As filed with the Securities and Exchange Commission on December 27, 2006
Registration No. ___
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM SB-2/A
REGISTRATION STATEMENT UNDER THE SECURITIES ACT OF 1933
ASIA BIOTECHNOLOGY GROUP INC.
(Exact name of registrant as specified in its charter)
| Delaware | N/A | N/A |
(State or other jurisdiction of organization) | (Primary Standard Industrial Classification Code Number) | (I.R.S. Employer Incorporation or Identification Number) |
No. 7, BohaiSanLu, Pingfang Industrial District,
Economic & Technological Development Area, Harbin, Heilongjiang Province, China
Telephone: +86 451 86812888
Fax: +86 451 86813999
(Address and telephone number of principal executive offices)
No. 7, BohaiSanLu, Pingfang Industrial District,
Economic & Technological Development Area, Harbin, Heilongjiang Province, China
(Address of principal place of business or intended principal place of business)
Mr. Xueliang Qiu, CEO
No. 7 BohaiSanLu, Pingfang Industrial District,
Economic & Technological Development Area, Harbin, Heilongjiang Province, China
Telephone: +86 451 86812888
Fax: +86 451 86813999
(Name, address and telephone number of agent for service)
Copies to:
Charles Law
King and Wood LLP
650 Page Mill Road Palo Alto, CA 94304
Tel. (650) 320-4563
Fax: (650) 494-1387
Approximate date of proposed sale to the public: As soon as practicable after the effective date of this registration statement.
If any of the securities being registered on this form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act, check the following box. x
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act Registration Statement number of the earlier effective Registration Statement for the same offering. o
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act Registration Statement number of the earlier effective Registration Statement for the same offering. o
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act Registration Statement number of the earlier effective Registration Statement for the same offering. o
If delivery of the prospectus is expected to be made pursuant to Rule 434, check the following box. o
CALCULATION OF REGISTRATION FEE
| Title of each class of securities to be registered | | Amount to be registered | | Proposed maximum offering price per unit(1) | | Proposed maximum aggregate offering price(1) | | Amount of registration fee | |
| Common Stock, $.001 par value | | | 23,444,000 Shares | | $ | 1(1 | ) | $ | 23,444,000 | | $ | 2,508.51 (1 | ) |
| Total | | | 23,444,000 Shares | | $ | 1(1 | ) | $ | 23,444,000 | | $ | 2,508.51 (1 | ) |
| (1) | Estimated solely for the purpose of calculating the registration fee in accordance with Rule 457( a) under the Securities Act of 1933. |
EXPLANATORY NOTE
A filing fee of $5,935.93 was paid upon the first filing of this registration statement on May 12, 2006 for 55,476,000 shares of common stock.
The offering price of $1.00 per share for the shares offered hereby has been arbitrarily determined by corporate management. The offering price was not established through any consideration of actual book value, earnings per share, past operating history, recent sales transactions, or any other recognized criteria of value.
The registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment which specifically states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933 or until the registration statement shall become effective on such date as the Commission, acting pursuant to said Section 8(a), may determine.
THE INFORMATION IN THIS PROSPECTUS IS NOT COMPLETE AND MAY BE CHANGED. WE MAY NOT SELL THESE SECURITIES UNTIL THE REGISTRATION STATEMENT FILED WITH THE SECURITIES AND EXCHANGE COMMISSION IS EFFECTIVE. THIS PROSPECTUS IS NOT AN OFFER TO SELL THESE SECURITIES, AND IT IS NOT SOLICITING AN OFFER TO BUY THESE SECURITIES IN ANY STATE WHERE THE OFFER OR SALE IS NOT PERMITTED.
SUBJECT TO COMPLETION, December 27, 2006
Asia Biotechnology Group, Inc.
23,444,000 Shares
Common Stock
This prospectus is an offering of up to 23,444,000 shares of our common stock by the selling shareholders.
These securities are more fully described in the section of this prospectus titled "Description of Securities".
These securities are being registered to permit public secondary trading of the securities offered by the selling shareholders named in this prospectus. We will not receive any of the proceeds from the sale of the securities by the selling shareholders.
The selling shareholders may, but are not obligated to, offer all or part of their shares of common stock for resale from time to time through public or private transactions, at either prevailing market prices or at privately negotiated prices. See "Plan of Distribution."
There is currently no public market for our common stock shares. We intend to request our common stock be quoted on the OTC Bulletin Board and trade our securities in the secondary market.
INVESTING IN OUR SECURITIES INVOLVES A HIGH DEGREE OF RISK. YOU SHOULD PURCHASE OUR SECURITIES ONLY IF YOU CAN AFFORD A COMPLETE LOSS OF YOUR INVESTMENT. SEE "RISK FACTORS" BEGINNING AT PAGE 8.
NEITHER THE SECURITIES AND EXCHANGE COMMISSION NOR ANY STATE SECURITIES COMMISSION HAS APPROVED OR DISAPPROVED OF THESE SECURITIES OR PASSED UPON THE ADEQUACY OR ACCURACY OF THIS PROSPECTUS. ANY REPRESENTATION TO THE CONTRARY IS A CRIMINAL OFFENSE.
The date of this prospectus is____, 2006
ASIA BIOTECHNOLOGY GROUP INC.
TABLE OF CONTENTS
| | Page |
| Prospectus Summary | 5 |
| Risk Factors | 8 |
| Use of Proceeds | 31 |
| Market for Common Equity and Related Shareholder Matters | 32 |
| Management’s Discussion and Analysis or Plan of Operation | 32 |
| Business | 49 |
| Management | 67 |
| Director and Executive Compensation | 68 |
| Certain Relationships and Related Transactions | 69 |
| Security Ownership of Certain Beneficial Owners and Management | 70 |
| Selling Security Holders | 71 |
| Description of Securities | 105 |
| Plan of Distribution | 108 |
| Legal Matters | 109 |
| Experts | 109 |
| Changes In and Disagreements With Accountants on Accounting and Financial Disclosure | |
| Where You Can Find Additional Information | |
| Financial Statements | F-1 |
OT, OT logo, OuTi, and OuTi logo are trademarks or logos of Harbin OT Pharmaceutical Co., Ltd., our indirect subsidiary in China ("OT Pharmaceutical"). All other brand names or trademarks appearing in this prospectus are the property of their respective holders.
You should rely only on the information contained in this prospectus in deciding whether to purchase the securities. We have not authorized anyone to provide information different from that contained in this prospectus. The information contained in the prospectus is accurate only as of the date of this prospectus, regardless of the time of delivery of this prospectus or of any sale of securities. Our business, financial condition, results of operations, and prospects may have changed since that date.
The information contained in this prospectus is not complete and is subject to change. The selling shareholders are not permitted to sell securities until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities, nor is it a solicitation of an offer to buy these securities in any state where the offer or sale is not permitted.
PROSPECTUS SUMMARY
This summary highlights selected information about Asia Biotechnology Group Inc. (“Asia Biotech” or the “Company”) and the offering that is contained elsewhere in this prospectus. You should read the entire prospectus before making an investment decision, especially the information presented under the heading "Risk Factors" on page 8 and the financial statements and related notes included elsewhere in this prospectus, as well as any other documents to which we refer you. Except as otherwise indicated by context, references in this prospectus to "we," "us," "our" or the "Company" are to the combined business of Asia Biotech, its wholly owned direct subsidiaries, Asia Biotechnology Group Inc. a BVI company ("ABG"), Harbin OT Pharmaceutical Co., Limited, a Samoa company ("OT Samoa"), and its indirect subsidiary in China, OT Pharmaceutical, and in each case do not include the selling shareholders. References to "China" or to the "PRC" are references to the People's Republic of China. This prospectus contains forward-looking statements and information about us. See "Forward-Looking Statements" on page 31.
OUR COMPANY
OVERVIEW
ASIA BIOTECHNOLOGY GROUP INC. (ASIA BIOTECH)
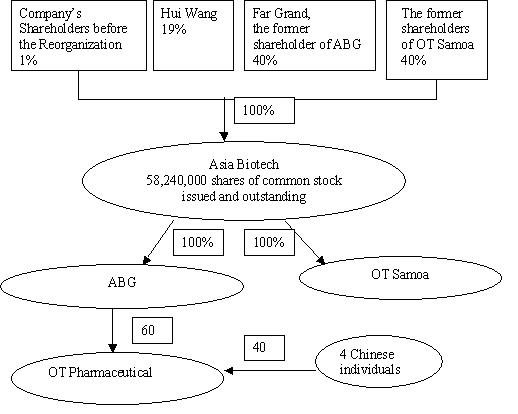
Asia Biotech was formerly named as Echelon Acquisition Corp., which was originally incorporated on July 27, 2004 under the laws of the State of Delaware and fell within the definition of a "blank check" corporation contained in Section (7) (b) (3) of the Securities Act of 1933, as amended. Since our inception we have intended to serve as a vehicle to effect an asset acquisition, merger, and the exchange of capital stock or other business combination with a domestic or foreign business. Prior to the acquisition with ABG and OT Samoa, we had no operations and only minimal liabilities.
On May 8, 2006, we entered into a triangular Agreement and Plan of Reorganization with the shareholders of ABG and OT Samoa. Pursuant to this agreement, we simultaneously acquired the one single issued and outstanding share of ABG from its sole shareholder in exchange for 23,296,000 shares of our common stock, and all of 20,000,000 issued and outstanding shares of OT Samoa from their shareholders in exchange for other 23,296,000 shares of our common stock. Immediately after the merger, the former shareholder of ABG and former shareholders of OT Samoa respectively own 40 percent of the shares of our common stock (the “Reorganization”). After the Reorganization, all of our business is conducted in China, and all of our management as well as our employees are located in China.
On July 19, 2006, we changed our name from Echelon Acquisition Corp. to Asia Biotechnology Group Inc. to reflect the change of our business scope.
There is currently no public market for our common stock. We intend to have our common stock quoted on the Over-The-Counter Bulletin Board.
Our principal executive office is located at No. 7 BohaiSanLu, Pingfang Industrial District, Economic & Technological Development Area, Harbin, Heilongjiang Province, China.
ASIA BIOTECHNOLOGY GROUP INC., BVI Company (ABG)
ABG is one of the Company’s wholly owned subsidiaries, which was incorporated on March 21, 2005 under the laws of the British Virgin Islands. Prior to the Reorganization, Far Grand Investment Limited, a company organized under the laws of Cayman Islands (“Far Grand”), was the sole shareholder of ABG. Mr. Wensheng Wang, a Chinese citizen, is the sole control person of Far Grand.
On November 3, 2005, ABG acquired 60% of the equity interests in OT Pharmaceutical under the relevant Chinese authorities’ approval.
HARBIN PHARMACEUTICAL CO., LTD., Chinese Company
(OT PHARMACEUTICAL)
On November 3, 2005, OT Pharmaceutical became ABG’s subsidiary. After the Reorganization, OT Pharmaceutical became Asia Biotech’s indirect subsidiary and the only operating entity of the Company.
ABG holds 60% of the equity interests in OT Pharmaceutical, the remaining 40% are held by four Chinese individuals.
OT Pharmaceutical's primary business is designing, manufacturing and marketing gynecological products and is mainly focused on the manufacturing and marketing of feminine suppositories.
HARBIN PHARMACEUTICAL CO., LTD., Samoa Company (OT SAMOA)
OT Samoa is the Company’s other wholly owned subsidiary which was incorporated on April 13, 2005 under the laws of Samoa. The shareholders of OT Samoa before the Reorganization were 1399 Chinese residents.
OT Samoa did not engage in any business other than sub-licensing a kind of gynecological treatment related proprietary technology and cooperating with ABG on the manufacturing of the technology related products.
The chart below illustrates our current corporate structure:
THE OFFERING
| Common stock outstanding prior to the offering | 58,240,000 shares |
| | |
| Common stock to be offered by the selling shareholders | 23,444,000 shares |
| | |
| Common stock to be outstanding after the offering | 58,240,000 shares |
| | |
| Use of Proceeds | We will receive no proceeds from the sale of shares of common stock in this offering. |
RISK FACTORS
This offering involves a high degree of risk. You should carefully consider the risks described below before making a decision to buy our common stock. If any of the following risks actually occurs, our business could be harmed. In that case, the trading price of our common stock could decline, and you may lose all or part of your investment. You should also refer to the other information in this prospectus, including our financial statements and the related notes. Except for historical information, the information in this prospectus contains "Forward-looking" statements about our expected future business and performance. Our actual operating results and financial performance may prove to be very different from what we have predicted as of the date of this prospectus. The risks described below address all material risks to us and our investors, as currently known to us. Notwithstanding the above, Section 27A of the Securities Act and Section 21E of the Securities Exchange Act expressly state that the safe harbor for forward looking statements does not apply to companies that issue securities that meet the definition of a penny stock, as such, the safe harbor for forward looking statements does not apply to us.
The following risk factors are mainly referring to the business of OT Pharmaceutical.
RISKS RELATED TO OUR BUSINESS AND INDUSTRY
WE HAVE A LIMITED OPERATING HISTORY WHICH MAKES EVALUATING OUR BUSINESS DIFFICULT
Our limited operating history may be insufficient for investors to evaluate our business and future prospects. Our indirect subsidiary, OT Pharmaceutical, was established and commenced business in 2001. Even though OT Pharmaceutical has commercialized nine medicines and one sanitary product since inception, it has historically lost money in the last two years and we cannot assure that it will not incur losses in the future. The other subsidiary, OT Samoa, cooperated with ABG on the manufacturing of certain products in the early fiscal year of 2006, but has not yet generated any revenues.
Our ability to generate revenue depends heavily on the successful commercialization and the market acceptance of our products. Nevertheless, we may not be able to validate and market products unless we successfully develop and commercialize our medicines and sanitary products and achieve success. Capital expenditures, marketing efforts and capital investment are significantly required in order to achieve such goals. If we do not successfully develop and commercialize our products, we will be unable to generate any revenue, or the revenue generated may not cover the expenditures. If we fail to generate sufficient revenue, we may be unable to continue our operations, our stock price would likely be lower, and investors may lose their entire investment.
WE EXPERIENCED HISTORICAL FINANCIAL LOSSES DURING THE PAST TWO YEARS
The net loss of OT Pharmaceutical was $545,093 by the end of June 30, 2006. We are unable to predict the extent of any future expenditure and the potential profitability. As all of our total revenues are from the sales of medicines and sanitary products, our ultimate success will depend on whether we are able to successfully market approved products. The historical financial losses and financial condition of the OT Pharmaceutical could make it difficult for us to obtain financing in the future or could reduce the value the market places on our common stock. Moreover, if we are unable to make a profit, the market value of our common stock will likely decline.
WE NEED TO RAISE ADDITIONAL CAPITAL TO FUND OUR OPERATIONS AND WE MAY BE UNABLE TO RAISE SUCH FUNDS
As an early-stage company, we need to raise additional capital for our future operations, especially for the development of new products or product lines, financing of general and administrative expenses, licensing or acquisition of additional technologies, and marketing of new or existing products. It has been estimated that OT Pharmaceutical may need to raise approximately US$100,000 in the research and development of its current 6 product candidates and approximately US$330,000 for the introduction of more production lines. Compared to the capital we need, our cash and cash equivalents at the end of June 30, 2006 was $37,923, which was not enough to meet OT Pharmaceutical’s operational needs and capital requirements. We need to raise additional capital through private placements and/or bank loans from a local bank by mortgaging or pledging OT Pharmaceutical’s assets.
There are no assurances that we will be able to raise the appropriate amount of capital needed for our future operations. In addition, we cannot assure you that financing will be available in amounts or on terms acceptable to us, if at all. Even though our assets were US$1,690,266 as of June 30, 2006, there are no assurances that any Chinese bank will accept our assets as collateral and loan us the necessary funding. Failure to obtain funding when needed may force us to delay, reduce, or eliminate our product development and manufacture programs. The incurrence of indebtedness would also result in increased debt service obligations and could result in operating and financing covenants that would restrict our operations or our ability to pay dividends to our shareholders.
WE RELY ON SUPPLIERS WHO ARE OUTSIDE OUR CONTROL AND ANY DISRUPTION WITH OUR SUPPLIERS COULD DELAY PRODUCT SHIPMENTS AND ADVERSELY AFFECT OUR BUSINESS OPERATIONS AND REVENUES
We rely upon certain suppliers who are outside our control. By the end of 2005, we had developed limited relationships with 21 suppliers for raw materials and 21 suppliers for drug packaging materials, including Shantou Sunda Plastic Factory, Nanning Shangren Plastic bags Co., Ltd., Harbin Gaomei Printing House Ltd., Gongxin Enterprise Ltd., and Jiangsu Qionghua Hi-tech Stock Co., Ltd. We cannot control the timing or resources that they devote to our products. Although we believe that alternative suppliers are available to supply materials, should any of these current suppliers fail to devote sufficient resources and time to our drug manufacture or increase their prices of materials supplied, it could delay product shipments and adversely affect our business operations and profitability. In addition, if the prices of raw materials needed for our products increase, and we cannot pass this price increase on to our end customers, our profit margins and operating results will suffer and we may be unable to produce certain products.
THE FAILURE TO MANAGE GROWTH EFFECTIVELY COULD HAVE AN ADVERSE EFFECT ON OUR BUSINESS, FINANCIAL CONDITION, AND RESULTS OF OUR OPERATIONS
Our management’s ability to successfully manage the business will be essential to achieving profitability. We may encounter difficulties associated with increasing production scale, including shortages of qualified personnel to operate our equipment, assemble our products or manage manufacturing operations, as well as shortages of key raw materials or components for our products. For effective growth management, we will be required to continue improving our operations, management, and financial systems and control. Our failure to manage growth effectively may lead to operational and financial inefficiencies that will have a negative effect on the Company's profitability.
WE ARE DEPENDENT ON CERTAIN KEY PERSONNEL AND LOSS OF THESE KEY PERSONNEL COULD HAVE A MATERIAL ADVERSE EFFECT ON OUR RESEARCH AND DEVELOPMENT, OPERATIONS AND REVENUES
Our success is, to a certain extent, attributable to the management, sales and marketing, and pharmaceutical factory operational expertise of key personnel. We are highly dependent on our senior management to manage our business and operations and upon our key research and development personnel for the development of new technologies and applications and the enhancement of our existing products. In particular, we are currently highly dependent upon the ability and experience of Mr. Xueliang Qiu, our CEO, President, Secretary and Chairman of the Board of Directors. Mr. Xueliang Qiu entered into an employment contract with OT Pharmaceutical for a term of three years from January 15, 2006 to January 14, 2008. Other senior management personnel of OT Pharmaceutical include senior management personnel include Ms. Meng Yan, Mr. Changfu Gong and Ms. Jieying Lu, who jointly perform key functions in the operation of OT Pharmaceutical. We also rely on our key research personnel such as Haitao Liu, Yuqiao Yang, Zhiming Chen. OT Pharmaceutical has entered into employment agreements with all of these individuals. Ms. Meng Yan will act as OT Pharmaceutical’s Assistant General Manager from January 1, 2006 to December 31, 2008. Mr. Changfu Gong will act as the Assistant General Manager for a term from January 1, 2006 to December 31, 2008. Ms. Jieying Lu will act as the chief engineer from January 1, 2006 to December 31, 2008. Haitao Liu is in charge of the production techniques, Yuqiao Yang is in charge of the toxin tests of candidate products, and Zhiming Chen is mainly in charge of the quality of raw materials.
We compete for qualified personnel with other pharmaceutical companies and research institutions. Intense competition for these personnel could cause our compensation costs to increase significantly, which could have a material adverse effect on our operations results. Our future success and ability to grow our business will depend in part on the continued service of these individuals and our ability to identify, hire and retain additional qualified personnel. If we are unable to attract and retain qualified employees, we may be unable to meet our business and financial goals. There can be no assurance that we will be able to retain these officers and key personnel after the term of their employment contracts expire. The loss of any one of these officers could have a material adverse effect upon our business, financial condition, and results of operations. We must attract, recruit and retain a sizeable workforce of technically competent employees. Our ability to effectively implement our business strategy will depend upon, among other factors, the successful recruitment and retention of additional highly skilled and experienced management and other key personnel. We cannot assure that we will be able to hire or retain such employees.
WE FACE COMPETITION IN THE PHARMACEUTICAL INDUSTRY AND SUCH COMPETITION COULD CAUSE OUR SALES REVENUE TO DECLINE
According to the State Food and Drug Administration of China, or the SFDA, there were approximately 5,071 pharmaceutical manufacturing companies in the PRC as of 30 June 2004, of which approximately 3,237 manufacturers obtained a GMP, or a Good Manufacturing Practice certificate. After GMP certification became a mandatory requirement on July 1, 2004, approximately 1,834 pharmaceutical manufacturers were forced to cease production (Source: Jilin Food and Drug Administration http://www.jlda.gov.cn/hyzx/showhyzx.x?id=789). Only the 3,237 pharmaceutical manufacturers with a GMP certificate were allowed to continue their manufacturing operations. The certificates, permits, and licenses required for pharmaceutical operation in the PRC create a potential barrier for new competitors seeking entrance into the market. Despite these obstacles, we face competitors who will attempt to create, or who are already marketing, products in the PRC that are similar to ours. The market of gynecological disease remedies is a segment with intense competition, where newly developed gynecological drugs for exterior use seem to emerge endlessly. Our subsidiary in China faces direct competition both domestically and internationally across all product lines and price points. The competitors are numerous and include major pharmaceutical companies such as Xian-Janssen Pharmaceutical Ltd., a foreign invested company, whose key gynecological product is Miconazole Nitrate Suppository, and Chengdu Enwei Group, a domestic company, whose major products include Skinpro Lotion.
Although OT Pharmaceutical focuses solely on R&D and manufacture of feminine suppositories, and one of its products, Shenqi Wenyang Suppository, has been labeled as a protected Chinese medicine by the PRC government, there is no assurance that OT Pharmaceutical’s products can successfully compete with other external gynecological products in China. Our competitors may succeed in developing technologies and products that are more advanced than the products we are developing. Many of our competitors have greater financial and other resources; larger varieties of products; more products that have received regulatory approvals; and greater pricing flexibility than we have. OT Pharmaceutical is also competing against their greater manufacturing efficiency and marketing capabilities, areas in which it has limited experience. For example, Skinpro Lotion produced by Chengdu Enwei Group took 17.65% market shares in Beijing, the capital of China, in 2001. Besides, compared to Chengdu Enwei’s more than 400 sales representatives and over 30 offices and agencies around China, OT Pharmaceutical only has 5 sales representatives and 21 sales distributors. Moreover, there can be no assurance that our products will be able to compete in price with those of our competitors. Consequently, we may be unable to offer products similar to, or more desirable than, those offered by our competitors, market our products as effectively as our competitors, or otherwise respond successfully to competitive pressures. As a result, as competition intensifies in the area of gynecological external remedies in China, we may lose customers and our sales may decline. There can be no assurance that our products will be either more effective in their therapeutic abilities and/or be able to compete in price with those of our competitors. Failure to do either of these may result in decreased revenues for our Company.
OUR DEVELOPMENT OF BUSINESS IS IN PART DEPENDENT ON CONTINUALLY DEVELOPING NEW AND ADVANCED PRODUCTS, TECHNOLOGIES, AND PROCESSES; FAILURE TO DO SO MAY CAUSE US TO LOSE OUR COMPETITIVENESS IN THE PHARMACEUTICAL INDUSTRY AND MAY CAUSE OUR REVENUES TO DECLINE
Through our subsidiaries, we have traditionally been committed to developing and manufacturing gynecological diseases treatments. To remain competitive in the pharmaceutical industry, it is important to continually develop new and advanced products, technologies, and processes. Currently, there are 6 product candidates in the development stage, and 3 pending patents under application. There is no assurance that the competitors' new products, technologies, and processes will not render our existing products obsolete or non-competitive. Our competitiveness in the pharmaceutical market therefore relies upon our ability to enhance our current products, introduce new products, and develop and implement new technologies and processes. Although OT Pharmaceutical owns 3 patents, and 3 pending patents in connection with gynecology disease treatments, our failure to technologically evolve and/or develop new or enhanced products may cause us to lose our competitiveness in the pharmaceutical industry and may cause our profits to decline.
THE SALE OF OUR PRODUCTS DEPENDS UPON THE DEGREE OF MARKET ACCEPTANCE AMONG THE MEDICAL COMMUNITY AND CUSTOMERS, AND FAILURE TO ATTAIN MARKET ACCEPTANCE AMONG THE MEDICAL COMMUNITY MAY HAVE AN ADVERSE IMPACT ON OUR OPERATIONS
The sale of our products depends upon the degree of market acceptance among the medical community and end customers. Out of our 9 medicines, these 3 medicines need a prescription from a physician, including Kushenjian Suppository, Shenqi Wenyang Suppository and Fu Ning Suppository. Even if our products are approved by the SFDA, there is no assurance that physicians will recommend our products to patients, or that patients will accept and use them. Furthermore, a product's prevalence and use at hospitals may be contingent upon our relationship with the medical community. The acceptance of our products among the medical community may depend upon several factors, including but not limited to, the product’s acceptance by physicians and patients as a safe and effective treatment, cost effectiveness, potential advantages over alternative treatments, and the prevalence and severity of side effects. Failure to attain market acceptance among the medical community may have an adverse impact on our operations.
WE ENJOY CERTAIN PREFERENTIAL TAX CONCESSIONS AND THE LOSS OF THESE PREFERENTIAL TAX CONCESSIONS WILL CAUSE OUR TAX LIABILITIES TO INCREASE
The income tax rate for domestic companies in the PRC is universally 33%. However, to attract more local enterprises to engage in high technology research and development in China, the PRC government has provided various incentives, such as reducing tax rates, to companies. Since OT Pharmaceutical owns certain technologies, patents and pending patents on the R&D and manufacturing of gynecological remedies, it was recognized as a high technology company by the local government in 2001, and accordingly enjoys preferential tax treatment, in the form of reduced tax rates and tax holidays. OT Pharmaceutical is now entitled to a 15% preferential corporate income tax rate compared to a rate of 33% applicable to most domestic companies, and it enjoyed the preferential policy of “two years of exemption” of corporate income tax from its inception. Moreover, OT Pharmaceutical is entitled to enjoy a 7-year cash refund equals to the building tax and land-use tax it has paid commencing from 2001, at the time the company was incorporated, to 2008.
These tax incentives have been granted by the municipal government of Heilongjiang Province and are subject to annual review by the municipal government. Continuing eligibility for the financial incentives OT Pharmaceutical receives requires that it continues to meet a number of criteria, such as its hi-tech company status, which is subject to the discretion of the municipal government. Moreover, either the central government or the municipal government of Heilongjiang could determine at any time to immediately eliminate or reduce these financial incentives, generally with prospective effect. As a result, in addition to any business or operations related factors we may otherwise experience, OT Pharmaceutical's profits, if they are generated, may decline because OT Pharmaceutical’s tax liabilities may increase if the preferential tax treatments are no longer applicable or available in the future.
WE ARE SUBJECT TO THE PRC'S PRICE CONTROL OF DRUGS WHICH MAY LIMIT OUR PROFITABILITY AND EVEN CAUSE US TO STOP MANUFACTURING CERTAIN PRODUCTS
The State Development and Reform Commission ("SDRC") of the PRC, and the price administration bureaus of the relevant provinces of the PRC in which the pharmaceutical products are manufactured, are responsible for the retail price control over our pharmaceutical products. The SDRC sets the price ceilings for certain pharmaceutical products in the PRC. The maximum prices of such pharmaceutical products are published by the state and provincial DRC from time to time. The upper limit of the prices of medicines subject to price control are set by the pricing authorities to create a reasonable profit margin for pharmaceutical enterprises, after taking into account the type and quality of the products, the production costs, the prices of substitute products, and other related factors. The prices of other medicines that are not subject to price control are determined by the company. Currently, four specifications of our two products, Econazole Nitrate Suppositories 1*3*0.15g, and 1*6*0.15g, Clotrimazole Suppositories 1*3*0.15g and 1*6*0.15g are subject to price control as of the date of this Prospectus. The price ceilings are above our production costs and we have not stopped manufacturing any products in the past due to price control. However, to the extent that we are subject to price control, our revenue, gross profit, gross margin and net income will be affected since the revenue we derive from our sales will be limited and we may face no limitation on our costs. Although our other products have not been subject to such price control as of the date of this Prospectus, there is no assurance that those products will remain unaffected by it. Further, if price controls affect both our revenue and our costs, our ability to be profitable and the extent of our profitability will be effectively subject to the determination of the applicable regulatory authorities in the PRC.
OUR CERTIFICATES, PERMITS, AND LICENSES ARE SUBJECT TO GOVERNMENTAL CONTROL AND RENEWAL; FAILURE TO OBTAIN RENEWAL WILL CAUSE ALL OR PART OF OUR OPERATIONS TO BE TERMINATED
OT Pharmaceutical is subject to various PRC laws and regulations pertaining to the pharmaceutical industry. OT Pharmaceutical has attained certificates, permits, and licenses required for the operation of a pharmaceutical enterprise and the manufacturing of pharmaceutical products in the PRC.
We obtained the Medicine Production Permit in January 2001, and it will be valid until December 31, 2010. If it does not renew the permit after 2010, OT Pharmaceutical will not be able to operate medicine production, which will cause its operations to be terminated. OT Pharmaceutical has obtained a GMP certificate which is effective through February 13, 2008. The pharmaceutical production permit and GMP certificate are valid for a term of five years and must be renewed before their expiration. During the renewal process, OT Pharmaceutical will be re-evaluated by the appropriate governmental authorities and must comply with the then prevailing standards and regulations which may change from time to time. In the event that OT Pharmaceutical is not able to renew the certificates, permits and licenses, all or part of our operations may be terminated. Furthermore, if escalating compliance costs associated with governmental standards and regulations restrict or prohibit any part of our operations, it may adversely affect our operation and profitability.
IF OUR PRODUCTS FAIL TO RECEIVE REGULATORY APPROVAL, WE MAY BE UNABLE TO RECOUP RESEARCH AND DEVELOPMENT EXPENDITURES
In China, a company wishing to enter the pharmaceutical industry must comply with the standards and regulations set forth by the government. In the PRC, the State Food and Drug Administration, or the SFDA is the authority that monitors and supervises the administration of the pharmaceutical industry including pharmaceutical products and medical appliances. Pharmaceutical manufacturing enterprises must obtain a Pharmaceutical Manufacturing Enterprise Permit issued by the relevant pharmaceutical administrative authorities and relevant health departments at the provincial level where the enterprise is located. Furthermore, all pharmaceutical products produced in the PRC, with the exception of Chinese herbal medicines in soluble form, must bear a registered number approved by the appropriate medicine administration authorities in the PRC. Lastly, in accordance with the World Health Organization, the PRC now requires compliance with GMP standards in pharmaceutical production in order to minimize the risks involved in any pharmaceutical production that cannot be eliminated through testing final products. As the regulatory approval process becomes more stringent, it also increases the industry's capital entry barrier.
The regulatory approval procedure for pharmaceuticals can be quite lengthy, costly, and uncertain. Depending upon the discretion of the SFDA, the approval process may be significantly delayed by additional clinical testing and require the expenditure of resources not currently available; in such an event, it may be necessary for us to abandon our application. Even where approval of the product is granted, it may contain significant limitations in the form of narrow indications, warnings, precautions, or contra-indications with respect to conditions of use.
Currently, OT Pharmaceutical has obtained SFDA approvals for 9 kinds of existing medicine products and has obtained the local health supervision authority’s approval for one sanitary product. The 6 candidate products under the R&D are sanitary products and are therefore not subject to the strict regulatory procedure on medicines. However, if OT Pharmaceutical intends to produce new medicines, it must comply with the relevant regulations, and if approval of our product is denied, abandoned, or severely limited in terms of the scope of product use, it may result in the inability to recoup research and development expenditures.
ALL ACTIVE INGREDIENTS OF OUR PRODUCTS ARE GENERIC DRUGS. OTHER COMPANIES MAY MAKE PRODUCTS SIMILAR TO OURS
Currently, OT Pharmaceutical does not need any licenses to produce the existing products. All active ingredients of OT Pharmaceutical’s products are generic drugs compliant with the Chinese Pharmacopoeia and Chinese Drug Standards. All patented ingredients should be subject to patent protection in China, but all active ingredients in OT Pharmaceutical’s products are not patented. However, we are exposed to risks that such generic drugs may be used by other companies for producing products similar to ours. They may capture market share by selling the similar products at low prices, which can reduce the market share held by our company. Although we believe that the complexity of our technologies may decrease the risks, there are no assurances that other companies may not make similar products.
OUR RESEARCH AND DEVELOPMENT MAY NEITHER BE SUCCESSFUL NOR COMPLETED WITHIN THE ANTICIPATED TIMEFRAME, IF EVER
There are six candidate products that are currently under research and development. The research and development of our new and existing products and their subsequent commercialization to some extent plays an important role in our success. The research and development of new products is costly and time consuming, and there are no assurances that our research and development of new products will either be successful or completed within the anticipated timeframe, if ever at all. There are also no assurances that if the product is developed, it will lead to successful commercialization. Currently, all the Company’s research and development is conducted by our key research personnel within OT Pharmaceutical. The Company needs to raise approximately US$ 100,000 for the development of our current 6 product candidates and approximately US$ 330,000 for the introduction of more production lines. Such funding will be derived from the income of OT Pharmaceutical. If the Company can not generate enough revenues in 2006, the funding contributed will have to be decreased. As a result, our research and development will be terminated.
The 6 product candidates are defined as disposable sanitary products under the related PRC regulations and, therefore, are not subject to the strict pharmaceutical related regulations. On May 27, 2004, The Company was granted permission to produce disposable sanitary products by the Ministry of Health of the PRC. The term of the permission is from May 27, 2004 to May 26, 2008.
WE CANNOT GUARANTEE THE PROTECTION OF OUR INTELLECTUAL PROPERTY RIGHTS AND IF INFRINGEMENT OR COUNTERFEITING OF OUR INTELLECTUAL PROPERTY RIGHTS OCCURS, OUR REPUTATION AND BUSINESS MAY BE ADVERSELY AFFECTED
Our success depends on our ability to protect our patents and trademarks. According to the PRC Patent Law, the protection period of patent right for inventions is twenty years, and the protection period of patent rights for utility models is ten years. No time extension is allowed. According to the PRC Trademark Law, a registered trademark is valid for a period of 10 years after approved, and it can be extended for another 10 years upon the application. Currently, OT Pharmaceutical owns 1 utility patent which was granted in 2004, 2 invention patents which were granted in 2006, and has 3 pending invention patents in China. To protect the reputation of our products, OT Pharmaceutical has registered and applied for registration of 21 trademarks or logos in the PRC. All of our products are sold under these trademarks. Please refer to the paragraph headed "Intellectual property rights" in the Business section of this Prospectus on page 35.
Although we believe our existing patents and pending patents (subject to approval of our applications) will provide a competitive advantage, the patent positions of pharmaceutical companies are highly uncertain and involve complex legal and factual questions. We may not be able to develop patentable products or processes, and may not be able to obtain patents from pending applications. Even if patent claims are allowed, the claims may not issue, or in the event of issuance, may not be sufficient to protect our technology. In addition, any patents or patent rights we obtain may be circumvented, challenged or invalidated by our competitors.
Regarding our trademarks, as of the date of this Prospectus, we have not experienced any infringements of such trademarks for sales of pharmaceutical products. However, there is no assurance that there will not be any infringement of our brand name or other registered trademarks or counterfeiting of our products in the future. Should any such infringement or counterfeiting occur, our reputation and business may be adversely affected. We may also incur significant expenses, and expend substantial amounts of time and effort to enforce our intellectual property rights in the future. Such a diversion of our resources may adversely affect our existing business and future expansion plans.
We may need to resort to litigation to enforce or defend our patents or trademarks in China. The experience and capabilities of PRC courts in handling intellectual property litigation varies and outcomes are unpredictable. Further, such litigation may require significant expenditures of cash and management efforts and could harm our business, financial condition and results of operations. An adverse determination in any such litigation will impair our intellectual property rights and may harm our business and reputation.
Due to the different legal systems and varying requirements in different countries, we have not applied for any patents outside China. The process of seeking patent protection can be lengthy and expensive, and we cannot assure you that our patent applications will be successful, or that our existing patent rights will provide us with sufficient protection or commercial advantage. We cannot assure you that our current or potential competitors do not have, and will not develop, products that will compete directly with our products despite our intellectual property rights.
WE MAY SUFFER AS A RESULT OF PRODUCT LIABILITY OR DEFECTIVE PRODUCTS
We may produce medicines which inadvertently have an adverse pharmaceutical effect on the health of individuals, despite proper testing. As existing PRC laws and regulations do not require us to maintain third party liability insurance to cover product liability claims and as the insurance industry in China is still in an early stage of development, business insurance is not readily available in the PRC. OT Pharmaceutical currently does not maintain any liability insurance. If a product liability claim is brought against us, it may, regardless of merit or eventual outcome, result in damage to our reputation, breach of contract with our customers, decreased demand for our products, costly litigation, or product recalls. In addition, since OT Pharmaceutical has to use its revenues to both defend any action and pay any claims that result from a settlement or judgment, it would incur significant expenses. If the nature or amount of any uninsured loss is significant, OT Pharmaceutical may be unable to continue in business. We currently are not aware of any existing or anticipated product liability claims with respect to our products.
BECAUSE A SIGNIFICANT ACCOUNT RECEIVABLE HAS BEEN GENERATED, THE FAILURE OF ITS COLLECTION COULD IMPAIR OUR FINANCIAL CONDITION
Our accounts receivable represents a large percentage of our assets. Our account receivable as of June 30, 2006 was US$424,092, representing approximately 25% of our total assets. This concentration of accounts receivable represents a significant credit risk. The failure of any of the distributors/customers to pay their obligations to us in a timely manner could have a material adverse effect upon our financial condition.
SUCCESSFUL COOPERATION BETWEEN OT SAMOA AND ABG IS UNCERTAIN. THERE CAN BE NO ASSURANCE THAT PRODUCTS WILL BE COMMERCIALIZED
As of January 22, 2006, OT Samoa executed a cooperation agreement with ABG on the manufacturing of certain pharmaceutical product. Products that appear promising in the early phases of development may fail to be commercialized. Moreover, our products may prove to be not accepted in the Chinese market. As a result, there can be no assurance that our products will be successfully commercialized.
RISKS RELATED TO DOING BUSINESS IN CHINA
Our revenues are derived from the operation in China. Accordingly, our business, financial condition, results of operation and prospects are affected significantly by economic, political and legal developments in China.
CHANGES IN CHINA’S ECONOMIC, POLITICAL AND SOCIAL CONDITION COULD ADVERSELY AFFECT OUR FINANCIAL CONDITION AND RESULTS OF OPERATION
We derive all of our revenues from our operations in China. Accordingly, our business, financial condition, results of operations and prospects are affected to a significant degree by economic, political and social conditions in China. The PRC economy differs from the economies of most developed countries in many respects, including the amount of government involvement, level of development, growth rate, control of foreign exchange and allocation of resources. The PRC government has implemented various measures to encourage economic growth and guide the allocation of resources. Some of these measures benefit the overall PRC economy, but may also have a negative effect on us. For example, our financial condition and results of operations may be adversely affected by changes in tax regulations applicable to us. The PRC government has implemented certain measures, including a recent interest rate increase, to control the pace of economic growth. These measures may cause decreased economic activity in China, including a slowing or decline in individual hospital spending, which in turn could adversely affect our financial condition and results of operations.
THE PRC LEGAL SYSTEM EMBODIES UNCERTAINTIES THAT COULD LIMIT THE LEGAL PROTECTIONS AVAILABLE TO YOU AND US
The PRC legal system is a civil law system based on written statutes. Unlike common law systems, it is a system in which decided legal cases have limited precedential value. In 1979, the PRC government began to promulgate a comprehensive system of laws and regulations governing economic matters in general. The overall effect of legislation over the past three decades has significantly increased the protections afforded to various forms of foreign investment in China. However, these laws, regulations, and legal requirements are relatively recent and are evolving rapidly, and their interpretation and enforcement involve uncertainties. These uncertainties could limit the legal protections available to foreign investors.
The practical effect of the PRC's legal system on our business operations in China can be viewed from two separate but intertwined considerations:
First, as a matter of the substantive law, the PRC laws regarding the Sino-foreign Equity Joint Ventures provide significant protection from government interference. In addition, these laws guarantee the full benefit of corporate articles and contracts to Sino-foreign Equity Joint Venture participants. These laws, however, do impose standards concerning corporate formation and governance, which are not qualitatively different from the corporation laws found in the United States. Similarly, PRC accounting laws mandate accounting practices which may not be consistent with the US Generally Accepted Accounting Principles. China accounting laws require that an annual "statutory audit" be performed in accordance with PRC accounting standards and that the books of account of Sino-foreign Equity Joint Venture be maintained in accordance with Chinese accounting laws. Article 78 of the Regulations for the Implementation of the Law of the People’s Republic of China on Chinese-foreign Equity Joint Ventures requires a Sino-foreign Equity Joint Venture to submit certain periodic fiscal reports and statements to designated financial and tax authorities.
Second, while the enforcement of substantive rights may appear less clear than United States procedures, Sino-foreign Equity Joint Ventures are Chinese registered companies that enjoy the same status as other Chinese registered companies in business-to-business dispute resolutions. The Chinese legal infrastructure is significantly different in operation from its United States counterpart, and may present a significant impediment to the operation of Sino-foreign Equity Joint Ventures.
Our PRC operating subsidiary, OT Pharmaceutical, is an equities joint venture enterprise and is subject to laws and regulations applicable to foreign investment in China, as well as laws and regulations applicable to foreign-invested enterprises. These laws and regulations change frequently, and their interpretation and enforcement involve uncertainties. For example, we may have to resort to administrative and court proceedings to enforce the legal protections that we enjoy either by law or contract. However, since PRC administrative and court authorities have significant discretion in interpreting and implementing statutory and contractual terms, it may be more difficult to evaluate the outcome of administrative and court proceedings and the level of legal protection we enjoy, than in more developed legal systems. These uncertainties may also impede our ability to enforce the contracts we have entered into. As a result, these uncertainties could materially and adversely affect our business and operations.
AS OUR MAIN OPERATION AND MANAGEMENT ARE OUTSIDE THE UNITED STATES, IT WILL BE DIFFICULT TO ACQUIRE JURISDICTION AND ENFORCE LIABILITIES AGAINST US AND OUR OFFICERS, DIRECTORS AND ASSETS BASED IN CHINA.
Our operating entity, OT Pharmaceutical is governed by PRC laws; all directors and officers reside outside of the United States, and our assets are and will continue to be located outside the United States. As a result, it may be difficult or impossible to effect service of process within the United States upon our directors or officers and our subsidiaries, or enforce against any of them court judgments obtained in United States’ courts, including judgments relating to United States federal securities laws. In addition, there is uncertainty as to whether the courts of the PRC and of other offshore jurisdictions would recognize or enforce judgments of United States’ courts obtained against us predicated upon the civil liability provisions of the securities laws of the United States or any state thereof, or be competent to hear original actions brought in the PRC or other offshore jurisdictions predicated upon the securities laws of the United States or any state thereof. Furthermore, because all of our assets are located in China, it would also be extremely difficult to access those assets to satisfy an award entered against us in a United States court.
As a result of all of the above, public shareholders may have more difficulty in protecting their interests in the face of actions taken by management, members of the board of directors or controlling shareholders than they would as public shareholders of a U.S. company.
RECENT PRC REGULATIONS RELATING TO ACQUISITIONS OF PRC COMPANIES BY FOREIGN ENTITIES MAY LIMIT OUR ABILITY TO ACQUIRE PRC COMPANIES AND ADVERSELY AFFECT THE IMPLEMENTATION OF OUR STRATEGY AS WELL AS OUR BUSINESS AND PROSPECTS
The PRC State Administration of Foreign Exchange, or SAFE, issued a public notice in January 2005 concerning foreign exchange regulations on mergers and acquisitions in China ("January Notice"). The public notice states that if an offshore company controlled by PRC residents intends to acquire a PRC company, such acquisition will be subject to strict examination by the relevant foreign exchange authorities. The public notice also states that the approval of the relevant foreign exchange authorities is required for any sale or transfer of a PRC company's assets or equity interests by the PRC residents to foreign entities, such as us, for the equity interests or assets of the foreign entities.
In April 2005, SAFE issued another public notice (the “April Notice”) further explaining the January notice. In accordance with the April notice, if an acquisition of a PRC company by an offshore company controlled by PRC residents has been confirmed by a Foreign Investment Enterprise Certificate prior to the promulgation of the January notice, the PRC residents must each submit a registration form to the local SAFE branch with respect to their respective ownership interests in the offshore company, and must also file an amendment to such registration if the offshore company experiences material events, such as changes in the share capital, share transfer, mergers and acquisitions, spin-off transactions or use of assets in China to guarantee offshore obligations. The April notice also provides that failure to comply with the registration procedures set forth therein may result in a restriction on the PRC company's ability to distribute profits to its offshore parent company. Pending the promulgation of detailed implementation rules, the relevant government authorities are reluctant to commence processing any registration or application for approval required under the SAFE notices.
In October 2005, SAFE promulgated another notice (“Decree No. 75) which vacates the above January Notice and April Notice. Decree No. 75 requires PRC residents and PRC corporate entities to register with and obtain approvals from relevant PRC government authorities in connection with their direct or indirect offshore investment activities. These regulations apply to our shareholders who are PRC residents in connection with any future offshore acquisitions.
The Decree No. 75 requires registration by March 31, 2006 of direct or indirect investments previously made by PRC residents in offshore companies prior to the implementation of the Decree No. 75 on November 1, 2005. If a PRC shareholder with a direct or indirect stake in an offshore parent company fails to make the required SAFE registration, the PRC subsidiaries of such offshore parent company may be prohibited from making distributions of profit to the offshore parent and from paying the offshore parent proceeds from any reduction in capital, share transfer or liquidation in respect of the PRC subsidiaries. Furthermore, failure to comply with the various SAFE registration requirements described above could result in liability under PRC law for foreign exchange evasion.
As it is uncertain how Decree No. 75 will be interpreted or implemented, we cannot predict how it will affect our business operations or future strategy. For example, we may be subject to a more stringent review and approval process with respect to our foreign exchange activities, such as the remittance of dividends and foreign-currency-denominated borrowings, which may adversely affect our results of operation and financial condition. In addition, if we decide to acquire a PRC company, we cannot assure that the owners of such company, as the case may be, will be able to complete the necessary approval, filings and registrations for the acquisition. This may restrict our ability to implement our acquisition strategy and adversely affect our business and prospects.
On August 8, 2006, six PRC regulatory agencies, including the Chinese Securities Regulatory Commission, or CSRC, promulgated a regulation (the “M&A Regulation”) that became effective on September 8, 2006. This regulation, among other things, has some provisions that purport to require that an offshore special purpose vehicle, or SPV, formed for listing purposes and controlled directly or indirectly by PRC companies or individuals, shall obtain the approval of the CSRC prior to the listing and trading of such SPV’s securities on an overseas stock exchange.
Because the M&A Regulation became effective very recently, it remains uncertain how the M&A Regulation will be interpreted and enforced by Chinese governmental authorities such as the CSRC and the Ministry of Commerce. We cannot predict how the M&A Regulation will affect our business operations or future strategy. If the CSRC requires that we obtain its approval, we may be unable to obtain a waiver of the CSRC approval requirements, if and when procedures are established to obtain such a waiver. Any uncertainties and/or negative publicity regarding this CSRC approval requirement could have a material adverse effect on the trading price of our common stock.
RESTRICTIONS ON CURRENCY EXCHANGE MAY LIMIT OUR ABILITY TO UTILIZE OUR REVENUES EFFECTIVELY
All of our revenues and operating expenses are denominated in Renminbi. The Renminbi is currently convertible under the “current account,” which includes dividends, trade and service-related foreign exchange transactions, but not under the “capital account,” which includes foreign direct investment and loans. Currently, OT Pharmaceutical may purchase foreign exchange for settlement of “current account transactions,” including payment of dividends to us, without the approval of SAFE. However, the relevant PRC governmental authorities may limit or eliminate our ability to purchase foreign currencies in the future. Since all of our future revenues will be denominated in Renminbi, any existing and future restrictions on currency exchange may limit our ability to utilize revenues generated in Renminbi to fund our business activities outside of China that are denominated in foreign currencies. Foreign exchange transactions under the capital account are still subject to limitations and require approvals from, or registration with, SAFE and other relevant PRC governmental authorities. This could affect the ability of OT Pharmaceutical to obtain foreign funding.
FLUCTUATIONS IN EXCHANGE RATE COULD RESULT IN FOREIGN CURRENCY EXCHANGE LOSSES
As all of our cash and cash equivalents are denominated in Renminbi. Fluctuations in exchange rates between US dollars and Renminbi will affect our balance sheet and earnings per share in US dollars. In addition, appreciation or depreciation in the value of the Renminbi relative to the US dollar would affect our financial results reported in US dollar terms without giving effect to any underlying change in our business, financial condition or results of operations. Since July 2005, the Renminbi is no longer pegged solely to the US dollar. Instead, the Renminbi is reported to be pegged against a basket of currencies, determined by the People’s Bank of China, against which it can rise or fall by as much as 0.3% each day. The Renminbi may appreciate or depreciate significantly in value against the US dollar in the long term, depending on the fluctuation of the basket of currencies against which it is currently valued, or it may be permitted to enter into a full float, which may also result in a significant appreciation or depreciation of the Renminbi against the US dollar. Fluctuations in the exchange rate will also affect the relative value of any dividend we issue after this offering which will be exchanged into US dollars, and earnings from and the value of any US dollar-denominated investments we make in the future.
Very limited hedging transactions are available in China to reduce our exposure to exchange rate fluctuations. To date, we have not entered into any hedging transactions in an effort to reduce our exposure to foreign currency exchange risk. While we may decide to enter into hedging transactions in the future, the availability and effectiveness of these hedges may be limited and we may not be able to successfully hedge our exposure at all. In addition, our currency exchange losses may be magnified by PRC exchange control regulations that restrict our ability to convert Renminbi into foreign currency.
CHINA HAS DIFFERENT CORPORATE DISCLOSURE, GOVERNANCE AND REGULATORY REQUIREMENTS THAN THOSE IN THE UNITED STATES WHICH MAY MAKE IT MORE DIFFICULT OR COMPLEX TO CONSUMMATE A BUSINESS COMBINATION
Companies in China are subject to accounting, auditing, regulatory and financial standards and requirements that differ, in some cases significantly, from those applicable to public companies in the United States, which may make it more difficult or complex to consummate a business combination. In particular, the assets and profits appearing on the financial statements of a Chinese company may not reflect its financial position or results of operations in the way they would be reflected had such financial statements been prepared in accordance with U.S. Generally Accepted Accounting Principles (GAAP). There is substantially less publicly available information about Chinese companies than there is about United States companies. Moreover, companies in China are not subject to the same degree of regulation as are United States companies with respect to such matters as insider trading rules, tender offer regulation, shareholder proxy requirements and the timely disclosure of information.
To meet the requirements of the United States Federal securities laws, which is necessary in order to seek stockholder approval of a business combination, a proposed target business will be required to have certain financial statements which are prepared in accordance with, or which can be converted to GAAP and audited in accordance with U.S. Generally Accepted Auditing Standards (GAAS). GAAP and GAAS compliance may limit the potential number of acquisition targets.
PRC ECONOMIC REFORM POLICIES OR NATIONALIZATION COULD RESULT IN A TOTAL INVESTMENT LOSS IN OUR COMMON STOCK
Since 1979, the Chinese government has reformed its economic policies. Because many reforms are unprecedented or experimental, they are expected to be refined and improved. Other political, economic and social factors, such as political changes, changes in the rates of economic growth, unemployment or inflation, or in the disparities in per capita wealth between regions within China, could lead to further readjustment of the reform measures. This refining and readjustment process may negatively affect our operations.
Although the Chinese government owns the majority of productive assets in China, in the past several years the government has implemented economic reform measures that emphasize decentralization and encourage private economic activity. Because these economic reform measures may be inconsistent or ineffectual, there are no assurances that:
| | · | We will be able to capitalize on economic reforms; |
| | · | The Chinese government will continue its pursuit of economic reform policies; |
| | · | The economic policies, even if pursued, will be successful; |
| | · | Economic policies will not be significantly altered from time to time; and |
| | · | Business operations in China will not become subject to the risk of nationalization. |
Over the last few years, China's economy has registered high growth rates. Recently, there have been indications that rates of inflation have increased. In response, the Chinese government recently has taken measures to curb this excessively expansive economy. These measures have included restrictions on the availability of domestic credit, reducing the purchasing capability of some of its customers. These austere measures alone may not succeed in slowing down the economy's excessive expansion or control inflation, and may result in severe dislocations in the Chinese economy. The Chinese government may adopt additional measures to further combat inflation, including the establishment of freezes or restraints on certain projects or markets. These measures may adversely affect our operations.
There can be no assurance that the reforms to China's economic system will continue or that we will not be adversely affected by changes in China's political, economic, and social conditions and by changes in policies of the Chinese government, such as changes in laws and regulations, measures which may be introduced to control inflation, changes in the rate or method of taxation, imposition of additional restrictions on currency conversion and remittance abroad, and reduction in tariff protection and other import restrictions.
ANY OCCURRENCE OF SERIOUS INFECTIOUS DISEASES, SUCH AS RECURRENCE OF SEVERE ACUTE RESPIRATORY SYNDROME (SARS) CAUSING WIDESPREAD PUBLIC HEALTH PROBLEMS, COULD ADVERSELY AFFECT OUR BUSINESS AND RESULTS OF OPERATIONS
A renewed outbreak of SARS or other widespread public health problems in China, where all of our revenue is derived, and in Heilongjiang, where our operations are headquartered, could have a negative effect on our operations. Our operations may be impacted by a number of public health-related factors, including the following:
| | · | quarantines or closures of our factories or subsidiaries which would severely disrupt its operations; |
| | · | the sickness or death of the key officers and employees; and |
| | · | general slowdown in the Chinese economy. |
Any of the foregoing events or other unforeseen consequences of public health problems could adversely affect our business and results of operations.
WE ARE SUBJECT TO THE ENVIRONMENTAL PROTECTION LAWS OF THE PRC
Our manufacturing process produces by-products such as effluent and noise, which are harmful to the environment. We are subject to multiple laws governing environmental protection as well as standards set by the relevant governmental bodies determining the classification of different wastes and proper disposal. OT Pharmaceutical properly passed the environmental examination on January 10, 2006, but there is no assurance that we will pass the environmental examinations in future.
China is experiencing substantial problems with environmental pollution. Accordingly, it is likely that the national, provincial and local governmental agencies will adopt stricter pollution controls. There can be no assurance that future changes in environmental laws and regulations will not impose costly compliance requirements on us or otherwise subject us to future liabilities. Our business's profitability may be adversely affected if additional or modified environmental control regulations are imposed upon us.
OUR BUSINESS MAY BE ADVERSELY AFFECTED AS A RESULT OF CHINA'S ENTRY INTO THE WORLD TRADE ORGANIZATION ("WTO") BECAUSE THE PREFERENTIAL TAX TREATMENTS AVAILABLE TO US MAY BE DISCONTINUED AND FOREIGN PHARMACEUTICAL MANUFACTURERS MAY COMPETE WITH US IN THE PRC PHARMACEUTIAL INDUSTRY
The PRC became a member of the WTO on December 11th, 2001. The current tax benefits enjoyed by OT Pharmaceutical may be regarded as unfair treatment by other members of the WTO. Accordingly, the preferential tax treatments available to our indirect subsidiary may be discontinued. In such circumstances, our profitability may be adversely affected. In addition, we may face additional competition from foreign pharmaceutical manufacturers if they set up their production facilities in the PRC or form Sino-foreign joint ventures with our competitors in the PRC. In the event that we fail to maintain our competitiveness against these competitors, our profitability may be adversely affected.
RISKS RELATED TO OUR COMMON STOCK
THERE IS NO MARKET FOR OUR COMMON STOCK
We intend to apply for a listing of our common stock on the Over-the-Counter Bulletin Board, but have not yet done so. To be listed on the OTCBB, we will have to comply with the requirement of material information regarding the issuer's finances, business and other information. There can be no assurance that the application for our common stock will be approved, or that if it is approved and listed, there can be no assurance that an active trading market will be maintained. We cannot assure you that our common stock will be included for trading on any stock exchange or through any other quotation system (including, without limitation, the NASDAQ Stock Market).
THE INITIAL OFFERING PRICE HAS BEEN ARBITRARILY SET BY OUR MANAGEMENT AND ACCORDINGLY DOES NOT INDICATE THE ACTUAL VALUE OF OUR BUSINESS
Until such time as our stock is quoted on the OTC Bulletin Board, the selling stockholders will offer the shares of common stock to which this registration statement relates at a price of $1.00 per share. The initial offering price is not based upon earnings or operating history, does not reflect our actual value, and bears no relation to our earnings, book values, net worth or any other recognized criteria of value. Accordingly, the offering price should not be regarded as an indication of any future price of our common stock.
THE NUMBER OF SHARES BEING REGISTERED FOR SALE IS SIGNIFICANT IN RELATION TO OUR TRADING VOLUME
All of the shares registered for sale on behalf of the selling stockholders are “restricted shares”, as defined in Rule 144 under the Securities Act. We have filed this registration statement to register 40% of all issued and outstanding shares of common stock of the company. Such an amount of shares, if sold in the market all at once or at about the same time, could depress the market price during the period the registration statement remains effective and also could affect our ability to raise equity capital. Except for those held by non-affiliates for a period of two years, calculated pursuant to Rule 144, any outstanding shares not sold by the selling stockholders pursuant to this prospectus will remain “restricted shares” in the possession of the stockholders.
THE MARKET PRICE FOR OUR COMMON STOCK MAY BE VOLATILE WHICH COULD RESULT IN A COMPLETE LOSS OF YOUR INVESTMENT
The market price for our common stock is likely to be highly volatile and subject to wide fluctuations in response to factors including the following:
| · | actual or anticipated fluctuations in our quarterly operating results, |
| · | announcements of new products by us or our competitors, |
| · | changes in financial estimates by securities analysts, |
| · | conditions in the pharmaceutical market, |
| · | changes in the economic performance or market valuations of other companies involved in pharmaceutical production, |
| · | announcements by our competitors of significant acquisitions, strategic partnerships, joint ventures or capital commitments, |
| · | additions or departures of key personnel, or |
In addition, the securities markets have, from time to time, experienced significant price and volume fluctuations that are not related to the operating performance of particular companies. These market fluctuations may also materially and adversely affect the market price of our common stock.
A LARGE PORTION OF OUR COMMON STOCK IS CONTROLLED BY A SMALL NUMBER OF SHAREHOLDERS AND, AS A RESULT, THESE SHAREHOLDERS ARE ABLE TO INFLUENCE THE OUTCOME OF SHAREHOLDER VOTES ON VARIOUS MATTERS
Currently, Far Grand and Mrs. Hui Wang respectively own 40% and 19% of Asia Biotech’s outstanding ordinary shares. As a result, these shareholders are able to influence the outcome of shareholder votes on various matters, including the election of directors and other corporate transactions including business combinations. In addition, the occurrence of sales of a large number of shares of our common stock, or the perception that these sales could occur, may affect our stock price and could impair our ability to obtain capital through an offering of equity securities. Furthermore, the current ratios of ownership of our common stock reduce the public float and liquidity of our common stock, which can in turn affect the market price of our common stock.
WE ARE LIKELY TO REMAIN SUBJECT TO "PENNY STOCK” REGULATIONS AND AS A CONSEQUENCE THERE ARE ADDITIONAL SALES PRACTICE REQUIREMENTS AND ADDITIONAL WARNINGS ISSUED BY THE SEC
As long as the trading price of our common stock is below $5.00 per share, the open-market trading of our common stock will be subject to the "penny stock" rules. The "penny stock" rules impose additional sales practice requirements on broker-dealers who sell securities to persons other than established customers and accredited investors (generally those with assets in excess of $1,000,000 or annual income exceeding $200,000 or $300,000 together with their spouse). For transactions covered by these rules, the broker-dealer must make a special suitability determination for the purchase of securities and have received the purchaser's written consent to the transaction before the purchase.
Additionally, for any transaction involving a penny stock, unless exempt, the broker-dealer must deliver, before the transaction, a disclosure schedule prescribed by the Securities and Exchange Commission relating to the penny stock market. The broker-dealer also must disclose the commissions payable to both the broker-dealer and the registered representative and current quotations for the securities. Finally, monthly statements must be sent disclosing recent price information on the limited market in penny stocks. These additional burdens imposed on broker-dealers may restrict the ability of broker-dealers to sell the common stock and may affect a shareholder's ability to resell the common stock.
Shareholders should be aware that, according to Securities and Exchange Commission Release No. 34-29093, the market for penny stocks has suffered in recent years from patterns of fraud and abuse. Such patterns include (i) control of the market for the security by one or a few broker-dealers that are often related to the promoter or issuer; (ii) manipulation of prices through prearranged matching of purchases and sales and false and misleading press releases; (iii) boiler room practices involving high-pressure sales tactics and unrealistic price projections by inexperienced sales persons; (iv) excessive and undisclosed bid-ask differential and markups by selling broker-dealers; and (v) the wholesale dumping of the same securities by promoters and broker-dealers after prices have been manipulated to a desired level, along with the resulting inevitable collapse of those prices and with consequential investor losses. Our management is aware of the abuses that have occurred historically in the penny stock market.
COMPLIANCE WITH RECENTLY ENACTED CHANGES IN SECURITIES LAWS AND REGULATIONS COULD COST HUNDREDS OF THOUSANDS OF DOLLARS, REQUIRE ADDITIONAL PERSONNEL AND REQUIRE HUNDREDS OF MAN HOURS OF EFFORT, AND THERE CAN BE NO ASSURANCE THAT WE WILL HAVE THE PERSONNEL, FINANCIAL RESOURCES OR EXPERTISE TO COMPLY WITH THESE REGULATIONS
Efforts to comply with recently enacted changes in securities laws and regulations will increase our costs and require additional management resources, and we still may fail to comply.
As directed by Section 404 of the Sarbanes-Oxley Act of 2002, the SEC adopted rules requiring public companies to include a report of management on the company's internal controls over financial reporting in their annual reports on Form 10-KSB. In addition, the public accounting firm auditing the company's financial statements must attest to and report on management's assessment of the effectiveness of the company's internal controls over financial reporting. These requirements are not presently applicable to us. If and when these regulations become applicable to us, and if we are unable to conclude that we have effective internal controls over financial reporting or if our independent auditors are unable to provide us with an unqualified report as to the effectiveness of our internal controls over financial reporting as required by Section 404 of the Sarbanes-Oxley Act of 2002, investors could lose confidence in the reliability of our financial statements, which could result in a decrease in the value of our securities. We have not yet begun a formal process to evaluate our internal controls over financial reporting. Given the status of our efforts, coupled with the fact that guidance from regulatory authorities in the area of internal controls continues to evolve, substantial uncertainty exists regarding our ability to comply by applicable deadlines.
FORWARD-LOOKING STATEMENTS
This prospectus contains forward-looking statements within the meaning of Section 27A of the Securities Act and Section 21E of the Securities Exchange Act (the "Exchange Act"). We have based these forward-looking statements largely on our current expectations and projections about future events and financial trends affecting the financial condition of our business. These forward-looking statements are subject to a number of risks, uncertainties, and assumptions, including, among other things:
| | · | general economic and business conditions, both internationally and in the PRC markets, |
| | · | our expectations and estimates concerning future financial performance, financing plans, and the impact of competition, |
| | · | our ability to implement our growth strategy, |
| | · | anticipated trends in our business, |
| | · | advances in technologies, and |
| | · | other risk factors set forth under "Risk Factors" in this prospectus. |
In addition, in this prospectus, we use words such as "anticipates," "believes," "plans," "expects," "future," "intends," and similar expressions to identify forward-looking statements.
We undertake no obligation to update publicly or revise any forward-looking statements, whether as a result of new information, future events or otherwise after the date of this prospectus. In light of these risks and uncertainties, the forward-looking events and circumstances discussed in this prospectus may not occur and actual results could differ materially from those anticipated or implied in the forward-looking statements.
Notwithstanding the above, Section 27A of the Securities Act and Section 21E of the Exchange Act expressly state that the safe harbor for forward looking statements does not apply to companies that issue penny stock. Because we issue penny stock, the safe harbor for forward looking statements does not apply to us.
USE OF PROCEEDS
This prospectus relates to shares of our common stock that may be offered and sold from time to time by the selling shareholders. We will not receive any proceeds from the sale of shares of common stock in this offering.
MARKET FOR COMMON EQUITY AND RELATED SHAREHOLDER MATTERS
There is currently no market for our common stock. We intend to apply for a listing of our common stock on the OTC Bulletin Board, but have not yet done so.
As of December 27, 2006, there were approximately 1446 shareholders of record of the Company’s common stock.
MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
DISCLAIMER REGARDING FORWARD LOOKING STATEMENTS
The following discussion should be read in conjunction with the Company’s financial statements, together with the notes to those statements, included elsewhere in this report. The following discussion contains forward-looking statements that involve risks, uncertainties, and assumptions. Words such as "plans," "intends," "hopes," "seeks," "anticipates," "expects, "and the like, often identify such forward looking statements, but are not the only indication that a statement is a forward-looking statement. Such forward-looking statements include statements concerning our plans and objectives with respect to our present and future operations, and statements which express or imply that such present and future operations will or may produce revenues, income or profits. These and other factors may cause our actual results to differ materially from any forward- looking statement. We caution you not to place undue reliance on these forward-looking statements. Although we base these forward-looking statements on our expectations, assumptions, and projections about future events, actual events and results may differ materially, and our expectations, assumptions, and projections may prove to be inaccurate. The forward-looking statements speak only as of the date hereof, and we expressly disclaim any obligation to publicly release the results of any revisions to these forward-looking statements to reflect events or circumstances after the date of this filing.
OVERVIEW
Asia Biotechnology Group Inc. (formerly known as Echelon Acquisition Corp.) (“Asia Biotech”)
Echelon Acquisition Corp., incorporated in the state of Nevada on July 27, 2004, changed its name to Asia Biotechnology Group Inc. (“Asia Biotech” or “the Company”) on July 19, 2006. The Company was initially formed as a "blank check" entity for the purpose of seeking a merger, acquisition or other business combination transaction with a privately owned entity seeking to become a publicly−owned entity.
On May 8, 2006, the Company completed a reverse acquisition transaction with Asia Biotechnology Group Inc. (the "ABG"), a corporation organized under the laws of British Virgin Islands and Harbin OT Pharmaceutical Co., Limited, a company organized under the laws of Samoa (the "OT Samoa “). The Company issued to the ABG Shareholder an aggregate of 23,296,000 shares of the Company’s common stock, and simultaneously issued to the OT Samoa Shareholders an aggregate of 23,296,000 shares of the Company’s common stock in exchange for all of the issued and outstanding shares of ABG and OT Samoa.
ABG and OT Samoa both thereby became the Company's wholly owned subsidiaries. The former shareholders of ABG (“Shareholders”) became the Company's major shareholders. This share exchange transaction resulted in those Shareholders obtaining a 40% significant voting interest in the Company. Generally accepted accounting principles require that the company whose shareholders retain the majority interest in a combined business be treated as the acquirer for accounting purpose, resulting in a reverse acquisition. Accordingly, the share exchange transaction has been accounted for as a recapitalization of the Company.
Asia Biotechnology Group Inc. (“ABG”)
Asia Biotechnology Group Inc. is a corporation organized under the laws of British Virgin Islands (the "ABG") on March 21, 2005. Far Grand Investment Limited plays as the sole shareholder under a trust agreement and the beneficiary of the trust is Mr. Mingshi Qiu. On November 3, 2005, ABG purchased 60% equity interest of Harbin OT Pharmaceutical Co., Ltd. (OT Pharmaceutical), a Chinese foreign owned enterprise incorporated in the PRC on April 13, 2001. OT Pharmaceutical is a feminine suppository manufacturer.
Harbin OT Pharmaceutical Co., Limited ("OT Samoa “)
Harbin OT Pharmaceutical Co., Limited is a company organized under the laws of Samoa (the "OT Samoa”) on April 13, 2005. OT Samoa was organized by Mr. Zhu Lei in April 2005 in anticipation of a business combination with ABG and a U.S. reporting company. Mr. Zhu funded OT Samoa by contributing $200,000. On January 22, 2006, Mr. Zhu licensed his proprietary technology/know-how related to the manufacture of certain medicines to OT Samoa. At the same day, OT Samoa entered into a Cooperation Agreement with ABG.The technology was not recorded on the books of OT Samoa, because the agreement consists only of an operating license and royalty arrangement with no transfer of ownership. OT Samoa did not engage in any business other than licensing the technology owned by Mr. Zhu and cooperating with ABG on the manufacturing of the know-how related products. On January 26, 2006, Mr. Zhu, the sole incorporator, owner and control person transferred his shares to 1,399 separate individuals. Mr. Zhu remained the sole director of OT Samoa and retained rights of rescission with regard to the transfer of his shares to the 1,399 individuals up until the time that those shares were exchanged for Asia Biotech shares.
The principal activities of the Company and its subsidiaries are the manufacturing and trading of feminine superiorities. All activities of the Company are principally conducted by subsidiaries operating in the PRC.
Harbin OT Pharmaceutical Co., Ltd. (OT Pharmaceutical)
Results of Operations - Years Ended December 31, 2005 and 2004
Harbin OT Pharmaceutical Co., Ltd. (OT Pharmaceutical), a Chinese foreign owned enterprise, incorporated in the People’s Republic of China (“PRC”) on April 13, 2001. On November 3, 2005, Asia Biotechnology Group Inc., organized under the laws of British Virgin Islands (the "ABG"), purchased 60% equity interest of OT Pharmaceutical. The principal activity of OT Pharmaceutical is the manufacturing and trading of feminine suppositories in the People's Republic of China.
The following table summarizes the results of OT Pharmaceutical during the years ended December 31, 2005 and 2004 and provides information regarding the dollar and percentage increase or (decrease) from 2005 to 2004.
Line Item | | 12/31/05 | | 12/31/04 | | $ Increase (Decrease) | | % Increase (Decrease) | |
| | | | | | | | | | |
| Revenues | | $ | 484,635 | | $ | 47,738 | | | 436,897 | | | 915 | |
Cost of Sales | | $ | 462,420 | | $ | 22,352 | | | 440,068 | | | 1,969 | |
| Gross Profit | | $ | 22,215 | | $ | 25,386 | | | (3,171 | ) | | (12.5 | ) |
| Operating Expenses | | $ | 246,391 | | $ | 245,638 | | | 753 | | | 0.3 | |
| Other Income | | $ | 10,814 | | $ | 78,485 | | | (67,671 | ) | | (86.2 | ) |
| Net Loss before minority interests | | $ | (213,362 | ) | $ | (141,767 | ) | | (71,595 | ) | | 50.5 | |
| Minority Interests | | $ | - | | $ | - | | | - | | | - | |
| Net Loss | | $ | (213,362 | ) | $ | (141,767 | ) | | (71,595 | ) | | 50.5 | |
| Loss per common share | | $ | 0.00 | | $ | 0.00 | | | - | | | - | |
| Weighted Average ordinary shares outstanding | | | N/A | | | N/A | | | - | | | | |
During the year ended December 31, 2005, we incurred net loss of $213,362, as compared to a net loss for the year ended December 31, 2004 of $141,767 representing a 50.5% increase.
Net Revenue
Net revenue for the year ended December 31, 2005 was $484,635 compared to $47,738 for the year ended December 31, 2004, an increase of $436,897, which is due primarily to the increased sales to one customer. Over 84% of the total sales were generated from Red Cross in 2005.
Gross Profit
Gross profit for the year ended December 31, 2005 was $22,215 compared to $25,386 for the year ended December 31, 2004. The decrease of $3,171 is due primarily to most of sales sold to non-profit organizations, including Red Cross. We intended to make initially small profit margins on our products as our products become better known to many people over the many provinces in PRC. Through our sales to Red Cross, we are building up our brand in order to make our products well known and popular throughout China.
Operating Expenses
For the year ended December 31, 2005, we incurred operating expenses of $246,391 compared to $245,638 per table for the year ended December 31, 2004. The $246,391 incurred as of December 31, 2005 included mainly salary expense of $43,096, depreciation expense of $40,082 other general operating expenses of $155,484.
Salary expense had a net increase of $22,518 for the year ended December 31, 2005 over the year ended December 31, 2004 due to the hiring of new labor for the expansion of production lines.
Depreciation expense had a net decrease of $48,181 for the year ended December 31, 2005 over the year ended December 31, 2004. The Company has more production lines throughout the year of 2005, and the deprecation for all plant and machinery in the production line and factory was provided and recorded under the cost of sales. The depreciation of the plant and machinery in non productive months in 2004 was provided and recorded under the operating expense. As a results, the depreciation of Year 2005 recorded under the operating expense was lower than 2004.
Other selling, general and administrative expenses had a net increase of $50,894 for the year ended December 31, 2005 over the year ended December 31, 2004, which included packing materials written off amounting to $52,743. All package materials with our prior business company name, were written off after we changed our company name in 2005. Other selling, general and administrative expenses included communication expenses, motor vehicles expenses, traveling expenses, repairs and maintenance, staff welfare, and miscellaneous expenses.
Other Income
Total other income for the year ended December 31, 2005 was $10,814 compared to $78,485 for the year ended December 31, 2004. Other Income for the year ended December 31, 2005 included a Government grant of $10,618, interest income of $74 and gain on disposal of fixed assets of $122. The decrease of $67,671 is due primarily to the decrease of the Government Grant from the $73,577 we received in 2004 to $10,618 in 2005.
Cash Flows -Years Ended December 31, 2005 and 2004
The following is a summary of cash flows from operating, investing, and financing activities during the years indicated:
| | | Years Ended December 31, | |
| | | 2005 | | 2004 | |
| Operating Activities | | $ | (58,101 | ) | $ | 2,200 | |
| Investing Activities | | $ | (20,568 | ) | $ | (16,803 | ) |
| Financing Activities | | $ | 96,105 | | $ | 12,042 | |
| Net effect on cash | | $ | 17,817 | | $ | (2,561 | ) |
Operation Activities
Net cash used in operations was $58,101 for the year ended December 31, 2005 compared to cash provided of $2,200 for the year ended December 31, 2004.
The net cash used was mainly attributable by the loss from operations and the increase in accounts receivable.
Investing Activities
Net cash used in investing was $20,568 for the year ended December 31, 2005 compared to cash used of $16,803 for the year ended December 31, 2004. During the year ended December 31, 2005, the Company bought plant & equipment of $20,568.
Financing Activities
Net cash provided by financing was $96,105 for the year ended December 31, 2005 compared to cash provided $12,042 for the year ended December 31, 2004. During the year ended December 31, 2005, the net increase of $84,063 for the year ended December 31, 2005 was mainly advanced from shareholders.
Liquidity and Capital Resources
We had $22,290 cash and cash equivalents as of December 31, 2005. As of such date, we also had total current assets of $715,781 and total current liabilities of $591,032 giving us working capital of $124,749. As of such date, we also had total assets of $1,701,273 and total liabilities of $591,032, we have accumulated a deficit (net loss) from operations of $1,094,363.
We believe that our currently-available working capital, should be adequate to sustain our operations at our current levels through the end of fiscal year 2006.
We currently have no material commitments for capital expenditures. Our future growth is dependent on our ability to raise capital for expansion, and to seek additional revenue sources. If we decide to pursue any acquisition opportunities or other expansion opportunities, we may need to raise additional capital, although there can be no assurance such capital-raising activities would be successful.
For the Research and Development activities, we anticipated we need $100,000 (RMB800,000) for the development of the six product candidates currently undergoing research and development. Also, we prepare to invest $330,000 (RMB 2,600,000) for the invention of a new assembly production line for the production in order to have mass production.
Our cash on hand as of December 31, 2005 was $22,290, and we believe this cash will be sufficient to fund our operations through December 31, 2006. Being defined as the hi-tech company by the local Chinese government, OT Pharmaceutical has been supported by the local authorities continuously from 2004 to 2005, and it had been granted funds from Harbin government up to $84,200. We may raise additional working capital through private placements and/or bank loans. Currently we do not have any debts, but if OT Pharmaceutical fails to obtain the funds from the local government or if revenue generated is not enough to support the expansion of our business, we may make loans from a local bank by mortgaging or pledging our assets.
There are no assurances that we will be able to raise the appropriate amount of capital needed for our future operations. Even though our plant and machinery as of December 31, 2005 was $882,533 there are no assurances that any Chinese bank will accept our assets as mortgage and make loans to us. Failure to obtain funding when needed may force us to delay, reduce, or eliminate our product development programs. We cannot assure you that our revenues will be sufficient to meet our operational needs and capital requirements. In addition, we cannot assure you that financing will be available in amounts or on terms acceptable to us, if at all. The incurrence of indebtedness would result in increased debt service obligations and could result in operating and financing covenants that would restrict our operations or our ability to pay dividends to our shareholders.
Contractual Obligations
The Company did not have any lease obligations as of December 31, 2005.
Off-Balance Sheet Arrangements
We do not have any off-balance sheet arrangement or commitment that will have a current effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources that is material to investors.
Inflation
We believe that inflation has not had a material impact on our results of operations for the year ended December 31, 2005.
Seasonality
We may experience seasonal variations in revenues and operating costs due to seasonality, however, we do not believe that these variations will be material.
Critical Accounting Policies
The Securities and Exchange Commission issued Financial Reporting Release No. 60, "Cautionary Advice Regarding Disclosure About Critical Accounting Policies" suggesting that companies provide additional disclosure and commentary on their most critical accounting policies. In Financial Reporting Release No. 60, the Securities and Exchange Commission has defined the most critical accounting policies as the ones that are most important to the portrayal of a company's financial condition and operating results, and require management to make its most difficult and subjective judgments, often as a result of the need to make estimates of matters that are inherently uncertain. Based on this definition, The Company has identified the following significant policies as critical to the understanding of its financial statements.
The preparation of financial statements in conformity with accounting principles generally accepted in the United States requires the Company’s management to make assumptions, estimates and judgments that affect the amounts reported in the financial statements, including the notes thereto, and related disclosures of commitments and contingencies, if any. The Company considers its critical accounting policies to be those that require the more significant judgments and estimates in the preparation of financial statements, including the following:
We recognize revenue in accordance with SEC Staff Accounting Bulletin No. 104. Product revenue is recognized when title and risk of ownership have been transferred, provided that persuasive evidence of an arrangement exists, the price is fixed and determinable, remaining obligations are insignificant and collectibility is reasonably assured. Transfer of title and risk of ownership occur when the product is shipped to the customer. Revenue is recorded at the invoiced amount net of discounts.
Our sales to the agent of Red Cross Society of China are recognized in accordance with SEC Staff Accounting Bulletin No. 104 and we recognize all revenues upon shipment for sales to our customers since we have no continuing obligations subsequent to shipment.
| | · | Product return policy and the assumptions used to estimate product returns of new products |
When we signed sales contracts with the Chinese Red Cross sales agent, we stated that there are no good returns except for the poor quality of the products. We ensured that all products we manufactured are under the GMP standard. The contract does not provide for return of good. The Management believes that the Company experienced few goods returns in 2006.
We have established a very low level of inventory for our short production process, efficient distribution channel and short estimated shelf life. The expiration date of the product is two years from the date of manufacture. The sales agent purchases the products from the Company once per month and all the sales agents keep a low level of inventory and we keep low inventory level also for our short production process.
| | · | Dilutions Estimates from revenue (e.g., product returns, chargebacks, incentives, fees, rebates, etc.,) |
The Company experienced few goods return, chargebacks, rebates and other significant dilution reserve in 2004 and 2005 and therefore, the Company believes that there will be insignificant goods return/chargebacks/rebates in the subsequent year.
| · | Contractual arrangements with Red Cross Society of China |
The Company signed sales contract with Red Cross Society of China and agreed that:
1. Having ascertained by Chinese Red Cross Health Assistance Office and examined by special review board”, All gynecological medicines (including Shehuang Suppository) of OT series produced by OT Pharmaceutical shall be the designated medicines in “Health Assistance——Red Cross Actions”.
2. OT Pharmaceutical should ensure to provide designated medicines for “Health Assistance Station” in “Health Assistance——Red Cross Actions” while performing other sales contract.
3. Settlement of accounts: The two parties shall check the total amount of medicine provided in 2005 before December 12, 2005. Health Assistance Office will pay OT Pharmaceutical after the Chinese government allocates sufficient grants to Health Assistance Office.
4. The term of the contract is temporarily designated as two years.
| · | Shipments made to Red Cross Society of China |
OT Pharmaceutical made no shipments to Red Cross wherein such shipments were as a result of incentives and/or in excess of the ordinary course of business inventory level. The Company recognized the revenue when the products shipped to Red Cross Society of China from the warehouse.
| · | Accounting on property, plant and equipment |
Property, plant and equipment are stated at cost including the cost of improvements. Depreciation and amortization are provided on the straight-line method based on the estimated useful lives of the assets as follows:
| Buildings | 20 years |
| Leasehold improvements | 20 years |
| Plant and machinery | 10 years |
| Motor vehicles | 5 years |
| Furniture, fixtures and equipment | 5 years |
| · | Accounting for allowance for doubtful accounts |
Trade accounts receivable are typically unsecured and derived from revenues earned from customers. Trade accounts receivable are recorded at the invoiced amount and do not bear interest. The allowance for doubtful accounts represents the Company’s best estimate of the amount of probable credit losses in the existing accounts receivable balance. The Company determines the allowance for doubtful accounts based upon historical write-off experience and current economic conditions. The Company reviews the adequacy of its allowance for doubtful accounts on a regular basis. Account balances are charged off against the allowance after all means of collection have been exhausted and the potential for recovery is considered remote.
Allowance for doubtful accounts was $36,096 and $0 at December 31, 2005 and December 31, 2004, respectively.
The Company signed a sales contract with Red Cross Society of China in which the terms of payment is longer than one year. As of December 31, 2005 and December 31, 2004, the accounts receivable with Red Cross was $426,124 and $0. The Company believes that there was no provision needed on this balance as of December 31, 2005.
| · | Research and development |
We expense product development costs as incurred until we determine that the product is productivity feasible and the product is successfully passed through some examinations. The research and development costs will then be capitalized and subject to amortization.
During the year ended December 31, 2005 and the year ended December 31, 2004, all expenditures for research activities relating to product development and improvement are $8,206 and $1,771 respectively and charged to expense under selling and administrative expenses.
We state our inventories at the lower of cost or market value, cost being determined on a standard cost basis (which approximates actual cost on a first−in, first−out basis) and market value being determined as the lower of replacement cost or net realizable value. Standard costs are monitored on a monthly basis and updated at least annually and as necessary to reflect changes in raw material costs and labor and overhead rates.
We write down inventory for estimated excess or obsolete inventory equal to the difference between the cost of inventory and the estimated market value based upon assumptions about future demand and market conditions. If actual market conditions are less favorable than those projected by management, additional inventory write−downs may be required. There is no inventory write−down for the year ended December 31, 2005 and the year ended December 31, 2004
We have established a very low level of inventory for our short production process, efficient distribution channel and short estimated shelf life. The expiration date of the product is two years from the date of manufacture. The sales agent purchases the products from the Company once per month and all the sales agents keep a low level of inventory and we keep low inventory level also for our short production process.
Management relies on historical experience, legal advice and on assumptions believed to be reasonable under the circumstances in making its judgment and estimates. Actual results could differ materially from those estimates.
Asia Biotechnology Group Inc. and its subsidiaries
Results of Operations - Six Months Ended June 30, 2006 and Six Months Ended June 30, 2005
Summary
The following table summarizes the results of the Company and its subsidiaries’ operations during the six months ended June 30, 2006 and the six months ended June 30, 2005 and provides information regarding the dollar and percentage increase or (decrease) from the 2006 six months to the 2005 six months.
Line Item | | 6/30/06 | | 6/30/05 | | $ Increase (Decrease) | | % Increase (Decrease) | |
| | | | | | | | | | |
| Revenues | | $ | 220,748 | | $ | 282,904 | | | (62,156 | ) | | (22.0 | ) |
Cost of Sales | | $ | 141,239 | | $ | 280,822 | | | (139,583 | ) | | (49.7 | ) |
| Gross Profit | | $ | 79,509 | | $ | 2,082 | | | 77,427 | | | 3718.9 | |
| Operating Expenses | | $ | 130,759 | | $ | 90,822 | | | 39,937 | | | 44.0 | |
| Other Income (Expanse) | | $ | (517,954 | ) | $ | 5 | | | (517,959 | ) | | 10,000,000 | |
| Net Loss before minority interests | | $ | 569,204 | | $ | 88,735 | | | (480,469 | ) | | 541.5 | |
| Minority Interests | | $ | 6,656 | | $ | 35,494 | | | (28,838 | ) | | 81.2 | |
| Net Loss | | $ | 562,548 | | $ | 53,241 | | | (509,307 | ) | | 956.6 | |
| Loss per common share | | $ | 0.02 | | $ | 0.00 | | $ | 0.02 | | | - | |
| Weighted Average ordinary shares oustanding | | | 33,528,221 | | | 23,296,000 | | | - | | | | |
During the six months ended June 30, 2006, we incurred consolidated net loss of $562,598, as compared to a net loss for the six months ended June 30, 2005 of $53,241 representing 956.6% increase.
Net Revenue
Net revenue for the six months ended June 30, 2006 was $220,748 compared to $282,904 for the six months ended June 30, 2005. The decrease of $62,156 was the result of a decrease in sales to the agent of the Red Cross in 2006.
Gross Profit
Gross profit for the six months ended June 30, 2006 was $79,509 compared to $2,082 for the six months ended June 30, 2005. The Company suffered large wastage of raw material for a new automatic production machine in 2005 and eventually, the Company had 36% gross profit margin during the six months ended June 30, 2006.
Operating Expenses
Total operating expenses for the six months ended June 30, 2006 increased by $39,937 or 44%, primarily for the reasons stated below. The individual components that attributed to the increase (decrease) in operating expenses for the six months ended June 30, 2006 compared to the six months ended June 30, 2005 consist primarily of the following:
| | · | salary expenses for the six months ended June 30, 2006 was $28,452 compared $16,844 for the six months ended June 30, 2005. The increase of $11,608 was the result of the hiring more staff. |
| | · | other selling, general and administrative fee expense for the six months ended June 30, 2006 was $79,034 compared $36,084 for the six months ended June 30, 2005. The increase of $42,950 was the result of the increased motor expense of $15,145 and overseas traveling of $24,388. |
Other Income (expense)
Total other expense for the six months ended June 30, 2006 increased by $517,959, representing the reorganization expense of $550,000 and the bad debt recovery of $31,970.
Cash Flows - Six Months Ended June 30, 2006 and Six Months Ended June 30, 2005
The following is a summary of cash flows from operating, investing, and financing activities during the periods indicated:
| | | Six Months Ended | |
| | | 2006 | | 2005 | |
| Operating Activities | | $ | (72,760 | ) | $ | (32,876 | ) |
| Investing Activities | | $ | (294,202 | ) | $ | (1,939 | ) |
| Financing Activities | | $ | (71,880 | ) | $ | 40,881 | |
| Net effect on cash | | $ | (421,387 | ) | $ | 6,066 | |
Operation Activities
Net cash used in operations was $72,760 for the six months ended June 30, 2006 compared to cash used of $32,876 for the six months ended June 30, 2005.
The net cash used was mainly attributable by the PRC subsidiary and was mainly due to the decrease in accounts payable and customer deposits.
Investing Activities
Net cash used in investing was $294,202 for the six months ended June 30, 2006 compared to cash used of $1,939 for the six months ended June 30, 2005. During the six months ended June 30, 2006, the Company bought a motor vehicle of $59,202 and paid reorganization expense of $235,000.
Financing Activities
Net cash used in financing activities was $71,880 for the six months ended June 30, 2006, representing repayment to a shareholder compared to cash provided of $40,881 representing the advance from a shareholder.
Liquidity and Capital Resources
We had $37,923 cash and cash equivalents as of June 30, 2006. As of such date, we also had total current assets of $724,208 and total current liabilities of $575,629, giving us working capital of $148,579. As of such date, we also had total assets of $1,690,266 and total liabilities of $575,629, we have accumulated a deficit (net loss) from operations of $627,400.
Major cash commitments in the next fiscal year are related to the funding of the Company’s business, corporate administration and operations, and proposed research activities. Our ability to generate revenue depends heavily on the successful commercialization and the market acceptance of our products. Capital expenditures, marketing efforts and capital investment are significantly required in order to achieve such goal. If we do not successfully develop and commercialize OT series suppositories, we will be unable to generate any revenue. For instance, one of our core products, Shenqi Wenyang Suppository just came to the Chinese market in 2005 and because of limited marketing, it only generated approximately USD8,500 revenue during that period. Our new product development and management’s ability to successful manage the business will be essential to achieving consistent profitability.
For the Research and Development activities, we will reserve 5% on gross sales for the research and development cost for the new products and there is no capital cost for the development of new products. We anticipated we need $100,000 (RMB800,000) for the development of the six product candidates currently undergoing research and development. Also, we prepare to invest $330,000 (RMB 2,600,000) for the invention of a new assembly production line for the production in order to have mass production.
We, through our subsidiaries, have traditionally been committed to manufacturing gynecological diseases treatment products. Currently OT Pharmaceutical will need to raise additional capital especially in the research and development of new products. We need to raise additional $100,000 in the development of our current 6 product candidates and approximately $330,000 in introducing more production lines. Compared to the capital we need, our cash on hand as of June 30, 2006 was $37,923. Being defined as a hi-tech company by the local Chinese government, OT Pharmaceutical had been supported by the local authorities continuously from 2004 to 2005, and it had been granted funds from Harbin government up to $88,200 (RMB705,600). We may raise additional working capital through private placements and/or bank loans. Currently we do not have any debts, but if OT Pharmaceutical fails to obtain the funds from the local government or if revenue generated is not enough to support the expansion of our business, we may make loans from a local bank by mortgaging or pledging our assets.
There are no assurances that we will be able to raise the appropriate amount of capital needed for our future operations. Even though our plant and machinery as of June 30, 2006 was $854,906, there are no assurances that any Chinese bank will accept our assets as mortgage and make loans to us. Failure to obtain funding when needed may force us to delay, reduce, or eliminate our product development programs. We cannot assure you that our revenues will be sufficient to meet our operational needs and capital requirements. In addition, we cannot assure you that financing will be available in amounts or on terms acceptable to us, if at all. The incurrence of indebtedness would result in increased debt service obligations and could result in operating and financing covenants that would restrict our operations or our ability to pay dividends to our shareholders.
Contractual Obligations
The Company did not have any lease obligations as of June 30, 2006.
Off-Balance Sheet Arrangements
We do not have any off-balance sheet arrangement or commitment that will have a current effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources that is material to investors.
Inflation
We believe that inflation has not had a material impact on our results of operations for the six months ended June 30, 2006.
Seasonality
We may experience seasonal variations in revenues and operating costs due to seasonality, however, we do not believe that these variations will be material.
Critical Accounting Policies
The Securities and Exchange Commission issued Financial Reporting Release No. 60, "Cautionary Advice Regarding Disclosure About Critical Accounting Policies" suggesting that companies provide additional disclosure and commentary on their most critical accounting policies. In Financial Reporting Release No. 60, the Securities and Exchange Commission has defined the most critical accounting policies as the ones that are most important to the portrayal of a company's financial condition and operating results, and require management to make its most difficult and subjective judgments, often as a result of the need to make estimates of matters that are inherently uncertain. Based on this definition, The Company has identified the following significant policies as critical to the understanding of its financial statements.
The preparation of financial statements in conformity with accounting principles generally accepted in the United States requires the Company’s management to make assumptions, estimates and judgments that affect the amounts reported in the financial statements, including the notes thereto, and related disclosures of commitments and contingencies, if any. The Company considers its critical accounting policies to be those that require the more significant judgments and estimates in the preparation of financial statements, including the following:
We recognize revenue in accordance with SEC Staff Accounting Bulletin No. 104. Product revenue is recognized when title and risk of ownership have been transferred, provided that persuasive evidence of an arrangement exists, the price is fixed and determinable, remaining obligations are insignificant and collectibility is reasonably assured. Transfer of title and risk of ownership occur when the product is shipped to the customer. Revenue is recorded invoiced amount net of discounts.
Our sales to Red Cross Society of China are recognized in accordance with SEC Staff Accounting Bulletin No. 104. and we recognize all revenues upon shipment for sales to our customers since we have no continuing obligations subsequent to shipment.
| | · | Product return policy and the assumptions used to estimate product returns of new products |
When we signed sales contracts with the Chinese Red Cross sales agent, we stated that there are no good returns except for the poor quality of the products. We ensured that all products we manufactured are under the GMP standard. The contract does not provide for return of good. The Management believes that the Company experienced few goods returns in 2006.
We have established a very low level of inventory for our short production process, efficient distribution channel and short estimated shelf life. The expiration date of the product is two years from the date of manufacture. The sales agent purchases the products from the Company once per month and all the sales agents keep a low level of inventory and we keep low inventory level also for our short production process.
| | · | Dilutions Estimates from revenue (e.g., product returns, chargebacks, incentives, fees, rebates, etc.,) |
The Company experienced few goods return, chargebacks, rebates and other significant dilution reserve in 2004 and 2005 and therefore, the Company believes that there will be insignificant goods return/chargebacks/rebates in the subsequent year.
| | · | Contractual arrangements with Red Cross Society of China |
The Company signed sales contract with Red Cross Society of China and agreed that:
1. Having ascertained by Chinese Red Cross Health Assistance Office and examined by special review board”, All gynecological medicines (including Shehuang Suppository) of OT series produced by OT Pharmaceutical shall be the designated medicines in “Health Assistance——Red Cross Actions”.
2. OT Pharmaceutical should ensure to provide designated medicines for “Health Assistance Station” in “Health Assistance——Red Cross Actions” while performing other sales contract.
3. Settlement of accounts: The two parties shall check the total amount of medicine provided in 2005 before December 12, 2005. Health Assistance Office will pay OT Pharmaceutical after the Chinese government allocates sufficient grants to Health Assistance Office.
4. The term of the contract is temporarily designated as two years.
| · | Shipments made to Red Cross Society of China |
OT Pharmaceutical made no shipments to Red Cross wherein such shipments were as a result of incentives and/or in excess of the ordinary course of business inventory level. The Company recognized the revenue when the products shipped to Red Cross Society of China from the warehouse.
| · | Accounting on property, plant and equipment |
Property, plant and equipment are stated at cost including the cost of improvements. Depreciation and amortization are provided on the straight-line method based on the estimated useful lives of the assets as follows:
| Buildings | 20 years |
| Leasehold improvements | 20 years |
| Plant and machinery | 10 years |
| Motor vehicles | 5 years |
| Furniture, fixtures and equipment | 5 years |
| · | Accounting for allowance for doubtful accounts |
Trade accounts receivable are typically unsecured and derived from revenues earned from customers. Trade accounts receivable are recorded at the invoiced amount and do not bear interest. The allowance for doubtful accounts represents the Company’s best estimate of the amount of probable credit losses in the existing accounts receivable balance. The Company determines the allowance for doubtful accounts based upon historical write-off experience and current economic conditions. The Company reviews the adequacy of its allowance for doubtful accounts on a regular basis. Account balances are charged off against the allowance after all means of collection have been exhausted and the potential for recovery is considered remote.
Allowance for doubtful accounts was $5,602 and $0 at June 30, 2006 and June 30, 2005, respectively. Doubtful account write-offs have been insignificant during the six months ended June 30, 2006 and the six months ended June 30, 2005.
The Company signed a sales contract with Red Cross Society of China in which the terms of payment is longer than one year. As of June 30, 2005 and June 30, 2006, the accounts receivable with Red Cross was $ 427,919 and the Company believe that there was no provision needed on this balance as of June 30, 2006.
| · | Research and development |
We expense product development costs as incurred until we determine that the product is productivity feasible and the product is successfully passed through some examinations. The research and development costs will then be capitalized and subject to amortization.
During the six month ended June 30, 2006 and the six months ended June 30, 2005, all expenditures for research activities relating to product development and improvement are $135 and $0 respectively and charged to expense under selling and administrative expenses.
We state our inventories at the lower of cost or market value, cost being determined on a standard cost basis (which approximates actual cost on a first−in, first−out basis) and market value being determined as the lower of replacement cost or net realizable value. Standard costs are monitored on a monthly basis and updated at least annually and as necessary to reflect changes in raw material costs and labor and overhead rates.
We write down inventory for estimated excess or obsolete inventory equal to the difference between the cost of inventory and the estimated market value based upon assumptions about future demand and market conditions. If actual market conditions are less favorable than those projected by management, additional inventory write−downs may be required. There is no inventory write−down for the six months ended June 30, 2006 and the six months ended June 30, 2005.
We have established a very low level of inventory for our short production process, efficient distribution channel and short estimated shelf life. The expiration date of the product is two years from the date of manufacture. The sales agent purchases the products from the Company once per month and all the sales agents keep a low level of inventory and we keep low inventory level also for our short production process.
Management relies on historical experience, legal advice and on assumptions believed to be reasonable under the circumstances in making its judgment and estimates. Actual results could differ materially from those estimates.
BUSINESS
OVERVIEW
ASIA BIOTECH
Asia Biotech, formerly known as Echelon Acquisition Corp., was originally incorporated on July 27, 2004 under the laws of the State of Delaware. It was a "blank check" corporation as defined by Section (7) (b) (3) of the Securities Act of 1933, as amended. Since inception, the Company has intended to serve as a vehicle to effect an asset acquisition, merger, exchange of capital stock or other business combination with a domestic or foreign business. Before the merger with ABG and OT Samoa, we had no operations and had only minimal liabilities.
On May 8, 2006, Asia Biotech entered into a triangular Agreement and Plan of Reorganization with shareholders of ABG and OT Samoa. Pursuant to this Agreement, Asia Biotech acquired the single issued and outstanding share of ABG from the sole Shareholder of ABG in exchange for 23,296,000 shares of our common stock, and all of the 20,000,000 issued and outstanding shares of OT Samoa from 1399 shareholders in exchange for a total of 23,296,000 shares of our common stock. Immediately after the merger, Asia Biotech owns 100% shares of ABG and OT Samoa, and the former shareholder of ABG and former shareholders of OT Samoa respectively own 40 percent of the shares of the Company’s common stock.
On July 19, 2006, the name of Echelon Acquisition Corp. was changed to Asia Biotechnology Group Inc.
Although OT Samoa’s only asset is identified as an intercompany account with ABG, OT Samoa is not controlled by ABG. Rather, it cooperates with ABG. OT Samoa does not engage in any business other than sub-licensing a kind of gynecological treatment related technology. On January 22, 2006, OT Samoa and ABG entered into a Cooperation Agreement, Pursuant to this agreement, these two companies cooperate to manufacture certain gynecological products by using the technology sub-licensed by OT Samoa. The business purpose for acquiring OT Samoa is that the Company may generate revenues by sub-licensing the proprietary technology granted by OT Samoa.
During the interim period ended June 30, 2006, the assets (US$2,240,266) of ABG were much more significant than the assets (US$200,000) of OT Samoa. However, ABG has not generated profits yet, because the only operating subsidiary of ABG, OT Pharmaceutical has not generated any profits. OT Pharmaceutical may continuously lose money in the near future. On the other hand, even though the assets of OT Samoa were relatively small, OT Samoa is unlikely to lose money since its only business is sub-licensing its granted technology. Moreover, OT Samoa and ABG cooperate on the manufacturing of certain medical product and the profit to be generated will be distributed equally.
Asia Biotech conducts its operations through its China-based indirect subsidiary, OT Pharmaceutical. Asia Biotech, ABG and OT Samoa have not conducted any revenue generating activities to date. After the Reorganization, all of Asia Biotech's business is conducted in China, and all of Asia Biotech's management as well as our employees are located in China.
Our principal executive office is located at No. 7 BohaiSanLu, Pingfang Industrial District, Economic & Technological Development Area, Harbin, Heilongjiang Province, China.
ABG
ABG was incorporated on March 21, 2005 under the laws of the British Virgin Islands. Far Grand, a company organized under the laws of Cayman Islands, acted as the sole shareholder of ABG. Mr. Wensheng Wang, a Chinese citizen, is the sole shareholder and director of Far Grand.
As of October 17, 2005, ABG entered into a stock transfer agreement with 5 individual shareholders of OT Pharmaceutical. Pursuant to this agreement, ABG acquired 60% of the equity interests in OT Pharmaceutical. Currently, 4 individual Chinese shareholders together hold the remaining 40% equity interests. After the acquisition, OT Pharmaceutical became a Sino-foreign joint venture as defined by PRC laws.
ABG and OT Samoa currently cooperate with each other on the manufacturing of certain gynecological products.
OT PHARMACEUTICAL
OT Pharmaceutical was established in 2001 under PRC laws. Since its inception, OT Pharmaceutical has focused primarily on designing, manufacturing and marketing gynecological products, and especially on the marketing of feminine suppositories. Since it was acquired by ABG, OT Pharmaceutical has been conducting ABG’s only business operation. After the Reorganization, OT Pharmaceutical became the only operation of Asia Biotech.
ABG holds 60% equity interests in OT Pharmaceutical, the remaining 40% equity interests are held by four Chinese nationals.
OT SAMOA
OT Samoa was incorporated on April 13, 2005 under the laws of Samoa. The shareholders of OT Samoa before the Reorganization were 1399 Chinese residents. It has no intention to manufacture or market products. It intends to sub-license the granted technology in exchange for sub-license fees and royalty payments and to continue to seek other in-licensing opportunities in pursuit of its business strategy.
Currently OT Samoa and ABG cooperate on the manufacturing of certain gynecological products.
MARKET FOCUS
We, through our subsidiaries, are principally focused on the manufacturing of gynecological medicines and sanitary products. All of our existing medicines are suppositories, among which certain suppositories are pure natural herbal medicine. Our goals are to develop gynecological treatment mainly based on traditional Chinese medicines and to increase the market penetration of our products in the PRC. To achieve these goals, we intend to continue to grow our business through the following strategies: developing our relationship with Chinese distributors to expand our distribution channels, expanding on the market acceptance in China, and increasing our technological advantages through focused research and development.
We believe our principal competitive strength is our advanced technologies, and we expect to generate revenues from marketing the products of OT Pharmaceutical in China and sub-licensing the proprietary technology granted to OT Samoa.
PRINCIPAL PRODUCTS
Pharmaceutical Products
At the date of this prospectus, our current portfolio of pharmaceutical products includes 9 suppositories targeting the treatment of gynecological diseases in China. All medicines have been cleared by Chinese State Drug Administration, or the CSDA, for sale in China. Out of 9 suppositories, these 3 suppositories need a prescription from a physician: Kushenjian Suppository, Shenqi Wenyang suppository and Fu Ning Suppository.
One of our products, Fu Ning Suppository, has been labeled by the CSDA as a Protected Traditional Chinese Medicine, and is accordingly enjoying a 7-year protection period commencing from 2001 to 2007. During this period, OT Pharmaceutical can exclusively produce Fu Ning Suppository and no other pharmaceutical manufacturers are allowed to produce identical suppositories.
The tables below list our 9 approved drugs and their detailed information:
| · | Table I Product Functions |
No. | | Product Names | | Functions |
| 1 | | Econazole Nitrate Suppository | | To treat monilial vaginitis |
| | | | | |
| 2 | | Shehuang Suppository | | To clear away pathogenic heat and dampness; to relieve itching; to treat thick, foul-smelling vaginal discharge caused by vaginitis |
| | | | | |
| 3 | | Clotrimazole Suppository | | To treat monilia vulvae infection |
| | | | | |
| 4 | | Compound Metrnidazole Suppository | | To treat trichomonad vaginitis and senile vaginitis |
| | | | | |
| 5 | | Compound Chlorhexidine Acetate Suppository | | To treat diseases caused by anaerobic organism; trichomonad in vagina |
| | | | | |
| 6 | | Kushenjian Suppository | | To treat monilial and trichomonad vaginitis, senile vaginitis chronic cervicitis, and pelvic infection |
| | | | | |
| 7 | | Metronidazole, Clotrimazole and Chlorhexidine Acetate Suppository | | To treat colpitis mycotica, trichomonas vaginalis, bacteroidal vaginitis, and mixed infection vaginitis |
| | | | | |
| 8 | | Shenqi Wenyang Suppository | | To treat pelvic pain, and thick vaginal discharge; to clear away pathogenic heat and dampness, and to relieve itching |
| | | | | |
| 9 | | Fu Ning Suppository | | To treat cervical erosion, and to dissolve the stagnant, trichomonas vaginalis and bacteroidal vaginitis |
| · | Table II Detailed Description of Products |
No. | | Product Names | | Specification | | Weight/Size | | Permission Number | | OTC/RX | | Chemical Medicine /Chinese Herbal Medicine |
| 1 | | Econazole Nitrate Suppository | | 3 suppositories /carton 7 suppositories /carton | | 0.15g per unit | | H23022433 | | OTC | | Chemical Medicine |
| | | | | | | | | | | | | |
| 2 | | Shehuang Suppository | | 3 suppositories/ carton | | Approximately 4.9g per unit | | B20020646 | | OTC | | Chinese Herbal Medicine |
| | | | | | | | | | | | | |
| 3 | | Clotrimazole Suppository | | 3 suppositories/ carton 7 suppositories /carton 10suppositories/carton | | 0.15g per unit | | H23022432 | | OTC | | Chemical Medicine |
| | | | | | | | | | | | | |
| 4 | | Compound Metrnidazole Suppository | | 3 suppositories /carton 7suppositories /carton 10suppositories/carton | | 0.5g per unit | | H1999218 | | OTC | | Chemical Medicine |
| | | | | | | | | | | | | |
| 5 | | Compound Chlorhexidine Acetate Suppository | | 7 suppositories /carton | | Compound | | H23023087 | | OTC | | Chemical Medicine |
| | | | | | | | | | | | | |
| 6 | | Kushenjian Suppository | | 3 suppositories/carton 7 suppositories/carton | | 0.5g per unit | | H23022711 | | RX | | Chemical Medicine |
| | | | | | | | | | | | | |
| 7 | | Metronidazole, Clotrimazole and Chlorhexidine Acetate Suppository | | 3 suppositories/ carton 7 suppositories/ carton | | Compound | | H10983208 | | OTC | | Chemical Medicine |
| | | | | | | | | | | | | |
| 8 | | Shenqi Wenyang Suppository | | 3 suppositories /carton | | 2.3 g per unit | | B20020476 | | RX | | Chinese Herbal Medicine |
| | | | | | | | | | | | | |
| 9 | | Fu Ning Suppository | | 3 suppositories/ carton 7 suppositories/ carton | | 3.59g per unit | | Z23022028 | | RX | | Chinese Herbal Medicine |
Sanitary Product
We also offer one sanitary product in the Chinese market. It is named as OT Partner Anti-bacterial Washing Liquid and has been approved by the local health supervision authority. This washing liquid is a supplementary gynecological product to relieve the itching caused by bacteria in the vagina.
Revenues
Our significant revenues are generated from the sales of the above products. The amount of revenues generated by each product in each of the last fiscal years and for the period ended June 30, 2006 is illustrated as below:
Table III: Revenues Generated
No. | | Product Names | | 2004 ( in US$) | | 2005 (in US$) | | June 30, 2006
(in US$) | |
| 1 | | Econazole Nitrate Suppository | | 2,450 | | 4,695 | | 23,699 | |
| 2 | | Shehuang Suppository | | 441 | | 3,866 | | 6,896 | |
| 3 | | Clotrimazole Suppository | | 5,879 | | 148,410 | | 23,482 | |
| 4 | | Compound Metrnidazole Suppository | | 7,932 | | 79,357 | | 27,856 | |
| 5 | | Compound Chlorhexidine Acetate Suppository | | 0 | | 12,454 | | 0 | |
| 6 | | Kushenjian Suppository | | 2,404 | | 4,309 | | 6,036 | |
| 7 | | Metronidazole, Clotrimazole and Chlorhexidine Acetate Suppository | | 15,447 | | 150,232 | | 69,822 | |
| 8 | | Shenqi Wenyang Suppository | | 0 | | 8,144 | | 0 | * |
| 9 | | Fu Ning Suppository | | 13,184 | | 72,912 | | 67,255 | |
| 10 | | OT Partner Anti-bacterial Washing Liquid | | 0 | | 256 | | 1,4832 | |
| | | Subtotal | | 47,737 | | 484,635 | | 226,528 | |
| | | | | | | | | (5,780 | )* |
| | | Total | | | | | | 220,748 | |
*Note: An amount of US$ 5,780 of goods was returned in 2006, because OT Pharmaceutical canceled its sales contract with a distributor of Shengqi Wenyang Suppository.
TECHNOLOGIES
Background
The gynecological conditions can range from chronic, repeated yeast infections (candidiasis), abnormal periods, vaginal warts, to cervical cancer. The use of vaginal suppositories is one of the commonly accepted methods to treat gynecological ailments, especially vaginal infections such as mycotic vaginitis, trichomonas vaginitis and candidiasis.
The soluble suppositories may not irritate the mucosa of the vagina and they are easy to dissolve in the vagina. Moreover, there are no known side-effects that would require treatment to be discontinued. The suppositories constitute a more attractive alternative to surgery than oral tablets or liquid treatments. (Source http://www.pslgroup.com/dg/120de2.htm )
Since all of our products are gynecological treatments, the advanced gynecological technologies are the foundation of our operation. The details of them are briefly described below:
OT Pharmaceutical’s Technologies
OT Pharmaceutical’s technologies relate to a novel pharmaceutical utility for the treatment of gynecological diseases which comprises of a therapeutic drug for the intra-vaginal area and a method of solubilizing the medical components in liquid promptly. One of OT Pharmaceutical’s proprietary technologies is the surface treatment of the suppository. After special treatment on the outer periphery or surface of the suppositories is complete, the suppositories’ surface and inner cotton pessary may be combined tightly to prevent the soluble medicine from spilling and to control the volume of the medicine. Moreover, such treatment may prevent the suppository from becoming deformed.
We believe that our patent can improve the therapeutic effectiveness of successful drug compounds, within a shortened period, and at a reduced cost.
OT Pharmaceutical is seeking patent protection for technological developments. The details of our patents and pending patents are described in Intellectual Property Section.
OT Samoa’s Technology
Pursuant to the Know-How License Agreement (“License Agreement”) executed between OT Samoa and Mr. Lei Zhu, OT Samoa acquired a world-wide, non-exclusive right and license to use the know-how (as described below) and the right to sublicense such know-how.
This proprietary know-how licensed to OT Samoa is a type of Broad-spectrum and Antibacterial Gynecological Suppository and Generic Technique of Manufacture for Ointment. This type of the suppository and ointment is made of ciprofloxacin, which is used for local application. Adopting ciprofloxacin to make the suppository and ointment makes the pharmacodynamic action work directly and creates no side-effect of stimulating the intestine and stomach. Also, using ciprofloxacin can diminish the toxicity and side effects of the suppository on the liver. In particular, this product provides convenience for the patients who do not want to take medicine by mouth or deglutition, and it is an effective medicine for treating various infectious diseases.
Ciprofloxacin belongs to the third generation of quinolones, which is broad spectrum and provides a sound antibacterial effect. This technique is aimed at providing a type of local application made from ciprofloxacin that is a suppository and ointment. It can work directly and avoids stimulating the stomach and intestine; it can also diminish the toxicity and side effects on the liver.
Since this technique of manufacturing the suppository and ointment adopts ciprofloxacin, which is broad-spectrum and has strong antibacterial qualities, application of the medicine results in direct access to tissue and mucous membrane, and can have direct effect to the condition. This method avoids the effect of stimulating the stomach and intestine with macrolide; it also directly gets into the general circulation without touching the liver, diminishing biochemical changes and reducing the toxicity and side effects on the liver.
This kind of medicine provides special convenience for patients who don’t want to take medicine by mouth or deglutition and it is an effective medicine for treating various infectious diseases. In particular, it is the first choice for treating diseases such as urethritis, which is caused by non-gonococcus, venereal diseases and chancroid.
INTELLECTUAL PROPERTY
The protection of our intellectual property is a strategic priority for our business. We rely primarily on a combination of trademark and patent protection in the PRC to safeguard our intellectual property (“IP”) and our brand. Our ability to protect and use our intellectual property rights in the continued development and commercialization of our technologies and products, to operate without infringing the IP rights of others, and to prevent others from infringing upon our IP rights, is crucial to our continued success.
Our policy is to seek patent protection for the inventions that are important to the development of our business. At the date of this Prospectus, we own 1 utility model patent right and 2 invention patent rights. OT Pharmaceutical has applied for 3 invention patent applications on the existing suppositories. We intend to apply for more patents to protect our core technologies and medicines in the future.
Table IV and table V below lists our patents and pending patents applied in China:
Table IV Patents
Patents | | Patent No. | | Approval Date | | Description |
| Utility Model | | Zl 200420115601.9 | | Dec 14, 2005 | | A kind of Two-way model for forming and packaging suppositories |
| | | | | | | |
| Invention | | ZL 031 19493.1 | | July 5, 2006 | | A kind of compound drug, and its producing method and its application on treatment of gynecological diseases |
| | | | | | | |
| Invention | | ZL 031 19492.3 | | July 5, 2006 | | A kind of compound drug, and its producing method and its application on treatment of gynecological diseases |
Table V Pending Patents
Pending Patents | | Application No. | | Description |
| Invention | | 200410091260.0 | | A kind of suppository, pessary with the special surface disposal, and the related producing method |
| | | | | |
| Invention | | 200410104481.7 | | A kind of Metronidazole, Clotrimazole and Chlorhexidine acetate suppository and its producing method |
| | | | | |
| Invention | | 200410104482.1 | | A kind of suppository, pessary with the special surface disposal, and the related producing method |
In China, applicants must submit patent applications to the State Intellectual Property Office of China for approval. The duration of patent right for inventions is 20 years and the duration of patent right for utility models is 10 years.
Due to its advanced and practical technologies and related patents, OT Pharmaceutical was qualified as a High-New Tech Enterprise by the local Chinese government in 2002, and accordingly enjoys certain preferable incentive programs by the Harbin local government, such as tax deduction and local government’s grants.
From 2000 to 2003, OT Pharmaceutical has registered 21 trademarks and logos including OT, O.T. and OuTi in China. According to the PRC Trademark Law, a registered trademark is valid for 10 years and it can be renewed for another 10 years upon application. Currently, all of our products are sold under registered trademarks and logos.
RESEARCH AND DEVELOPMENT
Although in relatively small amounts, OT Pharmaceutical is engaged in the research of sanitary products that consists of non-prescription traditional Chinese medicines and supplements. Table VI below lists 6 product candidates that are undergoing the research and development.
Table VI Product Candidates Undergoing R&D
No. | | Product Candidates | | Development Stage | | Estimated Developmental Timeline | | Funds Needed Approx |
| 1 | | OT Partners Effervescent Tablets(Sterilization) | | Technological test | | 8 months | | US$15000 |
| 2 | | OT Partners Effervescent Tablets(anti-bacteria) | | Technological test | | 8 months | | US$15000 |
| 3 | | OT Partners Tablets (Sterilization ) | | Toxicological test | | 6 months | | US$15000 |
| 4 | | OT Partners Suppository (Sterilization) | | Toxicological test | | 6 months | | US$15000 |
| 5 | | Washing Liquid (Nutritive ) | | Functional test | | 5 months | | US$20000 |
| 6 | | Washing Liquid (anti vaginal itching ) | | Functional test | | 5 months | | US$20000 |
| | | Total | | | | | | US$100,000 |
Our sanitary product candidates usually undergo the following phases: technological test, toxicological test and functional test. The R&D period is estimated for 10 months.
Currently, all our research and development is conducted by our key research personnel within OT Pharmaceutical. The research team is lead by capable Chinese traditional medicines experts in the company. As of December 22, 2006, 4 technicians, including 2 of our senior researchers at OT Pharmaceutical, were engaging in the research and development of new products. They were in charge of the quality control of the existing products.
We will dedicate 5% of our total annual revenues in the future in our research and development of new products.
MANUFACTURING
The manufacture of our product candidates for clinical trials and commercial purposes is subject to current good manufacturing practices and other regulations. Our production facilities were certified to be in compliance with Good Manufacturing Practice, or GMP standards. OT Pharmaceutical has also received the government approval for the production of sanitary products in 2004.
Currently OT Pharmaceutical has one factory and three production lines, and we intend to introduce more production lines throughout the year 2007 to compete in the suppositories market. To date, OT Pharmaceutical has 12 machines and 19 supporting parts for the manufacturing of suppositories.
OT Pharmaceutical purchases raw materials for manufacturing in the Chinese open market. Substantially all such materials are obtainable from numerous sources. By the end of 2005, we developed limited relationships with 21 suppliers for raw materials and 21 suppliers for drug packaging materials, including Shantou Sunda Plastic Factory, Nanning Shangren Plastic bags Co., Ltd., Harbin Gaomei Printing House Ltd., Gongxin Enterprise Ltd., and Jiangsu Qionghua Hi-tech Stock Co., Ltd.
DISTRIBUTION
OT Pharmaceutical’s sales and marketing activities are limited to the PRC, primarily to the Red-Cross and pharmaceutical distribution agents. To date, OT Pharmaceutical’s products are distributed in the mainland of China within 88 cities among 23 provinces, sovereignties, and autonomous regions.
Marketing of our products has been mainly accomplished through the use of our 10 sales representatives and 21 proxy agents. OT Pharmaceutical uses a flat distribution channel system of independent regional distributors. In a typical distribution contract, a distributor will be provided with certain sales targets for a particular period according to a set retail price. If the distributor completes the sales task within the prescribed period, the agent distributor will be given greater economic incentives. If the distributor fails to complete the sales task within the prescribed period, OT Pharmaceutical has the right to cancel its contract with the distributor and sign with other competent distributors. OT Pharmaceutical also sells the medicines through promotions to end users such as soliciting and advertising in pharmacies.
We intend to continue using independent distributors. Our growth in terms of revenue requires additional sales representatives, which is part of our business plan for 2007.
PRINCIPAL MARKETS IN THE PRC
In 2004, the PRC's pharmaceutical industry realized sales of US$42 billion (RMB347.6 billion) and net profits of US$3.7 billion (RMB30.64 billion); a 17.44% increase in realized sales and 11.74% increase in net profits from the previous year (Source:http://www.chinapharm.com.cn). According to a Chinese government report, China's pharmaceutical sales in 2005 are expected to be approximately US$ 45.5 billion (RMB 376.6 billion), growing 17% from the previous year (Source: http://www.biotech.org.cn/news/news/show.php ?id=21470). It is estimated that China's pharmaceutical industry will maintain at least a 12%-15% growth rate through the year 2010 (Source:http://www.511511.com/A1/200501/A100000391720050104093750375.shtml). According to analyst predictions, China is expected to become the world’s fifth-largest pharmaceuticals market by 2010, and the largest by 2050.(Source: Investing in China’s Pharmaceutical Industry by PricewaterhouseCooper http://www.pwchk.com/ )
Herbal Pharmaceutical Industry in the PRC
OT Pharmaceutical’s principal business falls into the Chinese herbal medicine manufacturing industry of the pharmaceutical manufacturing industry. As of 2002, there are 1,141 kinds of Chinese herbal medicine which have passed the national registration process, including 11.5% Level 1 new drugs, 6.5% Level 2 new drugs, 40% Level 3 new drugs, 40% Level 4 new drugs and 2% Level 5 new drugs (Source: the second sub-section of section three in ‘Overview of the Chinese Herbal Pharmaceutical Industry’, April 20, 2002, http://www.kangqiaonet.com/newspub/yanxianni.php?tid=27&id=1721).
The Chinese herbal medicine industry has grown in China in the past ten years. In 1999 there were a total of 1,033 Chinese herbal medicine manufactures in China which realized approximately US$ 4.18 billion (RMB 34.6 billion) revenue and approximately US$0.447 billion (RMB3.7 billion) profits (Source: the 1.4 section in ‘Researching Report on the Chinese Herbal Pharmaceutical Industry’, Feb 6, 2002, http://www.tysc.com.cn/news/news_detail.php?id=71560&cat_id=31). From 1996 to 2000, the Chinese herbal medicine finished products industry grew at an average rate of 24.6% compared with 18% of the total pharmaceutical industry in China (Source: the second paragraph of section one in ‘Overview of the Chinese Herbal Pharmaceutical Industry’, April 20, 2002, http://www.kangqiaonet.com/newspub/yanxianni.php?tid=27&id=1721).
The Detailed Market Sector in the PRC
The target consumers of our market are mainly female ages 21-45 years old. In recent years, the requirements and the sale amount of gynecological medications have increased gradually. It was calculated that sales of gynecological medications were approximately US$0.65 billion (RMB5.2 billion) in 2004, an increase of 16.84% compared with 2003. (Source: http://www.cpha.org.cn/html/content/xw/content_53_2348.htm )
The suppository plays an important role in gynecological medications. Compared to 2003, in the market of Beijing alone, the anti-infective suppositories occupied 40% of sales, and increased at a rate of 29% in 2004. (Source: http://www.cpha.org.cn/html/content/rdxw/content_52_1924.htm)
The share of gynecologic drugs on drug store market is considerable, according to statistical data of June, 2002, the sales amount of gynecologic drugs accounts for 10.11% of the total of Chinese patent drugs on retail market over the whole country, ranking fourth overall. The sales volume and sales amount of such products in the last three years remained at a firm growth rate of about 10%, while at the same time the average price increased progressively yet with small amplitude. The mean annual price of 2002-2005 is RMB 22.88 per unit. The market is still increasing, and it is estimated that the 2005 sales amount will break through RMB 1 billion.
The women's healthcare service network has gradually improved. By the end of 2004, there were 2,997 healthcare institutes for women and children throughout China, with 243,000 beds for women.(http://www.china.org.cn/e-white/20050824/6.htm)
The predicted growth is based upon the relaxation of trade barriers following China's accession to the World Trade Organization, advances in the Chinese economy, and China's large, female population.
COMPETITION
A company wishing to enter the industry must comply with the standards and regulations set forth by the government. In the PRC, the SFDA is the authority that monitors and supervises the administration of the pharmaceutical industry including pharmaceutical products, medical appliances, and equipment. Pharmaceutical manufacturing enterprises must obtain a Pharmaceutical Manufacturing Enterprise Permit issued by the relevant pharmaceutical administrative authorities and relevant health departments at the provincial level where the enterprise is located. Furthermore, all pharmaceutical products produced in the PRC, with the exception of Chinese herbal medicines in soluble form, must bear a registered number approved by the appropriate medicine administration authorities in the PRC. Lastly, in accordance with the World Health Organization, the PRC now requires compliance with GMP standards in pharmaceutical production in order to minimize the risks involved in any pharmaceutical production that cannot be eliminated through testing final products. As the regulatory approval process becomes more stringent, it also increases the industry's capital entry barrier.
Due to the variety of consumer demands within the pharmaceutical market, pharmaceutical companies have relatively dispersed product lines.
The market of gynecological external remedies is a segment with intense competition, where newly developed gynecological drugs for exterior use emerge often. The incidence rate of gynecopathy has been quite high. The market of gynecological external remedies has increased by 10% annually and Chinese material medical preparation is now facing the challenge of Western medicine. The target consumers of the market are mainly the female ages 21-45 years old. The apparent seasonality and high loyalty are characteristics of sales in the market. The major products in the market include the old-line Skinpro Lotion and the foreign-funded brand Miconazole Nitrate Suppository.
GOVERNMENT REGULATION
Currently, OT Pharmaceuticals has obtained SFDA approvals for 9 kinds of existing medicine products and obtained the local health supervision authority's approval for one sanitary product. The 6 candidate products under the R&D are sanitary products and are therefore not subject to the strict regulatory procedure on medicines. However, if OT Pharmaceutical intends to produce new medicines, it must comply with the relevant regulations.
The testing, approval, manufacturing, labeling, advertising and marketing, and post-approval safety reporting, among other things, of our products or are extensively regulated by governmental authorities in the PRC.
In the PRC, the State Food and Drug Administration, or the SFDA, regulates and supervises pharmaceutical products under the relevant pharmaceutical Laws and regulations. Each procedure of our pharmaceutical production is subject to the requirements for the manufacture and sale of pharmaceutical products as provided by these laws and regulations, including but not limited to, the standards of clinical testing, declaration, approval and transfer of new medicine registrations, applicable industry standards of manufacturing, distribution, packaging, advertising and pricing.
Preclinical Laboratory Tests and Animal Tests
Preclinical tests include in-vitro laboratory evaluation of the product candidate, as well as in-vivo animal studies to assess the potential safety and efficacy of the product candidate. We must submit the results of the preclinical tests, together with manufacturing information, analytical data, and the sample of product candidate to the provincial level SFDA as part of an investigational new drug, or IND, which must be approved before we may commence human clinical trials. We cannot assure that submission of an IND will result in the China SFDA allowing human clinical trials (see below) to begin, or that, once begun, issues will not arise that suspend such human clinical trials.
Human Clinical Trial
Clinical trials involve the administration of the product candidate to healthy volunteers or patients under the supervision of principal investigators, who are generally physicians or independent third parties not employed by us or under our control. Clinical trials typically are conducted in three sequential phases, but the phases may overlap or be combined. In Phase1, the initial introduction of the drug into human subjects, the drug is usually tested for safety (adverse effects), dosage tolerance, and pharmacologic action. Phase2 usually involves studies in a limited patient population to evaluate the preliminary efficacy of the drug for specific, targeted conditions; to determine dosage tolerance and appropriate dosage and to identify possible adverse effects and safety risks. Phase 3 trials generally further evaluate clinical efficacy and test further for safety within an expanded patient population. Either the company or the China SFDA may suspend clinical trials at any time on various grounds, including a finding that subjects are being exposed to an unacceptable health risk.
After three phases of human clinical trials, the company will submit to the provincial level SFDA a report containing the results of the preclinical and clinical studies, together with other detailed information, including information on the manufacture and composition of the product candidate, to apply for a new drug certificate. In the meantime, the company will submit raw materials of the product candidate to the China Medicine and Biological Products Examination Institute.
New Drug Certificate
The provincial level SFDA will conduct a preliminary examination of our application. Once it decides to accept the application, the provincial level SFDA will conduct an on-site examination based upon our application and draw sample products within 5 days. The medicine inspection institute will inspect the selected samples and later submit its inspection report to the SFDA. At the same time, the provincial level SFDA will submit its opinion, examination report on our product candidates, together with application materials to the SFDA. If the SFDA is satisfied with the examination results, it will issue the new drug certificate to us.
The new drug certificate entitles us to all intellectual property rights associated with the new drug provided by the PRC law. We consider obtaining a new drug certificate for our product candidates to be a milestone, since the issuance of a certificate signals the Chinese government’s protection of our intellectually property rights for the new drug.
Production Permit
Simultaneously with the application for new drug certificate, we also apply to the provincial level SDFA for a production license to manufacture the new drug. The Production Permit is valid for a term of five years and must be renewed before its expiration. During the renewal process, we will be re-evaluated by the appropriate governmental authorities and must comply with the then prevailing standards and regulations which may change from time to time.
OT Pharmaceutical has obtained a Production Permit which is valid from January 1, 2006 to December 31, 2010.
GMP Certificate
After receiving a new medicine certificate and production permit, we will further need to submit to the SFDA an application for a Good Manufacturing Practice, or GMP. A GMP certificate is used to approve the equipment and control of the manufacturing workshop of a particular drug. The PRC government authority issue GMP standards for pharmaceutical manufacturing enterprises in order to minimize the risks involved in any pharmaceutical production that cannot be eliminated through testing the final products. The process of GMP authorization requires about 3 months.
A GMP certificate is valid for five years, except that the certificate of a newly established enterprise is only valid for one year. We should apply for renewal of our GMP certificate no later than six months prior to the date of expiration of our GMP certificate. Newly established enterprises should apply for reassessment no later than three months prior to the expiration of their GMP certificates and, if eligible, will receive a five-year GMP certificate subject to reassessment by the relevant authority.
Having obtained a valid production permit and, business license, complied with the GMP requirements of the SFDA and obtained the new drug certificate, we can then commence with the manufacturing of the new medicine. In addition, the SFDA requires up to five years’ surveillance period to monitor approved products. Within this period, other enterprises are not allowed to manufacture our pharmaceutical products.
To commence manufacturing our other pharmaceutical products which have already met the Chinese pharmaceutical standards on safety and effectiveness, we are required to obtain a pharmaceutical approval number from the SFDA.
OT Pharmaceutical’s GMP certificate was obtained on February 12, 2003, and will expire on February 11, 2008.
Regulations on protection of Chinese Herbal Medicines
The Chinese government protects the legal rights and interests of enterprises engaged in the production of Chinese herbal medicine and the promotion and development of activities relating to Chinese herbal medicine. When a company obtains such protection ( usually five years), any similar product made by other companies will be considered infringement. Currently, Fu Ning Surppository has been granted Chinese Herbal Medicine Protected Certification by SFDA.
Registration of Pharmaceutical Products
All pharmaceutical products that are produced in the PRC must bear a registered number approved by the SFDA in the PRC, with the exception of Chinese herbs and Chinese herbal medicines in soluble form. The medicine manufacturing enterprises must obtain the medicine registration number before manufacturing any medicine.
All of our nine gynecological suppositories obtained a registration number in 2002.
Other Relevant Laws and Regulations
OT Pharmaceutical’s sanitary product candidates usually undergo the following phases: technological test, toxicological test and functional test. Currently in China there are no strict regulations and rules to guide every step of the R&D of sanitary products, but the commercialization of sanitary products in China is subject to the approval of the local public health supervision and examination center.
We are subject to a variety of laws and regulations administered by Chinese governmental authorities at the national and provincial levels. We believe we are currently in compliance with PRC laws and regulations; however, we may incur significant costs to comply with these laws and regulations now or in the future. We can not assure that the existing regulatory requirements under which we currently operate will not change and that such change could not have a material adverse effect on our business and anticipated operations.
LEGAL PROCEEDINGS
We have no pending legal proceedings. From time to time, we may be involved in various claims, lawsuits, disputes with third parties, and actions involving allegations of breach of contract or product liability actions incidental to the normal business operations.
DESCRIPTION OF PROPERTY
OT Pharmaceutical owns a use right of the land covering an area of 7,900.14 square meters located at No.7, Bohai Street, Development Zone Pingfang Industrial Area, Harbin, China. The main buildings on this land plot include a 729-square meter production workshop, a 445.02-squre meter warehouse a 964.22-square meter office building and a 250.90-square meter garage. The term of the land use right is 50 years commencing June 9, 2000. We believe this property is sufficient to support OT Pharmaceutical’s future business development.
EMPLOYEES
The Company currently has 19 regular employees. We provide routine employee benefits such as pension and health insurances to these employees. In addition, OT Pharmaceutical has 60 temporary workers performing production functions. None of these employees are covered by a collective bargaining agreement and they all reside in China. We believe relations with our employees are good.
MANAGEMENT
The following table sets forth certain information regarding our directors and executive officers as of December 27, 2006. The directors will serve until the next annual meeting of the stockholders or until their successors are elected or appointed and qualified. Executive officers will serve at the board's discretion.
Name and Address | | Age | | Position |
| | | | | |
| Xueliang Qiu | | 51 | | Director, Chairman of the Board,President Chief Executive Officer and Secretary |
| | | | | |
| Feng Yang | | 45 | | Director, Chief Financial Officer |
XUELIANG QIU
Director, Chairman of the Board, President and Chief Executive Officer
Mr. Qiu has been served as the Chairman of the Board of OT Pharmaceutical since 2001. Prior to joining OT Pharmaceutical, from 1996 to 2001, Mr. Qiu was the Chief Executive Officer and the Chairman of the Board of Harbin Tiangong Enterprise Group Limited, a PRC Company. From 1985 to 1996, Mr. Qiu was an officer in charge of medicines approval and management in Health Department, Heilongjiang Province. From 1983 to 1985, Mr. Qiu was a doctor in People Hospital, Guiling County, Heilongjiang province. Mr. Qiu has almost 20 years’ experience in the pharmaceutical industry. Xueling Qiu is a graduate of Heilongjiang University of Chinese Medicine and he also has studied in the West China Center of Medical Sciences, Shanghai No. 2 Medicine School, Harbin Medicine University.
FENG YANG
Director, Chief Financial Officer
From 2005, Mr. Yang has served as Chief Financial Officer of ABG. From 1986 to 2003, he has served in various management positions including Chief Financial Officer, Director, and Vice President for six different companies. Mr. Yang currently is the director of China Association of Chief Financial Officers, Beijing District. He has almost 20 years of financial management experience.
There are no family relationships among our directors or officers.
BOARD COMPOSITION AND COMMITTEES
The board of directors is currently composed of two members. All board actions require the approval of two directors in attendance at a meeting at which a quorum is present.
We currently have no committees of Audit, Compensation, or any other committees; therefore, the board will act in the capacity of the absent committees.
There are presently no material pending legal proceeding to which a director, officer, or employee of ours is a party. There is no pending litigation or legal proceeding involving one of our directors, officers, employees or other agents as to which indemnification is being sought, and we are not aware of any pending or threatened litigation that may result in claims for indemnification by any director, officer, employee or other agent.
CODE OF ETHICS
We do not yet have a Code of Ethics. We may adopt one after we expand our business and recruit more regular employees.
DIRECTOR AND EXECUTIVE COMPENSATION
No cash compensation was paid to our director for services as director since our Company was incorporated in 2004. We have no standard arrangement pursuant to which our board of directors is compensated for their services in their capacity as directors. The board of directors may award special remuneration to any director undertaking any special services on behalf of our Company other than those services ordinarily required of a director. All authorized out-of-pocket expenses incurred by a director on our behalf will be subject to reimbursement upon our receipt of required supporting document of such expenses. No director received and/or accrued any compensation for his services as a director, including committee participation and/or special assignments.
The following table provides compensation information for the period indicated with respect to the person who served as our President for the years ended December 31, 2005 and 2004, and as of December 27, 2006.
SUMMARY COMPENSATION TABLE
| | | | | | | | | | Long Term Compensation | | | | |
| | | | | | | Annual Compensation | | | Awards | | | Payouts | | | | |
| (a) | | | (b) | | | (c) | | | (d) | | | (e) | | | (f) | | | (g) | | | (h) | | | (i) | |
| Name and Principal Position | | | Year | | | Salary ($) | | | Bonus ($) | | | Other Annual Compensation | | | Restricted Stock Awards ($) | | | Securities Underlying Options/ SARs (#) | | | LTIP Payouts ($) | | | All Other Compensation ($) | |
Soloman Lam President, Secretary, Treasurer and Director | | | 2004 | | | 0 | | | 0 | | | 0 | | | 0 | | | 0 | | | 0 | | | 0 | |
William Tay President, Secretary, Treasurer, and Director | | | 2005 | | | 0 | | | 0 | | | 0 | | | 0 | | | 0 | | | 0 | | | 0 | |
Hui Wang President, Secretary, Treasurer, Director and Chief Accounting Officer | | | 2006 | | | 0 | | | 0 | | | 0 | | | 0 | | | 0 | | | 0 | | | 0 | |
Xueliang Qiu Chairman of the Board, President, Secretary, Chief Executive Officer | | | 2006 | | | 0 | | | 0 | | | 0 | | | 0 | | | 0 | | | 0 | | | 0 | |
Our management did not spend any material time working since we had no material business. Accordingly, we did not compensate any officer of director during that time period.
RELATED TRANSACTIONS
Cooperation Agreement between OT Samoa and ABG
Our subsidiaries, ABG and OT Samoa entered into a Cooperation Agreement on January 22, 2006. Pursuant to this Agreement, ABG and OT Samoa cooperate on the manufacturing of certain gynecological diseases treatment. OT Pharmaceutical and OT Samoa shall be distributed 50% of the profits generated from the Know-how Products respectively.
STOCK OPTION GRANTS AND EXERCISES
We currently have no option, retirement, pension, or profit sharing programs for the benefit of the directors, officers or other employees, but the board of directors may recommend adoption of one or more such programs in the future.
EMPLOYMENT, SEVERANCE AND CHANGE OF CONTROL AGREEMENTS
On January 15, 2005, our subsidiary OT Pharmaceutical entered into an employment agreement with Mr. Xueliang Qiu pursuant to which Mr. Qiu was hired as the General Manager of OT Pharmaceutical. Such agreement has a three year term and is renewable upon mutual agreements between the parties. Pursuant to the agreement, Mr. Qiu's annual salary will be determined based on his performance, but shall in no event be less than approximately $10,000 (RMB80,000).
DIVIDEND POLICY
Since inception, we have not paid, nor declared, any dividends and we do not intend to declare any such dividends in the foreseeable future. Our ability to pay dividends is subject to limitations imposed by Delaware law and the laws of the PRC.
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
The following table sets forth certain information known to us with respect to the beneficial ownership of our common stock as of December 22, 2006 and (1) all persons who are known to us to be beneficial owners of five percent of more of the common stock, (2) each of our directors, and (3) all current directors and executive officers as a group.
NAME AND ADDRESS OF BENEFICIAL OWNER | | SHARES BENEFICIALLY OWNED | | PERCENTAGE OF CLASS OWNED | |
Mingshi Qiu (1) Room3, Floor2, Buidling2, No.1 Zhenxing Street, Nangang District, Harbin, China | | | 23,296,000 | | | 40 | |
Far Grand George Town, Grand Cayman, Cayman Islands | | | 23,296,000 | | | 40 | |
Hui Wang Room 712, officer Tower1, Bright China Changan Building, No.7 Jianguomennei Avenue, Dongcheng District, Beijing, China 100005 | | | 11,065,600 | | | 19 | |
Jiaxin Yang (2) 138-3 Fanrong street Nangang district,Harbin,China | | | 5,678,400 | | | 9.75 | |
| Xueliang Qiu (3) | | | 0 | | | 0 | |
| Feng Yang(3) | | | 0 | | | 0 | |
| Current directors and executive officers as a group | | | 0 | | | 0 | |
| (1) | Mingshi Qiu is the beneficiary of a trust, of which Far Grand, as the trustee of such trust, holds 40% shares of the common stock of the Company. |
| (2) | Jiaxin Yang is one of the former OT Samoa Shareholders. |
| (3) | Address of all directors and executive officers is No. 7 BohaiSanLu, Pingfang Industrial District, Economic & Technological Development Area, Harbin, Heilongjiang Province, China |
Beneficial ownership is determined in accordance with the rules of the Securities and Exchange Commission and includes voting or investment power with respect to the securities. Unless otherwise indicated by footnote, the persons named in the table have sole voting and sole investment power with respect to all shares of common stock shown as beneficially owned by them, subject to applicable community property laws. Percentage of beneficial ownership is based on 58,240,000 shares of our common stock outstanding as of December 27, 2006.
SELLING SECURITY HOLDERS
We have prepared this prospectus to allow the selling shareholders or their pledgees, donees, transferees or other successors in interest, to sell up to 23,444,000 shares of our common stock. All of the common stock offered by this prospectus is being offered by the selling shareholders for their own accounts.
The following table sets forth the names of the selling shareholders and for each selling shareholder the number of shares of common stock beneficially owned as of December 27, 2006, and the number of shares being registered. All information with respect to share ownership has been furnished by the selling shareholders. The shares being offered are being registered to permit public secondary trading of the shares and each selling shareholder may offer all or part of the shares owned for resale from time to time. A selling shareholder is under no obligation, however, to sell any shares immediately pursuant to this prospectus, nor is a selling shareholder obligated to sell all or any portion of the shares at any time. Therefore, no estimate can be given as to the number of shares of common stock that will be sold pursuant to this prospectus or the number of shares that will be owned by the selling shareholders upon termination of the offering made hereby. We will file a supplement to this prospectus to name successors to any named selling shareholders who are able to use this prospectus to resell the securities registered hereby.
Selling Stockholder | | Shares of Common Stock Owned | | Percent of Class | | Shares of Common Stock to be Registered | | Percent of Common Stock owned after Completion of Offering | |
| WILLIAM TAY * | | | 148,000 | | | 0.25 | % | | 148,000 | | | 0 | |
| ZHANG ZHAO | | | 2,070,793 | | | 3.56 | % | | 2,070,793 | | | 0 | |
| CHEN YUZHUO* | | | 46,592 | | | 0.08 | % | | 46,592 | | | 0 | |
| LI YONGXIA* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| REN BAOYI* | | | 349,440 | | | 0.60 | % | | 349,440 | | | 0 | |
| WANG WENSHENG* | | | 116,480 | | | 0.20 | % | | 116,480 | | | 0 | |
| GUO YANMING* | | | 116,480 | | | 0.20 | % | | 116,480 | | | 0 | |
| LIN HAI* | | | 116,480 | | | 0.20 | % | | 116,480 | | | 0 | |
| QIU XUELI* | | | 116,480 | | | 0.20 | % | | 116,480 | | | 0 | |
| LV SHIYING* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| FU DONGMEI* | | | 232,960 | | | 0.40 | % | | 232,960 | | | 0 | |
| CHU DONGCHANG* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| ZHENG WANFU* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| JIANG YING* | | | 1,747 | | | 0.00 | % | | 1,747 | | | 0 | |
| WU XIAOFEI* | | | 1,747 | | | 0.00 | % | | 1,747 | | | 0 | |
| YAN ZENGYOU* | | | 1,747 | | | 0.00 | % | | 1,747 | | | 0 | |
| CHANG XIAOXIA* | | | 1,747 | | | 0.00 | % | �� | 1,747 | | | 0 | |
| JIANG HUI* | | | 3,844 | | | 0.01 | % | | 3,844 | | | 0 | |
| HE AILIAN* | | | 6,989 | | | 0.01 | % | | 6,989 | | | 0 | |
| ZHOU LIXING* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| WANG ZHENGANG* | | | 1,747 | | | 0.00 | % | | 1,747 | | | 0 | |
| TANG XIN* | | | 874 | | | 0.00 | % | | 874 | | | 0 | |
| XING JICHUN* | | | 3,844 | | | 0.01 | % | | 3,844 | | | 0 | |
| JIN ZHENGHUAN* | | | 6,989 | | | 0.01 | % | | 6,989 | | | 0 | |
| GAO SHIYUAN* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| WANG GUOJIE* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| ZHANG FEISHI* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| DONG MAOSEN* | | | 2,446 | | | 0.00 | % | | 2,446 | | | 0 | |
| WANG CHUNMING* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| ZHANG XIN* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| CUI QINGXIANG* | | | 17,472 | | | 0.03 | % | | 17,472 | | | 0 | |
| JI TIEGANG* | | | 5,242 | | | 0.01 | % | | 5,242 | | | 0 | |
| JIANG ZHURONG* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| LIU LIQIN* | | | 1,747 | | | 0.00 | % | | 1,747 | | | 0 | |
| YUAN ZHIGANG* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| SONG YIJING* | | | 23,296 | | | 0.04 | % | | 23,296 | | | 0 | |
| BO JINFENG* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| LIU GUIYING* | | | 1,747 | | | 0.00 | % | | 1,747 | | | 0 | |
| MENG WEI* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| SHI KAI* | | | 12,230 | | | 0.02 | % | | 12,230 | | | 0 | |
| FENG QIZHI* | | | 116,480 | | | 0.20 | % | | 116,480 | | | 0 | |
| PANG HONGRU* | | | 20,966 | | | 0.04 | % | | 20,966 | | | 0 | |
| ZHANG MINGFENG* | | | 27,955 | | | 0.05 | % | | 27,955 | | | 0 | |
| CAO JUMEI* | | | 20,966 | | | 0.04 | % | | 20,966 | | | 0 | |
| MA YUNHUI* | | | 34,944 | | | 0.06 | % | | 34,944 | | | 0 | |
| OUYANG YUNSHOU* | | | 1,747 | | | 0.00 | % | | 1,747 | | | 0 | |
| ZHANG ZHIHUA* | | | 6,989 | | | 0.01 | % | | 6,989 | | | 0 | |
| LV JINGZHAO* | | | 2,796 | | | 0.00 | % | | 2,796 | | | 0 | |
| ZHOU WEICHENG* | | | 5,242 | | | 0.01 | % | | 5,242 | | | 0 | |
| LI JINCHANG* | | | 5,242 | | | 0.01 | % | | 5,242 | | | 0 | |
| DONG YANPING* | | | 6,989 | | | 0.01 | % | | 6,989 | | | 0 | |
| AI LIGUO* | | | 6,989 | | | 0.01 | % | | 6,989 | | | 0 | |
| ZOU DELI* | | | 20,966 | | | 0.04 | % | | 20,966 | | | 0 | |
| LIANG QIZHI* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| LI WEI* | | | 4,193 | | | 0.01 | % | | 4,193 | | | 0 | |
| SONG JICHUN* | | | 1,747 | | | 0.00 | % | | 1,747 | | | 0 | |
| WANG QINGBIN* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| JIANG BIAO* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| LI YUHUA* | | | 1,398 | | | 0.00 | % | | 1,398 | | | 0 | |
| YANG LIHUA* | | | 1,747 | | | 0.00 | % | | 1,747 | | | 0 | |
| MAN YUHUA* | | | 1,747 | | | 0.00 | % | | 1,747 | | | 0 | |
| JIANG YUBIN* | | | 34,944 | | | 0.06 | % | | 34,944 | | | 0 | |
| ZHANG JIAWEI* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| MAN YUZHEN* | | | 1,747 | | | 0.00 | % | | 1,747 | | | 0 | |
| ZHANG DONG* | | | 2,097 | | | 0.00 | % | | 2,097 | | | 0 | |
| LIU XIANFENG* | | | 6,989 | | | 0.01 | % | | 6,989 | | | 0 | |
| DONG LI* | | | 13,978 | | | 0.02 | % | | 13,978 | | | 0 | |
| FU WENYAN* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| FENG YUJIE* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| CAI YONGQIN* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| WANG QIUPING* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| CAI YONGBO* | | | 34,944 | | | 0.06 | % | | 34,944 | | | 0 | |
| WANG ZHONGBIN* | | | 1,747 | | | 0.00 | % | | 1,747 | | | 0 | |
| WANG QIUBO* | | | 6,989 | | | 0.01 | % | | 6,989 | | | 0 | |
| YU XIAOYIN* | | | 1,747 | | | 0.00 | % | | 1,747 | | | 0 | |
| SUN QINGCHENG* | | | 17,472 | | | 0.03 | % | | 17,472 | | | 0 | |
| NIU YUYING* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| ZHANG HAIYAN* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| GUO WENTAO* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| YU CHUNHONG* | | | 6,989 | | | 0.01 | % | | 6,989 | | | 0 | |
| QIN YI* | | | 10,483 | | | 0.02 | % | | 10,483 | | | 0 | |
| ZHANG SHUSEN* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| ZHAO YIMING* | | | 699 | | | 0.00 | % | | 699 | | | 0 | |
| ZHAO ZHENJIANG* | | | 1,747 | | | 0.00 | % | | 1,747 | | | 0 | |
| LV ZHONGQIU* | | | 13,978 | | | 0.02 | % | | 13,978 | | | 0 | |
| ZHAN SHUZHEN* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| YU CHUNHUI* | | | 1,048 | | | 0.00 | % | | 1,048 | | | 0 | |
| ZHAO HUANYUN* | | | 1,747 | | | 0.00 | % | | 1,747 | | | 0 | |
| HU CHUNHUI* | | | 10,483 | | | 0.02 | % | | 10,483 | | | 0 | |
| WU TIEJUN* | | | 1,048 | | | 0.00 | % | | 1,048 | | | 0 | |
| WO JINYING* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| YANG ZICHUN* | | | 1,747 | | | 0.00 | % | | 1,747 | | | 0 | |
| SUN SHUFEN* | | | 1,747 | | | 0.00 | % | | 1,747 | | | 0 | |
| ZHANG XIAOJIE* | | | 17,472 | | | 0.03 | % | | 17,472 | | | 0 | |
| YU ZHIPING* | | | 1,747 | | | 0.00 | % | | 1,747 | | | 0 | |
| LI QIN* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| LIU YUXIA* | | | 6,989 | | | 0.01 | % | | 6,989 | | | 0 | |
| LIU YUHUA* | | | 10,483 | | | 0.02 | % | | 10,483 | | | 0 | |
| LIU GUOJIANG* | | | 27,955 | | | 0.05 | % | | 27,955 | | | 0 | |
| SHAO SHUJUAN* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| HAN SHUANG* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| YUAN CUILI* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| MA SUQIN* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| HU WEIBIN* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| HUANG QIJUN* | | | 1,747 | | | 0.00 | % | | 1,747 | | | 0 | |
| YU BIN* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| DANG SUYUN* | | | 2,446 | | | 0.00 | % | | 2,446 | | | 0 | |
| MAN YUZHU* | | | 1,747 | | | 0.00 | % | | 1,747 | | | 0 | |
| CHEN ANLI* | | | 1,747 | | | 0.00 | % | | 1,747 | | | 0 | |
| HU BAOSHAN* | | | 1,747 | | | 0.00 | % | | 1,747 | | | 0 | |
| WANG GUOYUN* | | | 6,989 | | | 0.01 | % | | 6,989 | | | 0 | |
| ZHANG YICHI* | | | 1,747 | | | 0.00 | % | | 1,747 | | | 0 | |
| LI YANG* | | | 1,747 | | | 0.00 | % | | 1,747 | | | 0 | |
| XU BIN* | | | 1,747 | | | 0.00 | % | | 1,747 | | | 0 | |
| LI LIQUN* | | | 1,747 | | | 0.00 | % | | 1,747 | | | 0 | |
| SUN JINGLAN* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| ZHANG FENGCHUN* | | | 6,989 | | | 0.01 | % | | 6,989 | | | 0 | |
| TANG XU* | | | 13,978 | | | 0.02 | % | | 13,978 | | | 0 | |
| DONG JUNDONG* | | | 1,747 | | | 0.00 | % | | 1,747 | | | 0 | |
| QIN SHIWEI* | | | 699 | | | 0.00 | % | | 699 | | | 0 | |
| ZHU XIUJIE* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| WANG ZHAOXIA* | | | 1,747 | | | 0.00 | % | | 1,747 | | | 0 | |
| YU JIE* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| ZHAO WEIFENG* | | | 31,450 | | | 0.05 | % | | 31,450 | | | 0 | |
| LIU LANLING* | | | 34,944 | | | 0.06 | % | | 34,944 | | | 0 | |
| PANG SHULAN* | | | 23,296 | | | 0.04 | % | | 23,296 | | | 0 | |
| HUANG JING* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| LUO GUIZHI* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| SHI PING* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| CAO YONGQIANG* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| WANG BINGJIE* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| YANG XINSHUANG* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| WANG ZHENGKAI* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| LUAN WEI* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| YU LIQING* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| JIANG XIN* | | | 8,736 | | | 0.02 | % | | 8,736 | | | 0 | |
| JIANG YONG* | | | 8,736 | | | 0.02 | % | | 8,736 | | | 0 | |
| GUO XIUZHU* | | | 2,912 | | | 0.01 | % | | 2,912 | | | 0 | |
| ZHANG NING* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| JING CHANG* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| WANG YONGJIANG* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| KONG LINGQUAN* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| GUO YING* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| WEN GUOCAI* | | | 1,747 | | | 0.00 | % | | 1,747 | | | 0 | |
| ZHANG REN* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| WANG CHANGYI* | | | 8,387 | | | 0.01 | % | | 8,387 | | | 0 | |
| LIU GUOQIANG* | | | 1,747 | | | 0.00 | % | | 1,747 | | | 0 | |
| MU ZHIMIN* | | | 17,472 | | | 0.03 | % | | 17,472 | | | 0 | |
| YAN YONGHUA* | | | 17,472 | | | 0.03 | % | | 17,472 | | | 0 | |
| HE XU* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| LIN GANG* | | | 4,193 | | | 0.01 | % | | 4,193 | | | 0 | |
| QIAO XIUAN* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| MA GUANGRUI* | | | 1,747 | | | 0.00 | % | | 1,747 | | | 0 | |
| LU SHUMIN* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| WANG GUIHUA* | | | 6,989 | | | 0.01 | % | | 6,989 | | | 0 | |
| TANG JINZHUANG* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| ZHANG ZHIPENG* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| DU JINGHONG* | | | 1,398 | | | 0.00 | % | | 1,398 | | | 0 | |
| DU JINGFEN* | | | 2,621 | | | 0.00 | % | | 2,621 | | | 0 | |
| YANG JIANZHONG* | | | 699 | | | 0.00 | % | | 699 | | | 0 | |
| SHEN YANHUA* | | | 6,989 | | | 0.01 | % | | 6,989 | | | 0 | |
| WANG DEHUAN* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| YANG MING* | | | 12,230 | | | 0.02 | % | | 12,230 | | | 0 | |
| XIA KEJUN* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| YU GUOZHEN* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| YANG GUOZHEN* | | | 6,989 | | | 0.01 | % | | 6,989 | | | 0 | |
| LI YANJUN* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| LIU YUNYING* | | | 6,989 | | | 0.01 | % | | 6,989 | | | 0 | |
| WANG XUEQIN* | | | 699 | | | 0.00 | % | | 699 | | | 0 | |
| LIANG HAO* | | | 1,747 | | | 0.00 | % | | 1,747 | | | 0 | |
| ZHANG WENXUE* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| YUAN HONGAN* | | | 699 | | | 0.00 | % | | 699 | | | 0 | |
| YI YONGBIN* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| JIN LI* | | | 1,747 | | | 0.00 | % | | 1,747 | | | 0 | |
| WANG ZHILI* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| SUN YINLI* | | | 1,747 | | | 0.00 | % | | 1,747 | | | 0 | |
| WANG HONGKAI* | | | 1,048 | | | 0.00 | % | | 1,048 | | | 0 | |
| WANG HONGGUANG* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| HU SHUANGYIN* | | | 1,398 | | | 0.00 | % | | 1,398 | | | 0 | |
| JIAO HONGBO* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| PU JIANPING* | | | 6,989 | | | 0.01 | % | | 6,989 | | | 0 | |
| LIU ZHIYING* | | | 6,989 | | | 0.01 | % | | 6,989 | | | 0 | |
| QIAO YONGKUAN* | | | 6,989 | | | 0.01 | % | | 6,989 | | | 0 | |
| LANG YAN* | | | 1,747 | | | 0.00 | % | | 1,747 | | | 0 | |
| XIA MAOZHUANG* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| CAI YUNGENG* | | | 699 | | | 0.00 | % | | 699 | | | 0 | |
| WU KAI* | | | 6,989 | | | 0.01 | % | | 6,989 | | | 0 | |
| ZHANG JUN* | | | 6,989 | | | 0.01 | % | | 6,989 | | | 0 | |
| SUN HONGMEI* | | | 1,747 | | | 0.00 | % | | 1,747 | | | 0 | |
| ZHOU YAKUN* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| WANG QIULI* | | | 1,747 | | | 0.00 | % | | 1,747 | | | 0 | |
| GUO CHUNXIA* | | | 2,446 | | | 0.00 | % | | 2,446 | | | 0 | |
| MENG FANGUANG* | | | 6,989 | | | 0.01 | % | | 6,989 | | | 0 | |
| YUAN XIURONG* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| YAN HUILING* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| ZHANG GUIZHI* | | | 1,048 | | | 0.00 | % | | 1,048 | | | 0 | |
| ZHANG LIBO* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| SUN HUIJUN* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| YI FUGUI* | | | 1,398 | | | 0.00 | % | | 1,398 | | | 0 | |
| LI YANJUN* | | | 6,989 | | | 0.01 | % | | 6,989 | | | 0 | |
| WANG MANLING* | | | 1,048 | | | 0.00 | % | | 1,048 | | | 0 | |
| LI YANMIN* | | | 1,048 | | | 0.00 | % | | 1,048 | | | 0 | |
| FENG XIAO* | | | 19,219 | | | 0.03 | % | | 19,219 | | | 0 | |
| TANG XIANCHENG* | | | 10,483 | | | 0.02 | % | | 10,483 | | | 0 | |
| LI AIHUA* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| WU BAIJIE* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| WU SHUHONG* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| WU CHANGLAN* | | | 3,844 | | | 0.01 | % | | 3,844 | | | 0 | |
| SI GUOZHEN* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| WANG BO* | | | 1,165 | | | 0.00 | % | | 1,165 | | | 0 | |
| SONG GUANGXIN* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| ZHANG JIANKUN* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| LIU LIHUA* | | | 1,747 | | | 0.00 | % | | 1,747 | | | 0 | |
| JIANG WEI* | | | 699 | | | 0.00 | % | | 699 | | | 0 | |
| ZHAO HONGMEI* | | | 699 | | | 0.00 | % | | 699 | | | 0 | |
| MA ZHAOPING* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| SHEN WEI* | | | 699 | | | 0.00 | % | | 699 | | | 0 | |
| CHI TIEZHONG* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| JING LIYING* | | | 1,747 | | | 0.00 | % | | 1,747 | | | 0 | |
| YIN WEIYING* | | | 1,747 | | | 0.00 | % | | 1,747 | | | 0 | |
| WANG LIANJIA* | | | 1,747 | | | 0.00 | % | | 1,747 | | | 0 | |
| WANG YONGMING* | | | 1,747 | | | 0.00 | % | | 1,747 | | | 0 | |
| ZHANG SUOHUA* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| LV BAOLIN* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| PEI XIAOYING* | | | 45,427 | | | 0.08 | % | | 45,427 | | | 0 | |
| ZHAI LIJUN* | | | 1,747 | | | 0.00 | % | | 1,747 | | | 0 | |
| BAI HUA* | | | 1,747 | | | 0.00 | % | | 1,747 | | | 0 | |
| YAN CHUNMEI* | | | 1,747 | | | 0.00 | % | | 1,747 | | | 0 | |
| LUO LI* | | | 6,989 | | | 0.01 | % | | 6,989 | | | 0 | |
| ZHANG FENGWEI* | | | 6,989 | | | 0.01 | % | | 6,989 | | | 0 | |
| YUE BAIYAN* | | | 1,747 | | | 0.00 | % | | 1,747 | | | 0 | |
| ZHANG GUIJUN* | | | 1,747 | | | 0.00 | % | | 1,747 | | | 0 | |
| DONG ZENGTIAN* | | | 349 | | | 0.00 | % | | 349 | | | 0 | |
| QIU FENGYUN* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| CHENG YAO* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| SHI YUHUAN* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| JIAO GUANGWEI* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| ZHU CHUNDI* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| ZHANG YADAN* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| YIN JIRONG* | | | 4,659 | | | 0.01 | % | | 4,659 | | | 0 | |
| LIU CUILIAN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| ZHANG YEJIAN* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| HAN DONGJUN* | | | 4,193 | | | 0.01 | % | | 4,193 | | | 0 | |
| YANG YUYING* | | | 13,978 | | | 0.02 | % | | 13,978 | | | 0 | |
| LIU YONGMEI* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| QU HONGWEI* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| ZENG XIANMIN* | | | 699 | | | 0.00 | % | | 699 | | | 0 | |
| LI JIA* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| WANG XIAOFANG* | | | 1,747 | | | 0.00 | % | | 1,747 | | | 0 | |
| PAN LIJUN* | | | 58,240 | | | 0.10 | % | | 58,240 | | | 0 | |
| LANG SHULAN* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| ZHAO ADAI* | | | 1,747 | | | 0.00 | % | | 1,747 | | | 0 | |
| CHEN YUANYUAN* | | | 9,318 | | | 0.02 | % | | 9,318 | | | 0 | |
| YIN FAN* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| ZHANG YANG* | | | 1,747 | | | 0.00 | % | | 1,747 | | | 0 | |
| ZHANG YUHAI* | | | 1,747 | | | 0.00 | % | | 1,747 | | | 0 | |
| WANG XIUZHEN* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| FENG YANHUA* | | | 1,747 | | | 0.00 | % | | 1,747 | | | 0 | |
| LIU YANQIU* | | | 1,747 | | | 0.00 | % | | 1,747 | | | 0 | |
| WANG HAOLIANG* | | | 1,747 | | | 0.00 | % | | 1,747 | | | 0 | |
| AN FAN* | | | 13,978 | | | 0.02 | % | | 13,978 | | | 0 | |
| YAN TIEHONG* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| GUO WEIZHONG* | | | 699 | | | 0.00 | % | | 699 | | | 0 | |
| CHEN JIANFENG* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| ZHOU DIANCAI* | | | 10,483 | | | 0.02 | % | | 10,483 | | | 0 | |
| WANG HANSHAN* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| LI WENMAO* | | | 1,398 | | | 0.00 | % | | 1,398 | | | 0 | |
| QIU FENGQIN* | | | 18,637 | | | 0.03 | % | | 18,637 | | | 0 | |
| CHENG ZHIHUA* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| WANG XIAOXIA * | | | 699 | | | 0.00 | % | | 699 | | | 0 | |
| XIAO LIMIN* | | | 1,048 | | | 0.00 | % | | 1,048 | | | 0 | |
| ZHANG YONGJUN* | | | 1,747 | | | 0.00 | % | | 1,747 | | | 0 | |
| WANG XIUYAN* | | | 23,296 | | | 0.04 | % | | 23,296 | | | 0 | |
| LI JING * | | | 1,398 | | | 0.00 | % | | 1,398 | | | 0 | |
| LI LI* | | | 699 | | | 0.00 | % | | 699 | | | 0 | |
| FU XIUWEN* | | | 1,747 | | | 0.00 | % | | 1,747 | | | 0 | |
| ZHANG JINGJING* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| ZHENG XIUZHEN* | | | 23,296 | | | 0.04 | % | | 23,296 | | | 0 | |
| XIU JIAN* | | | 1,398 | | | 0.00 | % | | 1,398 | | | 0 | |
| DONG NAIRU* | | | 349 | | | 0.00 | % | | 349 | | | 0 | |
| DONG NAIGUI* | | | 699 | | | 0.00 | % | | 699 | | | 0 | |
| DONG NAISEN* | | | 349 | | | 0.00 | % | | 349 | | | 0 | |
| WEN CHANGSHENG* | | | 1,747 | | | 0.00 | % | | 1,747 | | | 0 | |
| WEN XIURU* | | | 1,747 | | | 0.00 | % | | 1,747 | | | 0 | |
| LIU ZUJIE* | | | 6,989 | | | 0.01 | % | | 6,989 | | | 0 | |
| XU XIAOYAN* | | | 349 | | | 0.00 | % | | 349 | | | 0 | |
| WANG WEI* | | | 6,989 | | | 0.01 | % | | 6,989 | | | 0 | |
| MING YANG* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| WANG LILI* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| CHUAI XIUQIN* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| LI MAOWEN* | | | 2,097 | | | 0.00 | % | | 2,097 | | | 0 | |
| WANG PING* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| CHEN YUJIE* | | | 46,592 | | | 0.08 | % | | 46,592 | | | 0 | |
| TAN YUSEN* | | | 12,813 | | | 0.02 | % | | 12,813 | | | 0 | |
| LI XUAN* | | | 23,296 | | | 0.04 | % | | 23,296 | | | 0 | |
| WANG MINGGUANG* | | | 34,944 | | | 0.06 | % | | 34,944 | | | 0 | |
| CUI CHENGMING* | | | 34,944 | | | 0.06 | % | | 34,944 | | | 0 | |
| WANG AIRONG* | | | 34,944 | | | 0.06 | % | | 34,944 | | | 0 | |
| CAO WEI* | | | 23,296 | | | 0.04 | % | | 23,296 | | | 0 | |
| SUN BIN* | | | 10,483 | | | 0.02 | % | | 10,483 | | | 0 | |
| SUN XIAOLAN* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| SUN YING* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| YANG JIE* | | | 6,989 | | | 0.01 | % | | 6,989 | | | 0 | |
| WU NAISHI* | | | 1,048 | | | 0.00 | % | | 1,048 | | | 0 | |
| ZHANG XIAOJU* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| SUN ZUWU* | | | 1,747 | | | 0.00 | % | | 1,747 | | | 0 | |
| MA LIRU* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| XU SHUWEN* | | | 1,165 | | | 0.00 | % | | 1,165 | | | 0 | |
| LIU YING * | | | 17,472 | | | 0.03 | % | | 17,472 | | | 0 | |
| QU SHIMING* | | | 10,483 | | | 0.02 | % | | 10,483 | | | 0 | |
| YE HONG* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| CHAI LINA* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| SUN YING* | | | 4,659 | | | 0.01 | % | | 4,659 | | | 0 | |
| GAO SONGSHAN* | | | 23,296 | | | 0.04 | % | | 23,296 | | | 0 | |
| FU DONGSHENG* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| WEI XINGANG* | | | 1,165 | | | 0.00 | % | | 1,165 | | | 0 | |
| XU YANLU* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| XU LEI* | | | 17,472 | | | 0.03 | % | | 17,472 | | | 0 | |
| HAO QIAN* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| JIANG ZHUQIAN* | | | 10,483 | | | 0.02 | % | | 10,483 | | | 0 | |
| FU FENG* | | | 5,242 | | | 0.01 | % | | 5,242 | | | 0 | |
| WANG SHIYING* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| ZHANG WEIJUN* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| YANG LI* | | | 23,296 | | | 0.04 | % | | 23,296 | | | 0 | |
| WANG LIPENG* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| SHU JIE* | | | 34,944 | | | 0.06 | % | | 34,944 | | | 0 | |
| GUAN JIAOLIN* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| WANG SHIJIE* | | | 93,184 | | | 0.16 | % | | 93,184 | | | 0 | |
| LI XIAOBO* | | | 93,184 | | | 0.16 | % | | 93,184 | | | 0 | |
| LI ZHI* | | | 93,184 | | | 0.16 | % | | 93,184 | | | 0 | |
| SONG YAJUAN* | | | 93,184 | | | 0.16 | % | | 93,184 | | | 0 | |
| BAI YUJIE* | | | 93,184 | | | 0.16 | % | | 93,184 | | | 0 | |
| LIU XIUWEN* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| LIU ZHENGRONG* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| WANG YIQIU* | | | 13,395 | | | 0.02 | % | | 13,395 | | | 0 | |
| ZHONG JIGE* | | | 6,989 | | | 0.01 | % | | 6,989 | | | 0 | |
| JIANG WEI* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| GUO XUEMEI | | | 1,630,720 | | | 2.80 | % | | 1,630,720 | | | 0 | |
| ZHANG XINDONG* | | | 874 | | | 0.00 | % | | 874 | | | 0 | |
| YU RUI* | | | 1,747 | | | 0.00 | % | | 1,747 | | | 0 | |
| LI HUI* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| LIU CHENGMING* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| LI HEQING* | | | 23,296 | | | 0.04 | % | | 23,296 | | | 0 | |
| YU SHUKUN* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| LI JIHUA* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| XU SHAN* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| LIU HEXIANG* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| QU XIN* | | | 17,472 | | | 0.03 | % | | 17,472 | | | 0 | |
| QU YAZHUO* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| LIU RONGBIN* | | | 34,944 | | | 0.06 | % | | 34,944 | | | 0 | |
| ZHAO HUANXIONG* | | | 8,154 | | | 0.01 | % | | 8,154 | | | 0 | |
| JIANG SHUMEI* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| CHI LIHUA* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| ZHAO KUAN* | | | 8,154 | | | 0.01 | % | | 8,154 | | | 0 | |
| GUO YULAN* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| REN FENGQIN* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| YU YING* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| SHAO RUIXIA* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| ZHANG GUIFEN* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| LIU SHAOHUA* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| WANG XIUWEN* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| ZHUANG SHUXIAN* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| HAN LI* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| LIAO YAN* | | | 8,154 | | | 0.01 | % | | 8,154 | | | 0 | |
| LI GUIFEN* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| SUN JINGLAN* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| ZHANG JING* | | | 23,296 | | | 0.04 | % | | 23,296 | | | 0 | |
| XING GUIMEI* | | | 25,626 | | | 0.04 | % | | 25,626 | | | 0 | |
| YANG JUNWEN* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| XING QISHENG* | | | 23,296 | | | 0.04 | % | | 23,296 | | | 0 | |
| HOU YAN* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| LIU SU* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| MA YUEHUA* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| LI ZHEN* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| GAO JIANHONG* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| XIONG JIAJI* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| XIONG DEQIU* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| TAN BIGU* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| JIA WENHUAN* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| DUAN WENQIN* | | | 23,296 | | | 0.04 | % | | 23,296 | | | 0 | |
| LI LI* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| TAO PING* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| ZHANG SHUMING* | | | 27,175 | | | 0.05 | % | | 27,175 | | | 0 | |
| YU DEBO* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| LIU YUXIU* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| ZHENG HUAXIN* | | | 3,879 | | | 0.01 | % | | 3,879 | | | 0 | |
| ZHOU RUNJIAN* | | | 34,944 | | | 0.06 | % | | 34,944 | | | 0 | |
| TENG YANWEI* | | | 34,944 | | | 0.06 | % | | 34,944 | | | 0 | |
| GUO FENGHUA* | | | 145,600 | | | 0.25 | % | | 145,600 | | | 0 | |
| CHEN XI* | | | 104,832 | | | 0.18 | % | | 104,832 | | | 0 | |
| ZHAO XUEZHEN* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| WANG GUIYING* | | | 5,358 | | | 0.01 | % | | 5,358 | | | 0 | |
| TENG JIE* | | | 18,637 | | | 0.03 | % | | 18,637 | | | 0 | |
| PIAO YINSHU* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| SONG ZHAOLIAN* | | | 58,240 | | | 0.10 | % | | 58,240 | | | 0 | |
| WO JING* | | | 15,725 | | | 0.03 | % | | 15,725 | | | 0 | |
| LAN LI* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| YAN SHUMEI* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| YOU YUHUI* | | | 3,879 | | | 0.01 | % | | 3,879 | | | 0 | |
| LIU XINGZHENG* | | | 3,879 | | | 0.01 | % | | 3,879 | | | 0 | |
| ZHANG CHUNGUO* | | | 21,665 | | | 0.04 | % | | 21,665 | | | 0 | |
| YANG CHUNJI* | | | 34,944 | | | 0.06 | % | | 34,944 | | | 0 | |
| WANG HAN* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| YANG ZHINAN* | | | 76,877 | | | 0.13 | % | | 76,877 | | | 0 | |
| XU HONGZHI* | | | 9,318 | | | 0.02 | % | | 9,318 | | | 0 | |
| WANG XIUXIA* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| XU JIN* | | | 23,296 | | | 0.04 | % | | 23,296 | | | 0 | |
| LI TIANYUAN* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| LI ZHIFANG* | | | 7,688 | | | 0.01 | % | | 7,688 | | | 0 | |
| LIU CHUNMEI* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| SHAO YINGSHI* | | | 10,483 | | | 0.02 | % | | 10,483 | | | 0 | |
| QIAO QINGXIA* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| WANG WENJUN* | | | 4,659 | | | 0.01 | % | | 4,659 | | | 0 | |
| SUN LIHUA* | | | 4,659 | | | 0.01 | % | | 4,659 | | | 0 | |
| JIN YONGZHE* | | | 4,659 | | | 0.01 | % | | 4,659 | | | 0 | |
| BAI YUN* | | | 23,296 | | | 0.04 | % | | 23,296 | | | 0 | |
| LI CHENGXIE* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| JIN XIUMEI* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| YOU SHURONG* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| SUN XIAORUI* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| GAO LIWEI* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| GAO PING* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| KONG LINGQIN* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| LIU CHANGHUI* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| JING DEWEN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| GONG BING* | | | 4,077 | | | 0.01 | % | | 4,077 | | | 0 | |
| ZHANG XIUHUA* | | | 9,784 | | | 0.02 | % | | 9,784 | | | 0 | |
| GE LI* | | | 8,154 | | | 0.01 | % | | 8,154 | | | 0 | |
| MA SHUFANG* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| SHI LEI* | | | 15,142 | | | 0.03 | % | | 15,142 | | | 0 | |
| JIANG YURONG* | | | 6,989 | | | 0.01 | % | | 6,989 | | | 0 | |
| ZHAO YISHENG* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| YIN BINGJIE* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| LIU HANLIANG* | | | 6,989 | | | 0.01 | % | | 6,989 | | | 0 | |
| YIN CHUANFENG* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| JIANG JING* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| HAN XIAOMING* | | | 23,296 | | | 0.04 | % | | 23,296 | | | 0 | |
| YU CAIXIA* | | | 23,296 | | | 0.04 | % | | 23,296 | | | 0 | |
| GE YANBIN* | | | 8,154 | | | 0.01 | % | | 8,154 | | | 0 | |
| MA LIPING* | | | 23,296 | | | 0.04 | % | | 23,296 | | | 0 | |
| CAO QINGZHEN* | | | 9,901 | | | 0.02 | % | | 9,901 | | | 0 | |
| SONG PING* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| YAN FENGMING* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| CHE LANPING* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| JIANG ZERONG* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| JIN YING* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| LIU MEIJUN* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| FANG GUIFENG* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| LIU CHANGGUO* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| WANG ZHIGUANG* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| FAN BO* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| LI FUJUN* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| HUANG XIANGYUE* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| WEN SHUQI* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| XU HUILAN* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| JIA QINGBIN* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| GUAN XIUFANG* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| ZOU JINGWEN* | | | 34,944 | | | 0.06 | % | | 34,944 | | | 0 | |
| YANG LEI* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| GAO MENG* | | | 8,154 | | | 0.01 | % | | 8,154 | | | 0 | |
| MIU DAN* | | | 8,154 | | | 0.01 | % | | 8,154 | | | 0 | |
| XU ZHI* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| LU HONGYI* | | | 2,912 | | | 0.01 | % | | 2,912 | | | 0 | |
| LI SHUHUA* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| GAO LIMING* | | | 2,912 | | | 0.01 | % | | 2,912 | | | 0 | |
| ZHANG GUIYU* | | | 3,879 | | | 0.01 | % | | 3,879 | | | 0 | |
| ZHAO CHUNJING* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| ZHOU LIANYUAN* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| HUANG YANG* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| LIU DEXIN* | | | 1,165 | | | 0.00 | % | | 1,165 | | | 0 | |
| HE QINGFU* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| CHEN YAN* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| LI GUOQING* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| SUN LIQIN* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| LV JIANHUA* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| YIN LANYUE* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| LIN XI* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| ZHANG JING* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| LIU TENGFEI* | | | 55,910 | | | 0.10 | % | | 55,910 | | | 0 | |
| LIU TENGYUE* | | | 32,614 | | | 0.06 | % | | 32,614 | | | 0 | |
| FENG SHURONG* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| ZHAO GUIZHI* | | | 2,329 | | | 0.00 | % | | 2,329 | | | 0 | |
| WANG CHUNXIANG* | | | 4,659 | | | 0.01 | % | | 4,659 | | | 0 | |
| LV NING* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| FENG XUEDONG* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| ZHANG YAN* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| SHAN BINMEI* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| ZHOU AIYING* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| ZHANG WEI* | | | 9,120 | | | 0.02 | % | | 9,120 | | | 0 | |
| ZHANG WEI* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| GAO SHUJIE* | | | 35,410 | | | 0.06 | % | | 35,410 | | | 0 | |
| FU HONG* | | | 21,351 | | | 0.04 | % | | 21,351 | | | 0 | |
| CAI SHUXIA* | | | 6,208 | | | 0.01 | % | | 6,208 | | | 0 | |
| HAO FENGXIA* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| HE LIHUA* | | | 46,592 | | | 0.08 | % | | 46,592 | | | 0 | |
| JIN MEILIAN* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| LIU SHUYUN* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| ZHENG XIUFANG* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| CHEN YULAN* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| HE MINGLI* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| GONG JIANBING* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| LIU GUOMIN* | | | 3,879 | | | 0.01 | % | | 3,879 | | | 0 | |
| LI BAOZHEN* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| ZHANG JIEMIN* | | | 4,659 | | | 0.01 | % | | 4,659 | | | 0 | |
| ZHAO RENSHU* | | | 8,154 | | | 0.01 | % | | 8,154 | | | 0 | |
| GU YONGLU* | | | 3,879 | | | 0.01 | % | | 3,879 | | | 0 | |
| CHENG MINGYAO* | | | 7,956 | | | 0.01 | % | | 7,956 | | | 0 | |
| HUANG SHIXING* | | | 4,659 | | | 0.01 | % | | 4,659 | | | 0 | |
| LI YANYAN* | | | 4,659 | | | 0.01 | % | | 4,659 | | | 0 | |
| LI HUIXIN* | | | 12,813 | | | 0.02 | % | | 12,813 | | | 0 | |
| HUANG XIULI* | | | 3,879 | | | 0.01 | % | | 3,879 | | | 0 | |
| LENG YE* | | | 4,659 | | | 0.01 | % | | 4,659 | | | 0 | |
| HUANG XIJIANG* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| HAN QINGLI* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| WANG YINGLIAN* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| HAN JIGAO* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| LU CHUNHUA* | | | 4,659 | | | 0.01 | % | | 4,659 | | | 0 | |
| LV YANAN* | | | 4,659 | | | 0.01 | % | | 4,659 | | | 0 | |
| LI XIAOPING* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| FENG SHANGYU* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| WANG PENG* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| PIAO YINGAI* | | | 15,142 | | | 0.03 | % | | 15,142 | | | 0 | |
| LIU JUNYING* | | | 6,756 | | | 0.01 | % | | 6,756 | | | 0 | |
| JIN ZHEXIONG* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| ZHU GUANGLU* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| ZHANG YANQIU* | | | 5,940 | | | 0.01 | % | | 5,940 | | | 0 | |
| YANG XIUHUA* | | | 4,659 | | | 0.01 | % | | 4,659 | | | 0 | |
| LI YANHUA* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| JU SUJIE* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| SHAO SHAN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| NI HAO* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| LIU YUMEI* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| LIU CHANGQING* | | | 23,296 | | | 0.04 | % | | 23,296 | | | 0 | |
| ZHAO LIFENG* | | | 4,077 | | | 0.01 | % | | 4,077 | | | 0 | |
| LI LEI* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| LI YONG* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| WANG YINGJIE* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| JU SUYING* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| JING HONGLIAN* | | | 1,165 | | | 0.00 | % | | 1,165 | | | 0 | |
| CHEN GUANGCHUN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| ZHU JINGRONG* | | | 4,077 | | | 0.01 | % | | 4,077 | | | 0 | |
| FAN LIMING* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| JIANG JINLIAN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| XIA GUILING* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| CHEN JINGMING* | | | 23,296 | | | 0.04 | % | | 23,296 | | | 0 | |
| LI NING* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| LI SHUYUN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| SHI DANLI* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| SHI SAIYU* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| ZHENG SAIMIN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| XIA MINQIONG* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| DING YI* | | | 4,659 | | | 0.01 | % | | 4,659 | | | 0 | |
| ZHONG ZHENCHENG* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| YAN JIANPING* | | | 1,165 | | | 0.00 | % | | 1,165 | | | 0 | |
| ZHANG SHAOMING* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| GU YOULI* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| ZHAO YONG* | | | 3,879 | | | 0.01 | % | | 3,879 | | | 0 | |
| MING ZHAOHUAN* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| LI QING* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| GUO JING* | | | 2,329 | | | 0.00 | % | | 2,329 | | | 0 | |
| ZHANG YU* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| SHEN WEIWEN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| WEN JING* | | | 1,165 | | | 0.00 | % | | 1,165 | | | 0 | |
| KE HAIPING* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| ZHANG RONGGUI* | | | 1,165 | | | 0.00 | % | | 1,165 | | | 0 | |
| CHEN CHU* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| YE XINKANG* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| LIU MEIJUN* | | | 13,395 | | | 0.02 | % | | 13,395 | | | 0 | |
| ZHENG YILIN* | | | 1,164 | | | 0.00 | % | | 1,164 | | | 0 | |
| WU CUIRONG* | | | 1,165 | | | 0.00 | % | | 1,165 | | | 0 | |
| SHI ZHENYAN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| NI ZHENGLIN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| YAN XIAOJUN* | | | 10,483 | | | 0.02 | % | | 10,483 | | | 0 | |
| YU PING* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| FAN HONGMING* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| YANG JINMING* | | | 4,659 | | | 0.01 | % | | 4,659 | | | 0 | |
| ZHANG SHIYUN * | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| WANG XIN* | | | 4,659 | | | 0.01 | % | | 4,659 | | | 0 | |
| XU KENING* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| DAI QIUQIN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| WANG XINTIAN* | | | 12,813 | | | 0.02 | % | | 12,813 | | | 0 | |
| XIE ZHAOSHUN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| WU YING* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| CHEN XIAOYING* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| LIU XIAOBO* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| LIU ENFU* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| WANG QIAOLING* | | | 6,989 | | | 0.01 | % | | 6,989 | | | 0 | |
| ZU LILI* | | | 6,989 | | | 0.01 | % | | 6,989 | | | 0 | |
| HU MUFAN* | | | 6,989 | | | 0.01 | % | | 6,989 | | | 0 | |
| WANG QINGLING* | | | 4,659 | | | 0.01 | % | | 4,659 | | | 0 | |
| WANG HUILING* | | | 10,483 | | | 0.02 | % | | 10,483 | | | 0 | |
| TANG MING* | | | 8,154 | | | 0.01 | % | | 8,154 | | | 0 | |
| ZHU LEI* | | | 4,659 | | | 0.01 | % | | 4,659 | | | 0 | |
| XU JING* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| HU XIAOYAN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| CHEN YUJUAN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| CAO YU* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| ZHANG LIPING* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| YANG XUEZHONG* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| CAI JIANPING* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| GAO MINGYU* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| ZHAO GUANGHAI* | | | 2,329 | | | 0.00 | % | | 2,329 | | | 0 | |
| ZHANG YONGKANG* | | | 8,154 | | | 0.01 | % | | 8,154 | | | 0 | |
| LIU CONGJUN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| YUAN XINYU* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| FENG XIAOWEN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| FANG JIAQIANG* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| PU YONGCHUN* | | | 4,659 | | | 0.01 | % | | 4,659 | | | 0 | |
| ZHANG FENG* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| GU MIN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| LI YUANFU* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| WU GUIZHEN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| WU QINGZHI* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| LI GUOXI* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| QIU DAOYUAN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| LIU BAODONG* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| XU GUOTAI* | | | 4,659 | | | 0.01 | % | | 4,659 | | | 0 | |
| HUANG XIAOFEI* | | | 2,329 | | | 0.00 | % | | 2,329 | | | 0 | |
| WANG FEI* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| HUANG YUFANG* | | | 2,329 | | | 0.00 | % | | 2,329 | | | 0 | |
| LI LI* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| WANG WEIDONG* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| REN TIANYOU* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| HU HAILING* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| WEI FENGLAN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| ZHAO XIANG* | | | 2,329 | | | 0.00 | % | | 2,329 | | | 0 | |
| JIN YUJUAN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| YAN GUIFANG* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| LIU SUN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| GU ZHENGRONG* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| ZHANG LIHUA* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| LIN JIANJUN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| YU LIQI* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| ZHANG LIANG* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| HUANG YI* | | | 4,659 | | | 0.01 | % | | 4,659 | | | 0 | |
| WU RUIJUAN * | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| YE ZAIMIN* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| ZHANG TENGFEI* | | | 4,659 | | | 0.01 | % | | 4,659 | | | 0 | |
| XU SHISHENG* | | | 8,154 | | | 0.01 | % | | 8,154 | | | 0 | |
| CHEN ZONGLIN* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| CAI LIPING* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| ZHANG HULAN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| ZHANG GUANGYUN* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| LIN TIANYIN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| SONG GANG* | | | 4,659 | | | 0.01 | % | | 4,659 | | | 0 | |
| CHEN SHUANGPING* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| WANG TIANGANG* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| XIE WEIJUN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| LIU SHAOHUA* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| ZHU XIUHUA* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| GAO MINXIU* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| FAN JICHUN* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| ZHAO JING* | | | 2,329 | | | 0.00 | % | | 2,329 | | | 0 | |
| SHEN QIANG* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| QU CHEN* | | | 12,813 | | | 0.02 | % | | 12,813 | | | 0 | |
| LU JIANHUI* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| YU SHENG* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| DONG CHENGCHENG* | | | 4,659 | | | 0.01 | % | | 4,659 | | | 0 | |
| DU YONGNING* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| WANG FENGJUN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| XIA JUN* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| GAO HONG* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| MENG XIANTAO* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| XING XIUZHEN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| SONG LILI* | | | 4,659 | | | 0.01 | % | | 4,659 | | | 0 | |
| SONG YUANYUAN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| YU HUIZHOU* | | | 4,659 | | | 0.01 | % | | 4,659 | | | 0 | |
| REN YUHUA * | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| HU JIALIN* | | | 2,329 | | | 0.00 | % | | 2,329 | | | 0 | |
| LUO FUYI* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| ZHANG JINFENG* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| ZHOU YALI* | | | 2,329 | | | 0.00 | % | | 2,329 | | | 0 | |
| ZANG LINYAN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| LI FENGLIN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| SUN RUIZE* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| LIU YANQIN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| ZHANG SHUMEI* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| ZHANG WENCHAO* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| SUN PENGYUN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| CHEN YANHONG* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| YU WEIHUA * | | | 4,659 | | | 0.01 | % | | 4,659 | | | 0 | |
| XU SHILIANG* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| LIN LIMIN* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| CAO YIFENG* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| LIU AIFANG* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| CHEN WEI* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| XU YING* | | | 4,659 | | | 0.01 | % | | 4,659 | | | 0 | |
| YUAN HONG* | | | 6,057 | | | 0.01 | % | | 6,057 | | | 0 | |
| FU LIN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| ZHANG JINSONG* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| LI YANG* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| LI LIHUA* | | | 18,637 | | | 0.03 | % | | 18,637 | | | 0 | |
| JIN DONGBIN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| CAI LIHUA* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| HUANG GUOPING* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| LIU BO* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| HU LIPING* | | | 2,329 | | | 0.00 | % | | 2,329 | | | 0 | |
| CUI JIANJUN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| WANG PINGJI* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| ZHAN YANHUA* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| ZHANG FUTIAN* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| LIN WEI* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| HUANG YONGDING* | | | 9,318 | | | 0.02 | % | | 9,318 | | | 0 | |
| BAI YUQING* | | | 4,659 | | | 0.01 | % | | 4,659 | | | 0 | |
| LIU MIN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| ZHU QIAOYING* | | | 2,329 | | | 0.00 | % | | 2,329 | | | 0 | |
| WU JIAFENG* | | | 8,154 | | | 0.01 | % | | 8,154 | | | 0 | |
| CAO SHIRONG* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| YANG JINPING* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| GUO CUIFENG* | | | 2,329 | | | 0.00 | % | | 2,329 | | | 0 | |
| SONG CONGCONG* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| LIU ZHONGTIAN* | | | 4,659 | | | 0.01 | % | | 4,659 | | | 0 | |
| KONG LINGYAN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| DAI HONGTAO* | | | 1,164 | | | 0.00 | % | | 1,164 | | | 0 | |
| GAO ZHANCHUN* | | | 1,164 | | | 0.00 | % | | 1,164 | | | 0 | |
| ZHANG AIXIN* | | | 2,329 | | | 0.00 | % | | 2,329 | | | 0 | |
| KONG LINGWU* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| SHENG ZIQIN* | | | 1,165 | | | 0.00 | % | | 1,165 | | | 0 | |
| XIANG MINGYUE* | | | 1,165 | | | 0.00 | % | | 1,165 | | | 0 | |
| SUN WEI* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| WU CHENGLIN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| GAO XIANCAI* | | | 1,164 | | | 0.00 | % | | 1,164 | | | 0 | |
| ZHANG GUOXIANG* | | | 2,329 | | | 0.00 | % | | 2,329 | | | 0 | |
| XIANG LIANG* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| WANG JIAN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| ZHANG SHOUHAI* | | | 2,329 | | | 0.00 | % | | 2,329 | | | 0 | |
| XIE XIAOJUN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| LIU JIAN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| XU BINGGUI* | | | 4,659 | | | 0.01 | % | | 4,659 | | | 0 | |
| ZHAO HONGFANG* | | | 4,659 | | | 0.01 | % | | 4,659 | | | 0 | |
| LIU JIANHE* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| KUANG XIUYAN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| WU CHANGMING* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| MA JIANMEI* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| JIAO BO* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| DONG FENG* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| JIANG RUIYIN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| FU HUI* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| TIAN CHANGBO* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| YANG HONGWEI* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| LI ZHAOXIN* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| ZHANG ZHAO* | | | 4,659 | | | 0.01 | % | | 4,659 | | | 0 | |
| ZHANG JUN* | | | 2,329 | | | 0.00 | % | | 2,329 | | | 0 | |
| YUE JIANSHENG* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| YANG PING* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| CHEN BO* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| LU ZHENWEI * | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| GAO CHENGUANG* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| LU JINGXIA* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| CHEN JIANYUN* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| GE JIAQING* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| WANG FENGYUAN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| YIN XUDONG* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| LIU GUIPING* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| LI JIE* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| MAO JIANFANG* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| SUN JIE* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| CHEN SUPING* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| GU YI* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| LV DAOMING* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| YANG JIE* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| HAN WANG* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| CAI ZHENGHONG* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| CAI FAYING* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| YAN SHULONG* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| WANG CHUNYING* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| LIU ZHENYU* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| XIE FENGZU* | | | 10,483 | | | 0.02 | % | | 10,483 | | | 0 | |
| WANG DECHEN* | | | 12,230 | | | 0.02 | % | | 12,230 | | | 0 | |
| TANG FENG* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| ZHU XINGUO* | | | 2,329 | | | 0.00 | % | | 2,329 | | | 0 | |
| ZHOU XIAOLING* | | | 2,329 | | | 0.00 | % | | 2,329 | | | 0 | |
| ZHU JUN* | | | 2,329 | | | 0.00 | % | | 2,329 | | | 0 | |
| XIE PING* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| ZHOU QIUFANG* | | | 19,406 | | | 0.03 | % | | 19,406 | | | 0 | |
| LI XIUHUA* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| WANG PING* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| QIN SHUGUANG* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| WANG ZHEN* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| GE XIA* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| WANG XUEMEI* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| DING JUAN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| XIAO JIETING* | | | 1,165 | | | 0.00 | % | | 1,165 | | | 0 | |
| LIU QINGDUO* | | | 1,165 | | | 0.00 | % | | 1,165 | | | 0 | |
| LIU QINGCHUN* | | | 1,165 | | | 0.00 | % | | 1,165 | | | 0 | |
| YAO JINXIAN* | | | 4,659 | | | 0.01 | % | | 4,659 | | | 0 | |
| TONG YIDONG* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| YING BOQIN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| BAI QIU* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| YU JIALIN* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| YIN LIANG* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| WANG BIN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| WANG LEI* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| LIU MINGHUA* | | | 4,659 | | | 0.01 | % | | 4,659 | | | 0 | |
| GAO JIXU* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| SHI HAITAO* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| SONG ZHENYU* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| YU QINGHUA* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| SUN JUNJIE* | | | 4,659 | | | 0.01 | % | | 4,659 | | | 0 | |
| WANG YUZHEN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| LI YUBO* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| MA JIE* | | | 8,154 | | | 0.01 | % | | 8,154 | | | 0 | |
| MA XIANYUN* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| SUN SHAOJIE* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| WANG GUANSHAN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| HU JUN* | | | 2,329 | | | 0.00 | % | | 2,329 | | | 0 | |
| ZHANG RUNXI* | | | 2,329 | | | 0.00 | % | | 2,329 | | | 0 | |
| ZHANG LISHUN* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| MAO PINJIAO* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| LI HUA* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| WANG SHUGE* | | | 6,057 | | | 0.01 | % | | 6,057 | | | 0 | |
| XU YEPING* | | | 4,659 | | | 0.01 | % | | 4,659 | | | 0 | |
| TANG JIAFAN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| SUN WEICHUN* | | | 8,154 | | | 0.01 | % | | 8,154 | | | 0 | |
| ZHANG SHUGEN* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| WANG CHUNYAN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| ZHAO FUYUN* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| TONG AMIN* | | | 9,318 | | | 0.02 | % | | 9,318 | | | 0 | |
| LI BAODONG* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| HAN FUTONG* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| JU JINGCAI* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| WANG ZHIJUN* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| LI YUNJIE* | | | 1,165 | | | 0.00 | % | | 1,165 | | | 0 | |
| CHENG JUNYI* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| CHEN HONGFENG* | | | 34,944 | | | 0.06 | % | | 34,944 | | | 0 | |
| LONG XU* | | | 4,659 | | | 0.01 | % | | 4,659 | | | 0 | |
| KONG XIANZHEN* | | | 1,165 | | | 0.00 | % | | 1,165 | | | 0 | |
| XU SHUJUAN* | | | 1,165 | | | 0.00 | % | | 1,165 | | | 0 | |
| DU JIANJUN* | | | 4,659 | | | 0.01 | % | | 4,659 | | | 0 | |
| WANG MIN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| SONG LIMING* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| YUAN YINGHUA* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| ZHAO JIDI* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| YAO TINGJIANG* | | | 34,944 | | | 0.06 | % | | 34,944 | | | 0 | |
| LIU JINJUN* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| LANG YINGWEI* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| YE CHUNMEI* | | | 34,944 | | | 0.06 | % | | 34,944 | | | 0 | |
| XIANG SHENGRONG* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| HU ZHENGUO* | | | 2,329 | | | 0.00 | % | | 2,329 | | | 0 | |
| YIN GUOSHEN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| HUANG MINGGUANG* | | | 1,165 | | | 0.00 | % | | 1,165 | | | 0 | |
| MIAO JIANPING* | | | 1,747 | | | 0.00 | % | | 1,747 | | | 0 | |
| HOU XIAODONG* | | | 1,164 | | | 0.00 | % | | 1,164 | | | 0 | |
| CHEN YUNCHENG* | | | 1,165 | | | 0.00 | % | | 1,165 | | | 0 | |
| LIANG JIERU* | | | 4,659 | | | 0.01 | % | | 4,659 | | | 0 | |
| SUN JIYU* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| LIN XIA* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| PAN YUHUA* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| WANG DIANJIE* | | | 1,934 | | | 0.00 | % | | 1,934 | | | 0 | |
| JIANG YUQIN* | | | 3,879 | | | 0.01 | % | | 3,879 | | | 0 | |
| LI GUIXIANG* | | | 1,549 | | | 0.00 | % | | 1,549 | | | 0 | |
| ZHANG SHOUQIN* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| WANG SHUQIN* | | | 6,989 | | | 0.01 | % | | 6,989 | | | 0 | |
| LIU MINGFEN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| LIU SHUWEN* | | | 3,879 | | | 0.01 | % | | 3,879 | | | 0 | |
| ZHANG LIJUN* | | | 1,934 | | | 0.00 | % | | 1,934 | | | 0 | |
| ZHANG CAI* | | | 1,980 | | | 0.00 | % | | 1,980 | | | 0 | |
| WANG RU* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| SONG QUQING* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| SHAN YIGUO* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| HAO QIAO* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| SUI YUEMEI* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| ZHANG JIAN* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| WANG LINGXIU* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| ZHAO GUOYING* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| LIU CHUNFANG* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| SUN XUEHAI* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| GU YUEMING* | | | 4,659 | | | 0.01 | % | | 4,659 | | | 0 | |
| MA CHUNHAI* | | | 4,659 | | | 0.01 | % | | 4,659 | | | 0 | |
| WANG XIAOPING* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| CHEN JUN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| ZHONG PING* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| WANG JIANQIANG* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| CUI SHUYANG* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| HAO LINGYAN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| BAO AILING* | | | 6,989 | | | 0.01 | % | | 6,989 | | | 0 | |
| LI XIANGDONG* | | | 1,165 | | | 0.00 | % | | 1,165 | | | 0 | |
| LIU XIANGYING* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| DING LINQING* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| YANG QIUPING* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| CHOU BAOQING* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| ZHANG JIFAN* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| LIU DONGXUE* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| LIU XIAODONG* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| ZHANG LI* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| LIU JIE* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| JIANG FENGZHI* | | | 4,077 | | | 0.01 | % | | 4,077 | | | 0 | |
| YU JIANGPING* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| ZHANG NAN* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| HUA YONG* | | | 2,329 | | | 0.00 | % | | 2,329 | | | 0 | |
| WU JINYAN* | | | 1,165 | | | 0.00 | % | | 1,165 | | | 0 | |
| ZHANG JINGJUAN* | | | 1,165 | | | 0.00 | % | | 1,165 | | | 0 | |
| GAO SHUFEN* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| LIU YILI* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| LI ZHANGUO* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| ZHU JINYAN* | | | 2,329 | | | 0.00 | % | | 2,329 | | | 0 | |
| DONG BAOZHANG* | | | 1,164 | | | 0.00 | % | | 1,164 | | | 0 | |
| ZHENG ZHENQI* | | | 1,165 | | | 0.00 | % | | 1,164 | | | 0 | |
| WANG XIUXIN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| LIN JUYING* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| YANG HUI* | | | 4,659 | | | 0.01 | % | | 4,659 | | | 0 | |
| GAO WEI* | | | 1,164 | | | 0.00 | % | | 1,164 | | | 0 | |
| SHEN JUNCHANG* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| YIN XIANJUN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| HU MINXU* | | | 2,329 | | | 0.00 | % | | 2,329 | | | 0 | |
| HE LIJUAN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| SONG WENXIANG* | | | 1,165 | | | 0.00 | % | | 1,165 | | | 0 | |
| WANG GUICHEN* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| SHANG JUNHUA* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| SHANG JUNYUN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| LI BAOCAI* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| ZHANG LI* | | | 34,944 | | | 0.06 | % | | 34,944 | | | 0 | |
| HAN BINGSHUANG* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| ZHANG CHUNMEI* | | | 6,989 | | | 0.01 | % | | 6,989 | | | 0 | |
| ZHOU YUANSHENG* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| YANG YUMEI* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| YUAN ZHENXIANG* | | | 1,165 | | | 0.00 | % | | 1,165 | | | 0 | |
| LI HONGYING* | | | 1,165 | | | 0.00 | % | | 1,165 | | | 0 | |
| SONG BAOQIN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| HUANG TIANCAI* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| ZHANG QIUYING* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| GUO GUOFANG* | | | 2,329 | | | 0.00 | % | | 2,329 | | | 0 | |
| ZHANG LIXIANG* | | | 2,329 | | | 0.00 | % | | 2,329 | | | 0 | |
| ZHANG XINLING* | | | 2,329 | | | 0.00 | % | | 2,329 | | | 0 | |
| SI YUAI* | �� | | 1,165 | | | 0.00 | % | | 1,165 | | | 0 | |
| BAI XIANGBIN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| WANG HONGYAN* | | | 1,165 | | | 0.00 | % | | 1,165 | | | 0 | |
| WAN JIANBO* | | | 1,165 | | | 0.00 | % | | 1,165 | | | 0 | |
| YANG QING* | | | 8,154 | | | 0.01 | % | | 8,154 | | | 0 | |
| LU XINPING* | | | 1,165 | | | 0.00 | % | | 1,165 | | | 0 | |
| CHEN JIHONG* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| YANG CHUNFANG* | | | 1,165 | | | 0.00 | % | | 1,165 | | | 0 | |
| SHI YOUPENG* | | | 4,659 | | | 0.01 | % | | 4,659 | | | 0 | |
| ZHANG XIUZHEN* | | | 3,960 | | | 0.01 | % | | 3,960 | | | 0 | |
| QIN DAIHUA* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| CHEN YUANLI* | | | 4,659 | | | 0.01 | % | | 4,659 | | | 0 | |
| WANG DEZHI* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| REN FU* | | | 23,296 | | | 0.04 | % | | 23,296 | | | 0 | |
| SUN XINZENG* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| ZHAO XIUMIN* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| WANG PING* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| ZHANG FENGFEN* | | | 2,329 | | | 0.00 | % | | 2,329 | | | 0 | |
| LIN JING* | | | 1,165 | | | 0.00 | % | | 1,165 | | | 0 | |
| XIAN MINGYU* | | | 6,989 | | | 0.01 | % | | 6,989 | | | 0 | |
| YU LIJUAN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| PU DI* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| HUANG HEQING* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| LI LIN* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| LI WENYING* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| QIU ZIYAO* | | | 1,165 | | | 0.00 | % | | 1,165 | | | 0 | |
| QIU JIANHUA* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| CHEN HAIGUANG* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| XU RUILIAN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| MA JINGSHU* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| ZHANG QINYAN* | | | 2,329 | | | 0.00 | % | | 2,329 | | | 0 | |
| WANG XIZI* | | | 1,165 | | | 0.00 | % | | 1,165 | | | 0 | |
| ZHONG HANMING* | | | 1,164 | | | 0.00 | % | | 1,164 | | | 0 | |
| ZHANG RONG* | | | 2,329 | | | 0.00 | % | | 2,329 | | | 0 | |
| WU TINGYONG* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| DU BO* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| LIN ZHENHONG* | | | 9,318 | | | 0.02 | % | | 9,318 | | | 0 | |
| LIANG YIXIANG* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| BAO SHUFEN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| YANG QIANQING* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| SUN GUANGJUN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| CHEN LINGYAN* | | | 1,164 | | | 0.00 | % | | 1,164 | | | 0 | |
| LI YAFENG* | | | 3,844 | | | 0.01 | % | | 3,844 | | | 0 | |
| WEI GUOHUA* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| LIU YANFANG* | | | 4,077 | | | 0.01 | % | | 4,077 | | | 0 | |
| XING QUANXI* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| WANG LING * | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| ZHANG JIANZHONG* | | | 1,164 | | | 0.00 | % | | 1,164 | | | 0 | |
| LI DESEN* | | | 1,165 | | | 0.00 | % | | 1,165 | | | 0 | |
| LIU CONGYONG* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| SUN YONG* | | | 1,165 | | | 0.00 | % | | 1,165 | | | 0 | |
| HOU ZHONG* | | | 1,164 | | | 0.00 | % | | 1,164 | | | 0 | |
| YU CONGRONG* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| ZHAO JING* | | | 5,242 | | | 0.01 | % | | 5,242 | | | 0 | |
| SHI BING* | | | 17,472 | | | 0.03 | % | | 17,472 | | | 0 | |
| WU XINSHENG* | | | 4,659 | | | 0.01 | % | | 4,659 | | | 0 | |
| LI MENHUA* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| JIANG GE* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| ZHANG GUIXIA* | | | 4,077 | | | 0.01 | % | | 4,077 | | | 0 | |
| LU HUANYING * | | | 4,077 | | | 0.01 | % | | 4,077 | | | 0 | |
| XIAN MINGZI* | | | 10,483 | | | 0.02 | % | | 10,483 | | | 0 | |
| YUE HUI * | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| QI MEI * | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| WANG XIUHE * | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| WANG YANMEI* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| QU YANG* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| JIANG FENG * | | | 1,281 | | | 0.00 | % | | 1,281 | | | 0 | |
| ZHAO YAN* | | | 4,193 | | | 0.01 | % | | 4,193 | | | 0 | |
| WU XINLI* | | | 4,659 | | | 0.01 | % | | 4,659 | | | 0 | |
| ZHANG SHUYING* | | | 4,077 | | | 0.01 | % | | 4,077 | | | 0 | |
| YI QINGPING* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| LI MIN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| ZHOU HOUQUAN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| YAN XIAOLI* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| YAN BINGUI* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| LIN LI* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| XU SHAOBO* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| WANG JUN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| YU QI* | | | 17,472 | | | 0.03 | % | | 17,472 | | | 0 | |
| ZHENG PING * | | | 2,329 | | | 0.00 | % | | 2,329 | | | 0 | |
| LIANG HONG* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| LI MENGTAO* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| YU YANHONG * | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| LI CHANGXIN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| WANG YIHUA * | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| HAO YANLING* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| HE DAXIN* | | | 4,659 | | | 0.01 | % | | 4,659 | | | 0 | |
| YANG RUI* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| JU JIFAN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| FANG KAIQING* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| HE PEIYAN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| CHEN JIANPING * | | | 4,659 | | | 0.01 | % | | 4,659 | | | 0 | |
| CHEN XUEDI* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| DAI XUENI* | | | 4,659 | | | 0.01 | % | | 4,659 | | | 0 | |
| ZHANG DONGYAN* | | | 1,165 | | | 0.00 | % | | 1,165 | | | 0 | |
| DAI JUN* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| YANG HONGMEI* | | | 1,165 | | | 0.00 | % | | 1,165 | | | 0 | |
| TIAN HENGPING * | | | 1,165 | | | 0.00 | % | | 1,165 | | | 0 | |
| ZHAO LIHAI* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| XU LIJIE* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| YIN CUIYUN* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| LEI HONGWEN* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| SHI QINGYUAN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| CAO XIULAN* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| SUN ZAIHE* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| CHEN YIKANG* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| GAO LINGXIN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| ZHANG ENYING* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| CAO KEYI* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| LIN TIANMIN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| YANG MINXIANG* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| SU DIANLI* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| YANG GUOYING* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| ZHANG YING* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| YANG XIUYING* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| LI BAOJING* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| JIN YUEZHU* | | | 1,165 | | | 0.00 | % | | 1,165 | | | 0 | |
| ZHAO SHIJUN* | | | 2,329 | | | 0.00 | % | | 2,329 | | | 0 | |
| LEI DINGKUAN* | | | 1,165 | | | 0.00 | % | | 1,165 | | | 0 | |
| SUN XUE * | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| YANG HUIXIAN* | | | 4,659 | | | 0.01 | % | | 4,659 | | | 0 | |
| MA XIULI* | | | 1,165 | | | 0.00 | % | | 1,165 | | | 0 | |
| XUE SHUANG * | | | 10,483 | | | 0.02 | % | | 10,483 | | | 0 | |
| CHEN ZHONGMEI* | | | 1,164 | | | 0.00 | % | | 1,164 | | | 0 | |
| ZHOU YE* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| ZHU YOUJIAN* | | | 1,164 | | | 0.00 | % | | 1,164 | | | 0 | |
| LIU WEISHENG* | | | 1,165 | | | 0.00 | % | | 1,165 | | | 0 | |
| KONG FANHUA* | | | 1,165 | | | 0.00 | % | | 1,165 | | | 0 | |
| NI YANLING* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| ZHANG LI* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| ZHONG HONGFEI* | | | 2,329 | | | 0.00 | % | | 2,329 | | | 0 | |
| MA SHUZHI * | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| BI XUEYING* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| ZHANG LIJIE* | | | 1,164 | | | 0.00 | % | | 1,164 | | | 0 | |
| DENG XINGHAI* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| SHAO JINMING* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| SU GENYUAN* | | | 1,165 | | | 0.00 | % | | 1,165 | | | 0 | |
| LI SHULIAN* | | | 4,310 | | | 0.01 | % | | 4,310 | | | 0 | |
| YANG JIE * | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| ZHOU HONGJUN* | | | 19,406 | | | 0.03 | % | | 19,406 | | | 0 | |
| XIE JING * | | | 3,879 | | | 0.01 | % | | 3,879 | | | 0 | |
| MA GANG* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| ZHANG JING* | | | 9,318 | | | 0.02 | % | | 9,318 | | | 0 | |
| LIU HUANTING* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| TENG YOUSHENG* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| TANG JIMIN * | | | 1,165 | | | 0.00 | % | | 1,165 | | | 0 | |
| XU QING* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| WANG YINGHUA* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| SHEN YAN* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| TANG YINHUA* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| ZHANG JINLONG* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| LIU LIYING* | | | 1,165 | | | 0.00 | % | | 1,165 | | | 0 | |
| ZHAO JINCHENG* | | | 1,164 | | | 0.00 | % | | 1,164 | | | 0 | |
| WANG YOULAN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| ZHENG SHUJIN* | | | 2,329 | | | 0.00 | % | | 2,329 | | | 0 | |
| FANG XIAOLAN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| WU YUANFENG* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| ZHU HONGXI* | | | 6,989 | | | 0.01 | % | | 6,989 | | | 0 | |
| CAO WEIZHEN* | | | 12,230 | | | 0.02 | % | | 12,230 | | | 0 | |
| WANG MINGLI* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| MENG ZHAOYI* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| YANG YUMEI* | | | 2,796 | | | 0.00 | % | | 2,796 | | | 0 | |
| MA LIANYOU * | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| MA LIANZHEN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| QI SHULAN* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| WU LIXIN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| JIANG JING * | | | 6,989 | | | 0.01 | % | | 6,989 | | | 0 | |
| DONG SHUFEN* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| HAO ZHENQI* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| ZHANG ZHONGFAN* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| ZHANG LIJIE* | | | 11,683 | | | 0.02 | % | | 11,683 | | | 0 | |
| WEI DAN* | | | 19,802 | | | 0.03 | % | | 19,802 | | | 0 | |
| GU YOUYING* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| WANG YING* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| WANG YAN* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| DU DAILING* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| HU JINGHUA* | | | 2,329 | | | 0.00 | % | | 2,329 | | | 0 | |
| PIAO ZHENFU* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| GUO LIYA* | | | 2,329 | | | 0.00 | % | | 2,329 | | | 0 | |
| XU YALI* | | | 4,077 | | | 0.01 | % | | 4,077 | | | 0 | |
| WANG YURONG* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| WEI YIQING* | | | 1,165 | | | 0.00 | % | | 1,165 | | | 0 | |
| ZHANG GUIMIN* | | | 4,077 | | | 0.01 | % | | 4,077 | | | 0 | |
| DU LIPING* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| WEN GUIFEN* | | | 4,077 | | | 0.01 | % | | 4,077 | | | 0 | |
| FAN WEICHENG* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| ZHANG JING* | | | 4,659 | | | 0.01 | % | | 4,659 | | | 0 | |
| XIANG YAHONG* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| ZHAOYING* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| CUI YUMING* | | | 17,472 | | | 0.03 | % | | 17,472 | | | 0 | |
| ZHANG SHUJUN* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| SUN YULIU* | | | 1,165 | | | 0.00 | % | | 1,165 | | | 0 | |
| LI QINGQIN* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| TAO SHUFEN* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| ZHAO LIYING* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| WANG YINGHUI* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| QIU JIANHUA* | | | 30,285 | | | 0.05 | % | | 30,285 | | | 0 | |
| YANG XIANFENG* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| HUANG YUHUANG* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| ZHAO GUANGSONG* | | | 1,164 | | | 0.00 | % | | 1,164 | | | 0 | |
| XU XIAORAN* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| CAI GUOFENG* | | | 6,989 | | | 0.01 | % | | 6,989 | | | 0 | |
| WU FUYA* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| SU PING* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| LI SHU FEN* | | | 3,879 | | | 0.01 | % | | 3,879 | | | 0 | |
| WANG SONGMEI* | | | 3,879 | | | 0.01 | % | | 3,879 | | | 0 | |
| SHAO LIXIAN* | | | 3,879 | | | 0.01 | % | | 3,879 | | | 0 | |
| WANG XINYING* | | | 3,879 | | | 0.01 | % | | 3,879 | | | 0 | |
| LIU XIANZHOU* | | | 3,879 | | | 0.01 | % | | 3,879 | | | 0 | |
| SHEN XUBO* | | | 1,980 | | | 0.00 | % | | 1,980 | | | 0 | |
| ZHAO GUOHUI* | | | 3,879 | | | 0.01 | % | | 3,879 | | | 0 | |
| FU YUJIE* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| ZHAO BAOLING* | | | 3,879 | | | 0.01 | % | | 3,879 | | | 0 | |
| SHEN GUANGHUA* | | | 6,208 | | | 0.01 | % | | 6,208 | | | 0 | |
| WANG XUE* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| ZHANG JIANHUA* | | | 3,879 | | | 0.01 | % | | 3,879 | | | 0 | |
| ZHANG JINGBO* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| LIANG ENSHUN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| WANG GUOHUA* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| ZHANG CUIYING* | | | 4,077 | | | 0.01 | % | | 4,077 | | | 0 | |
| SUN SHUYUN* | | | 4,077 | | | 0.01 | % | | 4,077 | | | 0 | |
| ZHANG HAIKUAN* | | | 4,077 | | | 0.01 | % | | 4,077 | | | 0 | |
| WANG HAIGANG* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| YAO MINGCAI* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| SUN GUIYUN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| SUN XIUQING* | | | 1,165 | | | 0.00 | % | | 1,165 | | | 0 | |
| LIU ZHANMIN* | | | 3,879 | | | 0.01 | % | | 3,879 | | | 0 | |
| XU HUIZHEN* | | | 3,879 | | | 0.01 | % | | 3,879 | | | 0 | |
| LIU TIANE* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| WANG SHAOHUA* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| YV LIMIN* | | | 4,077 | | | 0.01 | % | | 4,077 | | | 0 | |
| ZHANG JINGRONG* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| QIU PING* | | | 1,165 | | | 0.00 | % | | 1,165 | | | 0 | |
| CUI CAIHONG* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| LI LIJIE* | | | 1,165 | | | 0.00 | % | | 1,165 | | | 0 | |
| WANG ZHIHONG* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| JIN JUXIU* | | | 1,165 | | | 0.00 | % | | 1,165 | | | 0 | |
| ZHANG JING* | | | 1,164 | | | 0.00 | % | | 1,164 | | | 0 | |
| WANG ZUOYV* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| DONG YVSHU* | | | 1,164 | | | 0.00 | % | | 1,164 | | | 0 | |
| LIU AIQIN* | | | 4,659 | | | 0.01 | % | | 4,659 | | | 0 | |
| DENG XIUPING* | | | 1,164 | | | 0.00 | % | | 1,164 | | | 0 | |
| DAI HONGJIE* | | | 1,164 | | | 0.00 | % | | 1,164 | | | 0 | |
| YAN MEISHENG* | | | 15,142 | | | 0.03 | % | | 15,142 | | | 0 | |
| LI YVQIANG* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| YANG LIANGCE* | | | 8,154 | | | 0.01 | % | | 8,154 | | | 0 | |
| LIU XIAOLING* | | | 1,165 | | | 0.00 | % | | 1,165 | | | 0 | |
| WU LIBO* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| YV HONGE* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| WANG GUIMEI* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| YAN GUOLIN* | | | 1,398 | | | 0.00 | % | | 1,398 | | | 0 | |
| ZHANG XIAOKUN* | | | 3,879 | | | 0.01 | % | | 3,879 | | | 0 | |
| WU GUANGYAN* | | | 4,659 | | | 0.01 | % | | 4,659 | | | 0 | |
| WANG ENPU* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| SUN ZHEN* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| YIN KAIWEI* | | | 23,296 | | | 0.04 | % | | 23,296 | | | 0 | |
| WANG LANXIANG* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| YANG ZHONGXIN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| AN JIULIAN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| ZHU BAILIN* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| ZHU BAIRONG* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| ZHANG ZUOPENG* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| YAO LI* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| ZHOU LIRONG* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| MA JINGYUN* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| YU JINGZHI* | | | 23,296 | | | 0.04 | % | | 23,296 | | | 0 | |
| LI SHUXIU * | | | 5,125 | | | 0.01 | % | | 5,125 | | | 0 | |
| SHA YUJING* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| CHENG JIANHUA* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| SONG XUEGUANG* | | | 1,165 | | | 0.00 | % | | 1,165 | | | 0 | |
| CHENG LI* | | | 1,164 | | | 0.00 | % | | 1,164 | | | 0 | |
| CHE HUI* | | | 1,165 | | | 0.00 | % | | 1,165 | | | 0 | |
| ZHAO SHILIN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| LV CHEN* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| YE FEI* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| LI SHURONG* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| LI CAILING* | | | 1,165 | | | 0.00 | % | | 1,165 | | | 0 | |
| CHEN YOU* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| KANG YANFANG* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| GU YUYING* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| LI SHUMEI* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| WANG SHENGLI* | | | 4,659 | | | 0.01 | % | | 4,659 | | | 0 | |
| CI JUAN* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| CHEN XIAOLING* | | | 9,318 | | | 0.02 | % | | 9,318 | | | 0 | |
| ZHANG WENJUN* | | | 6,989 | | | 0.01 | % | | 6,989 | | | 0 | |
| MA DONGMEI* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| CAO KUNLONG * | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| GAO XUEXIA* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| ZHEN YANHUA* | | | 2,329 | | | 0.00 | % | | 2,329 | | | 0 | |
| ZHANG LINA* | | | 1,164 | | | 0.00 | % | | 1,164 | | | 0 | |
| ZHANG JING* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| ZHU QING* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| JIN LIE* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| MAO XIAODI* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| CHE CHANG* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| LIU CAIXIA* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| SHEN YAN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| WEI SHOUREN* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| LIU NAN* | | | 36,342 | | | 0.06 | % | | 36,342 | | | 0 | |
| CHEN QIANG* | | | 12,813 | | | 0.02 | % | | 12,813 | | | 0 | |
| LIU SHU* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| WANG MINGJING* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| FENG LIAN* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| ZHANG ZHIXIN* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| XIAO RUIDONG* | | | 23,296 | | | 0.04 | % | | 23,296 | | | 0 | |
| LI LINA* | | | 4,659 | | | 0.01 | % | | 4,659 | | | 0 | |
| GAO SONG* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| LUAN HAILONG* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| SU WEIGUANG* | | | 6,989 | | | 0.01 | % | | 6,989 | | | 0 | |
| LUO CAIQIN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| MENG FANYU* | | | 12,813 | | | 0.02 | % | | 12,813 | | | 0 | |
| YANG YUBIN* | | | 15,142 | | | 0.03 | % | | 15,142 | | | 0 | |
| LV ZHUJUN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| ZHANG XIAOXIN* | | | 9,668 | | | 0.02 | % | | 9,668 | | | 0 | |
| ZHANG GUILAN* | | | 1,164 | | | 0.00 | % | | 1,164 | | | 0 | |
| LIU ZHONGWEI* | | | 1,165 | | | 0.00 | % | | 1,165 | | | 0 | |
| ZHANG SHULAN* | | | 1,164 | | | 0.00 | % | | 1,164 | | | 0 | |
| MENG XIANGYU* | | | 1,165 | | | 0.00 | % | | 1,165 | | | 0 | |
| WANG JIXIN* | | | 699 | | | 0.00 | % | | 699 | | | 0 | |
| MENG ZHAOMING* | | | 1,864 | | | 0.00 | % | | 1,864 | | | 0 | |
| CAO YING* | | | 699 | | | 0.00 | % | | 699 | | | 0 | |
| XIA ZHANKUN* | | | 699 | | | 0.00 | % | | 699 | | | 0 | |
| JI LING* | | | 1,165 | | | 0.00 | % | | 1,165 | | | 0 | |
| SUN YIYANG* | | | 1,165 | | | 0.00 | % | | 1,165 | | | 0 | |
| YANG SHUHUA* | | | 1,165 | | | 0.00 | % | | 1,165 | | | 0 | |
| ZHANG YUANXI* | | | 1,164 | | | 0.00 | % | | 1,164 | | | 0 | |
| LIU XIAOBO* | | | 699 | | | 0.00 | % | | 699 | | | 0 | |
| DANG CHANGPING* | | | 699 | | | 0.00 | % | | 699 | | | 0 | |
| SONG XIBIN* | | | 1,165 | | | 0.00 | % | | 1,165 | | | 0 | |
| ZHANG RONG* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| PAN JINYONG* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| PAN FUGUI* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| JIANG WAN* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| XIA ZHISHAN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| LI LI* | | | 58,240 | | | 0.10 | % | | 58,240 | | | 0 | |
| YAO YINGBIN* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| HUA AN* | | | 2,329 | | | 0.00 | % | | 2,329 | | | 0 | |
| YANG XIURU* | | | 17,472 | | | 0.03 | % | | 17,472 | | | 0 | |
| LI JINHUA* | | | 58,240 | | | 0.10 | % | | 58,240 | | | 0 | |
| JIA HONGXUE* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| ZHOU CHUNFANG* | | | 1,164 | | | 0.00 | % | | 1,164 | | | 0 | |
| WANG ZHAO* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| LI SHU* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| CHEN JIE* | | | 34,944 | | | 0.06 | % | | 34,944 | | | 0 | |
| SHI MEIPING* | | | 23,296 | | | 0.04 | % | | 23,296 | | | 0 | |
| YANG GUANGMIAO* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| XU JUE* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| LI WEI* | | | 23,296 | | | 0.04 | % | | 23,296 | | | 0 | |
| LI GUOYING* | | | 1,165 | | | 0.00 | % | | 1,165 | | | 0 | |
| ZHU JUNYI | | | 1,164,800 | | | 2.00 | % | | 1,164,800 | | | 0 | |
| ZHANG TAO* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| SUN QIUSHI* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| LI JUN* | | | 17,472 | | | 0.03 | % | | 17,472 | | | 0 | |
| YAO JUNYING* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| LI KEMING* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| LI YI* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| CHEN LEI* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| XU SHUQIN* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| WANG KEBIN* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| CAO RUIXUE* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| CAO YAN* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| LI GANGJIAN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| ZHU XU* | | | 17,239 | | | 0.03 | % | | 17,239 | | | 0 | |
| WANG JIANGHAI* | | | 1,165 | | | 0.00 | % | | 1,165 | | | 0 | |
| ZHANG LING* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| YANG YIXUAN* | | | 46,592 | | | 0.08 | % | | 46,592 | | | 0 | |
| CAO HACHEN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| HAN LING* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| LI SHIQIU* | | | 1,747 | | | 0.00 | % | | 1,747 | | | 0 | |
| ZHANG YING* | | | 1,747 | | | 0.00 | % | | 1,747 | | | 0 | |
| ZHANG GUILAN* | | | 1,747 | | | 0.00 | % | | 1,747 | | | 0 | |
| LI ZUOZHI* | | | 1,747 | | | 0.00 | % | | 1,747 | | | 0 | |
| DING NING* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| CAI LIWEN* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| SONG YUCHUAN* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| CHANG YAGUANG* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| ZOU SHUXIA* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| XU YUZHANG* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| ZHANG ZHIJIAN* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| REN GUOHUA* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| ZHANG YUANLONG* | | | 6,989 | | | 0.01 | % | | 6,989 | | | 0 | |
| ZHOU CHAO* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| LIANG QIUHUAN* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| SUN GUOJUN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| LIAO YANG* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| LI GUANGMING* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| JIANG CHAO* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| QI ZEYAN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| WANG ZHIJUN* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| WANG JIXING* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| FAN SHENGJIN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| JIN HONG* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| MENG XIANGYUN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| ZHANG MIN* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| ZHAO YING* | | | 116,480 | | | 0.20 | % | | 116,480 | | | 0 | |
| LI CHANGJIANG* | | | 1,165 | | | 0.00 | % | | 1,165 | | | 0 | |
| XU XIAOHUA* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| ZHANG SHULAN* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| WANG HONGYING* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| WANG JIEYING* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| LIU HUIPING* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| WU JINGHUI* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| HE YEGUANG* | | | 174,720 | | | 0.30 | % | | 174,720 | | | 0 | |
| SUN GUIFANG* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| WANG HUIYU* | | | 17,472 | | | 0.03 | % | | 17,472 | | | 0 | |
| WEI JIE* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| CHENG YANG* | | | 1,164 | | | 0.00 | % | | 1,164 | | | 0 | |
| WANG BIN* | | | 1,165 | | | 0.00 | % | | 1,165 | | | 0 | |
| WANG QINGLING* | | | 1,165 | | | 0.00 | % | | 1,165 | | | 0 | |
| YAN WENXUE* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| WANG JUNHUAN* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| LI XUEFEI* | | | 16,307 | | | 0.03 | % | | 16,307 | | | 0 | |
| KANG XIAOCHUN* | | | 108,326 | | | 0.19 | % | | 108,326 | | | 0 | |
| XIAO LU* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| WANG YANAN | | | 1,164,800 | | | 2.00 | % | | 1,164,800 | | | 0 | |
| YANG JIAXIN | | | 5,678,400 | | | 9.75 | % | | 5,678,400 | | | 0 | |
| YU DEQING* | | | 23,296 | | | 0.04 | % | | 23,296 | | | 0 | |
| ZHANG HAI* | | | 34,944 | | | 0.06 | % | | 34,944 | | | 0 | |
| WANG YANHUA* | | | 8,154 | | | 0.01 | % | | 8,154 | | | 0 | |
| WEN JI* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| LV YUXIA* | | | 1,631 | | | 0.00 | % | | 1,631 | | | 0 | |
| LI CAIYUN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| SONG LIPING* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| NI SONGZHI* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| JIN YANLI* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| LANG HONGMEI* | | | 15,142 | | | 0.03 | % | | 15,142 | | | 0 | |
| WANG YING* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| SHI YANMEI* | | | 9,551 | | | 0.02 | % | | 9,551 | | | 0 | |
| GUO LINGLI* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| ZHU XIAOYING* | | | 26,790 | | | 0.05 | % | | 26,790 | | | 0 | |
| ZHANG QIUMEI* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| SUN LEI* | | | 8,154 | | | 0.01 | % | | 8,154 | | | 0 | |
| DUAN TAIPING* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| WANG QUANYING* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| LIU LIJUAN* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| CAO CHUNYONG* | | | 8,154 | | | 0.01 | % | | 8,154 | | | 0 | |
| LI JIXIANG* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| ZHANG MEI* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| GUAN JINGLAN* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| QIU HONGSHENG* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| LIU ZHEQI* | | | 8,154 | | | 0.01 | % | | 8,154 | | | 0 | |
| LIU GUOMING* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| LI JING* | | | 69,888 | | | 0.12 | % | | 69,888 | | | 0 | |
| SHA JINGYUN* | | | 8,387 | | | 0.01 | % | | 8,387 | | | 0 | |
| JI ZHONGSHA* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| CHEN XIUYUN* | | | 4,193 | | | 0.01 | % | | 4,193 | | | 0 | |
| ZHANG SUXIA* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| LI RUNHUA* | | | 7,921 | | | 0.01 | % | | 7,921 | | | 0 | |
| LIU RUI* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| GONG CHANGFU* | | | 38,438 | | | 0.07 | % | | 38,438 | | | 0 | |
| YAN MENG* | | | 38,438 | | | 0.07 | % | | 38,438 | | | 0 | |
| YAO XIAOJUAN* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| LIU WENRU* | | | 3,844 | | | 0.01 | % | | 3,844 | | | 0 | |
| NA JIA* | | | 3,844 | | | 0.01 | % | | 3,844 | | | 0 | |
| SHEN YUE* | | | 3,844 | | | 0.01 | % | | 3,844 | | | 0 | |
| LI QIUJU* | | | 3,844 | | | 0.01 | % | | 3,844 | | | 0 | |
| ZHAO SHUYIN* | | | 3,844 | | | 0.01 | % | | 3,844 | | | 0 | |
| CHENG LIN* | | | 3,844 | | | 0.01 | % | | 3,844 | | | 0 | |
| CHEN CHEN | | | 582,400 | | | 1.00 | % | | 582,400 | | | 0 | |
| NIU GUIXIN* | | | 1,165 | | | 0.00 | % | | 1,165 | | | 0 | |
| WANG YANG* | | | 2,330 | | | 0.00 | % | | 2,330 | | | 0 | |
| ZHAO GUIRU* | | | 4,659 | | | 0.01 | % | | 4,659 | | | 0 | |
| TANG FANGJIE* | | | 1,165 | | | 0.00 | % | | 1,165 | | | 0 | |
| ZHANG GUANGHUA* | | | 1,164 | | | 0.00 | % | | 1,164 | | | 0 | |
| ZHAO QIANG* | | | 1,164 | | | 0.00 | % | | 1,164 | | | 0 | |
| HE YUNSHENG* | | | 1,164 | | | 0.00 | % | | 1,164 | | | 0 | |
| JIA XIAO* | | | 1,164 | | | 0.00 | % | | 1,164 | | | 0 | |
| LV XIANJIE* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| LV JIEYING* | | | 1,165 | | | 0.00 | % | | 1,165 | | | 0 | |
| YANG YVQIAO* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| CHEN ZHIMING* | | | 3,494 | | | 0.01 | % | | 3,494 | | | 0 | |
| LIU HAITAO* | | | 1,165 | | | 0.00 | % | | 1,165 | | | 0 | |
| YIN ZHAOGUO* | | | 2,912 | | | 0.01 | % | | 2,912 | | | 0 | |
| ZHAO QINGSHENG* | | | 11,648 | | | 0.02 | % | | 11,648 | | | 0 | |
| ZHUANG YINGCHUN* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| HUANG CHINAN* | | | 5,824 | | | 0.01 | % | | 5,824 | | | 0 | |
| Total | | | 23,444,000 | | | 40.25 | % | | 23,444,000 | | | 0 | |
* Less than 1% of the outstanding shares of common stock.
DESCRIPTION OF SECURITIES
The descriptions in this section and in other sections of this prospectus of our securities and various provisions of our certificate of incorporation and our bylaws are limited solely to descriptions of the material terms of our securities, articles of incorporation and bylaws.
Our authorized capital stock consists of 100,000,000 shares of common stock, $.001par value per share, 58,240,000 shares of which are issued and outstanding.
The holders of our common stock are entitled to equal dividends and distributions per share with respect to the common stock when and if declared by the Board of Directors from funds legally available therefore. No holder of any shares of our common stock has a pre-emptive right to subscribe for any of our securities. Upon liquidation, dissolution, or winding up of us, and after payment of creditors and preferred shareholders, the assets will be divided pro-rata on a share-for-share basis among the holders of the shares of common stock. All shares of common stock now outstanding are fully paid, validly issued, and non-assessable.
Each share of common stock is entitled to one vote with respect to the election of any director or any other matter upon which shareholders are required or permitted to vote. Holders of the common stock do not have cumulative voting rights, so the holders of more than 50% of the combined shares voting for the election of directors may elect all of the directors if they choose to do so, and, in that event, the holders of the remaining shares will not be able to elect any members to the board of directors.
ANTI-TAKEOVER EFFECTS OF VARIOUS PROVISIONS OF DELAWARE LAW AND OUR ARTICLES OF INCORPORATION AND BYLAWS
We are subject to Section 203 of the Delaware General Corporation Law. Subject to certain exceptions, this statute regulating corporate takeovers prohibits a Delaware corporation from engaging in any business combination with any interested shareholder for three years following the date that the shareholder became an interested shareholder.
Generally, a business combination includes a merger, asset or stock sale, or other transaction resulting in a financial benefit to the interested shareholder. An interested shareholder is a person who, together with affiliates and associates, owns or, within three years prior to the determination of interested shareholder status, did own 15% or more of a corporation's outstanding voting securities. We expect the existence of this provision to have an anti-takeover effect with respect to transactions our board of directors does not approve in advance. We also anticipate that Section 203 may discourage takeover attempts that might result in a premium over the market price for the shares of common stock held by shareholders.
Provisions of our certificate of incorporation and bylaws may have the effect of making it more difficult for a third party to acquire, or discourage a third party from attempting to acquire, control of our Company by means of a tender offer, a proxy contest or otherwise. These provisions may also make the removal of incumbent officers and directors more difficult. These provisions are intended to discourage certain types of coercive takeover practices and inadequate takeover bids and to encourage persons seeking to acquire control of us to first negotiate with us. These provisions could also limit the price that investors might be willing to pay in the future for shares of our common stock. These provisions may make it more difficult for shareholders to take specific corporate actions and could have the effect of delaying or preventing a change in control.
SHARES ELIGIBLE FOR FUTURE SALE
As of December 27, 2006, all 58,240,000 outstanding shares of our common stock are “restricted securities” as such term is defined under Rule 144, in that such shares were issued in private transactions not involving a public offering and may not be sold in the absence of registration other than in accordance with Rules 144, 144(k), or 701 promulgated under the Securities Act or another exemption from registration.
In general, under Rule 144 as currently in effect, a person, including an affiliate, who has beneficially owns shares for at least one year is entitled to sell, within any three-month period commencing 90 days after the date of this prospectus, a number of shares that does not exceed the greater of one percent of the then outstanding shares of our common stock or the average weekly trading volume in our common stock during the four calendar weeks preceding the date on which notice of such sale is filed, subject to various restrictions. In addition, a person who is not deemed to have been an affiliate of ours at any time during the 90 days preceding a sale and who has beneficially owned the shares proposed to be sold for at least two years would be entitled to sell those shares under Rule 144(k) without regard to the requirements described above.
To the extent that shares were acquired from an affiliate, such person's holding period for the purpose of affecting a sale under Rule 144 commences on the date of transfer from the affiliate. However, in the SEC's interpretive letter to the NASD, the SEC concluded that promoters or affiliates of blank check companies and their transferees would be deemed underwriters, under the Act, when reselling the securities of a blank check company. The letter goes on to state that the securities held by the persons referenced above can only be sold through a registered offering and not in reliance on Rule 144. From approximately 2004 through May 8, 2006, the date we completed the exchange, we were a blank check company. Based on the foregoing, and as of December 27, 2006, no share of our restricted shares was eligible for sale under Rule 144. Based on management's review of our stock transfer records, each of the holders of the afore-referenced shares acquired their securities prior to the time that we became a blank check company.
There has been very limited trading volume in our common stock to date. Sales of substantial amounts of our common stock under Rule 144, this prospectus, or otherwise, could adversely affect the prevailing market price of our common stock and could impair our ability to raise capital through the future sale of our securities.
TRANSFER AGENT AND REGISTRAR
The Transfer Agent and Registrar for our common stock is Securities Transfer Corporation, 2591 Dallas Parkway Ste 102, Frisco TX 75034 and its telephone number is 469-633-0101.
PLAN OF DISTRIBUTION
We are registering a total of 23,444,000 shares of our common stock that are being offered by the selling shareholders. As used in this prospectus, "selling shareholders" includes the pledgees, donees, transferees or others who may later hold the selling shareholders' interests in the common stock. We will pay the costs and fees of registering the common shares, but the selling shareholders will pay any brokerage commissions, discounts or other expenses relating to the sale of the common stock. We will not receive the proceeds from the sale of the shares by the selling shareholders.
The selling shareholders and any of their pledgees, assignees and successors-in-interest may, from time to time, sell any or all of their shares of common stock on any stock exchange, market, or trading facility on which the shares are traded or in private transactions. These sales may be at fixed or negotiated prices. The selling shareholders may use any one or more of the following methods when selling shares:
| · | Ordinary brokerage transactions and transactions in which the broker-dealer solicits purchasers; |
| · | Block trades in which the broker-dealer will attempt to sell the shares as agent but may position and resell a portion of the block as principal to facilitate the transaction; |
| · | Purchases by a broker-dealer as principal and resale by the broker-dealer for its account; |
| · | An exchange distribution in accordance with the rules of the applicable exchange; |
| · | Privately negotiated transactions; |
| · | Broker-dealers may agree with the selling shareholders to sell a specified number of such shares at a stipulated price per share; |
| · | A combination of any such methods of sale; and |
| · | Any other method permitted pursuant to applicable law. |
The selling shareholders may also sell shares under Rule 144 under the Securities Act, if available, rather than under this prospectus. Broker-dealers engaged by the selling shareholders may arrange for other brokers-dealers to participate in sales. Broker-dealers may receive commissions or discounts from the selling shareholders (or, if any broker-dealer acts as agent for the purchaser of shares, from the purchaser) in amounts to be negotiated. The selling shareholders do not expect these commissions and discounts to exceed what is customary in the types of transactions involved.
The selling shareholders may from time to time pledge or grant a security interest in some or all of the shares of common stock or warrants owned by them and, if they default in the performance of their secured obligations, the pledges, or secured parties may offer and sell the shares of common stock from time to time under this prospectus, or under an amendment to this prospectus under Rule 424(b)(3) or other applicable provision of the Securities Act, amending the list of selling shareholders to include the pledgee, transferee, or other successors in interest as selling shareholders under this prospectus.
LEGAL MATTERS
The validity of the registration for the resale of common stock will be passed upon for us by King & Wood LLP.
EXPERTS
The financial statements for Asia Biotechnology Group Inc. as of and for the years ended December 31, 2005 and 2004; the consolidated financial statements of Asia Biotechnology Group Inc. (BVI) as of and for the years ended December 31, 2005; the financial statements of Harbin OT Pharmaceutical Co., Ltd. (China) as of and for the years ended December 31,2005 and 2004 included in this prospectus have been audited by Child, Van Wagoner & Bradshaw, PLLC, independent registered public accounting firm, to the extent and for the periods set forth in their reports appearing elsewhere herein and are included in reliance upon such reports given upon the authority of that firm as experts in auditing and accounting.
CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
None.
WHERE YOU CAN FIND ADDITIONAL INFORMATION
We have filed with the SEC a registration statement on Form SB-2 under the Securities Act in connection with the offering of the common stock by the selling shareholders. This prospectus, which is part of the registration statement, does not contain all of the information included in the registration statement. Some information is omitted and you should refer to the registration statement and its exhibits. With respect to references made in this prospectus to any contract, agreement or other document of ours, such references are not necessarily complete and you should refer to the exhibits attached to the registration statement for copies of the actual contracts, agreements or other documents. You may review a copy of the registration statement, including exhibits, at the SEC's public reference room at 450 Fifth Street, N.W., Washington, D.C. 20549. The public may obtain information on the operation of the public reference room by calling the SEC at 1-800-SEC-0330.
We also file annual, quarterly and current reports, proxy statements and other information with the SEC. You may read and copy any reports, statements or other information on file at the public reference rooms. You can also request copies of these documents, for a copying fee, by writing to the SEC.
Financial Statements
Table of Contents
| | | Page | |
PART I AUDITED CONSOLIDATED FINANCIAL STATEMENTS FOR YEARS ENDED DECEMBER 31, 2005 & 2004 | | | |
| | | | |
| REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM | | | F-2 | |
| ASIA BIOTECHNOLOGY GROUP INC. (FORMERLY ECHELON ACQUISITION CORP) | | | F-3 | |
| BALANCE SHEET | | | F-3 | |
| STATEMENTS OF OPERATIONS | | | F-4 | |
| STATEMENTS OF SHAREHOLDERS' EQUITY | | | F-5 | |
| STATEMENTS OF CASH FLOWS | | | F-6 | |
| NOTES TO FINANCIAL STATEMETNS | | | F-7 | |
| | | | | |
| HARBIN OT PHARMACEUTICAL CO., LIMITED | | | F-12 | |
| CONSOLIDATED BALANCE SHEET | | | F-12 | |
| CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS | | | F-13 | |
| CONSOLIDATED STATEMENT OF SHAREHOLDERS' EQUITY | | | F-14 | |
| CONSOLIDATED STATEMENT OF CASH FLOW | | | F-15 | |
| NOTES TO FINANCIAL STATEMENTS | | | F-16 | |
| | | | | |
| REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM | | | F-21 | |
| ASIA BIOTECHNOLOGY GROUP INC | | | F-22 | |
| CONSOLIDATED BALANCE SHEET | | | F-22 | |
| CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS | | | F-23 | |
| CONSOLIDATED STATEMENT OF SHAREHOLDERS' EQUITY | | | F-24 | |
| CONSOLIDATED STATEMENT OF CASH FLOW | | | F-25 | |
| NOTES TO FINANCIAL STATEMENTS | | | F-26 | |
| | | | | |
| REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM | | | F-35 | |
| HARBIN OT PHARMACEUTICAL CO., LTD. | | | F-36 | |
| CONSOLIDATED BALANCE SHEET | | | F-36 | |
| CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS | | | F-37 | |
| CONSOLIDATED STATEMENT OF SHAREHOLDERS' EQUITY | | | F-38 | |
| CONSOLIDATED STATEMENT OF CASH FLOW | | | F-39 | |
| NOTES TO FINANCIAL STATEMENTS | | | F-40 | |
| | | | | |
PART II UNAUDITED PROFORMA CONSOLIDATED FINANCIAL STATEMENTS | | | | |
| | | | | |
| UNAUDITED PRO FORMA CONSOLIDATED STATEMENT OF OPERATIONS | | | F-51 | |
| NOTES TO UNAUDITED PRO FORMA CONSOLIDATED FINANCIAL STATEMENTS | | | F-52 | |
| | | | | |
PART III UNAUDITED CONSOLIDATED FINANCIAL STATEMENTS FOR PERIOD ENDED JUNE 30, 2006 | | | | |
| | | | | |
| ASIA BIOTECHNOLOGY GROUP INC. (FORMER ECHELON ACQUISITION CORP) | | | F-53 | |
| BALANCE SHEET | | | F-53 | |
| STATEMENTS OF OPERATIONS COMPREHENSIVE INCOME | | | F-54 | |
| STATEMENTS OF CASH FLOWS | | | F-55 | |
| NOTES TO FINANCIAL STATEMETNS | | | F-56 | |
Child, Van Wagoner & Bradshaw, PLLC
A PROFESSIONAL LIMITED LIABILITY COMPANY Of CERTIFIED PUBLIC ACCOUNTANTS
5296 S. Commerce Dr., Suite 300, Salt Lake City, UT 84107
PHONE: (801) 281-4700 FAX: (801) 281-4701
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To The Board of Directors
Asia Biotechnology Group Inc. (formerly known as Echelon Acquisition Corp.)
We have audited the accompanying balance sheet of Asia Biotechnology Group Inc. as of December 31, 2005, and the related statements of operations, shareholders’ equity (deficit), and cash flows for the year ended December 31, 2005 and for the period from July 27, 2004 (inception) to December 31, 2004. These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States of America). Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audits included consideration of internal control over financial reporting, as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of Asia Biotechnology Group Inc. as of December 31, 2005, and the results of its operations and its cash flows for the year ended December 31, 2005 and for the period from July 27, 2004 (inception) to December 31, 2004, in conformity with accounting principles generally accepted in the United States of America.
Child, Van Wagoner & Bradshaw, PLLC
Salt Lake City, Utah
May 8, 2006
ASIA BIOTECHNOLOGY GROUP INC. (FORMERLY KNOWN AS ECHELON ACQUISITION CORP.)
(A DEVELOPMENT STAGE COMPANY)
BALANCE SHEET
| | | December 31, 2005 | |
| ASSETS | | | |
| | | | |
| Current assets: | | | |
| Cash and cash equivalents | | $ | - | |
| Total assets | | $ | - | |
| | | | | |
| LIABILITIES AND SHAREHOLDERS’ DEFICIT | | | |
| | | | |
| Current liabilities: | | | |
| Accrued liabilities | | $ | 800 | |
| Total current liabilities | | | 800 | |
| | | | | |
| Total liabilities | | | 800 | |
| | | | | |
| Shareholders’ deficit: | | | | |
| Preferred Stock at $0.001 par value; authorized 20,000,000 shares; no shares issued and outstanding | | | - | |
Common stock at $0.001 par value; authorized 100,000,000 shares; 11,648,000 shares issued and outstanding | | | 11,648 | |
| Deficit accumulated during the development stage | | | (12,448 | ) |
| Total shareholders’ deficit | | | (800 | ) |
| Total liabilities and shareholders’ deficit | | $ | - | |
See accompanying notes to financial statements.
ASIA BIOTECHNOLOGY GROUP INC. (FORMERLY KNOWN AS ECHELON ACQUISITION CORP.)
(A DEVELOPMENT STAGE COMPANY)
STATEMENTS OF OPERATIONS
| | | For the Year ended December 31, | | Period from July 27, 2004 (inception) to December 31, | | Period from July 27, 2004 (inception) to December 31, | |
| | 2005 | | 2004 | | 2005 | |
| | | | | | | | |
| Revenue | | $ | - | | $ | - | | $ | - | |
| | | | | | | | | | | |
| General and administrative | | | | | | | | | | |
| Organization and related expenses | | | - | | | 148 | | | 148 | |
| General and administrative expenses | | | 11,500 | | | 800 | | | 12,300 | |
| Total general and administrative | | | 11,500 | | | 948 | | | 12,448 | |
| | | | | | | | | | | |
| Net loss | | $ | (11,500 | ) | $ | (948 | ) | $ | (12,448 | ) |
| | | | | | | | | | | |
| Net loss per share | | | | | | | | | | |
| - basic and fully diluted | | $ | (0.00 | ) | $ | (0.01 | ) | | | |
| | | | | | | | | | | |
| Weighted average ordinary shares outstanding | | | | | | | | | | |
| - basic and fully diluted | | | 9,946,630 | | | 148,000 | | | | |
See accompanying notes to financial statements.
ASIA BIOTECHNOLOGY GROUP INC. (FORMERLY KNOWN AS ECHELON ACQUISITION CORP.)
(A DEVELOPMENT STAGE COMPANY)
STATEMENTS OF SHAREHOLDERS' EQUITY
| | | Ordinary share | | Additional | | | | Accumulated other | | Total Share- | |
| | | Shares | | | | paid-in | | Accumulated | | comprehensive | | holders’ | |
| | | outstanding | | Amount | | capital | | Deficit | | income | | equity | |
| | | | | | | | | | | | | | |
| Shares issued to founder for organization cost and services on July 27, 2004 (inception) | | | 148,000 | | $ | 148 | | $ | - | | $ | - | | $ | - | | $ | 148 | |
| Net loss | | | | | | | | | - | | | (948 | ) | | - | | | (948 | ) |
| Balance at December 31, 2004 | | | 148,000 | | | 148 | | | - | | | (948 | ) | | - | | | (800 | ) |
| | | | | | | | | | | | | | | | | | | | |
| Shares issued for services rendered on February 23, 2005 | | | 11,500,000 | | | 11,500 | | | - | | | | | | - | | | 11,500 | |
| Net loss | | | | | | | | | | | | (11,500 | ) | | - | | | (11,500 | ) |
| Balance at December 31, 2005 | | | 11,648,000 | | $ | 11,648 | | $ | - | | $ | (12,448 | ) | $ | - | | $ | (800 | ) |
See accompanying notes to financial statements.
ASIA BIOTECHNOLOGY GROUP INC. (FORMERLY KNOWN AS ECHELON ACQUISITION CORP.)
(A DEVELOPMENT STAGE COMPANY)
STATEMENTS OF CASH FLOWS
| | | For the Year ended December 31, | | Period from July 27, 2004 (inception) to December 31, | | Period from July 27, 2004 (inception) to December 31, | |
| | | 2005 | | 2004 | | 2005 | |
| | | | | | | | |
| Cash flows from operating activities | | | | | | | |
| Net loss | | $ | (11,500 | ) | $ | (948 | ) | $ | (12,448 | ) |
| Issuance of stock for services rendered | | | 11,500 | | | 148 | | | 11,648 | |
| Increase in accrued liabilities | | | - | | | 800 | | | 800 | |
| Net cash generated from operating activities | | | - | | | - | | | - | |
| | | | | | | | | | | |
| Cash flows from investing activities | | | - | | | - | | | - | |
| | | | | | | | | | | |
| Cash flows from financing activities | | | - | | | - | | | - | |
| | | | | | | | | | | |
| Net increase (decrease) in cash and cash equivalents | | | - | | | - | | | - | |
| Cash and cash equivalents, beginning of year | | | - | | | - | | | - | |
| Cash and cash equivalents, end of year | | $ | - | | $ | - | | $ | - | |
| | | | | | | | | | | |
| Supplementary disclosures of cash flow information: | | | | | | | | | | |
| Cash paid (refund) during the year for: | | | | | | | | | | |
| Interest | | $ | - | | $ | - | | $ | - | |
| Income taxes | | $ | - | | $ | - | | $ | - | |
See accompanying notes to financial statements.
NOTE 1 BUSINESS DESCRIPTION AND ORGANIZATION
Asia Biotechnology Group Inc.(formerly known as Echelon Acquisition Corp.) (“Asia Biotech” or the "Company") was incorporated under the laws of the State of Delaware on July 27, 2004 and has been inactive since inception. The Company intends to serve as a vehicle to effect an asset acquisition, merger, exchange of capital stock or other business combination with a domestic or foreign business.
NOTE 2 SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of Presentation - Development Stage Company
The Company has not earned any revenue from operations. Accordingly, the Company's activities have been accounted for as those of a "Development Stage Enterprise" as set forth in Financial Accounting Standards Board Statement No. 7 ("SFAS 7"). Among the disclosures required by SFAS 7 are that the Company's financial statements be identified as those of a development stage company, and that the statements of operations, stockholders' equity and cash flows disclose activity since the date of the Company's inception.
A. Accounting Method
The Company's financial statements are prepared using the accrual method of accounting. The Company has elected a fiscal year ending on December 31.
B. Cash and cash equivalents
Cash and cash equivalents include cash on hand, cash accounts, interest bearing savings accounts and time certificates of deposit with a maturity of three months or less when purchased.
C. Foreign currency translation
Transactions and balances originally denominated in U.S. dollars are presented at their original amounts. Transactions and balances in other currencies are converted into U.S. dollars in accordance with Statement of Financial Accounting Standards (SFAS) No. 52, "Foreign Currency Translation," and are included in determining net income or loss.
For foreign operations with the local currency as the functional currency, assets and liabilities are translated from the local currencies into U.S. dollars at the exchange rate prevailing at the balance sheet date. Revenues, expenses and cash flows are translated at the average exchange rate for the period to approximate translation at the exchange rate prevailing at the dates those elements are recognized in the financial statements. Translation adjustments resulting from the process of translating the local currency financial statements into U.S. dollars are included in determining comprehensive loss.
D. Use of estimates
The preparation of financial statements in conformity with generally accepted accounting principles requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosures of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
E. Significant Estimates
Several areas require management's estimates relating to uncertainties for which it is reasonably possible that there will be a material change in the near term. The more significant areas requiring the use of management estimates related to valuation of the useful lives of the Company's equipment and valuation of contingent liabilities and the valuation of stock issued for services.
F. Income Taxes
The Company accounts for income taxes under the Financial Accounting Standards Board (FASB) Statement No. 109, "Accounting for Income Taxes" "Statement 109"). Under Statement 109, deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. Under Statement 109, the effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date. There were no current or deferred income tax expense or benefits due to the Company not having any material operations for the period ended December 31, 2005.
G. Basic Loss Per Common Share
Basic loss per common share has been calculated based on the weighted average number of shares outstanding during the period after giving retroactive effect to stock splits. There are no dilutive securities at December 31, 2005 for purposes of computing fully diluted earnings per share.
H. Stock Based Compensation
The Company accounts for stock options issued to employees in accordance with the provisions of the Accounting Principles Board ("APB") Opinion No. 25, "Accounting for Stock Issued to Employees," and related interpretations. As such, compensation cost is measured on the date of grant as the excess of the current market price of the underlying stock over the exercise price. Such compensation amounts, if any, are amortized over the respective vesting periods of the option grant. The Company adopted the disclosure provisions of SFAS No. 123, "Accounting for Stock-Based Compensation" and SFAS 148, "Accounting for Stock-Based Compensation -Transition and Disclosure", which permits entities to provide pro forma net income (loss) and pro forma earnings (loss) per share disclosures for employee stock option grants as if the fair-valued based method defined in SFAS No. 123 had been applied. The Company accounts for stock options and stock issued to non-employees for goods or services in accordance with the fair value method of SFAS 123.
I. Impact of New Accounting Standards
In December 2004, the FASB issued SFAS No. 123(R), “Share-Based Payment”. SFAS 123(R) is a revision of SFAS No., 123, “Accounting for Stock Based Compensation,” and supersedes Accounting Principles Board Opinion No. 25 (“APB 25”), “Accounting for Stock Issued to Employees.” Among other items SFAS 123(R) eliminates the use of APB 25 and the intrinsic value method of accounting, and requires companies to recognize the cost of employee services received in exchange for awards of equity instruments, based on the grant date fair value of those awards, in the financial statements. The effective date of SFAS 123 (R) is the first annual reporting period beginning after June 15, 2005. The adoption of SFAS 123 (R) is not expected to have a material impact on the Company’s financial position, results of operations or cash flows.
In March 2005, the SEC staff issued additional guidance on SFAS 123 (R) in the form of Staff Accounting Bulletin (“SAB”) No. 107. SAB 107 was issued to assist preparers by simplifying some of the implementation challenges of FAS 123 (R) while enhancing the information that investors receive. SAB 107 creates a framework that is premised on two themes: (a) considerable judgment will be required by preparers to successfully implement FAS 123 (R), specifically when valuing employee stock options; and (b) reasonable individuals, acting in good faith, may conclude differently on the fair value of employee share options. Key topics covered by SAB 107 include: (a) valuation models - SAB 107 reinforces the flexibility allowed by FAS 123 (R) to choose an option-pricing model that meets the standard’s fair value measurement objective; (b) expected volatility - the SAB provides guidance on when it would be appropriate to rely exclusively on either historical or implied volatility in estimating expected volatility; and (c) expected term - the new guidance includes examples and some simplified approaches to determining the expected term under certain circumstances. The Company will apply the principles of SAB 107 in conjunction with its adoption of SFAS 123 (R) but does not believe its adoption will have material impact on the Company’s financial statements or results of operations.
In December 2004, the FASB issued SFAS No. 153, Exchanges of Nonmonetary Assets, an amendment of APB Opinion No. 29. SFAS No. 153 addresses the measurement of exchanges of nonmonetary assets and redefines the scope of transactions that should be measured based on the fair value of the assets exchanged. SFAS No. 153 is effective for nonmonetary asset exchanges occurring in fiscal periods beginning after June 15, 2005. The adoption of SFAS No. 153 did not have a material impact on the Company's financial statements or results of operations.
In January 2003, the FASB issued FASB Interpretation No. 46, ("FIN 46"), Consolidation of Variable Interest Entities ("VIE"). Until this interpretation, the Company generally included entities in its consolidated financial statements only if it controlled the entity through voting interests. FIN No. 46 requires a variable interest entity, as defined, to be consolidated by a company if that company is subject to a majority of the risk of loss from the variable interest entity's activities or entitled to receive a majority of the entity's residual returns. FIN No. 46 is effective for reporting periods ending after December 15, 2003. The adoption of FIN No. 46 did not have a material impact on the Company's Consolidated Financial Statements as of December 30, 2005.
In March 2005, FASB issued FASB Interpretation (“FIN”) No. 47, “Accounting for Conditional Asset Retirement Obligations.” FIN 47 clarifies that the term “Conditional Asset Retirement Obligation” as used in FASB Statement No. 143, “Accounting for Asset Retirement Obligation,” refers to a legal obligation to perform an asset retirement activity in which the timing and/or method of settlement are conditional on a future event that may or may not be within the control of the entity. Accordingly, an entity is required to recognize a liability for the fair value of a Conditional Asset Retirement Obligation if the fair value of the liability can be reasonably estimated. FIN 47 is effective no later than the end of fiscal years ending after December 15, 2005. Management does not believe the adoption of FIN 47 will have a material affect on the Company’s financial position, results of operations or cash flows. In May 2005, the Financial Accounting Standards Board (“FASB”) issued SFAS No. 154, Accounting Changes and Error Corrections (“SFAS No. 154”), which replaced Accounting Principles Board Opinion No. 20, Accounting Changes and SFAS No. 3, Reporting Accounting Changes in Interim Financial Statements. SFAS No. 154 changes the requirements for the accounting for and reporting of a change in accounting principles. It requires retrospective application to prior periods’ financial statements of changes in accounting principles, unless it is impracticable to determine either the period-specific effects or the cumulative effect of the change. This statement is effective for accounting changes and corrections of errors made in fiscal years beginning after December 15, 2005. The impact on the Company’s operations will depend on future accounting pronouncements or changes in accounting principles.
In November 2004, the Financial Accounting Statements Board (FASB) issued SFAS Statement No. 151, “Inventory Costs,” an amendment of the Accounting Research Bulletin (ARB) No. 43, Chapter 4. Under FASB Statement No. 151, all abnormal amounts of idle facility expense, freight, handling costs, and wasted materials (spoilage) should be recognized as current-period charges by requiring the allocation of fixed production overheads to inventory based on the normal capacity of the production facilities. The adoption of this pronouncement is not expected to have a material impact on the Company’s financial statements, results of operations, or cash flows.
In May 2005, the Financial Accounting Standards Board (“FASB”) SFAS No. 154, Accounting Changes and Error Corrections (“SFAS No. 154”), which replaces Accounting Principles Board Opinion No. 20, Accounting Changes and SFAS No. 3, Reporting Accounting Changes in Interim Financial Statements. SFAS No. 154 changes the requirements for the accounting for and reporting of a change in accounting principles. It requires retrospective application to prior periods’ financial statements of changes in accounting principles, unless it is impracticable to determine either the period-specific effects or the cumulative effect of the change. This statement is effective for accounting changes and corrections of errors made in fiscal years beginning after December 15, 2005. The impact on the Company’s operations will depend on future accounting pronouncements or changes in accounting principles.
J. Off-Balance Sheet Arrangements
The Company does not have any off-balance sheet credit exposure related to its customers.
NOTE 3 SHAREHOLDER'S EQUITY
On July 27, 2004 (inception), the Board of Directors issued 148,000 shares of common stock for $148 in services to the founding shareholder of the Company to fund organizational start-up costs.
On February 23, 2005, the Board of Directors issued 11,500,000 shares of common stock for $11,500 in services rendered to an officer and director of the Company.
Common Stock
The holders of the Company's common stock:
* Have equal ratable rights to dividends from funds legally available for payment of dividends when, as and if declared by the board of directors;
* Are entitled to share ratably in all of the assets available for distribution to holders of common stock upon liquidation, dissolution or winding up of our affairs;
* Do not have preemptive, subscription or conversion rights, or redemption or access to any sinking fund; and
* Are entitled to one non-cumulative vote per share on all matters submitted to stockholders for a vote at any meeting of stockholders.
Preferred Stock
The Company has authorized, but not issued, 20,000,000 shares of preferred stock at $.001 per share. The board of directors has the authority to establish and fix the designation, powers, or preferences of preferred shares without further vote by the shareholders.
NOTE 4 SUBSEQUENT EVENTS
On May 8, 2006, an agreement and plan of reorganization was entered into among Asia Biotechnology Group Inc. (formerly known as Echelon Acquisition Corp.), a corporation organized under the laws of the State of Delaware (the "Asia Biotech"); Asia Biotechnology Group Inc., a corporation organized under the laws of British Virgin Islands (the "ABG"); Far Grand Investments Limited, a corporation organized under the laws of Cayman Islands, acting as the shareholder of ABG, (the “ABG Shareholder”); Harbin OT Pharmaceutical Co., Limited, a company organized under the laws of Samoa (the “OT Samoa”); and shareholders of OT Samoa ( collectively the “OT Samoa Shareholders”).
The respective Boards of Directors of Asia Biotech, ABG and OT Samoa have adopted resolutions pursuant to which all of the issued and outstanding shares of the common stock of ABG (the “ABG Share”) and all of the issued and outstanding shares of OT Samoa (the “OT Samoa Shares”) will be converted into the right to receive a specified number of shares of the common stock of Asia Biotech (the “Asia Biotech Shares”); and whereas, the sole consideration for the exchange of the ABG Share shall be the receipt by the ABG Shareholder of 23,296,000 Asia Biotech Shares, $0.001 par value per share; and the sole consideration for the exchange of the OT Samoa Shares shall be the receipt by the OT Samoa Shareholders of 23,296,000 Asia Biotech Shares, $0.001 par value per share.
The ABG Shareholder and the OT Samoa Shareholders individually agreed to transfer to Asia Biotech at the closing (the "Closing") the ABG Share and OT Samoa Shares, in exchange for newly issued and restricted shares of common stock of Asia Biotech. In connection with the acquisition of the ABG Share and the OT Samoa Shares, Asia Biotech shall issue to the ABG Shareholder an aggregate of Twenty Three Million Two Hundred and Ninety Six Thousand (23,296,000) shares of Asia Biotech common stock, and shall simultaneously issue to the OT Samoa Shareholders an aggregate of Twenty Three Million Two Hundred and Ninety Six Thousand (23,296,000) shares of Asia Biotech common stock. Such shares at the Closing shall equal eighty percent (80%) of the issued and outstanding shares of Asia Biotech. After the Closing, there will be 58,240,000 outstanding shares of common stock of the reorganized Asia Biotech.
ABG and OT Samoa both became Echelon's wholly owned subsidiaries and the former shareholders of ABG (“shareholders”) obtained effective operating control of the combined company after the share exchange. Generally accepted accounting principles require that ABG whose shareholders retain the majority interest in a combined business be treated as the acquirer for accounting purpose, resulting in a reverse acquisition. Accordingly, the share exchange transaction has been accounted for as a recapitalization of Echelon. The equity section of future financial statements will be been restated to reflect the recapitalization of Echelon due to the reverse acquisition as of the first day of the first period presented.
HARBIN OT PHARMACEUTICAL COMPANY LIMITED
(A DEVELOPMENT STAGE COMPANY)
UNAUDITED BALANCE SHEET
| | | | | December 31, 2005 | |
ASSETS | | | | | |
| Current assets: | | | | | |
| Cash | | | | | $ | 1 | |
| Total assets | | | | | $ | 1 | |
| | | | | | | | |
SHAREHOLDERS’ EQUITY | | | | | | | |
| | | | | | | | |
| Shareholders’ Equity: | | | | | | | |
| Common stock at $0.01 par value; authorized 100,000,000 shares; 100 shares issued and outstanding | | | | | | 1 | |
| Retained earnings during the development stage | | | | | | - | |
| Total shareholders’ equity | | | | | $ | 1 | |
See accompanying notes to unaudited financial statements.
HARBIN OT PHARMACEUTICAL COMPANY LIMITED
(A DEVELOPMENT STAGE COMPANY)
UNAUDITED STATEMENTS OF OPERATIONS
| | | Period from April 13, 2005 (inception) to December 31, | | Accumulated from April 13, 2005 (inception) to December 31, | |
| | | 2005 | | 2005 | |
| | | | | | |
| Revenue | | $ | - | | $ | - | |
| | | | | | | | |
| General and administrative | | | | | | | |
| Organization and related expenses | | | - | | | - | |
| General and administrative expenses | | | - | | | - | |
| Total general and administrative | | | - | | | - | |
| | | | | | | | |
| Net profits | | | - | | | - | |
| | | | | | | | |
| Net earnings per share | | | | | | | |
| - basic and fully diluted | | $ | 0.00 | | $ | 0.00 | |
| | | | | | | | |
| Weighted average ordinary shares outstanding | | | | | | | |
| - basic and fully diluted | | | 100 | | | 100 | |
See accompanying notes to unaudited financial statements.
HARBIN OT PHARMACEUTICAL COMPANY LIMITED
(A DEVELOPMENT STAGE COMPANY)
UNAUDITED STATEMENT OF SHAREHOLDERS' EQUITY
FOR THE PERIOD FROM APRIL 13, 2005 (INCEPTION DATE)
TO DECEMBER 31, 2005
| | | | | | | Retained | | | |
| | | Common stock | | | | Earnings during the | | | |
| | | Shares | | | | development | | Shareholders | |
| | | outstanding | | Amount | | stage | | equity | |
| Issuance of 100 shares of $0.01 par value at par | | | 100 | | $ | 1 | | $ | - | | $ | 1 | |
| Net income | | | - | | | - | | | - | | | - | |
| Balance at December 31, 2005 | | | 100 | | $ | 1 | | $ | - | | $ | 1 | |
See accompanying notes to unaudited financial statements.
HARBIN OT PHARMACEUTICAL COMPANY LIMITED
(A DEVELOPMENT STAGE COMPANY)
UNAUDITED STATEMENTS OF CASH FLOWS
| | | Period from April 13, 2005 (inception) to December 31, | | Accumulated from April 13, 2005 (inception) to December 31, | |
| | | 2005 | | 2005 | |
| | | | | | |
| Cash flows from operating activities | | | | | |
| Net loss | | $ | - | | $ | - | |
| Net cash generated from operating activities | | | - | | | - | |
| | | | | | | | |
| Cash flows from investing activities | | | - | | | - | |
| | | | | | | | |
| Cash flows from financing activities | | | | | | | |
| Issuance of share capital | | | 1 | | | 1 | |
| Net cash generated from financing activities | | | 1 | | | 1 | |
| | | | | | | | |
| Net increase in cash and cash equivalents | | | 1 | | | 1 | |
| Cash and cash equivalents, beginning of period | | | - | | | - | |
| Cash and cash equivalents, end of period | | $ | 1 | | $ | 1 | |
| | | | | | | | |
| Supplementary disclosures of cash flow information: | | | | | | | |
| Cash paid (refund) during the period for: | | | | | | | |
| Interest | | $ | - | | $ | - | |
| Income taxes | | $ | - | | $ | - | |
See accompanying notes to unaudited financial statements.
NOTE 1 BUSINESS DESCRIPTION AND ORGANIZATION
Harbin OT Pharmaceutical Co., Limited ("OT Samoa” or the "Company") is a company organized under the laws of Samoa. OT Samoa was organized by Mr. Zhu Lei in April 13, 2005 in anticipation of a business combination with Asia Biotechnology Group Inc., BVI ("ABG") and a U.S. reporting company. On January 22, 2006, Mr. Zhu licensed his proprietary technology/know-how related to the manufacture of certain medicines to OT Samoa . At the same day, OT Samoa entered into a Cooperation Agreement with ABG. The technology was not recorded on the books of OT Samoa, because the agreement consists only of an operating license and royalty arrangement with no transfer of ownership. OT Samoa did not engage in any business other than licensing the technology owned by Mr. Zhu and cooperating with ABG on the manufacturing of the know-how related products.
NOTE 2 SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of Presentation - Development Stage Company
The Company has not earned any revenue from operations. Accordingly, the Company's activities have been accounted for as those of a "Development Stage Enterprise" as set forth in Financial Accounting Standards Board Statement No. 7 ("SFAS 7"). Among the disclosures required by SFAS 7 are that the Company's financial statements be identified as those of a development stage company, and that the statements of operations, stockholders' equity and cash flows disclose activity since the date of the Company's inception.
A. Accounting Method
The Company's financial statements are prepared using the accrual method of accounting. The Company has elected a fiscal year ending on December 31.
B. Cash and cash equivalents
Cash and cash equivalents include cash on hand.
C. Foreign currency translation
Transactions and balances originally denominated in U.S. dollars are presented at their original amounts. Transactions and balances in other currencies are converted into U.S. dollars in accordance with Statement of Financial Accounting Standards (SFAS) No. 52, "Foreign Currency Translation," and are included in determining net income or loss.
For foreign operations with the local currency as the functional currency, assets and liabilities are translated from the local currencies into U.S. dollars at the exchange rate prevailing at the balance sheet date. Revenues, expenses and cash flows are translated at the average exchange rate for the period to approximate translation at the exchange rate prevailing at the dates those elements are recognized in the financial statements. Translation adjustments resulting from the process of translating the local currency financial statements into U.S. dollars are included in determining comprehensive loss.
D. Use of estimates
The preparation of financial statements in conformity with generally accepted accounting principles requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosures of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
E. Significant Estimates
Several areas require management's estimates relating to uncertainties for which it is reasonably possible that there will be a material change in the near term. The more significant areas requiring the use of management estimates related to valuation of the useful lives of the Company's equipment and valuation of contingent liabilities and the valuation of stock issued for services.
F. Income Taxes
The Company accounts for income taxes under the Financial Accounting Standards Board (FASB) Statement No. 109, "Accounting for Income Taxes" "Statement 109"). Under Statement 109, deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. Under Statement 109, the effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date. There were no current or deferred income tax expense or benefits due to the Company not having any material operations for the period ended December 31, 2005.
G. Basic Loss Per Common Share
Basic loss per common share has been calculated based on the weighted average number of shares outstanding during the period after giving retroactive effect to stock splits. There are no dilutive securities at December 31, 2005 for purposes of computing fully diluted earnings per share.
H. Stock Based Compensation
The Company accounts for stock options issued to employees in accordance with the provisions of the Accounting Principles Board ("APB") Opinion No. 25, "Accounting for Stock Issued to Employees," and related interpretations. As such, compensation cost is measured on the date of grant as the excess of the current market price of the underlying stock over the exercise price. Such compensation amounts, if any, are amortized over the respective vesting periods of the option grant. The Company adopted the disclosure provisions of SFAS No. 123, "Accounting for Stock-Based Compensation" and SFAS 148, "Accounting for Stock-Based Compensation -Transition and Disclosure", which permits entities to provide pro forma net income (loss) and pro forma earnings (loss) per share disclosures for employee stock option grants as if the fair-valued based method defined in SFAS No. 123 had been applied. The Company accounts for stock options and stock issued to non-employees for goods or services in accordance with the fair value method of SFAS 123.
I. Impact of New Accounting Standards
In January 2003, the FASB issued FASB Interpretation No. 46, ("FIN 46"), Consolidation of Variable Interest Entities ("VIE"). Until this interpretation, the Company generally included entities in its consolidated financial statements only if it controlled the entity through voting interests. FIN No. 46 requires a variable interest entity, as defined, to be consolidated by a company if that company is subject to a majority of the risk of loss from the variable interest entity's activities or entitled to receive a majority of the entity's residual returns. FIN No. 46 is effective for reporting periods ending after December 15, 2003. The adoption of FIN No. 46 did not have a material impact on the Company's Consolidated Financial Statements as of December 31, 2005.
In November 2004, the Financial Accounting Statements Board (FASB) issued SFAS Statement No. 151, “Inventory Costs,” an amendment of the Accounting Research Bulletin (ARB) No. 43, Chapter 4. Under FASB Statement No. 151, all abnormal amounts of idle facility expense, freight, handling costs, and wasted materials (spoilage) should be recognized as current-period charges by requiring the allocation of fixed production overheads to inventory based on the normal capacity of the production facilities. The adoption of this pronouncement is not expected to have a material impact on the Company’s financial statements, results of operations, or cash flows.
In December 2004, the FASB issued SFAS No. 123(R), “Share-Based Payment”. SFAS 123(R) is a revision of SFAS No., 123, “Accounting for Stock Based Compensation,” and supersedes Accounting Principles Board Opinion No. 25 (“APB 25”), “Accounting for Stock Issued to Employees.” Among other items SFAS 123(R) eliminates the use of APB 25 and the intrinsic value method of accounting, and requires companies to recognize the cost of employee services received in exchange for awards of equity instruments, based on the grant date fair value of those awards, in the financial statements. The effective date of SFAS 123 (R) is the first annual reporting period beginning after December 15, 2005. The adoption of SFAS 123 (R) is not expected to have a material impact on the Company’s financial position, results of operations or cash flows.
In December 2004, the FASB issued SFAS No. 153, Exchanges of Nonmonetary Assets, an amendment of APB Opinion No. 29. SFAS No. 153 addresses the measurement of exchanges of nonmonetary assets and redefines the scope of transactions that should be measured based on the fair value of the assets exchanged. SFAS No. 153 is effective for nonmonetary asset exchanges occurring in fiscal periods beginning after June 15, 2005. The adoption of SFAS No. 153 did not have a material impact on the Company's financial statements or results of operations.
In March 2005, the SEC staff issued additional guidance on SFAS 123 (R) in the form of Staff Accounting Bulletin (“SAB”) No. 107. SAB 107 was issued to assist preparers by simplifying some of the implementation challenges of FAS 123 (R) while enhancing the information that investors receive. SAB 107 creates a framework that is premised on two themes: (a) considerable judgment will be required by preparers to successfully implement FAS 123 (R), specifically when valuing employee stock options; and (b) reasonable individuals, acting in good faith, may conclude differently on the fair value of employee share options. Key topics covered by SAB 107 include: (a) valuation models - SAB 107 reinforces the flexibility allowed by FAS 123 (R) to choose an option-pricing model that meets the standard’s fair value measurement objective; (b) expected volatility - the SAB provides guidance on when it would be appropriate to rely exclusively on either historical or implied volatility in estimating expected volatility; and (c) expected term - the new guidance includes examples and some simplified approaches to determining the expected term under certain circumstances. The Company will apply the principles of SAB 107 in conjunction with its adoption of SFAS 123 (R) but does not believe its adoption will have material impact on the Company’s financial statements or results of operations.
In March 2005, FASB issued FASB Interpretation (“FIN”) No. 47, ”“Accounting for Conditional Asset Retirement Obligations.” FIN 47 clarifies that the term “Conditional Asset Retirement Obligation” as used in FASB Statement No. 143, “Accounting for Asset Retirement Obligation,” refers to a legal obligation to perform an asset retirement activity in which the timing and/or method of settlement are conditional on a future event that may or may not be within the control of the entity. Accordingly, an entity is required to recognize a liability for the fair value of a Conditional Asset Retirement Obligation if the fair value of the liability can be reasonably estimated. FIN 47 is effective no later than the end of fiscal years ending after December 15, 2005. Management does not believe the adoption of FIN 47 will have a material affect on the Company’s financial position, results of operations or cash flows.
In May 2005, the Financial Accounting Standards Board (“FASB”) issued SFAS No. 154, Accounting Changes and Error Corrections (“SFAS No. 154”), which replaced Accounting Principles Board Opinion No. 20, Accounting Changes and SFAS No. 3, Reporting Accounting Changes in Interim Financial Statements. SFAS No. 154 changes the requirements for the accounting for and reporting of a change in accounting principles. It requires retrospective application to prior periods’ financial statements of changes in accounting principles, unless it is impracticable to determine either the period-specific effects or the cumulative effect of the change. This statement is effective for accounting changes and corrections of errors made in fiscal years beginning after December 15, 2005. The impact on the Company’s operations will depend on future accounting pronouncements or changes in accounting principles.
In July 2006, the Financial Accounting Standards Board (“FASB”) has published FASB Interpretation No. 48 (“FIN No. 48”), Accounting for Uncertainty in Income Taxes, to address the noncomparability in reporting tax assets and liabilities resulting from a lack of specific guidance in FASB Statement of Financial Accounting Standards (“SFAS”) No. 109, Accounting for Income Taxes, on the uncertainty in income taxes recognized in an enterprise’s financial statements. FIN No. 48 will apply to fiscal years beginning after December 15, 2006, with earlier adoption permitted. The adoption of FIN 48 is not expected to have a material effect on the Company’s financial condition or results of operations.
J. Off-Balance Sheet Arrangements
The Company does not have any off-balance sheet credit exposure related to its customers.
NOTE 3 INCOME TAXES
The Company is incorporated in Samoa and is not subject to income taxes under the current laws of the Samoa.
NOTE 4 SHAREHOLDER'S EQUITY
On April 13, 2005 (inception), the Company issued 100 shares of common stock of $0.01 par value to Mr. Zhu Lei for cash of $1.
NOTE 5 SUBSEQUENT EVENTS
On January 19, 2006, the Company further issued 19,999,900 shares of common stock of $0.01 par value to Mr. Zhu Lei for cash of $199,999.
On January 26, 2006, Mr. Zhu Lei, the sole incorporator, owner and control person transferred his shares to 1,399 separate individuals. Mr. Zhu remained the sole director of OT Samoa and retained rights of rescission with regard to the transfer of his shares to the 1,399 individuals up until the time that those shares were exchanged for Asia Biotechnology Group Inc., US shares.
On May 8, 2006, an agreement and plan of reorganization was entered into among Asia Biotechnology Group Inc. , a corporation organized under the laws of the State of Delaware ("Asia Biotech"); Asia Biotechnology Group Inc., a corporation organized under the laws of British Virgin Islands ("ABG"); Far Grand Investments Limited, a corporation organized under the laws of Cayman Islands, acting as the shareholder of ABG, (the “ABG Shareholder”); Harbin OT Pharmaceutical Co., Limited, a company organized under the laws of Samoa ("OT Samoa”); and shareholders of OT Samoa ( collectively the “OT Samoa Shareholders”).
The respective Boards of Directors of Asia Biotech, ABG and OT Samoa have adopted resolutions pursuant to which all of the issued and outstanding shares of the common stock of ABG (the “ABG Share”) and all of the issued and outstanding shares of OT Samoa (the “OT Samoa Shares”) will be converted into the right to receive a specified number of shares of the common stock of Asia Biotech (the “Asia Biotech Shares”); and whereas, the sole consideration for the exchange of the ABG Share shall be the receipt by the ABG Shareholder of 23,296,000 Asia Biotech Shares, $0.001 par value per share; and the sole consideration for the exchange of the OT Samoa Shares shall be the receipt by the OT Samoa Shareholders of 23,296,000 Asia Biotech Shares, $0.001 par value per share.
The ABG Shareholder and the OT Samoa Shareholders individually agreed to transfer to Asia Biotech at the closing (the "Closing") the ABG Share and OT Samoa Shares, in exchange for newly issued and restricted shares of common stock of Asia Biotech. In connection with the acquisition of the ABG Share and the OT Samoa Shares, Asia Biotech shall issue to the ABG Shareholder 23,296,000 shares of Asia Biotech common stock, and shall simultaneously issue to the OT Samoa Shareholders an aggregate of 23,296,000 shares of Asia Biotech common stock. Such shares at the Closing shall equal 80% of the issued and outstanding shares of Asia Biotech. After the Closing, there will be 58,240,000 outstanding shares of common stock of the reorganized Asia Biotech.
Child, Van Wagoner & Bradshaw, PLLC
A PROFESSIONAL LIMITED LIABILITY COMPANY OF CERTIFIED PUBLIC ACCOUNTANTS
5296 S. Commerce Dr., Suite 300, Salt Lake City, UT 84107
PHONE: (801) 281-4700 FAX: (801) 281-4701
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To The Board of Directors
Asia Biotechnology Group Inc.
Beijing, PRC
We have audited the accompanying consolidated balance sheet of Asia Biotechnology Group Inc. as of December 31, 2005, and the related consolidated statements of operations and comprehensive loss, shareholders’ equity (deficit), and cash flows for the period from March 21, 2005 (Date of Inception) to December 31, 2005. These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States of America). Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audits included consideration of internal control over financial reporting, as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the consolidated financial position of Asia Biotechnology Group Inc. as of December 31, 2005, and the results of its operations and its cash flows for the period from March 21, 2005 (Date of Inception) to December 31, 2005, in conformity with accounting principles generally accepted in the United States of America.
Child, Van Wagoner & Bradshaw, PLLC
Salt Lake City, Utah
May 8, 2006
ASIA BIOTECHNOLOGY GROUP INC.
CONSOLIDATED BALANCE SHEET
| | | December 31, | |
| | | 2005 | |
| ASSETS | | | |
| | | | |
| Current assets | | | |
| Cash and cash equivalents | | $ | 459,310 | |
| Accounts receivable, less allowances for doubtful accounts of $ 36,095. | | | 426,124 | |
| Inventories | | | 247,314 | |
| Prepaid expense - reorganization expense | | | 315,000 | |
| Other current assets | | | 20,087 | |
| Total current assets | | | 1,467,835 | |
| | | | | |
| Property, plant and equipment, net | | | 860,021 | |
| Land use right, net | | | 100,362 | |
| Total assets | | $ | 2,428,218 | |
| | | | | |
| LIABILITIES AND SHAREHOLDERS’ DEFICIT | | | | |
| | | | |
| Current liabilities | | | | |
| Accounts payable | | $ | 107,658 | |
| Accrued expenses | | | 13,385 | |
| Customer deposits | | | 354,010 | |
| Due to shareholders | | | 1,577,050 | |
| Other current liabilities | | | 6,047 | |
| Total current liabilities | | | 2,058,150 | |
| | | | | |
| Minority interests | | | 434,032 | |
| | | | | |
| Shareholders’ deficit | | | | |
Ordinary share, par value $1 per share; authorized 50,000 shares, shares issued and outstanding 1 share | | | 1 | |
| Accumulated deficit | | | (64,052 | ) |
| Accumulated other comprehensive income | | | 87 | |
| Total shareholders’ deficit | | | (63,964 | ) |
| Total liabilities and shareholders’ deficit | | $ | 2,428,218 | |
See accompanying notes to consolidated financial statements.
ASIA BIOTECHNOLOGY GROUP INC.
CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS
| | | March 21, 2005 (Date of Inception) to December 31, 2005 | |
| | | | |
| Net sales | | $ | 37,972 | |
| | | | | |
| Cost of sales | | | 32,214 | |
| | | | | |
| Gross profit | | | 5,758 | |
| | | | | |
| Operating expenses | | | | |
| Allowance for bad debt | | | 3,614 | |
| Accounting and audit fee | | | 45,000 | |
| Salaries | | | 8,154 | |
| Depreciation | | | 4,549 | |
| Amortization of land use right | | | 373 | |
| Other selling, general and administrative | | | 31,420 | |
| | | | | |
| Total operating expenses | | | 93,110 | |
| | | | | |
| Loss from operations | | | (87,352 | ) |
| | | | | |
| Non-Operating Income | | | | |
| Government Grant | | | 10,618 | |
| Interest income | | | 47 | |
| | | | | |
| Total Non-Operating Expenses | | | 10,665 | |
| | | | | |
| Loss before income taxes and minority interests | | | (76,787 | ) |
| | | | | |
| Income taxes | | | - | |
| | | | | |
| Loss before minority interests | | | (76,787 | ) |
| | | | | |
| Minority interests | | | 12,635 | |
| | | | | |
| Net loss | | $ | (64,052 | ) |
| | | | | |
| Other comprehensive income | | | | |
| Foreign currency translation adjustment | | | 87 | |
| | | | | |
| Comprehensive loss | | $ | (63,965 | ) |
| | | | | |
| Loss per share - basic and diluted | | $ | (64,052 | ) |
| | | | | |
| Weighted average shares outstanding - basic and diluted | | | 1 | |
See accompanying notes to consolidated financial statements.
ASIA BIOTECHNOLOGY GROUP INC.
CONSOLIDATED STATEMENT OF SHAREHOLDERS' EQUITY (DEFICIT)
FOR THE PERIOD FROM MARCH 21, 2005 (DATE OF INCEPTION)
TO DECEMBER 31, 2005
| | | Ordinary share | | Additional | | | | Accumulated other | | | |
| | Shares | | | | paid-in | | Accumulated | | comprehensive | | equity | |
| | | outstanding | | Amount | | capital | | deficit | | income | | (deficit) | |
| | | | | | | | | | | | | | |
| Share capital | | | 1 | | $ | 1 | | $ | - | | $ | - | | $ | - | | $ | 1 | |
| Net loss | | | | | | | | | | | | (64,052 | ) | | | | | (64,052 | ) |
| Foreign currency translation gain | | | | | | | | | | | | | | | 87 | | | 87 | |
| | | | | | | | | | | | | | | | | | | | |
| Balance at December 31, 2005 | | | 1 | | $ | 1 | | $ | - | | $ | (64,052 | ) | $ | 87 | | $ | (63,964 | ) |
See accompanying notes to consolidated financial statements.
ASIA BIOTECHNOLOGY GROUP INC.
CONSOLIDATED STATEMENT OF CASH FLOW
FOR THE PERIOD FROM MARCH 21, 2005 (DATE OF INCEPTION)
TO DECEMBER 31, 2005
| | | March 21, 2005 (Date of Inception) to December 31, 2005 | |
| | | | |
| Cash flows from operating activities | | | |
| Net loss | | $ | (64,052 | ) |
| Adjustment to reconcile net income to net cash used in | | | | |
| operating activities: | | | | |
| Depreciation and amortization of property, plant and equipment | | | 18,284 | |
| Minority interests share of net loss | | | (12,635 | ) |
| Changes in current assets and liabilities (net of effects of acquisitions and disposals of entities) | | | | |
| Accounts receivable | | | 30,660 | |
| Inventories | | | 4,091 | |
| Prepaid expense - reorganization expense | | | (315,000 | ) |
| Other current assets | | | (10,693 | ) |
| Accounts payable | | | 5,732 | |
| Accrued expenses | | | 1,696 | |
| Customer deposits | | | 11,572 | |
| Other current liabilities | | | (13,467 | ) |
| Net cash used in operating activities | | | (343,812 | ) |
| | | | | |
| Cash flows from investing activities | | | | |
| Capital expenditure | | | (13,151 | ) |
| Cost of investment in subsidiary, net of cash acquired in acquisition | | | (666,389 | ) |
| Net cash used in investing activities | | | (679,540 | ) |
| | | | | |
| Cash flows from financing activities | | | | |
| Proceeds from share capital | | | 1 | |
| Advances from shareholders | | | 1,482,574 | |
| Net cash provided by financing activities | | | 1,482,575 | |
| | | | | |
| Effect of foreign currencies on cash flows | | | 87 | |
| | | | | |
| Net increase in cash and cash equivalents | | | 459,310 | |
| Cash and cash equivalents, beginning of period | | | - | |
| Cash and cash equivalents, end of period | | $ | 459,310 | |
See accompanying notes to consolidated financial statements.
NOTE 1 ORGANIZATION
Asia Biotechnology Group Inc. (“the Company” or “Asia Biotechnology” or “ABG”) is a limited liability company registered under the laws of the British Virgin Islands and was incorporated in British Virgin Islands on March 21, 2005.
Asia Biotechnology is an investment holding and it acquired 60% shareholding of a company called OT Pharmaceutical Co. Ltd. (“OT Pharmaceutical”) on November 3, 2005. All activities of the Group are principally conducted by subsidiary company operating in the People’s Republic of China (“PRC”).
OT Pharmaceutical Company Limited is a Chinese foreign owned enterprise incorporated in the People’s Republic of China (“PRC”) on April 13, 2001. The Company is a feminine suppository manufacturer and provides this suppository to clinics and the Red Cross Society of China.
NOTE 2 SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
A. Basis of presentation
The consolidated financial statements include the accounts of Asia Biotechnology Group Inc. and OT Pharmaceutical Company Limited. All material intercompany accounts and transactions have been eliminated in consolidation.
B. Fiscal year
These financial statements have been prepared using December 31 as the fiscal year end.
C. Minority interest in subsidiary
The Company records minority interest expense, which reflects the minority shareholders' 40% portion of the earnings or loss of OT Pharmaceutical Company Limited.
D. Control by principal stockholders
The directors, executive officers and their affiliates or related parties own, beneficially and in the aggregate, the majority of the voting power of the outstanding shares of the common stock of the Company. Accordingly, the directors, executive officers and their affiliates, if they voted their shares uniformly, would have the ability to control the approval of most corporate actions, including increasing the authorized capital stock of the Company and the dissolution, merger or sale of the Company's assets.
E. Cash and cash equivalents
Cash and cash equivalents include cash on hand, cash accounts, interest bearing savings accounts and time certificates of deposit with a maturity of three months or less when purchased.
F. Inventories
Inventories are stated at the lower of cost or market, determined by the weighted average method. Finished goods inventories consist of raw materials, direct labor and overhead associated with the manufacturing process.
G. Trade accounts receivable
Trade accounts receivable are recorded at the invoiced amount and do not bear interest. The allowance for doubtful accounts represents the Company’s best estimate of the amount of probable credit losses in the existing accounts receivable balance. The Company determines the allowance for doubtful accounts based upon historical write-off experience and current economic conditions. The Company reviews the adequacy of its allowance for doubtful accounts on a regular basis. Account balances are charged off against the allowance after all means of collection have been exhausted and the potential for recovery is considered remote. The Company signed a sales contract with Red Cross Society of China with the terms of payment is over than one year.
H. Credit Risk and Customers
I. Property, plant and equipment
Property, plant and equipment are stated at cost including the cost of improvements. Depreciation and amortization are provided on the straight-line method based on the estimated useful lives of the assets as follows:
| Buildings | | 20 years |
| Leasehold improvements | | 20 years |
| Plant and machinery | | 10 years |
| Motor vehicles | | 5 years |
| Furniture, fixtures and equipment | | 5 years |
J. Valuation of long-lived assets
The Company periodically evaluates the carrying value of long-lived assets to be held and used, including intangible assets subject to amortization, when events and circumstances warrant such a review. The carrying value of a long-lived asset is considered impaired when the anticipated undiscounted cash flow from such asset is separately identifiable and is less than its carrying value. In that event, a loss is recognized based on the amount by which the carrying value exceeds the fair market value of the long-lived asset. Fair market value is determined primarily using the anticipated cash flows discounted at a rate commensurate with the risk involved. Losses on long-lived assets to be disposed of are determined in a similar manner, except that fair market values are reduced for the cost to dispose.
The Company recognizes revenue when it is realized and earned. The Company considers revenue realized or realizable and earned when (1) it has persuasive evidence of an arrangement, (2) delivery has occurred, (3) the sales price is fixed or determinable, and (4) collectibility is reasonably assured. Delivery does not occur until products have been shipped to the client, risk of loss has transferred to the client and client acceptance has been obtained, client acceptance provisions have lapsed, or the Company has objective evidence that the criteria specified in client acceptance provisions have been satisfied. The sales price is not considered to be fixed or determinable until all contingencies related to the sale have been resolved.
L. Comprehensive income (loss)
Comprehensive income (loss) includes changes to equity accounts that were not the result of transactions with shareholders. Comprehensive income (loss) is comprised of net income (loss) and other comprehensive income and loss items. The Company’s comprehensive income and losses generally consist of changes in the fair value of changes in the cumulative foreign currency translation adjustment.
M. Foreign currency translation
Transactions and balances originally denominated in U.S. dollars are presented at their original amounts. Transactions and balances in other currencies are converted into U.S. dollars in accordance with Statement of Financial Accounting Standards (SFAS) No. 52, "Foreign Currency Translation," and are included in determining net income or loss.
For foreign operations with the local currency as the functional currency, assets and liabilities are translated from the local currencies into U.S. dollars at the exchange rate prevailing at the balance sheet date. Revenues, expenses and cash flows are translated at the average exchange rate for the period to approximate translation at the exchange rate prevailing at the dates those elements are recognized in the financial statements. Translation adjustments resulting from the process of translating the local currency financial statements into U.S. dollars are included in determining comprehensive loss.
The Company has determined the PRC Chinese Yuan Renminbi to be the functional currency of the Company. The financial statements of the Company are translated to United States dollars using year-end rates of exchange for assets and liabilities, and average rates of exchange for the period for revenues, costs, and expenses. Net gains and losses resulting from foreign exchange transactions are included in the consolidated statements of operations. The cumulative translation adjustment and effect of exchange rate changes at December 31, 2005 were $87.
N. Stockholder Loan
The caption "Due to shareholders" on the consolidated Balance Sheet consists of loans that are unsecured, non-interest bearing and have no fixed terms of repayment, and therefore, are deemed payable on demand.
O. Use of estimates
The preparation of financial statements in conformity with generally accepted accounting principles requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosures of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
P. Significant Estimates
Several areas require management's estimates relating to uncertainties for which it is reasonably possible that there will be a material change in the near term. The more significant areas requiring the use of management estimates related to valuation of the useful lives of the Company's equipment and valuation of contingent liabilities.
Q. Income Taxes
The Company accounts for income taxes under the Financial Accounting Standards Board (FASB) Statement No. 109, "Accounting for Income Taxes" "Statement 109"). Under Statement 109, deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. Under Statement 109, the effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date.
R. Basic Loss Per Common Share
Basic loss per common share has been calculated based on the weighted average number of shares outstanding during the period.
S. Off-Balance Sheet Arrangements
The Company does not have any off-balance sheet credit exposure related to its customers.
NOTE 3 INVENTORIES
Inventories by major categories, consist of the following at December 31, 2005:
| Raw materials | | $ | 182,250 | |
| Finished goods | | | 26,846 | |
| Packaging materials | | | 38,218 | |
| | | $ | 247,314 | |
NOTE 4 PROPERTY, PLANT AND EQUIPMENT
Property, plant and equipment, which is all located in the PRC, consist of the following at December 31, 2005:
| At cost: | | | |
| Buildings | | $ | 828,871 | |
| Leasehold improvements | | | 51,375 | |
| Plant and machinery | | | 298,042 | |
| Motor vehicles | | | 83,921 | |
| Furniture, fixtures and equipment | | | 44,012 | |
| Total | | | 1,306,221 | |
| Less: accumulated depreciation and amortization | | | (446,200 | ) |
| Net book value | | $ | 860,021 | |
NOTE 5 LAND USE RIGHT
Land use right for the land located in PRC, consists of the following at December 31, 2005:
| Land Use Right, cost | | $ | 110,718 | |
| Less: accumulated amortization | | | (10,356 | ) |
| Net book value | | $ | 100,362 | |
All the land in the PRC is owned by the PRC government. The government, according to PRC laws, may grant to entities the right to use of land for a specified period of time. Thus all of the Company’s land occupied in the PRC is considered to be leasehold land and amortized on a straight-line basis over the respective term of the right to use the land.
The subsidiary, OT Pharmaceutical is granted the right to use of land for 50 years and is amortized on a straight-line basis over 50 years of the right to use the land from the date of acquisition in 2001.
NOTE 6 INTELLECTUAL PROPERTY
The subsidiary, OT Pharmaceutical, owns patent rights and technical know-how which were contributed by minority shareholders. The costs to the minority shareholder for obtaining the patent right were not recorded on the balance sheet as the patent application costs were not significant.
NOTE 7 BUSINESS ACQUISITION
On November 3, 2005, the Company obtained a 60% interest in OT Pharmaceutical Co. Ltd. for $670,000 in cash.
The fair value of assets acquired are as follows:
| Cash | | $ | 3,611 | |
| Accounts receivable | | | 430,215 | |
| Inventory | | | 277,973 | |
| Prepaid expenses | | | 9,395 | |
| Property, plant and equipment | | | 965,515 | |
| Due to shareholder | | | (94,477 | ) |
| Liabilities assumed | | | (475,565 | ) |
| Net assets | | | 1,116,667 | |
| Less: minority interest @ 40% | | | 446,667 | |
| Net assets purchased | | $ | 670,000 | |
The financial statements for the period from March 21, 2005 (Date of Inception) to December 31, 2005 include the results of operations of the acquired company for the period November 3, 2005 to December 31, 2005.
NOTE 8 INCOME TAXES
The Company accounts for income taxes in accordance with the provisions of SFAS No. 109, "Accounting for Income Taxes."
Income tax expense is based on reported income before income taxes. Deferred income taxes reflect the effect of temporary differences between assets and liabilities that are recognized for financial reporting purposes and the amounts that are recognized for income tax purposes. In accordance with SFAS 109, these deferred income taxes are measured by applying currently enacted tax laws.
BRITISH VIRGIN ISLANDS
The Company is incorporated in the British Virgin Islands and, under the current laws of the British Virgin Islands, is not subject to income taxes.
PRC
Enterprises income tax in PRC is generally charged at 33%, in which 30% is for national tax and 3% is for local tax, of the assessable profit. The Company incorporated in PRC are subject to PRC enterprises income tax at the applicable tax rates on the taxable income as reported in their Chinese statutory accounts in accordance with the relevant enterprises income tax laws applicable to foreign enterprises. Pursuant to the same enterprises income tax laws, the subsidiaries are fully exempted from PRC enterprises income tax for two years starting from the first profit-making year, followed by a 50% tax exemption for the next three years.
According to the PRC’s applicable income tax laws, regulations, notices and decisions related to foreign investment enterprises and their investors, income such as dividends and profits distribution from the PRC derived from a foreign enterprise which has no establishment in the PRC is subject to a 10% withholding tax.
There are net operating loss carryforwards allowed under the China's governments' tax systems. In China, the previous five years net operating losses are allowed to be carryforward five years to offset future taxable income. The Company has available approximately $ 1,100,000 of unused operating loss carryforwards and based on a 33% tax rate has a deferred tax asset of approximately $363,000 in which the company recorded a valuation allowance for the same amount at December 31, 2005.
The company withholds and pays income taxes on its employees' wages, which funds the Chinese government's sponsored health and retirement programs of all the employees.
NOTE 9 SHAREHOLDERS’ EQUITY
The Company was incorporated with an authorized share capital of $50,000 divided into 50,000 shares of $1 each.
1 subscribers’ share was issued on inception of the Company.
NOTE 10 EMPLOYEE BENEFITS
The Company has established its own employee welfare plan in accordance with Chinese law and regulations. The Company makes annual contributions of 14% of all employees’ salaries to its employee welfare plan. The total expenses for the above plan were $1,142 for the period from March 21, 2005 (Inception Date) to December 31, 2005. The Company has recorded welfare payments in the amount of $13,385 as of December 31, 2005 in balance sheet.
NOTE 11 RECENT ACCOUNTING PRONOUNCEMENTS
In December 2004, the FASB issued SFAS No. 123(R), “Share-Based Payment”. SFAS 123(R) is a revision of SFAS No., 123, “Accounting for Stock Based Compensation,” and supersedes Accounting Principles Board Opinion No. 25 (“APB 25”), “Accounting for Stock Issued to Employees.” Among other items SFAS 123(R) eliminates the use of APB 25 and the intrinsic value method of accounting, and requires companies to recognize the cost of employee services received in exchange for awards of equity instruments, based on the grant date fair value of those awards, in the financial statements. The effective date of SFAS 123 (R) is the first annual reporting period beginning after June 15, 2005. The adoption of SFAS 123 (R) is not expected to have a material impact on the Company’s financial position, results of operations or cash flows.
In March 2005, the SEC staff issued additional guidance on SFAS 123 (R) in the form of Staff Accounting Bulletin (“SAB”) No. 107. SAB 107 was issued to assist preparers by simplifying some of the implementation challenges of FAS 123 (R) while enhancing the information that investors receive. SAB 107 creates a framework that is premised on two themes: (a) considerable judgment will be required by preparers to successfully implement FAS 123 (R), specifically when valuing employee stock options; and (b) reasonable individuals, acting in good faith, may conclude differently on the fair value of employee share options. Key topics covered by SAB 107 include: (a) valuation models - SAB 107 reinforces the flexibility allowed by FAS 123 (R) to choose an option-pricing model that meets the standard’s fair value measurement objective; (b) expected volatility - the SAB provides guidance on when it would be appropriate to rely exclusively on either historical or implied volatility in estimating expected volatility; and (c) expected term - the new guidance includes examples and some simplified approaches to determining the expected term under certain circumstances. The Company will apply the principles of SAB 107 in conjunction with its adoption of SFAS 123 (R) but does not believe its adoption will have material impact on the Company’s financial statements or results of operations.
In December 2004, the FASB issued SFAS No. 153, Exchanges of Nonmonetary Assets, an amendment of APB Opinion No. 29. SFAS No. 153 addresses the measurement of exchanges of nonmonetary assets and redefines the scope of transactions that should be measured based on the fair value of the assets exchanged. SFAS No. 153 is effective for nonmonetary asset exchanges occurring in fiscal periods beginning after June 15, 2005. The adoption of SFAS No. 153 did not have a material impact on the Company's financial statements or results of operations.
In January 2003, the FASB issued FASB Interpretation No. 46, ("FIN 46"), Consolidation of Variable Interest Entities ("VIE"). Until this interpretation, the Company generally included entities in its consolidated financial statements only if it controlled the entity through voting interests. FIN No. 46 requires a variable interest entity, as defined, to be consolidated by a company if that company is subject to a majority of the risk of loss from the variable interest entity's activities or entitled to receive a majority of the entity's residual returns. FIN No. 46 is effective for reporting periods ending after December 15, 2003. The adoption of FIN No. 46 did not have a material impact on the Company's Consolidated Financial Statements as of December 30, 2005.
In March 2005, FASB issued FASB Interpretation (“FIN”) No. 47, “Accounting for Conditional Asset Retirement Obligations.” FIN 47 clarifies that the term “Conditional Asset Retirement Obligation” as used in FASB Statement No. 143, “Accounting for Asset Retirement Obligation,” refers to a legal obligation to perform an asset retirement activity in which the timing and/or method of settlement are conditional on a future event that may or may not be within the control of the entity. Accordingly, an entity is required to recognize a liability for the fair value of a Conditional Asset Retirement Obligation if the fair value of the liability can be reasonably estimated. FIN 47 is effective no later than the end of fiscal years ending after December 15, 2005. Management does not believe the adoption of FIN 47 will have a material affect on the Company’s financial position, results of operations or cash flows. In May 2005, the Financial Accounting Standards Board (“FASB”) issued SFAS No. 154, Accounting Changes and Error Corrections (“SFAS No. 154”), which replaced Accounting Principles Board Opinion No. 20, Accounting Changes and SFAS No. 3, Reporting Accounting Changes in Interim Financial Statements. SFAS No. 154 changes the requirements for the accounting for and reporting of a change in accounting principles. It requires retrospective application to prior periods’ financial statements of changes in accounting principles, unless it is impracticable to determine either the period-specific effects or the cumulative effect of the change. This statement is effective for accounting changes and corrections of errors made in fiscal years beginning after December 15, 2005. The impact on the Company’s operations will depend on future accounting pronouncements or changes in accounting principles.
In November 2004, the Financial Accounting Statements Board (FASB) issued SFAS Statement No. 151, “Inventory Costs,” an amendment of the Accounting Research Bulletin (ARB) No. 43, Chapter 4. Under FASB Statement No. 151, all abnormal amounts of idle facility expense, freight, handling costs, and wasted materials (spoilage) should be recognized as current-period charges by requiring the allocation of fixed production overheads to inventory based on the normal capacity of the production facilities. The adoption of this pronouncement is not expected to have a material impact on the Company’s financial statements, results of operations, or cash flows.
In May 2005, the Financial Accounting Standards Board (“FASB”) SFAS No. 154, Accounting Changes and Error Corrections (“SFAS No. 154”), which replaces Accounting Principles Board Opinion No. 20, Accounting Changes and SFAS No. 3, Reporting Accounting Changes in Interim Financial Statements. SFAS No. 154 changes the requirements for the accounting for and reporting of a change in accounting principles. It requires retrospective application to prior periods’ financial statements of changes in accounting principles, unless it is impracticable to determine either the period-specific effects or the cumulative effect of the change. This statement is effective for accounting changes and corrections of errors made in fiscal years beginning after December 15, 2005. The impact on the Company’s operations will depend on future accounting pronouncements or changes in accounting principles.
NOTE 12 SUBSEQUENT EVENTS
On May 8, 2006, an agreement and plan of reorganization was executed among Echelon Acquisition Corp., a corporation organized under the laws of the State of Delaware (the "Asia Biotech"); Asia Biotechnology Group Inc., a corporation organized under the laws of British Virgin Islands (the "ABG"); Far Grand Investments Limited, a corporation organized under the laws of Cayman Islands, acting as the shareholder of ABG, (the “ABG Shareholder”); Harbin OT Pharmaceutical Co., Limited, a company organized under the laws of Samoa (the "OT Samoa”); and shareholders of OT Samoa ( collectively the “OT Samoa Shareholders”).
The respective Boards of Directors of Asia Biotech, ABG and OT Samoa have adopted resolutions pursuant to which all of the issued and outstanding shares of the common stock of ABG (the “ABG Share”) and all of the issued and outstanding shares of OT Samoa (the “OT Samoa Shares”) will be converted into the right to receive a specified number of shares of the common stock of Asia Biotech (the “Asia Biotech Shares”); and whereas, the sole consideration for the exchange of the ABG Share shall be the receipt by the ABG Shareholder of 23,296,000 Asia Biotech Shares, $0.001 par value per share; and the sole consideration for the exchange of the OT Samoa Shares shall be the receipt by the OT Samoa Shareholders of 23,296,000 Asia Biotech Shares, $0.001 par value per share.
The ABG Shareholder and the OT Samoa Shareholders individually agreed to transfer to Asia Biotech at the closing (the "Closing") the ABG Share and OT Samoa Shares, in exchange for newly issued and restricted shares of common stock of Asia Biotech. In connection with the acquisition of the ABG Share and the OT Samoa Shares, Asia Biotech shall issue to the ABG Shareholder an aggregate of Twenty Three Million Two Hundred and Ninety Six Thousand (23,296,000) shares of Asia Biotech common stock, and shall simultaneously issue to the OT Samoa Shareholders an aggregate of Twenty Three Million Two Hundred and Ninety Six Thousand (23,296,000) shares of Asia Biotech common stock. Such shares at the Closing shall equal eighty percent (80%) of the issued and outstanding shares of Asia Biotech. After the Closing, there will be 58,240,000 outstanding shares of common stock of the reorganized Asia Biotech.
ABG and OT Samoa both became Echelon's wholly owned subsidiaries and the former shareholders of ABG (“shareholders”) obtained effective operating control of the combined company after the share exchange. Generally accepted accounting principles require that ABG whose shareholders retain the majority interest in a combined business be treated as the acquirer for accounting purpose, resulting in a reverse acquisition. Accordingly, the share exchange transaction has been accounted for as a recapitalization of Echelon. The equity section of the future financial statements will be restated to reflect the recapitalization of Echelon due to the reverse acquisition as of the first day of the first period presented.
Child, Van Wagoner & Bradshaw, PLLC
A Professional Limited Liability Company of CERTIFIED PUBLIC ACCOUNTANTS
5296 S. Commerce Dr., Suite 300, Salt Lake City, UT 84107
PHONE: (801) 281-4700 FAX: (801) 281-4701
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To The Board of Directors
Harbin OT Pharmaceutical Company Ltd.
Beijing, PRC
We have audited the accompanying consolidated balance sheets of Harbin OT Pharmaceutical Company Ltd. as of December 31, 2005 and 2004, and the related consolidated statements of operations and comprehensive loss, shareholders’ equity, and cash flows for the years then ended. These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States of America). Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audits included consideration of internal control over financial reporting, as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the consolidated financial position of Harbin OT Pharmaceutical Company Ltd. as of December 31, 2005 and 2004, and the results of its operations and its cash flows for the years then ended, in conformity with accounting principles generally accepted in the United States of America.
Child, Van Wagoner & Bradshaw, PLLC
Salt Lake City, Utah
May 8, 2006
HARBIN OT PHARMACEUTICAL COMPANY LIMITED
CONSOLIDATED BALANCE SHEETS
| | | December 31, | | December 31, | |
| | | 2005 | | 2004 | |
| | | $ | | | |
ASSETS | | | | | |
| Current assets | | | | | |
| Cash and cash equivalents | | $ | 22,290 | | $ | 4,473 | |
Accounts receivable, less allowances for doubtful accounts of $36,096 and $29,755 respectively | | | 426,123 | | | 2,598 | |
| Inventories | | | 247,280 | | | 516,218 | |
| Other current assets | | | 20,088 | | | 28,115 | |
| Total current assets | | | 715,781 | | | 551,404 | |
| | | | | | | | |
| Property, plant and equipment, net | | | 882,533 | | | 937,204 | |
| Land use right, net | | | 102,959 | | | 102,599 | |
| Total assets | | $ | 1,701,273 | | $ | 1,591,207 | |
| | | | | | | | |
LIABILITIES AND SHAREHOLDERS’ EQUITY | | | | | |
| Current liabilities | | | | | |
| Accounts payable | | $ | 107,658 | | $ | 70,922 | |
| Accrued expenses | | | 13,385 | | | 5,346 | |
| Customer deposits | | | 354,010 | | | 209,197 | |
| Due to shareholders | | | 109,932 | | | 12,042 | |
| Other current liabilities | | | 6,047 | | | - | |
| Total current liabilities | | | 591,032 | | | 297,507 | |
| | | | | | | | |
| Shareholders’ equity | | | | | | | |
| Capital - at stated value, no authorized shares | | | 2,174,701 | | | 2,174,701 | |
| Accumulated deficit | | | (1,094,363 | ) | | (881,001 | ) |
| Accumulated other comprehensive income | | | 29,903 | | | - | |
| Total shareholders’ equity | | | 1,110,241 | | | 1,293,700 | |
| Total liabilities and shareholders’ equity | | $ | 1,701,273 | | $ | 1,591,207 | |
See accompanying notes to consolidated financial statements.
HARBIN OT PHARMACEUTICAL COMPANY LIMITED
CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS
FOR THE YEARS ENDED DECEMBER 31, 2005 AND 2004
| | | December 31, 2005 | | December 31, 2004 | |
| | | | | | |
| Net sales | | $ | 484,635 | | $ | 47,738 | |
| | | | | | | | |
| Cost of sales | | | 462,420 | | | 22,352 | |
| | | | | | | | |
| Gross profit | | | 22,215 | | | 25,386 | |
| | | | | | | | |
| Operating expenses | | | | | | | |
| Allowance for bad debt | | | 5,493 | | | 29,755 | |
| Salaries | | | 43,096 | | | 20,578 | |
| Depreciation | | | 40,081 | | | 87,096 | |
| Amortization of land use right | | | 2,237 | | | 2,214 | |
| Other selling, general and administrative | | | 155,484 | | | 105,995 | |
| | | | | | | | |
| Total operating expenses | | | 246,391 | | | 245,638 | |
| | | | | | | | |
| Loss from operations | | | (224,176 | ) | | (220,252 | ) |
| | | | | | | | |
| Non-operating income | | | | | | | |
| Government grant | | | 10,618 | | | 73,577 | |
| Gain on disposal | | | 122 | | | 4,823 | |
| Other Income | | | - | | | 70 | |
| Interest income | | | 74 | | | 15 | |
| | | | | | | | |
| Total non-operating income | | | 10,814 | | | 78,485 | |
| | | | | | | | |
| (Loss) before income taxes | | | (213,362 | ) | | (141,767 | ) |
| | | | | | | | |
| Income taxes | | | - | | | - | |
| | | | | | | | |
| Net (loss) | | $ | (213,362 | ) | $ | (141,767 | ) |
| | | | | | | | |
| Other comprehensive income | | | | | | | |
| Foreign currency translation adjustment | | | 29,903 | | | - | |
| | | | | | | | |
| Comprehensive (loss) | | $ | (183,459 | ) | $ | (141,767 | ) |
See accompanying notes to consolidated financial statements.
HARBIN OT PHARMACEUTICAL COMPANY LIMITED
STATEMENTS OF SHAREHOLDERS' EQUITY
FOR THE YEARS ENDED DECEMBER 31, 2005 AND 2004
| | | Capital | | Accumulated Deficit | | Accumulated Other Comprehensive Income | | Total Stockholders’ Equity | |
| | | | | | | | | | |
| Balance, December 31, 2003 | | $ | 2,174,701 | | $ | (739,234 | ) | $ | - | | $ | 1,435,467 | |
| | | | | | | | | | | | | | |
| Net loss for the year ended December 31, | | | | | | (141,767 | ) | | | | | (141,767 | ) |
| | | | | | | | | | | | | | |
| Comprehensive income - foreign currency translation adjustment | | | - | | | - | | | — | | | - | |
| | | | | | | | | | | | | | |
| Balance, December 31, 2004 | | | 2,174,701 | | | (881,001 | ) | | - | | | 1,293,700 | |
| | | | | | | | | | | | | | |
| Net loss for the year ended December 31, | | | - | | | (213,362 | ) | | | | | (213,362 | ) |
| | | | | | | | | | | | | | |
| Comprehensive income - foreign currency translation adjustment | | | - | | | - | | | 29,903 | | | 29,903 | |
| | | | | | | | | | | | | | |
| Balance, December 31, 2005 | | $ | 2,174,701 | | $ | (1,094,363 | ) | $ | 29,903 | | $ | 1,110,241 | |
See accompanying notes to consolidated financial statements.
HARBIN OT PHARMACEUTICAL COMPANY LIMITED
CONSOLIDATED STATEMENTS OF CASH FLOWS
FOR THE YEARS ENDED DECEMBER 31, 2005 AND 2004
| | | December 31, 2005 | | December 31, 2004 | |
| | | | | | |
| Cash flows from operating activities | | | | | |
| Net(loss) | | $ | (213,362 | ) | $ | (141,767 | ) |
| Adjustment to reconcile net loss to net cash provided by (used in) | | | | | | | |
| operating activities: | | | | | | | |
| Bad debt allowance | | | 5,493 | | | - | |
| Depreciation and amortization of property, plant and equipment | | | 100,326 | | | 89,310 | |
| Changes in current assets and liabilities (net of effects of acquisitions and disposals of entities) | | | | | | | |
| Accounts receivable | | | (422,544 | ) | | 24,440 | |
| Inventories | | | 277,908 | | | (31,355 | ) |
| Other current assets | | | 8,616 | | | (21,832 | ) |
| Accounts payable | | | 34,389 | | | 11,687 | |
| Accrued expenses | | | 7,127 | | | 2,008 | |
| Customer deposits | | | 137,337 | | | 69,049 | |
| Other current liabilities | | | 6,609 | | | 660 | |
| Net cash provided by (used in) operating activities | | | (58,101 | ) | | 2,200 | |
| | | | | | | | |
| Cash flows from investing activities | | | | | | | |
| Purchase of fixed assets | | | (20,568 | ) | | (16,803 | ) |
| Net cash used in investing activities | | | (20,568 | ) | | (16,803 | ) |
| | | | | | | | |
| Cash flows from financing activities | | | | | | | |
| Advance from shareholders | | | 96,105 | | | 12,042 | |
| Net cash provided by financing activities | | | 96,105 | | | 12,042 | |
| | | | | | | | |
| Effect of foreign currency translation on cash | | | 381 | | | - | |
| | | | | | | | |
| Net increase / (decrease) in cash and cash equivalents | | | 17,817 | | | (2,561 | ) |
| Cash and cash equivalents, beginning of year | | | 4,473 | | | 7,034 | |
| Cash and cash equivalents, end of year | | $ | 22,290 | | $ | 4,473 | |
| | | | | | | | |
| Cash paid for interest | | $ | - | | $ | $ - | |
| Cash paid for income taxes | | $ | - | | $ | - | |
See accompanying notes to consolidated financial statements.
NOTE 1 ORGANIZATION
Harbin OT Pharmaceutical Company Limited (“OT Pharmaceutical”) is a Chinese foreign owned enterprise incorporated in the People’s Republic of China (“PRC”) on April 13, 2001. The Company is a feminine suppository manufacturer and provides this suppository to clinics and the Red Cross Society of China in the PRC.
Asia Biotechnology Group Inc. acquired 60% shareholding of OT Pharmaceutical on November 3, 2005 and became the holding company of OT Pharmaceutical.
NOTE 2 SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
A. Accounting Method
The Company's financial statements are prepared using the accrual method of accounting. The Company has elected a fiscal year ending on December 31.
B. Control by principal stockholders
The directors, executive officers and their affiliates or related parties own, beneficially and in the aggregate, the majority of the voting power of the outstanding shares of the common stock of the Company. Accordingly, the directors, executive officers and their affiliates, if they voted their shares uniformly, would have the ability to control the approval of most corporate actions, including increasing the authorized capital stock of the Company and the dissolution, merger or sale of the Company's assets.
C. Cash and cash equivalents
Cash and cash equivalents include cash on hand, cash accounts, interest bearing savings accounts and time certificates of deposit with a maturity of three months or less when purchased.
D. Inventories
Inventories are stated at the lower of cost or market, determined by the weighted average method. Finished goods inventories consist of raw materials, direct labor and overhead associated with the manufacturing process.
E. Trade accounts receivable
Trade accounts receivable are recorded at the invoiced amount and do not bear interest. The allowance for doubtful accounts represents the Company’s best estimate of the amount of probable credit losses in the existing accounts receivable balance. The Company determines the allowance for doubtful accounts based upon historical write-off experience and current economic conditions. The Company reviews the adequacy of its allowance for doubtful accounts on a regular basis. Account balances are charged off against the allowance after all means of collection have been exhausted and the potential for recovery is considered remote. The Company signed a sales contract with Red Cross Society of China in which the terms of payment is longer than one year.
F. Credit Risk and Customers
We have a concentration of customers in sales of feminine suppositories. We are diligent in attempting to ensure that we issue credit to credit-worthy customers. However, our customer base is small and our accounts receivable balances are usually over 90 days outstanding, and that exposes us to significant credit risk. Therefore, a credit loss can be very large relative to our overall profitability.
During the year ended December 31, 2005, there is one customer that accounted for approximately 90% of our revenues, and totaling around $435,000 and other customers accounted for less than 10% of our total revenue. The loss of the major customer, could have a material impact on our results of operations.
G. Property, plant and equipment
Property, plant and equipment are stated at cost including the cost of improvements. Depreciation and amortization are provided on the straight-line method based on the estimated useful lives of the assets as follows:
| Buildings | | 20 years |
| Leasehold improvements | | 20 years |
| Plant and machinery | | 10 years |
| Motor vehicles | | 5 years |
| Furniture, fixtures and equipment | | 5 years |
H. Valuation of long-lived assets
The Company periodically evaluates the carrying value of long-lived assets to be held and used, including intangible assets subject to amortization, when events and circumstances warrant such a review. The carrying value of a long-lived asset is considered impaired when the anticipated undiscounted cash flow from such asset is separately identifiable and is less than its carrying value. In that event, a loss is recognized based on the amount by which the carrying value exceeds the fair market value of the long-lived asset. Fair market value is determined primarily using the anticipated cash flows discounted at a rate commensurate with the risk involved. Losses on long-lived assets to be disposed of are determined in a similar manner, except that fair market values are reduced for the cost to dispose.
I. Revenue recognition
The Company recognizes revenue when it is realized and earned. The Company considers revenue realized or realizable and earned when (1) it has persuasive evidence of an arrangement, (2) delivery has occurred, (3) the sales price is fixed or determinable, and (4) collectibility is reasonably assured. Delivery does not occur until products have been shipped to the client, risk of loss has transferred to the client and client acceptance has been obtained, client acceptance provisions have lapsed, or the Company has objective evidence that the criteria specified in client acceptance provisions have been satisfied. The sales price is not considered to be fixed or determinable until all contingencies related to the sale have been resolved.
J. Comprehensive income (loss)
Comprehensive income (loss) includes changes to equity accounts that were not the result of transactions with shareholders. Comprehensive income (loss) is comprised of net income (loss) and other comprehensive income and loss items. The Company’s comprehensive income and losses generally consist of changes in the fair value of changes in the cumulative foreign currency translation adjustment.
K. Foreign currency translation
Transactions and balances originally denominated in U.S. dollars are presented at their original amounts. Transactions and balances in other currencies are converted into U.S. dollars in accordance with Statement of Financial Accounting Standards (SFAS) No. 52, "Foreign Currency Translation," and are included in determining net income or loss.
For foreign operations with the local currency as the functional currency, assets and liabilities are translated from the local currencies into U.S. dollars at the exchange rate prevailing at the balance sheet date. Revenues, expenses and cash flows are translated at the average exchange rate for the period to approximate translation at the exchange rate prevailing at the dates those elements are recognized in the financial statements. Translation adjustments resulting from the process of translating the local currency financial statements into U.S. dollars are included in determining comprehensive loss.
The Company has determined the PRC Chinese Yuan Renminbi (“RMB”) to be the functional currency of the Company. The financial statements of the Company are translated to United States dollars using year-end rates of exchange for assets and liabilities, and average rates of exchange for the period for revenues, costs, and expenses. Net gains and losses resulting from foreign exchange transactions are included in the consolidated statements of operations. The cumulative translation adjustment and effect of exchange rate changes were $29,903 and nil as of December 31, 2005 and 2004 respectively.
L. Due to stockholder
The caption "Due to shareholders" on the condensed consolidated Balance Sheet consists of advances that are unsecured, non-interest bearing and have no fixed terms of repayment, and therefore, are deemed payable on demand.
M. Use of estimates
The preparation of financial statements in conformity with generally accepted accounting principles requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosures of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
N. Significant Estimates
Several areas require management's estimates relating to uncertainties for which it is reasonably possible that there will be a material change in the near term. The more significant areas requiring the use of management estimates related to valuation of the useful lives of the Company's equipment and valuation of contingent liabilities.
O. Income Taxes
The Company accounts for income taxes under Financial Accounting Standards Board (FASB) Statement No. 109, "Accounting for Income Taxes" ("Statement 109"). Under Statement 109, deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. Under Statement 109, the effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date.
P. Basic Income/Loss Per Common Share
No basic income/loss per common share has been calculated based on the weighted average number of shares outstanding during the period since the Company is a Chinese company with stated capital, without number of shares, and please see note 10.
P. Off-Balance Sheet Arrangements
The Company does not have any off-balance sheet credit exposure related to its customers.
NOTE 3 INVENTORIES
Inventories by major categories, consist of the following at December 31, 2005 and 2004:
| | | December 31, | | December 31, | |
| | | 2005 | | 2004 | |
| | | | | | |
| Raw materials | | $ | 182,216 | | $ | 99,195 | |
| Finished goods | | | 26,846 | | | 369,258 | |
| Packaging materials | | | 38,218 | | | 47,765 | |
| | | $ | 247,280 | | $ | 516,218 | |
NOTE 4 PROPERTY, PLANT AND EQUIPMENT
Property, plant and equipment, which is all located in the PRC, consist of the following at December 31, 2005 and 2004:
| | | December 31, | | December 31, | |
| | | 2005 | | 2004 | |
| At cost: | | | | | |
| Buildings | | $ | 850,002 | | $ | 823,795 | |
| Leasehold improvements | | | 52,693 | | | 51,375 | |
| Plant and machinery | | | 305,434 | | | 288,187 | |
| Motor vehicles | | | 86,074 | | | 83,921 | |
| Furniture, fixtures and equipment | | | 44,915 | | | 37,987 | |
| Total | | | 1,339,118 | | | 1,285,265 | |
| Less: accumulated depreciation and amortization | | | (456,585 | ) | | (348,061 | ) |
| Net book value | | $ | 882,533 | | $ | 937,204 | |
Depreciation expense was $98,089 and $87,096 for the years ended December 31, 2005 and 2004, respectively. Of those amounts, $58,008 and $0 was included in cost of sales.
NOTE 5 LAND USE RIGHT
Land use right for the land located in PRC, consists of the following at December 31, 2005 and 2004:
| | | December 31, | | December 31, | |
| | | 2005 | | 2004 | |
| | | | | | |
| Land use right, cost | | $ | 113,558 | | $ | 110,718 | |
| Less: accumulated amortization | | | (10,599 | ) | | (8,119 | ) |
| Net book value | | $ | 102,959 | | $ | 102,599 | |
All the land in the PRC is owned by the PRC government. The government, according to PRC laws, may grant to entities the right to use of land for a specified period of time. Thus all of the Company’s land occupied in the PRC is considered to be leasehold land and amortized on a straight-line basis over the respective term of the right to use the land.
The Company is granted the right to use of land for 50 years and is amortized on a straight-line basis over 50 years of the right to use the land from the date of acquisition in 2001. Amortization expense was $2,237 and $2,214 for the years ended December 31, 2005 and 2004, respectively.
NOTE 6 INTELLECTUAL PROPERTY
The transfers of nonmonetary assets to a company by its promoters or shareholders in exchange for stock prior to or at the time of the company’s initial public offering normally was recorded at the transferors’ historical cost basis determined under GAAP and in accordance with Topic 5(g) Transfers Of Nonmonetary Assets By Promoters Or Shareholders.
The patent rights which were contributed by the shareholders to OT Pharmaceutical, were valued at the historical cost. As at December 31, 2005, the Company is the registered owner of those patents, but the costs have been fully amortized in previous years.
NOTE 7 CUSTOMER DEPOSITS
The customer deposits were received from customers in connection with orders of products to be delivered in future periods. The customer deposits were $354,010 and $209,197 as of December 31, 2005 and December 31, 2004 respectively.
NOTE 8 GOVERNMENT GRANTS
The Company receives grants from the PRC Government to help fund the Company’s development of high technology projects. The grants are recognized as other income when the proceeds are received or collectible. The government grants were $10,618 and $73,577 for the year ended December 31, 2005 and December 31, 2004 respectively.
NOTE 9 INCOME TAXES
The Company accounts for income taxes in accordance with the provisions of SFAS No. 109, "Accounting for Income Taxes."
Income tax expense is based on reported income before income taxes. Deferred income taxes reflect the effect of temporary differences between assets and liabilities that are recognized for financial reporting purposes and the amounts that are recognized for income tax purposes. In accordance with SFAS 109, these deferred income taxes are measured by applying currently enacted tax laws.
PRC
Enterprises income tax in PRC is generally charged at 33%, in which 30% is for national tax and 3% is for local tax, of the assessable profit. The companies incorporated in PRC are subject to PRC enterprises income tax at the applicable tax rates on the taxable income as reported in their Chinese statutory accounts in accordance with the relevant enterprises income tax laws applicable to foreign enterprises.
According to the PRC’s applicable income tax laws, regulations, notices and decisions related to foreign investment enterprises and their investors, income such as dividends and profits distribution from the PRC derived from a foreign enterprise which has no establishment in the PRC is subject to a 10% withholding tax.
There are net operating loss carryforwards allowed under the Chinese governments' tax systems. In China, the previous five years net operating losses are allowed to be carryforward five years to offset future taxable income. The Company has available approximately $ 1,100,000 of unused operating loss carryforwards and based on a 33% tax rate has a deferred tax asset of approximately $363,000 in which the company recorded a valuation allowance for the same amount at December 31, 2005.
The company withholds and pays income taxes on its employees' wages, which funds the Chinese government's sponsored health and retirement programs of all the employees.
NOTE 10 CAPITAL
The Company was incorporated with capital of RMB30,000,000 ($3,624,501) which was paid up by plant and machinery of RMB8,400,000 ($1,014,860), patent of RMB6,000,000 ($724,900), technical know how of RMB6,000,000 ($724,900), and cash of RMB9,600,000 ($1,159,841). According to US GAAP, the patents and the technical know how contributed by the shareholders should be stated at the historical costs. Since these costs were previously expensed by their owners as incurred, no value was recorded by the Company. As a result, the capital was RMB18,000,000 and was represented by $2,174,701.
No basic income/loss per common share has been calculated based on the weighted average number of shares outstanding during the period since the Company is a Chinese company with stated capital, without number of shares.
NOTE 11 EMPLOYEE BENEFITS
The Company has established its own employee welfare plan in accordance with Chinese law and regulations. The Company makes annual contributions of 14% of all employees’ salaries to its employee welfare plan. The total expenses for the above plan were $6,033 and $3,147 for the year ended December 31, 2005 and December 31, 2004 respectively. The Company has recorded welfare payments payable in the amount of $13,385 and $5,346 as of December 31, 2005 and December 31, 2004 as accrued expenses on the balance sheet.
NOTE 12 RECENT ACCOUNTING PRONOUNCEMENTS
In December 2004, the FASB issued SFAS No. 123(R), “Share-Based Payment”. SFAS 123(R) is a revision of SFAS No. 123, “Accounting for Stock Based Compensation,” and supersedes Accounting Principles Board Opinion No. 25 (“APB 25”), “Accounting for Stock Issued to Employees.” Among other items SFAS 123(R) eliminates the use of APB 25 and the intrinsic value method of accounting, and requires companies to recognize the cost of employee services received in exchange for awards of equity instruments, based on the grant date fair value of those awards, in the financial statements. The effective date of SFAS 123 (R) is the first annual reporting period beginning after December 15, 2005. The adoption of SFAS 123 (R) is not expected to have a material impact on the Company’s financial position, results of operations or cash flows.
In March 2005, the SEC staff issued additional guidance on SFAS 123 (R) in the form of Staff Accounting Bulletin (“SAB”) No. 107. SAB 107 was issued to assist preparers by simplifying some of the implementation challenges of FAS 123 (R) while enhancing the information that investors receive. SAB 107 creates a framework that is premised on two themes: (a) considerable judgment will be required by preparers to successfully implement FAS 123 (R), specifically when valuing employee stock options; and (b) reasonable individuals, acting in good faith, may conclude differently on the fair value of employee share options. Key topics covered by SAB 107 include: (a) valuation models - SAB 107 reinforces the flexibility allowed by FAS 123 (R) to choose an option-pricing model that meets the standard’s fair value measurement objective; (b) expected volatility - the SAB provides guidance on when it would be appropriate to rely exclusively on either historical or implied volatility in estimating expected volatility; and (c) expected term - the new guidance includes examples and some simplified approaches to determining the expected term under certain circumstances. The Company will apply the principles of SAB 107 in conjunction with its adoption of SFAS 123 (R) but does not believe its adoption will have material impact on the Company’s financial statements or results of operations.
In December 2004, the FASB issued SFAS No. 153, “Exchanges of Nonmonetary Assets, an amendment of APB Opinion No. 29.” SFAS No. 153 addresses the measurement of exchanges of nonmonetary assets and redefines the scope of transactions that should be measured based on the fair value of the assets exchanged. SFAS No. 153 is effective for nonmonetary asset exchanges occurring in fiscal periods beginning after June 15, 2005. The adoption of SFAS No. 153 did not have a material impact on the Company's financial statements or results of operations.
In January 2003, the FASB issued FASB Interpretation No. 46, ("FIN 46"), “Consolidation of Variable Interest Entities ("VIE").” Until this interpretation, the Company generally included entities in its consolidated financial statements only if it controlled the entity through voting interests. FIN No. 46 requires a variable interest entity, as defined, to be consolidated by a company if that company is subject to a majority of the risk of loss from the variable interest entity's activities or entitled to receive a majority of the entity's residual returns. FIN No. 46 is effective for reporting periods ending after December 15, 2003. The adoption of FIN No. 46 did not have a material impact on the Company's Consolidated Financial Statements as of December 30, 2005.
In March 2005, FASB issued FASB Interpretation (“FIN”) No. 47, “Accounting for Conditional Asset Retirement Obligations.” FIN 47 clarifies that the term “Conditional Asset Retirement Obligation” as used in FASB Statement No. 143, “Accounting for Asset Retirement Obligation,” refers to a legal obligation to perform an asset retirement activity in which the timing and/or method of settlement are conditional on a future event that may or may not be within the control of the entity. Accordingly, an entity is required to recognize a liability for the fair value of a Conditional Asset Retirement Obligation if the fair value of the liability can be reasonably estimated. FIN 47 is effective no later than the end of fiscal years ending after December 15, 2005. Management does not believe the adoption of FIN 47 will have a material affect on the Company’s financial position, results of operations or cash flows. In May 2005, the Financial Accounting Standards Board (“FASB”) issued SFAS No. 154, “Accounting Changes and Error Corrections” (“SFAS No. 154”), which replaced Accounting Principles Board Opinion No. 20, Accounting Changes and SFAS No. 3, Reporting Accounting Changes in Interim Financial Statements. SFAS No. 154 changes the requirements for the accounting for and reporting of a change in accounting principles. It requires retrospective application to prior periods’ financial statements of changes in accounting principles, unless it is impracticable to determine either the period-specific effects or the cumulative effect of the change. This statement is effective for accounting changes and corrections of errors made in fiscal years beginning after December 15, 2005. The impact on the Company’s operations will depend on future accounting pronouncements or changes in accounting principles.
In November 2004, the Financial Accounting Statements Board (FASB) issued SFAS Statement No. 151, “Inventory Costs,” an amendment of the Accounting Research Bulletin (ARB) No. 43, Chapter 4. Under FASB Statement No. 151, all abnormal amounts of idle facility expense, freight, handling costs, and wasted materials (spoilage) should be recognized as current-period charges by requiring the allocation of fixed production overheads to inventory based on the normal capacity of the production facilities. The adoption of this pronouncement is not expected to have a material impact on the Company’s financial statements, results of operations, or cash flows.
In May 2005, the Financial Accounting Standards Board (“FASB”) issued SFAS No. 154, “Accounting Changes and Error Corrections” (“SFAS No. 154”), which replaces Accounting Principles Board Opinion No. 20, Accounting Changes and SFAS No. 3, Reporting Accounting Changes in Interim Financial Statements. SFAS No. 154 changes the requirements for the accounting for and reporting of a change in accounting principles. It requires retrospective application to prior periods’ financial statements of changes in accounting principles, unless it is impracticable to determine either the period-specific effects or the cumulative effect of the change. This statement is effective for accounting changes and corrections of errors made in fiscal years beginning after December 15, 2005. The impact on the Company’s operations will depend on future accounting pronouncements or changes in accounting principles.
NOTE 13 SUBSEQUENT EVENTS
On May 8, 2006, an agreement and plan of reorganization was entered into among Asia Biotechnology Group Inc. , a corporation organized under the laws of the State of Delaware ("Asia Biotech"); Asia Biotechnology Group Inc., a corporation organized under the laws of British Virgin Islands ("ABG"); Far Grand Investments Limited, a corporation organized under the laws of Cayman Islands, acting as the shareholder of ABG, (the “ABG Shareholder”); Harbin OT Pharmaceutical Co., Limited, a company organized under the laws of Samoa ("OT Samoa”); and shareholders of OT Samoa ( collectively the “OT Samoa Shareholders”).
The respective Boards of Directors of Asia Biotech, ABG and OT Samoa have adopted resolutions pursuant to which the issued and outstanding share of the common stock of ABG (the “ABG Share”) and all of the issued and outstanding shares of OT Samoa (the “OT Samoa Shares”) will be converted into the right to receive a specified number of shares of the common stock of Asia Biotech (the “Asia Biotech Shares”); and whereas, the sole consideration for the exchange of the ABG Share shall be the receipt by the ABG Shareholder of 23,296,000 Asia Biotech Shares, $0.001 par value per share; and the sole consideration for the exchange of the OT Samoa Shares shall be the receipt by the OT Samoa Shareholders of 23,296,000 Asia Biotech Shares, $0.001 par value per share.
The ABG Shareholder and the OT Samoa Shareholders individually agreed to transfer to Asia Biotech at the closing (the "Closing") the ABG Share and OT Samoa Shares, in exchange for newly issued and restricted shares of common stock of Asia Biotech. In connection with the acquisition of the ABG Share and the OT Samoa Shares, Asia Biotech shall issue to the ABG Shareholder 23,296,000 shares of Asia Biotech common stock, and shall simultaneously issue to the OT Samoa Shareholders an aggregate of 23,296,000 shares of Asia Biotech common stock. Such shares at the Closing shall equal 80% of the issued and outstanding shares of Asia Biotech. After the Closing, there will be 58,240,000 outstanding shares of common stock of the reorganized Asia Biotech.
ABG and OT Samoa both became Asia Biotech 's wholly owned subsidiaries and the former shareholders of ABG (“shareholders”) obtained effective operating control of the combined company after the share exchange. Generally accepted accounting principles require that ABG whose shareholders retain the majority interest in a combined business be treated as the acquirer for accounting purpose, resulting in a reverse acquisition. Accordingly, the share exchange transaction has been accounted for as a recapitalization of Asia Biotech. The equity section of the future financial statements will be restated to reflect the recapitalization of Asia Biotech due to the reverse acquisition as of the first day of the first period presented.
ASIA BIOTECHNOLOGY GROUP INC.
UNAUDITED PRO FORMA CONSOLIDATED FINANCIAL STATEMENTS
Basis of Presentation
On May 8, 2006, an agreement and plan of reorganization among Asia Biotechnology Group Inc. , a corporation organized under the laws of the State of Delaware ("Asia Biotech"); Asia Biotechnology Group Inc., a corporation organized under the laws of British Virgin Islands ("ABG"); Far Grand Investments Limited, a corporation organized under the laws of Cayman Islands, acting as the shareholder of ABG, (the “ABG Shareholder”); Harbin OT Pharmaceutical Co., Limited, a company organized under the laws of Samoa ("OT Samoa”); and shareholders of OT Samoa ( collectively the “OT Samoa Shareholders”).
The respective Boards of Directors of Asia Biotech, ABG and OT Samoa have adopted resolutions pursuant to which all of the issued and outstanding shares of the common stock of ABG (the “ABG Share”) and all of the issued and outstanding shares of OT Samoa (the “OT Samoa Shares”) will be converted into the right to receive a specified number of shares of the common stock of Asia Biotech (the “Asia Biotech Shares”); and whereas, the sole consideration for the exchange of the ABG Share shall be the receipt by the ABG Shareholder of 23,296,000 Asia Biotech Shares, $0.001 par value per share; and the sole consideration for the exchange of the OT Samoa Shares shall be the receipt by the OT Samoa Shareholders of 23,296,000 Asia Biotech Shares, $0.001 par value per share.
The ABG Shareholder and the OT Samoa Shareholders individually agreed to transfer to Asia Biotech at the closing (the "Closing") the ABG Share and OT Samoa Shares, in exchange for newly issued and restricted shares of common stock of Asia Biotech. In connection with the acquisition of the ABG Share and the OT Samoa Shares, Asia Biotech issued to the ABG Shareholder 23,296,000 shares of Asia Biotech common stock, and simultaneously issued to the OT Samoa Shareholders an aggregate of 23,296,000 shares of Asia Biotech common stock. Such shares at the Closing were equal to 80% of the issued and outstanding shares of Asia Biotech. After the Closing, there were 58,240,000 outstanding shares of common stock of the reorganized Asia Biotech.
On May 8, 2006, Asia Biotech completed an acquisition of ABG pursuant to the agreement and plan of reorganization. The acquisition was accounted for as a recapitalization effected by a share exchange, wherein ABG is considered the acquirer for accounting and financial reporting purposes.
The unaudited pro forma consolidated financial statements of ABG in the opinion of management include all material adjustments directly attributable to the share exchange contemplated by the Agreement. No pro forma consolidated balance sheet is provided because the accompanying financial statements dated June 30, 2006, include a consolidated balance sheet. The pro forma consolidated statement of operations includes the accounts of Asia Biotech, ABG, OT Samoa and Harbin OT Pharmaceutical Company Limited, a PRC company (“OT Pharmaceutical”). The statement was prepared as if the above mentioned reorganization and the acquisition of OT Pharmaceutical by ABT were consummated on January 1, 2005. These pro forma consolidated financial statements have been prepared for comparative purposes only and do not purport to be indicative of the results of operations which actually would have resulted had the transaction occurred on the date indicated and are not necessarily indicative of the results that may be expected in the future.
ASIA BIOTECHNOLOGY GROUP INC.
UNAUDITED PRO FORMA CONSOLIDATED STATEMENT OF OPERATIONS
FOR THE YEAR ENDED DECEMBER 31, 2005
| | | Asia Biotechnology Group, Inc. | | Asia Biotechnology Group, Inc. (BVI) | | Harbin OT Pharmaceutical Company Limited (PRC) | | Harbin OT Pharmaceutical Co., Limited (Samoa) | | | |
Pro Forma Total | |
| | | $ | | $ | | $ | | $ | | $ | | $ | |
| Net sales | | | - | | | - | | | 484,635 | | | - | | | | | | 484,635 | |
| Cost of sales | | | - | | | - | | | 462,420 | | | - | | | | | | 462,420 | |
| Gross profit | | | - | | | - | | | 22,215 | | | - | | | | | | 22,215 | |
| Operating expenses | | | | | | | | | | | | | | | | | | | |
| Allowance for bad debt | | | - | | | - | | | 5,493 | | | - | | | | | | 5,493 | |
| Accountant and audit fee | | | - | | | 45,000 | | | - | | | - | | | | | | 45,000 | |
| Salaries | | | - | | | - | | | 43,096 | | | - | | | | | | 43,096 | |
| Depreciation | | | - | | | - | | | 40,081 | | | - | | | | | | 40,081 | |
| Amortization of land use right | | | - | | | - | | | 2,237 | | | - | | | | | | 2,237 | |
| Other selling, general and administrative | | | 11,500 | | | 100 | | | 155,484 | | | - | | | | | | 167,084 | |
| Total operating expenses | | | 11,500 | | | 45,100 | | | 246,391 | | | - | | | | | | 302,991 | |
| Loss from operations | | | (11,500 | ) | | (45,100 | ) | | (224,176 | ) | | - | | | | | | (280,776 | ) |
| Non-operating income | | | | | | | | | | | | | | | | | | | |
| Government Grant | | | - | | | - | | | 10,618 | | | - | | | | | | 10,618 | |
| Gain on disposal | | | - | | | - | | | 122 | | | - | | | | | | 122 | |
| Interest income | | | - | | | - | | | 74 | | | - | | | | | | 74 | |
| Total non-operating income | | | - | | | - | | | 10,814 | | | - | | | | | | 10,814 | |
Loss before income taxes and minority interests | | | (11,500 | ) | | (45,100 | ) | | (213,362 | ) | | - | | | | | | (269,962 | ) |
| Income taxes | | | - | | | - | | | - | | | - | | | | | | - | |
| Loss before minority interest | | | (11,500 | ) | | (45,100 | ) | | (213,362 | ) | | - | | | | | | (269,962 | ) |
| Minority interest in loss | | | - | | | - | | | - | | | - | | | 85,345 | | | 85,345 | |
| Net loss | | | (11,500 | ) | | (45,100 | ) | | (213,362 | ) | | - | | | | | | (184,617 | ) |
Other comprehensive income Foreign currency translation gain | | | - | | | - | | | 29,903 | | | - | | | (11,961 | ) | | 17,942 | |
| Comprehensive loss | | | (11,500 | ) | | (45,100 | ) | | (183,459 | ) | | - | | | | | | (166,675 | ) |
| Basic and fully diluted loss per share | | | | | | | | | | | | | | | | | | (0.00 | ) |
Basic and fully diluted common shares outstanding | | | | | | | | | | | | | | | | | | 58,240,000 | |
ASIA BIOTECHNOLOGY GROUP INC.
NOTES TO UNAUDITED PRO FORMA CONSOLIDATED FINANCIAL STATEMENTS
The following adjustments to the unaudited pro financial statements are based on the assumption that the share exchange was consummated on December 31, 2005.
| | | DR | | CR | |
| | | | $ | | | $ | |
DESCRIPTION | | | | | | | |
| | | | | | | | |
| Minority interest | | | 85,345 | | | | |
| To record elimination of 40% of OT Pharmaceutical’s current period loss | | | | | | | |
| | | | | | | | |
| Minority interest | | | | | | 11,961 | |
| To record elimination of 40% of OT Pharmaceutical’s foreign currency translation adjustment | | | | | | | |
ASIA BIOTECHNOLOGY GROUP INC.
(FORMERLY KNOWN AS ECHELON ACQUISITION CORP.)
CONDENSED CONSOLIDATED BALANCE SHEETS
| | | June 30, | | December 31, | |
| | | 2006 | | 2005 | |
| | | Unaudited | | | |
ASSETS | | | | | |
| Current assets: | | | | | |
| Cash and cash equivalents | | $ | 37,923 | | $ | 459,310 | |
| Accounts receivable, less allowances for doubtful accounts of $ 5,602 | | | 424,092 | | | 426,124 | |
| Inventories | | | 248,872 | | | 247,314 | |
| Prepaid expense | | | - | | | 315,000 | |
| Other current assets | | | 13,321 | | | 20,087 | |
| Total current assets | | $ | 724,208 | | $ | 1,467,835 | |
| | | | | | | | |
| Property, plant and equipment, net | | | 854,906 | | | 860,021 | |
| Land use right, net | | | 111,152 | | | 100,362 | |
| Total assets | | $ | 1,690,266 | | $ | 2,428,218 | |
LIABILITIES AND SHAREHOLDERS’ DEFICIT | | | | | | | |
| Current liabilities: | | | | | | | |
| Accounts payable | | $ | 32,317 | | $ | 107,658 | |
| Accrued expenses | | | 21,382 | | | 13,385 | |
| Customer deposits | | | 307,165 | | | 354,010 | |
| Due to stockholder | | | 208,852 | | | 1,577,050 | |
| Other current liabilities | | | 5,913 | | | 6,047 | |
| Total current liabilities | | | 575,629 | | | 2,058,150 | |
| | | | | | | | |
| Minority interests | | | 427,376 | | | 434,032 | |
| | | | | | | | |
| Shareholders’ equity (deficit): | | | | | | | |
Preferred Stock at $0.001 par value; authorized 20,000,000 shares; no shares issued and outstanding | | | - | | | | |
Common stock at $0.001 par value; authorized 100,000,000 shares; 58,240,000 and 23,296,000 shares issued and outstanding | | | 58,240 | | | 23,296 | |
| Additional paid in capital | | | 1,238,879 | | | (23,295 | ) |
| Accumulated deficit | | | (627,400 | ) | | (64,052 | ) |
| Accumulated other comprehensive income | | | 17,542 | | | 87 | |
| Total shareholders’ equity (deficit) | | | 687,261 | | | (63,964 | ) |
| Total liabilities and shareholders’ equity (deficit) | | $ | 1,690,266 | | $ | 2,428,218 | |
See accompanying notes to financial statements.
ASIA BIOTECHNOLOGY GROUP INC.
(FORMERLY KNOWN AS ECHELON ACQUISITION CORP.)
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE INCOME
| | | For the six months ended June 30, | |
| | | 2006 | | 2005 | |
| | | Unaudited | | Unaudited | |
| | | | | | |
| Net sales | | $ | 220,748 | | $ | 282,904 | |
| | | | | | | | |
| Cost of sales | | | 141,239 | | | 280,822 | |
| | | | | | | | |
| Gross profit | | | 79,509 | | | 2,082 | |
| | | | | | | | |
| Operating expenses | | | | | | | |
| Salaries | | | 28,452 | | | 16,844 | |
| Depreciation | | | 22,128 | | | 36,787 | |
| Amortization of land use right | | | 1,145 | | | 1,107 | |
| Other selling, general and administrative | | | 79,034 | | | 36,084 | |
| Total operating expenses | | | 130,759 | | | 90,822 | |
| | | | | | | | |
| (Loss) from operations | | | (51,250 | ) | | (88,740 | ) |
| | | | | | | | |
| Non-Operating Income | | | | | | | |
| Reorganization cost | | | (550,000 | ) | | - | |
| Interest income | | | - | | | 5 | |
| Other income | | | 32,046 | | | - | |
| | | | | | | | |
| Total Non-Operating Income | | | (517,954 | ) | | 5 | |
| | | | | | | | |
| (Loss) before income taxes and minority interests | | | (569,204 | ) | | (88,735 | ) |
| | | | | | | | |
| Income taxes | | | - | | | - | |
| | | | | | | | |
| (Loss) before minority interests | | | (569,204 | ) | | (88,735 | ) |
| | | | | | | | |
| Minority interests | | | 6,656 | | | 35,494 | |
| | | | | | | | |
| Net (loss) | | $ | (562,548 | ) | $ | (53,241 | ) |
| | | | | | | | |
| Other comprehensive income | | | | | | | |
| Foreign currency translation adjustment | | | 17,455 | | | - | |
| Comprehensive income/ (loss) | | $ | (545,093 | ) | $ | (53,211 | ) |
| | | | | | | | |
Net loss per share - basic and fully diluted | | $ | (0.02 | ) | $ | (0.002 | ) |
| | | | | | | | |
Weighted average ordinary shares outstanding - basic and fully diluted | | | 33,528,221 | | | 23,296,000 | |
See accompanying notes to financial statements.
ASIA BIOTECHNOLOGY GROUP INC.
(FORMERLY KNOWN AS ECHELON ACQUISITION CORP.)
CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS
| | | For the six months ended June 30, 2006 | | For the six months ended June 30, 2005 | |
| | | Unaudited | | Unaudited | |
| | | | | | |
| Cash flows from operating activities | | | | | |
| Net loss | | $ | (562,548 | ) | $ | (53,241 | ) |
Adjustment to reconcile net income to net cash used in operating activities: | | | | | | | |
| Depreciation and amortization of property, plant and equipment | | | 53,526 | | | 58,376 | |
| Minority interests share of net (loss) | | | (6,656 | ) | | (35,494 | ) |
| Reorganization costs | | | 550,000 | | | - | |
Changes in current assets and liabilities (net of effects of acquisitions and disposals of entities) | | | | | | | |
| Accounts receivable | | | 2,032 | | | (271,838 | ) |
| Inventories | | | (1,558 | ) | | 158,221 | |
| Other current assets | | | 6,766 | | | 17,443 | |
| Accounts payable | | | (75,340 | ) | | (2,255 | ) |
| Accrued expenses | | | 7,997 | | | 1,744 | |
| Customer deposits | | | (46,845 | ) | | 60,169 | |
| Other current liabilities | | | (134 | ) | | 33,999 | |
| Net cash used in operating activities | | | (72,760 | ) | | (32,876 | ) |
| | | | | | | | |
| Cash flows from investing activities | | | | | | | |
| Cash acquired in reverse merger | | | - | | | 4,473 | |
| Cash paid in reorganization expenses | | | (235,000 | ) | | - | |
| Capital expenditure | | | (59,202 | ) | | (6,412 | ) |
| Net cash used in investing activities | | | (294,202 | ) | | (1,939 | ) |
Cash flows from financing activities Proceeds from share capital | | | | | | 1 | |
| (Repayment) / Proceeds from stockholder | | | (71,880 | ) | | 40,880 | |
| Net cash (used in) / provided by financing activities | | | (71,880 | ) | | 40,881 | |
| | | | | | | | |
| Effect of foreign currencies on cash flows | | | 17,455 | | | - | |
| | | | | | | | |
| Net (decrease) in cash and cash equivalents | | | (421,387 | ) | | 6,066 | |
| Cash and cash equivalents, beginning of period | | | 459,310 | | | - | |
| Cash and cash equivalents, end of period | | $ | 37,923 | | $ | 6,066 | |
Noncash Financing and Investing Activities Stockholder debt contributed as capital | | $ | 1,097,118 | | $ | - | |
| Supplementary disclosures of cash flow information: | | | | | | | |
| Cash paid (refund) during the period for: | | | | | | | |
| Interest | | $ | - | | $ | - | |
| Income taxes | | $ | - | | $ | - | |
See accompanying notes to financial statements.
NOTE 1 ORGANIZATION AND BASIS OF PREPARATION OF FINANCIAL STATEMENTS
Asia Biotechnology Group Inc. (formerly known as Echelon Acquisition Corp.) (“Asia Biotech”)
Asia Biotechnology Group Inc. (formerly known as Echelon Acquisition Corp.) (“Asia Biotech” or “the Company”) was originally incorporated in July 27, 2004 in accordance with the Laws of the State of Nevada under the name of Echelon Acquisition Corp. The Company was initially formed as a "blank check" entity for the purpose of seeking a merger, acquisition or other business combination transaction with a privately owned entity seeking to become a publicly−owned entity.
On May 8, 2006, the Company completed a reverse acquisition transaction with Asia Biotechnology Group Inc. ("ABG"), a corporation organized under the laws of British Virgin Islands and Harbin OT Pharmaceutical Co., Limited, a company organized under the laws of Samoa (the "OT Samoa”). The Company issued to the ABG Shareholder an aggregate of 23,296,000 shares of the Company’s common stock, and simultaneously issued to the OT Samoa Shareholders an aggregate of 23,296,000 shares of the Company’s common stock in exchange for all of the issued and outstanding shares of ABG and OT Samoa.
ABG and OT Samoa both thereby became the Company's wholly owned subsidiary. The former shareholders of ABG (“Shareholder”) became the Company's major shareholder. This share exchange transaction resulted in the Shareholder obtaining a 40% significant voting interest in the Company. Generally accepted accounting principles require that the company whose shareholders retain the majority interest in a combined business be treated as the acquirer for accounting purpose, resulting in a reverse acquisition. Accordingly, the share exchange transaction with ABG has been accounted for as a recapitalization of the Company and the share exchange transaction with OT Samoa was accounted for using the purchase method. Accordingly, the financial statements prior to May 8,2006 are primarily those of ABG presented with the capital structure of Asia Biotech.
On July 19, 2006, the Company changed its name to Asia Biotechnology Group Inc. (Asia Biotech).
Asia Biotechnology Group Inc. (“ABG”)
Asia Biotechnology Group Inc. is a corporation organized under the laws of British Virgin Islands (the "ABG") on March 21, 2005. Far Grand Investment Limited plays as the sole shareholder under a trust agreement and the beneficiary of the trust is Mr. Mingshi Qiu. On November 3, 2005, ABG purchased 60% equity interest of Harbin OT Pharmaceutical Co., Ltd. (OT Pharmaceutical), a Chinese foreign owned enterprise incorporated in the People’s Republic of China (“PRC”) on April 13, 2001. OT Pharmaceutical is a feminine suppository manufacturer.
Harbin OT Pharmaceutical Co., Limited ("OT Samoa”)
Harbin OT Pharmaceutical Co., Limited is a company organized under the laws of Samoa (the "OT Samoa”). OT Samoa was organized by Mr. Zhu Lei in April 2005 in anticipation of a business combination with ABG and a U.S. reporting company. Mr. Zhu funded OT Samoa by contributing $200,000. On January 22, 2006, Mr. Zhu licensed his proprietary technology/know-how related to the manufacture of certain medicines to OT Samoa . At the same day, OT Samoa entered into a Cooperation Agreement with ABG.The technology was not recorded on the books of OT Samoa, because the agreement consists only of an operating license and royalty arrangement with no transfer of ownership. OT Samoa did not engage in any business other than licensing the technology owned by Mr. Zhu and cooperating with ABG on the manufacturing of the know-how related products. On January 26, 2006, Mr. Zhu, the sole incorporator, owner and control person transferred his shares to 1,399 separate individuals. Mr. Zhu remained the sole director of OT Samoa and retained rights of rescission with regard to the transfer of his shares to the 1,399 individuals up until the time that those shares were exchanged for Asia Biotech shares.
The principal activities of the Company and its subsidiaries are the manufacturing and trading of feminine suppository. All activities of the Group are principally conducted by subsidiaries operating in the People's Republic of China (“PRC”).
Basis of Presentation
The accompanying unaudited financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America for interim financial information and with the instructions to Form 10−QSB of Regulation S−B. They do not include all information and footnotes required by accounting principles generally accepted in the United States of America for complete financial statements. However, except as disclosed herein, there has been no material change in the information disclosed in the notes to the financial statements for the year ended December 31, 2005 included in the Company's Annual Report on Form 10−KSB, as amended and filed with the Securities and Exchange Commission. The interim unaudited financial statements should be read in conjunction with those financial statements included in the Form 10−KSB. In the opinion of management, all adjustments considered necessary for a fair presentation, consisting solely of normal recurring adjustments, have been made. Operating results for the six months ended June 30, 2006 are not necessarily indicative of the results that may be expected for the year ending December 31, 2006.
NOTE 2 SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
A. Accounting Method
The Company's financial statements are prepared using the accrual method of accounting. The Company has elected a fiscal year ending on December 31.
B. Control by principal stockholders
The directors, executive officers and their affiliates or related parties own, beneficially and in the aggregate, the majority of the voting power of the outstanding shares of the common stock of the Company. Accordingly, the directors, executive officers and their affiliates, if they voted their shares uniformly, would have the ability to control the approval of most corporate actions, including increasing the authorized capital stock of the Company and the dissolution, merger or sale of the Company's assets.
C. Cash and cash equivalents
Cash and cash equivalents include cash on hand, cash accounts, interest bearing savings accounts and time certificates of deposit with a maturity of three months or less when purchased.
D. Inventories
Inventories are stated at the lower of cost or market, determined by the weighted average method. Finished goods inventories consist of raw materials, direct labor and overhead associated with the manufacturing process.
E. Trade accounts receivable
Trade accounts receivable are recorded at the invoiced amount and do not bear interest. The allowance for doubtful accounts represents the Company’s best estimate of the amount of probable credit losses in the existing accounts receivable balance. The Company determines the allowance for doubtful accounts based upon historical write-off experience and current economic conditions. The Company reviews the adequacy of its allowance for doubtful accounts on a regular basis. Account balances are charged off against the allowance after all means of collection have been exhausted and the potential for recovery is considered remote. The Company signed a sales contract with Red Cross Society of China in which the terms of payment is longer than one year.
F. Credit Risk and Customers
We have a concentration of customers in trading of feminine suppository. We are diligent in attempting to ensure that we issue credit to credit-worthy customers. However, our customer base is small and our accounts receivable balances are usually over 90 days outstanding, and that exposes us to significant credit risk. Therefore, a credit loss can be very large relative to our overall profitability.
During the six months ended June 30, 2006, there is one customer that accounted for more than 41% of our revenues, and totaling $91,295 and other customers accounted for less than 10% of our total revenue. The loss of the major customer,, could have a material impact on our results of operations.
G. Property, plant and equipment
Property, plant and equipment are stated at cost including the cost of improvements. Depreciation and amortization are provided on the straight-line method based on the estimated useful lives of the assets as follows:
| Buildings | | | 20 years | |
| Leasehold improvements | | | 20 years | |
| Plant and machinery | | | 10 years | |
| Motor vehicles | | | 5 years | |
| Furniture, fixtures and equipment | | | 5 years | |
H. Valuation of long-lived assets
The Company periodically evaluates the carrying value of long-lived assets to be held and used, including intangible assets subject to amortization, when events and circumstances warrant such a review. The carrying value of a long-lived asset is considered impaired when the anticipated undiscounted cash flow from such asset is separately identifiable and is less than its carrying value. In that event, a loss is recognized based on the amount by which the carrying value exceeds the fair market value of the long-lived asset. Fair market value is determined primarily using the anticipated cash flows discounted at a rate commensurate with the risk involved. Losses on long-lived assets to be disposed of are determined in a similar manner, except that fair market values are reduced for the cost to dispose.
I. Revenue recognition
The Company recognizes revenue when it is realized and earned. The Company considers revenue realized or realizable and earned when (1) it has persuasive evidence of an arrangement, (2) delivery has occurred, (3) the sales price is fixed or determinable, and (4) collectibility is reasonably assured. Delivery does not occur until products have been shipped to the client, risk of loss has transferred to the client and client acceptance has been obtained, client acceptance provisions have lapsed, or the Company has objective evidence that the criteria specified in client acceptance provisions have been satisfied. The sales price is not considered to be fixed or determinable until all contingencies related to the sale have been resolved.
J. Comprehensive income (loss)
Comprehensive income (loss) includes changes to equity accounts that were not the result of transactions with shareholders. Comprehensive income (loss) is comprised of net income (loss) and other comprehensive income and loss items. The Company’s comprehensive income and losses generally consist of changes in the fair value of changes in the cumulative foreign currency translation adjustment.
K. Foreign currency translation
Transactions and balances originally denominated in U.S. dollars are presented at their original amounts. Transactions and balances in other currencies are converted into U.S. dollars in accordance with Statement of Financial Accounting Standards (SFAS) No. 52, "Foreign Currency Translation," and are included in determining net income or loss.
For foreign operations with the local currency as the functional currency, assets and liabilities are translated from the local currencies into U.S. dollars at the exchange rate prevailing at the balance sheet date. Revenues, expenses and cash flows are translated at the average exchange rate for the period to approximate translation at the exchange rate prevailing at the dates those elements are recognized in the financial statements. Translation adjustments resulting from the process of translating the local currency financial statements into U.S. dollars are included in determining comprehensive loss.
The Company has determined the PRC Chinese Yuan Renminbi to be the functional currency of the Company. The financial statements of the Company are translated to United States dollars using year-end rates of exchange for assets and liabilities, and average rates of exchange for the period for revenues, costs, and expenses. Net gains and losses resulting from foreign exchange transactions are included in the consolidated statements of operations. The cumulative translation adjustment and effect of exchange rate changes were $17,542 and nil as of June 30, 2006 and June 30 2005 respectively.
L. Due to stockholder
The caption "Due to stockholder" on the condensed consolidated Balance Sheet consists of advances that are unsecured, non-interest bearing and have no fixed terms of repayment, and therefore, are deemed payable on demand.
M. Use of estimates
The preparation of financial statements in conformity with generally accepted accounting principles requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosures of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
N. Significant Estimates
Several areas require management's estimates relating to uncertainties for which it is reasonably possible that there will be a material change in the near term. The more significant areas requiring the use of management estimates related to valuation of the useful lives of the Company's equipment and valuation of contingent liabilities.
O. Income Taxes
The Company accounts for income taxes under the Financial Accounting Standards Board (FASB) Statement No. 109, "Accounting for Income Taxes" "Statement 109"). Under Statement 109, deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. Under Statement 109, the effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date.
P. Basic Income/Loss Per Common Share
Basic loss per common share has been calculated based on the weighted average number of shares outstanding during the period after giving retroactive effect to stock splits. There are no dilutive securities at June 30, 2006 for purposes of computing fully diluted earnings per share.
Q. Stock Based Compensation
In December 2004, the FASB issued SFAS No. 123R, “Share-Based Payments (revised 2004).” This statement replaces SFAS 123, “Accounting for Stock−Based Compensation” and supersedes Accounting Principles Board's Opinion No. 25 (ABP 25), “Accounting for Stock Issued to Employees.” SFAS 123R requires companies to measure the cost of employee services received in exchange for an award of equity instruments based on the grant date fair value of the award. That cost will be recognized over the period during which an employee is required to provide services in exchange for the award - the requisite service period (usually the vesting period). In March 2005, the SEC staff expressed their views with respect to SFAS No. 123R in Staff Accounting Bulletin No. 107, “Share-Based Payment,” (SAB 107). SAB 107 provides guidance on valuing options. We adopted SFAS 123R on January 1, 2006.
R. Off-Balance Sheet Arrangements
The Company does not have any off-balance sheet credit exposure related to its customers.
NOTE 3 INVENTORIES
Inventories by major categories, consist of the following at June 30, 2006:
| Raw materials | | $ | 161,971 | |
| Finished goods | | | 22,733 | |
| Packaging materials | | | 64,168 | |
| | | $ | 248,872 | |
NOTE 4 PROPERTY, PLANT AND EQUIPMENT
Property, plant and equipment, which are all located in the PRC, consist of the following at June 30, 2006:
| At cost: | | | |
| Buildings | | $ | 828,871 | |
| Leasehold improvements | | | 51,375 | |
| Plant and machinery | | | 302,950 | |
| Motor vehicles | | | 121,859 | |
| Furniture, fixtures and equipment | | | 48,436 | |
| Total | | | 1,353,491 | |
| Less: accumulated depreciation and amortization | | | (498,585 | ) |
| Net book value | | $ | 854,906 | |
NOTE 5 LAND USE RIGHT
Land use right for the land located in PRC, consists of the following at June 30, 2006:
| Land Use Right, cost | | $ | 122,650 | |
| Less: accumulated amortization | | | (11,498 | ) |
| Net book value | | $ | 111,152 | |
All the land in the PRC is owned by the PRC government. The government, according to PRC laws, may grant to entities the right to the use of land for a specified period of time. Thus all of the Company’s land occupied in the PRC is considered to be leasehold land and amortized on a straight-line basis over the respective term of the right to use the land.
The subsidiary, OT Pharmaceutical is granted the right to the use of land for 50 years and is amortized on a straight-line basis over the 50 years of the right to use the land from the date of acquisition in 2001.
NOTE 6 INTELLECTUAL PROPERTY
The transfers of nonmonetary assets to a company by its promoters or shareholders in exchange for stock prior to or at the time of the company’s initial public offering normally was recorded at the transferors’ historical cost basis determined under GAAP and in accordance with Topic 5(g) Transfers Of Nonmonetary Assets By Promoters Or Shareholders.
The patent rights which were contributed by the shareholders to the OT Pharmaceutical, were valued at the historical cost. As at June 30, 2006, the Company is registered owner of those patents, but the costs have been fully amortized in previous year.
NOTE 7 INCOME TAXES
United States
The Company is incorporated in the United States of America and are subject to United States of America tax law. No provisions for income taxes has been made as the Company has no taxable income for the period. The applicable income tax rate for the Company for the six months ended June 30, 2006 and 2005 is 34%.
British Virgin Islands
Asia Biotechnology Group Inc., a wholly owned subsidiary of the Company, incorporated in the British Virgin Islands and, under the current laws of the British Virgin Islands, is not subject to income taxes.
Samoa
Harbin OT Pharmaceutical Co., a wholly owned subsidiary of the Company, incorporated in the Samoa and, under the current laws of the Samoa, is not subject to income taxes.
PRC
Harbin OT Pharmaceutical Co., Ltd., a 60% owned subsidiary of the Company, incorporated in the People’s Republic of China as Chinese foreign owned enterprise is subject to enterprises income tax. No provisions for income taxes has been made as the Company’s subsidiary has incurred a loss for the period.
Enterprises income tax is generally charged at 33%, in which 30% is for national tax and 3% is for local tax, of the assessable profit. The Company incorporated in PRC is subject to PRC enterprises income tax at the applicable tax rates on the taxable income as reported in their Chinese statutory accounts in accordance with the relevant enterprises income tax laws applicable to foreign enterprises. Pursuant to the same enterprises income tax laws, the subsidiaries are fully exempted from PRC enterprises income tax for two years starting from the first profit-making year, followed by a 50% tax exemption for the next three years.
According to the PRC’s applicable income tax laws, regulations, notices and decisions related to foreign investment enterprises and their investors, income such as dividends and profits distribution from the PRC derived from a foreign enterprise which has no establishment in the PRC is subject to a 10% withholding tax.
There are net operating loss carryforwards allowed under the China's governments' tax systems. In China, the previous five years net operating losses are allowed to be carried forward five years to offset future taxable income. The Company has available approximately $ 1,100,000 of unused operating loss carryforwards and based on a 33% tax rate has a deferred tax asset of approximately $363,000 in which the company recorded a valuation allowance for the same amount at June 30, 2006.
The company withholds and pays income taxes on its employees' wages, which funds the Chinese government's sponsored health and retirement programs of all the employees.
NOTE 8 SHAREHOLDER’s EQUITY
On July 27, 2004 (inception), the Board of Directors issued 148,000 shares of common stock for $148 in services to the founding shareholder of the Company to fund organizational start-up costs.
On February 23, 2005, the Board of Directors issued 11,500,000 shares of common stock for $11,500 in services rendered to an officer and director of the Company.
On May 8, 2006, the Company issued to the ABG Shareholder an aggregate of 23,296,000 shares of common stock of the Company , and simultaneously issued to the OT Samoa Shareholders an aggregate of 23,296,000 shares of Asia Biotech common stock. Such shares at the Closing were equal to 80% of the issued and outstanding shares of Asia Biotech. After the Closing, there were 58,240,000 outstanding shares of common stock of the reorganized Asia Biotech.
On May 14, 2006, an agreement for the waiver of stockholder debt was signed between the stockholder and the Company and agreed that stockholder debt of $1,097,118 was contributed as capital. Consequently, the $1,097,118 was credited to additional paid in capital.
Common Stock
The holders of the Company's common stock:
* Have equal ratable rights to dividends from funds legally available for payment of dividends when, as and if declared by the board of directors;
* Are entitled to share ratably in all of the assets available for distribution to holders of common stock upon liquidation, dissolution or winding up of our affairs;
* Do not have preemptive, subscription or conversion rights, or redemption or access to any sinking fund; and
* Are entitled to one non-cumulative vote per share on all matters submitted to stockholders for a vote at any meeting of stockholders.
Preferred Stock
The Company has authorized, but not issued, 20,000,000 shares of preferred stock at $.001 per share. The board of directors has the authority to establish and fix the designation, powers, or preferences of preferred shares without further vote by the shareholders.
NOTE 9 EMPLOYEE BENEFITS
The Company has established its own employee welfare plan in accordance with Chinese law and regulations. The Company makes annual contributions of 14% of all employees’ salaries to its employee welfare plan. The total expenses for the above plan were $3,313 for the six months ended June 30, 2006 respectively. The Company has recorded welfare payments in the amount of $18,082 as of June 30, 2006 in balance sheet.
NOTE 10 RECENT ACCOUNTING PRONOUNCEMENTS
In December 2004, the FASB revised FASB Statement No. 123R, Accounting for Stock−Based Compensation. This Statement supersedes APB Opinion No. 25, Accounting for Stock Issued to Employees, and its related implementation guidance. This Statement establishes standards for the accounting for transactions in which an entity exchanges its equity instruments for goods or services. It also addresses transactions in which an entity incurs liabilities in exchange for goods or services that are based on the fair value of the entity’s equity instruments or that may be settled by the issuance of those equity instruments. This Statement focuses primarily on accounting for transactions in which an entity obtains employee services in share−based payment transactions. This Statement does not change the accounting guidance for share−based payment transactions with parties other than employees provided in Statement 123 as originally issued and EITF Issue No. 96−18, “Accounting for Equity Instruments That Are Issued to Other Than Employees for Acquiring, or in Conjunction with Selling, Goods or Services.” It applies in the first reporting period beginning after December 15, 2005. The adoption of Statement No. 123R (revised 2004) did not have a material impact on the Company’s financial position, liquidity, or results of operations.
In July 2006, the Financial Accounting Standards Board (“FASB”) has published FASB Interpretation No. 48 (“FIN No. 48”), Accounting for Uncertainty in Income Taxes, to address the noncomparability in reporting tax assets and liabilities resulting from a lack of specific guidance in FASB Statement of Financial Accounting Standards (“SFAS”) No. 109, Accounting for Income Taxes, on the uncertainty in income taxes recognized in an enterprise’s financial statements. FIN No. 48 will apply to fiscal years beginning after December 15, 2006, with earlier adoption permitted. The adoption of FIN 48 is not expected to have a material effect on the Company’s financial condition or results of operations.
PART II: INFORMATION NOT REQUIRED IN PROSPECTUS
ITEM 24. INDEMNIFICATION OF DIRECTORS AND OFFICERS.
Section 145 of the Delaware General Corporation Law authorizes a court to award, or a corporation's board of directors to grant, indemnity to directors and officers in terms sufficiently broad to permit such indemnification under certain circumstances for liabilities (including reimbursement for expenses incurred) arising under the Securities Act.
Our Amended and Restated Certificate of Incorporation and our Bylaws provide for the indemnification of a present or former director or officer. Such indemnification shall include expenses, including attorney's fees actually or reasonably incurred by him. We may indemnify such individuals against all costs, expenses, and liabilities incurred in a threatened, pending, or completed action, suit, or proceeding brought because such individual is one of our directors or officers.
ITEM 25. OTHER EXPENSES OF ISSUANCE AND DISTRIBUTION.
The following table sets forth an itemization of various expenses, all of which we will pay in connection with the sale and distribution of the securities being registered. All of the amounts shown are estimates, except the SEC registration fee.
| SEC Registration Fee | | $ | 5,935.93 | |
| Accounting Fees and Expenses | | $ | 45,000.00 | |
| Legal Fees and Expenses | | $ | 80,000.00 | |
| Miscellaneous | | $ | 0.00 | |
| Total | | $ | 130,935.93 | |
ITEM 27. EXHIBITS
| Exhibit No. | | | Description | |
| 2.1* | | | Agreement and Plan of Reorganization, dated as of May 8, 2006. | |
| 4.1 | | | Certificate of Amendment of Certificate of Incorporation | |
| 5.1 | | | Opinion regarding legality | |
| 10.1* | | | Certificate of land use | |
| 10.2 | | | Know-How License Agreement | |
| 10.3 | | | Cooperation Agreement | |
| 10.4 | | | Employment Contract | |
| 10.5 | | | Raw Material Supply Contract | |
| 10.6 | | | Red Cross Contract | |
| 21* | | | Subsidiaries of the Registrant | |
| 23.1 | | | Consent of King and Wood (Included in Exhibit 5.1) | |
| 23.2 | | | Consent of Child, Van Wagoner&Bradshaw, PLLC | |
| 99.1 | | | | |
* Provided previously in Form SB-2
The following Exhibits are attached hereto or incorporated herein by reference.
ITEM 28. UNDERTAKINGS
(a) Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the registrant pursuant to the provisions described under Item 14 above, or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act of 1933 and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act of 1933 and will be governed by the final adjudication of such issue.
(b) The undersigned registrant hereby undertakes:
(1) To file, during any period in which offers or sales are being made, a post-effective amendment to this Registration Statement;
(i) To include any prospectus required by section 10(a)(3) of the Securities Act of 1933;
(ii) To reflect in the prospectus any facts or events arising after the effective date of the Registration Statement (or the most recent post-effective amendment thereof) which, individually, or in the aggregate, represent a fundamental change in the information set forth in the Registration Statement; notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than a 20% change in the maximum aggregate offering price set forth in the "Calculation of Registration Fee" table in the effective Registration Statement; and
(iii) To include any material information with respect to the plan of distribution not previously disclosed in the Registration Statement or any material change to such information in the Registration Statement
(2) That, for the purpose of determining any liability under the Securities Act of 1933, each post-effective amendment shall be deemed to be a new Registration Statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof; and
(3) To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the Offering.
(c) The undersigned registrant hereby undertakes that, for purposes of determining any liability under the Securities Act, each filing of the registrant's annual report pursuant to Section 13(a) or Section 15(d) of the Securities Exchange Act of 1934 (and, where applicable, each filing of an employee benefit plan's annual report pursuant to Section 15(d) of the Securities Exchange Act of 1934) that is incorporated by reference in the Registration Statement shall be deemed to be a new Registration Statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the Registrant certifies that it has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in Beijing on December 27, 2006.
| | | |
| | Asia Biotechnology Group Inc. |
| | | |
| By: | Representative |
| | | /s/ Xueliang Qiu, President & CEO |
| |
/s/ Feng Yang, CFO
|
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
| SIGNATURE | | TITLE | | DATE |
| | | | | |
/s/ Xueliang Qiu
| | President & CEO Chairman of the Board | | December 27, 2006 |
| | | | | |
/s/ Feng Yang
| | CFO, Director | | December 27, 2006 |