UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 1-K
ANNUAL REPORT
ANNUAL REPORT PURSUANT TO REGULATION A OF THE SECURITIES ACT OF 1933
For the fiscal year ended December 31, 2021
CYTONICS CORPORATION
(Exact name of registrant as specified in its charter)
| Commission file number: | | 024-11196 |
| | | |
| Florida | | 20-8883869 |
(State or other jurisdiction of incorporation or organization) | | (I.R.S. Employer Identification No.) |
| | | |
658 West Indiantown Road, Suite 214 Jupiter, Florida | | 33458 |
| (Address of principal executive office) | | (Zip Code) |
(561) 406-2864
(Registrant’s telephone number, including area code)
Series C Preferred Stock
(Title of each class of securities issued pursuant to Regulation A)
PART II
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This annual report contains forward looking statements that are subject to various risk and uncertainties and that express our opinions, expectations, beliefs, plans, objectives, assumptions or projections regarding future events or future results, in contrast with statements that reflect historical facts. Many of these statements are contained under the headings “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and “Business.” Forward-looking statements are generally identifiable by use of forward-looking terminology such as “anticipate,” “intend,” “believe,” “estimate,” “plan,” “seek,” “project” or “expect,” “may,” “will,” “would,” “could” or “should,” or other similar words or expressions. Forward-looking statements are based on certain assumptions, discuss future expectations, describe future plans and strategies, or state other forward-looking information. Our ability to predict future events, actions, plans or strategies is inherently uncertain. Although we believe that the expectations reflected in our forward-looking statements are based on reasonable assumptions, actual outcomes could differ materially from those set forth or anticipated in our forward-looking statements. Factors that could cause our forward-looking statements to differ from actual outcomes include, but are not limited to, those described under the heading “Risk Factors.” Readers are cautioned not to place undue reliance on any of these forward-looking statements, which reflect our views as of the date of this annual report. Furthermore, except as required by law, we are under no duty to, and do not intend to, update any of our forward-looking statements after the date of this annual report, whether as a result of new information, future events or otherwise.
You should read thoroughly this annual report and the documents that we refer to herein with the understanding that our actual future results may be materially different from and/or worse than what we expect. We qualify all of our forward-looking statements by these cautionary statements including those made in Risk Factors appearing elsewhere in this annual report. Other sections of this annual report include additional factors which could adversely impact our business and financial performance. New risk factors emerge from time to time and it is not possible for our management to predict all risk factors, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements. Except for our ongoing obligations to disclose material information under the Federal securities laws, we undertake no obligation to release publicly any revisions to any forward-looking statements, to report events or to report the occurrence of unanticipated events. These forward-looking statements speak only as of the date of this annual report, and you should not rely on these statements without also considering the risks and uncertainties associated with these statements and our business.
Overview
CYTONICS CORPORATION, a Florida corporation (“we,” “us,” “ours” or the “Company”) was formed on July 19, 2006 as a Florida corporation, under the name Gamma Spine, Inc. and was renamed Cytonics Corporation on April 17, 2007. The Company was formed for the purpose of researching and developing, marketing and distributing analytic tools used to detect biomarkers associated with certain diseases referred to as “assays,” therapeutic drugs, and related instruments and disposables related to musculoskeletal diseases. We are a development stage research company dedicated to developing therapeutics based on the naturally-occurring protease inhibitor alpha-2-macroglobulin (A2M), a blood serum protein that has known cartilage-protecting effects and could potentially serve as a treatment for osteoarthritis. To this end, we have developed a number of diagnostic and therapeutic products aimed at treating joint pain and inflammation.
Our mission is to improve people’s lives by limiting the progression of chondral pathology, which is bone and cartilage degeneration, which leads to disabling pain, inflammation, and the development of arthritis. Our strategy has been to leverage the unique molecular characteristics of A2M to develop autologous (“self-derived”) and synthetic (manufactured in a laboratory) therapeutics. We have developed two autologous A2M therapies and have out-licensed the drugs to medical device distributors in the human and veterinary orthopedic markets. Our current focus is on the development of a synthetic A2M variant (“CYT-108”) that can be synthesized in a laboratory and purchased “off-the-shelf,” and can be delivered in high concentrations to damaged and inflamed joints by an orthopedist. We seek to maximize the value of the drugs we discover by putting them in the hands of leading pharmaceutical companies with late-stage development, commercialization and marketing expertise.
The Osteoarthritis (“OA”) market represents a $180 billion annual opportunity, which is currently attributable to treating pain symptoms and invasive surgery. According to the Centers for Disease Control and Prevention (the “CDC”), in the U.S., over 27 million people are treated for back pain every year, and osteoarthritis (OA) affects nearly 30 million people. Back pain is the #1 cause of missed work and the #1 healthcare expense in our country. Joint pain and OA account for millions of doctor office visits each year, and are rapidly rising as the average life expectancy increases. In all, musculoskeletal disease burdens our nation with human suffering, lost productivity, missed work, and excessive medical expenses.
Our Company has developed protein-based diagnostics and therapeutics for chronic orthopedic diseases. In 2010, our Company launched our flagship product, a first-in-kind biomarker assay that detects byproducts of cartilage degradation in joint fluid, called “FACT” (Fibronectin-Aggrecan Complex Test). The FACT diagnostic product is currently sold to physicians nationwide, and is used to assess the extent of cartilage damage in patients and determine the appropriate course of treatment. Samples of patient’s joint (synovial) fluid are delivered to Cytonics’ laboratory for testing, and the results are uploaded to a secure database accessible only to physicians. Over 900 kits were distributed to physicians in 2019, with roughly 934 kits distributed in 2020 and roughly 785 kits distributed in 2021. The decline in sales is related to the shutdown of many medical offices during COVID-19, and the hurdle of re-establishing relationships with this customer base upon re-opening.
We have developed a range of therapeutic products for the treatment of painful osteoarthritis, back, and joint pain based upon our understanding of Fibronectin-Aggrecan Complex formation and the role that A2M plays in regulating cartilage degradation. The Company’s first therapeutic product, APIC-PRP was cleared for sale via the 510(k) regulatory process in January 2014. This technology is predicated on Platelet Rich Plasma (PRP), a concentration of the plasma cells found in blood that is injected into joints that are painful and inflamed. The APIC-PRP system differs from PRP in that it selectively concentrates A2M while removing other blood proteins. The final preparation is a highly-concentrated, A2M-rich solution that can be injected into damaged, painful, and inflamed joints.
The Company has entered into licensing agreements with certain distributors of medical devices whereby the Company has granted sales, marketing manufacturing, and distribution licenses in the human and veterinary markets with exclusive rights to sell both domestically and internationally in the human and veterinary markets the APIC-PRP products and FACT products of the Company.
Our current focus is on the development of a synthetic A2M variant (“CYT-108”), based upon the protein structure of the naturally-occurring A2M molecule that has been shown to inhibit the enzymes that degrade cartilage in disease models of osteoarthritis. This synthetic variant, “CYT-108,” has been shown to inhibit cartilage degradation more than 2-fold over that of the naturally-occurring A2M molecule in small animal models of osteoarthritis (Zhang, Y., Wei, X., Browning, S., Scuderi, G., Hanna, L. S., & Wei, L. (2017)). Targeted designed variants of alpha-2-macroglobulin (A2M) attenuate cartilage degeneration in a rat model of osteoarthritis induced by anterior cruciate ligament transection. (Arthritis Research & Therapy, 19(1). doi: 10.1186/s13075-017-1363-4). Cytonics has contracted a Collaborative Manufacturing Organization and Collaborative Research Organization (Sinclair Research, Auxvasse, MO) to synthesize CYT-108 and perform pre-clinical tests ahead of human clinical trials. In 2019 we successfully purified CYT-108 for pre-clinical trials, which were initiated in October 2019 in an animal model of post-traumatic osteoarthritis. If CYT-108 is approved for sale by the FDA and if CYT-108 is successfully commercialized, we anticipate that CYT-108 will substantially reduce APIC sales as we believe that CYT-108 is a superior treatment option to APIC. We are currently working toward FDA approval by preparing for Phase 1 human clinical trials starting in Q1 of 2023.
For the fiscal years ended December 31, 2021 and 2020, we generated revenues of $307,500 and $591,056, respectively, and reported net losses of $2,544,189 and $995,850, respectively, and negative cash flow from operating activities of $2,784,503 and $585,103, respectively. As noted in our financial statements, we had an accumulated stockholders’ deficit of approximately $19,907,507 and recurring losses from operations as of December 31, 2021. These conditions raise substantial doubt about the Company’s ability to continue as a going concern. Please refer to Note 3 of our audited financial statements included elsewhere in this Annual Report on Form 1-K.
FACT DIAGNOSTIC PRODUCT
Cytonics’ first product was “FACT” (Fibronectin-Aggrecan Complex Test), a pilot-launched lab developed test diagnostic assay that can identify pain generators associated with degenerating cartilage such as in OA or degenerative disc disease. Cytonics demonstrated in top peer-review journals (ID of a Novel Fibronectin-Aggrecan Complex in Synovial Fluid of Knees w/Painful Meniscal Injury; JBJS, Feb 2011; Clinically Significant Improvement in Functional Outcome After Lumbar Epidural Steroid Injection for Radiculopathy is Predicted by Assay for Novel Fibronectin-Aggrecan Complex; Golish SR et al SPINE, August 2011) that “FAC” (Fibronectin-Aggrecan complex) is present in joints exhibiting pain from cartilage degeneration but not in asymptomatic joints. We believe FACT will become an important tool to improve joint and especially spine surgery results currently compromised by poor diagnostics.
FACT™ is an enzyme linked immunosorbent sandwich assay (ELISA) that measures the presence of a FAC in a fluid specimen taken from patients with spine or joint related pain. The presence of the Fibronectin-Aggrecan Complex (FAC) and other related biomarkers has been shown in clinical studies to be associated with inflammation due to tissue damage or degeneration. FACT™ is a Laboratory Developed Test that was developed, and its performance characteristics determined by Cytonics Corporation. The test has not been cleared or approved by the U.S. Food and Drug Administration. Federal law restricts the FACT diagnostic test to sale only by or on the order of a physician. FACT may be protected by U.S. Patent 8,338,572, which was issued to the Company on December 25, 2012 and other pending U.S. and foreign patent applications made by the Company as further described below.
The FACT™ diagnostic product was debuted at the North American Spine Society (NASS) Annual Meeting in October 2010 in Orlando, FL. We received a very positive response from physicians and were awarded “Best New Technology for Spine Care in 2010” and “Best New Technology in Diagnostics and Imaging in 2010.”
Following NASS, we initiated a pilot launch of the FACT testing service with a limited number of physicians, the majority of which were Cytonics’ investors.
In February 2011, Synthes Corporation, a leading orthopedic implant company, purchased 400,000 shares of our Series B Preferred Stock at $2.50 per share, along with the option to purchase additional equity of the Company. In June 2011, Synthes exercised the option to purchase additional equity and also purchased an additional 1,600,000 shares of Series B Preferred Stock at $2.50 per share. On April 2013, Johnson and Johnson (“J&J”), who purchased Synthes in 2012, purchased an additional 87,500 shares of Series B Preferred Stock at $4.00 per share.
In June 2011, Synthes also entered into an Exclusive Global Marketing and Distribution Agreement (“Agreement”) with the Company. In the Agreement, Synthes received an exclusive worldwide license to sell and market certain of the Company’s diagnostic products. As part of this Agreement, Synthes also received the option to negotiate for the purchase of an exclusive worldwide license to sell and market the Company’s autologous and non-autologous therapeutic products. The Synthes option to negotiate for the Company’s autologous therapeutic products expired in 2012. In April of 2013, the marketing and distribution agreement was terminated upon mutual agreement (with J&J). All options expired upon termination of the Agreement.
The FACT diagnostic product is currently sold to physicians nationwide, and is used to assess the extent of cartilage damage in patients and determine the appropriate course of treatment. Samples of patient’s joint (synovial) fluid are delivered to Cytonics’ laboratory for testing, and the results are uploaded to a secure database accessible only to physicians. Over 900 kits were distributed to physicians in 2019, with roughly 900 kits distributed in 2020 and roughly 785 kits distributed in 2021. The decline in sales is related to the shutdown of many medical offices during COVID-19, and the hurdle of re-establishing relationships with the customer base upon re-opening.
FACT REGULATORY STRATEGY
Cytonics testing service for FACT is regulated under the Clinical Laboratories Improvement Amendments of 1988. The FACT is considered a Lab Developed Test (LDT) and has not been cleared or approved by the U.S. Food and Drug Administration (FDA). The FDA does have enforcement authority over LDT's, but until now has not exercised its authority, except on a very limited basis.
The current FDA regulatory landscape of In Vitro Diagnostic (IVD) and LDTs remains unclear. However, based on the publicly available information, as well as our current regulatory experience, the FDA appears to be considering its authority of LDTs marketed without FDA clearance or approval at this point in time. Given that the FACT™ assay meets the definition of an LDT (i.e., IVD test created and used by the same CLIA-certified laboratory; “CLIA” means clinical laboratory improvement amendments), it is our understanding that FDA likely would allow our strategic partner to market the FACT™ assay without premarket clearance or approval under the agency’s enforcement discretion pending forthcoming guidance from FDA regarding the future regulation of LDTs. Thus, it is our view that until such time that FDA provides further guidance on its strategy for regulation of LDTs, we may offer the FACT™ assay as an LDT through our CLIA-certified laboratory without receiving FDA clearance or approval. However, there can be no assurance that our understanding of the foregoing is correct.
The FDA has detailed a potential risk-based classification scheme for the future regulation of LDTs. Based on our experience with currently regulated IVDs, it is our understanding that the agency’s proposed risk-based classification scheme, if pursued by FDA, likely will correlate to well-defined regulatory pathways. For example, makers of an LDT that could result in serious injury or death, result in difficulty detecting a false result, or have a high public health risk likely will be required to submit an FDA approval mechanism that requires randomized prospective data (“PMA”). Next, makers of an LDT that could result in a non-serious injury, result in a relatively easy to detect false result, or are an adjunctive test likely will be required to submit a 510(k) notice. Lastly, makers of an LDT that could result in little potential for injury, result in an easy to detect false result, or are a highly adjunctive test likely will be 510(k) exempt. However, the agency has acknowledged that there are a number of issues that remain undecided including, whether some tests will remain subject to enforcement discretion and the timeline for phasing in a new LDT regulatory scheme. It is our view that, similar to previous guidance issued by FDA where the agency seeks regulatory oversight regarding LDTs, there very likely will be a reasonable grace period for companies to comply with any new regulatory requirements.
It is our further view that our FACT™ assay will be considered by FDA to represent a moderate risk device. Moderate risk devices are often regulated through the 510(k) process if an appropriate “predicate” class I or class II device can be identified. In the event that our assay is subject to FDA regulations sometime in the future, we will implement a strategy that has the greatest chance that the FACT™ assay will be found to be subject to the lowest level of regulation. We will identify the appropriate class I or class II predicates but we will have to work with FDA through the pre-submission process to determine whether FDA will accept the company’s 510(k) substantial equivalence arguments.
In summary, while the FDA continues to consider implementation of enforcement over LDTs, we can currently offer the FACT™ assay through a CLIA-certified laboratory. Should our assay become subject to new FDA regulation, our strategy will be to request FDA oversight of the FACT™ assay at the lowest level of regulation. Finally, even with future FDA regulation of LDTs, it is likely that the agency will provide a grace period for LDTs currently offered through CLIA laboratories to comply with any new regulatory requirements. Accordingly, we believe that we are likely to have a period of time while offering the FACT™ assay as an LDT in which to seek FDA premarket clearance or approval, if required. However, there can be no assurance that the foregoing will occur as expected, and if the FDA decides to take different action that we expect, it could have a material adverse effect on the Company.
ORTHOPEDIC THERAPIES
Cytonics exploited its unique understanding of the cartilage degenerative/pain cascade to create therapeutic products for the treatment of painful osteoarthritic joint and spine disease. Its key scientific discovery is that Alpha-2-Macroglobulin (“A2M”), a protein that can be concentrated from a patient’s blood, inhibits catabolic proteases that have long been known to be implicated in cartilage breakdown secondary to OA. According to the CDC, OA affects over 30 million U.S. patients each year and while clinicians offer various injections to treat the associated pain, there is no definitive treatment except for expensive joint replacement. Cytonics’ development and clinical- regulatory projects have already brought two autologous A2M versions to the clinic to treat the disease as well as its painful symptoms and we believe will culminate in already-optimized recombinant off-the-shelf versions.
The Company’s first therapeutic product, Autologous Platelet Integrated Concentrate (“APIC”) received 510(k) clearance (FDA approval for medical devices) as platelet rich plasma (PRP) in January 2014 (BK-130060-0). Unlike other PRP products which proved only modest clinical benefit, APIC’s novel two-stage process follows traditional centrifugation with a second, filtration step, resulting in a plasma concentrate that is low in WBCs (which are harmful to intra-articular healing (Platelet-Rich Plasma Increases Matrix Metalloproteases in Cultures of Human Synovial Fibroblasts; Browning SR, Weiser Am, Scuderi GI, Carballo C, Hanna LS; JBJS Am 2012; 94(23); e1721-e1727) and which concentrates not just platelets but also alpha-2-macroglobulin, (A2M) a potent and broad-based protease inhibitor. The Company has entered into licensing agreements with certain distributors of medical devices whereby the Company has granted sales, marketing manufacturing, and distribution licenses in the human and veterinary markets with exclusive rights to sell both domestically and internationally in the human and veterinary markets the APIC-PRP products of the Company.
Inspired by Cytonics’ 2013 Orthopedic Research Society presentation of its pilot work with A2M, (Is There a Chondroprotective effect of Autologous Protease Inhibitor Concentrate on an Osteoarthritis Rabbit Model? A Pilot Study; Cuellar J and V, Browning S, Scuderi G, Golish R, Hanna L; presented 2013 ORS, San Antonio) independent investigators from Brown University supported by NIH and its Chinese counterpart published in July 2014 a seminal paper validating Cytonics’ work and concluding that “…. supplemental A2M provides chondral protection in post-traumatic OA.”(Identification of a2-Macroglobulin as a Master Inhibitor of Cartilage-Degrading Factors That Attenuates the Progression of Posttraumatic Osteoarthritis.; Wei et al; Arthritis and Rheumatology Vol 66 No. 7, July 2014). This team continues to pursue independent animal research demonstrating that A2M protects joints from chronic osteoarthritic degradation.
RECOMBINANT
Cytonics’ ultimate goal is to expand upon the therapeutic potential of the naturally-occurring (“wild-type”) A2M molecule by engineering a synthetic (“recombinant”) version of the wild-type A2M. We believe that this will allow us to create an off-the-shelf biologic drug that can be produced in high concentrations and in mass quantities. The creation of a recombinant A2M molecule involves three distinct steps: (1) Obtaining the DNA sequence that encodes for the A2M protein found in the body, (2) Engineering that DNA sequence to give the A2M variant improved functionality, and (3) Expressing the A2M variant DNA to create an A2M variant protein product (the final drug that has therapeutic function). This is accomplished using high speed screening and discovery techniques. Cytonics screened thousands of A2M variants and identified multiple optimized recombinant forms whose protease inhibition exceeds that of the naturally occurring, wild-type A2M. These select variants have been synthesized, validated in vitro, secured by an international patent portfolio, and have demonstrated cartilage-protective activity and tolerability in preclinical work in a small animal model at Brown University. We believe that these research efforts confirm Cytonics’ ability to engineer biologic variants of naturally-occurring molecules for therapeutic purposes. We have contracted with Goodwin Laboratory (Plantation, FL) for GLP/GMP production of our A2M variants and to move forward with pre-clinical trials. A pilot pre-clinical study was initiated in October 2019 at Sinclair Research (Auxvasse, MO) and concluded in December 2019.We concluded the study in Q1 2020 and it was determined that CYT-108 showed signs of activity in preserving cartilage and other joint tissues, as measured by an independent pathologist, and safety in an animal model of osteoarthritis.
APIC THERAPEUTIC PRODUCTS
Cytonics has developed autologous treatments for osteoarthritis, back, and joint pain. Based on Cytonics’ in depth knowledge of the pain cascade and our discovery of FAC, we have developed a method to concentrate proteins that are known to inhibit cartilage degeneration and potentially prevent the formation of FAC. We have developed the APIC PRP and APIC Mini Systems, which utilize a unique and proprietary process to concentrate platelets, growth factors, and/or protease inhibitors from a patient’s own blood for a range of orthopedic applications. The resulting concentrated formulas have been shown to slow the breakdown of cartilage in vitro and in vivo studies. To-date, over 8,000 patients suffering from joint pain and inflammation have been treated with Cytonics’ APIC system.
APIC REGULATORY STRATEGY
In January 2014, we received FDA 510k clearance for our APIC PRP System. This was a major milestone for the Company and allows us to sell and market the APIC System in the U.S. In 2020 we entered into an agreement with Christie Medical Holdings, the holding company of CareStream America, an international manufacturer and distributor of medical devices, for the exclusive manufacturing and sales right of the APIC system in the international human market. The agreement also included the first rights to develop our second autologous technology, the APIC Mini, a more cost efficient version of the APIC PRP System that is appropriate for treating small joints. CareStream America has completed the comparable system testing in preparation for a 510k submission in the first half of 2023.
We have received CE (“Conformité Européenne” which means European Conformity) mark approval for use of the APIC Systems in Europe. We have no plans currently to pursue sales and marketing of the APIC Systems for use in Europe.
We are supported in all FDA submissions by our regulatory affairs counsel.
ADDITIONAL PRODUCTS
The Company plans to develop additional products that include therapeutics that we plan to manufacture, outsource to contract manufacturing, or license to strategic partners. Products currently in development or envisioned include the following:
| | 1. | Recombinant Protein Therapeutic – A recombinant protein therapy delivered locally to the source of pain to reduce inflammation, eliminate pain, and to promote healing. |
| | 2. | APIC PRP for the treatment of chronic wounds. |
| | 3. | APIC Mini for the treatment of small joints in humans and animals. |
CHRONIC WOUNDS
A further development is that the same MMP and other proteases that are implicated in OA – and inhibited by A2M – are elevated in many chronic wounds, thus prevent their healing, (Analysis of the acute and chronic wound environments: the role of protease and their inhibitors; Trengove N, Schultz G et al; Wound Repair and Regenerative Medicine 1999 Nov-Dec; 7 (6); 442-451 and Protease Activity Levels Associated with Healing Status of Chronic Wounds; Serena, T et al; Poster 429, European Wound Management Association (EWMA) meeting Vienna, 2012) and that APIC has been shown to significantly decrease the protease levels that are upregulated in Elevated Protease Activity (“EPA”) wounds (Communications, Drs. Serena, Kirsner, Hanft). While this represents an enormous clinical need and market, Cytonics has elected to prioritize and pursue other clinical indications, such inflammatory joint disease, for their technologies.
LICENSING AGREEMENTS
A2MCyte, LLC
In October 2015, the Company entered into an Exclusive Sales, Marketing, Manufacturing and Distribution Agreement with A2MCyte, LLC, whereby the Company granted to A2MCyte an exclusive manufacturing, marketing and sales license of the Company’s APIC-PRP products during a 5-year period with an exclusive right to sell such products in the United States. In April 2020, prior to the expiration of the term of the agreement in October 2020, the Company exercised its option to terminate the agreement due to A2MCyte’ s continued failure to achieve aggregate product sales milestones since the effectiveness of the agreement. The total amount of payments made by A2MCyte to the Company under the agreement was $1,290,000 consisting of $500,000 of upfront payments and $790,000 minimum royalty payments. There are no more royalty obligations or other payment obligations of A2MCyte under this agreement since the termination of the agreement in April 2020.
Christie Medical Holdings, LLC
On October 8, 2019, the Company entered into a letter of intent, effective January 1, 2020, with Christie Medical Holdings, LLC (“Christie Medical Holdings”) for a grant of an exclusive manufacturing, marketing and sales license in the human markets with exclusive rights to sell both domestically and internationally in the human markets the Company’s APIC-PRP and FACT products. The terms and conditions of the letter of intent were superseded by that certain definitive Exclusive Sales, Marketing, Manufacturing and Distribution Agreement for Human Market, dated as of April 27, 2020, between the Company and Christie Medical Holdings. As of December 31, 2021, the total amount of payments made by Christie Medical Holdings to the Company was $430,000, consisting of $130,000 in upfront payments and $300,000 in royalty payments. To date, the total amount of payments made by Christie Medical Holdings to the Company is $690,000, consisting of $210,000 of upfront payments and $480,000 royalty payments.
Term and Termination Provisions
The term of the agreement is a 10-year term immediately following the effective date of the agreement (April 27, 2020), subject to automatic renewal for an additional successive 5-year term. Either party of the agreement has the right to terminate the agreement upon written notice to the other party in the event of the other party (i) materially breaches or fails to perform any of its obligations, representations or undertakings under the agreement or (ii) becomes insolvent, adjudged bankrupt, liquidates, dissolves or there is an assignment of the other party’s business for the benefit of creditors. The agreement may also be terminated at any time during the term of the agreement by written mutual agreement between the parties.
Upfront Payments
As to upfront payments, pursuant to the terms of the agreement, Christie Medical Holdings is to pay the Company a non-refundable payment in the amount of $450,000, of which (i) Christie Medical Holdings has already paid $210,000 to the Company and (ii) $240,000 is to be paid by Christie Medical Holdings to the Company in three equal payments ($80,000 per payment) at the beginning of each calendar year following the effectiveness of the agreement. As of December 31, 2021, the total amount of upfront payments made by Christie Medical Holdings was $130,000.
Royalty Payments
As to royalty payments, pursuant to the terms of the agreement, Christie Medical Holdings will pay the Company a portion of the revenue generated from sales and distribution of APIC-PRP and FACT products equal to 10% of the aggregate products sales per quarter, payable within 30 days after the end of each calendar quarter during a calendar year. Notwithstanding the foregoing, Christie Medical Holdings is required to pay the Company royalty guaranteed quarterly minimum payments as to APIC-PRP products as follows:
| Royalty Payment Period | | Minimum Due Per Quarter | |
| 3rd Quarter of 2020 through 2021 | | $ | 50,000 | |
| 2022 through 2023 | | $ | 60,000 | |
| 2024 | | $ | 65,000 | |
| 2025 | | $ | 70,000 | |
| 2026 through 2029 | | $ | 75,000 | |
Sales Milestones
There are no sales milestone requirements under this agreement.
Astaria Global, LLC
On June 30, 2019, the Company entered into an Exclusive License Agreement for Manufacturing, Sales, Marketing, and Distributing in the Veterinary Market with Astaria Global, LLC, a device distributor in the veterinary market, whereby the Company granted to Astaria Global, LLC an exclusive manufacturing, marketing and sales license of the Company’s APIC-PRP products in the veterinary markets with an exclusive right to sell such products in the veterinary markets both domestically and internationally. As of December 31, 2021, the total amount of payments made by Astaria Global, LLC to the Company was $290,000, $250,000 of which were upfront payments. To date, the total amount of payments made by Astaria Global, LLC to the Company is $372,500, consisting of $287,500 of upfront payments and $85,000 royalty payments.
Term and Termination Provisions
The term of the agreement is in perpetuity following the effectiveness of the agreement (June 30, 2019). Either party of the agreement has the right to terminate the agreement upon written notice to the other party in the event of the other party (i) materially breaches or fails to perform any of its obligations, representations or undertakings under the agreement or (ii) becomes insolvent, adjudged bankrupt, liquidates, dissolves or there is an assignment of the other party’s business for the benefit of creditors. The agreement may also be terminated at any time during the term of the agreement by written mutual agreement between the parties.
Upfront Payments
As to upfront payments, pursuant to the terms of the agreement, Astaria Global, LLC agreed to pay the Company a non-refundable licensing fee in the amount of $400,000, of which (i) Astaria Global, LLC has already paid $287,500 to the Company to date and (ii) the remainder is to be paid by Astaria Global, LLC to the Company in three equal payments ($37,500 per payment) payable within 15 days of the beginning of each of the third and second quarters of calendar years 2021, 2022, and 2023. As of December 31, 2021, the total amount received was $290,000, $250,000 of which were upfront payments.
Royalty Payments
As to royalty payments, pursuant to the terms of the agreement, Astaria Global, LLC will pay the Company a portion of the revenue generated from sales and distribution of APIC-PRP products equal to 2%, 3%, and 4% of the aggregate products sales per quarter for calendar year 2021, calendar year 2022, and calendar years 2023 and thereafter, respectively, payable within 15 days after the end of each calendar quarter during a calendar year. To date, $40,000 (including royalty guaranteed quarterly minimum payments) as to APIC-PRP products have been paid by Astaria Global, LLC to the Company.
Sales Milestones
There are no sales milestone requirements under the agreement.
COMPANY AND TECHNOLOGY
Cytonics is a research-driven company that has published 14 peer-review papers. We currently have seven U.S. patents (US 10,265,388, US 11,040,092, US 10,940,189, US 9,352,021, US 9,498,514, US 10,400,028, US 10,889,631), three U.K. patents (GB2501611, GB2503131 and GB2522561), two European patents (EP 2827882 and EP 3221341; each of which are validated in FR, DE, and GB), one Canadian patent (CA 2865170), two Australian patents (AU 2013222414, AU 2015349782), and one Japanese patent (JP 6861152). The Company also has five additional related pending patents applications. The Company has also achieved early revenue through the out-licensing of its autologous APIC-PRP technology to a US-based distributor of medical devices. It maintains its laboratory and office in Jupiter, Florida. Its first therapeutic product, APIC-PRP was cleared for sale via the 510(k) regulatory process in January 2014. Unlike traditional PRP or other injectable products that have been recently directed at orthopedic indications, Cytonics APIC products were conceived specifically for use in osteoarthritis (OA) joints, and, while not required by FDA, were validated in the lab prior to pilot launch. The Company has entered into certain licensing agreements with to certain distributors of medical devices whereby the Company has granted sales, marketing manufacturing, and distribution licenses in the human and veterinary markets with exclusive rights to sell both domestically and internationally in the human and veterinary markets the APIC-PRP and FACT products of the Company.
THE SCIENCE OF OSTEOARTHRITIS AND CYTONICS A2M
The cause and progression of osteoarthritis (OA) is complex. It is known that there are biomechanical and biochemical contributors as well as consequences to the disease but because this is still not fully understood there are no treatments that prevent or significantly slow its progression.
Currently available non-surgical therapies are nonsteroidal anti-inflammatory drugs (NSAIDs), corticosteroid injections and visco-supplementation which all treat pain and functional symptoms, temporarily. Joint replacement surgery and physical therapy following injury can address the biomechanical aspects of OA, but there are as yet no effective therapies to address the biochemical contributors. Any treatment that will delay and reduce the necessity of expensive joint replacement will be very beneficial for the patient and reduce the economic burden.
PROTEASES AND PROTEASE INHIBITORS
Cartilage degradation is the hallmark of OA and while it has been researched for years, inhibition of its progression has proven elusive. Many metabolic and physiologic processes in animals are regulated by an interactive balance between proteases and their inhibitors. Homeostasis (a healthy, steady state) is achieved by this carefully regulated balance and imbalance – such as that observed when cartilage cell physiology changes - can result in a disease state. A growing body of evidence agrees that cartilage catabolism (the breakdown of molecules and thus tissue) follows changes in the normal physiology of cartilage cells, leading to upregulation (and consequent “overbalance”) of metalloproteases (a large family of catabolic protein enzymes that break down cartilage).
Cytonics’ First Cartilage Degradation Discovery
A spine surgeon, Dr. Scuderi, in his initial Cytonics research program, sought to enable definitive identification of the pain generator in Degenerative Disc Disease (DDD). The current imperfect diagnosis techniques for DDD lead to suboptimal surgical results for debilitating procedures that cost patients and payers $40 billion per year. Cytonics demonstrated that Aggrecan fragments from degraded cartilage form complexes with Fibronectin from the synovial or disc fluid in vivo and that the complex can be assayed as a biomarker for pain associated with the degenerating cartilage. They named the complex “FAC” (Fibronectin-Aggrecan complex” and the assay for it “FACT”.)
They then reasoned that perhaps FAC was not just a byproduct of cartilage degeneration but was a mediator in the degenerative cascade. By this hypothesis, preventing its formation could stop the degenerative process or the associated pain and so this guided the next phase of Cytonics’ research.
Cartilage degradation in OA has been associated with increased activity of several classes of proteases, including A Disintegrin-like and Metalloproteinase with ThromboSpondin (ADAMTS)-4 and ADAMTS-5, matrix metalloproteinase’s (MMPs) 1, 5-7 and inflammatory proteases (elastase and cathepsin-G) 8-10. Proteases break down molecules (and thus tissues) by cleaving protein bonds. The cleaving of an Aggrecan fragment, which then forms a complex with fibronectin, is an example of such catabolism.
Alpha-2 Macroglobulin, a broad-based protease inhibitor
Cytonics’ next key understandings and discoveries relate to findings that Alpha-2-Macroglobulin (A2M) functions as a highly potent serine protease inhibitor throughout different tissues and extracellular spaces. A2M is a glycoprotein in blood plasma that can inhibit a broad range of MMPs and ADAMTSs, as well as other serine proteases such as elastases and cathepsins. It could thus inhibit OA progression.
While not the current focus of Cytonics’ clinical research, the use of A2M to also inhibit the pain and progression of disc degeneration via local delivery – which some believe to be the “holy grail” of DDD treatment, is another key clinical opportunity for Cytonics. The spine clinic has shown great interest in such a treatment, but multiple high visibility, high-cost programs to treat DDD pain via injection have all failed. Sixteen of the leading spine surgeons and interventionalists have expressed an interest in treating DDD with Cytonics’ products.
It has also been demonstrated in experimental models, and in pilot clinical experience, that A2M is capable of in inhibiting the proteases implicated in non-healing wounds with Elevated protease Activity (EPA), recently determined to represent as much as a third of chronic wounds.
How does A2M inhibit proteases that cause OA?
A2M is a “suicide inhibitor” that uses a highly potent ‘bait and trap’ approach to protease inhibition. A2M is a cage-like protein with a central “bait region” which is particularly subject to proteolytic cleavage (Figure 2.2 F1).
Fig 3.1: Diagram of A2M entrapment of proteases.
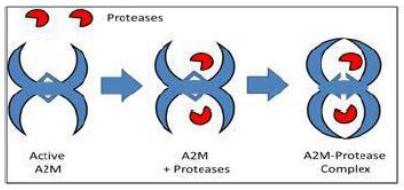
Once a protease enters the A2M “trap” and cleaves the “bait region” A2M undergoes a conformational change that closes the trap and prevents the protease from escaping {Feldman, 1985}. Additionally, the conformational change reveals a binding region to the LRP-1 receptor, which helps facilitate the removal of A2M-protease complexes.
A2M IS A POTENTIAL THERAPEUTIC AGENT
A2M is thus an appealing candidate to inhibit the proteases that break down cartilage in OA (and, it turns out, in some other disease states as well). Cytonics first demonstrated that the concentration of A2M in synovial fluid of a healthy person is less than 30 ug/ml, whereas in OA joints A2M is found to be 80 - 120 ug/ml. This is consistent with the idea that under natural conditions A2M in synovial fluid is not sufficient to stop the early initiation of OA. Thus, increases in A2M (for example, by addition of additional A2M) may be therapeutic.
Validation of A2M and APIC as Therapeutic Agents
Cytonics next conducted a series of experiments to demonstrate that A2M inhibits OA progression. It then repeated the experiments with the “product version” of A2M, Autologous Platelet Integrated Concentrate or APIC, which is now marketed in the U.S. Several of these studies were integral components of Cytonics’ 700-page Investigational New Drug Application to FDA, which was approved in 30 days with no questions, a highly unusual result and a tribute to the submission’s quality.
This series of experiments and their findings are summarized below.
| | 1. | A2M inhibits OA in ex vivo cartilage models. |
Cytonics first employed a series of traditional ex-vivo experiments. In these experiments, explanted specimens of bovine cartilage explants (“BCE”) were degraded by addition of a series of known OA-associated matrix metalloproteinases: (ADAMTS-4, ADAMTS-5, MMP-3, MMP-7, MMP-12, or MMP-13).
The experiment first quantified the degradation of the BCE by each protease, then repeated with the addition of A2M to demonstrate, in compelling and unambiguous results, that it greatly inhibits the degradation of bovine cartilage explants by each of the proteinases.
| | 2. | A2M inhibits indirect cartilage catabolism by pro inflammatory cytokines |
To further confirm and clarify its findings, Cytonics then tested in the same model, the impact of A2M on pro-inflammatory cytokines TNF-α and IL-1β, which act upon endogenous chondrocytes in the cartilage to produce proteases, also and “indirectly” breaking down cartilage resulting in increased sGAG in the media. A2M was shown to similarly inhibit these cytokines and their downstream proteases.
| | 3. | APIC inhibits OA in ex vivo cartilage models. |
Cytonics’ next series of experiments were created to evaluate not purified A2M as in earlier experiments, but a “product version” of A2M in the same models. Autologous Protease Inhibitor Concentrate (APIC) is Cytonics’ autologous biologic treatment obtained from the patient’s own blood and processed at point-of-service. The APIC process concentrates A2M from the patient’s plasma to 5-7 mg/ml and permits it to be used as treatment for mild to moderate OA. It similarly inhibited cartilage degradation.
| | 4. | APIC inhibits Post-Traumatic Osteoarthritis (PTOA) in a rabbit OA model |
Cytonics next progressed to a well-validated animal model, which mimics the development of early post-traumatic and/or established Osteoarthritis depending on the time point after surgically created ACL injury (“ACL-T”). These studies were key in proving A2M performance and were carried out by AccelLab (Montreal, Canada). AccelLab’s Institutional Animal Care and Use Committee (IACUC) approved all studies, which meet or exceeds all U.S. standards.
 | |
| |
| In this experiment, animals were allocated in 2 study groups and results summarized below: |
| · In treatment Group 1, 6 rabbits received ACL-T surgery and A2M treatment on the right knee, (green) and sham surgery on the left knee. |
| · In control Group 2, 6 rabbits received ACL-T surgery and saline treatment on the right knee, (red) and no sham surgery on the left knee. |
| |
| |
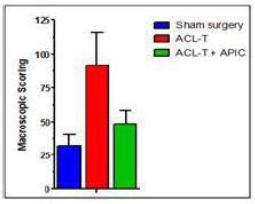
Macroscopic evaluation of the total femur, tibia and patella (below) using the Osteoarthritis Research Society International (OARSI) grading system demonstrated that A2M reduced cartilage degradation by 53 ±20% compared to untreated controls (p = 0.0086).
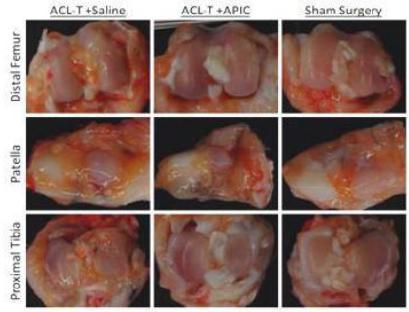 | | Fig 3.3 Macroscopic Images of rabbit knees 6 weeks after ACL Injury and treatment with Saline or APIC. Sham surgeries without ACL-T were performed as a control. |
| | 5. | A2M Confirmed as the Principle OA Therapeutic in APIC |
Despite this exciting conclusion in an animal model, Cytonics continued its characterization of the APIC formulation to confirm that among its multiple plasma components A2M is the active compound.
APIC, as an autologous blood products designed to inhibit cartilage catabolism, consists of all the constituents of plasma at some concentration. Therefore, other common blood protease inhibitors a1-anti-trypsin (AT), a1-anti-chymotrypsin, (ACT) and anti-thrombin III (ATIII) are present in APIC and were tested for their ability to inhibit cartilage catabolism in the Bovine Cartilage Explant (BCE) model. In summary, these experiments confirmed that while A2M is able to completely inhibit the pro-inflammatory cytokine-induced catabolism of cartilage (A, below) equal molar amounts of purified human AT, ACT, and heparinized ATIII did not. Further, the chondroprotective abilities of purified AT, ACT, and ATIII at the concentrations present in APIC were tested (B, below) with similar conclusion.
| | 6. | A2M inhibits FAC formation |
Coming full circle, Cytonics then ran novel experiments that used the culture media from BCE experiments (test series 1) to confirm that A2M does in fact inhibit the formation of FAC, the cartilage breakdown product that is the subject of its diagnostic product. It was demonstrated that treatment of the cartilage with A2M prevented cartilage catabolism and subsequently inhibited FAC formation. This finding inspired the compelling clinical project that won for Cytonics the Best Paper Award at 2015 ISIS, wherein Cytonics found that patients who are “FAC+” within the disc are more likely to demonstrate clinical improvement following intradiscal autologous A2M injection. The results of this investigation suggest that autologous A2M may be an efficacious biologic treatment in discogenic pain and that FAC may be an important biomarker in patient selection for this treatment.
Together, these studies demonstrate that A2M and autologous APIC are capable of treating OA with little to no side effects. Several of these studies were vital components of Cytonics’ 700-page IND submission to FDA, which can be reviewed in its entirety upon request and execution of a Non-Disclosure Agreement.
APIC-PRP AS A THERAPEUTIC FOR CHRONIC WOUNDS
Cytonics is fully focused on the treatment of OA but has previously established that other disease states can be treated with its existing APIC products.
An understanding in the treatment of chronic wounds is that many of those that are most resistant to healing are characterized by Elevated Protease Activity (EPA) (Treatment of Osteoarthritis of the Knee; Evidence-Based Guideline, 2nd Edition; AAOS, May 18, 2013). This has led to a growing interest in specialized methods to treat these wounds, and in new point-of-care diagnostics (Systematic Review for Effectiveness of Hyaluronic Acid in the Treatment of Severe Degenerative Joint Disease (DJD) of the Knee; AHRQ, Dec 14, 2014), that can identify these wounds in a matter of minutes so as to offer them special treatment. Elevated Protease Activity (EPA) contributes to non-healing wounds by digesting growth factors that assist in the healing process, degrading Extracellular Matrix (ECM) proteins, and disrupting cell-cell and cell-matrix communication. MMP-2, MMP-8, and Neutrophil Elastase have been identified as important factors in EPA non-healing wounds.
Currently there are no therapies that we are aware of that seek to treat these wounds by inhibiting the elevated proteases that characterize them. Rather, companies offer re-purposed collagen wound dressings, which have been a staple of wound care for decades. As the target proteases attack collagen binding sites as well as ECM and other wound healing factors, it is proposed that these aged collagen products compete for protease binding sites and thus lessen the impact of EPA.
As the proteinases implicated in EPA are closely related to those that APIC products inhibit in orthopedic joints, Cytonics sought to determine if they would be similarly inhibited in these wounds. In experiments carried out in conjunction with University of Florida-Gainesville, Cytonics demonstrated that both APIC and A2M inhibited protein digestion by Chronic Wound Fluid (WF) from a patient with a DFU, in a dose-dependent manner. This exciting result suggests a new treatment in heretofore hard-to-heal chronic wounds, and several leading wound care clinicians successfully treated difficult, long term non-healing wounds with APIC, and expressed interest in treating more of their chronic wound patients with APIC.
Cytonics is not pursuing this program at this time and believes that with the anticipated U.S. release of the first point-of-care EPA diagnostic, this first and only treatment that inhibits EPA proteases will be a valuable out licensing opportunity for the $6 billion Advanced Wound Care segment. It has been estimated that a third of the chronic wounds treated in this hard-to-heal segment are EPA wounds.
CYTONICS THERAPEUTIC PRODUCTS
1. APIC PRP
Cytonics’ first therapeutic product APIC PRP received FDA permission to market via the 510(k) process in January 2014. Like all other PRP systems and despite its distinguishing technology and performance, Cytonics APIC-PRP is indicated by its FDA-approved labeling for:
The Cytonics Autologous Platelet Integrated Concentrate (APIC) is indicated for the rapid preparation of autologous platelet rich plasma from a small sample of blood at the patient's point of care. The platelet rich plasma is mixed with autograft and/or allograft bone prior to application to a bony defect for improving bone graft handling characteristics.
This means that the single indication for which PRP products are approved is one that is rarely practiced and is irrelevant to current PRP or APIC use. While Cytonics’ goal is to create products to treat OA, and not necessarily to “be in the PRP business,” a strategic decision was made to gain quick APIC access to the clinic via this method, before pursuing the more time consuming Investigational New Drug process for its biologic drug candidate, “CYT-108”. Following pilot launch in 2014, Cytonics has seen increasing success and interest in its APIC system.
Like earlier PRP systems it incorporates a centrifuge to concentrate platelets, but APIC PRP (below) is distinguished from all other systems primarily by a second step that further concentrates the platelets as well as the protease inhibitor A2M.
Fig 4.1. Pictures of APIC PRP system hardware and disposable (below)
Because APIC products are conceived specifically to treat degenerating cartilage in joints and other inflammatory pathology related to protease activity, they have specific features that differentiate them from other systems. Key to all APIC products are features that concentrate A2M. After centrifugation, the APIC PRP System utilizes a hollow fiber Tangential Flow Filter (TFF, schematic in Fig 2.) to further concentrate platelets and concentrate A2M.


Fig. 4.2 Tangential Flow Filter
The resultant concentrate has a low RBC content, low WBC and a very high A2M concentration. Because it has been demonstrated that WBCs – often concentrated along with platelets – are actually harmful in the treatment of joint pain (Misharin, A. V., Cuda, C. M., Saber, R., Turner, J. D., Gierut, A. K., Haines, G. K., … Perlman, H. (2014). Nonclassical Ly6C− Monocytes Drive the Development of Inflammatory Arthritis in Mice. Cell Reports, 9(2), 591–604. doi: 10.1016/j.celrep.2014.09.032), Cytonics configured its system to have a WBC content barely higher than physiologic, unlike most other systems. (Figure 3.3)
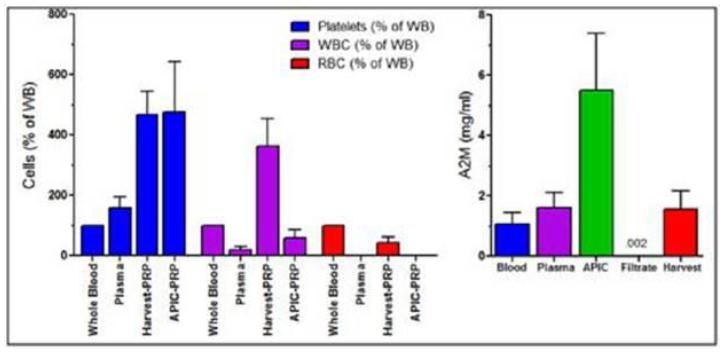
Fig. 4.3 Characterization of WBC, RBC, Platelet and A2M content of Cytonics and Harvest PRP Systems
2. APIC MINI
 | | The APIC Mini system was designed to provide an A2M concentration in smaller volumes for applications such as spinal discs and facet joints, small joints (i.e. wrist, hand), and podiatry. The APIC Mini System will provide the market with the only low cost A2M concentration System. This system requires a lower volume of blood and yields approximately 2cc’s of concentrated APIC. This product will not require a separate pump, as in APIC-PRP. There will be a 2-step process with centrifugation only utilizing a filter in the centrifugation step. Christie Medical Holdings has finished testing with our predicate device and are awaiting biocompatibility-testing results. They anticipate filing with 510k application for this product in the first half of 2023. We anticipate a similar labeling to APIC-PRP. |
Fig 4.4. Picture of APIC Mini system disposable kit
“The Cytonics Autologous Platelet Integrated Concentrate (APIC-Mini) is indicated for the rapid preparation of autologous platelet rich plasma from a small sample of blood at the patient's point of care. The platelet rich plasma is mixed with autograft and/or allograft bone prior to application to a bony defect for improving bone graft handling characteristics.”
3. Recombinant A2M Program
Cytonics’ ultimate goal with its A2M program is to bring recombinant, off-the-shelf products to the various clinics, bypassing the need to prepare APIC products onsite. Recombinant products offer multiple advantages including the ability to have variants that are more effective than “wild-type” and are the obvious culmination of this program.
Cytonics’ efforts to identify optimal recombinant variants began in 2011, where intense, targeted design and production of A2M variants were screened against “wild type” A2M using both in vitro protease digestion assays and ex vivo cartilage models of OA. As indicated below, several of the variants have demonstrated considerably more inhibitory effect than human “wild type” (also recombinantly produced) A2M. We have achieved success in a small animal model of OA at Brown University (REF). Our recombinant variant CYT-108 performed better than Wild Type A2M and showed evidence of cartilage upregulation in RT-PCR. This is an important accomplishment as it represents an IND enabling study for an eventual Biologic License Application (BLA). Our next step is protein development and scale to accomplish a large animal study, and toxicity study.
Fig. 4.7 Comparison of Candidate Inhibition Compared to Wild Type A2M. Fig. 4.8 Summary of animal data (N=77) CYT-108 statistically better then WT in preventing PTOA in ACL-T rat model | 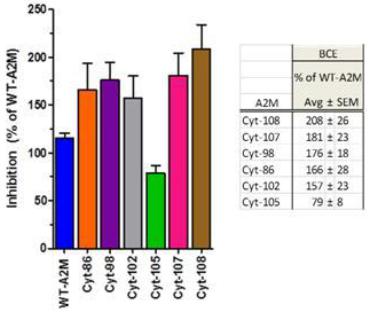 |
Fig. 4.9 Wild-type alpha-2-macroglobulin (WT-A2M) (left) and A2M variants CYT-98 and CYT-108 (right) inhibit cartilage catabolism induced by TNFα and IL-1β. *Compared with wt-A2M, P < 0.05. BCE, bovine articular cartilage explants, SGAG sulfated glycosaminoglycan | 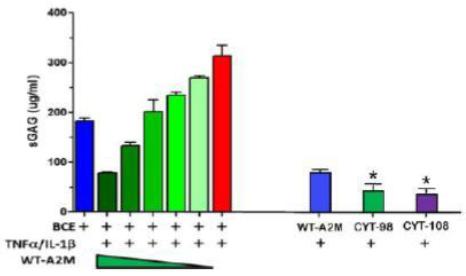 |
In 2020, we concluded a plot preclinical study to examine the effects of CYT-108 when administered subcutaneously and intra-articularly (in the joint). When injected subcutaneously at a 10x proposed dose (5mg per kg of body weight), we observed no adverse effects nor organ damage as determined by an independent pathologist. We also observed some signs of efficacy, although the data was not statistically significant due to the small sample size.
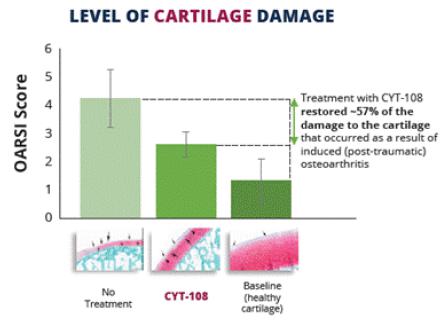
Figure 5.0. Intra-articular injection of CYT-108 at a dose of 0.5mg/kg (proposed effective dose) appears to prevent up to 57% of the damage to the cartilage tissue on the articular surface of the femur and tibia in the knee joint. Note: data is not statistically significant.
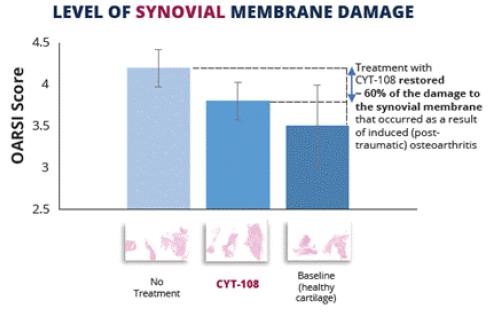
Figure 5.1. Intra-articular injection of CYT-108 at a dose of 0.5mg/kg (proposed effective dose) appears to prevent up to 60% of the damage to the synovial membrane encapsulating the joint. Note: data is not statistically significant.
Note: The data represented in Figures 5 and 5.1 (and the next slide) is from a preclinical experiment conducted by Sinclair Research Center LLC ("Sinclair"), a Contract Research Organization located in Auxvasse, MO. Sinclair was contracted by Cytonics to perform the preclinical studies measuring the effect of CYT-108 on cartilage integrity and other joint tissues. Data was analyzed by an independent pathologist.
Moreover, in 2016 we were issued the first patents in both the UK and US for our machined variants. We currently have seven U.S. patents (US 10,265,388, US 11,040,092, US 10,940,189, US 9,352,021, US 9,498,514, US 10,400,028, US 10,889,631), three U.K. patents (GB2501611, GB2503131 and GB2522561), two European patents (EP 2827882 and EP 3221341; each of which are validated in FR, DE, and GB), one Canadian patent (CA 2865170), two Australian patents (AU 2013222414, AU 2015349782), and one Japanese patent (JP 6861152). The Company also has five additional related pending patents applications.
THE MARKET FOR OA TREATMENT
Cytonics has focused its efforts on the treatment of osteoporosis, one of the largest, rapidly growing, and underserved global clinical markets.
Centers for Disease Control (CDC) define Osteoarthritis (OA) as “…a disease of the entire joint involving the cartilage, joint lining, ligaments, and underlying bone. The breakdown of these tissues eventually leads to pain and joint stiffness. The joints most commonly affected are the knees, hips, and those in the hands and spine. …. There is currently no cure for OA. Treatment for OA focuses on relieving symptoms and improving function, and can include a combination of patient education, physical therapy, weight control, use of medications, and eventually total joint replacement.”
Dramatically Rising Incidence and Cost
According to the CDC, OA is estimated to affect 72 million US adults in 2030. An aging US population and an increasing incidence of obesity has greatly increased the occurrence of OA, but the increasing desire of aging baby boomers to remain active, combined with improved techniques, has caused the number of joint replacement surgeries (virtually all of which are to treat OA) to rise at substantially faster rates. In its recent report, “Health, 2014, United States,” HHS reported that the incidence per 10,000 patients ages 45-64 of total knee and total hip replacements increased dramatically from 6.3 to 37.1, and from 6.2 to 18.2 (Health, United States, 2014; HHS, Library of Congress Catalog Number 76–641496). The American Academy of Orthopaedic Surgeons (AAOS) reported 680,150 total knee replacements and 370,770 total hip replacements in 2014 during their 2018 annual meeting, and the AOOS anticipates total knee replacements and total hip replacements to grow by 142% and 190%, respectively, by 2030.The estimated cost of treating all orthopedic pain conditions is more than $54 billion according to Blue Cross Blue Shield (2019) (Blue Cross Blue Shield, The Health of America Report 2019).
Current Non-Surgical OA Treatments
Prior to resorting to joint replacement for the treatment of knee and hip OA, clinicians had few options. They recommend weight loss, and exercise and treat the pain, but not the disease, with periodic injections of corticosteroids or hyaluronic acid (HA). Injection therapy represents a $1B a year market. The treatment of joint OA thus lacks any alternatives between a simple injection to temporarily relieve pain and a major joint replacement surgery.
Hyaluronic Acid (HA)
The CDC reports that in the US, HA is the pre-surgical treatment of choice and is approaching $1 billion annually in sales, with research showing that the global HA market is reported to exceed $15B by 2026.
While the average selling price for HA products is around $250-$300, corticosteroid injections are far less expensive. Almost all injections take place in the clinician’s office where the practice is allowed to charge (by CMS) a 6% premium over the cost of the HA injectate, but also bills for ancillary codes including office, diagnosis and injecting, making their total reimbursement $400-$500. In all cases, private payers reimburse considerably more than CMS.
Table 1. Hyaluronic Acid Injection Products and Competitors
| Product | | Seller/Manufacturer | | Est. Current Market Share | |
| Synvisc, Synvisc One | | Genzyme/Sanofi | | | 41.3 | % |
| Monovisc | | JNJ DePuy-Synthes/Anika | | | 20.5 | % |
| Euflexxa | | Ferring | | | 18.5 | % |
| Supartz | | Supartz/Sekagaku | | | 9.6 | % |
| Hyalgan | | Fidia Pharmaceuticals | | | 6.3 | % |
| Gel-One | | Zimmer/Sekagaku | | | 3.9 | % |
(Joint Fluid, US; SmarTRAK; July 2015; BioMed GPS).
Payer/Reimbursement Pressure on HA
The large full-line orthopedic biotech firms field large direct sales organizations to promote their offerings. Pain clinicians also treat joint pain with injections and have demonstrated more willingness to try new injection products; they are more likely to offer multiple corticosteroid injections and to rely on injections as the major revenue driver in their practices.
Very few medical drugs or devices are successful without reimbursement from public CMS and private payers. Starting in 2013, the use of HA has come under pressure. In May 2013, following years of recommending its use in OA knees, the American Academy of Orthopedic Surgeons (AAOS) reversed its position and recommended against such use, citing the lack of compelling evidence in a meta-analysis of the published data (Treatment of Osteoarthritis of the Knee; Evidence-Based Guideline, 2nd Edition; AAOS, May 18, 2013). In December 2014, CMS issued its own critical report, a Technology Assessment” (Systematic Review for Effectiveness of Hyaluronic Acid in the Treatment of Severe Degenerative Joint Disease (DJD) of the Knee; AHRQ, Dec 14, 2014) from sister Agency for Healthcare Research and Quality (AHRQ) in which it reported that, in its (Medicare/Medicaid) patient population the “…average effects (of HA) do not meet the minimum clinically important difference…”. This is seen as the first step in a CMS coverage decision to restrict/reduce or even end coverage for HA and indeed several private payers have already ended or restricted coverage as a result of these reports.
Many orthopedic surgeons still believe, despite their society’s recommendation and rationale, that HA works to temporarily relieve pain for at least some of their patients and continue to offer it to those patients (the majority) whose payers still cover it. Indeed, some payers who immediately suspended coverage of HA following the AAOS recommendation have returned to some level of coverage, because they have seen a resultant increase in total joint surgeries when HA was not offered. Orthopedists generally believe – and some publications support – that HA generally offers some pain relief at 4 weeks, peaks at 8 weeks, and then declines to show some residual effect even out to 24 weeks. The AAOS recommendation timing is fortuitous for Cytonics, as clinicians have begun to seek effective alternatives to HA.
New Non-Surgical OA Treatments
We believe that there is clearly an opportunity for new products and players who can deliver what payers are seeking. In its report, AHRQ/CMS clearly indicated their disappointment that no HA offerings demonstrated in their PMA (FDA approval mechanism that requires randomized prospective data) applications and clinical studies the ability to reduce or delay the incidence of joint replacement surgery, despite such claims by manufacturers. The strong implication (and common sense economic conclusion) is that such results would not warrant coverage.
Many new technologies are offered in this segment with relatively little market penetration – and no compelling randomized data – to date. They fall primarily into three categories: (1) Platelet Rich Plasma or “PRP” (without concentrated A2M) (2) “stem-cell” offerings derived from autologous adipose (fat) tissue or bone marrow and (3) placental-derived allograft products. Notably, the large orthopedic players have declined to participate in these segments because they would prefer to wait until a new technology is clinically proven in Level One studies, has specific FDA-cleared labeling claims and has gained reimbursement before acquiring at a premium. To date, none of the firms with autologous or allograft treatments have shown any indication that they will pursue this route, so Cytonics will remain as players in the niche cash-pay market for knee pain treatments.
Platelet-Rich-Plasma (PRP)
PRP has been offered for years by orthopedists for extra-articular (ligament, tendon) pain. Despite a complete lack of compelling clinical evidence, this treatment is safe and profitable for clinicians. As many clinicians have begun to get pushback from payers for HA treatments, they have begun to offer traditional PRP products for intra-articular (inside the joint, as a treatment for OA) use. There is little to no evidence that supports its clinical efficacy and in fact most PRPs have been shown to cause more pain due to high WBC content. Unlike HA, the majority of PRP sales today are NOT directed at intra-articular (OA pain relief) indications and the Company estimates the current U.S. market for OA-directed PRP at well under $50 million.
Stem Cell Treatments Directed at OA
Like PRP, so-called “stem cell treatments” have been offered largely for extra-articular soft-tissue orthopedic injuries (and many unrelated indications) but recently have begun to be used intra-articularly to treat OA pain. Clinicians and patients are enamored of the appeal of stem cells, which in theory can impact healing and other physiologic functions in many ways, or can differentiate into specific types of cells that are especially useful in the location into which they are injected. However, no studies have indicated that they can do either in any orthopedic indication, or that the cells are either alive, active or replicating once injected. Stem cells are typically derived in an office setting from the patient’s adipose (fat tissue) or from bone marrow. The ensuing preparation is time-consuming and labor intensive. Some firms offer “externally expanded” stem cells, typically mesenchymal stem cells (MSC’s that are capable of differentiating into skin, bone, cartilage, muscle) to vastly increase the number of cells; these clearly are heavily regulated by FDA and unavailable for sale in the U.S.
Because stem cell, PRP, and placental tissue injections are all unreimbursed and minimally regulated, clinicians are free to charge patients cash at whatever rate they desire; these treatments can thus be enormously profitable is some practices in wealthy areas. They tend to be offered more frequently by pain clinicians and “regenerative clinicians” than by practicing orthopedists and are sometimes accompanied by claims of “regenerating cartilage” to treat OA. The Company believes that, while it will in some cases compete with such treatments, they will never be able to demonstrate disease (or even pain) treatment and thus will not achieve reimbursement.
Placental-Derived products Directed at OA
In recent years allograft amniotic membranes, sourced from consenting C-Section mothers, have been found to be very effective in treating certain chronic wounds and, in certain surgeries to prevent post-surgical adhesions. (Ilic, D., Vicovac, L., Nikolic, M., & Ilic, E. L. (2016). Human amniotic membrane grafts in therapy of chronic non-healing wounds: Table 1. British Medical Bulletin, 117(1), 59–67. doi: 10.1093/bmb/ldv053). Some allograft tissues, like bone, skin and many of these amniotic membrane tissues have been classified by FDA as “361 Human Cellular and Tissue products” which, by virtue of minimal manipulation”, “for homologous use” and “non-reliance on cellular activity” are deemed inherently safe and exempted from pre-market approval and can thus be brought quickly to market. As a result of the recent criticisms of HA, many of these firms are promoting placental-derived tissue products as treatments for soft tissue and for OA orthopedic pathologies.
Changing FDA Rules for Amniotic and Other Allografts
FDA has indicated, and provided controversial guidance, for a much more limiting definition of “minimal manipulation” and promises new interpretation for “homologous use” as well. The clear intent, and possible outcome despite industry resistance, is the greatly reduce the number of new, lightly-regulated, “361 HCT/P” products. The products are, in order of frequency of use, (1) amniotic membrane, ground to minute particles and suspended in saline (2) stem cells derived from amniotic fluid suspended in saline or (3) processed amniotic fluid.
Ground Amnion in Suspension
Several firms offer ground up or “micronized” amniotic tissues in suspension. As claimed 361 HCT/P allograft tissues they are essentially unregulated by FDA but have no labeling claims and are always intended for “homologous use.” The market leader overall in placental tissues is Mimedx (NASDAQ, MDXG) by virtue of the commanding success in demonstrating intact membrane efficacy in chronic wounds. Their ground up (“micronized’) amnion injectable product was challenged by FDA in 2013 as not qualifying as a “361” allograft because the grinding was “more than minimal manipulation.” This product thus needs to go through an FDA pre-market approval process (like Cell-Free APIC) and MiMedx has continued to lobby the FDA for continued marketing of the injectable product while they pursue an Investigational New Drug (IND) application, the prospective randomized study in plantar fasciitis for which is underway. This will be the first “FDA study” for any placental tissue but expected, unlike Cytonics A2M products to treat just the pain associated with knee OA, thus competing with traditional HA.
Amniotic Fluid Stem Cells in Suspension
BioD and NuCel offer amniotic fluid claimed to have live stem cells, suspended in fluid for injection and BioD is the reported market leader in placental derived injectables with sales estimated between $20-30 million. No evidence of efficacy has been offered and there are many small emerging players in the segment as the technology (and currently, regulatory) bars are relatively low. Changing FDA perspectives on these allograft products may have a major impact on this segment will begin to be clarified following the three –day September workshop with FDA and industry representatives.
Processed Amniotic Fluid
Several firms offer injections made solely from processed amniotic fluid that seems to offer better pain reduction than other placental-derived injections, with perhaps somewhat longer durability; it has thus attracted new emerging competitors. As 361 HCT/P products, these offerings also have no specific labeling claim.
Unless they proceed like Cytonics through the rigorous IND or BLA regulatory process, we believe that all of these allograft products will continue to have no labeling claims and no pathway to reimbursement. It is unclear that any of the firms have an interest in that pathway, and most do not have the funding or capability.
Cytonics A2M products Strategy vs. Competition
AAOS and payers have made it clear that they are not impressed with HA and that they desire a treatment that can actually delay or preferably, reduce the need for total joint surgery. Like HA, all of the reviewed product types are intended to reduce the pain, and thus improve patient’s function, in OA joints, but none are targeting the disease itself, and all are just additional palliative treatments for a chronic progressive disease. We believe that none have a scientific rationale, tested and proven, to demonstrate a method of action, and physiologic effect, like APIC. Some of them, and APIC, will take a share from HA by virtue of offering increased cartilage-protecting abilities and longevity of use than that of the short-lived HA injections. Others may take a share because, as unreimbursed treatments, they enable the clinician to promote aggressively and charge unlimited amounts for such injections. We believe that only Cytonics’ APIC line of products treats the disease itself at a molecular level and is generating Level One, prospective randomized data, in an FDA-approved study with OA labeling claims to demonstrate long term relief of symptoms and attenuation of disease process. We believe that this pathway will in turn enable Cytonics to seek reimbursement and compete directly for the $1 billion U.S. hyaluronic acid market.
Summary of Current Status of Cytonics’ Products
Status of Cytonics Products and Programs:
Product
/Project | | Description | | Status | | Value |
| Recombinant A2M (“CYT-108”) | | Optimized | | GMP drug tested in IND-enabling toxicology and demonstrated an acceptable safety profile. The IND application for CYT-108 as a treatment for osteoarthritis of the knee is under construction and is anticipated to be submitted in 1Q23. | | Intended Vehicle for Cytonics Investor Exit Plan: Submit IND application for Phase 1a study of CYT-108 as a treatment for primary osteoarthritis of the knee in 1Q23. Begin Phase 1a study in the second half of 2023, with preliminary data expected in the first half of 2024. |
| APIC first generation | | PRP kit and hardware which concentrates platelets and A2M | | 510k cleared; sales $625k in 2017 with very small distribution footprint; Licensed to multi-national distributor for $2.97M (upfront fee plus royalty payments through 2029), with minimum $300,000 in annual royalties (human market) starting in 2020 through 2029. Recently licensed to veterinary distributor for $660,000 (upfront fee plus royalty payments through 2025). | | Active, Entered into agreement with Christie Medical Holdings for a 10-year license beginning in 2020 for an additional $450,000 and 10% royalties on in-market sales (total deal value ~$3M). Entered into a 6 year licensing deal with Astaria Global for exclusive manufacturing and sales rights in the veterinary market (total deal value ~$700,000. Plan: help grow revenue establish A2M/APIC brand |
| APIC MINI second generation | | Low cost PRP kit | | Application for 510(k) 2020 | | Proposed Active Licensed development, manufacturing, and sales rights to Christie Medical Holdings. CMH anticipates 510k filing by The first half of 2023. |
| APIC for Spine | | Same products directed at DDD | | On hold; should be paired with FACT. Needs clinical data | | Inactive. Minimal current clinical use. License opportunity |
| APIC for EPA wounds | | Same products directed at EPA wounds | | On hold. Small pilot demonstrated cartilage-protective activity and generated clinician advocates | | Inactive License opportunity |
| APIC for Veterinary Applications | | Same products directed animals | | Out-licensed to distributor in the equine veterinary industry, royalties on in-market sales starting in 2021 | | Active. Licensed to veterinary medical device manufacturer and distributor in Q2 2019 for $400,000 (the first tranche of which in the amount of $152,500 has been received by the Company and the remaining balance will be amortized over 2 years) and royalties on in-market sales. Plan: help grow the market for equine applications, then expand to companion animals. Currently negotiating a development, manufacturing, and sales license for CYT-108 for veterinary applications. |
| FACT | | Biomarker assay for the detection of cartilage damage | | Sold to physicians nationwide, often accompanying the sale of an APIC-PRP kit | | Provides physicians with a quantitative method to determine the extent of cartilage damage and promotes APIC-PRP treatment. Assisted Christie Medical Holdings (CMH) with setting up a CLIA-certified lab. All FACT tests are conducted in CMH’s lab. |
Our current focus is on the development of a synthetic A2M variant (“CYT-108”), based upon the protein structure of the naturally-occurring A2M molecule that has been shown to inhibit the enzymes that degrade cartilage in disease models of osteoarthritis. This synthetic variant, “CYT-108,” has been shown to inhibit cartilage degradation more than 2-fold over that of the naturally-occurring A2M molecule in small animal models of osteoarthritis (Zhang, Y., Wei, X., Browning, S., Scuderi, G., Hanna, L. S., & Wei, L. (2017)). Targeted designed variants of alpha-2-macroglobulin (A2M) attenuate cartilage degeneration in a rat model of osteoarthritis induced by anterior cruciate ligament transection. (Arthritis Research & Therapy, 19(1). doi: 10.1186/s13075-017-1363-4). Cytonics has contracted a Collaborative Manufacturing Organization and Collaborative Research Organization (Sinclair Research, Auxvasse, MO) to synthesize CYT-108 and perform pre-clinical tests ahead of human clinical trials. In 2019 we successfully purified CYT-108 for pre-clinical trials, which were initiated in October 2019 in an animal model of post-traumatic osteoarthritis. If CYT-108 is approved for sale by the FDA and if CYT-108 is successfully commercialized, we anticipate that CYT-108 will substantially reduce APIC sales as we believe that CYT-108 is a superior treatment option to APIC. We are currently working toward FDA approval by preparing for Phase 1 human clinical trials starting in Q1 of 2023.
Cytonics A2M products have many potential indications and the Company has chosen to focus on osteoarthritis treatment. Its primary focus is the execution of Recombinant A2M milestones, currently the large animal trial and IND application.
All other opportunities are on hold and are licensing/partnering opportunities with no planned Cytonics development or clinical work.
INDUSTRY OVERVIEW
According to the Wall Street Journal, nearly 30 million patients seek treatment for back pain annually. Back pain is the second-most common reason patients visit their physicians and the most common reason for missed work. However, according to Orthopedics Today, spine surgery is rare with approximately 3.5% of the most severely affected patients receiving surgery. Back pain is the number one cause of healthcare expenditures, resulting in more than $100 billion in medical expenses annually. According to the National Ambulatory Care Survey, the two most common musculoskeletal reasons for a patient to visit a physician were back pain, with 20 million visits, and knee pain, with 15 million visits annually. Of those visits, the second and third most common diagnoses were low back pain and osteoarthritis. One in five adults in the U.S. report being diagnosed by a physician with arthritis. By 2030, it is estimated by the NIH that approximately 70 million Americans will have doctor diagnosed arthritis compared to 42.7 million in 2002, a 58% increase for that period. The economic toll of arthritis in its various forms is estimated to result in excess of $86 billion in lost productivity and healthcare costs. This growth makes the spine and joint markets the fastest growing markets in orthopedics. These growing markets create a unique opportunity to commercialize innovative diagnostic and treatment technologies.
For patients with back or knee pain, making an accurate diagnosis is a substantial challenge for physicians. Both spine sciatic pain and knee or joint pain can have multiple etiologies. Various technologies exist to assist physicians to accurately diagnose these patients, but many are expensive and time consuming. MRI, CT scan, and Arthroscopy still require subjective judgment calls by the physician and radiologist. The costs of these diagnostic tests are staggering. The cost of an incorrect diagnosis is significant. Multiple trips to providers, duplicate tests, unnecessary physical therapy or treatments and days or weeks of lost work time are just a few of the compelling reasons providers and insurance carriers seek a less costly and more accurate diagnostic tool.
There are more than 4,500 domestic orthopedic surgeons and neuro-surgeons in the U.S. that perform spine procedures for back pain. Many of these surgeons currently perform epidural injections as a first level of treatment for back pain. Additional levels of treatment include spine surgery fusion, discectomies, stenosis and laminectomies. It is estimated that the average U.S. spine surgeon performs 200 procedures a year with fusions accounting for 45% of all procedures, discectomies 44%, and stenosis and laminectomies 11%.
Additionally, according to the American Academy of Pain Management, there are over 6,000 pain management specialists throughout the U.S. that diagnose and treat back and joint pain. Many of these physicians perform 10 to 20 pain reduction procedures, including epidural injections, each day.
The Company is focused on serving the existing demand for spine and joint pain diagnosis and treatment and is developing new and innovative diagnostic and treatment technologies.
TECHNOLOGY AND INTELLECTUAL PROPERTY
We will seek to commercialize certain technology related to the medical treatment of conditions affecting the human spine and other major joint spaces in the body, including certain technology subject to patent applications (the "Intellectual Property"). We currently have seven U.S. patents (US 10,265,388, US 11,040,092, US 10,940,189, US 9,352,021, US 9,498,514, US 10,400,028, US 10,889,631), three U.K. patents (GB2501611, GB2503131 and GB2522561), two European patents (EP 2827882 and EP 3221341; each of which are validated in FR, DE, and GB), one Canadian patent (CA 2865170), two Australian patents (AU 2013222414, AU 2015349782), and one Japanese patent (JP 6861152). The Company also has five additional related pending patents applications.
The Company’s Trademarks
On September 17, 2019, the following trademark (the “Trademark”) was registered by the Company with the United States Patent and Trademark Office under Ser No. 88-321,585:
CYTONICS
The Trademark consists of standard characters (“CYTONICS”, Serial #88321585) without claim to any particular font style, size or color. The Trademark has a duration of 10 years from issuance, until September 17, 2029.
The Company’s Patents
The Company has the following patents issued at this time:
Country
of
Issuance | Patent No.
/Issue Date | Patented Independent Claim(s) |
| US | 10,265,388 4/23/19 | 1. A liquid composition comprising: (a) an alpha-2-macroglobulin polypeptide (A2M) from a biological sample that is blood or bone marrow aspirate from an individual mammal, wherein the concentration of the A2M is at least 1.1 times higher than in the biological sample; (b) a non-A2M protein with a molecular weight of less than 500 kDa, wherein the concentration of the non-A2M protein with a molecular weight of less than 500 kDa is less than the concentration of the non-A2M protein with a molecular weight of less than 500 kDa in the biological sample, wherein the non-A2M protein with a molecular weight of less than 500 kDa is fibrinogen; (c) a non-A2M protein with a molecular weight of less than 100 kDa, wherein the concentration of the non-A2M protein with a molecular weight of less than 100 kDa is less than the concentration of the non-A2M protein with a molecular weight of less than 100 kDa in the biological sample, wherein the non-A2M protein with a molecular weight of less than 100 kDa is C X C motif chemokine receptor 2 (CXCR2) or ATP Binding Cassette Subfamily F Member 1 (ABCF1); (d) a fluid from the blood or bone marrow aspirate from the mammal; and (e) an effective amount of an anti-coagulant selected from the group consisting of EDTA, tri-sodium citrate, acidcitrate- dextrose (ACD), citrate-phosphate-dextrose (CPD), citrate-phosphate-double dextrose (CP2D), and citrate-phosphate-dextrose-adenine (CPDA1); wherein the liquid composition is not coagulated, is substantially free of white blood cells, and comprises less amount of non-A2M proteins with a molecular weight of less than 500 kDa than in the biological sample; and wherein the liquid composition is prepared by a process comprising: (i) passing a blood sample or bone marrow aspirate sample substantially free of white blood cells through a filter; (ii) retaining the retentate from (i); and (iii) adding the effective amount of the anti-coagulant to the retentate, wherein the filter has a molecular weight cut-off of about 500 kDa or less. |
Country
of
Issuance | Patent No.
/Issue Date | Patented Independent Claim(s) |
| US | 10,940,189 3/9/21 | 1. A composition comprising a recombinant alpha-2-macroglobulin (A2M) polypeptide comprising a non-natural bait region, wherein: (a) the non-natural bait region comprises: (i) a sequence with 100% sequence identity to at least 24 contiguous amino acid residues of SEQ ID NO: 47, and (ii) two or more protease recognition sequences; (b) the non-natural bait region replaces the bait region of a wild-type A2M polypeptide with a sequence according to SEQ ID NO: 3; and (c) the non-natural bait region has at least 70% identity to the entirety of SEQ ID NO: 47. |
| US | 11,040,092 | 1. A method of treating a disease or condition in a subject in need thereof, wherein the disease or condition (A) is associated with inflammation in a joint, spinal disc, bone, tendon, or ligament; (B) is associated with degeneration in a joint, spinal disc, bone, tendon, or ligament; (C) is associated with an injury of a joint, spinal disc, bone, tendon, or ligament; or (D) is associated with wound healing, the method comprising administering to the subject at an anatomic site in need of treatment a liquid composition comprising: (a) an alpha-2-macroglobulin polypeptide (A2M) from a blood sample from the subject, wherein the A2M is present at a concentration at least 3.5 times higher than the concentration of the A2M in the blood sample from the subject; and (b) platelet-rich plasma from the blood sample from the subject; |
| EP | EP2827882 4/8/20 | 1. A liquid composition comprising: (a) an alpha-2-macroglobulin polypeptide (A2M) isolated from a biological sample from a mammal, wherein a concentration of the A2M present in the liquid composition is at least 5 times higher than the concentration of A2M present in the biological sample, and (b) plasma from the biological sample from the mammal; wherein the liquid composition does not elicit an immune response in the mammal. |
| UK | GB2501611B 3/4/15 | 1. A liquid composition comprising: (a) alpha-2-macroglobulin (A2M) isolated from a biological sample from an individual mammal, wherein the A2M is present at a concentration of at least 1.1 times higher than the concentration of A2M present in the biological sample from the individual mammal, and wherein the liquid composition is non-immunogenic to the individual mammal; and (b) plasma, bone marrow aspirate (BMA), or another body fluid from the biological sample from the individual mammal. |
| UK | GB2503131B 11/18/15 | 1. A composition comprising an isolated recombinant A2M polypeptide, wherein the recombinant A2M polypeptide comprises one or more non-natural bait regions, wherein the one or more non-natural bait regions replaces a wild-type A2M bait region and comprises one or more protease recognition sites not present in a wild-type A2M polypeptide, and wherein the recombinant A2M polypeptide is characterized by at least a 10% enhanced inhibition of two or more different proteases selected from serine proteases, threonine proteases, cysteine proteases, aspartate proteases, glutamic acid proteases, and metalloproteases, compared to wild-type A2M. 20. A composition comprising an isolated recombinant A2M polynucleotide, wherein the recombinant A2M polynucleotide encodes for one or more non-natural bait regions, wherein the one or more non-natural bait regions replaces a wild-type A2M bait region and comprises one or more protease recognition sites not present in a wild-type A2M polypeptide, and wherein the encoded recombinant A2M polypeptide is characterized by at least a 10% enhanced inhibition of two or more different proteases selected from serine proteases, threonine proteases, cysteine proteases, aspartate proteases, glutamic acid proteases, and metalloproteases, compared to wild-type A2M. 23. A method for determining the enhanced inhibition of a protease by a recombinant A2M polypeptide comprising: (a) providing a recombinant A2M polypeptide comprising one or more non-natural bait regions, wherein the one or more non-natural bait regions replaces a wild-type A2M bait region and comprises one or more protease recognition sites not present in a wild-type A2M polypeptide, and wherein the recombinant A2M polypeptide is characterized by at least a 10% enhanced inhibition of two or more different proteases selected from serine proteases, threonine proteases, cysteine proteases, aspartate proteases, glutamic acid proteases, and metalloproteases, compared to wild-type A2M; (b) contacting the recombinant A2M polypeptide with the protease and a substrate cleaved by the protease; (c) contacting a wild-type A2M polypeptide with the protease and the substrate cleaved by the protease; and (d) comparing the amount of cleavage of the substrate from step (b) to the amount of cleavage of the substrate from step (c), thereby determining the enhanced inhibition of the protease by the recombinant A2M polypeptide. 24. A method for making a recombinant A2M polynucleotide comprising: (a) providing a vector containing a recombinant A2M polynucleotide comprising a sequence substantially similar to SEQ ID NO 2; (b) digesting the vector containing a recombinant A2M polynucleotide with restriction endonucleases to form a linear vector without a wild-type A2M bait region; (c) ligating one end of one or more polynucleotides encoding one or more nonnatural bait regions, wherein the one or more non-natural bait regions encode for one or more protease recognition sites not present in a wild-type A2M polypeptide, to one end of the linear vector; and (d) ligating the other end of the one or more polynucleotides encoding one or more of the non-natural bait regions to the other end of the linear vector, thereby forming a vector containing a recombinant A2M polynucleotide, and wherein a recombinant A2M polypeptide produced from the vector of (d) is characterized by at least a 10% enhanced inhibition of two or more different proteases selected from serine proteases, threonine proteases, cysteine proteases, aspartate proteases, glutamic acid proteases and metalloproteases, compared to wild-type A2M. |
Country
of
Issuance | Patent No.
/Issue Date | Patented Independent Claim(s) |
| UK | GB2522561B 9/21/16 | 1. An apparatus comprising: a flow filtration module comprising an inlet, an outlet, and one or more filters fluidly connected between the inlet and outlet; and a centrifuge, wherein a flow of a supernatant of a biological sample from a mammal obtained by centrifuging the biological sample with the centrifuge passes through the one or more filters to produce a composition comprising an alpha-2-macroglobulin (A2M) polypeptide at a concentration that is at least 1.1 times higher than the concentration of A2M polypeptide present in the biological sample; wherein the composition further comprises plasma, bone marrow aspirate (BMA), or another body fluid from the biological sample; and wherein the one or more filters has a pore size with a molecular weight cut-off of at most 500 kDa. 2. An apparatus comprising: a flow filtration module comprising an inlet, an outlet, and one or more filters fluidly connected between the inlet and outlet; and a pump in fluid connection with the filtration module, wherein the pump is coupled to the filtration module upstream of the inlet or downstream of the outlet, wherein activation of the pump produces a flow of the biological sample from the inlet to the outlet and through the one or more filters to produce a composition comprising an alpha-2-macroglobulin (A2M) polypeptide at a concentration that is at least 1.1 times higher than the concentration of A2M polypeptide present in the biological sample; wherein the composition further comprises plasma, bone marrow aspirate (BMA), or another body fluid from the biological sample; and wherein the one or more filters has a pore size with a molecular weight cut-off of at most 500 kDa. 9. A apparatus comprising: a flow filtration module comprising an inlet, an outlet, and two or more filters; wherein the two or more filters are fluidly connected in series between the inlet and outlet; wherein a flow of a biological sample from a mammal passes through the at least two filters to produce a composition comprising an A2M polypeptide at a concentration that is at least 1.1 times higher than the concentration of A2M present in the biological sample; wherein a first filter of the two or more filters has a pore size of at most 1 micron; and wherein a second filter of the two or more filters has a pore size with a molecular weight cut-off of at most 500 kDa, wherein the composition further comprises plasma, bone marrow aspirate (BMA), or another body fluid from the biological sample. |
| AU | 2013222414 3/31/18 | 1. A substantially non-immunogenic liquid composition comprising: (a) alpha-2-macroglobulin (A2M) isolated from a biological sample from a mammal, wherein the A2M is present at a concentration of at least 1.1 times higher than the concentration of A2M present in the biological sample from the mammal; and (b) plasma, bone marrow aspirate (BMA), or another body fluid from the biological sample. 10. A method for enrichment of A2M from a biological sample obtained from a mammal comprising: (a) flowing the sample through one or more filters, thereby separating the biological sample into a filtrate and a retentate; and (b) collecting the retentate, wherein the retentate is enriched for A2M, wherein a concentration in said retentate of a non-A2M protein with a molecular weight of less than about 500 kDa is less than 90% of a concentration of the non-A2M protein in the biological sample, wherein the retentate further comprises plasma, bone marrow aspirate (BMA), or another body fluid from the biological sample and wherein the retentate is substantially non-immunogenic. 18. A method for enrichment of A2M from a biological sample from a mammal comprising: (a) flowing or passing the biological sample through one or more first filters, thereby separating the biological sample into a first filtrate and a first retentate; (b) flowing the first filtrate through one or more second filters, thereby separating the sample into a second filtrate and a second retentate, wherein the second retentate is enriched in A2M; and (c) collecting the second retentate, wherein the second retentate further comprises plasma, bone marrow aspirate (BMA), or another body fluid from the biological sample, and wherein the second retentate is substantially non-immunogenic. |
Country
of
Issuance | Patent No.
/Issue Date | Patented Independent Claim(s) |
| CA | 2,865,170 12/22/20 | 1. A liquid composition comprising: (a) an alpha-2-macroglobulin polypeptide (A2M) isolated from a plasma or bone marrow aspirate (BMA) sample from a mammal, wherein a concentration of the A2M present in the liquid composition is at least 5 times higher than the concentration of the A2M present in the plasma or BMA sample from the mammal from which the A2M was isolated, and (b) plasma from the plasma sample from the mammal from which the A2M was isolated or BMA from the BMA sample from the mammal from which the A2M was isolated; wherein the liquid composition does not elicit an immune response in the mammal. |
| US | 9,352,021 5/31/16 | 1. A method for the treatment or prophylaxis of a chronic wound in a mammalian subject, comprising topically applying to the chronic wound an effective amount of a composition comprising an alpha-2-macroglobulin polypeptide (A2M) isolated from a biological sample from said mammalian subject wherein the composition does not elicit an immune response by the mammalian subject when topically applied to the chronic wound. 15. A method for the treatment or prophylaxis of a chronic wound in a mammalian subject, comprising topically applying to the chronic wound an effective amount of a composition comprising an alpha-2-macroglobulin polypeptide (A2M) isolated from a biological sample from said mammalian subject, wherein the composition comprises (a) the A2M at a concentration of at least 1.1 times higher than a concentration of A2M present in the biological sample, and (b) a body fluid from the biological sample, wherein the composition does not elicit an immune response by the mammalian subject when topically applied to the chronic wound. 20. A method for the treatment or prophylaxis of a chronic wound in a mammalian subject, comprising topically applying to the chronic wound an effective amount of a composition comprising an alpha-2-macroglobulin polypeptide (A2M) isolated from a biological sample from said mammalian subject, wherein the composition comprises a first and second plurality of non-A2M proteins, wherein the first plurality of non-A2M proteins have a molecular weight of more than 10 kDa and are present at a concentration at least 1.1 times higher than a concentration of those proteins in the biological sample; and the second plurality of non-A2M proteins have a molecular weight less than 500 kDa and are present at an amount of less than 90% of an amount of those proteins in the biological sample, wherein the composition does not elicit an immune response by the mammalian subject when topically applied to the chronic wound. |
| US | 9,498,514 11/22/16 | 1. A method for the treatment or prophylaxis of a chronic wound in a mammalian subject, comprising topically applying to the chronic wound an effective amount of a composition comprising a recombinant alpha-2-macroglobulin polypeptide (A2M) comprising a non-natural bait region that replaces a wild-type A2M bait region. 19. A wound dressing comprising an effective amount of a composition for the treatment or prophylaxis of a chronic wound in a mammalian subject, wherein the composition comprises a recombinant alpha-2-macroglobulin polypeptide (A2M) comprising a non-natural bait region that replaces a wild-type A2M bait region. |
| US | 10,400,028 9/3/19 | 1. A composition comprising a recombinant alpha-2-macroglobulin (A2M) polypeptide comprising a non-natural bait region, wherein the non-natural bait region comprises a sequence with at least 80% identity to SEQ ID NO: 20. |
| US | 10,889,631 1/12/21 | 1. A method of treating a mammalian subject with an inflammatory disease or condition, comprising administering to the subject a pharmaceutical composition comprising (a) a pharmaceutically effective amount of a recombinant alpha-2-macroglobulin (A2M) polypeptide comprising a non-natural bait region, wherein the non-natural bait region comprises a sequence with at least 80% identity to SEQ ID NO: 20; and (b) a pharmaceutically acceptable carrier. |
| EP** | EP3221341 7/29/20 | 1. A composition comprising a recombinant variant alpha-2-macroglobulin (A2M) polypeptide comprising a non-natural bait region comprising a plurality of protease recognition sequences, wherein: (i) the non-natural bait region comprises a sequence with at least 92% identity to a sequence selected from the group consisting of SEQ ID NOs 6-30, (ii) the recombinant variant A2M polypeptide comprises at least 80% sequence identity to SEQ ID NO 3, (iii) the recombinant variant A2M polypeptide comprises at least 500 amino acids, (iv) the recombinant variant A2M polypeptide is characterized by an enhanced inhibition of a protease selected from the group consisting of a serine protease, a threonine protease, a cysteine protease, an aspartate protease, a metalloprotease, a glutamic acid protease, and combinations thereof, compared to an inhibition of the protease by a wild-type A2M protein; and (v) the non-natural bait region has a sequence that does not comprise a sequence selected from the group consisting of SEQ ID NOs 84-143. |
Country
of
Issuance | Patent No.
/Issue Date | Patented Independent Claim(s) |
| JP | 6861152 3/31/21 | A composition comprising a recombinant variant alpha-2-macroglobulin (A2M) polypeptide comprising a non-natural bait region comprising a plurality of protease recognition sequences, wherein the non-natural bait region comprises a sequence selected from the group consisting of SEQ ID NOs 6-30, wherein the recombinant variant A2M polypeptide comprises at least 90% sequence identity to SEQ ID NO 3, wherein the recombinant variant A2M polypeptide is characterized by an enhanced inhibition of a protease selected from the group consisting of a serine protease, a threonine protease, a cysteine protease, an aspartate protease, a metalloprotease, a glutamic acid protease, and combinations thereof, compared to an inhibition of the protease by a wild-type A2M protein, and wherein the non-natural bait region has a sequence that does not comprise a sequence selected from the group consisting of SEQ ID NOs 84-143. |
| AU | 2015349782 11/26/20 | 1. A composition comprising a recombinant variant alpha-2-macroglobulin (A2M) polypeptide comprising a non-natural bait region comprising a plurality of protease recognition sequences, wherein the non-natural bait region comprises a sequence with at least 92% overall identity to a sequence selected from the group consisting of SEQ ID NOs: 6-30. |
* If the foregoing patents are issued, we expect their duration for be for 20 years from issuance, however this term could be extended if there is a delay in prosecution caused by the USPTO, and there can be no assurance that any of the foregoing patents will ever be granted.
** Divisional patents issued in DE, FR, and GB
RESEARCH
We currently have a staff of 1 full time employee and 7 technical consultants, including 2 PhD’s, 2 MD/PhD’s, and 3 MBA’s. These advisors serve as scientific, financial and regulatory consultants.
In 2013, our research team developed a series of recombinant variants of A2M which has the potential to be a treatment for osteoarthritis, back and joint pain. In vitro and in vivo testing on small animal models of trauma-induced arthritis have demonstrated substantial cartilage-protecting abilities and tolerability of our recombinant A2M product. We have outsourced drug development to Goodwin Biotechnology, a contract services provider with expertise in cell line development, scale up, and GMP manufacturing.
We have contracted with Goodwin Laboratory (Plantation, FL) for GLP/GMP production of our A2M variants and to move forward with pre-clinical trials.
We have successfully completed a pilot pre-clinical study in 2020 and used results to propose a GLP toxicity (safety) study to the FDA. The data acquired from the pilot pre-clinical study was used to inform the study parameters of a larger, GLP-compliant pre-clinical study initiated in 2020, an IND filing with the FDA in early 2023. We anticipate entering Phase 1 clinical trials in mid 2023.
MANUFACTURING
We do not intend to manufacture the majority of our products, but instead we will use outside suppliers and contract manufacturers. We will select suppliers that have a substantial track record of working with FDA regulated medical products and maintaining good manufacturing practices that comply with FDA requirements. Each potential supplier will be audited by our staff to ensure they satisfy our requirements.
MARKETING
Orthopedic, spine and neuro-surgeons, as well as pain management physicians, will be the decision makers for the use of our products. We plan to develop a sales and marketing program targeting these physicians in the US, where there may be a high interest in our products.
The development of our Company name and the APIC brand will be important for stimulating industry interest and recognition of our Company. We plan to issue press releases, place advertisements in industry journals/magazines, and engage in various forms of direct marketing (email, mailing, in person selling, attendance at trade shows, etc.).
COMPETITION
The spine and orthopedic markets are known for their intense competition; however, in the field of orthopedic diagnostics and biologic therapeutics there are very few competitors. Our primary competition will be companies that provide the existing standard of care including steroids, NSAIDs, viscosupplementation, and closed irrigation treatments. We believe that by proving a clinical and economic advantage over existing treatments, we will be able to compete effectively. We will, however, be competing for market share against other drug and device companies that may possess greater resources and experience than us.
Given the large market potential for therapies, it is likely that other pharmaceutical companies and orthopedic companies have internal development programs for therapeutic products that will compete with our products.
PRICING STRATEGY
Our pricing strategy is to position our products as premium priced products, however, to be less expensive than the current treatment algorithms for spine and joint pain. We will work closely with patients, insurance carriers and workman’s compensation providers to determine a fair price for our assay and therapeutic products.
DISTRIBUTION
The Company has licensed our APIC-PRP system to a national independent distributor. We limited the pilot launch of our products to regions of the U.S. with the highest sales potential by engaging a small number of independent agents. Some of the initial sales agents were also investors in our Company. We believe that as investors in the Company, these agents will be motivated to represent our product line and to help promote the features and benefits of our products to their local physicians. Our distributor network would be developed and managed by an internal sales and marketing staff.
REIMBURSEMENT
In the United States, any surgical product or treatment success will stem from the level of reimbursement available to the surgeon and hospital that performs the procedure. We plan on retaining the services of a reimbursement-consultant to assist us with gaining reimbursement allowance from private and government insurance entities for our products. Reimbursement allowance will be applied for following the appropriate clinical efficacy studies.
CORPORATE HISTORY
We were originally organized on July 19, 2006 in Florida as Gammaspine, Inc. On April 17, 2007, we changed our name from Gammaspine, Inc. to Cytonics Corporation.
Our authorized capital is 70,000,000 shares, of which (1) 50,000,000 shares are Common Stock, par value $0.001 per share (the "Common Stock"), and (2) 20,000,000 shares are Preferred Stock, par value $0.001 per share, which may, at the sole discretion of the Board of Directors, be issued in one or more series (“Preferred Stock”). The Board of Directors has designated (a) 150,000 shares as Initial Preferred Stock, par value $0.001 (the “Initial Preferred Stock”), (b) 1,500,000 shares as Series A Preferred Stock, par value $0.001 per share (the “Series A Preferred Stock”), (c) 6,000,000 shares as Series B Preferred Stock, par value $0.001 per share (the “Series B Preferred Stock”), and (d) 10,000,000 shares as Series C Preferred Stock, par value $0.0001 per shares (the “Series C Preferred Stock”).
Private Placements – Common Stock
On August 18, 2014, the Company issued 12,500 shares of Common Stock at a purchase price of $2.00 per share (for an aggregate of $25,000 of proceeds) to an accredited investor in a private placement under Rule 506(b) of Regulation D of the Securities Act.
During the period from April 20, 2007 to August 18, 2014, Cytonics issued an aggregate of 3,499,470 shares of Common Stock as compensation (for an aggregate of $0.00 of proceeds) to members of management in consideration of the recipient’s research and work contributions to the Company in reliance on Rule 506(b) of Regulation D of the Securities Act.
Private Placements – Preferred Stock
On November 30, 2009, the Company issued 150,000 shares of Initial Preferred Stock at a purchase price of $2.00 per share (for an aggregate of $300,000 of proceeds) to 3 accredited investors in a private placement under Rule 506(b) of Regulation D of the Securities Act.
On November 30, 2009, Cytonics issued an aggregate of 576,190 shares of Series A Preferred Stock at a purchase price of $4.00 per share (for an aggregate of $2,304,760 of proceeds) to 48 accredited investors in a private placement under Rule 506(b) of Regulation D of the Securities Act.
During the period from February 4, 2011 to June 9, 2011, Cytonics issued an aggregate of 1,779,000 shares of Series B Preferred Stock at an average purchase price of $2.50 per share (for an aggregate of $4,447,500 of proceeds) to 11 accredited investors in a private placement under Rule 506(b) of Regulation D of the Securities Act.
During the period from November 15, 2012 to January 15, 2016, Cytonics issued an aggregate of 795,865 shares of Series B Preferred Stock at an average purchase price of $4.00 per share (for an aggregate of $3,183,460 of proceeds) to 49 accredited investors in a private placement under Rule 506(b) of Regulation D of the Securities Act.
Private Placements – Convertible Notes
From June 30, 2018 to June 30, 2019, the Company issued an aggregate of $804,000 of convertible promissory notes in exchange for aggregate proceeds of $804,000 to 21 accredited investors in a private placement under Rule 506(c) of Regulation D of the Securities Act. The convertible promissory notes bear simple interest at the rate of 10% per annum payable by Cytonics on a quarterly basis with principal due on June 30, 2021 and are voluntarily convertible by the holder into shares of the Company’s common stock at a conversion price of $1.60 per share until the Company completes a public offering of its common stock for gross proceeds of at least $1,000,000 pursuant to an effective registration statement under the Securities Act, at which point the conversion price would be equal to the sale price in such public offering.
On October 31, 2019, the Company issued a convertible note in a private placement under Rule 506(b) of Regulation D of the Securities Act, in the amount of $100,000 to an investor, which is convertible into equity of the Company.
During 2020, the Company issued promissory notes in the principal amount of $715,000 (the “2020 Notes”), of which $40,000 was with related parties. In connection with the 2020 notes, the Company issued options to purchase 357,500 shares of common stock at a share price of $2.00.
The foregoing notes were either repaid or converted to equity as of the date of this filing.
2019 Regulation CF Offering and Regulation D Rule 506(c) Offering
On May 17, 2019, the Company issued an aggregate of $486,511 of convertible promissory notes (“Crowd Notes”) in exchange for aggregate proceeds of $463,344 to both accredited and non-accredited investors under Regulation CF and Rule 506(c) of Regulation D. In connection with the offering, which was facilitated by SI Securities, LLC as placement agent, the company issued to SI Securities, LLC $23,167 in Crowd Notes, in addition to offering expenses and commissions of $44,000. The Crowd Notes bear accrued interest of 5% payable upon maturity. The outstanding loan balance of the Crowd Notes will automatically convert if the Company completes a “Qualified Equity Financing” which means an offering of stock by the Company for gross proceeds of at least $1,000,000, or pursuant to an effective registration statement under the Securities Act, at which point the conversion price would be equal to the lesser of (i) sale price discounted by 20% in such public offering or (ii) the quotient resulting from dividing the valuation cap of $32,400,000 by the fully-diluted capitalization immediately prior to the closing of the Qualified Equity Financing (the “Conversion Price”). Commencing on the maturity date, the Crowd Note holders (by a decision of those Crowd Note holders holding a majority of the principal amount of the outstanding Crowd Notes) have the option to (a) require the Company to pay the outstanding loan balances or (b) convert the Crowd Notes into a number of the Company’s most senior class of shares equal to the quotient obtained by dividing the outstanding loan balance by the Conversion Price.
Appointment of Issuer Direct as Transfer Agent
On February 12, 2020, Cytonics entered into that certain Transfer Agent agreement with Issuer Direct, whereby Issuer Direct agreed to act as the transfer agent of Cytonics.
Employment Agreements
On May 15, 2018, the Company entered into a letter agreement with Joey Bose to engage his services as the President of the Company, with an annual base salary of $85,000 to be paid semi-monthly. Also pursuant to the letter agreement, the base salary is to be reviewed every twelve months and the Company also granted Mr. Bose an initial award of 424,800 stock options to purchase share of the Company’s common stock at an exercise price of $2.00 per share to vest over 3 years. Also according to the letter agreement, Mr. Bose will be awarded a performance bonus that will be tied to the completion of specific objectives.
Issuance of Stock Options
In April 2007, the Company’s shareholders adopted the 2007 Stock Incentive Plan providing for the grant of stock options and restricted stock awards to employees and non-employee directors selected by the Board of Directors or a committee of our Board of Directors. Options granted under the plan may include non-statutory stock options as well as incentive stock options intended to qualify under Section 422 of the Internal Revenue Code. The Company’s 2007 Stock Incentive Plan expired in April 2017, and no awards have been granted thereunder since such time.
In December 2017, the Company (i) granted new options to purchase 1,378,834 shares of common stock and (ii) modified existing options to purchase 461,300 shares of common stock. These granted and modified options are fully vested and carry an exercise price of $1.00 per share over a 5-year term.
On November 19, 2018, the Board of Directors adopted the 2018 Stock Incentive Plan (the “Plan”), under which the Board of Directors may grant stock-based compensation awards with respect to an aggregate of up to 5,000,000 shares of Common stock, subject to adjustment and increase pursuant to the terms of the Plan. The Plan permits the grant of stock options, restricted stock, restricted stock units and other forms of stock-based compensation to selected persons providing services to the Company (including non-employee directors).
During 2021, the Company granted options to the Company’s President to purchase 424,800 shares of common stock at an exercise price of $2.00 per share and a grant date fair value of $0.16.
Additionally, a debt holder was given an option purchase 15,000 shares of common stock at an exercise price of $2.00 per share that is fully vested on issuance and exercisable over five years.
During 2020, the Company granted options to purchase 357,500, 20,000 of which were issued to related parties, shares of common stock at an exercise price of $2.00 per share with a term of five years and a grant date fair value of approximately $0.08 in connection with the issuance of notes payable. The Company also granted options to two directors to purchase a total of 300,000 shares of common stock at an exercise price of $2.00 per share and a term of five years. Of the options granted, 200,000 options vest immediately, and 100,000 vest over one (1) year at 25,000 per quarter. The grant date fair value of the options was approximately $0.09.
At December 31, 2021, the Company has options outstanding to purchase 5,161,834 shares of common stock under the 2007 and 2018 Plans at exercise prices ranging from $0.05 to $2.00 per share and with remaining vesting periods of one to five (5) years.
The following is a summary of the Company’s stock option activity for the years ended December 31, 2020 and 2021:
| | | 2020 | | | 2021 | |
| | | Number of
Options | | | Weighted-
Average
Exercise Price | | | Number of
Options | | | Weighted-
Average
Exercise Price | |
| Outstanding at January 1 | | | 5,845,870 | | | $ | 0.78 | | | | 5,733,310 | | | $ | 0.94 | |
| Granted | | | 657,500 | | | $ | 2.00 | | | | 439,800 | | | $ | 2.00 | |
| Forfeited | | | - | | | $ | - | | | | (709,276) | | | $ | 0.38 | |
| Expired | | | (770,060 | ) | | $ | 1.31 | | | | (302,000 | ) | | $ | 1.00 | |
| Outstanding at December 31 | | | 5,733,310 | | | $ | 0.94 | | | | 5,161,834 | | | $ | 1.10 | |
| Exercisable at December 31 | | | 5,467,177 | | | $ | 0.90 | | | | 4,807,834 | | | $ | 1.04 | |
The following table summarizes stock option information at December 31, 2021:
| | | | | | | Weighted | | | | | | Weighted | |
| | | | | | | Average | | | | | | Average | |
| Exercise | | | | | | Contractual Life | | | | | | Contractual Life | |
| Price | | | Outstanding | | | (Years) | | | Exercisable | | | (Years) | |
| $ | 0.05 | | | | 800,000 | | | | 5.30 | | | | 800,000 | | | | 5.30 | |
| | 0.38 | | | | 185,000 | | | | 0.22 | | | | 185,000 | | | | 0.22 | |
| | 0.57 | | | | 496,000 | | | | 1.50 | | | | 496,000 | | | | 1.50 | |
| | 1.00 | | | | 2,058,734 | | | | 2.03 | | | | 2,058,734 | | | | 2.03 | |
| | 2.00 | | | | 1,622,100 | | | | 3.57 | | | | 1,268,100 | | | | 3.33 | |
| Total | | | | 5,161,834 | | | | | | | | 4,807,834 | | | | | |
Forward Stock Split
Our board of directors approved on February 13, 2018 a 2-for-1 forward split of our Common Stock, which was effected on February 16, 2018. The forward split divided each one share of our outstanding common stock into two shares of common stock and correspondingly adjusted the exercise prices of our common stock purchase warrants and options and conversion price of our convertible debt. All references to common stock, common stock purchase warrants, restricted stock, share data, per share data and related information have been retroactively adjusted, where applicable, to reflect the forward split of our common stock (and the corresponding adjustment of the exercise prices of our common stock purchase warrants) as if it had occurred at the beginning of the earliest period presented. As a result of the forward stock split, the number of shares of common stock issued and outstanding increased from 4,773,560 shares as of February 16, 2018, to approximately 9,547,120 shares. In connection with the forward stock split, we filed Articles of Amendment to our Articles of Incorporation to effect the forward stock split with the Secretary of State of Florida on February 16, 2018.
2019 Promissory Note
On October 31, 2019, the Company issued a promissory note with an unrelated individual in the amount of $100,000. The promissory note bears interest at 10% and is due October 31, 2024. The note holder may elect to convert all, but only all, of the outstanding principal and accrued interest into shares as follows: 1) prior to an initial public offering (IPO) at a conversion price of $2.00 or 2) at the completion of an IPO, at a conversion price equal to the share price paid in the IPO less a 10% discount. Further, the Company may elect to repay the principal and accrued interest in the form of equity shares as follows: 1) upon 15 days advance notice to the lender of the Company’s election to convert into equity shares at a conversion rate of $2 or 2) at any time following a public offering of stock that results in gross proceeds of at least $1,000,000, the Company may elect to require the lender to convert all outstanding principal and accrued interest into equity shares at a 10% discount to the offering price.
Conversion of the 2019 CF Offering Notes and 2019 Promissory Note
During the year ended December 31, 2021, the aggregate outstanding principal and accrued interest outstanding on the 2019 Notes and 2019 Promissory Note of $586,511 and $60,126, respectively, were converted to 570,541 shares of Series C Preferred Stock. The conversion of the 2019 Notes was triggered by a Qualified Equity Financing that occurred in 2021. The Company elected to require the holder of the 2019 Promissory Note to convert the outstanding principal and accrued interest into the Series C Preferred Stock after the sale of Series C Preferred Stock that resulted in gross proceeds in excess of $1,000,000. Since the conversion was in accordance with the original terms of the 2019 Notes and 2019 Promissory Note, no gain or loss was recorded upon conversion and the $646,637 of outstanding principal and accrued interest was classified to equity. As of December 31, 2021 and 2020, the total principal outstanding on the 2019 Notes and 2019 Promissory Note was $0 and $586,511, respectively.
2020 Promissory Notes and Subsequent Conversions of Certain Notes
During 2020, the Company issued promissory notes in the principal amount of $715,000 of which $40,000 was with related parties. In 2021, the Company issued an additional promissory note with a principal amount of $30,000 (collectively referred to as the “2020 Notes”). In connection with the 2020 notes, the Company issued options to purchase 372,500 shares of common stock at a share price of $2.00, which was recorded as a debt discount in the amount of $28,463 and additional paid- in-capital based on the relative fair value method. Certain holders of the 2020 Notes elected to convert their outstanding principal and accrued interest into shares of common stock. During the year ended December 31, 2021, $75,000 of outstanding principal and accrued interest of $13,000 of 2020 Notes were converted to 55,000 shares of common stock. Since the 2020 Notes were converted to shares of common stock, the Company recorded a gain on the extinguishment of the 2020 Notes in the amount of $55,165. During the year ended December 31, 2021, an aggregate of outstanding principal and accrued interest of $1,558,506 on the 2018 Notes, 2019 Notes and 2020 Notes was converted into 570,541 Series C Preferred Stock and 569,922 shares of common stock. This resulted in an aggregate gain on extinguishment of $571,629. At December 31, 2021 and 2020, aggregate outstanding principal and accrued interest on the 2018 Notes, 2019 Notes and 2020 Note was $0 and $2,105,511, including $140,000 with related parties, and $0 and $105,900, respectively.
Cares Act Paycheck Protection Program Loan
In April 2020, the Company entered into a promissory note evidencing an unsecured loan (the “Loan”) in the amount of $27,212 made to the Company under the Paycheck Protection Program (the “PPP”). The PPP was established under the CARES Act and is administered by the U.S. Small Business Administration. The promissory note matured in April 2022 and had interest at a rate of 1% per annum. In 2021, the Company received notification that Loan was forgiven and recorded gain on extinguishment of $27,408.
Regulation A Offering
On July 6, 2020, the Company began its offering up to 9,500,000 shares of its Series C Preferred Stock in a “Tier 2 Offering” under Regulation A (the “Reg A Offering”). The price per share of Series C Preferred Stock was $2.00 per share. The Series C Preferred Stock shares are convertible into shares of the Company’s common stock either at the discretion of the investor or automatically upon the occurrence of the consummation of an underwritten public offering of the Company resulting in at least $20,000,000 net proceeds to the Company. The total number of shares of the Common Stock into which the Series C Preferred Stock may be converted at the discretion of the investor is based on a conversion ratio of 1 for 1. The Company raised $4,659,638 funds in its Reg A Offering and issued 2,329,819 shares of its Series C Preferred Stock to 2,557 investors. The Reg A Offering was closed on April 30, 2021.
2022 Regulation Crowdfunding Offering
The Company is currently engaged in a Regulation Crowdfunding Offering of up to $5,000,000 worth of its Series C Preferred Stock for a price of $2.30 per share (the “2022 Reg CF Offering”). To date, the Company raised approximately $2,730,185 during the offering period in the 2022 Reg CF Offering.
ORGANIZATIONAL STRUCTURE
The Company does not have any subsidiaries.
EMPLOYEES
We currently have a staff of 1 full time employee, no part time employees and 7 technical consultants, including 2 PhD’s, 2 MD/PhD’s, and 3 MBA’s. These advisors serve as scientific, financial and regulatory consultants.
LEGAL PROCEEDINGS
From time to time, we are involved in various claims and legal actions arising in the ordinary course of business.
On January 8, 2018, we received a letter from the trustee for IRC Clinics, Inc. (“IRC”) in their ongoing bankruptcy case advising us that their filing had been converted from Chapter 11 to Chapter 7 on January 9, 2017 and that the trustee’s duty is to liquidate IRC and collect all monies contractually due to it, which it said also includes funds owed by the Company to IRC pursuant to a Master Services Agreement dated February 15, 2015. The Company had a discussion with the attorney for the trustee for IRC, and he advised that they were not aware of this dispute and the Company has not had any communications with the attorney for the trustee for IRC since June 21, 2018. There was no specific dollar amount demanded.
There are no legal proceedings currently pending against us which we believe would have a material effect on our business, financial position or results of operations and, to the best of our knowledge, there are no such legal proceedings contemplated or threatened.
PROPERTIES
Our corporate office is located at 658 West Indiantown Road, Suite 214, Jupiter, Florida 33458. Upon expiration of the Company’s office lease at this location in 2017, the Company began leasing this office space from the Company’s President on a month-to-month basis for $2,000 monthly through June 30, 2020. Total rent expense incurred on space leased from the Company’s President was $12,000 for the year ended December 31, 2020. Currently, Cytonics uses this address as its corporate office, however, the Company does not maintain a physical office space at this location as employees work from home at this time. We do not intend on leasing physical office space in the near future.
COVID-19
The Company’s operations may be affected by the ongoing outbreak of the coronavirus disease 2020 (COVID-19) which in March 2020, was declared a pandemic by the World Health Organization. The ultimate disruption which may be caused by the outbreak is uncertain; however, it may result in a material adverse impact on the Company’s financial position, operations and cash flows.
Possible areas that may be affected include, but are not limited to, Drug manufacturing, APIC sales, and clinical trials. In addition, the employees of affiliated companies that provide services to us could be medically or mentally affected by the pandemic and may be required to work remotely. This situation could cause a reduction in productivity or the inability to complete critical tasks for the Company.
The entire actual effects of the spread of COVID-19 are difficult to assess at this time as the actual effects will depend on many factors beyond the control and knowledge of the Company. However, the impact of COVID-19 may cause an overall decline in the economy as a whole and therefore may materially harm our business, results of operations and financial condition.
As of the date of this filing, the Company has experienced a reduction in APIC sales as a result of the COVID 19 pandemic.
RISK FACTORS
In addition to the other information contained in this annual report, you should carefully consider the following risks. The occurrence of any of the following risks might cause you to lose all or a part of your investment. Some statements in this annual report, including statements in the following risk factors, constitute forward-looking statements. Please refer to “Cautionary Note Regarding Forward-Looking Statements” for more information regarding forward-looking statements.
Risks Related to Our Business
We have a relatively limited operating history and thus are subject to the risks of business development. We are a development stage company and have sold limited products to date.
The likelihood of the Company’s success must be considered in light of the problems, expenses, difficulties, complications, and delays frequently encountered in connection with the expansion of a business, operation in a competitive industry, and the continued development of advertising, promotions and a corresponding customer base. There is a possibility that the Company could sustain losses in the future. We have sold a very limited quantity of products to date. We are subject to business risks that are typical of development stage companies, and “start-up” and “lead-time” factors are expected to affect the timing of our receipt of revenues. As a start-up entity, we are subject to many of the risks common to such enterprises, including inability to implement our business plan, under-capitalization, cash shortages, and limitations with respect to personnel, financing and other resources, as well as uncertainty of our ability to generate revenues. We cannot assure you that our activities will be successful or result in any revenues or profit for us, and the likelihood of our success must be considered in light of the stage of our development. Our success or failure will depend in large part upon decisions made by our management and the marketplace conditions, in addition to many factors beyond our control. Until we generate significant revenues, we will experience negative cash flows and financial losses. If we are unsuccessful, you may lose your entire investment. There can be no assurance that Cytonics’ efforts will result in successful commercialization or further development of Cytonics’ operations, that Cytonics’ marketing efforts will be successful, or that Cytonics will ever achieve significant (or any) revenue. If Cytonics fails to achieve its goals outlined, Cytonics may run out of money to run its operation and as such, Cytonics would need to raise additional working capital. Failure to do so could result with investors losing part or all of their money invested.
We have a history of operating losses.
To date, we have not been profitable and have incurred significant losses and cash flow deficits. For the fiscal years ended December 31, 2021 and 2020, we reported net losses of $2,544,189 and $995,850, respectively, and negative cash flow from operating activities of $2,784,503 and $585,103, respectively. As of December 31, 2021, we had an aggregate accumulated deficit of $19,907,507. To date, the Company has funded its research and development and operating activities through sales of debt and equity securities, grant funding and licenses of its products. The Company intends to continue to seek funding through investments by strategic partners and from private and public sales of securities until such time that the Company generates sufficient cash flow to sustain its operations. There is no guarantee that the Company will be able to raise sufficient capital or generate a level of revenues to sustain its operations. Management believes that the Company’s capital requirements depend on many factors, including liquidity necessary for the continued development and marketing of its products. These financial statements do not include any adjustments relating to the carrying amounts of recorded assets or the carrying amounts and classification of recorded liabilities that may be required should the Company be unable to continue as a going concern.
We expect to experience losses in the future and may not become profitable.
Pursuant to our business strategy, we expect to continue to make expenditures on research, clinical trials, and regulatory approvals, which will adversely affect operating results until revenues from sales of our products reach a level at which these costs are supported. Our recent operations have been financed and are expected to continue to be financed primarily through sales by us of our equity and through debt. Since the formation of our Company, we have generated limited revenues, with the exception of the federal grant income, income from the sale of an exclusive license to our diagnostic products, and quarterly royalties. We may experience quarterly and annual losses, and expect to do so at least through the end of the 2022 calendar year. We may never become profitable. If we do achieve profitability, we may not be able to sustain or increase profitability on a quarterly or annual basis. We will need to generate significant revenues to achieve and maintain profitability.
We will need additional capital to fund our operations, which, if obtained, could result in substantial dilution or significant debt service obligations. Our inability to procure additional financing, if required, may have a material adverse effect on us. We may not be able to obtain additional capital on commercially reasonable terms, which could adversely affect our liquidity and financial position.
We require additional equity and/or debt financing to continue our operations on a long-term basis. We will require additional financing in the future, and may not be able to secure any additional financing we may need on terms favorable to us, or at all.
Even if we are able to create additional revenues, we will need to raise additional capital. Although we believe that we have access to capital resources, there are no commitments in place for new financing as of the date of this document and there can be no assurance that we will be able to obtain funds on commercially acceptable terms, if at all. We expect to have ongoing needs for working capital in order to fund operations and to continue to expand our operations. To that end, we may be required to raise additional funds through equity or debt financing. In order to continue operating, we may need to obtain additional financing, either through borrowings, private offerings, public offerings, or some type of business combination, such as a merger, or buyout, and there can be no assurance that we will be successful in such pursuits. We may be unable to acquire the additional funding necessary to continue operating. However, there can be no assurance that we will be successful in securing additional capital. If we are unsuccessful, we may need to (a) initiate cost reductions; (b) forego business development opportunities; (c) seek extensions of time to fund liabilities, or (d) seek protection from creditors. Our inability to obtain any additional financing could have a material adverse effect upon us.
In addition, if we are unable to generate adequate cash from operations, and if we are unable to find sources of funding, it may be necessary for us to sell one or more lines of business or all or a portion of our assets, enter into a business combination, or reduce or eliminate operations. These possibilities, to the extent available, may be on terms that result in significant dilution to our investors or that result in our investors losing all of their investment in our Company.
If we are able to raise additional capital, we do not know what the terms of any such capital raising would be. In addition, any future sale of our equity securities could be at prices substantially below prices at which our shares are currently valued. To the extent we require additional financing and cannot raise it, we may have to limit our then-current operations, curtail all or certain portions of our business objectives and plans or terminate our operations. We may seek to increase our cash reserves through the sale of additional equity or debt securities. The sale of convertible debt securities or additional equity securities could result in additional and potentially substantial dilution to our investors. The incurrence of indebtedness would result in increased debt service obligations and could result in operating and financing covenants that would restrict our operations and liquidity. In addition, our ability to obtain additional capital on acceptable terms is subject to a variety of uncertainties. We cannot assure you that financing will be available in amounts or on terms acceptable to us, if at all. Any failure to raise additional funds on favorable terms could have a material adverse effect on our liquidity and financial condition.
Some of our products are ready for commercial sales, but there is no certainty that these products will be successfully marketed.
Our ability to develop and commercialize products based on our proprietary technology will depend on our ability to develop products internally and may depend upon key outside partnerships that may not materialize on a timely basis or at all. There is no certainty that products employing our technology will be successfully marketed or licensed. Our products and technologies may prove to be unworkable or economically unfeasible. Many medical and pharmaceutical products require long development and testing periods and large capital investments with no certainty that the product will be successfully marketed.
Some of our products are in the developmental stage and may require more testing.
Our technologies are based on extensive testing, but there are still important questions and additional testing and development that will need to occur prior to making our products available for broad sale to the medical community. The company may need to initiate expensive clinical studies to further support the use and market acceptance of our products. We have had limited technical input or evaluations with respect to our products from other physicians or medical professionals, and therefore have received limited validation of the potential efficacy of our products or their viability in the market from persons outside of our Company. Our limited resources in supporting our products could have a material adverse effect upon our business.
We may have a limited number of products.
We may not be able to afford to develop additional products. If the production or sales of any of our limited number of products do not meet our expectations, our dependence upon small numbers of products and our inability to quickly develop new products could have a material adverse effect upon our business, prospects, financial condition and results of operations.
The development and commercialization of the Company’s products and services are highly competitive.
The Company faces competition with respect to any products and services that it may seek to develop or commercialize in the future. Its competitors include major companies worldwide. The Biopharmaceutical market is an emerging industry where new competitors are entering the market frequently. Many of the Company’s competitors have significantly greater financial, technical and human resources and may have superior expertise in research and development and marketing approved services and thus may be better equipped than the Company to develop and commercialize services. These competitors also compete with the Company in recruiting and retaining qualified personnel and acquiring technologies. Smaller or early stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. Accordingly, the Company’s competitors may commercialize products more rapidly or effectively than the Company is able to, which would adversely affect its competitive position, the likelihood that its services will achieve initial market acceptance and its ability to generate meaningful additional revenues from its products and services.
The failure of our products to gain market acceptance would have an adverse effect upon our ability to generate revenues and attain profitability.
A significant challenge for us will be gaining market acceptance of our products. The participation and interest of practicing surgeons and other physicians will be critical. It may require significant time, effort and expense to attract sufficient numbers of physicians for our products to gain widespread acceptance. We cannot assure you that a sufficient number of physicians will invest the time required to gain familiarity with and be trained in the use of our products, or that, once trained, they will be committed to continued usage.
Similarly, other medical procedures and products can also treat certain of the medical indications that we believe can be treated by our products. The medical community widely accepts many alternative treatments, and certain of these other treatments have a long history of prior use. We cannot be certain that our products will gain acceptance over established treatments or that either physicians or the medical community in general will accept and use our products. Market acceptance of our products depends on many factors, including our ability to convince prospective customers that our technology is an attractive alternative to other technologies, to manufacture products in sufficient quantities and at an acceptable cost, and to supply and service sufficient quantities of our products directly or through our strategic alliances. The industry is subject to rapid and continuous change arising from, among other things, consolidation and technological improvements. One or more of these factors may vary unpredictably, which could have a material adverse effect upon our business, prospects, financial condition and results of operations.
We will be dependent upon third party suppliers and manufacturers.
Because of our limited resources, we will be dependent upon other companies to conduct research, supply key components and to manufacture our products. Our ability to develop and maintain relationships with these suppliers, as well as our ability to develop additional sources for key components and manufacturing capabilities, may be important for our long-term success. We cannot assure you that we will be able to establish or maintain relationships with third party suppliers and manufacturers that may be necessary for the execution of our business plan.
Our potential inability to contract with qualified distributors to represent us and promote our products could have a negative effect on our business and financial performance.
We will rely heavily on distributors to sell our products and to market our products to physicians. Our potential inability to establish distributor relationships could have a material adverse effect upon our business, prospects, financial condition and results of operations. If we are unable to establish relationships with qualified distributors, we will be unable to effectively compete for sales of our products in the medical industry.
Reimbursement levels allowed by private and government insurance entities will affect the market acceptance of our products and our potential revenue.
If we are unable to convince patients to pay cash or the health insurance entities to reimburse us for our products, it will very likely have a negative effect on our ability to generate significant revenues. Physicians and hospitals are each responsible for the costs associated with surgical procedures. In order for both parties to receive payment for performing these surgical services they each must seek reimbursement from the patient and the patient’s health care provider. The level of compensation is determined by reimbursement codes that are used to define each step of a surgical operation. Each code is defined by a detailed procedural description and associated level of monetary value. These industry standard codes are accepted throughout the healthcare industry and ultimately contribute to the success or failure of a new product introduction. Physicians use codes, referred to as Current Procedural Terminology (CPT) codes, which are recognized by health care providers. Physicians define or code each step of an operative procedure by using a different code. These codes vary by procedure and often are subjective to the physician’s specific definition of his procedure. These codes can be referenced from various sources such as the “Common Coding Scenarios for Comprehensive Spine Care,” which is published annually by the American Medical Association. This code notation is submitted to the insurer and notifies the health care provider of what service or procedure was provided. A pre-established fee for that code number is then reimbursed to the operating physician. The code reimbursement value will vary based on the patient’s insurance carrier as each carrier maintains different levels for reimbursement.
Not only does the surgeon submit for reimbursement for his surgery, but the hospital also provides a service requiring a separate code submission for reimbursement. Diagnosis-Related Group (“DRG”) codes are codes responsible for hospital reimbursement under the Medicare Prospective Payment System. New DRG codes are continuously being created to more clearly address different procedures necessary in the medical industry. Reimbursement for spine and orthopedic procedures will continue to change as the national healthcare system places pressure on the industry to lower costs. More specifically, manufacturers will continue to be under pressure to offer competitive pricing as health care providers look for alternatives to decrease costs.
Our products are not currently covered by any specific Current Procedural Terminology (“CPT”) code, and we do not expect that there will be any CPT code approved for reimbursement of our products any time in the near future.
The CPT code set is maintained by the American Medical Association through the CPT Editorial Panel. CPT codes are used to describe medical, surgical, and diagnostic services and are designed to communicate uniform information about medical services and procedures among physicians, coders, patients, accreditation organizations, and payers (including the Centers for Medicare and Medicaid Services and insurers) for administrative, financial (including reimbursement), and analytical purposes.
Our products are not currently covered by any specific CPT code, and we do not expect that there will be any CPT code approved for reimbursement of our products any time in the near future. Until such time that a CPT code is approved that covers one or more of our products, patients would be required to pay for our products out of pocket. The process of obtaining a new CPT code to cover a product can take years and is expensive and full of uncertainties. Our inability to obtain a CPT code for our products on a timely or acceptable basis could have a material adverse effect upon our business, prospects, financial condition and results of operations. Further, approval of a CPT code for our products may place substantial restrictions on the indications for which our products may be used or the persons with whom they may be used. To gain reimbursement for the use of a product for clinical indications other than those for which the product was initially approved or cleared or for significant changes to the product, further studies, including clinical trials and approvals, may be required.
Our operations are subject to extensive government regulation.
Medical products, devices and therapies are subject to extensive government regulation and review. These regulations are constantly changing. While we believe that our proposed operations will be conducted in material compliance with applicable laws, we have not received or applied for a legal opinion from counsel or from any federal or state judicial or regulatory authority to this effect, and many aspects of our business operations have not been the subject of state or federal regulatory interpretation. Interpretation and enforcement of existing federal and state laws regulating health care, and future laws regulating health care, could have a material adverse effect upon our business, prospects, financial condition and results of operations.
Our founder and Chairman of our Board of Directors, Gaetano Scuderi, M.D., is a physician. He owns a significant percentage of our issued and outstanding shares. A number of our other investors are physicians. All of our investor physicians may have a conflict of interest in working in the best interests of patients while recommending our products to those patients, because they may receive a financial benefit from sales of our products as a result of their ownership interest in our Company.
Physician Self-Referral Laws. The federal self-referral law (known as the “Stark Law”) imposes restrictions on physicians’ referrals for certain designated health services reimbursable by Medicare or Medicaid to entities with which any such physician (or an immediate family member) has a financial relationship, whether through an ownership, debt or compensation arrangement, and it prohibits an entity from billing Medicare or Medicaid, or any other federally funded health benefit program, for services rendered pursuant to a prohibited referral. Violations can result in denial and recoupment of payments for services, imposition of substantial civil or monetary penalties and exclusion from Medicare and Medicaid. In addition, several states have adopted self-referral laws, some of which are not limited to Medicare or Medicaid reimbursed services or to certain designated health services. Violation of any such laws may result in the imposition of penalties on the physicians pursuant to applicable regulations, including fines and suspension or revocation of the physician’s license. Investors should make their own determination as to the risk of violation of the Stark Law. Any sanctions imposed upon us could have a material adverse effect upon our business, prospects, financial condition and results of operations.
Fraud and Abuse. The anti-kickback provisions of the Social Security Act prohibit the solicitation, payment, receipt or offering of any direct or indirect remuneration in return for, or as an inducement for, referrals of patients for items or services reimbursable under Medicare, Medicaid or other federally funded health care benefit programs. Violation of these provisions, which are commonly known as the “Fraud and Abuse Law,” can lead to the imposition of civil and criminal penalties upon all parties involved, including substantial civil monetary penalties and exclusion from Medicare, Medicaid and other federally funded health care benefit programs. The scope of prohibited conduct under the Fraud and Abuse Law is broad and it extends to economic arrangements involving hospitals, physicians and their business partners, including arrangements such as those contemplated by us. Applicable state regulations may also prohibit the payment of remuneration in return for referrals. Violation of these state regulations may result in the imposition of monetary penalties on physicians, and suspension or revocation of a physician’s license. It is not certain that our operations will meet the requirements of a safe harbor under the Fraud and Abuse Law. We cannot assure you that our operations do not, or in the future will not, violate such law, and any sanctions imposed upon us could have a material adverse effect upon our business, prospects, financial condition and results of operations.
Licensure and Corporate Practice of Medicine. State laws and regulations combine to regulate the provision of health care services by prohibiting business entities, such as us, from practicing medicine or otherwise providing health care services without a license and from exercising control over the medical judgments or decisions of physicians and licensed entities. Violation of these requirements could result in civil and criminal sanctions against us. While we believe that our business as structured materially complies with these laws, we cannot assure you that the regulatory authorities or other parties will not assert that we are engaged in the corporate practice of medicine or the unlicensed provision of health care services, and if such an action were taken or such a determination were made, it could have a material adverse effect upon our business, prospects, financial condition and results of operations.
We may not be able to obtain the regulatory approvals necessary to market our products. Further, if we fail to comply with the extensive governmental regulations that affect our business, we could be subject to penalties and could be precluded from marketing our products.
Our research and development activities and the manufacturing, labeling, distribution and marketing of our products will be subject to regulation by numerous governmental agencies, including but not limited to the FDA, the State of Florida, HHS, and CMS. The United States Food and Drug Administration (“FDA”) imposes mandatory procedures and standards for the conduct of clinical trials and the production and marketing of products for diagnostic and human therapeutic use.
Our products are subject to approvals or clearances prior to marketing for commercial use. The process of obtaining necessary approvals or clearances can take years and is expensive and full of uncertainties. Our inability to obtain required regulatory approvals on a timely or acceptable basis could have a material adverse effect upon our business, prospects, financial condition and results of operations. Further, approvals or clearances may place substantial restrictions on the indications for which our products may be marketed or the persons to whom they may be marketed. To gain approval for the use of a product for clinical indications other than those for which the product was initially approved or cleared or for significant changes to the product, further studies, including clinical trials and approvals, may be required.
We believe that the most significant risk relates to the regulatory classification of certain of our products. In the filing of each application, we make a legal judgment about the appropriate form and content of the application. If the regulator disagrees with our judgment in any particular case and, for example, requires us to file a pre-market approval application rather than allowing us to market for approved uses while we seek broader approvals, or requires extensive additional clinical data, the time and expense required to obtain the required approval might be significantly increased or the approval might not be granted.
Approved products will be subject to continuing regulatory requirements relating to quality control and quality assurance, maintenance of records, reporting of adverse events, documentation, and labeling and promotion of medical devices.
The regulatory authorities require that our products be manufactured according to rigorous standards. These regulatory requirements may significantly increase our production or purchasing costs above currently expected levels and may even prevent us from making our products in quantities sufficient to meet market demand. If we change our approved manufacturing process, regulators may require a new approval before that process may be used. Failure to develop our manufacturing capability may mean that even if we develop promising new products, we may not be able to produce them profitably, as a result of delays and additional capital investment costs. Manufacturing facilities are also subject to inspections by or under the authority of the relevant regulator. In addition, failure to comply with applicable regulatory requirements could subject us to enforcement action, including product seizures, recalls, withdrawal of clearances or approvals, restrictions on or injunctions against marketing our product or products based on our technology, and civil and criminal penalties.
The Affordable Care Act and other payment and policy changes may have a material adverse effect on us.
The Patient Protection and Affordable Care Act enacted on March 23, 2010, as amended by the Health Care and Education Reconciliation Act of 2010 enacted on March 30, 2010, or, together, the Affordable Care Act, imposes a 2.3% excise tax on the sale of any taxable human medical device after December 31, 2012, subject to certain exclusions, by the manufacturer, producer or importer of such devices. The total cost to the industry was expected to be approximately $20 billion over ten years. This significant tax burden on our industry could have a material negative impact on the results of our operations and our cash flows. A significant portion of our sales will be considered medical device sales under this new legislation. Therefore, our products will be subject to this excise tax.
Further, the Affordable Care Act encourages hospitals and physicians to work collaboratively through shared savings programs, such as accountable care organizations, as well as other bundled payment initiatives, which may ultimately result in the reduction of medical device acquisitions and the consolidation of medical device suppliers used by hospitals. While passage of the Affordable Care Act may ultimately expand the pool of potential end-users of our products, the above-discussed changes could adversely affect the prices we are able to charge for our products or the amounts of reimbursement available for our products and could limit the acceptance and availability of our products. Each of these could have a material adverse effect on our financial position and the results of our operations.
Further, with the increase in demand for healthcare services, we expect both a strain on the capacity of the healthcare system and more proposals by legislators, regulators and third-party payers to keep healthcare costs down. Certain proposals, if passed, could impose limitations on the prices we will be able to charge for our products, or the amounts of reimbursement available from governmental agencies or third-party payers. These limitations could have a material adverse effect on our financial position and results of operations.
Federal healthcare reform continues to be a political issue, and it is unclear how federal elections may ultimately impact the effects of the Affordable Care Act. Various healthcare reform proposals have also emerged at the state level. We cannot predict what healthcare initiatives, if any, will be implemented at the federal or state level, or the effect any future legislation or regulation will have on us. However, an expansion in government’s role in the United States healthcare industry may lower reimbursements for our products, reduce medical procedure volumes and adversely affect our business, possibly materially.
Changes in the health care industry may require us to reduce the selling prices for our products or result in a reduction in the size of the market for our products, which could have a negative effect on our financial performance.
Trends toward managed care, health care cost containment and other changes in government and private sector initiatives are placing increased emphasis on the delivery of more cost-effective medical therapies that could adversely affect the sale or the prices of our products. For example:
| | · | Major third-party payers of hospital services, including Medicare and Medicaid and private health care insurers, have substantially revised their payment methodologies, which has resulted in stricter standards for reimbursement of hospital charges for certain medical procedures; |
| | · | Third-party payer cutbacks could create downward price pressure; |
| | · | Numerous legislative changes have been passed and others are being considered that would result in major reforms in the U.S. health care system that could have an adverse effect on our business; |
| | · | There has been a consolidation among health care facilities and purchasers of medical products in the United States and these entities, which prefer to limit the number of suppliers from which they purchase medical products, may decide not to purchase or to stop purchasing our products or demand discounts on our prices; |
| | · | There are proposed and existing laws and regulations in many markets regulating pricing and profitability of companies in the health care industry; and |
| | · | There have been initiatives by third-party payers to challenge the prices charged for medical products, which initiatives could affect our ability to sell products on a competitive basis. |
Both the pressure to reduce prices for our products in response to these trends and the decrease in the size of the market as a result of these trends could adversely affect our future levels of revenues and profitability of sales.
In addition, there are laws and regulations that regulate the means by which companies in the health care industry may compete by discounting the prices of their products. Although we intend to exercise care in structuring our customer discount arrangements to comply with those laws and regulations, we cannot assure you that:
| | · | Government officials charged with responsibility for enforcing those laws will not assert that our customer discount arrangements are in violation of those laws or regulations, or |
| | · | Government regulators or courts will interpret those laws or regulations in a manner consistent with our interpretation. |
We may be subject to material liability or litigation expenses for claims associated with our products or related instruments.
Because of the nature of our business, we may become a defendant in medical malpractice, products liability or similar lawsuits, and may become subject to the attendant risk of substantial damage awards. Direct claims, suits or complaints could be asserted against us. While we expect to address these risks through comprehensive general liability insurance, we cannot assure you that such insurance will be available to us, affordable for us, or that any claim asserted against us will be covered by insurance, or will not exceed the coverage limits of applicable insurance. Further, we cannot assure you that we will be able to obtain any such insurance in the future, or that the insurance, if available, will not be too costly to obtain or maintain. A claim against us that is not defended by an insurance carrier, or a successful claim against or settlement by us in excess of our insurance coverage, or our inability to obtain or maintain insurance, could have a material adverse effect upon our business, prospects, financial condition and results of operations.
Our current and planned business is inherently expensive, risky and may not be understood by or accepted in the marketplace, which could adversely affect our future value.
The business currently conducted by the Company is financially speculative. To date, very few companies have been successful in their efforts to commercialize such a business. Furthermore, the number of people who may use our services is difficult to forecast with accuracy. Our future success is dependent on the establishment of the market for our services and our ability to capture a share of this market with the services and offerings we plan to develop. Due to the groundwork laid by the founders, our costs have been kept at a minimum. This allows us to become profitable quickly with a very minute amount of the potential market using our tools and resources.
Negative publicity could adversely affect our business and operating results.
Negative publicity about our industry or our Company, including the utility of our services and offerings, even if inaccurate, could adversely affect our reputation and the confidence in, and the use of, our marketplace, which could harm our business and operating results. Harm to our reputation can arise from many sources, including employee misconduct, misconduct by our partners, outsourced service providers or other counter-parties, failure by us or our partners to meet minimum standards of service and quality and compliance failures and claims.
We are susceptible to adverse economic conditions and our business may be adversely affected by changes in general economic conditions.
While we intend to finance our operations and growth of our business from funding and cash flow from operations, if adverse global economic conditions persist or worsen, we could experience a decrease in cash flow from operations attributable to reduced demand for our tools, resources and services and as a result, we may need to acquire additional financing for our continued operation and growth. There can be no assurance that alternative financing on acceptable terms would be available to Cytonics.
Our financial performance will be significantly affected by changes in national and local economic conditions, including:
| | · | Changes in the price or availability of materials that we utilize in the manufacture of our products and instruments; |
| | · | Changes in purchasing or reimbursement policies by hospitals, hospital chains, insurance carriers or Medicare/Medicaid; |
| | · | Changes by insurance companies in reimbursement or coverage for our products; and |
| | · | Entry of lower cost alternatives to our products. |
Our business is highly dependent upon the volume of patients seeking treatment for back and joint pain conditions. In turn, the demand for our products is highly sensitive to the following factors:
| | · | The development of alternative procedures or pharmaceutical treatments; |
| | · | Seasonal demand in our principal markets; |
| | · | Changes in market demand for the product; and |
| | · | An increase in the cost of malpractice insurance for surgeons who perform spine and joint diagnosis and treatment, which increases the cost of procedures and reduces the number of procedures performed. |
Because these factors can be volatile, our revenue levels can also be volatile. We expect to offer our products for sale throughout the United States. Adverse economic conditions in the United States could have a material adverse effect upon our business, prospects, financial condition and results of operations.
We may not effectively execute our strategy.
Our business strategy requires that we successfully and simultaneously complete many tasks. To be successful, we will need to:
| | · | Raise sufficient capital to fund our financial requirements; |
| | · | Develop products that gain market acceptance and can be sold at competitive prices; |
| | · | Negotiate effective business relationships and licensing agreements with others in the pharmaceutical and medical device industry; |
| | · | Attract and retain qualified, professional employees; and |
| | · | Evolve our business to gain advantages in an increasingly competitive environment. |
We cannot assure you that we will be able to successfully execute any or all of the elements of our strategy. Our failure to successfully execute any one of the elements of our strategy may have a material adverse effect on our business and results of operations.
We may fail to implement our business plan.
Investors may lose their entire investment if we fail to implement our business plan. Our prospects must be considered in light of the risks, uncertainties, expenses, and difficulties frequently encountered by companies in their early stages of development. These risks include, without limitation, competition, the absence of ongoing revenue streams, inexperienced management and lack of brand recognition. We cannot guarantee that we will be successful in executing our business. If we fail to implement and create a base of operations for our proposed business, we may be forced to cease operations, in which case investors may lose their entire investment.
There may be unanticipated obstacles to execution of our business plan.
Our proposed plan of operation and prospects will depend largely upon our ability to successfully establish Cytonics’ presence in a timely fashion, retain and continue to hire skilled management, technical, marketing, and other personnel, and attract and retain significant numbers of quality business partners and corporate clients. There can be no assurance that we will be able to successfully implement our business plan or develop or maintain future business relationships, or that unanticipated expenses, problems or technical difficulties which would result in material delays in implementation will not occur.
Competition with third parties in our contemplated industry is intense, which could reduce our profitability and our ability to pay dividends or raise additional funds.
We compete with many other entities seeking to undertake similar activities as we do. Furthermore, we expect that our most significant competitors will be fully integrated and more established companies. These companies are developing competing services and products and they have significantly greater capital resources and research and development, manufacturing, testing, regulatory compliance, and marketing capabilities. As a result, our competitors may develop more competitive or affordable products or services, or achieve earlier product and service commercialization than we are able to achieve. Competitive products and services may render any services, products or product candidates that we may acquire or develop uneconomic or obsolete.
There is no assurance that we will be able to grow or maintain our market share or customer base. In addition, we expect that the markets in which we will compete will continue to attract new competitors and new technologies. Increased competition in our markets could lead to price reductions, reduced profits, or loss of market share. The current global economic conditions could also result in increased price competition for our products and services and or provide an opportunity for our products and services to underprice the competition.
To compete successfully, we need to maintain a successful research and development effort and continue to adapt our model and services based on the industry demand. If we fail to enhance our current products and develop new products in response to changes in technology and industry standards, or fail to bring product enhancements or new product developments to market quickly enough, or accurately predict future changes in our customers’ needs and our competitors develop new technologies or products, our products could become less competitive or obsolete.
We operate in a rapidly changing marketplace.
The markets in which we compete are rapidly changing and highly competitive, and we may not be able to compete effectively.
Our industry is highly competitive.
The healthcare industry is highly competitive. We will be competing for market share against established drug and device companies and other medical technology companies that possess greater resources and experience than we do. We may also face competition from other early-stage companies that have alternative technological solutions, as well as universities, research institutions and other non-profit entities. Many of our competitors will have access to greater financial, technical, research and development, marketing, manufacturing, sales, distribution and other resources than we do. Our more established competitors also will have substantially greater experience in conducting clinical trials and obtaining regulatory approvals. Further, our competitors may be more effective at developing commercially viable products based upon their technologies.
We may need to develop new applications for our products to become or remain competitive. Technological advances by one or more of our future competitors could render our present or future products obsolete or uneconomical. Further, the removal of regulatory barriers in the future may also result in new competitors entering our business. Our future success will depend upon our ability to compete effectively against current technology as well as to respond effectively to technological advances and regulatory changes. Competitive pressures could have a material adverse effect upon our business, prospects, financial condition and results of operations.
The volatile credit and capital markets could have a material adverse effect on our financial condition.
Our ability to manage our future debt will be dependent on our level of positive cash flow. An economic downturn may negatively impact our cash flows. Credit and capital markets can be volatile, which could make it more difficult for us to refinance our debt or to obtain additional debt or equity financings in the future. Such constraints could increase our costs of borrowing and could restrict our access to other potential sources of future liquidity. Our failure to have sufficient liquidity to make interest and other payments required by our debt could result in a default of such debt and acceleration of our borrowings, which would have a material adverse effect on our business and financial condition.
A prolonged economic downturn could materially affect us in the future.
The recent recession and prolonged economic downturn reduced consumer confidence to historic lows, impacting the public’s ability and desire to spend discretionary dollars as a result of job losses, home foreclosures, significantly reduced home values, investment losses, bankruptcies and reduced access to credit, resulting in lower levels of customer traffic. If the economy experiences another significant decline, our business and results of operations could be materially adversely affected.
Information technology system failures or breaches of our network security could interrupt our operations and adversely affect our business.
We rely on our computer systems and network infrastructure across our operations. Our operations depend upon our ability to protect our computer equipment and systems against damage from physical theft, fire, power loss, telecommunications failure or other catastrophic events, as well as from internal and external security breaches, viruses and other disruptive problems. Any damage or failure of our computer systems or network infrastructure that causes an interruption in our operations could have a material adverse effect on our business and subject us to litigation or to actions by regulatory authorities.
We are continuing to develop our information technology capabilities, if we are unable to successfully upgrade or expand our technological capabilities, we may not have the ability to take advantage of market opportunities, manage our costs and transactional data effectively, satisfy customer requirements, execute our business plan or respond to competitive pressures.
We are exposed to the risk of natural disasters, unusual weather conditions, pandemic outbreaks, political events, war and terrorism that could disrupt business and result in lower sales, increased operating costs and capital expenditures.
Our headquarters and company-operated locations, as well as certain of our vendors and customers, are located in areas which have been and could be subject to natural disasters such as floods, hurricanes, tornadoes, fires or earthquakes. Adverse weather conditions or other extreme changes in the weather, including resulting electrical and technological failures, may disrupt our business and may adversely affect our ability to continue our operations. These events also could have indirect consequences such as increases in the costs of insurance if they result in significant loss of property or other insurable damage. Any of these factors, or any combination thereof, could adversely affect our operations.
Public health epidemics or outbreaks could adversely impact our business.
In December 2019, a novel strain of coronavirus (COVID-19) emerged in Wuhan, Hubei Province, China. While initially the outbreak was largely concentrated in China and caused significant disruptions to its economy, it has now spread to several other countries and infections have been reported globally. The extent to which the coronavirus impacts our operations will depend on future developments, which are highly uncertain and cannot be predicted with confidence, including the duration of the outbreak, new information which may emerge concerning the severity of the coronavirus and the actions to contain the coronavirus or treat its impact, among others. In particular, the continued spread of the coronavirus globally could adversely impact our operations, including among others, our sales and marketing and clinical trial operations and could have an adverse impact on our business and our financial results.
Rapid growth may strain our resources.
We expect to experience significant and rapid growth in the scope and complexity of our business, which may place a significant strain on our senior management team and our financial and other resources. Such growth, if experienced, may expose us to greater costs and other risks associated with growth and expansion. We may be required to hire a broad range of additional employees, including other support personnel, among others, in order to successfully advance our operations. We may be unsuccessful in these efforts or we may be unable to project accurately the rate or timing of these increases.
Our ability to manage our growth effectively will require us to continue to improve our operations, to improve our financial and management information systems, and to train, motivate, and manage our future employees. This growth may place a strain on our management and operational resources. The failure to develop and implement effective systems, or to hire and retain sufficient personnel for the performance of all of the functions necessary to effectively service and manage our business, or the failure to manage growth effectively, could have a materially adverse effect on our business, financial condition, and results of operations. In addition, difficulties in effectively managing the budgeting, forecasting, and other process control issues presented by such a rapid expansion could harm our business, financial condition, and results of operations.
The Company projects aggressive growth in 2022.
Although the Company estimates that it has enough runway until end of year, they will be ramping up cash burn to advance R&D of their lead drug candidate through the FDA regulatory process and fund other Company operations after the raise. Doing so could require significant effort and expense or may not be feasible.
Our risk management efforts may not be effective which could result in unforeseen losses.
We could incur substantial losses and our business operations could be disrupted if we are unable to effectively identify, manage, monitor, and mitigate financial risks, such as credit risk, interest rate risk, prepayment risk, liquidity risk, and other market-related risks, as well as operational risks related to our business, assets and liabilities. Our risk management policies, procedures, and techniques, including our scoring methodology, may not be sufficient to identify all of the risks we are exposed to, mitigate the risks we have identified or identify additional risks to which we may become subject in the future. We plan to mitigate this risk by executing our business plan and creating cash flow in order to stem the need for debt acquisition to survive.
Our business is located in Florida and may be subject to interruptions caused by hurricanes and other natural disasters.
Our physical facilities and employees are all located in southeastern Florida and thus our business operations are more susceptible than businesses located in other parts of the country to interruptions caused by hurricanes and flooding. These natural disasters pose a risk of damage to our facilities and interruptions to our business, which could be prolonged, as a result of extended power outages, the unavailability of our employees during periods when they must attend to personal circumstances, and the inability of third parties to provide necessary services to us due to similar factors. Damage to our facilities and extended interruption of our business could result in lost revenues and other material adverse consequences for our business, which may not be adequately covered by insurance or for which insurance may not be available.
If we become a public reporting company in the future, we will be required to publicly report on an ongoing basis as an “emerging growth company” and will be subject to less rigorous public reporting requirements and cannot be certain if the reduced reporting requirements applicable to emerging growth companies will make our Company less attractive to investors.
If we become a public reporting company in the future, we will be required to publicly report on an ongoing basis as an “emerging growth company” (as defined in the Jumpstart Our Business Startups Act of 2012, which we refer to as the JOBS Act) under the reporting rules set forth under the Exchange Act. For so long as we remain an “emerging growth company”, we may take advantage of certain exemptions from various reporting requirements that are applicable to other Exchange Act reporting companies that are not “emerging growth companies”, including but not limited to:
| | ● | not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act; |
| | | |
| | ● | taking advantage of extensions of time to comply with certain new or revised financial accounting standards; |
| | | |
| | ● | being permitted to comply with reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements; and |
| | | |
| | ● | being exempt from the requirement to hold a non-binding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved. |
If we become a public reporting company in the future, we expect to take advantage of these reporting exemptions until we are no longer an emerging growth company. We could be an emerging growth company for up to five years, although circumstances could cause us to lose that status earlier, including if the market value of our common stock held by non-affiliates exceeds $700 million, if we issue $1 billion or more in non-convertible debt during a three-year period, or if our annual gross revenues exceed $1 billion. We would cease to be an emerging growth company on the last day of the fiscal year following the date of the fifth anniversary of our first sale of common equity securities under an effective registration statement or a fiscal year in which we have $1 billion in gross revenues. Finally, at any time we may choose to opt-out of the emerging growth company reporting requirements. If we choose to opt out, we will be unable to opt back in to being an emerging growth company.
If we do not become a public reporting company under the Exchange Act for any reason, we will continue to be required to publicly report on an ongoing basis under the reporting rules set forth in Regulation A for Tier 2 issuers. The ongoing reporting requirements under Regulation A are more relaxed than for “emerging growth companies” under the Exchange Act. The differences include, but are not limited to, being required to file only annual and semiannual reports, rather than annual and quarterly reports. Annual reports are due within 120 calendar days after the end of the issuer’s fiscal year, and semiannual reports are due within 90 calendar days after the end of the first six months of the issuer’s fiscal year.
In either case, we will be subject to ongoing public reporting requirements that are less rigorous than Exchange Act rules for companies that are not “emerging growth companies”, and our stockholders could receive less information than they might expect to receive from more mature public companies.
We cannot predict if investors will find our Company less attractive because we may rely on these exemptions. If some investors find our Company less attractive as a result, there may be a less active trading market for our securities.
We depend on our executive officers, the loss of whom could materially harm our business.
We rely upon the accumulated knowledge, skills and experience of our executive officers and significant employees. If they were to leave us or become incapacitated, we might suffer in our planning and execution of business strategy and operations, impacting our brand and financial results. We also do not maintain any key man life insurance policies for any of our employees.
If we are unable to recruit additional executives and personnel, we may not be able to execute our forecasted business strategy and our growth may be hindered; limited time availability.
Our success largely depends on the performance of our management team and other key personnel and our ability to continue to recruit qualified senior executives and other key personnel. Competition for senior management personnel is intense and there can be no assurance that we will be able to retain our personnel or attract additional qualified personnel. The loss of a member of senior management may require the remaining executive officers to divert immediate and substantial attention to fulfilling his or her duties and to seeking a replacement. We may not be able to continue to attract or retain such personnel in the future. Any inability to fill vacancies in our senior executive positions on a timely basis could impair our ability to implement our business strategy, which would harm our business and results of operations.
We may not be able to retain key personnel or replace them if they leave.
Our future success depends partially on our ability to attract and retain highly qualified personnel. Competition for personnel is intense and we cannot assure you that we will be able to retain key employees or that we will be able to attract and retain additional qualified personnel in the future. Our inability to attract and retain necessary personnel could have a material adverse effect upon our business, prospects, financial condition and results of operations.
Members of our Board and our executive officers will have other business interests and obligations to other entities, and certain officers and directors may have conflicts of interest.
None of our directors or our executive officers will be required to manage the Company as their sole and exclusive function and they may have other business interests and may engage in other activities in addition to those relating to the Company, provided that such activities do not compete with the business of the Company or otherwise breach their agreements with the Company. We are dependent on our directors and executive officers to successfully operate our Company. Their other business interests and activities could divert time and attention from operating our business. Of course, we believe that, the Cytonics opportunity is very good and we anticipate that those involved during our initial phases will provide these position holders the ability to turn to Cytonics full time.
Individuals serving on our Board of Directors or as our officers actively participate in other business ventures and investment opportunities, including those that may compete with us. A conflict of interest is likely to arise if a director or officer becomes affiliated with a business entity that is a competitor, customer, provider or supplier of, or otherwise does business with, our Company. Individuals with such conflicts are required to disclose any such conflicts to us pursuant to their fiduciary duty of loyalty to our Company as required by our corporate governance policies and Florida state law. However, we cannot be certain that our directors and officers will always make disclosures, and therefore we may not be able to determine if a conflict of interest exists.
The Company does not have formal advisor agreements in place with listed advisors.
Advisor agreements typically provide the expectation of the engagement, services, compensation, and other miscellaneous duties and rights of the Company and advisor. These individuals may not be compensated for their expertise and advice. There is no guarantee that advisor agreements will be entered into.
The Company had outstanding liabilities in 2020.
During 2018, the Company initiated a private placement offering for the issuance of $1,000,000 in aggregate principal convertible promissory notes (“2018 Notes”), resulting in the issuance of multiple notes in the aggregate principal amount of $794,000, inclusive of a $50,000 note to a principal stockholder and chairman of the board and $50,000 note to the former Company’s Chairman of the Board and the prior chief financial officer. During 2019, an additional $10,000 promissory note was issued with the same terms. The 2018 Notes bore interest at a rate of 10% per year, payable quarterly, on March 31, June 30, September 30 and December 31 of each year, with a maturity date of June 30, 2021. As of December 31, 2021 and 2020 the total principal outstanding on the 2018 Notes was $0 and $804,000, respectively. During 2019, the Company issued convertible promissory notes in the aggregate amount of $486,511 (the “2019 Notes"). The issuance of the 2019 Notes resulted in the Company receiving net proceeds of $418,593. The 2019 Notes bore interest at a rate of 5% compounded each calendar quarter commencing with June 30, 2019. All outstanding principal and accrued interest were due May 2021 ("Maturity Date''). During the years ended December 31, 2021 and 2020, the Company recognized aggregate amortization expense of $98,648 and $229,960 related to the beneficial conversion feature and debt issuance costs which is included on the statements of operations. As of December 31, 2021 and 2020, the aggregate unamortized debt discount was $0 and $98,648. On October 31, 2019, the Company issued a promissory note with an unrelated individual in the amount of $100,000. The promissory note bears interest at 10% and is due October 31, 2024. The note holder may elect to convert all, but only all, of the outstanding principal and accrued interest into shares as follows: 1) prior to an initial public offering (IPO) at a conversion price of $2.00 or 2) at the completion of an IPO, at a conversion price equal to the share price paid in the IPO less a 10% discount. As of December 31, 2021 and 2020, the total principal outstanding on the 2019 Notes and 2019 Promissory Note was $0 and $586,511, respectively. During 2020, the Company issued promissory notes in the principal amount of $715,000 of which $40,000 was with related parties. In 2021, the Company issued an additional promissory note with a principal amount of $30,000 (collectively referred to as the “2020 Notes”). In connection with the 2020 notes, the Company issued options to purchase 372,500 shares of common stock at a share price of $2.00, which was recorded as a debt discount in the amount of $28,463 and additional paid in- capital based on the relative fair value method. The debt discount was amortized over the terms of the 2020 notes of one (1) year. In 2021, $670,000, including $40,000 to related parties, of outstanding principal on the 2020 Notes were repaid in in cash and all accrued interest outstanding at that time At December 31, 2021 and 2020, aggregate outstanding principal and accrued interest on the 2018 Notes, 2019 Notes and 2020 Note was $0 and $2,105,511, including $140,000 with related parties, and $0 and $105,900, which is included in the caption accounts payable and accrued liabilities on the balance sheets, respectively. In April 2020, the Company entered into a promissory note evidencing an unsecured loan (the “Loan”) in the amount of $27,212 made to the Company under the Paycheck Protection Program (the “PPP”). The PPP was established under the CARES Act and is administered by the U.S. Small Business Administration. In 2021, the Company received notification that Loan was forgiven and recorded gain on extinguishment of $27,408.
Intellectual Property Risks
Our intellectual property rights may not provide meaningful commercial protection for our products, which could enable third parties to use our technology or very similar technology and could reduce our ability to compete successfully.
Our ability to compete effectively will depend, in part, on our ability to maintain the proprietary nature of our technologies, which includes our ability to obtain, protect and enforce patents on our technology and to protect our trade secrets. While our technology is subject to patent applications that cover significant aspects of our product line, our patent applications may not provide us with any significant competitive advantage. Others may challenge our patent applications and, as a result, our proprietary rights could be narrowed, invalidated or rendered unenforceable. Competitors may develop products similar to ours that our patent applications do not cover. Our current and future patent applications may not result in the issuance of patents. Further, there is a substantial backlog of patent applications in many patent offices and the approval or rejection of patent applications may take several years.
We are dependent upon a limited number of PhD’s and MD’s for the continued development of our proprietary technology, and if one or more of them were to become unavailable to us, our ability to continue the development of our technology could be adversely affected.
Dr. Gaetano Scuderi, Dr. Lewis Hanna, Dr. Robert Bowser, Dr. Shawn Browning, and Dr. John David Laughlin are the named inventors of all or part of our patented technology, and we depend to a substantial degree upon some of them for continued development of our technology and applications of our technology to the spine and joint markets. We have the right of ownership in any future technologies or products that they may develop while working for the Company or as consultants. They have assigned their rights in our proprietary technology to the Company, and as employees and/or shareholders in our Company, continue to have an economic incentive to work with us on the development and commercialization of our technology. Nevertheless, we do not have any agreements with them that obligate them to continue providing services or other assistance to us, including in the continued development and commercialization of our products and our technology. If they were to cease providing assistance to us in these areas, our business could be adversely affected.
We have disclosed detailed information regarding our intellectual property to certain of our competitors.
We have disclosed some of our Product designs and other proprietary information to certain potential partners who may also compete with us in the future. Although these parties have executed non-disclosure agreements with respect to the proprietary information, there can be no assurances that they will maintain the confidentiality of our intellectual property rights or refrain from infringing on our intellectual property rights.
Our proprietary technology includes unpatented trade secrets, which we may not be able to protect.
Our proprietary technology includes unpatented trade secrets, the competitive advantage of which is substantially dependent upon our ability to maintain their continued secrecy. Trade secrets are difficult to protect. We cannot assure you that others will not independently develop substantially equivalent proprietary information and techniques or otherwise gain access to our trade secrets, that those trade secrets will not be disclosed, or that we can effectively protect our unpatented trade secrets.
In an effort to protect our trade secrets, we have a policy of requiring our employees, consultants and advisors to execute proprietary information agreements upon commencement of employment or consulting relationships with us. We expect that these agreements will provide that all confidential information developed or made known to the individual during the course of his or her relationship with us must be kept confidential, except in specified circumstances. We cannot assure you, however, that these agreements will provide meaningful protection for our trade secrets or other proprietary information in the event of the unauthorized use or disclosure of confidential information.
Our success will depend partly on our ability to operate without infringing or misappropriating the proprietary rights of others.
We may be sued for infringing upon the intellectual property rights of others. In addition, we may find it necessary, if threatened, to initiate a lawsuit seeking a declaration from a court that we do not infringe upon the proprietary rights of others or that the other party’s rights are invalid or unenforceable. If we do not prevail in any litigation, in addition to any damages we might have to pay, we would be required to stop the infringing activity or obtain a license. Any required license may not be available to us on acceptable terms, or at all. In addition, some licenses may be nonexclusive, and, therefore, our competitors may have access to the same technology licensed to us. If we fail to obtain a required license or are unable to design around a patent, we may be unable to sell some of our products, which could have a material adverse effect upon our business, prospects, financial condition and results of operations.
We may be involved in lawsuits to protect or enforce our intellectual property rights, which may be expensive.
In order to protect or enforce our intellectual property rights, we may have to initiate legal proceedings against third parties, such as patent infringement suits or interference proceedings. Intellectual property litigation is expensive, and, even if we prevail, the expense of that litigation could adversely affect our financial position. In addition, litigation is time consuming and could divert our management’s time and attention from our business operations.
Risks Related to Ownership of Our Securities
There has been no active public market for our securities, and we do not intend to apply for a quotation of any of our securities on OTC Markets or on any stock exchange, which may adversely impact the market for our securities and make them difficult to sell.
There currently is not, an active market for our securities. We do not intend to apply for quotation of our securities on OTC Markets or on any stock exchange and a trading market for our securities may never develop which may make our securities difficult to sell.
The Company's existing investors have not waived their pre-emptive rights and may plan on exercising those rights.
The pre-emptive right entitles those investors to participate in this securities issuance on a pro rata basis. If those investors choose to exercise their pre-emptive right, it could dilute shareholders in this round. This dilution could reduce the economic value of the investment, the relative ownership resulting from the investment, or both.
The Company’s stock price may be volatile.
The price of the Company’s securities is likely to be highly volatile and could fluctuate widely in price in response to various potential factors, many of which will be beyond the Company’s control, including the following:
| | · | goods or services by the Company or its competitors; |
| | · | additions or departures of key personnel; |
| | · | the Company’s ability to execute its business plan; |
| | · | operating results that fall below expectations; |
| | · | loss of any strategic relationship; |
| | · | economic and other external factors; and |
| | · | period-to-period fluctuations in the Company’s financial results. |
In addition, the securities markets have from time to time experienced significant price and volume fluctuations that are unrelated to the operating performance of particular companies. These market fluctuations may also materially and adversely affect the market price of the Company’s securities.
We are required to publicly report on an ongoing basis under the reporting rules set forth in Regulation A for Tier 2 issuers and are accordingly subject to ongoing public reporting requirements that are less rigorous than Exchange Act rules for companies that are not "emerging growth companies", and our stockholders could receive less information than they might expect to receive from more mature public companies.
We are required to publicly report on an ongoing basis under the reporting rules set forth in Regulation A for Tier 2 issuers. The ongoing reporting requirements under Regulation A are more relaxed than for "emerging growth companies" under the Exchange Act. The differences include, but are not limited to, being required to file only annual and semiannual reports, rather than annual and quarterly reports. Annual reports are due within 120 calendar days after the end of the issuer's fiscal year, and semiannual reports are due within 90 calendar days after the end of the first six months of the issuer's fiscal year.
In such case, we are subject to ongoing public reporting requirements that are less rigorous than Exchange Act rules for companies that are not "emerging growth companies", and our stockholders could receive less information than they might expect to receive from more mature public companies.
The preparation of our financial statements involves the use of estimates, judgments and assumptions, and our financial statements may be materially affected if such estimates, judgments or assumptions prove to be inaccurate.
Financial statements prepared in accordance with accounting principles generally accepted in the United States of America ("GAAP") typically require the use of estimates, judgments and assumptions that affect the reported amounts. Often, different estimates, judgments and assumptions could reasonably be used that would have a material effect on such financial statements, and changes in these estimates, judgments and assumptions may occur from period to period over time. Significant areas of accounting requiring the application of management's judgment include, but are not limited to, determining the fair value of assets and the timing and amount of cash flows from assets. These estimates, judgments and assumptions are inherently uncertain and, if our estimates were to prove to be wrong, we would face the risk that charges to income or other financial statement changes or adjustments would be required. Any such charges or changes could harm our business, including our financial condition and results of operations and the price of our securities.
Certain provisions of our Articles of Incorporation may make it more difficult for a third party to effect a change-of-control.
Our amended and restated articles of incorporation authorizes the Board of Directors to issue up to 20,000,000 shares of preferred stock, of which 2,350,000 shares remain undesignated. Such undesignated shares of preferred stock may be issued in one or more subseries, the terms of which may be determined at the time of issuance by the Board of Directors without further action by the stockholders. These terms may include preferences as to dividends and liquidation, conversion rights, redemption rights and sinking fund provisions. The issuance of any preferred stock could diminish the rights of holders of our common stock, and therefore could reduce the value of such common stock. In addition, specific rights granted to future holders of preferred stock could be used to restrict our ability to merge with, or sell assets to, a third party. The ability of the Board of Directors to issue preferred stock could make it more difficult, delay, discourage, prevent or make it more costly to acquire or effect a change-in-control, which in turn could prevent our stockholders from recognizing a gain in the event that a favorable offer is extended.
Limitations on director and officer liability and indemnification of our Company’s officers and directors by us may discourage stockholders from bringing suit against an officer or director.
Our Company’s amended and restated articles of incorporation and amended and restated bylaws provide, with certain exceptions as permitted by governing state law, that a director or officer shall not be personally liable to us or our stockholders for breach of fiduciary duty as a director, except for acts or omissions which involve intentional misconduct, fraud or knowing violation of law, or unlawful payments of dividends. These provisions may discourage stockholders from bringing suit against a director for breach of fiduciary duty and may reduce the likelihood of derivative litigation brought by stockholders on our behalf against a director.
We are responsible for the indemnification of our officers and directors.
Should our officers and/or directors require us to contribute to their defense, we may be required to spend significant amounts of our capital. Our articles of incorporation and bylaws also provide for the indemnification of our directors, officers, employees, and agents, under certain circumstances, against attorney's fees and other expenses incurred by them in any litigation to which they become a party arising from their association with or activities on behalf of our Company. This indemnification policy could result in substantial expenditures, which we may be unable to recoup. If these expenditures are significant, or involve issues which result in significant liability for our key personnel, we may be unable to continue operating.
Cytonics is obligated to pay certain fees and expenses.
Cytonics will pay various fees and expenses related to its ongoing operations regardless of whether or not Cytonics’ activities are profitable. These fees and expenses will require dependence on third-party relationships. Cytonics is generally dependent on relationships with its strategic partners and vendors, and Cytonics may enter into similar agreements with future potential strategic partners and alliances. Cytonics must be successful in securing and maintaining its third-party relationships to be successful. There can be no assurance that such third parties may regard their relationship with Cytonics as important to their own business and operations, that they will not reassess their commitment to the business at any time in the future, or that they will not develop their own competitive services or products, either during their relationship with Cytonics or after their relations with Cytonics expire. Accordingly, there can be no assurance that Cytonics’ existing relationships or future relationships will result in sustained business partnerships, successful service offerings, or significant revenues for Cytonics.
An orthopedic implant company invested $4 million in the Company at a price of $2.50 per share and acquired the exclusive marketing and distribution rights to our diagnostic products. Although the implant company invested an additional $350,000 in the company in 2013, the marketing and distribution agreement has been terminated upon mutual agreement.
In February 2011, a leading orthopedic implant company purchased 400,000 shares of Series B Preferred Stock at $2.50 per share, along with the option to purchase additional equity of the Company. In June 2011, the orthopedic implant company exercised the option to purchase additional equity and purchased an additional 1,200,000 shares of Series B Preferred Stock at $2.50 per share. In April 2013, the orthopedic implant company purchased an additional 87,500 shares of Series B Preferred Stock at $4.00 per share.
In June 2011, the Company and the orthopedic implant company also entered into an Exclusive Global Marketing and Distribution Agreement. In this agreement, the orthopedic implant company received an exclusive worldwide license to sell and market certain of the Company’s diagnostic products. As part of this agreement, the orthopedic implant company also received the option to negotiate for the purchase of an exclusive worldwide license to sell and market the Company’s autologous and non-autologous therapeutic products. The orthopedic implant company’s option to negotiate for the Company’s autologous therapeutic products expired in 2012. In April of 2013, the marketing and distribution agreement was terminated upon mutual agreement. All options expired upon termination of the agreement.
We do not intend to pay dividends for the foreseeable future.
We have never declared or paid any cash dividends on our common stock and do not intend to pay any cash dividends in the foreseeable future. We anticipate that we will retain all of our future earnings for use in the development of our business and for general corporate purposes. Any determination to pay dividends in the future will be at the discretion of our board of directors. Accordingly, investors must rely on sales of their common stock after price appreciation, which may never occur, as the only way to realize any future gains on their investments.
A majority of the common stock of the Company is owned by a small number of owners who are also officers and directors of the Company.
The Company’s current officers and directors beneficially own 84.02% in the aggregate of the issued and outstanding common stock shares of the Company. Subject to any fiduciary duties owed to our other owners or investors under Florida law, the Company’s current officers and directors may be able to exercise significant influence over matters requiring approval, including the election of directors or managers and approval of significant Company transactions, and will have significant control over the Company’s management and policies. Some of these persons may have interests that are different from yours. For example, these persons may support proposals and actions with which you may disagree. The concentration of ownership could delay or prevent a change in control of the Company or otherwise discourage a potential acquirer from attempting to obtain control of the Company, which in turn could reduce the price potential investors are willing to pay for the Company. In addition, these persons could use their voting influence to maintain the Company’s existing management, delay or prevent changes in control of the Company, or support or reject other management and board proposals.
| Item 2. | Management’s Discussion and Analysis of Financial Condition and Results of Operations. |
You should read the following discussion and analysis of our consolidated financial condition and consolidated results of operations together with our consolidated financial statements and the related notes and other financial information included elsewhere in this annual report.
The following discussion of our financial condition and results of operations should be read in conjunction with the consolidated financial statements and the notes to those statements that are included elsewhere in this annual report. Our discussion includes forward-looking statements based upon current expectations that involve risks and uncertainties, such as our plans, objectives, expectations and intentions. Actual results and the timing of events could differ materially from those anticipated in these forward-looking statements as a result of a number of factors, including those set forth under the Risk Factors, Cautionary Note Regarding Forward-Looking Statements and Business sections in this annual report. We use words such as “anticipate,” “estimate,” “plan,” “project,” “continuing,” “ongoing,” “expect,” “believe,” “intend,” “may,” “will,” “should,” “could,” and similar expressions to identify forward-looking statements. Our future operating results, however, are impossible to predict and no guaranty or warranty is to be inferred from those forward-looking statements.
Overview
was formed on July 19, 2006 as a Florida corporation, under the name Gamma Spine, Inc. and was renamed Cytonics Corporation on April 17, 2007. The Company was formed for the purpose of researching and developing, marketing and distributing analytic tools used to detect biomarkers associated with certain diseases referred to as “assays,” therapeutic drugs, and related instruments and disposables related to musculoskeletal diseases. We are a development stage research company dedicated to developing therapeutics based on the naturally-occurring protease inhibitor alpha-2-macroglobulin (A2M), a blood serum protein that has known cartilage-protecting effects and could potentially serve as a treatment for osteoarthritis. To this end, we have developed a number of diagnostic and therapeutic products aimed at treating joint pain and inflammation.
Our mission is to improve people’s lives by limiting the progression of chondral pathology, which is bone and cartilage degeneration, which leads to disabling pain, inflammation, and the development of arthritis. Our strategy has been to leverage the unique molecular characteristics of A2M to develop autologous (“self-derived”) and synthetic (manufactured in a laboratory) therapeutics. We have developed two autologous A2M therapies and have out-licensed the drugs to medical device distributors in the human and veterinary orthopedic markets. Our current focus is on the development of a synthetic A2M variant (“CYT-108”) that can be synthesized in a laboratory and purchased “off-the-shelf,” and can be delivered in high concentrations to damaged and inflamed joints by an orthopedist. We seek to maximize the value of the drugs we discover by putting them in the hands of leading pharmaceutical companies with late-stage development, commercialization and marketing expertise.
For the fiscal years ended December 31, 2021 and 2020, we generated revenues of $307,500 and $591,056, respectively, and reported net losses of $2,544,189 and $995,850, respectively, and negative cash flow from operating activities of $2,784,503 and $585,103, respectively. As noted in our financial statements, we had an accumulated stockholders’ deficit of approximately $19,907,507 and recurring losses from operations as of December 31, 2021.
Results of Operations
Year Ended December 31, 2020 Compared to Year Ended December 31, 2021
The following discussion of results of operations refers to the year ended December 31, 2020 compared to the year ended December 31, 2021.
| | | | | | | | | Increase | |
| | | 2020 | | | 2021 | | | (Decrease) | |
| Revenues | | $ | 591,056 | | | $ | 307,500 | | | $ | (283,556 | ) |
| Cost of goods sold | | | N/A | | | | N/A | | | | | |
| Gross profit | | | N/A | | | | N/A | | | | | |
| Gross profit % | | | N/A | | | | N/A | | | | | |
| | | | | | | | | | | | | |
| Operating expenses: | | | | | | | | | | | | |
| Selling, general and administrative expenses | | | 258,881 | | | | 584,045 | | | | 325,164 | |
| Research and development expenses | | | 503,906 | | | | 2,205,358 | | | | 1,701,452 | |
| Payroll expenses | | | 219,473 | | | | 237,674 | | | | 18,201 | |
| Professional Fees | | | 170,667 | | | | 143,930 | | | | (26,737 | ) |
| Amortization | | | 31,503 | | | | 30,376 | | | | (1,127 | ) |
| Impairment loss | | | 34,276 | | | | - | | | | (34,276 | ) |
| Total Operating Expenses | | | 1,218,706 | | | | 3,201,383 | | | | 1,982,677 | |
| Loss from operations | | | (627,650 | ) | | | (2,893,883 | ) | | | (2,266,233 | ) |
| Other income (expenses): | | | | | | | | | | | | |
| Interest income | | | 9,274 | | | | 14,514 | | | | 5,240 | |
| Other income | | | 1,000 | | | | - | | | | (1,000 | ) |
| Paycheck protection program loan forgiveness | | | 0 | | | | 27,408 | | | | 27,408 | |
| Gain on extuishment of debt | | | 0 | | | | 571,629 | | | | 571,629 | |
| Interest Expense | | | (378,474 | ) | | | (263,857 | ) | | | 114,617 | |
| Total other income (expenses) | | | (368,200 | ) | | | 349,694 | | | | 18,506 | |
| | | | | | | | | | | | | |
| Loss before provision for income taxes | | | (995,850 | ) | | | (2,544,189 | ) | | | (1,548,339 | ) |
| | | | | | | | | | | | | |
| Income tax provision | | | - | | | | - | | | | - | |
| | | | | | | | | | | | | |
| Net loss | | $ | (995,850 | ) | | $ | (2,544,189 | ) | | $ | (1,548,339 | ) |
Revenue
Our revenue, net for the year ended December 31, 2020 was $591,056 compared to $307,500 for the year ended December 31, 2021. This decrease in revenue was due to a reduction in the amortized license acquisition fee payments.
Cost of Goods Sold
Cost of goods sold primarily consists of materials and freight costs. Considering that Cytonics does not source raw materials, manufacture, or distribute any of its proprietary products, Cost of Goods Sold is not relevant.
Selling, General, and Administrative Expenses
Selling, general, and administrative expenses totaled $258,881 and $584,045 for the years ended December 31, 2020 and 2021, respectively. Excluded in these expenses were payroll and related expenses of $219,473 and $237,674 during the years ended December 31, 2020 and 2021, respectively. Included in our payroll and related expenses are charges for stock-based compensation totaling $88,899 for the year ended December 31, 2020 and $33,248 for the year ended December 31, 2021. At December 31, 2021, there was approximately $55,094 of unrecognized compensation costs related to stock options outstanding which will be recognized through 2024. The Company will recognize forfeitures as they occur. Professional fees totaled $170,667 and $143,930 during the years ended December 31, 2020 and 2021, respectively. These expenses included legal, accounting and auditing expenses.
Research and Development Expenses
Research and development expenses totaled $503,906 and $2,205,358 for the years ended December 31, 2020 and 2021, respectively. The increase in the research and development expenses was attributable to the GMP manufacturing of CYT-108 and IND-enabling toxicity study.
Interest Income and Expense
Interest income totaled $9,274 for the year ended December 31, 2020 as compared to $14,514 for the year ended December 31, 2021. The increase in interest income was due to an increase in cash held in a money market account. Interest expenses totaled $378,474 during the year ended December 31, 2020 as compared to $263,857 for the year ended December 31, 2021. This figure represents the interest paid on the Company’s outstanding convertible and promissory notes.
Net Loss
Net loss totaled $995,850 and $2,544,189 during the years ended December 31, 2020 and 2021, respectively. Non-cash amounts reflected stock-based compensation, amortization, including amortization of debt discounts, and loss on impairment of intangible assets.
Liquidity and Capital Resources
Liquidity
We have primarily financed our operations through the sale of unregistered equity, promissory notes and warrants. As of December 31, 2021, our Company had cash totaling $967,757, current assets totaling $1,093,058, and total assets of $1,756,758. We had total liabilities of $554,929, all of which were current, and positive working capital of $538,129. Stockholders’ equity reflected a deficit of $19,907,507.
Sources and Uses of Cash for the Years Ended December 31, 2020 and December 31, 2021
The following table summarizes our cash flows for the year ended December 31, 2020 and 2021.
| | | 2020 | | | 2021 | |
| Net cash used in operating activities | | $ | 585,103 | | | $ | 2,784,503 | |
| Net cash used in investing activities | | | 78,651 | | | | 50,285 | |
| Net cash provided by financing activities | | | 663,516 | | | | 3,265,191 | |
| Net (decrease) increase in cash | | $ | (238 | ) | | $ | 430,403 | |
Net cash used by operating activities was $585,103 for the year ended December 31, 2020, and $2,784,503 for the year ended December 31, 2021. The increase in net cash used by operating activities was related to marketing expenses related to capital raising.
Net cash used by investing activities was $78,651 for the year ended December 31, 2020 as compared to $50,285 for the year ended December 31, 2021. The net cash used for investing activities can be attributed to the purchase of intangible assets, namely the prosecution of our patent portfolio.
Net cash provided by financing activities totaled $663,516 for the year ended December 31, 2020 as compared to $3,265,191 for the year ended December 31, 2021. The net cash provided by financing activities for the year ended December 31, 2020 was primarily attributed to the proceeds from the sale of simple notes in a bridge round of funding.
Availability of Additional Funds
Our capital requirements going forward will consist of financing our operations until we are able to reach a level of revenues and gross margins adequate to equal or exceed our ongoing operating expenses. Other than the borrowings from related and third parties, we do not have any credit agreement or source of liquidity immediately available to us.
Since inception our operations have primarily been funded through proceeds from existing shareholders in exchange for equity and debt.
Although we believe that we have access to capital resources, there are no commitments in place for new financing and there can be no assurance that we will be able to obtain funds on commercially acceptable terms, if at all. We expect to have ongoing needs for working capital in order to (a) fund operations; plus (b) research and development. To that end, we may be required to raise additional funds through equity or debt financing. However, there can be no assurance that we will be successful in securing additional capital. If we are unsuccessful, we may need to (a) initiate cost reductions; (b) forego business development opportunities; (c) seek extensions of time to fund its liabilities, or (d) seek protection from creditors.
In addition, if we are unable to generate adequate cash from operations, and if we are unable to find sources of funding, it may be necessary for us to sell all or a portion of our assets, enter into a business combination, or reduce or eliminate operations. These possibilities, to the extent available, may be on terms that result in significant dilution to our shareholders or that result in our shareholders losing all of their investment in our Company.
If we are able to raise additional capital, we do not know what the terms of any such capital raising would be. In addition, any future sale of our equity securities would dilute the ownership and control of your shares and could be at prices substantially below prices at which our shares currently trade. Our inability to raise capital could require us to significantly curtail or terminate our operations. We may seek to increase our cash reserves through the sale of additional equity or debt securities. The sale of convertible debt securities or additional equity securities could result in additional and potentially substantial dilution to our shareholders. The incurrence of indebtedness would result in increased debt service obligations and could result in operating and financing covenants that would restrict our operations and liquidity. In addition, our ability to obtain additional capital on acceptable terms is subject to a variety of uncertainties.
Our audited financial statements included elsewhere in this Annual Report have been prepared in conformity with accounting principles generally accepted in the United States of America (“U.S. GAAP”), which contemplate the realization of assets and the satisfaction of liabilities in the normal course of business. The carrying amounts of assets and liabilities presented in the financial statements do not necessarily purport to represent realizable or settlement values. The financial statements do not include any adjustment that might result from the outcome of this uncertainty.
On July 6, 2020, the Company began its offering up to 9,500,000 shares of its Series C Preferred Stock in a “Tier 2 Offering” under Regulation A (the “Reg A Offering”). The price per share of Series C Preferred Stock was $2.00 per share. The Series C Preferred Stock shares are convertible into shares of the Company’s common stock either at the discretion of the investor or automatically upon the occurrence of the consummation of an underwritten public offering of the Company resulting in at least $20,000,000 net proceeds to the Company. The total number of shares of the Common Stock into which the Series C Preferred Stock may be converted at the discretion of the investor is based on a conversion ratio of 1 for 1. The Company raised $4,659,638 funds in its Reg A Offering and issued 2,329,819 shares of its Series C Preferred Stock to 2,557 investors. The Reg A Offering was closed on April 30, 2021.
The Company is currently engaged in a Regulation Crowdfunding Offering of up to $5,000,000 worth of its Series C Preferred Stock for a price of $2.30 per share (the “2022 Reg CF Offering”). To date, the Company raised approximately $2,730,185 during the offering period in the 2022 Reg CF Offering.
Off-Balance Sheet Arrangements
We have not entered into any other financial guarantees or other commitments to guarantee the payment obligations of any third parties. We have not entered into any derivative contracts that are indexed to our shares and classified as stockholders’ equity or that are not reflected in our financial statements. Furthermore, we do not have any retained or contingent interest in assets transferred to an unconsolidated entity that serves as credit, liquidity or market risk support to such entity. We do not have any variable interest in any unconsolidated entity that provides financing, liquidity, market risk or credit support to us or engages in leasing, hedging or research and development services with us.
Critical Accounting Policies Involving Management Estimates and Assumptions
Basis of Presentation
The accompanying financial statements have been prepared in conformity with accounting principles generally accepted in the United States of America (U.S. GAAP).
During 2018, the Company affected a 2:1 split of its common stock. All shares of common stock have been adjusted to reflect post-split amounts for all periods presented.
Use of Estimates
To prepare financial statements in conformity with generally accepted accounting principles (“GAAP”), management must make estimates and assumptions that affect the amounts reported in the financial statements and accompanying notes. Actual results could differ from those estimates.
Cash and Cash Equivalents
We consider currency on hand, demand deposits and all highly liquid investments with an original or remaining maturity of three months or less to be cash and cash equivalents. As of December 31, 2020 and 2021, the Company had no cash equivalents.
Concentration of Credit Risk and Significant Customers
Financial instruments, which potentially subject the Company to concentrations of credit risk, consist primarily of cash and cash equivalents and accounts receivable. The Company limits its exposure to credit loss by placing its cash with high credit quality financial institutions. During the year ended December 31, 2021 and 2020 the Company generated revenues from two (2) customers, and three (3) customers, respectively. At December 31, 2021 and 2020, two (2) customers and one (1) customer accounted for 100% of total accounts receivable balance, respectively.
Accounts Receivable
Accounts receivable in the accompanying financial statements are stated at the amounts management expects to collect. The Company performs credit evaluations of its customers’ financial condition and may require a prepayment for a portion of the services to be performed. Management has determined that no allowance for uncollectible receivables is required at December 31, 2020 and 2021.
Intangible Assets
Intangible assets consist of costs paid to third parties for work related to the Company’s patents. The costs paid to third parties for the Companies’ assets are amortized using the straight-line method over the life of the underlying patents, which approximates 15 years. Intangible assets are tested for impairment whenever events or circumstances indicate that the carrying amount may not be recoverable.
Revenue Recognition
Revenue is recognized when obligations under the terms of a contract with a customer are satisfied; generally this occurs with the transfer of control or access of the company’s licenses or performance of services. Revenue is measured as the amount of consideration the company expects to receive in exchange for transferring goods or providing services. A performance obligation is a promise in a contract to transfer a distinct good or service to the customer, and is the unit of account in the contract. A contract’s transaction price is allocated to each distinct performance obligation and recognized as revenue when, or as, the performance obligation is satisfied.
Research and Development
Costs related to research and development are expensed as incurred and include costs associated with research and development of new products and enhancements to existing products. The amount of research and development costs for 2020 and 2021 were $503,906 and $2,205,358, respectively.
Income Taxes
The Company uses the asset and liability method to recognize deferred tax assets and liabilities for the expected future tax consequences of temporary differences between the carrying amounts and the tax basis of assets and liabilities.
Deferred tax assets and liabilities are determined using the effective tax rates for the years in which the tax assets and liabilities are expected to be realized. A valuation allowance is established when it is more likely than not that the future realization of all or some of the deferred tax assets will be achieved. The Company recorded a full valuation allowance on its net deferred tax assets at December 31, 2020 and 2021.
Quantitative and Qualitative Disclosure about Market Risk
Trade Policy Risk
Substantially all of the Company's products are manufactured outside the United States. Most products imported into the United States is subject to duty and restrictive quotas on the amount of products that can be imported from certain countries into the United States each year. Because of the duty rates and quotas, changes in U.S. trade policy as reflected in various legislation, trade preference programs and trade agreements have the potential to materially impact the Company's sourcing strategy and the competitiveness of its contract manufacturers. The Company manages this risk by continually monitoring U.S. trade policy, analyzing the impact of changes in such policy and adjusting its manufacturing and sourcing strategy accordingly.
Foreign Currency Risk
The Company receives United States dollars for all of its product sales. Substantially all inventory purchases from our contract manufacturer are also denominated in United States dollars; however, purchase prices for the Company's products may be impacted by fluctuations in the exchange rate between the United States dollar and the local currencies of the contract manufacturer, which may have the effect of increasing the Company's cost of goods in the future. The Company does not engage in hedging activities with respect to such exchange rate risk.
Commodity Price Risk
The Company is subject to commodity price risk arising from price fluctuations in the market prices of sourced titanium and steel products or the various raw materials components of its manufactured products. The Company is subject to commodity price risk to the extent that any fluctuations in the market prices of its purchased titanium and steel products and raw materials are not reflected by adjustments in selling prices of its products or if such adjustments significantly trail changes in these costs. The Company neither enters into significant long-term sales contracts nor enters into significant long-term purchase contracts. The Company does not engage in hedging activities with respect to such risk.
Credit Risk
Credit risk relates to the risk of loss resulting from non-performance or non-payment by counterparties pursuant to the terms of their contractual obligations. Risks surrounding counterparty performance and credit could ultimately impact the amount and timing of expected cash flows.
Certain financial instruments potentially subject the company to a concentration of credit risk. These financial instruments consist primarily of cash and cash equivalents and accounts and vendor receivables. We place our cash and cash equivalents with high-credit, quality financial institutions. The balances in these accounts exceed the amounts insured by the Federal Deposit Insurance Corporation.
| Item 3. | Directors and Officers. |
Directors and Executive Officers
The following table sets forth the names, positions and ages of our directors and executive officers as of the date of this Annual Report. Our directors are elected by our stockholders at annual meeting of the stockholders and serve until the next annual meeting of the stockholders or, in absence of such annual meeting, until their successors are elected and qualified. Officers are elected by our board of directors and their terms of office are at the discretion of our board.
| Name | | Age | | Positions Held | | Initial Term of
Office |
| Gaetano J. Scuderi, MD | | 60 | | Founder and Chairman of the Board | | July 2006 |
| | | | | | | |
| Joey Bose | | 33 | | Chief Executive and Financial Officer, President and Director | | May 2018 |
| | | | | | | |
| Lewis Hanna, PhD | | 76 | | Chief Scientific Officer | | February 2008 |
| | | | | | | |
| Gordon V. Ramseier | | 78 | | Director | | January 2018 |
| | | | | | | |
| Tracy Goeken, MD | | 48 | | Director | | November 2020 |
| | | | | | | |
| Phil LoGrasso, PhD | | 59 | | Director | | December 2020 |
Biographical information concerning the directors and executive officers listed above is set forth below.
Gaetano J. Scuderi, MD. Dr. Scuderi is the Founder and Chairman of the Board of Cytonics Corporation and has served as a director of Cytonics Corporation since July 2006. Dr. Scuderi previously served as the CEO of the Company from 2015 to 2018. Dr. Scuderi is a fellowship-trained spine surgeon and has practiced medicine since 1993 to the present. Dr. Scuderi currently practices orthopedic surgery in Jupiter, FL which he has been engaged in since 2013. He was previously Clinical Assistant Professor in the Department of Orthopedic Surgery of Stanford University from 2009 to 2012. Dr. Scuderi has published over 50 scientific articles and is a member of American Academy of Orthopedic Surgeons (AAOS). His paper entitled, “Improving Response to Treatment for Patients with DDD by the use of Molecular Markers” was awarded Best Paper at 2015’s annual meeting of the International Spine Intervention Society (ISIS). He graduated medical school from State University of New York at Buffalo, N.Y. in 1987 and completed his Residency and Internship at University of Miami School of Medicine, Jackson Memorial Medical Center. He then went on to a fellowship in spine surgery at UCSD.
Anjun K. (Joey) Bose. Mr. Bose is the President and Chief Executive Officer of the Company and has served in such capacity starting in May of 2018. Mr. Bose was appointed as a member of our Board of Directors in November of 2020. Mr. Bose was appointed as the Company’s Interim Chief Executive and Financial Officer on April 30, 2021. He was appointed Chief Executive Officer on January 1, 2022. Mr. Bose has over 10 years’ experience in biotechnology research development and investment banking. His principal activities include coordinating capital raising efforts, initiating clinical trials for two lead drug candidates, filing and maintaining patent protection of intellectual property, and identifying strategic buyers and out-licensing opportunities for the company. From August 2017 to May 2018, Mr. Bose served as the VP of Investment Banking from Affinia Capital, LLC. From August 2015 to August 2017, Mr. Bose served as an Associate of Investment Banking at CG Capital Markets, LLC. From August 2012 to August 2015, Mr. Bose was a graduate student engaged in academic research at Johns Hopkins University. Mr. Bose began his R&D career at the University of Virginia where he developed a novel assay to measure phosphatase activity in the context of cancer biology. He continued his graduate studies in protein engineering at Johns Hopkins University, where he elucidated cell signaling pathways dysregulated in blood cancers. He went on to pursue a career in biotechnology investment banking at a number of boutique banks in Palm Beach County, Florida. He holds a B.S. in Biomedical Engineering from the University of Virginia and a M.S. in Biomedical Engineering from Johns Hopkins University.
Lewis Hanna, PhD. Dr. Hanna has served as the Chief Scientific Officer of Cytonics Corporation since February 2008. Until 2004, Dr. Hanna was the director of process development at Alexion Pharmaceutical where he directed a group of 15 scientists developing and manufacturing therapeutic antibodies and single chain antibodies for multiple indications. Dr. Hanna also held position of group leader and principal scientist in Bristol Myers Squibb and R. W. Johnson Pharmaceutical Research Institute, from 1995 to 1997, and 1988 to 1994, respectively. While at Cytonics, Dr. Hanna directed proteomic research that led to the discovery of a protein complex biomarker for spine disc degeneration (“FAC”; patent allowed). He characterized the biomarker and developed an ELISA assay for the detection of the protein complex biomarker in spinal disc lavage. Further research studies of this biomarker resulted in deeper understanding and the discovery of a new therapeutic strategy for osteoarthritis. Dr. Hanna has over 28 years of research experience in pharmaceutical and biotechnology companies, focused on the structure and function of proteins including extensive experience working with therapeutic protein folding, purification, formulation, large-scale production, quality, and the regulatory requirements to obtain FDA new drug approval. He also is expert at quality and regulatory requirements to obtain FDA new drug approval and has guided Cytonics’ successful regulatory submissions. Dr. Hanna received his BS degree from Cairo University, received his PhD from City University of New York, and completed a post-doctoral fellowship at Cornell University.
Gordon V. Ramseier. Mr. Ramseier has served as a director of the Board of Directors of Cytonics Corporation, since January 2018. Since February 2018 to the present, he co-founded and currently serves as the President of BCI LifeSciences LLC, an advisory firm, comprised of distinguished senior level executives from the life sciences industry. From January 1995 to September of 2019, he founded and served as the Executive Director of the Sage Group, Inc. Earlier in his career, he served as Director of Marketing and Gynecological Products at G.D. Searle and Product Manager of Pfizer Laboratories. Mr. Ramseier has served as a non-executive chairman of Metacrine Sciences Inc. from 1997 to 1999. He serves as Director of Business Development and Member of Advisory Board at Vessel Metrics, LLC. He was a Partner at Booz, Allen and Hamilton Management Consultants from 1979 to 1986. From 1992 to 1995, Mr. Ramseier was President and Chief Executive Officer of OncoTherapeutics, an early stage, private biopharmaceutical company focusing on innovative, immunotherapeutic approaches to cancer, located in Cranbury, NJ. From 1986 to 1990, he was President and CEO of Immunetech Pharmaceuticals, Inc., of San Diego. From 1986 to 1990, he served as President and Chief Executive Officer at Dura. He has operated a private consulting company since 1994 and also performed consulting work from 1990 to 1992. He served as a Partner in the Healthcare Industries Practice providing strategic planning services to such diverse clients as Johnson & Johnson, Bayer, The National Wholesale Druggists Association and Blue Cross. His work with the National Wholesale Druggists Association produced a landmark study of the U.S. drug wholesaling industry. Prior to entering the emerging company arena, Mr. Ramseier was 9 years with Booz, Allen and Hamilton, a recognized international management consulting firm. He served as Founding Vice Chairman of the BCNJ. He has been Executive Director of The Sage Group, Inc. since 1995. He serves as a Member of the Board of Directors/Trustee of Biotechnology Council of New Jersey. He served as a Director of Dura Pharmaceuticals Inc. since 1986. He served as a Director of BioNJ Inc. He served on the boards of seven emerging LifeScience companies. Mr. Ramseier received M.B.A. (With Distinction) from the Amos Tuck School of Business Administration, Dartmouth College and B.S. in Chemistry from Washington & Lee University.
Tracy Goeken, MD. Dr. Goeken brings over 15 years’ experience as a Chief Medical Officer and Director of Clinical Development and Regulatory Affairs in the biopharma industry. He is currently the Chief Medical Officer of Linical Accelovance Group where he has held such position since 08/2018. Prior to that, he served as a Medical Consultant to Q2K Medical Consulting from 02/2018 to the present, Chief Medical Officer of Somahlution Inc. from 01/2013 to 01/2018, Vice President of Clinical and Medical Affairs at Nuron Biotech Inc. from 07/2010 to 01/2013, Director of Global Pharmacovigilance at Pharm-Olam International from 07/2006 to 07/2010, Department Manager, Cardiovascular Research at The Methodist Hospital Clinical Trial Support Unit from 08/2005 to 07/2006, and as a research associate at the SRB Research Institute from 2004 to 2005.
Phil LoGrasso, PhD. Dr. LoGrasso has spent the last three decades actively involved in forming relationships with Big Pharma, venture-backed biotech companies, academic researchers at the NIH, and biotech-focused hedge funds. We believe that this wealth of experience provides a unique, deep-seated knowledge into essential elements for scientific and financial success in the biopharma industry. He is currently a Senior Analyst at GQG Partners where he has held such position since 01/2019. Prior to that, he served as a Consultant at OPKO Health Inc. from 01/2011 to 12/2019, Sr. Health Care Analyst at Senzar Asset Management from 01/2013 to 10/2018, Professor of Molecular Therapeutics and Sr. Director of Drug Discovery at Scripps Research Institute from 07/2005 to 01/2016, Program Director at the NIH from 07/2004 to 07/2005, the Director of Preclinical Research and Development at Avera Pharmaceuticals from 07/2002 to 01/2004, a Research Fellow at Merck from 05/1994 to 07/2002, a Post-Doctoral Fellow at Sandoz from 02/1992 to 05/1994, and has held the position of Advisor and Director on multiple Boards.
Corporate Governance
Director Qualifications
Gaetano J. Scuderi, MD - Our Board believes that Dr. Scuderi’s qualifications to serve on our board include his extensive experience in the development of diagnostic assays and therapeutic drugs related to musculoskeletal disease.
Joey Bose - Our Board believes that Mr. Bose’s qualifications to serve on our board include his experience in the biotechnology industry.
Gordon V. Ramseier – Our Board believes that Mr. Ramseier’s qualifications to serve on our board include his vast experience in business and financial matters in the biotechnology industry.
Tracy Goeken, MD - Our Board believes that Dr. Goeken’s qualifications to serve on our board include his extensive experience in the biopharma industry.
Phil LoGrasso, PhD - Our Board believes that Dr. LoGrasso’s qualifications to serve on our board include his extensive experience in the biopharma industry.
Board of Directors and Board Committees
Our Board has not established any committees, including an audit committee, a compensation committee or a nominating committee, or any committee performing a similar function. The functions of those committees are being undertaken by our Board.
We do not have a policy regarding the consideration of any director candidates that may be recommended by our stockholders, including the minimum qualifications for director candidates, nor has our officers and directors established a process for identifying and evaluating director nominees. We have not adopted a policy regarding the handling of any potential recommendation of director candidates by our stockholders, including the procedures to be followed. Our officers and directors have not considered or adopted any of these policies as we have never received a recommendation from any stockholder for any candidate to serve on our Board of Directors.
Given our relative size, we do not anticipate that any of our stockholders will make such a recommendation in the near future. While there have been no nominations of additional directors proposed, in the event such a proposal is made, all current members of our Board will participate in the consideration of director nominees.
In the future, we intend to establish an audit committee of our Board of Directors. Our securities are not quoted on an exchange that has requirements that a majority of our Board members be independent and we are not currently otherwise subject to any law, rule or regulation requiring that all or any portion of our Board of Directors include “independent” directors, nor are we required to establish or maintain an audit committee or other committee of our Board.
Board Leadership Structure and Board’s Role in Risk Oversight
We decided to separate the positions of Chairman of the Board and Chief Executive Officer in August of 2018, relieving Dr. Scuderi as the Chief Executive Officer and appointed Antonio Carvalho in his place at that time, Mr. Carvalho has since then resigned from all positions with the Company. Dr. Scuderi still serves as our Chairman of the Board of Directors. Joey Bose now serves as our Chief Executive Officer. We believe that separating the positions of Chairman and Chief Executive Officer allows for focused leadership of our organization which benefits us in our relationships with investors, customers, suppliers, employees and other constituencies. We believe that distributing the leadership of the Company amongst highly-qualified individuals is the appropriate leadership structure for our Company and that any risks inherent in that structure are balanced by the oversight of our three independent directors (Gordon Ramseier, Tracy Goeken, MD and Phil LoGrasso, PhD) on our Board. However, no single leadership model is right for all companies and at all times. The Board recognizes that depending on the circumstances, other leadership models, such as the appointment of a lead independent director, might be appropriate. Accordingly, the Board may periodically review its leadership structure. The Board will only hold executive sessions in which only independent directors are present.
Our Board is generally responsible for the oversight of corporate risk in its review and deliberations relating to our activities. Our principal source of risk falls into two categories, financial and product commercialization. The Board oversees management of financial risks; our Board regularly reviews information regarding our cash position, liquidity and operations, as well as the risks associated with each. The Board regularly reviews plans, results and potential risks related to our business. The Board is also expected to oversee risk management as it relates to our compensation plans, policies and practices for all employees including executives and directors, particularly whether our compensation programs may create incentives for our employees to take excessive or inappropriate risks which could have a material adverse effect on the Company.
Compensation Committee
We currently do not have a compensation committee of our Board of Directors. The Board as a whole determines executive compensation.
Compensation Committee Interlocks and Insider Participation
Our Board does not have, and has not had, a compensation committee. None of our executive officers serves as a member of the board of directors or compensation committee of any entity which has one or more executive officers serving as a member of our Board.
Compensation of Directors
Our Board has the authority to fix the compensation of directors. Our current employee director does not receive separate compensation for his service on our Board of Directors.
On November 4, 2020, we granted 100,000 stock options to Tracy Goeken, MD, with a fair value at issuance of $8,013, with a final maturity date of May 21, 2023. On December 15, 2020, we granted 200,000 stock options to Phil LoGrasso, PhD with a fair value at issuance of $18,137, with a final maturity date of June 16, 2023.
We granted 200,000 stock options to Gordon Ramseier on January 1, 2018, with a fair value at issuance of $35,377 with a final maturity date of January 31, 2023.
During 2021, the Company granted options to the Company’s President, who is also a director, to purchase 424,800 shares of common stock at an exercise price of $2.00 per share and a grant date fair value of $0.16.
During 2020, the Company also granted options to two directors to purchase a total of 300,000 shares of common stock at an exercise price of $2.00 per share and a term of five years. Of the options granted, 200,000 options vest immediately, and 100,000 vest over one (1) year at 25,000 per quarter. The grant date fair value of the options was approximately $0.09.
Director Independence
Our Board of Directors is currently composed of five members, three of which qualify as an independent director (Gordon Ramseier, Phil LoGrasso, PhD and Tracy Goeken, MD) in accordance with the published listing requirements of the NASDAQ. The NASDAQ independence definition includes a series of objective tests, such as that the director is not, and has not been for at least three years, one of our employees and that neither the director, nor any of his family members has engaged in various types of business dealings with us. In addition, our Board has not made a subjective determination as to each director that no relationships exist which, in the opinion of our Board, would interfere with the exercise of independent judgment in carrying out the responsibilities of a director, though such subjective determination is required by the NASDAQ rules. Had our Board of Directors made these determinations, our Board would have reviewed and discussed information provided by the directors and us with regard to each director’s business and personal activities and relationships as they may relate to us and our management.
Code of Business Conduct and Ethics
We plan to adopt a code of business conduct and ethics that applies to all of our employees, officers and directors, including those officers responsible for financial reporting. Following the adoption of same, the code of business conduct and ethics will be available at our website at www.cytonics.com. We intend to post any amendments to the code, or any waivers of its requirements, on our website.
Relaxed Ongoing Reporting Requirements
If we become a public reporting company in the future, we will be required to publicly report on an ongoing basis as an “emerging growth company” (as defined in the Jumpstart Our Business Startups Act of 2012, which we refer to as the JOBS Act) under the reporting rules set forth under the Exchange Act. For so long as we remain an “emerging growth company”, we may take advantage of certain exemptions from various reporting requirements that are applicable to other Exchange Act reporting companies that are not “emerging growth companies”, including but not limited to:
| | ● | not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act; |
| | | |
| | ● | taking advantage of extensions of time to comply with certain new or revised financial accounting standards; |
| | | |
| | ● | being permitted to comply with reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements; and |
| | | |
| | ● | being exempt from the requirement to hold a non-binding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved. |
If we become a public reporting company in the future, we expect to take advantage of these reporting exemptions until we are no longer an emerging growth company. We could be an emerging growth company for up to five years, although circumstances could cause us to lose that status earlier, including if the market value of our common stock held by non-affiliates exceeds $700 million, if we issue $1 billion or more in non-convertible debt during a three-year period, or if our annual gross revenues exceed $1 billion. We would cease to be an emerging growth company on the last day of the fiscal year following the date of the fifth anniversary of our first sale of common equity securities under an effective registration statement or a fiscal year in which we have $1 billion in gross revenues. Finally, at any time we may choose to opt-out of the emerging growth company reporting requirements. If we choose to opt out, we will be unable to opt back in to being an emerging growth company.
If we do not become a public reporting company under the Exchange Act for any reason, we will continue to be required to publicly report on an ongoing basis under the reporting rules set forth in Regulation A for Tier 2 issuers. The ongoing reporting requirements under Regulation A are more relaxed than for “emerging growth companies” under the Exchange Act. The differences include, but are not limited to, being required to file only annual and semiannual reports, rather than annual and quarterly reports. Annual reports are due within 120 calendar days after the end of the issuer’s fiscal year, and semiannual reports are due within 90 calendar days after the end of the first six months of the issuer’s fiscal year.
In either case, we will be subject to ongoing public reporting requirements that are less rigorous than Exchange Act rules for companies that are not “emerging growth companies”, and our stockholders could receive less information than they might expect to receive from more mature public companies.
EXECUTIVE COMPENSATION
2021 Summary Compensation Table
The following summary compensation table provides information regarding the compensation paid during our fiscal years ended December 31, 2020 and December 31, 2021 to our chief executive officer/chief financial officer (our principal executive officer, principal financial officer and principal accounting officer) and our president (the only executive officer with total compensation of $100,000 or more). We refer to these individuals as our “named executive officers,” or “NEOs.”
| Name and Position | | Year | | | Salary
($) | | | Bonus
($) | | | Stock
Awards
($) | | | Option
Awards
($) | | | Non-
Equity
Incentive
Plan
Compensation
($) | | | Non-
qualified
Deferred
Compensation
Earnings
($) | | | All
Other
Compensation
($) | | | Total
($) | |
| Joey Bose (2) | | | 2021 | | | $ | 150,000 | | | $ | 45,000 | | | | — | | | $ | — | | | | — | | | | — | | | | — | | | $ | 195,000 | |
| President and CEO | | | 2020 | | | $ | 97,000 | | | $ | 0 | | | | | | | | | | | | | | | | | | | | | | | $ | 97,000 | |
| | (1) | Antonio Carvalho, former CEO, received 200,000 stock options with an exercise price of $2.00 per share on January 31, 2018. He received an additional 100,000 stock options with an exercise price of $2.00 per share on February 1, 2019. As of December 31, 2019, 229,167 of Mr. Carvalho’s shares have vested. Mr. Carvalho resigned from all positions with the Company on April 30, 2021. |
| | (2) | Joey Bose was appointed President of Cytonics in May 2018. He received 424,800 stock options on June 4, 2018 with an exercise price of $2.00 per share. As of December 31, 2021, 495,600 of Mr. Bose’s’ shares have vested. Mr. Bose was appointed as the Interim Chief Executive and Financial Officer of the Company on April 30, 2021. Mr. Bose was appointed Chief Executive Officer on January 1, 2022. |
Employment Agreements
On May 15, 2018, the Company entered into a letter agreement with Joey Bose to engage his services as the President of the Company, with an annual base salary of $85,000 to be paid semi-monthly. Also pursuant to the letter agreement, the base salary is to be reviewed every twelve months and the Company also granted Mr. Bose an initial award of 424,800 stock options to purchase share of the Company’s common stock at an exercise price of $2.00 per share to vest over 3 years. Also according to the letter agreement, Mr. Bose will be awarded a performance bonus that will be tied to the completion of specific objectives.
Other than the foregoing, there are no employment agreements between Cytonics Corporation and its executives.
Elements of Compensation
Antonio Carvalho, former CEO, received 200,000 stock options with an exercise price of $2.00 per share on January 31, 2018. He received an additional 100,000 stock options with an exercise price of $2.00 per share on February 1, 2019. As of December 31, 2019, 229,167 of Mr. Carvalho’s options have vested. During the year ended December 31, 2020 Mr. Carvalho received 0 new stock options. As of December 31, 2020, 267,500 of Mr. Carvalho’s options have vested. Mr. Carvalho resigned from all positions with the Company on April 30, 2021.
Mr. Bose was paid an annual salary of $85,000, as well as awarded 424,800 stock options on June 4, 2018 with an exercise price of $2.00 per share, and received $5,000 in bonuses in 2018. During the year ended December 31, 2020 Mr. Bose received $97,000 in total compensation. During the year ended December 31, 2021, Mr. Bose received $195,000 in total compensation. As of December 31, 2021, 495,600 of Mr. Bose’s options have vested. Mr. Bose was appointed as a Director of the Company in November of 2020 and was appointed as the Interim Chief Executive and Financial Officer of the Company on April 30, 2021. Mr. Bose was appointed Chief Executive Officer on January 1, 2022.
Base Salary
Mr. Bose received a fixed base salary in an amount determined by the Board of the Company based on a number of factors, including:
| | ● | The nature, responsibilities and duties of the officer’s position; |
| | | |
| | ● | The officer’s expertise, demonstrated leadership ability and prior performance; |
| | | |
| | ● | The officer’s salary history and total compensation, including annual cash bonuses and long-term incentive compensation; and |
| | | |
| | ● | The competitiveness of the market for the officer’s services. |
Mr. Bose’s base salary for 2020 and 2021 is listed in “—Summary Compensation Table” above.
Mr. Carvalho received no salary in 2020 and 2021.
Stock Option Grants
We granted 300,000 stock options, with a fair value at issuance of $28,300, to our directors and executive officers in 2020 as follows:
Phil LoGrasso – 200,000 stock options granted, vested immediately on December 15, 2020, exercise price of $2.00 per share, expire on December 15, 2025.
Tracy Goeken – 100,000 stock options granted, vested immediately on November 4, 2020, exercise price of $2.00 per share, expire on December 4, 2025.
In 2020, Joey Bose was granted 10,000 options with a $2.00 exercise price.
In 2020, Gaetano J. Scuderi, MD was granted 10,000 options with a $2.00 exercise price.
We granted 100,000 options, with a fair value at issuance of $24,600, to our directors and executive officers in 2019 as follows:
| | · | Antonio Carvalho was granted 100,000 options on February 1, 2019, with a final maturity date of February 1, 2029. |
We granted 624,800 stock options, with a fair value at issuance of $168,075, to our directors and executive officers in 2018 as follows:
| | · | Antonio Carvalho was granted 200,000 options on January 1, 2018, with a final maturity date of January 31, 2023. |
| | · | Joey Bose was granted 424,800 options on June 4, 2018, with a final maturity date of June 4, 2021. |
| | · | Gordon Ramseier was granted 200,000 options on January 1, 2018, with a final maturity date of January 31, 2023. |
During 2021, the Company granted options to the Company’s President, who is also a director, to purchase 424,800 shares of common stock at an exercise price of $2.00 per share and a grant date fair value of $0.16.
During 2020, the Company also granted options to two directors to purchase a total of 300,000 shares of common stock at an exercise price of $2.00 per share and a term of five years. Of the options granted, 200,000 options vest immediately, and 100,000 vest over one (1) year at 25,000 per quarter. The grant date fair value of the options was approximately $0.09
Compensation Discussion and Analysis
Equity Incentive Plans
In April 2007, the Company’s shareholders adopted the 2007 Stock Incentive Plan providing for the grant of stock options and restricted stock awards to employees and non-employee directors selected by the Board of Directors or a committee of our Board of Directors. Options granted under the plan may include non-statutory stock options as well as incentive stock options intended to qualify under Section 422 of the Internal Revenue Code. The Company’s 2007 Stock Incentive Plan expired in April 2017, and no awards have been granted thereunder since such time.
In December 2017, the Company (i) granted non-Plan based options to purchase 1,378,834 shares of common stock and (ii) modified existing Plan and non-Plan based options to purchase 461,300 shares of common stock. These granted and modified options are fully vested and carry an exercise price of $1.00 per share over a 5-year term.
On November 19, 2018, the Board of Directors adopted the 2018 Stock Incentive Plan (the “Plan”), under which the Board of Directors may grant stock-based compensation awards with respect to an aggregate of up to 5,000,000 shares of Common stock, subject to adjustment and increase pursuant to the terms of the Plan. The Plan permits the grant of stock options, restricted stock, restricted stock units and other forms of stock-based compensation to selected persons providing services to the Company (including non-employee directors).
The following is a summary of the Company’s stock option activity for the years ended December 31, 2020 and 2021:
| | | 2020 | | | 2021 | |
| | | Number of
Options | | | Weighted-
Average
Exercise Price | | | Number of
Options | | | Weighted-
Average
Exercise Price | |
| Outstanding at January 1 | | | 5,845,870 | | | $ | 0.78 | | | | 5,733,310 | | | $ | 0.94 | |
| Granted | | | 657,500 | | | $ | 2.00 | | | | 439,800 | | | $ | 2.00 | |
| Forfeited | | | - | | | $ | - | | | | (709,276 | ) | | | 0.38 | |
| Expired | | | (770,060 | ) | | $ | 1.31 | | | | (302,000 | ) | | $ | 1.00 | |
| Outstanding at December 31 | | | 5,733,310 | | | $ | 0.94 | | | | 5,161,834 | | | $ | 1.10 | |
| Exercisable at December 31 | | | 5,467,177 | | | $ | 0.90 | | | | 4,807,834 | | | $ | 1.04 | |
The following table summarizes stock option information at December 31, 2021:
| | | | | | | Weighted | | | | | | Weighted | |
| | | | | | | Average | | | | | | Average | |
| Exercise | | | | | | Contractual Life | | | | | | Contractual Life | |
| Price | | | Outstanding | | | (Years) | | | Exercisable | | | (Years) | |
| $ | 0.05 | | | | 800,000 | | | | 5.30 | | | | 800,000 | | | | 5.30 | |
| | 0.38 | | | | 185,000 | | | | 0.22 | | | | 185,000 | | | | 0.22 | |
| | 0.57 | | | | 496,000 | | | | 1.50 | | | | 496,000 | | | | 1.50 | |
| | 1.00 | | | | 2,058,734 | | | | 2.03 | | | | 2,058,734 | | | | 2.03 | |
| | 2.00 | | | | 1,622,100 | | | | 3.57 | | | | 1,268,100 | | | | 3.33 | |
| Total | | | | 5,161,834 | | | | | | | | 4,807,834 | | | | | |
Executive Compensation Philosophy
Our Board of Directors determines the compensation given to our executive officers in their sole determination. Our Board of Directors reserves the right to pay our executives or any future executives a salary, and/or issue them shares of common stock issued in consideration for services rendered and/or to award incentive bonuses which are linked to our performance, as well as to the individual executive officer’s performance. This package may also include long-term stock based compensation to certain executives, which is intended to align the performance of our executives with our long-term business strategies. Additionally, while our Board of Directors has not granted any performance base stock options to date, the Board of Directors reserves the right to grant such options in the future, if the Board in its sole determination believes such grants would be in the best interests of the Company.
Incentive Bonus
The Board of Directors may grant incentive bonuses to our executive officers and/or future executive officers in its sole discretion, if the Board of Directors believes such bonuses are in the Company’s best interest, after analyzing our current business objectives and growth, if any, and the amount of revenue we are able to generate each month, which revenue is a direct result of the actions and ability of such executives.
Long-Term, Stock Based Compensation
In order to attract, retain and motivate executive talent necessary to support the Company’s long-term business strategy we may award our executives and any future executives with long-term, stock-based compensation in the future, at the sole discretion of our Board of Directors.
| Item 4. | Security Ownership of Management and Certain Securityholders. |
The following table sets forth information about the beneficial ownership of our common stock on December 1 2022:
| | ● | each person known to us to be the beneficial owner of more than 5% of our common stock; |
| | | |
| | ● | each named executive officer; |
| | | |
| | ● | each of our directors; and |
| | | |
| | ● | all of our executive officers and directors as a group. |
Unless otherwise noted below, the address for each beneficial owner listed on the table is in care of Cytonics Corporation, 658 West Indiantown Road, Suite 214, Jupiter, Florida 33458. We have determined beneficial ownership in accordance with the rules of the SEC. We believe, based on the information furnished to us that the persons and entities named in the tables below have sole voting and investment power with respect to all shares of common stock that they beneficially own, subject to applicable community property laws. We have based our calculation of the percentage of beneficial ownership on 10,248,803 shares of our common stock outstanding as of December 1, 2022.
In computing the number of shares of common stock beneficially owned by a person and the percentage ownership of that person, we deemed outstanding shares of common stock subject to options, warrants, preferred stock or restricted stock units held by that person that are currently exercisable or convertible or exercisable or convertible within 60 days of December1, 2022. We did not deem these shares outstanding, however, for the purpose of computing the percentage ownership of any other person.
| | | Shares
Beneficially
Owned | | | Percentage
of Shares
Beneficially
Owned | |
| Name of Beneficial Owner | | | | | | | | |
| Directors and Named Executive Officers: | | | | | | | | |
| Gaetano J. Scuderi, MD(1) | | | 6,453,870 | | | | 62.97 | % |
| Anjun K. (Joey) Bose(2) | | | 637,200 | | | | 6.22 | % |
| Gordon V. Ramseier(3) | | | 370,000 | | | | 3.61 | % |
| Tracy Goeken, MD(4) | | | 150,000 | | | | 1.46 | % |
| Phil LoGrasso, PhD(5) | | | 250,000 | | | | 2.44 | % |
| Lewis Hanna | | | 750,000 | | | | 7.32 | % |
| All named executive officers and directors as a group (6 persons) | | | 8,611,070 | | | | 84.02 | % |
| 5% Holders | | | | | | | | |
| None. | | | | | | | | |
(1) Includes 6,453,870 shares of the Company’s common stock.
(2) Includes 637,200 shares of common stock issuable upon the exercise of options held by Mr. Bose.
(3) Includes 320,000 shares of the Company’s common stock issuable upon the exercise of options held by Mr. Ramseier.
(4) Includes 150,000 shares of the Company’s common stock issuable upon the exercise of options held by Dr. Goeken.
(5) Includes 250,000 shares of the Company’s common stock issuable upon the exercise of options held by Dr. LoGrasso.
| Item 5. | Interest of Management and Others in Certain Transactions. |
Policies and Procedures for Related Party Transactions
Currently our Board of Directors is and will be responsible for reviewing and approving, prior to our entry into any such transaction, all related party transactions and potential conflict of interest situations involving:
| | ● | any of our directors, director nominees or executive officers; |
| | | |
| | ● | any beneficial owner of more than 5% of our outstanding stock; and |
| | | |
| | ● | any immediate family member of any of the foregoing. |
Board of Directors will review any financial transaction, arrangement or relationship that:
| | ● | involves or will involve, directly or indirectly, any related party identified above and is in an amount greater than $0; |
| | | |
| | ● | would cast doubt on the independence of a director; |
| | | |
| | ● | would present the appearance of a conflict of interest between us and the related party; or |
| | | |
| | ● | is otherwise prohibited by law, rule or regulation. |
The Board of Directors will review each such transaction, arrangement or relationship to determine whether a related party has, has had or expects to have a direct or indirect material interest. Following its review, the Board of Directors will take such action as it deems necessary and appropriate under the circumstances, including approving, disapproving, ratifying, canceling or recommending to management how to proceed if it determines a related party has a direct or indirect material interest in a transaction, arrangement or relationship with us. Any member of the Board of Directors who is a related party with respect to a transaction under review will not be permitted to participate in the discussions or evaluations of the transaction; however, the Board of Directors member will provide all material information concerning the transaction to the Board of Directors.
Related Party Transactions
During 2018, the Company issued two (2) convertible notes, each in the principal amount of $50,000 to related parties, Dr. Scuderi, our founder and Chairman of the Board and Mr. Carvalho, our former Chief Executive Officer, Chief Financial officer and Director.
During 2020, the Company issued promissory notes in the principal amount of $715,000 (the “2020 Notes”), of which $40,000 was with related parties.
During 2020, the Company granted options to purchase 357,500, 20,000 of which were issued to related parties, shares of common stock at an exercise price of $2.00 per share with a term of five years and a grant date fair value of approximately $0.08 in connection with the issuance of notes payable. The Company also granted options to two directors to purchase a total of 300,000 shares of common stock at an exercise price of $2.00 per share and a term of five years. Of the options granted, 200,000 options vest immediately, and 100,000 vest over one (1) year at 25,000 per quarter. The grant date fair value of the options was approximately $0.09.
During 2021, the Company granted options to the Company’s President to purchase 424,800 shares of common stock at an exercise price of $2.00 per share and a grant date fair value of $0.16.
Upon expiration of the Company’s office lease in 2017, the Company began leasing space from the Company’s President on a month-to-month basis for $2,000 monthly through June 30, 2020. Total rent expense incurred on space leased from the Company’s President was $12,000 for the year ended December 31, 2020 which is included in selling, general and administrative expenses on the statement of operations.
| Item 6. | Other Information. |
None.
| Item 7. | Financial Statements. |
INDEX TO FINANCIAL STATEMENTS
Cytonics Corporation
Audited Financial Statements
December 31, 2021 and 2020

REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Cytonics Corporation
Opinion on the Financial Statements
We have audited the accompanying balance sheets of Cytonics Corporation (“the Company”) as of December 31, 2021 and 2020, and the related statements of operations, stockholders’ equity (deficit), and cash flows for the years then ended and the related notes to the financial statements (collectively referred to as the financial statements). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2021 and 2020, and the results of its operations and its cash flows for the years then ended, in conformity with accounting principles generally accepted in the United States of America.
Substantial Doubt Regarding Going Concern
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 3 of the financial statements, the Company suffered recurring losses from operations and has a significant accumulated deficit. In addition, the Company continues to experience negative cash flows from operations. These factors raise substantial doubt about the Company’s ability to continue as a going concern. Management’s plans regarding these matters are described in Note 3 of the financial statements. The accompanying financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB and in accordance with auditing standards generally accepted in the United States of America. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
D. Brooks and Associates CPAs, P.A.
We have served as the Company’s auditor since 2017.
Palm Beach Gardens, Florida
March 30, 2022

Cytonics Corporation
Balance Sheets
December 31, 2021 and 2020
| Assets |
| | | 2021 | | | 2020 | |
| Current assets: | | | | | | | | |
| Cash | | $ | 967,757 | | | $ | 537,354 | |
| Accounts receivable, net | | | 123,333 | | | | 80,000 | |
| Deferred offering costs | | | - | | | | 137,882 | |
| Prepaid expenses | | | 1,968 | | | | 1,903 | |
| Total current assets | | | 1,093,058 | | | | 757,139 | |
| | | | | | | | | |
| Accounts receivable, non-current, net | | | 217,860 | | | | 283,676 | |
| Intangible assets, net | | | 445,840 | | | | 425,931 | |
| | | | | | | | | |
| Total assets | | $ | 1,756,758 | | | $ | 1,466,746 | |
Liabilities and Stockholders' Equity (Deficit)
| Current liabilities: | | | | | | | | |
| Accounts payable and accrued liabilities | | $ | 554,929 | | | $ | 498,078 | |
| Convertible notes and notes payable, net of debt discounts | | | - | | | | 1,744,303 | |
| Paycheck protection program loan | | | - | | | | 19,710 | |
| Convertible notes and notes payable, related parties | | | - | | | | 138,570 | |
| Total current liabilities | | | 554,929 | | | | 2,400,661 | |
| | | | | | | | | |
| Convertible notes and notes payable, net of debt discounts | | | - | | | | 100,000 | |
| Paycheck protection program loan | | | - | | | | 7,502 | |
| Total liabilities | | | 554,929 | | | | 2,508,163 | |
| | | | | | | | | |
| Stockholders' equity (deficit): | | | | | | | | |
| Convertible Initial Preferred Stock, $.001 par value; 150,000 shares authorized, issued and outstanding | | | 150 | | | | 150 | |
| Convertible Series-A Preferred Stock, $.001 par value; 1,500,000 shares authorized; 576,190 shares issued and outstanding | | | 576 | | | | 576 | |
| Convertible Series-B Preferred Stock, $.001 par value; 6,000,000 shares authorized; 2,574,865 shares issued and outstanding | | | 2,575 | | | | 2,575 | |
| Convertible Series-C and C-1 Preferred Stock, $.001 par value; 10,000,000 shares authorized; 2,994,557 and nil shares issued and outstanding, respectively | | | 2,995 | | | | - | |
| Common Stock, $.001 par value; 50,000,000 shares authorized, 10,117,042 and 9,547,120 shares issued and outstanding, respectively | | | 10,117 | | | | 9,547 | |
| Additional paid-in capital | | | 21,092,923 | | | | 16,309,053 | |
| Accumulated deficit | | | (19,907,507 | ) | | | (17,363,318 | ) |
| | | | | | | | | |
| Total stockholders' equity (deficit) | | | 1,201,829 | | | | (1,041,417 | ) |
| | | | | | | | | |
| Total liabilities and stockholders' equity (deficit) | | $ | 1,756,758 | | | $ | 1,466,746 | |
See accompanying notes to the financial statements.
Cytonics Corporation
Statements of Operations
For the Years Ended December 31, 2021 and 2020
| | | 2021 | | | 2020 | |
| Revenues: | | | | | | | | |
| Service revenues | | $ | - | | | $ | 6,000 | |
| License and royalty revenues | | | 307,500 | | | | 585,056 | |
| Total revenues | | | 307,500 | | | | 591,056 | |
| | | | | | | | | |
| Operating Expenses: | | | | | | | | |
| Research and laboratory expenses | | | 2,205,358 | | | | 503,906 | |
| Payroll expense | | | 237,674 | | | | 219,473 | |
| Selling, general, and administrative expenses | | | 584,045 | | | | 258,881 | |
| Professional fees | | | 143,930 | | | | 170,667 | |
| Amortization | | | 30,376 | | | | 31,503 | |
| Impairment loss | | | - | | | | 34,276 | |
| Total operating expenses | | | 3,201,383 | | | | 1,218,706 | |
| | | | | | | | | |
| Loss from operations | | | (2,893,883 | ) | | | (627,650 | ) |
| | | | | | | | | |
| Other (expense) income: | | | | | | | | |
| Interest income | | | 14,514 | | | | 9,274 | |
| Other income | | | - | | | | 1,000 | |
| Paycheck protection program loan forgiveness | | | 27,408 | | | | - | |
| Gain on extuishment of debt | | | 571,629 | | | | - | |
| Interest expense | | | (263,857 | ) | | | (378,474 | ) |
| Total other expense, net | | | 349,694 | | | | (368,200 | ) |
| | | | | | | | | |
| Net loss before income taxes | | | (2,544,189 | ) | | | (995,850 | ) |
| | | | | | | | | |
| Income taxes | | | - | | | | - | |
| | | | | | | | | |
| Net loss | | $ | (2,544,189 | ) | | $ | (995,850 | ) |
| | | | | | | | | |
| Net loss per share, basic and diluted | | $ | (0.25 | ) | | $ | (0.10 | ) |
| | | | | | | | | |
| Weighted average shares outstanding, basic and diluted | | | 10,023,030 | | | | 9,547,120 | |
See accompanying notes to the financial statements.
Cytonics Corporation
Statements of Changes in Stockholders' Equity (Deficit)
For the Years Ended December 31, 2021 and 2020
| | | Initial Convertible
Preferred Stock | | | Series-A Convertible
Preferred Stock | | | Series-B Convertible
Preferred Stock | | | Series-C and C-1 Convertible
Preferred Stock | | | Common Stock | | | Additional | | | | | | Total in
Stockholders' | |
| | | Shares | | | Par Value | | | Shares | | | Par Value | | | Shares | | | Par Value | | | Shares | | | Par Value | | | Shares | | | Par Value | | | Paid-In Capital | | | Accumulated Deficit | | | Equity
(Deficit) | |
| Balance, December 31, 2019 | | | 150,000 | | | $ | 150 | | | | 576,190 | | | $ | 576 | | | | 2,574,865 | | | $ | 2,575 | | | | - | | | $ | - | | | | 9,547,120 | | | $ | 9,547 | | | $ | 16,191,691 | | | $ | (16,367,468 | ) | | $ | (162,929 | ) |
| Stock-based compensation | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | 88,899 | | | | - | | | | 88,899 | |
| Warrants issued in connection with issuance of indebtedness | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | 28,463 | | | | - | | | | 28,463 | |
| Net loss | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | (995,850 | ) | | | (995,850 | ) |
| Balance, December 31, 2020 | | | 150,000 | | | $ | 150 | | | | 576,190 | | | $ | 576 | | | | 2,574,865 | | | $ | 2,575 | | | | - | | | $ | - | | | | 9,547,120 | | | $ | 9,547 | | | $ | 16,309,053 | | | $ | (17,363,318 | ) | | $ | (1,041,417 | ) |
| Stock-based compensation | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | 33,248 | | | | - | | | | 33,248 | |
| Issuance preferred stock for cash, net of issuance costs | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | 2,424,016 | | | | 2,424 | | | | - | | | | - | | | | 3,764,885 | | | | - | | | | 3,767,309 | |
| Conversion of debt to equity | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | 570,541 | | | | 571 | | | | 569,922 | | | | 570 | | | | 985,737 | | | | - | | | | 986,878 | |
| Net loss | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | (2,544,189 | ) | | | (2,544,189 | ) |
| Balance, December 31, 2020 | | | 150,000 | | | $ | 150 | | | | 576,190 | | | $ | 576 | | | | 2,574,865 | | | $ | 2,575 | | | | 2,994,557 | | | $ | 2,995 | | | | 10,117,042 | | | $ | 10,117 | | | $ | 21,092,923 | | | $ | (19,907,507 | ) | | $ | 1,201,829 | |
See accompanying notes to the financial statements.
Cytonics Corporation
Statements of Cash Flows
For the Years Ended December 31, 2021 and 2020
| | | | | | | |
| | | 2021 | | | 2020 | |
| Cash flows from operating activities: | | | | | | | | |
| Net loss | | $ | (2,544,189 | ) | | $ | (995,850 | ) |
| Adjustments to reconcile net loss to net cash used in operating activities: | | | | | | | | |
| Amortization | | | 30,376 | | | | 31,503 | |
| Amortization of debt discounts | | | 122,639 | | | | 234,431 | |
| Stock-based compensation | | | 33,248 | | | | 88,899 | |
| Paycheck protection program loan and accrued interest forgiveness | | | (27,408 | ) | | | - | |
| Gain on extuishment of debt | | | (571,629 | ) | | | - | |
| Impairment loss | | | - | | | | 34,276 | |
| (Increases) decreases in assets: | | | | | | | | |
| Accounts receivable | | | 22,483 | | | | (345,259 | ) |
| Prepaid expenses | | | (65 | ) | | | 26,451 | |
| Increase in liabilities: | | | | | | | | |
| Accounts payable and accrued expenses | | | 150,042 | | | | 340,446 | |
| Net cash used in operating activities | | | (2,784,503 | ) | | | (585,103 | ) |
| | | | | | | | | |
| Cash flows from investing activities: | | | | | | | | |
| Purchase of intangible assets | | | (50,285 | ) | | | (78,651 | ) |
| Net cash used in investing activities | | | (50,285 | ) | | | (78,651 | ) |
| | | | | | | | | |
| Cash flows from financing activities: | | | | | | | | |
| Proceeds from issuance of notes payable | | | 30,000 | | | | 675,000 | |
| Proceeds from issuance of notes payable - related parties | | | - | | | | 40,000 | |
| Repayments of notes payable - related parties | | | (40,000 | ) | | | - | |
| Repayments of convertible notes payable | | | (630,000 | ) | | | - | |
| Proceeds from paycheck protection program loan | | | - | | | | 27,212 | |
| Proceeds from issuance of preferred stock, net of fees | | | 3,905,191 | | | | - | |
| Deferred offering costs | | | - | | | | (78,696 | ) |
| Net cash provided by financing activities | | | 3,265,191 | | | | 663,516 | |
| | | | | | | | | |
| Net increase (decrease) in cash | | | 430,403 | | | | (238 | ) |
| | | | | | | | | |
| Cash, beginning of year | | | 537,354 | | | | 537,592 | |
| | | | | | | | | |
| Cash, end of year | | $ | 967,757 | | | $ | 537,354 | |
| | | | | | | | | |
| Supplemental cash flow information: | | | | | | | | |
| Cash paid for interest | | $ | 153,183 | | | $ | - | |
| Cash paid for taxes | | $ | - | | | $ | - | |
| | | | | | | | | |
| Schedule of non-cash financing activity: | | | | | | | | |
| | | | | | | | | |
| Issuance of warrants with notes payable | | $ | - | | | $ | 28,463 | |
See accompanying notes to the financial statements.
Cytonics Corporation
Notes to the Financial Statements
Note 1 – Nature of Business
Cytonics Corporation (the “Company”) is a research and development company that develops therapies and diagnostics for back and joint pain, which it then licenses to unrelated third parties. The Company was incorporated in the State of Florida under the name Gamma Spine, Inc. on July 19, 2006 and was renamed Cytonics Corporation on April 17, 2007.
Note 2 – Summary of Significant Accounting Policies
Basis of Presentation
The accompanying financial statements have been prepared in conformity with accounting principles generally accepted in the United States of America (U.S. GAAP).
Use of Estimates
The preparation of the financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the amounts reported in the financial statements and accompanying Notes. Actual results could differ materially from those estimates. The Company’s most significant estimates include the useful life of intangible assets, fair value of stock-based compensation and stock and warrants issued with convertible notes and notes payable.
Cash and Cash Equivalents
Cash includes cash deposited in major financial institutions, which at times may exceed Federal Deposit Insurance Corporation insurance limits.
The Company considers highly liquid investments with maturities of three months or less from the date of purchase to be cash equivalents. These investments are carried at cost, which approximates fair value. As of December 31, 2021 and 2020 the Company had no cash equivalents.
Revenue Recognition
The Company recognizes revenue when obligations under the terms of a contract with a customer are satisfied; generally this occurs with the transfer of control or access of the Company’s licenses or performance of services. Revenue is measured as the amount of consideration the company expects to receive in exchange for transferring goods or providing services. A performance obligation is a promise in a contract to transfer a distinct good or service to the customer, and is the unit of account in the contract. A contract’s transaction price is allocated to each distinct performance obligation and recognized as revenue when, or as, the performance obligation is satisfied.
Contracts with customers consist of licensing arrangements and, to a lesser extent, research and development related services. Revenues from licensing and royalty fees are received from the granting of exclusive sales, marketing, manufacturing and distribution rights associated with the Company’s functional intellectual property (IP). The Company’s performance obligation is satisfied at a point in time (upon delivery to the customer), where the Company has no remaining obligation to support or maintain the intellectual property licensed to the customer. The Company typically requires a non-refundable license fee, paid over several years, and quarterly royalty payments based on a percentage of sales with minimum quarterly royalty guarantees. The Company applies a discount to license fees that represent earned revenues at the time of contract execution to be received over time.
Cytonics Corporation
Notes to the Financial Statements
Note 2 – Summary of Significant Accounting Policies, continued
Revenue Recognition, continued
Revenue from license fees are recognized at a point in time when the Company transfers the functional IP to the customer as long as management believes the total consideration owed by the customer for the license fee is probable of being received. Due to the financing component embedded in the license fee, the Company records the revenue and accounts receivable at its net present value using an estimated discount rate at the point in time the performance obligation associated with the license fee has been completed. Management applies a discount rate that reflects the customers’ creditworthiness and the amount that would have been received from the customer if the license fee was paid upon execution of the contract. The effects of the financial component is separately presented from revenue as interest income.
Minimum guaranteed royalty (MGRs) payments are not binding and are considered to be contingent on the customers’ ability to generate sales. The Company’s contracts include termination clauses for nonpayment by customers or mutual agreement. The termination clauses are likely to be triggered if the customer is unable to make the MGRs. The Company has historically made price concessions when needed by customers. Given the contractual nature of the MGRs and customary business practices, the Company recognizes revenue from MGRs pursuant to ASC 606-10-55-65, which requires recognition for a sales-based royalty promised in exchange for a license of intellectual property only when (or as) the later of the following events occurs:
| a. | The subsequent sale or usage occurs. |
| b. | The performance obligation to which some or all of the sales-based or usage-based royalty has been satisfied (or partially satisfied). |
The Company recognizes revenue from MGRs when they become due under the terms of the contract and the consideration has been received or is expected to be received.
License and royalties due under the contract not yet received have been reflected as accounts receivable on the balance sheets, net of any discounts
The Company also generates revenues for running diagnostic tests. The service is invoiced and the revenue is recognized upon completion of the test and after the test results are reported to the customer, which is at the point the Company has satisfied its performance obligation.
Except for the estimate of discount rate to be applied to license fees to be received over a period of years, the Company’s contracts do not include multiple performance obligations or variable consideration. Since the Company’s revenue is generated from a small number customer contracts, the Company does not have material contract assets or liabilities.
Cytonics Corporation
Notes to the Financial Statements
Note 2 – Summary of Significant Accounting Policies, continued
Revenue Recognition, continued
During the year ended December 31, 2020, the Company received consideration from a customer in connection with the granting of exclusive sales, marketing, manufacturing and distribution rights associated with the Company’s functional intellectual property. Upon execution of the contract, the Company required a $450,000 nonrefundable license fee, payable in installments over a period of years through 2025 which management believes is probable of collecting. In the event the contract is terminated prior to its ten year term, the customer is required to pay a portion of the license fee based on sliding scale and the year of termination. During the year ended December 31, 2020, the Company recorded $404,806 of license fee revenue net of a present value discount in the amount of $45,194 when the functional IP was transferred to the customer. As of December 31, 2020 $400,000 of the nonrefundable fee remains due which has been presented on the balance sheets as an accounts receivable in the amount of $363,676, net of a $36,324 discount. As of December 31, 2021 $320,000 of the nonrefundable fee remains due which has been presented on the balance sheets as an accounts receivable in the amount of $297,860, net of a $22,140 discount.
During the years ended December 31, 2021 and 2020, the Company recognized revenue from license fees and MGRs of $307,500 and $585,056, respectively, which is presented on the statement of operations as license and royalty revenues.
During the years ended December 31, 2021 and 2020, service revenues which are recognized at the completion of the service were none and $6,000 as presented on the statement of operations.
Intangible Assets
The Company’s intangible assets include seven U.S. patents (US 10,265,388, US 11,040,092, US 10,940,189, US 9,352,021, US 9,498,514, US 10,400,028, US 10,889,631), three U.K. patents (GB2501611, GB2503131 and GB2522561), two European patents (EP 2827882 and EP 3221341; each of which are validated in FR, DE, and GB), one Canadian patent (CA 2865170), two Australian patents (AU 2013222414, AU 2015349782), and one Japanese patent (JP 6861152). The Company also has five additional related pending patents applications. The cost of issued patents are capitalized and amortized over the life of the patents which is 20 years from the earliest filing date of the non- provisional or PCT application to which priority is claimed. The costs of patents in development are expensed as incurred. The unamortized costs associated with previously capitalized patents that have expired or abandoned are written off.
The Company assesses potential impairments to its imatngible assets when there is evidence that events or changes in circumstances indicate that the carrying amount of an asset may not be recovered. Any required impairment loss is measured as the amount by which the asset’s carrying value exceeds its fair value and is recorded as a reduction in the carrying value of the related asset and a charge to operating results. The Company had no and $34,276 impairment of intangible assets at December 31, 2021 and 2020, respectively.
Cytonics Corporation
Notes to the Financial Statements
Note 2 – Summary of Significant Accounting Policies, continued
Fair Value of Financial Instruments
The carrying amounts of cash, accounts receivable, accounts payable and accrued liabilities, and convertible notes approximate their fair values because of the short maturities and/or market interest of these financial instruments.
Extinguihsment of Debt
The Company evaluates the settlement of debt transactions in accordance with ASC 405 Liabilities and ASC 470 Debt. If debt is converted or exchanged into issuer’s equity shares on terms different from those at issuance, the transaction is accounted for as a debt extingushment with the difference between the carrying value of the debt and the fair value of equity shares being recorded in earnings as a gain or loss.. At December 31, 2021 the Company had $571,629 of gain on extinguishment due to the conversion of convertible notes payable and notes payable (see Note 5).
Contingencies
The Company records contingent liabilities resulting from asserted and unasserted claims when it is probable that a liability has been incurred and the amount of the loss is reasonably estimable. Contingent liabilities are disclosed when there is a reasonable possibility that the ultimate loss will exceed the recorded liability. The process of estimating probable losses requires professional judgment in the analysis of multiple factors, in some cases including judgments about the potential actions of third party claimants and courts.
Concentrations of Risk
In the normal course of business, the Company is potentially subject to concentrations of credit risk in its trade receivables. Although the Company is directly affected by the financial condition of its customers, management does not believe significant credit risks exist at December 31, 2021 or 2020. Generally, the Company does not require collateral or other securities to support its Trade Receivables.
Share-Based Payments
The Company measures the cost of services received in exchange for an award of equity instruments to employees and nonemployees based on the grant date fair value of the award, which is recognized as compensation expense over the vesting term.
Income Taxes
The Company accounts for income taxes under the asset and liability method, which requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been included in the financial statements. Under this method, deferred tax assets and liabilities are determined on the basis of the differences between the financial statement and tax bases of assets and liabilities using enacted tax rates in effect for the year in which the differences are expected to reverse. The effect of a change in tax rates on deferred tax assets and liabilities is recognized in income in the period that includes the enactment date.
Cytonics Corporation
Notes to the Financial Statements
Note 2 – Summary of Significant Accounting Policies, continued
Income Taxes, continued
The Company recognizes deferred tax assets to the extent that management believes these assets are more likely than not to be realized. In making such a determination, management considers all available positive and negative evidence, including future reversals of existing taxable temporary differences, projected future taxable income, tax-planning strategies, and results of recent operations. If we determine that the Company would be able to realize deferred tax assets in the future in excess of their net recorded amount, the Company would make an adjustment to the deferred tax asset valuation allowance, which would reduce the provision for income taxes.
Recently Issued Accounting Standards
Cash Flows
In August 2016, the FASB issued ASU No. 2016-15, “Classification of Certain Cash Receipts and Cash Payments”, which addresses eight specific cash flow issues with the objective of reducing diversity in practice. The Company adopted the provisions of ASU No. 2016-15 in 2020, which did not have an impact on it’s cash flow presentation.
Accounting for Certain Financial Instruments with Down Round Features
In July 2017, the FASB issued ASU No. 2017-11, Earnings Per Share (Topic 260); Distinguishing Liabilities from Equity (Topic 480); Derivatives and Hedging (Topic 815): (Part I) Accounting for Certain Financial Instruments with Down Round Features, (Part II) Replacement of the Indefinite Deferral for Mandatorily Redeemable Financial Instruments of Certain Nonpublic Entities and Certain Mandatorily Redeemable Noncontrolling Interests with a Scope Exception (ASU 2017-11).
Part I of ASU 2017-11 simplified the accounting for certain financial instruments with down round features, a provision in an equity-linked financial instrument (or embedded feature) that provides a downward adjustment of the current exercise or conversion price based on the price of future equity offerings. Previous accounting guidance created cost and complexity for organizations that issue financial instruments with down round features by requiring, on an ongoing basis, fair value measurement of the entire instrument or conversion option each reporting period.
ASU 2017-11 requires companies to disregard the down round feature when assessing whether the instrument is indexed to its own stock, for purposes of determining liability or equity classification. Companies that provide earnings per share (EPS) data will adjust their basic EPS calculation for the effect of the feature when triggered (i.e., when the exercise or conversion price of the related equity- linked financial instrument is adjusted downward because of the down round feature) and will recognize the effect of the trigger within equity.
ASU 2017-11 is effective for public business entities for fiscal years beginning after December 15, 2018, and interim periods within those annual periods. For all other entities, the amendments are effective for annual periods beginning after December 15, 2019, and interim periods within annual periods beginning after December 15, 2020.
The Company’s adoption of ASU 2017-11 related to financial instruments issued with down round features on January 1, 2020 did not have an impact on the Company’s financial condition or results of operations.
Cytonics Corporation
Notes to the Financial Statements
Note 2 – Summary of Significant Accounting Policies, continued
Accounting Standards Not Yet Adopted
On August 5, 2020, the FASB issued ASU 2020-06,1 (“ASU 2020”) which simplifies the accounting for certain financial instruments with characteristics of liabilities and equity, including convertible instruments and contracts on an entity’s own equity. The ASU is part of the FASB’s simplification initiative, which aims to reduce unnecessary complexity in U.S. GAAP. ASU 2020 is effective for fiscal years beginning after December 31, 2023. The Company feels the adoption of ASU 2020 will not have a material impact to the financial statements.
Accounting Standards Not Yet Adopted, continued
The amendments are effective for public business entities, that are not smaller reporting companies, in fiscal years beginning after December 15, 2021, and interim periods within those fiscal years. For all other entities, in fiscal years beginning after December 15, 2023, and interim periods within those fiscal years. The guidance may be early adopted for fiscal years beginning after December 15, 2020, and interim periods within those fiscal years.
Subsequent Events
Management has evaluated subsequent events and transactions for potential recognition or disclosure in the financial statements through March 30, 2021, the date these financial statements were available to be issued.
Note 3 –Going Concern
As shown in the accompanying financial statements, during the year ended December 31, 2021 the Company has sustained a net loss of approximately $2.5 million and had net cash used in operations of approximately $2.8 million. As of December 31, 2021, the Company had accumulated deficit of approximately $19.9 million. These conditions raise substantial doubt about the Company’s ability to continue as a going concern.
To date, the Company has funded its research and development and operating activities through sales of debt and equity securities, grant funding and licenses of its products.
The Company intends to continue to seek funding through investments by strategic partners and from private and public sales of securities until such time that the Company generates sufficient cash flow to sustain its operations.
There is no guarantee that the Company will be able to raise sufficient capital or generate a level of revenues to sustain its operations. Management believes that the Company’s capital requirements depend on many factors, including liquidity necessary for the continued development and marketing of its products. These financial statements do not include any adjustments relating to the carrying amounts of recorded assets or the carrying amounts and classification of recorded liabilities that may be required should the Company be unable to continue as a going concern.
Cytonics Corporation
Notes to the Financial Statements
Note 4 – Intangible Assets
The following is a summary of activity related to intangible assets, which consists of capitalized patent costs, for the years ended December 31, 2021 and 2020:
| | | Patents | |
| Carrying value at December 31, 2019 | | $ | 413,059 | |
| Additions | | | 78,651 | |
| Amortization | | | (31,503 | ) |
| Impairment | | | (34,276 | ) |
| Carrying value at December 31, 2020 | | | 425,931 | |
| Additions | | | 50,285 | |
| Amortization | | | (30,376 | ) |
| Carrying value at December 31, 2021 | | $ | 445,840 | |
Future amortization of intangible assets is as follows:
| 2022 | | $ | 30,376 | |
| 2023 | | | 30,376 | |
| 2024 | | | 30,376 | |
| 2025 | | | 27,573 | |
| 2026 | | | 27,573 | |
| Thereafter | | | 299,566 | |
| | | $ | 445,840 | |
Amortization expense was $30,376 and $31,503 for the years ended December 31, 2021 and 2020, respectively.
Note 5 – Convertible Notes and Notes Payable, Net
2018 Notes
During 2018, the Company initiated a private placement offering for the issuance of $1,000,000 in aggregate principal convertible promissory notes (“2018 Notes”), resulting in the issuance of multiple notes in the aggregate principal amount of $794,000, inclusive of a $50,000 note to a principal stockholder and chairman of the board and $50,000 note to the former Company’s Chairman of the Board and the prior chief financial officer. During 2019, an additional $10,000 promissory note was issued with the same terms. The 2018 Notes bore interest at a rate of 10% per year, payable quarterly, on March 31, June 30, September 30 and December 31 of each year, with a maturity date of June 30, 2021.
Cytonics Corporation
Notes to the Financial Statements
Note 5 – Convertible Notes and Notes Payable, Net, continued
2018 Notes, continued
Prior to the completion of an Initial Public Offering (“IPO”) of common stock, as defined, the holders of the 2018 Notes may elect to convert all outstanding principal and accrued interest into shares of common stock at a conversion price of $1.60 per share, and at a conversion price equal to the sale price of the common stock at any time following the completion of an IPO.
Subsequent to completion of an IPO of at least $1,000,000, the Company may elect to require holders of the 2018 Notes to convert all of the outstanding principal and accrued interest into shares of Common Stock at a conversion price equal to 80% of the sale price of the Common Stock in the IPO.
With the completion of a Series C Preferred Stock Offerring (“Series C Offerring”) which resulted in gross proceeds in excess of $1,000,000 during 2021, the Company required the holders of the 2018 notes to convert their outstanding principal and accrued interest into shares of common stock at a conversion rate of $1.60 (80% of the $2 price per share in the Series C Offerring). During the year ended December 31, 2021, $804,000 of principal and accrued interest of $19,870 were converted to 514,922 shares of common stock.
Since the 2018 Notes were converted to shares of common stock under terms that differed from the original conversion terms of the 2018 Notes, the Company recorded a gain on the extinguishment of the 2018 Notes in the amount of $516,464. The gain reflects the difference between the carrying amount of the 2018 Notes and the estimated fair value of the shares of common stock issued upon conversion.
In estimating the fair value of common stock, the Company used the backsolve method, which utilizes the option pricing method to calculate an implied value of $.597 per share of common stock. For purposes of the Backsolve method, the Company used the recent sales transactions of the Series C Preferred Stock which were purchased at a price of $2.00 per share (see Note 6). A summary of the key inputs used in the backsolve method at December 31, 2021 were volatility of 96%, risk free rate of .31 %, and an estimated three year period until a liquidity or an exit.
As of December 31, 2021 and 2020 the total principal outstanding on the 2018 Notes was $0 and $804,000, respectively.
2019 Notes
During 2019, the Company issued convertible promissory notes in the aggregate amount of $486,511 (the “2019 Notes"). The issuance of the 2019 Notes resulted in the Company receiving net proceeds of $418,593. The 2019 Notes bore interest at a rate of 5% compounded each calendar quarter commencing with June 30, 2019. All outstanding principal and accrued interest were due May 2021 ("Maturity Date'').
Of the total 2019 Notes, $23,167 was issued as consideration related to debt issuance costs. In addition, the Company paid debt issuance costs of $44,751 for total aggregate debt issuance of costs of $67,918 were recorded as discount on the debt to be amortized over the twenty -four-month term of the 2019 Notes.
Cytonics Corporation
Notes to the Financial Statements
Note 5 – Convertible Notes and Notes Payable, Net, continued
2019 Notes, continued
The 2019 Notes automatically will convert to equity upon the occurrence of: 1) the sale and issuance of preferred stock in which the Company receives gross proceeds of $ 1,000,000 or more ("Qualified Equity Financing”) or 2) a Corporate Transaction, as defined in the agreement. If automatic conversion does not occur by the Maturity Date, the 2019 Note holders can elect, by a majority, to require the Company to pay the outstanding balance or convert the 2019 Notes into shares.
Upon conversion, the conversion price shall be based on the following:
Qualified Equity Financing – the lower of: a) a 20% discount to the price paid per share for the preferred stock (the “Discount”) issued in the Qualified Equity Financing or b) the quotient resulting from dividing $32,400,000 (the "Valuation Cap”) by the fully-diluted shares outstanding immediately prior to the closing of the Qualified Equity Financing. Upon conversion, the 2019 Note holders will receive shares equivalent to shares issued in the Qualified Equity Financing except the liquidation preference per share shall equal the conversion price.
Corporate Transaction – the quotient resulting from dividing the Valuation Cap by the fully diluted shares outstanding immediately prior to the closing of a Corporate Transaction.
At Maturity – the quotient resulting from dividing the Valuation Cap by the fully diluted shares outstanding immediately prior to the closing of a Corporate Transaction.
If the Company issues convertible debt in any future series separate from the 2019 Notes at a lower pre-money valuation cap or higher discount, the Valuation Cap and/or Discount on the 2019 Notes will be automatically amended to the lower Valuation Cap and/or higher Discount, as applicable.
The above conversion features embedded in the 2019 Notes resulted in the Company recognizing a beneficial conversion feature, measured at its intrinsic value on issuance date, of approximately $392,000, which was recorded as discount to the debt and additional paid in capital. The debt discount is being amortized over the term of the 2019 Notes.
During the years ended December 31, 2021 and 2020, the Company recognized aggregate amortization expense of $98,648 and $229,960 related to the beneficial conversion feature and debt issuance costs which is included on the statements of operations. As of December 31, 2021 and 2020, the aggregate unamortized debt discount was $0 and $98,648.
2019 Promissory Note
On October 31, 2019, the Company issued a promissory note with an unrelated individual in the amount of $100,000. The promissory note bears interest at 10% and is due October 31, 2024. The note holder may elect to convert all, but only all, of the outstanding principal and accrued interest into shares as follows: 1) prior to an initial public offering (IPO) at a conversion price of $2.00 or 2) at the completion of an IPO, at a conversion price equal to the share price paid in the IPO less a 10% discount.
Cytonics Corporation
Notes to the Financial Statements
Note 5 – Convertible Notes and Notes Payable, Net, continued
2019 Promissory Note, continued
Further, the Company may elect to repay the principal and accrued interes in the form of equity shares as follows: 1) upon 15 days advance notice to the lender of the Company’s election to convert into equity shaers at a conversion rate of $2 or 2) at any time following a public offering of stock that results in gross proceeds of at least $1,000,000, the Company may elect to require the lender to convert all outstanding principal and accrued interest into equity shaers at a 10% discount to the offering price.
Conversion of the 2019 Notes and 2019 Promissory Note
During the year ended December 31, 2021, the aggregate outstanding principal and accrued interest outstanding on the 2019 Notes and 2019 Promissory Note of $586,511 and $60,126, respectively, were converted to 570,541 shares of Series C Preferred Stock. The conversion of the 2019 Notes was triggered by a Qualified Equity Financing that occurred in 2021. The Company elected to require the holder of the 2019 Promissory Note to convert the outstanding principal and accrued interest into the Series C Preferred Stock after the sale of Series C Preferred Stock that resulted in gross proceeds in excess of $1,000,000 (see Note 6). Since the conversion was in accordance with the original terms of the 2019 Notes and 2019 Promissory Note, no gain or loss was recorded upon conversion and the $646,637 of outstanding principal and accrued itnerest was classified to equity.
As of December 31, 2021 and 2020 , the total principal outstanding on the 2019 Notes and 2019 Promissory Note was $0 and $586,511, respectively.
2020 Notes
During 2020, the Company issued promissory notes in the principal amount of $715,000 of which $40,000 was with related parties. In 2021, the Company issued an additional promissory note with a principal amount of $30,000 (collectively referred to as the “2020 Notes”). In connection with the 2020 notes, the Company issued options to purchase 372,500 shares of common stock at a share price of $2.00, which was recorded as a debt discount in the amount of $28,463 and additional paid- in-capital based on the relative fair value method. The fair value of the options issued was determined using the blacksholes method, an application of the option pricing model, which uses the price established by a recent financing transaction of the Company’s equity participating securities to compute a total equity value for the Company (see Notes 6 and 7). The debt discount was amortized over the terms of the 2020 notes of one (1) year. In 2021, $670,000, including $40,000 to related parties, of outstanding principal on the 2020 Notes were repaid in in cash and all accrued interest outstanding at that time
Certain holders of the 2020 Notes elected to convert their outstanding principal and accrued interest into shares of common stock.
Cytonics Corporation
Notes to the Financial Statements
Note 5 – Convertible Notes and Notes Payable, Net, continued
2020 Notes, continued
During the year ended December 31, 2021, $75,000 of outstanding principal and accrued interest of $13,000 of 2020 Notes were converted to 55,000 shares of common stock. Since the 2020 Notes were converted to shares of common stock, the Company recorded a gain on the extinguishment of the 2020 Notes in the amount of $55,165. The gain reflects the difference between the carrying amount of the 2020 Notes and the estimated fair value of the shares of common stock issued upon conversion.
In estimating the fair value of common stock, the Company used the backsolve method, which utilizes the option pricing method to calculate an implied value of $.597 per share of common stock. For purposes of the Backsolve method, the company used the recent sales transactions of the Series C Preferred Stock which were purchased at a price of $2.00 per share (see Note 6). A summary of the key inputs used in the backsolve method at December 31, 2021 were volatility of 96%, risk free rate of .31 %, and an estimated period of three years till liquidity or an exit.
During the year ended December 31, 2021, an aggregate of outstanding principal and accrued interest of $1,558,506 on the 2018 Notes, 2019 Notes and and 2020 Notes was converted into 570,541 Series C Preferred Stock and 569,922 shares of common stock. This resulted in an aggregate gain on extinguishment of $571,629 which has been reflected on the statement of operations.
At December 31, 2021 and 2020, aggregate outstanding principal and accrued interest on the 2018 Notes, 2019 Notes and 2020 Note was $0 and $2,105,511, including $140,000 with related parties, and $0 and ,$105,900 which is included in the caption accounts payable and accrued liabilities on the balance sheets,respectively.
CARES Act Paycheck Protection Program Loan
In April 2020, the Company entered into a promissory note evidencing an unsecured loan (the “Loan”) in the amount of $27,212 made to the Company under the Paycheck Protection Program (the “PPP”). The PPP was established under the CARES Act and is administered by the U.S. Small Business Administration.
The promissory note matures in April 2022 and bears interest at a rate of 1% per annum. Beginning November 2020, the Company isrequired to make 18 monthly payments of principal and interest in the amount of approximately $1,600. The Loan may be prepaid by the Companyat any time prior to maturity with no prepayment penalties. The proceeds from the Loan may only be used for payroll costs (includingbenefits), interest on mortgage obligations, rent, utilities and interest on certain other debt obligations.
The Note contains customary events of default relating to, among other things, payment defaults, making materially false andmisleading representations to the lender or breaching the terms of the Loan documents. The occurrence of an event of default willresult in an increase in the interest rate to 18% per annum and provides the lender with customary remedies, including the right torequire immediate payment of all amounts owed under the promissory note. In 2021, the Company received notification that Loan was forgiven and recorded gain on extinguishment of $27,408.
Cytonics Corporation
Notes to the Financial Statements
Note 6 – Stockholders’ Equity
The Company is authorized to issue 50,000,000 shares of common stock with a part value of $0.001 per share and 20,000,000 shares of preferred stock with a part value of $0.001. The Board of Directors has designated (a) 150,000 shares as Initial Preferred Stock, (b) 1,500,000,000 shares of Series A Preferred Stock, and (c) 6,000,000 shares of Series B Preferred Stock, and (d) 10,000,000 shares of Series C Preferred Stock.
Common Stock
With the completion of a Series C Preferred Stock Offerring which resulted in gross proceeds in excess of $1,000,000, the Company had convertible notes and notes payable convert to 569,922 shares of common stock in 2021 (see Note 5).
At December 31, 2021 and 2020, the Company had 10,117,042 and 9,547,120 , respectively shares of common stock issued and outstanding. The holders of common stock are entitled to one vote for each share held of record upon such matters and in such manner as may be provided by law. Subject to preferences applicable to any shares of the Company’s outstanding Preferred Stock, the holders of common stock are entitled to receive ratably such dividends, if any, as may be declared by the Board of Directors out of funds legally available therefore. In the event of a liquidation, dissolution or winding up of the Company, the holders of common stock are entitled to share ratably in all assets remaining after payment of liabilities and liquidation preferences of any shares of the Company’s outstanding Preferred Stock. Holders of common stock have no pre-emptive rights or rights to convert their common stock into any other securities. There is no redemption or sinking fund provisions applicable to the common stock. All outstanding shares of common stock are fully paid and non-assessable.
Preferred Stock
At December 31, 2021 and 2020, the Company had (1) 150,000 shares of Initial Convertible Preferred Stock (Initial Preferred), (2) 576,190 shares of Series A Convertible Preferred Stock (Series A Preferred), (3) 2,574,865 shares of Series B Convertible Preferred Stock (Series B Preferred) issued and outstanding. The Initial Convertible Preferred stock has a liquidation preference of $2 per share ($300,000). The Series A Convertible Preferred Stock has a liquidation preference of $4 per share ($2,304,760). The Series B Convertible Preferred Stock has a liquidation preference in the amount paid by the holders (ranging from $2.50 to $4 per share, $7,360,960 in the aggregate). In the event of any liquidation event, the order of liquidation preference is as follows: the (1) Initial Preferred Stock (in parity with Series A Preferred), (2) Series A Preferred (in parity with Initial Preferred Stock) and (3) Series B Preferred.
Each share of Initial Preferred Stock is convertible into 2.4 shares of Common Stock, and both the Series A and Series B Preferred stock are each convertible into two (2) shares of Common Stock.
During 2020, the Company amended its Articles of Incorporation to designate 10,000,000 shares as Series C Preferred Stock of which 500,000 shares were designated as Series C-1 Preferred Stock. Each share of Series C-1 Preferred Stock (“Series C-1”) has a par value $0.001 and a stated value equal to its conversion rate.
Cytonics Corporation
Notes to the Financial Statements
Note 6 – Stockholders’ Equity, continued
Preferred Stock, continued
Each share of Series C Preferred Stock has a par value $0.001 and a stated value equal to $2.00 (“Series C Purchase Price”). All other rights and privaleges of the Series C Preferred Stock and Series C-1 are identical except the stated value as discussed above.
Holders of the Series C and C-1 Preferred Stock will vote with holders of common Stock as a single class, and will participate in all dividends that are declared and paid on Common Stock on the same basis as if each shares of Series C and C-01 Preferred Stock were converted into Common Stock.
Holders of the Series C and C-1 Preferred Stock may voluntarily convert the Series C and C-1 Preferred Stock into shares of Common Stock on a one-to-one ratio, and the Series C and C-1 Preferred Stock will automatically convertupon a Public Offering resulting in at least $20 million of net proceeds to the Company.
In the event of any liquidation event, the holders of the Series C and C-1 Preferred Stock will have a liquidation preference in parity with the holders of Initial Preferred Stock and Series A Preferred Stock. The Series C Convertible Preferred Stock has a liquidation preference of $5,989,114 (2,931,132 at $2 per share). The Series C-1 Convertible Preferred Stock have a liquidation preference of $532,472 (507,116 at $1.05 per share).
With the completion of a Series C Preferred Stock Offerring (“Offerring”) which resulted in gross proceeds in excess of $1,000,000, the Company issued 2,424,016 Series C Preferred Stock for $2.00 which generated $3,767,309 of net proceeds, net of $962,391 equity issuance costs. As of December 31, 2020, $137,882 of these issuance costs costs were classified on the balance sheet as deferred offering cost. Upon completion of the Series C Offerrign these deferred offering costs were recorded as a reduction of additional paid-in capital.
As further disclosed in Note 5, the completion of the Offerring resulted in convertible notes payable being converted into 570,541 of Series C-1 Preferred Stock.
At December 31, 2021 and 2020, the Company had issued and outstanding 2,994,557 and no shares of Series C and C-1 Preferred Stock, respectively.
Note 7 – Stock-Based Compensation
In April 2007, the Company’s shareholders adopted the 2007 Stock Incentive Plan (“2007 Plan”), providing for the grant of stock options and restricted stock awards to employees, non-employee service providers and Board members. Plan Options granted under the plan may include non- statutory stock options as well as incentive stock options intended to qualify under Section 422 of the Internal Revenue Code. Awards under the 2007 may be granted only during the ten years immediately following the effective date of the Plan.
Cytonics Corporation
Notes to the Financial Statements
Note 7 – Stock-Based Compensation, continued
During 2018, the Company’s Board adopted the 2018 Stock Incentive Plan (“2018 Plan”), effectively replacing the 2007 Plan, to provide for the issuance of up to 5,000,000 shares of stock through the grant of stock options, restricted stock or restricted stock units.
During 2021, the Company granted options to the Company’s President to purchase 424,800 shares of common stock at an exercise price of $2.00 per share and a grant date fair value of $0.16.
Additionally, a debt holder was given an option purchase 15,000 shares of common stock at an exercise price of $2.00 per share that is fully vested on issuance and exercisisable over five years (see Note 5).
During 2020, the Company granted options to purchase 357,500, 20,000 of which were issued to related parties, shares of common stock at an exercise price of $2.00 per share with a term of five years and a grant date fair value of approximately $0.08 in connection with the issuance of notes payable (see Note 5). The Company also granted options to two directors to purchase a total of 300,000 shares of common stock at an exercise price of $2.00 per share and a term of five years. Of the options granted, 200,000 options vest immediately, and 100,000 vest over one (1) year at 25,000 per quarter. The grant date fair value of the options was approximately $0.09.
At December 31, 2021, the Company has options outstanding to purchase 5,161,834 shares of common stock under the 2007 and 2018 Plans at exercise prices ranging from $0.05 to $2.00 per share and with remaining vesting periods of one to five (5) years.
The Company determined the grant date fair value of the options granted using the Black Scholes Method using the following assumptions:
| | | 2021 | | 2020 |
| Expected Volatility | | 93.60% | | 91.9%-94.2% |
| Expected Term | | 2.5 years | | 2.5 years |
| Risk Free Rate | | 0.16% | | 0.11% - 0.14% |
| Dividend Rate | | 0.00% | | 0.00% |
Cytonics Corporation
Notes to the Financial Statements
Note 7 – Stock-Based Compensation, continued
The following is a summary of the Company’s stock option activity:
| | | 2021 | | | 2020 | |
| | | Number of
Options | | | Weighted-Average
Exercise Price | | | Number of
Options | | | Weighted-Average
Exercise Price | |
| Outstanding at January 1 | | | 5,733,310 | | | $ | 0.94 | | | | 5,845,870 | | | $ | 0.78 | |
| Granted | | | 439,800 | | | $ | 2.00 | | | | 657,500 | | | $ | 2.00 | |
| Forfeited | | | (709,276 | ) | | $ | 0.38 | | | | - | | | $ | - | |
| Expired | | | (302,000 | ) | | $ | 1.00 | | | | (770,060 | ) | | $ | 1.31 | |
| Outstanding at December 31 | | | 5,161,834 | | | $ | 1.10 | | | | 5,733,310 | | | $ | 0.94 | |
| Exercisable at December 31 | | | 4,807,834 | | | $ | 1.04 | | | | 5,467,177 | | | $ | 0.90 | |
The following table summarizes stock option information at December 31, 2021:
Exercise
Price | | | Outstanding | | | Weighted Average
Contractual Life
(Years) | | | Exercisable | | | Weighted Average
Contractual Life
(Years) |
| $ | 0.05 | | | | 800,000 | | | | 5.30 | | | | 800,000 | | | 5.30 |
| | 0.38 | | | | 185,000 | | | | 0.22 | | | | 185,000 | | | 0.22 |
| | 0.57 | | | | 496,000 | | | | 1.50 | | | | 496,000 | | | 1.50 |
| | 1.00 | | | | 2,058,734 | | | | 2.03 | | | | 2,058,734 | | | 2.03 |
| | 2.00 | | | | 1,622,100 | | | | 3.57 | | | | 1,268,100 | | | 3.33 |
| Total | | | | 5,161,834 | | | | | | | | 4,807,834 | | | |
During the year ended December 31, 2021, 302,933 options vested with a weighted average grant date fair value of $.69. Stock compensation expense for the years ended December 31, 2021 and 2020 was $33,248 and $88,899, respectively, which is included in payroll expense on the statements of operations.
At December 31, 2021, there was 354,000 options unvested with an average grant date fair value of $.16 and $55,094 of unrecognized compensation costs related to stock options which will be recognized over the weighted average remaining years of 2.50.
Cytonics Corporation
Notes to the Financial Statements
Note 8 – Related Party Transactions
Upon expiration of the Company’s office lease in 2017, the Company began leasing space from the Company’s President on a month-to-month basis for $2,000 monthly through June 30, 2020. Total rent expense incurred on space leased from the Company’s President was $12,000 for the year ended December 31, 2020 which is included in selling, general and administrative expenses on the statement of operations. The Company did not occupy the office in 2021.
See Note 5 for convertible notes and notes payable with related parties.
Note 9 – Commitments and Contingencies
From time-to-time, the Company may become involved in various claims and legal proceedings of a nature considered normal to its business. While it is not feasible to predict or determine the financial outcome of any proceedings, management does not believe that the resolve of unasserted claims and proceedings will result in a material adverse effect on the Company’s financial position, results of operations or liquidity.
Note 10 – Concentration of Credit Risks
During the year ended December 31, 2021 and 2020 the Company generated revenues from two (2) customers, and three (3) customers, respectively. At December 31, 2021 and 2020, two (2) customers and one (1) customer accounted for 100% of total accounts receivable balance, respectively.
Financial instruments which potentially subject the Company to concentrations of credit risk consist principally of cash deposits in excess of the FDIC insured limit of $250,000. At December 31, 2021 and 2020, such cash balances were in excess of federally insured amounts by approximately $1,204,000 and $234,000, respectively.
During the year ended December 31, 2021 the Company purchased from one (1) vendor 98% of research and laboratory fees. At December 31, 2021 the vendor accounted for 68% of total accounts payable.
Cytonics Corporation
Notes to the Financial Statements
Note 11 – Income Taxes
Components of income tax benefit are as follows for the years ended December 31:
| | | 2021 | | | 2020 | |
| Current income tax expense (refund) - federal | | $ | - | | | $ | - | |
| Current income tax expense (refund) - state | | | - | | | | - | |
| Total current income tax expense (refund) | | | - | | | | - | |
| | | | �� | | | | | |
| Deferred income tax expense (benefit) - federal | | | 377,026 | | | | 246,389 | |
| Deferred income tax expense (benefit) - state | | | 65,792 | | | | 54,745 | |
| Total deferred income tax expense (benefit) | | | 442,818 | | | | 301,134 | |
| | | | | | | | | |
| Change in valuation allowance | | | (442,818 | ) | | | (301,134 | ) |
| | | | | | | | | |
| Total provision for income taxes | | $ | - | | | $ | - | |
The tax effects of temporary differences which give rise to the significant portions of deferred tax assets and liabilities are summarized as follows as of December 31:
| | | 2021 | | | 2020 | |
| Deferred Tax Assets: | | | | | | | | |
| Net operating loss | | $ | 3,260,443 | | | $ | 2,697,371 | |
| Stock options | | | 381,304 | | | | 406,997 | |
| Tax credits | | | 263,834 | | | | 263,835 | |
| Total deferred tax assets | | | 3,905,581 | | | | 3,368,203 | |
| | | | | | | | | |
| Deferred Tax Liabilities: | | | | | | | | |
| Amortization | | | 65,045 | | | | 101,175 | |
| Net deferred tax liability | | | 65,045 | | | | 101,175 | |
| | | | | | | | | |
| Less: Valuation Allowance | | | (3,970,626 | ) | | | (3,469,378 | ) |
| Total Net Deferred Tax Assets | | $ | - | | | $ | - | |
The Company will have approximately $13.7M of operating loss carry-forwards as of December 31, 2021. Net deferred tax assets are mainly comprised of temporary differences between financial statement carrying amount and tax basis of assets and liabilities.
Net deferred tax assets are mainly comprised of temporary differences between financial statement carrying amount and tax basis of assets and liabilities.
Cytonics Corporation
Notes to the Financial Statements
Note 11 – Income Taxes, continued
ASC 740 requires a valuation allowance to reduce the deferred tax assets reported if, based on the weight of the evidence, it is more likely than not that some portion or all of the deferred tax assets will not be realized. At December 31, 2021 and 2020, the Company’s deferred tax assets have been fully valued due to the uncertainty of the Company’s ability to generate taxable income and benefit from these deferred tax assets.
In addition, the Company performed a comprehensive review of its uncertain tax positions and determined that no adjustments were necessary relating to unrecognized tax benefits at December 31, 2021. The Company's federal and state income tax returns are subject to examination by taxing authorities for three years after the returns are filed, and the Company's federal and state income tax returns for 2018 through 2020 remain open to examination.
The reconciliation of the income tax benefit is computed at the U.S. federal statutory rate as follows at December 31:
| | | 2021 | | | 2020 | |
| Federal statutory income tax | | | 21.00 | % | | | 21.00 | % |
| Permanent Differences | | | 0.23 | % | | | 0.02 | % |
| Change in Tax Rate | | | -3.81 | % | | | -9.41 | % |
| Change in Valuation Allowance | | | -17.41 | % | | | -30.02 | % |
| State income taxes, net of federal benefit | | | 2.82 | % | | | 3.53 | % |
| Prior Year Adjustments | | | -2.83 | % | | | 14.88 | % |
| Total | | | 0.00 | % | | | 0.00 | % |
Note 12 – Subsequent Events
In January 2022, the Company issued 100,000 options to two Company directors that can be exercised for $2 which expire in 5 years and vest over 1 year at 25,000 per quarter. These options which will result in stock compensation of $16,100 over the versting period.
Index to Exhibits
| Exhibit No. | | Exhibit Description |
| | | |
| 2.1 | | Third Amended and Restated Articles of Incorporation of Cytonics Corporation filed with Florida Secretary of State on February 14, 2011. (Incorporated by reference to Exhibit 2.1 to the Form 1-A filed with the Securities and Exchange Commission on April 17, 2020). |
| | | |
| 2.2 | | Articles of Amendment to the Third Amended and Restated Articles of Incorporation of Cytonics Corporation filed with Florida Secretary of State on February 19, 2013. (Incorporated by reference to Exhibit 2.2 to the Form 1-A filed with the Securities and Exchange Commission on April 17, 2020). |
| 2.3 | | Articles of Amendment to the Articles of Incorporation of Cytonics Corporation filed with Florida Secretary of State on February 16, 2018. (Incorporated by reference to Exhibit 2.3 to the Form 1-A filed with the Securities and Exchange Commission on April 17, 2020). |
| | | |
| 2.4 | | Articles of Amendment to the Articles of Incorporation of Cytonics Corporation filed with Florida Secretary of State on March 9, 2020. (Incorporated by reference to Exhibit 2.4 to the Form 1-A filed with the Securities and Exchange Commission on April 17, 2020). |
| | | |
| 2.5 | | Articles of Amendment to the Articles of Incorporation of Cytonics Corporation filed with Florida Secretary of State on March 17, 2020. (Incorporated by reference to Exhibit 2.5 to the Form 1-A filed with the Securities and Exchange Commission on April 17, 2020). |
| 3.1 | | Term Sheet for Convertible Promissory Notes issued From June 30, 2018 to October 15, 2018. (Incorporated by reference to Exhibit 3.1 to the Form 1-A filed with the Securities and Exchange Commission on April 17, 2020). |
| | | |
| 3.2 | | Form of Convertible Promissory Notes issued on May 17, 2019 pursuant to Regulation CF Offering. (Incorporated by reference to Exhibit 3.2 to the Form 1-A filed with the Securities and Exchange Commission on April 17, 2020). |
| | | |
| 3.3 | | Convertible Promissory Note issued to JK Garvey Investment Co., L.P. on October 31, 2019. (Incorporated by reference to Exhibit 3.3 to the Form 1-A filed with the Securities and Exchange Commission on April 17, 2020). |
| | | |
| 4.1 | | Form of Subscription Agreement for U.S. Residents in connection with Convertible Promissory Note offering from June 30, 2018 to October 15, 2018. (Incorporated by reference to Exhibit 4.1 to the Form 1-A filed with the Securities and Exchange Commission on April 17, 2020). |
| | | |
| 4.2 | | Subscription Agreement of JK Garvey Investment Co., L.P. dated October 24, 2019. (Incorporated by reference to Exhibit 4.2 to the Form 1-A filed with the Securities and Exchange Commission on April 17, 2020). |
| | | |
| 6.1 | | Cytonics 2018 Stock Option and Stock Issuance Plan. †(Incorporated by reference to Exhibit 6.1 to the Form 1-A filed with the Securities and Exchange Commission on April 17, 2020). |
| | | |
| 6.2 | | Form of Nonqualified Stock Option Agreement. † (Incorporated by reference to Exhibit 6.2 to the Form 1-A filed with the Securities and Exchange Commission on April 17, 2020). |
| | | |
| 6.3 | | Employment Agreement with Anjun (Joey) Bose dated March 15, 2018. † (Incorporated by reference to Exhibit 6.7 to the Form 1-A filed with the Securities and Exchange Commission on April 17, 2020). |
| | | |
| 6.4 | | Exclusive Sales, Marketing, Manufacturing and Distribution Agreement between the Cytonics Corporation and A2MCyte, LLC dated October 30, 2015. (Incorporated by reference to Exhibit 6.4 to the Form 1-A filed with the Securities and Exchange Commission on April 17, 2020). |
| | | |
| 6.5 | | Exclusive License Agreement for Manufacturing, Sales, Marketing and Distribution in the Veterinary Market between Cytonics Corporation and Astaria Global, LLC dated June 30, 2019. (Incorporated by reference to Exhibit 6.5 to the Form 1-A filed with the Securities and Exchange Commission on April 17, 2020). |
| | | |
| 6.6 | | Exclusive Sales, Marketing, Manufacturing and Distribution Agreement for Human Market between Cytonics Corporation and Christie Medical Holdings, LLC dated April 27, 2020. + (Incorporated by reference to Exhibit 6.6 to Amendment No. 1 to the Form 1-A filed with the Securities and Exchange Commission on May 29, 2020). |
| | | |
| 6.7 | | Cytonics 2018 Stock Option and Stock Issuance Plan, as amended on May 28, 2021. + (Incorporated by reference to Exhibit 6.1 to the Form 1-U filed with the Securities and Exchange Commission on June 3, 2021). |
| | | |
| 15.1 | | Form D – December 3, 2018 filing with the Securities and Exchange Commission. (Incorporated by reference to the Form D filed with the Securities and Exchange Commission on December 3, 2018.) |
| 15.3 | | Form C-U Progress Update – May 24, 2019. (Incorporated by reference to the Form C-U filed with the Securities and Exchange Commission on May 24, 2019.) |
| | | |
| 15.4 | | Form D – June 10, 2019 filing with the Securities and Exchange Commission. (Incorporated by reference to the Form D filed with the Securities and Exchange Commission on June 10, 2019.) |
| | | |
| 15.5 | | Form D – June 19, 2019 filing with the Securities and Exchange Commission. (Incorporated by reference to the Form D filed with the Securities and Exchange Commission on June 19, 2019.) |
| | | |
| 15.6 | | Form C Offering Statement – April 6, 2022 filing with the Securities and Exchange Commission. (Incorporated by reference to the Form C filed with the Securities and Exchange Commission on April 6, 2022.) |
| | | |
| 15.7 | | Form C-U Progress Update– May 18, 2022. (Incorporated by reference to the Form C-U filed with the Securities and Exchange Commission on May 18, 2022.) |
| | | |
| 15.8 | | Form C-U Progress Update– June 10, 2022. (Incorporated by reference to the Form C-U filed with the Securities and Exchange Commission on June 10, 2022.) |
| | | |
| 15.9 | | Form C-U Progress Update– June 28, 2022. (Incorporated by reference to the Form C-U filed with the Securities and Exchange Commission on June 28, 2022.) |
| | | |
| 16 | | Form C-U Progress Update– July 12, 2022. (Incorporated by reference to the Form C-U filed with the Securities and Exchange Commission on July 12, 2022.) |
| | | |
| 16.1 | | Form C-U Progress Update– July 20, 2022. (Incorporated by reference to the Form C-U filed with the Securities and Exchange Commission on July 20, 2022.) |
| | | |
| 16.2 | | Form C-U Progress Update– August 2, 2022. (Incorporated by reference to the Form C-U filed with the Securities and Exchange Commission on August 2, 2022.) |
| | | |
| 16.3 | | Form C-U Progress Update– August 22, 2022. (Incorporated by reference to the Form C-U filed with the Securities and Exchange Commission on August 22, 2022.) |
| | | |
| 16.4 | | Form C-U Progress Update– September 6, 2022. (Incorporated by reference to the Form C-U filed with the Securities and Exchange Commission on September 6, 2022.) |
| | | |
| 16.5 | | Form C-U Progress Update– September 16, 2022. (Incorporated by reference to the Form C-U filed with the Securities and Exchange Commission on September 16, 2022.) |
| | | |
| 16.6 | | Form C-U Progress Update– September 30, 2022. (Incorporated by reference to the Form C-U filed with the Securities and Exchange Commission on September 30, 2022.) |
| | | |
| 16.7 | | Form C-U Progress Update– October 17, 2022. (Incorporated by reference to the Form C-U filed with the Securities and Exchange Commission on October 17, 2022.) |
| | | |
| 16.8 | | Form C-U Progress Update– October 31, 2022. (Incorporated by reference to the Form C-U filed with the Securities and Exchange Commission on October 31, 2022.) |
| 16.9 | | Form C Offering Statement – November 23, 2022 filing with the Securities and Exchange Commission. (Incorporated by reference to the Form C filed with the Securities and Exchange Commission on November 23, 2022.) |
*Filed herewith.
† Includes management contracts and compensation plans and arrangements
SIGNATURES
Pursuant to the requirements of Regulation A, the issuer has duly caused this report to be signed on its behalf by the undersigned, thereunto duly organized.
| Dated: December 1, 2022 | Cytonics Corporation |
| | |
| | By: | /s/ Joey Bose |
| | | Joey Bose |
| | | Chief Executive Officer, President, principal executive officer |
Pursuant to the requirements of Regulation A, this report has been signed below by the following persons on behalf of the issuer and in the capacities and on the dates indicated.
| Dated: December 1, 2022 | /s/ Joey Bose |
| | Joey Bose |
| | Chief Executive Officer, President (principal executive officer and principal financial and accounting officer) |
| | |
| Dated: December 1, 2022 | /s/ Gaetano Scuderi |
| | Gaetano Scuderi |
| | Founder & Chairman of the Board |
| Dated: December 1, 2022 | /s/ Phil LoGrasso |
| | Phil LoGrasso |
| | Director |
| | |
| Dated: December 1, 2022 | /s/ Tracey Goeken |
| | Tracey Goeken |
| | Director |
| Dated: December 1, 2022 | /s/ Gordon Ramseier |
| | Gordon Ramseier |
| | Director |