Preliminary Offering Circular Dated October 23, 2015
An offering statement pursuant to Regulation A relating to these securities has been filed with the Commission, which we refer to as the Commission. Information contained in this Preliminary Offering Circular is subject to completion or amendment. These securities may not be sold nor may offers to buy be accepted before the offering statement filed with the Commission is qualified. This Preliminary Offering Circular shall not constitute an offer to sell or the solicitation of an offer to buy nor may there be any sales of these securities in any state in which such offer, solicitation or sale would be unlawful before registration or qualification under the laws of any such state. We may elect to satisfy our obligation to deliver a Final Offering Circular by sending you a notice within two business days after the completion of our sale to you that contains the URL where the Final Offering Circular or the offering statement in which such Final Offering Circular was filed may be obtained.

APERION BIOLOGICS, INC.
2,500,000 Shares of Common Stock
This is our initial public offering. No public market currently exists for our shares. We are selling 2,500,000 shares of our common stock. We expect that the initial public offering price will be between $7.00 and $9.00 per share. We have applied to list our common stock on The NASDAQ Capital Market under the symbol “ZLIG.”
We are an “emerging growth company” as defined in the Jumpstart Our Business Startups Act, or the JOBS Act, and, as such, may elect to comply with certain reduced reporting requirements for this Offering Circular and future filings after this offering.
| THE OFFERING | | PER SHARE | | TOTAL
OFFERING | |
| Initial Public Offering Price | | | | | |
| Underwriting Discounts and Commissions | | | | | |
| Proceeds to us | | | | | |
(1) We refer you to “Underwriting” beginning on page 25 of this Offering Circular for additional information regarding total underwriter compensation.
See “Risk Factors” on page 9 to read about factors you should consider before buying shares of our common stock.
The underwriter has agreed to use its best efforts to procure potential purchasers for the shares of common stock offered pursuant to this Offering Circular.
The shares are being offered on an all or none basis. The offering will commence on the date of this Offering Circular. All investor funds received from the date of this Offering Circular to the closing date of this offering, which shall take place on , 2015, will be deposited into an escrow account until closing. The closing date is also the termination date of this offering. If, on the closing date, investor funds are not received for the full amount of shares to be sold in this offering, the offering will terminate and any funds received will be returned promptly, without interest.

The date of this Offering Circular is , 2015
This Offering Circular follows the disclosure format of Part I of Form S-1 pursuant to the general instructions of Part II(a)(1)(ii) of Form 1-A.
THE UNITED STATES COMMISSION DOES NOT PASS UPON THE MERITS OF OR GIVE ITS APPROVAL TO ANY SECURITIES OFFERED OR THE TERMS OF THE OFFERING, NOR DOES IT PASS UPON THE ACCURACY OR COMPLETENESS OF ANY OFFERING CIRCULAR OR OTHER SELLING LITERATURE. ANY REPRESENTATION TO THE CONTRARY IS UNLAWFUL.
THE SECURITIES HAVE NOT BEEN REGISTERED UNDER THE SECURITIES ACT OF 1933, AS AMENDED (THE “SECURITIES ACT”) OR APPLICABLE STATE SECURITIES LAWS, AND THESE SECURITIES ARE OFFERED PURSUANT TO AN EXEMPTION FROM REGISTRATION WITH THE COMMISSION. HOWEVER, THE COMMISSION HAS NOT MADE AN INDEPENDENT DETERMINATION THAT THE SECURITIES OFFERED HEREUNDER ARE EXEMPT FROM REGISTRATION.
THIS OFFERING CIRCULAR CONTAINS ALL OF THE REPRESENTATIONS BY THE COMPANY CONCERNING THIS OFFERING, AND NO PERSON SHALL MAKE DIFFERENT OR BROADER STATEMENTS THAN THOSE CONTAINED HEREIN. INVESTORS ARE CAUTIONED NOT TO RELY UPON ANY INFORMATION NOT EXPRESSLY SET FORTH IN THIS OFFERING CIRCULAR.
TABLE OF CONTENTS
OFFERING CIRCULAR SUMMARY
This summary highlights information contained elsewhere in this Offering Circular and does not contain all of the information that you should consider in making your investment decision. Before investing in our common stock, you should carefully read this entire Offering Circular, including our financial statements and the related notes and the information set forth under the headings “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” in each case included elsewhere in this Offering Circular. Unless otherwise stated, all references to “us,” “our,” “we,” the “Company” and similar designations refer to Aperion Biologics, Inc.
Overview
We are a commercial-stage medical device company addressing the significant need for an alternative to human-based sources of tissues to be used in surgical procedures. Our lead product, the Z-Lig ®, is produced by a patent-protected process for porcine tendons and its safety and performance was demonstrated in multicenter, prospective randomized trials in Europe and South Africa. In 2014, we received the CE Mark (the regulatory permission to market the product in Europe) for use initially as a knee joint anterior cruciate ligament (“ACL”) replacement in revision and multiligament procedures, which allows us to distribute the Z-Lig in any market that recognizes this approval. We have been building the appropriate distribution channels, primarily focusing on establishing a network of distributors that have strong relationships with sports medicine doctors.
Z-Lig is the only known biological alternative to human tissue for ACL replacement. Since Z-Lig is a biological implant that maintains a scaffold, it can become populated and remodeled with the patient’s own cells (similar to human-sourced tissue), referred to in the industry as “ligamentization”. This ligamentization is one of the keys to Z-Lig’s proven ability to be highly functional in patients many years post-implantation. Our propriety process is protected by an extensive portfolio of patents and patent applications, including 23 issued patents in the U.S. and internationally.
Our Solution
To solve for xenotransplant (transplants between the two different species) rejection problems, we developed the Z-Process, which is a proprietary process that immunochemically modifies animal tissue so that it is compatible with the human immune system. The Z-Process addresses both α-gal (galactose) and non-gal antigens on the xenograft (graft from another species) to create porcine tendons that can be safely used in humans while still maintaining their biological scaffold activity. Using the Z-Process, we developed the Z-Lig ® device as an immunocompatible, porcine-derived ACL reconstruction alternative that provides a readily available, off-the-shelf solution and is strong, sterile, cost-effective and consistent.
Our Product Candidate Pipeline
In addition to the ACL, our processing technology represents a platform that can also potentially be applied to opportunities involving cartilage, soft tissue patches (extracellular matrices), bone, heart valves, vascular grafts, collagen and other tissues throughout the body.
The Company has several product families in clinical and preclinical development and research stages that fall into four general groupings:
| | · | Z-Lig - family of devices designed for use in a range of ligament reconstruction procedures, in addition to the initial application for the ACL. |
| | · | Z-Patch - an extracellular matrix product used in soft tissue repair and augmentation procedures. |
| | · | Z-Fix - a bone-based product for biologic fixation in ACL reconstruction procedures which would be complementary to the Z-Lig device permitting an all-biologic ACL reconstruction. |
| | · | Z-Meniscus - a meniscus device which can be used in the repair or reconstruction of meniscal injuries and defects. |
Our Strategies
Our contact with clinicians confirmed that the initial market need for the Z-Lig is comprised of those revision and multiligament knee joint cases where a graft option is required to achieve the desired clinical result. The first phase of our European roll-out calls for the targeting of a select number of markets where human or synthetic graft options are accepted; in particular those markets whose sites participated in our European and South African (EUSA) clinical trials. Thus, the initial target markets for Z-Lig distribution include Italy, South Africa, Poland, Spain, UK, and the Benelux countries (Netherlands, Belgium, Luxembourg). We have identified and signed with a select group of distributors that have met our selection criteria, which include existing complementary product lines, market share, financial stability, and willingness to invest in the product line. In these markets, we are introducing the product through a targeted approach by focusing on clinicians chosen by our investigative surgeons and our distribution partners.
Supporting our indirect distribution efforts will be Aperion direct personnel specifically tasked with sales management and also clinical marketing specialists for training and technical/scientific field education.
The next distribution phase is expected to expand our application of use to include all ACL indications and furthermore to add European and Asian markets that accept the CE Mark, with a specific focus on high-volume clinical centers or those specializing in knee injuries. The third and final phase would include pan-European expansion into markets in the EU and continued introduction in markets outside the United States that accept the CE Mark and potential distribution into non-European nations whose regulatory bodies require additional regulatory approval.
We intend to build a sizable revenue base with the Z-Lig in overseas (outside U.S.) markets over the next several years. Our marketing efforts in the U.S. are contingent on obtaining FDA approval for the Z-Lig and other devices. The FDA has granted us an unconditional approval for a clinical trial of the Z-Lig in the United States. We plan to market our products in the U.S. after the trial is completed and FDA approval is obtained.
Our Risks
An investment in our common stock involves a high degree of risk. You should carefully consider the risks summarized below. These risks are discussed more fully in the “Risk Factors”
section immediately following this Offering Circular summary. These risks include, but are not limited to, the following:
| | · | We have incurred significant operating losses since inception and may continue to incur losses for the foreseeable future; |
| | · | We only recently received approval in Europe to commercialize our Z-Lig products, and we have no significant experience or capability to sell our products on a commercial scale; |
| | · | Our Z-Lig products may not gain market acceptance among surgeons, physicians, patients, healthcare payors and the medical community; |
| | · | We may not be able to generate long-term or additional positive clinical data to support or expand our commercialization efforts; |
| | · | We may not be able to protect our intellectual property; and |
| | · | CrossCart LLC, our controlling stockholder, controls all aspects of our business. |
Implications of Being an Emerging Growth Company
We qualify as an “emerging growth company” as defined in the Jumpstart Our Business Startups Act of 2012, as amended, or the JOBS Act. As an emerging growth company, we may take advantage of specified reduced disclosure and other requirements that are otherwise applicable generally to public companies. These provisions include:
| | · | only two years of audited financial statements in addition to any required unaudited interim financial statements with correspondingly reduced “Management’s Discussion and Analysis of Financial Condition and Results of Operations” disclosure; |
| | · | reduced disclosure about our executive compensation arrangements; |
| | · | no non-binding advisory votes on executive compensation or golden parachute arrangements; and |
| | · | exemption from the auditor attestation requirement in the assessment of our internal control over financial reporting. |
We may take advantage of these exemptions for up to five years or such earlier time that we are no longer an emerging growth company. We would cease to be an emerging growth company on the date that is the earliest of (i) the last day of the fiscal year in which we have total annual gross revenues of $1 billion or more; (ii) the last day of our fiscal year following the fifth anniversary of the date of the completion of this offering; (iii) the date on which we have issued more than $1 billion in nonconvertible debt during the previous three years; or (iv) the date on which we are deemed to be a large accelerated filer under the rules of the Commission. We may choose to take advantage of some but not all of these exemptions. We have taken advantage of reduced reporting requirements in this offering circular. Accordingly, the information contained herein may be different from the information you receive from other public companies in which you hold stock. We have irrevocably elected to ‘‘opt out’’ of the exemption for the delayed adoption of certain accounting standards and, therefore, will be subject to the same new or revised accounting standards as other public companies that are not emerging growth companies.
Company and Other Information
We were initially incorporated under the laws of the State of California as CrossCart, Inc., in 1996. In June 2008, CrossCart, Inc. was reincorporated under the laws of the State of Delaware, and in May 2009, CrossCart, Inc. changed its name to “Aperion Biologics, Inc.” Our principal executive office is located at 11969 Starcrest Dr. San Antonio, TX 78247, and our telephone number is (210) 858-7070. Our website address is www.aperionbiologics.com. We do not incorporate the information on or accessible through our website into this Offering Circular, and you should not consider any information on, or that can be accessed through, our website a part of this Offering Circular.
We own various U.S. federal trademark registrations and applications, and unregistered trademarks, including the following marks referred to in this Offering Circular: “Z-Lig” and “Aperion Biologics”. All other trademarks or trade names referred to in this Offering Circular are the property of their respective owners. Solely for convenience, the trademarks and trade names in this Offering Circular are referred to without the symbols ® and ™, but such references should not be construed as any indicator that their respective owners will not assert, to the fullest extent under applicable law, their rights thereto.
This Offering Circular summary highlights information contained elsewhere and does not contain all of the information that you should consider in making your investment decision. Before investing in our common stock, you should carefully read this entire Offering Circular, including our financial statements and the related notes included elsewhere in this Offering Circular. You should also consider, among other things, the matters described under “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” in each case appearing elsewhere in this Offering Circular.
The Offering
| Common stock offered by us | 2,500,000 Shares |
| | |
| |
| Common stock to be outstanding immediately after this offering | 6,465,396 Shares |
| | |
| Use of proceeds | We intend to use approximately $700,000 of net proceeds from this offering to pay certain accounts payable. We also intend to use a portion of the net proceeds to repay outstanding principal amounts and interest on certain demand notes and line of credit notes issued to CrossCart LLC in the amount of approximately $186,720, including accrued interest. We also intend to use approximately $300,000 of net proceeds to pay certain compensation owed to our employees as a result of the reduction of their salaries and fees. We intend to use the remaining net proceeds to launch our commercialization plan for Z-Lig products in Europe, South Africa and other countries that accept our approved CE Mark; fund post-market registry studies of Z-Lig; conduct pre-clinical and R&D activities for additional product candidates; and initiate clinical trials for Z-Lig products in the U.S. See “Use of Proceeds” on page 28. |
| | |
| Risk factors | You should carefully read “Risk Factors” on page 9 in this Offering Circular for a discussion of factors that you should consider before deciding to invest in our common stock. |
| | |
| Proposed NASDAQ Capital Market Symbol | “ZLIG” |
The number of shares of our common stock to be outstanding after this offering is based on 3,965,396 shares of our common stock outstanding as of June 30, 2015 and excludes:
| | · | 350,421 shares of common stock issuable upon the exercise of stock options outstanding as of June 30, 2015 at a weighted average exercise price of $1.47 per share; |
| | · | 659,937 shares of common stock issuable upon the exercise of warrants outstanding as of June 30, 2015 at a weighted average exercise price of $1.47 per share, which warrants prior to the completion of this offering are exercisable to purchase common stock or convertible preferred stock, assuming such warrants will not be exercised prior to the completion of this offering; |
| | · | 102,651 shares of common stock reserved for future issuance under our 2008 Stock Option/Stock Issuance Plan; |
| | | |
| | · | ______ shares of common stock reserved for future issuance under our 2015 Stock Option and Incentive Plan (the “2015 Plan”), which will become effective immediately prior to the completion of this offering. |
| · | 47,026 shares of common stock issued to our directors in October 2015 as payments to a portion of certain fees for board services which were deferred since July 2013, and 3,261 shares of common stock issued to outside contractors for services under consulting agreements. |
Unless otherwise indicated, all information in this Offering Circular reflects or assumes the following:
| | · | the filing of our amended and restated certificate of incorporation and the adoption of our amended and restated bylaws, which will occur immediately prior to the completion of this offering; |
| | · | the conversion of all of our outstanding shares of convertible preferred stock into an aggregate of 2,038,314 shares of common stock upon the completion of this offering, including 448,220 shares of common stock to be issued as cumulative dividends accrued under such preferred stock; |
| | · | the issuance of 1,704,713 shares of common stock upon the conversion of approximately an aggregate of $9,838,058 in outstanding principal and accrued interest on our convertible promissory notes (which is currently convertible into shares of our preferred stock but will be amended to be convertible into shares of our common stock in connection with the offering), upon the completion of this offering, at an average conversion price of $5.70 per share, assuming that the offering is completed on June 30, 2015; |
| | · | the issuance of additional shares of common stock as a result of anti-dilution adjustments provided in our certificate of incorporation; and |
| | · | a one-for-18.4 reverse split of our common stock, which became effective on 2015. |
| · | no exercise of options after June 30, 2015. |
Risk Factors
Investing in our common stock involves a high degree of risk. You should carefully consider the risks described below along with all of the other information contained in this Offering Circular, including our financial statements and the related notes, before deciding whether to purchase our common stock. If any of the adverse events described in the following risk factors, as well as other factors which are beyond our control, actually occurs, our business, results of operations and financial condition may suffer significantly. As a result, the trading price of our common stock could decline, and you may lose all or part of your investment in our common stock.
Risks Related to Our Business and Finance
We have incurred significant operating losses since inception, anticipate that we will continue to incur losses for the foreseeable future and will need to raise additional capital.
We have generated operating losses since we began operations in 2008, and our net losses attributable to common stockholders for the fiscal year ended September 30, 2014 and 2013 were $3.29 million and $5.45 million respectively. We expect to continue to incur additional operating losses for the foreseeable future as we plan the commercialization of Z-Lig products and continue our clinical and pre-clinical studies. Our recurring operating losses and our need for additional sources of capital to fund our ongoing operations raise substantial doubt about our ability to continue as a going concern. As a result, our independent registered public accounting firm included an explanatory paragraph in its report on our financial statements as of and for the year ended September 30, 2014 with respect to this uncertainty. If the time required to generate material revenues and achieve profitability is longer than we currently anticipate, or if we are unable to raise new capital through equity or obtain debt financing or other sources of funding, we may be forced to curtail or suspend our operations, which would have a material adverse effect on the value of your investment.
We expect capital outlays and operating expenditures to increase over the next several years as we establish and expand our marketing infrastructure to commercialize our Z-Lig products. Following the completion of this offering, we believe our financial resources will be adequate to sustain our current operations through the end of calendar year 2017. However, we will need to raise additional capital. We cannot be certain that we will be able to obtain financing on terms acceptable to us, or at all. Our failure to obtain adequate and timely funding will materially adversely affect our business and our ability to develop our products and would have a material adverse effect on the value of your investment.
We only recently received approval in Europe to commercialize our Z-Lig products, and we have no significant experience or capability to sell our products on a commercial scale.
Our future is significantly dependent on the commercial success of our Z-Lig family of products. We have only recently received a CE Mark in April 2014 that allowed us to conduct limited sales in select European markets beginning in February 2015. As a result, we have no significant history or experience in selling Z-Lig or any other products in Europe or in any other markets, and we have limited relationships with surgeons, physicians, clinicians and hospitals that may purchase or use our products. In order for us to commercialize our products successfully, we will need to develop, or obtain through outsourcing arrangements, the capability to market and
sell our products on a commercial scale. We may not have the ability and sufficient resources to establish the infrastructure and organizations needed to execute these functions, which can be complex and costly. Our effort to commercialize Z-Lig products is also subject to a number of additional risks, which could have a material adverse effect on the value of your investment, including:
| | · | competitors’ established relationships with our potential customers; |
| | · | limitations in our ability to demonstrate the advantages of our products compared to competing products and the relative safety, efficacy and ease of use of our products; |
| | · | our inability to convince doctors and hospitals to use our products; and |
| | · | the introduction and market acceptance of competing products and technologies. |
Our Z-Lig products may not gain market acceptance among surgeons, physicians, patients, healthcare payors and the medical community.
A critical element in our commercialization strategy is to persuade and educate the medical community on the safe and effective use of our products and how Z-Lig products differentiate from the existing supplies of surgical issues. Surgeons, physicians and hospitals may not perceive the benefits of our products and may be unwilling to change from the devices they are currently using. A number of factors may limit the market acceptance of our Z-Lig products, including the following:
| | · | rate of adoption by healthcare practitioners; |
| | · | rate of a product’s acceptance by the target population; |
| | · | timing of market entry relative to competitive products; |
| | · | availability of third-party reimbursement; |
| | · | government review and approval requirements; |
| | · | extent of marketing efforts by us and third-party distributors or agents retained by us; and |
| | · | side effects or unfavorable publicity concerning our products or similar products. |
Therefore, even after we have demonstrated the effectiveness of the Z-Lig products, we may not be able to commercialize these products successfully if we cannot achieve an adequate level of market acceptance. Our inability to successfully commercialize our products will have a material adverse effect on the value of your investment.
We may experience defects and manufacturing issues in our supplies of animal issues or raw materials.
As we continue to expand our clinical development and commercialization activities, we expect to scale up the manufacturing and supplies of animal tissues that are the main component of our Z-Lig products. We may not be able to control or maintain a consistent and high quality of
raw materials, such as porcine tissues, or uncover defects prior to implantation in humans. In the past, we observed and experienced contamination in porcine tissues resulting from the processing and handling of these materials during clinical trials, which led to bacterial infections associated with the grafts in patients implanted with Z-Lig in the trial. While we have implemented stringent validation, safety and corrective procedures and intend to conduct additional post-market studies to confirm the effectiveness of such preventive measures, there is no guarantee that we can eliminate this risk in the future. In addition, external factors outside of our control may affect the quality of our supplies of animal or porcine issues. For example, swine diseases or pandemics may severely reduce the supply of porcine tissues required to manufacture our Z-Lig products. Mishandling or lack of quality control at our third-party suppliers may also negatively impact our ability to obtain a sufficient and acceptable level of porcine tissues to meet our needs. Our failure to eliminate contamination and deficiency in the animal and porcine issues used in our products may require us to incur additional costs to implement preventive measures, or cause significant delays and disruptions in the development and commercialization of our products, which would have an adverse effect on our business operations and financial condition.
The safety and efficacy of our products is supported by limited clinical data, and we may not be able to generate long-term or additional positive clinical data to support or expand our commercialization efforts.
We have obtained a CE Mark to market and sell our Z-Lig products in Europe based on the 6- and 12-month data collected from our clinical studies in 2013 and the longer term (12 years) safety data from the U.S. pilot safety trial. We have committed to continue these clinical studies to support post-market commercial activities in Europe and to meet certain post-market approval requirements by European regulatory agencies. In addition, we intend to conduct additional clinical studies to support further commercialization efforts and expand the markets for our Z-Lig products. For example, the CE Mark we received in April 2014 is limited to revision and multiligament ACL surgeries and does not cover primary ACL surgeries. We have established post-market clinical plans to generate additional data required to expand the indication of Z-Lig products to cover primary ACL surgeries, which would allow us to reach a broader ACL reconstruction market. There is no guarantee that we can duplicate the positive results from our earlier trials for future studies or generate the required data to support the expansion of Z-Lig indication. Our failure to do so may result in the loss of CE Mark approval or other regulatory approvals, delays, failures in the adoption of our products by surgeons and physicians, damage to our reputation and legal claims against us.
In addition, any negative data from our post-market clinical studies may adversely impact our ability to conduct clinical trials and seek regulatory approval of our products from the FDA to market our products in the United States. Furthermore, we are developing several products based on our Z-process that would allow a commercialization path based on FDA’s Section 510(K) pre-market clearance procedures. This procedure is shorter and typically requires the submission of less supporting documentation than other FDA approval processes and does not require long-term clinical studies. As result, we may encounter difficulties and delays in marketing these products which otherwise may not be the case if the products have been proven safe and effective in more extensive and long-term clinical trials.
Our clinical studies of our current or future products may not produce results necessary to support regulatory clearance or approval in the United States or elsewhere.
We plan to seek FDA approval of Z-Lig by conducting pivotal clinical trials in the United States. While we have conducted a limited safety pilot study in the United States showing that Z-Lig was safe, and we have received a CE Mark based on the clinical studies in Europe and South Africa, there is no guarantee that the same results will be duplicated in a clinical trial with a larger patient population in the United States. Our inability to achieve acceptable results in future clinical studies would have a material adverse effect on our business operations and financial condition and the value of your investment.
If third-party payors fail to provide appropriate levels of reimbursement for the use of our products, our revenues could be adversely affected.
Sales of our products depend on the availability of adequate reimbursement from third-party payors. In each market in which we do business, our inability to obtain reimbursement approval or the failure of third-party payors to reimburse health care providers at a level which justifies the use of our products instead of cheaper alternatives will hurt our business.
Moreover, we are unable to predict what changes will be made to the reimbursement methodologies used by third-party payors in the future. We cannot be certain that under current and future payment systems, in which healthcare providers may be reimbursed a set amount based on the type of procedure performed, such as those utilized by Medicare and in many privately managed care systems, the cost of our products will be justified and incorporated into the overall cost of the procedure. A failure by third-party payors to provide appropriate levels of reimbursement for the use of our products will have a material adverse effect on our business operations and financial condition and the value of your investment.
As we expand into multiple international markets, we will face similar risks relating to adverse changes in coverage and reimbursement procedures and policies in those markets. Reimbursement and healthcare payment systems vary significantly among markets in different jurisdictions. Our inability to obtain international coverage and reimbursement approval, or any adverse changes in coverage and the reimbursement policies of foreign third-party payors, could negatively affect our ability to sell our products.
We rely on third party suppliers for our raw materials, including porcine tissues, and their inability to supply us with an adequate supply of materials could harm our business.
We rely on third-party suppliers to supply and manufacture the raw materials, including porcine tissues, for the development of our products. To be successful, our suppliers must be able to provide us with products and components in substantial quantities, in compliance with regulatory requirements (including ISO 22442), in accordance with agreed upon specifications, at acceptable cost and on a timely basis. Among other factors, our anticipated growth could strain the ability of suppliers to deliver an increasingly large supply of products, materials and components. If we are unable to obtain sufficient quantities of high quality components to meet customer demand on a timely basis, we could lose customers, our reputation may be harmed and our business could suffer.
In addition, we currently use one supplier for the supply of porcine tissues required to manufacture our Z-Lig products, biologics, and components. Our dependence on one supplier involves several risks, including limited control over pricing, availability, quality and delivery schedules. If any one or more of our suppliers cease to provide us with sufficient quantities of our components in a timely manner or on terms acceptable to us, we would have to seek alternative sources of supplies. We could incur significant delays while we locate and engage
alternative qualified suppliers, including delays associated with qualifying the new supplier to meet all applicable regulatory requirements in the United States, Europe and other markets in which we develop and sell our products. Even if we are able to identify an alternative supplier, we might not be able to negotiate or receive the same or more favorable terms as those provided by our existing suppliers. Any such disruption or increased expenses could harm our commercialization efforts and adversely affect our ability to generate revenue. Any inability to meet our customers’ demands for these products could lead to decreased sales and harm our reputation and result in the loss of customers, which would have a material adverse effect on our business operations and financial condition and the value of your investment.
Failure to attract, retain, and motivate skilled personnel may delay our commercialization plans and research and development efforts.
Our commercial success depends on our continued ability to attract, retain, and motivate highly qualified management and scientific personnel. Competition for skilled and qualified personnel in the medical device industry is intense. If we lose the services of personnel with the necessary skills, including the members of our senior management team, it could significantly impede our commercialization and research and development objectives. Replacing key personnel and consultants may be difficult and may take an extended period of time because of the limited number of individuals in our industry with the breadth of skills and experience required to develop, gain regulatory approval of and commercialize products successfully.
Our international operations subject us to certain risks.
We intend to market our Z-Lig products initially in select European countries and in South Africa, including Denmark, United Kingdom, Netherlands, Belgium, Spain, Italy and Poland. We also have plans to expand our commercialization activities to other non-European countries that accept the CE Mark, such as Canada, Turkey, Saudi Arabia, Australia and Korea. We will also pursue various clinical and pre-clinical activities in the United States and Europe. Our international operations will subject us to rules, regulations and customs of multiple jurisdictions, and compliance with these rules and regulations is costly and exposes us to penalties for non-compliance. Other laws and regulations that can significantly impact us include various anti-bribery laws, including the U.S. Foreign Corrupt Practices Act and anti-boycott laws, as well as export controls laws. Any failure to comply with applicable legal and regulatory obligations could impact us in a variety of ways that include, but are not limited to, significant criminal, civil and administrative penalties, denial of export privileges, seizure of shipments and restrictions on certain business activities. Also, the failure to comply with applicable legal and regulatory obligations could result in the disruption of our sales activities.
Our international operations expose us to additional risks, including:
| | · | difficulties in enforcing or defending intellectual property rights; |
| | · | pricing pressure that we may experience internationally; |
| | · | third-party reimbursement policies that may require some of the patients who receive our products to directly absorb medical costs or that may necessitate the reduction of the selling prices of our products; |
| | · | competitive disadvantage to competition with established business and customer relationships; |
| | · | the imposition of additional U.S. and foreign governmental controls or regulations; changes in duties and tariffs, license obligations and other non-tariff barriers to trade; |
| | · | foreign currency exchange rate fluctuations; |
| | · | difficulties in communicating with our employees, partners and collaborators, and maintaining consistency with our internal guidelines; |
| | · | difficulties in enforcing agreements and collecting receivables through certain foreign legal systems; and |
| | · | difficulties in establishing and maintaining an effective internal control system to ensure timely and accurate financial reporting. |
If we experience any of these risks, our sales in international countries may be harmed and our results of operations would suffer.
We may face product liability claims that could result in costly litigation and significant liabilities.
Manufacturing and marketing of our products, and clinical testing of our products under development, may expose us to product liability and other tort claims. Although we have, and intend to maintain, liability insurance, the coverage limits of our insurance policies may not be adequate and one or more successful claims brought against us may have a material adverse effect on our business and results of operations. Additionally, product liability claims could negatively affect our reputation, product sales, and our ability to obtain and maintain regulatory approval for our products.
We have identified material weaknesses in our internal control over financial reporting and may identify additional material weaknesses in the future or otherwise fail to maintain an effective system of internal controls.
Effective internal controls are necessary for us to provide reliable financial reports and prevent fraud. If we fail to maintain an effective system of internal controls, we might not be able to report our financial results accurately or prevent fraud; and in that case, our stockholders could lose confidence in our financial reporting, which would harm our business and could negatively impact the price of our stock. We have limited accounting personnel and other resources with which to address our internal controls and procedures. During the course of preparing for this offering, we determined that as of September 30, 2014 and through the date of this filing, we had a material weakness due to the lack of accounting personnel with sufficient experience with United States generally accepted accounting principles to address the accounting for complex debt and equity transactions. In addition,we have identified a material weakness due to the lack of segregation of duties in accounting personnel so that all journal entries and account reconciliations are reviewed by someone other than the preparer, heightening the risk of error or fraud.
A material weakness is a deficiency, or combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of our annual or interim financial statements will not be prevented or detected on a timely basis. We plan to remediate the material weaknesses primarily by implementing additional review procedures within the finance department and hiring additional personnel with the requisite accounting and financial reporting experience to enhance our internal computation and review processes. We cannot assure you that the measures we have taken to date, or any measures we may take in the future, will be sufficient to remediate the material weakness in our internal control over financial reporting or to avoid potential future material weaknesses. Even if we conclude that our internal control over financial reporting provides reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles, because of its inherent limitations, internal control over financial reporting may not prevent or detect fraud or misstatements. Failure to implement required new or improved controls, or difficulties encountered in their implementation, could harm our results of operations or cause us to fail to meet our future reporting obligations.
Our reporting obligations as a public company will place a significant strain on our management, operational and financial resources and systems for the foreseeable future. If we fail to timely achieve and maintain the adequacy of our internal control over financial reporting, we may not be able to produce reliable financial reports or help prevent fraud. Our failure to achieve and maintain effective internal control over financial reporting could prevent us from filing our periodic reports on a timely basis which could result in the loss of investor confidence in the reliability of our financial statements, harm our business, negatively impact the trading price of our common stock and cause our common stock to be delisted from NASDAQ.
The requirements of being a public company may strain our resources and divert management’s attention.
As a public company, we will be subject to the reporting requirements of the Securities Exchange Act of 1934, as amended, or the Exchange Act, the Sarbanes-Oxley Act, the Dodd-Frank Act, the continued listing requirements of The NASDAQ Capital Market and other applicable securities rules and regulations. Despite recent reforms made possible by the JOBS Act, compliance with these rules and regulations will nonetheless increase our legal and financial compliance costs, make some activities more difficult, time-consuming or costly and increase demand on our systems and resources, particularly after we are no longer an “emerging growth company.” The Exchange Act requires, among other things, that we file annual, quarterly, and current reports with respect to our business and operating results.
As a result of disclosure of information in this Offering Circular and in filings required of a public company, our business and financial condition will become more visible, which we believe may result in threatened or actual litigation, including by competitors and other third parties. If such claims are successful, our business and operating results could be harmed, and even if the claims do not result in litigation or are resolved in our favor, these claims, and the time and resources necessary to resolve them, could divert the resources of our management and adversely affect our business, brand and reputation and results of operations.
We also expect that being a public company and these new rules and regulations will make it more expensive for us to obtain director and officer liability insurance, and we may be required to accept reduced coverage or incur substantially higher costs to obtain coverage. These factors could also make it more difficult for us to attract and retain qualified members of our Board of Directors, particularly to serve on our audit committee and Compensation Committee, and qualified executive officers.
We are an “emerging growth company,” and we cannot be certain if the reduced reporting requirements applicable to emerging growth companies will make our common stock less attractive to investors.
We are an “emerging growth company,” as defined in the JOBS Act. For as long as we continue to be an emerging growth company, we may take advantage of exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies, including not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act, reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved. We could be an emerging growth company for up to five years, although circumstances could cause us to lose that status earlier, including if the market value of our common stock held by non-affiliates exceeds $700 million as of any March 31 before that time, in which case, we would no longer be an emerging growth company as of the following September 30. We cannot predict if investors will find our common stock less attractive because we may rely on these exemptions. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock and our stock price may be more volatile.
Risks Relating to Our Intellectual Property
If we are unable to protect our intellectual property, our business will be negatively affected.
The market for medical devices is subject to frequent litigation regarding patent and other intellectual property rights. It is possible that our patents or licenses may not withstand challenges made by others or protect our rights adequately.
Our success depends in large part on our ability to secure effective patent protection for our products and processes in Europe and the United States. We have filed and intend to continue to file patent applications to protect our proprietary technology. However, we face the risks that:
| | · | we may fail to secure necessary patents prior to or after obtaining regulatory clearances, thereby permitting competitors to market competing products; and |
| | · | our already-granted patents may be re-examined, invalidated or not extended. |
We also own trade secrets and confidential information that we try to protect by entering into confidentiality agreements with our employees and other parties. However, the confidentiality agreements may not be honored or, if breached, we may not have sufficient remedies to protect our confidential information. Further, our competitors may independently learn our trade secrets or develop similar or superior technologies. To the extent that our consultants, key employees
or others apply technological information to our projects that they develop independently or others develop, disputes may arise regarding the ownership of proprietary rights to such information, and such disputes may not be resolved in our favor. If we are unable to protect our intellectual property adequately, our business and commercial prospects will suffer.
Because it is difficult and costly to protect our proprietary rights, and third parties have filed patent applications that are similar to ours, we cannot ensure the proprietary protection of our technologies and products.
Our commercial success will depend in part on obtaining patent protection of our technology and successfully defending any of our patents that may be challenged. The patent positions of medical device companies can be highly uncertain and can involve complex legal and factual questions, and we cannot predict the breadth of claims allowed in patents we own or license.
We are a party to certain license agreements that give us rights under specified patents and patent applications, including a license agreement with the University of Missouri. Our current licenses, and our future licenses frequently will, contain performance obligations. If we fail to meet those obligations, the licenses could be terminated. If we are unable to continue to license these technologies on commercially reasonable terms, or at all, we may be forced to delay or terminate our product development and research activities.
We are unable to exercise the same degree of control over intellectual property that we license from third parties as we exercise over our internally developed intellectual property. We do not control the prosecution of certain of the patent applications that we license from third parties; therefore, the patent applications may not be prosecuted as we desire or in a timely manner.
The degree of future protection for our proprietary rights is uncertain, and we cannot ensure that:
| | · | we or our licensors will be the first to file patent applications for these inventions; |
| | · | the patents of others will not have an adverse effect on our ability to do business; |
| | · | others will not independently develop similar or alternative technologies or reverse engineer any of our products, processes or technologies; |
| | · | any of our pending patent applications will result in issued patents; |
| | · | any patents issued or licensed to us or our collaborators or strategic partners will provide a basis for commercially viable products or will provide us with any competitive advantages; |
| | · | any patents issued or licensed to us will not be challenged and invalidated by third parties; or |
| | · | we will develop additional products, processes or technologies that are patentable. |
Others have filed and in the future are likely to file patent applications that are similar to ours. The costs of litigating any infringement claim could be substantial. Moreover, we cannot predict whether we, our collaborators, or strategic partners would prevail in any actions. In addition, if the relevant patent claims were upheld as valid and enforceable and our products or processes
were found to infringe the patent or patents, we could be prevented from making, using, or selling the relevant product or process unless we could obtain a license. We can give no assurance that we will be able to obtain such license on commercially reasonable terms, or at all. Negotiation of licensing agreements in the biotechnology and life science industries can be intense and difficult, and we may not be able to negotiate terms with respect to royalty rates and milestone fees that are equivalent to or more favorable than what we have previously negotiated under our existing license agreements. If we cannot obtain a license agreement, we would be forced to design around the relevant patent claims, and there is no assurance that we can do so in a cost effective manner, or at all. There may be significant litigation in our industry regarding patent and other intellectual property rights, which could subject us to litigation. If we become involved in litigation, it could consume a substantial portion of our managerial and financial resources.
We rely on trade secrets to protect technology where we believe patent protection is not appropriate or obtainable. Trade secrets, however, are difficult to protect. While we require employees, and consultants to enter into confidentiality agreements, we may not be able to adequately protect our trade secrets or other proprietary information or enforce these confidentiality agreements.
We may infringe the intellectual property rights of others, which may prevent or delay our product development efforts.
Our success will depend in part on our ability to operate without infringing the proprietary rights of third parties. We cannot guarantee that our products, or manufacture or use of our product candidates, will not infringe third-party patents. Furthermore, a third party may claim that we are using inventions covered by the third party’s patent rights and may go to court to stop us from engaging in our normal operations and activities, including making or selling our product candidates. These lawsuits are costly and could affect our results of operations and divert the attention of managerial and scientific personnel. Some of these third parties may be better capitalized and have more resources than us. There is a risk that a court would decide that we are infringing the third party’s patents and would order us to stop the activities covered by the patents. In that event, we may not have a viable way around the patent and may need to halt commercialization of the relevant product candidate. In addition, there is a risk that a court will order us to pay the other party damages for having violated the other party’s patents. In addition, we may be obligated to indemnify our licensors and collaborators against certain intellectual property infringement claims brought by third parties, which could require us to expend additional resources. The pharmaceutical and biotechnology industries have produced a proliferation of patents, and it is not always clear to industry participants, including us, which patents cover various types of products or methods of use. The coverage of patents is subject to interpretation by the courts, and the interpretation is not always uniform.
Patent litigation is costly and time consuming, even in cases where the claims against us are without merit. We may not have sufficient resources to bring these actions to a successful conclusion. In addition, if we do not obtain a license, develop or obtain non-infringing technology, fail to defend an infringement action successfully or have infringed patents declared invalid, we may incur substantial monetary damages, encounter significant delays in bringing our product candidates to market and be precluded from manufacturing or selling our product candidates.
International patent protection is particularly uncertain, and if we are involved in opposition proceedings in foreign countries, we may have to expend substantial sums and management resources.
The laws of some foreign countries may not protect our intellectual property rights to the same extent as the laws of the United States. For example, certain countries do not grant patent claims that are directed to the treatment of humans. We may participate in opposition
proceedings to determine the validity of our foreign patents or our competitors’ foreign patents, which could result in substantial costs and diversion of our efforts.
The medical device industry is characterized by extensive patent litigation, and we could become subject to litigation that could be costly, result in the diversion of management’s attention, and harm our business and financial condition.
Our success depends in part on not infringing the patents or violating the other proprietary rights of others. Significant litigation regarding patent rights occurs in the medical industry, including among companies focused on regenerative medicine. It is possible that U.S. and foreign patents and pending patent applications controlled by third parties may be alleged to cover our Z-Process. Our competitors in Europe, the United States and abroad, many of which have substantially greater resources and have made substantial investments in patent portfolios and competing technologies, may have applied for or obtained or may in the future apply for and obtain, patents that will prevent, limit or otherwise interfere with our ability to make, use and sell our products. At any given time, we may be involved as either a plaintiff or a defendant in a number of patent infringement actions, the outcomes of which may not be known for prolonged periods of time.
The large number of patents, the rapid rate of new patent applications and issuances, the complexities of the technologies involved and the uncertainty of litigation significantly increase the risks related to any patent litigation. Any potential intellectual property litigation also could force us to do one or more of the following:
| | · | stop selling, making, or using products that use the disputed intellectual property; |
| | · | obtain a license from the intellectual property owner to continue selling, making, licensing, or using products, which license may require substantial royalty payments and may not be available on reasonable terms, or at all; |
| | · | incur significant legal expenses; |
| | · | pay substantial damages or royalties to the party whose intellectual property rights we may be found to be infringing; |
| | · | pay the attorney fees and costs of litigation to the party whose intellectual property rights we may be found to be infringing; or |
| | · | redesign those products that contain the allegedly infringing intellectual property, which could be costly, disruptive and/or infeasible. |
If any of the foregoing occurs, we may have to withdraw existing products from the market or may be unable to commercialize one or more of our products, all of which could have a material adverse effect on our business, results of operations and financial condition. Any litigation or claim against us, even those without merit, may cause us to incur substantial costs, and could place a significant strain on our financial resources, divert the attention of management from our core business and harm our reputation. Further, as the number of participants in the regenerative tissue industry grows, the possibility of intellectual property infringement claims against us increases.
Risks Related to Our Industry
We are in a highly competitive market segment, and if our competitors are better able to develop and market products that are safer, more effective, less costly, easier to use or otherwise superior than any products that we may develop, our business will be adversely impacted.
The medical device industry is highly competitive and subject to technological change. Our success depends, in part, upon our ability to maintain a competitive position in the development of technologies and products for use in the ACL knee reconstruction graft and other orthopaedic markets. Any product we develop that achieves regulatory clearance or approval will have to compete for market acceptance and market share. We believe that the primary competitive factors in ACL replacement graft markets are clinical effectiveness, product safety, reliability and durability, and eligible patient populations, physician experience and comfort with use of a particular device, ease of use, product support and service, sales force experience and relationships and price. We face significant competition in the United States and internationally, and we expect the intensity of competition will increase over time. We compete with companies that provide allograft and traditional xenograft solutions for orthopaedic surgical procedures. Many of the companies developing or marketing competing products enjoy several advantages to us, including:
| | · | greater financial and human resources for product development, sales and marketing; |
| | · | greater name recognition; |
| | · | long established relationships with physicians and hospitals; |
| | · | longer-term clinical trial data due to earlier regulatory approval; |
| | · | the ability to offer rebates or bundle multiple product offerings to offer greater discounts or incentives; and |
| | · | more established sales and marketing programs and distribution networks; and |
Our competitors may develop and patent processes or products competitive with us, obtain regulatory clearance or approvals for competing products more rapidly than us or develop more effective or less expensive products or technologies that render our technology or products obsolete or less competitive. We also face fierce competition in recruiting and retaining qualified sales, scientific, and management personnel, establishing clinical trial sites and enrolling patients in clinical studies. Our inability to successfully compete with existing and future market competitors will have a material adverse effect on our business operations and financial condition and the value of your investment.
Our business is subject to extensive governmental regulation that could make it more expensive and time consuming for us to introduce new or improved products.
Our products must comply with regulatory requirements imposed by various regulatory agencies. These requirements involve lengthy and detailed laboratory and clinical testing procedures, sampling activities, an extensive agency review process, and other costly and time-consuming procedures. It often takes several years to satisfy these requirements, depending on the complexity and novelty of the product. We also are subject to numerous additional licensing
and regulatory requirements relating to safe working conditions, manufacturing practices, environmental protection, fire hazard control, and disposal of hazardous or potentially hazardous substances. Some of the most important requirements we face include:
| | · | European Union CE mark requirements; |
| | · | Medical Device Quality Management System Requirements (ISO 13485:2003); and |
| | · | Occupational Safety and Health Administration requirements. |
Government regulation may impede our ability to conduct clinical studies and to manufacture our existing and future products. Government regulation also could delay our marketing of new products for a considerable period of time and impose costly procedures on our activities. The regulatory agencies may not approve any of our future products on a timely basis, if at all. Any delay in obtaining, or failure to obtain, such approvals could negatively impact our marketing of any future products and reduce our product revenues.
Our products remain subject to strict regulatory controls on manufacturing, marketing and use. We may be forced to modify or recall a product after release in response to regulatory action or unanticipated difficulties encountered in general use. Any such action could have a material effect on the reputation of our products and on our business and financial position.
Further, regulations may change, and any additional regulation could limit or restrict our ability to use any of our technologies, which could harm our business. We could also be subject to new international, federal, state or local regulations that could affect our research and development programs and harm our business in unforeseen ways. If this happens, we may have to incur significant costs to comply with such laws and regulations, which will harm our results of operations.
Risks Related to Our Common Stock and Corporate Structure
CrossCart LLC controls all aspects of our business.
CrossCart LLC (“CrossCart”) is our largest and controlling stockholder and currently beneficially owns approximately 77.7% of our outstanding shares of common stock on a fully-diluted basis assuming the conversion of all of our outstanding preferred stock and convertible notes. Following the completion of this offering, CrossCart will beneficially own approximately 48.2% of our outstanding shares of common stock. In addition, Dr. Kevin R. Stone, Chairman of our Board of Directors, is the controlling member and manager of CrossCart. In addition, CrossCart is our most significant creditor and historically we have depended on the funding of CrossCart and Dr. Stone to maintain our operations. As a result, CrossCart and Dr. Stone are and will be able to exercise significant control over our business operations and on all matters requiring stockholders’ approval, including the election of directors, approval of significant corporate transactions and the definition of rights and privileges of all securities. Due to CrossCart’s and Dr. Stone’s controlling position, we may take actions with respect to our business that may conflict with the desire of other stockholders.
An active trading market for our common stock may not develop and you may not be able to resell your shares at or above the initial offering price.
Prior to this offering, there has been no public market for shares of our common stock. In the absence of an active trading market for our common stock, investors may not be able to sell their common stock at or above the initial public offering price or at the time that they would like to sell. In addition, we intend to submit a listing application for our common stock to be listed on a national securities exchange, including the NASDAQ Capital Market (“NASDAQ”), and there is no guarantee that we can meet the listing standards or that such listing application will be accepted. Even if such application is accepted and our common stock is listed on NASDAQ or other exchanges, an active trading market for our common stock may never develop, which will adversely impact your ability to sell our shares. If shares of common stock are not listed on NASDAQ or another national securities exchange, we anticipate that our common stock will be quoted at over-the-counter, or OTC markets, following the completion of this offering. There is no assurance that an active trading market for our common stock will develop on the OTC market.
Our stock price may be influenced by public perception of alternative tissue replacement and government regulation of such technology.
External events in the field of ligament reconstruction or the use of animal-derived tissue in human transplantation may have a significant negative impact on the public perception and stock price of certain companies involved in this kind of work. Potential adverse events in this field may occur in the future that could result in increased governmental regulation of our Z-Lig device. These external events may have a negative impact on public perceptions of our business, which could cause our stock price to decline.
We do not intend to pay dividends on our common stock.
We have never paid dividends on our common stock and we currently intend to retain any future earnings and do not expect to pay any cash dividends on our common stock in the foreseeable future. The declaration and payment of all future dividends, if any, will be at the sole discretion of our Board of Directors, which retains the right to change our dividend policy at any time, and may be limited by our debt arrangements in place from time to time. Consequently, stockholders must rely on sales of their common stock after price appreciation, which may never occur, as the only way to realize any future gains on their investment.
New stockholders will incur substantial and immediate dilution as a result of this offering.
The initial public offering price is substantially higher than the book value per share of our outstanding common stock. As a result, investors purchasing common stock in this offering will incur substantial and immediate dilution. At the assumed initial public offering price of $8.00 per share, the midpoint of the price range set forth on the cover page of this Offering Circular, purchasers in this offering will experience immediate and substantial dilution of approximately $5.41 per share, representing the difference between our historical net tangible book value per share after giving effect to this offering and the initial public offering price. In addition, we have issued options to acquire common stock at prices significantly below the public offering price. To the extent such options are ultimately exercised, there will be further dilution to investors in this offering.
Anti-takeover provisions in our certificate of incorporation and Delaware law could make an acquisition of the Company more difficult and could prevent attempts by our stockholders to remove or replace current management.
Provisions of Delaware law and our amended and restated certificate of incorporation and amended and restated bylaws, which will be effective upon the completion of this offering, may discourage, delay or prevent a merger, acquisition or other change in control that stockholders may consider favorable, including transactions in which stockholders might otherwise receive a premium for their shares. These provisions may also prevent or delay attempts by stockholders to replace or remove our current management or members of our Board of Directors. These provisions include:
| | · | advance notice requirements for stockholder proposals and nominations; |
| | · | the inability of stockholders to act by written consent or to call special meetings; |
| | · | the ability of our Board of Directors to make, alter or repeal our amended and restated bylaws: and |
| | · | the authority of our Board of Directors to issue preferred stock with such terms as our Board of Directors may determine. |
The affirmative vote of the holders of at least 75% of our shares of capital stock entitled to vote, and not less than 75% of the outstanding shares of each class entitled to vote thereon as a class, is generally required to amend or repeal the above provisions that are contained in our amended and restated certificate of incorporation. In addition, absent approval of our Board of Directors, our amended and restated bylaws may only be amended or repealed by the affirmative vote of the holders of at least 75% of our shares of capital stock entitled to vote.
In addition, upon the completion of this offering, we will be subject to the provisions of Section 203 of the Delaware General Corporation Law, which limits business combination transactions with stockholders of 15% or more of our outstanding voting stock that our Board of Directors has not approved. These provisions and other similar provisions make it more difficult for stockholders or potential acquirers to acquire us without negotiation. These provisions may apply even if some stockholders may consider the transaction beneficial to them.
As a result, these provisions could limit the price that investors are willing to pay in the future for shares of our common stock. These provisions might also discourage a potential acquisition proposal or tender offer, even if the acquisition proposal or tender offer is at a premium over the then-current market price for our common stock.
Dilution
If you invest in our common stock in this offering, your interest will be diluted to the extent of the difference between the public offering price per share of our common stock and the pro forma as adjusted net tangible book value per share of our common stock immediately after this offering.
Our historical net tangible book value as of June 30, 2015 was ($28,398,077), or ($127.71) per share of our common stock. Historical net tangible book value per share represents our total tangible assets less total liabilities divided by the number of shares of our common stock outstanding. Our pro forma net tangible book value (deficit) as of June 30, 2015 was approximately ($1,388,463), or ($0.35) per share of common stock. Pro forma tangible book value per share represents our total tangible assets less total liabilities divided by the number of shares of common stock outstanding as of June 30, 2015 after giving effect to (i) the automatic conversion of all of the outstanding shares of our convertible preferred stock into an aggregate of 2,038,314 shares of our common stock upon the completion of this offering, including 448,220 shares of common stock to be issued as cumulative dividends accrued under such preferred stock and (ii) the conversion of approximately an aggregate of $9,838,058 in outstanding principal and accrued interest on our convertible promissory notes into shares of our common stock, upon the completion of this offering, at an average conversion price of $5.70 per share, assuming that the offering is completed on June 30, 2015. Pro forma as adjusted net tangible book value per share gives further effect to the issuance of 2,500,000 shares of our common stock at an assumed initial public offering price of $8.00 per share, the midpoint of the price range set forth on the cover page of this Offering Circular, and after deducting underwriting discounts and commissions and estimated offering expenses payable by us. Our pro forma, as
adjusted net tangible book value as of June 30, 2015 would have been $16,773,537, or $2.59 per share. This represents an immediate increase in pro forma net tangible book value of $2.94 per share to existing stockholders and an immediate dilution in pro forma net tangible book value of $5.41 per share to investors purchasing common stock in this offering.
The following table illustrates this per share dilution:
| Assumed initial public offering price per share | | | | | | $ | 8.00 | |
| Historical net tangible book value per share as of June 30, 2015 | | $ | (127.71 | ) | | | | |
| Increase attributable to the conversion of outstanding convertible preferred stock and convertible promissory notes | | | 127.36 | | | | | |
| Pro forma net tangible book value per share as of June 30, 2015 | | | (0.35 | ) | | | | |
| Increase in net tangible book value per share | | | 2.94 | | | | | |
| | | | | | | | | |
| Pro forma, as adjusted net tangible book value per share after this offering | | $ | 2.59 | | | | | |
| | | | | | | | | |
| Dilution per share to investors in this offering | | | | | | $ | 5.41 | |
The following table summarizes on an as adjusted basis as of June 30, 2015, the difference between the number of shares of common stock purchased from us, the total consideration paid and the average price per share paid by existing stockholders and by new investors, assuming an initial public offering price of $8.00 per share, the midpoint of the price range set forth on the cover page of this Offering Circular, and before deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us:
| | | Shares Purchased | | | Total Consideration (1) | | | Average
Price per | |
| | | Number | | | Percent | | | Amount | | | Percent | | | Share | |
| Existing stockholders | | | 3,965,396 | | | | 70.0 | % | | $ | 41,672,495 | | | | 67.6 | % | | $ | 10.51 | |
| New investors | | | 2,500,000 | | | | 30.0 | | | | 20,000,000 | | | | 32.4 | % | | | 8.00 | |
| | | | | | | | | | | | | | | | | | | | | |
| Total | | | 6,465,396 | | | | 100 | % | | $ | | | | | 100 | % | | $ | 9.54 | |
| (1) | A $1.00 increase (decrease) in the assumed initial public offering price of $8.00 per share, the midpoint of the price range set forth on the cover of this Offering Circular, would increase (decrease) the total consideration paid to us by new investors and total consideration paid to us by all stockholders by $2,362,500, assuming that the number of shares offered by us, as set forth on the cover page of this Offering Circular, remains the same and after deducting estimated placement agent fees and estimated offering expenses payable by us. An increase (decrease) of 1.0 million shares in the number of shares offered by us would increase (decrease) the total consideration paid |
to us by new investors and total consideration paid to us by all stockholders by $7,560,000, assuming the assumed initial public offering price of $8.00 per share remains the same and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us.
As of June 30, 2015, options to purchase 350,421 shares of common stock were outstanding at a weighted average exercise price of $1.47 per share and warrants to purchase 659,937 shares of common stock and preferred stock were outstanding at a weighted average exercise price of $1.47 per share. Assuming all of our outstanding options and warrants are exercised, new investors will own approximately 13.5% of our outstanding shares while contributing approximately 8.1% of the total amount paid to fund our company.
Underwriting
General
We and WR Hambrecht + Co, LLC (the underwriter) intend to enter into an underwriting agreement with respect to the shares being offered. Subject to certain conditions, the underwriter has agreed to use its best efforts to procure potential purchasers for the shares of our common stock offered hereby. This offering is being undertaken on a best efforts only basis. The underwriter is not required to take or pay for any specific number or dollar amount of our common stock.
The shares are being offered on an all or none basis. Investor funds will be deposited into an escrow account for the benefit of investors set up by American Stock Transfer and Trust Company (“AST”) at J.P. Morgan Chase Bank, N.A. The offering will not be completed unless we sell the number of shares specified on the cover page of this Offering Circular. All investor funds received prior to the closing will be deposited into the escrow account until closing. The escrow account will be opened on the date of this Offering Circular and will remain open until the closing date. All funds received into the escrow account after the pricing of the offering will be held in a non-interest bearing account in accordance with Rule 15c2-4 under the Exchange Act. The escrow account will not be opened until the date of this Offering Circular. All funds must be transmitted directly by wire to the specified bank account at J.P. Morgan Chase Bank, N.A. per the instructions disseminated at pricing by the underwriter. AST will not accept or handle any funds. On the closing date, the escrow agent will notify the underwriter whether the full amount necessary to purchase the shares to be sold in this offering has been received. If, on the closing date, investor funds are not received in respect of the full amount of shares to be sold in this offering, then all investor funds that were deposited into the escrow account will be returned promptly to investors, and the offering will terminate. The following table shows the per share and total underwriting discounts and commissions to be paid to the underwriter.
| | | Per
Share | | | Total | |
| Public offering price | | $ | | | | $ | | |
| Underwriting discounts and commissions payable by us(1) | | $ | | | | $ | | |
| Proceeds, before expenses, to us | | $ | | | | $ | | |
| (1) | The underwriting discounts and commissions do not include the financial advisory fees, reimbursable expenses, or underwriter’s warrants described below. |
Shares sold to the public will initially be offered at the initial public offering price set forth on the cover of this Offering Circular. Any shares sold to securities dealers may be sold at a discount of up to $ per share from the initial public offering price. After the initial offering of the shares, the offering price and the other selling terms may be subject to change. The offering of the shares is subject to receipt and acceptance and subject to the right to reject any order in whole or in part.
Engagement Agreement with the Underwriter
We are currently party to an engagement agreement with the underwriter. The term of the engagement agreement began on June 2, 2015 and will continue for one year, until June 2, 2016, unless one of the following events occurs prior to June 2, 2016, in which case the engagement agreement would be terminated early:
| (i) | we and the underwriter mutually agree to terminate the engagement agreement; |
| (ii) | either we or the underwriter unilaterally decide to terminate the engagement agreement upon thirty days’ prior written notice to the other party; |
| (iii) | we execute a definitive underwriting agreement with the underwriter; |
| (iv) | we terminate the engagement agreement due to the underwriter’s material failure to provide the services contemplated by the engagement agreement; or |
| (v) | we decide not to proceed with the offering or withdraw any offering statement filed with the Commission. |
Compensation for Advisory Services. As part of the engagement agreement, the underwriter agreed to provide us with financial advice and assistance concerning our business. As compensation for these advisory services, we agreed to pay the underwriter $5,000 per month during the term of the engagement agreement. If the engagement agreement were to stay in place for its full one-year term, we would pay the underwriter a total of $60,000 as compensation for its advisory services.
Offering Expenses. Under the terms of the engagement agreement, we will be responsible for all offering fees and expenses pursuant to the terms of the definitive underwriting agreement. These expenses will include, but are not limited to, the following:
| (i) | fees and disbursements of our legal counsel, accountants and other professionals we engage; |
| (ii) | fees and expenses incurred for producing offering documents, including printing costs; |
| (iv) | all legal fees related to the registration and qualification of the shares under state securities laws and FINRA clearance (such fees not to exceed $30,000); and |
| (v) | transportation, accommodation, and other road show expenses. |
To the extent any of our fees and expenses are paid by the underwriter with our approval, we will reimburse the underwriter for such amounts. This reimbursement obligation applies whether or not the offering closes.
Reimbursable Expenses. In the event the offering does not close or the engagement agreement is terminated for any reason other than because of the underwriter’s material failure to provide the services contemplated by the engagement agreement, we have agreed to reimburse the underwriter for all unreimbursed, reasonable, documented, out-of-pocket fees, expenses, and disbursements, including the underwriter’s legal fees, up to $25,000. However, the amount of reimbursable expenses to be paid to the underwriter will be reduced by the amount of financial advisory fees we have paid to the underwriter. For a description of the financial advisory fees, see “Compensation for Advisory Services” above.
Termination Fee. If we terminate the engagement agreement and then consummate a public offering in which WR Hambrecht + Co does not serve as the underwriter or placement agent within six months of such termination, then we have agreed to pay WR Hambrecht + Co a termination fee equal to $25,000. However, the termination fee will be reduced by the amount of reimbursable expenses and financial advisory fees we have paid to WR Hambrecht + Co. See “Reimbursable Expenses” and “Compensation for Advisory Services” above. The termination fee is not payable in the event we terminate the engagement agreement due to the underwriter’s material failure to provide the services contemplated by the engagement agreement.
Underwriting Commission. We have agreed that the definitive underwriting agreement will provide for us to pay a commission of 5.5% of the gross offering proceeds to the underwriter as compensation immediately upon consummation of the offering.
Underwriter’s Warrants. Upon the closing of this offering, we have also agreed to issue to the underwriter warrants to purchase a number of shares of our common stock equal to 4.5% of the total shares of our common stock offered in the final offering statement. The underwriter’s warrants are exercisable commencing on the qualification date of the offering statement related to this offering, and will be exercisable for five years. The underwriter’s warrants are not redeemable by us. The underwriter’s warrants also provide for “piggyback” registration rights with respect to the registration of the shares of common stock underlying the underwriter’s warrants. These piggyback registration rights commence 180 days after the qualification date of the offering statement and terminate on the second anniversary of such date.
The underwriter’s warrants and the shares of common stock underlying the underwriter’s warrants have been deemed compensation by the Financial Industry Regulatory Authority, or FINRA, and are therefore subject to a 180-day lock-up pursuant to Rule 5110(g)(1) of FINRA. The underwriter, or permitted assignees under such rule, may not sell, transfer, assign, pledge, or hypothecate the underwriter’s warrants or the shares of common stock underlying the underwriter’s warrants, nor will the underwriter, or permitted assignees engage in any hedging, short sale, derivative, put, or call transaction that would result in the effective economic disposition of the underwriter’s warrants or the underlying shares of common stock for a period of 180 days from the qualification date of the offering statement, except that they may be transferred, in whole or in part, by operation of law or by reason of our reorganization, or to any underwriter or selected dealer participating in the offering and their officers or partners if the underwriter’s warrants or the underlying shares of our common stock so transferred remain subject to the foregoing lock-up restrictions for the remainder of the time period. The underwriter’s warrants will provide for adjustment in the number and price of the underwriter’s warrants and the shares of common stock underlying such underwriter’s warrants in the event of recapitalization, merger, stock split, or other structural transaction, or a future financing undertaken by us.
Lock-Up Agreements
We and our officers, directors, and substantially all of our stockholders have agreed, or will agree, with the underwriter, subject to certain exceptions, that, without the prior written consent of the underwriter, we and they will not, directly or indirectly, during the period ending 90 days after the date of the Offering Circular:
| | · | offer, pledge, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant for the sale of, or otherwise dispose of or transfer any shares of the our common stock or any securities convertible into or exchangeable or exercisable for our common stock, whether now owned or |
hereafter acquired by the undersigned or with respect to which the undersigned has or hereafter acquires the power of disposition; or
| · | enter into any swap or any other agreement or any transaction that transfers, in whole or in part, the economic consequence of ownership of our common stock, whether any such swap or transaction is to be settled by delivery of our common stock or other securities, in cash or otherwise |
This agreement does not apply, in our case, to securities issued pursuant to existing employee benefit plans or securities issued upon exercise of options and other exceptions, and in the case of our officers, directors and other holders of our securities, exercise of stock options issued pursuant to a stock option or similar plans, and other exceptions.
Determination of Offering Price
Prior to the offering, there has been no public market for the shares. The initial public offering price will be determined by negotiation between us and the underwriter. The principal factors to be considered in determining the initial public offering price will include:
| | · | the information set forth in this Offering Circular and otherwise available to the underwriter; |
| | · | our history and prospects and the history of and prospects for the industry in which we compete; |
| | · | our past and present financial performance; |
| | · | our prospects for future earnings and the present state of our development; |
| | · | the general condition of the securities markets at the time of this offering; |
| | · | the recent market prices of, and demand for, publicly traded common stock of generally comparable companies; and |
| | · | other factors deemed relevant by the underwriter and us. |
Exchange Listing
We have applied with the NASDAQ Capital Market to list shares of our common under the symbol “ZLIG.” In order to meet one of the requirements for listing our common stock on NASDAQ, the underwriter intends to sell lots of 100 or more shares to a minimum of 300 beneficial holders.
Indemnification
We have agreed to indemnify the underwriter against certain liabilities, including liabilities under the Securities Act. The underwriter and its affiliates are engaged in various activities, which may include securities trading, commercial and investment banking, financial advisory, investment management, investment research, principal investment, hedging, financing and brokerage activities. The underwriter and its affiliates may in the future perform various financial advisory and investment banking services for us, for which they received or will receive customary fees and expenses.
In the ordinary course of their various business activities, the underwriter and its affiliates may make or hold a broad array of investments and actively trade debt and equity securities (or related derivative securities) and financial instruments (including bank loans) for their own account and for the accounts of their customers, and such investment and securities activities may involve securities and/or instruments of the issuer. The underwriter and its affiliates may also make investment recommendations and/or publish or express independent research views in respect of such securities or instruments, or recommend to clients that they acquire, long and/or short positions in such securities and instruments.
Use of Proceeds
We estimate that the net proceeds to us from the sale of 2,500,000 shares of common stock in this offering will be approximately $18,162,000 based upon an assumed initial public offering price of $8 per share, the midpoint of the price range set forth on the cover page of this Offering Circular after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us.
We anticipate that we will use approximately $700,000 of net proceeds from the offering to repay certain outstanding accounts payable, including trade payables, consulting and professional fees. We also intend to repay outstanding principal amounts and accrued interest owed under our demand notes (the “Demand Notes”) and a line of credit note (the “LOC Note”) issued to CrossCart LLC. The outstanding principal of each Demand Note carries an interest rate of 8% per annum, which may be increased to 10% if an event of default occurs. As of June 30, 2015, the total amount outstanding under the Demand Notes was $125,093,
including accrued interest. The LOC Note bears interest at a rate of 10% per annum, and all principal and accrued interest will be due and payable on May 1, 2016. The LOC Note contains customary events of default. If the Company fails to pay all amounts due by May 1, 2016, the interest on the remaining outstanding balance will accrue at a rate of 12% per annum. As of June 30, 2015, the total amount outstanding under the LOC Note was $61,627, including accrued interest.
We also intend to use approximately $300,000 of the net proceeds to pay certain compensation owed to our employees as a result of the reduction of their salaries, including approximately $210,000 of reduced compensation owed to our Chief Executive Officer and Chief Financial Officer. For a description of the salary reduction, see Executive and Director Compensation on page 65.
We anticipate that we will use the remaining net proceeds, approximately $16,975,280, from this offering to:
| | · | launch our commercialization plan for Z-Lig products in Europe, South Africa and other countries that accept our approved CE Mark, including the establishment of sales, marketing and distribution infrastructure, expansion of our processing and manufacturing capacity to a commercial scale and hiring of additional personnel; |
| | · | fund post-market registry studies of Z-Lig in Europe, South Africa and other select markets; |
| | · | conduct pre-clinical and R&D activities for product candidates; and |
| | · | initiate clinical trials for Z-Lig products in the U.S. |
We anticipate using the remaining amounts, if any, for general corporate purposes.
The expected use of net proceeds from this offering represents our intentions based upon our current plans and business conditions, which could change in the future as our plans and business conditions evolve. The amounts and timing of our actual expenditures may vary significantly depending on numerous factors, including the progress of our commercialization effort, the results from clinical trials and preclinical studies, the amount of cash available from other sources and any unforeseen cash needs. As a result, our management will retain broad discretion over the allocation of the net proceeds from this offering.
Dividend Policy
We have never declared or paid any dividends on our common stock. We currently intend to retain any future earnings to finance the growth and development of our business, and we do not anticipate that we will declare or pay any cash dividends on our common stock in the foreseeable future. Any future determination to pay cash dividends will be at the discretion of our Board of Directors, subject to applicable laws, and will be dependent upon our financial condition, results of operations, capital requirements, and other factors our Board of Directors deems relevant. Investors should not purchase our common stock with the expectation of receiving cash dividends.
Description of Business
Overview
Aperion Biologics, Inc. (“ABI”), headquartered in San Antonio, Texas, is a commercial-stage medical device company addressing the significant need for an alternative to human-based sources of tissues to be used in surgical procedures. Our lead product, the Z-Lig, is a patent-protected porcine tendon currently in limited commercial release for use initially as an anterior cruciate ligament (ACL) replacement in revision and multiligament knee joint procedures (see Figure 1). Z-Lig is the only known biological alternative to human tissue for ACL replacement. Since the Z-Lig is a biological implant that maintains a scaffold, it can become populated and remodeled with the patient’s own cells (similar to human-sourced tissue), referred to in the industry as “ligamentization”. This ligamentization is one of the keys to Z-Lig’s proven ability to be highly functional in patients for many years after implantation. Our propriety process is protected by an extensive portfolio of patents and patent applications, including 14 issued patents in the U.S. and internationally.
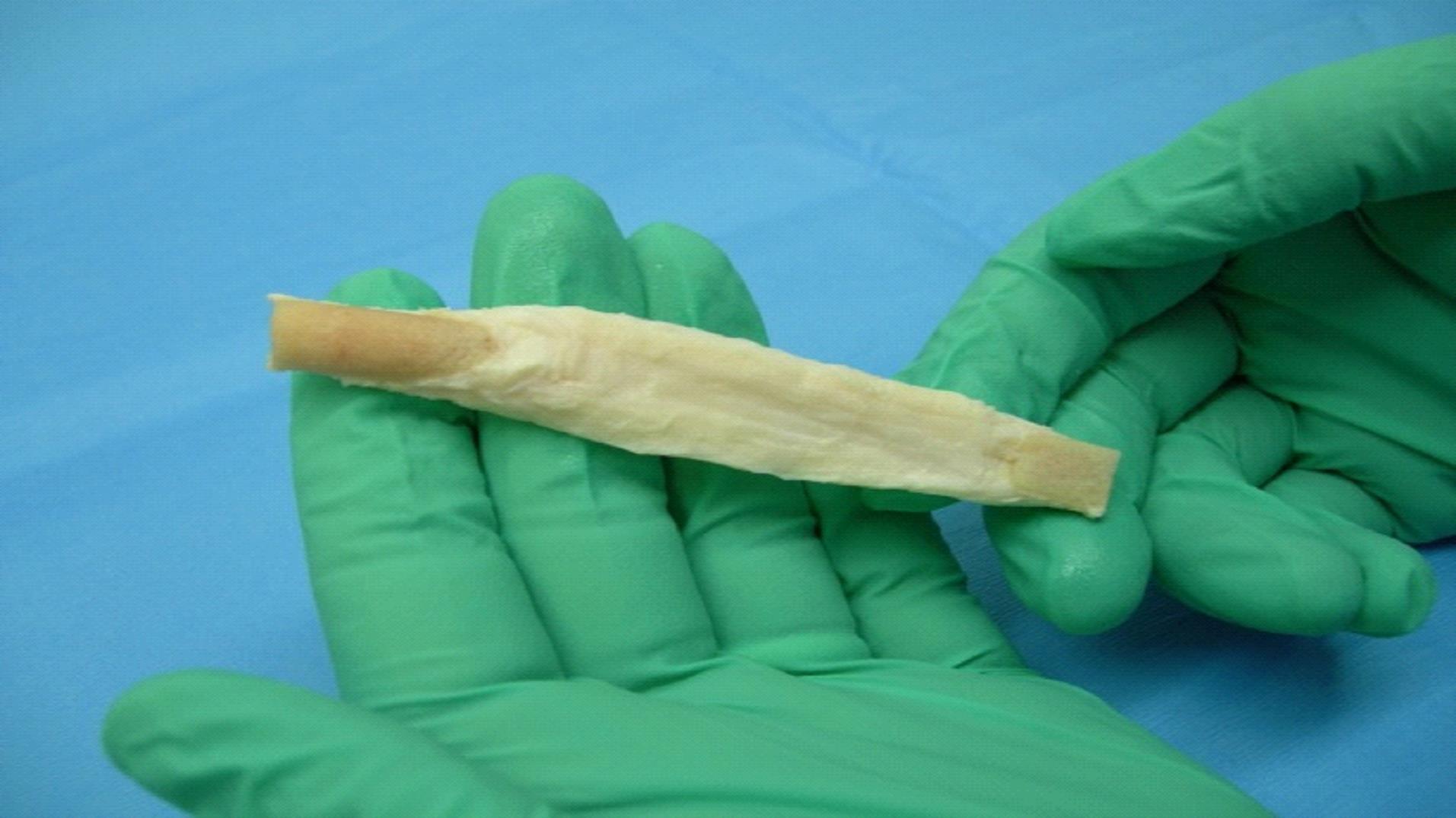 |  |
Figure 1: ABI’s Z-Lig ® (in product packaging on right) for use in ACL replacement surgery.
With the exception of synthetic ACL replacements, which are deemed inadequate by most orthopaedic surgeons, the only current ACL replacement choices (see Figure 2) include using tendon harvested from another part of the patient’s own body or tendon from a human cadaver (autograft and allograft, respectively). These existing choices have a number of shortcomings. As for autografts, the main clinical issue is the need for a second surgical site for harvesting. This results in longer operation times, increased patient pain and morbidity, longer rehabilitation time and reduction of function at the harvest site. Many clinicians believe autograft harvesting actually causes “harm” to patients by sacrificing one part of the human body to fix another.
As for allografts, the main clinical issue is the lack of supply of tissue of adequate quality. Allografts are harvested from cadaveric donors. The quality of allografts can be quite variable due to the age, sex, size, genetics, and medical condition of the donors and how the allograft tissue is processed. Because tissue donation is not commonplace, the supply of allograft tissue can be quite variable. Even in the U.S. with over an estimated 300,000 ACL replacements performed annually, less than 25% of these procedures were performed using allograft. The remaining patients must have the graft harvested from their own bodies.
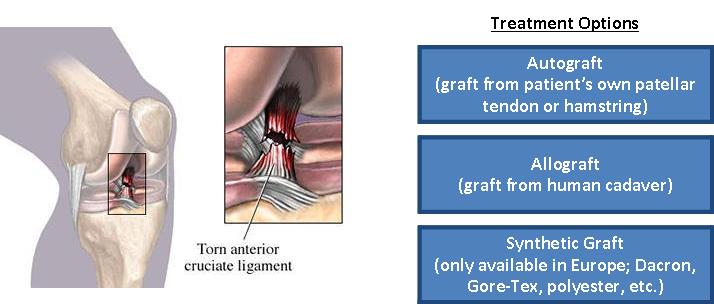
Figure 2: Torn ACLs can be replaced with human-based or synthetic grafts.
The need for an alternative to human-sourced grafts has been recognized in the medical community. The only non-human commercialized grafts are synthetics which tend to quickly wear, degenerate and fail. Synthetic grafts are not approved for use in the U.S. and we are not aware of any in clinical development in the U.S. In Europe, synthetic grafts have extremely limited commercial acceptance and are primarily used for patients who wish to return to sport in the shortest time period possible. We believe that the lack of durability of a synthetic graft can mainly be attributed to its inability to regenerate (as tissue-based implants do) after wear from use. The only synthetic graft in development in Europe is a resorbable polymer based scaffold graft currently in clinical safety trials and will require an additional clinical study prior to commercial availability.
Given the issues with synthetic grafts, we believe that animal tissue is the only viable alternative to human-sourced ACL grafts. No other company sells or has in human clinical development an animal-based (xenograft) ACL product.
The xenograft market is a well-accepted segment of the medical device industry. Xenografts from porcine, bovine and equine sources have been used as heart valves, extracellular matrices (patches), injectable collagen and other replacement/augmentation tissues for many years. Our products are different from currently commercialized xenografts in that they (i) act as biological scaffolds (unlike, for example, heart valves) and (ii) do not illicit an immunological reaction (unlike, for example, extracellular matrices and injectable collagen).
We were originally incorporated in the State of Delaware under the name “CrossCart, Inc.” CrossCart Inc. was established in 1996 to develop xenograft replacement tissues including cartilage, meniscus cartilage, soft tissue devices, and ligaments. Its efforts to do so were then focused on ligaments due to the ability to measure objectively the outcomes, the presence of an FDA ligament guidance document, and the large unmet needs. The Company developed the Z-Lig process to be flexibly applied to each of these tissue devices. Due to the limitations of the CrossCart name, the Company was renamed Aperion Biologics Inc. in 2008 and established a manufacturing facility in San Antonio.
CrossCart, Inc. is a separate and distinct entity from CrossCart LLC, which was formed in 2013 as an investment vehicle to purchase outstanding securities the Company held by a previous controlling stockholder. In addition, Dr. Kevin R. Stone, a member of our Board of Directors, is the controlling member and manager of CrossCart LLC.
Industry and Market Opportunities
We believe that regenerative medicine is one of the markets with the highest growth potential within the medical device industry. The potential size of the tissue engineering market totals $74 billion according to industry research. Based on industry reports and our studies, we estimate that the market expenditures for ACL autografts and allografts total over $2 billion worldwide, including $750 million in the U.S., $623 million in Europe and $625 million in the rest of the world. These figures include both the cost of allografts (approximately $2,500 per procedure based on our calculation) and the direct surgical costs of the autograft harvesting and subsequent direct incremental rehabilitation and medication costs related to the second surgical site (approximately $2,500 per procedure in total based on our calculation).
We believe that the global supply of quality allografts does not sufficiently meet the demand for knee ligament procedures. Furthermore, the demand is expected to be driven higher by the expanding aging and active population suffering from degenerative ailments. Over 250,000 ACL reconstruction surgeries are performed in Europe annually. The vast majority of such surgeries were performed using autografts. Throughout most of Europe, allografts are available on a very limited basis and the need for an alternative option is more acute.
We have also conducted a study to estimate of the size of the potential markets applicable to our earlier stage pipeline, including Z-Patch, Z-Fix, and Z-Meniscus. Based on this study, we believe that the potential market sizes for extracellular matrix that can be addressed by Z-Patch are $1.0 billion in U.S. and $1.2 billion in Europe; the potential market sizes for bone replacement that can be partially addressed by Z-Fix are $1.0 billion in U.S. and $1.1 billion in Europe; and the potential market sizes for meniscus replacement that can be addressed by Z-Meniscus are $150 million in U.S. and $150 million in Europe.
Current Treatment and Limitations
The major problems with the existing ACL replacement graft market are (i) autografts require a second surgical site which causes significant additional cost and morbidity; (ii) allografts are in short supply, are highly inconsistent in quality and can transmit human disease; (iii) synthetic grafts are unable to regenerate and therefore fail due to the continuous abrasion from regular use; and (iv) traditional xenografts have been plagued with rejection or lose their ability to be populated with the host’s cells.
Below are the specific issues within each category of graft:
Problems with Human-Sourced Grafts (Autografts and Allografts)
Autografts
As shown in Figure 3 below, during an autograft procedure, a second surgical site is chosen on the patient’s body and tendon (patellar tendon or hamstring) is harvested. Consequently, the patient suffers from additional pain, morbidity and scarring at the harvest site. The requirement for a second surgical site prolongs operating time and causes a more complex and extended recovery period. Most patients have impaired function, including muscle weakness and many even develop arthritis at the autograft harvest site. The direct surgical costs of the autograft harvesting and the subsequent direct incremental rehabilitation and medication costs are high and it is approximately $2,500 per procedure. In addition, patients’ own tendons available for harvesting are of limited supply and do not grow back. Given that many active patients reinjure their repaired ACL or injure their other ACL, autografts may not be a viable follow-on procedure.
Figure 3: Harvesting of autograft patellar tendon
Allografts
Supply of cadaver tissue remains extremely constrained and demand for high quality tissue significantly outstrips the supply. One allograft tendon is needed for each ACL replacement. Only half of the 25,000 cadavers available in the U.S. annually provide tissue meeting the specific requirements of orthopaedic tissue donation and, of those that meet the requirements, the yield is only about six harvested grafts suitable for ACL replacement per cadaver. Therefore, of the over 300,000 ACL replacements performed annually in the U.S., approximately 20-25% of these procedures were performed using allograft. Outside the U.S., the availability of allografts is even more limited on a country by country basis. Of these procedures performed with an allograft, we believe a significant portion were performed with a graft that would generally be considered by most surgeons to be less than ideal, typically because the graft was not: (i) the preferred type (bone-tendon-bone, bone-tendon or soft tissue); (ii) the preferred length; or (iii) from a young enough donor.
Allografts have been shown to have inconsistent characteristics due to the broad range of ages of the tissue donors. Published studies document the biomechanical properties of human allografts based on age (see Figure 4). Tensile testing has been performed on allografts from different aged donors. Tensile testing to failure from different allograft donors, as shown in the chart below, demonstrate that the effect of donor age on the strength of allografts can be significant. For example, an allograft from a 66-75 year old donor can only withstand (i) approximately 60% of the stress load that an 18-35 year old allograft can withstand or (ii) approximately 40% of the stress load that a Z-Lig can withstand. Surgeons and certain patients are aware of the importance of the age of the cadaver from which the allograft is derived and given the preference for young donors, the supply shortage of quality allografts is more pronounced.
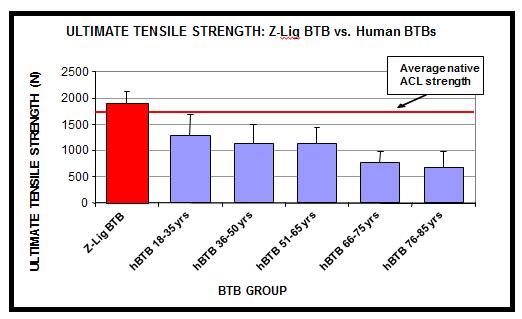
Figure 4:Tensile Strength of the Z-Lig BTB and BTB Allografts from Donors of Various Age Ranges
Allografts are produced in tissue banks which are regulated differently than medical devices by the FDA. Product from tissue banks is not subject to the FDA approval process and is therefore not subject to the same controls and regulations as is a medical device. Despite a range of improvements in industry monitoring and oversight made beginning predominantly in 2005, soft tissue allograft infections in patients continue to be reported and have resulted in recalls of allografts from several tissue banks. While allografts are generally safe, the risk of human disease transmission continues to exist.
In addition to the disadvantage discussed above, the availability of allograft is even more restricted in Europe and other international markets. These patients are often left with little to no choice but to use autografts because the allograft market in these markets is severely underdeveloped due to legal, cultural and religious issues. Allografts in some international markets are only available from hospital-based or regional tissue banks. The quality, processing and consistency of these grafts have even greater variability and thus limits allograft acceptance.
Problems with Non-Human Grafts (Synthetic Grafts and Traditional Xenografts)
Synthetic Grafts
Numerous attempts have been made to develop artificial ligament devices. Ligaments made from Dacron, carbon fiber, Gore-Tex and other chemicals/fibers have been tested by competitors. However, we believe that no synthetic device to date has possessed the necessary biological and biomechanical properties to achieve long-term success because they not have supported meaningful cellular remodeling by the human’s own cells. The only graft that has survived the test of time in the ACL indication are biologic grafts. Regardless of these limitations, a limited number of synthetic devices are available outside the U.S. but the acceptance of such devices by surgeons has been very low. Combination devices, which include a synthetic device augmented with a human allograft, have seen only limited technical success and they do not address most of the issues discussed above regarding human allograft sourcing and use.
Xenografts
Specific types of antibodies in humans, known as anti-gal antibodies, bind to the α-gal antigen
on animal-based grafts and initiate a rapid immune rejection process. The α-gal-mediated rejection may be prevented by: (i) removing or deactivating the antigens found on the xenograft or (ii) sourcing the xenograft from an animal that has been genetically modified to remove or eliminate α-gal antigens.
Deactivation of antigens involves crosslinking — a chemical manipulation of the xenograft to make it acceptable to the human body. However, too much crosslinking results in a xenograft that is incapable of acting as a biological scaffold and thereby incapable of becoming populated with the patient’s own cells (no ligamentization occurs). The ability of a xenograft to become remodeled and incorporated into the host has been shown to be vital to the long-term performance of the implant. Too little crosslinking results in the patient’s immune system attacking the xenograft and rejecting it either acutely or over the longer-term (see Figure 4 below). Historical attempts by competitors to use xenografts have ended mostly with failure. Companies have attempted to develop animal-based products for the ACL market using high concentrations of glutaraldehyde to attenuate recognition of antigens on the graft, previously successfully used to “fix” porcine heart valves, but were abandoned due to the frequent failure of subsequent implants.
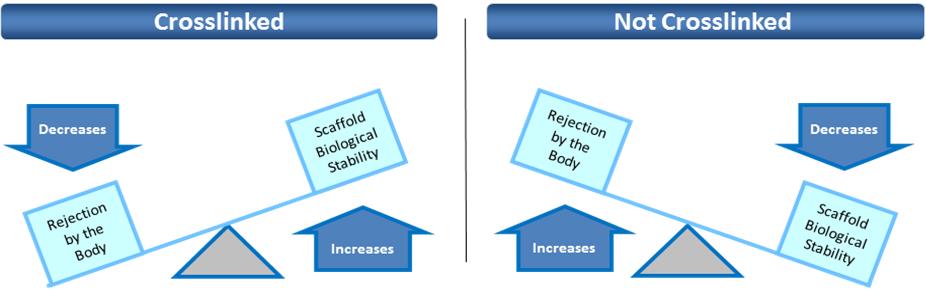
Figure 5: Impact Chemical Cross Linking on Biologic Properties
We believe that previous failure of knee-related xenograft was mainly due to local toxicity and synovitis caused by residuals of the chemical crosslinking agent leaching into the surrounding joint areas and not rapidly dissipating. Knee capsules, unlike heart valves and rotator cuffs, generally have very little turnover of surrounding fluid, and, as a result, residual crosslinking agent does not have the opportunity to meaningfully dissipate or be removed.
An alternative method to prevent α-gal-mediated rejection is by using genetically altered animals. Genetic alteration has been shown to be technically feasible in pigs. However, scaled-up production of such pigs has not occurred because genetically modified pigs have not been found to be completely void of the α-gal antigen nor reliably reproduce offspring free of the α-gal antigen. We believe the resultant production cost and regulatory hurdles will be very significant, are we are not aware of any company that is attempting to scale-up production of genetically modified pigs for commercial tissue use.
Our proprietary approach has been shown to solve both the rejection as well as the compatibility issues. Currently, we believe we are the only company that is pursuing an animal-derived ACL implant. See “Our Solutions and Advantages” section for additional details.
Our Solutions and Advantages
To solve the xenotransplant rejection problems mentioned above, we developed the Z-Process ®. The Z-Process is a proprietary process that immunochemically modifies animal tissue so that it is compatible with the human immune system (see Figure 6 below). The Z-Process addresses both α-gal and non-gal antigens on the xenograft. Our Z-Process also creates porcine tendons for safe use in humans while still maintaining biological scaffold activity of the implant. Using our Z-Process, we developed the Z-Lig device as an immunocompatible, porcine-derived ACL reconstruction alternative which provides a readily available, off-the-shelf solution that is strong, sterile, and reproducible. The process and resultant product is protected by our extensive portfolio of patents and patent applications. See “—Intellectual Property”
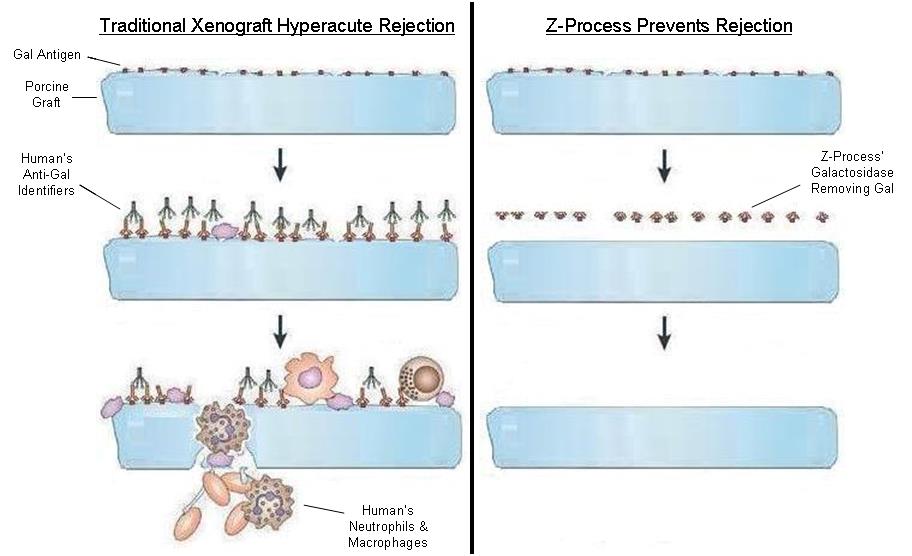
Figure 6: Z-Process Effect on Immune Response
We use α (alpha) -galactosidase enzyme to cleave the terminal galactose of the gal antigen so that the carbohydrate chain remaining on the animal tissue is the same in its structure as the carbohydrate chains present in humans. Importantly, our technology uses α-galactosidase enzyme to remove α-gal antigens from non-primate animal tissues, including ligaments, cartilage, bone, heart valves, vascular tissue, injectable collagen and other tissues. We are not aware of any competing technology that acts as a replacement for α-galactosidase for cleaving the gal antigen while still maintaining the tissue’s biological scaffold. Therefore, we believe that competitors are prevented from deactivating the α-gal antigen from specific animal tissues without either (i) infringing ABI’s patents or (ii) making the xenograft non-functional or inert and incapable of incorporating the host’s own cells.
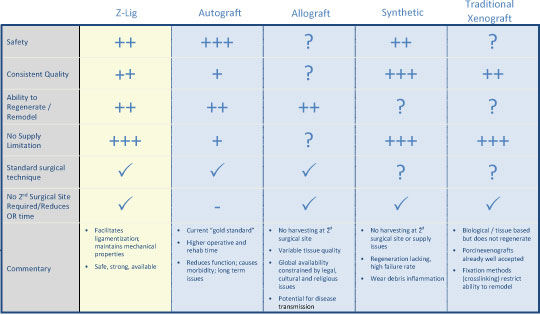
Figure 7:Advantages of the Z-Lig versus Other Graft Options
While the α-galactosidase enzyme treatment solves the α-gal rejection issue, it has no known effect on the non-gal immune response. To address the non-gal response, low level chemical crosslinking is utilized to control the response due to the non-gal antigens. This multi-step process was developed by our team and refined over many years to the final, highly specific and reproducible process used in the clinical trials. Our process patents cover the crosslinking treatment of tissues for non-gal antigens and subsequent sterilization using forms of e-beam irradiation.
Outside of autografts and allografts, Z-Lig represents a new and unique method in the industry in that it incorporates the host’s cells. When first implanted, Z-Lig contains no live cells. Z-Lig is gradually populated and remodeled by the patient’s own cells, eventually yielding a mature human ligament through the process of ligamentization. We believe that Z-Lig remodels at a similar rate as human allografts, with substantial ligamentization occurring within 6 to 12 months and complete remodeling within two to three years. This remodeling is key to Z-Lig’s long-term functionality.
In regenerative medicine much of the work with stem cells, growth factors and bioactive agents is also focused on the development of an ideal scaffold. Many different techniques have been employed to replicate the architecture and organization of normal tissue and organs, i.e. non-woven and woven materials, development of porous materials, 3-D printing, etc. All of these efforts may have shortcomings in terms of the architecture, mechanical properties and biological-materials interactions, among others. We believe scaffolds produced using the Z-Process may offer optimal properties in terms of the natural architecture, target mechanical properties, and inherent material properties of a biological substrate. The use of scaffolds produced using the Z-Process could represent a sizeable advance in the development of regenerative therapies.
Product Candidates and Pipeline
The Company’s product pipeline is summarized in Figure 8 below:
The Company currently has four product candidates in commercial, clinical and preclinical development and research stages. Below is the current status, clinical results and regulatory strategy for the product candidates in the portfolio:

Figure 8: Product Pipeline
Z-Lig ®Family - The Z-Lig family of devices are designed for use in a range of ligament reconstruction procedures, especially the initial application for the anterior cruciate ligament (ACL). The device is sourced from animal tissue but is sized and has the requisite biological and mechanical properties for use in these type procedures. A key advantage of this device is that little training is required for its use. The use of the device does not require the surgeon to change his surgical technique, his preferred fixation system, nor his rehabilitation protocols.
We received the CE Mark for the BT+ (bone-tendon) and BTB (bone-tendon-bone) versions of the Z-Lig Device Family for revision and multiligament ACL reconstruction (ACLR) procedures. We have developed a portfolio of data supportive of clinical safety and performance to enable the opportunity to market the device in the EU. We also have plans to provide additional clinical data required to expand our approved indication to the full ACL indication to cover all ACL procedures, including primary ACL surgeries. The Z-Lig has thus been evaluated to demonstrate its suitability as a biocompatible graft alternative to allograft and/or autograft through bench testing and extensive in vivo testing. We plan to expand indications as well as introduce a ST (soft tissue) version to complete the spectrum of ligament reconstruction devices for the Z-Lig Device Family.
Z-Patch - an extracellular matrix product used in soft tissue repair and augmentation procedures, such as hernia and Achilles tendon repair. Sourced from porcine dermis, this product has potential applications in orthopaedics, general, reconstructive, plastic and wound care indications. In the U.S., this product may be developed and approved through the 510(k) clearance route under the FDA process.
Z-Fix - a xenograft bone based product which is a biologic fixation device for ACL reconstruction and would be complementary to the Z-Lig device (all biologic ACL reconstruction). In the U.S., this product may be developed and approved through the 510(k) clearance route. Designs using bone could be expanded to include indications such as bone graft repair and spacer implants used in spine surgery.
Z-Meniscus - a xenograft meniscus device which can be used in the repair or reconstruction of meniscal injuries and defects. In the U.S. and markets outside the U.S., this device would require additional clinical data prior to regulatory approval and commercialization.
Our Current Status - Clinical Results with the Z-Lig
After extensive review of our regulatory dossier, we were granted the CE Mark for the Z-Lig device for multiligament and revision ACL procedures in April 2014. The dossier was an extensive summary of the science, preclinical, validation data and included the data collected from the European and South African multicenter study. We are also committed to plans for continued clinical and medical evaluation of this device family to insure the processing related issues have been addressed (post-market controls, reporting / vigilance, and long-term safety and performance). The additional data on 20 to 30 subjects at one year period will be submitted to support the indication expansion to the full ACL indication. In addition, we have committed to post-market safety evaluations and plans to continue Z-Lig Subject follow-up for an extended period of time (up to 60 month follow-up) for post-market surveillance purposes, which will generate the additional clinical data for market expansion opportunities both in the existing markets covered by the CE Mark and in the U.S.
In 2012, we initiated our multicenter, prospective, controlled (allograft), randomized and blinded clinical study in Europe and South Africa and subjects enrolled in the trial have surpassed the 36 month long-term follow-up period. This study enrolled sixty-six subjects (n=66). All time points to complete the endpoint analysis for Primary Performance and Efficacy have been achieved, therefore the study continues for the purpose of collecting additional post-market safety data. Generally, the failure of ACL grafts occurs within the early post-implantation window (3 months after the operation), when the ligamentization process has initiated and graft strength is most vulnerable. Our assessment with the Primary Performance measure (primary endpoint for CE Marking) supported the conclusion of non-inferiority of Z-Lig versus allograft, with 100% success in all subjects at 6 months follow-up in the PP population. Assessment of clinically relevant success outcomes such as patient activity, patient satisfaction, stability, functional and effusion resulted in compelling data showing comparable performance to the allograft control.
Surgeons using the device (our study Principal Investigators) have reported consistent, comparable or superior quality of the Z-Lig graft materials in comparison to allograft controls. Surgeons have reported that the Z-Lig graft has a superior bone plug material, is easily used and managed during surgery, and requires no change to their standard surgical procedure.
Within the initial 12 months, bacterial infections associated with the graft were reported in 6 subjects implanted with Z-Lig in this study. We conducted a proactive and extensive safety investigation which concluded that the infections were caused by contamination of incoming tissue and processing deficiencies. Excepting these cases of bacterial infection, it was concluded that the reported complications are consistent with those found in the medical and scientific literature and were unrelated to the graft. We have implemented a robust corrective action plan to address the sources of bacterial contamination, and these validation activities
have been completed to verify that the corrective actions were sufficient and effective and that sterility can be assured. Our corrective action plan and validation data were reviewed by the Notified Body for CE Marking and they required no additional action by us. We plan to execute additional post-market study to confirm the effectiveness of the corrective actions.
U.S. Human Clinical Results
Z-Lig is currently under development in the U.S. under an Investigational Device Exemption (IDE) with the FDA. Z-Lig’s development trials follow the FDA guidance document on Intra-Articular Knee Ligament Reconstruction Devices. The safety testing modules conducted for CE Mark approval can also support FDA approval of Z-Lig as well as 510(k) approvals of follow-on products.
The results of the U.S. safety pilot study demonstrated that the Z-Lig was safe (see Figure 7 below). Data from the human clinical study demonstrated the long-term safety and feasibility of the Z-Lig device. The study included 10 subjects followed for 2 years under the protocol. This group represented a highly active subject population, including subjects with high pre-injury Tegner activity levels (competitive athletes with Tegner ratings 8-10), subjects with clinically significant comorbidities, and subjects undergoing revision ACL surgeries. These are all known risk factors to outcomes following ACL surgery. The device performed as intended during the surgical procedure and post-surgically through progressive weight bearing and return to normal activities. The grafts were well-tolerated without observation of a deleterious immunogenic response. In 4 of the 10 subjects, non-device related adverse events were experienced either due to vigorous sports activities (e.g., sports injury, rupture) or events related to the initial procedure (e.g., fixation, procedure error). No systemic diseases or secondary infections resulted from the implantation of the device and there was no acute immune response or long-term rejection response exhibited by any of the subjects. The 12-month results of the pilot study described below were submitted to the FDA, leading to unconditional acceptance by the FDA of a pivotal clinical trial protocol. As assessed by the operating surgeon and two non-affiliated orthopaedic knee specialists, five of six evaluable subjects presented with functional grafts at the 24-month post-operative time point and satisfied all success criteria, including effusion, knee laxity (KT 1000), pivot shift, Lachmans and Anterior Drawer tests. Assessment by MRI also showed significant remodeling and maturation of the functioning grafts. One of six evaluable patients that presented without a functional graft had a tibial bone fixation that loosened.
Figure 9: Five Year Outcomes from U.S. Safety Pilot Study
The above figure (Figure 9) describes the outcomes of subjects five years postsurgery. Recently, we completed 12-year follow-up on the five evaluable patients with functional grafts. Safety of the devices continued to be exhibited throughout the 12-year interval, and all evaluable patients continued to demonstrate graft function with knee stability. The nature and rate of complications and adverse events seen during this feasibility study were similar to those cited in the literature. In April 2014, we received unconditional approval from FDA to move forward with its pivotal efficacy trial.
The pivotal efficacy trial will involve 326 patients at up to 10 clinical sites. The study will be controlled, randomized and evaluator-blinded. Half of the patients will receive Z-Lig while the other half will receive an allograft control. The study’s primary endpoint is non-inferiority of function of Z-Lig (relative to allograft) at 12 months and 24 months post-implant. We plan to continue discussions with the FDA regarding ways to optimize the study through randomization schemes, the number of clinical sites and discussions surrounding the data gathered during the European trial.
The body of data in support of the safety and performance of the Z-Lig Device Family is thus consistent and extensive, with multiple studies concluding that the Z-Lig Device Family performs in accordance with design criteria and has demonstrated adequate safety and performance in support of its intended use. Studies for long-term efficacy analysis have been completed (reported herein) and post-market surveillance studies are in effect for ongoing assessment.
Preclinical Studies
Prior to the clinical experience, we conducted controlled studies in 20 Rhesus monkeys to evaluate Z-Lig’s safety and performance prior to use in humans. Our studies showed that knee joints of monkeys implanted with a Z-Lig displayed normal motion and laxity within a few weeks post implantation. This normal motion and laxity was maintained for the entire one-year period of the study. Serological studies of these monkeys showed a transient production of non-gal
antibodies that was deemed to have substantially decreased at approximately nine months post-implantation due to the gradual replacement of the pig tissue and matrix by the recipient’s own cells and tissue. Biomechanical analysis of the implants after one year in monkeys demonstrated characteristics similar to those of the control (monkey allograft) and a significant return to normal leg function (as also seen in the allograft-implanted monkey). Histological studies of the implants one year post-implantation revealed effective replacement of the porcine tissue components with those of the recipient, demonstrating that the device served as a scaffold that supported gradual remodeling with the monkey’s own cells. In addition to these animal studies, we also successfully completed initial safety and performance studies, including cytotoxicity, acute systemic toxicity, pyrogenicity, residual risk assessment, viral safety, viral inactivation, tensile strength and dynamic abrasion.
Our Marketing & Distribution Strategy
Markets and Methods of Distribution
The orthopaedic surgery marketplace is fragmented with many clinicians focused on certain types of surgical procedures, such as trauma, total joints, spine, sports medicine. Our marketing efforts will be focused on developing awareness of the Z-Lig to stakeholders treating sports or activity related injuries. The scope of our marketing and distribution efforts includes the patients who desire their specific expertise and suppliers who service their needs. The identification of this market segment and the key opinion leaders enable a more efficient, focused sales and marketing effort. Additionally since the Z-Lig device does not require changes to surgical technique and thus avoids additional costs for training, marketing activities are focused on educating on the science, technology and the previous clinical experiences.
The following describes our plans to initiate marketing and distribution activities as an independent organization through a distribution model. With the CE Mark approval, we intend to introduce the Z-Lig ACLR device through a focused, phased market introduction (see Figure 10). We believe the initial market need for the Z-Lig are revision and multiligament cases where a graft option is typically required to achieve the desired clinical result. Initially we intend to target a select number of markets where human or synthetic graft options are accepted, in particular those nations whose sites participated in our European and South African (EUSA) clinical trials, which includes Italy, South Africa, Poland, Spain, UK, and the Benelux countries (Netherlands, Belgium, Luxemburg). We will utilize indirect distribution channels who have met our selection criteria (i.e. existing/complementary product lines, market share, financial, willing to invest in the product line, among others). In these select markets, we will introduce the product through a targeted approach, clinicians chosen by our investigative surgeons and/or our distribution partners. Supporting the efforts of our indirect distribution channel will be direct personnel specifically tasked with sales management and clinical marketing specialists for training and technical/scientific field education. The next distribution phase will expand our distribution reach to additional European and Asian markets that accept the CE Mark, with a specific focus on high-volume clinical centers or those specializing in knee injuries. The third and final phase would include pan-European expansion into markets in the EU and continued global introduction in markets outside the United States who accept the CE Mark and potential distribution into non-European nations whose regulatory bodies require additional regulatory approval.
The following describes the main elements our proposed structured rollout in each region:
| | · | Identify Targets in Each Country |
| | · | Adapt Brand Messaging and Marketing Communication |
| | o | Marketing Materials adapted for local markets |
| | o | Product Training material: develop Sales and Surgeon training (product and technology) presentations. |
| | o | Sales Operations: Ordering and shipping processes |
| | · | Seed and Expansion stage |
| | § | Sales training meetings in each market with distributors |
| | § | Distributors identify 4-10 “launch” clinicians |
| | • | Tradeshows - national/in-country - European Society of Sports Traumatology, Knee Surgery, & Arthroscopy (ESSKA), International Society of Arthroscopy, Knee Surgery and Orthopaedic Sports Medicine (ISAKOS), European Federation of National Associations of Orthopaedic Sports Traumatology (EFOST), and others. |
| | • | “Surgeons selling surgeons” - Company sponsored symposia |
| | • | Post Sale Monitoring and Partnering |

Figure 10: Z-Lig Phased Launch Plan
Phase 1 (launch to 12-18 months)
Distribution efforts will be in 6 to 10 markets which have accepted options for ACL reconstruction (allograft and synthetic), have reimbursement pathways for graft alternatives, and/or are countries where we have conducted our clinical evaluations. Some of these initial markets may be characterized as:
| | · | Major markets which utilize graft options such as allograft, xenograft or synthetic; clinicians who are receptive to the use of alternatives. |
| | · | Markets with minimal coding restraints. |
| | · | Markets where we have conducted our clinical evaluations to take advantage of our clinical successes and leverage our study clinicians and key opinion leaders (KOLs) to introduce and penetrate the local markets. |
| | · | Collaboration with our distributors or clinical investigators; initial distribution in each market will focus on a select number of thought leader clinicians to effect an orderly launch of our product and gain clinical experience and feedback, verify current sizing and surgical techniques, and help insure success and traction in the marketplace. |
| | · | This limited release will enable us to gather first hand data, establish successes and help fine tune training and marketing activities. |
| | · | In some markets release will take the form of a registry to establish a clinical database for the local market and obtain product performance feedback. The data will also be used for broader marketing and reimbursement activities in the future. |
| | · | Presence at key international medical events with exhibit supplemented with symposia or presentations. |
Phase 2 (12-18 months to 24-36 months)
Efforts will focus on expanding our customer base in existing countries and extend our distribution to additional European markets with a specific focus on high-volume clinical centers or those specializing in knee injuries. This phase will also involve markets outside of Europe that accept the CE Mark (i.e. Canada, Turkey, Saudi Arabia, Australia and Korea). Working with these centers may require implementation of additional “mini-studies” for clinical acceptance and reimbursement. Continue our presence at international meetings and assist our distributors at national events. Continue to add and train distributors is expansion markets.
Phase 3 (24 months and beyond)
Efforts will continue in the expansion of our customer base in markets with established distribution. In addition to pan-European expansion in the EU markets, this phase will also involve continued introduction in markets outside the United States who accept the CE Mark. These non-European markets would include those whose regulatory bodies require additional regulatory submissions and approvals. We would also make efforts to develop access, advocacy and adoption in the non-European markets.
Our Distributors
We require that each indirect distributor purchase stock of our products and store grafts and provide local distribution, billing, and collection for its designated markets. Initially distributors will be directed to identify a select group of qualified clinicians to become users and potential key opinion leaders of the Z-Lig within their territory.
Choosing the right distribution partners as we move into the various international markets is key. Some of the issues that have to be explored and expectations established with any potential business partner before distribution can begin are:
| | · | A current presence in the geographic region in the orthopedic, sports medicine or sports traumatology market with existing products |
| | · | The experience, ability, or the desire to handle a regenerative medicine/tissue-based product (store, monitor, distribute, invoice, collect, etc) |
| | · | Presence in the target market including accounts and relationships with clinicians |
| | · | Presence at local, regional, and national logical meetings |
| | · | A willingness to invest in training their sales force and customers, and a willingness to invest in marketing, training and promotional activities that will drive distribution and acceptance of their products |
| | · | A willingness to invest in activities needed to establish products in their markets, including those related to establishing reimbursement. Local knowledge of regulatory barriers and time to market conditions are valuable. |
| | · | Distributors with adequate financial resources |
Quality of the product, safety, efficacy, and price are important, but the value of surgeon relationships and customer service are equally important factors for success.
Our Management, Direct Sales and Marketing Team
As we continue to advance our commercialization efforts and expand our operations, we expect to hire additional management members to execute our strategies. These additional hires may include senior management positions overseeing our business development and regulatory affairs. We also intend to hire additional personnel to enhance our financial reporting, accounting and auditing capabilities, with the goal of ensuring that we comply with any ongoing reporting requirements under the rules and regulations of the SEC. We expect to increase our headcount by about 10 to 15 persons in the next year following the offering.
We will initially use an indirect distribution model. We intend to increase our headcounts by hiring additional sales and marketing employees, including a Vice President of European, Middle East and Africa Distribution Management responsible for establishing our distribution network, initiating and coordinating our distribution activities within the distribution network, and driving distribution objectives; and Clinical Marketing Specialist(s) who will be focused on both surgeon and sales representative education on the science and technology surrounding the Z-Process, Z-Lig product, and our company.
Marketing Approach
The introduction of an innovative technology, especially one for ligament reconstruction, will meet an unmet market need but will also encounter the challenges of clinical acceptance. Our efforts to execute a Level I clinical trial with KOLs in Europe represent a significant step towards gaining scientific and clinical credibility. The study design includes post-marketing surveillance out to 5 years as a means of providing continual product performance feedback. We will focus on peer-to-peer marketing activities as a rapid and effective means of establishing credibility and market acceptance of our new technology.
Direct marketing efforts at logical meetings, symposiums and others are highly effective, especially those with clinician-to-clinician interactions. For example, we have conducted lunchtime symposiums or have had invited lectures at the ISAKOS (International Society of Arthroscopy, Knee Surgery and Orthopaedic Sports Medicine) and ESSKA ( European Society for Sports Traumatology, Knee Surgery and Arthroscopy) meetings to introduce surgeons to the Z-Lig in a peer-to-peer environment. We plan to repeat this successful format at additional international meetings. Independent surgeon training symposia with KOLs will be encouraged/supported. Exhibiting at major international meetings is planned and supporting local distributors to exhibit at in-country meetings will be encouraged. Marketing efforts will also be expected by the local distribution channels to augment company efforts.
Use of Initial Market Feedback
In some markets, post launch market feedback will be collected from clinicians who have had the opportunity to use the product. Data will be used to confirm safety and performance expectations as well as provide information for product enhancements, market reception and education or training opportunities. We anticipate surveys to be sent to approximately 20-24 clinicians who have used the product in a minimum of 6 cases and can provide follow up assessments 6 and 12 months post-operative. These surveys will be conducted with surgeons who have been targeted in our launch activities.
The Advantage of Clinical Registries
In addition to the ongoing multicenter trial (post-market surveillance), performance of smaller, local market studies as registries will be encouraged. These additional studies have been proposed to supplement our CE Mark approval and may be needed to encourage clinical Z-Lig use in new markets (those outside of trial countries). These studies can explore issues such as cost (reimbursement), performance versus autograft, overall operative time savings, and other advantages.
Competition
As has been mentioned elsewhere in the business section, the principal competition for our Z-Lig device arises from other types of ACL graft materials: allograft (from cadaveric tissue) and synthetic (polymers that are designed to replicate the physical, strength characteristics of native human ligaments. We do not believe that there are other xenograft (from other species) or biologic ligaments either in the market or under development by the established orthopaedic companies. In some global markets, synthetic devices such as those produced by LARS, Xiros (Neoligaments) and Telos have limited clinical use due to their less than satisfactory clinical history. Currently, the only synthetic device under clinical testing that we are aware of is a resorbable polymer-based scaffold that is being developed by Soft Tissue Regeneration.
Intellectual Property
Our product candidates and Z-Process technology are protected by an extensive intellectual property portfolio. As of June 30, 2015, we held 23 issued patents in the U.S. and internationally, consisting of 17 issued patents in the U.S. and 6 issued patents in Europe and Japan. In addition, we currently hold 3 patent applications pending in the U.S. and Europe. These issued patents will expire between 2015 and 2024. In September 2015, nine (9) issued patents in the U.S. have expired, and these patents covered the following claims: 5 patents relates to ligament xenografts and 4 patents relate to articular cartilage xenografts. Consequently, as of September 30, 2015, we held a total of 14 issued patents, consisted of 8 issued patents in the U.S. and 6 issued patents in Europe and Japan. In addition, one U.S. patent will expire in February 2016, which relates to meniscus xenograft. We do not expect that the expiration of the nine (9) patents in September 2015 and the patent expiring in February 2016 will have any significant impact on our marketing and commercialization plans for Z-Lig based on the CE Mark in Europe, because these expiring patents are all U.S. patents, and our patent protection in Europe remains fully effective and valid. In addition, we believe that the expiration of these U.S. patents will not have a material negative impact on our business operations in the U.S. as we continue to develop our product candidates and conduct clinical trials in the U.S.. Three (3) of the remaining eight (8) U.S. patents cover the Z-Lig device, and the other five (5) cover other tissue types to allow protection for other product candidates. We believe these the remaining eight (8) U.S. patents will provide us with sufficient protection to develop and market Z-Lig and other product candidates in U.S.
In general, our patent portfolio includes claims relating to treatment and application of specific tissues for a variety of medical and surgical uses. The intellectual property portfolio includes combined patents of process and material composition that support deantigenation, sterilization and viral inactivation of a wide range of biological tissues. ABI has exclusive rights to the only known method for humanizing non-primate animal grafts for replacement of ligaments and bones in humans without making the graft biologically inert.
In January 2009, we entered into a licensing agreement with the Curators of University of Missouri pursuant to which we obtained an exclusive right and license to use five U.S. Patents covering an alpha-galactosidase. The license was obtained to ensure a viable source of alpha-galactosidase to use the Z-Process. Under this license agreement, we are required to pay royalty ranging from 1.5% to 1.9% on net sales less than $10 million and 1.2% to 1.6% on net sales greater than $10 million. In addition, we are required to pay an annual minimum royalty payment of $35,000. All payments under this agreement will be terminated if the aggregate amount paid under the agreement (including both sale royalties and minimum royalty payments) equals to or exceeds $3,500,000.
Manufacturing
Z-Lig raw materials are sourced from suppliers that meet ABI’s strict quality and performance requirements and are compliant with ISO 22442 standards. Through simple, predictable ramp-up of the breeding and processing of the pigs and supply chain redundancies, we believe we can readily meet all of the expected market demand. Accordingly, we have not entered into any formal supply agreements with our suppliers to provide a guaranteed volume of raw materials. In addition, by utilizing varying ages and strains of pig, we established a flexible manufacturing process that permits us to adjust the size of our Z-Lig products to accommodate the demand of customers.
Government Regulation
Our products are medical devices that are subject to extensive regulation by government authorities in the European Union (the “EU”), the United States and in other countries and jurisdictions, including South Africa, and non-EU European countries. These governmental authorities regulate the marketing and distribution of medical devices in their respective geographies. The regulations cover the entire life cycle of the product, including the research, development, testing, manufacture, quality control, packaging, storage, labeling, advertising and
promotion of the devices. In addition, post-approval monitoring and reporting, as well as import and export of medical devices, are subject to regulatory requirements.
Review and approval of medical devices in the EU
The EU has a coordinated system for the authorization of medical devices. The EU Medical Devices Directive (Council Directive 93/42/EEC, as amended) sets out the basic regulatory framework for medical devices in the European Union. In the EU our medical devices must comply with the Essential Requirements in Annex I to the EU Medical Devices Directive, which we refer to as the Essential Requirements. Compliance with these requirements is a prerequisite to be able to affix the Certificate of Conformity mark, or CE Mark, to our medical devices, without which they cannot be marketed or sold in the European Economic Area, or EEA. To demonstrate compliance with the Essential Requirements we must undergo a conformity assessment procedure, which varies according to the type of medical device and its classification. Except for low risk medical devices (Class I with no measuring function and which are not sterile), where the manufacturer can issue a CE Declaration of Conformity based on a self-assessment of the conformity of its products with the Essential Requirements, a conformity assessment procedure requires the intervention of a third-party organization designated by competent authorities of an EU country to conduct conformity assessments, which is referred to as a Notified Body. The Notified Body would typically audit and examine products' technical file and the quality system for the manufacture, design and final inspection of the devices before issuing a CE Certificate of Conformity demonstrating compliance with the relevant Essential Requirements. To date, we have used the CE Marking process to satisfy the conformity standards required to market and sell our Z-Lig products in the EU.
With respect to implantable devices or devices classified as Class III in the EU, the manufacturer must conduct clinical studies to obtain the required clinical data, unless reliance on existing clinical data from similar devices can be justified. As part of the conformity assessment process, depending on the type of devices, the Notified Body will review the manufacturer's clinical evaluation process, assess the clinical evaluation data of a representative sample of the devices' subcategory or generic group, or assess all the clinical evaluation data, verify the manufacturer's assessment of that data and assess the validity of the clinical evaluation report and the conclusions drawn by the manufacturer. To date, we have conducted clinical studies in Europe and South Africa to obtain clinical data as part of the clinical evaluation process.
Even after we receive a CE Certificate of Conformity enabling us to affix the CE Mark on a product and to sell our product in the EEA countries, a Notified Body or a competent authority may require post-marketing studies of our product. Failure to comply with such requirements in a timely manner could result in the withdrawal of our CE Certificate of Conformity and the recall or withdrawal of our product from the market in the EU, which would prevent us from generating revenue from sales of that product in the EEA. Moreover, each CE Certificate of Conformity is valid for a maximum of five years, but more commonly three years. Our current CE Certificates of Conformity are valid through 2019 for our Z-Lig products. At the end of each period of validity we are required to apply to the Notified Body for a renewal of the CE Certificate of Conformity. There may be delays in the renewal of the CE Certificate of Conformity or the Notified Body may require modifications to our products or to the related Technical Files before it agrees to issue the new CE Certificate of Conformity.
In addition, we must inform the Notified Body that carried out the conformity assessment of the medical devices we market or sell in the EEA of any planned substantial changes to our devices
that could affect compliance with the Essential Requirements or the devices' intended purpose. The Notified Body will then assess the changes and verify whether they affect the products' conformity with the Essential Requirements or the conditions for the use of the devices. If the assessment is favorable, the Notified Body will issue a new CE Certificate of Conformity or an addendum to the existing CE Certificate of Conformity attesting compliance with the Essential Requirements. If it is not, we may not be able to continue to market and sell the product in the EEA.
On September 26, 2012, the European Commission adopted a package of legislative proposals designed to replace the existing regulatory framework for medical devices in the EU. These proposals provide for a revision of the current regulatory framework for medical devices in the EU to strengthen patient safety, transparency and product traceability. The proposals, for instance, include reinforced rules governing clinical evaluation throughout the life of the device, improved traceability of devices in the supply chain, including a phased and risk-based introduction of unique device identification, or UDI, improved market surveillance and vigilance, as well as better co-ordination between national regulators, increased powers for Notified Bodies to undertake unannounced inspections and strengthened supervision of Notified Bodies by member states. The European Commission's proposals may undergo significant amendments as they are reviewed by the European Council and European Parliament as part of the EU legislative process. If and when adopted, the proposed new legislation may prevent or delay the EU approval or clearance of our products under development or may impact our ability to modify our currently EU approved or cleared products on a timely basis and impose additional costs relating to clinical evaluation, vigilance and product traceability.
Marketing and sales considerations in the EU
In the EU, medical devices may be promoted only for the intended purpose for which the devices have been CE Marked. Failure to comply with this requirement could lead to the imposition of penalties by the competent authorities of the EU Member States. The penalties could include warnings, orders to discontinue the promotion of the medical device, seizure of the promotional materials and fines. Promotional materials must also comply with various laws and codes of conduct developed by medical device industry bodies in the EU governing promotional claims, comparative advertising, advertising of medical devices reimbursed by the national health insurance systems and advertising to the general public.
Post-approval monitoring in the EU
Additionally, all manufacturers placing medical devices into the market in the EU are legally bound to report any serious or potentially serious incidents involving devices they produce or sell to the competent authority in whose jurisdiction the incident occurred. In the EU, manufacturers must comply with the EU Medical Device Vigilance System. Under this system, incidents must be reported to the relevant authorities of the EU countries, and manufacturers are required to take field safety corrective actions to reduce a risk of death or serious deterioration in the state of health associated with the use of a medical device that is already placed on the market.
Third-party reimbursement
In the United States and most other major joint implant markets, third-party payors, including government health programs, commercial health insurers and managed care organizations, reimburse hospitals and other medical facilities an aggregate amount for all elements of a joint
replacement procedure, including operating room time, patient care and the joint replacement product. As a result, our products generally are not reimbursed separately, but instead are subject to the limits imposed by third-party payors on the coverage and reimbursement of procedures that utilize our products.
Sales of our products will depend, in part, on the extent to which the costs of such procedures involving the use of our products cleared by the EU and other jurisdictions will be covered by third-party payors, including government health programs in the United States, such as Medicare and Medicaid, commercial health insurers and managed care organizations. The process for determining whether a payor will provide coverage for a particular procedure may be separate from the process for setting the price or reimbursement rate that the payor will pay for the procedure once coverage is approved. Third party payors may limit coverage to particular procedures on an approved list, or formulary, which might not include all of the approved procedures involving the use of our products for a particular indication.
In the EU, pricing and reimbursement schemes vary widely from country to country. In many foreign markets, pricing of medical devices is subject to governmental control. In the United States, there have been, and we expect that there will continue to be, a number of federal and state proposals to limit payments by governmental payors for medical devices, and the procedures in which medical devices are used. While we cannot predict whether such legislative or regulatory proposals will be adopted, the adoption of such proposals could have a material adverse effect on our business, financial condition and profitability.
Review, approval and clearance of medical devices in the United States
Medical devices in the United States are strictly regulated by the FDA. Unless an exemption applies, a medical device may not be marketed in the United States unless it has been cleared through filing of a 510(k) premarket notification, or 510(k), or approved by the FDA pursuant to a premarket approval application, or PMA. The information that must be submitted to the FDA in order to obtain clearance or approval to market a new medical device varies depending on how the medical device is classified by the FDA. Medical devices are classified into one of three classes depending on the level of control necessary to assure the safety and effectiveness of the device. Class I devices have the lowest level or risk associated with them, and are subject to general controls, including labeling, premarket notification and adherence to the Quality System Regulations (“QSRs”). Class II devices are subject to general controls and special controls, including performance standards. Class III devices, which have the highest level of risk associated with them, are subject to most of the aforementioned requirements as well as to premarket approval. Most Class I devices and some Class II devices are exempt from the 510(k) requirement, although manufacturers of these devices are still subject to registration, listing, labeling and QSR requirements.
The PMA process is more complex, costly and time consuming than the 510(k) clearance procedure. A PMA must be supported by extensive data, including technical, preclinical, clinical, manufacturing, control and labeling information, that demonstrate the safety and effectiveness of the device for its intended use. After a PMA is submitted, the FDA has 45 days to determine whether it is sufficiently complete to permit a substantive review. If the PMA is complete, the FDA will file the PMA. The FDA is subject to performance goal review times for PMAs and may issue a decision letter as a first action on a PMA.
In evaluating a 510(k), the FDA will determine whether the device has the same intended use as the predicate device, and (a) has the same technological characteristics as the predicate
device, or (b) has different technological characteristics, and (1) the data supporting substantial equivalence contains information, including appropriate clinical or scientific data, if deemed necessary by the FDA, that demonstrates that the device is as safe and as effective as a legally marketed device, and (2) does not raise different questions of safety and effectiveness than the predicate device. Most 510(k)s do not require clinical data for clearance, but the FDA may request such data. The FDA seeks to review and act on a 510(k) within 90 days of submission, but it may take longer if the agency finds that it requires more information to review the 510(k).
Our Z-Lig products are classified as Class III medical devices. We intend to obtain regulatory approval of our Z-Lig products by filing a PMA with the FDA. The PMA process for the Z-Lig may be subject to additional clinical evaluation under an Investigational Device Exemption (IDE) to collect more human clinical data prior to filing a PMA. We intend to rely on the 510(k) premarket notification process to obtain FDA clearance for our product candidates which may pursue this regulatory pathway.
Investigational device exemption
A clinical trial to support safety and effectiveness of the device is typically required for a PMA and, in a small percentage of cases, the FDA may require a clinical study in support of a 510(k) submission. A manufacturer that wishes to conduct a clinical study involving the device is subject to the FDA's Investigational Device Exemption, or IDE, regulation. The IDE regulation distinguishes between significant and nonsignificant risk device studies and the procedures for obtaining approval to begin the study differ accordingly. Also, some types of studies are exempt from the IDE regulations. A significant risk device presents a potential for serious risk to the health, safety, or welfare of a subject. Significant risk devices are devices that are substantially important in diagnosing, curing, mitigating or treating disease or in preventing impairment to human health. Studies of devices that pose a significant risk require both FDA approval and an approval of an independent institutional review Board, or IRB, prior to initiation of a clinical study. Nonsignificant risk devices are devices that do not pose a significant risk to the human subjects. A nonsignificant risk device study requires only IRB approval prior to initiation of a clinical study.
An IDE application is considered approved 30 days after it has been received by the FDA, unless the FDA otherwise informs the sponsor prior to 30 calendar days from the date of receipt, that the IDE is granted (“approved”), granted with conditions, or disapproved. The clinical trial must be conducted in accordance with applicable regulations, including but not limited to the FDA's IDE regulations and current good clinical practices. A clinical trial may be suspended by the FDA, the IRB or the sponsor at any time for various reasons, including a belief that the risks to the study participants outweigh the benefits of participation in the trial.
Post-marketing restrictions and enforcement
After a device is placed on the market, numerous regulatory requirements apply. These include: compliance with the QSR and other compliance requirements; labeling regulations, which prohibit the promotion of products for uncleared or unapproved or "off-label" uses and impose other restrictions on labeling; and medical device reporting obligations, which require that manufacturers investigate and report to the FDA adverse events, including deaths, or serious injuries that may have been or were caused by a medical device and malfunctions in the device that would likely cause or contribute to a death or serious injury if it were to recur.
Facilities
We lease a 4,000 square foot facility in San Antonio, Texas, under a lease agreement that was recently renewed and will expire on December 31, 2018, at our option. The facility has a 1,000-square foot Class 8 clean room area which contains four 100-square foot modular clean rooms of Class 6 to Class 7 certification. We believe that we have adequate capacity to support Z-Lig and other production requirements for clinical trials through commercial launch in Europe and the U.S. The remainder of ABI’s facility is used for product development, distribution and general and administrative purposes.
Legal Proceedings
We may from time to time be involved in various claims and legal proceedings of a nature we believe are normal and incidental to a medical device business. These matters may include product liability, intellectual property, employment, and other general claims. We accrue for contingent liabilities when it is probable that a liability has been incurred and the amount can be reasonably estimated. We are not presently a party to any legal proceedings that, in the opinion of our management, are likely to have a material adverse effect on our business. Regardless of outcome, litigation can have an adverse impact on us because of defense and settlement costs, diversion of management resources and other factors.
Management’s Discussion and Analysis of
Financial Condition and Results of Operations
You should read the following discussion and analysis of our financial condition and results of our operations together with our financial statements and related notes appearing at the end of this Offering Circular. This discussion contains forward-looking statements reflecting our current expectations that involve risks and uncertainties. Actual results and the timing of events may differ materially from those contained in these forward-looking statements due to a number of factors, including those discussed in the section entitled "Risk Factors" and elsewhere in this Offering Circular.
Aperion Biologics, Inc. (“we” or the “Company”) is a commercial-stage medical device company addressing the significant unmet need for an alternative to human-based sources of tissues to be used in surgical procedures. Our core technology platform is an enzymatic stripping of the key carbohydrate antigens followed by a unique conversion process that both “humanizes” and sterilizes animal tissues without unduly affecting their biomechanical or biological properties. The Company’s lead product, the Z-Lig, is a patent-protected porcine tendon currently in limited commercial release for use initially as an anterior cruciate ligament (ACL) replacement in revision and multiligament knee joint procedures. Z-Lig is the only known biological alternative to human tissue for ACL replacement. Since the Z-Lig is a biological implant that maintains a scaffold, it can become populated and remodeled with the patient’s own cells (similar to human-sourced tissue), referred to in the industry as “ligamentization.” This ligamentization is one of the keys to Z-Lig’s proven ability to be highly functional in patients for many years after implantation. Our propriety process is protected by an extensive portfolio of patents and patent applications, including 23 issued patents in the U.S. and internationally.
The Company’s primary activities since its inception have been the development and implementation of its business plans, performing research on its platform technology, conducting clinical trials on its products in both animals and humans, pursuing regulatory approvals to market its product in U.S. and international markets, and raising capital. In April 2014 the Company received the CE Mark to allow commercial sales of the Z-Lig in foreign markets that accept the CE Mark (primarily Europe). The first commercial sale took place in February 2015.
The Company was founded in October 1996 under the name CrossCart, Inc. The Company changed its name to Aperion Biologics, Inc. (a Delaware corporation) in May 2009. To date, the Company has funded its business primarily with the proceeds from private placements of equity and debt securities.
Results of Operations
The Nine Months ended June 30, 2015 as compared to June 30, 2014
As mentioned above, the Company commenced its first commercial sale of Z-Lig in February 2015 and recognized $27,000 of sales during the nine months ended June 30, 2015. There were no sales during the nine months ended June 30, 2014.
Costs related to clinical studies decreased by $38,833 from $165,896 for the nine months ended June 30, 2014 to $127,063 for the nine months ended June 30, 2015. The decrease was primarily driven by lower expenses associated with the Company’s clinical trials outside of the United States.
Research and development expenses decreased by $16,429 from $56,345 for the nine months ended June 30, 2014 to $39,916 for the nine months ended June 30, 2015, primarily due to completion of an R&D consulting contract which reduced expenses $15,174 between the periods.
Sales, general and administrative expenses increased by $19,421 from $1,099,724 for the nine months ended June 30, 2014 to $1,119,145 for the nine months ended June 30, 2015. Sales and marketing activities related to the launch of the Z-Lig product increased $103,451. In addition, consulting activities related to fundraising resulted in an increase of $100,734 for the period. Offsetting these increases were a decrease of $55,262 in salary and personnel costs in operations, a decrease of $48,567 in legal fees and a decrease of $84,469 and in regulatory expenses due to the completion of the CE Mark application process.
Other expenses increased for the period primarily as a result of higher interest expense related to the Company’s borrowings to support its activities, as interest expense increased $91,564 between the periods. This increase was driven by the increase in the total amount of average interest bearing debt from $6,400,014 for the nine months ended June 30, 2014 to $7,956,327 for the nine months ended June 30, 2015.
Fiscal Year ended September 30, 2014 as compared to September 30, 2013
Operating expenses decreased by $1,537,299 from $3,389,580 in fiscal year 2013 to $1,852,281 in fiscal year 2014. The decrease was primarily due to the completion of the Company’s clinical trials outside of the U.S. and related follow-up examinations, data gathering and compilation activities and reduced salary and personnel expenses.
Research and development expenses decreased by $358,599 from $417,844 in fiscal year 2013 to $59,245 in fiscal year 2014, primarily due to a reduction of salary and personnel expenses and reduced use of consultants.
Sales, general and administrative expenses decreased by $604,906 from $2,101,434 in fiscal year 2013 to $1,496,528 in fiscal year 2014. The decrease was primarily attributed to a $226,098 reduction in spending in our Quality Assurance department related to the completion of significant activities relating to our CE Mark application and the adverse event investigation in our clinical trials, a $133,404 decrease in salary and personnel expenses in the Sales department, and a $178,832 decrease in salary and personnel expenses in our Operations Department as a result of the completion of process validation production, which was offset by an increase of $57,411 in fees related to fundraising activities and an increase of $62,499 in fees to Board members due to increasing the size of the Board of Directors.
Other expenses increased for the period primarily as a result of higher interest expense related to the Company’s borrowings to support its activities, as interest expense increased $234,931 between the periods. This increase was driven by the increase in the total amount of average interest bearing debt from $4,537,000 for fiscal year 2013 to $6,600,014 for fiscal year 2014.
Critical Accounting Policies and Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America ("U.S. GAAP") requires our management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates. The Company's significant estimates and assumptions include the fair value of the Company's stock, stock-based compensation, estimated useful lives of depreciable assets, and the valuation allowance relating to the Company's deferred tax assets.
Contingencies
Certain conditions may exist as of the date the financial statements are issued, which may result in a loss to the Company, but which will only be resolved when one or more future events occur or fail to occur. The Company's management and its legal counsel assess such contingent liabilities, and such assessment inherently involves an exercise of judgment. In assessing loss contingencies related to legal proceedings that are pending against the Company or unasserted claims that may result in such proceedings, the Company's legal counsel evaluates the perceived merits of any legal proceedings or unasserted claims, as well as the perceived merits of the amount of relief sought or expected to be sought therein.
If the assessment of a contingency indicates it is probable that a material loss has been incurred and the amount of the liability can be estimated, then the estimated liability would be accrued in the Company's financial statements. If the assessment indicates a potentially material loss contingency is not probable, but is reasonably possible, or is probable, but cannot be estimated, then the nature of the contingent liability, together with an estimate of the range of possible loss, if determinable and material, would be disclosed.
Loss contingencies considered remote are generally not disclosed unless they involve guarantees, in which case the guarantees would be disclosed.
Stock-Based Payments
Stock-based compensation expense for all stock-based payment awards is based on the estimated fair value of the award. For employees and directors, the award is measured on the grant date. For non-employees, the award is measured on the grant date and is then remeasured at each vesting date and financial reporting date. The Company recognizes the estimated fair value of the award as compensation cost over the requisite service period of the award, which is generally the option vesting term. The Company generally issues new shares of common stock to satisfy option and warrant exercises.
Fair Value Measurement
The Company measures the fair value of financial assets and liabilities based on the guidance of Accounting Standard Codification 820 "Fair Value Measurements and Disclosures" ("ASC 820") which defines fair value, establishes a framework for measuring fair value, and expands disclosures about fair value measurements.
ASC 820 defines fair value as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. ASC 820 also establishes a fair value hierarchy, which requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value. ASC 820 describes three levels of inputs that may be used to measure fair value:
Level 1 — quoted prices in active markets for identical assets or liabilities
Level 2 — quoted prices for similar assets and liabilities in active markets or inputs that are observable
Level 3 — inputs that are unobservable (for example, cash flow modeling inputs based on assumptions)
The fair value of the Company's cash and accounts payable approximates the carrying amounts of such instruments due to their short maturity. The fair value of the convertible promissory notes approximates the carrying amount because the rate and terms currently available to the Company approximate the rate and terms on the existing debt.
Convertible Instruments
U.S. GAAP requires companies to bifurcate conversion options from their host instruments and account for them as free standing derivative financial instruments according to certain criteria. The criteria include circumstances in which (a) the economic characteristics and risks of the embedded derivative instrument are not clearly and closely related to the economic characteristics and risks of the host contract, (b) the hybrid instrument that embodies both the embedded derivative instrument and the host contract is not re-measured at fair value under otherwise applicable generally accepted accounting principles with changes in fair value reported in earnings as they occur and (c) a separate instrument with the same terms as the embedded derivative instrument would be considered a derivative instrument. An exception to this rule is when the host instrument is deemed to be conventional as that term is described under applicable U.S. GAAP.
When the Company has determined that the embedded conversion options should not be bifurcated from their host instruments, the Company records, when necessary, discounts to convertible notes for the intrinsic value of conversion options embedded in debt instruments based upon the differences between the fair value of the underlying common stock at the commitment date of the note transaction and the effective conversion price embedded in the note. Debt discounts under these arrangements are amortized over the term of the related debt to their stated date of redemption. The Company also records, when necessary, deemed dividends for the intrinsic value of conversion options embedded in preferred shares based upon the differences between the fair value of the underlying common stock at the commitment date of the transaction and the effective conversion price embedded in the preferred shares.
Preferred Stock
The Company applies the guidance enumerated in theDistinguishing Liabilities from Equity Topic of the Accounting Standards Codification (ASC 480) when determining the classification and measurement of preferred stock. Preferred shares subject to mandatory redemption (if any) are classified as liability instruments and are measured at fair value. The Company classifies conditionally redeemable preferred shares, which includes preferred shares that feature redemption rights that are either within the control of the holder or subject to redemption upon the occurrence of uncertain events not solely within the Company's control, as temporary equity. At all other times, the Company classifies its preferred shares in stockholders' deficiency.
Stock Warrants
The Company classifies as equity any contracts that (i) require physical settlement or net-share settlement or (ii) provides the Company with a choice of net-cash settlement or settlement in its own shares (physical settlement or net-share settlement) provided that such contracts are indexed to the Company's own stock. The Company classifies as assets or liabilities any contracts that (i) require net-cash settlement (including a requirement to net cash settle the contract if an event occurs and if that event is outside the Company's control), or (ii) gives the counterparty a choice of net-cash settlement or settlement in shares (physical settlement or net-share settlement). The Company assesses classification of its stock warrants at each reporting date to determine whether a change in classification between assets and liabilities is required. The warrants to purchase the Company’s preferred and common stock were issued in connection with certain of its convertible promissory notes and preferred stock. The Company evaluated the common stock warrants to assess their proper classification using the applicable criteria enumerated under U.S. GAAP and determined that the common stock warrants meet the criteria for equity classification in the balance sheet as of September 30, 2014 and 2013. The Company evaluated the Series C and Series C2 preferred stock warrants to assess their proper classification using the applicable criteria enumerated under U.S. GAAP and determined that the Series C and Series C2 preferred stock warrants meet the criteria for liability classification, as they are exercisable into a redeemable security, as of September 30, 2014 and 2013. These warrants are marked to market at each reporting period. The Company has determined that the value of such liabilities are de minimis.
Recently Issued Accounting Pronouncements
In June 2014, the FASB issued ASU No. 2014-10, "Development Stage Entities (Topic 915): Elimination of Certain Financial Reporting Requirements, Including an Amendment to Variable Interest Entities Guidance in Topic 810, Consolidation," ("ASU 2014-10"). ASU 2014-10 removes the definition of a development stage entity from the Master Glossary of the ASC, thereby removing the financial reporting distinction between development stage entities and other reporting entities from U.S. GAAP. In addition, ASU 2014-10 eliminates the requirements for development stage entities to (1) present inception-to-date information in the statements of operations, cash flows, and stockholders' equity, (2) label the financial statements as those of a development stage entity, (3) disclose a description of the development stage activities in which the entity is engaged, and (4) disclose in the first year in which the entity is no longer a development stage entity that in prior years it had been in the development stage. ASU 2014-10 is effective for annual reporting periods beginning after December 15, 2014, and interim periods therein. Early adoption is permitted. The Company adopted ASU 2014-10 during the year ended September 30, 2014 which resulted in the removal of previously required development stage disclosures. The Company's activities are subject to significant risks and uncertainties, which are detailed in Note 2 in the financial statements for the fiscal years ended September 30, 2014 and 13.
In June 2014, the FASB issued ASU No. 2014-12, "Compensation - Stock Compensation (Topic 718): Accounting for Share-Based Payments When the Terms of an Award Provide that a Performance Target Could be Achieved after the Requisite Service Period," ("ASU 2014-12"). The amendments in ASU 2014-12 require that a performance target that affects vesting and that could be achieved after the requisite service period be treated as a performance condition. A reporting entity should apply existing guidance in ASC Topic No. 718, "Compensation - Stock Compensation" as it relates to awards with performance conditions that affect vesting to account for such awards. The amendments in ASU 2014-12 are effective for annual periods and interim periods within those annual periods beginning after December 15, 2015. Early adoption is permitted. Entities may apply the amendments in ASU 2014-12 either: (a) prospectively to all awards granted or modified after the effective date; or (b) retrospectively to all awards with performance targets that are outstanding as of the beginning of the earliest annual period presented in the financial statements and to all new or modified awards thereafter. The Company does not anticipate that the adoption of ASU 2014-12 will have a material impact on its financial statements.
In August 2014, the FASB issued ASU No. 2014-1, "Presentation of Financial Statements - Going Concern (Subtopic 205-40): Disclosure of Uncertainties about an Entity's Ability to Continue as a Going Concern" ("ASU 2014-15"). ASU 2014-15, which is effective for annual reporting periods ending after December 15, 2016, extends the responsibility for performing the going-concern assessment to management and contains guidance on how to perform a going-concern assessment and when going-concern disclosures would be required under U.S. GAAP. The Company does not anticipate that the adoption of ASU 2014-12 will have a material impact on its financial statements. Management's evaluations regarding the events and conditions that raise substantial doubt regarding the Company's ability to continue as a going concern have been disclosed in Note 2 in the financial statements for the fiscal years ended September 30, 2014 and 13.
JOBS Act
On April 5, 2012, the Jumpstart Our Business Startups Act of 2012, or the JOBS Act, was enacted. Section 107 of the JOBS Act provides that an “emerging growth company” can take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act for complying with new or revised accounting standards. In other words, an “emerging growth company” can delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. We have irrevocably elected not to avail ourselves of this extended transition period and, as a result, we will adopt new or revised accounting standards on the relevant dates on which adoption of such standards is required for other public companies.
We are in the process of evaluating the benefits of relying on other exemptions and reduced reporting requirements provided by the JOBS Act. Subject to certain conditions set forth in the JOBS Act, as an “emerging growth company,” we intend to rely on certain of these exemptions, including without limitation, (i) providing an auditor’s attestation report on our system of internal controls over financial reporting pursuant to Section 404(b) of the Sarbanes-Oxley Act and (ii) complying with any requirement that may be adopted by the Public Company Accounting Oversight Board regarding mandatory audit firm rotation or a supplement to the auditor’s report providing additional information about the audit and the financial statements, known as the auditor discussion and analysis. We will remain an “emerging growth company” until the earliest of (a) the last day of the fiscal year in which we have total annual gross revenues of $1 billion or more, (b) the last day of our fiscal year following the fifth anniversary of the date of the completion of this offering, (c) the date on which we have issued more than $1 billion in non-convertible debt during the previous three years or (d) the date on which we are deemed to be a large accelerated filer under the rules of the Commission.
Liquidity and Capital Resources
We had cash of $124,769 at September 30, 2014 and $258,564 at June 30, 2015, sourced primarily from the proceeds received in private placements of convertible promissory notes.
As of September 30, 2014, the Company had working capital deficit and a stockholders’ deficiency of $12,132,432 and $25,990,881, respectively. During the year ended September 30, 2014, the Company incurred a net loss attributable to common stockholders of $3,291,838. The Company has not generated any material revenues and has incurred net losses since inception. Our recurring operating losses and our need for additional sources of capital to fund our ongoing operations raise substantial doubt about our ability to continue as a going concern. As a result, our independent registered public accounting firm included an explanatory paragraph in its report on our financial statements as of and for the year ended September 30, 2014 with respect to this uncertainty.
Since August 2013, the Company has relied primarily on the issuance of convertible promissory notes to fund its continuing operations. Most of this funding has been provided by CrossCart, LLC, which is the Company’s largest shareholder and controlled by Dr. Kevin R. Stone, who is the Chairman of the Board of Directors of the Company. As of June 30, 2015 the Company had borrowed $6,825,615 from CrossCart, LLC and other related parties. As of June 30, 2015, we had a total of $8,156,027 in outstanding principal amount of convertible promissory notes, the majority of which, or $6,669,000 in outstanding principal amount, have matured and are due and payable, and we have not been able to make payments due to lack of funding. Pursuant to the terms of the notes, the interest rates on these notes were increased from 8% to 10% per annum as a result of the event of default. The Company has reflected the increased interest rates in its income statement and balance sheets for the periods presented in this Offering Circular. In October 2015, we entered into an agreement with each noteholder, including CrossCart, pursuant to which (i) the noteholders waived any default resulting from such non-payment of notes and this waiver will expire on March 31, 2016 and (ii) all outstanding amounts due under the convertible promissory notes will be converted into shares of our common stock immediately following the completion of the offering at the initial conversion price.
We believe that the net proceeds from this offering of approximately $18,162,000 will be sufficient to fund our current operations through the end of calendar year 2017, and thereafter we may need additional capital to continue our operations. We do not expect to be able to satisfy our cash requirements solely through sales of the Z-Lig in the near future, therefore we expect to rely on equity and debt financing to fund our operations. The sale of additional equity securities could result in additional dilution to our stockholders and those securities may have rights senior to those of our common stock. The incurrence of indebtedness would result in increased debt service obligations and could result in operating and financing covenants that would restrict our operations.
We cannot assure that we will have sufficient capital to finance our growth and business operations or that such capital will be available on terms that are favorable to us or at all. We are currently incurring operating deficits that are expected to continue for the foreseeable future, and as we begin to prosecute our marketing plan, we expect our operating deficit will continue to grow initially until we begin to generate a sufficient level of revenue from our commercialization efforts.
Plan of Operations
Since the receipt of the CE Mark in April 2014, the Company has developed a detailed marketing plan that will initially focus on distribution efforts of its Z-Lig products in 6 to 10 markets that have accepted options for ACL reconstruction (allograft and synthetic), have reimbursement pathways for graft alternatives, and/or are countries where we have conducted our clinical trials. We intend to use an indirect distribution model and focus on peer-to-peer marketing activities as a rapid and effective means of establishing credibility and market acceptance of our new technology. We intend to increase our headcount by hiring additional sales and marketing employees, including a Vice President of EMEA Distribution Management responsible for establishing our distribution network, initiating and coordinating our distribution
activities within the distribution network, and driving distribution objectives. We also intend to hire Clinical Marketing Specialist(s) who will be focused on both surgeon and sales representative education on the science and technology surrounding the Z-Process, Z-Lig product, and our Company. In later years the Company intend to expand into other countries to broaden the reach of Z-Lig.
The Company recognizes that following the completion of this offering, it will need to raise additional capital in order to meet its obligations and execute its business plan within the next two years. If the Company is unable to raise sufficient additional funds through this offering, it will be required to develop and implement an alternative plan to further extend payables, reduce overhead or scale back its business plan until sufficient additional capital is raised to support further operations. There can be no assurance that such a plan will be successful.
Off-Balance Sheet Arrangements
We did not have during the periods presented, and we do not currently have, any off-balance sheet arrangements, as defined in the rules and regulations of the SEC.
Quantitative and Qualitative Disclosures about Market Risks
We are exposed to market risks in the ordinary course of our business. Our cash and cash equivalents include cash in readily available checking and money market accounts. These investments are not dependent on interest rate fluctuations that may cause the principal amount of these investments to fluctuate, although we do not expect such fluctuation will have a material impact on our financial conditions. In addition, as we continue our commercialization efforts internationally, we may generate revenue and incur expenses denominated in currencies other than the U.S. dollar, a majority of which we expect to be denominated in Euros. As a result, our revenue may be significantly impacted by fluctuations in foreign currency exchange rates. Our international selling, marketing and administrative costs related to these sales are largely denominated in the same foreign currencies, which mitigates our foreign currency exchange risk exposure.
Management
The following table sets forth information regarding our executive officers, directors and significant employees, including their ages as of June 30, 2015:
Directors, Executive Officers and Key Employees
The following table sets forth information regarding our directors, executive officers and key employees as of June 30, 2015:
| Name | | Age | | | | Position |
| Directors and Executive Officers | | | | | | |
| Daniel R. Lee | | 54 | | | | Chief Executive Officer and President |
| David Cocke | | 50 | | | | Chief Financial Officer |
| David W. Anderson(1)(2) | | 62 | | | | Director |
| France Dixon Helfer(2) | | 62 | | | | Director |
| Alfred G. Holcomb(1) | | 63 | | | | Director |
| Kevin R. Stone, M.D. | | 60 | | | | Chairman and Director |
| Mike Ward(1) | | 44 | | | | Director |
| | | | | | | |
| Significant Employee | | | | | | |
| Lance Johnson | | 49 | | | | Vice President Quality |
| (1) | Member of audit committee. |
| (2) | Member of Compensation Committee. |
Executive Officers
Daniel R. Lee. Mr. Lee has been serving as our Chief Executive Officer since August 2008. Prior to joining us, from July 2006 to August 2008, Mr. Lee was Director of Marketing at Smith & Nephew Endoscopy, a sports medicine technology company, responsible for its TRUREPAIR business unit. From November 2003 to July 2006, Mr. Lee was Director of Marketing at OsteoBiologics, Inc., a medical supply company providing off-the-shelf bio-absorbable implant for articular cartilage, which was acquired by Smith & Nephew Endoscopy in 2006. Prior to joining OsteoBiologics, Inc., Mr. Lee was the Director of Marketing for Regeneration Technologies, Inc (“RTI”), a leading allograft tissue processor for orthopaedic surgical applications, from 1999 to 2003. Prior to joining RTI, he was the Director of the Sports Medicine Research and Development group at Surgical Dynamics, a subsidiary of U.S. Surgical Corporation. He is a member of the Society for Biomaterials and the American Association of
Tissue Banks where he holds a Certified Tissue Bank Specialist certification. He currently holds thirteen (13) patents on implants and instruments used in orthopaedic and general surgery. Mr. Lee holds an M.S. in Biomedical Engineering from the University of Alabama at Birmingham and a B.A. in Materials Science and Engineering from the Johns Hopkins University. We believe Mr. Lee’s extensive executive, business and marketing experiences in managing biotechnology companies and his knowledge and expertise in the field of biologics qualifies him to serve as a member of our board of directors. In addition, Mr. Lee’s day-to-day management and intimate knowledge of our business and operations provide our board with an in-depth understanding of the Company.
David Cocke. Mr. Cocke has been serving as our Chief Financial Officer since September 2008. Since 1997, Mr. Cocke has been serving as a General Manager of NuPak Medical, Ltd., an ISO 13485-certified contract manufacturing medical device company. He was responsible for all aspects of management, including sales, finance and operations. Prior to that, from November 1993 to May 1996, Mr. Cocke was Chief Financial Officer of NuTech, Inc., a technology incubator subsidiary for KCI, a leader in tissue-based products for surgical procedures and wound healing. From 1991 to 1993, he was Director at the Corporate Development department at KCI. Prior to KCI, David was employed by GE Capital in its Corporate Finance Group and Salomon Brothers Inc in its Investment Banking Group. Mr. Cocke holds an MBA from the University of Virginia’s Darden Graduate School of Business Administration and a BBA with High Honors from the University of Texas at Austin.
Directors
David W. Anderson. Mr. Anderson has been our director since February 2012. Since September 2014, Mr. Anderson has been serving as the Chief Executive Officer of Orteq Ltd., an orthopedic medical device and regenerative medicine company focused on sports medicine. He also serves as Chairman of the Board of Gentis, Inc. a clinical stage spinal implant company which he joined in 2004. From 1999 to 2004, he was the founder and a director of Replication Medical, Inc., an orthopedic medical device company focused on spine surgeries. From 1994 to 1999, he served as the Chief Executive Officer of Bionx Implants, Inc., a leading manufacturer and marketer of reinforced polymer implants. From 1986 to 1989, he was the founder and Executive Vice President of Osteotech, Inc., a global leader in providing biologic solutions for regenerative medicine, which was acquired by Medtronic Inc. in 2010. Mr. Anderson also served as the Chief Executive Officer of Kensey Nash Corporation, a medical device company, from 1992 to 1994, and Sterilox Technologies, a manufacturer of non-toxic disinfectants, from 2000 to 2004. Mr. Anderson holds a B.S. in Chemical Engineering from Cornell University. Mr. Anderson brings to the Board over 25 years of experiences in entrepreneurship, strategic transactions and financing in the medical device industry.
France Dixon Helfer. Ms. Helfer has been our director since February 2014. Ms. Helfer is currently the President and Chief Executive Officer of TinyKicks LLC, a University of California healthcare technology company based in Irvine, California, since January 2015. From September 2011 to September 2014, Ms. Helfer was the President, Chief Executive Officer and Director of Halo Healthcare Inc., a medical device and diagnostics company focused on the detection and screening of breast cancer. Prior to that, from August 2004 to January 2008, Ms. Helfer was the President and Founder of Pegasus Biologics, Inc., which was acquired by Baxter Healthcare Inc. Prior to that, Ms. Helfer held a number of executive positions at various biotechnology companies,including Medtronic, Xenotech Labs, SORIN Bimedica, Eclipse Surgical and MMDDataDirect. Ms. Helfer serves on the Boards of various business organizations and academic institutions, including Chapman University’s Crean College of Health and Behavioral Sciences. Ms. Helfer holds a B.A. in Biologic Sciences from California State University, Fullerton. Ms. Helfer brings to the Board over 20 years of experience as a senior level executive in the medical device industry.
Alfred G. Holcomb. Mr. Holcomb has been our director since 2008. Since 2004, Mr. Holcomb has been serving as the Vice President of Acquisition and Divestutures at Lewis Energy Corp., an oil drilling company,where he led the completion of numerous strategic transactions. From 1978 to 2004, Mr. Holcomb was a partner at the law firm of Schoenbum, Curphy & Scanlan. Mr. Holcomb has been an active speaker on the Eagle Ford in conferences around the world. Mr. Holcomb holds his B.A. in Finance in from University of Texas, a J.D. from St. Mary's University School of Law and a LL.M. in Taxation from New York University. Mr. Holcomb brings to the Board extensive experience and knowledge on strategic, legal and financial matters.
Kevin R. Stone, M.D. Dr. Stone is the founding scientist of our company and served as our Chief Executive Officer from 1996 to 2008, as a director since our inception and as our Chairman of the Board since 2014. Dr. Stone is a renowned orthopaedic surgeon and founded the Stone Clinic in 1988, an orthopedic clinic focused on biologic approaches to treating joint injuries. He is also the Founder and Chairman of Stone Research Foundation, an independent research institution investigating new techniques for joint health, arthritis and human performance, since 1996. Dr. Stone is the controlling member and manager of CrossCart LLC, our controlling stockholder. Dr. Stone is also an attending staff at the California Pacific Medical Center and the San Francisco Surgery Center. From 1999 to 2003, Dr. Stone served as the Chief Executive Officer and Chairman of the Board of Joint Juice, Inc. (now Post Holdings, Inc.). Dr. Stone is also the founder and Chief Executive Officer of Rescue Reel, LLC since 2006 and the founder of ProprioSense Holdings, LLC since 2012. Dr. Stone holds an A.B.cum laude in biology from Harvard College and an M.D. from the University of North Carolina School of Medicine, residency training in general surgery at Stanford University and orthopaedics at Harvard and Fellowship trained in knee surgery and sports medicine. Dr. Stone is recognized internationally as a leading expert and authority on advanced orthopedic surgical and rehabilitation techniques to repair damaged cartilage and ligaments. He brings to the Board extensive expertise, knowledge and experience in the field of orthobiologic medicine and provides us with significant management, operational and financial support.
Mike Ward. Mr. Ward has been our director since March 2014. Mr. Ward has been serving as the Vice President, Corporate Development at BioNano Genomics, Inc., a genomic mapping company, since March 2014. Prior to joining BioNano Genomics, Inc., from 2009 to 2013, Mr. Ward was a Vice President at Lurie Investment Fund, LLC, the venture capital arm of Ann and Robert H. Lurie Foundation, where he was responsible for managing the life sciences venture capital and private equity investments of the foundation. Prior to 2009, Mr. Ward worked for more than 15 years in the investment banking industry, holding various positions at Leerink Partners, Credit Suisse, Dresdner Kleinwort Wasserstein, BMO and Vector Securities. Mr. Ward also serves on the Boards of the following healthcare companies: Nanosphere, Inc., a publicly traded molecular diagnostics company and CytoPherx, Inc., an acute renal failure-focused therapeutic medical device company. Mr. Ward holds a B.S. in finance from the University of Illinois. Mr. Ward brings to the Board over 20 years of life-sciences dedicated investment banking, venture capital and private equity experiences.
Significant Employee
Lance Johnson. Mr. Johnson has been serving as our Vice President, Quality since November 2010. Prior to joining us, he was the Quality Manager at Zimmer Spine, the orthopaedic spine division of Zimmer, Inc., from March 2009 to October 2010. From March 2005 to November 2009, Mr. Johnson was the Associate Quality Engineering Manager for Abbott Spine, an orthopaedic spine company, working specifically with new product development teams. In addition, he served as an investigator with the FDA from 1989 to 2005. From 1994 to 2005, he
was the resident investigator in charge of the Austin field office and as contributor to the FDA international cadre. During his time at the FDA, he audited medical device firms in the US, Europe and Canada. Mr. Johnson received his Medical Device Level II certification in 1998 and received his Level II Auditor certification in 2003. Lance trained and certified FDA, Texas, and BSI auditors during his career with the FDA. Mr. Johnson holds a B.S. in Biotechnology from Oklahoma State University.
Board Composition
Controlled Company and Director Independence
Upon completion of this offering, CrossCart LLC will continue to control a majority of the voting power of our outstanding common stock. As a result, we will be a “controlled company” under NASDAQ corporate governance standards. As a controlled company, we do not have to comply with certain corporate governance requirements under NASDAQ rules, including the following:
| · | A majority of our Board of Directors to consist of “independent directors” as defined by the applicable rules and regulations of NASDAQ; |
| · | The compensation of our executive officers to be determined, or recommended to the Board of Directors for determination, by independent directors constituting a majority of the independent directors of the Board in a vote in which only independent directors participate or by a Compensation Committee comprised solely of independent directors; and |
| · | That director nominees to be selected, or recommended to the Board of Directors for selection, by independent directors constituting a majority of the independent directors of the Board in a vote in which only independent directors participate or by a nomination committee comprised solely of independent directors. |
While we are exempt from these corporate governance requirements as a controlled company, we intend to comply voluntarily with some of these requirements. For example, our Board has determined that all of our directors are “independent” under applicable NASDAQ rules except Mr. Daniel Lee and Dr. Kevin R. Stone, therefore the majority of our Board will consist of independent directors following completion of this offering. In addition, the Board intends to establish a Compensation Committee consisting solely of independent directors, as described in more detail below.
As a result of us being a “controlled company,” you may not have the same protections afforded to stockholders of companies that are subject to all of the corporate governance requirements of NASDAQ. In the event that we cease to be a controlled company, we will be required to comply with these provisions within the transition periods specified in the applicable NASDAQ rules. As a controlled company, however, we will not be exempt from the independence requirements for our audit committee, and we intend to comply with the applicable requirements of the Sarbanes−Oxley Act and NASDAQ rules with respect to our audit committee within the applicable time frame.
Board Leadership Structure and Board’s Role in Risk Oversight
Our Board has a Chairman, Dr. Kevin R. Stone, who is also our controlling stockholder. The Chairman has authority, among other things, to preside over Board meetings and set the agenda for Board meetings. Accordingly, the Chairman has substantial ability to shape the work of our Board. We currently believe that separation of the roles of Chairman and Chief Executive Officer ensures appropriate oversight by the Board of our business and affairs. However, no single leadership model is right for all companies and at all times. The Board recognizes that depending on the circumstances, other leadership models, such as the appointment of a lead independent director, might be appropriate. Accordingly, the Board may periodically review its leadership structure. In addition, following the completion of the offering, the Board will hold executive sessions in which only independent directors are present.
Our Board is generally responsible for the oversight of corporate risk in its review and deliberations relating to our activities. Our principal source of risk falls into two categories, financial and product commercialization. The audit committee oversees management of financial risks; our Board regularly reviews information regarding our cash position, liquidity and operations, as well as the risks associated with each. The Board regularly reviews plans, results and potential risks related to our product development, commercialization efforts and clinical trial progress. Our Compensation Committee is expected to oversee risk management as it relates to our compensation plans, policies and practices for all employees including executives and directors, particularly whether our compensation programs may create incentives for our employees to take excessive or inappropriate risks which could have a material adverse effect on the Company.
Committees of the Board of Directors
Upon completion of this offering our Board of Directors will have an audit committee (the “Audit Committee”) and a Compensation Committee (the “Compensation Committee”). The composition and function of each committee are described below.
Audit Committee
Upon the completion of this offering, the Audit Committee will be comprised of Messrs. David Anderson, Alfred Holcomb and Mike Ward. The Board has also determined that Mr. Ward qualifies as an “audit committee financial expert” as defined under applicable SEC rules. Our Audit Committee is expected to adopt a written charter, and following this offering, a copy of this charter will be posted on the Corporate Governance section of our website, at www.aperionbiologics.com.
Our audit committee is authorized to:
| · | approve and retain the independent auditors to conduct the annual audit of our financial statements; |
| · | review the proposed scope and results of the audit; |
| · | review and pre-approve audit and non-audit fees and services; |
| · | review accounting and financial controls with the independent auditors and our financial and accounting staff; |
| · | review and approve transactions between us and our directors, officers and affiliates; |
| · | recognize and prevent prohibited non-audit services; and |
| · | establish procedures for complaints received by us regarding accounting matters; oversee internal audit functions, if any. |
Compensation Committee
Upon the completion of this offering, the Compensation Committee will be comprised of David Anderson and France Helfer. Our Compensation Committee is authorized to:
| · | review and determine the compensation arrangements for management; |
| · | establish and review general compensation policies with the objective to attract and retain superior talent, to reward individual performance and to achieve our financial goals; |
| · | administer our stock incentive and purchase plans; |
| · | oversee the evaluation of the Board of Directors and management; and |
| · | review the independence of any compensation advisers. |
Our Compensation Committee is expected to adopt a written charter, and following this offering, a copy of this charter will be posted on the Corporate Governance section of our website, atwww.aperionbiologics.com
Compensation Committee Interlocks and Insider Participation
None of the members of our Compensation Committee, at any time, has been one of our officers or employees. None of our executive officers currently serves, or in the past year has served, as a member of the Board of Directors or Compensation Committee of any entity that has one or more executive officers on our Board of Directors or Compensation Committee. For a description of transactions between us and members of our Compensation Committee and affiliates of such members, please see “Certain Relationship and Related Party Transactions”.
Code of Business Conduct and Ethics
Prior to the completion of the offering, we will adopt a code of business conduct and ethics that applies to all of our employees, officers and directors, including those officers responsible for financial reporting. The code of business conduct and ethics will be available at our website atwww.aperionbiologics.com. We expect that any amendments to the code, or any waivers of its requirement, will be disclosed on our website.
Executive and Director Compensation
Summary Compensation Table
The following table provides information regarding the compensation paid during our fiscal years ended September 30, 2015 and September 30, 2014 to our principal executive officer and our principal financial officer. We refer to these individuals as our named executive officers. There are no other executive officers.
| Name and Principal Position | | Fiscal Year | | Salary ($) | | | Bonus ($) | | | All Other Compensation ($) | | | Total ($) | |
| Daniel R. Lee | | 2015 | | | 140,021 | (1) | | | — | | | | 1,078 | (2) | | | 141,099 | |
| Chief Executive Officer | | 2014 | | | 164,500 | (1) | | | — | | | | — | | | | 164,500 | |
| David Cocke | | 2015 | | | | | | | | | | | 79,825 | (3) | | | 79,825 | |
| Chief Financial Officer | | 2014 | | | | | | | | | | | 42,000 | (3) | | | 42,000 | |
| (1 | ) | Represents base salary actually paid following the reduction as described below. |
| | |
| (2) | Represents life insurance policy premiums paid by the Company. |
| | |
| (3 | ) | Represents the consulting fees actually paid following the reduction as described below. |
Narrative to Summary Compensation Table
We are currently evaluating various compensation programs to implement upon the completion of this offering. The disclosures below describe our historical compensation practices, except as specifically noted.
In setting the compensation of our named executive officers and our other employees, we consider compensation for comparable positions in the market, the historical compensation levels of our executives, individual performance as compared to our expectations and objectives, our desire to motivate our employees to achieve short- and long-term results that are in the best interests of our stockholders, and a long-term commitment to our company. We do not target a specific competitive position or a specific mix of compensation among base salary, bonus or long-term incentives.
Our board of directors has historically determined the compensation for our executive officers; effective with the date of this offering, the board will delegate this authority to the compensation committee.
Annual Salary
The base salary of our Chief Executive Officer was originally established under his employment agreement with the company dated July 22, 2008. His base salary was initially set at $175,000 per year subject to review and increase at the board’s discretion. His base salary has been reviewed and adjusted periodically. His base salary was increased to $235,000 per year in January 2012, but subsequently reduced as described below. In fiscal year 2015, he received a base salary of $140,021. It is expected that following this offering, our Chief Executive Officer’s base salary will be increased to $235,000.
Our Chief Financial Officer has been providing services pursuant to the Independent Consultant Agreement with the Company effective as of September 1, 2008 which entitled him to receive a consulting fee at the rate of $100 per hour up to a maximum of $4,000 per month. His consulting fee was increased to a rate of $125 per hour up to a maximum of $5,000 per month in August 2011, but subsequently reduced as described below. Effective as of August 17, 2015, his consulting fees have been increased to $5,000 per week. It is expected that following the completion of this offering, he will enter into an employment agreement with the Company that will provide appropriate compensation arrangement for a position of a Chief Financial Officer.
As part of our effort to preserve cash, since July 2013, we have reduced, in full or partially, the salaries of our Chief Executive Officer and other full time employees and the consulting fees payable to our Chief Financial Officer until the Company has received a certain amount of financing or a liquidation event (as more fully described under the Management Retention Plan below). It was agreed that base salaries and consulting fees would be adjusted back to their respective pre-July 2013 levels upon completion of such financing or liquidation event.
Management Retention Plan
In connection with the reduction of salaries and consulting fees and in an effort to retain our employees and service providers, we implemented a management retention plan in July 2013 pursuant to which our named executive officers were each entitled to a bonus in connection with certain financing and liquidation events subject to continued service up to such financing or liquidity event. Upon an external financing (beyond CrossCart LLC) of at least $2 million funding or signing of a license/distribution deal with at least $2 million of upfront payments or milestones achievable within 12 months of signing, Mr. Lee was entitled to receive a bonus in an amount equal to $35,000 plus the aggregate reduction in his salary since July 2013 and Mr. Cocke was entitled to receive a bonus in an amount equal to $15,000 plus the aggregate reduction in his consulting fees since July 2013. The bonus payable on liquidation events was based on the amount of proceeds from the event plus the aggregate reduction in salaries and consulting fees.
In October 2015, the plan was amended so that upon completion of this offering, Mr. Lee will receive a cash bonus in an amount equal to the aggregate reduction in his salary since July 2013 plus a bonus of $35,000 payable in the form of shares of our common stock (with the value based on the initial public offering price set forth on the cover page of this Offering Circular) and Mr. Cocke will receive a cash bonus in an amount equal to the aggregate reduction in his consulting fees since July 2013 plus a bonus of $15,000 payable in the form of shares of common stock (with the value based on the initial public offering price set forth on the cover page of this Offering Circular). As of September 30, 2015, the aggregate amount of reduced salary and fees for the Chief Executive Officer and Chief Financial Officer was $183,104 and $39,250, respectively. In addition, the plan was amended in October 2015 so that upon completion of this offering, the participants will not be eligible to receive any bonuses upon the occurrence of a liquidation event under the plan.
Long-Term Incentives
Our 2008 Stock Option/Stock Issuance Plan (the “2008 Plan”) authorizes us to make grants to eligible recipients of options, stock awards, and restricted stock units. To date, we have granted only stock options to our named executive officers under the 2008 Plan. We have adopted the 2015 Equity Incentive Plan which serves as the successor to the 2008 Plan and which will be the only plan under which equity-based awards will be made. Prior to adoption of the 2008 Plan, we had granted options on a limited basis to certain service providers.
We typically grant stock options at the start of employment or service to each named executive officer and our other employees. To date, we have not maintained a practice of granting additional equity on an annual basis, but we have granted additional equity following significant equity financings, and we have retained discretion to provide additional targeted grants in certain circumstances. Our named executive officers received their initial grants in connection with commencement of their services which, for our Chief Executive Officer reflected the grant for 3% of the outstanding shares that he was entitled to under his employment agreement. Both named executive officers received additional grants in 2011 and 2012 as retention grants.
We set the option exercise price based on our per-share valuation on the date of grant. Grants to our named executive officers typically vest 12.5% on the first six-month anniversary of the vesting commencement date and in equal monthly installments thereafter over the next 42-months.
Outstanding Equity Awards at September 30, 2015
The following table sets forth information regarding equity awards held by the named executive officers as of September 30, 2015.
| | Option Awards | | Stock Awards | |
| Name | | Number of Securities Underlying Unexercised Options (#) Exercisable (1) | | | Number of Securities Underlying Unexercised Options (#) Unexercisable (2) | | | Option
Exercise Price ($) | | | Option Expiration Date | | Number of Units of Stock That Have Not Vested (#) | | | Market Value of Units of Stock That Have Not Vested ($) | |
| Daniel R. Lee | | | 51,960 | | | | — | | | | 0.18 | | | 4/14/2020 | | | | | | | — | |
| | | | 54,348 | | | | — | | | | 1.90 | | | 10/2/2021 | | | | | | | — | |
| | | | 4,528 | | | | 906 | | | | 4.05 | | | 5/14/2022 | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | |
| | | | — | | | | — | | | | — | | | — | | | | | | | | |
| Total | | | 110,836 | | | | 906 | | | | — | | | — | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | |
| David Cocke | | | 1,957 | | | | — | | | | 0.18 | | | 4/14/2020 | | | | | | | — | |
| | | | 23,043 | | | | | | | | 1.90 | | | 10/2/2021 | | | | | | | — | |
| | | | 3,398 | | | | 678 | | | | 4.05 | | | 5/14/2022 | | | | | | | — | |
| | | | — | | | | | | | | — | | | — | | | | | | | | |
| Total | | | 28,398 | | | | 678 | | | | — | | | — | | | | | | | | |
| (1) | Each stock option was granted pursuant to our 2008 Stock Option/Stock Issuance Plan. Each option is immediately exercisable on the date of grant subject to vesting over a four-year period, with 12.5% of the shares to vest upon completion of six months of service measured from the vesting commencement date and the balance to vest in 36 successive equal monthly installments upon the completion of each additional month of service thereafter. Each option is subject to accelerated vesting in connection with a change in control as described in “Agreements Regarding Change in Control and Termination of Employment” below. This column represents the vested shares subject to the option notwithstanding the fact that the entire option is currently exercisable. |
| (2) | This column represents the unvested shares subject to the option notwithstanding the fact that the entire option is currently exercisable. |
Agreements Regarding Change in Control and Termination of Employment
Under the terms of his employment agreement, should Mr. Lee’s employment be terminated other than for cause, Mr. Lee will be entitled to receive (i) salary continuation for a period of nine (9) months and (ii) continuation of health care coverage under our group health care plans for himself and his eligible dependents for a period of nine (9) months. The severance payments and benefits are conditioned on Mr. Lee executing a general release of claims against the Company.
Mr. Lee is also subject to certain non-compete and non-solicitation covenants generally for a period of two (2) years following termination of employment. In the event of a breach of the covenants, Mr. Lee will forfeit all vested options granted under his employment agreement and the Company has the right to repurchase all shares acquired upon exercise of options granted under his employment agreement at the original exercise price paid for the shares.
In the event of a change in control, outstanding awards will accelerate unless assumed, replaced or otherwise continued. See “Equity Incentive Plans” below.
Director Compensation for Fiscal Year 2015
Our non-employee board members are entitled to receive an annual retainer of $25,000 except that the chairman of the board is entitled to receive an annual retainer of $75,000. However, as part of our effort to preserve cash, we deferred payment of the annual retainer effective July 2013. As of September 30, 2015, an aggregate of $333,749 in retainer fees had been deferred. In October 2015, we entered into an agreement with each director pursuant to which we paid 70% of the deferred fees by issuing shares of our common stock based at $4.97 per share, the then fair market value of our shares, and agreed that the remaining 30% of such deferred fees will be paid in cash following the completion of this offering. We issued an aggregate of 47,026 shares of common stock as payments for 70% of such deferred fees for all directors.
Historically we have not established any formal policy to grant equity awards to newly appointed directors. Effective with the date of this offering, our non-employee board members will be eligible to receive equity awards annually in accordance with the automatic grant program for non-employee directors under the 2015 Equity Incentive Plan. See “Equity Incentive Plans” below.
The following table sets forth information regarding the compensation of each individual who served as a non-employee member of our Board of Directors during fiscal year 2015.
| Name | | Fees Earned or Paid (1) | |
| Kevin R. Stone | | $ | 75,000 | |
| David W. Anderson | | $ | 25,000 | |
| France Dixon Helfer | | $ | 25,000 | |
| Alfred G. Holcomb | | $ | 25,000 | |
| Mike Ward | | $ | 25,000 | |
| (1) | As described above, the payment of the fees had been deferred; in October 2015, we paid 70% of such deferred fees in the form of shares of common stock. No stock option was granted to any non-employee director in fiscal year 2015. As of September 30, 2015, our non-employee directors held outstanding options as follows: |
Name | Number of Shares Subject to Options |
| Kevin R. Stone | 5,435 | |
| David W. Anderson | 39,565 | |
| France Dixon Helfer | 8,967 | |
| Alfred G. Holcomb | ----- | |
| Mike Ward | 8,967 | |
Equity Incentive Plans
The principal features of our equity incentive plans, including our proposed new 2015 Equity Incentive Plan to be approved by the board and stockholders, are summarized below. These summaries are qualified in their entirety by reference to the actual text of the plans, which will be filed as exhibits to the offering statement of which this offering circular is a part.
Prior to adoption of the 2008 Plan, we had granted options on a limited basis; of those option grants, only the option granted to Dr. Stone covering 5,435 shares of common stock remains outstanding.
2008 Stock Option/Stock Issuance Plan
Our board of directors adopted our 2008 Stock Option/Stock Issuance Plan or the 2008 Plan on January 14, 2009 and our stockholders approved it on May 20, 2009. Our 2008 Plan provides for the grant of options, stock awards and restricted stock units. We will cease issuing awards under the 2008 Plan upon the implementation of the 2015 Equity Incentive Plan, which is described below. Following this offering, we will grant equity awards under our 2015 Stock Incentive Plan.
An aggregate of 494,565 shares of our common stock were reserved for issuance under the 2008 Plan. As of June 30, 2015, options to purchase 328,682 shares remained outstanding and 102,651 shares remained available for future grant. No other awards have been made under the 2008 Plan. All options outstanding and the remaining share reserve under the 2008 Plan will be transferred to the 2015 Equity Incentive Plan.
The terms of the options granted under the 2008 Plan are substantially similar to the terms of the options granted under the 2015 Equity Incentive Plan.
2015 Equity Incentive Plan
We expect our board of directors and our stockholders to approve our 2015 Equity Incentive Plan prior to the completion of the offering and it will become effective immediately following thecompletion of the offering. The Incentive Plan is intended to serve as a successor to our 2008 Plan. The Incentive Plan became effective upon its adoption by the board of directors.
Type of Awards. Under the Incentive Plan, eligible persons may, at the discretion of the plan administrator, be granted options, stock appreciation rights, stock awards and restricted stock units. In addition, the Incentive Plan includes an automatic grant program for our non-employee directors. The principal features of each type of award are described below.
Administration . The compensation committee of our board of directors will have the exclusive authority to administer the Incentive Plan with respect to awards made to our executive officers and non-employee board members and will also have the authority to make awards to all other eligible individuals. However, our board of directors may at any time appoint a secondary committee of one or more board members to have authority to make awards under the Incentive Plan to individuals other than executive officers and non-employee board members. The term “plan administrator,” as used in this summary, will mean our compensation committee or any secondary committee, to the extent each such entity is acting within the scope of its administrative authority under the Incentive Plan.
Eligibility . Employees, including officers, and non-employee board members, as well as consultants and independent advisors, in our employ or service or in the employ or service of our parent or subsidiary companies (whether now existing or subsequently established) will be eligible to participate in the Incentive Plan.
Securities Subject to Incentive Plan . Subject to adjustment as described below, [ ] shares of our common stock have been reserved for issuance over the term of the Incentive Plan. Such share reserve is comprised of 102,651 shares remaining available for issuance under the 2008 Plan upon adoption of the Incentive Plan and an increase of [ ] shares.
The shares of our common stock subject to outstanding awards (including options transferred from the 2008 Plan) will be available for subsequent award and issuance to the extent those awards subsequently expire, are forfeited or cancelled or terminate for any reason prior to the issuance of the shares of common stock subject to those awards. Unvested shares issued under the Incentive Plan and subsequently forfeited or repurchased by the Company will be added back to the reserve and available for subsequent award and issuance. Should the exercise price of an option be paid in shares of our common stock (whether through the withholding of a portion of the otherwise issuable shares or through the tender of outstanding shares), then the number of shares reserved for issuance will be reduced by the net number of shares issued under the exercised option. Upon the exercise of any stock appreciation right, the share reserve will be reduced by the net number of shares actually issued upon such exercise. Should shares of common stock otherwise issuable under the Incentive Plan be withheld by us in satisfaction of the withholding taxes incurred in connection with the issuance, exercise, vesting or settlement of an award, then the number of shares of common stock available for issuance will be reduced by the net number of shares actually issued after any such share withholding.
Subject to adjustment as described below, the maximum number of shares of common stock which may be issued under the Incentive Plan pursuant to options intended to qualify as incentive stock options under the federal tax laws may not exceed [ ] shares in the aggregate.
Award Limitations.A participant in the Incentive Plan may not receive (i) options or stock appreciation rights denominated in shares of our common stock at the time of grant (whether payable in such common stock, cash or a combination of both) for more than [ ] shares of our common stock in the aggregate in any calendar year or (ii) stock awards or restricted stock units for more than [ ] shares of our common stock in the aggregate in any calendar year.
Awards. The plan administrator will have complete discretion to determine which eligible individuals are to receive awards, the time or times when those awards are to be granted, the number of shares subject to each such award, the vesting and exercise schedule (if any) to be in effect for the award, the cash consideration (if any) payable per share subject to the award, the form of settlement of the awards, the maximum term for which the award is to remain outstanding and the status of any granted option as either an incentive stock option or a non-statutory option under the federal tax laws.
Options. Each granted option will have an exercise price per share determined by the plan administrator, but the exercise price will not be less than one hundred percent of the fair market value of the option shares on the grant date. No granted option will have a term in excess of ten years. Each option will generally vest and become exercisable for the underlying shares in one or more installments over a specified period of service measured from the grant date. Upon cessation of service, the optionee will have a limited period of time in which to exercise his or her outstanding options to the extent they are at the time exercisable for vested shares. The plan administrator will have complete discretion to extend the period following the optionee’s cessation of service during which his or her outstanding options may be exercised, provide for continued vesting during the applicable post-service exercise period and/or to accelerate the exercisability or vesting of such options in whole or in part. Such discretion may be exercised at any time while the options remain outstanding.
Stock Appreciation Rights. The Incentive Plan allows the issuance of two types of stock appreciation rights:
| · | Tandem stock appreciation rights granted in conjunction with options which provide the holders with the right to surrender the related option grant for an appreciation distribution from us in an amount equal to the excess of (i) the fair market value of the vested shares of common stock subject to the surrendered option over (ii) the aggregate exercise price payable for those shares. |
| · | Stand-alone stock appreciation rights which allow the holders to exercise those rights as to a specific number of shares of our common stock and receive in exchange an appreciation distribution from us in an amount equal to the excess of (i) the fair market value of the shares of common stock as to which those rights are exercised over (ii) the aggregate exercise price in effect for those shares. The exercise price per share may not be less than the fair market value per share of our common stock on the date the stand-alone stock appreciation right is granted, and the right may not have a term in excess of ten years. |
The appreciation distribution on any exercised stock appreciation right may be paid in (i) cash, (ii) shares of our common stock or (iii) a combination of cash and shares of the Company’s common stock. Upon cessation of service, the holder of a stock appreciation right will have a limited period of time in which to exercise such right to the extent exercisable at that time. The plan administrator will have complete discretion to extend the period following the holder’s cessation of service during which his or her outstanding stock appreciation rights may be exercised, provide for continued vesting during the applicable post-service exercise period and/or to accelerate the exercisability or vesting of those stock appreciation rights in whole or in part. Such discretion may be exercised at any time while the stock appreciation right remains outstanding.
Repricing . The board has the discretionary authority to: (i) cancel outstanding options or stock appreciation rights in return for new options or stock appreciation rights with an exercise or base price per share based on the fair market value per share on the new grant date, (ii) cancel outstanding options or stock appreciation rights for consideration payable in cash, shares of common stock or other equity awards and (iii) reduce the exercise or base price in effect for outstanding options or stock appreciation rights or issue new options or stock appreciation rights with a lower exercise or base price in cancellation of outstanding options or stock appreciation rights with a higher exercise or base price.
Stock Awards and Restricted Stock Units. Shares may be issued under the Incentive Plan subject to performance or service vesting requirements established by the plan administrator. Shares may also be issued as a fully-vested bonus for past services without any cash outlay required of the recipient.
Shares of our common stock may also be issued pursuant to restricted stock units which entitle the recipients to receive those shares upon the attainment of designated performance goals or the completion of a prescribed service period or upon the expiration of a designated time period following the vesting of those units, including (without limitation), a deferred distribution date following the termination of the recipient’s service with us. Restricted stock units subject to performance vesting may be structured so that the award converts into shares of our common stock at a rate based on the attainment level of performance for each performance objective.
Outstanding stock awards will be forfeited and restricted stock units will automatically terminate if the performance goals or service requirements established for such awards are not attained. However, the plan administrator will have the discretionary authority to vest or make payments in satisfaction of one or more outstanding awards as to which the designated performance goals or service requirements are not attained.
Restricted stock units may be settled in cash, shares of our common stock or a combination of both, as determined by the plan administrator. Dividend equivalents may be paid or credited, whether in cash or in actual or phantom shares of our common stock, on outstanding restricted stock units, upon such terms and conditions as determined by the plan administrator.
Non-employee director automatic grant program. Our non-employee directors will receive automatic grants under the terms set forth below.
Each individual who is first elected or appointed as a non-employee director other than at a regular annual stockholders meeting at any time after the date of this offering will automatically be granted, on the date of such initial election or appointment (the “Initial Grant Date”), an award in the form of an option or restricted stock units with a value determined by multiplying the Initial Applicable Amount by a fraction, the numerator of which is the number of months (including any partial month, expressed as a fraction) that will elapse between the Initial Grant Date and the date of the next regular annual stockholders meeting following such Initial Grant Date, and the denominator of which is twelve (12) months (the “Initial Grant”). On the date of each regular annual stockholders meeting, beginning with the 2016 annual stockholders meeting, each non-employee director will automatically be granted an award in the form of an option or restricted stock units with a value equal to the Annual Applicable Amount for that year (the “Annual Grant”).
Our compensation committee will have the sole discretion to determine the amount and type of awards within the foregoing limitations. For purposes of the Initial Grant and the Annual Grant, the value of an award will be calculated as follows: (A) the value of an option share will be equal to the fair value of an option share as estimated on the date of grant for purposes of our financial statements and (B) the value of a restricted stock unit will be equal to the fair market value per share of common stock on the date of grant. The Initial Applicable Amount and the Annual Applicable Amount will be determined by the compensation committee on or before the grant date of the award, but in no event will exceed $[ ] and $[ ], respectively.
The shares subject to each Initial Grant will vest on the date of the next regular annual stockholders meeting following the award grant date, provided the participant continues in board service up to such date. The shares subject to each Annual Grant will vest on the date of the next regular annual stockholders meeting following the award grant date, provided the participant continues in board service up to such date. However, the shares subject to each award will immediately vest upon a change in control or upon the board member’s cessation of board service by reason of death or disability.
The exercise price per share of any option granted under this program will be equal to one hundred percent (100%) of the fair market value per common share on the option grant date and each option will have a maximum term of ten (10) years measured from the option grant date, subject to earlier termination following the non-employee board member’s cessation of board service.
The shares of common stock underlying restricted stock units granted which vest in accordance with the applicable vesting provisions will be issued as they vest. However, awards may be structured so that the issuance of the shares of common stock which vest under those awards is deferred, in accordance with the applicable requirements of Code Section 409A and the regulations thereunder, beyond the vesting date to a designated date or the occurrence of any earlier event such as cessation of board service or a change in control. Each restricted stock unit will include a dividend equivalent right pursuant to which a book account will be established for the non-employee board member and credited from time to time with each dividend or distribution, whether in cash, securities or other property (other than shares of common stock) which is made per issued and outstanding share of common stock during the period the share of common stock underlying that restricted stock unit remains unissued. The amount credited to the book account with respect to such restricted stock unit will be paid to the non-employee board member concurrently with the issuance of the share of common stock underlying that unit.
Change in Control . In the event the Company should experience a change in control, each outstanding award may be assumed, cancelled and substituted or otherwise continued in effect by the successor corporation or replaced with a cash incentive program which preserves the intrinsic value of the award and provides for the subsequent vesting and payout of that value in accordance with the same vesting schedule in effect for that award. In the absence of such assumption, substitution, continuation or replacement of the award, the award will automatically accelerate and vest in full immediately prior to the change in control. The plan administrator will have complete discretion to grant one or more awards which will vest upon a change in control or in the event the individual’s service with the Company or the successor entity terminates within a designated period following a change in control transaction.
Unless the definition of change in control is otherwise set out forth in an individual award agreement, a change in control will be deemed to occur in the event (a) the Company is acquired by merger or sells all or substantially all of its assets, (b) there occurs any transaction or series of related transactions pursuant to which any person or group of related persons acquires directly or indirectly beneficial ownership of securities possessing (or convertible into or exercisable for securities possessing) more than fifty percent (50%) of the total combined voting power of the Company’s outstanding securities or (c) the composition of our board changes over a period of twelve (12) consecutive months or less such that a majority of the board ceases to be comprised of individuals who either (1) have been board members continuously since the beginning of such period, or (2) have been elected or nominated for election as board members during such period by at least a majority of the board members described in clause (1) who were still in office at the time the board approved such election or nomination.
Adjustment . In the event any change is made to the outstanding shares of the Company’s common stock by reason of any stock split, stock dividend, recapitalization, reincorporation, combination of shares, exchange of shares, spin-off transaction or other change affecting the outstanding common stock as a class without the Company’s receipt of consideration or should the value of the Company’s outstanding shares of common stock be substantially reduced by reason of a spin-off transaction or extraordinary dividend or distribution, or should there occur any change in control transaction or any other merger, consolidation or other reorganization, equitable adjustments will be made to: (i) the maximum number and/or class of securities issuable under the Incentive Plan; (ii) the maximum number and/or class of securities that may be issued pursuant to incentive stock options; (iii) the maximum number and/or class of securities for which any one person may be granted common stock-denominated awards per calendar year; (iv) the number and/or class of securities and the exercise price per share in effect for outstanding options and stock appreciation rights; (v) the number and/or class of securities subject to each outstanding award (other than options and stock appreciation rights) and the cash consideration (if any) payable per share; and (vi) the number and/or class of securities subject to forfeiture or our outstanding repurchase rights and the repurchase price payable per share. Such adjustments will be made in such manner as the plan administrator deems appropriate, and such adjustments will be final, binding and conclusive.
Transferability and Shareholder Rights . Awards are generally not transferable and may only be exercised by the participant. No participant will have any shareholder rights with respect to any award until such award is exercised or vests and the underlying shares are issued.
Amendment and Termination . Our board may amend or modify the Incentive Plan at any time subject to any stockholder approval requiredunder applicable law or regulation or pursuant to the listing standards of the stock exchange on which our common stock is at the time primarily traded.
Unless sooner terminated by our board, the Incentive Plan will terminate on the earliest of (i) , 2025, (ii) the date on which all shares available for issuance under the Incentive Plan have been issued as fully-vested shares or (iii) the termination of all outstanding awards in connection with certain changes in control or ownership.
Security Ownership of Management and Certain Securityholders
The following table sets forth certain information known to us regarding beneficial ownership of our capital stock as of September 30, 2015 for (i) each named executive officer, (ii) each director, (iii) all named executive officers and directors as a group and (iv) each person, or group of affiliated persons, known by us to be the beneficial owner of more than five percent (5%) of our capital stock. The percentage of beneficial ownership in the table below is based on 4,096,867 shares of common stock deemed to be outstanding as of September 30, 2015 and gives effect to (i) the conversion of all outstanding shares of our convertible preferred stock into an aggregate of 2,085,910 shares of our common stock following the completion of this offering, including shares of common stock to be issued as cumulative dividends accrued under such preferred stock; (ii) the issuance of 1,788,588 shares of common stock upon automatic conversion of approximately an aggregate of $10,284,946 in outstanding principal and accrued interest under our convertible promissory notes upon the completion of this offering, assuming that the offering is completed on September 30, 2015; and (iii) no exercise of any warrant to purchase shares of preferred stock or common stock. In addition, shares of common stock that may be acquired by the stockholder within 60 days of September 30, 2015, pursuant to the exercise of stock options are deemed to be outstanding for the purpose of computing the percentage ownership of such shareholder, but are not deemed to be outstanding for the purpose of computing the percentage ownership of any other person shown in the table.
| | | Shares Beneficially Owned
Prior to the Offering | | | Percentage of Shares
Beneficially Owned | |
| | | | | | Before Offering | | | After Offering | |
| Name of Beneficial Owner | | | | | | | | | | | | |
| 5% Stockholders: | | | | | | | | | | | | |
| CrossCart LLC(1) | | | 4,096,867 | | | | 77.7 | % | | | 48.2 | % |
| | | | | | | | | | | | | |
| Directors and Named Executive Officers: | | | | | | | | | | | | |
| Daniel Lee(2) | | | 111,742 | | | | 2.7 | % | | | 1.7 | % |
| David Cocke(3) | | | 30,807 | | | | * | | | | * | |
| David W. Anderson(4) | | | 39,565 | | | | 1.0 | | | | * | |
| France Dixon Helfer(5) | | | 8,967 | | | | * | | | | * | |
| Alfred G. Holcomb(6) | | | 45,521 | | | | 1.1 | | | | * | |
| Dr. Kevin R. Stone(1) (7) (8) | | | 3,371,675 | | | | 82.2 | % | | | 51.1 | % |
| Mike Ward(9) | | | 8,967 | | | | * | | | | * | |
| All named executive officers and directors as a group (7 persons)(10) | | | 3,617,245 | | | | 84.1 | % | | | 53.3 | % |
* Represents less than 1%
(1) Dr. Kevin R. Stone, a director of the Company, is the controlling member and manager of CrossCart LLC. As such, Dr. Stone may be deemed to have voting and dispositive power of all securities beneficially owned by CrossCart LLC reported herein. Dr. Stone disclaims beneficial ownership of all shares held by CrossCart LLC. The mailing address of CrossCart LLC and Dr. Stone is 3727 Buchanan Street, San Francisco, CA 94123.
(2) Includes 111,742 shares of common stock issuable upon exercise of stock options, of which 111,062 shares are fully vested within 60 days of September 30, 2015. The remaining 680 shares do not vest within 60 days of September 30, 2015 and would be subject to repurchase upon termination of employment if exercised prior to vesting.
(3) Includes 29,076 shares of common stock issuable upon exercise of stock options, of which 28,568 shares are fully vested within 60 days of September 30, 2015. The remaining 509 shares do not vest within 60 days of September 30, 2015 and would be subject to repurchase upon termination of employment if exercised prior to vesting.
(4) Includes 39,565 shares of common stock issuable upon exercise of stock options, of which 38,819 shares are fully vested within 60 days of September 30, 2015. The remaining 746 shares do not vest within 60 days of September 30, 2015 and would be subject to repurchase upon termination of employment if exercised prior to vesting. Excluded are 8,865 shares of common stock issued as payments for certain fees for board services that were deferred since July 2013.
(5) Includes 8,967 shares of common stock issuable upon exercise of stock options, of which 7,846 shares are fully vested within 60 days of September 30, 2015. The remaining 1,121 shares do not vest within 60 days of September 30, 2015 and would be subject to repurchase upon termination of employment if exercised prior to vesting. Excluded are 6,164 shares of common stock issued as payments for certain fees for board services that were deferred since July 2013.
(6) Excluded are 7,926 shares of common stock issued as payments for certain fees for board services that were deferred since July 2013.
(7) Includes 1,435,690 shares of common stock issuable upon automatic conversion of an aggregate of $8,255,668 in principal amount and accrued interest under certain convertible promissory notes held by CrossCart LLC, assuming that the offering is completed on September 30, 2015; and excludes 289,523 shares of common stock issuable upon exercises of warrants to purchase shares of our common stock held by CrossCart LLC.
(8) Excludes an aggregate of 129,796 shares of common stock issuable upon the exercise of certain warrants to purchase common stock held by Dr. Stone in his individual capacity, and such warrants are not exercisable until the earlier of (i) the completion of this offering and (ii) March 31, 2016; and includes 5,435 shares of common stock issuable upon exercise of stock options, all of which are vested as of September 30, 2015. Excluded are 17,906 shares of common stock issued as payments for certain fees for board services that were deferred since July 2013. All shares of common stock, warrants and stock options reported in this footnote (8) are held directly by the Stone Family Revocable Trust dated 9/20/90 (the “Trust”) for which Dr. Stone and Susan Stone serve as co-trustees. As the co-trustee of the Trust, Dr. Stone is deemed to have beneficial ownership of all securities held by the Trust.
(9) Includes 8,967 shares of common stock issuable upon exercise of stock options, of which 7,473 shares are fully vested within 60 days of September 30, 2015. The remaining 1,495 shares do not vest within 60 days of September 30, 2015 and would be subject to repurchase upon termination of employment if exercised prior to vesting. Excluded are 6,164 shares of common stock issued as payments for certain fees for board services that were deferred since July 2013.
(10) Includes 203,753 shares of common stock issuable upon exercise of stock options, of which 199,203 shares are fully vested within 60 days of September 30, 2015. The remaining 4,550 shares do not vest within 60 days of September 30, 2015 and would be subject to repurchase upon termination of employment if exercised prior to vesting. Excluded are 47,026 shares of common stock issued as payments for certain fees for board services that were deferred since July 2013.
Certain Relationships and Related Party Transactions
Transactions with CrossCart and Dr. Kevin Stone
Convertible Note Financing
Dr. Kevin Stone, a director of the Company, is a controlling member and the manager of CrossCart LLC, a California limited liability company (“CrossCart”). Between August 19, 2013 and May 23, 2014, CrossCart loaned an aggregate of $1,520,015 to the Company in exchange for the issuance of eight (8) separate and identical convertible promissory notes in the principal amount of $200,000, $200,000, $150,000, $250,000, $250,000, $150,000, $150,000,$150,000, and $20,015 respectively (the “CrossCart Series D Notes”). Each CrossCart Series D Note bears an interest of 8% per annum, which rate increases to 10% per annum upon maturity or if an event of default occurs. In general, all principal and interest accrued under each CrossCart Series D Note is due and payable, upon demand at the sole option of CrossCart, following each such note’s maturity dates, all of which have occurred with the exception of the CrossCart Series D Note with a principal amount of $20,015 which matures on May 23, 2016. The CrossCart Series D Notes contain customary events of default. The principal and any accrued interest under the CrossCart Series D Notes are convertible, at CrossCart’s discretion, into shares of Series D Preferred Stock of the Company at an initial conversion price of $4.60 per share. Under the terms of the CrossCart Series D Notes, the Company is required to use reasonable efforts to cause a sufficient number of shares of Series D Preferred Stock to be authorized and reserved for the conversion of such notes. As of June 30, 2015 the total outstanding amount due under the CrossCart Series D Notes was $3,260,851, including accrued interest. In October 2015, we entered into an agreement with CrossCart to provide that upon completion of this offering, all outstanding amounts due under the 2013 CrossCart Series D Notes will be automatically converted into shares of our common stock at the initial conversion price of $4.60 per share. In addition, under this agreement, CrossCart agrees to waive all defaults under the notes as a result of non-payments of any amount due under the notes and this waiver expires on March 31, 2016.
Demand Notes
On December 18, 2014, the Company issued a demand note to CrossCart for an aggregate principal amount of $120,000 (the “Demand Note”). The outstanding principal of each Demand Note carries an interest rate of 8% per annum, which may be increased to 10% if an event of default occurs. All principal and accrued interest under the Demand Note is due and payable, upon demand, at the sole option and discretion of CrossCart. The Demand Note includes customary events of default. The outstanding balance on the Demand Note as of June 30, 2015 was $125,093, including accrued interest.
In addition, as inducement to enter into the Demand Note, the Company granted CrossCart certain royalty rights (the “Royalty Right”), pursuant to which the Company agrees to pay a royalty equal to 5% of any net sales from products manufactured using the Company’s Z-Process on the first $12,000,000 of net sales. The Royalty Right will expire on December 31,
2016 or when $12,000,000 in net sales has been achieved. The Royalty Right was further amended in connection with the execution of the Line of Credit Note as described below. Furthermore, as inducement to enter into the Demand Note, the Company agreed to pay CrossCart a transaction bonus equal to 3% of the gross proceeds received from any control change transaction of the Company (“Transaction Bonus”). In September 2015, the Company and CrossCart entered into an agreement to provide that CrossCart’s right to receive the Transaction Bonus will terminate and expire upon completion of this offering. As of June 30, 2015, the total amount outstanding under the Demand Note was $125,093, including accrued interest.
Line of Credit Note
On May 1, 2015, the Company entered into a Line of Credit Note with CrossCart that provides the Company with a credit line up to $100,000. During the term of this note, the Company may request borrowings, with a minimum amount of $10,000 for each request, from CrossCart, and such request may be granted or denied at CrossCart’s sole discretion. The Company may repay the outstanding amount of this note at its discretion. The note bears interest at rate of 10% per annum, and all principal amount and accrued interest will be due and payable on May 1, 2016. The note contains customary events of default. If the Company fails to pay all amounts due by May 1, 2016, the interest on the remaining outstanding balance will accrue at a rate of 12% per annum. As of June 30, 2015, the total amount outstanding under the Line of Credit Note was $61,627, including accrued interest. In addition, as inducement to enter into the Line of Credit Note, the Company amended the Royalty Right such that CrossCart will be entitled to receive a 5% royalty on all net sales in excess of $12,000,000 without an expiration date (the “Additional Royalty Rights”). In September 2015, the Company and CrossCart entered into an agreement to provide that the Additional Royalty Rights will terminate and expire upon completion of this offering.
Extension of Warrants and Stock Options and Amendment of Warrants and Notes
On or about December 23, 2013, the Company entered into a letter agreement (the “Letter Agreement”) with CrossCart and Dr. Stone to extend the expiration dates of the following warrants held by Dr. Stone (the “Series C Warrants”):
| | · | A warrant to purchase 577 shares of Series C Preferred Stock issued on July 25, 2007 having an exercise price of $8.83 per share and an expiration date of July 25, 2012. |
| | · | A warrant to purchase 340 shares of Series C Preferred Stock issued on August 14, 2007 having an exercise price of $8.83 per share and an expiration date of August 14, 2012. |
The expiration dates of the two Series C Warrants were extended five (5) years from the initial expiration dates, such that the expiration dates are now July 25, 2017 and August 14, 2017, respectively.
In addition, the Letter Agreement extended the expiration date of stock options to purchase 5,435 shares of common stock of the Company, which was issued to Dr. Stone on February 11, 2003 with an exercise price of $8.28 per share. The expiration date of such stock option was extended to February 11, 2018.
In October 2015, the Company entered into an agreement with CrossCart to amend (i) certain warrants to purchase an aggregate of 174,883 shares of common stock and (ii) certain warrants to purchase $1,012,500 worth of Series C preferred stock. The amendment (i) prohibits the holder from exercising these warrants prior to the completion of the offering; and (ii) removes the provision in certain preferred stock warrants that would cause such warrants to expire upon completion of the this offering, thus allowing such warrants to continue to be outstanding after closing of this offering.
In October 2015, the Company entered into an agreement with Dr. Kevin Stone to amend (i) certain warrants to purchase an aggregate of 128,878 shares of common stock and (ii) Series C Warrants. The amendment prohibits the holder from exercising these warrants prior to the completion of the offering.
In October 2015, the Company entered into an agreement with CrossCart to amend certain convertible promissory notes in the principal amount of $6,520,015. The amendment provides that these notes will automatically convert into such number of shares of the Company’s common stock equal to the quotient obtained by dividing (A) the outstanding principal amount and accrued interests due under the note and (B) the applicable conversion price of the note. Furthermore, pursuant to the amendment, CrossCart LLC waives all defaults under the notes as a result of any non-payment of the notes by the Company and this waiver expires on March 31, 2016.
Transaction between CrossCart and Lurie Investment
On August 19, 2013, CrossCart, Dr. Stone, Lurie Investment Fund, L.L.C. (“Lurie”) and Alfa-Tech, L.L.C. (“Alfa-Tech” and, together with “Lurie”, the “Lurie Entities”) entered into a Stock and Note Purchase Agreement (the “Lurie Purchase Agreement”), pursuant to which CrossCart purchased from the Lurie Entities the following securities of the Company held by Lurie: (i) an aggregate of 703,963 shares of Series B Preferred Stock; (ii) an aggregate of 384,964 shares of Series C Preferred Stock; (iii) an aggregate of 50,957 shares of Series C-2 Preferred Stock; (iv) warrants to purchase an aggregate of 50,951 shares of Series C-1 Preferred Stock at an exercise price of $8.83 per share; (v) warrants to purchase an aggregate of 63,689 shares of Series C-2 Preferred Stock at an exercise price of $8.83 per share; (vi) warrants to purchase an aggregate of 174,883 shares of common stock at an exercise price of 0.18 per share; and (vi) convertible promissory notes in the aggregate principal amount of $5,000,000 (collectively, the “Lurie Securities”). In exchange for the Lurie Securities, CrossCart agreed to pay the Lurie Entities cash consideration of $1.00 and certain contingent consideration upon the consummation of a sale of the Company, including the occurrence of a “deemed liquation event” as defined in the Company’s Certificate of Incorporation (the “Lurie Contingent Consideration”). In general, the Lurie Contingent Consideration shall equal to 20% of the gross proceeds received by CrossCart from the Company in such sale to the extent the proceeds exceed CrossCart’s capital contribution made by the purchaser to the Company.
Change of Control Bonus
On March 3, 2012, the Company awarded Dr. Stone a bonus (the “Stone Bonus Agreement”) payable in the event of an acquisition of the Company. An acquisition is defined as a sale, merger, consolidation, transfer or other disposition of all or substantially all (more than 80%) of the Company to another entity (the “Acquisition”). An initial public offering of the Company’s common stock pursuant to an effective registration statement filed with the Commission under the Securities Act of 1933, as amended (the “Securities Act”), does not constitute an Acquisition. Dr. Stone was entitled to receive a bonus award in an amount equal to 1% of the proceeds received in the Acquisition that occurred on or prior to December 16, 2013. On December 16, 2013, the Board amended and extended the Stone Bonus Agreement to cover any Acquisition that occurs on or before the completion of an initial public offering of the Company’s stock pursuant to an effective registration statement filed with the Commission under the Securities Act. In September 2015, the Company and Dr. Stone entered into an amendment to the Stone Bonus Agreement, pursuant to which the Stone Bonus Agreement will be terminated, and no bonus will be paid to Dr. Stone, upon the completion of this offering.
Transactions with Bruce Stone
Between May 22, 2014 and June 2, 2015, the Company issued three convertible promissory notes in the amount of $50,000, $35,000 and $40,000, respectively, to Mr. Bruce Stone, who is the brother of Dr. Kevin Stone, pursuant to Note Purchase Agreements dated April 17, 2014, November 12, 2014 and May 20, 2015, respectively (collectively, the “Bruce Stone Notes”). Each note bears interest at a rate of 8% per annum, which may be increased to 10% per annum if an event of default occurs. In general, all principal and interest accrued under each Bruce Stone Note are due and payable, upon demand at the sole option of Mr. Stone, on or after two years after the date of issuance. The notes contain customary events of default. The principal and any accrued interest under the Bruce Stone Notes are convertible, at Mr. Stone’s sole discretion, into shares of Series D Preferred Stock and in the case of the notes issued on November 12, 2014, into shares of Series E Preferred Stock, of the Company at an initial conversion price of $4.60 per share. Under the terms of the Bruce Stone Notes, the Company is required to use reasonable efforts to cause a sufficient number of shares of Series D Preferred Stock
authorized and reserved for issuance upon conversion of the notes. As of June 30, 2015 the total amount outstanding under the Bruce Stone Notes was $131,407, including accrued interest. In addition, in October 2015 we entered into an agreement with Mr. Stone to provide that upon completion of this offering, all outstanding amount due under the Bruce Stone Notes will be automatically converted into shares of our common stock at the initial conversion price.
Management Retention Plan
On December 16, 2013, the Board approved the Management Retention Plan (the “Plan”), pursuant to which certain key employees, including Mr. Lee and Mr. Cocke, are entitled to receive a bonus upon the occurrence of specified events, provided that the participant must continue as an employee of the Company through the occurrence of the triggering event. Under the Plan, upon an external financing (beyond any financing from CrossCart) of at least $2.0 million or a signing of a license or distribution agreement with at least $2.0 million of upfront payments or milestone payments achievable within 12 months of signing, Mr. Lee is entitled to receive a bonus equal to $35,000 plus a salary delta amount (the “Salary Delta Amount”), and Mr. Cocke is entitled to receive a bonus equal to $15,000 plus the Salary Delta Amount. The Salary Delta Amount is calculated as the difference between the salary actually received after July 1, 2013 and the amount of salary such person would have received if the salary rate in effect during June 2013 had continued from July 2013 until the bonus is earned. In October 2015, the Board amended the Plan to provide that all bonuses (but not the Salary Delta Amount) under the Plan shall be paid in shares of common stock based on the public offering price in this offering.
In addition, the Plan also provides that upon closing of a deemed liquidation events specified in the Company’s certificate of incorporation or a sale of the Company’s securities representing 50% or more of total voting power of outstanding securities (the “Acquisition”), Mr. Lee and Mr. Cocke are entitled to receive certain bonus payments based, in part, on the amount of proceeds received by the Company in the Acquisition (the “Acquisition Bonus”). In October 2015, the Board amended the Plan to provide that the Acquisition Bonus will terminate and expire upon the completion of this offering.
Transactions with Directors
In December 2013, the Company entered into a letter agreement with each of David W. Anderson and Alfred G. Holcomb, two of our directors, to provide certain acquisition bonus payments (the “Director Bonus Agreement”), Under the Director Bonus Agreement, in the event of an acquisition of the Company or the occurrence of a “deemed liquidation” event under our certificate of incorporation (the “Acquisition”), each director will be eligible to receive the following cash bonus payments (the “Director Acquisition Bonuses”):
| | · | if the proceeds of the Acquisition is at least $15 million and less than $25 million, a cash bonus of $100,000; |
| | · | if the proceeds of the Acquisition is at least $25 million and less than $100,000 million, a cash bonus of $250,000; and |
| | · | if the proceeds of the Acquisition is at least $100 million, a cash bonus equal to 10% of 4% of such proceeds. |
In October 2015 we entered into an agreement with each of Mr. Anderson and Mr. Holcom to provide that the Director Acquisition Bonuses will terminate and expire immediately upon
completion of this offering.
Indemnification Agreements
We intend to enter into indemnification agreements with or have contractual obligations to provide indemnification to each of our directors and intend to enter into such agreements with certain of our executive officers. These agreements require us, among other things, to indemnify these individuals for certain expenses (including attorneys’ fees), judgments, fines and settlement amounts reasonably incurred by such person in any action or proceeding, including any action by or in our right, on account of any services undertaken by such person on behalf of our company or that person’s status as a member of our Board of Directors to the maximum extent allowed under Delaware law.
Policies and Procedures for Related Party Transactions
Following this offering, pursuant to the written charter of our Audit Committee, the Audit Committee will be responsible for reviewing and approving, prior to our entry into any such transaction, all related party transactions and potential conflict of interest situations involving a principal stockholder, a member of the Board of Directors or senior management.
Description of Capital Stock
The following descriptions are summaries of the material terms of our amended and restated certificate of incorporation and amended and restated bylaws, which will be effective upon completion of this offering. The descriptions of the common stock and preferred stock give effect to changes to our capital structure that will occur immediately prior to the completion of this offering. We refer in this section to our amended and restated certificate of incorporation as our certificate of incorporation, and we refer to our amended and restated bylaws as our bylaws.
General
Upon completion of this offering, our authorized capital stock will consist of 48,000,000 shares of common stock, par value $0.001 per share, and 2,000,000 shares of preferred stock, par value $0.001 per share, all of which shares of preferred stock will be undesignated.
As of June 30, 2015, 3,965,395 shares of our common stock were outstanding and held by 61 stockholders of record. This amount assumes (i) the conversion of all outstanding shares of our convertible preferred stock into common stock, which will occur immediately prior to the completion of this offering and (ii) the issuance of shares of common stock upon the conversion of all principal amount and accrued interests under our Notes (as discussed below) upon the completion of this offering, assuming that the offering is completed on September 30, 2015. In addition, as of June 30, 2015, we had outstanding options to purchase 350,421 shares of our common stock under our 2008 Stock Option Plan, and a predecessor plan, at a weighted average exercise price of $1.47 per share, of which options to purchase an aggregate of 328,468 were exercisable.
Common Stock
The holders of our common stock are entitled to one vote for each share held on all matters submitted to a vote of the stockholders. The holders of our common stock do not have any cumulative voting rights. Holders of our common stock are entitled to receive ratably any dividends declared by the Board of Directors out of funds legally available for that purpose, subject to any preferential dividend rights of any outstanding preferred stock. Our common stock has no preemptive rights, conversion rights or other subscription rights or redemption or sinking fund provisions.
In the event of our liquidation, dissolution or winding up, holders of our common stock will be entitled to share ratably in all assets remaining after payment of all debts and other liabilities and any liquidation preference of any outstanding preferred stock. The shares to be issued by us in this offering will be, when issued and paid for, validly issued, fully paid and non-assessable.
Preferred Stock
Immediately prior to the completion of this offering, we expect that all outstanding shares of our convertible preferred stock will be converted into shares of our common stock. Immediately prior to the completion of this offering, our amended and restated certificate of incorporation will be amended and restated to delete all references to such shares of convertible preferred stock. Upon the completion of this offering, our Board of Directors will have the authority, without
further action by our stockholders, to issue up to 2,000,000 shares of preferred stock in one or more series and to fix the rights, preferences, privileges and restrictions thereof. These rights, preferences and privileges could include dividend rights, conversion rights, voting rights, terms of redemption, liquidation preferences, sinking fund terms and the number of shares constituting, or the designation of, such series, any or all of which may be greater than the rights of common stock. The issuance of our preferred stock could adversely affect the voting power of holders of common stock and the likelihood that such holders will receive dividend payments and payments upon our liquidation. In addition, the issuance of preferred stock could have the effect of delaying, deferring or preventing a change in control of our company or other corporate action. Immediately after completion of this offering, no shares of preferred stock will be outstanding, and we have no present plan to issue any shares of preferred stock.
Convertible Promissory Notes
In fiscal 2012, we borrowed $3,250,000 through the issuance of certain convertible promissory notes to existing stockholders. These notes accrue interest at 8% per year and are convertible into shares of the Company’s Series C-2 Preferred Stock at a conversion price of $8.83 per share (the “Series C-2 Notes”). In fiscal 2013 and 2014, we borrowed $4,181,027 through the issuance of certain convertible promissory notes to new and existing investors. These notes accrue interest at 8% and are due upon demand of the holder. The holders may, in their sole discretion, convert all principal and accrued interest under these notes into shares of the Company’s Series D Preferred Stock at a conversion price of $4.60 per share (the “Series D Notes”). In 2015, we borrowed $780,000 through the issuance of certain convertible promissory notes to existing and new investors. These notes accrue interest at a rate of 8% per year and are convertible into shares of the Company’s Series D or Series E Preferred Stock at a conversion price of $4.60 per share (the “Series E Notes”, and together with the Series C-2 Notes and Series D Notes, the “Notes”).
In October 2015 we entered into an agreement with each holder of the Notes to provide that upon the completion of this offering all principal and accrued interest under the Notes will be converted automatically into shares of common stock, based on the initial conversion price. In addition, under this agreement, each noteholder agreed to waive any default under the notes as a result of any non-payment by the Company of the amount due under the notes and this waiver will expire on March 31, 2016. For more information on the Notes, see “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Liquidity and Capital Resources—Convertible Note Financings.”
Warrants
As of June 30, 2015, we had outstanding warrants to purchase 51,868 shares of Series C convertible preferred stock and 63,689 shares of Series C-2 convertible preferred stock, together which are exercisable for an aggregate of 115,557 shares of common stock upon the completion of this offering. In addition, as of June 30, 2015, we had outstanding warrants to purchase an aggregate of 544,380 shares of our common stock.
On August 19, 2013, our largest stockholder, CrossCart LLC purchased preferred stock, promissory notes and warrants for common stock and preferred stock previously held by Lurie Investment Fund, LLC, another stockholder of the Company. The warrants have a net exercise provision and contain provisions for the adjustment of the exercise price and the number of shares issuable upon the exercise of the warrant in the event of certain stock dividends, stock splits, recapitalizations, reclassifications and consolidations. The warrants for preferred stock are exercisable until their expiration on either (i) the earlier of certain dates in 2020 or a sale or merger of the Company, or (ii) the earlier of certain dates in 2022 or the conversion of the
promissory notes as noted above. The warrants for common stock are exercisable until their expiration on June 9, 2018. Pursuant to the terms of the preferred stock warrants, these warrants will be converted automatically into warrants to purchase shares of common stock upon completion of this offering. In October 2015, we entered into agreements with our warrant holders pursuant to which the holders agreed not to exercise these warrants prior to the completion of this offering.
Registration Rights
Upon the completion of this offering, the holders of approximately 4.3 million shares of our common stock, including shares issuable upon the conversion of our convertible preferred stock and Notes, or their permitted transferees, are entitled to rights with respect to the registration of these securities under the Securities Act, which we refer to as our registrable securities. These rights are provided under the terms of an investor rights agreement between us and certain holders of our common stock, Series A convertible preferred stock, Series B convertible preferred stock, and Series C convertible preferred stock. The investor rights agreement includes demand registration rights, short-form registration rights and piggyback registration rights. All fees, costs and expenses of underwritten registrations under these agreements will be borne by us and all selling expenses, including underwriting discounts and selling commissions, will be borne by the holders of the shares being registered.
Demand Registration Rights
Beginning 180 days after the completion of this offering, the holders of at least 60% of our Series C convertible preferred stock are entitled to demand registration rights. Under the terms of the investor rights agreement, upon the written request of such holders to sell registrable securities with an anticipated aggregate offering price (net of underwriting discounts and commissions) of at least $5.0 million, we will be required to use our best efforts to file a registration statement covering the offering and sale of such securities and use reasonable, diligent efforts to effect the registration of all or a portion of these securities for public resale. We are required to effect only two registrations pursuant to this provision of the investor rights agreement. In the event we register securities in connection with an underwritten offering, the underwriters will have the right to limit the number of shares included in such offering.
Short-Form Registration Rights
Upon the completion of this offering, the holders of at least 30% of our registrable securities are also entitled to short form registration rights. Pursuant to the investor rights agreement, if we are eligible to file a registration statement on Form S-3, upon the written request of such holders to sell registrable securities, we will be required to use our best efforts to effect a registration of such securities. In the event we register securities in connection with an underwritten offering, the underwriters will have the right to limit the number of shares included in such offering.
Piggyback Registration Rights
Upon the completion of this offering, the holders of our registrable securities are entitled to piggyback registration rights. If we register any of our securities either for our own account or for the account of other security holders, such holders are entitled to include their shares in the registration. In the event we register securities in connection with an underwritten offering, the underwriters will have the right to limit the number of shares included in such offering.
Indemnification
Our investor rights agreement contains customary cross-indemnification provisions, under which we are obligated to indemnify holders of registrable securities in the event of material
misstatements or omissions in the registration statement attributable to us, and they are obligated to indemnify us for material misstatements or omissions attributable to them.
Expiration of Registration Rights
The registration rights granted under the investor rights agreement will terminate after the earlier of (i) the fifth anniversary of the Company’s first underwritten public offering of its common stock under the Securities Act, (ii) with respect to any holder of registrable securities, the date on which all of such holder’s shares can be sold during a three-month period without registration in reliance on Rule 144 under the Securities Act, (iii) the closing of a deemed liquidation event or (iv) termination of the agreement upon consent of the parties.
Anti-Takeover Effects of Our Certificate of Incorporation and Bylaws and Delaware Law
Our certificate of incorporation and bylaws include a number of provisions that may have the effect of delaying, deferring or preventing another party from acquiring control of us and encouraging persons considering unsolicited tender offers or other unilateral takeover proposals to negotiate with our Board of Directors rather than pursue non-negotiated takeover attempts. These provisions include the items described below.
Board Composition and Filling Vacancies
Our certificate of incorporation provides for the division of our Board of Directors into three classes serving staggered three-year terms, with one class being elected each year. Our certificate of incorporation also provides that directors may be removed only for cause and then only by the affirmative vote of the holders of 75% or more of the shares then entitled to vote at an election of directors. Furthermore, any vacancy on our Board of Directors, however occurring, including a vacancy resulting from an increase in the size of our Board, may only be filled by the affirmative vote of a majority of our directors then in office even if less than a quorum. The classification of directors, together with the limitations on removal of directors and treatment of vacancies, has the effect of making it more difficult for stockholders to change the composition of our Board of Directors.
No Written Consent of Stockholders
Our certificate of provides that all stockholder actions are required to be taken by a vote of the stockholders at an annual or special meeting, and that stockholders may not take any action by written consent in lieu of a meeting. This limit may lengthen the amount of time required to take stockholder actions and would prevent the amendment of our bylaws or removal of directors by our stockholders without holding a meeting of stockholders.
Meetings of Stockholders
Our certificate of incorporation and bylaws provide that only a majority of the members of our Board of Directors then in office may call special meetings of stockholders and only those matters set forth in the notice of the special meeting may be considered or acted upon at a special meeting of stockholders. Our bylaws limit the business that may be conducted at an annual meeting of stockholders to those matters properly brought before the meeting.
Advance Notice Requirements
Our bylaws establish advance notice procedures with regard to stockholder proposals relating to the nomination of candidates for election as directors or new business to be brought before meetings of our stockholders. These procedures provide that notice of stockholder proposals must be timely given in writing to our corporate secretary prior to the meeting at which the action is to be taken. Generally, to be timely, notice must be received at our principal executive offices not less than 90 days nor more than 120 days prior to the first anniversary date of the annual meeting for the preceding year. Our bylaws specify the requirements as to form and content of all stockholders’ notices. These requirements may preclude stockholders from bringing matters before the stockholders at an annual or special meeting.
Amendment to Certificate of Incorporation and Bylaws
Any amendment of our certificate of incorporation must first be approved by a majority of our Board of Directors, and if required by law or our certificate of incorporation, must thereafter be approved by a majority of the outstanding shares entitled to vote on the amendment and a majority of the outstanding shares of each class entitled to vote thereon as a class, except that the amendment of the provisions relating to stockholder action, Board composition, limitation of liability and the amendment of our bylaws and certificate of incorporation must be approved by not less than 75% of the outstanding shares entitled to vote on the amendment, and not less than 75% of the outstanding shares of each class entitled to vote thereon as a class. Our bylaws may be amended by the affirmative vote of a majority of the directors then in office, subject to any limitations set forth in the bylaws; and may also be amended by the affirmative vote of at least 75% of the outstanding shares entitled to vote on the amendment, or, if our Board of Directors recommends that the stockholders approve the amendment, by the affirmative vote of the majority of the outstanding shares entitled to vote on the amendment, in each case voting together as a single class.
Undesignated Preferred Stock
Our certificate of incorporation provides for 2,000,000 authorized shares of preferred stock. The existence of authorized but unissued shares of preferred stock may enable our Board of Directors to discourage an attempt to obtain control of us by means of a merger, tender offer, proxy contest or otherwise. For example, if in the due exercise of its fiduciary obligations, our Board of Directors were to determine that a takeover proposal is not in the best interests of our stockholders, our Board of Directors could cause shares of preferred stock to be issued without stockholder approval in one or more private offerings or other transactions that might dilute the voting or other rights of the proposed acquirer or insurgent stockholder or stockholder group. In this regard, our certificate of incorporation grants our Board of Directors broad power to establish the rights and preferences of authorized and unissued shares of preferred stock. The issuance of shares of preferred stock could decrease the amount of earnings and assets available for distribution to holders of shares of common stock. The issuance may also adversely affect the rights and powers, including voting rights, of these holders and may have the effect of delaying, deterring or preventing a change in control of us.
Section 203 of the Delaware General Corporation Law
Upon completion of this offering, we will be subject to the provisions of Section 203 of the Delaware General Corporation Law. In general, Section 203 prohibits a publicly held Delaware corporation from engaging in a “business combination” with an “interested stockholder” for a three-year period following the time that this stockholder becomes an interested stockholder, unless the business combination is approved in a prescribed manner. Under Section 203, a
business combination between a corporation and an interested stockholder is prohibited unless it satisfies one of the following conditions:
| | · | before the stockholder became interested, our Board of Directors approved either the business combination or the transaction which resulted in the stockholder becoming an interested stockholder; |
| | · | upon consummation of the transaction which resulted in the stockholder becoming an interested stockholder, the interested stockholder owned at least 85% of the voting stock of the corporation outstanding at the time the transaction commenced, excluding for purposes of determining the voting stock outstanding shares owned by persons who are directors and also officers, and employee stock plans, in some instances, but not the outstanding voting stock owned by the interested stockholder; or |
| | · | at or after the time the stockholder became interested, the business combination was approved by our Board of Directors and authorized at an annual or special meeting of the stockholders by the affirmative vote of at least two-thirds of the outstanding voting stock which is not owned by the interested stockholder. |
Section 203 defines a business combination to include:
| | · | any merger or consolidation involving the corporation and the interested stockholder; |
| | · | any sale, transfer, lease, pledge or other disposition involving the interested stockholder of 10% or more of the assets of the corporation; |
| | · | subject to exceptions, any transaction that results in the issuance or transfer by the corporation of any stock of the corporation to the interested stockholder; |
| | · | subject to exceptions, any transaction involving the corporation that has the effect of increasing the proportionate share of the stock of any class or series of the corporation beneficially owned by the interested stockholder; and |
| | · | the receipt by the interested stockholder of the benefit of any loans, advances, guarantees, pledges or other financial benefits provided by or through the corporation. |
In general, Section 203 defines an interested stockholder as any entity or person beneficially owning 15% or more of the outstanding voting stock of the corporation and any entity or person affiliated with or controlling or controlled by the entity or person.
Shares Eligible for Future Sale
Prior to this offering, there has been no public market for our common stock, and we cannot predict the effect, if any, that market sales of shares of our common stock or the availability of shares of our common stock for sale will have on the market price of our common stock prevailing from time to time. Nevertheless, sales of substantial amounts of our common stock, including shares issued upon exercise of outstanding options or warrants, or the perception that these sales could occur in the public market after this offering could adversely affect market prices prevailing from time to time and could impair our ability to raise capital through the sale of our equity securities.
Based on the number of shares of common stock outstanding as of June 30, 2015, upon the closing of this offering, 6,465,396 shares of common stock will be outstanding. The number of shares outstanding upon completion of this offering assumes:
| | · | the conversion of all of our outstanding shares of convertible preferred stock into an aggregate of 2,038,314 shares of common stock upon the completion of this offering, including shares of common stock to be issued as cumulative dividends accrued under such preferred stock; |
| | · | the issuance of 1,704,713 shares of common stock upon the conversion of approximately an aggregate of $9,838,058 in outstanding principal and accrued interest on our convertible promissory notes (which is currently convertible into shares of our preferred stock but will be amended to be convertible into shares of our common stock in connection with the offering), upon the completion of this offering, at an average conversion price of $5.71 per share, assuming that the offering is completed on September 30, 2015; and |
| | · | no exercise of outstanding options or warrants. |
However, because the number of shares of common stock that will be issued upon conversion of the convertible notes depends in part on the closing date of this offering, the actual number of shares issuable upon such conversion may be different from the amount we have assumed for purposes of this discussion. See “Offering Circular Summary - The Offering.”
All of the shares sold in this offering will be freely tradable unless purchased by our affiliates. The remaining 3,965,396 shares of common stock outstanding after this offering will be restricted as a result of securities laws or lock-up agreements as described below. Following the expiration of the lock-up period, all shares will be eligible for resale in compliance with Rule 144 or Rule 701 to the extent these shares have been released from any repurchase option that we may hold.
Rule 144
In general, under Rule 144 as currently in effect, any person who is or has been an affiliate of ours during the 90 days immediately preceding the sale and who has beneficially owned shares for at least six months is entitled to sell, within any three-month period commencing 90 days after the date of this Offering Circular, a number of shares that does not exceed the greater of:
| | · | 1% of the then-outstanding shares of common stock, which will equal approximately 64,654 shares immediately after this offering; and |
| | · | the average weekly trading volume during the four calendar weeks preceding the sale, subject to the filing of a Form 144 with respect to the sale. |
Sales under Rule 144 by our affiliates are also subject to certain manner of sale provisions and notice requirements and to the availability of current public information about us.
A person who is not deemed to have been an affiliate of ours at any time during the 90 days immediately preceding the sale and who has beneficially owned his or her shares for at least six months is entitled to sell his or her shares under Rule 144 without regard to the limitations described above, subject only to the availability of current public information about us during the six months after the initial six-month holding period is met. After a non-affiliate has beneficially owned his or her shares for one year or more, he or she may freely sell his or her shares under Rule 144 without complying with any Rule 144 requirements.
We are unable to estimate the number of shares that will be sold under Rule 144, since this will depend on the market price for our common stock, the personal circumstances of the sellers and other factors. Prior to the offering, there has been no public market for the common stock, and there can be no assurance that a significant public market for the common stock will develop or be sustained after the offering. Any future sale of substantial amounts of the common stock in the open market may adversely affect the market price of the common stock offered by this Offering Circular.
Rule 701
In general, under Rule 701 under the Securities Act, any of our employees, directors, consultants or advisors who purchased shares from us in connection with a qualified compensatory stock or option plan or other written agreement and in compliance with Rule 701, is eligible to resell those shares 90 days after the effective date of this offering in reliance on Rule 144, but without compliance with the various restrictions, including the holding period, contained in Rule 144.
Lock-up and Market Stand-Off Agreements
We, our directors and executive officers, and certain of our other stockholders have agreed that, subject to certain exceptions, they will not sell any common stock without the prior written consent of W.R. Hambrecht + Co., LLC for a period of 90 days from the date of this Offering Circular.
In addition to the restrictions contained in the lock-up agreements described above, we have entered into agreements with certain security holders, including the Second Amended and Restated Investor Rights Agreement and our standard form of stock option agreement, that contain certain market stand-off provisions imposing restrictions on the ability of such security holders to offer, sell, or transfer our equity securities for a period of 180 days following the date of this Offering Circular.
LEGAL MATTERS
The validity of the issuance of the shares of common stock offered by this Offering Circular will be passed upon for us by Morgan Lewis & Bockius, LLP, San Francisco, California. Certain legal matters in connection with this offering will be passed upon for the underwriter by Wyrick Robbins Yates & Ponton LLP, Raleigh, North Carolina.
EXPERTS
The financial statements as of September 30, 2014 and 2013 and for each of the two years in the period ended September 30, 2014, included in this Offering Circular have been so included in reliance on the report (which contains an explanatory paragraph relating to our ability to continue as a going concern as described in Note 2 to the Financial Statements), by Marcum LLP, an independent registered public accounting firm, given on the authority of such firm as experts in auditing and accounting.
WHERE YOU CAN FIND MORE INFORMATION
We have filed an offering statement on Form 1-A with the SEC under the Securities Act with respect to the common stock offered by this Offering Circular. This Offering Circular, which constitutes a part of the offering statement, does not contain all of the information set forth in the offering statement or the exhibits and schedules filed therewith. For further information with respect to us and our common stock, please see the offering statement and the exhibits and schedules filed with the offering statement. Statements contained in this Offering Circular regarding the contents of any contract or any other document that is filed as an exhibit to the offering statement are not necessarily complete, and each such statement is qualified in all respects by reference to the full text of such contract or other document filed as an exhibit to the offering statement. The offering statement, including its exhibits and schedules, may be inspected without charge at the public reference room maintained by the SEC, located at 100 F Street, N.E., Room 1580, Washington, D.C. 20549, and copies of all or any part of the offering statement may be obtained from such offices upon the payment of the fees prescribed by the SEC. Please call the SEC at 1-800-SEC-0330 for further information about the public reference room. The SEC also maintains an Internet website that contains reports, proxy and information statements and other information regarding registrants that file electronically with the SEC. The address of the site iswww.sec.gov.
Upon completion of this offering, we will become subject to the information and periodic reporting requirements of the Exchange Act, and, in accordance therewith, will file periodic reports, proxy statements and other information with the SEC. Such periodic reports, proxy statements and other information will be available for inspection and copying at the public reference room and on the SEC website referred to above.
We also maintain a website at www.aperionbiologics.com. Upon completion of this offering, you may access these materials at our website free of charge as soon as reasonably practicable after they are electronically filed with, or furnished to, the SEC. Information contained on our website is not a part of this Offering Circular and the inclusion of our website address in this Offering Circular is an inactive textual reference only.
Aperion Biologics, Inc.
Financial Statements
September 30, 2014 and 2013
Aperion Biologics, Inc. Table of Contents |
Report of Independent Registered Public Accounting Firm
To the Audit Committee of the Board of Directors and Stockholders ofAperion Biologics, Inc.
We have audited the accompanying balance sheets of Aperion Biologics, Inc. (the "Company") as of September 30, 2014 and 2013, and the related statements of operations, changes in stockholders' deficiency, and cash flows for the fiscal years then ended, and the related notes to the financial statements. These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements based on our audit.
We conducted our audit in accordance with standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free from material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company's internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audit provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of Aperion Biologics, Inc. as of September 30, 2014 and 2013, and the results of its operations and its cash flows for the fiscal years then ended in accordance with accounting principles generally accepted in the United States of America.
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 2 to the financial statements, the Company has not generated any material revenues and has incurred net losses since inception. These conditions raise substantial doubt about the Company's ability to continue as a going concern. Management's plans in regard to these matters are also described in Note 2. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Marcum LLP
New York, NY
September 21, 2015, except for Note 1A, as to which the date is ____________, 2015.
The foregoing report is in the form that will be signed upon the completion of the reverse stock split described in Note 1A to the financial statements.
/s/ Marcum LLP
Marcum LLP
New York, NY
October 23, 2015
Aperion Biologics, Inc. Balance Sheets September 30, 2014 and 2013 |
| | | 2014 | | | 2013 | |
| Assets | | | | | | | | |
| | | | | | | | | |
| Current Assets | | | | | | | | |
| Cash | | $ | 124,769 | | | $ | 38,126 | |
| Inventory | | | 62,054 | | | | 62,175 | |
| Prepaid expenses and other current assets | | | 8,185 | | | | 99,324 | |
| | | | | | | | | |
| Total current assets | | | 195,008 | | | | 199,625 | |
| | | | | | | | | |
| Property and Equipment - net | | | 61,453 | | | | 53,266 | |
| | | | | | | | | |
| Total Assets | | $ | 256,461 | | | $ | 252,891 | |
| | | | | | | | | |
| | | | | | | | | |
| Liabilities and Stockholders' Deficiency | | | | | | | | |
| | | | | | | | | |
| Current Liabilities | | | | | | | | |
| Accounts payable | | $ | 607,395 | | | $ | 784,832 | |
| Convertible promissory notes | | | 6,669,000 | | | | 5,624,000 | |
| Advances payable | | | 100,000 | | | | 100,000 | |
| Accrued dividends | | | 3,363,015 | | | | 2,568,789 | |
| Payroll liabilities | | | 157,035 | | | | 31,407 | |
| Accrued liabilities | | | 1,430,995 | | | | 717,253 | |
| | | | | | | | | |
| Total current liabilities | | | 12,327,440 | | | | 9,826,281 | |
| | | | | | | | | |
| Convertible promissory notes, net of current maturities | | | 707,027 | | | | - | |
| Total liabilities | | | 13,034,467 | | | | 9,826,281 | |
| | | | | | | | | |
| Commitments and contingencies | | | | | | | | |
| | | | | | | | | |
| Redeemable convertible preferred stock - $0.001 par value | | | | | | | | |
| Series C - liquidation preference of $8,723,271 and $8,441,231, at September 30, 2014 and 2013, respectively, 10,000,000 shares designated, 399,173 shares issued and outstanding at September 30, 2014 and 2013 | | | 6,860,823 | | | | 6,860,823 | |
| Series C-2 - liquidation preference of $8,093,071 and $7,580,885, at September 30, 2014 and 2013, respectively, 18,000,000 shares designated, 724,901 shares issued and outstanding at September 30, 2014 and 2013 | | | 6,352,052 | | | | 6,352,052 | |
| | | | | | | | | |
| Total redeemable convertible preferred stock | | | 13,212,875 | | | | 13,212,875 | |
| | | | | | | | | |
| Stockholders' Deficiency | | | | | | | | |
| Convertible preferred stock - $0.001 par value, 35,250,000 shares authorized | | | | | | | | |
| Series A - liquidation preference of $669,453, 750,000 shares designated, 36,383 shares issued and outstanding at September 30, 2014 and 2013 | | | 669,453 | | | | 669,453 | |
| Series B - liquidation preference of $13,617,853, 6,500,000 shares designated, 161,674 shares issued and outstanding at September 30, 2014 and 2013 | | | 13,617,853 | | | | 13,617,853 | |
| Common stock - $0.001 par value | | | | | | | | |
| 60,000,000 shares authorized, 219,651 shares issued and outstanding at September 30, 2014 and 2013 | | | 4,042 | | | | 4,042 | |
| Additional paid-in capital | | | 322,260 | | | | 235,038 | |
| Accumulated deficit | | | (40,604,489 | ) | | | (37,312,651 | ) |
| Total stockholders' deficiency | | | (25,990,881 | ) | | | (22,786,265 | ) |
| | | | | | | | | |
| Total Liabilities and Stockholders' Deficiency | | $ | 256,461 | | | $ | 252,891 | |
The accompanying notes are an integral part of these financial statements
Aperion Biologics, Inc. Statements of Operations Years Ended September 30, 2014 and 2013 |
| | | 2014 | | | 2013 | |
| | | | | | | |
| Revenue | | $ | - | | | $ | - | |
| | | | | | | | | |
| Costs and expenses: | | | | | | | | |
| Clinical studies | | | 296,508 | | | | 870,302 | |
| Research and development | | | 59,245 | | | | 417,844 | |
| Sales, general, and administrative | | | 1,496,528 | | | | 2,101,434 | |
| Total costs and expenses | | | 1,852,281 | | | | 3,389,580 | |
| | | | | | | | | |
| Operating loss | | | (1,852,281 | ) | | | (3,389,580 | ) |
| | | | | | | | | |
| Other income (expense): | | | | | | | | |
| Interest income | | | - | | | | 20 | |
| Interest expense | | | (645,331 | ) | | | (410,400 | ) |
| Total other expense | | | (645,331 | ) | | | (410,380 | ) |
| | | | | | | | | |
| Net loss | | | (2,497,612 | ) | | | (3,799,960 | ) |
| Preferred stock: | | | | | | | | |
| Series C convertible contractual dividends | | | (282,040 | ) | | | (282,040 | ) |
| Series C convertible deemed dividends | | | - | | | | (838,753 | ) |
| Series C-2 convertible contractual dividends | | | (512,186 | ) | | | (529,141 | ) |
| | | | | | | | | |
| Loss attributable to common stockholders | | $ | (3,291,838 | ) | | $ | (5,449,894 | ) |
| | | | | | | | | |
| Per share data: | | | | | | | | |
| Net loss - basic and diluted | | $ | (11.41 | ) | | $ | (17.30 | ) |
| Series C convertible contractual dividends | | | (1.29 | ) | | | (1.29 | ) |
| Series C convertible deemed dividends | | | - | | | | (3.86 | ) |
| Series C-2 convertible contractual dividends | | | (2.21 | ) | | | (2.39 | ) |
| | | | | | | | | |
| Net loss attributable to common stockholders - basic and diluted | | $ | (14.90 | ) | | $ | (24.84 | ) |
| | | | | | | | | |
| Weighted average number of common shares outstanding - basic and diluted | | | 219,651 | | | | 219,267 | |
The accompanying notes are an integral part of these financial statements
Aperion Biologics, Inc. Statements of Changes in Stockholders' Deficiency Years Ended September 30, 2014 and 2013 |
| | | Preferred Stock | | | | | | Additional | | | | | | | |
| | | Series A | | | Series B | | | Common Stock | | | Paid-In | | | Accumulated | | | | |
| | | Shares | | | Amount | | | Shares | | | Amount | | | Shares | | | Amount | | | Capital | | | Deficit | | | Total | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Balance at September 30, 2012 | | | 36,383 | | | $ | 669,453 | | | | 161,674 | | | $ | 13,617,853 | | | | 218,919 | | | $ | 4,028 | | | $ | 131,442 | | | $ | (31,862,757 | ) | | $ | (17,439,981 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Exercise of stock options | | | - | | | | - | | | | - | | | | - | | | | 733 | | | | 14 | | | | 642 | | | | - | | | | 656 | |
| Stock-based compensation | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | 102,954 | | | | - | | | | 102,954 | |
| Preferred stock dividends | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | (811,181 | ) | | | (811,181 | ) |
| Preferred stock accretion | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | (838,753 | ) | | | (838,753 | ) |
| Net loss - year ended September 30, 2013 | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | (3,799,960 | ) | | | (3,799,960 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Balance at September 30, 2013 | | | 36,383 | | | | 669,453 | | | | 161,674 | | | | 13,617,853 | | | | 219,651 | | | | 4,042 | | | | 235,038 | | | | (37,312,651 | ) | | | (22,786,265 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Stock-based compensation | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | 87,222 | | | | - | | | | 87,222 | |
| Preferred stock dividends | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | (794,226 | ) | | | (794,226 | ) |
| Net loss - year ended September 30, 2014 | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | (2,497,612 | ) | | | (2,497,612 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Balance at September 30, 2014 | | | 36,383 | | | $ | 669,453 | | | | 161,674 | | | $ | 13,617,853 | | | | 219,651 | | | $ | 4,042 | | | | $ 322,260 | | | $ | (40,604,489 | ) | | $ | (25,990,881 | ) |
The accompanying notes are an integral part of these financial statements
Aperion Biologics, Inc. Statements of Cash Flows Years Ended September 30, 2014 and 2013 |
| | | 2014 | | | 2013 | |
| | | | | | | |
| Cash Flows From Operating Activities | | | | | | | | |
| Net loss | | $ | (2,497,612 | ) | | $ | (3,799,960 | ) |
| | | | | | | | | |
| Adjustments to reconcile net loss to net cash used in operating activities: | | | | | | | | |
| Stock-based compensation | | | 87,222 | | | | 102,954 | |
| Depreciation and amortization | | | 33,928 | | | | 59,274 | |
| Changes in operating assets and liabilities: | | | | | | | | |
| Inventory | | | 121 | | | | 42,215 | |
| Prepaid expenses and other current assets | | | 91,139 | | | | (39,581 | ) |
| Accounts payable | | | (177,437 | ) | | | 326,949 | |
| Accrued liabilities | | | 839,370 | | | | 512,555 | |
| Net cash used in operating activities | | | (1,623,269 | ) | | | (2,795,594 | ) |
| | | | | | | | | |
| Cash Flows From Investing Activities | | | | | | | | |
| Purchase of property and equipment | | | (42,115 | ) | | | (15,876 | ) |
| Net cash used in investing activities | | | (42,115 | ) | | | (15,876 | ) |
| | | | | | | | | |
| Cash Flows From Financing Activities | | | | | | | | |
| Proceeds from the issuance of convertible promissory notes | | | 1,807,027 | | | | 2,374,000 | |
| Payments of convertible promissory notes | | | (55,000 | ) | | | - | |
| Stock options exercised | | | - | | | | 656 | |
| Net cash provided by financing activities | | | 1,752,027 | | | | 2,374,656 | |
| | | | | | | | | |
| Net increase (decrease) in cash | | | 86,643 | | | | (436,814 | ) |
| | | | | | | | | |
| Cash at beginning of period | | | 38,126 | | | | 474,940 | |
| | | | | | | | | |
| Cash at end of period | | $ | 124,769 | | | $ | 38,126 | |
| | | | | | | | | |
| Supplemental Disclosures of Cash Flow Information | | | | | | | | |
| | | | | | | | | |
| Cash paid for interest | | $ | 3,190 | | | $ | - | |
| | | | | | | | | |
| Supplemental Disclosures of Noncash Flow Information | | | | | | | | |
| | | | | | | | | |
| Accrual of preferred stock dividends | | $ | 794,226 | | | $ | 811,181 | |
| | | | | | | | | |
| Accretion of preferred stock to liquidation value | | $ | - | | | $ | 838,753 | |
The accompanying notes are an integral part of these financial statements
Aperion Biologics, Inc. Notes to Financial Statements September 30, 2014 and 2013 |
| 1. | Summary of Significant Accounting Policies |
Business Organization and Nature of Operations
Aperion Biologics, Inc. ("ABI" or the "Company") is a clinical-stage medical device company that develops patented technology to make animal tissues usable for human medical applications without causing rejection by the body. The core platform technology is an enzymatic stripping of the key carbohydrate antigens followed by a unique conversion process that both "humanizes" and sterilizes the tissues without unduly affecting their biomechanical or biological properties.
The Company's primary activities since its inception have been the development and implementation of its business plans, performing research on its platform technology, conducting clinical trials on its products in both animals and humans, pursuing regulatory approvals to market its product in US and international markets, and raising capital. To date, the Company has not generated any material revenues from operations.
The Company was founded in October 1996 under the name CrossCart, Inc. The Company changed its name to Aperion Biologics, Inc. (a Delaware corporation) in May 2009. To date, the Company has funded its business with the proceeds of private placements of equity and debt securities.
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America ("U.S. GAAP") requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates. The Company's significant estimates and assumptions include the fair value of the Company's stock, stock-based compensation, estimated useful lives of depreciable assets, and the valuation allowance relating to the Company's deferred tax assets.
Cash and Cash Equivalents
The Company considers all highly liquid investments with an original maturity of three months or less when purchased to be cash equivalents. As of September 30, 2014 and 2013, the Company did not have any cash equivalents.
Inventory
Inventory is stated at the lower of cost (first-in, first-out method) or market (net realizable value).
Depreciation and Amortization
Property and equipment are stated at cost. Depreciation and amortization are calculated on the straight-line method based on the following estimated useful lives: machinery and equipment - 5 years; leasehold improvements - 4 years; computer equipment and software - 3 years; and furniture and fixtures - 5 years. Amortization of leasehold improvements is computed using the straight-line method over the shorter of the remaining lease term or the estimated useful lives of the improvements.
Repairs and maintenance are expensed as incurred. Major replacements and betterments are capitalized.
Aperion Biologics, Inc. Notes to Financial Statements September 30, 2014 and 2013 |
| 1. | Summary of Significant Accounting Policies (Continued) |
Impairment of Long-Lived Assets
The Company reviews the carrying value of property and equipment for impairment whenever events and circumstances indicate the carrying value of an asset may not be recoverable from the estimated future cash flows expected to result from its use and eventual disposition. In cases where undiscounted expected future cash flows are less than the carrying value, an impairment loss is recognized equal to an amount by which the carrying value exceeds the fair value of assets. The factors considered by management in performing this assessment include current operating results, trends and prospects, and the effects of obsolescence, demand, competition, and other economic factors. The Company has not identified any such impairment loss during the years ended September 30, 2014 and 2013.
Income Taxes
Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases. Deferred tax assets, including tax loss and credit carryforwards, and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date. Deferred income tax expense represents the change during the period in the deferred tax assets and deferred tax liabilities. The components of the deferred tax assets and liabilities are individually classified as current and noncurrent based on their characteristics. Deferred tax assets are reduced by a valuation allowance when, in the opinion of management, it is more likely than not that some portion or all the deferred tax assets will not be realized. As of September 30, 2014 and 2013, management evaluated the positive and negative evidence bearing upon the realizability of its deferred tax assets and determined that it is more likely than not that the Company will not recognize the benefits of the deferred tax assets. As a result, a full valuation allowance was recorded. The valuation allowance increased by approximately $851,000 and $1,651,000 during the years ended September 30, 2014 and 2013, respectively.
U.S. GAAP prescribes a recognition threshold and measurement process for financial statement recognition and measurement of a tax position taken or expected to be taken in a tax return.
Management has evaluated and concluded that there were no material uncertain tax positions requiring recognition in the Company's financial statements as of September 30, 2014 and 2013. The Company does not expect any significant changes in the unrecognized tax benefits within twelve months of the reporting date.
The Company classifies interest expense and any related penalties related to income tax uncertainties as a component of income tax expense. No interest or penalties have been recognized during the years ended September 30, 2014 and 2013.
Contingencies
Certain conditions may exist as of the date the financial statements are issued, which may result in a loss to the Company, but which will only be resolved when one or more future events occur or fail to occur. The Company's management and its legal counsel assess such contingent liabilities, and such assessment inherently involves an exercise of judgment. In assessing loss contingencies related to legal proceedings that are pending against the Company or unasserted claims that may result in such proceedings, the Company's legal counsel evaluates the perceived merits of any legal proceedings or unasserted claims, as well as the perceived merits of the amount of relief sought or expected to be sought therein.
Aperion Biologics, Inc. Notes to Financial Statements September 30, 2014 and 2013 |
| 1. | Summary of Significant Accounting Policies (Continued) |
If the assessment of a contingency indicates it is probable that a material loss has been incurred and the amount of the liability can be estimated, then the estimated liability would be accrued in the Company's financial statements. If the assessment indicates a potentially material loss contingency is not probable, but is reasonably possible, or is probable, but cannot be estimated, then the nature of the contingent liability, together with an estimate of the range of possible loss, if determinable and material, would be disclosed.
Contingencies (Continued)
Loss contingencies considered remote are generally not disclosed unless they involve guarantees, in which case the guarantees would be disclosed.
Research and Development
Research and development costs are charged to operations when incurred. The amounts charged in 2014 and 2013 were $59,245 and $417,844, respectively.
Stock-Based Payments
Stock-based compensation expense for all stock-based payment awards is based on the estimated fair value of the award. For employees and directors, the award is measured on the grant date. For non-employees, the award is measured on the grant date and is then remeasured at each vesting date and financial reporting date. The Company recognizes the estimated fair value of the award as compensation cost over the requisite service period of the award, which is generally the option vesting term. The Company generally issues new shares of common stock to satisfy option and warrant exercises.
Fair Value Measurement
The Company measures the fair value of financial assets and liabilities based on the guidance of ASC 820 "Fair Value Measurements and Disclosures" ("ASC 820") which defines fair value, establishes a framework for measuring fair value, and expands disclosures about fair value measurements.
ASC 820 defines fair value as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. ASC 820 also establishes a fair value hierarchy, which requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value. ASC 820 describes three levels of inputs that may be used to measure fair value:
Level 1 — quoted prices in active markets for identical assets or liabilities
Level 2 — quoted prices for similar assets and liabilities in active markets or inputs that are observable
Level 3 — inputs that are unobservable (for example, cash flow modeling inputs based on assumptions)
The fair value of the Company's cash and accounts payable approximates the carrying amounts of such instruments due to their short maturity. The fair value of the convertible promissory notes approximates the carrying amount because the rate and terms currently available to the Company approximate the rate and terms on the existing debt.
Aperion Biologics, Inc. Notes to Financial Statements September 30, 2014 and 2013 |
| 1. | Summary of Significant Accounting Policies (Continued) |
Convertible Instruments
U.S. GAAP requires companies to bifurcate conversion options from their host instruments and account for them as free standing derivative financial instruments according to certain criteria. The criteria include circumstances in which (a) the economic characteristics and risks of the embedded derivative instrument are not clearly and closely related to the economic characteristics and risks of the host contract, (b) the hybrid instrument that embodies both the embedded derivative instrument and the host contract is not re-measured at fair value under otherwise applicable generally accepted accounting principles with changes in fair value reported in earnings as they occur and (c) a separate instrument with the same terms as the embedded derivative instrument would be considered a derivative instrument. An exception to this rule is when the host instrument is deemed to be conventional as that term is described under applicable U.S. GAAP.
When the Company has determined that the embedded conversion options should not be bifurcated from their host instruments, the Company records, when necessary, discounts to convertible notes for the intrinsic value of conversion options embedded in debt instruments based upon the differences between the fair value of the underlying common stock at the commitment date of the note transaction and the effective conversion price embedded in the note. Debt discounts under these arrangements are amortized over the term of the related debt to their stated date of redemption. The Company also records, when necessary, deemed dividends for the intrinsic value of conversion options embedded in preferred shares based upon the differences between the fair value of the underlying common stock at the commitment date of the transaction and the effective conversion price embedded in the preferred shares. The Company has analyzed the conversion feature of the convertible promissory notes and determined that there was no beneficial conversion feature associated with such notes. The Company issued certain warrants in connection with the issuance of certain convertible notes, and the fair value of such warrants was determined to be de-minimus (see Note 9 to the Financial Statements). Accordingly, no discount was recorded related to the convertible notes.
Preferred Stock
The Company applies the guidance enumerated inDistinguishing Liabilities from Equity Topic of the Accounting Standards Codification (ASC 480) when determining the classification and measurement of preferred stock. Preferred shares subject to mandatory redemption (if any) are classified as liability instruments and are measured at fair value. The Company classifies conditionally redeemable preferred shares, which includes preferred shares that feature redemption rights that are either within the control of the holder or subject to redemption upon the occurrence of uncertain events not solely within the Company's control, as temporary equity. At all other times, the Company classifies its preferred shares in stockholders' deficiency.
Stock Warrants
The Company classifies as equity any contracts that (i) require physical settlement or net-share settlement or (ii) provides the Company with a choice of net-cash settlement or settlement in its own shares (physical settlement or net-share settlement) provided that such contracts are indexed to the Company's own stock. The Company classifies as assets or liabilities any contracts that (i) require net-cash settlement (including a requirement to net cash settle the contract if an event occurs and if that event is outside the Company's control), or (ii) gives the counterparty a choice of net-cash settlement or settlement in shares (physical settlement or net-share settlement). The Company assesses classification of its stock warrants at each reporting date to determine whether a change in classification between assets and liabilities is required. The Company's warrants to purchase preferred and common stock were issued in connection with certain of its convertible promissory notes and preferred stock. The Company evaluated the common stock warrants to assess their proper classification using the applicable criteria enumerated under U. S. GAAP and determined that the common stock warrants meet the criteria for equity classification in the balance sheet as of September 30, 2014 and 2013. The Company evaluated the Series C and Series C2 preferred stock warrants to assess their proper classification using the applicable criteria enumerated under U. S. GAAP and determined that the Series C and Series C2 preferred stock warrants meet the criteria for liability classification, as they are exercisable into a redeemable security, as of September 30, 2014 and 2013. These warrants are marked to market at each reporting period. The Company has determined that the value of such liabilities are de minimis.
Aperion Biologics, Inc. Notes to Financial Statements September 30, 2014 and 2013 |
| 1. | Summary of Significant Accounting Policies (Continued) |
Sequencing Policy
The Company follows a sequencing policy for which in the event partial reclassification of contracts subject to ASC 815-40-25 is necessary, due to the Company's inability to demonstrate it has sufficient authorized shares, shares will be allocated on the basis of earliest issuance date of potentially dilutive instruments with the earliest grants receiving first allocation of shares. See Note 6.
Net Earnings (Loss) Per Share of Common Stock
Basic net earnings (loss) per share is computed by dividing net earnings (loss) attributable to common stockholders by the weighted average number of common shares outstanding during the period. Diluted net earnings (loss) per share reflects the potential dilution that could occur if securities or other instruments to issue common stock were exercised or converted into common stock. Potentially dilutive securities are excluded from the computation of diluted net earnings (loss) per share if their inclusion would be anti-dilutive and consist of the following:
| | | September 30, | |
| | | 2014 | | | 2013 | |
| | | | | | | |
| Options | | | 340,231 | | | | 304,497 | |
| Warrants to purchase common stock | | | 544,380 | | | | 544,380 | |
| Warrants to purchase preferred stock | | | 115,557 | | | | 115,557 | |
| Series A convertible preferred stock | | | 36,383 | | | | 36,383 | |
| Series B convertible preferred stock | | | 230,330 | | | | 230,330 | |
| Series C convertible preferred stock | | | 631,778 | | | | 596,540 | |
| Series C-2 convertible preferred stock | | | 979,133 | | | | 915,142 | |
| Convertible promissory notes, including accrued interest | | | 1,225,857 | | | | 644,846 | |
| | | | | | | | | |
| Total potentially dilutive shares | | | 4,103,649 | | | | 3,387,675 | |
Recently Issued Accounting Pronouncements
In June 2014, the FASB issued ASU No. 2014-10, "Development Stage Entities (Topic 915): Elimination of Certain Financial Reporting Requirements, Including an Amendment to Variable Interest Entities Guidance in Topic 810, Consolidation," ("ASU 2014-10"). ASU 2014-10 removes the definition of a development stage entity from the Master Glossary of the ASC, thereby removing the financial reporting distinction between development stage entities and other reporting entities from U.S. GAAP. In addition, ASU 2014-10 eliminates the requirements for development stage entities to (1) present inception-to-date information in the statements of operations, cash flows, and stockholders' equity, (2) label the financial statements as those of a development stage entity, (3) disclose a description of the development stage activities in which the entity is engaged, and (4) disclose in the first year in which the entity is no longer a development stage entity that in prior years it had been in the development stage. ASU 2014-10 is effective for annual reporting periods beginning after December 15, 2014, and interim periods therein. Early adoption is permitted. The Company adopted ASU 2014-10 during the year ended September 30, 2014 which resulted in the removal of previously required development stage disclosures. The Company's activities are subject to significant risks and uncertainties, which are detailed in Note 2.
Aperion Biologics, Inc. Notes to Financial Statements September 30, 2014 and 2013 |
| 1. | Summary of Significant Accounting Policies (Continued) |
Recently Issued Accounting Pronouncements (Continued)
In June 2014, the FASB issued ASU No. 2014-12, "Compensation - Stock Compensation (Topic 718): Accounting for Share-Based Payments When the Terms of an Award Provide that a Performance Target Could be Achieved after the Requisite Service Period," ("ASU 2014-12"). The amendments in ASU 2014-12 require that a performance target that affects vesting and that could be achieved after the requisite service period be treated as a performance condition. A reporting entity should apply existing guidance in ASC Topic No. 718, "Compensation - Stock Compensation" as it relates to awards with performance conditions that affect vesting to account for such awards. The amendments in ASU 2014-12 are effective for annual periods and interim periods within those annual periods beginning after December 15, 2015. Early adoption is permitted. Entities may apply the amendments in ASU 2014-12 either: (a) prospectively to all awards granted or modified after the effective date; or (b) retrospectively to all awards with performance targets that are outstanding as of the beginning of the earliest annual period presented in the financial statements and to all new or modified awards thereafter. The Company does not anticipate that the adoption of ASU 2014-12 will have a material impact on its financial statements.
In August 2014, the FASB issued ASU No. 2014-15,"Presentation of Financial Statements - Going Concern (Subtopic 205-40): Disclosure of Uncertainties about an Entity's Ability to Continue as a Going Concern" ("ASU 2014-15"). ASU 2014-15, which is effective for annual reporting periods ending after December 15, 2016, extends the responsibility for performing the going-concern assessment to management and contains guidance on how to perform a going-concern assessment and when going-concern disclosures would be required under U.S. GAAP. The Company does not anticipate that the adoption of ASU 2014-12 will have a material impact on its financial statements. Management's evaluations regarding the events and conditions that raise substantial doubt regarding the Company's ability to continue as a going concern have been disclosed in Note 2.
Subsequent Events
Management has evaluated subsequent events to determine if events or transactions occurring through September 21, 2015, the date on which the financial statements were available to be issued require adjustment or disclosure in the Company's financial statements.
Effective as of __________, 2015, the Company amended its Articles of incorporation and effected a 18.4-for-1 reverse stock split. All per share amounts and number of shares in the financial statements and related notes have been retroactively restated to reflect the reverse stock split.
| 2. | Going Concern and Management's Liquidity Plans |
As of September 30, 2014, the Company had working capital deficit and a stockholders' deficiency of $12,132,432 and $25,990,881, respectively. During the year ended September 30, 2014, the Company incurred a net loss of $2,497,612. The Company has not generated any material revenues and has incurred net losses since inception. Furthermore, as of the date of this report, the Company has $6,724,000 of convertible promissory notes that have matured and are in default. These conditions raise substantial doubt about the Company's ability to continue as a going concern.
The Company recognizes it will need to raise additional capital in order to meet its obligations and execute its business plan for at least the next twelve month period. There is no assurance that additional financing will be available when needed or that management will be able to obtain such financing on terms acceptable to the Company and that the Company will become profitable and generate positive operating cash flow in the future. If the Company is unable to raise sufficient additional funds, it will have to develop and implement a plan to further extend payables, reduce overhead or scale back its business plan until sufficient additional capital is raised to support further operations. There can be no assurance that such a plan will be successful.
Accordingly, the accompanying financial statements have been prepared in conformity with U.S GAAP, which contemplate continuation of the Company as a going concern and the realization of assets and the satisfaction of liabilities in the normal course of business. The carrying amounts of assets and liabilities presented in the financial statements do not necessarily represent realizable or settlement values. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Aperion Biologics, Inc. Notes to Financial Statements September 30, 2014 and 2013 |
Inventory consists of alpha-galactosidase, a raw material enzyme used in the manufacturing of the Company's core platform technology.
Property and equipment consist of the following:
| | | September 30, | |
| | | 2014 | | | 2013 | |
| Machinery and equipment | | $ | 248,731 | | | $ | 206,616 | |
| Leasehold improvements | | | 126,768 | | | | 126,768 | |
| Computer equipment and software | | | 56,735 | | | | 56,735 | |
| Furniture and fixtures | | | 23,122 | | | | 23,122 | |
| | | | 455,356 | | | | 413,241 | |
| Less accumulated depreciation and amortization | | | 393,903 | | | | 359,975 | |
| Net property and equipment | | $ | 61,453 | | | $ | 53,266 | |
Depreciation and amortization expense for the years ended September 30, 2014 and 2013 was $33,928 and $59,274, respectively.
In 2007, the Company received a $100,000 advance payable from a potential strategic partner, which is due on demand. This obligation bears interest of 9.5% per annum. At September 30, 2014 and 2013 the balance of this note was $100,000. Accrued interest payable related to the note totaled $65,602 and $56,102 at September 30, 2014 and 2013, respectively, and is included as accrued liabilities on the balance sheets.
| 6. | Convertible Promissory Notes |
Related Parties
Dr. Kevin Stone, a director and majority beneficial shareholder of the Company, is the managing member and a controlling person of CrossCart LLC, a California limited liability company (CrossCart). The Company issued convertible promissory notes to CrossCart and its predecessor-in-interest, Lurie Investments, Inc., totaling $1,120,015 during the year ended September 30, 2014 and $2,150,000 during the year ended September 30, 2013. These notes bear interest of 8.0% per annum, which increases to 10% per annum if an event of default occurs. These notes have thirty days to two year terms and mature on various dates through May 2016. Interest is being accrued at the default rate of 10% on notes that have passed their maturity date. At September 30, 2014 and 2013, the balance of these notes totaled $6,520,015 and $5,400,000, respectively. Accrued interest payable related to these notes totaled $1,093,186 and $493,428, at September 30, 2014 and 2013, respectively, and is included as accrued liabilities on the balance sheets.
Bruce Stone is the brother of Dr. Kevin Stone. The Company issued a convertible promissory note to Bruce Stone in the amount of $50,000 during the year ended September 30, 2014. This note bears interest of 8.0% per annum, which increases to 10% per annum if an event of default occurs. At September 30, 2014, the balance of this note totaled $50,000. Accrued interest payable related to this note totaled $1,433 at September 30, 2014 and is included as accrued liabilities on the balance sheets.
Aperion Biologics, Inc. Notes to Financial Statements September 30, 2014 and 2013 |
| 6. | Convertible Promissory Notes (Continued) |
Related Parties (Continued)
As of September 30, 2014, the Company had $3,750,000 and $2,820,015 issued and outstanding principal amount of convertible promissory notes, plus $802,972 and $291,647 in accrued interest, that are convertible into Series C2 and Series D convertible redeemable preferred stock, respectively. As of the date of this report, Series D was not effected. As of September 30, 2013, the Company had $3,750,000 issued and outstanding principal amount, plus $430,056 in accrued interest, and $1,650,000 issued and outstanding principal amount, plus $63,372 in accrued interest, of convertible promissory notes that are convertible into Series C2 and Series D convertible redeemable preferred stock, respectively. The Series C2 Notes are convertible at $8.83 per share, subject to adjustment for stock-splits and similar transactions. All of these notes are convertible at the option of the holder. The Series D Notes are convertible at $4.60 per share, subject to adjustment for stock splits or similar transactions. Of the notes convertible into Series D convertible redeemable preferred stock, $1,050,000 are mandatorily convertible upon a qualified financing (as defined) and at the option of the holder and $1,770,015 are convertible solely at the option of the holder.
As of September 30, 2014, the Company had $6,500,000 issued and outstanding principal amount of convertible promissory notes classified as current liabilities, and $70,015 issued and outstanding principal amount of convertible promissory notes classified as long term liabilities. As of September 30, 2013, the Company had $5,400,000 issued and outstanding principal amount of convertible promissory notes classified as current liabilities.
If all convertible instruments were to convert at the same time, the Company would not have sufficient common shares authorized. All of the notes contain language that provide that the conversion of the notes may be delayed until the proper number of shares is authorized and reserved, with the Company obligated to use its best efforts to cause a sufficient number of shares to be authorized and reserved as soon as practicable.
Unrelated Parties
The Company issued convertible promissory notes totaling $637,012 during the year ended September 30, 2014 and $224,000 during the year ended September 30, 2013. These notes bear interest of 8.0% per annum, which increases to 10% per annum if an event of default occurs, and mature at various dates through September 3, 2016. After maturity, outstanding principal amount of the notes may be converted into shares of Series C-2 or Series D Convertible Preferred Stock. As of the date of this report, Series D was not effected. During the year ended September 30, 2014, $55,000 of these notes was paid in full. At September 30, 2014 and 2013 the balance of these notes totaled $806,012 and $224,000, respectively. Accrued interest payable related to the notes totaled $39,535 and $8,138, at September 30, 2014 and 2013, respectively, and is included as accrued liabilities on the balance sheets.
As of September 30, 2014, the Company had $806,012 issued and outstanding principal amount of convertible promissory notes, plus $39,535 in accrued interest, that are convertible into Series D preferred stock. As of September 30, 2013, the Company had $224,000 issued and outstanding principal amount, plus $8,138 in accrued interest, of convertible promissory notes convertible into Series D preferred stock. The Series D notes are convertible at $4.60 per share, subject to adjustment for stock-splits and similar transactions. All of these notes are convertible at the option of the holder.
As of September 30, 2014, the Company had $169,000 issued and outstanding principal amount of convertible promissory notes classified as current liabilities and $637,012 issued and outstanding principal amount of convertible promissory notes classified as long term liabilities. As of September 30, 2013, the Company had $224,000 issued and outstanding principal amount of convertible promissory notes classified as current liabilities.
If all convertible instruments were to convert at the same time, the Company would not have sufficient common shares authorized. All of the notes contain language that provide that the conversion of the Notes may be delayed until the proper number of shares is authorized and reserved, with the Company obligated to use its best efforts to cause a sufficient number of shares to be authorized and reserved as soon as practicable.
| 7. | Stockholders' Deficiency |
According to the Restated Certificate of Incorporation, as amended on May 18, 2011, the Company is authorized to issue 95,250,000 shares of all classes of stock, consisting of (1) 60,000,000 shares of common stock, par value $0.001 per share, and (2) 35,250,000 shares of preferred stock, par value $0.001 per share.
Aperion Biologics, Inc. Notes to Financial Statements September 30, 2014 and 2013 |
| 7. | Stockholders' Deficiency (Continued) |
Preferred Stock
Of the 35,250,000 shares of preferred stock at September 30, 2014 and 2013, 750,000 shares are designated as Series A convertible preferred stock, 6,500,000 shares are designated as Series B convertible preferred stock, 10,000,000 shares are designated as Series C convertible preferred stock and 18,000,000 are designated as Series C-2 convertible preferred stock.
The Series A convertible preferred stock, Series B convertible preferred stock, Series C convertible redeemable preferred stock and Series C2 convertible redeemable preferred stock are entitled to vote on an as-converted basis together with the holders of common stock as a single class, except as provided by law or other provisions of the certificate of incorporation.
The Series C and C-2 convertible preferred stock have a liquidation preference over the Series B convertible preferred stock. The Series B convertible preferred stock has a liquidation preference over the Series A convertible preferred stock and would share in assets on a pro-rata basis. The Series A convertible preferred stock has a liquidation preference over common stock and would share in assets on a pro-rata basis.
The preferred stock is subject to mandatory conversion upon the occurrence of either (a) the closing of the sales of shares of common stock at a price of at least $44.16 per share (subject to adjustment) in a public offering pursuant to the Securities Act of 1933, resulting in at least $50 million in gross proceeds to the company or (b) upon the majority of vote of the holders of the Series C preferred stock (voting together as a class on an as-converted basis.)
Series A Convertible Preferred Stock
As of September 30, 2014 and 2013, 36,383 shares of $0.001 par value Series A convertible preferred stock were issued and outstanding. The holders of Series A convertible preferred stock are entitled to receive, out of any funds legally available, dividends at the rate of 8% of the Series A original issue price per share per annum when and as declared by the Company's Board of Directors (the "Board"). Each share of Series A convertible preferred stock may be converted at the option of the holder into the number of shares of common stock determined by dividing the Series A original issue price by the Series A conversion price of $18.40. As Series A Shares are not subject to redemption, they are classified in stockholders' deficiency. From inception through the date of this report, the Board has not declared any Series A convertible preferred stock dividends.
Series B Convertible Preferred Stock
As of September 30, 2014 and 2013, 161,674 shares of $0.001 par value of Series B convertible preferred stock were issued and outstanding. The holders of Series B convertible preferred stock are entitled to receive, out of any funds legally available, dividends at the rate of 8% of the Series B original issue price per share per annum when and as declared by the Company's Board. Each share of Series B convertible preferred stock may be converted at the option of the holder into the number of shares of common stock determined by dividing the Series B original issue price by the Series B conversion price of $59.06. As Series B Shares are not subject to redemption, they are classified in stockholders' deficiency. From inception through the date of this report, the Board has not declared any Series B convertible preferred stock dividends.
Aperion Biologics, Inc. Notes to Financial Statements September 30, 2014 and 2013 |
| 7. | Stockholders' Deficiency (Continued) |
Series C Convertible Redeemable Preferred Stock
As of September 30, 2014 and 2013, 399,173 shares of $0.001 par value Series C convertible redeemable preferred stock were issued and outstanding. Subsequent to April 29, 2013, the holders of a majority of the outstanding shares of Series C preferred stock may demand the Company redeem all or any portion of such stockholders' shares of Series C convertible redeemable preferred stock for a cash amount per share equal to the greater of two times the Series C original issue price plus any accrued unpaid dividends, whether or not declared, or the per share amount that would have been payable had all shares of Series C convertible redeemable preferred stock been converted into common stock. Series C convertible preferred stock accrues dividends at the rate of 8% of the original Series C issue price from the date of issuance. Each share of Series C convertible preferred stock, including accrued dividends, may be converted at the option of the holder into shares of common stock at an initial conversion rate of $8.83 per share. The conversion rate is subject to adjustment for certain dilutive issuances of common stock and equivalents.
Since the Series C Shares are redeemable in certain circumstances which are considered to be outside the control of the Company, such shares have been classified as temporary equity. The Series C shares were assessed under ASC 480, and determined that the contingency is not within the scope of ASC 480 because the shares are convertible at the option of the investor prior to redemption date and are not considered mandatorily redeemable. Because the preferred stock may be converted into common stock by the holder at any time, classification as mezzanine equity securities is required under SEC guidance because the redemption or conversion is at the option of the holder. The Company has also analyzed the conversion option related to the Series C Shares and deemed that since an active market for such shares does not exist, the conversion option was not deemed readily convertible to cash. Accordingly, the conversion option did not qualify as a derivative and was not bifurcated from the host instrument.
The Company has calculated accretion using the interest method, at a rate of 8%, through the earliest redemption date of April 30, 2013. The value of the shares is being accreted to the redemption value at April 29, 2013. During the year ended September 30, 2013, the Company recorded accretion as a deemed dividend of $838,753. The accumulated accretion as of September 30, 2014 and 2013 totaled $3,525,500.
Aperion Biologics, Inc. Notes to Financial Statements September 30, 2014 and 2013 |
| 7. | Stockholders' Deficiency (Continued) |
Series C-2 Convertible Redeemable Preferred Stock
As of September 30, 2014 and 2013, 724,901 shares of $0.001 par value redeemable preferred stock were outstanding). Subsequent to April 29, 2013, the holders of a majority of the outstanding shares of Series C-2 convertible redeemable preferred stock may demand the Company redeem all or any portion of such stockholders' shares of Series C-2 convertible redeemable preferred stock for a cash amount per share equal to the greater of Series C original issue price plus, any accrued unpaid dividends, whether or not declared, or the per share amount that would have been payable had all shares of Series C convertible redeemable preferred stock been converted into common stock. Series C-2 convertible redeemable preferred stock accrues dividends at the rate of 8% of the original Series C issue price from the date of issuance. Each share of Series C-2 convertible redeemable preferred stock, including accrued dividends, may be converted at the option of the holder into shares of common stock at an initial conversion rate of $8.83 per share. The conversion rate is subject to adjustment for certain dilutive issuances of common stock and equivalents.
Since the Series C Shares are redeemable in certain circumstances which are considered to be outside the control of the Company, such shares have been classified as temporary equity. The Series C shares were assessed under ASC 480, and determined that the contingency is not within the scope of ASC 480 because the shares are convertible at the option of the investor prior to redemption date and are not considered mandatorily redeemable. Because the preferred stock may be converted into common stock by the holder at any time, classification as mezzanine equity securities is required under SEC guidance because the redemption or conversion is at the option of the holder. The Company has also analyzed the conversion option related to the Series C Shares and deemed that since an active market for such shares does not exist, the conversion option was not deemed readily convertible to cash. Accordingly, the conversion option did not qualify as a derivative and was not bifurcated from the host instrument.
Preferred Stock Dividends
No dividends with respect to the Series A preferred stock and Series B preferred stock accrue. At the discretion of the Board, dividends may be paid at the rate of 8% of the Series A and Series B original issue price.
Series C and Series C-2 preferred stock accrue cumulative dividends at the rate of 8% per annum of the original Series C issue price per share from the date of issuance. Dividends accrue whether or not such dividends are earned or declared and whether or not sufficient funds are legally available.
Common Stock
Common stock stockholders have voting rights equal to one vote for each share of common stock held and will participate in dividends declared by the Board, subject to preferences of preferred stock stockholders. Similarly, common stock stockholders share ratably in all distributions upon liquidation of the Company subject to preferences of preferred stock stockholders.
On November 17, 2012, an employee of the Company exercised options to purchase 305 shares of common stock in exchange for cash proceeds of $577, which was the exercise price in accordance with the applicable employee stock option agreement.
Aperion Biologics, Inc. Notes to Financial Statements September 30, 2014 and 2013 |
| 7. | Stockholders' Deficiency (Continued) |
Common Stock (Continued)
On July 22, 2013, an employee of the Company exercised options to purchase 427 shares of common stock in exchange for cash proceeds of $79, which was the exercise price in accordance with the applicable employee stock option agreement.
The Company's 2008 Stock - Option/Stock Issuance Plan (the "Plan") authorizes the grant of incentive stock options to employees, directors, and consultants of the Company. As of September 30, 2014 and 2013, a total of 431,333 and 432,065 shares, respectively, of common stock of the Company have been reserved for issuance under the Plan and as of September 30, 2014, 112,841 shares were available for issuance. Not included in this reserve are certain options, which were granted prior to and are outside of the 2008 Plan, and, as of September 30, 2014 and 2013, the number of shares of common stock issued outside the Plan totaled 21,739 and 32,609, respectively. Awards that are forfeited generally become available for grant under the Plan. Shares issued under the Plan are subject to transfer restrictions.
Stock options expire ten years from grant date and generally vest within two to four years with an exercise price fixed by the Plan administrator, but not less than the fair market value of the Company's shares of common stock on the option grant date. During the year ended September 30, 2014, the Company issued 35,734 stock options to employees and consultants with an exercise price ranging from of $0.29 to $0.37 and fair values of $0.20 and $0.37 per share, respectively. The stock options granted in 2014 are subject to vesting over two to four years and have a term of ten years. During the year ended September 30, 2013, the Company issued 53,424 stock options to employees and consultants with an exercise price ranging from of $0.29 to $4.05 and fair values of $2.67 and $3.02 per share, respectively. The stock options granted in 2013 are subject to vesting over two to four years and have a term of ten years.
During the years ended September 30, 2014 and 2013, 0 and 12,311 options were forfeited, respectively, due to the termination of employees. The forfeited options were added back to the available pool for future issuance.
The following table represents stock option activity for the years ended September 30, 2014 and 2013:
| | | | | | | | | Weighted Average | |
| | | Stock Options | | | Exercise Price | | | Fair Value | | | Contractual | | | Aggregate | |
| | | Outstanding | | | Exercisable | | | Outstanding | | | Exercisable | | | Vested | | | Life (Years) | | | Intrinsic Value | |
| Balance - September 30, 2012 | | | 264,117 | | | | 157,711 | | | $ | 1.84 | | | $ | 1.47 | | | $ | 1.47 | | | | 10.00 | | | | | |
| Granted | | | 53,424 | | | | | | | | 1.84 | | | | | | | | | | | | | | | | | |
| Exercised | | | (733 | ) | | | | | | | .92 | | | | | | | | | | | | | | | | | |
| Forfeited | | | (12,311 | ) | | | | | | | 7.73 | | | | | | | | | | | | | | | | | |
| Balance - September 30, 2013 | | | 304,497 | | | | 196,677 | | | | 1.66 | | | | 1.47 | | | | 1.47 | | | | 10.00 | | | | | |
| Granted | | | 35,734 | | | | | | | | .37 | | | | | | | | | | | | | | | | | |
| Exercised | | | - | | | | | | | | - | | | | | | | | | | | | | | | | | |
| Forfeited | | | - | | | | | | | | - | | | | | | | | | | | | | | | | | |
| Balance - September 30, 2014 | | | 340,231 | | | | 281,363 | | | $ | 1.47 | | | $ | 1.47 | | | $ | 1.47 | | | | 10.00 | | | $ | 8,854 | |
Aperion Biologics, Inc. Notes to Financial Statements September 30, 2014 and 2013 |
| 8. | Stock Options (Continued) |
The following table summarizes information on stock options outstanding and exercisable as of September 30, 2014:
Exercise
Price | | | Number
Outstanding | | | Weighted Average
Remaining Contractual
Term (years) | | | Weighted Average Exercise
Price | | | Number
Exercisable | | | Weighted Average Exercise
Price | |
| $ | 0.18 | | | | 96,508 | | | | 4.75 | | | $ | 0.18 | | | | 95,965 | | | $ | 0.18 | |
| | 0.29 | | | | 66,332 | | | | 9.04 | | | | 0.29 | | | | 41,940 | | | | 0.29 | |
| | 1.90 | | | | 118,967 | | | | 7.08 | | | | 1.90 | | | | 105,754 | | | | 1.90 | |
| | 4.05 | | | | 52,989 | | | | 7.81 | | | | 4.05 | | | | 32,270 | | | | 4.05 | |
| $ | 8.28 | | | | 5,435 | | | | 3.37 | | | $ | 8.28 | | | | 5,435 | | | $ | 8.28 | |
| | | | | | 340,231 | | | | | | | | | | | | 281,364 | | | | | |
On December 23, 2013, the Company extended the expiration date of 5,435 options to purchase common stock (exercise price of 8.28 per share) granted to Dr. Kevin Stone from January 31, 2014 to February 11, 2018. This action was taken by unanimous written consent of the Board of Directors of the Company. The Company has evaluated the compensation expense of these extensions and judged them to be immaterial.
Stock-Based Compensation
For the years ended September 30, 2014 and 2013, stock-based compensation related to stock options totaled approximately $87,200 and $102,900, respectively. As of September 30, 2014, total unrecognized stock option compensation expense is $77,353 which will be recognized as those options vest over a period of approximately four years. The amount of future stock option compensation expense could be affected by any future option grants or by any option holders leaving the Company before their grants are fully vested.
The fair value of each option grant is estimated at the date of grant using the Black-Scholes pricing model. Volatility is based on average historical volatilities for public companies in similar industries over the expected term of the options. The expected term of employee options represents the period of time that options granted are expected to be outstanding using the "plain vanilla" method. For non-employee options, the contractual term is used. The risk-free rate is for periods within the contractual life of options and is based on the United States Treasury yield curve in effect at the time of grant. In addition, the Company is required to estimate the expected forfeiture rate and only recognize expense for those shares expected to vest. In estimating the Company's forfeiture rate, the Company analyzed its historical forfeiture rate, the remaining lives of unvested options, and the number of vested options as a percentage of total options outstanding. If the Company's actual forfeiture rate is materially different from its estimate, or if the Company reevaluates the forfeiture rate in the future, the stock-based compensation expense could be significantly different from what the Company has recorded in the current period.
The weighted-average Black-Scholes assumptions are as follows:
| | | September 30, | |
| | | 2014 | | | 2013 | |
| Term | | 5.5 to 10 years | | | 7 to 10 years | |
| Risk-free interest rate | | | 1.07 | % | | | 1.07 | % |
| Volatility | | | 49.80 | % | | | 57.80 | % |
| Expected dividend yield | | | - | | | | - | |
| Forfeiture rate | | | - | | | | - | |
Aperion Biologics, Inc. Notes to Financial Statements September 30, 2014 and 2013 |
Common Stock
During 2008, the Company issued warrants to purchase shares of common stock. The warrants are exercisable through June 9, 2018. The number of shares of common stock issuable under outstanding stock warrants at the end of September 30, 2014 and 2013 totaled 544,380 at an exercise price $0.18 per share.
The following table represents common stock warrant activity for the years ended September 30, 2014 and 2013:
| | | | | | | | | Weighted Average | |
| | | Stock Options | | | Exercise Price | | | Fair Value | | | Contractual | | | Aggregate | |
| | | Outstanding | | | Exercisable | | | Outstanding | | | Exercisable | | | Vested | | | Life (Years) | | | Intrinsic Value | |
| Balance - September 30, 2012 | | | 544,380 | | | | 544,380 | | | $ | 0.18 | | | $ | 0.18 | | | $ | 0.18 | | | | 10.00 | | | | | |
| Granted | | | - | | | | | | | | - | | | | | | | | | | | | | | | | | |
| Exercised | | | - | | | | | | | | - | | | | | | | | | | | | | | | | | |
| Cancelled/forfeited | | | - | | | | | | | | - | | | | | | | | | | | | | | | | | |
| Balance - September 30, 2013 | | | 544,380 | | | | 544,380 | | | | 0.18 | | | | 0.18 | | | | 0.18 | | | | 10.00 | | | | | |
| Granted | | | - | | | | | | | | - | | | | | | | | | | | | | | | | | |
| Exercised | | | - | | | | | | | | - | | | | | | | | | | | | | | | | | |
| Cancelled/forfeited | | | - | | | | | | | | - | | | | | | | | | | | | | | | | | |
| Balance - September 30, 2014 | | | 544,380 | | | | 544,380 | | | $ | 0.18 | | | $ | 0.18 | | | $ | 0.18 | | | | 10.00 | | | $ | 60,100 | |
Series C and C-2 Preferred Stock
During 2006 through 2013, the Company issued warrants to purchase shares of Series C and C-2 preferred stock. The warrants expire at various dates through 2023. The number of shares of Series C preferred stock issuable under outstanding warrants at the end of September 30, 2014 and 2013 totaled 51,868 at an exercise price of $8.83 per share. The number of shares of Series C-2 preferred stock issuable under outstanding warrants at the end of September 30, 2014 and 2013 totaled 63,689 at an exercise price of $8.83 per share. Pursuant to the terms of the preferred stock warrants, these warrants will be converted automatically into warrants to purchase shares of common stock upon completion of an offering, as defined.
In December 2012, the Company issued 8,492 warrants to CrossCart in connection with issuance of a convertible note. The warrants have an exercise price of $8.83 and a fair value of $3.97 per warrant. The warrants expire in ten years from date of issuance. The Company accounted for this warrant issuance as a liability when issued. The value of the derivative is determined to be de minimis.
The following table represents Series C and C-2 preferred stock warrant activity for the years ended September 30, 2014 and 2013:
| | | | | | | | | Weighted Average | |
| | | Stock Options | | | Exercise Price | | | Fair Value | | | Contractual | | | Aggregate | |
| | | Outstanding | | | Exercisable | | | Outstanding | | | Exercisable | | | Vested | | | Life (Years) | | | Intrinsic Value | |
| Balance - September 30, 2012 | | | 107,065 | | | | 107,065 | | | $ | 8.83 | | | $ | 8.83 | | | $ | 8.83 | | | | 10.00 | | | | | |
| Granted | | | 8,492 | | | | | | | | 8.83 | | | | | | | | | | | | | | | | | |
| Exercised | | | - | | | | | | | | - | | | | | | | | | | | | | | | | | |
| Cancelled/forfeited | | | - | | | | | | | | - | | | | | | | | | | | | | | | | | |
| Balance - September 30, 2013 | | | 115,557 | | | | 115,557 | | | | 8.83 | | | | 8.83 | | | | 8.83 | | | | 10.00 | | | | | |
| Granted | | | - | | | | | | | | - | | | | | | | | | | | | | | | | | |
| Exercised | | | - | | | | | | | | - | | | | | | | | | | | | | | | | | |
| Cancelled/forfeited | | | - | | | | | | | | - | | | | | | | | | | | | | | | | | |
| Balance - September 30, 2014 | | | 115,557 | | | | 115,557 | | | $ | 8.83 | | | $ | 8.83 | | | $ | 8.83 | | | | 10.00 | | | $ | - | |
Aperion Biologics, Inc. Notes to Financial Statements September 30, 2014 and 2013 |
On December 23, 2013 the Company extended the expiration date on 577 warrants to purchase Series C preferred stock (exercise price of $8.83 per share) from January 31, 2014 to July 25, 2017, and on 340 warrants to purchase Series C preferred stock (exercise price of $8.83 per share) from January 31, 2014 to August 14, 2017. This action was taken by unanimous written consent of the Board of Directors of the Company. The Company has evaluated the compensation expense of these extensions and deemed them to be immaterial.
The tax effects of temporary differences that give rise to deferred tax assets are as follows:
| | | September 30, | |
| | | 2014 | | | 2013 | |
| | | | | | | |
| Current | | | | | | | | |
| Accrued expenses | | $ | 385,609 | | | $ | 170,532 | |
| Non-current: | | | | | | | | |
| Net operating loss carryforward | | | 6,992,613 | | | | 6,429,889 | |
| Research and development credit carryforward | | | 296,589 | | | | 294,069 | |
| Patent costs | | | 125,903 | | | | 88,721 | |
| Property and equipment | | | 10,438 | | | | 6,942 | |
| Stock compensation | | | 64,660 | | | | 35,004 | |
| | | | | | | | | |
| Total deferred tax asset | | | 7,875,812 | | | | 7,025,157 | |
| Valuation allowance | | | (7,875,812 | ) | | | (7,025,157 | ) |
| | | | | | | | | |
| Deferred tax asset, net of valuation allowance | | $ | | | | $ | — | |
A reconciliation of the statutory federal income tax rate to the Company's effective tax rate is as follows:
| | | Years Ended
September 30, | |
| | | 2014 | | | 2013 | |
| U.S. statutory federal rate | | | - 34.00 | % | | | - 34.00 | % |
| Permanent difference | | | 0.04 | % | | | 0.08 | % |
| R&D Tax Credit | | | - 0.10 | % | | | - 0.61 | % |
| Change in valuation allowance | | | 34.06 | % | | | 43.43 | % |
| True-up/other | | | 0.00 | % | | | - 8.90 | % |
| | | | | | | | | |
| Income tax provision | | | 0.00 | % | | | 0.00 | % |
Aperion Biologics, Inc. Notes to Financial Statements September 30, 2014 and 2013 |
| 10. | Income Taxes (Continued) |
The income tax provision consists of the following:
| | | Years Ended
September 30, | |
| | | 2014 | | | 2013 | |
| Federal | | | | | | | | |
| Current | | $ | - | | | $ | - | |
| Deferred | | | (850,652 | ) | | | (1,650,476 | ) |
| State and local | | | | | | | | |
| Current | | | - | | | | - | |
| Deferred | | | - | | | | - | |
| Change in valuation allowance | | | 850,652 | | | | 1,650,476 | |
| | | | | | | | | |
| Income tax provision (benefit) | | $ | - | | | $ | - | |
The Company files a United States federal income tax return, as well as franchise tax returns in various state jurisdictions. The Company has available at September 30, 2014, an aggregated $20,566,389 of unused operating loss carryforwards that may be applied against future taxable income and that expire on various dates through 2034.
The net operating loss carryforwards will be subject to limitation by Internal Revenue Code Section 382. If an ownership change occurs under Code Section 382, the use of existing NOLs will be limited to an annual limitation. The Company estimates the amount of NOLs generated prior to May 31, 2013 and available to utilize against future taxable income is limited to an annual limitation of $318,655. The Company has not yet determined the exact amount of such limitation, and its estimate of the annual limitation may be subject to change. Such NOLs will be available to offset future taxable income until utilized. NOLs generated after June 1, 2013 are fully available to offset taxable income and begin expiring in 2021.
The Company has available at September 30, 2014, an aggregated $296,589 of unused research and development credits that may provide future tax benefits and that expire on various dates through 2034.
Aperion Biologics, Inc. Notes to Financial Statements September 30, 2014 and 2013 |
| 11. | Commitments and Contingent Liabilities |
Lease Commitments
The Company leases office space and certain equipment under noncancellable operating lease agreements. The leases generally require the Company to pay certain maintenance, insurance, and other operating expenses. Rental expense under operating leases totaled approximately $35,784 and $35,793 for the years ended September 30, 2014 and 2013, respectively.
Future minimum lease payments under noncancellable operating leases as of September 30, 2014 are as follows:
| Year ending September 30, | | | |
| 2015 | | $ | 27,336 | |
| 2016 | | | 1,740 | |
| 2017 | | | 1,740 | |
| 2018 | | | 1,740 | |
| 2019 | | | 1,160 | |
| | | | | |
| | | $ | 33,716 | |
Royalty Commitments
The Company has entered into a license agreement whereby the Company may use certain inventions and discoveries with its products. The license agreement calls for sales royalties ranging from 1.5% to 1.9% on net sales less than $10 million and 1.2% to 1.6% on net sales greater than $10 million, with a minimum annual royalty of $35,000, which shall be creditable against the sales royalties. The cumulative royalty payments to be paid under this agreement shall not exceed $3,500,000. Unless terminated earlier under the provisions of the agreement, the license agreement will remain in force during the life of the patents for the products included in the agreement. Royalty expense under this agreement totaled $35,000 for years ended September 30, 2014 and 2013.
The Company entered into a license agreement in 2003 which called for the payment of royalties and milestone payments related to the use of a certain enzyme. The Company has paid a total of $190,000 in royalty payments to date under this agreement, and has accrued $50,000 in additional royalty payments contingent upon the delivery of certain product documentation. The Company has not used the licensed enzymes since 2008. In 2014 the Company received a letter from the licensor alleging that Company owed additional payments under the license agreement; however, it is the opinion of the Company that no payments other than the amounts accrued are owed under this contract.
Transaction Bonus
In December 2014, the Company issued a demand note to CrossCart for an aggregate principal amount of $120,000 (the "Demand Note"). As an inducement to enter into the Demand Note, the Company agreed to pay CrossCart a transaction bonus (the "Transaction Bonus") equal to 3% of the gross proceeds received from any control change transaction of the Company.
Aperion Biologics, Inc. Notes to Financial Statements September 30, 2014 and 2013 |
| 11. | Commitments and Contingent Liabilities (Continued) |
Change of Control Bonus
In March 2012, the Company awarded Dr. Stone a bonus (the "Stone Bonus Agreement") in the event of an acquisition of the Company. An acquisition is defined as a sale, merger, consolidation, transfer or other disposition of all or substantially all (more than 80%) of the Company to another entity (the "Acquisition"). An initial public offering of the Company's common stock pursuant to an effective registration statement filed with the Commission under the Securities Act of 1933, as amended (the "Securities Act"), does not constitute an Acquisition. Dr. Stone is entitled to receive a bonus award in an amount equal to 1% of the proceeds received in the Acquisition that occurred on or prior to the completion of an initial public offering of the Company's stock pursuant to an effective registration statement filed with the Commission under the Securities Act.
Management Retention Plan
In December 2013, the Board approved the Management Retention Plan (the "Plan"), pursuant to which certain executives are entitled to receive a bonus upon the occurrence of specified events, provided that the participant must continue as an employee of the Company through the occurrence of the triggering event. Under the Plan, upon a significant external financing (beyond any financing from CrossCart) or a signing of a significant license or distribution agreement, the executives are to receive bonuses and any deferred compensation that would have been paid had full salaries been in effect from July 2013 through the date of the external financing/licensing agreement. All unpaid salaries have been accrued in the Company's financial statements as of September 30, 2014 and 2013.
In addition, the Plan also provides that upon closing of a deemed liquidation event specified in the Company's certificate of incorporation or a sale of the Company's securities representing 50% or more of total voting power of outstanding securities, the executives are entitled to receive bonus payments based, in part, on the amount of proceeds received by the Company in the Acquisition.
Transactions with Directors
In December 2013, the Company entered into a letter agreement with two of its directors, to provide certain acquisition bonus payments (the "Director Bonus Agreement"), Under the Director Bonus Agreement, in the event of a deemed liquidation event specified in the Company's certificate of incorporation or a sale of the Company's securities representing 50% or more of total voting power of outstanding securities, the directors are entitled to receive bonus payments based, in part, on the amount of proceeds received by the Company in the Acquisition.
The Company is dependent on a third party to supply inventory. The loss or significant reduction in product availability from the supplier could have a material adverse effect on the Company. The Company believes that its relationship with the supplier is satisfactory.
Payable Settlement
In March 2015, the Company settled an outstanding balance with a supplier totaling $105,000 by issuing a Convertible Promissory Note.
Aperion Biologics, Inc. Notes to Financial Statements September 30, 2014 and 2013 |
| 13. | Subsequent Events (Continued) |
Royalty Agreement Amendment
On September 7, 2015, CrossCart, LLC, Dr. Kevin Stone, and the Company agreed to terminate CrossCart's unlimited royalty on sales of products produced by the Company's proprietary manufacturing process, subject to completion of the Company's initial public offering of securities. The royalty will now be capped after the first $12,000,000 of sales.
Convertible Promissory Notes
On September 1, 2015, the Company completed the sale of $200,000 of convertible promissory notes. The notes convert into the Series E preferred stock at $4.97 per share at the option of the holder. As of the date of this report, the Series E shares have not yet been effected.
Bonus Termination
On September 7, 2015, CrossCart, LLC, Dr. Kevin Stone, and the Company agreed to terminate the Transaction Bonus and Stone Bonus Agreement upon completion of this offering.
Lease Commitment
On September 17, 2015 the Company received notice that its offer to extend its commercial lease on the property at 11969 Starcrest Drive was accepted by the landlord for a term beginning October 1, 2015 and ending December 31, 2018.
Aperion Biologics, Inc.
Financial Statements
June 30, 2015 and 2014
Aperion Biologics, Inc. Condensed Balance Sheets June 30, 2015 and September 30, 2014 |
| | | June 30, 2015
(unaudited) | | | September 30,
2014 | |
| | | | | | | |
| Assets | | | | | | | | |
| Current Assets | | | | | | | | |
| Cash | | $ | 258,564 | | | $ | 124,769 | |
| Inventory | | | 67,684 | | | | 62,054 | |
| Prepaid expenses and other current assets | | | 25,869 | | | | 8,185 | |
| | | | | | | | | |
| Total current assets | | | 352,117 | | | | 195,008 | |
| | | | | | | | | |
| Property and Equipment - net | | | 50,079 | | | | 61,453 | |
| | | | | | | | | |
| Total Assets | | $ | 402,196 | | | $ | 256,461 | |
| | | | | | | | | |
| Liabilities and Stockholders' Deficiency | | | | | | | | |
| Current Liabilities | | | | | | | | |
| Accounts payable | | $ | 768,031 | | | $ | 607,395 | |
| Convertible promissory notes | | | 6,976,027 | | | | 6,669,000 | |
| Demand note | | | 120,000 | | | | - | |
| Line of credit | | | 60,600 | | | | - | |
| Advances payable | | | 100,000 | | | | 100,000 | |
| Accrued dividends | | | 3,958,681 | | | | 3,363,015 | |
| Payroll liabilities | | | 283,932 | | | | 157,035 | |
| Accrued liabilities | | | 2,140,127 | | | | 1,430,995 | |
| | | | | | | | | |
| Total current liabilities | | | 14,407,398 | | | | 12,327,440 | |
| | | | | | | | | |
| Convertible promissory notes, net of current maturities | | | 1,180,000 | | | | 707,027 | |
| | | | | | | | | |
| Total liabilities | | | 15,587,398 | | | | 13,034,467 | |
| | | | | | | | | |
| Commitments and contingencies | | | | | | | | |
| | | | | | | | | |
| Redeemable convertible preferred stock - $0.001 par value | | | | | | | | |
| Series C - liquidation preference of $8,934,801 and $8,723,271, at June 30, 2015 and September 30, 2014, respectively, 10,000,000 shares designated, 399,173 shares issued and outstanding at June 30, 2015 and September 30, 2014 | | | 6,860,823 | | | | 6,860,823 | |
| Series C-2 - liquidation preference of $8,477,207 and $8,093,071, at June 30, 2015 and September 30, 2014, respectively 18,000,000 shares designated, 724,901 shares issued and outstanding at June 30, 2015 and September 30, 2014 | | | 6,352,052 | | | | 6,352,052 | |
| Total redeemable convertible preferred stock | | | 13,212,875 | | | | 13,212,875 | |
| | | | | | | | | |
| Stockholders' Deficiency | | | | | | | | |
| Preferred stock - $0.001 par value, 35,250,000 authorized | | | | | | | | |
| Series A - liquidation preference of $669,453, 750,000 shares designated, 36,383 shares issued and outstanding at June 30, 2015 and September 30, 2014 | | | 669,453 | | | | 669,453 | |
| Series B - liquidation preference of $13,617,853, 6,500,000 shares designated, 161,674 shares issued and outstanding at June 30, 2015 and September 30, 2014 | | | 13,617,853 | | | | 13,617,853 | |
| Common stock - $0.001 par value | | | | | | | | |
| 60,000,000 shares authorized, 222,369 and 219,651 shares issued and outstanding at June 30, 2015 and September 30, 2014, respectively | | | 4,042 | | | | 4,042 | |
| Additional paid-in capital | | | 371,533 | | | | 322,260 | |
| Accumulated deficit | | | (43,060,958 | ) | | | (40,604,489 | ) |
| Total stockholders' deficiency | | | (28,398,077 | ) | | | (25,990,881 | ) |
| | | | | | | | | |
| Total Liabilities and Stockholders' Deficiency | | $ | 402,196 | | | $ | 256,461 | |
The accompanying notes are an integral part of these condensed financial statements.
Aperion Biologics, Inc. Condensed Statements of Operations Nine Months Ended June 30, 2015 and 2014
(unaudited) |
| | | 2015 | | | 2014 | |
| | | | | | | |
| Revenue | | $ | 27,000 | | | $ | - | |
| | | | | | | | | |
| Cost of goods sold | | | 12,946 | | | | - | |
| | | | | | | | | |
| Gross profit | | | 14,054 | | | | - | |
| | | | | | | | | |
| Costs and expenses: | | | | | | | | |
| Clinical studies | | | 127,063 | | | | 165,896 | |
| Research and development | | | 39,916 | | | | 56,345 | |
| Sales, general, and administrative | | | 1,119,145 | | | | 1,099,724 | |
| Total costs and expenses | | | 1,286,124 | | | | 1,321,965 | |
| | | | | | | | | |
| Operating loss | | | (1,272,070 | ) | | | (1,321,965 | ) |
| | | | | | | | | |
| Other income (expense): | | | | | | | | |
| Royalty expense | | | (27,600 | ) | | | - | |
| Interest expense | | | (561,132 | ) | | | (469,568 | ) |
| Total other expense | | | (588,732 | ) | | | (469,568 | ) |
| | | | | | | | | |
| Net loss | | | (1,860,802 | ) | | | (1,791,533 | ) |
| Preferred stock: | | | | | | | | |
| Series C convertible contractual dividends | | | (211,530 | ) | | | (211,530 | ) |
| Series C-2 convertible contractual dividends | | | (384,136 | ) | | | (384,137 | ) |
| | | | | | | | | |
| Loss attributable to common stockholders | | $ | (2,456,468 | ) | | $ | (2,387,200 | ) |
| | | | | | | | | |
| Per share data: | | | | | | | | |
| Net loss - basic and diluted | | $ | (8.46 | ) | | $ | (8.10 | ) |
| Series C convertible contractual dividends | | | (0.92 | ) | | | (0.92 | ) |
| Series C-2 convertible contractual dividends | | | (1.66 | ) | | | (1.84 | ) |
| | | | | | | | | |
| Net loss attributable to common stockholders - basic and diluted | | $ | (11.04 | ) | | $ | (10.86 | ) |
| | | | | | | | | |
| Weighted average number of common shares outstanding - basic and diluted | | | 219,981 | | | | 219,651 | |
The accompanying notes are an integral part of these condensed financial statements.
Aperion Biologics, Inc. Condensed Statements of Cash Flows Nine Months Ended June 30, 2015 and 2014
(unaudited) |
| | | 2015 | | | 2014 | |
| | | | | | | |
| Cash Flows From Operating Activities | | | | | | | | |
| Net loss | | $ | (1,860,802 | ) | | $ | (1,791,533 | ) |
| | | | | | | | | |
| Adjustments to reconcile net loss to net cash used in operating activities | | | | | | | | |
| Stock-based compensation | | | 49,273 | | | | 64,852 | |
| Depreciation and amortization | | | | | | | | |
| Changes in operating assets and liabilities: Inventory | | | 11,374 | | | | 28,861 | |
| Inventory | | | (5,630 | ) | | | - | |
| Prepaid expenses and other current assets | | | (17,685 | ) | | | 85,737 | |
| Accounts payable | | | 265,636 | | | | (228,752 | ) |
| Accrued liabilities | | | 836,029 | | | | 583,950 | |
| Net cash used in operating activities | | | (721,805 | ) | | | (1,256,885 | ) |
| | | | | | | | | |
| Cash Flows From Investing Activities | | | | | | | | |
| Purchase of property and equipment | | | - | | | | (42,115 | ) |
| Net cash used in investing activities | | | - | | | | (42,115 | ) |
| | | | | | | | | |
| Cash Flows From Financing Activities | | | | | | | | |
| Proceeds from the issuance of convertible promissory notes | | | 675,000 | | | | 1,407,027 | |
| Payments of convertible promissory notes | | | - | | | | (55,000 | ) |
| Proceeds from demand note | | | 120,000 | | | | - | |
| | | | | | | | | |
| Proceeds from line of credit | | | 60,600 | | | | - | |
| Net cash provided by financing activities | | | 855,600 | | | | 1,352,027 | |
| | | | | | | | | |
| Net increase in cash | | | 133,795 | | | | 53,027 | |
| | | | | | | | | |
| Cash at beginning of period | | | 124,769 | | | | 38,126 | |
| | | | | | | | | |
| Cash at end of period | | $ | 258,564 | | | $ | 91,153 | |
| | | | | | | | | |
| Supplemental Disclosures of Cash Flow Information | | | | | | | | |
| Cash paid for interest | | $ | - | | | $ | 3,190 | |
| | | | | | | | | |
| Supplemental Disclosures of Noncash Flow Information | | | | | | | | |
| Accrual of preferred stock dividends | | $ | 595,666 | | | $ | 595,667 | |
| | | | | | | | | |
| Accretion of preferred stock to liquidation value | | $ | - | | | $ | - | |
| | | | | | | | | |
| Conversion of accounts payable to a convertible promissory note | | $ | 105,000 | | | $ | - | |
The accompanying notes are an integral part of these condensed financial statements.
Aperion Biologics, Inc. Notes to Condensed Financial Statements June 30, 2015 and 2014
(unaudited) |
| 1. | Summary of Significant Accounting Policies |
Business Organization and Nature of Operations
Aperion Biologics, Inc. ("ABI" or the "Company") is a commercial-stage medical device company that develops patented technology to make animal tissues usable for human medical applications without causing rejection by the body. The core platform technology is an enzymatic stripping of the key carbohydrate antigens followed by a unique conversion process that both "humanizes" and sterilizes the tissues without unduly affecting their biomechanical or biological properties.
The Company's primary activities since its inception have been the development and implementation of its business plans, performing research on its platform technology, conducting clinical trials on its products in both animals and humans, pursuing regulatory approvals to market its product in US and international markets, and raising capital. The Company has not generated any material revenues from operations. To date, the Company has funded its business with the proceeds of private placements of equity and debt securities.
The accompanying unaudited condensed financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America ("U.S. GAAP") for interim financial information. Accordingly, they do not include all of the information and disclosures required by U.S. GAAP for annual financial statements. In the opinion of management, such statements include all adjustments (consisting only of normal recurring items) that are considered necessary for a fair presentation of the condensed financial statements of the Company as of June 30, 2015 and 2014 and for the nine months ended June 30, 2015 and 2014. The results of operations for the nine months ended June 30, 2015 and 2014 are not necessarily indicative of the operating results for the full year ending September 30, 2015, or any other period. These condensed financial statements should be read in conjunction with the audited financial statements and related disclosures of the Company as of September 30, 2014 and for the year then ended, which are included elsewhere in this document.
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates. The Company's significant estimates and assumptions include the fair value of the Company's stock, stock-based compensation, estimated useful lives of depreciable assets, and the valuation allowance relating to the Company's deferred tax assets.
Revenue
The Company recognizes revenues when its obligations to a customer are fulfilled relative to a specific product or service offering and all of the following conditions are satisfied: (i) persuasive evidence of an arrangement exists; (ii) the price is fixed or determinable; (iii) collectability is reasonably assured; and (iv) delivery has occurred or services have been rendered.
Revenue from products is recognized when the risks and rewards of ownership have been transferred to the customer, which is upon shipment.
Aperion Biologics, Inc. Notes to Condensed Financial Statements June 30, 2015 and 2014
(unaudited) |
| 1. | Summary of Significant Accounting Policies (Continued) |
Contingencies
Certain conditions may exist as of the date the financial statements are issued, which may result in a loss to the Company, but which will only be resolved when one or more future events occur or fail to occur. The Company's management and its legal counsel assess such contingent liabilities, and such assessment inherently involves an exercise of judgment. In assessing loss contingencies related to legal proceedings that are pending against the Company or unasserted claims that may result in such proceedings, the Company's legal counsel evaluates the perceived merits of any legal proceedings or unasserted claims, as well as the perceived merits of the amount of relief sought or expected to be sought therein.
If the assessment of a contingency indicates it is probable that a material loss has been incurred and the amount of the liability can be estimated, then the estimated liability would be accrued in the Company's financial statements. If the assessment indicates a potentially material loss contingency is not probable, but is reasonably possible, or is probable, but cannot be estimated, then the nature of the contingent liability, together with an estimate of the range of possible loss, if determinable and material, would be disclosed.
Loss contingencies considered remote are generally not disclosed unless they involve guarantees, in which case the guarantees would be disclosed.
Research and Development
Research and development costs are charged to operations when incurred. The amounts charged for the nine months ended June 30, 2015 and 2014 were $39,916 and $56,345, respectively.
Net Earnings (Loss) Per Share of Common Stock
Basic net earnings (loss) per share is computed by dividing net earnings (loss) attributable to common stockholders by the weighted average number of common shares outstanding during the period. Diluted net earnings (loss) per share reflect the potential dilution that could occur if securities or other instruments to issue common stock were exercised or converted into common stock. Potentially dilutive securities are excluded from the computation of diluted net earnings (loss) per share if their inclusion would be anti-dilutive and consist of the following:
| | | June 30, | |
| | | 2015 | | | 2014 | |
| Options | | | 350,421 | | | | 340,231 | |
| Warrants to purchase common stock | | | 544,380 | | | | 544,380 | |
| Warrants to purchase preferred stock | | | 115,557 | | | | 115,557 | |
| Series A convertible preferred stock | | | 36,383 | | | | 36,383 | |
| Series B convertible preferred stock | | | 230,330 | | | | 230,330 | |
| Series C convertible preferred stock | | | 662,574 | | | | 627,337 | |
| Series C-2 convertible preferred stock | | | 1,035,059 | | | | 971,068 | |
| Convertible promissory notes, including accrued interest | | | 1,635,360 | | | | 1,260,885 | |
| | | | | | | | | |
| Total potentially dilutive shares | | | 4,610,065 | | | | 4,126,171 | |
Aperion Biologics, Inc. Notes to Condensed Financial Statements June 30, 2015 and 2014
(unaudited) |
Summary of Significant Accounting Policies (Continued)
Recently Issued Accounting Pronouncements
Recent accounting standards that have been issued or proposed by the FASB or other standards-setting bodies that do not require adoption until a future date are not expected to have a material impact on the Company's financial statements upon adoption.
Subsequent Events
Management has evaluated subsequent events to determine if events or transactions occurring through September 21, 2015, the date on which the condensed financial statements were available to be issued require adjustment or disclosure in the Company's financial statements.
Effective as of _________, 2015, the Company amended its Certificate of Incorporation and effected a 18.4-for-1 reverse stock split. All per share amounts and number of shares in the condensed financial statements and related notes have been retroactively restated to reflect the reverse stock split.
| 2. | Going Concern and Management's Liquidity Plans |
As of June 30, 2015, the Company had working capital deficit and a stockholders' deficiency of $14,055,281 and $28,398,077, respectively. During the nine months ended June 30, 2015, the Company incurred a net loss of $1,860,802. The Company has not generated any material revenues and has incurred net losses since inception. Furthermore, as of the date of this report, the Company has $6,724,000 of convertible promissory notes that have matured and are in default. These conditions raise substantial doubt about the Company's ability to continue as a going concern.
The Company recognizes it will need to raise additional capital in order to meet its obligations and execute its business plan for at least the next twelve month period. There is no assurance that additional financing will be available when needed or that management will be able to obtain such financing on terms acceptable to the Company and that the Company will become profitable and generate positive operating cash flow in the future. If the Company is unable to raise sufficient additional funds, it will have to develop and implement a plan to further extend payables, reduce overhead or scale back its business plan until sufficient additional capital is raised to support further operations. There can be no assurance that such a plan will be successful.
Accordingly, the accompanying financial statements have been prepared in conformity with U.S. GAAP, which contemplate continuation of the Company as a going concern and the realization of assets and the satisfaction of liabilities in the normal course of business. The carrying amounts of assets and liabilities presented in the financial statements do not necessarily represent realizable or settlement values. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Inventory raw materials include alpha-galactosidase, an enzyme used in the manufacturing of the Company's core platform technology, and untreated porcine tissue. Finished goods include porcine tissue that has been sterilized and packaged.
Inventory consists of the following:
| | | June 30,
2015 | | | September 30,
2014 | |
| | | | | | | |
| Raw materials | | $ | 35,156 | | | $ | 62,084 | |
| Finished goods | | | 32,528 | | | | - | |
| | | | | | | | | |
| | | $ | 67,684 | | | $ | 62,084 | |
Aperion Biologics, Inc. Notes to Condensed Financial Statements June 30, 2015 and 2014
(unaudited) |
| 4. | Convertible Promissory Notes |
Related Parties
Dr. Kevin Stone, a director and majority beneficial shareholder of the Company, is the managing member and a controlling person of CrossCart LLC, a California limited liability company (CrossCart). The Company previously issued convertible promissory notes to CrossCart and its predecessor-in-interest, Lurie Investments, Inc. At June 30, 2015 and September 30, 2014, the balance of these notes totaled $6,520,015. Accrued interest payable related to these notes totaled $1,572,975 and $1,093,186, at June 30, 2015 and September 30, 2014, respectively, and is included as accrued liabilities on the condensed balance sheets.
Bruce Stone is the brother of Dr. Kevin Stone. The Company issued convertible promissory notes to Bruce Stone totaling $75,000 during the nine months ended June 30, 2015. These notes bear interest of 8% per annum, which increases to 10% per annum if an event of default occurs. At the noteholder's discretion, outstanding principal and any unpaid interest amounts of the notes may be converted into shares of Series D or Series E convertible preferred stock. At June 30, 2015 and September 30, 2014, the balance of these notes totaled $125,000 and $50,000, respectively. Accrued interest payable related to these notes totaled $6,407 and $1,433 at June 30, 2015 and September 30, 2014, respectively, and is included as accrued liabilities on the balance sheets.
As of June 30, 2015, the Company had $3,750,000, $2,860,015 and $35,000 issued and outstanding principal amount of convertible promissory notes, plus $1,082,139, $495,485 and $1,758 in accrued interest, that are convertible into Series C2, Series D and Series E convertible redeemable preferred stock, respectively. As of September 30, 2014, the Company had $3,750,000 and $2,820,015 issued and outstanding principal amount of convertible promissory notes, plus $802,972 and $291,647 in accrued interest, that are convertible into Series C2 and Series D convertible redeemable preferred stock, respectively. As of the date of this report, Series D and Series E were not effected. The Series C2 notes are convertible at $8.83 per share, subject to adjustment for stock-splits and similar transactions. All of these notes are convertible at the option of the holder. The Series D notes are convertible at $4.60 per share, subject to adjustment. Of the Series D amount above, $1,050,000 are contingently convertible upon a qualified financing (as defined) and $1,770,015 are convertible at the option of the holder. The Series E notes are convertible at $4.60/share, subject to adjustment. All of the Series E notes are convertible at the option of the holder.
As of June 30, 2015, the Company had $6,570,015 issued and outstanding principal amount of convertible promissory notes classified as current liabilities and $75,000 issued and outstanding principal amount of convertible promissory notes classified as long term liabilities. As of September 30, 2014, the Company had $6,500,000 issued and outstanding principal amount of convertible promissory notes classified as current liabilities and $70,015 issued and outstanding principal amount of convertible promissory notes classified as long term liabilities.
If all convertible instruments were to convert at the same time, the Company would not have sufficient common shares authorized. All of the notes contain language that provide that the conversion of the notes may be delayed until the proper number of shares is authorized and reserved, with the Company obligated to use its best efforts to cause a sufficient number of shares to be authorized and reserved as soon as practicable.
Unrelated Parties
The Company issued convertible promissory notes totaling $705,000 during the nine months ended June 30, 2015. These notes bear interest of 8% per annum, which increases to 10% per annum if an event of default occurs, and mature at various dates through September 3, 2016. At the noteholder's discretion, outstanding principal and any unpaid interest amounts of the notes may be converted into shares of Series D, or Series E convertible preferred stock. At June 30, 2015 and September 30, 2014 the balance of these notes totaled $1,511,012 and $806,012, respectively. Accrued interest payable related to the notes totaled $102,649 and $39,535, at June 30, 2015 and September 30, 2014, respectively, and is included as accrued liabilities on the balance sheets.
Aperion Biologics, Inc. Notes to Condensed Financial Statements June 30, 2015 and 2014
(unaudited) |
| 4. | Convertible Promissory Notes (Continued) |
Unrelated Parties (Continued)
As of June 30, 2015, the Company had $1,106,012 and $405,000 issued and outstanding principal amount of convertible promissory notes, plus $90,732 and $11,917 in accrued interest, that are convertible into Series D and E convertible preferred stock, respectively. As of September 30, 2014 the Company had issued and outstanding $806,012 of convertible promissory notes, plus $39,535 in accrued interest, that are convertible into Series D convertible preferred stock. The Series D notes are convertible at $4.60 per share, subject to adjustment for stock-splits and similar transactions. The Series E notes are convertible at $4.60 per share, subject to adjustment for stock-splits and similar transactions. The Series D and E notes are convertible at the option of the holder. As of the date this report, the Series D and Series E shares were not effected.
As of June 30, 2015, the company had $406,012 issued and outstanding principal amount of convertible promissory notes classified as current liabilities and $1,105,000 issued and outstanding principal amount of convertible promissory notes classified as long term liabilities. As of September 30, 2014, the Company had $169,000 issued and outstanding principal amount of convertible promissory notes classified as current liabilities and $637,012 issued and outstanding principal amount of convertible promissory notes classified as long term liabilities.
If all convertible instruments were to convert at the same time, the Company would not have sufficient common shares authorized. All of the notes contain language that provide that the conversion of the notes may be delayed until the proper number of shares is authorized and reserved, with the Company obligated to use its best efforts to cause a sufficient number of shares to be authorized and reserved as soon as practicable.
| 5. | Demand Note and Line of Credit |
Related Parties
On December 18, 2014 the Company entered into a demand note with CrossCart totaling $120,000. This note bears interest at 8%, which increases to 10% per annum if an event of default occurs. At June 30, 2015, the balance of this note totaled $120,000. Accrued interest payable related to this note totaled $5,093 at June 30, 2015 and is included as accrued liabilities on the balance sheet.
During fiscal 2015, the Company entered into a line of credit totaling $100,000 with CrossCart. This note matures on August 31, 2016 and bears interest at 10% per annum, which increases to 12% per annum if an event of default occurs. At June 30, 2015, the balance of this note totaled $60,600. Accrued interest payable related to this note totaled $518 at June 30, 2015 and is included as accrued liabilities on the balance sheet.
| 6. | Stockholders' Deficiency |
Preferred Stock Dividends
No dividends with respect to the Series A preferred stock and Series B preferred stock accrue. At the discretion of the Board, dividends may be paid at the rate of 8% of the Series A and Series B original issue price. From inception through the date of this report, the Board has not declared any Series A or Series B convertible preferred stock dividends.
Series C and Series C-2 preferred stock accrue cumulative dividends at the rate of 8% per annum of the original Series C issue price per share from the date of issuance. Dividends accrue whether or not such dividends are earned or declared and whether or not sufficient funds are legally available. For the nine months ended June 30, 2015, $211,530 and $384,136 of Series C and Series C-2 dividends, respectively, were accrued and expensed.
Common Stock
On May 28, 2015, the Company issued 2,717 shares of common stock to a consultant with a de minimis value in accordance with a service agreement.
Aperion Biologics, Inc. Notes to Condensed Financial Statements June 30, 2015 and 2014
(unaudited) |
During the nine months ended June 30, 2015, the Company issued 40,761 stock options to a consultant with an exercise price of $0.37 and a fair value of 0.22 per share. The stock options granted are subject to vesting over two years and have a term of ten years.
During the nine months ended June 30, 2015, 30,571 options were forfeited due to the termination of services. The forfeited options were added back to the available pool for future issuance.
The following table represents stock option activity for the nine months ended June 30, 2015:
| | | | | | | | | Weighted Average | |
| | | Stock Options | | | Exercise Price | | | Fair Value | | | Contractual | | | Aggregate | |
| | | Outstanding | | | Exercisable | | | Outstanding | | | Exercisable | | | Vested | | | Life (Years) | | | Intrinsic Value | |
| Balance - September 30, 2014 | | | 340,231 | | | | 281,363 | | | $ | 1.47 | | | $ | 1.47 | | | $ | 1.47 | | | | 10.00 | | | | | |
| Granted | | | 40,761 | | | | | | | | 0.37 | | | | | | | | | | | | | | | | | |
| Exercised | | | - | | | | | | | | - | | | | | | | | | | �� | | | | | | | |
| Forfeited | | | (30,571 | ) | | | | | | | 0.37 | | | | | | | | | | | | | | | | | |
| Balance - June 30, 2015 | | | 350,421 | | | | 328,468 | | | | 1.47 | | | | 1.47 | | | | 1.47 | | | | 10.00 | | | $ | 8,854 | |
The following table summarizes information on stock options outstanding and exercisable as of June 30, 2015:
Exercise Price | | | Number Outstanding | | | Weighted Average
Remaining Contractual
Term (years) | | | Weighted Average Exercise
Price | | | Number Exercisable | | | Weighted Average Exercise
Price | |
| $ | 0.18 | | | | 96,508 | | | | 4.00 | | | $ | 0.18 | | | | 95,965 | | | $ | 0.18 | |
| | 0.29 | | | | 66,332 | | | | 8.26 | | | | 0.29 | | | | 59,980 | | | | 0.29 | |
| | 0.37 | | | | 10,190 | | | | 9.88 | | | | 0.37 | | | | 10,190 | | | | 0.37 | |
| | 1.90 | | | | 118,967 | | | | 6.33 | | | | 1.90 | | | | 115,305 | | | | 1.90 | |
| | 4.05 | | | | 52,989 | | | | 7.06 | | | | 4.05 | | | | 41,594 | | | | 4.05 | |
| $ | 8.28 | | | | 5,435 | | | | 2.62 | | | $ | 8.28 | | | | 5,435 | | | $ | 8.28 | |
| | | | | | 350,421 | | | | | | | | | | | | 328,468 | | | | | |
Stock-Based Compensation
For the nine months ended June 30, 2015 and 2014, stock-based compensation totaled approximately $43,719 and $57,919, respectively. As of June 30, 2015, total unrecognized stock option compensation expense is $38,222, which will be recognized as those options vest over a period of approximately four years.
The fair value of each option grant is estimated at the date of grant using the Black-Scholes pricing model. Volatility is based on average historical volatilities for public companies in similar industries over the expected term of the options. The expected term of options represents the period of time that options granted are expected to be outstanding. The risk-free rate is for periods within the contractual life of options and is based on the United States Treasury yield curve in effect at the time of grant. In addition, the Company is required to estimate the expected forfeiture rate and only recognize expense for those shares expected to vest. In estimating the Company's forfeiture rate, the Company analyzed its historical forfeiture rate, the remaining lives of unvested options, and the number of vested options as a percentage of total options outstanding. If the Company's actual forfeiture rate is materially different from its estimate, or if the Company reevaluates the forfeiture rate in the future, the stock-based compensation expense could be significantly different from what the Company has recorded in the current period.
Aperion Biologics, Inc. Notes to Condensed Financial Statements June 30, 2015 and 2014
(unaudited) |
| 7. | Stock Options (Continued) |
Stock-Based Compensation (Continued)
The weighted-average Black-Scholes assumptions are as follows:
| | | June 30,
2015 | | | June 30,
2014 | |
| | | | | | | |
| Term | | 5.5 to 10 years | | | 5.5 to 10 years | |
| Risk-free interest rate | | | 1.00 | % | | | 1.00 | % |
| Volatility | | | 49.30 | % | | | 49.80 | % |
| Expected dividend yield | | | - | | | | - | |
| Forfeiture rate | | | - | | | | - | |
| 8. | Commitments and Contingent Liabilities |
Lease Commitments
The Company leases office space and certain equipment under noncancellable operating lease agreements. The leases generally require the Company to pay certain maintenance, insurance, and other operating expenses. Rental expense under operating leases totaled approximately $28,535 and $26,557 for the nine months ended June 30, 2015 and 2014, respectively.
Future minimum lease payments under noncancellable operating leases as of June 30, 2015 are as follows:
| Year ending June 30, | | | |
| 2016 | | $ | 8,139 | |
| 2017 | | | 1,740 | |
| 2018 | | | 1,740 | |
| 2019 | | | 1,595 | |
| | | | | |
| | | $ | 13,214 | |
Royalty Commitments
The Company has entered into a license agreement whereby the Company may use certain inventions and discoveries with its products. The license agreement calls for sales royalties ranging from 1.5% to 1.9% on net sales less than $10 million and 1.2% to 1.6% on net sales greater than $10 million, with a minimum annual royalty of $35,000, which shall be creditable against the sales royalties. The cumulative royalty payments to be paid under this agreement shall not exceed $3,500,000. Unless terminated earlier under the provisions of the agreement, the license agreement will remain in force during the life of the patents for the products included in the agreement. Royalty expense under this agreement totaled $26,250 for nine months ended June 30, 2015 and 2014. In March 2015, the Company converted the outstanding payable of $105,000 into a convertible promissory note.
The Company entered into a license agreement in 2003 which called for the payment of royalties and milestone payments related to the use of a certain enzyme. The Company has paid a total of $190,000 in royalty payments to date under this agreement, and has accrued $50,000 in additional royalty payments contingent upon the delivery of certain product documentation. The Company has not used the licensed enzymes since 2008. In 2014 the Company received a letter from the licensor alleging that Company owed additional payments under the license agreement; however, it is the opinion of the Company that no payments other than the amounts accrued are owed under this contract.
Aperion Biologics, Inc. Notes to Condensed Financial Statements June 30, 2015 and 2014
(unaudited) |
| 8. | Commitments and Contingent Liabilities (Continued) |
Royalty Commitments - Related Party
The Company has an agreement with CrossCart to pay CrossCart a royalty of 5% of sales of products produced by the Z-Process and is payable on these sales in perpetuity. Total royalty expense charged to operations was $1,350 for the nine months ended June 30, 2015. Royalty expense payable at June 30, 2015 was $1,350 and is included as accrued liabilities on the balance sheet. In September 2015, the Company entered into an agreement with CrossCart and Dr. Kevin Stone, under which such royalty will terminate immediately following the completion of this offering. See “Subsequent Event” in Note 10 below.
Transaction Bonus
In December 2014, the Company issued a demand note to CrossCart for an aggregate principal amount of $120,000 (the "Demand Note"). As an inducement to enter into the Demand Note, the Company agreed to pay CrossCart a transaction bonus (the "Transaction Bonus") equal to 3% of the gross proceeds received from any control change transaction of the Company.
Change of Control Bonus
In March 2012, the Company awarded Dr. Stone a bonus (the "Stone Bonus Agreement") in the event of an acquisition of the Company. An acquisition is defined as a sale, merger, consolidation, transfer or other disposition of all or substantially all (more than 80%) of the Company to another entity (the "Acquisition"). An initial public offering of the Company's common stock pursuant to an effective registration statement filed with the Commission under the Securities Act of 1933, as amended (the "Securities Act"), does not constitute an Acquisition. Dr. Stone is entitled to receive a bonus award in an amount equal to 1% of the proceeds received in the Acquisition that occurred on or prior to the completion of an initial public offering of the Company's stock pursuant to an effective registration statement filed with the Commission under the Securities Act.
Management Retention Plan
In December 2013, the Board approved the Management Retention Plan (the "Plan"), pursuant to which certain executives are entitled to receive a bonus upon the occurrence of specified events, provided that the participant must continue as an employee of the Company through the occurrence of the triggering event. Under the Plan, upon a significant external financing (beyond any financing from CrossCart) or a signing of a significant license or distribution agreement, the executives are to receive bonuses and any deferred compensation that would have been paid had full salaries been in effect from July 2013 through the date of the external financing/licensing agreement. All unpaid salaries have been accrued in the company's financial statements.
In addition, the Plan also provides that upon closing of a deemed liquidation event specified in the Company's certificate of incorporation or a sale of the Company's securities representing 50% or more of total voting power of outstanding securities, the executives are entitled to receive bonus payments based, in part, on the amount of proceeds received by the Company in the Acquisition.
Transactions with Directors
In December 2013, the Company entered into a letter agreement with two of its directors, to provide certain acquisition bonus payments (the "Director Bonus Agreement"), Under the Director Bonus Agreement, in the event of a deemed liquidation event specified in the Company's certificate of incorporation or a sale of the Company's securities representing 50% or more of total voting power of outstanding securities, the directors are entitled to receive bonus payments based, in part, on the amount of proceeds received by the Company in the Acquisition.
Aperion Biologics, Inc. Notes to Condensed Financial Statements June 30, 2015 and 2014
(unaudited) |
The Company is dependent on a third party to supply inventory. The loss or significant reduction in product availability from the supplier could have a material adverse effect on the Company. The Company believes that its relationship with the supplier is satisfactory.
Royalty Agreement Amendment
On September 7, 2015, CrossCart, LLC, Dr. Kevin Stone, and the Company agreed to terminate CrossCart's unlimited royalty on sales of products produced by the Company's proprietary manufacturing process, subject to completion of the Company's initial public offering of securities. The royalty will now be capped after the first $12,000,000 of sales.
Bonus Termination
On September 7, 2015, CrossCart, LLC, Dr. Kevin Stone, and the Company agreed to terminate the Transaction Bonus and Stone Bonus Agreement, subject to completion of the Company's initial public offering of securities.
Lease Commitment
On September 17, 2015 the Company received notice that its offer to extend its commercial lease on the property at 11969 Starcrest Drive was accepted by the landlord for a term beginning October 1, 2015 and ending December 31, 2018.
Convertible Promissory Notes
On September 1, 2015, the Company completed the sale of $200,000 of convertible promissory notes. The notes convert into the Series E preferred stock at $4.97 per share at the option of the holder. As of the date of this report, the Series E shares were not effected.
On September 22, 2015 the Company completed the sale of $100,000 of convertible promissory notes. The notes convert into the Series E preferred stock at $4.97 per share at the option of the holder. As of the date of this report, the Series E shares were not effected.
On October 2, 2015 the Company completed the sale of $25,000 of convertible promissory notes. The notes convert into the Series E preferred stock at $4.97 per share at the option of the holder. As of the date of this report, the Series E shares were not effected.
On October 16, 2015, the Company completed the sale of $100,000 of convertible promissory notes. The notes convert into the Series E preferred stock at $4.97 per share at the option of the holder. As of the date of this report, the Series E shares were not effected.
Option Exercise
On October 1, 2015 a consultant exercised options to purchase 2,717 shares of common stock in exchange for cash proceeds of $500, which was the exercise price in accordance with the applicable stock option agreement.
On October 1, 2015 a consultant exercised options to purchase 5,435 shares of common stock in exchange for cash proceeds of $1,000, which was the exercise price in accordance with the applicable stock option agreement.
On October 2, 2015 a consultant exercised options to purchase 2,717 shares of common stock in exchange for cash proceeds of $500, which was the exercise price in accordance with the applicable stock option agreement.
Management Retention Plan Modification
On October 16, 2015 the Board modified, by unanimous written consent, the Management Retention Plan, subsequent to the completion of the Company’s initial public offering of securities. The bonus payments due in event of a deemed liquidation event were eliminated, and a portion of the bonuses due in the event of an external financing or similar transaction will be paid in Company stock at the public offering price.
Payment of Deferred Board Fees
On October 16, 2015 the Board modified, by unanimous written consent, the payment terms of its fee agreements with its non-employee directors. The payments due to non-employee directors will be made by issuing shares of common stock at $4.97 per share for 70% of the amounts due, with the balance of 30% being paid in cash subsequent to the completion of the offering. The Company also entered into an agreement with each director to provide for the modified fee arrangement.
Amendments to Convertible Promissory Notes
In October 2015, all of the Company’s Convertible Promissory Note holders agreed to automatically convert their Notes into common stock of the Company at the respective Series D ($4.60 per share) or Series E price per share ($4.60 or $4.97) per share as applicable.) In addition, a limited waiver of default was granted which expires on March 31, 2016.
Notice of Conversion
On October 19, 2015, the holder of a majority of the outstanding shares of the Company’s Series C preferred Stock issued a notice to elect to convert its shares into common stock in the event of the completion of the Company’s initial public offering. This election will trigger mandatory conversion of all Preferred Stock, including accrued dividends, upon the completion of the offering.
Director Bonus Agreement Termination
On October 12 and 18, 2015 two directors of the Company agreed to terminate the Director Bonus Agreement, subject to completion of the Company’s initial public offering of securities.
PART III—EXHIBITS
| Exhibit | | Description of Document |
| | | |
| 1.1 | | Form of Underwriting Agreement* |
| | | |
| 2.1 | | Amended and Restated Certificate of Incorporation of the Issuer, as currently in effect+ |
| | | |
| 2.2 | | Second Amended and Restated Certificate of Incorporation, as in effect following the offering+ |
| | | |
| 2.3 | | Bylaws of the Issuer, as currently in effect+ |
| | | |
| 2.4 | | Amended and Restated Bylaws, as in effect following the offering+ |
| | | |
| 2.5 | | Certificate of Amendment |
| | | |
| 3.1 | | Second Amended and Restated Investors’ Rights Agreement+ |
| | | |
| 3.2 | | Form of Convertible Promissory Note+ (previously filed as Exhibit 3.3) |
| | | |
| 3.3 | | Form of Warrant to purchase Preferred Stock of the Company+ (previously filed as Exhibit 3.5) |
| | | |
| 3.4 | | Form of Warrant to purchase Common Stock of the Company+ (previously filed as Exhibit 3.6) |
| | | |
| 3.5 | | Form of Underwriter’s Warrant to Purchase Common Stock of the Company |
| | | |
| 3.6 | | Form of Amendment to Convertible Promissory Note |
| | | |
| 6.1 | | License Agreement dated January 8, 2009 between the Company and The Curators of the University of Missouri† |
| | | |
| 6.2 | | Letter Agreement dated December 23, 2013 by and among the Company, CrossCart LLC and Dr. Kevin R. Stone+ |
| | | |
| 6.3 | | Aperion Management Retention Plan+ |
| | | |
| 6.4 | | Aperion Amended and Restated Management Retention Plan |
| | | |
| 6.5 | | Lease Agreement dated December 17, 2008 by and among the Company and Titan Mac Fund I, LP, as amended on June 21, 2012.+ |
| | | |
| 6.6 | | Independent Consultant Agreement effective September 1, 2008 between the Company and David Cocke+ |
| | | |
| 6.7 | | 2008 Stock Option/Stock Issuance Plan+ |
| | | |
| 6.8 | | Transaction Bonus Forfeiture Agreement by and between the Company and David W. Anderson |
| | | |
| 6.9 | | Transaction Bonus Forfeiture Agreement by and between the Company and Alfred G. Holcomb |
| | | |
| 6.10 | | Omnibus Amendment to Warrants by and between the Company and CrossCart, LLC |
| | | |
| 6.11 | | Omnibus Amendment to Warrants by and between the Company and Kevin Stone, MD |
| | | |
| 6.12 | | Omnibus Amendment to Convertible Promissory Notes by and between the Company and CrossCart, LLC |
| | | |
| 6.13 | | Employment Agreement by and between the Company and Daniel R. Lee |
| | | |
| 6.14 | | Royalty Right and Transaction Bonus Forfeiture Agreement by and among the Company, Dr. Kevin Stone and CrossCart LLC, dated September 10, 2015 |
| | | |
| 6.15 | | Form of Demand Promissory Note issued to CrossCart LLC+ (previously filed as Exhibit 3.2) |
| | | |
| 6.16 | | Line of Credit Note issued to CrossCart LLC+ (previously filed as Exhibit 3.4) |
| | | |
| 8.1 | | Escrow Agreement* |
| | | |
| 10.1 | | Power of Attorney+ |
| | | |
| 11.1 | | Consent of Marcum LLP |
| 12.1 | | Opinion Of Morgan, Lewis & Bockius LLP* |
| | | |
| 13.1 | | Testing the water materials† |
* To be filed by amendment.
| † | Confidential treatment has been requested for certain information contained in this document. Such information has been omitted and filed separately with the Commission |
SIGNATURES
Pursuant to the requirements of Regulation A, the issuer certifies that it has reasonable grounds to believe that it meets all of the requirements for filing on Form 1-A and has duly caused this offering statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the city of San Antonio, State of Texas, on October 23, 2015.
| | APERION BIOLOGICS, INC. |
| | |
| | By: | /s/ Daniel R. Lee |
| | | Daniel R. Lee |
| | | Chief Executive Officer |
Pursuant to the requirements of the Securities Act of 1933, as amended, this Offering Statement has been signed by the following persons in the capacities indicated.
| Signature | | Title | | Date |
| | | | | |
| /s/ Daniel R. Lee | | Chief Executive Officer and Director | | October 23, 2015 |
| Daniel R. Lee | | (Principal Executive Officer) | | |
| | | | | |
| /s/ David Cocke | | Chief Financial Officer | | October 23, 2015 |
| David Cocke | | (Principal Financial Officer and Principal Accounting Officer) | | |
| | | | | |
| * | | Director | | October 23, 2015 |
| * | | Director | | October 23, 2015 |
| France Dixon Helfer | | | | |
| | | | | |
| * | | Director | | October 23, 2015 |
| Alfred Holcomb | | | | |
| | | | | |
| * | | Director | | October 23, 2015 |
| Kevin R. Stone, M.D. | | | | |
| | | | | |
| * | | Director | | October 23, 2015 |
| Mike Ward | | | | |
| * | /s/ Daniel R. Lee | | | |
| | Daniel R. Lee | | | |
| | Attorney-in-fact | | | |












