (0.1 | ) | | (1.0 | ) |
At December 31, 2004 | | | – | | | 8.6 | | | 2.0 | | | 1.3 | | | 11.9 | |
Net book value | | | | | | | | | | | | | | | | |
At January 1, 2004 | | | 0.6 | | | 15.1 | | | 6.4 | | | 1.4 | | | 23.5 | |
At December 2004 | | | 0.6 | | | 11.8 | | | 4.8 | | | 1.3 | | | 18.5 | |
The Company does not have any property, plant and equipment.
11 | SUBSIDIARIES AND JOINT VENTURES |
INVESTMENT IN SUBSIDIARIES
| | 2005
£m | | Company
2004
£m |
At January 1, | | 15.5 | | 15.1 |
Deemed capital contribution | | 0.4 | | 0.4 |
At December 31, | | 15.9 | | 15.5 |
The consolidated financial statements include the financial statements of Acambis plc and the following subsidiaries:
Company name | | Main business | | Country of
incorporation | | Parent
company | | %
owned |
| | | | | | | | |
Acambis Research Limited | | Corporate administration and sales | | England and Wales | | Acambis plc | | 100 |
Acambis Inc. | | R&D, sales and manufacturing | | US | | Acambis plc | | 100 |
Berna Products Corporation | | Sales, marketing and distribution | | US | | Acambis Inc. | | 100 |
Smallpox Biosecurity Limited | | Marketing | | England and Wales | | Acambis plc | | 100 |
JOINT VENTURE
As described in note 21, the Group has an interest in a Joint Venture. Since May 1999, Acambis has performed a pre-agreed work program on behalf of the Joint Venture. Costs incurred by the Group on behalf of the Joint Venture and corresponding turnover received from the Joint Venture have been included in the Group’s financial statements.
Page 85 of 122
12 | FINANCIAL ASSETS: AVAILABLE-FOR-SALE INVESTMENTS |
| | 2005
£m | | Group
2004
£m |
At January 1, | | – | | – |
Additions | | 0.4 | | – |
Revaluation surplus transfer to equity (note 24) | | 0.2 | | – |
At December 31, | | 0.6 | | – |
In May 2005, the Group sold information and rights of a previous R&D project to Cambridge Biostability Limited, an unquoted UK company, in exchange for 1,425,200 shares. The investment represents less than a 20% shareholding in that company.
The Company does not have any available-for-sale investments
13 | OTHER NON-CURRENT ASSETS |
| | 2005
£m | | Group
2004
£m | | 2005
£m | | Company
2004
£m |
Prepayments and accrued income | | – | | 0.1 | | – | | – |
Settlement of Canton agreement | | – | | 2.4 | | – | | 0.6 |
| | – | | 2.5 | | – | | 0.6 |
The discounted interest rate used to value the Canton settlement receivable was 8.0%.
| | 2005
£m | | Group
2004
£m |
Raw materials | | 0.4 | | 0.4 |
Work in progress | | 0.5 | | 2.7 |
Finished goods | | 2.7 | | 2.9 |
| | 3.6 | | 6.0 |
Inventory is stated net of a provision of £2.5m (2004 - £3.6m).The amount of inventory write-down recognized as an expense in 2005 was £1.8m (2004– £4.7m). This expense is included in the cost of sales line.
At December 31, 2005 and December 31, 2004, the Company did not hold any inventory.
15 | TRADE AND OTHER RECEIVABLES |
| | 2005
£m | | Group
2004
£m | | 2005
£m | | Company 2004
£m |
Trade receivables | | 12.4 | | 8.2 | | – | | – |
Other receivables | | 0.5 | | 0.7 | | 0.5 | | 0.2 |
Prepayments and accrued income | | 4.8 | | 2.2 | | 0.3 | | 0.4 |
Settlement of Canton agreement | | 2.9 | | 2.6 | | 1.7 | | 0.6 |
| | 20.6 | | 13.7 | | 2.5 | | 1.2 |
Trade receivables are non-interest-bearing and are generally on terms of 30 to 60 days. There was a provision against trade receivables of £0.1m at December 31, 2005 (2004 – £nil).
Page 86 of 122
The Group’s financial instruments comprise primarily cash and liquid resources, a finance lease facility, an overdraft facility, foreign currency contracts, short- and long-term debtors receivable under the Canton settlement and various items, such as trade debtors and trade creditors, which arise directly from its operations. The main purpose of these financial instruments is to provide working capital for the Group’s operations.
The main risks arising from the Group’s activities and involving the use of financial instruments are foreign currency risk, interest rate risk and liquidity risk. The Board reviews and agrees the Group’s objectives and policies for managing each of these risks. Details of the Group’s objectives and policies, both during the year and since the year-end, are set out below, along with numerical disclosures for each category of financial instrument. Except where indicated, these disclosures are indicative of the situation throughout the year.
FOREIGN CURRENCY RISK
The Group has subsidiaries that operate and trade in the US, with revenues, expenses and financing denominated principally in US dollars. Through these overseas operations, the Group is subject to foreign exchange risk, including the risk of fluctuations in the Group’s net investment in, and reported profits from, foreign subsidiaries when translated into sterling. In addition, the UK trading subsidiary enters into contracts in a variety of foreign currencies.
The Group had overall surplus cash funds throughout the year but had to determine in which currency to hold cash available for working capital and surplus funds. This was done with reference to anticipated future expenditure patterns and relative returns on funds held in different currencies. The Group’s current policy is to hold surplus funds in sterling over the long term, which currently achieves a higher interest rate return whilst mitigating the risk of fluctuations in the Group’s net assets, when reported in sterling.
From time to time, the Group makes use of forward contracts in order to reduce uncertainty over the sterling value of anticipated US dollar receipts, thereby reducing uncertainty over the level of the Group’s profits when reported in sterling. Typically, in 2005 the Group took out forward contracts for known significant foreign currency transactions only. There were no forward contracts outstanding at the year-end.
During the year, the Group also used dual currency deposits for both euro and US dollar deposits, allowing an enhanced interest rate to be earned, which may, at maturity, be converted into sterling or dollars at the banks’ discretion, at a rate previously agreed. The Group had no dual currency deposits outstanding at the year-end.
Where Group companies have monetary assets and liabilities denominated in currencies other than their functional currency, these balances are translated into that subsidiary’s functional currency. With the exception of gains and losses on those inter-Company balances that are considered to be ‘as permanent as equity’ and recorded in reserves, foreign exchange gains and losses arising are recorded immediately in the income statement. These amounts include sterling-denominated cash balances held in the US, US dollar- and euro-denominated balances held by the Company and a US dollar-denominated overdraft facility held by a UK subsidiary. In addition, the Group has other current assets and liabilities denominated in foreign currencies, which the Board does not consider to be significant.
LIQUIDITY RISK
The Board monitors the level of cash and liquid resources on a regular basis, and management monitors the level on a daily basis, to ensure that the Group has sufficient liquid funds to enable it to meet its commitments as they fall due. This is achieved through the production and review of cash forecasts, including sensitivity analyses. Approximately 60% of the Group’s cash and liquid resources are managed on a discretionary basis by a third party within strict parameters that have been set by the Board. The remainder is invested in managed funds or invested in bank deposits within the parameters set by the Board. These parameters include the requirement that the institutions used must have a minimum rating of Aa2 long-term or P-1 short-term, and a maximum investment with any one counter-party of £20m.
Page 87 of 122
INTEREST RATE RISK
The Group finances its operations predominantly through cash and liquid resources generated through operating activities, from the issuance of equity shares, through finance leases and through an overdraft facility. It is the Group’s policy to invest surplus cash on deposit or in money market funds managed by professional money managers. The performance of the investments is reviewed by management on a regular basis to ensure that competitive rates of return are being achieved, subject to the Board’s requirement relating to the accessibility of funds and standing of financial institutions used. The Board reviews regularly the financing facilities available to the Group to ensure competitive rates of interest are being obtained. During the year, the Group invested in a cash deposit which accrues interest dependent on the sterling LIBOR rate. This deposit of £10.0m was outstanding at the year-end and was valued at £10.0m (2004 – deposit £5.8m, valued at £5.7m).
The following table sets out the carrying value by maturity, for each financial instrument that is exposed to interest rate risk.
| | Group | | Company |
2005 | | Within
one year
£m | | One-two
years
£m | | Total
£m | | Within
one year
£m | | One-two
years
£m | | Total
£m |
Floating rate: | | | | | | | | | | | | |
Cash | | 11.0 | | — | | 11.0 | | 6.5 | | — | | 6.5 |
Fixed rate: | | | | | | | | | | | | |
Short-term deposits | | 38.2 | | — | | 38.2 | | 36.0 | | — | | 36.0 |
Liquid investments | | 8.8 | | 10.0 | | 18.8 | | 8.8 | | 10.0 | | 18.8 |
Obligations under finance leases | | (7.1 | ) | — | | (7.1 | ) | — | | — | | — |
2004 | | Within
one year
£m | | One-two
years
£m | | Total
£m | | Within
one year
£m | | One-two
years
£m | | Total
£m |
Floating rate | | | | | | | | | | | | |
Cash | | 30.2 | | — | | 30.2 | | 27.8 | | — | | 27.8 |
| | | | | | | | | | | | |
Fixed rate | | | | | | | | | | | | |
Cash | | 0.7 | | — | | 0.7 | | 0.7 | | — | | 0.7 |
Short-term deposits | | 50.1 | | — | | 50.1 | | 41.8 | | — | | 41.8 |
Liquid investments | | 20.8 | | — | | 20.8 | | 17.8 | | — | | 17.8 |
Obligations under finance leases | | (3.1 | ) | (6.3 | ) | (9.4 | ) | — | | — | | — |
The Group’s main customer is the US Government and therefore it assesses the credit risk as low. There are no other significant concentrations of credit risk.
FAIR VALUES OF FINANCIAL ASSETS AND FINANCIAL LIABILITIES
There is no material difference between the book values and fair values of the Group’s financial assets and liabilities as at December 31, 2005. Fair values have been calculated by discounting cash flows at prevailing interest rates.
| | Group | | Company | |
| | 2005
£m | | 2004
£m | | 2005
£m | | 2004
£m | |
Assets: | | | | | | | | | |
Foreign currency contracts | | 0.1 | | — | | 0.1 | | — | |
Liabilities: | | | | | | | | | |
Foreign currency contracts | | — | | (0.1 | ) | — | | (0.1 | ) |
Page 88 of 122
In accordance with IAS39, the Group has reviewed all contracts for embedded derivatives that are required to be separately accounted for if they do not meet certain requirements set out in the standard. This derivative is fair valued based on discounted future cash flows with gains and losses passing through the income statement as hedge accounting is not available.
The Group has an embedded derivative deposit which accrues interest dependent on UK LIBOR (the London Interbank Offered Rate).
During the year, the Group also used dual currency deposits for both euro and US dollar deposits, allowing an enhanced interest rate to be earned, which may, at maturity, be converted into sterling or dollars at the banks’ discretion, at a rate previously agreed. The Group had no dual currency deposits outstanding at the year-end (2004 – none).
From time to time, the Group makes use of forward contracts in order to reduce uncertainty over the sterling value of anticipated US dollar receipts, thereby reducing uncertainty over the level of the Group’s profits when reported in sterling. Typically, in 2005 the Group took out forward contracts for known significant foreign currency only. The Group had no forward contracts outstanding at the year-end (2004 – a forward contract to sell dollars and buy sterling outstanding at the year-end).
17 | CASH AND CASH EQUIVALENTS |
| | 2005
£m | | Group
2004
£m | | 2005
£m | | Company
2004
£m |
Cash | | 11.0 | | 30.9 | | 6.5 | | 28.5 |
Short-term deposits | | 38.2 | | 50.1 | | 36.0 | | 41.8 |
| | 49.2 | | 81.0 | | 42.5 | | 70.3 |
The weighted average interest rate received in the year was 3.9% for cash at bank. Short-term deposits are made for varying periods of between one day and three months, (the weighted average maturity being 14 days) and have earned interest at 4.7%.
The Group had cash and liquid resources of £68.0m at December 31, 2005 (2004 – £101.8m). Of this amount, deposits with an original maturity of more than three months of £18.8m (2004 – £20.8m) have been classified as liquid investments. The majority of these resources are invested in managed funds or on bank deposit, denominated in sterling, US dollars and euros. Approximately 16% of the Group’s cash and liquid resources is available for use with a day’s notice (2004 – 30%), with the remainder being invested on deposits of up to 18 months. The Group had £0.7m of restricted cash on deposit at the year-end (2004 – £0.4m).
| | 2005
£m | | Group
2004
£m | | 2005
£m | | Company
2004
£m |
Current: | | | | | | | | |
Short-term borrowings | | 4.0 | | 3.6 | | — | | — |
Short-term financial liabilities – obligations under finance leases | | 7.1 | | 3.1 | | — | | — |
Other financial liabilities | | 0.1 | | — | | — | | — |
Derivative financial liabilities | | — | | 0.1 | | — | | — |
| | 11.2 | | 6.8 | | — | | — |
Non-current: | | | | | | | | |
Other financial liabilities | | 1.6 | | 6.3 | | — | | — |
Page 89 of 122
SHORT-TERM BORROWINGS
Under the terms of the agreement between Acambis and Evans Vaccines Limited (a subsidiary of Chiron Corporation, which has been acquired by Novartis AG) given certain conditions the obligation under the bank overdraft facility of £4.0m (2004 – £3.6m) for part of the costs incurred on the ARILVAX project may be repayable within one year. The facility is underwritten by Chiron. Chiron has granted to Acambis 100% of the marketing rights to ARILVAX in the US, whilst retaining an option to buy back 50% of the profits from the US sales in return for refunding to Acambis the costs that Acambis has incurred on the ARILVAX program. The overdraft facility was fully utilized at December 31, 2005 (2004 – fully utilized) and was renewed in January 2006 for a further year.
During the year, an exchange loss of £0.4m (2004 – gain of £0.3m) was recorded in the income statement, resulting from the revaluation of this US dollar-denominated facility.
OBLIGATIONS UNDER FINANCE LEASES
The Group has a $40m (c. £21m) finance lease facility. This was arranged through Baxter and was approved by shareholders in December 2001. In 2001, the Group drew down $18.6m (£14.0m) and has made no further draw-downs from the facility. The repayment schedule for the lease financing required that interest only was repaid in 2003 and capital and interest are repayable over 2004 to 2006. The Group had an option to repurchase all of the facility’s assets in December 2003, and on each anniversary thereafter, for the capital balance outstanding at that time, plus any accrued but unpaid interest due at the time, and a make-whole payment (discounted to present value) equal to the projected future interest stream payable to the end of the lease term.
In December 2001, the Group committed to a finance lease, repayable within five years, relating to the purchase and sale-and-leaseback of capital assets within the manufacturing plant.
OTHER FINANCIAL LIABILITIES
In May 2005, the Group purchased a fill/finish facility for c. £1.8m ($3m) upfront and a further c. £2.6m ($4.5m) in equal installments between 2006 and 2017. The balance relating to the discounted value of future payments is £1.7m at December 31, 2005 (2004 – £nil). £0.1m is included in ‘current other financial liabilities’ (2004 – £nil), and £1.6m in ‘non-current other financial liabilities’ (2004 – £nil).
TRADE AND OTHER PAYABLES
| | 2005
£m | | Group
2004
£m | | 2005
£m | | Company
2004
£m | |
Trade payables | | 16.0 | | 5.8 | | – | | 0.1 | |
Other taxation and social security | | 0.1 | | 0.1 | | – | | – | |
Other payables | | – | | 0.7 | | – | | – | |
Deferred and contingent consideration | | – | | 1.7 | | – | | – | |
| | 16.1 | | 8.3 | | – | | 0.1 | |
In August 2005 BN filed legal actions against Acambis in the US in relation to IP on its MVA smallpox vaccine. A further suit was filed in Austria in February 2006. BN alleges use of trade secrets, misappropriation and patent infringement. Acambis strongly believes these allegations are without foundation and is vigorously defending its position. A current provision of £2.3m (2004 – £nil) has been recognized in relation to future legal costs relating to the MVA litigation. The Company has no provisions.
Page 90 of 122
21 | INVESTMENT IN JOINT VENTURE |
The Group has a 50% interest in the Pasteur Mérieux-OraVax joint venture, as described in note 21 (the Joint Venture), whose principal business is to develop, manufacture, market and sell immunotherapeutic and preventative vaccines against H. pylori infection in humans. The Joint Venture represents collaboration between two partnerships, Mérieux-OraVax SNC and OraVax-Mérieux Co., incorporated in Delaware, US. These partnerships were formed in March 1995 between the companies now known as Acambis Inc. and sanofi pasteur. The Joint Venture trades under the name of Pasteur Mérieux-OraVax and its accounting year-end is December 31, The R&D budgets of the two partnerships are established by joint committees in which each of the parties has an equal participation and role. The parties pay approximately equal shares of the agreed budgets. The Joint Venture is being wound down.
The following information is given in respect of the Group’s share of the Joint Venture:
| | 2005
£m | | 2004
£m | |
Loss before tax | | – | | – | |
Current assets | | 0.7 | | 0.6 | |
Liabilities due within one year | | (1.0 | ) | (0.9 | ) |
| | (0.3 | ) | (0.3 | ) |
Due to the nature of this Joint Venture as collaboration between two partners, the following table provides an alternative analysis of the amounts shown above:
| | 2005
£m | | 2004
£m | |
Share of cumulative amounts invested by the partners | | 17.0 | | 15.2 | |
Share of cumulative losses incurred by the Joint Venture | | (17.3 | ) | (15.5 | ) |
| | (0.3 | ) | (0.3 | ) |
22 | OTHER NON-CURRENT LIABILITIES | |
| | | | | | | |
| | 2005
£m | | Group
2004
£m | | 2005
£m | | Company
2004
£m | |
Deferred and contingent consideration | | – | | 0.5 | | – | | – | |
23 | CALLED-UP SHARE CAPITAL | |
| | | | | | | | | | | |
GROUP AND COMPANY
| | Number | | 2005
£m | | Number | | 2004
£m |
Authorized shares of 10p each | | | | | | | | |
At January 1, and December 31, | | 140,000,000 | | 14.0 | | 140,000,000 | | 14.0 |
Allotted, called-up and fully paid ordinary shares of 10p each | | | | | | | | |
At January 1, | | 107,219,329 | | 10.7 | | 105,637,848 | | 10.6 |
Exercise of share options | | 132,078 | | – | | 1,581,481 | | 0.1 |
At December 31, | | 107,351,407 | | 10.7 | | 107,219,329 | | 10.7 |
All shares have equal voting rights.
As described in note 24, Acambis Employees Trustees Limited holds 84,972 shares, which will be used to satisfy awards made under the LTIP. Consideration received in 2005 through the exercise of share options amounted to £0.2m (2004 – £1.9m).
Page 91 of 122
24 | STATEMENT OF CHANGES IN EQUITY |
GROUP
| | Share
capital
£m | | Share
premium
account
£m | | Retained
earnings
£m | | Other
reserves
£m | | Total
£m | |
At January 1, 2005 | | 10.7 | | 97.8 | | 1.5 | | (2.5 | ) | 107.5 | |
Gain on foreign currency exchange | | – | | – | | – | | 1.6 | | 1.6 | |
Total income and expense recognized directly in equity | | – | | – | | – | | 1.6 | | 1.6 | |
Loss for the year | | – | | – | | (27.0 | ) | – | | (27.0 | ) |
Total income and expense recognized | | – | | – | | (27.0 | ) | 1.6 | | (25.4 | ) |
Issue of new shares | | – | | 0.2 | | – | | – | | 0.2 | |
Purchase of treasury shares | | – | | – | | (0.2 | ) | – | | (0.2 | ) |
Revaluation of available-for-sale investment (net of deferred tax) | | – | | – | | 0.1 | | – | | 0.1 | |
Credit in respect of employee share schemes | | – | | – | | 0.8 | | – | | 0.8 | |
At December 31, 2005 | | 10.7 | | 98.0 | | (24.8 | ) | (0.9 | ) | 83.0 | |
GROUP
| | Share
capital
£m | | Share
premium
account
£m | | Retained
earnings
£m | | Other
reserves
£m | | Total
£m | |
At January 1, 2004 | | 10.6 | | 96.0 | | (20.1 | ) | – | | 86.5 | |
Loss on foreign currency exchange | | – | | – | | – | | (2.5 | ) | (2.5 | ) |
Deferred tax on share options | | – | | – | | 1.2 | | – | | 1.2 | |
Total income and expense recognized directly in equity | | – | | – | | 1.2 | | (2.5 | ) | (1.3 | ) |
Profit for the year | | – | | – | | 19.7 | | – | | 19.7 | |
Total income and expense recognized | | – | | – | | 20.9 | | (2.5 | ) | 18.4 | |
Issue of new shares | | 0.1 | | 1.8 | | – | | – | | 1.9 | |
Credit in respect of employee share schemes | | – | | – | | 0.7 | | – | | 0.7 | |
At December 31, 2004 | | 10.7 | | 97.8 | | 1.5 | | (2.5 | ) | 107.5 | |
The amount shown in ‘other reserves’ relates to foreign currency translation.
COMPANY
| | Share
capital
£m | | Share
premium
account
£m | | Retained
earnings
£m | | Total
£m | |
At January 1, 2005 | | 10.7 | | 97.6 | | 5.2 | | 113.5 | |
Profit for the year | | – | | – | | 9.1 | | 9.1 | |
Total income and expense recognized for the year | | – | | – | | 9.1 | | 9.1 | |
Issue of new shares | | – | | 0.2 | | – | | 0.2 | |
Purchase of treasury shares | | – | | – | | (0.2 | ) | (0.2 | ) |
Credit in respect of employee share schemes | | – | | – | | 0.4 | | 0.4 | |
Deemed capital contribution | | – | | – | | 0.4 | | 0.4 | |
At December 31, 2005 | | 10.7 | | 97.8 | | 14.9 | | 123.4 | |
Page 92 of 122
COMPANY
| | Share | | Share premium | | Retained | | |
| | capital | | account | | earnings | | Total |
| | £m | | £m | | £m | | £m |
At January 1, 2004 | | 10.6 | | 95.8 | | 1.0 | | 107.4 |
Deferred tax on share option awards | | – | | – | | 0.4 | | 0.4 |
Total income and expense recognized directly in equity | | – | | – | | 0.4 | | 0.4 |
Profit for the year | | – | | – | | 3.2 | | 3.2 |
Total income and expense recognized for the year | | – | | – | | 3.6 | | 3.6 |
Issue of new shares | | 0.1 | | 1.8 | | – | | 1.9 |
Credit in respect of employee share schemes | | – | | – | | 0.2 | | 0.2 |
Deemed capital contribution | | – | | – | | 0.4 | | 0.4 |
At December 31, 2004 | | 10.7 | | 97.6 | | 5.2 | | 113.5 |
At December 31, 2005, Acambis Employees’ Trustees Limited held 84,972 (2004 – 62,190) ordinary shares in the Company with a total nominal value of £0.01m (2004 – £0.01m). The cost of these shares of £0.2m (2004 – £0.1m) is shown as a deduction to retained earnings. The total market value of these shares at December 31, 2005 is £0.2m (2004 – £0.2m). All shares held by the Trust have been allocated to long-term incentive awards and a charge has been made in respect of all of these awards. All costs relating to the administration of the Trust are included within the accounts of the Company as they arise.
SUMMARY OF SHARE SCHEMES IN OPERATION DURING THE YEAR
Acambis had the following share-based payment schemes in operation during the year.
1996 AND 1999 SCHEMES
The 1996 Scheme and the 1999 Plan involve the grant of market-value share options to participants. The options are subject to a market-based performance condition (Acambis’ TSR performance against a comparator group). The options granted have a maximum contractual life of 10 years with the exception of the 15 October 2005 and 28 October 2003 options granted to employees which have a maximum contractual life of four years. For all options granted after January 1, 2004 (to employees or Directors) performance is measured over three years and there is no retesting of the performance condition. Further information regarding the operation of the scheme can be found in the remuneration report.
LTIP
The LTIP involves the grant of nil-cost share options to participants. The options are subject to a market-based performance condition (Acambis’ TSR performance against a comparator group). The options granted have a maximum contractual life of three years and six months. For all options granted under the LTIP performance is measured over three years and there is no retesting of the performance condition. Further information regarding the operation of the scheme can be found in the remuneration report.
SAYE SCHEME
The SAYE Scheme is based on a three-year monthly savings contract and eligible employees are granted share options with an exercise price of up to 20% below the share price when the invitation is issued. The options granted have a maximum contractual life of three years and six months. Vesting of the options is not subject to the achievement of a performance target.
ESPP
The ESPP is based on a two-year monthly savings contract and eligible employees are granted share options with an exercise price of up to a 15% discount to the share price at the time of invitation. The options granted have a maximum contractual life of two years and three months. Vesting of the options is not subject to the achievement of a performance target.
Page 93 of 122
Options outstanding under all schemes are as follows:
| | January 1, | | | | | | | | December 31, |
| | 2005 | | Granted | | Exercised | | Lapsed | | 2005 |
Scheme | | ’000 | | ’000 | | ’000 | | ’000 | | ‘000 |
1996 | | 233 | | 36 | | (10 | ) | (59 | ) | 200 |
1999 | | 3,173 | | 806 | | (104 | ) | (342 | ) | 3,533 |
SAYE | | 105 | | 38 | | (12 | ) | (50 | ) | 81 |
ESPP | | 85 | | 50 | | – | | (60 | ) | 75 |
1990 US1 | | 121 | | – | | – | | (107 | ) | 14 |
1995 US2 | | 155 | | – | | – | | (28 | ) | 127 |
Total | | 3,872 | | 930 | | (126 | ) | (646 | ) | 4,030 |
| | January 1, | | | | | | | | December 31, |
| | 2004 | | Granted | | Exercised | | Lapsed | | 2004 |
Scheme | | ’000 | | ’000 | | ’000 | | ’000 | | ’000 |
1995 | | 5 | | – | | (5 | ) | – | | – |
1996 | | 318 | | 70 | | (113 | ) | (42 | ) | 233 |
1999 | | 3,925 | | 864 | | (1,277 | ) | (339 | ) | 3,173 |
SAYE | | 192 | | 24 | | (105 | ) | (6 | ) | 105 |
ESPP | | 79 | | 20 | | – | | (14 | ) | 85 |
1990 US1 | | 167 | | – | | (46 | ) | – | | 121 |
1995 US2 | | 190 | | – | | (35 | ) | – | | 155 |
Total | | 4,876 | | 978 | | (1,581 | ) | (401 | ) | 3,872 |
NOTES
1 | The OraVax 1990 Stock Incentive Plan |
2 | The OraVax 1995 Stock Incentive Plan |
The following table shows outstanding options, divided into ranges to help assess the number and timing of additional shares that may be issued and the cash that may be received upon exercise of those options.
Year of grant | | Weighted average
exercise price | | Period exercisable in
normal circumstances | | Number
outstanding |
1996 | | $26.02 | | 1999-2006 | | 30,547 |
1996 | | £1.70 | | 1999-2006 | | 17,685 |
1997 | | $4.89 | | 2000-2007 | | 105,443 |
1999 | | $1.68 | | 2002-2009 | | 5,090 |
1999 | | £0.36 | | 2002-2009 | | 85,434 |
2000 | | £0.92 | | 2003-2006 | | 250,000 |
2000 | | £0.96 | | 2003-2010 | | 3,600 |
2001 | | £1.25 | | 2004-2006 | | 208,000 |
2001 | | £3.33 | | 2004-2006 | | 19,520 |
2001 | | £1.38 | | 2004-2011 | | 258,201 |
2002 | | £2.46 | | 2005-2006 | | 237,152 |
2002 | | £1.80 | | 2005-2006 | | 16,065 |
2002 | | £2.62 | | 2005-2012 | | 363,419 |
2003 | | £3.26 | | 2006-2007 | | 265,716 |
2003 | | £2.74 | | 2006-2007 | | 8,346 |
2003 | | £3.00 | | 2006-2013 | | 359,265 |
2004 | | £2.65 | | 2006 | | 24,798 |
2004 | | £2.81 | | 2007-2008 | | 377,340 |
2004 | | £2.36 | | 2007-2008 | | 17,896 |
2004 | | £2.91 | | 2007-2014 | | 462,949 |
Page 94 of 122
Year of grant | | Weighted average
exercise price | | Period exercisable in
normal circumstances | | Number
outstanding |
2005 | | £1.87 | | 2007 | | 50,652 |
2005 | | £2.46 | | 2008-2009 | | 344,440 |
2005 | | £2.01 | | 2008-2009 | | 38,414 |
2005 | | £2.34 | | 2008-2015 | | 479,895 |
Total | | | | | | 4,029,867 |
Whilst they have no present intention of utilizing such authority, at the AGM to be held on 23 June 2006 the Directors will seek authority from the shareholders to allot shares up to an aggregate nominal value of £3,264,670 (32,646,703 ordinary shares of 10p each), being the un-issued ordinary shares of the Company at 21 April 2006. Currently, the Directors have authority to allot shares up to an aggregate nominal value of £3,276,481.
The Group operates an HM Revenue and Customs-approved SAYE scheme in the UK and an ESPP scheme in the US.
CHARGE IN THE INCOME STATEMENT
In accordance with the transitional provisions of IFRS2, Acambis has recognized an expense in respect of all grants under these plans made after 7 November 2002 which remained unvested at December 31, 2004. Acambis recognized a total expense of £0.8m in 2005 (2004 – £0.7m) in accordance with IFRS2.
FINANCIAL DETAILS OF SHARE OPTIONS
Options were exercised on a regular basis during the year. The average share price during 2005 was £2.35.
The weighted average fair values for grants made in the year are as noted in the table below. Grants made to employees and Directors under the 1996 and 1999 Plans are shown separately since different inputs have been used for these grants.
Weighted average fair value | | 2005
£ | | 2004
£ | |
1996 Plan (Employee grants) | | 0.83 | | 1.12 | |
1996 Plan (Director grants) | | N/A | | 1.36 | |
1999 Plan (Employee grants) | | 0.68 | | 1.03 | |
1999 Plan (Director grants) | | 0.84 | | 1.19 | |
LTIP | | 1.41 | | 2.37 | |
ESPP | | 0.62 | | 1.36 | |
SAYE | | 0.88 | | 1.16 | |
The assumptions used in the calculation of the fair values in the above table are:
• | Expected volatility was based on the historical volatility of the Company’s share price: |
| – | over the three years prior to the grant date for employee grants under the 1996 and 1999 Plan and all grants under the SAYE Scheme and LTIP; – over the four years prior to the grant date for Director grants under the 1996 and 1999 Plan; and |
| – | over the two years prior to the grant date for all grants under the ESPP. |
• | A zero dividend yield assumption has been used in the calculation of these fair values. |
1996 PLAN, 1999 PLAN AND LTIP
The fair value of shares awarded under the 1996 Plan and 1999 Plan is calculated using a binomial option pricing model adjusted to reflect the TSR market-based performance condition. The awards were calculated using the following assumptions:
Page 95 of 122
1996 PLAN (EMPLOYEE GRANTS)
| | 2005 | | 2004 | |
Weighted average share price (£) | | 2.58 | | 3.00 | |
Weighted average exercise price (£) | | 2.58 | | 2.93 | |
Weighted average volatility (%) | | 41.4 | | 51.1 | |
Weighted average correlation (%) | | 5.0 | | 14.8 | |
Weighted average expected life (years) | | 3.5 | | 3.5 | |
Weighted average risk-free interest rate (%) | | 4.6 | | 4.7 | |
1996 PLAN (DIRECTOR GRANTS)
| | 2005 | | 2004 | |
Weighted average share price (£) | | N/A | | 3.46 | |
Weighted average exercise price (£) | | N/A | | 3.46 | |
Weighted average volatility (%) | | N/A | | 53.6 | |
Weighted average correlation (%) | | N/A | | 14.3 | |
Weighted average expected life (years) | | N/A | | 4.00 | |
Weighted average risk-free interest rate (%) | | N/A | | 4.6 | |
1999 PLAN (EMPLOYEE GRANTS)
| | 2005 | | 2004 | |
Weighted average share price (£) | | 2.41 | | 2.85 | |
Weighted average exercise price (£) | | 2.41 | | 2.84 | |
Weighted average volatility (%) | | 36.9 | | 50.2 | |
Weighted average correlation (%) | | 4.3 | | 14.9 | |
Weighted average expected life (years) | | 3.1 | | 3.5 | |
Weighted average risk-free interest rate (%) | | 4.3 | | 4.7 | |
1999 PLAN (DIRECTOR GRANTS)
| | 2005 | | 2004 | |
Weighted average share price (£) | | 2.34 | | 3.05 | |
Weighted average exercise price (£) | | 2.34 | | 3.04 | |
Weighted average volatility (%) | | 47.9 | | 52.0 | |
Weighted average correlation (%) | | 4.5 | | 14.5 | |
Weighted average expected life (years) | | 4.0 | | 3.7 | |
Weighted average risk-free interest rate (%) | | 4.3 | | 4.7 | |
LTIP
| | 2005 | | 2004 | |
Weighted average share price (£) | | 2.19 | | 3.46 | |
Weighted average exercise price (£) | | – | | – | |
Weighted average volatility (%) | | 40.8 | | 53.3 | |
Weighted average correlation (%) | | 5.0 | | 15.0 | |
Weighted average expected life (years) | | 3.0 | | 3.0 | |
Weighted average risk-free interest rate (%) | | 4.3 | | 4.5 | |
The 1996 Plan, 1999 Plan and the LTIP have a TSR market-based performance condition, such that the Company’s TSR over the performance period will be compared with the TSR of the comparator companies on the date of grant. The maximum number of shares would vest if Acambis were ranked in the upper quartile of the comparator group, being prorated down to a 30% vesting at a ranking of the median. No shares vest if Acambis’ ranking falls below the median. The fair value of options under the 1996 Plan, 1999 Plan and LTIP has been adjusted to take into account this market-based performance condition using a pricing model based on expectations about volatility and the correlation of share price returns in the group of comparator companies and which incorporates into the valuation the interdependency between share price performance and TSR vesting.
Page 96 of 122
ESPP AND SAYE GRANTS
The fair value of options granted under the ESPP and SAYE scheme are calculated using a binomial option pricing model with the following assumptions:
ESPP
| | 2005 | | 2004 | |
Weighted average share price (£) | | 2.17 | | 3.44 | |
Weighted average exercise price (£) | | 1.87 | | 2.65 | |
Weighted average volatility (%) | | 32.4 | | 44.8 | |
Expected life (years) | | 2.0 | | 2.0 | |
Weighted average risk-free interest rate (%) | | 4.4 | | 5.0 | |
| | 2005 | | 2004 | |
Weighted average share price (£) | | 2.39 | | 3.51 | |
Weighted average exercise price (£) | | 2.01 | | 2.74 | |
Weighted average volatility (%) | | 34.7 | | 55.2 | |
Expected life (years) | | 3.3 | | 3.3 | |
Risk-free interest rate (%) | | 4.3 | | 4.9 | |
FAIR VALUE OF OPTIONS GRANTED IN 2003
The charge under IFRS2 for the current period includes a charge for options granted under the above schemes during the year ended December 31, 2003 and the year ended December 31, 2002 with the following weighted average grant date fair values:
| | 2003
£ | | 2002
£ | |
1996 Plan (Employee grants) | | 1.25 | | 1.11 | |
1996 Plan (Director grants) | | N/A | | N/A | |
1999 Plan (Employee grants) | | 1.23 | | 1.13 | |
1999 Plan (Director grants) | | 1.33 | | N/A | |
LTIP | | 2.24 | | N/A | |
ESPP | | 1.66 | | N/A | |
SAYE | | 1.78 | | N/A | |
The fair values for the 2003 grants were calculated using the binomial model (adjusted for the TSR performance condition where relevant).
| | 1996 Plan
(Employee
grants) | | 1996 Plan
(Director
grants) | | 1999 Plan
(Employee
grants) | | 1999 Plan
(Director
grants) | | LTIP | | ESPP | | SAYE | |
| | | | | | | | | | | | | | | |
Weighted average share price (£) | | 3.34 | | N/A | | 3.21 | | 2.94 | | 3.23 | | 3.72 | | 3.51 | |
Weighted average exercise price (£) | | 3.34 | | N/A | | 3.17 | | 2.94 | | – | | 3.08 | | 2.74 | |
Weighted average volatility (%) | | 55.0 | | N/A | | 55.8 | | 63.7 | | 55.2 | | 59.2 | | 55.2 | |
Weighted average expected life (years) | | 3.2 | | N/A | | 3.3 | | 4.0 | | 3.0 | | 2.0 | | 3.3 | |
Weighted average risk-free interest rate (%) | | 4.5 | | N/A | | 4.4 | | 4.4 | | 3.8 | | 3.4 | | 4.9 | |
The fair values for the 2002 grants were calculated using the binomial model (adjusted for the TSR performance condition where relevant).
Page 97 of 122
| | 1996 Plan
(Employee
grants) | | 1996 Plan
(Director
grants) | | 1999 Plan
(Employee
grants) | | 1999 Plan
(Director
grants) | | LTIP | | ESPP | | SAYE |
Weighted average share price (£) | | 2.47 | | N/A | | 2.52 | | N/A | | N/A | | N/A | | N/A |
Weighted average exercise price (£) | | 2.47 | | N/A | | 2.52 | | N/A | | N/A | | N/A | | N/A |
Weighted average volatility (%) | | 66.8 | | N/A | | 66.7 | | N/A | | N/A | | N/A | | N/A |
Weighted average expected life (years) | | 3.5 | | N/A | | 3.5 | | N/A | | N/A | | N/A | | N/A |
Weighted average risk-free interest rate (%) | | 4.3 | | N/A | | 4.4 | | N/A | | N/A | | N/A | | N/A |
For the options granted under the 1996 Plan and 1999 Plan prior to January 1, 2004 where the TSR condition is retested at the end of year four (if not met at the end of year three) and/or at the end of year five (if not met at the end of year four), a three years and six months vesting period has been used to approximate the impact of the retesting condition on the fair value. This retesting condition applies to a limited number of option grants and does not apply to new option grants.
i) LEASE COMMITMENTS
The minimum lease payments under operating leases are as follows:
| | Land and buildings | | Group
Plant and
machinery |
| | 2005 | | 2004 | | 2005 | | 2004 |
| | £m | | £m | | £m | | £m |
Total commitments under operating lease: | | | | | | | | |
Due within one year | | 2.3 | | 1.7 | | 0.1 | | 0.1 |
Due within one to five years | | 9.6 | | 3.1 | | 0.2 | | 0.1 |
Due beyond five years | | 8.2 | | 7.7 | | – | | – |
| | 20.1 | | 12.5 | | 0.3 | | 0.2 |
| | Land and buildings | | Company
Plant and
machinery |
| | 2005 | | 2004 | | 2005 | | 2004 |
| | £m | | £m | | £m | | £m |
Total commitments under operating lease: | | | | | | | | |
Due within one year | | 0.6 | | 0.6 | | – | | – |
Due within one to five years | | 2.3 | | 2.3 | | – | | – |
Due beyond five years | | 7.1 | | 7.7 | | – | | – |
| | 10.0 | | 10.6 | | – | | – |
In March 2000, the Group entered into a sub-lease with Medivir UK Limited with respect to a part of the facility at Peterhouse Technology Park in the UK. In December 2003, this sub-lease was amended, such that 45% of the facility was rented to Medivir until November 2004. During 2004, Medivir contributed £0.2m in operating lease rentals relating to land and buildings (2005 – £nil).
ii) CAPITAL COMMITMENTS
At the end of the year, capital commitments contracted but not provided for were £0.1m (2004 – £0.2m).
Page 98 of 122
iii) PENSION ARRANGEMENTS
The Group provides pension benefits to all full-time employees on a defined contribution basis. The Company operates a self-administered, HM Revenue and Customs-approved pension scheme for UK Executive Directors. Other employees may operate private personal pension schemes. The normal age of retirement for UK staff is 65 years. In the US, the Group offers a ‘401k Savings and Retirement Plan’ for all employees, including Executive Directors. The Group pension cost (including 401k costs) for the year was £0.4m (2004 – £0.4m). At the year-end, the Group owed £0.2m (2004 – £0.2m) to the pension schemes. This amount is shown in the balance sheet under ‘accruals and deferred income’.
27 | RELATED PARTY TRANSACTIONS |
For the year ended December 31, 2005, the Group has included turnover of £nil (2004 – £0.1m) in respect of costs incurred in performing services for the Joint Venture and a loss of £nil (2004 – £0.1m) within its Group financial statements. At December 31, 2005, the amounts the Group owed to the Joint Venture amounted to £0.4m (2004 – £0.3m).
Amounts owed by the Joint Venture to the Group at December 31, 2005 were £0.3m (2004 – £0.3m).
In 2005, the Company settled transactions on behalf of subsidiaries of £33.6m (2004 – nil). The inter-company balances outstanding at December 31, are detailed on the Company balance sheet. In 2005 the Company credited £3.6m to subsidiaries relating to management charges (2004 – charge of £22.7m) and charged £3.4m to subsidiaries relating to interest (2004 – £1.7m).
DIRECTORS’ REMUNERATION, INTERESTS AND TRANSACTIONS
Full disclosure of Directors’ remuneration, interests and transactions is given in that part of the remuneration report that is required to be audited. Aggregate gains made by Directors on the exercise of share options were £0.1m (2004 – £1.8m).
KEY MANAGEMENT COMPENSATION
The remuneration received by key management personnel, including the Directors, is as follows:
| | 2005 | | 2004 |
| | £m | | £m |
Salaries and short-term employee benefits | | 1.6 | | 1.5 |
Post-employment benefits | | 0.1 | | 0.1 |
Other long-term benefits | | — | | — |
Termination benefits | | — | | 0.2 |
Share-based payments | | 0.3 | | 0.2 |
| | 2.0 | | 2.0 |
Key management personnel are those persons having authority and responsibility for planning, directing and controlling the activities of the Group, directly or indirectly, including all Executive and Non-executive Directors. The number of key management personnel whose remuneration is included above is 11 (2004 – 12).
DIRECTORS’ INTERESTS
No Director or key management personnel had any disclosable related party transactions with the Group during the year.
28 | RECONCILIATION OF EQUITY AND PROFIT UNDER UK GAAP TO IFRS |
With effect from January 1, 2005, Acambis has prepared consolidated financial statements under IFRS. The comparative information for the year to December 31, 2004 that was previously reported under UK GAAP has been restated in accordance with IFRS. In order to understand the impact of transition to IFRS, this note provides reconciliations of certain information previously presented under UK GAAP to the amounts restated in accordance with IFRS.
Page 99 of 122
EXPLANATORY NOTES
The notes below explain the impact that the adoption of IFRS has had on the Group’s consolidated results.
i) IFRS1 ‘FIRST TIME ADOPTION OF IFRS’
The Group has taken advantage of the following exemptions available under IFRS1:
• | To apply IFRS3 ‘Business combinations’ from August 2003; |
• | To treat all at cumulative translation differences on overseas subsidiaries as zero at the date of transition to IFRS; and |
• | Not to apply IFRS2 ‘Share based payments’ to awards made before 7 November 2002, and that had not vested at December 31, 2004. |
ii) IFRS3 ‘BUSINESS COMBINATIONS’
Under UK GAAP, the excess of consideration over the fair value of net assets acquired was recognized as goodwill, and amortized over its useful economic life. Under IFRS, intangible assets acquired in a business combination are recognized at their fair value subject to meeting the definition of an intangible asset as set out in IAS38 ‘Intangible assets’. The residual goodwill is not subject to amortization, and is tested annually for impairment along with any other indefinite life assets in accordance with IAS36 ‘Impairment of assets’. Intangible assets acquired in a business combination are tested for impairment when there are indicators that the asset is impaired.
The adoption of IFRS3 has had the following impact:
• | Amortization of goodwill for Acambis Inc. ceases from January 2004; |
• | Amortization of goodwill for BPC ceases from August 2003; and |
• | The application of IFRS to the BPC acquisition in 2003 has resulted in the creation of an intangible asset and associated deferred tax liability and a reduction in the carrying amount of goodwill. |
iii) IFRS2 ‘SHARE-BASED PAYMENTS’
Acambis offers share options to employees as an employment benefit. Under UK GAAP, no accounting charge is made for share options issued at market value. Under IFRS, a fair value must be calculated and recognized as an expense over the vesting period, with a corresponding increase in equity. Deferred tax is recognized on share options where there is a temporary timing difference, which arises when the accounting book value and the tax book value of the options differ.
The charge previously made relating to UITF 17 (Revised 2003) ‘Employee Share Schemes’ has been reversed, as it is replaced by the IFRS2 charge. Deferred tax is calculated based on the expected tax deduction on exercise of the options compared to the accounting charge on grant of the option. Acambis has not provided for any increase in a deferred tax asset. Under IFRS, income tax relating to items recognized directly in equity is recognized in equity and not in the income statement, resulting in a movement between the tax charge under UK GAAP and equity under IFRS.
In the Company accounts, the granting of options to employees of subsidiaries is deemed a capital contribution.
iv) IAS38 ‘INTANGIBLE ASSETS’
IAS38 has had the following impact:
• | Capitalized software has been reclassified from ‘property plant and equipment’ to ‘intangible assets’; and |
• | Certain intangible assets acquired in a business are recognized at their value as described in explanatory note (ii). |
IAS38 also requires capitalization of development costs incurred on an individual project if, and only if, specific criteria are met. Previously under UK GAAP, this was an alternative treatment. Management has reviewed these criteria and it is our opinion that it is not possible to satisfy the requirement to demonstrate the technical feasibility of a project, and that it will generate probable future economic benefits, until final submission for regulatory approval has been obtained. Therefore, the Group has not capitalized any internally generated development costs to date.
Page 100 of 122
v) IAS12 ‘INCOME TAX’
Under IFRS deferred tax is recognized on taxable temporary differences arising between the tax base and the accounting base of balance sheet items. The scope of IAS12 is wider than the corresponding UK GAAP standards, and means that deferred tax is recognized on certain temporary differences that would not have given rise to deferred tax under UK GAAP. For Acambis, the main differences on adoption of IFRS arise in relation to intangible assets acquired in a business combination and share-based payments.
vi) IAS17 ‘LEASES’
Under IAS17, the presentation of the Canton finance lease facility differs from that under UK GAAP. Under IFRS the asset is restated to the net present value of the minimum lease payments, with a corresponding entry recorded as a lease creditor. This is unwound over the period of the lease.
vii) IAS21 ‘THE EFFECTS OF CHANGES IN FOREIGN EXCHANGE RATES’
Cumulative exchange differences arising on the retranslation of the Group’s overseas subsidiaries are reported as a separate component of equity under IFRS. There is no impact on the balance at transition as Acambis has taken advantage of the exemption available under IFRS1, as described above. The exchange differences on permanent-as-equity loans are recorded through the income statement in the Company’s accounts.
viii) IAS7 ‘CASH FLOW STATEMENT’
The following differences have arisen between the consolidated cash flow statement presented under UK GAAP, and the consolidated statement of cash flows prepared under IFRS:
• | Reclassification of certain liquid investments as cash and cash equivalents; and |
• | Reduction in the amounts disclosed as ‘purchases of liquid investments’ and ‘sale of liquid investments’. The adoption of IFRS had no material impact on the underlying cash flows of the Group. |
ix) IAS39 ‘RECOGNITION AND MEASUREMENT OF FINANCIAL INSTRUMENTS’
Under IFRS, derivative financial instruments are recorded at fair value, which has resulted in a net decrease in assets of £0.1m at January 1, 2005.
RECONCILIATION OF PROFIT FOR YEAR ENDED DECEMBER 31, 2004
| | | | | | | | Group | |
| | | | UK GAAP | | Adjustment | | IFRS | |
| | Note | | £m | | £m | | £m | |
Revenue | | (ii) | | 85.5 | | – | | 85.5 | |
Cost of sales | | | | (34.3 | ) | (0.7 | ) | (35.0 | ) |
Gross profit | | | | 51.2 | | (0.7 | ) | 50.5 | |
Research and development costs | | (iii) | | (28.9 | ) | (0.4 | ) | (29.3 | ) |
Sales and marketing costs | | (iii) | | (2.7 | ) | (0.1 | ) | (2.8 | ) |
Administration costs including costs relating to Canton plant impairment and restructuring costs | | (i), (ii), (iii) | | (7.7 | ) | 2.2 | | (5.5 | ) |
Other operating income – settlement of Canton agreement | | | | 10.2 | | – | | 10.2 | |
Operating profit | | | | 22.1 | | 1.0 | | 23.1 | |
Non-operating income | | | | 0.2 | | (0.2 | ) | – | |
Finance income | | | | 4.8 | | – | | 4.8 | |
Finance costs | | | | (0.9 | ) | – | | (0.9 | ) |
Profit before tax | | | | 26.2 | | 0.8 | | 27.0 | |
Taxation | | (v) | | (6.4 | ) | (0.9 | ) | (7.3 | ) |
Profit for the year attributable to shareholders | | | | 19.8 | | (0.1 | ) | 19.7 | |
Earnings per ordinary share (basic) | | | | 18.6 | p | (0.1 | )p | 18.5 | p |
Earnings per ordinary share (fully diluted) | | | | 18.2 | p | (0.1 | )p | 18.1 | p |
Page 101 of 122
RECONCILIATION OF PROFIT FOR YEAR ENDED DECEMBER 31, 2004
| | | Company | |
| | | | £m | |
Profit for the Company under UK GAAP | | | | 5.5 | |
IFRS2 share based payments | | (iii) | | 0.1 | |
Tax effect of IFRS2 | | (iii) | | (0.4 | ) |
IAS39 financial liabilities | | (ix) | | (0.1 | ) |
IAS21 foreign currency loss | | (vii) | | (1.9 | ) |
Retained profit under IFRS for 2004 | | | | 3.2 | |
RECONCILIATION OF EQUITY DECEMBER 31, 2004
| | | | | | | Group | |
| | | UK GAAP | | Adjustment | | IFRS | |
| Note | | £m | | £m | | £m | |
Assets | | | | | | | | |
Non-current assets | | | | | | | | |
Goodwill | (ii | ) | 16.0 | | (0.6 | ) | 15.4 | |
Other intangible assets | (ii), (iv | ) | – | | 4.1 | | 4.1 | |
Property, plant and equipment | (iv), (vi | ) | 17.5 | | 1.0 | | 18.5 | |
Other non-current assets | | | 2.5 | | – | | 2.5 | |
| | | 36.0 | | 4.5 | | 40.5 | |
Current assets | | | | | | | | |
Inventory | | | 6.0 | | – | | 6.0 | |
Trade and other receivables | | | 13.7 | | – | | 13.7 | |
Current tax assets | | | 1.9 | | – | | 1.9 | |
Liquid investments | (viii | ) | 70.9 | | (50.1 | ) | 20.8 | |
Cash and cash equivalents | (viii | ) | 30.9 | | 50.1 | | 81.0 | |
| | | 123.4 | | – | | 123.4 | |
Liabilities | | | | | | | | |
Current liabilities | | | | | | | | |
Interest-bearing loans and borrowings | | | (6.7 | ) | – | | (6.7 | ) |
Trade and other payables | | | (8.3 | ) | – | | (8.3 | ) |
Accruals and deferred income | (vi | ) | (26.6 | ) | (1.3 | ) | (27.9 | ) |
Derivative financial instruments | (ix | ) | – | | (0.1 | ) | (0.1 | ) |
Income tax payable | | | (4.6 | ) | – | | (4.6 | ) |
| | | (46.2 | ) | (1.4 | ) | (47.6 | ) |
Net current assets | | | 77.2 | | (1.4 | ) | 75.8 | |
Non-current liabilities | | | | | | | | |
Investment in Joint Venture | | | (0.3 | ) | – | | (0.3 | ) |
Long-term financial liabilities | | | (6.3 | ) | – | | (6.3 | ) |
Other non-current liabilities | | | (0.5 | ) | – | | (0.5 | ) |
Deferred income tax liabilities | (ii | ) | (0.1 | ) | (1.6 | ) | (1.7 | ) |
| | | (7.2 | ) | (1.6 | ) | (8.8 | ) |
Net assets | | | 106.0 | | 1.5 | | 107.5 | |
Shareholders’ equity | | | | | | | | |
Share capital | | | 10.7 | | – | | 10.7 | |
Share premium | | | 97.8 | | – | | 97.8 | |
Other reserves | (vii | ) | – | | (2.5 | ) | (2.5 | ) |
Retained earnings | | | (2.5 | ) | 4.0 | | 1.5 | |
Total shareholders’ equity | | | 106.0 | | 1.5 | | 107.5 | |
Page 102 of 122
RECONCILIATION OF EQUITY AT 1 JANUARY 2004
| | | | | | | | Group | |
| | | | UK GAAP | | Adjustment | | IFRS | |
| | Note | | £m | | £m | | £m | |
Assets | | | | | | | | | |
Non-current assets | | | | | | | | | |
Goodwill | | (ii | ) | 18.4 | | (2.9 | ) | 15.5 | |
Other intangible assets | | (ii), (iv | ) | 0.8 | | 4.9 | | 5.7 | |
Property, plant and equipment | | (iv), (vi | ) | 21.0 | | 2.6 | | 23.6 | |
Deferred tax assets | | | | 2.1 | | – | | 2.1 | |
Other non-current assets | | | | 0.1 | | – | | 0.1 | |
| | | | 42.4 | | 4.6 | | 47.0 | |
Current assets | | | | | | | | | |
Inventory | | | | 18.2 | | – | | 18.2 | |
Trade and other receivables | | | | 10.2 | | – | | 10.2 | |
Liquid investments | | (viii) | | 62.0 | | (44.2 | ) | 17.8 | |
Cash and cash equivalents | | (viii) | | 63.2 | | 44.2 | | 107.4 | |
| | | | 153.6 | | – | | 153.6 | |
Liabilities | | | | | | | | | |
Current liabilities | | | | | | | | | |
Trade and other payables | | | | (15.4 | ) | – | | (15.4 | ) |
Interest-bearing loans and borrowings | | | | (6.9 | ) | – | | (6.9 | ) |
Accruals and deferred income | | (vi | ) | (74.3 | ) | (1.4 | ) | (75.7 | ) |
Income tax payable | | | | (0.3 | ) | – | | (0.3 | ) |
| | | | (96.9 | ) | (1.4 | ) | (98.3 | ) |
Net current assets | | | | 56.7 | | (1.4 | ) | 55.3 | |
Non-current liabilities | | | | | | | | | |
Investment in Joint Venture | | | | (0.3 | ) | – | | (0.3 | ) |
Long-term financial liabilities | | | | (9.6 | ) | – | | (9.6 | ) |
Accruals and deferred income | | (vi | ) | (0.1 | ) | (1.4 | ) | (1.5 | ) |
Other non-current liabilities | | | | (2.6 | ) | – | | (2.6 | ) |
Deferred income tax liabilities | | (ii | ) | – | | (1.8 | ) | (1.8 | ) |
| | | | (12.6 | ) | (3.2 | ) | (15.8 | ) |
Net assets | | | | 86.5 | | – | | 86.5 | |
Shareholders’ equity | | | | | | | | | |
Share capital | | | | 10.6 | | – | | 10.6 | |
Share premium | | | | 96.0 | | – | | 96.0 | |
Retained earnings | | | | (20.1 | ) | – | | (20.1 | ) |
Total shareholders’ equity | | | | 86.5 | | – | | 86.5 | |
Page 103 of 122
RECONCILIATION OF EQUITY AT DECEMBER 31, 2004
| | | | | | | | Company | |
| | | | UK GAAP | | Adjustment | | IFRS | |
| | Note | | £m | | £m | | £m | |
Non-current assets | | | | | | | | | |
Investments in subsidiaries | | (iii | ) | 15.0 | | 0.5 | | 15.5 | |
Amounts owed by subsidiary undertaking | | | | 26.1 | | – | | 26.1 | |
Other non-current assets | | | | 0.6 | | – | | 0.6 | |
| | | | 41.7 | | 0.5 | | 42.2 | |
Current assets | | | | | | | | | |
Trade and other receivables | | | | 1.2 | | – | | 1.2 | |
Liquid investments | | (viii | ) | 53.9 | | (36.1 | ) | 17.8 | |
Cash and cash equivalents | | (viii | ) | 34.2 | | 36.1 | | 70.3 | |
| | | | 89.3 | | – | | 89.3 | |
Current liabilities | | | | | | | | | |
Trade and other payables | | | | (0.1 | ) | – | | (0.1 | ) |
Amounts owed by subsidiary undertakings | | | | (16.0 | ) | – | | (16.0 | ) |
Accruals and deferred income | | | | (0.7 | ) | – | | (0.7 | ) |
Financial liabilities: derivative financial instruments | | (ix | ) | – | | (0.1 | ) | (0.1 | ) |
Income tax payable | | | | (1.1 | ) | – | | (1.1 | ) |
| | | | (17.9 | ) | (0.1 | ) | (18.0 | ) |
Net current assets | | | | 71.4 | | (0.1 | ) | 71.3 | |
Net assets | | | | 113.1 | | 0.4 | | 113.5 | |
Shareholders’ equity | | | | | | | | | |
Share capital | | | | 10.7 | | – | | 10.7 | |
Share premium | | | | 97.6 | | – | | 97.6 | |
Retained earnings | | | | 4.8 | | 0.4 | | 5.2 | |
Total shareholders’ equity | | | | 113.1 | | 0.4 | | 113.5 | |
RECONCILIATION OF EQUITY AT JANUARY 1, 2004
| | | | UK GAAP | | Adjutmrnt | | Company | |
| | Note | | £m | | £m | | IFRS | |
Non-current assets | | | | | | | | | |
Investments in subsidiaries | | (iii | ) | 15.0 | | 0.1 | | 15.1 | |
Amounts owed by subsidiary undertaking | | | | 28.0 | | – | | 28.0 | |
| | | | 43.0 | | 0.1 | | 43.1 | |
Current assets | | | | | | | | | |
Liquid investments | | | | 35.0 | | (24.0 | ) | 11.0 | |
Cash and cash equivalents | | | | 43.9 | | 24.0 | | 67.9 | |
| | | | 78.9 | | – | | 78.9 | |
Current liabilities | | | | | | | | | |
Trade and other payables | | | | (0.2 | ) | – | | (0.2 | ) |
Amounts owed by subsidiary undertakings | | | | (13.9 | ) | – | | (13.9 | ) |
Accruals and deferred income | | | | (0.5 | ) | – | | (0.5 | ) |
| | | | (14.6 | ) | – | | (14.6 | ) |
Net current assets | | | | 64.3 | | – | | 64.3 | |
Net assets | | | | 107.3 | | 0.1 | | 107.4 | |
Shareholders’ equity | | | | | | | | | |
Share capital | | | | 10.6 | | – | | 10.6 | |
Share premium | | | | 95.8 | | – | | 95.8 | |
Retained earnings | | | | 0.9 | | 0.1 | | 1.0 | |
Total shareholders’ equity | | | | 107.3 | | 0.1 | | 107.4 | |
Page 104 of 122
29 | RECONCILIATION TO US ACCOUNTING PRINCIPLES |
SUMMARY OF SIGNIFICANT DIFFERENCES BETWEEN IFRS FOLLOWED BY THE GROUP AND US GAAP
The Group’s financial statements have been prepared under IFRS, which differs in certain significant respects from US GAAP. The principal differences between the Group’s accounting policies under IFRS and US GAAP are set out below. As noted in Note 1 of the financial statements, the consolidated financial statements of Acambis have been prepared, for the first time, in accordance with IFRS.
a) MULTIPLE-ELEMENT ARRANGEMENTS
The $428m ACAM2000 contract awarded in November 2001 consists of two principal components: to manufacture 155 million doses of smallpox vaccine; and to take the vaccine through clinical trials to FDA licensure.
Under IFRS, this contract has been accounted for as a single-element arrangement. As detailed in note 1 of the financial statements, turnover and profits are recognized according to the extent of performance under the contract, based on the proportion of costs incurred to date divided by the total estimated costs to complete. All research and development costs are expensed as incurred and manufacturing costs are held on the balance sheet as inventory until relevant criteria for revenue recognition are met.
Under US GAAP, the Group treats this contract as a multiple-element arrangement, resulting in a different allocation of revenue compared to the IFRS treatment. The two primary elements of the contract (the manufacturing and the development portions) each are separable deliverables which have value to the CDC on a standalone basis and have fair values which are objectively and reliably determinable. In addition, each of the deliverables is accounted for under SAB 104, not under a percentage to complete model.
For purposes of US GAAP, the Group has determined the fair value of the development and manufacturing portions of the contract and has allocated the total contract value to the development and manufacturing using the relative fair value method as prescribed by Emerging Issues Task Force (EITF) Issue No. 00-21, ‘Accounting for Revenue Arrangements with Multiple Deliverables’.
The development portion of the contract consists of the completion of Phase I/II clinical trials, completion of Phase III clinical trials, completion and submission of a Biologics License Application with the FDA and post FDA approval activities. The manufacturing portion consists of the manufacture and delivery of smallpox vaccine to the CDC.
The fair values were determined using the US regulations and guidance applicable to government contracts utilizing specifically the contracting regulations stipulated in the Federal Acquisition Regulation (FAR), together with the evidence provided by the Group’s ACAM1000 contract. After determining the fair value of the two elements of the contract, as noted above, the Group allocated the arrangement consideration to the separate units of accounting using the relative fair value method. $152m of the ACAM2000 contract was allocated to the research and development element and $276m allocated to the manufacturing element.
The table below shows total revenue recognized relating to each major class under the $428m contract in 2005 and 2004:
| | Year ended Dec 31 | |
| | 2005
£m | | 2004
£m | |
Development | | 20.3 | | 21.6 | |
Manufacturing | | – | | 33.7 | |
Total revenue under US GAAP for the contract | | 20.3 | | 55.3 | |
Page 105 of 122
Under US GAAP, with respect to the development work, revenue is recognized under the proportional performance method as services are provided under the contract. The development work has been further broken down into three distinct phases consisting of:
| • | four phase I/II clinical trials; |
| • | two phase III clinical trials; and |
| • | submitting IND and obtaining FDA approval. |
The development revenue was allocated to these three distinct phases using the relative fair value method using the same methodology to determine fair value that was applied to the development services as a whole as noted above. The Group determined this was necessary because the level of effort was not consistent throughout each of these phases. Therefore, after allocating the revenue to each of these phases, such revenue was recognized on a straight-line basis over the expected performance period of each phase. Relevant cost of revenue is recorded on an accrual basis as actual costs are incurred; this contrasts with the IFRS treatment, where revenues are recognized as costs are incurred, using the principles of long-term contract accounting.
Manufacturing costs were held on the balance sheet as ‘inventory’ until the relevant criteria for revenue recognition are met. The Group recognized revenue on the manufacturing portion of the contract as smallpox vaccine was delivered to the CDC. Thus, manufacturing revenue and costs were recognized in the income statement on the transfer of the risks and rewards of ownership, typically through delivery of product to the customer’s premises, assuming all other revenue recognition criteria were met at this time.
Costs associated with expected re-labeling to be undertaken in the future were being accrued as smallpox vaccine was delivered to the CDC. Costs and revenue associated with providing storage or vaccine disposal services are only included when, and if, it is deemed probable that such services will be performed.
b) INVENTORY SUBJECT TO DEFERRED REVENUE ARRANGEMENTS
In the case of inventory subject to deferred revenue arrangements, the criteria specified for determining whether the risks and rewards of ownership have transferred differ between IFRS and US GAAP.
At December 31, 2004, a proportion of revenue relating to certain batches of smallpox vaccine were required to be recognized under IFRS the relevant recognition criteria having been met. US GAAP stipulates additional criteria, including that all components of the vaccine have been delivered. As at December 31, 2004 certain batches did not meet this criterion, with the result that the costs and revenues were not recognizable in the year ended December 31, 2004 under US GAAP. Accordingly, the cost of inventory of £0.3m which was expensed under IFRS remained capitalized as inventory under US GAAP at December 31, 2004, and related revenue of £0.5m was not recognized under US GAAP at December 31, 2004.
During 2005 the batches met the additional criteria, in that all components of the vaccine were delivered. Therefore the £0.3m was expensed from inventory to cost of revenue and the related revenue of £0.5m was recognized.
c) LICENSE FEES
Under IFRS certain license fees were recognized when paid, where such payments were not refundable. Under US GAAP the Group follows SAB104, ‘Revenue Recognition in Financial Statements’, and where such license fees are not refundable and are not credited against associated R&D activities, these fees are considered inseparable from the associated R&D effort. As such, those license fees are deferred and recognized over the period of the license term or over the period of the R&D agreement. Deferred revenues relating to research programs terminated during this period are released to revenue on termination of the program.
Page 106 of 122
| | Year ended Dec 31 | |
| | 2005
£m | | 2004
£m | |
Revenue recognized under IFRS | | 40.9 | | 85.5 | |
Multiple-element arrangements | | 7.5 | | 17.5 | |
Deferred revenue arrangements | | 0.5 | | (0.5 | ) |
License fees | | 0.9 | | – | |
Revenue recognized under US GAAP | | 49.8 | | 102.5 | |
d) TAX
i) EFFECTS OF REVENUE ADJUSTMENTS
As the majority of the profit in relation to the development portion of the $428m ACAM2000 contract is recorded in the Group’s UK operations, any timing difference on the recognition of profit gives rise to a temporary difference at the UK tax rate of 30%.
As the profit in relation to the manufacturing portion of the $428m ACAM2000 contract and any profit that is associated with ‘deferred inventory arrangements’ and license fees are recorded in the US operations, any timing difference on the recognition of profit gives rise to a temporary difference at the marginal US tax rate of 41%. These temporary differences are included within ‘net tax effect of US GAAP adjustments’ in the reconciliations of net profit and shareholders’ equity. A valuation allowance may be recorded against deferred tax assets arising from operating loss carry forwards in accordance with the criteria described under ‘Deferred Tax’ below.
ii) TAX ON EMPLOYEE SHARE OPTIONS
The Group is entitled to a tax deduction for the amount treated as compensation under US and UK tax rules for certain employee share options.
The deduction arises on exercise of the option, while any compensation expense is recorded over the vesting period of the option.
Under US GAAP, the tax benefit arising on Incentive Stock Options in the US (and on options with similar characteristics granted outside the US) is recorded, in equity, in the period in which the option is exercised and the tax benefit arises. In respect of non-qualifying options in the US (and on options with similar characteristics granted outside the US) deferred tax assets are calculated by multiplying the compensation expense recorded in respect of these options by the prevailing tax rate in the relevant tax jurisdiction. Valuation allowances are recorded against such deferred tax assets unless utilization of the assets against future taxable profits is more likely than not, as assessed in accordance with the criteria in SFAS 109. Where, on exercise of the relevant option, the tax benefit obtained exceeds the deferred tax asset in relation to the relevant options, the excess is recorded in additional paid-in capital. Where the tax benefit is less than the deferred tax asset, the write-down of the deferred tax asset is recorded against additional paid-in capital to the extent of previous excess tax benefits recorded in this account, with any remainder recorded in the income statement.
Under IFRS the deferred tax asset arising is calculated by comparing the estimated amount of tax deduction to be obtained in the future (based on the Company’s share price at the balance sheet date) with the cumulative amount of compensation expense recorded in the income statement. If the amount of estimated future tax deduction exceeds the cumulative amount of the remuneration expense at the statutory rate, the excess is recorded directly in equity, against the profit and loss reserve. Deferred tax assets are recognised only if the utilisation of the assets against future taxable profits is more likely than not.
e) COMPENSATION COSTS UNDER VARIABLE PLAN ACCOUNTING FOR SHARE OPTIONS AND SAYE PLAN DISCOUNT
Acambis has granted share options to employees that will vest upon the attainment of certain targets.
Page 107 of 122
Under IFRS, the cost of these equity-settled transactions with employees is measured by reference to the fair value at the date of grant. The fair value is determined using a binomial option pricing model, as described in note 1 of the financial statements. Under US GAAP, APB Opinion No. 25, ‘Accounting for Stock Issued to Employees’ (APB25), the Group is required to follow variable plan accounting for these grants and measure compensation expense as the difference between the exercise price and the fair market value of the related share under option during each accounting period over the vesting period of the options. Increases in fair market value of the share result in a charge to operations and decreases in the fair market value of the share result in a credit to operations, limited to the cumulative amount previously expensed.
Under US GAAP, in accounting for new offers made under the SAYE plan since January 24, 2002, Acambis follows the requirements of pronouncement EITF 00-23 ‘Issues relating to the Accounting for Stock Compensation under APB25 and FIN44’ (Financial Accounting Standards Board Interpretation Number (FIN) 44, ‘Accounting for Certain Transactions Involving Stock Compensation’, (FIN44)). The compensation charge under US GAAP in respect of such plans is calculated using one of the two following methods:
1) | Under normal circumstances, where the plan qualifies as a fixed plan, the compensation charge is the difference between the market price of the shares at the date of grant and the exercise price of the option and is recorded on a straight-line basis over the vesting period. |
2) | Where a SAYE scheme is offered at a lower price than a previous scheme, variable accounting commences for all existing higher priced awards when the offer is made. For those awards that are retained by the employees because the new lower-priced offer is declined, variable accounting continues until the awards are exercised, are forfeited, or expire unexercised. New awards are accounted for as variable to the extent that previous higher priced options are cancelled. |
The table shows the (credit)/charge made to the group income statement under US GAAP.
| | Year ended Dec 31 | |
| | 2005
£m | | 2004
£m | |
(Credited)/charged to the Group income statement | | (0.4 | ) | 0.6 | |
f) PURCHASE PRICE ACCOUNTING, GOODWILL AND INTANGIBLES
Under both IFRS and US GAAP, goodwill is the amount by which the fair value of the purchase consideration exceeds the fair value of assets acquired. As explained in note 28 of the financial statements, on adoption of IFRS in 2005, the Group took advantage of IFRS 1 and has applied IFRS 3 to business combinations from August 2003, freezing the existing UK GAAP goodwill balances at their January 1, 2004 levels for acquisitions before that date.
i) ACQUISITION OF ACAMBIS INC.
During 1999, Acambis acquired Acambis Inc. Under superseded UK GAAP, in-process R&D did not meet the criteria for recognition as a separate intangible asset and, thus, such amounts were subsumed within goodwill. As permitted under IFRS transition rules, Acambis elected not to restate goodwill for acquisitions prior to August 2003. Therefore the IFRS goodwill relating to Acambis Inc. has not been restated and is based on the UK GAAP value of the goodwill at 1 January 2004, and is deemed a sterling denominated asset. Under US GAAP at the date of acquisition, in accordance with APB Opinion No. 16, ‘Business Combinations’, and No. 17, ‘Intangible Assets’, in-process R&D is separately identified and analyzed to determine the fair market value at the date of acquisition. In-process R&D is identified in accordance with the definition within Statement of Financial Accounting Standard (SFAS) No.2, ‘Accounting for Research and Development Costs’. Following identification of qualifying R&D projects within Acambis Inc, their value was determined by estimating the costs to develop the purchased in-process R&D into commercially viable products, estimating the resulting net cash flows from the projects and discounting the net cash flows to their present value.
As a result of the valuation of in-process R&D under US GAAP, the fair value of assets acquired is different from that calculated under IFRS, resulting in differing values of goodwill. Under US GAAP, in-process R&D is written off to the income statement when incurred.
Page 108 of 122
ii) ACQUISITION OF BERNA PRODUCTS CORPORATION (BPC)
In August 2003, Acambis acquired BPC, a sales, promotion and distribution organization based in Miami, US and Toronto, Canada in order to enhance the marketing function of the Group.
The consideration paid for BPC includes amounts contingent on the future performance of that organization. Under IFRS, a reasonable estimate of the fair value of amounts expected to be payable in the future is included in the cost of the acquisition and such amounts are re-assessed at each reporting date. The fair value of contingent consideration payable in cash is taken to be the estimated amount of cash payable discounted to its present value. The discount is unwound through the income statement so that the full liability is recognized at the time it is due to be paid. Under US GAAP, contingent consideration is recognized when the contingency is resolved, and the consideration becomes payable. Accordingly, contingent consideration paid in 2004 and 2005 was recognized at that time.
iii) AMORTIZATION OF GOODWILL AND INTANGIBLES
As under US GAAP, IFRS goodwill is not systematically amortized, but is also reviewed annually for impairment. Intangible assets acquired as part of a business combination are amortized under US GAAP and IFRS. The annual impairment review carried out this year did not reveal any indication of impairment.
g) CAPITALIZATION OF INTEREST
Under IFRS, Acambis’ accounting policy is that interest is not capitalized. US GAAP requires interest incurred as part of the cost of constructing fixed assets to be capitalized and amortized over the life of the asset.
h) AVAILABLE FOR SALE INVESTMENTS
Under US GAAP, investments in available-for-sale securities are marked to market where the market value is readily determinable and gains and losses, net of deferred taxation, are recorded in ‘Other comprehensive income’. Where an impairment is considered to be other than temporary, the security is written down through the income statement to a new cost basis represented by the fair value of the security on the date the impairment was determined. Under IFRS, the Group’s accounting policy is to carry such investments at market value, with the movement net of deferred tax recorded though the ‘Statement of recognised income and expense’. The basis for determining a readily available market value differs between US GAAP and IFRS, such that, under US GAAP, no revaluation of the group’s unlisted investment has been recorded.
i) RESTRICTED CASH
US GAAP requires that any cash that is restricted as to its use be excluded from cash and cash equivalents. Acambis has escrow bank accounts of £0.7m (2004: £0.4m) that meet the restriction criteria. If Acambis reported a US GAAP balance sheet, these would appear separately on that balance sheet.
j) DEFERRED TAX
Under IAS 12 ‘Income taxes’ deferred tax is provided on a full liability method, on all temporary differences at the balance sheet date between the tax bases of assets and liabilities and their carrying amounts for financial reporting purposes. Under US GAAP, SFAS 109 ‘Deferred tax’ deferred tax is provided on a full liability basis. Future tax benefits are recognized as deferred tax assets to the extent that their realization is more likely than not as determined by the evaluation of certain criteria.
k) IN-PROCESS R&D
Under IAS 38 ‘Intangible assets’ it is necessary to capitalise any acquired in-process R&D. Amortisation commences once the acquired technology is brought into use. Un-amortised amounts are subject to impairment reviews annually and on occurrence of a trigger event. Under US GAAP acquired in-process R&D is expensed immediately to the income statement.
l) CAPITALIZED SOFTWARE
Under IFRS, computer software used in the group is classified as an intangible asset. Under US GAAP, such computer software is classified as a tangible asset. There is no impact on shareholders’ equity. At the end of 2005 there was software with a carrying value of £0.3m (2004 - £0.3m).
Page 109 of 122
RECONCILIATION OF NET (LOSS)/PROFIT FROM IFRS TO US GAAP
Based on the differences detailed above, the following table shows the reconciliation of the Group’s net (loss)/profit for the past two years:
| | Year ended Dec 31 | |
| | Notes | | 2005
£m | | 2004
£m | |
Net (loss)/profit as reported under IFRS | | | | (27.0 | ) | 19.7 | |
Adjustments for: | | | | | | | |
Revenue recognition | | a,b,c | | 8.9 | | 17.0 | |
Inventory subject to deferred revenue arrangements | | b | | – | | 0.3 | |
Recognition of costs previously deferred | | b | | (0.3 | ) | (9.4 | ) |
Other income relating to license fees | | c | | (0.4 | ) | – | |
Compensation costs under variable plan accounting for share options and SAYE plan discount | | e | | 1.2 | | 0.1 | |
Purchase price accounting adjustments | | f | | 0.1 | | 0.1 | |
Depreciation and impairment of capitalized interest | | g | | (0.2 | ) | (0.2 | ) |
Capitalization of interest | | g | | 0.5 | | – | |
Available for sale investments | | h | | – | | 0.5 | |
In-process R&D | | k | | (0.4 | ) | – | |
Net tax effect of US GAAP adjustments | | d | | (2.7 | ) | (4.5 | ) |
Net (loss)/profit as reported under US GAAP | | | | (20.3 | ) | 23.6 | |
(LOSS)/PROFIT PER SHARE UNDER US GAAP
| | Year ended Dec 31 | |
| | 2005 | | 2004 | |
Basic (loss)/profit per ordinary share in pence | | (18.9 | ) | 22.2 | |
Shares used in computing basic (loss)/profit per ordinary share | | 107,211,367 | | 106,300,080 | |
Diluted (loss)/profit per ordinary share in pence | | (18.9 | ) | 21.7 | |
Shares used in computing diluted (loss)/profit per ordinary share | | 107,211,367 | | 108,649,389 | |
Anti-dilutive securities | | 2,474,352 | | 942,032 | |
OTHER COMPREHENSIVE INCOME
In addition to the net loss for the year, there is also other comprehensive income relating to foreign currency gains, which amounted to £2.6m (2004 – loss of £2.8m).
RECONCILIATION OF SHAREHOLDERS’ EQUITY FROM IFRS TO US GAAP
| | As at Dec 31 | |
| | Notes | | 2005
£m | | 2004
£m | |
Shareholders’ equity as reported under IFRS | | | | 83.0 | | 107.5 | |
Revenue recognition | | a,b,c | | 3.1 | | (5.5 | ) |
Inventory subject to deferred revenue arrangements | | b | | — | | 0.3 | |
Goodwill of Acambis Inc. | | f | | (7.6 | ) | (8.2 | ) |
Goodwill of BPC | | f | | 0.3 | | (1.3 | ) |
Contingent consideration | | f | | — | | 1.4 | |
Capitalization of interest | | g | | 0.8 | | 0.5 | |
Available for sale investments | | h | | (0.2 | ) | — | |
In-process R&D | | k | | (0.4 | ) | — | |
Net tax effect of US GAAP adjustments | | d | | (0.8 | ) | 1.6 | |
Shareholders’ equity as reported under US GAAP | | | | 78.2 | | 96.3 | |
Page 110 of 122
RECENT ACCOUNTING PRONOUNCEMENTS
US GAAP
In November 2004, the FASB issued FASB Statement No. 151 ‘Inventory Costs — An Amendment of ARB No. 43, Chapter 4’ (‘FAS 151’). FAS 151 amends the guidance in ARB No. 43, Chapter 4 ‘Inventory Pricing,’ to clarify the accounting for abnormal amounts of idle facility expense, freight, handling costs, and wasted material (spoilage). Among other provisions, the new rule requires that items such as idle facility expense, excessive spoilage, double freight, and re-handling costs be recognized as current-period charges regardless of whether they meet the criterion of ‘so abnormal’ as stated in ARB No. 43. Additionally, FAS 151 requires that the allocation of fixed production overheads to the costs of conversion be based on the normal capacity of the production facilities. FAS 151 is effective for fiscal years beginning after June 15, 2005.
The Group will adopt FAS 151 in 2006 but does not expect the adoption of the new standard to have a material impact.
In December 2004, the FASB issued FASB Statement No. 153 ‘Exchanges of Nonmonetary Assets — An Amendment of APB Opinion No. 29, Accounting for Nonmonetary Transactions’ (‘FAS 153’). FAS 153 eliminates the exception from fair value measurement for non monetary exchanges of similar productive assets in paragraph 21(b) of APB Opinion No. 29, ‘Accounting for Nonmonetary Transactions,’ and replaces it with an exception for exchanges that do not have commercial substance. FAS 153 specifies that a non-monetary exchange has commercial substance if the future cash flows of the entity are expected to change significantly as a result of the exchange. FAS 153 is effective for the fiscal periods beginning after June 15, 2005. The Group will adopt FAS 153 in 2006 but does not expect the adoption of the new standard to have a material impact.
In December 2004, the FASB issued FASB Statement No. 123 (revised 2004) ‘Share-Based Payment’ (‘FAS 123(R)’), which replaces FAS No. 123 ‘Accounting for Stock-Based Compensation’ (‘FAS 123’) and supersedes APB Opinion No. 25 ‘Accounting for Stock Issued to Employees.’ FAS 123(R) requires all share-based payments to employees, including grants of employee stock options, to be recognized in the financial statements based on their fair values beginning with the first interim or annual period after June 15, 2005, with early adoption encouraged. The pro forma disclosures previously permitted under FAS 123 no longer will be an alternative to financial statement recognition. The Group is currently assessing the impact of FAS 123(R).
In March 2005, the FASB issued FASB Interpretation No. 47 ‘Accounting for Conditional Asset Retirement Obligations — an interpretation of FASB Statement No. 143’ (‘FIN 47’). FIN 47 clarifies the timing of liability recognition for legal obligations associated with the retirement of a tangible long-lived asset when the timing and/or settlement are conditional on a future event. FIN 47 is effective for the fiscal periods ending after December 15, 2005. The adoption of FIN 47 did not have a material impact on the Group.
In May 2005, the FASB issued Statement No. 154, ‘Accounting Changes and Error Corrections — A replacement of APB Opinion No. 20 and FASB Statement No. 3’ (‘FAS 154’). This statement requires retrospective application to prior periods’ financial statements of changes in accounting principles unless it is impracticable to determine either the period-specific effects or the cumulative effect of the change. This statement applies to all voluntary changes in accounting principles and changes required by an accounting pronouncement that does not include specific transition provisions. FAS 154 is required to be adopted in fiscal years beginning after December 15, 2005. FAS 154 would not have had a material effect on the financial position, results of operations or cash flows of the Group under US GAAP as at December 31, 2005.
In October 2005, the FASB issued FASB Staff Position (FSP) 13-1 ‘Accounting for Rental Costs Incurred during a Construction Period’ (‘FSP 13-1’). FSP 13-1 requires rental costs associated with ground or building operating leases that are incurred during a construction period to be recognized as rental expense and included in income from continuing operations. FSP 13-1 is effective for the fiscal periods beginning after December 15, 2005. The Group will adopt FSP 13-1 in 2006 but does not expect the adoption of the new standard to have a material impact.
Page 111 of 122
In January 2006 the FASB issued FASB Statement No. 155 ‘Accounting for Certain Hybrid Financial Instruments — an amendment of FASB Statements No. 133 and 140’ (‘FAS 155’). FAS 155 provides entities with relief from having to separately determine the fair value of an embedded derivative that would otherwise be required to be bifurcated from its host contract. FAS 155 is effective for all financial instruments acquired, issued or subject to a re-measurement event occurring after the beginning of an entity’s first fiscal year that begins after September 15, 2006. The Group is currently evaluating the impact the adoption of FAS 155 will have, but does not expect it to have a material impact.
Exhibit Number | Description of Exhibit |
| |
1.1† | Memorandum of Association of the Company (incorporated herein by reference to Exhibit 3.1 to the Company’s Registration Statement on Form F-4, as filed with the Securities and Exchange Commission on February 10, 1999 (File No. 333-72077)). |
| |
1.2† | Articles of Association of the Company (incorporated herein by reference to Exhibit 1.2 to the Company’s Annual Report on Form 20-F, as filed with the Securities and Exchange Commission on June 30, 2005 (File No. 000-30126)). |
| |
2.1† | Deposit Agreement between and among Acambis plc and The Bank of New York, as Depositary (incorporated herein by reference to the Company’s Registration Statement on Form F-6 as filed with the Securities and Exchange Commission on February 13, 2001 (File No. 333-13166)). |
| |
4.1† | Director’s Service Agreement between Acambis plc and Gordon Cameron, dated February 23, 2004 (incorporated herein by reference to Exhibit 4.2 to the Company’s Annual Report on Form 20-F, as filed with the Securities and Exchange Commission on June 28, 2004 (File No. 000-30126)). |
| |
4.2 | Letter of Appointment between Acambis plc and Thomas Monath, dated June 23, 2006. |
| |
4.3** | Letter of Appointment between Acambis plc and Alan Smith, dated March 22, 2005. |
| |
4.4 | Letter of Appointment between Acambis plc and Alan Dalby, dated March 22, 2005. |
| |
4.5 | Letter of Appointment between Acambis plc and Michael Lytton, dated March 22, 2005. |
| |
4.6† | Letter of Appointment between Acambis plc and Ross Graham, dated March 24, 2004 (incorporated herein by reference to Exhibit 4.8 to the Company’s Annual Report on Form 20-F, as filed with the Securities and Exchange Commission on June 28, 2004 (File No. 000-30126)). |
| |
4.7**† | Director’s Service Agreement between Acambis plc and David Lawrence, dated July 8, 2004 (incorporated herein by reference to Exhibit 4.9 to the Company’s Annual Report on Form 20-F, as filed with the Securities and Exchange Commission on June 30, 2005 (File No. 000-30126)). |
| |
4.8** | Letter of Appointment between Acambis plc and Peter Fellner, dated February 2, 2006. |
Page 112 of 122
Exhibit Number | Description of Exhibit |
| | |
4.9† | Sublease, dated December 21, 2001, between Baxter Capital Corporation and Acambis Inc. (incorporated herein by reference to Exhibit 4.12 to the Company’s Annual Report Form 20-F/A, as filed with the Securities and Exchange Commission on July 15, 2003 (File No. 000-30126)). |
| |
4.10† | First Amendment to Sublease, dated April 16, 2003, between Baxter Capital Corporation and Acambis Inc. (incorporated herein by reference to Exhibit 4.13 to the Company’s Annual Report Form 20-F/A, as filed with the Securities and Exchange Commission on July 15, 2003 (File No. 000-30126)). |
| |
4.11**† | Vero Cell Know-How License, between and among Baxter AG and Oravax Inc., dated as of September 19, 2000 (incorporated herein by reference to Exhibit 4.15 to the Company’s Annual Report Form 20-F/A, as filed with the Securities and Exchange Commission on April 1, 2005 (File No. 000-30126)). |
| |
4.12**† | License Agreement between Baxter Vaccine AG and Acambis Inc., dated December 20, 2002 (incorporated herein by reference to Exhibit 4.16 to the Company’s Annual Report Form 20-F/A, as filed with the Securities and Exchange Commission on April 1, 2005 (File No. 000-30126)). |
| |
4.13**† | ACAM2000 Prime Contract, dated November 28, 2001, between U.S. Government and Acambis Inc. (incorporated herein by reference to Exhibit 4.18 to the Company’s Annual Report Form 20-F/A, as filed with the Securities and Exchange Commission on April 1, 2005 (File No. 000-30126)). |
| |
4.14**† | Modification 0001, dated December 12, 2001, of ACAM2000 Prime Contract between U.S. Government and Acambis Inc. (incorporated herein by reference to Exhibit 4.19 to the Company’s Annual Report Form 20-F/A, as filed with the Securities and Exchange Commission on April 1, 2005 (File No. 000-30126)). |
| |
4.15**† | Modification 0002, dated December 12, 2001, of ACAM2000 Prime Contract between U.S. Government and Acambis Inc. (incorporated herein by reference to Exhibit 4.20 to the Company’s Annual Report Form 20-F/A, as filed with the Securities and Exchange Commission on April 1, 2005 (File No. 000-30126)). |
| |
4.16**† | Modification 0003, dated December 31, 2001, of ACAM2000 Prime Contract between U.S. Government and Acambis Inc. (incorporated herein by reference to Exhibit 4.21 to the Company’s Annual Report Form 20-F/A, as filed with the Securities and Exchange Commission on April 1, 2005 (File No. 000-30126)). |
| |
4.17**† | Modification 0004, dated January 31, 2002, of ACAM2000 Prime Contract between U.S. Government and Acambis Inc. (incorporated herein by reference to Exhibit 4.22 to the Company’s Annual Report Form 20-F/A, as filed with the Securities and Exchange Commission on April 1, 2005 (File No. 000-30126)). |
| |
4.18**† | Modification 0005, dated January 3, 2003, of ACAM2000 Prime Contract between U.S. Government and Acambis Inc. (incorporated herein by reference to Exhibit 4.23 to the Company’s Annual Report Form 20-F/A, as filed with the Securities and Exchange Commission on April 1, 2005 (File No. 000-30126)). |
| |
4.19**† | Modification 0006, dated February 28, 2003, of ACAM2000 Prime Contract between U.S. Government and Acambis Inc. (incorporated herein by reference to Exhibit 4.24 to the Company’s Annual Report Form 20-F/A, as filed with the Securities and Exchange Commission on April 1, 2005 (File No. 000-30126)). |
| |
Page 113 of 122
Exhibit Number | Description of Exhibit |
| | |
4.20**† | Modification 0007, dated May 30, 2003, of ACAM2000 Prime Contract between U.S. Government and Acambis Inc. (incorporated herein by reference to Exhibit 4.25 to the Company’s Annual Report Form 20-F/A, as filed with the Securities and Exchange Commission on April 1, 2005 (File No. 000-30126)). |
| |
4.21** | Modification 0008, dated February 15, 2005, of ACAM2000 Prime Contract between U.S. Government and Acambis Inc. |
| |
4.22**† | Modification 0009, dated November 4, 2003, of ACAM2000 Prime Contract between U.S. Government and Acambis Inc. (incorporated herein by reference to Exhibit 4.21 to the Company’s Annual Report on Form 20-F, as filed with the Securities and Exchange Commission on June 28, 2004 (File No. 000-30126)). |
| |
4.23**† | Distribution, Manufacturing and License Agreement between Acambis Research Limited, Baxter Healthcare SA and Baxter Healthcare Corporation, dated January 13, 2003 (incorporated herein by reference to Exhibit 4.26 to the Company’s Annual Report Form 20-F/A, as filed with the Securities and Exchange Commission on April 1, 2005 (File No. 000-30126)). |
| |
4.24**† | Modification 0001 to the Distribution, Manufacturing and License Agreement between Acambis Research Limited, Baxter Healthcare SA and Baxter Healthcare Corporation, dated May 13, 2003 (incorporated herein by reference to Exhibit 4.27 to the Company’s Annual Report Form 20-F/A, as filed with the Securities and Exchange Commission on April 1, 2005 (File No. 000-30126)). |
| |
4.25**† | Subcontract, between Acambis Inc. and Baxter Healthcare SA, dated November 14, 2001 (incorporated herein by reference to Exhibit 4.28 to the Company’s Annual Report Form 20-F/A, as filed with the Securities and Exchange Commission on April 1, 2005 (File No. 000-30126)). |
| |
4.26**† | Modification 0001 to Subcontract, between Acambis Inc. and Baxter Healthcare SA, dated May 9, 2002 (incorporated herein by reference to Exhibit 4.29 to the Company’s Annual Report Form 20-F/A, as filed with the Securities and Exchange Commission on April 1, 2005 (File No. 000-30126)). |
| |
4.27**† | Modification 0002 to Subcontract, between Acambis Inc. and Baxter Healthcare SA, dated May 9, 2002 (incorporated herein by reference to Exhibit 4.30 to the Company’s Annual Report Form 20-F/A, as filed with the Securities and Exchange Commission on April 1, 2005 (File No. 000-30126)). |
| |
4.28**† | Modification 0003 to Subcontract, between Acambis Inc. and Baxter Healthcare SA, dated December 20, 2002 (incorporated herein by reference to Exhibit 4.31 to the Company’s Annual Report Form 20-F/A, as filed with the Securities and Exchange Commission on April 1, 2005 (File No. 000-30126)). |
| |
4.29**† | Modification 0004 to Subcontract, between Acambis Inc. and Baxter Healthcare SA, dated December 20, 2002 (incorporated herein by reference to Exhibit 4.32 to the Company’s Annual Report Form 20-F/A, as filed with the Securities and Exchange Commission on April 1, 2005 (File No. 000-30126)). |
| |
4.30**† | Modification 0005 to Subcontract, between Acambis Inc. and Baxter Healthcare SA, dated May 29, 2003 (incorporated herein by reference to Exhibit 4.33 to the Company’s Annual Report Form 20-F/A, as filed with the Securities and Exchange Commission on July 15, 2003 (File No. 000-30126)). |
| |
Page 114 of 122
Exhibit Number | Description of Exhibit |
| | |
4.31**† | Modification 0006 to Subcontract, between Acambis Inc. and Baxter Healthcare SA, dated October 14, 2003 (incorporated herein by reference to Exhibit 4.30 to the Company’s Annual Report on Form 20-F, as filed with the Securities and Exchange Commission on June 28, 2004 (File No. 000-30126)). |
| |
4.32**† | Modification 0007 to Subcontract, between Acambis Inc. and Baxter Healthcare SA, dated May 14, 2004 (incorporated herein by reference to Exhibit 4.31 to the Company’s Annual Report on Form 20-F, as filed with the Securities and Exchange Commission on June 28, 2004 (File No. 000-30126)). |
| |
4.33** | Modification 0008 to Subcontract, between Acambis Inc. and Baxter Healthcare SA, dated December 27, 2005. |
| |
4.34**† | Distribution Agreement, between Berna Biotech Ltd. and Berna Products Corp., dated June 28, 2001 (incorporated herein by reference to Exhibit 4.40 to the Company’s Annual Report on Form 20-F, as filed with the Securities and Exchange Commission on June 28, 2004 (File No. 000-30126)). |
| |
4.35**† | MVA Prime Contract between U.S. Government and Acambis Inc., dated February 13, 2003 (incorporated herein by reference to Exhibit 4.41 to the Company’s Annual Report on Form 20-F, as filed with the Securities and Exchange Commission on June 28, 2004 (File No. 000-30126)). |
| |
4.36**† | Modification 0001 to MVA Prime Contract between U.S. Government and Acambis Inc., dated July 14, 2003 (incorporated herein by reference to Exhibit 4.42 to the Company’s Annual Report on Form 20-F, as filed with the Securities and Exchange Commission on June 28, 2004 (File No. 000-30126)). |
| |
4.37** | Modification 0002 to MVA Prime Contract between U.S. Government and Acambis Inc., dated September 22, 2004. |
| |
4.38** | Modification 0003 to MVA Prime Contract between U.S. Government and Acambis Inc., dated September 28, 2005. |
| |
4.39**† | MVA Baxter Subcontract between Baxter Healthcare SA and Acambis Inc., dated April 15, 2003 (incorporated herein by reference to Exhibit 4.43 to the Company’s Annual Report on Form 20-F, as filed with the Securities and Exchange Commission on June 28, 2004 (File No. 000-30126)). |
| |
4.40**† | Modification 0001 to MVA Baxter Subcontract between Baxter Healthcare SA and Acambis Inc., dated March 24, 2003 (incorporated herein by reference to Exhibit 4.44 to the Company’s Annual Report on Form 20-F, as filed with the Securities and Exchange Commission on June 28, 2004 (File No. 000-30126)). |
| |
4.41**† | Modification 0002 to MVA Baxter Subcontract between Baxter Healthcare SA and Acambis Inc., dated July 14, 2003 (incorporated herein by reference to Exhibit 4.45 to the Company’s Annual Report on Form 20-F, as filed with the Securities and Exchange Commission on June 28, 2004 (File No. 000-30126)). |
| |
4.42**† | Modification 0003 to MVA Baxter Subcontract between Baxter Healthcare SA and Acambis Inc., dated December 17, 2003 (incorporated herein by reference to Exhibit 4.46 to the Company’s Annual Report on Form 20-F, as filed with the Securities and Exchange Commission on June 28, 2004 (File No. 000-30126)). |
| |
Page 115 of 122
Exhibit Number | Description of Exhibit |
| | |
4.43**† | Modification 0004 to MVA Baxter Subcontract between Baxter Healthcare SA and Acambis Inc., dated February 6, 2004 (incorporated herein by reference to Exhibit 4.47 to the Company’s Annual Report on Form 20-F, as filed with the Securities and Exchange Commission on June 28, 2004 (File No. 000-30126)). |
| |
4.44** | Modification 0005 to MVA Baxter Subcontract between Baxter Healthcare SA and Acambis Inc., dated September 13, 2004. |
| |
4.45**† | Agency and Development Agreement between Cangene Corporation and Acambis Research Limited, dated March 3, 2003 (incorporated herein by reference to Exhibit 4.48 to the Company’s Annual Report on Form 20-F, as filed with the Securities and Exchange Commission on June 28, 2004 (File No. 000-30126)). |
| |
4.46**† | Sub-Agency Agreement between Acambis Research Limited and Baxter Healthcare S.A., dated September 12, 2003 (incorporated herein by reference to Exhibit 4.49 to the Company’s Annual Report on Form 20-F, as filed with the Securities and Exchange Commission on June 28, 2004 (File No. 000-30126)). |
| |
4.47**† | MVA Prime Contract between the National Institutes of Health and Acambis Inc., dated September 30, 2004 (incorporated herein by reference to Exhibit 4.51 to the Company’s Annual Report on Form 20-F, as filed with the Securities and Exchange Commission on June 30, 2005 (File No. 000-30126)). |
| |
4.48**† | Subcontract, between Acambis Inc. and Baxter Healthcare SA, dated September 30, 2004 (incorporated herein by reference to Exhibit 4.52 to the Company’s Annual Report on Form 20-F, as filed with the Securities and Exchange Commission on June 30, 2005 (File No. 000-30126)). |
| |
4.49**† | Modification 001 to Subcontract, between Acambis Inc. and Baxter Healthcare SA, dated September 30, 2004 (incorporated herein by reference to Exhibit 4.53 to the Company’s Annual Report on Form 20-F, as filed with the Securities and Exchange Commission on June 30, 2005 (File No. 000-30126)). |
| |
4.50† | Asset Purchase Agreement, between BioReliance Corporation and Acambis Inc., dated May 6, 2005 (incorporated herein by reference to Exhibit 4.54 to the Company’s Annual Report on Form 20-F, as filed with the Securities and Exchange Commission on June 30, 2005 (File No. 000-30126)). |
| |
8.1 | List of Significant Subsidiaries of Acambis plc. |
| |
12.1 | Section 302 Certification of Gordon Cameron. |
| |
12.2 | Section 302 Certification of David Lawrence. |
| |
13.1 | Certification of Gordon Cameron pursuant to 18 U.S.C. Section 1350, as adopted by Section 906 of the Sarbanes-Oxley Act of 2002. |
| |
13.2 | Certification of David Lawrence pursuant to 18 U.S.C. Section 1350, as adopted by Section 906 of the Sarbanes-Oxley Act of 2002. |
| |
15.1 | Consent of PricewaterhouseCoopers LLP. |
| |
** | Certain portions of this exhibit have been omitted and filed separately with the Commission pursuant to an application for confidential treatment under Rule 24b-2 promulgated under the Securities Exchange Act of 1934, as amended. |
Page 116 of 122
SIGNATURES
The registrant hereby certifies that it meets all of the requirements for filing on Form 20-F and that is has duly caused and authorized the undersigned to sign this annual report on its behalf.
| | | ACAMBIS PLC |
| | | | By: |
/s/ Gordon Cameron
|
| | | | Name: | Gordon Cameron |
| | | | Title: | Chief Executive Officer |
| | | | | | |
| Date: June 26, 2006 | | | |
Page 117 of 122
Exhibit Index
Exhibit
Number | Description of Exhibit |
| |
1.1† | Memorandum of Association of the Company (incorporated herein by reference to Exhibit 3.1 to the Company’s Registration Statement on Form F-4, as filed with the Securities and Exchange Commission on February 10, 1999 (File No. 333-72077)). |
| |
1.2† | Articles of Association of the Company (incorporated herein by reference to Exhibit 1.2 to the Company’s Annual Report on Form 20-F, as filed with the Securities and Exchange Commission on June 30, 2005 (File No. 000-30126)). |
| |
2.1† | Deposit Agreement between and among Acambis plc and The Bank of New York, as Depositary (incorporated herein by reference to the Company’s Registration Statement on Form F-6 as filed with the Securities and Exchange Commission on February 13, 2001 (File No. 333-13166)). |
| |
4.1† | Director’s Service Agreement between Acambis plc and Gordon Cameron, dated February 23, 2004 (incorporated herein by reference to Exhibit 4.2 to the Company’s Annual Report on Form 20-F, as filed with the Securities and Exchange Commission on June 28, 2004 (File No. 000-30126)). |
| |
4.2 | Letter of Appointment between Acambis plc and Thomas Monath, dated June 23, 2006. |
| |
4.3** | Letter of Appointment between Acambis plc and Alan Smith, dated March 22, 2005. |
| |
4.4 | Letter of Appointment between Acambis plc and Alan Dalby, dated March 22, 2005. |
| |
4.5 | Letter of Appointment between Acambis plc and Michael Lytton, dated March 22, 2005. |
| |
4.6† | Letter of Appointment between Acambis plc and Ross Graham, dated March 24, 2004 (incorporated herein by reference to Exhibit 4.8 to the Company’s Annual Report on Form 20-F, as filed with the Securities and Exchange Commission on June 28, 2004 (File No. 000-30126)). |
| |
4.7**† | Director’s Service Agreement between Acambis plc and David Lawrence, dated July 8, 2004 (incorporated herein by reference to Exhibit 4.9 to the Company’s Annual Report on Form 20-F, as filed with the Securities and Exchange Commission on June 30, 2005 (File No. 000-30126)). |
| |
4.8** | Letter of Appointment between Acambis plc and Peter Fellner, dated February 2, 2006. |
| |
4.9† | Sublease, dated December 21, 2001, between Baxter Capital Corporation and Acambis Inc. (incorporated herein by reference to Exhibit 4.12 to the Company’s Annual Report Form 20-F/A, as filed with the Securities and Exchange Commission on July 15, 2003 (File No. 000-30126)). |
| |
4.10† | First Amendment to Sublease, dated April 16, 2003, between Baxter Capital Corporation and Acambis Inc. (incorporated herein by reference to Exhibit 4.13 to the Company’s Annual Report Form 20-F/A, as filed with the Securities and Exchange Commission on July 15, 2003 (File No. 000-30126)). |
| |
4.11**† | Vero Cell Know-How License, between and among Baxter AG and Oravax Inc., dated as of September 19, 2000 (incorporated herein by reference to Exhibit 4.15 to the Company’s Annual Report Form 20-F/A, as filed with the Securities and Exchange Commission on |
| |
Page 118 of 122
Exhibit
Number | Description of Exhibit |
| |
| April 1, 2005 (File No. 000-30126)). |
| |
4.12**† | License Agreement between Baxter Vaccine AG and Acambis Inc., dated December 20, 2002 (incorporated herein by reference to Exhibit 4.16 to the Company’s Annual Report Form 20-F/A, as filed with the Securities and Exchange Commission on April 1, 2005 (File No. 000-30126)). |
| |
4.13**† | ACAM2000 Prime Contract, dated November 28, 2001, between U.S. Government and Acambis Inc. (incorporated herein by reference to Exhibit 4.18 to the Company’s Annual Report Form 20-F/A, as filed with the Securities and Exchange Commission on April 1, 2005 (File No. 000-30126)). |
| |
4.14**† | Modification 0001, dated December 12, 2001, of ACAM2000 Prime Contract between U.S. Government and Acambis Inc. (incorporated herein by reference to Exhibit 4.19 to the Company’s Annual Report Form 20-F/A, as filed with the Securities and Exchange Commission on April 1, 2005 (File No. 000-30126)). |
| |
4.15**† | Modification 0002, dated December 12, 2001, of ACAM2000 Prime Contract between U.S. Government and Acambis Inc. (incorporated herein by reference to Exhibit 4.20 to the Company’s Annual Report Form 20-F/A, as filed with the Securities and Exchange Commission on April 1, 2005 (File No. 000-30126)). |
| |
4.16**† | Modification 0003, dated December 31, 2001, of ACAM2000 Prime Contract between U.S. Government and Acambis Inc. (incorporated herein by reference to Exhibit 4.21 to the Company’s Annual Report Form 20-F/A, as filed with the Securities and Exchange Commission on April 1, 2005 (File No. 000-30126)). |
| |
4.17**† | Modification 0004, dated January 31, 2002, of ACAM2000 Prime Contract between U.S. Government and Acambis Inc. (incorporated herein by reference to Exhibit 4.22 to the Company’s Annual Report Form 20-F/A, as filed with the Securities and Exchange Commission on April 1, 2005 (File No. 000-30126)). |
| |
4.18**† | Modification 0005, dated January 3, 2003, of ACAM2000 Prime Contract between U.S. Government and Acambis Inc. (incorporated herein by reference to Exhibit 4.23 to the Company’s Annual Report Form 20-F/A, as filed with the Securities and Exchange Commission on April 1, 2005 (File No. 000-30126)). |
| |
4.19**† | Modification 0006, dated February 28, 2003, of ACAM2000 Prime Contract between U.S. Government and Acambis Inc. (incorporated herein by reference to Exhibit 4.24 to the Company’s Annual Report Form 20-F/A, as filed with the Securities and Exchange Commission on April 1, 2005 (File No. 000-30126)). |
| |
4.20**† | Modification 0007, dated May 30, 2003, of ACAM2000 Prime Contract between U.S. Government and Acambis Inc. (incorporated herein by reference to Exhibit 4.25 to the Company’s Annual Report Form 20-F/A, as filed with the Securities and Exchange Commission on April 1, 2005 (File No. 000-30126)). |
| |
4.21** | Modification 0008, dated February 15, 2005, of ACAM2000 Prime Contract between U.S. Government and Acambis Inc. |
| |
4.22**† | Modification 0009, dated November 4, 2003, of ACAM2000 Prime Contract between U.S. Government and Acambis Inc. (incorporated herein by reference to Exhibit 4.21 to the Company’s Annual Report on Form 20-F, as filed with the Securities and Exchange Commission on June 28, 2004 (File No. 000-30126)). |
| |
Page 119 of 122
Exhibit
Number | Description of Exhibit |
| |
4.23**† | Distribution, Manufacturing and License Agreement between Acambis Research Limited, Baxter Healthcare SA and Baxter Healthcare Corporation, dated January 13, 2003 (incorporated herein by reference to Exhibit 4.26 to the Company’s Annual Report Form 20-F/A, as filed with the Securities and Exchange Commission on April 1, 2005 (File No. 000-30126)). |
| |
4.24**† | Modification 0001 to the Distribution, Manufacturing and License Agreement between Acambis Research Limited, Baxter Healthcare SA and Baxter Healthcare Corporation, dated May 13, 2003 (incorporated herein by reference to Exhibit 4.27 to the Company’s Annual Report Form 20-F/A, as filed with the Securities and Exchange Commission on April 1, 2005 (File No. 000-30126)). |
| |
4.25**† | Subcontract, between Acambis Inc. and Baxter Healthcare SA, dated November 14, 2001 (incorporated herein by reference to Exhibit 4.28 to the Company’s Annual Report Form 20-F/A, as filed with the Securities and Exchange Commission on April 1, 2005 (File No. 000-30126)). |
| |
4.26**† | Modification 0001 to Subcontract, between Acambis Inc. and Baxter Healthcare SA, dated May 9, 2002 (incorporated herein by reference to Exhibit 4.29 to the Company’s Annual Report Form 20-F/A, as filed with the Securities and Exchange Commission on April 1, 2005 (File No. 000-30126)). |
| |
4.27**† | Modification 0002 to Subcontract, between Acambis Inc. and Baxter Healthcare SA, dated May 9, 2002 (incorporated herein by reference to Exhibit 4.30 to the Company’s Annual Report Form 20-F/A, as filed with the Securities and Exchange Commission on April 1, 2005 (File No. 000-30126)). |
| |
4.28**† | Modification 0003 to Subcontract, between Acambis Inc. and Baxter Healthcare SA, dated December 20, 2002 (incorporated herein by reference to Exhibit 4.31 to the Company’s Annual Report Form 20-F/A, as filed with the Securities and Exchange Commission on April 1, 2005 (File No. 000-30126)). |
| |
4.29**† | Modification 0004 to Subcontract, between Acambis Inc. and Baxter Healthcare SA, dated December 20, 2002 (incorporated herein by reference to Exhibit 4.32 to the Company’s Annual Report Form 20-F/A, as filed with the Securities and Exchange Commission on April 1, 2005 (File No. 000-30126)). |
| |
4.30**† | Modification 0005 to Subcontract, between Acambis Inc. and Baxter Healthcare SA, dated May 29, 2003 (incorporated herein by reference to Exhibit 4.33 to the Company’s Annual Report Form 20-F/A, as filed with the Securities and Exchange Commission on July 15, 2003 (File No. 000-30126)). |
| |
4.31**† | Modification 0006 to Subcontract, between Acambis Inc. and Baxter Healthcare SA, dated October 14, 2003 (incorporated herein by reference to Exhibit 4.30 to the Company’s Annual Report on Form 20-F, as filed with the Securities and Exchange Commission on June 28, 2004 (File No. 000-30126)). |
| |
4.32**† | Modification 0007 to Subcontract, between Acambis Inc. and Baxter Healthcare SA, dated May 14, 2004 (incorporated herein by reference to Exhibit 4.31 to the Company’s Annual Report on Form 20-F, as filed with the Securities and Exchange Commission on June 28, 2004 (File No. 000-30126)). |
| |
4.33** | Modification 0008 to Subcontract, between Acambis Inc. and Baxter Healthcare SA, dated December 27, 2005. |
| |
Page 120 of 122
Exhibit
Number | Description of Exhibit |
| |
4.34**† | Distribution Agreement, between Berna Biotech Ltd. and Berna Products Corp., dated June 28, 2001 (incorporated herein by reference to Exhibit 4.40 to the Company’s Annual Report on Form 20-F, as filed with the Securities and Exchange Commission on June 28, 2004 (File No. 000-30126)). |
| |
4.35**† | MVA Prime Contract between U.S. Government and Acambis Inc., dated February 13, 2003 (incorporated herein by reference to Exhibit 4.41 to the Company’s Annual Report on Form 20-F, as filed with the Securities and Exchange Commission on June 28, 2004 (File No. 000-30126)). |
| |
4.36**† | Modification 0001 to MVA Prime Contract between U.S. Government and Acambis Inc., dated July 14, 2003 (incorporated herein by reference to Exhibit 4.42 to the Company’s Annual Report on Form 20-F, as filed with the Securities and Exchange Commission on June 28, 2004 (File No. 000-30126)). |
| |
4.37** | Modification 0002 to MVA Prime Contract between U.S. Government and Acambis Inc., dated September 22, 2004. |
| |
4.38** | Modification 0003 to MVA Prime Contract between U.S. Government and Acambis Inc., dated September 28, 2005. |
| |
4.39**† | MVA Baxter Subcontract between Baxter Healthcare SA and Acambis Inc., dated April 15, 2003 (incorporated herein by reference to Exhibit 4.43 to the Company’s Annual Report on Form 20-F, as filed with the Securities and Exchange Commission on June 28, 2004 (File No. 000-30126)). |
| |
4.40**† | Modification 0001 to MVA Baxter Subcontract between Baxter Healthcare SA and Acambis Inc., dated March 24, 2003 (incorporated herein by reference to Exhibit 4.44 to the Company’s Annual Report on Form 20-F, as filed with the Securities and Exchange Commission on June 28, 2004 (File No. 000-30126)). |
| |
4.41**† | Modification 0002 to MVA Baxter Subcontract between Baxter Healthcare SA and Acambis Inc., dated July 14, 2003 (incorporated herein by reference to Exhibit 4.45 to the Company’s Annual Report on Form 20-F, as filed with the Securities and Exchange Commission on June 28, 2004 (File No. 000-30126)). |
| |
4.42**† | Modification 0003 to MVA Baxter Subcontract between Baxter Healthcare SA and Acambis Inc., dated December 17, 2003 (incorporated herein by reference to Exhibit 4.46 to the Company’s Annual Report on Form 20-F, as filed with the Securities and Exchange Commission on June 28, 2004 (File No. 000-30126)). |
| |
4.43**† | Modification 0004 to MVA Baxter Subcontract between Baxter Healthcare SA and Acambis Inc., dated February 6, 2004 (incorporated herein by reference to Exhibit 4.47 to the Company’s Annual Report on Form 20-F, as filed with the Securities and Exchange Commission on June 28, 2004 (File No. 000-30126)). |
| |
4.44** | Modification 0005 to MVA Baxter Subcontract between Baxter Healthcare SA and Acambis Inc., dated September 13, 2004. |
| |
4.45**† | Agency and Development Agreement between Cangene Corporation and Acambis Research Limited, dated March 3, 2003 (incorporated herein by reference to Exhibit 4.48 to the Company’s Annual Report on Form 20-F, as filed with the Securities and Exchange Commission on June 28, 2004 (File No. 000-30126)). |
| |
4.46**† | Sub-Agency Agreement between Acambis Research Limited and Baxter Healthcare S.A., |
| |
Page 121 of 122
Exhibit
Number | Description of Exhibit |
| |
| dated September 12, 2003 (incorporated herein by reference to Exhibit 4.49 to the Company’s Annual Report on Form 20-F, as filed with the Securities and Exchange Commission on June 28, 2004 (File No. 000-30126)). |
| |
4.47**† | MVA Prime Contract between the National Institutes of Health and Acambis Inc., dated September 30, 2004 (incorporated herein by reference to Exhibit 4.51 to the Company’s Annual Report on Form 20-F, as filed with the Securities and Exchange Commission on June 30, 2005 (File No. 000-30126)). |
| |
4.48**† | Subcontract, between Acambis Inc. and Baxter Healthcare SA, dated September 30, 2004 (incorporated herein by reference to Exhibit 4.52 to the Company’s Annual Report on Form 20-F, as filed with the Securities and Exchange Commission on June 30, 2005 (File No. 000-30126)). |
| |
4.49**† | Modification 001 to Subcontract, between Acambis Inc. and Baxter Healthcare SA, dated September 30, 2004 (incorporated herein by reference to Exhibit 4.53 to the Company’s Annual Report on Form 20-F, as filed with the Securities and Exchange Commission on June 30, 2005 (File No. 000-30126)). |
| |
4.50† | Asset Purchase Agreement, between BioReliance Corporation and Acambis Inc., dated May 6, 2005 (incorporated herein by reference to Exhibit 4.54 to the Company’s Annual Report on Form 20-F, as filed with the Securities and Exchange Commission on June 30, 2005 (File No. 000-30126)). |
| |
8.1 | List of Significant Subsidiaries of Acambis plc. |
| |
12.1 | Section 302 Certification of Gordon Cameron. |
| |
12.2 | Section 302 Certification of David Lawrence. |
| |
13.1 | Certification of Gordon Cameron pursuant to 18 U.S.C. Section 1350, as adopted by Section 906 of the Sarbanes-Oxley Act of 2002. |
| |
13.2 | Certification of David Lawrence pursuant to 18 U.S.C. Section 1350, as adopted by Section 906 of the Sarbanes-Oxley Act of 2002. |
| |
15.1 | Consent of PricewaterhouseCoopers LLP. |
| |
† Previously filed.
** Certain portions of this exhibit have been omitted and filed separately with the Commission pursuant to an application for confidential treatment under Rule 24b-2 promulgated under the Securities Exchange Act of 1934, as amended.
Page 122 of 122



