Exhibit 13.1

Title Text 1 Investment Overview Daniel Lee, CEO David Cocke, CFO Kevin Stone, MD

Overview 2 Disclaimer The information contained herein is provided solely for the purpose of acquainting the reader with Aperion biologics, inc. (The “company” or “we”) and its executive personnel, and to solicit any indication of interest in a potential offering of securities by the company. It is not an offer to sell nor is it a solicitation of any offer to buy any securities and conveys no right, title or interest in the company or the products of its business activities. No money or other consideration is being solicited in connection this meeting and presentation, and if sent in response, will not be accepted. No offer to buy the securities can be accepted and no part of the purchase price can be received until an offering statement on form 1 - a is qualified pursuant to regulation a of the securities act of 1933, as amended, and any such offer may be withdrawn or revoked, without obligation or commitment of any kind, at any time before notice of its acceptance given after the qualification date. Any person’s indication of interest in the meeting involves no obligation or commitment of any kind. The information contained herein is confidential and strictly proprietary. By accepting this document, the recipient agrees not to reproduce its contents nor disclose or distribute same to any person or entity without the expressed prior consent of the company. This presentation does not purport to be all - inclusive or to contain all of the information you or any prospective investor may desire. You should make your own decision on whether this investment opportunity meets your investment objectives and risk tolerance level. Any person considering entering into a proposed transaction should seek its own independent financial and legal advice . IMPORTANT: An offering statement relating to our securities has been filed with the Securities and Exchange Commission but has not yet become qualified. These securities may not be sold nor may offers be accepted prior to the time the offering statement becomes qualified. You can access a copy of the preliminary offering circular by clicking on this link: http://www.sec.gov/Archives/edgar/data/1439026/000114420415060614/v420878_ partiiandiii.htm You can also call or write the underwriter of the proposed offering, W.R. Hambrecht & Co., to obtain a copy of the preliminary offering circular, at 1 - 800 - 673 - 6476, fax 1 - 415 - 551 - 3123; 909 Montgomery Street, 3rd Floor, San Francisco, CA 94133 Forward Looking Statements This presentation contains forward - looking statements that are based on our management’s belief and assumptions and on information currently available to our management. Although we believe that the expectations reflected in these forward - looking statements are reasonable, these statements involve known and unknown risks, uncertainties and other factors that may cause our actual results, levels or performance or achievements expressed or implied by these forward - looking statements. Accordingly, you should not place undue reliance on these statements. Forward - looking statements in this presentation include, but are not limited to, statements relating to the projected growth of ACL markets; the anticipated commercialization of our Z - Lig products; the potential applications of our Z - process and technology; our strategies to realize development opportunities; the timing and status of clinical trials for our product candidates; and our ability to successfully launch and market our products. These forward looking statements are subject to various risk factors that may cause actual results to differ materially from our current expectations. We have no current intention of updating the forward - looking statements contained in this presentation except to the extent required by law. You should therefore not rely on these forward - looking statements as representing our views as of any date subsequent to the date of this presentation.

ACL Ruptures – A major clinical problem: ▪ Athletes? Active individuals? ▪ 300,000 of these annually in the US, similar number outside the US Current patient options far from optimal: ▪ Auto – painful, 2nd surgery, re - hab , permanent weakness ▪ Allo – supply, inconsistent product, disease risk ▪ Xeno – animal tissue healthy & strong – problem is rejection 3 A new solution for ACL Injuries Z - Lig ® fixes that. Aperion’s 1st approved product is designed to be a better ACLR solution, for both patients and doctors.

The Aperion Solution – Z - Process ▪ Tissue processing technology platform ▪ Removes the key antigens - Enzymatic cleavage ▪ Applicable to many tissues ▪ Solves the problem regarding availability and supply of replacement grafts A broad platform for use with other tissues and indications 4 We’ve made animal tissue safe for human use Aperion is an innovator in biologic orthopedic implants
• Z - Lig ® has actual data behind it ▪ Rare in orthopaedics - Level 1 prospective double blind trial in Europe and South Africa, plus 12 year U.S. safety data ▪ Level 1 patients three years post implantation – non - inferior to allograft • Z - Lig ® received CE - Mark for commercial use in 2014 ▪ Commercial introduction underway in Europe, other countries ▪ Unconditional FDA approval received to commence US pivotal trial • $2b market in ACL alone, worldwide ▪ Only competition is current methods ▪ High Gross Margins >75% 5 Commercialization Underway Capital raise will fund Z - Lig ® commercialization: sales and marketing, inventory production, and US trial
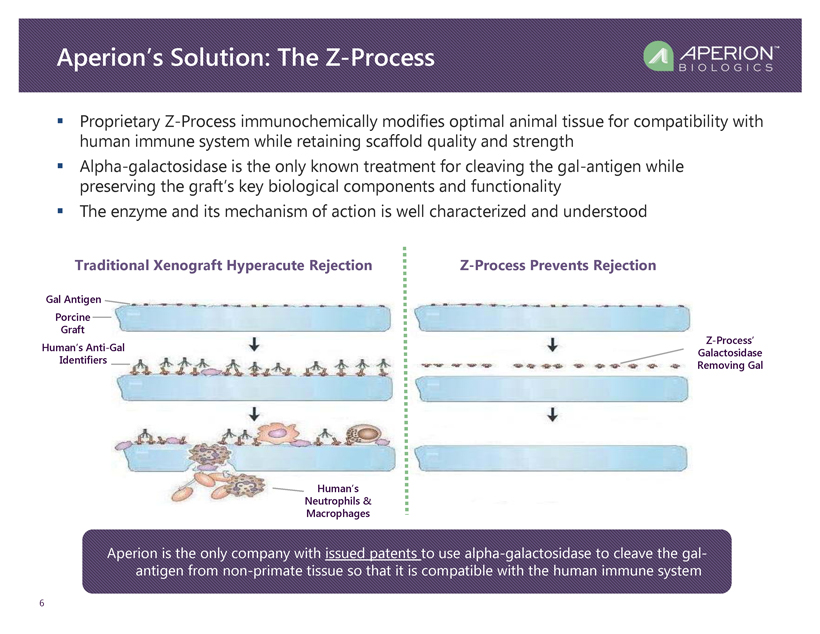
Aperion’s Solution: The Z - Process 6 ▪ Proprietary Z - Process immunochemically modifies optimal animal tissue for compatibility with human immune system while retaining scaffold quality and strength ▪ Alpha - galactosidase is the only known treatment for cleaving the gal - antigen while preserving the graft’s key biological components and functionality ▪ The enzyme and its mechanism of action is well characterized and understood Traditional Xenograft Hyperacute Rejection Z - Process Prevents Rejection Gal Antigen Porcine Graft Human’s Anti - Gal Identifiers Human’s Neutrophils & Macrophages Z - Process’ Galactosidase Removing Gal Aperion is the only company with issued patents to use alpha - galactosidase to cleave the gal - antigen from non - primate tissue so that it is compatible with the human immune system
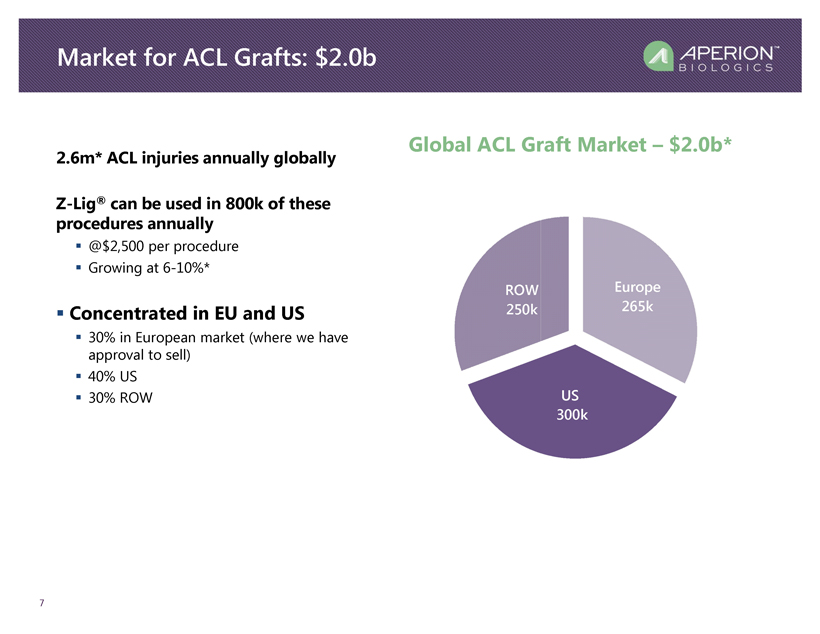
2.6m* ACL injuries annually globally Z - Lig ® can be used in 800k of these procedures annually ▪ @$2,500 per procedure ▪ Growing at 6 - 10%* ▪ Concentrated in EU and US ▪ 30% in European market (where we have approval to sell) ▪ 40% US ▪ 30% ROW 7 Market for ACL Grafts: $ 2. 0b Europe 265k US 300k ROW 250k Global ACL Graft Market – $ 2. 0 b *

Market is Growing Globally 8 (1) Figures are from third party research and are projected 2012 estimates. ROW includes markets that can be penetrated in the n ear term via CE Mark approval. Note: These figures include: (i) the sales of allografts and synthetic grafts and (ii) the direct surgical costs of the autogra ft harvesting and subsequent direct incremental rehabilitation and medication costs related to the second surgical site (assumed to be $2,500 per procedure in total). ACL reconstruction estimated to outpace hip, knee and spine growth 0.0 0.4 0.7 1.1 1.5 2015 2016 2017 2018 2019 2020 2021 Number of Procedures Annually US EU ROW

9 Z - Lig ® Product Advantages Z - Lig ® is an alternative knee ligament reconstruction tissue that is designed to be better for patients and better for surgeons . Z - Lig ® is a young, strong, healthy tendon that becomes the patient’s own ligament over time . Available on demand, when surgeons need it Designed for improved immunologic compatibility by patients Requires no changes to surgical technique, or rehabilitation Cost effective 14 patents worldwide CE Approved and commercially available
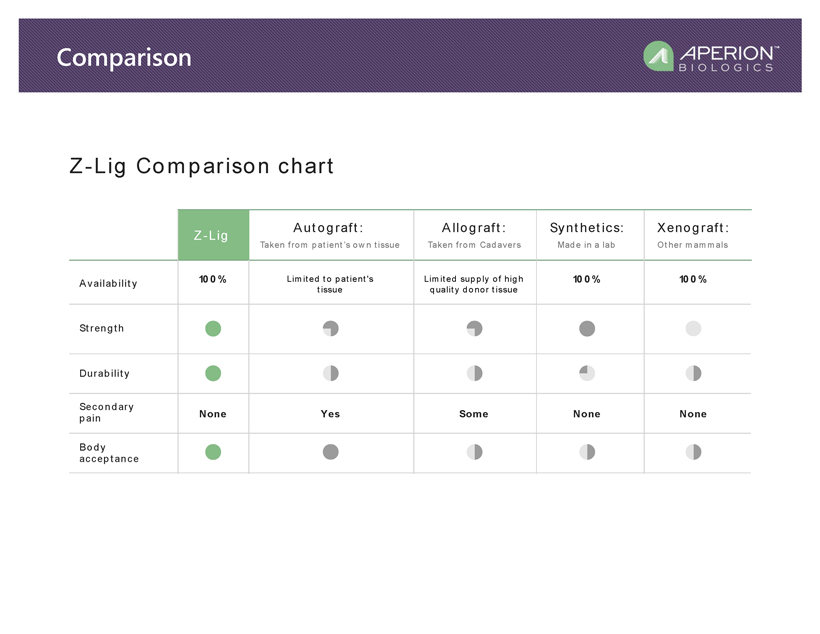
Availability None None NoneYes Some 100% 100%100% Z-Lig Autograft: Allograft: Synthetics: Xenograft: Strength Durability Secondary pain Body acceptance Taken from pa tient ’s own tis sue Limit ed to patient's tissue Limit ed supply of high quality donor tis sue Taken from Cada vers Made in a lab Other mammals Z-Lig Comparison chart Comparison

Main C ompetition is Autograft (Patient harvested) 11 Autograft 228,000 76% Allograft 72,000 24% US ACL Graft Market by Type (300k procedures) European ACL Graft Market by Type (265k procedures) Autograft 257,000 97% Allograft 5,200 2% Synthetics 2,600 1%

12 Performance Tegner SF - 36 Patients return to a high level of activity and demonstrate increased satisfaction with their outcomes over time 0. 2. 4. 6. 8. 10. 0 3 6 12 24 Allograft Z-Lig 40. 45. 50. 55. 60. 0 3 6 12 24 Allograft Z-Lig 0% 25% 50% 75% 100% Baseline 6 12 24 Allograft Z-Lig One - Legged Hop Percentage achieving > 90% functional performance of operated knee when compared to uninjured knee . . . Z - Lig enables return to normal functional performance Tegner SF - 36

Outcomes - Composite Stability 13 6 Months 12 Months 24 Months Demonstrated success better than literature reports with allograft – a clinical “standard of care” 91 % 9 % Success Fa i lure 92% 8% Success Fa i lure 9 6 % 4 % Success Fa i lure 85% Success: the “ Standard of Care ”

Market Launch: Immediate Impact 14 The right people, right place and right data to sell in Europe Z - Lig will be immediately impactful in Europe + + Support of key clinical opinion leaders + Key Market Launch Plan Elements + Phased launch in select markets Initial target: multi - ligament and revision ACL reconstruction surgeries - 15% of total Work with key clinicians and “thought leader” clinical investigators Working with 1 leading distributor in each country: stocking inventory, invoicing, billing Registries in select markets to collect additional clinical experience Compelling European clinical data Restrictions on allograft importation Limited local market allograft supply Need for immediate alternative to autograft

15 Multiyear Rollout Plan Phase 1: Launch to 12 to 18 months • Start with 10 Centers in selected initial countries: Benelux ( B elgium, Netherlands, Luxemburg) , R epublic of S outh Africa , I taly , S pain , Nordic (Denmark, Norway, Sweden, Finland) , but also United Kingdom, Germany , Poland , Switzerland/Austria • Initiate regulatory process for: T urkey , Russia, Australia , S . Korea, and others Phase 2: next 12 to 18 months • Add >20 centers • Deepen operations in Initial markets • Extend markets throughout Europe and other countries Phase 3: > 24 months and beyond • Existing markets expand and stabilize • Further countries granting regulator y approval: China, S. Korea , Singapore, Brazil , etc . • New ligament applications Develop One Key Distributor Relationship per Country - Often Legally Required (Invoicing, etc.) • Rationalizing/optimizing based on new personnel Champions and Clinicians in each Country • Clinical investigators will continue to use/advocate for Z - Lig • For Phase 1, Additional 4 to 10 New Clinicians in Each Country to be identified in conjunction with new distributor
Leadership 16 Name Background Daniel Lee Chief Executive Officer • Joined Aperion in 2008; 20+ years experience in medical device industry • Responsible for TruRepair business at Smith & Nephew Endoscopy, responsible for Global Marketing Activities at OsteoBiologics, Director of Marketing at Regeneration Technologies and Director of Sports Medicine R&D group at Surgical Dynamics • Certified Tissue Bank Specialist . 13 patents on implants and instruments used in orthopaedic and general surgery • Received Masters of Science in Biomedical Engineering from University of Alabama at Birmingham and Bachelor of Science degree in Materials Science and Engineering from Johns Hopkins University Kevin Stone Founder • Founding scientist and Chairman of the Board • Dr. Stone is a leading expert and authority of advanced orthopedic surgical and rehabilitation techniques to repair damaged cartilage and ligaments. • Founded Stone Clinic in 1988, an orthopedic clinic focused on biologic approaches to treating joint injuries. • CEO and Chairman of the Board of Joint Juice, Inc. (now Premier Nutrition Inc.), founder and CEO of Rescue Reel, LLC, and the founder of ProprioSense Holdings, LLC. • Dr. Stone holds an A.B. cum laude in biology from Harvard College and an M.D. from the University of North Carolina School of Medicine. David Cocke Chief Financial Officer • Joined Aperion in 2008 • Prior Experience: GM at NuPak Medical, CFO at NuTech, managed Corporate Development Department at KCI, employed at Corporate Finance group of GE Capital and Investment Banking group of Salomon Brothers • Received MBA degree from University of Virginia’s Darden Graduate School of Business Administration and BBA degree with High Honors from the University of Texas at Austin Lance Johnson VP, Quality Systems • Joined Aperion Biologics in November 2010; 20+ years of experience in FDA Requirements and Quality Systems • Prior Experience: Quality Manager at Zimmer Spine, Quality Engineering Manager at Abbott Spine and 16 years of experience as investigator with FDA; Received Level II Auditor certification in 2003 and Medical Device Level II certification in 1998 • Received Bachelor of Science degree in Biotechnology from Oklahoma State University

Intellectual Property 17 14 issued patents in the U.S. and internationally and 3 patent applications: ▪ E xclusive rights to use alpha - galactosidase enzyme to remove gal antigens from non - primate animal tissues, including ligaments, cartilage, bone, heart valves, vascular tissue, collagen and many other tissues o Material Composition for Z - processed tissues o The Process used to create the Z - products ▪ Additional extensive coverage of immunochemical modifications of dermal - and tendon - derived injectable collagen formulations Exceptionally solid IP estate providing Aperion with a powerful position in removal of α - Gal in key tissues

Title Text FUTURE PRODUCTS
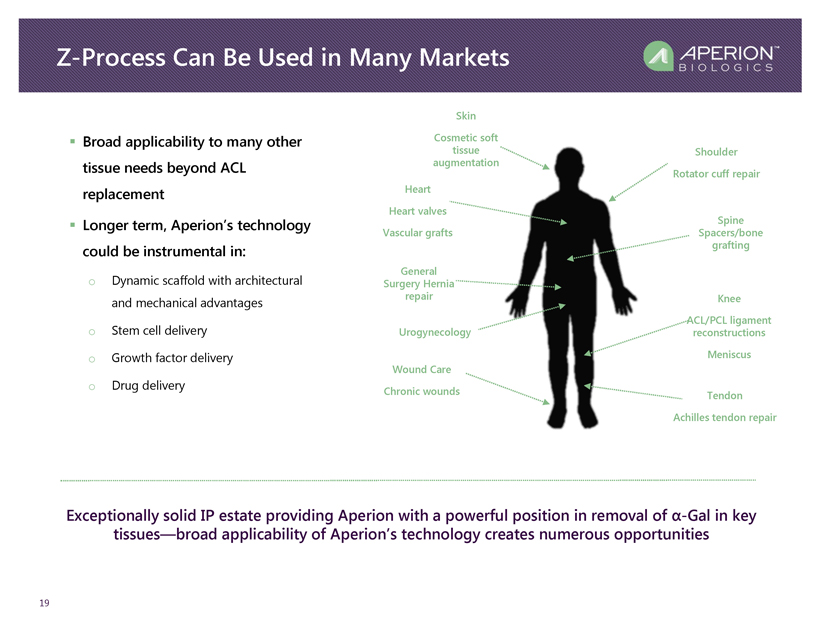
Z - Process Can Be Used in Many Markets 19 ▪ Broad applicability to many other tissue needs beyond ACL replacement ▪ Longer term, Aperion’s technology could be instrumental in: o Dynamic scaffold with architectural and mechanical advantages o Stem cell delivery o Growth factor delivery o Drug delivery Shoulder Rotator cuff repair Knee ACL/PCL ligament reconstructions Meniscus Spine Spacers/bone grafting Heart Heart valves Vascular grafts Tendon Achilles tendon repair Skin Cosmetic soft tissue augmentation General Surgery Hernia repair Wound Care Chronic wounds Urogynecology Exceptionally solid IP estate providing Aperion with a powerful position in removal of α - Gal in key tissues — broad applicability of Aperion’s technology creates numerous opportunities

Product Pipeline 20 Z - Patch for soft tissue repair/augmentation Z - Lig family of devices: Z - Lig BT+, Z - Lig BTB, Z - Lig ST Z - Fix for biologic fixation in bone grafts, ligaments, more

Near Term Product Pipeline: $ 6b Market 21 Current Development Status Product Name Product Category Initial Regulatory Pathway Research Preclinical Pilot Pivotal Marketed Clinical Detail EU Market U.S. Market Z - Lig® ACL CE Mark • 6 & 12 months post implantation in 60 patient EUSA trial • 100% of remaining subjects met 36 month endpoint in 4/2015 1Q:15 $ 0. 63 b PMA • FDA has unconditionally approved U.S. pivotal trial protocol • 10 - patient U.S. pilot safety trial completed 2018 - 9 $ 0. 75 b Z - Patch Extracellular Matrix CE Mark • Completion of developmental testing and validation 1H:17 • CE Mark approval by 1H:18 2018 $1.0b 510 (k) • 510(k) approval by 1H:18 • U.S. post - market tevaluation to follow 2018 $1.2b

Near Term Product Pipeline: $ 6b Market 22 Current Development Status Product Name Product Category Initial Regulatory Pathway Research Preclinical Pilot Pivotal Marketed Clinical Detail EU Market U.S. Market Z - Fix Bone CE Mark • CE Mark approval by 2H:18 2018 $ 1 .0b 510 (k) • Preclinical testing to be completed in 2H:16 2018 $ 1 . 1 b Z - Meniscus Meniscus CE Mark • Preclinical testing to be conducted 2H:17 2019 - 20 $ 1 50m PMA • Preclinical testing to be conducted 2H:17 2020 $ 1 50m

23 Use of funds 1H 2016 2H 2015 2H 2016 1H 2017 2H 2017 • Scale - up • Staffing • Expanded indication study • Launch Core EU • Expansion EU • N on - EU markets • Regulatory submissions for non - EU markets • Initiate US IDE study • Introduce soft tissue ACL product • Introduce dermis product • Expansion into other OUS markets • Full ACL indication Reg A+ IPO $20M

Why Aperion ? Why Now? 24 ✓ Z - Lig will be the first commercialized and clinically tested, “off - the - shelf” biological ACL replacement ✓ Addresses shortcomings of existing market alternatives – Z - Lig is Healthy and Strong, Every Time First & Only Tissue - Based Graft Product ✓ Entered into commercialization in select EU markets in Q12015 ✓ FDA unconditional approval for US pivotal IDE clinical trial Commercialization ✓ Disruptive IP protected technology facilitating successful implantation of processed animal tissues ✓ Broad range of additional biologic applications – ligament, matrix, bone, valves, etc. Proprietary Product Platform ✓ First biologic graft to address >$2 billion global ACL market – a high - growth market in orthopedics ✓ Early market opportunity in Europe Large Global Market Opportunity ✓ 96% success at 6 months and 92% at 12 months in ongoing Level 1 randomized, controlled OUS clinical trial ✓ Performance better than the standard of care ✓ Clinical results validate 1 2 year data from US human pilot safety study Compelling Clinical Data

Title Text 25 APPENDIX 1

Preclinical Efficacy Studies 26 Serological studies – Support immunocompatibility; transient production of gal and non - gal antibodies substantially decreased at approximately 9 months Biomechanical testing – Demonstrates graft functional efficacy comparable to the allograft and autograft reconstructions Histopathology – Advanced graft remodeling and maturation – Functional host integration of both tendon and bone – No adverse synovial response Rhesus Allograft (+ control) Untreated Porcine ( - control) Z - Lig 1 2 3 Aperion conducted studies in 20 Rhesus monkeys; treated with Z - Lig , allograft and unprocessed porcine xenograft All Z - Lig results were substantially similar to the allograft - implanted monkey control

US Pilot Trial Overview 27 • Z - Lig safety pilot was conducted in 10 humans under an IDE with the FDA • As assessed in five of six evaluable subjects: – Presented with functional grafts at the 12 - and 24 - month post - operative time points – Satisfied all study success criteria including effusion, KT - 1000, Pivot Shift, Lachman and Anterior Drawer tests – Via MRI, showed significant remodeling and maturation of the functioning grafts ▪ Mean Age 41 Years (range 21 - 51) ▪ 7 males / 3 females ▪ 5 of 10 chronic ACL injury (>3 months post injury) ▪ Operative / injured knee previous surgeries: ▪ 2 ACL revisions ▪ 1 ACL repair ▪ 3 Medial meniscal repairs ▪ Contralateral surgeries: ▪ 3 ACL reconstructions ▪ Extremely athletic population – Pre - injury Tegner average = 8.0 Patient Demographics Continued safety and graft function in patients from pilot safety study 12 years post - op

FDA Overview and timeline 28 ✓ Current team optimizes manufacturing and sourcing operations for long - term commercial viability • Receives unconditional approval to commence IDE pivotal non - inferiority trial • We plan to begin this trial in 2016 with funds from this offering ✓ 2010 - 201 4 FDA Timeline ✓ Optimize US pivotal trial protocol ✓ Preparation for U.S. pivotal trial Planned Optimization Benefit Randomization Change subject randomization from 1:1 to 2:1 Reduces N from 326 to 245 Clinical Sites Increase number of clinical sites from 10 to 20 Reduces recruitment period from 30 months to 15 months EUSA Trial Data Consider EUSA trial data in US trial Potentially reduces N or reduces focus on longer term data Screw Selection Company remains screw manufacturer agnostic Allow multiple manufacturers’ screws to be used
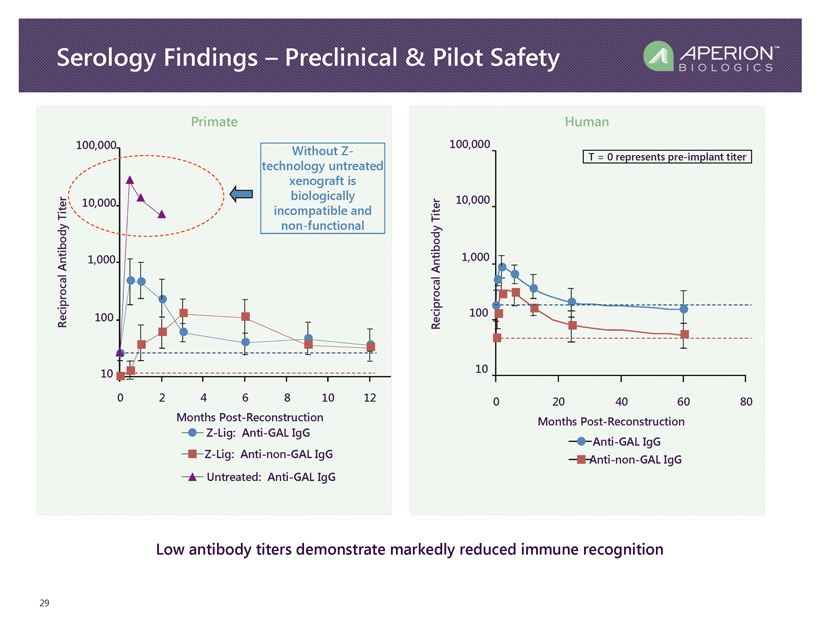
Serology Findings – Preclinical & Pilot Safety 29 Low antibody titers demonstrate markedly reduced immune recognition 10 100 1,000 10,000 100,000 0 2 4 6 8 10 12 Months Post - Reconstruction Reciprocal Antibody Titer Z - Lig: Anti - GAL IgG Z - Lig: Anti - non - GAL IgG Untreated: Anti - GAL IgG 10 100 1,000 10,000 100,000 0 20 40 60 80 Months Post - Reconstruction Anti - GAL IgG Anti - non - GAL IgG Reciprocal Antibody Titer T = 0 represents pre - implant titer Without Z - technology untreated xenograft is biologically incompatible and non - functional Primate Human
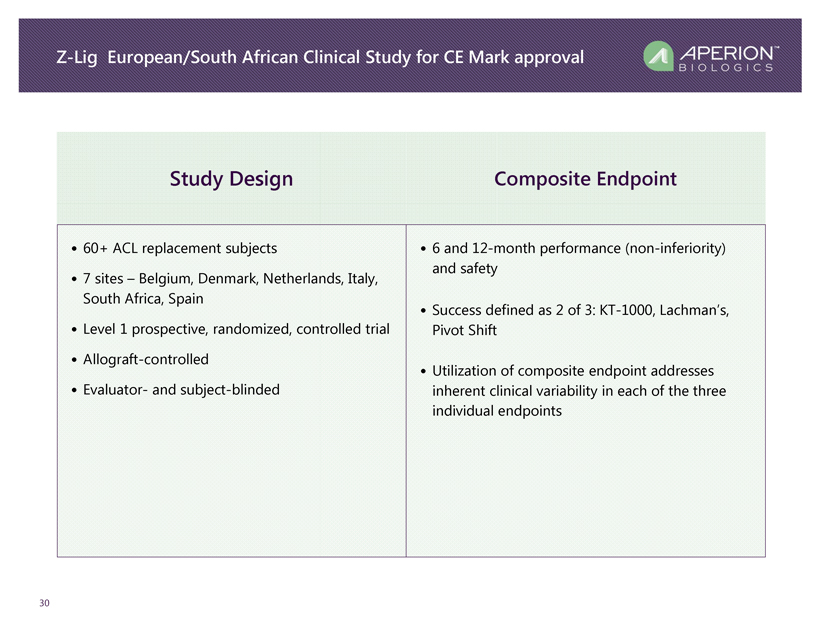
Z - Lig European/South African Clinical Study for CE Mark approval 30 Study Design Composite Endpoint • 60+ ACL replacement subjects • 7 sites – Belgium, Denmark, Netherlands, Italy, South Africa, Spain • Level 1 prospective, randomized, controlled trial • Allograft - controlled • Evaluator - and subject - blinded • 6 and 12 - month performance (non - inferiority) and safety • Success defined as 2 of 3: KT - 1000, Lachman’s, Pivot Shift • Utilization of composite endpoint addresses inherent clinical variability in each of the three individual endpoints

CE Trial Investigators & Sites 31 Physician Institution Leading Opinion Leaders René Verdonk, MD, PhD Peter Verdonk, MD, PhD Fredrik Almqvist, MD, PhD Gent University Hospital Dept. of Orthopaedic Surgery & Traumatology Gent, Belgium • One of the most highly respected orthopedic/sports medicine research centers in Europe • Prof. René Verdonk was inducted into the Hall of Fame for the American Orthopaedic Society for Sports Medicine, one of the highest honors given to a member of this American society Willem van der Merwe, MD Cape Town, South Africa Sport Science Institute of South Africa Cape Town, South Africa • The Sports Science Institute is a leading center in sports medicine for South Africa • Dr. van der Merwe is a member of the ACL Study group, a peer - selected international group focused on advanced ACL treatments Maurilio Marcacci, MD Stefano Zaffagnini, MD Instituti Ortopedici Rizzoli - Bologna University Sports Traumatology Dept. Bologna, Italy • One of the most highly respected orthopedic/sports medicine research centers in Europe • Considered to be the top orthopedic center in Italy Ramón Cugat, MD, PhD Montserrat García - Balletbó, MD, PhD Hospital Quirón Artroscopia GC Barcelona, Spain • Dr. Cugat is orthopedic consultant for FC Barcelona and medical assessor for the Catalan Football Federation (130,000 players in the Catalan region) • Considered one of the top ACL clinicians in Spain Martin Lind, MD, PhD Peter Faunoe, MD Aarhus University Hospital Dept. of Orthopaedic Surgery & Traumatology Aarhus, Denmark • Dr. Lind is responsible for the Scandinavian ACL Registry in Denmark • Scandinavian ACL Registry is recognized as one of the most comprehensive ACL registries Kees van Egmond, MD Rutger Zuurmond, MD Isala Klinieken Department of Orthopaedic Surgery Zwolle, Netherlands • The Isala Klinieken is the leading training center in the Netherlands for orthopedic surgeons Pedro Guillen, MD Clínica Cemtro Orthopaedic Surgery & Traumatology Madrid, Spain • Clinica Cemtro is a private hospital founded by Prof. Guillen which performs over 1,000 ACL reconstructions annually • Considered one of the top ACL clinicians in Spain

Key Clinical Takeaways 32 ✓ Key endpoint met for non - inferiority evaluation by Notified Body ✓ Compelling 6, 12 and 24 month safety and performance data versus “standard of care” – study control are performed well above historical results ✓ Objective measurements - stability, ROM ✓ Functional - one legged hop ✓ Subjective - SF - 36, Tegner ✓ All of study subjects have met 36 month milestone, many are beyond 48 months Clinical Analysis Z - Lig performed extremely well and demonstrated success at 6, 12 and 24 months Six subjects suffered SAEs deemed related to device — external experts confirm all device - related SAEs caused by infection from water system — remedied by corrective action and validation of improvement s to m anufacturing process

Title Text 33 The Science Behind Z - Lig APPENDIX 2

Immune Challenge: alpha - galactosidase (α - Gal) 34 • Lower species express specific carbohydrate epitopes called α - Gal • Humans (and some primates) have evolved to produce antibodies that bind to the α - Gal antigen to initiate a rapid immune rejection process • 1% of circulating human antibodies are anti - Gal

Aperion’s Solution: The Z - Process 35 Tissue cleaning process α - Galactosidase enzyme treatment • Solves α - Gal rejection issue by cleaving the terminal galactose of the α - Gal antigen Stabilization While alpha - galactosidase enzyme treatment solves the gal rejection issue, it has no effect on the non - α - Gal immune response • Crosslinking provides additional ability to tailor implant with desired remodeling properties Sterilization and packaging • Terminally sterilized by irradiation • Packaged in a ready - to - use graft configuration minimizing operating room time A patented, reproducible process that preserves the graft’s ability to remodel, providing long - term functionality 1 Biological and Mechanical Properties maintained 2 3 4

Aperion’s Manufacturing Facility 36 Unlike allografts, Aperion’s production process is highly scalable • Readily available and consistent raw material • Porcine tissue sourced in accordance with ISO/TR 22442 and FDA regulations Significantly higher gross margins than currently commercialized allografts 4,000 - square foot facility in San Antonio, Texas • 1,000 - square feet of Class 6 to Class 8 clean rooms • Received ISO 13485 certification of its quality systems in January 2010, recertified in June 2011, transferred to new notified body October 2013 • Supports commercial sales for next 2 - 4 years Aperion’s headquarters in San Antonio, Texas has Class 6 to Class 8 manufacturing clean rooms

Title Text 37 Daniel Lee d lee@aperionbiologics.com David Cocke dcocke@aperionbiologics.com Kevin Stone kstonemd@stoneclinic.com CONTACT




































