UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark One)
| x | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2008
| ¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission file number 001-33657
Abraxis BioScience, Inc.
(Exact name of registrant as specified in its charter)
| | |
Delaware | | 30-0431735 |
(State of incorporation) | | (I.R.S. Employer Identification No.) |
| |
11755 Wilshire Boulevard, Suite 2000 Los Angeles, CA 90025 | | (310) 883-1300 |
| (Address of principal executive offices, including zip code) | | (Registrant’s telephone number, including area code) |
Securities registered pursuant to Section 12(b) of the Act:
| | |
Common Stock, par value $0.001 per share | | The NASDAQ Stock Market LLC |
| (Title of Class) | | (Name of each exchange on which registered) |
Securities registered pursuant to Section 12(g) of the Act:None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes¨ Nox
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes¨ Nox
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yesx No¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained to the best of the registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K.x
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
| | | | | | |
| ¨ Large accelerated filer | | x Accelerated filer | | ¨ Non-accelerated filer | | ¨ Smaller reporting company |
| | | | (Do not check if a smaller
reporting company) | | |
Indicate by check mark whether the registrant is a shell company (as determined by rule 12b-2 of the Exchange Act). Yes¨ Nox
Shares of the registrant’s common stock was first distributed to the stockholders of APP Pharmaceuticals, Inc. on November 13, 2007 and commenced trading on November 14, 2007. Prior to that date, there was no public market for the registrant’s common stock.
The approximate aggregate market value of voting and non-voting stock held by non-affiliates of the registrant was $438 million as of June 30, 2008. All executive officers and directors of the registrant have been deemed, solely for the purpose of the foregoing calculation, to be “affiliates” of the registrant.
The number of shares of common stock outstanding as of February 24, 2009 was 40,070,846
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the Proxy Statement for the registrant’s 2009 Annual Meeting of Stockholders are incorporated by reference into Part III of this Annual Report on Form 10-K.
Abraxis BioScience, Inc.
FORM 10-K
For the Year Ended December 31, 2008
TABLE OF CONTENTS
2
PART I
Item 1. BUSINESS
Unless the context otherwise requires, references to “New Abraxis,” “Abraxis BioScience,” “Abraxis,” “we,” “us” and “our” refer to Abraxis BioScience, Inc. (formerly New Abraxis, Inc.) and its subsidiaries, including our operating subsidiary Abraxis BioScience, LLC; references to “Old Abraxis” refer to Abraxis BioScience, Inc. (formerly American Pharmaceuticals, Inc.) prior to the 2007 separation; references to “APP” refer to APP Pharmaceuticals, Inc. and its subsidiaries, including its operating subsidiary APP Pharmaceuticals Partners, LLC (which we sometimes refer to as APP LLC); and references to the “distribution,” “separation,” “2007 separation” or “spin-off” refer to the transactions in which we separated from Old Abraxis and became an independent publicly-traded company in 2007.
Note Regarding Forward-Looking Statements
This Annual Report on Form 10-K and other materials filed or to be filed by us with the Securities and Exchange Commission, or the SEC, as well as information included in oral statements or other written statements made or to be made by us, contain forward-looking statements within the meaning of federal securities laws. We intend these forward-looking statements to be covered by the safe harbor provisions for forward-looking statements in the federal securities laws. These forward-looking statements are not historical facts but rather are based on current expectations, estimates and projections about our industry, our beliefs and assumptions. These risks and uncertainties include those described in “Risk Factors” and elsewhere in this Annual Report on Form 10-K. Forward-looking statements, whether express or implied, are not guarantees of future performance and are subject to risks and uncertainties, which can cause actual results to differ materially from those currently anticipated, due to a number of factors, which include, but are not limited to:
| | • | | the amount and timing of costs associated with the continuing launch of Abraxane®; |
| | • | | our ability to maintain and/or improve sales and earnings performance; |
| | • | | the actual results achieved in further clinical trials of Abraxane® may or may not be consistent with the results achieved to date; |
| | • | | the market adoption of any new pharmaceutical products; |
| | • | | the difficulty in predicting the timing or outcome of product development efforts and regulatory approvals; |
| | • | | our ability and that of our suppliers to comply with laws, regulations and standards, and the application and interpretation of those laws, regulations and standards, that govern or affect the pharmaceutical industry, the non-compliance with which may delay or prevent the sale of their products; |
| | • | | the availability and price of acceptable raw materials and components from third-party suppliers; |
| | • | | any adverse outcome in litigation; |
| | • | | general economic, political and business conditions that adversely affect our company or our suppliers, distributors or customers; |
| | • | | changes in costs, including changes in labor costs, raw material prices or advertising and marketing expenses; |
| | • | | inventory reductions or fluctuations in buying patterns by wholesalers or distributors; |
| | • | | the impact on our products and revenues of patents and other proprietary rights licensed or owned by us, our competitors and other third parties; |
| | • | | the ability to successfully manufacture our products in an efficient, time-sensitive and cost effective manner; and |
| | • | | the impact of recent legislative changes to the governmental reimbursement system. |
3
You should read this Annual Report on Form 10-K with the understanding that actual future results may be materially different from expectations. Readers should carefully review the factors described in“Item 1A: Risk Factors”below and other documents we file from time to time with the SEC for a more detailed description of these risks and other factors that may affect the forward-looking statements. All forward-looking statements made in this Annual Report on Form 10-K are qualified by these cautionary statements. These forward-looking statements are made only as of the date of this Annual Report on Form 10-K, and we do not undertake any obligation (and we expressly disclaim any such obligation), other than as may be required by law, to update or revise any forward-looking statements to reflect changes in assumptions, the occurrence of unanticipated events or changes in future operating results over time or otherwise. We use words such as “should,” “anticipate,” “expect,” “intend,” “plan,” “believe,” “seek,” “estimate” and variations of these words and similar expressions to identify forward-looking statements, but these are not the exclusive means of identifying forward-looking statements.
Available Information
Our Internet address is www.abraxisbio.com. Our Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K, proxy statements and other information are available free of charge on our website as soon as reasonably practical after they are electronically filed or furnished to the SEC. The information found on our website shall not be deemed incorporated by reference by any general statement into any filing under the Securities Act of 1933 or under the Securities Exchange Act of 1934, except to the extent we specifically incorporate the information found on our website by reference, and shall not be deemed filed under such Acts.
The SEC also maintains an Internet site that contains reports, proxy and information statements, and other information about issuers that file reports electronically with the SEC. The address of that site is www.sec.gov.
2007 Separation
On November 13, 2007, Abraxis BioScience, Inc. (formerly “American Pharmaceutical Partners, Inc.”) (“Old Abraxis”) was separated into two independent publicly-traded companies: one holding the former Abraxis Pharmaceutical Products business (which is referred to as the “hospital-based business”); and the other holding the former Abraxis Oncology and Abraxis Research businesses (which is referred to as the “proprietary business”). Following the separation, the proprietary business changed its name from New Abraxis, Inc. to Abraxis BioScience, Inc. and the hospital-based business was operated under the name APP Pharmaceuticals, Inc.
In connection with the separation, stockholders of Old Abraxis as of November 13, 2007 received one share of our company for every four shares of Old Abraxis held as of that date. In addition, in connection with the separation, we entered into a separation and distribution agreement that provides for, among other things, the principal corporate transactions required to effect the separation and other specified terms governing our relationship with APP after the spin-off. We also entered into various agreements with APP, including (i) a transition services agreement pursuant to which we and APP agreed to continue to provide each other with various services on an interim, transitional basis, for periods up to 24 months depending on the particular service; (ii) a manufacturing agreement whereby we and APP agreed to manufacture Abraxane® and certain other products and to provide other manufacturing-related services for a period of four or five years; (iii) an employees matters agreement providing for each company’s respective obligations to employees and former employees who are or were associated with their respective businesses, and for other employment and employee benefit matters; (iv) various real estate leases; and (v) a tax allocation agreement. Also, in connection with the separation, APP contributed $700 million in cash to us.
4
Proposed 2009 Spin-Off
In January 2009, we announced that our board of directors approved a plan to spin-off a newly-formed subsidiary Abraxis Health, Inc. as a new independent, stand-alone company holding our drug discovery, pilot manufacturing and development business. If the spin-off occurs, our stockholders would own (i) shares of Abraxis Health and (ii) shares of our common stock, and we would continue to operate our existing business, excluding the drug discovery, pilot manufacturing and development business to be held by Abraxis Health. In connection with the proposed spin-off, Abraxis Health would enter into several agreements with us related to, among other things, manufacturing, transition services, product development and research, tax allocations, clinical development and a number of ongoing commercial relationships.
The proposed spin-off is subject to a number of closing conditions, including final approval by our board of directors and the effectiveness of the registration statement registering the common stock of Abraxis Health to be distributed to our stockholders in connection with the spin-off. Approval by our stockholders is not required as a condition to the consummation of the proposed spin-off. In connection with the proposed spin-off, Abraxis Health filed a registration statement on Form 10 with the SEC. Stockholders are urged to read Abraxis Health’s Form 10 registration statement carefully because it contains important information about the proposed spin-off. SeeItem 8—Financial Statements and Supplementary Data, Note 15—Subsequent Events for further discussion.
Overview
We are a Delaware corporation that was formed in June 2007. Our business was conducted as part of Old Abraxis prior to the separation. References in this Annual Report on Form 10-K to the historical assets, liabilities, products, businesses or activities of our company are generally intended to refer to the historical assets, liabilities, products, businesses or activities of the business as it was conducted as part of Old Abraxis prior to the separation.
We are one of the few fully integrated biotechnology companies, with a breakthrough marketed product (Abraxane®), global ownership of our proprietary technology platform and clinical pipeline, dedicated nanoparticle manufacturing capabilities for worldwide supply integrated with seasoned in-house capabilities, including discovery, clinical drug development, regulatory and sales and marketing.
We are dedicated to the discovery, development and delivery of next-generation therapeutics and core technologies that offer patients safer and more effective treatments for cancer and other critical illnesses. Our portfolio includes the world’s first and only protein-based nanoparticle chemotherapeutic compound (Abraxane®) which is based on our proprietary tumor targeting technology known as thenab® technology platform. Thisnab® technology platform is the first to exploit the tumor’s biology against itself, taking advantage of an albumin-specific, receptor-mediated transport system and allowing the delivery of a drug from the vascular space across the blood vessel wall to the underlying tumor tissue. Abraxane® is the first clinical and commercial validation of ournab® technology platform. From the discovery and research phase to development and commercialization, we are committed to rapidly enhancing our product pipeline and accelerating the delivery of breakthrough therapies that will transform the lives of patients who need them.
We own the worldwide rights to Abraxane®, a next-generation, nanometer-sized, solvent-free taxane that was approved by the U.S. Food and Drug Administration, or the FDA, in January 2005 for its initial indication in the treatment of metastatic breast cancer and launched in February 2005. We believe the successful launch of Abraxane® validates ournab® tumor targeting technology, a novel biologically interactive (receptor-mediated) system to deliver chemotherapeutic agents in high concentrations preferentially to all tumors secreting a protein called SPARC, which attracts and binds to albumin. Abraxane® revenue for the year ended December 31, 2008 and 2007 was $ 335.6 million and $324.7 million, respectively, which included the recognition of previously deferred revenue related to our June 2006 co-promotion agreement with AstraZeneca UK Limited. In January 2009, we re-acquired from AstraZeneca the exclusive rights to market Abraxane® in the United States, thereby ending the co-promotion agreement.
5
Our research and development approach is based on the integration of ournab® tumor targeting technology, our natural product drug discovery platform, our multi-functional chemistry capabilities with our in-house clinical trial and regulatory strengths, combined with our unique nanoparticle manufacturing capabilities. Our product pipeline includes over five clinical oncology and cardiovascular product candidates in various stages of testing and development, including Abraxane® and Coroxane™ for various indications. We also have over 45 discovery product candidates and novel chemical entities for various diseases, including cancer, multiple sclerosis and Alzheimer’s. We believe the application of ournab® technology will serve as the platform for the development of numerous drugs for the treatment of cancer and other critical illnesses. To leverage in-house manufacturing, clinical trial and regulatory expertise, we will continue to supplement our discovery efforts through technology acquisitions and external collaborations with academia and start-up biotechnology companies.
Abraxane®, A Proprietary Injectable Oncology Product
Overview
Abraxane® is a next-generation, nanometer-sized, solvent-free taxane that was approved by the FDA in January 2005 for its initial indication in the treatment of metastatic breast cancer and launched in February 2005. As of December 2008, Abraxane was approved for marketing in 36 countries. Taxanes are one of the most widely used chemotherapy agents. We believe the successful launch of Abraxane® validates ournab® tumor targeting technology described below. Effective January 1, 2006, we received a unique reimbursement “J” code for Abraxane®, which facilitates reimbursement from Medicare and Medicaid as well as private payors.
First clinical and commercial validation of our nab® tumor targeting technology
Abraxane® represents the first in a new class of protein-bound drug particles that takes advantage of albumin, a natural carrier of water insoluble molecules (e.g., various nutrients, vitamins and hormones) found in humans. Because tumors have an increased need for nutrients to support rapid malignant growth, albumin complexes accumulate preferentially in tumors. Recent molecular analyses have identified receptor-mediated pathways (gp60) facilitating active transport of albumin complexes from the blood into tissues and resulting in preferential accumulation in tumors by tumor-secreted proteins (SPARC), which attract albumin. Abraxane® consists only of paclitaxel nanometer-sized albumin-bound particles and, in a pivotal 460 patient Phase III clinical trial, demonstrated an almost doubling of the response rate, a longer time to tumor progression in metastatic breast cancer as well as an increased survival in patients previously treated for metastatic disease when compared to Taxol®. We believe that ournab® tumor targeting technology and these albumin pathways allow more rapid delivery of paclitaxel to the cancer cells, with preferential accumulation while at the same time allowing less drug indiscriminately into normal, healthy cells. This is supported by the clinical findings demonstrated by Abraxane®.
Overcoming the obstacles and toxicity of solvents
Many oncology drugs are water insoluble and thus require solvents to formulate the drugs for injection. Taxol® and its generic solvent-based equivalents contain the active ingredient paclitaxel dissolved in the solvent Cremophor. The toxicity of Cremophor limits the dose of Taxol® that can be administered, potentially limiting the efficacy of the drug. Furthermore, patients receiving Taxol® require pre-medication with steroids and antihistamines to prevent the toxic side effects associated with Cremophor and, in some cases, require a growth factor such as G-CSF to overcome low white blood cell levels resulting from chemotherapy.
Abraxane® utilizes ournab® tumor targeting technology to encapsulate an amorphous form of paclitaxel in albumin, a human protein found in blood and avoids the need for Cremophor. Because it contains no Cremophor solvent, this next-generation taxane product enables the administration of 50% more chemotherapy with a well- tolerated safety profile, requires no routine premedication to prevent hypersensitivity reactions, can be given over a shorter infusion time using standard IV tubing and reduces the need for G-CSF support.
6
Abraxane® clinical development, label expansion and global commercialization plans
We will continue to pursue an aggressive and comprehensive clinical development plan to maximize the commercial potential and clinical knowledge of Abraxane®. As of December 31, 2008, approximately 25 company-sponsored Abraxane® clinical studies and approximately 62 investigator-initiated Abraxane® clinical studies were planned or underway for various indications, of which more than 42 had patient participation. The investigator-initiated studies generally are intended to advance the clinical knowledge of Abraxane® and support our clinical development program.
Results from five company-sponsored studies of Abraxane® were presented at the 31st Annual San Antonio Breast Cancer Symposium in December 2008. Preliminary results from a single-arm, open-label, Phase II clinical trial evaluating Abraxane® in combination with gemcitabine and epirubicin for the treatment of patients with locally advanced breast cancer (neoadjuvant treatment) indicated that 18 percent of patients (n=23) given a regimen of Abraxane®, gemcitabine and epirubicin achieved a complete pathologic response (the disappearance of all target lesions), and 68 percent (n=84) achieved a partial response (a 30 percent or greater decrease in size of target lesions). The company also presented pre-clinical data evaluating the potential antitumor activity of nab®-paclitaxel given in combination with two investigational therapies that utilize our proprietary nanoparticle technology, nab®-rapamycin and nab®-IDN5404.
We will continue to study the use of Abraxane® in a variety of oncology settings and intend to focus our Phase III trials in first-line non-small cell lung cancer (NSCLC), melanoma and pancreatic cancer, all of which are expected to be superiority trials utilizing weekly dosing schedules of Abraxane®. The Phase III NSCLC pivotal trial is a randomized, open-label trial comparing weekly 100 mg/m2 Abraxane® (days 1, 8 and 15 of each cycle) and 200 mg/m2 Taxol® (paclitaxel) injection every three weeks. Carboplatin will be administered at AUC=6 on day 1 of each cycle repeated every three weeks in both treatment arms. The study will enroll over 1,000 patients with Stage IIIb and IV non-small cell lung cancer. The primary endpoint of the study is overall response rate and the detection of meaningful differences in progression-free survival (PFS). Patient enrollment of this trial began in the fourth quarter of 2007 and is presently 60% enrolled. The Phase III melanoma trial is expected to begin in the first half of 2009. Based on encouraging Phase I/II clinical trial data of Abraxane®in combination with gemcitabine in the treatment of metastatic pancreatic cancer, we filed with the FDA a Phase III randomized trial of Abraxane®for use in both first and second line pancreatic cancer. A presentation of the Phase I/II study on use of Abraxane®in treatment of pancreatic cancer was presented at the American Society of Clinical Oncology Annual Meeting in May 2008. We continue to expand our clinical experience with Abraxane® and its potential in treating multiple tumor types as a single agent and in combination.
With regard to the global commercialization, Abraxane® is presently approved in 36 countries worldwide. Subsequent to the FDA approval of Abraxane® in the US in January 2005, Abraxis received regulatory approval from the Therapeutic Products Directorate of Health Canada to market Abraxane® for the treatment of metastatic breast cancer in Canada in June 2006. In October 2007, Abraxis received regulatory approval from India’s Drug Controller General to market Abraxane® for the treatment of breast cancer after failure of combination chemotherapy for metastatic disease or relapse within 6 months of adjuvant chemotherapy. In January 2008, the European Commission granted marketing approval in 30 European countries (via the centralized procedure) for Abraxane® in patients who have failed first-line treatment for metastatic disease and for whom standard, anthracycline containing therapy is not indicated. In March 2008, we received regulatory approval with the Korean FDA to market Abraxane® in South Korea for the treatment of breast cancer after failure of combination chemotherapy for metastatic disease. In June 2008, we received regulatory approval with the State Food and Drug Administration of the People’s Republic of China to market Abraxane® for the treatment of breast cancer after failure of combination chemotherapy for metastatic disease or relapse within 6 months of adjuvant chemotherapy. Prior therapy should have included an anthracycline unless clinically contraindicated. In September 2008, we received regulatory approval from the Therapeutic Goods Administration, Australia to market Abraxane® powder for injection (suspension) for the treatment of metastatic carcinoma of the breast after failure of anthracycline therapy. We launched Abraxane® in India (through our partner Biocon) in July 2008, in
7
the United Arab Emirates with partner Neobiocon in October 2008, in the United Kingdom in December 2008, in Germany in February 2009 and in Australia in collaboration with Specialised Therapeutics Australia Pty Ltd. in February 2009. Additionally, in 2009, we expect to launch Abraxane® in South Korea with partner Green Cross Corporation.
Marketing authorization applications have been submitted to several countries including Russia in June 2006, Japan in February 2008, and via the centralized procedure, in the Gulf Corporation Council countries (Saudi Arabia, Bahrain, Sultanate of Oman and Kuwait) in December 2008. In 2009, we further plan to submit marketing authorization applications for regulatory approval of Abraxane® (for the metastatic breast cancer indication) in New Zealand, Bhutan, Sri Lanka, Brazil and Taiwan.
nab® Tumor Targeting Technology Platform
We have identified a biological pathway specific to tumors through which tumors preferentially accumulate albumin-bound complexes. This preferential accumulation of albumin appears to be facilitated through a tumor’s secretion of a protein called SPARC, which binds to and accumulates albumin. A body of research has suggested that the secretion of SPARC occurs in most solid tumors that are difficult to treat, including in breast, lung, ovarian, head and neck, melanoma, gastric, esophageal, glioma and cervical tumors. Exploiting these tumors’ attraction for albumin, we have developed a novel mechanism to deliver chemotherapy agents in high concentrations preferentially to all tumors secreting SPARC. This is accomplished through ournab® tumor targeting technology, which encapsulates chemotherapy agents in nanometer-sized particles of albumin that is approximately 1/100th the size of a single red blood cell. Millions of these particles can be injected in a single dose of medication. This albumin-bound medication enters the bloodstream where it is transported across the blood vessel wall through a specific albumin receptor (gp60) on the blood vessel wall and targeted by SPARC secreted from the tumor cell.
The following diagram depicts ournab® tumor targeting technology and the proposed mechanism by which high concentrations of chemotherapy agents are delivered preferentially to tumors through the biological gp60 receptor pathway and through albumin binding to SPARC.
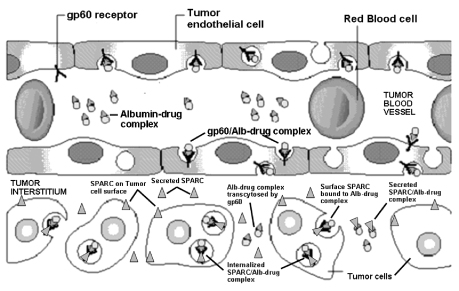
In April 2007, at the 98th Annual Meeting of the American Association for Cancer Research (AACR), we announced results from various pre-clinical studies that support the role of SPARC and ournab® tumor targeting
8
technology. The results from one pre-clinical study demonstrated that SPARC is an albumin-binding protein and that the level of the SPARC expression could be correlated with the response of tumors to Abraxane®. This provides concrete support for the earlier hypothesis that higher levels of SPARC protein may lead to enhanced anti-tumor effect of Abraxane®. The pre-clinical study results also provided evidence for chemotherapy induced angiogenesis and a rationale for combining Abraxane® with VEGF inhibitor drugs like Avastin® . We continue to explore the role of ournab® technology for targeting SPARC and other biological pathways, as well as for the development of new therapeutic candidates, including our clinical product candidates described below.
We believe we can apply ournab® tumor targeting technology to numerous chemotherapy agents. By exploiting the abnormal vascular growth (angiogenesis) and the overexpression of albumin-binding proteins (gp60 and SPARC) in advanced tumor cells and by overcoming water insolubility of many active chemotherapy agents, we believe that our technology may revolutionize the delivery of chemotherapy agents to cancer patients. As shown below, Abraxane® represents the first clinical and commercial validation of ournab® technology that takes advantage of albumin, a natural carrier of water insoluble molecules (e.g., various nutrients, vitamins and hormones) found in humans. Because tumors have an increased need for nutrients to support rapid malignant growth, albumin complexes accumulate preferentially in tumors.

We believe that ournab® tumor targeting technology will serve as the platform for the development of numerous other drugs for the treatment of cancer and other critical illnesses. We are committed to maximizing the potential application of our technology.
9
Select Clinical Product Candidates
We intend to continue to leverage ournab® tumor targeting technology and our internal clinical development and regulatory expertise to develop numerous drug candidates for the treatment of cancer and other critical illnesses. The following table is a selection of certain of our clinical product candidates and shows the status of these clinical product candidates.
| | | | |
Compound | | Critical Illness | | Status |
| | |
Abraxane® | | First-Line Non-Small Cell Lung Cancer | | Phase III began in the fourth quarter of 2007 and is 60% enrolled. |
| | |
Abraxane® | | Malignant Melanoma | | Phase III planned to begin in the first half of 2009. |
| | |
ABI-008 (nab®-docetaxel) | | Solid Tumors | | Phase I completed in hormone-refractory prostate cancer (HRPC). Phase II started in 2008. |
| | |
ABI-009 (nab®-rapamycin) | | Solid Tumors | | Phase I in progress. |
| | |
ABI-010 (nab®-17AAG) | | Solid Tumors | | IND filed in the first quarter of 2008. Phase I planned to begin in the first half of 2009. |
| | |
ABI-011 (nab®-thiocolchicine dimer) | |
Solid Tumors | | IND filing planned for 2009. |
| | |
Coroxane™ | | Coronary Artery Restenosis | | Phase II. Seeking strategic partner. |
| | |
Coroxane™ | | Peripheral Artery Restenosis | | Phase II. Seeking strategic partner. |
ABI-008 (nab®-docetaxel). ABI-008 is a solvent-free, Tween®-free nanometer-sized form of docetaxel. Docetaxel is the active ingredient in the currently largest selling taxane, Taxotere®. An investigational new drug application, or IND, was filed for ABI-008 in the fourth quarter of 2006. ABI-008 is currently in development for the first-line treatment of hormone-refractory prostate cancer (HRPC). Phase I studies have been completed and Phase II studies started in the second half of 2008. A Phase I/II trial in metastatic breast cancer is expected to begin later in 2009. Additionally, two Phase III ABI-008 trials, in HRPC and 2nd line non-small cell lung cancer (NSCLC), are planned to be filed with the FDA in 2009.
ABI-009 (nab®-rapamycin). Rapamycin, a protein kinase inhibitor, inhibits downstream signals from mTOR, a pathway that promotes tumor growth. Currently, it is marketed as an orally bioavailable immunosuppresive drug and no intravenous form is available because of the water insolubility of this molecule. The anti-cancer effects of rapamycin are limited by the immunosuppression associated with chronic oral administration. ABI-009 is a nanometer-sized, albumin-bound form of rapamycin able to be administered intravenously or by inhalation. At the 98th Annual Meeting of the AACR in April 2007, first-time data were presented from a pre-clinical study evaluating the toxicity and anti-tumor effect of ABI-009. These results demonstrated that ABI-009 was well-tolerated, showed linear pharmacokinetics, and was highly effective against a number of tumor models in vivo. The Phase I study is currently ongoing with ABI-009 in patients with solid tumors.
ABI-010 (nab®-17AAG). 17AAG is a polyketide inhibitor of Hsp90 (heat shock protein 90) and interrupts several biological processes implicated in cancer cell growth and survival. Hsp90 is a protein chaperone that
10
binds to several sets of signaling proteins, known as “client proteins.” 17AAG binds to Hsp90 and causes its dissociation from, and consequent degradation of, the client proteins. Because the Hsp90 client proteins are so important in signal transduction and in transcription (processes critical to the growth and survival of cancer cells), 17AAG may serve as a chemotherapy agent in the treatment of multiple cancers. Current formulations of 17AAG require solvents such as Cremophor and DMSO. ABI-010 is a solvent-free albumin-bound form of this drug The IND fornab®-17AAG and the clinical development plan was approved by the FDA in May 2008.
ABI-011 (nab® -thiocolchicine dimer).Thiocolchicine dimers are novel thiocolchicines with dual mechanisms of action showing both microtubule destabilization and the disruption of topoisomerase-1 activity.nab®-thiocolchicine dimer is a nanoparticle, albumin-bound form of the water insoluble dimer. ABI-011 was selected for its cytotoxic activity in nanomole range concentrations on several human tumor cell lines and, in particular, on a tumor cell line resistant to cisplatin and topotecan. This particular behavior was related to a dual mechanism of action. ABI-011 has the additional oncotherapeutic property of inhibiting topoisomerase-1 nuclear enzymes in a different manner than the classic topo-1 inhibitors, camptothecins. Pre-clinical studies of ABI-011 have shown that it also possesses anti-angiogenic and vascular disrupting activity. We anticipate that an IND fornab® thiocolchicine dimer will be filed in 2009.
Coroxane™ (nanometer-sized paclitaxel, Abraxane®, under the trade name Coroxane™).Coroxane™ is currently closing its Phase II clinical studies for coronary restenosis as well as peripheral artery (superficial femoral artery) restenosis. The SNAPIST series of studies examines the use of Coroxane™ in the treatment of coronary artery restenosis, including the use of Coroxane™ in patients receiving bare metal stents. Coroxane™ administered with bare metal stents may address the issue of incomplete re-endothelialization and acute thrombosis associated with drug-eluting stents. Coroxane™ administered following balloon angioplasty in the superficial femoral artery may help reduce the incidence of restenosis in these patients. We currently intend to seek a strategic partner for the further development and marketing of Coraxane™.
Research and Development
Our research and development efforts are based on the tight integration and rapid translation of discovery from the bench to the clinic among its chemistry, biology, pharmaceutical, regulatory and clinical development groups. We have recruited seasoned discovery scientists, medicinal chemists, formulation scientists and integrated these talents with a team of clinical development and regulatory experts. Our approach is based on the integration of various capabilities and resources, including:
| | • | | ournab® tumor targeting technology; |
| | • | | our proprietary natural product drug discovery platform; |
| | • | | our multi-functional chemistry capabilities; and |
| | • | | internal clinical trial and regulatory competencies. |
Natural Product Drug Discovery Platform
Almost half of today’s best-selling drugs originate from natural products or their derivatives. A key feature of natural products is their enormous structural and chemical diversity that is not represented in combinatorial libraries of synthetic compounds. This makes natural products a fertile resource for finding novel compounds that interact with new targets for drug discovery. In addition, we believe ournab® tumor targeting technology platform is ideally suited to the formulation and delivery of natural products, which tend to be more water insoluble, larger and more complex than typical synthetic drugs.
We have established a proprietary natural product library of large chemical diversity for drug discovery. The library currently represents approximately 100,000 semi-purified screening samples derived from microorganisms (over 65,000 strains of actinomycetes, fungi and bacteria) retrieved from over 38,000 soil
11
samples representing geographic, habitat and genetic diversity from all over the world. New strains of microorganisms are continuously being added from our soil sample collection, and we believe the millions of microorganisms in each soil sample provide us with an almost limitless resource for continuing to create new and targeted libraries of natural product chemical diversity for drug discovery. For example,nab®-17AAG was developed based on extracts from our natural product library.
Natural products are an important component of our drug discovery strategy and, with the capability to overcoming water insolubility through the use of ournab® technology, we believe we have the unique opportunity to translate water insoluble compounds discovered from our natural product libraries into clinical applications.
Multi-functional Chemistry Capabilities. We possess a full range of chemistry capabilities, including organic and medicinal chemistry, analytical chemistry, formulation, process development and natural product isolation chemistry. Our approach, which involves chemistry-driven discovery combined with biology-driven validation and integration with ournab® technology, has been applied successfully on many drug discovery programs.
Internal Clinical Trial and Regulatory Competencies. We have established key internal clinical development and regulatory competencies to execute rapid clinical translation of our technology in the most cost-efficient manner. Clinical Operations, Safety Reporting, Data Management and Biostatistics are located in our Durham, North Carolina office. Clinical Operations manages all of our company-sponsored clinical trials by overseeing the study conduct at each site and verifying data integrity. Data Management enters all clinical trial data into an in-house database, which is then analyzed by our biostatistics team who produce reports for FDA submissions. Safety reporting and post-marketing safety surveillance are carried out using a Drug Safety Reporting System. The regulatory operations team is responsible for making submissions to the FDA and global regulatory authorities. This seasoned team accomplished the first electronic submission of an NDA with the FDA of an oncology product, resulting in FDA approval of Abraxane®. An important strategy underway is the development of informatics and electronic systems to streamline our clinical trial management processes, including establishing alliances to enhance patient accrual and provide efficient methods to capture data.
Drug Discovery Strategies
Our drug discovery strategies are based upon the following:
| | • | | our nab® tumor targeting technology; |
| | • | | our natural product drug discovery platform; |
| | • | | biomarkers and molecular profiling; |
| | • | | cyclodextrin-based delivery technology; |
| | • | | nucleic acid complementation technology; |
| | • | | pluripotent adult stem cells; |
| | • | | our cancer drug discovery targeting the tumor suppressor p53; |
| | • | | immunotherapeutics and related assay systems; |
| | • | | Abraxis translational molecular bioscience at California NanoSystems Institute; |
| | • | | central nervous system therapies. |
12
SPARC
SPARC (Secreted Protein Acidic Rich in Cysteine) appears to be the intratumoral target of ournab® technology platform due to its secretion by a variety of tumors and the affinity of albumin for SPARC. In addition, it has been discovered that the SPARC protein, when administered systemically in combination with chemotherapy, can greatly sensitize a chemotherapy resistant tumor in xenograft tumor models. Our scientists, in collaboration with university scientists, are exploring the therapeutic potential of SPARC in the treatment of chemo-resistant tumors. We will continue to explore the role of ournab® technology for targeting SPARC and other biological pathways, as well as for the development of new therapeutic candidates.
Biomarkers and Molecular Profiling
In May 2007, we entered into a license agreement with the University of Southern California (USC) under which we licensed the exclusive worldwide development and commercialization rights for an intellectual property portfolio of diagnostic protein biomarkers for therapy response, therapy toxicity and disease recurrence in colorectal cancers. The intellectual property licensed is based on USC research by Associate Professor of Medicine Heinz-Joseph Lenz and colleagues. We believe these licensed biomarkers are consistent with our strategy to identify compounds with specific activity linked to specific biological markers related to particular disease states and may be useful predictors of response to treatments (evaluated through clinical response, toxicity and time to disease progression) and prognostic markers which are important in determining the aggressiveness of cancers as well as other diseases.
Cyclodextrin-Based Delivery Technology
Through our wholly-owned subsidiary Shimoda Biotech (Pty) Limited (Shimoda), we are developing cyclodextrin-based formulations of existing therapeutics to improve their bioavailability characteristics. Shimoda has used cyclodextrin formulations to improve the dissolution rate and bioavailability of orally-deliverable drugs, such as diclofenac sodium, to increase aqueous solubility of poorly water-soluble drugs, to increase the chemical and physical stability of injectable drugs and to mask the unpleasant taste of oral drugs. Cyclodextrin is a water-soluble complex oligosaccharide that structurally forms a unique nanoparticle capable of delivering hydrophobic compounds via multiple routes, including oral ingestion and intravenous injection.
Shimoda’s first cyclodextrin-based product, Dyloject® (diclofenac sodium solution for injection), is an injectable painkiller for the treatment of post-surgical pain. Dyloject® is the world’s first solubilized intravenous formulation of diclofenac. Diclofenac is a non-steroidal anti-inflammatory drug (NSAID). Dyloject® was launched in December 2007 in the United Kingdom by Javelin Pharmaceuticals under an exclusive worldwide license agreement pursuant to which Shimoda will receive milestone payments and royalties.
Nucleic Acid Complementation Technology
We are pursuing the development of an engineered split-toxin platform which leads to the functional reconstitution of a cytotoxin from multiple parts under very specific conditions (for example, in cancer cells arising from known genetic anomalies or in virus-infected cells). This strategy does not require traditional high-throughput drug screening methodologies and has the added capability of being further modified to serve as a diagnostic tool for monitoring patients’ responses to therapy. This research is being pursued through Dithera, Inc. (Dithera). In December 2008, we purchased preferred stock of Dithera representing 50% of Dithera’s outstanding capital stock and were required under GAAP to consolidate Dithera in our 2008 financial statements.
Pluripotent Adult Stem Cells
In September of 2006, we entered into a license with the University of Maryland under which we exclusively licensed the worldwide intellectual property rights to the CD34+ non-adherent adult bone marrow stem cell technology. This technology may present an opportunity to reverse the effects of neurodegenerative
13
diseases such as macular degeneration, Alzheimer’s and Parkinson’s diseases, and multiple sclerosis, as well as to rehabilitate non-CNS diseased organs such as the pancreas for autologous production of insulin in diabetic patients.
Cancer Drug Discovery Targeting the Tumor Suppressor p53
In July 2007, we entered into a license agreement with the Buck Institute for Age Research under which we exclusively licensed the worldwide intellectual property rights for technologies designed to generate novel therapeutics and identify new drug discovery targets. There are no up-front or milestone payments required by us under the license agreement. If products are successfully developed incorporating any of the licensed technologies, we will pay the Buck Institute royalties based on a percentage of net sales. The term of the agreement will continue until the last patent claiming a licensed product under the agreement expires. However, the license agreement may be terminated earlier by either party upon the other party’s failure to timely cure a material breach under the agreement or upon the other party’s bankruptcy or insolvency.
Through this license agreement, we own the rights to a proprietary discovery platform designed to discover new chemical entities that remediate the signaling activities of the tumor suppressor p53 in p53-dysfunctional cancer cells. Loss of p53 activity is associated with one-half of all human tumors, often rendering these cancer cells resistant to conventional therapies. The licensed technologies have made the discovery of reactivators of appropriate p53 signaling behavior possible. Inherent in the design of the technologies is a strategy to develop therapeutics that selectively stimulate programmed cell death in p53-dysfunctional cancer cells and that would leave healthy cells expressing normal p53 unaffected.
The therapies developed in this program will target a specific population of aberrant (tumor) cells and forge a novel class of chemotherapeutics which have the potential to be much more potent than general inhibitors of cell proliferation or inducers of cell death. In the era of personalized medicine, and in combination with our emerging diagnostic methodologies, we believe this program will generate a novel pipeline of drugs that promise cancer patients greater proficiency with fewer side effects.
Immunotherapeutics and Related Assay Systems
The technologies licensed from the Buck Institute also included a novel immunotherapeutic/anti-cancer compound (T9) and highly sensitive cell-based assay systems for the discovery of additional immune-modulating drugs. Immune-modulating drugs represent an emerging class of therapies with broad clinical application in the treatment of cancer, allergies, inflammation, autoimmunity and tissue transplantation. T9 is a highly potent bi-functional molecule with the ability to kill cancer cells and to activate the immune response to recognize cancer cells in a manner analogous to childhood vaccination.
T9 was originally discovered using robust ultra-sensitive cell-based assay systems which respond to minute amounts of potential immune-modulating drugs. Over time, these systems were further modified to allow for their use in high throughput screens for the identification of compounds that can control the magnitude and quality of the immune response. The immune-modulating high throughput screening systems (IMHTSS) technologies are important tools for the discovery of novel agents which modulate the immune response through controlling the type and degree of inflammation. “Hits” resulting from the screening of synthetic and natural product libraries using the IMHTSS technologies are anticipated to be further developed to act as immune adjuvants in improving existing vaccination platforms or to inhibit the immune response in the context of allergies (such as asthma) or autoimmune diseases (such as type I diabetes, multiple sclerosis and lupus erythematosus).
Translational Molecular Bioscience at CNSI
The California NanoSystems Institute (CNSI) is a multidisciplinary research center at UCLA whose mission is to encourage university-industry collaboration and to enable the rapid commercialization of discoveries in
14
nanosystems. CNSI members include some of the world’s preeminent scientists, and the work conducted at the institute represents world-class expertise in five targeted areas of nanosystems-related research: renewable energy, environmental nanotechnology and nanotoxicology, nanobiotechnology and biomaterials, nanomechanical and nanofluidic systems, and nanoelectronics, photonics and architectonics.
In July 2007, we entered into a research collaboration agreement with CNSI under which the parties agreed to collaborate on early research in nanobiotechnology for the advancement of new technologies in medicine. Under the agreement, we committed to fund up to $10 million over ten years in two tranches of $5 million for research projects selected by a committee comprised equally of our and CNSI representatives. Any intellectual property resulting from the projects will be owned by CNSI. However, we have first right to negotiate an option or license to all of such intellectual property on commercially reasonable terms, including royalties. In addition, if the parties are unable to successfully negotiate a license, CNSI may not license the intellectual property to another party at a lower rate than offered to us for a specified period of time. The term of the agreement is ten years, but either party can terminate on 30 days notice and the funding commitment for the second $5 million is subject to mutual agreement within 30 days of the end of the fifth year of the agreement.
This partnership provides CNSI and our researchers the opportunity to jointly pursue innovative approaches to the diagnosis and treatment of life-threatening diseases, leveraging the complementary resources and skills of both organizations. Working side by side with CNSI researchers, our scientists will focus on rapidly and seamlessly translating early scientific discovery into practical application. The Abraxis/CNSI Research Collaboration Lab has been designed to integrate and support multidisciplinary science, including cellular and molecular biology (including high-throughput discovery), nanodetection methodologies and tools for diagnostic discoveries, medicinal and synthetic chemistry, computational structural biology (including rational approaches to drug discovery) and bioengineering of nanodevices and nanomaterials.
Biosimilars
In June 2007, we entered into a license agreement with Biocon Limited under which we licensed the right to develop and commercialize a biosimilar version of G-CSF (granulocyte-colony stimulating factor) in North America and the European Union. G-CSF is an haematopoietic growth factor that works by encouraging the bone marrow to produce more white blood cells. Therapeutic G-CSF is primarily used for the treatment of neutropenia, the lowering of the white blood cells that fight infections. Biocon has received regulatory approval of its G-CSF from the Drugs Controller General of India (DCGI) for the treatment of neutropenia in cancer patients. The biological activity of Biocon’s G-CSF used in clinical trials was evaluated by the National Institute of Biological Standards and Control (NIBSC), UK, which provides independent testing of biological medicines. The NIBSC found that the potency of Biocon’s drug met the necessary requirements of a biosimilar G-CSF.
In November 2007, we entered into a license agreement with Green Cross Corporation under which we licensed the right to develop and commercialize five biosimilars for the United States and Canada on an exclusive basis: Enbrel® (etanercept), pegylated G-CSF, recombinant Factor VIII, Interferon-Alpha and Erythropoietin. Etanercept is a chimeric protein designed to neutralize tumor necrosis factor (TNF), an important regulator of local inflammatory response. Pegylated G-CSF, like G-CSF, is primarily used for the treatment of neutropenia, but its half-life is greatly increased compared to G-CSF because of the polyethylene glycol treatment (a process also commonly referred to as “pegylating”), thereby reducing the frequency and dose of the injections required to sustain a patient’s neutrophil levels. Recombinant Factor VIII is a clotting factor that is essential for reestablishing hemostasis in hemophiliac patients. Interferon-Alpha is a powerful, multifunctional protein produced by innate cells of the immune system to combat viral infections. Erythropoietin is a protein produced mainly by cells of the kidneys to maintain red blood cell levels by inhibiting their ability to commit cellular “suicide” and stimulate their production from precursors and is approved for patients experiencing anemia due to a variety of mechanisms including chronic renal failure and certain anti-cancer chemotherapies.
15
Central Nervous System Therapies
In April 2007, we formed a joint venture with Cenomed, Inc. (Cenomed) to create Cenomed BioSciences, LLC. This venture is designed to further the research and development of novel drugs that interact with the central nervous system focused on psychiatric and neurological diseases. Substantially all of the assets of Cenomed were contributed to this joint venture. The previous focus of the research by Cenomed included the development of drugs for the treatment of schizophrenia, neuroprotection, mild cognitive impairment and memory and attention impairments associated with aging, attention deficit hyperactivity disorder and pain. We hold a 70% membership interest in this joint venture, which is consolidated in our financial results of operations.
Sales and Marketing
We have a dedicated sales and marketing group in North America which targets key segments of the oncology market: specifically, leading oncologists, cancer centers and the oncology distribution channel. This group is comprised of approximately 136 sales, marketing and medical professionals and support staff members. With respect to sales and marketing of Abraxane® in countries outside of North America, we are continuing to establish our infrastructure and are evaluating developing our own sales and marketing expertise as well as exploring possible strategic relationships for other jurisdictions. Sales force and commercial services in our European market will be provided by Innovex, a unit of Quintiles Transnational Corp., which is the leading global commercial solutions provider to the pharmaceutical, biotech, and medical device industries.
Strategic Relationships
Taiho Pharmaceutical Co., Ltd.
In May 2005, we entered into a license agreement with Taiho Pharmaceutical Co., Ltd. under which we granted to Taiho the exclusive rights to market and sell Abraxane® in Japan. In March 2008, Taiho filed a Japanese New Drug Application (J-NDA) with the Ministry of Health, Labour and Welfare to market Abraxane® for the treatment of breast cancer in Japan. We established a joint steering committee with Taiho to oversee the development of Abraxane® in Japan for the treatment of breast, lung and gastric cancer and other solid tumors. Under this license agreement, Taiho paid us a non-refundable, upfront payment and will make additional payments to us upon achievement of various clinical, regulatory and sales milestones, with total potential payments in excess of $50 million. In addition, we will receive royalties from Taiho based on net sales under the license agreement. The agreement will remain in effect until terminated by one of the parties in accordance with its terms. The agreement may be terminated: (a) at any time by mutual agreement of the parties; (b) by either party if the other party fails to timely cure a material breach under the agreement or the other party is bankrupt or insolvent; (c) by Taiho upon nine months notice if Taiho determines, for safety, legal or other reasons, not to continue pursuing the development and commercialization of Abraxane® in Japan; (d) by Taiho if we fail to timely remedy certain manufacturing deficiencies; and (e) at any time by us if Taiho sells (i) Abraxane® while concurrently selling Abraxane® in a generic form or (ii) an injectable formulation of a taxane compound, including paclitaxel and docetaxel.
Biocon Limited
In June 2007, we entered into a license agreement with Biocon Limited under which we granted Biocon the right to market and sell Abraxane® in India, Pakistan, Bangladesh, Sri Lanka, United Arab Emirates, Saudi Arabia, Kuwait and certain other South Asian and Persian Gulf countries. Biocon’s rights are exclusive with respect to India, Pakistan, Bangladesh, Nepal, Bhutan, Sri Lanka and the Maldive Islands and are co-exclusive with us and our licensees with respect to all other countries. If Biocon fails to achieve a specified target market share by a specified time in any exclusive country, then we have the right to make Biocon’s license in that country non-exclusive unless Biocon makes a payment to us equal approximately to the royalty that would have been payable had Biocon achieved the target market share. If Biocon fails to achieve a minimum market share by
16
a specified time in any exclusive country for two consecutive calendar years, then we also have the right to terminate Biocon’s rights with respect to such country. The agreement will remain in effect until terminated by one of the parties in accordance its terms. We may terminate the agreement if Biocon (a) fails to timely cure a material breach; (b) becomes bankrupt; or (c) sells Abraxane® while concurrently selling a generic form of Abraxane®. Biocon may terminate the agreement if we fail to timely cure a material breach. We will receive payments from Biocon based on the higher of a percentage net sales or a specified profit split under the license agreement. In October 2007, we received regulatory approval from India’s Drug Controller General to market Abraxane® for the treatment of metastatic breast cancer in India. In July 2008, we launched Abraxane in India for the treatment of metastatic breast cancer.
ProMetic Life Sciences Inc
In September 2008, we entered into agreements with ProMetic Life Sciences Inc. (ProMetic) to develop and commercialize four biopharmaceutical products targeting underserved medical conditions. We entered into the following agreements: (i) a securities purchase agreement, (ii) a license agreement, (iii) a supply and license agreement (iv) an exclusive manufacturing agreement and (v) a services agreement. The transaction included an initial investment in ProMetic of $7 million and optional future investment rights of up to $25 million. Of the $7 million initial investment, $5.2 million was allocated to the purchase of ProMetic’s common stock and $1.8 million was allocated to the future investment rights option. We will have access to ProMetic’s proprietary protein technologies to commercialize the biopharmaceuticals and will fund all development costs to regulatory approval. In consideration, we will pay potential milestone and royalty payments to ProMetic and royalties on the net sales of the four products. Additionally, ProMetic will perform product development activities on behalf of Abraxis under the service agreement.
Competition
Competition among biotechnology, pharmaceutical and other companies that research, develop, manufacture or market proprietary pharmaceuticals is intense and is expected to increase. We compete with these entities in all areas of business, including competing to attract and retain qualified scientific and technical personnel.
Our products’ and product candidates’ competitive position among other pharmaceutical products may be based on, among other things, patent position, product efficacy, safety, reliability, availability, patient convenience/delivery devices and price, as well as, the development and marketing of new competitive products. Certain of our products and product candidates may face substantial competition from products marketed by large pharmaceutical companies, many of which have greater clinical, research, regulatory, manufacturing, marketing, financial and human resources, and experience than we do. In addition, the introduction of new products or the development of new processes by competitors or new information about existing products may result in product replacements or price reductions, even for products protected by patents.
Some of our competitors are actively engaged in research and development in areas where we are developing product candidates. The competitive marketplace for our product candidates is significantly dependent upon the timing of entry into the market. Early entry may have important advantages in gaining product acceptance and market share and, as a result, may contribute significantly to the product’s eventual success and profitability. Accordingly, in some cases, the relative speed with which we can develop products, complete the testing, receive approval, and supply commercial quantities of the product to the market is expected to be important to our competitive position.
In addition, we compete with large pharmaceutical and biotechnology companies when entering into cooperative arrangements with smaller companies and research organizations in the biotechnology industry for the development and commercialization of products and product candidates. Small companies, academic institutions, governmental agencies and other public and private research organizations conduct a significant
17
amount of research and development in the biotechnology industry. These entities may seek to enter into licensing arrangements to collect royalties for use of technology or for the sale of products they have discovered or developed. We may face competition in licensing or acquisition activities from pharmaceutical companies and large biotechnology companies that also seek to acquire technologies or product candidates from these entities.
We believe that Abraxane® competes, directly or indirectly, with the primary taxanes in the market place, including Bristol-Myers Squibb’s Taxol® and its generic equivalents, Sanofi-Aventis’ Taxotere® and other cancer therapies. Many pharmaceutical companies have developed and are marketing, or are developing, alternative formulations of paclitaxel and other cancer therapies that may compete directly or indirectly with Abraxane®.
Regulatory Considerations
Proprietary pharmaceutical products are subject to extensive pre- and post-market regulation by the FDA, including regulations that govern the testing, manufacturing, safety, efficacy, labeling, storage, record keeping, advertising and promotion of the products under the Federal Food Drug and Cosmetic Act and the Public Health Services Act, and by comparable agencies in foreign countries. FDA approval is required before any dosage form of any drug can be marketed in the United States. All applications for FDA approval of drugs must contain information relating to pharmaceutical formulation, stability, manufacturing, processing, packaging, labeling and quality control.
The process required by the FDA before a new drug may be marketed in the United States generally involves:
| | • | | completion of pre-clinical laboratory and animal testing; |
| | • | | submission of an investigational new drug application, or IND, which must become effective before trials may begin; |
| | • | | performance of adequate and well-controlled human clinical trials to establish the safety and efficacy of the proposed drug product’s intended use; and |
| | • | | submission to and approval by the FDA of a new drug application, or NDA. |
Clinical trials are typically conducted in three sequential phases that may overlap. These phases generally include:
| | • | | Phase I during which the drug is introduced into healthy human subjects or, on occasion, patients, and generally is tested for safety, stability, dose tolerance and metabolism; |
| | • | | Phase II during which the drug is introduced into a limited patient population to determine the efficacy of the product in specific targeted diseases, to determine dosage tolerance and optimal dosage and to identify possible adverse effects and safety risks; and |
| | • | | Phase III during which the clinical trial is expanded to a more diverse patient group in geographically dispersed trial sites to further evaluate clinical efficacy, optimal dosage and safety. |
The drug sponsor, the FDA or the Institutional Review Board at each institution at which a clinical trial is being performed may suspend a clinical trial at any time for various reasons, including a belief that the subjects are being exposed to an unacceptable health risk.
The results of product development, preclinical animal studies and human studies are submitted to the FDA as part of the NDA. The NDA also must contain extensive manufacturing information. The FDA may approve on the basis of the submission made or disapprove the NDA if applicable FDA regulatory criteria are not satisfied. The FDA may also require additional clinical data. Under certain circumstances, drug sponsors may obtain approval pursuant to Section 505(b)(2) of the Federal Food, Drug & Cosmetic Act based in part upon literature or
18
an FDA finding and/or effectiveness for another approved product, even where the products are not duplicates in terms of formulation and bioequivalence. Once approved, the FDA may withdraw the product approval if compliance with pre- and post-market regulatory standards is not maintained or if problems occur after the product reaches the marketplace. In addition, the FDA may require post-marketing studies to monitor the effect of approved products and may limit further marketing of the product based on the results of these post-marketing studies. The FDA has broad post-market regulatory and enforcement powers, including the ability to levy fines and civil penalties, suspend or delay issuance of approvals, seize or recall products, and withdraw approvals.
Satisfaction of FDA pre-market approval requirements typically takes several years and the actual time required may vary substantially based upon the type, complexity and novelty of the product or disease. Government regulation may delay or prevent marketing of potential products for a considerable period of time and impose costly procedures upon a manufacturer’s activities. Success in early stage clinical trials does not assure success in later stage clinical trials. Data obtained from clinical activities is not always conclusive and may be susceptible to varying interpretations that could delay, limit or prevent regulatory approval. Even if a product receives regulatory approval, later discovery of previously unknown problems with a product may result in restrictions on the product or even complete withdrawal of the product from the market.
In addition to regulating and auditing human clinical trials, the FDA regulates and inspects equipment, facilities, laboratories and processes used in the manufacturing and testing of such products prior to providing approval to market a product. If after receiving clearance from the FDA, a material change is made in manufacturing equipment, location or process, additional regulatory review may be required. We and our suppliers also must adhere to current good manufacturing practice and product-specific regulations enforced by the FDA through its facilities inspection program. The FDA also conducts regular, periodic visits to re-inspect equipment, facilities, laboratories and processes following the initial approval. If, as a result of these inspections, the FDA determines that the equipment, facilities, laboratories or processes do not comply with applicable FDA regulations and conditions of product approval, the FDA may seek civil, criminal or administrative sanctions and/or remedies against our manufacturers, including the suspension of manufacturing operations.
While the above process describes the regulatory process in the United States, similar processes for drug development and approval to market generally apply internationally.
We are also subject to various federal and state laws pertaining to health care “fraud and abuse,” including anti-kickback laws and false claims laws. Anti-kickback laws make it illegal to solicit, offer, receive or pay any remuneration in exchange for, or to induce, the referral of business, including the purchase or prescription of a particular drug. The federal government has published regulations that identify “safe harbors” or exemptions for certain arrangements that do not violate the anti-kickback statutes. Due to the breadth of the statutory provisions and the absence of guidance in the form of regulations or court decisions addressing some practices, it is possible that our practices might be challenged under anti-kickback or similar laws. False claims laws prohibit anyone from knowingly and willingly presenting, or causing to be presented for payment to third-party payers (including Medicare and Medicaid), claims for reimbursed drugs or services that are false or fraudulent, claims for items or services not provided as claimed or claims for medically unnecessary items or services. The activities of our strategic partners relating to the sale and marketing of our products may be subject to scrutiny under these laws. Violations of fraud and abuse laws may be punishable by criminal and/or civil sanctions, including fines and civil monetary penalties, as well as the possibility of exclusion from federal health care programs (including Medicare and Medicaid). If the government were to allege against or convict us of violating these laws, there could be a material adverse effect on us. Our activities could be subject to challenge for the reasons discussed above and due to the broad scope of these laws and the increasing attention being given to them by law enforcement authorities.
We are also subject to regulation under the Occupational Safety and Health Act, the Toxic Substances Control Act, the Resource Conservation and Recovery Act, and other current and potential future federal, state, or local laws, rules and/or regulations. Our research and development activities involve the controlled use of
19
chemicals, biological materials and other hazardous materials. We believe that our procedures comply with the standards prescribed by federal, state and local laws, rules and/or regulations; however, the risk of injury or accidental contamination cannot be completely eliminated.
Manufacturing
We have manufacturing facilities in Melrose Park, Illinois, Phoenix, Arizona and Barbengo, Switzerland. For the length of the applicable lease, our manufacturing facility in Melrose Park has been leased to APP and APP has agreed to provide certain contract manufacturing services to us in accordance with the terms of the manufacturing agreement. In addition, we leased from APP a portion of APP’s Grand Island, New York manufacturing facility to enable us to perform our responsibilities under the manufacturing agreement with APP for its term. The initial term of the manufacturing agreement will expire on December 31, 2011, but the term of the manufacturing agreement will be automatically extended for one year if either APP exercises its option to extend the lease for our Melrose Park manufacturing facility for an additional year or we exercise our option to extend the lease for APP’s Grand Island manufacturing facility for an additional year.
In addition, as part of the separation agreement, APP’s Puerto Rico facility was divided into two separate facilities and we took ownership of one part of the facility. The portion of the Puerto Rico facility that is owned by us following the separation is the 90,000 square foot active pharmaceutical ingredients manufacturing plant currently leased to Pfizer for a term expiring in February 2012. Pfizer has notified us that it intends to vacate the premises in May 2009. Pfizer will not incur any penalty for the early termination of the lease.
We are required to comply with the applicable FDA manufacturing requirements contained in the FDA’s current Good Manufacturing Practice, or cGMP, regulations. cGMP regulations require quality control and quality assurance as well as the corresponding maintenance of records and documentation. Our manufacturing facilities must meet cGMP requirements to permit us to manufacture our products. We are subject to the periodic inspection of our facilities, procedures and operations and/or the testing of our products by the FDA, the Drug Enforcement Administration and other authorities to assess our compliance with applicable regulations.
Failure to comply with the statutory and regulatory requirements subjects the manufacturer to possible legal or regulatory action, including the seizure or recall of products, injunctions, consent decrees placing significant restrictions on or suspending manufacturing operations, and civil and criminal penalties. Adverse experiences with a product must be reported to the FDA and could result in the imposition of market restriction through labeling changes or in product removal. Product approvals may be withdrawn if compliance with regulatory requirements is not maintained or if problems concerning safety or efficacy of the product occur following approval.
Raw Materials
The manufacture of our products requires raw materials and other components that must meet stringent FDA requirements. Some of these raw materials and other components currently are available only from a limited number of sources. Additionally, regulatory approvals for a particular product denote the raw materials and components, and the suppliers for such materials that may be used for that product. Even when more than one supplier exists, we may elect to list, and in some cases have only listed, one supplier in our applications with the FDA. Any change in or addition of a supplier not previously approved must then be submitted through a formal approval process with the FDA. From time to time, it is necessary to maintain increased levels of certain raw materials due to the anticipation of raw material shortages or in response to market opportunities.
Intellectual Property
We rely on trade secrets, unpatented proprietary know-how, continuing technological innovation and patent protection to preserve our competitive position. We have over 100 issued U.S. and foreign patents, including patents relating to Abraxane® and the technology surrounding Abraxane®. The issued patents covering
20
Abraxane®, and methods of use and preparation of Abraxane®, currently expire through 2018, but we have additional pending U.S. and foreign patent applications covering Abraxane® that could extend the expiration dates.
Our success depends on our ability to operate without infringing the patents and proprietary rights of third parties. We cannot determine with certainty whether patents or patent applications of other parties will materially affect our ability to make, use, offer to sell or sell any products. A number of pharmaceutical companies, biotechnology companies, universities and research institutions may have filed patent applications or may have been granted patents that cover aspects of our or our licensors’ products, product candidates or other technologies.
Intellectual property protection is highly uncertain and involves complex legal and factual questions. Our patents and those for which we have or will have licensed rights may be challenged, invalidated, infringed or circumvented, and the rights granted in those patents may not provide proprietary protection or competitive advantages to us. We and our licensors may not be able to develop patentable products. Even if patent claims are allowed, the claims may not issue, or in the event of issuance, may not be sufficient to protect the technology owned by or licensed to us.
Third-party patent applications and patents could reduce the coverage of the patents licensed, or that may be licensed to or owned by us. If patents containing competitive or conflicting claims are issued to third parties, we may be enjoined from commercialization of products or be required to obtain licenses to these patents or to develop or obtain alternative technology. In addition, other parties may duplicate, design around or independently develop similar or alternative technologies to our licensors or our technologies.
Litigation may be necessary to enforce patents issued or licensed to us or to determine the scope or validity of another party’s proprietary rights. U.S. Patent and Trademark Office interference proceedings may be necessary if we and another party both claim to have invented the same subject matter. Similarly, our patents and patent applications, or those of our licensors, could face other challenges, such as opposition proceedings and reexamination proceedings. Any such challenge, if successful, could result in the invalidation of, or a narrowing of scope of, any such patents and patent applications. We could incur substantial costs and our management’s attention would be diverted if:
| | • | | patent litigation is brought by third parties; |
| | • | | we are a party to or participate in patent suits brought against or initiated by us or our licensors; |
| | • | | we are a party to or participate in an interference proceeding; or |
| | • | | we are a party to an opposition or reexamination proceeding. |
In addition, we may not prevail in any of these actions or proceedings.
We are currently involved in a patent infringement lawsuit with Élan Pharmaceutical Int’l Ltd. See “Item 3—Legal Proceedings” below.
Employees
As of December 31, 2008, we had a total of approximately 734 full-time employees, of which approximately 310 were dedicated to research and development activities, including clinical trials, approximately 160 were in manufacturing, approximately 136 were in sales and marketing and approximately 128 were in administration and finance. None of our employees are represented by a labor union or subject to a collective bargaining agreement. We have not experienced any work stoppage and consider our relations with our employees to be good.
21
Environment
We believe that our operations comply in all material respects with applicable laws and regulations concerning the environment. While it is impossible to predict accurately the future costs associated with environmental compliance and potential remediation activities, compliance with environmental laws is not expected to require significant capital expenditures and has not had, and is not expected to have, a material adverse effect on our financial condition or competitive position.
Item 1A. RISK FACTORS
You should carefully consider the risks described below before investing in our publicly traded securities. The risks described below are not the only ones facing us. Our business is also subject to the risks that affect many other companies, such as competition and, general economic conditions. Additional risks not currently known to us or that we currently believe are immaterial also may impair our business operations and our liquidity.
Risks Related to our Business and Industry
We are highly dependent upon the strong market acceptance of Abraxane®.
Our future profitability is highly dependent on the strong market acceptance of Abraxane®. For the years ended December 31, 2008 and 2007, total Abraxane® revenue was $335.6 million and $324.7 million, respectively, in each case including $36.4 million of recognized deferred revenue relating to the co-promotion agreement with AstraZeneca. The co-promotion agreement ended in January 2009. Because Abraxane® is our only approved product, Abraxane® revenue represented approximately 97.2% and 97.3% of our revenues for the year ended December 31, 2008 and 2007, respectively. We anticipate that sales of Abraxane® will remain a substantial portion of our total revenue over the next several years. However, a number of pharmaceutical companies are working to develop alternative formulations of paclitaxel and other cancer drugs and therapies, any of which may compete directly or indirectly with Abraxane® and which might adversely affect the commercial success of Abraxane®.
Abraxane® could lose market share or revenue due to numerous factors, many of which are beyond our control, including:
| | • | | lower prices offered on similar products by other manufacturers, including generic forms of Taxol; |
| | • | | substitute or alternative products or therapies; |
| | • | | development by others of new pharmaceutical products or treatments that are more effective than Abraxane®; |
| | • | | introduction of other generic equivalents or products which may be therapeutically interchanged with Abraxane®; |
| | • | | interruptions in manufacturing or supply; |
| | • | | changes in the prescribing practices of physicians; |
| | • | | changes in third-party reimbursement practices; and |
| | • | | migration of key customers to other manufacturers or sellers. |
In addition, we will continue to make a significant investment in Abraxane®, including costs associated with conducting clinical trials and obtaining necessary regulatory approvals for the use of Abraxane® in other indications and settings and in other jurisdictions, expansion of our marketing, sales and manufacturing staff, the acquisition of paclitaxel raw material, expansion of manufacturing facilities and the manufacture of finished product. The success of Abraxane® in the Phase III trial for metastatic breast cancer and other clinical trials may
22
not be representative of future clinical trial results for Abraxane® with respect to other clinical indications. The results from clinical, pre-clinical studies and early clinical trials conducted to date may not be predictive of results to be obtained in later clinical trials, including those ongoing at present. Further, the commencement and completion of clinical trials may be delayed by many factors that are beyond our control, including:
| | • | | slower than anticipated patient enrollment; |
| | • | | difficulty in finding and retaining patients fitting the trial profile or protocols; and |
| | • | | adverse events occurring during the clinical trials. |
Proprietary product development efforts may not result in commercial products.
We intend to maintain an aggressive research and development program for proprietary pharmaceutical products. Successful product development in the biotechnology industry is highly uncertain, and statistically very few research and development projects produce a commercial product. Product candidates that appear promising in the early phases of development, such as in early stage human clinical trials, may fail to reach the market for a number of reasons, including:
| | • | | the product candidate did not demonstrate acceptable clinical trial results even though it demonstrated positive pre-clinical trial results; |
| | • | | the product candidate was not effective in treating a specified condition or illness; |
| | • | | the product candidate had harmful side effects in humans or animals; |
| | • | | the necessary regulatory bodies, such as the FDA, did not approve a product candidate for an intended use; |
| | • | | the product candidate was not economical to manufacture and commercialize; |
| | • | | other companies or people have or may have proprietary rights to a product candidate, such as patent rights, and will not let the product candidate be sold on reasonable terms, or at all; or |
| | • | | the product candidate is not cost effective in light of existing therapeutics. |
We may be required to perform additional clinical trials or change the labeling of our products if side effects or manufacturing problems are identified after our products are on the market.
If side effects are identified after any of our products are on the market, or if manufacturing problems occur, regulatory approval may be withdrawn and product recalls, reformulation of products, additional clinical trials, changes in labeling of products, and changes to or re-approvals of our manufacturing facilities may be required, any of which could have a material adverse effect on sales of the affected products and on our business and results of operations.
After products are approved for commercial use, we or regulatory bodies could decide that changes to the product labeling are required. Label changes may be necessary for a number of reasons, including the identification of actual or theoretical safety or efficacy concerns by regulatory agencies or the discovery of significant problems with a similar product that implicates an entire class of products. Any significant concerns raised about the safety or efficacy of our products could also result in the need to recall products, reformulate those products, to conduct additional clinical trials, to make changes to the manufacturing processes, or to seek re-approval of the manufacturing facilities. Significant concerns about the safety and effectiveness of a product could ultimately lead to the revocation of our marketing approval. The revision of product labeling or the regulatory actions described above could be required even if there is no clearly established connection between the product and the safety or efficacy concerns that have been raised. The revision of product labeling or the regulatory actions described above could have a material adverse effect on sales of the affected products and on our business and results of operations.
23
If we or our suppliers are unable to comply with ongoing and changing regulatory standards, sales of our products could be delayed or prevented.
Virtually all aspects of our business, including the development, testing, manufacturing, processing, quality, safety, efficacy, packaging, labeling, record-keeping, distribution, storage and advertising and promotion of our products and disposal of waste products arising from these activities, are subject to extensive regulation by federal, state and local governmental authorities in the United States, including the FDA and the Department of Health and Humans Services Office of Inspector General (OIG). Our business is also subject to regulation in foreign countries. Compliance with these regulations is costly and time-consuming.
Our manufacturing facilities and procedures and those of our suppliers are subject to ongoing regulation, including periodic inspection by the FDA and foreign regulatory agencies. For example, manufacturers of pharmaceutical products must comply with detailed regulations governing current good manufacturing practices, including requirements relating to quality control and quality assurance. We must spend funds, time and effort in the areas of production, safety, quality control and quality assurance to ensure compliance with these regulations. We cannot assure that our manufacturing facilities or those of our suppliers will not be subject to regulatory action in the future.
Our products generally must receive appropriate regulatory clearance before they can be sold in a particular country, including the United States. We may encounter delays in the introduction of a product as a result of, among other things, insufficient or incomplete submissions to the FDA for approval of a product, objections by another company with respect to our submissions for approval, new patents by other companies, patent challenges by other companies which result in a 180-day exclusivity period, and changes in regulatory policy during the period of product development or during the regulatory approval process. The FDA has the authority to revoke drug approvals previously granted and remove from the market previously approved products for various reasons, including issues related to current good manufacturing practices for that particular product or in general. We may be subject from time to time to product recalls initiated by us or by the FDA. Delays in obtaining regulatory approvals, the revocation of a prior approval, or product recalls could impose significant costs on us and adversely affect our ability to generate revenue.
Our inability or the inability of our suppliers to comply with applicable FDA and other regulatory requirements can result in, among other things, warning letters, fines, consent decrees restricting or suspending our manufacturing operations, delay of approvals for new products, injunctions, civil penalties, recall or seizure of products, total or partial suspension of sales and criminal prosecution. Any of these or other regulatory actions could materially adversely affect our business and financial condition.
We depend on third parties to supply raw materials and other components and may not be able to obtain sufficient quantities of these materials, which will limit our ability to manufacture our products on a timely basis and harm our operating results.
The manufacture of Abraxane® requires, and our product candidates will require, raw materials and other components that must meet stringent FDA requirements. Some of these raw materials and other components are available only from a limited number of sources. We have entered into agreement with Indena SpA and ScinoPharm Taiwan, Ltd. to supply us with paclitaxel, the active pharmaceutical ingredient in Abraxane®. One of the suppliers is currently in the process of being qualified by the appropriate regulatory authorities. We believe there are more than 15 potential commercial suppliers in the process of being qualified by the appropriate regulatory authorities. We have not historically experienced any paclitaxel supply shortages. Additionally, we maintain a safety stock supply of paclitaxel to mitigate any supply disruption. Our regulatory approvals for each particular product denote the raw materials and components, and the suppliers for such materials, we may use for that product. Obtaining approval to change, substitute or add a raw material or component, or the supplier of a raw material or component, can be time consuming and expensive, as testing and regulatory approval are necessary. If our suppliers are unable to deliver sufficient quantities of these materials on a timely basis or we
24
encounter difficulties in our relationships with these suppliers, the manufacture and sale of our products may be disrupted, and our business, operating results and reputation could be adversely affected.
The injectable pharmaceutical products markets are highly competitive, and if we are unable to compete successfully, our revenue will decline and our business will be harmed.
The markets for injectable pharmaceutical products are highly competitive, rapidly changing and undergoing consolidation. Abraxane® competes, directly or indirectly, with the primary taxanes in the market place, including Bristol-Myers Squibb’s Taxol® and its generic equivalents, Sanofi-Aventis’ Taxotere® and other cancer therapies. Many pharmaceutical companies have developed and are marketing, or are developing, alternative formulations of paclitaxel and other cancer therapies that may compete directly or indirectly with Abraxane®. Many of our competitors have significantly greater research and development, financial, sales and marketing, manufacturing, regulatory and other resources than us. As a result, they may be able to devote greater resources to the development, manufacture, marketing or sale of their products, receive greater resources and support for their products, initiate or withstand substantial price competition, more readily take advantage of acquisition or other opportunities, or otherwise more successfully market their products.
Any reduction in demand for our products could lead to a decrease in prices, fewer customer orders, reduced revenues, reduced margins, reduced levels of profitability, or loss of market share. These competitive pressures could adversely affect our business and operating results.
Other companies may claim that we infringe on their intellectual property or proprietary rights, which could cause us to incur significant expenses or prevent us from selling our products.
Our success depends in part on our ability to operate without infringing the patents and proprietary rights of third parties. The manufacture, use and sale of new products with conflicting patent rights have been subject to substantial litigation in the pharmaceutical industry. These lawsuits relate to the validity and infringement of patents or proprietary rights of third parties. A number of pharmaceutical companies, biotechnology companies, universities and research institutions may have filed patent applications or may have been granted patents that cover aspects of our products or our licensors’ products, product candidates or other technologies.
Future or existing patents issued to third parties may contain claims that conflict with our products. We may be subject to infringement claims from time to time in the ordinary course of our business, and third parties could assert infringement claims against us in the future with respect to Abraxane®, products that we may develop or products that we may license. We are in the process of appealing the jury ruling in the patent infringement lawsuit with Élan Pharmaceutical Int’l Ltd. See “Item 3 —Legal Proceedings”. Litigation or interference proceedings could force us to:
| | • | | stop or delay selling, manufacturing or using products that incorporate or are made using the challenged intellectual property; |
| | • | | enter into licensing or royalty agreements that may not be available on acceptable terms, if at all. |
Any litigation or interference proceedings, regardless of their outcome, would likely delay the regulatory approval process, be costly and require significant time and attention of key management and technical personnel.
Our inability to protect our intellectual property rights in the United States and foreign countries could limit our ability to manufacture or sell our products.
We rely on trade secrets, unpatented proprietary know-how, continuing technological innovation and patent protection to preserve our competitive position. We have over 100 issued U.S. and foreign patents, including
25
patents relating to Abraxane® and the technology surrounding Abraxane®. The issued patents covering Abraxane®, and methods of use and preparation of Abraxane®, currently expire through 2018, but we have additional pending U.S. and foreign patent applications pending that could extend the expiration dates. Our patents and those for which we have licensed or will license rights, including for Abraxane®, may be challenged, invalidated, infringed or circumvented, and the rights granted in those patents may not provide proprietary protection or competitive advantages to us. We and our licensors may not be able to develop patentable products. Even if patent claims are allowed, the claims may not issue, or in the event of issuance, may not be sufficient to protect the technology owned by or licensed to us. Third-party patents could reduce the coverage of the patents licensed, or that may be licensed to or owned by us. If patents containing competitive or conflicting claims are issued to third parties, we may be prevented from commercializing the products covered by such patents, or may be required to obtain or develop alternate technology. In addition, other parties may duplicate, design around or independently develop similar or alternative technologies.
We may not be able to prevent third parties from infringing or using our intellectual property. We generally control and limit access to, and the distribution of, our product documentation and other proprietary information. Despite our efforts to protect this proprietary information, however, unauthorized parties may obtain and use information that we regard as proprietary. Other parties may independently develop similar know-how or may even obtain access to these technologies.
The laws of some foreign countries do not protect proprietary information to the same extent as the laws of the United States, and many companies have encountered significant problems and costs in protecting their proprietary information in these foreign countries.
The U.S. Patent and Trademark Office and the courts have not established a consistent policy regarding the breadth of claims allowed in pharmaceutical patents. The allowance of broader claims may increase the incidence and cost of patent interference proceedings and the risk of infringement litigation. On the other hand, the allowance of narrower claims may limit the value of our proprietary rights.
The strategy to license rights to or acquire and commercialize proprietary, biological injectable or other specialty injectable products may not be successful, and we may never receive any return on our investment in these products.
We may license rights to or acquire products or technologies from third parties. Other companies, including those with substantially greater financial and sales and marketing resources, will compete with us to license rights to or acquire these products and technologies. We may not be able to license rights to or acquire these proprietary or other products or technologies on acceptable terms, if at all. Even if we obtain rights to a pharmaceutical product and commit to payment terms, including, in some cases, significant up-front license payments, we may not be able to generate product sales sufficient to create a profit or otherwise avoid a loss.
A product candidate may fail to result in a commercially successful drug for other reasons, including the possibility that the product candidate may:
| | • | | be found during clinical trials to be unsafe or ineffective; |
| | • | | fail to receive necessary regulatory approvals; |
| | • | | be difficult or uneconomical to produce in commercial quantities; |
| | • | | be precluded from commercialization by proprietary rights of third parties; or |
| | • | | fail to achieve market acceptance. |
The marketing strategy, distribution channels and levels of competition with respect to any licensed or acquired product may be different from those of Abraxane®, and we may not be able to compete favorably in any new product category.
26
We may become subject to federal false claims or other similar litigation brought by private individuals and the government.
The Federal False Claims Act allows persons meeting specified requirements to bring suit alleging false or fraudulent Medicare or Medicaid claims and to share in any amounts paid to the government in fines or settlement. These suits, known as qui tam actions, have increased significantly in recent years and have increased the risk that a health care company will have to defend a false claim action, pay fines and/or be excluded from Medicare and Medicaid programs. Federal false claims litigation can lead to civil monetary penalties, criminal fines and imprisonment and/or exclusion from participation in Medicare, Medicaid and other federally funded health programs. Other alternate theories of liability may also be available to private parties seeking redress for such claims. A number of parties have brought claims against numerous pharmaceutical manufacturers, and we cannot be certain that such claims will not be brought against us, or if they are brought, that such claims might not be successful.
We may need to change our business practices to comply with changes to, or may be subject to charges under, the fraud and abuse laws.
We are subject to various federal and state laws pertaining to health care fraud and abuse, including the federal Anti-Kickback Statute and its various state analogues, the federal False Claims Act and marketing and pricing laws. Violations of these laws are punishable by criminal and/or civil sanctions, including, in some instances, imprisonment and exclusion from participation in federal and state health care programs such as Medicare and Medicaid. We may have to change our advertising and promotional business practices, or our existing business practices could be challenged as unlawful due to changes in laws, regulations or rules or due to administrative or judicial findings, which could materially adversely affect our business.
We face uncertainty related to pricing and reimbursement and health care reform.
In both domestic and foreign markets, sales of our products will depend in part on the availability of reimbursement from third-party payors such as government health administration authorities, private health insurers, health maintenance organizations and other health care-related organizations. Effective January 1, 2006, we received a unique reimbursement “J” code for Abraxane® from the Centers for Medicare and Medicaid Services. That code allows providers to bill Medicare for the use of Abraxane®. We believe that most major insurers reimburse providers for Abraxane® use consistent with the FDA-approved indication, which we believe is consistent with the reimbursement practices of major insurers for other drugs containing paclitaxel for the same indication. However, reimbursement by such payors is presently undergoing reform, and there is significant uncertainty at this time how this will affect sales of certain pharmaceutical products. There is possible U.S. legislation or regulatory action affecting, among other things, pharmaceutical pricing and reimbursement, including under Medicaid and Medicare, the importation of prescription drugs that are marketed outside the U.S. and sold at prices that are regulated by governments of various foreign countries.
Medicare, Medicaid and other governmental reimbursement legislation or programs govern drug coverage and reimbursement levels in the United States. Federal law requires all pharmaceutical manufacturers to rebate a percentage of their revenue arising from Medicaid-reimbursed drug sales to individual states. For Abraxane® and any other proprietary products, which are marketed and sold under new drug applications, manufacturers are required to rebate the greater of (a) 15.1% of the average manufacturer price or (b) the difference between the average manufacturer price and the lowest manufacturer price for products sold during a specified period.
Both the federal and state governments in the United States and foreign governments continue to propose and pass new legislation, rules and regulations designed to contain or reduce the cost of health care. Existing regulations that affect the price of pharmaceutical and other medical products may also change before any of our products are approved for marketing. Cost control initiatives could decrease the price that we receive for any product we develop in the future. In addition, third-party payers are increasingly challenging the price and cost-effectiveness of medical products and services and litigation has been filed against a number of pharmaceutical
27
companies in relation to these issues. Additionally, significant uncertainty exists as to the reimbursement status of newly approved injectable pharmaceutical products. Our products may not be considered cost effective or adequate third-party reimbursement may not be available to enable us to maintain price levels sufficient to realize an adequate return on our investment.
State pharmaceutical marketing compliance and reporting requirements may expose us to regulatory and legal action by state governments or other government authorities.
In recent years, several states, including California, Vermont, Maine, Minnesota, New Mexico and West Virginia, in addition to the District of Columbia, have enacted legislation requiring pharmaceutical companies to establish marketing compliance programs and file periodic reports on sales, marketing, pricing and other activities. Similar legislation is being considered in other states. Many of these requirements are new and uncertain, and available guidance is limited. We are continuing to assess our compliance with these state laws. Unless we are in full compliance with these laws, we could face enforcement action and fines and other penalties and could receive adverse publicity, all of which could harm our business.
We may be required to defend lawsuits or pay damages for product liability claims.
Product liability is a major risk in testing and marketing biotechnology and pharmaceutical products. We may face substantial product liability exposure in human clinical trials and for products that we sell after regulatory approval. We maintain insurance coverage for product liability claims in the aggregate amount of $100 million, including primary and excess coverages, which we believe is reasonably adequate coverage. Product liability claims, regardless of their merits, could exceed policy limits, divert management’s attention and adversely affect our reputation and the demand for these products.
We depend heavily on the principal members of our management and research and development teams, the loss of whom could harm our business.
We depend heavily on the principal members of our management and research and development teams, including Dr. Patrick Soon-Shiong, Chief Executive Officer, David O’Toole, Chief Financial Officer, Bruce Wendel, Executive Vice President of Corporate Operations and Development, Neil P. Desai, Senior Vice President of Research and Development, Lex H.T. Van Der Ploeg, Senior Vice President of Integrative Medicine and Translational Science, and Nicholas Everett, Chief Scientist, Drug Discovery. Each of the members of the executive management team is employed “at will.” The loss of the services of any member of the executive management team may significantly delay or prevent the achievement of product development or business objectives.
To be successful, we must attract, retain and motivate key employees, and the inability to do so could seriously harm our business and operations.
To be successful, we must attract, retain and motivate executives and other key employees. We face competition for qualified scientific, technical and other personnel, which may adversely affect our ability to attract and retain key personnel. We also must continue to attract and motivate employees and keep them focused on our strategies and goals.
If we are unable to integrate successfully potential future acquisitions, our business may be harmed.
As part of our business strategy and growth plan, we may acquire businesses, technologies or products that we believe will complement our business. The process of integrating an acquired business, technology or product may result in unforeseen operating difficulties and expenditures and may require significant management attention that would otherwise be available for ongoing development of our existing business. In addition, we may not be able to maintain the levels of operating efficiency that any acquired company achieved or might have achieved separately. Successful integration of the companies that we may acquire will depend upon our ability to,
28
among other things, eliminate redundancies and excess costs. As a result of difficulties associated with combining operations, we may not be able to achieve cost savings and other benefits that the company might hope to achieve with acquisitions. Future acquisitions could result in potentially dilutive issuances of equity securities, the incurrence of debt and contingent liabilities or have an undesirable impact on our financial statements.
We are subject to risks associated with international operations, which could harm both our domestic and international operations.
As part of our business strategy and growth plan, we plan to expand our international sales as we obtain regulatory approvals to market our Abraxane and other product candidates in foreign countries, including countries in the European Union and Asia. Expansion of our international operations could impose substantial burdens on our resources, divert management’s attention from domestic operations and otherwise harm our business. In addition, international operations are subject to risks, including:
| | • | | regulatory requirements of differing nations; |
| | • | | inadequate protection of intellectual property; |
| | • | | difficulties and costs associated with complying with a wide variety of complex domestic and foreign laws and treaties; |
| | • | | legal uncertainties regarding, and timing delays associated with, tariffs, export licenses and other trade barriers; and |
Any of these or other factors could adversely affect our ability to compete in international markets and our operating results.
Risks Relating to Our Separation from Old Abraxis
We have limited history operating as an independent company, and we may be unable to make the changes necessary to operate successfully as an independent company.
Prior to the separation, our business was operated by Old Abraxis as part of its broader corporate organization rather than as a stand-alone company. Old Abraxis assisted us by providing financing and corporate functions such as human resources, information technology, internal audit, tax and accounting functions. APP has no obligation to provide assistance to us other than certain interim services. These interim services include, among other things, manufacturing services, information technology services, accounting and finance services and human resources support. Because our business has not recently been operated as an independent company, we cannot assure you that we will be able to successfully implement the changes necessary to operate independently or that we will not incur additional costs operating independently that would have a negative effect on our business, results of operations and financial condition.
In addition, prior to the separation, our business was able to leverage Old Abraxis’ size, relationships and purchasing power in procuring goods, services and technology (including office supplies, computer software licenses and equipment), travel and all employee benefits plans for which per employee cost was based on number of lives covered. Our separation from Old Abraxis has had a significant impact on the per employee cost for certain coverage such as health care and disability.
We are in the process of creating our own, or engaging third parties to provide, systems and business functions to replace many of the systems and business functions Old Abraxis provided to us. We will also need to make significant investments to develop our independent ability to operate without Old Abraxis’ existing operational and administrative infrastructure. These initiatives will be costly to implement, and we may not be successful in implementing these systems and business functions.
29
Our historical financial information may not be representative of our future results as an independent company.
The historical financial information for periods prior to our separation on November 13, 2007 may not reflect what our results of operations, financial position and cash flows would have been had we been an independent company for those periods. This is primarily because:
| | • | | our historical financial information reflects allocations for services historically provided to us by Old Abraxis, which allocations may not reflect the costs we would have incurred for similar services as an independent company; and |
| | • | | our historical financial information does not reflect changes that we incurred or expect to incur as a result of our separation from Old Abraxis, including changes in the cost structure, personnel needs, financing and operations of the contributed business as a result of the separation from Old Abraxis and from reduced economies of scale. |
In addition, we are now responsible for the additional costs associated with being an independent public company, including costs related to corporate governance and listed and registered securities. Therefore, our historical financial statements before the separation on November 13, 2007 may not be indicative of our current or future performance as an independent company.
Our separation from Old Abraxis may present significant challenges.
There is a significant degree of difficulty and management distraction inherent in having separated from Old Abraxis. These difficulties include:
| | • | | preserving customer, supplier and other important relationships; |
| | • | | the potential difficulty in retaining key officers and personnel; and |
| | • | | separating corporate infrastructure, including systems, insurance, accounting, legal, finance, tax and human resources. |
We anticipate that it generally will take up to 24 months from the separation date to completely separate from Old Abraxis, with the exception of manufacturing activities which APP will undertake for us and certain lease arrangements, which will last for a period of four or five years. Our separation from Old Abraxis may not be successfully or cost-effectively completed. The failure to do so could have an adverse effect on our business, financial condition and results of operations.
The process of separating operations could cause an interruption of, or loss of momentum in, the activities of one or more of our businesses. Members of our senior management may be required to devote considerable amounts of time to this separation process, which will decrease the time they will have to manage our business, service existing customers, attract new customers and develop new products or strategies. If our senior management is not able to manage effectively the separation process, or if any significant business activities are interrupted as a result of the separation process, our business could suffer.
We may be required to indemnify APP and may not be able to collect on indemnification rights from APP.
Under the terms of the separation and distribution agreement, we have agreed to indemnify APP from and after the distribution with respect to all liabilities of Old Abraxis not related to its hospital-based products business and the use by APP of any trademarks or other source identifiers owned by us. Similarly, APP has agreed to indemnify us from and after the distribution with respect to all liabilities of Old Abraxis related to its hospital-based products business and the use by us of any trademarks or other source identifiers owned by APP.
Under the terms of the tax allocation agreement, we agreed to indemnify APP against all tax liabilities to the extent they relate to the proprietary products business, and APP agreed to indemnify us against all tax liabilities
30
to the extent they relate to the hospital-based products business. The tax allocation agreement also generally allocates between us and APP any liability for taxes that may arise in connection with the distribution. In September, 2008, APP entered into a merger agreement with Fresenius Kabi, a subsidiary of Fresenius SE. Pursuant to the merger agreement, Fresenius acquired all of the outstanding common stock of APP. Pursuant to the tax allocation agreement and the merger agreement, APP received a tax opinion, in form and substance reasonably acceptable to us, that the acquisition should not affect the qualification of the distribution under Section 355 and Section 368(a)(1)(D) of the Internal Revenue Code and the nonrecognition of gain to APP in the distribution. Under the terms of the tax allocation agreement, we are generally liable for, and are required to indemnify APP against, any tax liability arising as a result of the distribution failing to qualify for tax-free treatment unless, notwithstanding such tax opinion, such tax liability is imposed as a result of an acquisition of APP, including the acquisition of APP by Fresenius, or certain other specified acts of APP.
Under the terms of the manufacturing agreement, we have agreed to indemnify APP from any damages resulting from a third-party claim caused by or alleged to be caused by (i) our failure to perform our obligations under the manufacturing agreement; (ii) any product liability claim arising from the negligence, fraud or intentional misconduct of us or any of our affiliates or any product liability claim arising from our manufacturing obligations (or any failure or deficiency in our manufacturing obligations) under the manufacturing agreement; (iii) any claim that the manufacture, use or sale of Abraxane® or our pipeline products infringes a patent or any other proprietary right of a third party; or (iv) any recall, product liability claim or other third-party claim not arising from the gross negligence or bad faith of, or intentional misconduct or intentional breach of the manufacturing agreement by APP, by reason of the $100 million limitation of liability described below. We have also agreed to indemnify APP for liabilities that it becomes subject to as a result of its activities under the manufacturing agreement and for which it is not responsible under the terms of the manufacturing agreement. APP has agreed to indemnify us from any damages resulting from a third-party claim caused by or alleged to be caused by (i) APP’s gross negligence, bad faith, intentional misconduct or intentional failure to perform its obligations under the manufacturing agreement; or (ii) any product liability claim arising from the gross negligence or bad faith of, or intentional misconduct or intentional breach of the manufacturing agreement by APP. APP generally will not have any liability for monetary damages to us or third parties in connection with the manufacturing agreement for damages in excess of $100 million in the aggregate.
There are no time limits on when an indemnification claim must be brought and no other monetary limits on the amount of indemnification that may be provided. These indemnification obligations could be significant. Our ability to satisfy any of these indemnification obligations will depend upon the future financial strength of our company. We cannot determine whether we will have to indemnify APP for any substantial obligations. We also cannot assure you that, if APP becomes obligated to indemnify us for any substantial obligations, APP will have the ability to satisfy those obligations. Any indemnification payment by us, or any failure by APP to satisfy its indemnification obligations, could have a material adverse effect on our business.
We will be dependent upon APP to manufacture Abraxane® for a remaining term of three or four years, and the manufacture of pharmaceutical products is highly regulated.
In connection with the separation and distribution agreement, we entered into a manufacturing agreement with APP for the manufacture of Abraxane® and our pipeline products whereby APP agreed to undertake certain of the tasks necessary to manufacture Abraxane® and our pipeline products until December 31, 2011, with this agreement automatically extended by one year if either APP elects to exercise its option to extend the lease on our Melrose Park manufacturing facility or we elect to exercise our option to extend the lease on APP’s Grand Island manufacturing facility. Accordingly, we will be dependent upon APP to manufacture our products. The amount and timing of resources that APP devotes to the manufacture of our products is not within our direct control. Further, in the event of capacity constraints at the manufacturing facilities, the manufacturing agreement provides that the available capacity will be prorated between us and APP according to the parties’ then current use of manufacturing capacity at the relevant facilities. Any loss in manufacturing capacity pursuant to these proration provisions could be detrimental to our business and operating results. While the manufacturing
31
agreement allows us to override these proration provisions, we may only do so by paying APP additional fees under the manufacturing agreement. If we are forced to pay APP additional fees to retain our capacity rights under the manufacturing agreement, it could be detrimental to our operating results.
The manufacture of pharmaceutical products is highly exacting and complex, due in part to strict regulatory requirements and standards which govern both the manufacture of a particular product and the manufacture of these types of products in general. Problems may arise during their manufacture due to a variety of reasons, including equipment malfunction, failure to follow specific protocols and procedures and environmental factors. If problems arise during the production of a batch of product, that batch of product may have to be discarded. This could, among other things, lead to loss of the cost of raw materials and components used and lost revenue. If such problems are not discovered before the product is released to the market, recall costs may also be incurred. Under the terms of the manufacturing agreement, we have the final responsibility for release of the products manufactured pursuant to the manufacturing agreement and will bear all expenses in connection with any recall of products, unless the recall is a result of APP’s gross negligence, bad faith, intentional misconduct or intentional breach, in which case APP would bear all costs and expenses related to such product recall, subject to the $100 million limit on liability under the manufacturing agreement. To the extent APP encounters difficulties or problems with respect to the manufacture of our pharmaceutical products, including Abraxane®, this may be detrimental to our business, operating results and reputation.
Risks Related To An Investment In Our Common Stock
Our Chief Executive Officer and entities affiliated with him collectively own a significant percentage of our common stock and could exercise significant influence over matters requiring stockholder approval, regardless of the wishes of other stockholders.
Our Chief Executive Officer and entities affiliated with him collectively own approximately 80% of our outstanding common stock. Accordingly, they have the ability to significantly influence all matters requiring stockholder approval, including the election and removal of directors and approval of significant corporate transactions such as mergers, consolidations and sales of assets. This concentration of ownership could have the effect of delaying, deferring or preventing a change in control or impeding a merger or consolidation, takeover or other business combination, which could cause the market price of our common stock to fall or prevent our stockholders from receiving a premium in such a transaction. This significant concentration of stock ownership may adversely affect the market for and trading price of our common stock if investors perceive that conflicts of interest may exist or arise.
Substantial sales of our common stock could depress the market price of our common stock.
All of the shares of our common stock issued in connection with the separation, other than shares issued to our affiliates, are eligible for immediate resale in the public market. Although shares issued to our affiliates are not immediately freely tradable, we have granted registration rights to the former ABI shareholders, including our Chief Executive Officer. Under the registration rights agreement, the former ABI shareholders will have the right to require us to register all or a portion of the shares of our common stock they received in connection with the separation. In addition, the former ABI shareholders may require us to include their shares in future registration statements that we file and our Chief Executive Officer may require us to register shares for resale on a Form S-3 registration statement. Upon registration, the registered shares generally will be freely tradeable in the public market without restriction. However, in connection with any underwritten offering, the holders of registrable securities will agree to lock up any other shares for up to 90 days and will agree to a limit on the maximum number of shares that can be registered for the account of the holders of registrable securities under so-called “shelf” registration statements.
Substantial sales of our shares, or the perception that such sales might occur, could depress the market price for our shares. Our Chief Executive Officer and entities affiliated with him own over 80% of our outstanding
32
common stock. In connection with Old Abraxis requesting from the Internal Revenue Service a private letter ruling regarding the U.S. federal income tax consequences of the transactions, our Chief Executive Officer, his wife and certain entities affiliated with him have represented to the Internal Revenue Service that they have no current plan or intention to sell their shares after the separation.
Our stock price may be volatile in response to market and other factors.
The market price for our common stock may be volatile and subject to price and volume fluctuations in response to market and other factors, including the following, some of which are beyond our control:
| | • | | variations in our quarterly operating results from the expectations of securities analysts or investors; |
| | • | | revisions in securities analysts’ estimates; |
| | • | | announcements of technological innovation or new products by us or our competitors; |
| | • | | announcements by us or our competitors of significant acquisition, strategic partnerships, joint ventures or capital commitments; |
| | • | | general technological, market or economic trends; |
| | • | | investor perception of our industry or our prospects; |
| | • | | insider selling or buying; |
| | • | | investors entering into short sale contracts; |
| | • | | regulatory developments affecting our industry; and |
| | • | | additions or departures of key personnel. |
We are not subject to the provisions of Section 203 of the Delaware General Corporation Law, which could negatively affect your investment.
We elected in our certificate of incorporation to not be subject to the provisions of Section 203 of the Delaware General Corporation Law. In general, Section 203 prohibits a publicly held Delaware corporation from engaging in a “business combination” with an “interested stockholder” for a period of three years after the date of the transaction in which the person became an interested stockholder, unless the business combination is approved in a prescribed manner. A “business combination” includes a merger, asset sale or other transaction resulting in a financial benefit to the interested stockholder. An “interested stockholder” is a person who, together with affiliates and associates, owns (or, in certain cases, within three years prior, did own) 15% or more of the corporation’s voting stock. Our decision not to be subject to Section 203 will allow, for example, our Chief Executive Officer (who with members of his immediate family and entities affiliated with him own approximately 80% of our common stock) to transfer shares in excess of 15% of our voting stock to a third-party free of the restrictions imposed by Section 203. This may make us more vulnerable to takeovers that are completed without the approval of our board of directors and/or without giving us the ability to prohibit or delay such takeovers as effectively. These provisions could also limit the price that investors would be willing to pay in the future for shares of our common stock.
Item 1B. UNRESOLVED STAFF COMMENTS
None.
33
Item 2. PROPERTIES
We operate various facilities in the United States, Puerto Rico, Canada and Switzerland, which have an aggregate size of approximately 0.9 million square feet. We believe that our existing facilities are sufficient to meet our expected needs. The location and uses of our principal facilities as of December 31, 2008 were as follows:
| | | | | | | | |
Location | | Use | | Own/
Lease | | Lease Expiration | | Approximate
Square Feet |
Los Angeles, CA | | Principal executive office | | Lease | | July 2012 | | 60,900 |
Marina Del Rey, CA | | Research & regulatory affairs | | Lease | | August 2011 | | 50,700 |
Bridgewater, NJ | | Administration | | Lease | | September 2011 | | 33,000 |
Elk Grove Village, Illinois | | Warehouse | | Own | | — | | 90,000 |
Melrose Park, Illinois(1) | | Manufacturing | | Own | | — | | 122,000 |
Melrose Park, Illinois(2) | | Research and development and warehouse | | Own | | — | | 147,000 |
Grand Island, New York(3) | | Manufacturing | | Lease | | December 2011 | | 5,700 |
Barbengo, Switzerland | | Manufacturing | | Own | | — | | 25,000 |
Phoenix, Arizona | | Manufacturing | | Own | | — | | 200,000 |
Barceloneta, Puerto Rico(4) | | Manufacturing | | Own | | — | | 90,000 |
Durham, North Carolina | | Clinical trial management | | Lease | | April 2016 | | 36,000 |
Auburn, California | | Research and development | | Lease | | April 2010 | | 12,800 |
Ontario, Canada | | Administration | | Lease | | October 2013 | | 13,600 |
George, South Africa(5) | | Research and development | | Lease | | November 2011 | | 6,500 |
| | | | | | | | |
| | | | | | | | 893,200 |
| | | | | | | | |
| (1) | For the length of the applicable lease, the manufacturing facility in Melrose Park has been leased to APP, and APP has agreed to provide certain contract manufacturing services to us in accordance with the terms of the manufacturing agreement. |
| (2) | We have leased approximately 71,000 square feet of our warehouse space located at this Melrose Park facility to APP for a term initially expiring in December 2011. We have also leased approximately 48,000 square feet of our research and development space located at this Melrose Park facility to APP for a term expiring in December 2010. |
| (3) | We have leased from APP a portion of APP’s Grand Island, New York manufacturing facility to enable us to perform our responsibilities under the manufacturing agreement for its term. The initial term of the manufacturing agreement will expire on December 31, 2011, but the term of the manufacturing agreement will be automatically extended for one year if either APP exercises its option to extend the lease for our Melrose Park manufacturing facility for an additional year or we elect to exercise our option to extend the lease for APP’s Grand Island manufacturing facility for an additional year. |
| (4) | APP’s Puerto Rico facility was divided into two separate facilities, and we took ownership of one part of the facility. The portion of the Puerto Rico facility owned by us following the separation is the 90,000 square foot active pharmaceutical ingredients manufacturing plant currently leased to Pfizer for a term expiring in February 2012. Pfizer has notified us that it intends to vacate the premises in May 2009. Pfizer will not incur any penalty for the early termination of the lease. |
| (5) | Through our wholly-owned subsidiary, Shimoda Biotech (Pty) Limited, we also lease approximately 250 square feet of office space in Port Elizabeth, South Africa under a lease expiring in July 2010. |
Item 3. LEGAL PROCEEDINGS
On July 19, 2006, Élan Pharmaceutical Int’l Ltd. filed a lawsuit against Old Abraxis in the U.S. District Court for the District of Delaware alleging that Old Abraxis willfully infringed upon two of their patents by asserting that Abraxane® uses technology protected by Élan-owned patents. Élan sought unspecified damages
34
and an injunction. In August 2006, Old Abraxis filed a response to Élan’s complaint contending that it did not infringe on the Élan patents and that the Élan patents are invalid. On June 13, 2008, the jury ruled that we infringed upon one of Élan’s patents, which runs until 2011, and awarded Élan $55.2 million in damages for sales of Abraxane® through the judgment date. We have filed various post-trial motions and are appealing the jury ruling. We assumed any liability of Old Abraxis relating to this litigation in connection with the separation.
We are from time to time subject to claims and litigation arising in the ordinary course of business. We intend to defend vigorously any such litigation that may arise under all defenses that would be available to us. In the opinion of management, the ultimate outcome of proceedings of which management is aware, even if adverse to us, would not have a material adverse effect on our consolidated financial position or results of operations.
Item 4. SUBMISSION OF MATTERS TO A VOTE OF SECURITY HOLDERS
Our 2008 annual meeting of stockholders was held on November 5, 2008.
| (a) | Our stockholders voted as follows with respect to elect five directors to serve until our next annual meeting of stockholders or until their successors are duly elected and qualified: |
| | | | |
DIRECTORS | | FOR | | Authority
Withheld |
Patrick Soon-Shiong, M.D. | | 36,013,428 | | 3,157,271 |
Kirk K. Calhoun | | 39,141,929 | | 28,770 |
Stephen D. Nimer, M.D. | | 39,142,216 | | 28,483 |
Leonard Shapiro | | 39,141,970 | | 28,729 |
David S. Chen, Ph.D. | | 39,142,216 | | 28,483 |
| (b) | Our stockholders voted as follows with respect to a proposal to ratify the appointment of Ernst & Young LLP as our independent registered public accounting firm for the fiscal year ended December 31, 2008. |
| | | | | | |
FOR | | AGAINST | | ABSTENTIONS | | BROKER NON-VOTES |
39,153,201 | | 15,636 | | 1,862 | | -0- |
35
PART II
Item 5. MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Market for Common Stock
Our common stock is listed and traded on The Nasdaq Global Market under the symbol “ABII.” Regular-way trading of our common stock commenced on November 14, 2007, the day after our separation from Old Abraxis. The following table sets forth the prices for our common stock as reported by NASDAQ for fiscal years 2008 and 2007:
| | | | | | | | | | | | |
| | | 2008
Price Per Share | | 2007(a)
Price Per Share |
| For the quarter ended: | | High | | Low | | High | | Low |
March 31 | | $ | 69.00 | | $ | 53.00 | | | n/a | | | n/a |
June 30 | | $ | 69.91 | | $ | 58.33 | | | n/a | | | n/a |
September 30 | | $ | 78.95 | | $ | 59.03 | | | n/a | | | n/a |
December 31 | | $ | 74.50 | | $ | 46.28 | | $ | 73.44 | | $ | 33.00 |
(a) No trading occurred prior to November 14, 2007. Figures for the quarter ended December 31, 2007 are as reported on Nasdaq between November 14, 2007 and December 31, 2007.
As of February 24, 2009, there were approximately 70 stockholders of record of our common stock and the closing price of our stock as reported by NASDAQ on that date was $73.01.
Dividend Policy
We have never declared or paid a cash dividend and we have no current intention of paying cash dividends. We currently anticipate that we will retain future earnings to support our growth strategy. We do not anticipate paying regular cash dividends on our common stock in the foreseeable future
Issuer Purchases of Equity Securities
We did not repurchase any of our equity securities during the fourth quarter of 2008.
Securities Authorized for Issuance under Equity Compensation Plans
Please see Part III, Item 12 of this Annual Report on Form 10-K for disclosure relating to our equity compensation plans. Such information is incorporated by reference from our Proxy Statement for our 2009 Annual Meeting of Stockholders.
36
Performance GraphNotwithstanding anything to the contrary set forth in any of our previous filings under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended, that might incorporate future filings, including this Annual Report of Form 10-K, in whole or in part, the following graph shall not be deemed to be incorporated by reference into any such filings.
The following graph compares the percentage change in the cumulative total stockholder return on our common stock as compared with the NASDAQ Pharmaceutical Index and the NASDAQ stock market (U.S. Index). The comparison assumes an investment of $100 on November 14, 2007 (the date on which our common stock first began to be publicly traded) in our common stock and in each of the foregoing indices, and assumes reinvestment of dividends, of which we paid none.The stock performance shown on the graph below is not necessarily indicative of future price performance.
Abraxis BioScience, Inc.
NASDAQ Pharmaceutical Index
NASDAQ Stock Market (U.S. Index)
37
Item 6. SELECTED FINANCIAL DATA
We derived the following selected consolidated and combined financial data for the five years ended December 31, 2008 from our audited consolidated and combined financial statements. You should read the data in conjunction with “Item 7: Management’s Discussion and Analysis of Financial Condition and Results of Operations,” our consolidated and combined financial statements, related notes and other financial information included herein. Historical results of operations and financial position are not necessarily indications of the results that may be expected in future periods.
| | | | | | | | | | | | | | | | | | | | |
| | | Year ended December 31, | |
| | | 2008 | | | 2007 | | | 2006 | | | 2005 | | | 2004 | |
| | | (in thousands, except share and per share data) | |
Consolidated and Combined Statement of Operations Data: | | | | | | | | | | | | | | | | | | | | |
Abraxane revenue | | $ | 335,631 | | | $ | 324,692 | | | $ | 174,906 | | | $ | 133,731 | | | $ | — | |
Other revenue | | | 9,678 | | | | 8,994 | | | | 7,381 | | | | 1,944 | | | | 237 | |
| | | | | | | | | | | | | | | | | | | | |
Net revenue | | | 345,309 | | | | 333,686 | | | | 182,287 | | | | 135,675 | | | | 237 | |
Cost of sales | | | 39,068 | | | | 34,450 | | | | 21,183 | | | | 24,066 | | | | 340 | |
| | | | | | | | | | | | | | | | | | | | |
Gross profit (loss) | | | 306,241 | | | | 299,236 | | | | 161,104 | | | | 111,609 | | | | (103 | ) |
Operating expenses: | | | | | | | | | | | | | | | | | | | | |
Research and development | | | 103,590 | | | | 88,717 | | | | 63,073 | | | | 50,121 | | | | 34,896 | |
Selling, general and administrative | | | 216,804 | | | | 230,031 | | | | 119,462 | | | | 69,239 | | | | 41,645 | |
Reacquisition costs | | | 158,909 | | | | — | | | | — | | | | — | | | | — | |
Amortization of intangible assets | | | 39,429 | | | | 38,615 | | | | 27,349 | | | | — | | | | — | |
In-process research and development | | | 13,900 | | | | — | | | | 83,447 | | | | — | | | | — | |
Merger related costs | | | — | | | | — | | | | 16,722 | | | | — | | | | — | |
Litigation costs | | | 57,635 | | | | — | | | | — | | | | — | | | | — | |
Impairment charge | | | 9,214 | | | | — | | | | — | | | | — | | | | — | |
Equity in net income of Drug Source Company, LLC | | | (908 | ) | | | (3,771 | ) | | | (2,776 | ) | | | (1,843 | ) | | | (2,373 | ) |
| | | | | | | | | | | | | | | | | | | | |
Total operating expense | | | 598,573 | | | | 353,592 | | | | 307,277 | | | | 117,517 | | | | 74,168 | |
| | | | | | | | | | | | | | | | | | | | |
Loss from operations | | | (292,332 | ) | | | (54,356 | ) | | | (146,173 | ) | | | (5,908 | ) | | | (74,271 | ) |
Interest income | | | 18,809 | | | | 4,990 | | | | 399 | | | | 287 | | | | 70 | |
Other expense | | | (4,999 | ) | | | — | | | | — | | | | — | | | | — | |
Interest expense | | | (187 | ) | | | (190 | ) | | | (4,741 | ) | | | (6,563 | ) | | | (2,100 | ) |
| | | | | | | | | | | | | | | | | | | | |
Loss before income taxes | | | (278,709 | ) | | | (49,556 | ) | | | (150,515 | ) | | | (12,184 | ) | | | (76,301 | ) |
(Benefit) provision for income taxes | | | (1,938 | ) | | | (7,952 | ) | | | (25,964 | ) | | | 478 | | | | — | |
| | | | | | | | | | | | | | | | | | | | |
Net loss | | $ | (276,771 | ) | | $ | (41,604 | ) | | $ | (124,551 | ) | | $ | (12,662 | ) | | $ | (76,301 | ) |
| | | | | | | | | | | | | | | | | | | | |
Basic and diluted loss per common share | | $ | (6.91 | ) | | $ | (1.04 | ) | | $ | (3.11 | ) | | $ | (0.32 | ) | | $ | (1.91 | ) |
| | | | | | | | | | | | | | | | | | | | |
Weighted-average common shares outstanding(1): | | | | | | | | | | | | | | | | | | | | |
Basic | | | 40,032 | | | | 39,991 | | | | 39,990 | | | | 39,990 | | | | 39,990 | |
| | | | | | | | | | | | | | | | | | | | |
Diluted | | | 40,032 | | | | 39,991 | | | | 39,990 | | | | 39,990 | | | | 39,990 | |
| | | | | | | | | | | | | | | | | | | | |
Consolidated balance sheet data: | | | | | | | | | | | | | | | | | | | | |
Working capital (deficit) | | $ | 386,148 | | | $ | 735,181 | | | $ | 25,093 | | | $ | 55,232 | | | $ | (11,540 | ) |
Total assets | | | 1,438,584 | | | | 1,502,255 | | | | 764,783 | | | | 179,080 | | | | 115,993 | |
Total debt | | | — | | | | — | | | | — | | | | 190,000 | | | | — | |
Total stockholder’s equity (deficit) | | | 929,472 | | | | 1,197,387 | | | | 459,021 | | | | (65,644 | ) | | | 47,492 | |
Other data: | | | | | | | | | | | | | | | | | | | | |
Cash flow (used in) provided by operating activities | | $ | (315,468 | ) | | $ | (2,893 | ) | | $ | 170,870 | | | $ | (22,272 | ) | | $ | (88,671 | ) |
Purchases of property plant and equipment | | | (43,729 | ) | | | (40,581 | ) | | | (64,431 | ) | | | (17,212 | ) | | | (26,016 | ) |
Cash flow provided by (used in) financing activities | | | 2,360 | | | | 752,082 | | | | (94,398 | ) | | | 40,728 | | | | 119,458 | |
| (1) | As of the completion of the separation on November 13, 2007, we had 40.0 million common shares outstanding. The same number of shares is being used for both diluted earnings per share and basic earnings per share for all periods prior to the separation date. All potentially dilutive employee stock awards were excluded from the computation of diluted loss per common share for all periods as the effect on net loss per share was anti-dilutive. |
38
Item 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
OVERVIEW
The following management’s discussion and analysis of financial condition and results of operations, or MD&A is intended to assist the reader in understanding our company. The MD&A is provided as a supplement to, and should be read in conjunction with the other sections of this Annual Report on Form 10-K, including “Item 1: Business”; “Item 1A: Risk Factors”, “Item 6: Selected Financial Data”; and “Item 8: Financial Statements and Supplemental Data.”
Background
We are one of the few fully integrated global biotechnology companies, with a breakthrough marketed product (Abraxane®), global ownership of our proprietary technology platform and clinical pipeline, dedicated nanoparticle manufacturing capabilities for worldwide supply integrated with seasoned in-house capabilities, including discovery, clinical drug development, regulatory and sales and marketing.
We are dedicated to the discovery, development and delivery of next-generation therapeutics and core technologies that offer patients safer and more effective treatments for cancer and other critical illnesses. Our portfolio includes the world’s first and only protein-based nanoparticle chemotherapeutic compound (Abraxane®) which is based on our proprietary tumor targeting technology known as thenab® technology platform. This nab® technology platform is the first to exploit the tumor’s biology against itself, taking advantage of an albumin-specific, receptor-mediated transport system and allowing the delivery of a drug from the vascular space across the blood vessel wall to the underlying tumor tissue. Abraxane® is the first clinical and commercial validation of ournab® technology platform. From the discovery and research phase to development and commercialization, we are committed to rapidly enhancing our product pipeline and accelerating the delivery of breakthrough therapies that will transform the lives of patients who need them.
We own the worldwide rights to Abraxane®, a next generation, nanometer-sized, solvent-free taxane that was approved by the U.S. Food and Drug Administration, or the FDA, in January 2005 for its initial indication in the treatment of metastatic breast cancer and launched in February 2005. We believe the successful launch of Abraxane® validates ournab™ tumor targeting technology, a novel biologically interactive (receptor-mediated) system to deliver chemotherapeutic agents in high concentrations preferentially to all tumors secreting a protein called SPARC, which attracts and binds to albumin.
Proposed 2009 Spin-Off
In January 2009, we announced that our board of directors approved a plan to spin-off a newly-formed subsidiary Abraxis Health, Inc. as a new independent, stand-alone company holding our drug discovery, pilot manufacturing and development business. If the spin-off occurs, our stockholders would own (i) shares of Abraxis Health and (ii) shares of our common stock, and we would continue to operate our existing business, excluding the drug discovery, pilot manufacturing and development business to be held by Abraxis Health. In connection with the proposed spin-off, Abraxis Health would enter into several agreements with us related to, among other things, manufacturing, transition services, product development and research, tax allocations, clinical development and a number of ongoing commercial relationships.
The proposed spin-off is subject to a number of closing conditions, including final approval by our board of directors and the effectiveness of the registration statement registering the common stock of Abraxis Health to be distributed to our stockholders in connection with the spin-off. Approval by our stockholders is not required as a condition to the consummation of the proposed spin-off. In connection with the proposed spin-off, Abraxis Health filed a registration statement on Form 10 with the SEC. Stockholders are urged to read the Form 10 registration statement carefully because it contains important information about the proposed spin-off. SeeNote 15—Subsequent Events for further discussion.
39
2007 Separation
On November 13, 2007, Old Abraxis was separated into two independent publicly-traded companies: one holding the former Abraxis Pharmaceutical Products business, or the hospital-based business; and our company, which holds the former Abraxis Oncology and Abraxis Research businesses, or the proprietary business, which we refer to as the “2007 Separation”. In connection with the separation, we changed our name from New Abraxis, Inc. to Abraxis BioScience, Inc. (and the hospital-based business is operated under the name APP Pharmaceuticals, Inc.).
In connection with the separation, stockholders of Old Abraxis as of November 13, 2007 received one share of our company for every four shares of Old Abraxis held as of that date. In addition, in connection with the separation, we entered into a separation and distribution agreement that provides for, among other things, the principal corporate transactions required to effect the separation and other specified terms governing our relationship with APP after the spin-off. We also entered into various agreements with APP, including: (i) a transition services agreement pursuant to which we and APP agreed to continue to provide each other with various services on an interim, transitional basis, for periods up to 24 months depending on the particular service; (ii) a manufacturing agreement whereby we and APP agreed to manufacture Abraxane® and certain other products and to provide other manufacturing-related services for a period of four or five years; (iii) an employees matters agreement providing for each company’s respective obligations to employees and former employees who are or were associated with their respective businesses, and for other employment and employee benefit matters; (iv) various real estate leases; and (v) a tax allocation agreement. Also, in connection with the separation, APP contributed $700 million in cash to us.
2006 Merger
On April 18, 2006, American Pharmaceutical Partners, Inc. or “Old APP,” completed a merger with American BioScience, Inc., or ABI, Old APP’s former parent, pursuant to the terms of the Agreement and Plan of Merger dated November 27, 2005, which we refer to as the “2006 Merger.” APP’s certificate of incorporation was amended to change its name to Abraxis BioScience, Inc. (Old Abraxis).
For accounting purposes, the 2006 Merger was treated as a downstream merger with ABI viewed as the surviving entity, although Old APP was the surviving entity for legal purposes. As such, effective as of the merger date, the 2006 Merger was accounted for as an implied acquisition of Old APP’s minority interests using the purchase method of accounting. Therefore, the historical book value of the minority interests was stepped up to its estimated fair values and the historical shareholders’ equity of the accounting acquirer, ABI, was retroactively restated for the equivalent number of shares received in the 2006 Merger. The purchase price attributed to minority interests was allocated to the minority interests’ pro-rata share of Old APP’s tangible and identifiable intangible assets and liabilities based on their estimated fair values at the acquisition date. The excess of the purchase price attributed to minority interests over the minority interests’ pro-rata share of the fair values of APP’s assets and liabilities was recorded as goodwill. As part of the 2007 Separation, we were allocated $241.4 million in goodwill from the 2006 Merger that is associated with our operations.
40
RESULTS OF OPERATIONS
Overview
The following table sets forth the results of our operations for each of the three years ended December 31, 2008, and forms the basis for the following discussion of our operating activities:
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | Change Favorable (Unfavorable) | |
| | | Year Ended December 31, | | | 2008 vs. 2007 | | | 2007 vs. 2006 | |
| | | 2008 | | | 2007 | | | 2006 | | | $ | | | % | | | $ | | | % | |
| | | (in thousands, except per share data) | |
Consolidated and combined statements of operations data: | | | | | | | | | | | | | | | | | | | | | | | | | | |
Revenue: | | | | | | | | | | | | | | | | | | | | | | | | | | |
Abraxane | | $ | 335,631 | | | $ | 324,692 | | | $ | 174,906 | | | $ | 10,939 | | | 3 | % | | $ | 149,786 | | | 86 | % |
Other | | | 9,678 | | | | 8,994 | | | | 7,381 | | | | 684 | | | 8 | % | | | 1,613 | | | 22 | % |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Net revenue | | | 345,309 | | | | 333,686 | | | | 182,287 | | | | 11,623 | | | 3 | % | | | 151,399 | | | 83 | % |
Cost of sales | | | 39,068 | | | | 34,450 | | | | 21,183 | | | | (4,618 | ) | | (13 | )% | | | (13,267 | ) | | (63 | )% |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Gross profit | | | 306,241 | | | | 299,236 | | | | 161,104 | | | | 7,005 | | | 2 | % | | | 138,132 | | | 86 | % |
Operating expenses: | | | | | | | | | | | | | | | | | | | | | | | | | | |
Research and development | | | 103,590 | | | | 88,717 | | | | 63,073 | | | | (14,873 | ) | | (17 | )% | | | (25,644 | ) | | (41 | )% |
Selling, general and administrative | | | 216,804 | | | | 230,031 | | | | 119,462 | | | | 13,227 | | | 6 | % | | | (110,569 | ) | | (93 | )% |
Reacquisition costs | | | 158,909 | | | | — | | | | — | | | | (158,909 | ) | | — | | | | — | | | — | |
Amortization of intangible assets | | | 39,429 | | | | 38,615 | | | | 27,349 | | | | (814 | ) | | (2 | )% | | | (11,266 | ) | | (41 | )% |
Acquired in-process research and development | | | 13,900 | | | | — | | | | 83,447 | | | | (13,900 | ) | | — | | | | 83,447 | | | — | |
Merger related costs | | | — | | | | — | | | | 16,722 | | | | — | | | — | | | | 16,722 | | | — | |
Litigation costs | | | 57,635 | | | | — | | | | — | | | | (57,635 | ) | | — | | | | — | | | — | |
Impairment charge | | | 9,214 | | | | — | | | | — | | | | (9,214 | ) | | — | | | | — | | | — | |
Equity in net income of Drug Source Co., LLC | | | (908 | ) | | | (3,771 | ) | | | (2,776 | ) | | | (2,863 | ) | | (76 | )% | | | 995 | | | 36 | % |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Total operating expense | | | 598,573 | | | | 353,592 | | | | 307,277 | | | | (244,981 | ) | | (69 | )% | | | (46,315 | ) | | (15 | )% |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Loss from operations | | | (292,332 | ) | | | (54,356 | ) | | | (146,173 | ) | | | (237,976 | ) | | (438 | )% | | | 91,817 | | | 63 | % |
Interest income | | | 18,809 | | | | 4,990 | | | | 399 | | | | 13,819 | | | 277 | % | | | 4,591 | | | 1,151 | % |
Other expense | | | (4,999 | ) | | | — | | | | — | | | | 4,999 | | | — | | | | — | | | — | |
Interest expense | | | (187 | ) | | | (190 | ) | | | (4,741 | ) | | | 3 | | | 2 | % | | | 4,551 | | | 96 | % |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Loss before income taxes | | | (278,709 | ) | | | (49,556 | ) | | | (150,515 | ) | | | (229,153 | ) | | (462 | )% | | | 100,959 | | | 67 | % |
Benefit for income taxes | | | (1,938 | ) | | | (7,952 | ) | | | (25,964 | ) | | | (6,014 | ) | | (76 | )% | | | (18,012 | ) | | (69 | )% |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Net loss | | $ | (276,771 | ) | | $ | (41,604 | ) | | $ | (124,551 | ) | | $ | (235,167 | ) | | (565 | )% | | $ | 82,947 | | | 67 | % |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Basic and diluted loss per common share | | $ | (6.91 | ) | | $ | (1.04 | ) | | $ | (3.11 | ) | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Weighted-average common shares outstanding: | | | | | | | | | | | | | | | | | | | | | | | | | | |
Basic | | | 40,032 | | | | 39,991 | | | | 39,990 | | | | | | | | | | | | | | | |
Diluted | | | 40,032 | | | | 39,991 | | | | 39,990 | | | | | | | | | | | | | | | |
41
Operating Results
Net Revenue
Net revenue for the year ended December 31, 2008 increased $11.6 million, or 3.5%, to $345.3 million as compared to $333.7 million in 2007. Included in net revenue for the year ended December 31, 2008 was $39.5 million of recognized deferred revenue relating to the co-promotion agreement with AstraZeneca (“Co-Promotion Agreement”) and the license agreements with Taiho and Green Cross as compared to $39.4 million for the year ended December 31, 2007. The Co-Promotion Agreement was terminated effective January 2009.
Abraxane® revenue for the year ended December 31, 2008 increased $10.9 million to $335.6 million as compared to $324.7 million in 2007. The increase was primarily the result of a price increase and increased unit sales of Abraxane®. Excluding the recognition of deferred revenue relating to the Co-Promotion Agreement, total Abraxane® revenue for the year ended December 31, 2008 increased 3.8% to $299.3 million compared to $288.3 from the prior year. Abraxane® revenue represented 97.2% of net revenue for the year ended December 31, 2008 as compared to 97.3% in 2007.
Other revenue increased to $9.7 million in 2008 from $9.0 million in 2007, primarily due to higher management fees revenue from our Melrose Park manufacturing and R&D facilities, and service revenue from our Phoenix plant, partially offset by lower licensing revenues due to a milestone payment received in 2007.
Net revenue in 2007 increased $151.4 million, or 83.0%, to $333.7 million as compared to the prior year. Excluding the recognition of deferred revenue relating to the Co-Promotion Agreement, Abraxane® revenue increased 84.0%, to $288.3 million compared to the prior year. The increase in Abraxane® sales was primarily due to product sales from increased market penetration. Abraxane® revenue represented 97.3% of net revenue for the year ended December 31, 2007 as compared to 96.0% in 2006.
Other revenue increased to $9.0 million in 2007 from $7.4 million in 2006, primarily due to $1.3 million of contract manufacturing revenue from the Phoenix manufacturing facility.
Gross Profit
Gross profit for the year ended December 31, 2008 was $306.2 million as compared to $299.2 million in 2007. Included in gross profit was the recognition of $36.4 million in each of 2008 and 2007 of deferred revenue under the Co-Promotion Agreement. Excluding the recognized deferred revenue from the Co-Promotion Agreement, gross profit as a percentage of net revenue for the year ended December 31, 2008 was 87.3% versus 88.4% in 2007. The decrease in gross margin percentage over the same period of 2007 was primarily due to a milestone payment received in 2007, increased sales of lower margin products associated with our contract manufacturing in Phoenix, offset partially by a pricing increase of Abraxane in 2008.
Gross profit for the year ended December 31, 2007 was $299.2 million as compared to $161.1 million in 2006. Included in gross profit was the recognition of $36.4 million in 2007 and $18.2 million in 2006 of deferred revenue under the Co-Promotion Agreement. Excluding the recognized deferred revenue from the Co-Promotion Agreement, gross profit as a percentage of net revenue for the year ended December 31, 2007 was 88.4% versus 87.1% in 2006. The increase in gross margin percentage over the same period of 2006 was primarily the result of efficiencies due to higher volume of Abraxane® sales.
Research and Development
Research and development expense for the year ended December 31, 2008 increased $14.9 million, or 16.8%, to $103.6 million as compared to $88.7 million in 2007. The increase was primarily due to increased investments in research and development companies, and increased spending in clinical and investigator sponsored studies, offset partially by reductions in stock compensation expense.
42
Research and development expense increased by $25.6 million, or 40.7%, in 2007 to $88.7 million as compared to $63.1 million in 2006. The increase was primarily due to increased spending in biosimilar development, stock compensation expense, legal costs and costs associated with the start-up of our Phoenix, Arizona manufacturing facility, partially offset by decreased clinical trial spending as a result of the timing of Phase III clinical trials.
Selling, General and Administrative
Selling, general and administrative expense for year ended December 31, 2008 decreased $13.2 million to $216.8 million, or 62.8% of net revenue, from $230.0 million, or 68.9% of net revenue in 2007. The decrease was primarily the result of a legal charge taken in 2007, and overall lower domestic expenditures of marketing expenses including costs associated with the sharing provisions of the Co-Promotion Agreement. Partially offsetting the decrease in selling, general and administrative expense was increased international spending with the launch of Abraxane, additional administration costs associated with the 2007 Separation from APP Pharmaceutical, and expenses related to the preparation for the spin-off of Abraxis Health.
Selling, general and administrative expense increased $110.6 million, or 92.6%, in 2007 to $230.0 million as compared to $119.5 million in 2006. As a percentage of net revenue, selling, general and administrative expenses in 2007 increased to 68.9% as compared to 65.5% in 2006. The increase was due primarily to increased legal-related expense, activities supporting the increase in Abraxane® product sales, which includes costs for commission expense relating to the Co-Promotion Agreement, increased marketing activities relating to Abraxane® and costs associated with increased market penetration of Abraxane® in Canada. To a lesser extent, the increase was also impacted by increased stock compensation expense relating to the RSU plans assumed in connection with the 2006 Merger and professional fees. The increase in selling, general and administrative expense was partially offset by the cost sharing agreement provisions of the Co-Promotion Agreement.
Reacquisition costs
In November 2008, we entered into an agreement under which we would, subject to the terms and conditions of the agreement, reacquire exclusive rights to market Abraxane® in the United States. In accordance with this agreement, the Co-Promotion Agreement ended effective January 2009 and we agreed to pay AstraZeneca a $268.0 million fee by March 31, 2009. For the year ended December 31, 2008, we recorded a charge of $158.9 million representing the net amount of previously deferred revenue associated with the Co-Promotion Agreement included in our balance sheet and the $268.0 million termination payment.
Amortization of Intangible Assets
Amortization of intangibles assets totaled $39.4 million, $38.6 million and $27.3 million for the years ended December 31, 2008 2007 and 2006, respectively, and included $31.5 million in each of 2008 and 2007 and $22.2 million in 2006 of amortization associated with the U.S. rights to Abraxane®. The increase in 2008 amortization of intangible assets from 2007 relates to incremental amortization associated with the acquisition of Shimoda Biotech and Platco Technologies in April 2008 and the increase in the 2007 amortization from 2006 relates to the fact that 2006 did not include a full year of amortization associated with the U.S. rights to Abraxane.
Acquired in-Process Research and Development
In connection with the purchase of Shimoda Biotech and Platco Technologies in April 2008, we acquired certain research and development projects that were required to be expensed in accordance with generally accepted accounting principles. Approximately $13.9 million of the purchase price was expensed as in-process research and development for projects that, as of the acquisition date, had not yet reached technological or regulatory feasibility and had no alternative future uses in their current states.
43
The $83.4 million of in-process research and development in 2006 resulted from the 2006 Merger. Research and development activities that had not yet reached technological or regulatory feasibility and had no alternative future use in their current state at the merger date were expensed.
Merger Related Costs
Merger related costs of $16.7 million in 2006 included (i) non-cash stock compensation expense related to shares issued to ABI employees in the 2006 Merger and (ii) direct merger and transaction-related costs.
Litigation Costs
On June 13, 2008, a jury ruled that we infringed upon one of Élan’s patent, which runs until 2011, and awarded Élan $55.2 million in damages for sales of Abraxane® through the judgment date. We have filed various post-trial motions and are appealing the jury ruling (SeeNote 13 – Contingenciesto the condensed consolidated financial statements for further discussion). For the year ended December 31, 2008, we expensed $57.6 million for this matter, which includes $2.4 million for interest expense.
Impairment Charge
We recorded a property, plant and equipment impairment charge totaling $9.2 million as a result of an anticipated sale of certain property, plant and equipment,.
Equity Income in Drug Source Company, LLC
Drug Source Company, LLC is 50% owned by us and is a selling agent of raw material to the pharmaceutical industry. Our investment in Drug Source Company is intended to both generate a return on our investment and to strengthen our future strategic sourcing capabilities over time. Because our 50% ownership interest in Drug Source Company does not provide financial or operational control of Drug Source Company, we account for our interest in Drug Source Company under the equity method. Our equity income in Drug Source Company for the years ended December 31, 2008, 2007 and 2006 was $0.9 million, $3.8 million and $2.8 million, respectively. The decrease in 2008 was due primarily to higher sales of lower margin products.
Other Non-Operating Items
Interest income consists primarily of interest earned on invested cash . The increase in interest income in 2008 of $13.8 million was primarily due to a full year’s interest income in 2008 versus only one and one-half months of interest income in 2007. Interest income of $5.0 million in 2007 was primarily related to interest earned on the $700 million of cash contributed to us in connection with the separation in comparison to $0.4 million for 2006.
Other expense was $5.0 million for the year ended December 31, 2008 compared to $0 in 2007. The increase was primarily related to a write-down of marketable securities whose values were determined to be impaired. Interest expense remained consistent for 2008 and 2007. For the year ended December 31, 2007, there was $0.2 million of interest expense in comparison to $4.7 million in 2006. The decrease in interest expense in 2007 was primarily the result of interest expense from Old Abraxis debt that was allocated to us in connection with the 2007 Separation.
Provision for Income Taxes
At December 31, 2008, we were in a net deferred tax asset position. Management believes it is likely that a portion of the deferred tax assets will not be realized. Therefore, we recorded a valuation allowance of $103.6 million against ending deferred assets. For 2008, we reported an income tax benefit of $1.9 million on a pre-tax loss from continuing operations of $278.7 million.
44
At December 31, 2007, we were in a net deferred tax asset position. In the judgment of management, it is not likely that a portion of these assets will be realized. Therefore, we recorded a valuation allowance of $2.3 million against our deferred tax assets at December 31, 2007.
Prior to the second quarter of 2006, we maintained a valuation allowance against our net deferred tax assets, including net operating loss carryforwards, as it was not likely that they would be realized. In the second quarter of 2006, deferred income tax liabilities attributable to us resulted from the step up of certain assets in the 2006 Merger. These liabilities will reverse and create sources of taxable income in the future. Therefore, we eliminated the valuation allowance at the time of the 2006 Merger. The elimination of the valuation allowance had no effect on our combined statements of operations.
LIQUIDITY AND CAPITAL RESOURCES
Overview
The following tables summarize key elements of our financial position and sources and (uses) of cash and cash equivalents for the three years ended December 31, 2008:
| | | | | | | | | | | | |
| | | December 31, | |
| | | 2008 | | | 2007 | | | 2006 | |
| | | (in thousands) | |
Summary Financial Position | | | | | | | | | | | | |
Cash and cash equivalents | | $ | 306,390 | | | $ | 705,125 | | | $ | 525 | |
Cash collateral for reacquisition of agreement | | | 300,631 | | | | — | | | | — | |
Working capital | | | 386,148 | | | | 735,181 | | | | 25,093 | |
Total assets | | | 1,438,584 | | | | 1,502,255 | | | | 764,783 | |
Total stockholders’ equity | | | 929,472 | | | | 1,197,387 | | | | 459,021 | |
| |
| | | Year Ended December 31, | |
| | | 2008 | | | 2007 | | | 2006 | |
| | | (in thousands) | |
Summary of (Uses) and Sources of Cash and Cash Equivalents: | | | | | | | | | | | | |
Operating activities | | $ | (315,468 | ) | | $ | (2,893 | ) | | $ | 170,870 | |
Purchases of property, plant and equipment | | | (43,729 | ) | | | (40,581 | ) | | | (64,431 | ) |
Purchases of investments and marketable securities | | | (19,730 | ) | | | (150 | ) | | | — | |
Cash paid for acquisition, net of cash acquired | | | (14,998 | ) | | | — | | | | — | |
Purchases of investments and other assets | | | (7,105 | ) | | | (4,174 | ) | | | (14,800 | ) |
Financing activities | | | 2,360 | | | | 752,082 | | | | (94,398 | ) |
(Uses) and Sources of Cash
Operating Activities
Net cash used in operating activities was $315.5 million for the year ended December 31, 2008 compared to cash used in operating activities of $2.9 million in 2007. The increase in cash used in operations of $312.6 million was primarily due to cash collateral set aside for the $268.0 million payment to reacquire the exclusive rights to market Abraxane in the U.S. and estimated remaining payments under the Co-Promotion Agreement of $18 million. Cash outflow for prepaid expenses, accounts payable and accrued expenses accounted for additional increases in cash used for operating activities. The uses of cash were partially offset by cash provided from decreases in accounts receivable, inventory and higher interest income earned on our cash balance in 2008.
Net cash used in operating activities was $2.9 million for the year ended December 31, 2007 compared to cash provided by operating activities of $170.9 million in 2006. The $173.8 million decrease in cash provided by
45
operating activities in 2007 was primarily due to the receipt in 2006 of a $200 million upfront fee associated with the Co-Promotion Agreement. This payment was recorded as deferred revenue and is being recognized over the period of the agreement. Additionally, the decrease in cash provided by operating activities was impacted by an increase in accounts receivable and other current assets offset by an increase in accounts payable.
Investing Activities
Our investing activities have included capital expenditures necessary to expand and maintain our manufacturing capabilities and infrastructure and to acquire various intellectual property rights. Additionally, we purchased investments in marketable securities, which we classify as available-for-sale securities.
Net cash used for the acquisition of property, plant and equipment for the year ended December 31, 2008, 2007 and 2006 totaled $43.7 million, $40.6 million and $64.4 million, respectively. The expenditures in 2008 related primarily to the modernization of our Melrose Park, Illinois and Phoenix, Arizona manufacturing facilities. In 2007, we increased capital investments in our core manufacturing and development capabilities including our 90,000 square foot active pharmaceutical ingredients plant in Puerto Rico and our Phoenix, Arizona manufacturing facility. Expenditures in 2006 related primarily to the purchase of the corporate aircraft.
In 2008, we purchased $19.7 million in marketable securities, which included $5.2 million for our investments in ProMetic Life Sciences, Inc, $5.4 million in other equity marketable securities and $9.1 million in debt marketable securities. Additionally, in 2008, we purchased $2.6 million in warrants and investment rights and $4.5 million in other investments. We acquired 100% of the equity of both Shimoda Biotech (Pty) Ltd and Platco Technologies (Pty) Ltd. for $15.1 million.
Net cash used for the acquisition of product license rights and other non-current assets totaled $4.1 million and $14.8 million for the years ended December 31, 2007 and 2006. The decrease from 2006 was due to the purchase of an office building and land in Culver City, California in 2006.
Financing Activities
Net cash provided in financing activities was $2.4 million for the year ended December 31, 2008 compared to $752.1 million in 2007. Net cash provided from financing activities in 2008 represented cash proceeds received from the exercise of stock options. Net cash provided by financing activities in 2007 related to net transactions with Old Abraxis prior to the separation, including the $700 million in cash that was contributed to us. Net cash used in 2006 was $94.4 million which represented a net $190.0 million repayment of debt under our credit facility in connection with the 2006 Merger, partially offset by $96.5 million provided by net transactions with Old Abraxis.
Sources of Financing and Capital Requirements
We have historically funded our research and development activities through product license fees, including milestones, and borrowings, whereas our primary sources of liquidity have been cash flow from operations and funding from Old Abraxis with which we had inter-company transactions prior to the separation. We received a $700 million cash contribution in connection with the separation and related transactions in November 2007.
Capital Requirements
Our future capital requirements will depend on numerous factors, including:
| | • | | expansion of product sales into international markets; |
| | • | | working capital requirements and production, sales, marketing and development costs required to support Abraxane®; |
46
| | • | | research and development, including clinical trials, spending to develop further product candidates and ongoing studies; |
| | • | | the need for manufacturing expansion and improvement; |
| | • | | the requirements of any potential future acquisitions, asset purchases or equity investments; and |
| | • | | the amount of cash generated by operations, including potential milestone and license revenue. |
We believe our cash and cash equivalents on hand, together with any cash generated from operations, will be sufficient to finance our operations for at least the next twelve months. In the event we engage in future acquisitions or significant capital projects, we may have to raise additional capital through additional borrowings or the issuance of debt or equity securities.
Contractual Obligations
Contractual obligations represent future cash commitments and liabilities under agreements with third parties, and exclude contingent liabilities for which we cannot reasonably predict future payment. Accordingly, the table below excludes contractual obligations relating to milestones and royalty payments due to third parties contingent upon certain future events. Such events could include, but are not limited to, development milestones, regulatory approvals, and product sales. The following information summarizes our contractual obligations and other commitments as of December 31, 2008:
| | | | | | | | | | | | | | | |
| | | Payments due by period |
Contractual Obligations | | Total | | Less than
1 year | | 1-3 years | | 3-5 years | | More than
5 years |
| | | (in thousands) |
Unconditional purchase obligations | | $ | 221,288 | | $ | 19,733 | | $ | 85,534 | | $ | 85,584 | | $ | 30,437 |
Operating lease obligations | | | 23,307 | | | 6,644 | | | 13,459 | | | 2,914 | | | 290 |
Reacquisition payable | | | 268,000 | | | 268,000 | | | — | | | — | | | — |
Other long term obligations | | | 19,789 | | | 4,270 | | | 15,519 | | | — | | | — |
| | | | | | | | | | | | | | | |
Total contractual cash obligations | | $ | 532,384 | | $ | 298,647 | | $ | 114,512 | | $ | 88,498 | | $ | 30,727 |
| | | | | | | | | | | | | | | |
Collaborative Agreements and Other Licensing Obligations
We have entered into various collaborative agreements under which we have agreed to provide funding to academic and clinical trial organizations of up to $65.5 million for research and other projects over a period of time of up to five years. Although we cannot predict the timing, we generally can control the timing and amount of spending as the projects require our approval in advance of funding.
Clinical Trial and Other Commitments
We have entered into various clinical trial agreements with third parties for the management, planning and execution of clinical trials. These agreements generally include milestone payments based on the number of patients enrolled in the clinical study. If all milestones in these agreements were achieved, related milestone commitments to be paid by us in 2009 through 2013 would approximate $42.6 million. In addition, we own a library of natural drug discovery soil samples and related strains acquired from around the world, which is intended for use in the discovery of new chemical entities. We are committed to paying up to $4.2 million upon the achievement of certain milestones related to this library.
Off-Balance Sheet Arrangements
We provided irrevocable standby letters of credits to secure our obligations under certain lease agreements and other obligations. These standby letters of credit are generally for a term of one-year or less, in no case with
47
maturities exceeding December 31, 2009. However, the standby letters may be extended to match the term of our obligation. As of December 31, 2008, there were $3.8 million of these letters of credit outstanding. In addition, in connection with the reacquisition of the exclusive rights to market Abraxane® in the U.S. (seeItem 8—Financial Statement and Supplementary Data, Note 3—Acquisitions and Other Transactions for further details), we issued $286.0 million in irrevocable standby letters of credit to secure the future payments under the termination agreement. These letters of credits are collateralized by $300.6 million of our cash balance and expire in April 2009. The termination liability is recorded in our balance sheet under the caption “Reacquisition payable”.
CRITICAL ACCOUNTING POLICIES AND ESTIMATES
The preparation of financial statements in conformity with accounting principles generally accepted in the United States requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenue and expense during the reporting period. The most significant estimates in our combined financial statements are discussed below. Actual results could vary from those estimates.
Revenue Recognition
Abraxane® Revenue Recognition
We recognize revenue from the sale of a product when title and risk of loss have transferred to the customer, collection is reasonably assured and we have no further performance obligation. This is typically when the product is received by the customer. At the time of sale, as further described below, we reduce sales and provide for estimated chargebacks, customer or Medicaid rebates, product returns and customer credits and cash discounts. Our methodology used to estimate and provide for these sales provisions was consistent across all periods presented. Accruals for sales provisions are presented in our combined financial statements as a reduction of net revenue and accounts receivable and, for customer or Medicaid rebates, an increase in accrued liabilities. Our sales provisions totaled $32.8 million, $30.5 million and $18.5 million for the years ended December 31, 2008, 2007 and 2006, respectively, and related reserves totaled $9.2 million and $7.9 million at December 31, 2008 and 2007, respectively. The increase in reserves is primarily the result of increased sales of Abraxane®, as well as the timing of the related payments. We regularly review information related to these estimates and adjust our reserves accordingly if, and when, actual experience differs from estimates.
We have internal historical information on chargebacks, rebates and customer returns and credits which we use as the primary factor in determining the related reserve requirements. Due to the nature of our product and its primary use in hospital and clinical settings with generally consistent demand, we believe that this internal historical data, in conjunction with periodic review of available third-party data and updated for any applicable changes in available information, provides a reliable basis for such estimates.
Research Revenue Recognition
Research revenue generally consists of amounts earned from the development and research to advance the commercial application of pharmaceutical compounds for third parties. Such research revenue may include upfront license fees, milestone payments and reimbursement of development and other costs, and royalties. Non-refundable upfront license fees under which we have continuing involvement in the form of development, manufacturing or commercialization are recognized as revenue ratably over either the development period, if the development risk is significant, or the estimated product useful life, if development risk has been substantially eliminated. Achievement-based milestone payments are recognized as revenue when the milestone objective is attained because the earnings process relating to such payments is complete upon attainment and because the payments are non-refundable and are not dependent upon future activities or the achievement of future objectives. These milestone payments are at risk and require substantive activity to achieve. Reimbursement of development and other costs are recognized as revenue as the related costs are incurred. Royalties are recognized
48
as earned in accordance with the contract terms when royalties from licensees can be reliably measured and collectibility is reasonably assured. Royalty estimates are made in advance of amounts collected using historical and forecasted trends.
Chargebacks
Following industry practice, we typically sell our product to independent pharmaceutical wholesalers at wholesale list price. The wholesaler in turn sells our product to an end user, normally a hospital or alternative healthcare facility, at a lower contractual price previously established between us and the end user via a group purchasing organization, or GPO. GPOs enter into collective purchasing contracts with pharmaceutical suppliers to secure more favorable product pricing on behalf of their end-user members.
Our initial sale to the wholesaler, and the resulting receivable, are recorded at our wholesale list price. However, as most of these selling prices will be reduced to a lower end-user contract price, at the time of sale revenue is reduced by, and a provision recorded for, the difference between the list price and estimated end-user contract price. When the wholesaler ultimately sells the product to the end user at the end-user contract price, the wholesaler charges us, a chargeback, for the difference between the list price and the end-user contract price and such chargeback is offset against our initial estimated contra asset. The most significant estimates inherent in the initial chargeback provision relate to wholesale units pending chargeback and the ultimate end-user contract selling price. We base our estimation for these factors primarily on internal sales and chargeback processing experience, estimated wholesaler inventory stocking levels, current contract pricing and our expectation for future contract pricing changes.
Our net chargeback reserve totaled $1.3 million and $1.2 million at December 31, 2008 and 2007, respectively. Due to information constraints in the distribution channel, it has not been practical, and has not been necessary, for us to capture and quantify the impact of current versus prior year activity on the chargeback provision. Information constraints within the distribution channel primarily relate to our inability to track product through the channel on a unit or specific lot basis. In addition, for the most part, we do not receive information from our customers with respect to what level of their sales are subject to chargeback. The lack of information on a specific lot basis precludes us from tracking actual chargeback activity to the period of our initial sale. As a result, we rely on internal data, external IMS data and management estimates in order to estimate the amount of inventory in the channel subject to future chargeback. The amount of inventory in the channel is comprised of physical inventory at the distributor and that the distributor has yet to report as end user sales. Physical inventory in the channel is determined by the difference between our sales less end user sales as reported by IMS. As IMS data is reported approximately one month after end user sales, the last month of end user sales is determined by a mathematical trend incorporating IMS data for prior months. We estimate yet to be reported end user sales based on a historical average number of days to process charge back activities from the date of the end user sale. We also review current year chargeback activity to determine whether material changes in the provision relate to prior period sales; such changes have not been material to our statements of operations. As a proprietary product, Abraxane® does not require significant chargeback reserves.
Rebates, Returns and Credits
Rebates or administrative fees, are offered to certain wholesale customers, GPOs and end-user customers, consistent with pharmaceutical industry practices. Settlement of rebates and fees may generally occur from one to 15 months from date of sale. We provide a general provision for rebates at the time of sale based on the contracted rates and historical redemption rates. Upon receipt of chargeback, due to the availability of product and customer specific information on these programs, we then establish a specific provision for fees or rebates based on the specific terms of each agreement. Our rebate reserve totaled $5.5 million and $6.2 million at December 31, 2008 and 2007, respectively. Rebates are reflected in the combined financial statements as a reduction of net revenue and as a current accrued liability.
49
Consistent with industry practice, our return policy permits our customers to return product within a window of time before and after the expiration of product dating. We provide for product returns and other customer credits at the time of sale by applying historical experience factors generally based on our historic data on credits issued by credit category or product, relative to related sales and we provide specifically for known outstanding returns and credits. Our reserve for customer credits and product returns totaled $0.7 million and $0.4 million at December 31, 2008 and 2007, respectively.
Inventories
Inventories consist of products currently approved for marketing and may include certain products pending regulatory approval. From time to time, we capitalize inventory costs associated with products prior to regulatory approval based on our judgment of probable future commercial success and realizable value. Such judgment incorporates our knowledge and best judgment of where the product is in the regulatory review process, our required investment in the product, market conditions, competing products and our economic expectations for the product post-approval relative to the risk of manufacturing the product prior to approval. In evaluating the market value of inventory pending regulatory approval as compared to its cost, we considered the market, pricing and demand for competing products, its anticipated selling price for the product and the position of the product in the regulatory review process. If final regulatory approval for such products is denied or delayed, we may need to provide for and expense such inventory. No inventory held at December 31, 2008 and 2007 was pending regulatory approval.
We routinely review our inventory and establish reserves when the cost of the inventory is not expected to be recovered or our product cost exceeds realizable market value. In instances where inventory is at or approaching expiry, is not expected to be saleable based on our quality and control standards or for which the selling price has fallen below cost a reserve is established. We reserve for any inventory impairment based on the specific facts and circumstances. In evaluating the market value of inventory pending regulatory approval as compared to its cost, we consider the market, pricing and demand for competing products, our anticipated selling price for the product and the position of the product in the regulatory review process. Provisions for inventory reserves are reflected in the combined financial statements as an element of cost of sales with inventories presented net of related reserves.
Allocated Expenses
Up to the date of the separation and related transactions on November 13, 2007, our product development and general and administrative functions were fully integrated with Old Abraxis, including product development, accounting, treasury, payroll, internal audit, information technology, corporate income tax, legal services and investor relations. To the extent that an asset, liability, revenue, or expense is directly associated with us, it is reflected in the accompanying consolidated and combined financial statements. In addition, Old Abraxis provided manufacturing services to us at cost. After the distribution, certain of these arrangements have continued on a temporary basis for a period generally not to exceed 24 months (or four or five years with respect to the manufacturing arrangement and real estate leases). The accompanying consolidated and combined financial statements reflect the application of certain estimates and allocations for periods before November 13, 2007 and management believes the methods used to allocate these operating expenses are reasonable. The allocation methods include relative head count, sales, square footage and management knowledge. These allocated operating expenses totaled $33.3 million and $25.8 million for the years ended December 31, 2007 and 2006, respectively. The financial information in these consolidated and combined financial statements does not include all the expenses that would have been incurred had we been a separate, stand-alone entity for the periods prior to November 13, 2007. As such, the consolidated and combined financial statements for periods prior to and including November 13, 2007 may not be indicative of our future performance and do not necessarily reflect what our consolidated and combined results of operations, financial position, and cash flows would have been had we operated as an independent, publicly-traded company during the periods presented, including changes in our capitalization as a result of the separation and related transactions.
50
Stock-Based Compensation
We account for stock based compensation in accordance with FAS 123R, which requires the recognition of compensation cost for all share-based payments (including employee stock options) at fair value. We use the straight-line attribution method to recognize share-based compensation expenses over the applicable vesting period of the award. Options currently granted generally expire ten years from the grant date and vest ratably over a four-year period. RSU’s granted under the 2001 Stock Incentive Plan generally vest ratably over a four-year period, while awards under the RSU II plan generally vest with respect to one half of the units on the second anniversary of the 2006 Merger and with respect to the remaining one half of the units on the fourth anniversary of the 2006 Merger.
To determine stock-based compensation, we use the Black-Scholes option pricing model to estimate the fair value of options granted under equity incentive plans and rights to acquire stock granted under our stock participation plan. Awards under RSU Plan I and RSU Plan II entitle the holders on each vesting date to convert the vested portion of their awards into that number of shares of our common stock equal to the value of the vested portion of the award divided by the lower of (i) the average closing price of our common stock over the three consecutive trading days ending on and including the second full trading day preceding the vesting date and (ii) $66.63 (which is the adjusted price following the separation). Accordingly, compensation expense related to these RSU plans is based on the lower of the market price or $66.63 and is expensed under the liability method in accordance with FAS 123(R) on a straight-line basis over the applicable vesting period. Compensation expense associated with RSU awards under the 2001 Stock Incentive Plan is accounted for under the equity method in accordance with FAS 123(R).
Acquisitions
Our consolidated financial statements and results of operations reflect an acquired business after the completion of the acquisition. We account for acquired businesses using the purchase method of accounting, which requires that the assets acquired and liabilities assumed be recorded at the date of acquisition at their respective fair values. Any excess of the purchase price over the estimated fair values of the net assets acquired is recorded as goodwill. Amounts allocated to acquired IPR&D are expensed at the date of acquisition. When we acquire net assets that do not constitute a business under generally accepted accounting principles in the U.S. (GAAP), no goodwill is recognized.
The judgments made in determining the estimated fair value assigned to each class of assets acquired and liabilities assumed, as well as asset lives, can materially impact our results of operations. Accordingly, for significant items, we typically obtain assistance from third-party valuation specialists. The valuations are based on information available near the acquisition date and are based on expectations and assumptions that have been deemed reasonable by management.
Determining the useful life of an intangible asset also requires judgment, as different types of intangible assets will have different useful lives and certain assets may even be considered to have indefinite useful lives. For example, the useful life of the right associated with a pharmaceutical product’s exclusive patent will be finite and will result in amortization expense being recorded in our results of operations over a determinable period. However, the useful life associated with a brand that has no patent protection but that retains, and is expected to retain, a distinct market identity could be considered to be indefinite and the asset would not be amortized.
Impairment of long-lived assets
We review all of our long-lived assets, including goodwill and other intangible assets, for impairment indicators at least annually and we perform detailed impairment testing for goodwill annually and for all other long-lived assets whenever impairment indicators are present. Examples of those events or circumstances that may be indicative of impairment include:
| | • | | A significant adverse change in legal factors or in the business climate that could affect the value of the asset, including, by way of example, a successful challenge of our patent rights that could result in generic competition earlier than expected. |
51
| | • | | A significant adverse change in the extent or manner in which an asset is used, including, by way of example, restrictions imposed by the FDA or other regulatory authorities that could affect our ability to manufacture or sell a product. |
| | • | | A projection or forecast that demonstrates losses associated with an asset, including, by way of example, a change in a government reimbursement program that could result in an inability to sustain projected product revenues or profitability or the introduction of a competitor’s product that could result in a significant loss of market share. |
The value of intangible assets is determined primarily using the “income method,” which starts with a forecast of all expected future net cash flows. Accordingly, the potential for impairment for intangible assets may exist if actual revenues are significantly less than those initially forecasted or actual expenses are significantly more than those initially forecasted. Some of the more significant estimates and assumptions inherent in the intangible asset impairment estimation process include: the amount and timing of projected future cash flows; the discount rate selected to measure the risks inherent in the future cash flows; and the assessment of the asset’s life cycle and the competitive trends impacting the asset, including consideration of any technical, legal, regulatory, or economic barriers to entry as well as expected changes in standards of practice for indications addressed by the asset.
RECENT ACCOUNTING PRONOUNCEMENTS
In December 2007, the FASB issued Statement No. 141(R),Business Combination (SFAS 141R) and Statement No. 160,Accounting and Reporting of Noncontrolling Interest in Consolidated Financial Statements, an Amendment of ARB No. 51 (SFAS 160). These new standards will significantly change the accounting for and reporting of business combination transactions and noncontrolling (minority) interests in the consolidated financial statements. Pursuant to SFAS 141(R), an acquiring entity will be required to recognize all assets acquired and liabilities assumed in a transaction at the acquisition date fair value, with limited exceptions, and all transaction related costs will be expensed. In addition, SFAS 141(R) will have an impact on the goodwill impairment test associated with acquisitions. SFAS 141R and SFAS 160 are required to be adopted simultaneously and are effective for the first annual reporting period beginning on or after December 15, 2008. Earlier application is not permitted. SFAS 141R and SFAS 160 could have a significant impact on our accounting for future business combinations and other business arrangements depending on the nature, terms and size of our business combinations that occur in the future.
In December 2007, the FASB ratified EITF No. 07-1,Accounting for Collaborative Agreements (EITF 07-1). EITF 07-1 provides guidance regarding financial statement presentation and disclosure of collaborative arrangements, as defined. EITF 07-1 requires that nonrefundable advance payments for goods and services that will be used or rendered in future research and development activities pursuant to executory contractual arrangements be deferred and recognized as a expense in the period that the related goods are delivered or services provided. EITF 07-1 will be applicable to arrangements we may enter into regarding development and commercialization of products and product candidates. EITF 07-1 is effective for us as of January 1, 2009, and its adoption is not expected to have a material impact on our consolidated results of operations or financial position.
In April 2008, the FASB issued Staff Position 142-3 (FSP 142-3),Determination of the Useful Life of Intangible Assets. FSP 142-3 amends the factors that should be considered in developing renewal or extension assumptions used to determine the useful life of a recognizable intangible asset under FASB Statement No. 142,Goodwill and Other Intangible Assets. The intent of FSP 142-3 is to improve the consistency between the useful life of a recognized intangible asset under Statement 142 and the period of expected cash flows used to measure the fair value of the asset under SFAS 141R and other U.S. generally accepted accounting principles. FSP 142-3 is effective for us beginning on January 1, 2009 and will be applied prospectively to all intangible assets recognized as of, and subsequent to, the effective date. The adoption of FSP 142-3 could have a significant impact on our accounting for future recognized intangible assets.
52
In October 2008, the FASB issued FSP 157-3, Determining the Fair Value of a Financial Asset When the Market for That Asset is Not Active. FSP 157-3 clarifies the application of SFAS No. 157, Fair Value Measurements, in a market that is not active and provides an example to illustrate key considerations in determining fair value of financial assets when the market for that financial asset is not active. FSP 157-3 applies to financial assets within the scope of accounting pronouncements that require or permit fair value measurements in accordance with SFAS No. 157. FSP 157-3 was effective upon issuance and included prior periods for which financial statements had not been issued. The application of FSP 157-3 did not have a material impact on our consolidated results of operations or financial position.
Item 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
We are exposed to market risks associated with changes in interest rates and foreign currency exchange rates. Interest rate changes affect primarily our investments in marketable securities and our debt obligations. Changes in foreign currency exchange rates can affect our operations outside of the United States.
Foreign Currency Risk: We have operations in Canada, Switzerland and other parts of the world; however, both revenue and expenses of those operations are typically denominated in the currency of the country of operations, providing a partial hedge. Nonetheless, these foreign operations are presented in our combined financial statement in U.S. dollars and can be impacted by foreign currency exchange fluctuations through both (i) translation risk, which is the risk that the financial statements for a particular period or as of a certain date depend on the prevailing exchange rates of the various currencies against the U.S. dollar, and (ii) transaction risk, which is the risk that the currency impact of transactions denominated in currencies other than the subsidiaries functional currency may vary according to currency fluctuations.
With respect to translation risk, even though there may be fluctuations of currencies against the U.S. dollar, which may impact comparisons with prior periods, the translation impact is included in accumulated other comprehensive income, a component of stockholders’ equity, and does not affect the underlying results of operations. Gains and losses related to transactions denominated in a currency other than the functional currency of the countries in which we operate are included in the consolidated statements of operations. We do not hedge our investments in our foreign subsidiaries.
Investment Risk: The primary objectives of our investment program are the safety and preservation of principal, maintaining liquidity to meet operating and projected cash flow requirements, and maximizing return on invested funds, while diversifying risk. Some of the securities that we invest in may have interest rate risk. This means that a change in prevailing interest rates may cause the fair value of the principal amount of the investment to fluctuate. For example, if we hold a security that was issued with a fixed interest rate at the prevailing rate and the prevailing rate later rises, the fair value of the principal amount of our investment will probably decline.
To minimize this risk, we maintain an investment portfolio of cash equivalents and short-term investments consisting of high credit quality securities, including money market funds, commercial paper, government and non-government debt securities. We do not use derivative financial instruments.
We are also exposed to equity price risks on marketable equity securities included in our portfolio of investments entered into for the promotion of business and strategic objectives. These investments are generally in small capitalization stocks in the biotechnology industry sector. We regularly review the market prices of these investments for impairment purposes. For the year ended December 31, 2008, we realized other than temporary losses of $4.7 million on available for sale securities whose values, based on market quotation, had declined below their carrying value. No losses were recognized in 2007 and 2006. As of December 31, 2008 and 2007 our investment in marketable equity securities totaled $6.2 million and $3.7 million, respectively.
Interest Rate Risk: As of December 31, 2008, we had no debt obligations outstanding. Consequently, we have minimal current exposure to changes in interest rates on borrowings.
53
Item 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
Report of Independent Registered Public Accounting Firm
The Board of Directors and Stockholders of Abraxis BioScience, Inc.
We have audited the accompanying consolidated balance sheets of Abraxis BioScience, Inc. (formerly known as New Abraxis, Inc.) as of December 31, 2008 and 2007, and the related consolidated and combined statements of operations, stockholders’ equity, and cash flows for each of the three years in the period ended December 31, 2008. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management and evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the consolidated financial position of Abraxis BioScience, Inc. at December 31, 2008 and 2007, and the consolidated and combined results of its operations and its cash flows for each of the three years in the period ended December 31, 2008, in conformity with U.S. generally accepted accounting principles.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), Abraxis BioScience, Inc.’s internal control over financial reporting as of December 31, 2008, based on criteria established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission and our report dated March 2, 2009 expressed an unqualified opinion thereon.
/s/ Ernst & Young LLP
Los Angeles, California
March 2, 2009
54
Abraxis BioScience, Inc.
Consolidated Balance Sheets
| | | | | | | |
| | | December 31, |
| | | 2008 | | | 2007 |
| | | (in thousands, except par
and share data) |
Assets | | | | | | | |
Current assets: | | | | | | | |
Cash and cash equivalents | | $ | 306,390 | | | $ | 705,125 |
Cash collateral for reacquisition of agreement | | | 300,631 | | | | — |
Accounts receivable, net of chargebacks of $1,258 in 2008 and $1,172 in 2007 and credit returns of $695 in 2008 and $414 in 2007 | | | 37,011 | | | | 43,944 |
Related party receivable | | | 1,915 | | | | 1,958 |
Inventories | | | 63,506 | | | | 73,677 |
Prepaid expenses and other current assets | | | 33,795 | | | | 18,572 |
Deferred income taxes | | | 65,585 | | | | 33,696 |
| | | | | | | |
Total current assets | | | 808,833 | | | | 876,972 |
Property, plant and equipment, net | | | 166,720 | | | | 145,120 |
Investment in Drug Source Company, LLC | | | 10,183 | | | | 9,275 |
Intangible assets, net of accumulated amortization of $105,113 in 2008 and $67,136 in 2007 | | | 175,291 | | | | 205,231 |
Goodwill | | | 241,361 | | | | 241,361 |
Other non-current assets | | | 36,196 | | | | 24,296 |
| | | | | | | |
Total assets | | $ | 1,438,584 | | | $ | 1,502,255 |
| | | | | | | |
Liabilities and stockholders’ equity | | | | | | | |
Current liabilities: | | | | | | | |
Accounts payable | | $ | 39,142 | | | $ | 33,579 |
Accrued liabilities | | | 53,020 | | | | 54,927 |
Accrued litigation costs | | | 57,635 | | | | — |
Reacquisition payable | | | 268,000 | | | | — |
Related party payable | | | — | | | | 6,986 |
Income taxes payable | | | 679 | | | | 5,010 |
Deferred revenue | | | 4,209 | | | | 41,289 |
| | | | | | | |
Total current liabilities | | | 422,685 | | | | 141,791 |
Deferred income taxes, non-current | | | 62,685 | | | | 32,396 |
Long-term portion of deferred revenue | | | 8,223 | | | | 121,138 |
Other non-current liabilities | | | 15,519 | | | | 9,543 |
| | | | | | | |
Total liabilities | | | 509,112 | | | | 304,868 |
Stockholders’ equity: | | | | | | | |
Common stock—$0.001 par value; 100,000,000 shares authorized; issued and outstanding—40,066,451 shares in 2008 and 39,993,684 shares in 2007 | | | 40 | | | | 40 |
Additional paid-in capital | | | 1,203,092 | | | | 1,192,461 |
(Accumulated deficit) retained earnings | | | (272,689 | ) | | | 4,082 |
Accumulated other comprehensive (loss) income | | | (971 | ) | | | 804 |
| | | | | | | |
Total stockholders’ equity | | | 929,472 | | | | 1,197,387 |
| | | | | | | |
Total liabilities and stockholders’ equity | | $ | 1,438,584 | | | $ | 1,502,255 |
| | | | | | | |
See accompanying notes to consolidated and combined financial statements
55
Abraxis BioScience, Inc.
Consolidated and Combined Statements of Operations
| | | | | | | | | | | | |
| | | Year ended December 31, | |
| | | 2008 | | | 2007 | | | 2006 | |
| | | (in thousands, except per share data) | |
Abraxane revenue | | $ | 335,631 | | | $ | 324,692 | | | $ | 174,906 | |
Other revenue | | | 9,678 | | | | 8,994 | | | | 7,381 | |
| | | | | | | | | | | | |
Net revenue | | | 345,309 | | | | 333,686 | | | | 182,287 | |
Cost of sales | | | 39,068 | | | | 34,450 | | | | 21,183 | |
| | | | | | | | | | | | |
Gross profit | | | 306,241 | | | | 299,236 | | | | 161,104 | |
Operating expenses: | | | | | | | | | | | | |
Research and development | | | 103,590 | | | | 88,717 | | | | 63,073 | |
Selling, general and administrative | | | 216,804 | | | | 230,031 | | | | 119,462 | |
Reacquisition costs | | | 158,909 | | | | — | | | | — | |
Amortization of intangible assets | | | 39,429 | | | | 38,615 | | | | 27,349 | |
Acquired in-process research and development | | | 13,900 | | | | — | | | | 83,447 | |
Merger related costs | | | — | | | | — | | | | 16,722 | |
Litigation costs | | | 57,635 | | | | — | | | | — | |
Impairment charge | | | 9,214 | | | | — | | | | — | |
Equity in net income of Drug Source Company, LLC | | | (908 | ) | | | (3,771 | ) | | | (2,776 | ) |
| | | | | | | | | | | | |
Total operating expense | | | 598,573 | | | | 353,592 | | | | 307,277 | |
| | | | | | | | | | | | |
Loss from operations | | | (292,332 | ) | | | (54,356 | ) | | | (146,173 | ) |
Interest income | | | 18,809 | | | | 4,990 | | | | 399 | |
Other expense | | | (4,999 | ) | | | — | | | | — | |
Interest expense | | | (187 | ) | | | (190 | ) | | | (4,741 | ) |
| | | | | | | | | | | | |
Loss before income taxes | | | (278,709 | ) | | | (49,556 | ) | | | (150,515 | ) |
Benefit for income taxes | | | (1,938 | ) | | | (7,952 | ) | | | (25,964 | ) |
| | | | | | | | | | | | |
Net loss | | $ | (276,771 | ) | | $ | (41,604 | ) | | $ | (124,551 | ) |
| | | | | | | | | | | | |
Basic and diluted loss per common share | | $ | (6.91 | ) | | $ | (1.04 | ) | | $ | (3.11 | ) |
| | | | | | | | | | | | |
Stock-based compensation is included above in the following classification: | | | | | | | | | | | | |
Cost of sales | | $ | 252 | | | $ | 1,068 | | | $ | 1,705 | |
Research and development | | | 3,997 | | | | 10,453 | | | | 3,776 | |
Selling, general and administrative | | | 9,025 | | | | 10,772 | | | | 9,624 | |
Other merger related costs | | | — | | | | — | | | | 11,115 | |
| | | | | | | | | | | | |
| | $ | 13,274 | | | $ | 22,293 | | | $ | 26,220 | |
| | | | | | | | | | | | |
See accompanying notes to consolidated and combined financial statements
56
Abraxis BioScience, Inc.
Consolidated and Combined Statements of Stockholders’ Equity
Year Ended December 31, 2008, 2007 and 2006
(in thousands)
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Common Stock | | Additional
Paid-in
Capital | | | (Accumulated
Deficit)
Retained
Earnings | | | Accumulated
Other
Comprehensive
(Loss) Income | | | Parent Company
Investment | | | Total | |
| | | Shares | | Amount | | | | | |
Balance, January 1, 2006 | | — | | $ | — | | $ | — | | | $ | — | | | $ | 952 | | | $ | (66,596 | ) | | $ | (65,644 | ) |
Net transactions with parent company. | | — | | | — | | | — | | | | — | | | | — | | | | 157,178 | | | | 157,178 | |
Effect of 2006 merger | | — | | | — | | | — | | | | — | | | | — | | | | 492,134 | | | | 492,134 | |
Comprehensive (loss) income: | | | | | | | | | | | | | | | | | | | | | | | | | |
Net loss | | | | | | | | | | | | — | | | | — | | | | (124,551 | ) | | | (124,551 | ) |
Unrealized loss on available-for-sale securities, net of tax | | | | | | | | | | | | — | | | | (1,089 | ) | | | — | | | | (1,089 | ) |
Foreign currency translation adjustments | | | | | | | | | | | | — | | | | 993 | | | | — | | | | 993 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
Comprehensive loss | | | | | | | | | | | | | | | | | | | | | | | | (124,647 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | |
Balance, December 31, 2006 | | — | | $ | — | | $ | — | | | $ | — | | | $ | 856 | | | $ | 458,165 | | | $ | 459,021 | |
Net transactions with parent company. | | — | | | — | | | — | | | | — | | | | — | | | | 779,187 | | | | 779,187 | |
Issuance of common stock in connection with separation and related transactions | | 39,990 | | | 40 | | | 1,191,626 | | | | — | | | | — | | | | (1,191,666 | ) | | | — | |
Exercise of stock options | | 3 | | | — | | | 59 | | | | — | | | | — | | | | — | | | | 59 | |
Repurchase of common stock relating to the vesting of restricted units, net | | 1 | | | — | | | (9 | ) | | | — | | | | — | | | | — | | | | (9 | ) |
Equity-based compensation | | | | | | | | 785 | | | | — | | | | — | | | | — | | | | 785 | |
Comprehensive (loss) income: | | | | | | | | | | | | | | | | | | | | | | | | | |
Net loss | | | | | | | | | | | | 4,082 | | | | — | | | | (45,686 | ) | | | (41,604 | ) |
Unrealized loss on available-for-sale securities, net of tax | | | | | | | | | | | | — | | | | (368 | ) | | | — | | | | (368 | ) |
Foreign currency translation adjustments | | | | | | | | | | | | — | | | | 316 | | | | — | | | | 316 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
Comprehensive loss | | | | | | | | | | | | | | | | | | | | | | | | (41,656 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | |
Balance, December 31, 2007 | | 39,994 | | $ | 40 | | $ | 1,192,461 | | | $ | 4,082 | | | $ | 804 | | | $ | — | | | $ | 1,197,387 | |
Exercise of stock options | | 46 | | | — | | | 2,360 | | | | — | | | | — | | | | — | | | | 2,360 | |
Net settlement of vested restricted stocks | | 26 | | | — | | | (946 | ) | | | — | | | | — | | | | — | | | | (946 | ) |
Other | | | | | | | | (354 | ) | | | — | | | | — | | | | — | | | | (354 | ) |
Equity-based compensation | | | | | | | | 9,571 | | | | — | | | | — | | | | — | | | | 9,571 | |
Comprehensive (loss) income: | | | | | | | | | | | | | | | | | | | | | | | | | |
Net loss | | | | | | | | | | | | (276,771 | ) | | | — | | | | — | | | | (276,771 | ) |
Unrealized loss on available-for-sale securities, net of tax | | | | | | | | | | | | — | | | | (2,883 | ) | | | — | | | | (2,883 | ) |
Foreign currency translation adjustments | | | | | | | | | | | | — | | | | 1,108 | | | | — | | | | 1,108 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
Comprehensive loss | | | | | | | | | | | | | | | | | | | | | | | | (278,546 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | |
Balance, December 31, 2008 | | 40,066 | | $ | 40 | | $ | 1,203,092 | | | $ | (272,689 | ) | | $ | (971 | ) | | $ | — | | | $ | 929,472 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
See accompanying notes to consolidated and combined financial statements
57
Abraxis BioScience, Inc.
Consolidated and Combined Statements of Cash Flows
| | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2008 | | | 2007 | | | 2006 | |
| | | (in thousands) | |
Cash flows from operating activities: | | | | | | | | | | | | |
Net loss | | $ | (276,771 | ) | | $ | (41,604 | ) | | $ | (124,551 | ) |
Adjustments to reconcile net loss to net cash (used in) provided by operating activities: | | | | | | | | | | | | |
Depreciation | | | 13,571 | | | | 10,443 | | | | 5,882 | |
Amortization of intangible assets | | | 39,429 | | | | 38,702 | | | | 28,032 | |
Amortization of merger related inventory step-up | | | — | | | | — | | | | 737 | |
Acquired in-process research and development | | | 13,900 | | | | — | | | | 83,447 | |
Equity-based compensation | | | 13,274 | | | | 22,293 | | | | 26,220 | |
Loss on disposal of property, plant and equipment | | | — | | | | 481 | | | | 124 | |
Impairment charges | | | 9,214 | | | | — | | | | — | |
Other than temporary loss on marketable securities | | | 4,658 | | | | — | | | | — | |
Loss on derivatives | | | 1,664 | | | | — | | | | — | |
Deferred income taxes | | | (3,457 | ) | | | (12,981 | ) | | | (44,486 | ) |
Non-cash legal expense | | | — | | | | 14,763 | | | | — | |
Equity in net income of Drug Source Company, LLC | | | (908 | ) | | | (3,771 | ) | | | (2,776 | ) |
Changes in operating assets and liabilities: | | | | | | | | | | | | |
Accounts receivable, net | | | 6,861 | | | | (16,345 | ) | | | 6,837 | |
Cash collateral for reacquisition of agreement | | | (300,631 | ) | | | — | | | | — | |
Inventories | | | 10,101 | | | | 6,444 | | | | (11,768 | ) |
Prepaid expenses and other current assets | | | (5,126 | ) | | | (11,430 | ) | | | (3,768 | ) |
Accounts payable and accrued expenses | | | (8,894 | ) | | | 18,210 | | | | 29,099 | |
Deferred revenue | | | (149,996 | ) | | | (34,933 | ) | | | 178,956 | |
Accrued litigation costs | | | 57,635 | | | | — | | | | — | |
Reacquisition payable | | | 268,000 | | | | — | | | | — | |
Related party | | | (6,943 | ) | | | 5,251 | | | | (765 | ) |
Other non-current liabilities | | | (1,049 | ) | | | 1,584 | | | | (350 | ) |
| | | | | | | | | | | | |
Net cash (used in) provided by operating activities | | | (315,468 | ) | | | (2,893 | ) | | | 170,870 | |
Cash flows from investing activities: | | | | | | | | | | | | |
Purchases of property, plant and equipment | | | (43,729 | ) | | | (40,581 | ) | | | (64,431 | ) |
Purchases of available-for-sale investments | | | (19,730 | ) | | | (150 | ) | | | — | |
Equity investment in unconsolidated entity | | | — | | | | — | | | | (3,000 | ) |
Cash paid for acquisition, net of cash acquired | | | (14,998 | ) | | | — | | | | — | |
Proceeds from pay-down of notes receivable | | | — | | | | — | | | | 1,280 | |
Purchase of warrants and investment rights | | | (2,591 | ) | | | — | | | | — | |
Purchases of investments and other assets | | | (4,514 | ) | | | (4,174 | ) | | | (13,080 | ) |
| | | | | | | | | | | | |
Net cash used in investing activities | | | (85,562 | ) | | | (44,905 | ) | | | (79,231 | ) |
Cash flows from financing activities: | | | | | | | | | | | | |
Proceeds from the exercise of stock options | | | 2,360 | | | | 59 | | | | — | |
Issuance of restricted stocks | | | — | | | | (9 | ) | | | — | |
Proceeds from issuance of debt | | | — | | | | — | | | | 24,000 | |
Repayment of borrowings | | | — | | | | — | | | | (214,000 | ) |
Payment of deferred financing costs | | | — | | | | — | | | | (852 | ) |
Net transactions with parent company | | | — | | | | 752,032 | | | | 96,454 | |
| | | | | | | | | | | | |
Net cash provided by (used in) financing activities | | | 2,360 | | | | 752,082 | | | | (94,398 | ) |
Effect of exchange rates on cash and cash equivalents | | | (65 | ) | | | 316 | | | | 993 | |
| | | | | | | | | | | | |
Net (decrease) increase in cash and cash equivalents | | | (398,735 | ) | | | 704,600 | | | | (1,766 | ) |
Cash and cash equivalents, beginning of year | | | 705,125 | | | | 525 | | | | 2,291 | |
| | | | | | | | | | | | |
Cash and cash equivalents, end of year | | $ | 306,390 | | | $ | 705,125 | | | $ | 525 | |
| | | | | | | | | | | | |
See accompanying notes to consolidated and combined financial statements
58
Abraxis BioScience, Inc.
Notes to Consolidated and Combined Financial Statements
December 31, 2008
1. Description of Business
Abraxis BioScience, Inc. (formerly known as New Abraxis, Inc.) is a Delaware corporation that was formed in June 2007. Unless the context requires otherwise, references to “we,” “us” or “our” refer to Abraxis BioScience, Inc. and its subsidiaries, including its operating subsidiary Abraxis BioScience, LLC, references to “APP” refer to APP Pharmaceuticals, Inc. and its subsidiaries, including its operating subsidiary APP Pharmaceuticals, LLC (which we sometimes refer to as “APP LLC”), references to “Old Abraxis” refer to Abraxis BioScience, Inc. prior to the 2007 separation. We are a fully integrated biotechnology company, with a marketed product (Abraxane®), global ownership of our proprietary technology platform and clinical pipeline, dedicated nanoparticle manufacturing capabilities for worldwide supply integrated with in-house capabilities, including discovery, clinical drug development, regulatory and sales and marketing.
We are dedicated to the discovery, development and delivery of next-generation therapeutics and core technologies that offer patients safer and more effective treatments for cancer and other critical illnesses. Our portfolio includes a protein-based nanoparticle chemotherapeutic compound (Abraxane®) which is based on our proprietary tumor targeting technology known as the nab® technology platform. This nab® technology platform exploits the tumor’s biology against itself, taking advantage of an albumin-specific, receptor-mediated transport system and allowing the delivery of a drug from the vascular space across the blood vessel wall to the underlying tumor tissue. Abraxane® is the first commercial validation of this nab® technology platform. From the discovery and research phase to development and commercialization, we are committed to rapidly enriching its product pipeline and accelerating the delivery of breakthrough therapies that will transform the lives of patients who need them.
We own the worldwide rights to Abraxane® , a next-generation, nanometer-sized, solvent-free taxane that was approved by the U.S. Food and Drug Administration, or the FDA, in January 2005 for its initial indication in the treatment of metastatic breast cancer and launched in February 2005.
2007 Separation
On November 13, 2007, Abraxis BioScience, Inc. (“Old Abraxis”) was separated into two independent publicly-traded companies: one holding the former Abraxis Pharmaceutical Products business (which is referred to as the “hospital-based business”); and the other holding the former Abraxis Oncology and Abraxis Research businesses (which is referred to as the “proprietary business”). Following the separation, the proprietary business changed its name from New Abraxis, Inc. to Abraxis BioScience, Inc. and the hospital-based business is operated under the name APP Pharmaceuticals, Inc. References to the “separation” or “spin-off” refer to the transactions in which the proprietary business and hospital-based business of Old Abraxis were separated into two independent public companies. References to the historical assets, liabilities, products, businesses or activities of our company are generally intended to refer to the historical assets, liabilities, products, businesses or activities of the business as it was conducted as part of Old Abraxis prior to the separation.
In connection with the separation, stockholders of Old Abraxis as of November 13, 2007 received one share of our company for every four shares of Old Abraxis held as of that date. In addition, in connection with the separation, we entered into a separation and distribution agreement that provides for, among other things, the principal corporate transactions required to effect the separation and other specified terms governing our relationship with APP after the spin-off. We also entered into various agreements with APP, including (i) a transition services agreement pursuant to which we and APP agreed to continue to provide each other with various services on an interim, transitional basis, for periods up to 24 months depending on the particular service; (ii) a manufacturing agreement whereby we and APP agreed to manufacture Abraxane® and certain other products and to provide other manufacturing-related services for a period of four or five years; (iii) an employees
59
matters agreement providing for each company’s respective obligations to employees and former employees who are or were associated with their respective businesses, and for other employment and employee benefit matters; (iv) various real estate leases; and (v) a tax allocation agreement.
2006 Merger
On April 18, 2006, American Pharmaceutical Partners, Inc. or “Old APP,” completed a merger with American BioScience, Inc., or ABI, Old APP’s former parent, pursuant to the terms of the Agreement and Plan of Merger dated November 27, 2005, which we refer to as the “2006 Merger.” APP’s certificate of incorporation was amended to change its name to Abraxis BioScience, Inc. (Old Abraxis).
For accounting purposes, the 2006 Merger was treated as a downstream merger with ABI viewed as the surviving entity, although Old APP was the surviving entity for legal purposes. As such, effective as of the merger date, the 2006 Merger was accounted for as an implied acquisition of Old APP’s minority interests using the purchase method of accounting. Therefore, the historical book value of the minority interests was stepped up to its estimated fair values and the historical shareholders’ equity of the accounting acquirer, ABI, was retroactively restated for the equivalent number of shares received in the 2006 Merger. The purchase price attributed to minority interests was allocated to the minority interests’ pro-rata share of Old APP’s tangible and identifiable intangible assets and liabilities based on their estimated fair values at the acquisition date. The excess of the purchase price attributed to minority interests over the minority interests’ pro-rata share of the fair values of APP’s assets and liabilities was recorded as goodwill. As part of the 2007 separation, we were allocated $241.4 million in goodwill from the 2006 Merger that is associated with our operations.
2. Summary of Significant Accounting Policies
Basis of Consolidation and Combination
The accompanying consolidated and combined financial statements reflect the consolidated operations of Abraxis BioScience and its subsidiaries as an independent, publicly-traded company as of and subsequent to November 13, 2007 and a combined reporting entity comprising the assets and liabilities that constituted the proprietary business of Old Abraxis for periods prior to November 13, 2007. The consolidated and combined financial statements include the assets, liabilities and results of operations of our wholly-owned and majority-owned operating subsidiaries and variable interest entities which we are the primary beneficiary. Additionally, the consolidated and combined statements include our investment in Drug Source Company, LLC, which is accounted for using the equity method. All material intercompany balances and transactions were eliminated in consolidation and combination.
For variable interest entities, we assess the terms of our interest in the entity to determine if we are the primary beneficiary as prescribed by FASB Interpretation 46R,Consolidation of Variable Interest Entities, an Interpretation of Accounting Research Bulletin No. 51 (FIN 46R). The primary beneficiary of a variable interest entity is the party that absorbs a majority of the entity’s expected losses, receives a majority of its expected residual returns, or both, as a result of holding variable interest. Variable interests are ownership, contractual, or other pecuniary interests in an entity that change with changes in the fair value of the entity’s net assets excluding variable interests. We have one variable interest entity and since we are the primary beneficiary the variable interest entiry is consolidated in our financial statements.
The consolidated and combined financial statements for periods prior to and including November 13, 2007 do not necessarily reflect what our consolidated and combined results of operations, financial position, and cash flows would have been had we operated as an independent, publicly-traded company during the periods presented, including changes in our capitalization as a result of the separation and related transactions. To the extent that an asset, liability, revenue, or expense is directly associated with us, it is reflected in the accompanying consolidated and combined financial statements. Certain general corporate overhead and other
60
expenses for periods prior to the separation have been allocated to us. Management believes such allocations were reasonable; however, they may not be indicative of our actual results had we been operating as an independent, publicly traded company for the periods presented. SeeNote 7—Related Party Transactions for further information regarding allocated expenses.
Reclassifications
Certain amounts have been reclassified to conform to current year presentations. The reclassifications did not impact net loss or total stockholders’ equity.
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States requires management to make estimates and assumptions that affect the reported amount of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the consolidated financial statements. Estimates may also affect the reported amounts of revenues and expenses during the reporting period. Actual results could differ from these estimates.
Cash and Cash Equivalents
It is our policy to include cash and investments having maturity of three months or less at the time of acquisition in cash and cash equivalents.
Cash Collateral for Reacquisition of Agreement
As part of the agreement to reacquire the exclusive rights to market Abraxane in the U.S. (seeNote 3—Acquisitions and Other Transactions), we provided $286 million ($268 million for the termination payment and $18 million for estimated final payments due under the Co-Promotion Agreement) in irrevocable standby letters of credit to secure the future payments under the agreement. The letters of credits are collateralized by $300.6 million of cash and expire in April 2009. The $300.6 million of cash collateral is held in a depository account that is insured up to a $250,000 limit.
Accounts Receivable and Concentration of Credit Risk
We typically have multi-year contractual agreements with specialty and traditional group purchasing organizations (GPOs) and comprehensive academic cancer centers to supply our product to end-users. As is traditional in the pharmaceutical industry, a significant amount of our branded pharmaceutical products are sold to end users under GPO contracts through a relatively small number of drug wholesalers and specialty distributors, which comprise the primary pharmaceutical distribution chain in the United States. Four wholesalers collectively represented approximately 79%, 76% and 84% of our revenue in 2008, 2007 and 2006, respectively. A single wholesaler accounted for 35%, 50% and 45% of our revenue in 2008, 2007 and 2006, respectively, with the next largest wholesaler accounting for 17%, 13% and 16% in 2008, 2007 and 2006, respectively. These four wholesalers also represented 82% of accounts receivable at December 31, 2008 and 2007. We have a policy to routinely monitor the creditworthiness of customers, review outstanding customer balances and record allowances for bad debts as necessary. Historical credit losses have been insignificant.
61
Inventories
Inventories are valued at the lower of cost or market as determined under the first-in, first-out, or FIFO, method, as follows:
| | | | | | |
| | | December 31, |
| | | 2008 | | 2007 |
| | | (in thousands) |
Finished goods | | $ | 14,477 | | $ | 982 |
Work in process | | | 9,615 | | | 8,194 |
Raw materials | | | 39,414 | | | 64,501 |
| | | | | | |
| | $ | 63,506 | | $ | 73,677 |
| | | | | | |
Inventories consist of products currently approved for marketing and may from time to time, include certain products pending regulatory approval. Occasionally, we capitalize inventory costs associated with products prior to regulatory approval based on our judgment of probable future commercial success and realizable value. Such judgment incorporates our knowledge and best judgment of where the product is in the regulatory review process, our required investment in the product, market conditions, competing products and our economic expectations for the product post-approval relative to the risk of manufacturing the product prior to approval. In evaluating the market value of inventory pending regulatory approval as compared to its cost, we considered the market, pricing and demand for competing products, its anticipated selling price for the product and the position of the product in the regulatory review process. If final regulatory approval for such products is denied or delayed, we may need to provide for and expense such inventory. No inventory held at December 31, 2008 and 2007 was pending regulatory approval.
We routinely review our inventory and establish reserves when the cost of the inventory is not expected to be recovered or its product cost exceeds realizable market value. In instances where inventory is at or approaching expiry, is not expected to be saleable based on quality and control standards or for which the selling price has fallen below cost, we reserve for any inventory impairment based on the specific facts and circumstances. Provisions for inventory reserves are reflected in the financial statements as an element of cost of sales with inventories presented net of related reserves.
Investment in Drug Source Company, LLC
Drug Source Company, LLC is a joint venture with three other partners established in June 2000 to purchase raw materials for resale to pharmaceutical companies. Since our 50% interest in Drug Source Company does not provide financial or operational control of the entity, we account for our interest in Drug Source Company under the equity method. Our equity in the net income of Drug Source Company is classified in operating expenses in the consolidated and combined statements of operations.
Property, Plant and Equipment
Property, plant and equipment is stated on the basis of cost or allocated fair value relative to our acquisitions. Provisions for depreciation are computed for financial reporting purposes using the straight-line method over the estimated useful life of the related asset and for leasehold improvements the lesser of the estimated useful life of the related asset or the term of the related lease as follows:
| | |
Buildings and improvements | | 10-30 years |
Corporate aircraft | | 15 years |
Machinery and equipment | | 3-10 years |
Furniture and fixtures | | 5-7 years |
62
Depreciation expense was $13.6 million, $10.4 million and $5.9 million for the years ended December 31, 2008, 2007 and 2006, respectively. Old Abraxis utilized certain assets owned by us and accordingly, the related depreciation expense of $2.2 million and $1.4 million for the years ended December 31, 2007 and 2006, respectively, was not allocated to our operating results.
Property, plant, and equipment consist of the following:
| | | | | | | | |
| | | December 31, | |
| | | 2008 | | | 2007 | |
| | | (in thousands) | |
Land | | $ | 10,594 | | | $ | 10,516 | |
Building and improvements | | | 83,421 | | | | 79,545 | |
Machinery and equipment | | | 30,242 | | | | 26,656 | |
Corporate aircraft | | | 46,552 | | | | 46,552 | |
Furniture and fixtures | | | 11,234 | | | | 10,603 | |
Construction in progress | | | 42,352 | | | | 5,622 | |
| | | | | | | | |
| | | 224,395 | | | | 179,494 | |
Less accumulated depreciation | | | (57,675 | ) | | | (34,374 | ) |
| | | | | | | | |
| | $ | 166,720 | | | $ | 145,120 | |
| | | | | | | | |
Impairment Charge
We review our property, plant and equipment for impairment whenever events or changes in circumstance indicate that the carrying amount of an asset may not be recoverable. For the year ended December 31, 2008, we recorded an asset impairment charge of $9.2 million as a result of an anticipated sale of certain property, plant and equipment. The amount is included in “impairment charge” in the consolidated and combined statements of operations.
Goodwill and Intangible Assets
We are required to perform impairment tests related to goodwill under SFAS No. 142,Goodwill and Other Intangible Assetsannually, which we perform on October 1, and whenever events or changes in circumstances suggest that it is more likely than not that the fair value of the reported units are below their carrying values. We completed the annual impairment tests for our $241.4 million of goodwill and determined that goodwill was not impaired and that no impairment charges were required in 2008.
Intangible assets are recorded at acquisition cost or allocated fair value relative to our acquisitions and are amortized on a straight-line basis over the period estimated revenues are expected to be derived from such assets (weighted average amortization period of approximately five years). We review our intangible assets for impairment whenever events or changes in circumstance indicate that the carrying amount of an intangible asset may not recoverable. We recognized no impairment charges in 2008 for any intangible assets.
Marketable Securities
We determine the appropriate classification of our investments in marketable securities at the time of purchase and re-evaluate such determinations at each balance sheet date. We consider our marketable securities to be available-for-sale as defined in Statement of Financial Accounting Standards No. 115,Accounting for Certain Investments in Debt and Equity Securities. Accordingly, these investments are recorded at fair value and the unrealized gains and losses are included in accumulated other comprehensive loss in the statement of stockholders’ equity. As of December 31, 2008, we had $15.3 million of marketable securities that were designated as available-for-sale securities, of which, $3.7 million is included in “Prepaid expenses and other
63
current assets” and $11.6 million is included in “Other non-current assets”. At December 31, 2007, we had $3.7 million of marketable securities designated as available-for-sale included in “Other non-current assets”. Available for sale securities consisted of the following:
| | | | | | | | | | | | | | | | | | | |
| | | December 31, 2008 | | December 31, 2007 |
| | | Adjusted
Cost | | Unrealized
gain (loss) | | | Estimated
Fair Market
Value | | Adjusted
Cost | | Unrealized
gain (loss) | | Estimated
Fair Market
Value |
| | | (in thousands) |
Marketable equity securities | | $ | 8,613 | | $ | (2,434 | ) | | $ | 6,179 | | $ | 2,650 | | $ | 1,089 | | $ | 3,739 |
Marketable debt securities | | | 9,131 | | | — | | | | 9,131 | | | — | | | — | | | — |
| | | | | | | | | | | | | | | | | | | |
Total available for sale securities | | $ | 17,744 | | $ | (2,434 | ) | | $ | 15,310 | | $ | 2,650 | | $ | 1,089 | | $ | 3,739 |
| | | | | | | | | | | | | | | | | | | |
In May 2008, we purchased notes having an aggregate principal amount of $5.0 million from a privately held company. The notes are senior secured convertible notes due 2018 and earn interest at nine percent per annum. The notes are convertible into an ownership interest of up to 49% of the privately held company. In addition, at the option of the privately held company, subject to certain restrictions, we may be required to purchase additional notes having an aggregate principal amount of up to $5.0 million within six months from the closing date. This option was subsequently extended to May 2009 during the third quarter of 2008 and to December 31, 2009 (or such earlier date after June 30, 2009 as we designate on 30 days notice) in the first quarter of 2009. The $5.0 million convertible notes are designated as available-for-sale securities and are measured at fair value each reporting period.
In November 2008, we purchased notes having an aggregate principal amount of $5.0 million from a publicly-held company. The convertible notes are due in 2015 and bear interest at nine percent per annum. The notes are convertible into common shares at a conversion price of $1.25 per share. The notes have a further provision for warrants representing the right to purchase additional common stock of the company in two tranches. The first tranche is exercisable in whole or in part at $2 per share, which could result in our having a 40% ownership interest in the company. The second tranche is exercisable in whole or in part at $3 per share which could result in an increase of our ownership interest from 40% to 50%, assuming full conversion of the notes. The $5.0 million convertible notes are designated as available-for-sale securities and are measured at fair value each reporting period.
We review quarterly, our available-for-sale securities for other than temporary declines in fair value and whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. This review includes an analysis of the facts and circumstances of each individual investment such as the severity of loss, the length of time the fair value has been below cost, the expectation for that security’s performance and the creditworthiness of the issuer. For the year ended December 31, 2008, we realized other than temporary losses of $4.7 million on available-for-sale securities whose values, based on market quotation, had declined significantly below their carrying value and we assessed the decline as other-than-temporary. The loss is included in “Other expense” in the consolidated and combined statements of operations. There were no such losses in 2007 and 2006. As of December 31, 2008, we have one investment in a marketable equity security with an unrealized loss of $3.4 million. As of December 31, 2008, we concluded that the unrealized loss on this investment is temporary in nature. We believe that the change in the unrealized loss on the security was caused by general market conditions and an other-than-temporary impairment does not exist. Additionally, we have the intent and ability to hold this investment for the time necessary for fair value to recover above cost.
Fair Value Measurement
We adopted the provisions of Statement of Financial Accounting Standards No. 157,Fair Value Measurements(SFAS 157), effective January 1, 2008, for our financial assets and liabilities. Under this standard, fair value is defined as the price that would be received to sell an asset or paid to transfer a liability (i.e., the “exit price”) in an orderly transaction between market participants at the measurement date. The adoption of SFAS 157 did not have a material impact on our consolidated financial statements.
64
SFAS 157 establishes a hierarchy for inputs used in measuring fair value that maximizes the use of observable inputs and minimizes the use of unobservable inputs by requiring that observable inputs be used when available. Observable inputs are inputs that market participants would use in pricing the asset or liability based on market data obtained from sources independent of us. Unobservable inputs are inputs that reflect our assumptions about the inputs that market participants would use in pricing the asset or liability and are developed based on the best information available in the circumstances. The fair value hierarchy is broken down into three levels based on the source of inputs as follows:
| | |
Level 1 – | | Valuations based on unadjusted quoted prices in active markets that are accessible at the measurement date for identical, unrestricted assets or liabilities; |
| |
Level 2 – | | Valuations based on quoted prices in markets that are not active, or financial instruments for which all significant inputs are observable; either directly or indirectly; and |
| |
Level 3 – | | Valuations based on prices or valuation techniques that require inputs that are both significant to the fair value measurement and unobservable; thus, reflecting assumptions about the market participants. |
The following fair value hierarchy table presents information about each major category of our financial assets measured at fair value on a recurring basis as of December 31, 2008:
| | | | | | | | | | | | |
| | | Basis of fair value measurements |
| | | Quoted prices in
active markets for
identical assets
(Level 1) | | Significant other
observable
inputs
(Level 2) | | Significant
unobservable
inputs
(Level 3) | | Balance as of
December 31, 2008 |
| | | (in thousands) |
Marketable equity securities | | $ | 6,179 | | $ | — | | $ | — | | $ | 6,179 |
Marketable debt securities | | | — | | | — | | | 9,131 | | | 9,131 |
Derivatives | | | — | | | 1,143 | | | — | | | 1,143 |
| | | | | | | | | | | | |
Total | | $ | 6,179 | | $ | 1,143 | | $ | 9,131 | | $ | 16,453 |
| | | | | | | | | | | | |
Gains or losses considered to be temporary are included in accumulated other comprehensive loss at each measurement date. Other than temporary losses are included in other expense in the statement of operations.
The level 3 marketable securities represent convertible notes that were purchased in May and November 2008 (see above). The value of the convertible notes at December 31, 2008 approximates their purchase price. We had no level two or three investments at December 31, 2007.
Fair Value of Financial Instruments
Our financial instruments consist mainly of cash and cash equivalents, accounts receivable and accounts payable. Cash equivalents include investments with maturities of three months or less at the time of acquisition. At December 31, 2008 and 2007, the carrying amounts of items comprising current assets and current liabilities approximate fair value due to the short-term nature of these financial instruments.
Income Taxes
In accordance with Statement of Financial Accounting Standards No. 109,Accounting for Income Taxes, deferred tax assets and liabilities are recognized for future tax consequences attributable to differences between the consolidated and combined financial statement carrying amounts of existing assets and liabilities and their respective tax bases as well as net operating loss and capital loss carry forwards. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect of deferred tax assets and liabilities of a change in tax rates is recognized in the financial statements in the period that includes the legislative enactment date.
65
We were not a taxable entity prior to the separation on November 13, 2007; however, income taxes are provided in the accompanying consolidated and combined financial statements as if we were filing a separate return. Old Abraxis will indemnify us for all tax liabilities to the extent they relate to the hospital-based generic injectable products business arising prior to the separation date.
Stock-Based Compensation
We account for stock-based compensation in accordance with FAS 123(R)Share-Based Payments, which requires the recognition of compensation cost for all share-based payments (including employee stock options) at fair value. We use the straight-line attribution method to recognize share-based compensation expense over the vesting period of the award. Options currently granted generally expire ten years from the grant date and vest ratably over a four-year period, while restricted stock units currently granted generally vest ratably over a four-year period. Awards currently granted under the RSU Plan II generally vest with respect to one half of the units on the second anniversary of the 2006 Merger and with respect to the remaining one half of the units on the fourth anniversary of the 2006 Merger.
Stock-based compensation recognized under FAS 123(R), as well as expense associated with the retrospective application, uses the Black-Scholes option pricing model to estimate the fair value of options granted under equity incentive plans and rights to acquire stock granted under a stock participation plan. Compensation expense related to equity awards of restricted stock units is based upon the market price on the date of grant. Additionally, expense related to the RSU Plan II awards is based on the lower of the market price or $66.63 and is expensed under the liability method in accordance with FAS 123(R) over the applicable vesting period. Pre-tax stock-based compensation costs for the years ended December 31, 2008, 2007 and 2006, is $13.3 million, $22.3 million and $26.2 million, respectively. SeeNote 11— Stock-Based Compensations for a more detailed discussion.
Foreign Currency Translation
The financial statements of our consolidated foreign subsidiaries are prepared using the subsidiaries currency as the functional currency. Balance sheet amounts are translated at the end of period exchange rate. Income statement and cash flow amounts are translated at the average exchange rate for the period. Adjustments from translating these financial statements into U.S. dollars are recognized in the equity section of the balance sheet under the caption, “Accumulated other comprehensive (loss) income.”
Abraxane® Revenue
We recognize revenue from the sale of Abraxane® when title and risk of loss have transferred to the customer, collection is reasonably assured and we have no further performance obligation. This is typically when the wholesalers receive the product. At the time of sale, as further described below, we reduce sales and provide for estimated chargebacks, bad debts, customer or Medicaid rebates, product returns and customer credits. Our methodology used to estimate and provide for theses sales provisions was consistent across all periods presented. Accruals for sales allowance are presented in our financial statements as a reduction of sales and accounts receivable and, for customer or Medicaid rebates, in accrued liabilities. We regularly review information related to these estimates and adjust reserves accordingly if, and when, actual experience differs from estimates.
We have internal historical information on chargebacks, rebates and customer returns and credits, which we use as the primary factor in determining the related reserve requirements. We believe that this internal historical data, in conjunction with periodic review of available third-party data and updated for any applicable changes in available information, provides a reliable basis for such estimates.
Our sales provisions totaled $32.8 million, $30.5 million and $18.5 million in 2008, 2007 and 2006 respectively. Related reserves totaled $9.2 million and $7.9 million at December 31, 2008 and 2007, respectively.
66
Co-Promotion Agreement:On April 26, 2006, we entered into a Co-Promotion and Strategic Marketing Services Agreement with AstraZeneca UK Limited, a wholly owned subsidiary of AstraZeneca PLC (seeNote 3—Acquisitions and Other Transactions). The parties entered into an agreement to end the Co-Promotion Agreement in November 2008, with the agreement effectively ending in January 2008. We recorded revenue of $36.4 million in each of the years ended December 31, 2008 and 2007 and $18.2 million in 2006 relating to deferred revenue associated with an up-front cash payment from AstraZeneca of $200 million in 2006 under the Co-Promotion Agreement. At December 31, 2008 and 2007, unearned revenue associated with the Co-Promotion Agreement of $0 and $145.5 million, respectively, was recorded as deferred revenue on the balance sheet.
Other Revenue
Other revenue generally consists of amounts earned from the development and research to advance the commercial application of pharmaceutical compounds for third parties. Such research revenue may include up-front license fees, milestone payments and reimbursement of development and other costs, and royalties. Non-refundable up-front license fees under which we have continuing involvement in the form of development, manufacturing or commercialization are recognized as revenue ratably over either: the development period if the development risk is significant; or the estimated product useful life if the development risk has been substantially eliminated. Achievement-based milestone payments are recognized as revenue when the milestone objective is attained because the earnings process relating to such payments is complete upon attainment and because the payments are non-refundable and are not dependent upon future activities or the achievement of future objectives. These milestone payments are at risk and require substantive activity to achieve. Reimbursement of development and other costs are recognized as revenue as the related costs are incurred. Royalties are recognized as earned in accordance with the contract terms when royalties from licensees can be reliably measured and collectibility is reasonably assured. Royalty estimates are made in advance of amounts collected using historical and forecasted trends.
On May 27, 2005, American BioScience, Inc. (“ABI”), the former parent of Old Abraxis, licensed the rights to Abraxane® in Japan to Taiho Pharmaceutical Co., Ltd, or Taiho, a subsidiary of Otsuka Pharmaceutical Ltd. Under the agreement, we and Taiho established a steering committee, comprised of three representatives from each of us and Taiho, to oversee the preclinical and clinical development of Abraxane® in Japan for the treatment of breast, lung, gastric, and other solid tumors. The steering committee is required to meet at least once annually. In addition, under the agreement, the steering committee established a development working group, comprised of an equal number of representatives from both us and Taiho, who is required to meet at least once quarterly until regulatory approval of Abraxane® is first obtained in Japan. The license provides for a non-refundable $20.0 million up-front payment, $38.5 million in potential milestone payments contingent upon the achievement of various clinical, regulatory and sales objectives, royalties based on net sales under the agreement; and an agreement under which we will supply Taiho with Abraxane®. Due to our continuing involvement under the agreement through the steering committee and the development working group, the $20.0 million non-refundable up-front payment has been deferred. At the time the up-front payment was made, we estimated that it would take approximately five years to receive regulatory approval for the first indication of Abraxane® in Japan (following which approval, the steering committee’s responsibilities would be substantially diminished) and that Abraxane® could begin to face competition in Japan as early as seven years after entering into the agreement. We believe these estimates remain accurate. Accordingly, the up-front payment is being recognized as research revenue ratably over seven years.
In each of 2007 and 2006, we recorded revenue of $3.0 million relating to milestone payments received from Taiho. We did not receive a milestone payment in 2008. In addition, we recognized deferred revenue of $3.1 million in 2008 and $2.9 million in each of 2007 and 2006 for the amortization of the $22.0 million in deferred up-front payments, of which $20.0 million related to the Taiho up-front payment. At December 31, 2008 and 2007, we had deferred revenue of $11.4 million and $14.5 million, respectively, recorded in our balance sheet.
67
Chargebacks
Following industry practice, we typically sell our product to independent pharmaceutical wholesalers at wholesale list price. The wholesaler in turn sells our product to an end-user, normally a hospital or alternative healthcare facility, at a lower contractual price previously established between us and the end-user via a group purchasing organization, or GPO. GPOs enter into collective purchasing contracts with pharmaceutical suppliers to secure more favorable product pricing on behalf of their end-user members.
Our initial sale to the wholesaler, and the resulting receivable, are recorded at our wholesale list price. As most of these list selling prices will be reduced to a lower end-user contract price, we then reduce reported sales and receivables by the difference between the list price and estimated end-user contract price. Then, when the wholesaler ultimately sells the product to the end-user at the end-user contract price, the wholesaler charges us a chargeback for the difference between the list price and the end-user contract price and such chargeback is offset against our initial estimated provision. On a monthly basis, we compare the unit and price components of our recorded chargeback provision to estimated ending wholesale units in the distribution channel pending chargeback under end-user contracts and estimated end-user contract prices and adjust the chargeback provision as necessary.
The provision for chargebacks is presented in our financial statements as a reduction of sales. Our provision for chargebacks during the each of the three years ended December 31, 2008 was as follows:
| | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2008 | | | 2007 | | | 2006 | |
| | | (in thousands) | |
Balance at beginning of year | | $ | 1,172 | | | $ | 1,491 | | | $ | 2,115 | |
Provision for chargebacks | | | 10,871 | | | | 9,578 | | | | 11,579 | |
Credit or checks issued to third parties | | | (10,785 | ) | | | (9,897 | ) | | | (12,203 | ) |
| | | | | | | | | | | | |
Balance at end of year | | $ | 1,258 | | | $ | 1,172 | | | $ | 1,491 | |
| | | | | | | | | | | | |
Rebates
Rebates or administrative fees are offered to certain wholesale customers, GPOs and end-user customers, consistent with pharmaceutical industry practices. Settlement of rebates and fees may generally occur from one to 15 months from date of sale. We accrue for customer rebates at the time of sale based on the contracted rates and historical redemption rates. Upon receipt of chargeback, due to the availability of product and customer specific information on these programs, we establish a provision for customer rebates based on the specific terms of each agreement. Rebates are reflected in the financial statements as a current accrued liability. Our provision for customer rebates during each of the three years ended was as follows:
| | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2008 | | | 2007 | | | 2006 | |
| | | (in thousands) | |
Balance at beginning of year | | $ | 6,206 | | | $ | 3,296 | | | $ | 5,439 | |
Provision for customer rebates | | | 19,096 | | | | 17,721 | | | | 7,537 | |
Credit issued to third parties | | | (19,750 | ) | | | (14,811 | ) | | | (9,680 | ) |
| | | | | | | | | | | | |
Balance at end of year | | $ | 5,552 | | | $ | 6,206 | | | $ | 3,296 | |
| | | | | | | | | | | | |
Returns and Credits, Cash Discounts and Bad Debts
Consistent with industry practice, our return policy permits our customers to return product within a window of time before and after the expiration of product dating. We provide for product returns and other customer credits at the time of sale by applying historical experience factors, generally based on our historic data on credits
68
issued by credit category or product, relative to related sales and we provide specifically for known outstanding returns and credits. The accrual for customer credits and product returns is presented in the financial statements as a reduction of revenue and accounts receivable. Our provision for customer credits and product returns during each of the three years ended was as follows:
| | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2008 | | | 2007 | | | 2006 | |
| | | (in thousands) | |
Balance at beginning of year | | $ | 414 | | | $ | 465 | | | $ | 657 | |
Provision for customer credits and product returns | | | 569 | | | | 129 | | | | (128 | ) |
Credit issued to third parties | | | (288 | ) | | | (180 | ) | | | (64 | ) |
| | | | | | | | | | | | |
Balance at end of year | | $ | 695 | | | $ | 414 | | | $ | 465 | |
| | | | | | | | | | | | |
We establish a reserve for bad debts based on general and identified customer credit exposure. Our actual loss due to bad debts in each of the three years ended December 31, 2008 was less than $0.2 million.
Shipping and Handling Fees and Costs
Shipping and handling fees billed to customers are recognized in net revenue. Shipping and handling costs are included in cost of sales.
Research and Development Costs
Research and development costs are expensed as incurred or consumed and consist of pre-production manufacturing scale-up and related salaries and other personnel-related expenses, depreciation, facilities, raw material and production costs and professional services.
Acquired In-Process Research and Development
For acquisitions prior to January 1, 2009, the estimated fair value of acquired in-process research and development projects, which had not reached technological feasibility at the date of acquisition and which did not have an alternative future use, were immediately expensed. Beginning January 1, 2009, with the adoption of FASB Statement No. 141R, Business Combination, the fair value of acquired in-process research and development projects will be capitalized at acquisition date (see “Recent Accounting Pronouncements” below.
Clinical Trial Agreements
We have entered into various clinical trial agreements with third parties for the planning, management and execution of clinical trials. We record accruals based on patient enrollment for estimated clinical study costs related to work performed by participating hospitals and clinics. We also account for other costs associated with the clinical trials as incurred. These costs include activities provided by contract research organizations, incremental patient medical costs and costs associated with laboratory services.
Per Share Information
Basic loss per common share is computed by dividing net loss by the weighted-average number of common shares outstanding during the reporting period. Diluted loss per common share is computed by dividing net loss by the weighted-average number of common shares used for the basic calculations plus any potentially dilutive shares for the portion of the year that the shares were outstanding, unless the impact is anti-dilutive. For the periods prior to the separation on November 13, 2007, basic and diluted loss per share were computed using the number of shares of our common stock outstanding on November 13, 2007. All potentially dilutive employee
69
stock awards were excluded from the computation of diluted loss per common share for all periods, as the effect on net loss per share was anti-dilutive. Calculations of basic and diluted loss per common share information are based on the following:
| | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2008 | | | 2007 | | | 2006 | |
| | | (in thousands, except per share data) | |
Basic and dilutive numerator: | | | | | | | | | | | | |
Net loss | | $ | (276,771 | ) | | $ | (41,604 | ) | | $ | (124,551 | ) |
| | | | | | | | | | | | |
Denominator: | | | | | | | | | | | | |
Weighted average basic and diluted common shares outstanding | | | 40,032 | | | | 39,991 | | | | 39,990 | |
| | | | | | | | | | | | |
Loss per common share—basic and diluted | | $ | (6.91 | ) | | $ | (1.04 | ) | | $ | (3.11 | ) |
| | | | | | | | | | | | |
Potentially dilutive shares not included | | | 243 | | | | — | | | | — | |
| | | | | | | | | | | | |
Supplemental Disclosure of Cash Flow Information
During 2008, we made income tax payments of $5.9 million. In 2007 and 2006 we were not a separate entity subject to income taxes as we were a subsidiary of Old Abraxis.
Recent Accounting Pronouncements
In December 2007, the FASB issued Statement No. 141(R),Business Combination (SFAS 141R) and Statement No. 160,Accounting and Reporting of Noncontrolling Interest in Consolidated Financial Statements, an Amendment of ARB No. 51 (SFAS 160). These new standards will significantly change the accounting for and reporting of business combination transactions and noncontrolling (minority) interests in the consolidated financial statements. Pursuant to SFAS 141(R), an acquiring entity will be required to recognize all assets acquired and liabilities assumed in a transaction at the acquisition date fair value, with limited exceptions, and all transaction related costs will be expensed. In addition, SFAS 141(R) will have an impact on the goodwill impairment test associated with acquisitions. SFAS 141R and SFAS 160 are required to be adopted simultaneously and are effective for the first annual reporting period beginning on or after December 15, 2008. Earlier application is not permitted. SFAS 141R and SFAS 160 could have a significant impact on our accounting for future business combinations and other business arrangements depending on the nature, terms and size of our business combinations that occur in the future.
In December 2007, the FASB ratified EITF No. 07-1,Accounting for Collaborative Agreements (EITF 07-1). EITF 07-1 provides guidance regarding financial statement presentation and disclosure of collaborative arrangements, as defined. EITF 07-1 requires that nonrefundable advance payments for goods and services that will be used or rendered in future research and development activities pursuant to executory contractual arrangements be deferred and recognized as a expense in the period that the related goods are delivered or services provided. EITF 07-1 will be applicable to arrangements we may enter into regarding development and commercialization of products and product candidates. EITF 07-1 is effective for us as of January 1, 2009, and its adoption is not expected to have a material impact on our consolidated results of operations or financial position.
In April 2008, the FASB issued Staff Position 142-3 (FSP 142-3),Determination of the Useful Life of Intangible Assets. FSP 142-3 amends the factors that should be considered in developing renewal or extension assumptions used to determine the useful life of a recognizable intangible asset under FASB Statement No. 142,Goodwill and Other Intangible Assets. The intent of FSP 142-3 is to improve the consistency between the useful life of a recognized intangible asset under Statement 142 and the period of expected cash flows used to measure the fair value of the asset under SFAS 141R and other U.S. generally accepted accounting principles. FSP 142-3
70
is effective for us beginning on January 1, 2009 and will be applied prospectively to all intangible assets recognized as of, and subsequent to, the effective date. The adoption of FSP 142-3 could have a significant impact on our accounting for future recognized intangible assets.
In October 2008, the FASB issued FSP 157-3,Determining the Fair Value of a Financial Asset When the Market for That Asset is Not Active. FSP 157-3 clarifies the application of SFAS No. 157,Fair Value Measurements, in a market that is not active and provides an example to illustrate key considerations in determining fair value of financial assets when the market for that financial asset is not active. FSP 157-3 applies to financial assets within the scope of accounting pronouncements that require or permit fair value measurements in accordance with SFAS No. 157. FSP 157-3 was effective upon issuance and included prior periods for which financial statements had not been issued. The application of FSP 157-3 did not have a material impact on our consolidated results of operations or financial position.
3. Acquisitions and Other Transactions
Acquisition of Shimoda Biotech and Platco Technologies
In April 2008, we acquired Shimoda Biotech (Pty) Ltd and its subsidiary, Platco Technologies (Pty) Ltd, located in Plettenberg Bay, South Africa. Shimoda Biotech focuses on the development of new pharmaceutical products by combining successful off-patent molecules with a novel cyclodextrin drug delivery platform, seeking to exploit the faster onset and improved bioavailability characteristics of that platform. Platco Technologies focuses on the development of novel platinum-based anti-cancer drugs. Shimoda’s first cyclodextrin-based product, Dyloject® (diclofenac sodium solution for injection), is an injectable painkiller for the treatment of post-surgical pain. Dyloject® is a solubilized intravenous formulation of diclofenac. Diclofenac is a non-steroidal anti-inflammatory drug (NSAID). Dyloject® was launched in December 2007 in the United Kingdom by Javelin Pharmaceuticals under an exclusive worldwide license agreement pursuant to which Shimoda Biotech will receive milestone payments and royalties.
Under the terms of the agreement, we acquired 100% of the equity of both Shimoda Biotech and Platco Technologies for an initial upfront payment at closing of $15.1 million, plus potential additional payments of up to $14.6 million upon the achievement of specified milestones. We accounted for the purchase as a business combination and the purchase price paid, including transaction costs of $0.3 million, was allocated to the net assets and liabilities acquired based on their respective fair values at the acquisition date, which included $8.0 million of intangible assets associated with Dyloject® and $6.9 million in liabilities relating to net milestones payments to be paid in the future. The $8.0 million of identifiable intangible assets represents existing technology with an estimated amortizable life of seventeen years. Additionally, $13.9 million of the purchase price was expensed as in-process research and development for projects that, as of the acquisition date, had not yet reached technological or regulatory feasibility and had no alternative future uses in their current states. The results of Shimoda Biotech and Platco Technologies have been included in the condensed consolidated financial statements as of the acquisition date.
Investment and License Agreements with ProMetic Life Sciences Inc.
In September 2008, we entered into agreements with ProMetic Life Sciences Inc. (ProMetic) to develop and commercialize four biopharmaceutical products targeting underserved medical conditions. We entered into the following agreements: (i) a securities purchase agreement, (ii) a license agreement, (iii) a supply and license agreement, (iv) an exclusive manufacturing agreement, and (v) a services agreement. The transaction included an initial investment in ProMetic of $7 million and optional future investment rights of up to $25 million. Of the $7 million initial investment, $5.2 million was allocated to the purchase of ProMetic’s common stock and $1.8 million was allocated to the future investment rights option. We will have access to ProMetic’s proprietary protein technologies to commercialize the biopharmaceuticals and will fund all development costs to regulatory approval. For the year ended December 31, 2008, we incurred $1.1 million in development costs under the license agreements. We may also make potential payments upon achievement of specified milestones and make royalty payments to ProMetic based on the net sales of the four potential new products. Additionally, ProMetic will perform product development activities on behalf of Abraxis under the service agreement.
71
Various Other 2008 Agreements
In December 2008, we entered into an agreement to provide a grant to a non-profit US-based incubator for biomedical start-ups. The transaction included an initial grant by us of $4 million, which was charged to research and development expense and additional funding of $0.6 million per quarter for a total of $14 million over 5 years subject to our approval. Additionally, an optional future payment of $3.5 million is possible upon approval of an additional project by us. Our chief executive officer serves on the board of this non-profit entity.
In December 2008, we purchased preferred stock of DiThera, Inc. representing 50% of DiThera’s outstanding capital stock for $5 million. We have determined that DiThera is a variable interest entity and we are the primary beneficiary. Therefore, we have consolidated DiThera in our financial statements.
Acquisition of Puerto Rico Manufacturing Facility
In February 2007, Old Abraxis completed the acquisition of the Pfizer Inc. Cruce Davila manufacturing facility in Barceloneta, Puerto Rico for $32.5 million in cash. This 56-acre site consists of a 172,000 square foot validated manufacturing plant with capabilities of producing EU- and US-compliant injectable pharmaceuticals, as well as protein-based biologics and metered-dosed inhalers. In addition, the acquisition included a state-of-the-art, computer-controlled 90,000 square foot active pharmaceutical ingredients manufacturing plant, and two support facilities with quality assurance and laboratories, totaling 262,000 square feet. Under the terms of the agreement, the active pharmaceutical ingredients plant is leased back to Pfizer. The acquisition was accounted for as an asset purchase, and the purchase price was allocated based upon independent fair values of the acquired assets. In connection with the 2007 separation, this entire facility was contributed to APP, and APP conveyed back to us the active pharmaceutical ingredients manufacturing plant currently leased to Pfizer. The property, plant and equipment and lease liability that was conveyed to us in 2008 has been included in our financial statements since the acquisition.
Cenomed Joint Venture
In April 2007, we formed a joint venture with Cenomed, Inc. to create Cenomed BioSciences, LLC. This venture is designed to further the research and development of novel drugs that interact with the central nervous system focused on psychiatric and neurological diseases, including the treatment of schizophrenia, neuroprotection, mild cognitive impairment and memory/attention impairment associated with aging, attention deficit hyperactivity disorder and pain. Pursuant to the operating agreement, we agreed to contribute up to $6 million to the joint venture to fund the research and development of these drugs. Cenomed BioSciences, LLC is consolidated in our financial statements.
Exclusive License of Intellectual Property Portfolio
In May 2007, we entered into an agreement with the University of Southern California (USC) that provides us with the exclusive worldwide development and commercialization rights for an intellectual property portfolio of diagnostic protein biomarkers for therapy response, therapy toxicity and disease recurrence in colorectal cancers (CRCs).
St. John’s Health Center
In November 2007, we entered into a master grant agreement with St. John’s Health Center and the John Wayne Cancer Institute to build and support a clinical trial infrastructure and clinical trial program and to support certain research efforts. As of December 31, 2008, we had a total of $47.8 million in commitments, subject to our approval, through December 31, 2012 under this agreement. In October 2008, we entered into a service agreement with St. John’s Health Center to provide tissue banking services for certain clinical trials for a period of up to five years. Timing and amount of funding under these agreements requires approval by our Company.
72
We made payments to St. John’s Health Center of $2.2 million and $0 in 2008 and 2007, respectively. Our chief executive officer is on the Board of Trustees for the St. John’s Health Center.
Biocon Agreements
In June 2007, we entered into an agreement with Biocon Limited under which we licensed the right to develop and commercialize a biosimilar version of G-CSF (granulocyte-colony stimulating factor) in North America and the European Union. G-CSF is an haematopoietic growth factor that works by encouraging the bone marrow to produce white blood cells. Therapeutic G-CSF is primarily used for the treatment of neutropenia, the lowering of the white blood cells that fight infections. Biocon has received regulatory approval from the Indian DCGI for its G-CSF product for the treatment of neutropenia in cancer patients. Under the terms of the agreement, we paid Biocon a $7.5 million licensing fee, which was recorded as research and development expense in 2007, and, following regulatory approval in the licensed territories, we will pay royalties to Biocon based on a percentage of net sales under the agreement.
In June 2007, we also entered into an agreement with Biocon Limited under which we granted Biocon the right to market and sell Abraxane® in India, Pakistan, Bangladesh, Sri Lanka, United Arab Emirates, Saudi Arabia, Kuwait and certain other South Asian and Persian Gulf countries. We will receive payments from Biocon based on the higher of a percentage of net sales or a specified profit split under the license agreement. In October 2007, we received regulatory approval from India’s Drug Controller General to market Abraxane® for the treatment of metastic breast cancer in India. In July 2008, we launched Abraxane in India for the treatment of breast cancer after failure of combination therapy for metastatic disease or relapse within six months of adjuvant chemotherapy.
Acquisition of Manufacturing Facility in Phoenix, Arizona
In July 2007, we acquired Watson Pharmaceuticals, Inc.’s sterile injectable manufacturing facility in Phoenix, Arizona. This fully-equipped facility, comprising approximately 200,000 square feet, includes manufacturing as well as chemistry and microbiology laboratories and has the ability to manufacture lyophilized powders, suspension products, and aqueous and oil solutions. In connection with the acquisition, we have agreed to contract manufacture certain injectable products for and on behalf of Watson for a specified period of time.
California NanoSystems Institute
In July 2007, we entered into a research collaboration agreement with the California NanoSystems Institute, or CNSI, at UCLA under which the parties agreed to collaborate on early research in nanobiotechnology for the advancement of new technologies in medicine. Under the agreement, we committed to fund up to $10 million over ten years in two tranches of $5 million for research projects selected by a committee comprised equally of CNSI and our representatives. We generally can control the timing and amount of funding under this agreement because we, in effect, need to approve any projects to be funded. The term of the agreement is ten years, but either party can terminate the agreement on 30 days notice, and the funding commitment for the second $5 million is subject to mutual agreement within 30 days of the end of the fifth year of the agreement. The partnership provides CNSI and our researchers the opportunity to jointly pursue innovative approaches to the diagnosis and treatment of life-threatening diseases, leveraging the complementary resources and skills of both organizations. Our chief executive officer serves on the board of CNSI. We made payments to CNSI of $1.3 million and $0.3 million in 2008 and 2007, respectively.
AstraZeneca UK Limited
In 2006, we entered into a co-promotion and strategic marketing services agreement with AstraZeneca UK Limited, a wholly-owned subsidiary of AstraZeneca PLC, to co-promote Abraxane® in the United States (Co-Promotion Agreement). Under the terms of the agreement, AstraZeneca paid an up-front fee of $200 million
73
(which was being deferred and amortized into income over the term of the co-promotion agreement) and equally shared in costs associated with advertising and promoting in the United States and certain clinical trials that are part of the overall clinical development program. The co-promotion agreement, which began on July 1, 2006, was to run for five and one half years. AstraZeneca received a 22% commission on U.S. net sales of Abraxane® during the term of the agreement, with a trailing commission of ten percent for the first year and five percent for the second year following the five and one half year term.
In November 2008, we entered into an agreement with AstraZeneca UK Limited under which we would, subject to the terms and conditions of the agreement, re-acquire the exclusive rights to market Abraxane® in the United States. In accordance with this agreement, the co-promotion agreement was terminated effective January 2009, and we agreed to pay AstraZeneca a $268 million fee by March 31, 2009. The $268 million fee is accrued in our consolidated balance sheet as of December 31, 2008 and the remainder of the previously deferred revenue of $109.1 million was charged to income and netted against the $268 million fee charge reflected in our 2008 consolidated statement of operations as the negotiations were substantively concluded in December 2008 and standby letters of credit were obtained to cover the obligations.
4. 2006 Merger
On April 18, 2006, Old APP, completed a merger with ABI, Old APP’s former parent, pursuant to the terms of the Agreement and Plan of Merger dated November 27, 2005. For accounting purposes, the 2006 Merger was treated as a downstream merger with ABI viewed as the surviving entity, although APP was the surviving entity for legal purposes. As such, effective as of the merger date, the 2006 Merger was accounted for as an implied acquisition of Old APP’s minority interests using the purchase method of accounting. The purchase price attributed to minority interests was allocated to the minority interests’ pro-rata share of Old APP’s tangible and identifiable intangible assets and liabilities based on their estimated fair values at the acquisition date. The excess of the purchase price attributed to minority interests over the minority interests’ pro-rata share of the fair values of Old APP’s assets and liabilities was reflected as goodwill.
Approximately $83.4 million of the estimated fair value related to us was allocated to in-process research and development and represents the minority interests’ share of the estimated fair value of our in-process research and development assets for projects that, as of the acquisition date, had not yet reached technological or regulatory feasibility and had no alternative future uses in their current states. All of the $83.4 million of in-process research and development was expensed in connection with the 2006 Merger. The estimated fair values of these projects as of the acquisition date were determined based on a discounted cash flow model prepared by an independent third-party. The estimated cash flows for each project were probability adjusted to take into account the risks associated with the successful commercialization of the projects.
5. Intangible Assets
As of December 31, 2008 and 2007, our goodwill had a carrying value of $241.4 million resulting from the 2006 Merger and the carrying value has not changed since that date. Goodwill is not deductible for income tax purposes.
All of our intangible assets, other than goodwill, are subject to amortization. Amortization expense on intangible assets was $39.4 million, $38.7 million and $27.3 million for the years ended December 31, 2008, 2007 and 2006, respectively. At December 31, 2008, the weighted average expected lives of intangibles was approximately 4.8 years.
74
The following table reflects the components of intangible assets, which have finite lives:
| | | | | | | | | | | | | | | | | | | | |
| | | Weighted-
average
Remaining
Life | | December 31, 2008 | | December 31, 2007 |
| | | | Gross
Carrying
Amount | | Accumulated
Amortization | | Net Book
Value | | Gross
Carrying
Amount | | Accumulated
Amortization | | Net Book
Value |
| | | (in thousands) |
Core technology | | 4.3 yrs | | $ | 220,202 | | $ | 85,197 | | $ | 135,005 | | $ | 220,202 | | $ | 53,740 | | $ | 166,462 |
Customer relationships | | 4.3 yrs | | | 23,588 | | | 9,127 | | | 14,461 | | | 23,588 | | | 5,757 | | | 17,831 |
Tradename | | 4.3 yrs | | | 26,478 | | | 10,245 | | | 16,233 | | | 26,478 | | | 6,462 | | | 20,016 |
Other | | 14.1 yrs | | | 10,136 | | | 544 | | | 9,592 | | | 2,099 | | | 1,177 | | | 922 |
| | | | | | | | | | | | | | | | | | | | |
Total | | | | $ | 280,404 | | $ | 105,113 | | $ | 175,291 | | $ | 272,367 | | $ | 67,136 | | $ | 205,231 |
| | | | | | | | | | | | | | | | | | | | |
Estimated amortization expense for identifiable intangible assets, other than goodwill, for each of the four succeeding years (2009 – 2012) is $39.2 million and for the fifth year in 2013 it is $11.9 million.
6. Accrued Liabilities
Accrued liabilities consisted of the following at:
| | | | | | |
| | | December 31, |
| | | 2008 | | 2007 |
| | | (in thousands) |
Sales and marketing | | $ | 13,125 | | $ | 15,466 |
Payroll and employee benefits | | | 14,797 | | | 12,249 |
Legal and insurance | | | 17,632 | | | 12,234 |
RSU Plan II | | | — | | | 12,614 |
Milestone | | | 3,950 | | | — |
Other | | | 3,516 | | | 2,364 |
| | | | | | |
| | $ | 53,020 | | $ | 54,927 |
| | | | | | |
7. Related Party Transactions
Receivables from related parties totaled $1.9 million and $2.0 million at December 31, 2008 and 2007, respectively and payables to related parties totaled $0 and $7.0 million at December 31, 2008 and 2007, respectively. Receivables from related parties at December 31, 2008 included $1.6 million in receivables from Drug Source Company and $0.3 million from APP. At December 31, 2007, we had receivables of $2.0 million from Drug Source Company and $7.0 million payable to APP.
Until May 2008, our chief executive officer and chairman of our board of directors, Patrick Soon-Shiong, M.D., and our former chief financial officer, were also the chief executive officer and chief financial officer, respectively of APP. Dr. Soon-Shiong also owned approximately 80% of the outstanding capital stock of APP and served as its chairman of the board until September 2008.
In September 2008, APP was acquired by Fresenius Kabi Pharmaceutical Holding, Inc., a subsidiary of Fresenius SE. With the purchase, our CEO ceased being a shareholder of and having a controlling interest in APP. However, he became a non-controlling member of the Fresenius Kabi Board of Directors. APP continues to be a party to the shared services, the manufacturing and other agreements and leases entered into in connection with the separation of us and APP.
75
Transactions with APP Pharmaceuticals, Inc.
In connection with the separation on November 13, 2007, we entered into a number of agreements that governs the relationship between APP and us for a period of time after the separation. The agreements were entered into while we were still a wholly owned subsidiary of Old Abraxis. These agreements include: (i) a tax allocation agreement, (ii) a dual officer agreement, (iii) an employee matters agreement, (iv) a transition services agreement, (v) a manufacturing agreement, and (vi) various real estate leases. Transactions relating to these agreements recorded in our consolidated and combined statement of operations are summarized in the following table:
| | | | | | | |
| | | Year Ended
December 31, | |
| | | 2008 | | 2007 | |
| | | (in thousands) | |
Other revenue: | | | | | | | |
Net rental income | | $ | 2,588 | | $ | 216 | |
Cost of sales: | | | | | | | |
Manufacturing and distribution | | | 1,676 | | | 33 | |
Facility management fees | | | 3,000 | | | 250 | |
Selling, general and administrative expenses: | | | | | | | |
Net general and administrative | | | 549 | | | (111 | ) |
Tax Allocation Agreement
The tax allocation agreement allocates the liability for taxes. Under the tax allocation agreement, APP is responsible for and has agreed to indemnify us against all tax liabilities to the extent they relate to APP’s hospital-based business, and we are responsible for and have agreed to indemnify APP against all tax liabilities to the extent they relate to our proprietary products business. The tax allocation agreement also provide the extent to which, and the circumstances under which, the parties would be liable if the distribution were not to constitute a tax-free distribution under Section 355 and Section 368(a)(1)(D) of the Internal Revenue Code. In general, we are required to indemnify APP for any taxes resulting from a failure of the distribution to so qualify, unless such failures results solely from APP’s specified acts.
Dual Officer Agreement
We entered into an agreement with APP under which we and APP acknowledged and agreed that our chief executive officer and former chief financial officer may serve as an officer of both companies and receive compensation from either or both companies. APP also acknowledged and agreed in this agreement that neither our chief executive officer nor our former chief financial officer will have any obligation to present to APP any business or corporate opportunity that may come to his or her attention, other than certain business opportunities. This agreement was effectively terminated when our chief executive officer and former chief financial officer resigned from APP.
Employee Matters Agreement
We entered into an employee matters agreement with APP, providing our respective obligations to employees and former employees who are or were associated with our respective businesses, and for other employment and employee benefit matters. Under the terms of the employee matters agreement, APP generally assumed all liabilities and obligations related to employee benefits for current and former employees who are or were associated with the hospital-based business, and we generally assumed all liabilities and obligations related to employee benefits for current and former employees who are or were associated with the proprietary products business. Under the employee matters agreement, the parties have agreed to reimburse one another for all indemnifiable losses that each may incur on behalf of the other as a result of any of the benefit plans or any of the termination or severance obligations.
76
Transition Services Agreement
Pursuant to the transition services agreement we and APP will continue to provide to one another various services on an interim, transitional basis, for periods up to 24 months depending on the particular service. Services that we provide to APP include legal services (e.g., assistance with SEC filings, labor and employment matters, and litigation support), financial services and corporate development. Services that APP provide to us include information technology support, tax services, legal services, accounts payable services, internal audit services, accounts receivable services, general accounting related assistance, corporate insurance and franchise tax services, human resources, customer operations, sales and marketing support, corporate purchasing and facility services and regulatory support (including assistance with state and federal license renewals). Payments made under the transition services agreement are based on the providing party’s actual costs of providing a particular service. This agreement will terminate after a period of 24 months, but generally may be terminated earlier by either party as to specific services on certain conditions.
Manufacturing Agreement
We entered into a manufacturing agreement with APP for the manufacture of Abraxane® and certain of our pipeline products by APP for us at the Melrose Park and Grand Island manufacturing facilities. Under the terms of the manufacturing agreement, we will perform certain manufacturing activities with respect to Abraxane® or other pipeline products, principally related to the formulation and compounding of the active pharmaceutical ingredients in such products, and APP will undertake the remainder of the manufacturing processes. As part of the manufacturing services, APP will also provide us with training related to proper manufacturing practices and other related training, assistance with quality assurance and control and information technology support related to manufacturing operations. With regard to the Melrose Park and Grand Island facilities, APP is responsible for obtaining and maintaining necessary approvals to manufacture Abraxane® or other pipeline products in compliance with the regulatory requirements applicable as to the jurisdictions in which such products are sold, subject to the right to receive reimbursement from us for the costs of such approvals in certain circumstances. We are responsible for obtaining and maintaining the remainder of the regulatory approvals required to sell and distribute Abraxane® both in the United States and in other jurisdictions and we are responsible for the final release of the product for sale at the completion of the manufacturing process. In the manufacturing agreement, APP agreed with us to cap the manufacturing growth over the term of the agreement of Abraxane® and other pipeline products that APP is required to manufacture under the agreement. Further, in the event of capacity constraints at the Melrose Park or Grand Island facilities, the manufacturing agreement will provide that the available capacity will be prorated between us and APP according to the parties’ then current use of APP���s manufacturing capacity. While the manufacturing agreement allows us to override these proration provisions, we may only do so by paying APP additional fees under the manufacturing agreement. The fee we would be required to pay APP to override the proration provisions of the manufacturing agreement is equal to the profit lost by APP as a result of our election to override the proration provisions.
We will pay APP a customary margin on its manufacturing costs. In addition, during each of the first three years of the manufacturing agreement, we will pay APP a facility management fee equal to $3 million. The amount of this fee may be offset to the extent APP employees are transferred to us based upon the amount of compensation paid to such transferred employees. The term of the manufacturing agreement will end on December 31, 2011, which will be automatically extended for one year if either APP exercises its right to extend the lease on our Melrose Park manufacturing facility for an additional year or we exercise our right to extend the lease for APP’s Grand Island manufacturing facility for an additional year. (SeeReal Estate Leases below).
The manufacturing agreement includes customary confidentiality provisions pursuant to which we and APP will keep confidential all information of the other party, subject to certain exceptions.
In addition, the manufacturing agreement contains the following indemnification provisions. We will indemnify APP, its affiliates and its officers, directors and employees and agents (which are referred to as the “APP indemnified parties”) from any damages incurred by or assessed against them resulting from a third-party
77
claim caused by or alleged to be caused by (i) our failure to perform our obligations under the manufacturing agreement (ii) any product liability claim arising from the negligence, fraud or intentional misconduct of our or any of our affiliates or any product liability claim arising from our manufacturing obligations (or any failure or deficiency in our manufacturing obligations) under the manufacturing agreement; (iii) any claim that the manufacture, use or sale of Abraxane® or our pipeline products infringes a patent or any other proprietary right of a third party; or (iv) any recall, product liability claim or other third-party claim not arising from the gross negligence or bad faith of, or intentional misconduct or intentional breach of the manufacturing agreement by, the APP indemnified parties by reason of the $100 million limitation of liability described below. We will also indemnify the APP indemnified parties for liabilities that they become subject to as a result of their activities under the manufacturing agreement and for which they are not responsible under the terms of the manufacturing agreement. APP will indemnify us, and our affiliates, and their respective officers, directors, employees and agents from any damages incurred or assessed against them resulting from a third-party claim caused by or alleged to be caused by (i) APP’s gross negligence, bad faith, intentional misconduct or intentional failure to perform its obligations under the manufacturing agreement; and (ii) any product liability claim arising from the gross negligence or bad faith of, or intentional misconduct or intentional breach of the manufacturing agreement by, APP or its affiliates. The APP indemnified parties will not have any liability for monetary damages to our affiliates or third parties in connection with the manufacturing agreement for damages in excess of $100 million in the aggregate, except to the extent that the damages are the result of (i) one of APP’s or our executive officers in bad faith affirmatively instructing their employees to intentionally breach APP’s obligation to manufacture Abraxane® or pipeline products under the manufacturing agreement or (ii) any intentional failure by APP, in bad faith, to cure any material breach of its obligation to manufacture Abraxane® or pipeline products under the manufacturing agreement following notice of this breach.
Real Estate Leases
Following the 2007 separation, we own the manufacturing facility located on Ruby Street, Melrose Park, Illinois, and the research and development and the warehouse facility, both located in the same building on N. Cornell Avenue, Melrose Park, Illinois. APP owns the manufacturing facility located in Grand Island, New York. In connection with the separation, the parties entered into a series of lease agreements to facilitate continued production of their respective pharmaceutical products while a manufacturing transition plan is being implemented. Under the terms of the lease agreements, we lease the Ruby Street facility, consisting of approximately 122,000 square feet of office, warehouse and pharmaceutical manufacturing space, to APP. The initial term of the lease expires on December 31, 2011 and may be renewed at the option of APP for one additional year if APP is manufacturing a certain level of its products at the Ruby Street facility in the period prior to the expiration of the lease. During the term of the lease, we will have access to the Ruby Street facility to perform certain elements of the manufacturing processes of Abraxane® and other nab® technology product candidates under the manufacturing agreement as well as, under certain circumstances, to provide contract manufacturing services to third parties. In order to provide sufficient warehouse space to APP during the term of the Ruby Street lease, we also leased the Cornell Warehouse facility, consisting of approximately 71,000 square feet of warehouse space, to APP. The initial term of the lease will be until December 31, 2011, and may be renewed at APP’s option for one additional year if the lease for the Ruby Street manufacturing facility is extended. We also leased the Cornell R&D facility, consisting of approximately 48,000 square feet of research and development space, to APP. The initial term of the lease will be until December 31, 2010 and may be terminated upon twelve months written notice from and after January 1, 2009. This lease has no option to extend the term of the lease.
We leased a portion of APP’s Grand Island facility, consisting of approximately 5,700 square feet of pharmaceutical manufacturing space, to allow us to perform our obligations under the manufacturing agreement. The initial term of the lease will be until December 31, 2011, and may be renewed at our option for one additional year if we have not received regulatory approvals from the EMEA to manufacture Abraxane® in at least two facilities (not including the Grand Island facility) by June 30, 2011.
78
Allocated Expenses
Prior to the separation, our product development and general and administrative functions were fully integrated with Old Abraxis, including product development, accounting, treasury, payroll, internal audit, information technology, corporate income tax, legal services and investor relations. In addition, Old Abraxis provided manufacturing services to us at cost. After the 2007 separation, certain of these arrangements (see above) continue on a temporary basis for a period generally not to exceed 24 months, or four or five years in the case of manufacturing-related activities and lease arrangements. For periods prior to the 2007 separation, the consolidated and combined financial statements reflect the application of certain estimates and allocations and management believes the methods used to allocate these operating expenses are reasonable. The allocation methods include relative head count, sales, square footage and management knowledge. These allocated operating expenses totaled $33.3 million and $25.8 million for the years ended December 31, 2007 and 2006, respectively.
8. Leases and Commitments
Leases
We have entered into various operating lease agreements for warehouses, office space, automobiles, communications, information technology equipment and software and office equipment, including real estate leases as described inNote 7—Related Party Transactions. Rental expense amounted to $10.1 million, $5.6 million and $3.3 million for the years ended December 31, 2008, 2007 and 2006, respectively.
As of December 31, 2008, future annual minimum lease payments related to non-cancelable operating leases, including amounts pertaining to related party leases are as follows:
| | | | | | |
Year | | Total
Amount | | Related
Party |
| | | (in thousands) |
2009 | | $ | 6,644 | | $ | 39 |
2010 | | | 5,961 | | | 39 |
2011 | | | 4,968 | | | 39 |
2012 | | | 2,530 | | | 39 |
2013 | | | 1,213 | | | — |
Thereafter | | | 1,991 | | | — |
| | | | | | |
| | $ | 23,307 | | $ | 156 |
| | | | | | |
Purchase Obligations
As of December 31, 2008, we purchased all of our approved paclitaxel supply from one supplier. We believe our supply risk with respect to this supplier is minimal given that the supplier is well-regarded within the industry and we have not historically experienced supply shortages with this supplier. Additionally, we maintain an adequate safety stock supply of paclitaxel to mitigate any supply disruption. As of December 31, 2008 and 2007, our inventory included $34.4 million and $35.9 million, respectively, of paclitaxel. As of December 31, 2008, we have no commitment agreement to purchase any paclitaxel in 2009.
We also have other material supply agreements that require us to purchase approximately $26.6 million of material supply in each of the next five years.
Clinical Trial and Other Commitments
We have entered into various clinical trial agreements with third parties for the management, planning and execution of clinical trials. These agreements generally include milestone payments based on the number of patients enrolled in the clinical study. If all milestones in these agreements were achieved, related milestone
79
commitments to be paid by us in 2009 through 2013 would approximate $42.6 million. Based on our current estimates of patient enrollment, approximately $8 million would be payable in 2009, approximately $13.6 million would be payable in 2010, approximately $9 million would be payable in 2011, approximately $6 million would be payable in 2012 and approximately $6 million would be payable in 2013.
We own a library of natural drug discovery soil samples and related strains acquired from around the world, which is intended for use in the discovery of new chemical entities. We are committed to paying up to $4.2 million upon the achievement of certain milestones related to these libraries, which we expect to occur within the next five years.
We have entered into various collaborative agreements under which we have agreed to provide funding to academic and clinical trial organizations of up to $65.5 million for research and other projects over the next five years. Even though we cannot predict the timing, we generally can control the timing and amount of spending as the projects require our approval in advance of funding.
9. Employee Benefit Plan
Following the 2007 separation, we adopted and our employees became eligible to participate in a defined contribution plan intended to be tax-qualified. APP cooperated with us to transfer the account balances of our employees to the defined contribution plan adopted by us. We adopted the defined contribution plan in the first quarter of 2008. Prior to the 2007 separation, Old Abraxis sponsored a 401(k) defined-contribution plan, or 401(k) Plan, covering substantially all eligible employees. Employee contributions to the 401(k) Plan are voluntary. Participants’ contributions are limited to their annual tax deferred contribution limit as allowed by the Internal Revenue Service. Our total matching contributions to our 401(k) Plan in 2008 and Old Abraxis’ 401(k) Plan in 2007 and 2006 were $1.8 million, $1.4 million and $0.9 million for the years ended December 31, 2008, 2007 and 2006, respectively.
10. Stockholders’ Equity
For all periods prior to November 13, 2007, Old Abraxis’ investment in our proprietary products businesses is shown as parent company investment in the consolidated and combined financial statements. Parent company investment represents the historical investment of capital in us, our accumulated net earnings after taxes, and the net effect of transactions with and allocations from Old Abraxis.See Note 7—Related Party Transactions for additional information regarding the allocation to us of various expenses incurred by Old Abraxis.
On November 13, 2007, Old Abraxis completed a distribution of one share of our common stock (par value of $0.001) for every four shares of Old Abraxis common stock. Following the separation, we had 40.0 million shares of common stock outstanding. After the separation adjustments were recorded on November 13, 2007, the remaining parent company investment balance, which includes all earnings prior to the separation, was transferred to additional paid in capital. Net (loss) income subsequent to the separation is included in retained earnings.
Registration Rights
Pursuant to a registration rights agreement, Old Abraxis granted registration rights to the former ABI stockholders (including our Chief Executive Officer and entities affiliated with him) with respect to all 86,096,523 shares of Old Abraxis common stock they received in the 2006 Merger. Under the terms of the registration rights agreement, any securities issued or issuable in respect of Old Abraxis common stock (including our common stock) would have the benefits of the registration rights agreement. Accordingly, in connection with the separation, we entered into a new registration rights agreement with the former ABI shareholders with respect to 21,524,130 shares of our common stock containing provisions substantially identical to the registration rights agreement entered into in connection with the 2006 Merger.
80
These stockholders have the right to require us to register all or a portion of the shares of our common stock they receive in the distribution. In addition, these stockholders may require us to include their shares in future registration statements that we file and may require us to register their shares for resale on a Form S-3 registration statement. Upon registration, the registered shares generally will be freely tradeable in the public market without restriction. However, in connection with any underwritten offering, the stockholders will agree to lock up any other shares for up to 90 days and will agree to a limit on the maximum number of shares that can be registered for the account of these holders under so-called “shelf” registration statements.
Except for underwriters discounts and commissions and certain marketing expenses, we will be obligated to pay all expenses for the first eight registration statements filed upon the request of a holder of registrable securities, and any other or additional expenses of registration are to be borne by the holders of registrable securities on a pro rata basis. These registration rights are subject to some conditions and limitations, among them the right of the underwriters of an offering to limit the number of shares included in registration. We will be obligated to indemnify the holders of these registration rights, and each selling holder will be obligated to indemnify us, against specified liabilities under the Securities Act of 1933, the Securities Exchange Act of 1934 and other applicable federal and state laws.
11. Stock-Based Compensation
2007 Stock Incentive Plan
We adopted the 2007 Stock Incentive Plan, which was approved by the sole stockholder subsequent to the 2007 separation. We have reserved 6,000,000 shares of our common stock for issuance under our 2007 Stock Incentive Plan, subject to adjustments for stock splits, stock dividends or other similar changes in our common stock or our capital structure. The number of shares initially reserved for issuance or transfer pursuant to awards under the 2007 Stock Incentive Plan will be increased by the number of shares represented by awards that are forfeited or cancelled, or that expire or terminate, including any shares that are forfeited by the award recipient or repurchased by us pursuant to the terms of the relevant award agreement.
Our 2007 Stock Incentive Plan provides for the grant of (a) incentive stock options to our employees, including officers and employee directors, (b) non-qualified stock options to our employees, directors and consultants and (c) other types of awards, collectively referred to as “awards.”
No more than 1,000,000 shares under options can be granted to an individual optionee in any given fiscal year. We may grant up to an additional 750,000 shares, which will not count against the limit set forth in the previous sentence, in connection with an optionee’s initial commencement of service with our company.
Our board of directors or a committee designated by our board of directors administers our 2007 Stock Incentive Plan and has authority to determine the terms and conditions of awards, including the types of awards, the number of shares subject to each award, the vesting schedule of the awards and the selection of grantees.
The exercise price of all options granted under our 2007 Stock Incentive Plan will be determined by our board of directors or a committee designated by our board of directors, but in no event may this price be less than the fair market value of our common stock on the date of grant, unless otherwise determined by our board of directors with respect to non-qualified stock options. If, however, incentive stock options are granted to a person who owns stock possessing more than 10% of the voting power of all our classes of stock, the exercise price of such option must equal at least 110% of the fair market value our common stock on the grant date and the maximum term of these incentive stock options must not exceed five years. The maximum term of an incentive stock option granted to any person who does not own stock possessing more than 10% of the voting power of all our classes of stock must not exceed ten years. Our board of directors or a committee designated by our board of directors determines the term of all other awards granted under our 2007 Stock Incentive Plan. The consideration for the option may consist of cash, check, shares of our common stock, the assignment of part of the proceeds from the sale of shares acquired upon exercise of the option, through a same-day sale and exercise procedure facilitated by a brokerage firm, loan or any combination of these forms of consideration.
81
In the event of one of the following, options under our 2007 Stock Incentive Plan will terminate and be cancelled, unless the successor corporation assumes or substitutes the options:
| | • | | a dissolution, liquidation or sale of all or substantially all of our assets, |
| | • | | a merger or consolidation in which we are not the surviving entity, |
| | • | | a corporate transaction in which our stockholders give up a majority of their equity interest in our company, or |
| | • | | an acquisition of 50% or more of our stock by any individual or entity, including by tender offer or a reverse merger. |
However, in lieu of the termination of options upon occurrence of the above events, the terms of some individual award agreements may otherwise provide for full acceleration of an optionee’s outstanding options immediately upon occurrence of the above events or full acceleration of any outstanding options that are not assumed or substituted by the surviving corporation, and full acceleration of any otherwise assumed or substituted options if optionee’s employment is terminated for cause or by optionee for good reason within twelve months of the occurrence of such events.
Unless terminated sooner, our 2007 Stock Incentive Plan will automatically terminate in 2017. Our board of directors has authority to amend or terminate our 2007 Stock Incentive Plan, subject to stockholder approval of some amendments. However, no action may be taken which will affect any shares of common stock previously issued and sold or any option previously granted under our 2007 Stock Incentive Plan, without the grantee’s consent.
Converted Stock-Based Compensation Plans
Following the 2007 separation, and pursuant to the provisions of the respective plan documents, holders of Old Abraxis restricted stock units (RSUs) and stock options who are current and former directors or employees of Old Abraxis whose employment related to our proprietary products business had their Old Abraxis BioScience RSUs and stock options converted into newly-issued RSUs and stock options of our company pursuant to a conversion formula that was intended to preserve the intrinsic value and maintained their pre-distribution RSUs and stock options. Except for adjustments made in connection with the conversion, the RSUs and stock options so issued had substantially the same terms, including expiration date and vesting schedule, as the converted Old Abraxis RSUs and stock options. Additionally, we assumed the American BioScience Restricted Stock Unit Plan I and American BioScience Restricted Stock Unit Plan II. We also assumed the restricted units previously granted under these plans. In connection with the separation, we adopted the 2007 Stock Incentive Plan.
For the periods prior to the 2007 separation on November 13, 2007, our employees held stock compensation awards in the following plans sponsored by Old Abraxis:
1997 Stock Option Plan
Under the 1997 Stock Option Plan, or the 1997 Plan, options to purchase shares of Old Abraxis’ common stock were granted to certain employees and the directors with an exercise price equal to the estimated fair market value of Old Abraxis’ common stock on the date of grant. The 1997 Plan stock options vested upon completion of Old Abraxis’ initial public offering in December 2001.
2001 Stock Incentive Plan
The 2001 Stock Incentive Plan, or the 2001 Plan, provided for the grant of incentive stock options and restricted stock to employees, including officers and employee directors, non-qualified stock options to employees, directors and consultants and other types of awards.
82
The compensation committee of Old Abraxis’ board of directors administered the 2001 Plan and had the authority to determine the terms and conditions of awards, including the types of awards, the number of shares subject to each award, the vesting schedule of the awards and the selection of the grantees. The exercise price of all options granted under the 2001 Plan were determined by the compensation committee of Old Abraxis’ board of directors, but in no event was this price less than the fair market value of Old Abraxis’ common stock on the date of grant.
2001 Non-Employee Director Stock Option Program
The 2001 Non-Employee Director Stock Option Program, or the 2001 Program, was adopted as part, and is subject to the terms and conditions, of the 2001 Plan. The 2001 Program established a program for the automatic grant of awards to non-employee directors at the time that they initially join the board and at each annual meeting of the stockholders at which they are elected.
Restricted Unit Plan
In connection with the 2007 separation, we assumed the American BioScience Restricted Unit Plan I and the American BioScience Restricted Unit Plan II (the “Plans”). We also assumed the restricted units previously granted under these plans to our employees.
Shares of our common stock will be issuable upon the vesting of these units. The units granted under American BioScience Restricted Unit Plan I vested upon the completion of the 2006 Merger. The units issued under the American BioScience Restricted Unit Plan II generally vested one-half on April 18, 2008 (which is the second anniversary of the closing of the 2006 Merger). The balance of the shares generally will vest on April 18, 2010. The units entitle their holders to receive a number of our shares of common stock determined on each vesting date based on the notional price that vests on such date divided by our average trading price over the three days prior to vesting; except that if the average trading price of our stock price is less than $66.63, then the notional price is divided by $66.63. The maximum number of our shares that may be issuable under this restricted unit plan is 367,100 shares.
We also assumed an agreement between Old Abraxis and RSU Plan LLC (“RSU LLC”). Under the terms of this agreement, RSU LLC has agreed that prior to the date on which restricted stock units issued pursuant to American BioScience Restricted Unit Plan II become vested, RSU LLC will deliver, or cause to be delivered, to us the number of our shares of common stock or cash (or a combination thereof) in an amount sufficient to satisfy the obligations to participants under the American BioScience Restricted Unit Plan II of the vested restricted units. We are required to satisfy our obligations under the American BioScience Restricted Unit Plan II by paying to the participants in the American BioScience Restricted Unit Plan II cash and/or shares of our common stock in the same proportion as was delivered by the RSU LLC. The intention of this agreement is to have RSU LLC satisfy our obligations under American BioScience Restricted Unit Plan II so that there would not be any further dilution to our stockholders as a result of our assumption of the American BioScience Restricted Unit Plan II.
As of December 31, 2008, we recorded a long-term liability of $11.0 million for awards relating to the Plans. We had a current liability of $12.6 million and a long-term liability of $3.7 million at December 31, 2007.
Employee Stock Purchase Plan
Certain of our employees participated in the Old Abraxis’ 2001 Employee Stock Purchase Plan, or the ESPP. Eligible employees contributed up to 10% of their base earnings toward the semi-annual purchase and issuance of Old Abraxis common stock. The employees’ purchase price was the lesser of 85% of the fair market value of the stock on the first business day of the offer period or 85% of the fair market value of the stock on the last business day of the semi-annual purchase period. Employees could purchase no more than 625 shares of
83
Abraxis BioScience common stock within any given purchase period. The Compensation Committee of Old Abraxis indefinitely suspended the ESPP from and after the purchase period that ended on July 31, 2007.
Equity Compensation
Restricted Stock
At December 31, 2008, 248,631 restricted stock units were outstanding under our 2007 and 2001 Stock Incentive Plan with a weighted average per share market value of $61.28 and with vesting dates ranging from March 2009 to March 2012. Upon vesting, each restricted stock unit entitles the holder to one share of our common stock. Compensation expense related to restricted stock unit grants is based upon the market price on the date of grant and is charged to earnings on a graded amortization basis over the applicable vesting period. Restricted stock units currently granted generally vest ratably over a four year period.
Stock-based compensation costs for all RSU awards (including liability awards) for the years ended December 31, 2008, 2007 and 2006 were $8.1 million, $17.2 million and $19.2 million, respectively. As of December 31, 2008, there was $13.6 million of total unrecognized compensation expense related to restricted stocks granted under our stock-based compensation plans, which is expected to be recognized over a weighted average period of 1.7 years.
Following is a reconciliation of unvested restricted stock unit awards as of December 31, 2008:
| | | | | | | | |
| | | Number of
Awards | | | Weighted-
Average
Grant Date
Fair Value(1) | | Weighted-
Average
Remaining
Amortization
Period (Years) |
Outstanding unvested awards at December 31, 2007 | | 133,410 | | | $ | 51.93 | | |
Granted | | 179,714 | | | | 64.99 | | |
Exercised | | (41,191 | ) | | | 52.15 | | |
Forfeited | | (23,302 | ) | | | 52.48 | | |
| | | | | | | | |
Outstanding unvested awards at December 31, 2008 | | 248,631 | | | $ | 61.28 | | 1.71 |
| | | | | | | | |
| (1) | Grant date fair values are based on our stock price on the grant date. |
Stock Options
We account for stock-based compensation in accordance with FAS 123(R), which requires the recognition of compensation cost for all share-based payments, including employee stock options, at fair value. We use the straight-line and graded attribution methods to recognize share-based compensation expense over the vesting period of the award. Options currently granted generally expire ten years from the grant date and vest ratably over a four-year period.
In accordance with FAS 123(R), we adjust stock-based compensation on a quarterly basis for revisions to the estimated rate of equity award forfeitures based on actual forfeiture experience. During the fourth quarter of 2007, we revised the forfeiture rate with respect to stock options. The effect of the change in the forfeiture rate resulted in a reduction in stock compensation expense of $1.0 million for 2007. No changes in estimated rate of forfeitures were made during 2008 or 2006.
As of December 31, 2008, we had $8.0 million of total unrecognized compensation expense related to stock options granted under the equity incentive plans which is expected to be recognized over a weighted average period of 1.9 years.
84
The fair values of options granted during the years ended December 31, 2008, 2007 and 2006 were determined using the Black-Scholes option pricing model, which incorporates various assumptions. The risk-free rate of interest for the average contractual life of the option is based on the U.S. Treasury yield curve in effect at the time of grant. The dividend yield is zero as we do not and Old Abraxis did not anticipate paying any dividends. The expected term of awards granted is based upon the historical exercise patterns of the participants in the plans and expected volatility is based on the historical volatility of the stock price over the expected term of the award. The weighted average estimated values of employee stock option grants and rights granted under Old Abraxis’ employee stock purchase plan as well as the weighted average assumptions that were used in calculating such values during the last three years were based on estimates at the date of grant as follows:
| | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2008 | | | 2007 | | | 2006 | |
| | | (dollars in thousands, except per share data) | |
Stock Options | | | | | | | | | | | | |
Risk-free rate | | | 2.5 | % | | | 3.8 | % | | | 3.9 | % |
Dividend yield | | | — | | | | — | | | | — | |
Expected life in years | | | 5 | | | | 5 | | | | 5 | |
Volatility | | | 45 | % | | | 52 | % | | | 59 | % |
Weighted average grant date fair value of options granted | | $ | 27.55 | | | $ | 26.44 | | | $ | 12.71 | |
Our stock options outstanding that have vested and are expected to vest as of December 31, 2008 were as follows:
| | | | | | | | | | |
| | | Number of
Shares | | Weighted
Average
Exercise
Price | | Weighted
Average
Remaining
Contractual
Term | | Aggregate
Intrinsic
Value(1) |
Vested | | 556,033 | | $ | 50.47 | | 5.05 | | $ | 8,590,710 |
Expected to vest | | 364,357 | | | 65.08 | | 1.85 | | | 306,060 |
| | | | | | | | | | |
Total | | 920,390 | | | $56.25 | | 6.54 | | | $8,896,770 |
| | | | | | | | | | |
| (1) | These amounts represent the difference between the exercise price and $65.92, the closing price of our common stock on December 31, 2008 for in the money options. |
The above options outstanding that are expected to vest are net of estimated future option forfeitures in accordance with the provisions of FAS 123(R). Options with grant date fair values of $5.9 million and $5.2 million and with weighted average grant date fair values of $33.19 and $36.15 vested during the years ended December 31, 2008 and 2007, respectively. Options with a fair value of $1.3 million and $0.2 million, weighted average grant date fair values of $27.46 and $8.96 and aggregate intrinsic values of $0.7 million and $1.0 million were exercised during the year ended December 31, 2008 and 2007, respectively. Non-vested options totaling 0.4 million and 0.4 million shares with weighted average grant date fair values of $29.00 and $31.29 were outstanding at December 31, 2008 and 2007, respectively.
85
Additional information with respect to our stock option activity is as follows:
| | | | | | | | | | | |
| | | Options Outstanding | | Exercisable Options |
| | | Shares | | | Weighted-
Average
Exercise
Price | | Shares | | Weighted-
Average
Exercise
Price |
Outstanding at January 1, 2006 | | 592,799 | | | $ | 56.42 | | 313,465 | | $ | 24.61 |
| | | | | | | | | | | |
Granted | | 391,565 | | | | 65.45 | | | | | |
Exercised | | (25,698 | ) | | | 34.69 | | | �� | | |
Forfeited | | (97,494 | ) | | | 85.39 | | | | | |
| | | | | | | | | | | |
Outstanding at December 31, 2006 | | 861,172 | | | | 57.98 | | 364,366 | | $ | 37.50 |
| | | | | | | | | | | |
Granted | | 79,287 | | | | 55.02 | | | | | |
Exercised | | (23,488 | ) | | | 15.63 | | | | | |
Forfeited | | (14,336 | ) | | | 72.05 | | | | | |
Expired | | (4,509 | ) | | | 76.54 | | | | | |
| | | | | | | | | | | |
Outstanding at December 31, 2007 | | 898,126 | | | | 55.95 | | 463,021 | | $ | 46.99 |
| | | | | | | | | | | |
Granted | | 179,714 | | | | 65.05 | | | | | |
Exercised | | (46,707 | ) | | | 50.51 | | | | | |
Forfeited | | (99,429 | ) | | | 67.46 | | | | | |
Expired | | (11,314 | ) | | | 97.04 | | | | | |
| | | | | | | | | | | |
Outstanding at December 31, 2008 | | 920,390 | | | | 56.25 | | 556,033 | | $ | 50.47 |
| | | | | | | | | | | |
In connection with separation, stock options of our employees were converted to reflect the adjusted value of the options based on our stock price after the 2007 separation. The number of options outstanding on the separation date and the associated exercise prices were converted using a rate of 0.4243, which was based on Old Abraxis’s stock price for the 10 consecutive trading days preceding the separation date and our stock price for the 10 consecutive trading days following the separation. No additional stock compensation resulted from the modifications under the provisions of FAS 123(R) because it was determined the changes were required by the terms of the awards.
The following table summarizes information about our stock options outstanding at December 31, 2008:
| | | | | | | | | | | | |
| | | Options Outstanding | | Options Exercisable |
Exercise Price Ranges | | Number of
Shares | | Weighted-
Average
Remaining
Contractual
Life | | Weighted-
Average
Exercise
Price | | Number of
Shares | | Weighted-
Average
Exercise
Price |
$ 0.00 – $ 12.43 | | 173,622 | | 1.8 | | $ | 7.88 | | 173,622 | | $ | 7.88 |
$ 12.44 – $ 24.87 | | 13,521 | | 3.0 | | | 15.90 | | 13,521 | | | 15.90 |
$ 24.88 – $ 37.30 | | 26,289 | | 3.8 | | | 30.10 | | 26,289 | | | 30.14 |
$ 37.31 – $ 49.74 | | 47,683 | | 7.8 | | | 47.77 | | 28,374 | | | 47.49 |
$ 49.75 – $ 62.17 | | 131,569 | | 8.4 | | | 55.52 | | 71,955 | | | 55.87 |
$ 62.18 – $ 74.61 | | 399,909 | | 8.4 | | | 67.19 | | 128,120 | | | 68.86 |
$ 74.62 – $ 87.04 | | 23,313 | | 5.8 | | | 80.62 | | 21,351 | | | 80.67 |
$ 87.05 – $ 99.48 | | 17,365 | | 6.5 | | | 95.02 | | 17,365 | | | 95.02 |
$ 99.49 – $111.91 | | 82,810 | | 5.8 | | | 107.43 | | 72,892 | | | 107.24 |
$ 111.92 – $124.35 | | 4,309 | | 6.2 | | | 120.80 | | 2,544 | | | 121.06 |
| | | | | | | | | | | | |
| | 920,390 | | 6.5 | | | 56.25 | | 556,033 | | | 50.47 |
| | | | | | | | | | | | |
86
12. Income Taxes
Deferred tax assets and liabilities consist of the following:
| | | | | | | | |
| | | December 31, | |
| | | 2008 | | | 2007 | |
| | | (in thousands) | |
Deferred tax assets: | | | | | | | | |
Inventory | | $ | 5,022 | | | $ | 3,261 | |
Deferred revenue | | | 3,795 | | | | 60,937 | |
Net operating loss and credit carryforwards | | | 3,638 | | | | — | |
Stock based compensation | | | 12,315 | | | | 9,502 | |
Activity from partnerships | | | 2,189 | | | | 84 | |
Other accruals and reserves | | | 3,250 | | | | 3,674 | |
Accrued legal | | | 28,034 | | | | 1,431 | |
Reacquisition payable | | �� | 104,013 | | | | — | |
Unrealized losses on marketable securities | | | 1,660 | | | | — | |
Impairments of investments and other assets | | | 5,296 | | | | — | |
| | | | | | | | |
Total deferred tax assets | | | 169,212 | | | | 78,889 | |
Deferred tax liabilities: | | | | | | | | |
Intangible amortization | | | (55,374 | ) | | | (69,167 | ) |
Purchase accounting intangible | | | (2,235 | ) | | | — | |
Depreciation | | | (5,076 | ) | | | (3,529 | ) |
Unrealized gains on marketable securities | | | — | | | | (2,597 | ) |
| | | | | | | | |
Total deferred tax liabilities | | | (62,685 | ) | | | (75,293 | ) |
| | | | | | | | |
| | | 106,527 | | | | 3,596 | |
Valuation allowance | | | (103,627 | ) | | | (2,296 | ) |
| | | | | | | | |
Net deferred tax assets | | $ | 2,900 | | | $ | 1,300 | |
| | | | | | | | |
Deferred tax assets are reduced by a valuation allowance if, based on the weight of available evidence, it is more likely than not that some portion or all of the deferred tax assets will not be realized. Both positive and negative evidence are considered in forming our judgment as to whether a valuation allowance is appropriate, and more weight is given to evidence that can be objectively verified. Valuation allowances are reassessed whenever there are changes in circumstances that may cause a change in judgment.
As of December 31, 2008, management believed it is more likely than not that a portion of the deferred tax assets will not be realized. The net change in the valuation allowance balance from 2007 to 2008 was $101.3 million. The net increase in the valuation allowance resulted from the increases to the deferred tax asset balances relating to both disallowed legal fees and accrued fees relating to the reacquisition of the exclusive rights to market Abraxane in the U.S.
At December 31, 2008, we had a net deferred tax asset of $2.9 million compared to $1.3 million in 2007. The $2.9 million net deferred tax asset consists of a deferred tax asset in the U.S. of $4.4 million and a net deferred tax liability of $1.5 million from Shimoda Biotech. The $4.4 million deferred tax assets in the U.S reflect the carryback of future losses to offset taxable income for both 2008 and 2007.
During 2008, we acquired Shimoda Biotech, Ltd, a South African corporation. As part of our purchase price allocation, Shimoda’s existing intangibles were grossed up to fair market value. Since the purchase price allocation to intangibles is not recognized under South African tax rules, a deferred tax liability was established.
As of December 31, 2008, we had research and development tax credits carryforward for federal tax purposes of $696,000 which will expire beginning in 2029.
87
We were not a taxable entity prior to the separation on November 13, 2007; however, income taxes are provided in the accompanying combined and consolidated financial statements as if we were filing a separate return. Old Abraxis will indemnify us for all tax liabilities to the extent they relate to the hospital-based generic injectable products business arising prior to the separation date.
The provision (benefit) for income taxes consists of the following:
| | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2008 | | | 2007 | | | 2006 | |
| | | (in thousands) | |
Current: | | | | | | | | | | | | |
Federal | | $ | 678 | | | $ | 4,168 | | | $ | 15,847 | |
State | | | 841 | | | | 861 | | | | 2,675 | |
| | | | | | | | | | | | |
Total current | | | 1,519 | | | | 5,029 | | | | 18,522 | |
Deferred: | | | | | | | | | | | | |
Federal | | | (91,078 | ) | | | (11,766 | ) | | | (38,060 | ) |
State | | | (9,863 | ) | | | (3,511 | ) | | | (6,426 | ) |
Foreign | | | (2,565 | ) | | | — | | | | — | |
Valuation allowance | | | 100,049 | | | | 2,296 | | | | — | |
| | | | | | | | | | | | |
Total deferred | | | (3,457 | ) | | | (12,981 | ) | | | (44,486 | ) |
| | | | | | | | | | | | |
Total benefit for income taxes | | $ | (1,938 | ) | | $ | (7,952 | ) | | $ | (25,964 | ) |
| | | | | | | | | | | | |
Pursuant to APB 23,Accounting for Income Taxes- Special Areas, a provision should be made for the tax effect of the earnings of the foreign subsidiaries that are not permanently invested outside the United States. It is our intention that earnings of our foreign subsidiaries are permanently invested outside the U.S., therefore no deferred tax asset was set up against the cumulative earnings of our foreign subsidiaries.
A reconciliation of the federal statutory rate to our effective tax rate is as follows:
| | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2008 | | | 2007 | | | 2006 | |
Tax provision at statutory federal rate | | (35.0 | )% | | (35.0 | )% | | (35.0 | )% |
State income taxes | | (3.5 | ) | | (4.2 | ) | | (3.6 | ) |
In-process research and development | | 1.8 | | | — | | | 21.5 | |
Research and development credits | | (0.4 | ) | | (4.6 | ) | | (1.3 | ) |
Stock based compensation | | 0.4 | | | 2.4 | | | (0.3 | ) |
Net operating losses utilized by APP Pharmaceuticals, Inc. | | — | | | 17.9 | | | — | |
Other | | 0.1 | | | 2.9 | | | 1.4 | |
Change in valuation allowance | | 35.9 | | | 4.6 | | | — | |
| | | | | | | | | |
Effective tax rate | | (0.7 | )% | | (16.0 | )% | | (17.3 | )% |
| | | | | | | | | |
88
We adopted the provisions of FASB Interpretation No. 48,Accounting for Uncertainty in Income Taxes (FIN 48) on January 1, 2007. This interpretation clarifies what criteria must be met prior to the recognition of the financial statement benefit of a position taken in a tax return in accordance with FASB Statement No. 109, Accounting for Income Taxes. Following is a reconciliation of our total gross unrecognized tax benefits from January 1, 2007 to December 31, 2008 (in thousands).
| | | |
Balance, December 31, 2006 | | $ | — |
Additions for tax positions related to current year | | | 5,170 |
Additions for tax positions related to prior years | | | — |
Reductions for tax periods of prior years | | | — |
| | | |
Balance, December 31, 2007 | | $ | 5,170 |
Additions for tax positions related to current year | | | — |
Additions for tax positions related to prior years | | | 354 |
Reductions for tax periods of prior years | | | — |
| | | |
Balance, December 31, 2008 | | $ | 5,524 |
| | | |
The majority of this $5.5 million of gross unrecognized tax benefits, if and when recognized, would affect our effective tax rate. We do not anticipate that any of the total tax benefits of $5.5 million will reverse in the next twelve months.
We recognize interest and penalties related to unrecognized tax benefits in our income tax expense. At December 31, 2008, we had no accrued interest or penalties.
13. Contingencies
On July 19, 2006, Élan Pharmaceutical Int’l Ltd. filed a lawsuit against Old Abraxis in the U.S. District Court for the District of Delaware alleging that Old Abraxis willfully infringed upon two of their patents by asserting that Abraxane® uses technology protected by Élan-owned patents. Élan sought unspecified damages and an injunction. In August 2006, Old Abraxis filed a response to Élan’s complaint contending that it did not infringe on the Élan patents and that the Élan patents are invalid. On June 13, 2008, the jury ruled that we infringed upon one of Élan’s patents, which runs until 2011, and awarded Élan $55.2 million in damages for sales of Abraxane® through the judgment date. We have filed various post-trial motions and are appealing the jury ruling. We assumed any liability of Old Abraxis relating to this litigation in connection with the separation. For the year ended December 31, 2008, we recorded litigation costs of $57.6 million for this matter, which includes $2.4 million of interest.
We are from time to time subject to claims and litigation arising in the ordinary course of business. We intend to defend vigorously any such litigation that may arise under all defenses that would be available to us. In the opinion of management, the ultimate outcome of proceedings of which management is aware, even if adverse to us, would not have a material adverse effect on our consolidated financial position or results of operations.
We record accruals for contingencies to the extent that we conclude their occurrence is probable and the related damages are estimable. These assessments involve complex judgments about future events and rely on estimates and assumptions. Although we believe we have substantial defenses in these matters, litigation is inherently unpredictable and we could in the future incur judgments or enter into settlements that could have a material adverse effect on our results of operations.
14. Segment Information
We operate in one business segment. Therefore, the results of operations are reported on a combined basis for purpose of segment reporting.
89
15. Subsequent Events
In January 2009, we announced that our board of directors approved a plan to spin-off its newly-formed subsidiary, Abraxis Health, Inc. as a new independent, stand-alone company holding our drug discovery, pilot manufacturing and development business. If the spin-off occurs, our stockholders would own (i) shares of Abraxis Health and (ii) shares of our common stock, and we would continue to operate our existing business, excluding the drug discovery, pilot manufacturing and development business to be held by Abraxis Health. In connection with the proposed spin-off, Abraxis Health would enter into several agreements with us related to, among other things, manufacturing, transition services, product development and research, tax allocations, clinical development and a number of ongoing commercial relationships.
The proposed spin-off is subject to a number of closing conditions, including final approval by our board of directors and the effectiveness of the registration statement registering the common stock of Abraxis Health to be distributed to our stockholders in connection with the spin-off. Approval by our stockholders is not required as a condition to the consummation of the proposed spin-off. In connection with the proposed spin-off, Abraxis Health filed a registration statement on Form 10 with the SEC. Stockholders are urged to read the Form 10 registration statement carefully because it contains important information about the proposed spin-off.
16. Unaudited Quarterly Financial Data
Selected quarterly data for years end December 31, 2008 and 2007 was as follows:
| | | | | | | | | | | | | | | | |
| | | Year Ended December 31, 2008 | |
| | First | | | Second | | | Third | | | Fourth | |
| | | (in thousands, except per share data) | |
Net revenue | | $ | 82,142 | | | $ | 77,622 | | | $ | 93,381 | | | $ | 92,164 | |
Gross profit | | | 73,535 | | | | 67,677 | | | | 82,253 | | | | 82,776 | |
Net income (loss) | | | 4,309 | | | | (84,143 | ) | | | (15,059 | ) | | | (181,878 | ) |
Income (loss) per common share: | | | | | | | | | | | | | | | | |
Basic and diluted | | $ | 0.11 | | | $ | (2.10 | ) | | $ | (0.38 | ) | | $ | (4.54 | ) |
| | | | | | | | | | | | | | | | |
| |
| | | Year Ended December 31, 2007 | |
| | First | | | Second | | | Third | | | Fourth | |
| | | (in thousands, except per share data) | |
Net revenue | | $ | 71,893 | | | $ | 83,217 | | | $ | 87,791 | | | $ | 90,785 | |
Gross profit | | | 64,234 | | | | 76,318 | | | | 76,173 | | | | 82,511 | |
Net loss | | | (5,671 | ) | | | (13,971 | ) | | | (16,772 | ) | | | (5,190 | ) |
Loss per common share(1): | | | | | | | | | | | | | | | | |
Basic and diluted | | $ | (0.14 | ) | | $ | (0.35 | ) | | $ | (0.42 | ) | | $ | (0.13 | ) |
| | | | | | | | | | | | | | | | |
| (1) | As of the completion of the separation, on November 13, 2007, we had 40.0 million common shares outstanding. The same number of shares is being used for both diluted earnings per share and basic earnings per share for all periods prior to the separation date. |
90
Item 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
None.
Item 9A. CONTROLS AND PROCEDURES
(a) Evaluation of disclosure controls and procedures.
We maintain disclosure controls and procedures, as such term is defined under Exchange Act Rules 13a-15(e) and 15d-15(e), that are designed to ensure that information required to be disclosed by us in the reports that we file or submit under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms, and that such information is accumulated and communicated to management, including our Chief Executive Officer and our Chief Financial Officer, to allow timely decisions regarding required disclosures. In designing and evaluating our disclosure controls and procedures, we recognize that any controls and procedures, no matter how well designed and operated, can only provide reasonable assurance of achieving the desired control objectives and in reaching a reasonable level of assurance we necessarily are required to apply judgment in evaluating the cost-benefit relationship of possible controls and procedures. Our management, with participation of our Chief Executive Officer and Chief Financial Officer evaluated the effectiveness of our disclosure controls and procedures as of the end of the fiscal year covered by this report. We relied in part on the system of internal controls over financial reporting performed by APP on behalf of our company in accordance with the terms and conditions of the transition services agreement executed between the parties in connection with the separation. Based on their evaluation and subject to the foregoing, management concluded that our disclosure controls and procedures were effective as of December 31, 2008.
(b) Management’s Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting as defined in Rules 13a-15(f) under the Securities Exchange Act of 1934. Our internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with accounting principles generally accepted in the United States. Internal control over financial reporting includes those written policies and procedures that:
| | • | | pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of our assets; |
| | • | | provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with accounting principles generally accepted in the United States of America; |
| | • | | provide reasonable assurance that our receipts and expenditures are being made only in accordance with authorization of our management and directors; and |
| | • | | provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of assets that could have a material effect on the consolidated financial statements. |
Internal control over financial reporting includes the controls themselves, monitoring and internal auditing practices and actions taken to correct deficiencies as identified.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
91
Management assessed the effectiveness of our internal control over financial reporting as of December 31, 2008. In making this assessment, management used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) in Internal Control—Integrated Framework. Management’s assessment included an evaluation of the design of our internal control over financial reporting and testing of the operational effectiveness of its internal control over financial reporting. Management reviewed the results of its assessment with the Audit Committee of our Board of Directors.
Based on this assessment, management believes that we maintained effective internal control over financial reporting as of December 31, 2008, based on those criteria.
The effectiveness of the Company’s internal control over financial reporting as of December 31, 2008 has been audited by Ernst & Young LLP, an independent registered public accounting firm, as stated in their report below.
(c) Attestation Report of Independent Registered Public Accounting Firm
92
Report of Independent Registered Public Accounting Firm
The Board of Directors and Stockholders of Abraxis BioScience, Inc.
We have audited Abraxis BioScience, Inc.’s internal control over financial reporting as of December 31, 2008, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (the COSO criteria). Abraxis BioScience, Inc.’s management is responsible for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting included above under the heading “Management’s Report on Internal Control over Financial Reporting.” Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, Abraxis BioScience, Inc. maintained, in all material respects, effective internal control over financial reporting as of December 31, 2008, based on the COSO criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the December 31, 2008 consolidated financial statements of Abraxis BioScience, Inc. and subsidiaries and our report dated March 2, 2009 expressed an unqualified opinion thereon.
/s/ Ernst & Young LLP
Los Angeles, California
March 2, 2009
93
(d) Changes in internal controls.
Management has determined that, as of December 31, 2008, there were no changes in our internal control over financial reporting, as such term is defined in Exchange Act Rules 13a-15(f) and 15d-15(f), that occurred during our most recent fiscal quarter that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Item 9B. OTHER INFORMATION
None.
94
PART III
Item 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
The information required by this item will be contained in the Proxy Statement for our 2009 Annual Meeting of Stockholders and is incorporated herein by reference.
Item 11. EXECUTIVE COMPENSATION
The information required by this item will be contained in the Proxy Statement for our 2009 Annual Meeting of Stockholders and is incorporated herein by reference.
Item 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
The information required by this item will be contained in the Proxy Statement for our 2009 Annual Meeting of Stockholders and is incorporated herein by reference.
Item 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
The information required by this item will be contained in the Proxy Statement for our 2009 Annual Meeting of Stockholders and is incorporated herein by reference.
Item 14. PRINCIPAL ACCOUNTANT FEES AND SERVICES
The information required by this item will be contained in the Proxy Statement for our 2009 Annual Meeting of Stockholders and is incorporated herein by reference.
95
PART IV
Item 15. EXHIBITS, FINANCIAL STATEMENTS AND FINANCIAL STATEMENT SCHEDULE
a. (1) Financial Statements
The following consolidated and combined financial statements of Abraxis BioScience, Inc. are included in Part II, Item 8 of this Report:
Report of Independent Registered Public Accounting Firm
Consolidated Balance Sheets at December 31, 2008 and 2007
Consolidated and Combined Statements of Operations for the Years Ended December 31, 2008, 2007 and 2006
Consolidated and Combined Statements of Stockholders’ Equity for the Years Ended December 31, 2008, 2007 and 2006
Consolidated and Combined Statements of Cash Flows for the Years Ended December 31, 2008, 2007 and 2006
Notes to Consolidated and combined Financial Statements
a. (2) Financial Statement Schedule
All schedules are omitted because the required information is not present or is not present in amounts sufficient to require submission of the schedule or because the information required is given in the consolidated and combined financial statements or the notes thereto.
a. (3) Exhibits
The exhibits are as set forth in the Exhibit Index.
96
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Los Angeles, State of California on the 6th day of March, 2009.
| | |
| ABRAXIS BIOSCIENCE, INC. |
| |
| By: | | /s/ PATRICK SOON-SHIONG, M.D. |
| | | Patrick Soon-Shiong, M.D. |
| | Chairman of the Board of Directors and Chief Executive Officer |
POWER OF ATTORNEY
Each person whose signature appears below constitutes and appoints Patrick Soon-Shiong, M.D. and David O’Toole, and each of them, as attorneys-in-fact, each with the power of substitution, for him or her in any and all capacities, to sign any amendment to this Annual Report on Form 10-K and to file the same, with exhibits thereto and other documents in connection therewith, with the Commission, granting to said attorneys-in-fact, and each of them, full power and authority to do and perform each and every act and thing requisite and necessary to be done in connection therewith, as fully to all intents and purposes as he or she might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact or any of them, or their or his substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the Partnership in the capacities and on the dates indicated.
| | | | |
Signature | | Title | | Date |
| | |
/s/ PATRICK SOON-SHIONG, M.D. Patrick Soon-Shiong, M.D. | | Chairman of the Board of Directors and Chief Executive Officer (Principal Executive Officer) | | March 6, 2009 |
| | |
/s/ DAVID O’TOOLE David O’Toole | | Executive Vice President and Chief Financial Officer (Principal Financial and Accounting Officer) | | March 6, 2009 |
| | |
/s/ KIRK K. CALHOUN Kirk K. Calhoun | | Director | | March 6, 2009 |
| | |
/s/ DAVID S. CHEN, PH.D. David S. Chen Ph.D. | | Director | | March 6, 2009 |
| | |
/s/ STEPHEN D. NIMER, M.D. Stephen D. Nimer, M.D. | | Director | | March 6, 2009 |
| | |
/s/ LEONARD S. SHAPIRO Leonard S. Shapiro | | Director | | March 6, 2009 |
97
EXHIBIT INDEX
Exhibit Index
| | |
Exhibit Number | | Description |
| |
| 2.1 | | Separation and Distribution Agreement among APP Pharmaceuticals, Inc., Abraxis BioScience, LLC, APP Pharmaceuticals, LLC and the Registrant (incorporated by reference to Exhibit 2.1 to the Registrant’s Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on December 20, 2007) |
| |
| 3.1 | | Amended and Restated Certificate of Incorporation of the Registrant (incorporated by reference to Exhibit 3.1 to the Registrant’s Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on December 20, 2007) |
| |
| 3.2 | | Amended and Restated Bylaws of the Registrant (incorporated by reference to Exhibit 3.2 to the Registrant’s Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on December 20, 2007) |
| |
| 3.3 | | Certificate of Amendment to Amended and Restated Certificate of Incorporation of the Registrant (incorporated by reference to Exhibit 3.3 to the Registrant’s Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on December 20, 2007) |
| |
| 4.1 | | Reference is made to Exhibits 3.1, 3.2 and 3.3 |
| |
| 4.2 | | Specimen Stock Certificate of the Registrant (incorporated by reference to Exhibit 4.2 to the Registrant’s Amendment #2 to Form 10 Registration Statement with the Securities and Exchange Commission on October 24, 2007) |
| |
| 4.3 | | Registration Rights Agreement by and among the Registrant and certain stockholders of the Registrant as set forth therein (incorporated by reference to Exhibit 4.3 to the Registrant’s Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on December 20, 2007) |
| |
| 10.1 | | Separation and Distribution Agreement among APP Pharmaceuticals, Inc., Abraxis BioScience, LLC, APP Pharmaceuticals, LLC and the Registrant (Reference is made to Exhibit 2.1 hereto) |
| |
| 10.2 | | Tax Allocation Agreement among APP Pharmaceuticals, Inc., Abraxis BioScience, LLC, APP Pharmaceuticals, LLC and the Registrant (incorporated by reference to Exhibit 10.2 to the Registrant’s Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on December 20, 2007) |
| |
| 10.3 | | Transition Services Agreement between APP Pharmaceuticals, Inc. and the Registrant (incorporated by reference to Exhibit 10.3 to the Registrant’s Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on December 20, 2007) |
| |
| 10.4 | | Employee Matters Agreement among APP Pharmaceuticals, Inc., APP Pharmaceuticals, LLC, Abraxis BioScience, LLC and the Registrant (incorporated by reference to Exhibit 10.4 to the Registrant’s Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on December 20, 2007) |
| |
| 10.5* | | Manufacturing Agreement between APP Pharmaceuticals, LLC and the Registrant (incorporated by reference to Exhibit 10.5 to the Registrant’s Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on December 20, 2007) |
| |
| 10.6 | | Lease Agreement between APP Pharmaceuticals, LLC and Abraxis BioScience, LLC for the premises located at 2020 Ruby Street, Melrose Park, Illinois (incorporated by reference to Exhibit 10.6 to the Registrant’s Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on December 20, 2007) |
98
| | |
Exhibit Number | | Description |
| |
| 10.7 | | Lease Agreement between APP Pharmaceuticals, LLC and Abraxis BioScience, LLC for the warehouse facilities located at 2045 N. Cornell Avenue, Melrose Park, Illinois (incorporated by reference to Exhibit 10.7 to the Registrant’s Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on December 20, 2007) |
| |
| 10.8 | | Lease Agreement between APP Pharmaceuticals, LLC and Abraxis BioScience, LLC for the research and development facility located at 2045 N. Cornell Avenue, Melrose Park, Illinois (incorporated by reference to Exhibit 10.8 to the Registrant’s Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on December 20, 2007) |
| |
| 10.9 | | Lease Agreement between Abraxis BioScience, LLC and APP Pharmaceuticals, LLC for the premises located at 3159 Staley Road, Grand Island, New York (incorporated by reference to Exhibit 10.9 to the Registrant’s Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on December 20, 2007) |
| |
| 10.10 | | Form of Indemnification Agreement between the Registrant and each of its executive officers and directors (incorporated by reference to Exhibit 10.10 to the Registrant’s Amendment #1 to Form 10 Registration Statement with the Securities and Exchange Commission on October 5, 2007) |
| |
| 10.11 | | Employment Agreement, dated January 25, 2006, between the Registrant and Carlo Montagner (incorporated by reference to Old Abraxis’ Quarterly Report on Form 10-Q/A filed with the Securities and Exchange Commission on August 10, 2006) |
| |
| 10.12 | | Standard Form Office Lease, dated March 24, 2006, between the Registrant and California State Teacher’s Retirement System, as amended on May 26, 2006, for the premises located at 11755 Wilshire Boulevard, Los Angeles, California (incorporated by reference to Old Abraxis’ Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on November 9, 2006) |
| |
| 10.13 | | Co-Promotion Strategic Marketing Services Agreement, dated April 26, 2006, between the Registrant and AstraZeneca UK Limited (incorporated by reference to Old Abraxis’ Quarterly Report on Form 10-Q/A filed with the Securities and Exchange Commission on August 10, 2006) |
| |
| 10.14 | | Abraxis BioScience, Inc. 2007 Stock Incentive Plan, including forms of agreement thereunder (incorporated by reference to Exhibit 10.15 to the Registrant’s Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on December 20, 2007) |
| |
| 10.15 | | American BioScience, Inc. Restricted Stock Unit Plan I, including a form of agreement thereunder (incorporated by reference to Old Abraxis’ Registration Statement on Form S-8 (File No. 333-133364) filed with the Securities and Exchange Commission on April 18, 2006) |
| |
| 10.16 | | American BioScience, Inc. Restricted Stock Unit Plan II, including a form of agreement thereunder (incorporated by reference to Old Abraxis’ Registration Statement on Form S-8 (File No. 333-133364) filed with the Securities and Exchange Commission on April 18, 2006) |
| |
| 10.17 | | Agreement, dated April 18, 2006, between the Registrant and RSU LLC (incorporated by reference to Old Abraxis’ Quarterly Report on Form 10-Q/A filed with the Securities and Exchange Commission on August 10, 2006) |
| |
| 10.18* | | Aircraft Purchase and Sale Agreement (incorporated by reference to Old Abraxis’ Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on November 9, 2006) |
| |
| 10.19 | | Escrow Agreement, dated April 18, 2006, by and among Abraxis BioScience, our chief executive officer and Fifth Third Bank (Incorporated by reference to Old Abraxis’ Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on May 10, 2006). |
99
| | |
Exhibit Number | | Description |
| |
| 10.20* | | License Agreement, dated as of May 27, 2005, between the Registrant and Taiho Pharmaceutical Co., Ltd. (incorporated by reference to Exhibit 10.10 to the Registrant’s Amendment #3 to Form 10 Registration Statement with the Securities and Exchange Commission on November 2, 2007) |
| |
| 10.21 | | Agreement between APP Pharmaceuticals, Inc. and the Registrant (incorporated by reference to Exhibit 10.22 to the Registrant’s Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on December 20, 2007) |
| |
| 10.22 | | Employment Agreement, dated as of May 22, 2008, between the Registrant and David O’Toole (incorporated by reference to Exhibit 10.22 to the Registrant’s Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on August 14, 2008) |
| |
| 10.23 | | Employment Agreement, dated as of October 6, 2008, between the Registrant and Edward Geehr, M.D. (incorporated by reference to Exhibit 10.23 to the Registrant’s Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on November 14, 2008) |
| |
| 10.24† | | Termination Agreement dated as of November 19, 2008, between Abraxis BioScience, LLC and AstraZeneca UK Limited |
| |
| 14.1 | | Code of Business Conduct (incorporated by reference to Exhibit 14.1 to Registrant’s Annual Report on Form 10-K filed with the Securities and Exchange Commission on March 31, 2008) |
| |
| 21.1† | | List of Subsidiaries |
| |
| 23.1† | | Consent of Independent Registered Public Accounting Firm |
| |
| 24.1 | | Power of Attorney (included on signature page) |
| |
| 31.1† | | Certification of Chief Executive Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 |
| |
| 31.2† | | Certification of Chief Financial Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 |
| |
| 32.1† | | Certification of Chief Executive Officer pursuant to 18 U.S.C. Section 1350, as adopted by Section 906 of the Sarbanes-Oxley Act of 2002 |
| |
| 32.2† | | Certification of Chief Financial Officer pursuant to 18 U.S.C. Section 1350, as adopted by Section 906 of the Sarbanes-Oxley Act of 2002 |
| * | Certain portions of this exhibit have been omitted pursuant to a request for confidential treatment filed with the Securities and Exchange Commission. |
100


