Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes No ?X
State the aggregate market value of the voting and non-voting common equity held by non-affiliates computed by reference to the price at which the common equity was last sold, or the average bid and asked price of such common equity, as of the last business day of the registrant’s most recently computed second fiscal quarter. $6,380,000
The number of shares of the issuer’s common stock issued and outstanding as of June 30, 2010 was 127,500,000 shares.
Documents InPersonNamecorporated By Reference: None
| | | | |
PART I | | | | |
ITEM 1. | | BUSINESS | | |
ITEM 1A. | | RISK FACTORS | | |
ITEM 2. | | PROPERTIES | | |
ITEM 3. | | LEGAL PROCEEDINGS | | |
ITEM 4. | | SUBMISSION OF MATTERS TO A VOTE OF SECURITY HOLDERS | | |
PART II
|
|
|
|
|
ITEM 5. | | MARKET FOR REGISTRANT'S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES | | |
ITEM 6. | | SELECTED FINANCIAL DATA | | |
ITEM 7. | | MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS | | |
ITEM 7A. | | QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK | | |
ITEM 8. | | FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA | | |
ITEM 9. | | CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE | | |
ITEM 9A. | | CONTROLS AND PROCEDURES | | |
ITEM 9B. | | OTHER INFORMATION | | |
PART III
|
|
|
|
|
ITEM 10. | | DIRECTORS, EXECUTIVE OFFICERS, PROMOTERS, CONTROL PERSONS AND CORPORATE GOVERNANCE COMPLIANCE WITH SECTION 16(a) OF THE EXCHANGE ACT. | | |
ITEM 11. | | EXECUTIVE COMPENSATION | | |
ITEM 12. | | SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS | | |
ITEM 13. | | CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR placeCityINDEPENDENCE | | |
ITEM 14. | | PRINCIPAL ACCOUNTANT FEES AND SERVICES | | |
PART IV
|
|
|
|
|
ITEM 15. | | EXHIBITS | | |
|
|
SIGNATURES
|
| |
PART I
Certain statements contained in this Annual Report on Form 10-K constitute “forward-looking statements.” These statements, identified by words such as “plan,” “anticipate,” “believe,” “estimate,” “should,” “expect,” and similar expressions include our expectations and objectives regarding our future financial position, operating results and business strategy. These statements reflect the current views of management with respect to future events and are subject to risks, uncertainties and other factors that may cause our actual results, performance or achievements, or industry results, to be materially different from those described in the forward-looking statements. Such risks and uncertainties include those set forth under the caption “Management’s Discussion and Analysis or Plan of Operation� 48; and elsewhere in this Annual Report. We advise you to carefully review the reports and documents we file from time to time with the Securities and Exchange Commission (the “SEC”), particularly our Quarterly Reports on Form 10-Q and our Current Reports onForm 8-K.
As used in this Annual Report, the terms “we,” “us,” “our,” “Fero” and the “Company” mean Fero Industries, Inc., unless otherwise indicated. All dollar amounts in this Annual Report are expressed in U.S. dollars, unless otherwise indicated.
ITEM 1. Business.
Corporate History
We were inPersonNamecorporated in the State of Colorado on December 11, 2000 under the name Fero Industries, Inc. and were dormant until December 2006. During this time we were a development stage company that had not commenced any operations other than initial PersonNamecorporate formation and capitalization, the building of a central website, www.oil-n-gasbrokerage.net, and the acquisition of our domain names and the development of our business plan. It was our initial intention to create a web portal whereby we planned to serve as an all inclusive information provider for anyone worldwide who was looking to buy, sell or lease anything to do with the exploration and/or production of oil and gas.
In October, 2009, we abandoned the original business plan and began seeking out potential acquisition candidates in the healthcare industry.
On October 13, 2009 Fero Industries, Inc. (the “Registrant”) entered into a Definitive Share Exchange Agreement to acquire Pyro Pharmaceuticals, Inc. of Irvine California. On December 8, 2009 the registrant and Pyro Pharmaceuticals, Inc. mutually terminated the Share Exchange Agreement and Entered in to an Asset Purchase Agreement. Pyro Pharmaceuticals, Inc. is engaged in the business of developing therapeutics against multi-drug resistant infectious microorganisms. The Definitive Agreement provides for the Purchase of Pyro’s assets for shares of the Company’s common stock, with Fero remaining as the parent entity and Pyro as a subsidiary. It is anticipated that a final closing to occur upon completion of Pyro’s audited financial Statements and will be subject to certain terms and conditions as set forth in the definitive agreement. This transaction has been terminated.
On May 23, 2010, Fero Industries, Inc., a Colorado PersonNamecorporation, (the "Company") entered that certain Asset Acquisition Agreement (the "Agreement") with Gvest, Inc., (“Gvest”) an Ontario, Canada PersonNamecorporation. Pursuant to the terms and conditions of the Agreement, the Company acquired certain assets directly related to the manufacturing, sale and distribution of that certain product known as Sucanon, which is an herbal remedy for Type II Diabetes.
4
The acquired assets include all of the intellectual property rights, training, and “know how” to manufacture and produce Sucanon, including sources and suppliers of Sucanon ingredients and mixing equipment; certain associated trademarks and patents ("Acquired Assets"). The Acquired Assets include the exclusive world-wide rights to manufacture, sell and distribute Sucanon. The Company purchased the Acquired Assets for an aggregate purchase price of $250,000. The Agreement contained customary representations and warranties and pre- and post-closing covenants of each party and customary closing conditions. Breaches of the representations and warranties were subject to customary indemnification provisions, subject to specified aggregate limits of liability.
On June 29, 2010, the Company and Gvest determined that all closing conditions had been met and that the transaction should therefore close based on the terms and conditions thereof. On July 1, 2010, the Company received such documents necessary to conduct complete and full due diligence relating to the Acquired Assets.
On July 7, 2010, the Company determined, based on a full review and the completion of due diligence related to the Acquired Assets, the Company has determined that the Acquired Assets will allow the Company to move forward with its new business plan to make, manufacture and distribute Sucanon world-wide.
Overview
Fero’s mission is to acquire healthcare related companies, products, and technologies that have large market potential, improve the quality of care, or have unmet needs. Fero is focused on the medical device, biotechnology, pharmaceutical, nutraceutical, and healthcare IT industries. Fero's mission is to create and enhance shareholder value via a growth-by-acquisition strategy by acquiring synergistic companies, products, and technologies that have large market potential, improve the quality of care, or have unmet needs.
Fero’s initial focus is on diabetes. The Company has acquired the intellectual property and other exclusive world-wide rights related to the production, marketing, and distribution of Sucanon also known as Diab II, a treatment for Type II diabetes.
Sucanon is approved and is sold as an Over-The-Counter ("OTC") herbal remedy for Type II diabetes in Mexico and as a prescription pharmaceutical in Peru. Sucanon has also undergone clinical trials in China and Brazil. Application for United States Food and Drug Administration (“FDA”) approval of Sucanon has not been made. As such, it is not offered for sale nor approved for sale in the United States at this time. The Company will consider applying for FDA approval in the near future.
Current Product
Sucanon is one of only several drugs in the world, belonging to a class of diabetic medications called insulin sensitizers. Insulin sensitizers lower blood sugar by increasing the muscle, fat and liver’s sensitivity to insulin. Insulin sensitizers are blood sugar normalizing or euglycemic drugs that help return the blood sugar to the normal range without the risk of low blood sugars.
5
| |
| 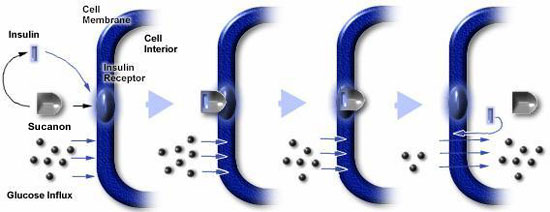
1. Insulin binding to receptors and entering the cell, (which is impeded in NIDDM patients), is essential for the uptake of glucose;
2. Sucanon increases the binding of insulin to its receptors;
3. Sucanon increases the internalization of insulin; and,
4. As a result, Sucanon increases the intracellular level of insulin, which then increases the uptake of glucose. |
Sucanon is a medication that helps the body make better use of its own insulin, the hormone that controls blood sugar levels. Type II Diabetics produce insulin, but their cells gradually lose the ability to absorb and use insulin to get sugar out of the blood stream. Sucanon transports sugar out of the blood stream and into cells where it can be burned. Sucanonparticularly helps muscle cells use insulin and thus draws sugar out of the blood stream.
Sucanon increases sensitivity to insulin which leads to decreased blood sugar levels and a reduction of a wide range of Type II Diabetes symptoms, including: weight gain, fatigue, excess thirst and excess urination. The reduction in blood sugar levels also reduces the possibility of peripheral nerve damage; this damage caused to peripheral nerves by chronic high blood sugar can ultimately lead to impotence in men and amputation of limbs in both men and women.
Sucanon is an herbal medication. It is derived from the combination of the dried root oftricosanthis and molybdenum, a light metal. Sucanon’s chemical name is manitolatodimolybdate.
Clinical Trial Summary:
Clinical trials on Sucanon were performed in China. After submission of a New Drug Application ("NDA") Sucanon was approved by the State Food and Drug Administration (SFDA) of country-regionplaceChina. Subsequent clinical trials were also performed on Sucanon in Brazil, yet the trials have not yet been completed.
Sucanon clinical trials (see Clinical Resultsfor more detailed information on trial results) were shown to reduce the problems and symptoms of Type II Diabetes:
High blood sugar: Clinical studies have shown that Sucanon reduces blood sugar readings by about 25% - 30% and brings high blood sugar back into the normal range (non-fasting blood sugar is above 200 mg/dL (milligrams per deciliter) or fasting blood sugar is above 126 mg/dL).
6
Fatigue: Clinical studies have shown that Sucanon reduces fatigue. Fatigue is a frequent symptom of Type II Diabetes or a pre-diabetic condition called Impaired Glucose Tolerance.
Weight gain: Clinical studies have shown that people who have taken Sucanon report weight loss along with increased energy. Very often, people who are diabetic or pre-diabetic gain weight because their insulin-resistance leads to sugar being converted into fat instead of being burned to produce energy.
Excess thirst and urination: Clinical studies have shown that Sucanon reduces excess thirst and excess urination. Higher-than-normal levels of blood sugar instigate thirst, which in turn leads to increased frequency of urination.
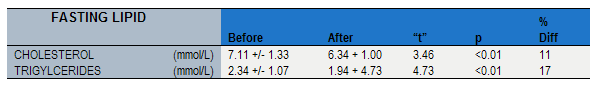
High cholesterol and triglyceride levels: Clinical studies have shown that Sucanon reduces the levels of cholesterol and triglycerides. People who are diabetic or pre-diabetic often have elevated cholesterol and triglyceride levels. Elevated cholesterol and triglycerides significantly increase the risk of heart disease.
Side effects: Clinical studies have shown that Sucanon showed no side effects. This setsSucanon apart from many other anti-diabetic products, which can have effects on digestion, the liver, or the heart.
Toxicity: Clinical studies have shown that Sucanon toxicity was undistinguishable from the placebo. In addition, Sucanon showed no carcinogenicity, mutagenicity, and teratogenicity in mice.
Clinical Results
Clinical Experience
The clinical benefits of Sucanon were convincingly demonstrated in a double-blind, randomized, placebo- & Glibenclamide-controlled, multi-center, efficacy and safety study in 370 adult patients with Type II diabetes. Sucanon was administered as tablets, one in the morning and one in the evening. The duration of the study was 6 months: four months treatment, preceded by one month screening evaluation, and followed by one month post-treatment follow-up. Glibenclamide is a commonly prescribed sulfonylurea; its benefits and limitations have been well known to diabetologists for over a decade. The parameters of response to therapy included an evaluation of the changes in clinical signs and symptoms of diabetes, an alteration in the blood and urine measurements of glucose metabolism, and an alteration in blood lipid levels.
The results indicated that the parameters of disease activity in patients receiving either Glibenclamide or Sucanon responded in a highly relevant clinical manner and that the differences from baseline measurements were statistically highly significant (p values <0.01). The lack of response in the group of patients who were randomized to receive placebo was also unequivocal, where the effect of administration was clinically small or non-existent, and the baseline to treatment difference was statistically insignificant (p value >0.05). An extract of the data is summarized in the following graphs and tables.
7
Table 1
Changes in glucose abnormalities in 370 Type II diabetic patients in 3 treatment groups of the randomized, double-blind, controlled study (before treatment and at the end of treatment analyses)
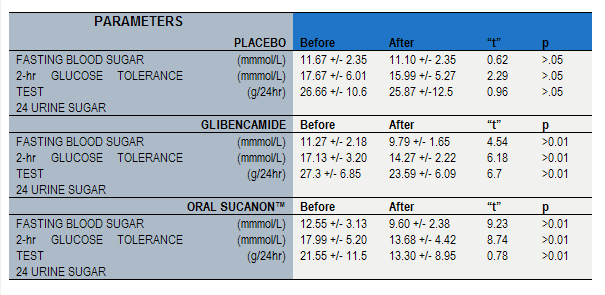
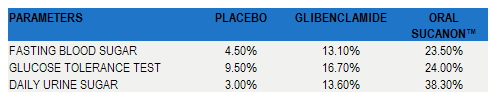
Table 2
Results from table 1 expressed as “Percent Improvement” (baseline to end of treatment)
Response to therapy was documented not only by a loss of, or a reduction in, disease related symptoms which included polyuria, polvdipsia, polyphagia, and fatigue, but also by the improvement in objective parameters of disease, namely, a reduction to normal or near normal levels in the elevated fasting blood glucose, and urinary sugar, and a normalization of the 100 g - oral glucose tolerance test. The objective results are given in table 1 above where the mean and standard deviations for these Values are listed, as well as the calculated "t" and “p" values. Given that the coefficient of variance of baseline values for the three treatment groups is small, and the patient number per group relatively large (n = 123), a between treatment group comparison is not unreasonable. These calculations (not shown) reveal that the improvements associated with therapy for both the Glibenclamide group of patients and the Sucanon group of patients were both better than placebo for all objective parameters measured to a level that was statistically significant (p Values <0.05 to <0.01 respectively. This was not surprising from the t values listed in table 1. The difference in reduction of fasting blood glucose between the latter treatment groups was not statistically significant (p value >.0.05).
Table 3
Sucanon associated improvements in blood lipid levels
8
Pre-clinical pharmacology
Pre-clinical in vivo and in vitro studies have identified that intravenous and oral Sucanon is pharmacodynamically active in diabetic rats, and out-performed all biguanides and sulfonylureas tested in those models. When added to rat muscle cells, its critical influence commences in seconds as it up-regulates insulin receptors, in a manner not yet understood, with the resultant increase in insulin endocytosis, uptake of glucose, and L-leucine effects, all of which last more than an hour.
In single-dose rat studies, peak response in lowering blood glucose takes 2 to 4 hours to occur, and the effect is lost by about 10 hours. Multiple oral dosing in rats (48 days) and up to 4 months in man, shows no loss of activity. Clear-cut pharmacological dose-response features were documented. Sucanon is also superior to other hypoglycemic agents in these models.
Toxicity
The therapeutic index is so large (10,000 in mice) that its margin of safety must be unique in the armamentarium of drugs for the treatment of diabetes. Carcinogenicity, mutagenicity, and teratogenicity toxicities were not found in mice. Chronic dosing in dogs and rats at 2000 times the therapeutic dose was free of any toxicity.
Sucanon Regulatory Approvals:
Sucanon has been approved for prescription sale in Peru and has been approved as an over-the-counter (non-prescription) product in Mexico. Application for United States FDA regulatory approval has not yet been made. Thus, doctors cannot prescribe nor purchase Sucanon in the United States. However, Type II diabetics can buy Sucanon for their own use and have it delivered to them from Mexico under the U.S. FDA’s “personal importation” guidelines. A similar program exists for Type II diabetics in Canada who wish to buy Sucanon for their own use.
Growth Strategy
With this acquisition completed, Fero's strategy is to expand its product offering by acquiring other products in the bioceutical market place.
Fero's growth-by-acquisition strategy is critical to its current operations. Growing an existing business using strategies to increase sales and improve operations (i.e., organic growth) should be a constant, on-going goal of any business.
The benefits of growing through acquisition include:
·
Acquisitions can produce desired growth results much quicker than traditional organic growth strategies;
·
Effective means of developing a competitive advantage by acquiring companies to address existing weaknesses or needs;
·
Proven method of rapidly growing sales and income by acquiring new customers;
·
Strategic acquisitions can be an effective means to diversify your customer base and/or enter new markets to reduce reliance on any one customer or industry;
·
Acquisitions can be accomplished on a leveraged basis with financing to produce a return-on-investment superior to traditional organic growth results;
·
Acquiring a business can be an excellent way to develop a competitive advantage;
·
Acquiring a niche competitor that provides a related service your business doesn't currently offer can provide an immediate competitive advantage; and
·
Acquisition of another business can be an effective means of quickly increasing revenue and income while strategically adding customers to diversify your customer base.
9
In summary, the combination of the following key parameters differentiates Fero from other companies on the market:
·
To maximize benefits to shareholders, our PersonNamecorporate focus is devoted to the acquisition of synergistic companies, products, and technologies which bring value added revenue and market opportunities;
·
An experienced Management Team and Advisory Board directing all activities;
·
A pipeline of potential acquisition candidates;
·
A well-implemented strategy of acquiring candidates which have large market potential and significant unmet needs;
·
A highly flexible organization and low fixed overheads due to our innovative outsourcing strategy; and
·
A highly professional approach characterized by swift, responsive and well thought out decision-making and ability to redirect business efforts quickly and at low cost, to maximize partnering activities.
Competition
Our competitors include fully integrated pharmaceutical companies, nutraceutical, and biotechnology companies, universities and public and private research institutions. Many of the organizations competing with us have substantially greater capital resources, larger research and development staffs and facilities, greater experience in drug development and in obtaining regulatory approvals, and greater manufacturing and marketing capabilities than we do.
Insurance
Due to the nature of the business conducted by the Company in the past, we do not currently maintain any insurance. However, we intend to acquire and maintain insurance appropriate to our new activities in the future on such terms that management shall deem to be commercially reasonable.
Patents, Trademarks, Licenses, Franchises, Concessions, Royalty Agreements and Labor Contracts
Pursuant to the Acquisition Agreement, we acquired the following intellectual property rights including patents and trademarks Intellectual Property.
Trademarks:
| | | |
MARK |
REG./APP. NO |
REG./ APP. DATE |
JURISDICTION |
Sucanon | 3255374 | June 26, 2007 | United States |
Sucanon | 720203 | August 2, 2001 | Mexico |
Sucanon Premix* | 1421376 | December 10, 2008 | Canada |
10
We previously applied for the trademark for Sucanon Premix in the International class 1 which consists of pharmaceutical preparations for the treatment of Type I and Type II diabetes. In February of 2010 we received a default notice and the application was thereby deemed abandoned in May of 2010. We are in the process of reviving the patent.
Patents:
| | |
ABSTRACT |
PATENT NO. |
ISSUE DATE |
Method and composition for the treatment of diabetes | 6,153,632 | November 28, 2000 |
Employees
We currently have no employees other than our officers/directors. However, we intend to seek out and identify qualified persons to assist the Company in implementing its new business plan and operations.
Government Regulation
The development, manufacture and commercialization of pharmaceutical products is generally subject to extensive regulation by various federal and state governmental entities. The FDA, which is the principal U.S. regulatory authority over pharmaceutical products, has the power to seize adulterated or misbranded products and unapproved new drugs, to request their recall from the market, to enjoin further manufacture or sale, to publicize certain facts concerning a product and to initiate criminal proceedings. As a result of federal statutes and FDA regulations pursuant to which new pharmaceuticals are required to undergo extensive and rigorous testing, obtaining pre-market regulatory approval requires extensive time and expenditures. Under the Federal Food, Drug, and Cosmetic Act, or FFDCA, as amended (21 U.S.C. 301 et. seq.), a new drug may not be commercialized or otherwise distributed in the U.S. without the prior approval of the FDA or p ursuant to an applicable exemption from the FFDCA.
We have incurred losses since our inception. For the year ended June 30, 2010, we generated revenue of $0, and incurred a net loss of $269,655. At June 30, 2010 we had working capital deficit of $292,405 and an accumulated deficit of $329,005. Our auditors, have expressed substantial doubt about our ability to continue as going concern.
Our principal offices are located at 254-16 Midlake Boulevard SE Calgary Alberta Canada and our telephone number is (403) 827-7936. We are a PersonNamePersonNameColorado PersonNamecorporation.
ITEM 1A. Risk Factors.
We currently rely on one product, and have no product sales to date, and cannot give assurance that there will be any sales in the future.
Our only product, Sucanon has just recently been approved for sale to the general public in Mexico and Peru; yet, no revenues have been generated to date from product sales. There is no guarantee that we will ever be able to market and promote Sucanon. To become profitable, we will have to successfully market and promote Sucanon and obtain regulatory approvals in other jurisdictions. There can be no assurance that our marketing and promotion efforts will be successful; that we will be able to obtain all required regulatory approvals; that we will be able to manufacture our products at an acceptable cost and with acceptable quality; or that our products can be successfully marketed in the future. We currently do not expect to receive significant revenues from the sale of any of our products for at least the next several years.
11
We will need additional funds to conduct our planned research and development efforts; we may not be able to obtain such funds and may never become profitable.
We have accumulated a large deficit since inception that has primarily resulted from the significant research and development expenditures we have made in seeking to identify and validate new asset acquisitions. We expect our losses and operating expenses will continue to be substantial until we can market and sell Sucanon. We have substantially less money than we need to further our marketing efforts to develop a definitive market for Sucanon.
We have incurred losses since inception and anticipate that we will continue to incur losses for the foreseeable future and there is a question about our ability to continue as a going concern.
We are a development stage company with a limited operating history. We expect to continue to incur significant and increasing operating losses, in the aggregate and on a per share basis, for the next several years. These losses, among other things, have had and will continue to have an adverse effect on our stockholders’ equity, net current assets and working capital. Because of the numerous risks and uncertainties associated with developing new drugs, we are unable to predict the extent of any future losses or when we will become profitable, if at all. Currently, we have not generated any product revenue. We have financed our operations and internal growth primarily through the sale of equity securities. We have devoted substantially all of our efforts to asset acquisition. Our Financial Statements includes an explanatory paragraph that indicates the financial statements are affected by conditions and events that cast substantial doubt on our ability to continue as a going concern. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
The current global economic environment poses severe challenges to our business strategy, which relies on access to capital from the markets and our collaborators, and creates other financial risks for us.
The global economy, including credit markets and the financial services industry, has been experiencing a period of substantial turmoil and uncertainty. These conditions have generally made equity and debt financing more difficult to obtain, and may negatively impact our ability to complete financing transactions. The duration and severity of these conditions is uncertain, as is the extent to which they may adversely affect our business and the business of current and prospective collaborators and vendors. If the global economy does not improve or worsens, we may be unable to secure additional funding to sustain our operations or to find suitable partners to advance our internal programs, even if we receive positive results from our research and development or business development efforts.
If we do not partner or raise additional funds, we may have to further curtail our activities.
While we believe our strategy will conserve resources, our ability to advance our marketing efforts is limited. Without additional capital or funding from prospective partners, we will need to significantly curtail some of our current and planned activities and expenditures.
We may suffer losses from product liability claims.
It is possible that Sucanon could cause adverse events to patients, such as immunologic or allergic reactions. These reactions may not be observed in clinical trials, but may nonetheless occur after commercialization. If any of these reactions occur, they may render Sucanon ineffective in some patients and our sales would suffer. We may be susceptible to product liability lawsuits from events arising out of the use of Sucanon. If product liability lawsuits are brought against us, we may incur substantial liabilities and may be required to limit commercialization of our products. Our business exposes us to potential product liability risks, which are inherent in the testing, manufacturing, marketing and sale of bioceutical products. We may not be able to avoid product liability claims.
12
Product liability insurance for the pharmaceutical and biotechnology industries is generally expensive, if available at all. If we are unable to protect against potential product liability claims, we may be unable to market Sucanon A successful product liability claim brought against us in excess of our insurance coverage may cause us to incur substantial liabilities and, as a result, our business may fail.
Our commercial success depends significantly on our ability to develop and commercialize our potential products without infringing the intellectual property rights of third parties.
Our commercial success will depend, in part, on our not infringing the patents or proprietary rights of third parties. Third parties that believe we are infringing on their rights could bring actions against us claiming damages and seeking to enjoin clinical testing, manufacturing and marketing of the affected product or products. If we become involved in any litigation, it could consume a substantial portion of our resources, regardless of the outcome of the litigation. If any of these actions are successful, we could be required, in addition to any potential liability for damages, to obtain a license to continue to manufacture or market the affected product, in which case we may be required to pay substantial royalties or grant cross-licenses to our patents. However, any such license may not be available on acceptable terms or at all. Ultimately, we could be prevented from commercializing a product, or forced to cease some aspect of our business operations, as a result of patent infringement claims, which would harm our business. We may enter into licensing agreements with third party intellectual property owners for use of their property in connection with our potential products in order to ensure that such third party’s rights are not infringed.
Although we are not aware that any of our intended potential products would materially infringe the rights of others, a claim of infringement may be asserted against us and any such assertion may result in costly litigation or may require us to obtain a license in order to make, use, or sell our products. Third parties may assert infringement claims against us in the future with respect to current or future products. Any such claims or litigation, with or without merit, could be costly and a diversion of management’s attention, which could have a material adverse effect on our business, operating results and financial condition. Adverse determinations in such claims or litigation could harm our business, operating results and financial condition.
We may experience delays in obtaining required regulatory approvals in the U.S. to market our potential proposed product candidates.
Delays in regulatory approval, limitations in regulatory approval and withdrawals of regulatory approval may have a negative impact on our results of operations. If we experience significant delays in testing or approvals, our product development costs, or our ability to license product candidates, will increase. If the FDA grants regulatory approval of a product, this approval will be limited to those disease stages and conditions for which the product has been demonstrated through clinical trials to be safe and effective. Any product approvals that we receive in the future could also include significant restrictions on the use or marketing of our products. Product approvals, if granted, can be withdrawn for failure to comply with regulatory requirements or upon the occurrence of adverse events following commercial introduction of the potential products.
Failure to comply with applicable FDA or other applicable regulatory requirements may result in criminal prosecution, civil penalties, recall or seizure of products, total or partial suspension of production or injunction, as well as other regulatory action against our product candidates or us. If approvals are withdrawn for a potential product, or if a potential product were seized or recalled, we would be unable to sell or license that product and our revenues would suffer. In addition, outside the U.S., our ability to market any of our potential products is contingent upon receiving market application authorizations from the appropriate regulatory authorities and these foreign regulatory approval processes include all of the risks associated with the FDA approval process described above.
13
If competitors develop and market products that offer advantages as compared to our product candidates, our commercial opportunities will be limited.
Other companies have product candidates in development to treat the conditions we are seeking to ultimately treat. If these competitors are able to develop products that are more effective, have fewer side effects, are less expensive or offer other advantages as compared to our product candidates, our commercial opportunities will be limited. Furthermore, if our competitors commercialize competing products before we do, then our ability to penetrate the market and sell our products may be impaired. Our competitors include fully integrated pharmaceutical companies and biotechnology companies, universities and public and private research institutions. Many of the organizations competing with us have substantially greater capital resources, larger research and development staffs and facilities, greater experience in drug development and in obtaining regulatory approvals, and greater manufacturing and marketing capabilities than we do.
Research and development programs are expensive, time consuming, uncertain and susceptible to change, interruption, delay or termination.
Drug research and development programs are very expensive, time consuming and difficult to design and implement. Our projects are in various stages of research and development and are prone to the risks of failure inherent in drug research and development. We will need to complete additional research, development, clinical trials and preclinical studies before we can demonstrate that our drug candidates will be safe and effective to the satisfaction of the FDA and similar non-US regulatory authorities. These trials are expensive and uncertain processes that take years to complete. Failure can occur at any stage of the process, and successful early clinical or preclinical trials do not ensure that later trials or studies will be successful. In addition, the commencement or completion of our planned clinical trials could be substantially delayed or prevented by several factors, including:
·
limited number of, and competition for, suitable patients required for enrollment in our clinical trials;
·
limited number of, and competition for, suitable sites to conduct our clinical trials;
·
delay or failure to obtain FDA approval or agreement to commence a clinical trial;
·
delay or failure to obtain sufficient supplies of our drug candidates for our clinical trials;
·
delay or failure to reach agreement on acceptable clinical trial agreement terms or clinical trial protocols with prospective sites or investigators; and
·
delay or failure to obtain institutional review board, or IRB, approval to conduct a clinical trial at a prospective site.
Even if the results of our research and development programs are favorable, the development programs for our prospective drug candidates may take significantly longer than expected to complete, if they are completed at all.
We may engage in new partnerships and other strategic transactions that could impact our liquidity, increase our expenses and present significant distractions to our management.
From time to time we consider strategic transactions, such as out-licensing or in-licensing of compounds or technologies, acquisitions of companies and asset purchases. Additional potential transactions we may consider include a variety of different business arrangements, including strategic partnerships, joint ventures, spin-offs, restructurings, divestitures, business combinations and investments. Any such transaction may require us to incur non-recurring or other charges, may increase our near- and long-term expenditures and may pose significant integration challenges, require additional expertise or disrupt our management or business, which could harm our operations and financial results.
14
As part of an effort to enter into significant transactions, we conduct business, legal and financial due diligence with the goal of identifying and evaluating material risks involved in the transaction. Despite our efforts, we ultimately may be unsuccessful in ascertaining or evaluating all such risks and, as a result, might not realize the intended advantages of the transaction. If we fail to realize the expected benefits from any transaction we may consummate, whether as a result of unidentified risks, integration difficulties, regulatory setbacks or other events, our business, results of operations and financial condition could be adversely affected.
Drug discovery and development is intensely competitive in the therapeutic areas on which we focus. If our competitors develop treatments that are approved faster, marketed better, less expensive or demonstrated to be more effective or safer than our drug candidates, our commercial opportunities will be reduced or eliminated.
Many companies are pursuing the development of new drugs that target the same diseases and conditions that we target. Many of our competitors, particularly large pharmaceutical companies, have substantially greater research, development and marketing capabilities and greater financial, scientific and human resources than we do. Companies that complete clinical trials, obtain required regulatory agency approvals, and commence commercial sale of their drugs before we do for the same indication may achieve a significant competitive advantage, including certain patent and FDA marketing exclusivity rights. In addition, our competitors may develop drugs with fewer side effects, more desirable characteristics (such as route of administration or frequency of dosing) or better efficacy than our drug candidates or drugs, if any, for the same indication. Our competitors may also market generic or other drugs that compete with our drugs at a lower pri ce than our drugs, which may negatively impact our drug sales, if any. Any results from our research and development efforts, or from our joint efforts with our existing or any future collaborators, may not compete successfully with existing or newly discovered products or therapies.
Collaborative relationships may lead to disputes and delays in drug development and commercialization.
We may in the future have conflicts with our prospective collaborators, such as conflicts concerning the interpretation of preclinical or clinical data, the achievement of milestones, or the ownership of intellectual property. If any conflicts arise with prospective collaborators, such collaborators may act in a manner that is adverse to our interests. Any such disagreement could result in one or more of the following, each of which could delay, or lead to termination of, development or commercialization of our partnered drug candidates, and in turn prevent us from generating revenues:
·
unwillingness on the part of a collaborator to pay us research funding, milestone payments or royalties that we believe are due to us under a collaboration;
·
uncertainty regarding ownership of intellectual property rights arising from our collaborative activities, which could prevent us from entering into additional collaborations;
·
unwillingness on the part of a collaborator to keep us informed regarding the progress of its development and commercialization activities or to permit public disclosure of the results of those activities;
·
slowing or cessation of a collaborator’s development or commercialization efforts with respect to our drug candidates; or
·
litigation or arbitration.
15
Even if we receive regulatory approval to commercialize Sucanon, our ability to generate revenues from any resulting products will be subject to a variety of risks, many of which are out of our control.
Even if our potential drug candidate obtains regulatory approval, the resulting product may not gain market acceptance among physicians, patients, healthcare payers or the medical community. We believe that the degree of market acceptance and our ability to generate revenues from such products will depend on a number of factors, including:
·
timing of market introduction of competitive drugs;
·
efficacy and safety of our drug candidates;
·
prevalence and severity of any side effects;
·
potential or perceived advantages or disadvantages over alternative treatments;
·
strength of sales, marketing and distribution support;
·
price of our future products, both in absolute terms and relative to alternative treatments;
·
the effect of current and future healthcare laws on our drug candidates;
·
availability of coverage and reimbursement from government and other third-party payers; and
·
product labeling or product insert requirements of the FDA or other regulatory authorities.
If our potential approved drugs, if any, fail to achieve market acceptance, we may not be able to generate significant revenue to achieve or sustain profitability.
Our operations might be interrupted by the occurrence of a natural disaster or other catastrophic event.
We depend on our facilities and on our collaborators, contractors and vendors for the continued operation of our business. Natural disasters or other catastrophic events, including terrorist attacks, interruptions in the supply of natural resources, political and governmental changes, wildfires and other fires, explosions, actions of animal rights activists, earthquakes and wars could disrupt our operations or those of our collaborators, contractors and vendors. Even though we believe we carry commercially reasonable business interruption and liability insurance, and our contractors may carry liability insurance that protect us in certain events, we might suffer losses as a result of business interruptions that exceed the coverage available under our and our contractors’ insurance policies or for which we or our contractors do not have coverage. For example, we are not insured against a terrorist attack. Any natural disaster or catastrophic event could have a significant negative impact on our operations and financial results. Moreover, any such event could delay our research and development programs.
The Company’s stock price may be volatile.
The market price of the Company’s common stock is likely to be highly volatile and could fluctuate widely in price in response to various factors, many of which are beyond the Company’s control, including the following:
·
services by the Company or its competitors;
·
additions or departures of key personnel;
·
the Company’s ability to execute its business plan;
·
operating results that fall below expectations;
·
loss of any strategic relationship;
·
industry developments;
·
economic and other external factors; and
·
period-to-period fluctuations in the Company’s financial results.
16
In addition, the securities markets have from time to time experienced significant price and volume fluctuations that are unrelated to the operating performance of particular companies. These market fluctuations may also materially and adversely affect the market price of the Company’s common stock.
FINRA sales practice requirements may limit a stockholder’s ability to buy and sell our stock.
The Financial Industry Regulatory Authority (“FINRA”) has adopted rules that relate to the application of the SEC’s penny stock rules in trading our securities and require that a broker/dealer have reasonable grounds for believing that the investment is suitable for that customer, prior to recommending the investment. Prior to recommending speculative, low priced securities to their non-institutional customers, broker/dealers must make reasonable efforts to obtain information about the customer’s financial status, tax status, investment objectives and other information.
Under interpretations of these rules, FINRA believes that there is a high probability that speculative, low priced securities will not be suitable for at least some customers. FINRA’s requirements make it more difficult for broker/dealers to recommend that their customers buy our common stock, which may have the effect of reducing the level of trading activity and liquidity of our common stock. Further, many brokers charge higher transactional fees for penny stock transactions. As a result, fewer broker/dealers may be willing to make a market in our common stock, reducing a shareholder’s ability to resell shares of our common stock.
The Company’s common stock is currently deemed to be “penny stock”, which makes it more difficult for investors to sell their shares.
The Company’s common stock is and will be subject to the “penny stock” rules adopted under section 15(g) of the Exchange Act. The penny stock rules apply to companies whose common stock is not listed on the NASDAQ Stock Market or other national securities exchange and trades at less than $5.00 per share or that have tangible net worth of less than $5,000,000 ($2,000,000 if the company has been operating for three or more years). These rules require, among other things, that brokers who trade penny stock to persons other than “established customers” complete certain documentation, make suitability inquiries of investors and provide investors with certain information concerning trading in the security, including a risk disclosure document and quote information under certain circumstances. Many brokers have decided not to trad e penny stocks because of the requirements of the penny stock rules and, as a result, the number of broker-dealers willing to act as market makers in such securities is limited. If the Company remains subject to the penny stock rules for any significant period, it could have an adverse effect on the market, if any, for the Company’s securities. If the Company’s securities are subject to the penny stock rules, investors will find it more difficult to dispose of the Company’s securities.
17
The elimination of monetary liability against the Company’s directors, officers and employees under Colorado law and the existence of indemnification rights to the Company’s directors, officers and employees may result in substantial expenditures by the Company and may discourage lawsuits against the Company’s directors, officers and employees.
The Company’s certificate of inPersonNamecorporation contains a specific provision that eliminate the liability of directors for monetary damages to the Company and the Company’s stockholders; further, the Company is prepared to give such indemnification to its directors and officers to the extent provided by Colorado law. The Company may also have contractual indemnification obligations under its employment agreements with its executive officers. The foregoing indemnification obligations could result in the Company incurring substantial expenditures to cover the cost of settlement or damage awards against directors and officers, which the Company may be unable to recoup. These provisions and resultant costs may also discourage the Company from bringing a lawsuit against d irectors and officers for breaches of their fiduciary duties and may similarly discourage the filing of derivative litigation by the Company’s stockholders against the Company’s directors and officers even though such actions, if successful, might otherwise benefit the Company and its stockholders.
ITEM 2. Description of Property.
The mailing address of our business is 254-16 Midlake Boulevard SE Calgary AB Canada Our President provides office space to us at no charge. The cost of the donated premises is valued at $0 per month on our financial statements.
We own no real estate holdings and we have no policy to acquire assets for possible capital gain or income.
ITEM 3. Legal Proceedings
We are not aware of any legal proceedings for and or against us.
ITEM 4. Submission of Matters to a Vote of Securities Holders.
As of June 30, 2010 there were no submissions of matters that require a vote of shareholders.
PART II
Item 5. Market for Registrants Common Equity, Related Stockholder Matters and Issuer Purchase of Equity Securities.
Our shares of common stock commenced trading on the OTC Bulletin Board under the symbol FERO on August 7th, 2008 On December 5, 2008 the company completed a 5 to 1 forward split bring the total issued and outstanding to 25,500,000 and the change of our trading symbol from FERO to FROI on the Over the Counter Bulletin Board.
| | |
QUARTER | HIGH ($) | LOW ($) |
1st Quarter 2010 | $.13 | $.08 |
2nd Quarter 2010 | $.11 | $.05 |
3rd Quarter 2010 | $.05 | $.03 |
18
Holders of Common Stock
As of June 30, 2010, there were 14 registered shareholders of our common stock.
Dividends
On April 15th, 2010 the Board of Directors of Fero Industries (the Registrant) passed a resolution declaring a stock dividend of four (4) shares of common stock for each share held, of record as of April 20, 2010. The common shares of the Registrant will be considered Ex Dividend on April 21, 2010. Upon issuance of the dividend shares, this will bring the total issued and outstanding shares to: 127,500,000.
Recent Sales Of Unregistered Securities
On December 11, 2000 we issued 15,000,000 shares of our common stock to our President and Chief Executive Officer, Kyle Schlosser for $600 in return for his time effort and expense of forming the company and keeping it in good standing. These shares were issued pursuant to the exemption provided by Regulation 4(2) and Regulation S of the Securities Act of 1933.
On December 28, 2006 we issued 12,500,000 shares of our common stock to our Secretary/Treasurer and Chief Financial Officer, Leigh-Ann Squire for $500 in return for his agreement to join our Board of Directors, become an officer of the registrant and his agreement to provide the computer and internet expertise in constructing our websites and providing the server for operation of the sites, at no charge. These shares were issued in reliance on the exemption provided by Section 4(2), Rule 506 and Regulation S of the Act.
On April 20, 2007 we issued 12,500,000 shares of our common stock to Mr. Jerry Capehart of Grand Prairie, Texas for $500 as partial payment for seventeen domain names relating to the oil and gas industry.
On April 30, 2007 we issued 77,500,000 shares of our common stock to thirty-one (31) non-US persons for $31,000. These shares were issued in reliance on the exemption provided by Regulation S promulgated under the Securities Act of 1933, as amended.
On May 10, 2007 we issued 10,000,000 shares of our common stock to three US individuals. These shares were issued in reliance on the exemption provided by Section 4(2) of the Securities Act of 1933, as amended.
* No advertising or general solicitation was employed in offering the securities. The offerings and sales were made to a limited number of persons, all of whom were accredited investors, business associates of the Company or executive officers of the Company, and transfer was restricted by the Company in accordance with the requirements of the Securities Act of 1933. In addition to representations by the above-referenced persons, we have made independent determinations that all of the above-referenced persons were accredited or sophisticated investors, and that they were capable of analyzing the merits and risks of their investment, and that they understood the speculative nature of their investment.
19
ITEM 6. Selected Financial Data.
Summary of Statements of Operations of Fero
Year Ended June 30, 2010 and 2009
| | | | |
| | June 30, 2010 | | June 30, 2009 |
| | | | | | |
Sales | | $ | Nil | | $ | Nil |
Gross Profit | | $ | Nil | | $ | Nil |
Net Income | | $ | Nil | | $ | Nil |
Net Income Per Share, diluted | | $ | Nil | | $ | Nil |
Summary of Balance Sheets of Fero as at June 30, 2010 and 2009
| | | | |
| | June 30, 2010 | | June 30, 2009 |
| | | | | | |
Working Capital | | $ | (292,405) | | | (22,750) |
Total Assets | | $ | - | | | - |
Stockholders’ Equity | | $ | (292,405) | | | (22,750) |
ITEM 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations.
Forward-Looking Statements
The information in this report contains forward-looking statements. All statements other than statements of historical fact made in report are forward looking. In particular, the statements herein regarding industry prospects and future results of operations or financial position are forward-looking statements. These forward-looking statements can be identified by the use of words such as “believes,” “estimates,” “could,” “possibly,” “probably,” anticipates,” “projects,” “expects,” “may,” “will,” or “should” or other variations or similar words. No assurances can be given that the future results anticipated by the forward-looking statements will be achieved. Forward-looking statements reflect management’s current expectations and are inherently uncertain. Our ac tual results may differ significantly from management’s expectations.
The following discussion and analysis should be read in conjunction with our financial statements, included herewith. This discussion should not be construed to imply that the results discussed herein will necessarily continue into the future, or that any conclusion reached herein will necessarily be indicative of actual operating results in the future. Such discussion represents only the best present assessment of our management.
We have incurred recurring losses to date. Our financial statements have been prepared assuming that we will continue as a going concern and, accordingly, do not include adjustments relating to the recoverability and realization of assets and classification of liabilities that might be necessary should we be unable to continue in operation.
We expect we will require additional capital to meet our long term operating requirements. We expect to raise additional capital through, among other things, the sale of equity or debt securities.
20
Liquidity and Capital Resources
As of June 30, 2010 we had no available cash. We plan to continue to provide for our capital needs by issuing debt or equity securities or receiving advances from shareholders or our officers and directors.
We will require additional financing in order to complete our stated plan of operations for the next twelve months. We believe that we will require additional financing to carry out our intended objectives during the next twelve months. There can be no assurance, however, that such financing will be available or, if it is available, that we will be able to structure such financing on terms acceptable to us and that it will be sufficient to fund our cash requirements until we can reach a level of profitable operations and positive cash flows. If we are unable to obtain the financing necessary to support our operations, we may be unable to continue as a going concern. We currently have no firm commitments for any additional capital.
The downturn in the United States stock and debt markets could make it more difficult to obtain financing through the issuance of equity or debt securities. Even if we are able to raise the funds required, it is possible that we could incur unexpected costs and expenses, fail to collect significant amounts owed to us, or experience unexpected cash requirements that would force us to seek alternative financing. Further, if we issue additional equity or debt securities, stockholders may experience additional dilution or the new equity securities may have rights, preferences or privileges senior to those of existing holders of our shares of common stock. If additional financing is not available or is not available on acceptable terms, we will have to curtail our operations.
We presently do not have any available credit, bank financing or other external sources of liquidity. Due to our brief history and historical operating losses, our operations have not been a source of liquidity. We will need to obtain additional capital in order to expand operations and become profitable. In order to obtain capital, we may need to sell additional shares of our common stock or borrow funds from private lenders. There can be no assurance that we will be successful in obtaining additional funding.
To date, we have generated no revenues and have incurred operating losses in every quarter. These factors among others may raise substantial doubt about our ability to continue as a going concern.
Results of Operations
For the Year Ended June 30, 2010 compared to the Year Ended June 30, 2009
We are a development stage company and have no revenues to date.
We incurred operating expenses of $269,655 and $13,150 for the years ended June 30, 2010 and 2009, respectively. The increase of $256,505 is a result of the acquisition of Sucanon, increase in professional fees and general and administrative expenses over the prior period. The increase in our operating expenses for the year ended June 30, 2010 was a result of increased administrative expenses and acquisition costs in connection with our ongoing development efforts.
During the year ended June 30, 2010, we recognized a net loss of $269,655 compared to a net loss of $13,150 for the year ended June 30, 2009. The increase was a result of the increase in acquisition and operational expenses as discussed above.
Liquidity and Capital Resources
At June 30, 2010, we had total assets of $0. At June 30, 2010, we had total current liabilities of $292,405, consisting of accounts payable of $6,150, advances from shareholders of $36,255 and a promissory note payable of $250,000.
21
During the year ended June 30, 2010, we used cash of $23,755 in operations, and during the year ended June 30, 2009, we used cash in operations of $2,900.
During the year ended June 30, 2010 and 2009, we did not have any cash flows from investing activities.
During the year ended June 30, 2010, we received $23,755 from our financing activities. During the Year ended June 30, 2009, we received $2,500 from our financing activities.
ITEM 7A. Quantitative and Qualitative Disclosures About Market Risk.
We do not hold any derivative instruments and do not engage in any hedging activities. Because most of our purchases and sales will made in Canadian dollars, any exchange rate change affecting the value of the in Canadian dollar relative to the U.S. dollar could have an effect on our financial results as reported in U.S. dollars. If the in Canadian dollar were to depreciate against the U.S. dollar, amounts reported in U.S. dollars would be correspondingly reduced. If the in Canadian dollar were to appreciate against the U.S. dollar, amounts reported in U.S. dollars would be correspondingly increased.
ITEM 8. Financial Statements and Supplementary Data.
. FINANCIAL STATEMENTS.
Index to Financial Statements:
Audited financial statements as of June 30, 2010, including:
| |
1. | Reports of Independent Registered Public Accounting Firm; |
2. | Balance Sheets as of June 30, 2010 |
3. | Statements of Operations for the years ended June 30, 2010 and 2009and for the period from inceptiononDecember 11, 2000, to June 30, 2010; |
4. | Statements of Cash Flows for the years ended June 30, 2010 and 2009 and for the period from inception on December 11, 2000to June 30, 2010; |
5. | Statement of Stockholders’ Equity (Deficiency) for the period from inception on December 11, 2000 through June 30, 2010; and |
6. | Notes to Financial Statements. |
22
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and
Stockholders of Fero Industries Inc.
(a Development Stage Company)
We have audited the accompanying balance sheets of Fero Industries Inc. (a Development Stage Company) (the Company) as of June 30, 2010 and 2009, and the related statements of operations, stockholders’ equity, and cash flows for each of the years in the two-year period ended June 30, 2010. The Company’s management is responsible for these financial statements. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements , assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of Fero Industries Inc. (a Development Stage Company) as of June 30, 2010 and 2009, and the results of its operations and its cash flows for each of the years in the two-year period ended June 30, 2010 in conformity with accounting principles generally accepted in the United States of America.
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. The Company does not have the necessary working capital to service its debt and for its planned activity, which raises substantial doubt about its ability to continue as a going concern. Management’s plans in regard to these matters are described in the notes to the financial statements. These financial statements do not include any adjustments that might result from the outcome of this uncertainty.
|
Madsen & Associates CPA’s, Inc. Murray, Utah December 13, 2010 |
F2
FERO INDUSTRIES INC.
(A Development Stage Company)
Balance Sheets
| | | |
| June 30, | | June 30, |
| 2010 | | 2009 |
ASSETS | | | |
| | | |
Current Assets: | | | |
Cash | $ - | | $ - |
Total Current Assets | $ - | | $ - |
| | | |
Total Assets | $ - | | $ - |
| | | |
| | | |
LIABILITIES AND STOCKHOLDERS' EQUITY (DEFICIT) | | | |
| | | |
Current Liabilities: | | | |
Accounts payable | $ 6,150 | | $ 10,250 |
Total Current Liabilities | 6,150 | | 10,250 |
| | | |
Advances from shareholder | 36,255 | | 12,500 |
Promissory note payable | 250,000 | | - |
Total liabilties | 292,405 | | 22,750 |
| | | |
Stockholders' Equity (Deficit): | | | |
Preferred stock, $.001 par value; authorized 10,000,000, none issued | - | | - |
Common stock, $.001 par value; 500,000,000 shares authorized | | | |
127,500,000 shares issued and outstanding at | | | |
June 30, 2010 and June 30, 2009 | 127,500 | | 127,500 |
Additional paid in capital | (90,900) | | (90,900) |
Accumulated deficit | (329,005) | | (59,350) |
| | | |
Total Stockholders' Equity (Deficit) | (292,405) | | (22,750) |
| | | |
Total Liabilities and Stockholders' Equity (Deficit) | $ - | | $ - |
| | | |
F3
FERO INDUSTRIES INC.
(A Development Stage Company)
Statement of Operations
| | | |
| | | From |
| | | December 11, |
| (Restated) | (Restated) | 2000 |
| For the | For the | (Date of |
| year | year | inception) |
| ended | ended | to |
| June 30, | June 30, | June 30, |
| 2010 | 2009 | 2010 |
| | | |
Revenue: | $ - | $ - | $ - |
Total Revenue | - | - | - |
| | | |
Operating Expenses: | | | |
General & administrative | 19,655 | 13,150 | 79,005 |
Impairment of bioceutical assets | 250,000 | | 250,000 |
Total Operating Expenses | 269,655 | 13,150 | 329,005 |
| | | |
NET LOSS | $ (269,655) | $ (13,150) | $ (329,005) |
| | | |
Weighted Average Shares | | | |
Common Stock Outstanding | 127,500,000 | 127,500,000 | |
| | | |
Net Loss Per Share | | | |
(Basic and Fully Dilutive) | $ - | $ - | |
| | | |
F4
FERO INDUSTRIES INC.
(A Development Stage Company
Statement of Shareholders Equity
F6
FERO INDUSTRIES INC.
(A Development Stage Company)
NOTES TO THE FINANCIAL STATEMENTS
(RESTATED)
NOTE 1 – ORGANIZATION AND BASIS OF PRESENTATION
NATURE OF OPERATIONS
Fero Industries, Inc. (the “Company”) was inPersonNamecorporated under the laws of the State of Colorado on December 11, 2000. The Company’s activities to date have been limited to organization and capital formation. The Company is a “development stage company”
BASIS OF PRESENTATION
These financial statements and related notes are presented in accordance with accounting principles generally accepted in the United States, and are expressed in US dollars.
NOTE 2 – NATURE OF SIGNIFICANT ACCOUNTING POLICIES
CASH AND CASH EQUIVALENTS
The Company considers all highly liquid debt instruments purchased with maturity of three months or less to be cash equivalents.
REVENUE RECOGNITION
The Company recognizes revenue at the time services are performed.
USE OF ESTIMATES
The preparation of the Company’s financial statements requires management to make estimates and assumptions that affect the amounts reported in the financial statements and accompanying notes. Actual results could differ from these estimates.
FAIR VALUE OF FINANCIAL INSTRUMENTS
The Company’s short-term financial instruments consist of cash and cash equivalents and accounts payable. The carrying amounts of these financial instruments approximate fair value because of their short-term maturities. The Company does not hold or issue financial instruments for trading purposes nor does it hold or issue interest rate or leveraged derivative financial instruments.
EARNINGS PER SHARE
Basic Earnings per Share (“EPS”) is computed by dividing net income available to common stockholders by the weighted average number of common stock shares outstanding during the year. Diluted EPS is computed by dividing net income available to common stockholders by the weighted-average number of common stock shares outstanding during the year plus potential dilutive instruments such as stock options and warrants. Basic and diluted EPS are the same for the Company, as of June 30, 2010, as the Company does not have any common share equivalents outstanding.
F7
FERO INDUSTRIES INC.
(A Development Stage Company)
NOTES TO THE FINANCIAL STATEMENTS
(RESTATED)
FAIR VALUE OF FINANCIAL INSTRUMENTS
The Company’s short-term financial instruments consist of cash and cash equivalents and accounts payable. The carrying amounts of these financial instruments approximate fair value because of their short-term maturities. The Company does not hold or issue financial instruments for trading purposes nor does it hold or issue interest rate or leveraged derivative financial instruments.
EARNINGS PER SHARE
Basic Earnings per Share (“EPS”) is computed by dividing net income available to common stockholders by the weighted average number of common stock shares outstanding during the year. Diluted EPS is computed by dividing net income available to common stockholders by the weighted-average number of common stock shares outstanding during the year plus potential dilutive instruments such as stock options and warrants. Basic and diluted EPS are the same for the Company, as of June 30, 2010, as the Company does not have any common share equivalents outstanding.
INCOME TAXES
The Company uses the asset and liability method of accounting for income taxes, which requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of temporary differences between the carrying amounts and the tax basis of certain assets and liabilities. Deferred income tax assets and liabilities are computed annually for the difference between the financial statement and tax bases of assets and liabilities that will result in taxable or deductible amounts in the future, based on enacted tax laws and rates applicable to the periods in which the differences are expected to affect taxable income. Valuation allowances are established when necessary to reduce deferred tax assets to the amount expected to be realized. Income tax expense is the tax payable or refundable for the period, plus or minus the change during the period in deferred tax assets and liabilities.
Deferred income taxes may arise from temporary differences resulting from income and expanse items reported for financial accounting and tax purposes in different periods. Deferred taxes are classified as current or non-current, depending on the classification of the assets and liabilities to which they relate. Deferred taxes arising from temporary differences that are not related to an asset or liability are classified as current or non-current depending on the periods in which the temporary differences are expected to reverse. As of June 30, 2010, the Company has recorded a valuation allowance to fully offset the deferred tax asset of approximately $112,000 related to its cumulative net operating losses of $329,005.
CONCENTRATION OF CREDIT RISK
Financial instruments that potentially subject the Company to a concentration of credit risk consist principally of cash. During the year the Company did not maintain cash deposits at financial institution in excess of the $100,000 limit covered by the Federal Deposit Insurance Corporation.
F8
FERO INDUSTRIES INC.
(A Development Stage Company)
NOTES TO THE FINANCIAL STATEMENTS
(RESTATED)
RECENT ACCOUNTING PRONOUNCEMENTS
The Company does not expect that the adoption of recent accounting pronouncements will have a material impact on its financial statements.
NOTE 3 – ACQUISITION OF DOMAIN NAMES AND DEPOSITS
On April 20, 2007, the Company entered into an asset purchase and sale agreement with Mr. Jerry Capehart of Grand Prairie, Texas whereby he sold to us a 100% undivided right title and interest in seventeen internet domain names for a total purchase price of $180,000. Terms of the purchase are as follows: $5,000 to Mr. Capehart and 2,500,000 shares of our common stock as a non-refundable deposit (recorded as Deposits on the Balance Sheet) and an additional $75,000 on or before June 30, 2008. All domain names are fully valid and registered and ready for construction. The Company did not fulfill its duties in the contract. As such, the Company has not recorded the liability or corresponding asset related to this sale agreement in its financial statements.
NOTE 4– ACQUISITION OF SUCANON
On May 23, 2010, Fero Industries, Inc., a Colorado PersonNamecorporation, (the "Company") entered that certain Asset Acquisition Agreement (the "Agreement") with Gvest, Inc., (“Gvest”) an Ontario, Canada PersonNamecorporation. Pursuant to the terms and conditions of the Agreement, the Company acquired certain assets directly related to the manufacturing, sale and distribution of that certain product known as Sucanon, which is an herbal remedy for Type II Diabetes. The acquired assets include all of the intellectual property rights, training, and “know how” to manufacture and produce Sucanon, including sources and suppliers of Sucanon ingredients and mixing equipment; certain associated trademarks and patents ("Acquired Assets"). The Acquired Assets include the exclusive world-wide rights to manufacture, sell and distribute Sucanon. The Company purchased the Acquired Assets for an aggregate purchase pri ce of $250,000. The Agreement contained customary representations and warranties and pre- and post-closing covenants of each party and customary closing conditions. Breaches of the representations and warranties were subject to customary indemnification provisions, subject to specified aggregate limits of liability. As of June 30, 2010 this transaction was not closed.
NOTE 5 – COMMON STOCK
On December 11, 2000 the Company issued 15,000,000 shares of its common stock to its President and Chief Executive Officer, Kyle Schlosser for $600 in return for his time effort and expense of forming the company and keeping it in good standing.
On December 28, 2006 the Company issued 12,500,000 shares of our common stock to our Secretary/Treasurer and Chief Financial Officer, Leigh-Ann Squire for $500 in return for her agreement to join our Board of Directors, become an officer of the registrant and his agreement to provide the computer and internet expertise in constructing our websites and providing the server for operation of the sites, at no charge.
F9
FERO INDUSTRIES INC.
(A Development Stage Company)
NOTES TO THE FINANCIAL STATEMENTS
(RESTATED)
As referred to in Note 4 above, on April 20, 2007 the Company issued 12,500,000 shares of our common stock to Mr. Jerry Capehart of Grand Prairie, Texas for $500, as a good faith deposit for seventeen domain names relating to the oil and gas industry.
On April 30, 2007 the Company issued 77,500,000 shares of our common stock to thirty-one non US persons for cash of $31,000.
On May 10, 2007 the Company issued 10,000,000 shares of our common stock to three US individuals (one representing a Grandchildren’s Trust), for cash of $4,000.
On November 18, 2008 the Board of Directors of the registrant passed unanimously a resolution authorizing a forward split of the authorized and issued and outstanding common shares on a three to one (5 – 1) basis bringing the total common shares issued and outstanding to 25,500,000. The forward split has been retroactively recorded in the financial statements of the Company as if the forward split had occurred at the inception of the Company and the authorized common shares have increased to 500,000,000.
On April 15, 2010 the Board of Directors of Fero Industries (the Registrant) passed a resolution declaring a stock dividend of four (4) shares of common stock for each share held, of record as of April 20, 2010. The common shares of the Registrant will be considered Ex Dividend on April 21, 2010. This brings the total issued and outstanding common shares to 127,500,000. All share references in these financial statements have been retroactively adjusted for this stock dividend.
NOTE 6- PROMISSORY NOTE
On June 24, 2010, we issued a Two Hundred Fifty Thousand Dollars ($250,000) Promissory Note (the “Note”) in favor of Mr. Peter Hogendoorn (the “Lender”). The Note contains standard representations, and warranties and affirmative and negative covenants, and is described in greater detail below. The Note memorializes a loan made by the Lender to the Company, in order for the Company to close that certain Asset Acquisition Agreement with Gvest. The Note accrues simple interest at a rate equal to 1% over the average Canadian Prime Rate and is due 30 days from the date executed, or thereafter by mutual agreement of the parties hereto, the principal and all accrued interest thereon shall be due and payable within ten (10) days of written demand by Holder. Additionally, the Note may be repaid in whole or in part by the Company without penalty or premium at any time and from time to time prior to the Maturity Date.
NOTE 7– GOING CONCERN
The accompanying financial statements have been prepared assuming the Company will continue as a going concern. As shown in the accompanying financial statements, the Company has no sales and has incurred a net loss of $329,005 since inception. The future of the Company is dependent upon its ability to obtain financing and upon future profitable operations from the development of its mineral properties. Management has plans to seek additional capital through a private placement and public offering of its common stock. The financial statements do not include any adjustments relating to the recoverability and classifications of recorded assets, or the amounts of and classification of liabilities that might be necessary in the event the Company cannot continue in existence.
F10
FERO INDUSTRIES INC.
(A Development Stage Company)
NOTES TO THE FINANCIAL STATEMENTS
(RESTATED)
NOTE 8- RESTATEMENT
The Company is restating its June 30, 2010 balance sheet to reclassify the Advances from shareholder and Promissory note payable from long term to current liabilities. The Company is restating its statements of operations to reflect the correct number of weighted average number of shares outstanding, which previously did not give retroactive effect to the April 1, 2010 stock dividend. The Company is restating its statement of stockholders’ equity to disclose the correct net loss for the year ended June 30, 2010. The Company is also adjusting its statement of stockholders’ equity to correctly present the stock dividend that occurred on April 15, 2010 (retroactive adjustment). These adjustments had no effect on the results of operations.
The Company is also restating its footnotes to add a subsequent events footnote for the Asset Acquisition Agreement that closed on July 7, 2010.
NOTE 9- SUBSEQUENT EVENTS (RESTATED)
On July 7, 2010, the Company closed its Asset Acquisition Agreement that will allow the Company to move forward with its new business plan to make, manufacture, and distribute Sucanon world-wide. The acquired assets include:
·
Intellectual property rights, training, and “know how” to manufacture and produce Sucanon
·
Sources and suppliers of Sucanon ingredients and mixing equipment
·
Certain associated trademarks and patents ("Acquired Assets")
·
Inventory
·
World-wide rights to manufacture, sell and distribute Sucanon
·
75% ownership of Smith Rothe Pharmaceutical (“Smith Rothe”)
·
98% ownership of Pharmaroth SA de CV, a Mexican PersonNamecorporation (“Pharmaroth”)
·
100% of 314202 B.C. Ltd., a Canadian PersonNamecorporation
The purchase price of these assets was $250,000. The Company performed a subsequent impairment test on the acquired assets and determined that there was an impairment. Therefore, the Company decided to report an impairment loss of $250,000 as of June 30, 2010. Also, since the acquired companies had no significant operations, assets, or liabilities, no pro-forma information is presented.
On September 22, 2010 and October 1, 2010 the Company issued a total of 5,850,000 shares of its common stock at $.04 per share for legal and consulting services.
The Company has evaluated subsequent events through December 13, 2010.
F11
ITEM 9. Changes and Disagreements With Accountants on Accounting and Financial Disclosure.
There has not been any changes in and or disagreements with our principal independent accountants.
We have engaged Madsen & Associates CPA’s, Inc. as our independent auditors since May 2007.
During the years ended June 30, 2010 and 2009 and subsequent to June 30, 2010 through to the date hereof, neither we, nor anyone on our behalf, has consulted with Madsen & Associates CPA’s, Inc. regarding the application of accounting principles to a specified transaction, whether completed or proposed, or the type of audit opinion that might be rendered on our financial statements, nor has Madsen & Associates CPA’s, Inc. provided to us a written report or oral advice regarding such principles or audit opinion or any matter that was the subject of a disagreement or any reportable events as set for in Item 304(a)(3) of Regulation SB.
ITEM 9A (T). CONTROLS AND PROCEDURES
MANAGEMENT'S ANNUAL REPORT ON INTERNAL CONTROL OVER FINANCIAL REPORTING.
The Company maintains a system of disclosure controls and procedures that are designed for the purposes of ensuring that information required to be disclosed in our SEC reports is recorded, processed, summarized, and reported within the time periods specified in the SEC rules and forms, and that such information is accumulated and communicated to our management, including the Chief Executive Officer as appropriate to allow timely decisions regarding required disclosure.
Management, after evaluating the effectiveness of the Company's disclosure controls and procedures as defined in Exchange Act Rules 13a-14(c) as of May 31, 2009 (the "Evaluation Date") concluded that as of the Evaluation Date, the Company's disclosure controls and procedures were effective to ensure that material information relating to the Company would be made known to them by individuals within those entities, particularly during the period in which this annual report was being prepared and that information required to be disclosed in our SEC reports is recorded, processed, summarized, and reported within the time periods specified in the SEC's rules and forms.
Our management is responsible for establishing and maintaining adequate internal control over financial reporting for the company in accordance with as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act. Our internal control over financial reporting is designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. Our internal control over financial reporting includes
those policies and procedures that:
(1) pertain to the maintenance of records that, in reasonable detail,
accurately and fairly reflect the transactions and dispositions of our
assets;
(2) provide reasonable assurance that transactions are recorded as
necessary to permit preparation of financial statements in accordance
with generally accepted accounting principles, and that our receipts
and expenditures are being made only in accordance with authorizations
of our management and directors; and
(3) provide reasonable assurance regarding prevention or timely detection
of unauthorized acquisition, use or disposition of our assets that
could have a material effect on our financial statements.
23
Management's assessment of the effectiveness of the registrant's internal control over financial reporting is as of the year ended June 30, 2010 We believe that internal control over financial reporting is effective at providing this reasonable level of assurance as of the period covered, due to the fact that we have two officers and directors with accounting and public company experience. In the future the company will endeavor to add other directors with sufficient SEC and accounting related expertise, to provide adequate segregation of duties and financial accounting and reporting controls.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
This annual report does not include an attestation report of the Company's registered public accounting firm regarding internal control over financial reporting. Management's report was not subject to attestation by the Company's registered public accounting firm pursuant to temporary rules of the Securities and Exchange Commission that permit the Company to provide only management's report in this annual report.
There was no change in our internal control over financial reporting that occurred during the fiscal year ended June 30, 2010, that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
ITEM 9B. OTHER INFORMATION.
None.
PART III
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS, PROMOTERS AND CONTROL PERSONS; COMPLIANCE WITH SECTION 16(A) OF THE EXCHANGE ACT.
DIRECTORS AND EXECUTIVE OFFICERS
Our executive officers and directors and their respective ages and positions as of March 17, 2007 are
as follows:
| | |
Name | Age | Position |
Kyle Schlosser | 48 | President, Chief Executive Officer and Director |
Leigh-Ann Squire | 41 | Chief Financial Officer, Secretary, Treasurer and Director |
Executive Biographies
Kyle Schlosser –Chief Executive Officer and President : Mr. Schlosser has served as our President since our inception in December 2000. Since August 2006, Mr. Schlosser has been the founder and president of Jack’s Custom Shacks Inc., manufacturer of portable office sleeping and cooking structures for the oil and gas and mining industries. From April 2004 through June 2006, Mr. Schlosser served as an independent oil and gas consult with assignments allover the PersonNameUnited States, PersonNameCanada and the PersonNameMiddle East. From June 2002 to December 2004, Mr. Schlosser served as Chairman and interim president of Micromining Technologies Ltd. (MMZ-TSX) a PersonNamecorporation involved with environmental clean-up of oilfield and mining sites. From 1980 through 2002 he served as pre sident of KWS Enterprises Inc., a Seismic Drilling contractor, supplying seismic drilling for major oil companies.
24
Leigh-Ann Squire –Chief Financial Officer, Secretary and Treasurer : Ms. Squire has served as of Chief Financial Officer, Treasurer and Secretary since December 28, 2006. Since 1997, Ms Squire has been Benefit Coordinator for Fauth Financial Group of PersonNamePersonNameCalgary, PersonNameAB a firm providing investment consulting and management and management of benefit plans for over 220 companies. She has a extensive background in computer programming and organization. Prior to 1997 Ms. Squire was employed by The Great West Life Assurance Company as an investment and Benefits coordinator.
Significant Employees and Consultants
We have no significant employees other than Mr. Schlosser and Ms. Squires.
Committees of The Board Of Directors
Our Board of Directors does not maintain a separately-designated standing audit committee. As a result, our entire Board of Directors acts as our audit committee. Our Board of Directors has determined that we do not presently have a director who meets the definition of an “audit committee financial expert.” We believe that the cost related to appointing a financial expert to our Board of Directors at this time is prohibitive. Our Board of Directors presently do not have a compensation committee, nominating committee, an executive committee of our board of directors, stock plan committee or any other committees.
Terms of Office
Our directors are appointed for one-year terms to hold office until the next annual general meeting of the holders of our common stock or until removed from office in accordance with our by-laws. Our officers are appointed by our Board of Directors and hold office until removed by our Board of Directors.
Code of Ethics
We have adopted a Code of Ethics applicable to our officers and directors which is a "code of ethics" as defined by applicable rules of the SEC. If we make any amendments to our Code of Ethics other than technical, administrative, or other non-substantive amendments, or grant any waivers, including implicit waivers, from a provision of our Code of Ethics to our Chief Executive Officer, Chief Financial Officer, or certain other finance executives, we will disclose the nature of the amendment or waiver, its effective date and to whom it applies in a current report on Form 8-K filed with the SEC.
Compliance with Section 16(A) Of The Securities Exchange Act
Section 16(a) of the Exchange Act requires our executive officers, directors, and persons who beneficially own more than ten percent of our equity securities (collectively, the “Reporting Persons”) to file reports of ownership and changes in ownership with the SEC. Reporting Persons are required by SEC regulation to furnish us with copies of all Section 16(a) forms they file. Based on our review of the copies of such forms received by us, there were no Reporting Persons who failed to file on a timely basis, reports required by Section 16(a) of the Exchange Act during the most recent fiscal year.
25
ITEM 11. Executive Compensation.
Summary Compensation Table



