Exhibit 99.1
PRESS RELEASE
| 
| Paris, July 30, 2015 |
Sanofi delivers solid sales and business EPS(1) growth in Q2 2015
Sales growth broad based across geographies and businesses
· Group sales(2) increased 4.9% (+16.1% on a reported basis) to €9,378 million
· Slightly lower Diabetes sales (-3.8%) consistent with previous quarter and significant U.S. market access already granted for Toujeo®
· Genzyme delivered 26.6% growth; rare disease products increased 9.1% and the Multiple Sclerosis franchise sales more than doubled
· Animal Health recorded another strong quarter (+14.2%) driven by NexGard®
· Vaccines sales grew 8.6% led by influenza and booster vaccines
· Emerging Markets(3) sales increased 7.5%
Strong financial performance
· Business net income grew 4.2% at CER (+19.7% on a reported basis) to €1,840 million
· Business EPS increased 5.1% at CER to €1.41 and grew 20.5% on a reported basis
Significant progress in advancing innovative products
· Praluent® approved on July 24, in the U.S. and recommended for approval in the EU
· Positive Phase III topline results announced for LixiLan in diabetes in July and sarilumab in rheumatoid arthritis in May
· Phase IIIb ELIXA study results demonstrated cardiovascular safety of lixisenatide supporting the U.S. regulatory filing
· Olipudase alfa for Niemann-Pick type B advanced to Phase II and Breakthrough Therapy designation granted by FDA
New strategic collaboration with Regeneron in immuno-oncology
· Collaboration includes a PD-1 inhibitor in Phase I and other immuno-oncology antibodies currently in Preclinical development, including LAG3, GITR and PD-L1
2015 financial guidance
· The performance of the second quarter is in line with the full year guidance announced on February 5, 2015. Sanofi expects 2015 Business EPS(1) to be stable to slightly growing versus 2014(4) at constant average exchange rates, barring major unforeseen adverse events
· In addition, the positive currency impact on 2015 full-year business EPS is estimated to be approximately +10%, under the assumption that exchange rates remain stable in the following two quarters at the average rates of June 2015
Sanofi Chief Executive Officer, Olivier Brandicourt commented:
“In the second quarter, Sanofi delivered solid growth on both the top and bottom lines that was consistent with our expectations. We continue to execute on multiple product launches and are excited about the recent approval of Praluent®. We are also investing in our commercial infrastructure, biologic capabilities and R&D programs including in immuno-oncology. Recently, we announced a new organizational structure which will be implemented beginning in January 2016 and will simplify and focus Sanofi to optimize future growth.”
(1) In order to facilitate an understanding of operational performance, Sanofi comments on the business net income statement. Business net income is a non-GAAP financial measure (See Appendix 10 for definitions of financial indicators). The consolidated income statement for Q2 2015 is provided in Appendix 4 and a reconciliation of business net income to IFRS net income reported in Appendix 3; (2) Growth in net sales is expressed at constant exchange rates (CER) unless otherwise indicated (see Appendix 10 for a definition); (3) See page 8; (4) 2014 business EPS was €5.20
Investor Relations: (+) 33 1 53 77 45 45 - E-mail: IR@sanofi.com - Media Relations: (+) 33 1 53 77 46 46 - E-mail: MR@sanofi.com
Web site: www.sanofi.com Mobile app: SANOFI IR available on the App Store and Google Play
1
2015 second-quarter and first-half key figures
| | Q2 2015 | | Change
(reported) | | Change
(CER) | | H1 2015 | | Change
(reported) | | Change
(CER) | |
Net sales | | € | 9,378 | m | +16.1 | % | +4.9 | % | € | 18,188 | m | +14.3 | % | +3.6 | % |
Business net income(1) | | € | 1,840 | m | +19.7 | % | +4.2 | % | € | 3,566 | m | +15.6 | % | +2.9 | % |
Business EPS(1) | | € | 1.41 | | +20.5 | % | +5.1 | % | € | 2.73 | | +16.7 | % | +3.8 | % |
IFRS net income reported | | € | 1,302 | m | +67.6 | % | | | € | 2,325 | m | +24.9 | % | | |
IFRS EPS reported | | € | 1.00 | | +69.5 | % | | | € | 1.78 | | +26.2 | % | | |
2015 second-quarter and first-half sales
Unless otherwise indicated, all sales growth figures in this press release are stated at constant exchange rates(1).
In the second quarter of 2015, sales of Sanofi increased 16.1% on a reported basis to €9,378 million. Exchange rate movements had a positive effect of 11.2 percentage points reflecting mainly the strength of the U.S. dollar against the Euro. At constant exchange rates, sales increased 4.9% reflecting the sustained strength of our diversified businesses.
First-half sales reached €18,188 million, an increase of 14.3% on a reported basis. Exchange rate movements had a favorable effect of 10.7 percentage points.
€ million | | Q2 2015
net sales | | Change
(CER) | | H1 2015
net sales | | Change
(CER) | |
Pharmaceuticals | | 7,800 | | +3.7 | % | 15,255 | | +3.0 | % |
Diabetes | | 1,988 | | -3.8 | % | 3,825 | | -3.5 | % |
Genzyme | | 907 | | +26.6 | % | 1,728 | | +28.6 | % |
Consumer Healthcare (CHC) | | 890 | | +1.3 | % | 1,869 | | +3.4 | % |
Generics | | 520 | | +9.2 | % | 998 | | +9.7 | % |
Oncology | | 390 | | +3.6 | % | 747 | | -1.9 | % |
Established Rx Products | | 3,105 | | +3.1 | % | 6,088 | | +0.8 | % |
Vaccines | | 887 | | +8.6 | % | 1,584 | | +2.5 | % |
Animal Health | | 691 | | +14.2 | % | 1,349 | | +13.9 | % |
Total net sales | | 9,378 | | +4.9 | % | 18,188 | | +3.6 | % |
Pharmaceuticals
Second-quarter sales for Pharmaceuticals were €7,800 million, an increase of 3.7%, driven mainly by Genzyme and Generics, which were partially offset by lower sales of Diabetes. First-half sales for Pharmaceuticals grew 3.0% to €15,255 million.
(1) See Appendix 10 for definitions of financial indicators.
2
Diabetes
€ million | | Q2 2015
net sales | | Change
(CER) | | H1 2015
net sales | | Change
(CER) | |
Lantus® | | 1,709 | | -5.8 | % | 3,293 | | -5.4 | % |
Amaryl® | | 109 | | 0.0 | % | 206 | | +0.5 | % |
Apidra® | | 93 | | +11.7 | % | 184 | | +11.2 | % |
Insuman® | | 34 | | 0.0 | % | 67 | | +1.5 | % |
BGM (Blood Glucose Monitoring) | | 16 | | 0.0 | % | 32 | | 0.0 | % |
Toujeo® | | 13 | | — | | 20 | | — | |
Lyxumia® | | 10 | | +50.0 | % | 18 | | +54.5 | % |
Afrezza® | | 2 | | — | | 3 | | — | |
Total Diabetes | | 1,988 | | -3.8 | % | 3,825 | | -3.5 | % |
Second-quarter sales of the Diabetes division were €1,988 million, a decrease of 3.8% reflecting lower sales of Lantus® in the U.S. as expected and in line with the previous quarter. In the U.S., sales of Diabetes were €1,134 million (down 14.0%) and accounted for 57% of worldwide Diabetes sales. Sales of Diabetes outside the U.S. were €854 million, an increase of 11.2% and represented 43% of worldwide Diabetes sales. First-half sales for the Diabetes division were €3,825 million down 3.5%.
Second-quarter sales of Lantus® were €1,709 million down 5.8%. In the U.S., sales of Lantus® decreased 15.4% to €1,086 million due to increased rebates granted to maintain favorable formulary positions with key payers. Sales of Lantus® outside the U.S. grew 13.0% over the period. In Emerging Markets, second-quarter sales of Lantus® were up 21.4% to €305 million, driven by Latin America, China and Middle East. In Western Europe, sales of the product were up 6.1% to €230 million over the period driven by double-digit growth in France and Germany. A biosimilar of Lantus® was launched in some small Eastern European markets in July. First-half sales of Lantus® reached €3,293 million, down 5.4%.
Toujeo®, a next-generation basal insulin, was launched in the U.S. market at the end of March and significant market access has already been achieved. Indeed, as of August, 73% of lives are covered by commercial plans and have unrestricted access to Toujeo®, including 45% of lives with preferred Tier 2 access. In Medicare Part D, 91% of lives are covered with unrestricted access to Toujeo®. Following EU approval in April, Toujeo® was launched in Germany, the Netherlands and some Nordic countries. Toujeo® was also recently approved in Japan, Canada and Australia. Total sales of the product were €13 million and €20 million in the second quarter and the first half of 2015, respectively.
Second-quarter sales of Amaryl® were stable at €109 million, of which €90 million were generated in Emerging Markets (up 1.3%). First-half sales of Amaryl® were relatively stable at €206 million.
Sales of Apidra® were up 11.7% to €93 million in the second quarter, driven by Emerging Markets (up 27.8% to €24 million). In Western Europe sales were up 8.3% (to €26 million), while in the U.S. sales of the product remained stable (at €34 million). First-half sales of Apidra® were up 11.2% to €184 million.
Second-quarter sales of Lyxumia® (lixisenatide) were €10 million. The results of the Phase IIIb ELIXA cardiovascular outcomes study of Lyxumia® were presented at American Diabetes Association’s 75th Scientific Sessions in June and were included in the U.S. New Drug Application for lixisenatide submitted at the end of July. First-half sales of Lyxumia® were €18 million.
Afrezza®, a new rapid-acting inhaled insulin therapy (partnered with MannKind), was launched in the U.S. in February 2015. Sales of the product were €2 million and €3 million in the second quarter and the first half of 2015, respectively.
3
Genzyme
€ million | | Q2 2015
net sales | | Change
(CER) | | H1 2015
net sales | | Change
(CER) | |
Cerezyme® | | 199 | | +6.3 | % | 388 | | +5.5 | % |
Myozyme® / Lumizyme® | | 165 | | +14.3 | % | 321 | | +16.5 | % |
Fabrazyme® | | 146 | | +4.9 | % | 287 | | +14.9 | % |
Aldurazyme® | | 50 | | +4.4 | % | 98 | | +7.0 | % |
Cerdelga® | | 16 | | — | | 26 | | | |
Total Rare Diseases | | 647 | | +9.1 | % | 1,260 | | +12.3 | % |
Aubagio® | | 204 | | +80.4 | % | 374 | | +84.0 | % |
Lemtrada™ | | 56 | | ns | | 94 | | ns | |
Total Multiple Sclerosis | | 260 | | +118.4 | % | 468 | | +118.3 | % |
Total Genzyme | | 907 | | +26.6 | % | 1,728 | | +28.6 | % |
Sales of Genzyme increased 26.6% to €907 million in the second quarter sustained by the strong performance of Aubagio® and the launch progress of Lemtrada®. Genzyme recorded another quarter with double-digit sales growth in all territories; U.S. sales increased 37.0% to €398 million, Western Europe sales grew 24.9% to €265 million and Emerging Markets sales were up 18.0% to €162 million. First-half sales of Genzyme increased 28.6% to €1,728 million.
Second-quarter sales of Rare Diseases grew 9.1% to €647 million.
Sales of the Gaucher franchise grew 13.1% to €215 million in the second-quarter. Sales of Cerezyme® increased 6.3% to €199 million driven by strong performance in Emerging Markets (up 28.1% to €76 million). In the U.S., sales of Cerezyme® were down 11.1% reflecting the launch of Cerdelga®, the only first-line oral therapy for Gaucher disease type 1 patients. In the U.S., sales of the Gaucher franchise were up 15.6% to €64 million in the second quarter. First-half sales of Cerezyme® increased 5.5% to €388 million. Cerdelga® recorded sales of €16 million in the second quarter and €26 million in the first-half.
Sales of Fabrazyme® reached €146 million, an increase of 4.9% in the second quarter. The performance recorded by the product in the U.S. (up 9.1% to €76 million), Western Europe, (up 14.3% to €33 million) and the rest of the world (up 25.0% to €19 million) was partially offset by decreased sales in Emerging Markets (down 29.2% to €18 million) reflecting an unfavorable phasing effect in Brazil. First-half sales of Fabrazyme® increased 14.9% to €287 million.
Second-quarter sales of Myozyme®/Lumizyme® increased 14.3% to €165 million, largely driven by new patient accruals. In the U.S., growth (up 21.2% to €51 million) was partially due to the conversion of clinical study patients in late 2014. In Emerging Markets, sales were up 20.0% to €31 million. First-half sales of Myozyme®/Lumizyme® increased 16.5% to €321 million.
Sales of Multiple Sclerosis products increased 118.4% to €260 million in the second quarter.
For the first time, Aubagio® became Genzyme largest brand in terms of quarterly sales. Sales of Aubagio® grew 80.4% to €204 million driven by sales in the U.S. (up 59.7% to €142 million) and Western Europe (€46 million versus €21 million in the same period of 2014) with strong performance in France. First-half sales of Aubagio® increased 84.0% to €374 million.
Second-quarter sales of Lemtrada® were €56 million including €21 million in Western Europe (mainly in Germany and the UK) and €29 million in the U.S. where the product was introduced late last year. First-half sales of Lemtrada® were €94 million compared to €11 million in the first half of 2014.
4
Consumer Healthcare
€ million | | Q2 2015
net sales | | Change
(CER) | | H1 2015
net sales | | Change
(CER) | |
Allegra® | | 116 | | +6.4 | % | 258 | | +11.6 | % |
Doliprane® | | 70 | | 0.0 | % | 155 | | -1.9 | % |
Essentiale® | | 45 | | -18.2 | % | 95 | | -12.4 | % |
Lactacyd® | | 42 | | +12.5 | % | 68 | | +5.3 | % |
Enterogermina® | | 36 | | -5.6 | % | 93 | | +16.2 | % |
Nasacort® | | 32 | | 0.0 | % | 74 | | -10.3 | % |
Maalox® | | 26 | | +13.0 | % | 54 | | +8.0 | % |
No Spa® | | 22 | | -4.0 | % | 44 | | -1.9 | % |
Dorflex® | | 20 | | -18.5 | % | 43 | | -10.0 | % |
Magne B6® | | 21 | | +22.2 | % | 41 | | +15.0 | % |
Other CHC Products | | 460 | | +2.9 | % | 944 | | +4.9 | % |
Total Consumer Healthcare | | 890 | | +1.3 | % | 1,869 | | +3.4 | % |
In the second quarter, sales of Consumer Healthcare (CHC) products increased 1.3% to €890 million driven by Allegra®, Lactacyd®, Maalox® and Magne B6®. Sales of CHC in the U.S. were up 7.9% to €237 million over the period sustained by Allegra® and Other CHC Products. Sales of CHC in Emerging Markets were down slightly (2.1% to €432 million) reflecting lower sales in Russia due to softer demand following price increases implemented to partially offset the Ruble depreciation. In Western Europe, sales were broadly stable (down 0.6% to €161 million) despite a price decrease of Doliprane® in France in January 2015. First-half sales of CHC reached €1,869 million, an increase of 3.4%.
Generics
Second-quarter sales of Generics were up 9.2% to €520 million driven by sales in Japan (Rest of the World sales were €27 million, up 225.0%) where Sanofi and its partner Nichi-Iko Pharmaceuticals launched an authorized generic of Plavix® at the end of the quarter. In Emerging Markets, sales of Generics increased 6.0% to €297 million led by Eastern Europe. In the U.S. and Western Europe, second-quarter sales of generics were up 5.3% (to €50 million) and 4.4% (to €146 million), respectively. First-half sales of Generics increased 9.7% to €998 million.
Oncology
€ million | | Q2 2015
net sales | | Change
(CER) | | H1 2015
net sales | | Change
(CER) | |
Jevtana® | | 82 | | +13.6 | % | 159 | | +10.6 | % |
Thymoglobulin® | | 69 | | +13.0 | % | 124 | | +2.8 | % |
Taxotere® | | 62 | | -16.4 | % | 115 | | -24.3 | % |
Eloxatin® | | 57 | | +6.4 | % | 111 | | +5.4 | % |
Mozobil® | | 35 | | +19.2 | % | 69 | | +19.6 | % |
Zaltrap® | | 20 | | +20.0 | % | 40 | | +19.4 | % |
Total Oncology | | 390 | | +3.6 | % | 747 | | -1.9 | % |
Sales of Oncology grew 3.6% to €390 million with the performance of Jevtana®, Thymoglobulin®, Mozobil® and Zaltrap® partially offset by the continued impact of generics on Taxotere® in Japan. First-half sales of Oncology were €747 million, down 1.9%.
Second-quarter sales of Jevtana® grew 13.6% to €82 million led by the U.S. (up 18.2% to €33 million) and Japan where the product was launched in September 2014. First-half sales of Jevtana® increased 10.6% to €159 million.
5
Sales of Thymoglobulin® increased 13.0% to €69 million in the second quarter driven by the U.S. (up 14.8%). First-half sales of Thymoglobulin® increased 2.8% to €124 million.
Second-quarter sales of Taxotere® decreased 16.4% (to €62 million), mainly due to generic competition in Japan. Over the same period, sales of Eloxatin® increased 6.4% (to €57 million) sustained by growth in China. First-half sales of Taxotere® and Eloxatin® were down 24.3% (€115 million) and up 5.4% (€111 million), respectively.
Sales of Mozobil® increased 19.2% to €35 million in the second quarter, driven by growth in the U.S. (up 21.4% to €21 million). First-half sales of Mozobil® increased 19.6% to €69 million.
Second-quarter sales of Zaltrap® grew 20.0% to €20 million, led by sales in Western Europe offseting lower sales in the U.S. First-half sales of Zaltrap® increased 19.4% to €40 million.
Established Rx Products
€ million | | Q2 2015
net sales | | Change
(CER) | | H1 2015
net sales | | Change
(CER) | |
Plavix® | | 545 | | +15.3 | % | 1,028 | | +2.1 | % |
Lovenox® | | 433 | | -0.5 | % | 871 | | +0.4 | % |
Renvela®/Renagel® | | 231 | | +43.1 | % | 457 | | +26.5 | % |
Aprovel®/Avapro® | | 224 | | +1.0 | % | 425 | | +0.5 | % |
Synvisc® /Synvisc-One® | | 116 | | +4.3 | % | 201 | | +3.7 | % |
Multaq® | | 87 | | +10.6 | % | 170 | | +2.9 | % |
Myslee®/Ambien®/Stilnox® | | 74 | | -6.8 | % | 149 | | -9.3 | % |
Allegra® | | 37 | | -10.3 | % | 117 | | -6.7 | % |
Other | | 1,358 | | -3.0 | % | 2,670 | | -1.9 | % |
Total Established Rx Products | | 3,105 | | +3.1 | % | 6,088 | | +0.8 | % |
Total sales of Established Rx Products increased 3.1% to €3,105 million and 0.8% to €6,088 million in the second quarter and the first half of 2015, respectively.
Second-quarter sales of Plavix® were up 15.3% to €545 million and benefited from a low second quarter 2014 comparison basis in Japan due to favorable buying patterns in the first quarter of 2014. As expected, multiple generic versions of Plavix® entered the market in Japan at the end of June 2015 and will impact Plavix® sales in the second half of 2015. The performance of Plavix® was driven by Japan (up 39.2% to €208 million) and Emerging Markets (up 9.4% to €278 million), which largely offset the decrease in Western Europe (-16.7% to €45 million). First-half sales of Plavix® increased 2.1% to €1,028 million.
Sales of Lovenox® were €433 million (down 0.5%) in the second quarter. The performance of the product in Emerging Markets (up 5.4% to €163 million) and Western Europe (up 2.7% to €230 million) was offset by lower sales in the U.S. (down 55.2% to €17 million) due to generic competition. Sanofi is aware of competitors that have filed marketing authorisation applications for biosimilar enoxaparin with health authorities in Europe. First-half sales of Lovenox® were €871 million (up 0.4%).
Second-quarter sales of Renvela®/Renagel® were up 43.1% to €231 million driven by the growth in the U.S. (up 59.1% to 174 million) benefited from a low second quarter 2014 comparison due to a limited allotment of generic Renvela® tablets granted to Impax. Renvela®/Renagel® also recorded strong sales growth in Emerging Markets (up 90.9% to €23 million) in the quarter. Generics are currently marketed in some European countries and we continue to expect potential generic approvals in the U.S. First-half sales of Renvela®/Renagel® were up 26.5% to €457 million.
Sales of Aprovel®/Avapro® were €224 million, up 1.0% in the second quarter. The performance in Emerging Markets (up 19.8% to €143 million) was driven by Latin America and offset lower sales in Western Europe (down 26.9% to €38 million) due to generic competition. First-half sales of Aprovel®/Avapro® were €425 million (up 0.5%).
6
Vaccines
€ million | | Q2 2015
net sales | | Change
(CER) | | H1 2015
net sales | | Change
(CER) | |
Polio/Pertussis/Hib Vaccines (incl. Pentacel®, Pentaxim® and Imovax®) | | 273 | | -14.1 | % | 555 | | -1.4 | % |
Meningitis/Pneumonia Vaccines (incl. Menactra®) | | 145 | | +7.0 | % | 242 | | +19.3 | % |
Adult Booster Vaccines (incl. Adacel ®) | | 118 | | +21.7 | % | 213 | | +10.4 | % |
Influenza Vaccines (incl. Vaxigrip® and Fluzone®) | | 114 | | +88.1 | % | 136 | | -32.0 | % |
Travel and Other Endemic Vaccines | | 97 | | -14.6 | % | 179 | | -9.0 | % |
Other Vaccines | | 140 | | +52.7 | % | 259 | | +47.2 | % |
Total Vaccines (consolidated sales) | | 887 | | +8.6 | % | 1,584 | | +2.5 | % |
Second-quarter consolidated sales of Sanofi Pasteur increased 8.6% to €887 million driven by influenza vaccines in the Southern Hemisphere, adult booster vaccines and VaxServe (a Sanofi Pasteur company and U.S. specialty supplier of vaccines, sales reported in the line «Other Vaccines »). Second-quarter sales of Sanofi Pasteur grew 7.6% (to €452 million) in the U.S. and 11.3% (to €344 million) in Emerging Markets. First-half sales of Sanofi Pasteur were up 2.5% to €1,584 million.
Sales of Polio/Pertussis/Hib vaccines decreased 14.1% to €273 million in the second quarter reflecting lower sales of Pentacel® in the U.S. due to a high basis for comparison in the second quarter of 2014 and Pentaxim® in China due to phasing effects. Over the period, Shantha sold €7 million of Shan5TM, its pediatric pentavalent vaccine, to supply global health organizations. First-half sales of Polio/Pertussis/Hib vaccines decreased 1.4% to €555 million.
Second-quarter sales of Menactra® were up 5.7% to €133 million driven by sales in Emerging market (up 30.8% to €19 million). First-half sales of Menactra® increased 19.6% to €220 million.
Sales of Adult Booster vaccines increased 21.7% to €118 million in the second quarter led by the U.S. (up 16.9% to €86 million) which benefited from a supply ramp up of Adacel®. First-half sales of Adult Booster vaccines increased 10.4% to €213 million.
Second-quarter sales of Influenza vaccines grew 88.1% to €114 million, benefiting from the delay of the Southern Hemisphere influenza campaign due to the two new strains included in influenza vaccines this year. First-half sales of Influenza vaccines decreased 32.0% to €136 million reflecting lower sales in Brazil due to supply ramp up of the Butantan Institute as a result of the technology transfer agreement with Sanofi Pasteur and sales of pandemic flu vaccines in the U.S. in the first half of 2014.
Second-quarter and first-half sales of Travel and Other Endemic Vaccines declined 14.6% to €97 million and 9.0% to €179 million, respectively.
Sales of Sanofi Pasteur MSD (not consolidated), the joint venture with Merck & Co. in Europe, were €161 million (up 3.6% on a reported basis) and €300 million (down 4.2% on a reported basis) in the second quarter and first half, respectively.
7
Animal Health
€ million | | Q2 2015
net sales | | Change
(CER) | | H1 2015
net sales | | Change
(CER) | |
Companion Animal | | 464 | | +16.2 | % | 907 | | +14.7 | % |
Production Animal | | 227 | | +10.4 | % | 442 | | +12.3 | % |
Total Animal Health | | 691 | | +14.2 | % | 1,349 | | +13.9 | % |
of which Vaccines | | 205 | | +5.0 | % | 391 | | +8.1 | % |
of which fipronil products | | 194 | | +1.8 | % | 387 | | +1.5 | % |
of which avermectin products | | 131 | | +16.3 | % | 288 | | +17.0 | % |
Animal Health delivered another quarter of double-digit growth with sales up 14.2% to €691 million in the second quarter continuing the impressive recovery of this business. In the U.S. and Emerging Markets, Animal Health sales grew 20.3% (to €345 million) and 16.3% (to €164 million), respectively. First-half sales of Animal Health increased 13.9% to €1,349 million.
Sales of the Companion Animals segment increased 16.2% to €464 million in the second quarter, reflecting the success of NexGard® and the performance of Heartgard®, which continued to benefit from a limited supply of competitor products. Sales of NexGard®, Merial’s next generation flea and tick product for dogs, more than doubled compared to the second quarter of 2014. Sales of the Frontline® family of products were stable over the period. In July, Merial launched Oravet®, dental hygiene chews for dogs in the U.S. Oravet®, provides a unique, easy-to-administer option, and joins Merial’s extensive product portfolio for pets. First-half sales of Companion Animals segment grew 14.7% to €907 million.
Second-quarter sales of the Production Animals segment were up 10.4% to €227 million driven by the continued recovery of the Avian business in Emerging Markets as well as the Ruminant business, which benefited from the success of LongRange® in the U.S. and IvermecGold® in Brazil. First-half sales of the Production Animals segment grew 12.3% to €442 million.
Net sales by geographic region
€ million | | Q2 2015
net sales | | Change
(CER) | | H1 2015
net sales | | Change
(CER) | |
United States | | 3,250 | | +2.1 | % | 6,226 | | +1.5 | % |
Emerging Markets(a) | | 3,182 | | +7.5 | % | 6,041 | | +7.4 | % |
of which Latin America | | 982 | | +7.1 | % | 1,828 | | +7.1 | % |
of which Asia | | 934 | | +9.3 | % | 1,783 | | +9.0 | % |
of which Eastern Europe, Russia and Turkey | | 616 | | +3.0 | % | 1,202 | | +5.7 | % |
of which Africa and Middle East | | 602 | | +9.0 | % | 1,132 | | +6.0 | % |
Western Europe(b) | | 1,998 | | +3.4 | % | 4,029 | | +1.9 | % |
Rest of the world(c) | | 948 | | +8.2 | % | 1,892 | | +2.0 | % |
of which Japan | | 545 | | +9.1 | % | 1,114 | | -0.5 | % |
TOTAL | | 9,378 | | +4.9 | % | 18,188 | | +3.6 | % |
(a) World less the U.S., Canada, Western Europe, Japan, South Korea, Australia and New Zealand;
(b) France, Germany, UK, Italy, Spain, Greece, Cyprus, Malta, Belgium, Luxembourg, Portugal, Netherlands, Austria, Switzerland, Sweden, Ireland, Finland, Norway, Iceland, Denmark;
(c) Japan, South Korea, Canada, Australia and New Zealand;
Second-quarter sales in the U.S. were €3,250 million, up 2.1%. The performance of Genzyme (up 37.0%), Animal Health (up 20.3%), Vaccines (up 7.6%), Oncology (up 8.7%) and CHC (up 7.9%) were partially offset by lower sales of Lantus® (down 15.4%). First-half sales in the U.S. increased 1.5% to €6,226 million.
In Emerging Markets, second-quarter sales grew 7.5% to €3,182 million led by Diabetes (up 17.0%), Genzyme (up 18.0%), vaccines (up 11.3%) and Animal Health (up 16.3%). In Asia, second-quarter
8
sales were up 9.3% led by Pharmaceuticals (up 10.6%). Sales in China grew 9.2% to €537 million driven by Lantus® and Plavix®, which were partially offset by lower Vaccines sales. In Latin America, second-quarter sales grew 7.1% to €982 million driven by Vaccines (up 23.8%) and Animal Health (up 12.9%). Latin American sales benefited from continued higher sales in Venezuela (up 31.1% to €199 million) due to buying patterns associated with local market conditions (Sanofi does not expect this increased demand to be replicated throughout 2015). Sales in Brazil were €334 million (down 5.2%) where sales growth of Diabetes and Vaccines were offset by lower CHC and rare disease sales. Sales in Eastern Europe, Russia and Turkey were up 3.0% to €616 million in the second quarter with the performance in Turkey and Poland was partially offset by lower sales in Russia. Sales in Russia were €159 million, down 11.6% impacted by the local economic and currency situation. In Africa and Middle-East, sales increased 9.0% to €602 million. In the Emerging Markets, first-half sales increased 7.4% to €6,041 million.
In the second quarter, sales in Western Europe increased 3.4% to €1,998 million, which included strong performance of Genzyme (up 24.9%) and 6.5% growth of Diabetes sales. In Western Europe, first-half sales increased 1.9% to €4,029 million.
In Japan, second-quarter sales grew 9.1% to €545 million, reflecting a low basis for comparison for Plavix® and strong contribution from generics, which largely offset lower sales of Vaccines and generic Taxotere® competition. In Japan, first-half sales decreased 0.5% to €1,114 million.
R&D update
Consult Appendix 8 for full overview of Sanofi’s R&D pipeline
Regulatory update
Regulatory updates since the publication of the first quarter results on April 30, 2015 include the following:
· In late July, a New Drug Application (NDA) for lixisenatide, a once-daily GLP-1 analogue, was submitted to the U.S. Food and Drug Administration (FDA) for the treatment of Diabetes.
· In July, the U.S. FDA approved Praluent® (alirocumab, collaboration with Regeneron), the first FDA-approved treatment in a new class of drugs known as PCSK9 inhibitors. Praluent® is indicated as an adjunct to diet and maximally tolerated statin therapy for the treatment of adults with heterozygous familial hypercholesterolemia or clinical atherosclerotic cardiovascular disease, who require additional lowering of low-density lipoprotein (LDL) cholesterol. In parallel, the CHMP also issued an opinion recommending the approval of Praluent® in EU in July. The final EMA decision is expected in late September.
· In July, the Ministry of Health, Labor and Welfare (MHLW) in Japan granted a marketing authorization for Lantus® XR (known as Toujeo® in the U.S. and Europe), a next-generation basal insulin for the treatment of type 1 and type 2 diabetes. Toujeo® was also approved in Canada in May and Australia in June.
· In June, the FDA granted Breakthrough Therapy designation to olipudase alfa. This enzyme replacement therapy is being investigated by Genzyme for the treatment of patients with Niemann-Pick disease Type B.
At the end of July 2015, the R&D pipeline contained 36 pharmaceutical new molecular entities (excluding Life Cycle Management) and vaccine candidates in clinical development of which 12 are in Phase III or have been submitted to the regulatory authorities for approval.
Collaboration
At the end of July, Sanofi and Regeneron entered into a new global collaboration to discover, develop and commercialize new antibody cancer treatments in the emerging field of immuno-oncology. As part of the agreement, the two companies will jointly develop a programmed cell death protein 1 (PD-1) inhibitor currently in Phase 1 testing, and plan to initiate clinical trials in 2016 with new therapeutic
9
candidates based on ongoing, innovative preclinical programs. Beyond PD-1, other targets in preclinical development include antibodies to lymphocyte-activation gene 3 (LAG3), glucocorticoid-induced tumornecrosis-factor-receptor-related protein (GITR) and a programmed death-ligand 1 (PD-L1) inhibitor.
Portfolio update
Phase III:
· In June, Sanofi and Regeneron announced that the Phase III ODYSSEY JAPAN trial of Praluent® (alirocumab) met its primary endpoint. At week 24, patients in the Praluent group experienced an average 64 percent greater reduction from baseline in their LDL-C, when added to current standard of care including statins, compared to standard of care alone (p<0.0001).
· The full results of the Phase IIIb ELIXA study, which was designed to assess the cardiovascular (CV) safety of Lyxumia® (lixisenatide) in adults with type 2 diabetes and high CV risk were presented in June and support the recent U.S. NDA for lixisenatide submitted to the FDA.
· In June the results from the EDITION JP 1 and EDITION JP 2 extension studies where Japanese participants (with type 1 and type 2 diabetes, respectively) received Toujeo® or Lantus® for a total of 12 months, were presented and showed that Toujeo® maintained similar blood sugar control, with fewer people experiencing night-time low blood sugar events, compared with Lantus®.
· In May, Sanofi and Regeneron announced that, SARIL-RA-TARGET, a Phase III study of sarilumab, an investigational, fully-human IL6 receptor antibody, met its co-primary efficacy endpoints in patients with rheumatoid arthritis (RA) who were inadequate responders to or intolerant of TNF-alpha inhibitors (TNF-IR). Two additional trials from the Phase III program, SARIL-RA-EASY and SARIL-RA-ASCERTAIN evaluating the usability of the sarilumab autoinjector device and a safety calibrator study designed to assess the safety of two subcutaneous doses of sarilumab and tocilizumab infusion, respectively, also met their primary endpoints. Detailed results from all three SARIL-RA trials will be presented at future medical congresses
Phase II:
· Additional positive results from an interim analysis of a pivotal Phase IIb study of dupilumab (in collaboration with Regeneron) in adult patients with moderate-to-severe asthma who are uncontrolled despite treatment with inhaled corticosteroids and long-acting beta agonists, were presented in May. The study met its primary endpoint of improving lung function in asthma patients with high blood eosinophils counts. Based on discussions with the U.S. FDA, this Phase IIb study may be considered one of two pivotal efficacy studies required for a potential dupilumab biologics license application (BLA) in asthma. A Phase III clinical trial of dupilumab in patients with uncontrolled persistent asthma has been initiated.
· Olipudase alfa, GZ402665, an investigational enzyme replacement therapy for Niemann-Pick type B, moved into Phase II.
· Sanofi decided to opt out the anti-GDF8 monoclonal antibody (SAR391786, collaboration with Regeneron) evaluated in Sarcopenia.
· The Proof of Concept study of fresolimumab in focal segmental glomerulosclerosis was not achieved.
Phase I:
· A TrkA antagonist GR389988, entered Phase I in osteo-arthritis.
10
2015 second-quarter and first-half financial results
Business Net Income(5)
In the second quarter of 2015, Sanofi generated net sales of €9,378 million, an increase of 16.1% on a reported basis (up 4.9% at constant exchange rates). First-half sales were €18,188 million, an increase of 14.3% on a reported basis (up 3.6% at constant exchange rates).
Other revenues were €83 million, up 16.9% and €163 million, up 5.8% in the second quarter and the first half, respectively. At constant exchange rates, other revenues were stable in the second quarter and down 7.8% in the first half, reflecting lower royalties received on Enbrel® sales in Europe.
Second-quarter gross profit increased 17.8% (up 4.8% at constant exchange rates) to €6,523 million. The gross margin ratio improved by 1.0 percentage points to 69.6% versus the second quarter of 2014. This improvement was driven essentially by the favorable currency effect. The positive impact from Genzyme and Renagel® was offset by Vaccines mix and Lantus® U.S. In the first half of 2015, the gross margin ratio improved by 0.6 percentage points to 69.4% versus the first half of 2014. Sanofi continues to expect that the gross margin for 2015 will be between 68% and 69%.
Second-quarter Research and development expenses were up 8.6% (down 0.3% at constant exchange rates) to €1,290 million reflecting lower spend in Diabetes, alirocumab and Oncology but higher spend on dupilumab. First-half R&D expenses increased 7.0% (down 1.0% at constant exchange rates) to €2,489 million. In the first half of 2015, the ratio of R&D to net sales was 0.9 percentage points lower at 13.7%.
Selling and general expenses (SG&A) increased 17.4% to €2,648 million in the second quarter. At constant exchange rates, SG&A grew 5.9% driven by launches in Multiple Sclerosis, Animal Health and Diabetes. The ratio of SG&A to net sales was 0.3 percentage points higher to 28.2% compared with the second quarter of 2014. First-half SG&A expenses were €5,086 million, an increase of 17.4% (up 6.2% at constant exchange rates). In the first half of 2015, the ratio of selling and general expenses to net sales was 0.8 percentage points higher to 28.0% compared with the first half of 2014.
Other current operating income net of expenses was -€20 million in the second quarter versus €54 million in the second quarter of 2014 which included a payment of €62 million related to the return of Eligard® U.S. rights to Tolmar Pharmaceuticals. First-half other current operating income net of expenses was -€87 million compared to €29 million for the same period of 2014. In the first half of 2015, this line included a foreign exchange loss of €100 million in connection with our Venezuelan operations.
The share of profits from associates was €30 million in the second quarter versus €26 million in the second quarter of 2014 and included Sanofi’s share in Regeneron profit recorded under the equity method since the beginning of April 2014 as well as Sanofi’s share of profit in Sanofi Pasteur MSD (the Vaccines joint venture with Merck & Co. in Europe). In the first half, the share of profits from associates was €61 million versus €39 million for the same period of 2014.
Non-controlling interests were -€29 million in the second quarter (versus -€30 million in the second quarter of 2014). First-half non-controlling interests were -€62 million versus -€65 million for the same period of 2014.
Business operating income(6) increased 19.6% to €2,566 million in the second quarter. At constant exchange rates, business operating income grew 4.7%. The ratio of business operating income to net sales was 0.8 percentage points higher to 27.4% versus the same period of last year. First-half business operating income was €4,964 million, up 15.7% (or up 3.4% at constant exchange rates). In the first half of 2015, the ratio of business operating income to net sales improved by 0.3 percentage points to 27.3%.
(5) See Appendix 10 for definitions of financial indicators, and Appendix 3 for reconciliation of business net income to IFRS net income reported
(6) Business operating income is the segment result used by the Group. The consolidated income statement for Q2 2015 is provided in Appendix 4
11
Net financial expenses were €112 million in the second quarter compared to €94 million in the second quarter of 2014. In the second quarter 2014, this line included a capital gain of €31 million before tax linked to the sales of some financial investments. First-half net financial expenses were €209 million versus €170 million in the first half of 2014.
Second-quarter and first-half effective tax rates were 25%, which were stable versus the same periods of 2014.
Second-quarter business net income(5) was up 19.7% to €1,840 million. At constant exchange rates, business net income increased 4.2%. The ratio of business net income to net sales was 19.6% in the second quarter versus 19.0% in the second quarter of 2014. First-half business net income increased 15.6% to €3,566 million, (up 2.9% at constant exchange rates). The ratio of business net income to net sales improved by 0.2 percentage points to 19.6% compared to the first half of 2014.
In the second quarter of 2015, business earnings per share(5) (EPS) was €1.41, up 20.5% on a reported basis and up 5.1% at constant exchange rates. The average number of shares outstanding was 1,305.9 million in the second quarter versus 1,314.5 million in the same period in 2014. In the first half of 2015, business earnings per share(1) was €2.73, up 16.7% on a reported basis and up 3.8% at constant exchange rates. The average number of shares outstanding was 1,307.2 million in the first half versus 1,317.2 million in the first half of 2014.
From business net income to IFRS net income reported (see Appendix 3)
In the first half of 2015, the main reconciling items between business net income and IFRS net income reported were:
· A €1,229 million amortization charge related to fair value remeasurement on intangible assets of acquired companies (primarily Aventis: €354 million, Genzyme: €449 million and Merial: €241 million) and to acquired intangible assets (licenses/products: €57 million). A €611 million amortization charge on intangible assets related to fair value remeasurement of acquired companies (primarily Aventis: €176 million, Genzyme: €223 million and Merial: €122 million), and to acquired intangible assets (licenses/products: €29 million) was booked in the second quarter. These items have no cash impact on the Group.
· An impairment of intangible assets of €28 million (of which €1 million in the second quarter of 2015). This item has no cash impact on the Group.
· An income of €71 million mainly reflecting a decrease in the fair value of contingent considerations related to the CVRs (€18 million, of which a charge of €5 million in the second quarter of 2015) and a decrease of Bayer contingent considerations (€39 million, of which €59 million in the second quarter of 2015) linked to Lemtrada®.
· Restructuring costs of €381 million (including €28 million in the second quarter mainly related to continuation of transformation in Europe).
· A €561 million tax effect arising from the items listed above, comprising €431 million of deferred taxes generated by amortization charged against intangible assets, €135 million associated with restructuring costs, €10 million associated with impairment of intangible assets and a charge of €15 million associated with fair value remeasurement of contingent consideration liabilities The second quarter tax effect was €206 million, including €214 million of deferred taxes generated by amortization charged against intangible assets and a charge of €22 million associated with fair value remeasurement of contingent consideration liabilities (see Appendix 3).
· A tax of €111 million on dividends paid to shareholders of Sanofi.
(5) See Appendix 10 for definitions of financial indicators, and Appendix 3 for reconciliation of business net income to IFRS net income reported
12
· In “Share of profits/losses from associates”, a charge of €127 million, net of tax, (of which €65 million in the second quarter of 2015) mainly relating to the share of the fair-value re-measurements on assets and liabilities as part of the acquisition of associates and to the share of amortization of intangible assets of joint-ventures. This item has no cash impact on the Group.
Capital Allocation
In the first half of 2015, net cash generated by operating activities increased 29% to €3,084 million after capital expenditures of €667 million and an increase in working capital by €181 million. This net Cash Flow has contributed to finance a share repurchase (€1,243 million), partially offset by proceeds from the issuance of new shares (€462 million), dividend paid by Sanofi (€3,694 million), acquisitions and partnerships net of disposals (€363 million) and restructuring costs (€345 million). As a consequence, net debt increased from €7,171 million at December 31, 2014 to €9,726 million at the end of June 2015 (amount net of €4,701 million cash and cash equivalents) and included the translation impact of the debt, which represented €391 million, mainly related to the debt held in U.S. dollars.
Forward-Looking Statements
This press release contains forward-looking statements as defined in the Private Securities Litigation Reform Act of 1995, as amended. Forward-looking statements are statements that are not historical facts. These statements include projections and estimates and their underlying assumptions, statements regarding plans, objectives, intentions and expectations with respect to future financial results, events, operations, services, product development and potential, and statements regarding future performance. Forward-looking statements are generally identified by the words “expects”, “anticipates”, “believes”, “intends”, “estimates”, “plans” and similar expressions. Although Sanofi’s management believes that the expectations reflected in such forward-looking statements are reasonable, investors are cautioned that forward-looking information and statements are subject to various risks and uncertainties, many of which are difficult to predict and generally beyond the control of Sanofi, that could cause actual results and developments to differ materially from those expressed in, or implied or projected by, the forward-looking information and statements. These risks and uncertainties include among other things, the uncertainties inherent in research and development, future clinical data and analysis, including post marketing, decisions by regulatory authorities, such as the FDA or the EMA, regarding whether and when to approve any drug, device or biological application that may be filed for any such product candidates as well as their decisions regarding labelling and other matters that could affect the availability or commercial potential of such product candidates, the absence of guarantee that the product candidates if approved will be commercially successful, the future approval and commercial success of therapeutic alternatives, the Group’s ability to benefit from external growth opportunities, trends in exchange rates and prevailing interest rates, the impact of cost containment policies and subsequent changes thereto, the average number of shares outstanding as well as those discussed or identified in the public filings with the SEC and the AMF made by Sanofi, including those listed under “Risk Factors” and “Cautionary Statement Regarding Forward-Looking Statements” in Sanofi’s annual report on Form 20-F for the year ended December 31, 2014. Other than as required by applicable law, Sanofi does not undertake any obligation to update or revise any forward-looking information or statements.
13
Appendices
List of appendices
Appendix 1: | | 2015 second-quarter and 2015 first-half consolidated net sales by geographic region and product |
| | |
Appendix 2: | | 2015 second-quarter and 2015 first-half business net income statement |
| | |
Appendix 3: | | Reconciliation of business net income to IFRS net income reported |
| | |
Appendix 4: | | 2015 second-quarter and 2015 first-half consolidated income statement |
| | |
Appendix 5: | | Change in net debt |
| | |
Appendix 6: | | Simplified consolidated balance sheet |
| | |
Appendix 7: | | 2015 currency sensitivity |
| | |
Appendix 8: | | R&D pipeline |
| | |
Appendix 9: | | Expected R&D milestones |
| | |
Appendix 10: | | Definitions |
14
Appendix 1: Net sales by geographic region and product
Q2 2015 net sales
(€ million) | | Total | | % CER | | %
reported | | Western
Europe | | % CER | | United
States | | % CER | | Emerging
Markets | | % CER | | Rest of
the
World | | % CER | |
Lantus | | 1,709 | | -5.8 | % | 9.8 | % | 230 | | 6.1 | % | 1,086 | | -15.4 | % | 305 | | 21.4 | % | 88 | | 6.7 | % |
Apidra | | 93 | | 11.7 | % | 20.8 | % | 26 | | 8.3 | % | 34 | | 0.0 | % | 24 | | 27.8 | % | 9 | | 25.0 | % |
Amaryl | | 109 | | 0.0 | % | 13.5 | % | 4 | | 0.0 | % | 0 | | -100.0 | % | 90 | | 1.3 | % | 15 | | 0.0 | % |
Insuman | | 34 | | 0.0 | % | 3.0 | % | 20 | | 5.3 | % | 1 | | 0.0 | % | 14 | | 8.3 | % | -1 | | -200.0 | % |
BGM | | 16 | | 0.0 | % | 0.0 | % | 15 | | 0.0 | % | 0 | | — | | 0 | | — | | 1 | | 0.0 | % |
Lyxumia | | 10 | | 50.0 | % | 66.7 | % | 5 | | 25.0 | % | 0 | | — | | 1 | | 0.0 | % | 4 | | 200.0 | % |
Afrezza | | 2 | | — | | — | | 0 | | — | | 2 | | — | | 0 | | — | | 0 | | | |
Toujeo | | 13 | | — | | — | | 1 | | — | | 11 | | — | | 0 | | — | | 1 | | | |
Other Diabetes | | 2 | | -33.3 | % | -33.3 | % | 0 | | — | | 0 | | — | | 1 | | — | | 1 | | -66.7 | % |
Diabetes | | 1,988 | | -3.8 | % | 11.2 | % | 301 | | 6.5 | % | 1,134 | | -14.0 | % | 435 | | 17.0 | % | 118 | | 4.8 | % |
Taxotere | | 62 | | -16.4 | % | -7.5 | % | 2 | | -75.0 | % | 1 | | -50.0 | % | 38 | | 6.1 | % | 21 | | -32.1 | % |
Jevtana | | 82 | | 13.6 | % | 24.2 | % | 35 | | 2.9 | % | 33 | | 18.2 | % | 8 | | -10.0 | % | 6 | | | |
Eloxatine | | 57 | | 6.4 | % | 21.3 | % | 1 | | 0.0 | % | 1 | | — | | 33 | | 16.0 | % | 22 | | -4.8 | % |
Thymoglobulin | | 69 | | 13.0 | % | 27.8 | % | 9 | | 25.0 | % | 38 | | 14.8 | % | 18 | | 6.7 | % | 4 | | 0.0 | % |
Mozobil | | 35 | | 19.2 | % | 34.6 | % | 9 | | 12.5 | % | 21 | | 21.4 | % | 3 | | 50.0 | % | 2 | | 0.0 | % |
Zaltrap | | 20 | | 20.0 | % | 33.3 | % | 12 | | 22.2 | % | 6 | | -16.7 | % | 2 | | 100.0 | % | 0 | | -100.0 | % |
Other Oncology | | 65 | | -6.6 | % | 6.6 | % | 13 | | 0.0 | % | 41 | | 0.0 | % | 5 | | -25.0 | % | 6 | | -28.6 | % |
Oncology | | 390 | | 3.6 | % | 16.1 | % | 81 | | 3.9 | % | 141 | | 8.7 | % | 107 | | 6.4 | % | 61 | | -9.8 | % |
Aubagio | | 204 | | 80.4 | % | 110.3 | % | 46 | | 123.8 | % | 142 | | 59.7 | % | 7 | | 250.0 | % | 9 | | 200.0 | % |
Lemtrada | | 56 | | 733.3 | % | 833.3 | % | 21 | | 300.0 | % | 29 | | — | | 2 | | — | | 4 | | 200.0 | % |
Cerezyme | | 199 | | 6.3 | % | 13.7 | % | 62 | | 1.7 | % | 49 | | -11.1 | % | 76 | | 28.1 | % | 12 | | -7.7 | % |
Cerdelga | | 16 | | — | | — | | 1 | | — | | 15 | | — | | 0 | | — | | 0 | | | |
Myozyme | | 165 | | 14.3 | % | 24.1 | % | 73 | | 6.0 | % | 51 | | 21.2 | % | 31 | | 20.0 | % | 10 | | 37.5 | % |
Fabrazyme | | 146 | | 4.9 | % | 18.7 | % | 33 | | 14.3 | % | 76 | | 9.1 | % | 18 | | -29.2 | % | 19 | | 25.0 | % |
Aldurazyme | | 50 | | 4.4 | % | 11.1 | % | 18 | | 6.3 | % | 11 | | -11.1 | % | 17 | | 6.3 | % | 4 | | 25.0 | % |
Other Rare Diseases products | | 71 | | -1.6 | % | 10.9 | % | 11 | | 0.0 | % | 25 | | 9.5 | % | 11 | | 11.1 | % | 24 | | -18.2 | % |
Genzyme | | 907 | | 26.6 | % | 41.1 | % | 265 | | 24.9 | % | 398 | | 37.0 | % | 162 | | 18.0 | % | 82 | | 12.1 | % |
Plavix | | 545 | | 15.3 | % | 28.2 | % | 45 | | -16.7 | % | 0 | | -100.0 | % | 278 | | 9.4 | % | 222 | | 35.0 | % |
Lovenox | | 433 | | -0.5 | % | 2.9 | % | 230 | | 2.7 | % | 17 | | -55.2 | % | 163 | | 5.4 | % | 23 | | 0.0 | % |
Renagel / Renvela | | 231 | | 43.1 | % | 68.6 | % | 29 | | -15.2 | % | 174 | | 59.1 | % | 23 | | 90.9 | % | 5 | | 40.0 | % |
Aprovel | | 224 | | 1.0 | % | 16.1 | % | 38 | | -26.9 | % | 5 | | -40.0 | % | 143 | | 19.8 | % | 38 | | -5.7 | % |
Allegra | | 37 | | -10.3 | % | -5.1 | % | 3 | | 0.0 | % | 0 | | — | | 1 | | -50.0 | % | 33 | | -8.8 | % |
Myslee / Ambien / Stilnox | | 74 | | -6.8 | % | 1.4 | % | 9 | | -10.0 | % | 17 | | -22.2 | % | 17 | | 7.1 | % | 31 | | -3.2 | % |
Synvisc / Synvisc One | | 116 | | 4.3 | % | 24.7 | % | 8 | | 0.0 | % | 90 | | -2.7 | % | 14 | | 44.4 | % | 4 | | 100.0 | % |
Multaq | | 87 | | 10.6 | % | 31.8 | % | 10 | | -16.7 | % | 74 | | 15.4 | % | 2 | | 0.0 | % | 1 | | | |
Depakine | | 108 | | 2.0 | % | 9.1 | % | 35 | | 0.0 | % | 0 | | — | | 70 | | 6.6 | % | 3 | | -50.0 | % |
Tritace | | 70 | | -10.7 | % | -6.7 | % | 30 | | -9.1 | % | 0 | | — | | 38 | | -10.0 | % | 2 | | -50.0 | % |
Lasix | | 47 | | 0.0 | % | 4.4 | % | 20 | | -5.0 | % | 1 | | — | | 16 | | 7.7 | % | 10 | | 0.0 | % |
Targocid | | 42 | | 2.6 | % | 10.5 | % | 20 | | -4.8 | % | 0 | | — | | 19 | | 13.3 | % | 3 | | 0.0 | % |
Orudis | | 43 | | -16.7 | % | -10.4 | % | 5 | | 0.0 | % | 0 | | — | | 37 | | -19.0 | % | 1 | | 0.0 | % |
Cordarone | | 33 | | -3.0 | % | 0.0 | % | 6 | | 0.0 | % | 0 | | — | | 19 | | 0.0 | % | 8 | | -11.1 | % |
Xatral | | 23 | | -8.7 | % | 0.0 | % | 9 | | 0.0 | % | 0 | | — | | 12 | | -15.4 | % | 2 | | 0.0 | % |
Actonel | | 6 | | -75.0 | % | -70.0 | % | 0 | | -100.0 | % | 0 | | — | | 4 | | -62.5 | % | 2 | | -71.4 | % |
Auvi-Q / Allerject | | 35 | | 81.3 | % | 118.8 | % | 0 | | — | | 32 | | 92.3 | % | 0 | | — | | 3 | | 33.3 | % |
Other Rx Drugs | | 951 | | -2.4 | % | 2.6 | % | 391 | | 5.2 | % | 83 | | -29.3 | % | 385 | | 1.9 | % | 92 | | -18.8 | % |
Total Other Rx Drugs | | 3,105 | | 3.1 | % | 12.1 | % | 888 | | -1.8 | % | 493 | | 4.7 | % | 1,241 | | 5.3 | % | 483 | | 6.3 | % |
Consumer Healthcare | | 890 | | 1.3 | % | 9.1 | % | 161 | | -0.6 | % | 237 | | 7.9 | % | 432 | | -2.1 | % | 60 | | 14.6 | % |
Generics | | 520 | | 9.2 | % | 11.6 | % | 146 | | 4.4 | % | 50 | | 5.3 | % | 297 | | 6.0 | % | 27 | | 225.0 | % |
Pharmaceuticals | | 7,800 | | 3.7 | % | 14.4 | % | 1,842 | | 3.5 | % | 2,453 | | -1.0 | % | 2,674 | | 6.5 | % | 831 | | 8.2 | % |
Polio / Pertussis / Hib | | 273 | | -14.1 | % | -3.9 | % | 10 | | 66.7 | % | 89 | | -20.0 | % | 147 | | -8.8 | % | 27 | | -32.5 | % |
Adult Booster Vaccines | | 118 | | 21.7 | % | 42.2 | % | 8 | | 0.0 | % | 86 | | 16.9 | % | 19 | | 63.6 | % | 5 | | 20.0 | % |
Meningitis/Pneumonia | | 145 | | 7.0 | % | 26.1 | % | 0 | | — | | 113 | | -1.1 | % | 30 | | 31.8 | % | 2 | | | |
Influenza Vaccines | | 114 | | 88.1 | % | 93.2 | % | 1 | | 0.0 | % | 0 | | — | | 102 | | 81.8 | % | 11 | | 233.3 | % |
Travel And Other Andemics Vaccines | | 97 | | -14.6 | % | -5.8 | % | 6 | | -25.0 | % | 31 | | -16.1 | % | 44 | | -19.2 | % | 16 | | 16.7 | % |
Other Vaccines | | 140 | | 52.7 | % | 89.2 | % | -1 | | 0.0 | % | 133 | | 59.7 | % | 2 | | -60.0 | % | 6 | | 66.7 | % |
Vaccines | | 887 | | 8.6 | % | 23.5 | % | 24 | | 9.1 | % | 452 | | 7.6 | % | 344 | | 11.3 | % | 67 | | 1.6 | % |
Fipronil products | | 194 | | 1.8 | % | 14.8 | % | 52 | | 2.0 | % | 104 | | -4.5 | % | 33 | | 34.8 | % | 5 | | -28.6 | % |
Vaccines | | 205 | | 5.0 | % | 13.9 | % | 45 | | -2.2 | % | 51 | | 7.9 | % | 94 | | 4.8 | % | 15 | | 25.0 | % |
Avermectin products | | 131 | | 16.3 | % | 33.7 | % | 10 | | -16.7 | % | 83 | | 17.5 | % | 18 | | 41.7 | % | 20 | | 17.6 | % |
Others | | 161 | | 53.3 | % | 78.9 | % | 25 | | 9.1 | % | 107 | | 79.2 | % | 19 | | 31.3 | % | 10 | | 75.0 | % |
Animal Health | | 691 | | 14.2 | % | 28.7 | % | 132 | | 0.0 | % | 345 | | 20.3 | % | 164 | | 16.3 | % | 50 | | 17.5 | % |
Total Group | | 9,378 | | 4.9 | % | 16.1 | % | 1,998 | | 3.4 | % | 3,250 | | 2.1 | % | 3,182 | | 7.5 | % | 948 | | 8.2 | % |
15
H1 2015 net sales
(€ million) | | Total | | % CER | | %
reported | | Western
Europe | | % CER | | United
States | | % CER | | Emerging
Markets | | % CER | | Rest of
the
World | | % CER | |
Lantus | | 3,293 | | -5.4 | % | 9.6 | % | 453 | | 6.2 | % | 2,093 | | -14.3 | % | 581 | | 19.7 | % | 166 | | 4.1 | % |
Apidra | | 184 | | 11.2 | % | 21.1 | % | 51 | | 8.5 | % | 69 | | 1.8 | % | 46 | | 29.4 | % | 18 | | 12.5 | % |
Amaryl | | 206 | | 0.5 | % | 13.2 | % | 8 | | -20.0 | % | 1 | | 0.0 | % | 168 | | 6.5 | % | 29 | | -18.2 | % |
Insuman | | 67 | | 1.5 | % | 3.1 | % | 38 | | -5.0 | % | 1 | | 0.0 | % | 28 | | 16.7 | % | 0 | | — | |
BGM | | 32 | | 0.0 | % | 0.0 | % | 30 | | 3.4 | % | 0 | | — | | 1 | | 0.0 | % | 1 | | -50.0 | % |
Lyxumia | | 18 | | 54.5 | % | 63.6 | % | 10 | | 42.9 | % | 0 | | — | | 3 | | 200.0 | % | 5 | | 33.3 | % |
Afrezza | | 3 | | — | | — | | 0 | | — | | 3 | | — | | 0 | | — | | 0 | | — | |
Toujeo | | 20 | | — | | — | | 1 | | — | | 18 | | — | | 0 | | — | | 1 | | — | |
Other Diabetes | | 2 | | -33.3 | % | -33.3 | % | 0 | | — | | 0 | | — | | 1 | | — | | 1 | | -66.7 | % |
Diabetes | | 3,825 | | -3.5 | % | 10.9 | % | 591 | | 5.6 | % | 2,185 | | -13.0 | % | 828 | | 17.7 | % | 221 | | -0.5 | % |
Taxotere | | 115 | | -24.3 | % | -15.4 | % | 4 | | -62.5 | % | 3 | | -40.0 | % | 69 | | -11.6 | % | 39 | | -33.3 | % |
Jevtana | | 159 | | 10.6 | % | 20.5 | % | 73 | | 0.0 | % | 60 | | 16.7 | % | 16 | | -5.9 | % | 10 | | 800.0 | % |
Eloxatine | | 111 | | 5.4 | % | 19.4 | % | 2 | | 0.0 | % | 2 | | 0.0 | % | 65 | | 12.0 | % | 42 | | -2.5 | % |
Thymoglobulin | | 124 | | 2.8 | % | 17.0 | % | 18 | | 12.5 | % | 70 | | 14.0 | % | 27 | | -18.8 | % | 9 | | 0.0 | % |
Mozobil | | 69 | | 19.6 | % | 35.3 | % | 19 | | 12.5 | % | 39 | | 18.5 | % | 8 | | 60.0 | % | 3 | | 0.0 | % |
Zaltrap | | 40 | | 19.4 | % | 29.0 | % | 25 | | 50.0 | % | 12 | | -28.6 | % | 3 | | 50.0 | % | 0 | | -100.0 | % |
Other Oncology | | 129 | | -13.7 | % | -1.5 | % | 27 | | -6.9 | % | 79 | | -17.9 | % | 13 | | -20.0 | % | 10 | | 11.1 | % |
Oncology | | 747 | | -1.9 | % | 9.9 | % | 168 | | 3.1 | % | 265 | | -0.5 | % | 201 | | -4.2 | % | 113 | | -7.9 | % |
Aubagio | | 374 | | 84.0 | % | 113.7 | % | 82 | | 115.8 | % | 265 | | 64.1 | % | 13 | | 300.0 | % | 14 | | 333.3 | % |
Lemtrada | | 94 | | 663.6 | % | 754.5 | % | 39 | | 280.0 | % | 45 | | — | | 4 | | — | | 6 | | 400.0 | % |
Cerezyme | | 388 | | 5.5 | % | 13.1 | % | 123 | | 2.5 | % | 99 | | -10.0 | % | 142 | | 22.3 | % | 24 | | 0.0 | % |
Cerdelga | | 26 | | — | | — | | 1 | | — | | 25 | | — | | 0 | | — | | 0 | | — | |
Myozyme | | 321 | | 16.5 | % | 26.4 | % | 142 | | 6.9 | % | 99 | | 25.0 | % | 59 | | 27.3 | % | 21 | | 31.3 | % |
Fabrazyme | | 287 | | 14.9 | % | 29.9 | % | 64 | | 18.9 | % | 147 | | 12.3 | % | 37 | | 12.9 | % | 39 | | 19.4 | % |
Aldurazyme | | 98 | | 7.0 | % | 14.0 | % | 35 | | 6.3 | % | 20 | | 0.0 | % | 33 | | 10.3 | % | 10 | | 11.1 | % |
Other Rare Diseases products | | 140 | | 4.2 | % | 17.6 | % | 23 | | 9.5 | % | 55 | | 15.0 | % | 19 | | 18.8 | % | 43 | | -14.3 | % |
Genzyme | | 1,728 | | 28.6 | % | 42.9 | % | 509 | | 24.6 | % | 755 | | 37.4 | % | 307 | | 25.5 | % | 157 | | 16.1 | % |
Plavix | | 1,028 | | 2.1 | % | 12.7 | % | 89 | | -24.1 | % | 0 | | -100.0 | % | 504 | | 5.1 | % | 435 | | 7.0 | % |
Lovenox | | 871 | | 0.4 | % | 4.1 | % | 461 | | 1.3 | % | 43 | | -42.6 | % | 320 | | 8.2 | % | 47 | | 0.0 | % |
Renagel / Renvela | | 457 | | 26.5 | % | 47.9 | % | 62 | | -6.2 | % | 339 | | 36.6 | % | 45 | | 31.3 | % | 11 | | 20.0 | % |
Aprovel | | 425 | | 0.5 | % | 14.2 | % | 75 | | -30.2 | % | 8 | | -33.3 | % | 268 | | 19.3 | % | 74 | | 0.0 | % |
Allegra | | 117 | | -6.7 | % | -1.7 | % | 6 | | 0.0 | % | 0 | | — | | 1 | | -50.0 | % | 110 | | -6.3 | % |
Myslee / Ambien / Stilnox | | 149 | | -9.3 | % | -1.3 | % | 19 | | -9.5 | % | 35 | | -14.7 | % | 33 | | 7.1 | % | 62 | | -13.2 | % |
Synvisc / Synvisc One | | 201 | | 3.7 | % | 23.3 | % | 15 | | 7.1 | % | 155 | | -0.8 | % | 24 | | 29.4 | % | 7 | | 20.0 | % |
Multaq | | 170 | | 2.9 | % | 22.3 | % | 20 | | -9.1 | % | 144 | | 4.5 | % | 5 | | 0.0 | % | 1 | | 100.0 | % |
Depakine | | 212 | | 4.7 | % | 11.0 | % | 70 | | 1.5 | % | 0 | | — | | 135 | | 8.6 | % | 7 | | -25.0 | % |
Tritace | | 145 | | -2.8 | % | 1.4 | % | 60 | | -7.7 | % | 0 | | — | | 82 | | 4.1 | % | 3 | | -40.0 | % |
Lasix | | 89 | | 4.9 | % | 9.9 | % | 38 | | -7.5 | % | 2 | | 0.0 | % | 30 | | 8.0 | % | 19 | | 33.3 | % |
Targocid | | 82 | | 2.7 | % | 9.3 | % | 40 | | -2.4 | % | 0 | | — | | 38 | | 13.3 | % | 4 | | -25.0 | % |
Orudis | | 93 | | 3.6 | % | 12.0 | % | 9 | | -10.0 | % | 0 | | — | | 82 | | 5.6 | % | 2 | | 0.0 | % |
Cordarone | | 67 | | 0.0 | % | 3.1 | % | 12 | | 0.0 | % | 0 | | — | | 39 | | 5.6 | % | 16 | | -11.8 | % |
Xatral | | 48 | | -6.4 | % | 2.1 | % | 18 | | -5.3 | % | 0 | | — | | 27 | | -7.7 | % | 3 | | 0.0 | % |
Actonel | | 13 | | -70.7 | % | -68.3 | % | 1 | | -88.9 | % | 0 | | — | | 8 | | -55.6 | % | 4 | | -78.6 | % |
Auvi-Q / Allerject | | 52 | | 65.4 | % | 100.0 | % | 1 | | 0.0 | % | 45 | | 71.4 | % | 0 | | — | | 6 | | 50.0 | % |
Other Rx Drugs | | 1,869 | | -2.7 | % | 1.8 | % | 785 | | 0.4 | % | 164 | | -31.5 | % | 736 | | 5.1 | % | 184 | | -12.4 | % |
Total Other Rx Drugs | | 6,088 | | 0.8 | % | 8.9 | % | 1,781 | | -4.1 | % | 935 | | -0.5 | % | 2,377 | | 7.1 | % | 995 | | -2.1 | % |
Consumer Healthcare | | 1,869 | | 3.4 | % | 9.9 | % | 363 | | 0.0 | % | 496 | | 6.6 | % | 887 | | 2.1 | % | 123 | | 15.0 | % |
Generics | | 998 | | 9.7 | % | 12.5 | % | 284 | | 0.7 | % | 91 | | 10.6 | % | 574 | | 9.1 | % | 49 | | 161.1 | % |
Pharmaceuticals | | 15,255 | | 3.0 | % | 12.9 | % | 3,696 | | 1.7 | % | 4,727 | | -1.8 | % | 5,174 | | 8.4 | % | 1,658 | | 2.2 | % |
Polio / Pertussis / Hib | | 555 | | -1.4 | % | 12.1 | % | 17 | | 41.7 | % | 207 | | 1.2 | % | 279 | | 6.7 | % | 52 | | -38.0 | % |
Adult Booster Vaccines | | 213 | | 10.4 | % | 29.9 | % | 13 | | -18.8 | % | 159 | | 4.9 | % | 31 | | 61.1 | % | 10 | | 42.9 | % |
Meningitis/Pneumonia | | 242 | | 19.3 | % | 41.5 | % | 1 | | — | | 187 | | 16.0 | % | 50 | | 29.7 | % | 4 | | 0.0 | % |
Influenza Vaccines | | 136 | | -32.0 | % | -29.9 | % | 1 | | 0.0 | % | -2 | | -104.8 | % | 122 | | -25.6 | % | 15 | | 8.3 | % |
Travel And Other Andemics Vaccines | | 179 | | -9.0 | % | 0.6 | % | 14 | | 7.7 | % | 50 | | -10.9 | % | 84 | | -14.1 | % | 31 | | 3.7 | % |
Other Vaccines | | 259 | | 47.2 | % | 79.9 | % | -1 | | -200.0 | % | 246 | | 50.8 | % | 6 | | -16.7 | % | 8 | | 80.0 | % |
Vaccines | | 1,584 | | 2.5 | % | 17.7 | % | 45 | | 4.7 | % | 847 | | 11.1 | % | 572 | | -3.1 | % | 120 | | -15.8 | % |
Fipronil products | | 387 | | 1.5 | % | 13.8 | % | 121 | | 6.3 | % | 192 | | -4.9 | % | 57 | | 20.0 | % | 17 | | -15.8 | % |
Vaccines | | 391 | | 8.1 | % | 17.1 | % | 87 | | -1.1 | % | 94 | | 5.6 | % | 173 | | 7.2 | % | 37 | | 59.1 | % |
Avermectin products | | 288 | | 17.0 | % | 35.8 | % | 27 | | -3.6 | % | 191 | | 25.0 | % | 29 | | 17.4 | % | 41 | | 5.4 | % |
Others | | 283 | | 46.4 | % | 68.5 | % | 53 | | 15.9 | % | 175 | | 59.6 | % | 36 | | 29.6 | % | 19 | | 125.0 | % |
Animal Health | | 1,349 | | 13.9 | % | 28.0 | % | 288 | | 4.4 | % | 652 | | 17.8 | % | 295 | | 13.0 | % | 114 | | 25.6 | % |
Total Group | | 18,188 | | 3.6 | % | 14.3 | % | 4,029 | | 1.9 | % | 6,226 | | 1.5 | % | 6,041 | | 7.4 | % | 1,892 | | 2.0 | % |
16
Appendix 2: Business net income statement
Second
quarter | | Group Total | | Pharmaceuticals | | Vaccines | | Animal Health | | Others | |
2015
€ million | | Q2
2015 | | Q2 2014 | | Change | | Q2
2015 | | Q2 2014 | | Change | | Q2
2015 | | Q2 2014 | | Change | | Q2
2015 | | Q2 2014 | | Change | | Q2
2015 | | Q2
2014 | |
Net sales | | 9,378 | | 8,075 | | 16.1 | % | 7,800 | | 6,820 | | 14.4 | % | 887 | | 718 | | 23.5 | % | 691 | | 537 | | 28.7 | % | — | | — | |
Other revenues | | 83 | | 71 | | 16.9 | % | 67 | | 58 | | 15.5 | % | 8 | | 7 | | 14.3 | % | 8 | | 6 | | 33.3 | % | — | | — | |
Cost of sales | | (2,938 | ) | (2,608 | ) | 12.7 | % | (2,252 | ) | (2,058 | ) | 9.4 | % | (450 | ) | (350 | ) | 28.6 | % | (236 | ) | (200 | ) | 18.0 | % | — | | — | |
As % of net sales | | (31.3 | )% | (32.3 | )% | | | (28.9 | )% | (30.2 | )% | | | (50.7 | )% | (48.8 | )% | | | (34.2 | )% | (37.2 | )% | | | | | | |
Gross profit | | 6,523 | | 5,538 | | 17.8 | % | 5,615 | | 4,820 | | 16.5 | % | 445 | | 375 | | 18.7 | % | 463 | | 343 | | 35.0 | % | — | | — | |
As % of net sales | | 69.6 | % | 68.6 | % | | | 72.0 | % | 70.7 | % | | | 50.2 | % | 52.2 | % | | | 67.0 | % | 63.9 | % | | | | | | |
Research & Development expenses | | (1,290 | ) | (1,188 | ) | 8.6 | % | (1,104 | ) | (1,030 | ) | 7.2 | % | (142 | ) | (123 | ) | 15.4 | % | (44 | ) | (35 | ) | 25.7 | % | — | | — | |
As % of net sales | | (13.8 | )% | (14.7 | )% | | | (14.2 | )% | (15.1 | )% | | | (16.0 | )% | (17.1 | )% | | | (6.4 | )% | (6.5 | )% | | | | | | |
Selling and general expenses | | (2,648 | ) | (2,255 | ) | 17.4 | % | (2,216 | ) | (1,930 | ) | 14.8 | % | (188 | ) | (142 | ) | 32.4 | % | (244 | ) | (183 | ) | 33.3 | % | — | | — | |
As % of net sales | | (28.2 | )% | (27.9 | )% | | | (28.4 | )% | (28.3 | )% | | | (21.2 | )% | (19.8 | )% | | | (35.3 | )% | (34.1 | )% | | | | | | |
Other current operating income/expenses | | (20 | ) | 54 | | | | (11 | ) | 42 | | | | 1 | | 3 | | | | 5 | | 11 | | | | (15 | ) | (2 | ) |
Share of profit/loss of associates(1) and joint ventures | | 30 | | 26 | | | | 29 | | 25 | | | | 1 | | 1 | | | | — | | — | | | | — | | — | |
Net income attributable to non-controlling interests | | (29 | ) | (30 | ) | | | (29 | ) | (30 | ) | | | — | | — | | | | — | | — | | | | — | | — | |
Business operating income | | 2,566 | | 2,145 | | 19.6 | % | 2,284 | | 1,897 | | 20.4 | % | 117 | | 114 | | 2.6 | % | 180 | | 136 | | 32.4 | % | (15 | ) | (2 | ) |
As % of net sales | | 27.4 | % | 26.6 | % | | | 29.3 | % | 27.8 | % | | | 13.2 | % | 15.9 | % | | | 26.0 | % | 25.3 | % | | | | | | |
Financial income and expenses | | (112 | ) | (94 | ) | | | | | | | | | | | | | | | | | | | | | | | | |
Income tax expense | | (614 | ) | (514 | ) | | | | | | | | | | | | | | | | | | | | | | | | |
Tax rate(2) | | 25.0 | % | 25.0 | % | | | | | | | | | | | | | | | | | | | | | | | | |
Business net income | | 1,840 | | 1,537 | | 19.7 | % | | | | | | | | | | | | | | | | | | | | | | |
As % of net sales | | 19.6 | % | 19.0 | % | | | | | | | | | | | | | | | | | | | | | | | | |
Business earnings per share(3) (in euros) | | 1.41 | | 1.17 | | 20.5 | % | | | | | | | | | | | | | | | | | | | | | | |
(1) Net of tax
(2) Determined on the basis of Business income before tax, associates and non-controlling interests
(3) Based on an average number of shares outstanding of 1,305.9 million in the second quarter of 2015 and 1,314.5 million in the second quarter of 2014
17
First Half | | Group Total | | Pharmaceuticals | | Vaccines | | Animal Health | | Others | |
2015
€ million | | H1
2015 | | H1 2014 | | Change | | H1
2015 | | H1 2014 | | Change | | H1
2015 | | H1 2014 | | Change | | H1
2015 | | H1 2014 | | Change | | H1
2015 | | H1
2014 | |
Net sales | | 18,188 | | 15,917 | | 14.3 | % | 15,255 | | 13,517 | | 12.9 | % | 1,584 | | 1,346 | | 17.7 | % | 1,349 | | 1,054 | | 28.0 | % | — | | — | |
Other revenues | | 163 | | 154 | | 5.8 | % | 129 | | 126 | | 2.4 | % | 14 | | 14 | | — | | 20 | | 14 | | 42.9 | % | — | | — | |
Cost of sales | | (5,724 | ) | (5,124 | ) | 11.7 | % | (4,442 | ) | (4,046 | ) | 9.8 | % | (826 | ) | (700 | ) | 18.0 | % | (456 | ) | (378 | ) | 20.6 | % | — | | — | |
As % of net sales | | (31.5 | )% | (32.2 | )% | | | (29.1 | )% | (29.9 | )% | | | (52.1 | )% | (52.0 | )% | | | (33.8 | )% | (35.8 | )% | | | | | | |
Gross profit | | 12,627 | | 10,947 | | 15.3 | % | 10,942 | | 9,597 | | 14.0 | % | 772 | | 660 | | 17.0 | % | 913 | | 690 | | 32.3 | % | — | | — | |
As % of net sales | | 69.4 | % | 68.8 | % | | | 71.7 | % | 71.0 | % | | | 48.7 | % | 49.0 | % | | | 67.7 | % | 65.5 | % | | | | | | |
Research& Development expenses | | (2,489 | ) | (2,327 | ) | 7.0 | % | (2,143 | ) | (2,025 | ) | 5.8 | % | (262 | ) | (230 | ) | 13.9 | % | (84 | ) | (72 | ) | 16.7 | % | — | | — | |
As % of net sales | | (13.7 | )% | (14.6 | )% | | | (14.0 | )% | (15.0 | )% | | | (16.5 | )% | (17.1 | )% | | | (6.2 | )% | (6.8 | )% | | | | | | |
Selling and general expenses | | (5,086 | ) | (4,333 | ) | 17.4 | % | (4,310 | ) | (3,721 | ) | 15.8 | % | (344 | ) | (271 | ) | 26.9 | % | (432 | ) | (341 | ) | 26.7 | % | — | | — | |
As % of net sales | | (28.0 | )% | (27.2 | )% | | | (28.3 | )% | (27.5 | )% | | | (21.7 | )% | (20.1 | )% | | | (32.0 | )% | (32.4 | )% | | | | | | |
Other current operating income/ expenses | | (87 | ) | 29 | | | | (39 | ) | 19 | | | | 2 | | 1 | | | | 5 | | 17 | | | | (55 | ) | (8 | ) |
Share of profit/loss of associates(1) and joint ventures | | 61 | | 39 | | | | 61 | | 33 | | | | — | | 6 | | | | — | | — | | | | — | | — | |
Net income attributable to non-controlling interests | | (62 | ) | (65 | ) | | | (62 | ) | (65 | ) | | | — | | — | | | | — | | — | | | | — | | — | |
Business operating income | | 4,964 | | 4,290 | | 15.7 | % | 4,449 | | 3,838 | | 15.9 | % | 168 | | 166 | | 1.2 | % | 402 | | 294 | | 36.7 | % | (55 | ) | (8 | ) |
As % of net sales | | 27.3 | % | 27.0 | % | | | 29.2 | % | 28.4 | % | | | 10.6 | % | 12.3 | % | | | 29.8 | % | 27.9 | % | | | | | | |
Financial income and expenses | | (209 | ) | (170 | ) | | | | | | | | | | | | | | | | | | | | | | | | |
Income tax expense | | (1,189 | ) | (1,036 | ) | | | | | | | | | | | | | | | | | | | | | | | | |
Tax rate(2) | | 25.0 | % | 25.0 | % | | | | | | | | | | | | | | | | | | | | | | | | |
Business net income | | 3,566 | | 3,084 | | 15.6 | % | | | | | | | | | | | | | | | | | | | | | | |
As % of net sales | | 19.6 | % | 19.4 | % | | | | | | | | | | | | | | | | | | | | | | | | |
Business earnings per share(3) (in euros) | | 2.73 | | 2.34 | | 16.7 | % | | | | | | | | | | | | | | | | | | | | | | |
(1) Net of tax
(2) Determined on the basis of Business income before tax, associates and non-controlling interests
(3) Based on an average number of shares outstanding of 1,307.2 million in the first semester of 2015 and 1,317.2 million in the first semester of 2014
18
Appendix 3: Reconciliation of Business net income to IFRS net income reported
€ million | | Q2 2015 | | Q2 2014 | | Change | |
| | | | | | | |
Business net income | | 1,840 | | 1,537 | | 19.7 | % |
| | | | | | | |
Amortization of intangible assets(1) | | (611 | ) | (624 | ) | | |
| | | | | | | |
Impairment of intangible assets | | (1 | ) | (71 | ) | | |
| | | | | | | |
Fair value remeasurement of contingent consideration liabilities | | 70 | | (124 | ) | | |
| | | | | | | |
Restructuring costs | | (28 | ) | (84 | ) | | |
| | | | | | | |
Other gains and losses, and litigation | | — | | — | | | |
| | | | | | | |
Tax effect of items listed above: | | 206 | | 274 | | | |
| | | | | | | |
Amortization of intangible assets | | 214 | | 207 | | | |
Impairment of intangible assets | | — | | 25 | | | |
Fair value remeasurement of contingent consideration liabilities | | (22 | ) | 13 | | | |
Other gains and losses, and litigation | | — | | — | | | |
Restructuring costs | | 14 | | 29 | | | |
| | | | | | | |
Other tax items(2) | | (111 | ) | (110 | ) | | |
| | | | | | | |
Share of items listed above attributable to non-controlling interests | | 2 | | 3 | | | |
| | | | | | | |
Restructuring costs of associates and joint ventures, and expenses arising from the impact of acquisitions on associates and joint ventures | | (65 | ) | (24 | ) | | |
| | | | | | | |
IFRS net income reported (3) | | 1,302 | | 777 | | 67.6 | % |
| | | | | | | |
IFRS earnings per share(4) reported (in euros) | | 1.00 | | 0.59 | | | |
(1) Of which related to amortization expense generated by the remeasurement of intangible assets as part of business combinations: €582 million in the second quarter of 2015 and €601 million in the second quarter of 2014
(2) Tax on dividends paid to shareholders of Sanofi
(3) Net income attributable to equity holders of Sanofi
(4) Based on an average number of shares outstanding of 1,305.9 million in the second quarter of 2015 and 1,314.5 in the second quarter of 2014
See page 12 for comments on the reconciliation of business net income to consolidated net income.
19
€ million | | H1 2015 | | H1 2014 | | Change | |
| | | | | | | |
Business net income | | 3,566 | | 3, 084 | | 15.6 | % |
| | | | | | | |
Amortization of intangible assets(1) | | (1,229 | ) | (1,301 | ) | | |
| | | | | | | |
Impairment of intangible assets | | (28 | ) | (74 | ) | | |
| | | | | | | |
Fair value remeasurement of contingent consideration liabilities | | 71 | | (132 | ) | | |
| | | | | | | |
Restructuring costs | | (381 | ) | (135 | ) | | |
| | | | | | | |
Other gains and losses, and litigation | | — | | 35 | | | |
| | | | | | | |
Tax effect of items listed above: | | 561 | | 522 | | | |
| | | | | | | |
Amortization of intangible assets | | 431 | | 451 | | | |
Impairment of intangible assets | | 10 | | 26 | | | |
Fair value remeasurement of contingent consideration liabilities | | (15 | ) | 14 | | | |
other gains and losses, and litigation | | — | | (13 | ) | | |
Restructuring costs | | 135 | | 44 | | | |
| | | | | | | |
Other tax items(2) | | (111 | ) | (110 | ) | | |
| | | | | | | |
Share of items listed above attributable to non-controlling interests | | 3 | | 4 | | | |
| | | | | | | |
Restructuring costs of associates and joint ventures, and expenses arising from the impact of acquisitions on associates and joint ventures | | (127 | ) | (32 | ) | | |
| | | | | | | |
IFRS net income reported(3) | | 2,325 | | 1,861 | | 24.9 | % |
| | | | | | | |
IFRS earnings per share(4) reported (in euros) | | 1.78 | | 1.41 | | | |
(1) Of which related to amortization expense generated by the remeasurement of intangible assets as part of business combinations: €1,172 million in the first semester of 2015 and €1,258 million in the first semester of 2014
(2) Tax on dividends paid to shareholders of Sanofi
(3) Net income attributable to equity holders of Sanofi
(4) Based on an average number of shares outstanding of 1,307.2 million in the first semester of 2015 and 1,317.2 in the first semester of 2014
See page 12 for comments on the reconciliation of business net income to IFRS net income reported.
20
Appendix 4: Consolidated income statement
€ million | | Q2 2015 | | Q2 2014 | | H1 2015 | | H1 2014 | |
| | | | | | | | | |
Net sales | | 9,378 | | 8,075 | | 18,188 | | 15,917 | |
| | | | | | | | | |
Other revenues | | 83 | | 71 | | 163 | | 154 | |
| | | | | | | | | |
Cost of sales | | (2,938 | ) | (2,608 | ) | (5,724 | ) | (5,124 | ) |
| | | | | | | | | |
Gross profit | | 6,523 | | 5,538 | | 12,627 | | 10,947 | |
| | | | | | | | | |
Research and development expenses | | (1,290 | ) | (1,188 | ) | (2,489 | ) | (2,327 | ) |
| | | | | | | | | |
Selling and general expenses | | (2,648 | ) | (2,255 | ) | (5,086 | ) | (4,333 | ) |
| | | | | | | | | |
Other operating income | | 47 | | 106 | | 83 | | 116 | |
| | | | | | | | | |
Other operating expenses | | (67 | ) | (52 | ) | (170 | ) | (87 | ) |
| | | | | | | | | |
Amortization of intangible assets | | (611 | ) | (624 | ) | (1,229 | ) | (1,301 | ) |
| | | | | | | | | |
Impairment of intangible assets | | (1 | ) | (71 | ) | (28 | ) | (74 | ) |
| | | | | | | | | |
Fair value remeasurement of contingent consideration liabilities | | 70 | | (124 | ) | 71 | | (132 | ) |
| | | | | | | | | |
Restructuring costs | | (28 | ) | (84 | ) | (381 | ) | (135 | ) |
| | | | | | | | | |
Operating income | | 1,995 | | 1,246 | | 3,398 | | 2,674 | |
| | | | | | | | | |
Financial expense | | (134 | ) | (145 | ) | (267 | ) | (292 | ) |
| | | | | | | | | |
Financial income | | 22 | | 51 | | 58 | | 157 | |
| | | | | | | | | |
Income before tax and associates and joint ventures | | 1,883 | | 1,152 | | 3,189 | | 2,539 | |
| | | | | | | | | |
Income tax expense(1) | | (519 | ) | (350 | ) | (739 | ) | (624 | ) |
| | | | | | | | | |
Share of profit/loss of associates and joint ventures | | (35 | ) | 2 | | (66 | ) | 7 | |
| | | | | | | | | |
Net income | | 1,329 | | 804 | | 2,384 | | 1,922 | |
| | | | | | | | | |
Net income attributable to non-controlling interests | | 27 | | 27 | | 59 | | 61 | |
| | | | | | | | | |
Net income attributable to equity holders of Sanofi | | 1,302 | | 777 | | 2,325 | | 1,861 | |
| | | | | | | | | |
Average number of shares outstanding (million) | | 1,305.9 | | 1,314.5 | | 1,307.2 | | 1,317.2 | |
| | | | | | | | | |
Earnings per share (in euros) | | 1.00 | | 0.59 | | 1.78 | | 1.41 | |
(1) In 2015, including a tax on dividends paid to shareholders of Sanofi: (111) M€ compared to (110) M€ in 2014
21
Appendix 5: Change in net debt
€ million | | H1 2015 | | H1 2014 | |
| | | | | |
Business net income | | 3,566 | | 3,084 | |
| | | | | |
Depreciation amortization and impairment of property. plant and equipment and software | | 628 | | 582 | |
| | | | | |
Net gains and losses on disposals of non-current assets, net of tax | | (44 | ) | (97 | ) |
| | | | | |
Other non-cash items | | (218 | ) | (98 | ) |
| | | | | |
Operating cash flow before changes in working capital (1) | | 3,932 | | 3,471 | |
| | | | | |
Changes in working capital (1) | | (181 | ) | (552 | ) |
| | | | | |
Acquisitions of property, plant and equipment and software | | (667 | ) | (529 | ) |
| | | | | |
Free cash flow (1) | | 3,084 | | 2,390 | |
| | | | | |
Acquisitions of intangibles, excluding software | | (280 | ) | (108 | ) |
| | | | | |
Acquisitions of investments, including assumed debt | | (169 | ) | (1,679 | ) |
| | | | | |
Restructuring costs paid | | (345 | ) | (382 | ) |
| | | | | |
Proceeds from disposals of property, plant and equipment, intangibles, and other non-current assets, net of tax(1) | | 86 | | 179 | |
| | | | | |
Issuance of Sanofi shares | | 462 | | 240 | |
| | | | | |
Dividends paid to shareholders of Sanofi | | (3,694 | ) | (3,676 | ) |
| | | | | |
Acquisition of treasury shares | | (1,243 | ) | (1,010 | ) |
| | | | | |
Transactions with non-controlling interests including dividends | | (16 | ) | (6 | ) |
| | | | | |
Foreign exchange impact | | (391 | ) | (37 | ) |
| | | | | |
Other items | | (49 | ) | (62 | ) |
| | | | | |
Change in net debt | | (2,555 | ) | (4,151 | ) |
(1) Excluding restructuring costs
22
Appendix 6: Simplified consolidated balance sheets
ASSETS
€ million | | 06/30/15 | | 12/31/14 | | LIABILITIES & EQUITY
€ million | | 06/30/15 | | 12/31/14 | |
| | | | | | | | | | | |
Property, plant and equipment | | 10,540 | | 10,396 | | Equity attributable to equity-holders of sanofi | | 56,618 | | 56,120 | |
| | | | | | | | | | | |
Intangible assets (including goodwill) | | 55,062 | | 53,740 | | Equity attributable to non-controlling interests | | 156 | | 148 | |
| | | | | | | | | | | |
Non-current financial assets, investments in associates | | 5,373 | | 4,959 | | Total equity | | 56,774 | | 56,268 | |
| | | | | | | | | | | |
Deferred tax assets | | 4,923 | | 4,860 | | Long-term debt | | 10,770 | | 13,276 | |
| | | | | | | | | | | |
| | | | | | Non-current liabilities related to business combinations and to non-controlling interests | | 1,132 | | 1,133 | |
| | | | | | | | | | | |
Non-current assets | | 75,898 | | 73,955 | | Provisions and other non-current liabilities | | 9,206 | | 9,578 | |
| | | | | | | | | | | |
| | | | | | Deferred tax liabilities | | 3,742 | | 4,105 | |
| | | | | | | | | | | |
Inventories, accounts receivable and other current assets | | 17,308 | | 16,086 | | Non-current liabilities | | 24,850 | | 28,092 | |
| | | | | | | | | | | |
Cash and cash equivalents | | 4,701 | | 7,341 | | Accounts payable and other current liabilities | | 12,192 | | 11,363 | |
| | | | | | | | | | | |
| | | | | | Current liabilities related to business combinations and to non-controlling interests | | 141 | | 131 | |
| | | | | | | | | | | |
| | | | | | Short-term debt and current portion of long-term debt | | 3,962 | | 1,538 | |
| | | | | | | | | | | |
Current assets | | 22,009 | | 23,427 | | Current liabilities | | 16,295 | | 13,032 | |
| | | | | | | | | | | |
Assets held for sale or exchange | | 16 | | 10 | | Liabilities related to assets held for sale or exchange | | 4 | | — | |
| | | | | | | | | | | |
Total ASSETS | | 97,923 | | 97,392 | | Total LIABILITIES & EQUITY | | 97,923 | | 97,392 | |
23
Appendix 7: 2015 currency sensitivity
2015 Business EPS currency sensitivity
Currency | | Variation | | Business EPS Sensitivity | |
U.S. Dollar | | -0.05 USD/EUR | | +EUR 0.10 | |
Japanese Yen | | +5 JPY/EUR | | -EUR 0.03 | |
Russian Ruble | | +10 RUB/EUR | | -EUR 0.06 | |
Currency exposure on Q2 2015
Currency | | Q2 2015 | |
US $ | | 35.6 | % |
Euro € | | 23.0 | % |
Japanese Yen | | 5.5 | % |
Chinese Yuan | | 5.4 | % |
Brazilian Real | | 3.2 | % |
British Pound | | 2.0 | % |
Russian Ruble | | 1.6 | % |
Canadian $ | | 1.5 | % |
Australian $ | | 1.5 | % |
Mexican Peso | | 1.4 | % |
Others | | 19.3 | % |
Currency average rates
| | Q2 2014 | | Q2 2015 | | Change | | Average June
2015 | |
€/$ | | 1.37 | | 1.10 | | -20 | % | 1.12 | |
€/Yen | | 140.03 | | 134.14 | | -4 | % | 138.74 | |
€/Yuan | | 8.54 | | 6.85 | | -20 | % | 6.96 | |
€/Ruble | | 47.96 | | 58.12 | | +21 | % | 61.24 | |
24
Appendix 8: R&D Pipeline
Registration
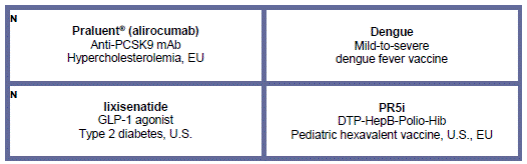
Phase III
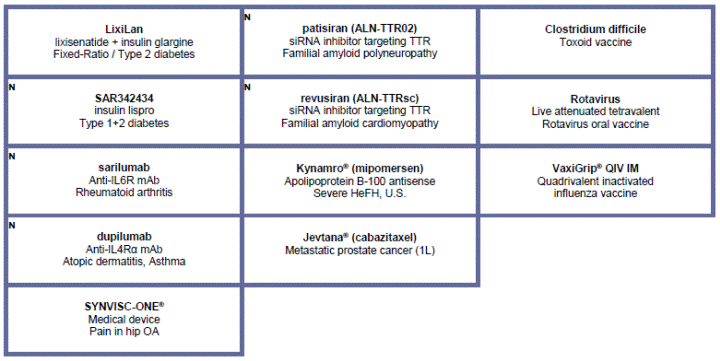
Phase II
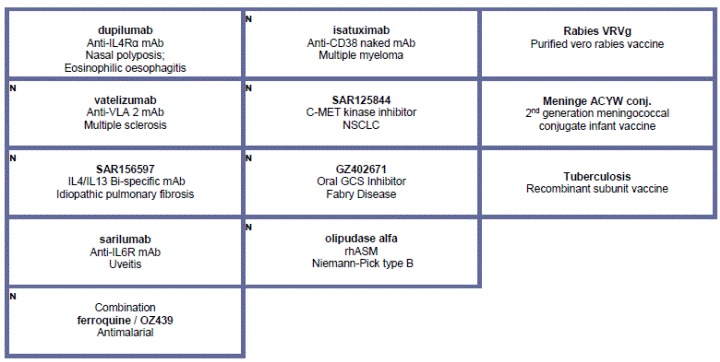
25
Phase I
N : New molecular entity
26
Appendix 9: Expected R&D milestones
Product | | Event | | Timing |
| | | | |
Praluent® (alirocumab) | | Expected EC regulatory approval in Hypercholesterolemia | | Q3 2015 |
| | | | |
LixiLan | | Expected Phase III top line results in Diabetes | | Q3 2015 |
| | | | |
Vaxigrip® QIV IM (3+ years) | | Expected EU regulatory submission | | Q4 2015 |
| | | | |
Dengue vaccine | | Expected regulatory decision in endemic countries | | Q4 2015 |
| | | | |
LixiLan | | Expected U.S. regulatory submission in Diabetes | | Q4 2015 |
| | | | |
Sarilumab | | Expected U.S. regulatory submission in Rheumatoid Arthritis | | Q4 2015 |
| | | | |
PR5i vaccine (DTP-HepB-Polio-Hib) | | Expected U.S. regulatory decision | | Q4 2015 |
| | | | |
LixiLan | | Expected EU regulatory submission in Diabetes | | Q1 2016 |
| | | | |
Dupilumab | | Expected start of Phase III trial in Nasal Polyposis | | Q1 2016 |
| | | | |
PR5i vaccine (DTP-HepB-Polio-Hib) | | Expected EU regulatory decision | | Q1 2016 |
| | | | |
Meningitis ACYW conj. vaccine | | Expected start of Phase III trial | | Q1 2016 |
| | | | |
Dupilumab | | Expected Phase III top line results in Atopic Dermatitis | | Q1 2016 |
| | | | |
Rotavirus vaccine | | Expected regulatory submission in India | | Q2 2016 |
| | | | |
Insulin lispro | | Expected Phase III top line results in Diabetes | | Q2 2016 |
| | | | |
Lixisenatide | | Expected U.S. regulatory decision in Diabetes | | Q3 2016 |
27
Appendix 10: Definitions of non-GAAP financial indicators
Net sales at constant exchange rates (CER)
When we refer to changes in our net sales “at constant exchange rates” (CER), this means that we exclude the effect of changes in exchange rates.
We eliminate the effect of exchange rates by recalculating net sales for the relevant period at the exchange rates used for the previous period.
Reconciliation of reported net sales to net sales at constant exchange rates for the second quarter and the first half of 2015
€ million | | Q2 2015 | | H1 2015 | |
Net sales | | 9,378 | | 18,188 | |
Effect of exchange rates | | -910 | | -1,692 | |
Net sales at constant exchange rates | | 8,468 | | 16,496 | |
Net sales on a constant structure basis
We eliminate the effect of changes in structure by restating prior-period net sales as follows:
· by including sales from the acquired entity or product rights for a portion of the prior period equal to the portion of the current period during which we owned them, based on sales information we receive from the party from whom we make the acquisition;
· similarly, by excluding sales in the relevant portion of the prior period when we have sold an entity or rights to a product;
· for a change in consolidation method, by recalculating the prior period on the basis of the method used for the current period.
Business net income
Sanofi publishes a key non-GAAP indicator. This indicator “Business net income”, replaced “adjusted net income excluding selected items”.
Business net income is defined as net income attributable to equity holders of Sanofi excluding:
· amortization of intangible assets,
· impairment of intangible assets,
· fair value remeasurement of contingent consideration liabilities related to business combinations,
· other impacts associated with acquisitions (including impacts of acquisitions on associates),
· restructuring costs(1),
· other gains and losses (including gains and losses on disposals of non-current assets(1)),
· costs or provisions associated with litigation(1),
· tax effects related to the items listed above as well as effects of major tax disputes.
· tax (3%) on dividends paid to Sanofi shareholders.
(1) Reported in the line items Restructuring costs and Gains and losses on disposals, and litigation, which are defined in Note B.20. to our consolidated financial statements.
28




