Exhibit 13.3


Aperion Biologics, Inc. 11969 Starcrest Drive San Antonio, TX 78247 Phone: (210) 858-7070 Fax: (210) 495-0239 www.aperionbiologics.com 
| | | Company Description Aperion Biologics, Inc. (or “the Company”) is a medical device company seeking to improve the supply, cost, and performance of new types of tissue graft material, as is used in knee reconstruction among other procedures. The Company has developed and patented the Z-Process® technology, which enables immunochemical modifications to animal tissue to make it compatible with the human immune system. Aperion’s lead product, the Z-Lig®, targets the $2 billionanterior cruciate ligament (ACL) reconstruction market. There are an estimated 2.6 million ACL injuries worldwide every year. Z-Lig® may be the first “off-the-shelf,” biologicxenograft (porcine-derived) to provide a consistently healthy and strong tissue graft option for ACL reconstruction, as Z-Lig® has been shown to maintain a biological scaffold that supports the regeneration of a new ligament. This is a key development as existing non-humansynthetic graft options (which are to date available only in parts of Europe) cannot regenerate native tissue and thus ultimately have a high failure rate. Aperion’s Z-Lig® received aCE Mark in April 2014 for revision and multiligament procedures and is available to registered physicians and surgical facilities through distribution partners in Europe and South Africa. Aperion has FDA clearance to begin pivotal U.S. trials of Z-Lig® and has completed a pilot safety study in the U.S. with favorable results. In addition, the Company has a pipeline of product candidates in development, based on the Z-Process® technology for reducing the likelihood of xenograft tissue rejection, for applications in orthopaedics, general and urogynecological surgery, bone grafting, wound healing, and heart valve replacement. Key Points n Unlike existing human sources of tissue graft material (e.g.,autografts andallografts), Z-Lig® production is highly scalable, since it is derived from a readily available and consistent source material. The device also overcomes the donor site pain of autografts and is believed to offer more reliable quality (strength, durability) and lead to faster recovery times than current autograft options. n The Z-Process® platform entails an enzymatic “stripping” of key carbohydrate antigens followed by a novel conversion process that both humanizes and sterilizes (immunochemically modifying) animal tissue without affecting its biomechanical or biological properties. n Ongoing clinical trials of Z-Lig® have shown success rates at 6, 12, and 24 months—equal to or better than the standard of care. All patients have met the 36-month point (with some patients over 48 months post implantation). The pilot safety study in 10 individuals showed no infections or acute or long-term immune responses. n Aperion’s product candidates and Z-Process® are protected by an extensive intellectual property portfolio, with 14 issued patents globally and a number of pending applications. As well, to the Company’s knowledge, it holds rights to the only patented method for humanizing non-primate animal grafts to replace human tissue. n As of June 30, 2015, Aperion’s cash position was $258,564. |
| †BOLDWORDS IN CONTEXT ARE REFERENCED IN THE GLOSSARY ON PAGES 57-58.See insidefor applicable disclosures. |

Table of Contents
| Investment Highlights | |
| | |
| Executive Overview | 5 |
| | |
| Milestones | |
| | |
| Intellectual Property | |
| | |
| Leadership | |
| | |
| Core Story | |
| | |
| Anterior Cruciate Ligament (ACL) Injuries: Background, Graft Options, and Market Dynamics | |
| | |
| Aperion's Technology and Product Development | 22 |
| | |
| Marketing and Distribution | 32 |
| | |
| Product Pipeline | 35 |
| | |
| Potential Competition | |
| | |
| Historical Financial Results | |
| | |
| Risks and Disclosures | |
| | |
| Glossary | |
| CRYSTAL RESEARCH ASSOCIATES, LLC | EXECUTIVE INFORMATIONAL OVERVIEW® | PAGE 2 |

Investment Highlights
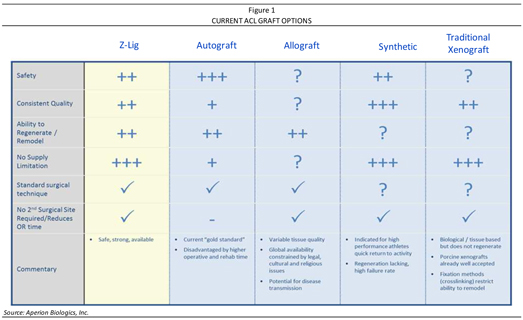
| n | There is an unmet need for new technology alternatives for ACL reconstruction procedures as nearly all surgeons performing this procedure utilize only two graft types—autograft (taken from the patient) and allograft (taken from a cadaver)—with each graft type having their respective shortcomings. Autografts have been associated with an increase in operation time, complications, morbidity, and rehabilitation time, and a decrease in function at the harvest site. Allografts may suffer from supply constraints, unpredictable and variable tissue quality (along with the possibility for disease transmission), as well as a lack of availability in many global markets. Furthermore, allografts are generally not accessible in a majority of European and other international markets for a variety of reasons—either legal, religious, cultural, or otherwise—thus making an alternative technology for ACL reconstruction all the more necessary. The only non-human products available for ACL reconstruction are synthetic grafts, which are only available and approved in parts of Europe or other international markets (with limitations) and are not able to regenerate (thus, they eventually fail due to durability issues). |
| n | Aperion has developed what it believes to be the first “off-the-shelf” biologic xenograft (porcine-derived) ACL reconstruction product that has proven to be consistently healthy and strong, while minimizing the potential for rejection. When initially implanted, Z-Lig® contains no live cells, and over time, is gradually populated and remodeled using a patient’s own cells to yield a mature human ligament through the process of “ligamentization.” The Z-Lig® is believed to remodel at a similar rate as human allografts, with substantial ligamentization occurring over 6 to 12 months and complete remodeling over two to three years—with remodeling believed to be key to long-term functionality of the Z-Lig®. |
| n | The Company believes that the Z-Lig® is novel in the ACL industry due to its ability to successfully maintain a biological scaffold, where the scaffold functions and supports the regeneration of a new ligament. Accordingly, the Z-Lig® could transform the standard of care for ACL reconstructions and disrupt the allograft industry, where there is a perceived lack of competition from any FDA-approved product. |
| n | While the pilot safety study was initially conducted to assess Z-Lig®’s safety and implantability, its original participants have now had their Z-Lig® implanted for over 12 years and continue to demonstrate safety and efficacy.The safety pilot study in 10 individuals showed no infections or acute or long-term immune responses. In addition, ongoing European clinical trials have demonstrated success rates at 6, 12, and 24 months, which are equal to or better than the standard of care. All patients have met the 36-month time point (with some patients over 48 months post implantation). |
| n | Market expenditures for ACL autografts and allografts are estimated at over $2 billion worldwide—including $750 million in the U.S. (300,000 ACL procedures annually), $623 million in Europe (265,000 ACL procedures annually), and $625 million in the rest of the world (250,000 procedures annually). These expenditures include both the cost of allografts and the direct surgical costs of the autograft harvesting and subsequent direct incremental rehabilitation and medication costs related to the second surgical site. Within the medical device arena, the xenograft market—in which the Z-Lig® participates—has shown to be well accepted. Derived from porcine, bovine, and equine sources, xenografts have been used as heart valves, extracellular matrices (patches), injectable collagen, and other replacement/augmentation tissues. |
| n | Aperion’s Z-Lig® is priced between $1,500 to $2,500 (versus $1,800 to $3,500 for allograft and autograft), with its production being highly scalable, given that it is derived from a readily available and consistent source material. The Company believes that production of Z-Lig® may provide support for higher gross margins than current allograft products. |
| CRYSTAL RESEARCH ASSOCIATES, LLC | EXECUTIVE INFORMATIONAL OVERVIEW® | PAGE 3 |

| n | The Z-Process® has broad applicability to many other tissue needs beyond ACL replacement.The main complication that historically has prevented animal-derived tissue from being used in human transplantation is the potential for rejection. If this immune response is mitigated, as Aperion’s Z-Process® seeks to do, animal tissue can be incorporated without having to suppress a patient’s immune system. Using its technology platform, Aperion is also developing product candidates for use in orthopaedics, general and urogynecological surgery, bone grafting, wound healing, and heart valve replacement. |
| n | Aperion’s product candidates and processes are covered by 14 issued U.S. and international patents and five pending patents. The issued patents cover the treatment of most tissues, such as tendons, ligaments, cartilage, vessels, valves, bone, and others, derived from any species of non-primate animal, such as pig, sheep, cow, and horse (including any genetically modified versions), noting that to the Company’s knowledge, it holds the rights to the only patented method for humanizing non-primate animal grafts to replace tissue in humans. |
| n | With a broad patent portfolio and a lack of any biologic candidates that the Company believes could be considered viable competitors in clinical development, Aperion aims to be the only entity marketing a tissue-based alternative to human-sourced ACL grafts for many years to come. While most major orthopaedic medical device companies have established divisions dedicated to orthopaedic biologics (orthobiologics) and/or have demonstrated an intent to expand into such markets, Aperion’s patent portfolio may serve to prevent such companies from attempting to independently develop animal-based products within its respective category. |
| n | The Company was founded by Dr. Kevin Stone, who is the founding scientist of Aperion and served as its chief executive officer from 1996 to 2008, as a director since its inception, and as chairman of the board since 2014.Dr. Stone is an established orthopaedic surgeon who founded the Stone Clinic (http://www.stoneclinic.com/dr-stone) in 1988, which is an orthopaedic clinic focused on biologic approaches to treating joint injuries. He is also the founder and chairman of Stone Research Foundation, an independent research institution investigating new techniques for joint health, arthritis, and human performance. |
| CRYSTAL RESEARCH ASSOCIATES, LLC | EXECUTIVE INFORMATIONAL OVERVIEW® | PAGE 4 |

Executive Overview
COMPANY OVERVIEW
Aperion Biologics, Inc. (“Aperion” or “the Company”) is a commercial-stage medical device company addressing the significant need for an alternative to human-based sources of tissues to be used in surgical procedures. The Company has developed and patented a technique, called the Z-Process®, which makes animal tissue compatible for human applications. The core platform to its technology is an enzymatic “stripping” of key carbohydrate antigens followed by a conversion process that both humanizes as well as sterilizes (immunochemically modifying) animal tissue without affecting its biomechanical or biological properties. The resulting tissue scaffold provides mechanical stability and functionality while being biologically integrated and remodeled. The Company’s lead product, Z-Lig®, is a patented animal tendon currently in limited commercial release for use as an anterior cruciate ligament (ACL) replacement in revision and multiligament procedures.
The Z-Lig® consists of specially treated and sterilized porcine tissue designed to serve as a cell-friendly, functional scaffold once implanted into a patient without concern of rejection. Z-Lig® received a CE Mark in April 2014 for revision and multiligament procedures and is available to registered physicians and surgical facilities through Aperion’s distribution partners in Europe and South Africa. As well, the Company has been cleared by the FDA to commence pivotal U.S. trials of Z-Lig®. The Z-Process® can be applied to a variety of tissues, ranging from orthopedic ligaments, bone, and meniscus, to valves and vessels, as well as to soft tissue grafts for augmentation and repair.
LIGAMENT RECONSTRUCTION
There are over 800,000 knee ligament reconstruction procedures performed annually—virtually all of which employ only two graft types: (1) autograft, where tissue material is harvested from a location in the patient’s own body and is reimplanted into the knee; and (2) allograft, where the tissue material is harvested from a human cadaver for implantation into the patient. There are also other less frequently used synthetic grafts, which are non-human, tend to quickly wear, degenerate, and fail, and are used with significant limitations (noting these are not in use in the U.S. and have only limited commercial acceptance in Europe and other international markets). With a need for more graft options widely recognized within the medical community, Aperion is developing a viable xenograft (derived from animal tissue) alternative to human-sourced ACL grafts. An overview of each currently available tissue grafting option for ACL reconstruction is summarized below and on page 6, with greater details provided in the Core Story on page 15-35, followed by a description of Aperion’s solution—the Z-Lig® ACL reconstruction device.
Human-Sourced Grafts (Autografts and Allografts)
Autograft
During an autograft procedure for ACL reconstruction, a second surgical site is required on the patient’s own body where either a patellar tendon or hamstring tendon is harvested. The patellar tendon bone-tendon-bone (BTB) graft has been the “gold standard” graft choice for clinicians for ACL reconstructions since it was made popular during the 1980s, and has been used extensively by surgeons since that time. To date, it remains the graft of choice for a high number of orthopaedic surgeons who perform ACL reconstruction surgery on a constant basis as it has consistently shown excellent surgical outcomes with a 90% to 95% success rate in terms of returning to pre-injury level of sports. However, harvesting tendons results in the patient suffering from additional pain, morbidity, and scarring at the harvest site, and since a second surgical site is necessary, it is associated with a prolonged operating time, which potentially subjects the patient to further risk of infection and may lead to a longer, more difficult recovery.
| CRYSTAL RESEARCH ASSOCIATES, LLC | EXECUTIVE INFORMATIONAL OVERVIEW® | PAGE 5 |

Allograft
With allografts, since the tissue is harvested from cadaver donors, quality can vary based on age, sex, size, genetics, and medical condition of the donors—with the inconsistent nature of allografts stemming largely from the broad age range from donor tissue, as documented in published studies relating to biomechanical properties of human allografts based on age. Moreover, since tissue donation is not commonplace around the world, supply of allograft tissue can be limited. For example, even in the U.S. where roughly 300,000 ACL replacements are performed annually, less than 25% of these procedures can be performed using allografts. The remaining 225,000 patients must have the graft harvested from their own bodies (autograft). Importantly, regulation of products made from tissue banks is not subject to the medical device regulatory review and approval nor subject to the same controls and regulations as a commercial device. Thus, while largely safe, there does remain a risk of human disease transmission with allografts.
Non-Human Grafts (Synthetic and Xenografts)
The only non-human commercialized graft option is synthetic, which quickly wear, degenerate, and fail, likely because of the inability to regenerate (as tissue-based implants do). Because of this, synthetic grafts are not approved for use in the U.S. and have extremely limited commercial acceptance in Europe and other international markets. They are primarily used for patients who wish to return to their sport in the shortest time period possible. Xenografts may therefore provide a better alternative to human-sourced ACL grafts (autograft or allograft), with Aperion holding the only xenograft-based ACL graft under development (to the Company’s knowledge). Xenografts from porcine, bovine, and equine sources have long been used as heart valves, extracellular matrices (patches), injectable collagen, and other replacement/augmentation tissues. Aperion’s products are distinct from currently commercialized xenografts, however, in that they accomplish the following: (1) act as biological scaffolds (unlike, for example, heart valves); and (2) do not illicit an immunological reaction (unlike, for example, extracellular matrices and injectable collagen).
A summary of the currently available graft options for ACL reconstruction is provided in Figure 1, followed by a brief overview of Aperion’s Z-Lig® on page 7, which received a CE Mark in April 2014 for revision and multiligament procedures and is available to registered physicians and surgical facilities through the Company’s distribution partners in Europe and South Africa.
| CRYSTAL RESEARCH ASSOCIATES, LLC | EXECUTIVE INFORMATIONAL OVERVIEW® | PAGE 6 |

Aperion’s Z-Lig® Anterior Cruciate Ligament (ACL) Replacement Device
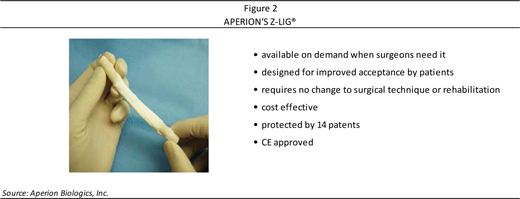
Aperion has developed the Z-Lig® ACL reconstruction device (as shown in Figure 2) as an immunocompatible, porcine-derived ACL reconstruction alternative. The Z-Lig® is a porcine graft that may be used to rebuild the ACL in a human knee. Z-Lig® has been developed for off-the-shelf use with no change for the surgeon in surgical technique. Created using animal tissue harvested from ISO (International Organization for Standardization)-compliant facilities, the tissue is put through the Company’s proprietary and patented Z-Process®, where it is “humanized” in an enzyme bath and is stripped of its carbohydrate antigens—the alpha-gal carbohydrate—which are the antigens found in animal tissue that cause acute rejection. Additional processing steps are used to address the non-gal antigens. At the end of the Z-Process®, the Z-Lig® tissue can be used in surgery as it is now “humanized” and sterilized for acceptance in the human body. Following ACL reconstructive surgery, the Z-Lig® tissue remodels itself over time to be indistinguishable from the patient’s own tissue, with substantial ligamentization occurring within predictable timeframes. Z-Lig® has demonstrated to be strong, sterile, and reproducible (with reliable quality, as it can be consistently taken from a young healthy pig). A summary of the key attributes of Aperion’s Z-Lig® is provided in Figure 2.
European CE Mark Approved Clinical Trial
Aperion concluded enrollment of the Z-Lig® European clinical trial in the second quarter of 2012. It was a prospective, double blinded randomized and controlled trial conducted with 66 patients undergoing ACL replacement at seven trial locations (sites located in Belgium, Denmark, Netherlands, Italy, South Africa, and Spain). The control arm was treatment with an allograft. Primary endpoints to the trial were performance and safety over two years. Aperion collected 6- and 12-month data for all patients in the study in the second quarter of 2013. Based on the review of the clinical performance on the 6- and 12-month data, the Company submitted an application for CE Mark approval, which Aperion was granted in April 2014. Z-Lig® is now available to registered physicians and surgical facilities through Aperion’s distribution partners in Europe and South Africa. Importantly, this was a Level 1 Study, where the Company felt that it was essential to run a high quality study to meet the scientific and clinical expectations of the community—for an ACL device, there is a high bar that needs to be met to convince physicians to switch from their current standard of care. A summary of the trial investigators and sites is provided in Figure 27 (page 30).
| CRYSTAL RESEARCH ASSOCIATES, LLC | EXECUTIVE INFORMATIONAL OVERVIEW® | PAGE 7 |

U.S. Pilot Safety Study
Under anInvestigational Device Exemption (IDE) with the U.S. FDA, Aperion conducted a pilot safety study in which Z-Lig® was implanted as an ACL replacement in 10 people, where the endpoints were effusion and knee stability. As part of this study, no systemic issues or secondary infections arose from the device’s implantation, nor was there any acute immune response or longer-term rejection response from any of the subjects, and the study’s objectives of safety and implantability were met. After submitting the 24-month results to the FDA, the Company received approval for a pivotal non-inferiority clinical trial protocol. While this pilot study was to assess the safety and implantability of Z-Lig®, Aperion was able to collect favorable five, ten, and twelve-year efficacy data.
MARKET SIZE
As one of the few high-growth markets in orthopaedics, ACL injuries are quite common, with approximately 2.6 million reported globally every year. This amounts to a market of roughly $2 billion in ACL surgeries, based on 800,000 targetable procedures annually, at an estimated cost of $2,500 per procedure. The market is forecast to expand between 6% and 10% through 2020. The majority of this market is concentrated in the EU and U.S. In the EU (where Aperion is now approved to sell Z-Lig®), roughly 265,000 ACL reconstruction procedures take place every year (30% of the market). In the U.S., roughly 300,000 ACL procedures are performed every year (40% of the market), and in the rest of the world, there are roughly 250,000 ACL procedures annually (30% of the market). Demand for these procedures is driven by an increasingly more active younger population and older sports injuries in those who want to continue to participate in their activity of choice.
ADDITIONAL DEVELOPMENT EFFORTS
Along with development and beginning to market (in Europe) the Company’s Z-Lig® device, Aperion has stated that it expects the second stage of development for its ACL device to include therapeutics, i.e., stem cells, autologous, or exogenous growth factors, or gene therapy. A new paradigm in ACL reconstruction could be the dynamic scaffold capable of delivering a therapeutic agent. Static scaffolds (which are largely polymer-based) are not indicated in ACL repair, require time and conditions to facilitate tissue generation, and deliver therapeutics only. Dynamic scaffolds would enable instant function while concurrently delivering the bioactive agent, supporting tissue regeneration, and reducing rehabilitation requirements, while accelerating a patient’s return to normal activities. Combining a functional tissue scaffold with a therapeutic agent could be a key development in regenerative technologies, specifically leveraging Aperion’s ability to humanize porcine tissue into functional implants and scaffolds and reducing the time required to restore function.
Aperion’s technology platform may also have applications in other areas, such as cartilage, soft tissue patches (extracellular matrices), bone, heart valves, vascular grafts, collagen, and other tissues. These developments include product candidates in clinical and preclinical development and research stages, such as the Z-Patch, Z-Fix, and Z-Meniscus. U.S. and European markets for extracellular matrix that can be addressed by Z-Patch are $1.0 billion in U.S. and $1.2 billion in Europe; the potential market sizes for bone replacement that can be partially addressed by Z-Fix are $1.0 billion in U.S. and $1.1 billion in Europe; and the potential market sizes for meniscus replacement that can be addressed by Z-Meniscus are $150 million in U.S. and $150 million in Europe.
PATENT PORTFOLIO
Aperion’s product candidates and Z-Process® technology are protected by an extensive intellectual property portfolio. There are currently 14 U.S. and international patents that have been issued, and three patents pending. The Company’s patent portfolio includes claims relating to treatment and application of specific tissues for a variety of medical and surgical uses. The intellectual property portfolio includes combined patents of process and material composition that support deantigenation, sterilization, and viral inactivation of a wide range of biological tissues. Aperion also has exclusive rights to the only known method for humanizing non-primate animal grafts for replacement of ligaments, bone, etc. in humans without making the graft inert.
| CRYSTAL RESEARCH ASSOCIATES, LLC | EXECUTIVE INFORMATIONAL OVERVIEW® | PAGE 8 |

Z-Process® patents comprise coverage of key immunochemical modification and sterilization techniques as applied to a variety of animal-derived tissues, including connective tissue grafts (tendon, ligament, articular cartilage, and fibrocartilage); cardiovascular tissues (heart valve and pericardium); and calcified and de-calcified bone (granular bone matrix, cortical, and cancellous structural bone for struts, cages, and machined implants). The patent portfolio further includes immunochemical modifications of solubilized and homogenized dermal- and tendon-derived injectable collagen formulations.
EMPLOYEES, HEADQUARTERS, FACILITIES, AND INFRASTRUCTURE
Formerly known as CrossCart, Inc. (changing its name to Aperion Biologics on May 28, 2009), Aperion is now based in San Antonio, Texas, and currently employs 3.5 full-time equivalent (FTE) employees and expects to increase its headcount by about 10 to 15 individuals in the next year, following its proposed public offering. The Company leases a 4,000-square foot facility in San Antonio, with a 1,000-square foot Class 8 clean room area (shown in Figure 9) that contains four 100-square foot modular clean rooms of Class 6 to Class 7 certification. The Company received ISO 13485 (the International Organization for Standardization) certification of its quality systems in January 2010. The processing facility is scaled to have suitable capability and capacity to support Z-Lig® and other manufacturing requirements from clinical trials through commercial launch in Europe and the U.S. Aperion uses the remaining part of its facility for product development, distribution, and general and administrative purposes.

| CRYSTAL RESEARCH ASSOCIATES, LLC | EXECUTIVE INFORMATIONAL OVERVIEW® | PAGE 9 |

Milestones
In seeking to become a leading tissue products company, Aperion has to date achieved the milestones as listed below. Moving forward, the Company has established objectives (potential milestones) over the next 12-18 months as it seeks broader product expansion for Z-Lig® in European, South African, and other markets, and moves the product closer toward U.S. product approval. As well, the Company expects to continue to progress in the development of its other pipeline candidates.
Recent Milestones
| n | Received CE Mark for Z-Lig® |
| n | Completed enrollment in Z-Lig® performance trial and collected two year clinical data |
| n | Initiated limited distribution in select European markets |
| n | Commercial scale enzyme order |
Potential Milestones
| n | Management appointments in anticipation of European launch |
| n | Execute targeted launch strategy |
| n | Continue to launch Z-Lig® across the European Union and South African markets |
| n | Potential U.S. approval for Z-Lig® by 2018-2019 |
| n | Submit applications for market approvals outside U.S. accepting Company’s CE Mark |
| n | Expand indications for ACL use in CE Marked countries and registry studies in select markets |
| n | Develop follow-on products such as a soft tissue patch collaborative research and development activities |
Upcoming Scheduled Events
| n | November 27, 2015 – November 28, 2015, EFOST 2015 – 8th European Sports Trauma Societies Congress 2015, Torino, Italy |
| n | December 2, 2015 – December 5, 2015, Orthopaedic Summit 2015: Evolving Techniques, Las Vegas, United States |
| n | January 29, 2016 – January 30, 2016, ICRS Focus Meeting – Allograft, Brussels, Belgium |
| n | March 1, 2016 – March 5, 2016, American Academy of Orthopaedic Surgeons (AAOS) Annual Meeting, Orlando, United States |
| n | May 4, 2016 – May 7 2016, 17th European Society of Sports Traumatology, Knee Surgery & Arthroscopy (ESSKA) Congress, Barcelona, Spain |
| CRYSTAL RESEARCH ASSOCIATES, LLC | EXECUTIVE INFORMATIONAL OVERVIEW® | PAGE 10 |

Intellectual Property
Aperion Biologics holds a portfolio of 14 issued patents with applicability to the U.S., Europe, and Japan, as listed in Figure 4. These patents generally relate to the Company’s product candidates and core technology platform, the Z-Process®, and include coverage for a number of Aperion’s key competencies, including immunochemical modification and sterilization techniques as applied to a variety of animal-derived tissues, bone, and solubilized and homogenized dermal- and tendon-derived injectable collagen formulations. Importantly, to the Company’s knowledge, it holds exclusive rights to the only known method for humanizing non-primate animal grafts to replace ligaments, bone, and other material in humans without making the graft inert. Many of the patents listed in Figure 4 are held under “CrossCart, Inc.”, which is now Aperion Biologics, with the listed inventor being Dr. Kevin Stone. Dr. Stone is currently the chairman of Aperion (biography provided on page 14). He is also the founding scientist of the Company and was CEO from 1996 to 2008. Of note is that Z-Lig® has patent coverage until 2024.
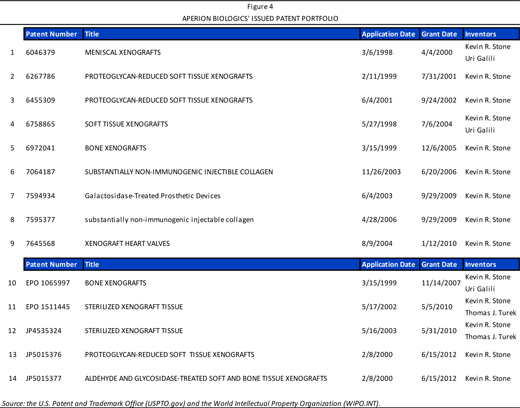
In the course of developing its technology and product candidates, Aperion has also publicly showcased its scientific data through a number of publications, presentations, and poster sessions, as summarized in Figure 35 (page 55) of the Appendix. Readers may consult these reference documents for additional information regarding the Company’s product development.
| CRYSTAL RESEARCH ASSOCIATES, LLC | EXECUTIVE INFORMATIONAL OVERVIEW® | PAGE 11 |

Leadership
Management
Members of Aperion’s executive management, as profiled below, have particular experience in biologics, sports medicine, and orthopaedics.
Daniel Lee, Chief Executive Officer
Mr. Lee has over 25 years of experience in the medical device industry. Prior to joining Aperion in 2008, Mr. Lee was responsible for the TRUREPAIR business unit at Smith & Nephew Endoscopy (SNE). Prior to Smith & Nephew, he was responsible for global marketing activities at OsteoBiologics, Inc. (OBI), which provided the only off-the-shelf bioabsorbable implant for articular cartilage repair in Europe. OBI was acquired by Smith & Nephew in 2006. Prior to joining OBI, Mr. Lee was the director of marketing for Regeneration Technologies, Inc. (RTI), a leading allograft tissue processor for orthopaedic, spinal, craniofacial, and urologic surgical applications, which went public in 2000. While at RTI, he played a key role in creating and establishing RTI’s Sports Medicine business unit. Prior to joining RTI, he was the director of the Sports Medicine Research and Development group at Surgical Dynamics, a subsidiary of U.S. Surgical Corporation. Much of his experience at U.S. Surgical focused on using resorbable materials for orthopaedic products. Mr. Lee is a member of the Society for Biomaterials and the American Association of Tissue Banks (AATB), where he holds a Certified Tissue Bank Specialist (CTBS) certification. He currently holds eleven patents on implants and instruments used in orthopaedic and general surgery. Mr. Lee received a Master of Science in Biomedical Engineering from the University of Alabama at Birmingham and a Bachelor of Science degree in Materials Science and Engineering from the Johns Hopkins University.
David Cocke, Chief Financial Officer
Mr. Cocke has served as chief financial officer since September 2008. Since 1997, he has been serving as a general manager of NuPak Medical, Ltd., an ISO 13485-certified contract manufacturing medical device company. He was responsible for all aspects of management, including sales, finance and operations. Prior to that, from November 1993 to May 1996, Mr. Cocke was chief financial officer of NuTech, Inc., a technology incubator subsidiary for KCI, a leader in tissue-based products for surgical procedures and wound healing. From 1991 to 1993, he was director at the Corporate Development department at KCI. Prior to KCI, Mr. Cocke was employed by GE Capital in its Corporate Finance Group and Salomon Brothers Inc in its Investment Banking Group. Mr. Cocke holds an MBA from the University of Virginia’s Darden Graduate School of Business Administration and a BBA with High Honors from the University of Texas at Austin.
Lance Johnson, Vice President, Quality Systems
Mr. Johnson has over 20 years of experience in FDA Requirements and Quality Systems. Prior to joining Aperion in November 2010, he was the quality manager at Zimmer Spine. Prior to Zimmer Spine, Mr. Johnson was the quality engineering manager for Abbott Spine, working specifically with new product development teams. In addition to his industry experience, he spent 16 years as an investigator with the FDA. Mr. Johnson specialized in medical device compliance and worked in both the San Francisco and Dallas districts. He spent 12 years as the resident in charge of the Austin field office and as contributor to the FDA international cadre. During his FDA career, Mr. Johnson audited medical device firms in the U.S., Europe, and Canada. He received a Medical Device Level II certification in 1998 and received a Level II Auditor certification in 2003. Mr. Johnson trained and certified FDA, Texas, and BSI auditors during his career with the FDA. He received his Bachelor of Science degree in Biotechnology from Oklahoma State University.
| CRYSTAL RESEARCH ASSOCIATES, LLC | EXECUTIVE INFORMATIONAL OVERVIEW® | PAGE 12 |

Board of Directors
Members of Aperion’s board of directors are listed in Figure 5 and profiled thereafter.

David W. Anderson
Mr. Anderson is the chairman and chief executive officer of Orteq Ltd., an orthopedic medical device company focused on sports medicine. He also serves as chairman of the Board of Gentis, Inc. a clinical stage spinal implant company which he joined in 2004. From 1999 to 2004, he was the founder and a director of Replication Medical, Inc., an orthopedic medical device company focused on spine surgeries. From 1994 to 1999, he served as the chief executive officer of Bionx Implants, Inc., a leading manufacturer and marketer of reinforced polymer implants. From 1986 to 1989, he was the founder and Executive Vice President of Osteotech, Inc., a global leader in providing biologic solutions for regenerative medicine, which was acquired by Medtronic Inc. in 2010. Mr. Anderson also served as the chief executive officer of Kensey Nash Corporation, a medical device company, from 1992 to 1994, and Sterilox Technologies, a manufacturer of non-toxic disinfectants, from 2000 to 2004. Mr. Anderson holds a B.S. in Chemical Engineering from Cornell University. Mr. Anderson brings to the Board over 25 years of experiences in entrepreneurship, strategic transactions, and financing in the medical device industry.
France Dixon Helfer
Ms. Helfer is currently the president and chief executive officer of TinyKicks LLC, a University of California healthcare technology company based in Irvine, California, since January 2015. From September 2011 to September 2014, Ms. Helfer was the president, chief executive officer and director of Halo Healthcare Inc., a medical device and diagnostics company focused on the detection and screening of breast cancer. Prior to that, from August 2004 to January 2008, Ms. Helfer was the president and founder of Pegasus Biologics, Inc., which was acquired by Baxter Healthcare Inc. Prior to that, Ms. Helfer held a number of executive positions at various biotechnology companies, including Medtronic, Xenotech Labs, SORIN Biomedica, Eclipse Surgical, and MMDDataDirect. Ms. Helfer serves on the boards of various business organizations and academic institutions, including Chapman University’s Crean College of Health and Behavioral Sciences. Ms. Helfer holds a B.A. in Biologic Sciences from California State University, Fullerton. Ms. Helfer brings to the Board over 20 years of experience as a senior level executive in the medical device industry.
Alfred G. Holcomb
Mr. Holcomb is the vice president of Acquisition and Divestitures at Lewis Energy Corp., an oil drilling company, since 2004, where he led the completion of numerous strategic transactions. From 1978 to 2004, Mr. Holcomb was a partner at the law firm of Schoenbum, Curphy & Scanlan. Mr. Holcomb has been an active speaker on the Eagle Ford shale formation in conferences around the world. Mr. Holcomb holds a B.A. in Finance in from University of Texas, a J.D. from St. Mary's University School of Law, and a LL.M. in Taxation from New York University. Mr. Holcomb brings to the Board extensive experience and knowledge on strategic, legal, and financial matters.
| CRYSTAL RESEARCH ASSOCIATES, LLC | EXECUTIVE INFORMATIONAL OVERVIEW® | PAGE 13 |

Kevin R. Stone, Chairman of the Board
Dr. Stone is the founding scientist of the company and served as its chief executive officer from 1996 to 2008, as a director since its inception and as chairman of the board since 2014. Dr. Stone is a renowned orthopaedic surgeon and founded the Stone Clinic in 1988, an orthopedic clinic focused on biologic approaches to treating joint injuries. He is also the founder and chairman of Stone Research Foundation, an independent research institution investigating new techniques for joint health, arthritis, and human performance, since 1996. Dr. Stone is the managing member of CrossCart, LLC, Aperion’s controlling stockholder. He is also an attending staff at the California Pacific Medical Center and the San Francisco Surgery Center. From 1999 to 2003, Dr. Stone served as the chief executive officer and chairman of the board of Joint Juice, Inc. (now Premier Nutrition, a part of Post Holdings Inc.). He is also the founder and chief executive officer of Rescue Reel, LLC since 2006 and the founder of ProprioSense Holdings, LLC since 2012. Dr. Stone holds an A.B. cum laude in biology from Harvard College and an M.D. from the University of North Carolina School of Medicine, Chapel Hill. He is recognized internationally as a leading expert and authority of advanced orthopedic surgical and rehabilitation techniques to repair damaged cartilage and ligaments. He brings to the Board extensive expertise, knowledge, and experience in the field of orthobiologic medicine and provides Aperion with significant management, operational, and financial support.
Mike Ward
Mr. Ward has been a member of Aperion’s board of directors since March 2014. He is the vice president, corporate development at BioNano Genomics, Inc., a genomic mapping company. Prior to joining BioNano Genomics, Inc., from 2009 to 2013, Mr. Ward was a vice president at Lurie Investment Fund, LLC, the venture capital arm of Ann and Robert H. Lurie Foundation, where he was responsible for managing the life sciences venture capital and private equity investments of the foundation. Prior to 2009, he worked for more than 15 years in the investment banking industries, holding various positions at Leerink Partners, Credit Suisse, Dresdner Kleinwort Wasserstein, BMO, and Vector Securities. Mr. Ward holds a B.S. in finance from the University of Illinois. He brings to the Board over 20 years of life-sciences dedicated investment banking, venture capital, and private equity experiences.
| CRYSTAL RESEARCH ASSOCIATES, LLC | EXECUTIVE INFORMATIONAL OVERVIEW® | PAGE 14 |

Core Story
ANTERIOR CRUCIATE LIGAMENT (ACL) INJURIES: BACKGROUND, GRAFT OPTIONS, AND MARKET DYNAMICS
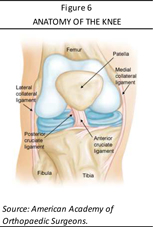
One of the most common knee injuries is an ACL sprain or tear. The knee is a hinge joint where the femur (thigh bone) meets the tibia (shin bone). This important joint is held together by four ligaments (fibrous tissues) that connect the bones to one another: anterior cruciate ligament (ACL); medial collateral ligament (MCL); lateral collateral ligament (LCL); and posterior cruciate ligament (PCL). The knee ligament or ACL runs diagonally between the (thigh bone) femur and the tibia (shin bone). It keeps the tibia from slipping in front of the femur and provides stability to the knee when it rotates from side to side (shown in Figure 6). Athletes who participate in high demand/high impact sports such as soccer, football, skiing, hockey, and basketball are more likely to injure their ACLs—with the majority of these injuries occurring without impact with another player (according to the American Academy of Orthopedic Surgeons [AAOS]). The most common mechanism for tearing the ACL is when an athlete has his or her foot planted and twists the knee or is hit on the outer side of the knee, causing the knee to buckle (a pop may be felt and/or heard), followed by immediate swelling and difficulty bearing weight. As well, the knee may feel unstable or may buckle. An ACL that is severely torn may be surgically reconstructed through ACL reconstruction surgery so that the thigh and shin bones are reconnected with a ligament or tendon.
ACL Reconstruction Surgery
Reconstruction of the ACL is a surgery designed to restore knee movement and strength after the ligament has been torn and to prevent injury or degeneration to other knee structures. While not all ACL tears require surgery, a physician can determine the best course of action—whether to send a patient to rehab only or to surgery plus rehab. Typically surgery is needed if a patient has completely torn the ACL or has a partial tear and the knee is very unstable. As well, it may be needed if a patient is very active in sports or has a job that requires knee strength and stability and wants his/her knee to be as strong and stable as it was before the injury. Further, individuals with chronic ACL deficiency (when the knee is unstable and affects an individual’s quality of life) are candidates for surgery. Other candidates for surgery include patients who have injured other parts of the knee, such as the cartilage or meniscus or other knee ligaments or tendons.
Arthroscopic Surgery
Once it is determined that an individual needs surgery, the torn ligament must be removed and replaced. In performing ACL reconstruction surgery, the majority of orthopedic surgeons perform the procedure arthroscopically (creating a small incision in the knee and inserting instruments for surgery through these incisions) versus open surgery (which requires cutting a large incision in the knee). The arthroscopic method provides greater ease with which to see and work on knee structures, provides smaller incisions than open surgery, can be performed at the same time as diagnostic arthroscopy (using arthroscopy to find out about the injury or damage to the knee), and is likely to pose fewer risks than open surgery, with the surgery performed either under regional (spinal) anesthesia or general anesthesia.
| CRYSTAL RESEARCH ASSOCIATES, LLC | EXECUTIVE INFORMATIONAL OVERVIEW® | PAGE 15 |

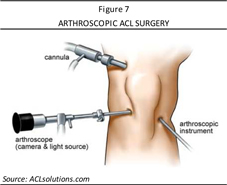
Arthroscopic ACL reconstruction involves a surgeon initially making several small incisions (typically two or three) around the knee (as shown in Figure 7). Sterile physiologic (salt) solution is pumped into the knee through one incision to expand it and wash blood from the area, which also provides the surgeon a clearer view of the knee structures. An arthroscope is then inserted into one of the other incisions, where a camera at the end of the arthroscope transmits pictures from inside the knee to a monitor inside the operating room. The other small incisions have small drills inserted through holes into the upper and lower leg bones, where these bones join at the knee joint. The holes form tunnels through which the replacement tissue graft (either an autograft, allograft, synthetic graft, or xenograft [in the case of Aperion’s Z-Lig®]) can be anchored. The graft is pulled through the two tunnels that were drilled in the upper and lower leg bones and the surgeon secures the graft with hardware, such as screws or staples, and then closes the incisions with stitches or tape. The patient is then bandaged and taken to recovery.
Overview of Tissue Graft Options
The topic of ACL reconstruction is highly debated among sports medicine orthopaedists who perform regular arthroscopic reconstructive knee surgeries. One reason is that research has yet to demonstrate the perfect ACL graft, as all grafts have their advantages and disadvantages. Additionally, every knee is unique and there are situations in which the same graft is not best suited to one patient versus another. That said, there are several options available to orthopaedic surgeons and their respective patients. An ideal graft should have comparable characteristics to the natural ACL, be incorporated in the tibial and femoral tunnels with proper fixation characteristics, heal fast, and uphold its biomechanical features for a long period of time with minimal adverse effects on the extensor mechanism.
ACL replacement grafts currently come from either tendon harvested from another part of the patient’s own body (autograft) or tendon from a human cadaver (allograft). Of note is that synthetic ACL replacements made of Dacron, Gore-Tex, polyester, etc., have been used, though these grafts have been met with mixed results by the majority of orthopedic surgeons and accordingly are only approved on a very limited basis in certain parts of Europe and other markets (and are not approved in the U.S.). A summary of the tissue graft options available for ACL reconstruction is provided in Figure 8 as a point of reference to the accompanying sections along with the main shortcomings that exist with each respective graft option, as further discussed in the accompanying section.

| CRYSTAL RESEARCH ASSOCIATES, LLC | EXECUTIVE INFORMATIONAL OVERVIEW® | PAGE 16 |

Alternatives to human-sourced grafts continue to be sought by the medical community since the only non-human commercialized grafts—synthetics—have demonstrated to wear quickly, degenerate, and eventually fail. While having limited commercial acceptance in Europe, they are largely employed by patients wanting to return to their respective activities/sport as quickly as possible. Aperion believes that the lack of durability of a synthetic graft is largely due to its inability to regenerate (compared to tissue-based implants which do regenerate). Due to issues stemming from the use of synthetic grafts and the lack of allograft in the global marketplace, the Company operates under the premise that animal tissue is the only practical alternative to human-sourced ACL grafts, with Aperion being the only company to its knowledge to sell or have for commercial distribution an animal-based ACL product.
Greater details, including advantages and disadvantages of current tissue grafting options are provided in the accompanying section, followed by details of Aperion’s animal-based tissue alternative graft, Z-Lig®, which received a CE Mark in April 2014 for revision and multiligament procedures and is available to registered physicians and surgical facilities through Aperion’s distribution partners in Europe and South Africa. As well, Aperion has received FDA clearance for Z-Lig® to begin U.S. pivotal trials.
Human-Sourced Grafts (Autografts and Allografts)
Autografts
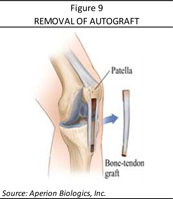
During an autograft procedure (Figure 9), a second surgical site is selected on the patient’s body—patellar tendon or hamstring tendon—which is then harvested from the patient. This harvesting results in added pain, morbidity, and scarring at the site and since a second surgical site is required, leads not only to prolonged operating times but also a more compounded and extended recovery for the patient. The majority of patients have diminished function, including muscle weakness, and many also develop arthritis at the harvest site. Direct surgical costs of the autograft harvesting and the following direct incremental rehabilitation and medication costs are estimated at $2,500 to $3,500 per procedure. As well, since the patient’s own tendons available for harvesting are in limited supply (and do not regrow), many active patients who reinjure their repaired ACL or injure their other ACL are limited in the number of autografts for follow-on procedures.
Allografts

During an allograft procedure, cadaver tissue supply is used as the ACL replacement (Figure 10). With supply extremely constrained, with one allograft tendon required per ACL replacement, roughly half of the 25,000 cadavers available in the U.S. annually provide tissue that meets the specific requirements for donation of orthopaedic tissue, and among the cadavers that meet those requirements, only approximately six harvested grafts are produced that are suitable for ACL replacement per cadaver. As a result, of the 300,000 ACL replacements performed every year in the U.S., only 20% to 25% of these procedures are performed via allograft (with the remaining performed using autografts, as described above). Beyond the U.S., accessibility to allografts is even more limited, and Aperion considers a large number of the procedures being performed as less than ideal due to the fact that the graft was either not the preferred type (bone-tendon-bone, bone-tendon, or soft tissue), the preferred length, or from a younger donor.
| CRYSTAL RESEARCH ASSOCIATES, LLC | EXECUTIVE INFORMATIONAL OVERVIEW® | PAGE 17 |

Age of Donor versus Strength of Allograft
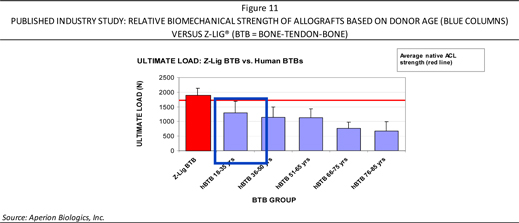
The inconsistent nature of allografts stems largely from the broad age range from donor tissue, as documented in published studies relating to biomechanical properties of human allografts based on age. Specifically,tensile testing has been performed on allografts from different aged donors. The effect of donor age on the strength of allografts can be significant, as illustrated in Figure 11. This Figure compares for a point of reference, Aperion’s Z-Lig® (which is a xenograft tissue graft) with human bone-tendon-bones of differing ages (19-35; 36-50; 51-65; and 76-85). For instance, an allograft from a 66-75 year old donor can only withstand approximately 60% of the stress load that an 18-35 year old allograft can withstand or (approximately 40% of the stress load that Aperion’s Z-Lig® can withstand). Surgeons are cognizant of the importance that the age and health issues of the cadaver has and from where the allograft is derived—thus making such a shortage of quality allografts even more evident—and potentially providing more support for Aperion’s Z-Lig® xenograft-based device.
Governing FDA Regulations for Allografts versus Other Medical Devices
Since allografts are produced in tissue banks, they are regulated differently by the FDA than other medical devices. Allografts are not subject to the FDA approval process and are therefore not subject to the same controls and regulations as would be a medical device. While there has been improvement with regard to monitoring and oversight in this industry, soft tissue allograft infections in patients continue to be reported, which have resulted in recalls. Thus, while generally safe, there remains a risk of human disease transmission when using allograft tissue.
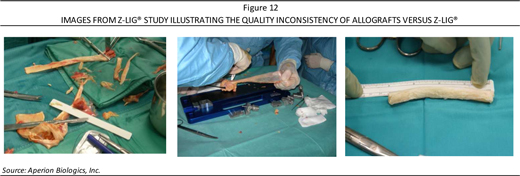
Worth noting is that the use of allografts is largely among U.S. markets since most of Europe as well as other international markets largely employ autografts due to either legal, cultural, and/or religious issues surrounding allografts. While available in a selection of international markets from hospital- or regional-based tissue banks, the quality, processing, and consistency of allografts holds larger variability, which limits its acceptance (Figure 12, page 19). As such, Aperion’s off-the-shelf Z-Lig® product could capture demand in this particular market.
| CRYSTAL RESEARCH ASSOCIATES, LLC | EXECUTIVE INFORMATIONAL OVERVIEW® | PAGE 18 |

Non-Human Grafts (Synthetic and Xenografts)
Synthetic Grafts

Countless efforts have been undertaken to create artificial ligament products, as shown in Figure 13. Such developments have included ligaments made from Dacron, carbon fiber, Gore-Tex, and other chemicals/fibers. Yet, synthetic devices continue to lack the required biological and biomechanical properties to do well long-term since they do not provide for sustained meaningful cellular remodeling by a human’s own cells—with the only graft to survive in the ACL indication over time being those which are biologic. Nonetheless, there are a limited number of synthetic devices marketed outside the U.S. (albeit with overall low acceptance by surgeons). Of note is that a newer synthetic augmentation graft, Artelon®, which is a series of woven polymer fibers with similar mechanical properties to ligaments, is under study. This material is biocompatible and has shown to serve as a scaffold to allow native tissue to gradually grow in over three to four years. However, it has also shown to slowly weaken over time, which gives the new biologic tissue a progressive load stimulus that allows it to adapt and strengthen to its ultimate maturity. There are currently, however, no prosthetic ligaments in the U.S. approved by the FDA for ACL reconstruction surgery.
Synthetic grafts are not approved for use in the U.S. and have extremely limited commercial acceptance in Europe and other international markets. Their use largely centers on patients who wish to return to their activities/sports within the shortest possible time frame. Aperion believes that the synthetic graft’s lack of durability is largely due to its inability to regenerate (compared to tissue-based implants which do regenerate) and thus the reason it wears out from use. Issues with synthetic grafts have led Aperion to believe that animal tissue is a viable alternative to human-sourced ACL grafts, and accordingly the basis for its xenograft-based development efforts.
Xenograft
Defined as the transplantation of organs or tissues from other animal species into humans, xenotransplantation is under investigation for a range of conditions, including replacement of the heart, lungs, liver, and kidneys. It is also being developed for non-whole organ scenarios, such as diabetes, neurodegenerative disorders, chronic pain control, and ex vivo perfusion events. Research has focused on pig organs and tissues as its biochemical profile is similar to that of human organs. There remains substantial discussion as to the pros and cons of xenotransplantation, with the U.S. and UK having taken regulatory steps to foster this debate and to control clinical trials. Companies such as Aperion are pursuing xenotransplantation within regulatory frameworks, as the potential that exists within the ACL reconstruction market is believed to be significant.
| CRYSTAL RESEARCH ASSOCIATES, LLC | EXECUTIVE INFORMATIONAL OVERVIEW® | PAGE 19 |

The xenograft market has become a well-accepted segment of the medical device industry. Derived from porcine (pig), bovine (cow), and equine (horse) sources, xenografts have been used as heart valves, extracellular matrices (patches), injectable collagen, and other replacement/augmentation tissues for years. Aperion’s products are distinct from currently commercialized xenografts in that they act as biological scaffolds (unlike, for example, heart valves) and do not illicit an immunological reaction (unlike, for example, extracellular matrices and injectable collagen) due to its Z-Process®. There is not believed to be another company selling or in human clinical development for an animal-based (xenograft) product specifically for ACL tissue grafting.
Why Xenotransplantation Works
Xenotransplantation is the transplantation of living cells, tissues, or organs from one species to another, where such cells, tissues, or organs are called xenografts (versus allotransplantation, where the transplant comes from the same species). Certain procedures aim to use cells or tissues from other species to treat life-threatening and debilitating illnesses, such as cancer, diabetes, liver failure, and Parkinson’s disease.
Over time, companies have attempted to develop animal-based products for the ACL market. Perhaps the most well-known of these efforts to create an animal-derived ligament was made by XenoTech, LLC in the 1980s. The Company believed that becauseglutaraldehyde (an oily liquid that is used to sterilize medical and dental equipment, and used for industrial water treatment as well as for a preservative) had been successfully used to “fix” porcine heart valves, bovine tissue which was treated in a similar manner would work for ligament replacement. Unfortunately, the resulting implants were largely unsuccessful due to the complete crosslinking of the graft which is thought to have prevented the host’s own cells from penetrating and assimilating into the device—thus, similar to glutaraldehyde fixed heart valves, the grafts eventually failed. The company eventually ended its development efforts in this space.
Certain type of human antibodies, known as anti-gal antibodies, binded to the alpha-gal antigen on animal-based grafts and set off a rapid immune rejection process. Other antibodies attach to non-gal antigens. Preventing the rejection can be done by either removing or deactivating the antigens found on the xenograft or sourcing the xenograft from a genetically modified animal. Removing antigens is done by Aperion’s patented enzyme wash. Deactivating remaining antigens involves crosslinking—a chemical manipulation of the xenograft that when done at a low level that makes it acceptable to the human body, noting that excessive crosslinking results in a xenograft that is incapable of acting as a biological scaffold and thus unable of become populated with the patient’s own cells (no “ligamentization”). Remodeling and incorporating a xenograft into a host has demonstrated to be vital in terms of the implant’s long-term performance. Too little crosslinking leads to an individual’s immune system attacking the xenograft and rejecting it (either acutely or over the longer-term), as demonstrated in Figure 14.
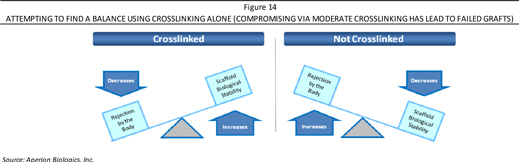
With a balance of deactivating the antigens and maintaining biological function, Aperion is using an animal tissue (pig) and altering it via its proprietary Z-Process® to be accepted by the human immune system. By immunochemically modifying animal tissue to make it compatible with the human immune system, both α-gal and non-gal antigens on the xenograft are deactivated, which then may make porcine tendons safe for use in humans while retaining the implant’s biological scaffold activity.
| CRYSTAL RESEARCH ASSOCIATES, LLC | EXECUTIVE INFORMATIONAL OVERVIEW® | PAGE 20 |

Market Size
Soft Tissue Repair Market
In 2013, the global orthopedic soft tissue repair market was valued at $5.6 billion and is expected to grow at a CAGR of 7.2% from 2013 to 2019, to reach an estimated value of $8.5 billion in 2019 (Source: Transparency Market Research’sOrthopedic Soft Tissue Repair Market [Surgical Procedures-ACL/PCL Reconstruction, Meniscal Repair, Rotator Cuff Repair, Hip Arthroscopy, Biceps Tenodesis and Shoulder Labrum Repair] Global Industry Analysis, Size, Share, Growth, Trends and Forecast, 2013-2019). Some of the key factors driving this market include an aging population (which diminishes body functions and movements, making it more prone to injuries), increased obesity rates, a higher rate of soft tissue damage and sports injuries, increasing healthcare expenditures, and a lack of substitutes for soft tissue repair surgery. As well, participation in sports activities is growing, which is resulting in a higher incidence of orthopedic sports injuries. In August 2013,USA Today reported that more than 1.35 million children/adolescents face severe sports injuries in the U.S. every year, with the European Injury Database (EU IDB) catalogue reporting that, on average, 6.1 million people in the EU annually are treated in hospitals for sports injuries.
ACL/PCL reconstruction is the largest practiced procedure in the orthopedic soft tissue repair market, with this procedure expected to grow at a CAGR of about 8.3% from 2013 to 2019. Some of the other major procedures for orthopedic soft tissue repair include meniscal repair, hip arthroscopy, rotator cuff, shoulder labrum, and biceps tenodesis. Worldwide, the majority of knee injury incidences are reported during sports followed by shoulder and hip injuries. Aging is considered as one of the main factors leading to a rise in orthopedic soft tissue injuries, including knee injuries. The reason for this is that aging causes weakening of bones, loss of proper bodily functions, decreased movements, and flexibility—all leading to more cases of knee injury.
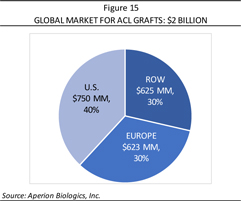
Aperion believes that regenerative medicine is one of the highest growth potential markets in the medical device industry for treating individuals with soft tissue injuries, such as ACL injuries. This growth has created a market valued at roughly $2 billion for ACL grafts, made up of $750 million in the U.S., $623 million in Europe, and $625 million in the rest of the world (ROW), as shown in Figure 15. The size of the markets are represented not only by the cost of autografts, at an estimated $2,500 per procedure, but as well in the surgical costs of harvesting and subsequent incremental rehabilitation and medication costs (noting that the majority of these procedures are performed using autografts, as allografts are only available on a limited basis). The greater part of this market is concentrated in the EU and U.S., where roughly 30% or 265,000 ACL reconstruction procedures take place in European markets (where Aperion is now approved to sell Z-Lig®) and 40% or 300,000 ACL procedures are performed in the U.S. (where the Company has FDA approval to commence U.S. pivotal trials for Z-Lig®).
| CRYSTAL RESEARCH ASSOCIATES, LLC | EXECUTIVE INFORMATIONAL OVERVIEW® | PAGE 21 |

APERION’S TECHNOLOGY AND PRODUCT DEVELOPMENT
Seeking to become a leading tissue product company, Aperion is addressing the need for alternatives to human-based grafts by using animal-based tissue technology. Having developed and patented a technique, called the Z-Process®, animal tissue can be immunochemically modified to be made compatible with the human immune system, thereby avoiding rejection. This facilitates successful implanting of animal tissues into humans, where the tissue is able to regenerate and repair itself over time (compared to synthetic material, which cannot, and will eventually wear out over time).
Technology’s History
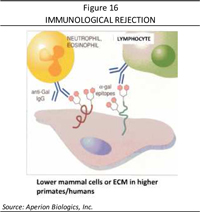
Starting in 1995, a sequence of studies was done by Dr. Stone and others to try to determine how to use intact animal tissue and avoid rejection. Since all animals have specific carbohydrates that humans and old world primates do not have, the tissue is recognized as foreign when transplanted to people. The recognition is an acute rejection response. A strategy was designed to cleave the epitope and thus “humanize” the tissue, as shown in Figure 16.
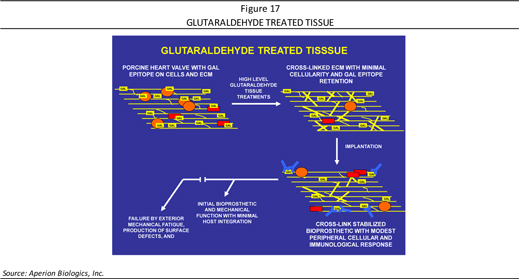
What typically happens in pig tissue (such as a heart valve) is that it contains glutaraldehyde and it is transplanted into a body where it functions for a while. However, due to the extensive crosslinking by the aldehyde, no remodeling occurs, and the tissue becomes brittle and eventually fails. For this reason, young patients do not receive pig heart valves but older people may, as the valve may not fail due to the shortened lifespan of the patient. However, if the carbohydrate epitope could be cleaved off, then less glutaraldehyde fixation can be used, remodeling can occur, and the graft may last a lifetime—such is the strategy behind Aperion’s technology (shown in Figure 17).
| CRYSTAL RESEARCH ASSOCIATES, LLC | EXECUTIVE INFORMATIONAL OVERVIEW® | PAGE 22 |

Beginning with a patellar tendon from a pig, where the tissue is young, healthy, and strong (with the appropriate collagen morphology), the biomechanics matches the human biomechanics, with the static tensile testing and dynamic abrasive bending testing all matching human data. It is believed that young healthy pig tissue may be better than allograft since with each year of aging, the human tissue loses 1% of biomechanics (i.e., 40 year old tissue is not as good as 20 year old tissue, and where tissue from a young healthy pig at six months is in fact stronger than even tissue from a young human patient). Thus, with a primate reconstruction model (which is the only model that can be used for xenografts to show the immunologic rejection model against the galactocele epitope), appropriate functioning of the graft was demonstrated where it was shown to be immunocompatible and safe with no adverse histopathology and biomechanics that were observed to be like the normal allograft (where they would rupture mid substance) and where the tissue ingrowth of the primate into the pig tissue was acceptable. Such research is the foundation for Aperion and critical evidence of the technology’s efficacy.
Z-Process® Technology
To address the complications of xenotransplantation rejection, Aperion developed the Z-Process® to immunochemically modify animal tissue and make it compatible with the human immune system, controlling both α-gal and non-gal antigens on the xenograft (as illustrated in Figure 18), and making the porcine tendons safe for use in humans while retaining the implant’s biological scaffold activity. The core platform to its technology is an enzymatic “stripping” of the key carbohydrate antigens followed by a conversion process that both humanizes as well as sterilizes animal tissue without affecting its biomechanical or biological properties.
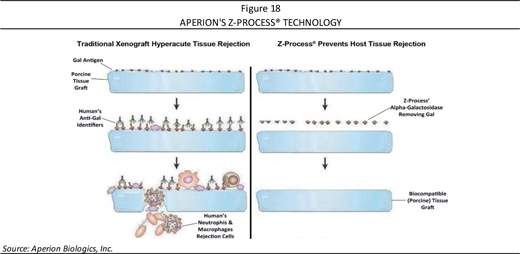
Employing α (alpha)-galactosidase enzyme, Aperion is able to cleave the terminal galactose of the gal antigen such that the carbohydrate chain remaining on the animal tissue is the same in its structure as the carbohydrate chains present in humans. This tissue scaffold provides mechanical stability and function while being biologically integrated and remodeled. Importantly, the Company owns the exclusive rights (with issued patents) to use α-galactosidase enzyme to remove α-gal antigens from non-primate animal tissues (including ligaments, cartilage, bone, heart valves, vascular tissue, collagen, and many other tissues) and is unaware of any other technology that could act as a replacement for α-galactosidase for cleaving the gal antigen while still maintaining the tissue’s biological scaffold.
| CRYSTAL RESEARCH ASSOCIATES, LLC | EXECUTIVE INFORMATIONAL OVERVIEW® | PAGE 23 |

Market Potential
Aperion’s Z-Process® platform has broad applicability beyond ACL replacement across many other tissue needs, including extracellular matrix; wound healing; bone replacement; and heart valves (illustrated in Figure 19). It is possible as well that the technology could have application in dynamic scaffold with architectural and mechanical advantages; stem cell delivery; growth factor delivery; and drug delivery. Aperion’s intellectual property may afford the Company a solid position in removal of α-gal in key tissues with broad applicability.

Z-Lig® ACL Replacement Device
Using its Z-Process®, Aperion developed the Z-Lig® BTB ACL replacement device to be an off-the-shelf, readily available, immunocompatible, porcine-derived ACL reconstruction alternative, which is resilient, sterile, and reproducible (as shown in Figure 20 and 21 [page 25]). When initially implanted, Z-Lig® contains no live cells, and over time is gradually populated and remodeled using the patient’s own cells to yield a mature human ligament through the process of “ligamentization,” a fundamental process for being highly functional for many years following implantation. The Z-Lig® is believed to remodel at a similar rate as human allografts, with substantial ligamentization occurring over 6 to 12 months and complete remodeling over two to three years—with remodeling believed to be key to long-term functionality of the Z-Lig®.
Through bench testing and extensive in vivo testing, Z-Lig® was evaluated to demonstrate its suitability as a biocompatible graft alternative to allograft and/or autograft. The Company has stated that it plans to expand indications for the Z-Lig® and introduce a soft tissue version to complete the spectrum of ligament reconstruction devices. In April 2014, Aperion received the CE Mark for the BT+ (bone-tendon) and BTB (bone-tendon-bone) versions of the Z-Lig® device family for revision and multiligament ACL reconstruction procedures.
| CRYSTAL RESEARCH ASSOCIATES, LLC | EXECUTIVE INFORMATIONAL OVERVIEW® | PAGE 24 |


In addition to developing the Z-Lig® for ACL reconstruction, Aperion’s technology platform may also have applications to other areas, such as cartilage, soft tissue patches (extracellular matrices), bone, heart valves, vascular grafts, collagen, and other tissues. These developments include product candidates in preclinical development and research stages, such as the Z-Patch, Z-Fix, and Z-Meniscus. U.S. and European markets for extracellular matrix that can be addressed by Z-Patch are $1.0 billion in U.S. and $1.2 billion in Europe; the potential market sizes for bone replacement that can be partially addressed by Z-Fix are $1.0 billion in U.S. and $1.1 billion in Europe; and the potential market sizes for meniscus replacement that can be addressed by Z-Meniscus are $150 million in U.S. and $150 million in Europe. Importantly, Aperion believes that potential competitors (such as those described on pages 36-39) are likely to be prevented from using animal tissue for an ACL replacement without either infringing the Company’s patents (described on page 11) or making the graft inert and incapable of incorporating the host’s own cells.
Source Material
Source material for Z-Lig® comes from suppliers that not only meet the Company’s quality and performance requirements but as well are compliant with ISO 22442 standards (standards that apply to medical devices other than in vitro diagnostic medical devices and that are manufactured utilizing materials of animal origin, which are non-viable or have been rendered non-viable). Using a predictable ramp-up of the breeding and processing of the pigs and supply chain redundancies, the Company believes that the anticipated market demand for its Z-Lig® can be satisfied. As well, the Company can accommodate various size requirements of its Z-Lig® product through changing the age and strain of the animal.
Development Status
In 2012, a multicenter, prospective, blinded, allograft-controlled, randomized, and blinded clinical study in EU and South Africa completed enrollment. Currently all remaining subjects have surpassed the 36-month long-term follow-up period. Having enrolled 66 subjects (n=66), the 12-month time point to complete endpoint analysis for primary performance and efficacy were achieved with the study continuing to follow subjects for post-market safety. ACL graft failure largely happens within the early post-implantation window, which is at three to six months post-operative, at which point ligamentization has started and where graft strength is most susceptible. The Primary Performance stability assessment (primary endpoint for CE Marking) supported the conclusion of non-inferiority of Z-Lig® versus allograft, with comparable success between groups at six months follow-up.
| CRYSTAL RESEARCH ASSOCIATES, LLC | EXECUTIVE INFORMATIONAL OVERVIEW® | PAGE 25 |

Clinical results from trials in the EU and South Africa have enabled the Company to receive a CE Mark. The non-inferiority trial was performed at seven different centers in six countries, and was performed in the EU as well as South Africa. Surgeons as well as evaluators were blinded. This was level 1 study, where the Company believed that it was very important to run a high quality study to meet the scientific and clinical expectations of the community—for an ACL device, the burden of proof needs to be met to convince physicians to switch from their current standard of care. Clinical trial investigators were chosen very carefully, where the Company was fortunate to engage some of the premier sports medicine/knee surgeons working in Europe. Not only were these investigators for the Company but as Aperion goes to market, these investigators are anticipated to be key opinion leaders in their respective market and help drive acceptance and adoption of the Z-Lig® device in the various markets.
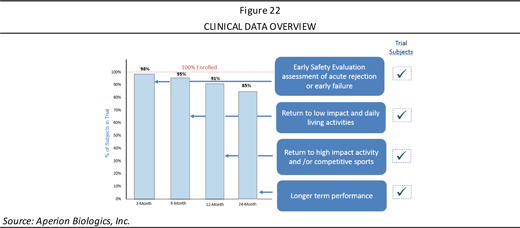
Clinical data over time shows the number of patients that were able to be retained in the study, where at 24 months, the Company was still able to get follow up data on 85% of patients (Figure 22). Each of the time points represents key data points, where at 3 months, an immune response would be expected; 6 months if the grafts are not offering stability, patients will not be released to daily living; and 12 and 24 months is continued performance of the device in providing knee stability. Data being shown are the most meaningful outcomes to the clinicians, documented in a paper published in 2013, where clinicians identified the most important parameters to success outcomes in ACL reconstruction surgery.
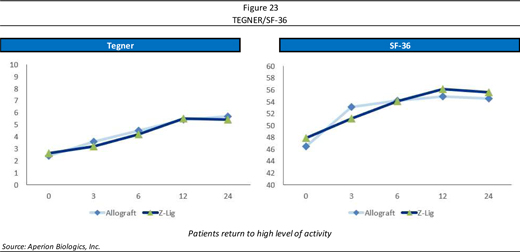
One of the most important things are that patients want to return to their normal level of activity but also want to be satisfied, which is what the Tegner and SF-36 scores reflect (Figure 23, page 27). Tegner is an activity score; whereas SF-36 is a patient satisfaction score. Comparing Z-Lig® to Allograft, this was a non-inferiority study, designed to show that Z-Lig® was equivalent to one of the standards of care (allograft)—where the lines are on top of one another—showing Z-Lig® to be as good or better than one of the current standards of care.
| CRYSTAL RESEARCH ASSOCIATES, LLC | EXECUTIVE INFORMATIONAL OVERVIEW® | PAGE 26 |

The composite endpoint (which in this case refers to stability) used for the study were three metrics called KT-1000 Lachmans, Pivot Shift—which are all commonly accepted stability assessments by the orthopaedic surgeon. At 6, 12, and 24 months, when compared to the historical standard of care, 85% is expected from autograft as well as allograft, where Z-Lig® is performing as good or better than the standard of care, as shown in Figure 24.
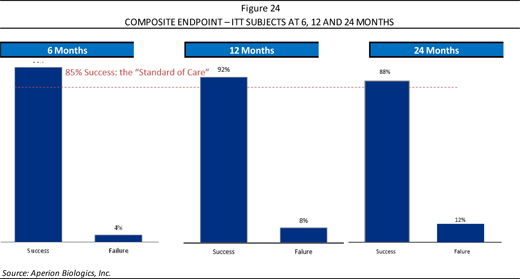
| CRYSTAL RESEARCH ASSOCIATES, LLC | EXECUTIVE INFORMATIONAL OVERVIEW® | PAGE 27 |

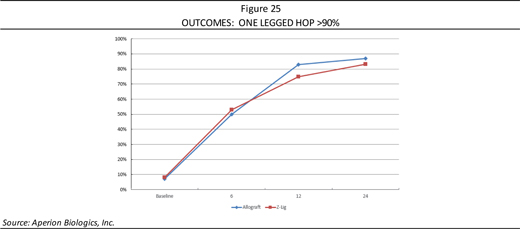
In the one legged hop functional test, which is a standard performance test where the patient can compare the ability to hop on the operated knee versus the knee which was not injured, if a patient can achieve greater than 90% of the performance, it is considered successful. Figure 25 shows the number of patients who achieved greater than 90% one legged hop versus their unoperated hop, demonstrating that the Z-Lig® performs well compared to the allograft and enables return to normal functional performance.
Preclinical Findings
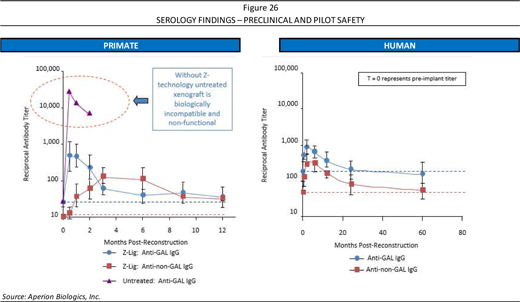
Prior to Z-Lig®’s use in humans, Aperion conducted controlled studies in 20 Rhesus monkeys to evaluate the product’s safety and performance. These preclinical studies demonstrated that knee joints of monkeys implanted with a Z-Lig® showed normal motion and laxity after several weeks post implantation, which was upheld for a year following the study.Serological studies of these monkeys demonstrated a short-lived production of non-gal antibodies, which was considerably reduced at roughly nine months after implantation due to the steady replacement of the pig tissue and matrix by the recipient’s own cells and tissue.
As shown in Figure 26 (page 29), if a piece of animal tissue that has not been treated with the Z-Process® is used, there is a huge spike on an antibody response, which is shown to the left with a circle. However, if a piece of pig tissue implanted into the primate is treated with the Z-Process®, there may be a small elevation at the early time frames for the alpha-gal antibody, as well as other antigens that may cause an antibody response, as well as a bit of an elevation—but all goes back down to a baseline with time. As illustrated in the right of Figure 26, this was experienced in the pilot human trial (the safety trial) conducted in the U.S. at Dr. Stone’s clinic. Figure 26 illustrates that the immunologic response of the alpha-gal as well as the non-gal antigens is behaving as predicted from the preclinical animal studies.
Biomechanical analysis of the implants in monkeys after one year showed characteristics similar to those of the control (monkey allograft) and a substantial return to intact ACL strength. One year post-implantation, histological studies of the implants showed that the effective replacement of the porcine tissue components with those of the recipient served as a scaffold to support slow and steady remodeling with the monkey’s own cells. Aperion has further completed initial safety and performance studies (including cytotoxicity, acute systemic toxicity, pyrogenicity, residual risk assessment, viral safety, viral inactivation, tensile strength, and dynamic abrasion).
| CRYSTAL RESEARCH ASSOCIATES, LLC | EXECUTIVE INFORMATIONAL OVERVIEW® | PAGE 28 |

Summary of the CE Trial Investigators and Sites
Measuring clinically relevant success outcomes, which include patient activity, patient satisfaction, stability, function, and effusion, lead to data which demonstrated performance equivalent to the allograft control. When compared to allograft controls, surgeons using the device reported consistent, comparable, or superior quality of the Z-Lig® graft materials, and even reported that the Z-Lig® graft showed superior bone plug material, which was easily used and managed during surgery, and required no change to the standard surgical technique. A summary of the CE trial investigators and sites is provided in Figure 27 (page 30).
Of note, bacterial infections were reported in six patients implanted with Z-Lig® in this study within the initial 12 months. Following these infections, an extensive safety investigation concluded that the infections all stemmed from contamination of incoming tissue as well as processing deficiencies. With the exception of these cases of bacterial infections, reported complications were consistent with those found in the medical and scientific literature and were unrelated to the Z-Lig® graft. Aperion has stated that it has now put into place a vigorous corrective action plan to deal with the sources of bacterial contamination. Furthermore, validation activities have been completed to verify that the corrective actions were adequate and that sterility can be guaranteed. Aperion has also stated that it intends to conduct supplementary post market studies to satisfy the effectiveness of the corrective actions it has taken.
In September 2013, the dossier to include the data collected from the European and South African multicenter study was submitted to a Notified Body (BSI), which is one of the top tier notified bodies in the EU. Following widespread analysis of the science, preclinical, clinical, and validation data, Aperion was granted the CE Mark for the Z-Lig® device for multiligament and revision ACL procedures. As well, plans were committed for the continued clinical and medical evaluation of this device family to ensure the processing-related issues have been addressed. Further data on 20 to 30 subjects at one year will be submitted to support the indicated expansion to the full ACL indication. Additionally, Aperion expects to conduct post-market safety evaluations as well as continue with Z-Lig® subject follow-up for a prolonged time period for post-market surveillance purposes (with a goal of generating further clinical data, which could be utilized to expand its markets).
| CRYSTAL RESEARCH ASSOCIATES, LLC | EXECUTIVE INFORMATIONAL OVERVIEW® | PAGE 29 |


U.S. Human Clinical Results: Z-Lig® Pilot Study Results Demonstrate Safety
In the U.S., Z-Lig® is being developed via an Investigational Device Exemption (IDE) with the FDA, where the product’s development trials follow the FDA’s guidance on Intra-Articular Knee Ligament Reconstruction Devices. Safety testing modules for CE Mark approval will be used to support FDA approval of Z-Lig® along with 510(k) approvals of follow-on products—with no systemic diseases or secondary infections linked to the device’s implantation, nor were there any acute immune response or long-term rejection response.
The pilot study’s results showed the Z-Lig® to be safe, with data from a completed U.S. human clinical study confirming long-term safety and feasibility of the device. With 10 subjects followed per protocol for two years, the Company believes that this was a highly active subject population, as it included subjects with high pre-injuryTegner activity levels (competitive athletes with Tegner ratings 8-10); subjects with clinically significant comorbidities, and subjects undergoing revision ACL surgeries (which are all known risk factors to outcomes following ACL surgery). Accordingly, Aperion believes that this cohort of subjects represented a highly robust test of the device.
During the surgical procedure and thereafter, the device performed as anticipated through progressive weight bearing and then upon returning to normal activities. Furthermore, the grafts were well-tolerated and devoid of any damaging immunogenic response. Four of the 10 subjects experienced non-device related adverse events due to either vigorous sports activities (e.g., sports injury, rupture) or events related to the initial procedure (e.g., fixation, procedure error). The study’s 12-month results, as described in the accompanying section were submitted to the FDA, leading to acceptance by the FDA of a pivotal clinical trial protocol.
| CRYSTAL RESEARCH ASSOCIATES, LLC | EXECUTIVE INFORMATIONAL OVERVIEW® | PAGE 30 |

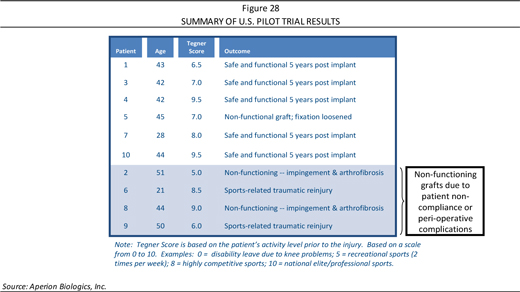
Following an evaluation by the implanting surgeon and two non-affiliated orthopaedic knee specialists, five of six subjects presented with functional grafts at the 24-month post-operative time point. These individuals were satisfied with all success criteria including effusion, knee laxity (KT 1000), pivot shift,Lachmans, andAnterior Drawer tests. MRI assessment as well demonstrated substantial remodeling and maturation of the functioning grafts (noting that one of the six evaluable patients presenting without a functional graft had a tibial bone fixation that loosened). Aperion subsequently completed a twelve-year follow-up on the five evaluable patients with functional grafts, showing the continued safety of the devices throughout a twelve-year interval, with all evaluable patients showing graft function with knee stability. Thus, the study showed proof of concept of the Company’s Z-Lig® for the replacement of ruptured human ACL in a relevant clinical setting.
Clearance to Proceed with Pivotal Efficacy Trial
On the U.S. front, the Company has unconditional approval to begin the U.S. trial, which is expected to involve 326 patients at up to 10 clinical sites. Aperion has not yet commenced the trial as the Company does not have the resources to conduct this U.S. clinical trial and it requires two years of follow up. Having the U.S. regulatory pathway addressed helps reduce the risk for getting U.S. regulatory approval.
| CRYSTAL RESEARCH ASSOCIATES, LLC | EXECUTIVE INFORMATIONAL OVERVIEW® | PAGE 31 |

MARKETING AND DISTRIBUTION
Aperion has stated that it expects to begin marketing and distributing its Z-Lig® as an independent organization. The Company seeks to introduce the Z-Lig® device through a focused and tiered market rollout. Based on the Company’s clinician contacts, Aperion believes there is immediate market need and that they will likely embrace the Z-Lig® for revision and multiligament cases, where a graft option is frequently needed to attain the preferred clinical results. The Company seeks to address a select number of markets, which already have accepted human or synthetic graft options—specifically those countries whose sites participated in the European and South African clinical trial. Accordingly, the initial target markets for Z-Lig® distribution include, but are not limited to, South Africa, Denmark, the UK, Netherlands, Belgium, Spain, Italy, and Poland. Aperion expects to be able to use indirect distribution channels who have met the Company’s selection criteria (i.e. existing/complementary product lines, market share, willing to invest in the product line, etc.), where the product is to be introduced via a targeted approach, with clinicians chosen by Aperion’s surgeons and/or its distribution partners.
Efforts of this indirect distribution channel are expected to be led by individuals tasked with sales management and clinical specialists for training and technical/scientific field education. The subsequent distribution phase is slated to expand distribution reach into additional European as well as Asian markets, which accept the CE Mark (specifically concentrating on high-volume clinical centers or those specializing in knee injuries). For the final phase, Aperion has stated that it intends to include pan-European expansion into markets in the EU and continued introduction into markets outside the U.S.—in particular, those who accept the CE Mark—with possible distribution into non-European nations whose regulatory bodies require additional regulatory approval. A summary of the Company’s distribution strategy is outlined in Figure 29 and detailed in the accompanying section.
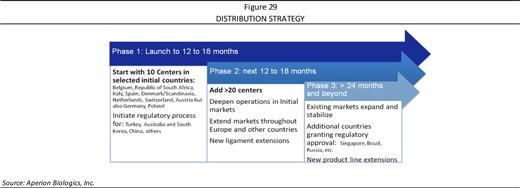
Phase 1 (Launch to 12-18 Months)
Aperion has outlined a distribution strategy whereby it expects to be in 6 to 10 markets within 12 to 18 months following launch. These markets are anticipated to show the following criteria: (1) accepted options for ACL reconstruction (allograft and synthetic); (2) reimbursement pathways for graft alternatives; and/or (3) areas where the Company has conducted its clinical evaluations—with these markets likely to become early adopters. Figure 30 (page 33) goes into greater detail on characteristics of initial markets. The Company also plans to submit regulatory applications to markets outside of the EU, including China, Korea, India, Australia, and others, which accept the CE Mark approval. Review in these markets may take 6 to 24 months.
| CRYSTAL RESEARCH ASSOCIATES, LLC | EXECUTIVE INFORMATIONAL OVERVIEW® | PAGE 32 |


Phase 2 (12-18 Months to 24-36 Months)
During the second phase of launch, the Company expects to focus on expanding its customer base in countries where its product is already available, as well as widen the distribution into additional European markets—focusing specifically on high-volume clinical centers and/or centers that specialize in knee injuries. As well, Aperion has stated that it expects to market the Z-Lig® outside of Europe in countries that accept the CE Mark (i.e., Canada, Turkey, Saudi Arabia, Australia, and Korea)—noting that working with these centers may necessitate additional mini-studies to gain clinical acceptance and/or reimbursement. Additionally, the Company expects to continue its presence at international meetings, to assist its distributors at national events, and to continue to add and train distributors to expand markets.
Phase 3 (24 Months and Beyond)
Aperion expects to continue expanding its customer base into markets with proven distribution. Beyond the pan-European expansion in the EU markets, Aperion seeks to introduce the Z-Lig® into markets outside the U.S. (specifically those that accept the CE Mark). Non-European markets being targeted could include those where regulatory bodies require additional regulatory submissions and approvals.
U.S. Market Regulatory and Commercial Progress
The Company recently received approval to move forward with its pivotal U.S. trial, which is significant as it suggests the FDA is comfortable with changes made by the Company in moving to San Antonio and in its transition toward becoming a commercially viable process. The next step in the U.S. approval pathway is protocol optimization.
| CRYSTAL RESEARCH ASSOCIATES, LLC | EXECUTIVE INFORMATIONAL OVERVIEW® | PAGE 33 |

Distributors
Each indirect distribution channel is required to stock and store grafts as well as to provide local distribution, billing, and collection for their selected markets. At first, distributors of the Z-Lig® are expected to be directed to identify a select group of qualified clinicians to become users and potential key opinion leaders within their territory. This is important as the Company’s expands internationally. Before distribution can begin with any potential business partner, the Company will likely require a current presence in the geographic region in the orthopedic, sports medicine, or sports traumatology market with existing products. As well, the partner must have the experience, ability, or the desire to handle a regenerative medicine/tissue-based product (store, monitor, distribute, invoice, collect, etc.). The partner must further have a presence in the target market, including accounts and relationships with clinicians, with a presence at local, regional, and national logical meetings. Partners are also expected to have a willingness to invest in training their sales force and customers, and a willingness to invest in marketing, training, and promotional activities that are intended to drive distribution and acceptance of these products. Moreover, there must be a desire to invest in necessary activities to establish products in these respective markets (including related to establishing reimbursement), where they hold a knowledge of regulatory barriers and time to market conditions.
| CRYSTAL RESEARCH ASSOCIATES, LLC | EXECUTIVE INFORMATIONAL OVERVIEW® | PAGE 34 |

PRODUCT PIPELINE
As described in Figure 31, Aperion has four product candidates in clinical and preclinical development and research stages. The most advanced is Z-Lig® for ACL reconstruction (which has received a CE Mark in Europe and is being made available on a limited basis). In addition, Aperion’s technology platform may have applications to other areas, such as cartilage, soft tissue patches (extracellular matrices), bone, heart valves, vascular grafts, collagen, and other tissues. Early stage product candidates include the Z-Patch (an extracellular matrix), Z-Fix (for biologic ACL fixation using bone), and Z-Meniscus (for meniscus).

The Anabolic Era of Tissue Cells and Factors
Without resulting in a negative immune response, xenografts can be applied as a tissue matrix for other growth factors. For example, the xenograft could be applied to the meniscus cartilage, since so often patients are seen with a segmented defect which does not warrant a full transplantation—thus a patient could receive a segmented reconstruction for a meniscus. Overall, the model is to eventually put in dead tissue with a patient’s own cells/factors, give it a boost, and eventually change the one year remodeling time to either shorten it or provide for higher outcomes in any tissue transplant performed. Ultimately, Aperion seeks to apply the xenograft tissue model for ACL replacement to a range of other tissues.
| CRYSTAL RESEARCH ASSOCIATES, LLC | EXECUTIVE INFORMATIONAL OVERVIEW® | PAGE 35 |

Potential Competition
As detailed on pages 36-39, conventional ACL reconstruction surgeries largely employ autografts, where the graft tissue is taken from within the patient’s own body to repair the ACL. This practice has been in use for decades, with variations largely attributed to the manner by which the graft is attached in the body. A de facto “gold standard” for ACL surgery is the patellar tendon bone-tendon-bone (BTB) graft harvested from the patient’s patellar tendon. It is known to create a strong bond and requires only two interference screws to hold the bone in place in the knee. Today, interference screws are available in bioresorbable materials that dissolve within the bone over two to three years. However, the BTB approach has been associated with considerable post-operative pain, pain when resuming athletic activities, a larger incision site, and a loss of sensation. Other approaches use grafts from the hamstring or quadriceps tendons instead of the patellar tendon, which are increasingly used due to advancements in the technologies for attaching these grafts to the knee (Source: OA Centers for Orthopaedics).
Beyond autografts, ACL reconstruction procedures performed in the U.S. today also use allograft tissue, which entails tendons (Patellar, hamstring, Achilles, and so on) harvested from cadavers and used in place of or in combination with the patient’s own tissue. Autografts are generally used for first-time ACL patients, though allografts may be required in patients who are undergoing a complicated multiple ligament reconstruction who need several grafts (such as simultaneous ACL and PCL reconstructions after a dislocated knee) or who have had multiple injuries and whose own tendon availability is limited.
There are multiple opportunities to improve upon these existing ACL repair techniques, including the development of synthetic (man-made) grafts, xenografts (derived from another species), or bioengineering entirely new materials to mimic the ACL. However, to date, Aperion is one of the few entities working to introduce a new graft product that is neither autograft nor allograft. Major orthopaedic and medical device companies are rather focused on refining their surgical technologies for improving patient outcomes in ACL reconstruction in terms of incorporating bone growth stimulants, bioresorbable materials, faster healing times, better range of motion, and so on. The summaries presented below are not intended to be an exhaustive collection of potential competitors to Aperion; however, they are believed to be representative of the type of ACL surgical products that are currently in use or are in development and that the Company may encounter as it seeks to promote adoption of its Z-Lig® solution in the knee and other body regions.
Artelon, Inc.
http://www.artelon.com/
Nashville, Tennessee-based Artelon is a medical technology company working to commercialize degradable implants that reinforce and support surgical repairs to restore body functions. Artelon® is a proprietary, degradable biomaterial that incorporates a series of woven polymer fibers with similar mechanical properties to ligaments. It is the product of almost 20 years of research, and thousands of patients have been treated with an Artelon® implant to date. The synthetic Artelon® serves as a temporary scaffold for the body’s own cells while it degrades and integrates with the body over a six-year period. It further stabilizes and relieves load during the healing period, and has shown favorable biocompatibility. The company is currently focused on both expanding the use of Artelon® into multiple clinical arenas as well as creating new products based on the Artelon® platform. Artelon® holds both the CE Mark and FDA clearance as a tissue reinforcement for use in general surgical procedures for reinforcement of soft tissue at points of weakness. It has been used to reinforce the rotator cuff, patella, Achilles tendon, bicep, and quadriceps. To date, it is not approved as a prosthetic ligament for ACL reconstruction surgery though concepts under study for the biomaterial include long-term reinforcement support and replenishment of soft tissue (assisting with the building of new tissue by providing a scaffold for tissue growth).
| CRYSTAL RESEARCH ASSOCIATES, LLC | EXECUTIVE INFORMATIONAL OVERVIEW® | PAGE 36 |

Arthrex, Inc.
https://www.arthrex.com/
Headquartered in Naples, Florida, Arthrex is a global medical device company specializing in orthopaedics. Arthrex has developed more than 8,500 products and surgical procedures for minimally invasive orthopaedics procedures and believes that it has pioneered the field of arthroscopy, a procedure for diagnosing and treating joint problems. In the knees, the company offers an “All-inside” minimally invasive surgical technique that is completely arthroscopic and designed to reduce post-operative pain. In addition, Arthrex supplies a number of conventional knee surgery products and techniques designed to facilitate either autograft or allograft ACL reconstruction. These include Hamstring or BTB Graft Harvest Preparation, ACL RetroConstruction/FlipCutter or All-Inside ACL Reconstruction/FlipCutter Medial Portal Femoral Socket Preparation, Transtibial ACL Reconstruction, ACL Soft Tissue Graft Fixation, and ACL BTB Graft Fixation. Beyond the ACL, Arthrex’s other knee procedures include cartilage repair and transplants, ligament reconstruction, limb alignments, osteotomies, meniscal repair or transplant, PCL reconstruction, knee resurfacing, and more. The company also offers orthosurgical products targeting the shoulder, elbow, hand and wrist, foot and ankle, and hip as well as orthobiologics that include soft tissue xenografts patches made to replace or reinforce injured human tissue with a graft from another species (desired by physicians because they are readily available) and synthetic bone grafts.
Cayenne Medical, Inc.
http://cayennemedical.com/
Founded in 2005, Cayenne Medical designs, develops, and markets soft tissue reconstruction solutions for the knee, shoulder, and extremities. The company is headquartered in Scottsdale, Arizona. Its knee products include two specifically for ACL surgeries: (1) AperFix®, stated by Cayenne to be the only soft tissue multi-ligament system with rigid aperture fixation; and (2) iFix® for bone-tendon bone (BTB) fixation. Cayenne also recently expanded its product portfolio to include the Mirror™ Partial Knee System, a ligament-sparing surgical device to balance the knee throughout the entire range of motion. AperFix® is a femoral implant to fix soft tissue in place. It mimics the BTB construct used for decades but is believed to better restore native knee kinematics. The iFix® entails the interference screws used to attach BTB grafts during arthroscopic or open ACL reconstruction procedures.
CONMED Corporation (CNMD-NASDAQ)
http://www.conmed.com/
CONMED is a global medical technology company specialized in the development and sale of surgical and patient monitoring products and services for the following specialties: orthopaedic, sports medicine, general surgery, gynecology, gastroenterology, pulmonology, and anesthesiology. CONMED has headquarters in Utica, New York, with its Arthroscopy, Orthopedic Powered Surgical Instruments and medical video systems division’s operating out of Largo, Florida. CONMED’s orthopaedics work has been in place since the early 1980s, and the company today owns well-known brands Hall®, Concept®, Shutt®, and Linvatec®. Its Largo, Florida, facility also houses a Center for Orthopaedic Education, which has additional location in New York and Germany. CONMED has a strategic partnership with the Musculoskeletal Transplant Foundation (MTF), through which the company offers allografts, biological solutions and fixation for ligament reconstruction, cartilage repair, and meniscal transplants to surgeons and their patients. This partnership also enables access to MTF’s graft matching process for anatomically matching allografts to each unique patient, which is intended to minimize the need for extensive modifications to the allograft at the time of surgery. For ACL surgeries specifically, CONMED has developed the Pinn-ACL® CrossPin System instruments and implants for transverse femoral fixation of soft tissue grafts in ACL reconstruction. This uses bioabsorbable implants and biomaterials to help place and secure the soft tissue graft.
| CRYSTAL RESEARCH ASSOCIATES, LLC | EXECUTIVE INFORMATIONAL OVERVIEW® | PAGE 37 |

MedShape Solutions, Inc.
www.medshape.com
Founded in 2005, MedShape is an orthopedic device company developing shape memory biomaterials that can transform and adapt inside the body in order to improve the technology of soft tissue fixation, fracture repair, and joint fusions. The company also uses superelastic alloys for self-adapting implants for joint fusion and fracture repair. MedShape is based in Atlanta, Georgia, and is affiliated with the Georgia Institute of Technology. For ACL repair, the company has recently introduced the Exoshape™ ACL Fixation System which is made out of MedShape’s proprietary shape memory PEEK Altera®. It offers non-rotational aperture soft tissue graft interference fixation for both the femoral and tibial sides. Exoshape™ is marketed as a way to address issues of traditional interference screws and “sheath-and-screw” devices, which require manual torque. MedShape’s device incorporates a deployment gun to expand the PEEK Altera® shape memory sheath without rotational motion.
Northwestern University McCormick School of Engineering
http://www.mccormick.northwestern.edu/biomedical/
A research team from the Northwestern University McCormick School of Engineering’s Department of Biomedical Engineering is working on development of a synthetic ACL graft using braided polymers (braided to increase strength and toughness) combined with calcium nanoparticles. The Northwestern design has three main components: (1) polyester fibers; (2) an antioxidant, porous biomaterial; and (3) calcium nanoparticles. The resulting graft seeks to mimic the mechanical characteristics of the ACL, and the anti-inflammatory material helps reduce reactions from the body. Moreover, the team may have found a way to integrate the artificial ligament with native bone by impregnating pores in the anti-inflammatory material with hydroxyapatite to form a nanocomposite that can integrate with bone. Initial results in animal studies have been favorable, showing that animals could bear weight in the knees and function after six weeks. Further research is needed.
RTI Surgical
http://www.rtix.com/
Florida-based RTI Surgical is a global surgical implant provider focused on regenerative medicine and is believed to be one of the largest processors of biologic implants. The company, which also has a portfolio of metal and synthetic implants, has operations across the U.S. as well as in the Netherlands and Germany. Among its products, RTI Surgical offers a line of BioCleanse®-processed allografts for ACL reconstruction surgery. The BioCleanse® technology, along with RTI Surgical’s Tutoplast® and Cancelle® SP DBM, entail tissue sterilization processes that have been shown to address donor-to-recipient disease transmission risk while preserving tissue strength and biocompatibility. These processes have a record of more than five million implants sterilized with zero incidence of allograft-associated infection, according to RTI Surgical’s website. In its BioCleanse® line of donated, sterilized tissues for ligament reconstruction, RTI Surgical offers the following products: implants from Achilles Tendons, Anterior and Posterior Tibialis Tendons, and Patellar Tendon; Adjustable Length BTB implants; BTB Select® (which is a BTB allograft product enabling precision size-matching relative to the intra-articular length of the patient); Gracilis, Peroneus, and Semitendinosus (non-bone tendons greater than or equal to 220mm in length with varying folded widths); and Pre-shaped BTB implants. Beyond applications in sports medicine, RTI Surgical’s allograft, metal, and synthetic products also address needs in cardiothoracic care, wound care, other extremities and soft tissues, the spine, and orthobiologics for filling bone voids, repairing fractures, and skeletal defects.
| CRYSTAL RESEARCH ASSOCIATES, LLC | EXECUTIVE INFORMATIONAL OVERVIEW® | PAGE 38 |

Smith & Nephew (SNN-NYSE)
http://www.smith-nephew.com/
London, England-based Smith & Nephew is a diversified advanced medical technology business operating in more than 100 countries. Among its specialties, the company believes it is particularly skilled in orthopaedic reconstruction, advanced wound management, sports medicine (knee/shoulder/hip), and trauma. Its sports medicine business offers specialized devices and fixation systems to repair damaged tissue; fluid management equipment for surgical access; digital cameras, digital image capture, scopes, light sources and monitors to assist with visualization; radiofrequency wands, electromechanical and mechanical blades, and hand instruments for resecting damaged tissue. For ACL reconstruction, Smith & Nephew markets the ENDOBUTTON™ CL BTB Fixation System which uses a bone-tendon-bone (BTB) graft harvested from the patient’s own patella tendon with bone plugs at each end. The ENDOBUTTON™ fixation device is a pre-threaded, continuous loop of suture that holds the graft in place. Smith & Nephew states that its system provides a stronger repair than previous procedures for ACL reconstruction, and allows surgeons to adjust the length of the graft, depending on the patient’s size. The ENDOBUTTON™ CL BTB system is also intended to eliminate the complications associated with using interference screws, which can break, enter the bone at the wrong angle, or tear the graft. The ENDOBUTTON™ CL BTB system eliminates the need for a screw at the femoral end, in many cases.
Soft Tissue Regeneration, Inc.
http://softtissueregeneration.com/
Soft Tissue Regeneration (STR), based in New Haven, Connecticut, is a medical device company focused on the application of resorbable polymer scaffold technology to regenerate and repair ligament and tendon tissue. The company’s devices are based on proprietary fiber, braid, and mesh designs comprised of an established resorbable polymer. STR is developing a synthetic product for ACL repair called the L-C Ligament®, which is currently in clinical trials in Europe. L-C Ligament® is neither autograft nor allograft. Rather, it is a biocompatible and bioresorbable synthetic scaffold designed to facilitate re-growth of the ACL. The STR soft tissue engineered matrices regenerate native tissue and aim to actually allow a new, native ACL to regrow, while only requiring the same open and minimally invasive surgical techniques as presently used for autograft and allograft tissues. The L-C Ligament® has shown in animal studies to be capable of successfully regenerating a native ligament intra-articularly. Clinical studies in human patients commenced in Europe in 2013 and are ongoing. STR is developing this technology platform into complementary products as well, including STR GRAFT™ for soft tissue augmentation, repair of tendons and ligaments, and rotator cuff repair.
Stryker Corporation (SYK-NYSE)
http://www.stryker.com/
Headquartered in Kalamazoo, Michigan, Stryker is a global medical technology company focused on products and services in orthopaedics, medical and surgical, and neurotechnology and spine specialties in over 100 countries worldwide. The company offers an entire suite of instrumentation for ACL repair and reconstruction surgeries. Stryker’s Universal ACL Instrumentation System and endoscopic surgical technique is intended to enable surgeons to obtain more reproducible anatomical graft placement and stable fixation in ACL reconstruction. It includes femoral aimers for precise anatomical placement of femoral tunnels, a tibial guide, the Stryker Universal Wedge Screw (a titanium screw developed specifically for ACL injuries), and a variety of endoscopes, light sources, cameras, instrumentation, and power tools for use in the operating room in support of ACL surgeries. In addition to its devices, Stryker invests in development of biological technologies to foster regeneration and healing for patients suffering from bone, cartilage, and soft tissue injuries and defects. To date, Stryker offers regenerative biological products for trauma, spine, craniomaxillofacial, and joint replacement.
| CRYSTAL RESEARCH ASSOCIATES, LLC | EXECUTIVE INFORMATIONAL OVERVIEW® | PAGE 39 |

Historical Financial Results
Aperion’s key historical financial statements: the Statements of Operations, Balance Sheets, and Statements of Cash Flows, are provided in Figures 32, 33, and 34, as well as on the Company’s most recent SEC filing.
http://www.sec.gov/Archives/edgar/data/1439026/000114420415060614/v420878_partiiandiii.htm
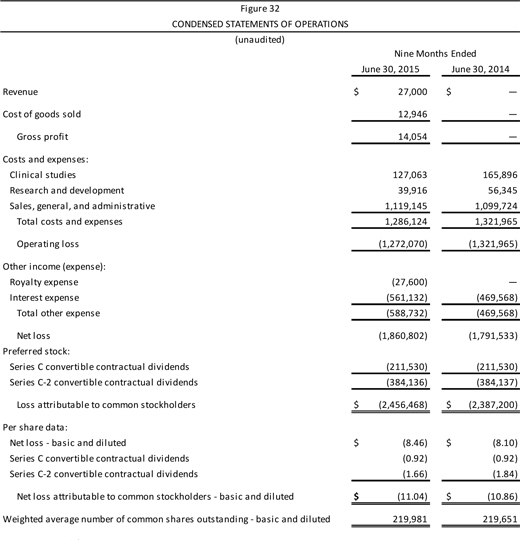
Source: Aperion Biologics, Inc. Form 1-A filed October 23, 2015. Share and per share data presented herein reflect an 18.4:1 reverse stock split that has not been effected as of the date of this report.
| CRYSTAL RESEARCH ASSOCIATES, LLC | EXECUTIVE INFORMATIONAL OVERVIEW® | PAGE 40 |

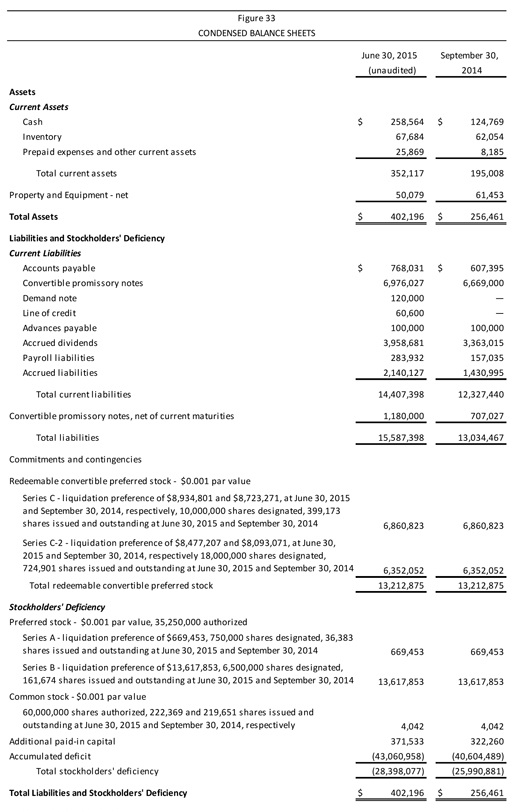
Source: Aperion Biologics, Inc. Form 1-A filed October 23, 2015. Share and per share data presented herein reflect an 18.4:1 reverse stock split that has not been effected as of the date of this report.
| CRYSTAL RESEARCH ASSOCIATES, LLC | EXECUTIVE INFORMATIONAL OVERVIEW® | PAGE 41 |

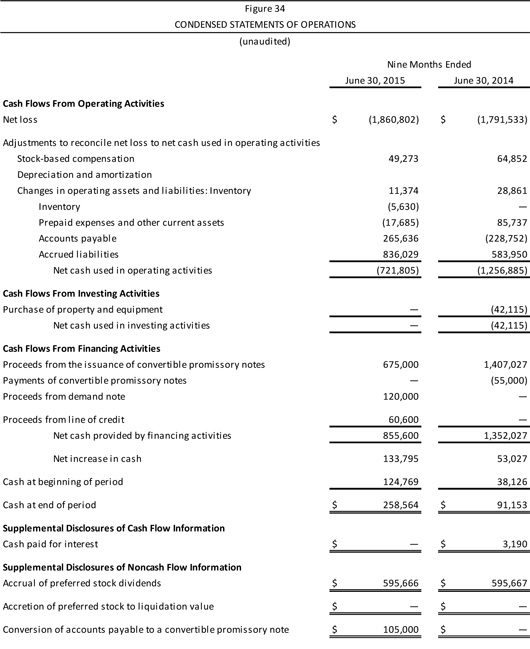
Source: Aperion Biologics, Inc. Form 1-A filed October 23, 2015. Share and per share data presented herein reflect an 18.4:1 reverse stock split that has not been effected as of the date of this report.
| CRYSTAL RESEARCH ASSOCIATES, LLC | EXECUTIVE INFORMATIONAL OVERVIEW® | PAGE 42 |

Risks and Disclosures
This Executive Informational Overview® (EIO) has been prepared by Crystal Research Associates, LLC (“CRA”) with the assistance of Aperion Biologics, Inc. (“Aperion” or “the Company”) based upon information provided by the Company. CRA has not independently verified such information. Some of the information in this EIO® relates to future events or future business and financial performance. Such statements constitute forward-looking information within the meaning of the Private Securities Litigation Act of 1995. Such statements can only be predictions and the actual events or results may differ from those discussed due to the risks described in Aperion’s statements in its public and investor materials as well as regulatory forms filed from time to time.
The content of this report with respect to Aperion has been compiled primarily from information available to the public released by the Company through news releases, investor presentations, and other materials released from time to time. Aperion is solely responsible for the accuracy of this information. Information as to other companies has been prepared from publicly available information and has not been independently verified by Aperion or CRA. Certain summaries of activities and outcomes have been condensed to aid the reader in gaining a general understanding. CRA assumes no responsibility to update the information contained in this report. In addition, CRA has been compensated in cash of US$42,500 for its services in creating this report and updates.
IMPORTANT: An offering statement relating to these securities has been filed with the Securities and Exchange Commission but has not yet become qualified. These securities may not be sold nor may offers be accepted prior to the time the offering statement becomes qualified. You can access a copy of the preliminary offering circular included in the offering statement by clicking on this link:
http://www.sec.gov/Archives/edgar/data/1439026/000114420415060614/v420878_partiiandiii.htm
You can also call or write the underwriter of the proposed offering, W.R. Hambrecht & Co., to obtain a copy of the preliminary offering circular, at 1-800-673-6476, fax 1-415-551-3123; 909 Montgomery Street, 3rd Floor, San Francisco, CA 94133.
No money or other consideration is being solicited in connection with this information, and if sent in response, will not be accepted. No offer to buy the securities can be accepted and no part of the purchase price can be received until an offering statement on Form 1-A is qualified pursuant to Regulation A of the Securities Act of 1933, as amended, and any such offer may be withdrawn or revoked, without obligation or commitment of any kind, at any time before notice of its acceptance given after the qualification date. Any person's indication of interest involves no obligation or commitment of any kind.
Investors should carefully consider the risks and information about Aperion’s business, as described below. Investors should not interpret the order in which considerations are presented in this or other filings as an indication of their relative importance. The risks and uncertainties overviewed below are not the only risks that the Company faces. Additional risks and uncertainties not presently known to Aperion or that it currently believes to be immaterial may also adversely affect the Company’s business. If any of such risks and uncertainties develops into an actual event, Aperion’s business, financial condition, and results of operations could be materially and adversely affected, and the trading price of the Company’s shares could decline.
This report is published solely for information purposes and is not to be construed as an offer to sell or the solicitation of an offer to buy any security in any state. Past performance does not guarantee future performance. Additional information about Aperion, as well as copies of this report, can be obtained by calling(210) 858-7070.
Investing in Aperion’s common stock involves a high degree of risk. An investor should carefully consider the risks described below along with all of the other available Company information, including its financial statements and the related notes, before deciding whether to purchase Aperion’s common stock. If any of the adverse events described in the following risk factors, as well as other factors which are beyond the Company’s control, actually occurs, Aperion’s business, results of operations, and financial condition may suffer significantly. As a result, the trading price of its common stock could decline, and an investor may lose all or part of an investment in the Company’s common stock.
| CRYSTAL RESEARCH ASSOCIATES, LLC | EXECUTIVE INFORMATIONAL OVERVIEW® | PAGE 43 |

Risks Related to the Company’s Business and Finance
Aperion has incurred significant operating losses since inception, anticipates that it will continue to incur losses for the foreseeable future and will need to raise additional capital.
Aperion has generated operating losses since the Company began operations in 1996, and its net losses for the fiscal year ended September 30, 2014 and 2013 were $3,291,838 and $5,449,894, respectively. The Company expects to continue to incur additional operating losses for the foreseeable future as it plans the commercialization of Z-Lig® products and continues its clinical and pre-clinical studies. If the time required to generate revenues and achieve profitability is longer than it currently anticipates, or if the Company is unable to raise new capital through equity or obtain debt financing or other sources of funding, Aperion may be forced to curtail or suspend its operations, which would have a material adverse effect on the value of one’s investment.
The Company expects capital outlays and operating expenditures to increase over the next several years as it establishes and expands its marketing infrastructure to commercialize its Z-Lig® products. Following the completion of an offering, the Company believes that its financial resources will be adequate to sustain its current operations at least through the end of calendar year 2017. In the event, Aperion will need to raise additional capital, the Company cannot be certain that it will be able to obtain financing on terms acceptable to it, or at all. Failure to obtain adequate and timely funding will materially adversely affect its business and its ability to develop its technology products and would have a material adverse effect on the value of one’s investment.
Aperion only recently received approval in Europe to commercialize its Z-Lig® products, and has no significant experience or capability to sell its products on a commercial scale.
The Company’s future is significantly dependent on the commercial success of its Z-Lig® family of products. Aperion has only recently received CE Mark in April 2014 that allowed it to conduct limited sales in select European markets beginning in February 2015. As a result, the Company has no significant history or experience in selling Z-Lig® or any other products in Europe or in any other markets, and has limited relationships with surgeons, physicians, clinicians, and hospitals that may purchase or use its products. In order for the Company to commercialize its products successfully, Aperion will need to develop, or obtain through outsourcing arrangements, the capability to market and sell its products on a commercial scale. The Company may not have the ability and sufficient resources to establish the infrastructure and organizations needed to execute these functions, which can be complex and costly. Aperion’s efforts to commercialize Z-Lig® products is also subject to a number of additional risks, which could have a material adverse effect on the value of one’s investment, including:
| · | competitors’ established relationships with the Company’s potential customers; |
| · | limitations in Aperion’s ability to demonstrate the advantages of its products compared to competing products and the relative safety, efficacy, and ease of use of its products; |
| · | the Company’s inability to convince doctors and hospitals to use its products; and |
| · | the introduction and market acceptance of competing products and technologies. |
Aperion’s Z-Lig® products may not gain market acceptance among surgeons, physicians, patients, healthcare payers, and the medical community.
A critical element in the Company’s commercialization strategy is to persuade and educate on the safe and effective use of its products and how Z-Lig® products differentiates from the existing supplies of surgical issues. Surgeons, physicians, and hospitals may not perceive the benefits of Aperion’s products and may be unwilling to change from the devices they are currently using. A number of factors may limit the market acceptance of the Z-Lig® products,including the following:
| · | rate of adoption by healthcare practitioners; |
| CRYSTAL RESEARCH ASSOCIATES, LLC | EXECUTIVE INFORMATIONAL OVERVIEW® | PAGE 44 |

| · | rate of a product’s acceptance by the target population; |
| · | timing of market entry relative to competitive products; |
| · | availability of third-party reimbursement; |
| · | extent of marketing efforts by Aperion and third-party distributors or agents retained by the Company; and |
| · | side effects or unfavorable publicity concerning its products or similar products. |
Therefore, even after Aperion has demonstrated the effectiveness of the Z-Lig® products, the Company may not be able to commercialize these products successfully if it cannot achieve an adequate level of market acceptance. Aperion’s inability to successfully commercialize its products will have a material adverse effect on the value of one’s investment.
Aperion may experience defects and manufacturing issues in its supplies of animal tissues or raw materials.
As the Company continues to expand its clinical development and commercialization activities, it expects to scale up the manufacturing and supplies of animal tissues, which are the main component of the Z-Lig® products. The Company may not be able to control or maintain a consistent and high quality of raw materials, such as porcine tissues, or uncover defects prior to implantation in humans. In the past, Aperion observed and experienced contamination in porcine tissues resulting from the processing and handling of these materials during clinical trials, which led to bacterial infections associated with the grafts in patients implanted with Z-Lig® in the trial. While the Company has implemented stringent validation, safety, and corrective procedures and intends to conduct additional post-market studies to confirm the effectiveness of such preventive measures, there is no guarantee that Aperion can eliminate this risk in the future. In addition, external factors outside of the Company’s control may affect the quality of its supplies of animal or porcine issues. For example, swine diseases or pandemics may severely reduce the supply of porcine tissues required to manufacture the Z-Lig® products. Mishandling or lack of quality control at Aperion’s third-party suppliers may also negatively impact its ability to obtain sufficient and acceptable level of porcine tissues to meet its needs. The Company’s failure to eliminate contamination and deficiency in the animal and porcine tissues used in its products may require the Company to incur additional costs to implement preventive measures, or cause significant delays and disruptions in the development and commercialization of its products, which would have an adverse effect on Aperion’s business operations and financial conditions.
The safety and efficacy of Aperion’s products is supported by limited clinical data, and the Company may not be able to generate long-term or additional positive clinical data to support or expand its commercialization efforts.
Aperion has obtained a CE Mark to market and sell its Z-Lig® products in Europe based on the 6- and 12-month-data collected from its clinical studies in 2013 and the longer term (12 years) safety data from the U.S. pilot safety trial. The Company has committed to continue these clinical studies to support post-market commercial activities in Europe and to meet certain post-market approval requirements by European regulatory agencies. In addition, Aperion intends to conduct additional clinical studies to support further commercialization efforts and expand the markets for its Z-Lig® products. For example, the CE Mark that was received in April 2014 is limited to revision and multiligament ACL surgeries and does not cover primary ACL surgeries. The Company has established post-market clinical plans to generate additional data required to expand the indication of Z-Lig® products to cover primary ACL surgeries, which would allow Aperion to reach a broader ACL reconstruction market. There is no guarantee that the Company can duplicate the positive results from its earlier trials for future studies or generate the required data to support the expansion of Z-Lig® indication. Failure to do so may result in the loss of CE Mark approval or other regulatory approvals, delays, failures in the adoption of its products by surgeons and physicians, damage to the Company’s reputation, and legal claims against Aperion.
| CRYSTAL RESEARCH ASSOCIATES, LLC | EXECUTIVE INFORMATIONAL OVERVIEW® | PAGE 45 |

In addition, any negative data from the Company’s post-market clinical studies may adversely impact Aperion’s ability to conduct clinical trials and seek regulatory approval of its products from the FDA to market in the U.S. Furthermore, Aperion is developing several products based on its Z-Process® that would allow a commercialization path based on FDA’s Section 510(K) pre-market clearance procedures. This procedure is shorter and typically requires the submission of less supporting documentation than other FDA approval processes and does not require long-term clinical studies. As result, Aperion may encounter difficulties and delays in marketing these products which otherwise may not be the case if the products had been proven safe and effective in more extensive and long-term clinical trials.
Aperion’s clinical studies of its current or future products may not produce results necessary to support regulatory clearance or approval in the U.S. or elsewhere.
The Company plans to seek FDA approval of Z-Lig® by conducting pivotal clinical trials in the U.S. While Aperion has conducted a limited safety pilot study in the U.S. showing that Z-Lig® was safe, and the Company has received a CE Mark based on the clinical studies in Europe and South Africa, there is no guarantee that the same results will be duplicated in a clinical trial with a larger patient population in the U.S. The Company’s inability to achieve acceptable results in future clinical studies would have a material adverse effect on its business operations and financial condition and the value of one’s investment.
If third-party payors fail to provide appropriate levels of reimbursement for the use of Aperion’s products, its revenues could be adversely affected.
Sales of Aperion’s products depend on the availability of adequate reimbursement from third-party payers. In each market in which the Company does business, Aperion’s inability to obtain reimbursement approval or the failure of third-party payers to reimburse healthcare providers at a level which justifies the use of its products instead of cheaper alternatives will hurt the Company’s business. Moreover, Aperion is unable to predict what changes will be made to the reimbursement methodologies used by third-party payors in the future. The Company cannot be certain that under current and future payment systems, in which healthcare providers may be reimbursed a set amount based on the type of procedure performed, such as those utilized by Medicare and in many privately managed care systems, the cost of the Company’s products will be justified and incorporated into the overall cost of the procedure. A failure by third-party payors to provide appropriate levels of reimbursement for the use of Aperion’s products will have a material adverse effect on its business operations and financial condition and the value of one’s investment. As Aperion expands into multiple international markets, it will face similar risks relating to adverse changes in coverage and reimbursement procedures and policies in those markets. Reimbursement and healthcare payment systems vary significantly among markets in different jurisdictions. The Company’s inability to obtain international coverage and reimbursement approval, or any adverse changes in coverage and the reimbursement policies of foreign third-party payors, could negatively affect Aperion’s ability to sell its products.
Aperion relies on third party suppliers for its raw materials, including porcine tissues, and their inability to supply the Company with an adequate supply of materials could harm Aperion’s business.
The Company relies on third-party suppliers to supply and manufacture the raw materials, including porcine tissues, for the development of its products. To be successful, Aperion’s suppliers must be able to provide the Company with products and components in substantial quantities, in compliance with regulatory requirements (including ISO 22442), in accordance with agreed upon specifications, at acceptable cost, and on a timely basis. Among other factors, the Company’s anticipated growth could strain the ability of suppliers to deliver an increasingly large supply of products, materials, and components. If unable to obtain sufficient quantities of high quality components to meet customer demand on a timely basis, Aperion could lose customers, its reputation may be harmed, and its business could suffer. In addition, Aperion currently uses one supplier for the supply of porcine tissues required to manufacture its Z-Lig® products, biologics, and components. The dependence on one supplier involves several risks, including limited control over pricing, availability, quality, and delivery schedules. If any one or more of Aperion’s suppliers cease to provide the Company with sufficient quantities of its components in a timely manner or on terms acceptable to Aperion, the Company would have to seek alternative sources of supplies.
| CRYSTAL RESEARCH ASSOCIATES, LLC | EXECUTIVE INFORMATIONAL OVERVIEW® | PAGE 46 |

Aperion could incur significant delays while it locates and engages alternative qualified suppliers, including delays associated with qualifying the new supplier to meet all applicable regulatory requirements in the U.S., Europe, and other markets in which Aperion develops and sell its products. Even if the Company is able to identify an alternative supplier, Aperion might not be able to negotiate or receive the same or more favorable terms as those provided by its existing suppliers. Any such disruption or increased expenses could harm commercialization efforts and adversely affect the Company’s ability to generate revenue. Any inability to meet its customers’ demands for these products could lead to decreased sales and harm Aperion’s reputation and result in the loss of customers to its competitors, which could cause the market price of the Company’s common stock to decline.
Failure to attract, retain, and motivate skilled personnel may delay Aperion’s commercialization plans and research and development efforts.
The Company’s commercial success depends on its continued ability to attract, retain, and motivate highly qualified management and scientific personnel. Competition for skilled and qualified personnel in the medical device industry is intense. If Aperion loses the services of personnel with the necessary skills, including the members of its senior management team, this could significantly impede the Company’s commercialization and research and development objectives. Replacing key personnel and consultants may be difficult and may take an extended period of time because of the limited number of individuals in the industry with the breadth of skills and experience required to develop, gain regulatory approval of, and commercialize products successfully.
The Company’s international operations subject it to certain risks
Aperion intends to market its Z-Lig® products initially in select European countries and in South Africa, including Denmark, United Kingdom, Netherlands, Belgium, Spain, Italy, and Poland. The Company also has plans to expand its commercialization activities to other non-European countries that accept CE Mark, such as Canada, Turkey, Saudi Arabia, Australia, and Korea. The Company also expects to pursue various clinical and pre-clinical activities in the U.S. and Europe. Its international operation will subject it to rules, regulations, and customs of multiple jurisdictions, and compliance with these rules and regulations is costly and exposes Aperion to penalties for non-compliance. Other laws and regulations that can significantly impact the Company include various anti-bribery laws, including the U.S. Foreign Corrupt Practices Act and anti-boycott laws, as well as export controls laws. Any failure to comply with applicable legal and regulatory obligations could impact Aperion in a variety of ways that include, but are not limited to, significant criminal, civil, and administrative penalties, denial of export privileges, seizure of shipments, and restrictions on certain business activities. Also, the failure to comply with applicable legal and regulatory obligations could result in the disruption of its sales activities.
The Company’s international operations expose it to additional risks, including:
| · | difficulties in enforcing or defending intellectual property rights; |
| · | pricing pressure internationally; |
| · | third-party reimbursement policies that may require some of the patients who receive the Company’s products to directly absorb medical costs or that may necessitate the reduction of the selling prices of its products; |
| · | competitive disadvantage to competition with established business and customer relationships; |
| · | the imposition of additional U.S. and foreign governmental controls or regulations, changes in duties and tariffs, license obligations, and other non-tariff barriers to trade; |
| · | foreign currency exchange rate fluctuations; |
| · | difficulties in communicating with Company employees, partners and collaborators, and maintaining consistency with its internal guidelines; |
| CRYSTAL RESEARCH ASSOCIATES, LLC | EXECUTIVE INFORMATIONAL OVERVIEW® | PAGE 47 |

| · | difficulties in enforcing agreements and collecting receivables through certain foreign legal systems; and |
| · | difficulties in establishing and maintaining an effective internal control system to ensure timely and accurate financial reporting. |
If Aperion experiences any of these risks, the sales in international countries may be harmed and the Company’s results of operations would suffer.
Aperion may face product liability claims that could result in costly litigation and significant liabilities.
Manufacturing and marketing of the Company’s products, and clinical testing of its products under development, may expose it to product liability and other tort claims. Although Aperion has, and intends to maintain, liability insurance, the coverage limits of its insurance policies may not be adequate and one or more successful claims brought against it may have a material adverse effect on its business and results of operations. Additionally, product liability claims could negatively affect Aperion’s reputation, product sales, and its ability to obtain and maintain regulatory approval for its products.
Risks Relating to the Company’s Intellectual Property
If Aperion is unable to protect its intellectual property, the Company’s business will be negatively affected.
The market for medical devices is subject to frequent litigation regarding patent and other intellectual property rights. It is possible that Aperion’s patents or licenses may not withstand challenges made by others or protect its rights adequately. The Company’s success depends in large part on its ability to secure effective patent protection for its products and processes in Europe and the U.S. Aperion has filed and intends to continue to file patent applications to protect its proprietary technology. However, the Company faces the risks that:
| · | it may fail to secure necessary patents prior to or after obtaining regulatory clearances, thereby permitting competitors to market competing products; and |
| · | its already-granted patents may be re-examined, invalidated, or not extended. |
Aperion also owns trade secrets and confidential information that it tries to protect by entering into confidentiality agreements with its employees and other parties. However, the confidentiality agreements may not be honored or, if breached, the Company may not have sufficient remedies to protect its confidential information. Further, Aperion’s competitors may independently learn its trade secrets or develop similar or superior technologies. To the extent that Aperion’s consultants, key employees, or others apply technological information to its projects that they develop independently or others develop, disputes may arise regarding the ownership of proprietary rights to such information, and such disputes may not be resolved in Aperion’s favor. If the Company is unable to protect its intellectual property adequately, its business and commercial prospects will suffer.
Because it is difficult and costly to protect the Company’s proprietary rights, and third parties have filed patent applications that are similar, Aperion cannot ensure the proprietary protection of its technologies and products.
The Company’s commercial success will depend in part on obtaining patent protection of its technology and successfully defending any of its patents that may be challenged. The patent positions of medical device companies can be highly uncertain and can involve complex legal and factual questions, and Aperion cannot predict the breadth of claims allowed in patents it owns or licenses. Aperion is a party to certain license agreements that give it rights under specified patents and patent applications, including its license agreement with University of Missouri. The Company’s current licenses, as its future licenses frequently will, contain performance obligations. If Aperion fails to meet those obligations, the licenses could be terminated. If unable to continue to license these technologies on commercially reasonable terms, or at all, Aperion may be forced to delay or terminate its product development and research activities.
| CRYSTAL RESEARCH ASSOCIATES, LLC | EXECUTIVE INFORMATIONAL OVERVIEW® | PAGE 48 |

Aperion is unable to exercise the same degree of control over intellectual property that it licenses from third parties as it exercises over its internally developed intellectual property. The Company does not control the prosecution of certain of the patent applications that it license from third parties; therefore, the patent applications may not be prosecuted as it desires or in a timely manner. The degree of future protection for its proprietary rights is uncertain, and the Company cannot ensure that:
| · | it or its licensors were the first to file patent applications for these inventions; |
| · | the patents of others will not have an adverse effect on the Company’s ability to do business; |
| · | others will not independently develop similar or alternative technologies or reverse engineer any of the Company’s products, processes or technologies; |
| · | any of the Company’s pending patent applications will result in issued patents; |
| · | any patents issued or licensed to it or its collaborators or strategic partners will provide a basis for commercially viable products or will provide the Company with any competitive advantages; |
| · | any patents issued or licensed to the Company will not be challenged and invalidated by third parties; or |
| · | the Company will develop additional products, processes, or technologies that are patentable. |
Others have filed and in the future are likely to file patent applications that are similar to Aperion’s. The costs of litigating any infringement claim could be substantial. Moreover, the Company cannot predict whether it, its collaborators, or strategic partners would prevail in any actions. In addition, if the relevant patent claims were upheld as valid and enforceable and the Company’s products or processes were found to infringe the patent or patents, the Company could be prevented from making, using, or selling the relevant product or process unless it could obtain a license or were able to design around the patent claims. Aperion can give no assurance that such a license would be available on commercially reasonable terms, or at all, or that it would be able to successfully design around the relevant patent claims. There may be significant litigation in the genomics industry regarding patent and other intellectual property rights, which could subject the Company to litigation. If Aperion becomes involved in litigation, it could consume a substantial portion of its managerial and financial resources.
Aperion relies on trade secrets to protect technology where it believes patent protection is not appropriate or obtainable. Trade secrets, however, are difficult to protect. While the Company requires employees and consultants to enter into confidentiality agreements, it may not be able to adequately protect its trade secrets or other proprietary information or enforce these confidentiality agreements.
Aperion may infringe the intellectual property rights of others, which may prevent or delay its product development efforts.
The Company’s success will depend, in part, on its ability to operate without infringing the proprietary rights of third parties. Aperion cannot guarantee that its products, or manufacture or use of its product candidates, will not infringe third-party patents. Furthermore, a third party may claim that the Company is using inventions covered by the third party’s patent rights and may go to court to stop Aperion from engaging in its normal operations and activities, including making or selling its product candidates. These lawsuits are costly and could affect Aperion’s results of operations and divert the attention of managerial and scientific personnel.
Some of these third parties may be better capitalized and have more resources than the Company. There is a risk that a court would decide that the Company is infringing the third party’s patents and would order Aperion to stop the activities covered by the patents. In that event, Aperion may not have a viable way around the patent and may need to halt commercialization of the relevant product candidate. In addition, there is a risk that a court will order the Company to pay the other party damages for having violated the other party’s patents.
| CRYSTAL RESEARCH ASSOCIATES, LLC | EXECUTIVE INFORMATIONAL OVERVIEW® | PAGE 49 |

In addition, Aperion may be obligated to indemnify its licensors and collaborators against certain intellectual property infringement claims brought by third parties, which could require Aperion to expend additional resources. The pharmaceutical and biotechnology industries have produced a proliferation of patents, and it is not always clear to industry participants, including the Company, which patents cover various types of products or methods of use. The coverage of patents is subject to interpretation by the courts, and the interpretation is not always uniform.
Patent litigation is costly and time consuming, even in cases where the claims against a Company are without merit. Aperion may not have sufficient resources to bring these actions to a successful conclusion. In addition, if the Company does not obtain a license, develop or obtain non-infringing technology, fails to defend an infringement action successfully, or has infringed patents declared invalid, it may incur substantial monetary damages, encounter significant delays in bringing its product candidates to market, and be precluded from manufacturing or selling its product candidates.
International patent protection is particularly uncertain, and if Aperion is involved in opposition proceedings in foreign countries, it may have to expend substantial sums and management resources.
The laws of some foreign countries may not protect Aperion’s intellectual property rights to the same extent as the laws of the U.S. For example, certain countries do not grant patent claims that are directed to the treatment of humans. Aperion may participate in opposition proceedings to determine the validity of its foreign patents or its competitors’ foreign patents, which could result in substantial costs and diversion of management’s efforts.
The medical device industry is characterized by extensive patent litigation, and Aperion could become subject to litigation that could be costly, result in the diversion of management’s attention, and harm its business and financial condition.
The Company’s success depends, in part, on not infringing the patents or violating the other proprietary rights of others. Significant litigation regarding patent rights occurs in the medical industry, including among companies focused on regenerative medicine. It is possible that U.S. and foreign patents and pending patent applications controlled by third parties may be alleged to cover the Company’s Z-Process®. Aperion’s competitors in Europe, the U.S., and abroad, many of which have substantially greater resources and have made substantial investments in patent portfolios and competing technologies, may have applied for or obtained or may in the future apply for and obtain, patents that will prevent, limit, or otherwise interfere with Aperion’s ability to make, use, and sell its products. At any given time, Aperion may be involved as either a plaintiff or a defendant in a number of patent infringement actions, the outcomes of which may not be known for prolonged periods of time.
The large number of patents, the rapid rate of new patent applications and issuances, the complexities of the technologies involved and the uncertainty of litigation significantly increase the risks related to any patent litigation. Any potential intellectual property litigation also could force Aperion to do one or more of the following:
| · | stop selling, making, or using products that use the disputed intellectual property; |
| · | obtain a license from the intellectual property owner to continue selling, making, licensing, or using products, which license may require substantial royalty payments and may not be available on reasonable terms, or at all; |
| · | incur significant legal expenses; |
| · | pay substantial damages or royalties to the party whose intellectual property rights the Company may be found to be infringing; |
| · | pay the attorney fees and costs of litigation to the party whose intellectual property rights the Company may be found to be infringing; or |
| CRYSTAL RESEARCH ASSOCIATES, LLC | EXECUTIVE INFORMATIONAL OVERVIEW® | PAGE 50 |

| · | redesign those products that contain the allegedly infringing intellectual property, which could be costly, disruptive and/or infeasible. |
If any of the foregoing occurs, the Company may have to withdraw existing products from the market or may be unable to commercialize one or more of its products, all of which could have a material adverse effect on Aperion’s business, results of operations, and financial condition. Any litigation or claim against it, even those without merit, may cause the Company to incur substantial costs, and could place a significant strain on the Company’s financial resources, divert the attention of management from its core business and harm its reputation. Further, as the number of participants in the regenerative tissue industry grows, the possibility of intellectual property infringement claims against it increases.
Risks Related to the Company’s Industry
Aperion is in a highly competitive market segment, and if its competitors are better able to develop and market products that are safer, more effective, less costly, easier to use, or otherwise superior than any products that the Company may develop, its business will be adversely impacted.
The medical device industry is highly competitive and subject to technological change. Aperion’s success depends, in part, upon its ability to maintain a competitive position in the development of technologies and products for use in the ACL knee reconstruction graft and other orthopaedic markets. Any product the Company develops that achieves regulatory clearance or approval will have to compete for market acceptance and market share. Aperion believes that the primary competitive factors in ACL replacement graft markets are clinical effectiveness, product safety, reliability and durability, and eligible patient populations, physician experience and comfort with use of a particular device, ease of use, product support and service, sales force experience and relationships, and price. The Company faces significant competition in the U.S. and internationally, and Aperion expects the intensity of competition will increase over time. Aperion competes with companies that provide allograft and traditional xenograft solutions for orthopaedic surgical procedures. Many of the companies developing or marketing competing products enjoy several advantages to it, including:
| · | greater financial and human resources for product development, sales, and marketing; |
| · | greater name recognition; |
| · | long established relationships with physicians and hospitals; |
| · | longer-term clinical trial data due to earlier regulatory approval; |
| · | the ability to offer rebates or bundle multiple product offerings to offer greater discounts or incentives; and |
| · | more established sales and marketing programs and distribution networks. |
Aperion’s competitors may develop and patent processes or products competitive with it, obtain regulatory clearance or approvals for competing products more rapidly than it, or develop more effective or less expensive products or technologies that render the Company’s technology or products obsolete or less competitive. Aperion also faces fierce competition in recruiting and retaining qualified sales, scientific, and management personnel, establishing clinical trial sites and enrolling patients in clinical studies. The Company’s inability to successfully compete with existing and future market competitors will have a material adverse effect on its business operations and financial condition and the value of one’s investment.
| CRYSTAL RESEARCH ASSOCIATES, LLC | EXECUTIVE INFORMATIONAL OVERVIEW® | PAGE 51 |

Aperion’s business is subject to extensive governmental regulation that could make it more expensive and time consuming for it to introduce new or improved products.
The Company’s products must comply with regulatory requirements imposed by various regulatory agencies. These requirements involve lengthy and detailed laboratory and clinical testing procedures, sampling activities, an extensive agency review process, and other costly and time-consuming procedures. It often takes several years to satisfy these requirements, depending on the complexity and novelty of the product. The Company is also subject to numerous additional licensing and regulatory requirements relating to safe working conditions, manufacturing practices, environmental protection, fire hazard control, and disposal of hazardous or potentially hazardous substances. Some of the most important requirements faced include:
| · | European Union CE mark requirements; |
| · | Medical Device Quality Management System Requirements (ISO 13485:2003); and |
| · | Occupational Safety and Health Administration (OSHA) requirements. |
Government regulation may impede its ability to conduct clinical studies and to manufacture existing and future products. Government regulation also could delay Aperion’s marketing of new products for a considerable period of time and impose costly procedures on its activities. The regulatory agencies may not approve any of the Company’s future products on a timely basis, if at all. Any delay in obtaining, or failure to obtain, such approvals could negatively impact the Company’s marketing of any future products and reduce its product revenues.
Aperion’s products remain subject to strict regulatory controls on manufacturing, marketing, and use. The Company may be forced to modify or recall a product after release in response to regulatory action or unanticipated difficulties encountered in general use. Any such action could have a material effect on the reputation of its products and on its business and financial position. Further, regulations may change, and any additional regulation could limit or restrict Aperion’s ability to use any of its technologies, which could harm the Company’s business. Aperion could also be subject to new international, federal, state, or local regulations that could affect its research and development programs and harm its business in unforeseen ways. If this happens, Aperion may have to incur significant costs to comply with such laws and regulations, which will harm the results of its operations.
Risks Related to the Company’s Common Stock and Corporate Structure
CrossCart LLC controls all aspects of its business.
CrossCart LLC (“CrossCart”) is the Company’s largest and controlling stockholder and beneficially owns approximately 80% of its outstanding shares of common stock on a fully-diluted basis assuming the conversion of all of the Company’s preferred stock and convertible notes. In addition, Dr. Kevin R. Stone, a member of Aperion’s Board of Directors, is the controlling member and manager of CrossCart. CrossCart is the Company’s most significant creditor and historically Aperion depended on the funding of CrossCart and Dr. Stone to maintain its operations. As a result, CrossCart and Dr. Stone are and will be able to exercise significant control over the Company’s business operations and on all matters requiring stockholders approval, including the election of directors and approval of significant corporate transactions. Due to CrossCart’s and Dr. Stone’s controlling position, Aperion may take actions with respect to its business that may conflict with the desire of other stockholders.
| CRYSTAL RESEARCH ASSOCIATES, LLC | EXECUTIVE INFORMATIONAL OVERVIEW® | PAGE 52 |

An active trading market for the Company’s Common Stock may not develop and an investor may not be able to resell his/her shares at or above the initial offering price.
Prior to this offering, there has been no public market for shares of the Company’s common stock. In the absence of an active trading market for its common stock, investors may not be able to sell their common stock at or above the initial public offering price or at the time that they would like to sell. In addition, Aperion intends to submit a listing application for its common stock to be listed on a national securities exchange, including the NASDAQ Stock Market (“NASDAQ”), and there is no guarantee that the Company can meet the listing standards or that such listing application will be accepted. Even if such application is accepted and Aperion’s common stock is listed on NASDAQ or other exchanges, an active trading market for the Company’s common stock may never develop, which will adversely impact one’s ability to sell his/her shares. If shares of common stock are not listed on NASDAQ or another national securities exchange, the Company anticipates that its common stock will be quoted at over-the-counter, or OTC markets, following the completion of an offering. There is no assurance that an active trading market for its common stock will develop on the OTC market.
Aperion’s stock price may be influenced by public perception of alternative tissue replacement and government regulation of such technology.
External events in the field of ligament reconstruction or the use of animal derived tissue in human transplantation may have a significant negative impact on the public perception and stock price of certain companies involved in this kind of work. Potential adverse events in this field may occur in the future that could result in increased governmental regulation of Aperion’s Z-Lig® device. These external events may have a negative impact on public perceptions of the Company’s business, which could cause its stock price to decline.
The Company does not intend to pay dividends on its common stock.
Aperion has never paid dividends on its common stock and currently intends to retain any future earnings and does not expect to pay any cash dividends on its common stock in the foreseeable future. The declaration and payment of all future dividends, if any, will be at the sole discretion its board of directors, which retains the right to change the Company’s dividend policy at any time, and may be limited by its debt arrangements in place from time to time. Consequently, stockholders must rely on sales of their common stock after price appreciation, which may never occur, as the only way to realize any future gains on their investment.
Anti-takeover provisions in Aperion’s certificate of incorporation and Delaware law could make an acquisition of the Company more difficult and could prevent attempts by stockholders to remove or replace current management.
Provisions of Delaware law and the Company’s amended and restated certificate of incorporation and amended and restated bylaws, which are expected to become effective upon the Company completing a planned offering, may discourage, delay, or prevent a merger, acquisition, or other change in control that stockholders may consider favorable, including transactions in which stockholders might otherwise receive a premium for their shares. These provisions may also prevent or delay attempts by stockholders to replace or remove its current management or members of its board of directors. These provisions include:
| · | advance notice requirements for stockholder proposals and nominations; |
| · | the inability of stockholders to act by written consent or to call special meetings; |
| · | the ability of Aperion’s board of directors to make, alter, or repeal its amended and restated bylaws: and |
| · | the authority of the Company’s board of directors to issue preferred stock with such terms as its board of directors may determine. |
| CRYSTAL RESEARCH ASSOCIATES, LLC | EXECUTIVE INFORMATIONAL OVERVIEW® | PAGE 53 |

The affirmative vote of the holders of at least 75% of Aperion’s shares of capital stock entitled to vote, and not less than 75% of the outstanding shares of each class entitled to vote thereon as a class, is generally necessary to amend or repeal the above provisions that are contained in the Company’s amended and restated certificate of incorporation. In addition, absent approval of Aperion’s board of directors, its amended and restated bylaws may only be amended or repealed by the affirmative vote of the holders of at least 75% of the Company’s shares of capital stock entitled to vote.
In addition, upon the completion a planned offering, the Company will be subject to the provisions of Section 203 of the Delaware General Corporation Law, which limits business combination transactions with stockholders of 15% or more of its outstanding voting stock that the Company’s board of directors has not approved. These provisions and other similar provisions make it more difficult for stockholders or potential acquirers to acquire the Company without negotiation. These provisions may apply even if some stockholders may consider the transaction beneficial to them. As a result, these provisions could limit the price that investors are willing to pay in the future for shares of Aperion’s common stock. These provisions might also discourage a potential acquisition proposal or tender offer, even if the acquisition proposal or tender offer is at a premium over the then-current market price for its common stock.
| CRYSTAL RESEARCH ASSOCIATES, LLC | EXECUTIVE INFORMATIONAL OVERVIEW® | PAGE 54 |

Appendix
In the course of developing its technology and product candidates, Aperion has publicly showcased its scientific data through a number of publications, presentations, and poster sessions, as summarized in Figure 35 (continued onto page 56). Readers may consult these reference documents for additional information regarding the Company’s product development.
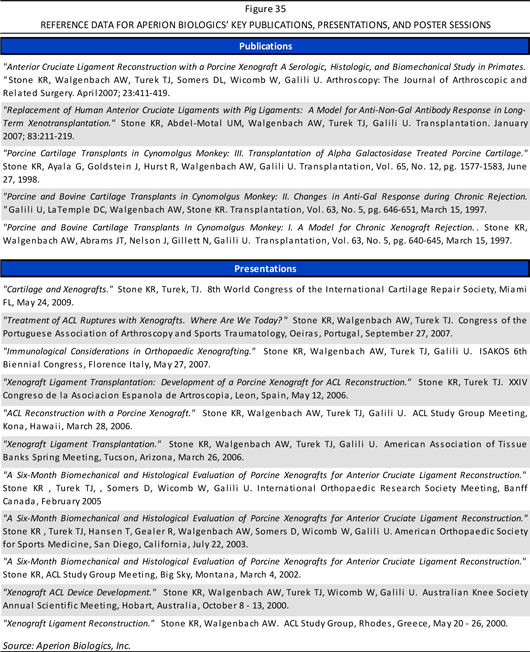
| CRYSTAL RESEARCH ASSOCIATES, LLC | EXECUTIVE INFORMATIONAL OVERVIEW® | PAGE 55 |


| CRYSTAL RESEARCH ASSOCIATES, LLC | EXECUTIVE INFORMATIONAL OVERVIEW® | PAGE 56 |

Glossary
Allografts—The transplant of an organ or tissue from one individual to another of the same species with a different genotype. For example, a transplant from one person to another, but not an identical twin, is an allograft. Allografts account for many human transplants, including those from cadaveric, living related, and living unrelated donors. Also known as an allogeneic graft or a homograft.
Anterior Cruciate Ligament (ACL)—One of a pair of cruciate ligaments (the other being the posterior cruciate ligament) in the human knee. They are also called cruciform ligaments as they are arranged in a crossed formation. In the quadruped stifle joint (analogous to the knee), based on its anatomical position, it is also referred to as the cranial cruciate ligament. The ACL is one of the four main ligaments of the knee, and the ACL provides 85% of the restraining force to anterior tibial displacement at 30 degrees and 90 degrees of knee flexion.
Anterior Drawer Tests—A commonly used test in orthopedic examinations to check for ACL integrity. This is one of the most well known and most used special tests in orthopedics and is also one of the easiest to perform.
Autografts—A tissue or an organ grafted into a new position in or on the body of the same individual.
Biomechanical—Study of the structure and function of biological systems such as humans, animals, plants, organs, and cells by means of the methods of mechanics.
CE Mark—Formerly EC mark, CE Mark is a mandatory conformity marking for certain products sold within the European Economic Area (EEA) since 1985. The CE marking is also found on products sold outside the EEA that are manufactured in, or designed to be sold in, the EEA. The letters "CE" are the abbreviation of French phrase "Conformité Européene" which literally means "European Conformity".
Glutaraldehyde—An organic compound with the formula CH₂ (CH₂CHO)₂. A pungent colorless oily liquid, glutaraldehyde is used to sterilize medical and dental equipment. It is also used for industrial water treatment and as a preservative.
Investigational Device Exemption (IDE)—Allows the investigational device to be used in a clinical study in order to collect safety and effectiveness data required to support a Premarket Approval (PMA) application or a Premarket Notification [510(k)] submission to Food and Drug Administration (FDA). Clinical studies are most often conducted to support a PMA. Only a small percentage of 510(k)’s require clinical data to support the application. Investigational use also includes clinical evaluation of certain modifications or new intended uses of legally marketed devices. All clinical evaluations of investigational devices, unless exempt, must have an approved IDE before the study is initiated.
Lachmans Test—A clinical test used to diagnose injury of the anterior cruciate ligament (ACL). It is recognized as reliable, sensitive, and usually superior to the anterior drawer test. The knee is flexed at 20-30 degrees with the patient supine.
Osteoarthritis—The most common form of arthritis, affecting millions of people worldwide. It occurs when the protective cartilage on the ends of your bones wears down over time. Although osteoarthritis can damage any joint in the body, the disorder most commonly affects joints in your hands, knees, hips, and spine. Osteoarthritis often gradually worsens, and no cure exists. But staying active, maintaining a healthy weight and other treatments may slow progression of the disease and help improve pain and joint function.
| CRYSTAL RESEARCH ASSOCIATES, LLC | EXECUTIVE INFORMATIONAL OVERVIEW® | PAGE 57 |

Serological—Serology is the scientific study of serum and other bodily fluids, where the term usually refers to the diagnostic identification of antibodies in the serum. Such antibodies are typically formed in response to an infection (against a given microorganism), against other foreign proteins (in response, for example, to a mismatched blood transfusion), or to one's own proteins (in instances of autoimmune disease). Serological tests may be performed for diagnostic purposes when an infection is suspected, in rheumatic illnesses, and in many other situations, such as checking an individual’s blood type.
Tegner Activity Levels—Tegner activity level scale is a scale that aims to provide a standardized method of grading work and sporting activities. It was developed to complement the Lysholm scale after it was observed that that limitations in function scores in Lysholm scale may be masked by a decrease in activity level. Tegner activity level scale is used in conjunction with the Lysholm Knee Scoring Scale, originally in patients with ACL injury.
Tensile testing—Also known as tension testing, tensile testing is a fundamental materials science test in which a sample is subjected to a controlled tension until failure. The results from the test are commonly used to select a material for an application, for quality control, and to predict how a material will react under other types of forces. Properties that are directly measured via a tensile test are ultimate tensile strength, maximum elongation, and reduction in area.
Viscoelastic—The property of materials that exhibit both viscous and elastic characteristics when undergoing deformation. Viscous materials, like honey, resist shear flow and strain linearly with time when a stress is applied.
| CRYSTAL RESEARCH ASSOCIATES, LLC | EXECUTIVE INFORMATIONAL OVERVIEW® | PAGE 58 |

Intentionally Blank.
| CRYSTAL RESEARCH ASSOCIATES, LLC | EXECUTIVE INFORMATIONAL OVERVIEW® | PAGE 59 |









































