Filed by Corgentech Inc. Pursuant to Rule 425
Under the Securities Act of 1933
And Deemed Filed Pursuant to Rule 14a-12
Under the Securities Exchange Act of 1934
Subject Company: AlgoRx Pharmaceuticals, Inc.
Commission File No. of Subject Company: 000-51146
*** *** ***
The following presentation was presented to AlgoRx Pharmaceuticals, Inc. employees on September 26, 2005.

Corgentech and AlgoRx Announce Merger Agreement
Presentation to AlgoRx Employees
September 26, 2005

FORWARD LOOKING STATEMENTS
This presentation includes “forward-looking statements” within the meaning of the safe harbor provisions of the United States Private Securities Litigation Reform Act of 1995. Words such as “expect,” “estimate,” “project,” “budget,” “forecast,” “anticipate,” “intend,” “plan,” “may,” “will,” “could,” “should,” “believes,” “predicts,” “potential,” “continue,” and similar expressions are intended to identify such forward-looking statements. Forward-looking statements in this presentation include, without limitation, forecasts of product development, FDA filings, benefits of the proposed merger, and other matters that involve known and unknown risks, uncertainties and other factors that may cause actual results, levels of activity, performance or achievements to differ materially from results expressed or implied in this presentation. Such risk factors include, among others: difficulties encountered in integrating merged businesses; uncertainties as to the timing of the merger; approval of the transaction by the stockholders of the companies; the satisfaction of closing conditions to the transaction, including the receipt of regulatory approvals; whether certain market segments grow as anticipated; the competitive environment in the biotechnology industry; and whether the companies can successfully develop new products and the degree to which these gain market acceptance. Actual results may differ materially from those contained in the forward-looking statements in this presentation. Additional information concerning these and other risk factors is contained in Corgentech’s Form 10-K/A for the year ended December 31, 2004 and most recently filed Form10-Q.
Corgentech and AlgoRx undertake no obligation and do not intend to update these forward-looking statements to reflect events or circumstances occurring after this press release. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date of this presentation. All forward-looking statements are qualified in their entirety by this cautionary statement.
2

FORWARD LOOKING STATEMENTS (2)
Additional Information and Where to Find It
Corgentech Inc. intends to file a registration statement on Form S-4, and Corgentech and AlgoRx Pharmaceuticals, Inc. intend to file a related joint proxy statement/prospectus, in connection with the merger transaction involving Corgentech and AlgoRx. Investors and security holders are urged to read the registration statement on Form S-4 and the related joint proxy/prospectus when they become available because they will contain important information about the merger transaction. Investors and security holders may obtain free copies of these documents (when they are available) and other documents filed with the SEC at the SEC’s web site at www.sec.gov. In addition, investors and security holders may obtain free copies of the documents filed with the SEC by contacting Corgentech Investor Relations at the email address: investors@corgentech.com.
Corgentech, AlgoRx and their directors and executive officers may be deemed to be participants in the solicitation of proxies from the stockholders of Corgentech and AlgoRx in connection with the merger transaction. Information regarding the special interests of these directors and executive officers in the merger transaction will be included in the joint proxy statement/prospectus of AlgoRx and Corgentech described above. Additional information regarding the directors and executive officers of Corgentech is also included in Corgentech’s proxy statement for its 2005 Annual Meeting of Stockholders, which was filed with the SEC on April 27, 2005. This document is available free of charge at the SEC’s web site at www.sec.gov and from Investor Relations at Corgentech as described above.
CREATING LATE-STAGE COMPANY
Multiple late-stage product development programs focused on significant clinical problems
Pain management
Inflammatory diseases
Brings together development and commercial expertise in critical areas
Marketing, commercialization, regulatory affairs and clinical trials management
Financial strength and flexibility
All discovery & development programs 100% owned

AGENDA
About Corgentech
Product Pipeline of Combined Company
Terms and Timelines
Communications
Summary
CORGENTECH
Public biotechnology company founded in 1999
~70 employees all based in South San Francisco
Developing transcription factor decoys for inflammatory diseases and cancer and aptamers for antibiotic resistance
Two Phase 1/2 trials under way for NF-kB Decoy for eczema
Experience in marketing, commercialization, regulatory affairs and clinical trials management
Phase 3 trial failures of E2F decoy for coronary artery bypass grafts in December and March
Prompted us to look for other late-stage product opportunities Financial strength and flexibility
Over $95 million in cash at the end of the second quarter
All discovery & development programs 100% owned
6
ACCOMPLISHMENTS
Research
NF-kB Decoy lead compound in 6 months
Demonstrated efficacy in multiple preclinical models of arthritis, asthma, IBD and eczema
Developed internally topical formulation equivalent to best of Dow
All IND-enabling preclinical studies for NF-kB Decoy for eczema accomplished internally in 10 months Development
Peripheral Phase 3 trial of 1400 patients at 80 sites enrolled in 18 months
CABG Phase 3 trial of 2400 patients at 100 sites enrolled in 12 months
Both databases queried, cleaned and locked on time
7
ACCOMPLISHMENTS
Regulatory
First drug with Fast Track status in CardioRenal Division
In Phase 3 CABG trial, persuaded FDA to accept 2,400 patient trial w/graft failure surrogate endpoint and one year f/u in lieu of FDA proposal of 13,000 patient trial w/mortality clinical endpoint lasting several years
When first Phase 3 trial failed, persuaded FDA to accept single trial (second Phase 3) as basis for approval
Experienced with electronic submissions
510(k) for device filed
Filed preclinical module and FDA had NO comments
CMC module was 98% complete and would have been filed in two weeks – BMS review stated it to be high quality
Third party manufacturers were mostly ready for FDA plant inspection
Corgentech timeline for filing modules was six months shorter than proposed by BMS and met
8

ACCOMPLISHMENTS
Manufacturing
Managed drug supply, and device design and supply
Drug and device at commercial scale in mid-Phase 3
All validation batches were completed in 2004
Execution of long term supply agreements pending clinical data
Commercial
Separate ICD-9 code in May 2004 – “impossible before clinical data”
Pricing conversations with CMS supported $5,000-8,000 reimbursement, public statements were $2,500-3,500
Reimbursement code available at launch, not one or more years later Marketing team built
Pre-launch and launch plans complete pending clinical data
Key Sales management identified
Sales territories mapped and prepared to initiate rep recruitment
Human Resources
HR team experienced with corporate integrations
9

ACCOMPLISHMENTS
BMS Deal
$45 MM upfront
50/50 US profit split
$570 MM milestone payments
12-20% ex-US royalties
65/35 expense split until commercialization
Third best Phase 3 deal at time
CGTK retained control and drove process to
CGTK timeline, not longer BMS timeline
10
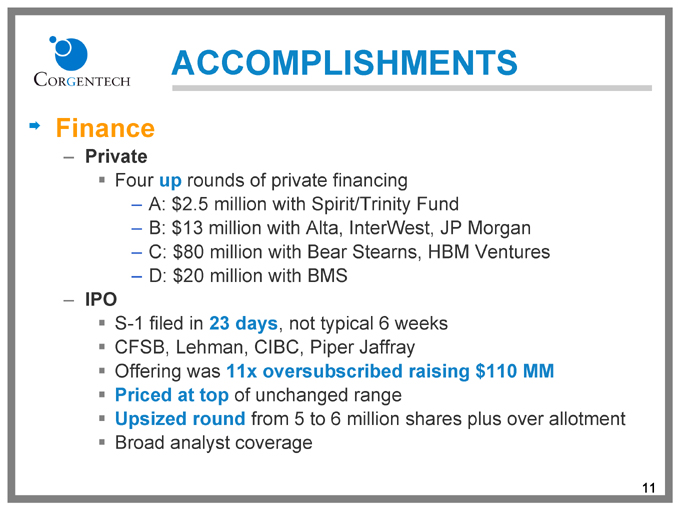
ACCOMPLISHMENTS
Finance
Private
Four up rounds of private financing
A: $2.5 million with Spirit/Trinity Fund
B: $13 million with Alta, InterWest, JP Morgan
C: $80 million with Bear Stearns, HBM Ventures
D: $20 million with BMS
IPO
S-1 filed in 23 days, not typical 6 weeks
CFSB, Lehman, CIBC, Piper Jaffray
Offering was 11x oversubscribed raising $110 MM
Priced at top of unchanged range
Upsized round from 5 to 6 million shares plus over allotment
Broad analyst coverage
11

DIVERSE TEAM
12

AGENDA
About Corgentech
Product Pipeline of Combined Company
Terms and Timelines
Communications
Summary
13
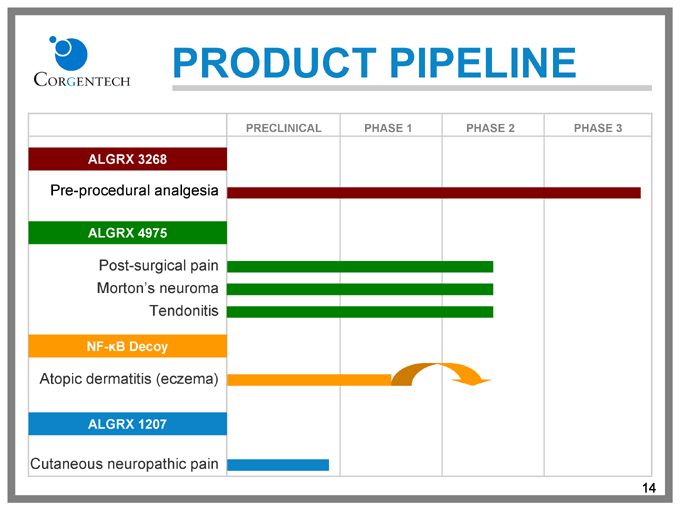
PRODUCT PIPELINE
PRECLINICAL PHASE 1 PHASE 2 PHASE 3
ALGRX 3268
Pre-procedural analgesia
ALGRX 4975
Post-surgical pain
Morton’s neuroma
Tendonitis
NF-kB Decoy
Atopic dermatitis (eczema)
ALGRX 1207
Cutaneous neuropathic pain
14
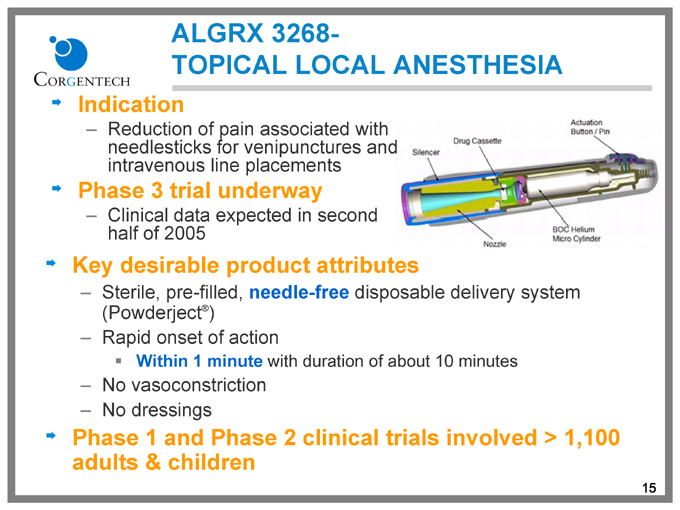
ALGRX 3268-
TOPICAL LOCAL ANESTHESIA
Indication
Reduction of pain associated with needlesticks for venipunctures and intravenous line placements
Phase 3 trial underway
Clinical data expected in second half of 2005
Key desirable product attributes
Sterile, pre-filled, needle-free disposable delivery system (Powderject®)
Rapid onset of action
Within 1 minute with duration of about 10 minutes
No vasoconstriction
No dressings
Phase 1 and Phase 2 clinical trials involved > 1,100 adults & children
15
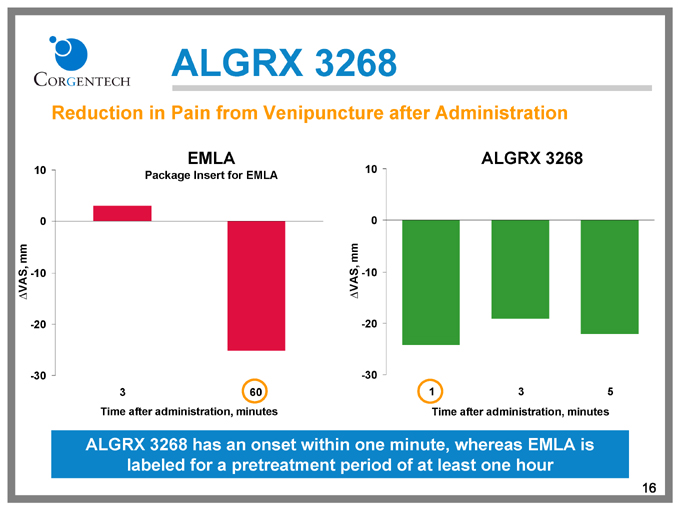
ALGRX 3268
Reduction in Pain from Venipuncture after Administration
EMLA
Package Insert for EMLA
ALGRX 3268
10
0
VAS, mm -10
-20
-30
10
0
VAS, mm -10
-20
-30
3 60
Time after administration, minutes
1 3 5
Time after administration, minutes
ALGRX 3268 has an onset within one minute, whereas EMLA is labeled for a pretreatment period of at least one hour
16
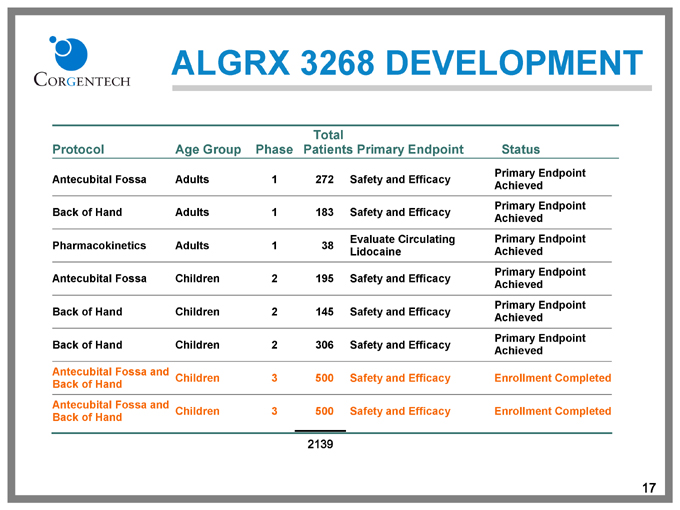
ALGRX 3268 DEVELOPMENT
Protocol Age Group Phase Total Patients Primary Endpoint Status Primary Endpoint
Antecubital Fossa Adults 1 272 Safety and Efficacy Achieved Primary Endpoint
Back of Hand Adults 1 183 Safety and Efficacy Evaluate Circulating Achieved Primary Endpoint
Pharmacokinetics Adults 1 38 Lidocaine Achieved Primary Endpoint
Antecubital Fossa Children 2 195 Safety and Efficacy Achieved Primary Endpoint
Back of Hand Children 2 145 Safety and Efficacy Achieved Primary Endpoint
Back of Hand Children 2 306 Safety and Efficacy Achieved
Antecubital Fossa and Back of Hand Children 3 500 Safety and Efficacy Enrollment Completed
Antecubital Fossa and Back of Hand Children 3 500 2139 Safety and Efficacy Enrollment Completed
17
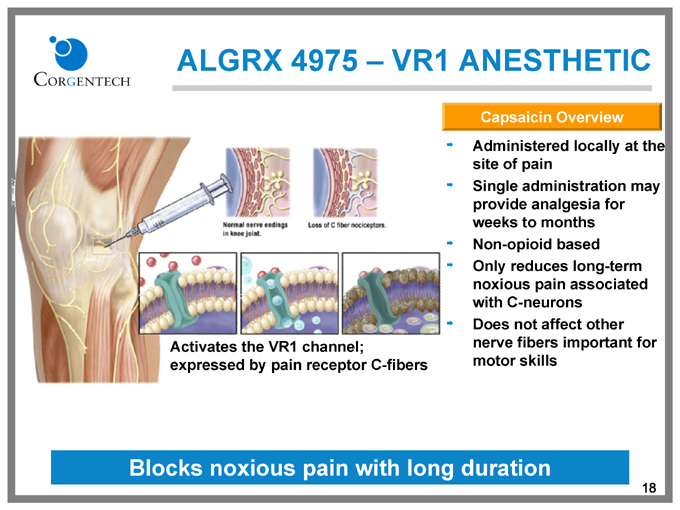
ALGRX 4975 – VR1 ANESTHETIC
Capsaicin Overview
Administered locally at the site of pain
Single administration may provide analgesia for weeks to months
Non-opioid based
Only reduces long-term noxious pain associated with C-neurons
Does not affect other nerve fibers important for motor skills
Activates the VR1 channel; expressed by pain receptor C-fibers
Blocks noxious pain with long duration
18
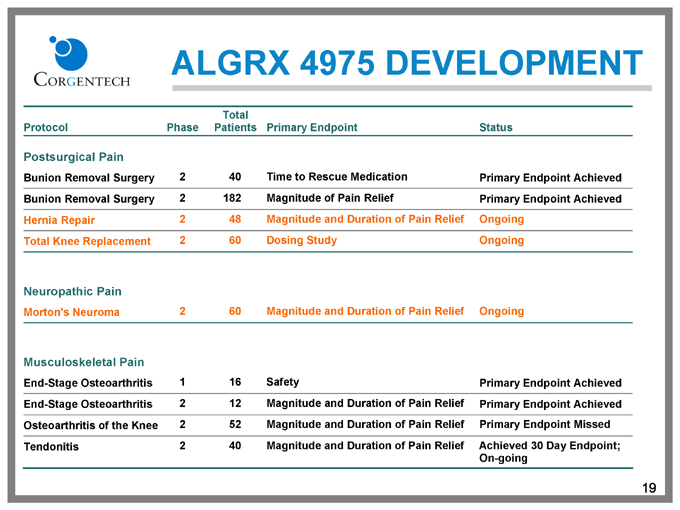
ALGRX 4975 DEVELOPMENT
Protocol Phase Total Patients Primary Endpoint Status
Postsurgical Pain
Bunion Removal Surgery 2 40 Time to Rescue Medication Primary Endpoint Achieved
Bunion Removal Surgery 2 182 Magnitude of Pain Relief Primary Endpoint Achieved
Hernia Repair 2 48 Magnitude and Duration of Pain Relief Ongoing
Total Knee Replacement 2 60 Dosing Study Ongoing
Neuropathic Pain
Morton’s Neuroma 2 60 Magnitude and Duration of Pain Relief Ongoing
Musculoskeletal Pain
End-Stage Osteoarthritis 1 16 Safety Primary Endpoint Achieved
End-Stage Osteoarthritis 2 12 Magnitude and Duration of Pain Relief Primary Endpoint Achieved
Osteoarthritis of the Knee 2 52 Magnitude and Duration of Pain Relief Primary Endpoint Missed
Tendonitis 2 40 Magnitude and Duration of Pain Relief Achieved 30 Day Endpoint; On-going
19
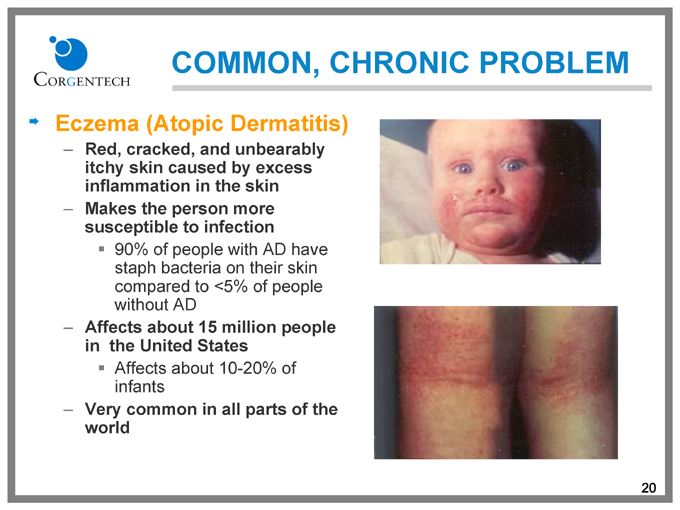
COMMON, CHRONIC PROBLEM
Eczema (Atopic Dermatitis)
Red, cracked, and unbearably itchy skin caused by excess inflammation in the skin
Makes the person more susceptible to infection
90% of people with AD have staph bacteria on their skin compared to <5% of people without AD
Affects about 15 million people in the United States
Affects about 10-20% of infants
Very common in all parts of the world
20
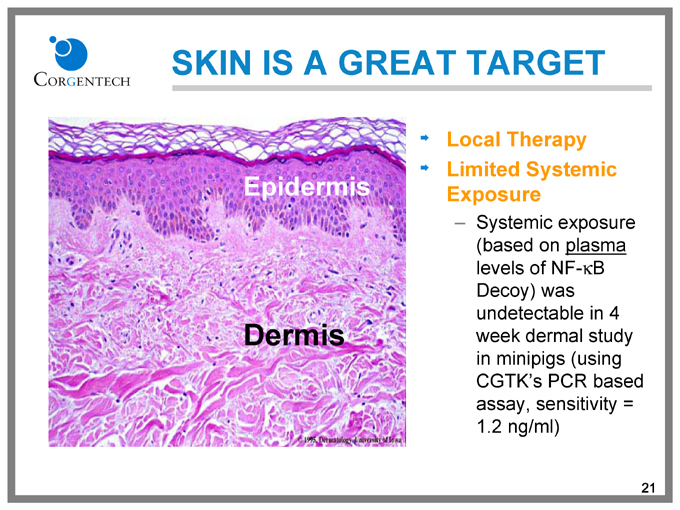
SKIN IS A GREAT TARGET
Local Therapy
Limited Systemic Exposure
Systemic exposure (based on plasma levels of NF-kB Decoy) was undetectable in 4 week dermal study in minipigs (using CGTK’s PCR based assay, sensitivity = 1.2 ng/ml)
Epidermis
Dermis
21
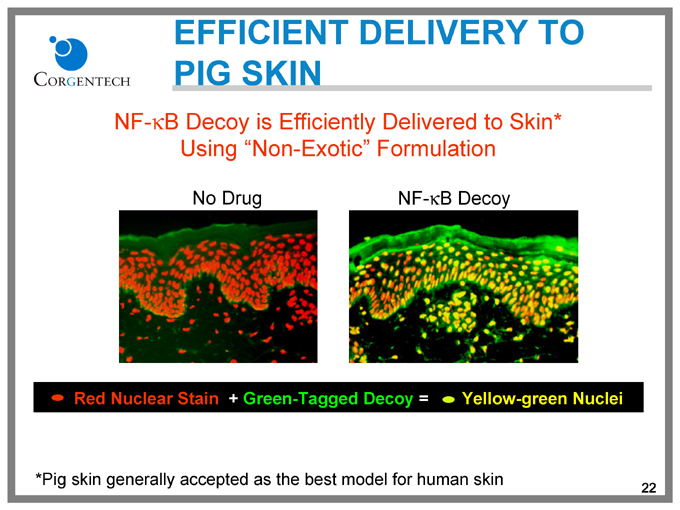
EFFICIENT DELIVERY TO PIG SKIN
NF-kB Decoy is Efficiently Delivered to Skin* Using “Non-Exotic” Formulation
No Drug
NF-kB Decoy
Red Nuclear Stain + Green-Tagged Decoy = Yellow-green Nuclei
*Pig skin generally accepted as the best model for human skin
22
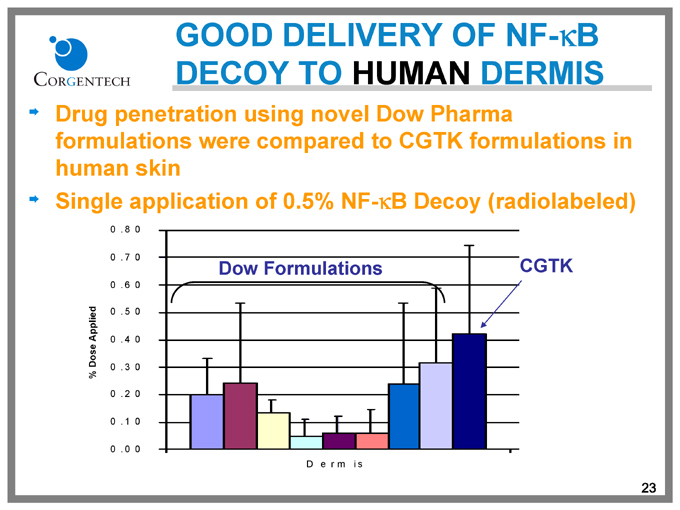
GOOD DELIVERY OF NF-kB DECOY TO HUMAN DERMIS
Drug penetration using novel Dow Pharma formulations were compared to CGTK formulations in human skin
Single application of 0.5% NF-kB Decoy (radiolabeled)
0 . 8 0
0 . 7 0
0 . 6 0
0 . 5 0
0 . 4 0
% Dose Applied 0 . 3 0
0 . 2 0
0 . 1 0
0 . 0 0
Dow Formulations
CGTK
Dermis
23
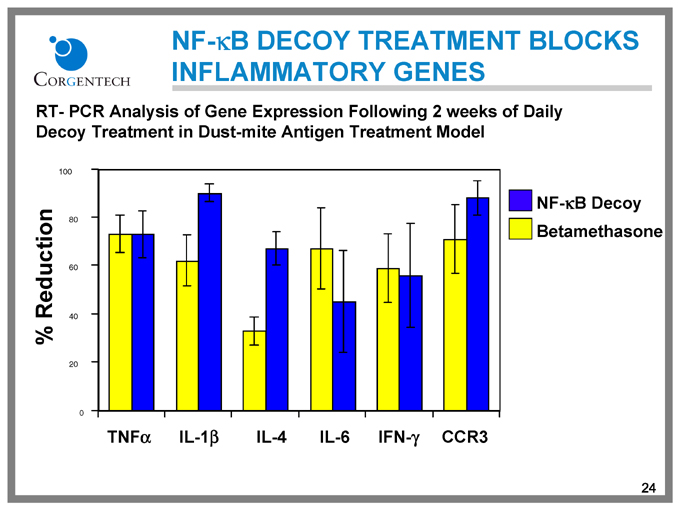
NF-kB DECOY TREATMENT BLOCKS INFLAMMATORY GENES
RT- PCR Analysis of Gene Expression Following 2 weeks of Daily Decoy Treatment in Dust-mite Antigen Treatment Model
100
80
60
% Reduction 40
20
0
NF-kB Decoy Betamethasone
TNFa IL-1ß IL-4 IL-6 IFN-g CCR3
24
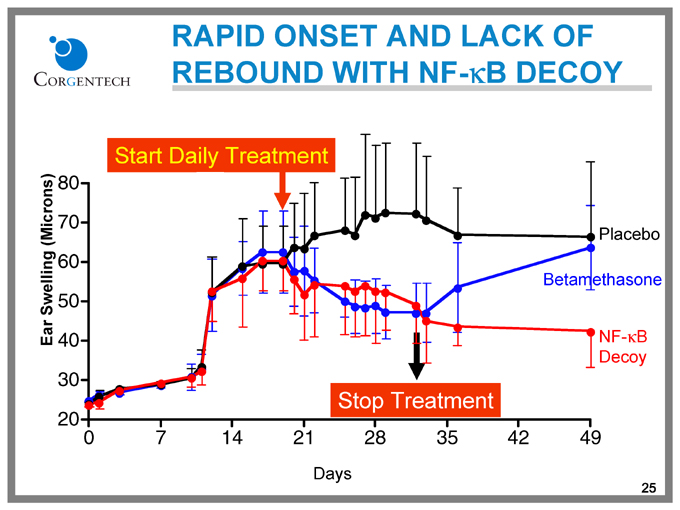
RAPID ONSET AND LACK OF REBOUND WITH NF-kB DECOY
Ear Swelling (Microns)
80
70
60
50
40
30
20
0 7 14 21 28 35 42 49
Start Daily Treatment
Placebo
Betamethasone
NF-kB Decoy
Stop Treatment
Days
25
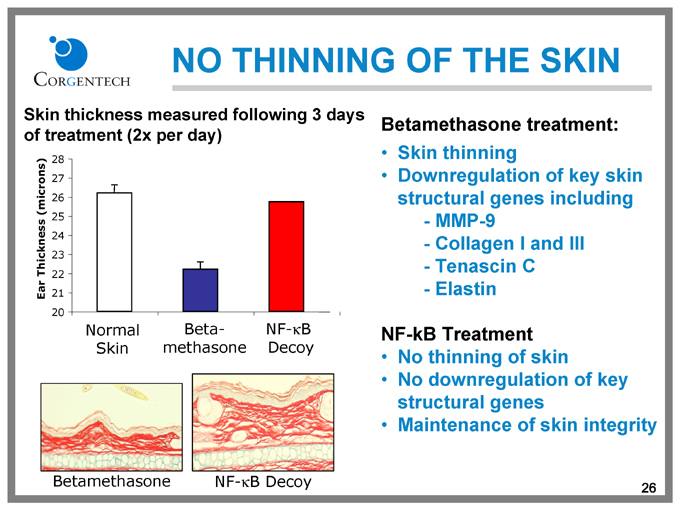
NO THINNING OF THE SKIN
Skin thickness measured following 3 days of treatment (2x per day)
Ear Thickness (microns)
28
27
26
25
24
23
22
21
20
Normal Beta- NF-KB
Skin methasone Decoy
Betamethasone treatment:
Skin thinning
Downregulation of key skin structural genes including
MMP-9
Collagen I and III Tenascin C Elastin
NF-kB Treatment
No thinning of skin No downregulation of key structural genes Maintenance of skin integrity
Betamethasone
NF-kB Decoy
26
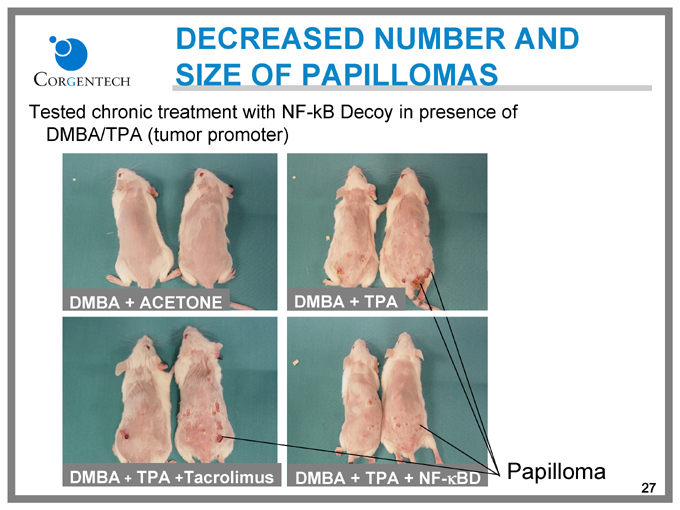
DECREASED NUMBER AND SIZE OF PAPILLOMAS
Tested chronic treatment with NF-kB Decoy in presence of DMBA/TPA (tumor promoter)
DMBA + ACETONE
DMBA + TPA
DMBA + TPA + Tacrolimus
DMBA + TPA + NF-kBD
Papilloma
27
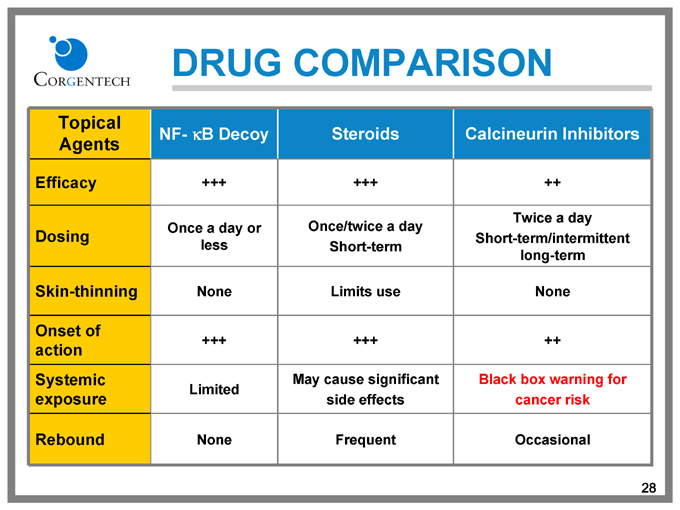
DRUG COMPARISON
Topical Agents NF- kB Decoy Steroids Calcineurin Inhibitors
Efficacy +++ +++ ++
Dosing Once a day or less Once/twice a day Short-term Twice a day Short-term/intermittent long-term
Skin-thinning None Limits use None
Onset of action +++ +++ ++
Systemic exposure Limited May cause significant side effects Black box warning for cancer risk
Rebound None Frequent Occasional
28
PHASE 1/2’s: PROOF OF CONCEPT
Adults with mild-moderate eczema 200 cm2 affected area (treated area will be moderate) Randomized, double blind Endpoints
Safety, tolerability and PK
Multiple endpoints to assess preliminary efficacy
Physician and patient assessments of target area (signs and symptoms)
29
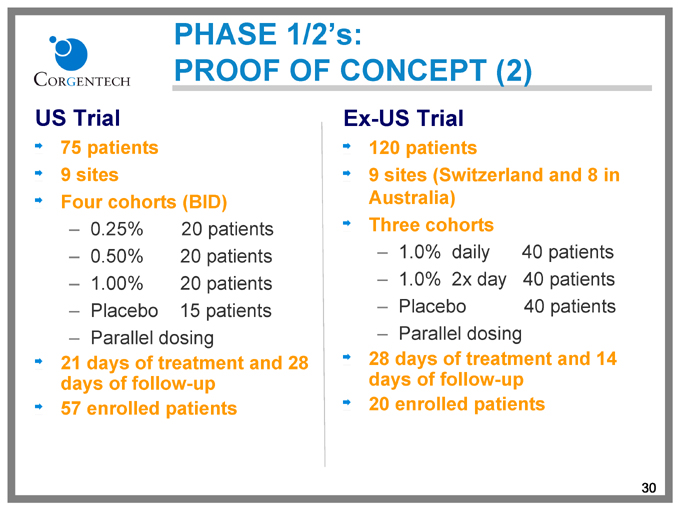
PHASE 1/2’s:
PROOF OF CONCEPT (2)
US Trial
75 patients 9 sites Four cohorts (BID)
0.25% 20 patients
0.50% 20 patients
1.00% 20 patients
Placebo 15 patients
Parallel dosing
21 days of treatment and 28 days of follow-up 57 enrolled patients
Ex-US Trial
120 patients
9 sites (Switzerland and 8 in Australia) Three cohorts
1.0% daily 40 patients
1.0% 2x day 40 patients
Placebo 40 patients
Parallel dosing
28 days of treatment and 14 days of follow-up 20 enrolled patients
30

ALGRX 1207
IND-enabling studies underway
New chemical class of local anesthetic for topical analgesia
Deep, rapid penetration of the skin and long duration of action Addresses a wide variety of procedures including:
Neuropathic pain Dermatological surgery Surgical incisions
31
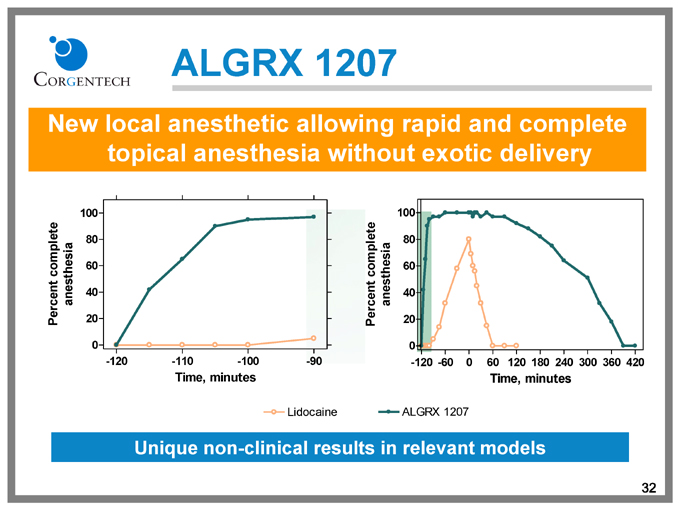
ALGRX 1207
New local anesthetic allowing rapid and complete topical anesthesia without exotic delivery
Percent complete anesthesia
100
80
60
40
20
0
-120 -110 -100 -90
Time, minutes
Percent complete anesthesia
100
80
60
40
20
0
-120 -60 0 60 120 180 240 300 360 420
Time, minutes
Lidocaine
ALGRX 1207
Unique non-clinical results in relevant models
32
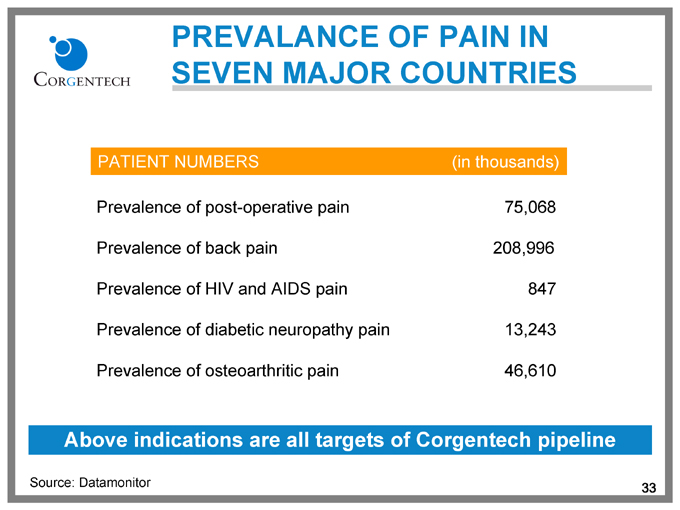
PREVALANCE OF PAIN IN SEVEN MAJOR COUNTRIES
PATIENT NUMBERS (in thousands)
Prevalence of post-operative pain 75,068
Prevalence of back pain 208,996
Prevalence of HIV and AIDS pain 847
Prevalence of diabetic neuropathy pain 13,243
Prevalence of osteoarthritic pain 46,610
Above indications are all targets of Corgentech pipeline
Source: Datamonitor
33

3268: TOPICAL LOCAL
ANESTHESIA MARKET DYNAMICS
Strong focus of pain management including treatment protocols and guidelines Increase in dedicated pediatric hospitals and specialized pediatric departments in larger general hospitals Continuing pressure on cost reduction and patient through-put in hospitals Reimbursement for TLAs a significant challenge for office-based procedures Fundamental market need for easy to use, fast onset TLA
Current market dynamics provide an excellent opportunity for a fast acting, easy to use TLA product like ALGRX 3268
34
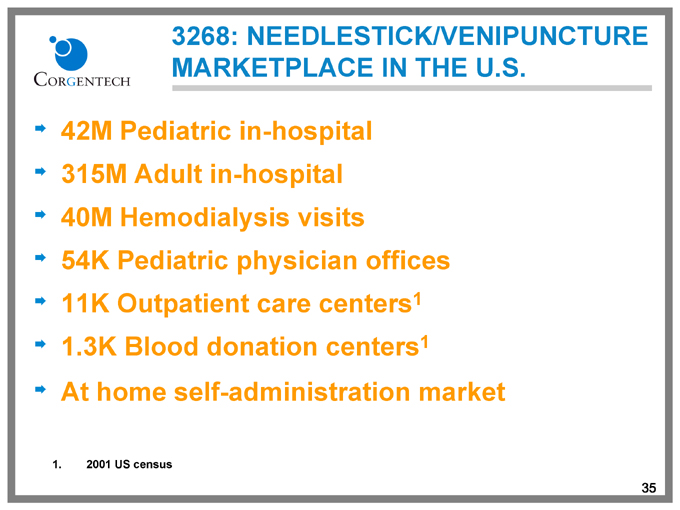
3268: NEEDLESTICK/VENIPUNCTURE MARKETPLACE IN THE U.S.
42M Pediatric in-hospital 315M Adult in-hospital 40M Hemodialysis visits 54K Pediatric physician offices 11K Outpatient care centers1 1.3K Blood donation centers1 At home self-administration market
1. 2001 US census
35
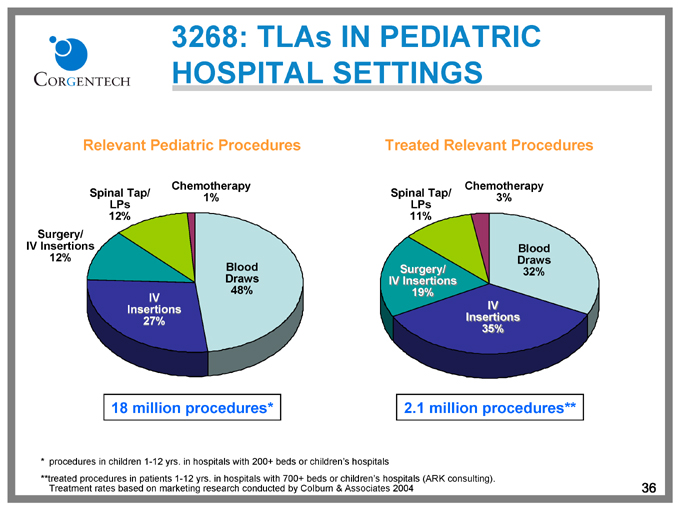
3268: TLAs IN PEDIATRIC HOSPITAL SETTINGS
Relevant Pediatric Procedures
Spinal Tap/ LPs 12%
Chemotherapy 1%
Surgery/ IV Insertions 12%
IV Insertions 27%
Blood Draws 48%
18 million procedures*
Treated Relevant Procedures
Spinal Tap/ LPs 11%
Chemotherapy 3%
Surgery/ IV Insertions 19%
IV Insertions 35%
Blood Draws 32%
2.1 million procedures**
* | | procedures in children 1-12 yrs. in hospitals with 200+ beds or children’s hospitals |
**treated procedures in patients 1-12 yrs. in hospitals with 700+ beds or children’s hospitals (ARK consulting).
Treatment rates based on marketing research conducted by Colburn & Associates 2004
36
3268: CURRENT CLINICAL PRACTICE OF TLAs IN HOSPITAL SETTING
Many hospitals offer TLA agents as an option to reduce needlestick pain
Existence of established protocols for TLA use is mixed Nurses rather than physicians usually decision maker
Some ERs apply TLAs, however use is often impractical due to delayed onset of action TLAs are most commonly offered to pediatric patients ages 3-10
Parents and sometimes children over 10 occasionally request TLA to reduce needlestick pain Patient and parent anxiety levels are key factors driving the use of TLAs Previously treated children frequently request TLA
37
3268: PHASED MARKETING APPROACH
Physcian Offices, Labs, Blood donation centers
Hemodialysis Centers In-hospital Pediatric 18M
38
4975: POST-OPERATIVE PAIN MARKET
US post-operative pain market: $1.7 billion Virtually all patients experience some level of pain post operatively
50% indicate lack of adequate pain control
Patients’ post-surgical pain experiences between 1993 and 1999 have not improved Europe also under treats post-operative pain
Pain in hospital associated with increased length of stay, longer recovery times, poorer patient outcomes
39

4975: POTENTIAL TARGETS—HIGH VOLUME SURGICAL PROCEDURES
Procedure Volume
Caesarean Section 1,163,893
Hysterectomy (all) 1,042,715
Episiotomy 417,699
Arthroplasty (knee) 399,139
Hip Replacement, 343,554
total/partial
CABG 316,471
Colorectal resection 280,969
40

CURRENT POST-OP PAIN MEDICATIONS VS. ALGRX 4975
Epidurals
Use declining significantly due to contra-indication in combination use with anticoagulants
Opioids
Nausea, vomiting, respiratory depression and constipation Sedation may limit the ability of the patient to ambulate and prolong time to discharge
Decreased bowel function can lead to an ileus which causes morbidity and prolongs hospitalization
Concern about addiction leads to insufficient pain relief which has physiologic consequences
Non-narcotics
Unacceptable anesthesia for moderate to severe pain
ALGRX 4975
Unlikely to be contra-indicated for use in conjunction with anticoagulants Site-specific with limited systemic exposure and unlikely to cause nausea, vomiting, respiratory depression or sedation No addiction potential Long-acting with single administration and ideal for moderate to severe pain
41
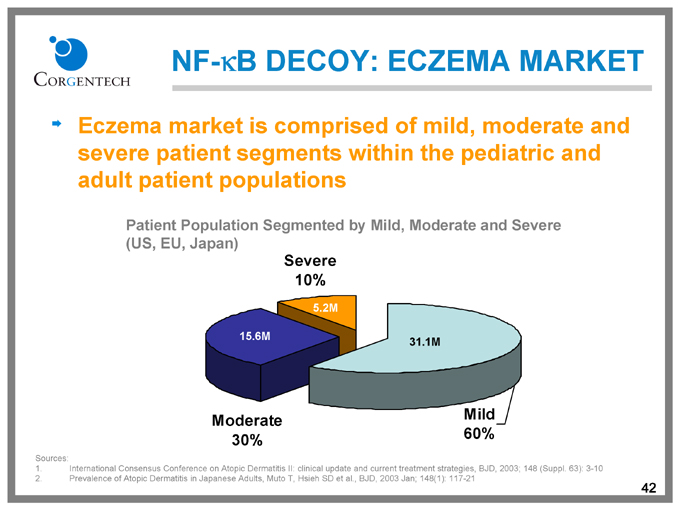
NF-kB DECOY: ECZEMA MARKET
Eczema market is comprised of mild, moderate and severe patient segments within the pediatric and adult patient populations
Patient Population Segmented by Mild, Moderate and Severe (US, EU, Japan)
Severe 10%
5.2M
15.6M
31.1M
Moderate 30%
Mild 60%
Sources:
1. International Consensus Conference on Atopic Dermatitis II: clinical update and current treatment strategies, BJD, 2003; 148 (Suppl. 63): 3-10
2. Prevalence of Atopic Dermatitis in Japanese Adults, Muto T, Hsieh SD et al., BJD, 2003 Jan; 148(1): 117-21
42
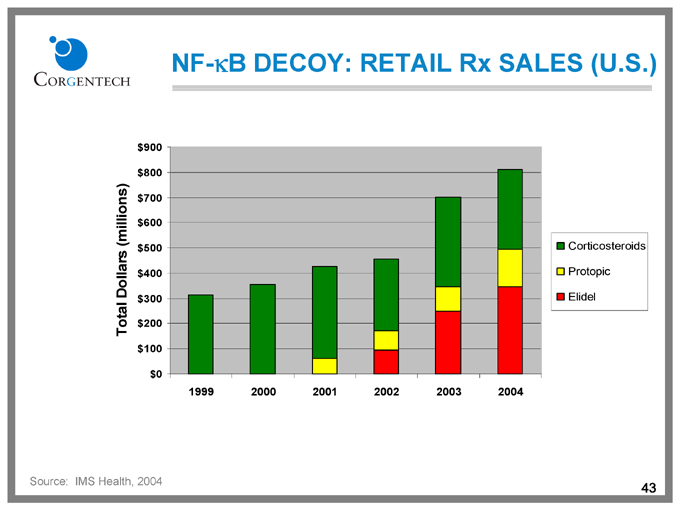
NF-kB DECOY: RETAIL Rx SALES (U.S.)
Total Dollars (millions)
$900
$800
$700
$600
$500
$400
$300
$200
$100
$0
1999 2000 2001 2002 2003 2004
Corticosteroids Protopic Elidel
Source: IMS Health, 2004
43
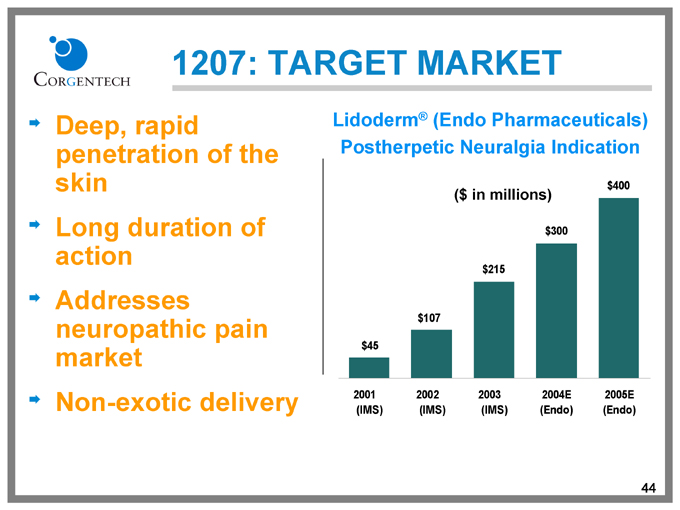
1207: TARGET MARKET
Deep, rapid penetration of the skin Long duration of action Addresses neuropathic pain market Non-exotic delivery
Lidoderm® (Endo Pharmaceuticals) Postherpetic Neuralgia Indication
($ in millions)
$45
2001
(IMS)
$107
2002
(IMS)
$215
2003
(IMS)
$300
2004E
(Endo)
$400
2005E
(Endo)
44

AGENDA
About Corgentech Product Pipeline of Combined Company Terms and Timelines Communications Summary
45

TERMS OF MERGER AGREEMENT
Both Secaucus and South San Francisco facilities to remain open
Headquarters: South San Francisco
Combined management team will work to integrate the companies and to identify synergies, redundancies and needs across the combined organization
Corgentech and AlgoRx currently employ ~95 people total Goal is to complete assessment over next three weeks
Board and Management
New Board of Directors composed of: Richard Brewer, Dr. Charles Cohen, Thomas Colligan, Carter Eckert, Dr. Rodney Ferguson, John McLaughlin, Dr. Arnold Oronsky and Dr. Michael Powell
A ninth board member to be identified and added soon after deal approval
Management
John McLaughlin as CEO
Dr. Ronald Burch as VP of Development Richard Powers as CFO
James Huang as President with primary responsibility for commercial and other operations, key liaison to AlgoRx
Stock Transaction
As of the date of closing of the merger agreement, Corgentech would issue sufficient shares such that AlgoRx shareholders would receive, in a tax-free exchange, 62% of the combined company and Corgentech shareholders would own 38% of the combined company
46
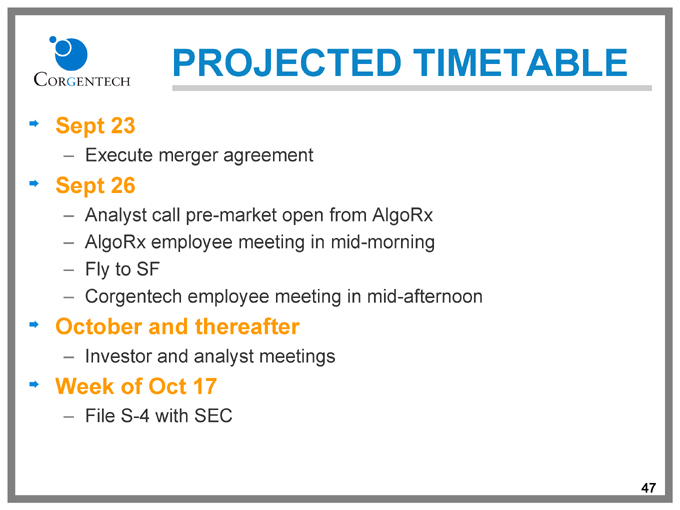
PROJECTED TIMETABLE
Sept 23
Execute merger agreement
Sept 26
Analyst call pre-market open from AlgoRx AlgoRx employee meeting in mid-morning Fly to SF
Corgentech employee meeting in mid-afternoon
October and thereafter
Investor and analyst meetings
Week of Oct 17
File S-4 with SEC
47
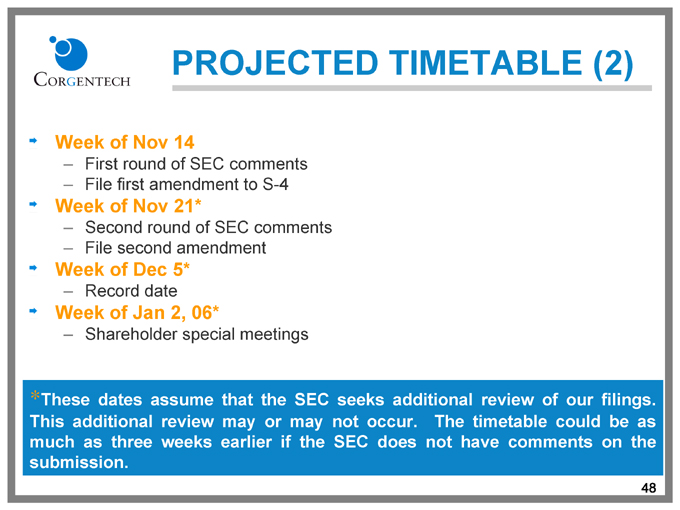
PROJECTED TIMETABLE (2)
Week of Nov 14
First round of SEC comments File first amendment to S-4
Week of Nov 21*
Second round of SEC comments File second amendment
Week of Dec 5*
Record date
Week of Jan 2, 06*
Shareholder special meetings
* | | These dates assume that the SEC seeks additional review of our filings. |
This additional review may or may not occur. The timetable could be as much as three weeks earlier if the SEC does not have comments on the submission.
48

AGENDA
About Corgentech Product Pipeline of Combined Company Terms and Timelines Communications Summary
49

COMMUNICATIONS
Identify key individual contacts in Secaucus, Sunnyvale and SSF to assure clear communication, answer questions, etc. during transition period Project teams
Good venue to share information, discuss issues, solve problems, allocate resources Minutes within 36 hours – good means to update, share decisions, advise on timeline Regularly scheduled video-conferences for teams and subteams Encourage ad hoc meetings to brainstorm solutions Important with four project teams, filing NDA, etc.
50

AGENDA
About Corgentech Product Pipeline of Combined Company Terms and Timelines Communications Summary
51

STRONG COMBINED COMPANY
Increased development resources (personnel and financial) assure timely execution of multiple clinical trials
Additional regulatory, manufacturing and quality resources assure timely FDA submissions, increased likelihood of successful plant inspections needed for FDA approval and negotiation of long term contracts for uninterrupted product supply Commercial resources assure proper positioning, sales roll out and reimbursement for maximizing sales Multiple technological approaches offer risk diversification in product portfolio Business development resources allow enhanced shareholder return through strategic product partnering Substantial financial resources
52



















































