UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 20-F
| o | | REGISTRATION STATEMENT PURSUANT TO SECTION 12(b) OR (g) OF THE SECURITIES EXCHANGE ACT OF 1934 |
| |
| | | -OR- |
| |
| x | | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 for the fiscal year ended December 31, 2002 |
| |
| | | -OR- |
| |
| o | | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 (NO FEE REQUIRED) for the transition period from to
Commission File Number: 000-30126 |
Acambis plc
(Exact name of Registrant as specified in its charter)
Not Applicable
(Translation of Registrant’s name into English)
England and Wales
(Jurisdiction of incorporation or organization)
Peterhouse Technology Park, 100 Fulbourn Road, Cambridge, CB1 9PT England
(Address of principal executive office)
Securities registered or to be registered pursuant to Section 12(b) of the Act: None
Securities registered or to be registered pursuant to Section 12(g) of the Act:
ORDINARY SHARES OF 10 PENCE EACH
(Title of Class)
Indicate the number of outstanding shares of each of the Registrant’s classes of capital or
common stock as of the close of period covered by this Annual Report —
93,011,883 ordinary shares of 10p each as of December 31, 2002
Indicate by check mark whether the Registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the Registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days:
Indicate by check mark which financial statement item the Registrant has elected to follow:
TABLE OF CONTENTS

| | | | | | | | | | | | | | | | | | | | | |
| Our business | | | Financial information | | Corporate governance |
| |
| 1 | | Profits | | | 16 | | | Directors’ responsibilities | | | 49 | | | Board of Directors |
| 2 | | Products | | | 16 | | | Independent auditors’ report | | | 50 | | | Directors' report |
| 6 | | Prospects | | | 18 | | | Group profit and loss account | | | 51 | | | Corporate governance statement |
| | | | | | | | 18 | | | Group statement of total recognised | | | 54 | | | Remuneration report |
| 10 | | Responsibility | | | | | | gains and losses | | | 61 | | | Shareholder information |
| 12 | | Operating review | | | 19 | | | Group balance sheet | | | | | | | | |
| 14 | | Financial review | | | 20 | | | Company balance sheet | | | | | | | | |
| | | | | | | | 21 | | | Group cash flow statement | | | General information |
| | | | | | | | 22 | | | Notes to Group financial statements | | | | | | | | |
| | | | | | | | 42 | | | Further information required by Form 20-F | | | 63 | | | Company information and advisors |
| | | | | | | | 47 | | | Summarised Group statements | | | 64 | | | Index |
| | | | | | | | | | | | | | | | 65 | | | Cross-reference to Form 20-F |
Acambis is a UK public limited company with shares listed on the London Stock Exchange and, in the form of American Depositary Receipts, on Nasdaq. This is the Annual Report and Form 20-F for the year ended 31 December 2002. It contains the Annual Report and Financial Statements in accordance with UK regulations and incorporates the Annual Report on Form 20-F for the US Securities and Exchange Commission (“SEC”) to meet US regulations. A cross-reference for the Form 20-F is provided on the fold-out flap inside the back cover of this report. References to the Group and Acambis throughout this document relate to Acambis plc and all of its subsidiary and associated undertakings. References to the Company are to Acambis plc, the ultimate holding company.
Cautionary statement regarding
forward-looking statements
Under the safe harbour provisions of the US Private Securities Litigation Reform Act of 1995, the Company cautions investors that any forward-looking statements or projections made in this document are subject to risks and uncertainties that may cause actual results to differ materially from those projected. These forward-looking statements are based on estimates and assumptions made by the management of Acambis and are believed to be reasonable, though are inherently uncertain and difficult to predict. Actual results or experience could differ materially from the forward-looking statements. Factors that may affect the Group’s operations are discussed in the operating review, financial review and the corporate governance statement contained within this Annual Report and Form 20-F, and in documents as filed with the US Securities and Exchange Commission from time to time.

(4a)
(4b) | | I am delighted to report that Acambis is now a profitable company, recording a pre-tax profit of £9.6m for the year ended 31 December 2002. This major milestone for the Group is the result of the significant progress we have made on the US Government’s smallpox vaccine contracts. We produced the first doses of smallpox vaccine for the Government stockpile within just 10 months of being awarded the contract. Such a development and production timescale is unprecedented, and was made possible by |
the continued dedication and enthusiasm of all our employees.
Now that we are profitable, our goal is to sustain profitability. Our strategy to achieve this is outlined in the “Prospects” section of this report. Most critically, we are focusing our resources on significant near-term revenue-generating opportunities. As part of this, we are enhancing the commercial strength of the Group, including establishing a focused marketing capability, to complement
our already strong research, development and manufacturing functions.
With a good balance of short, medium and long-term projects and the right infrastructure and resources to capitalise on the opportunities available, we are in a good position to remain profitable.
ALAN SMITH
Chairman
2

We have around300employees
based at locations in the UK and the US
Acambis is one of the world’s leading vaccines companies and is recognised internationally as the foremost producer of smallpox vaccines. We have around 300 employees based at locations in the UK and the US who provide not only research and development expertise but also all the key disciplines that are essential to a successful vaccines business — clinical operations, regulatory affairs, quality control, quality assurance, development, manufacturing, sales and marketing.
Our two major contracts with the US Government have established us as the world leader in the field of smallpox vaccines. Under these contracts, which date from September 2000 and November 2001, we have developed a new, second-generation smallpox vaccine and are contributing to a stockpile of smallpox vaccine sufficient to provide a dose of vaccine for every man, woman and child in the US. The stockpile, intended for emergency use, gives the US the ability rapidly to protect its population against this terrible disease if smallpox virus were used as a bioterrorist weapon.
Other governments have also recognised the need to establish a stockpile of smallpox vaccine, and 10 have already placed orders with us for the vaccine. Unusually, this product is being sold to governments even while the clinical trials are being conducted. We plan to submit the vaccine to the regulators for licensure in 2004.
We have added to our smallpox vaccine two related products, Modified Vaccinia Ankara (“MVA”) for the immunocompromised and vaccinia immune globulin (“VIG”) for treatment of reactions to the vaccine, to create a smallpox vaccine franchise. Together, these three products represent those required by governments looking to protect all their citizens against the threat of smallpox virus being used as a bioterrorist weapon.
PRODUCTS IN CLINICAL DEVELOPMENT
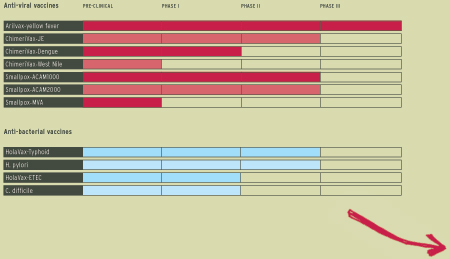
Research and development
Our smallpox vaccine is just one product in a broad pipeline of vaccines that we are currently taking through clinical development. We have a total of nine anti-viral and anti-bacterial vaccines that are undergoing clinical trials. These vaccines either target diseases for which no vaccine currently exists or offer improvements over those vaccines currently available.
3
4
Clinical trials, quality control and the regulatory environment
When a new vaccine is developed, it needs to undergo rigorous testing to establish whether it is safe and how effective it is in generating the right response in the body’s natural immune system. As these products are intended for human use, the testing and licensure processes are highly regulated. It is critical to our success that we employ people with the right knowledge and experience to enable us to meet the requirements of the regulators. In recent times, both in response to the requirements of our smallpox vaccine project and as our other vaccines have progressed into latter stage clinical trials, we have increased our clinical operations and regulatory affairs teams, and also the quality assurance and quality control functions that oversee the processes and procedures involved in the development and manufacture of vaccines.
Control ofmanufacture
is critical to our success
Manufacturing
In the world of biological products such as vaccines, developing the manufacturing process is as important as the scientific research. Currently, there is a worldwide shortage of manufacturing capacity for biological products, which can impact timelines and product availability. Control of manufacture is, therefore, critical to our success.
We have been putting in place this strategically important manufacturing capability. We started to reactivate our US-based manufacturing facility early in 2001 and will complete the programme in 2003. Parts of the facility are already fully up and running, in particular the suites producing smallpox vaccine. Once complete, the facility will be capable of producing both anti-viral and anti-bacterial vaccines and will meet the manufacturing needs of many of our products. Recognising how critical this capability is to our business, we regularly review our requirements for manufacturing and the potential for expansion. For instance, we have recently started to put in place a small-scale manufacturing capability for our C. difficile project.
Our first product is being
submitted for licensure
in2003
Commercialisation
The next important step for us is to increase our ability to successfully commercialise the products we develop and manufacture. Historically, biotechnology companies have tended to out-license the rights to sell the products they develop to pharmaceutical companies in return for the pharmaceutical company funding the costs of development. For this, the biotechnology company typically received a small royalty on the sales. Acambis is in the fortunate position that we have the in-house capability and resources, including sufficient funds, to enable us to develop the products ourselves, thereby retaining greater value within the company. In some cases, we might look to partner a product if we believe a partner can target areas of the world or specific distribution channels more effectively than we can. In other areas, though, we are looking to establish our own sales teams and have recently put in place a sales and marketing function to manage this new stage in our development. With our first product being submitted for licensure in 2003, we are building on the significant strengths we have already established to turn Acambis from a development company into one that is capable of both developing and selling vaccines.
5
6

Smallpox vaccine sales made us a profitable company in 2002. This was principally driven by the major $428m (c.£270m) contract we have with the US Centers for Disease Control and Prevention (“CDC”), under which revenues are expected to be recognised between 2002 and 2005, with the majority (between $240m (c.£150m) and $280m (c.£175m) under United Kingdom generally accepted accounting principles) expected in 2003. This is an exceptional contract as it is of a size that is unlikely to be matched anywhere in the world for smallpox vaccine. The result is that shareholders can expect to see an exceptional spike in revenues and profits in 2003.
To grow revenues and profits from 2004 onwards, our focus is on maximising our near-term revenue-generating opportunities whilst maintaining a broad research and development (“R&D”) pipeline for the medium and longer term. We see four main value drivers for Acambis going forward:
1the opportunities surrounding our smallpox vaccine franchise which can generate additional revenues from 2003 onwards;
2the establishment of a travel vaccines franchise, initially in the US through the planned launch of Arilvax® in 2004 and followed by ChimeriVax-JE during 2006;
3near-term revenue-generating opportunities both from within our existing pipeline, such as ChimeriVax- West Nile, and new opportunities; and
4sustained long-term growth from the continued progression of products in our development pipeline.
We are already recognised as the world’s
leading producerof smallpox vaccine
| | | Smallpox vaccine franchise |
| | | We are already recognised as the world’s leading producer of smallpox vaccine through our contracts with the US and other governments and have been working to leverage this position by establishing a strong smallpox vaccine franchise. This will enable us to offer governments a portfolio of three related products required to protect their citizens against the threat of smallpox: |
| a) | | smallpox vaccine for the majority; |
| b) | | Modified Vaccinia Ankara (“MVA”) for the immunocompromised; and |
| c) | | vaccinia immune globulin (“VIG”), which is a treatment available for use in the rare event of severe reactions to the vaccine. |
| | | We see a number of additional opportunities for our smallpox vaccine. |
| | | The US Government’s strategy is to have a stockpile of smallpox vaccines for every member of the US population. |
That represents around 290 million doses. It is envisaged that Acambis will maintain the US stockpile through the replacement of expired doses, thereby providing a steady revenue stream to Acambis over the medium to long-term.
Following completion of the US Government orders to create the initial stockpile, we will be in a position to supply other governments. With a number of governments, we are seeing interest in agreeing “placeholder” orders that enable them to order a relatively small number of doses in the short-term to provide an instant emergency stockpile, with a view to placing additional, larger orders when supply and budgets allow. In addition to the US, we have signed contracts with 10 governments to date.
Day by day, the strength of our position in supplying governments increases both as additional production capability becomes available with the fulfilment of the US’s requirements and as new clinical data on the vaccine is produced from our clinical trial programme. Having already secured several contracts, we are aggressively pursuing further orders in conjunction with our marketing partner, Baxter.

7
8

We plan to submit our vaccine to both the US and European regulatory authorities in 2004. If approved, we would then have the world’s only licensed second-generation smallpox vaccine. This would strengthen our competitive position with governments and also open up the opportunity for sales to private individuals, for which we have seen considerable interest. The latter is another opportunity for us and we are currently exploring how best to maximise our position.
MVA
There is a proportion of the population that is more at risk of suffering adverse reactions to the smallpox vaccine because they have compromised immune system disorders such as HIV, eczema or other allergies. MVA is a more attenuated form of the current generation of smallpox vaccines and, as such, should reduce the risk of side effects in “at risk” people who would otherwise be unable to be vaccinated against smallpox. In addition to being used as a vaccine in its own right, MVA could also be used as a pre-vaccine to prime the immune systems of these “at risk” individuals ahead of receiving the regular vaccine.

We intend to tender for the contract to supply the US Government with 30 million doses of MVA for an emergency stockpile. This represents a significant opportunity for us and, as with the smallpox vaccine, we also intend to market MVA vaccine to other governments.
VIG
VIG can be used to treat adverse reactions that may arise as a result of vaccination against smallpox. It is produced by our partner, Cangene Corporation (“Cangene”), by vaccinating professional plasma donors with smallpox vaccine and collecting the antibodies they generate. Acambis has an exclusive marketing partnership with Cangene to sell Cangene’s VIG in markets outside North America and Israel.
Today, we are the only company with all three smallpox-related products. This puts us in a uniquely strong position for selling to governments around the world that are implementing bio-defence programmes.
Travel vaccines
The adult vaccine market, of which travel vaccines are a part, is expected to be one of the fastest-growing segments of the vaccines sector over the next decade. In many cases in this market, there is either no licensed vaccine available or, where a vaccine is already licensed, ours is differentiated by potential improvements in safety, efficacy or routes of administration. In the US, travel vaccines represent a strong market that is well served through travel clinics.
We are in the process of completing a paediatric trial on Arilvax®, a yellow fever vaccine to which we have US marketing rights, with results expected to be available in April 2003. We plan to submit a Biologics License Application to the US Food and Drug Administration around the middle of this year. If Arilvax® is approved, we can begin to establish a presence in this field and capitalise on it with the other travel vaccines in our development pipeline.
Our next travel vaccine is ChimeriVax-JE, which could be available during 2006. Process development and manufacturing scale-up for ChimeriVax-JE is almost complete
Today, we are the
only company
with all three smallpox-related products
and we will be conducting a bridging trial with this new material during 2003 to confirm that it produces clinical results equivalent to those seen with the material used in previous trials. We plan to begin the Phase III trial in the first quarter of 2004.
The other travel vaccines we have in development would follow thereafter. These include oral vaccines against typhoid fever and travellers’ diarrhoea caused by enterotoxigenic E. coli (“ETEC”). A bridging study will be carried out for HolaVax-Typhoid during 2003, and a challenge study of HolaVax-ETEC is underway.
Other near-term revenue-generating opportunities
We are also focusing on near-term revenue-generating opportunities, both those within our existing portfolio and also some new opportunities.
Internally, we are already directing significant resources to our programme to develop a vaccine against West Nile virus. This virus has become a major problem since it arrived in the US in 1999. In 2002, the virus spread to a total of 41 US States, where 3,873 cases of West Nile infection were diagnosed and there were 246 deaths. We have been developing a vaccine for three years and, in the first half of this year, we plan to become the first company to take a West Nile vaccine into human clinical trials. Results from these trials should be available in the second half of this year. The scale of this problem and the need to provide an effective counter-measure means that there is considerable demand to make available a safe, efficacious vaccine as quickly as possible. This level of demand and the impetus for rapid progression of the vaccine’s development have encouraged us to take steps to accelerate the clinical testing and manufacture of the vaccine.
We are also actively exploring other revenue-generating opportunities that could contribute to near-term revenue and profits growth. We are focusing our efforts on opportunities that complement our areas of expertise and strategic focus.
R&D pipeline
Sustained growth of the Company in the medium to long-term will come from the new opportunities offered by our broad R&D pipeline of products. In addition to those referred to above, we continue to progress our dengue project with our partner Aventis Pasteur, and our H. pylori and C. difficile programmes. We also have a number of other opportunities currently at the research stage that could contribute to our strong development pipeline.
Altogether, this adds up to a significant number of opportunities that we are currently pursuing, any of which can make an important contribution to our plans for continuing as a profitable company and achieving growth in revenues and profits from the 2004 baseline onwards. These are exciting times for Acambis. Having established firm foundations in the elements of our business that we consider to be key to our success, we are ideally placed to exploit the opportunities before us and maximise the value we generate for shareholders.
9
| | | |
| | | |
| THE MANAGEMENT TEAM | | 10 |
| | | |
 | | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | | |
| | | BOARD OF DIRECTORS | |
| | |  |
| | | Executive Directors
1 Gordon Cameron,Chief
Financial Officer and President of
Acambis Inc. |
| | | 2 Nicolas Higgins,Chief Business
Officer |
| | | 3 Dr Thomas Monath,Chief
Scientific Officer |
| | | 4 Dr John Brown,Chief Executive
Officer |
| | | |
| | | |
| | | |
AS WE OUTLINED IN THE “PROSPECTS” SECTION OF THIS REPORT, WE HAVE AMBITIOUS PLANS FOR THE CONTINUED GROWTH OF ACAMBIS. TO ENSURE THAT WE CAN ACHIEVE OUR OBJECTIVES, A STRONG INFRASTRUCTURE NEEDS TO EXIST, INCLUDING A BALANCED BOARD OF DIRECTORS, A HIGH-QUALITY, EXPERIENCED SENIOR MANAGEMENT TEAM, ROBUST CORPORATE GOVERNANCE STRUCTURES AND A COMMITMENT TO OUR CORPORATE SOCIAL RESPONSIBILITIES.
Board of Directors
During the last year, we continued to enhance our Board of Directors with the appointment of Dr Thomas Monath, our Chief Scientific Officer, to the Board. Today, we have four Executive Directors and four Non-executive Directors. We are seeking to strengthen the Non-executive Director representation on the Board.
During the year, we promoted Elizabeth Brown to the role of Company Secretary, who took over the role from our Chief Financial Officer, Gordon Cameron. This change was to give greater independence to the role by assigning it to an individual who does not also hold a position on the Board. Miss Brown has been with Acambis since 1996 and also heads up the UK finance function.
Senior management
During the last two years, we have strengthened the senior management team through a number of appointments and promotions:
| • | | Clinical Operations and Regulatory Affairs:Dr Philip Bedford, who has been with Acambis since 1997, was promoted to Senior Vice President, Clinical Operations and Regulatory Affairs, reflecting the increasing importance of this team. |
| • | | Commercial:Stephen Atkinson was promoted to Vice President, Commercial Development in March 2002. He has been with Acambis since 1993. |
| • | | Government contracts:Roger McAvoy (previously Chief of the Acquisition Law Division at the Electronic Systems Center of the US Air Force Material Command) joined in 2001 as Vice President, Government Contracts and Legal Affairs. |
| • | | Manufacturing:Dr Joe Caldwell joined us in 2002 as our Senior Vice President, Operations, with responsibility for manufacturing. He was previously Managing Director of UK-based manufacturer Evans Vaccines and worked for GlaxoSmithKline for 25 years. |
| • | | Marketing:In November 2002, Dr Christian Loucq, who has previously worked with European vaccines company Rhein Biotech, and the predecessors to GlaxoSmithKline and Aventis Pasteur, joined as Vice President, Sales & Marketing to establish that function. |
| • | | Research:Dr Mike Darsley was promoted to Vice President, Bacterial Research. He joined Acambis in 1996. Dr Dennis Trent (previously Principal Scientist at Aventis Pasteur) was recruited as Vice President, Viral Research. |
Corporate social responsibility
The issue of corporate social responsibility (“CSR”) has continued to increase in importance and the Board recognises this as a significant issue for the Group to address. We believe that we are already strong in a number of key areas relating to CSR, including:
| • | | transparency and accountability in our communication with shareholders; |
| • | | equal and fair treatment of our employees; |
| • | | rigorous implementation of health and safety policies and working practices; |
| • | | involvement in both our industry and the communities in which our operations are based; and |
| • | | responsible management of our impact on the environment. |
| | | Work is underway, and will continue during 2003, to assess our current position, to formalise the relevant policies, structures and procedures, and to initiate a system of reporting to shareholders our activities. We anticipate being able to provide our first report on CSR in our 2003 Annual Report. |
| | | | | |
| Non-executive Directors |
| |
| 1 | | Alan Smith,Chairman |
| | 2 | | Alan Dalby,Non-executive Director |
| | 3 | | Michael Lytton,Non-executive Director |
| | 4 | | Victor Schmitt,Non-executive Director |
| |
| |  |
11
OPERATING REVIEW

Smallpox vaccine update
US Government contracts
We have two contracts with the US Centers for Disease Control and Prevention (“CDC”). Contract 1 dates from September 2000 and relates to the ACAM1000 smallpox vaccine. Contract 2 dates from November 2001 and relates to the ACAM2000 smallpox vaccine. Under these contracts, we have strict confidentiality obligations that limit our ability to give details on the status, quantity or timing of delivery of smallpox vaccine. We are, however, delighted to be able to report that we are well on course to complete delivery of 155 million doses of ACAM2000 smallpox vaccine to the CDC in the first half of 2003. In addition, the ACAM1000 programme is proceeding in line with the CDC’s expectations and requirements.
Other government contracts
We have the world’s most advanced second-generation smallpox vaccines. Marketing of ACAM2000 to governments around the world is being carried out in conjunction with our strategic partner and major shareholder, Baxter Healthcare Corporation (“Baxter”). We have seen considerable interest among governments, and have 10 contracts in place to date in addition to the US Government contract. Of these, six are with European governments. These are small in comparison with the US contracts. They are expected to have a positive impact on earnings in 2003.
ACAM2000 trial results
On 11 March 2003, we published results from a Phase I trial of ACAM2000. The trial tested the safety, tolerability and immunogenicity of ACAM2000 in adult subjects who had not previously been inoculated against smallpox. All 100 subjects in the open-label trial were vaccinated with ACAM2000.
The currently accepted indication of protective immunogenicity in the case of smallpox vaccination is the development of a pock-mark on the skin, known as a “take”. This was the primary endpoint of the trial. A “take” was seen in 99% of the subjects. A secondary endpoint was the development of a neutralising antibody response, which occurred in 96% of subjects. No vaccine-associated serious adverse events were observed. Under our fast-track development programme, Phase II trials of ACAM2000 are
| | | | | |
| 2002 HIGHLIGHTS | 12 |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |  |
already well underway and we anticipate that the Phase III trials will begin later this year.
MVA
As announced on 25 February 2003, we have been awarded a $9.2m contract by the US National Institute of Allergy and Infectious Diseases, to develop a Modified Vaccinia Ankara (“MVA”) vaccine. MVA is a weakened form of the current generation of smallpox vaccines and, as such, should allow the safe inoculation of “at risk” people with weakened immune systems, who would otherwise be unable to be vaccinated against smallpox.
For this contract, Acambis is acting as the prime contractor and Baxter is our subcontractor, enabling us to leverage each other’s strengths and capabilities. Under this initial contract, we will develop the vaccine, manufacture several thousand doses and conduct a Phase I clinical trial in healthy adults.
The US Government has also declared its intention to issue a “Request for Proposals” for a stockpile of 30 million doses of MVA later this year. Given our experience in delivering large quantities of vaccine to the US Government in a short period of time and having won one of the two initial contracts awarded, we are well placed to bid for this potentially valuable supply contract.
VIG
We announced on 11 March 2003 that Acambis has entered into an exclusive marketing partnership with Cangene Corporation (“Cangene”) to market its vaccinia immune globulin (“VIG”) product in markets outside North America and Israel. We will work together to make the product available to other countries. Cangene is currently supplying VIG under contract to the CDC.
VIG is used in treating severe reactions that may be brought on by the administration of smallpox vaccine. VIG is an antibody product manufactured from human plasma collected from individuals who have been vaccinated with a smallpox vaccine. Cangene is conducting clinical trials in order to obtain US Food and Drug Administration (“FDA”) licensure of the product.
Research and development update
Arilvax®
We are in the process of completing a paediatric trial in Peru on Arilvax® , a yellow fever vaccine, with results expected to be available in April. A Biologics License Application will be submitted to the FDA around the middle of this year.
ChimeriVax-JE
A second Phase II trial of our ChimeriVax-JE vaccine against Japanese encephalitis was successfully completed during the year. It showed that ChimeriVax-JE was well tolerated at all the dose levels tested and that 98% of subjects developed JE-neutralising antibodies within one month of vaccination. We have almost completed process development and manufacturing scale-up of the vaccine and will be conducting bridging trials with this new material during 2003 to confirm that it produces clinical results equivalent to those seen in trials using the original material. We plan to begin Phase III trials in the first quarter of 2004.
ChimeriVax-Dengue
We announced results from a successful initial Phase I trial of one of the four components (ChimeriVax-DEN2) that make up our ChimeriVax-Dengue vaccine against dengue fever. The 56-subject trial showed that ChimeriVax-DEN2 was well-tolerated at both of the dose levels tested, 100% of subjects developed neutralising antibodies to the homologous Dengue 2 virus serotype within one month of vaccination and 96% developed neutralising antibodies to a wild-type Dengue 2 virus. There were no serious adverse events. A Phase I trial of our tetravalent (four-component) ChimeriVax-Dengue vaccine will be undertaken in 2003.
ChimeriVax-West Nile
Data from pre-clinical trials of ChimeriVax-West Nile, our vaccine against West Nile virus, showed that the vaccine was safe and induced high levels of neutralising antibodies and demonstrated the ability of ChimeriVax-West Nile to protect against challenge with wild-type West Nile virus. A Phase I trial of our vaccine candidate will start in the first half of 2003, with results expected to be available in the second half of the year.HolaVax-Typhoid
Process development and manufacturing of our oral typhoid vaccine by our partners, Berna Biotech, is nearing completion and a clinical bridging trial will begin in the first half of 2003.
HolaVax-ETEC
A proof-of-principle challenge study is underway to test the effectiveness of the first of the five components of our HolaVax-ETEC vaccine against E. coli-related travellers’ diarrhoea in protecting vaccinated volunteer subjects when exposed to wild-type ETEC. The first of a series of Phase I trials to test the other four strains in our pentavalent vaccine began early in 2003.
H. pylori
Additional trials completed towards the end of 2002 put us in a position to identify the optimal vaccine candidate to take into further clinical development.
C. difficile
We are exploring two different products to prevent and/or treat C. difficile-associated diarrhoea. The passive treatment and active prophylactic vaccine both make use of a toxoid vaccine comprising inactivated C. difficile toxins. In a trial for the passive treatment, a lower level of antibody generation was seen than had been expected, resulting, we discovered, from the toxoid vaccine having lost some of its potency. We decided not to proceed further with the trial, but are now aggressively pursuing manufacture of new toxoid vaccine. We expect to return to clinical trials around the end of 2003.
DR JOHN BROWN
Chief Executive Officer
| | | | | |
|  | |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| | 13 |
| |
| | | |
(5a)
(5c)
(5d) | | Trading results
Revenue for the year increased significantly to £79.7m (2001 — £8.9m) and arose primarily from the two ongoing smallpox vaccine contracts with the CDC. Activity on these two contracts increased sharply in 2002 as we undertook manufacture of smallpox vaccine for the US Government stockpile. During the year, we also continued to receive revenues from Aventis Pasteur for our ChimeriVax-Dengue vaccine programme. |
| | | |
| | | 2002 saw a new category on the face of the profit and loss account for cost of sales, representing costs incurred on the two CDC contracts. Cost of sales in 2002 increased sharply to £49.2m (2001 — £5.1m) in line with the increased activity. The re-classification of 2001 costs from research and development (“R&D”) costs to cost of sales, which was made to reflect better the nature of the agreements with the CDC, amounted to £5.1m. |
| | | |
| | | Expenditure on R&D increased to £16.3m (2001 — £12.6m). During 2002, we expensed certain one-off start-up costs in relation to the reactivation of our manufacturing plant. In future periods, we anticipate that all costs relating to the manufacturing plant will be classified within cost of sales when the plant is fully utilised for production activities. In addition, expenditure on our internally funded projects increased marginally during the year as the products within our pipeline progressed through clinical development. |
| | | |
| | | In 2002, we received the first income from Baxter under the contract manufacturing agreement entered into between us in December 2000. £1.3m was received as a contribution to our commissioning costs incurred in activating our manufacturing facility. This income has been netted off against R&D costs. |
| | | |
| | | Administrative costs, including amortisation of goodwill, increased to £4.3m (2001 — £3.5m), reflecting increased headcount compared to 2001. Interest receivable reduced marginally to £0.7m (2001 — £0.9m) as a result of lower average levels of cash held throughout the period. Interest payable increased significantly to £1.2m (2001 — £0.2m) as a result of the lease-financing facility secured in December 2001 for the reactivation of our manufacturing plant. Under the terms of the agreement, interest on this |
| | | | | |
| | 14 |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| Revenue | |
| Revenue in 2002 increased by over 800% to £79.7m when comparing to the 2001 level of £8.9m. This sharp increase was as a result of the activity under both of the smallpox vaccine contracts with the CDC. | |
| |
| SOURCES OF REVENUE | |
| 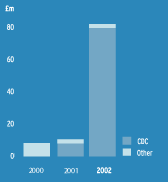 | |
| |
| |
| |
| |
| |
| |
| |
| |
| | |
| |
facility was accrued during 2002. Repayment of interest will commence in 2003. During 2002, an exchange gain of £0.4m (2001 — loss of £0.1m) was recorded as a result of the revaluation of the amounts outstanding under our US dollar-denominated debt facility for our Arilvax® programme.
In accordance with Financial Reporting Standard 11, “Impairment of Fixed Assets and Goodwill”, in 2002 we recorded an exceptional loss of £0.1m (2001 — £0.4m) in respect of the impairment write-down of the investment held in Medivir AB. At 31 December 2002, the book value of the investment was £0.3m (2001 — £0.4m).
The pre-tax profit for 2002 was £9.6m (2001 — loss of £12.6m), the improvement achieved primarily as a result of increased revenues under our smallpox vaccine programmes.
The tax charge relating to 2002 was negligible due to the availability of tax losses within the Group. During 2003, we hope that we may be able to utilise the remaining brought-forward losses, lowering the effective tax rate.
Capital expenditure
Fixed asset additions for 2002 increased to £ 11.5m (2001 — £8.4m). £9.1m of the expenditure in 2002 related to the investment made to reactivate our manufacturing plant. This process was substantially complete at the end of 2002 and we therefore anticipate capital expenditure levels to be lower in 2003.
Balance sheet highlights
Cash/debtors
The cash balance of the Group (including short-term investments) at 31 December 2002 amounted to £11.8m (2001 — £22.2m). We received two significant payments two days after the year-end which, had they been received two days earlier, would have boosted the cash position significantly. These large post year-end receipts are reflected in the large debtors balance at 31 December 2002 of £59.0m (2001 — £13.8m). The decrease in cash over 2001 is a result of the major working capital requirements arising from the ACAM2000 CDC contract.
In June 2002, the third instalment of £7.0m in respect of its equity subscription was received from Baxter International, Inc., giving it a shareholding at that time of 16.9%. The final subscription of a further £7.0m was made on 27 March 2003, and has increased Baxter’s shareholding to 20.6%. We anticipate that the cash balance will rise significantly over the coming months as the proceeds from the CDC contracts increase and accelerate. We expect to have over £100m in cash by the end of the year.
Stock/creditors: amounts falling due within one year
Stock held at 31 December 2002 amounted to £48.3m (2001 — £nil). This balance principally represents work-in-progress and finished goods in relation to work being carried out under the ACAM2000 CDC contract. Payments for certain stock items do not take place until after delivery of the vaccine stocks to the US Government. This results in a substantial increase in trade creditors, included within the total Creditors: amounts falling due within one year, of £88.5m (2001 — £16.6m). The levels of both stock and trade creditors will reduce during the first half of 2003 as we complete deliveries of smallpox vaccine.Our adopted method for recognising revenue under the ACAM2000 contract with the CDC, the percentage of cost-to-completion method, continues to give rise to a significant difference between invoices submitted and amounts recognised as revenue. At the year-end, the amount recorded as deferred income (included within the total creditors number above) under the contract was around £21.1m (2001 — £3.9m).
Lease financing and overdraft facilities
During 2002, we did not make any drawdowns from the lease-financing facility secured via Baxter in December 2001 for the reactivation of our manufacturing plant. The balance on the facility at 31 December 2002 was £14.0m (2001 — £14.3m). During 2002, interest accrued on the facility was rolled into the outstanding balance. Interest accruing on the facility during 2003 will be repaid from cash. The balance on the Arilvax® overdraft facility at 31 December 2002 was £4.3m (2001 — £4.8m).
Employees
At 31 December 2002, Group headcount had increased to 291 (2001 — 178). The significant increase seen in 2002 was as a result of putting the appropriate level of resources in place at our manufacturing plant in making it a fully operational facility, and the need for further support within the clinical, quality and regulatory functions to manage the number of clinical trials now being performed by the Group. We anticipate that there will be some additional growth in employee numbers in each of the above areas during 2003 and Group headcount is expected to reach around 325 by the end of the year.
GORDON CAMERON
Chief Financial Officer
| | | | | |
| | |
| |
| Pre-tax profit | |
| The financial results for 2002 marked a turning point for Acambis with the announcement of our maiden pre-tax profit of £9.6m. This was principally as a result of the activity level seen on the second smallpox vaccine contract that we have with the CDC. The $428m (c.£270m) contract contributed almost $90m (c.£55m) to Acambis’ revenue in 2002. |
| |
| |
| Key financial indicators | |
| | | | | | | | | | |
| | | 2002 | | 2001 | |
| |
| |
| | Revenue | | £79.7m | | | £8.9m | |
| | Profit/(loss)
before tax | | £9.6m | | | £(12.6)m | |
| | Earnings/(loss) per
share – basic | | 10.0p | | | (13.7)p | |
| | Earnings/(loss) per
ADR – basic | | $ | 1.61 | | | $ | (1.99) | |
| | Operating cash outflow | | £(6.2)m | | | £(8.0)m | |
| | Cash at end of period (including short- term investments) | | £11.8m | | | £22.2m | |
| |
| | Although we reported our maiden pre-tax profit in 2002, operating cashflow was still negative in the year. This was as a result of the major working capital requirement under the second smallpox vaccine contract with the CDC. | |
| |
| | We expect that our cash balance at the end of 2003 will be over £100m. | |
15
| (17a) | | DIRECTORS’ RESPONSIBILITIES |
Financial statements, including adoption of going concern basis
Company law requires the Directors to prepare financial statements for each financial year which give a true and fair view of the state of affairs of the Company and the Group and of the profit or loss of the Group for that period.
After making enquiries, the Directors have a reasonable expectation that the Company and the Group have adequate resources to continue in operational existence for the foreseeable future. For this reason, they continue to adopt the going concern basis in preparing the financial statements.
In preparing the financial statements, the Directors are required to:
| • | | select suitable accounting policies and then apply them consistently; |
| • | | make judgments and estimates that are reasonable and prudent; |
| • | | state whether applicable accounting standards have been followed, subject to any material departures disclosed and explained in the financial statements; and |
| • | | prepare the financial statements on the going concern basis unless it is inappropriate to presume that the Group will continue in business. |
The Directors are responsible for keeping proper accounting records which disclose with reasonable accuracy at any time the financial position of the Company and the Group and enable them to ensure that the financial statements comply with the Companies Act 1985. They are also responsible for safeguarding the assets of the Company and the Group and hence for taking reasonable steps for the prevention and detection of fraud and other irregularities.
The Directors are responsible for the maintenance and integrity of the Group’s website. Uncertainty regarding legal requirements is compounded as information published on the internet is accessible in many countries with different legal requirements relating to the preparation and dissemination of financial statements.
| (17b) | | INDEPENDENT AUDITORS’ REPORT TO THE MEMBERS OF ACAMBIS PLC DIRECTORS’ RESPONSIBILITIES |
We have audited the financial statements which comprise the Group profit and loss account, the Group and Company balance sheets, the Group cash flow statement, the Group statement of total recognised gains and losses and the related notes numbered 1 to 29. We have also audited the disclosures required by Part 3 of Schedule 7A to the Companies Act 1985 contained in the Directors’ remuneration report (“the auditable part”).
Respective responsibilities of Directors and auditors
The Directors’ responsibilities for preparing the annual report, the Directors’ remuneration report and the financial statements in accordance with applicable United Kingdom law and accounting standards are set out in the statement of Directors’ responsibilities.
Our responsibility is to audit the financial statements and the auditable part of the Directors’ remuneration report in accordance with relevant legal and regulatory requirements, United Kingdom Auditing Standards issued by the Auditing Practices Board and United States Auditing Standards.
We report to you our opinion as to whether the financial statements give a true and fair view and whether the financial statements and the auditable part of the Directors’ remuneration report have been properly prepared in accordance with the Companies Act 1985. We also report to you if, in our opinion, the Directors’ report is not consistent with the financial statements, if the Group has not kept proper accounting records, if we have not received all the information and explanations we require for our audit, or if information specified by law regarding Directors’ remuneration and transactions is not disclosed.
We read the other information contained in the annual report and consider the implications for our report if we become aware of any apparent misstatements or material inconsistencies with the financial statements. The other information comprises only the sections entitled “Our business” (encompassing the chairman’s statement, the operating review and the financial review), “Further information required by Form 20-F”, “Summarised Group statements”, “Shareholder information”, “Board of Directors” and “General information” as well as the unaudited comments in the right margin adjacent to the financial statements, the Directors’ report, the unaudited part of the Directors’ remuneration report and the corporate governance statement.
We review whether the corporate governance statement reflects the Group’s compliance with the seven provisions of the Combined Code specified for our review by the Listing Rules of the Financial Services Authority, and we report if it does not. We are not required to consider whether the Board’s statements on internal control cover all risks and controls, or to form an opinion on the effectiveness of the Group’s corporate governance procedures or its risk and control procedures.
| | | | | |
| 16 |
| |
| |
| In this section of the report, this margin is being used to highlight key information or to explain aspects of the financial data. |
| |
| |
| |
| (A) | COMMENTARY |
| |
| The item that is referred to is indicated with the symbol shown on the left. Please note that the information contained in this column has not been audited, but is consistent with other information in the document that has been audited. |
| |
| |
| FORM 20-F |
| |
| Our principal listing is on the London Stock Exchange, but our shares are also quoted on the Nasdaq National Market as American Depository Receipts and we have a number of US shareholders. US-domestic companies file their annual business overview with their regulator, the US Securities and Exchange Commission, under a Form 10-K. A foreign issuer, such as Acambis, files its equivalent document under a Form 20-F. Since much of the information required in a Form 20-F is already contained in our Annual Report, we have combined the two documents and included a section that incorporates any additional information required over and above that provided in the Annual Report. |
| |
| | |
| |
| (1a) | For those US shareholders who are used to reading a Form 20-F, we have added a fold-out flap inside the back cover of the document that provides a cross-reference of Form 20-F items to the information in the Report. We have denoted this Form 20-F information by using this symbol on the left where the number denotes the specific item on the cross-reference. |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| THE INFORMATION CONTAINED IN THIS
COLUMN HAS NOT BEEN AUDITED. |
| |
| |
Basis of audit opinion
We conducted our audit in accordance with United Kingdom Auditing Standards issued by the Auditing Practices Board, and with generally accepted auditing standards in the United States. An audit includes examination, on a test basis, of evidence relevant to the amounts and disclosures in the financial statements and the auditable part of the directors’ remuneration report. It also includes an assessment of the significant estimates and judgments made by the Directors in the preparation of the financial statements, and of whether the accounting policies are appropriate to the Group’s circumstances, consistently applied and adequately disclosed.
We planned and performed our audit so as to obtain all the information and explanations which we considered necessary in order to provide us with sufficient evidence to give reasonable assurance that the financial statements and the auditable part of the Directors’ remuneration report are free from material misstatement, whether caused by fraud or other irregularity or error. In forming our opinion we also evaluated the overall adequacy of the presentation of information in the financial statements.
Opinion
In our opinion:
| • | | the financial statements give a true and fair view of the state of affairs of the Company and the Group at 31 December 2002 and of the profit and cash flows of the Group for the year then ended; |
| • | | the financial statements have been properly prepared in accordance with the Companies Act 1985; |
| • | | those parts of the Directors’ remuneration report required prepared in accordance with the by Part 3 of Schedule 7A to the Companies Act 1985; and |
| • | | the consolidated financial statements present fairly, in all material respects, the financial position of Acambis plc and its subsidiaries at 31 December 2002, and the results of their operations and their cash flows for the year then ended in conformity with accounting principles generally accepted in the United Kingdom. |
The financial statements of Acambis plc as of 31 December 2001, and for each of the two years in the period ended 31 December 2001, were audited by other independent accountants who have ceased operations. Those independent accountants expressed an unqualified opinion on those financial statements in their report dated 15 April 2002.
Reconciliation to US GAAP
Accounting principles generally accepted in the United Kingdom vary in certain important respects from accounting principles generally accepted in the United States of America. The application of the latter would have affected the determination of consolidated net income for each of the three years in the period ended 31 December 2002 and the determination of consolidated shareholders’ equity at 31 December 2002 and 2001 to the extent summarised in note 29 to the consolidated financial statements.
PricewaterhouseCoopers LLP
Chartered Accountants and Registered Auditors
Abacus House
Castle Park
Cambridge CB3 0AN
UK
27 March 2003
| (17c) | | GROUP PROFIT AND LOSS ACCOUNT |
| | | | | | | | | | | | | | | | | | |
| | |
|
| | | | | | | | | | | 2001 | | 2000 |
| | | | | | | 2002 | | (restated) | | (restated) |
| (A) | | | Notes | £’000 | | £’000 | | £’000 |
| |
|
| (B) | Turnover | | | 2 | | | | 79,716 | | | | 8,914 | | | | 6,264 | |
| (A) | Cost of sales | | | | | | | (49,249 | ) | | | (5,063 | ) | | | (517 | ) |
| |
|
| | Gross profit | | | | | | | 30,467 | | | | 3,851 | | | | 5,747 | |
| | Research and development costs | | | | | | | (16,299 | ) | | | (12,594 | ) | | | (12,195 | ) |
| | Administrative costs (including amortisation of goodwill) | | | 3 | | | | (4,257 | ) | | | (3,499 | ) | | | (2,949 | ) |
| |
|
| | Group operating profit/(loss) | | | 4 | | | | 9,911 | | | | (12,242 | ) | | | (9,397 | ) |
| | Share of loss of joint venture | | | 14(d | ) | | | (171 | ) | | | (410 | ) | | | (2,138 | ) |
| |
|
| | Total operating profit/(loss) before exceptional items (Group and joint venture) | | | | | | | 9,740 | | | | (12,652 | ) | | | (11,535 | ) |
| | Exceptional items: | | | | | | | | | | | | | | | | |
| | Profit on disposal of fixed asset investment | | | | | | | – | | | | – | | | | 221 | |
| | Profit on sale of discontinued operations | | | | | | | – | | | | – | | | | 414 | |
| | Amounts written off fixed asset investment | | | 5 | | | | (85 | ) | | | (423 | ) | | | (670 | ) |
| |
|
| | Profit/(loss) on ordinary activities before finance charges | | | | | | | 9,655 | | | | (13,075 | ) | | | (11,570 | ) |
| |
|
| | Interest receivable | | | | | | | 685 | | | | 857 | | | | 983 | |
| | Interest payable and similar charges | | | 6 | | | | (1,211 | ) | | | (214 | ) | | | (216 | ) |
| | Exchange gain/(loss) on foreign currency borrowings | | | 18 | | | | 449 | | | | (126 | ) | | | (271 | ) |
| |
|
| (B) | Profit/(loss) on ordinary activities before taxation | | | 7 | | | | 9,578 | | | | (12,558 | ) | | | (11,074 | ) |
| |
|
| (C) | Taxation | | | 10 | | | | (3 | ) | | | 131 | | | | – | |
| |
|
| | Profit/(loss) on ordinary activities after taxation (being retained profit/(loss) for the financial year) | | | | | | | 9,575 | | | | (12,427 | ) | | | (11,074 | ) |
| |
|
| | Earnings/(loss) per ordinary share (basic) | | | 11 | | | | 10.0 | p | | | (13.7 | )p | | | (13.9 | )p |
| | Earnings/(loss) per ordinary share (fully diluted) | | | 11 | | | | 9.7 | p | | | (13.7 | )p | | | (13.9 | )p |
| |
|
| | | A statement of movements on reserves is given in note 22.
The accompanying notes are an integral part of this Group profit and loss account.
All amounts in 2002 arise from continuing operations (see note 4). |
(17d)
| | GROUP STATEMENT OF TOTAL RECOGNISED GAINS AND LOSSES
For the year ended 31 December |
| | | | | | | | | | | | | |
| | |
|
| | 2002 | | 2001 | | 2000 |
| | £’000 | | £’000 | | £’000 |
|
| Profit/(loss) for the year | | | 9,575 | | | | (12,427 | ) | | | (11,074 | ) |
| Gain/(loss) on foreign currency translation | | | 1,276 | | | | (314 | ) | | | (817 | ) |
|
| Total recognised gains and losses for the year | | | 10,851 | | | | (12,741 | ) | | | (11,891 | ) |
|
| | | The accompanying notes are an integral part of this Group statement of total recognised gains and losses. |
| | | | | |
| 18 |
| |
| |
| (A) | Cost of sales — restatement |
| The Group has begun to classify the costs directly attributable to funded research and vaccine manufacture programmes as cost of sales, rather than research and development expenditure. This is because the Directors believe this classification more appropriately reflects the nature of the arrangements into which the Group has entered. In the three financial years shown, cost of sales represent only those costs incurred on the two CDC smallpox vaccine contracts. The restatement of 2001 and 2000 costs from R&D costs to cost of sales amounted to £5.1m and £0.1m respectively. There is no impact on net profit. |
| |
| (B) | Turnover and profit |
| The increase in turnover and the recording of a maiden profit in 2002 has principally been driven by the second smallpox vaccine contract with the US Government agency, the Centers for Disease Control and Prevention (“CDC”). |
| |
| (C) | | Taxation |
| The tax charge relating to 2002 was negligible due to the availability of tax losses within the Group. We hope that when the remaining tax losses can be utilised, this will have the effect of lowering the effective tax rate. |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| THE INFORMATION CONTAINED IN THIS COLUMN HAS NOT BEEN AUDITED. |
| |
| (17e | ) | GROUP BALANCE SHEET
At 31 December |
| | | | | | | | | | | | | | |
| | | |
| |
| | | | | | | | 2002 | | | 2001 | |
| | | | Notes | | | £'000 | | | £'000 | |
| |
|
| | Fixed assets | | | | | | | | | | | | |
| | Intangible assets: goodwill | | | 12 | | | | 13,641 | | | | 14,845 | |
| | Tangible assets | | | 13 | | | | 19,988 | | | | 12,255 | |
| | Investment in joint ventures: | | | 14 | | | | | | | | | |
| | — share of gross assets | | | | | | | – | | | | 915 | |
| | — share of gross liabilities | | | | | | | – | | | | (848 | ) |
| |
|
| | | | | | | | | – | | | | 67 | |
| | Other investments | | | 14 | | | | 1,100 | | | | 1,640 | |
| |
|
| | | | | | | | | 34,729 | | | | 28,807 | |
| |
|
| | Current assets | | | | | | | | | | | | |
(D) | Stock | | | 15 | | | | 48,343 | | | | – | |
(E) | Debtors: amounts receivable within one year | | | 16 | | | | 54,040 | | | | 7,542 | |
| | Debtors: amounts receivable after one year | | | 17 | | | | 4,925 | | | | 6,235 | |
| | Short-term investments | | | | | | | 105 | | | | 120 | |
(F) | Cash at bank and in hand | | | | | | | 11,672 | | | | 22,093 | |
| |
|
| | | | | | | | | 119,085 | | | | 35,990 | |
| |
|
(G) | Creditors: amounts falling due within one year | | | 18 | | | | (88,460 | ) | | | (16,603 | ) |
| |
|
| | Net current assets | | | | | | | 30,625 | | | | 19,387 | |
| |
|
| | Total assets less current liabilities | | | | | | | 65,354 | | | | 48,194 | |
| | Creditors: amounts falling due after one year | | | 19 | | | | (18,897 | ) | | | (20,534 | ) |
| | Provisions for liabilities and charges | | | | | | | | | | | | |
| | Investment in joint ventures: | | | 14 | (d) | | | | | | | | |
| | — share of assets | | | | | | | 941 | | | | – | |
| | — share of liabilities | | | | | | | (1,147 | ) | | | – | |
| |
|
| | | | | | | | | (206 | ) | | | – | |
| |
|
| | Net assets | | | | | | | 46,251 | | | | 27,660 | |
| |
|
| | Capital and reserves | | | | | | | | | | | | |
| | Called-up share capital | | | 21 | | | | 9,901 | | | | 9,308 | |
| | Share premium account | | | 22 | | | | 87,745 | | | | 80,598 | |
| | Profit and loss account | | | 22 | | | | (51,395 | ) | | | (62,246 | ) |
| |
|
| | Shareholders’ funds — all equity | | | 23 | | | | 46,251 | | | | 27,660 | |
| |
|
| | | The accompanying notes are an integral part of this Group balance sheet. |
| |
| (D) | Stock
2002 is the first year that Acambis has recognised stock on the balance sheet. This largely reflects work-in-progress and finished goods under the second CDC smallpox vaccine contract.
|
|
| (E) | Debtors: amounts receivable within one year
This includes amounts invoiced but not received at the year-end of £46.1m, principally from the CDC.
|
| (F) | Cash at bank and in hand
Cash at the year-end was £11.7m. We received two significant payments two days after the year-end which, had they been received two days earlier, would have increased the cash position significantly. These large post year-end receipts are reflected in the large debtors balance at 31 December 2002 of £59.0m.
We expect to have over £100m in cash by the end of 2003.
|
| (G) | Creditors: amounts falling due within one year
The increase in trade creditors and deferred revenue has arisen principally from the second CDC smallpox vaccine contract.
|
| | |
| | THE INFORMATION CONTAINED IN THIS COLUMN HAS NOT BEEN AUDITED. | 19 |
| (17f | ) | COMPANY BALANCE SHEET |
| (A) | | At 31 December |
| | | | | | | | | | | | | |
| | |
| |
| | | | | | | 2002 | | | 2001 | |
| | | Notes | | | £’000 | | | £'000 | |
|
| Fixed assets | | | | | | | | | | | | |
| Investments | | | 14 | | | | 15,131 | | | | 15,586 | |
|
| Current assets | | | | | | | | | | | | |
| Debtors: amounts receivable within one year | | | 16 | | | | 589 | | | | 70 | |
| Debtors: amounts receivable after one year | | | 17 | | | | 71,643 | | | | 60,170 | |
| Short-term investments | | | | | | | - | | | | – | |
| Cash at bank and in hand | | | | | | | 12,217 | | | | 18,884 | |
|
| | | | | | | | 84,449 | | | | 79,124 | |
Creditors: amounts falling due within one year | | | 18 | | | | (429 | ) | | | (219 | ) |
|
| Net current assets | | | | | | | 84,020 | | | | 78,905 | |
|
| Total assets less current liabilities | | | | | | | 99,151 | | | | 94,491 | |
|
| Net assets | | | | | | | 99,151 | | | | 94,491 | |
|
| Capital and reserves | | | | | | | | | | | | |
| Called-up share capital | | | 21 | | | | 9,901 | | | | 9,308 | |
| Share premium account | | | 22 | | | | 87,611 | | | | 80,464 | |
| Profit and loss account | | | 22 | | | | 1,639 | | | | 4,719 | |
|
| Shareholders’ funds — all equity | | | | | | | 99,151 | | | | 94,491 | |
|
Signed on behalf of the Board
Dr John Brown,Chief Executive Officer
Gordon Cameron,Chief Financial Officer
27 March 2003
The accompanying notes are an integral part of this Company balance sheet.
20
| | |
| (A) | Company balance sheet
The Company information relates to Acambis plc, the holding company that owns the the principal ones of which are Acambis Research Limited in the UK and Acambis Inc. in the US. The Company’s accounts are consolidated with those of the subsidiaries to produce the Group’s accounts.
The structure of the principal trading subsidiaries of the Group is as follows:

THE INFORMATION CONTAINED IN THIS COLUMN HAS NOT BEEN AUDITED.
|
| | | | | | | | | | | | | | | | | | |
| (17g) | GROUP CASH FLOW STATEMENT
For the year ended 31 December |
| | | |
| |
| | | | | | | | 2002 | | | 2001 | | | 2000 | |
| | | | Notes | | | £000 | | | £000 | | | £000 | |
| |
|
| | Net cash outflow from operating activities | | | 24 | | | | (6,182 | ) | | | (7,959 | ) | | | (9,207 | ) |
| |
|
| | Returns on investments and servicing of finance | | | | | | | | | | | | | | | | |
| | Interest received | | | | | | | 685 | | | | 1,219 | | | | 852 | |
| | Interest paid | | | | | | | (94 | ) | | | (224 | ) | | | (207 | ) |
| | Interest element of finance lease payments | | | | | | | – | | | | – | | | | (5 | ) |
| |
|
| | Net cash inflow from returns on investments and servicing of finance | | | | | | | 591 | | | | 995 | | | | 640 | |
| |
|
| | Taxation | | | | | | | 131 | | | | – | | | | – | |
| |
|
| | Capital expenditure and financial investment | | | | | | | | | | | | | | | | |
(B) | Purchase of tangible fixed assets | | | | | | | (11,464 | ) | | | (8,427 | ) | | | (363 | ) |
| | Sale of tangible fixed assets | | | | | | | – | | | | – | | | | 7 | |
| | Funds advanced to joint venture | | | | | | | – | | | | (520 | ) | | | (2,332 | ) |
| | Proceeds from sale of trade investments | | | | | | | – | | | | – | | | | 221 | |
| |
|
| | Net cash outflow from capital expenditure and financial investment | | | | | | | (11,464 | ) | | | (8,947 | ) | | | (2,467 | ) |
| |
|
| | Acquisitions and disposals | | | | | | | | | | | | | | | | |
| | Disposal costs on sale of business | | | | | | | – | | | | – | | | | (243 | ) |
| |
|
| | Net cash outflow from acquisitions and disposals | | | | | | | – | | | | – | | | | (243 | ) |
| |
|
| | Net cash outflow before management of liquid resources and financing | | | | | | | (16,924 | ) | | | (15,911 | ) | | | (11,277 | ) |
| |
|
| | Management of liquid resources | | | 25 | | | | 5 | | | | 19,834 | | | | (1,894 | ) |
| |
|
| | Financing | | | | | | | | | | | | | | | | |
| | Net proceeds from issue of new shares | | | | | | | | | | | | | | | | |
(C) | — Baxter subscription | | | | | | | 6,954 | | | | 3,477 | | | | 9,458 | |
| | — Other | | | | | | | 786 | | | | 785 | | | | 890 | |
| | Overdraft facility | | | | | | | – | | | | – | | | | 2,502 | |
| | Exercise of options over issued shares held by ESOP | | | | | | | – | | | | – | | | | 43 | |
| | Capital element of finance lease payments | | | | | | | – | | | | (13 | ) | | | (73 | ) |
| | Proceeds from new finance lease commitment | | | | | | | – | | | | 12,738 | | | | – | |
| |
|
| | Net cash inflow from financing | | | | | | | 7,740 | | | | 16,987 | | | | 12,820 | |
| |
|
| | (Decrease)/increase in cash for the year | | | 25 | | | | (9,179 | ) | | | 20,910 | | | | (351 | ) |
| |
|
| | The accompanying notes are an integral part of this Group cash flow statement. |
(B) Purchase of tangible fixed assets
The expenditure in 2002 represents the continued investment being made to reactivate our manufacturing facility in the US. This process is now largely complete.
(C) Baxter subscription
Baxter’s 28m subscription in new Acambis shares is scheduled as follows:
| | | | | | | | | |
| | | | | | | Approximate |
| | | | | | | aggregate |
| Date | | Amount | | | shareholding |
|
| 2000 | | | £10.4m | | | | 10 | % |
| 2001 | | | £3.5m | | | | 13 | % |
| 2002 | | | £7.0m | | | | 17 | % |
| 2003 | | | £7.0m | | | | 21 | % |
On 27 March 2003, Baxter accelerated its fourth and final subscription, taking its shareholding to 20.6%.
THE INFORMATION CONTAINED IN THIS COLUMN HAS NOT BEEN AUDITED.
21
| (17h) | | NOTES TO GROUP FINANCIAL STATEMENTS |
| | | A summary of the more important accounting policies, which have been reviewed by the Board of Directors in accordance with Financial Reporting Standard (“FRS”) 18, “Accounting Policies”, and have been consistently applied, is set out below. FRS 19, “Deferred Tax”, has been adopted in the year, but has had no impact on the amounts included in the profit and loss account and balance sheet. |
| | | The preparation of our financial statements requires us to make estimates and judgments that affect the reported amount of net assets at the date of our financial statements and the reported amounts of revenues and expenses during the period. |
| | | The financial statements have been prepared under the historical cost convention and in accordance with the Companies Act 1985 and United Kingdom generally accepted accounting principles (“UK GAAP”). Where there are significant differences to United States generally accepted accounting principles (“US GAAP”) these have been discussed in note 29. |
| | | The Group financial statements include and consolidate the financial statements of Acambis plc and each of its subsidiary undertakings. Acquisitions made by the Group are accounted for under the acquisition method of accounting and the Group financial statements include the results of such subsidiaries from the relevant date of acquisition. Intra-group transactions and profits are eliminated fully on consolidation. The loss for the financial year, dealt with in the financial statements of the Company, was £1.6m (2001 — profit of £4.6m). Under the provisions of Section 230 of the Companies Act 1985, no profit and loss account is presented for the Company. |
| | | Group turnover comprises the value of sales (excluding VAT and similar taxes, trade discounts and intra-group transactions) and income derived from product sales, licence fees, contract research fees and development milestone payments receivable from third parties in the normal course of business. Revenue from product sales is recognised on delivery of the product. Non-refundable licence fees are recognised over the term of the licence. Where the Group is required to undertake research and development activities and the fee is creditable against services provided by the Group, that licence fee is deferred and recognised over the period over which the services are performed. Contract research fees are recognised in the accounting period in which the related work is carried out. Access fees and milestones receivable are recognised when they fall contractually due. |
| |
| | | Profit is recognised on long-term contracts when the final outcome can be assessed with reasonable certainty by including in the profit and loss account, turnover and related costs as contract activity progresses. Turnover is recognised according to the stage reached in the contract by reference to the costs incurred in relation to total estimated expected costs. |
| | | The second smallpox vaccine contract with the US Centers for Disease Control & Prevention (“CDC”) is a fixed fee arrangement requiring the delivery of products as well as a concurrent research and development (“R&D”) programme. This arrangement has been treated as a single long-term contract, whose elements have not been accounted for separately. Turnover and profits are recognised according to the stage reached in the contract, as described above. Manufacturing costs are deemed to be incurred on delivery of products; R&D costs are recognised as incurred. |
| | | The Group has classified costs which are directly attributable to funded research and vaccine manufacture programmes as cost of sales. Certain research and development costs were, in prior years, classified as R&D expenditure. The Directors believe that the new classification more appropriately reflects the nature of the arrangements the Group has entered into. This reclassification has been applied to the two CDC smallpox vaccine contracts. The financial information for 2001 and 2000 has been re-presented so that cost classifications are shown on a comparable basis. The monetary impact of this re-classification in 2001 and 2000 is £5.1m and £0.5m respectively, which was previously included within R&D costs. There is no impact on net profit. |
| | | R&D costs are written off in the period in which they are incurred. |
22
(A) Notes to Group financial statements
The notes to Group financial statements are intended to provide additional analysis and detail required to meet statutory obligations.
THE INFORMATION CONTAINED IN THIS COLUMN HAS NOT BEEN AUDITED.
| | | Grants, which are non-refundable, are intended to contribute towards specific costs and are recognised in line with the proportion of those costs incurred and are netted off against R&D costs. |
| | | All schemes are defined contribution schemes and pension contributions are charged to the profit and loss account in the year to which they relate. Any difference between amounts charged to the profit and loss account and contributions paid are shown in the balance sheet under prepayments or creditors falling due within one year. |
| | | Current tax, including UK corporation tax and foreign tax, is provided at amounts expected to be paid or recovered using the tax rates and laws that have been enacted or substantially enacted by the balance sheet date. Provision is made for all deferred tax assets and liabilities in accordance with FRS 19, using full provision accounting, when an event has taken place by the balance sheet date which gives rise to an increased or reduced tax liability in the future. Deferred tax is measured at the average tax rates that are expected to apply in the periods in which the timing differences are expected to reverse, based on tax rates and laws that have been enacted or substantially enacted by the balance sheet date. Deferred tax assets are recognised to the extent that they are regarded as recoverable. Deferred tax assets and liabilities are not discounted. |
| | | Intangible assets — goodwill |
| | | Goodwill arising on the acquisition of subsidiary undertakings, representing the excess of fair value of the consideration given over the fair value of the identified assets and liabilities acquired, is capitalised and written off on a straight-line basis over its useful economic life. The Directors are of the opinion that the useful economic life of goodwill will not exceed 15 years. The carrying values of goodwill and intangible assets are subject to review and any impairment is charged to the profit and loss account. |
| | | Fixed assets are stated at original historical cost, net of depreciation and any provision for impairment. Depreciation is provided on all tangible fixed assets at rates calculated to write off the cost of each asset on a straight-line basis over its expected useful life, or the period of the lease if shorter, to its residual value based on prices prevailing at the date of acquisition, as follows: |
| | | |
| Leasehold land and buildings | | — 15 years or term of lease if shorter |
| Laboratory and manufacturing equipment | | — 4 to 7 years |
| Office equipment | | — 3 to 5 years |
| | | The carrying values of tangible fixed assets are subject to review and any impairment is charged to the profit and loss account. The Group does not capitalise interest charges on loans to fund the purchase of tangible fixed assets. |
| | | Shares in the Company purchased for employee share options are held under trust and are included as a fixed asset investment until the interest in the shares is unconditionally transferred to the employees. Provision is made for any permanent impairment in the value of the shares held by the trust. The Group’s other fixed asset investments are shown at cost less any provision for impairment. |
| | | Joint venture undertakings |
| | | Joint ventures are dealt with under the gross equity method. The Group’s share of revenues and operating losses for the joint venture is included in the Group profit and loss account and the Group’s share of gross assets and liabilities is included in the Group balance sheet. |
| | | Bank deposits, which are not repayable on demand, are treated as short-term investments in accordance with FRS 1, “Cash flow statements”. Movements in such investments are included under “management of liquid resources” in the Group’s cash flow statement. |
| | | Stock, excluding long-term contracts |
| | | Stock is stated at the lower of cost and net realisable value. In general, cost is determined on a first-in-first-out basis and includes transport and handling costs. Where necessary, provision is made for obsolete, slow-moving and defective stock. |
| | | NOTES TO GROUP FINANCIAL STATEMENTS |
| | | Assets acquired under finance leases are included in the balance sheet as tangible fixed assets and are depreciated over the shorter of the lease period or their useful lives. The capital elements of future lease payments are recorded as liabilities, while the interest elements are charged to the profit and loss account over the period of the leases to give a constant charge on the balance of the capital repayments outstanding. The cost of operating leases is charged to the profit and loss account on a straight-line basis over the lease term, even if rental payments are not made on such a basis. |
| | | Transactions denominated in foreign currencies are recorded in the local currency at actual exchange rates as at the date of the transaction. Monetary assets and liabilities denominated in foreign currencies are translated at the rates ruling at the balance sheet date. Any gain or loss arising from a change in exchange rates subsequent to the date of the transaction is included as an exchange gain or loss in the profit and loss account. |
| | | Assets and liabilities of overseas subsidiary and joint venture undertakings are translated into sterling at rates of exchange ruling at the balance sheet date. The results and cash flows of overseas subsidiary and joint venture undertakings are translated into sterling using average rates of exchange. Exchange adjustments arising when the opening net assets and the losses for the year retained by overseas subsidiary and joint venture undertakings are translated into sterling are taken directly to reserves and reported in the statement of total recognised gains and losses. |
| | | Where financing of a foreign subsidiary through long-term loans and deferred trading balances is intended to be as permanent as equity, such loans and inter-company balances are treated as part of the net investment and, as such, any exchange differences arising are dealt with as adjustments to reserves. |
| | | The Group attempts to reduce its foreign currency exposure using forward planning of currency requirements for US dollars and UK sterling, and entering into spot rate currency contracts as appropriate. The Group does not enter into derivative transactions. |
| | | Employee share option schemes |
| | | In accordance with Urgent Issues Task Force (“UITF”) Abstract 17, “Employee Share Schemes” (“UITF 17”), the cost of awards to employees of share options is charged to the profit and loss account on a straight-line basis over the period to which the performance relates, based on an assessment of the probability of the performance criteria being met. The cost of such awards is calculated as the difference between the fair value of the shares at the date of the grant and the exercise price of the option. Any liability relating to National Insurance payments due on exercise of options that has not been transferred to the employees is also accrued over the performance period of the option. |
| (4b) | | The Group’s turnover comprises product sales, licence fees, contract research fees, royalties and milestone payments. One customer, the CDC, accounted for 95%, 64% and 9% of Group turnover in 2002, 2001 and 2000 respectively. The Directors are of the opinion that the Group has only one class of business. |
| | | The geographical analysis of turnover by origin and destination, operating profit/(loss) and net assets/(liabilities) and share of loss of the joint venture is as follows: |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | Europe | | | North America | |
| | | |
| | |
| |
| | | | 2002 | | | 2001 | | | 2000 | | | 2002 | | | 2001 | | | 2000 | |
| | | | £000 | | | £'000 | | | £'000 | | | £'000 | | | £'000 | | | £'000 | |
| |
|
| | Turnover by customer location | | | 785 | | | | 73 | | | | 265 | | | | 78,931 | | | | 8,841 | | | | 5,999 | |
| | Turnover by origin | | | 785 | | | | 73 | | | | 265 | | | | 78,931 | | | | 8,841 | | | | 5,999 | |
| | Profit/(loss) on ordinary activities before taxation | | | (2,741 | ) | | | 601 | | | | (5,942 | ) | | | 12,319 | | | | (13,159 | ) | | | (5,132 | ) |
| | Net assets/(liabilities) | | | 53,537 | | | | 55,805 | | | | 35,377 | | | | (7,286 | ) | | | (28,145 | ) | | | 762 | |
| | Share of loss of Joint Venture | | | – | | | | – | | | | – | | | | (171 | ) | | | (410 | ) | | | (2,138 | ) |
| |
|
| | | In 2002, sales to Europe represented 1% and sales to North America represented 99% of total sales. The figures shown on the face of the Group profit and loss account on page 18 analyse the operating profit/(loss) before finance charges and taxation. The net liabilities of the joint venture with Aventis Pasteur of £0.2m (2001 — net assets of £0.1m, see note 14(d)) are included within North America. |
24
| | | |
| (A) | | Turnover
Our turnover represents product sales and revenues for research and development.
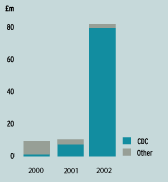
Other sources include revenues receivable from Aventis Pasteur (for our ChimeriVax-Dengue project) and our H. pylori joint venture. |
THE INFORMATION CONTAINED IN THIS COLUMN HAS NOT BEEN AUDITED.
| | | | | | | | | | | | | | | |
| | | |
|
| | | | | 2002 | | 2001 | | 2000 |
| | | | | £'000 | | £'000 | | £'000 |
| | |
|
| | | Administrative costs | | | 3,053 | | | | 2,295 | | | | 1,745 |
(B) | | Amortisation of goodwill | | | 1,204 | | | | 1,204 | | | | 1,204 |
| | |
|
| | | | | | 4,257 | | | | 3,499 | | | | 2,949 |
| | |
|
| 4 | | Discontinued operations |
| | | During 2000, the Group completed the disposal of its interest in its drug discovery business, Mimetrix, to Medivir AB, a Swedish biotechnology company listed on the Stockholm Stock Exchange. The results of Mimetrix up to 31 March 2000 (the date from which Medivir AB assumed the obligations of Mimetrix’s operating activities) are as follows: |
| | | | | | | |
| | | |
|
| | | | | 2000 |
| | | | | £'000 |
| | |
|
| | | Turnover | | | – | |
| | | Research and development costs | | | (643 | ) |
| | | Administrative costs | | | (81 | ) |
| | |
|
| | | Operating loss | | | (724 | ) |
| | |
|
| | | The Mimetrix activities were all UK-based. |
| 5 | | Amounts written off fixed asset investment |
| | | During the year, the investment of shares held in Medivir AB, which were acquired in 2000 in exchange for the assets of Mimetrix Limited, were written down to reflect the fall in market value during the year. This resulted in an exceptional loss of £0.1m (2001 — £0.4m). This adjustment was in accordance with FRS 11, “Impairment of Fixed Assets and Goodwill”. |
| 6 | | Interest payable and similar charges |
| | | This note details the interest payable on the Arilvax® overdraft facility (see note 18 for more information), as well as interest from assets held under finance leases. |
| | | | | | | | | | | | | | | |
| | | |
|
| | | | | 2002 | | 2001 | | 2000 |
| | | | | £'000 | | £'000 | | £'000 |
| | |
|
| | | On bank overdrafts | | | 93 | | | | 214 | | | | 211 |
(C) | | Interest element of finance leases | | | 1,118 | | | | – | | | | 5 |
| | |
|
| | | | | | 1,211 | | | | 214 | | | | 216 |
| | |
|
| 7 | | Profit/(loss) on ordinary activities before taxation |
| | | Profit/(loss) on ordinary activities before taxation is stated: |
| | | | | | | | | | | | | | | | | | | |
| | | |
|
| | | | | | | | | 2002 | | 2001 | | 2000 |
| | | | | Notes | | £'000 | | £'000 | | £'000 |
| | |
|
| | | After crediting: | | | | | | | | | | | | | | | | |
| | | Grant income | | | | | | | 1,892 | | | | 1,142 | | | | 165 |
| | |
|
| | | And after charging: | | | | | | | | | | | | | | | | |
| | | Amortisation of goodwill | | | 12 | | | | 1,204 | | | | 1,204 | | | | 1,204 |
| | | Depreciation of tangible fixed assets | | | 13 | | | | | | | | | | | | | |
| | | — owned | | | | | | | 1,148 | | | | 851 | | | | 855 |
| | | — held under finance leases | | | | | | | 262 | | | | – | | | | 73 |
| | | Operating lease charges | | | 26(a | ) | | | | | | | | | | | | |
| | | — hire of plant and machinery | | | | | | | 13 | | | | 5 | | | | 77 |
| | | — other | | | | | | | 1,379 | | | | 1,582 | | | | 1,630 |
| | | Auditors’ remuneration | | | | | | | | | | | | | | | | |
| | | — audit services (Company: £3,000) | | | | | | | 77 | | | | 58 | | | | 60 |
| | |
|
| | | In 2002, the Group paid amounts to the auditors of £0.1m to Arthur Andersen (2001 — £0.1m, 2000 — £0.1m) and £0.1m to PricewaterhouseCoopers LLP in respect of non-audit services (primarily tax compliance and advisory services). |
| | | |
| (B) | | Amortisation of goodwill
This goodwill arose when Acambis Inc. was acquired in 1999. It is being written off over 15 years, resulting in an annual charge to the profit and loss account of £1.2m (see note 12). |
| |
| (C) | | Interest element of finance leases
During 2001, the Group put in place a $40 million (c.£25m) lease-finance facility to fund the reactivation of our manufacturing plant. The repayment obligations for the lease financing are as follows: |
| • | | None in 2002; |
| • | | Interest only in 2003; and |
| • | | Capital and interest during 2004 to 2006. |
| |
| | | Consequently, interest that accrued in 2002 has been added into the outstanding balance. |
THE INFORMATION CONTAINED IN THIS COLUMN HAS NOT BEEN AUDITED.
25
| | | NOTES TO GROUP FINANCIAL STATEMENTS |
| | | The average monthly number of employees during the year (including Executive Directors) was: |
| | | | | | | | | | | | | | | | | | | | | | | |
| | | | |
|
| | | | | UK | | | US | | | 2002 | | | 2001 | | | 2000 | |
| | | | | Number | | | Number | | | Number | | | Number | | | Number | |
| | |
|
| | | Research and development | | | 30 | | | | 87 | | | | 117 | | | | 88 | | | | 71 | |
| | | Manufacturing | | | – | | | | 63 | | | | 63 | | | | 12 | | | | – | |
| | | Administration | | | 23 | | | | 39 | | | | 62 | | | | 50 | | | | 35 | |
| | |
|
| | | | | | 53 | | | | 189 | | | | 242 | | | | 150 | | | | 106 | |
| | |
|
| | | The Group had 291 employees at 31 December 2002 (2001 — 178). The Company had four employees, all of whom were Directors, at 31 December 2002 (2001 — three, 2000 — four). |
| | | The cost to the Group of paying its staff was: |
| | | | | | | | | | | | | | | |
| | | | |
|
| | | | | 2002 | | | 2001 | | | 2000 | |
| | | | | £'000 | | | £'000 | | | £'000 | |
| | |
|
| | | Wages and salaries | | | 11,341 | | | | 7,505 | | | | 4,862 | |
| | | Social security costs | | | 1,942 | | | | 1,008 | | | | 433 | |
| | | Other pension and 401K costs (see note 26(c)) | | | 447 | | | | 226 | | | | 235 | |
| | |
|
| | | | | | 13,730 | | | | 8,739 | | | | 5,530 | |
| | |
|
| | | During 2002, a third-party company paid a share of the Group’s administrative costs, including £0.3m, 2000 — £0.2m) for staff costs. These costs are included in the figures shown above. |
| 9 | | Directors’ remuneration, interests and transactions |
| | | Full disclosure of Directors’ remuneration, interests and transactions is given in the “auditable” part of the remuneration report. Aggregate gains made by Directors on the exercise of share options were £0.7m (2001 — £0.7m). |
| (B) | | Tax is charged annually, on profits made, in the country where each company is based. |
| | | Tax on profit on ordinary activities |
| | | | | | | | | | | | | | | |
| | | | |
|
| | | | | 2002 | | 2001 | | 2000 |
| | | | | £'000 | | £'000 | | £'000 |
| | |
|
| | | UK corporation tax at 30% (2001 — 30%; 2000 — 30%): | | | (100 | ) | | | – | | | | – | |
| | | Foreign taxation | | | 201 | | | | – | | | | – | |
| | | Over provision in respect of prior year | | | (98 | ) | | | (131 | ) | | | – | |
| | |
|
| | | | | | 3 | | | | (131 | ) | | | – | |
| | |
|
26
| | | |
| (A) | | Employee numbers
As shown in our graph below, our average number of employees rose significantly in 2002, principally as a result of reactivating the manufacturing facility and the work on the CDC smallpox vaccine contracts. |
| |
| | | 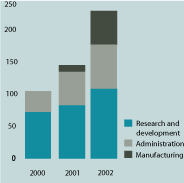 |
| |
| | | We estimate that the headcount will increase during 2003 up to around 325. |
| |
| (B) | | Taxation
Loss-making R&D companies traditionally generate taxable losses which can, in future, be offset against trading profits. For as long as it remains uncertain that the Group will have taxable profits against which these taxable losses can be offset, a deferred tax asset (historic taxable losses) is not recognised in the accounts. |
| |
| | | 2002 has seen a small tax charge due to state taxation liability arising in the US which is largely offset by an R&D tax credit claimed in the UK. |
THE INFORMATION CONTAINED IN THIS COLUMN HAS NOT BEEN AUDITED.
| | | The tax assessed for the year is different from the standard rate of corporation tax in the UK of 30%. The differences are explained below: |
| | | | | | | | | | | | | | | |
| | | | |
|
| | | | | 2002 | | 2001 | | 2000 |
| | | | | £'000 | | £'000 | | £'000 |
| | |
|
| | | Profit/(loss) on ordinary activities before tax | | | 9,578 | | | | (12,558 | ) | | | (11,074 | ) |
| | |
|
| | | Profit/(loss) on ordinary activities multiplied by the standard rate of corporation tax in the UK of 30% (2001 and 2000: R&D tax credit rate of 16%) | | | 2,873 | | | | (2,009 | ) | | | (1,772 | ) |
| | | Effects of: | | | | | | | | | | | | |
| | | Utilisation of tax losses | | | (7,122 | ) | | | – | | | | – |
| | | Expenses not deductible for tax purposes | | | 663 | | | | 10 | | | | (58 | ) |
| | | Losses arising in the year | | | 548 | | | | 1,869 | | | | 1,831 |
| | | Difference in tax rates used compared to UK standard rate | | | 664 | | | | – | | | | – |
| | | Difference between capital allowances and depreciation | | | (143 | ) | | | – | | | | – |
| | | Prior year R&D tax credit | | | (98 | ) | | | – | | | | – |
| | | Other short-term timing differences | | | 2,618 | | | | (1 | ) | | | (1 | ) |
| | |
|
| | | Current tax charge/(credit) for year | | | 3 | | | | (131 | ) | | | – |
| | |
|
| | | | | | | | | | | |
| | | | |
|
| | | | | 2002 | | | 2001 | |
| | | | | £'000 | | | £'000 | |
| | |
|
| | | Unrecognised asset in respect of accelerated capital allowances | | | 274 | | | | 450 | |
| | | Unrecognised tax losses | | | 8,535 | | | | 14,020 | |
| | | Unrecognised asset on short-term timing differences | | | 3,966 | | | | 1,515 | |
| | |
|
| | | Total unrecognised deferred tax asset | | | 12,775 | | | | 15,985 | |
| | |
|
| 11 | | Earnings per ordinary share |
| | | Basic earnings per share is calculated by dividing the earnings attributable to ordinary shareholders by the weighted average number of ordinary shares in issue during the year, excluding those held in the employee share trust (see note 14(c)) which are treated as cancelled until the shares vest unconditionally with the employees. |
| | | For diluted earnings per share, the weighted average numbers of shares used in the calculations are set out below: |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | |
|
| | | | | | | | | | | | | 2002 | | | | | | | | | | 2001 | | | | | | | | | | 2000 |
| | | | | | | | | Weighted | | Per- | | | | Weighted | | Per- | | | | Weighted | | Per- |
| | | | | | | | | average | | share | | | | average | | share | | | | average | | share |
| | | | | Earnings | | number | | amount | | Earnings | | number | | amount | | Earnings | | number | | amount |
| | | | | £'000 | | of shares | | pence | | £'000 | | of shares | | pence | | £'000 | | of shares | | pence |
| | |
|
| | | Basic EPS | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Earnings attributable to ordinary shareholders | | | 9,575 | | | | 96,101,507 | | | | 10.0 | | | | (12,427 | ) | | | 91,027,463 | | | | (13.7 | ) | | | (11,074 | ) | | | 79,638,484 | | | | (13.9 | ) |
| | | Effect of dilutive securities | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Options | | | – | | | | 2,875,375 | | | | (0.3 | ) | | | – | | | | – | | | | – | | | | – | | | | – | | | | – |
| | |
|
| | | Diluted EPS | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Adjusted earnings | | | 9,575 | | | | 98,976,882 | | | | 9.7 | | | | (12,427 | ) | | | 91,027,463 | | | | (13.7 | ) | | | (11,074 | ) | | | 79,638,484 | | | | (13.9 | ) |
| | |
|
| | | For diluted earnings per share, the weighted average ordinary shares in issue is adjusted to assume conversion of all dilutive potential ordinary shares. The Group’s dilutive securities consist of: those share options without performance conditions granted to employees where the exercise price is less than the average market price of the Company’s ordinary shares during the year, and those share options with performance criteria and contingently issuable shares, which are included in the diluted earnings per share calculation where the related performance conditions have been met at the year-end. |
| | | This goodwill arose when Acambis Inc. was acquired in 1999. It is being written off over 15 years, resulting in an annual charge to the profit and loss account. |
| | | | | | | | | | | |
| | | | |
|
| | | | | 2002 | | | 2001 | |
| | | | | £'000 | | | £'000 | |
| | |
|
| | | Cost at 1 January and 31 December | | | 18,055 | | | | 18,055 | |
| | |
|
| | | Amortisation | | | | | | | | |
| | | At 1 January | | | 3,210 | | | | 2,006 | |
| | | Charge for the year | | | 1,204 | | | | 1,204 | |
| | |
|
| | | At 31 December | | | 4,414 | | | | 3,210 | |
| | |
|
| | | Net book value | | | | | | | | |
| | | At 31 December | | | 13,641 | | | | 14,845 | |
| | |
|
| | | NOTES TO GROUP FINANCIAL STATEMENTS |
| | | Physical assets held for continuing use in the business. |
| | | | | | | | | | | | | | | | | | | |
| | | | |
|
| | | | | | | | | Laboratory and | | | | |
| | | | | Short leasehold | | manufacturing | | Office | | |
| | | | | land and buildings | | equipment | | equipment | | Total |
| | | Group | | £'000 | | £'000 | | £'000 | | £'000 |
| | |
|
| | | Cost | | | | | | | | | | | | | | | | |
| | | At 1 January 2002 | | | 10,111 | | | | 3,859 | | | | 869 | | | | 14,839 |
| | | Additions | | | 4,963 | | | | 4,519 | | | | 1,246 | | | | 10,728 |
| | | Disposals | | | – | | | | (31 | ) | | | (281 | ) | | | (312 | ) |
(A) | | Exchange movement | | | (1,170 | ) | | | (765 | ) | | | (126 | ) | | | (2,061 | ) |
| | |
|
| | | At 31 December 2002 | | | 13,904 | | | | 7,582 | | | | 1,708 | | | | 23,194 | |
| | |
|
| | | Depreciation | | | | | | | | | | | | | | | | |
| | | At 1 January 2002 | | | 1,274 | | | | 829 | | | | 481 | | | | 2,584 | |
| | | Charge for year | | | 482 | | | | 651 | | | | 277 | | | | 1,410 | |
| | | Disposals | | | – | | | | (22 | ) | | | (281 | ) | | | (303 | ) |
(A) | | Exchange movement | | | (190 | ) | | | (250 | ) | | | (45 | ) | | | (485 | ) |
| | |
|
| | | At 31 December 2002 | | | 1,566 | | | | 1,208 | | | | 432 | | | | 3,206 | |
| | |
|
| | | Net book value | | | | | | | | | | | | | | | | |
| | | At 1 January 2002 | | | 8,837 | | | | 3,030 | | | | 388 | | | | 12,255 | |
| | |
|
| | | At 31 December 2002 | | | 12,338 | | | | 6,374 | | | | 1,276 | | | | 19,988 | |
| | |
|
| | | Net book value of assets held under
finance lease included above: |
| | |
|
| | | At 1 January 2002 | | | 5,220 | | | | 2,208 | | | | – | | | | 7,428 | |
| | |
|
| | | At 31 December 2002 | | | 4,633 | | | | 1,923 | | | | – | | | | 6,556 | |
| | |
|
| | | The Company has no tangible fixed assets. |
| 14 | | Fixed asset investments |
| | | These are assets including shares that are held as an ongoing investment. |
| | | | | | | | | | | | | | | | | | | |
| | | | |
|
| | | | | | | | | Group | | | | | | Company |
| | | | | 2002 | | 2001 | | 2002 | | 2001 |
| | | | | £'000 | | £'000 | | £'000 | | £'000 |
| | |
|
| | | a) Subsidiary undertakings | | | – | | | | – | | | | 14,988 | | | | 14,988 | |
| | | b) Trade investments | | | 303 | | | | 388 | | | | – | | | | – | |
| | | c) Investment in own shares | | | 797 | | | | 1,252 | | | | 143 | | | | 598 | |
| | |
|
| | | | | | 1,100 | | | | 1,640 | | | | 15,131 | | | | 15,586 | |
| | |
|
| | | d) Joint venture | | | (206 | ) | | | 67 | | | | – | | | | – | |
| | |
|
| (B) | | (a) Subsidiary undertakings |
| (4c) | | The Company, Acambis plc, owns 100% of the ordinary share capital of Acambis Research Limited and Acambis Inc., incorporated in England and Wales and the US respectively. The main activity of Acambis Research Limited is R&D. The main activities of Acambis Inc. are R&D and manufacturing. Other companies held are not significant to the Group. All subsidiary undertakings have been consolidated.
The cost of the investments in the subsidiary undertakings in the books of the Company is as follows: |
| | | | | | | |
| | | | |
|
| | | | | £'000 | |
| | |
|
| | | Cost and net book value at 1 January and 31 December 2002 | | | 14,988 | |
| | |
|
28
| | | |
| (A) | | Exchange movement
During 2002, the monthly closing US $/£ exchange rate has varied between 1.4555 and 1.6095. This has given rise to an exchange rate movement on the assets located in the US, which has an impact on both asset cost and accumulated depreciation. |
| |
| (B) | | Subsidiary undertakings
The principal trading subsidiaries of the Group are Acambis Research Limited and Acambis Inc. The structure of the principal trading subsidiaries of the Group is as follows: |
| |
| | | 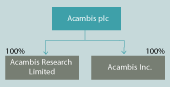 |
THE INFORMATION CONTAINED IN THIS COLUMN HAS NOT BEEN AUDITED.
| | | (b) Trade investments
The investments held during 2002 are shares the Group holds in a non-related overseas listed company, Medivir AB. |
| | | | | | | | | | | |
| | | | |
|
| | | | | 2002 | | | 2001 | |
| | | | | £'000 | | | £'000 | |
| | |
|
| | | Cost
At 1 January | | | 1,481 | | | | 1,481 | |
| | |
|
| | | At 31 December | | | 1,481 | | | | 1,481 | |
| | |
|
| | | Amounts provided | | | | | | | | |
| | | At 1 January | | | 1,093 | | | | 670 | |
| | | Provided in the year | | | 85 | | | | 423 | |
| | |
|
| | | At 31 December | | | 1,178 | | | | 1,093 | |
| | |
|
| | | Net book value | | | | | | | | |
| | | At 1 January | | | 388 | | | | 811 | |
| | |
|
| | | At 31 December | | | 303 | | | | 388 | |
| | |
|
| | | The market value of the investment in Medivir AB at 31 December 2002 was £0.3m (2001 — £0.4m).
(c) Investment in own shares
These are shares in Acambis plc that have been bought by an employee trust. The shares may be issued to certain employees and Directors as share options or long-term incentive awards are exercised. Provision has been made for those shares that are likely to be issued. |
| | | | | | | | | | | | | | | | | | | |
| | | | | | | | | Group | | | | | | | Company | |
| | | | |
|
| | | | | 2002 | | 2001 | | | 2002 | | 2001 | |
| | | | | £'000 | | £'000 | | | £'000 | | £'000 | |
| | |
|
| | | Cost | | | | | | | | | | | | | | | | |
| | | At 1 January | | | 1,606 | | | | 1,606 | | | | 952 | | | | 952 | |
| | | Disposals | | | (287 | ) | | | – | | | | (287 | ) | | | – | |
| | |
|
| | | At 31 December | | | 1,319 | | | | 1,606 | | | | 665 | | | | 952 | |
| | |
|
| | | Amounts provided | | | | | | | | | | | | | | | | |
| | | At 1 January | | | 354 | | | | 161 | | | | 354 | | | | 161 | |
| | | Provided in the year | | | 420 | | | | 193 | | | | 420 | | | | 193 | |
| | | Release of previously provided amounts | | | (252 | ) | | | – | | | | (252 | ) | | | – | |
| | |
|
| | | At 31 December | | | 522 | | | | 354 | | | | 522 | | | | 354 | |
| | |
|
| | | Net book value | | | | | | | | | | | | | | | | |
| | | At 1 January | | | 1,252 | | | | 1,445 | | | | 598 | | | | 791 | |
| | |
|
| | | At 31 December | | | 797 | | | | 1,252 | | | | 143 | | | | 598 | |
| | |
|
| | | At 31 December 2002, Acambis Employees’ Trustees Limited held 589,685 (2001 — 723,396) ordinary shares in the Company with a total market value of £1.6m (2001 — 589,685 (2001 — £2.5m) on behalf of the Acambis Employees’ Trust. All shares held by the Trust have been allocated to long-term incentive awards and a provision has been made in respect of all of these shares. All costs relating to the administration of the Trust are dealt with in the accounts of the Company as they arise.
Disposals of £0.3m (2001 — £nil) during the year relate to share options granted in accordance with the rules of the Acambis Share Incentive Plan (“LTIP”) having vested as a result of the performance criteria having been met at the exercise date. During the year, a provision of £0.4m (2001 — £0.2m) was made in relation to share options that have been granted in accordance with the rules of the LTIP, as a result of the performance criteria having been met at 31 December 2002. As a result of certain share options becoming exercisable during the year, this gave rise to a release of £0.3m (2001 — £nil) being provisions made in previous years (see remuneration report on page 62 for further details). |
| |
| (C) | | (d) Joint ventures
The Group has an interest in the Pasteur Mérieux-OraVax joint venture (“Joint Venture”), whose principal business is to develop, manufacture, market and sell immunotherapeutic and preventative vaccines against H. pylori infection in humans. The Joint Venture represents a collaboration between two partnerships, Mérieux-OraVax SNC and OraVax-Mérieux Co., incorporated in Delaware, US. These partnerships were formed in March 1995 between Acambis Inc. and Aventis Pasteur. The Joint Venture trades under the name of Pasteur Mérieux-OraVax and its accounting year-end is 31 December. The R&D budgets of the two partnerships are established by joint committees in which each of the parties has an equal participation and role. The parties pay approximately equal shares of the agreed budgets. |
| | | |
| (C) | | Joint ventures
The structure of the H. pylori joint venture is shown in the following diagram: |
| |
| | |  |
| |
| * | | Through the Pasteur Mérieux-OraVax joint venture, Acambis owns 50.1% of Mérieux-OraVax SNC, a French partnership, and 49.9% of OraVax-Mérieux Co., a Massachusetts General partnership. Aventis Pasteur owns the remaining balances of the two partnerships.
Whilst Acambis’ and Aventis Pasteur’s interests in each of the partnerships are very slightly different, the collaboration is structured such that all costs, capital contributions, profits and losses are shared equally by the parties. |
THE INFORMATION CONTAINED IN THIS COLUMN HAS NOT BEEN AUDITED.
29
| | | NOTES TO GROUP FINANCIAL STATEMENTS |
| 14 | | Fixed asset investments |
| | | (d) Joint ventures (continued) |
| | | The following information is given in respect of the Group’s share of the Joint Venture: |
| | | | | | | | | | | |
| | | | |
|
| | | | | 2002 | | 2001 |
| | | | | £'000 | | £'000 |
| | |
|
| | | Loss before tax | | | (171 | ) | | | (410 | ) |
| | |
|
| | | Current assets | | | 941 | | | | 915 | |
| | | Liabilities due within one year | | | (1,147 | ) | | | (848 | ) |
| | |
|
| | | | | | (206 | ) | | | 67 | |
| | |
|
| | | Due to the nature of this Joint Venture, being a collaboration between two partnerships, the following table provides an alternative analysis of the amounts shown above: |
| | | | | | | | | | | |
| | | | |
|
| | | | | 2002 | | 2001 |
| | | | | £'000 | | £'000 |
| | |
|
| | | Share of cumulative amounts invested in the partnerships | | | 18,169 | | | | 20,090 | |
| | | Share of cumulative losses incurred by the partnerships | | | (18,375 | ) | | | (20,023 | ) |
| | |
|
| | | | | | (206 | ) | | | 67 | |
| | |
|
| | | | | | | | | | | | | | | | | | | |
| (A) | | | | | | | | Group | | | | | | | Company | |
| | | | |
|
| | | | | 2002 | | | 2001 | | | 2002 | | | 2001 | |
| | | | | £'000 | | | £'000 | | | £'000 | | | £'000 | |
| | |
|
| | | Raw materials | | | 1,131 | | | | – | | | | – | | | | – | |
| | | Work in progress | | | 34,934 | | | | – | | | | – | | | | – | |
| | | Finished goods | | | 12,278 | | | | – | | | | – | | | | – | |
| | |
|
| | | | | | 48,343 | | | | – | | | | – | | | | – | |
| | |
|
| 16 | | Debtors: amounts receivable within one year |
| | | | | | | | | | | | | | | | | | | |
| | | | | | | | | Group | | | | | | | Company | |
| | | | |
|
| | | | | 2002 | | | 2001 | | | 2002 | | | 2001 | |
| | | | | £'000 | | | £'000 | | | £'000 | | | £'000 | |
| | |
|
(B) | | Trade debtors | | | 46,090 | | | | 5,918 | | | | – | | | | – | |
| | | Other debtors | | | 2,406 | | | | 285 | | | | 589 | | | | 42 | |
(C) | | Corporation tax | | | 198 | | | | 131 | | | | – | | | | – | |
| | | Prepayments and accrued income | | | 5,346 | | | | 1,208 | | | | – | | | | 28 | |
| | |
|
| | | | | | 54,040 | | | | 7,542 | | | | 589 | | | | 70 | |
| | |
|
| 17 | | Debtors: amounts receivable after one year |
| | | | | | | | | | | | | | | | | | | |
| | | | | | | | | Group | | | | | | | Company | |
| | | | |
|
| | | | | 2002 | | | 2001 | | | 2002 | | | 2001 | |
| | | | | £'000 | | | £'000 | | | £'000 | | | £'000 | |
| | |
|
| | | Amounts owed by subsidiary undertakings | | | – | | | | – | | | | 71,643 | | | | 60,170 | |
| | | Prepayments and accrued income | | | 4,925 | | | | 6,235 | | | | – | | | | – | |
| | |
|
| | | | | | 4,925 | | | | 6,235 | | | | 71,643 | | | | 60,170 | |
| | |
|
| | | Acambis is required to prepay insurance premiums, on behalf of the CDC, relating to the first CDC smallpox vaccine contract, secured in September 2000. At 31 December 2002, the total amount prepaid was £5.6m (2001 — £7.0m), of which £0.7m (2001 — £0.8m) is included within “Debtors: amounts receivable within one year” and £4.9m (2001 — £6.2m) is included within “Debtors: amounts receivable after one year”. The total of £5.6m (2001 — £7.0m) is offset by an equal amount in creditors (see notes 18 and 19). Both balances will reduce over the term of the insurance cover, which was up to 20 years at inception in 2000. |
30
| | | |
| (A) | | Stock
Stock is a new category on the balance sheet and consists solely of stock relating to the production of smallpox vaccine. |
| |
| (B) | | Trade debtors
Amounts owed by the CDC under the two smallpox vaccine contracts are included within trade debtors at the year-end. |
| |
| (C) | | Corporation tax
See note 10 on pages 26 and 27 for details of the corporation tax calculation. |
THE INFORMATION CONTAINED IN THIS COLUMN HAS NOT BEEN AUDITED.
| 18 | | Creditors: amounts falling due within one year |
| | | | | | | | | | | | | | | | | | | |
| | | | | | | | | Group | | | | | | | Company | |
| | | | |
|
| | | | | 2002 | | | 2001 | | | 2002 | | | 2001 | |
| | | | | £'000 | | | £'000 | | | £'000 | | | £'000 | |
| | |
|
| | | Overdraft facility (see below) | | | 4,349 | | | | 4,809 | | | | – | | | | – | |
(D) | | Trade creditors | | | 54,769 | | | | 3,674 | | | | 58 | | | | 47 | |
| | | Amounts owed to Joint Venture | | | – | | | | 496 | | | | – | | | | – | |
| | | Corporation tax | | | 201 | | | | – | | | | – | | | | – | |
| | | Other taxes and social security | | | 87 | | | | 69 | | | | – | | | | – | |
| | | Other creditors | | | 58 | | | | 44 | | | | 49 | | | | – | |
(E) | | Accruals and deferred income | | | 28,996 | | | | 7,511 | | | | 322 | | | | 172 | |
| | |
|
| | | | | | 88,460 | | | | 16,603 | | | | 429 | | | | 219 | |
| | |
|
| | | Under the terms of the agreement between Acambis and Evans Vaccines Limited, given certain conditions, the obligation under the bank overdraft facility of £4.3m (2001 — £4.8m) for the costs incurred on the Arilvax® project may be repayable within one year. The facility is underwritten by PowderJect Pharmaceuticals Plc (“PowderJect”). PowderJect has granted to Acambis 100% of the marketing rights to Arilvax® in the US, whilst retaining an option to buy back 50% of the profits from the US sales in return for refunding to the Group the costs the Group has incurred on the Arilvax® programme. The overdraft facility was renewed in January 2003 for a further year. Interest is charged as disclosed within “Financial liabilities” in note 20. |
| | | During the year an exchange gain of £0.4m (2001 — exchange loss of £0.1m) was recorded on the face of the Group profit and loss account, resulting from the revaluation of this US dollar-denominated facility. |
| 19 | | Creditors: amounts falling after one year |
| | | | | | | | | | | | | | | | | | | |
| | | | | | | | | Group | | | | | | | Company | |
| | | | |
|
| | | | | 2002 | | | 2001 | | | 2002 | | | 2001 | |
| | | | | £'000 | | | £'000 | | | £'000 | | | £'000 | |
| | |
|
| | | Obligations under finance leases | | | 13,972 | | | | 14,299 | | | | – | | | | – | |
| | | Accruals and deferred income | | | 4,925 | | | | 6,235 | | | | – | | | | – | |
| | |
|
| | | | | | 18,897 | | | | 20,534 | | | | – | | | | – | |
| | |
|
| | | The amount in accruals and deferred income relates to the insurance premiums, payable on behalf of the CDC, relating to the first CDC smallpox vaccine contract, the equivalent figure being included in Debtors, as referred to in note 17 above. |
| | | In December 2001, the Group committed to a finance lease, repayable within five years, relating to the purchase and sale-and-leaseback of capital assets within the manufacturing plant. Further details regarding this finance lease facility are given within “Financial liabilities” in note 20. |
| (11) | | The Group’s financial instruments comprise primarily cash and liquid resources, a long-term finance lease, an overdraft facility and various items, such as trade debtors and trade creditors, that arise directly from its operations. The main purpose of these financial instruments is to provide working capital for the for the Group’s operations.
The Group holds funds in both US dollars and sterling. The Group’s current policy is to hold surplus funds in sterling over the long term, which currently achieves a higher interest rate return, whilst also mitigating exchange rate exposure.
The main risks arising from the Group’s financial instruments are interest rate risk, liquidity risk and foreign currency risk. The Board reviews and agrees policies for managing each of these risks. These policies have remained unchanged since the beginning of 2000. All of the Group’s short-term debtors and creditors are excluded from these disclosures, other than currency risk disclosures. |
| | | The Group finances its operations predominantly through cash, liquid resources and lease financing. It is the Group’s policy to invest surplus cash in money market funds, managed by professional money managers. The performance of the funds invested is reviewed by the Board on a regular basis to ensure that competitive rates of return are being achieved. The Board reviews regularly the financing facilities available to the Group to ensure competitive rates of interest are being obtained. |
| | | The Board monitors the level of cash and liquid resources on a regular basis, and management on a daily basis, to ensure that the Group has sufficient liquid funds to enable it to continue as a going concern. This is achieved through the production and review of cash forecasts, including sensitivity analyses. The Group’s liquid resources are managed on a discretionary basis by a third party within strict parameters, which have been set by the Board. |
| | | |
| (D) | | Trade creditors
This includes amounts payable to suppliers, largely for payments of stock. |
| |
| (E) | | Accruals and deferred income
Deferred income represents the difference between invoices submitted to the CDC before the year-end and amounts recognised as revenue. |
THE INFORMATION CONTAINED IN THIS COLUMN HAS NOT BEEN AUDITED.
31
| | | NOTES TO GROUP FINANCIAL STATEMENTS |
| 20 | | Financial instruments (continued) |
| | | The Group has one principal subsidiary that operates and trades overseas — Acambis Inc. It operates in the US and its revenues, expenses and financing are denominated principally in US dollars. |
| | | The Group has a US dollar-denominated overdraft facility, as described in note 18, which is held in the UK and is subject to exchange rate movements. These are recognised as incurred. In addition, the Group has other current assets and liabilities denominated in foreign currencies, which the Board does not consider to be significant. |
| | | The tables below show the extent to which Group companies have monetary assets and liabilities in currencies other than their local currency. |
| | | Net foreign currency monetary assets/(liabilities) |
| | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | 2001 | |
| | | | |
|
| | | | | Sterling | | | US dollar | | | Euros | | | Total | |
| | | | | £000 | | | £000 | | | £000 | | | £000 | |
| | |
|
| | | Functional currency of Group operation: | | | | | | | | | | | | | | | | |
| | | Sterling | | | – | | | | 28,782 | | | | – | | | | 28,782 | |
| | | US dollars | | | – | | | | – | | | | – | | | | – | |
| | |
|
| | | | | | – | | | | 28,782 | | | | – | | | | 28,782 | |
| | |
|
| | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | 2002 | |
| | | | |
|
| | | | | Sterling | | | US dollar | | | Euros | | | Total | |
| | | | | £000 | | | £000 | | | £000 | | | £000 | |
| | |
|
| | | Functional currency of Group operation: | | | | | | | | | | | | | | | | |
| | | Sterling | | | – | | | | 27,399 | | | | 755 | | | | 28,154 | |
| | | US dollars | | | – | | | | – | | | | – | | | | – | |
| | |
|
| | | | | | – | | | | 27,399 | | | | 755 | | | | 28,154 | |
| | |
|
| | | The Group had cash and liquid resources of £11.8m at 31 December 2002 (2001 — £22.2m). The majority of these resources are invested in managed funds, denominated in sterling and US dollars, and are available for use with a day’s notice. During 2002, these funds achieved weighted average returns of 4.1% (2001 — 5.4%) for funds invested in UK sterling, and 1.9% (2001 — 4.7%) for funds invested in US dollars. The Group also holds shares in Medivir AB (see note 14(b)). This investment does not subject the Group to interest rate risk as it has no maturity date. |
| | | The Group’s overdraft facility, which is denominated in US dollars and is underwritten by PowderJect, is explained in note 18. At 31 December 2002, the Group had fully utilised the overdraft facility (2001 — fully utilised). Interest on the facility is charged at 0.35% per annum above the bank-lending rate for US dollars. During 2002, the weighted average interest payable on the facility was 2.0% (2001 — 4.5%). |
| | | The only other financial liability of the Group at 31 December 2002 was a long-term lease-finance facility, which matures within four years. This $40m (c. £25m) lease-finance facility, arranged through Baxter International, Inc. (“Baxter”), was approved by shareholders in December 2001. The Group secured financing of $10m (c. £6.8m) for the sale-and-leaseback of the land and shell of the manufacturing plant, and up to $25m (c. £17m) to lease-finance the capital expenditure items purchased to reactivate the manufacturing plant. In December 2001, the Group received $18.6m (c. £12.8m), which represented $8.6m (c. £6.0m) in respect of capital expenditure already incurred throughout 2001, plus $10m (c. £6.8m) representing the net proceeds from sale-and-leaseback of the land and shell of the building. To facilitate this transaction, the land and shell were purchased for $2.3m (c. £1.5m). This amount is included in the outstanding facility. During 2002, no further draw downs were made from the facility, although interest of $1.7m (£1.1m) accrued during the year and has converted into the capital balance owing on the facility. The financing has been reflected in the financial statements as a long-term creditor. |
| | | During 2002, interest was charged to the facility at 3% above the prime rate of Banc One Corporation, a US-based bank, resulting in an interest charge of £1.1m (2001 — £0.03m) being added to the facility. From January 2003, the interest percentage payable in respect of the facility has been fixed at 6.25% until the end of the life of the lease. |
| | | The repayment schedule for the lease financing requires that no interest or capital were repayable during 2002, interest only need be repaid in 2003 and capital and interest are repayable over 2004 to 2006. The Group has an option to repurchase all of the facility’s assets from the end of 2003, and on each anniversary thereafter, for the capital balance outstanding at that time, plus any accrued but unpaid interest due at the time, plus a make-whole payment (discounted to present value) equal to the projected future interest stream payable to the end of the lease term. |
| | | At 31 December 2002, the balance on this facility was $22.5m (£14.0m), resulting in $17.5m (c. £10.9m) not being used at that time (2001 — $19.2m). The facility is denominated in US dollars. |
| (A) | | Profile of the Group’s financial liabilities |
| | | The maturity profile of the overdraft facility, together with the future minimum finance lease obligations (net of finance charges) to which the Group is committed are as follows: |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | |
|
| | | Overdraft | | Finance | | 2002 | | Overdraft | | Finance | | 2001 |
| | | faciity | | lease | | | | faciity | | lease | | |
| | | £’000 | | £’000 | | £’000 | | £’000 | | £’000 | | £’000 |
|
| Within one and two years | | | 4,349 | | | | 4,428 | | | | 8,777 | | | | 4,809 | | | | – | | | | 4,809 | |
| Between two and five years | | | – | | | | 9,544 | | | | 9,544 | | | | – | | | | 14,299 | | | | 14,299 | |
|
| | | | 4,349 | | | | 13,972 | | | | 18,321 | | | | 4,809 | | | | 14,299 | | | | 19,108 | |
|
| | | Fair values of financial assets and financial liabilities |
| | | In the opinion of the Directors, there is no material difference between the book values and fair values of the Group’s financial assets and liabilities as at 31 December 2002. Fair values have been calculated by discounting cash flows at prevailing interest rates. |
| 21 | | Called-up share capital |
| | | | | | | | | | | | | | | | | |
| | | Year ending 31 December 2002 | | | Year ending 31 December 2001 | |
| | |
|
| | | Number | | | £’000 | | | Number | | | £’000 | |
|
Authorised ordinary shares of 10p each
At 1 January | | | 140,000,000 | | | | 14,000 | | | | 140,000,000 | | | | 14,000 | |
| At 31 December | | | 140,000,000 | | | | 14,000 | | | | 140,000,000 | | | | 14,000 | |
Allotted, called-up and fully paid ordinary shares of 10p each
At 1 January | | | 93,081,919 | | | | 9,308 | | | | 88,675,680 | | | | 8,868 | |
| Baxter subscription | | | 4,967,562 | | | | 497 | | | | 2,674,841 | | | | 267 | |
| Other — exercise of share options | | | 962,402 | | | | 96 | | | | 1,731,398 | | | | 173 | |
|
| At 31 December | | | 99,011,883 | | | | 9,901 | | | | 93,081,919 | | | | 9,308 | |
|
| | | In 2000, an alliance was formed with Baxter involving a series of agreements, including a subscription by Baxter in Acambis. The subscription is payable in instalments between December 2000 and, at the latest, June 2003. The subscription price in 2002 was £1.40 (2001 — £1.30) per ordinary share. As described within “Post balance sheet events” on page 37, on 27 March 2003, Baxter accelerated its fourth and final subscription, taking its shareholding to 20.6%. |
| | | The Group operates several share option schemes. Options outstanding under the various schemes, as defined in the remuneration report on pages 59 to 61, are as follows: |
| | | | | | | | | | | | | | | | | | | | | |
| | |
|
| | | 1 January 2000 | | | Granted | | | Exercised | | | Lapsed | | | 31 December 2000 | |
| Scheme | | ’000 | | | ’000 | | | ’000 | | | ’000 | | | ’000 | |
|
19941 | | | 981 | | | | – | | | | (7 | ) | | | – | | | | 974 | |
| 1995 | | | 2,796 | | | | – | | | | (682 | ) | | | (289 | ) | | | 1,825 | |
| 1996 | | | 1,247 | | | | 28 | | | | (349 | ) | | | (361 | ) | | | 565 | |
| 1999 | | | 787 | | | | 1,722 | | | | – | | | | (117 | ) | | | 2,392 | |
| SAYE | | | 827 | | | | 42 | | | | (106 | ) | | | (368 | ) | | | 395 | |
| 1990 US | | | 261 | | | | – | | | | (1 | ) | | | (20 | ) | | | 240 | |
| 1995 US | | | 201 | | | | – | | | | – | | | | (10 | ) | | | 191 | |
|
| Total | | | 7,100 | | | | 1,792 | | | | (1,145 | ) | | | (1,165 | ) | | | 6,582 | |
|
| | |
| (A) | Profile of Group’s financial liabilities — finance lease
This relates to the financing put in place for our manufacturing plant in 2001. We have an option to repurchase the plant and all of its assets in December 2003 on the second anniversary of the establishment of the agreement, and on each anniversary thereafter, for the outstanding capital balance.
|
| (B) | Share option schemes
We recognise that our people are one of our most important assets and encourage all employees to share in the growth of the Group by offering share options to every employee.
THE INFORMATION CONTAINED IN THIS COLUMN HAS NOT BEEN AUDITED. |
33
| | | NOTES TO GROUP FINANCIAL STATEMENTS |
| 21 | | Called-up share capital
Share option schemes (continued) |
| | | | | | | | | | | | | | | | | | | | | |
| | |
|
| | | 1 January 2001 | | | Granted | | | Exercised | | | Lapsed | | | 31 December 2001 | |
| Scheme | | '000 | | | '000 | | | '000 | | | '000 | | | '000 | |
|
19941 | | | 974 | | | | – | | | | (966 | ) | | | – | | | | 8 | |
| 1995 | | | 1,825 | | | | – | | | | (411 | ) | | | (48 | ) | | | 1,366 | |
| 1996 | | | 565 | | | | 78 | | | | (268 | ) | | | (65 | ) | | | 310 | |
| 1999 | | | 2,392 | | | | 1,503 | | | | – | | | | (286 | ) | | | 3,609 | |
| SAYE | | | 395 | | | | 61 | | | | (30 | ) | | | (38 | ) | | | 388 | |
| 1990 US | | | 240 | | | | – | | | | (57 | ) | | | – | | | | 183 | |
| 1995 US | | | 191 | | | | – | | | | – | | | | – | | | | 191 | |
|
| Total | | | 6,582 | | | | 1,642 | | | | (1,732 | ) | | | (437 | ) | | | 6,055 | |
|
| | | | | | | | | | | | | | | | | | | | | |
| | |
|
| | | 1 January 2002 | | | Granted | | | Exercised | | | Lapsed | | | 31 December 2002 | |
| Scheme | | '000 | | | '000 | | | '000 | | | '000 | | | '000 | |
|
19941 | | | 8 | | | | – | | | | (8 | ) | | | – | | | | – | |
| 1995 | | | 1,366 | | | | – | | | | (421 | ) | | | – | | | | 945 | |
| 1996 | | | 310 | | | | 135 | | | | (126 | ) | | | – | | | | 319 | |
| 1999 | | | 3,609 | | | | 873 | | | | (265 | ) | | | (163 | ) | | | 4,054 | |
| SAYE | | | 388 | | | | 72 | | | | (141 | ) | | | (1 | ) | | | 318 | |
| 1990 US | | | 183 | | | | – | | | | (2 | ) | | | – | | | | 181 | |
| 1995 US | | | 191 | | | | – | | | | – | | | | – | | | | 191 | |
|
| Total | | | 6,055 | | | | 1,080 | | | | (963 | ) | | | (164 | ) | | | 6,008 | |
|
| | | A breakdown of the total options outstanding at 31 December 2002 is as follows: |
| | | | | | | | | | | | | |
|
| | | | | | | | | | | Period in which |
| | | | | | | Weighted average | | exercisable in |
| Scheme | | Number | | exercise price | | normal circumstances |
|
| 19952 | | | 945 | | | | £1.25 | | | September 1999 — April 2006 |
| 1996 | | | 319 | | | | £1.53 | | | July 1999 — December 2012 |
| 1999 | | | 4,054 | | | | £1.42 | | | November 2002 — December 2012 |
| SAYE | | | 318 | | | | £0.95 | | | November 2002 — April 2005 |
| 1990 US | | | 181 | | | | $3.74 | | | September 1999 — June 2009 |
| 1995 US | | | 191 | | | | $8.61 | | | September 1999 — June 2007 |
|
| Total | | | 6,008 | | | | | | | | | |
|
| | | Whilst they have no present intention of utilising such authority, at the Annual General Meeting to be held on 13 May 2003, the Directors will seek authority from the shareholders to allot shares up to an aggregate nominal value of £3,457,941 (34,579,416 ordinary shares of 10p each) being one third of the issued ordinary shares of the Company. |
| | 1) The Peptide Therapeutics Group plc 1994 Unapproved Share Option Scheme. |
| | 2) Parallel options under the 1995 Scheme existed over 14,015 of the options outstanding at 31 December 2002. Option holders may elect to exercise either option, at which time the alternative option lapses. |
| | | The Group operates an Inland Revenue-approved Save-As-You-Earn (“SAYE”) scheme in the UK and has taken advantage of the exemption given in UITF 17 from recognising a charge in the profit and loss account for the discount on those options. |
| | | At the Annual General Meeting to be held on 13 May 2003, the Directors will seek the authority of shareholders to introduce a new all-employee share plan for US-based employees, which is similar to the SAYE scheme in the UK. |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | 2002 | | | | | | | 2001 | | | | | | | 2000 | |
| | |
|
| | | Share | | | Profit | | | Share | | | Profit | | | Share | | | Profit | |
| | | premium | | | and loss | | | premium | | | and loss | | | premium | | | and loss | |
| | | account | | | account | | | account | | | account | | | account | | | account | |
| Group reserves | | £’000 | | | £’000 | | | £’000 | | | £’000 | | | £’000 | | | £’000 | |
|
| At 1 January | | | 80,598 | | | | (62,246 | ) | | | 76,776 | | | | (49,505 | ) | | | 67,456 | | | | (37,614 | ) |
| Issue of new shares | | | 7,147 | | | | – | | | | 3,822 | | | | – | | | | 9,320 | | | | – | |
| Exchange adjustments | | | – | | | | 1,276 | | | | – | | | | (314 | ) | | | – | | | | (817 | ) |
| Retained profit/(loss) for the year | | | – | | | | 9,575 | | | | – | | | | (12,427 | ) | | | – | | | | (11,074 | ) |
|
| At 31 December | | | 87,745 | | | | (51,395 | ) | | | 80,598 | | | | (62,246 | ) | | | 76,776 | | | | (49,505 | ) |
|
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | 2002 | | | | | | | 2001 | | | | | | | 2000 | |
| | |
|
| | | Share | | | Profit | | | Share | | | Profit | | | Share | | | Profit | |
| | | premium | | | and loss | | | premium | | | and loss | | | premium | | | and loss | |
| | | account | | | account | | | account | | | account | | | account | | | account | |
| Company reserves | | £’000 | | | £’000 | | | £’000 | | | £’000 | | | £’000 | | | £’000 | |
|
| At 1 January | | | 80,464 | | | | 4,719 | | | | 76,642 | | | | 99 | | | | 67,322 | | | | 132 | |
| Issue of new shares | | | 7,147 | | | | – | | | | 3,822 | | | | – | | | | 9,320 | | | | – | |
| Exchange adjustments | | | – | | | | (1,459 | ) | | | – | | | | – | | | | – | | | | – | |
| Retained (loss)/profit for the year | | | – | | | | (1,621 | ) | | | – | | | | 4,620 | | | | – | | | | (33 | ) |
|
| At 31 December | | | 87,611 | | | | 1,639 | | | | 80,464 | | | | 4,719 | | | | 76,642 | | | | 99 | |
|
| 23 | | Reconciliation of movements in Group shareholder’s funds |
| | | | | | | | | |
| | |
|
| | | 2002 | | | 2001 | |
| | | £’000 | | | £’000 | |
|
| Retained profit/(loss) for the period | | | 9,575 | | | | (12,427 | ) |
| Gain/(loss) on foreign currency exchange | | | 1,276 | | | | (314 | ) |
| New share capital subscribed | | | 7,740 | | | | 4,262 | |
|
| Net increase/(decrease) in shareholders’ funds | | | 18,591 | | | | (8,479 | ) |
| Opening shareholders’ funds | | | 27,660 | | | | 36,139 | |
|
| Closing shareholders’ funds | | | 46,251 | | | | 27,660 | |
|
| 24 | | Reconciliation of the operating profit/(loss) to net cash outflow from operating activities |
| | | | | | | | | | | | | |
| | |
|
| | | 2002 | | | 2001 | | | 2000 | |
| | | £’000 | | | £’000 | | | £’000 | |
|
| Operating profit/(loss) | | | 9,911 | | | | (12,242 | ) | | | (9,397 | ) |
| Depreciation and amortisation | | | 2,614 | | | | 1,942 | | | | 2,132 | |
| Increase in stock | | | (52,631 | ) | | | – | | | | – | |
| Increase in debtors | | | (50,602 | ) | | | (3,685 | ) | | | (8,558 | ) |
| Increase in creditors | | | 81,798 | | | | 5,685 | | | | 6,607 | |
| Loss on sale of tangible fixed assets | | | 9 | | | | – | | | | 6 | |
| Exchange differences arising on inter-company balances | | | 1,274 | | | | (499 | ) | | | (691 | ) |
| Other | | | 1,445 | | | | 840 | | | | 694 | |
|
| Net cash outflow from operating activities | | | (6,182 | ) | | | (7,959 | ) | | | (9,207 | ) |
|
| | | In 2001 and 2002, all cash flows arose from continuing operating activities. |
| | |
| (A) | Share premium account
Acambis ordinary shares have a nominal value of 10p. When new shares are issued, either through fundraisings, share option exercises or corporate transactions such as the subscription agreement with Baxter, the price paid for the shares in excess of the 10p nominal value is recognised as share premium.
|
THE INFORMATION CONTAINED IN THIS COLUMN HAS NOT BEEN AUDITED.
35
| | | NOTES TO GROUP FINANCIAL STATEMENTS |
| 25 | | Analysis and reconciliation of net funds/(debt) |
| | | | | | | | | | | | | | | | | | | | | | |
| | | |
|
| | | | 1 January 2001 | | | Cash | | | Non-cash | | | Exchange | | | 31 December 2001 | | |
| | | | | | | flow | | | movements | | | movement | | | | |
| | | | £’000 | | | £’000 | | | £’000 | | | £’000 | | | £’000 | |
| |
|
| | Cash | | | 1,179 | | | | 20,910 | | | | – | | | | 4 | | | | 22,093 | |
| | Liquid resources | | | 19,938 | | | | (19,834 | ) | | | – | | | | 16 | | | | 120 | |
| | Overdraft facility | | | (4,686 | ) | | | – | | | | – | | | | (124 | ) | | | (4,810 | ) |
| | | | | | | | |
| | | | | | | | | | | | | |
| | | | | | | | | 1,076 | | | | | | | | | | | | | |
| | Finance lease | | | (13 | ) | | | (12,725 | ) | | | (1,546 | ) | | | (15 | ) | | | (14,299 | ) |
| |
|
| | Net funds | | | 16,418 | | | | (11,649 | ) | | | (1,546 | ) | | | (119 | ) | | | 3,104 | |
| |
|
| | | | | | | | | | | | | | | | | | | | | | |
| | | |
|
| | | | 1 January 2002 | | | Cash | | | Non-cash | | | Exchange | | | 31 December 2002 | |
| | | | | | | flow | | | movements | | | movement | | | | |
| | | | £’000 | | | £’000 | | | £’000 | | | £’000 | | | £’000 | |
| |
|
| | Cash | | | 22,093 | | | | (9,179 | ) | | | – | | | | (1,242 | ) | | | 11,672 | |
| | Liquid resources | | | 120 | | | | (5 | ) | | | – | | | | (10 | ) | | | 105 | |
| | Overdraft facility | | | (4,810 | ) | | | – | | | | – | | | | 461 | | | | (4,349 | ) |
| | | | | | | | |
| | | | | | | | | | | | | |
| | | | | | | | | (9,184 | ) | | | | | | | | | | | | |
| | Finance lease | | | (14,299 | ) | | | – | | | | (1,118 | ) | | | 1,445 | | | | (13,972 | ) |
| |
|
(A) | Net debt | | | 3,104 | | | | (9,184 | ) | | | (1,118 | ) | | | 654 | | | | (6,544 | ) |
| |
|
| | | Liquid resources at 31 December 2002 of £0.1m represents amounts held by Acambis Inc. as a deposit against property rentals. |
| | | | | | | | | | | | | |
| | |
|
| | | 2002 | | | 2001 | | | 2000 | |
| | | £’000 | | | £’000 | | | £’000 | |
|
| (Decrease)/increase in cash | | | (9,179 | ) | | | 20,910 | | | | (351 | ) |
| (Decrease)/increase in liquid resources | | | (5 | ) | | | (19,834 | ) | | | 1,894 | |
|
| (Decrease)/increase in cash and liquid resources | | | (9,184 | ) | | | 1,076 | | | | 1,543 | |
| Net cash outflow from lease financing | | | – | | | | 13 | | | | 29 | |
| Proceeds from new finance lease | | | – | | | | (12,738 | ) | | | – | |
| Net increase in overdraft facility | | | – | | | | – | | | | (2,502 | ) |
|
| Change in net (debt)/funds arising from cash flows | | | (9,184 | ) | | | (11,649 | ) | | | (930 | ) |
| Non-cash element of finance lease | | | (1,118 | ) | | | (1,546 | ) | | | – | |
| Exchange adjustments | | | 654 | | | | (119 | ) | | | (59 | ) |
|
| Movement in net (debt)/funds | | | (9,648 | ) | | | (13,314 | ) | | | (989 | ) |
| Net funds at 1 January | | | 3,104 | | | | 16,418 | | | | 17,407 | |
|
| Net (debt)/funds at 31 December | | | (6,544 | ) | | | 3,104 | | | | 16,418 | |
|
| | | The minimum annual rentals payable by the Group under non-cancellable operating leases are as follows: |
| | | | | | | | | | | | | | | | | |
| | | Land and buildings | | | Equipment | |
| | |
|
| | | 2002 | | | 2001 | | | 2002 | | | 2001 | |
| | | £’000 | | | £’000 | | | £’000 | | | £’000 | |
|
| Operating leases which expire: | | | | | | | | | | | | | | | | |
| Within one year | | | 31 | | | | – | | | | 13 | | | | – | |
| Within two to five years | | | 1,640 | | | | 962 | | | | – | | | | 6 | |
| After more than five years | | | – | | | | 520 | | | | – | | | | – | |
|
| | | | 1,671 | | | | 1,482 | | | | 13 | | | | 6 | |
|
| | | At 31 December 2002, the Company had no operating leases (2001 — £nil). |
36
| | |
| (A) | Net debt
As a result of the major working capital requirements for the second CDC smallpox vaccine contract, cash and liquid resources at the year-end of £11.8 million was less than our obligations under the finance lease and overdraft facilities. However, this was simply the result of timing differences in payments. Two days following the 2002 year-end our cash was such that net funds of the Group were once again positive. |
THE INFORMATION CONTAINED IN THIS COLUMN HAS NOT BEEN AUDITED.
| | | In March 2000, the Group entered into a sub-lease with Medivir UK Limited in respect of 50% of the facility at Peterhouse Technology Park in the UK. The sub-lease expires in September 2003, and provides that Medivir UK Limited will pay Acambis £0.3m per annum in operating lease rentals relating to land and buildings. In September 1999, the Group entered a further sub-lease with Millennium Pharmaceuticals, Inc. in respect of 25% of the facility at 38 Sidney Street in Cambridge, Massachusetts, USA. This sub-lease expired in December 2002 at which point Acambis re-occupied all of this space. |
| | | At the end of the year, capital commitments contracted but not provided for were £0.1m (2001 — £6.4m). |
| | (c) Pension arrangements
The Group provides pension benefits to all full-time employees on a defined contribution basis. The Company operates a self-administered, Inland Revenue-approved pension scheme for UK Executive Directors. Other employees may operate private personal pension schemes. In the US the Group offers a “401k Savings and Retirement Plan” for all employees, including Executive Directors. The Group pension cost (including 401k costs) for the year was £0.5m (2001 — £0.2m). At the year-end, the Group owed £0.2m (2001 — £0.01m) to the pension schemes. This amount is shown in the balance sheet under “Creditors: amounts falling due within one year”. |
| 27 | | Related party transactions |
| (7b) | | Under the provisions of FRS 8, “Related Party Disclosures”, it is not necessary to disclose related party transactions between the Company, Acambis Research Limited and Acambis Inc. because they are eliminated on preparation of the Group’s financial statements. |
| | | As described in note 14(d), the Group has an interest in the Joint Venture. Since May 1999, Acambis has performed a pre-agreed work programme on behalf of the Joint Venture. Costs incurred by the Group on behalf of the Joint Venture and corresponding turnover received from the Joint Venture have been included in the Group’s financial statements. For the year ended 31 December 2002, the Group has included turnover of £0.3m (2001 — £0.5m) in respect of costs incurred in performing services for the Joint Venture within its Group financial statements. 50% of the loss incurred by Pasteur Mérieux-OraVax in 2002, amounting to £0.2m (2001 — £0.4m), is shown on the face of the Group profit and loss account on page 18. At 31 December 2002, the amounts the Group owed to the Joint Venture amounted to £nil (2001 — £0.5m). Amounts owed by the Joint Venture to the Group at 31 December 2002 were £nil (2001 — £nil). |
| | | In 2002, the Group took the view that, taking into account the increase in its shareholding in Acambis and the presence of a representative from Baxter on the Board, Baxter’s influence on Acambis became significant, and therefore Baxter is a related party. Baxter’s subscriptions in the shares of the Company are set out in note C on page 21, and the Group’s long-term lease-finance facility with Baxter is described within “Financial liabilities” in note 20. The Group made sales to Baxter of £0.7m in 2002 (2001 — £nil), of which £0.7m (2001 — £nil) was unpaid at the year-end, and made purchases of goods and services from Baxter of £45.7m (2001 — £nil), of which £42.7m (2001 — £nil) was unpaid at the year-end. |
| 28 | | Post balance sheet events |
| | | Under a prior agreement with BTG International Limited (“BTG”), Acambis was required to pay to BTG an annual turnover tax of 2% of the Group’s gross turnover on an ongoing basis. Following a decision by the High Court of Justice Queen’s Bence Division in the UK in February 2003, it was confirmed that this turnover tax covers all subsidiaries of the Company. This decision has no effect on the financial statements of the Group. |
| | | On 27 March 2003, Baxter International, Inc. accelerated its fourth and final subscription of equity shares from the original subscription date of no later than 1 June 2003. These shares were traded from 27 March 2003. This subscription of 4.6 million shares at a subscription amount of £7.0m increased Baxter’s shareholding in the Company to 20.6%. |
| | | NOTES TO GROUP FINANCIAL STATEMENTS |
| 29 | | Reconciliation to US accounting principles |
| | | Summary of significant differences between UK GAAP followed by the Group and US GAAP |
| | | The Group’s financial statements have been prepared under UK GAAP, which differs in certain respects from US GAAP. The principal differences between the Group’s accounting policies under UK GAAP and US GAAP are set out below. |
| | | Revenues represent sales and income derived from product sales, licence fees, contract research fees and development milestone payments. Under UK GAAP, these revenues are recognised using the accounting policies as set out in note 1. The Directors have selected the accounting policies they believe to be the most appropriate in the circumstances, as required by FRS 18 “Accounting Policies”. In making this judgment, they have taken into account recent publications by the Accounting Standards Board (“ASB”), specifically the discussion paper entitled “Revenue Recognition” and the proposed additional application note to FRS 5, “Substance of Transactions”. |
| | | For US purposes, the Group adopted Staff Accounting Bulletin (“SAB”) 101 with effect from 1 January 2000. |
| | | Prior to 2001, under UK GAAP, certain licence fees were recognised when paid, where such payments were not refundable. Following the Group’s adoption of SAB101 under US GAAP, where licence fees are not refundable and are not creditable against associated R&D activities, these fees are considered inseparable from the associated R&D effort. As such, those licence fees are deferred and recognised over the period of the licence term or over the period of the R&D agreement. |
| | | Multiple element arrangements |
| | | In November 2001, Acambis was awarded a second contract by the CDC. This contract is divided into two principal components: to manufacture 155 million doses of smallpox vaccine; and to take the vaccine through clinical trials to US Food and Drug Administration (“FDA”) licensure. Under UK GAAP, this contract has been accounted for as a single-element arrangement. Under US GAAP, the Group treats this contract as a multiple element arrangement resulting in a different allocation of revenue compared to the UK GAAP treatment. The Group has determined the fair value of the development and manufacturing portions of the contract and has allocated the total contract value to the development and manufacturing using the relative fair value method as prescribed by Emerging Issues Task Force (“EITF”) Issue No. 00-21, “Accounting for Revenue Arrangements with Multiple Deliverables.” The development portion of the contract consists of the completion of Phase I/II clinical trials, completion of Phase III clinical trials including completion and submission of a Biologics Licenses Application with the FDA and post FDA approval activities. The manufacturing portion consists of the manufacture and delivery of smallpox vaccine to the CDC. The Group recognises revenue for each significant development activity on a straight-line basis over the expected duration of each respective activity unrelated to the costs incurred over that period; this contrasts with the UK GAAP treatment, where revenues are recognised as costs are incurred, using the principles of long-term contract accounting. The Group recognises revenue on the manufacturing portion of the contract as smallpox vaccine is delivered to the CDC and all risks and rewards of ownership have transferred to the CDC. Costs associated with expected re-labelling to be undertaken in the future are being accrued as smallpox vaccine is delivered to the CDC. Costs and revenue associated with providing storage or vaccine disposal services are only included when, and if, it is deemed probable that such services will be performed. |
| (A) | | Compensation costs under variable plan accounting for stock options and SAYE plan discount |
| | | Acambis has granted stock options to employees that will vest upon the attainment of certain targets. Under UK GAAP, there is no accounting for these grants after the initial grant date. Under US GAAP, APB Opinion No. 25, “Accounting for Stock Issued to Employees” (“APB25”), the Company would be required to follow variable plan accounting for these grants and measure compensation expense as the difference between the exercise price and the fair market value of the stock during each accounting period over the vesting period of the options. Increases in fair market value of the stock would result in a charge to operations and decreases in the fair market value of the stock would result in a credit to operations, subject to the cumulative amount previously expensed. |
| | | Under UK GAAP, Acambis has taken advantage of the exemption provided by UITF 17, not to recognise any compensation charge in respect of options granted under SAYE plans. Under US GAAP, in accounting for new offers made since 24 January 2002, Acambis would follow the requirements of pronouncement EITF 00-23 “Issues relating to the Accounting for Stock Compensation under APB25 and FIN44” (Financial Accounting Standards Board Interpretation Number (“FIN”) 44, “Accounting for Certain Transactions Involving Stock Compensation”, (“FIN44”)) which does not permit such an exemption in respect of plans with certain characteristics. The compensation charge under US GAAP in respect of such plans would be calculated as the difference between the market price of the shares at the date of grant and the exercise price of the option and would be recorded on a straight-line basis over the savings period. |
38
| | | |
| (A) | | Compensation costs under variable plan accounting for stock options and SAYE plan discount
Under US GAAP, a company is required to put a charge through its profit and loss account for share options not yet exercised but that are likely to be exercised. The charge is calculated as the difference between the market value of the shares at the year-end and the exercise price. |
THE INFORMATION CONTAINED IN THIS COLUMN HAS NOT BEEN AUDITED.
| | | The table below shows the additional charge or credit that would have been made to the Group profit and loss account under US GAAP. |
| | | | | | | | | | | | | |
| | | Year ended 31 December | |
| | |
| |
| | | 2002 | | | 2001 | | | 2000 | |
| | | £'000 | | | £'000 | | | £'000 | |
|
| Charged to the Group profit and loss account | | | 1,212 | | | | 5,210 | | | | 312 | |
|
| | | Provision against own shares held |
| | | Under UK GAAP, own shares held by the Acambis Employees’ Trustees Limited are accounted for as fixed asset investments and provisions made against these investments to reduce them to the recoverable amount are charged in the profit and loss account. Under US GAAP, own shares are recorded at cost, are not reviewed for impairment and are accounted for within shareholders’ equity. |
| | | Purchase price accounting for in-process R&D and goodwill amortisation |
| | | During 1999, Acambis acquired Acambis Inc. Under both UK GAAP and US GAAP, goodwill is identified as being the amount that the fair value of the consideration exceeds the fair value of assets acquired. However, under UK GAAP no value is attributed to in-process R&D, which is not the case under US GAAP. Under US GAAP, in accordance with APB Opinion No.16, “Business Combinations”, and No.17, “Intangible Assets”, in-process R&D is separately identified and analysed to determine the fair market value at the date of acquisition. In-process R&D is identified in accordance with the definition within Statement of Financial Accounting Standard (“SFAS”) No.2, “Accounting for Research and Development Costs”. Following identification of qualifying R&D projects, their value was determined by estimating the costs to develop the purchased in-process R&D into commercially viable products, estimating the resulting net cash flows from the projects and discounting the net cash flows to their present value. |
| | | As a result of the valuation of in-process R&D under US GAAP, the fair value of assets acquired is different from that calculated under UK GAAP, resulting in differing values of goodwill. The table below shows the difference in the goodwill calculation and the associated amortisation charge in the Group profit and loss account. Under US GAAP, in-process R&D is written off to the profit and loss account when incurred. |
| | | Under UK GAAP, Acambis amortises goodwill on a straight-line basis over an estimate of the time the Group is expected to benefit from it. Until 1 January 2002, this was also Acambis’ accounting policy under US GAAP. Following the provisions of SFAS 142, “Goodwill and Other Intangible Assets” (“SFAS 142”), the carrying value of goodwill in US GAAP was frozen and became subject to annual impairment reviews. The transitional impairment review carried out on adoption of SFAS 142 did not reveal any indication of impairment. |
| | | | | | | | | | | | | | | | | |
| | | Year ended 31 December 2002 | | | Year ended 31 December 2001 | |
| | |
| | |
| |
| | | UK GAAP | | | US GAAP | | | UK GAAP | | | US GAAP | |
| | | £'000 | | | £'000 | | | £'000 | | | £'000 | |
|
| Goodwill on acquisition included on balance sheet (cost) | | | 18,055 | | | | 6,155 | | | | 18,055 | | | | 6,155 | |
| Amortisation brought forward | | | (3,210 | ) | | | (1,094 | ) | | | (2,006 | ) | | | (684 | ) |
| Amortisation charged to Group profit and loss account for the year | | | (1,204 | ) | | | – | | | | (1,204 | ) | | | (410 | ) |
|
| Goodwill (net book value) | | | 13,641 | | | | 5,061 | | | | 14,845 | | | | 5,061 | |
|
| | | Capitalisation of interest |
| | | Under UK GAAP, Acambis’ accounting policy is that interest is not capitalised. US GAAP would require interest incurred as part of the cost of constructing fixed assets to be capitalised and amortised over the life of the asset. |
| | | NOTES TO GROUP FINANCIAL STATEMENTS |
| 29 | | Reconciliation to US accounting principles (continued) |
| | | Reconciliation of net loss from UK GAAP to US GAAP |
| | | Based on the differences detailed above, the following table shows the reconciliation of the Group’s net loss for the past three years: |
| | | | | | | | | | | | | |
| | | Year ended 31 December | |
| | |
| |
| | | 2002 | | | 2001 | | | 2000 | |
| | | £'000 | | | £'000 | | | £'000 | |
|
| Net profit/(loss) as reported under UK GAAP | | | 9,575 | | | | (12,427 | ) | | | (11,074 | ) |
| Adjustments for: | | | | | | | | | | | | |
| Revenue recognition | | | (24,124 | ) | | | 1,995 | | | | (570 | ) |
| Compensation (costs)/credit under variable plan accounting for stock options and SAYE plan discount | | | (1,212 | ) | | | (5,210 | ) | | | (312 | ) |
| Provision against own shares held | | | 420 | | | | – | | | | – | |
| Amortisation charge for goodwill | | | 1,204 | | | | 794 | | | | 794 | |
| Capitalisation of interest | | | 560 | | | | – | | | | – | |
|
| Net loss as reported under US GAAP before cumulative effect of accounting change | | | (13,577 | ) | | | (14,848 | ) | | | (11,162 | ) |
| Cumulative effect of accounting change: revenue recognition | | | – | | | | – | | | | (2,572 | ) |
|
| Net loss as reported under US GAAP after cumulative effect of accounting change | | | (13,577 | ) | | | (14,848 | ) | | | (13,734 | ) |
|
| | | Loss per share under US GAAP |
| | | Under US GAAP, the Group would compute loss per share under SFAS No.128 “Earnings per Share” (“SFAS 128”). Under SFAS 128, basic net loss per ordinary share is computed using the weighted average number of shares of common stock outstanding during the period. Under US GAAP, diluted net loss per ordinary share for Acambis is the same as basic net loss per ordinary share, as the effects of the Company’s potential ordinary share equivalents are anti-dilutive. Under UK GAAP the basis of calculation is the same. |
| | | Because the Group has recorded a net profit under UK GAAP, certain securities which are dilutive under UK GAAP are anti-dilutive under US GAAP. The net loss per share under US GAAP is presented below: |
| | | | | | | | | | | | | |
| | | Year ended 31 December | |
| | |
| |
| | | 2002 | | | 2001 | | | 2000 | |
| | | £'000 | | | £'000 | | | £'000 | |
|
| Basic loss per ordinary share in pence before cumulative effect of accounting change | | | (14.1 | ) | | | (16.3 | ) | | | (14.0 | ) |
| Basic loss per share in pence of cumulative effect of accounting change | | | – | | | | – | | | | (3.2 | ) |
|
| Basic loss per share in pence | | | (14.1 | ) | | | (16.3 | ) | | | (17.2 | ) |
|
| Shares used in computing basic loss per ordinary share | | | 96,101,507 | | | | 91,027,463 | | | | 79,638,484 | |
| Diluted loss per ordinary share in pence | | | (14.1 | ) | | | (16.3 | ) | | | (17.2 | ) |
|
| Shares used in computing diluted loss per ordinary share | | | 96,101,507 | | | | 91,027,463 | | | | 79,638,484 | |
|
| Anti-dilutive securities, not included above | | | 3,100,226 | | | | 2,294,366 | | | | 1,565,251 | |
|
| | | Anti-dilutive securities represent stock options outstanding, which have not been included in the calculation of loss per ordinary share as the impact of including such shares in the calculation of loss per share would be anti-dilutive. |
| | | Income and expense transactions with the same party |
| | | In accordance with EITF Issue 02-16, “Accounting by a Reseller for Cash Consideration Received from a Vendor”, certain transactions with the same party, which are shown gross under UK GAAP, are netted under US GAAP. These adjustments have no impact on net income. The individual line items affected are cost of sales (which under US GAAP in 2002 is £48.0m) and R&D costs included within operating expenses (2002 — £21.5m). |
| | | As discussed in note 4, the Group completed the disposal of its interest in its drug discovery business, Mimetrix, during 2000. Under US GAAP, the operating results of discontinued operations should be reported separately in a single caption after income from continuing operations, net of income tax effects, for all periods presented. This presentation difference has no impact on the net loss for the periods presented. |
Reconciliation of shareholders’ equity from UK GAAP to US GAAP
| | | | | | | | | | | | | |
| | | As at 31 December | |
| | |
| |
| | | 2002 | | | 2001 | | | 2000 | |
| | | £’000 | | | £’000 | | | £’000 | |
|
| Shareholders’ equity as reported under UK GAAP | | | 46,251 | | | | 27,660 | | | | 36,139 | |
| Goodwill | | | (8,580 | ) | | | (9,784 | ) | | | (10,578 | ) |
| Revenue recognition (cumulative) | | | (25,272 | ) | | | (1,147 | ) | | | (3,142 | ) |
| Capitalisation of interest | | | 560 | | | | – | | | | – | |
| Reclassification of net book value of investment in Group shares | | | (797 | ) | | | (1,252 | ) | | | (1,445 | ) |
|
| Shareholders’ equity as reported under US GAAP | | | 12,162 | | | | 15,477 | | | | 20,974 | |
|
Group statement of cash flows
The Group statement of cash flows prepared under UK GAAP presents substantially the same information as that required under US GAAP by SFAS No.95, “Statement of Cash Flows”. These standards differ, however, with regard to classification of items within the statements and the definition of cash and cash equivalents.
Under UK GAAP, cash comprises only cash in hand and deposits repayable on demand. Deposits are repayable on demand if they can be withdrawn at any time without notice and without penalty or if a maturity or period of notice of not more than 24 hours or one working day has been agreed. Under US GAAP, cash equivalents are short-term highly liquid investments, with a maturity of three months or less at inception, that are readily convertible to known amounts of cash and present insignificant risk of changes in value because of changes in interest rates.
Under UK GAAP, cash flows are presented separately for operating activities, returns on investments and servicing of finance, taxation, capital expenditure and financial investment, management of liquid resources and financing activities. US GAAP requires only three categories of cash flow activity to be reported: operating, investing and financing. Cash flows from taxation and returns on investments and servicing of finance under UK GAAP would, with the exception of dividends paid, be shown under operating activities under US GAAP. The payment of dividends and the payment to acquire own shares (treasury stock) would be included as a financing activity under US GAAP. Management of liquid resources under UK GAAP would be included as cash and cash equivalents under US GAAP to the extent that the amounts involved have a maturity of less than three months and are convertible into known amounts of cash. Summary statements of cash flow presented under US GAAP are given below:
| | | | | | | | | | | | | |
| | | Year ended 31 December | |
| | |
| |
| | | 2002 | | | 2001 | | | 2000 | |
| | | £’000 | | | £’000 | | | £’000 | |
|
| Net cash used in operating activities | | | (5,459 | ) | | | (6,968 | ) | | | (8,782 | ) |
| Net cash used in investing activities | | | (11,460 | ) | | | 5,803 | | | | (4,488 | ) |
| Net cash provided by/(used in) financing activities | | | 7,740 | | | | 16,987 | | | | 12,819 | |
| Effect of foreign exchange on cash and cash equivalents | | | (1,242 | ) | | | – | | | | – | |
|
| (Decrease)/increase in cash and cash equivalents | | | (10,421 | ) | | | 15,822 | | | | (451 | ) |
| Opening cash and cash equivalents | | | 22,093 | | | | 6,271 | | | | 6,722 | |
|
| Closing cash and cash equivalents | | | 11,672 | | | | 22,093 | | | | 6,271 | |
|
Statement of other comprehensive income
| | | | | | | | | | | | | |
| | |
| |
| | | 2002 | | | 2001 | | | 2000 | |
| | | £’000 | | | £’000 | | | £’000 | |
|
| Net loss | | | (13,577 | ) | | | (14,848 | ) | | | (13,734 | ) |
| Other comprehensive income: foreign currency translation adjustment net of tax | | | 1,276 | | | | (314 | ) | | | (817 | ) |
|
| Total comprehensive income | | | (12,301 | ) | | | (15,162 | ) | | | (14,551 | ) |
|
NOTES TO GROUP FINANCIAL STATEMENTS
31 December 2002
| 29 | | Reconciliation to US accounting principles (continued) |
| | | Recently issued pronouncements |
| | | In June 2002, the Financial Accounting Standards Board (“FASB”) issued SFAS No.146, “Accounting for costs associated with exit or disposal activities” (“SFAS 146”). SFAS 146 addresses financial accounting and reporting for costs associated with exit or disposal activities and nullifies EITF 94-3, “Liability recognition for certain employee termination benefits and other costs to exit an activity (including certain costs incurred in a restructuring)”. SFAS 146 requires that a liability for a cost associated with an exit or disposal activity be recognised when the liability is incurred. Under EITF 94-3, a liability for an exit cost was recognised at the date of the commitment to an exit plan. SFAS 146 states that a commitment to a plan, by itself, does not create an obligation that meets the definition of a liability. Therefore, SFAS 146 eliminates the definition and requirement for recognition of exit costs in EITF 94-3. It also established that fair value is the objective for initial measurement of the liability. SFAS 146 is to be applied prospectively to exit or disposal activities initiated after 31 December 2002. Management does not expect the adoption of SFAS 146 to have a material effect on its consolidated financial statements. |
| | | In November 2002, the FASB issued FASB Interpretation Number (“FIN”) No.45, “Guarantor’s accounting and disclosure requirements for guarantees, including indirect guarantees of indebtedness of others” (“FIN 45”). FIN 45 requires the guarantor to recognise, at the inception of a guarantee, a liability for the fair value of the obligation undertaken in issuing the guarantee. It also elaborates on the disclosure to be made by a guarantor in its financial statements about its obligation under certain guarantees that it has issued and to be made in regard of product warranties. The initial recognition and movement provisions of this interpretation are applicable on a prospective basis to guarantees issued or modified after 31 December 2002, while the provisions of the disclosure requirements are effective for financial statements of interim or annual periods ending after 15 December 2002. Management does not expect the adoption of FIN 45 to have a material effect on its consolidated financial statements. |
| | | In November 2002, the EITF reached a consensus on “Accounting for revenue arrangements with multiple deliverables” (“Issue 00-21”). This addresses certain aspects of the accounting by a vendor for arrangements under which it will perform multiple revenue-generating activities. Specifically, Issue 00-21 addresses how to determine whether an arrangement involving multiple deliverables contains more than one unit of accounting and how arrangement consideration should be measured and allocated to the separate units of accounting in the arrangement. The provisions of Issue 00-21 are effective for revenue arrangements entered into in fiscal periods beginning after 15 June 2003, with early application permitted. Management have applied the provisions of EITF 00-21 to existing multiple element arrangements. |
| | | In January 2003, the FASB issued FIN No.46, “Consolidation of variable interest entities” (“FIN 46”). FIN 46 clarifies the application of Accounting Research Bulletin No. 51, “Consolidated financial statements,” to certain entities in which equity investors do not have the characteristics of a controlling financial interest or do not have sufficient equity at risk for the entity to finance its activities without additional subordinated financial support from other parties. FIN 46 applies immediately to variable interest entities created after 31 January 2003, and to variable interest entities in which an enterprise obtains an interest after that date. It applies in the first fiscal year or interim period beginning after 15 June 2003, to variable interest entities in which an enterprise holds a variable interest that it acquired before 1 February 2003. FIN 46 applies to public enterprises as of the beginning of the applicable interim or annual period. Management does not expect the adoption of FIN 46 to have a material effect on its consolidated financial statements. |
| (A) | | FURTHER INFORMATION REQUIRED BY FORM 20-F
(unaudited) |
| | | Management’s discussion and analysis of financial condition and results of operations |
| (4b) | | Overview
The financial statements contained within this Annual Report and Form 20-F have been prepared in accordance with UK GAAP. A reconciliation to US GAAP is set out in note 29 to the financial statements. The principal differences between UK GAAP and US GAAP accounting arise on revenue recognition timing differences (which has resulted in lower revenue being recorded under US GAAP in 2002 which will reverse in 2003 and 2004), accounting for stock options and the treatment of goodwill. These differences are described within note 29 to the financial statements. |
| | | Critical accounting policies
The preparation of our financial statements requires us to make estimates and judgments that affect the reported amount of net assets at the date of our financial statements and the reported amounts of revenues and expenses during the period. |
| | | Critical accounting policies are those that have a significant impact on the Group’s results and require the most difficult, subjective or complex judgments by management. For a full description of our accounting policies please refer to note 1. Our critical accounting policies include the following: |
42
| | | |
| (A) | | Further information required by Form 20-F
This section contains the information that the US Securities and Exchange Commission requires in a Form 20-F that does not appear elsewhere in our Annual Report. |
| | | Revenue on long-term contracts is recognised according to estimates by management of the progress of the contract and the estimated total costs. This requires that the extent of progress towards completion of contracts may be estimated with reasonable certainty. Revisions in these estimates may cause the Group’s revenues to fluctuate from period to period. |
| | | The Group’s US GAAP revenue recognition accounting policy for multiple element arrangements, which is described more fully on page 38, requires the determination of the fair value of the individual elements of multiple element arrangements and the allocation of the total contract value amongst the elements identified using the relative fair value method. Under the Group’s UK GAAP accounting policy, contracts are accounted for as long-term contracts and are only segmented into multiple elements where the Group is capable of attributing a reliable fair value to each element by reference to individual transactions (which might be the case, for instance, where separate tenders were submitted for each element). The Group therefore makes significant judgments in determining whether a particular multiple element arrangement is capable of segmentation under the requirements of US GAAP and following best practice under UK GAAP. Where, under US GAAP, segmentation is required, significant assumptions are made in selecting appropriate similar contracts to provide evidence of the fair value of the individual elements. Changes in estimates of fair value of elements in an arrangement would have a significant impact on the timing of revenue recognition. |
| | | Differences in revenue recognition policies under UK GAAP and US GAAP give rise to a significant reconciling adjustment. The size and direction of this reconciling amount in the reconciliation of UK to US GAAP net income will vary considerably from period to period based on the nature of contract activity in the period. |
| | | Deferred tax
The Group has significant brought forward operating losses in a particular tax jurisdiction, against which, at 31 December 2002, the Group has maintained a full valuation allowance. In determining the appropriateness of this valuation allowance, estimates are made of the Group’s ability to use these losses, based on the estimated taxable profit or loss in that jurisdiction, which include estimates of future revenues and costs, as well as the jurisdiction in which they will arise. Revisions in these estimates may cause the valuation allowance recorded and the Group’s tax charge to fluctuate from period to period. |
| | | Valuation of long-lived assets and goodwill
Depreciation and amortisation rates are determined based on management estimates of the expected useful economic lives of the assets concerned. The most appropriate life is determined separately for each asset on acquisition, subject to prescribed ranges of asset lives, as disclosed in note 1 to the financial statements. Changes to the useful lives selected could have a material impact on the Group’s net profit. |
| | | The Group assesses the carrying value of long-lived assets and goodwill whenever events or changes in circumstances indicate that such carrying value may not be recoverable. When we determine that the carrying value of long-lived assets and goodwill may not be recoverable, we measure any impairment based on a projected discounted cash flow method using a discount rate determined by our management to be commensurate with the risk inherent in our current business model. This review is based upon our projections of anticipated timing and amount of future cash flows and appropriate discount rates. While we believe that our assumptions are appropriate, such estimates and assumptions could differ materially from actual results and accordingly result in a material impact on the carrying value of long-lived assets and goodwill. |
| (5a) | | Results of operations |
| (5d) | | The information contained within the financial review on pages 14 and 15 covers the results for the year ended 31 December 2002 (prepared under UK GAAP) compared to the prior year ended 31 December 2001. The following information compares the results (under UK GAAP) for the year ended 31 December 2001 to the prior year ended 31 December 2000, and the Group’s financial position at 31 December 2002 and 2001. |
| | | 2001 year versus 2000 year
The financial results for the year 2000 include data for the three-month period ended 31 March 2000 for the discontinued operation of Mimetrix Limited. |
| | | Turnover for the year 2001 was £8.9m (2000 — £6.3m), which comprised R&D funding primarily from ongoing contracts with the CDC and Aventis Pasteur. The increase in revenues reflects a full year of revenue receivable from the first contract with the CDC in 2001 compared to only three months in 2000. |
| (5c) | | Expenditure on R&D increased in 2001 to £17.7m (2000 — £12.7m). There was an increase in the number of product programmes, each of which has continued to advance towards the next stage of development and/or clinical trials. Our share of the expenditure on the H. pylori joint venture with Aventis Pasteur was lower in 2001 at £0.4m (2000 — £2.1m), as expenditure on the project was largely restricted to continuing a number of small clinical trials. |
| | | FURTHER INFORMATION REQUIRED BY FORM 20-F |
| | | Results of operations (continued)
Administrative costs increased during 2001 to £3.5m (2000 — £2.9m), primarily as a result of expansion of the Company’s activities, in particular the reactivation of the manufacturing facility. Administrative costs include £1.2m (2000 — £1.2m) of amortisation of the goodwill which arose as a result of the acquisition of Acambis Inc. in 1999. Interest receivable decreased marginally to £0.9m in 2001 (2000 — £1.0m), primarily as a result of lower rates of interest available in the period. Interest payable of £0.2m (2000 — £0.2m), relating entirely to the Arilvax® overdraft facility, remained constant. During 2001, an exchange loss of £0.1m (2000 — £0.3m) arose as a result of the revaluation of the amount outstanding under the US dollar-denominated Arilvax® facility. |
| | | In accordance with FRS 11, “Impairment of Fixed Assets and Goodwill”, during 2001 the Group recorded an exceptional loss of £0.4m (2000 — £0.7m) relating to the impairment write-down of the investment held in Medivir AB, which the Group acquired on the sale of the Mimetrix business in 2000. |
| | | During 2001, the Group elected to claim an R&D tax credit of £0.1m (2000 — £nil) in relation to qualifying R&D expenditure in the UK. |
| | | The net loss for the year increased to £12.4m (2000 — £11.1m), primarily as a result of increased R&D and operating costs at the new Massachusetts manufacturing facility, both of which were largely offset by increased turnover. |
| (4b) | | Liquidity and capital resources |
| (5b) | | The Group had aggregate cash and liquid resources of £11.8m at 31 December 2002 (2001 — £22.2m), a decrease of £10.4m since the start of the year (2001 — increase of £1.1m). During the year, Acambis received £7.7m (2001 — £4.3m) from the issue of new shares, primarily being attributable to the net proceeds receivable from the third equity subscription for new shares by Baxter. Cash used by operations during the year, principally to support R&D, was £6.2m (2001 — £8.0m). |
| | | During 2002, Acambis made additions of short leasehold land and buildings of £5.0m (2001 — £6.9m, 2000 — £0.2m), acquired £4.5m of laboratory and manufacturing equipment (2001 — £2.7m, 2000 — £0.1m) and £1.2m of office equipment (2001 — £0.4m, 2000 — £0.1m). Additions in 2002 and 2001 were primarily in relation to the reactivation of our manufacturing facility. |
| | | From incorporation through to 31 December 2002, Acambis has financed its operations primarily from equity issuances totalling £85.3m, licence fees and milestone payments totalling £3.1m, R&D contract fees and sales of product totalling £101.6m and government grants totalling £3.9m. At 31 December 2002, Acambis held investments in Medivir AB (market value at 31 December 2002 of £0.3m, 2001 — £0.4m) and in Acambis ordinary shares through Acambis’ Employee Share Ownership Trust (market value at 31 December 2002 of £1.6m, 2001 — £2.5m). Under the terms of the strategic alliance set up with Baxter in 2000, the Group has received cash of £7.0m in 2002 (2001 — £3.5m, 2000 — £10.4m) in exchange for new shares in Acambis. On 27 March 2003, the Group issued 4.6 million new shares in relation to the final subscription to Baxter. |
| | | At 31 December 2002, the balance on the Arilvax® overdraft facility was £4.3m ($7.0m) (2001 — £4.8m, 2000 — £4.7m). At 31 December 2002, the Group did not have any undrawn borrowing facilities in respect of this overdraft facility (2001 — £nil, 2000 — £nil). Given certain circumstances, this facility which is fully described in note (d) on page 46, “Evans Vaccines agreement”, may be repayable within one year. In December 2001, the Group secured lease financing for up to $40m (c.£28m) with Baxter in respect of Acambis’ manufacturing facility. At 31 December 2002, the Group had an outstanding liability of £14.0m ($22.5m) under this financing facility (2001 — £14.3m, 2000 — £nil). At 31 December 2002, the Group had $17.5m (c. £10.9m) undrawn borrowing facilities in respect of this financing facility (2001 — £13.2m, 2000 — £nil). The repayment terms of this facility are described in note 18. |
| | | The Group does not have any other financial liabilities. |
| | | Since inception, Acambis’ cash expenditures have exceeded revenues on an annualised basis. Acambis’ future capital requirements will depend on many factors, including, but not limited to, the expenditure required to maintain the manufacturing facility, the progress of R&D programmes, pre-clinical and clinical testing, the time and costs involved in obtaining regulatory approvals, the cost of filing, prosecuting and enforcing any patent claims and other intellectual property rights, competing technological and market developments, changes in Acambis’ existing research relationships, the ability of Acambis to establish collaborative arrangements, receipt of any licence fees and royalties and the acquisition of additional facilities and capital expenditure. |
| | | As a result of the $40m (c. £28m) lease-financing facility secured in December 2001 and the award of the second US Government smallpox vaccine contract in November 2001, Acambis believes that there will be sufficient cash to fund its operations through 2003. Changes in R&D plans or other events affecting Acambis’ operations, however, may result in accelerated or unexpected expenditures. If additional funds are raised by issuing equity securities, dilution to existing shareholders may result and future investors may be granted rights superior to those of existing shareholders. |
| (5c) | | Collaboration agreements |
| (10c) | | (a) US CDC agreements
The Group has been awarded two distinct contracts (“Contract 1” and “Contract 2”) by the CDC to develop and manufacture smallpox vaccines for the purposes of countering the threat of bioterrorism. |
| | | Contract 1
This contract was awarded in September 2000, and incorporates two elements. First, to develop a new smallpox vaccine and, second, to produce and maintain a stockpile of vaccine doses. At the time of the initial award, the estimated value of the 20-year contract was $343m. This value made certain assumptions concerning price per dose, the shelf-life of the vaccine and that a 40 million-dose stockpile of the vaccine would be produced and maintained for 20 years. Following the terrorist events of 11 September 2001, the CDC issued a change order, increasing the number of doses to be delivered from 40 million to 54 million. |
| | | The Group is required to sustain a production capability throughout the 20-year life of the contract, both to replace outdated doses and to be able to respond to increased demand as required. The exclusive commercial rights to the smallpox vaccine developed under this contract with the CDC are retained by Acambis. |
| | | During 2002, the Group recorded turnover of £17.7m (2001 — £5.7m, 2000 — £0.6m) for R&D costs incurred by the Group and funded by the CDC. |
| | | Contract 2
In November 2001, Acambis was awarded a second contract by the CDC to develop, manufacture, deliver and store 155 million doses of smallpox vaccine. The value of this contract, which carries a fixed price, is $428m (c.£270m). Contract 2 is divided into two principal components: funding to take the vaccine through clinical trials to US FDA licensure; and manufacture of the vaccine. The funding for the R&D element will be received over 2002 and 2003, whereas the funding for the manufacture will be received on delivery of the vaccine, which started during 2002 and is continuing into 2003. |
| | | Turnover has been recorded in 2002 in relation to this contract of £58.2m (2001 — £nil, 2000 — £nil). |
| | | (b) Baxter agreements
In December 2000, Acambis entered into the following series of agreements with Baxter: |
| • | | Baxter has invested £27.8m in new Acambis equity. This subscription was payable in four instalments between December 2000 and, at the latest, June 2003 at an average price of 130p per share. The first instalment of £10.4m was made in December 2000, the second instalment of £3.5m was made in June 2001 and the third instalment of £7.0m was made in June 2002. The fourth and final instalment totalling £7.0m was made on 27 March 2003; |
| • | | Acambis has the exclusive rights to manufacture components of certain of Baxter’s vaccines at Acambis’ manufacturing facility; |
| • | | Acambis granted Baxter an option to be its marketing partner for the yellow fever vaccine, Arilvax®, in the US (subject to approval from PowderJect Pharmaceuticals plc (“PowderJect”)); and |
| • | | Baxter licensed to Acambis the rights to use Baxter’s novel Vero-cell technology in exchange for royalty payments to Baxter. This currently applies to the production of Acambis’ ChimeriVax-JE, ChimeriVax-West Nile and smallpox vaccines. |
| | | Under the terms of the agreement entered into in December 2000, in 2002, the Group received income of £1.3m (2001 — £nil) in relation to Baxter’s share of the commissioning of Acambis’ manufacturing plant. This income has been netted off against R&D costs on the face of the profit and loss account. No turnover was recorded in 2002 (2001 — £nil, 2000 — £nil) under this agreement. |
| | | In November 2001, Acambis entered into a sub-contract with Baxter for the purchase of crude bulk smallpox vaccine and the subsequent assembly of various components into multi-dose kits of smallpox vaccine. |
| | | In December 2001, Acambis entered into a long-term lease-finance facility with Baxter in respect of Acambis’ manufacturing plant. Further details of this facility are described within “Financial liabilities” in note 20. |
| | | In December 2002, Acambis entered into a distribution, manufacturing and licence agreement with Baxter, giving Baxter exclusive distribution rights for ACAM2000 smallpox vaccine in all countries of the world excluding the US and the UK. In 2002, turnover of £0.7m (2001 — £nil, 2000 — £nil) was recorded under this agreement. |
| | | (c) Aventis Pasteur agreements |
| | | H. pylori
The arrangements relating to the Joint Venture, called Pasteur Mérieux-OraVax, are described in notes 14(d) and 27. The Group’s share of the net liabilities of Pasteur Mérieux-OraVax at 31 December 2002 was £0.2m (2001 — net assets of £0.07m, 2000 — net liabilities of £0.04m). During 2002, the Group recorded turnover of £0.3m (2001 — £0.5m, 2000 — £2..4m) for R&D costs incurred by the Group and funded by Pasteur Mérieux-OraVax. |
| | | The Group will share any revenues from sales of the final product in equal proportions with Aventis Pasteur. |
| | | FURTHER INFORMATION REQUIRED BY FORM 20-F |
| | | ChimeriVax-Dengue
The Group has a licensing and collaboration agreement with Aventis Pasteur to employ the ChimeriVax™ technology to develop a vaccine against dengue fever. Aventis Pasteur fully funds all R&D costs incurred on the project. Previously, Aventis Pasteur also funded the Group’s Japanese encephalitis project, the rights for which were returned to Acambis during 2000. During 2002, the Group has recorded turnover of £3.3m (2001 — £2.6m, 2000 — £3.0m) for R&D costs incurred by the Group and funded by Aventis Pasteur under this agreement. |
| | | The Group has the ability to receive clinical and regulatory milestones through to product licensure in addition to royalties on sales of the actual product. |
| | | (d) Evans Vaccines agreement
In September 1999, the Group entered into an agreement with Medeva Pharma Limited to obtain regulatory approval in the US for Arilvax®. Medeva Pharma Limited assigned this agreement to Evans Vaccines Limited, a wholly-owned subsidiary of PowderJect, in October 2000. The Group is funding 100% of the costs of the clinical trials and regulatory submission. As described in note 18, “Creditors: amounts falling due within one year”, the costs are being partly financed through an overdraft facility up to a maximum of $7m, being underwritten by PowderJect. PowderJect has granted to Acambis 100% of the marketing rights to Arilvax® in the US, whilst still retaining an option to buy back 50% of the profits from the US sales in return for refunding to the Group the costs the Group has incurred on the Arilvax® programme. No turnover was recorded in 2002 (2001 — £nil, 1999 — £nil) under this agreement. |
| (10e) | | UK tax consequences of owning Acambis shares |
| | | Taxation of dividends
There is no withholding tax on dividends paid by a UK company. Under the provisions of the income tax convention currently in force between the UK and the US, a US holder who is an individual or a corporate portfolio holder, who does not own 10% or more of the Acambis shares (a “Corporate Portfolio Holder”), will be entitled to receive a payment from the UK Inland Revenue equal to the tax credit in respect of the dividend (the “Tax Treaty Payment”) minus a withholding of 15% of the aggregate of the dividend and the tax credit (one-ninth of the dividend), meaning that the amount of the withholding will effectively eliminate the amount of Tax Treaty Payment. Thus, a US individual or US Corporate Portfolio Holder who is entitled to an £80 dividend paid by Acambis will receive £80. |
| | | UK taxation of capital gains
A US holder who is not resident, or ordinarily resident, for tax purposes in the UK will not be liable for UK tax on capital gains on the disposal of Acambis shares unless the US holder carries on a trade, profession or vocation in the UK through a branch or agency and the Acambis shares are or have been used by, held by, or acquired for use by or for the purposes of such trade, profession, vocation, branch or agent. |
| | | UK inheritance tax on estates and gifts
The estate and gift tax convention in force between the US and the UK provides that the UK tax to which the convention applies is capital transfer tax and that it will also apply to identical or substantially similar taxes which are imposed subsequently. Capital transfer tax in the UK has been replaced by inheritance tax. It is understood that, in practice, the US tax authorities and the UK Inland Revenue apply the convention on the basis that inheritance tax has replaced capital transfer tax as the tax to which the convention now applies, although the convention has not been amended to that effect. |
| | | On the basis of that practice, Acambis shares held in the US by an individual who is domiciled for the purposes of the estate and gift tax convention in the US and is not for the purposes of the convention a national of the UK, will not be subject to inheritance tax on the individual’s death or on a transfer of the Acambis shares during the individual’s lifetime. However, special rules apply in the case of Acambis shares held in trust or as part of the business property of a permanent establishment in the UK or related to the fixed base in the UK of a person providing independent personal services. |
| | | UK stamp duty and stamp duty reserve tax
Any transfer of Acambis shares will result in the stamp duty liability at the rate of 0.5% (rounded to the nearest £5) of the consideration (which liability is generally payable by the purchaser of the Acambis shares). There is no charge to ad valorem stamp duty on gifts. On a transfer of Acambis shares from a nominee to the beneficial owner, if the nominee has at all times held the Acambis shares on behalf of the transferee, under which no beneficial interest passes and which is neither a sale, nor arises under or following a contract of sale, nor is in contemplation of sale, a fixed £5 stamp duty will be payable. |
| | | Stamp duty reserve tax, generally at a rate of 0.5% on the consideration, is currently payable on any agreement to transfer ordinary shares or any interest therein unless: |
| • | | an instrument transferring the shares is duly executed and stamped within the appropriate time limits; and |
| • | | stamp duty, generally at a rate of 0.5% (rounded to the nearest £5) of consideration (or part thereof), is paid. |
| | | Increased UK stamp duty and stamp duty reserve tax charges will apply if the Acambis shares are issued or transferred to a custodian for a clearing system or to a depositary who issues depositary receipts in respect of such shares. These are generally at the rate of 1.5% of the consideration paid or the market value of the Acambis shares depending on the circumstances. |
| | | The above summary is not intended to constitute a complete analysis of all of the UK tax consequences of the ownership or disposition of Acambis shares. This discussion is included for general information purposes only and may not apply to a particular stockholder in light of such stockholder’s particular circumstances. Stockholders are urged to consult their own tax advisers as to the particular tax consequences to them of the ownership or disposition of Acambis shares, including the application of state, local and other foreign tax laws. |
| (4d) | | Description of property |
| | | The following table summarises the premises which Acambis currently leases: |
| | | | | | | | | | | |
| | | | |
|
| | | Location | | Use | | Approximate area | | Lease dated | | Lease term |
| | |
|
| | | Peterhouse Technology Park, |
| | | Cambridge, UK1 | | R&D/office | | 30,000 sq ft | | December 1998 | | 25 years |
| | | Cambridge, MA, USA2 | | R&D/office | | 53,000 sq ft | | January 1996 | | 10 years |
| | | Cambridge, MA, USA | | Office | | 2,700 sq ft | | March 2002 | | 18 months |
| | | Canton MA, USA | | Manufacturing | | 47,000 sq ft | | December 2001 | | 5 years |
| | | Canton MA, USA | | Office/warehousing | | 27,000 sq ft | | April 2002 | | 5 years |
| | |
|
| | | 1) Approximately 15,000 sq ft is sub-leased to a third-party. |
| | | 2) Until 31 December 2002, approximately 13,000 sq ft was sub-leased to a third-party. Acambis reoccupied the space from the start of 2003. |
| | | The Group believes its properties to be adequately maintained and suitable for their intended use. |
| (10d) | | Exchange controls and other limitations affecting security holders |
| | | There are no UK government laws, decrees or regulations restricting the import or export of capital or affecting the remittance of dividends or other payments to holders of the Company’s ordinary shares who are non-residents of the UK. There are no limitations relating only to non-residents of the UK under English law or the Company’s Memorandum and Articles of Association on the right to be a holder of, and to vote in respect of, the Company’s ordinary shares. |
| | | Exchange rate information |
| | | References in this report to “US dollars”, “$”, or “¢” are to the currency of the United States. References to pounds “sterling”, “pounds”, “£”, “pence” or “p” are to the currency of the United Kingdom. There are 100 pence to each pound. Solely for your convenience, this report contains translations of certain pounds sterling amounts into US dollars at specified rates. These translations should not be taken as assurances that the pounds sterling amounts actually represent such US dollar amounts or could be converted into US dollars at the rate indicated or at any other rate. |
| | | The table below sets forth, for the periods and dates indicated, the exchange rate for the US dollar against the pound based on the noon buying rate, expressed in dollars per pound sterling. The period average is based on the average of the noon buying rates on the last day of each month during the period. |
| | | | | | | | | | | | | | | | | | | |
| | | | | Period | | | Period | | | | | | | |
| | | | | Average | | | End | | | High | | | Low | |
| | |
|
| | | Year ended 31 December | | | | | | | | | | | | | | | | |
| | | 1998 | | | 1.6573 | | | | 1.6628 | | | | 1.7222 | | | | 1.6114 | |
| | | 1999 | | | 1.6172 | | | | 1.6150 | | | | 1.6765 | | | | 1.5515 | |
| | | 2000 | | | 1.5163 | | | | 1.4938 | | | | 1.6522 | | | | 1.4002 | |
| | | 2001 | | | 1.4400 | | | | 1.4555 | | | | 1.5047 | | | | 1.3726 | |
| | | 2002 | | | 1.5035 | | | | 1.6095 | | | | 1.6095 | | | | 1.4092 | |
| | |
|
| | | Monthly high and low rates (for the last full six months) are: | | | | | | | | | | | | | | |
| | | September 2002 | | | | | | | | | | | 1.5726 | | | | 1.5347 | |
| | | October 2002 | | | | | | | | | | | 1.5699 | | | | 1.5442 | |
| | | November 2002 | | | | | | | | | | | 1.5893 | | | | 1.5439 | |
| | | December 2002 | | | | | | | | | | | 1.6095 | | | | 1.5552 | |
| | | January 2003 | | | | | | | | | | | 1.6472 | | | | 1.5977 | |
| | | February 2003 | | | | | | | | | | | 1.6469 | | | | 1.5721 | |
| | | As of 11 March 2003, the noon buying rate for pounds sterling was $1.6062. |
| | | SUMMARISED GROUP STATEMENTS |
| (A) | | Selected financial information (in thousands, except per share data) |
| (3a) | | The following selected financial information for each of the fiscal years in the five-year period ended 31 December 2002 has been derived from Acambis’ audited Group financial statements. The selected financial data has been prepared in accordance with UK GAAP, which differs in certain respects from US GAAP. The Group financial statements for the three-year period ended 31 December 2002 are included elsewhere in this Annual Report and Form 20-F. The principal differences between UK GAAP and US GAAP are summarised in note 29. |
| | | Translation of pounds sterling into US dollars for the year ended 31 December 2002 has been made at the rate of £1.00=$1.6095 based upon the mid-point closing rate at 31 December 2002. This translation is provided solely for the convenience of the reader and does not reflect financial information in accordance with generally accepted accounting principles for foreign currency translations. Such translations should not be construed as representations that the sterling amounts represent, or have been or could be so converted into US dollars at that rate or any other rate. The following information should be read in conjunction with the financial review on pages 14 and 15 and Acambis’ Group financial statements and the notes thereto appearing elsewhere in this document. |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | Year ended 31 December |
| | | | |
|
| | | | | 2002 | | | 2002 | | | 2001 | | | 2000 | | | 1999 | | | 1998 | |
| | | | | | | | | | | (restated) | | | (restated) | | | | | | | |
| | | | | $'000 | | | £'000 | | | £'000 | | | £'000 | | | £'000 | | | £'000 | |
| | |
|
| | | Statement of operations data:
UK GAAP
Turnover (revenues) | | | 128,303 | | | | 79,716 | | | | 8,914 | | | | 6,264 | | | | 5,584 | | | | 731 | |
| | | Cost of sales | | | (79,266 | ) | | | (49,249 | ) | | | (5,063 | ) | | | (517 | ) | | | – | | | | – | |
| | |
|
| | | Gross profit | | | 49,037 | | | | 30,467 | | | | 3,851 | | | | 5,747 | | | | 5,584 | | | | 731 | |
| | | Operating expenses: | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Research and development costs | | | 26,233 | | | | 16,299 | | | | 12,594 | | | | 12,195 | | | | 14,124 | | | | 8,107 | |
| | | Administrative costs (including amortisation of goodwill) | | | 6,852 | | | | 4,257 | | | | 3,499 | | | | 2,949 | | | | 2,757 | | | | 1,277 | |
| | |
|
| | | Total operating expenses | | | 33,085 | | | | 20,556 | | | | 16,093 | | | | 15,144 | | | | 16,881 | | | | 9,384 | |
| | |
|
| | | Operating profit/(loss) | | | 15,952 | | | | 9,911 | | | | (12,242 | ) | | | (9,397 | ) | | | (11,297 | ) | | | (8,653 | ) |
| | | Share of operating loss of joint venture | | | (275 | ) | | | (171 | ) | | | (410 | ) | | | (2,138 | ) | | | (2,292 | ) | | | – | |
| | | Interest receivable, net | | | (847 | ) | | | (526 | ) | | | 643 | | | | 767 | | | | 1,096 | | | | 1,118 | |
| | | Preference dividend receivable | | | – | | | | – | | | | – | | | | – | | | | (22 | ) | | | 22 | |
| | | Exceptional items | | | (137 | ) | | | (85 | ) | | | (423 | ) | | | (35 | ) | | | – | | | | – | |
| | | Exchange gain/(loss) on US currency borrowings | | | 723 | | | | 449 | | | | (126 | ) | | | (271 | ) | | | – | | | | – | |
| | | Taxation | | | (4 | ) | | | (3 | ) | | | 131 | | | | – | | | | – | | | | – | |
| | |
|
| | | Profit/(loss) on ordinary activities after taxation | | | 15,412 | | | | 9,575 | | | | (12,427 | ) | | | (11,074 | ) | | | (12,515 | ) | | | (7,513 | ) |
| | |
|
| | | Basic earnings/(loss) per ordinary share | | | $0.16 | | | | £0.10 | | | | £(0.14 | ) | | | £(0.14 | ) | | | £(0.19 | ) | | | £(0.21 | ) |
| | |
|
| | | Number of shares — weighted average | | | 96,101,507 | | | | 96,101,507 | | | | 91,027,463 | | | | 79,638,484 | | | | 65,979,689 | | | | 36,233,240 | |
| | |
|
| | | Diluted earnings/(loss) per ordinary share | | | $0.16 | | | | £0.10 | | | | £(0.14 | ) | | | £(0.14 | ) | | | £(0.19 | ) | | | £(0.21 | ) |
| | |
|
| | | Number of shares — weighted average | | | 98,976,882 | | | | 98,976,882 | | | | 91,027,463 | | | | 79,638,484 | | | | 65,979,689 | | | | 36,233,240 | |
| | |
|
48
| (A) | | Selected financial information
To assist our Form 20-F readers, comparative financial information for 2002 is given in US dollars on the following two pages. |
THE INFORMATION CONTAINED IN THIS COLUMN HAS NOT BEEN AUDITED.
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Year ended 31 December |
| | |
|
| | | 2002 | | 2002 | | 2001 | | 2000 | | 1999 | | 1998 |
| | | | | | | (restated) | | (restated) | | | | |
| | | $'000 | | £'000 | | £'000 | | £'000 | | £'000 | | £'000 |
|
| US GAAP | | | | | | | | | | | | | | | | | | | | | | | | |
| Turnover (revenues) | | | 89,475 | | | | 55,592 | | | | 10,909 | | | | 5,694 | | | | 5,584 | | | | 731 | |
| Cost of sales | | | (77,266 | ) | | | (48,006 | ) | | | (5,063 | ) | | | (517 | ) | | | – | | | | – | |
|
| Gross profit | | | 12,209 | | | | 7,586 | | | | 5,846 | | | | 5,177 | | | | 5,584 | | | | 731 | |
| Operating expenses | | | (34,559 | ) | | | (21,472 | ) | | | (20,509 | ) | | | (14,662 | ) | | | (16,292 | ) | | | (8,611 | ) |
|
| Operating loss | | | (22,350 | ) | | | (13,886 | ) | | | (14,663 | ) | | | (9,485 | ) | | | (10,708 | ) | | | (7,880 | ) |
| Loss per share (basic and diluted) | | | $(0.23 | ) | | | £(0.14 | ) | | | £(0.16 | ) | | | £(0.12 | ) | | | £(0.16 | ) | | | £(0.22 | ) |
|
| Retained loss before cumulative effect of accounting changes | | | (21,853 | ) | | | (13,577 | ) | | | (14,848 | ) | | | (11,162 | ) | | | (23,826 | ) | | | (6,740 | ) |
| Cumulative effect of accounting change: | | | | | | | | | | | | | | | | | | | | | | | | |
| Revenue recognition | | | – | | | | – | | | | – | | | | (2,572 | ) | | | – | | | | – | |
|
| Net loss (being retained loss for the year) | | | (21,853 | ) | | | (13,577 | ) | | | (14,848 | ) | | | (13,734 | ) | | | (23,826 | ) | | | (6,740 | ) |
|
| Net loss from continuing operations | | | (21,853 | ) | | | (13,577 | ) | | | (14,848 | ) | | | (10,438 | ) | | | (20,897 | ) | | | (4,394 | ) |
| Net loss per share (basic and diluted) from continuing operations | | | $(0.23 | ) | | | £(0.14 | ) | | | £(0.16 | ) | | | £(0.13 | ) | | | £(0.32 | ) | | | £(0.12 | ) |
|
| Net loss per share (basic and diluted) before cumulative effect of accounting change | | | $(0.23 | ) | | | £(0.14 | ) | | | £(0.16 | ) | | | £(0.14 | ) | | | £(0.36 | ) | | | £(0.19 | ) |
|
| Net loss per share (basic and diluted) showing cumulative effect of accounting change | | | – | | | | – | | | | – | | | | £(0.03 | ) | | | – | | | | | |
|
| Net loss per share (basic and diluted) | | | $(0.23 | ) | | | £(0.14 | ) | | | £(0.16 | ) | | | £(0.17 | ) | | | £(0.36 | ) | | | £(0.19 | ) |
|
| | | | | | | | | | | | | | | | | | | | | | | | | |
| The Group has not paid dividends in any of the years shown above |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | As at 31 December |
| | |
|
| | | | 2002 | | | | 2002 | | | | 2001 | | | | 2000 | | | | 1999 | | | | 1998 | |
| | | | $'000 | | | | £'000 | | | | £'000 | | | | £'000 | | | | £'000 | | | | £'000 | |
|
| Balance sheet data: | | | | | | | | | | | | | | | | | | | | | | | | |
| UK GAAP | | | | | | | | | | | | | | | | | | | | | | | | |
| Cash and liquid resources | | | 18,955 | | | | 11,777 | | | | 22,213 | | | | 21,117 | | | | 19,459 | | | | 9,754 | |
| Working capital (including debtors due after one year) | | | 49,291 | | | | 30,625 | | | | 19,387 | | | | 21,237 | | | | 16,309 | | | | 8,660 | |
| Fixed assets (tangible) | | | 32,171 | | | | 19,988 | | | | 12,255 | | | | 3,185 | | | | 4,633 | | | | 3,875 | |
| Fixed assets (intangible) | | | 21,955 | | | | 13,641 | | | | 14,845 | | | | 16,049 | | | | 17,253 | | | | – | |
| Total assets | | | 260,023 | | | | 153,814 | | | | 64,797 | | | | 52,781 | | | | 44,332 | | | | 20,006 | |
| Long-term obligations | | | (30,415 | ) | | | (18,897 | ) | | | (20,534 | ) | | | (6,546 | ) | | | (1,989 | ) | | | – | |
| Called-up share capital | | | 15,936 | | | | 9,901 | | | | 9,308 | | | | 8,868 | | | | 7,840 | | | | 3,658 | |
| Shareholders’ equity (net assets) | | | 74,441 | | | | 46,251 | | | | 27,660 | | | | 36,139 | | | | 37,682 | | | | 16,204 | |
|
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | As at 31 December |
| | |
|
| | | | 2002 | | | | 2002 | | | | 2001 | | | | 2000 | | | | 1999 | | | | 1998 | |
| | | | $'000 | | | | £'000 | | | | £'000 | | | | £'000 | | | | £'000 | | | | £'000 | |
|
| US GAAP | | | | | | | | | | | | | | | | | | | | | | | | |
| Cash, cash equivalents and short-term investments | | | 18,955 | | | | 11,777 | | | | 22,213 | | | | 21,117 | | | | 19,459 | | | | 9,754 | |
| Working capital (including debtors due after one year) | | | 8,617 | | | | 5,354 | | | | 19,215 | | | | 16,553 | | | | 16,309 | | | | 8,660 | |
| Fixed assets | | | 41,216 | | | | 25,608 | | | | 17,316 | | | | 8,656 | | | | 10,514 | | | | 3,875 | |
| Total assets | | | 233,371 | | | | 144,996 | | | | 53,589 | | | | 37,616 | | | | 31,301 | | | | 18,322 | |
| Shareholders’ equity (net assets) | | | 19,575 | | | | 12,162 | | | | 15,477 | | | | 20,974 | | | | 24,651 | | | | 14,520 | |
|
| | | |
 | | Alan Smith, Chairman*
Mr Smith, 58, a member of the Chartered Institute of Public Finance and Accountancy, joined the board of Acambis on 3 November 1995 as a Non-executive Director and was appointed non-executive Chairman on 20 May 1999. He was Group Managing Director of Anglian Water plc until December 1997 and is currently Chairman of Avlar Bioventures Limited. He is Chairman of the Nominations Committee. The Board considers Mr Smith to be an independent Non-executive Director. |
| |
 | | Dr John Brown, Chief Executive Officer**
Dr Brown, 48, was appointed to the Board of Acambis on 28 April 1995 as Finance Director and was appointed Chief Executive Officer on 1 March 1997. He holds a PhD in Pharmacology and an MBA. Prior to joining Acambis, he worked at Glaxo plc, PA Consulting Group, Bell Lawrie White and Sutherland and Partners. Dr Brown is Chairman of the Roslin Institute (Edinburgh). |
| |
 | | Gordon Cameron, Chief Financial Officer and President of Acambis Inc.**
Mr Cameron, 36, is a Chartered Accountant. He joined Acambis in 1996 from the corporate finance department at N M Rothschild where he had advised Acambis on its listing on the London Stock Exchange. Prior to that, he worked for Hill Samuel Bank and Ernst & Young. He was appointed to the Board as Finance Director on 1 March 1997 and was additionally appointed as President of Acambis Inc. on 31 March 2001. Mr Cameron served as Company Secretary for the Group from 28 February 1998 until 1 July 2002. |
| |
 | | Nicolas Higgins, Chief Business Officer**
Mr Higgins, 46, has a BSc in Biochemistry, an MSc in Biochemical Engineering and an MSc in Management of Intellectual Property. He joined Acambis in 1994 with responsibility for managing its intellectual property, became Licensing Director in 1996 and was appointed to the Board as Chief Business Officer (formerly Commercial Director) on 3 October 1996. He previously worked for Unilever, PA Consulting Group and Porton International. Mr Higgins is a Non-executive Director of Intercytex Limited. |
| |
 | | Dr Thomas Monath, Chief Scientific Officer**
Dr Monath, 62, joined the Group in 1992,prior to which he worked at the US Centers for Disease Control and Prevention, and was Colonel-in-Chief of the Virology Division of the US Army Medical Research Institute of Infectious Diseases. He is a qualified medical doctor. Dr Monath was appointed to the Board as Chief Scientific Officer on 12 March 2002. |
| |
 | | Alan Dalby, Non-executive Director*
Mr Dalby, 66, became a Non-executive Director of Acambis on 1 May 1998. He was an executive director of SmithKline, a predecessor company to GlaxoSmithKline plc, and retired from the role of Chairman of Reckitt Benckiser plc in 2001. He is a director of Alteon, Inc., a US-based biotechnology company. He is the senior independent Non-executive Director. The Board considers Mr Dalby to be an independent Non-executive Director. Mr Dalby is the Chairman of the Remuneration Committee. |
| |
 | | Michael Lytton, Non-executive Director*
Mr Lytton, 45, was appointed to the Board of Acambis as a Non-executive Director on 12 March 2001. He is a General Partner of Oxford Bioscience Partners, a US-based life sciences venture capital fund. Prior to this, he was a Partner of the Boston-based law firm of Palmer & Dodge LLP, where he had a particular emphasis on biotechnology and healthcare. He holds a JD and an MSc in Epidemiology and Medical Statistics. He is a member of the Board of GPC Biotech AG, ActivX, Inc., AnaVios Pharmaceuticals, Inc., Descartes Therapeutics, Inc., Graffinity Pharmaceuticals AG, Psychiatric Genomics, Inc., Rib-X Pharmaceuticals, Inc. and Therascope Pharmaceuticals AG. The Board considers Mr Lytton to be an independent Non-executive Director. Mr Lytton is the Chairman of the Audit Committee. |
| |
 | | Victor Schmitt, Non-executive Director
Mr Schmitt, 54, is President, Venture Management at Baxter Healthcare Corporation, where he is responsible for the creation and management of its interests in development stage biotechnology companies, and for business development for its biosciences division, which contains Baxter’s vaccines business. He joined the Board of Acambis on 4 December 2000. The Board does not consider Mr Schmitt to be an independent Non-executive Director given that he represents Baxter’s interest on the Acambis Board. |
* Member of the Audit Committee, Remuneration Committee and Nominations Committee
** Member of the Group Executive Committee
50
Directors information
The Directors who served during the year were:
Executive:
Dr John Brown
Gordon Cameron
Nicolas Higgins
Dr Thomas Monath
— appointed 12 March 2002
Non-executive:
Alan Smith
Alan Dalby
Michael Lytton
Dr Geoffrey Porges
— resigned 22 January 2003
Sir Brian Richards
— retired 30 May 2002
Victor Schmitt
During 2002, of the six Board meetings held, full attendance by all Board Directors was achieved at four of these meetings.
The usual business address of all of the Directors is the registered office of the Company, except Gordon Cameron and Dr Thomas Monath whose usual business address is that of Acambis Inc. at 38 Sidney Street, Cambridge, MA in the US.
In accordance with the Company’s Articles of Association, Nicolas Higgins will retire by rotation at this year’s Annual General Meeting and, being eligible, offers himself for re-election.
Company Secretary information
On 1 July 2002, Gordon Cameron stepped down from his role as Company Secretary with Elizabeth Brown filling the position from this date. Miss Brown, a certified accountant, joined Acambis in 1996. In addition to her role as Company Secretary, she is Director of Finance & Planning responsible for the UK finance function.

| | | For the year ended 31 December 2002 |
| | | The Directors’ report on the affairs of the Group, is presented below. The Group financial statements and auditors’ report, for the year ended 31 December 2002 are presented within this document. |
| | | Principal activities and business review
A review of the business and future developments of the Group is set out on pages 1 to 15. The principal activities of the Group are the research, development, manufacture and sale of vaccines to prevent and treat infectious diseases. |
| | | Results and dividends
The profit for the year after taxation amounted to £9.6m (2001 — loss of £12.4m). The Directors do not recommend a final dividend for the year (2001 — £nil). In the year ended 31 December 2002, the Group generated revenues of £79.7m (2001 — £8.9m). |
| | | Research and development (“R&D”)
The Group incurred R&D costs of £16.3m (2001 — £12.6m) during the year which have been written off to the profit and loss account in accordance with the Group’s accounting policy. |
| (6a) | | Directors and their interests |
| | | The Directors who served during the year are shown on page 50. The interests of the Directors in the Company’s shares and options to purchase shares in the Company are shown in the remuneration report on pages 59 to 63. At 31 December 2002, the Directors held an aggregate 698,092 shares, representing 0.71% of the current issued capital. None of the Directors had an interest in a contract of significance to which the Company or any of its subsidiary undertakings was party during the year. |
| (A) | | Policy on payment of creditors |
| | | It is the Group’s policy that payments to suppliers are made in accordance with those terms and conditions agreed between the Group and its suppliers, provided that all trading terms and conditions have been met. At 31 December 2002, the Company had an average of 21 days (2001 — 19 days) of purchases outstanding in trade creditors. |
| | | Corporate social responsibility
The Directors recognise the importance of corporate social responsibility and are in the process of reviewing current activities, formalising policies and preparing a fuller report on our activities and commitments in this area. We will report back to our shareholders on progress in our 2003 Annual Report. |
| | | Political and charitable donations
During the year, the Group made contributions amounting to £880 (2001 — £4,000) to local and national charitable organisations. No political donations were made during the year (2001 — £nil). Employees participated in various charitable fundraising activities during the year in aid of local and national charities. |
| | | Employees
Acambis seeks to involve employees in its corporate objectives, plans, performance and on other relevant matters of interest to employees through various communication methods including regular Company meetings. Employees of Acambis are not part of any labour unions. The Directors consider there to be a good relationship between employees and management. The Group is an equal opportunities employer and does not discriminate in the recruitment and promotion of staff, including applicants who are disabled. If an employee becomes disabled it is the policy, wherever practicable, to provide continued employment. All employees are encouraged to share in the growth of the Group, being eligible to participate in share option schemes. |
| | | Health, safety and environmental issues
In the UK, Acambis is a member of the British Safety Council and is committed to high standards and aims for continuous improvement in health, safety and environmental performance. In the US, Acambis contracts with Mount Auburn Hospital’s Occupational Health Service to provide medical surveillance, and prevention and treatment of work-related injuries and illnesses, including administering of appropriate immunisations. The Group has an excellent health and safety record and has never been required to report any accidents or injuries to the Health and Safety Executive in the UK or the Occupational Health and Safety Administration in the US. The Group seeks to minimise the environmental impact of its activities. Waste materials are recycled, where possible, and hazardous waste is handled by specialist disposal companies. |
| | | By order of the Board
Elizabeth Brown,Company Secretary
27 March 2003 |
| | | |
| (A) | | Policy on payment of creditors
At 31 December 2002, the Group had an average of 178 days (2001 — 54 days) of purchases outstanding in trade creditors. |
| |
| | | This number includes outstanding trade creditors payable to Baxter, which, under the terms of our agreement with them, we are not required to pay until we have been paid by the CDC. Excluding these amounts payable to Baxter the Group had an average of 66 days (2001 — 54 days) of purchases outstanding in trade creditors. |
| |
| | | Other information
Information regarding the substantial shareholders of Acambis, this year’s Annual General Meeting, the appointment of the Group’s auditors and special business to be conducted at the Annual General Meeting is contained within the shareholder information section of this document on pages 64 to 66. |
THE INFORMATION CONTAINED IN THIS COLUMN HAS NOT BEEN AUDITED.
51
| (6c) | | CORPORATE GOVERNANCE STATEMENT |
| | | Corporate governance focuses on the direction and control of companies and, in particular, the role of the Board of Directors and the need to establish a framework of effective accountability. The following statement describes the main principles of corporate governance and how they have been applied by Acambis. |
| | | Compliance with the Code of Best Practice
The Company has complied throughout the year with the provisions of the Code of Best Practice set out in Section 1 of the Combined Code, published by the Hampel Committee and the London Stock Exchange. |
| | | Statement of applying the Principles of Good Governance
The Company has applied the Principles of Good Governance set out in Section 1 of the Combined Code by complying with the Code of Best Practice as reported above. Further explanation of how the principles have been applied is set out below and, in connection with Directors’ remuneration, in the remuneration report. |
| | | The BioIndustry Association Code of Best Practice (“BIA Code”)
The BIA Code was introduced in 1999 and the Company has adopted this code since that time and applies it throughout the Group. The BIA Code includes principles and provisions relating to corporate governance matters, access to external advice, confidentiality, dealings in a company’s shares and standards of public announcements. It is intended to operate by reference to the particular circumstances of bioscience companies in support of the Combined Code, Principles of Good Governance and Code of Best Practice, the Turnbull Report and the rules of the Financial Services Authority. The BIA Code was obligatory for all BIA members from 1 January 2000. The Company, being a member of the BIA, complies with the principles in the BIA Code and maintains and develops procedures to support compliance with its specific provisions. |
| | | The Board and Committees
Board of Directors
The Board of Directors meets, on average, six times a year and also on an ad-hoc basis as required. It is responsible for the business and commercial strategy, monitoring progress, the approval of major transactions, and the approval of financial statements and operating and capital expenditure budgets. The information provided to the Board includes strategic and operational reviews, management accounting summaries and ad-hoc reports which provide details in respect of the ongoing running of the business. The Executive Directors of the Company are fully involved with the management of the Group at all levels, retaining overall direction and control of the Group. The Board delegates the day-to-day responsibility of managing the Group to a number of committees, details of which are set out below. |
| | | Audit Committee
The Audit Committee is made up of the Chairman and the Non-executive Directors, except Victor Schmitt, and is chaired by Michael Lytton. It examines and reviews, on behalf of the Board, internal financial controls, financial and accounting policies and practices, the form and content of financial reports and statements, compliance with corporate governance best practice, and the work of the external auditors. The Chief Executive Officer, the Chief Financial Officer and the external auditors may be in attendance at meetings. The Audit Committee meets, at a minimum, four times a year and at least once during the year without any Executive Directors present. |
| | | The Nasdaq National Market in the US is currently revising its corporate governance standards to comply with provisions of the recently adopted Sarbanes-Oxley Act in the US. Acambis will undertake a review of its compliance with the corporate governance standards and audit committee requirements once Nasdaq’s revised rules are finalised in order to ensure Acambis’ compliance with Nasdaq’s requirements. |
| | | Remuneration Committee
The Remuneration Committee is made up of the Chairman and the Non-executive Directors, except Victor Schmitt, and is chaired by Alan Dalby. It determines, on behalf of the Board, the Group’s policy for executive remuneration and the individual remuneration packages for the Executive Directors of the Group. The Chief Executive Officer attends the meetings except when his own remuneration is being considered. The Committee has access to professional advice, both inside and outside the Company, in the furtherance of its duties. The remuneration report is set out on pages 56 to 63. |
| | | Nominations Committee
The Nominations Committee comprises the Chairman and the Non-executive Directors, except Victor Schmitt, and is chaired by Alan Smith. It has responsibility for proposing to the Board, in the first instance, any new appointments of both Executive and Non-executive Directors. The Chief Executive Officer may be in attendance at Nominations Committee meetings. |
| | | Group Executive Committee
The Group Executive Committee is made up of the Executive Directors and several other members of the senior management team. It is chaired by the Chief Executive Officer, meets on a fortnightly basis and is responsible for the executive management of the Group. It makes recommendations to the Board as appropriate. |
| | | Communication with shareholders
The Board attaches high priority to communications with shareholders. In addition to our obligation to provide an Annual Report and financial statements, we also send quarterly results statements to all shareholders. The Directors meet regularly with institutional shareholders and there is an opportunity for individual shareholders to question the Directors at the Annual General Meeting. In addition, the Group has an Internet website (www.acambis.com) to aid communications. All of the Group’s press releases, as well as other information on the Group including the current share price, can be accessed through the website. |
| | | Internal controls
The Board has applied Principle D.2 of the Combined Code by establishing a continuous process for identifying, evaluating and managing the significant risks the Group faces. The Board regularly reviews the process, which has been in place since the start of 2000. The process in place is in accordance with Internal Control: Guidance for Directors on the Combined Code published in September 1999. The Board is responsible for the Group’s system of internal control and for reviewing its effectiveness. Such a system is designed to manage rather than eliminate the risk of failure to achieve business objectives, and can only provide reasonable and not absolute assurance against material misstatement or loss. |
| | | In 2000, the Group established a working committee specifically tasked to review and evaluate the risks to which the business is exposed. This committee is made up of members of the Executive Directors of the Board as well as certain members of senior management, all of whom assume different operating responsibilities within the business. Each member participates in the ongoing risk-management process and, ultimately, its findings are reported to the Board. |
| | | In compliance with Provision D.2.1 of the Combined Code, the Board continuously reviews the effectiveness of the Group’s system of internal control. The Board’s monitoring covers all controls, including financial, operational and compliance controls and risk management. It is based, principally, on reviewing reports from management to consider whether significant risks are identified, evaluated, managed and controlled and whether any significant weaknesses are promptly remedied or indicate a need for more extensive monitoring. The Board has also performed a specific assessment for the purpose of this Annual Report. This assessment considers all significant aspects of internal control arising during the period covered by the report, including the need to have an internal audit function. The Audit Committee assists the Board in discharging its review responsibilities. |
| | | As of the date of this Annual Report, based on the assessment of the Board of Directors, there were no changes in the Group’s internal controls or in other factors that could significantly affect these controls subsequent to the date of their evaluation, including any corrective actions with regard to significant deficiencies and material weaknesses. |
| | | Based on their review during the 90-day period prior to the date of this Annual Report and Form 20 —F of the Group’s disclosure controls and procedures, the Chief Executive Officer and Chief Financial Officer have concluded that the Group’s current disclosure controls and procedures are effective to ensure that material information by the Group is recorded, processed, summarised and reported in a timely manner and that the information is accumulated and communicated to management to allow timely decisions regarding required disclosure. |
| | | CORPORATE GOVERNANCE STATEMENT |
| (4b) | | There are risks and uncertainties relevant to the Group’s business. The factors listed below are those that the Group believes could cause the Group’s actual results to differ materially from expected and historical results. Other factors besides those listed here could also adversely affect the Group. |
| | | To date, the Group has not completed the full clinical development of any product candidate. If a product fails in clinical studies, regulatory approval and consequent sales would be delayed. The difficulties, uncertainties and the high level of investment associated with new product development are inherently uncertain. A product can fail at any stage of the process, and late-stage product candidates could fail to receive regulatory approval. New product candidates may appear promising in development but fail to reach the market because of efficacy or safety concerns, the inability to obtain necessary regulatory approvals, the difficulty or excessive cost to manufacture and/or the infringement of patents or intellectual property rights of others. The Group faces the risk that its patent applications may be denied, or that issued patents may be challenged or otherwise not provide protection for any commercially viable products developed. The success of the Group’s products will depend on being able to produce the product at a reasonable cost, convincing doctors to prescribe the product, the patients accepting the product, and the product being more effective than its competitors. Third-party reimbursement and healthcare cost containment may constrain future revenues. A significant proportion of future revenue may depend on payments by third-party payers, including government health administration authorities and private health insurers. The Group may not be able to sell its products profitably if reimbursement is unavailable or limited. |
| | | Until the 2002 financial year, Acambis had a history of operating losses. To fund future operations, Acambis may need additional financing, and this financing may not be available, or may only be available on terms that dilute our shareholders. If the Group is unable to raise additional funds as needed, it may be required to delay, reduce or eliminate some of its development programmes. |
| | | Legal factors, including product liability claims, environmental concerns and patent disputes with competitors, could preclude commercialisation of products or negatively affect the profitability of such products, or give rise to liabilities for which the Group may have no, or only limited, insurance coverage. Recent loss experience within the insurance industry as a whole, including pharmaceutical product liability exposures, has increased the cost of insurance coverage generally for biotechnology companies, including Acambis. |
| | | Acambis has entered into several significant commercial agreements, and the success of products is, therefore, highly dependent on collaborators. These collaborators have significant discretion over the resources they devote, and Acambis cannot guarantee that third parties will devote adequate resources to the collaborations, or that those products can be successfully commercialised without those collaborators. |
| | | Regulation imposed by government agencies imposes significant costs and restrictions on the development, testing, approval and manufacturing of pharmaceutical products for human use. Lost market opportunities may result from delays and uncertainties in the approval process of the US FDA, the European Agency for the Evaluation of Medicinal Products and comparable agencies in other foreign countries. In some countries, including the US and those of the European Union, regulatory controls have become increasingly demanding, increasing not only the cost of product development but also the time required to reach the market and the uncertainty of successfully doing so. The Group expects that this trend will continue and will expand to other countries. |
| | | The loss of key employees could weaken the Group’s scientific expertise and delay the development of products. Biotechnology companies, such as Acambis, are highly dependent on employees who have an in-depth and long-term understanding of Acambis’ technologies, products, programmes, collaborative relationships and strategic goals. The loss of these employees could have a negative impact on the business and prospects of the Group. |
| | | The Group’s business may be negatively affected by the intense competition it faces from pharmaceutical and specialised biotechnology companies engaged in the development of vaccines and other drugs in areas in which the Group is engaged. Competitors in the biotechnology and pharmaceutical industries may have superior products, manufacturing capabilities or marketing expertise. Many of Acambis’ competitors may have greater financial and human resources and more experience in research and development. If the Group fails to obtain adequate intellectual property rights for its product candidates, competitors will be able to take advantage of Acambis’ R&D efforts. |
| | | The biotechnology field is characterised by significant and rapid technological change. Research and discoveries by others may result in medical insights or breakthroughs which render the Group’s product candidates obsolete before they generate any revenue. |
| | | The Group has no control over changes in inflation and interest rates, foreign currency exchange rates and controls or other economic factors affecting its business or the possibility of political unrest, legal and regulatory changes or nationalisation in jurisdictions in which the Group operates. These factors could materially affect the Group’s future results of operations. |
| | | The Group conducts a substantial proportion of its operations outside the United Kingdom. Fluctuations in the exchange rate between sterling and other currencies, especially the US dollar, materially affect the Group’s reported revenues and operating results. |
| | | New or revised accounting standards and rules introduced from time to time by UK, US or international accounting standard setting boards could have a material adverse impact on the Group’s reported financial results. |
| | | In 2002 the Group reported its maiden profit. The effective tax rate on the Group’s earnings is affected by the tax rates applicable in the UK and the US. Changes in tax laws or in their application with respect to matters, such as transfer pricing, that relate to the proportion of the Group’s earnings which may be taxed at more favourable rates could increase the Group’s effective tax rate and adversely affect its net earnings. |
| | | Acambis has a major contract with the US Government to manufacture 155 million doses of smallpox vaccine and to take that vaccine through to licensure. The costs associated with this programme, and the revenue recognition thereon, will have a material impact on the financial results of the Group until the product is approved. |
| | | Acambis has one manufacturing facility, in the US. The loss of this facility may have a significant impact on the results of the Group. |
| | | This list should not be considered an exhaustive statement of all potential risks and uncertainties. |
| | | The Board has complied with the provisions of the Combined Code as discussed within the Company’s corporate governance statement on pages 52 to 55, and has applied the Principles of Good Governance relating to Directors’ remuneration as described below. |
| | | In August 2002, the Directors’ Remuneration Report Regulations 2002 (the “Regulations”) came into force. The Regulations, which amend part VII (Accounts and Audit) of the Companies Act 1985, introduce a new statutory disclosure regime on directors’ remuneration which is to be applied alongside the Combined Code and the requirements of the Listing Rules. |
| | | The Regulations also introduced a new requirement that a resolution be put each year to shareholders to approve the Remuneration Committee’s report. A resolution putting this report to such a vote is included in the Notice of Annual General Meeting. |
| | | Those sections which our auditors, PricewaterhouseCoopers LLP, have audited have been specifically identified within this report. |
| | | Remuneration Committee
The current members of the Remuneration Committee (“the Committee”), all independent Non-executive Directors, are Alan Dalby (Chairman), Alan Smith and Michael Lytton. Sir Brian Richards and Dr Geoffrey Porges also sat on the Committee up to the dates of their respective retirement and resignation from the Board. Sir Brian Richards chaired the Committee until the date of his retirement. The remit of the Committee is to determine, on behalf of the Board, the remuneration and other benefits of all Executive Directors, including base salary, benefits, pension contributions, bonus payments, share based long-term incentives and service contracts. |
| | | During the year, the Committee appointed New Bridge Street Consultants, an independent professional organisation specialising in providing advice on executive remuneration issues, employee share schemes and pensions, as its advisors. The advice provided by New Bridge Street Consultants materially assisted the Committee. Additionally, New Bridge Street Consultants provided advice to the Company on the day-to-day operation of the Company’s share option plans. |
| | | The Chief Executive Officer and the Company Secretary also materially assisted the Committee in its discussions except in relation to their own remuneration. |
| | | Policy on Executive Director remuneration
The Committee is aware that it must both attract and retain individuals of the highest calibre. It therefore aims to ensure that remuneration packages are competitive with comparable publicly listed companies and that they fairly and responsibly reward individuals for their contribution to the success of the Group. A significant proportion of directors’ remuneration is performance related through annual bonus, share options and long-term incentive plan awards. |
| | | Components of Executive Directors’ remuneration
Basic salary and benefits
In determining the basic salary of each Director, the Committee takes into account the individual’s responsibilities and pay levels are set in the light of independent assessment of market practices. Base salaries for Executive Directors are reviewed annually and compared to salary levels in a group of comparably sized biotechnology companies placing Acambis at or about the median. For US-based Executive Directors, salary levels in companies of a similar size to Acambis Inc. are also reviewed for comparative purposes. Salary reviews take account of all responsibility changes. Benefits comprise car allowance, private healthcare, life assurance, permanent health insurance, private telephone and the use of company assets. |
| | | Annual bonus
Bonuses are non-pensionable and based on a percentage of basic salary, up to a maximum of 50%. Bonuses are paid, at the discretion of the Committee, in recognition of each Director’s contribution to the success of the Group. Objectives are set that are considered to be both challenging and realistic. For 2003, the performance metrics on which bonus payments will be assessed are a mix of financial, product development and business development targets. The Acambis Share Incentive Plan (see page 62) permits Directors to elect to receive up to 50% of their annual bonus in Acambis shares. Such shares must be held for at least one year and, after such time, the Directors will be entitled to receive one matching share for every four shares held. No performance conditions are applied to these matching shares as the amount of any bonus invested has itself already been performance tested. Matching at this level is offered to encourage Executive Directors to participate in, and to link their annual bonus to, share ownership. |
| | | Long-term incentives
The Committee principally seeks to incentives Executive Directors by offering participation in share-based long term incentive schemes. |
| | | Executive Directors currently participate in grants of share options under the Acambis 1999 Share Option Plan and in grants of performance shares under the Acambis Share Incentive Plan. These plans and the performance conditions that apply to awards under these plans are described in more detail on pages 59 and 62. |
The Committee has established a policy that it believes is balanced whereby Executive Directors can receive an annual grant of options of up to one times base salary per annum (granted in two half-yearly tranches) and an annual grant of performance shares of up to one times base salary per annum. The Committee intends to consult leading institutional shareholders should it wish to alter this policy in future to allow additional grants to be made.
The Committee encourages Executive Directors to build and maintain interests in Acambis shares, thereby aligning their interests with other shareholders. In 2001 and 2002, Executive Directors voluntarily retained the balance of shares acquired through share option and performance share exercises, taking account of liabilities arising from the exercise of awards, including tax. It is the Remuneration Committee’s intention to consider introducing formal shareholding guidelines for Executive Directors during 2003.
Shareholders’ approval is being sought at the 2003 Annual General Meeting for amendments to the Company’s share option plans. The amendments will include a new individual limit for the Company’s share option plans such that an employee or Executive Director may be granted discretionary options in any year over shares with a market value, at the date of option grant, of up to two times base salary. This limit may be extended if the participant has been recruited, in which case the limit will be four times base salary within 12 months of joining, (or exceptionally at the discretion of the Committee eight times base salary if the participant is based in the US and forfeits vested options with a previous employer by reason of joining Acambis). The new limit will enable the Company to continue to make grants of options in line with its policy of making regular annual grants of options at levels up to one times base salary per annum. The proposed two times base salary annual limit is generally in line with UK market practice where companies seek to operate option plans on a phased annual basis. The Company wishes, however, to retain the flexibility to make higher awards in the case of recruitment.
Further amendments are proposed to introduce a new limit on the number of shares that are available for all share plans so that during the five years from the date of the AGM a further 5% of issued share capital may be used for the Company’s share plans and to allow the Company’s share plans to operate during that period. This new 5% in five years limit will give the Company the flexibility it needs to continue to operate its share plans over a foreseeable period and will allow the Company to continue to offer meaningful participation in share plans to all employees.
Pension scheme
In the UK, the Company operates a self-administered, defined contribution, Inland Revenue-approved pension scheme for the Executive Directors (including Gordon Cameron who is currently based in the US). The Company contributes 18% of base salary into this scheme on behalf of each Executive Director. No other benefits are pensionable. In the US, the Group offers a 401k Savings and Retirement Plan for all employees, including Executive Directors. Participants may contribute up to 15% of their annual compensation into the plan. The Company can make discretionary matching contributions, up to a maximum of 3% of base salary. Pension costs for each Director are shown on page 58.
Directors’ service contracts
Details of the service contracts of those who served as Directors during the year are:
| | | | | | | | | |
| | |
|
| Director | | Contract date | | Notice period |
|
| Executive: | | | | | | | | |
| Dr John Brown | | 1 March 1997 | | 12 months |
| Gordon Cameron | | 1 March 1997 | | 12 months |
| Nicolas Higgins | | 29 November 1996 | | 12 months |
| Dr Thomas Monath | | 12 March 2002 | | 12 months |
|
| Non-executive: | | | | | | | | |
| Alan Smith | | 1 January 1998 | | 3 months |
| Alan Dalby | | 25 March 1998 | | 3 months |
| Michael Lytton | | 12 March 2001 | | 3 months |
| Dr Geoffrey Porges1 | | 12 March 2001 | | 3 months |
| Sir Brian Richards2 | | 1 March 1997 | | 3 months |
| Victor Schmitt | | 4 December 2000 | | See note 3 |
|
| 1) | | Dr Porges resigned from the Board on 22 January 2003. |
| 2) | | Sir Brian Richards retired from the Board on 30 May 2002. |
| 3) | | Mr Schmitt’s appointment would end under certain circumstances in conjunction with the reduction of Baxter’s shareholding in Acambis,or the termination or breach of the subscription agreement between Baxter and the Company dated 19 September 2000. |
All Executive Directors have contracts with twelve-month notice periods in line with the Combined Code recommendations. On early termination of contract, an Executive Director would be entitled to basic salary and benefits for the notice period.
The Committee believes that in the event of early termination of an Executive Director’s contract it is appropriate to examine the specific circumstances of each case. Where appropriate, the Committee may agree to a phased payment of compensation over a fixed term. During this term, if the Executive Director were to find a new position payments would cease. The Committee does, however, reserve the right to make a payment in lieu of any period of notice.
Non-executive Directors are entitled to their fees during any notice period.
REMUNERATION REPORT
Components of Executive Directors’ remuneration (continued)
External appointments
The Committee recognises that Executive Directors may be invited to take up non-executive directorships or public service appointments and that these can broaden the experience and knowledge of the Director, from which the Company will benefit. Accordingly, subject to Board approval, they may accept non-executive appointments, as long as these are not likely to lead to a conflict of interest and are allowed to retain any fees paid.
Non-executive Directors’ fees and terms
The Non-executive Directors’ fees are determined by the Board on the basis of independent advice on current levels in similar businesses. Fees are reviewed periodically. Non-executive Directors are not eligible for pensions, incentives or any similar payments other than out-of-pocket travel and accommodation costs in connection with the performance of their duties. Non-executive Directors do not participate in the Company’s long-term incentive schemes, nor do they receive pension contributions or a bonus.
Directors’ remuneration (audited)
The total remuneration of the Directors for the year ended 31 December 2002 was £1.0m (2001 — £1.2m) and comprised salaries, benefits, bonuses, pension contributions and Non-executive Director fees. The remuneration received by each Director who served during the year was as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | |
|
| | | Basic salary/ | | | | | | Bonus | | | Total | | | Total | | | Pension | | | Pension | |
| | | fees | | | Benefits | | | payment | | | 2002 | | | 2001 | | | 2002 | | | 2001 | |
| Directors | | £ | | | £ | | | £ | | | £ | | | £ | | | £ | | | £ | |
|
| Executive: | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Dr John Brown1 | | | 266,500 | | | | 14,554 | | | | 30,030 | | | | 311,084 | | | | 439,927 | | | | 47,970 | | | | 44,622 | |
Gordon Cameron2 | | | 184,334 | | | | 11,086 | | | | 19,785 | | | | 215,205 | | | | 342,302 | | | | 33,806 | | | | 31,583 | |
| Nicolas Higgins | | | 158,875 | | | | 12,055 | | | | 17,903 | | | | 188,833 | | | | 263,790 | | | | 28,598 | | | | 26,373 | |
Dr Thomas Monath3 | | | 143,543 | | | | 12,089 | | | | 19,477 | | | | 175,109 | | | | – | | | | 3,474 | | | | – | |
|
| Total | | | 753,252 | | | | 49,784 | | | | 87,195 | | | | 890,231 | | | | 1,046,019 | | | | 113,848 | | | | 102,578 | |
|
| Non-executive: | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Alan Smith | | | 60,000 | | | | – | | | | – | | | | 60,000 | | | | 40,000 | | | | – | | | | – | |
| Alan Dalby | | | 28,000 | | | | – | | | | – | | | | 28,000 | | | | 20,000 | | | | – | | | | – | |
| Michael Lytton | | | 28,000 | | | | – | | | | – | | | | 28,000 | | | | 14,580 | | | | – | | | | – | |
Dr Geoffrey Porges4 | | | 28,000 | | | | – | | | | – | | | | 28,000 | | | | 14,580 | | | | – | | | | – | |
Sir Brian Richards5 | | | 11,667 | | | | – | | | | – | | | | 11,667 | | | | 20,000 | | | | – | | | | – | |
Victor Schmitt6 | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
|
| Total | | | 155,667 | | | | – | | | | – | | | | 155,667 | | | | 109,160 | | | | – | | | | – | |
|
Former Directors7 | | | – | | | | – | | | | – | | | | – | | | | 64,546 | | | | – | | | | 3,291 | |
|
| Total | | | 908,919 | | | | 49,784 | | | | 87,195 | | | | 1,045,898 | | | | 1,219,725 | | | | 113,848 | | | | 105,869 | |
|
| 1) | | Under the rules of the LTIP Dr Brown elected to receive 50% of his 2002 bonus in the form of Acambis shares (as described within the “Annual Bonus” on page 56). |
| 2) | | During the year Mr Cameron received £41,168 in addition to the above remuneration, in relation to his living expenses whilst located in the US. |
| 3) | | Dr Monath was appointed to the Board on 12 March 2002. Amounts in 2002 represent the period from the date of his appointment. |
| 4) | | Dr Porges resigned from the Board on 22 January 2003. |
| 5) | | Sir Brian Richards retired from the Board on 31 May 2002. Amounts in 2002 represent the period up to the date of his retirement. |
| 6) | | Mr Schmitt, under the terms of his appointment, does not receive fees. |
| 7) | | Payments to former Directors relates to amounts paid to Dr Gordon during 2001. Dr Gordon resigned from the Board on 31 March 2001. |
| | | | | | | | | | | | | |
| (6e) | | Directors’ interests
The Directors who held office at 31 December 2002 had the following beneficial interests in the shares of the Company: |
| | |
|
| |
| | | | | | | Number of ordinary 10p shares | | Number of ordinary 10p shares |
| | | | | | | held 31 December 2002 | | held 31 December 2001 |
| |
|
| | Dr John Brown | | | 305,245 | | | 239,602 |
| | | Gordon Cameron1 | | | 163,849 | | | 22,750 |
| | | Alan Dalby | | | 5,000 | | | 5,000 |
| | | Nicolas Higgins | | | 205,978 | | | 201,936 |
| | | Michael Lytton | | | 8,120 | | | 5,420 |
| | | Dr Thomas Monath | | | – | | | – |
| | | Dr Geoffrey Porges2 | | | 8,100 | | | 5,420 |
| | | Victor Schmitt | | | – | | | – |
| | | Alan Smith | | | 1,800 | | | 1,800 |
| |
|
|
|
|
|
| | | Notes | | | | | | |
| | | 1) 133,711 of the shares owned by Mr. Cameron in Trust on his behalf by the Trustees of the |
| | | Acambis Employees’ Trust. |
| | | 2) Dr Porges resigned from the Board on 22 January 2003. |
| |
| | | Individually, each of the Directors beneficially owns less than 1% of the total issued share capital. As at 31 December 2002, the Directors had no interests in shares of any other Group company. There have been no changes in the interests of the current Directors in the share capital of the Company since 31 December 2002. The Executive Directors also have an interest as potential beneficiaries in 589,685 ordinary shares held at 27 March 2003 by the Trustees of the Acambis Employees’ Trust. |
| |
| (6e) | | Share options (audited)
All Executive Directors are eligible to participate in the Company’s share option schemes. |
|
|
|
|
| | The share option schemes consist of an Inland Revenue-approved executive scheme and unapproved executive schemes. The grant of options under the current executive schemes (the “1996 Scheme” and the“1999 plan”) as defined below is at the discretion of the Committee and their exercise is subject to performance conditions. |
|
|
|
|
| | | Grants to be made in 2003 will be subject to performance conditions relating to the performance of Acambis’ total shareholder return (“TSR”) compared to a comparator group of other companies within the industry. These companies are: |
| | | | | |
Antisoma plc
Axis-Shield plc
Cambridge Antibody Technology Group plc
Celltech Group PLC
GW Pharmaceuticals plc
Galen Holdings PLC
GeneMedix plc | | Goldshield Group PLC
Intercare Group PLC
NeuTec Pharma PLC
Oxford Glycosciences plc
Phytopharm plc
PowderJect Pharmaceuticals Plc
Proteome Sciences plc | | Protherics PLC
Shire Pharmaceuticals Group plc
SkyePharma PLC
Vernalis Group plc
Xenova Group plc |
| |
| These companies represent all of the constituents of the FTSE Pharmaceuticals and Biotech Index with a market capitalisation greater than £30 million but excluding AstraZeneca Plc and GlaxoSmithkline Plc. The Committee has chosen this group as being the most appropriate for Acambis given that Acambis is a constituent of this sector. The Committee did consider the appropriateness of including selected US biotechnology companies, and will continue to review this in future years. |
| | | Components of Executive Directors’ remuneration (continued)
Share options (audited) (continued) |
| | | The TSR condition seeks to align the interests of executives with the interests of shareholders by requiring superior relative TSR performance compared with direct biotechnology competitors before options can be exercised. The maximum allocation of shares would be achieved if Acambis is ranked in the upper quartile of the comparator group, being prorated down to a 30% allocation at a ranking at the median (for options granted from September 2001 until the end of 2002, the proportion of shares vesting at the median was 50%). No allocation will be made if Acambis’ ranking falls below the median. The performance condition is measured initially over a three-year period beginning at the date of grant and, if not initially achieved in full, can be further measured over a four-year period measured from the same fixed base point. Options granted from September 2001 until the end of 2002 also allow for re-testing over a five-year period. For the purposes of TSR calculation, the Company’s TSR will be averaged over the three months preceding the commencement of the period and the three months preceding a measurement date to ensure that results are not influenced by short-term volatility. From 2003, grants to Executive Directors will be subject to an additional performance condition that requires the Committee to be satisfied that there has been improvement in the Company’s underlying financial performance over the relevant performance period. |
| | | The Company also operates an Inland Revenue-approved savings-related scheme, which is available generally to all UK employees, provided they enter into savings contracts. |
| | | The Directors who held office at 31 December 2002 hold options to acquire ordinary shares of the Company under the Acambis 1995 Unapproved Share Option Scheme (“1995 Scheme”), the Acambis 1996 Approved Share Option Scheme (“1996 Scheme”), the Acambis 1995 Savings-Related Share Option Scheme (“SAYE Scheme”), the Acambis 1999 Share Option Plan (“1999 Plan”), the OraVax 1990 Stock Incentive Plan (“1990 US Scheme”) and the OraVax 1995 Stock Incentive Plan (“1995 US Scheme”), as shown in the table below. Following the introduction of the 1999 Plan in October 1999, no further issues of options will be made under the 1995 Scheme, the 1990 US Scheme or the 1995 US Scheme. |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| |
|
| | | | | | | | | | | | | | | | | | | | | | | Exercise | | Earliest date | | |
| Director | | Scheme | | 1 January 2002 | | Granted | | Exercised | | 31 December 2002 | | price | | of exercise | | Expiry Date |
|
| Dr John Brown | | | 19951,3 | | | 35,370 | | | – | | | (35,370 | ) | | – | | | £1.70 | | 28 Nov 98 | | 28 Nov 02 |
| | | | 19953 | | | 91,153 | | | – | | | – | | | 91,153 | | | £1.74 | | 11 Sep 99 | | 11 Sep 03 |
| | | | 19953 | | | 350,000 | | | – | | | – | | | 350,000 | | | £0.97 | | 01 Apr 02 | | 01 Apr 09 |
| | | | 19963 | | | 16,681 | | | – | | | – | | | 16,681 | | | £1.80 | | 09 Jul 99 | | 09 Jul 06 |
| | | | 19993 | | | 250,000 | | | – | | | – | | | 250,000 | | | £0.92 | | 28 Sep 03 | | 28 Sep 10 |
| | | | 19994 | | | 208,000 | | | – | | | – | | | 208,000 | | | £1.25 | | 24 Sep 04 | | 24 Sep 11 |
| | | | 19994 | | | 19,520 | | | – | | | – | | | 19,520 | | | £3.33 | | 31 Dec 04 | | 31 Dec 11 |
| | | | 19994 | | | – | | | 42,763 | | | – | | | 42,763 | | | £3.04 | | 26 Apr 05 | | 26 Apr 12 |
| | | | 19994 | | | – | | | 58,584 | | | – | | | 58,584 | | | £2.33 | | 26 Sep 05 | | 26 Sep 12 |
| | | SAYE2,5 | | | 30,273 | | | – | | | (30,273 | ) | | – | | | £0.32 | | 01 Dec 02 | | 01 Jun 03 |
| | | SAYE5 | | | – | | | 5,250 | | | – | | | 5,250 | | | £1.80 | | 01 Dec 05 | | 01 Jun 06 |
|
| Total | | | | | | | 1,000,997 | | | 106,597 | | | (65,643 | ) | | 1,041,951 | | | | | | | | | | | | |
|
|
|
|
|
| Gordon Cameron | | | 19953 | | | 170,954 | | | – | | | – | | | 170,954 | | | £1.70 | | 20 Dec 99 | | 20 Dec 03 |
| | | | 19951,3 | | | 160,000 | | | – | | | (160,000 | ) | | – | | | £0.97 | | 01 Apr 02 | | 01 Apr 09 |
| | | | 19963 | | | 17,685 | | | – | | | – | | | 17,685 | | | £1.70 | | 20 Dec 99 | | 20 Dec 03 |
| | | | 19993 | | | 150,000 | | | – | | | – | | | 150,000 | | | £0.92 | | 28 Sep 03 | | 28 Sep 10 |
| | | | 19994 | | | 147,990 | | | – | | | – | | | 147,990 | | | £1.25 | | 24 Sep 04 | | 24 Sep 11 |
| | | | 19994 | | | 13,911 | | | – | | | – | | | 13,911 | | | £3.33 | | 31 Dec 04 | | 31 Dec 11 |
| | | | 19994 | | | – | | | 30,545 | | | – | | | 30,545 | | | £3.04 | | 26 Apr 05 | | 26 Apr 12 |
| | | | 19994 | | | – | | | 39,116 | | | – | | | 39,116 | | | £2.33 | | 26 Sep 05 | | 26 Sep 12 |
| | | SAYE2,5 | | | 30,273 | | | – | | | (30,273 | ) | | – | | | £0.32 | | 01 Dec 02 | | 01 Jun 03 |
| | | SAYE5 | | | – | | | 5,250 | | | – | | | 5,250 | | | £1.80 | | 01 Dec 05 | | 01 Jun 06 |
|
| Total | | | | | | | 690,813 | | | 74,911 | | | (190,273 | ) | | 575,451 | | | | | | | | | | | | |
|
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| |
|
| | | | | | | | | | | | | | | | | | | | | | | Exercise | | Earliest date | | |
| Director | | Scheme | | 1 January 2002 | | Granted | | Exercised | | 31 December 2002 | | price | | of exercise | | Expiry Date |
|
| Nicolas Higgins | | | 19951 | ,3 | | 23,580 | | | – | | | (23,580 | ) | | – | | | £1.70 | | 28 Nov 98 | | 28 Nov 02 |
| | | | 19953 | | | 80,513 | | | – | | | – | | | 80,513 | | | £1.74 | | 11 Sep 99 | | 11 Sep 03 |
| | | | 19953 | | | 238,835 | | | – | | | – | | | 238,835 | | | £0.97 | | 01 Apr 02 | | 01 Apr 09 |
| | | | 19963 | | | 16,681 | | | – | | | – | | | 16,681 | | | £1.80 | | 09 Jul 99 | | 09 Jul 06 |
| | | | 19993 | | | 150,000 | | | – | | | – | | | 150,000 | | | £0.92 | | 28 Sep 03 | | 28 Sep 10 |
| | | | 19994 | | | 124,000 | | | – | | | – | | | 124,000 | | | £1.25 | | 24 Sep 04 | | 24 Sep 11 |
| | | | 19994 | | | 11,637 | | | – | | | – | | | 11,637 | | | £3.33 | | 31 Dec 04 | | 31 Dec 11 |
| | | | 19994 | | | – | | | 25,493 | | | – | | | 25,493 | | | £3.04 | | 26 Apr 05 | | 26 Apr 12 |
| | | | 19994 | | | – | | | 34,925 | | | – | | | 34,925 | | | £2.33 | | 26 Sep 05 | | 26 Sep 12 |
| | | SAYE | | | 6,250 | | | – | | | – | | | 6,250 | | | £1.52 | | 29 Oct 04 | | 29 Apr 05 |
|
| Total | | | | | | | 651,496 | | | 60,418 | | | (23,580 | ) | | 688,334 | | | | | | | | | | | | |
|
| Dr Thomas Monath | | | 19993 | | | 84,529 | | | – | | | – | | | 84,529 | | | £0.36 | | 30 Nov 02 | | 30 Nov 09 |
| | | | 19994 | | | 100,000 | | | – | | | – | | | 100,000 | | | £0.92 | | 28 Sep 03 | | 28 Sep 10 |
| | | | 19994 | | | 147,110 | | | – | | | – | | | 147,110 | | | £1.25 | | 24 Sep 04 | | 24 Sep 11 |
| | | | 19994 | | | – | | | 30,403 | | | – | | | 30,403 | | | £3.04 | | 26 Apr 05 | | 26 Apr 12 |
| | | | 19994 | | | – | | | 38,575 | | | – | | | 38,575 | | | £2.33 | | 26 Sep 05 | | 26 Sep 12 |
|
| Total | | | | | | | 331,639 | | | 68,978 | | | – | | | 400,617 | | | | | | | | | | | | |
|
Notes | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | 1) | | The market value of these shares exercised by Dr Brown, Mr Cameron and Mr Higgins at the time of exercise was 238p per share. |
| | 2) | | The market value of these shares exercised by Dr Brown and Mr Cameron at the time of exercise was 247p per share. |
| | 3) | | The performance condition for those options granted under the 1995 scheme, the 1996 scheme and the 1999 plan is either: |
| | a) | | that the percentage growth in the Company’s share price over the three years from the date of grant must exceed the percentage growth in the total return for the FTSE All-Share index over that three year period; or |
| | b) | | that the average percentage share price movements of the Company over each of the three years beginning on a date not earlier than the grant date and ending on the date of exercise must exceed the average movements in the FTSE All-Share Index over each of those three years. |
| | 4) | | The performance condition for those options granted under the 1999 plan compares the Company’s TSR to the TSR of a chosen group of biotechnology companies over a three-year period. A median ranking must be achieved before any part of the option may be exercised (50% of the option) and an upper quartile ranking must be achieved for the option to vest in full. This condition if not initially achieved in full can be further measured over a four or five-year period measured from the same fixed base point. |
| | 5) | | No performance conditions apply to SAYE options. |
| | 6) | | All of the above options were granted for nil consideration, and are held over 10p ordinary shares in the Company. |
| | 7) | | The market price of shares at 31 December 2002 was 277p and the range during the year was 181p to 379p per share. |
| | Further information on each of the Company’s share option schemes, including the number of options outstanding, exercise prices and exercise periods, is set out in note 21 to the financial statements. |
| (6e) | | Long-Term Share Incentive Plan (audited)
The Acambis Share Incentive Plan (“LTIP”) has been established for Executive Directors and certain senior employees. The plan is designed to encourage participants to focus their efforts on longer-term growth in shareholder value and to encourage commitment to remain with the Acambis Group.
Long-term incentive awards are made, upon the recommendation of the Committee, by the Trustees of the Acambis Employees’ Trust and comprise performance shares being a right to acquire, at no cost, a fixed maximum number of shares in the Company. The right to acquire shares can only be exercised after three years and is subject to a performance target.
Awards made from September 2001 onwards are subject to performance conditions relating to the performance of Acambis’ TSR compared to a comparator group of other companies within the industry over a single three-year period with no opportunity for re-testing. For grants in 2003 this condition will apply and the comparator companies will be as detailed above in the section on share options. The maximum allocation of shares would be achieved if Acambis is ranked in the upper quartile of the comparator group, being prorated down to a 30% allocation at a ranking of the median. No allocation will be made if Acambis’ ranking falls below the median. The performance condition is measured over a three-year period beginning at date of award. For the purposes of TSR calculation, the Company’s TSR will be averaged over the three months preceding the commencement of the period and the three months preceding a measurement date to ensure that results are not influenced by short-term volatility.
From 2003, awards to Executive Directors will be subject to an additional performance condition that requires the Committee to be satisfied that there has been improvement in the Company’s underlying financial performance over the relevant performance period.
The Directors who held office at 31 December 2002 hold shares of the Company under the LTIP as follows: |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| |
|
| | | | | | | | | | | | | | | | | | | Exercise | | Earliest date | | |
| Directors | | 1 January 2002 | | Granted | | Exercised1 | | 31 December 2002 | | price2 | | of exercise | | Expiry Date |
|
| Dr John Brown | | | 195,488 | 4 | | | – | | | | – | | | | 195,488 | | Nil | | 27 Sep 04 | | 27 Mar 05 |
| | | | – | | | | 83,112 | 3,4 | | | – | | | | 83,112 | | | Nil | | 22 Apr 05 | | 22 Oct 05 |
|
| Total | | | 195,488 | | | | 83,112 | | | | – | | | | 278,600 | | | | | | | | | | | | |
|
| Gordon Cameron | | | 123,711 | | | | – | | | | (123,711 | ) | | | – | | | Nil | | 06 Apr 02 | | 06 Oct 02 |
| | | | 139,084 | 4 | | | – | | | | – | | | | 139,084 | | | Nil | | 27 Sep 04 | | 27 Mar 05 |
| | | | – | | | | 2,500 | 5,7 | | | – | | | | 2,500 | | | Nil | | 19 Apr 03 | | 19 Oct 03 |
| | | | – | | | | 30,928 | 6,7 | | | – | | | | 30,928 | | | Nil | | 06 Apr 04 | | 06 Oct 04 |
| | | | – | | | | 59,366 | 3,4 | | | – | | | | 59,366 | | | Nil | | 22 Apr 05 | | 22 Oct 05 |
|
| Total | | | 262,795 | | | | 92,794 | | | | (123,711 | ) | | | 231,878 | | | | | | | | | | | | |
|
| Nicolas Higgins | | | 116,541 | 4 | | | – | | | | – | | | | 116,541 | | | Nil | | 27 Sep 04 | | 27 Mar 05 |
| | | | – | | | | 49,547 | 3,4 | | | – | | | | 49,547 | | | Nil | | 22 Apr 05 | | 22 Oct 05 |
|
| Total | | | 116,541 | | | | 49,547 | | | | – | | | | 166,088 | | | | | | | | | | | | |
|
| Dr Thomas Monath | | | – | | | | 59,090 | 3,4 | | | – | | | | 59,090 | | | Nil | | 22 Apr 05 | | 22 Oct 05 |
|
| Total | | | – | | | | 59,090 | | | | – | | | | 59,090 | | | | �� | | | | | | | | |
|
| 1) | | The market value of these shares at the time of the award, 6 April 1999, was 99p per share. The market value of these shares at the date of vesting, 8 April 2002, was 323.5p per share and at the date of exercise, 25 September 2002, was 239p per share. The performance target attached to this award measured the increase in market capitalisation of the Company over a three-year period. Given the market capitalisation in the Company over the three-year period had exceeded the maximum target of a 100% increase, 100% of this award vested. |
| 2) | | The exercise price for all awards made under the LTIP is £1.00 in total for the exercise of any number of shares comprised in an award. All LTIP awards are held over ordinary 10p shares in the Company. |
| 3) | | The market value of these shares at the date of the award, 22 April 2002, was 321p per share. The performance target attached to these awards are as described for awards made from September 2001 onwards. |
| 4) | | The performance condition for these awards compare the Company’s TSR to the TSR of a chosen group of biotechnology companies over a three-year period. A median ranking must be achieved before any part of the award may vest (30% of the award) and an upper quartile ranking must be achieved for the award to vest in full. After three years, vested plan shares may be left in the Trust and participants can then receive a grant of a further one matching share for each four plan shares so deposited. The matching shares will vest provided the participant remains employed and does not withdraw his plan shares for a further two years. |
| 5) | | In accordance with the rules of the LTIP, Mr Cameron elected to receive one third of his 2001 bonus (10,000 shares) in the form of Acambis shares (see annual bonus on page 56). These shares are disclosed within the table of Directors interests’ on page 59. This award represents the one matching share for every four shares to which Mr Cameron would be entitled if the basic award (10,000 shares) were held by the Trust for a period of one year from award. |
| 6) | | Following the vesting of Mr Cameron’s award during the year (see note 1), he elected to leave the balance of these plan shares (after paying applicable taxes) with the Trust. Provided Mr Cameron remains employed and does not withdraw his plan shares for a further two years, he can then receive an additional one matching share for each four plan shares so deposited. |
| 7) | | These awards are not subject to performance conditions as the bonus awards invested and the plan shares deposited have already been performance tested. |
| | | Gains made by Directors on share options and LTIPs (audited)
The table below shows gains made by individual Directors from the exercise of share options and LTIPs. The gains are calculated as at the exercise date, although the shares may have been retained. |
| | | | | | | | | |
| |
|
| | | 2002 | | 2001 |
| | | £'000 | | £'000 |
|
| Dr John Brown | | | 89 | | | 330 |
| Gordon Cameron | | | 585 | | | – |
| Nicolas Higgins | | | 16 | | | 343 |
|
| Total gains on share options & LTIPs | | | 690 | | | 673 |
|
| | | Acambis’ Total Shareholder Return (“TSR”) performance
Acambis’ TSR performance is shown against the FTSE All-Share Pharmaceuticals & Biotech Index. This index has been chosen as Acambis is a constituent of this sector. |
| | 31 December 1997 = 0
On behalf of the Board
Alan Dalby,Non-executive Director
27 March 2003 |
| (7a) | | Substantial shareholdings
The shareholdings in the table set out below represent the shareholdings amounting to 3% or more of the ordinary share capital of the Company that had been notified to the Company in accordance with Sections 198 to 208 of the Companies Act 1985, at the time of publication of the 2000, 2001 and 2002 Annual Reports.
The figures in the columns entitled “2001 Annual Report” and “2000 Annual Report” do not necessarily represent the current shareholdings or percentages held by the respective shareholders. |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | As at 11 March 2003 | | 2001 Annual Report | | 2000 Annual Report |
| | |
|
| | | Number of | | | | | | Number of | | | | | | Number of | | |
| | | shares held | | Percentage | | shares held | | Percentage | | shares held | | Percentage |
|
Baxter International, Inc.1 | | | 16,713,603 | | | 16.87 | % | | | 11,746,041 | | | 12.62 | % | | | 9,071,200 | | | 10.23 | % |
| Fidelity Management & Research Company | | | 6,010,194 | | | 6.06 | % | | | – | | | – | | | – | | | – |
| Barclays PLC | | | 4,950,803 | | | 5.00 | % | | | – | | | – | | | – | | | – |
| Morley Fund Management Limited | | | 4,022,012 | | | 4.06 | % | | | 3,650,893 | | 3.92 | % | | | – | | | – |
| Legal & General Investment Management Limited | | | 3,067,331 | | | 3.10 | % | | | – | | | – | | | – | | | – |
| Philips & Drew Life Limited | | | – | | | – | | | – | | | – | | | 4,122,260 | | | 4.65 | % |
|
| | | 1) | | In December 2000, Acambis entered into a strategic alliance with Baxter, as a result of which at 11 March 2003 equity subscriptions of £19.9m (net of expenses) had been received, representing 16.7 million new ordinary shares as shown above. The remaining subscription totalling £7.0m, in respect of a further 4.6 million new shares, was received on 27 March 2003, taking Baxter’s holding to 21.3 million new ordinary shares representing a 20.6% holding at that date. |
| | | As far as is known to the Directors, the Company is not directly or indirectly owned or controlled by another corporation or by any other government and the only shareholder directly or indirectly owning more than 10% of the Company is shown in the above table. All shareholders have the same voting rights. |
| | | Analysis of share register at 11 March 2003 |
| | | | | | | | | | | | | | | | | |
| |
|
| | Number | | Percentage of | | Number | | Percentage of issued |
| Shareholding | | of holders | | total holders | | of shares | | share capital |
|
| 1 — 1,000 | | | 1,752 | | | 56.1 | | | 939,545 | | | 0.9 |
| 1,001 — 5,000 | | | 914 | | | 29.3 | | | 2,068,550 | | | 2.1 |
| 5,001 — 100,000 | | | 351 | | | 11.2 | | | 8,593,069 | | | 8.7 |
| 100,001 — 500,000 | | | 65 | | | 2.1 | | | 15,912,344 | | | 16.0 |
| 500,001 — 1,000,000 | | | 17 | | | 0.6 | | | 10,858,348 | | | 11.0 |
| 1,000,001 and over | | | 23 | | | 0.7 | | | 60,730,002 | | | 61.3 |
|
| | | | 3,122 | | | 100.0 | | | 99,101,858 | | | 100.0 |
|
| | | As at 11 March 2003, US record holders, including Baxter and American Depository Receipt (“ADR”) holders, held approximately 21.3% of the issued share capital of ordinary 10p shares. |
| (9a) | | Nature of trading market |
| | | Comparative market price information |
| (9c) | | Acambis shares are traded on the London Stock Exchange under the symbol “ACM” and on the US Nasdaq National Market in the form of ADRs under the symbol “ACAM”.
The following tables set out the high and low closing mid-market prices for Acambis’ shares and close prices for ADRs: |
| | | | | | | | | | | | | | | | | | | |
| | | | | | | | | Shares | | | | | | ADRs |
| | | | |
|
| | | | | High | | Low | | High | | Low |
| | | | |
|
| | | Calendar year | | Pence per ordinary share | | Dollars per ADR |
| | |
|
| | | 1998 | | | 262.9 | | | 65.7 | | | n/a | | | n/a |
| | | 1999 | | | 125.0 | | | 36.0 | | | n/a | | | n/a |
| | | 2000 | | | 134.0 | | | 51.5 | | | n/a | | | n/a |
| | | 2001 | | | | | | | | | | | | | | | | |
| | | First quarter | | | 136.0 | | | 103.5 | | | n/a | | | n/a |
| | | Second quarter | | | 137.5 | | | 104.5 | | | 18.05 | | | 16.64 |
| | | Third quarter | | | 153.5 | | | 114.0 | | | 23.80 | | | 16.50 |
| | | Fourth quarter | | | 353.0 | | | 147.0 | | | 51.09 | | | 22.76 |
| |
| | | 2002 | | | | | | | | | | | | | | | | |
| | | First quarter | | | 379.0 | | | 317.5 | | | 55.03 | | | 45.01 |
| | | Second quarter | | | 339.5 | | | 208.5 | | | 46.70 | | | 31.66 |
| | | Third quarter | | | 287.5 | | | 181.0 | | | 44.84 | | | 28.37 |
| | | Fourth quarter | | | 291.0 | | | 226.5 | | | 47.46 | | | 35.42 |
| |
| | | Monthly high and low prices (for the last full six months) are as follows: | | | | | | | | | | | | | | |
| | | September 2002 | | | 266.0 | | | 225.0 | | | 43.45 | | | 34.97 |
| | | October 2002 | | | 261.5 | | | 226.5 | | | 41.20 | | | 35.42 |
| | | November 2002 | | | 262.0 | | | 246.0 | | | 41.50 | | | 38.55 |
| | | December 2002 | | | 291.0 | | | 242.5 | | | 47.46 | | | 37.21 |
| | | January 2003 | | | 281.0 | | | 207.5 | | | 45.44 | | | 33.26 |
| | | February 2003 | | | 230.0 | | | 207.5 | | | 37.15 | | | 32.35 |
| | |
|
| | | As of 11 March 2003, the mid-market price of an Acambis share was 222p and the close price of an Acambis ADR was $35.15. The number of outstanding ordinary shares of 10p each at that date was 99,101,858. |
| | | Comparative dividend information
Acambis has never paid any cash dividends on its shares and does not anticipate paying cash dividends for the foreseeable future. |
| | | The Annual General Meeting of the Company will be held at 10 a.m. on 13 May 2003 at the offices of Weil, Gotshal & Manges, One South Place, London EC2M 2WG. The Notice of the Annual General Meeting accompanies this Annual Report and Form 20-F.
|
| | | Special business
The special business to be conducted at the Annual General Meeting (“AGM”) is detailed in the Notice of the AGM. In addition to the reappointment of PricewaterhouseCoopers LLP as auditors (as discussed below) authority is being sought:
|
| • | | to change certain rules within the Acambis share option and share incentive plans (the “Share Plans”). The Directors will seek approval to amend the Share Plans: |
| a) | | to provide that during the five years following the date of the 2003 AGM the number of shares which may be issued in connection with share options or long-term incentive awards may not exceed 5% of the ordinary share capital of the Company in issue from time to time. This will replace the current dilution limits applying under the Share Plans;
|
| b) | | to amend the Share Plans so that the Company has the authority to update these plans during the five-year period following the date of the AGM; and
|
| c) | | such that a qualifying employee or Director may, in any financial year, be granted discretionary share options over shares with a market value, as at the date of grant, of up to two-times the participant’s base annual salary. This limit could be extended if the participant had been recruited, in which case the limit shall be four-times his/her base annual salary within 12 months of joining or, exceptionally, at the discretion of the Remuneration Committee, eight-times his/her base annual salary if the participant is based in the US and forfeits vested options with an immediate previous employer by reason on joining Acambis; |
| • | | to introduce a new all-employee share plan for US-based employees. The new plan is similar in style to the Acambis 1995 Savings-Related Share Option Scheme for UK employees; |
| • | | to give the Company the authority to purchase up to 10% of its own issued ordinary shares at a price of not less than 10 pence per share and not more than 5% above the average of the middle market quotations of the Company’s shares as shown in the London Stock Exchange Daily Official List for the five dealing days before the purchase is made. This authority shall expire at the conclusion of the Company’s next AGM or 15 months from the passing of this resolution, whichever is the earlier; and |
| • | | to disapply the statutory pre-emption rights in respect of the allotment of new shares pursuant to rights issues or otherwise for cash up to an aggregate nominal value of £518,691, being 5% of the currently issues ordinary shares of the Company in accordance with the current guidelines of the Investment Committee of the Association of British Insurers and the National Association of Pension Funds. This authority shall expire at the conclusion of the Company’s next AGM or 15 months from the passing of this resolution, whichever is the earlier. |
| | | Auditors
Following the conversion of our auditors PricewaterhouseCoopers to a limited liability partnership (“LLP”) from 1 January 2003, PricewaterhouseCoopers resigned and the Directors appointed its successor, PricewaterhouseCoopers LLP, as auditors. A resolution to reappoint PricewaterhouseCoopers LLP as auditors to the Company will be proposed at the AGM. This authority shall expire at the conclusion of the Company’s next General Meeting. PricewaterhouseCoopers were appointed as auditors of the Company in March 2002, at which time the current audit partner was also appointed.
|
| (10b) | | Memorandum and Articles of Association
A summary of the principal provisions of the Group’s Memorandum and Articles of Association can be found by referring to the Registration Statement on Form F-4 (filed with the Securities and Exchange Commission in April 1999) relating to the acquisition of Acambis Inc., as well as the document (filed in November 2000) in relation to the proposed subscription by Baxter.
A copy of both the Memorandum and Articles of Association of the Company has been filed with the Registrar of Companies. The Memorandum contains the fundamental provisions of the Company’s constitution. The Articles contain the rules for the internal management and control of the Company.
|
| (10h) | | Documents on display
Certain documents referred to in this Annual Report and Form 20-F are available for inspection at the registered office of the Company. |
| | | | | | | | | | | | | |
| | | COMPANY INFORMATION AND ADVISERS | | | | | |
| |
| | Company Secretary, registered | | Announcements | | Registrars |
| | | office and Group headquarters | | First quarter results – May | | Capita Registrars |
| | | Elizabeth Brown | | Second quarter/interim results – September | | The Registry |
| | | Peterhouse Technology Park | | Third quarter results – November | | 34 Beckenham Road |
| | | 100 Fulbourn Road | | Final results – March | | Beckenham |
| | | Cambridge CB1 9PT | | | | | | Kent BR3 4TU |
| | | UK | | Corporate advisers | | UK |
| | | Tel: +44 (0) 1223 275 300 | | | | | | | | |
| | | | | | | Financial advisers and stockbrokers | | Auditors |
| (4a) | | Registered number: 2863682 | | Deutsche Bank | | PricewaterhouseCoopers LLP |
| | | Date of incorporation: 19 October 1993 | | Winchester House | | Abacus House |
| | | Country of jurisdiction: England and Wales | | 1 Great Winchester Street | | Castle Park |
| | | | | | | London EC2N 2DB | | Cambridge CB3 0AN |
| | | US operations | | UK | | UK |
| | | 38 Sidney Street | | | | | | | | |
| | | Cambridge | | Legal advisers | | | | |
| | | Massachusetts 02139 | | Weil, Gotshal & Manges | | | | |
| | | USA | | One South Place | | | | |
| | | Tel: +1 617 761 4200 | | London EC2M 2WG | | | | |
| | | | | | | UK | | | | |
| | | Shareholder information | | | | | | | | |
| | | The share price is obtainable in most UK and | | Patent Agents | | | | |
| | | US national newspapers and on Acambis' | | Reddie & Grose | | | | |
| | | website atwww.acambis.com | | Daedalus House | | | | |
| | | | | | | Station Road | | | | |
| | | London Stock Exchange mnemonic – ACM | | Cambridge CB1 2RE | | | | |
| | | US Nasdaq National Market ticker
symbol – ACAM | | UK | | | | |
| | | Reuters reference – ACM.L | | Clark & Elbing LLP | | | | |
| | | | | | | 176 Federal Street | | | | |
| | | Analyst coverage of Acambis | | Boston | | | | |
| | | Altium Capital | | Massachusetts 02110 | | | | |
| | | Cazenove | | USA | | | | |
| | | C. E. Unterberg, Towbin | | | | | | | | |
| | | Commerzbank | | Bankers | | | | |
| | | Credit Suisse First Boston | | Barclays Bank PLC | | | | |
| | | Deutsche Bank | | Corporate Banking | | | | |
| | | Evolution Beeson Gregory | | PO Box 885 | | | | |
| | | Goldman Sachs | | Mortlock House | | | | |
| | | ING Barings | | Vision Park | | | | |
| | | KBC Peel Hunt | | Histon | | | | |
| | | Merrill Lynch | | Cambridge CB4 9DE | | | | |
| | | Nomura | | UK | | | | |
| | | Teather & Greenwood | | | | | | | | |
| | | UBS Warburg | | | | | | | | |
| | | WestLB Panmure | | | | | | | | |
| | | Williams de Broë | | | | | | | | |
INDEX
| | | | | | | | | | | | | | | | | | | | | |
| Accounting policies | | | 22-24 | | | Goodwill | | | 23,27 | | | Senior management | | | 11 | |
| Administrative costs | | | 25 | | | Group Executive Committee | | | 53 | | | Share capital | | | 33-34 | |
| ADR pricing | | | 65 | | | Government grants | | | 23 | | | Share options | | | 24,33-34,59-61 | |
| Annual General Meeting | | | 66 | | | | | | | | | | | Share pricing | | | 65 | |
| Arilvax® | | | 8,13 | | | Health and safety | | | 51 | | | Share register analysis | | | 64 | |
| Audit Committee | | | 52 | | | H. pylori | | | 9,13 | | | Shareholder information | | | 64-66 | |
| Auditors | | | 16-17,66 | | | HolaVax-ETEC | | | 9,13 | | | Shareholders’ | | | 35 | |
| Aventis Pasteur | | | 29-30,45 | | | HolaVax-Typhoid | | | 9,13 | | | Smallpox | | | 2-3,6-8,12-13 | |
| | | | | | | | | | | | | | | Staff costs | | | 26 | |
| Balance sheets | | | 19,20 | | | Independent auditors’ | | | 16-17 | | | Statement of total recognised gains and losses report | | | 18 | |
| Baxter | | | 7,12,45 | | | Interest | | | 25 | | | Stock | | | 23,30 | |
| Berna Biotech | | | 13 | | | Internal controls | | | 53 | | | Stock options (see Share options) | | | | |
| Board of Directors | | | 10-11,49,50,52 | | | Investments | | | 23,28-30 | | | Strategy | | | 6-9 | |
| | | | | | | | | | | | | | | Subsidiaries | | | 28 | |
| Capital expenditure | | | 15 | | | Japanese encephalitis | | | | | | Substantial shareholdings | | | 64 | |
| Cash | | | 15 | | | (see ChimeriVax-JE) | | | | | | Summarised Group statements | | | 48-49 | |
| Cash flow | | | 21,40-41 | | | Joint venture | | | 23,29-30,45 | | | | | | | | | |
| CDC | | | 44-45 | | | | | | | | | | | Taxation | | | 15,23,26,46 | |
| Chairman’s statement | | | 1 | | | Leases | | | 24,31,33 | | | Trade investments | | | 29 | |
| Charitable donations | | | 51 | | | | | | | | | | | Trading results | | | 14-15 | |
| ChimeriVax-Dengue | | | 13 | | | Manufacturing | | | 4-5,14-15 | | | Travel vaccines | | | 8-9 | |
| ChimeriVax-JE | | | 8-9,13 | | | MD&A | | | 42-47 | | | | | | | | | |
| ChimeriVax-West Nile | | | 9,13 | | | Memorandum and Articles of | | | | | | US GAAP reconciliation | | | 37-42 | |
| C. difficile | | | 9,13 | | | Association | | | 66 | | | | | | | |
| Clinical operations | | | 4 | | | MVA | | | 3,7,8,12-13 | | | VIG | | | 3,8,12 | |
| Collaboration agreements | | | 44-46 | | | | | | | | | | | | | | | | | |
| Company information | | | | | | Nominations Committee | | | 53 | | | | | | | | | |
| and advisers | | | 67 | | | Notes to Group financial statements | | | 22-42 | | | West Nile virus | | | 9,13 | |
| Corporate governance statement | | | 52-55 | | | | | | | | | | | | | | | |
| Corporate social responsibility | | | 11,51 | | | | | | | | | | | | | | | | | |
| Cost of sales | | | 14,22 | | | Operating review | | | 12-13 | | | | | | | | | |
| Creditors | | | 31 | | | Organisational structure | | | 28 | | |
| | | | | | | | | | | | Abbreviations used in this document |
| Debtors | | | 30 | | | Product update | | | 12-13 | | | | | | | |
| Directors’ interests | | | 51,59 | | | Profit and loss account | | | 18 | | | ADR | American Depositary Receipt |
| Directors’ remuneration | | | 26,58 | | | Pensions | | | 23,37,57,58 | | | APB | Accounting Principles Board |
| Directors’ report | | | 51 | | | Political donations | | | 51 | | | BIA | BioIndustry Association |
| Directors’ | | | | | | Post balance sheet | | | | | | CDC | US Centers for Disease Control and |
| responsibilities | | | 16 | | | events | | | 37 | | | | Prevention |
| Discontinued operations | | | 25 | | | Property | | | 47 | | | EITF | Emerging Issues Task Force |
| Dividends | | | 51 | | | | | | | | | | | FASB | Financial Accounting Standards Board |
| | | | | | | R&D | | | 3,12-13 | | | FDA | US Food and Drug Administration |
| Earnings per share | | | 27 | | | Reconciliation to US GAAP | | | 37-42 | | | FIN | FASB Interpretation Number |
| Employees | | | 15,26,51 | | | Registrars | | | 67 | | | FRS | Financial Reporting Standard |
| Environment | | | 51 | | | Related party transactions | | | 37 | | | R&D | Research and development |
| Evans Vaccines Limited | | | 46 | | | Remuneration Committee | | | 52,56 | | | SEC | US Securities and Exchange Commission |
| Exchange rate information | | | 47 | | | Remuneration report | | | 56-63 | | | SFAS | Statement of Financial Accounting |
| | | | | | | Reserves | | | 35 | | | | Standard |
| Financial commitments | | | 36-37 | | | Risk factors | | | 54-55 | | | UITF | Urgent Issues Task Force |
| Financial instruments | | | 31-33 | | | | | | | | | | | UK GAAP | United Kingdom generally accepted |
| Financial review | | | 14-15 | | | Sales and marketing | | | 5 | | | | accounting principles |
| Fixed asset investments | | | 23,28,30 | | | Segmental information | | | 24 | | | US GAAP | United States generally accepted |
| Foreign currencies | | | 24,32,47 | | | Selected financial information | | | 48-49 | | | | accounting principles |
68
CROSS-REFERENCE TO FORM 20-FThis cross-reference to Form 20-F is provided to assist those who are more familiar with the contents and layout of an Annual Report on Form 20-F. We have denoted this Form 20-F information by using this symbol (1a), where the number denotes the specific item on the cross-reference. The information in this document that is referred to in the following table shall be deemed to be filed with the Securities and Exchange Commission for all purposes.
| | | | | | | |
| Item | | | | Page | |
|
| 1 | | Identity of directors, senior management and advisers | | | n/a | |
|
|
|
|
| 2 | | Offer statistics and expected timetable | | | n/a | |
|
|
|
|
| 3 | | Key Information | | | | |
| a) | | Selected financial data | | | 48-49 | |
| b) | | Capitalisation and indebtedness | | | n/a | |
| c) | | Reasons for the offer and use of proceeds | | | n/a | |
| d) | | Risk factors | | | 54-55 | |
|
|
|
|
| 4 | | Information on the Company | | | | |
| a) | | History and development of the Company | | | 1-15, 67 | |
| b) | | Business overview | | | 1-15, 24, 43-44, 54-55 | |
| c) | | Organisational structure - note 14(a) | | | 28 | |
| d) | | Property, plants and equipment | | | 47 | |
|
|
|
|
| 5 | | Operating and financial review and prospects | | | | |
| a) | | Operating results | | | 14-15, 43 | |
| b) | | Liquidity and capital resources | | | 44 | |
| c) | | Research and development, patents and licences etc | | | 14, 43, 44-46 | |
| d) | | Trend information | | | 14-15, 43 | |
|
|
|
|
| 6 | | Directors, senior management and employees | | | | |
| a) | | Directors and senior management | | | 50-51 | |
| b) | | Compensation | | | 56-63 | |
| c) | | Board practices | | | 52-53 | |
| d) | | Employees — note 8 | | | 26 | |
| e) | | Share ownership | | | 59-62 | |
|
|
|
|
| 7 | | Major shareholders and related party transactions | | | | |
| a) | | Major shareholders | | | 64 | |
| b) | | Related party transactions | | | 37 | |
| c) | | Interests of experts and counsel | | | n/a | |
|
|
|
|
| 8 | | Financial information | | see Item 17 |
|
|
|
|
| 9 | | The offer and listing | | | | |
| a) | | The offer and listing details | | | 65 | |
| b) | | Plan of distribution | | | n/a | |
| c) | | Markets | | | 65 | |
| d) | | Selling shareholders | | | n/a | |
| e) | | Dilution | | | n/a | |
| f) | | Expenses of the issue | | | n/a | |
|
|
|
|
| 10 | | Additional information | | | | |
| a) | | Share capital | | | n/a | |
| b) | | Memorandum and Articles of Association | | | 66 | |
| c) | | Material contracts | | | 44-46 | |
| d) | | Exchange controls | | | 47 | |
| e) | | Taxation | | | 46 | |
| f) | | Dividends and paying agents | | | n/a | |
| g) | | Statement by experts | | | n/a | |
| h) | | Documents on display | | | 66 | |
| i) | | Subsidiary information | | | n/a | |
|
|
|
|
| 11 | | Quantitative and qualitative disclosure about market risk | | | | |
| | Financial instruments | | | 31-33 | |
|
|
|
|
| 12 | | Description of securities other than equity securities | | | n/a | |
|
|
|
|
| 13 | | Defaults, dividend arrears and delinquencies | | none |
|
|
|
|
| 14 | | Material modifications to the rights of security holders | | | n/a | |
|
|
|
|
| 15 | | Controls and procedures | | | 53 | |
|
|
|
|
| 16 | | Reserved item | | | n/a | |
|
|
|
|
| 17 | | Financial statements | | | | |
| a) | | Directors' responsibilities | | | 16 | |
| b) | | Independent auditors' report to members of Acambis plc | | | 16-17 | |
| c) | | Group profit and loss account | | | 18 | |
| d) | | Group statement of total recognised gains and losses | | | 18 | |
| e) | | Group balance sheet | | | 19 | |
| f) | | Company balance sheet | | | 20 | |
| g) | | Group cash flow statement | | | 21 | |
| h) | | Notes to Group financial statements | | | 22-49 | |
|
|
|
|
| 18 | | Financial statements | | see Item 17 |
|
|
|
|
| 19 | | Exhibits | | | n/a | |
| | | | | | | 69 |
Acambis plc
Peterhouse Technology Park
100 Fulbourn Road
Cambridge CB1 9PT UK
Tel: +44 (0) 1223 275 300
Fax: +44 (0) 1223 416 300
E-mail: acambis@acambis.com
www.acambis.com
Item 19. Exhibits
| | | |
| Exhibit | | |
| Number | | Description of Exhibit |
| |
|
| 1.1* | | Memorandum and Articles of Association of the Company (incorporated herein by reference to Exhibit 3.1 to the Company’s Registration Statement on Form F-4, as filed with the Securities and Exchange Commission on February 10, 1999 (File No. 333-72077)). |
| 2.1* | | Deposit Agreement between and among Acambis plc and The Bank of New York, as Depositary (incorporated herein by reference to the Company’s Registration Statement on Form F-6 as filed with the Securities and Exchange Commission on February 13, 2001 (File No. 333-13166)). |
| 4.1* | | Option Agreement between Peptide Therapeutics Limited and the joint ventures between OraVax, Inc. and Pasteur Mérieux Serums et Vaccins known as Pasteur Mérieux OraVac S.N.C. and OraVax Mérieux Co., dated April 17, 1998 (incorporated herein by reference to Exhibit 10.2 to the Company’s Registration Statement on Form F-4, as filed with the Securities and Exchange Commission on April 9, 1999 (File No. 333-72077)). |
| 4.2* | | Overview Agreement between Peptide Therapeutics Limited and Pasteur Mérieux Serums et Vaccins S.A., dated January 25, 1999 (incorporated herein by reference to Exhibit 10.10 to the Company’s Registration Statement on Form F-4, as filed with the Securities and Exchange Commission on April 9, 1999 (File No. 333-72077)). |
| 4.3* | | Standstill Agreement between Peptide Therapeutics Limited and Pasteur Mérieux Serums et Vaccins S.A., dated January 25, 1999 (incorporated herein by reference to Amendment No. 2 to Schedule 13D filed on January 28, 1999 by Peptide Therapeutics Group plc (File No. 005-48363)). |
| 4.4* | | Director’s Service Agreement between Peptide Therapeutics Group plc and John Brown, dated March 1, 1997 (incorporated herein by reference to Exhibit 10.18 to the Company’s Registration Statement on Form F-4, as filed with the Securities and Exchange Commission on February 10, 1999 (File No. 333-72077)). |
| 4.5* | | Director’s Service Agreement between Peptide Therapeutics Group plc and Gordon Cameron, dated March 1, 1997, as amended September 18, 1998 (incorporated herein by reference to Exhibit 10.17 to the Company’s Registration Statement on Form F-4, as filed with the Securities and Exchange Commission on February 10, 1999 (File No. 333-72077)). |
| * | | Incorporated by reference. |
| | | |
| Exhibit | | |
| Number | | Description of Exhibit |
| |
|
| 4.6* | | Director’s Service Agreement between Peptide Therapeutics Group plc and Nicholas Higgins, dated November 29, 1996, as amended September 18, 1998 (incorporated herein by reference to Exhibit 10.16 to the Company’s Registration Statement on Form F-4, as filed with the Securities and Exchange Commission on February 10, 1999 (File No. 333-72077)). |
| 4.7 | | Letter of Appointment between Acambis plc and Thomas Monath, dated March 11, 2002, as amended February 13, 2003, and Employment Agreement between OraVax, Inc. and Thomas Monath, dated October 16, 1991. |
| 4.8* | | Letter of Appointment between Peptide Therapeutics Group plc and Alan Smith, dated January 8, 1998, as amended April 30, 1998 (incorporated herein by reference to Exhibit 10.20 to the Company’s Registration Statement on Form F-4, as filed with the Securities and Exchange Commission on February 10, 1999 (File No. 333-72077)). |
| 4.9* | | Letter of Appointment between Peptide Therapeutics Group plc and Alan Dalby, dated March 25, 1998 (incorporated herein by reference to Exhibit 10.19 to the Company’s Registration Statement on Form F-4, as filed with the Securities and Exchange Commission on February 10, 1999 (File No. 333-72077)). |
| 4.10 | | Letter of Appointment between Acambis plc and Michael Lytton, dated March 12, 2001. |
| 4.11 | | Letter of Appointment between Acambis plc and Victor Schmitt, dated December 4, 2000. |
| 8.1 | | List of Significant Subsidiaries of Acambis plc |
| 10.1 | | Consent of PricewaterhouseCoopers LLP |
| 12.1 | | Certification of John Brown pursuant to 18 U.S.C. Section 1350, as adopted by Section 906 of the Sarbanes-Oxley Act of 2002 |
| 12.2 | | Certification of Gordon Cameron pursuant to 18 U.S.C. Section 1350, as adopted by Section 906 of the Sarbanes-Oxley Act of 2002 |
| * | | Incorporated by reference. |
SIGNATURES
The registrant hereby certifies that it meets all of the requirements for filing on Form 20-F and that is has duly caused and authorized the undersigned to sign this annual report on its behalf.
| | | |
| | | |
| | | |
| | ACAMBIS PLC |
| | | |
| | By: | /s/ Gordon Cameron |
| | |
|
| | | Name: Gordon Cameron
Title: Chief Financial Officer |
Date: June 30, 2003
CERTIFICATION
I, John Brown, certify that:
1. I have reviewed this annual report on Form 20-F of Acambis plc;
2. Based on my knowledge, this annual report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this annual report;
3. Based on my knowledge, the financial statements, and other financial information included in this annual report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this annual report;
4. The registrant’s other certifying officers and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-14 and 15d-14) for the registrant and have:
| | a. | | designed such disclosure controls and procedures to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this annual report is being prepared; |
| |
| | b. | | evaluated the effectiveness of the registrant’s disclosure controls and procedures as of a date within 90 days prior to the filing date of this annual report (the “Evaluation Date”); and |
| |
| | c. | | presented in this annual report our conclusions about the effectiveness of the disclosure controls and procedures based on our evaluation as of the Evaluation Date; |
5. The registrant’s other certifying officers and I have disclosed, based on our most recent evaluation, to the registrant’s auditors and the audit committee of registrant’s board of directors (or persons performing the equivalent function):
| | a. | | all significant deficiencies in the design or operation of internal controls which could adversely affect the registrant’s ability to record, process, summarize and report financial data and have identified for the registrant’s auditors any material weaknesses in internal controls; and |
| |
| | b. | | any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant’s internal controls; and |
6. The registrant’s other certifying officers and I have indicated in this annual report whether or not there were significant changes in internal controls or in other factors that could significantly affect internal controls subsequent to the date of our most recent evaluation, including any corrective actions with regard to significant deficiencies and material weaknesses.
| | | |
| | | |
| | | |
| | By: | /s/ John Brown |
| | |
|
| | | Name: John Brown
Title: Chief Executive Officer |
Date: June 30, 2003
CERTIFICATION
I, Gordon Cameron, certify that:
1. I have reviewed this annual report on Form 20-F of Acambis plc;
2. Based on my knowledge, this annual report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this annual report;
3. Based on my knowledge, the financial statements, and other financial information included in this annual report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this annual report;
4. The registrant’s other certifying officers and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-14 and 15d-14) for the registrant and have:
| | a. | | designed such disclosure controls and procedures to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this annual report is being prepared; |
| |
| | b. | | evaluated the effectiveness of the registrant’s disclosure controls and procedures as of a date within 90 days prior to the filing date of this annual report (the “Evaluation Date”); and |
| |
| | c. | | presented in this annual report our conclusions about the effectiveness of the disclosure controls and procedures based on our evaluation as of the Evaluation Date; |
5. The registrant’s other certifying officers and I have disclosed, based on our most recent evaluation, to the registrant’s auditors and the audit committee of registrant’s board of directors (or persons performing the equivalent function):
| | a. | | all significant deficiencies in the design or operation of internal controls which could adversely affect the registrant’s ability to record, process, summarize and report financial data and have identified for the registrant’s auditors any material weaknesses in internal controls; and |
| |
| | b. | | any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant’s internal controls; and |
6. The registrant’s other certifying officers and I have indicated in this annual report whether or not there were significant changes in internal controls or in other factors that could significantly affect internal controls subsequent to the date of our most recent evaluation, including any corrective actions with regard to significant deficiencies and material weaknesses.
| | | |
| | | |
| | | |
| | By: | /s/ Gordon Cameron |
| | |
|
| | | Name: Gordon Cameron
Title: Chief Financial Officer |
Date: June 30, 2003
Exhibit Index
| | | |
| Exhibit | | |
| Number | | Description of Exhibit |
| |
|
| 1.1* | | Memorandum and Articles of Association of the Company (incorporated herein by reference to Exhibit 3.1 to the Company’s Registration Statement on Form F-4, as filed with the Securities and Exchange Commission on February 10, 1999 (File No. 333-72077)). |
| 2.1* | | Deposit Agreement between and among Acambis plc and The Bank of New York, as Depositary (incorporated herein by reference to the Company’s Registration Statement on Form F-6 as filed with the Securities and Exchange Commission on February 13, 2001 (File No. 333-13166)). |
| 4.1* | | Option Agreement between Peptide Therapeutics Limited and the joint ventures between OraVax, Inc. and Pasteur Mérieux Serums et Vaccins known as Pasteur Mérieux OraVac S.N.C. and OraVax Mérieux Co., dated April 17, 1998 (incorporated herein by reference to Exhibit 10.2 to the Company’s Registration Statement on Form F-4, as filed with the Securities and Exchange Commission on April 9, 1999 (File No. 333-72077)). |
| 4.2* | | Overview Agreement between Peptide Therapeutics Limited and Pasteur Mérieux Serums et Vaccins S.A., dated January 25, 1999 (incorporated herein by reference to Exhibit 10.10 to the Company’s Registration Statement on Form F-4, as filed with the Securities and Exchange Commission on April 9, 1999 (File No. 333-72077)). |
| 4.3* | | Standstill Agreement between Peptide Therapeutics Limited and Pasteur Mérieux Serums et Vaccins S.A., dated January 25, 1999 (incorporated herein by reference to Amendment No. 2 to Schedule 13D filed on January 28, 1999 by Peptide Therapeutics Group plc (File No. 005-48363)). |
| 4.4* | | Director’s Service Agreement between Peptide Therapeutics Group plc and John Brown, dated March 1, 1997 (incorporated herein by reference to Exhibit 10.18 to the Company’s Registration Statement on Form F-4, as filed with the Securities and Exchange Commission on February 10, 1999 (File No. 333-72077)). |
| 4.5* | | Director’s Service Agreement between Peptide Therapeutics Group plc and Gordon Cameron, dated March 1, 1997, as amended September 18, 1998 (incorporated herein by reference to Exhibit 10.17 to the Company’s Registration Statement on Form F-4, as filed with the Securities and Exchange Commission on February 10, 1999 (File No. 333-72077)). |
| * | | Incorporated by reference. |
| | | |
| Exhibit | | |
| Number | | Description of Exhibit |
| |
|
| 4.6* | | Director’s Service Agreement between Peptide Therapeutics Group plc and Nicholas Higgins, dated November 29, 1996, as amended September 18, 1998 (incorporated herein by reference to Exhibit 10.16 to the Company’s Registration Statement on Form F-4, as filed with the Securities and Exchange Commission on February 10, 1999 (File No. 333-72077)). |
| 4.7 | | Letter of Appointment between Acambis plc and Thomas Monath, dated March 11, 2002, as amended February 13, 2003, and Employment Agreement between OraVax, Inc. and Thomas Monath, dated October 16, 1991. |
| 4.8* | | Letter of Appointment between Peptide Therapeutics Group plc and Alan Smith, dated January 8, 1998, as amended April 30, 1998 (incorporated herein by reference to Exhibit 10.20 to the Company’s Registration Statement on Form F-4, as filed with the Securities and Exchange Commission on February 10, 1999 (File No. 333-72077)). |
| 4.9* | | Letter of Appointment between Peptide Therapeutics Group plc and Alan Dalby, dated March 25, 1998 (incorporated herein by reference to Exhibit 10.19 to the Company’s Registration Statement on Form F-4, as filed with the Securities and Exchange Commission on February 10, 1999 (File No. 333-72077)). |
| 4.10 | | Letter of Appointment between Acambis plc and Michael Lytton, dated March 12, 2001. |
| 4.11 | | Letter of Appointment between Acambis plc and Victor Schmitt, dated December 4, 2000. |
| 8.1 | | List of Significant Subsidiaries of Acambis plc |
| 10.1 | | Consent of PricewaterhouseCoopers LLP |
| 12.1 | | Certification of John Brown pursuant to 18 U.S.C. Section 1350, as adopted by Section 906 of the Sarbanes-Oxley Act of 2002 |
| 12.2 | | Certification of Gordon Cameron pursuant to 18 U.S.C. Section 1350, as adopted by Section 906 of the Sarbanes-Oxley Act of 2002 |
| * | | Incorporated by reference. |