Exhibit 13.1















RELIEF FOR OSTEOARTHRITIS

DISCLAIMER & FORWARD LOOKING STATEMENTS THE COMPANY IS “TESTING THE WATERS” UNDER REGULATION A UNDER THE SECURITIES ACT OF 1933. THIS PROCESS ALLOWS COMPANIES TO DETERMINE WHETHER THERE MAY BE INTEREST IN AN EVENTUAL OFFERING OF ITS SECURITIES. THE COMPANY IS NOT UNDER ANY OBLIGATION TO MAKE AN OFFERING UNDER REGULATION A. IT MAY CHOOSE TO MAKE AN OFFERING TO SOME, BUT NOT ALL, OF THE PEOPLE WHO INDICATE AN INTEREST IN INVESTING, AND THAT OFFERING MIGHT NOT BE MADE UNDER REGULATION A. IF THE COMPANY DOES GO AHEAD WITH AN OFFERING, IT WILL ONLY BE ABLE TO MAKE SALES AFTER IT HAS FILED AN OFFERING STATEMENT WITH THE SECURITIES AND EXCHANGE COMMISSION (SEC) AND THE SEC HAS “QUALIFIED” THE OFFERING STATEMENT. THE INFORMATION IN THAT OFFERING STATEMENT WILL BE MORE COMPLETE THAN THE INFORMATION THE COMPANY IS PROVIDING NOW, AND COULD DIFFER IN IMPORTANT WAYS. YOU MUST READ THE DOCUMENTS FILED WITH THE SEC BEFORE INVESTING. NO MONEY OR OTHER CONSIDERATION IS BEING SOLICITED, AND IF SENT IN RESPONSE, WILL NOT BE ACCEPTED. NO OFFER TO BUY THE SECURITIES CAN BE ACCEPTED AND NO PART OF THE PURCHASE PRICE CAN BE RECEIVED UNTIL THE OFFERING STATEMENT FILED BY THE COMPANY WITH THE SEC HAS BEEN QUALIFIED BY THE SEC. ANY SUCH OFFER MAY BE WITHDRAWN OR REVOKED, WITHOUT OBLIGATION OR COMMITMENT OF ANY KIND, AT ANY TIME BEFORE NOTICE OF ACCEPTANCE GIVEN AFTER THE DATE OF QUALIFICATION. AN INDICATION OF INTEREST INVOLVES NO OBLIGATION OR COMMITMENT OF ANY KIND. AN OFFERING STATEMENT REGARDING THIS OFFERING HAS BEEN FILED WITH THE SEC. YOU MAY OBTAIN A COPY OF THE PRELIMINARY OFFERING CIRCULAR THAT IS PART OF THAT OFFERING STATEMENT FROM: CYTONICS CORPORATION 561 - 406 - 2864 658 W. Indiantown Road, Suite 214, Jupiter, FL 33458 OR AT https://www.sec.gov/Archives/edgar/data/1421744/000110465920048004/0001104659 - 20 - 048004 - index.htm THIS PRESENTATION CONTAINS OFFERING MATERIALS PREPARED SOLELY BY CYTONICS CORPORATION WITHOUT THE ASSISTANCE OF SI SECURITIES, AND NOT SUBJECT TO FINRA RULE 2210. IN ADDITION, THIS PRESENTATION MAY CONTAIN FORWARD - LOOKING STATEMENTS AND INFORMATION RELATING TO, AMONG OTHER THINGS, THE COMPANY, ITS BUSINESS PLAN AND STRATEGY, AND ITS INDUSTRY. THESE STATEMENTS REFLECT MANAGEMENT’S CURRENT VIEWS WITH RESPECT TO FUTURE EVENTS BASED INFORMATION CURRENTLY AVAILABLE AND ARE SUBJECT TO RISKS AND UNCERTAINTIES THAT COULD CAUSE THE COMPANY’S ACTUAL RESULTS TO DIFFER MATERIALLY. INVESTORS ARE CAUTIONED NOT TO PLACE UNDUE RELIANCE ON THESE FORWARD - LOOKING STATEMENTS AS THEY CONTAIN HYPOTHETICAL ILLUSTRATIONS OF MATHEMATICAL PRINCIPLES, ARE MEANT FOR ILLUSTRATIVE PURPOSES, AND THEY DO NOT REPRESENT GUARANTEES OF FUTURE RESULTS, LEVELS OF ACTIVITY, PERFORMANCE, OR ACHIEVEMENTS, ALL OF WHICH CANNOT BE MADE. MOREOVER, NO PERSON NOR ANY OTHER PERSON OR ENTITY ASSUMES RESPONSIBILITY FOR THE ACCURACY AND COMPLETENESS OF FORWARD - LOOKING STATEMENTS, AND IS UNDER NO DUTY TO UPDATE ANY SUCH STATEMENTS TO CONFORM THEM TO ACTUAL RESULTS. 488334.v2
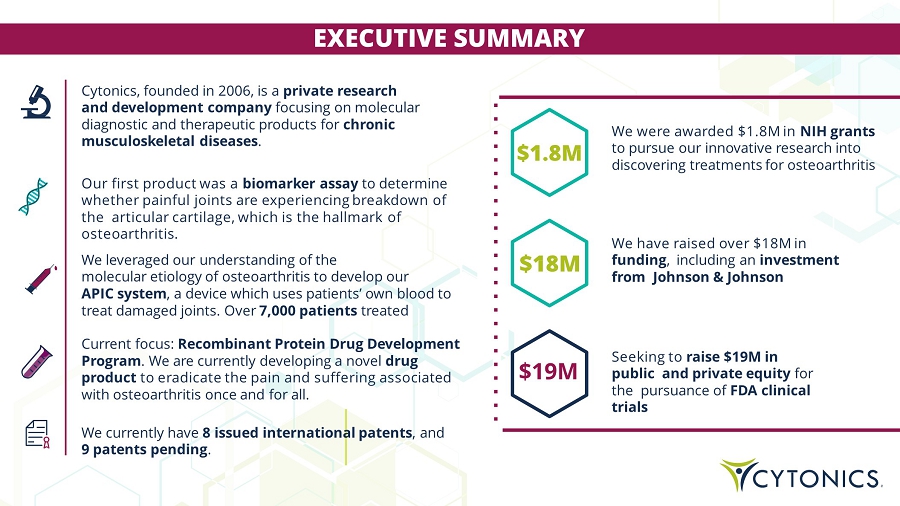
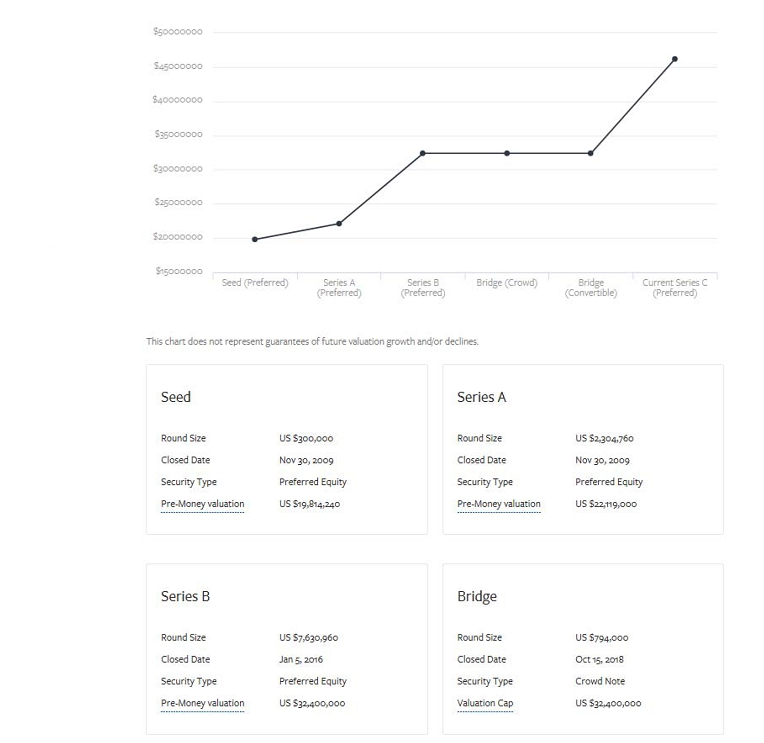
Cytonics, founded in 2006, is a private research and development company focusing on molecular diagnostic and therapeutic products for chronic musculoskeletal diseases . Our first product was a biomarker assay to determine whether painful joints are experiencing breakdown of the articular cartilage, which is the hallmark of osteoarthritis. We leveraged our understanding of the molecular etiology of osteoarthritis to develop our APIC system , a device which uses patients’ own blood to treat damaged joints. Over 7,000 patients treated Current focus: Recombinant Protein Drug Development Program . We are currently developing a novel drug product to eradicate the pain and suffering associated with osteoarthritis once and for all. We currently have 8 issued international patents , and 9 patents pending . EXECUTIVE SUMMARY We were awarded $1.8M in NIH grants to pursue our innovative research into discovering treatments for osteoarthritis We have raised over $18M in funding , including an investment from Johnson & Johnson Seeking to raise $20M in public and private equity for the pursuance of FDA clinical trials $19M
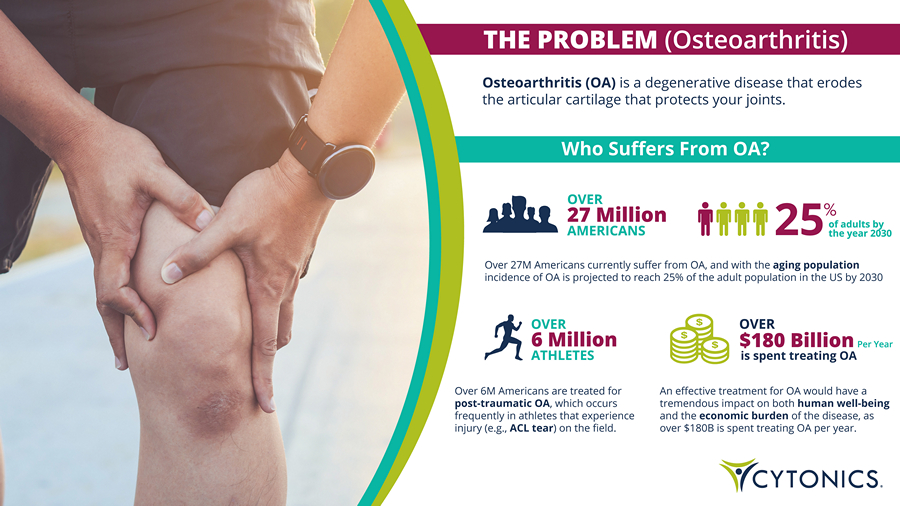
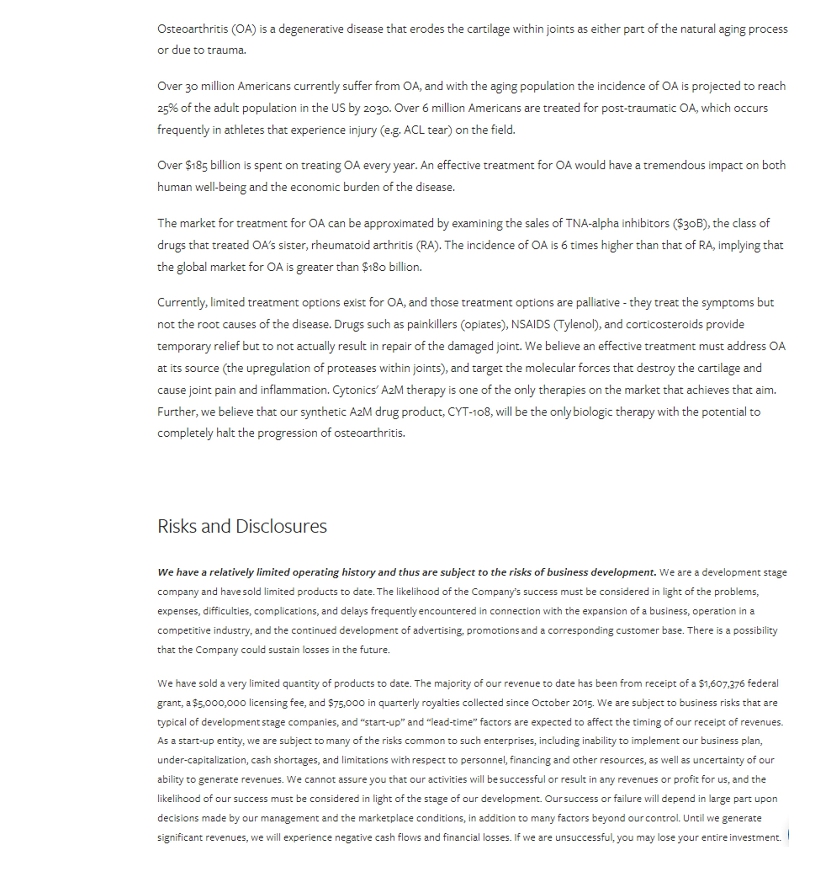
THE PROBLEM (Osteoarthritis) Osteoarthritis (OA) is a degenerative disease that erodes the articular cartilage that protects your joints. Over 27M Americans currently suffer from OA, and with the aging population incidence of OA is projected to reach 25% of the adult population in the US by 2030 Over 6M Americans are treated for post - traumatic OA , which occurs frequently in athletes that experience injury (e.g., ACL tear ) on the field. An effective treatment for OA would have a tremendous impact on both human well - being and the economic burden of the disease, as over $180B is spent treating OA per year. Who Suffers From OA? is spent treating OA
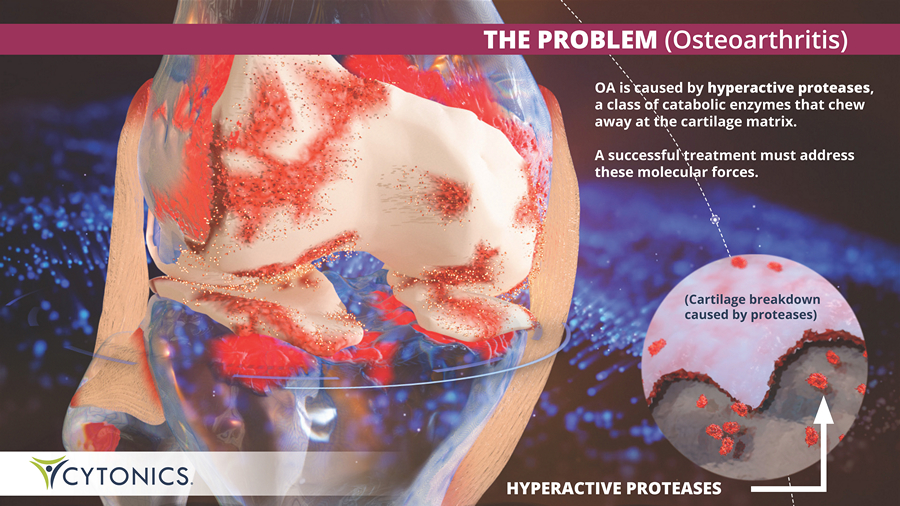
THE PROBLEM (Osteoarthritis) OA is caused by hyperactive proteases , a class of catabolic enzymes that chew away at the cartilage matrix. A successful treatment must address these molecular forces. (Cartilage breakdown caused by proteases) HYPERACTIVE PROTEASES
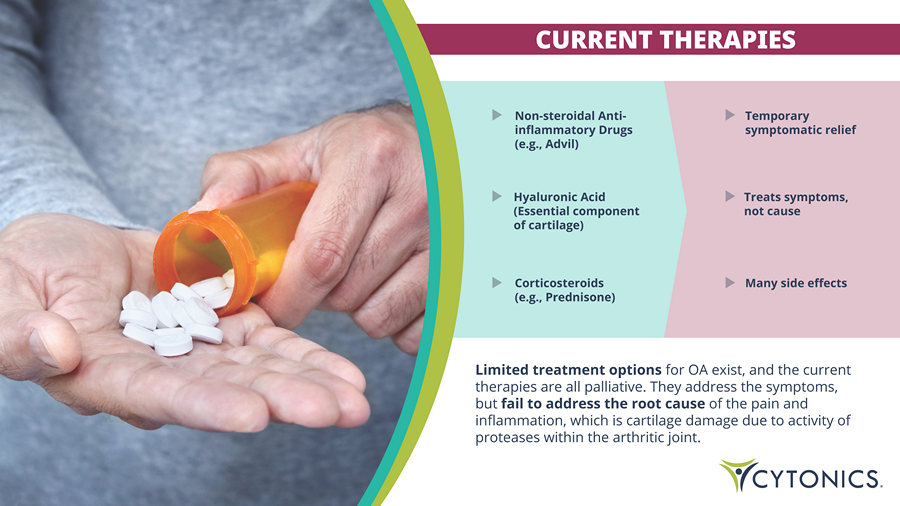
CURRENT THERAPIES Limited treatment options for OA exist, and the current therapies are all palliative. They address the symptoms, but fail to address the root cause of the pain and inflammation, which is cartilage damage due to activity of proteases within the arthritic joint. Non - steroidal Anti - inflammatory Drugs (e.g., Advil) Temporary symptomatic relief Corticosteroids (e.g., Prednisone) Many side effects Hyaluronic Acid (Essential component of cartilage) Treats symptoms, not cause
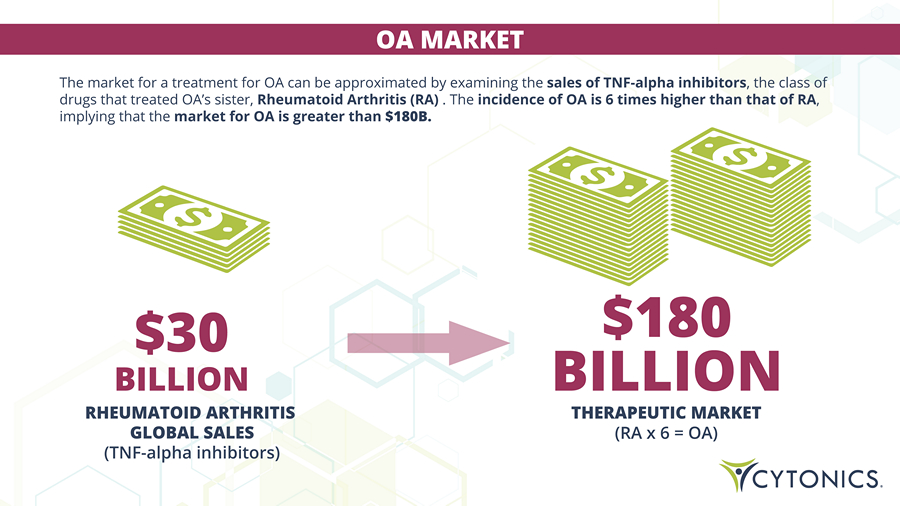
OA MARKET RHEUMATOID ARTHRITIS GLOBAL SALES (TNF - alpha inhibitors) THERAPEUTIC MARKET (RA x 6 = OA) The market for a treatment for OA can be approximated by examining the sales of TNF - alpha inhibitors , the class of drugs that treated OA’s sister, Rheumatoid Arthritis (RA) . The incidence of OA is 6 times higher than that of RA , implying that the market for OA is greater than $180B.
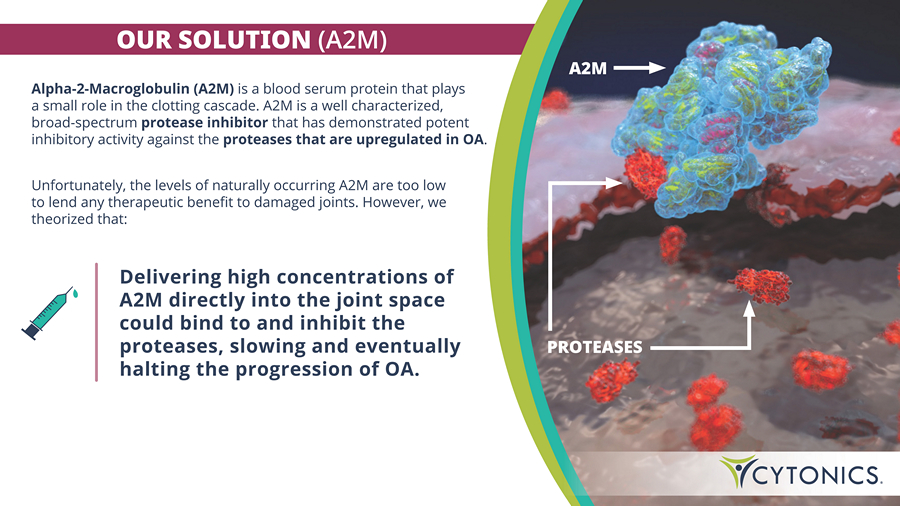
OUR SOLUTION (A2M) PROTEASES A2M Alpha - 2 - Macroglobulin (A2M) is a blood serum protein that plays a small role in the clotting cascade. A2M is a well characterized, broad - spectrum protease inhibitor that has demonstrated potent inhibitory activity against the proteases that are upregulated in OA . Unfortunately, the levels of naturally occurring A 2 M are too low to lend any therapeutic benefit to damaged joints . However, we theorized that : Delivering high concentrations of A2M directly into the joint space could bind to and inhibit the proteases, slowing and eventually halting the progression of OA.

OUR INNOVATION - THE APIC SYSTEM We developed the APIC system to concentrate the A 2 M found naturally in the bloodstream . This is achieved by drawing and centrifuging patient’s blood, then filtering out all of the proteins that could cause damage to the joint (such as proteases and inflammatory cytokines) . Our system selectively enriches for A2M, delivering high concentrations of the therapeutic A2M to the joint and eliminating all of the damaging molecules. Our APIC technology is often incorrectly compared to existing PRP (platelet rich plasma) therapies. PRP systems concentrate all the proteins in the blood, delivering a mix of potentially therapeutic and deleterious molecules to the joint. APIC SYSTEM - Selectively concentrates the A2M found within the bloodstream 4 - 6x above naturally occurring levels . Our proprietary filtration process removes the harmful proteins that remain in PRP formulations.

OUR INNOVATION - THE APIC SYSTEM Our APIC system has been used to successfully treat over 7,000 patients nationwide Our technology has been proven to slow cartilage degradation, alleviate pain, eventually halt the progression of OA and allow the body’s regenerative mechanisms to heal the damaged tissue. This observation has been independently verified by a number of academic groups. “As a busy spine surgeon for the last 25 years the direction that Cytonics is proceeding in attempting to minimize clinical failures through their Autologous Platelet Integrated Concentration (APIC) System is breathtaking and timely.” - Alexander R Vaccaro, MD, PhD, MBA

PHYSICIAN TESTIMONIALS I have been using Cytonics’ alpha2 - macroglobulin kits to treat various joint pains mostly in the knee. This is part of my regenerative medicine practice. I’ve seen remarkable results such that I have suggested that my wife and my son undergo treatments as well as patients. The treatments were remarkably successful in both of them. I am very pleased and I’m looking forward to having this product available more easily off - the - shelf and approved by insurance. I expect a huge demand for it. Thank you. - Laurence Rosenfield, MD “I was an early investor in Cytonics as the technology is timely in unraveling the etiology of Low back pain. The future will be assaying for specific biomarkers to determine not only the cause of pain but the potential for improvement with certain interventions. As a busy spine surgeon for the last 25 years the direction that Cytonics is proceeding in attempting to minimize clinical failures through their Autologous Platelet Integrated Concentration (APIC) System is breathtaking and timely.” - Alexander R Vaccaro, MD, PhD, MBA “Cytonics’ recombinant drug development program is anchored in robust preclinical data indicating that the proteinase inhibitor alpha - 2 - macroglobulin critically inhibits cartilage breakdown in models of osteoarthritis. Cytonics has developed a lead recombinant drug candidate, a variant of human alpha - 2 - macroglobulin that possesses a unique and improved bioactivity profile. Cytonics’ strategic efforts are exciting as they target the development of a first biologic therapy for patients suffering from osteoarthritis.” - Martin Angst, MD

PATIENT TESTIMONIALS “I suffered prolonged pain from a partial tear in my right Achilles tendon. I am very familiar with this pain as I ruptured and had my left Achilles surgically repaired. After almost eight months of therapy and various treatments, Richard Grossman, MD told me about Cytonics and the available A2M treatment. I received my first injection in April of 2018 and within weeks the large nodule in my Achilles had shrunk significantly. While I was feeling much better and able to start playing basketball and tennis again for the first time in ten months, I still felt a little pain. I went back for a 2nd injection in November of 2018 and the pain has been reduced to only minor pain with NO LIMITATIONS. The A2M therapy has given me my sports and mobility life back and I have recommended this treatment to all of my friends.” - Daryle Bobb “I partially tore my ACL in a skiing accident in Switzerland. After an unnecessary arthroscopy revealed I was not a candidate for ACL reconstruction, my knee was swollen and stiff for 6 weeks. Then I had a single treatment of Cytonics A2M therapy, APIC. Within 2 days the swelling and stiffness was gone and hasn’t returned 6 months later. I was so impressed with these results that I have been evangelizing for APIC treatment to my doctors and friends ever since. Even if I need another treatment soon, a couple APIC injections per year with no noticeable side effects and no drugs is closer to a miracle - treatment than I imagined possible before my experience with Cytonics’ product. Joint injuries can be physically and emotionally debilitating, but medical advancements like this make now the best time in history to tear one’s ACL. Thanks to Cytonics for developing this product!” - Gabe “[Dr. Scuderi] took out some of my blood and he put it into the centrifuge and they did what they had to do and then he reinjected the A2M protein back into my knee. Before he did the procedure, I could not bend my knee, I could not walk upstairs. I really couldn't do anything. In fact, I was using a brace on my knee just to give me some support because the whole knee felt like it was going to cave in. A few days after the procedure I was walking and we were walking the dogs and the swelling seemed to have been going down.” - Gail Lynn “I came with Gail when she discovered Dr. Scuderi and what he can do for arthritis. I went for an x - ray. Very simply, he did the same procedure. He took blood from my arm and put it in a centrifuge and got the protein out and injected it in my shoulder. And I’ve been great. We had nothing but success with this protein shot.” - Robert Lynn
THE NEXT GENERATION: PROTEOMICS Recent innovation in protein engineering has enabled researchers to “Edit” proteins , giving them special functions that result in therapeutic effects . Over the last decade, molecular biologists have made tremendous strides towards identifying and characterizing the thousands of proteins that exist in the human body. This line of inquiry gave birth to the field of “ Proteomics .” Proteomics allows scientists to study the structure and function of proteins, and discover how they malfunction in diseases .
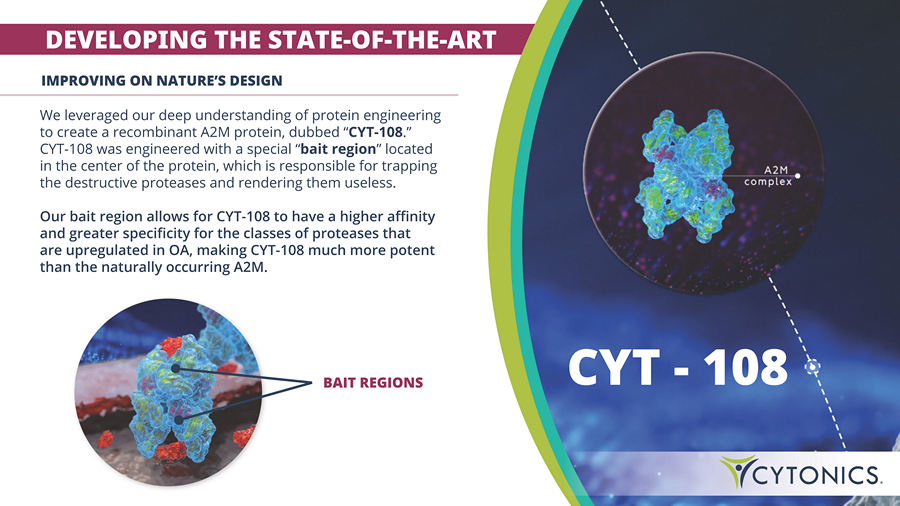
DEVELOPING THE STATE - OF - THE - ART BAIT REGIONS IMPROVING ON NATURE’S DESIGN We leveraged our deep understanding of protein engineering to create a recombinant A2M protein, dubbed “ CYT - 108 .” CYT - 108 was engineered with a special “ bait region ” located in the center of the protein, which is responsible for trapping the destructive proteases and rendering them useless. Our bait region allows for CYT - 108 to have a higher affinity and greater specificity for the classes of proteases that are upregulated in OA, making CYT - 108 much more potent than the naturally occurring A2M.
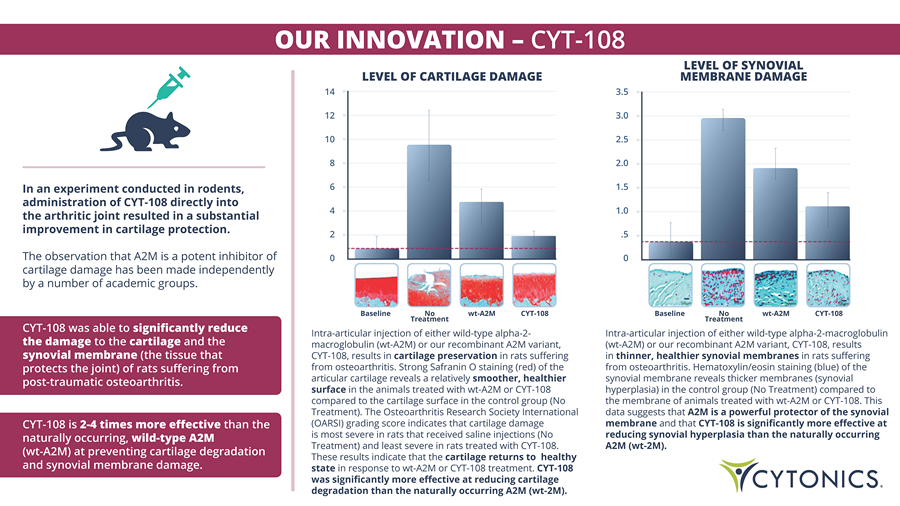
OUR INNOVATION – CYT - 108 In an experiment conducted in rodents, administration of CYT - 108 directly into the arthritic joint resulted in a substantial improvement in cartilage protection. The observation that A 2 M is a potent inhibitor of cartilage damage has been made independently by a number of academic groups . Intra - articular injection of either wild - type alpha - 2 - macroglobulin (wt - A2M) or our recombinant A2M variant, CYT - 108, results in cartilage preservation in rats suffering from osteoarthritis. Strong Safranin O staining (red) of the articular cartilage reveals a relatively smoother, healthier surface in the animals treated with wt - A2M or CYT - 108 compared to the cartilage surface in the control group (No Treatment). The Osteoarthritis Research Society International (OARSI) grading score indicates that cartilage damage is most severe in rats that received saline injections (No Treatment) and least severe in rats treated with CYT - 108. These results indicate that the cartilage returns to healthy state in response to wt - A2M or CYT - 108 treatment. CYT - 108 was significantly more effective at reducing cartilage degradation than the naturally occurring A2M (wt - 2M). Intra - articular injection of either wild - type alpha - 2 - macroglobulin (wt - A2M) or our recombinant A2M variant, CYT - 108, results in thinner, healthier synovial membranes in rats suffering from osteoarthritis. Hematoxylin/eosin staining (blue) of the synovial membrane reveals thicker membranes (synovial hyperplasia) in the control group (No Treatment) compared to the membrane of animals treated with wt - A2M or CYT - 108. This data suggests that A2M is a powerful protector of the synovial membrane and that CYT - 108 is significantly more effective at reducing synovial hyperplasia than the naturally occurring A2M (wt - 2M). Baseline No Treatment wt - A2M CYT - 108 Baseline Treatment No wt - A2M CYT - 108 CYT - 108 was able to significantly reduce the damage to the cartilage and the synovial membrane (the tissue that protects the joint) of rats suffering from post - traumatic osteoarthritis. CYT - 108 is 2 - 4 times more effective than the naturally occurring, wild - type A2M (wt - A2M) at preventing cartilage degradation and synovial membrane damage. 0 2 0 .5 LEVEL OF CARTILAGE DAMAGE LEVEL OF SYNOVIAL MEMBRANE DAMAGE 14 3.5 12 3.0 10 2.5 8 2.0 6 1.5 4 1.0
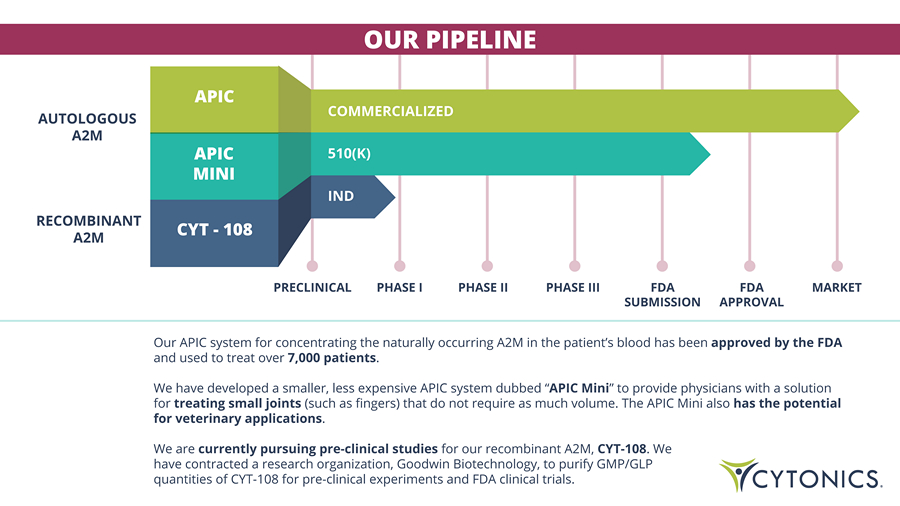
Our APIC system for concentrating the naturally occurring A2M in the patient’s blood has been approved by the FDA and used to treat over 7,000 patients . We have developed a smaller, less expensive APIC system dubbed “ APIC Mini ” to provide physicians with a solution for treating small joints (such as fingers) that do not require as much volume. The APIC Mini also has the potential for veterinary applications . We are currently pursuing pre - clinical studies for our recombinant A2M, CYT - 108 . We have contracted a research organization, Goodwin Biotechnology, to purify GMP/GLP quantities of CYT - 108 for pre - clinical experiments and FDA clinical trials. AUTOLOGOUS A2M RECOMBINANT A2M PHASE I PRECLINICAL PHASE II PHASE III FDA SUBMISSION FDA APPROVAL MARKET APIC APIC MINI CYT - 108 COMMERCIALIZED 510(K) IND OUR PIPELINE
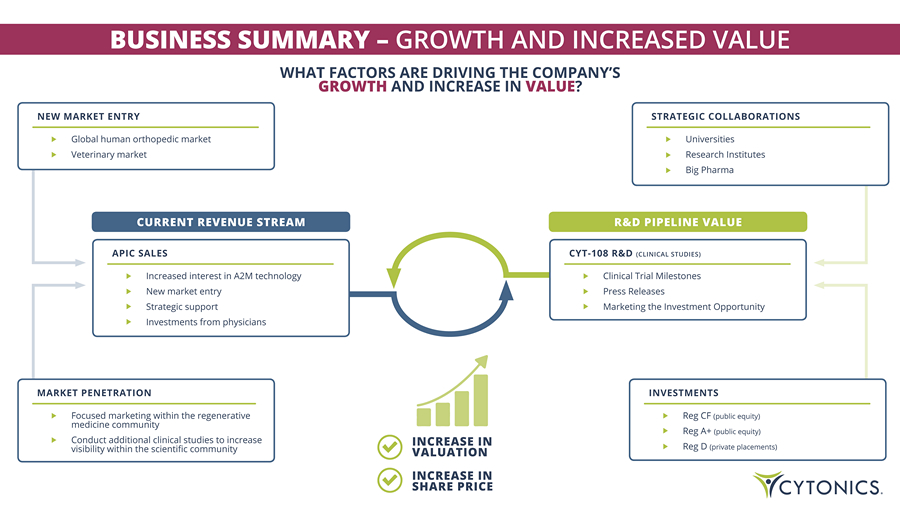
BUSINESS SUMMARY – GROWTH AND INCREASED VALUE WHAT FACTORS ARE DRIVING THE COMPANY’S GROWTH AND INCREASE IN VALUE ? APIC SALES Increased interest in A2M technology New market entry Strategic support Investments from physicians CURRENT REVENUE STREAM CYT - 108 R&D (CLINICAL STUDIES) Clinical Trial Milestones Press Releases Marketing the Investment Opportunity R&D PIPELINE VALUE NEW MARKET ENTRY Global human orthopedic market Veterinary market MARKET PENETRATION Focused marketing within the regenerative medicine community Conduct additional clinical studies to increase visibility within the scientific community STRATEGIC COLLABORATIONS Universities Research Institutes Big Pharma INVESTMENTS Reg CF (public equity) Reg A+ (public equity) Reg D (private placements) INCREASE IN VALUATION INCREASE IN SHARE PRICE

BUSINESS SUMMARY – APIC FORECAST Cytonics receives 10% of APIC sales as royalties. CYT - 108 clinical success will drive APIC sales , as media attention will increase Cytonics’ visibility within the regenerative medicine community. APIC sales will rapidly decline if CYT - 108 is approved by the FDA and if it is successfully commercialized . APIC Sales will be cannibalized by CYT - 108, a superior treatment option. Forecast Parameters and Assumptions Further penetration into the human orthopedic market Expansion into the veterinary market Expansion into global markets How will we drive future growth? - 500,000 1 , 00 0 , 0 00 1 , 50 0 , 0 00 2 , 00 0 , 0 00 2 , 50 0 , 0 00 $ 3,000,000 201 9 202 0 202 1 202 2 202 3 202 4 202 5 202 6 202 7 202 8 202 9 203 0 203 1 2032 (CYT - 108 Approved) APIC SALES HOW DOES THE COMPANY CURRENTLY MAKE MONEY? These statements reflect management’s current views based information currently available and are subject to risks and uncertainties that could cause the company’s actual results to differ materially. Investors are cautioned not to place undue reliance on these forward - looking statements as they are meant for illustrative purposes and they do not represent guarantees of future results, levels of activity, performance, or achievements, all of which cannot be made. Moreover, no person nor any other person or entity assumes responsibility for the accuracy and completeness of forward - looking statements, and is under no duty to update any such statements to conform them to actual results.
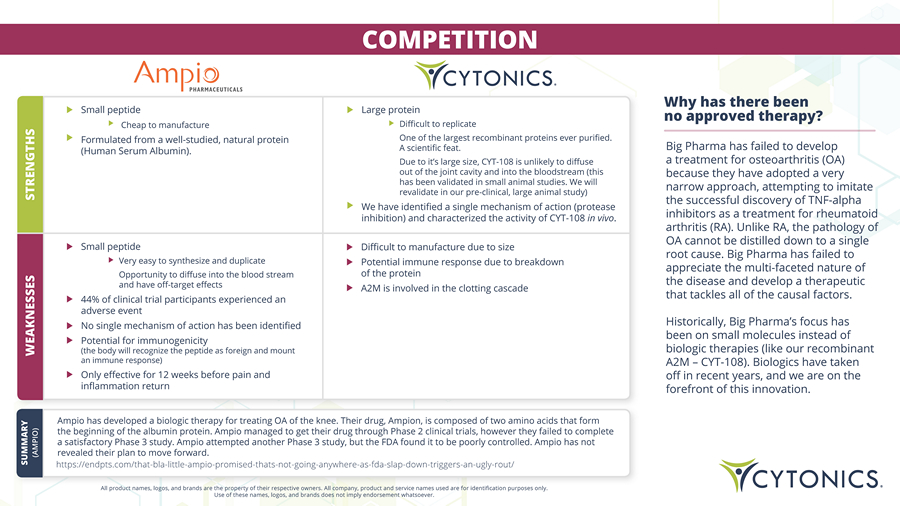
COMPETITION All product names, logos, and brands are the property of their respective owners. All company, product and service names used are for identification purposes only. Use of these names, logos, and brands does not imply endorsement whatsoever. Why has there been no approved therapy? Big Pharma has failed to develop a treatment for osteoarthritis (OA) because they have adopted a very narrow approach, attempting to imitate the successful discovery of TNF - alpha inhibitors as a treatment for rheumatoid arthritis (RA). Unlike RA, the pathology of OA cannot be distilled down to a single root cause. Big Pharma has failed to appreciate the multi - faceted nature of the disease and develop a therapeutic that tackles all of the causal factors. Historically, Big Pharma’s focus has been on small molecules instead of biologic therapies (like our recombinant A2M – CYT - 108). Biologics have taken off in recent years, and we are on the forefront of this innovation. Ampio has developed a biologic therapy for treating OA of the knee. Their drug, Ampion, is composed of two amino acids that form the beginning of the albumin protein. Ampio managed to get their drug through Phase 2 clinical trials, however they failed to complete a satisfactory Phase 3 study. Ampio attempted another Phase 3 study, but the FDA found it to be poorly controlled. Ampio has not revealed their plan to move forward. https://endpts.com/that - bla - little - ampio - promised - thats - not - going - anywhere - as - fda - slap - down - triggers - an - ugly - rout/ SUMMARY (AMPIO) WEAKNESSE S STRENGTHS Small peptide Cheap to manufacture Formulated from a well - studied, natural protein (Human Serum Albumin). Large protein Difficult to replicate One of the largest recombinant proteins ever purified. A scientific feat. Due to it’s large size, CYT - 108 is unlikely to diffuse out of the joint cavity and into the bloodstream (this has been validated in small animal studies. We will revalidate in our pre - clinical, large animal study) We have identified a single mechanism of action (protease inhibition) and characterized the activity of CYT - 108 in vivo . Small peptide Very easy to synthesize and duplicate Opportunity to diffuse into the blood stream and have off - target effects 44% of clinical trial participants experienced an adverse event No single mechanism of action has been identified Potential for immunogenicity (the body will recognize the peptide as foreign and mount an immune response) Only effective for 12 weeks before pain and inflammation return Difficult to manufacture due to size Potential immune response due to breakdown of the protein A2M is involved in the clotting cascade
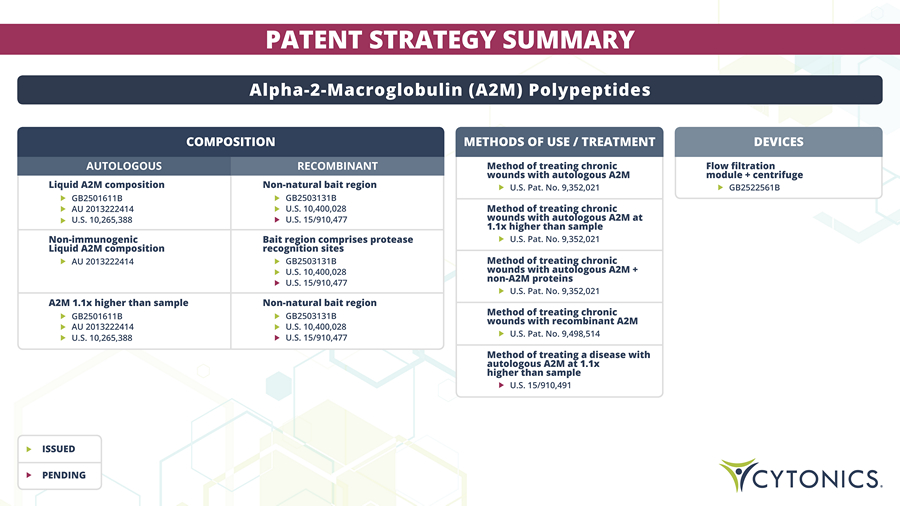
PATENT STRATEGY SUMMARY Alpha - 2 - Macroglobulin (A2M) Polypeptides DEVICES Flow filtration module + centrifuge GB2522561B METHODS OF USE / TREATMENT Method of treating chronic wounds with autologous A2M U.S. Pat. No. 9,352,021 Method of treating chronic wounds with recombinant A2M U.S. Pat. No. 9,498,514 Method of treating chronic wounds with autologous A2M at 1.1x higher than sample U.S. Pat. No. 9,352,021 Method of treating a disease with autologous A2M at 1.1x higher than sample U.S. 15/910,491 Method of treating chronic wounds with autologous A2M + non - A2M proteins U.S. Pat. No. 9,352,021 COMPOSITION AUTOLOGOUS Liquid A2M composition GB2501611B AU 2013222414 U.S. 10,265,388 RECOMBINANT Non - natural bait region GB2503131B U.S. 10,400,028 U.S. 15/910,477 Non - immunogenic Liquid A2M composition AU 2013222414 Bait region comprises protease recognition sites GB2503131B U.S. 10,400,028 U.S. 15/910,477 A2M 1.1x higher than sample Non - natural bait region GB2501611B AU 2013222414 U.S. 10,265,388 GB2503131B U.S. 10,400,028 U.S. 15/910,477 ISSUED PENDING
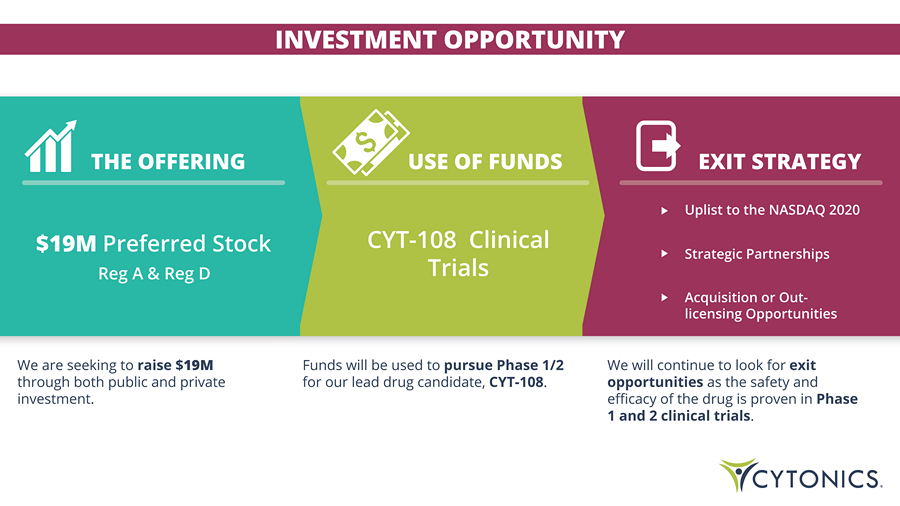
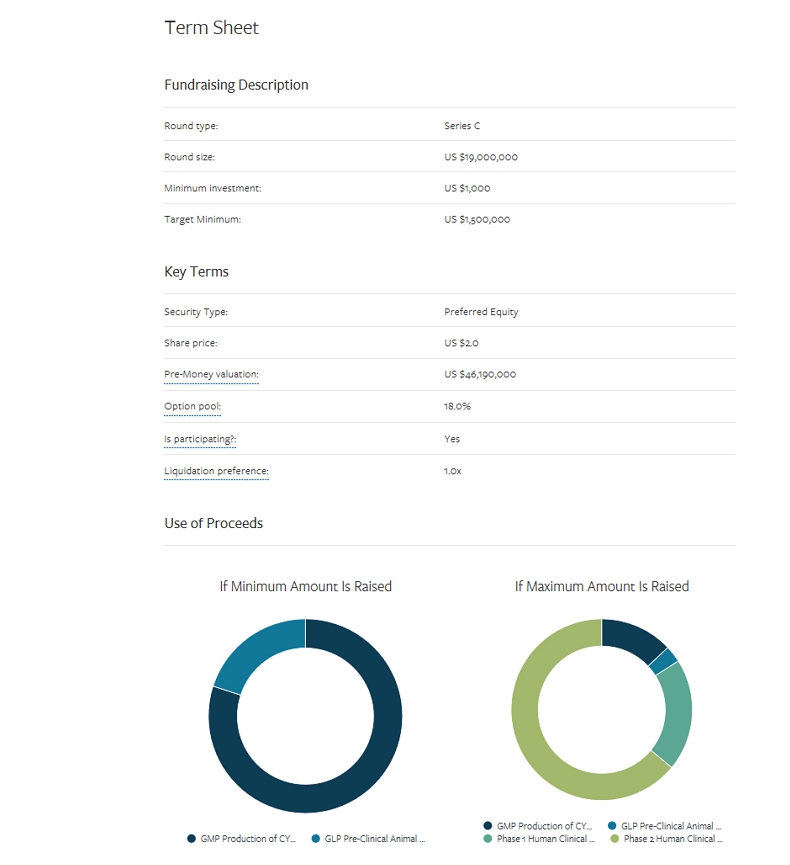
INVESTMENT OPPORTUNITY THE OFFERING Uplist to the NASDAQ 2020 Strategic Partnerships Acquisition or Out - licensing Opportunities CYT - 108 Clinical Trials USE OF FUNDS EXIT STRATEGY We are seeking to raise $ 19M through both public and private investment. Funds will be used to pursue Phase 1/2 for our lead drug candidate, CYT - 108 . We will continue to look for exit opportunities as the safety and efficacy of the drug is proven in Phase 1 and 2 clinical trials . $19M Preferred Stock Reg A & Reg D
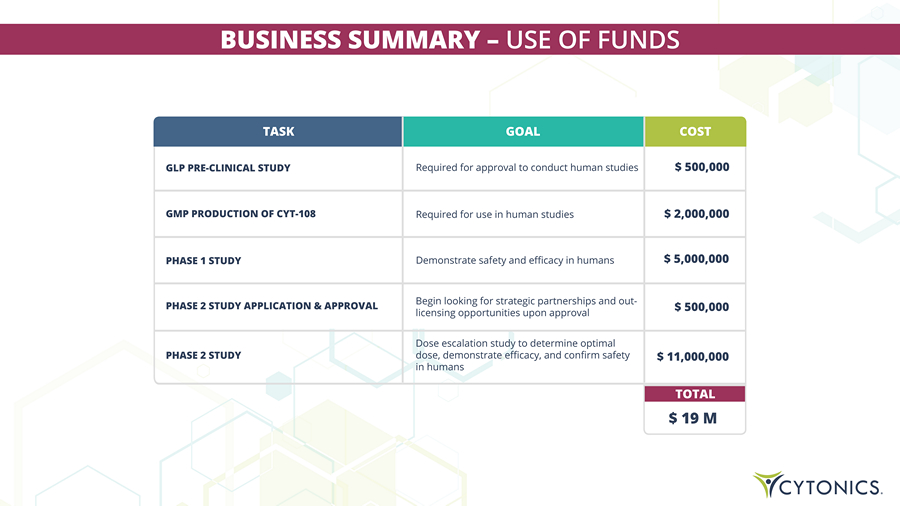
BUSINESS SUMMARY – USE OF FUNDS $ 19 M TOTAL TASK GLP PRE - CLINICAL STUDY GOAL Required for approval to conduct human studies COST $ 500,000 GMP PRODUCTION OF CYT - 108 Required for use in human studies $ 2,000,000 PHASE 1 STUDY Demonstrate safety and efficacy in humans $ 5,000,000 PHASE 2 STUDY APPLICATION & APPROVAL Begin looking for strategic partnerships and out - licensing opportunities upon approval $ 500,000 PHASE 2 STUDY Dose escalation study to determine optimal dose, demonstrate efficacy, and confirm safety in humans $ 11,000,000
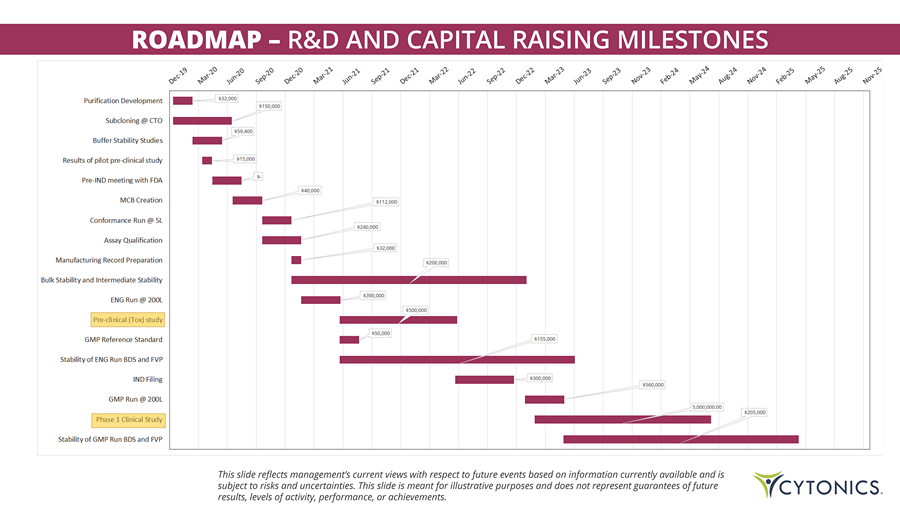
ROADMAP – R&D AND CAPITAL RAISING MILESTONES This slide reflects management’s current views with respect to future events based on information currently available and is subject to risks and uncertainties. This slide is meant for illustrative purposes and does not represent guarantees of future results, levels of activity, performance, or achievements.
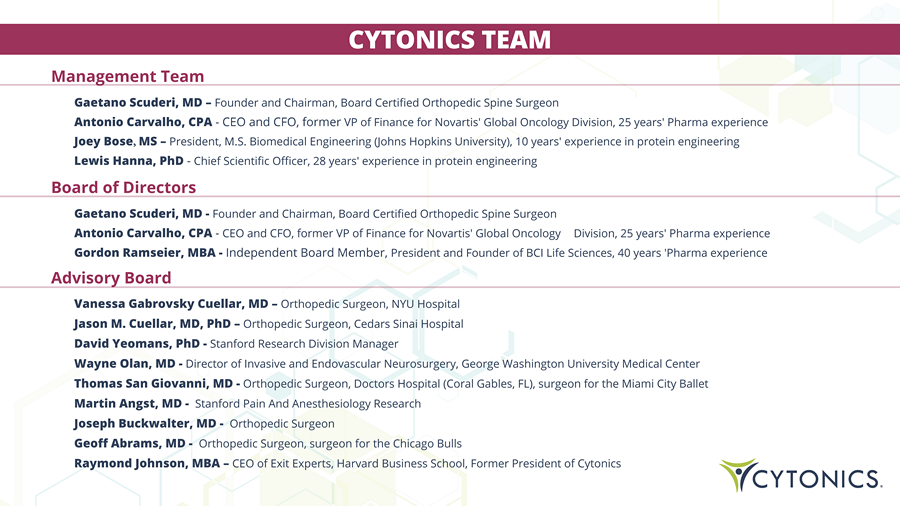
CYTONICS TEAM Management Team Gaetano Scuderi, MD – Founder and Chairman , Board Certified Orthopedic Spine Surgeon Antonio Carvalho, CPA - CEO and CFO, former VP of Finance for Novartis' Global Oncology Division , 25 years ' Pharma experience Joey Bose , MS – President, M.S. Biomedical Engineering (Johns Hopkins University), 10 years' experience in protein engineering Lewis Hanna, PhD - Chief Scientific Officer, 28 years ' experience in p rotein e ngineering Board of Directors Gaetano Scuderi, MD - Founder and Chairman, Board Certified Orthopedic Spine Surgeon Antonio Carvalho, CPA - CEO and CFO, former VP of Finance for Novartis' Global Oncology Division, 25 years' Pharma experience Gordon Ramseier, MBA - Independent Board Member, President and Founder of BCI Life Sciences, 40 years ' Pharma experience Advisory Board Vanessa Gabrovsky Cuellar, MD – Orthopedic Surgeon, NYU Hospital Jason M . Cuellar, MD, PhD – Orthopedic Surgeon, Cedars Sinai Hospital David Yeomans, PhD - Stanford Research Division Manager Wayne Olan, MD - Director of Invasive and Endovascular Neurosurgery, George Washington University Medical Center Thomas San Giovanni, MD - Orthopedic Surgeon, Doctors Hospital (Coral Gables, FL), surgeon for the Miami City Ballet Martin Angst, MD - Stanford Pain And Anesthesiology Research Joseph Buckwalter, MD - Orthopedic Surgeon Geoff Abrams, MD - Orthopedic Surgeon, surgeon for the Chicago Bulls Raymond Johnson, MBA – CEO of Exit Experts, Harvard Business School, Former President of Cytonics
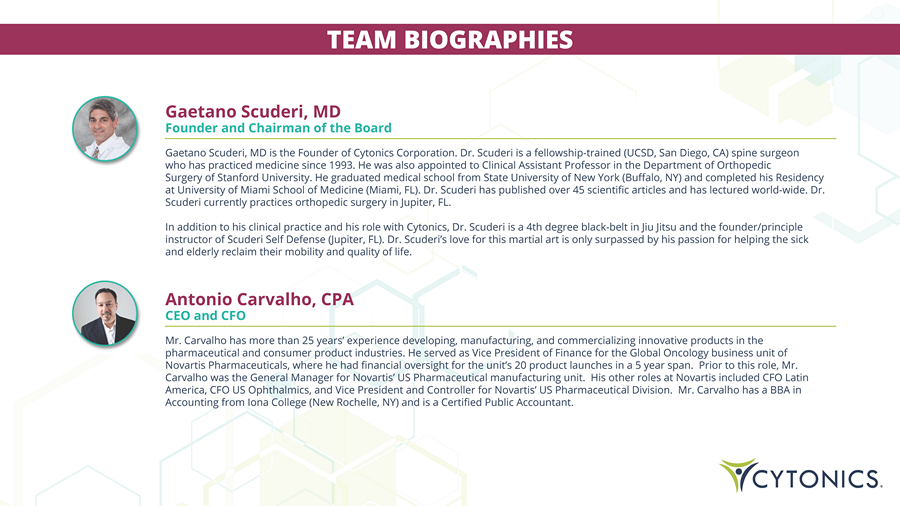
TEAM BIOGRAPHIES Gaetano Scuderi, MD Founder and Chairman of the Board Gaetano Scuderi, MD is the Founder of Cytonics Corporation . Dr . Scuderi is a fellowship - trained (UCSD, San Diego, CA) spine surgeon who has practiced medicine since 1993 . He was also appointed to Clinical Assistant Professor in the Department of Orthopedic Surgery of Stanford University . He graduated medical school from State University of New York (Buffalo, NY) and completed his Residency at University of Miami School of Medicine (Miami, FL) . Dr . Scuderi has published over 45 scientific articles and has lectured world - wide . Dr . Scuderi currently practices orthopedic surgery in Jupiter, FL . In addition to his clinical practice and his role with Cytonics, Dr. Scuderi is a 4th degree black - belt in Jiu Jitsu and the founder/principle instructor of Scuderi Self Defense (Jupiter, FL). Dr. Scuderi’s love for this martial art is only surpassed by his passion for helping the sick and elderly reclaim their mobility and quality of life. Antonio Carvalho, CPA CEO and CFO Mr. Carvalho has more than 25 years’ experience developing, manufacturing, and commercializing innovative products in the pharmaceutical and consumer product industries. He served as Vice President of Finance for the Global Oncology business unit of Novartis Pharmaceuticals, where he had financial oversight for the unit’s 20 product launches in a 5 year span. Prior to this role, Mr. Carvalho was the General Manager for Novartis’ US Pharmaceutical manufacturing unit. His other roles at Novartis included CFO Latin America, CFO US Ophthalmics, and Vice President and Controller for Novartis’ US Pharmaceutical Division. Mr. Carvalho has a BBA in Accounting from Iona College (New Rochelle, NY) and is a Certified Public Accountant.

TEAM BIOGRAPHIES Joey Bose, MS President Mr. Bose has over 10 years’ experience in biotechnology research development and healthcare investment banking. He began his career as a systems biology researcher at the University of Virginia and Johns Hopkins University, advancing the field of proteomics and elucidating the molecular drivers of cancers. Mr. Bose worked for two boutique healthcare investment banks/consultancies in the south Florida region, bringing his expertise in translational medicine to the deal diligencing team. As President of Cytonics, his primary responsibilities include coordinating capital raising efforts, initiating clinical trials for the company’s lead drug candidate (CYT - 108), filing and maintaining patent protection of intellectual property, and identifying strategic buyers and out - licensing opportunities for the company. He holds a BS in Biomedical Engineering from the University of Virginia (Charlottesville, VA) and an MS in Biomedical Engineering from Johns Hopkins University (Baltimore, MD). Lewis Hanna, PhD Chief Scientific Officer Dr. Hanna has served as Chief Scientific Officer of Cytonics since February 2008. Dr. Hanna has over 28 years’ experience in pharmaceutical research and development, specializing in the development of recombinant protein therapies. He has extensive knowledge of protein folding, purification, formulation, large - scale production, quality, and the regulatory requirements to obtain FDA new drug approval. Until 2004, Dr. Hanna was the Director of Process Development at Alexion Pharmaceutical, and prior to that he was a Group Leader at Bristol - Myers Squibb Pharmaceutical Research Institute. He also served a Principal Research Scientist at R.W. Johnson Pharmaceutical Research Institute (Raritan, NJ) for 7 years. Dr. Hanna received his BS degree from Cairo University (Giza, Egypt), received his PhD from City University of New York (New York City, NY), and completed a post - doctoral fellowship at Cornell University (Ithaca, NY).

KEYS TO SUCCESS MASSIVE MARKET POTENTIAL FOR EFFECTIVE DIAGNOSTICS AND ORTHOPEDIC PAIN RELIEF THERAPEUTICS $18 0 B market for effective osteoarthritis treatments BROAD PATENT COVERAGE World - renowned Wilson Sonsini Patent Attorneys 8 issued international patents, 9 pending MAJOR BREAKTHROUGH DISCOVERIES Fibronectin - Aggrecan Complex biomarker for osteoarthritis Purified one of the largest recombinant proteins to - date TRACK RECORD OF SUCCESS Over 7 , 000 patients treated with APIC therapy Successful 510 k approval for APIC technology CE mark designation POSSESS CORE COMPETENCIES TO ACHIEVE MILESTONES Hogen - Lovells Regulatory Attorneys Over 100 years’ combined experience in Pharma R&D OUTSTANDING TEAM OF MBAs, MDs, AND PhDs

OUR COLLABORATORS

Abrams, Geoffrey D., et al. “Fibronectin – Aggrecan Complex as a Marker for Cartilage Degradation in Non - Arthritic Hips.” Knee Surgery, Sports Traumatology, Arthroscopy, vol. 22, no. 4, 2014, pp. 768 – 773., doi:10.1007/s00167 - 014 - 2863 - 2. Bedi, Asheesh, et al. “The Effect of Matrix Metalloproteinase Inhibition on Tendon - to - Bone Healing in a Rotator Cuff Repair Model.” Journal of Shoulder and Elbow Surgery, vol. 19, no. 3, 2010, pp. 384 – 391., doi:10.1016/j.jse.2009.07.010. Browning, Shawn R, et al. “Platelet - Rich Plasma Increases Matrix Metalloproteinases in Cultures of Human Synovial Fibroblasts.” The Journal of Bone and Joint Surgery - American Volume, vol. 94, no. 23, 2012, doi:10.2106/jbjs.k.01501. Cuellar, Jason M. “Intradiscal Injection of an Autologous Alpha - 2 - Macroglobulin (A2M) Concentrate Alleviates Back Pain in FAC - Positive Patients.” Orthopedics and Rheumatology Open Access Journal, vol. 4, no. 2, Mar. 2017, doi:10.19080/oroaj.2017.04.555634. Demirag, Burak, et al. “The Effect of Alpha - 2 Macroglobulin on the Healing of Ruptured Anterior Cruciate Ligament in Rabbits.” Connective Tissue Research, vol. 45, no. 1, 2004, pp. 23 – 27., doi:10.1080/03008200490278115. Demirag, Burak. “Enhancement of Tendon - Bone Healing of Anterior Cruciate Ligament Grafts by Blockage of Matrix Metalloproteinases.” The Journal of Bone and Joint Surgery (American), vol. 87, no. 11, Jan. 2005, p. 2401., doi:10.2106/jbjs.d.01952. Gettins, Peter, and Leon W. Cunningham. “Identification of Proton Resonances from the Bait Region of Human .Alpha.2 - Macroglobulin and Effects of Proteases and Methylamine.” Biochemistry, vol. 25, no. 18, 1986, pp. 5011 – 5017., doi:10.1021/bi00366a007. Luan, Y., et al. “Inhibition of ADAMTS - 7 and ADAMTS - 12 Degradation of Cartilage Oligomeric Matrix Protein by Alpha - 2 - Macroglobulin.” Osteoarthritis and Cartilage, vol. 16, no. 11, 2008, pp. 1413 – 1420., doi:10.1016/j.joca.2008.03.017. Marynen, P., et al. “A Genetic Polymorphism in a Functional Domain of Human Pregnancy Zone Protein: the Bait Region.” FEBS Letters, vol. 262, no. 2, 1990, pp. 349 – 352., doi:10.1016/0014 - 5793(90)80226 - 9. Tortorella, Micky D., et al. “α2 - Macroglobulin Is a Novel Substrate for ADAMTS - 4 and ADAMTS - 5 and Represents an Endogenous Inhibitor of These Enzymes.” Journal of Biological Chemistry, vol. 279, no. 17, July 2004, pp. 17554 – 17561., doi:10.1074/jbc.m313041200. Zhang, Yang, et al. “Targeted Designed Variants of Alpha - 2 - Macroglobulin (A2M) Attenuate Cartilage Degeneration in a Rat Model of Osteoarthritis Induced by Anterior Cruciate Ligament Transection.” Arthritis Research & Therapy, vol. 19, no. 1, 2017, doi:10.1186/s13075 - 017 - 1363 - 4. APPENDIX – PUBLICATIONS
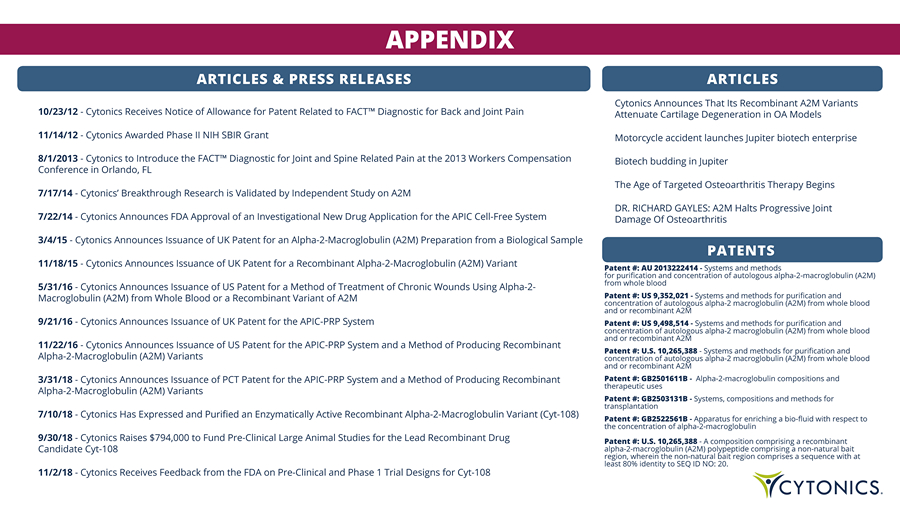
APPENDIX ARTICLES & PRESS RELEASES ARTICLES Cytonics Announces That Its Recombinant A2M Variants Attenuate Cartilage Degeneration in OA Models Motorcycle accident launches Jupiter biotech enterprise Biotech budding in Jupiter The Age of Targeted Osteoarthritis Therapy Begins DR. RICHARD GAYLES: A2M Halts Progressive Joint Damage Of Osteoarthritis 10/23/12 - Cytonics Receives Notice of Allowance for Patent Related to FACT™ Diagnostic for Back and Joint Pain 11/14/12 - Cytonics Awarded Phase II NIH SBIR Grant 8/1/2013 - Cytonics to Introduce the FACT™ Diagnostic for Joint and Spine Related Pain at the 2013 Workers Compensation Conference in Orlando, FL 7/17/14 - Cytonics’ Breakthrough Research is Validated by Independent Study on A2M 7/22/14 - Cytonics Announces FDA Approval of an Investigational New Drug Application for the APIC Cell - Free System 3/4/15 - Cytonics Announces Issuance of UK Patent for an Alpha - 2 - Macroglobulin (A2M) Preparation from a Biological Sample 11/18/15 - Cytonics Announces Issuance of UK Patent for a Recombinant Alpha - 2 - Macroglobulin (A2M) Variant 5/31/16 - Cytonics Announces Issuance of US Patent for a Method of Treatment of Chronic Wounds Using Alpha - 2 - Macroglobulin (A2M) from Whole Blood or a Recombinant Variant of A2M 9/21/16 - Cytonics Announces Issuance of UK Patent for the APIC - PRP System 11/22/16 - Cytonics Announces Issuance of US Patent for the APIC - PRP System and a Method of Producing Recombinant Alpha - 2 - Macroglobulin (A2M) Variants 3/31/18 - Cytonics Announces Issuance of PCT Patent for the APIC - PRP System and a Method of Producing Recombinant Alpha - 2 - Macroglobulin (A2M) Variants 7/10/18 - Cytonics Has Expressed and Purified an Enzymatically Active Recombinant Alpha - 2 - Macroglobulin Variant (Cyt - 108) 9/30/18 - Cytonics Raises $794,000 to Fund Pre - Clinical Large Animal Studies for the Lead Recombinant Drug Candidate Cyt - 108 11/2/18 - Cytonics Receives Feedback from the FDA on Pre - Clinical and Phase 1 Trial Designs for Cyt - 108 PATENTS Patent #: AU 2013222414 - Systems and methods for purification and concentration of autologous alpha - 2 - macroglobulin (A2M) from whole blood Patent #: US 9,352,021 - Systems and methods for purification and concentration of autologous alpha - 2 macroglobulin (A2M) from whole blood and or recombinant A2M Patent #: US 9,498,514 - Systems and methods for purification and concentration of autologous alpha - 2 macroglobulin (A2M) from whole blood and or recombinant A2M Patent #: U.S. 10,265,388 - Systems and methods for purification and concentration of autologous alpha - 2 macroglobulin (A2M) from whole blood and or recombinant A2M Patent #: GB2501611B - Alpha - 2 - macroglobulin compositions and therapeutic uses Patent #: GB2503131B - Systems, compositions and methods for transplantation Patent #: GB2522561B - Apparatus for enriching a bio - fluid with respect to the concentration of alpha - 2 - macroglobulin Patent #: U.S. 10,265,388 - A composition comprising a recombinant alpha - 2 - macroglobulin (A2M) polypeptide comprising a non - natural bait region, wherein the non - natural bait region comprises a sequence with at least 80% identity to SEQ ID NO: 20.

658 W. Indiantown Rd. Suite 214 Jupiter, FL 33458 joey.bose@cytonics.com 443 - 827 - 8135 cytonics.com RELIEF FOR OSTEOARTHRITIS

Cytonics Welcome Back Letter
Hi James,
This is Joey Bose, President of Cytonics. We’re excited to be back on SeedInvest after our first successful round last year. We just launched a Reg A+ reservation campaign to accept indications of interest for our Series C round as we await qualification by the SEC.I want to give you thefirst look at the campaign before it launches publicly.

https://crm.private.seedinvest.com/email_campaigns/email_campaign/6853 | 1/3 |
Our core mission is to disrupt the regenerative medicine field with our proprietary and innovative biologic therapies for osteoarthritis. As we previously successfully raised on SeedInvest in 2019, I wanted to share with you some specific highlights of this new round:
| • | We initiated a pilot pre-clinical study in late 2019 for our lead drug candidate CYT-108 - this was a key milestone on the path to Phase 1 human clinical trials. |
| • | We have executed licensing agreements for sale of our FACT and APIC technologies in the human and veterinary markets, with a value up to $7mm and 10% royalties on net sales. |
| • | Our IP portfolio consists of 8 issued U.S. and international patents with 9 patents pending. |
| • | Our founder is a leading orthopedic surgeon (21 years of experience) plus the company is backed by renowned physicians, researchers, and biotech investors. |
With the funds raised from this round, we plan to pursue drug development, pre-clinical studies, and Phase 1 clinical trials for CYT-108.You can read more about our Series C fundraise, keep up to date on campaign updates, write me any questions, and reserve shares on our profile here.
Regards,
Joey Bose
President of Cytonics
https://crm.private.seedinvest.com/email_campaigns/email_campaign/6853 | 2/3 |
Questions?Email Us. We're happy to help.
You are receiving this email because you are a registered user on SeedInvest. If you would like to stop receiving emails about new deal launches,unsubscribe here.
If you would like to stop receiving all SeedInvest marketing emails, including deal introductions, newsletters, event invitations, and new product announcements, pleaseunsubscribe here. Please note you will still receive investment confirmation emails and all other transactional emails related to activities on your account.
Cytonics is accepting reservations for an Offering under Tier II of Regulation A. No money or other consideration is being solicited, and if sent in response, it will not be accepted. No sales of securities will be made or commitment to purchase accepted until qualification of the offering statement by the Securities and Exchange Commission (the “Commission”) and approval of any other required government or regulatory agency. A reservation is non-binding and involves no obligation or commitment of any kind. No offer to buy securities can be accepted and no part of the purchase price can be received without an Offering Statement that has been qualified by the Commission. A Preliminary Offering Circular that forms a part of the Offering Statement has been filed with the Commission, a copy of which may be obtained from Cytonics:https://www.seedinvest.com/cytonics
Copyright © 2020 Circle Internet Financial Limited (“Circle”), All rights reserved. This communication is intended solely for the use of the individual(s) to whom it was intended to be addressed. If you are not the intended recipient of this message you are hereby notified that any review, dissemination, distribution or copying of this message is strictly prohibited. This communication is for information purposes only and should not be regarded as a recommendation of, or an offer to sell or as a solicitation of an offer to buy, any financial product. Investments are offered only via definitive transaction documents and any potential investor should read such documents carefully, including all risks, before investing. Startup investments involve a high degree of risk and those investors who cannot hold an investment for the long term (at least 5-7 years) or afford to lose their entire investment should not invest in startups. All securities-related activity is conducted by SI Securities, LLC dba SeedInvest, an affiliate of Circle, and a registered broker-dealer, and member FINRA/SIPC, located at 61 Broadway, Suite 1705, New York, NY 10006. To learn more about investing in startups and its risks visitwww.seedinvest.com/academy.
https://crm.private.seedinvest.com/email_campaigns/email_campaign/6853 | 3/3 |
Cytonics Webinar Transcript -1
[00:00] Lauren: Hi, everyone. Thank you so much for taking the time to join us today. Cytonics is our latest Reg A plus reservation campaign to.
The company is developing diagnostics and therapeutics for osteoarthritis. The company has completed a successful seeding invest raise last year in 2019. Here we have the president joining us to talk more about the company and answer questions from investors. If you have any specific questions that you would like for him to answer. Feel free to type them in the right-hand side control. With that, I will hand it to Joey.
[01:13] Joey: Lauren, thank you so much. As introduced, my name's Joey Bose. I'm the president of Cytonics. I've been president for two years now. I'll give a brief introduction about the company. So Cytonics was founded in 2006 by Dr. Gaetano Scuderi, a practicing spinal surgeon. He’d also done some research in regenerative medicine at Stanford. And the focus of his research was on musculoskeletal diseases such as osteoarthritis. And while he was doing this research, he discovered that there is a biomarker that can detect the extent of cartilage decay or degradation as a result of the progression of osteoarthritis.
And what he ended up doing was figuring out why does that biomarker even form? Maybe if we can stop the formation of that biomarker, it could halt the progression of osteoarthritis and solve the disease. And so with that in mind, he created the APIC system, which is autologous, meaning that it derives from yourself, a system for purifying out this molecule called alpha-2-macroglopbulin, which it just so happens, can protect cartilage from degradation.
We can concentrate it and inject it back into a damaged joint, it can actually reverse the effects of osteoarthritis. And so that's what we did. We developed the APIC system and it was first license in 2015 to a medical device distributor. And since then, we've treated over 7000 patients in the human space and are just getting into the veterinary space.
Now, the natural extension of that technology is to create a synthetic form of the A2M. Why rely on the blood? What we can create is a synthetic variant that we can produce in a lab at scale and is more cost efficient and more effective. That's exactly what we did. So the evolution of this company kind of went from an idea to the observation that there is some sort of biomarker. That's an indication of the presence of osteoarthritis. The solution to the disease lies in figuring out why does a biomarker even exist, then using nature's own design to concentrate something that comes naturally from your body.
And then creating a more effective form in the dish in a lab. And so we've currently we currently have eight patents that cover all of this technology. And we've had great fundraising success to date, including investment from Johnson & Johnson as well as our previous raise that we did on SeedInvest.
In this round, we're looking to raise about 20 million dollars to pursue clinical trials. Osteoarthritis is really a big deal. It affects over twenty seven million Americans and it's getting more prevalent as our population gets older. And also as we stay more active, global population is becoming more active, more and more interested in sports. And people are able to enjoy longer lifespans that also feature a higher quality of life. Osteoarthritis is inevitable.
And so there are over six million athletes in the United States alone that get post-traumatic stress from damage to their joints, like an ACL tear. And it represents a huge economic burden. The global market is about one hundred eighty billion dollars. And it's the number one cause of missed work. And it's really debilitating. It really ruins quality of life. Our solution would address this enormous market as well as bringing a lot of relief.
So the problem is overactive enzymes that lead to cartilage degradation. That's the origin of osteoarthritis. And it's a result of these catabolic proteases that chew up the cartilage matrix. A2M binds to these proteases and inactivates them, before being excreted by the immune system.
So the current therapies don't address this root costs, which these overactive proteases, chewing the cartilage, they're really just bandaids. They're really three big modalities of treatment. One is non-steroidal anti-inflammatory drugs like aspirin, which are just temporary anti inflammation, pain relief. Second is hyaluronic acid, which is what's considered a viscosupplementation, meaning you're taking a viscous substance like molasses and putting into the joint giving a little extra cushion.
Well, that doesn’t stay that forever and it gets cleared out. So, again, it's just a Band-Aid. And then last thing, corticosteroids, which do bring down inflammation, but are very painful to administer. And, you know, they are not going to last forever either. And the cartilage stays in its eroded state. So a successful treatment would feature something that actually addresses that root cause of disease, that cartilage damage.
So we theorize that if we could deliver high concentrations of A2M directly into damaged joints, we can slow and eventually halt the progression of osteoarthritis. And that's exactly what we did. It works beautifully.
So we're able to create this hyper concentrate of A2M in a very small volume that can accommodate your joint space. So it's not painful. And this technology has been widely successful in the human market. We treat the elderly, athletes, and basically anyone in between. And we have a number of testimonials from both physicians and patients. I encourage you to go to the website and kind of look a little bit more. We have some really nice videos that also explain how this technology works.
And also testimonials from people that have benefited from it and have used it. So as I alluded to earlier, the next step is to ask, why take it from the body when we can actually generate a synthetic form and that falls under the field of proteomics, which is engineering these proteins to have special function. So we took the DNA from the natural the human A2M DNA, and we edited it to give it special functions that make it bind to those proteases.
So less is needed to get the same effect as the natural version. And so we dubbed this molecule CYT-108. And we two bodies of evidence to suggest that it works and that it's non-toxic.
And you'll see without treatment, there's a significant amount of cartilage damage. And then when you add the wild type A2M which is that protein, you get it reduced by about 50 percent. Instead, if you treat it with CYT-108, it gets reduced by an additional 50 percent. Now it's now it's decreased by almost a quarter. And this was done in a rat model of osteoarthritis.
And so our next experiment, which actually just concluded, I don't have the data published yet, but it was conducted by Third Party Independent Collaborative Research Organization. We actually tested the safety of CYT-108 and in a large animal model of osteoarthritis. And as soon as that data is out, it will be published and distributed. Preliminary data suggests that it did not cause an immune response and it also didn’t affect the organs negatively.
That's a big deal. It means that if you administered into the joint, it's well-tolerated. And so that's what we're going to present to the FDA and use as are our basis for an IND submission to get into human. Phase one, clinical trials. So as an investor, your calculus is to determine whether an investment is attractive by weighing the upside potential against potential risk. Now, Cytonics is kind of interesting in that as an early stage biotech company, we actually generate revenue and we have a business model that works.
The market's enormous and we're constantly building it out in the human global space as well as the veterinary space. We actually just signed two deals. One is for global distribution in the human space. And the other one is for global distribution in the veterinary space. Primarily the equine space.
And we expect as those markets that are built out will increase somewhat exponentially until 2028 when we expect CYT-108 away to be approved upon which they'll cannibalize the sales. So this is an example of a business model that works. It addresses a huge market that we're able to build out, not just for APIC, but for CYT-108.
So that ties into the second proposition for the upside, which is the clinical success of CYt-108That's the biggest driver of the valuation. Now, biotech investments are inherently risky and it's very difficult to assign a dollar value to the stock. How much is an early stage therapeutic asset worth? There's really two ways to compute. And the first is to look at discounted cash flow that's adjusted for the clinical trial risk at each stage and assign a value based on that.
So the second part of that calculus is, is assessing the risk. And there's really two major risks in something like this. One is that someone beats us to it. Now, that's highly unlikely because Big Pharma has historically failed to develop a treatment for osteoarthritis because they’re thinking has been very narrow and trying to replicate the logic for developing a treatment for rheumatoid arthritis. And the big difference is that rheumatoid arthritis is a very simple, linear pathway in the way that works.
Osteoarthritis is multifactorial. You have to address many more factors. Our technology does that.. So Big Pharma historically has sort of given up on this. There is a small company called Apmpio that was developing a biologic treatment and they failed in phase three clinical studies. The FDA found the study poorly controlled. So that sort of ties into the second risk, which is the inherent risk of clinical trials. And a lot of trials fail simply because they weren't designed correctly.
So, you know, we intend to manage that risk by maintaining close contact with the FDA. Every time we have new data come out, we schedule a meeting with the FDA to talk about it. See what they think about it, and what they want to see as the next step. So that as we move through the process, we're not going to be suddenly surprised by the FDA, come back and say your experiments weren't designed correctly. Now, the second thing that really risks this project is the fact that we actually have each one technology that works.
So, as mentioned, we we're raising money for the pursuance of clinical trials for CYT-108, primarily phase one, phase two, as well as manufacturing the drug at scale for those trials with the ultimate ideas of developing this drug to the point where a big pharmaceutical company, like Johnson & Johnson, might be interested in partnering or acquiring. Now there's two big exits for early stage biotech. One is just a straight acquisition. The second is an out licensing agreement where the development is performed by a large pharmaceutical company in exchange for an upfront fee and royalties on sales.
So Cytonics management team is really, really strong. As I mentioned, it was founded by Gaetano's Scuderi, a practicing surgeon, who really was the genius that came up with this technology. We're currently led by Antonio Carvalho, the CEO and CFO, who was a former V.P. of finance for Novartis and very sharp guy for the business development aspect of pharmaceutical development. My background is a biomedical engineering. I worked in corporate finance and investment banking in the life sciences for a number of years. Lewis Hanna was the brains behind CYT-108, and he has over at this point over 30 years experience in protein engineering. We are supported by an advisory board that stacked with experts in all stages of development process, from basic research to clinical trial design, as well as the regulatory aspects of drug development. And so, you know, we're really in a great spot right now. I think the company is undervalued at its current price. We have a very rich patent portfolio that covers both the APIC system and the CYT-108 drug.
We have a track record of success with our A2M- based technologies. We know that they work and they're selling. We're building out this market so that once CYT-108 is ready the potential will be realized. People already understand the potential of the core technology, and we possess the core competencies and the team of industry experts to be able to get us to an exit opportunity. So with that, I want to thank you and we'll take questions.
[16:13] Lauren: Thanks so much. We'll get right into questions. First question, what risks, if any, do you see to your patent portfolio protecting site went away and other modalities for osteoarthritis?
[16:32] Joey: So the way that we went about doing this is that we try to make it look as broad as possible, as well as a very narrow, specific claims. And so what we did is the IP is really centered on figuring out how those proteases bind to the win and then patenting all those binding sites. So any technology that uses synthetic A2M to bind to proteases will essentially be in violation of our patent because there are specific binding sites that we've identified and that that's the crux of the intellectual property.
[17:46] Lauren: Do you use CRISPR to edit the proteins?
[17:50] Joey: No. No, we did it. That was originally done. In 2014, 2015, before CRISPR was on the map. It wasn't used quite as much, but no, we did not; we used a pretty standard recombinant protein engineering.
[18:20] Lauren: What portion of the race will be used for continuing R&D of CYT-108? And what will the R&D specifically focus on?
[18:31] Joey: Right. So the vast majority, if you actually go and see the SeedInvest profile, you'll see it broken down. The sole focus right now is advancing site went a way through clinical trials. That is the biggest driver of value. And so we're a hundred percent focused on that, which is why we out license APIC, we don't actually sell ourselves. We collect royalties so we can stay focused on the research and development of CYT-108.The first use of funds is the manufacturing of the drug for four broad use for clinical trials. A lot of it needs to be made. The second is qualifying the drug to the FDA. Qualifying means showing that it has certain characteristics. It's pure, it's active, and it works the way we think it does. And the third is actually using those in clinical trials, recruiting the patients, designing the trial and administering the drug and then following up with them. That's a very expensive process. And so that really will take up the vast majority of these funds.
[19:10] Lauren: When do you expect FDA approval? So we. FDA approval will not be for a number of years.
[19:15] Joey: The exact timeline will really be dependent upon how longitudinal the FDA wants the study to be. Meaning how long after treatment does the FDA want to have us measure the effects of the drug? And that could that could vary quite a bit. And that's one of the things that we're talking with the FDA about. We think it will be a phase one clinical trials by 2023. So we expect to exit somewhere between phase one and phase two. So we're looking at about three to four years. That's our goal for an exit event, for our investors to see a return on their investment. Without the intention of actually taking it all the way to commercialization.
[20:10] Lauren: Who are your top competitors?
[20:16] Joey: So. Honestly, there really aren't any competitors. And the reason for that is nobody is actually looking to create a solution, they are looking to create pain management systems and anti-inflammatory technology. A lot of the research for osteoarthritis is being directed to non-opiate solutions. But again, those are bandaids. That one company, Ampio, was the only one we're aware of that actually is creating a biologic treatment. And even they aren't exactly sure how it works. It just so happens that it showed activity in laboratory models. So really, this is a wide open space for actual treatment that addresses the cause of osteoarthrits. And that's one of the beautiful things. Nobody really understood how this worked until we discovered that FACT biomarker, which is sort of an indication of how on a molecular level, osteoarthritis is functioning. And that was, you know, relatively recently in the grand scheme of things. And so other companies haven't really been able to catch up to that idea and create a competing technology that once again addresses the cause and isn't some sort of pain management system for the effects of the disease.
[21:56] Lauren: Are you using a partner for marketing the APIC product or will you be using multiple channel partners to sell the product?
[22:05] Joey: So currently we have an exclusive, a single exclusive licensing agreement with a company called CareStream America. That deal was just signed last month for the exclusive global distribution, marketing, manufacturing, primarily in the United States of Canada. But they're also working in the European market and we're seeking to see approval for sale over there. Secondly, we have a deal with a company called Astaria Global, which is a veterinary medical device distributor, and they're building out an equire market because this this technology works beautifully in horses. And we have the data others actually conducted at Boise State that that substantiates that. And we have a number of horse breeders and trainers that are using this product and race horses. And so those two companies are the ones that are building out these markets. And our concerned with the marketing and the business development side of things. So once again, we're staying focused on the development of CYT-108 and advancing clinical trials without being distracted by trying to sell APIC.
[24:36] Lauren: Are there any side effects with CYT-108?
[24:40] Joey: So I can't say for sure because it's not being used in humans. In our large animals study we didn't observe any sort of immune response, nor did we observe any sort of damage to the organs. What this suggests to me is that if it worked in animals and it's a variant of the human protein, well, then it should show with same, if not even better, safety profile in humans. That's not substantiated by data yet. But that's simply the logic. So when it comes to side effects, the reason that I don't believe that that's really going to be a big issue is because the drug is administered directly to a joint and not put in the systemic circulation. Now, this drug is very big. Physically, it's a big molecule, which means it's difficult to diffuse out of the joint into the bloodstream. So if it was in the bloodstream. You'd expect something. The body's going to recognize it as a foreign agent and it's going to up regulate some antibodies. We saw none of that.
[26:45] Lauren: Great. We have time for one more question. Why are you seeking funding via this route and not VC? Right.
[26:50] That's a great question. And so VC tends to be highly dilutive as well as very controlling. And so at this stage, it is not attractive to raise money at the valuation they would propose. Nor do we want a venture capital firm to accommodate and sort of restructure our board and put people in places of management. We've succeeded to date with a very lean business model. So it's unique. It's certainly not traditional, but as many people, you know, on this conference call have experienced, it's sort of a unique opportunity. And venture capital can be kind of stifling for early stage companies.
[28:26] Lauren: Great. Thank you so much, Joey. And thank you to everyone who asked questions. If your question was not answered, we will be following up with unanswered questions to the company, who can then post answers to the discussion board in the coming days. So please be there. Thank you again, everyone, for joining. This concludes today's webinar.
[28:30] Joey: Thank you so much. Appreciate it.