QuickLinks -- Click here to rapidly navigate through this document
Exhibit 4.1

SQI DIAGNOSTICS INC.
ANNUAL INFORMATION FORM
June 15, 2011
TABLE OF CONTENTS
| | |
| | Page |
|---|
FORWARD-LOOKING STATEMENTS | | 3 |
CORPORATE STRUCTURE | | 5 |
GENERAL DEVELOPMENT OF THE BUSINESS | | 5 |
DESCRIPTION OF THE BUSINESS | | 7 |
RISK FACTORS | | 23 |
DIVIDENDS AND DISTRIBUTIONS | | 36 |
DESCRIPTION OF CAPITAL STRUCTURE | | 36 |
MARKET FOR SECURITIES | | 37 |
DIRECTORS AND OFFICERS | | 39 |
LEGAL PROCEEDINGS AND REGULATORY ACTIONS | | 43 |
TRANSFER AGENTS AND REGISTRARS | | 43 |
EXPERTS | | 43 |
ADDITIONAL INFORMATION | | 43 |
2
SQI DIAGNOSTICS INC.
ANNUAL INFORMATION FORM
In this annual information form ("Annual Information Form"), unless otherwise indicated, all dollar amounts are expressed in Canadian dollars and the statistical and financial data are presented as of June 15, 2011.
FORWARD-LOOKING STATEMENTS
This Annual Information Form, including the documents incorporated by reference into this Annual Information Form, contains forward-looking statements. These statements relate to future events or future performance and reflect our expectations and assumptions regarding our growth, results of operations, performance and business prospects and opportunities. Such forward-looking statements reflect our current beliefs and are based on information currently available to us. In some cases, forward-looking statements can be identified by terminology such as "our goal", "may", "would", "could", "will", "should", "expect", "plan", "intend", "anticipate", "believe", "estimate", "predict", "potential", "continue" or the negative of these terms or other similar expressions concerning matters that are not historical facts. The forward-looking statements in this Annual Information Form, including any documents incorporated by reference into this Annual Information Form, include, among others, statements regarding our future operating results, economic performance and product development efforts, and statements in respect of:
- •
- our expected future losses and accumulated deficit levels;
- •
- our requirement for, and our ability to obtain, future funding on favourable terms or at all;
- •
- market competition and technological advances of competitive products;
- •
- our expectations regarding the acceptance of our products by the market;
- •
- our expectations regarding the progress and the successful and timely completion of the various stages of the regulatory clearance process;
- •
- our strategy to develop new products and to enhance the capabilities of existing products;
- •
- our strategy with respect to research and development;
- •
- our dependence on expanding our customer base;
- •
- our plans to market, sell and distribute our products;
- •
- our plans in respect of strategic partnerships for research and development;
- •
- our ability to obtain a sufficient supply of the components needed for our systems;
- •
- our plans to retain and recruit personnel;
- •
- our ability to satisfy customer demand for our systems;
- •
- our plans to correct defects or errors in our systems;
- •
- the effect of litigation on our business;
- •
- our strategy with respect to the protection of our intellectual property; and
- •
- our expectations with respect to existing and future corporate alliances and licensing transactions with third parties, and the receipt and timing of any payments to be made by us or to us in respect of such arrangements.
A number of factors could cause actual events, performance or results, including those in respect of the foregoing items, to differ materially from the events, performance and results discussed in the forward-looking
3
statements. Factors that could cause actual events, performance or results to differ materially from those set forth in the forward-looking statements include, but are not limited to:
- •
- the extent of our future losses;
- •
- our ability to obtain the capital required to fund development and operations;
- •
- development or commercialization of similar products by our competitors;
- •
- our ability to develop and market our products;
- •
- our ability to comply with applicable governmental and securities regulations and standards;
- •
- our ability to develop and commercialize our technologies;
- •
- delays or failures in our ability to develop and implement new diagnostic products;
- •
- our reliance on a few key and significant customers;
- •
- the impact of changes in the business strategies and development priorities of our strategic partners;
- •
- loss of suppliers or increases to the cost of the components of our systems;
- •
- the impact of legislative changes to the healthcare system and regulatory process;
- •
- our ability to attract and retain skilled and experienced personnel;
- •
- damage to our manufacturing facility or its failure to accommodate future sales growth;
- •
- the impact of unknown defects or errors and product liability claims;
- •
- the impact of liability from the use of hazardous and biological materials and other claims;
- •
- our ability to successfully manage fluctuations in revenue;
- •
- foreign currency fluctuations;
- •
- our ability to obtain patent protection and protect our intellectual property rights and not infringe on the intellectual property rights of others;
- •
- the expense and potential harm to our business of intellectual property litigation;
- •
- stock market volatility;
- •
- changing market conditions;
- •
- the fact that further equity financing may substantially dilute the interests of our shareholders; and
- •
- other risks detailed from time-to-time in our ongoing quarterly filings, annual information forms, annual reports and annual filings with applicable securities regulators, and those which are discussed under the heading "Risk Factors".
Although the forward-looking statements contained in this Annual Information Form and in the documents incorporated by reference are based on what we consider to be reasonable assumptions based on information currently available to us, there can be no assurance that actual events, performance or results will be consistent with these forward-looking statements, and our assumptions may prove to be incorrect. These forward-looking statements are made as of the date of this Annual Information Form.
Forward-looking statements made in a document incorporated by reference into this Annual Information Form are made as of the date of the original document and have not been updated by us except as expressly provided for in this Annual Information Form. Except as required under applicable securities legislation, we undertake no obligation to publicly update or revise forward-looking statements, whether as a result of new information, future events or otherwise.
4
CORPORATE STRUCTURE
The principal and registered office of SQI Diagnostics Inc. ("SQI" or the "Company" and, in this Annual Information Form, "we", "us" and "our" refer to the Company unless the context otherwise requires) is located at 36 Meteor Drive, Toronto, ON M9W 1A4. SQI has a single subsidiary, SQI Diagnostics Systems Inc. ("SQIDS"), which is wholly-owned.
Emblem Capital Inc., the predecessor to SQI, was incorporated on September 11, 2003 pursuant to theCanada Business Corporation Act (the "CBCA") and filed articles of amendment to change its name to "SQI Diagnostics Inc."
On April 20, 2007, an amalgamation between Umedik Inc. and 670194 Canada Inc., a wholly-owned subsidiary of Emblem Capital Inc., was completed and the amalgamated company changed its name to "SQI Diagnostics Systems Inc." on September 7, 2007.
The predecessor to Umedik Inc. was formed on April 19, 1999 pursuant to theBusiness Corporations Act (Ontario) under the corporate name of Pockit Corporation. Pockit Corporation filed articles of amendment on June 9, 1999 to change its name to Poc • Kit Corporation. Poc • Kit Corporation was continued under the CBCA on December 1, 1999 under the name of e-umedik Inc. and e-umedik Inc. filed articles of amendment on October 20, 2000 to change its name to Umedik Inc. 6701914 Canada Inc. was incorporated on January 12, 2007 pursuant to the CBCA.
GENERAL DEVELOPMENT OF THE BUSINESS
Our business was founded in 1999 on the concept that more sensitive, timely and less costly disease diagnostics based on antigen, protein and antibody detection would benefit healthcare providers. We believed that diagnostic testing based on enzyme-linked immunosorbent assay ("ELISA") technology, a test involving an enzyme and an antibody, the diagnostic technology used at the time, had not changed significantly since its development in the 1950s and lacked accuracy and sensitivity, while the testing procedures were lengthy, difficult to execute and labour intensive. We also believed that DNA microarray technology, which was instrumental throughout the 1990s in mapping the human genome, could provide the basis for new antigen, protein and antibody detection technology that could advance beyond the limitations imposed by ELISA technology. However, antigen, protein and antibody microarrays present a very different set of challenges from the DNA microarrays used for the human genome project.
Until 1999, there was little published research on the use of microarrays for antigen, protein and antibody detection for humanin-vitro diagnostics ("IVD") applications. Our founders believed the problem stemmed from the following scientific problems:
- •
- Microarray surface coatings and printing technology were not sufficiently advanced to enable antigen, protein and antibody microarrays to be reliably printed with diagnostic grade signal;
- •
- Users could not reliably or consistently measure multiple biomarkers (specifically, antigens, proteins and antibodies) in the same test because these biomarkers have very different surface charge and binding characteristics; and
- •
- High levels of variability were likely related to the lack of standardization and control of titer plate-based ELISA tests leading to inaccurate or less predictable outcomes.
We believe that we have addressed these problems with our platform that allows us to detect and measure multiple antibodies and their different sub-types in the same two-dimensional planar microarray. Our use of specialty surface treatments and surface-coating processes allows us to create microarrays with consistent spot characteristics and limited background noise (in other words, with high signal to noise ratios). Our two dimensional planar array design securely fixes all reagents used in an assay to the glass surface independently of one another, which eliminates unwanted biochemical interactions. Our IgXPLEX technology can detect different antibody isotypes (IgA, IgG, IgM) simultaneously from a single microarray spot using multiple detection wavelengths of light. Our calibration technology adjusts each microarray for every test, which reduces the variability that may result from environmental factors or from the use of external calibrators. We have enhanced the precision of our microarrays through the use of our system, which uses a statistically valid number of
5
replicate spots for each biomarker being tested in combination with complex software algorithms. We believe that our technology delivers consistent, repeatable, precise test results and can measure the concentration of multiple biomarkers in a single test. Our automated technology can analyze multiple patient samples simultaneously using less time, effort, and consumables than existing titer plate technology.
Three Year History
The list below describes the development of the Company's business over the last three completed financial years.
| | |
The Company completed a private placement of 2,439,500 shares at a price of $1.50 per share for cash proceeds of $3,349,700 and also issued broker warrants for 194,200 common shares. | | June 2008 |
The Company received its license from Health Canada for the SQiDworks Diagnostics Platform. See "— Government Regulation". | |
November 2008 |
The Company received its license from Health Canada for the QuantiSpot Rheumatoid Arthritis Assay. See "— Government Regulation". | |
November 2008 |
The Company completed the first tranche of a non-brokered private placement of 2,400,000 common shares at a price of $1.25 per share for gross proceeds of $3,000,000. | |
December 2008 |
The Company completed the second tranche of a non-brokered private placement of 1,331,500 common shares at a price of $1.25 per share for gross proceeds of $1,664,375 and also issued 106,520 warrants. | |
January 2009 |
The Company's IgX PLEX Quantitative Assay and SQiDworks Diagnostics Platform were European Conformity ("CE") marked and registered. See "— Government Regulation". | |
February 2009 |
The Company's 510(k) submission for IgX PLEX Rheumatoid Arthritis Qualitative Assay and SQiDworks Diagnostics Platform was cleared by the U.S. Food and Drug Administration ("FDA"). See "— Government Regulation". | |
October 2009 |
The Company completed a private placement of 2,398,104 units at a price of $2.75 per unit for gross proceeds of $6,594,786. Each unit was comprised of one common share and one half common share purchase warrant. See "Market for Securities". | |
December 2009 |
The Company completed a private placement of 2,280,000 units at a price of $2.50 per unit for gross proceeds of $5,700,000. Each unit was comprised of one common share and one half common share purchase warrant. See "Market for Securities". | |
August 2010 |
The Company received its license from Health Canada for the IgX PLEX Celiac Qualitative Assay. See "— Government Regulation". | |
September 2010 |
The Company received its license from Health Canada for the IgX PLEX Celiac Panel. See "— Government Regulation". | |
April 2011 |
The Company received confirmation that its IgX PLEX Celiac quantitative assay had been CE marked and registered. See "— Government Regulation". | |
June 2011 |
The Company's 510(k) submission for IgX PLEX Celiac Qualitative Assay and SQiDworks Diagnostics Platform was cleared by the FDA. See "— Government Regulation". | |
June 2011 |
6
DESCRIPTION OF THE BUSINESS
General
We are a life sciences company that develops and commercializes proprietary technologies and products for advanced microarray diagnostics. Our goal is to become a leader in the development and commercialization of microarray and multiplexed diagnostics by offering our customers a comprehensive "turnkey" solution that increases the efficiency and ease of diagnostic testing and test development.
Our target customers — clinical, academic and diagnostic development laboratories — require diagnostic processing equipment and consumable tests ("systems") that are capable of processing large numbers of patient samples at low cost and with minimal labour requirements ("high-throughput systems"). High-throughput systems have not been widely employed in autoimmune disease, allergen or companion diagnostics testing and only limited use of high-throughput systems exists in infectious disease testing. To our knowledge, no fully-automated high-throughput systems exist that are capable of addressing the combined multiplex testing needs of these markets. A fully-automated system capable of providing multiple biomarker measurements in a single test array has the potential to increase a laboratory's throughput with significantly less labour, consumables and other costs.
Our proprietary microarray tests and fully-automated instruments are designed to simplify antigen, protein and antibody testing workflow, increase throughput and reduce costs, all while providing excellent data quality. In many instances, our technology enables analysis that was traditionally unavailable.
Our principal lines of business include:
- •
- the development, manufacturing, and marketing of fully-automated diagnostic instruments (our "platform");
- •
- the development and marketing of tests for the autoimmune, allergen, infectious disease and companion diagnostics testing markets (our "assays"); and
- •
- the commercialization of microarray printing technology (our "printing solutions").
Our Platform
Our FDA-cleared SQiDworks platform is comprised of three key elements: a proprietary one-time-use consumable microarray device, a validated and integrated instrument system that fully-automates the processing of patient samples in the microarray device, and a proprietary software processing system that processes and analyses the array and reports the presence or absence of a biomarker ("qualitative testing") and/or the amount of biomarker present ("quantitative testing"). Our SQiDworks platform can perform qualitative or quantitative testing for multiple biomarkers simultaneously. Our SQiDworks platform is currently the only FDA-cleared, fully-automated, microarray system and multiplexing solution in our target markets and we believe there are significant barriers to entry in these markets. We believe that our SQiDworks platform addresses many of the key challenges faced by our customers today by delivering accurate patient results in less time and with significantly reduced labour, consumables and other costs.
We are developing SQiDlite, our fully-automated, bench-top diagnostic platform for both IVD and research use only ("RUO") applications. We are also currently commercializing SQiDman, our small semi-automated platform for processing microarrays, to be used by research customers or development partners for RUO applications in the microarray development process.
Our Assays and the Development of Our Test Menu
We have received regulatory clearance to market our qualitative rheumatoid arthritis and celiac assays in the United States, our qualitative and quantitative rheumatoid arthritis and celiac assays in Canada, and our quantitative rheumatoid arthritis and celiac assays in the European Union for use on our SQiDworks platform. We have a robust pipeline of autoimmune tests and one companion diagnostic test in development, and plan to develop a broad test menu for autoimmune disease, companion diagnostics, infectious disease and allergen testing markets in the future. Our current in-development tests include multiplexed panels to aid in the diagnosis
7
of vasculitis, lupus, Crohn's disease and inflammatory bowel disease ("IBD") and in the management of treatment of autoimmune affected patients with a class of drugs referred to as "anti-TNF drugs" such as Remicade®, Enbrel® and Humira®. We are currently developing more sensitive rheumatoid arthritis and celiac assays that measure additional biomarkers.
We have targeted these testing markets because we believe:
- •
- healthcare providers require the measurement of multiple biomarkers to aid in the diagnosis and therapeutic monitoring of these diseases;
- •
- these testing markets are underserved by fully-automated, high-throughput, multiplexed systems;
- •
- tests in these markets are generally run at sufficiently high volumes in larger test facilities that would benefit from both multiplexing and automation; and
- •
- these tests typically qualify for private and public reimbursement in Canada, the United States, and the European Union.
One of our key operational goals is to continue to develop and seek regulatory approval for additional tests, as we believe that expanding our "test menu" will drive adoption of our platform and products by customers.
The development of our test menu is augmented by our partnerships with leading research institutions, including Beth Israel Deaconess Medical Center, the Cleveland Clinic, and the University of North Carolina. Our partnerships provide us with many advantages, including the rights to approximately 10,800 patient samples, which aid us in the development and validation of our assays.
We also offer development services and manufacturing of diagnostic kits and microarrays to our customers. We will convert content of laboratories and third parties into microarray products, manufacture microarray test kits for sale to these customers and, in the case of third parties, enable them to re-sell our high throughput systems on which these proprietary assays can be run. Our laboratory customers may use these custom microarray components to perform diagnostic test services using our platforms and may subsequently sell the results to their customers.
The Market for Our Products
The diagnostic products and services market is increasing in importance, complexity, breadth and size. Diagnostics are critical to high-quality healthcare and guide a majority of clinical decisions.
Over the last forty years, the number of biomarkers that can be measured by commercial laboratories has grown dramatically, with less than 4,000 different types of biomarker measured in the 1970s, more than doubling to approximately 10,000 with automation in the 1980s, and increasing to approximately 25,000 in the early 2000s.
Since 1976, the number of biomarkers measured by assays cleared through the FDA's 510(K) process to aid in the diagnosis of the six autoimmune diseases that comprise our target market has increased from four to 48. This represents an increase in the average number of biomarkers per disease state in our autoimmune disease target market from 0.7 to 8. We believe that this increase in biomarkers is indicative of a healthcare trend whereby healthcare providers are seeking to run diagnostic tests for increasing numbers of biomarkers to assist in the diagnosis of disease.
Researchers and laboratories are accelerating the rate at which new biomarkers are being commercialized for the known set of diseases being diagnosed today. It is estimated that an additional 30,000 biomarkers (molecular and protein) in various stages of development have been identified and not yet commercialized. We believe that the increased use of existing assays and the introduction of new assays will drive growth of the diagnostics testing market, a market that is estimated to have a growth rate of approximately five to nine percent annually from 2009 to 2014.
Our strategy is to focus initially on the immunoassay segment of the diagnostic test market since there are no multiplexed, microarray, IVD solutions in the immunoassay space. The 2012 global immunoassay diagnostic test market is estimated to be US$10.3 billion, of which approximately US$4.5 billion is within our strategic
8
market focus, which includes tests for autoimmune disease, infectious disease, and allergen tests. These three markets are estimated to be US$1.5 billion, US$2.4 billion and US$0.6 billion, respectively.
Autoimmune Disease Tests
Our primary testing market in the immunoassay segment is for products that aid in the diagnosis of autoimmune disease, which is estimated to be approximately US$1.5 billion per year. We have targeted the autoimmune testing segment for the following reasons:
- •
- there are ten autoimmune disease states, which include the autoimmune diseases in our target market, that command most of the blood testing revenues for the entire autoimmune testing segment;
- •
- the most common tests for autoimmune diseases currently evaluate approximately 70 biomarkers, representing an average of approximately seven biomarkers per disease;
- •
- these tests are generally run at high volumes at larger test facilities and in batches and are not typically run on a "one off" basis;
- •
- generally, autoimmune disease tests have an established reimbursement model from private and public healthcare payers;
- •
- each of the autoimmune diagnostic tests we are developing has "predicate" technologies with FDA clearance on older, single biomarker, manual titer plate technology that we intend to replace;
- •
- regulatory clearance for the majority of autoimmune assays is through the FDA 510(k) process, which is well established; and
- •
- autoimmune disease tests are regulated as Class II devices by the FDA. Class II diagnostic clearance is generally obtained following a shorter review period than for a Class III device. See "— Government Regulation".
Infectious Disease and Allergen Tests
We also plan to enter the immunoassay segment of the infectious disease diagnostics market, which is estimated to be approximately US$2.4 billion for 2012, followed by the immunoassay segment of the allergen diagnostics market, which is approximately US$0.6 billion for 2012.
Print Services
According to a press release issued by F. Hoffmann-La Roche Ltd., in 2007 the estimated market for protein and molecular microarray print equipment and services was approximately US$600 million per year.
RUO and Lab -Developed Tests
Approximately 34% of the US$3.7 billion U.S. annual market for RUO and lab-developed tests is directly addressable by our proprietary technologies. Additionally, we believe that the US$2.2 billion European market for RUO and lab-developed tests has approximately the same segmentation as the U.S. market. As such, we anticipate that the combined U.S. and European markets for RUO and lab-developed tests that are directly addressable by our proprietary technologies to be approximately US$2 billion.
Companion Diagnostics
We believe that the commercialization of "companion diagnostics" tests is a meaningful market opportunity for us. Autoimmune diseases are increasingly being treated with antibody-based or other biologic drugs. Often, there are different variants of the antibody or biologic drugs. For example, anti-TNF-based drugs are used to treat rheumatoid arthritis, Crohn's disease and IBD. The market for anti-TNF drugs was estimated to be approximately US$16 billion per year in 2008. Anti-TNF drugs are currently marketed under brand names such as Remicade®, Enbrel® and Humira®, which represented more than 99% of the anti-TNF drug market in 2008. The effectiveness of the treatment of autoimmune patients with these drugs may be enhanced by the monitoring of the concentration of these drugs in a patient's blood by a "companion diagnostic" test. Our multiplexing
9
technology allows us to combine the tests for both the diagnosis and therapeutic monitoring of a patient's disease. We expect this test combination to result in significantly less labour, consumables and other costs and provide us with a large market opportunity.
Diagnostics
Assays are used world-wide to assist healthcare providers in preventing, diagnosing and treating diseases. In order to be able to determine whether someone has a particular disease, a typical assay will test for "biomarkers", a term used to refer to a specific antigen, protein or antibody. The amount or "concentration" of the biomarker in a person's blood usually indicates one or more of the following: the predisposition or risk of getting the disease; presence or absence of the disease; and severity of the disease. For example, assays have been used for more than 50 years to detect the presence of rheumatoid factors, which are diagnostic markers for rheumatoid arthritis. The use of assays to detect rheumatoid factors first received 510(k) clearance by the FDA approximately 35 years ago. Rheumatoid factors can be detected in the blood of a patient before the first symptoms appear, can indicate the progression of the disease, and provide information that may guide a healthcare provider's determination of a patient's treatment plan.
Diagnostics "platforms" refer to the specific technology or instrument system on which a consumable device is "run" to generate test results. There are a number of different platforms on which assays can be run. For example, ELISA plates and readers, ELISA bead technology and polymerase chain reaction are all platforms for running assays.
A microarray is a device that is used to perform multiple tests simultaneously. A microarray is comprised of two principal elements.
- •
- A surface upon which capture molecules are printed in the form of spots. The surface is typically glass or plastic and is chemically coated to more easily "accept" and bind the molecules being printed without damaging the molecules.
- •
- A physical superstructure to separate individual wells. Each well contains numerous spots. Each of the spots within an individual well is analogous to a traditional microtiter plate test well.
The biological and chemical reactions that occur in each test well generate multiple test results. Each of these test results is a billable event for our customers.
Limitations of Current Technologies
Clinical, academic, and diagnostic development laboratories are faced with increasing numbers of tests or experiments required to support a diagnostic conclusion or development decision. In the diagnostic setting, there are a growing number of biomarkers that are used for each patient to aid in the diagnosis and monitoring of many diseases. This has led to a growing workload of increasingly complex and costly diagnostic tests for laboratories. Additionally, laboratories face pressure to reduce overall healthcare system costs.
Our target customers — clinical, academic and diagnostic development laboratories — require diagnostic processing equipment and consumable tests that are capable of processing large numbers of patient samples at low cost and with minimal labour requirements. High-throughput systems have not been widely employed in autoimmune disease or allergen testing and limited use of high-throughput systems exists in infectious disease testing. To our knowledge, no high-throughput systems exist that are capable of addressing the combined multiplex testing needs of these markets. A fully-automated system capable of providing multiple biomarker measurements in a single test array has the potential to increase a laboratory's throughput with significantly less labour, consumables, and other costs. We believe these cost savings would be realized because, among other things:
- •
- a fully-automated system reduces "hands-on" technician time;
- •
- the volume of liquid reagent is less and the number of other consumables, such as pipette tips, required to process one microarray is fewer when compared to traditional methods of running multiple tests in multiple titer plates;
10
- •
- the amount of serum required per patient to produce results on a 12 biomarker test is approximately 240 times greater using titer plates than using microarray plates;
- •
- a laboratory does not incur the expense of sending some tests of a larger multiplex panel to other labs;
- •
- the cost of management of multiple samples being tested by multiple technicians and combining the results to a single report would be largely eliminated by multiplexing capability; and
- •
- reducing the number of hands-on steps through automation would potentially reduce error rates.
Microtiter Plates
Laboratories almost exclusively use microtiter plates to perform immunoassay tests. The use of microtiter plates restricts an immunoassay test to the analysis of a single biomarker. Microtiter plates are predominantly processed with a high contribution of direct labour. This increases the time to produce a test result and the potential for error. Since equipment available to reduce labour is limited, the use of microtiter plates restricts the throughput of a laboratory. We estimate that 60% of our target customers' costs to produce a diagnostic result are due to direct labour.
Skilled laboratory labour capable of performing diagnostic testing in our target markets is limited and is expected to contribute to laboratories' growing cost structure at an increasing rate. The United States Department of Health and Human Services reported in 2009 that by 2012, 138,000 lab professionals, including medical technicians and laboratory assistants, will be needed, but fewer than 50,000 will be trained.
Multiplex Diagnostic Tests
A solution to the cost and labour constraints of immunoassay testing for single biomarkers is to perform diagnostic tests for multiple biomarkers simultaneously in a single assay (a "multiplex diagnostic test"). The processes used to complete a single test within a diagnostic panel of several biomarkers are complex and technically difficult. Such complexity has limited the throughput and efficiency of multiplex diagnostic testing, and technical challenges have restricted the widespread adoption of automated systems. Most laboratories do not use automated processing equipment to augment their workforce in our key target markets where the measurement of multiple antigens, proteins or antibodies is required.
Bead-Based Array Systems
Bead-based array systems were initially developed for DNA-based testing and were subsequently adapted for protein-based and antibody-based testing. Bead-based methods, however, have faced limitations that reduce their utility, particularly for multiplex diagnostics and experimentation. In particular, the workflow for bead arrays is complex, time consuming and costly. For example, standard protocols for a nine-biomarker bead array require multiple complex operations including approximately 190 steps and approximately five hours of "hands-on" time to complete.
Automated Systems
While laboratories use automated systems for many types of blood tests, to our knowledge, there are no fully-automated high-throughput microarray systems. A number of diagnostics companies have developed automated single-plex fully-automated, bench-top systems for autoimmune testing and we are only aware of one bead-based automated multiplex system.
11
Antigen, Protein and Antibody Microarrays and Their Technical Challenges
An alternate to bead-based array systems is a microarray printed on a two dimensional substrate. These antigen, protein and antibody microarrays solve several limitations of bead-based systems such as interactions between beads, but are challenging to develop as we believe there are several technological obstacles, including:
- •
- precision and accuracy of printing;
- •
- cross interference of similar molecules in the system;
- •
- calibration and standardization of multiple separate molecular signals in a single microarray well; and
- •
- complex software systems required to measure and analyze the multiple signals measured within the microarray.
Precision and Accuracy of Printing
Microarray printing has been traditionally done with contact printers that, like a quill pen, physically "spot" the biomarker capture molecules onto the microarray surface. These contact methods of printing are imprecise and lead to microarrays with high degrees of variability and significant background noise. This reduces the commercial applications of contact spotted microarrays. Contact spotting does not typically lead to IVD capable microarrays in antigen, protein and antibody applications. Some earlier versions of non-contact printers are also used today, but these technologies have similar drawbacks to contact spot printers.
Cross Interference
The ability to discriminate among different "isotypes" (types) of individual target antibodies and different antigens and proteins is important to the measurement of biomarkers and it has proven challenging to develop multiplex tests that discriminate between these different entities in a single microarray.
Calibration and Standardization
The processing of multiplex arrays is complex and is prone to high degrees of variation between test arrays. This error potentially leads to reduced test performance and has restricted the use of multiplex arrays almost exclusively to the research market where there is a tolerance for less accurate measurements.
Diagnostic approaches for measuring multiple antigen, protein or antibody biomarkers for large numbers of patients are generally unavailable or are not cleared or approved as IVD tests. Multiplexing, as it is available today, does not adequately address antigen, protein and antibody focused measurement. Most multiplexing technologies have been developed and commercialized for the processing of high-density genetic or molecular arrays. These technologies generally work from a different set of technological principles than those needed to measure multiple antigens, proteins or antibodies. Further, these technologies are used in a research setting and are generally inadequate for use in commercial diagnostic testing.
Software Systems
The sophistication of the automated systems and processing software required to analyze microarrays has restricted the commercial viability of microarrays in diagnostic laboratories.
Our Solution
Our proprietary microarray tests and automated instruments are designed to simplify antigen, protein and antibody testing workflow, increase throughput, reduce costs and provide excellent data quality. In many instances, our technology enables analysis that was traditionally unavailable.
Traditional biomarker testing methods using microtiter plates require approximately four minutes on average of technician "hands-on" time per biomarker per patient ("test effort"). For example, a four biomarker test for celiac on samples from 74 patients would, on average, require eight microtiter plates, assuming each patient's blood sample is tested in duplicate using standard good laboratory practices. This would have traditionally required approximately 20 hours of direct "hands-on" labour using predicate technology.
12
Our Fully-Automated SQiDworks Diagnostic Platform
Our high-throughput SQiDworks diagnostic platform is a fully-automated microarray processing and analytical system capable of 888 results per hour. By way of example, for a celiac assay that measures four biomarkers, our SQiDworks platform requires approximately 30 seconds of technician "hands-on" time per patient per panel compared to approximately 16 minutes of technician "hands-on" time using microtiter plate technology, and uses one microarray kit instead of 8 single biomarker microtiter kits to generate diagnostic results for the four biomarkers. The difference between the approximately 20 hours of direct hands-on labour using microtiter plate technology and the approximately 30 minutes of direct hands-on labour using our system results in significant cost savings, as does using one microarray kit rather than eight microtiter kits.
Additionally, the cost savings demonstrated above increase as the number of biomarkers per diagnostic test increase. For example, for a lupus test that measures twelve biomarkers, our SQiDworks platform requires the same amount of technician "hands-on" time as the celiac assay, and also requires only one microarray kit. However, microtiter plate technology would require approximately 48 minutes of technician "hands-on" time per patient per panel and the use of 24 single biomarker microtiter kits. This equates to a reduction of approximately 59 hours of direct hands-on labour using our system as well as greater savings with respect to consumables.
Our IgXPLEXMicroarrays
Our IgXPLEX microarrays have the ability to discriminate between individual isotypes of antibodies and antigens and proteins within a single well of a microarray, resulting in the measurement of multiple biomarkers in a single test.
Our microarray technology requires the use of significantly less patient blood than traditional methods. Our system requires a ten microliter drop of blood to be processed from a single sample tube, compared to the multiple milliliters of blood used by traditional methods. Our system dispenses the single drop of blood once per patient in a microarray well. Traditional methods require multiple blood samples to be manipulated into multiple test vessels over many steps. The reduction in processing steps and the ability to generate simultaneous multiplexed measurements from a single drop of blood increase the predictive value of the test. The increased predictive value of the test would allow the healthcare provider to choose a treatment plan earlier in the course of the disease.
Our IgXPLEXCHEX Technology
Our IgXPLEXCHEX technology provides multiple in-microarray checks to ensure that the test has been completed without system, control, calibration or microarray-related errors. These tests reduce errors that are common to microtiter and microarray tests.
Our proprietary multiplex assay development processes and microarray manufacturing capabilities, combined with our automated instruments, are designed to significantly reduce the complexity and cost to our customers to commercialize microarray tests using their own biomarkers. Customers with a need to perform large numbers of experiments may benefit from the use of our microarrays in their screening and development programs when traditional methods may be too onerous due to the large number of single tests that would be required.
Our Key Competitive Strengths
We believe that our key competitive strengths include the following:
- •
- Only FDA Cleared, Fully-Automated Microarray Processing System. We have received marketing clearance from the FDA, a Canadian regulatory medical device licence, and have CE marked our SQiDworks instrument system. SQiDworks is the only system for automatically processing, analyzing and providing test results in protein-based and antibody-based microarrays to achieve these regulatory clearances.
- •
- Fully-Integrated, High-Throughput Solution Dramatically Reduces Workflow and Cost. Our proprietary microarray tests and fully-automated instruments are designed to significantly simplify antigen, protein
13
and antibody workflow, increase throughput and reduce costs, all while providing excellent data quality. For example, competing systems require hundreds of steps to produce the same number of test results as can be produced by our SQiDworks fully-integrated diagnostics system in five steps.
- •
- Expertise in Microarray Assay Development. We have clinically validated development experience and have created processes, systems and development algorithms to simplify the commercialization of our products. We believe our expertise will reduce the time required to complete the commercial development of our pipeline products. We also believe that our experience can be applied to RUO products to allow us to commercialize our own and other's content in significantly less time than our competition.
- •
- Focus on Penetrating Existing Markets that Are Technically Challenging and Have Established Reimbursement. We focus on disease testing markets that have existing reimbursement and payment programs in place, which allows us to quickly bring our products into established markets. Additionally, antigen, protein and antibody multiplex tests are more technically challenging to develop, which may restrict others from easily entering these markets.
- •
- Partnerships with Leading Institutions. We have entered into agreements with leading institutions and healthcare providers to collaborate with us in developing and validating our assays including providing us with access to approximately 10,800 clinically validated patient blood samples.
- •
- Significant Intellectual Property Portfolio. Our intellectual property covers key areas of our commercial business as well as our pipeline, including microarray surfaces, multiplexing, microarray analytics, and microarray processing. We have issued patents and pending patent applications that cover aspects of our microarray technology for analyzing, identifying and quantifying analytes. We are actively seeking protection for our methods of achieving high-throughput quantitative analysis for our proprietary methods for making microarray substrates and for the systems that carry out these methods for manufacturing microarray substrates. In the course of improving our products, from time to time we develop processes and systems that provide us with a competitive advantage and the Company will keep such developments confidential.
Products
SQI Platforms
| | | | | | | | |
| |
| | Regulatory Status |
|---|
| | Development
Status |
|---|
Product | | Canada | | United States | | Europe |
|---|
SQiDworks | | Complete | | Licensed | | Cleared as a system with IgXPLEXRA | | CE Marked |
SQiDman | | Development RUO | | Not Required — RUO | | Not Required — RUO | | Not Required — RUO |
SQiDlite RUO | | In development | | Not Required — RUO | | Not Required — RUO | | Not Required — RUO |
SQiDlite IVD | | In development | | To be filed | | To be filed | | To be filed |
SQiDworks
We have developed our fully-automated SQiDworks platform to enable laboratory customers to generate multiple patient test results with less than one unit of traditional "test effort". "Test effort" refers to the number of steps required to perform a test using microtiter plate technology.
We have received marketing clearance from the FDA, a medical device license from Health Canada, and have CE marked our SQiDworks instrument system. SQiDworks is the only fully-automated microarray processing system to achieve these regulatory clearances.
Our SQiDworks platform integrates a microfluidics station, an automated microarray scanner, our drying device and our proprietary processing and analytic software.
The microfluidics station automatically performs sample handling and processing of the patient serum sample and prepares our IgXPLEXmicroarray for scanning and analysis.
14
Our dryer technology dries the microarray surface, which uniformly enhances the signal to noise ratios of our microarrays. The microarray scanner reads the various IgXPLEXmicroarray devices.
Our proprietary software uses complex algorithms to locate the array of spots containing reagents and to process the spots to measure each of the biomarkers of interest. This measurement can be either qualitative or quantitative.
SQI Assays
Our four "cleared" or "approved" assays, two for rheumatoid arthritis and two for celiac, test for two of the more common autoimmune diseases. Rheumatoid arthritis is a chronic inflammatory disease, the cause of which is unknown. The incidence of rheumatoid arthritis in North America is 1.4 cases per 100,000 in men and 3.6 cases per 100,000 in women. The prevalence of rheumatoid arthritis is approximately 2% world wide. It is estimated that total size of the patient market in the United States for rheumatoid arthritis diagnostic testing for 2010 was approximately six million patients. Celiac is an inherited autoimmune disorder that affects the digestive process of the small intestine. It is estimated that the total size of the patient market in the United States for celiac diagnostic testing for 2010 was approximately 3.5 million patients.
Our currently cleared or approved assays are as follows:
| | | | | | | | |
| |
| | Clearance/Approval Status |
|---|
| | Development
Status |
|---|
Product | | Canada | | United States | | Europe |
|---|
IgXPLEXRA
(Qualitative) | | Complete | | Licensed | | Cleared | | N/A |
IgXPLEXRA*
(Quantitative) | | Complete | | Licensed | | To be filed | | CE Marked |
IgXPLEXCeliac
(Qualitative) | | Complete | | Licensed | | Cleared | | N/A |
IgXPLEXCeliac
(Quantitative) | | Complete | | Licensed | | To be filed | | CE Marked |
- *
- Marketed in Canada under the name "QuantiSpot Rheumatoid Arthritis"
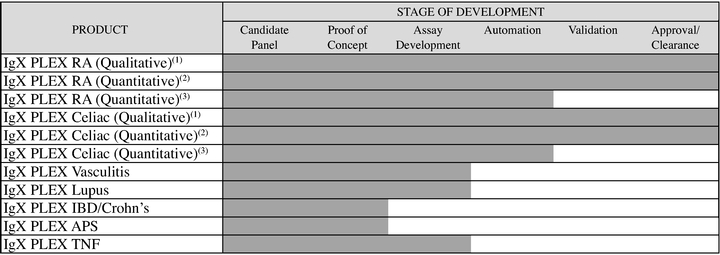
We also have IgXPLEXLupus, IgXPLEXVasculitis and IgXPLEXTNF products in the development stage and IgXPLEXIBD/Crohn's and IgXPLEXAntiphospholipid Syndrome ("APS") products at the proof of concept stage. We also have rheumatoid arthritis and celiac assays with greater sensitivity that measure additional biomarkers in development.
The table below sets out estimates by Frost and Sullivan of the total number of patients in the United States who required diagnostic testing for diseases for which we are developing diagnostic tests.
| | | | | | | | | | | | | |
Autoimmune Segment | | Lupus | | Vasculitis | | Anti-TNF | | IBD/Crohn's | |
|---|
Estimated Total Patient Market 2010 (US) | | | 5,000,000 | | | 400,000 | | | 3,100,000 | | | 550,000 | |
Research and Development
We have assembled an experienced research and development team at our Toronto facility with the scientific, microarray printing, immunoassay, engineering, software, and process development expertise that we believe is necessary to grow our business.
Platform Development
SQiDman is our small semi-automated platform that we are commercialising for RUO purposes, and SQiDlite is our fully-automated, bench-top diagnostic platform.
SQiDlite. Our engineering development team is currently focused on the near term delivery of SQiDlite, our fully-automated, bench-top diagnostic platform. This platform will be a fully-automated microarray
15
processing and analytic platform. We expect that SQiDlite will be able to process multiple sizes of microarray devices from single eight-well strips up to a single 96-well microarray plate.
SQiDlite is designed to serve the following markets:
- •
- IVD Customers who wish to run IgXPLEX assays in flexible batch sizes up to and including 96 patients per test kit. We believe that many hospitals and other laboratories would benefit from SQiDlite's more flexible batch size capability. SQiDlite is designed to run tests in volumes per run of eight to 96 patients in multiples of eight. We believe that a platform that allows for scalable testing volumes for our assays would increase the number of our potential hospital customers in North America from the largest 1,000 to the largest 5,000 by diagnostic testing volume.
- •
- Customers who seek a customizable automated microarray analyzer for RUO purposes. We believe that many RUO customers would benefit from an automated microarray analyzer that can be used in a configurable manner to optimize the development of assays. However, once assays are developed, many RUO customers seek to "lock" the settings to prevent modifications by the platform's operator. Our SQiDlite platform has been designed to accommodate these RUO customers and provide this functionality.
SQiDman. We are currently commercializing SQiDman on a pilot basis. SQiDman, our small semi-automated platform for processing microarrays, is intended to be used by research customers or development partners for RUO purposes in the microarray development process. We currently use SQiDman systems for in-house development and quality control processes. We expect the full commercial launch of SQiDman for RUO purposes in late 2011, following the enhancement of its user interface software.
Microarray Assay Development
The largest component of our current research and development efforts is microarray development. We plan to continue to focus on the commercialization of microarray content that can be run by our customers on our diagnostic platforms. Our research and development efforts are focused on the following areas:
Autoimmune Disease. We have a broad menu of autoimmune disease tests in our microarray development pipeline to augment our current product offerings. For example, in addition to our products for which we have marketing clearance or approval, we have IgXPLEX Vasculitis, IgXPLEX Lupus, and IgXPLEX TNF products in the development stage and IgXPLEX IBD/Crohn's and IgXPLEX APS at the proof of concept stage. Additionally, we have rheumatoid arthritis and celiac assays with greater sensitivity that measure additional biomarkers in development. See "— SQI Assays" for a description of the markets for the autoimmune disease tests in our microarray development pipeline.
Infectious Disease. We plan to develop tests in the area of infectious disease. Infectious disease panels leverage our strengths in multiplexing, antibodies and high-throughput diagnostic systems. We have scientists and assay development specialists with experience in infectious disease assay development.
Allergen Testing. Allergen tests are very similar to, and depend upon many of the same technological advances as, autoimmune disease tests. Because allergen panels have large numbers of biomarkers, we believe this is an excellent area of opportunity to apply our multiplexing and microarray technology.
Custom Assay Development and Print Services. We plan to add assay design and print services to our product offerings. This expansion is intended to enable our laboratory and diagnostic customers to expand their use of our platforms by converting their content to microarrays. We believe that applying our in-house processes and systems to develop microarray formatted tests incorporating customers' content will allow them to reduce their assay costs with less development risk and effort by purchasing their microarrays and development services directly from us. For example, our customers will be able to add requested target biomarkers to an existing panel of biomarkers or they may request an entire panel of protein-based or antibody-based biomarkers to be developed into a RUO microarray that they may use as a lab-developed test.
16
- (1)
- Approved or cleared in the U.S. and Canada.
- (2)
- Approved or cleared in Canada and Europe.
- (3)
- Development status for clearance in the U.S.
Our Strategy
Our goal is to become an industry leader in the development and commercialization of microarray and multiplexing IVD medical systems. We intend to accomplish this goal through:
- •
- Product development efforts. We intend to continue to focus our research and development on high-volume, high-margin, multiplexed tests for diseases for which there are existing tests that have reimbursement programs in place.
- •
- Strategic market penetration. We have identified and are marketing our turnkey SQiDworks platform and approved assays to a specific group of laboratories that process high volumes of tests and typically adopt new technologies to gain market share. Please see "— Sales and Marketing" for a description of our target laboratories.
- •
- Leveraging our core technology and expertise to access new markets and new customers. Our technology and microarray development processes enable us to provide customized third party microarray formatted test development and manufacturing services.
- •
- Seeking partnerships and strategic acquisitions. We intend to continue to seek partnerships that will enable us to expand into new markets, broaden and deepen our lines of business and develop and strengthen our relationships with our customers.
- •
- Leading through innovation. We intend to continue our research and development in each of our lines of business in order to become the industry leader in multiplexing microarrays.
Strategic Alliances
We have a number of collaboration agreements with leading global research and treatment institutes. These collaborations improve our ability to develop products by providing us with access to patient serum required for assay development, verification of products in development and final product clinical validation.
17
The following table provides an overview of our partnering collaborations and the relevant pipeline product as at June 15, 2011:
| | | | |
Partner Institute | | Pipeline Product | | Purpose |
|---|
Cleveland Clinic | | Rheumatoid arthritis, IBD | | Serum Samples
Clinical Validation
Collaboration |
Beth Israel Deaconess Medical Center | | Celiac, anti-TNF | | Serum Samples
Collaboration / Publication |
Hospital Clinic De Barcelona, Spain | | Vasculitis | | Serum Samples
Collaboration / Publication |
University Hospital Maastricht, The Netherlands | | Vasculitis | | Serum Samples
Collaboration |
The University of North Carolina at Chapel Hill | | Vasculitis | | Collaboration
Serum Samples
Clinical Validation |
We collaborate with these partners to assist our development of new candidate biomarkers for future tests and intend to continue to seek additional alliances and partnerships. We have been active in strategically publishing our progress and successes both alone and in collaboration with certain of our partners.
Sales and Marketing
We currently have a three-person marketing team. Our marketing efforts are focused on generating sales of our currently cleared or approved system, which includes SQiDworks, IgXPLEX RA and IgXPLEX Celiac, to targeted, high-volume laboratories in North America and Europe.
We have identified the 400 largest laboratories, by testing volume, of the approximately 14,000 laboratories that provide blood testing services in North America and a select group of approximately 30 European laboratories. We believe this group contains laboratories that would readily adopt new technologies, look to obtain an economic advantage over their competitors, and attempt to increase their market share of diagnostic testing services. We believe that this initial target group has sufficient testing volume to support the use of SQiDworks, our currently cleared or approved tests, and the tests that we anticipated to be cleared or approved by the end of 2011. We use various marketing strategies to provide incentives to the laboratories in our initial target group that are among the first to adopt our technology. As we expand our test menu, we intend to broaden our target market and reduce the incentives provided to the early adopters of our products.
Customers
We focus on two customer markets:
- •
- Diagnostic Markets — this market consists of reference and other diagnostic laboratories that provide for-profit diagnostic blood tests.
Customers in this market seek to purchase a full diagnostics solution, including automated platforms and IVD assays. These customers sell the results produced by running our IVD assays on our automated platforms. In addition, customers in this market seek to purchase automated platforms and RUO assays that they intend to convert into lab-developed tests that incorporate their biomarkers. These customers would run these lab-developed tests on our automated platforms and sell the results to their customers.
- •
- Life Sciences Markets — this market consists of customers seeking to commercialize their biomarkers as turnkey multiplexed products delivered by our contract development and manufacturing services. The customers in this market are expected to buy our automated platform and consumable microarray test kits, and to pay a royalty to us on their sales.
18
There are two types of customers in the life sciences markets:
- •
- Contract Manufacturing and Development Customers — these customers seek expertise for pilot and scale up microarray printing, and have needs in the molecular or protein/antibody areas; and
- •
- OEM customers — these customers seek full contract development and manufacturing services to commercialize biomarkers owned by them and seek high-throughput automated microarray analyzers to sell through their channels.
Manufacturing
Our manufacturing facility is located in Toronto, Canada. We manufacture all of our microarray kits for commercial sale and internal research and development in our facility.
We operate a "current good manufacturing practices", or "cGMP", facility that has been certified ISO 13485:2003 compliant, which enables us to meet both regulatory requirements and customer expectations. This is an international standard developed by the International Organization for Standardization that specifies the requirements for a quality management system for organizations providing medical devices and related services. Our microarray production facility is operated to ISO Class 7 clean room specifications, commonly referred to as a Class 10,000 operating level, which defines the maximum level of particles permitted per square meter.
Based on our microarray manufacturing forecasts, we expect that there is adequate space in the current location to expand our manufacturing capacity to meet our expected needs until the end of 2012. Until a facility upgrade is completed, we intend to undertake equipment upgrades to ensure sufficient manufacturing capacity to meet our expected requirements for commercial sales and our internal validation studies until the end of 2012.
To manufacture consumable tests, we acquire raw materials and custom molecules for our assays from well-established vendors who are either ISO certified or who have an established quality system. Each incoming reagent ingredient undergoes quality testing as required and scrutiny before being released to the manufacturing unit, and the reagent ingredients are then incorporated into finished goods as an IgXPLEX kit. Each kit contains the printed diagnostic array and several self-contained wet reagents that are loaded into the SQiDworks platform prior to use by the customer. We have been producing products suitable for validation studies since the beginning of 2007.
Certain key components of our SQiDworks platform are manufactured by third parties in FDA or ISO certified facilities and we manufacture one component. We assemble the components and deliver them to our customers, where they undergo a series of quality acceptance tests.
Competition
We compete with both established and development-stage life sciences companies that design, manufacture and market basic ELISA technology, lab automation products or multiplexing technologies. For example, companies such as INOVA Diagnostics, Inc., Phadia AB, Axis-Shield plc and Hycor Biomedical, Inc. have immunoassay products based on basic ELISA technology. Companies such as Roche Diagnostics Corporation, bioMérieux SA, Siemens AG and Abbott Laboratories Inc. provide laboratory automation technology. Companies such as Luminex Corporation and Bio-Rad Laboratories Inc. also provide bead-based multiplexing technology. These companies provide products that compete in certain segments in which we sell our products. In addition, a number of other companies and academic groups are in the process of developing novel technologies for life science markets.
Intellectual Property Strategy and Position
Our core intellectual property consists of patents that cover the following:
- •
- multiplexed qualification of antigens and antibodies;
- •
- internal (in-array) calibration; and
- •
- methods to create diagnostic level spot morphology.
19
We originated our core technology. Our patent strategy is to seek broad patent protection on new developments in microarray technology, tests and systems and then later file patent applications covering new implementations of the technology and new microarray platforms utilizing the technology. As these technologies are implemented and tested, we file new patent applications covering scientific methodologies enabled by our technology.
We have developed our own portfolio of issued patents, patent applications and design patents directed to our methodologies, commercial products and technologies in development. For our core technology relating to multiplexed qualification of antigens and antibodies and internal (in-array) calibration, we have obtained issued patents and approvals in certain jurisdictions from the patent family having the title "Method to Measure Dynamic Internal Calibration True Dose Response Curves". This patent family is directed to our calibration technology that adjusts each microarray for every test, which reduces the variability that may result from environmental factors or from the use of external calibrators. Other patent applications are currently pending. Patents issuing from this patent family will be in force until July 20, 2025, provided that all maintenance fees are paid.
We have also obtained either an issued patent or an allowance in some jurisdictions directed to our core technology relating to methods to create diagnostic level spot morphology. The patent family for this core technology has the title "Method and Device to Optimize Analyte and Antibody Substrate Binding by Least Energy Adsorption". The patent family is directed to the use of specialty surface treatments and surface coating process means that can create microarrays with consistent spot characteristics and limited background noise (in other words, with high signal to noise ratios). Other patent applications are currently pending. Patents issuing from this patent family will be in force until July 20, 2025, provided that all maintenance fees are paid.
We have filed patent applications that are currently pending in several jurisdictions directed to our qualitative and quantitative rheumatoid arthritis assays. In addition, patent applications are pending in several jurisdictions directed to our products and methodologies including patent families entitled "Method for Double-Dip Substrate Spin Optimization of Coated Micro-Array Supports", "Method and Device to Remove Fluid and Vapor", "Array Fluorescence Equalization Method", "Methods for Multiplex Analyte Detection and Quantification" and "Multiplex Microarrays and Methods for Quantification of Analytes".
Government Regulation
We believe that our major markets are the United States, Canada and the European Union. The following describes the regulatory clearance or approval process for diagnostic systems in each of those jurisdictions.
United States
Research Use Only
"Research use only" components are those components in the laboratory research phase of development that are not represented as effective IVD products. These components must be labelled "For Research Use Only. Not for use in diagnostic procedures." These components are exempt from regulatory oversight in the United States. Manufacturers frequently sell these components to laboratories certified under the Clinical Laboratory Improvement Amendments, or "CLIA", which are the United States federal regulatory standards that apply to clinical laboratory testing performed on human specimens in the United States with certain exceptions, including clinical trials and basic research.
Lab-Developed Tests
CLIA-compliant labs frequently develop and validate an assay from RUO components, sold to them by manufacturers, but the lab must validate these tests and assume all liability for use on patient samples. These tests are regulated as lab-developed tests.
Diagnostic Tests
Diagnostic tests or assays, known as "in vitro diagnostic", or "IVD", products by the FDA, are those reagents, instruments and systems intended for use in the diagnosis of disease or other conditions, including a
20
determination of a person's state of health, in order to cure, mitigate, treat or prevent disease or its sequelae. These tests are intended for use in the collection, preparation and examination of specimens taken from the human body.
Diagnostic tests are classified into one of three categories for FDA clearance or market approval. These categories are based on the degree of risk they pose to humans and their importance in preventing impairment in human health.
All IVD tests are subject to the following "general" requirements or controls, as well as, in the case of Class II and Class III diagnostic tests, additional requirements (except where a particular device is expressly exempt from one or more of such requirements). General controls include:
- •
- Registering the manufacturer with the FDA;
- •
- Listing the product to be marketed with the FDA;
- •
- Manufacturing the products in accordance with quality systems regulation (also known as current good manufacturing practices);
- •
- Labelling the test in accordance with labelling regulations;
- •
- Submitting a pre-market notification (also known as a "510(k)") before marketing a test; and
- •
- Reporting of adverse events and product recalls (corrections and removal).
Class I
Class I tests are subject to the least regulatory control as they present minimal potential for harm to the user. Most Class I devices and IVD products are exempt from the pre-market notification and/or good manufacturing practices regulation. Examples of Class I IVD products include pregnancy or cholesterol tests.
Class II
The FDA defines Class II test as those for which "general controls" alone are insufficient to assure safety and effectiveness, and existing methods are available to provide such assurances. In addition to complying with general controls, Class II devices are also subject to special controls.
Class II tests often require pre-market notification under the FDA's 501(k) process, in connection with which the manufacturer submits data to the FDA to demonstrate that its test is substantially equivalent to a legally marketed test (known as a "predicate" test).
Special controls may include special labelling requirements, mandatory performance standards and post-market monitoring. Examples of Class II tests include most blood tests, such as blood tests for rheumatoid arthritis, vasculitis, lupus and other immunological tests.
Class III
Class III is the most stringent regulatory category for tests. Class III tests are those for which the FDA believes insufficient information exists to assure safety and effectiveness solely through general or special controls.
Class III devices are usually those that support or sustain human life, are of substantial importance in preventing impairment of human health, or which present a potential, unreasonable risk of illness or injury.
The FDA typically requires "pre-market approval" for these tests to ensure the safety and effectiveness of Class III tests. This type of approval would require the submission and FDA review of clinical data to assess the safety and effectiveness of the test.
Examples of Class III tests include tests for the diagnosis of many infectious diseases and cancer.
21
European Economic Area
The European Economic Area, which consists of the 27 member states of the European Union and the European Free Trade Association countries of Iceland, Norway, Switzerland and Liechtenstein, requires what is called "CE" approval (the letters "CE" stand for "conformité Européenne" ("European Conformity") for, among other things, IVD tests). Under the CE regulations, a manufacturer does a preliminary self-assessment to determine whether the products comply with all relevant legislative requirements. At its most basic, the manufacturer must do a conformity assessment, set up a technical file and sign an EC declaration of conformity. This documentation must be made available to authorities upon request. The relevant European Union directive may also require that the product be examined by a conformity assessment body.
We have completed CE marking for our IgXPLEX RA quantitative assay, IgX PLEX Celiac quantitative assay, and the SQiDworks platform, which allows us to market these assays and SQiDworks platform in the European Union. We must also implement and maintain an ISO quality management system. We received our initial ISO 13485 certification in June 2008 and have maintained this certification to date.
As in the United States, components sold without any representation of performance claims may be labelled for "research use only," and are exempt from regulatory oversight in the European Union.
Canada
The Canadian Health Protection Branch regulatory regime requires approvals and submissions similar in timing and scope to European Union CE approvals. TheMedical Devices Regulations (the Regulations), promulgated under theFood and Drugs Act (Canada), set out, among other things, the requirements for the licensing of an "in vitro diagnostic device" or "IVDD".In vitro diagnostic devices include reagents, assays and equipment used for examining specimens taken from the body. IVDDs are designated as Class I, II, III and IV, based on the degree of risk associated with their use. For example, a blood test that detects bacterial meningitis is categorized as a Class III IVDD because of the risk that a false-negative test result may cause death or long-term disability due to delayed diagnosis. Class IV IVDDs include donor screening tests for transmissible viruses such as HIV and hepatitis, which present a high public health risk.
For Class I IVDDs, all that is required is an establishment licence to manufacture the IVDD. For Class II, III and IV IVDDs, the Medical Devices Regulations require the IVDDs to meet ISO standard 13485:2003, which provides both design and manufacturing standards.
An application for a Class II medical device includes, in addition to information regarding the manufacturer and specific document, the following:
- •
- a description of the medical conditions, purposes and uses for which the device is manufactured, sold or represented;
- •
- a list of the standards complied with in the manufacture of the device to satisfy the safety and effectiveness requirements;
- •
- an attestation by a senior official of the manufacturer that the manufacturer has objective evidence to establish that the device meets the safety and effectiveness requirements;
- •
- an attestation by a senior official of the manufacturer that the device label meets the applicable labelling requirements of the Regulations;
- •
- in the case of a near patientin vitro diagnostic device, an attestation by a senior official of the manufacturer that investigational testing has been conducted on the device using human subjects representative of the intended users and under conditions similar to the conditions of use; and
- •
- a copy of the quality management system certificate certifying that the quality management system under which the device is manufactured satisfies National Standard of Canada CAN/CSA-ISO 13485:03, Medical devices — Quality management systems — Requirements for regulatory purposes.
As in the United States and Europe, components sold without any representation of performance claims may be labelled for "research use only," and are exempt from regulatory oversight in Canada.
22
Approvals/Clearances
Health Canada
We have received licenses for all applications which have been made to Health Canada to date as listed in the following table.
| | | | | | | | |
| Catalog Number | | Product | | Medical Device License | | Date of Issuance |
|---|
| | 10005 | | QuantiSpot Rheumatoid Arthritis Assay* | | | 78512 | | November 2008 |
| | 10105 | | IgX PLEX Rheumatoid Arthritis Qualitative Assay | | | 81016 | | October 2009 |
| | 10505 | | IgX PLEX Celiac Qualitative Assay | | | 83945 | | September 2010 |
| | 10515 | | IgX PLEX Celiac Panel* | | | 85930 | | April 2011 |
| | 01003 | | SQiDworks Diagnostics Platform | | | 78513 | | November 2008 |
United States
Our 510(k) submission for IgX PLEX Rheumatoid Arthritis Qualitative Assay and SQiDworks Diagnostics Platform was cleared by the FDA in October 2009 and our 510(k) submission for IgX PLEX Celiac Qualitative Assay and SQiDworks Diagnostics Platform was cleared by the FDA in June 2011.
European Union
Our IgX PLEX Celiac quantitative assay (Catalog #10515), IgX PLEX RA quantitative assay (Catalog #10005) and SQiDworks Diagnostics Platform (Catalog #01004) have been CE marked and registered with the competent authority for our authorized representative.
Specific registrations for each product will be made with the notified body of each country in which commercialization is anticipated once applicable label translations have been completed.
Employees
As of May 31, 2011, we had 54 employees, of which three were in sales and marketing, 31 were in research and development, 14 were in manufacturing and operations and six were in administration. Our employees have specialized knowledge in areas such as multiplexing, immunology, microarray design and manufacture, assay development, systems engineering and medical-systems sales and servicing.
We have never experienced a work stoppage or other labour disturbance. To our knowledge, none of our employees belongs to, or is represented by, a labour union.
RISK FACTORS
An investment in our common shares involves a number of risks. In addition to the other information contained in this Annual Information Form and in the documents incorporated by reference into this Annual Information Form, including our consolidated financial statements and related notes, you should give careful consideration to the following risk factors. Any of the matters highlighted in these risk factors could have a material adverse effect on our business, results of operations and financial condition, causing an investor to lose all, or part of, its, his or her investment.
The risks and uncertainties described below are not the only ones we face. Additional risks and uncertainties that we are not aware of or focused upon, or that we currently deem to be immaterial, may also impair our business operations and cause the trading price of our common shares to decline.
23
Risks Related to Our Business and Strategy
We have incurred losses since inception, and we expect to continue to incur losses for the foreseeable future.
We have a limited operating history and have incurred significant losses in each fiscal year since our inception, including net losses of $3.8 million, $5.9 million, $8.1 million and $4.1 million during fiscal 2008, 2009, 2010 and the six months ended March 31, 2011, respectively. As of March 31, 2011, we had an accumulated deficit of $37.6 million. These losses have resulted principally from costs incurred in our research and development programs and from our selling, general and administrative expenses. We expect to continue to incur operating and net losses and negative cash flow from operations, which may increase, for the foreseeable future due in part to anticipated increases in expenses for research and product development and expansion of our sales and marketing capabilities. We anticipate that our business will generate operating losses until we successfully implement our commercial development strategy and generate significant additional revenues to support our level of operating expenses. Because of the numerous risks and uncertainties associated with our commercialization efforts and future product development, we are unable to predict when we will become profitable, and we may never become profitable. Even if we do achieve profitability, we may not be able to sustain or increase our profitability.
Our future capital needs are uncertain and we may need to raise additional funds in the future, which may not be available on a timely basis or on commercially reasonably terms.
We believe that our existing cash and cash equivalents, will be sufficient to meet our anticipated cash requirements for at least the next 10 months. However, we may need to raise substantial additional capital to:
- •
- expand the commercialization of our products;
- •
- manufacture our platforms in advance of placing them with our customers;
- •
- fund our operations; and
- •
- further our research and development.
Our future funding requirements will depend upon many factors, including:
- •
- development of new and existing products;
- •
- market acceptance of our products;
- •
- the cost of our research and development activities;
- •
- the cost of potential licensing of technologies patented by others;
- •
- the cost of filing and prosecuting patent applications;
- •
- the cost of defending, in litigation or otherwise, any claims that we infringe third party patents or violate other intellectual property rights;
- •
- the cost and timing of regulatory clearances or approvals;
- •
- the cost and timing of establishing additional sales, marketing and distribution capabilities;
- •
- the cost and timing of establishing additional technical support capabilities;
- •
- the effect of competing technological and market developments; and
- •
- the extent to which we acquire or invest in businesses, products and technologies, although we currently have no commitments or agreements relating to any of these types of transactions.
If we raise additional funds by issuing equity securities, our shareholders may experience dilution. Debt financing, if available, may involve covenants restricting our operations or our ability to incur additional debt. Any debt or additional equity financing may contain terms that are not favourable to us or our shareholders. If we raise additional funds through collaboration and licensing arrangements with third parties, it may be
24
necessary to relinquish some rights to our technologies or our products, or grant licenses on terms that are not favourable to us.
If we do not have, or if we are unable to timely obtain additional funds on acceptable terms, or at all, we may have to delay development or commercialization of our products or license to third parties the rights to commercialize products or technologies that we would otherwise seek to commercialize. We also may have to liquidate some or all of our assets, reduce the scope of or eliminate some or all of our development programs, reduce marketing, customer support or other resources devoted to our products, or cease operations. Any of these factors could harm our business, financial condition and results of operations.
Market competition and technological advances of similar diagnostics products could reduce the attractiveness of our products or render them obsolete.
The markets for our products are characterized by rapidly changing technology, evolving industry standards, changes in customer needs, emerging competition, new product introductions and strong price competition. We compete with both established and development stage companies, universities, research institutions, governmental agencies and healthcare providers that design, manufacture and market similar diagnostic products. Many of our current competitors have significantly greater name recognition, greater financial and human resources, broader product lines and product packages, larger sales forces, larger existing installed bases, larger intellectual property portfolios and greater experience and capabilities in researching, developing and testing products, in obtaining FDA and other regulatory approvals or clearances, and in manufacturing, marketing and distribution, than we have. For example, companies such as Bio-Rad Laboratories Inc., Phadia AB, Axis-Shield plc, and INOVA Diagnostics, Inc. have products that compete in certain segments of the market in which we sell our products, including immunoassays. In addition, a number of other companies and academic groups are in the process of developing novel products and technologies for diagnostics markets.
Our competitors may be able to respond more quickly and effectively than we can to new or changing opportunities, technologies, standards or customer requirements. In light of these advantages, even if our technology is more effective than the product or service offerings of our competitors, current or potential customers might accept competitive products and services in lieu of purchasing our technology. We anticipate that we will face increased competition in the future as existing companies and competitors develop new or improved products and as new companies enter the market with new technologies. We may not be able to compete effectively against these organizations. Increased competition is likely to result in pricing pressures, which could harm our sales, profitability or market share. Our failure to compete effectively could materially and adversely affect our business, financial condition and results of operations.
If our products fail to achieve and sustain sufficient market acceptance, our revenue will be adversely affected.
We currently have one customer and our success depends, in part, upon our ability to develop and market products that are recognized and accepted as reliable, accurate, timely and cost effective by physicians, lab technicians and administrators. Most of our potential customers already use expensive diagnostic products and systems in their laboratories and may be reluctant to replace those systems. Market acceptance of our products and technologies will depend upon many factors, including our ability to provide a broad test menu of assays to potential customers, and our ability to convince potential customers that our systems are an attractive cost- and time-saving alternative to existing technologies. Compared to most competing technologies, our microarray assay technology is relatively new, and most potential customers have limited knowledge of, or experience with, our products. Prior to adopting our microarray assay technology, some potential customers may need to devote time and effort to testing and validating our systems. Any failure of our systems to meet these customer benchmarks could result in customers choosing to retain their existing systems or to purchase systems other than ours.
25
We are subject to complex regulatory compliance requirements and the failure to obtain, or the withdrawal of, regulatory clearance or approval for our products could adversely affect our ability to market our products and/or require us to incur significant costs to comply with such requirements.
We operate in a highly regulated industry and we are subject to the authority of certain regulatory agencies, including Health Canada, the FDA, European Conformity (CE) and applicable health authorities in other countries, with regard to the development, testing, manufacturing, marketing and sale of our diagnostic products. The process of obtaining such clearances or approvals can be costly and time-consuming, and if we are unable to timely obtain or maintain regulatory clearances or approvals, it would have a material adverse effect on our business. Clearance by regulatory authorities can be suspended or revoked, or we could be fined, based on a failure to continue to comply with applicable standards. Any failure to obtain (or significant delay in obtaining) or maintain applicable regulatory clearances or approvals (or, to a lesser extent, approval of applicable health authorities in other countries) for our new or existing products could materially affect our ability to market its products successfully and could therefore have a material adverse effect on our business. Additionally, the authority of the regulatory agencies or the application of certain regulations may be expanded or otherwise changed in such a manner that would place additional regulatory burdens on us or our customers. Such a change in our industry could have a material adverse effect on our business.
We must manufacture products in compliance with regulatory requirements, in sufficient quantities and on a timely basis, while maintaining product quality and acceptable manufacturing costs. If we or our suppliers are unable to manufacture or contract for such capabilities on acceptable terms, our plans for commercialization could be materially adversely affected.
Our manufacturing facilities are subject to periodic regulatory inspections by the regulatory agencies and these facilities are subject to quality standards requirements of the applicable regulatory authorities. We, or our contractors, may not satisfy such regulatory or standards requirements, and any failure to do so may have a material adverse effect on our business, financial condition and results of operations.
We may not be able to develop new products or enhance the capabilities of our existing diagnostics products to keep pace with rapidly changing technology and customer requirements.
The field of diagnostics is characterized by rapidly changing and developing technologies that include new products that could render our diagnostic processing equipment and consumable tests obsolete at any time and thereby adversely affect our financial condition and future prospects. Our success depends upon our ability to develop new products with improved performance and cost effectiveness in existing and new markets. New technologies, techniques or products could emerge that might offer better combinations of price and performance than our current or future product lines. It is critical to our success for us to anticipate changes in technology and customer requirements and to successfully introduce new, enhanced and competitive technology to meet our prospective customers' needs on a timely basis.
Developing and marketing new products and services will require us to incur substantial development costs and we may not have adequate resources available to be able to successfully introduce new versions of, or enhancements to, our products. We cannot guarantee that we will be able to maintain technological advantages over emerging technologies in the future. While we plan to continue to make improvements to our current and future cleared or approved and marketed diagnostic processing equipment and consumable tests, we may not be able to successfully implement these improvements. If we fail to keep pace with emerging technologies, demand for our products will not grow, and our business, revenue, financial condition and operating results could suffer materially. Even if we successfully implement some or all of these planned improvements, we cannot guarantee that potential customers will find our enhanced products to be an attractive alternative to existing technologies, including our current products.
Research and development of diagnostic products requires significant testing and investment and may not result in commercially viable products within the timeline anticipated, if at all.
New diagnostic products, and improvements to existing diagnostic products, require significant research, development, testing and investment prior to any final commercialization. Our business depends upon the continued development and improvement of our existing products, our development of new products to serve
26
existing markets and our development of new products to create new markets and applications that were previously not practical with existing systems. We believe that the adoption of our platform by potential customers depends, in part, upon our ability to provide a test menu of assays to potential customers. To date, we have obtained regulatory approval for only a few diagnostic assays.
We intend to devote significant personnel and financial resources to research and development activities designed to advance the capabilities of our diagnostic technology and, in the case of our IVD business, to obtain regulatory approval of additional assays. In the past, our product development projects have been delayed. We may have similar delays in the future, and we may not obtain any benefits from our research and development activities. Any delay or failure by us to develop new products or enhance existing products would have a material adverse effect on our business and results of operations. If we are unable to successfully develop these products, accomplish such improvements, receive applicable regulatory clearances or approvals, produce the products in commercial quantities at reasonable costs, or successfully market the products, it would have a material adverse effect on our business and results of operations. Our long-term success must be considered in light of the expenses, difficulties and delays frequently encountered in connection with the development of new technology and the competitive and highly regulated environment in which we operate.
We may need additional capacity to meet our manufacturing needs at the end of 2012.
Based on our microarray manufacturing forecasts, we expect that there is adequate capacity in our current location to expand our manufacturing capacity to meet our expected needs until the end of 2012. Until a facility upgrade is completed, we intend to undertake equipment upgrades to ensure sufficient manufacturing capacity to meet our expected requirements for commercial sales and our internal validation studies until the end of 2012. Pending the contemplated upgrades to, or expansion of, our facility, we are leasing our facility under short-term arrangements. We do not anticipate any requirement to relocate any of our operations. However, we may need to relocate our facility upon one months' notice from our landlord.
Our inability to obtain our required manufacturing space in a timely manner and on terms acceptable to us will result in delays which could have a material adverse effect on our financial condition and results of operations.
Even if we are able to enter into a lease for a new facility, we must also implement and maintain an international standard ISO quality management system for such new facility. Any delay in implementing an ISO quality management system will have a material adverse effect on our financial condition and results of operations.
Our future success depends upon our ability to expand our customer base and introduce new products and services.
Our success will depend upon our ability to gain acceptance, and then increase our market share, among our customers, attract additional customers outside of our initial target markets, and bring to market new products and services. Attracting new customers and introducing new products and services requires substantial time and expense. For example, it may be difficult to identify, engage and market to customers who are unfamiliar with the benefits of our products and services. Any failure to establish and expand our existing customer base or launch new products or services would adversely affect our ability to increase our revenues.
We have limited experience in marketing, selling and distributing our products, and we need to expand our internal and external sales and marketing force and distribution capabilities to successfully commercialize and sell our products.
As we are in the early stages of commercializing and marketing our products, we have limited experience in marketing, selling and distributing our products. We may not be able to market, sell and distribute our products effectively enough to support our planned growth. We intend to market, sell and distribute our products directly through our own sales force in North America, Europe and elsewhere. Our future sales will depend in large part upon our ability to develop and substantially expand our direct sales force and to increase the scope of our marketing efforts.
27
Our products are technically complex and used for specialized applications. Our ability to market our products effectively will depend, in part, upon our ability to convince laboratories that our products will deliver accurate patient results in less time and with significantly reduced labour, consumables and other costs. As a result, we believe it is necessary to develop a direct sales force that includes people with specific scientific backgrounds and expertise and a marketing group with technical sophistication. Competition for such employees is intense. We may not be able to attract and retain personnel, or be able to build an efficient and effective sales and marketing force, which could negatively impact sales of our products, and reduce any future revenues and profitability.
If our sales, marketing and distribution efforts are not successful, our technologies and products may not gain market acceptance, which would materially impact our business operations.
We rely on strategic partnerships for research and development and commercialization of our products.
We have entered into and may continue to enter into strategic partnerships with a number of medical institutions. For example, we have entered into strategic agreements with the Cleveland Clinic, Beth Israel Deaconess Medical Center, Hospital Clinic De Barcelona, University Hospital Maastricht, and The University of North Carolina at Chapel Hill. If any of our strategic partners were to change their business strategies or development priorities, they may no longer be willing or able to participate in such strategic partnerships which could have a material adverse effect on the timing of our future development efforts. In addition, we may not control the strategic partnerships in which we participate. We may also have certain obligations with regard to our strategic partnerships, in addition to the obligation to pay money, such as an obligation to publish the results of research.
If any of our strategic partners terminate their relationship with us or fail to perform their obligations in a timely manner, or if we fail to perform our obligations in a timely manner, the development or commercialization of our technology in potential products may be affected, delayed or terminated.
We depend upon key suppliers for some of the components and materials used in our platform technologies and our microarrays, and the loss of any of these suppliers could harm our business.
We rely on key suppliers for certain components and materials used in our platform technologies, including our SQiDworks diagnostic platform and our microarrays. We do not have agreements with these key suppliers to supply us with components in the future. The loss of any of these key suppliers would require significant time and effort to locate and qualify an alternative source of supply. There are a limited number of suppliers who can manufacture the highly specialized equipment that forms a part of our SQiDworks system.
Our first set of assays being commercialized requires a highly specific mono-layer coating on the glass surface which is used to bond each of the microarray "spots". We have worked closely with these manufacturers to extend the capabilities of their standard products to support the unique needs of our platform technologies and microarray devices. Any change in any component that forms a part of our SQiDworks system will require additional testing to ensure that it performs in a substantially similar manner to the existing component.
Our reliance on these suppliers also subjects us to other risks that could harm our business, including the following:
- •
- we may be subject to increased component costs;
- •
- we may not be able to ensure that any component that we change performs in a substantially similar manner to the existing component;
- •
- we may not be able to obtain adequate supply in a timely manner or on commercially reasonable terms;
- •
- our suppliers may make errors in manufacturing components that could negatively affect the efficacy of our systems or cause delays in shipment of our systems; and
- •
- our suppliers may encounter financial hardships unrelated to our demand for components, which could inhibit their ability to fulfill our orders and meet our requirements.
28
We may not be able to quickly establish additional or replacement suppliers, particularly for our single source components. Any interruption or delay in the supply of components or materials, or our inability to obtain components or materials from alternate sources at acceptable prices in a timely manner, could impair our ability to meet the demand of our strategic partners and future customers.
Future legislative or regulatory changes to the healthcare system, including reimbursement, may adversely affect our business.
The healthcare regulatory environments in the jurisdictions in which we operate and plan to operate may change in a way that restricts our ability to market our diagnostic testing products due to medical coverage or reimbursement limits. Sales of our diagnostic systems will depend, in part, upon the extent to which the costs to patients of such tests are paid by health maintenance, managed care, and similar healthcare management organizations, or reimbursed by government health payor administration authorities, private health coverage insurers and other third party payors. These healthcare management organizations and third party payors are increasingly challenging the prices charged for medical products and services. The containment of healthcare costs has become a priority of governments. Accordingly, our potential products may not be considered cost effective, and reimbursement to the ultimate patient may not be available or sufficient to allow us to sell our products on a competitive basis. Legislation and regulations affecting reimbursement for our products may change at any time and in ways that are difficult to predict and these changes may be adverse to us. For example, a reduction in U.S. Medicare, Medicaid or other third party payor reimbursements for diagnostic services could have a negative effect on our operating results. In June 2011, the FDA issued draft guidance that sets forth the FDA's proposed interpretation of laws regarding the marketing of IVD products labelled as RUO products that could be used forin vitro diagnostic purposes. Among other things, the draft guidance suggests that it is generally inappropriate for a manufacturer to sell RUO products to clinical laboratories that the manufacturer knows, or has reason to know, use the products for clinical diagnostic uses. Given that the guidance is in draft form and has only recently been issued by the FDA, it is not clear how the FDA will interpret this guidance. As a result, we cannot be certain what impact, if any this guidance will have on our business.
We rely on certain key personnel and our ability to successfully grow our business would be adversely affected by their departure from our company.
Our performance depends substantially upon the performance of our senior management and key scientific and technical personnel, including our Chief Executive Officer, Claude Ricks, our Chief Financial Officer, Andrew Morris, and our Chief Scientific Officer, Dr. Peter Lea. Retaining these key personnel and recruiting additional qualified personnel in the future will be critical to our success. We believe there are only a limited number of individuals with the requisite skills to serve in many of our key positions. Competition for qualified personnel in the diagnostics industry is intense and recruiting and retaining qualified personnel with experience in our industry is very difficult. We compete for key personnel with other medical equipment and software manufacturers and technology companies, as well as universities and research institutions.
If we are unable to attract and retain skilled and experienced personnel, or if we lose the services of any member of our senior management or our scientific or technical staff, we could experience significant delays in, or we could be unable to, complete the development and commercialization of our products or achieve our other business objectives, and such a development could require our management to divert its attention to transition matters and identification of suitable replacements, if any. Such a development could have a material adverse effect on our business, financial condition and results of operations.
We do not maintain, and do not intend to obtain, key employee life insurance on any of our personnel.
A portion of our compensation to our key employees is in the form of stock option grants. A prolonged decline in our share price could make it difficult for us to retain our employees and recruit additional qualified personnel.
If we cannot provide quality technical support, we could lose customers and our operating results could suffer.
The placement of our products and the introduction of our technology into our customers' existing operations and on-going customer support can be complex. Accordingly, we need highly trained technical
29
support personnel. To effectively support new customers, we will need to substantially expand our technical support staff. If we are unable to attract, train or retain the number of highly qualified technical services personnel that our business will require, our business, financial condition and results of operations will suffer.
We may experience development or manufacturing problems or delays that could limit the growth of our revenue or increase our losses.
We have been developing our core technologies in our facilities in Toronto, Canada, which we believe has adequate space to expand the manufacturing capacity to our expected needs for the foreseeable future. However, we may encounter unforeseen situations at this facility that would result in delays or shortfalls in our development and production. In addition, our development and production processes and assembly methods may have to change to accommodate any significant future expansion of our manufacturing capacity. If we are unable to keep up with development of or demand for our products, our revenue could be impaired, market acceptance for our products could be adversely affected and our customers might instead purchase our competitors' products. Our inability to successfully manufacture our products would have a material adverse effect on our business, financial condition and results of operations.
Our products could have unknown defects or errors, which may give rise to claims against us and adversely affect market adoption of our systems.
Our products utilize complex technology applied on a small scale, and our systems may develop or contain undetected defects or errors. As our production levels increase, material performance problems, defects or errors could arise. We may determine to correct any defects or errors in response to customer concerns, in order to preserve customer relationships, and to help foster continued adoption and use of our systems. The costs incurred in correcting any defects or errors may be substantial and could adversely affect our operating margins.
In manufacturing our products, we depend upon third parties for the supply of various components. Many of these components require a significant degree of technical expertise to produce. If our suppliers fail to produce components to specification, or if the suppliers, or we, use defective materials or workmanship in the manufacturing process, the reliability and performance of our products will be compromised.
If our products contain defects, we may experience:
- •
- a failure to achieve market acceptance or expansion of our product sales;
- •
- loss of customer orders and delay in order fulfillment;
- •
- damage to our brand reputation;
- •
- increased cost of our warranty program due to product repair or replacement;
- •
- product recalls or replacements;
- •
- inability to attract new customers;
- •
- diversion of resources from our manufacturing and research and development departments into our service department; and
- •
- legal claims against us, including product liability claims, which could be costly and time consuming to defend and result in substantial damages.
The occurrence of any one or more of the foregoing could negatively affect our business, financial condition and results of operations.
We operate in an industry where there is the potential for substantial product liability claims that would cause us to incur significant costs, create adverse publicity and/or prevent us from commercializing our products.
We may be subject to claims of personal injury and could become liable to clinical laboratories, hospitals, physicians and patients for harm resulting from use of our products. We could suffer financial loss due to defects in our products, and such financial loss and potential litigation expenses could have a material adverse effect on our business, financial condition and results of operations. If our product liability insurance is not adequate to
30
cover all claims or if we are unable to maintain such insurance at reasonable cost, it would have a material adverse effect on our business, financial condition and results of operations.
We use hazardous chemicals and biological materials in our business. Any claims relating to improper handling, storage or disposal of these materials could be time consuming and costly.
Our research and development and manufacturing processes involve the controlled use of hazardous materials, including flammables, toxics, corrosives and biologics. We maintain a level 2 biohazard laboratory (a laboratory that has established specific procedures for handling bacteria and viruses that may pose a risk of mild disease in humans) and our operations produce hazardous biological and chemical waste products. We cannot eliminate the risk of accidental contamination or discharge and any resultant injury from these materials. We may be sued for any injury or contamination that results from our use or the use by third parties of these materials. We do not currently maintain separate environmental liability coverage and any such contamination or discharge could result in significant cost to us in penalties, damages and suspension of our operations.
We may be unable to successfully manage fluctuations in revenue, which could impede our ability to successfully develop, market and sell our products.
Our quarterly and annual revenues may fluctuate due to several factors, including customer order patterns, the rate of acceptance of our products, regulatory uncertainties or delays, costs and timing associated with business development activities, including potential licensing of technologies, and international market conditions. The impact of one, or a combination of several, of these factors could have a significant adverse effect on our business, financial condition and results of operations.
Our future financial results may be adversely affected by foreign exchange fluctuations.
We expect that a significant portion of our future revenues will be denominated in U.S. and European currencies, and, therefore, we will be subject to fluctuations in exchange rates. There is a risk that significant fluctuations in exchange rates would negatively affect our operating margins and would therefore have an adverse effect on our future results of operations.
Risks Related to Intellectual Property
Our ability to protect our intellectual property and proprietary technology through patents and other means is uncertain.
Our commercial success depends in part upon our ability to protect our intellectual property and proprietary technologies. We rely on patent protection, where appropriate and available, as well as a combination of copyright, trade secret and trademark laws, and nondisclosure, confidentiality and other contractual restrictions to protect our proprietary technology. However, these legal means afford only limited protection and may not adequately protect our rights or permit us to gain or keep any competitive advantage. Our pending Canadian, U.S. and foreign patent applications may not issue as patents or may not issue in a form that will be sufficient to protect our proprietary technology and gain or keep our competitive advantage. Any patents we have obtained or do obtain may be subject to re-examination, reissue, opposition or other administrative proceeding, or may be challenged in litigation, and such challenges could result in a determination that the patent is invalid or unenforceable. In addition, competitors may be able to design alternative methods or devices that avoid infringement of our patents. To the extent our intellectual property, including licensed intellectual property, offers inadequate protection, or is found to be invalid or unenforceable, we are exposed to a greater risk of direct competition. If our intellectual property does not provide adequate protection against our competitors' products, our competitive position could be adversely affected, as could our business, financial condition and results of operations. Both the patent application process and the process of managing patent disputes can be time consuming and expensive. Furthermore, the laws of some foreign countries may not protect our intellectual property rights to the same extent as do the laws of Canada and the United States.
31
The patent positions of companies in the diagnostics industry can be highly uncertain and involve complex legal and factual questions for which important legal principles remain unresolved. No consistent policy regarding the breadth of claims allowed in such companies' patents has emerged to date in Canada and the United States. The laws of some non-Canadian countries do not protect intellectual property rights to the same extent as the laws of Canada, and many companies have encountered significant problems in protecting and defending such rights in foreign jurisdictions. The legal systems of certain countries, particularly certain developing countries, do not favour the enforcement of patents and other intellectual property protection, which could make it difficult for us to stop the infringement of our patents. Proceedings to enforce our patent rights in foreign jurisdictions could result in substantial cost and divert our efforts and attention from other aspects of our business. Changes in either the patent laws or in interpretations of patent laws in the United States, Canada or other countries may diminish the value of our intellectual property. We cannot predict the breadth of claims that may be allowed or enforced in our patents or in third party patents. For example:
- •
- we might not have been the first to make the inventions covered by each of our pending patent applications;
- •
- we might not have been the first to file patent applications for these inventions;
- •
- others may independently develop similar or alternative products and technologies or duplicate any of our products and technologies;
- •
- it is possible that none of our pending patent applications will result in issued patents, and even if they issue as patents, they may not provide a basis for commercially viable products, or may not provide us with any competitive advantages, or may be challenged and invalidated by third parties;
- •
- we may not develop additional proprietary products and technologies that are patentable;
- •
- the patents of others may have an adverse effect on our business; and
- •
- we may fail to apply for patents on important products and technologies in a timely fashion or at all.
In addition to pursuing patents on our technology, we take steps to protect our intellectual property and proprietary technology by entering into confidentiality agreements and intellectual property assignment agreements with our employees, consultants, corporate partners and, when needed, our advisors. Such agreements may not be enforceable or may not provide meaningful protection for our trade secrets or other proprietary information in the event of unauthorized use or disclosure or other breaches of the agreements, and we may not be able to prevent such unauthorized disclosure. Monitoring unauthorized disclosure is difficult, and we do not know whether the steps we have taken to prevent such disclosure are, or will be, adequate. If we were to enforce a claim that a third party had illegally obtained and was using our trade secrets, it would be expensive and time consuming, and the outcome would be unpredictable. In addition, courts outside Canada and the United States may be less willing to protect trade secrets.
We may be involved in lawsuits to protect or enforce our patents and proprietary rights, to determine the scope, coverage and validity of others' proprietary rights, or to defend against third party claims of intellectual property infringement that could require us to spend significant time and money and could prevent us from selling our products or services or impact our share price.
The diagnostics industry relies heavily upon patented technology. We follow a patent program to protect our technology and take precautions to avoid infringement against the technology of others. Litigation may be necessary for us to enforce our patent and proprietary rights and/or to determine the scope, coverage and validity of others' proprietary rights. Litigation on these matters has been prevalent in our industry and we expect that this will continue. To determine the priority of inventions, we may have to initiate and participate in interference proceedings declared by the Canadian Intellectual Property Office, the U.S. Patent and Trademark Office, and patent offices in other countries that could result in substantial legal fees and could substantially affect the scope of our patent protection. Also, our intellectual property may be subject to significant administrative and litigation proceedings such as invalidity, unenforceability, re-examination and opposition proceedings against our patents. The outcome of any litigation or other proceeding is inherently uncertain and might not be favourable to us, and we might not be able to obtain licenses to technology that we require on
32
commercially acceptable terms or at all. We could therefore incur substantial costs related to royalty payments for licenses obtained from third parties. Further, we could encounter delays in product introductions, or interruptions in product sales, as we develop alternative methods or products.
In addition, if we resort to legal proceedings to enforce our intellectual property rights or to determine the validity, scope and coverage of the intellectual property or other proprietary rights of others, the proceedings could be burdensome and expensive, even if we were to prevail.
Our commercial success may depend in part upon our non-infringement of the patents or proprietary rights of third parties. Numerous significant intellectual property issues have been litigated, and will likely continue to be litigated, between existing and new participants in the diagnostics market and competitors may assert that our products infringe their intellectual property rights as part of a business strategy to impede our successful entry into that market. Third parties may assert that we are employing their proprietary technology without authorization.
In addition, our competitors and others may have patents or may in the future obtain patents and claim that use of our products infringes these patents. As we move into new markets and applications for our products, incumbent participants in such markets may assert their patents and other proprietary rights against us as a means of slowing our entry into such markets or as a means to extract substantial license and royalty payments from us.
Patent infringement suits can be expensive, lengthy and disruptive to business operations. We could incur substantial costs and divert the attention of our management and technical personnel in prosecuting or defending against any claims, and may harm our reputation. There can be no assurance that we will prevail in any suit initiated against us by third parties. Furthermore, parties making claims against us may be able to obtain injunctive or other relief, which could block our ability to develop, commercialize and sell products, and could result in the award of substantial damages against us, including treble damages and attorneys' fees and costs in the event that we are found to be a willful infringer of third party patents.
In the event of a successful claim of infringement against us, we may be required to obtain one or more licenses from third parties, which we may not be able to obtain at a reasonable cost, if at all. In addition, we could encounter delays in product introductions while we attempt to develop alternative methods or products to avoid infringing third party patents or proprietary rights. Defense of any lawsuit or failure to obtain any required licenses on favourable terms could prevent us from commercializing our products, and the risk of a prohibition on the sale of any of our products could adversely affect our ability to grow and gain market acceptance for our products.
Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation. In addition, during the course of this kind of litigation, there could be public announcements of the results of hearings, motions or other interim proceedings or developments. If securities analysts or investors perceive these results to be negative, it could have a substantial adverse effect on the price of our common shares.
In addition, our agreements with suppliers, customers, and other entities with whom we do business, may require us to defend or indemnify these parties to the extent they become involved in infringement claims against us, including the claims described above. We could also voluntarily agree to defend or indemnify third parties in instances where we are not obligated to do so if we determine it would be important to our business relationships. If we are required or agree to defend or indemnify any of these third parties in connection with any infringement claims, we could incur significant costs and expenses that could adversely affect our business, financial condition or operating results.
We may in the future depend upon licensed technology to conduct our business. We might not control these technologies and any loss of our rights to them could in the future prevent us from selling our products.
We may in the future rely on licenses in order to be able to use various proprietary technologies that become material to our business. We might not own the patents that underlie these licenses. This may subject us to certain risks that might not be present if we develop the technology and intellectual property independently.
33
In many potential third party licenses, we might not control the prosecution, maintenance, or filing of the patents to which we may in the future hold licenses, or the enforcement of these patents against third parties. We cannot be certain that drafting and/or prosecution of the licensed patents by the licensors will be conducted in compliance with applicable laws and regulations or will result in valid and enforceable patents and other intellectual property rights. As a result, we may depend upon the licensor to diligently pursue and prosecute these intellectual property rights. The failure of the licensor to diligently pursue such protection could adversely affect our business and financial condition.
Our rights to use the technology we might license may be subject to the validity of the owner's intellectual property rights. Enforcement of such licensed patents or defense of any claims asserting the invalidity of these patents is often subject to the control or cooperation of our licensors. Legal action could be initiated against the owners of the intellectual property that we may license. Even if we are not a party to these legal actions, an adverse outcome could harm our business because it might prevent these other companies or institutions from continuing to license intellectual property that we may need to operate our business.
Our rights to use the technology we may in the future license are subject to the negotiation of, continuation of and compliance with the terms of those licenses. Specifically, license agreements typically subject us to milestone obligations and royalty payments. Some of these obligations may be substantial and may obligate us to obtain certain regulatory clearances or approvals by a specified date or exercise diligence in bringing potential products to market. The failure to meet these obligations typically results in the termination of the license and the loss of rights to the technology. Any such termination could prevent us from marketing some or all of our products and could adversely affect our business, financial condition and results of operations.
Because of the complexity of our products and the patents we may license, determining the scope of such licenses and related royalty obligations can be difficult and can lead to disputes between us and the licensor. An unfavourable resolution of such a dispute could lead to an increase in the royalties payable pursuant to the license. If a licensor believed we were not paying the royalties due under the license or were otherwise not in compliance with the terms of the license, the licensor might attempt to revoke the license. If such an attempt were successful, we might be barred from producing and selling some or all of our products.
Finally, many potential third party licenses of technology expire when the patents underlying the technology expire or at some period of time after expiration. As a result, our ability to exploit and fully commercialize the licensed technology over time may be limited. This may adversely affect our business, financial condition and results of operations.
We may be subject to damages resulting from claims that we or our employees have wrongfully used or disclosed alleged trade secrets of our employees' former employers.
Many of our employees were previously employed at universities or other life sciences companies, including our competitors or potential competitors. Although no claims against us are currently pending, we may be subject to claims that these employees or we have inadvertently or otherwise used or disclosed trade secrets or other proprietary information of their former employers. Litigation may be necessary to defend against these claims. If we fail in defending such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel. A loss of key research personnel or their work product could hamper or prevent our ability to commercialize potential products, which could severely harm our business. Even if we are successful in defending against these claims, litigation could result in substantial costs and be a distraction to management.
Risks Related to Our Common Shares
We expect that our share price will fluctuate significantly, and you may not be able to resell your common shares at or above the current price.
Our common shares are listed for trading on the TSX Venture Exchange ("TSXV"). We cannot predict the extent to which investor interest in our company will lead to the development of an active trading market on the TSXV, or how liquid that market might become. If an active trading market does not develop, you may have difficulty selling any of our common shares that you buy. The market price of our common shares on the TSXV,
34
like the share prices of many publicly traded life sciences companies, has been highly volatile, and the trading price of our common shares may remain volatile in response to various factors, some of which are beyond our control. These factors could include, for example:
- •
- actual or anticipated quarterly variation in our results of operations or the results of our competitors;
- •
- announcements by us or our competitors of technological innovations, new commercial products, significant contracts, commercial relationships or capital commitments;
- •
- developments or disputes concerning our intellectual property or other proprietary rights;
- •
- commencement of, or our involvement in, litigation;
- •
- market conditions in the diagnostics sector;
- •
- regulatory developments in the U.S., Canada and other countries;
- •
- failure to complete significant sales;
- •
- issuance of new or changed securities analysts' reports or recommendations for our common shares;
- •
- any future sales of our common shares or other securities;
- •
- any major change to the composition of our board of directors or management; and
- •
- general economic conditions and slow or negative growth of our markets.
The stock market in general, and market prices for the securities of technology-based companies like ours in particular, have from time to time experienced volatility that often has been unrelated to the operating performance of the underlying companies. A certain degree of stock price volatility can be attributed to being a newly public company. These broad market and industry fluctuations may adversely affect the market price of our common shares, regardless of our operating performance. In several recent situations where the market price of an issuer's shares has been volatile, holders of those shares have instituted securities class action litigation against the company that issued the shares. If any of our shareholders were to bring a lawsuit against us, the defense and disposition of the lawsuit could be costly, and could divert the time and attention of our management and harm our operating results.
If securities or industry analysts do not publish research or publish unfavourable research about our business, our share price and trading volume could decline.
The trading market for our common shares will rely in part on the research and reports that equity research analysts may publish about us and our business. We do not currently have and may never obtain research coverage by equity research analysts. Equity research analysts may elect not to provide research coverage of our common shares, and such lack of research coverage may adversely affect the market price of our common shares. If we obtain equity research analyst coverage, we will not have any control over the analysts or the content and opinions included in their reports. The price of our common shares could decline if one or more equity research analysts downgrade our common shares or issue other unfavourable commentary or research. If one or more equity research analysts ceases coverage of our company or fails to publish reports on us regularly, demand for our common shares could decrease, which in turn could cause our share price or trading volume to decline.
Our directors and executive officers will continue to have substantial control over us and could limit your ability to influence the outcome of key transactions, including changes of control.
Our executive officers, directors and their affiliates beneficially own or control approximately 21.45% of our outstanding common shares. Accordingly, these executive officers, directors and their affiliates, acting as a group, have substantial influence over the outcome of corporate actions requiring shareholder approval, including the election of directors, any merger, consolidation or sale of all or substantially all of our assets or any other significant corporate transactions or agreements. These shareholders may also delay or prevent a change of control of our company, even if such a change of control would benefit our other shareholders. The significant
35
concentration of share ownership may adversely affect the trading price of our common shares due to investors' perception that conflicts of interest may exist or arise.
We have never paid dividends on our common shares, and we do not anticipate paying any cash dividends in the foreseeable future.
To date, we have not paid any dividends and do not expect to do so in the foreseeable future. We currently intend to retain all future earnings for the operation and expansion of our business. Dividends on our common shares are declared at the discretion of our board of directors and will depend on our results of operations, capital requirements, financial condition, prospects, contractual arrangements and other factors that our board determines is relevant.
DIVIDENDS AND DISTRIBUTIONS
To date, we have not paid any dividends and do not expect to do so in the foreseeable future. We currently intend to retain all future earnings for the operation and expansion of our business. Dividends on our common shares are declared at the discretion of our board of directors and will depend on our results of operations, capital requirements, financial condition, prospects, contractual arrangements and other factors that our board determines is relevant.
DESCRIPTION OF CAPITAL STRUCTURE
Common Shares
We are authorized to issue an unlimited number of common shares. The holders of common shares are entitled to one vote per share at meetings of shareholders, to receive such dividends as declared by our board for directors and to receive the remaining property and assets of upon our dissolution or winding up. The common shares are not subject to any future call or assessment and there are no pre-emptive, conversion or redemption rights attached to such shares.
Warrants
As of May 31, 2011, we had outstanding warrants to purchase an aggregate of 2,632,852 common shares at exercise prices ranging from $1.90 per share to $5.00 per share. These warrants will expire at various times between December 4, 2011 and August 12, 2012. In the event of a distribution of dividends, a stock split, a reorganization, a reclassification, a consolidation, or a similar event, each warrant provides for adjustment of the exercise price and the number of shares issuable upon exercise.
Options
We maintain a stock option plan for the benefit of directors, officers, employees and consultants. The aggregate number of common shares reserved for issuance under the plan, together with any other employee stock option plans, options for services and employee share purchase plans, may not exceed 10% of the issued and outstanding common shares at the time of the option grant (on a non-diluted basis). Options granted pursuant to the plan must have terms not to exceed five years, and are granted at an option price that may not be less than the fair market price at the time the options are granted. All options granted to individual optionees, other than consultants, generally vest in three equal instalments over a period of 36 months.
36
MARKET FOR SECURITIES
Trading Price and Volume
Our common shares are listed and posted for trading on the TSXV under the symbol "SQD". The following table sets out, for the periods indicated, the reported high and low sales prices and aggregate volume of trading of our common shares on the TSXV for the periods indicated.
2009
| | | | | | | | | | |
Month | | Volume
(#) | | High Trading
Price
(C$) | | Low Trading
Price
(C$) | |
|---|
October | | | 105,441 | | | 2.50 | | | 1.81 | |
November | | | 764,708 | | | 3.67 | | | 2.50 | |
December | | | 179,564 | | | 3.00 | | | 2.40 | |
2010
| | | | | | | | | | |
Month | | Volume
(#) | | High Trading
Price
(C$) | | Low Trading
Price
(C$) | |
|---|
January | | | 169,691 | | | 2.60 | | | 2.25 | |
February | | | 438,181 | | | 2.40 | | | 2.12 | |
March | | | 153,377 | | | 2.27 | | | 1.60 | |
April | | | 315,620 | | | 2.47 | | | 2.21 | |
May | | | 199,865 | | | 2.41 | | | 2.05 | |
June | | | 1,097,152 | | | 2.47 | | | 2.00 | |
July | | | 354,891 | | | 2.42 | | | 2.21 | |
August | | | 644,310 | | | 3.13 | | | 2.30 | |
September | | | 604,825 | | | 3.18 | | | 2.76 | |
October | | | 744,468 | | | 2.97 | | | 2.65 | |
November | | | 193,705 | | | 2.70 | | | 2.42 | |
December | | | 104,220 | | | 2.75 | | | 2.50 | |
2011
| | | | | | | | | | |
Month | | Volume
(#) | | High Trading
Price
(C$) | | Low Trading
Price
(C$) | |
|---|
January | | | 253,844 | | | 3.20 | | | 2.70 | |
February | | | 442,792 | | | 3.42 | | | 2.90 | |
March | | | 177,537 | | | 3.38 | | | 3.00 | |
April | | | 100,731 | | | 3.25 | | | 2.95 | |
May | | | 414,333 | | | 3.45 | | | 3.09 | |
June(1) | | | 93,824 | | | 3.60 | | | 3.35 | |
- (1)
- As at market close on June 14, 2011.
Prior Sales
Common Shares
On August 12, 2010, pursuant to a private placement, we issued 2,280,000 units, each comprised of one common share and one-half common share purchase warrant, at a price of $2.50 per unit, resulting in gross proceeds of $5,700,000.
37
On December 4, 2009, pursuant to a private placement, we issued 2,398,104 units, each comprised of one common share and one-half common share purchase warrant, at a price of $2.75 per unit, resulting in gross proceeds of $6,595,000.
Since October 1, 2009, we have issued the following common shares upon the exercise of warrants.
| | | | | | | |
Date of Issue | | Number of
Common Shares
Issued | | Purchase Price
per Common Share | |
|---|
November 9, 2009 | | | 5,000 | | | $2.40 | |
November 16, 2009 | | | 1,563 | | | $2.40 | |
November 23, 2009 | | | 2,500 | | | $2.40 | |
January 18, 2010 | | | 13,288 | | | $0.60 | |
April 12, 2010 | | | 30,000 | | | $2.40 | |
April 23, 2010 | | | 186,205 | | | $0.60 | |
May 06, 2010 | | | 101,000 | | | $2.40 | |
May 14, 2010 | | | 65,000 | | | $2.40 | |
June 3, 2010 | | | 194,200 | | | $1.50 | |
June 22, 2010 | | | 15,000 | | | $2.40 | |
June 25, 2010 | | | 217,500 | | | $2.40 | |
June 29, 2010 | | | 139,000 | | | $2.40 | |
January 21, 2011 | | | 106,520 | | | $1.25 | |
Since October 1, 2009, we have issued the following common shares upon the exercise of stock options.
| | | | | | | |
Date of Issue | | Number of
Common Shares
Issued | | Exercise Price | |
|---|
April 14, 2010 | | | 141,670 | | | $1.20 | |
April 14, 2010 | | | 166,670 | | | $0.60 | |
April 21, 2010 | | | 5,000 | | | $1.74 | |
June 18, 2010 | | | 16,667 | | | $1.20 | |
August 23, 2010 | | | 10,000 | | | $1.75 | |
September 1, 2010 | | | 10,000 | | | $1.75 | |
December 17, 2010 | | | 7,500 | | | $1.74 | |
January 21, 2011 | | | 2,500 | | | $1.30 | |
Feb 14, 2011 | | | 8,334 | | | $1.20 | |
March 30, 2011 | | | 30,000 | | | $1.74 | |
April 26, 2011 | | | 33,334 | | | $1.20 | |
Options
The following table sets forth the details for all options that we granted under our option plan during the 12-month period prior to October 1, 2009.
| | | | | | | |
Date of Grant | | Number of
Common Shares
Under
Options Granted | | Exercise Price | |
|---|
November 4, 2009 | | | 25,000 | | | $3.26 | |
February 22, 2010 | | | 124,000 | | | $2.25 | |
May, 27, 2010 | | | 60,000 | | | $2.10 | |
August 16, 2010 | | | 175,000 | | | $2.50 | |
October 4, 2010 | | | 100,000 | | | $2.90 | |
January 31, 2011 | | | 75,000 | | | $2.85 | |
38
Warrants
The following table sets forth the details for all warrants that we issued since October 1, 2009.
| | | | | | | |
Date of Issue | | Number of
Common Shares
Under
Warrants Granted | | Exercise Price | |
|---|
December 4, 2009 | | | 143,886 | | | $2.75 | |
December 4, 2010 | | | 1,199,052 | | | $4.00 | |
August 12, 2010 | | | 1,140,000 | | | $5.00 | |
August 12, 2010 | | | 57,000 | | | $2.50 | |
DIRECTORS AND OFFICERS
The following table sets forth the names, municipalities of residence, positions held with us and principal occupations of our directors and executive officers and, if a director, the month and year in which the person became a director. Our directors hold office until the next annual meeting of our shareholders or until their successors are appointed.
| | | | | | | | | |
Name, Place of Residence and
Director Since (if applicable) | | Principal Occupation | | Number of Common
Shares Beneficially
Owned or Controlled | | Percentage of
Common Shares | |
|---|
Claude Ricks
Barrie, ON
May, 2007 | | Currently and since May 2007, Mr. Ricks has been the President, Chief Executive Officer and a director of the Company Prior to that, Mr. Ricks was the Chief Executive Officer of SQIDS. | | | 1,992,157 | | | 6% | |
Dr. Peter Lea
Toronto, ON
May, 2007 | | Currently and since May 2007, Dr. Lea has been the Chief Science Officer and a director of the Company. Prior to that, Dr. Lea was Chief Science Officer and director of SQIDS. | | | 2,173,904 | | | 6% | |
Eric Schneider (1) (2)
Waterloo, ON
May, 2007 | | Currently and for the past five years, Mr. Schneider is a partner in the law firm Miller Thomson LLP and its predecessors. | | | 380,585 | | | 1% | |
Saied Nadjafi (2)
Toronto, ON
May, 2007 | | Currently and for the past five years Mr. Nadjafi is an independent business man. | | | 1,931,475 | | | 6% | |
David Williams (1)
Toronto, ON
May, 2007 | | Currently and for the past five years, Mr. Williams is the President of Roxborough Holdings Limited, a Toronto based investment company. | | | 477,225 | | | 1% | |
Paul J. Mountain, (2)
Welland, ON
August, 2007 | | Currently and since August 2007, Mr. Mountain has been an independent consultant. Prior to that and since January 1990 Mr. Mountain was Vice President of Science and Technology at MDS Inc., a Toronto-based diversified healthcare company. | | | Nil | | | —
| |
39
| | | | | | | | | |
Name, Place of Residence and
Director Since (if applicable) | | Principal Occupation | | Number of Common
Shares Beneficially
Owned or Controlled | | Percentage of
Common Shares | |
|---|
Peter Winkley (1)
Mississauga, ON
November, 2007 | | Currently and since September 2010, Mr. Winkley is the Vice President and Chief Financial Officer of Algoma Capital Corporation. Prior to that, Mr. Winkley was the Chief Financial Officer of Therapure Biopharma Inc. a Mississauga-based biologics contract manufacturer. Prior to this, Mr. Winkley was Vice-President, Corporate Finance at MDS Inc. | | | Nil | | | — | |
Andrew Morris
Oakville, ON | | Currently and since 2004, Mr. Morris is the Chief Financial Officer of the Company. | | | 283,340 | | | 0.8% | |
Catherine Smith
Toronto, ON | | Currently and since January 2005, Ms. Smith is the Vice President, Technology of the Company. | | | 42,168 | | | 0.1% | |
Jaymie R. Sawyer
Thornhill, ON | | Currently and since October 2010, Ms. Sawyer is the Vice President, R&D of the Company. Prior to that and since 2002, Ms. Sawyer was the Director, R&D at BD Biosciences | | | Nil | | | —
| |
- (1)
- Member of the audit committee (the "Audit Committee") (appointed annually).
- (2)
- Member of the compensation committee (the "Compensation Committee") (appointed annually).
As of June 15, 2011 as a group, our directors and executive officers beneficially owned, directly or indirectly, or exercised control over, 7,280,854 common shares, which represented 21.45% of the outstanding common shares. Additionally, as of June 15, 2011 as a group, our directors and executive officers beneficially owned, directly or indirectly, or exercised control over, options to purchase up to 1,158,339 common shares.
Except as disclosed below, each of our directors and executive officers has been engaged for five years in his present principal occupation or in other capacities with the company or organization (or predecessor thereof) in which he currently holds his principal occupation. The information provided below has been provided to us by the individuals themselves and has not been independently verified by us.
Board Committees
Our board of directors has established an Audit Committee and a Compensation Committee to assist the directors in efficiently carrying out their responsibilities.
Audit Committee
Our board of directors has appointed an Audit Committee consisting of three directors, being Eric Schneider, David Williams and Peter Winkley, the Chair of the Audit Committee all of whom are "financially literate" within the meaning of Multilateral Instrument 52-110 (Audit Committees) have accounting or related financial expertise, and are "independent" within the meaning of applicable Canadian securities laws. The responsibilities and mandate of the Audit Committee are set out in an Audit Committee Charter. The primary purposes of the Audit Committee are to:
- (a)
- assist the board's oversight of:
- (i)
- the integrity of our financial statements and other financial reporting;
40
- (ii)
- the external auditor's qualifications and independence;
- (iii)
- the performance of our internal audit functions and internal auditor, if and when one is appointed;
- (iv)
- our compliance with legal and regulatory requirements; and
- (v)
- any other matters as defined by the board;
- (b)
- manage, on behalf of the shareholders, the relationship between us and the external auditors by:
- (i)
- recommending to the board the nomination and remuneration of the external auditors;
- (ii)
- overseeing the work of the external auditors for the purpose of preparing or issuing an auditor's report or performing other audit, review or attest services for us, including the resolution of any disagreements between management and the external auditor regarding financial reporting;
- (iii)
- approving fees for all audit and audit-related services and pre-approving all non-audit services to be provided to us by our external auditor;
- (iv)
- managing the relationship and facilitating communication between us and the external auditors; and
- (c)
- oversee the preparation of any report that is required by any applicable securities regulatory authority to be included in the annual proxy statement, annual information form or any other of our public disclosure documents.
Compensation Committee
Our board of directors has appointed a Compensation Committee consisting of three directors, being Eric Schneider, the Chair, Saied Nadjafi and Paul Mountain who are "independent" under applicable Canadian securities laws.
The responsibilities and mandate of the Compensation Committee are set out in a Compensation Committee Charter. The primary purposes of the Compensation Committee are to:
- •
- assist the board in discharging the board's oversight responsibilities relating to the compensation, development, succession and retention of the Chief Executive Officer, President and senior management, and
- •
- establish fair and competitive compensation and performance incentive plans.
Corporate Cease Trade Orders, Penalties and Bankruptcies
Other than as disclosed below, to our knowledge, no proposed director:
- (i)
- is, as at the date of this Annual Information Form, or has been, within 10 years before the date of this Annual Information Form, a director or executive officer of any company (including our company) that, while that person was acting in that capacity,
- A.
- was the subject of a cease trade or similar order or an order that denied the relevant company access to any exemption under securities legislation, for a period of more than 30 consecutive days;
- B.
- was subject to an event that resulted, after the director or executive officer ceased to be a director or executive officer, in the company being the subject of a cease trade or similar order or an order that denied the relevant company access to any exemption under securities legislation, for a period of more than 30 consecutive days; or
- C.
- within a year of that person ceasing to act in that capacity, became bankrupt, made a proposal under any legislation relating to bankruptcy or insolvency or was subject to or instituted any proceedings, arrangement or compromise with creditors or had a receiver, receiver manager or trustee appointed to hold its assets; or
41
- (ii)
- has, within the 10 years before the date of this Annual Information Form, become bankrupt, made a proposal under any legislation relating to bankruptcy or insolvency, or become subject to or instituted any proceedings, arrangement or compromise with creditors, or had a receiver, receiver manager or trustee appointed to hold the assets of the proposed director.
Mr. Nadjafi was a director and officer of TelcoPlus Enterprises Inc. when the British Columbia and Alberta securities commissions issued a cease trade order against the company in June, 2003. The cease trade order was issued due to the fact that the company was a shell company that had no operating business. The cease trade order was revoked in December 2003.
Mr. Williams was a director of Octagon Industries Inc. ("Octagon") from November 1993 to 2005. Octagon was subject to cease trade orders issued by the British Columbia Securities Commission ("BCSC") on May 29, 2001 (revoked on August 28, 2001) and on June 24, 2004, and by the Alberta Securities Commission on June 8, 2004, for failure to file its required financial statements. Octagon was delisted from the NEX (a separate exchange from the TSXV) for default of paying its listing fees for the third quarter of 2004. On August 12, 2001, the trustees of Octagon sent a proposal to unsecured creditors of Octagon pursuant to theBankruptcy and Insolvency Act (Canada). A majority of the unsecured creditors approved the proposal at a general meeting of the creditors held on August 25, 2001.
Mr. Williams also served as a director of RoaDor Industries Inc. ("RoaDor"), a reporting issuer in the Provinces of British Columbia, Alberta and Ontario, when on February 18, 2011, the BCSC and the Ontario Securities Commission each issued a cease trade order against RoaDor for failure to file its financial statements and management's discussion and analysis related thereto for the year ended September 30, 2010. The cease trade orders remain in effect as of the date of this Annual Information Form.
Mr. Winkley became a director and the corporate secretary of 1608557 Ontario Inc. ("1608557") (formerly Hemosol Corp.) in November 2008 as a part of that company's emergence from CCAA protection. In December 2008 and March 2009, the applicable Canadian securities regulatory authorities issued cease trade orders against that company as a result of the failure to file financial statements for periods during the 2005, 2006, 2007 and 2008 fiscal years. Mr. Winkley ceased to be a director and officer of 1608557 in December 2009.
Personal Bankruptcies
To our knowledge, none of our existing or proposed directors have, within the 10 years before the date of this Annual Information Form, become bankrupt, made a proposal under any legislation relating to bankruptcy or insolvency, or been subject to or instituted any proceedings, arrangements or compromise with creditors, or had a receiver, receiver manager or trustee appointed to hold the assets of that person.
Conflicts of Interest
Our directors are required by law to act honestly and in good faith with a view to our best interests and to disclose any interests that they may have in any project or opportunity of SQI. If a conflict of interest arises at a meeting of the board, any director in a conflict must disclose his interest and abstain from voting on such matter.
To our knowledge, and other than disclosed herein, there are no known existing or potential conflicts of interest among SQI, our promoters, directors and officers or other members of management of SQI or of any proposed promoter, director, officer or other member of management as a result of their outside business interests, except that certain of the directors and officers serve as directors and officers of other companies, and therefore it is possible that a conflict may arise between their duties to SQI and their duties as a director or officer of such other companies.
42
LEGAL PROCEEDINGS AND REGULATORY ACTIONS
From time to time we are involved in various claims and legal proceedings of a nature considered normal to our business. While it is not feasible to predict or determine the outcome of these proceedings, management believes any current actions to be without merit, and no provision in respect of these matters has been made in our financial statements.
TRANSFER AGENTS AND REGISTRARS
The transfer agent and registrar for our common shares is Equity Financial Trust Company at its principal offices located in Toronto, Ontario.
EXPERTS
Collins Barrow Toronto LLP, Licensed Public Accountants, are the auditors of the Company and have performed audits in respect of the audited annual consolidated financial statements of the Company as at and for the years ended September 30, 2010 and 2009. Collins Barrow Toronto LLP, Licensed Public Accountants is an independent auditor within the meaning of the Rules of Professional Conduct of the Institute of Chartered Accountants of Ontario.
ADDITIONAL INFORMATION
Additional information relating to the Company may be found on the System for Electronic Document Analysis and Retrieval which can be accessed at www.sedar.com. Additional information, directors' and officers' remuneration and indebtedness, principal holders of common shares authorized for issuance under equity compensation plans, if applicable, will be contained in the Company's information circular for its annual and special meeting of shareholders anticipated to be held in July 2011. Additional financial information is also provided in the Company's financial statements and management's discussion and analysis for the year ended September 30, 2010.
43
QuickLinks
FORWARD-LOOKING STATEMENTSCORPORATE STRUCTUREGENERAL DEVELOPMENT OF THE BUSINESSDESCRIPTION OF THE BUSINESSDIVIDENDS AND DISTRIBUTIONSDESCRIPTION OF CAPITAL STRUCTUREMARKET FOR SECURITIESDIRECTORS AND OFFICERSLEGAL PROCEEDINGS AND REGULATORY ACTIONSTRANSFER AGENTS AND REGISTRARSEXPERTSADDITIONAL INFORMATION

