UNITED STATES SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 10-K
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d)
OF THE SECURITIES EXCHANGE ACT OF 1934
| | | |
| þ | | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d)
OF THE SECURITIES EXCHANGE ACT OF 1934 |
| | | For the Fiscal Year Ended June 30, 2007 |
or |
| | | |
| | | |
| o | | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d)
OF THE SECURITIES EXCHANGE ACT OF 1934 |
| | | For the transition period from to |
Commission file number 000-51445
Adams Respiratory Therapeutics, Inc.
(Exact name of Registrant as specified in its Charter)
| | | |
Delaware | | 75-2725552 |
(State of incorporation) | | (I.R.S. Employer Identification No.) |
4 Mill Ridge Lane | | 07930 |
Chester, New Jersey | | (Zip Code) |
(Address of principal executive offices) | | |
(908) 879-1400
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Securities Exchange Act of 1934:
Common Stock, Par Value $0.01 Per Share
(Title of class)
Securities registered pursuant to Section 12(g) of the Securities Exchange Act of 1934:
None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes þ No o
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes o No þ
Indicate by check mark whether the registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes þ No o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 ofRegulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of thisForm 10-K or any amendment to thisForm 10-K. o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, or a non-accelerated filer. See definition of “accelerated filer and large accelerated filer” inRule 12b-2 of the Exchange Act. (Check one):
Large Accelerated Filer þ Accelerated Filer o Non-Accelerated Filer o
Indicate by check mark whether the registrant is a shell company (as defined inRule 12b-2 of the Act). Yes o No þ
The aggregate market value of the voting and non-voting common equity held by non-affiliates of the registrant, based on the last sale price for such stock on December 29, 2006 as reported on that date was $1,358,631,665.16.
The number of shares of registrant’s common stock outstanding on August 28, 2007 was 35,848,177.
Portions of the registrant’s definitive Proxy Statement for the 2007 Annual Meeting of Stockholders of the registrant to be held December 14, 2007 are incorporated by reference into Part III of thisForm 10-K.
TABLE OF CONTENTS
A, Adams, A Adams Respiratory Therapeutics, Adams Respiratory Therapeutics, Delsym, Humibid, Junior Mucus, Kid and Clock design, MiniMelts, Mini-Melts, Mucinex, Mucinex Full Force, Mucinex IN...Mucus OUT, Mucinex Moisture Smart, Mr. Mucus, Mrs. Mucus, Nothing Lasts Longer, Opening New Pathways to Respiratory Relief, Turn off the Cough and the Junior Mucus, Mr. Mucus, Mrs. Mucus and XL Mucus characters are our registered trademarks or are the subject of pending U.S. trademark applications. Each of the other trademarks, trade names or service marks appearing in this document belongs to its respective holder.
PART I
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
Some of the statements made under the headings “Business” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and elsewhere in this Annual Report onForm 10-K contain forward-looking statements that reflect our plans, beliefs and current views with respect to, among other things, future events and financial performance. We often identify these forward-looking statements by the use of forward-looking words such as “believe,” “expect,” “potential,” “continue,” “may,” “will,” “should,” “could,” “would,” “seek,” “predict,” “intend,” “plan,” “estimate,” “anticipate” or the negative version of those words or other comparable words. Specifically, this Annual Report contains, among others, forward-looking statements regarding:
| | |
| | • | our course of action related to, and the FDA’s response to, an abbreviated new drug application filing by Perrigo R&D Co.; |
| |
| | • | FTC and DOJ approval of our settlement agreement with Mutual Pharmaceutical Company; |
| |
| | • | our involvement in litigation having an adverse impact on our business, financial condition, results of operations or cash flows; |
| |
| | • | our obligations related to product returns and royalty payments; |
| |
| | • | our plans with respect to the commercialization of products under the Humibid brand; |
| |
| | • | our ability to obtain sufficient quantities of raw materials, including guaifenesin and dextromethorphan; |
| |
| | • | our expectations of the seasonality of our product sales and related revenue fluctuations; |
| |
| | • | our intentions to market additional products, grow our business and expand our product portfolio; |
| |
| | • | the need to conduct clinical trials to develop product candidates; |
| |
| | • | the conversion of prescriptions for long-acting guaifenesin combination products into sales of Mucinex DM and Mucinex D and their maximum strength versions as a result of the FDA’s enforcement action against unapproved products; |
| |
| | • | the effect of our April 2007 product recall on our business; |
| |
| | • | our ability to meet our anticipated operating needs with revenues, existing cash and our credit facility; |
| |
| | • | the timing of the USPTO’s reexamination of the patentability of our delivery system for guaifenesin and our ability to prevail in the reexamination process; |
| |
| | • | our reliance on and continued consolidation of our top customers; |
| |
| | • | our expectations of increased pricing pressures; |
| |
| | • | our ability to leverage our brand names; |
| |
| | • | our intentions with respect to our consumer and professional marketing efforts; |
| |
| | • | our ability to develop a reputation as an expert and effectively compete in the respiratory therapeutics market; and |
| |
| | • | our exposure to credit rate and interest rate risk. |
Any forward-looking statements contained in this Annual Report are based upon our historical performance and on our current plans, estimates and expectations. You should not regard the inclusion of this forward-looking information as a representation by us or any other person that we will achieve the future plans, estimates or expectations contained in this Annual Report. Such forward-looking statements are subject to various risks and uncertainties. In addition, there are or will be important factors that could cause our actual results to differ materially from those in the forward-looking statements. We believe these factors include, but are not limited to, those described in Part I, Item IA. Risk Factors in this Annual Report onForm 10-K.
1
These cautionary statements should not be construed as exhaustive and should be read in conjunction with the other cautionary statements that are included in this Annual Report. Moreover, we operate in a continually changing business environment, and new risks and uncertainties emerge from time to time. Management cannot predict these new risks or uncertainties, nor can it assess the impact, if any, that any such risks or uncertainties may have on our business or the extent to which any factor, or combination of factors, may cause actual results to differ from those projected in any forward-looking statement. Accordingly, the risks and uncertainties to which we are subject can be expected to change over time, and we undertake no obligation to update publicly or review the risks or uncertainties described in this Annual Report. We also undertake no obligation to update publicly or review any of the forward-looking statements made in this Annual Report, whether as a result of new information, future developments or otherwise.
If one or more of the risks or uncertainties referred to in this Annual Report materialize, or if our underlying assumptions prove to be incorrect, actual results may vary materially from what we have projected. Any forward-looking statements contained in this Annual Report reflect our current views with respect to future events and are subject to these and other risks, uncertainties and assumptions relating to our operations, financial condition, growth strategy and liquidity. You should specifically consider the factors identified in this Annual Report that could cause actual results to differ. We qualify all of our forward-looking statements by these cautionary statements. In addition, with respect to all of our forward-looking statements, we claim the protection of the safe harbor for forward-looking statements contained in the Private Securities Litigation Reform Act of 1995.
As used herein, except as otherwise indicated by the context, references to “we,” “us,” “our,” or the “Company” refer to Adams Respiratory Therapeutics, Inc. and its subsidiaries.
Overview
Our Company. We are a specialty pharmaceutical company focused on the late-stage development, commercialization and marketing of over-the-counter, or OTC, and prescription pharmaceuticals for the treatment of respiratory disorders. We currently market six oral-solid OTC products for adults under our Mucinex brand, six products in our Mucinex line of products for children, two Mucinex nasal spray products, and two products under our Delsym brand. We are a Delaware corporation, and we initially incorporated in Texas on September 12, 1997.
We operate in one business segment, specialty pharmaceuticals. All of our revenues are attributed to sales of our products in the United States. Our net revenues were $331.6 million, $239.1 million and $160.2 million for the fiscal years ended June 30, 2007, 2006 and 2005, respectively. Our net income was $30.5 million, $46.4 million and $27.0 million for the fiscal years ended June 30, 2007, 2006 and 2005, respectively. Our total assets were $318.5 million, $258.1 million and $67.2 million at June 30, 2007, 2006 and 2005, respectively. At June 30, 2007 and 2006, our long-lived assets were $142.6 million and $133.0 million, respectively, and primarily consisted of intangible assets and property, plant and equipment, all of which are located in the United States.
All of our products treat symptoms associated with respiratory disorders, which allows us to maximize resources and concentrate on identifying the best strategies for marketing and product development. By focusing on the respiratory therapeutics market, we believe that we can develop our reputation as an expert in that area, which will allow us to more effectively compete with other respiratory therapeutics products. Further, we believe that our focus on the respiratory therapeutics market allows us to identify additional products that will complement and expand our portfolio of respiratory therapeutics.
The Respiratory Therapeutics Market. Respiratory disorders include serious conditions for which patients seek professional medical treatment, such as emphysema, pneumonia, chronic obstructive pulmonary disease, or COPD, chronic bronchitis and sinusitis, as well as less serious disorders, which patients often diagnose and treat by themselves, including the common cold, bronchitis, and allergies. Respiratory therapeutics to treat these disorders range from prescription pharmaceuticals prescribed by a physician, to OTC
2
pharmaceuticals purchased by the consumer, often as the result of a recommendation by a healthcare professional.
According to IMS Health-National Sales Perspectivestm (retail and non-retail), for the 12 months ended June 30, 2007, the prescription market in the United States for all respiratory disorders was approximately $17.0 billion, while the prescription cough and cold market in the United States was approximately $1.1 billion. According to Information Resources, Inc., or IRI, consumers in the United States spent approximately $3.6 billion on OTC cough, cold, allergy, sinus and nasal remedies during the 52 weeks ended August 12, 2007, which includes approximately $2.8 billion of adult and children’s cold, allergy and sinus remedies, $440 million of adult and children’s cough/congestion liquid remedies, and approximately $411 million of nasal remedies. In total, approximately $423 million of this OTC cough, cold, allergy and sinus market represents sales of pediatric products, according to IRI.
Respiratory disorders have different causes and symptoms, but the production of excess or thick mucus is a common factor in many of these disorders. Excess mucus often exacerbates respiratory disorders, such as acute respiratory infections, bronchitis, common cold, cough, and sinusitis. Guaifenesin, the principal active ingredient in our adult oral-solid Mucinex products, is the only expectorant approved by the Food and Drug Administration, or the FDA. It helps loosen mucus and thin bronchial secretions to rid the bronchial passageways of mucus and make coughs more productive. Guaifenesin is available in both long-acting and short-acting formulations and as a single ingredient or in combination with other active ingredients. Long-acting formulations are typically in tablet form and dosed every 12 hours. Short-acting or immediate-release formulations are often in liquid form and are dosed every four to six hours. Prior to the FDA approval of Mucinex in July 2002, long-acting formulations were available in the United States by prescription only, while short-acting formulations were available over the counter.
Market, Ranking and Other Data
The data included in this annual report regarding market share, historical sales, market size, and ranking, including our position and the position of our competitors within these markets, is based on data generated by the independent market research firms IRI and IMS Health Incorporated, or IMS Health.
IRI data reports non-prescription retail sales in the food, drug and mass merchandise markets. IRI data for the mass merchandise market does not include Wal-Mart, which ceased providing sales data to IRI in 2001. Although Wal-Mart represents a significant portion of the mass merchandise market for us, as well as our competitors, we believe that Wal-Mart’s exclusion from IRI data does not significantly change our market share or ranking relative to our competitors. As used in this annual report, the U.S. OTC cough, cold, allergy and sinus market as reported by IRI includes both the adult and children’s segments of the cough, cold, allergy and sinus market but excludes the U.S. OTC nasal products market in which our Mucinex nasal spray products are reported. We believe our products compete against products comprising both of these market segments.
IMS Health reports data from various sources, including drug manufacturers, wholesalers, retailers, pharmacies, mail services, long-term care facilities, and hospitals. We rely on IMS Health-NPAtm for historical retail prescription sales data related to extended-release, single-ingredient and combination guaifenesin products. We rely on IMS Health-National Sales Perspectivestm for retail and non-retail prescription sales data related to the remainder of our business. We believe this prescription and non-retail data is not reported in the data we receive from IRI.
3
Our Products
| | | | | | | |
| | | | | Fiscal
| |
Product | | Active Ingredients | | Launch Year | |
| |
| Mucinex SE | | 600 mg guaifenesin | | | 2003 | |
| Mucinex DM | | 600 mg guaifenesin/30 mg dextromethorphan HBr | | | 2005 | |
| Mucinex D | | 600 mg guaifenesin/60 mg pseudoephedrine HCl | | | 2006 | |
| Delsym Orange (Adult & Children) | | 5 ml dextromethorphan polistirex
(equivalent to 30 mg dextromethorphan HBr/5 ml) | | | 2006 | * |
| Delsym Grape (Adult & Children) | | 5 ml dextromethorphan polistirex
(equivalent to 30 mg dextromethorphan HBr/5 ml) | | | 2008 | ** |
| Maximum Strength Mucinex SE | | 1200 mg guaifenesin | | | 2008 | |
| Maximum Strength Mucinex DM | | 1200 mg guaifenesin/60 mg dextromethorphan HBr | | | 2008 | |
| Maximum Strength Mucinex D | | 1200 mg guaifenesin/120 mg pseudoephedrine HCl | | | 2008 | |
| Mucinex Mini-Melts | | 50 mg guaifenesin | | | 2007 | |
| | | 100 mg guaifenesin | | | 2007 | |
| Mucinex Cough Mini-Melts | | 100 mg guaifenesin/5 mg dextromethorphan (HBr) | | | 2008 | |
| Children’s Mucinex Liquid | | 100 mg/5 ml guaifenesin | | | 2007 | |
| Children’s Mucinex Cough Liquid | | 100 mg/5 ml guaifenesin
5 mg/5 ml dextromethorphan (HBr) | | | 2007 | |
| Children’s Mucinex Cold Liquid | | 100 mg/5 ml guaifenesin
2.5 mg/5 ml phenylephrine (HCl) | | | 2008 | |
| Mucinex Moisture Smart Nasal Spray | | 0.05% oxymetazoline/actuation | | | 2008 | |
| Mucinex Full ForceNasal Spray | | 0.05% oxymetazoline/actuation | | | 2008 | |
| | |
| * | | Acquired by Adams in June 2006 |
| |
| ** | | Proposed fiscal launch year |
Mucinex SE. Mucinex SE is an extended-release, single-ingredient 600 mg guaifenesin product, which we launched in July 2002 after receiving FDA approval of our New Drug Application, or NDA. For the fiscal years ended June 30, 2007, 2006 and 2005, our net sales of Mucinex SE were $131.9 million, $158.6 million and $117.4 million, respectively. Sales of Mucinex SE accounted for approximately 39.8%, 66.3% and 73.3% of our revenue in fiscal 2007, 2006 and 2005, respectively. According to IRI, for the 52-week period ended August 12, 2007, our Mucinex SE 20-count and 40-count stock keeping units, or SKUs, ranked number two and six, respectively, in terms of retail dollar sales in the approximate $3.2 billion U.S. OTC cough, cold, allergy and sinus market.
Mucinex DM. Mucinex DM combines the long-acting expectorant properties of guaifenesin with the cough suppressant dextromethorphan. The FDA approved our NDA for Mucinex DM in April 2004, and we launched Mucinex DM during August 2004. For the fiscal years ended June 30, 2007, 2006 and 2005, our net sales of Mucinex DM were $94.6 million, $59.2 million and $38.3 million, respectively. Sales of Mucinex DM accounted for approximately 28.5%, 24.8% and 23.9% of our revenue in fiscal 2007, 2006 and 2005, respectively. According to IRI, for the 52-week period ended August 12, 2007, our Mucinex DM20-count and 40-count SKUs were the number one and 16 SKUs, respectively, in terms of retail dollar sales.
4
Mucinex D. Mucinex D adds the decongestant pseudoephedrine to the expectorant properties of long-acting guaifenesin. The FDA approved our NDA for Mucinex D in June 2004; we began marketing Mucinex D in October 2005. As of September 2006, all products containing pseudoephedrine, including Mucinex D, are limited to behind-the-counter sales. For the fiscal years ended June 30, 2007 and 2006, our net sales for Mucinex D were $28.2 million and $19.3 million. According to IRI, for the 52-week period ended August 12, 2007, our Mucinex D 18-count and 36-count SKUs were the number 17 and 77 SKUs, respectively, in terms of retail dollar sales.
Maximum Strength Mucinex. We have also received FDA approval for three maximum strength 1200 mg long-acting, guaifenesin-based OTC products, which we began marketing under the Mucinex brand in July 2007. These three products are Maximum Strength Mucinex SE, a single-ingredient guaifenesin product; Maximum Strength Mucinex DM, a guaifenesin/dextromethorphan combination product; and Maximum Strength Mucinex D, a guaifenesin/pseudoephedrine combination product. Each of these maximum strength products has twice the amount of active ingredients in each tablet as its regular-strength Mucinex counterpart.
Delsym. Delsym is the only FDA-approved12-hour liquid cough suppressant available without a prescription. Delsym utilizes the cough suppressant dextromethorphan combined with patented Pennkinetic® technology to provide the longest-lasting OTC cough relief in a single dose on the market. Delsym has no private-label equivalent. We acquired the U.S. marketing and sales rights to Delsym from UCB, Inc., or UCB, in June 2006. We currently market two orange-flavored products in the Delsym line consisting of two adult SKUs and two children’s SKUs. In the first half of fiscal 2008, we plan to introduce adult and children’s grape-flavored additions to our Delsym line that include the same ingredients and dosing as our existing Delsym products. For the fiscal years ended June 30, 2007 and 2006, our net sales for Delsym totaled $48.3 million and $0.7 million. Sales of Delsym accounted for approximately 14.6% of our revenue in fiscal 2007. According to IRI, for the 52-week period ended August 12, 2007, Delsym was the number two brand in the approximate $440 million U.S. OTC liquid cough/congestion segment, with a 12.4% market share, based on retail dollar sales.
Mucinex Products for Children. In August 2006, we introduced a line of four immediate-release guaifenesin products under the Mucinex brand for children, including two liquid products and two products utilizing a proprietary licensed delivery system that we market as Mini-Melts. Mini-Melts are easy-to-take, quick-melting, coated granules of active pharmaceutical ingredients, saliva stimulants and flavors delivered in pre-measured packets. In July 2007, we introduced two additional products in our Mucinex products for children line: Mucinex Cough Mini-Melts in orange crème flavor, which combines guaifenesin with the cough suppressant dextromethorphan, and Mucinex Cold Liquid in mixed berry flavor, which combines guaifenesin with the nasal decongestant phenylephrine. According to IRI, for the 52-week period ended August 12, 2007, the Mucinex line of products for children was the sixth best-selling brand in the approximate $423 million pediatric segment of the U.S. cough, cold, allergy and sinus category, with a 5.9% market share. For the fiscal year ended June 30, 2007, our net sales of the Mucinex line of products for children was $29.2 million.
Mucinex Nasal Sprays. In July 2007, we expanded the Mucinex franchise with our first nasal spray product, Mucinex Full Force Nasal Spray. In August 2007, we also began to market a second nasal spray product, Mucinex Moisture Smart Nasal Spray. Both nasal spray products contain the decongestant oxymetazoline HCl, which offers immediate topical relief from nasal congestion and lasts for 12 hours. These products compete in the U.S. OTC nasal products market, which according to IRI for the 52-week period ended August 12, 2007, equaled $411 million.
Product Pipeline. All of our adult oral-solid Mucinex products, including their maximum strength versions, utilize our patented delivery system for guaifenesin, which has both immediate-release and extended-release components to provide immediate relief and long-lasting effect. We also intend to utilize this technology to develop new products that combine guaifenesin with prescription ingredients for the treatment of respiratory disorders. In December 2006, we filed a NDA with the FDA for the first of these products, Mucinex with Codeine, which the FDA accepted in March 2007 for filing and formal review. The FDA has set October 27, 2007 as the Prescription Drug User Fee Act, or PDUFA, date, which is the FDA’s goal date to review and act on the NDA submission. There is no guarantee that final marketing approval will be granted by
5
the PDUFA date. If approved by the FDA, Mucinex with Codeine, which combines oral-solid extended-release guaifenesin with codeine to treat cough, will be our first prescription product. According to IMS Health-National Sales Perspectivestm (retail and non-retail), as of the 12 months ended June 30, 2007, the prescription market for narcotic products treating cough consisted of approximately 23 million prescriptions written and dispensed by U.S. physicians, with approximately 10.3 million prescriptions written in the codeine combination cough market. If approved, Mucinex with Codeine will be the only 12-hour codeine combination product available to meet this demand. We also have two other oral solid extended-release guaifenesin combination products in development, of which at least one would be for prescription use.
In addition, we are actively reviewing opportunities to in-license or acquire prescription and OTC products for the respiratory markets. We intend to focus on prescription products in the later stages of development and OTC brands that we believe can expand our presence in the cough, cold, allergy and sinus market. In May 2005, we in-licensed erdosteine from Edmond Pharma SRL, or Edmond; erdosteine is currently approved for use in Europe, South Africa and Asia for the symptomatic treatment of respiratory infections, bronchitis and COPD. In December 2006, we completed our phase IIb erdosteine clinical trial; the preliminary results suggested that the two different erdosteine treatment groups do not seem to break statistically from the placebo. However, subsequent analysis has resulted in more promising data. We hope to conduct a meeting with the FDA before the end of calendar year 2007 to discuss these clinical results, before we determine the ultimate outcome of the erdosteine program. We cannot assure you that these trials will ultimately be successful or that the FDA or other relevant regulatory agencies will accept the results and approve or clear erdosteine for sale. In March 2007, we entered into a license and collaboration agreement with MonoSol Rx, LLC, or MonoSol, a drug delivery company specializing in proprietary rapidly dissolving thin film pharmaceutical products. Under the terms of our agreement with Monosol, we received an exclusive, royalty-bearing, non-transferable license from MonoSol to use its proprietary rapidly dissolving thin-film drug delivery technology to develop and market two or more respiratory products in North America.
Our product development expenses totaled approximately $23.9 million, $18.9 million and $7.4 million for the fiscal years ended June 30, 2007, 2006 and 2005, respectively.
Other Products. We discontinued sales of our AlleRxtm products in February 2005 when we entered into an agreement with Cornerstone Biopharma, Inc., or Cornerstone, to assign our AlleRxtm assets to Cornerstone in exchange for the Humibid trademark. For the fiscal years ended June 30, 2005 and 2004, our net sales for AlleRxtm products were $4.5 million and $8.3 million, respectively. Humibid sales were $(0.6) million and $1.3 million during the fiscal years ended June 30, 2007 and 2006, respectively. Humibid sales for fiscal 2007 included a returns allowance of $0.9 million related to inventory that has expired or is approaching expiration. We are currently evaluating the future commercialization of Humibid.
The FDA’s Removal of Competing Products. Mucinex SE, Mucinex DM and Mucinex D and their maximum strength versions are the only long-acting guaifenesin-based products approved by the FDA. The FDA’s policy, finalized in June 2006, is to seek removal of unapproved products from the market once it has approved a similar product. Following approval of Mucinex SE, the FDA took enforcement action in December 2003 to remove all other long-acting, single-ingredient guaifenesin products from the market. As a result, Mucinex SE and its maximum strength version are currently the exclusive alternatives to what we estimate, based on IMS Health-NPAtm data, were formerly 10.5 million prescriptions dispensed for long-acting, single-ingredient guaifenesin products. Prior to the FDA’s enforcement action, physicians continued to prescribe and pharmacies continued to dispense long-acting, single-ingredient guaifenesin as prescription products, and we believe less than 5% of the prescriptions written resulted in retail sales of Mucinex SE, despite Mucinex SE being the only FDA-approved product. After the FDA’s enforcement action, we believe that a majority of physician prescriptions for long-acting, single-ingredient guaifenesin were replaced by OTC retail sales of Mucinex SE.
On May 25, 2007, the FDA announced that companies must stop manufacturing and distributing unapproved timed-release dosage forms containing guaifenesin in combination with other active ingredients. The FDA indicated that companies marketing these unapproved products are expected to stop manufacturing them by August 27, 2007 and must cease shipping them in interstate commerce by November 26, 2007. We
6
believe that as a result of the FDA’s enforcement action, a majority of the estimated 8.5 million prescriptions, based on IMS Health-National Sales Perspectivestm (retail and non-retail) data for the 12 months ended June 30, 2007, currently dispensed for products containing long-acting guaifenesin in combination with either dextromethorphan, pseudoephedrine or phenylephrine, will be switched to OTC sales of Mucinex DM and Mucinex D and their maximum strength versions.
Our Sales and Marketing Strategy
We utilize a dual marketing strategy for our OTC Mucinex products, targeting both the physicians and pharmacists who recommend these products and consumers directly. While guaifenesin in short-acting or immediate-release formulations is a common ingredient in numerous OTC cough, cold and sinus remedies, long-acting guaifenesin was available only by prescription until the FDA’s approval of Mucinex SE as an OTC product. We seek to capitalize on the historic prescription nature of the market for long-acting, single-ingredient and combination guaifenesin products by continuing to develop physician and pharmacist support for these products. In addition, we advertise our Mucinex products in an effort to educate consumers about the unique benefits of Mucinex and encourage consumers to try our Mucinex products.
Our dual approach is also well suited to driving the Delsym opportunity. When we acquired the Delsym brand, it enjoyed fairly good support in the professional community but suffered from a lack of awareness among consumers. We leverage our existing sales and marketing infrastructure to market our Delsym brand of cough suppressant liquids to physicians and pharmacists, and we market directly to consumers through dedicated advertising campaigns.
Like our currently marketed OTC Mucinex and Delsym products, we intend to market our new OTC Mucinex products, including the maximum strength Mucinex products and the Mucinex nasal spray products, using dedicated consumer advertising campaigns and our existing sales and marketing infrastructure to drive further brand awareness and market penetration. In contrast, once approved by the FDA, we will market our first prescription product, Mucinex with Codeine, only to physicians and healthcare professionals, and we do not intend to market this product directly to consumers.
Professional Marketing. Our professional marketing campaign attempts to educate physicians, pharmacists and other healthcare professionals on the benefits of our products to encourage them to recommend our products to their patients and customers. Our physician marketing efforts focus primarily on primary care physicians and secondarily on respiratory specialist physicians such as allergists, otolaryngologists, and pulmonologists. Our125-person professional sales force details our Delsym and adult oral-solid Mucinex products to approximately 20,000 healthcare professionals; we believe these healthcare professionals have traditionally written most of the prescriptions for products similar to Delsym and our adult oral-solid Mucinex products. In addition, our professional sales force calls on approximately 6,500 pediatricians to detail the benefits of Delsym and our Mucinex products for children. As we develop additional respiratory products, we intend to utilize and further expand our sales force to market these products to physicians.
Consumer Marketing. We introduced our OTC Mucinex and Delsym products to consumers in an effort to expand the available market and capture a meaningful share of the OTC cough, cold, allergy and sinus market, which, we believe, based on IRI data, represented approximately $3.2 billion during the 52 weeks ended August 12, 2007. In November 2004, we launched our initial advertising campaign featuring “Mr. Mucus” and utilizing the tag line “Mucinex IN. Mucus OUT.” In October 2005, we began airing a new execution of this campaign, which introduced “Mrs. Mucus”, and in October 2006, we continued with a refreshed campaign featuring “Mr. Mucus” and his companions in support of Mucinex DM. Also in October 2006, we introduced “Junior Mucus” as an element in our advertising to support the launch of Mucinex Mini-Melts. In November 2006, we launched our initial advertising campaign for our Delsym product line. We intend to launch new advertising featuring the Mucus family of characters for both our adult and children’s Mucinex products and an updated and refreshed advertising campaign for Delsym in Fall 2007.
In November 2006, we won two Gold Awards at the 2006 Medical Marketing & Media Awards Show. The Mr. Mucus campaign was recognized as the Best Total Integration Program (for client companies under $5 billion sales) and the Best Branded TV Campaign. Judged by a panel of senior executives in healthcare
7
marketing and advertising, the MM&M Awards are presented annually byMedical Marketing & Media Magazineto showcase both marketing creativity and effectiveness in healthcare communications. Mr. Mucus beat more than 600 submissions from the pharmaceutical industry in these categories. In August 2007, MM&M announced the finalists for its 2007 awards and Mr. Mucus was named a finalist for Best Over-The-Counter Product Advertisement/Campaign.
Strength of our Mucinex and Delsym Brands. In addition to sales growth, we gauge the effectiveness of our advertising campaigns by measuring aided brand awareness. Aided brand awareness is a consumer’s ability to acknowledge awareness of our product after being told the product’s name from a list of brands. Based on research we sponsored, aided brand awareness of Mucinex increased from 14% prior to the launch of our first advertising campaign to 78% during the peak of the 2006/2007 cough and cold season. During the first year of consumer advertising support, Delsym brand awareness grew from 17% to 42%, according to research we sponsored.
Mucinex SE and Delsym ranked number one in pharmacist recommendations in their respective OTC therapeutic categories, as determined in the Pharmacy Times 2007 Over-the-Counter (OTC) Survey of Pharmacist Recommendations, with Mucinex receiving 62.6% percent of pharmacist recommendations in the adult expectorant category and Delsym receiving 34.3% and 41.4% of pharmacist recommendations in the children and adult cough categories, respectively.
Mucinex D was named the “DIANA” award winner at the 2006 Healthcare Distribution Management Association, or HDMA, Annual Leadership Forum. The award winner is chosen by HDMA’s members based on sales and market share achieved, overall category sales impact, and the quality and impact of marketing support programs. We were also awarded the 2007 Supplier Performance Awards by Retail Category, or SPARC, Silver Circle Award in the OTC Medication Category sponsored byRetailing Today Magazine. The SPARC award recognizes us as an important supplier in the OTC Medication Category, and the award is chosen by buyers and merchandise managers of the leading power retailers.
Seasonality
The launch of our advertising coincides with the traditional onset of the cough and cold season in the United States. According to the American Lung Association, adults contract two to four colds per year, primarily between September and May. As a result, we expect retail demand for our products to be higher between October 1 and March 31.
8
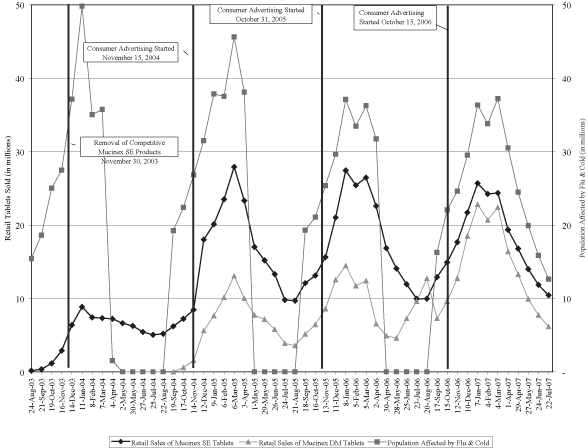
The following graph plots the retail tablet sales of Mucinex SE and Mucinex DM for each four-week period since their respective launches, based on data from IRI, and shows the effects of our consumer advertising campaigns and the FDA’s removal of products competitive with Mucinex SE from the market. To illustrate the development of the flu and cold season, the graph also shows a four-week average of the number of people in the United States who experienced cold or flu symptoms in the preceding seven days, as reported by Surveillance Data Inc., or SDI. Until April 2007, SDI did not track cold and flu symptoms from April through August of each year.
Trade Sales and Distribution
Our customers consist of drug wholesalers, retail drug stores, mass merchandisers, and grocery stores in the United States. We believe that each of these channels is important to our business, and we continue to seek opportunities for growth in each sector. The following table sets forth the percentage of gross sales for all of our customers for the last three fiscal years, across our major distribution channels:
| | | | | | | | | | | | | |
| | | Percentage of Gross Sales | |
Channel of Distribution | | 2007 | | | 2006 | | | 2005 | |
| |
| Wholesale Drug | | | 14.1 | % | | | 25.7 | % | | | 34.4 | % |
| Drug | | | 33.5 | | | | 30.1 | | | | 32.5 | |
| Mass | | | 33.2 | | | | 26.9 | | | | 19.0 | |
| Food | | | 18.6 | | | | 16.5 | | | | 13.4 | |
| Other | | | 0.6 | | | | 0.9 | | | | 0.6 | |
9
Certain drug wholesale customers distribute our products to non-retail institutions, such as federal facilities, long-term care facilities, hospitals, clinics, and HMOs. For the 12 months ended June 30, 2007, based on IMS Health-National Sales Perspectivestm data, we believe approximately 4.8% of our sales were directed to non-retail channels.
Our top 10 customers accounted for approximately 74%, 74% and 82% of our gross sales for the fiscal years ended June 30, 2007, 2006 and 2005, respectively. The following table sets forth a list of our principal customers in each of our distribution channels:
| | | |
Channel of Distribution | | Customers |
| |
| Wholesale Drug | | AmerisourceBergen |
| | | Cardinal Health |
| | | McKesson |
| Drug | | CVS* |
| | | Brooks/Eckerd |
| | | Rite Aid |
| | | Walgreens* |
| Food | | Peyton’s/Kroger |
| | | Publix Supermarkets |
| | | Safeway |
| Mass | | Kmart |
| | | Target |
| | | Wal-Mart* |
| | | Sam’s Club |
| | |
| * | | Represents customers who each accounted for greater than 10% of our gross sales for the fiscal year ended June 30, 2007. |
Consistent with industry practice, we maintain a return policy that allows our customers to return product within a specified period prior and subsequent to the expiration date. Occasionally, we also provide extended payment terms and greater discounts to our customers to ensure adequate distribution of our products.
Our trade sales force calls on national and regional retail accounts and wholesale distribution companies. The primary focus of our trade sales force is to maximize our shelf presence at retail drug, food and mass merchandise stores to support the efforts of our professional sales representatives and consumer advertising campaigns. For the more fragmented food channel and for smaller chains and individual stores, we rely on a national network of regional brokers to provide retail support. Our trade sales force performs analysis that helps both our sales representatives and our customers understand sales patterns and create appropriate promotions and merchandising aids for our products.
Since December 2003, we have expanded our trade sales force from one to 12 professionals as well as our shelf presence at food, drug and mass merchandiser stores. We believe a product’s importance to major retailers and attractiveness to consumers can be measured by All Commodity Volume, or ACV, as reported by IRI. ACV measures the weighted sales volume of stores that sell a particular product out of all the stores that sell products in that market segment generally. In the case of our products, ACV measures the percentage of retailers that sell our products out of all retailers that sell cough, cold, allergy and sinus remedies (on a weighted sales volume basis). The following table summarizes our ACV for each of our products:
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | ACV 52 Weeks Ended July 15, | |
| | | 2007 | | | 2006 | |
| | | Drug | | | Food | | | Food/Drug/Mass | | | Drug | | | Food | | | Food/Drug/Mass | |
| |
| Mucinex SE | | | 99.5 | % | | | 88.2 | % | | | 95.5 | % | | | 99.5 | % | | | 83.8 | % | | | 93.9 | % |
| Mucinex DM | | | 99.4 | % | | | 86.4 | % | | | 95.0 | % | | | 98.7 | % | | | 78.5 | % | | | 90.9 | % |
| Mucinex D | | | 93.7 | % | | | 46.7 | % | | | 76.3 | % | | | 91.9 | % | | | 38.3 | % | | | 66.5 | % |
| Children’s Mucinex | | | 96.8 | % | | | 79.8 | % | | | 91.2 | % | | | — | | | | — | | | | — | |
| Delsym | | | 98.8 | % | | | 82.5 | % | | | 93.4 | % | | | 97.7 | % | | | 72.1 | % | | | 88.7 | % |
10
In April 2004, we entered into an exclusive distribution and logistics agreement with Cardinal Health PTS, LLC, or Cardinal Health, which it assigned to Cardinal Health 105, Inc., or CH 105, in October 2005. Under this agreement, CH 105 is responsible for warehouse inventory operations, logistics, shipping, billing, and customer collections on a fee-for-service basis. CH 105 also serves as the exclusive distribution agent for commercial sales of Mucinex SE, Mucinex DM, Mucinex D and our other pharmaceutical products agreed to by CH 105 and us.
Competition
We currently compete in the OTC cough, cold, allergy and sinus and nasal product pharmaceutical markets, and we intend to compete in the prescription pharmaceutical market. The pharmaceutical industry is characterized by rapidly advancing technologies and intense competition. Our competitors include pharmaceutical companies, biotechnology companies, specialty pharmaceutical and generic drug companies, academic institutions, government agencies, and research institutions. All of these competitors currently engage or may engage in the future in the development, manufacture and commercialization of new pharmaceuticals, some of which may compete with our present or future products. The key competitive factors affecting the success of our brands are likely to be consumer awareness, physician and pharmacist acceptance, and value.
The OTC Cough, Cold, Allergy and Sinus Market and the OTC Nasal Products Market. The OTC cough, cold, allergy and sinus and nasal spray markets include products that consumers purchase over the counter to address the symptoms associated with mild respiratory disorders such as the common cold, cough and allergic rhinitis. These mild respiratory disorders often have multiple symptoms including nasal, sinus and bronchial congestion, cough, runny nose, and fever. Many of the products in the cough, cold, allergy and sinus and nasal spray markets contain more than one active ingredient, such as acetaminophen, dextromethorphan, diphenhydramine, guaifenesin, loratadine, oxymetazoline, phenylephrine, and pseudoephedrine, in order to be effective against several different cough and cold symptoms. Guaifenesin, in short-acting formulations, is an ingredient in many of these cough and cold OTC products. Familiar brand names in the OTC cough, cold, allergy and sinus and nasal markets include Afrin®, Vicks®, Dayquil®/Nyquil®, Tylenol®, Benadryl®, Sudafed®, Claritin®, and Robitussin®. We believe our current primary competitors in these markets are:
| | |
| | • | The Procter & Gamble Company (Dayquil®, Nyquil®, Vicks 44®, and Vicks Sinex®); |
| |
| | • | McNeil Consumer and Specialty Pharmaceuticals, a division of McNeil-PPC, Inc., which is an operating company of Johnson & Johnson (Tylenol®, Children’s Motrin®, Sudafed®, Benadryl®, PediaCare® and Children’s Tylenol®); |
| |
| | • | Wyeth (Robitussin®, Dimetapp® and Advil Cold and Sinus®); |
| |
| | • | Novartis Consumer Health, a division of Novartis AG (Theraflu®, Triaminic®, Comtrex® and 4Way®); |
| |
| | • | Schering-Plough Corp. (Claritin®, Coricidin® HBP, Drixoral® and Afrin®); |
| |
| | • | Bayer AG (Alka Seltzer Plus® and Aleve Cold and Sinus®); |
| |
| | • | Matrixx Initiatives, Inc. (Zicam® and Nasal Comforttm); and |
| |
| | • | Airborne, Inc. (Airborne®). |
In addition, we face substantial competition from private label brands, such as the CVS, Rite Aid, and Walgreens brands, which are often less expensive. We are aware that some private label brand companies have begun to market products containing immediate-release guaifenesin in tablet form.
The OTC cough, cold, allergy and sinus and nasal markets are fairly fragmented with 80 brands, including our Mucinex and Delsym products. Our Mucinex products accounted for approximately 9% of dollar sales during the 52-week period ended August 12, 2007, according to IRI. During this same period, according to IRI, private label products represented approximately 20% of this market.
11
The table below identifies brand rank and market share by dollar sales for the leading brands in the OTC cough, cold, allergy and sinus market, as reported by IRI for the 52, 12 and four weeks ended August 12, 2007.
| | | | | | | | | | | | | | | | | | | |
| | | Brand Rank(1) | | Market Share(1) | |
| | | 52 Weeks
| | 12 Weeks
| | 4 Weeks
| | 52 Weeks
| | | 12 Weeks
| | | 4 Weeks
| |
Brand | | Ended | | Ended | | Ended | | Ended | | | Ended | | | Ended | |
| |
| Private label | | | | | | | | | 19.9 | % | | | 24.2 | % | | | 25.0 | % |
Claritin® | | 1 | | 1 | | 1 | | | 10.7 | | | | 16.3 | | | | 14.2 | |
Tylenol® | | 2 | | 3 | | 3 | | | 10.0 | | | | 8.3 | | | | 8.5 | |
Vicks® | | 3 | | 5 | | 5 | | | 9.2 | | | | 6.2 | | | | 6.4 | |
Mucinex | | 4 | | 4 | | 4 | | | 8.7 | | | | 7.7 | | | | 7.8 | |
Benadryl® | | 5 | | 2 | | 2 | | | 5.9 | | | | 9.2 | | | | 9.8 | |
Robitussin® | | 6 | | 7 | | 7 | | | 5.7 | | | | 4.2 | | | | 4.1 | |
Sudafed® | | 7 | | 6 | | 6 | | | 5.1 | | | | 5.2 | | | | 5.5 | |
| Airborne | | 8 | | 8 | | 8 | | | 3.6 | | | | 2.2 | | | | 2.4 | |
Theraflu® | | 9 | | 10 | | 10 | | | 2.7 | | | | 1.5 | | | | 1.6 | |
Alka Seltzer Plus® | | 10 | | 9 | | 9 | | | 2.3 | | | | 1.6 | | | | 1.7 | |
Triaminic® | | 11 | | 11 | | 11 | | | 2.2 | | | | 1.4 | | | | 1.4 | |
Zicam® | | 12 | | 16 | | 16 | | | 1.8 | | | | 0.8 | | | | 0.9 | |
Delsym | | 13 | | 12 | | 12 | | | 1.7 | | | | 1.1 | | | | 1.1 | |
Dimetapp® | | 14 | | 13 | | 14 | | | 1.5 | | | | 1.0 | | | | 1.0 | |
Coricidin® | | 15 | | 15 | | 15 | | | 1.1 | | | | 0.8 | | | | 0.9 | |
| Others | | 16 through 60 | | through 60 | | through 60 | | | 7.9 | | | | 8.3 | | | | 7.7 | |
| | | | | | | | | | | | | | | | | | | |
| Total | | | | | | | | | 100.0 | % | | | 100.0 | % | | | 100.0 | % |
| | | | | | | | | | | | | | | | | | | |
| | |
| (1) | | Includes products sold in the OTC cough, cold, allergy and sinus market and does not include other products marketed under these brand names. |
Our Mucinex products for children also compete in the children’s segment of the OTC cough, cold, allergy and sinus market. Familiar brands in this market include Benadryl®, Dimetapp®, PediaCare®, Robitussin®, Triaminic®, and Tylenol®. According to IRI, for the 52-week period ended August 12, 2007, the Mucinex line of products for children was the sixth best-selling brand in the approximate $423 million children’s segment of the U.S. cough, cold, allergy and sinus category, with a 5.9% market share. Patient compliance with the products in the pediatric market is often a greater concern than with those products in the adult market because of issues with proper pediatric dosing and product taste and acceptability. We believe that products like our Mucinex Mini-Melts for children, which provide convenient and accurate dosing and taste good, have a competitive advantage in the OTC pediatric market.
In addition, our Mucinex Full Force Nasal Spray and Mucinex Moisture Smart Nasal Spray compete in the U.S. OTC nasal products market, which according to IRI for the 52-week period ended August 12, 2007, equaled $411 million.
The Prescription Cough Market. According to IMS Health-National Sales Perspectivestm (retail and non-retail), there were approximately 23 million prescriptions written during the 12 months ending June 30, 2007 for narcotic cough medications. Approximately 10.3 million of those prescriptions involved products combining codeine with another active ingredient. We believe that Mucinex with Codeine, if approved by the FDA, will primarily compete in this market subsegment. Of the 10.3 million codeine combination prescriptions that were written and dispensed, approximately 4.8 million were written for codeine in combination with guaifenesin and approximately 4.8 million were written for codeine in combination with promethazine, a first generation prescription anthihistamine. All of the currently available codeine combination products are short-acting products and nearly all of them are available only in liquid form. Mucinex with Codeine will be the
12
only12-hour codeine combination product available on the market. We believe the long-acting benefit delivered in our12-hour patented oral-solid dual-portion platform, combined with the convenient tablet dosing will provide a marketplace advantage to Mucinex with Codeine.
License Granted to Mutual. On August 23, 2006, we received notice from Mutual Pharmaceutical Co. and United Research Laboratories, Inc., wholly owned subsidiaries of Pharmaceutical Holdings Corp., which we refer to collectively as Mutual, that they had submitted an abbreviated new drug application, or ANDA, for 600 mg and 1200 mg single-ingredient, extended-release formulations of guaifenesin. On March 21, 2007, we entered into an agreement with Mutual to settle our litigation arising out of Mutual’s ANDA filing. Under the terms of the settlement agreement, we granted Mutual a non-exclusive, royalty-free license to sell certain 600 mg and 1200 mg single-ingredient and combination extended-release guaifenesin products in the United States, subject to the following conditions:
| | |
| | • | If Mutual receives FDA approval to market certain 600 mg single-ingredient extended-release guaifenesin products, we have agreed to grant Mutual a license to sell such products beginning no earlier than July 1, 2012, with the following exceptions: (i) if Mutual has final FDA approval for the 600 mg product, and prior to July 1, 2012, a third party files an ANDA, certifies against and successfully challenges our patents, and gains FDA approval for the 600 mg product, Mutual may begin selling its product 60 days prior to the date of first sale by the third party; or (ii) if Mutual has not received FDA approval to market the 600 mg product at the time the third party commences sale of an FDA-approved 600 mg product, Mutual may enter into a supply agreement with us to purchase tablets at full cost plus a 10% royalty, based on Mutual’s net sales of the licensed product; and Mutual may begin selling such product 90 days after the first sale by the third party. |
| |
| | • | If Mutual receives FDA approval to market certain 1200 mg single-ingredient, extended-release guaifenesin products or the 600 mg or 1200 mg combination extended-release guaifenesin products, which we refer to as the combination products, we will grant Mutual a license to sell such products, upon the occurrence of the following events: (i) if Mutual has final FDA approval for the 1200 mg single-ingredient product or a combination product, a third party files an ANDA, certifies against and successfully challenges our patents, and gains FDA approval for the 1200 mg single-ingredient product or a combination product, as the case may be, Mutual may begin selling its product 60 days prior to the date of first sale by the third party; or (ii) if Mutual has not received FDA approval to market the 1200 mg single-ingredient product or a combination product, as the case may be, at the time the third party commences sale of such an FDA-approved product, Mutual may enter into a supply agreement with us to purchase tablets at full cost plus a 10% royalty, based on Mutual’s net sales of the licensed product; and Mutual may begin selling product 90 days after the first sale by the third party. |
The settlement agreement is subject to review by the Federal Trade Commission and the U.S. Department of Justice. For a further discussion of the litigation with Mutual, see Part I. Item 3. Legal Proceedings in this Annual Report onForm 10-K.
Perrigo ANDA Filing. On August 20, 2006, we received a letter from Perrigo R&D Co., or Perrigo, notifying us that Perrigo had filed an ANDA for a single ingredient, extended-release formulation of guaifenesin that is the generic equivalent to our Mucinex SE product. In its letter, Perrigo notified us of its assertion that its proposed products would not infringe our patents that protect Mucinex SE, or alternatively that certain of our patent claims are not valid.
We are evaluating this matter and have requested from Perrigo additional data to evaluate their claims. If we determine that Perrigo’s products infringe our patents, we intend to vigorously defend our intellectual property rights. If we file a patent infringement lawsuit prior to October 4, 2007, the FDA will stay its approval of Perrigo’s ANDA until the earlier of 30 months or a court’s determination that Perrigo’s product does not infringe our patents or that certain of our patent claims are invalid. At this time, we cannot, however, predict whether we will file a patent infringement lawsuit, the duration of any resulting litigation or the stay in the FDA’s approval of Perrigo’s ANDA, or whether we would prevail in any such lawsuit.
13
In order for the FDA to approve Perrigo’s ANDA, Perrigo must demonstrate the bioequivalence of its product to Mucinex SE and that such product is “the same as” Mucinex SE with regard to conditions of use, active ingredients, route of administration, dosage form, strength, and labeling. Bioequivalence generally means that no significant difference exists in the rate and extent to which the active ingredients enter the bloodstream and become available at the site of drug action. Bioequivalence, however, does not mean that the products must be identical in all respects. The FDA has broad discretion to determine whether Perrigo’s product meets the FDA’s ANDA approval standards. We are unable to evaluate Perrigo’s claim that its product meets the ANDA bioequivalence approved standards, and we are unable to predict when or if the FDA will approve Perrigo’s ANDA. Furthermore, in connection with the FDA’s approval of our Mucinex SE NDA, the FDA required that we meet rigorous scientific standards. We believe the FDA should apply these same rigorous scientific standards to Perrigo’s products, but there can be no assurance that the FDA will apply such stricter standards.
If the FDA approves Perrigo’s ANDA, we may lose our effective market exclusivity of Mucinex SE and we may face stronger and more direct competition, which could negatively impact our business and operating results. Furthermore, the approval and launch of a generic competitor to Mucinex SE may trigger certain rights that Mutual has under our March 21, 2007 Settlement and License Agreement with Mutual. Such approval may also encourage others to file ANDAs covering products that compete with other of our adult oral-solid Mucinex products and products that combine guaifenesin with other ingredients. As a result, we may face greater competition from more competitors across our line of extended-release guaifenesin products, which could have a material adverse impact on our revenues, profitability and cash flows.
Government Regulation
The manufacture and marketing of prescription and OTC pharmaceutical products in the United States are subject to extensive regulation by the federal government, primarily the FDA, under the Federal Food, Drug and Cosmetic Act, or FDCA, the Controlled Substances Act and other federal statutes and regulations.
FDA Approval Process
Most drug products obtain FDA marketing approval pursuant to a NDA or an ANDA. A third alternative is a special type of NDA, commonly referred to as a Section 505(b)(2) NDA or 505(b)(2) application, which enables the applicant to rely, in part, on the safety and efficacy data of an existing product, or published literature, in support of its application. Our adult oral-solid Mucinex products were approved pursuant to Section 505(b)(2) NDAs. While we may seek approval of future products through any of these processes, we expect to seek approval for most of our future products using NDAs and Section 505(b)(2) NDAs.
| | |
| | • | New Drug Applications. NDAs are the standard applications required for new drug products and require extensive original clinical data demonstrating the safety and efficacy of the product candidate. Typically, NDAs require three phases of human clinical trials. In Phase I, the product candidate is introduced into humans and tested for safety, dose ranges and pharmacokinetics. In Phase II, the product candidate is introduced into a slightly larger patient population to assess efficacy for specific indications, assess response rates tolerance, determine optimal dose, and identify safety risks and adverse effects. In Phase III, the product candidate is introduced in an expanded patient population at multiple geographically dispersed sites to further test for safety and clinical efficacy. In addition, prior to beginning the human clinical trial work required for either a NDA or Section 505(b)(2) NDA, an applicant must obtain approval to begin this clinical testing by submitting an Investigational New Drug application, or IND, which includes the results of preclinical animal studies, to the FDA. |
| |
| | • | Abbreviated New Drug Applications. An ANDA is a type of application in which approval is based on a showing of “sameness” to an already approved drug product. ANDAs do not contain full reports of safety and effectiveness as required in NDAs but rather demonstrate that their proposed products are “the same as” reference products with regard to their conditions of use, active ingredients, route of administration, dosage form, strength, and labeling. ANDA applicants must demonstrate the bioequivalence of their products to the reference product. Bioequivalence generally means that no significant |
14
| | |
| | | difference exists in the rate and extent to which the active ingredients enter the blood-stream and become available at the site of drug action. Bioequivalence, however, does not mean that the products must be identical in all respects. Furthermore, the FDA has broad discretion to determine whether a product meets the ANDA approval standards. |
| | |
| | • | 505(b)(2) Applications. If a proposed product represents a change from an already approved product, and therefore does not qualify for an ANDA, the applicant may be able to submit a Section 505(b)(2) NDA or 505(b)(2) application. A 505(b)(2) application is made pursuant to Section 505(b)(2) of the FDCA and relies on one or more investigations conducted by a party other than the applicant in connection with an existing approved product. The FDA has determined that 505(b)(2) applications may be submitted for products that represent changes from approved products in conditions of use, active ingredients, route of administration, dosage form, strength, or bioavailability. A 505(b)(2) applicant must reference an approved product as well as the related safety data on which it proposes to rely. The applicant must also provide the FDA with any additional clinical data necessary to demonstrate the safety and effectiveness of the product with the proposed changes. Consequently, while an applicant avoids duplication of preclinical and certain clinical safety and efficacy studies through the use of a 505(b)(2) application, the FDA usually requires the applicant to perform at least one additional human clinical study in support of the application. |
In seeking approval for a drug through a NDA or 505(b)(2) application, applicants must list with the FDA each patent with claims that cover the drug or method of using the applicant’s product. Upon approval of a drug, each of the patents listed in the application for the drug are then published in the FDA’s Approved Drug Products with Therapeutic Equivalence Evaluations list, commonly known as the Orange Book. Applicants that file an ANDA or 505(b)(2) application must certify, with respect to each product referenced in their applications, that no patent exists in the Orange Book for that reference product, that the listed patents have expired, that the application may be approved upon the date of expiration of the listed patents, or that the patents listed in the Orange Book for the reference product are invalid or will not be infringed by the marketing of the applicant’s product. When an applicant submits an application containing a certification that a reference product’s patents are invalid or not infringed, the applicant must also provide notice to the owner of the reference product’s patent. If the owner of the reference product determines that the applicant’s product would infringe a valid patent listed in the Orange Book for the reference drug and files suit within 45 days of receiving notice of the application, the FDA will stay its approval of the applicant’s product until the earlier of 30 months or a court’s determination that the applicant’s product does not infringe the reference drug’s patent or that certain of the reference drug’s patent claims are invalid.
In addition, the FDA must inspect and find that manufacturing facilities comply with current good manufacturing practices, or cGMP, before it will approve a drug application. After the FDA approves a drug application, if any material change in the manufacturing process, equipment or location occurs that would necessitate additional data, then the FDA must review and approve such change before the product may be marketed with that material change.
Even after approval by the FDA, all marketed products and their manufacturers continue to be subject to annual reporting, facility inspection and continued governmental review. Subsequent discovery of previously unrecognized problems or failure to comply with applicable regulatory requirements could result in restrictions on manufacturing or marketing of the product, product recall or withdrawal, fines, seizure of product, injunction or criminal prosecution, as well as withdrawal or suspension of regulatory approvals. In addition, the advertising of all marketed OTC products are subject to the Federal Trade Commission and state consumer protection regulations.
Some products intended for OTC marketing require FDA approval through one of the three processes described in the preceding paragraphs. Many OTC drugs, however, may be commercially distributed without prior FDA approval by following the FDA’s OTC monographs. The OTC monographs classify certain drug ingredients as safe and effective for specified uses and establish categorical requirements for the marketing of drugs containing such ingredients without pre-approval. Our Mucinex line of products for children and our Mucinex nasal spray products are marketed pursuant to OTC monographs. Our adult oral-solid Mucinex and
15
the maximum strength versions of those products contain a new formulation of sustained-release guaifenesin, are not considered OTC monograph drugs, and therefore were approved pursuant to Section 505(b)(2) NDAs.
Approval of Our Existing Long-Acting Guaifenesin Products. The following table sets forth the timeline of the FDA’s approval of the 505(b)(2) applications for our existing long-acting guaifenesin products:
| | | | | | | |
| | | | | 505(b)(2)
| | |
| | | | | Application or
| | |
| | | | | Supplement
| | |
Product | | IND Filing | | Submission | | FDA Approval |
| |
| Mucinex SE | | June 1998 | | June 2000 | | July 2002 |
| Maximum Strength Mucinex SE | | June 1998 | | August 2002 | | December 2002 |
| Mucinex DM | | September 2000 | | June 2003 | | April 2004 |
| Maximum Strength Mucinex DM | | September 2000 | | June 2003 | | April 2004 |
| Mucinex D | | September 2000 | | January 2003 | | June 2004 |
| Maximum Strength Mucinex D | | September 2000 | | January 2003 | | June 2004 |
In 2002, the FDA approved our 505(b)(2) application for Mucinex SE as an OTC long-acting guaifenesin product. Prior to our 505(b)(2) application for Mucinex SE, only short-acting guaifenesin products had been marketed OTC, while long-acting guaifenesin products were marketed as prescription drugs, despite their lack of formal approval by the FDA. Under the Durham Humphrey Act of 1951, the FDA established that no drug may simultaneously be sold as a non-prescription product and as a prescription product at the same dose for the same indication. Any products that violate this rule are subject to FDA regulatory action and removal from the market.
On October 11, 2002, the FDA issued warning letters to 66 manufacturers, distributors, marketers, and retailers of single-ingredient guaifenesin extended-release products. The letters stated that such prescription products require FDA approval, and without FDA approval, they could no longer be marketed legally. A number of the manufacturers and distributors that received a warning letter from the FDA filed a “Citizens Petition,” which is similar to an appeal, with the FDA requesting that the agency either elect not to enforce existing regulatory policies requiring removal of the drugs from the market or delay such enforcement. On February 25, 2003, the FDA issued a letter in response to the Citizens Petition to the 66 recipients of the original warning letter, reiterating that following the FDA’s approval of Mucinex SE in July 2002, all other single-ingredient guaifenesin extended-release drug products may no longer be marketed legally absent FDA approval. The FDA decided, however, to allow a grace period for the manufacturers and distributors to remove such drugs from the market as follows:
| | |
| | • | the FDA required that the warning letter recipients cease manufacturing unapproved single-ingredient guaifenesin extended-release products no later than May 21, 2003; |
| |
| | • | no distribution (including distribution by secondary wholesalers or other distributors) could occur after October 23, 2003; and |
| |
| | • | no retail sales could occur after November 30, 2003. |
Historically, long-acting prescription guaifenesin products and, according to the FDA, several thousand other drugs were marketed without FDA approval. Resource limitations prevented FDA enforcement actions against many unapproved prescription and OTC drugs. In October 2003, the FDA published a draft compliance policy guide articulating its existing informal policy regarding drugs marketed in the United States that do not have required FDA approval. In June 2006, the FDA announced that it had finalized its policy, under which the FDA is more likely to take enforcement action against unapproved drugs once the FDA has approved a similar drug, whether the similar drug is prescription or OTC. In publishing the policy guide, the FDA publicly affirmed the actions it took relating to long-acting, single-ingredient guaifenesin products. On May 25, 2007, the FDA also announced that companies must stop manufacturing and distributing unapproved timed-release dosage forms containing guaifenesin in combination with other ingredients. The FDA indicated that companies marketing unapproved products containing guaifenesin in timed-release forms are expected to stop manufacturing them by August 27, 2007, and must cease shipping them in interstate commerce by November 26, 2007.
16
The FDA had previously approved Mucinex DM and Maximum Strength Mucinex DM, as well as Mucinex D and Maximum Strength Mucinex D, pursuant to Section 505(b)(2) NDAs.
Other Regulation
We are subject to additional regulation. The Prescription Drug Marketing Act, or the PDMA, imposes requirements and limitations upon the provision of drug samples to physicians and prohibits states from licensing distributors of prescription drugs unless the state licensing program meets certain federal guidelines that include minimum standards for storage, handling and record keeping. In addition, the PDMA sets forth civil and criminal penalties for violations. Some states are still implementing various sections of the PDMA.
Manufacturers of marketed drugs must comply with other applicable laws and regulations required by the FDA, the Drug Enforcement Administration, or the DEA, the Environmental Protection Agency, and other state and federal regulatory agencies. Failure to do so could lead to sanctions, which may include an injunction that would suspend manufacturing, the seizure of drug products and the refusal to approve additional marketing applications. Manufacturers of products containing listed chemicals or controlled substances are also subject to the licensing, quota and regulatory requirements of the Controlled Substances Act, including recordkeeping and storage requirements to ensure product security and prevent against diversion. Failure to comply with the Controlled Substances Act and the regulations promulgated thereunder could subject us to loss or suspension of those licenses and to civil or criminal penalties. We are currently developing products that contain listed controlled substances. Should these products receive FDA marketing approval, we will be subject to full compliance with the DEA’s regulatory authority.
Reimbursement
In the United States, sales of pharmaceutical products depend in part on the availability of reimbursement to the patient from third-party payors, such as government health administrative authorities, managed care providers and private insurance plans. Third-party payors are increasingly challenging the prices charged for medical products and services and examining their cost-effectiveness. Generally, such payors do not cover OTC products.
Medicaid, a state health program for certain low-income individuals, does not generally cover the cost of OTC products. However, 28 state Medicaid programs (three of which require prior approval of reimbursement from the state Medicaid program, or PA) have covered and continue to cover the cost of Mucinex SE, 22 state Medicaid programs (three of which require PA) have covered and continue to cover the cost of Mucinex DM, 18 state Medicaid programs (three of which require PA) have covered and continue to cover the cost of Mucinex D, 13 state Medicaid programs (two of which require PA) have covered and continue to cover the cost of Mucinex Children’s Mini-Melts, 21 state Medicaid programs (three of which require PA) have covered and continue to cover the cost of Mucinex Children’s Liquid, and 16 state Medicaid programs (two of which require PA) have covered and continue to cover the cost of Delsym. In addition, we are obligated to pay rebates on sales of our products to Medicaid beneficiaries. We estimate that sales to Medicaid beneficiaries represented approximately 2% and 3% of retail sales of our products during fiscal 2007 and 2006, respectively.
Manufacturing
Manufacture and Packaging. On July 31, 2006, we entered into an Asset Purchase Agreement with Cardinal Health, pursuant to which we reacquired certain rights and assets used in the manufacture of our adult oral-solid Mucinex brand products, which we refer to as the manufacturing assets repurchase. We had originally sold the manufacturing rights and operations in Fort Worth to Cardinal Health in 2004 in a transaction that resulted in Cardinal Health becoming the exclusive manufacturer of our products. We decided to reacquire the manufacturing operations to directly control the operations and increase our capacity to meet forecasted volumes resulting from the significant growth of the Mucinex product line. In connection with the Asset Purchase Agreement, Cardinal Health assigned to us its lease for the manufacturing facility located in Fort Worth, Texas.
17
Additionally, as contemplated by the Asset Purchase Agreement, on July 31, 2006, we entered into a three-year commercial manufacturing agreement with Cardinal Health under which they are committed to provide us with defined levels of guaifenesin granulation capacity. The Blackstone Group, L.P. subsequently acquired Cardinal Health in April 2007, and Cardinal Health is now known as Catalent Pharma Solutions, Inc., or Catalent. We are contractually obligated to purchase at least 80% of the capacity commitment from Catalent. We also entered into a three year packaging agreement with Cardinal Health (now Catalent), which provides us with guaranteed minimum levels of packaging capacity. We will make capacity reservation payments to Catalent throughout the term of the agreement. Such payments are creditable against the price of the units purchased. In accordance with the new commercial manufacturing agreement and packaging agreement, we will continue to rely on Catalent to perform certain aspects of the manufacture and packaging of Mucinex SE, Mucinex DM, Mucinex D, and the maximum strength versions of these products.
On October 26, 2006, we entered into a three-year renewable agreement with PharmPro, a division of Fluid Air, Inc., for additional processing and granulation of guaifenesin. Under this agreement, we are required to purchase 50 metric tons of finished granulation of guaifenesin during each contract year. PharmPro also agreed to reserve capacity to enable the manufacture of an additional 100% of the contracted volume, which we may use at our option.
On June 12, 2006, pursuant to a Product Purchase Agreement, dated May 24, 2006, among us, one of our wholly-owned subsidiaries Adams Respiratory Operations Sub, Inc., UCB, Inc. and UCB Manufacturing, Inc., we consummated the acquisition of certain rights and assets related to the manufacture and sale of the Delsym product line. Additionally, on June 12, 2006, we entered into a manufacturing supply agreement with UCB, pursuant to which UCB will manufacture and package the Delsym product line for us at its own manufacturing facility. The Delsym manufacturing agreement has an initial term of six years, with automatic one year renewals after expiration of the initial term.
We rely on separate third party contract manufacturers to produce and package our Mucinex line of products for children and our Mucinex nasal spray products.
Raw Material Sourcing Arrangements. We currently depend on Boehringer Ingelheim Chemicals, Inc., or Boehringer Ingelheim, and Delta Synthetic Co., LTD, or Delta, for all of the raw guaifenesin used in our adult oral-solid Mucinex products. In connection with the manufacturing assets repurchase, Cardinal Health assigned to us the contracts related to the manufacture of the adult oral-solid Mucinex products, including the guaifenesin supply arrangements with Delta and Boehringer Ingelheim. We entered into a new supply agreement with Boehringer Ingelheim in July 2006, pursuant to which we have agreed to purchase from Boehringer Ingelheim the lesser of 500 metric tons or 100% of our guaifenesin requirements during each contract year. We can purchase volumes in excess of 500 metric tons each contract year from other suppliers. In 2005, we received FDA approval to begin using Delta as a supplier of guaifenesin for use in Mucinex SE, and Cardinal Health began using the Delta material in the manufacture of Mucinex SE in November 2005. We are currently seeking FDA approval of the Delta material for use in our other Mucinex products. We believe that Boehringer Ingelheim and Delta will provide us with sufficient quantities of guaifenesin to meet our manufacturing needs.
Cardinal Health also assigned us its agreement with its sole dextromethorphan HBr supplier as part of the manufacturing assets repurchase. During fiscal 2004, this supplier of dextromethorphan HBr notified Cardinal Health that it intended to exit the dextromethorphan HBr manufacturing business, but in January 2006, this supplier agreed to provide Cardinal Health with an additional supply of dextromethorphan HBr. We also received FDA approval in December 2006 to obtain dextromethorphan from a secondary supplier. We believe these two suppliers will provide us with sufficient quantities to meet our manufacturing needs. We are actively pursuing additional suppliers of dextromethorphan HBr and guaifenesin as well as other active and inactive ingredients.
We currently have one approved supplier of codeine, which we believe will be able to meet our demand if the FDA approves Mucinex with Codeine, and we are seeking FDA approval of a secondary supplier.
18
Under the terms of our supply agreements with UCB and the manufacturers of our Mucinex products for children and Mucinex nasal spray products, those manufacturers are each responsible for obtaining, at their own expense, the raw material used in the manufacture of those products for us.
Intellectual Property
Our success depends in part on our ability to obtain and maintain proprietary protection for our product candidates, technology and know-how, to operate without infringing on the proprietary rights of others and to prevent others from infringing on our proprietary rights. We protect our proprietary position by, among other methods, filing U.S. and foreign patent applications related to our proprietary technology, inventions and improvements that are important to the development of our business. We also rely on trade secrets, know-how, continuing technological innovation, and in-licensing opportunities to develop and maintain our proprietary position.
The United States Patent and Trademark Office, or USPTO, has granted us U.S. patent no. 6,372,252, which expires in April 2020 and contains claims encompassing a guaifenesin product having an immediate-release portion and an extended-release portion. The USPTO has also granted us a second patent, U.S. patent no. 6,955,821, which expires in May 2020 and contains claims encompassing the combination of a long-acting guaifenesin product, including an immediate-release portion and an extended-release portion with another active pharmaceutical ingredient that yields a certain pharmacokinetic profile. Both of our single-ingredient products, Mucinex SE and its maximum strength version, utilize our patented technology in a dual-portion tablet providing both immediate and long-acting guaifenesin to patients. The two tablet layers combine the benefits of the fast onset of action of immediate-release guaifenesin with the convenient dosing and reliable12-hour blood levels produced by the extended-release guaifenesin tablet layer. The same bi-phasic guaifenesin release pattern also applies to our currently approved adult oral-solid Mucinex products and most likely any future combination product line extensions. The active ingredients in our products and most of our product candidates, including guaifenesin, dextromethorphan and pseudoephedrine, are chemical compounds that have been in existence for many years and, therefore, are not by themselves patentable.
On April 20, 2005, an anonymous third party filed a request for reexamination with the USPTO of our U.S. patent no. 6,372,252, which contains claims covering a long-acting guaifenesin product, including an immediate-release portion and an extended-release portion that yields a certain pharmacokinetic profile. On June 23, 2005, the USPTO denied the request for reexamination and found that the third party did not raise a substantial new question of patentability based on prior art. On July 22, 2005, the third party who filed the request for reexamination sought review of the USPTO’s denial of its request for reexamination by petition to the Director of the USPTO. The USPTO advised us on August 18, 2005 that the Director had granted the petition and ordered reexamination, and on December 29, 2005, the USPTO advised us of its initial, non-final determination to reject the claims of our U.S. Patent no. 6,372,252. Under typical procedural practices at the USPTO, this preliminary finding was made prior to our presentation of arguments in favor of affirming the claims under this patent. On March 21, 2006, we presented our arguments to the USPTO examiner in a personal interview, and on March 23, 2006 we filed a written response to the USPTO’s initial determination setting out those arguments. On June 20, 2006, the USPTO advised us that it had decided to continue to reject some claims of our U.S. Patent No. 6,372,252 but to confirm that several claims of this patent were patentable. In response to this communication from the USPTO, which is designated a final action, on August 21, 2006, we filed a request for reconsideration of some aspects of this action. On November 20, 2006, we filed a notice of appeal. On January 8, 2007, the USPTO confirmed that five of the 58 claims in the reexamination were patentable, and on January 22, 2007, we filed an appeal brief with the USPTO regarding the claims that the USPTO had continued to reject.
Under a reexamination proceeding and upon completion of the proceeding, the USPTO may leave the patent in its present form, narrow the scope of the claims of the patent or cancel all of the claims of the patent. Pursuant to this reexamination, the USPTO will reconsider the patentability of our delivery system for guaifenesin. From this point forward the reexamination could take up to three additional years, including the potential for an additional appeal should the pending appeal be unsuccessful.
19
We intend to vigorously defend our patent position and believe we will prevail in the reexamination process. We may not be successful, however, in maintaining our patent or the scope of its claims during reexamination and can offer no assurance as to the outcome of a reexamination proceeding.
We have filed patent applications in a number of foreign countries and we are currently seeking additional U.S. patent protection for each of our FDA-approved products.
A, Adams, A Adams Respiratory Therapeutics, Adams Respiratory Therapeutics, Delsym, Humibid, Junior Mucus, Kid and Clock design, MiniMelts, Mini-Melts, Mucinex, Mucinex Full Force, Mucinex IN...Mucus OUT, Mucinex Moisture Smart, Mr. Mucus, Mrs. Mucus, Nothing Lasts Longer, Opening New Pathways to Respiratory Relief, Turn off the Cough, and the Junior Mucus, Mr. Mucus, Mrs. Mucus and XL Mucus characters are our registered trademarks or are the subject of pending U.S. trademark applications. The marks may also be the subject of pending U.S. trademark applications.
In April 1999, we entered into a sublicense agreement with JMED Pharmaceuticals, Inc., or JMED, which gave us an exclusive license to manufacture and market AlleRxtm in exchange for royalty payments to JMED. Subsequently, we granted JMED the right to exchange its on-going royalty interest in the sublicense agreement into our common stock in the event of a public offering or change of control. In December 2004, we received the right to assign our sublicense agreement with JMED to Cornerstone. Pursuant to our 2004 assignment agreement with JMED, we paid JMED $2.0 million. Additionally, the assignment agreement provided that prior to March 31, 2005, a valuation would be performed on JMED’s on-going royalty interest in the sublicense agreement and JMED would have the right to receive the value of the royalty above the $2.0 million previously paid. The parties waived the March 31, 2005 deadline and in December 2006, a third-party valuation was completed and the royalty interest was valued at $4.5 million. Based upon the valuation, JMED notified us of their intent to convert the excess $2.5 million into shares of our common stock, and on January 15, 2006, we issued 147,058 shares of our common stock to JMED. We have begun to actively market the on-going royalty interest in the sublicense agreement to outside parties.
Pursuant to a February 2005 agreement with Cornerstone, Cornerstone assigned the rights to the Humibid trademark to us in exchange for our assignment of the AlleRxtm assets to Cornerstone. Under this agreement, we each agreed to release the other party from all claims and damages in a lawsuit that we filed against Cornerstone in 2004. Additionally, we assumed the financial obligation for future product returns of up to $1.0 million of AlleRxtm products sold by us prior to February 15, 2005 and returned to Cornerstone within the subsequent 18 months. Cornerstone assumed the same financial obligation with respect to Humibid product returns during that period. As of June 30, 2006, the first $1.0 million of Humibid inventory had been returned, and we now bear the responsibility for Humibid product returns and Cornerstone bears the same responsibility for AlleRxtm returns. We are currently in litigation with Cornerstone with regard to these returns. In addition, the agreement provides that we will pay Cornerstone a royalty ranging from 1% to 2% of net Humibid sales for a period of three years beginning February 15, 2005, subject to an annual minimum of $50,000. We launched Humibid SE in March 2006, and we paid the annual $50,000 minimum to Cornerstone in February 2006 and 2007. We are currently evaluating the commercialization of products using the Humibid name.
Employees
As of June 30, 2007, we had 463 employees. Currently, 78 of our employees work at our Chester, New Jersey facility and 247 work at our Fort Worth, Texas facility. One hundred and sixty-eight of our employees work in sales and marketing. Three of our employees are part-time employees. None of our employees are subject to collective bargaining agreements. We consider our relationships with our employees to be good.
Access to Our Filings with the Securities and Exchange Commission
Our website address iswww.adamsrt.com. The information on our website is not a part of, or incorporated into, this Annual Report onForm 10-K. We make our Annual Reports onForm 10-K, Quarterly Reports onForm 10-Q, Current Reports onForm 8-K, and amendments to those reports, which are filed or furnished pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934, or the Exchange Act,
20
available without charge on our website as soon as reasonably practicable after they are filed electronically with, or otherwise furnished to, the Securities and Exchange Commission, or the SEC.
The public may read and copy any material we file with the SEC at the SEC’s Public Reference Room at 100 F Street, NE, Washington, D.C. 20549. You can obtain information on the operation of the Public Reference Room by calling the SEC at1-800-SEC-0330. In addition, the SEC maintains an Internet site atwww.sec.gov, from which you can electronically access information regarding issuers that file electronically.
Risks Relating to Our Business
Mutual and Perrigo have filed ANDAs for single-ingredient, extended-release formulations of guaifenesin with the FDA. If the FDA approves either of these ANDAs or another ANDA filed by a third party for generic versions of our products, we may face more direct competition, which could negatively impact our sales.
On August 23, 2006, we received notice from Mutual that they had submitted an ANDA for 600 mg and 1200 mg single-ingredient, extended-release formulations of guaifenesin. On October 4, 2006, we sued Mutual for patent infringement based on Mutual’s ANDA filing, and on March 21, 2007, we entered into an agreement with Mutual settling that litigation. According to the terms of the settlement agreement, if Mutual obtains FDA approval of its 600 mg ANDA product, we have granted Mutual a license allowing it to sell generic 600 mg product commencing July 1, 2012, subject to certain exceptions in the event the FDA approves a third party ANDA for the 600 mg product. Additionally, Mutual may be able to sell generic versions of 1200 mg guaifenesin and guaifenesin combination products if the FDA approves a third party ANDA for a 1200 mg or combination product.
The settlement agreement with Mutual does not prevent third parties from filing an ANDA that seeks to sell generic versions of our products and asserts that our patents are not infringed, invalid or unenforceable.
On August 20, 2007, we received notice from Perrigo that they had submitted and received FDA acceptance of the filing of an ANDA for a single-ingredient, extended-release formulation of guaifenesin. The drug related to this ANDA could be a generic competitor or licensed as a branded competitor of Mucinex SE.
Perrigo has asserted that its proposed products do not infringe our patents that protect Mucinex SE, or alternatively that certain of our patent claims are not valid. We have not decided what course of action we will take in order to protect our intellectual property. We may accept Perrigo’s assertion that its products do not infringe our patents or we may determine that its products do, in fact, infringe our patents.
Additionally, we are unable to evaluate Perrigo’s claim that its products meet the ANDA bioequivalence approved standards, and we are unable to predict when or if the FDA will approve Perrigo’s ANDA. In connection with the FDA’s approval of our Mucinex SE NDA, the FDA required that we meet rigorous scientific standards. We believe the FDA should apply these same rigorous scientific standards to Perrigo’s products, but there can be no assurance that the FDA will apply such stricter standards.
We intend to vigorously defend our exclusive market position for Mucinex SE. We may not be successful, however, in maintaining our exclusive market position and can offer no assurance as to the outcome of Perrigo’s ANDA filing. If the FDA approves Perrigo’s ANDA, then our competitive position could be weakened and we may face stronger and more direct competition, which would negatively impact our business and operating results. Furthermore, the approval and launch of a generic competitor to Mucinex SE may trigger certain rights that Mutual has under our March 21, 2007 Settlement and License Agreement with Mutual. Such approval may also encourage Perrigo or others to file ANDAs covering products that compete with other of our adult oral-solid Mucinex products and products that combine guaifenesin with other ingredients. As a result, we may face greater competition from more competitors across our line of extended-release guaifenesin products, which could have a material adverse impact on our revenues, profitability and cash flows.
21
If another third party successfully challenges our patents and obtains FDA approval of a proposed generic product, our competitive position could be weakened, and we may face stronger and more direct competition, which could negatively impact our business and operating results. Such potential competition could have a material adverse impact on our revenues, profitability and cash flows.
If the FDA approves generic products that compete with any of our products, sales of our products may be adversely affected.
Our patents will not protect our products if competitors devise ways of making products that compete with ours without legally infringing our patents. The FDCA and FDA regulations and policies provide incentives to manufacturers to create modified, non-infringing versions of a drug in order to facilitate the approval of ANDAs for generic substitutes. These same types of incentives encourage manufacturers to submit NDAs that rely on literature and clinical data not prepared for or by such manufacturers. Manufacturers might only be required to conduct a relatively inexpensive study to show that their product has the same active ingredient(s), dosage form, strength, route of administration, and conditions of use (labeling) as our product and that the generic product is absorbed in the body at the same rate and to the same extent as our product, a comparison known as bioequivalence. Such products would be significantly less costly than ours to bring to market and could lead to the existence of multiple lower-priced competitive products, which would substantially limit our ability to obtain a return on the investments we have made in those products.
Although generic pharmaceuticals must meet the same quality standards as branded pharmaceuticals, companies that produce generic equivalents are generally able to offer their products at lower prices.
After the introduction of a generic competitor, a significant percentage of the sales of a branded product are typically lost to sales of the generic product. Accordingly, competition from generic equivalents could have a material adverse impact on our revenues, profitability and cash flows.
We face substantial competition that may prevent us from maintaining or increasing market share for our existing products and gaining market acceptance of our future products. Our competitors may develop or commercialize products before or more successfully than us.
Our competitors may develop products that are superior to ours or may more effectively market products that are competitive with ours. We believe that our Mucinex and Delsym products compete primarily with products with strong brand awareness marketed by large pharmaceutical companies, such as:
| | |
| | • | The Procter & Gamble Company (Dayquil®, Nyquil®, Vicks 44®, and Vicks Sinex®); |
| |
| | • | McNeil Consumer and Specialty Pharmaceuticals, a division of McNeil-PPC, Inc., which is an operating company of Johnson & Johnson (Tylenol®, Children’s Motrin®, Sudafed®, Benadryl®, PediaCare® and Children’s Tylenol®); |
| |
| | • | Wyeth (Robitussin®, Dimetapp® and Advil Cold and Sinus®); |
| |
| | • | Novartis Consumer Health, a division of Novartis AG (Theraflu®, Triaminic®, Comtrex® and 4Way®); |
| |
| | • | Schering-Plough Corp. (Claritin®, Coricidin® HBP, Drixoral® and Afrin®); |
| |
| | • | Bayer AG (Alka Seltzer Plus® and Aleve Cold and Sinus®); |
| |
| | • | Matrixx Initiatives, Inc. (Zicam® and Nasal Comforttm); and |
| |
| | • | Airborne, Inc. (Airborne®). |
We also face substantial competition from companies that market private label brands to our largest customers, which are typically sold at lower prices than our products. We are aware that some private label brand companies have begun to market products containing immediate-release guaifenesin in tablet form; however, we do not believe any long-acting guaifenesin-based products have been introduced in the OTC market.
22
With respect to all of our existing and future drug products, regardless of whether we market such products in the prescription or OTC market, we will compete with companies working to develop products and technologies that are more effective, safer or less costly than our products and technologies. Our competitors may also obtain FDA or other regulatory approval for their products more rapidly than us, have larger or more skilled sales forces to promote their products and develop more comprehensive protection for their technologies. Many of our competitors have substantially greater financial, technical and human resources than we do. Moreover, additional mergers and acquisitions in the pharmaceutical industry may result in our competitors having an even greater concentration of resources. We may not be able to maintain market acceptance of our products or successfully introduce new products if our competitors develop different or more advanced products, bring such products to market before we do or market their products more effectively in the OTC and prescription markets.
With respect to our current products and our future OTC products, we also compete for brand recognition and product availability at retail stores. In addition, we compete with our competitors on price. Advertising, promotion, merchandising, packaging, and the timing of new product introductions and line extensions have a significant impact on consumer buying decisions and, as a result, on our sales. The large pharmaceutical companies we compete against in the OTC market have considerably greater financial resources than we do and likely spend more on trade promotions and advertising. These competitors also likely benefit from greater purchasing power, stronger vendor relationships and broader distribution channels.
Sales of our products also affect in-store position, display space and inventory levels in retail outlets. If we are not able to maintain sufficient inventory levels of our products, maintain or improve in-store positioning of our products in retail stores, conduct effective advertising campaigns and other consumer and professional promotional programs, or maintain distribution and supply arrangements on competitive terms, we risk losing market share to our competitors in the OTC market.
We depend heavily on the success of two of our existing products, Mucinex SE and Mucinex DM, and the strength of the Mucinex brand. If we are unable to continue to successfully commercialize Mucinex SE and Mucinex DM and build the Mucinex and Delsym brands with the introduction of new products, our results of operations and future prospects will suffer.
Sales of Mucinex SE accounted for approximately 39.8%, 66.3% and 73.3% of our revenue in fiscal 2007, 2006 and 2005, respectively. Sales of Mucinex DM accounted for approximately 28.5%, 24.8% and 23.9% of our revenue in fiscal 2007, 2006 and 2005, respectively. Sales of our other Mucinex products, including Mucinex D and the Mucinex line of products for children, accounted for approximately 17.3% and 8.1% of our revenue in fiscal 2007 and 2006, respectively. Sales of Delsym products accounted for approximately 14.6% of our revenue for fiscal 2007. In the near term, we anticipate that our ability to generate revenues and establish our Mucinex and Delsym brands will depend largely on the continued success of Mucinex SE and Mucinex DM and the successful growth of Mucinex D, the Mucinex line of products for children, Delsym and future products that utilize the Mucinex and Delsym brand names. Any failure or delay in our efforts to successfully commercialize our products could have a negative impact on our revenues and ability to execute our business strategy.
We rely on two suppliers for guaifenesin, the active ingredient we require to manufacture Mucinex SE, Mucinex DM, Mucinex D, and the maximum strength versions of these products, and we have historically had difficulty obtaining the amount of guaifenesin we have required. We also rely on two suppliers for dextromethorphan, the additional active ingredient in Mucinex DM.
Currently, we obtain all of the raw guaifenesin for Mucinex SE, Mucinex DM, Mucinex D and the maximum strength versions of these products from two suppliers, Boehringer Ingelheim and Delta. We entered into a new agreement with Boehringer Ingelheim in July 2006, which lasts through June 2011, and obligates Boehringer Ingelheim to supply us with a minimum of 500 metric tons of guaifenesin per contract year. According to the terms of our agreement with Boehringer Ingelheim, if we do not purchase at least the contractual amount of guaifenesin in any12-month period, we must purchase 100% of our oral-solid guaifenesin requirements from Boehringer Ingelheim. Although Boehringer Ingelheim has had difficulty in the
23
past supplying us with the amount of guaifenesin we have requested, they have advised us that they are confident in their ability to meet their guaifenesin obligations for the remainder of the contract term. Under our agreement with Boehringer Ingelheim, they have also committed to using their commercially reasonable efforts to supply us with guaifenesin in excess of the contractual amount if we request such additional supply, but they have no obligation to provide us with such additional amounts. We currently also purchase guaifenesin from Delta, which the FDA has approved to supply the guaifenesin we use in Mucinex SE. Even with the Boehringer Ingelheim supply, we expect to continue to purchase additional guaifenesin from Delta for use in products for which Delta guaifenesin is qualified to be used. If Boehringer Ingelheim and Delta have difficulty supplying us with our requirements for guaifenesin, we may be unable to produce sufficient quantities of Mucinex SE, Mucinex DM, Mucinex D and the maximum strength versions of these products to meet demand.
We rely on two suppliers to provide us with dextromethorphan, the additional active ingredient in Mucinex DM. Our original supplier reduced its production of dextromethorphan in 2004 and indicated that it intended to exit the business. In January 2006, this supplier agreed to provide us with an additional supply of dextromethorphan. In December 2006, the FDA approved a second supplier of dextromethorphan. We believe these two suppliers will provide us with sufficient quantities of dextromethorphan to meet our manufacturing needs. If these suppliers have difficulty providing us with our requirements for dextromethorphan, we may be unable to produce sufficient quantities of Mucinex DM to meet demand.
We currently have one approved supplier of codeine, which we believe will be able to meet our demand if the FDA approves Mucinex with Codeine, and we are seeking FDA approval of a secondary supplier. If we do not qualify a secondary supplier for codeine, or if our existing supplier is unable or unwilling to provide us with sufficient supply, we will not be able to meet commercial demand for Mucinex with Codeine.
A limited number of manufacturers operating under cGMP regulations are capable of manufacturing guaifenesin, dextromethorphan, pseudoephedrine or codeine to our specifications. We may be unable to utilize alternative manufacturing sources for these ingredients or to obtain such manufacturing on commercially reasonable terms or on a timely basis. Any transfer of our sources of supply to other manufacturers will require the satisfaction of various regulatory requirements, which could cause us to experience significant delays in receiving adequate supplies of guaifenesin, dextromethorphan, pseudoephedrine, codeine or all of these ingredients. Any delays in the manufacturing process may adversely impact our ability to meet commercial demand on a timely basis, which would negatively impact our revenues, reputation and business strategy.
We rely on one manufacturing facility to manufacture most aspects of our adult oral-solid Mucinex products. We have limited experience manufacturing our own products and may experience difficulties with our manufacturing operations.
On July 31, 2006, we repurchased our manufacturing facility and operations in Fort Worth. We rely on this one facility to manufacture most aspects of our adult oral-solid Mucinex products, including Mucinex SE, Mucinex DM and Mucinex D. If we experience difficulties with our manufacturing operations, our ability to meet customer demand for these products could adversely impact our business and revenues.
Additionally, because we lack manufacturing experience, we could experience production delays and difficulties in maintaining and expanding our manufacturing operations, including difficulties:
| | |
| | • | maintaining production yields; |
| |
| | • | maintaining quality control; |
| |
| | • | maintaining costs; |
| |
| | • | hiring and retaining qualified personnel; and |
| |
| | • | complying with the FDA’s cGMP regulations and guidelines. |
If we encounter such difficulties in maintaining our manufacturing facility, our ability to manufacture our products will be limited, which could seriously harm our business and results of operations.
24
We recently acquired the Delsym product line. If we are unable to effectively integrate and market products under the Delsym brand, our results of operations could suffer.
On June 12, 2006, we consummated the acquisition of certain rights and assets related to the manufacture and sale of the Delsym product line from UCB. As a result of this acquisition, we may experience:
| | |
| | • | difficulties in integrating the Delsym product line into our existing business; |
| |
| | • | delays in realizing the benefits of the Delsym product line acquisition; |
| |
| | • | diversion of our management’s time and attention from other business concerns; |
| |
| | • | higher costs of integration than we anticipated; |
| |
| | • | difficulties in retaining key employees who are necessary to manage the Delsym product line; or |
| |
| | • | difficulties in maintaining uniform standards, controls, procedures and policies throughout our product lines. |
In connection with this acquisition, we entered into supply agreements with UCB pursuant to which UCB will manufacture the Delsym products for us. As a result, we are vulnerable to any interruptions in our supply from UCB. If UCB is unable to supply enough product to meet the demand for Delsym products, our results of operations may suffer.
Further, we may not be successful in our marketing of the Delsym product line and we can offer no assurance that this acquisition will be profitable.
We rely on Catalent to perform certain aspects of the manufacture and packaging of Mucinex SE, Mucinex DM, Mucinex D, and the maximum strength versions of these products. We do not have the manufacturing capacity to manufacture our Delsym products, our Mucinex line of products for children or our Mucinex nasal spray products and rely on other manufacturers to manufacture those products. If those manufacturers are unable to supply enough product to meet our customers’ demand, our results of operations may suffer.
We rely on Catalent, formerly known as Cardinal Health, to perform certain aspects of the manufacture and packaging of Mucinex SE, Mucinex DM, Mucinex D, and the maximum strength versions of these products. We rely on a single third party, UCB, to manufacture the Delsym product line, and we rely on several other manufacturers to manufacture our Mucinex line of products for children and Mucinex nasal spray products.
If our demand increases and we are unable to increase manufacturing capacity or unable to obtain additional capacity on reasonable economic terms to meet that demand, our revenues and operating results may be negatively impacted. The addition of capacity on unfavorable terms could also affect our revenue and profitability. In addition, any damage to, or disruption at, our manufacturers’ facilities could halt production of our products and materially harm our business.
Reliance on third party manufacturers entails risks to which we would not be subject if we performed all aspects of the manufacturing process, including:
| | |
| | • | the possibility that third parties may not comply with the FDA’s cGMP regulations, other regulatory requirements and quality assurance; |
| |
| | • | the possible breach of manufacturing agreements by third parties due to factors beyond our control; and |
| |
| | • | the possibility of termination or nonrenewal of an agreement by a third party manufacturer, based on its own business priorities, at a time that is costly or inconvenient for us. |
In the event of a supply disruption or a deterioration in our product quality from a third party manufacturer, we would have to rely on alternative manufacturing sources or identify and qualify new manufacturers. We may not be able to identify or qualify new manufacturers in a timely manner or obtain a sufficient allocation of their capacity to meet our requirements. In addition, alternative vendors must comply
25
with product validation and stability testing, which may involve additional manufacturing expense, delay in production or required regulatory approvals. Any resulting delays in meeting demand could negatively impact our inventory levels, sales, profitability, and reputation.
We cannot ensure that the FDA’s removal from the market of the existing prescription long-acting guaifenesin combination products similar to Mucinex DM and Mucinex D will result in increased sales of Mucinex DM or Mucinex D. Additionally, other companies may receive FDA approval and introduce products into the OTC market containing long-acting guaifenesin that are competitive with our products.
As described more fully under Item 1. Business — Government Regulation in this Annual Report onForm 10-K, following its approval of Mucinex SE, the FDA took enforcement action to remove all existing long-acting, single-ingredient guaifenesin products from the market, pursuant to its draft compliance policy guide articulating the FDA’s existing informal policy regarding drugs marketed in the United States that do not have required FDA approval. We believe that sales of Mucinex SE increased as a result of the FDA’s action, which caused Mucinex SE to be the only drug of its kind approved by the FDA for marketing and sale in the United States. On May 25, 2007, the FDA announced that companies must also stop manufacturing effective as of August 27, 2007, and distributing via interstate commerce as of November 26, 2007, unapproved time-released dosage forms containing guaifenesin. This action will result in the removal from the market of all existing, long-acting guaifenesin and dextromethorphan combination products similar to Mucinex DM, and all existing, long-acting guaifenesin and pseudoephedrine combination products similar to Mucinex D. We can offer no assurance that this action will result in increased sales of our Mucinex DM and Mucinex D products.
In addition, as described more fully under the heading “Government Regulation” in this Annual Report onForm 10-K, based on our patent position and regulatory requirements, we estimate that the process of developing and obtaining the necessary FDA approvals for competitive long-acting guaifenesin products would take two to three years from the start of the process. We believe that competitors may have already begun the process of developing and obtaining FDA approval for products competitive with Mucinex SE or our other products. As a result, the effective market exclusivity that we currently enjoy for Mucinex SE, and will enjoy once the FDA’s recent enforcement action becomes effective for Mucinex DM and Mucinex D, may not continue. The FDA’s approval of competitive long-acting guaifenesin OTC products would slow our growth and adversely affect our results of operations.
Many of our products and product candidates rely on guaifenesin, which is an expectorant. If our competitors develop a superior expectorant, our products and our patented technology may be rendered obsolete.
Guaifenesin is a fundamental component in most of our marketed products and many of our product candidates. Guaifenesin and the other active ingredients in our products and product candidates have been used for many years. Our competitors may develop new chemicals or compounds that render guaifenesin, our patented delivery system or our products obsolete. We can offer no assurance that our development efforts will be able to lead or keep pace with discoveries or technological advances that yield superior compounds or products or that we will recover our investment in our products before any such advancements render them obsolete.
We depend on a limited number of customers for a large portion of our sales, and demands made by, or the loss of, one or more of these customers could significantly reduce our margins or sales and adversely affect our business and financial results.
For fiscal 2007, our top five and top ten customers accounted for an aggregate of approximately 57.7% and 73.7% of our gross sales, respectively. CVS, Walgreens, and Wal-Mart/Sam’s Club each accounted for greater than 10% of our gross sales for fiscal 2007. In future periods, we expect that our top five and top ten customers will, in the aggregate, continue to account for a large portion of our sales. In addition, retailers have demanded, and may continue to demand, increased service and other accommodations, as well as price concessions. As a result, we may face downward pressure on our prices and increased expenses to meet these demands, which would reduce our margins. Given the growing trend toward consolidation of retailers, we
26
expect demands by customers and the concentration of our sales in a small number of customers to increase. The loss of one or more of our top customers, any significant decrease in sales to these customers, pricing concessions or other demands made by these customers, or any significant decrease in our retail display space in any of these customers’ stores could reduce our sales and margins and have a material adverse effect on our business, financial condition and results of operations.
Adverse publicity associated with us or our products could have a material adverse effect on us.
We are highly dependent upon consumer perceptions of us, our products, and the safety and quality of our products. We could be adversely affected if we or our product brands are subject to negative publicity. We could also be adversely affected if any of our products or any similar products distributed by other companies prove to be, or are asserted to be, harmful to consumers. Also, because of our dependence upon consumer perceptions, any adverse publicity associated with illness or other adverse effects resulting from consumers’ use or misuse of our products, or any similar products distributed by other companies, could have a material adverse impact on our results of operations.
Product liability lawsuits could divert our resources, result in substantial liabilities and reduce the commercial potential of our products.
Our business exposes us to the risk of product liability claims that is inherent in the manufacturing, testing and marketing of drugs and related products. These lawsuits may divert the attention of our management from pursuing our business strategy and may be costly to defend. In addition, if we are held liable in any of these lawsuits, we may incur substantial liabilities and may be forced to limit or forgo further commercialization of those products. Although we maintain general liability and product liability insurance in an amount that we believe is reasonably adequate to insulate us from potential claims, this insurance may not fully cover potential liabilities. In addition, our inability to obtain or maintain sufficient insurance coverage at an acceptable cost or to otherwise protect against potential product liability claims could prevent or inhibit the commercial production and sale of our products, which could adversely affect our business.
If we fail to obtain an adequate level of reimbursement for our products by Medicaid, our business may be adversely affected. Additionally, many state Medicaid programs do not cover the costs of our products and we cannot ensure that any Medicaid programs will continue to reimburse us for our products.
The availability and levels of reimbursement by Medicaid affect the market for both our current and future products. Medicaid continually attempts to contain or reduce the costs of healthcare by challenging the prices charged for pharmaceuticals. For example, we are obligated to provide rebates to the state Medicaid programs on sales of our products to Medicaid beneficiaries. We expect to continue to experience pricing pressures in connection with the sale of our current and future products due to potential increases in rebates and other downward trends in reimbursement aimed at reducing healthcare costs, as well as legislative proposals.
Medicaid does not generally cover the costs of OTC products. However, 28 state Medicaid programs (three of which require prior approval of reimbursement from the state Medicaid program, or PA) have covered and continue to cover the cost of Mucinex SE, 22 state Medicaid programs (three of which require PA) have covered and continue to cover the cost of Mucinex DM, 18 state Medicaid programs (three of which require PA) have covered and continue to cover the cost of Mucinex D, 13 state Medicaid programs (two of which require PA) have covered and continue to cover the cost of Mucinex Children’s Mini-Melts, 21 state Medicaid programs (three of which require PA) have covered and continue to cover the cost of Mucinex Children’s Liquid, and 16 state Medicaid programs (two of which require PA) have covered and continue to cover the cost of Delsym. We can offer no assurance that any Medicaid program will cover any of our new products or will continue to cover our current products.
27
Seasonal fluctuations in demand for our products may cause our operating results to vary significantly from quarter to quarter.
We expect retail demand for our products to be higher between October 1 and March 31 due to the prevalence of cough, cold and flu symptoms during that period. As a result, our shipments, and therefore revenues, are expected to be higher between July 1 and March 31 to support the retail demand through the cough, cold and flu season. We generally expect our revenues during the quarter ended June 30 to be lower than the other quarters. In addition, fluctuations in the severity of the annual cough, cold and flu season may cause our operating results to vary from year to year. Due to these seasonal fluctuations in demand, our operating results in any particular quarter may not be indicative of the results for any other quarter or for the entire year.
Risks Related to Product Development
We recently completed our phase IIb erdosteine clinical trial and are reviewing the final results of this trial. If we determine the results were unsuccessful, or if the FDA or other regulatory agencies do not accept or approve the results of such studies, our erdosteine product may not successfully come to market and our business prospects may suffer.
In May of 2005, we in-licensed erdosteine, a mucoregulator that is currently approved for use in Europe, South Africa and Asia for symptomatic treatment of respiratory infections, bronchitis and chronic obstructive pulmonary disease. In December 2006, we completed our phase IIb erdosteine clinical trial and are reviewing the final analysis of the trial’s results. The preliminary results suggested that the two different erdosteine treatment groups do not seem to break statistically from the placebo. However, subsequent analysis has resulted in more promising data. We hope to conduct a meeting with the FDA before the end of calendar year 2007 to discuss these clinical results, before we determine the ultimate outcome of the erdosteine program. We cannot assure you that these trials will ultimately be successful or that the FDA or other relevant regulatory agencies will accept the results and approve or clear erdosteine for sale. Further, we continue to evaluate the potential financial benefits and costs of erdosteine. If we determine that the results of this trial would prevent regulatory approval or the costs associated with attaining regulatory approval of erdosteine exceed the potential financial benefits, or if the projected development timeline is inconsistent with our investment horizon, we may choose to discontinue the development of erdosteine.
We may not be successful in our efforts to expand our portfolio of products.
We intend to expand our portfolio of products by:
| | |
| | • | developing and commercializing line extensions of Mucinex by combining long-acting guaifenesin with other ingredients to address various respiratory conditions; |
| |
| | • | developing and commercializing our Mucinex products for children; |
| |
| | • | developing and commercializing line extensions of Delsym; |
| |
| | • | developing and commercializing prescription products to address additional segments of the respiratory market using our platform technology for extended-release guaifenesin; and |
| |
| | • | acquiring or in-licensing additional technologies and additional pharmaceutical products or product candidates in the respiratory therapeutics market. |
Our failure to expand our portfolio of products in both the prescription and OTC respiratory therapeutic markets, or any unexpected delays in launching new products, will impair our ability to execute our growth strategy, which will negatively affect our financial position and results of operations.
28
We intend to conduct clinical trials on product candidates we develop or acquire in the future, which will be costly, take years to complete and may not ultimately be successful.
As part of our business strategy, we intend to pursue product candidates that must undergo preclinical studies and clinical trials as a condition to regulatory approval. Preclinical studies and clinical trials are expensive and, because we do not have the ability to conduct our own clinical trials, we will hire third parties to run the trials, which will lessen our control over the process. Clinical trials may take several years to complete and may not be successful. The commencement and completion of clinical trials may be delayed by many factors, including:
| | |
| | • | our inability to obtain materials sufficient for use in preclinical studies and clinical trials; |
| |
| | • | delays in patient enrollment and variability in the number and types of patients available for clinical trials; |
| |
| | • | difficulty in maintaining contact with patients after treatment, resulting in incomplete data; |
| |
| | • | inability to demonstrate effectiveness of product candidates during clinical trials; |
| |
| | • | unforeseen safety issues or side effects; |
| |
| | • | governmental or regulatory delays and changes in regulatory requirements, policy and guidelines; or |
| |
| | • | varying interpretation of data by the FDA. |
Although we have not been required to conduct extensive clinical trials to obtain FDA approval of our existing products, we expect that many of our future product candidates may require extensive clinical trials. We may not successfully complete clinical trials for our product candidates. Accordingly, we may not receive the regulatory approvals needed to market our product candidates. Any failure or delay in commencing or completing clinical trials or obtaining regulatory approvals for our product candidates would delay or prevent the commercialization of such product candidates, which could negatively impact our financial position.
Regulatory Risks
Our products are subject to recalls even after receiving FDA regulatory clearance or approval. Recalls could harm our reputation and business.
We are subject to ongoing reporting regulations that require us to report to the FDA if our products cause or contribute to a death or serious injury. These reports can lead to stricter safety warnings on product labeling, voluntary company recalls or withdrawal of the product from the market. In addition, if we become aware of adverse event reports, manufacturing defects or insufficient labeling, we may voluntarily elect to recall our products.
On April 2, 2007, we initiated a voluntary recall of specific lots of Children’s Mucinex liquid products. We recalled these lots because of possible confusion in determining the proper dose of medication for children, which could have occurred due to the dosing cup included in the product packaging. Although we do not expect the April 2007 recall to have a material adverse effect on our business, any product recalls, which we must report to the FDA, would divert managerial and financial resources and could harm our reputation with our customers and with the health care professionals who recommend our products.
Products approved for marketing remain subject to regulation. Complying with such regulation can be costly, and failure to comply could result in a loss of approvals or suspension of product sales.
We are subject to extensive regulation by the FDA and, to a lesser extent, by other applicable federal agencies, such as the Consumer Product Safety Commission, the Drug Enforcement Administration, the Federal Trade Commission, or FTC, the Environmental Protection Agency, and state government agencies. The FDCA, the Controlled Substances Act and other federal statutes and rules regulate the testing, manufacture, packaging, labeling, storage, record keeping, promotion, distribution, and sale of our products. If we or our manufacturers fail to comply with those regulations, we could become subject to significant penalties or
29
claims, which could materially and adversely affect our operating results or our ability to conduct our business. In addition, the adoption of new regulations or changes in the interpretations of existing regulations may result in significant compliance costs or discontinuation of product sales and may adversely affect our revenue and the marketing of our products.
In accordance with the FDCA and FDA regulations, our manufacturing processes and the manufacturing processes of our third party manufacturers must also comply with cGMP. The FDA inspects our facilities and the facilities of our third party manufacturers periodically to determine if we and our third party manufacturers are complying with cGMP. If the FDA implements additional regulations with which we and our third party manufacturers have to comply, our expenses would increase.
Additionally, if we or our third party manufacturers fail to comply with federal or state regulations, we could be required to:
| | |
| | • | suspend manufacturing operations; |
| |
| | • | change product formulations; |
| |
| | • | suspend the sale of products with non-complying specifications; |
| |
| | • | initiate product recalls; or |
| |
| | • | change product labeling, packaging or advertising or take other corrective action. |
Any of these actions could materially and adversely affect our financial results.
Further, our failure to comply with the FDA, FTC or state regulations relating to our product claims and advertising may result in enforcement actions and imposition of penalties or otherwise materially and adversely affect our marketing strategy and product sales.
We may not be able to obtain marketing approval for any of the products resulting from our development efforts and failure to obtain these approvals could materially harm our business.
The FDA must approve all new drugs before they can be marketed and sold in the United States. The FDA typically requires successfully completing extensive clinical trials and demonstrating manufacturing capability to obtain approval, as described more fully under Item 1. Business — Government Regulation in this Annual Report onForm 10-K. Clinical development is expensive, uncertain and lengthy, often taking a number of years for an applicant to file a NDA and for the FDA to approve it. Of the large number of drugs in development, only a small percentage result in the submission of a NDA to the FDA, and the FDA approves even fewer for commercialization.
We may need to successfully address a number of challenges in order to complete the development of our future products. For example, to obtain marketing approval for a new product candidate, we and our third party manufacturers will be required to consistently produce the active pharmaceutical ingredient in commercial quantities and of specified quality on a repeated basis. This requirement is referred to as process validation. If we are unable to satisfy this process validation requirement for a future product candidate, through our third party manufacturers or otherwise, we will not receive approval to market such product.
In addition, the FDA and other regulatory agencies may apply new standards for safety, manufacturing, packaging, and distribution of future product candidates. Complying with such standards may be time consuming or expensive and could result in delays in our obtaining marketing approval for future product candidates, or possibly preclude us from obtaining such approval. Such a delay could also increase our commercialization costs, possibly materially.
Furthermore, our future products may not be effective or may prove to have undesirable or unintended side effects, toxicities or other characteristics that may preclude us from obtaining regulatory approval or prevent or limit commercial use. Even if we do obtain regulatory approval, such regulatory approvals may be subject to limitations on the indicated uses for which we may market a product, which may limit the size of the market for such product.
30
The manufacture and packaging of pharmaceutical products such as our Mucinex and Delsym products are subject to FDA requirements. If we or our third party manufacturers fail to satisfy these requirements, our product development and commercialization efforts may be materially harmed.
As indicated above, an approved drug and its manufacturer are subject to continual review, including review and approval of the manufacturing facilities. Changes in the manufacturing process or procedure, including a change in the location where the product is manufactured or a change of a third party manufacturer, may require prior FDA review or approval or revalidation of the manufacturing process and procedures in accordance with cGMP. This review or revalidation may be costly and time consuming and could delay or prevent the launch or delivery of a product. To effect a change of site, we and the manufacturer must transfer the relevant manufacturing technology to the new site. This process is detailed and time consuming. If we change the manufacturing site, the FDA will likely require us to perform analytical tests to demonstrate that changing the manufacturing location will not affect the characteristics of the product. If we cannot establish to the satisfaction of the FDA that the products manufactured at the new site are equivalent to those manufactured at the prior site, we may not obtain, or may be delayed in obtaining, approval to manufacture our products at the new site. In addition, if we elect to manufacture products at the facility of another third party, we would need to ensure that the new facility and the manufacturing process are in compliance with cGMP. Any such new facility would be subject to a preapproval inspection by the FDA.
Furthermore, in order to obtain approval by the FDA of new products, we need to complete testing on both the active pharmaceutical ingredient and on the finished product in the packaging we propose for commercial sales. This testing includes testing of stability, identification of impurities and testing of other product specifications by validated test methods. If the required testing is delayed or produces unfavorable results, we may not obtain approval to launch the product or the FDA may delay its approval.
Our current and future products may be associated with certain transient side effects. If we or others identify additional, more severe side effects associated with our current or future products, we may be required to withdraw our products from the market, perform lengthy additional clinical trials or change the labeling of our products, any of which would hinder or preclude our ability to generate revenues.
Our current guaifenesin-based products may be associated with mild and transient side effects including upset stomach, nausea, vomiting, diarrhea, headache, dizziness, confusion, skin rash (including hives), constipation, and drowsiness. Mucinex D and Children’s Mucinex Cold Liquid may additionally be associated with unusual paleness, increased sweating, weakness, trouble in sleeping, nervousness, restlessness, fast or pounding heartbeat, trembling and difficulty urinating. Our other Mucinex products for children are not expected to be associated with additional side effects or problems than those associated with our other guaifenesin-based products. Our Delsym products may be associated with confusion, constipation, dizziness, drowsiness, headache, nausea or vomiting, and stomach pain. The Mucinex nasal sprays’ topical side effects may include nasal burning, nasal stinging, sneezing, increased discharge; may cause nasal congestion to recur or worsen; overuse may cause rebound congestion; potential for misuse if rebound congestion develops; and if absorbed, systemic side effects may include fast, irregular or pounding heartbeat, headache, lightheadedness, nervousness, trembling and trouble in sleeping. If our Mucinex with Codeine is approved for marketing by the FDA, its side effects may include nausea, vomiting, sedation, dizziness, constipation, allergic reactions, central nervous system depression, respiratory depression, circulatory depression; toxicity may include confusion, convulsions, hallucinations, mental depression, hepatotoxicity, and paradoxical excitation in children.
If we or others identify side effects after any of our products are on the market:
| | |
| | • | regulatory authorities may withdraw their approvals; |
| |
| | • | we may be required to reformulate our products, conduct additional clinical trials, change the labeling of our products, or implement changes to obtain new approvals of manufacturers’ facilities; |
| |
| | • | we may recall the affected products from the market; |
| |
| | • | we may experience a significant drop in sales of the affected products; |
31
| | |
| | • | our reputation in the marketplace may suffer; |
| |
| | • | we may become the target of lawsuits, including class action suits; and |
| |
| | • | we may be required to withdraw our products from the market and not be able to re-introduce them into the market. |
Any of these events could harm or prevent sales of the affected products or could substantially increase the costs and expenses of commercializing or marketing these products, which would adversely affect our results of operations and financial position.
The sale of products containing pseudoephedrine is restricted by additional federal and state government regulations, which may negatively impact our sales of Mucinex D. If the sale of dextromethorphan undergoes similar restrictions, it could negatively impact sales of our products containing dextromethorphan.
Our Mucinex D product contains pseudoephedrine HCl, a FDA-approved ingredient for the relief of nasal congestion. We understand that pseudoephedrine has been used in the illicit manufacture of methamphetamine, a dangerous and addictive drug. On March 9, 2006, President Bush signed into law the Combat Methamphetamine Epidemic Act of 2005, which provides that among other things: (i) effective April 9, 2006, consumers are prohibited from purchasing more than (a) 3.6 grams of pseudoephedrine base per day, which equates to four packages of Mucinex D 18-count and two packages of Mucinex D 36-count per day, and (b) 9.0 grams of pseudoephedrine base per month, which equates to ten packages of Mucinex D 18-count and five packages of Mucinex D 36-count per month; and (ii) effective September 30, 2006, retailers are required to (a) place products containing pseudoephedrine, including Mucinex D and Maximum Strength Mucinex D, behind the counter or in locked cabinets in the main section of their store, and (b) keep an electronic record of all pseudoephedrine sales. We believe that, to date, most states have also enacted regulations concerning the sale of pseudoephedrine, some of which include more restrictive sales limits than the new federal regulations, including limiting the amount of these products that can be purchased at one time, making pseudoephedrine a prescription product, or requiring that these products be located behind the counter, with the stated goal of deterring the illicit/illegal manufacture of methamphetamine. We believe that these restrictions have and will continue to negatively impact our sales of Mucinex D and will negatively impact Maximum Strength Mucinex D. If additional FDA-approved ingredients such as dextromethorphan, undergo similar restrictions, this could negatively impact sales of our products containing dextromethorphan.
Risks Related to Managing Growth
We depend on our key personnel, and if we are not able to retain those individuals or recruit additional technical personnel, our business will suffer.
We are highly dependent on the principal members of our management. The continued service of our Chief Executive Officer and President, Michael J. Valentino, is critical to our success. Mr. Valentino is the only member of our management team with whom we have entered into an employment agreement, but he may terminate it on short or no notice. In addition, Mr. Valentino’s employment agreement expires August 2008, and we can offer no assurance that we will be able to renew Mr. Valentino’s employment agreement. Further, we can offer no assurance that we will be able to hire a new highly qualified Chief Executive Officer in a timely manner.
The loss of any of our other executive officers, including our Chief Financial Officer and Treasurer, Rita M. O’Connor; our Chief Operating Officer, Robert D. Casale; our Executive Vice President, Sales and Corporate Accounts, John S. Thievon; our Executive Vice President, General Counsel, Chief Compliance Officer and Secretary, Walter E. Riehemann; our Executive Vice President, Human Resources, Peter D. Wentworth, Ph.D.; or our Senior Vice President of Research and Development, Helmut H. Albrecht, M.D. could cause disruption in our business. We do not carry key man life insurance on any of our key personnel.
In addition, our growth has required us to hire a significant number of qualified technical personnel. Intense competition exists among other companies and research and academic institutions for qualified personnel. If we cannot continue to attract and retain, on acceptable terms, the qualified personnel necessary
32
for the continued development of our business, we may not be able to sustain our operations or grow our business.
We may undertake strategic acquisitions of technologies and products. Integration of such technologies and products will involve a variety of costs, and we may never realize the anticipated benefits of such acquisitions.
We intend to pursue opportunities to acquire technologies, brands and products that would allow us to leverage our professional sales force or our marketing and development expertise or enhance our product portfolio or brand recognition in the OTC and prescription markets. We have limited experience in identifying and completing such acquisitions. Further, acquisitions typically entail many risks, including risks related to the integration of the technologies and products. In attempting to integrate such technologies and products, we may experience unexpected integration costs and delays, which may divert management and employee attention and disrupt our ability to develop and introduce new products. If we are not able to successfully integrate our acquisitions, we may not be able to realize the intended benefits of the acquisition.
As a result of acquiring products or entering into other significant transactions, we have experienced, and will likely continue to experience, significant charges to earnings for acquisitions and related expenses, including transaction costs, closure costs or acquired in-process product development charges. These costs may include substantial fees for investment bankers, attorneys, accountants, and financial printing costs. Charges that we may incur in connection with acquisitions could adversely affect our results of operations for particular quarterly or annual periods. In addition, we may lack the required funds or resources to carry out such acquisitions.
Risks Related to Intellectual Property
Our U.S. patent no. 6,372,252 is the subject of a request for reexamination, which the United States Patent and Trade Mark Office, or USPTO, granted upon petition to the USPTO Director. If the USPTO cancels our patent or substantially narrows the claims of our patent such that it no longer protects our products from competition, our business will be materially harmed.
On April 20, 2005, an anonymous third party filed a request for reexamination with the USPTO of our U.S. patent no. 6,372,252, which contains claims covering a long-acting guaifenesin product, including an immediate-release portion and an extended-release portion that yields a certain pharmacokinetic profile. On June 23, 2005, the USPTO denied the request for reexamination and found that the third party did not raise a substantial new question of patentability based on prior art. On July 22, 2005, the third party who filed the request for reexamination sought review of the USPTO’s denial of its request for reexamination by petition to the Director of the USPTO. The USPTO advised us on August 18, 2005 that the Director had granted the petition and ordered reexamination, and on December 29, 2005, the USPTO advised us of its initial, non-final determination to reject the claims of our U.S. Patent no. 6,372,252. Under typical procedural practices at the USPTO, this preliminary finding was made prior to our presentation of arguments in favor of affirming the claims under this patent. On March 21, 2006, we presented our arguments to the USPTO examiner in a personal interview, and on March 23, 2006, we filed a written response to the USPTO’s initial determination setting out those arguments. On June 20, 2006, the USPTO advised us that it had decided to continue to reject some claims of our U.S. Patent No. 6,372,252 but to confirm that several claims of this patent were patentable. In response to this communication from the USPTO, which is designated a final action, on August 21, 2006, we filed a request for reconsideration of some aspects of this action. On September 28, 2006, the USPTO responded to this request for reconsideration, adhering to its prior positions and declining to enter certain proposed amendments. On November 20, 2006, we filed a notice of appeal. On January 8, 2007, the USPTO confirmed that five of the 58 claims in the reexamination were patentable, and on January 22, 2007, we filed an appeal brief with the USPTO regarding the claims that the USPTO had continued to reject.
Under a reexamination proceeding and upon completion of the proceeding, the USPTO may leave the patent in its present form, narrow the scope of the claims of the patent or cancel all of the claims of the patent. Pursuant to this reexamination, the USPTO will reconsider the patentability of our delivery system for
33
guaifenesin. From this point forward the reexamination could take up to three additional years, including the potential for an additional appeal should the pending appeal be unsuccessful.
We intend to vigorously defend our patent position, and we believe we will prevail in the reexamination process. We may not be successful, however, in maintaining our patent or the scope of its claims during reexamination and can offer no assurance as to the outcome of a reexamination proceeding. If the USPTO does not confirm our patent or substantially narrows the claims of our patent following a reexamination, then our competitive position could be weakened and we may face stronger and more direct competition, which would negatively impact our business and operating results.
If our patent position does not adequately protect our products and future products, others will be able to compete against us more directly, which may harm our business.
Our patent portfolio includes two U.S. patents, eight foreign patents and several patent applications. Our most significant patents are currently our two U.S. patents, which contain claims covering: a long-acting guaifenesin product, including an immediate-release portion and an extended-release portion that yields a certain pharmacokinetic profile; and the combination of a long-acting guaifenesin product, including an immediate-release portion and an extended-release portion, with another active pharmaceutical ingredient that yields a certain pharmacokinetic profile. The active ingredients in our products and most of our product candidates, including guaifenesin, dextromethorphan and pseudoephedrine, are chemical compounds that have been in existence for many years and are not covered by patents that claim these chemical compounds. Our patents cover a formulation of a product that delivers guaifenesin with a bi-phasic release pattern. They do not and will not contain compound claims for the chemicals in these products. We can offer no assurance that our patents will effectively exclude competitors from introducing similar or equivalent products.
Our success will depend, in large part, on our ability to obtain additional patents in the United States, maintain our existing patent position and obtain and maintain adequate protection for the other intellectual property incorporated into our products. Our patents may be challenged, narrowed, invalidated, or circumvented, which could limit our ability to stop competitors from marketing similar products or limit the length of term of patent protection we may have for our products. We can offer no assurance that we will receive patents for any of our pending patent applications or any patent applications we may file in the future. In addition, our patents may not afford us protection against competitors with similar technology. Because patent applications in the United States and many foreign jurisdictions are typically not published until 18 months after filing, or in some cases not at all, and because publications of discoveries in the scientific literature often lag behind actual discoveries, we cannot be certain that we were the first to make the inventions claimed in issued patents or pending patent applications or that we were the first to file for protection of the inventions set forth in these patent applications. If we do not adequately protect our intellectual property, competitors may be able to use our technologies and erode or negate any competitive advantage we may have, which could harm our business and financial results.
If we are unable to protect the intellectual property rights related to our brands, our ability to compete effectively in the markets for our products could be negatively impacted.
A significant part of our business strategy is to position Mucinex and our Mucinex line of products for children as preferred brands for relief of respiratory congestion for the OTC cough, cold, allergy and sinus market and to position Delsym as a preferred brand for cough suppression. We believe that familiarity with our brands is an important competitive advantage and that the growth and sustainability of our market share for our product lines will depend to a significant extent upon the goodwill associated with our related trademarks and trade names. We intend to use the trademarks and trade names on our products to convey that the products we sell are “brand name” products, and we believe consumers ascribe value to our brands. We own the material trademark and trade name rights used in connection with the packaging, marketing and sale of our products. This ownership prevents our competitors or new entrants to the market from using our brand names. Therefore, we view trademark and trade name protection as critical to our business. Although most of our trademarks are registered in the United States, we may not be successful in asserting trademark or trade name protection. If we were to lose the exclusive right to use the Mucinex or Delsym brand name or other
34
brand names we establish or acquire in the future, our sales and operating results could be materially and adversely affected. We could also incur substantial costs to prosecute legal actions relating to the use of our trademarks and trade names, which could have a material adverse effect on our business, results of operations or financial condition.
Additionally, other parties may infringe on our property rights in our trademarks and trade names, which may dilute the value of our brands in the marketplace. Our competitors may also introduce brands that cause confusion with our brands in the marketplace, which could adversely affect the value that our customers associate with our brands and thereby negatively impact our sales. Any such infringement of our intellectual property rights would also likely result in a commitment of our time and resources to protect those rights through litigation or otherwise. In addition, third parties may assert claims against our trademark and trade name rights, and we may not be able to successfully resolve these claims. In such event, we may lose our ability to use the brand names that are the subject of these claims, which could have a material adverse impact on our sales and operating results. We could also incur substantial costs to defend even those claims that are not ultimately successful, which could materially adversely affect our business, results of operations or financial condition.
If we are unable to protect the confidentiality of our trade secrets and proprietary information, our technology and information may be used by others to compete against us.
In addition to patented technology, we rely upon unpatented proprietary technology, processes and know-how. We seek to protect this information in part by confidentiality agreements with our employees, consultants and third parties. These agreements may be breached, and we may not have adequate remedies for any such breach. In addition, our trade secrets may otherwise become known or be independently discovered by competitors. If we do not adequately protect our trade secrets and proprietary information, competitors may be able to use our technologies and erode or negate any competitive advantage we may have, which could harm our business and financial results.
Legal proceedings or third party claims of intellectual property infringement may require us to spend time and money and could prevent us from developing or commercializing products.
Our technologies, products or potential products in development may infringe rights under patents or patent applications of third parties. Third parties may own or control these patents and patent applications in the United States and abroad. These third parties could bring claims against us that would cause us to incur substantial expenses and, if successful, could cause us to pay substantial damages. Further, if a patent infringement suit were brought against us, we could be forced to stop or delay research, development, manufacturing, or sales of the product or product candidate that is the subject of the suit.
As a result of patent infringement claims, or to avoid potential claims, we may choose to seek, or be required to seek, a license from the third party and would most likely be required to pay license fees or royalties or both. These licenses may not be available on acceptable terms, or at all. Even if we were able to obtain a license, the rights may be nonexclusive, which would give our competitors access to the same intellectual property. Ultimately, we could be prevented from commercializing a product or be forced to cease some aspect of our business operations if, as a result of actual or threatened patent infringement claims, we are unable to enter into licenses on acceptable terms. This inability to enter into licenses could harm our business significantly.
The pharmaceutical industry has experienced substantial litigation and other proceedings regarding patent and other intellectual property rights. In addition to infringement claims against us, we may become a party to other patent litigation and other proceedings, including interference proceedings declared by the USPTO and opposition proceedings in the European Patent Office, regarding intellectual property rights with respect to our products and technology. The cost to us of any patent litigation or other proceeding, even if resolved in our favor, could be substantial. Some of our competitors may be able to sustain the costs of such litigation or proceedings more effectively because of their substantially greater financial resources. Uncertainties resulting from the initiation and continuation of patent litigation or other proceedings could have a material adverse
35
effect on our ability to compete in the marketplace. Patent litigation and other proceedings may also absorb significant management time.
Risks Relating to Future Financing Needs
We may need additional financing, which may be difficult to obtain. Our failure to obtain necessary financing or doing so on unattractive terms could adversely affect our marketing and development programs and other operations.
We will require substantial funds to commercialize our products, launch new products, promote our brand, and conduct development, including preclinical testing and clinical trials, of our potential products. We believe that our existing credit facility and existing cash, coupled with cash flow from product sales, will be sufficient to fund our anticipated levels of operations through at least the next two years. However, our future capital requirements will depend on many factors, including:
| | |
| | • | the success of our commercialization of our products and the costs associated with related marketing, promotional and sales efforts; |
| |
| | • | the timing of new product launches, product development and advancement of other product candidates into development; |
| |
| | • | potential acquisitions or in-licensing of other products or technologies; |
| |
| | • | the timing of, and the costs involved in, obtaining regulatory approvals; |
| |
| | • | the cost of manufacturing activities, including raw material sourcing and regulatory compliance; and |
| |
| | • | the costs involved in establishing and protecting our patent, trademark and other intellectual property rights. |
Additional financing may not be available to us when we need it or on favorable terms. If we are unable to obtain adequate financing on a timely basis, we may be required to significantly curtail one or more of our marketing, development, licensing, or acquisition programs. We could be required to seek funds through arrangements with others that may require us to relinquish rights to some of our technologies, product candidates or products that we would otherwise pursue on our own. If we raise additional funds by issuing equity securities, our then-existing stockholders will experience dilution and the terms of any new equity securities may have preferences over our common stock.
Risks Related to Our Common Stock
Our stock price is volatile and purchasers of our common stock could incur substantial losses.
Our stock price is volatile. From our initial public offering in July 2005 through August 28, 2007, the trading price of our common stock has ranged from $21.50 to $49.75 per share. The stock market in general, and the market for specialty pharmaceutical companies in particular, have experienced extreme volatility that has often been unrelated to the operating performance of particular companies. As a result of this volatility, investors may not be able to sell their common stock at or above their respective purchase prices. The market price for our common stock may be influenced by many factors, including:
| | |
| | • | the regulatory status of potentially competitive products; |
| |
| | • | regulatory developments; |
| |
| | • | developments or disputes concerning patents or other proprietary rights; |
| |
| | • | our ability to manufacture products to commercial standards; |
| |
| | • | public concern over our drugs; |
| |
| | • | litigation; |
| |
| | • | the departure of key personnel; |
36
| | |
| | • | future sales of our common stock; |
| |
| | • | variations in our financial results or those of companies that are perceived to be similar to us; |
| |
| | • | investors’ perceptions and expectations of us; and |
| |
| | • | general economic, industry and market conditions. |
If there are substantial sales of our common stock, our stock price could decline.
If our existing stockholders sell a large number of shares of our common stock or the public market perceives that existing stockholders might sell shares of common stock, the market price of our common stock could decline significantly. All of the shares sold in our initial public offering in July 2005, in our secondary offering in December 2005 and in our secondary offering in September 2006 are freely tradable without restriction or further registration under the federal securities laws, unless purchased by our “affiliates” as that term is defined in Rule 144 under the Securities Act of 1933, as amended, or the Securities Act.
On August 18, 2006, we filed a registration statement onForm S-3 covering 10,025,235 shares of common stock held by GAMI Investments, Inc., GVI Holdings, Inc., SZ Investments, L.L.C., Tullis-Dickerson Capital Focus III, L.P., TD Origen Capital Fund, L.P., TD Lighthouse Capital Fund, L.P., and Perseus-Soros BioPharmaceutical Fund, L.P. We filed the registration statement pursuant to our existing contractual obligations to register the shares following the first anniversary of our initial public offering in July 2005. On September 15, 2006, we filed a prospectus supplement to our prospectus in our registration statement onForm S-3, with respect to the offer and sale of 3,000,000 shares of common stock, and an additional 450,000 shares of common stock sold solely to cover over-allotments, held by SZ Investments, L.L.C. and Perseus-Soros BioPharmaceutical Fund, L.P.
We have registered approximately 3,243,310 and 2,737,802 shares of common stock that are authorized for issuance under our 1999 Long-Term Incentive Plan and 2005 Incentive Plan, respectively. After the completion of our initial public offering in July 2005, our board of directors decided not to grant any additional awards pursuant to the 1999 Long-Term Incentive Plan. As of August 27, 2007, 2,452,335 shares were subject to outstanding options, 972,765 of which are vested and exercisable. As of August 27, 2007, 125,169 restricted stock units and 251,301 performance shares were issued and outstanding, none of which are vested and exercisable. Because they are registered, the shares authorized for issuance under our stock plans can be freely sold in the public market upon issuance, subject to the restrictions imposed on our affiliates under Rule 144.
Our executive officers, directors and major stockholders have the ability to control all matters submitted to stockholders for approval.
Our executive officers, directors and stockholders who own more than 5% of our outstanding common stock, in the aggregate, currently beneficially own shares representing approximately 52.25% of our capital stock. As a result, if these stockholders choose to act together, they would have significant influence over all matters submitted to our stockholders for approval, as well as our management and affairs. This concentration of voting power could delay or prevent an acquisition of our company on terms that other stockholders may desire.
Provisions in our certificate of incorporation and under Delaware law may prevent or frustrate attempts by our stockholders to change our management and hinder efforts to acquire a controlling interest in us.
Provisions of our certificate of incorporation and bylaws may discourage, delay or prevent a merger, acquisition or other change in control that stockholders may consider favorable, including transactions in which investors might otherwise receive a premium for their shares. These provisions may also prevent or frustrate attempts by our stockholders to replace or remove our management. These provisions include:
| | |
| | • | a classified board of directors; |
| |
| | • | limitations on the removal of directors; |
37
| | |
| | • | advance notice requirements for stockholder proposals and nominations; |
| |
| | • | the inability of stockholders to act by written consent or to call special meetings; and |
| |
| | • | the ability of our board of directors to designate the terms of and issue new series of preferred stock without stockholder approval. |
The affirmative vote of the holders of at least 662/3% of our shares of capital stock entitled to vote is necessary to amend or repeal the above provisions of our certificate of incorporation. In addition, absent approval of our board of directors, our bylaws may only be amended or repealed by the affirmative vote of the holders of at least 662/3% of our shares of capital stock entitled to vote.
In addition, Section 203 of the Delaware General Corporation Law prohibits a publicly held Delaware corporation from engaging in a business combination with an interested stockholder, generally a person who, together with its affiliates, owns or within the last three years has owned 15% of our voting stock, for a period of three years after the date of the transaction in which the person became an interested stockholder, unless the business combination is approved in a prescribed manner. Accordingly, Section 203 may discourage, delay or prevent a change in control of our company.
Other than the $45 million cash dividend declared by our board of directors on June 2, 2005, we have not paid cash dividends and do not expect to pay dividends in the future, which means that our stockholders may not be able to realize the value of our shares except through sale.
Other than the $45 million cash dividend declared by our board of directors on June 2, 2005, we have never declared or paid cash dividends. We expect to retain earnings for our business and do not anticipate paying dividends on our common stock at any time in the foreseeable future. Because we do not anticipate paying dividends in the future, a sale of shares likely is the only opportunity our stockholders will have to realize the value of our common stock. Our board of directors will decide whether to pay dividends on common stock from time to time in the exercise of its business judgment, subject to contractual restrictions.
| |
| Item 1B. | Unresolved Staff Comments |
None.
Our corporate headquarters, comprising approximately 40,000 square feet, is located at 4 and 6 Mill Ridge Lane in Chester, New Jersey. We operate our corporate headquarters under two long-term leases. Our lease for the property located at 4 Mill Ridge Lane expires March 31, 2018, and our rent for this property totals $816,000 per year until March 31, 2009. In April 2009, our rent increases to $872,000 per year until March 31, 2012, increases in April 2012 to $920,000 per year until March 31, 2015 and, in April 2015, increases to $1.0 million until March 31, 2018. Our lease for the property located at 6 Mill Ridge Lane expires in September 2018. Our rent for this property totals $238,000 per year for the first two years of the lease term and subsequently increases by $8,000 per year during the remaining term of the lease.
We also continue to lease the building located at our former corporate headquarters in Chester, New Jersey. The lease for our former corporate headquarters expires in August 2014, and our annual rent equals approximately $410,850 and increases each year. We have sublet a portion of our former corporate headquarters, and we are evaluating our options for the remainder of that facility.
We lease approximately 130,000 square feet in Fort Worth, Texas to house our manufacturing operations for our adult oral-solid Mucinex brand products. The Fort Worth facility also serves as the center of our development and customer service operations. Our lease agreement for our Fort Worth, Texas property expires March 31, 2011. Under the Fort Worth, Texas lease, we pay approximately $37,500 per month (plus expenses) until March 31, 2008, when the monthly rent will increase to approximately $39,500 per month (plus expenses).
38
We also lease approximately 132,000 square feet of warehouse space in Irving, Texas. Our lease for the Irving facility expires September 30, 2012, and the lease payments for this facility, which begin on October 15, 2007, total $38,837 per month (plus expenses).
| |
| Item 3. | Legal Proceedings |
On October 4, 2006, we filed a complaint against Mutual in the United States District Court for the District of Pennsylvania, Civ. Act.No. 2:06-cv-04418-PD, asserting that Mutual’s proposed generic products would infringe our U.S. Patent No. 6,372,252, or the ‘252 Patent.
On March 21, 2007, we entered into a settlement agreement with Mutual, under which we agreed with Mutual to dismiss without prejudice all patent infringement claims and all counterclaims in the Pennsylvania action. Under the terms of the settlement agreement, Mutual admitted that the ‘252 Patent is valid and enforceable, and that the single-ingredient and combination extended-release guaifenesin-based products set forth in the ANDA filed by Mutual with the FDA infringe the ‘252 Patent. Under the settlement agreement, we granted Mutual a non-exclusive, royalty-free license under the ‘252 Patent to sell the Company’s 600 mg and 1200 mg single-ingredient and combination extended-release guaifenesin products in the United States, subject to the conditions outlined below.
If Mutual receives FDA approval to market the 600 mg single-ingredient, extended-release guaifenesin products, we agreed to grant Mutual a license to sell such products beginning no earlier than July 1, 2012, with the following exceptions: (i) if Mutual has final FDA approval for the 600 mg product, and prior to July 1, 2012, a third party files an ANDA, certifies against and successfully challenges our patents, and gains FDA approval for the 600 mg product, Mutual may begin selling its product 60 days prior to the date of first sale by the third party; or (ii) if Mutual has not received FDA approval to market the 600 mg product at the time the third party commences sale of an FDA-approved 600 mg product, Mutual may enter into a supply agreement with us to purchase tablets at full cost plus a 10% royalty, based on Mutual’s net sales of the licensed product; and Mutual may begin selling such product 90 days after the first sale by the third party.
If Mutual receives FDA approval to market the 1200 mg single-ingredient, extended-release guaifenesin product or the 600 mg or 1200 mg combination extended-release guaifenesin products, which we refer to as the combination products, the Company will grant Mutual a license to sell such products, upon the occurrence of the following events: (i) if Mutual has final FDA approval for the 1200 mg single-ingredient product or a combination product, and a third party files an ANDA, certifies against and successfully challenges our patents, and gains FDA approval for the 1200 mg single-ingredient product or a combination product, as the case may be, Mutual may begin selling its product 60 days prior to the date of first sale by the third party; or (ii) if Mutual has not received FDA approval to market the 1200 mg single-ingredient product or a combination product, as the case may be, at the time the third party commences sale of such an FDA-approved product, Mutual may enter into a supply agreement with us to purchase tablets at full cost plus a 10% royalty, based on Mutual’s net sales of the licensed product; and Mutual may begin selling product 90 days after the first sale by the third party.
The settlement agreement is subject to review by the Federal Trade Commission and the U.S. Department of Justice. For a further discussion of the lawsuits previously filed by us against Mutual, see Part II. Item 1. Legal Proceedings in our Quarterly Reports onForm 10-Q for the periods ended September 30, 2006 and December 31, 2006.
We are also a party to various claims and suits arising out of matters occurring in the normal course of business. However, we do not believe that any of these other proceedings are material.
| |
| Item 4. | Submission of Matters to a Vote of Security Holders |
No matters were submitted to a vote of security holders during the fourth quarter of fiscal 2007.
39
| |
| Item 5. | Market For Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities |
Market Information and Holders
Our common stock has traded on the NASDAQ Global Select Market, or NASDAQ, under the symbol “ARxT” since July 3, 2006. Prior to the creation of the NASDAQ Global Select Market, our common stock traded on the NASDAQ National Market. Our common stock began trading on the NASDAQ National Market on July 21, 2005 upon our initial public offering. Prior to our initial public offering, no established trading market existed for our common stock. Accordingly, no sales price information is available for our common stock for the periods prior to July 21, 2005. The following tables set forth the quarterly high and low closing sales prices of our common stock for the periods indicated, as reported by NASDAQ.
| | | | | | | | | | | |
Fiscal 2007 | | | | High Close | | | Low Close | |
| |
| First Quarter | | July 1- September 30, 2006 | | $ | 45.10 | | | $ | 36.59 | |
| Second Quarter | | October 1- December 31, 2006 | | $ | 43.93 | | | $ | 35.16 | |
| Third Quarter | | January 1- March 31, 2007 | | $ | 46.27 | | | $ | 28.22 | |
| Fourth Quarter | | April 1- June 30, 2007 | | $ | 45.95 | | | $ | 33.44 | |
| | | | | | | | | | | |
Fiscal 2006 | | | | High Close | | | Low Close | |
| |
| First Quarter | | July 21- September 30, 2005 | | $ | 34.32 | | | $ | 25.75 | |
| Second Quarter | | October 1- December 31, 2005 | | $ | 48.54 | | | $ | 32.95 | |
| Third Quarter | | January 1- March 31, 2006 | | $ | 46.05 | | | $ | 35.13 | |
| Fourth Quarter | | April 1- June 30, 2006 | | $ | 49.05 | | | $ | 38.97 | |
As of August 28, 2007, we had 72 stockholders of record.
Dividends
We have not declared or paid cash dividends on our common stock in the last two fiscal years. We currently intend to retain all future earnings to finance our development efforts, the development of our proprietary technologies, the in-licensing or acquisition of specialty pharmaceutical products and trademarks, and the expansion of our business. We do not intend to declare or pay cash dividends on our capital stock in the foreseeable future. Any future determination to pay dividends will be at the discretion of our board of directors and will depend upon our results of operations, financial condition, contractual restrictions, restrictions imposed by applicable law, and other factors our board of directors deems relevant. Additionally, our senior secured revolving credit facility, or the Credit Facility, restricts our ability to pay dividends. The terms of the Credit Facility do not permit us to pay dividends other than (i) dividends payable only in our common stock, for so long as no event of default exists under the Credit Facility and (ii) dividends from time to time in an amount not to exceed (A) 50% of our cumulative positive consolidated net income for the period from July 1, 2006 to the date of the end of the most recent fiscal quarter prior to which the dividends are proposed to be made, plus (B) 100% of the net proceeds of any issuance of stock not used to make an acquisition, so long as we are in pro forma compliance with the financial covenants of, and no event of default exists or would occur under, the Credit Facility after giving effect to the dividends. The Credit Facility is more fully described in Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations — Liquidity and Capital Resources in this Annual Report on Form 10-K.
40
Securities Authorized for Issuance Under Equity Compensation Plans
We maintain a 1999 Long-Term Incentive Plan and a 2005 Incentive Plan adopted by our board of directors and approved by our stockholders prior to our initial public offering. We do not have any equity compensation plans that have not been approved by our stockholders. The following table sets forth, as of June 30, 2007, information with respect to our equity compensation plans.
| | | | | | | | | | | | | |
| | | | | | | | | Number of
| |
| | | | | | | | | Securities
| |
| | | Number of
| | | Weighted-
| | | Remaining Available
| |
| | | Securities to be
| | | Average Exercise
| | | for Future Issuance
| |
| | | Issued Upon
| | | Price of
| | | Under Equity
| |
| | | Exercise of
| | | Outstanding
| | | Compensation Plans
| |
| | | Outstanding
| | | Options,
| | | (Excluding
| |
| | | Options, Warrants
| | | Warrants and
| | | Securities Reflected
| |
Plan Category | | and Rights | | | Rights | | | in Second Column) | |
| |
| Equity compensation plans approved by security holders | | | 2,430,725 | | | $ | 12.94 | | | | 1,454,881 | (1) |
| | | | | | | | | | | | | |
| Equity compensation plans not approved by security holders | | | — | | | | — | | | | — | |
| | | | | | | | | | | | | |
| Total | | | 2,430,725 | | | $ | 12.94 | | | | 1,454,881 | |
| | | | | | | | | | | | | |
| | |
| (1) | | Securities available for issuance under the 2005 Incentive Plan. No awards are available for issuance under the 1999 Long-Term Incentive Plan. |
Use of Proceeds from Registered Securities
We have used all of the proceeds from our initial public offering in July 2005.
Recent Sales of Unregistered Securities
The following information relates to all securities issued or sold by us in the fiscal year ended June 30, 2007 that were not registered under the Securities Act.
On December 18, 2006, upon the exercise of warrants, we issued an aggregate of 1,019 shares of common stock with a weighted average exercise price of $0.03.
On January 15, 2006, we issued 147,058 unregistered shares of our common stock to JMED in connection with JMED’s conversion of its royalty interest in the AlleRxtm product. JMED had previously earned royalty payments from us under an April 1999 sublicense agreement that gave us an exclusive license to manufacture and market AlleRxtm. We subsequently assigned the AlleRxtm license agreement to a third party, and we now receive the AlleRxtm royalties. See Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations — AlleRxtm Royalty Interest in this Annual Report on Form 10-K for a further discussion of the terms of JMED’s conversion of its royalty interest into our common stock.
No underwriters were involved in the foregoing sales of securities. The share issuances described above were issued to U.S. investors in reliance upon the exemption from the registration requirements of the Securities Act, as set forth in Section 4(2) under the Securities Act and Rule 506 of Regulation D promulgated thereunder, related to sales by an issuer not involving any public offering, to the extent an exemption from such registration was required.
41
Performance Graph
The following performance graph and related information shall not be deemed “soliciting material” or to be “filed” with the Securities and Exchange Commission, nor shall such information be incorporated by reference into any future filing under the Securities Act of 1933 or Securities Exchange Act of 1934, each as amended, except to the extent that the Company specifically incorporates it by reference into such filing.
The following graph shows a comparison of cumulative total returns for our common stock, the NASDAQ Stock Market (U.S. Companies) Index and the NASDAQ Pharmaceutical Index for the period from July 21, 2005, the date our common stock began to trade on NASDAQ, through June 30, 2007.
Total stockholder return assumes that an investment of $100 was made on July 21, 2005 in our common stock, the NASDAQ Stock Market (U.S. Companies) Index and the NASDAQ Pharmaceutical Index, with reinvestment of any dividends. We did not pay any dividends during the period indicated.
The comparison in the graph below is based on historical data and is not necessarily indicative of future performance of the Company’s common stock.
| | | | | | | | | | | | | | | | | | | | | |
| | | 07/21/05 | | | 12/30/05 | | | 06/30/06 | | | 12/29/06 | | | 06/29/07 | |
| |
| Adams Respiratory Therapeutics, Inc. | | | 100.0 | | | | 157.9 | | | | 173.3 | | | | 158.5 | | | | 153.0 | |
| NASDAQ Stock Market (U.S. Companies) Index | | | 100.0 | | | | 101.4 | | | | 100.3 | | | | 111.4 | | | | 119.5 | |
| NASDAQ Pharmaceutical Index | | | 100.0 | | | | 103.6 | | | | 95.2 | | | | 101.4 | | | | 103.6 | |
42
| |
| Item 6. | Selected Financial Data |
The following table presents our selected financial information, which you should read in conjunction with, and is qualified in its entirety by reference to, our historical financial statements, the notes to those statements and Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations in this Annual Report on Form 10-K. The selected financial information set forth below as of June 30, 2007 and 2006 and for the years ended June 30, 2007, 2006 and 2005 has been derived from our audited financial statements included herein. The selected financial information as of June 30, 2005, 2004 and 2003 and for the years ended June 30, 2004 and 2003 have been derived from our audited financial statements, which are not included in this annual report.
| | | | | | | | | | | | | | | | | | | | | |
| | | Years Ended June 30, | |
| | | 2007 | | | 2006 | | | 2005 | | | 2004 | | | 2003 | |
| | | | | | (In thousands, except per share amounts) | | | | |
| |
Statements of Operations Data | | | | | | | | | | | | | | | | | | | | |
| Net sales | | $ | 331,603 | | | $ | 239,105 | | | $ | 160,210 | | | $ | 61,295 | | | $ | 14,038 | |
| Cost of goods sold | | | 95,799 | | | | 49,358 | | | | 31,126 | | | | 11,928 | | | | 5,252 | |
| | | | | | | | | | | | | | | | | | | | | |
| Gross margin | | | 235,804 | | | | 189,747 | | | | 129,084 | | | | 49,367 | | | | 8,786 | |
| | | | | | | | | | | | | | | | | | | | | |
| Selling, marketing and administrative(1) | | | 162,861 | | | | 98,998 | | | | 78,044 | | | | 23,286 | | | | 23,310 | |
| Product development | | | 23,855 | | | | 18,904 | | | | 7,392 | | | | 3,181 | | | | 4,542 | |
AlleRxtm charge | | | 2,699 | | | | — | | | | — | | | | — | | | | — | |
| Other, net | | | (1,187 | ) | | | (4,307 | ) | | | (789 | ) | | | 3,198 | | | | 3,572 | |
| | | | | | | | | | | | | | | | | | | | | |
| Income/(loss) before income taxes | | | 47,576 | | | | 76,152 | | | | 44,437 | | | | 19,702 | | | | (22,638 | ) |
| Provision for/ (benefit from) for income taxes | | | 17,047 | | | | 29,801 | | | | 17,438 | | | | (16,124 | ) | | | — | |
| | | | | | | | | | | | | | | | | | | | | |
| Net income/ (loss) | | | 30,529 | | | | 46,351 | | | | 26,999 | | | | 35,826 | | | | (22,638 | ) |
| Accretion of preferred stock | | | — | | | | — | | | | (202,566 | ) | | | (28,100 | ) | | | 8,204 | |
| Dividend paid to preferred stockholders | | | — | | | | — | | | | (30,033 | ) | | | — | | | | — | |
| | | | | | | | | | | | | | | | | | | | | |
| Net income/ (loss) applicable to common stockholders | | $ | 30,529 | | | $ | 46,351 | | | $ | (205,600 | ) | | $ | 7,726 | | | $ | (14,434 | ) |
| | | | | | | | | | | | | | | | | | | | | |
| Income/ (loss) per common share Basic | | $ | 0.86 | | | $ | 1.42 | | | $ | (32.97 | ) | | $ | 1.64 | | | $ | (4.66 | ) |
| | | | | | | | | | | | | | | | | | | | | |
| Diluted | | $ | 0.82 | | | $ | 1.28 | | | $ | (32.97 | ) | | $ | 0.90 | | | $ | (4.66 | ) |
| | | | | | | | | | | | | | | | | | | | | |
| | |
| (1) | | Selling, marketing and administrative expenses includes non-cash stock-based compensation expense as follows: |
| | | | | | | | | | | | | | | | | | | | | |
| | | Years Ended June 30, | |
| | | 2007 | | | 2006 | | | 2005 | | | 2004 | | | 2003 | |
| | | (In thousands) | |
| |
| | | $ | 4,378 | | | $ | 4,134 | | | $ | 477 | | | $ | 686 | | | $ | 880 | |
| | | | | | | | | | | | | | | | | | | | | |
43
The following table contains summary balance sheet data:
| | | | | | | | | | | | | | | | | | | | | |
| | | At June 30, | |
| | | 2007 | | | 2006 | | | 2005 | | | 2004 | | | 2003 | |
| | | (In thousands) | |
| |
Balance Sheet Data | | | | | | | | | | | | | | | | | | | | |
| Cash and cash equivalents | | $ | 46,809 | | | $ | 40,320 | | | $ | 28,024 | | | $ | 44,422 | | | $ | 9,405 | |
| Working capital | | | 124,928 | | | | 72,870 | | | | 34,787 | | | | 47,360 | | | | 5,538 | |
| Total assets | | | 318,465 | | | | 258,095 | | | | 67,209 | | | | 72,066 | | | | 20,382 | |
| Long-term liabilities | | | 706 | | | | 2,010 | | | | 2,281 | | | | 2,731 | | | | 14,873 | |
| Redeemable convertible preferred stock | | | — | | | | — | | | | 316,455 | | | | 110,851 | | | | 60,647 | |
| Accumulated deficit | | | (212,313 | ) | | | (242,842 | ) | | | (289,193 | ) | | | (68,626 | ) | | | (76,352 | ) |
| Total stockholders’ equity/(deficit) | | | 275,348 | | | | 222,273 | | | | (273,625 | ) | | | (56,997 | ) | | | (64,169 | ) |
44
| |
| Item 7. | Management’s Discussion and Analysis of Financial Condition and Results of Operations |
You should read the following discussion and analysis of our financial condition and results of operations together with our financial statements and related notes appearing elsewhere in this Annual Report onForm 10-K. Some of the information contained in this discussion and analysis or set forth elsewhere in this Annual Report onForm 10-K, including information with respect to our plans and strategy for our business and related financing, includes forward-looking statements that involve risks and uncertainties. You should review Item 1A. Risk Factors of this Annual Report onForm 10-K for a discussion of important factors that could cause our actual results to differ materially from the results described in or implied by the forward-looking statements contained in the following discussion and analysis.
Overview
We are a specialty pharmaceutical company focused on the late-stage development, commercialization and marketing of OTC and prescription pharmaceuticals for the treatment of respiratory disorders. We currently market six oral-solid OTC products for adults under our Mucinex brand, six products in our Mucinex products for children line, two Mucinex nasal spray products, and two products under our Delsym brand.
Products
Mucinex SE. Mucinex SE is a long-acting, single-ingredient guaifenesin OTC product and, along with its maximum strength version, is the only single-ingredient, long-acting guaifenesin product approved by the FDA. The FDA approved Mucinex SE in July 2002.
Mucinex DM. Mucinex DM is an OTC product containing long-acting guaifenesin and the cough suppressant dextromethorphan and, along with its maximum strength version, is the only FDA-approved, long-acting guaifenesin and dextromethorphan combination product. The FDA approved Mucinex DM in April 2004, and we launched Mucinex DM in August 2004.
Mucinex D. Mucinex D is an OTC product containing long-acting guaifenesin and the decongestant pseudoephedrine and, along with its maximum strength version, is the only FDA-approved, long-acting guaifenesin and pseudoephedrine combination product. The FDA approved Mucinex D in June 2004, and we began to market Mucinex D in October 2005.
Maximum Strength Mucinex. We have also received FDA approval for three maximum strength 1200 mg extended-release, guaifenesin-based OTC products, which we began marketing in July 2007. Maximum Strength Mucinex SE, a 1200 mg long-acting, single-ingredient guaifenesin OTC product, was approved in December 2002. Maximum Strength Mucinex DM, an OTC product containing 1200 mg of long-acting guaifenesin and dextromethorphan, was approved in April 2004. Maximum Strength Mucinex D, an OTC product containing 1200 mg of long-acting guaifenesin and pseudoephedrine, was approved in October 2005. Along with Mucinex SE, Mucinex DM and Mucinex D, these maximum strength products are the only long-acting guaifenesin-based products approved by the FDA.
Delsym. Delsym is a long-acting, single-ingredient OTC product containing dextromethorphan and is the only FDA-approved OTC liquid cough suppressant that can deliver 12 hours of cough relief in a single dose. We acquired Delsym from UCB in June 2006. We currently market two orange-flavored products in the Delsym line consisting of two adult SKUs and two children’s SKUs. In the first half of fiscal 2008, we plan to introduce a grape-flavored addition to our Delsym line that includes the same ingredients and dosing as our existing Delsym products.
Mucinex Products for Children. In August 2006, we began to market a line of four immediate-release guaifenesin products under the Mucinex brand for children, including two liquid products and two Mini-Melts products. In July 2007, we introduced two new Mucinex products for children: Mucinex Cough Mini-Melts in orange crème flavor, which combines guaifenesin with the cough suppressant dextromethorphan, and Mucinex Cold Liquid in mixed berry flavor, which combines guaifenesin with the decongestant phenylephrine for the treatment of nasal congestion and cough.
45
Mucinex Nasal Sprays. Mucinex Full Force Nasal Spray and Mucinex Moisture Smart Nasal Spray are long-acting OTC nasal decongestant sprays containing oxymetazoline HCl, which provides immediate topical relief from nasal congestion and lasts for 12 hours. We began to market Mucinex Full Force Nasal Spray in July 2007 and Mucinex Moisture Smart Nasal Spray in August 2007.
Other Products. Humibid SE is a 1200 mg long-acting, single-ingredient guaifenesin OTC product that we began to market in March 2006. We are currently evaluating the future commercialization of this product.
Future Products. In December 2006, we submitted a NDA to the FDA for Mucinex with Codeine, an oral-solid extended-release guaifenesin and codeine combination product for the prescription treatment of cough. In March 2007, we were notified by the FDA that our NDA had been accepted for filing and is under formal review by the FDA. The PDUFA date for this NDA is October 27, 2007, the date the FDA has set as a goal to review and act on the NDA submission. There is no guarantee that final marketing approval for this product will be granted by the PDUFA date. If approved by the FDA, this will be the first prescription product in our current portfolio of respiratory products.
Revenue Growth
Our net revenues have grown to $331.6 million for the fiscal year ended June 30, 2007 from $239.1 million and $160.2 million for the fiscal years ended June 30, 2006 and 2005, respectively. Our revenue growth has been primarily driven by sales from the Delsym product line, which we acquired in June 2006, as well as increased sales of our Mucinex products. We believe that our new consumer advertising campaign for Delsym substantially contributed to Delsym’s revenue growth during fiscal 2007. The key factors underlying the growth of revenues for our Mucinex and Delsym products included:
| | |
| | • | Consumer advertising campaigns. Prior to the FDA’s approval of Mucinex SE as an OTC drug, long-acting single-ingredient guaifenesin and long-acting guaifenesin combination products were available only by prescription. We launched our initial Mucinex and Delsym consumer advertising campaigns in November 2004 and 2006, respectively, and our strategy has been to educate consumers about the unique benefits of Mucinex and Delsym to encourage trial of our products. |
| |
| | • | Our professional marketing efforts to physicians, pharmacists and other healthcare professionals. Our professional sales force targets high prescribers of long-acting guaifenesin products and encourages them to recommend Mucinex SE, Mucinex DM and Mucinex D to their patients. Our professional sales force also educates physicians, pharmacists and other healthcare professionals about the benefits of long-acting guaifenesin. Since December 2004, we have expanded our sales force from 50 to 125 representatives. |
| |
| | • | Expansion of our trade sales department and trade development efforts. Our trade sales force calls on national and regional retail accounts and wholesale distribution companies. The primary focus of our trade sales force is to maximize our shelf presence at retail drug, food and mass merchandise stores to support the efforts of our professional sales representatives and consumer advertising campaigns. Since December 2003, we have grown our trade sales force from one to 12 professionals. |
| |
| | • | The FDA’s removal of competitive long-acting, single-ingredient guaifenesin products after November 2003. The FDA’s removal from the market of all other long-acting single-ingredient guaifenesin products resulted in Mucinex SE’s status as the only long-acting, single-ingredient guaifenesin product available in the United States. Based on data from IMS Health — NPAtm, we estimate that, for the 12 months ended June 30, 2003, approximately 10.5 million prescriptions were filled for long-acting, single-ingredient guaifenesin products. After November 2003, we believe that a majority of prescriptions written for long-acting, single-ingredient guaifenesin resulted in OTC sales of our Mucinex SE. |
Results of Operations
During the fiscal years ended June 30, 2007, 2006 and 2005, we reported net income of $30.5 million, $46.4 million and $27.0 million, respectively.
46
Seasonality
We expect retail demand for our products to be higher between October 1 and March 31 due to the prevalence of cough, cold and flu. As a result, our shipments, and therefore revenues, are expected to be higher between July 1 and March 31 to support the retail demand through that season. We generally expect our revenues during the quarter ending June 30 to be lower than the other quarters.
Future Growth
We believe that our future growth will be driven by professional and consumer marketing efforts to create increased awareness of the Mucinex and Delsym brands and the benefits of long-acting guaifenesin, as well as new product launches, including Maximum Strength Mucinex, our expanded line of immediate-release guaifenesin products for children, our new nasal sprays, and our new Delsym flavor. In addition, we believe that the FDA’s recent action to remove from the market all other unapproved timed-release guaifenesin combination products will have a beneficial impact to our business. In May 2007, the FDA took action to remove from the market all unapproved timed-release guaifenesin combination products, similar to Mucinex DM and Mucinex D. The FDA has ordered the companies marketing these unapproved products to stop manufacturing them within 90 days of the FDA’s enforcement action and stop shipping them in interstate commerce within 180 days.
We plan to continue to invest in the commercialization of our current products and the development of our pipeline products, as well as to pursue in-licensing or acquisitions of new product candidates. Our future profitability is dependent upon the successful commercialization of our current Mucinex and Delsym products, as well as the introduction of new products.
Critical Accounting Policies and Estimates
Our consolidated financial statements are presented on the basis of U.S. generally accepted accounting principles. We have taken into consideration all professional accounting standards that are effective as of the date of these financial statements. Included within these policies are our “critical accounting policies.” Critical accounting policies are those policies that are most important to the preparation of our financial statements and require management’s most subjective and complex judgments due to the need to make estimates about matters that are inherently uncertain. Although we believe that our estimates and assumptions are reasonable, actual results may differ significantly from these estimates. Changes in estimates and assumptions based upon actual results may have a material impact on our results of operations and financial condition.
While our critical accounting policies are more fully described in Note 1 to our consolidated financial statements contained in this Annual Report onForm 10-K, we believe that the following accounting policies relating to revenue recognition, sales returns and allowances, Cardinal Health profit share, stock-based compensation charges, income taxes, and accretion of preferred stock are most critical in fully understanding and evaluating our reported financial results.
Revenue Recognition. We recognize product sales when the product is delivered to our customers, estimated provisions for product returns, rebates, chargebacks, cash discounts, trade promotions and other sales allowances are reasonably determinable and collectability is reasonably assured. Accruals for these estimated provisions are presented in our consolidated financial statements as reductions to sales. At times, we may provide extended payment terms for initial orders of new products we launch. Such special dating terms do not affect the Company’s ability to determine collectibility.
Sales Returns and Allowances. When we sell our products, we reduce the amount of revenue recognized from sales by an estimate of future product returns and other sales allowances. Other sales allowances include cash discounts, rebates including Medicaid rebates, chargebacks, and trade promotions relating to products sold in the current period. Factors that we consider in our estimates of future product returns include an estimate of the amount of product in the trade channel, competitive products, the remaining time to expiration of the product, the historical rate of returns, as well as industry trends and regulatory developments. Consistent with industry practice, we maintain a return policy that allows our customers to return product within a
47
specified period prior to and subsequent to the expiration date. Factors that we consider in our estimates regarding other sales allowances include historical payment experience in relationship to revenues, estimated customer inventory levels and current contract prices and terms with both direct and indirect customers. If actual future payments for product returns and other sales allowances exceed the estimates we made at the time of sale, our financial position, results of operations and cash flow would be negatively impacted.
The following table shows, at each balance sheet date, the balances of liabilities and accounts receivable valuation accounts resulting from sales returns and allowances:
| | | | | | | | | |
| | | June 30, | |
| | | 2007 | | | 2006 | |
| | | (In thousands) | |
| |
| Product returns | | $ | 6,152 | | | $ | 3,150 | |
| Chargebacks | | | 577 | | | | 941 | |
| Rebates and other | | | 1,205 | | | | 1,610 | |
| | | | | | | | | |
| Accrued returns, chargebacks, rebates and other(1) | | $ | 7,934 | | | $ | 5,701 | |
| | | | | | | | | |
| Cash discounts(2) | | $ | 336 | | | $ | 495 | |
| Trade promotions(2) | | $ | 2,830 | | | $ | 2,796 | |
| | |
| (1) | | Accrued returns, chargebacks, rebates and other sales allowances are reported under current liabilities on the consolidated balance sheets. |
| |
| (2) | | Cash discounts and trade promotions are reported as valuation allowances against accounts receivable on the consolidated balance sheets. |
The following table summarizes the activity of accrued returns, chargebacks and other sales allowances:
| | | | | | | | | | | | | | | | | |
| | | | | | | | | Rebates and
| | | Total Accrued
| |
| | | | | | | | | Other Sales
| | | Returns and Other
| |
| | | Returns | | | Chargebacks | | | Allowances | | | Sales Allowances | |
| | | (In thousands) | |
| |
| Balance at June 30, 2004 | | $ | 3,622 | | | $ | 2,332 | | | $ | 1,000 | | | $ | 6,954 | |
| Assumed liability for Humibid returns | | | 3,000 | | | | — | | | | — | | | | 3,000 | |
| Provision made to sales during the period | | | 1,054 | | | | 3,443 | | | | 3,205 | | | | 7,702 | |
| Provision/(benefit) related to sales made during prior periods | | | 649 | | | | (635 | ) | | | (410 | ) | | | (396 | ) |
| Payments/credits | | | (2,441 | ) | | | (2,667 | ) | | | (2,205 | ) | | | (7,313 | ) |
| | | | | | | | | | | | | | | | | |
| Balance at June 30, 2005 | | | 5,884 | | | | 2,473 | | | | 1,590 | | | | 9,947 | |
| Provision made to sales during the period | | | 2,643 | | | | 3,028 | | | | 3,212 | | | | 8,883 | |
| Benefit related to sales made during prior periods | | | (188 | ) | | | (2,226 | ) | | | (365 | ) | | | (2,779 | ) |
| Payments/credits | | | (5,189 | ) | | | (2,334 | ) | | | (2,827 | ) | | | (10,350 | ) |
| | | | | | | | | | | | | | | | | |
| Balance at June 30, 2006 | | | 3,150 | | | | 941 | | | | 1,610 | | | | 5,701 | |
| Provision made to sales during the period | | | 9,749 | | | | 2,402 | | | | 2,840 | | | | 14,991 | |
| Benefit related to sales made during prior periods | | | (1,012 | ) | | | — | | | | (731 | ) | | | (1,743 | ) |
| Payments/credits | | | (5,735 | ) | | | (2,766 | ) | | | (2,514 | ) | | | (11,015 | ) |
| | | | | | | | | | | | | | | | | |
| Balance at June 30, 2007 | | $ | 6,152 | | | $ | 577 | | | $ | 1,205 | | | $ | 7,934 | |
| | | | | | | | | | | | | | | | | |
48
Product Returns. The expiration period for our products generally ranges between 23 and 35 months, and it is our policy to accept returns for expired products up to 12 months after the expiration date. As of June 30, 2007, our product returns liability included the remaining liability of $0.8 million for certain formulations of Humibid that were manufactured prior to our acquisition of Humibid from Cornerstone in February 2005. As of June 30, 2006, our product returns liability included $2.1 million for products and formulations that we did not market at that time (AlleRxtm, Aquatab and certain formulations of Humibid). In connection with our acquisition of the Humibid trademark from Cornerstone, we assumed an estimated liability of $3.0 million for product returns of Humibid sold by Cornerstone prior to the acquisition. Included in the $2.1 million liability for products that we did not market at June 30, 2006 was approximately $31,000 associated with the first $1.0 million of returned AlleRxtm products that we were contractually obligated to assume during the18-month period from February 15, 2005 through August 15, 2006 in accordance with the settlement agreement with Cornerstone, as discussed under Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations — Commitments and Contractual Obligations in this Annual Report onForm 10-K. Our accrued liability for returns of products and formulations that we did not market as of June 30, 2006 reflected over 90% of Aquatab and Cornerstone’s Humibid products in the sales channel.
For products that we marketed as of June 30, 2007 (Mucinex SE, Mucinex DM, Mucinex D, Mucinex products for children, Humibid SE, and Delsym), our liability for product returns was approximately $5.4 million. Our estimates for returns of currently marketed products are based upon retail and non-retail sales data as reported by IRI and IMS Health-National Sales Perspectivestm, our estimates of the amount of product in the sales channel, historical and recent returns activity. The IRI data provides weekly retail unit sales by SKUs on a national basis. IMS Health-National Sales Perspectivestm data provides aggregate weekly prescriptions by SKU. Our estimates for returns of currently marketed products are generally significantly lower than for products and formulations that we currently do not market, with the exception of our returns liability for Humibid and our Mucinex products for children, discussed below. Based upon internal records and industry data, we believe that approximately $72.7 million of Mucinex SE, Mucinex DM, Mucinex D, Mucinex products for children, Humibid SE and Delsym exists in the sales channel at June 30, 2007. The entire $72.7 million could potentially be returned by our customers to us for credit. However, based upon our analysis of the above factors, we estimate that approximately $67.3 million will ultimately be purchased by consumers.
The $5.4 million returns liability for marketed products as of June 30, 2007 includes $3.9 million relating to Mucinex products for children, resulting in an estimated return rate of 36% for Mucinex products for children in the sales channel. We estimate that the rate of return will be higher for Mucinex products for children than for our adult oral-solid Mucinex products due to the $2.4 million liability associated with our April 2, 2007 voluntary recall of specific lots of Children’s Mucinex liquid products. Also contributing to the higher estimated return rate for Mucinex products for children is excess Mini-Melts inventory in the sales channel. The $5.4 million liability for marketed products also includes $0.9 million in estimated product returns for Humibid SE. We estimate that the return rate for Humibid SE to be between 90% and 100% of the products in the sales channel based on low consumption projections and our introduction of three Mucinex maximum strength products in July 2007.
Our remaining returns liability balance of $0.6 million for currently marketed products relates to our adult oral-solid Mucinex and Delsym products and results in an estimated return rate of 1% for these products in the sales channel. Based upon industry information, we believe that the product returns for marketed products similar to ours range between 1% and 3% of sales. Considering the historical low rate of returns for Mucinex SE, Mucinex DM, Mucinex D and Delsym, we believe that a range of 1% to 3% is a reasonable range for what may ultimately be returned. If our actual returns of currently marketed products vary from the 1% estimated return rate and result in either a 0% or 2% return rate, our income before taxes could increase or decrease by up to $0.6 million.
Chargebacks. Chargebacks represent the difference between our published list price per unit and the contractual prices under government contracts. Sales of our products to our wholesale customers are generally based on our published list price. Some of our wholesale customers sell our products to certain government agencies that are entitled to a discount of approximately 25% from our published list price. At the time we
49
sell our products to our wholesale customers, we estimate the amount that they will sell to their customers who are entitled to a discount pursuant to a government agency contract, resulting in a chargeback to us by our wholesale customers.
At June 30, 2007 and 2006, the Company’s chargeback liability did not increase in proportion to the increase in sales activity. We believe that our increase in sales volume has not been attributed to government purchases but has resulted from increased retail consumer demand, largely driven by our consumer advertising campaigns. Therefore, chargebacks have become a lower percentage of our total sales. During fiscal 2006, we recognized a benefit in the amount of $2.2 million from the reduction of our reserves for sales made during prior periods, due to the fact that chargebacks were a lower percentage of our total sales and were processed more quickly than originally estimated.
Our liability for chargebacks relies primarily on two estimates. The lag factor is the time between the date we ship product to our customer and the date the chargeback is presented to us for credit, which impacts the estimated percentage of sales that have historically been subject to chargebacks. We then apply this percentage to the estimated units in the sales channel to determine our current liability. The lag factor and percentage vary by customer and depend upon their individual buying and inventory holding patterns, which can fluctuate from quarter to quarter due to seasonality or other customer factors. We use historical information to determine the lag factor and percentage. If our estimates of the lag factor and percentage at June 30, 2007 varied by one month from actual future results, our income before income taxes would be impacted by approximately $13,000.
Rebates and Other Sales Allowances. We offer mail-in and point-of-sale rebates to retail consumers, Medicaid rebates to states covering our products under their Medicaid programs, and other sales allowances. The liability for rebates is based upon historical and current rebate redemption and utilization rates. For mail-in and point-of-sale rebates, we utilize third party processing companies. Such companies have experience in predicting rebate redemption rates based upon the value of the rebate in relation to the retail purchase price of the product. Other sales allowances include expected customer deductions for shortages and discrepancies, which amounts we do not believe are material. Considering the multitude of estimates made by us, as well as estimates prepared by third party companies that are necessary to evaluate the required liability balance for the various activities within rebates and other sales allowances, we believe that this liability balance could fluctuate by up to 20%. Therefore, if actual future results varied within a range of 20% from our June 30, 2007 balance of $1.2 million, our income before income taxes would be impacted by up to $0.2 million.
The following table summarizes the activity relating to cash discounts and trade promotions:
| | | | | | | | | |
| | | Reserve
| | | Reserve
| |
| | | for Cash
| | | for Trade
| |
| | | Discounts | | | Promotions | |
| | | (In thousands) | |
| |
| Balance at June 30, 2004 | | $ | 143 | | | $ | 988 | |
| Provision made to sales during the period | | | 3,441 | | | | 6,611 | |
| Provision/(benefit) related to sales made in prior periods | | | — | | | | (613 | ) |
| Deductions from reserves | | | (3,414 | ) | | | (4,878 | ) |
| | | | | | | | | |
| Balance at June 30, 2005 | | | 170 | | | | 2,108 | |
| Provision made to sales during the period | | | 5,196 | | | | 6,786 | |
| Provision/(benefit) related to sales made in prior periods | | | — | | | | 111 | |
| Deductions from reserves | | | (4,871 | ) | | | (6,209 | ) |
| | | | | | | | | |
| Balance at June 30, 2006 | | | 495 | | | | 2,796 | |
| Provision made to sales during the period | | | 7,144 | | | | 11,525 | |
| Provision/(benefit) related to sales made in prior periods | | | — | | | | (331 | ) |
| Deductions from reserves | | | (7,303 | ) | | | (11,160 | ) |
| | | | | | | | | |
| Balance at June 30, 2007 | | $ | 336 | | | $ | 2,830 | |
| | | | | | | | | |
50
Cash Discounts. We generally bill our accounts receivable on anet 30-day basis, with a 2% discount if paid within 30 days. Based upon historical experience, we estimate that customer cash discounts will approximate 2% of our current accounts receivable balance.
Trade Promotions. During fiscal year 2004, we began offering industry-standard trade promotion allowances to our trade customers. Currently, our trade promotion allowances approximate 3% of our published selling prices. Based upon our historical experience, we believe that this rate is appropriate for estimating the accrued liability for trade promotion allowances. As a result of the increase in sales volume during the fiscal year ended June 30, 2007, the valuation allowance against our accounts receivable balance has increased accordingly.
Cardinal Health Profit Share. In April 2004, we sold substantially all of our manufacturing assets, raw materials and in-process inventory located in Fort Worth, Texas to Cardinal Health. At the time of the 2004 asset sale, we also entered into a supply agreement with Cardinal Health, which we refer to as the 2004 Supply Agreement, pursuant to which Cardinal Health manufactured and supplied all of our drug products. Under the 2004 Supply Agreement, Cardinal Health was required to segregate direct manufacturing costs from indirect manufacturing costs. As finished goods were completed and shipped to a warehouse we designated, Cardinal Health billed us for the actual direct manufacturing costs incurred plus amark-up. Thismark-up was merely provided for interim billing and cash flow purposes and the actual amount payable to Cardinal Health was calculated at the end of each calendar quarter under a profit sharing formula. Profit for this purpose was calculated as net sales less the actual direct manufacturing cost of products sold during the calendar quarter, less freight and other logistics costs. The resulting profit was subject to profit sharing rates that declined as the total value of this profit increased, subject to a minimum profit share amount. At the end of each calendar quarter, a reconciliation was completed and a billing adjustment was made to the extent that the actual profit share differed from the totalmark-up paid to Cardinal Health during the calendar quarter.
The accounting policy with regard to this arrangement was to record the actual direct manufacturing cost and the effective profit share amount as inventory, as that equaled our final cost to purchase the inventory. The difference between the billedmark-up and the effective profit share amount was reflected on our consolidated balance sheet as a receivable from or payable to Cardinal Health.
Each month, as product was sold, the actual direct manufacturing cost plus an estimate of the profit share amount earned by Cardinal Health was charged to cost of goods sold. The estimated profit share amount considered for each contract year (i) our projected net product sales and gross profit, (ii) the projected profit share and (iii) the contractual minimum profit share amount.
Assuming our net sales per unit and the actual direct manufacturing cost per unit were constant during the contract year, an increase in unit sales would have resulted in a lower effective profit share amount per unit for the contract year. Conversely, if unit sales were lower than our initial estimates, the effective profit share per unit would have increased. At each contract year-end (March 31), a final reconciliation was performed and the estimates were adjusted to the actual results.
On July 31, 2006, we repurchased the Fort Worth, Texas manufacturing assets from Cardinal Health, and the profit share arrangement with Cardinal Health terminated. See Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations — Commitments and Contractual Obligations and Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations — Results of Operations in this Annual Report onForm 10-K for a description of our new arrangement with Cardinal Health, now known as Catalent.
51
The following activity has been recorded for the contract years ended March 31, 2006 and 2007, prior to the termination of the profit-share arrangement with Cardinal Health upon the manufacturing assets repurchase:
| | | | | | | | | |
| | | Contract Year Ended
| | | Contract Year Ended
| |
| | | March 31, 2006 | | | March 31, 2007 | |
| | | (In millions) | |
| |
| Total profit share payments to Cardinal Health | | $ | 17.6 | | | $ | 3.8 | |
| Profit share earned by Cardinal Health — FY 2004(1) | | | — | | | | — | |
| Profit share earned by Cardinal Health — FY 2005(1) | | | 1.9 | | | | — | |
| Profit share earned by Cardinal Health — FY 2006(1) | | | 8.3 | | | | 1.9 | |
| Profit share included in ending inventory(2) | | | 0.7 | | | | 1.5 | |
| | | | | | | | | |
| Excess profit share payments made to Cardinal Health | | | 6.7 | | | | 0.4 | |
| | | | | | | | | |
| Payments made by Cardinal Health | | | (6.7 | ) | | | — | |
| | | | | | | | | |
| Balance due from Cardinal Health June 30, 2006(3) | | $ | — | | | $ | 0.4 | |
| | | | | | | | | |
| | |
| (1) | | Earned profit share is included in cost of goods sold. For the 2007 contract year, the estimated effective profit share for the fourth quarter of fiscal 2006 was used to record the profit share earned. |
| |
| (2) | | Amounts included in ending inventory at March 31, 2006 for the 2006 contract year and at June 30, 2006 for the 2007 contract year. |
| |
| (3) | | Excess profit share payments due from Cardinal Health were included in prepaid expenses and other assets at June 30, 2006. This balance was eliminated in conjunction with the manufacturing assets repurchase. |
Stock-Based Compensation. In December 2004, the Financial Accounting Standards Board, or the FASB, issued Statement of Financial Accounting Standards, or SFAS, No. 123(R),Share-Based Payment, or SFAS No. 123(R), to expand and clarify SFAS No. 123,Accounting for Stock-Based Compensation,or SFAS No. 123, as amended by SFAS No. 148,Accounting for Stock-Based Compensation — Transition and Disclosure.SFAS No. 123(R) requires companies to recognize compensation expense in an amount equal to the fair value of all share-based payments granted to employees. Effective July 1, 2005, we adopted SFAS No. 123(R) and elected the prospective method for all future grants. Prior to our adoption of SFAS 123(R), we accounted for stock-based compensation in accordance with the fair value recognition provisions of SFAS No. 123. As a result, the adoption of SFAS No. 123(R) did not have a material impact on our operations, financial position or cash flows. We use the graded-vesting methodology to record compensation expense for stock options over the related vesting period, which generally ranges from three to five years. This methodology results in a greater amount of expense recognized towards the beginning of the vesting period as opposed to the straight-line method. For performance shares, stock compensation expense is recognized separately for each vesting tranche of the award, based on the closing price of our common stock on the date of grant, if the related financial targets are expected to be met. Because subjective input assumptions can materially affect the fair value estimate, in management’s opinion, the existing methods do not necessarily provide a reliable single measure of the fair value of our stock compensation arrangements.
Prior to fiscal 2006, we calculated our stock-based compensation using the minimum value method as permitted for nonpublic companies under SFAS No. 123. However, since filing our initial registration statement on March 25, 2005, we were no longer considered nonpublic and had to consider a volatility assumption in the calculation of fair value. Because we do not have sufficient history as a public company to support an estimate of future volatility, we use a combination of peer companies in our industry with similar business cycles. We use this volatility assumption on options granted after March 25, 2005. The addition of this assumption materially increased the fair value of subsequent option grants.
Income Taxes. Income taxes are accounted for in accordance with SFAS No. 109,Accounting for Income Taxes, or SFAS No. 109. Under this method, deferred tax assets and liabilities are recognized for the expected future tax consequences of temporary differences between the financial statement and tax basis of assets and liabilities using enacted tax rates in effect for the years in which the differences are expected to
52
reverse. Our net deferred tax assets relate primarily to sales and inventory reserves, nonqualified stock options, and net operating loss carryforwards, or NOLs. In assessing the realizability of deferred tax assets, we consider whether it is more likely than not that some portion or all of the deferred tax assets will not be realized. This assessment requires significant judgment and estimates. The ultimate realization of the deferred tax assets is dependent upon the generation of future taxable income during the period in which those temporary differences become deductible. We consider our history of losses, scheduled reversal of deferred tax assets and liabilities and projected future taxable income over the periods in which the deferred tax items are deductible. In addition, Internal Revenue Code Sections 382 and 383 contain provisions that may limit the NOL available to be used in any given year upon the occurrence of certain events, including significant changes in ownership interest.
In July 2006, the FASB issued FASB Interpretation No. 48,Accounting for Uncertainty in Income Taxes, or FIN 48, an interpretation of SFAS No. 109. FIN 48 provides measurement and recognition guidance related to accounting for uncertainty in income taxes by prescribing a recognition threshold for tax positions. FIN 48 also requires extensive disclosures about uncertainties in the income tax positions taken. We adopted FIN 48 on July 1, 2007, as required, and are currently evaluating the impact of FIN 48 on our financial statements. We are currently undergoing a federal tax audit for fiscal 2005, which may have an impact on our analysis. No material adjustments have been proposed by the Internal Revenue Service to date.
Inventories. Our inventories are stated at the lower of cost or market, using thefirst-in, first-out method. The manufacturing assets repurchase in July 2006 eliminated our profit share arrangement with Cardinal Health under the 2004 Supply Agreement. Upon the manufacturing assets repurchase, we began to manufacture our own adult oral-solid Mucinex products and to carry raw materials andwork-in-progress in our inventory, in addition to finished goods. Inventories are recorded net of reserves for obsolete inventory, which were $3.9 million and $24,000 as of June 30, 2007 and June 30, 2006, respectively. The reserves for obsolete inventory increased at June 30, 2007 due to an increase in Children’s Mini-Melts finished goods inventory approaching expiration and increased reserves relating to scrapped raw materials. Our reserves for inventory obsolescence are determined using estimates based on sales trends, historical experience and type and age of inventory. If actual conditions are less favorable than expected, additional inventory reserves may be required. However, we do not expect that this will have a material impact on our consolidated financial statements.
Accretion of Preferred Stock. Prior to the conversion to common stock upon the closing of our initial public offering in July 2005, we adjusted the carrying value of our Series A redeemable convertible preferred stock, or Series A Preferred Stock, our Series B redeemable convertible preferred stock, or Series B Preferred Stock, and our Series C redeemable convertible preferred stock, or Series C Preferred Stock, to redemption value. For Series A Preferred Stock and Series B Preferred Stock, redemption value equaled fair value. For Series C Preferred Stock, redemption value equaled the greater of 200% of the original per share purchase price or the fair value. All classes of preferred stock were redeemable at the option of the holder on specified dates. Accretion of Series C Preferred Stock up to liquidation value was recorded as a reduction of net income/(loss) applicable to common stockholders.
To the extent that the fair value was greater than the accreted liquidation value at the balance sheet date, the preferred stock was adjusted to reflect the fair market value, with the offset charged to accumulated deficit within stockholders’ equity/(deficit), and reflected a reduction of net income/(loss) applicable to common stockholders. Upon the closing of our initial public offering in July 2005, our preferred stock automatically converted into shares of common stock and, as a result, there was no further accretion.
Through July 2001, we incurred approximately $0.9 million and $1.2 million of issuance costs in connection with the issuance of Series A and B Preferred Stock, respectively. Such costs were recorded as a reduction of the carrying amount of the preferred stock and were accreted through a charge to accumulated deficit up to the original redemption date, using the effective interest method and included in net income/(loss) applicable to common stockholders. There were no issuance costs associated with the Series C Preferred Stock.
53
Operating Expenses
Product Development. Product development expenses have historically consisted of formulation, clinical and analytical development work with existing and well-established drugs and pharmaceutical ingredients. These products required development ofscale-up manufacturing, stability data and human pharmacokinetic studies to establish bioavailability and bioequivalence data for our products versus FDA reference drugs. The data from these efforts was used in the preparation and filing of NDAs.
Generally, our formulation, chemistry and analytical manufacturing controls and stability work has been performed utilizing our own employees and from April 2004, in cooperation with Cardinal Health. Upon our acquisition of Delsym in June 2006, we also began to perform development work in cooperation with UCB. Product development expenses include salary and benefits, raw materials and supplies, facilities, depreciation, and other allocated expenses associated with the performance of the above work and functions. The associated pharmacokinetic studies, clinical trials and certain support functions in preparing protocols, reports and other regulatory documents are performed primarily by scientific consultants and third party contract research organizations and are also included within product development expenses.
All of our prescription drug development other than development related to the erdosteine program has been limited to Phase I pharmacokinetic clinical qualification to support the associated NDAs. We have also recently completed a Phase IV clinical study involving Mucinex D in combination with antibiotics, and we have submitted the results of this study for presentation at an upcoming medical conference. We are continuing to analyze data from the phase IIb clinical study for erdosteine, which was completed in December 2006. We plan to request a meeting with the FDA to discuss the clinical results before we determine the ultimate outcome of the erdosteine program. The costs related to the erdosteine NDA program and the phase I studies for the pipeline prescription drugs in development are performed primarily by scientific consultants and third party contract research organizations and are also included within product development expenses.
Selling, Marketing and Administrative. Selling, marketing and administrative expenses include professional sales and marketing, consumer marketing, trade sales and distribution activities, and administrative expenses.
Our professional selling and marketing expenses are comprised primarily of (i) our professional sales representatives and the related management function, which includes salary, commission, benefits (including stock-based compensation), and business related expenses, (ii) physician samples, (iii) sales force training, (iv) sales force information technology, and (v) market research and advertising agency fees.
Our consumer marketing expenses are comprised of costs related to (i) media, including television, radio and print advertising, (ii) market research, (iii) website operations, (iv) commercial production, and (v) advertising agency fees.
Our trade sales and distribution expenses are primarily comprised of costs associated with our national and regional trade sales personnel and their related territory operations and outsourced warehouse and shipping operations paid to CH 105.
Administrative expenses include salaries and benefits (including stock-based compensation), professional fees and facility costs.
Results of Operations
Fiscal Year Ended June 30, 2007 Compared to Fiscal Year Ended June 30, 2006
Net Sales. Net sales increased by $92.5 million to $331.6 million for the fiscal year ended June 30, 2007, as compared to $239.1 million for the fiscal year ended June 30, 2006. The increase in net sales for fiscal 2007 is primarily due to incremental sales from the Delsym product line that we acquired in June 2006, the continued market penetration of Mucinex DM and Mucinex D, and the launch of our line of Mucinex products for children in August 2006. Net sales of Mucinex SE decreased during the year ended June 30, 2007, primarily due to the availability of and patient conversion to Mucinex DM. Overall, net sales for our products were also affected by the lower severity of upper respiratory conditions during the2006-2007 cough/
54
cold season. Net sales during the fiscal year ended June 30, 2007 approximated 91.3% of gross sales, as compared to approximately 92.9% for the fiscal year ended June 30, 2006. The decrease in net sales as a percentage of gross sales was primarily due to increases in reserves relating to the non-recurrence of the 2006 benefit from lower than estimated chargebacks and due to estimated product returns associated with our April 2007 voluntary recall of certain lots of Mucinex products for children.
The following table sets forth our net sales for the fiscal year ended June 30, 2007 and June 30, 2006:
| | | | | | | | | | | | | |
| | | Year Ended June 30, | |
| | | | | | | | | Increase
| |
Product | | 2007 | | | 2006 | | | (Decrease) | |
| | | (In thousands) | |
| |
| Mucinex SE | | $ | 131,871 | | | $ | 158,575 | | | $ | (26,704 | ) |
| Mucinex DM | | | 94,633 | | | | 59,235 | | | | 35,398 | |
| Mucinex D | | | 28,204 | | | | 19,347 | | | | 8,857 | |
| Delsym | | | 48,344 | | | | 652 | | | | 47,692 | |
| Mucinex products for children | | | 29,190 | | | | — | | | | 29,190 | |
| Humibid SE(1) | | | (639 | ) | | | 1,296 | | | | (1,935 | ) |
| | | | | | | | | | | | | |
| Net Sales | | $ | 331,603 | | | $ | 239,105 | | | $ | 92,498 | |
| | | | | | | | | | | | | |
| | |
| (1) | | Humibid SE’s sales for fiscal 2007 includes an allowance for sales returns of $0.9 million relating to retail inventory of Humibid that has expired or is approaching expiration. |
Cost of Goods Sold. Cost of goods sold increased by $46.4 million to $95.8 million for the fiscal year ended June 30, 2007, as compared to $49.4 million for the fiscal year ended June 30, 2006. Cost of goods sold increased in dollar terms primarily as a result of the increase in sales of our products. As a percentage of net sales, cost of goods sold during the fiscal years ended June 30, 2007 and 2006 totaled 28.9% and 20.6%, respectively. The increase in the percentage was primarily due to $9.2 million of non-recurring expenses recorded during fiscal 2007 relating to the manufacturing assets repurchase for items such as termination fees and the reversal of the deferred gain from the 2004 sale of the manufacturing assets to Cardinal Health. The increase in the percentage was also due to increased sales of lower-margin products, including Delsym, the Mucinex products for children, Mucinex DM and Mucinex D, lower sales of higher-margin products, such as Mucinex SE, increased raw material costs, and charges related to excess, scrapped and returned products. The increase in cost of goods sold was partially offset by the nonrecurrence of $10.2 million of profit share earned by Cardinal Health.
Selling, Marketing and Administrative. Selling, marketing and administrative expenses increased by $63.9 million to $162.9 million for the fiscal year ended June 30, 2007, as compared to $99.0 million for the fiscal year ended June 30, 2006. The increase for the fiscal year ended June 30, 2007 was primarily due to: (i) approximately $45.8 million associated with various sales, promotional and marketing programs, including increased spending on our consumer advertising campaigns, increased business development expenses and spending on product samples, increased sales force size, and increased promotions by our trade sales force; (ii) $13.9 million of general and administrative expenses, primarily related to increased legal and accounting fees, increased headcount, stock-based compensation, severance and insurance and occupancy costs; and (iii) approximately $5.4 million of additional expense related to distribution and shipping on the increased sales and higher storage fees from increased inventory. The increases in selling, marketing and administrative expenses for fiscal 2007 were partially offset by the nonrecurrence of $0.7 million in legal, printing, accounting and other costs related to our secondary offering in December 2005 and the nonrecurrence of a $0.5 million fee we paid and expensed in July 2005 to terminate our development and license agreement with Pharmaceutical Design L.L.C., or PD.
Product Development. Product development expenses increased by $5.0 million to $23.9 million during the fiscal year ended June 30, 2007, as compared to $18.9 million for the fiscal year ended June 30, 2006. The increase for the fiscal year ended June 30, 2007 was primarily due to increased spending on development of certain Mucinex product line extensions, a Phase IV clinical study involving Mucinex D in combination with
55
antibiotics, and increased expenses related to the Phase IIb clinical program for erdosteine. These increases in product development expenses were partially offset by a decrease in process improvement related costs and the nonrecurrence of a $0.7 million milestone payment made to Edmond for erdosteine during fiscal 2006.
AlleRxtmCharge. In December 2006, we recorded a non-cash pretax charge of $2.7 million relating to excess valuation of the AlleRxtm royalty interest, which we received upon the conversion of the AlleRxtm royalty interest into shares of our common stock in January 2007. In April 1999, we entered into a sublicense agreement with JMED, which provided us with an exclusive right to manufacture and market AlleRxtm in exchange for royalty payments to JMED. Subsequently, we granted JMED the right to exchange its on-going royalty interest in the sublicense agreement into our common stock in the event of a public offering or change of control. In December 2004, we entered into an agreement with JMED to assign our AlleRxtm sublicense agreement to Cornerstone, pursuant to which we paid JMED $2.0 million in January 2005, which we recorded as an intangible asset. Additionally, the assignment agreement provided that a valuation would be performed on JMED’s on-going royalty interest in the sublicense agreement and JMED would have the right to receive the value of the royalty above the $2.0 million previously paid. In December 2006, a third-party valuation was completed and the royalty interest was valued at $4.5 million. Because the appraisal value exceeded the $2.0 million we previously paid, JMED had the right to exchange the excess value of its royalty interest of $2.5 million into 147,058 unregistered shares of our common stock at our initial public offering price of $17.00.
On December 29, 2006, JMED notified us of its intention to convert its royalty interest, and we recorded a liability of $5.2 million, representing the total market value of the 147,058 shares of our common stock at the $40.81 closing market price of our common stock on the date we received notice from JMED, discounted to reflect the fact that we issued unregistered shares to JMED with a minimum holding period of one year. The fair value was determined using the Black-Scholes option pricing model for a put option with a one year holding period. We also increased the intangible asset relating to the AlleRxtm interest by $2.5 million, representing the excess value of the royalty interest per the valuation. Because the $4.5 million appraised value of this intangible asset is below the $7.2 million in total value we have paid to JMED ($2.0 million in January 2005 and $5.2 million in stock issued in January 2007), we expensed $2.7 million in December 2006, representing the appreciation in our common stock from our initial public offering through the date we received the notice of conversion. This non-cash pretax expense was included in a separate line item titled “AlleRxtm charge” in our consolidated statements of operations for the fiscal year ended June 30, 2007. After issuing shares of our common stock to JMED in January 2007, we began to actively market the AlleRxtm royalty interest and have reclassified the related intangible asset to held-for-sale.
Other, net. Other, net decreased $3.1 million to income of $1.2 million during the fiscal year ended June 30, 2007, as compared to income of $4.3 million during the fiscal year ended June 30, 2006. The decrease for the fiscal year ended June 30, 2007 was primarily due to the $2.5 million impairment charge recorded during fiscal 2007 relating to our Humibid intangible asset, decreased interest income of $1.8 million during fiscal 2007, primarily reflecting lower cash balances and partially mitigated by increased investment rates during fiscal 2007, and interest expense of $0.4 million recorded during fiscal 2007, primarily related to our past borrowings under the Credit Facility. These decreases in other, net were partially offset by the non-recurrence of a one-time pretax loss of $1.6 million recorded during fiscal 2006 in connection with the move of our corporate headquarters in Chester, New Jersey and by AlleRxtm royalty income of $0.5 million recorded during fiscal 2007.
Income Taxes. Income tax expense for the fiscal year ended June 30, 2007 was $17.0 million, as compared to $29.8 million for the fiscal year ended June 30, 2006. Our effective tax rates for the fiscal years ended June 30, 2007 and 2006 were 35.8% and 39.1%, respectively. The decline in our effective tax rate was primarily due to a tax deduction provided to U.S. manufacturers for which we are now eligible as a result of the manufacturing assets repurchase and the shift in our stock compensation expense from non-deductible incentive stock options to deductible non-qualified stock options. In addition, the decline in our effective tax rate was due to an adjustment recorded during fiscal 2007 to align our tax liability with our 2006 tax return, as filed during the third quarter of fiscal 2007, which reduced our fiscal 2007 effective tax rate by 0.8%. We
56
do not believe that this adjustment is material to our consolidated financial statements during any of the periods presented.
Fiscal Year Ended June 30, 2006 Compared to Fiscal Year Ended June 30, 2005
Net Sales. Net sales increased by $78.9 million to $239.1 million for the fiscal year ended June 30, 2006, as compared to $160.2 million for the fiscal year ended June 30, 2005. The increase in net sales for fiscal 2006 was primarily due to our consumer advertising campaign, which resulted in continued market penetration of Mucinex SE and Mucinex DM, and due to the launches of Mucinex D and Humibid SE. Net sales during the fiscal year ended June 30, 2006 approximated 93% of gross sales, as compared to approximately 90% for the fiscal year ended June 30, 2005. Two and a half percent of the increase was due to the lower than expected utilization rate of our trade promotion programs, and the remaining difference was primarily due to lower than expected government rebates as compared to the prior year. The change in the expected utilization rates was primarily attributable to additional historical experience following our initial consumer advertising campaign in November 2004.
In August 2004, we discontinued the manufacture and sale of our Aquatab product lines to focus on building the Mucinex brand. In February 2005, we acquired the Humibid trademark and transferred our AlleRxtm product line in exchange for the Humibid name.
The following table sets forth our net sales for the fiscal year ended June 30, 2006 and June 30, 2005:
| | | | | | | | | | | | | |
| | | Year Ended June 30, | |
| | | | | | | | | Increase/
| |
Product | | 2006 | | | 2005 | | | (Decrease) | |
| | | (In thousands) | |
| |
| Mucinex SE | | $ | 158,575 | | | $ | 117,369 | | | $ | 41,206 | |
| Mucinex DM | | | 59,235 | | | | 38,278 | | | | 20,957 | |
| Mucinex D | | | 19,347 | | | | — | | | | 19,347 | |
| Delsym | | | 652 | | | | — | | | | 652 | |
| Humibid SE | | | 1,296 | | | | — | | | | 1,296 | |
| Other products | | | — | | | | 4,563 | | | | (4,563 | ) |
| | | | | | | | | | | | | |
| Net sales | | $ | 239,105 | | | $ | 160,210 | | | $ | 78,895 | |
| | | | | | | | | | | | | |
Cost of Goods Sold. Cost of goods sold increased $18.3 million to $49.4 million for the fiscal year ended June 30, 2006, as compared to $31.1 million for the fiscal year ended June 30, 2005. Cost of goods sold increased in dollar terms primarily as a result of the increase in Mucinex SE and Mucinex DM sales and the launches of Mucinex D and Humibid SE. As a percentage of net sales, cost of goods sold during the fiscal years ended June 30, 2006 and 2005 totaled 20.6% and 19.4%, respectively. The increase in the percentage was due to the increased sales of higher cost products, such as Mucinex D and products packaged within display units. For the fiscal years ended June 30, 2006 and 2005, cost of goods sold included $10.2 million and $9.5 million, respectively, of profit share earned by Cardinal Health.
Selling, Marketing and Administrative. Selling, marketing and administrative expenses increased by $21.0 million to $99.0 million for the fiscal year ended June 30, 2006, as compared to $78.0 million for the fiscal year ended June 30, 2005. The increase for the fiscal year ended June 30, 2006 was primarily due to: (i) approximately $11.9 million associated with various sales and marketing programs, including increased spending on our consumer advertising campaign, the increase in our sales force in December 2004, expansion of the trade sales department, and professional marketing expenses; (ii) $7.7 million of general and administrative expenses, primarily related to new headcount, insurance, taxes, legal and accounting fees; (iii) an increase of $3.4 million for non-cash stock-based compensation, primarily due to the consideration of a volatility assumption in the calculation of fair value for fiscal 2006 stock option grants; (iv) approximately $1.8 million of additional expense related to distribution and shipping on the increased volume of adult oral-solid Mucinex sales; and (v) $0.7 of legal, printing and audit costs related to our secondary offering in December 2005. These increases in selling, marketing and administrative expenses were partially offset by:
57
(i) a decrease of $4.1 million relating to the non-recurrence of the additional discretionary performance bonus recorded during fiscal 2005 and (ii) a net decrease of $0.4 million relating to our decision not to go forward with the development and license agreement with PD. To terminate the PD agreement, we paid $0.5 million, which we expensed to selling, marketing and administrative expenses in July 2005. The related intangible asset with a net book value of $0.9 million was written off to selling, marketing and administrative expenses as of June 30, 2005.
Product Development. Product development expenses increased by $11.5 million to $18.9 million during the fiscal year ended June 30, 2006, as compared to $7.4 million for the fiscal year ended June 30, 2005. The increase for the fiscal year ended June 30, 2006 was primarily attributable to expenses related tostart-up costs of the erdosteine Phase IIb clinical program and spending on Mucinex line extensions. The fiscal year ended June 30, 2006 also included a $0.7 million milestone payment made to Edmond for erdosteine and approximately $0.7 million for non-cash stock-based compensation.
Other, net. Other, net increased $3.5 million to income of $4.3 million during the fiscal year ended June 30, 2006, as compared with income of $0.8 million during the fiscal year ended June 30, 2005. The increase for the fiscal year ended June 30, 2006 was primarily attributable to higher interest income reflecting earnings on the increased cash balances, which includes cash received from our initial public offering, coupled with higher interest rates. This increase was partially offset by a one-time pretax loss of $1.6 million recorded in fiscal 2006 in connection with the move of our corporate headquarters in Chester, New Jersey.
Income Taxes. Income tax expense for the fiscal year ended June 30, 2006 was $29.8 million, as compared to $17.4 million for the fiscal year ended June 30, 2005. Our effective tax rates for the years ended June 30, 2006 and 2005 were 39.1% and 39.2%, respectively. At June 30, 2006, we had approximately $2.3 million of NOL carryforwards that expire in fiscal 2012.
Accretion of Preferred Stock. Accretion of our redeemable convertible preferred stock for the fiscal year ended June 30, 2005 was $202.6 million, reflecting an adjustment made during fiscal 2005 to accrete all redeemable convertible stock to fair value. Upon the closing of our initial public offering in July 2005, our redeemable convertible preferred stock converted into shares of common stock. As a result, there was no accretion for the fiscal year ended June 30, 2006.
Dividend Paid to Preferred Stockholders. We paid a $45.0 million dividend on our stock during fiscal 2005, of which $30.0 million was paid to preferred stockholders on an “as converted” to common stock basis and is reflected as a reduction of net income/(loss) applicable to common stockholders.
Cash Flows
Fiscal Year Ended June 30, 2007 Compared to Fiscal Year Ended June 30, 2006
Net cash provided by operating activities was $6.3 million for the fiscal year ended June 30, 2007 as compared to $49.8 million for the fiscal year ended June 30, 2006. The decrease in net cash provided by operating activities during the fiscal year ended June 30, 2007 was primarily due to our July 2006 manufacturing assets repurchase, which led to a substantial increase in our inventory balance, as well as lower net income during fiscal 2007. The decrease in net cash provided by operating activities was partially offset by a decrease in the accounts receivable balance, primarily driven by the timing of sales and cash receipts.
Net cash used in investing activities was $11.1 million for the fiscal year ended June 30, 2007 compared to $167.0 million for the fiscal year ended June 30, 2006. The decrease in net cash used in investing activities primarily related to a decrease of $446.9 million in purchases of investments during fiscal 2007 and $122.0 million paid during fiscal 2006 to purchase the Delsym12-Hour OTC liquid cough suppressant product line from UCB. These decreases in net cash used in investing activities were partially offset by decreased proceeds from sales of investments of $404.5 million and increased property, plant and equipment purchases of $8.5 million, which included approximately $7.0 million of manufacturing assets we acquired in connection with the manufacturing assets repurchase.
58
Net cash provided by financing activities was $11.4 million during the fiscal year ended June 30, 2007, compared to net cash provided by financing activities of $129.6 million during the fiscal year ended June 30, 2006. Net cash provided by financing activities for the fiscal year ended June 30, 2006 included $107.8 million of net proceeds from the issuance of common stock in our initial public offering and $19.0 million of excess tax benefits from the exercise of nonqualified stock options and disqualifying dispositions of incentive stock options, as compared to $8.6 million of excess tax benefits for the fiscal year ended June 30, 2007.
Fiscal Year Ended June 30, 2006 Compared to Fiscal Year Ended June 30, 2005
Net cash provided by operating activities was $49.8 million the fiscal year ended June 30, 2006 as compared to $28.1 million for the fiscal year ended June 30, 2005. The increase in net cash provided by operating activities during fiscal 2006 was primarily due to the overall growth in our business, which resulted in increased net income, accounts payable and income taxes payable, partially offset by increased accounts receivable and inventories and a decrease in deferred tax assets.
Net cash used in investing activities was $167.0 million for the fiscal year ended June 30, 2006 as compared to $3.8 million for the fiscal year ended June 30, 2005. The increase in net cash used in investing activities was primarily attributable to $122.0 million paid in June 2006 to purchase the Delsym12-Hour cough suppressant liquid product line from UCB, as well as $474.9 million reflecting purchases of investments, partially offset by $436.6 million we received from sales of investments during fiscal 2006. Our long-term investments for the fiscal year ended June 30, 2006 included $1.0 million of restricted cash held as collateral for our letter of credit for our new office building in Chester, New Jersey.
Net cash provided by financing activities was $129.6 million during the fiscal year ended June 30, 2006 as compared to net cash used in financing activities of $40.7 million during the fiscal year ended June 30, 2005. During the fiscal year ended June 30, 2006, net cash provided by financing activities primarily consisted of $107.8 million of net proceeds from the issuance of common stock in our initial public offering and $19.0 million of excess tax benefits from the exercise of nonqualified stock options, as well as $2.8 million of proceeds from the exercise of stock options and warrants to purchase common stock. Net cash used in financing activities during the year ended June 30, 2005 included a dividend payment of $45.0 million, partially offset by $5.4 million of proceeds from the exercise of options and warrants to purchase common stock.
Liquidity and Capital Resources
In July 2005, we completed our initial public offering of common stock, which generated gross proceeds to us of approximately $117.0 million. From our inception through the completion of our initial public offering, we financed our operations primarily through the net proceeds from private placements of common stock, redeemable convertible preferred stock, notes convertible into redeemable convertible preferred stock, a revolving bank line of credit, and cash generated from our product sales.
On June 2, 2005, our board of directors declared a cash dividend totaling $45.0 million on shares of our common stock and shares of our preferred stock on an “as converted” basis (in accordance with our Certificate of Designations, Rights and Preferences of the Series A Convertible Preferred Stock, Series B Convertible Preferred Stock and Series C Convertible Preferred Stock of the Certificate of Incorporation). We paid the dividend on June 22, 2005.
Prior to the manufacturing assets repurchase, purchases of our finished product inventory from Cardinal Health were paid pursuant to a profit sharing formula, which was initially billed to us at an amount equal to Cardinal Health’s actual manufacturing cost plus amark-up. Themark-up payments to Cardinal Health were trued up each March 31st to the actual profit share amount. Any excess of themark-up payments over the actual profit share amount was refunded to us. As a result of the manufacturing assets repurchase, this profit-share arrangement terminated.
As of June 30, 2007, we had approximately $46.8 million of cash and cash equivalents, $33.0 million of short-term investments and working capital of $124.9 million, as compared to cash and cash equivalents of
59
$40.3 million, short-term investments of $22.2 million, working capital of $72.9 million, and long-term investments of $14.9 million as of June 30, 2006. The increase in cash and cash equivalents was primarily due to cash generated by our operations, partially offset by approximately $28.0 million that we paid during fiscal 2007 relating to the manufacturing assets repurchase. Our working capital increased primarily due to a substantial increase in our inventory balance, primarily as a result of the manufacturing assets repurchase and the overall growth in our business. We had no outstanding debt as of June 30, 2007.
In July 2006, we entered into the five-year $50.0 million Credit Facility, which may be increased by up to an additional $100.0 million, subject to compliance with certain conditions, should we need additional financing in the future. Prior to the closing of the Credit Facility, we were provided with a bridge facility with immediately available borrowings of up to $25.0 million. In July 2006, we drew $20.0 million from the bridge facility in connection with the manufacturing assets repurchase, which we repaid in full and terminated, partially using proceeds from the Credit Facility. In October 2006, we repaid the remaining outstanding balance under the Credit Facility. We intend to use any future borrowings under the Credit Facility to finance working capital requirements, capital expenditures and acquisitions and for other general corporate purposes. In November 2006, we issued a $1.5 million letter of credit as a security deposit on the operating lease we assumed from Cardinal Health as a result of the manufacturing assets repurchase. As a result, we currently have $48.5 million available under the Credit Facility.
The Credit Facility terminates on September 26, 2011, unless terminated earlier pursuant to the terms of the agreement. Borrowings under the Credit Facility bear interest at the higher of the prime rate established by Royal Bank of Canada or 0.50% per annum above the weighted-average federal funds rate, subject to quarterly adjustments based on our debt to EBITDA ratio, or the Leverage Ratio, as defined in the Credit Facility. The Credit Facility also requires the payment of an unused commitment fee equal to 0.20% per annum, subject to quarterly adjustments in accordance with the Leverage Ratio, ranging from 0.20% to 0.40% on the unused commitment under the Credit Facility.
The Credit Facility requires us to maintain a Leverage Ratio of not greater than 3.5 to 1.0, a senior secured leverage ratio of not greater than 2.0 to 1.0, and a fixed charge coverage ratio of not less than 2.0 to 1.0. We are currently in compliance with these covenants.
Our principal liquidity requirements are to meet the operating expenses of our growing business. Our operating expenses include selling, marketing and administrative and product development expenses and contractual commitments related to operating leases, raw material and finished goods purchase commitments, and royalty payments on our Mucinex, Delsym and Humibid products.
We expect to increase our selling, marketing and administrative expenses. We anticipate our selling and marketing expenses to increase as we seek to (i) continue to switch long-acting single-ingredient guaifenesin and combination prescription products into sales of our Mucinex products; (ii) expand our share of the market for long-acting guaifenesin and combination products; (iii) market the Mucinex products for children line, our Mucinex nasal spray products and our Delsym products, and (iv) increase our share of the OTC cough, cold, allergy and sinus and nasal spray markets. We intend to continue to increase our consumer advertising spending. We anticipate that our administrative expenses will increase to support our current growth plans, including additional headcount, and to defend our trademark and patent portfolio. Our product development expenses will include (i) expanding the Mucinex product line with OTC and prescription line extensions, (ii) expanding our Delsym product line; (iii) in-licensing or acquiring specialty pharmaceutical respiratory products and trademarks that may require additional development expenditures to achieve FDA marketing approval. We believe that our cash outflows related to acquiring products and entering into licensing agreements may increase as we pursue our product portfolio expansion initiative.
We believe that the Credit Facility and existing cash, coupled with cash flow from operations, will be sufficient to meet our anticipated operating needs for at least the next two fiscal years. We will require substantial funds to commercialize our products, launch new products, promote our brands and conduct development, including preclinical testing and clinical trials, of our potential products. We continually evaluate new opportunities for late-stage or currently-marketed complementary product candidates and, if and when appropriate, intend to pursue such opportunities through the acquisitions of companies, products or
60
technologies and our own development activities. Our ability to execute on such opportunities may be dependent, in part, upon our ability to raise additional capital on commercially reasonable terms. There can be no assurance that funds will be available when needed or on terms favorable to us or our stockholders. We could be required to seek funds through arrangements with others that may require us to relinquish rights to some of our technologies, product candidates or products that we would otherwise pursue on our own. If additional funds are raised by issuing equity securities, stockholders may experience dilution or such equity securities may provide for rights, preferences or privileges senior to those of the holders of our common stock.
Commitments and Contractual Obligations
Our major outstanding contractual obligations relate to operating leases, raw material purchase commitments, royalty payments on our Mucinex, Delsym and Humibid products, and payments under consulting agreements with former employees. These contractual obligations as of June 30, 2007 are as follows:
| | | | | | | | | | | | | | | | | | | | | |
| | | | | | Less Than
| | | | | | | | | More Than
| |
| | | Total | | | 1 Year | | | 1-3 Years | | | 3-5 Years | | | 5 Years | |
| | | (In thousands) | |
| |
| Operating leases(1) | | $ | 19,252 | | | $ | 3,091 | | | $ | 4,896 | | | $ | 3,700 | | | $ | 7,565 | |
| Purchase obligations | | | | | | | | | | | | | | | | | | | | |
| Manufacturing contracts(2) | | | 144,439 | | | | 38,688 | | | | 65,983 | | | | 39,768 | | | | — | |
| Marketing contracts | | | 2,500 | | | | 1,556 | | | | 944 | | | | — | | | | — | |
| Research contracts(3) | | | 440 | | | | 440 | | | | — | | | | — | | | | — | |
| Other purchase obligations(4) | | | 577 | | | | 532 | | | | 45 | | | | — | | | | — | |
| Royalty payments(5) | | | 3,500 | | | | 500 | | | | 1,000 | | | | 1,000 | | | | 1,000 | |
| Consulting payments | | | 59 | | | | 48 | | | | 11 | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | | | | | |
| Total | | $ | 170,767 | | | $ | 44,855 | | | $ | 72,879 | | | $ | 44,468 | | | $ | 8,565 | |
| | | | | | | | | | | | | | | | | | | | | |
| | |
| (1) | | Includes minimum rental payments for our corporate office buildings in Chester, New Jersey, our manufacturing facility in Fort Worth, Texas and our warehouse facility in Irving, Texas; office equipment leases and automobile lease payments for the sales force. |
| |
| (2) | | Consists of commitments to purchase raw materials and finished products. |
| |
| (3) | | In addition to the amounts set forth in the table, we may be required to make potential future milestone payments to third-parties as part of its development programs. These amounts are only payable upon achievement of certain development, regulatory, and commercial milestones. Because such payments are contingent upon occurrence of certain events and are uncertain in nature, they have not been included in the above table. |
| |
| (4) | | Consists of commitments related to technology agreements. |
| |
| (5) | | Represents minimum royalty payments to CellTech relating to the April 2004 settlement. See below for a further discussion of the CellTech settlement. |
In June 2007, we entered into an11-year operating lease for a new office facility in Chester, New Jersey, which is scheduled to commence on or prior to October 1, 2007. Our future minimum lease commitments under this lease are $0.2 million for each of fiscal 2008 through 2010, $0.3 million for each of fiscal 2011 through 2012 and an aggregate of $1.8 million thereafter.
In March 2007, we entered into a64-month operating lease for a warehouse facility in Irving, Texas, which commenced on May 15, 2007. In accordance with the provisions of this lease, we will receive free rent for the first five months of the lease. Our future minimum lease commitments under this operating lease are $0.3 million for fiscal 2008, $0.5 million for each of fiscal 2009 through 2012, and $0.1 million for fiscal 2013.
On October 26, 2006, we entered into a three-year renewable agreement with PharmPro, a division of Fluid Air, Inc., for processing and granulation of guaifenesin. Under this agreement, we are required to
61
purchase 50 metric tons of finished granulation of guaifenesin during each contract year. The remaining contracted amount under this agreement was $1.4 million as of June 30, 2007. PharmPro also agreed to reserve capacity to enable the manufacture of an additional 100% of the contracted volume, which we may use at our option. In exchange for this reservation of manufacturing capacity, we have prepaid $0.5 million to PharmPro, which is rebated back to us at a rate of $1.67 per kilogram of approved product produced. Any remaining balance relating to this prepayment at the end of the three-year period will be retained by PharmPro.
On July 31, 2006, we repurchased the Fort Worth, Texas manufacturing assets from Cardinal Health for approximately $28 million. As a result of the manufacturing assets repurchase, we were required to make an escrow deposit in the amount of $2.2 million, representing the remaining obligation for the operating lease on the Fort Worth, Texas building. In November 2006, this escrow deposit was refunded to us and the operating lease for this facility was amended to include us as the lessee. In connection with this amendment, we issued an irrevocable letter of credit in the amount of $1.5 million as a security deposit on the lease. Our future minimum lease commitments under this operating lease are $0.5 million for each of fiscal 2008, 2009 and 2010, and $0.4 million for fiscal 2011.
Upon the manufacturing assets repurchase, we entered into a packaging agreement with Cardinal Health (now Catalent) under which, in exchange for a guaranteed amount of packaging capacity, we are committed to pay Catalent non-refundable capacity reservation payments of $3.0 million in each year during the contract years ending June 30, 2007 and 2008 and $1.5 million in the contract year ending June 30, 2009. We also entered into a three year take-or-pay manufacturing agreement with Cardinal Health (now Catalent) for the granulation of guaifenesin, beginning on August 1, 2006. Under this manufacturing agreement, we are obligated to purchase or pay for 80% of committed volume at a specified price. The remaining contracted amount under the granulation agreement is $3.9 million as of June 30, 2007. We have the ability to use any other vendor with whom we may decide to contract above the committed volume.
As a result of the manufacturing assets repurchase, Cardinal Health assigned to us its January 2006 agreement with its sole supplier of dextromethorphan, which obligated Cardinal Health to purchase 45 metric tons of dextromethorphan through 2009. As of June 30, 2007, the remaining commitment for the entire contract was approximately $10.5 million. In December 2006, the FDA approved a second supplier of dextromethorphan. We believe these two suppliers will provide us with sufficient quantities of dextromethorphan to meet our manufacturing needs.
We depend on Boehringer Ingelheim and Delta for all of the raw guaifenesin used in our adult oral-solid Mucinex products. In connection with the manufacturing assets repurchase, Cardinal Health assigned us the contracts related to the manufacture of the adult oral-solid Mucinex products, including the guaifenesin supply arrangements with Boehringer Ingelheim and Delta. In July 2006, we entered into a new five-year supply agreement with Boehringer Ingelheim, pursuant to which we have agreed to purchase from Boehringer Ingelheim the lesser of 500 metric tons or 100% of our guaifenesin requirements during each contract year. Assuming we purchase 500 metric tons per year from Boehringer Ingelheim, our remaining commitment under this agreement is $65.0 million as of June 30, 2007. We may purchase volumes in excess of 500 metric tons from other suppliers. We believe that Boehringer Ingelheim and Delta will provide us with sufficient quantities of guaifenesin to meet our manufacturing needs.
In June 2006, we consummated the acquisition of the Delsym12-Hour OTC liquid cough suppressant product line from UCB. In addition to the cash purchase price of $122.0 million, we are committed to pay a 2.5% royalty to UCB on net sales of Delsym for a period of five years for the intellectual property associated with the Delsym product. UCB will continue to manufacture the Delsym product for us from raw materials to finished goods under a six-year renewable standard supply agreement, which is based on UCB’s manufacturing costs plus a markup, with a contract minimum of $11.8 million in each year. The remaining commitment under this agreement as of June 30, 2007 is $58.8 million. The agreement defines cost as UCB’s cost of manufacturing finished product, limited to raw materials, packaging materials, labor time, quality assurance time, and overhead. We believe that the cost plus markup amount is representative of fair value, based on our manufacturing agreement with Cardinal Health (now Catalent) and other manufacturing arrangements our
62
management has experienced in the industry. The agreement with UCB also provides for the potential technology transfer to us after five years.
We also entered into a five-year development agreement with UCB for certain product development projects, including additional product formulations of Delsym. Pursuant to this agreement, we pay UCB actual development costs plus an established markup percentage. We believe this agreement is considered to be at fair value, as it is consistent with the terms of our research and development agreement with Cardinal Health (now Catalent) for these services.
In October 2005, we entered into a12-year operating lease for a new office facility in Chester, New Jersey, which commenced on April 1, 2006. The first three years’ rent is fixed at $0.8 million per year, increases to $0.9 million in years four through nine, and $1.0 million in years 10 through 12.
In February 2005, we entered into an agreement with Cornerstone, in which we received the Humibid trademarks from Cornerstone and Cornerstone received the AlleRxtm trademarks from us. Additionally, we and Cornerstone released each other from all claims and damages in a previously filed lawsuit. As part of this arrangement, we now have the responsibility for all Humibid product returns, whether sold by us or Cornerstone, and Cornerstone bears the same liability for AlleRxtm products. In connection with this agreement, we are obligated to pay Cornerstone a royalty ranging from 1% to 2% of net Humibid sales for a period of three years after February 15, 2005, with an annual minimum royalty payment of $50,000.
In March 2003, CellTech Pharmaceuticals, Inc., CellTech US, Inc. and CellTech Americas, Inc., which we refer to collectively as CellTech, brought suit against us, alleging misappropriation of trade secrets and breach of confidentiality. We agreed to settle the lawsuit in April 2004 for $2.0 million. In connection with the settlement that we reached with CellTech during April 2004, we entered into a royalty-bearing license agreement with CellTech for our Mucinex products, subject to an annual maximum of $0.5 million and an annual minimum of $0.2 million until December 31, 2013.
Legal Proceedings
On October 4, 2006, we filed a complaint against Mutual in the United States District Court for the District of Pennsylvania, Civ. Act.No. 2:06-cv-04418-PD, asserting that Mutual’s proposed generic products would infringe our ’252 Patent. On March 21, 2007, we entered into a settlement agreement with Mutual, under which we agreed with Mutual to dismiss all patent infringement claims and all counterclaims in the lawsuit. In the settlement agreement, Mutual admitted that the ’252 Patent is valid and enforceable and that the single-ingredient and combination generic extended-release guaifenesin-based products set forth in the ANDA filed by Mutual with the FDA infringe the ’252 Patent. Under the settlement agreement, we granted Mutual a non-exclusive, royalty-free license under the ’252 Patent to sell our 600 mg and 1200 mg single-ingredient and combination extended-release guaifenesin products in the United States, subject to certain restrictions. The settlement agreement is subject to review by the Federal Trade Commission and the U.S. Department of Justice. For a further discussion of the terms of the settlement agreement with Mutual, please see Item 3. Legal Proceedings in this Annual Report onForm 10-K.
Subsequent Events
On August 20, 2006, we received a letter from Perrigo notifying us that Perrigo had filed an ANDA for a single ingredient, extended-release formulation of guaifenesin that is the generic equivalent to our Mucinex SE product. In its letter, Perrigo notified us of its assertion that its proposed products would not infringe our patents that protect Mucinex SE, or alternatively that certain of our patent claims are not valid.
We are evaluating this matter and have requested from Perrigo additional data to evaluate their claims. If we determine that Perrigo’s products infringe our patents, we intend to vigorously defend our intellectual property rights. If we file a patent infringement lawsuit prior to October 4, 2007, the FDA will stay its approval of Perrigo’s ANDA until the earlier of 30 months or a court’s determination that Perrigo’s product does not infringe our patents or that certain of our patent claims are invalid. We cannot, however, at this time
63
predict whether we will file a patent infringement lawsuit, the duration of any resulting litigation or the stay in the FDA’s approval of Perrigo’s ANDA, or whether we would prevail in any such lawsuit.
Inflation
Generally, inflation has not had, and we do not expect it to have, a material impact on net sales or income from continuing operations. However, there can be no assurance that our business will not be affected by inflation in the future.
Off-Balance Sheet Arrangements
We do not have any off-balance sheet arrangements.
| |
| Item 7A. | Quantitative and Qualitative Disclosure About Market Risk |
Our exposure to market risk is confined to our cash and cash equivalents. We invest in high-quality financial instruments, primarily money market funds, and government agency securities, with no security with an effective duration in excess of one year. As a result, we believe are subject to limited credit risk. We currently do not hedge interest rate exposure. Due to the short-term nature of our investments, we do not believe that we have any material exposure to interest rate risk arising from our investments.
Most of our transactions are conducted in U.S. dollars, although we do have some development and commercialization agreements with vendors located outside the United States. Transactions under certain of these agreements are conducted in U.S. dollars, subject to adjustment based on significant fluctuations in currency exchange rates. Transactions under certain other of these agreements are conducted in the local foreign currency. If the exchange rate undergoes a change of 10%, we do not believe that it would have a material impact on our results of operations or cash flows.
| |
| Item 8. | Financial Statements and Supplementary Data |
The information required by this item appears on pages F-1 through F-34 of this report.
| |
| Item 9. | Changes in and Disagreements with Accountants on Accounting and Financial Disclosure |
None.
| |
| Item 9A. | Controls and Procedures |
Evaluation of Disclosure Controls and Procedures
We carried out an evaluation, under the supervision and with the participation of management, including our Chief Executive Officer and Chief Financial Officer, of the effectiveness of the design and operation of our disclosure controls and procedures as of the end of the period covered by this report pursuant to Exchange ActRule 13a-15. Based on that evaluation, our Chief Executive Officer and Chief Financial Officer concluded that our disclosure controls and procedures were effective in ensuring that information required to be disclosed by us (including our consolidated subsidiaries) on reports that we file or submit under the Securities Exchange Act of 1934, as amended, is recorded, processed, summarized and reported in a timely basis.
Management’s Annual Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as defined inRule 13a-15(f) of the Exchange Act. Internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles.
Our internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and dispositions
64
of the assets of the Company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the Company are being made only in accordance with authorizations of management and directors of the Company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the Company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate. All internal control systems, no matter how well designed, have inherent limitations. Therefore, even those systems determined to be effective can provide only reasonable assurance with respect to financial statement preparation and presentation.
Our management performed an evaluation of the effectiveness of our internal control over financial reporting as of June 30, 2007 based on the criteria established by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) inInternal Control — Integrated Framework. Based on its assessment, our management believes that internal control over financial reporting was effective as of June 30, 2007.
Management’s assessment relating to the effectiveness of internal control over financial reporting was audited by our independent registered public accounting firm, Ernst & Young LLP, and Ernst & Young LLP has issued a report on management’s assessment and the effectiveness of internal control over financial reporting, which is set forth below.
Changes in Internal Control Over Financial Reporting
There has been no change in our internal control over financial reporting that occurred during the fourth quarter of fiscal 2007 that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
Report of Independent Registered Public Accounting Firm
The Board of Directors and Stockholders of
Adams Respiratory Therapeutics, Inc.
We have audited management’s assessment, included in the accompanying Management’s Annual Report on Internal Control over Financial Reporting, that Adams Respiratory Therapeutics, Inc. maintained effective internal control over financial reporting as of June 30, 2007, based on criteria established in Internal Control — Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (the COSO criteria). Adams Respiratory Therapeutics, Inc.’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting. Our responsibility is to express an opinion on management’s assessment and an opinion on the effectiveness of the company’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, evaluating management’s assessment, testing and evaluating the design and operating effectiveness of internal control, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over
65
financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements. Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, management’s assessment that Adams Respiratory Therapeutics, Inc. maintained effective internal control over financial reporting as of June 30, 2007, is fairly stated, in all material respects, based on the COSO criteria. Also, in our opinion, Adams Respiratory Therapeutics, Inc. maintained, in all material respects, effective internal control over financial reporting as of June 30, 2007, based on the COSO criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated balance sheets as of June 30, 2007 and 2006, and the related consolidated statements of operations, stockholders’ equity (deficit), and cash flows for each of the three years in the period ended June 30, 2007 of Adams Respiratory Therapeutics, Inc. and our report dated August 24, 2007 expressed an unqualified opinion thereon.
/s/ Ernst & Young LLP
MetroPark, New Jersey
August 24, 2007
| |
| Item 9B. | Other Information |
None.
| |
| Item 10. | Directors, Executive Officers and Corporate Governance |
The information required by this Item with respect to directors and executive officers is incorporated herein by reference from the information contained in our definitive proxy statement for our 2007 Annual Meeting of Stockholders, which we refer to as the Proxy Statement.
The information required by this Item regarding compliance with Section 16(a) of the Exchange Act appears under the heading “Other Matters-Section 16(a) Beneficial Ownership Reporting Compliance” in the Proxy Statement. That portion of the Proxy Statement is incorporated by reference into this Annual Report onForm 10-K.
The information required by this Item with respect to corporate governance and our Code of Conduct and Ethics is incorporated herein by reference from the information contained in the Proxy Statement under the heading “The Board of Directors and Corporate Governance”.
| |
| Item 11. | Executive Compensation |
The information required by this Item regarding executive compensation is incorporated herein by reference from the information contained in the Proxy Statement under the headings “Compensation of Executive Officers” and “Director Compensation”.
66
| |
| Item 12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
The information required by this Item regarding security ownership of certain beneficial owners and management is incorporated herein by reference from the information contained in the Proxy Statement under the heading “Stock Ownership of Certain Beneficial Owners and Management”. The information required by this Item regarding securities authorized for issuance under equity compensation plans appears under the heading “Equity Compensation Plan Information” in the Proxy Statement and is incorporated herein by reference.
| |
| Item 13. | Certain Relationships and Related Transactions, and Director Independence |
The information required by this Item about certain relationships and related transactions appears under the heading “Certain Relationships and Related Transactions” in the Proxy Statement and is incorporated herein by reference. The information required by this Item regarding director independence is incorporated herein by reference from the information contained in the Proxy Statement under the heading “The Board of Directors and Corporate Governance”.
| |
| Item 14. | Principal Accountant Fees and Services |
Information about principal accountant fees and services as well as related pre-approval policies appears under the heading “Audit and Related Fees” in the Proxy Statement and is incorporated herein by reference.
| |
| Item 15. | Exhibits, and Consolidated Financial Statement Schedules |
(a) Financial Statements and Schedules.
| | |
| | (1) | The following consolidated financial statements of the Company and Report of Ernst & Young LLP are included in this report: |
| | | | | |
| | | F-2 | |
| | | F-3 | |
| | | F-4 | |
| | | F-5 | |
| | | F-6 | |
| | | F-8 | |
| | | F-34 | |
Consolidated financial statement schedules not filed in this Annual Report onForm 10-K have been omitted either because they are not applicable or the required information is shown in Notes to Consolidated Financial Statements in this Annual ReportForm 10-K.
(3) Exhibits
The Exhibits listed in the Index of Exhibits appearing at pages 65 to 67 are included in this Annual Report onForm 10-K.
(b) Exhibits.
See Item 15(a)(3), above.
(c) Financial Statement Schedules.
See Item 15(a)(2), above.
67
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this Report to be signed on its behalf by the undersigned, thereunto duly authorized, in Chester, New Jersey on August 29, 2007.
ADAMS RESPIRATORY THERAPEUTICS, INC.
By: Michael J. Valentino
| | |
| | Its: | Chief Executive Officer |
Pursuant to the requirements of the Securities Exchange Act of 1934, this Report has been signed below by the following persons in the capacities and on the dates indicated.
| | | | | | | |
Name | | Title | | Date |
| |
| | | | | |
/s/ Michael J. Valentino
Michael J. Valentino | | Chief Executive Officer and Director (principal executive officer) | | August 29, 2007 |
| | | | | |
/s/ Rita M. O’Connor
Rita M. O’Connor | | Chief Financial Officer (principal financial and accounting officer) | | August 29, 2007 |
| | | | | |
/s/ Kirk K. Calhoun
Kirk K. Calhoun | | Director | | August 29, 2007 |
| | | | | |
/s/ Jane Delgado, Ph.D., M.S.
Jane Delgado, Ph.D., M.S. | | Director | | August 29, 2007 |
| | | | | |
/s/ Dr. Alan W. Dunton
Dr. Alan W. Dunton | | Director | | August 29, 2007 |
| | | | | |
/s/ Donald J. Liebentritt
Donald J. Liebentritt | | Director | | August 29, 2007 |
| | | | | |
John N. Lilly | | Director | | |
| | | | | |
/s/ Joan P. Neuscheler
Joan P. Neuscheler | | Director | | August 29, 2007 |
| | | | | |
/s/ Harold F. Oberkfell
Harold F. Oberkfell | | Director | | August 29, 2007 |
| | | | | |
/s/ Mark R. Sotir
Mark R. Sotir | | Director | | August 29, 2007 |
68
EXHIBIT INDEX
| | | | | |
Exhibit
| | | | |
Number | | | | Description |
| |
| 2.1 | | — | | Agreement of Merger between Adams Respiratory Therapeutics, Inc. and Adams Merger Sub, Inc. dated June 1, 2005, filed as Exhibit 2.1 to the Company’s Registration Statement onForm S-1 (RegistrationNo. 333-123585), and incorporated herein by reference. |
| 2.2 | | — | | Product Purchase Agreement, dated May 24, 2006, among UCB, Inc., UCB Manufacturing, Inc., Adams Respiratory Operations Sub, Inc. and Adams Respiratory Therapeutics, Inc., filed on May 31, 2006 as Exhibit 2.1 to the Company’s Current Report onForm 8-K, and incorporated herein by reference. |
| 2.3† | | — | | Asset Purchase Agreement, dated July 27, 2006, by and between Cardinal Health PTS, LLC and Adams Respiratory Operations, Inc., filed on August 2, 2006 as Exhibit 2.1 to the Company’s Current Report onForm 8-K, and incorporated herein by reference. |
| 3.1 | | — | | Certificate of Incorporation of Adams Respiratory Therapeutics, Inc., as amended, filed as Exhibit 3.1 to the Company’s Annual Report onForm 10-K for the fiscal year ended June 30, 2005, and incorporated herein by reference. |
| 3.2 | | — | | Amended and Restated Bylaws of Adams Respiratory Therapeutics, Inc.* |
| 4.1 | | — | | Specimen Stock Certificate of Adams Respiratory Therapeutics, Inc.’s Common Stock, par value $0.01 per share, filed as Exhibit 4.1 to the Company’s Registration Statement onForm S-1 (RegistrationNo. 333-123585), and incorporated herein by reference. |
| 4.2 | | — | | Reference is made to Exhibits 3.1 and 3.2. |
| 10.1 | | — | | Lease Agreement dated April 1, 2004 between Adams Laboratories, Inc. and William J. Adams, Jr. for Adams Respiratory Therapeutics, Inc.’s office in Chester, New Jersey, filed as Exhibit 10.1 to the Company’s Registration Statement onForm S-1 (RegistrationNo. 333-123585), and incorporated herein by reference. |
| 10.2† | | — | | Exclusive Distribution Agreement dated April 1, 2004 between Adams Laboratories, Inc. and Cardinal Health PTS, LLC, filed as Exhibit 10.3 to the Company’s Registration Statement onForm S-1 (RegistrationNo. 333-123585), and incorporated herein by reference. |
| 10.3‡ | | — | | 1999 Long-Term Incentive Plan, filed as Exhibit 10.6 to the Company’s Registration Statement onForm S-1 (RegistrationNo. 333-123585), and incorporated herein by reference. |
| 10.4‡ | | — | | Form of Award under the 1999 Long-Term Incentive Plan, filed as Exhibit 10.7 to the Company’s Registration Statement onForm S-1 (RegistrationNo. 333-123585), and incorporated herein by reference. |
| 10.5‡ | | — | | Amendment to the Adams Laboratories, Inc. 1999 Long-Term Incentive Plan, filed on August 28, 2006 as Exhibit 99.4 to the Company’s Current Report onForm 8-K, and incorporated herein by reference. |
| 10.6‡ | | — | | Adams Respiratory Therapeutics, Inc. 2005 Incentive Plan, filed as Exhibit 10.21 to the Company’s Registration Statement onForm S-1 (RegistrationNo. 333-123585), and incorporated herein by reference. |
| 10.7‡ | | — | | Amendment to the Adams Respiratory Therapeutics 2005 Incentive Plan, filed on August 28, 2006 as Exhibit 99.3 to the Company’s Current Report onForm 8-K, and incorporated herein by reference. |
| 10.8‡ | | — | | Form of Restricted Stock Award under Adams Respiratory Therapeutics, Inc. 2005 Incentive Plan, filed as Exhibit 10.22 to the Company’s Registration Statement onForm S-1 (RegistrationNo. 333-123585), and incorporated herein by reference. |
| 10.9‡ | | — | | Form of Incentive Stock Option Award under Adams Respiratory Therapeutics, Inc. 2005 Incentive Plan, filed as Exhibit 10.23 to the Company’s Registration Statement onForm S-1 (RegistrationNo. 333-123585), and incorporated herein by reference. |
| 10.10‡ | | — | | Form of Non-Statutory Stock Option Award under Adams Respiratory Therapeutics, Inc. 2005 Incentive Plan, filed as Exhibit 10.24 to the Company’s Registration Statement onForm S-1 (RegistrationNo. 333-123585), and incorporated herein by reference. |
69
| | | | | |
Exhibit
| | | | |
Number | | | | Description |
| |
| 10.11‡ | | — | | Form of Non-Statutory Stock Option Award under Adams Respiratory Therapeutics, Inc. 2005 Incentive Plan, filed on August 28, 2006 as Exhibit 99.1 to the Company’s Current Report onForm 8-K, and incorporated herein by reference. |
| 10.12‡ | | — | | Form of Performance-Based Restricted Stock Unit Certificate, filed on August 28, 2006 as Exhibit 99.2 to the Company’s Current Report onForm 8-K, and incorporated herein by reference. |
| 10.13 | | — | | Adams Respiratory Therapeutics, Inc. Director Compensation Plan, filed as Exhibit 10.8 to the Company’s Registration Statement onForm S-1 (RegistrationNo. 333-123585), and incorporated herein by reference. |
| 10.14 | | — | | Forms of Awards under Adams Respiratory Therapeutics, Inc. Director Compensation Plan, filed as Exhibit 10.9 to the Company’s Registration Statement onForm S-1 (RegistrationNo. 333-123585), and incorporated herein by reference. |
| 10.15‡ | | — | | Adams Laboratories, Inc. Retirement Savings Plan, filed as Exhibit 10.10 to the Company’s Registration Statement onForm S-1 (RegistrationNo. 333-123585), and incorporated herein by reference. |
| 10.16‡ | | — | | Employment Agreement with Michael J. Valentino dated August 7, 2003, filed as Exhibit 10.11 to the Company’s Registration Statement onForm S-1 (RegistrationNo. 333-123585), and incorporated herein by reference. |
| 10.17‡ | | — | | Separation and Independent Consulting Agreement, dated March 1, 2007, by and between the Company and David P. Becker, filed on March 7, 2007 as Exhibit 99.1 to the Company’s Current Report onForm 8-K, and incorporated herein by reference. |
| 10.18‡ | | — | | Form Change in Control Agreement, filed on June 13, 2007 as Exhibit 10.1 to the Company’s Current Report onForm 8-K, and incorporated herein by reference. |
| 10.19‡ | | — | | Adams Respiratory Therapeutics, Inc. Severance Pay Plan, filed on June 13, 2007 as Exhibit 10.2 to the Company’s Current Report onForm 8-K, and incorporated herein by reference. |
| 10.20 | | — | | Form of Indemnity Agreement between Adams Respiratory Therapeutics, Inc. and Directors and Certain Officers, filed as Exhibit 10.18 to the Company’s Registration Statement onForm S-1 (RegistrationNo. 333-123585), and incorporated herein by reference. |
| 10.21 | | — | | Lease Agreement, dated October 31, 2005, by and between WR&E — Chester, LLC and Adams Respiratory Therapeutics, Inc., filed on November 3, 2005 as Exhibit 10.1 to the Company’s Current Report onForm 8-K, and incorporated herein by reference. |
| 10.22 | | — | | Settlement Agreement dated January 14, 2005 by and among Adams Laboratories, Inc., Carolina Pharmaceuticals, Inc. and Cornerstone Biopharma, Inc., filed as Exhibit 10.20 to the Company’s Registration Statement onForm S-1 (RegistrationNo. 333-123585), and incorporated herein by reference. |
| 10.23 | | — | | Senior Revolving Credit Agreement, dated September 26, 2006, among Adams Respiratory Therapeutics, Inc., the Subsidiary Guarantors named therein, the Initial Lenders named therein, the Issuing Bank named therein and Royal Bank of Canada, filed on September 28, 2006 as Exhibit 10.27 to the Company’s Annual Report onForm 10-K for the fiscal year ended June 30, 2006, and incorporated herein by reference. |
| 10.24 | | — | | Security Agreement, dated July 11, 2006, from Adams Respiratory Therapeutics and the other Grantors named therein to Royal Bank of Canada, filed on July 17, 2006 as Exhibit 10.2 to the Company’s Current Report onForm 8-K, and incorporated herein by reference. |
| 10.25 | | — | | Security Agreement Supplement, dated September 26, 2006, among Adams Respiratory Products, Inc., Adams Respiratory Operations, Inc., Adams Respiratory Operations Sub, Inc., and Royal Bank of Canada, filed on September 28, 2006 as Exhibit 10.29 to the Company’s Annual Report onForm 10-K for the fiscal year ended June 30, 2006, and incorporated herein by reference. |
| 10.26 | | — | | Settlement Agreement, dated March 21, 2007, between Adams Respiratory Therapeutics, Inc. and Mutual Pharmaceutical Co. and United Research Laboratories, Inc., wholly owned subsidiaries of Pharmaceutical Holdings Corp., filed on May 15, 2007 as Exhibit 10.1 to the Company’s Quarterly Report onForm 10-Q for the quarter ended March 30, 2007, and incorporated herein by reference. |
70
| | | | | |
Exhibit
| | | | |
Number | | | | Description |
| |
| 14 | | — | | Code of Business Conduct and Ethics, filed as Exhibit 14 to the Company’s Annual Report onForm 10-K for the fiscal year ended June 30, 2005, and incorporated herein by reference. |
| 21* | | — | | Subsidiaries. |
| 23* | | — | | Consent of Ernst & Young LLP. |
| 31.1* | | — | | Certification of the Chief Executive Officer of Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
| 31.2* | | — | | Certification of the Chief Financial Officer of Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
| 32.1* | | — | | Certification of the Chief Executive Officer Pursuant to 18 U.S.C. Section 1350, As Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
| 32.2* | | — | | Certification of the Chief Financial Officer Pursuant to 18 U.S.C. Section 1350, As Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
| |
| * | Filed with this Annual Report. |
| |
| † | Portions of the exhibit have been omitted pursuant to a request for confidential treatment. The confidential portions have been filed with the SEC. |
| |
| ‡ | Management compensation contract or plan. |
71
Adams Respiratory Therapeutics, Inc.
Index to Consolidated Financial Statements and Schedule
| | | | | |
| | | F-2 | |
| | | F-3 | |
| | | F-4 | |
| | | F-5 | |
| | | F-6 | |
| | | F-8 | |
| | | F-34 | |
F-1
Report of Independent Registered Public Accounting Firm
The Board of Directors and Stockholders of
Adams Respiratory Therapeutics, Inc.
We have audited the accompanying consolidated balance sheets of Adams Respiratory Therapeutics, Inc. as of June 30, 2007 and 2006, and the related consolidated statements of operations, stockholders’ equity (deficit), and cash flows for each of the three years in the period ended June 30, 2007. Our audits also included the financial statement schedule listed in the Index at Item 15(a). These financial statements and schedule are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements and schedule based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the consolidated financial position of Adams Respiratory Therapeutics, Inc. at June 30, 2007 and 2006, and the consolidated results of its operations and its cash flows for each of the three years in the period ended June 30, 2007, in conformity with U.S. generally accepted accounting principles. Also, in our opinion, the related financial statement schedule, when considered in relation to the basic financial statements taken as a whole, presents fairly in all material respects the information set forth therein.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the effectiveness of Adams Respiratory Therapeutics, Inc.’s internal control over financial reporting as of June 30, 2007, based on criteria established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission and our report dated August 24, 2007 expressed an unqualified opinion thereon.
/s/ Ernst & Young LLP
MetroPark, New Jersey
August 24, 2007
F-2
Adams Respiratory Therapeutics, Inc.
Consolidated Balance Sheets
(Amounts in thousands, except per share amounts)
| | | | | | | | | |
| | | June 30, | |
| | | 2007 | | | 2006 | |
| |
| ASSETS |
| Current assets: | | | | | | | | |
| Cash and cash equivalents | | $ | 46,809 | | | $ | 40,320 | |
| Short-term investments | | | 32,972 | | | | 22,223 | |
| Accounts receivable, net of allowances for doubtful accounts of $54 at June 30, 2007 and 2006 | | | 15,936 | | | | 19,444 | |
| Inventories, net | | | 52,875 | | | | 10,603 | |
| Prepaid expenses and other assets | | | 3,335 | | | | 4,857 | |
AlleRxtm royalty interest held-for-sale | | | 3,771 | | | | 1,531 | |
| Income taxes receivable | | | 3,463 | | | | 4,045 | |
| Deferred tax assets | | | 8,178 | | | | 3,659 | |
| | | | | | | | | |
| Total current assets | | | 167,339 | | | | 106,682 | |
| Property, plant and equipment, net of accumulated depreciation | | | 19,763 | | | | 7,388 | |
| Deferred tax assets | | | 5,041 | | | | 2,545 | |
| Intangible assets, net of accumulated amortization of $490 and $565 at June 30, 2007 and 2006, respectively | | | 122,864 | | | | 125,597 | |
| Other assets | | | 3,458 | | | | 15,883 | |
| | | | | | | | | |
| Total assets | | $ | 318,465 | | | $ | 258,095 | |
| | | | | | | | | |
| |
| LIABILITIES AND STOCKHOLDERS’ EQUITY |
Current liabilities: | | | | | | | | |
| Accounts payable and accrued expenses | | $ | 24,010 | | | $ | 20,694 | |
| Accrued compensation and related items | | | 8,218 | | | | 5,769 | |
| Accrued returns, chargebacks, rebates and other sales allowances | | | 7,934 | | | | 5,701 | |
| Other current liabilities | | | 2,249 | | | | 1,648 | |
| | | | | | | | | |
| Total current liabilities | | | 42,411 | | | | 33,812 | |
| Long-term liabilities: | | | | | | | | |
| Deferred gain on sale of plant assets | | | — | | | | 1,309 | |
| Accrued royalties | | | 706 | | | | 701 | |
| | | | | | | | | |
| Total liabilities | | | 43,117 | | | | 35,822 | |
| | | | | | | | | |
| Commitments and contingencies (Note 14) | | | | | | | | |
| | | | | | | | | |
| Stockholders’ equity: | | | | | | | | |
| Preferred stock, $0.01 par value: Authorized shares — 50,000, Issued and outstanding — none | | | — | | | | — | |
| Common stock, Class A, $0.01 par value: | | | | | | | | |
| Authorized shares — 100,000, Issued and outstanding shares 35,695 and 34,874 at June 30, 2007 and 2006, respectively | | | 357 | | | | 349 | |
| Additional paid-in capital | | | 487,322 | | | | 464,877 | |
| Accumulated deficit | | | (212,313 | ) | | | (242,842 | ) |
| Accumulated other comprehensive loss | | | (18 | ) | | | (111 | ) |
| | | | | | | | | |
| Total stockholders’ equity | | | 275,348 | | | | 222,273 | |
| | | | | | | | | |
| Total liabilities and stockholders’ equity | | $ | 318,465 | | | $ | 258,095 | |
| | | | | | | | | |
See Notes to Consolidated Financial Statements which are an integral part of these statements.
F-3
Adams Respiratory Therapeutics, Inc.
Consolidated Statements of Operations
(Amounts in thousands, except per share amounts)
| | | | | | | | | | | | | |
| | | Year Ended June 30, | |
| | | 2007 | | | 2006 | | | 2005 | |
| |
| Net sales | | $ | 331,603 | | | $ | 239,105 | | | $ | 160,210 | |
| Cost of goods sold | | | 95,799 | | | | 49,358 | | | | 31,126 | |
| | | | | | | | | | | | | |
| Gross margin | | | 235,804 | | | | 189,747 | | | | 129,084 | |
| | | | | | | | | | | | | |
| Selling, marketing and administrative | | | 162,861 | | | | 98,998 | | | | 78,044 | |
| Product development | | | 23,855 | | | | 18,904 | | | | 7,392 | |
AlleRxtm charge | | | 2,699 | | | | — | | | | — | |
| Other, net | | | (1,187 | ) | | | (4,307 | ) | | | (789 | ) |
| | | | | | | | | | | | | |
| | | | 188,228 | | | | 113,595 | | | | 84,647 | |
| | | | | | | | | | | | | |
| Income before income taxes | | | 47,576 | | | | 76,152 | | | | 44,437 | |
| Provision for income taxes | | | 17,047 | | | | 29,801 | | | | 17,438 | |
| | | | | | | | | | | | | |
| Net income | | | 30,529 | | | | 46,351 | | | | 26,999 | |
| | | | | | | | | | | | | |
| Accretion of preferred stock | | | — | | | | — | | | | (202,566 | ) |
| Dividend paid to preferred stockholders | | | — | | | | — | | | | (30,033 | ) |
| | | | | | | | | | | | | |
| Net income/(loss) applicable to common stockholders | | $ | 30,529 | | | $ | 46,351 | | | $ | (205,600 | ) |
| | | | | | | | | | | | | |
| Income/(loss) per common share | | | | | | | | | | | | |
| Basic | | $ | 0.86 | | | $ | 1.42 | | | $ | (32.97 | ) |
| Diluted | | $ | 0.82 | | | $ | 1.28 | | | $ | (32.97 | ) |
| Weighted-average of common shares used in income/(loss) per share calculation | | | | | | | | | | | | |
| Basic | | | 35,340 | | | | 32,616 | | | | 6,236 | |
| Diluted | | | 37,113 | | | | 36,349 | | | | 6,236 | |
See Notes to Consolidated Financial Statements which are an integral part of these statements.
F-4
Adams Respiratory Therapeutics, Inc.
Consolidated Statements of Stockholders’ Equity/(Deficit)
(Amounts in thousands)
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | Accumulated
| | | Total
| |
| | | | | | | | | Additional
| | | | | | Other
| | | Stockholders’
| |
| | | Common Stock | | | Paid-In
| | | Accumulated
| | | Comprehensive
| | | Equity/
| |
| | | Shares | | | Amount | | | Capital | | | Deficit | | | Loss | | | (Deficit) | |
| |
| Balance as of June 30, 2004 | | | 5,210 | | | $ | 52 | | | $ | 11,577 | | | $ | (68,626 | ) | | | — | | | $ | (56,997 | ) |
| Stock compensation expense | | | — | | | | — | | | | 477 | | | | — | | | | — | | | | 477 | |
| Exercise of stock options | | | 384 | | | | 4 | | | | 1,200 | | | | — | | | | — | | | | 1,204 | |
| Exercise of warrants | | | 3,143 | | | | 31 | | | | 1,164 | | | | — | | | | — | | | | 1,195 | |
| Dividend | | | — | | | | — | | | | — | | | | (45,000 | ) | | | — | | | | (45,000 | ) |
| Tax benefit of stock options | | | — | | | | — | | | | 1,063 | | | | — | | | | — | | | | 1,063 | |
| Accretion of preferred stock | | | — | | | | — | | | | — | | | | (202,566 | ) | | | — | | | | (202,566 | ) |
| Comprehensive and net income | | | — | | | | — | | | | — | | | | 26,999 | | | | — | | | | 26,999 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Balance as of June 30, 2005 | | | 8,737 | | | | 87 | | | | 15,481 | | | | (289,193 | ) | | | — | | | | (273,625 | ) |
| Comprehensive income: | | | | | | | | | | | | | | | | | | | | | | | | |
| Net income | | | — | | | | — | | | | — | | | | 46,351 | | | | — | | | | 46,351 | |
| Unrealized loss on marketable securities, net of tax of $65 | | | — | | | | — | | | | — | | | | — | | | | (111 | ) | | | (111 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Total comprehensive income | | | | | | | | | | | | | | | | | | | | | | | 46,240 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Stock compensation expense | | | — | | | | — | | | | 4,549 | | | | — | | | | — | | | | 4,549 | |
| Restricted stock units | | | — | | | | — | | | | 255 | | | | — | | | | — | | | | 255 | |
| Exercise of stock options | | | 1,340 | | | | 14 | | | | 2,034 | | | | — | | | | — | | | | 2,048 | |
| Exercise of warrants | | | 373 | | | | 3 | | | | 714 | | | | — | | | | — | | | | 717 | |
| Tax benefit of stock options | | | — | | | | — | | | | 19,036 | | | | — | | | | — | | | | 19,036 | |
| Conversion of preferred stock | | | 17,534 | | | | 176 | | | | 316,279 | | | | — | | | | — | | | | 316,455 | |
| Initial public offering | | | 6,890 | | | | 69 | | | | 106,529 | | | | — | | | | — | | | | 106,598 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Balance as of June 30, 2006 | | | 34,874 | | | | 349 | | | | 464,877 | | | | (242,842 | ) | | | (111 | ) | | | 222,273 | |
| Comprehensive income: | | | | | | | | | | | | | | | | | | | | | | | | |
| Net income | | | — | | | | — | | | | — | | | | 30,529 | | | | — | | | | 30,529 | |
| Unrealized gain on marketable securities, net of tax of $55 | | | — | | | | — | | | | — | | | | — | | | | 93 | | | | 93 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Total comprehensive income | | | | | | | | | | | | | | | | | | | | | | | 30,622 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Stock compensation expense | | | — | | | | — | | | | 5,523 | | | | — | | | | — | | | | 5,523 | |
| Exercise of stock options and warrants | | | 674 | | | | 7 | | | | 3,101 | | | | — | | | | — | | | | 3,108 | |
| Tax benefit of stock options | | | — | | | | — | | | | 8,623 | | | | — | | | | — | | | | 8,623 | |
AlleRxtm conversion | | | 147 | | | | 1 | | | | 5,198 | | | | — | | | | — | | | | 5,199 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Balance as of June 30, 2007 | | | 35,695 | | | $ | 357 | | | $ | 487,322 | | | $ | (212,313 | ) | | $ | (18 | ) | | $ | 275,348 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
See Notes to Consolidated Financial Statements which are an integral part of these statements.
F-5
Adams Respiratory Therapeutics, Inc.
Consolidated Statements of Cash Flows
(Amounts in thousands)
| | | | | | | | | | | | | |
| | | Year Ended June 30, | |
| | | 2007 | | | 2006 | | | 2005 | |
| |
Operating Activities | | | | | | | | | | | | |
| Net income | | $ | 30,529 | | | $ | 46,351 | | | $ | 26,999 | |
| Adjustments to reconcile net income to net cash provided by operating activities: | | | | | | | | | | | | |
| Depreciation and amortization | | | 3,210 | | | | 1,259 | | | | 978 | |
| Stock compensation expense | | | 5,523 | | | | 4,804 | | | | 477 | |
AlleRxtm charge | | | 2,699 | | | | — | | | | — | |
| Impairment of intangible assets | | | 2,542 | | | | — | | | | 900 | |
| Loss on disposal of property, plant and equipment | | | 371 | | | | 926 | | | | 9 | |
| Loss on lease, net of sublease income | | | — | | | | 631 | | | | — | |
| Deferred tax assets | | | (6,960 | ) | | | 2,040 | | | | 8,549 | |
| Changes in operating assets and liabilities: | | | | | | | | | | | | |
| Accounts receivable | | | 3,508 | | | | (10,913 | ) | | | (4,357 | ) |
| Inventories | | | (42,272 | ) | | | (7,496 | ) | | | (591 | ) |
| Prepaid expenses and other current assets | | | 1,522 | | | | (130 | ) | | | (3,544 | ) |
| Intangible and other assets | | | (2,283 | ) | | | — | | | | — | |
| Accounts payable | | | 3,316 | | | | 10,507 | | | | 4,591 | |
| Income taxes payable (receivable) | | | 582 | | | | 945 | | | | (4,195 | ) |
| Accrued expenses and other liabilities | | | 3,979 | | | | 828 | | | | (1,679 | ) |
| | | | | | | | | | | | | |
Net cash provided by operating activities | | | 6,266 | | | | 49,752 | | | | 28,137 | |
Investing Activities | | | | | | | | | | | | |
| Purchases of property, plant and equipment | | | (15,314 | ) | | | (6,812 | ) | | | (1,731 | ) |
| Proceeds from disposal of property, plant and equipment | | | — | | | | — | | | | 33 | |
| Purchases of investments and increase in restricted cash | | | (28,000 | ) | | | (474,861 | ) | | | — | |
| Maturities of investments | | | 32,173 | | | | 436,644 | | | | — | |
| Purchases of intangible assets | | | — | | | | (122,000 | ) | | | (2,100 | ) |
| | | | | | | | | | | | | |
Net cash used in investing activities | | | (11,141 | ) | | | (167,029 | ) | | | (3,798 | ) |
Financing Activities | | | | | | | | | | | | |
| Borrowings on line of credit | | | 20,000 | | | | — | | | | — | |
| Repayments of line of credit | | | (20,000 | ) | | | — | | | | — | |
| Debt issuance costs | | | (367 | ) | | | — | | | | — | |
| Proceeds from exercise of warrants | | | — | | | | 717 | | | | 4,233 | |
| Proceeds from exercise of stock options | | | 3,108 | | | | 2,048 | | | | 1,204 | |
| Excess tax benefit from exercise of stock options | | | 8,623 | | | | 19,036 | | | | — | |
| Proceeds from issuance of common stock | | | — | | | | 107,772 | | | | — | |
| Payments of initial public offering costs | | | — | | | | — | | | | (1,174 | ) |
| Dividend payments | | | — | | | | — | | | | (45,000 | ) |
| | | | | | | | | | | | | |
Net cash provided by/(used in) financing activities | | | 11,364 | | | | 129,573 | | | | (40,737 | ) |
Net increase/(decrease) in cash and cash equivalents | | | 6,489 | | | | 12,296 | | | | (16,398 | ) |
Cash and cash equivalents at beginning of year | | | 40,320 | | | | 28,024 | | | | 44,422 | |
| | | | | | | | | | | | | |
Cash and cash equivalents at end of year | | $ | 46,809 | | | $ | 40,320 | | | $ | 28,024 | |
| | | | | | | | | | | | | |
Supplemental Disclosure of Cash Flow Information | | | | | | | | | | | | |
| Cash paid during the year for interest | | $ | 264 | | | $ | — | | | $ | — | |
| Cash paid during the year for income taxes | | $ | 15,935 | | | $ | 10,732 | | | $ | 13,084 | |
F-6
Supplemental Schedule of Noncash Financing Activity
On January 10, 2007, JMED converted its AlleRxtm royalty interest into shares of the Company’s common stock. In conjunction with this transaction, the Company increased its AlleRx intangible asset as follows:
| | | | | |
| Fair value of restricted common stock issued to JMED | | $ | 5,199 | |
AlleRxtm charge recorded in the Company’s Consolidated Statement of Income | | | 2,699 | |
| | | | | |
Increase in AlleRxtm royalty interest held-for sale | | $ | 2,500 | |
| | | | | |
See Notes to Consolidated Financial Statements which are an integral part of these statements.
F-7
Adams Respiratory Therapeutics, Inc.
Notes to Consolidated Financial Statements
($ in thousands, except per share amounts)
| |
| 1. | Nature of Operations and Summary of Significant Accounting Policies |
Nature of Operations
Adams Respiratory Therapeutics, Inc., a Delaware corporation (“the Company”), is a specialty pharmaceutical company focused on the late-stage development, commercialization and marketing of over-the-counter (“OTC”) and prescription pharmaceuticals for the treatment of respiratory disorders. As of the fiscal year ended June 30, 2007, the Company marketed seven OTC products under its Mucinex brand, including four products under its Mucinex products for children line, two products under its Delsym brand and one product under its Humibid brand. The Company’s corporate offices are located in Chester, New Jersey.
Basis of Presentation
The Company operates in one business segment, specialty pharmaceuticals. The Company’s “fiscal year” is from July 1 through June 30. Certain prior year amounts have been reclassified to conform to the current year presentation.
Principles of Consolidation
The consolidated financial statements include the accounts of Adams Respiratory Therapeutics, Inc. and its subsidiaries. All significant intercompany accounts and transactions have been eliminated.
Use of Estimates
The financial statements have been prepared in accordance with U.S. generally accepted accounting principles, which require the use of judgments and estimates by management that affect the amounts reported in the consolidated financial statements and accompanying notes. Actual results may differ from those estimates.
Cash Equivalents
The Company considers all highly liquid investments with a maturity date of three months or less at the date of acquisition to be cash equivalents.
Investments
Investments are comprised of marketable auction rate securities consisting primarily of federal-sponsored, state and municipal debt securities. Investments with maturities of less than one year from the balance sheet date are classified as short-term investments. Long-term investments that have maturities greater than one year from the balance sheet date and are not expected to be used in current operations are classified within other assets on the Consolidated Balance Sheet. The classification of auction rate securities is based on the Company’s expected holding period.
In June 2006, due to the utilization of $122,000 in connection with the Company’s purchase of Delsym and its planned repurchase of the Fort Worth, Texas manufacturing assets from Cardinal Health, the Company reclassified all of its investments from held-to-maturity to available-for-sale, in accordance with Statement of Financial Accounting Standards (“SFAS”) No. 115,Accounting for Certain Investments in Debt and Equity Securities. Previously, the Company classified its investments as held-to-maturity, recorded them at amortized cost and adjusted for the amortization of premiums and accretion of discounts to maturity. Such amortization, along with any interest earned on the securities, was recorded as interest income. After changing the investment classification, the Company’s available-for-sale securities are reported at fair value with unrealized
F-8
Adams Respiratory Therapeutics, Inc.
Notes to Consolidated Financial Statements — (Continued)
($ in thousands, except per share amounts)
gains and losses, net of tax, included in accumulated other comprehensive loss within the equity section of the Consolidated Balance Sheet.
Derivative Financial Instruments
The Company holds no derivative financial instruments and does not currently engage in hedging activities.
Accounts Receivable
Accounts receivable are generally billed on anet 30-day basis with a 2% discount if paid within 30 days. Occasionally, the Company provides extended payment terms and greater discounts to its customers to ensure adequate distribution of its products. The Company maintains a reserve for customer accounts that reduces receivables to amounts that are expected to be collected. In estimating the reserve, the Company considers historical experience with write-offs, specific customer risks and the level of past-due accounts. The provision for doubtful accounts is included in selling, marketing and administrative expenses. Reserves for cash discounts and trade promotional programs that are expected to be deducted from payments from the Company’s customers are recorded as a reduction to sales when the revenue is recorded. Reserves for cash discounts were $336 and $495 as of June 30, 2007 and 2006, respectively. Reserves for trade promotions were $2,830 and $2,796 as of June 30, 2007 and 2006, respectively.
Inventories
Inventories are stated at the lower of cost or market, using thefirst-in, first-out method. As a result of the Company’s July 31, 2006 repurchase of the manufacturing assets in Fort Worth, Texas from Cardinal Health (the “manufacturing assets repurchase”), the Company began to manufacture its own adult oral-solid Mucinex and Humibid products during fiscal 2007, thus carrying raw materials and work in progress in its inventory, in addition to finished goods.
Cardinal Health Profit Share. In April 2004, the Company sold substantially all of its manufacturing assets, raw materials and in-process inventory located in Fort Worth, Texas to Cardinal Health PTS, LLC (“Cardinal Health”). Pursuant to a supply agreement with Cardinal Health (the “2004 Supply Agreement”) entered into in connection with the sale of the manufacturing assets to Cardinal Health, Cardinal Health manufactured and supplied all of the Company’s drug products. Under the 2004 Supply Agreement, Cardinal Health was required to segregate direct manufacturing costs from indirect manufacturing costs, as defined in the 2004 Supply Agreement. As finished goods were completed and shipped to a Company-designated warehouse, Cardinal Health billed the Company for the actual direct manufacturing costs incurred plus amark-up. Themark-up was strictly provided for interim billing and cash flow purposes, and the final amount payable to Cardinal Health was calculated at the end of each contract year (March 31st) under a profit-sharing formula. The amount subject to the profit sharing was calculated as net sales, as defined in the 2004 Supply Agreement, less the actual manufacturing cost of the goods sold during the contract year less freight and other logistics costs. The resulting gross profit was subject to profit sharing rates that declined as the total value of gross profit increased. At the end of the contract year, a reconciliation was completed and a billing adjustment was made to the extent that the actual calculated profit share was greater or less than the totalmark-up paid to Cardinal Health during the contract year. At June 30, 2006, the Company had a receivable of $424 as a result of amark-up billed by Cardinal Health that exceeded the estimated March 31, 2007 contract year actual profit-share amount, which was included in prepaid expenses and other assets and eliminated in conjunction with the manufacturing assets repurchase. See Note 2 for more information relating to the manufacturing assets repurchase.
F-9
Adams Respiratory Therapeutics, Inc.
Notes to Consolidated Financial Statements — (Continued)
($ in thousands, except per share amounts)
The accounting policy with regard to the 2004 Supply Agreement with Cardinal Health was to record the actual direct manufacturing cost and estimated profit share as inventory, as that equaled the final cost to purchase inventory. Each month as product was sold, the actual direct manufacturing cost plus the estimate of the profit share amount earned by Cardinal Health was charged to cost of goods sold. The estimated profit share amount considered for each contract year included: (i) the Company’s projected net product sales and gross profit, (ii) the projected profit share and (iii) the contractual minimum profit share amount.
Property, Plant and Equipment
Property, plant and equipment is recorded at cost and depreciated using the straight-line method over estimated useful lives ranging from three to ten years. Leasehold improvements are amortized over the estimated useful lives of the assets or related lease terms, whichever is shorter. In accordance with Statement of Position (“SOP”)98-1,Accounting for the Costs of Computer Software Developed or Obtained for Internal Use, the Company capitalizes software costs incurred during the software development stage and amortizes them over the estimated useful life of the asset. Any preliminary or post-implementation software costs are expensed as incurred. Expenditures for repairs and maintenance are charged to expense as incurred; betterments that materially prolong the lives of assets are capitalized. The Company reviews its long-lived assets to assess recoverability using undiscounted cash flows. When necessary, charges for impairments of long-lived assets are recorded for the amount by which the carrying value of the assets exceeds the related present value of future cash flows. Upon retirement or other disposal of property, plant and equipment, the related cost and accumulated depreciation are eliminated from the asset and accumulated depreciation accounts, respectively. The difference, if any, between the net asset value and the proceeds is recorded as a realized gain or loss within other, net, or cost of goods sold if related to production equipment.
Useful lives of the Company’s property, plant and equipment are as follows:
| | | | | |
| | | Years | |
| |
| Machinery and equipment | | | 7-10 | |
| Furniture and fixtures | | | 5-7 | |
| Software | | | 3-5 | |
Intangible Assets
Intangible assets that have finite useful lives are amortized over their useful lives. Impairment of finite-lived intangibles and other assets is reviewed at least annually or when events and circumstances warrant an earlier review in accordance with SFAS No. 144,Accounting for the Impairment or Disposal of Long-Lived Assets(“SFAS No., 144”). Impairment is determined when estimated future undiscounted cash flows associated with an intangible asset are less than the asset’s carrying value. If such assets are considered to be impaired, the impairment recognized is measured by the amount by which the carrying amount of the assets exceeds the fair value of the assets on a discounted cash flow basis.
The Company’s Delsym trademark has an indefinite life and is not subject to amortization. In accordance with SFAS No. 142, Goodwill and Other Intangible Assets(“SFAS No. 142”),non-amortizable intangible assets are assessed for impairment on an annual basis, or sooner if an indicator of impairment is present. See Note 6 for additional information relating to the Company’s intangible assets.
Revenue Recognition
The Company recognizes product sales when the product is delivered to the customer, estimated provisions for product returns, rebates, chargebacks, discounts, trade promotions and other sales allowances
F-10
Adams Respiratory Therapeutics, Inc.
Notes to Consolidated Financial Statements — (Continued)
($ in thousands, except per share amounts)
are reasonably determinable, and collectibility is reasonably assured. Accruals for these provisions are presented in the financial statements as reductions to sales. At times, the Company may provide extended payment terms for initial orders of new products it launches. Such extended payment terms do not affect the Company’s ability to determine collectibility.
Sales Returns and Allowances
When the Company’s products are sold, the Company reduces the amount of revenue recognized from the sale by an estimate of future product returns and other sales allowances. Other sales allowances include cash discounts, rebates including Medicaid rebates, chargebacks, trade promotions and sales incentives relating to product sold in the current period.
Factors considered in the estimates of future product returns include an estimate of the amount of product in the trade channel, competitive products, the remaining time to expiration of the product, and the historical rate of returns. As of June 30, 2007 and 2006, allowances for sales returns of $6,152 and $3,150, respectively, were included within accrued returns, chargebacks, rebates and other. The allowance for sales returns as of June 30, 2007 included $2,353 relating to the Company’s April 2, 2007 voluntary recall of specific lots of Mucinex products for children due to the possible confusion in determining the proper dose of medication for children, which may have occurred due to the dosing cup included in the product packaging. The allowance for sales returns as of June 30, 2007 also included additional reserves for Mucinex line of products for children relating to excess inventory of Mini-Melt products and estimated product returns for Humibid SE due to low consumption projections for this product.
Consistent with industry practice, the Company maintains a return policy that allows its customers to return product within a specified period prior to and subsequent to the expiration date.
Factors considered in the estimates regarding other sales allowances include historical payment experience in relation to revenues, estimated customer inventory levels and current contract prices and terms with both direct and indirect customers.
The provision for chargebacks represents the amount payable in the future to a wholesaler for the difference between the invoice price paid to the Company by the wholesaler for a particular product and the negotiated contract price that the wholesaler’s customer pays for that product. The chargeback estimates take into consideration the current average chargeback rates by product and estimates of trade inventory level. The Company continually monitors its assumptions, giving consideration to current pricing trends and estimated trade inventory levels and makes adjustments to these estimates when it believes that the actual chargeback amounts payable in the future will differ from its original estimate. As of June 30, 2007 and 2006, reserves for chargebacks of $577 and $941, respectively, were included within accrued returns, chargebacks, rebates and other. At June 30, 2006 and 2007, the Company’s chargeback liability did not increase in proportion to the increase in sales activity. The Company believes that its increase in sales volume has not been attributed to government purchases but has resulted from increased retail consumer demand, largely driven by the Company’s consumer advertising campaigns. Therefore, chargebacks have become a lower percentage of the Company’s total sales during the fiscal years ended June 30, 2007 and 2006.
Rebates and sales incentives are recognized as a reduction of sales at the later of (i) the date at which the related revenue is recorded or (ii) the date at which the incentives are offered. Trade promotions include co-operative advertising arrangements and are recorded as a reduction of sales when the related revenue is recorded. The Company estimates the cost of rebates, sales incentives and trade promotions based on its historical experience with similar programs.
F-11
Adams Respiratory Therapeutics, Inc.
Notes to Consolidated Financial Statements — (Continued)
($ in thousands, except per share amounts)
If actual future payments for product returns and other sales allowances exceed the estimates the Company made at the time of sale, its financial position, results of operations and cash flow would be negatively impacted.
Advertising
Costs associated with television and radio advertising are expensed in the period the advertising airs and are included in selling, marketing and administrative expenses. Agency fees and production costs are expensed in the period they are incurred. Total advertising expense was $71,399, $37,652 and $32,043 for the years ended June 30, 2007, 2006 and 2005, respectively. As of June 30, 2007, the Company had prepaid advertising of $95. The Company had no prepaid advertising expense as of June 30, 2006.
Shipping and Handling Costs
The Company classifies shipping and handling costs within its selling, marketing and administrative expenses. Shipping and handling costs were $15,195, $7,604 and $3,905 for the years ended June 30, 2007, 2006 and 2005, respectively. Shipping and handling costs include distribution and storage fees related to the Company’s distribution services agreement, which it entered into with Cardinal Health in April 2004. In October 2005, Cardinal Health assigned the distribution services agreement to Cardinal Health 105, Inc. (“CH 105”).
Accounting for Stock-Based Compensation
In December 2004, the Financial Accounting Standards Board (“FASB”), issued SFAS No. 123(R),Share-Based Payment(“SFAS No. 123(R)”), to expand and clarify SFAS 123,Accounting for Stock-Based Compensation(“SFAS No. 123”), as amended by SFAS No. 148,Accounting for Stock-Based Compensation — Transition and Disclosure.SFAS No. 123(R) requires companies to recognize compensation expense in an amount equal to the fair value of all share-based payments granted to employees. Effective July 1, 2005, the Company adopted SFAS No. 123(R) and elected the prospective method for all future grants. Prior to the adoption of SFAS No. 123(R), the Company accounted for stock-based compensation in accordance with the fair value recognition provisions of SFAS No. 123. As a result, the adoption of SFAS No. 123(R) did not have a material impact on the Company’s operations, financial position or cash flows. The Company uses the graded-vesting methodology to record compensation expense for stock options over the vesting period, which generally ranges from three to five years. This methodology results in a greater amount of expense recognized towards the beginning of the vesting period as opposed to the straight-line method. For performance shares, stock compensation expense is recognized separately for each vesting tranche of the award, based on the closing price of the Company’s common stock on the date of grant, if the related financial targets are expected to be met. Because subjective input assumptions can materially affect the fair value estimate, in management’s opinion, the existing methods do not necessarily provide a reliable single measure of the fair value of the Company’s stock compensation arrangements.
Prior to fiscal 2006, the Company accounted for its stock-based compensation using the minimum value method as permitted for nonpublic companies under SFAS No. 123. However, since filing its initial registration statement on March 25, 2005, the Company is no longer considered “nonpublic” and must consider a volatility assumption in the calculation of fair value. Because the Company does not have much history as a public company to support its estimate of future volatility, a combination of peer companies in its industry with similar business cycles is used. This volatility assumption is used on options granted after March 25, 2005. The addition of this assumption materially increased the fair value of subsequent option grants.
F-12
Adams Respiratory Therapeutics, Inc.
Notes to Consolidated Financial Statements — (Continued)
($ in thousands, except per share amounts)
Product Development
Product development expenses have historically consisted of formulation, clinical and analytical development work with existing and well-established drugs and pharmaceutical ingredients. These products required development ofscale-up manufacturing, stability data and human pharmacokinetic studies to establish bioavailability and bioequivalence data for the Company’s products versus Food and Drug Administration (“FDA”) reference drugs. The data from these efforts was used in the preparation and filing of New Drug Applications (“NDAs”).
Generally, the Company’s formulation, chemistry and analytical manufacturing controls and stability work has been performed utilizing the Company’s own employees and since April 2004, in cooperation with Cardinal Health. Upon the Company’s acquisition of Delsym in June 2006, the Company also began to perform development work in cooperation with UCB, Inc. (“UCB”). Product development expenses include salary and benefits, raw materials and supplies, facilities, depreciation, and other allocated expenses associated with the performance of the above work and functions. The associated pharmacokinetic studies, clinical trials and certain support functions in preparing protocols, reports and other regulatory documents are primarily performed by scientific consultants and third party contract research organizations and are also included within product development expenses.
All of the Company’s prescription drug development other than development related to the erdosteine program has been limited to Phase I pharmacokinetic clinical qualification to support the associated NDAs. The Company has also recently completed a Phase IV clinical study involving Mucinex D in combination with antibiotics, and it has submitted the results of this study for presentation at an upcoming medical conference. The Company is continuing to analyze data from the phase IIb clinical study for erdosteine, which was completed in December 2006. The Company plans to request a meeting with the FDA to discuss the clinical results, before it determines the ultimate outcome of the erdosteine program. The costs related to the erdosteine NDA program and the phase I studies for the pipeline prescription drugs in development are performed primarily by scientific consultants and third party contract research organizations and are also included within product development expenses.
Income Taxes
Income taxes are accounted for in accordance with SFAS No. 109,Accounting for Income Taxes(“SFAS No. 109”). Under this method, deferred tax assets and liabilities are recognized for the expected future tax consequences of temporary differences between the financial statement and tax basis of assets and liabilities using enacted tax rates in effect for the years in which the differences are expected to reverse. In assessing the realizability of deferred tax assets, the Company considers whether it is more likely than not that some portion or all of the deferred tax assets will not be realized. This assessment requires significant judgment and estimates. The ultimate realization of the deferred tax assets is dependent upon the generation of future taxable income during the period in which those temporary differences become deductible. The Company considers its history of losses, scheduled reversal of deferred tax assets and liabilities and projected future taxable income over the periods in which the deferred tax items are deductible. Internal Revenue Code Sections 382 and 383 contain provisions that may limit the net operating loss carryforward (“NOL”) available to be used in any given year.
In July 2006, the FASB issued FASB Interpretation No. 48,Accounting for Uncertainty in Income Taxes(“FIN 48”), an interpretation of SFAS No. 109. FIN 48 provides measurement and recognition guidance related to accounting for uncertainty in income taxes by prescribing a recognition threshold for tax positions. FIN 48 also requires extensive disclosures about uncertainties in the income tax positions taken. The Company adopted FIN 48 on July 1, 2007, as required, and is currently evaluating the impact of its adoption of FIN 48
F-13
Adams Respiratory Therapeutics, Inc.
Notes to Consolidated Financial Statements — (Continued)
($ in thousands, except per share amounts)
on its financial statements. The Company is currently undergoing a federal tax audit for fiscal 2005, which may have an impact on its analysis. No material adjustments have been proposed by the Internal Revenue Service to date.
Accretion of Preferred Stock
Prior to the conversion to common stock upon the closing of the Company’s initial public offering in July 2005, the Company adjusted the carrying value of its Series A redeemable convertible preferred stock (“Series A Preferred Stock”), its Series B redeemable convertible preferred stock (“Series B Preferred Stock”) and its Series C redeemable convertible preferred stock (“Series C Preferred Stock”) to redemption value. For Series A Preferred Stock and Series B Preferred Stock, redemption value equaled fair value. For Series C Preferred Stock, redemption value equaled the greater of 200% of the amounts invested or fair value. All classes of preferred stock were redeemable at the option of the holder on specified dates. Accretion of Series C Preferred Stock was recorded as a reduction of net income/(loss) applicable to common stockholders. To the extent that the fair value was greater than the accreted liquidation value at the balance sheet date, the preferred stock was adjusted to reflect the fair market value, with the offset charged to accumulated deficit within stockholders’ equity/(deficit) and reflected a reduction of net income/(loss) applicable to common stockholders. Upon the closing of the Company’s initial public offering in July 2005, the Company’s preferred stock automatically converted into shares of common stock and, as a result, there was no further accretion.
Through July 2001, the Company incurred approximately $900 and $1,200 of issuance costs in connection with the issuance of Series A Preferred Stock and Series B Preferred Stock, respectively. Such costs were recorded as a reduction of the carrying amount of the preferred stock and were accreted through a charge to accumulated deficit up to the original redemption date, using the effective interest method and included in net income/(loss) applicable to common stockholders. There were no issuance costs associated with the Series C Preferred Stock. There was no preferred stock outstanding as of June 30, 2006.
Income/(Loss) per Common Share
Basic net income/(loss) per common share (“Basic EPS”) is computed by dividing net income/(loss) applicable to common stockholders by the weighted-average number of common shares outstanding. Diluted net income/(loss) per common share (“Diluted EPS”) is computed by dividing net income/(loss) applicable to common stockholders by the weighted-average number of common shares outstanding, plus potential dilutive common shares.
| |
| 2. | Manufacturing Assets Repurchase |
On July 31, 2006, the Company repurchased certain Fort Worth, Texas manufacturing assets from Cardinal Health for $28,000, $24,000 of which was paid upon closing with the remainder paid quarterly during fiscal 2007. The $28,000 purchase price includes the acquisition of $11,000 in inventory and $7,000 in manufacturing assets. The purchase price also includes $9,700 of non-recurring expenses for items such as termination fees, exit costs and impaired assets, as well as the reversal of the deferred gain from the 2004 sale of the manufacturing assets to Cardinal Health, which were recorded primarily within cost of goods sold during the fiscal year ended June 30, 2007. In connection with the manufacturing assets repurchase, the Company also entered into a commercial manufacturing agreement for guaifenesin granulation and a packaging agreement with Cardinal Health. (See Note 14 for additional information related to these agreements.) In April 2007, the Blackstone Group, L.P. acquired Cardinal Health, and Cardinal Health was renamed Catalent Pharma Solutions, Inc. (“Catalent”). In accordance with the new granulation and packaging agreements, the Company will continue to rely on Catalent to perform certain aspects of the manufacture and
F-14
Adams Respiratory Therapeutics, Inc.
Notes to Consolidated Financial Statements — (Continued)
($ in thousands, except per share amounts)
packaging of Mucinex SE, Mucinex DM and Mucinex D, as well as the maximum strength versions of these products, which the Company launched in July 2007.
| |
| 3. | Supplementary Financial Information |
Inventories
As a result of the manufacturing assets repurchase, the Company began to manufacture its own adult oral-solid Mucinex and Humibid products during fiscal 2007, thus carrying raw materials and work in progress in its inventory, in addition to finished goods, some of which were previously purchased from Cardinal Health (now Catalent).
The composition of the Company’s inventories as of June 30, 2007 and 2006 is as follows:
| | | | | | | | | |
| | | June 30,
| | | June 30,
| |
| | | 2007 | | | 2006 | |
| |
| Raw materials | | $ | 8,033 | | | $ | — | |
| Work in progress | | | 12,443 | | | | — | |
| Finished goods | | | 36,253 | | | | 10,627 | |
| | | | | | | | | |
| | | | 56,729 | | | | 10,627 | |
| Less: reserve for obsolescence(1) | | | (3,854 | ) | | | (24 | ) |
| | | | | | | | | |
| Inventories, net | | $ | 52,875 | | | $ | 10,603 | |
| | | | | | | | | |
| | |
| (1) | | The June 30, 2007 balance primarily relates to Children’s Mini-Melts inventory approaching expiration. |
Other Assets
Other assets as of June 30, 2006 primarily consisted of long-term investments of $14,883. As of June 30, 2007, all remaining investments are scheduled to mature within one year and have been reclassified to short-term. Other assets as of June 30, 2007 primarily consist of rabbi trust assets of $1,606 related to the Company’s deferred compensation plan. (See Note 9 for additional information related to the Company’s deferred compensation plan.) Also included within other assets is the restricted cash balance of $1,000 in both periods, which consists of a certificate of deposit of $1,000 representing cash held as collateral for the Company’s letter of credit for its office facility in Chester, New Jersey.
Accrued Compensation and Related Items
As of June 30, 2007 and 2006, the balance of accrued compensation and related items primarily consisted of a bonus accrual relating to fiscal 2007 and 2006. Also included within accrued compensation and related items as of June 30, 2007 and 2006 are accrued commissions, vacation and severance. The June 30, 2007 balance also includes a liability relating to the Company’s deferred compensation plan.
Other, net
For the fiscal year ended June 30, 2007, other, net primarily consisted of interest income of $3,894 and AlleRxtm royalty income of $453, which were partially offset by an impairment charge of $2,542 relating to the Company’s Humibid intangible asset (see Note 6) and interest expense of $478, primarily related to borrowings from the Company’s senior secured revolving credit facility, which the Company fully repaid in October 2006. For the fiscal year ended June 30, 2006, other, net primarily consisted of interest income of
F-15
Adams Respiratory Therapeutics, Inc.
Notes to Consolidated Financial Statements — (Continued)
($ in thousands, except per share amounts)
$5,677, partially offset by a one-time pretax loss of $1,557 recorded in connection with the move of the Company’s corporate headquarters in Chester, New Jersey, which was comprised of $926 associated with the write-off of leasehold improvements and fixtures not moved to the new facility and $631 representing the present value of the cash flows associated with abandoned lease, adjusted for expected sublease income.
The following table summarizes the Company’s investments as of June 30, 2007 and 2006, all of which are federal or state government and agency securities.
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | As of June 30,
| | | As of June 30,
| |
| | | 2007 | | | 2006 | |
| | | | | | Government
| | | | | | | | | Government
| | | | |
| | | U.S. State
| | | Sponsored
| | | | | | U.S. State
| | | Sponsored
| | | | |
| | | Agency Bonds | | | Bonds | | | Total | | | Agency Bonds | | | Bonds | | | Total | |
| |
| Amortized cost | | $ | 18,000 | | | $ | 15,000 | | | $ | 33,000 | | | $ | 12,282 | | | $ | 25,000 | | | $ | 37,282 | |
| Gross unrealized losses | | | — | | | | 28 | | | | 28 | | | | 13 | | | | 163 | | | | 176 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Estimated fair market value | | $ | 18,000 | | | $ | 14,972 | | | $ | 32,972 | | | $ | 12,269 | | | $ | 24,837 | | | $ | 37,106 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
The above marketable debt securities consist of auction rate securities with contractual ultimate maturities of up to 33 years. The auction rate securities have periodic re-set dates, at which point they are sold by the Company. All re-set dates were within one year of June 30, 2007. The Company classifies its investments in marketable securities as “available for sale” and records them at their fair value with any unrealized gains and losses reported in other comprehensive income. The Company did not realize any gains or losses on its investments during the fiscal years ended June 30, 2007 or 2006, as all investments were sold at their re-set maturity dates, yielding face value. Any gains or losses to be recognized by the Company upon the sale of a marketable security are specifically identified by investment. The Company did not have any other-than-temporary declines in the fair value of its investments.
| |
| 5. | Property, Plant and Equipment |
As of June 30, 2007 and 2006, property, plant and equipment and the accumulated depreciation were as follows:
| | | | | | | | | |
| | | June 30, | |
| | | 2007 | | | 2006 | |
| |
| Property, plant and equipment: | | | | | | | | |
| Machinery and equipment | | $ | 10,764 | | | $ | 390 | |
| Software | | | 2,991 | | | | 274 | |
| Leasehold improvements | | | 4,210 | | | | 2,360 | |
| Furniture and fixtures | | | 3,719 | | | | 2,760 | |
| Construction in progress | | | 1,354 | | | | 2,311 | |
| | | | | | | | | |
| | | | 23,038 | | | | 8,095 | |
| Less: accumulated depreciation and amortization | | | (3,275 | ) | | | (707 | ) |
| | | | | | | | | |
| | | $ | 19,763 | | | $ | 7,388 | |
| | | | | | | | | |
F-16
Adams Respiratory Therapeutics, Inc.
Notes to Consolidated Financial Statements — (Continued)
($ in thousands, except per share amounts)
Depreciation and amortization expense for property, plant and equipment was $2,567, $423 and $279 for the fiscal years ended June 30, 2007, 2006 and 2005, respectively.
Indefinite Life Asset — Delsym Trademark
In June 2006, the Company paid $122,000 to purchase the Delsym12-Hour cough suppressant liquid product line from UCB. In addition, the Company agreed to pay a 2.5% royalty to UCB over the next five years for the intellectual property associated with the Delsym product. In connection with this transaction, the entire purchase price of $122,000 has been recorded as an intangible asset representing the purchased U.S. trademarks and U.S. NDA No. 18658 for Delsym, which provided the Company with an exclusive right to market Delsym in the United States. UCB still owns the manufacturing process and will continue to manufacture the Delsym product for the Company from raw materials to finished goods under a six-year renewable supply agreement, which may be renewed for consecutive one-year periods. However, UCB is prohibited from manufacturing a similar product, branded or generic. The Company also entered into a technology transfer agreement with UCB that provides the Company with the ability to transfer the Delsym manufacturing process to a third party after five years.
Delsym was originally launched in the United States in December 1982. Since competitors have not been able to replicate the manufacturing process for this product and introduce competing products to the market and there are no indicators of diminishing cash flows for Delsym in the foreseeable future, this asset was deemed to have an indefinite life and thus is not being amortized. This intangible asset is reviewed for impairment at least annually under SFAS 142. The Company completed the annual impairment test for Delsym for fiscal 2007 in the fiscal fourth quarter and concluded that this asset is not impaired. Future impairment tests will be performed annually in the fiscal fourth quarter, or sooner if an indicator of impairment is present.
Amortizable Intangible Assets
The Company’s amortizable intangible assets as of June 30, 2007 and June 30, 2006 consist of the following:
| | | | | | | | | |
| | | June 30,
| | | June 30,
| |
| | | 2007 | | | 2006 | |
| |
| Amortizable intangible assets | | $ | 1,354 | | | $ | 4,162 | |
| Accumulated amortization | | | (490 | ) | | | (565 | ) |
| | | | | | | | | |
Net balance | | $ | 864 | | | $ | 3,597 | |
| | | | | | | | | |
The Company’s amortizable intangible assets are carried at cost less accumulated amortization, which is calculated on a straight-line basis over the estimated useful life of the asset, generally between five and fifteen years. Amortization expense was $643, $836 and $699 for fiscal 2007, 2006 and 2005, respectively. The
F-17
Adams Respiratory Therapeutics, Inc.
Notes to Consolidated Financial Statements — (Continued)
($ in thousands, except per share amounts)
estimated aggregate amortization expense for the next five fiscal years and thereafter with regard to the Company’s trade names and license agreements is:
| | | | | |
| Fiscal 2008 | | $ | 155 | |
| Fiscal 2009 | | | 155 | |
| Fiscal 2010 | | | 155 | |
| Fiscal 2011 | | | 155 | |
| Fiscal 2012 | | | 155 | |
| Thereafter | | | 89 | |
| | | | | |
| | | $ | 864 | |
| | | | | |
In February 2005, the Company entered into an agreement with Cornerstone Biopharma Inc. (“Cornerstone”), pursuant to which the Company received the Humibid trademark from Cornerstone and Cornerstone received the AlleRxtm assets from the Company. Additionally, the parties released each other from all claims and damages in a lawsuit that the Company filed in 2004. As part of this arrangement, the Company is contractually obligated to assume the financial responsibility for the first $1,000 of returned AlleRxtm product sold by the Company prior to February 15, 2005 and returned to Cornerstone during the18-month period beginning February 15, 2005. Conversely, Cornerstone assumed the same financial responsibility for the first $1,000 of Humibid product returns for the same18-month period. After the $1,000 threshold is met, the Company has the responsibility for all Humibid product returns, whether sold by it or Cornerstone, and Cornerstone bears the same liability for AlleRxtm product returns. In connection with this agreement, the Company is obligated to pay to Cornerstone a royalty ranging from 1% to 2% of net Humibid sales for a period of three years that began on February 15, 2005 with an annual minimum royalty payment of $50.
The Company recorded a $3,000 intangible asset, which represented the fair value of the Humibid trademark and a corresponding $3,000 liability that the Company assumed in the transaction during fiscal 2005. The liability assumed represents the assumed returns liability in excess of the $1,000 threshold. The remaining liability is recorded in accrued returns, chargebacks, rebates and other sales allowances. As of June 30, 2006, the first $1,000 of Humibid inventory had been returned and was recorded in prepaid expenses and other assets as a receivable due from Cornerstone. The Company is currently in litigation with Cornerstone with regard to these returns.
The $3,000 Humibid intangible asset was being amortized over its remaining estimated useful life of 15 years. In June 2007, the Company determined that the projected cumulative undiscounted cash flows to be generated for Humibid are less than the carrying value of the related intangible asset. As such, the Company believes that the Humibid intangible asset is impaired. As a result, the Company recorded an impairment charge of $2,542, representing the difference between the fair value using the projected discounted cash flow method and the carrying value of the related intangible asset as of June 30, 2007, which is included within other, net in the Company’s Consolidated Statement of Income for fiscal 2007.
During fiscal 2004, the Company paid $1,250 to enter into a development and license agreement with Pharmaceuticals Design L.L.C. (“PD”), for the rights to market respiratory products in patent-protected packaging configurations. In July 2005, the Company decided not to go forward with the development and license agreement with PD. To terminate this agreement, the Company paid PD $500, which was expensed to selling, marketing and administrative expenses in July 2005.
In April 2004, the Company entered into a royalty-bearing license agreement with Celltech Pharmaceuticals, Inc. As part of this agreement, the Company recorded a liability and a related asset of approximately $1,270, which represented the present value of the minimum amount due under the license agreement. This
F-18
Adams Respiratory Therapeutics, Inc.
Notes to Consolidated Financial Statements — (Continued)
($ in thousands, except per share amounts)
asset is amortized as additional royalty expense over the ten-year term of the license agreement. See Note 14 for further discussion of this license agreement.
AlleRxtm Royalty Interest Held-For-Sale
In April 1999, the Company entered into a sublicense agreement with JMED Pharmaceuticals, Inc. (“JMED”), which provided the Company with an exclusive right to manufacture and market AlleRxtm in exchange for royalty payments to JMED. Subsequently, the Company granted JMED the right to exchange its on-going royalty interest in the sublicense agreement into shares of the Company’s common stock in the event of a public offering or change of control. In December 2004, the Company received the right to assign its AlleRxtm sublicense agreement with JMED to Cornerstone. Pursuant to the 2004 assignment agreement with JMED, the Company paid JMED $2,000 in January 2005, which was recorded as an intangible asset. Additionally, the assignment agreement provided that a valuation would be performed on JMED’s on-going royalty interest in the sublicense agreement and JMED would have the right to receive the value of the royalty above the $2,000 previously paid. In December 2006, a third-party valuation was completed and the royalty interest was valued at $4,500. Because the appraisal value exceeded the $2,000 previously paid by the Company, JMED had the right to exchange the excess value of its royalty interest of $2,500 into 147,058 unregistered shares of the Company’s common stock at the initial public offering price of $17.00.
On December 29, 2006, JMED notified the Company of its intention to convert its royalty interest, and the Company recorded a liability of $5,199, representing the total market value of the 147,058 shares of its common stock at the $40.81 closing market price of the Company’s common stock on the date it received notice from JMED, discounted to reflect the fact that the Company issued unregistered shares to JMED with a minimum holding period of one year. The fair value was determined using the Black-Scholes option pricing model for a put option with a one year holding period. In addition, the Company increased the intangible asset relating to the AlleRxtm interest by $2,500, representing the excess value of the royalty interest per the valuation. Because the $4,500 appraised value of this intangible asset is below the $7,199 in total value paid to JMED ($2,000 in January 2005 and $5,199 in stock issued in January 2007), the Company expensed $2,699 in December 2006, representing the appreciation in its common stock from its initial public offering through the date it received the notice of conversion. This non-cash pretax expense was included in a separate line item titled “AlleRxtm charge” in the Company’s Consolidated Statements of Operations for the fiscal year ended June 30, 2007. On January 10, 2007, the Company issued 147,058 shares of its common stock to JMED and began to actively market the AlleRxtm royalty interest to outside parties due to the fact that this product is not consistent with its business strategy. Accordingly, the Company reclassified the related intangible asset to held-for-sale at its net carrying value of $3,771 and suspended its amortization, in accordance with SFAS No. 144. The Company expects to sell the AlleRxtm royalty interest within the next six months.
| |
| 7. | Redeemable Convertible Preferred Stock |
In connection with the Company’s initial public offering in July 2005, all of the Company’s redeemable convertible preferred stock was converted into shares of the Company’s common stock.
The following amounts were outstanding for each series of redeemable convertible preferred stock through June 30, 2006:
| | | | | | | | | | | | | |
| | | Series A | | | Series B | | | Series C | |
| |
| Balance at June 30, 2005 | | | 98,705 | | | | 98,251 | | | | 119,499 | |
| Conversion to common stock | | | (98,705 | ) | | | (98,251 | ) | | | (119,499 | ) |
| | | | | | | | | | | | | |
| Balance at June 30, 2006 | | $ | — | | | $ | — | | | $ | — | |
| | | | | | | | | | | | | |
F-19
Adams Respiratory Therapeutics, Inc.
Notes to Consolidated Financial Statements — (Continued)
($ in thousands, except per share amounts)
| |
| 8. | Stockholders’ Equity (Deficit) |
Initial Public Offering
In July 2005, the Company completed its initial public offering of 9,142,500 shares of common stock at a price of $17.00 per share. The offering consisted of 6,889,500 newly issued shares sold by the Company and 2,253,000 shares sold by selling stockholders. The offering generated gross proceeds of approximately $117,000 and net proceeds of $106,598. Upon completion of the initial public offering, all three series of redeemable convertible preferred stock were converted into 17,533,696 shares of common stock.
Secondary Offering
In December 2005, the Company completed a secondary offering of 5,660,890 shares of its common stock. All of the shares were sold by selling stockholders. The Company did not sell any shares in, or receive any proceeds from, the secondary offering. The Company paid approximately $700 of legal, printing, audit and other costs related to this secondary offering, which are included in selling, marketing and administrative expense for the fiscal year ended June 30, 2006.
On August 17, 2006, the Company registered 10,025,235 shares of common stock pursuant to its existing contractual obligation to register shares following the first anniversary of its initial public offering. On September 15, 2006, the Company filed a prospectus supplement with respect to 3,000,000 shares of its common stock offered by selling stockholders and an additional 450,000 shares of its common stock to cover over-allotments.
The Company did not sell any shares or receive any proceeds from this registration or the secondary offerings made pursuant to the prospectus supplement. The Company paid approximately $268 in legal, printing, accounting and other costs related to this secondary offering, which are included in selling, marketing and administrative expenses for the fiscal year ended June 30, 2007.
The Company provides a 401(k) benefit plan (the “Plan”) covering substantially all of the Company’s employees. Employees are eligible to participate in the Plan upon attaining the age of 18 and completing six months of service with the Company, and can contribute up to 80% of their compensation each year, subject to certain Internal Revenue Code limitations. The Company’s board of directors approved a match on employee contributions made during calendar years beginning in 2005, contingent upon an established sales threshold for the fiscal year ending during that calendar year. For the fiscal years ended June 30, 2007 and 2006, $434 and $190, respectively, was recorded on employee matches, primarily within selling, marketing and administrative expenses.
The Company also provides a deferred compensation plan, which allows certain of its highly compensated employees to defer their salaries, bonuses or commissions to a future date. The Company’s other assets as of June 30, 2007 include rabbi trust assets of $1,606 relating to this arrangement, with a corresponding liability of $1,547 recorded within accrued compensation and related items.
| |
| 10. | Stock Compensation Plan |
In March 2005, the Company’s board of directors adopted, and in May 2005 the Company’s stockholders adopted, the 2005 Incentive Plan. Under the terms of the 2005 Incentive Plan, employees, directors and others designated by the Company’s board of directors may be granted awards in the form of incentive stock options, nonqualified stock options, stock appreciation rights, performance awards, restricted stock, stock units,
F-20
Adams Respiratory Therapeutics, Inc.
Notes to Consolidated Financial Statements — (Continued)
($ in thousands, except per share amounts)
dividend equivalents or other stock-based awards at the discretion of the compensation committee of the Company’s board of directors. The Company reserved 2,737,802 shares for issuance under the 2005 Incentive Plan. As of June 30, 2007, 1,454,881 shares remained available for issuance under the 2005 Incentive Plan. Prior to the Company’s initial public offering, the Company issued awards under the 1999 Long-Term Incentive Plan, which had similar features to the 2005 Incentive Plan. The Company’s board of directors decided not to grant any additional awards pursuant to the 1999 Long-Term Incentive Plan after completion of the Company’s initial public offering.
The 2005 Incentive Plan is administered by the compensation committee of the Company’s board of directors. The compensation committee has the authority to designate participants, determine the type or types of awards to be granted to each participant and the number, terms and conditions of awards. Options granted are subject to a vesting term, generally over three to five years from the grant date. Options are granted for a fixed number of shares with an exercise price equal to the NASDAQ Stock Market closing price at the date of grant. New shares are issued upon exercise of the stock options.
On August 14, 2006, the Company’s board of directors amended the Company’s 2005 Incentive Plan and the Company’s 1999 Long-Term Incentive Plan to require mandatory anti-dilution adjustments for all equity restructurings, including stock dividends, stock splits, spin-offs, rights offerings, or large nonrecurring cash dividends, as well as to continue to permit discretionary adjustments. The board of directors also approved new long-term incentive awards, including stock options and performance-based restricted stock units under the Company’s 2005 Incentive Plan, as amended.
The Company currently accounts for its stock-based compensation in accordance with the provisions of SFAS No. 123(R). Prior to filing its initial registration statement on March 25, 2005, the Company calculated its stock-based compensation for stock options using the minimum value method, which did not include a volatility assumption in the calculation of fair value, as permitted for nonpublic companies under SFAS 123. Because the Company does not have sufficient history as a public company to support its estimate of future volatility, the historical volatility of a combination of peer companies of similar nature and size are used to calculate the volatility assumption for grants after March 25, 2005. The addition of the volatility assumption significantly increased the stock compensation expense in fiscal 2007 and 2006, as compared to fiscal 2005.
The Company recorded stock compensation expense of $5,523, $4,804 and $477 for the fiscal years ended June 30, 2007, 2006 and 2005, respectively, primarily in selling, marketing and administrative expenses. The tax benefit recognized on the stock compensation expense for the fiscal years ended June 30, 2007 and 2006 was $8,623 and $19,036, respectively, and related to the nonqualified stock options exercised during the periods and disqualifying dispositions upon the exercise of incentive stock options. Compensation cost not yet recognized as of June 30, 2007 was $5,662 and is expected to be recognized over the next weighted average of 1.1 years.
Stock Options
In estimating the expected life of the options, the Company considered the vesting period of the grants, historical exercise patterns, the expected volatility of the stock, and employees’ ages and years of service. The Company reduced the expected life of the options from six years for fiscal 2005 and prior to approximately five years for those grants in fiscal 2006 subsequent to the initial public offering. Under the 2005 Incentive Plan, as amended, the vesting term for stock options was lowered to three years. As a result, the Company lowered the expected life of the options granted under the new provisions to three years in calculating the stock option compensation expense. The Company assumed that options would be exercised sooner, because there is now a market for the stock and the increased stock volatility is expected to result in increased exercise activity in future periods.
F-21
Adams Respiratory Therapeutics, Inc.
Notes to Consolidated Financial Statements — (Continued)
($ in thousands, except per share amounts)
The Company uses the graded vesting methodology to record compensation expense for stock options over the vesting period. This methodology results in a greater amount of expense recognized towards the beginning of the vesting period as opposed to the straight-line method. Because subjective input assumptions can materially affect the fair value estimate, in management’s opinion, the existing methods do not necessarily provide a reliable single measure of the fair value of its stock compensation arrangements.
The following weighted-average assumptions were used in the calculation of fair value of options granted in the respective periods:
| | | | | | | | | | | | | |
| | | Year Ended June 30, | |
| | | 2007 | | | 2006 | | | 2005 | |
| |
| Expected life of the option (in years) | | | 3 | | | | 5 | | | | 6 | |
| Risk-free interest rate | | | 4.9 | % | | | 4.1 | % | | | 3.4 | % |
| Volatility | | | 42.8 | % | | | 65.3 | % | | | 0 | % |
| Expected dividend yield | | | 0 | % | | | 0 | % | | | 0 | % |
| Weighted-average fair value of stock options granted | | $ | 15.09 | | | $ | 11.84 | | | $ | 1.14 | |
A summary of the Company’s stock option activity and related information is as follows:
| | | | | | | | | |
| | | | | | Weighted-
| |
| | | | | | Average
| |
| | | | | | Exercise
| |
| | | Shares | | | Price | |
| | | (Shares in thousands) | |
| |
| Options outstanding at June 30, 2005 | | | 3,247 | | | $ | 1.68 | |
| Granted | | | 955 | | | | 20.74 | |
| Canceled | | | (26 | ) | | | 11.15 | |
| Exercised | | | (1,338 | ) | | | 1.53 | |
| | | | | | | | | |
| Options outstanding at June 30, 2006 | | | 2,838 | | | | 8.08 | |
| Granted | | | 349 | | | | 42.70 | |
| Canceled | | | (183 | ) | | | 17.76 | |
| Exercised | | | (672 | ) | | | 4.63 | |
| | | | | | | | | |
| Options outstanding at June 30, 2007 | | | 2,332 | | | $ | 13.48 | |
| | | | | | | | | |
F-22
Adams Respiratory Therapeutics, Inc.
Notes to Consolidated Financial Statements — (Continued)
($ in thousands, except per share amounts)
The following table summarizes significant ranges of outstanding and exercisable options as of June 30, 2007:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Options Outstanding | | | Options Exercisable | |
| | | | | | Weighted-
| | | | | | | | | | | | Weighted-
| | | | | | | |
| | | | | | Average
| | | | | | | | | | | | Average
| | | | | | | |
| | | | | | Years
| | | Weighted-
| | | | | | | | | Years
| | | Weighted-
| | | | |
| | | | | | Remaining
| | | Average
| | | | | | | | | Remaining
| | | Average
| | | | |
| | | | | | on
| | | Exercise
| | | Aggregate
| | | | | | on
| | | Exercise
| | | Aggregate
| |
Ranges of Exercise
| | Number
| | | Contractual
| | | Price per
| | | Intrinsic
| | | Number
| | | Contractual
| | | Price
| | | Intrinsic
| |
Prices | | Outstanding | | | Life | | | Option | | | Value | | | Exercisable | | | Life | | | per Option | | | Value | |
| | | (Shares in thousands) | |
| |
| $0.40 | | | 805 | | | | 6.1 | | | $ | 0.40 | | | $ | 31,386 | | | | 705 | | | | 6.0 | | | $ | 0.40 | | | $ | 27,484 | |
| $1.42 — $2.22 | | | 208 | | | | 6.7 | | | | 1.64 | | | | 7,856 | | | | 33 | | | | 6.8 | | | | 1.95 | | | | 1,220 | |
| $3.02 — $4.56 | | | 107 | | | | 6.7 | | | | 3.17 | | | | 3,892 | | | | 27 | | | | 4.8 | | | | 3.62 | | | | 981 | |
| $5.01 — $11.40 | | | 160 | | | | 7.3 | | | | 7.51 | | | | 5,093 | | | | 13 | | | | 6.3 | | | | 8.42 | | | | 389 | |
| $17.00 | | | 593 | | | | 8.1 | | | | 17.00 | | | | 13,291 | | | | 44 | | | | 8.1 | | | | 17.00 | | | | 993 | |
| $28.50-42.73 | | | 169 | | | | 8.8 | | | | 38.13 | | | | 439 | | | | 40 | | | | 8.4 | | | | 40.65 | | | | 46 | |
| $42.96 | | | 188 | | | | 9.1 | | | | 42.96 | | | | — | | | | — | | | | — | | | | — | | | | — | |
| $43.10-48.00 | | | 102 | | | | 9.2 | | | | 45.58 | | | | — | | | | 6 | | | | 8.9 | | | | 47.98 | | | | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | 2,332 | | | | | | | | | | | $ | 61,957 | | | | 868 | | | | | | | | | | | $ | 31,113 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
The aggregate intrinsic value in the preceding table represents the total pretax intrinsic value based on the Company’s closing price for its common stock of $39.39 as of June 29, 2007, which would have been received by the option holders had all option holders exercised their options as of that date. The total intrinsic value of options exercised during fiscal 2007 was $24,515.
The weighted-average grant date fair value per option for the following groups of options as of June 30, 2007 was:
| | | | | | | | | |
| | | | | | Weighted Average
| |
| | | Number of
| | | Grant Date
| |
| | | Options | | | Fair Value per Option | |
| | | (Shares in thousands) | |
| |
| Nonvested options at June 30, 2006 | | | 2,022 | | | $ | 5.72 | |
| Granted | | | 349 | | | | 15.09 | |
| Vested | | | (724 | ) | | | 3.91 | |
| Forfeited | | | (182 | ) | | | 7.65 | |
| | | | | | | | | |
| Nonvested options at June 30, 2007 | | | 1,465 | | | $ | 8.60 | |
| | | | | | | | | |
The fair value of options vested during fiscal 2007 was $2,831.
Performance Shares
During fiscal 2007, 184,861 performance shares were granted to certain of the Company’s employees. Under the 2005 Incentive Plan, as amended, performance shares have a vesting term of three years. The performance-based restricted stock units represent rights to earn shares of the Company’s common stock. Depending on the Company’s level of attainment of specified targets for earnings per share and pretax margin for the two consecutive-fiscal-year period beginning on July 1, 2006 and ending on June 30, 2008, the holder of a performance share award may earn from 0% to 150% of the target award. One-half of the units earned will be paid in shares of the Company’s common stock at the end of the two-year performance period, with the remainder paid one year later, provided that the holder is still employed by the Company. The related stock compensation expense is recognized separately for each vesting tranche of the award, with one-half of the expense recognized over two years and the second half recognized over three years. The fair value of performance shares is determined by using the grant date closing market price of the Company’s common
F-23
Adams Respiratory Therapeutics, Inc.
Notes to Consolidated Financial Statements — (Continued)
($ in thousands, except per share amounts)
stock. For the fiscal year ended June 30, 2007, the Company did not record any stock compensation expense for its fiscal 2007 performance share grants due to its determination that the related performance conditions over the two-year measurement period ended June 30, 2008 will not be achieved.
The weighted-average grant date fair value of performance shares as of June 30, 2007 was:
| | | | | | | | | |
| | | | | | Weighted Average
| |
| | | Number of
| | | Grant Date
| |
| | | Options | | | Fair Value per Option | |
| | | (Shares in thousands) | |
| |
| Nonvested performance shares at June 30, 2006 | | | — | | | $ | — | |
| Granted | | | 185 | | | | 44.70 | |
| Vested | | | — | | | | — | |
| Forfeited | | | (21 | ) | | | 43.91 | |
| | | | | | | | | |
| Nonvested performance shares at June 30, 2007 | | | 164 | | | $ | 44.80 | |
| | | | | | | | | |
Restricted Stock Units
During fiscal 2007 and 2006, 9,164 and 8,375 restricted stock units, respectively, were granted to certain of the Company’s board members at weighted-average fair values of $37.80 per unit and $30.45 per unit, respectively. During fiscal 2007, 674 restricted stock units with a weighted-average fair value of $37.12 per unit were forfeited upon resignation of a board member. During fiscal 2006, 491 restricted stock units with a fair value of $17.00 per unit were forfeited upon a board member’s retirement. The restricted stock units vest in full at the first annual stockholders’ meeting following the date of grant. Upon vesting, the restricted stock units will automatically convert into deferred stock units, which are not converted into the Company’s common stock until six months following a director’s termination of board service. The fair value of the restricted stock units is determined by using the grant date closing market price of the Company’s common stock. Stock compensation expense included $321 and $255, respectively, of expense associated with the restricted stock units for the fiscal years ended June 30, 2007 and 2006.
Warrants
At June 30, 2007 and 2006, the Company had the following outstanding warrants to purchase common stock:
| | | | | | | | | | | | | | | |
| As of June 30, |
| 2007 | | 2006 |
Outstanding
| | Exercise
| | Outstanding
| | Exercise
|
Warrants | | Price | | Warrants | | Price |
| |
| | 324 | | | $ | 4.56 | | | | 324 | | | $ | 4.56 | |
| | — | | | $ | 0.03 | | | | 1,020 | | | $ | 0.03 | |
| | | | | | | | | | | | | | | |
| | 324 | | | | | | | | 1,344 | | | | | |
| | | | | | | | | | | | | | | |
In connection with the Company’s initial public offering in July 2005, all outstanding Series B preferred warrants with an exercise price of $4.56 were converted into common stock warrants.
Deferred income taxes reflect the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes.
F-24
Adams Respiratory Therapeutics, Inc.
Notes to Consolidated Financial Statements — (Continued)
($ in thousands, except per share amounts)
The net deferred tax assets relate primarily to sales returns and allowances and nonqualified stock options. Federal NOLs approximated $1,826 at June 30, 2007 and will expire in fiscal 2012. State NOLs were approximately $15,550 as of June 30, 2007 and will expire between fiscal 2013 and 2026. During the fiscal year ended June 30, 2007, the Company utilized $430 of Federal NOLs and $13,535 of state NOLs. The Company utilized all of its existing research credits as of June 30, 2007. The NOLs and research credits are subject to limitations under Internal Revenue Code Sections 382 and 383, which contain provisions that limit the NOLs and research credits available to be used in any given year upon the occurrence of certain events, including significant changes in ownership interest.
The provision for income taxes consists of the following:
| | | | | | | | | | | | | |
| | | June 30, | |
| | | 2007 | | | 2006 | | | 2005 | |
| |
| Current provision: | | | | | | | | | | | | |
| Federal | | $ | 20,278 | | | $ | 25,450 | | | $ | 6,743 | |
| State | | | 3,837 | | | | 2,248 | | | | 2,146 | |
| Deferred: | | | | | | | | | | | | |
| Federal | | | (4,952 | ) | | | 1,457 | | | | 7,767 | |
| State | | | (2,116 | ) | | | 646 | | | | 782 | |
| | | | | | | | | | | | | |
| Total provision | | $ | 17,047 | | | $ | 29,801 | | | $ | 17,438 | |
| | | | | | | | | | | | | |
A reconciliation of the provision based on the Federal statutory income tax rate to the Company’s effective income tax rate is as follows:
| | | | | | | | | | | | | |
| | | June 30, | |
| | | 2007 | | | 2006 | | | 2005 | |
| |
| Provision at Federal statutory rate | | | 35.0 | % | | | 35.0 | % | | | 35.0 | % |
| State income taxes, net of Federal benefit | | | 2.4 | | | | 2.6 | | | | 4.3 | |
| Section 199 deduction | | | (0.9 | ) | | | — | | | | — | |
| Tax-exempt interest | | | (0.6 | ) | | | — | | | | — | |
| Research credit | | | (0.2 | ) | | | (0.1 | ) | | | (0.4 | ) |
| Incentive stock options | | | 0.4 | | | | 1.4 | | | | 0.3 | |
| Other | | | (0.3 | ) | | | 0.2 | | | | — | |
| | | | | | | | | | | | | |
| Effective tax rate | | | 35.8 | % | | | 39.1 | % | | | 39.2 | % |
| | | | | | | | | | | | | |
F-25
Adams Respiratory Therapeutics, Inc.
Notes to Consolidated Financial Statements — (Continued)
($ in thousands, except per share amounts)
The significant components of the net deferred tax assets are as follows:
| | | | | | | | | |
| | | June 30, | |
| | | 2007 | | | 2006 | |
| |
| Deferred tax assets — current: | | | | | | | | |
| Federal net operating loss carryforwards | | $ | 151 | | | $ | 151 | |
| Sales returns and allowances | | | 4,417 | | | | 2,971 | |
| Research credit | | | — | | | | 200 | |
| Inventory | | | 1,656 | | | | 9 | |
| Accrued bonuses | | | 1,888 | | | | — | |
| Other | | | 66 | | | | 328 | |
| | | | | | | | | |
| Total deferred tax assets — current | | | 8,178 | | | | 3,659 | |
| | | | | | | | | |
| Deferred tax assets — non-current: | | | | | | | | |
| Federal net operating loss carryforwards | | | 489 | | | | 639 | |
| State net operating loss carryforwards | | | 964 | | | | — | |
| Deferred compensation | | | 639 | | | | — | |
| Deferred gain on sale of plant assets | | | — | | | | 491 | |
| Nonqualified stock options | | | 2,608 | | | | 1,292 | |
| Patent defense costs | | | 1,730 | | | | — | |
| Humibid returns | | | — | | | | 400 | |
| Intangibles | | | (1,429 | ) | | | (623 | ) |
| Other | | | 40 | | | | 346 | |
| | | | | | | | | |
| Total deferred tax assets — non-current | | | 5,041 | | | | 2,545 | |
| | | | | | | | | |
| Total net deferred tax assets | | $ | 13,219 | | | $ | 6,204 | |
| | | | | | | | | |
F-26
Adams Respiratory Therapeutics, Inc.
Notes to Consolidated Financial Statements — (Continued)
($ in thousands, except per share amounts)
| |
| 12. | Income/(Loss) per Common Share |
The following table sets forth the computation of basic and diluted income/(loss) per common share:
| | | | | | | | | | | | | |
| | | June 30, | |
| | | 2007 | | | 2006 | | | 2005 | |
| | | (Shares in thousands) | |
| |
Net income | | $ | 30,529 | | | $ | 46,351 | | | $ | 26,999 | |
| Accretion of preferred stock | | | — | | | | — | | | | (202,566 | ) |
| Dividend paid to preferred stockholders | | | — | | | | — | | | | (30,033 | ) |
| | | | | | | | | | | | | |
| Net income/(loss) applicable to common stockholders | | $ | 30,529 | | | $ | 46,351 | | | $ | (205,600 | ) |
| | | | | | | | | | | | | |
| Average shares outstanding — basic | | | 35,340 | | | | 32,616 | | | | 6,236 | |
| Weighted-average dilutive stock options | | | 1,682 | | | | 2,571 | | | | — | |
| Weighted-average assumed conversion of redeemable convertible preferred stock | | | — | | | | 961 | | | | — | |
| Weighted-average dilutive warrants and restricted stock units | | | 13 | | | | 201 | | | | — | |
| Weighted-average dilutive JMED rights (see Note 6) | | | 78 | | | | — | | | | — | |
| | | | | | | | | | | | | |
| Average shares outstanding — diluted | | | 37,113 | | | | 36,349 | | | | 6,236 | |
| | | | | | | | | | | | | |
| Income/(loss) per common share: | | | | | | | | | | | | |
| Basic | | $ | 0.86 | | | $ | 1.42 | | | $ | (32.97 | ) |
| Diluted | | | 0.82 | | | $ | 1.28 | | | $ | (32.97 | ) |
The following table shows common share equivalents outstanding for the period, which are not included in the above historical calculations, as the effect of their inclusion is anti-dilutive during each period:
| | | | | | | | | | | | | |
| | | June 30, | |
| | | 2007 | | | 2006 | | | 2005 | |
| | | (Shares in thousands) | |
| |
| Warrants | | | — | | | | — | | | | 2,713 | |
| Stock options | | | 424 | | | | 135 | | | | 2,703 | |
| | | | | | | | | | | | | |
| | | | 424 | | | | 135 | | | | 5,416 | |
| | | | | | | | | | | | | |
| |
| 13. | Senior Revolving Credit Facility |
In September 2006, the Company entered into a new five-year $50,000 Senior Secured Revolving Credit Facility (the “Credit Facility”), which may be increased by up to an additional $100,000, subject to compliance with certain conditions, should the Company need additional financing in the future. Prior to closing the Credit Facility, the Company was provided with a bridge facility with immediately available borrowings of up to $25,000. In July 2006, the Company drew $20,000 from the bridge facility in connection with the manufacturing assets repurchase, which was repaid in full and terminated using proceeds from the Credit Facility. In October 2006, the Company repaid the remaining outstanding balance under the Credit Facility. Unamortized deferred debt issuance costs of $312 associated with the Credit Facility have been recorded primarily within other assets as of June 30, 2007.
The Credit Facility terminates on September 26, 2011, unless terminated earlier pursuant to the terms of the agreement. Borrowings under the Credit Facility bear interest at the higher of the prime rate established by the Royal Bank of Canada or 0.50% per annum above the weighted-average federal funds rate, subject to
F-27
Adams Respiratory Therapeutics, Inc.
Notes to Consolidated Financial Statements — (Continued)
($ in thousands, except per share amounts)
quarterly adjustments based on the Company’s debt to EBITDA ratio (“Leverage Ratio”), as defined in the Credit Facility. The Credit Facility also requires the payment of an unused commitment fee equal to 0.20% per annum, subject to quarterly adjustments in accordance with the Company’s Leverage Ratio, ranging from 0.20% to 0.40% on the unused commitment under the Credit Facility.
The Credit Facility contains certain dividend restrictions. The Company may only pay dividends either in its common stock as long as no event of default exists under the Credit Facility, or dividends in an amount not to exceed 50% of the Company’s cumulative positive consolidated net income from July 1, 2006 to the date of the end of the most recent fiscal quarter prior to which the dividends are proposed to be made, plus 100% of the net proceeds of any issuance of stock not used to make an acquisition, as long as the Company is still in compliance with the financial covenants after giving effect to such dividends.
The Credit Facility contains financial covenants that require the Company to maintain a Leverage Ratio of not greater than 3.5 to 1.0, a senior secured leverage ratio of not greater than 2.0 to 1.0, and a fixed charge coverage ratio of not less than 2.0 to 1.0. As of June 30, 2007, the Company was in compliance with these covenants.
| |
| 14. | Commitments and Contingencies |
The Company has obligations under noncancelable operating leases for buildings, certain office equipment and automobiles for its sales force expiring in various years through August 2018. The Company records the related rental expenses on a straight-line basis over the lease term. For the fiscal years ended June 30, 2007, 2006 and 2005, total rental expense was $3,136, $1,644 and $1,254, respectively.
In June 2007, the Company entered into an11-year operating lease for a new office facility in Chester, New Jersey, which is scheduled to commence on or prior to October 1, 2007. The Company’s future minimum lease commitments under this lease are $159 for fiscal 2008, $238 for fiscal 2009, $244 for fiscal 2010, $252 for fiscal 2011, $260 for fiscal 2012 and an aggregate of $1,806 thereafter.
In March 2007, the Company entered into a64-month operating lease for a warehouse facility in Irving, Texas, which commenced on May 15, 2007. In accordance with the provisions of this lease, the Company will receive free rent for the first five months of the lease. Future minimum lease commitments under this operating lease are $350 for fiscal 2008, $466 for each of fiscal 2009 through 2012, and $78 for fiscal 2013.
On July 31, 2006, the Company repurchased the Fort Worth, Texas manufacturing assets from Cardinal Health. As a result of the manufacturing assets repurchase, the Company was also required to make an escrow deposit in the amount of $2,169, representing the remaining obligation for the operating lease on the Fort Worth, Texas building. In November 2006, this escrow deposit was refunded to the Company and the operating lease for this facility was amended to include the Company as the lessee. In connection with this amendment, the Company issued an irrevocable letter of credit as a security deposit on the lease in the amount of $1,500. The Company’s future minimum lease commitments under this operating lease are $455 for fiscal 2008, $474 for each of fiscal 2009 and 2010, and $355 for fiscal 2011.
In October 2005, the Company entered into a12-year operating lease for a new office facility in Chester, New Jersey, which commenced on April 1, 2006. The first three years’ rent is fixed at $816 per year and increases to $872 in years four through six; $920 in years seven through nine; and $1,000 in years 10 through 12.
F-28
Adams Respiratory Therapeutics, Inc.
Notes to Consolidated Financial Statements — (Continued)
($ in thousands, except per share amounts)
The Company’s future minimum lease commitments under its operating leases, net of sublease income of $2,723 are as follows:
| | | | | |
| Fiscal 2008 | | $ | 3,091 | |
| Fiscal 2009 | | | 2,654 | |
| Fiscal 2010 | | | 2,242 | |
| Fiscal 2011 | | | 2,021 | |
| Fiscal 2012 | | | 1,679 | |
| Thereafter | | | 7,565 | |
| | | | | |
| | | $ | 19,252 | |
| | | | | |
On October 26, 2006, the Company entered into a three-year renewable agreement with PharmPro, a division of Fluid Air, Inc., for processing and granulation of guaifenesin. Under this agreement, the Company is required to purchase 50 metric tons of finished granulation of guaifenesin during each contract year, or approximately $715 per year. During fiscal 2007, the Company purchased $501 of finished granulation guaifenesin toward the contract year ended October 26, 2006. The remaining contracted amount under this agreement was $1,445 as of June 30, 2007. PharmPro also agreed to reserve capacity to enable the manufacture of an additional 100% of the contracted volume, which the Company may use at its option. In exchange for this reservation of manufacturing capacity, the Company has prepaid $500 to PharmPro, which is rebated back to the Company at a rate of $1.67 per kilogram of approved product produced. Any remaining balance relating to this prepayment at the end of the three-year period will be retained by PharmPro.
Upon the manufacturing assets repurchase, the Company entered into a packaging agreement with Cardinal Health (now Catalent), under which, in exchange for a guaranteed amount of packaging capacity, the Company is committed to pay Catalent non-refundable capacity reservation payments of $3,000 in each year during the contract years ending June 30, 2007 and 2008 and $1,500 for the contract year ending June 30, 2009. During the fiscal year ended June 30, 2007, the Company recorded $3,000 relating to the packaging agreement. In connection with the manufacturing assets repurchase, the Company also entered into a three year take-or-pay manufacturing agreement with Cardinal Health (now Catalent) for the granulation of guaifenesin. Under this manufacturing agreement, the Company is obligated to purchase or pay for 80% of committed volume at a specified price, beginning on August 1, 2006. During the fiscal year ended June 30, 2007, the Company purchased $2,372 of granulated product under this agreement. The remaining contracted amount under the manufacturing agreement is $3,896 as of June 30, 2007. The Company also has the ability to use any other vendor with whom it may decide to contract.
As a result of the Company’s manufacturing assets repurchase, Cardinal Health assigned to the Company its January 2006 agreement with its sole supplier of dextromethorphan, which obligated Cardinal Health to purchase 45 metric tons of dextromethorphan through 2009, or $4,200 in raw materials per year. During fiscal 2007, the Company purchased $2,121 in dextromethorphan from this supplier, subsequent to exhausting the previous contract. As of June 30, 2007, the remaining commitment for the entire contract was approximately $10,500. The FDA approved a second supplier of dextromethorphan in December 2006. The Company believes these two suppliers will provide it with sufficient quantities of dextromethorphan to meet its manufacturing needs.
The Company depends on Boehringer Ingelheim Chemicals, Inc. (“Boehringer Ingelheim”) and Delta Synthetic Co., LTD (“Delta”) for all of the raw guaifenesin used in its adult oral-solid Mucinex products. In connection with the manufacturing assets repurchase, Cardinal Health assigned the contracts related to the manufacture of the adult oral-solid Mucinex products, including the guaifenesin supply arrangements with
F-29
Adams Respiratory Therapeutics, Inc.
Notes to Consolidated Financial Statements — (Continued)
($ in thousands, except per share amounts)
Boehringer Ingelheim and Delta, to the Company. In July 2006, the Company entered into a new five-year supply agreement with Boehringer Ingelheim, pursuant to which the Company has agreed to purchase from Boehringer Ingelheim the lesser of 500 metric tons or 100% of its guaifenesin requirements during each contract year. During the fiscal year ended June 30, 2007, the Company purchased $13,204 of guaifenesin from Boehringer Ingelheim. Assuming the Company purchases 500 metric tons per year from Boehringer Ingelheim for the remainder of the contract, or $16,250 per year, its remaining commitment under this agreement is $65,000 as of June 30, 2007. The Company may purchase volumes in excess of 500 metric tons from other suppliers. The Company believes that Boehringer Ingelheim and Delta will provide it with sufficient quantities of guaifenesin to meet its manufacturing needs.
In June 2006, the Company consummated the acquisition of the Delsym12-Hour cough suppressant liquid product line from UCB. In addition to the cash purchase price of $122,000, the Company is committed to pay a 2.5% royalty to UCB for a period of five years for the intellectual property associated with the Delsym product. UCB will continue to manufacture the Delsym product for the Company from raw materials to finished goods under a six-year renewable standard supply agreement, which is based on UCB’s manufacturing costs plus a markup, with a contract minimum of $11,800 in each year. During fiscal 2007, the Company purchased $24,825 in finished goods from UCB, exceeding the contract minimum. The remaining commitment under this agreement as of June 30, 2007 is $58,795. The supply agreement defines cost as UCB’s cost of manufacturing finished product, limited to raw materials, packaging materials, labor time, quality assurance time, and overhead. The Company believes that the cost plus markup amount is representative of fair value, based on its manufacturing agreement with Catalent and other manufacturing arrangements its management has experienced in the industry. The agreement with UCB also provides for the potential technology transfer to the Company after five years.
The Company also entered into a five-year development agreement with UCB for certain product development projects, including additional product formulations of Delsym. Pursuant to this agreement, the Company pays UCB actual development costs plus an established markup percentage. The Company believes that this agreement is considered to be at fair value, as it is consistent with the terms of its research and development agreement with CH 105 for these services.
The Company is a party to various claims and suits arising out of matters occurring in the normal course of business. However, as of June 30, 2007, there was no current proceeding or litigation involving the Company that the Company believes will have a material adverse impact on its business, financial condition, results of operations or cash flows.
On October 4, 2006, the Company filed a complaint against Mutual in the United States District Court for the District of Pennsylvania, Civ. Act.No. 2:06-cv-04418-PD, asserting that Mutual’s proposed generic products would infringe our ’252 Patent. On March 21, 2007, the Company entered into a settlement agreement with Mutual, under which the Company agreed with Mutual to dismiss all patent infringement claims and all counterclaims in the lawsuit. In the settlement agreement, Mutual admitted that the ’252 Patent is valid and enforceable and that the single-ingredient and combination generic extended-release guaifenesin-based products set forth in the ANDA filed by Mutual with the FDA infringe the ’252 Patent. Under the settlement agreement, the Company granted Mutual a non-exclusive, royalty-free license under the ’252 Patent to sell the Company’s 600 mg and 1200 mg single-ingredient and combination extended-release guaifenesin products in the United States, subject to certain restrictions. The settlement agreement is subject to review by the Federal Trade Commission and the U.S. Department of Justice.
On May 7, 2004, the Company filed a complaint in the U.S. District Court for the Southern District of New York against Carolina Pharmaceuticals, Inc. (“Carolina”). The Company filed an amended complaint on October 25, 2004, which added Cornerstone as a defendant. The amended complaint alleged trademark
F-30
Adams Respiratory Therapeutics, Inc.
Notes to Consolidated Financial Statements — (Continued)
($ in thousands, except per share amounts)
infringement, false advertising and unfair competition claims, and sought damages and injunctive relief. Carolina and Cornerstone filed counterclaims against the Company, alleging that the Company’s activities with respect to its single-entity Mucinex product violated the anti-monopoly provisions of the federal antitrust laws, that the Company had engaged in false advertising in violation of federal law with respect to single-entity Mucinex and the Company’s AlleRxtm and Aquatab products, and that the Company had violated state law in competing with the products of Carolina and Cornerstone.
In February 2005, the Company entered into an agreement with Cornerstone in which the Company received the Humibid trademarks from Cornerstone and Cornerstone received the AlleRxtm trademarks from the Company. Additionally, the parties released each other from all claims and damages in the lawsuit. As part of this arrangement, the Company now has the responsibility for all Humibid product returns, whether sold by the Company or Cornerstone, and Cornerstone bears the same liability for AlleRxtm products. In connection with the Cornerstone agreement, the Company is obligated to pay Cornerstone a royalty ranging from 1% to 2% of net Humibid sales for a period of three years after February 15, 2005, with an annual minimum royalty payment of $50.
On March 28, 2003, CellTech Pharmaceuticals, Inc., CellTech US, Inc. and CellTech Americas, Inc. (collectively, “CellTech”) brought suit against the Company and certain current and former employees in the U.S. District Court for the Western District of New York. CellTech alleged that the Company (and certain individuals) misappropriated trade secrets and breached confidentiality provisions in a manufacturing contract and in the individuals’ past employment contracts with CellTech.
The Company agreed to settle the lawsuit on April 14, 2004 for the amount of $2,000. Additionally, the Company entered into a royalty-bearing license agreement whereby the Company makes monthly payments to Celltech based on a percentage of sales of certain of the Company’s products. The license agreement is for a ten-year period commencing December 31, 2003. Payments made under the license agreement are subject to an annual minimum of $200 and an annual maximum of $500. The Company recorded a liability, and a related other asset, of approximately $1,270, which represented the present value of the minimum amount due under the license agreement of ten annual payments of $200 per year, discounted at 10% per year. The other asset is amortized as additional royalty expense over the ten-year term of the license agreement.
On June 2, 2005, the Company’s board of directors approved the payment of a cash dividend of $45,000 on shares of the Company’s common stock and shares of the Company’s preferred stock on an “as converted” basis (in accordance with the Company’s Certificate of Designations, Rights and Preferences of the Series A Convertible Preferred Stock, Series B Convertible Preferred Stock and Series C Convertible Preferred Stock of the Company’s Certificate of Incorporation). The dividend was paid on June 22, 2005. The portion of the dividend that was paid on the “as converted” preferred stock was $30,033 and is reflected as an increase to net loss applicable to common stockholders.
F-31
Adams Respiratory Therapeutics, Inc.
Notes to Consolidated Financial Statements — (Continued)
($ in thousands, except per share amounts)
| |
| 16. | Net Sales Information |
Net Sales by Product
The following table details the Company’s net sales by product for the years ended June 30, 2007, 2006 and 2005.
| | | | | | | | | | | | | |
| | | Year Ended June 30, | |
Product | | 2007 | | | 2006 | | | 2005 | |
| | | (In thousands) | |
| |
| Mucinex SE | | $ | 131,871 | | | $ | 158,575 | | | $ | 117,369 | |
| Mucinex DM | | | 94,633 | | | | 59,235 | | | | 38,278 | |
| Mucinex D | | | 28,204 | | | | 19,347 | | | | — | |
| Mucinex products for children | | | 29,190 | | | | — | | | | — | |
| Humibid SE(1) | | | (639 | ) | | | 1,296 | | | | — | |
| Delsym | | | 48,344 | | | | 652 | | | | — | |
| Other products | | | — | | | | — | | | | 4,563 | |
| | | | | | | | | | | | | |
| Net sales | | $ | 331,603 | | | $ | 239,105 | | | $ | 160,210 | |
| | | | | | | | | | | | | |
| | |
| (1) | | Humibid SE’s sales for fiscal 2007 include allowance for sales returns of $874 relating to retail inventory of Humibid that has expired or is approaching expiration. |
Concentration of Credit Risk
The Company sells its products principally to wholesalers and retailers, including mass merchandisers, grocery stores, membership clubs, and drug stores throughout the United States. The Company performs ongoing credit evaluations of its customers’ financial condition and generally requires no collateral from its customers. Beginning in fiscal 2005, as a result of increased Mucinex sales, the Company reduced its concentration levels with certain customers and now sells to a combination of wholesalers, major retailers and mass merchandisers. The table below outlines the percentage of gross sales made to the following customers:
| | | | | | | | | | | | | |
| | | Year Ended June 30, | |
| | | 2007 | | | 2006 | | | 2005 | |
| |
| Wal-Mart Stores, Inc./Sam’s Club | | | 25 | % | | | 19 | % | | | 16 | % |
| Walgreen Co. | | | 13 | % | | | 12 | % | | | 14 | % |
| CVS Caremark Corporation | | | 13 | % | | | 10 | % | | | 11 | % |
| McKesson Corp. | | | 6 | % | | | 11 | % | | | 14 | % |
F-32
Adams Respiratory Therapeutics, Inc.
Notes to Consolidated Financial Statements — (Continued)
($ in thousands, except per share amounts)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | First Quarter | | | Second Quarter | | | Third Quarter | | | Fourth Quarter | |
| | | 2007 | | | 2006 | | | 2007 | | | 2006 | | | 2007 | | | 2006 | | | 2007 | | | 2006 | |
| | | (Unaudited) | |
| |
| Net sales | | $ | 90,142 | | | $ | 47,017 | | | $ | 110,576 | | | $ | 63,247 | | | $ | 84,024 | | | $ | 76,002 | | | $ | 46,861 | | | $ | 52,839 | |
| Cost of goods sold | | | 29,358 | | | | 8,521 | | | | 26,598 | | | | 13,920 | | | | 23,691 | | | | 16,166 | | | | 16,152 | | | | 10,751 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Gross margin | | | 60,784 | | | | 38,496 | | | | 83,978 | | | | 49,327 | | | | 60,333 | | | | 59,836 | | | | 30,709 | | | | 42,088 | |
| Selling, marketing and administrative | | | 29,983 | | | | 15,869 | | | | 55,963 | | | | 29,693 | | | | 52,629 | | | | 28,440 | | | | 24,286 | | | | 24,996 | |
| Product development | | | 6,287 | | | | 3,426 | | | | 6,727 | | | | 4,096 | | | | 5,043 | | | | 3,837 | | | | 5,798 | | | | 7,545 | |
AlleRxtm charge | | | — | | | | — | | | | 2,699 | | | | — | | | | — | | | | — | | | | — | | | | — | |
| Other, net | | | (879 | ) | | | (926 | ) | | | (823 | ) | | | (1,862 | ) | | | (1,165 | ) | | | (80 | ) | | | 1,680 | | | | (1,439 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Income/(loss) before income taxes | | | 25,393 | | | | 20,127 | | | | 19,412 | | | | 17,400 | | | | 3,826 | | | | 27,639 | | | | (1,055 | ) | | | 10,986 | |
| Provision/(benefit) for income taxes | | | 9,215 | | | | 7,739 | | | | 6,889 | | | | 6,734 | | | | 962 | | | | 10,696 | | | | (19 | ) | | | 4,632 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Net income/(loss) | | $ | 16,178 | | | $ | 12,388 | | | $ | 12,523 | | | $ | 10,666 | | | $ | 2,864 | | | $ | 16,943 | | | $ | (1,036 | ) | | $ | 6,354 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Income/(loss) per common share — diluted | | $ | 0.44 | | | $ | 0.36 | | | $ | 0.34 | | | $ | 0.29 | | | $ | 0.08 | | | $ | 0.46 | | | $ | (0.03 | ) | | $ | 0.17 | |
On August 20, 2007, the Company received a letter from Perrigo R&D Co. (“Perrigo”) that Perrigo had filed an abbreviated new drug application (“ANDA”) for a single-ingredient, extended-release formulation of guaifenesin that is the generic equivalent to the Company’s Mucinex SE product. In its letter, Perrigo notified the Company of its assertion that its proposed products would not infringe the Company’s patents that protect Mucinex SE, or alternatively that certain of the Company’s patent claims are not valid.
The Company is evaluating this matter and has requested from Perrigo additional data to evaluate their claims. If the Company determines that Perrigo’s products infringe the Company’s patents, the Company intends to vigorously defend its intellectual property rights. If the Company files a patent infringement lawsuit prior to October 4, 2007, the FDA will stay its approval of Perrigo’s ANDA until the earlier of 30 months or a court’s determination that Perrigo’s product does not infringe the Company’s patents or that certain of the Company’s patent claims are invalid. The Company cannot predict whether it will file a patent infringement lawsuit, the duration of any resulting litigation or the stay in the FDA’s approval of Perrigo’s ANDA, or whether the Company would prevail in any such lawsuit.
F-33
Schedule II
Adams Respiratory Therapeutics, Inc.
Valuation and Qualifying Accounts
($ in thousands)
Valuation and qualifying accounts deducted from assets to which they apply:
| | | | | | | | | | | | | |
| | | Allowance for Accounts Receivable | |
| | | Reserve for
| | | Reserve
| | | Reserve for
| |
| | | Doubtful
| | | for Cash
| | | Trade
| |
| | | Accounts | | | Discounts | | | Promotions | |
| |
| For year ended June 30, 2005: | | | | | | | | | | | | |
| Balance at the beginning of the year | | $ | 203 | | | $ | 143 | | | $ | 988 | |
| Provision made to sales during the period | | | (4 | ) | | | 3,441 | | | | 6,611 | |
| Benefit related to sales made in prior periods | | | — | | | | — | | | | (613 | ) |
| Deductions from reserves | | | (33 | ) | | | (3,414 | ) | | | (4,878 | ) |
| | | | | | | | | | | | | |
| Balance at the end of period | | $ | 166 | | | $ | 170 | | | $ | 2,108 | |
| | | | | | | | | | | | | |
| For year ended June 30, 2006: | | | | | | | | | | | | |
| Balance at the beginning of the year | | $ | 166 | | | $ | 170 | | | $ | 2,108 | |
| Provision made to sales during the period | | | (112 | ) | | | 5,196 | | | | 6,786 | |
| Provision related to sales made in prior periods | | | — | | | | — | | | | 111 | |
| Deductions from reserves | | | — | | | | (4,871 | ) | | | (6,209 | ) |
| | | | | | | | | | | | | |
| Balance at the end of period | | $ | 54 | | | $ | 495 | | | $ | 2,796 | |
| | | | | | | | | | | | | |
| For year ended June 30, 2007: | | | | | | | | | | | | |
| Balance at the beginning of the year | | $ | 54 | | | $ | 495 | | | $ | 2,796 | |
| Provision made to sales during the period | | | — | | | | 7,144 | | | | 11,525 | |
| Benefit related to sales made in prior periods | | | — | | | | — | | | | (331 | ) |
| Deductions from reserves | | | — | | | | (7,303 | ) | | | (11,160 | ) |
| | | | | | | | | | | | | |
| Balance at the end of period | | $ | 54 | | | $ | 336 | | | $ | 2,830 | |
| | | | | | | | | | | | | |
| | |
| (1) | | Charges related to cash discounts and trade promotions are reflected as reductions of sales to customers. |
F-34