QuickLinks -- Click here to rapidly navigate through this documentAs filed with the Securities and Exchange Commission on September 8, 2011
Registration No. 333-175382
U.S. SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Amendment No. 1
To
Form F-10
REGISTRATION STATEMENT UNDER
THE SECURITIES ACT OF 1933
SQI DIAGNOSTICS INC.
(Exact name of Registrant as specified in its charter)
| | | | |
| Canada | | 2835 | | N/A |
(Province or Other Jurisdiction of
Incorporation or Organization) | | (Primary Standard Industrial
Classification Code Number) | | (I.R.S. Employer Identification
Number, if any) |
36 Meteor Drive
Toronto, Ontario
Canada M9W 1A4
(416) 674-9500
(Address and telephone number of Registrant's principal executive offices)
Puglisi & Associates
850 Library Avenue, Suite 204
Neward, DE 19711
(302) 738-6680
(Name, address (including zip code) and telephone number (including area code) of agent for service in the United States)
Copies to:
| | | | | | | | | | | | |
Daniel M. Miller
Dorsey & Whitney LLP
Suite 1605, 777 Dunsmuir Street
P.O. Box 10444, Pacific Centre
Vancouver, B.C.
Canada V7Y 1K4
(604) 630-5199 | | Andrew Morris
Chief Financial Officer
SQI Diagnostics Inc.
36 Meteor Drive
Toronto, Ontario
Canada M9W 1A4
(416) 674-9500 | | Vanessa Grant
McCarthy Tetrault LLP
Box 48, Suite 5300
Toronto Dominion Bank Tower
Toronto, Ontario
Canada M5K 1E6
(416) 601-7525 |
Patrick O'Brien
Ropes & Gray LLP
Prudential Tower
800 Boylston Street
Boston, MA 02199-3600
(617) 951-7000 |
|
|
|
Gordon Raman
Borden Ladner Gervais LLP
Scotia Plaza
40 King Street West, 44th Floor
Toronto, Ontario
Canada M5H 3Y4
(416) 367-6000
|
Approximate date of commencement of proposed sale to the public:
As soon as practicable after the effective date of this Registration Statement.
Province of Ontario, Canada
(Principal jurisdiction regulating this offering)
It is proposed that this filing shall become effective (check appropriate box below):
| | | | | | |
| A. | | o | | upon filing with the Commission, pursuant to Rule 467(a) (if in connection with an offering being made contemporaneously in the United States and Canada). |
| B. | | ý | | at some future date (check appropriate box below) |
| | | 1. | | o | | pursuant to Rule 467(b) on ( ) at ( ) (designate a time not sooner than seven calendar days after filing). |
| | | 2. | | o | | pursuant to Rule 467(b) on ( ) at ( ) (designate a time seven calendar days or sooner after filing) because the securities regulatory authority in the review jurisdiction has issued a receipt or notification of clearance on ( ). |
| | | 3. | | o | | pursuant to Rule 467(b) as soon as practicable after notification of the Commission by the Registrant or the Canadian securities regulatory authority of the review jurisdiction that a receipt or notification of clearance has been issued with respect hereto. |
| | | 4. | | ý | | after the filing of the next amendment to this Form (if preliminary material is being filed).
|
If any of the securities being registered on this form are to be offered on a delayed or continuous basis pursuant to the home jurisdiction's shelf prospectus offering procedures, check the following box. o
The Registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the Registration Statement shall become effective as provided in Rule 467 under the Securities Act of 1933 or on such date as the Commission, acting pursuant to Section 8(a) of the Act, may determine.
PART I
INFORMATION REQUIRED TO BE DELIVERED TO OFFEREES OR PURCHASERS
The information in this prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and it is not soliciting an offer to buy these securities in any state where the offer or sale is not permitted.
Subject to Completion, dated September 7, 2011
Preliminary Prospectus
US$30,000,000
• common shares

SQI DIAGNOSTICS INC.
Common Shares
We are offering • of our common shares. This is the initial public offering of our common shares in the United States and includes a new issue of our common shares in Canada.
Our common shares are listed and posted for trading on the TSX Venture Exchange under the symbol "SQD-V". Our common shares have been approved for listing on the NYSE Amex LLC under the symbol "SQD". On September 6, 2011, the closing sale price of our common shares on the TSXV was C$2.71, or US$2.73 based on the U.S.-Canadian dollar noon exchange rate on September 6, 2011, as quoted by the Bank of Canada.
Investing in our common shares involves risks. See "Risk Factors" beginning on page • of this prospectus and see the risk factors incorporated by reference into this prospectus.
We are permitted, under a multi-jurisdictional disclosure system adopted by the United States, to prepare this prospectus in accordance with Canadian disclosure requirements, which are different from those of the United States. We prepare our financial statements, which are included in, and incorporated by reference into, this prospectus, in accordance with Canadian generally accepted accounting principles, and they are subject to Canadian auditing and auditor independence standards. Our financial statements may not be comparable to the financial statements of United States companies.
Acquiring, holding and disposing of our common shares may subject you to tax consequences both in the United States and Canada. This prospectus may not describe these tax consequences fully. You should read the tax discussion in this prospectus.
Your ability to enforce civil liabilities under United States federal securities laws may be adversely affected because we are organized under the laws of Canada, the majority of our officers and directors and the experts named in this prospectus are residents of Canada, and all or a substantial portion of our assets and the assets of those officers, directors and experts are located outside of the United States.
Neither the Securities and Exchange Commission nor any state securities regulator has approved or disapproved these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
| | | | | | |
| | | | | | |
| |
| | Price to Public
| | Underwriters' Fees
| | Net Proceeds to SQI, before expenses
|
|---|
| |
Per common share | | US$ | | US$ | | US$ |
| |
Total | | US$ | | US$ | | US$ |
|
The purchase price for investors in Canada will be payable in Canadian dollars and the purchase price for investors in the United States will be payable in U.S. dollars unless the underwriters otherwise agree. All of the proceeds of the offering will be paid by the underwriters in U.S. dollars based on the U.S. dollar offering price. The Canadian dollar offering price is the equivalent of the U.S. dollar offering price, based upon the U.S.-Canadian dollar noon exchange rate on , 2011, as quoted by the Bank of Canada.
We will pay all expenses of the offering.
The underwriters may also purchase up to an additional • common shares from us at the public offering price, less the underwriters' commission, within 30 days after the closing of this offering to cover over-allotments, if any, and for market stabilization purposes. If the underwriters exercise the option in full, the total underwriters' commission paid by us will be US$ • and the net proceeds to us, before expenses, will be US$ • .
The common shares will be ready for delivery in New York, New York on or about , 2011.
| | |
| Leerink Swann | | Rodman & Renshaw, LLC |
Kingsdale Capital Markets Inc. |
The date of this prospectus is , 2011
TABLE OF CONTENTS
| | | |
| | Page |
|---|
GENERAL MATTERS | | 1 |
FORWARD-LOOKING STATEMENTS | | 2 |
CURRENCY PRESENTATION AND EXCHANGE RATE INFORMATION | | 3 |
PROSPECTUS SUMMARY | | 5 |
| | Corporate Information | | 11 |
| | Details of the Offering | | 12 |
| | Selected Financial Information | | 13 |
RISK FACTORS | | 15 |
CAPITALIZATION | | 30 |
USE OF PROCEEDS | | 30 |
BUSINESS OF THE COMPANY | | 31 |
LEGAL PROCEEDINGS AND REGULATORY ACTIONS | | 53 |
ACQUISITION OF SCIENION AG | | 53 |
SELECTED CONSOLIDATED FINANCIAL INFORMATION | | 54 |
MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS | | 55 |
DIRECTORS AND OFFICERS | | 85 |
DESCRIPTION OF CAPITAL STRUCTURE | | 90 |
DIVIDEND POLICY | | 90 |
MARKET FOR SECURITIES | | 91 |
| | Prior Sales | | 91 |
PRINCIPAL SHAREHOLDERS | | 93 |
PLAN OF DISTRIBUTION | | 94 |
DOCUMENTS INCORPORATED BY REFERENCE | | 98 |
WHERE YOU CAN FIND MORE INFORMATION | | 99 |
AUDITORS, TRANSFER AGENTS AND REGISTRARS | | 99 |
CERTAIN CANADIAN FEDERAL INCOME TAX CONSIDERATIONS | | 99 |
CERTAIN MATERIAL UNITED STATES FEDERAL INCOME TAX CONSIDERATIONS | | 102 |
DOCUMENTS FILED AS PART OF THE U.S. REGISTRATION STATEMENT | | 107 |
ENFORCEABILITY OF CIVIL LIABILITIES | | 107 |
EXPERTS | | 108 |
LEGAL MATTERS | | 108 |
MATERIAL CONTRACTS | | 108 |
EXEMPTIONS FROM THE INSTRUMENT | | 108 |
INTEREST OF EXPERTS | | 109 |
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS OF SQI DIAGNOSTICS INC. | | SQI-F-1 |
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS OF SCIENION AG | | SC-F-1 |
INDEX TO PRO FORMA FINANCIAL STATEMENTS | | PF-1 |
GENERAL MATTERS
Unless otherwise noted or the context otherwise indicates, in this preliminary and amended and restated short form base PREP prospectus (the "prospectus"), the terms "SQI", "we", "us", "our" or "the Company" refer to SQI Diagnostics Inc. and our wholly-owned subsidiary, SQI Diagnostics Systems Inc., through which we conduct our business. Our trademarks include "SQI Diagnostics™", "SQiDWorks™", "IgXPLEX™", "QuantiSpot™" and others. Our assays for rheumatoid arthritis are referred to in this prospectus as "IgXPLEX RA". Our IgXPLEX RA quantitative assay is marketed in Canada under the name QuantiSpot Rheumatoid Arthritis. This prospectus contains company names, product names, trade names, trademarks and service marks of other organizations, all of which are the property of their respective owners.
Documents incorporated by reference into this prospectus may include market share information and industry data and forecasts obtained from independent industry publications and surveys. References in such documents to research reports, surveys or articles should not be construed as depicting the complete findings of the entire referenced report, survey or article. The information in any such report, survey or article is not incorporated by reference into this prospectus. Although we believe these sources are reliable, we have not independently verified any of the data nor ascertained the underlying economic assumptions relied upon in such reports, surveys or articles. Some data is also based on our estimates, which are derived from our review of our internal surveys, as well as independent sources. We cannot and do not provide you with any assurance as to the accuracy or completeness of such information. Market forecasts, in particular, are likely to be inaccurate, especially in respect of emerging markets, such as those for our products, or over long periods of time.
The information in this prospectus regarding the financial statements and business of Scienion AG has been furnished by Scienion.
You should rely only on the information contained in or incorporated by reference into this prospectus. Neither we nor the underwriters, have authorized any other person to provide you with different information.
You should not assume that the information contained in this prospectus is accurate as of any date other than the date on the front of this prospectus, unless otherwise noted in this prospectus or as required by law. It should be assumed that the information appearing in this prospectus and the documents incorporated by reference into this prospectus are accurate only as of their respective dates. Our business, financial condition, results of operations or prospects may have changed since those dates.
Information contained on our website, www.sqidiagnostics.com, is not part of this prospectus and is not incorporated by reference into this prospectus and may not be relied upon by prospective purchasers for the purpose of determining whether to invest in the common shares.
1
FORWARD-LOOKING STATEMENTS
This prospectus, including the documents incorporated by reference into this prospectus, contains forward-looking statements. These statements relate to future events or future performance and reflect our expectations and assumptions regarding our growth, results of operations, performance and business prospects and opportunities. Such forward-looking statements reflect our current beliefs and are based on information currently available to us. In some cases, forward-looking statements can be identified by terminology such as "our goal", "may", "would", "could", "will", "should", "expect", "plan", "intend", "anticipate", "believe", "estimate", "predict", "potential", "continue" or the negative of these terms or other similar expressions concerning matters that are not historical facts. The forward-looking statements in this prospectus, including any documents incorporated by reference into this prospectus, include, among others, statements regarding our future operating results, economic performance and product development efforts, and statements in respect of:
- •
- our expected future losses and accumulated deficit levels;
- •
- our requirement for, and our ability to obtain, future funding on favourable terms or at all;
- •
- market competition and technological advances of competitive products;
- •
- our expectations regarding the acceptance of our products by the market;
- •
- our expectations regarding the progress and the successful and timely completion of the various stages of the regulatory clearance process;
- •
- our strategy to develop new products and to enhance the capabilities of existing products;
- •
- our strategy with respect to research and development;
- •
- our dependence on expanding our customer base;
- •
- our plans to market, sell and distribute our products;
- •
- our plans in respect of strategic partnerships for research and development;
- •
- our ability to obtain a sufficient supply of the components needed for our systems;
- •
- the effect of operating as a public company in the United States and our plans for compliance;
- •
- our plans to acquire Scienion;
- •
- our plans to retain and recruit personnel;
- •
- our ability to satisfy customer demand for our systems;
- •
- our plans to correct defects or errors in our systems;
- •
- the effect of litigation on our business;
- •
- our strategy with respect to the protection of our intellectual property; and
- •
- our expectations with respect to existing and future corporate alliances and licensing transactions with third parties, and the receipt and timing of any payments to be made by us or to us in respect of such arrangements.
A number of factors could cause actual events, performance or results, including those in respect of the foregoing items, to differ materially from the events, performance and results discussed in the forward- looking statements. Factors that could cause actual events, performance or results to differ materially from those set forth in the forward-looking statements include, but are not limited to:
- •
- the extent of our future losses;
- •
- our ability to obtain the capital required to fund development and operations;
- •
- development or commercialization of similar products by our competitors;
- •
- our ability to develop and market our products;
- •
- our ability to comply with applicable governmental and securities regulations and standards;
2
- •
- our ability to develop and commercialize our technologies;
- •
- delays or failures in our ability to develop and implement new diagnostic products;
- •
- our reliance on a few key and significant customers;
- •
- our ability to attract and retain skilled and experienced personnel;
- •
- the impact of changes in the business strategies and development priorities of our strategic partners;
- •
- loss of suppliers or increases to the cost of the components of our systems;
- •
- the impact of legislative changes to the healthcare system and regulatory process;
- •
- the expense of compliance initiatives as a result of operating as a public company in the United States;
- •
- our ability to maintain effective internal control over financial reporting;
- •
- our ability to complete the proposed acquisition of Scienion and successfully integrate its business;
- •
- damage to our manufacturing facility or its failure to accommodate future sales growth;
- •
- the impact of unknown defects or errors and product liability claims;
- •
- the impact of liability from the use of hazardous and biological materials and other claims;
- •
- our ability to successfully manage fluctuations in revenue;
- •
- foreign currency fluctuations;
- •
- our ability to obtain patent protection and protect our intellectual property rights and not infringe on the intellectual property rights of others;
- •
- the expense and potential harm to our business of intellectual property litigation;
- •
- stock market volatility;
- •
- changing market conditions;
- •
- the fact that further equity financing may substantially dilute the interests of our shareholders; and
- •
- other risks detailed from time-to-time in our ongoing quarterly filings, annual information forms, annual reports and annual filings with applicable securities regulators, and those which are discussed under the heading "Risk Factors".
Although the forward-looking statements contained in this prospectus and in the documents incorporated by reference are based on what we consider to be reasonable assumptions based on information currently available to us, there can be no assurance that actual events, performance or results will be consistent with these forward-looking statements, and our assumptions may prove to be incorrect. These forward-looking statements are made as of the date of this prospectus.
Forward-looking statements made in a document incorporated by reference into this prospectus are made as of the date of the original document and have not been updated by us except as expressly provided for in this prospectus. Except as required under applicable securities legislation, we undertake no obligation to publicly update or revise forward-looking statements, whether as a result of new information, future events or otherwise.
CURRENCY PRESENTATION AND EXCHANGE RATE INFORMATION
In this prospectus, unless otherwise specified or the context otherwise requires, all dollar amounts are expressed in Canadian dollars. All references to "dollars", "C$" or "$" are to Canadian dollars and all references to "US$" are to United States dollars. For the amounts referenced under "Use of Proceeds" and "Plan of Distribution", the rate of exchange was US$1.00 = C$0.9910 or C$1.00 = US$1.0091, each based on the Bank of Canada's noon exchange rate on September 6, 2011.
The following table sets out (1) the high and low rate of exchange for one Canadian dollar in U.S. dollars during the indicated periods, (2) the average of the rate of exchange during those periods, and (3) the exchange
3
rate in effect as at the end of each of those periods, each based on the noon exchange rate published by the Bank of Canada. On September 6, 2011, the Bank of Canada's noon exchange rate was US$1.00 = C$1.0091 or C$1.00 = US$0.9910.
| | | | | | | | | | | | | | |
| | High | | Low | | Average | | End of
Period | |
|---|
Nine Month Periods ended June 30, | | | | | | | | | | | | | |
| | 2011 | | | 1.0542 | | | 0.9690 | | | 1.0115 | | | 1.0370 | |
| | 2010 | | | 1.0039 | | | 0.9221 | | | 1.0412 | | | 1.0606 | |
Fiscal Years Ended | | | | | | | | | | | | | |
| | September 30, 2010 | | | 1.0039 | | | 0.9221 | | | 0.9604 | | | 0.9429 | |
| | September 30, 2009 | | | 0.9426 | | | 0.7692 | | | 0.8472 | | | 0.9327 | |
4
PROSPECTUS SUMMARY
This summary highlights information contained in greater detail elsewhere in this prospectus. This summary may not contain all the information that you should consider before investing in our common shares. You should read the entire prospectus carefully, including "Risk Factors" starting on page 15 and our consolidated financial statements and related notes included elsewhere in this prospectus, before making an investment decision. Unless otherwise indicated, the terms "SQI", "we", "us", "our" and "the Company" refer to SQI Diagnostics Inc. and our wholly-owned subsidiary, SQI Diagnostics Systems Inc., through which we conduct our business.
SQI Diagnostics Inc.
Overview
We are a life sciences company that develops and commercializes proprietary technologies and products for advanced microarray diagnostics. Our goal is to become a leader in the development and commercialization of microarray and multiplexed diagnostics by offering our customers a comprehensive "turnkey" solution that increases the efficiency and ease of diagnostic testing and test development.
Our target customers — clinical, academic and diagnostic development laboratories — require diagnostic processing equipment and consumable tests ("systems") that are capable of processing large numbers of patient samples at low cost and with minimal labor requirements ("high-throughput systems"). High-throughput systems have not been widely employed in autoimmune disease, allergen or companion diagnostics testing and only limited use of high-throughput systems exists in infectious disease testing. To our knowledge, no fully-automated high-throughput systems exist that are capable of addressing the combined multiplex testing needs of these markets. A fully-automated system capable of providing multiple biomarker measurements in a single test array has the potential to increase a laboratory's throughput with significantly less labor, consumables and other costs.
Our proprietary microarray tests and fully-automated instruments are designed to simplify diagnostic testing workflow, increase throughput and reduce costs, all while providing excellent data quality. In many instances, our technology enables analysis that was traditionally unavailable.
Our Principal Business Lines
- •
- The development, manufacturing and marketing of fully-automated diagnostics instruments
SQiDworks is the only FDA-cleared, fully-automated, microarray system and multiplexing solution in our target markets, which can perform qualitative or quantitative testing for multiple biomarkers simultaneously. We also have a prototype of a fully-automated, bench-top diagnostic platform as well as a small, semi-automated system.
- •
- The development and marketing of tests for the autoimmune, allergen, infectious disease and companion diagnostics testing markets
We have received regulatory clearance to market our qualitative rheumatoid arthritis and celiac assays in the United States, our qualitative and quantitative rheumatoid arthritis and celiac assays in Canada, and our quantitative rheumatoid arthritis and celiac assays in the European Union. One of our key operational goals is to continue to develop and seek regulatory approval for additional tests, as we believe that expanding our "test menu" will drive adoption of our platform and products by customers. We also offer development services and manufacturing of diagnostic kits to our customers.
- •
- Our Printing Solutions
5
Acquisition of Scienion AG
On July 4, 2011, we announced the proposed acquisition of all of the issued and outstanding shares of Scienion, a German company that we believe is a market leader for microarray development, production arrayer printing systems and contract print and development services for the life sciences industry.
Scienion develops and markets a range of commercial microarray printing equipment and provides custom print and commercial print services.
We believe that the combination of our platform, our assays and the development of our test menu and, if the acquisition is completed, Scienion's industry leading print technologies, will enable the combined company to become a leader in sales of end-to-end microarray-based diagnostic systems, microarray printing and assay development services.
Our Target Markets
Diagnostics are critical to high-quality healthcare and guide a majority of clinical decisions. Over the last forty years, the number of biomarkers measured by laboratories has increased dramatically, and we believe there is a trend whereby healthcare providers are seeking to run diagnostic tests for an increasing number of biomarkers to aid in the diagnosis of disease. These tests are becoming increasingly complex and costly.
The 2012 global immunoassay diagnostic test market is estimated to be US$10.3 billion, of which we are strategically focusing on a directly addressable market of approximately US$4.5 billion, which includes immunological tests for autoimmune disease, infectious disease, and allergenecity. These three markets are estimated to be US$1.5 billion, US$2.4 billion and US$0.6 billion, respectively.
We have targeted these testing markets because we believe:
- •
- healthcare providers require the measurement of multiple biomarkers to aid in the diagnosis and therapeutic monitoring of these diseases;
- •
- these testing markets are typically underserved by fully-automated, high-throughput, multiplexed systems;
- •
- tests in these markets are generally run at sufficiently high volumes in larger test facilities which would benefit from both multiplexing and automation; and
- •
- these tests typically qualify for private and public reimbursement in Canada, the United States and the European Union.
We will also address selected areas of the companion diagnostics market, the "research use only" or "RUO" and lab-developed test markets and the market for microarray print and assay development tools.
Autoimmune Disease Tests
We have initially targeted the autoimmune testing segment for several reasons, which include:
- •
- there are ten autoimmune disease states that command most of the blood testing revenues for the entire autoimmune testing segment;
- •
- the most common tests for autoimmune diseases currently evaluate approximately 70 biomarkers, representing an average of approximately seven biomarkers per disease;
- •
- these tests are generally run at high volumes at larger test facilities and in batches and are not typically run on a "one off" basis;
- •
- each of the autoimmune diagnostic tests we are developing has predicate technologies with FDA clearance on older, single biomarker, manual titer plate technology that we intend to replace; and
- •
- regulatory clearance for the majority of autoimmune assays is through the FDA 510(k) process, which is well established.
6
Infectious Disease and Allergen Tests
We also plan to enter the immunoassay segment of the infectious disease diagnostics market, which is estimated to be approximately US$2.4 billion for 2012, followed by the immunoassay segment of the allergen diagnostics market, which is approximately US$0.6 billion for 2012.
Companion Diagnostics
Autoimmune diseases, such as rheumatoid arthritis, Crohn's disease and inflammatory bowel disease ("IBD"), are increasingly being treated with antibody-based or other biologic drugs. We believe that the effectiveness of the treatment of autoimmune patients with these drugs may be enhanced by the monitoring of the concentration of these drugs in a patient's blood by a "companion diagnostic" test. Our multiplexing technology allows us to combine the tests for both the diagnosis and therapeutic monitoring of a patient's disease.
RUO and Lab-Developed Tests
In addition to thein-vitro diagnostics ("IVD") market, we are targeting the market for RUO products and custom microarrays for lab-developed tests. Approximately one-third of the US$3.7 billion U.S. market for RUO and lab-developed tests is directly addressable by our proprietary technologies. We believe the combined U.S. and European markets for RUO and lab-developed tests that are directly addressable by our proprietary technologies to be approximately US$2 billion per year.
Microarray Print and Assay Development Tools
The market for protein and molecular microarray print equipment and services has been estimated to be approximately US$600 million per year.
Limitations of Current Technology
There are an increasing number of complex and costly diagnostic tests used to aid in the diagnosis and monitoring of many diseases. Our target customers — clinical, academic and diagnostic development laboratories — require diagnostic processing equipment and consumable tests that are capable of processing large numbers of patient samples at low cost and with minimal labor requirements. To our knowledge, no high-throughput systems exist that are capable of addressing the combined multiplex testing needs of these markets. A fully-automated system capable of providing multiple biomarker measurements in a single test array has the potential to increase a laboratory's throughput with significantly less labor, consumables, and other costs. We believe these cost savings would be realized because, among other things:
- •
- a fully-automated system reduces "hands-on" technician time;
- •
- the volume of liquid reagent is less and the number of other consumables, such as pipette tips, required to process one microarray is fewer when compared to traditional methods of running multiple tests in multiple titer plates;
- •
- a laboratory does not incur the expense of sending some tests (e.g. a large multiplex panel) to other labs;
- •
- the cost of managing multiple samples being tested by multiple technicians and combining the results to a single report would be largely eliminated by multiplexing capability; and
- •
- multiplexing and reducing the number of hands-on steps through automation would potentially reduce error rates.
Microtiter Plates
Laboratories almost exclusively use microtiter plates to perform immunoassay tests. The use of microtiter plates restricts an immunoassay test to the analysis of a single biomarker. Microtiter plates are predominantly
7
processed with a high contribution of direct labor. This increases the time to produce a test result and the potential for error and restricts the throughput of a laboratory.
Multiplex Diagnostic Tests
The processes used to complete a single test within a diagnostic panel of several biomarkers are complex and technically difficult. Such complexity has limited the throughput and efficiency of multiplex diagnostic testing, and technical challenges have restricted the widespread adoption of automated systems.
Bead-Based Array Systems
Bead-based array systems were initially developed for DNA-based testing and were subsequently adapted for protein-based and antibody-based testing. Bead-based methods, however, have faced limitations that reduce their utility, particularly for multiplex diagnostics and experimentation. In particular, the workflow for bead arrays is complex, time consuming and costly.
Automated Systems
While laboratories use automated systems for many types of blood tests, to our knowledge, there are no fully-automated high-throughput microarray systems for our target markets.
Antigen, Protein and Antibody Microarrays and Their Technical Challenges
Antigen, protein and antibody microarrays printed on a two-dimensional substrate solve several limitations of bead-based systems such as interactions between beads, but are challenging to develop as we believe there are several technological obstacles, including:
- •
- precision and accuracy of printing;
- •
- cross interference of similar molecules in the system;
- •
- calibration and standardization of multiple separate molecular signals; and
- •
- complex software systems required to analyze and measure the multiple signals.
Our Solution
Our proprietary microarray tests and automated systems are designed to simplify antigen, protein and antibody testing workflow, increase throughput, reduce costs and provide excellent data quality. In many instances, our technology enables analysis that was traditionally unavailable.
Our high-throughput SQiDworks diagnostic platform is a fully-automated microarray processing and analytical instrument, which provides significant cost savings and other benefits over existing technologies. Additionally, the incremental cost savings of tests run on our fully-automated platform versus existing technologies increase as the complexity of the test increases.
Our IgXPLEX microarrays have the ability to accurately measure multiple biomarkers in a single test. Additionally, our microarray technology uses less patient blood and has fewer steps than traditional methods, which increases the predictive value of the test. The increased predictive value of the test may allow the healthcare provider to choose a treatment plan earlier in the course of the disease.
Our IgXPLEX CHEX technology provides multiple in-microarray checks to ensure that the test has been completed without system, control, calibration or microarray-related errors. These tests reduce undetected errors that are common to microtiter and microarray tests.
Our proprietary multiplex assay development processes and microarray manufacturing capabilities, combined with our automated systems, are designed to significantly reduce the complexity and cost to our customers to commercialize microarray tests using their own biomarkers.
8
Products
SQI Platforms
| | | | | | | | |
| |
| | Clearance/Approval Status |
|---|
| | Development
Status |
|---|
Product | | Canada | | United States | | Europe |
|---|
SQiDworks | | Complete | | Licensed | | Cleared as a system with
IgXPLEX RA | | CE Marked |
SQiDman | | Development RUO | | Not Required — RUO | | Not Required — RUO | | Not Required — RUO |
SQiDlite RUO | | Prototype | | Not Required — RUO | | Not Required — RUO | | Not Required — RUO |
SQiDlite IVD | | Prototype | | To be filed | | To be filed | | To be filed |
We have developed our fully-automated SQiDworks platform to enable our customers to generate numerous patient test results with significantly less labor, consumables and other costs, while maintaining excellent data quality. We have received marketing clearance from the FDA and Canadian regulatory approval for, and have CE marked, our SQiDworks instrument system. SQiDworks is the only fully-automated microarray processing system to achieve these regulatory clearances.
SQiDman is our small semi-automated platform that we are commercialising for RUO purposes, and SQiDlite is our fully-automated, bench-top diagnostic platform.
SQI Assays
Our currently cleared or approved assays are as follows:
| | | | | | | | |
| |
| | Clearance/Approval Status |
|---|
| | Development
Status |
|---|
Product | | Canada | | United States | | Europe |
|---|
IgXPLEX RA (Qualitative) | | Complete | | Licensed | | Cleared | | N/A |
IgXPLEX RA* (Quantitative) | | Complete | | Licensed | | To be filed | | CE Marked |
IgXPLEX Celiac (Qualitative) | | Complete | | Licensed | | Cleared | | N/A |
IgXPLEX Celiac (Quantitative) | | Complete | | Licensed | | To be filed | | CE Marked |
- *
- Marketed in Canada under the name QuantiSpot Rheumatoid Arthritis.
Our four cleared or approved assays test for two of the more common autoimmune diseases. Rheumatoid arthritis is a chronic inflammatory disease. The prevalence of rheumatoid arthritis is approximately 2% world wide. It is estimated that total size of the patient market in the United States for rheumatoid arthritis diagnostic testing for 2010 was approximately six million patients. Celiac is an inherited autoimmune disorder that affects the digestive process of the small intestine. It is estimated that the total size of the patient market in the United States for celiac diagnostic testing for 2010 was approximately 3.5 million patients.
We also have IgXPLEX Lupus, IgXPLEX Vasculitis and IgXPLEX TNF products in the development stage and IgXPLEX IBD/Crohn's and IgXPLEX Antiphospholipid Syndrome products at the proof of concept stage. In addition, we are developing rheumatoid arthritis and celiac assays with greater sensitivity that measure additional biomarkers.
Scienion Products
Scienion offers a range of microarray printing equipment and associated services. Scienion's sciFLEXARRAYER range of microarray printing equipment is targeted to address research customers seeking bench-top equipment up to large customers seeking commercial-scale automated microarray printing equipment
9
as described in the table below. Scienion also markets contract microarray manufacturing and microarray development services.
| | | | |
Application | | Product | | Description |
|---|
Printing | | sciFLEXARRAYER S1 / DW | | Entry-level model of Scienion's sciFLEXARRAYER product line |
| | sciFLEXARRAYER S3 | | Automated non-contact dispensing system of ultra-low volumes designed for academic and research and development labs |
| | sciFLEXARRAYER S5 and S11 | | Automated non-contact dispensing systems of ultra-low volumes designed to manufacture high quality arrays |
| | sciFLEXARRAYER Sx | | Scalable robotic dispensing platforms that enable users to choose size of working area |
| | sciFLEXARRAYER S100 | | High-throughput array and biosensor production instrument |
Microarray Platform | | sciPLEXPLATE | | A specifically developed plate to array RNA, DNA, proteins, antigens and antibodies |
Liquid Handling | | sciSWIFT | | Storable and disposable liquid dispensing pens |
Key Competitive Strengths
We believe that our key competitive strengths include the following:
- •
- Only FDA Cleared, Fully-Automated Microarray Processing System. We have received marketing clearance from the FDA, Canadian regulatory approval for, and have CE marked, our SQiDworks instrument system as well as diagnostic tests for rheumatoid arthritis and celiac. SQiDworks is the only system for automatically processing, analyzing and providing test results in protein-based and antibody-based microarrays to achieve these regulatory clearances.
- •
- Fully-Integrated, High-Throughput Solution Dramatically Reduces Workflow and Cost. Our proprietary microarray tests and fully-automated systems are designed to significantly simplify antigen, protein and antibody workflow, increase throughput and reduce costs, all while providing excellent data quality. For example, competing systems require hundreds of steps to produce the same number of test results as can be produced by our SQiDworks fully-integrated diagnostics system in five steps.
- •
- Expertise in Microarray Assay Development. We have clinically validated development experience and have created processes, systems and development algorithms to simplify the commercialization of our products. We believe our expertise will reduce the time required to complete the commercial development of our pipeline products.
- •
- Focus on Penetrating Existing Markets that Are Technically Challenging and Have Established Reimbursement. We focus on disease testing markets that have existing reimbursement and payment programs in place, which allows us to quickly bring our products into established markets. Additionally, antigen, protein and antibody multiplex tests are more technically challenging to develop, which may restrict others from easily entering these markets.
10
- •
- Partnerships with Leading Institutions. We have entered into agreements with leading institutions and healthcare providers to collaborate with us in developing and validating our assays including providing us with access to approximately 10,800 clinically validated patient blood samples.
- •
- Significant Intellectual Property Portfolio. Our intellectual property covers key areas of our commercial business as well as our pipeline, including microarray surfaces, multiplexing, microarray analytics, and microarray processing.
Strategy
Our goal is to become an industry leader in the development and commercialization of microarray and multiplexing diagnostic systems. We intend to accomplish this goal through:
- •
- Product development efforts. We are continuing to focus our research and development on high-volume, high-margin, multiplexed tests for diseases for which there are existing tests that have reimbursement programs in place and predicate technologies that have received FDA clearance.
- •
- Strategic market penetration. We have identified and are marketing our turnkey SQiDworks platform and approved assays to a specific group of laboratories which process high volumes of tests and typically adopt new technologies to gain market share. Please see "— Sales and Marketing" for a description of our target laboratories.
- •
- Leveraging our core technology and expertise to access new markets and new customers. Our technology and microarray development processes enable us to provide customized third party microarray formatted test development and manufacturing services.
- •
- Seeking partnerships and strategic acquisitions. With our proposed acquisition of Scienion, we intend to become the industry leader in printing solutions, which will enable us to quickly expand our third party development and microarray manufacturing services. We intend to continue to seek partnerships that will enable us to expand into new markets, broaden and deepen our lines of business and develop and strengthen our relationships with our customers.
- •
- Leading through innovation. We intend to continue our research and development in each of our lines of business in order to become the industry leader in multiplexing microarrays.
Corporate Information
Our principal and registered office is located at 36 Meteor Drive, Toronto, ON M9W 1A4, and our telephone number is (416) 674-9500. We have a single subsidiary, SQI Diagnostics Systems Inc., which is wholly-owned.
Emblem Capital Inc., the predecessor to SQI Diagnostics Inc. was incorporated on September 11, 2003 pursuant to theCanada Business Corporation Act (the "CBCA") and filed articles of amendment to change its name to "SQI Diagnostics Inc."
On April 20, 2007, an amalgamation between Umedik Inc. and 670194 Canada Inc., a wholly-owned subsidiary of Emblem Capital Inc., was completed and the amalgamated company changed its name to "SQI Diagnostics Systems Inc." on September 7, 2007.
The predecessor to Umedik Inc. was formed on April 19, 1999 pursuant to theBusiness Corporations Act (Ontario) under the corporate name of Pockit Corporation. Pockit Corporation filed articles of amendment on June 9, 1999 to change its name to Poc•Kit Corporation. Poc•Kit Corporation was continued under the CBCA on December 1, 1999 under the name of e-umedik Inc. and e-umedik Inc. filed articles of amendment on October 20, 2000 to change its name to Umedik Inc. 6701914 Canada Inc. was incorporated on January 12, 2007 pursuant to the CBCA.
11
Details of the Offering
| | |
| Common Shares Offered: | | • common shares. |
Offering Price: |
|
US$ • (C$ • ) per common share. |
Size of Offering: |
|
US$ • (C$ • ) |
Common Shares Outstanding Assuming Completion of the Offering: |
|
As of September 6, 2011, we had 33,946,258 common shares outstanding (38,437,444 on a fully diluted basis). There will be • common shares outstanding immediately following the closing of the offering ( • on a fully diluted basis, including the shares issuable in connection with our proposed acquisition of Scienion). These calculations assume no exercise of the over-allotment option. See "Capitalization". |
Offering Type: |
|
Offering in the U.S. under the multi-jurisdictional disclosure system and in each of the Provinces of Ontario and British Columbia. |
Use of Proceeds: |
|
The estimated net proceeds of the offering of common shares to us, assuming no exercise of the over-allotment option, after deducting the underwriters' commission and our estimated expenses will be $ • . We intend to use approximately $15 million of the net proceeds in satisfaction of the cash portion of the purchase price payable for the Scienion acquisition, a portion for sales and marketing initiatives, a portion for research and development activities, a portion for the expansion of our manufacturing operations and the balance for working capital and other general corporate purposes. See "Use of Proceeds". |
Over-Allotment Option: |
|
We have granted the underwriters the over-allotment option exercisable for a period of 30 days from the closing of the offering to purchase up to an additional • common shares equal to 15% of the offered shares at the offering price to cover over-allotments, if any, and for market stabilization purposes. See "Underwriting". |
Dividend Policy: |
|
To date, we have not paid any dividends and do not expect to do so in the foreseeable future. We currently intend to retain all future earnings for the operation and expansion of our business. See "Dividend Policy". |
Listing: |
|
Our outstanding common shares are listed and posted for trading on the TSXV under the symbol "SQD". Our common shares have been approved for listing on NYSE Amex under the trading symbol "SQD". |
Risk Factors: |
|
There are certain risk factors inherent in an investment in our common shares and in our activities. You should carefully review the information set out under "Risk Factors" and all other information in and incorporated by reference into this prospectus before making an investment in our common shares. |
Lock-up Agreements: |
|
Our directors and officers have agreed to enter into lock-up agreements with the underwriters. These lock-up agreements restrict the ability of our directors and these officers to sell or transfer their common shares for a period of 90 days after the date of the final prospectus. |
12
Selected Financial Information
The following table sets forth selected financial information for the dates and periods indicated. Our selected financial information as of September 30, 2010 and 2009 has been derived from our audited consolidated financial statements for those annual periods, which are included elsewhere in this prospectus and incorporated by reference into this prospectus, and our selected financial information as of June 30, 2011 and 2010 has been derived from our unaudited interim consolidated financial statements for the nine month periods ended June 30, 2011 and 2010, which are included elsewhere in this prospectus and incorporated by reference into this prospectus. Our consolidated financial statements have been prepared in Canadian dollars and in accordance with Canadian generally accepted accounting principles. For a reconciliation of the significant differences in our consolidated annual financial statements between Canadian GAAP and U.S. GAAP, see "Supplemental Note Regarding the Reconciliation of the Consolidated Financial Statements for the Years Ended September 30, 2010 and 2009 with U.S. GAAP", which is included elsewhere in this prospectus and incorporated by reference into this prospectus. For a reconciliation of the significant differences in our interim consolidated financial statements between Canadian GAAP and U.S. GAAP, see the "Supplemental Note Regarding the Reconciliation of the Interim Consolidated Financial Statements for the Three and Nine Month Periods Ended June 30, 2011 and 2010 with U.S. GAAP", which is included elsewhere in this prospectus and incorporated by reference into this prospectus. Operating results for the nine month period ended June 30, 2011 are not necessarily indicative of the results that may be expected for the fiscal year ended September 30, 2011 or any other future period. The summary financial information below should be read in conjunction with our financial statements and the notes thereto and "Management's Discussion and Analysis of Financial Condition and Results of Operations" included elsewhere in this prospectus and incorporated by reference into this prospectus.
| | | | | | | | | | | | | | |
| | Year Ended
September 30, | | 9 Months Ended
June 30, | |
|---|
Consolidated Statement of Operations Data
C$ in 000's except per share data | | 2009 | | 2010 | | 2010 | | 2011 | |
|---|
Revenue: | | | | | | | | | | | | | |
| | Product sales | | $ | 5 | | $ | 5 | | $ | — | | $ | 22 | |
| | Consulting fees | | | 27 | | | 30 | | | 21 | | | 9 | |
| | | | | | | | | | |
Total Revenue | | | 32 | | | 35 | | | 21 | | | 31 | |
| | | | | | | | | | |
Expenses | | | | | | | | | | | | | |
| | Salaries and wages | | | 469 | | | 556 | | | 404 | | | 607 | |
| | General and administrative | | | 447 | | | 471 | | | 324 | | | 449 | |
| | Professional and consulting | | | 436 | | | 659 | | | 437 | | | 669 | |
| | Sales and marketing | | | 409 | | | 474 | | | 309 | | | 329 | |
| | Stock based compensation | | | 380 | | | 402 | | | 184 | | | 372 | |
| | Research and Development | | | 3,362 | | | 5,059 | | | 3,438 | | | 4,056 | |
| | Amortization — property and equipment | | | 417 | | | 415 | | | 310 | | | 337 | |
Amortization — patent and trademark | | | 98 | | | 100 | | | 85 | | | 88 | |
| | | | | | | | | | |
Total expenses | | | 6,018 | | | 8,136 | | | 5,491 | | | 6,907 | |
| | | | | | | | | | |
Operating loss before interest | | | (5,986 | ) | | (8,101 | ) | | (5,470 | ) | | (6,876 | ) |
Interest Income | | | 121 | | | 32 | | | 20 | | | 56 | |
Interest Expense | | | (45 | ) | | (4 | ) | | (2 | ) | | — | |
| | | | | | | | | | |
Net loss | | $ | (5,910 | ) | $ | (8,073 | ) | $ | (5,452 | ) | $ | (6,820 | ) |
| | | | | | | | | | |
Basic and diluted loss per share | | $ | (0.23 | ) | $ | (0.27 | ) | $ | (0.18 | ) | $ | (0.20 | ) |
Weighted average number of shares | | | 25,158 | | | 30,349 | | | 29,553 | | | 33,849 | |
13
| | | | | | | | | | |
Consolidated Balance Sheet Data | | September 30,
2009 | | September 30,
2010 | | June 30,
2011 | |
|---|
Cash and cash equivalents | | $ | 3,180 | | $ | 9,408 | | $ | 2,817 | |
Working capital | | | 3,280 | | | 8,930 | | | 2,238 | |
Total assets | | | 6,205 | | | 13,134 | | | 7,488 | |
Total shareholders' equity | | | 5,836 | | | 12,162 | | | 5,960 | |
14
RISK FACTORS
An investment in our common shares involves a number of risks. In addition to the other information contained in this prospectus and in the documents incorporated by reference into this prospectus, including our consolidated financial statements and related notes, you should give careful consideration to the following risk factors. Any of the matters highlighted in these risk factors could have a material adverse effect on our business, results of operations and financial condition, causing an investor to lose all, or part of, its, his or her investment.
The risks and uncertainties described below are not the only ones we face. Additional risks and uncertainties that we are not aware of or focused upon, or that we currently deem to be immaterial, may also impair our business operations and cause the trading price of our common shares to decline.
If our proposed acquisition of Scienion is completed, our combined businesses will face similar risks to those described below.
Risks Related to Our Business and Strategy
We have incurred losses since inception, and we expect to continue to incur losses for the foreseeable future.
We have a limited operating history and have incurred significant losses in each fiscal year since our inception, including net losses of $3.8 million, $5.9 million, $8.1 million and $6.8 million during fiscal 2008, 2009, 2010 and the nine months ended June 30, 2011, respectively. As of June 30, 2011, we had an accumulated deficit of $40.3 million. These losses have resulted principally from costs incurred in our research and development programs and from our selling, general and administrative expenses. We expect to continue to incur operating and net losses and negative cash flow from operations, which may increase, for the foreseeable future due in part to anticipated increases in expenses for research and product development and expansion of our sales and marketing capabilities. Additionally, following this offering, we expect that our selling, general and administrative expenses will increase due to the additional operational and reporting costs associated with being a SEC reporting company. We anticipate that our business will generate operating losses until we successfully implement our commercial development strategy and generate significant additional revenues to support our level of operating expenses. Because of the numerous risks and uncertainties associated with our commercialization efforts and future product development, we are unable to predict when we will become profitable, and we may never become profitable. Even if we do achieve profitability, we may not be able to sustain or increase our profitability.
Our future capital needs are uncertain and we may need to raise additional funds in the future, which may not be available on a timely basis or on commercially reasonably terms.
We believe that the net proceeds from this offering remaining after the completion of our proposed acquisition of Scienion described in this prospectus, together with our existing cash and cash equivalents, will be sufficient to meet our anticipated cash requirements for at least the next 18 months. However, we may need to raise substantial additional capital to:
- •
- expand the commercialization of our products;
- •
- manufacture our platforms in advance of placing them with our customers;
- •
- fund our operations; and
- •
- further our research and development.
Our future funding requirements will depend upon many factors, including:
- •
- development of new and existing products;
- •
- market acceptance of our products;
- •
- the cost of our research and development activities;
- •
- the cost of potential licensing of technologies patented by others;
- •
- the cost of filing and prosecuting patent applications;
15
- •
- the cost of defending, in litigation or otherwise, any claims that we infringe third party patents or violate other intellectual property rights;
- •
- the cost and timing of regulatory clearances or approvals;
- •
- the cost and timing of establishing additional sales, marketing and distribution capabilities;
- •
- the cost and timing of establishing additional technical support capabilities;
- •
- the effect of competing technological and market developments; and
- •
- the extent to which we acquire or invest in businesses, products and technologies, although we currently have no commitments or agreements relating to any of these types of transactions, other than our proposed acquisition of Scienion.
If we raise additional funds by issuing equity securities, our shareholders may experience dilution. Debt financing, if available, may involve covenants restricting our operations or our ability to incur additional debt. Any debt or additional equity financing may contain terms that are not favorable to us or our shareholders. If we raise additional funds through collaboration and licensing arrangements with third parties, it may be necessary to relinquish some rights to our technologies or our products, or grant licenses on terms that are not favorable to us.
If we do not have, or if we are unable to timely obtain additional funds on acceptable terms, or at all, we may have to delay development or commercialization of our products or license to third parties the rights to commercialize products or technologies that we would otherwise seek to commercialize. We also may have to liquidate some or all of our assets, reduce the scope of or eliminate some or all of our development programs, reduce marketing, customer support or other resources devoted to our products, or cease operations. Any of these factors could harm our business, financial condition and results of operations.
Market competition and technological advances of similar diagnostics products could reduce the attractiveness of our products or render them obsolete.
The markets for our products are characterized by rapidly changing technology, evolving industry standards, changes in customer needs, emerging competition, new product introductions and strong price competition. We compete with both established and development stage companies, universities, research institutions, governmental agencies and healthcare providers that design, manufacture and market similar diagnostic products. Many of our current competitors have significantly greater name recognition, greater financial and human resources, broader product lines and product packages, larger sales forces, larger existing installed bases, larger intellectual property portfolios and greater experience and capabilities in researching, developing and testing products, in obtaining FDA and other regulatory approvals or clearances, and in manufacturing, marketing and distribution, than we have. For example, companies such as Bio-Rad Laboratories Inc., Phadia AB, Axis-Shield plc, and INOVA Diagnostics, Inc. have products that compete in certain segments of the market in which we sell our products, including immunoassays. In addition, a number of other companies and academic groups are in the process of developing novel products and technologies for diagnostics markets.
With respect to our proposed acquisition of Scienion, the largest array print manufacturers that are also offering custom array services and whom we believe represent Scienion's most significant competitors include, in the United States, Arrayit Corporation, Bio-Synthesis Inc., Full Moon Biosystems, Inc., Microarrays Inc., Aushon BioSystems, Inc., and Applied Microarrays, Inc. and, in Europe, GeSiM mbH, and Arrayjet Limited.
Our competitors may be able to respond more quickly and effectively than we can to new or changing opportunities, technologies, standards or customer requirements. In light of these advantages, even if our technology is more effective than the product or service offerings of our competitors, current or potential customers might accept competitive products and services in lieu of purchasing our technology. We anticipate that we will face increased competition in the future as existing companies and competitors develop new or improved products and as new companies enter the market with new technologies. We may not be able to compete effectively against these organizations. Increased competition is likely to result in pricing pressures, which could harm our sales, profitability or market share. Our failure to compete effectively could materially and adversely affect our business, financial condition and results of operations.
16
If our products fail to achieve and sustain sufficient market acceptance, our revenue will be adversely affected.
We currently have one customer and our success depends, in part, upon our ability to develop and market products that are recognized and accepted as reliable, accurate, timely and cost effective by physicians, lab technicians and administrators. Most of our potential customers already use expensive diagnostic products and systems in their laboratories and may be reluctant to replace those systems. Market acceptance of our products and technologies will depend upon many factors, including our ability to provide a broad test menu of assays to potential customers, and our ability to convince potential customers that our systems are an attractive cost- and time-saving alternative to existing technologies. Compared to most competing technologies, our microarray assay technology is relatively new, and most potential customers have limited knowledge of, or experience with, our products. Prior to adopting our microarray assay technology, some potential customers may need to devote time and effort to testing and validating our systems. Any failure of our systems to meet these customer benchmarks could result in customers choosing to retain their existing systems or to purchase systems other than ours.
We are subject to complex regulatory compliance requirements and the failure to obtain, or the withdrawal of, regulatory clearance or approval for our products could adversely affect our ability to market our products and/or require us to incur significant costs to comply with such requirements.
We operate in a highly regulated industry and we are subject to the authority of certain regulatory agencies, including Health Canada, the FDA, European Conformity (CE) and applicable health authorities in other countries, with regard to the development, testing, manufacturing, marketing and sale of our diagnostic products. The process of obtaining such clearances or approvals can be costly and time-consuming, and if we are unable to timely obtain or maintain regulatory clearances or approvals, it would have a material adverse effect on our business. Clearance by regulatory authorities can be suspended or revoked, or we could be fined, based on a failure to continue to comply with applicable standards. Any failure to obtain (or significant delay in obtaining) or maintain applicable regulatory clearances or approvals (or, to a lesser extent, approval of applicable health authorities in other countries) for our new or existing products could materially affect our ability to market its products successfully and could therefore have a material adverse effect on our business. Additionally, the authority of the regulatory agencies or the application of certain regulations may be expanded or otherwise changed in such a manner that would place additional regulatory burdens on us or our customers. Such a change in our industry could have a material adverse effect on our business.
We must manufacture products in compliance with regulatory requirements, in sufficient quantities and on a timely basis, while maintaining product quality and acceptable manufacturing costs. If we or our suppliers are unable to manufacture or contract for such capabilities on acceptable terms, our plans for commercialization could be materially adversely affected.
Our manufacturing facilities are subject to periodic regulatory inspections by the regulatory agencies and these facilities are subject to quality standards requirements of the applicable regulatory authorities. We, or our contractors, may not satisfy such regulatory or standards requirements, and any failure to do so may have a material adverse effect on our business, financial condition and results of operations.
We may not be able to develop new products or enhance the capabilities of our existing diagnostics products to keep pace with rapidly changing technology and customer requirements.
The field of diagnostics is characterized by rapidly changing and developing technologies that include new products that could render our diagnostic processing equipment and consumable tests obsolete at any time and thereby adversely affect our financial condition and future prospects. Our success depends upon our ability to develop new products with improved performance and cost effectiveness in existing and new markets. New technologies, techniques or products could emerge that might offer better combinations of price and performance than our current or future product lines. It is critical to our success for us to anticipate changes in technology and customer requirements and to successfully introduce new, enhanced and competitive technology to meet our prospective customers' needs on a timely basis.
Developing and marketing new products and services will require us to incur substantial development costs and we may not have adequate resources available to be able to successfully introduce new versions of, or
17
enhancements to, our products. We cannot guarantee that we will be able to maintain technological advantages over emerging technologies in the future. While we plan to continue to make improvements to our current and future cleared or approved and marketed diagnostic processing equipment and consumable tests, we may not be able to successfully implement these improvements. If we fail to keep pace with emerging technologies, demand for our products will not grow, and our business, revenue, financial condition and operating results could suffer materially. Even if we successfully implement some or all of these planned improvements, we cannot guarantee that potential customers will find our enhanced products to be an attractive alternative to existing technologies, including our current products.
Research and development of diagnostic products requires significant testing and investment and may not result in commercially viable products within the timeline anticipated, if at all.
New diagnostic products, and improvements to existing diagnostic products, require significant research, development, testing and investment prior to any final commercialization. Our business depends upon the continued development and improvement of our existing products, our development of new products to serve existing markets and our development of new products to create new markets and applications that were previously not practical with existing systems. We believe that the adoption of our platform by potential customers depends, in part, upon our ability to provide a test menu of assays to potential customers. To date, we have obtained regulatory approval for only a few diagnostic assays.
We intend to devote significant personnel and financial resources to research and development activities designed to advance the capabilities of our diagnostic technology and, in the case of ourin vitro diagnostics business, to obtain regulatory approval of additional assays. In the past, our product development projects have been delayed. We may have similar delays in the future, and we may not obtain any benefits from our research and development activities. Any delay or failure by us to develop new products or enhance existing products would have a material adverse effect on our business and results of operations. If we are unable to successfully develop these products, accomplish such improvements, receive applicable regulatory clearances or approvals, produce the products in commercial quantities at reasonable costs, or successfully market the products, it would have a material adverse effect on our business and results of operations. Our long-term success must be considered in light of the expenses, difficulties and delays frequently encountered in connection with the development of new technology and the competitive and highly regulated environment in which we operate.
We may need additional capacity to meet our manufacturing needs at the end of 2012.
Based on our microarray manufacturing forecasts, we expect that there is adequate capacity in our current location to expand our manufacturing capacity to meet our expected needs until the end of 2012. Until a facility upgrade is completed, we intend to undertake equipment upgrades to ensure sufficient manufacturing capacity to meet our expected requirements for commercial sales and our internal validation studies until the end of 2012.
Our inability to obtain our required manufacturing space in a timely manner and on terms acceptable to us will result in delays which could have a material adverse effect on our financial condition and results of operations.
If it becomes necessary to relocate our operations even if we are able to enter into a lease for a new facility, we must implement and maintain an international standard "ISO" quality management system for such new facility. Any delay in implementing an ISO quality management system will have a material adverse effect on our financial condition and results of operations.
Our future success depends upon our ability to expand our customer base and introduce new products and services.
Our success will depend upon our ability to gain acceptance, and then increase our market share, among our customers, attract additional customers outside of our initial target markets, and bring to market new products and services. Attracting new customers and introducing new products and services requires substantial time and expense. For example, it may be difficult to identify, engage and market to customers who are unfamiliar with the benefits of our products and services. Any failure to establish and expand our existing customer base or launch new products or services would adversely affect our ability to increase our revenues.
18
We have limited experience in marketing, selling and distributing our products, and we need to expand our internal and external sales and marketing force and distribution capabilities to successfully commercialize and sell our products.
As we are in the early stages of commercializing and marketing our products, we have limited experience in marketing, selling and distributing our products. We may not be able to market, sell and distribute our products effectively enough to support our planned growth. We intend to market, sell and distribute our products directly through our own sales force in North America, Europe and elsewhere. Our future sales will depend in large part upon our ability to develop and substantially expand our direct sales force and to increase the scope of our marketing efforts.
Our products are technically complex and used for specialized applications. Our ability to market our products effectively will depend, in part, upon our ability to convince laboratories that our products will deliver accurate patient results in less time and with significantly reduced labor, consumables and other costs. As a result, we believe it is necessary to develop a direct sales force that includes people with specific scientific backgrounds and expertise and a marketing group with technical sophistication. Competition for such employees is intense. We may not be able to attract and retain personnel, or be able to build an efficient and effective sales and marketing force, which could negatively impact sales of our products, and reduce any future revenues and profitability.
If our sales, marketing and distribution efforts are not successful, our technologies and products may not gain market acceptance, which would materially impact our business operations.
We rely on strategic partnerships for research and development and commercialization of our products.
We have entered into and may continue to enter into strategic partnerships with a number of medical institutions. For example, we have entered into strategic agreements with the Cleveland Clinic, Beth Israel Deaconess Medical Center, Hospital Clinic De Barcelona, University Hospital Maastricht, and The University of North Carolina at Chapel Hill. If any of our strategic partners were to change their business strategies or development priorities, they may no longer be willing or able to participate in such strategic partnerships which could have a material adverse effect on the timing of our future development efforts. In addition, we may not control the strategic partnerships in which we participate. We may also have certain obligations with regard to our strategic partnerships, in addition to the obligation to pay money, such as an obligation to publish the results of research.
If any of our strategic partners terminate their relationship with us or fail to perform their obligations in a timely manner, or if we fail to perform our obligations in a timely manner, the development or commercialization of our technology in potential products may be affected, delayed or terminated.
We depend upon key suppliers for some of the components and materials used in our platform technologies and our microarrays, and the loss of any of these suppliers could harm our business.
We rely on key suppliers for certain components and materials used in our platform technologies, including our SQiDworks diagnostic platform and our microarrays. We do not have agreements with these key suppliers to supply us with components in the future. The loss of any of these key suppliers would require significant time and effort to locate and qualify an alternative source of supply. There are a limited number of suppliers who can manufacture the highly specialized equipment that forms a part of our SQiDworks system.
Our first set of assays being commercialized requires a highly specific mono-layer coating on the glass surface which is used to bond each of the microarray "spots". We have worked closely with these manufacturers to extend the capabilities of their standard products to support the unique needs of our platform technologies and microarray devices. Any change in any component that forms a part of our SQiDworks system will require additional testing to ensure that it performs in a substantially similar manner to the existing component.
Our reliance on these suppliers also subjects us to other risks that could harm our business, including the following:
- •
- we may be subject to increased component costs;
19
- •
- we may not be able to ensure that any component that we change performs in a substantially similar manner to the existing component;
- •
- we may not be able to obtain adequate supply in a timely manner or on commercially reasonable terms;
- •
- our suppliers may make errors in manufacturing components that could negatively affect the efficacy of our systems or cause delays in shipment of our systems; and
- •
- our suppliers may encounter financial hardships unrelated to our demand for components, which could inhibit their ability to fulfill our orders and meet our requirements.
We may not be able to quickly establish additional or replacement suppliers, particularly for our single source components. Any interruption or delay in the supply of components or materials, or our inability to obtain components or materials from alternate sources at acceptable prices in a timely manner, could impair our ability to meet the demand of our strategic partners and future customers.
Future legislative or regulatory changes to the healthcare system, including reimbursement, may adversely affect our business.
The healthcare regulatory environments in the jurisdictions in which we operate and plan to operate may change in a way that restricts our ability to market our diagnostic testing products due to medical coverage or reimbursement limits. Sales of our diagnostic systems will depend, in part, upon the extent to which the costs to patients of such tests are paid by health maintenance, managed care, and similar healthcare management organizations, or reimbursed by government health payor administration authorities, private health coverage insurers and other third party payors. These healthcare management organizations and third party payors are increasingly challenging the prices charged for medical products and services. The containment of healthcare costs has become a priority of governments. Accordingly, our potential products may not be considered cost effective, and reimbursement to the ultimate patient may not be available or sufficient to allow us to sell our products on a competitive basis. Legislation and regulations affecting reimbursement for our products may change at any time and in ways that are difficult to predict and these changes may be adverse to us. For example, a reduction in U.S. Medicare, Medicaid or other third party payor reimbursements for diagnostic services could have a negative effect on our operating results. In June 2011, the FDA issued draft guidance that sets forth the FDA's proposed interpretation of laws regarding the marketing of IVD products labelled as RUO products that could be used forin vitro diagnostic purposes. Among other things, the draft guidance suggests that it is generally inappropriate for a manufacturer to sell RUO products to clinical laboratories that the manufacturer knows, or has reason to know, use the products for clinical diagnostic uses. Given that the guidance is in draft form and has only recently been issued by the FDA, it is not clear how the FDA will interpret this guidance. As a result, we cannot be certain what impact, if any, this guidance will have on our business.
We will incur significant increased costs as a result of operating as a public company in the United States, and our management will be required to devote substantial time to new compliance initiatives.
As a new public company in the United States, we will incur significant legal, accounting and other expenses that we did not incur previously. In addition, the United States Sarbanes-Oxley Act, as well as new rules subsequently implemented by the SEC, have imposed various new requirements on public companies, including requiring changes in corporate governance practices. We expect to become subject to additional rules as a result of the adoption of the United States Dodd-Frank Wall Street Reform and Consumer Protection Act. Our management and other personnel will need to devote a substantial amount of time to these compliance initiatives. Moreover, these rules and regulations will increase our legal and financial compliance costs and will make some activities more time-consuming and costly. For example, we expect these rules and regulations to make it more difficult and more expensive for us to obtain director and officer liability insurance and we may be required to incur substantial costs to maintain the same or similar coverage.
20
If we fail to maintain effective internal control over financial reporting in the future, the accuracy and timing of our financial reporting may be impaired, which could adversely affect our business and our share price.
The Sarbanes-Oxley Act requires, among other things, that we perform system and process evaluation and testing of our internal control over financial reporting to allow our management to report on the effectiveness of our internal control over financial reporting, as required by Section 404 of the Sarbanes-Oxley Act. Our testing, or the subsequent testing by our independent registered public accounting firm, may reveal deficiencies in our internal control over financial reporting that are deemed to be material weaknesses. Our compliance with Section 404 will require that we incur substantial accounting expense and expend significant management time on compliance-related issues. If we are not able to comply with the requirements of Section 404 in a timely manner, or if we or our independent registered public accounting firm identifies deficiencies in our internal control over financial reporting that are deemed to be material weaknesses, the market price of our shares could decline and we could be subject to sanctions or investigations by the SEC or other regulatory authorities, which would require additional financial and management resources to address.
Failure to complete our planned Scienion acquisition or to successfully integrate Scienion's business with ours could negatively affect our business, financial condition and results of operations.
The completion of our acquisition of Scienion is subject to the satisfaction of certain closing conditions, including the closing of the offering for gross proceeds of at least $30,000,000. If we do not complete our proposed acquisition of Scienion, we will not realize any of the expected benefits of the acquisition. Based on current exchange rates, if the full offering is sold, we expect to waive the condition precedent. To the extent that the offering does not raise the anticipated proceeds of US$30 million, we will consider waiving this condition precedent, depending on the amount of the actual proceeds.
Our pending Scienion acquisition may consume a significant amount of our management resources. The success of the acquisition will depend, in part, upon our ability to realize the anticipated benefits from integrating Scienion's business with our existing businesses. The integration process may be complex, costly and time-consuming. To realize these anticipated benefits, we must successfully combine the business of Scienion in an efficient and effective manner. If we are not able to achieve these objectives within the anticipated time frame, or at all, the anticipated benefits of the acquisition may not be realized fully, or at all, or may take longer to realize than expected. We will also incur significant costs in connection with the completion of the Scienion acquisition. Most of these costs will be non-recurring transaction expenses.
We rely on certain key personnel and our ability to successfully grow our business would be adversely affected by their departure from our company.
Our performance depends substantially upon the performance of our senior management and key scientific and technical personnel, including our Chief Executive Officer, Claude Ricks, our Chief Financial Officer, Andrew Morris, and our Chief Scientific Officer, Dr. Peter Lea. Retaining these key personnel and recruiting additional qualified personnel in the future will be critical to our success. We believe there is only a limited number of individuals with the requisite skills to serve in many of our key positions. Competition for qualified personnel in the diagnostics industry is intense and recruiting and retaining qualified personnel with experience in our industry is very difficult. We compete for key personnel with other medical equipment and software manufacturers and technology companies, as well as universities and research institutions.
If we are unable to attract and retain skilled and experienced personnel, or if we lose the services of any member of our senior management or our scientific or technical staff, we could experience significant delays in, or we could be unable to, complete the development and commercialization of our products or achieve our other business objectives, and such a development could require our management to divert its attention to transition matters and identification of suitable replacements, if any. Such a development could have a material adverse effect on our business, financial condition and results of operations.
We do not maintain, and do not intend to obtain, key employee life insurance on any of our personnel.
21
A portion of our compensation to our key employees is in the form of stock option grants. A prolonged decline in our share price could make it difficult for us to retain our employees and recruit additional qualified personnel.
Additionally, following our proposed acquisition of Scienion, we expect that the current Chief Executive Officer of Scienion, Dr. Holger Eickhoff, will enter into an employment agreement with us to develop and commercialize Scienion's and our products. If we lose the services of Dr. Eickhoff, we may experience significant delays in connection with the integration of Scienion's business with our existing business or we may fail to realize some of the expected benefits of the acquisition which would have a material adverse effect on our business, financial condition and results of operations.
If we cannot provide quality technical support, we could lose customers and our operating results could suffer.
The placement of our products and the introduction of our technology into our customers' existing operations and on-going customer support can be complex. Accordingly, we need highly trained technical support personnel. To effectively support new customers, we will need to substantially expand our technical support staff. If we are unable to attract, train or retain the number of highly qualified technical services personnel that our business will require, our business, financial condition and results of operations will suffer.
We may experience development or manufacturing problems or delays that could limit the growth of our revenue or increase our losses.
We have been developing our core technologies in our facilities in Toronto, Canada, which we believe has adequate space to expand the manufacturing capacity to our expected needs for the foreseeable future. However, we may encounter unforeseen situations at this facility that would result in delays or shortfalls in our development and production. In addition, our development and production processes and assembly methods may have to change to accommodate any significant future expansion of our manufacturing capacity. If we are unable to keep up with development of or demand for our products, our revenue could be impaired, market acceptance for our products could be adversely affected and our customers might instead purchase our competitors' products. Our inability to successfully manufacture our products would have a material adverse effect on our business, financial condition and results of operations.
Our products could have unknown defects or errors, which may give rise to claims against us and adversely affect market adoption of our systems.
Our products utilize complex technology applied on a small scale, and our systems may develop or contain undetected defects or errors. As our production levels increase, material performance problems, defects or errors could arise. We may determine to correct any defects or errors in response to customer concerns, in order to preserve customer relationships, and to help foster continued adoption and use of our systems. The costs incurred in correcting any defects or errors may be substantial and could adversely affect our operating margins.
In manufacturing our products, we depend upon third parties for the supply of various components. Many of these components require a significant degree of technical expertise to produce. If our suppliers fail to produce components to specification, or if the suppliers, or we, use defective materials or workmanship in the manufacturing process, the reliability and performance of our products will be compromised.
If our products contain defects, we may experience:
- •
- a failure to achieve market acceptance or expansion of our product sales;
- •
- loss of customer orders and delay in order fulfillment;
- •
- damage to our brand reputation;
- •
- increased cost of our warranty program due to product repair or replacement;
- •
- product recalls or replacements;
- •
- inability to attract new customers;
22
- •
- diversion of resources from our manufacturing and research and development departments into our service department; and
- •
- legal claims against us, including product liability claims, which could be costly and time consuming to defend and result in substantial damages.
The occurrence of any one or more of the foregoing could negatively affect our business, financial condition and results of operations.
We operate in an industry where there is the potential for substantial product liability claims that would cause us to incur significant costs, create adverse publicity and/or prevent us from commercializing our products.
We may be subject to claims of personal injury and could become liable to clinical laboratories, hospitals, physicians and patients for harm resulting from use of our products. We could suffer financial loss due to defects in our products, and such financial loss and potential litigation expenses could have a material adverse effect on our business, financial condition and results of operations. If our product liability insurance is not adequate to cover all claims or if we are unable to maintain such insurance at reasonable cost, it would have a material adverse effect on our business, financial condition and results of operations.
We use hazardous chemicals and biological materials in our business. Any claims relating to improper handling, storage or disposal of these materials could be time consuming and costly.
Our research and development and manufacturing processes involve the controlled use of hazardous materials, including flammables, toxics, corrosives and biologics. We maintain a level 2 biohazard laboratory (a laboratory that has established specific procedures for handling bacteria and viruses that may pose a risk of mild disease in humans) and our operations produce hazardous biological and chemical waste products. We cannot eliminate the risk of accidental contamination or discharge and any resultant injury from these materials. We may be sued for any injury or contamination that results from our use or the use by third parties of these materials. We do not currently maintain separate environmental liability coverage and any such contamination or discharge could result in significant cost to us in penalties, damages and suspension of our operations.
We may be unable to successfully manage fluctuations in revenue, which could impede our ability to successfully develop, market and sell our products.
Our quarterly and annual revenues may fluctuate due to several factors, including customer order patterns, the rate of acceptance of our products, regulatory uncertainties or delays, costs and timing associated with business development activities, including potential licensing of technologies, and international market conditions. The impact of one, or a combination of several, of these factors could have a significant adverse effect on our business, financial condition and results of operations.
Our future financial results may be adversely affected by foreign exchange fluctuations.
We expect that a significant portion of our future revenues will be denominated in U.S. and European currencies, and, therefore, we will be subject to fluctuations in exchange rates. There is a risk that significant fluctuations in exchange rates would negatively affect our operating margins and would therefore have an adverse effect on our future results of operations.
Risks Related to Intellectual Property
Our ability to protect our intellectual property and proprietary technology through patents and other means is uncertain.
Our commercial success depends in part upon our ability to protect our intellectual property and proprietary technologies. We rely on patent protection, where appropriate and available, as well as a combination of copyright, trade secret and trademark laws, and nondisclosure, confidentiality and other contractual restrictions to protect our proprietary technology. However, these legal means afford only limited protection and may not adequately protect our rights or permit us to gain or keep any competitive advantage. Our pending Canadian, U.S. and foreign patent applications may not issue as patents or may not issue in a form
23
that will be sufficient to protect our proprietary technology and gain or keep our competitive advantage. Any patents we have obtained or do obtain may be subject to re-examination, reissue, opposition or other administrative proceeding, or may be challenged in litigation, and such challenges could result in a determination that the patent is invalid or unenforceable. In addition, competitors may be able to design alternative methods or devices that avoid infringement of our patents. To the extent our intellectual property, including licensed intellectual property, offers inadequate protection, or is found to be invalid or unenforceable, we are exposed to a greater risk of direct competition. If our intellectual property does not provide adequate protection against our competitors' products, our competitive position could be adversely affected, as could our business, financial condition and results of operations. Both the patent application process and the process of managing patent disputes can be time consuming and expensive. Furthermore, the laws of some foreign countries may not protect our intellectual property rights to the same extent as do the laws of Canada and the United States.
The patent positions of companies in the diagnostics industry can be highly uncertain and involve complex legal and factual questions for which important legal principles remain unresolved. No consistent policy regarding the breadth of claims allowed in such companies' patents has emerged to date in Canada and the United States. The laws of some non-Canadian countries do not protect intellectual property rights to the same extent as the laws of Canada, and many companies have encountered significant problems in protecting and defending such rights in foreign jurisdictions. The legal systems of certain countries, particularly certain developing countries, do not favor the enforcement of patents and other intellectual property protection, which could make it difficult for us to stop the infringement of our patents. Proceedings to enforce our patent rights in foreign jurisdictions could result in substantial cost and divert our efforts and attention from other aspects of our business. Changes in either the patent laws or in interpretations of patent laws in the United States, Canada or other countries may diminish the value of our intellectual property. We cannot predict the breadth of claims that may be allowed or enforced in our patents or in third party patents. For example:
- •
- we might not have been the first to make the inventions covered by each of our pending patent applications;
- •
- we might not have been the first to file patent applications for these inventions;
- •
- others may independently develop similar or alternative products and technologies or duplicate any of our products and technologies;
- •
- it is possible that none of our pending patent applications will result in issued patents, and even if they issue as patents, they may not provide a basis for commercially viable products, or may not provide us with any competitive advantages, or may be challenged and invalidated by third parties;
- •
- we may not develop additional proprietary products and technologies that are patentable;
- •
- the patents of others may have an adverse effect on our business; and
- •
- we may fail to apply for patents on important products and technologies in a timely fashion or at all.
In addition to pursuing patents on our technology, we take steps to protect our intellectual property and proprietary technology by entering into confidentiality agreements and intellectual property assignment agreements with our employees, consultants, corporate partners and, when needed, our advisors. Such agreements may not be enforceable or may not provide meaningful protection for our trade secrets or other proprietary information in the event of unauthorized use or disclosure or other breaches of the agreements, and we may not be able to prevent such unauthorized disclosure. Monitoring unauthorized disclosure is difficult, and we do not know whether the steps we have taken to prevent such disclosure are, or will be, adequate. If we were to enforce a claim that a third party had illegally obtained and was using our trade secrets, it would be expensive and time consuming, and the outcome would be unpredictable. In addition, courts outside Canada and the United States may be less willing to protect trade secrets.
24
We may be involved in lawsuits to protect or enforce our patents and proprietary rights, to determine the scope, coverage and validity of others' proprietary rights, or to defend against third party claims of intellectual property infringement that could require us to spend significant time and money and could prevent us from selling our products or services or impact our share price.
The diagnostics industry relies heavily upon patented technology. We follow a patent program to protect our technology and take precautions to avoid infringement against the technology of others. Litigation may be necessary for us to enforce our patent and proprietary rights and/or to determine the scope, coverage and validity of others' proprietary rights. Litigation on these matters has been prevalent in our industry and we expect that this will continue. To determine the priority of inventions, we may have to initiate and participate in interference proceedings declared by the Canadian Intellectual Property Office, the U.S. Patent and Trademark Office, and patent offices in other countries that could result in substantial legal fees and could substantially affect the scope of our patent protection. Also, our intellectual property may be subject to significant administrative and litigation proceedings such as invalidity, unenforceability, re-examination and opposition proceedings against our patents. The outcome of any litigation or other proceeding is inherently uncertain and might not be favorable to us, and we might not be able to obtain licenses to technology that we require on commercially acceptable terms or at all. We could therefore incur substantial costs related to royalty payments for licenses obtained from third parties. Further, we could encounter delays in product introductions, or interruptions in product sales, as we develop alternative methods or products.
In addition, if we resort to legal proceedings to enforce our intellectual property rights or to determine the validity, scope and coverage of the intellectual property or other proprietary rights of others, the proceedings could be burdensome and expensive, even if we were to prevail.
Our commercial success may depend in part upon our non-infringement of the patents or proprietary rights of third parties. Numerous significant intellectual property issues have been litigated, and will likely continue to be litigated, between existing and new participants in the diagnostics market and competitors may assert that our products infringe their intellectual property rights as part of a business strategy to impede our successful entry into that market. Third parties may assert that we are employing their proprietary technology without authorization.
In addition, our competitors and others may have patents or may in the future obtain patents and claim that use of our products infringes these patents. As we move into new markets and applications for our products, incumbent participants in such markets may assert their patents and other proprietary rights against us as a means of slowing our entry into such markets or as a means to extract substantial license and royalty payments from us.
Patent infringement suits can be expensive, lengthy and disruptive to business operations. We could incur substantial costs and divert the attention of our management and technical personnel in prosecuting or defending against any claims, and may harm our reputation. There can be no assurance that we will prevail in any suit initiated against us by third parties. Furthermore, parties making claims against us may be able to obtain injunctive or other relief, which could block our ability to develop, commercialize and sell products, and could result in the award of substantial damages against us, including treble damages and attorneys' fees and costs in the event that we are found to be a willful infringer of third party patents.
In the event of a successful claim of infringement against us, we may be required to obtain one or more licenses from third parties, which we may not be able to obtain at a reasonable cost, if at all. In addition, we could encounter delays in product introductions while we attempt to develop alternative methods or products to avoid infringing third party patents or proprietary rights. Defense of any lawsuit or failure to obtain any required licenses on favorable terms could prevent us from commercializing our products, and the risk of a prohibition on the sale of any of our products could adversely affect our ability to grow and gain market acceptance for our products.
Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation. In addition, during the course of this kind of litigation, there could be public announcements of the results of hearings, motions or other interim proceedings or developments. If securities
25
analysts or investors perceive these results to be negative, it could have a substantial adverse effect on the price of our common shares.
In addition, our agreements with suppliers, customers, and other entities with whom we do business, may require us to defend or indemnify these parties to the extent they become involved in infringement claims against us, including the claims described above. We could also voluntarily agree to defend or indemnify third parties in instances where we are not obligated to do so if we determine it would be important to our business relationships. If we are required or agree to defend or indemnify any of these third parties in connection with any infringement claims, we could incur significant costs and expenses that could adversely affect our business, financial condition or operating results.
We may in the future depend upon licensed technology to conduct our business. We might not control these technologies and any loss of our rights to them could in the future prevent us from selling our products.
We may in the future rely on licenses in order to be able to use various proprietary technologies that become material to our business. We might not own the patents that underlie these licenses. This may subject us to certain risks that might not be present if we develop the technology and intellectual property independently. In particular, our proposed acquisition of Scienion may require us to rely on licenses that Scienion depends on to be able to use its proprietary technologies that are material to its business.
In many potential third party licenses, we might not control the prosecution, maintenance, or filing of the patents to which we may in the future hold licenses, or the enforcement of these patents against third parties. We cannot be certain that drafting and/or prosecution of the licensed patents by the licensors will be conducted in compliance with applicable laws and regulations or will result in valid and enforceable patents and other intellectual property rights. As a result, we may depend upon the licensor to diligently pursue and prosecute these intellectual property rights. The failure of the licensor to diligently pursue such protection could adversely affect our business and financial condition.
Our rights to use the technology we might license may be subject to the validity of the owner's intellectual property rights. Enforcement of such licensed patents or defense of any claims asserting the invalidity of these patents is often subject to the control or cooperation of our licensors. Legal action could be initiated against the owners of the intellectual property that we may license. Even if we are not a party to these legal actions, an adverse outcome could harm our business because it might prevent these other companies or institutions from continuing to license intellectual property that we may need to operate our business.
Our rights to use the technology we may in the future license are subject to the negotiation of, continuation of and compliance with the terms of those licenses. Specifically, license agreements typically subject us to milestone obligations and royalty payments. Some of these obligations may be substantial and may obligate us to obtain certain regulatory clearances or approvals by a specified date or exercise diligence in bringing potential products to market. The failure to meet these obligations typically results in the termination of the license and the loss of rights to the technology. Any such termination could prevent us from marketing some or all of our products and could adversely affect our business, financial condition and results of operations.
Because of the complexity of our products and the patents we may license, determining the scope of such licenses and related royalty obligations can be difficult and can lead to disputes between us and the licensor. An unfavorable resolution of such a dispute could lead to an increase in the royalties payable pursuant to the license. If a licensor believed we were not paying the royalties due under the license or were otherwise not in compliance with the terms of the license, the licensor might attempt to revoke the license. If such an attempt were successful, we might be barred from producing and selling some or all of our products.
Finally, many potential third party licenses of technology expire when the patents underlying the technology expire or at some period of time after expiration. As a result, our ability to exploit and fully commercialize the licensed technology over time may be limited. This may adversely affect our business, financial condition and results of operations.
26
We may be subject to damages resulting from claims that we or our employees have wrongfully used or disclosed alleged trade secrets of our employees' former employers.
Many of our employees were previously employed at universities or other life sciences companies, including our competitors or potential competitors. Although no claims against us are currently pending, we may be subject to claims that these employees or we have inadvertently or otherwise used or disclosed trade secrets or other proprietary information of their former employers. Litigation may be necessary to defend against these claims. If we fail in defending such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel. A loss of key research personnel or their work product could hamper or prevent our ability to commercialize potential products, which could severely harm our business. Even if we are successful in defending against these claims, litigation could result in substantial costs and be a distraction to management.
Risks Related to Our Common Shares and This Offering
We expect that our share price will fluctuate significantly, and you may not be able to resell your common shares at or above the offering price.
Prior to this offering, our common shares have been listed for trading on the TSXV in Canada. Following this offering, we expect that our common shares will be listed on NYSE Amex. We cannot predict the extent to which investor interest in our company will lead to the development of an active trading market on the TSXV or NYSE Amex, or how liquid those markets might become. If an active trading market does not develop, you may have difficulty selling any of our common shares that you buy. We and the underwriters will determine the public offering price of our common shares through negotiation. This price will not necessarily reflect the price at which investors in the market will be willing to buy and sell our common shares following this offering. In addition, the market price of our common shares on the TSXV in Canada, like the share prices of many publicly traded life sciences companies, has been highly volatile, and the trading price of our common shares following this offering may remain volatile in response to various factors, some of which are beyond our control. These factors could include, for example:
- •
- actual or anticipated quarterly variation in our results of operations or the results of our competitors;
- •
- announcements by us or our competitors of technological innovations, new commercial products, significant contracts, commercial relationships or capital commitments;
- •
- developments or disputes concerning our intellectual property or other proprietary rights;
- •
- commencement of, or our involvement in, litigation;
- •
- market conditions in the diagnostics sector;
- •
- regulatory developments in the U.S., Canada and other countries;
- •
- failure to complete significant sales;
- •
- issuance of new or changed securities analysts' reports or recommendations for our common shares;
- •
- any future sales of our common shares or other securities;
- •
- any major change to the composition of our board of directors or management; and
- •
- general economic conditions and slow or negative growth of our markets.
The stock market in general, and market prices for the securities of technology-based companies like ours in particular, have from time to time experienced volatility that often has been unrelated to the operating performance of the underlying companies. A certain degree of stock price volatility can be attributed to being a newly public company. These broad market and industry fluctuations may adversely affect the market price of our common shares, regardless of our operating performance. In several recent situations where the market price of an issuer's shares has been volatile, holders of those shares have instituted securities class action litigation against the company that issued the shares. If any of our shareholders were to bring a lawsuit against
27
us, the defense and disposition of the lawsuit could be costly, and could divert the time and attention of our management and harm our operating results.
If securities or industry analysts do not publish research or publish unfavorable research about our business, our share price and trading volume could decline.
The trading market for our common shares will rely in part on the research and reports that equity research analysts may publish about us and our business. We do not currently have and may never obtain research coverage by equity research analysts. Equity research analysts may elect not to provide research coverage of our common shares after the completion of this offering, and such lack of research coverage may adversely affect the market price of our common shares. If we obtain equity research analyst coverage, we will not have any control over the analysts or the content and opinions included in their reports. The price of our common shares could decline if one or more equity research analysts downgrade our common shares or issue other unfavorable commentary or research. If one or more equity research analysts ceases coverage of our company or fails to publish reports on us regularly, demand for our common shares could decrease, which in turn could cause our share price or trading volume to decline.
Purchasers in this offering will experience immediate and substantial dilution in the book value of their investment.
The offering price of our common shares is substantially higher than the net tangible book value per common share immediately prior to this offering. Therefore, if you purchase our common shares in this offering, you will incur an immediate dilution of $ • in pro forma as adjusted net tangible book value per share as of June 30, 2011. In addition, new investors who purchase common shares in this offering will contribute approximately • % of the total amount of equity capital raised by us through the date of this offering, but will only own approximately • % of the outstanding share capital and approximately • % of the voting rights. In addition, we have issued options and warrants to acquire common shares at prices below the offering price. To the extent outstanding options and warrants are ultimately exercised, there will be further dilution to investors who purchase common shares in this offering. In addition, if the underwriters exercise their over-allotment option or if we issue additional equity securities, investors purchasing common shares in this offering will experience additional dilution. For a further description of the dilution that you will experience immediately after this offering, see "Dilution."
Future sales of common shares by existing shareholders could cause our share price to decline.
If our existing shareholders sell, or indicate an intention to sell, substantial amounts of our common shares in the public market, the trading price of our common shares could decline. Based on the number of our common shares outstanding as of September • , 2011, upon completion of this offering, we will have outstanding a total of • common shares, assuming no exercise of the underwriters' over-allotment option. Certain of our directors and officers will enter into lock-up agreements with the underwriters that restrict their ability to sell or transfer their common shares. The lock-up agreements pertaining to this offering will expire 90 days after the date of the final prospectus, although they may be extended for up to an additional 18 days under certain circumstances. Our underwriters, however, may, in their sole discretion, permit our officers and directors to sell common shares prior to the expiration of the lock-up agreements. After the lock-up agreements expire, based on common shares outstanding as of • , 2011, up to an additional • common shares will be eligible for sale in the public market. If these additional common shares are sold, or it is perceived that they will be sold in the public market, the trading price of our common shares could decline.
Our directors and executive officers will continue to have substantial control over us after this offering and could limit your ability to influence the outcome of key transactions, including changes of control.
Following the completion of this offering, our executive officers, directors and their affiliates will beneficially own or control approximately • % of our outstanding common shares assuming no exercise of the underwriters' over-allotment option. Accordingly, these executive officers, directors and their affiliates, acting as a group, would have substantial influence over the outcome of corporate actions requiring shareholder approval, including the election of directors, any merger, consolidation or sale of all or substantially all of our assets or any other significant corporate transactions or agreements. These shareholders may also delay or
28
prevent a change of control of our company, even if such a change of control would benefit our other shareholders. The significant concentration of share ownership may adversely affect the trading price of our common shares due to investors' perception that conflicts of interest may exist or arise.
We have broad discretion in the use of the net proceeds from this offering and may not use them effectively.
We will have broad discretion in the application of the net proceeds from this offering and could spend the proceeds in ways that do not improve our results of operations or enhance the value of our common shares. We intend to use the net proceeds from this offering to complete the acquisition of Scienion, sales and marketing initiatives, research and development activities, the expansion of our facilities and manufacturing operations as well as for working capital and other general corporate purposes. We may use a portion of our net proceeds to acquire and invest in complementary products, technologies or businesses; however, we currently have no agreements or commitments to complete any such transaction (other than the acquisition of Scienion). We have not allocated these net proceeds for any specific purposes. We might not be able to yield a significant return, if any, on any investment of these net proceeds. You will not have the opportunity to influence our management's decisions on how to use the net proceeds from this offering, and our failure to apply these funds effectively could have a material adverse effect on our business, delay the development of our products and cause the price of our common shares to decline.
We have never paid dividends on our common shares, and we do not anticipate paying any cash dividends in the foreseeable future.
To date, we have not paid any dividends and do not expect to do so in the foreseeable future. We currently intend to retain all future earnings for the operation and expansion of our business. Dividends on our common shares are declared at the discretion of our board of directors and will depend on our results of operations, capital requirements, financial condition, prospects, contractual arrangements and other factors that our board determines is relevant.
If we are a "passive foreign investment company," adverse U.S. federal income tax consequences will likely result for U.S. holders
Based on our current business plans and financial projections, we believe that we should not be classified as a passive foreign investment company ("PFIC") during our tax year ending September 30, 2012 but that we may be classified as a PFIC during our current tax year ending September 30, 2011. However, no assurance can be provided that we will not become a PFIC for any taxable year during which a "U.S. holder" (as defined in "Certain Material United States Federal Income Tax Considerations" below) holds common shares. If we are classified as a PFIC for any year during a U.S. holder's holding period, then such U.S. holder generally will be required to treat any gain realized upon a disposition of our common shares, or any so-called "excess distribution" received on our common shares, as ordinary income, and to pay an interest charge on a portion of such gain or distributions, unless the shareholder makes a timely and effective "qualified electing fund" election ("QEF Election") or a "mark-to-market" election with respect to the common shares. We do not currently intend to provide U.S. holders with the information required to make a QEF Election effective in the event we are a PFIC. A U.S. holder who makes the mark-to-market election generally must include as ordinary income each year the excess of the fair market value of the U.S. holder's common shares over the U.S. holder's basis therein. This paragraph is qualified in its entirety by the discussion below under the heading "Certain Material United States Federal Income Tax Considerations." Each U.S. holder should consult its own tax advisor regarding the PFIC rules and the U.S. federal income tax consequences of the acquisition, ownership, and disposition of common shares.
29
CAPITALIZATION
The table below sets out our capitalization as of June 30, 2011 on an actual basis, and on a pro forma basis to give effect to the sale of • of our common shares in this offering at the initial public offering price of US$ • per share and the issuance of common shares in connection with our proposed acquisition of Scienion after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us.
| | | | | | | |
| | As of June 30, 2011 | |
|---|
Capitalization | | Actual | | Pro Forma | |
|---|
Common Shares — 33,946,258 shares issued and outstanding unlimited authorized (actual), • shares issued and outstanding unlimited authorized (pro forma) | | $ | 35,377 | | | • | |
Warrants — 2,632,852 warrants convertible into 2,632,852 shares | | | 1,614 | | | • | |
Contributed Surplus | | | 9,274 | | | • | |
Accumulated Deficit | | | (40,305 | ) | | • | |
| | | | | | |
Total shareholder's equity | | $ | 5,960 | | | • | |
| | | | | | |
The table above excludes 1,859,000 common shares issuable upon exercise of stock options outstanding as of June 30, 2011, at a weighted average exercise price of $1.85 per share, and 493,493 common shares reserved for future issuance under our 2007 stock-based compensation plans, as amended June 8, 2011. The stock option plan approved by our shareholders at our annual general meeting held on July 7, 2011 provides that the number of common shares reserved for issuance under our stock option plan is equal to 10% of our issued and outstanding common shares.
USE OF PROCEEDS
We estimate that the net proceeds from the offering will be US$ • , after deducting estimated underwriting commissions of US$ • and estimated offering expenses of US$ • . If the underwriters' overallotment option is exercised in full, we estimate that we will receive aggregate net proceeds of US$ • .
We expect to use the net proceeds that we will receive from this offering as follows:
- •
- $ • million in satisfaction of the cash portion of the purchase price payable for our acquisition of Scienion (see "Acquisition of Scienion AG");
- •
- US$ • for sales and marketing initiatives to support the ongoing commercialization of our systems, including expanding our sales force;
- •
- US$ • for research and development activities, including US$ • for the continued build-out of our diagnostic test menu and US$ • to expand our line of diagnostic platforms;
- •
- US$ • for the expansion of our manufacturing operations; and
- •
- the balance for working capital and other general corporate purposes.
Our research and development program is conducted internally by our employees and consultants, supplemented by our partners who are used primarily to conduct third-party validation studies at the completion of a development program. We intend to continue the development of our SQiDlite platform for near term delivery and our SQiDman platform for full commercial launch for RUO purposes in late 2011. Additionally, we intend to use a portion of the proceeds to continue the development of the microarray tests in our development pipeline over the next 18 months. See "Business of the Company — Research and Development".
As of the date of this prospectus, we cannot specify with certainty all of the particular uses for the net proceeds to be received upon the completion of this offering. The actual use of the net proceeds of the offering will depend on operating and capital needs and the progress of all research and development programs. We may also use a portion of our net proceeds to acquire and invest in complementary products, technologies or businesses. However, other than the Scienion acquisition, we currently have no agreements or commitments to
30
complete any such transaction and are not involved in negotiations to do so. Accordingly, we will have broad discretion in the application of the net proceeds and potential investors will be relying on the judgment of our management regarding the application of the proceeds from this offering. Pending these uses, we intend to invest the net proceeds of the offering in investment grade, short-term, interest bearing securities.
For our fiscal year ended September 30, 2010, we had negative operating cash flow. To the extent required, the net proceeds from the offering will be used to fund negative operating cash flow in future periods.
BUSINESS OF THE COMPANY
Overview
We are a life sciences company that develops and commercializes proprietary technologies and products for advanced microarray diagnostics. Our goal is to become a leader in the development and commercialization of microarray and multiplexed diagnostics by offering our customers a comprehensive "turnkey" solution that increases the efficiency and ease of diagnostic testing and test development.
Our target customers — clinical, academic and diagnostic development laboratories — require diagnostic processing equipment and consumable tests ("systems") that are capable of processing large numbers of patient samples at low cost and with minimal labor requirements ("high-throughput systems"). High-throughput systems have not been widely employed in autoimmune disease, allergen or companion diagnostics testing and only limited use of high-throughput systems exists in infectious disease testing. To our knowledge, no fully- automated high-throughput systems exist that are capable of addressing the combined multiplex testing needs of these markets. A fully-automated system capable of providing multiple biomarker measurements in a single test array has the potential to increase a laboratory's throughput with significantly less labor, consumables and other costs.
Our proprietary microarray tests and fully-automated instruments are designed to simplify antigen, protein and antibody testing workflow, increase throughput and reduce costs, all while providing excellent data quality. In many instances, our technology enables analysis that was traditionally unavailable.
Our principal lines of business include:
- •
- the development, manufacturing, and marketing of fully-automated diagnostic instruments (our "platform");
- •
- the development and marketing of tests for the autoimmune, allergen, infectious disease and companion diagnostics testing markets (our "assays"); and
- •
- the commercialization of microarray printing technology (our "printing solutions").
Our Platform
Our FDA-cleared SQiDworks platform is comprised of three key elements: a proprietary one-time-use consumable microarray device, a validated and integrated instrument system that fully-automates the processing of patient samples in the microarray device, and a proprietary software processing system that processes and analyses the array and reports the presence or absence of a biomarker ("qualitative testing") and/or the amount of biomarker present ("quantitative testing"). Our SQiDworks platform can perform qualitative or quantitative testing for multiple biomarkers simultaneously. Our SQiDworks platform is currently the only FDA-cleared, fully-automated, microarray system and multiplexing solution in our target markets and we believe there are significant barriers to entry in these markets. We believe that our SQiDworks platform addresses many of the key challenges faced by our customers today by delivering accurate patient results in less time and with significantly reduced labor, consumables and other costs.
31
We have a prototype of SQiDlite, our fully-automated, bench-top diagnostic platform for bothin vitro diagnostic ("IVD") and research use only ("RUO") applications. We have also begun initial commercialization of SQiDman, our small semi-automated platform for processing microarrays, to be used by research customers or development partners for RUO applications in the microarray development process.
Our Assays and the Development of Our Test Menu
We have received regulatory clearance to market our qualitative rheumatoid arthritis and celiac assays in the United States, our qualitative and quantitative rheumatoid arthritis and celiac assays in Canada, and our quantitative rheumatoid arthritis and celiac assays in the European Union for use on our SQiDworks platform. We have a robust pipeline of autoimmune tests and one companion diagnostic test in development, and plan to develop a broad test menu for autoimmune disease, companion diagnostics, infectious disease and allergen testing markets in the future. Our current in-development tests include multiplexed panels to aid in the diagnosis of vasculitis, lupus, antiphospholipid syndrome ("APS"), Crohn's disease and inflammatory bowel disease ("IBD") and in the management of treatment of autoimmune affected patients with a class of drugs referred to as "anti-TNF drugs" such as Remicade®, Enbrel® and Humira®.We are currently developing more sensitive rheumatoid arthritis and celiac assays that measure additional biomarkers.
We have targeted these testing markets because we believe:
- •
- healthcare providers require the measurement of multiple biomarkers to aid in the diagnosis and therapeutic monitoring of these diseases;
- •
- these testing markets are underserved by fully-automated, high-throughput, multiplexed systems;
- •
- tests in these markets are generally run at sufficiently high volumes in larger test facilities that would benefit from both multiplexing and automation; and
- •
- these tests typically qualify for private and public reimbursement in Canada, the United States, and the European Union.
One of our key operational goals is to continue to develop and seek regulatory approval for additional tests, as we believe that expanding our "test menu" will drive adoption of our platform and products by customers.
The development of our test menu is augmented by our partnerships with leading research institutions, including Beth Israel Deaconess Medical Center, the Cleveland Clinic, and the University of North Carolina. Our partnerships provide us with many advantages, including the rights to approximately 10,800 patient samples, which aid us in the development and validation of our assays.
We also offer development services and manufacturing of diagnostic kits and microarrays to our customers. We will convert content of laboratories and third parties into microarray products, manufacture microarray test kits for sale to these customers and, in the case of third parties, enable them to re-sell our high throughput systems on which these proprietary assays can be run. Our laboratory customers may use these custom microarray components to perform diagnostic test services using our platforms and may subsequently sell the results to their customers.
Our Printing Solutions and Our Proposed Acquisition of Scienion
On July 4, 2011, we announced that we entered into a definitive agreement to acquire all of the issued and outstanding shares of Scienion, which we believe is a market leader in microarray printing development, production arrayer systems and contract print and development services for the life sciences industry. We believe our proposed acquisition of Scienion will further our entry into the market for providing enabling technologies and processes for multiplexed protein, antibody, antigen and molecular (genetic) microarrays. We believe Scienion is a leader in the commercialization of non-contact array printing equipment, as well as the "in-array" development and manufacturing services to a broad set of customers in all microarray printing markets. See "— Proposed Acquisition of Scienion" and "Acquisition of Scienion AG".
32
The Market for Our Products
The diagnostic products and services market is increasing in importance, complexity, breadth and size. Diagnostics are critical to high-quality healthcare and guide a majority of clinical decisions.
Over the last forty years, the number of biomarkers that can be measured by commercial laboratories has grown dramatically, with less than 4,000 different types of biomarker measured in the 1970s, more than doubling to approximately 10,000 with automation in the 1980s, and increasing to approximately 25,000 in the early 2000s.
Since 1976, the number of biomarkers measured by assays cleared through the FDA's 510(k) process to aid in the diagnosis of the six autoimmune diseases that comprise our target market has increased from four to 48. This represents an increase in the average number of biomarkers per disease state in our autoimmune disease target market from 0.7 to 8. We believe that this increase in biomarkers is indicative of a healthcare trend whereby healthcare providers are seeking to run diagnostic tests for increasing numbers of biomarkers to assist in the diagnosis of disease.
Researchers and laboratories are accelerating the rate at which new biomarkers are being commercialized for the known set of diseases being diagnosed today. It is estimated that an additional 30,000 biomarkers (molecular and protein) in various stages of development have been identified and not yet commercialized. We believe that the increased use of existing assays and the introduction of new assays will drive growth of the diagnostics testing market, a market that is estimated to have a growth rate of approximately five to nine percent annually from 2009 to 2014.
Our strategy is to focus initially on the immunoassay segment of the diagnostic test market since there are no multiplexed, microarray, IVD solutions in the immunoassay space. The 2012 global immunoassay diagnostic test market is estimated to be US$10.3 billion, of which approximately US$4.5 billion is within our strategic market focus, which includes tests for autoimmune disease, infectious disease, and allergen tests. These three markets are estimated to be US$1.5 billion, US$2.4 billion and US$0.6 billion, respectively.
Autoimmune Disease Tests
Our primary testing market in the immunoassay segment is for products that aid in the diagnosis of autoimmune disease, which is estimated to be approximately US$1.5 billion per year. We have targeted the autoimmune testing segment for the following reasons:
- •
- there are ten autoimmune disease states, which include the autoimmune diseases in our target market, that command most of the blood testing revenues for the entire autoimmune testing segment;
- •
- the most common tests for autoimmune diseases currently evaluate approximately 70 biomarkers, representing an average of approximately seven biomarkers per disease;
- •
- these tests are generally run at high volumes at larger test facilities and in batches and are not typically run on a "one off" basis;
- •
- generally, autoimmune disease tests have an established reimbursement model from private and public healthcare payers;
- •
- each of the autoimmune diagnostic tests we are developing has predicate technologies with FDA clearance on older, single biomarker, manual titer plate technology that we intend to replace;
- •
- regulatory clearance for the majority of autoimmune assays is through the FDA 510(k) process, which is well established; and
- •
- autoimmune disease tests are regulated as Class II devices by the FDA. Class II diagnostic clearance is generally obtained following a shorter review period than for a Class III device. See — Government Regulation.
Infectious Disease and Allergen Tests
We also plan to enter the immunoassay segment of the infectious disease diagnostics market, which is estimated to be approximately US$2.4 billion for 2012, followed by the immunoassay segment of the allergen diagnostics market, which is approximately US$0.6 billion for 2012.
33
| | |
SQI's Addressable Market —
Global Immunoassay Diagnostic
Test Market | | SQI's Strategic Market Focus —
Global Autoimmune, Infectious
Disease and Allergen Diagnostic
Testing Market |
US$10.3 Billion* |
|
US$4.5 Billion* |
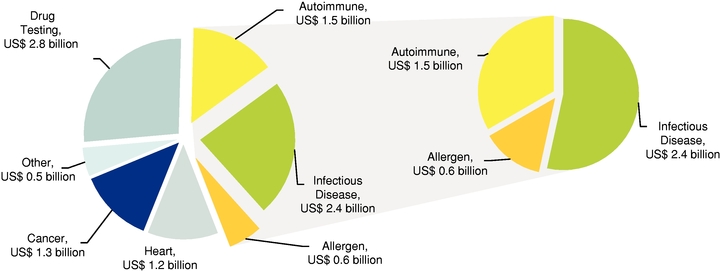
*Annual estimate for 2012
Source: Freedonia 2011
Print Services
According to a press release issued by F. Hoffmann-La Roche Ltd., in 2007, the estimated market for protein and molecular microarray print equipment and services was approximately US$600 million per year.
RUO and Lab-Developed Tests
Approximately 34% of the US$3.7 billion U.S. annual market for RUO and lab-developed tests is directly addressable by our proprietary technologies. Additionally, we believe that the US$2.2 billion European market for RUO and lab-developed tests has approximately the same segmentation as the U.S. market. As such, we anticipate that the combined U.S. and European markets for RUO and lab-developed tests that are directly addressable by our proprietary technologies to be approximately US$2 billion.
| | |
U.S. Annual Market for RUO and
Lab-Developed Test | | Global Annual Market for RUO and
Lab-Developed Tests |
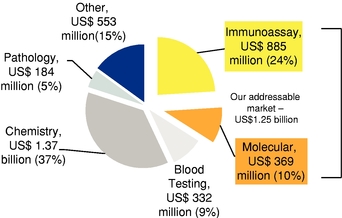 |
|
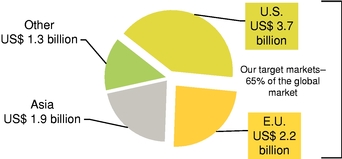
|
*Source: Kalorama 2008
34
Companion Diagnostics
We believe that the commercialization of "companion diagnostics" tests is a meaningful market opportunity for us. Autoimmune diseases are increasingly being treated with antibody-based or other biologic drugs. Often, there are different variants of the antibody or biologic drugs. For example, anti-TNF-based drugs are used to treat rheumatoid arthritis, Crohn's disease and IBD. The market for anti-TNF drugs was estimated to be approximately US$16 billion per year in 2008. Anti-TNF drugs are currently marketed under brand names such as Remicade®, Enbrel® and Humira® which represented more than 99% of the anti-TNF drug market in 2008. The effectiveness of the treatment of autoimmune patients with these drugs may be enhanced by the monitoring of the concentration of these drugs in a patient's blood by a "companion diagnostic" test. Our multiplexing technology allows us to combine the tests for both the diagnosis and therapeutic monitoring of a patient's disease. We expect this test combination to result in significantly less labor, consumables and other costs and provide us with a large market opportunity.
Diagnostics
Assays are used world-wide to assist healthcare providers in preventing, diagnosing and treating diseases. In order to be able to determine whether someone has a particular disease, a typical assay will test for "biomarkers", a term used to refer to a specific antigen, protein or antibody. The amount or "concentration" of the biomarker in a person's blood usually indicates one or more of the following: the predisposition or risk of getting the disease; presence or absence of the disease; and severity of the disease. For example, assays have been used for more than 50 years to detect the presence of rheumatoid factors, which are diagnostic markers for rheumatoid arthritis. The use of assays to detect rheumatoid factors first received 510(k) clearance by the FDA approximately 35 years ago. Rheumatoid factors can be detected in the blood of a patient before the first symptoms appear, can indicate the progression of the disease, and provide information that may guide a healthcare provider's determination of a patient's treatment plan.
Diagnostics "platforms" refer to the specific technology or instrument system on which a consumable device is "run" to generate test results. There are a number of different platforms on which assays can be run. For example, ELISA (enzyme-linked immunosorbent assay, a test involving an enzyme and an antibody) plates and readers, ELISA bead technology and polymerase chain reaction-based systems are all platforms for running assays.
A microarray is a device that is used to perform multiple tests simultaneously. A microarray is comprised of two principal elements.
- •
- A surface upon which capture molecules are printed in the form of spots. The surface is typically glass or plastic and is chemically coated to more easily "accept" and bind the molecules being printed without damaging the molecules.
- •
- A physical superstructure to separate individual wells. Each well contains numerous spots. Each of the spots within an individual well is analogous to a traditional microtiter plate test well.
The biological and chemical reactions that occur in each test well generate multiple test results. Each of these test results is a billable event for our customers.
Limitations of Current Technologies
Clinical, academic, and diagnostic development laboratories are faced with increasing numbers of tests or experiments required to support a diagnostic conclusion or development decision. In the diagnostic setting, there are a growing number of biomarkers that are used for each patient to aid in the diagnosis and monitoring of many diseases. This has led to a growing workload of increasingly complex and costly diagnostic tests for laboratories. Additionally, laboratories face pressure to reduce overall healthcare system costs.
Our target customers — clinical, academic and diagnostic development laboratories — require diagnostic processing equipment and consumable tests that are capable of processing large numbers of patient samples at low cost and with minimal labor requirements. High-throughput systems have not been widely employed in autoimmune disease or allergen testing and limited use of high-throughput systems exists in infectious disease
35
testing. To our knowledge, no high-throughput systems exist that are capable of addressing the combined multiplex testing needs of these markets. A fully-automated system capable of providing multiple biomarker measurements in a single test array has the potential to increase a laboratory's throughput with significantly less labor, consumables, and other costs. We believe these cost savings would be realized because, among other things:
- •
- a fully-automated system reduces "hands-on" technician time;
- •
- the volume of liquid reagent is less and the number of other consumables, such as pipette tips, required to process one microarray is fewer when compared to traditional methods of running multiple tests in multiple titer plates;
- •
- the amount of serum required per patient to produce results on a 12 biomarker test is approximately 240 times greater using titer plates than using microarray plates;
- •
- a laboratory does not incur the expense of sending some tests of a larger multiplex panel to other labs;
- •
- the cost of management of multiple samples being tested by multiple technicians and combining the results to a single report would be largely eliminated by multiplexing capability; and
- •
- reducing the number of hands-on steps through automation would potentially reduce error rates.
Microtiter Plates
Laboratories almost exclusively use microtiter plates to perform immunoassay tests. The use of microtiter plates restricts an immunoassay test to the analysis of a single biomarker. Microtiter plates are predominantly processed with a high contribution of direct labor. This increases the time to produce a test result and the potential for error. Since equipment available to reduce labor is limited, the use of microtiter plates restricts the throughput of a laboratory. We estimate that 60% of our target customers' costs to produce a diagnostic result are due to direct labor.
Skilled laboratory labor capable of performing diagnostic testing in our target markets is limited and is expected to contribute to laboratories' growing cost structure at an increasing rate. The United States Department of Health and Human Services reported in 2009 that by 2012, 138,000 lab professionals, including medical technicians and laboratory assistants, will be needed, but fewer than 50,000 will be trained.
Multiplex Diagnostic Tests
A solution to the cost and labor constraints of immunoassay testing for single biomarkers is to perform diagnostic tests for multiple biomarkers simultaneously in a single assay (a "multiplex diagnostic test"). The processes used to complete a single test within a diagnostic panel of several biomarkers are complex and technically difficult. Such complexity has limited the throughput and efficiency of multiplex diagnostic testing, and technical challenges have restricted the widespread adoption of automated systems. Most laboratories do not use automated processing equipment to augment their workforce in our key target markets where the measurement of multiple antigens, proteins or antibodies is required.
Bead-Based Array Systems
Bead-based array systems were initially developed for DNA-based testing and were subsequently adapted for protein-based and antibody-based testing. Bead-based methods, however, have faced limitations that reduce their utility, particularly for multiplex diagnostics and experimentation. In particular, the workflow for bead arrays is complex, time consuming and costly. For example, standard protocols for a nine-biomarker bead array require multiple complex operations including approximately 190 steps and approximately five hours of "hands-on" time to complete.
Automated Systems
While laboratories use automated systems for many types of blood tests, to our knowledge, there are no fully-automated high-throughput microarray systems. A number of diagnostics companies have developed
36
automated single-plex fully-automated, bench-top systems for autoimmune testing and we are only aware of one bead-based automated multiplex system.
Antigen, Protein and Antibody Microarrays and Their Technical Challenges
An alternative to bead-based array systems is a microarray printed on a two-dimensional substrate. These antigen, protein and antibody microarrays solve several limitations of bead-based systems such as interactions between beads, but are challenging to develop as we believe there are several technological obstacles, including:
- •
- precision and accuracy of printing;
- •
- cross interference of similar molecules in the system;
- •
- calibration and standardization of multiple separate molecular signals in a single microarray well; and
- •
- complex software systems required to measure and analyze the multiple signals measured within the microarray.
Precision and Accuracy of Printing
Microarray printing has been traditionally done with contact printers that, like a quill pen, physically "spot" the biomarker capture molecules onto the microarray surface. These contact methods of printing are imprecise and lead to microarrays with high degrees of variability and significant background noise. This reduces the commercial applications of contact spotted microarrays. Contact spotting does not typically lead to IVD capable microarrays in antigen, protein and antibody applications. Some earlier versions of non-contact printers are also used today, but these technologies have similar drawbacks to contact spot printers.
Cross Interference
The ability to discriminate among different "isotypes" (types) of individual target antibodies and different antigens and proteins is important to the measurement of biomarkers and it has proven challenging to develop multiplex tests that discriminate between these different entities in a single microarray.
Calibration and Standardization
The processing of multiplex arrays is complex and is prone to high degrees of variation between test arrays. This error potentially leads to reduced test performance and has restricted the use of multiplex arrays almost exclusively to the research market where there is a tolerance for less accurate measurements.
Diagnostic approaches for measuring multiple antigen, protein or antibody biomarkers for large numbers of patients are generally unavailable or are not cleared or approved as IVD tests. Multiplexing, as it is available today, does not adequately address antigen, protein and antibody focused measurement. Most multiplexing technologies have been developed and commercialized for the processing of high-density genetic or molecular arrays. These technologies generally work from a different set of technological principles than those needed to measure multiple antigens, proteins or antibodies. Further, these technologies are used in a research setting and are generally inadequate for use in commercial diagnostic testing.
Software Systems
The sophistication of the automated systems and processing software required to analyze microarrays has restricted the commercial viability of microarrays in diagnostic laboratories.
Our Solution
Our proprietary microarray tests and automated instruments are designed to simplify antigen, protein and antibody testing workflow, increase throughput, reduce costs and provide excellent data quality. In many instances, our technology enables analysis that was traditionally unavailable.
Traditional biomarker testing methods using microtiter plates require approximately four minutes on average of technician "hands-on" time per biomarker per patient ("test effort"). For example, a four biomarker
37
test for celiac on samples from 74 patients would, on average, require eight microtiter plates, assuming each patient's blood sample is tested in duplicate using standard good laboratory practices. This would have traditionally required approximately 20 hours of direct "hands-on" labor using predicate technology.
Our Fully-Automated SQiDworks Diagnostic Platform
Our high-throughput SQiDworks diagnostic platform is a fully-automated microarray processing and analytical system capable of 888 results per hour. By way of example, for a celiac assay that measures four biomarkers, our SQiDworks platform requires approximately 30 seconds of technician "hands-on" time per patient per panel compared to approximately 16 minutes of technician "hands-on" time using microtiter plate technology, and uses one microarray kit instead of 8 single biomarker microtiter kits to generate diagnostic results for the four biomarkers. The difference between the approximately 20 hours of direct hands-on labor using microtiter plate technology and the approximately 30 minutes of direct hands-on labor using our system results in significant cost savings, as does using one microarray kit rather than eight microtiter kits.
Additionally, the cost savings demonstrated above increase as the number of biomarkers per diagnostic test increase. For example, for a lupus test that measures twelve biomarkers, our SQiDworks platform requires the same amount of technician "hands-on" time as the celiac assay, and also requires only one microarray kit. However, microtiter plate technology would require approximately 48 minutes of technician "hands-on" time per patient per panel and the use of 24 single biomarker microtiter kits. This equates to a reduction of approximately 59 hours of direct hands-on labor using our system as well as greater savings with respect to consumables.
Our IgXPLEX Microarrays
Our IgXPLEX microarrays have the ability to discriminate between individual isotypes of antibodies and antigens and proteins within a single well of a microarray, resulting in the measurement of multiple biomarkers in a single test.
Our microarray technology requires the use of significantly less patient blood than traditional methods. Our system requires a ten microliter drop of blood to be processed from a single sample tube, compared to the multiple milliliters of blood used by traditional methods. Our system dispenses the single drop of blood once per patient in a microarray well. Traditional methods require multiple blood samples to be manipulated into multiple test vessels over many steps. The reduction in processing steps and the ability to generate simultaneous multiplexed measurements from a single drop of blood increase the predictive value of the test. The increased predictive value of the test would allow the healthcare provider to choose a treatment plan earlier in the course of the disease.
Our IgXPLEX CHEX Technology
Our IgXPLEX CHEX technology provides multiple in-microarray checks to ensure that the test has been completed without system, control, calibration or microarray-related errors. These tests reduce errors that are common to microtiter and microarray tests.
Our proprietary multiplex assay development processes and microarray manufacturing capabilities, combined with our automated instruments, are designed to significantly reduce the complexity and cost to our customers to commercialize microarray tests using their own biomarkers. Customers with a need to perform large numbers of experiments may benefit from the use of our microarrays in their screening and development programs when traditional methods may be too onerous due to the large number of single tests that would be required.
Proposed Acquisition of Scienion
On July 4, 2011, we announced the proposed acquisition of all of the issued and outstanding shares of Scienion, a German company that we believe is a market leader for microarray development, production arrayer systems and contract print and development services for the life sciences industry. We believe that the proposed acquisition represents a compelling opportunity to capitalize on the global trend towards miniaturisation and
38
parallelisation of tests for diagnostics and pharmaceutical applications, instruments, application development, service and software.
Scienion develops and markets a range of microarray printing equipment and provides custom print and commercial print services. Scienion's equipment prints microarray spots with high precision and positional accuracy on a variety of surfaces. Scienion's equipment is able to print with volumes of biomarkers as small as ten picolitres. This enables the printing of very small diameter microarray spots, which allows more spots to be printed in a given area and more data to be produced from a single microarray. Scienion has developed advanced "in-process" methods to measure the mass and position of spots as they are being printed. This design feature results in a more reliable and reproducible print process and more accurate microarrays.
We believe that the combination of our platform, our assays and the development of our test menu and, if the acquisition is completed, Scienion's industry leading print technologies, will enable the combined company to become a leader in sales of end-to-end diagnostic systems, microarray printing and assay development services.
We believe that the proposed acquisition will:
- •
- enable us to combine our assay commercialization capabilities, our range of cleared and in-development processing and analytical systems for running microarray tests and the expertise of the two companies for highly technical microarray printing capabilities;
- •
- strengthen our technology leadership position in microarray printing, surface chemistry, and automation of microarray processes;
- •
- accelerate the commercialization of our pipeline of RUO and IVD systems through the use of Scienion's arrayer equipment and complete "print solutions";
- •
- provide us with access to Scienion's base of more than 400 customers, many of whom are potential customers for our service offerings and RUO and IVD products;
- •
- provide sales support for Scienion's equipment, products and services in North America;
- •
- give us access to Scienion's sales and engineering field service to leverage our suite of diagnostic products and services in Europe and Asia;
- •
- diversify our revenues and add financial strength. See "Index to Consolidated Financial Statements of Scienion AG".
Our Key Competitive Strengths
We believe that our key competitive strengths include the following:
- •
- Only FDA Cleared, Fully-Automated Microarray Processing System. We have received marketing clearance from the FDA, a Canadian regulatory medical device licence, and have CE marked, our SQiDworks instrument system. SQiDworks is the only system for automatically processing, analyzing and providing test results in protein-based and antibody-based microarrays to achieve these regulatory clearances.
- •
- Fully-Integrated, High-Throughput Solution Dramatically Reduces Workflow and Cost. Our proprietary microarray tests and fully-automated instruments are designed to significantly simplify antigen, protein and antibody workflow, increase throughput and reduce costs, all while providing excellent data quality. For example, competing systems require hundreds of steps to produce the same number of test results as can be produced by our SQiDworks fully-integrated diagnostics system in five steps.
- •
- Expertise in Microarray Assay Development. We have clinically validated development experience and have created processes, systems and development algorithms to simplify the commercialization of our products. We believe our expertise will reduce the time required to complete the commercial development of our pipeline products. We also believe that our experience can be applied to custom products to allow us to commercialize our own and other's content in significantly less time than our competition.
39
- •
- Focus on Penetrating Existing Markets that Are Technically Challenging and Have Established Reimbursement. We focus on disease testing markets that have existing reimbursement and payment programs in place, which allows us to quickly bring our products into established markets. Additionally, antigen, protein and antibody multiplex tests are more technically challenging to develop, which may restrict others from easily entering these markets.
- •
- Partnerships with Leading Institutions. We have entered into agreements with leading institutions and healthcare providers to collaborate with us in developing and validating our assays including providing us with access to approximately 10,800 clinically validated patient blood samples.
- •
- Significant Intellectual Property Portfolio. Our intellectual property covers key areas of our commercial business as well as our pipeline, including microarray surfaces, multiplexing, microarray analytics, and microarray processing. We have issued patents and pending patent applications that cover aspects of our microarray technology for analyzing, identifying and quantifying analytes. We are actively seeking protection for our methods for making microarray substrates. In the course of improving our products, from time to time we develop processes and systems that provide us with a competitive advantage and we will keep such developments confidential.
Products
SQI Platforms
| | | | | | | | |
| |
| | Regulatory Status |
|---|
| | Development
Status |
|---|
Product | | Canada | | United States | | Europe |
|---|
SQiDworks | | Complete | | Licensed | | Cleared as a system with
IgXPLEX RA | | CE Marked |
SQiDman | | Development RUO | | Not Required — RUO | | Not Required — RUO | | Not Required — RUO |
SQiDlite RUO | | Prototype | | Not Required — RUO | | Not Required — RUO | | Not Required — RUO |
SQiDlite IVD | | Prototype | | To be filed | | To be filed | | To be filed |
SQiDworks
We have developed our fully-automated SQiDworks platform (shown below) to enable laboratory customers to generate multiple patient test results with less than one unit of traditional "test effort". "Test effort" refers to the number of steps required to perform a test using microtiter plate technology.
We have received marketing clearance from the FDA, a medical device license from Health Canada, and have CE marked, our SQiDworks instrument system. SQiDworks is the only fully-automated microarray processing system to achieve these regulatory clearances.
Our SQiDworks platform integrates a microfluidics station, an automated microarray scanner, our drying device and our proprietary processing and analytic software.
The microfluidics station automatically performs sample handling and processing of the patient serum sample and prepares our IgXPLEX microarray for scanning and analysis.
Our dryer technology dries the microarray surface, which uniformly enhances the signal to noise ratios of our microarrays. The microarray scanner reads the various IgXPLEX microarray signals.
Our proprietary software uses complex algorithms to locate the array of spots containing reagents and to process the spots to measure each of the biomarkers of interest. This measurement can be either qualitative or quantitative.
40

41
SQI Assays
Our four cleared or approved assays, two for rheumatoid arthritis and two for celiac, test for two of the more common autoimmune diseases. Rheumatoid arthritis is a chronic inflammatory disease, the cause of which is unknown. The incidence of rheumatoid arthritis in North America is 1.4 cases per 100,000 in men and 3.6 cases per 100,000 in women. The prevalence of rheumatoid arthritis is approximately 2% world wide. It is estimated that total size of the patient market in the United States for rheumatoid arthritis diagnostic testing for 2010 was approximately six million patients. Celiac is an inherited autoimmune disorder that affects the digestive process of the small intestine. It is estimated that the total size of the patient market in the United States for celiac diagnostic testing for 2010 was approximately 3.5 million patients.
Our currently cleared or approved assays are as follows:
| | | | | | | | |
| |
| | Clearance/Approval Status |
|---|
| | Development
Status |
|---|
Product | | Canada | | United States | | Europe |
|---|
IgXPLEX RA (Qualitative) | | Complete | | Licensed | | Cleared | | N/A |
IgXPLEX RA* (Quantitative) | | Complete | | Licensed | | To be filed | | CE Marked |
IgXPLEX Celiac (Qualitative) | | Complete | | Licensed | | Cleared | | N/A |
IgXPLEX Celiac (Quantitative) | | Complete | | Licensed | | To be filed | | CE Marked |
- *
- Marketed in Canada under the name "QuantiSpot Rheumatoid Arthritis".
We also have IgXPLEX Lupus, IgXPLEX Vasculitis and IgXPLEX TNF products in the development stage and IgXPLEX IBD/Crohn's and IgXPLEX Antiphospholipid Syndrome ("APS") products at the proof of concept stage. We also have rheumatoid arthritis and celiac assays with greater sensitivity that measure additional biomarkers in development.
The table below sets out estimates by Frost and Sullivan of the total number of patients in the United States who required diagnostic testing for diseases for which we are developing diagnostic tests.
| | | | | | | | | | | | | |
Autoimmune Segment | | Lupus | | Vasculitis | | Anti-TNF | | IBD/Crohn's | |
|---|
Estimated Total Patient Market 2010 (US) | | | 5,000,000 | | | 400,000 | | | 3,100,000 | | | 550,000 | |
Scienion Products
Scienion offers a range of microarray printing equipment and associated services. Scienion's sciFLEXARRAYER range of microarray printing equipment is targeted to address research customers seeking bench-top equipment up to large customers seeking commercial-scale automated microarray printing equipment
42
as described in the table below. Scienion also markets contract microarray manufacturing and microarray development services.
| | | | |
Application | | Product | | Description |
|---|
Printing | | sciFLEXARRAYER S1 / DW | | Entry-level model of Scienion's sciFLEXARRAYER product line |
| | sciFLEXARRAYER S3 | | Automated non-contact dispensing system of ultra-low volumes designed for academic and research and development labs |
| | sciFLEXARRAYER S5 and S11 | | Automated non-contact dispensing systems of ultra-low volumes designed to manufacture high quality arrays |
| | sciFLEXARRAYER Sx | | Scalable robotic dispensing platforms that enable users to choose size of working area |
| | sciFLEXARRAYER S100 | | High-throughput array and biosensor production instrument |
Microarray Platform | | sciPLEXPLATE | | A specifically developed plate to array RNA, DNA, proteins, antigens and antibodies |
Liquid Handling | | sciSWIFT | | Storable and disposable liquid dispensing pens |
History of the Company
Our business was founded in 1999 on the concept that more sensitive, timely and less costly disease diagnostics based on antigen, protein and antibody detection would benefit healthcare providers. We believed that diagnostic testing based on ELISA technology, the diagnostic technology used at the time, had not changed significantly since its development in the 1950s and lacked accuracy and sensitivity, while the testing procedures were lengthy, difficult to execute and labor intensive. We also believed that DNA microarray technology, which was instrumental throughout the 1990s in mapping the human genome, could provide the basis for new antigen, protein and antibody detection technology that could advance beyond the limitations imposed by ELISA technology. However, antigen, protein and antibody microarrays present a very different set of challenges from the DNA microarrays used for the human genome project.
Until 1999, there was little published research on the use of microarrays for antigen, protein and antibody detection for human IVD applications. Our founders believed the problem stemmed from the following scientific problems:
- •
- Microarray surface coatings and printing technology were not sufficiently advanced to enable antigen, protein and antibody microarrays to be reliably printed with diagnostic grade signal;
- •
- Users could not reliably or consistently measure multiple biomarkers (specifically, antigens, proteins and antibodies) in the same test because these biomarkers have very different surface charge and binding characteristics; and
- •
- High levels of variability were likely related to the lack of standardization and control of titer plate-based ELISA tests leading to inaccurate or less predictable outcomes.
We believe that we have addressed these problems with our platform that allows us to detect and measure multiple antibodies and their different sub-types in the same two-dimensional planar microarray. Our use of specialty surface treatments and surface-coating processes allows us to create microarrays with consistent spot
43
characteristics and limited background noise (in other words, with high signal to noise ratios). Our two dimensional planar array design securely fixes all reagents used in an assay to the glass surface independently of one another, which eliminates unwanted biochemical interactions. Our IgXPLEX technology can detect different antibody isotypes (IgA, IgG, IgM) simultaneously from a single microarray spot using multiple detection wavelengths of light. Our calibration technology adjusts each microarray for every test, which reduces the variability that may result from environmental factors or from the use of external calibrators. We have enhanced the precision of our microarrays through the use of our system, which uses a statistically valid number of replicate spots for each biomarker being tested in combination with complex software algorithms. We believe that our technology delivers consistent, repeatable, precise test results and can measure the concentration of multiple biomarkers in a single test. Our automated technology can analyze multiple patient samples simultaneously using less time, effort, and consumables than existing titer plate technology.
Research and Development
We have assembled an experienced research and development team at our Toronto facility with the scientific, microarray printing, immunoassay, engineering, software, and process development expertise that we believe is necessary to grow our business.
Platform Development
SQiDman is our small semi-automated platform that we are commercialising for RUO purposes, and SQiDlite is our fully-automated, bench-top diagnostic platform.
SQiDlite. Our engineering development team is currently focused on the near term delivery of SQiDlite, our fully-automated, bench-top diagnostic platform. This platform, shown below in concept form, will be a fully-automated microarray processing and analytic platform. We expect that SQiDlite will be able to process multiple sizes of microarray devices from single eight-well strips up to a single 96-well microarray plate.

SQiDlite is designed to serve the following markets;
- •
- IVD Customers who wish to run IgXPLEX assays in flexible batch sizes up to and including 96 patients per test kit. We believe that many hospitals and other laboratories would benefit from SQiDlite's more flexible batch size capability. SQiDlite is designed to run tests in volumes per run of eight to 96 patients in multiples of eight. We believe that a platform that allows for scalable testing volumes for our assays would
44
SQiDman. We are currently commercializing SQiDman on a pilot basis. SQiDman, our small semi-automated platform for processing microarrays, is intended to be used by research customers or development partners for RUO purposes in the microarray development process. We currently use SQiDman systems for in-house development and quality control processes. We expect the full commercial launch of SQiDman for RUO purposes in late 2011, following the enhancement of its user interface software.
Microarray Assay Development
The largest component of our current research and development efforts is microarray development. We plan to continue to focus on the commercialization of microarray content that can be run by our customers on our diagnostic platforms. Our research and development efforts are focused on the following areas:
Autoimmune Disease. We have a broad menu of autoimmune disease tests in our microarray development pipeline to augment our current product offerings. For example, in addition to our products for which we have marketing clearance or approval, we have IgXPLEX Vasculitis, IgXPLEX Lupus, and IgXPLEX TNF products in the development stage and IgXPLEX IBD/Crohn's and IgXPLEX APS at the proof of concept stage. Additionally, we have rheumatoid arthritis and celiac assays with greater sensitivity that measure additional biomarkers in development. Refer to "— SQI Assays" for a description of the markets for the autoimmune disease tests in our microarray development pipeline.
Infectious Disease. We plan to develop tests in the area of infectious disease. Infectious disease panels leverage our strengths in multiplexing, antibodies and high-throughput diagnostic systems. We have scientists and assay development specialists with experience in infectious disease assay development.
Allergen Testing. Allergen tests are very similar to, and depend upon many of the same technological advances as, autoimmune disease tests. Because allergen panels have large numbers of biomarkers, we believe this is an excellent area of opportunity to apply our multiplexing and microarray technology.
45
Custom Assay Development and Print Services. We plan to add assay design and print services to our product offerings. This expansion is intended to enable our laboratory and diagnostic customers to expand their use of our platforms by converting their content to microarrays. We believe that applying our in-house processes and systems to develop microarray formatted tests incorporating customers' content will allow them to reduce their assay costs with less development risk and effort by purchasing their microarrays and development services directly from us. For example, our customers will be able to add requested target biomarkers to an existing panel of biomarkers or they may request an entire panel of protein-based or antibody-based biomarkers to be developed into a RUO microarray that they may use as a lab-developed test.
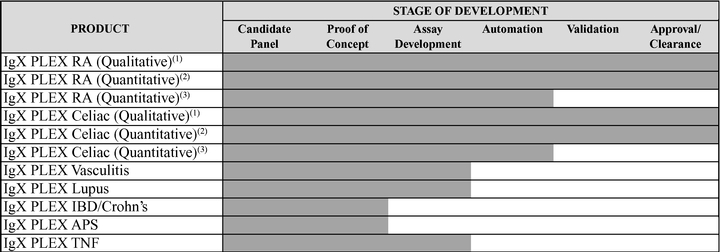
- (1)
- Approved or cleared in the U.S. and Canada.
- (2)
- Approved or cleared in Canada and Europe.
- (3)
- Development status for clearance in the U.S.
Our Strategy
Our goal is to become an industry leader in the development and commercialization of microarray and multiplexing IVD medical systems. We intend to accomplish this goal through:
- •
- Product development efforts. We intend to continue to focus our research and development on high-volume, high-margin, multiplexed tests for diseases for which there are existing tests that have reimbursement programs in place.
- •
- Strategic market penetration. We have identified and are marketing our turnkey SQiDworks platform and approved assays to a specific group of laboratories that process high volumes of tests and typically adopt new technologies to gain market share. Please see "— Sales and Marketing" for a description of our target laboratories.
- •
- Leveraging our core technology and expertise to access new markets and new customers. Our technology and microarray development processes enable us to provide customized third party microarray formatted test development and manufacturing services.
- •
- Seeking partnerships and strategic acquisitions. With our proposed acquisition of Scienion, we intend to become the industry leader in printing solutions, which will enable us to quickly expand our third party development and microarray manufacturing services. We intend to continue to seek partnerships that will enable us to expand into new markets, broaden and deepen our lines of business and develop and strengthen our relationships with our customers.
- •
- Leading through innovation. We intend to continue our research and development in each of our lines of business in order to become the industry leader in multiplexing microarrays.
46
Strategic Alliances
We have a number of collaboration agreements with leading global research and treatment institutes. These collaborations improve our ability to develop products by providing us with access to patient serum required for assay development, verification of products in development and final product clinical validation.
The following table provides an overview of our partnering collaborations and the relevant pipeline product as at September 1, 2011:
| | | | |
Partner Institute | | Pipeline Product | | Purpose |
|---|
Cleveland Clinic | | Rheumatoid arthritis, IBD | | Serum Samples
Clinical Validation
Collaboration |
Beth Israel Deaconess Medical Center | | Celiac, anti-TNF | | Serum Samples
Collaboration / Publication |
Hospital Clinic De Barcelona, Spain | | Vasculitis | | Serum Samples
Collaboration / Publication |
University Hospital Maastricht, The Netherlands | | Vasculitis | | Serum Samples
Collaboration |
The University of North Carolina at Chapel Hill | | Vasculitis | | Collaboration
Serum Samples
Clinical Validation |
We collaborate with these partners to assist our development of new candidate biomarkers for future tests and intend to continue to seek additional alliances and partnerships. We have been active in strategically publishing our progress and successes both alone and in collaboration with certain of our partners.
Sales and Marketing
We currently have a three-person marketing team. Our marketing efforts are focused on generating sales of our currently cleared or approved system, which includes SQiDworks, IgXPLEX RA and IgXPLEX Celiac, to targeted, high-volume laboratories in North America and Europe.
We have identified the 400 largest laboratories, by testing volume, of the approximately 14,000 laboratories that provide blood testing services in North America and a select group of approximately 30 European laboratories. We believe this group contains laboratories that would readily adopt new technologies, look to obtain an economic advantage over their competitors, and attempt to increase their market share of diagnostic testing services. We believe that this initial target group has sufficient testing volume to support the use of SQiDworks, our currently cleared or approved tests, and the tests that we anticipate to be cleared or approved by the end of 2011. We use various marketing strategies to provide incentives to the laboratories in our initial target group that are among the first to adopt our technology. As we expand our test menu, we intend to broaden our target market and reduce the incentives provided to the early adopters of our products.
We believe our proposed acquisition of Scienion and Scienion's four person sales team will enable us to accelerate our penetration into European markets.
Customers
We focus on two customer markets:
- •
- Diagnostic Markets — this market consists of reference and other diagnostic laboratories that provide for-profit diagnostic blood tests.
Customers in this market seek to purchase a full diagnostics solution, including automated platforms and IVD assays. These customers sell the results produced by running our IVD assays on our automated platforms. In addition, customers in this market seek to purchase automated platforms and RUO assays that they intend to
47
convert into lab-developed tests that incorporate their biomarkers. These customers would run these lab-developed tests on our automated platforms and sell the results to their customers.
- •
- Life Sciences Markets — this market consists of customers seeking to commercialize their biomarkers as turnkey multiplexed products delivered by our contract development and manufacturing services. The customers in this market are expected to buy our automated platform and consumable microarray test kits, and to pay a royalty to us on their sales.
There are two types of customers in the life sciences markets:
- •
- Contract Manufacturing and Development Customers — these customers seek expertise for pilot and scale up microarray printing, and have needs in the molecular or protein/antibody areas; and
- •
- OEM Customers — these customers seek full contract development and manufacturing services to commercialize biomarkers owned by them and seek high-throughput automated microarray analyzers to sell through their channels.
Manufacturing
Our manufacturing facility is located in Toronto, Canada. We manufacture all of our microarray kits for commercial sale and internal research and development in our facility.
We operate a "current good manufacturing practices", or "cGMP", facility that has been certified ISO 13485:2003 compliant, which enables us to meet both regulatory requirements and customer expectations. This is an international standard developed by the International Organization for Standardization that specifies the requirements for a quality management system for organizations providing medical devices and related services. Our microarray production facility is operated to ISO Class 7 clean room specifications, commonly referred to as a Class 10,000 operating level, which defines the maximum level of particles permitted per cubic foot.
Based on our microarray manufacturing forecasts, we expect that there is adequate space in the current location to expand our manufacturing capacity to meet our expected needs until the end of 2012. Until a facility upgrade is completed, we intend to undertake equipment upgrades to ensure sufficient manufacturing capacity to meet our expected requirements for commercial sales and our internal validation studies until the end of 2012.
To manufacture consumable tests, we acquire raw materials and custom molecules for our assays from well-established vendors who are either ISO certified or who have an established quality system. Each incoming reagent ingredient undergoes quality testing as required and scrutiny before being released to the manufacturing unit, and the reagent ingredients are then incorporated into finished goods as a IgXPLEX kit. Each kit contains the printed diagnostic array and several self-contained wet reagents that are loaded into the SQiDworks platform prior to use by the customer. We have been producing products suitable for validation studies since the beginning of 2007.
Certain key components of our SQiDworks platform are manufactured by third parties in FDA or ISO certified facilities and we manufacture one component. We assemble the components and deliver them to our customers, where they undergo a series of quality acceptance tests.
Competition
We compete with both established and development-stage life sciences companies that design, manufacture and market basic ELISA technology, lab automation products or multiplexing technologies. For example, companies such as INOVA Diagnostics, Inc., Phadia AB, Axis-Shield plc and Hycor Biomedical, Inc. have immunoassay products based on basic ELISA technology. Companies such as Roche Diagnostics Corporation, bioMérieux SA, Siemens AG and Abbott Laboratories Inc. provide laboratory automation technology. Companies such as Luminex Corporation and Bio-Rad Laboratories Inc. also provide bead-based multiplexing technology. These companies provide products that compete in certain segments in which we sell our products. In addition, a number of other companies and academic groups are in the process of developing novel technologies for life science markets.
48
With respect to our proposed acquisition of Scienion, the largest array print manufacturers that are also offering custom array services and whom we believe represent Scienion's most significant competitors include, in the United States, Arrayit Corporation, Bio-Synthesis Inc., Full Moon Biosystems, Inc., Microarrays Inc., Aushon BioSystems, Inc., and Applied Microarrays, Inc. and, in Europe, GeSiM mbH, and Arrayjet Limited.
Intellectual Property Strategy and Position
Our core intellectual property consists of patent applications or patents in certain jurisdictions that cover the following:
- •
- multiplexed quantification of antigens and antibodies;
- •
- internal (in-array) calibration; and
- •
- methods to create diagnostic level spot morphology.
We originated our core technology. Our patent strategy is to seek broad patent protection on new developments in microarray technology, tests and systems and then later file patent applications covering new implementations of the technology and new microarray platforms utilizing the technology. As these technologies are implemented and tested, we file new patent applications covering scientific methodologies enabled by our technology.
We have developed our own portfolio of issued patents, patent applications and design patents directed to our methodologies, commercial products and technologies in development. For our core technology relating to multiplexed quantification of antigens and antibodies and internal (in-array) calibration, we have obtained issued patents and approvals in certain jurisdictions from the patent family having the title "Method to Measure Dynamic Internal Calibration True Dose Response Curves". This patent family is directed to our calibration technology that adjusts each microarray for every test, which reduces the variability that may result from environmental factors or from the use of external calibrators. Other patent applications are currently pending. Patents issuing from this patent family will be in force until July 20, 2025, provided that all maintenance fees are paid.
We have also obtained either an issued patent or an allowance in some jurisdictions directed to our core technology relating to methods to create diagnostic level spot morphology. The patent family for this core technology has the title "Method and Device to Optimize Analyte and Antibody Substrate Binding by Least Energy Adsorption". The patent family is directed to the use of specialty surface treatments and surface coating process means that can create microarrays with consistent spot characteristics and limited background noise (in other words, with high signal to noise ratios). Other patent applications are currently pending. Patents issuing from this patent family will be in force until July 20, 2025, provided that all maintenance fees are paid.
We have filed patent applications that are currently pending in several jurisdictions directed to our qualitative and quantitative rheumatoid arthritis assays. In addition, patent applications are pending in several jurisdictions directed to our products and methodologies including patent families entitled "Method for Double-Dip Substrate Spin Optimization of Coated Micro-Array Supports", "Method and Device to Remove Fluid and Vapor", "Array Fluorescence Equalization Method", "Methods for Multiplex Analyte Detection and Quantification" and "Multiplex Microarrays and Methods for Quantification of Analytes".
Our proposed acquisition of Scienion allows us to acquire the right to commercialize technology relating to the printing of multiplexed molecular (genetic) microarrays. Scienion's core technology is picolitre liquid handling systems in the area of the life sciences. This technology is particularly advantageous for applications involving miniturization and printing of microarrays for diagnostic assays.
Scienion's core technology includes microarray printing equipment. Scienion's equipment prints microarray spots with high precision and positional accuracy on a variety of surfaces. This equipment is able to print with volumes of biomarkers as small as ten picolitres. Printing with such small volumes enables the printing of very small diameter microarray spots, which allows more spots to be printed in a given area and more data to be produced from a single microarray. Scienion has developed advanced "in-process" methods to measure the mass and position of spots as they are being printed.
49
Scienion has in-licensed patent applications and patents from the Max Planck Institute of Germany. The key in-licensed patents and patent applications relate to means of aspirate dispensing. This patented technology is relevant to microarray printing development, production arrayer systems and contract print and development services for the life sciences. Seven patent families have been in-licensed from the Max Planck Institute.
The patents in-licensed from Max Planck Institute of Germany will be in force until June 2, 2019 for the earliest filed of these patent families ranging to November 4, 2025 for the latest filed of these patent families, provided that all maintenance fees are paid.
Scienion has also filed patent applications directed to equipment for printing microarray spots that it has developed and owns. The technology that Scienion has developed relates to storage and dispensing modes. In particular, Scienion has filed patent applications directed to a microdispenser that can be stored and/or transported with the filled sample container independently of and fluidically separated from the actual dispensing device, without the sample liquid escaping from the sample container during storage or during transport. The filling of the sample container with the sample liquid takes place separately from the dispensing operation, whereas the filling operation in the case of the known microdispensers using a flexible supply line takes place immediately before or during the dispensing operation. In practice, the sample container of the microdispenser is usually filled prior to the dispensing operation, whereas no refilling is provided during the subsequent dispensing operation.
Scienion has focused its patent filings on key jurisdictions where it carries on business, namely the United States, Germany and the UK.
Patents that issue from the patent applications filed by Scienion will be in force until October 20, 2024 for the earliest filed of these patent families ranging to August 18, 2029 for the latest filed of these patent families, provided that all maintenance fees are paid.
Government Regulation
We believe that our major markets are the United States, Canada and the European Union. The following describes the regulatory clearance or approval process for diagnostic systems in each of those jurisdictions.
United States
Research Use Only
"Research use only" components are those components in the laboratory research phase of development that are not represented as effective IVD products. These components must be labeled "For Research Use Only. Not for use in diagnostic procedures." These components are exempt from regulatory oversight in the United States. Manufacturers frequently sell these components to laboratories certified under the Clinical Laboratory Improvement Amendments, or "CLIA", which are the United States federal regulatory standards that apply to clinical laboratory testing performed on human specimens in the United States with certain exceptions, including clinical trials and basic research.
Lab-Developed Tests
CLIA-compliant labs frequently develop and validate an assay from RUO components, sold to them by manufacturers, but the lab must validate these tests and assume all liability for use on patient samples. These tests are regulated as lab-developed tests.
Diagnostic Tests
Diagnostic tests or assays, known as "in vitro diagnostic", or "IVD", products by the FDA, are those reagents, instruments and systems intended for use in the diagnosis of disease or other conditions, including a determination of a person's state of health, in order to cure, mitigate, treat or prevent disease or its sequelae. These tests are intended for use in the collection, preparation and examination of specimens taken from the human body.
50
Diagnostic tests are classified into one of three categories for FDA clearance or market approval. These categories are based on the degree of risk they pose to humans and their importance in preventing impairment in human health.
All IVD tests are subject to the following "general" requirements or controls, as well as, in the case of Class II and Class III diagnostic tests, additional requirements (except where a particular device is expressly exempt from one or more of such requirements). General controls include:
- •
- Registering the manufacturer with the FDA;
- •
- Listing the product to be marketed with the FDA;
- •
- Manufacturing the products in accordance with quality systems regulation (also known as current good manufacturing practices);
- •
- Labelling the test in accordance with labelling regulations;
- •
- Submitting a pre-market notification (also known as a "510(k)") before marketing a test; and
- •
- Reporting of adverse events and product recalls (corrections and removal).
Class I
Class I tests are subject to the least regulatory control as they present minimal potential for harm to the user. Most Class I devices and IVD products are exempt from the pre-market notification and/or good manufacturing practices regulation. Examples of Class I IVD products include pregnancy or cholesterol tests.
Class II
The FDA defines Class II test as those for which "general controls" alone are insufficient to assure safety and effectiveness, and existing methods are available to provide such assurances. In addition to complying with general controls, Class II devices are also subject to special controls.
Class II tests often require pre-market notification under the FDA's 501(k) process, in connection with which the manufacturer submits data to the FDA to demonstrate that its test is substantially equivalent to a legally marketed test (known as a "predicate" test).
Special controls may include special labelling requirements, mandatory performance standards and post-market monitoring. Examples of Class II tests include most blood tests, such as blood tests for rheumatoid arthritis, vasculitis, lupus and other immunological tests.
Class III
Class III is the most stringent regulatory category for tests. Class III tests are those for which the FDA believes insufficient information exists to assure safety and effectiveness solely through general or special controls.
Class III devices are usually those that support or sustain human life, are of substantial importance in preventing impairment of human health, or which present a potential, unreasonable risk of illness or injury.
The FDA typically requires "pre-market approval" for these tests to ensure the safety and effectiveness of Class III tests. This type of approval would require the submission and FDA review of clinical data to assess the safety and effectiveness of the test.
Examples of Class III tests include tests for the diagnosis of many infectious diseases and cancer.
European Economic Area
The European Economic Area, which consists of the 27 member states of the European Union and the European Free Trade Association countries of Iceland, Norway, Switzerland and Liechtenstein, requires what is called "CE" approval (the letters "CE" stand for "conformité Européenne" ("European Conformity") for, among other things, IVD tests). Under the CE regulations, a manufacturer does a preliminary self-assessment to
51
determine whether the products comply with all relevant legislative requirements. At its most basic, the manufacturer must do a conformity assessment, set up a technical file and sign an EC declaration of conformity. This documentation must be made available to authorities upon request. The relevant European Union directive may also require that the product be examined by a conformity assessment body.
We have completed CE marking for our IgXPLEX RA quantitative assay, IgX PLEX Celiac quantitative assay, and the SQiDworks platform, which allows us to market these assays and SQiDworks platform in the European Union. We must also implement and maintain an ISO quality management system. We received our initial ISO 13485 certification in June 2008 and have maintained this certification to date.
As in the United States, components sold without any representation of performance claims may be labeled for "research use only," and are exempt from regulatory oversight in the European Union.
Canada
The Canadian Health Protection Branch regulatory regime requires approvals and submissions similar in timing and scope to European Union CE approvals. TheMedical Devices Regulations (the "Regulations"), promulgated under theFood and Drugs Act (Canada), set out, among other things, the requirements for the licensing of an "in vitro diagnostic device" or "IVDD".In vitro diagnostic devices include reagents, assays and equipment used for examining specimens taken from the body. IVDDs are designated as Class I, II, III and IV, based on the degree of risk associated with their use. For example, a blood test that detects bacterial meningitis is categorized as a Class III IVDD because of the risk that a false-negative test result may cause death or long-term disability due to delayed diagnosis. Class IV IVDDs include donor screening tests for transmissible viruses such as HIV and hepatitis, which present a high public health risk.
For Class I IVDDs, all that is required is an establishment licence to manufacture the IVDD. For Class II, III and IV IVDDs, the Medical Devices Regulations require the IVDDs to meet ISO standard 13485:2003, which provides both design and manufacturing standards.
An application for a Class II medical device includes, in addition to information regarding the manufacturer and specific document, the following:
- •
- a description of the medical conditions, purposes and uses for which the device is manufactured, sold or represented;
- •
- a list of the standards complied with in the manufacture of the device to satisfy the safety and effectiveness requirements;
- •
- an attestation by a senior official of the manufacturer that the manufacturer has objective evidence to establish that the device meets the safety and effectiveness requirements;
- •
- an attestation by a senior official of the manufacturer that the device label meets the applicable labelling requirements of the Regulations;
- •
- in the case of a near patientin vitro diagnostic device, an attestation by a senior official of the manufacturer that investigational testing has been conducted on the device using human subjects representative of the intended users and under conditions similar to the conditions of use; and
- •
- a copy of the quality management system certificate certifying that the quality management system under which the device is manufactured satisfies National Standard of Canada CAN/CSA-ISO 13485:03, Medical devices — Quality management systems — Requirements for regulatory purposes.
As in the United States and Europe, components sold without any representation of performance claims may be labeled for "research use only," and are exempt from regulatory oversight in Canada.
52
Approvals/Clearances
Health Canada
We have received licenses for all applications which have been made to Health Canada to date as listed in the following table.
| | | | | | | | |
Catalog
Number | | Product | | Medical Device
License | | Date of Issuance |
|---|
| | 10005 | | QuantiSpot Rheumatoid Arthritis Assay* | | | 78512 | | November 2008 |
| | 10105 | | IgX PLEX Rheumatoid Arthritis Qualitative Assay | | | 81016 | | October 2009 |
| | 10505 | | IgX PLEX Celiac Qualitative Assay | | | 83945 | | September 2010 |
| | 10515 | | IgX PLEX Celiac Panel* | | | 85930 | | April 2011 |
| | 01003 | | SQiDworks Diagnostics Platform | | | 78513 | | November 2008 |
United States
Our 510(k) submission for IgX PLEX Rheumatoid Arthritis Qualitative Assay and SQiDworks Diagnostics Platform was cleared by the FDA in October 2009 and our 510(k) submission for IgX PLEX Celiac Qualitative Assay and SQiDworks Diagnostics Platform was cleared by the FDA in June 2011.
European Union
Our IgXPLEX Celiac quantitative assay (Catalog #10515), IgXPLEX RA quantitative assay (Catalog #10005) and SQiDworks Diagnostics Platform (Catalog #01004) have been CE marked and registered with the competent authority for our authorized representative.
Specific registrations for each product will be made with the notified body of each country in which commercialization is anticipated once applicable label translations have been completed.
Employees
As of Aug 31, 2011, we had 52 employees, of which three were in sales and marketing, 30 were in research and development, 13 were in manufacturing and operations and six were in administration. Our employees have specialized knowledge in areas such as multiplexing, immunology, microarray design and manufacture, assay development, systems engineering and medical-systems sales and servicing.
We have never experienced a work stoppage or other labor disturbance. To our knowledge, none of our employees belongs to, or is represented by, a labor union.
LEGAL PROCEEDINGS AND REGULATORY ACTIONS
From time to time we are involved in various claims and legal proceedings of a nature considered normal to our business. While it is not feasible to predict or determine the outcome of these proceedings, management believes any current actions to be without merit, and no provision in respect of these matters has been made in our financial statements.
ACQUISITION OF SCIENION AG
On July 4, 2011 we entered into an agreement with those of Scienion's shareholders having the required percentage ownership of share capital to "drag along" the other shareholders pursuant to the Scienion Shareholders' agreement (the "Vendors") to purchase all of the shares of Scienion AG ("Scienion") for aggregate consideration to be satisfied by a closing cash payment of $15,000,000 (of which, approximately $7,700,000 will be utilized to satisfy certain debt settlements and $1,750,000 will be placed in escrow pursuant to the terms of a holdback escrow agreement) and the issuance of 735,294 SQI common shares.
The completion of our acquisition of Scienion is subject to the satisfaction of certain closing conditions, including the closing of the offering for gross proceeds of at least $30,000,000.
53
The acquisition agreement may be terminated if the closing does not occur on or before September 30, 2011. Additionally, either party may terminate the acquisition upon payment of a break fee in the amount of $250,000 if a party terminates its obligations as a result of the non-terminating party's breach of its obligations or failure to use commercially reasonable efforts to satisfy any conditions precedent within such non-terminating party's control.
SELECTED CONSOLIDATED FINANCIAL INFORMATION
The following table sets forth selected financial information for the dates and periods indicated. Our selected financial information as of September 30, 2010 and 2009 has been derived from our audited consolidated financial statements for those annual periods, which are included elsewhere in this prospectus and incorporated by reference into this prospectus, and our selected financial information as of June 30, 2011 and 2010 has been derived from our unaudited interim consolidated financial statements for the nine month periods ended June 30, 2011 and 2010, which are included elsewhere in this prospectus and incorporated by reference into this prospectus. Our consolidated financial statements have been prepared in Canadian dollars and in accordance with Canadian generally accepted accounting principles. For a reconciliation of the significant differences in our consolidated annual financial statements between Canadian GAAP and U.S. GAAP, see "Supplemental Note Regarding the Reconciliation of the Consolidated Financial Statements for the Years Ended September 30, 2010 and 2009 with U.S. GAAP", which is included elsewhere in this prospectus and incorporated by reference into this prospectus. For a reconciliation of the significant differences in our interim consolidated financial statements between Canadian GAAP and U.S. GAAP, see the "Supplemental Note Regarding the Reconciliation of the Interim Consolidated Financial Statements for the Three and Nine Month Periods Ended June 30, 2011 and 2010 with U.S. GAAP", which is included elsewhere in this prospectus and incorporated by reference into this prospectus. Operating results for the nine month period ended June 30, 2011 are not necessarily indicative of the results that may be expected for the fiscal year ended September 30, 2011 or any other future period. The summary financial information below should be read in conjunction with our financial statements and the notes thereto and "Management's Discussion and Analysis of Financial Condition and Results of Operations" included elsewhere in this prospectus and incorporated by reference into this prospectus.
| | | | | | | | | | | | | | |
| | Year Ended
September 30, | | 9 Months Ended
June 30, | |
|---|
Consolidated Statement of Operations Data
C$ in 000's except per share data | | 2009 | | 2010 | | 2010 | | 2011 | |
|---|
Revenue: | | | | | | | | | | | | | |
| | Product sales | | $ | 5 | | $ | 5 | | $ | — | | $ | 22 | |
| | Consulting fees | | | 27 | | | 30 | | | 21 | | | 9 | |
| | | | | | | | | | |
Total Revenue | | | 32 | | | 35 | | | 21 | | | 31 | |
| | | | | | | | | | |
Expenses | | | | | | | | | | | | | |
| | Salaries and wages | | | 469 | | | 556 | | | 404 | | | 607 | |
| | General and administrative | | | 447 | | | 471 | | | 324 | | | 449 | |
| | Professional and consulting | | | 436 | | | 659 | | | 437 | | | 669 | |
| | Sales and marketing | | | 409 | | | 474 | | | 309 | | | 329 | |
| | Stock based compensation | | | 380 | | | 402 | | | 184 | | | 372 | |
| | Research and Development | | | 3,362 | | | 5,059 | | | 3,438 | | | 4,056 | |
| | Amortization — property and equipment | | | 417 | | | 415 | | | 310 | | | 337 | |
Amortization — patent and trademark | | | 98 | | | 100 | | | 85 | | | 88 | |
| | | | | | | | | | |
Total expenses | | | 6,018 | | | 8,136 | | | 5,491 | | | 6,907 | |
| | | | | | | | | | |
Operating loss before interest | | | (5,986 | ) | | (8,101 | ) | | (5,470 | ) | | (6,876 | ) |
Interest Income | | | 121 | | | 32 | | | 20 | | | 56 | |
Interest Expense | | | (45 | ) | | (4 | ) | | (2 | ) | | — | |
| | | | | | | | | | |
Net loss | | $ | (5,910 | ) | $ | (8,073 | ) | $ | (5,452 | ) | $ | (6,820 | ) |
| | | | | | | | | | |
Basic and diluted loss per share | | $ | (0.23 | ) | $ | (0.27 | ) | $ | (0.18 | ) | $ | (0.20 | ) |
Weighted average number of shares | | | 25,158 | | | 30,349 | | | 29,553 | | | 33,849 | |
| | | | | | | | | | |
Consolidated Balance Sheet Data | | September 30,
2009 | | September 30,
2010 | | June 30,
2011 | |
|---|
Cash and cash equivalents | | $ | 3,180 | | $ | 9,408 | | $ | 2,817 | |
Working capital | | | 3,280 | | | 8,930 | | | 2,238 | |
Total assets | | | 6,205 | | | 13,134 | | | 7,488 | |
Total shareholders' equity | | | 5,836 | | | 12,162 | | | 5,960 | |
54
MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND
RESULTS OF OPERATIONS
For the purposes of this Management's Discussion and Analysis of Financial Condition and Results of Operations only, capitalized terms have the meaning given to them in this section.
This discussion and analysis covers the unaudited financial statements for the quarters ended June 30, 2011 and 2010, prepared in accordance with Canadian generally accepted accounting principles ("Canadian GAAP"). The fiscal year end of SQI Diagnostics Inc. ("SQI" or "Company") is September 30th of each calendar year.
All amounts are expressed in Canadian dollars unless otherwise indicated.
This discussion and analysis was prepared by management using information available as at August 25, 2011.
OVERVIEW
SQI Diagnostics Inc. is a life sciences Company that develops and commercializes proprietary technologies and products for advanced microarray diagnostics. Our goal is to become a leader in the development and commercialization of microarray and multiplexed diagnostics by offering our customers a comprehensive "turnkey" solution that increases the efficiency and ease of diagnostic testing and test development.
Our target customers — clinical, academic and diagnostic development laboratories — require diagnostic processing equipment and consumable tests ("systems") that are capable of processing large numbers of patient samples at low cost and with minimal labour requirements ("high-throughput systems"). High-throughput systems have not been widely employed in autoimmune disease, allergen or companion diagnostics testing and only limited use of high-throughput systems exists in infectious disease testing. To our knowledge, no fully-automated high-throughput systems exist that are capable of addressing the combined multiplex testing needs of these markets. A fully-automated system capable of providing multiple biomarker measurements in a single test array has the potential to increase a laboratory's throughput with significantly less labour, consumables and other costs.
Our proprietary microarray tests and automated systems are designed to simplify antigen, protein and antibody testing workflow, increase throughput, reduce costs and provide excellent data quality. In many instances, our technology enables analysis that was traditionally unavailable.
Our high-throughput SQiDworks diagnostic platform is a fully-automated microarray processing and analytical instrument, which provides significant cost savings and other benefits over existing technologies. Additionally, the incremental cost savings of tests run on our fully-automated platform versus existing technologies increase as the complexity of the test increases.
Our IgX PLEX microarrays have the ability to accurately measure multiple biomarkers in a single test. Additionally, our microarray technology uses less patient blood and has fewer steps than traditional methods, which increases the predictive value of the test. The increased predictive value of the test may allow the healthcare provider to choose a treatment plan earlier in the course of the disease.
Our proprietary multiplex assay development process and microarray manufacturing capabilities, combined with our automated systems, are designed to significantly reduce the complexity and cost to our customers to commercialize microarray tests using their own biomarkers.
The Company has been primarily involved in research, development and commercialization activities related to its core technology platform since 2003. The Company has expended significant resources to create and protect its technology platform through the filing of patents and in building an automated instrument and multiplexed assay platform. The Company has invested in fostering partnerships with clinicians who are leaders in our disease areas of focus and with potential novel biomarker collaborators. The Company has also incurred costs associated with gathering market intelligence concerning prospective customers, developing a direct sales platform and in marketing and selling to prospective customers.
The Company has developed its fully automated SQiDworks and semi-automated SQiDman™ microarray-based test platforms that enable laboratory customers to generate multiple patient test results with less than one
55
unit of traditional 'test effort'. The Company has received marketing clearance from the United States Food & Drug Administration ("FDA"), Canadian regulatory approval for, and has CE Marked its fully automated, high throughput SQiDworks platform. SQiDworks is the only fully-automated microarray processing system to achieve these regulatory clearances.
The SQiDworks platforms are to be used to run a menu of tests used to aid in the diagnosis of a wide range of diseases in targeted market segments. The Company has received clearance from the FDA and Canadian regulatory approval for qualitative rheumatoid arthritis (RA) test kits used to detect and measure a panel of biomarkers to aid in the diagnosis of rheumatoid arthritis. The Company has also received clearance from the FDA and Canadian regulatory approval for qualitative celiac disease test kits. Thequantitative RA assay has been licensed in Canada and CE Marked in Europe. The quantitative celiac assay has been licensed in Canada and CE Marked in Europe.
The Company is currently marketing its quantitative celiac assay during the remainder of the 2011 calendar year.
The Company is focusing on the continued development of a pipeline of other tests that can be processed on the SQiDworks platform. The Company is moving these assays through the development pipeline and expects to advance additional test kits through the regulatory process during fiscal 2011 as discussed further in this document. The Company is also focused on the release of SQiDlite, our second generation diagnostic platform. This platform will be a fully-automated microarray processing and analytic platform. It is intended to be a bench-top system able to process multiple sizes of microarray devices from single 8-well strips up to a single 96 well microarray plate. This system is based on the same technology and uses many of the same components as our SQiDworks system. It is targeted at small to medium sized diagnostic customers. Subsequent to the fiscal period end, the Company previewed a prototype of the SQiDlite system at the American Association for Clinical Chemistry Annual Conference (24-28 July 2011).
We plan to add additional services targeted at laboratory and other customers to leverage our expertise in assay design and microarray printing. This initiative is intended to enable our customers to expand the use of our SQiDworks and SQiDlite platforms by converting their content to microarrays. Subsequent to the period end, we presented the results of a collaborative research study where we included biomarkers of interest to a target customer to our in-development vasculitis panel. Our additional services will enable customers to add target biomarkers to an existing panel of biomarkers that they can then offer to their customers, or they may request an entire panel of protein-based biomarkers to be developed into a Research Use Only (RUO) microarray for which they may decide to seek Lab Developed Test regulatory clearance.
Subsequent to quarter-end, the Company announced the proposed acquisition of all of the issued and outstanding shares of Scienion AG, a German company that we believe is a market leader for microarray development, production arrayer printing systems and contract print and development services for the life science industry. Scienion develops and markets a range of commercial microarray printing equipment and provides custom print and commercial print services. The Company believes that the combination of its platform, assays and, as developed, its test menu with, if the acquisition is completed, Scienion's industry leading print technologies, will enable the combined Company to become a leader in end-to-end microarray-based diagnostic systems, microarray printing and assay development services.
56
Status of Development Program
The Company's development program includes several major components which the Company expects will advance its commercialization strategy. The status of each component is summarized and discussed in further detail below:
| | | | | | | | |
| |
| | Approval Status |
|---|
Product | | Development Status | | Canada | | United States | | Europe |
|---|
| SQiDworks™ Diagnostics Platform | | Complete | | Licensed | | Cleared as a system with IgX PLEX RA Assay | | CE Marked |
| SQiDlite Platform | | Development | | | | | | |
| SQiDman Analyzer | | Development — RUO | | Not required — RUO | | Not required — RUO | | Not required — RUO |
| IgX PLEX Rheumatoid Arthritis Assay (Qualitative) | | Complete | | Licensed | | Cleared | | |
| IgX PLEX Rheumatoid Arthritis Assay (Quantitative)* | | Complete | | Licensed | | | | CE Marked |
| IgX PLEX Celiac (Qualitative) | | Complete | | Licensed | | Cleared | | |
| IgX PLEX Celiac (Quantitative) | | Complete | | Licensed | | | | CE Marked |
| IgX PLEX Vasculitis Panel (Quantitative) | | Final Development | | | | | | |
| IgX PLEX Celiac DGP Panel (Quantitative) | | Final Development | | | | | | |
| IgX PLEX Rheumatoid Arthritis Panel with expanded markers (Quantitative) | | Final Development | | | | | | |
| IgX PLEX Lupus Panel (Quantitative) | | Development | | | | | | |
| IgX PLEX TNF Assay (Quantitative) | | Development | | | | | | |
| IgX PLEX IBD — Crohn's Disease (Quantitative) | | Proof of Concept | | | | | | |
| IgX PLEX APS (Quantitative) | | Proof of Concept | | | | | | |
- *
- Marketed in Canada under the name "QuantiSpot Rheumatoid Arthritis"
The Company's SQiDworks and SQiDman platforms are also capable of running RUO and Investigational Use Only (IUO) test kits and the Company is exploring sales opportunities related to these applications of its platform with the Company's products as well as through the potential development of target customers' content. Delivering RUO/IUO product based on customer owned content would require collaboration and assay development though this effort would be materially less than that experienced with the Company's pipeline of regulatory-cleared products. This creates additional new revenue opportunities for the Company.
The Company continues to focus on its in-market tests and believes that it must continuously improve and update its products. The Company has identified and has moved into development enhancements to the existing RA and celiac test panels. These enhancements includefully quantitative IgX PLEX microarray technology and expanded biomarker content. The Health Canada approval for IgX PLEX Celiac Quantitative test kit represents the first approval of the Company's second generation fully quantitative test. The enhancements to the RA panel are in the advanced stages of development.
All in-development tests will utilize this second generation, fully quantitative multiplexing technology; the Company believes these enhancements will provide significant market advantages compared to our competitors.
The Company's development pipeline includes multiplexed test for vasculitis, lupus and IBD-Crohn's. These tests are advancing through the development pipeline with the goal of moving some if not all of these tests into the regulatory filed stage during the remainder of fiscal 2011 and 2012.
Status of Commercialization Activities and Other Events in the Fiscal Year to Date
During the quarter-ended June 30, 2011, the Company invested in its sales and marketing team, its science, commercialization, and regulatory groups, and in infrastructure. The Company's sales efforts are focusing on the
57
North American market and European targets to generate sales to targeted customers of the currently approved system, including the SQiDworks fully automated analytical platform and RA and celiac tests.
Following is an overview of the Company's achievements for the fiscal year to date:
- (a)
- The Company continued to develop its commercial relationship with Gamma Dynacare Medical Labs (GDML) during the quarter-ended June 30, 2011 and achieved additional sales of our RA product in this quarter. The Company is working closely with GDML to develop its multiplexed RA business and in January 2011 GDML released its newsletter that focused on our RA multiplexed product. This marketing material described the benefits of multiplexing rheumatoid arthritis biomarkers on an automated platform to all of GDML's customers and is featured on GDML's website. The Company believes that this and similar marketing efforts will drive the continued growth of our RA product at GDML.
- (b)
- The Company has provided quantitative celiac test kits on an investigational use only basis to GDML enabling their internal review of the product's performance. The internal review by GDML, which is progressing well, is expected to lead to the expansion of the current contract to include the sale to them of our quantitative celiac test kits. GDML has released another newsletter with Celiac 4 plex quantitative being the focus of attention.
- (c)
- Progressed a number of pipeline diagnostic tests through our discovery and development program;
- (i)
- During the quarter-ended June 30, 2011, the Company received FDA clearance for its automated SQiDworks diagnostics platform and its IgX PLEX celiac qualitative assay for marketing in the United States and Health Canada approval for our quantitative celiac test.
- (ii)
- The Company's vasculitis assay continues to progress through the assay development pipeline and is expected to complete clinical validation in the fourth quarter of fiscal 2011. Collaborative studies demonstrating the utility of the Company's assays were presented at the 15th International Vasculitis and ANCA Workshop May 15th – 18th, 2011.
- (iii)
- The Company's quantitative lupus test panel is in the assay development stage. Moving the lupus panel through development is a significant achievement. The current development results show that SQI is able to effectively multiplex up to 16 protein biomarkers, including double stranded DNA. This is our largest panel to date and management believes that, if approved, it will provide SQI with the only such product in the market. Management also believes that the successful completion and clearance of the lupus product will be transformative to the Company's commercial position. The Company expects to initiate clinical validation of this product in the second calendar quarter of 2011, and to complete regulatory filings shortly thereafter.
- (iv)
- The Company's IBD-Crohn's candidate test panel is in the proof of concept stage and is being actively developed with the expectation of being completed and filed for regulatory approvals during calendar 2012.
- (v)
- Development continued on the anti-TNF test candidate, based on the expanded performance requirements requested by our partner, Mount Sinai Services. The Company continues to complete the commercialization of this product and expects to have a commercial product available by the end of calendar 2011.
- (vi)
- Initiated the platform development program for SQiDlite and continued platform development to commercialize our SQiDman platform with a target to complete development on a timeline to coincide with customer requirements for potential collaborations. The SQiDman platform is currently not targeted at IVD applications but in the near-term may be used by our collaborators and customers to assist in the development of their content into SQI-developed microarray RUO/IUO or LDT products. The development of the SQiDlite platform addresses the needs of smaller and mid-market IVD customers and of the research market. The SQiDlite platform will be developed as an automated device with the flexibility to process and analyze varying sizes of consumables up to the current 96-well consumable used in the SQiDworks;
58
The following table provides an overview of our partnering collaborations and the relevant pipeline product as at the period end:
| | | | |
| |
Partner Institute
| | Pipeline Product
| | Purpose
|
|---|
| |
| Cleveland Clinic | | Rheumatoid arthritis, IBD | | Serum Samples
Clinical Validation
Collaboration |
| |
Beth Israel
Deaconess Medical
Center | | Celiac, anti-TNF | | Serum Samples
Collaboration/Publication |
| |
Hospital Clinic
De Barcelona, Spain | | Vasculitis | | Serum Samples
Collaboration/Publication |
| |
University Hospital
Maastricht,
The Netherlands | | Vasculitis | | Serum Samples
Collaboration |
| |
The University of
North Carolina at
Chapel Hill | | Vasculitis | | Collaboration
Serum Samples
Clinical Validation |
| |
- *
- All Partnering Institutes are located in the USA unless otherwise annotated.
CORPORATE FINANCING TRANSACTIONS
During the three months ended June 30, 2011 a total of 33,334 employee stock options were exercised at a price of $1.20 for total proceeds of $40,000.
CRITICAL ACCOUNTING POLICIES AND SIGNIFICANT ESTIMATES
These financial statements are prepared in accordance with Canadian GAAP.
The significant accounting policies that management believes are the most critical in fully understanding and evaluating the reported financial results include the following:
Patents and Trademarks
The costs relating to patent and trademark fees are deferred and amortized over 10 years on a straight-line basis. Patents and trademarks are recorded net of accumulated amortization of $715,000 (September 30, 2010 — $627,000).
Research and Development Costs
Research costs are charged to earnings in the period in which they are incurred. Development costs are expensed as incurred or deferred if they meet the criteria for deferral under Canadian generally accepted accounting principles and are expected to provide future benefits with reasonable certainty. At June 30, 2011, the Company has developed a pipeline of novel tests for its diagnostic system. Deferral criteria have not yet been met, and accordingly, all development costs have been expensed.
Stock-Based Compensation and Other Stock-Based Payments
The Company applies a fair value based method of accounting for all stock-based payments. Accordingly, stock-based payments are measured at the fair value of the consideration received or the fair value of the equity instruments issued or liabilities incurred, whichever is more reliably measurable. Stock-based compensation is charged to operations over the vesting period and the offset is credited to contributed surplus. Consideration received upon the exercise of stock options is credited to share capital, at which time, the related contributed surplus is transferred to share capital.
59
Share Issuance Costs
Costs incurred in connection with the issuance of capital stock are netted against the proceeds received.
Income Taxes
The Company follows the liability method of accounting for income taxes. Under this method, future income tax assets and liabilities are determined based on temporary differences between financial reporting and tax bases of assets and liabilities, as well as for the benefit of losses available to be carried forward to future years for tax purposes. Future income tax assets and liabilities are measured using enacted or substantively enacted tax rates and laws that will be in effect when the differences are expected to reverse. Future income tax assets are recorded in the financial statements if realization is considered more likely than not.
Use of Estimates
The preparation of financial statements in conformity with Canadian generally accepted accounting principles requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amount of revenue and expenses during the year. Actual results could differ from those estimates. Significant areas requiring the use of management estimates relate to the determination of the useful lives of property and equipment and patents and trademarks for amortization purposes, valuation of investment tax credits receivable, valuation of stock options and warrants and valuation allowance on future tax assets.
Recent Accounting Pronouncements
- (a)
- Adoption of New Accounting Pronouncements
Business Combinations
In January 2009, the CICA issued Section 1582, Business Combinations, which replaces former guidance on business combinations. Section 1582 establishes principles and requirements of the acquisition method for business combinations and related disclosures. In addition, the CICA issued Sections 1601, Consolidated Financial Statements, and 1602, Non-Controlling Interests, which replaces the existing guidance. Section 1601 establishes standards for the preparation of consolidated financial statements, while section 1602 provides guidance on accounting for a non-controlling interest in a subsidiary in consolidated financial statements subsequent to a business combination.
These standards apply prospectively to business combinations for which the acquisition date is on or after the beginning of the first annual reporting period beginning on or after January 1, 2011 with earlier application permitted. The Company adopted the new standards on October 1, 2010.
- (b)
- Recent Accounting Pronouncements Issued and Not Yet Applied
International Financial Reporting Standards (IFRS)
Effective January 1, 2011 the CICA has adopted International Financial Reporting Standards ("IFRS"). The Company will be required to adopt IFRS for its 2012 fiscal year and will be required to provide IFRS comparative information for the previous fiscal year. The Company continues assess and plan for the conversion to IFRS. We have identified the main differences between existing Canadian GAAP and IFRS standards and begun quantifying the reporting differences. The Company has a conversion plan in place and believes it has the
60
resources in place to meet the conversion timelines. The following are the main differences and the expected impact on our business processes and information systems:
| | |
| |
Key Accounting Areas
| | Expected impact on the Company
|
|---|
| |
| IFRS 1 First time adoption of IFRS | | The Company has selected the applicable exemptions under IFRS.
Additional reconciliations and disclosure upon the initial conversion to IFRS will be included in the initial statements presented under IFRS commencing in the first quarter of fiscal 2012. The Company has begun the process of assessing and preparing the additional reconciliation. The Company is reviewing the disclosure requirements including the disclosure of other corporations which have already adopted IFRS. |
| |
| IAS 16 Property Plant and Equipment | | The Company has commenced a study of the useful life of each component of property plant and equipment and will restate, if applicable, the historic amortization expense. Based on the preliminary results of the study the Company does not expect a material adjustment upon adoption of IFRS. |
| |
| IAS 36 Impairment of Assets | | The Company will evaluate potential impairments using discounted cash flow analysis as required under IFRS. Based on our preliminary review the Company does not expect a material adjustment upon adoption of IFRS. |
| |
| IAS 12 Income Tax | | The Company has accumulated non-capital losses, undeducted scientific research and development costs, and investment tax credits that have not been reflected in the financial statements. These items will need to be assessed based on the IFRS criteria to ensure proper classification on the balance sheet. The Company believes that these items will not meet the criteria for inclusion on the balance sheet and will continue to be disclosed in the notes to the annual financial statements. |
| |
| IFRS 2 Share based payments | | IFRS 2 requires that each tranche of options with graded vesting be treated as a separate award. IFRS 2 also requires an estimate of forfeitures to be factored into the determination of compensations costs.
The Company expects to utilize the exemptions under IFRS 1 when converting to the new standard. The Company has begun to calculate the impact on all unvested tranches of options at the date of transition. Based on our preliminary calculation we expect the adjustment to increase the opening deficit by $180,000. |
| |
| IAS 1 Financial Statement Presentation | | Additional disclosure required as well as selection between presentation alternative will be addressed in the initial statements presented under IFRS.
The Company is analyzing the impact of the changes on its financial statements through a review of the standards as well as a review of the financial reports of corporations with earlier adoption dates.
The Company does not believe there will be a material change to its financial statement presentation. |
| |
The Company believes it has the financial reporting expertise in place to complete the transition to IFRS and does not believe the transition will materially impact its business activities. As the review of accounting policies is further finalized, we will review internal controls over financial reporting and the disclosure controls and policies and, where necessary, changes will be made.
At this point, the only area of impact is expected to result from IFRS 2 Share based payments. The implementation of the changes as a result of adopting IFRS 2 is not expected to have a material impact on the Company's internal controls over financial reporting or its disclosure controls and policies.
61
SELECTED FINANCIAL INFORMATION
Third Quarter Commentary
The table below summarizes quarterly financial information for the 3 month periods shown.
| | | | | | | | | | | | | |
| | |
| | June 30, 2011
(000s)
| | March 31, 2011
(000s)
| | December 31, 2010
(000s)
| | September 30, 2010
(000s)
| |
|---|
| | |
Revenue | | $ | 9 | | $ | 4 | | $ | 18 | | $ | 14 | |
| | |
Net Loss | | $ | 2,691 | | $ | 1,874 | | $ | 2,255 | | $ | 2,621 | |
| | |
Net Loss Per Share | | $ | (0.08 | ) | $ | (0.07 | ) | $ | (0.08 | ) | $ | (0.06 | ) |
| | |
Weighted Average Shares | | | 33,936 | | | 33,852 | | | 33,759 | | | 32,705 | |
| | |
| | | | | | | | | | | | | |
| | |
| | June 30, 2010
(000s)
| | March 31, 2010
(000s)
| | December 31, 2009
(000s)
| | September 30, 2009
(000s)
| |
|---|
| | |
Revenue | | $ | 6 | | $ | 10 | | $ | 5 | | $ | 7 | |
| | |
Net Loss | | $ | 1,812 | | $ | 2,020 | | $ | 1,620 | | $ | 1,616 | |
| | |
Net Loss Per Share | | $ | (0.06 | ) | $ | (0.07 | ) | $ | (0.06 | ) | $ | (0.06 | ) |
| | |
Weighted Average Shares | | | 30,790 | | | 29,917 | | | 27,930 | | | 27,271 | |
| | |
Revenue for the quarter-ended June 30, 2011 was $9,000 compared to $6,000 for the quarter-ended June 30, 2010. Revenue for the nine months ended June 30, 2011 was $31,000 compared to $21,000 for the nine months ended June 30, 2010. Revenue for the three and nine months ended June 30, 2011 included sales of its QuantiSpot RA test kits, there were no product sales during the same period in 2010. Revenue in the three and nine months ended June 30, 2010 resulted from consulting services provided to a related party; these services were not performed in the quarters ended March 31, 2011 and June 30, 2011.
For the quarter-ended June 30, 2011, the Company recorded a net loss of $2,691,000 ($0.08 net loss per share)compared to a net loss of$1,812,000 ($0.06 net loss per share) for the quarter-ended June 30, 2010. Per share values are based on the weighted average shares outstanding in the period. The net loss for the nine months ended June 30, 2011 was $6,820,000 ($0.20 net loss per share) compared to a net loss of $5,452,000 ($0.18 net loss per share) for the nine months ended June 30, 2010. For the quarter-ended June 30, 2011 there was an average of 33,936,000 shares outstanding (nine months ended June 30, 2011 — 33,849,000).
The net loss was greater for the three and nine months ended June 30, 2011 compared to the three and nine months ended June 30, 2010. The Company's intensified R&D efforts that began in the 2010 fiscal year have resulted, year to date, in increases in all R&D costs. These costs are primarily related to expenses in the discovery efforts for and development of assays as detailed below. Additional other expenses incurred included ordinary increases in wage and wage-related expenses owing to an increase in personnel, increased lab expenditures to support the greater number of projects, and other direct costs including serum acquisition and development and validation partner costs. Sales and marketing expense was higher in the nine months ended June 30, 2011 owing to additional travel and contract resources in sales and marketing as the Company continued to increase its sales effort for approved IgX PLEX assays in Canada and the United States and in anticipation of further product approvals. Professional and consulting costs were higher in the three and nine months ended June 30, 2011 due to legal and accounting costs incurred related to the acquisition of Scienion AG.
R&D expenditures for the quarter-ended June 30, 2011 were $1,443,000 compared to the $1,111,000 for the quarter-ended June 30, 2010. R&D expenditures for the nine month period ended June 30, 2011 were $4,056,000 compared to the $3,438,000 for the nine month period ended June 30, 2010. The increase in R&D expense for the three month period ended June 30, 2011 compared to the three months ending June 30, 2010 resulted from the SRED investment tax credit being recorded in the quarter-ended June 30, 2010, whereas the 2011 credit was recorded in the quarter-ended March 31, 2011. Increased R&D costs in the three months ended June 30, 2011 were offset by reductions in R&D recruiting and reduced regulatory costs compared to the same
62
quarter in 2010. The increase in R&D expense for the nine month period ended June 30, 2011 compared to the nine months ending June 30, 2010 resulted from increased R&D activity with an increased number of assay panels in development and to continued regulatory validation efforts related to the celiac products. In the nine months ended June 30, 2011, in addition to the celiac assay in regulatory validation, the Company had five panels in development and 1 additional panel in early discovery and development. In the third quarter of fiscal 2010 the Company had 5 panels in discovery and development.Also contributing to the increase in R&D costs is the annualized effect in the nine months ended June 30, 2011 of personnel additions made over the nine months ended June 30, 2010 as the Company accelerated its R&D efforts.
Corporate expenses include, primarily, salaries and related expenses (including benefits and payroll taxes) of the Company other than salaries and related expenses paid to personnel engaged in research and development. General and Administrative expenses include facility costs, insurance costs, and foreign exchange expenses. Corporate and general expenses totalled $400,000 for the three months ended June 30, 2011 compared to $262,000 for the three months ended June 30, 2010. Corporate and general expenses totalled $1,056,000 for the nine months ended June 30, 2011 compared to $728,000 for the nine months ended June 30, 2010. The increase from the three and nine months ended June 30, 2010 compared to the same periods in 2011 was primarily a result of higher salary costs, increased personnel and increased occupancy costs. Corporate expenses also included a loss on the disposition of equipment in the nine months ended June 30, 2011; there was no similar loss in the nine months ended June 30, 2010.
Professional consulting (legal, accounting, Board of Directors compensation, recruiting, administrative contractor, and investor relations) costs in the three months ended June 30, 2011 were $469,000 compared to $140,000 for the three months ended June 30, 2010. Professional consulting costs were $669,000 for the nine months ended June 30, 2011 compared $437,000 for the nine months ended June 30, 2010. The increase in professional and consulting costs in the three and nine months ended June 30, 2011 were primarily related to legal and consulting costs related to the acquisition of Scienion.
Sales and Marketing expenses were primarily related to sales and marketing consultant fees and to travel related to selling activities in the quarter. Sales and marketing expenses totalled $114,000 for the three months ended June 30, 2011 compared to $116,000 for the three months ended June 30, 2010. Sales and marketing expenses totalled $329,000 for the nine months ended June 30, 2011 compared to $309,000 for the nine months ended June 30, 2010. The increase in sales and marketing expenses for the nine months ended June 30, 2011 compared to the nine months ended June 30, 2010 were primarily related to additional consulting costs paid to increase staffing to support product pipeline and commercialization efforts. Sales and Marketing costs in the quarter-ended June 30, 2010 included costs for an additional sales contractor; this cost was not incurred in 2011.
Operational expenses were partially offset by interest income earned on short-term investments of $14,000 for the three ended June 30, 2011 (nine months — $56,000) compared to $8,000 for three months ended June 30, 2010 (nine months — $20,000). The Company invests its cash in variable term cashable government investment certificates and short-term money market deposits.
Non-cash stock based compensation charges totalled $144,000 for the three months ended June 30, 2011 (nine months — $372,000) compared to $69,000 for the three months ended June 30, 2010 (nine months — $184,000). The related stock option issuances are described further below in the Outstanding Share Capital section.
Outlook
Management expects losses to continue for the current fiscal year as investment continues in product development and commercialization efforts on its pipeline of autoimmune test kits and platforms, as well as investment in sales and marketing. During the remainder of the 2011 fiscal year the Company intends to focus on sales of its growing autoimmune test menu and placing SQiDworks systems in Canadian, US and European customers for system evaluation. We expect that some, or all, of these evaluation placements will lead to commercial acceptance and revenues from sales of consumable test kits in the future. The Company delivered one such evaluation placement in the 2010 fiscal year to GDML. Subsequent to GDML's 90 day internal acceptance and validation testing the Company executed a commercial agreement and has generated revenue since the fourth quarter of fiscal 2010. GDML is presently evaluating the Company's quantitative celiac test kits
63
and management is optimistic that this evaluation will lead to additional revenues from GDML attributed to IgX PLEX Quantitative Celiac kits in fiscal 2011. The Company also intends to focus on its expanding tools and services product offerings to generate additional sales opportunities during the remainder of the 2011 fiscal year.
Related Party Transactions
Transactions with related parties occur in the normal course of business and are measured at the exchange amount. Related party transactions are described below, unless they have been disclosed elsewhere in the financial statements.
Included in general and administrative expense for the three month period ended June 30, 2011 is $13,000 (three month period ended June 30, 2010 — $12,000) compared to $38,000 for the nine month period ended June 30, 2011 (nine month period ended June 30, 2010 $37,000), related to recovery of occupancy costs from a corporation in which an officer of the Company is also an officer. Consulting fee revenue of NIL for the three month ended June 30, 2011 (three month ended June 30, 2010 — $6,000) was earned from this corporation compared to $9,000 for the nine month period ended June 30, 2011 (nine month period ended June 30, 2010 $21,000). At quarter-end, $1,000 (September 30, 2010 — $1,000) due from this corporation is included in amounts receivable.
Sources and Uses of Cash
Operational activities for the quarter-ended June 30, 2011 were financed by cash on hand.
At June 30, 2011, current assets were $3,766,000 compared to $9,902,000 at September 30, 2010. Working capital as at June 30, 2011 was $2,238,000 compared to $8,930,000 at September 30, 2010.
Cash used in investing activities for the quarter-ended June 30, 2011 was $130,000 compared to $287,000 for the quarter-ended June 30, 2010. Increased investing activities in the three months ended June 30, 2010 was a result of additional laboratory equipment purchased to expand research and development efforts. Cash used in investing activities for the nine months ended June 30, 2011 was $557,000 compared to $468,000 for the nine months ended June 30, 2010. Increased additions to property and equipment in the current niine month period reflect the Company's investment in (1) an overhaul of its out-dated network and data storage infrastructure to expand its data storage capacity required to support the research and development program and to enhance its disaster recovery system to protect the vast amounts of data generated through product development and validation, and (2) a SQiDworks platform for internal use for platform development activities.
During the nine months ended June 30, 2011 a total of 81,668 options were exercised at an average price of $1.45 for total proceeds of $119,000.
During the nine months ended June 30, 2011 106,520 warrants with an expiry date of January 22, 2011 were exercised for total proceeds of $133,000.
See "Use of Proceeds".
We may need to raise additional capital through the issuance of securities in the near future in order to fund our on-going operations if we are unable to generate sufficient cash from operations to satisfy our on-going liquidity requirements. See "Risk Factors".
Risks
The Company is subject to various risks. Factors that could cause results or events to differ materially from management's current expectations include, but are not limited to:
- •
- We have incurred losses and expect to do soin the foreseeable future;
- •
- Our future capital needs are uncertain and we will need to raise additional funds in the future, which may not be available on a timely basis or on commercially reasonable terms;
- •
- Market competition and technological advances of similar diagnostics products could reduce the attractiveness of our products or render them obsolete;
64
- •
- If our products fail to achieve and sustain sufficient market acceptance, our revenue will be adversely affected;
- •
- We are subject to complex regulatory compliance requirements and the failure to obtain, or the withdrawal of, regulatory clearance or approval for our products could adversely affect our ability to market our products and/or require us to incur significant costs to comply with such requirements;
- •
- We may not be able to develop new products or enhance the capabilities of our existing diagnostics products to keep pace with rapidly changing technology and customer requirements;
- •
- Research and development of diagnostic products requires significant testing and investment and may not result in commercially viable products within the timeline anticipated, if at all;
- •
- We may need additional capacity to meet our manufacturing needs at the end of 2012; and
- •
- Our future success depends upon our ability to expand our customer base and introduce new products and services.
For a complete discussion of risks and uncertainties please refer to the information under the heading "Risk Factors" located elsewhere in this prospectus.
The Company's SQiDworks automated analytical platform and its lead IgX PLEX RA multiplexed test kit, which have received regulatory clearances in Canada, Europe and the United States, are believed to be the first microarray technologies in the autoimmune disease market to receive such clearances. The Company has continued to build on this regulatory success with the Health Canada licensing of its IgX PLEX Celiac Qualitative test and its IgX PLEX Celiac Quantitative test, also CE Marked in Europe. With the Health Canada approval for the first second generation fully quantitative assay, the Company anticipates that its quantitative celiac product line will progress commercially, later this year with the release of a 6-plex quantitative panel that adds additional emerging biomarkers for markets in Canada, the US and Europe.
Our tests are designed to run only on the SQiDworks platform. In order to obtain approval for the SQiDworks platform and the Company's consumable tests for sale in the United States, our largest target market, the FDA typically requires the conduct of clinical validation studies to compare the performance of a new test to predicate tests currently approved for sale in the US. Upon successful completion of validation studies conducted at both SQI's labs and at multiple third party labs, the data derived are then presented to the FDA in the form of a 510(k) Pre-market Notification. It is typical for the external validation studies to take several months to complete and upon receipt of a completed 510(k) submission the FDA may take up to 180 days to render a decision on the application, not including any "time-outs" which the Company may take to prepare responses to various inquiries from the FDA. The Company believes the experience gained in obtaining the clearance of the SQiDworks, RA test and celiac test, will enable it to complete and file applications for clearance of the subsequently developed pipeline of assays more efficiently. This in turn may result in shorter review periods at the FDA than was experienced with the SQiDworks-RA system. The timing of such clearances is dependent on several factors some of which are not controlled by the Company.
During the current reporting period the Company did not earn significant revenues from its test kits or SQiDworks platform. Management believes that material revenues from the sale of its test kits may be achieved in the 2011 calendar year; this is subject to certain risks including without limitation, the continued success of the development program and regulatory approvals of the products. The continuation of the Company's research, development and commercialization activities along with investment in marketing and sales is dependent upon the Company's ability to successfully manage its growth, investment in continued pipeline development and its cash requirements.
We are exposed to market risks related to changes in interest rates and foreign currency exchange rates, which could affect the value of our current assets and liabilities. We do not believe that the results of operations or cash flows would be affected to any significant degree by a sudden change in market interest rates relative to our cash investment, due to the prime interest rate based nature of the investment. We have not entered into any forward currency contracts or other financial derivatives to hedge foreign exchange risk. We are subject to foreign exchange rate changes that could have a material effect on future operating results or cash flows.
65
Management will continue to review the Company's financial needs and to seek additional capital financing as required from sources that may include equity financing, debt financing, collaborative projects and licensing arrangements; however, there can be no assurance that such additional funding will be available or if available, whether acceptable terms will be offered. On July 07, 2011, management announced that the Company had filed a preliminary short form base PREP prospectus with the Ontario Securities Commission and a registration statement on Form F-10 with the U.S. Securities and Exchange Commission in connection with a proposed offering of common shares.
Outstanding Share Capital
As at June 30, 2011, there were 33,946,000 common shares issued and outstanding.
The following tables describe the securities that have been issued that are convertible under certain conditions into common shares:
The Company had the following warrants outstanding at June 30, 2011:
| | | | | | |
Number of Warrants | | Purchase Price | | Expiry Date |
|---|
| | 1,199,000 | | $ | 4.00 | | December 4, 2011 |
| | 237,000 | | $ | 1.90 | | December 23 2011 |
| | 1,140,000 | | $ | 5.00 | | August 12, 2012 |
| | 57,000 | | $ | 2.50 | | August 12, 2012 |
| | | | | | |
| | 2,633,000 | | | | | |
| | | | | | |
The Company had the following stock options outstanding under the Plan at June 30, 2011:
| | | | | | |
Number of Options (000s) | | Exercise Price | | Expiry Date |
|---|
| | 58,000 | | $ | 1.20 | | August 29, 2011 |
| | 143,000 | | $ | 1.74 | | August 7, 2012 |
| | 50,000 | | $ | 1.50 | | October 23, 2012 |
| | 758,000 | | $ | 1.60 | | February 15, 2013 |
| | 255,000 | | $ | 1.75 | | August 26, 2013 |
| | 78,000 | | $ | 1.30 | | May 21, 2014 |
| | 25,000 | | $ | 3.26 | | November 3, 2014 |
| | 107,000 | | $ | 2.25 | | February 22, 2015 |
| | 50,000 | | $ | 2.10 | | May 27, 2015 |
| | 175,000 | | $ | 2.50 | | August 16, 2015 |
| | 100,000 | | $ | 2.90 | | October 4, 2015 |
| | 60,000 | | $ | 2.85 | | January 31, 2016 |
| | | | | | |
| | 1,859,000 | | | | | |
| | | | | | |
Off-Balance Sheet Arrangements
The Company has no off-balance sheet arrangements.
Future Prospects
In its current state of evolution, management believes that the Company has assembled the necessary intellectual, financial, and human capital to advance its current pipeline of autoimmune test panels and the SQiDworks system through the completion of clinical validation studies and regulatory filings in Canada, the U.S. and Europe. The Company believes that completion of its quantitative RA and celiac products justifies the current intensified investment in development and commercialization of its pipeline of an additional group of at least eight autoimmune microarray diagnostic panels over the next eighteen months. It further believes that successful completion of these tests in development, and collaborations with its partners, may lead to the
66
identification and commercialization of other test panels, not currently contemplated or in development, addressing unmet medical needs in the diagnosis or therapies for autoimmune, infectious disease and allergy management.
Management believes that the successful completion of its acquisition of Scienion, announced July 4, 2011 will enable us to:
- •
- combine our assay commercialization capabilities, our range of cleared and in-development processing and analytical systems for running microarray tests and the expertise of the two companies for highly technical microarray printing capabilities;
- •
- strengthen our technology leadership position in microarray printing, surface chemistry, and automation of microarray processes;
- •
- accelerate the commercialization of our pipeline of RUO and IVD systems through the use of Scienion's arrayer equipment and complete "print solutions";
- •
- provide us with access to Scienion's base of more than 400 customers, many of whom are potential customers for our service offerings and RUO and IVD products;
- •
- provide sales support for Scienion's equipment, products and services in North America;
- •
- give us access to Scienion's sales and engineering field service to leverage our suite of diagnostic products and services in Europe and Asia; and
- •
- diversify our revenues and add financial strength.
SQI's operational objectives are straightforward: generate revenue from products in the regulatory jurisdictions for which we have acquired regulatory approvals or licenses; continue successful commercialization and continuous improvement of a menu of autoimmune test kits; and, expand partnerships and other strategic relationships to enhance our product offerings or revenues. Success in these steps will allow the Company to further validate its multiplexing model, value proposition and to roll-out and sell its products to customers in its target markets.
Our goal is to become an industry leader in the development and commercialization of microarray and multiplexing diagnostic systems. We intend to accomplish this goal through:
- •
- Product development efforts. We are continuing to focus our research and development on high-volume, high-margin, multiplexed tests for diseases for which there are existing tests that have reimbursement programs in place and predicate technologies that have received FDA clearance.
- •
- Strategic market penetration. We have identified and are marketing our turnkey SQiDworks platform and approved assays to a specific group of laboratories which process high volumes of tests and typically adopt new technologies to gain market share.
- •
- Leveraging our core technology and expertise to access new markets and new customers. Our technology and microarray development processes enable us to provide customized third party microarray formatted test development and manufacturing services.
- •
- Seeking partnerships and strategic acquisitions. With our proposed acquisition of Scienion, we intend to become the industry leader in printing solutions, which we expect will enable us to quickly expand our third party development and microarray manufacturing services. We intend to continue to seek partnerships that will enable us to expand into new markets, broaden and deepen our lines of business and develop and strengthen our relationships with our customers.
- •
- Leading through innovation. We intend to continue our research and development in each of our lines of business in order to become the industry leader in multiplexing microarrays.
The Company will continue to invest in research and development activities related to its core platform technologies of microarray-based assays and detection devices.
67
Disclosure Controls and Procedures, and Internal Control Over Financial Reporting
The accompanying financial statements have been prepared by management in accordance with Canadian generally accepted accounting principles. For quarterly reporting periods, the Company's financial statements are approved by the Audit Committee and the Board of Directors. For annual reporting periods, the Company's financial statements are approved by the Board of Directors upon recommendation by the Audit Committee. The integrity and objectivity of these financial statements are the responsibility of management. In addition, management is responsible for all other information in this report and for ensuring that this information is consistent, where appropriate, with the information contained in the financial statements.
In support of this responsibility, management maintains a system of internal controls to provide reasonable assurance as to the reliability of financial information and the safeguarding of assets.
In particular, the CEO and CFO are responsible for establishing and maintaining disclosure controls and procedures ("DC&Ps") and internal controls over financial reporting ("ICFRs") for the Company, and have:
- (a)
- designed such DC&Ps, or caused them to be designed under supervision, to provide reasonable assurance that material information is made known during the period in which the annual and quarterly filings are being prepared;
- (b)
- designed such ICFRs, or caused them to be designed under supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with Canadian GAAP;
- (c)
- evaluated the design and effectiveness of the Company's DC&Ps as of the quarter-ended June 30, 2011;
- (d)
- have concluded that a material design weakness in the ICFRs may exist in terms of the inadequate segregation of certain duties, which is typical of development stage companies; mitigating factors, including dual-payment authorization policies and transparent internal financial transaction reporting processes, serve to minimize the risk that such design weakness could result in a material misstatement of results for the quarter-ended June 30, 2011; and
- (e)
- have concluded that, other than the item described above in sub-point (d), there are no additional material design weaknesses in the DC&Ps or ICFRs, and that the effectiveness of the DC&Ps is sufficient to expect the prevention or detection of material misstatements of results.
The financial statements include amounts that are based on the best estimates and judgments of management. The Board of Directors is responsible for ensuring that management fulfills its responsibility for financial reporting and internal control. The Board of Directors exercises this responsibility principally through the Audit Committee. The Audit Committee consists of three directors, all of whom are independent and not involved in the daily operations of the Company. The Audit Committee meets with management and the external auditors to satisfy itself that management's responsibilities are properly discharged and to review the financial statements prior to their presentation to the Board of Directors for approval.
68
MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND
RESULTS OF OPERATIONS
For the purposes of this Management's Discussion and Analysis of Financial Condition and Results of Operations only, capitalized terms have the meaning given to them in this section.
This discussion and analysis covers the audited financial statements for the years ended September 30, 2010 and 2009, prepared in accordance with Canadian generally accepted accounting principles (Canadian GAAP). The fiscal year end of SQI Diagnostics Inc. ("SQI" or "Company") is September 30 of each calendar year. This discussion and analysis was prepared by management using information available as at January 11, 2011.
All amounts are expressed in Canadian dollars unless otherwise indicated.
OVERVIEW
SQI Diagnostics Inc. is a medical systems company that develops proprietary human diagnostic technology through multiplexing, miniaturization and automation. Our technologies enable laboratories to analyze multiple biomarkers simultaneously, deliver accurate and quantitative patient results in less time, significantly reduce labour costs, and increase profits when compared with current diagnostic instrumentation. The Company's proprietary SQiDworks™ instrument and IgXPLEX consumable tests together form an immunoassay system capable of providing many of the diagnostic tests currently performed in reference laboratories engaged in testing human blood for a wide range of disease markers.
The Company has been primarily involved in research, development and commercialization activities related to its core technology platform since 2003. The Company has expended significant resources to create and protect its technology platform through the filing of patents and in building an automated instrument and multiplexed IgXPLEX assay platform. The Company has invested in fostering partnerships with clinicians who are leaders in our disease areas of focus and with potential novel biomarker collaborators. The Company has also incurred costs associated with gathering market intelligence concerning prospective customers, developing a direct sales platform and in marketing and selling to prospective customers.
The Company's strategy is to develop and commercialize test kits for the autoimmune disease market as further described below, and plans to pursue commercialization of tests in infectious disease and allergen testing in the future. The Company also plans to explore in-licensing opportunities to expand its product pipeline as well as to continuously improve its in-market product through the addition of novel biomarkers to the existing diagnostic panels of tests. To execute this strategy the Company plans to seek regulatory approvals to sell these additional tests globally starting with the North American markets. Following the successful commercialization of several IgXPLEX test panels, management will evaluate selling in additional markets starting with countries in the European Union.
During the fiscal year ended September 30, 2010, the Company's strategic focus was to initiate the transition from a development Company to a commercial Company. During fiscal 2010, the Company entered into a contract with Gamma Dynacare Medical Laboratories (GDML) to supply them with a SQiDworks assay processing and analytical system and QuantiSpot RA™ test kits. GDML has also agreed to evaluate the Company's IgXPLEX Celiac test kits in the 2011 fiscal year and the Company expects to deliver validation IgXPLEX Celiac products to them in the second quarter of fiscal 2011. GDML is a key customer for the Company; it processes significant test volumes of both rheumatoid arthritis and celiac disease tests.
The Company is currently in the process of obtaining US FDA clearance of its IgXPLEX Celiac test. The Company believes the clearance of this product will significantly enhance its US market position. The Company is also following up with and is optimistic of winning additional Canadian customers based on Health Canada licensing of its IgXPLEX Celiac product during 2010.
The Company is focusing on the continued development of a pipeline of assays and SQiDworks and SQiDman assay processing and analytical platforms. The Company expects to advance additional test kits through the regulatory process during fiscal 2011 as discussed further in this document.
69
Status of Development Program
The Company's development program includes several major components which the Company expects will advance its commercialization strategy. The status of each component is summarized and discussed in further detail below:
| | | | | | | | |
| |
| | Approval Status |
|---|
Product | | Development Status | | Canada | | United States | | Europe |
|---|
| SQiDworks™ Diagnostics Platform | | Complete | | Licensed | | Cleared as a
system with
IgX PLEX RA Assay | | CE Marked* |
| SQiDlite Platform | | Development | | | | | | |
| SQiDman Analyzer | | Development — RUO | | Not required — RUO | | Not required — RUO | | Not required — RUO |
| IgX PLEX Rheumatoid Arthritis Assay (Qualitative) | | Complete | | Licensed | | Cleared | | |
| QuantiSpot™ Rheumatoid Arthritis Assay (Quantitative) | | Complete | | Licensed | | | | CE Marked* |
| IgX PLEX Celiac Qualitative Assay | | Complete | | Licensed | | Filed | | |
| IgX PLEX Celiac Panel (Quantitative) | | Complete | | Licensed (on April 27, 2011 subsequent to quarter end) | | IUO | | CE Marked* |
| IgX PLEX Vasculitis Panel | | Final Development | | | | | | |
| IgX PLEX Celiac DGP Panel | | Final Development | | | | | | |
| IgX PLEX Rheumatoid Arthritis Panel with expanded markers | | Final Development | | | | | | |
| IgX PLEX Lupus Panel | | Development | | | | | | |
| IgX PLEX TNF Assay | | Development | | | | | | |
| IgX PLEX IBD — Crohn's Disease | | Proof of Concept | | | | | | |
- *
- Devices were self-certified or re-self-certified, in May 2011 following a change of our authorized representative.
- 1.
- The Company has developed fully automated SQiDworks and semi-automated SQiDman™ microarray-based test platforms that enable laboratory customers to generate multiple patient test results with less than one unit of traditional 'test effort'. The Company has received marketing clearance from the United States Food & Drug Administration ("FDA"), Canadian regulatory approval for, and has CE Marked its fully automated, high throughput SQiDworks platform. SQiDworks is the only such platform to achieve these regulatory clearances.
The SQiDworks platforms are to be used to run a menu of tests used to aid in the diagnosis of a wide range of diseases in targeted market segments. The Company has received clearance from the FDA, Canadian regulatory approval for, and has CE Marked its IgXPLEX RA test kits used to detect and measure a panel of biomarkers to aid in the diagnosis of rheumatoid arthritis. The Company has also received Canadian regulatory approval for, and has CE marked, its fully quantitative QuantiSpot RA™ tests kits, run on the SQiDworks platforms. The QuantiSpot RA test kits provide fully quantitative information to further aid in the diagnosis and diagnostic monitoring of rheumatoid arthritis.
The Company has received Canadian regulatory approval for its IgXPLEX Celiac test kits and filed for US FDA regulatory clearance to market this product.
The Company's SQiDworks and SQiDman platforms are also capable of running Research Use Only (RUO) and Investigational Use Only (IUO) test kits and the Company is exploring sales opportunities related to these applications of its platform with the Company's products as well as through the potential development of target customer's content. Delivering RUO/IUO product based on customer owned content would require collaboration and assay development though this effort would be materially less than that experienced with the Company's pipeline of regulatory-cleared products.
70
- 2.
- The Company continues to focus on its in-market tests and believes that it must continuously improve and update its products. The company has identified and has moved into development enhancements to the existing IgXPLEX RA and IgXPLEX Celiac test panels. These improvements, requiring regulatory approvals, will represent second generation, fully quantitative IgXPLEX microarray technology and include expanded biomarker content for IgXPLEX RA and IgXPLEX Celiac. All in-development tests will utilize this second generation, fully quantitative multiplexing technology; the Company believes these enhancements will provide significant market advantages compared to our competitors.
- 3.
- The Company has completed proof of concept for a multiplexed test to measure the presence of anti-TNF-based biologic drugs used to treat a variety of autoimmune diseases. The Company believes that a test to measure these biological drugs in patient serum will provide important clinical and treatment management tools for clinicians treating autoimmune diseases. The Company also believes that the development of thiscompanion diagnostic product will be used as a basis to develop other companion diagnostic products that could be delivered and sold as RUO or IUO kits to a broader market including but not limited to the pharmaceutical development and academic research markets. During the year, the Company completed development of the prototype of the IgXPLEX TNF and provided the prototype to its development partner, Mount Sinai Services (Toronto), to enable validation studies of the product and to provide initial performance feedback to the Company. The validation studies were completed, the performance criteria were met, and the Company's partner's feedback was received and is in the process of being implemented. The Company believes that it will deliver a commercial product to its partner during the 2011 calendar year.
- 4.
- During the fiscal year ended September 2010, the Company entered into an OEM development agreement with Silliker Inc. to complete development of a multiplexed botulism assay. Owing to SQI project prioritization, and minor changes to the scope of the project, the Company decided to hold development of this project. The Company, in association with Silliker will re-assess the viability of the project over the coming quarter and will prioritize it relative to the other development projects that are currently being focussed on.
Status of Commercialization Activities and Other Events in the Year
During the year ended September 30, 2010, the Company invested in its sales and marketing team, its science, commercialization, and regulatory groups, and in infrastructure. The Company's sales efforts are focusing on the North American market to generate sales to targeted customers of the currently approved system, including the SQiDworks fully automated analytical platform, IgXPLEX RA panel, QuantiSpot RA panel, and IgXPLEX Celiac panel (Canada). The science, commercialization, and regulatory groups are focusing on the continued development of pipeline assays and SQiDworks and SQiDman platforms.
Following is an overview of the Company's achievements for the year to date:
- (a)
- During the year ended 2010, the Company entered into a sales agreement with Gamma Dynacare Medical Laboratories (GDML) for the supply of its QuantiSpot RA test kits and SQiDworks platform. GDML has also requested that the Company provide to it sufficient IgXPLEX Celiac test kits upon the Company's successful validation of the product such that GDML can perform its internal review of the product's performance. The Company expects to deliver these test kits to GDML during the second quarter of 2011. Successful internal review by GDML is expected to lead to the expansion of the current contract to include IgXPLEX Celiac test kits;
- (b)
- Subsequent to the year ended September 30, 2010 the Company executed an evaluation agreement with a significant US-based reference laboratory and technology opinion-leader. The Company believes that the conversion of this target customer will result in a very positive general market reaction and demand for its products. The Company expects to deliver the SQiDworks platform to this target customer in the second fiscal quarter of 2011;
- (c)
- Completed an in-depth market survey of high potential target customers generating numerous high quality sales leads during the year;
71
- (d)
- Completed 50 sales calls with potential customers from our target list drawn from the top 100 diagnostic labs (based on volume) during the second, third and fourth quarters. The Company believes that it has generated significant interest from these target customers and that there is a high level of interest to evaluate the SQiDworks platform and available test kits upon the clearance of its IgXPLEX Celiac test kit;
- (e)
- Obtained the patent "Method to Measure Dynamic Internal Calibration True Dose Response Curves". Management believes this patent to be a significant achievement in its intellectual property portfolio establishing enhanced protection of in-array calibration and normalization; and,
- (f)
- Progressed a number of pipeline diagnostic tests through our discovery and development program;
- (i)
- the Company obtained clearance from the FDA to market and sell the IgXPLEX RA and SQiDwoks system; the first ever cleared multiplexed/microarray product. Health Canada licenses were also obtained to market the IgXPLEX RA product in addition to the previously licensed QuantiSpot RA product and SQiDworks platform;
- (ii)
- Applied for and obtained Health Canada license to market the IgXPLEX Celiac 4-PLEX panel;
- (iii)
- Applied for US FDA regulatory clearance of its IgXPLEX Celiac 4-PLEX panel. Subsequent to the fiscal year ended September 30, 2010, the Company received and responded to questions from the FDA regarding this panel. The questions and responses are described by the Company as those expected in the normal course of its regulatory processes.
- (iv)
- Based on market information obtained through in-depth market surveys and sales calls with potential customers the Company has identified enhancements to its existing RA and Celiac products. These enhancements, requiring regulatory approvals, are in development and include transition from qualitative to fully quantitative reporting and the expansion of additional biomarkers that the Company believes will be relevant in the market.
- (v)
- The IgXPLEX Vasculitis assay was advanced to the Assay Development stage and is expected to complete clinical validation in the second quarter of 2011.
- (vi)
- Advanced the IgXPLEX Lupus test kits to the proof of concept stage and subsequent to the year end it was progressed to the assay development stage. The Company expects to initiate clinical validation of this product in the second calendar quarter of 2011, and complete regulatory filings shortly thereafter.
- (vii)
- IgXPLEX IBD was progressed into the proof of concept stage and is being actively developed with the expectation of being completed and filed for regulatory approvals in 2011.
- (viii)
- Continued development of IgXPLEX TNF test kits based on the expanded performance requirements requested by our partner, Mount Sinai Services. During this time, the R&D team also made significant technical improvements in the assay. The IgXPLEX TNF test is intended to be used to measure and monitor the concentration of anti-TNF-based drugs including Remicade™, Humira™ and Enbrel™ in patients with a range of autoimmune and inflammatory disorders including but not limited to rheumatoid arthritis, crohn's disease, plaque psoriasis and ankylosing spondylitis. The Company believes that the cost-effective measurement of these drugs would provide clinicians enhanced treatment options. The Company expects that it will be able to complete commercialization of an IgXPLEX TNF test that will be able to be cleared for IVD sale in Canada. It will remain as a Research Use Only (RUO) product with limited performance claims in the US until expanded clinical studies are performed. However, the Company continues to believe that, as there are no predicate tests currently approved to test for the presence and concentration of anti-TNF, there is a viable RUO market for the product in the US.
- (ix)
- Initiated platform development for SQiDman with a target to complete development that is expected to coincide with customer requirements for potential research use collaborations. Subsequent to the year end this SQiDman platform became available for RUO/IUO applications. This platform is currently not targeted at IVD applications until the Company is actively
72
- (g)
- Partnering Successes in 2010
During and subsequent to the year ended 2010, the Company was successful in entering into multiple collaboration agreements with the leading institutes highlighting the market interest in multiplexing and the Company's achievements to date. These collaborations significantly improve the Company's ability to progress its products through the development process through obtaining access to patient sera needed for assay development, verification of in-development products and final product clinical validation.
The following table provides an overview of our partnering collaborations and the relevant pipeline product:
| | | | | | |
| |
Partnering Institute*
| | Principal
Investigator
| | Pipeline Product
| | Purpose
|
|---|
| |
| Cleveland Clinic (i) | | Dr. Tubbs | | IBD | | Serum Samples
Collaboration |
| |
Beth Israel Deaconess
Medical Center | | Dr. Kelly | | Celiac | | Serum Samples
Collaboration/Publication |
| |
Beth Israel Deaconess
Medical Center | | Dr. Moss | | Anti-TNF | | Serum Samples
Collaboration/Publication |
| |
Hospital Clinic de
Barcelona, Spain | | Dr. Cervera | | Vasculitis | | Serum Samples
Collaboration/Publication |
| |
| Cleveland Clinic | | Dr. Wang | | All
Rheumatoid Arthritis
Various | | Clinical Validation
Collaboration
Serum Samples |
| |
| University of Maryland | | Dr. Fasano | | Celiac | | Serum Samples |
| |
University Hospital
Maastricht, the
Netherlands | | Dr. Damoiseaux | | Vasculitis | | Serum Samples
Collaboration |
| |
University North
Carolina Kidney
Center (ii) | | Dr. Falk | | Vasculitis | | Collaboration
Serum Samples
Clinical Validation |
| |
- *
- All Partnering Institutes are located in the USA unless otherwise annotated.
- (i)
- Completed an "IBD Multiplex Panel Development Collaboration" agreement with Dr. Tubbs of the Department of Molecular Pathology, Cleveland Clinic. This agreement gives the Company access to a significant resource for professional collaboration of its IBD (Crohn's Disease) IgXPLEX product and next generation biomarkers as well as access to a valuable bank of characterized patient serum for development and clinical validation.
- (ii)
- Subsequent to the fiscal year end, the Company entered into a clinical validation agreement with the University North Carolina Kidney Center (UNCKC), Chapel Hill, with Dr. Falk acting as the principal investigator. This agreement gives the Company access to a significant bank of prospective and stored patient serum samples for characterized positive vasculitis patients. These serum samples substantially improve the Company's ability to develop and validate its IgXPLEX Vasculitis product. The collaboration agreement also provides the Company professional expertise from the world leader in diagnosing vasculitis disease. The Chapel Hill Protocol is the most recognized diagnostic algorithm used to diagnose vasculitis disease. As part of the agreement, the Company is collaborating to establish a center of excellence for microarray-based multiplex testing of vasculitis with Dr. Falk through the
73
collaboration and delivery of a SQiDworks platform at Chapel Hill. The agreement calls for co-publishing of the results of the collaborative efforts.
The Company anticipates that this platform can be used for expanded purposes alongside the collaboration with Dr. Falk including, but not limited to validation of additional products in its pipeline.
CORPORATE FINANCING TRANSACTIONS
During the fiscal year ended 2010 the Company was successful in raising significant capital to sustain its operations from the exercise of warrants and options and from two private placements, in total generating $13,997,000 in net cash.
On December 4, 2009, the Company completed a private placement resulting in the issuance of 2,398,104 units at a price of $2.75 per share for gross proceeds of $6,595,000 (net of cash costs — $6,109,000). Each unit was comprised of one common share and one half common share purchase warrant. Each whole warrant entitles the holder to purchase one additional common share at a price of $4.00, expiring December 4, 2011. The total share issuance costs were $611,000.
On August 12, 2010, the Company complete another private placement resulting in the issuance of 2,280,000 units at a price of $2.50 per unit for gross proceeds of $5,700,000 (net of cash costs — $5,399,000). Each unit was comprised of one common share and one half common share purchase warrant. Each whole warrant entitles the holder to purchase one additional common share at a price of $5.00, expiring on August 12, 2012. The total share issuance costs were $363,000.
During the fiscal year ended 2010 199,493 warrants with an expiry of April 26, 2010 were exercised resulting in the issuance of 199,493 shares and net proceeds of $120,000. During the fiscal year ended 194,200 warrants with an expiry of June 3, 2010 were exercised resulting in the issuance of 194,200 shares and net proceeds of $291,000. In addition, 576,563 warrants with an expiry date of June 29, 2010 were exercised resulting in the issuance of 576,563 shares and net proceeds of $1,384,000 the remaining 1,207,213 warrants with an expiry date of June 29, 2010 expired unexercised.
During the fiscal year ended 2010 the Company also issued 916,683 shares resulting from the exercise of options (850,017 of these option were issued under the Stock Option Plan). The exercise of these options resulted in gross proceeds of $694,000.
CRITICAL ACCOUNTING POLICIES AND SIGNIFICANT ESTIMATES
Financial statements are prepared in accordance with Canadian GAAP.
The significant accounting policies that management believes are the most critical in fully understanding and evaluating the reported financial results include the following:
Patents and Trademarks
The costs relating to patent and trademark fees are deferred and amortized over 10 years on a straight-line basis. Patents and trademarks are recorded net of accumulated amortization of $627,000 (September 30, 2009 — $527,000).
Research and Development Costs
Research costs are charged to earnings in the period in which they are incurred. Development costs are expensed as incurred or deferred if they meet the criteria for deferral under Canadian generally accepted accounting principles and are expected to provide future benefits with reasonable certainty. At September 30, 2010, the Company was in development of its pipeline of novel tests for its diagnostic system. Deferral criteria have not yet been met, and accordingly, all development costs have been expensed.
74
Stock-Based Compensation and Other Stock-Based Payments
The Company applies a fair value based method of accounting for all stock-based payments. Accordingly, stock-based payments are measured at the fair value of the consideration received or the fair value of the equity instruments issued or liabilities incurred, whichever is more reliably measurable. Stock-based compensation is charged to operations over the vesting period and the offset is credited to contributed surplus. Consideration received upon the exercise of stock options is credited to share capital and the related contributed surplus is transferred to share capital.
Share Issuance Costs
Costs incurred in connection with the issuance of capital stock are netted against the proceeds received.
Income Taxes
The Company follows the liability method of accounting for income taxes. Under this method, future income tax assets and liabilities are determined based on temporary differences between financial reporting and tax bases of assets and liabilities, as well as for the benefit of losses available to be carried forward to future years for tax purposes. Future income tax assets and liabilities are measured using enacted or substantively enacted tax rates and laws that will be in effect when the differences are expected to reverse. Future income tax assets are recorded in the financial statements if realization is considered more likely than not.
Use of Estimates
The preparation of financial statements in conformity with Canadian generally accepted accounting principles requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amount of revenue and expenses during the year. Actual results could differ from those estimates. Significant areas requiring the use of management estimates relate to the determination of the useful lives of property and equipment and patents and trademarks for amortization purposes, valuation of ITC's receivable, valuation of stock options and warrants and valuation allowance on future tax assets.
Recent Accounting Pronouncements
Business Combinations
In January 2009, the CICA issued Section 1582, Business Combinations, which replaces former guidance on business combinations. Section 1582 establishes principles and requirements of the acquisition method for business combinations and related disclosures. In addition, the CICA issued Sections 1601, Consolidated Financial Statements, and 1602, Non-Controlling Interests, which replaces the existing guidance. Section 1601 establishes standards for the preparation of consolidated financial statements, while section 1602 provides guidance on accounting for a non-controlling interest in a subsidiary in consolidated financial statements subsequent to a business combination.
These standards apply prospectively to business combinations for which the acquisition date is on or after the beginning of the first annual reporting period beginning on or after January 1, 2011 with earlier application permitted. The Company is currently evaluating the new sections to determine the potential impact of any future transactions on its consolidated financial statements.
International Financial Reporting Standards (IFRS)
The CICA plans to converge Canadian Generally Accepted Accounting Principles with International Financial Reporting Standards ("IFRS") over a transition period expected to end in 2011, when IFRS will be fully adopted. The Company will required to adopt IFRS for its 2012 fiscal year end and will be required to provide IFRS comparative information for the previous fiscal year. The Company continues to monitor and assess the impact of the convergence of Canadian GAAP and IFRS on its financial statements. We have identified the main differences between existing Canadian GAAP and IFRS standards. The Company has a
75
conversion plan in place and believes it has the resources in place to meet the conversion timelines. The following are the main differences and the expected impact on our business processes and information systems:
| | |
| |
Key Accounting Areas
| | Difference with potential impact on the Company
|
|---|
| |
| IFRS 1 First time adoption of IFRS | | The Company is in the process of selecting the applicable exemptions under IFRS. |
| | | Required reconciliations and disclosure upon the initial conversion to IFRS will be included in the initial statements presented under IFRS commencing in the first quarter of fiscal 2012. |
| |
| IAS 16 Property Plant and Equipment | | The Company will re-evaluate the useful life of each component of property plant and equipment and will restate, if applicable, the historic amortization expense. |
| |
| IAS 36 Impairment of Assets | | The Company will evaluate potential impairments using discounted cash flow analysis as required under IFRS |
| |
| IAS 12 Income Tax | | The Company has accumulated non-capital losses, undeducted scientific research and development costs, and investment tax credits that have not been reflected in the financial statements. These items will need to be assessed based on the IFRS criteria to ensure proper classification on the balance sheet. |
| |
| IFRS 2 Share based payments | | The Company will treat all options with graded vesting as separate option awards as required under IFRS 2. The Company will utilize the exemptions under IFRS 1 when converting to the new standard. |
| |
| IAS 1 Financial Statement Presentation | | Additional disclosure required as well as selection between presentation alternative will be addressed in the initial statements presented under IFRS. |
| |
SELECTED FINANCIAL INFORMATION
The table below summarizes annual financial information for the fiscal years ending September 30, 2010 and 2009. Certain comparative information has been reclassified to conform to the current year's presentation.
| | | | | | | |
| | |
| | Year ending
September 30, 2010
(000s)
| | Year ending
September 30, 2009
(000s)
| |
|---|
| | |
Revenue | | $ | 35 | | $ | 32 | |
| | |
Net loss | | $ | 8,073 | | $ | 5,910 | |
| | |
Net loss per share | | $ | (0.27 | ) | $ | (0.23 | ) |
| | |
Weighted average shares | | | 30,349 | | | 25,158 | |
| | |
Total Assets | | $ | 13,134 | | $ | 6,205 | |
| | |
The increasing net loss trend between the year ended September 30, 2009 and the fiscal year ended September 30, 2010 was primarily related to increased development activity and expenses for the scientific discovery and development of several IgXPLEX assays, the regulatory approval, and the commercialization of the QuantiSpot RA, IgXPLEX RA and IgXPLEX Celiac test panels and SQiDworks system. In addition a number of diagnostic tests continued progression through our discovery and development program including IgXPLEX Vasculitis and, IgXPLEX SLE (Lupus), IgXPLEX TNF and initial development of SQiDman. The Company also invested in the development of its second generation fully quantitative and expanded biomarker panels for IgXPLEX RA and Celiac products as previously discussed.
Differences in the net loss for 2010 versus 2009 were also related to increased professional and consulting fees including recruiting fees for personnel additions, legal and accounting fees, increased stock-based
76
compensation expenses, and expansion of infrastructure resulting in increased personnel and occupancy costs in the year ended September 30, 2010.
Gross research and development ("R&D") costs, which include R&D salaries, laboratory consumables and operating expenses, clinical studies, scientific consultants and clinical partner costs were $5,354,000 for the year ending September 30, 2010 compared to $3,449,000 for the year ending September 30, 2009. The principle difference in Gross R&D expenses of $1,905,000 between the two periods was due to:
- •
- an expansion of development activities owing to an expanded number of autoimmune products in advanced stages of development in 2010 compared to 2009. During 2009 IgXPLEX RA and Celiac were in the development stage with 2 other panels in early discovery. During 2010 the company advanced IgXPLEX Celiac to the regulatory approval stage, obtained the regulatory approval of IgXPLEX Celiac in Canada, had 5 additional panels in the development stage (second generation IgXPLEX Celiac and RA, IgPLEX Vasculitis, IgXPLEX Lupus, and IgPLEX TNF) and one product in the early discovery stage (IgPLEX IBD);
- •
- Initiated development efforts toward the SQiDman platform and enhancements to the SQiDworks platform and software allowing multi-plate/multi-product runs, pick and pay capability and Lab Information Management Systems ("LIMS") integration;
- •
- a greater level of activity and personnel additions in 2010 to complete the required work;
- •
- cost related to Autoimmune assay validation services;
- •
- new expenditures for internal and third party clinical studies including increased acquisition costs for patient serum tested in these studies.
In the fiscal period ending 2010 the Company had a peak of 53 employees, 44 involved directly in R&D compared to the 2009 fiscal year when the Company had a peak of 36 employees with 29 involved directly in R&D. R&D salaries and payroll costs increased from $2,372,000 for the period ending September 30, 2009 to $3,159,000 for the year ending September 30, 2010. R&D consumable costs rose in proportion to the number of R&D employees and development projects being prosecuted, and these expenses increased by $1,118,000 in the year ending September 30, 2010 above the expenses incurred in the period ending September 30, 2009.
Gross R&D expenses were offset by the recognition of Scientific Research and Experimental Development ("SRED") cash refunds received in the 2009 fiscal year of $87,000 resulting in net R&D expenses of $3,362,000 and by $295,000 in the 2010 fiscal period resulting in net R&D expenses of $5,059,000.
General and administrative ("G&A") expenses include occupancy costs (rent, maintenance and utilities), office supplies as well as other general operating costs and bank charges. G&A expenses increased in the year ending September 30, 2010 compared to the period ending September 30, 2009 from $447,000 to $471,000. The primary reasons for the $24,000 increase were an increase in travel related to corporate development activities, increased occupancy costs due to the increase in the number of employees, and other costs related to the general growth of the corporate activities and related overheads to operate the business. General and administrative expenses included a $97,000 patent write down in 2009; there was no similar write down in the current year. Amounts for sales and marketing included in general and administrative expenses in the prior year have been reclassified to sales and marketing expenses.
Sales and marketing costs were primarily related to sales and marketing consultant fees and to travel related to selling activities. Sales and marketing expenses totalled $474,000 for the year ended September 30, 2010 compared to $409,000 for the year ended September 30, 2009. The increase of $65,000 was primarily a result of additional consultant costs as the company expanded its commercialization efforts.
77
Fourth Quarter Commentary
The table below summarizes quarterly financial information for the 3 month periods shown.
| | | | | | | | | | | | | |
| | |
| | September 30, 2010
(000s)
| | June 30, 2010
(000s)
| | March 31, 2010
(000s)
| | December 31, 2009
(000s)
| |
|---|
| | |
Revenue | | $ | 14 | | $ | 6 | | $ | 10 | | $ | 5 | |
| | |
Net Loss | | $ | 2,621 | | $ | 1,811 | | $ | 2,021 | | $ | 1,620 | |
| | |
Net Loss Per Share | | $ | (0.08 | ) | $ | (0.06 | ) | $ | (0.07 | ) | $ | (0.06 | ) |
| | |
Weighted Average Shares | | | 32,705 | | | 30,790 | | | 29,917 | | | 27,930 | |
| | |
| | | | | | | | | | | | | |
| | |
| | September 30, 2009
(000s)
| | June 30, 2009
(000s)
| | March 31, 2009
(000s)
| | December 31, 2008
(000s)
| |
|---|
| | |
Revenue | | $ | 7 | | $ | 8 | | $ | 7 | | $ | 10 | |
| | |
Net Loss | | $ | 1,616 | | $ | 1,354 | | $ | 1,473 | | $ | 1,467 | |
| | |
Net Loss Per Share | | $ | (0.06 | ) | $ | (0.05 | ) | $ | (0.06 | ) | $ | (0.07 | ) |
| | |
Weighted Average Shares | | | 27,271 | | | 26,326 | | | 25,837 | | | 22,448 | |
| | |
Revenue for the quarter ended September 30, 2010 was $14,000 compared to $7,000 for the quarter ended September 30, 2009. In the last quarter of 2010 the Company completed its first sale of its QuantiSpot RA test kit to a 3rd party. Other revenue for the three month periods ending September 30, 2010 and 2009 was from service-based revenue provided to a related party.
For the quarter ended September 30, 2010, the Company recorded a net loss of $2,621,000 ($0.08 net loss per share) compared to a net loss of $1,616,000 ($0.06 net loss per share) for the quarter ended September 30, 2009. Per share values are based on the weighted average shares outstanding in the period. For the quarter ended September 30, 2010 there was an average of 32,705,000 shares outstanding.
Net loss and net loss per share were greater for the quarter ended September 30, 2010 compared to September 30, 2009. The increased loss for the three months ending September 30, 2010 was primarily related to increases in activity and expenses in the discovery efforts for and development of several IgXPLEX assays, including ordinary increases in wage and wage-related expenses owing to an increase in personnel, increased lab expenditures to support the greater number of projects, and other direct costs including serum acquisition and development and validation partner costs. Sales and Marketing expense was higher in the quarter ended 2010 owing to additional travel and contract resources in sales and marketing as the Company increase its sales effort for approved IgXPLEX panels in Canada and the United States and in anticipation of further approval.
R&D expenditures for the three month period ended September 30, 2010 were $1,621,000 compared to the $872,000 (before the effect of SR&ED tax credits) for the three month period ended September 30, 2009. The increase in R&D expense for the three month period ended September 30, 2010 compared to the three months ending September 30, 2009 resulted from increased R&D activity with an increased number of assay panels in development and to regulatory validation efforts related to the IgXPLEX Celiac assay. In the fourth quarter of 2009 the Company had 1 panel in development and one panel in the regulatory approval process. In the last quarter of 2010 in addition to the IgXPLEX Celiac assay in regulatory validation, the company had 5 panels in development and 1 additional panel in early discovery and development. The company has also expended developments efforts toward the SQiDman platform and enhancements to the SQiDworks platform and software. The company also incurred costs related to autoimmune assay validation services and expenditures for internal and third party clinical studies.
Corporate expenses include, primarily, all salaries and related expenses (including benefits and payroll taxes) of the Company other than salaries and related expenses paid to personnel engaged in research and development. General and Administrative expenses include facility costs, insurance costs, and foreign exchange expenses. Corporate and general expenses totalled $299,000 for the quarter ended September 30, 2010 compared to $290,000 for the quarter ended September 30, 2009. During the fourth quarter of 2010 corporate
78
expenses increase as a result of higher salary costs, including bonuses and increased personnel and increased occupancy costs. Corporate expenses included a write down for abandoned patents in the quartered ended September 30, 2009, there was no similar write down in 2010.
Professional consulting (legal, accounting, Board of Directors compensation, recruiting, administrative contractor, and investor relations) costs in the quarter ended September 30, 2010 were $223,000 compared to $179,000 from the quarter ended September 30, 2009. The increase in professional and consulting costs in the quarter ended September 30, 2010 were primarily related to the use of multiple experts in the areas of professional recruiting for science, regulatory, engineering and technical professionals, laboratory cost analysis, competitive and product intelligence, and work-flow management. Professional recruiting costs were incurred primarily to increase staffing to support product pipeline and commercialization efforts. Amounts for sales and marketing included in professional and consulting expenses in 2009 been reclassified to sales and marketing expenses to conform with the current year's presentation.
Sales and Marketing expenses were primarily related to sales and marketing consultant fees and to travel related to selling activities in the quarter. Sales and marketing expenses totalled $165,000 for the quarter ended September 30, 2010 compared to $82,000 for the quarter ended September 30, 2009. The increase in sales and marketing expenses were primarily related to additional consulting costs paid to increase staffing to support product pipeline and commercialization efforts.
Operational expenses were partially offset by interest income earned on short-term investments. The interest income was $32,000 and $12,000 for the year and quarter ended September 30, 2010 respectively. The interest earned for the year and quarter ended September 30, 2009 was $121,000 and $3,000. Interest in 2009 included interest earned on ITC credits outstanding. The Company invests its cash in variable term cashable government investment certificates and short-term money market deposits.
Non-cash stock based compensation charges totalled $218,000 for the quarter ended September 30, 2010 ($402,000 — year ended September 30, 2010) compared to $89,000 for the quarter ended September 30, 2009 ($380,000 — year ended September 30, 2009). The related stock option issuances are described further below in the Outstanding Share Capital section.
Outlook
Management expects losses to continue for the current fiscal year as investment continues in product development and commercialization efforts on its pipeline of autoimmune test kits as well as investment in sales and marketing. During the 2011 fiscal year the Company will focus on sales and placing SQiDworks systems in Canadian and US-based customers for system evaluation and expects that of the majority of these evaluation placements will lead to commercial acceptance and revenues from sales of consumable test kits. The Company delivered one such evaluation placement in January 2010 to GDML. This system was installed and handed off to the customer in February following training of its operational personnel. From the date of hand-off the customer was provided 90 days to run its internal acceptance validation. During the quarter ending June 30, 2010, the Company converted the positive validation of the platform to a commercial agreement for commercial use by the customer, and generated revenue to the Company in the quarter ended Sept 30, 2010.
During the 2010 fiscal year the Company utilized its significant positive customer feedback relating to the commercial feasibility of its system and consumable tests from this market survey generated in the first two quarters of the 2010 fiscal year to focus on high-value customers with higher volumes of rheumatoid arthritis testing. Based on this market feedback, the Company believes that its strategy of focusing development, commercialization and marketing efforts on panels of autoimmune assays targeted at medium and large reference laboratory customers continues to be sound and aligns with customer demand. As well, the Company generated a significant number of qualified sales leads from its survey activity and is in the process of conducting both initial and follow-up sales meetings with approximately 50 qualified prospective customers.
Our analysis of the market would indicate that there are over 315 laboratories in the United States with sufficient volume of rheumatoid arthritis testing to be target customers for the SQiDworks / IgXPLEX RA system. Management believes that the addressable market is sufficiently large and that with the completion of additional IgXPLEX panels, including IgXPLEX Celiac, licensed in Canada and currently under FDA review,
79
the company will be well-positioned for wider scale commercial acceptance of our platforms during the 2011 fiscal year, and beyond. Management believes the number of potential customers upon regulatory clearance of multiple IgXPLEX products (ex IgXPLEX RA and IgXPLEX Celiac) in the US, to greatly exceed those currently targeted with only one approved product in the US. Further, completion of SQiDworks Lite in 2011 for IVD and RUO/IUO applications will greatly enhance the addressable market into the 1,000s of potential customers.
Based on its successful FDA clearance, its Health Canada licenses and EU authorization, management increased the intensity of the development and commercialization of several new IgXPLEX test kits in 2010 and expects this development to result in the submission of a continuous flow of autoimmune test kit applications to the US, Canadian and EU regulatory bodies during the 2011 calendar year. This activity will generate similar R&D expenses in 2011 as was experienced in the second half of the 2010 fiscal year related to internal development, internal verification and validation studies and third party validation studies. This activity is expected to continue in the foreseeable future as the company completes the autoimmune pipeline, continues to improve in-market tests and initiates development in new clinical areas.
During the 2011 calendar year the Company believes it to be strategic to expand its marketing and sales program to RUO/IUO customers that conduct research in the relevant disease markets or that have an interest in companion testing during drug development and that are targeted in our clinical areas of interest. The Company would target lower throughput customers conducting research with SQiDman platform and RUO/IUO products, higher throughput customers with our SQiDworks platform or SQiDworks Lite, the Company's intermediate platform expected to start in development in the first half of calendar 2011 and anticipated to be completed with nine months after initiation. Management believe that SQiDworks Lite will be an important system for future clinical areas and non-reference lab customers. SQiDworks Lite is expected to be fully automated, allow smaller batch sizes and to have equivalent analytical performance when compared to the current fully automated SQiDworks Platform.
It will also be necessary to invest in expanding the Company's customer service and administrative elements to support our customers and sales, as we are successful in growing our placement of SQiDworks platforms across Canada and the United States, and increase our product menu available to our customers. Management will add these expenses as needed to support forecasted customer installations of SQiDworks platforms and sales of consumable kits.
Management will continue to monitor the cash burn rate in relation to the capital available to it and will manage cash flows as required in the context of the capital markets. Management believes that it may, at some point, seek additional capital to advance and accelerate the number of tests under development and being validated for regulatory submissions, to expand our areas of focus beyond autoimmune disease at the appropriate times, expand our fully marketed analytical platform system portfolio enabling us to address a broader market, to build SQiDworks platforms to address customer demand, as well as to expand our sales team and its efforts in the United States and other jurisdictions as appropriate.
Related Party Transactions
Transactions with related parties occur in the normal course of business and are measured at the exchange amount. Related party transactions are described below, unless they have been disclosed elsewhere in the financial statements.
Included in general and administrative expense for the year ended September 30, 2010 is $49,000 (September 30, 2009 — $50,000), related to recovery of occupancy costs, from a corporation in which an officer of the Company was also an officer. Consulting fee revenue of $30,000 for the year ended September 30, 2010 (year ended September 30, 2009 — $27,000) was earned from this corporation. At year end, $1,000 (September 30, 2008 — $6,000) due from this corporation is included in amounts receivable.
Sources and Uses of Cash
Operational activities for the year ended September 30, 2010 were financed by cash on hand.
80
During the year ended September 30, 2010 the Company:
- 1.
- Completed two non-brokered private placement for combined gross proceeds of $12,295,000 through the issuance of 4,678,000 shares; and,
- 2.
- Received net proceeds of $1,795,000 following the exercise 970,256 warrants resulting in the issuance of 970,256 shares.
- 3.
- Received $694,000 for new shares issued upon the exercise of 916,684 options.
At September 30, 2010, current assets were $9,902,000 compared to $3,649,000 at September 30, 2009. Working capital as at September 30, 2010 was $8,928,000 compared to $3,280,000 at September 30, 2009.
Management believes that cash on hand at September 30, 2010, and cash generated from revenues will be sufficient to fund Company operations for at least 12 months. The continued successful commercial launch and generation of revenue in the 2011 fiscal years will extend this period.
Risks
The Company is subject to various risks. Factors that could cause results or events to differ materially from management's current expectations include, but are not limited to:
- •
- changing competitive technology and market conditions;
- •
- the Company's ability to successfully commercialize additional IgXPLEX tests in the autoimmune disease market;
- •
- the successful and timely completion of clinical validation studies at partner sites;
- •
- the failure to obtain requisite regulatory approvals (including the clearance of the FDA) for the Company's diagnostic tests in a timely manner, if at all, and other inherent uncertainties related to the regulatory approval process;
- •
- the ability to generate sufficient acceptance of the Company's platforms and sales of test kits; and,
- •
- the ability of the Company to fund its cash requirements from internal and external sources.
Management seeks to mitigate these risks, and others, primarily by retaining experienced employees and advisors who have expertise in the scientific, medical business, regulatory, manufacturing and operational disciplines of automated platform integration and immunoassay diagnostic test development.
The Company's SQiDworks automated analytical platform and its lead IgXPLEX RA test kit used to detect and quantify a panel of biomarkers to aid in the diagnosis of rheumatoid arthritis was cleared and licensed to be sold and marketed in Canada during the quarter ended December 31, 2008 and in the United States in November of 2009, and in the quarter ended March 31, 2009 were authorized to be CE Marked and to be sold in Europe. To the best of the Company's knowledge, this was the first and remains the only multiplexed microarray test in the autoimmune disease market to have been successfully cleared by the FDA or, in combination with IgXPLEX Celiac, the only tests of this nature licensed in Canada. The Company sought regulatory approvals and clearances for its IgXPLEX Celiac test kit in the fourth quarter and received Health Canada approval prior to year end. The Company is awaiting FDA clearance.
IgXPLEX and QuantiSpot tests are designed to run only on the SQiDworks platform. In order to obtain approval for the SQiDworks platform and the Company's consumable tests for sale in the United States, our largest target market, the FDA typically requires the conduct of clinical validation studies to compare the performance of a new test to predicate tests currently approved for sale in the US. Upon successful completion of validation studies conducted at both SQI's labs and at multiple third party labs, the data derived are then presented to the FDA in the form of a 510(k) Pre-market Notification. It is typical for the external validation studies to take several months to complete and upon receipt of a completed 510(k) submission the FDA may take up to 180 days to render a decision on the application, not including any "time-outs" which the Company may take to prepare responses to various inquiries from the FDA. The Company believes the experience gained in obtaining the clearance of the SQiDworks-IgXPLEX RA system will enable it to complete and file
81
applications for clearance of subsequently developed pipeline IgXPLEX assays more efficiently. This in turn may result in shorter review periods at the FDA than was experienced with the SQiDworks-IgXPLEX RA system. The timing of such clearances is dependent on several factors some of which are not controlled by the Company.
The IgXPLEX multiplexed test panels used to detect and quantify a panel of biomarkers to aid in the diagnosis of lupus, vasculitis, and Crohn's disease, are currently in the Company's discovery and development pipeline along with the IgXPLEX TNF panel to detect the drug, anti-TNF, that is used in the management of multiple autoimmune diseases. The IgXPLEX TNF panel is used to measure the quantity of therapeutic agents in the body and the information from this test could be used by clinicians in the management of several autoimmune diseases, including but not limited to rheumatoid arthritis, vasculitis and irritable bowel disease.
The Company is expecting one, or all of its pipeline of new multiplexed test panels in the autoimmune disease market under development (Vasculitis, Lupus, IBD (Crohn's), and the second generation and expanded IgXPLEX RA and Celiac tests, and the SQiDworks platform, together each a system, to be commercially ready to file applications with the applicable regulatory jurisdictions in calendar 2011. Management believes that the IgXPLEX TNF test kits will be available for commercial sale for diagnostic use in Canada and research use in the US prior to the end of the 2011 calendar year.
During the current reporting period the Company did not earn material revenues from its test kits or SQiDworks platform. The continuation of the Company's research, development and commercialization activities along with investment in marketing and sales is dependent upon the Company's ability to successfully manage its growth, investment in continued pipeline development and its cash requirements. Management believes that it has sufficient cash reserves to support the development, validation and commercialization of Vasculitis, Lupus, IBD (Crohn's), and the second generation and expanded IgXPLEX RA and Celiac tests and SQiDworks in North America.
We are exposed to market risks related to changes in interest rates and foreign currency exchange rates, which could affect the value of our current assets and liabilities. We do not believe that the results of operations or cash flows would be affected to any significant degree by a sudden change in market interest rates relative to our cash investment, due to the prime interest rate based nature of the investment. We have not entered into any forward currency contracts or other financial derivatives to hedge foreign exchange risk. We are subject to foreign exchange rate changes that could have a material effect on future operating results or cash flows.
Management will continue to review the Company's financial needs and to seek additional capital financing as required from sources that may include equity financing, debt financing, collaborative projects and licensing arrangements. However, there can be no assurance that such additional funding will be available or if available, whether acceptable terms will be offered.
Outstanding Share Capital
As at September 30, 2010, there were 33,758,000 common shares issued and outstanding.
The following tables describe the securities that have been issued that are convertible under certain conditions into common shares:
The Company had the following warrants outstanding at September 30, 2010:
| | | | | | |
| Number of Warrants | | Purchase Price | | Expiry Date |
|---|
| | 144,000 | | $ | 2.750 | | December 4, 2010 |
| | 107,000 | | $ | 1.250 | | January 22, 2011 |
| | 1,199,000 | | $ | 4.000 | | December 4, 2011 |
| | 237,000 | | $ | 1.900 | | December 23, 2011 |
| | 1,140,000 | | $ | 4.000 | | August 12, 2012 |
| | 57,000 | | $ | 2.500 | | August 12, 2012 |
| | | | | | |
| | 2,884,000 | | | | | |
| | | | | | |
82
The Company had the following stock options outstanding under the Plan at September 30, 2010:
| | | | | | |
Number of Options (000s) | | Exercise Price | | Expiry Date |
|---|
| | 33,000 | | $ | 1.200 | | June 29, 2011 |
| | 67,000 | | $ | 1.200 | | August 29, 2011 |
| | 180,000 | | $ | 1.740 | | August 7, 2012 |
| | 50,000 | | $ | 1.500 | | October 23, 2012 |
| | 758,000 | | $ | 1.600 | | February 15, 2013 |
| | 269,000 | | $ | 1.750 | | August 26, 2013 |
| | 80,000 | | $ | 1.300 | | May 21, 2014 |
| | 25,000 | | $ | 3.260 | | November 3, 2014 |
| | 68,000 | | $ | 2.250 | | February 22, 2015 |
| | 60,000 | | $ | 2.100 | | May 27, 2015 |
| | 175,000 | | $ | 2.500 | | August 16, 2015 |
| | | | | | |
| | 1,764,000 | | | | | |
| | | | | | |
Off-Balance Sheet Arrangements
The Company has no off-balance sheet arrangements.
Future Prospects
In its current state of evolution, management believes that the Company has assembled the necessary intellectual, financial, and human capital to advance its current pipeline of autoimmune test panels and the SQiDworks system through the completion of clinical validation studies and regulatory filings in Canada, the U.S. and Europe. The Company believes that completion of its lead product IgXPLEX RA and its clearance in the United States plus the filing for clearance in the United States of IgXPLEX Celiac, the licensing in Canada of QuantiSpot RA, IgXPLEX RA and IgXPLEX Celiac, and authorization to CE Mark it in Europe of QuantiSpot RA justifies the current intensified investment in development and commercialization of its pipeline of an additional group of at least eight autoimmune microarray diagnostic panels over the next eighteen months, with four novel and two second generation tests currently in the Company's discovery or assay development processes. It further believes that successful completion of these pipeline tests may lead to the development and commercialization of other test panels addressing unmet medical needs in the detection of known analytes used in the diagnosis or therapies for autoimmune, infectious disease and allergy management.
SQI's operational objectives are straightforward: generate revenue from products in the regulatory jurisdictions for which we have acquired regulatory approvals or licenses; continued successful commercialization and continuous improvement of a menu of autoimmune test kits; and, expansion of partnerships and other strategic relationships to enhance our product offerings or revenues. Success in these steps will allow the Company to further validate its multiplexing model, value proposition and to roll-out and sell its products to customers in its target markets.
The Company plans to execute on the following components of its operational strategy:
- •
- Generate, maintain and grow customer sales in North America;
- •
- Complete commercialization and regulatory filings (where appropriate) for, vasculitis, lupus, IBD, anti-TNF and second generation product extensions for RA and Celiac panels;
- •
- Work with our partners to enhance our product offerings;
- •
- Provide world-class customer support and service to ensure satisfaction; and,
- •
- Publish scientific papers to broaden our product value proposition.
The Company will continue to invest in research and development activities related to its core platform technologies of microarray-based assays and detection devices.
83
Disclosure Controls and Procedures, and Internal Control Over Financial Reporting
The accompanying financial statements have been prepared by management in accordance with Canadian generally accepted accounting principles. For quarterly reporting periods, the Company's financial statements are approved by the Audit Committee and the Board of Directors. For annual reporting periods, the Company's financial statements are approved by the Board of Directors upon recommendation by the Audit Committee. The integrity and objectivity of these financial statements are the responsibility of management. In addition, management is responsible for all other information in this report and for ensuring that this information is consistent, where appropriate, with the information contained in the financial statements.
In support of this responsibility, management maintains a system of internal controls to provide reasonable assurance as to the reliability of financial information and the safeguarding of assets.
In particular, the CEO and CFO are responsible for establishing and maintaining disclosure controls and procedures ("DC&Ps") and internal controls over financial reporting ("ICFRs") for the Company, and have:
- (a)
- designed such DC&Ps, or caused them to be designed under supervision, to provide reasonable assurance that material information is made known during the period in which the annual and quarterly filings are being prepared;
- (b)
- designed such ICFRs, or caused them to be designed under supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with Canadian GAAP;
- (c)
- evaluated the design and effectiveness of the Company's DC&Ps as of the quarter ended September 30, 2010;
- (d)
- have concluded that a material design weakness in the ICFRs may exist in terms of the inadequate segregation of certain duties, which is typical of development stage companies; mitigating factors, including dual-payment authorization policies and transparent internal financial transaction reporting processes, serve to minimize the risk that such design weakness could result in a material misstatement of results for the quarter ended September 30, 2010; and
- (e)
- have concluded that, other than the item described above in sub-point (d), there are no additional material design weaknesses in the DC&Ps or ICFRs, and that the effectiveness of the DC&Ps is sufficient to expect the prevention or detection of material misstatements of results.
The financial statements include amounts that are based on the best estimates and judgments of management. The Board of Directors is responsible for ensuring that management fulfills its responsibility for financial reporting and internal control. The Board of Directors exercises this responsibility principally through the Audit Committee. The Audit Committee consists of three directors, all of whom are independent and not involved in the daily operations of the Company. The Audit Committee meets with management and the external auditors to satisfy itself that management's responsibilities are properly discharged and to review the financial statements prior to their presentation to the Board of Directors for approval.
84
DIRECTORS AND OFFICERS
The following table sets forth the names, municipalities of residence, positions held with us and principal occupations of our directors and executive officers and, if a director, the month and year in which the person became a director. Our directors hold office until the next annual meeting of our shareholders or until their successors are appointed.
| | | | | | | | | |
Name, Place of
Residence and
Director Since
(if applicable) | | Principal Occupation | | Number of Common
Shares Beneficially
Owned or Controlled(1) | | Percentage of
Common Shares | |
|---|
Claude Ricks
Barrie, ON
May, 2007 | | Currently and since May 2007, Mr. Ricks has been the President, Chief Executive Officer and a director of the Company Prior to that, Mr. Ricks was the Chief Executive Officer of SQI Diagnostics Systems Inc., a wholly-owned subsidiary of the Company. | | | 1,992,157 | | | 5.87% | |
Dr. Peter Lea
Toronto, ON
May, 2007 | | Currently and since May 2007, Dr. Lea has been the Chief Science Officer and a director of the Company. Prior to that, Dr. Lea was Chief Science Officer and director of SQI Diagnostics Systems Inc., a wholly-owned subsidiary of the Company. | | | 2,173,904 | | | 6.40% | |
Eric Schneider(2)(3)
Waterloo, ON
May, 2007 | | Currently and for the past five years, Mr. Schneider is a partner in the law firm Miller Thomson LLP and its predecessors. | | | 380,585 | | | 1.12% | |
Saied Nadjafi(3)
Toronto, ON
May, 2007 | | Currently and for the past five years Mr. Nadjafi is an independent business man. | | | 1,931,475 | | | 5.69% | |
David Williams(2)
Toronto, ON
May, 2007 | | Currently and for the past five years, Mr. Williams is the President of Roxborough Holdings Limited, a Toronto based investment company. | | | 477,225 | | | 1.40% | |
Paul J. Mountain(3)
Welland, ON
August, 2007 | | Currently and since August 2007, Mr. Mountain has been an independent consultant. Prior to that and since January 1990 Mr. Mountain was Vice President of Science and Technology at MDS Inc., a Toronto-based diversified healthcare company. | | | — | | | — | |
Peter Winkley(2)
Mississauga, ON
November, 2007 | | Currently and since September 2010, Mr. Winkley is the Vice President and Chief Financial Officer of Algoma Central Corporation. Prior to that, Mr. Winkley was the Chief Financial Officer of Therapure Biopharma Inc. a Mississauga-based biologics contract manufacturer. Prior to this, Mr. Winkley was Vice-President, Corporate Finance at MDS Inc. | | | — | | | — | |
Andrew Morris
Oakville, ON | | Currently and since 2004, Mr. Morris has been the Chief Financial Officer of the Company or SQI Diagnostics Systems Inc., a wholly-owned subsidiary of the Company. | | | 283,340 | | | 0.83% | |
Catherine Smith
Toronto, ON | | Currently and since January 2005, Ms. Smith is the Vice President, Technology of the Company. | | | 42,168 | | | 0.12% | |
Jaymie R. Sawyer
Thornhill, ON | | Currently and since October 2010, Ms. Sawyer is the Vice President, R&D of the Company. Prior to that and since 2002, Ms. Sawyer was the Director, R&D at BD Biosciences | | | — | | | —
| |
- (1)
- As of September 7, 2011. Options not included.
- (2)
- Member of the audit committee (the "Audit Committee") (appointed annually).
85
- (3)
- Member of the compensation committee (the "Compensation Committee") (appointed annually).
- (4)
- Member of the Nominating and Corporate Governance Committee (appointed annually).
As of September 7, 2011 as a group, our directors and executive officers beneficially owned, directly or indirectly, or exercised control over, 7,280,854 common shares, which represented 21.45% of the outstanding common shares. Additionally, as of September 7, 2011 as a group, our directors and executive officers beneficially owned, directly or indirectly, or exercised control over, options to purchase up to 841,669 common shares.
Except as disclosed below, each of our directors and executive officers has been engaged for five years in his present principal occupation or in other capacities with the company or organization (or predecessor thereof) in which he currently holds his principal occupation. The information provided below has been provided to us by the individuals themselves and has not been independently verified by us.
Board Committees
Our board of directors has established an Audit Committee, a Compensation Committee and a Nominating and Corporate Governance Committee to assist the directors in efficiently carrying out their responsibilities. The below descriptions of our board policies reflects the policies which we have adopted and will have effect upon the closing of the offering.
Audit Committee
Our board of directors has appointed an Audit Committee consisting of three directors, being Eric Schneider, David Williams and Peter Winkley, the Chair of the Audit Committee, all of whom are "financially literate" within the meaning of Multilateral Instrument 52-110 (Audit Committees) and applicable U.S. securities laws, have accounting or related financial expertise, and are "independent" within the meaning of applicable Canadian and United States securities laws and as may be defined by the TSXV and the NYSE Amex. The responsibilities and mandate of the Audit Committee are set out in an Audit Committee Charter. The primary purposes of the Audit Committee are to:
- (a)
- assist the board of directors in fulfilling its oversight of our financial integrity, specifically by assisting the board of directors' oversight of:
- (i)
- the integrity of our financial statements and other financial reporting;
- (ii)
- the external auditor's qualifications and independence;
- (iii)
- the performance of our internal audit function and internal auditor, if and when one is appointed;
- (iv)
- our compliance with legal and regulatory requirements;
- (v)
- the process by which we assess and manage risk; and
- (vi)
- any other matters as defined by the board of directors;
- (b)
- manage, on behalf of the shareholders, the relationship between our company and the external auditor by:
- (i)
- having direct responsibility for appointing, retaining and determining the compensation of the external auditor (in the Audit Committee's capacity as a committee of the board of directors and subject to the rights of shareholders and applicable law);
- (ii)
- overseeing the work of the external auditor engaged for the purpose of preparing or issuing an auditor's report or performing other audit, review or attest services for our company, including the resolution of any disagreements between management and the external auditor regarding financial reporting;
- (iii)
- determining and approving compensation for all audit and audit-related services and pre-approving all audit and permitted non-audit services to be provided to us or our subsidiaries by our external auditor;
86
- (iv)
- ensuring that the external auditor reports directly to the Audit Committee and meets with the Audit Committee or the board of directors without management present at each regularly scheduled meeting of the Audit Committee or the board of directors;
- (v)
- facilitating communication between our company and the external auditor; and
- (vi)
- overseeing the preparation of any report that is required by any applicable securities regulatory authority to be included in the annual proxy statement, annual information form or any other public disclosure document of our company.
Compensation Committee
Our board of directors has appointed a Compensation Committee consisting of three directors, being Saied Nadjafi, Paul Mountain and Eric Schneider, the Chair of the Compensation Committee, who are "independent" within the meaning of applicable Canadian and United States securities laws and as may be defined by the TSXV and the NYSE Amex.
The responsibilities and mandate of the Compensation Committee are set out in a Compensation Committee Charter. The primary purpose of the Compensation Committee is to assist the board of directors in discharging its oversight responsibilities for the executive compensation programs of the Company and determining compensation for the Company's directors and elected officers. The Compensation Committee is responsible for:
- (a)
- evaluating and providing recommendations to the board of directors regarding our equity-based and incentive compensation plans and programs;
- (b)
- evaluating and providing recommendations to the board of directors regarding compensation, incentive compensation and equity-based plans and programs for our executive officers and directors;
- (c)
- evaluating and providing recommendations to the board of directors regarding executive development and succession planning for our senior executives; and
- (d)
- producing an annual report on executive compensation for inclusion in our management proxy circular.
Nominating and Corporate Governance Committee
Our board of directors has appointed a Nominating and Corporate Governance Committee consisting of two directors, being David Williams and Eric Schneider, who are "independent" within the meaning of applicable Canadian and United States securities laws and as may be defined by the TSXV and the NYSE Amex.
The responsibilities and mandate of the Nominating and Corporate Governance Committee are set out in a Nominating and Corporate Governance Committee Charter. The primary purpose of the Nominating and Corporate Governance Committee is to discharge the responsibilities of the board of directors with respect to certain nominating and corporate governance matters. The Nominating and Corporate Governance Committee will also:
- (a)
- evaluate and make recommendations regarding the composition and governance of the board of directors and its committees;
- (b)
- assess the performance of members of the board and committee members;
- (c)
- recommend desired qualifications for board of director membership and conduct searches for potential board of directors members; and
- (d)
- review and make recommendations with regard to our corporate governance policies and procedures.
87
Corporate Cease Trade Orders, Penalties and Bankruptcies
Other than as disclosed below, to our knowledge, no proposed director:
- (i)
- is, as at the date of this prospectus, or has been, within 10 years before the date of this prospectus, a director or executive officer of any company (including our company) that, while that person was acting in that capacity,
- (A)
- was the subject of a cease trade or similar order or an order that denied the relevant company access to any exemption under securities legislation, for a period of more than 30 consecutive days;
- (B)
- was subject to an event that resulted, after the director or executive officer ceased to be a director or executive officer, in the company being the subject of a cease trade or similar order or an order that denied the relevant company access to any exemption under securities legislation, for a period of more than 30 consecutive days; or
- (C)
- within a year of that person ceasing to act in that capacity, became bankrupt, made a proposal under any legislation relating to bankruptcy or insolvency or was subject to or instituted any proceedings, arrangement or compromise with creditors or had a receiver, receiver manager or trustee appointed to hold its assets; or
- (ii)
- has, within the 10 years before the date of this prospectus, become bankrupt, made a proposal under any legislation relating to bankruptcy or insolvency, or become subject to or instituted any proceedings, arrangement or compromise with creditors, or had a receiver, receiver manager or trustee appointed to hold the assets of the proposed director.
- •
- Mr. Nadjafi was a director and officer of TelcoPlus Enterprises Inc. when the British Columbia and Alberta securities commissions issued a cease trade order against the company in June, 2003. The cease trade order was issued due to the fact that the company was a shell company that had no operating business. The cease trade order was revoked in December 2003.
- •
- Mr. Williams was a director of Octagon Industries Inc. ("Octagon") from November 1993 to 2005. Octagon was subject to cease trade orders issued by the British Columbia Securities Commission ("BCSC") on May 29, 2001 (revoked on August 28, 2001) and on June 24, 2004, and by the Alberta Securities Commission on June 8, 2004, for failure to file its required financial statements. Octagon was delisted from the NEX (a separate exchange from the TSXV) for default of paying its listing fees for the third quarter of 2004. On August 12, 2001, the trustees of Octagon sent a proposal to unsecured creditors of Octagon pursuant to theBankruptcy and Insolvency Act (Canada). A majority of the unsecured creditors approved the proposal at a general meeting of the creditors held on August 25, 2001.
- •
- Mr. Williams also served as a director of RoaDor Industries Inc. ("RoaDor"), a reporting issuer in the Provinces of British Columbia, Alberta and Ontario, when on February 18, 2011, the BCSC and the Ontario Securities Commission each issued a cease trade order against RoaDor for failure to file its financial statements and management's discussion and analysis related thereto for the year ended September 30, 2010. The cease trade orders remain in effect as of the date of this prospectus.
- •
- Mr. Winkley became a director and the corporate secretary of 1608557 Ontario Inc. ("1608557") (formerly Hemosol Corp.) in November 2008 as a part of that company's emergence from protection under theCompanies' Creditors Arrangement Act (Canada). In December 2008 and March 2009, the applicable Canadian securities regulatory authorities issued cease trade orders against that company as a result of the failure to file financial statements for periods during the 2005, 2006, 2007 and 2008 fiscal years. Mr. Winkley ceased to be a director and officer of 1608557 in December 2009.
Personal Bankruptcies
To our knowledge, none of our existing or proposed directors have, within the 10 years before the date of this prospectus, become bankrupt, made a proposal under any legislation relating to bankruptcy or insolvency, or been subject to or instituted any proceedings, arrangements or compromise with creditors, or had a receiver, receiver manager or trustee appointed to hold the assets of that person.
88
Executive Compensation for the Fiscal Year Ended September 30, 2010
Detailed information concerning the compensation of our executive officers and directors for the fiscal year ended September 30, 2010 is contained in pages 11 to 16 of our management proxy circular, which is incorporated by reference into this prospectus. For ease of reference, we have set out below the key tables relating to the compensation for the fiscal year ended September 30, 2010 for our Chief Executive Officer, our Chief Financial Officer, and our Chief Science Officer (collectively, the "Named Executive Officers" or "NEOs"). These tables have been extracted from our management proxy circular.
Summary Compensation Table
The following table sets forth all compensation earned by the Named Executive Officers during our last two fiscal years.
| | | | | | | | | | | | | | | | | | | | | |
| |
| | Annual Compensation | | Long Term Compensation | |
| | Total |
|---|
| |
| |
| |
| |
| | Awards | |
| |
| |
|
|---|
| |
| |
| |
| |
| | Securities
Under
Option/
SARs
Granted
(#) | |
| |
| |
| |
|
|---|
| |
| |
| |
| | Other
Annual
Compen-
sation
($) | |
| | Payouts | |
| |
|
|---|
| |
| |
| |
| |
| | All Other
Compen-
sation
($) | |
|
|---|
NEO
Name and
Principal Position | | Year | | Salary
($) | | Bonus
($) | | LTIP
Payouts
($) | | LTIP
Payouts
($) | | ($) |
|---|
Claude Ricks(1)
President and Chief Executive Officer | | | 2010
2009 | | | 182,308
171,923 | | | 12,308
Nil | | Nil
Nil | | Nil
Nil | | Nil
Nil | | Nil
Nil | | Nil
Nil | | 194,616
171,923 |
Andrew Morris(2)
Chief Financial Officer | | | 2010
2009 | | | 166,731
160,961 | | | 9,231
22,500 | | Nil
Nil | | Nil
Nil | | Nil
Nil | | Nil
Nil | | Nil
Nil | | 175,962
183,461 |
Dr. Peter Lea(3)
Chief Science Officer | | | 2010
2009 | | | 151,154
150,000 | | | 6,154
Nil | | Nil
Nil | | Nil
Nil | | Nil
Nil | | Nil
Nil | | Nil
Nil | | 157,308
150,000 |
- (1)
- Mr. Ricks was appointed Chief Executive Officer of the Company in April, 2007. Prior to that, Mr. Ricks was the Chief Executive Officer of SQI Diagnostics Systems Inc., the wholly-owned subsidiary of the Company.
- (2)
- Mr. Morris was appointed Chief Financial Officer of the Company in April, 2007. Prior to that, Mr. Morris was the Chief Financial Officer of SQI Diagnostics Systems Inc., the wholly-owned subsidiary of the Company.
- (3)
- Mr. Lea was appointed Chief Science Officer of the Company in April, 2007. Prior to that, Mr. Lea was the Chief Science Officer of SQI Diagnostics Systems Inc., the wholly-owned subsidiary of the Company.
Outstanding Option-Based Awards
We had the following option-based awards outstanding for Named Executive Officers at September 30, 2010.
| | | | | | | | | | | | |
Name | | Number of Securities
Underlying
Unexercised Options
(#) | | Option Exercise
Price
($) | | Option Expiration
Date | | Value of Unexercised in-
the-Money Options(1)
($) | |
|---|
Claude Ricks | | | 150,000 | | | 1.60 | | February 26, 2013 | | | 198,000 | |
Andrew Morris | | | 250,000 | | | 1.60 | | February 26, 2013 | | | 330,000 | |
Peter Lea | | | 150,000 | | | 1.60 | | February 26, 2013 | | | 198,000 | |
- (1)
- Closing market value on September 30, 2010 was $2.92.
89
Incentive Plan Awards — Value Vested or Earned During Year Ended September 30, 2010
The following incentive plan awards for Named Executive Officers vested during the year ended September 30, 2010.
| | | | | | | |
Name | | Option-Based Awards — Value
Vested During the Year ended
September 30, 2010(1) | | Non-Equity Incentive Plan
Compensation — Value
Vested During the Year ended
September 30, 2010 | |
|---|
Claude Ricks | | $ | 66,000 | | $ | 12,308 | |
Andrew Morris | | $ | 49,500 | | $ | 9,231 | |
Peter Lea | | $ | 66,000 | | $ | 6,154 | |
- (1)
- Closing market value on September 30, 2010 was $2.92.
Securities Authorized for Issuance under Equity Compensation Plans
The following table gives certain information as of September 30, 2010, being the our most recently completed financial year, with respect to our stock option plan under which our equity securities are authorized for issuance (see "Capitalization").
| | | | | | | | | | |
Plan Category | | Number of securities to be
issued upon exercise of
outstanding options | | Weighted-average
exercise price of
outstanding options | | Number of securities remaining
available for future issuance under
equity compensation plans | |
|---|
Equity compensation plans approved by security holders | | | 1,764,000 | | $ | 1.75 | | | 424,701 | |
DESCRIPTION OF CAPITAL STRUCTURE
Common Shares
We are authorized to issue an unlimited number of common shares. The holders of common shares are entitled to one vote per share at meetings of shareholders, to receive such dividends as declared by our board of directors and to receive the remaining property and assets of our company upon our dissolution or winding up. The common shares are not subject to any future call or assessment and there are no pre-emptive, conversion or redemption rights attached to such shares.
Warrants
As of September 7, 2011, we had outstanding warrants to purchase an aggregate of 2,632,852 common shares at exercise prices ranging from $1.90 per share to $5.00 per share. These warrants will expire at various times between December 4, 2011 and August 12, 2012. In the event of a distribution of dividends, a share split, a reorganization, a reclassification, a consolidation, or a similar event, each warrant provides for adjustment of the exercise price and the number of shares issuable upon exercise.
Options
We maintain a stock option plan for the benefit of directors, officers, employees and consultants. The aggregate number of common shares reserved for issuance under the plan, together with any other employee stock option plans, options for services and employee share purchase plans, may not exceed 10% of the issued and outstanding common shares at the time of the option grant (on a non-diluted basis). Options granted pursuant to the plan must have terms not to exceed five years, and are granted at an option price that may not be less than the fair market price at the time the options are granted. All options granted to individual optionees, other than consultants, generally vest in three equal installments over a period of 36 months.
DIVIDEND POLICY
To date, we have not paid any dividends and do not expect to do so in the foreseeable future. We currently intend to retain all future earnings for the operation and expansion of our business. Dividends on our common shares are declared at the discretion of our board of directors and will depend on our results of operations, capital requirements, financial condition, prospects, contractual arrangements and other factors that our board determines is relevant.
90
MARKET FOR SECURITIES
Trading Price and Volume
Our common shares are listed and posted for trading on the TSXV under the symbol "SQD". The following table sets out, for the periods indicated, the reported high and low sales prices and aggregate volume of trading of our common shares on the TSXV for the periods indicated.
2010
| | | | | | | | | | |
Month | | Volume
(#) | | High Trading Price
(C$) | | Low Trading Price
(C$) | |
|---|
January | | | 169,691 | | | 2.60 | | | 2.25 | |
February | | | 438,181 | | | 2.40 | | | 2.12 | |
March | | | 153,377 | | | 2.27 | | | 1.60 | |
April | | | 315,620 | | | 2.47 | | | 2.21 | |
May | | | 199,865 | | | 2.41 | | | 2.05 | |
June | | | 1,097,152 | | | 2.47 | | | 2.00 | |
July | | | 354,891 | | | 2.42 | | | 2.21 | |
August | | | 644,310 | | | 3.13 | | | 2.30 | |
September | | | 604,825 | | | 3.18 | | | 2.76 | |
October | | | 744,468 | | | 2.97 | | | 2.65 | |
November | | | 193,705 | | | 2.70 | | | 2.42 | |
December | | | 104,220 | | | 2.75 | | | 2.50 | |
2011
| | | | | | | | | | |
Month | | Volume
(#) | | High Trading Price
(C$) | | Low Trading Price
(C$) | |
|---|
January | | | 253,844 | | | 3.20 | | | 2.70 | |
February | | | 442,792 | | | 3.42 | | | 2.90 | |
March | | | 177,537 | | | 3.38 | | | 3.00 | |
April | | | 100,731 | | | 3.25 | | | 2.95 | |
May | | | 414,333 | | | 3.45 | | | 3.09 | |
June | | | 138,626 | | | 3.60 | | | 3.31 | |
July | | | 113,853 | | | 3.60 | | | 3.25 | |
August | | | 83,090 | | | 3.30 | | | 2.75 | |
September(1) | | | 2,930 | | | 2.71 | | | 2.71 | |
- (1)
- As at market close September 6, 2011.
Our common shares have been approved for listing on NYSE Amex under the trading symbol "SQD".
Prior Sales
Common Shares
On August 12, 2010, pursuant to a private placement, we issued 2,280,000 units, each comprised of one common share and one-half common share purchase warrant, at a price of $2.50 per unit, resulting in gross proceeds of $5,700,000.
On December 4, 2009, pursuant to a private placement, we issued 2,398,104 units, each comprised of one common share and one-half common share purchase warrant, at a price of $2.75 per unit, resulting in gross proceeds of $6,595,000.
91
During the 24-month period prior to September 7, 2011, we issued the following common shares upon the exercise of warrants.
| | | | | | | |
Date of Issue | | Number of Common Shares Issued | | Purchase Price per Common Share | |
|---|
November 9, 2009 | | | 5,000 | | $ | 2.40 | |
November 16, 2009 | | | 1,563 | | $ | 2.40 | |
November 23, 2009 | | | 2,500 | | $ | 2.40 | |
January 18, 2010 | | | 13,288 | | $ | 0.60 | |
April 12, 2010 | | | 30,000 | | $ | 2.40 | |
April 23, 2010 | | | 186,205 | | $ | 0.60 | |
May 06, 2010 | | | 101,000 | | $ | 2.40 | |
May 14, 2010 | | | 65,000 | | $ | 2.40 | |
June 3, 2010 | | | 194,200 | | $ | 1.50 | |
June 22, 2010 | | | 15,000 | | $ | 2.40 | |
June 25, 2010 | | | 217,500 | | $ | 2.40 | |
June 29, 2010 | | | 139,000 | | $ | 2.40 | |
January 21, 2011 | | | 106,520 | | $ | 1.25 | |
During the 24-month period prior to September 7, 2011, we issued the following common shares upon the exercise of stock options.
| | | | | | | |
Date of Issue | | Number of Common Shares Issued | | Exercise Price | |
|---|
April 14, 2010 | | | 141,670 | | $ | 1.20 | |
April 14, 2010 | | | 166,670 | | $ | 0.60 | |
April 21, 2010 | | | 5,000 | | $ | 1.74 | |
June 18, 2010 | | | 16,667 | | $ | 1.20 | |
August 23, 2010 | | | 10,000 | | $ | 1.75 | |
September 1, 2010 | | | 10,000 | | $ | 1.75 | |
December 17, 2010 | | | 7,500 | | $ | 1.74 | |
January 21, 2011 | | | 2,500 | | $ | 1.30 | |
Feb 14, 2011 | | | 8,334 | | $ | 1.20 | |
March 30, 2011 | | | 30,000 | | $ | 1.74 | |
April 26, 2011 | | | 33,334 | | $ | 1.20 | |
Options
The following table sets forth the details for all options that we granted under our stock option plan during the 24-month period prior to September 7, 2011.
| | | | | | | |
Date of Grant | | Number of Common Shares
Under Options Granted | | Exercise Price | |
|---|
November 4, 2009 | | | 25,000 | | $ | 3.26 | |
February 22, 2010 | | | 174,000 | | $ | 2.25 | |
May 27, 2010 | | | 60,000 | | $ | 2.10 | |
August 16, 2010 | | | 175,000 | | $ | 2.50 | |
October 4, 2010 | | | 100,000 | | $ | 2.90 | |
January 31, 2011 | | | 75,000 | | $ | 2.85 | |
92
Warrants
The following table sets forth the details for all warrants that we issued during the 24-month period prior to September 7, 2011.
| | | | | | | |
Date of Issue | | Number of Common Shares
Under Warrants Granted | | Exercise Price | |
|---|
December 4, 2009 | | | 143,886 | | $ | 2.75 | |
December 4, 2010 | | | 1,199,052 | | $ | 4.00 | |
August 12, 2010 | | | 1,140,000 | | $ | 5.00 | |
August 12, 2010 | | | 57,000 | | $ | 2.50 | |
PRINCIPAL SHAREHOLDERS
The following table shows information known to us with respect to the beneficial ownership of our common shares as of September 7, 2011, as adjusted, where applicable, to reflect the sale of common shares offered hereby, by:
- •
- each of our directors;
- •
- the Named Executive Officers who remain employees as of the date of this prospectus; and
- •
- all of our directors and Named Executive Officers as a group.
| | | | | | | | | | | | | |
| | Beneficial Ownership
Prior to the Offering† | | Beneficial Ownership
After the Offering*† | |
|---|
Name of Beneficial Owner | | Shares | | Percentage | | Shares | | Percentage | |
|---|
Directors and Named Executive Officers | | | | | | | | | | | | | |
Dr. Peter Lea | | | 2,173,904 | | | 6.40% | | | • | | | • | |
Andrew Morris | | | 283,340 | | | 0.83% | | | • | | | • | |
Paul J. Mountain | | | — | | | — | | | | | | | |
Saied Nadjafi | | | 1,931,475 | | | 5.69% | | | • | | | • | |
Claude Ricks | | | 1,992,157 | | | 5.87% | | | • | | | • | |
Eric Schneider | | | 380,585 | | | 1.12% | | | • | | | • | |
David A. Williams | | | 477,225 | | | 1.40% | | | • | | | • | |
Peter Winkley | | | — | | | — | | | | | | | |
All Directors and Named Executive Officers as a Group | | | 7,238,686 | | | 21.32% | | | • | | | •
| |
- †
- Options not included.
- *
- Assumes the over-allotment option is exercised in full.
Conflicts of Interest
Our directors are required by law to act honestly and in good faith with a view to our best interests and to disclose any interests that they may have in any project or opportunity of SQI. If a conflict of interest arises at a meeting of the board, any director in a conflict must disclose his interest and abstain from voting on such matter.
To the best of our knowledge, and other than disclosed herein, there are no known existing or potential conflicts of interest among SQI, our promoters, directors and officers or other members of management of SQI or of any proposed promoter, director, officer or other member of management as a result of their outside business interests, except that certain of the directors and officers serve as directors and officers of other companies, and therefore it is possible that a conflict may arise between their duties to SQI and their duties as a director or officer of such other companies.
93
PLAN OF DISTRIBUTION
Subject to the terms and conditions of the underwriting agreement, dated September • , 2011, between us and Leerink Swann LLC, as representative for each of the underwriters named below, we have agreed to sell to the underwriters, and each of the underwriters has agreed, severally and not jointly, to purchase from us, the number of common shares indicated opposite its name in the table below at the offering price, on or about the date of closing:
| | | | |
Underwriter | | Number of
Common Shares | |
|---|
Leerink Swann LLC | | | • | |
Rodman & Renshaw, LLC | | | • | |
Kingsdale Capital Markets Inc. | | | • | |
| | | | |
Total | | | • | |
| | | | |
The underwriters are offering the common shares subject to their acceptance of the common shares from us and subject to prior sale. The underwriting agreement provides that the obligations of the several underwriters are subject to certain conditions precedent, such as the receipt by the underwriters of officers' certificates and legal opinions and approval of certain legal matters by their counsel, including the validity of the common shares. The underwriting agreement provides that the underwriters have agreed, severally and not jointly, to take up and pay for all of the shares if any of them are purchased. However, the underwriters are not obligated to purchase any common shares covered by the underwriters' over-allotment option described below. If an underwriter defaults, the underwriting agreement provides that the purchase commitments of the non-defaulting underwriters may be increased or the underwriting agreement may be terminated. The obligations of the underwriters under the underwriting agreement may be terminated at the discretion of the representative of the underwriters on the basis of its assessment of the effect that certain changes in the United States', Canada's or international political, financial or economic conditions may have on the market for the common shares. The obligations of the underwriters may also be terminated upon the occurrence of certain stated events.
We have agreed to indemnify the underwriters and certain of their controlling persons against certain liabilities, including liabilities under applicable securities legislation, or to contribute to payments that the underwriters may be required to make in respect of those liabilities.
The underwriters reserve the right to withdraw, cancel or modify offers to the public and to reject orders in whole or in part. In addition, the underwriters have advised us that they do not intend to confirm sales to any account over which they exercise discretionary authority.
The offering is being made concurrently in the United States and Canada pursuant to the multi-jurisdictional disclosure system. The common shares will be offered in the United States through Leerink Swann LLC and Rodman & Renshaw, LLC, and in Ontario and British Columbia through Kingsdale Capital Markets Inc. either directly or through their respective broker-dealer affiliates or agents, as applicable. No securities will be offered or sold in any jurisdiction except by or through brokers or dealers duly registered under the applicable securities laws of that jurisdiction, or in circumstances where an exemption from such registered dealer requirements is available. Subject to applicable law, the underwriters may offer the common shares outside of the United States and Canada pursuant to prospectus exemptions.
The offering price of the common shares will be determined by negotiation between us and the underwriters.
The offering price of the common shares for investors in the United States will be payable in U.S. dollars and the offering price of the common shares for investors in Canada will be in Canadian dollars unless the underwriters otherwise agree.
Subscriptions for the common shares will be received subject to rejection or allotment, in whole or in part, and the right is reserved to close the subscription books at any time without notice. We expect to arrange for an instant deposit of the common shares to or for the account of the underwriters with CDS Clearing and Depository Services Inc. and The Depository Trust Company on the date of closing, which is expected to take
94
place on or about • , 2011, or such other date as may be agreed upon by us and the underwriters, but in any event no later than • , 2011. In any event, the common shares are to be taken up by the underwriters, if at all, on or before a date not later than 42 days after the date of the receipt of the final prospectus.
Commission and Expenses
The representative has advised us that the underwriters propose initially to offer the common shares to the public at the offering price set forth on the cover page of this prospectus and to dealers at that price less a concession not in excess of US$ • per common share. The underwriters may allow, and certain dealers may reallow, a discount from the concession not in excess of US$ • per common share to certain brokers and dealers. After the initial offering, the offering price, concession and reallowance to dealers may be reduced by the representative. No such reduction will change the amount of proceeds to be received by us as set forth on the cover page of this prospectus. The offering price has been determined by negotiation between us and the underwriters.
We offer no assurances that the offering price will correspond to the price at which the common shares will trade in the public market subsequent to the offering.
For purposes of the offering, if all of the common shares have not been sold after the underwriters have made a reasonable effort to sell the common shares at the offering price disclosed in this prospectus, the underwriters may from time to time decrease or change the offering price and the other selling terms provided that the price for the common shares shall not exceed the offering price and further provided that the compensation that is realized by the underwriters will be decreased by the amount that the aggregate price paid by the purchasers for the common shares is less than the gross proceeds paid by the underwriters to us. Any such reduction in the offering price will not affect the proceeds received by us.
The following table shows the offering price, the underwriting discounts and commissions that we are to pay the underwriters and the proceeds, before expenses, to us in connection with this offering. Such amounts are shown assuming both no exercise and full exercise of the underwriters' option to purchase additional shares described below.
| | | | | | | | | | | | | |
| | Per Share | | Total | |
|---|
| | Without
Option to
Purchase
Additional
Shares | | With
Option to
Purchase
Additional
Shares | | Without
Option to
Purchase
Additional
Shares | | With
Option to
Purchase
Additional
Shares | |
|---|
Public offering price | | US$ | • | | US$ | • | | US$ | • | | US$ | • | |
Underwriting discounts and commissions paid by us | | US$ | • | | US$ | • | | US$ | • | | US$ | • | |
Proceeds to us, before expenses | | US$ | • | | US$ | • | | US$ | • | | US$ | • | |
We estimate expenses payable by us in connection with this offering, other than the underwriting discounts and commissions referred to above, will be approximately US$ • .
Listing
Our common shares are listed on the TSXV under the trading symbol "SQD". Our common shares have been approved for listing on NYSE Amex under the trading symbol "SQD".
Option to Purchase Additional Shares
We have granted to the underwriters an option, exercisable for 30 days from the closing of the offering, to purchase up to an aggregate of • additional common shares at the offering price set forth on the cover page of this prospectus, less underwriting discounts and commissions. If the underwriters exercise this option, each underwriter will be obligated, subject to specified conditions, to purchase the number of additional common shares proportionate to that underwriter's initial purchase commitment as indicated in the table above. This option may be exercised only if the underwriters sell more common shares than the total number set forth on the cover page of the prospectus. We will pay the expenses associated with the exercise of this option.
95
No Sales of Similar Securities
We and certain of our officers and directors have agreed, subject to specified exceptions, not to directly or indirectly:
- •
- issue, offer, sell, agree to issue, offer or sell, solicit offers to purchase, grant any call option, warrant or other right to purchase, purchase any put option or other right to sell, pledge, borrow or otherwise dispose of any common shares;
- •
- will not establish or increase any "put equivalent position" or liquidate or decrease any "call equivalent position" (in each case within the meaning of Section 16 of the Exchange Act and the Rules and Regulations) with respect to any common shares; or
- •
- otherwise enter into any swap, derivative or other transaction or arrangement that transfers to another, in whole or in part, any economic consequence of ownership of common shares; or
- •
- publicly announce an intention to do any of the foregoing without the prior written consent of Leerink Swann LLC.
This restriction terminates after the close of trading of the common shares on and including the 90th day after the date of the final prospectus. However, subject to certain exceptions, in the event that either:
- •
- during the last 17 days of the 90-day restricted period, we issue an earnings release or material news or a material event relating to us occurs, or
- •
- prior to the expiration of the 90-day restricted period, we announce that we will release earnings results during the 16-day period beginning on the last day of the 90-day restricted period,
then in either case the expiration of the 90-day restricted period will be extended until the expiration of the 18-day period beginning on the date of the issuance of an earnings release or the occurrence of the material news or event, as applicable, unless Leerink Swann LLC waives, in writing, such an extension.
Leerink Swann LLC may, in its sole discretion and at any time or from time to time before the termination of the 90-day period, without public notice, release all or any portion of the securities subject to lock-up agreements. There are no existing agreements between the underwriters and any of our officers, directors or shareholders who will execute a lock-up agreement, providing consent to the sale of shares prior to the expiration of the lock-up period.
Stabilization
In connection with the sale of our common shares in the United States, the underwriters may sell more common shares than they are required to purchase in this offering or effect transactions which stabilize or maintain the market price of the common shares at levels other than those which otherwise might prevail on the open market. The underwriters have advised us that, pursuant to Regulation M under the Exchange Act, certain persons participating in the offering may engage in transactions, including over-allotment, stabilizing bids, syndicate covering transactions or the imposition of penalty bids, which may have the effect of stabilizing or maintaining the market price of the common shares at a level above that which might otherwise prevail in the open market. Over-allotment involves syndicate sales in excess of the offering size, which creates a syndicate short position.
Until the distribution of the common shares is completed, SEC rules may limit the underwriters from bidding for and purchasing the common shares sold in the offering. However, the representative may engage in transactions that stabilize the market price of the common shares, such as bids or purchases to peg, fix or maintain that price so long as stabilizing transactions do not exceed a specified maximum.
In accordance with the rules of the Ontario Securities Commission, the British Columbia Securities Commission and the Universal Market Integrity Rules for Canadian Marketplaces administered by the Investment Industry Regulatory Organization of Canada, the underwriters may not, at any time during the period of distribution, bid for or purchase common shares. This restriction is, however, subject to exceptions where the bid or purchase is not made for the purpose of creating actual or apparent active trading in, or raising
96
the price of, the common shares. These exceptions include a bid or purchase permitted under the Universal Market Integrity Rules for Canadian Marketplaces relating to market stabilization and passive market making activities and a bid or purchase made for and on behalf of a customer where the order was not solicited during the period of distribution.
"Covered" short sales are sales made in an amount not greater than the underwriters' option to purchase additional common shares in this offering. The underwriters may close out any covered short position by either exercising their option to purchase additional common shares or purchasing common shares in the open market. In determining the source of shares to close out the covered short position, the underwriters will consider, among other things, the price of shares available for purchase in the open market as compared to the price at which they may purchase shares through the option to purchase additional shares.
"Naked" short sales are sales in excess of the option to purchase additional common shares. The underwriters must close out any naked short position by purchasing shares in the open market. A naked short position is more likely to be created if the underwriters are concerned that there may be downward pressure on the price of the common shares in the open market after pricing that could adversely affect investors who purchase in this offering.
A stabilizing bid is a bid for the purchase of common shares on behalf of the underwriters for the purpose of fixing or maintaining the price of the common shares. A syndicate covering transaction is the bid for or the purchase of common shares on behalf of the underwriters to reduce a short position incurred by the underwriters in connection with the offering. A penalty bid is an arrangement permitting the underwriters to reclaim the selling concession otherwise accruing to a syndicate member in connection with the offering if the common shares originally sold by such syndicate member are purchased in a syndicate covering transaction and therefore have not been effectively placed by such syndicate member.
Neither we nor any of the underwriters make any representation or prediction as to the direction or magnitude of any effect that the transactions described above may have on the price of our common shares. The underwriters are not obligated to engage in these activities and, if commenced, any of the activities may be discontinued at any time.
Electronic Offer, Sale and Distribution of Common Shares
In connection with the offering, a prospectus in electronic format may be made available by e-mail or on the web sites or through online services maintained by one or more of the underwriters or their affiliates. In those cases, prospective investors may view offering terms online and may be allowed to place orders online. The underwriters may agree with us to allocate a specific number of common shares for sale to online brokerage account holders. Any such allocation for online distributions will be made by the underwriters on the same basis as other allocations. Other than the prospectus in electronic format, the information on the underwriters' web sites and any information contained in any other web site maintained by any of the underwriters is not part of this prospectus, and should not be relied upon by investors.
Affiliations
The underwriters and certain of their respective affiliates are full service financial institutions engaged in various activities, which may include securities trading, commercial and investment banking, financial advisory, investment management, investment research, principal investment, hedging, financing and brokerage activities. Some of the underwriters and certain of their respective affiliates have, from time to time, performed, and may in the future perform, various financial advisory and investment banking services for us, for which they received or will receive customary fees and commissions.
In the ordinary course of their various business activities, the underwriters and certain of their affiliates may make or hold a broad array of investments and actively trade debt and equity securities (or related derivative securities) and financial instruments (including bank loans) for their own account and for the accounts of their customers, and such investment and securities activities may involve securities and/or instruments of ours or our affiliates.
97
The underwriters and certain of their respective affiliates may also make investment recommendations and/or publish or express independent research views in respect of such securities or instruments and may at any time hold, or recommend to clients that they acquire, long and/or short positions in such securities and instruments.
DOCUMENTS INCORPORATED BY REFERENCE
Information has been incorporated by reference into this prospectus from documents filed with securities commissions or similar regulatory authorities in Canada. Copies of the documents incorporated by reference may be obtained on request without charge from our Chief Financial Officer at 36 Meteor Drive, Toronto, ON M9W 1A4, telephone: (416) 674-7500. These documents are also available through the Internet on SEDAR, which can be accessed at www.sedar.com.
We file annual and quarterly financial information, material change reports and other information with the securities commissions or similar regulatory authorities in Canada, which allow us to "incorporate by reference" the information we file with them, which means that we can disclose important information to you by referring you to those documents. Information that is incorporated by reference is an important part of this prospectus. The following documents previously filed with the securities commissions or similar regulatory authorities in Canada are specifically incorporated by reference into and form an integral part of this prospectus:
- (a)
- our annual information form dated June 15, 2011;
- (b)
- our management proxy circular dated June 8, 2011 prepared in connection with the annual and special meeting of our shareholders held on July 7, 2011;
- (c)
- our audited consolidated financial statements for the years ended September 30, 2010 and 2009, together with the auditors' report thereon;
- (d)
- the supplemental note regarding the reconciliation of our audited consolidated financial statements for the years ended September 30, 2010 and 2009 with U.S. GAAP, together with the auditors' report thereon;
- (e)
- our management's discussion and analysis of consolidated results of operations and financial condition for the fiscal year ended September 30, 2010;
- (f)
- our unaudited interim consolidated financial statements for the three and nine months ended June 30, 2011;
- (g)
- the supplemental note regarding the reconciliation of our unaudited interim consolidated financial statements for the three and nine month periods ended June 30, 2011 and 2010 with U.S. GAAP;
- (h)
- our management's discussion and analysis of consolidated results of operations and financial condition for the three and nine months ended June 30, 2011; and
- (i)
- our material change report, as amended, dated July 14, 2011 in respect of the proposed acquisition of Scienion.
Any annual information form, annual or interim financial statements and related management's discussion and analysis, material change report (other than a confidential material change report), business acquisition report, information circular or disclosure document filed by us with any securities commission or similar regulatory authority in Canada subsequent to the date of this prospectus and prior to the termination of the offering shall be deemed to be incorporated by reference into this prospectus, as well as any other document so filed by us that expressly states it to be incorporated by reference into this prospectus. Any report filed by us with the SEC or Report of Foreign Private Issuer on Form 6-K furnished by us to the SEC after the date of this prospectus until the termination of the distribution under this prospectus shall be deemed to be incorporated by reference as an exhibit to the registration statement of which this prospectus forms a part, if and to the extent expressly provided in such report. The documents incorporated or deemed to be incorporated by reference into this prospectus contain meaningful and material information relating to us and you should review all information contained in this prospectus and the documents incorporated or deemed to be incorporated by reference into this prospectus.
98
Any statement contained in this prospectus or in a document incorporated or deemed to be incorporated by reference into this prospectus shall be deemed to be modified or superseded, for purposes of this prospectus, to the extent that a statement contained in this prospectus or in any other subsequently filed document that also is or is deemed to be incorporated by reference into this prospectus modifies or supersedes that statement. Any such modifying or superseding statement need not state that it has modified or superseded a prior statement or include any other information set forth in the document that it modifies or supersedes. The making of a modifying or superseding statement shall not be deemed an admission for any purposes that the modified or superseded statement, when made, constituted a misrepresentation, an untrue statement of a material fact or an omission to state a material fact that is required to be stated or that is necessary to make a statement not misleading in light of the circumstances in which it was made. Any statement so modified or superseded shall not be considered in its unmodified or superseded form to constitute part of this prospectus; rather only such statement as so modified or superseded shall be considered to constitute part of this prospectus.
WHERE YOU CAN FIND MORE INFORMATION
This prospectus does not contain all of the information contained in the registration statement we have filed with the SEC, certain items of which are contained in the exhibits to the registration statement as permitted by the rules and regulations of the SEC. Statements included or incorporated by reference in this prospectus about the contents of any contract, agreement or other documents referred to are not necessarily complete, and in each instance, if such document is filed as an exhibit, you should refer to the exhibits for a more complete description of the matter involved. Each such statement is qualified in its entirety by such reference. Upon effectiveness of the registration statement, we will become subject to the informational requirements of the Exchange Act and in accordance therewith will be required to file reports and other information with the SEC. Under the multi-jurisdictional disclosure system adopted by the United States and Canada, we may prepare our reports and other information in accordance with the disclosure requirements of Canada, which are different from those of the United States. Prospective investors may read any document we file or furnish to the SEC, including those documents that are incorporated by reference in this prospectus, which are filed as exhibits to our registration statement on Form F-10, at the SEC's website at www.sec.gov. You may also read and copy any document we file at the SEC's Public Reference Room at 100 F Street, N.E., Washington, D.C. 20549. Please call the SEC at 1-800-SEC-0330 for further information on the operation of the Public Reference Room. We are also subject to filing requirements prescribed by the securities legislation of British Columbia, Alberta, and Ontario. These filings are electronically available from SEDAR at www.sedar.com.
As a "foreign private issuer" under the Exchange Act, we are exempt from the rules under the Exchange Act prescribing the furnishing and content of proxy statements, and our officers, directors and principal shareholders are exempt from the reporting and "short-swing profit" recovery provisions contained in Section 16 of the Exchange Act. In addition, we are not required to publish financial statements as promptly as United States companies.
AUDITORS, TRANSFER AGENTS AND REGISTRARS
Our auditors are Collins Barrow Toronto LLP. The transfer agent and registrar for the common shares is Computershare Investor Services Inc. at its principal offices located in Toronto, Ontario.
CERTAIN CANADIAN FEDERAL INCOME TAX CONSIDERATIONS
The following summary describes the principal Canadian federal income tax considerations generally applicable to a prospective investor who, for purposes of the Income Tax Act (Canada) (the "Tax Act") and at all relevant times, acquires common shares ("Shares") of the Company pursuant to the offering described in this prospectus and holds its Shares as capital property, deals at arm's length with the Company, and is not affiliated with the Company (a "Shareholder"). Generally, Shares will be capital property to a shareholder unless the Shares are held or were acquired in the course of carrying on a business of buying and selling securities or as part of an adventure or concern in the nature of trade. Certain shareholders who are residents of Canada for purposes of the Tax Act and whose Shares might not otherwise be capital property may, in some circumstances, be entitled to make an irrevocable election in accordance with subsection 39(4) of the Tax Act to have such Shares and every other "Canadian security" (as defined in the Tax Act) owned by them deemed to be capital
99
property in the taxation year of the election and in all subsequent taxation years. Such shareholders should consult their own tax advisors for advice with respect to whether an election under subsection 39(4) of the Tax Act is available or advisable in their particular circumstances.
This summary is not applicable to a Shareholder (a) that is a "financial institution" for purposes of the mark-to-market property rules contained in the Tax Act; (b) that is a "specified financial institution" (as defined in the Tax Act); (c) that has elected to report its tax results in a functional currency (which excludes Canadian currency); or (d) an interest in which is a "tax shelter investment" (as defined in the Tax Act). Such Shareholders should consult their own tax advisors.
This summary is based upon the current provisions of the Tax Act, the regulations thereunder (the "Regulations"), all specific proposals to amend the Tax Act and the Regulations publicly announced by or on behalf of the Minister of Finance (Canada) prior to the date hereof (the "Proposed Amendments"), and an understanding of the current administrative policies and assessing practices published in writing by the Canada Revenue Agency ("CRA") prior to the date hereof. This summary assumes that all Proposed Amendments will be enacted in the form proposed. However, no assurances can be given that the Proposed Amendments will be enacted as proposed, or at all. This summary does not otherwise take into account or anticipate any changes in law or administrative policies or assessing practices, whether by legislative, regulatory, administrative or judicial action or decision, nor does it take into account other federal or provincial, territorial or foreign tax legislation or considerations, which may differ materially from those described in this summary. This summary assumes that, at all relevant times, the Shares will be listed on a designated stock exchange (as defined in the Tax Act), which currently includes the TSXV.
This summary is of a general nature only and is not, and is not intended to be, and should not be construed to be, legal or tax advice to any particular Shareholder. This summary is not exhaustive of all Canadian federal income tax considerations. Accordingly, Shareholders should consult their own tax advisors with respect to the Canadian federal income tax consequences of acquiring, holding and disposing of Shares having regard to their own particular circumstances.
Residents of Canada
The following portion of this summary is generally applicable to a Shareholder who, for purposes of the Tax Act and any applicable income tax treaty, at all relevant times, is or is deemed to be resident in Canada (a "Resident Shareholder").
Dividends (including deemed dividends) received on the Shares by an individual Resident Shareholder (other than certain trusts) will be included in the individual's income and will be subject to the gross-up and dividend tax credit rules applicable to taxable dividends received by individuals from taxable Canadian corporations, including the enhanced dividend tax credit rules applicable to any dividends designated by the Company as "eligible dividends" in accordance with the Tax Act. Dividends received by an individual may give rise to alternative minimum tax.
Dividends (including deemed dividends) received on the Shares by a Resident Shareholder that is a corporation will be included in computing its income and will generally be deductible in computing its taxable income. A Resident Shareholder that is a "private corporation" or a "subject corporation", each as defined in the Tax Act, may be liable to pay a 331/3% refundable tax under Part IV of the Tax Act on dividends received (or deemed to be received) on the Shares to the extent such dividends are deductible in computing taxable income.
A disposition or deemed disposition of a Share by a Resident Shareholder will generally give rise to a capital gain (or capital loss) equal to the amount by which the proceeds of disposition of the Share exceed (or are less than) the aggregate of the adjusted cost base of the Share to the Resident Shareholder and any reasonable costs of disposition. For this purpose, the adjusted cost base to a Resident Shareholder of a Share
100
will be determined by averaging the cost of such Share with the adjusted cost base of any other Shares owned by the Resident Shareholder as capital property. Such capital gain (or capital loss) will be subject to the treatment described below under "Taxation of Capital Gains and Capital Losses".
Generally, one-half of any capital gain (a taxable capital gain) realized by a Resident Shareholder in a taxation year must be included in a Resident Shareholder's income for that year and one-half of any capital loss (an allowable capital loss) realized by a Resident Shareholder in a taxation year is required to be deducted against taxable capital gains realized by the Resident Shareholder in that year. Allowable capital losses in excess of taxable capital gains for a particular taxation year generally may be carried back and deducted in any of the three (3) preceding taxation years or carried forward and deducted in any subsequent taxation year against net taxable capital gains realized by the Resident Shareholder in such years, to the extent and in the circumstances described in the Tax Act.
The amount of any capital loss realized by a Resident Shareholder that is a corporation on the disposition of a Share may be reduced by the amount of any dividends received (or deemed to be received) by it on such Share to the extent and under the circumstances described in the Tax Act. Similar rules may apply where a Share is owned by a partnership or trust of which a corporation, trust or partnership is a member or beneficiary.
A Resident Shareholder that is throughout the year a "Canadian-controlled private corporation" (as defined in the Tax Act) may be liable for a refundable tax of 62/3% on its "aggregate investment income", which is defined to include an amount in respect of taxable capital gains.
Capital gains realized by an individual or a trust, other than certain trusts, may give rise to alternative minimum tax.
Non-Resident Shareholders
The following portion of this summary is applicable to a Shareholder who, for purposes of the Tax Act and at all relevant times, (i) has not been and is not resident or deemed to be resident in Canada, and (ii) does not use or hold and is not deemed to use or hold Shares in connection with carrying on a business in Canada (a "Non-Resident Shareholder"). Special rules, which are not discussed in this summary, may apply to a Non-Resident Shareholder that is either an insurer carrying on business in Canada and elsewhere or an "authorized foreign bank" (as defined in the Tax Act). Such Non-Resident Shareholders should consult their own tax advisors.
Dividends paid or credited (or deemed to be paid or credited) to a Non-Resident Shareholder by the Company will be subject to Canadian withholding tax at the rate of 25%, subject to reduction under the terms of an applicable tax treaty or convention. In the case of a Non-Resident Shareholder who is a resident of the United States for purposes of the Canada-U.S. Income Tax Convention (the "Convention"), who is the beneficial owner of the dividend, and who qualifies for the benefits of such Convention the rate of such withholding tax generally will be reduced to 15%. Not all persons who are residents of the U.S. for the purposes of the Convention will qualify for the benefits of the Convention. Non-Resident Shareholders are urged to consult their own tax advisors to determine their entitlement to any tax treaty relief as well as their ability to claim foreign tax credits with respect to any Canadian withholding tax, based on their particular circumstances.
A Non-Resident Shareholder will not be subject to tax under the Tax Act on any capital gain realized on a disposition or deemed disposition of Shares unless the Shares constitute "taxable Canadian property" (within the meaning of the Tax Act) to the Non-Resident Shareholder at the time of the disposition and such gain is not otherwise exempt from tax under the Tax Act pursuant to the provisions of an applicable tax treaty or convention.
101
Shares acquired pursuant to the offering described herein, generally, will not constitute taxable Canadian property to a Non-Resident Shareholder at the time of the disposition provided that the Shares are listed on a designated stock exchange (which currently includes the TSXV) at that time, and the Non-Resident Shareholder, persons with whom the Non-Resident Shareholder does not deal at arm's length, or the Non-Resident Shareholder together with all such persons, has not owned 25% or more of the issued shares of any class or series of the capital stock of the Company at any time during the sixty (60) month period that ends at the time of the disposition. Notwithstanding the foregoing, Shares may be deemed to be taxable Canadian property in certain circumstances specified in the Tax Act.
In the event that the Shares constitute or are deemed to constitute taxable Canadian property to a Non-Resident Shareholder, such Non-Resident Shareholder will generally be subject to tax as described above under the heading "Residents of Canada — Disposition of Common Shares" unless the Non-Resident Shareholder is entitled to relief under an applicable tax treaty or convention. Non-Resident Shareholders whose Shares may be taxable Canadian property should consult their own tax advisors.
CERTAIN MATERIAL UNITED STATES FEDERAL INCOME TAX CONSIDERATIONS
This section describes the material United States federal income tax considerations of the acquisition, ownership and disposition of the common shares. This summary addresses only persons or entities that are "U.S. holders" (as defined below). This summary is for general information purposes only and does not purport to be a complete analysis or listing of all potential U.S. federal income tax consequences that may apply to a U.S. holder arising from or relating to the acquisition, ownership, and disposition of the common shares. In addition, this summary does not take into account the individual facts and circumstances of any particular U.S. holder that may affect the U.S. federal income tax consequences to such U.S. holder. Accordingly, this summary is not intended to be, and should not be construed as, legal or U.S. federal income tax advice with respect to any U.S. holder. This summary does not address the U.S. state and local, U.S. federal estate and gift, or foreign tax consequences or the alternative minimum tax provisions of the Code (as defined below). Each U.S. holder should consult its own financial advisor, legal counsel, or accountant regarding the U.S. federal income, U.S. state and local, and foreign tax consequences arising from and relating to the acquisition, ownership, and disposition of common shares.
This section does not apply to you if you are a member of a class of holders subject to special rules, including, but not limited to:
- •
- U.S. holders that are tax-exempt organizations, qualified retirement plans, individual retirement accounts, or other tax-deferred accounts;
- •
- U.S. holders that are financial institutions, insurance companies, real estate investment trusts, or regulated investment companies;
- •
- U.S. holders that are dealers in securities or currencies or U.S. holders that are traders in securities that elect to apply a mark-to-market accounting method;
- •
- U.S. holders that have a "functional currency" other than the US dollar;
- •
- U.S. holders that own common shares as part of a straddle, hedging transaction, conversion transaction, constructive sale, or other arrangement involving more than one position;
- •
- U.S. holders who use common shares as security for a loan;
- •
- U.S. holders that acquired common shares in connection with the exercise of employee stock options or otherwise as compensation for services;
- •
- U.S. holders that hold common shares other than as capital assets within the meaning of Section 1221 of the Code;
- •
- U.S. expatriates or former long-term residents of the U.S.; and
- •
- U.S. holders that own (directly, indirectly, or by attribution) 10% or more of the total combined voting power of our outstanding shares.
102
This section is based on the Internal Revenue Code of 1986, as amended (the "Code"), its legislative history, existing and proposed regulations under the Code, published rulings and court decisions, the Convention Between Canada and the United States of America with Respect to Taxes on Income and on Capital, signed September 26, 1980, as amended (the "Canada-U.S. Tax Convention"), all as in effect on the date of this offering, and all of which are subject to change, possibly with retroactive effect. This summary does not discuss the potential effects, whether adverse or beneficial, of any proposed legislation that, if enacted, could be applied on a retroactive basis.
This summary is not binding upon the Internal Revenue Service (the "IRS") and no rulings have been or will be sought from the IRS regarding any matters discussed in this summary. In that regard, there can be no assurance that one or more of the tax considerations described in this summary will not be challenged by the IRS or a U.S. court.
If a partnership (or other entity or arrangement treated as a partnership for United States federal income tax purposes) holds the common shares, the United States federal income tax treatment of a partner (or member) will generally depend on the status of the partner and the tax treatment of the partnership (or other entity). This summary does not address the tax consequences to any such partner. A partner in a partnership (or member of such other entity) holding the common shares should consult its own tax advisor with regard to the U.S. federal income tax treatment of an investment in the common shares.
You are a U.S. holder if you are a beneficial owner of a common share and you are:
- •
- an individual who is a citizen or resident (including a lawful permanent resident alien holding a green card) of the United States as determined for U.S. federal income tax purposes;
- •
- a corporation (or other entity treated as a corporation for U.S. federal income tax purposes) created or organized in or under the laws of the United States or any state thereof or the District of Columbia;
- •
- an estate whose income is subject to U.S. federal income tax regardless of its source; or
- •
- a trust if it (1) is subject to the primary supervision of a federal, state or local court within the United States and one or more United States persons are authorized to control all substantial decisions of the trust or (2) has a valid election in effect under applicable Treasury Regulations to be treated as a U.S. person.
The United States federal income tax consequences of the acquisition, ownership and disposition of the common shares can be very complex and, in certain cases, uncertain or potentially unfavorable to a U.S. holder. Accordingly, each prospective investor considering an acquisition of, or who acquires common shares pursuant to this offering, is strongly urged to consult its own tax advisor with respect to the United States federal, state or local income and alternative minimum tax, estate or gift, or foreign tax consequences of such acquisition, ownership and disposition of common shares in light of its own particular facts and circumstances.
U.S. Holders
Taxation of Distributions
Under the U.S. federal income tax laws, and subject to the passive foreign investment company ("PFIC") rules discussed below, if you are a U.S. holder, a distribution of cash, if any, paid on a common share, including a constructive distribution, generally will be included in your gross income as a dividend (without reduction for any amounts withheld in respect of Canadian federal income tax) to the extent of our current or accumulated "earnings and profits" (as computed under United States federal income tax rules). We do not intend to calculate our earnings and profits under United States federal income tax rules. Accordingly, U.S. holders should expect that a distribution generally will be treated as a dividend for United States federal income tax purposes. U.S. holders should consult their own tax advisors with respect to the appropriate U.S. federal income tax treatment of any distribution received from us.
If you are a non-corporate U.S. holder, dividends paid to you in taxable years beginning before January 1, 2013 that constitute "qualified dividend income" will be taxable to you at a maximum federal tax rate of 15%, provided that you hold the common shares for more than 60 days during the 121-day period beginning 60 days
103
before the ex-dividend date and meet other requirements. Dividends we pay with respect to the common shares should be qualified dividend income provided that we are not a PFIC in the year the dividend is paid or the previous taxable year. Because we may have been a PFIC in the tax year ending on September 30, 2011, distributions paid in the following tax year may not constitute qualified dividend income eligible for preferential tax rates, even though we do not anticipate being a PFIC in the tax year beginning on October 1, 2011 and do not anticipate U.S. holders being subject to the PFIC rules.
You must include any Canadian tax withheld from the dividend payment in this gross amount even though you do not in fact receive the withheld amount. The dividend is taxable to you when you receive the dividend, actually or constructively. The dividend will not be eligible for the dividends-received deduction generally allowed to U.S. corporations in respect of dividends received from other U.S. corporations. The amount of the dividend distribution that you must include in your income as a U.S. holder will be the U.S. dollar value of the Canadian dollar payments made, determined at the spot Canadian dollar/U.S. dollar rate on the date the dividend distribution is includible in your income, regardless of whether the payment is in fact converted into U.S. dollars. Generally, any gain or loss resulting from currency exchange fluctuations during the period from the date you include the dividend payment in income to the date you convert the payment into U.S. dollars will be treated as ordinary income or loss. The gain or loss generally will be income or loss from sources within the United States for foreign tax credit limitation purposes.
To the extent that a distribution paid on the common shares is in excess of our current and accumulated "earnings and profits" (as determined for U.S. federal income tax purposes), such distribution generally will be treated as a non-taxable return of capital to the extent of your adjusted tax basis in the common shares, with any excess treated as a gain from the sale or exchange of the Shares.
A U.S. holder that pays (whether directly or through withholding) Canadian income tax with respect to distributions by us generally will be entitled, at the election of such U.S. holder, to receive either a deduction or a credit for such Canadian income tax paid. Complex limitations apply to the foreign tax credit, including the general limitation that the credit cannot exceed the proportionate share of a U.S. holder's U.S. federal income tax liability that such U.S. holder's "foreign source" taxable income bears to such U.S. holder's worldwide taxable income. In applying this limitation, a U.S. holder's various items of income and deduction must be classified, under complex rules, as either "foreign source" or "U.S. source." Special rules apply in determining the foreign tax credit limitation with respect to dividends that are subject to the maximum 15% tax rate.
For foreign tax credit purposes, dividends will be income from sources outside the United States and will, depending on your circumstances, be either "passive category" or "general category" income for purposes of computing the foreign tax credit allowable to you. However, the amount of a distribution with respect to the common shares that is treated as a "dividend" may be lower for U.S. federal income tax purposes than it is for Canadian federal income tax purposes, potentially resulting in a reduced foreign tax credit allowance to a U.S. holder. The foreign tax credit rules are complex, and each U.S. holder should consult its own U.S. tax advisor regarding the foreign tax credit rules.
Taxation of Sale, Exchange or other Taxable Disposition
Subject to the PFIC rules discussed below, if you are a U.S. holder and you sell or otherwise dispose of your common shares, you will recognize gain or loss for United States federal income tax purposes equal to the difference between the United States dollar value of the amount that you realize and your tax basis, determined in United States dollars, in your common shares. Any such gain or loss will be capital gain or loss if the common shares are held by you as capital assets. Any such gain or loss generally will be United States source income or loss for purposes of applying the United States foreign tax credit rules unless the gain is subject to tax in Canada and is re-sourced as "foreign source" under the Canada-U.S. Tax Convention and such U.S. holder elects to treat such gain as "foreign source." Capital gain of a non-corporate U.S. holder that is recognized in taxable years beginning before January 1, 2013 is generally taxed at a maximum federal rate of 15% where the U.S. holder has a holding period greater than one year. Deductions for capital losses are subject to limitations.
104
New Healthcare Legislation
Under newly enacted legislation, certain U.S. holders who are individuals, estates or trusts will be required to pay an additional 3.8% tax on, among other things, dividends on and capital gains from the sale or other disposition of stock for taxable years beginning after December 31, 2012. U.S. holders should consult their own tax advisors regarding the effect, if any, of this legislation on their ownership and disposition of the common shares.
PFIC Rules
If we are considered to be a PFIC, as that term is defined in Section 1297 of the Code, at any time during a U.S. holder's holding period, the following sections will generally describe the United States federal income tax consequences to a U.S. holder in relation to the acquisition, ownership and disposition of common shares.
We generally will be regarded as a PFIC for United States federal income tax purposes if, for a taxable year, either (i) 75% or more of our gross income for such taxable year is passive income or (ii) on the basis of a quarterly average, 50% or more of the assets held by us either produce passive income or are held for the production of passive income, based on the fair market value of such assets. "Passive income" includes, for example, dividends, interest, certain rents and royalties, certain gains from the sale of stock and securities, and certain gains from commodities transactions. If a foreign corporation (such as SQI) owns, directly or indirectly, at least 25% by value of the stock of another corporation, the foreign corporation is treated for purposes of the PFIC tests described above as owning its proportionate share of the assets of the other corporation, and as receiving directly its proportionate share of the other corporation's income. In addition, under certain attribution rules, if we are a PFIC, U.S. holders will be deemed to own their proportionate share of the stock of our subsidiaries which are PFICs (such subsidiaries referred to as "Subsidiary PFICs"), and will be subject to U.S. federal income tax on (i) a distribution on the shares of a Subsidiary PFIC and (ii) a disposition of shares of a Subsidiary PFIC, both as if the holder directly held the shares of such Subsidiary PFIC.
Based on current business plans and financial expectations, we believe that the common shares should not be treated as interests in a PFIC for U.S. federal income tax purposes for the tax year ending September 30, 2012 but that the common shares may be treated as interests in a PFIC for the tax year ending September 30, 2011. However, PFIC classification is fundamentally factual in nature, generally cannot be determined until the close of the taxable year in question, and is determined annually. In addition, whether any corporation will be a PFIC for any taxable year depends on the assets and income of such corporation over the course of each such taxable year. Consequently, there can be no assurance that we will not become a PFIC for any taxable year during which U.S. holders hold common shares. In any given taxable year for which we are classified as a PFIC, during which a U.S. holder holds common shares, a U.S. holder would be subject to increased tax liability (possibly including a non-deductible interest charge) (i) with respect to gain realized upon the sale or other disposition of the common shares or (ii) upon the receipt of certain distributions treated as "excess distributions," unless such U.S. holder elects to be taxed currently, by making a Mark-to-Market or a Qualified Electing Fund ("QEF") Election. An excess distribution generally would be any distribution to a U.S. holder with respect to common shares during a single taxable year that is greater than 125% of the average annual distributions received by such U.S. holder with respect to the common shares during the three preceding taxable years or, if shorter, during such U.S. holder's holding period for the common shares. In addition, certain special, generally adverse rules would apply to the common shares if we are a PFIC. For example, under certain proposed Treasury regulations, a "disposition" for this purpose may include, under certain circumstances, transfers at death, gifts, pledges, transfers pursuant to tax-deferred reorganizations and other transactions with respect to which gain ordinarily would not be recognized. In addition, if we are treated as a PFIC, dividends paid by us will not be eligible for taxation at the preferential tax rates described above under the heading "Taxation of Distributions."
If a U.S. holder owns the common shares during any year in which we are a PFIC, such U.S. holder generally must also file an annual report with the IRS (on IRS Form 8621 or other form specified by the U.S. Department of Treasury) regardless of whether such holder makes a Mark-to-Market or QEF Election.
105
Under the PFIC rules:
- •
- the gain or excess distribution will be allocated rateably over your holding period for the common shares;
- •
- the amount allocated to the taxable year in which you realized the gain or excess distribution and the amount allocated to any taxable year prior to the first taxable year in which the company is a PFIC will be taxed as ordinary income; and
- •
- the amount allocated to each prior year during which we were classified as a PFIC, with certain exceptions, will be taxed at the highest tax rate in effect for that year and an interest charge generally applicable to underpayments of tax will be imposed in respect of the tax attributable to each such year. A U.S. holder that is not a corporation must treat such interest as non-deductible personal interest.
If we were a PFIC, a U.S. holder might be able to make a Mark-to-Market Election with respect to the common shares to mitigate some of these adverse tax consequences. A U.S. holder that holds stock of a PFIC generally may make a Mark-to-Market Election with respect to its stock if the stock constitutes "marketable stock." Marketable stock is stock that is regularly traded (other than in de minimis quantities) on a U.S. exchange that is registered with the SEC or the national market system, or a non-U.S. exchange or other market that the U.S. Treasury Department determines has trading volume, listing, financial disclosure, and other rules adequate to carry out the purposes of the Mark-to-Market Election. The common shares should constitute marketable stock with respect to which a Mark-to-Market Election could be made. The common shares are expected to be traded on the NYSE Amex, which should be a "qualified exchange" for purposes of the Mark-to-Market Election. In such case, a U.S. holder would generally include as ordinary income or loss the difference between the fair market value of the U.S. holder's common shares at the end of the taxable year and the U.S. holder's adjusted tax basis in the common shares (but loss could only be included to the extent of the net amount of previously included income as a result of the Mark-to-Market Election). If a U.S. holder made the Mark-to-Market Election, the U.S. holder's tax basis in the common shares would be adjusted to reflect any such income or loss amounts. Any gain recognized on the sale or other disposition of common shares would be treated as ordinary income, and any losses incurred on the sale or other disposition of the common shares would be treated as ordinary losses to the extent of any net mark-to-market gains for prior taxable years. A U.S. holder should consult its own tax advisors regarding the availability and advisability of making a Mark-to-Market Election with respect to the common shares in its particular circumstances in the event that we are or become a PFIC. A Mark-to-Market Election would not be available for any stock of a Subsidiary PFIC that a U.S. holder is deemed to own.
A Mark-to-Market Election will be effective for the taxable year for which the election is made and all subsequent taxable years. The election cannot be revoked without the consent of the IRS unless the common shares cease to be marketable, in which case the election is automatically terminated. If we are classified as a PFIC for any taxable year in which a U.S. holder owns common shares but before a Mark-to-Market Election is made, the interest charge rules described above will apply to any mark-to-market gain recognized in the year the election is made.
The QEF Election, if available, generally would allow a U.S. holder to avoid the consequences of the PFIC rules but would require such holder to include its pro rata share of our ordinary income and net capital gain in income currently, whether or not distributed. However, we do not intend to provide U.S. holders with such information as may be required to make a QEF Election effective in the event we are a PFIC.
The PFIC rules are complex and in certain cases, uncertain. Each U.S. holder should consult its own tax advisor regarding the application and operation of the PFIC rules, including the availability and advisability of, and procedure for, making the Mark-to-Market and/or QEF Elections.
Backup Withholding and Information Reporting
Under U.S. federal income tax law and Treasury regulations, certain categories of U.S. holders must file information returns with respect to their investment in, or involvement in, a foreign corporation. Penalties for failure to file certain of these information returns are substantial. U.S. holders who acquire common shares through the offering and hold common shares should consult with their own tax advisors regarding the requirements of filing information returns, and if applicable, any Mark-to-Market Election or QEF Election.
106
If the holder is a non-corporate U.S. holder, then payments to it made within the United States, or by a United States payor or United States middleman, of dividends on and/or proceeds arising from the sale or other taxable disposition of common shares, generally will be subject to information reporting and backup withholding (currently at the rate of 28%), where such U.S. holder fails to furnish its correct United States taxpayer identification number (generally on IRS Form W-9), and to make certain certifications, or otherwise fails to establish an exemption from backup withholding. Any amounts withheld under the backup withholding rules from a payment to a U.S. holder generally may be refunded (or credited against its United States federal income tax liability, if any) provided the required information is furnished to the IRS in a timely manner. Each U.S. holder should consult its own tax advisor regarding the backup withholding tax.
DOCUMENTS FILED AS PART OF THE U.S. REGISTRATION STATEMENT
We have filed or will file the following documents with the SEC as part of the registration statement of which this prospectus forms a part: the documents listed under the heading "Documents Incorporated by Reference"; consents of auditors and counsel; powers of attorney; and the form of underwriting agreement.
ENFORCEABILITY OF CIVIL LIABILITIES
We are a corporation existing under theCanada Business Corporations Act. All of our directors and officers and the experts named in this prospectus are residents of Canada, and all or a substantial portion of their assets, and a substantial portion of our assets, are located outside the United States. We have appointed an agent for service of process in the United States (as set forth below), but it may be difficult for holders of common shares who reside in the United States to effect service within the United States upon those directors, officers and experts who are not residents of the United States. It may also be difficult for holders of common shares who reside in the United States to realize in the United States upon judgments of courts of the United States predicated upon our civil liability and the civil liability of our directors, officers and experts under the United States federal securities laws.
We filed with the SEC, concurrently with our registration statement on Form F-10 of which this prospectus is a part, an appointment of agent for service of process on Form F-X. Under the Form F-X, we appointed Puglisi & Associates as our agent for service of process in the United States in connection with any investigation or administrative proceeding conducted by the SEC, and any civil suit or action brought against or involving us in a United States court, arising out of or related to or concerning the offering of the common shares under this prospectus and the registration statement.
107
EXPERTS
Our consolidated financial statements for the fiscal years ended September 30, 2010 and September 30, 2009 have been audited by Collins Barrow Toronto LLP, chartered accountants, as stated in their report dated November 19, 2010 (except as to the supplemental note regarding the reconciliation of such financial statements for the years ended September 30, 2010 and 2009 with U.S. GAAP, which is dated July 4, 2011). The financial statements for Scienion for the fiscal years ended December 31, 2010 and December 31, 2009 have been audited by Ernst & Young GmbH, Germany as stated in their report dated August 31, 2011.
LEGAL MATTERS
Certain legal matters related to the common shares are being passed upon on our behalf by McCarthy Tétrault LLP, with respect to Canadian legal matters and Dorsey & Whitney LLP, with respect to U.S. legal matters. The underwriters are being represented in this offering by Borden Ladner Gervais LLP with respect to Canadian legal matters and by Ropes & Gray LLP with respect to U.S. legal matters. As of the date hereof, the partners and associates of each of McCarthy Tétrault LLP, Dorsey & Whitney LLP, Borden Ladner Gervais LLP and Ropes & Gray LLP, in each case as a group, beneficially own, directly or indirectly, less than 1%, respectively, of our outstanding securities.
MATERIAL CONTRACTS
The only material contracts entered into by us to which we are or will become a party on or prior to the closing of this offering are as follows:
- 1.
- the purchase agreement referred to under "Acquisition of Scienion AG"; and
- 2.
- the underwriting agreement referred to under "Plan of Distribution".
EXEMPTIONS FROM THE INSTRUMENT
Website Materials
We may make available certain materials describing the offering (the "Website Materials") on the website of one or more commercial services such as www.retailroadshow.com or www.netroadshow.com under the heading "SQI Diagnostics Inc." during the period prior to obtaining a final receipt for the final prospectus relating to this offering (the "Final Prospectus") from the securities regulatory authorities in Ontario and British Columbia. In order to give purchasers in Ontario and British Columbia the same unrestricted access to the Website Materials as provided to U.S. purchasers, we have applied for and obtained, in a decision dated July 15, 2011, exemptive relief from the securities regulatory authority in Ontario and will apply for similar relief from the securities regulatory authority in British Columbia. Pursuant to the terms of that exemptive relief, we and the Canadian underwriter have agreed that, in the event that the Website Materials contained any untrue statement of a material fact or omitted to state a material fact required to be stated or necessary in order to make any statement therein not misleading in the light of the circumstances in which it was made (a "misrepresentation"), a purchaser resident in Ontario and British Columbia who purchases the shares offered hereby pursuant to the Final Prospectus during the period of distribution shall have, without regard to whether the purchaser relied on the misrepresentation, rights against us and the Canadian underwriter with respect to such misrepresentation as are equivalent to the rights under section 130 of theSecurities Act (Ontario) and the equivalent legislation in British Columbia, subject to the defences, limitations and other terms thereof, as if such misrepresentation were contained in the Final Prospectus.
108
Financial Statements of Scienion
We have applied to the Ontario securities regulatory authority and expect to apply to the British Columbia and Alberta securities regulatory authorities for an exemption from section 5.1 of National Instrument 52-107,Acceptable Accounting Principles, Auditing Standards ("NI 52-107") for a decision that we be exempted from the following requirements of NI 52-107:
- •
- section 4.11 (4) of NI 52-107 which requires Scienion's audited annual financial statements be reconciled to the accounting principles used to prepare our financial statements and the requirement to provide the disclosure required by section 4.11 (4) in the notes to those financial statements;
- •
- section 4.12 (1) of NI 52-107 which requires Scienion's audited annual financial statements be audited in accordance with Canadian or United States generally accepted auditing standards, so as to permit such financial statements to be audited in accordance with International Standards on Auditing ("ISA");
- •
- section 4.12 (2)(a) of NI 52-107 that the auditor's report for Scienion's financial statements be accompanied by a statement by the auditor describing any material differences in the form and content of the auditor's report as compared to an auditor's report prepared in accordance with generally accepted auditing standards determined with reference to the Handbook of the Canadian Institute of Chartered Accountants ("Canadian GAAS"), and indicating that an auditor's report prepared in accordance with such standards would express an unmodified opinion; and
- •
- section 5.1 of NI 52-107 for a decision that we be exempted from the following requirements of NI 52-107:
- •
- section 4.11 (4) of NI 52-107 that Scienion's audited annual financial statements which are required to be included in the business acquisition report to be filed in respect of the acquisition of Scienion (the "BAR"), in accordance with section 8.2 of National Instrument 51-102,Continuous Disclosure Obligations ("NI 51-102"), be reconciled to the accounting principles used to prepare our financial statements and the requirement to provide the disclosure required by section 4.11 (4) in the notes to such financial statements;
- •
- section 4.12 (1) of NI 52-107 that Scienion's audited annual financial statements to be included in the BAR be audited in accordance with Canadian or United States generally accepted auditing standards, so as to permit such financial statements to be audited in accordance with ISA; and
- •
- section 4.12 (2)(a) of NI 52-107 that the auditor's report for Scienion's financial statements to be included in the BAR be accompanied by a statement by the auditor describing any material differences in the form and content of the auditor's report as compared to an auditor's report prepared in accordance with Canadian GAAS, and indicating that an auditor's report prepared in accordance with such standards would express an unmodified opinion.
We expect to receive the above noted exemptive relief from the appropriate securities regulatory authorities.
INTEREST OF EXPERTS
Collins Barrow Toronto LLP, Licensed Public Accountants is an independent auditor within the meaning of the Rules of Professional Conduct of the Institute of Chartered Accountants of Ontario.
109
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS OF SQI DIAGNOSTICS INC.
| | | | |
| 1. | | Unaudited interim consolidated financial statements for the three and nine months ended June 30, 2011 | | SQI-F-2 |
2. |
|
Supplemental Note regarding the Reconciliation of the Interim Consolidated Financial Statements for the Three and Nine Month Periods Ended June 30, 2011 and 2010 with U.S. GAAP |
|
SQI-F-13 |
3. |
|
Auditors' Report |
|
SQI-F-20 |
4. |
|
Audited consolidated financial statements for the years ended September 30, 2010 and 2009 |
|
SQI-F-21 |
5. |
|
Auditors' Report |
|
SQI-F-35 |
6. |
|
Supplemental Note Regarding the Reconciliation of the Consolidated Financial Statements for the Years Ended September 30, 2010 and 2009 with U.S. GAAP |
|
SQI-F-36 |
SQI-F-1
SQI Diagnostics Inc.
INTERIM CONSOLIDATED BALANCE SHEETS
(Unaudited)
(Amounts are in thousands of dollars)
| | | | | | | | | | |
| | Note | | As at
June 30,
2011 | | As at
September 30,
2010 | |
|---|
| |
| | (Unaudited)
| | (audited)
| |
|---|
ASSETS | | | | | | | | | | |
Current | | | | | | | | | | |
Cash and cash equivalents | | | | | $ | 2,817 | | $ | 9,408 | |
Amounts receivable | | | | | | 12 | | | 3 | |
Inventory | | | 4 | | | 512 | | | 260 | |
Prepaids and deposits | | | | | | 425 | | | 165 | |
Due from related party | | | 5 | | | — | | | 66 | |
| | | | | | | | | |
| | | | | | 3,766 | | | 9,902 | |
Due from related party | | | 5 | | | — | | | 32 | |
Deferred costs | | | 17 | | | 433 | | | — | |
Property and equipment | | | 6 | | | 2,717 | | | 2,713 | |
Patents and trademarks | | | | | | 572 | | | 469 | |
| | | | | | | | | |
| | | | | $ | 7,488 | | $ | 13,134 | |
| | | | | | | | | |
Liabilities | | | | | | | | | | |
Current | | | | | | | | | | |
Accounts payable and accrued liabilities | | | | | $ | 1,528 | | $ | 972 | |
Shareholders' Equity | | | | | | | | | | |
Capital stock | | | 8 | | | 35,377 | | | 35,026 | |
Warrants | | | 9 | | | 1,614 | | | 1,799 | |
Employee share purchase loan | | | 8 | | | — | | | (10 | ) |
Contributed surplus | | | 11 | | | 9,274 | | | 8,8832 | |
Deficit | | | | | | (40,305 | ) | | (33,485 | ) |
| | | | | | | | | |
| | | | | | 5,960 | | | 12,162 | |
| | | | | | | | | |
| | | | | $ | 7,488 | | $ | 13,134 | |
| | | | | | | | | |
Subsequent Events (Note 17)
Contingencies (Note 14)
| | | | |
| Approved by the Board | | | | |
|
|
(Signed)PETER WINKLEY
Director |
|
(Signed)CLAUDE RICKS
Director |
See accompanying notes
SQI-F-2
SQI Diagnostics Inc.
INTERIM CONSOLIDATED STATEMENT OF OPERATIONS AND DEFICIT
(Unaudited)
(Amounts are in thousands of dollars except per share amounts)
| | | | | | | | | | | | | | | | |
| | Note | | Three Month
Period
Ended
June 30,
2011 | | Three Month
Period
Ended
June 30,
2010 | | Nine Month
Period
Ended
June 30,
2011 | | Nine Month
Period
Ended
June 30,
2010 | |
|---|
Revenue | | | | | | | | | | | | | | | | |
Product sales | | | | | $ | 9 | | $ | — | | $ | 22 | | $ | — | |
Consulting fees | | | 7 | | | — | | | 6 | | | 9 | | | 21 | |
| | | | | | | | | | | | | |
| | | | | | 9 | | | 6 | | | 31 | | | 21 | |
| | | | | | | | | | | | | |
Expenses | | | | | | | | | | | | | | | | |
Salaries and wages | | | | | | 275 | | | 139 | | | 607 | | | 404 | |
General and administrative | | | 7 | | | 125 | | | 123 | | | 449 | | | 324 | |
Professional and consulting fees | | | 17 | | | 469 | | | 140 | | | 669 | | | 437 | |
Sales and marketing | | | | | | 114 | | | 116 | | | 329 | | | 309 | |
Stock-based compensation | | | 13 | | | 144 | | | 69 | | | 372 | | | 184 | |
Research and development costs | | | | | | 1,443 | | | 1,111 | | | 4,056 | | | 3,438 | |
Amortization — patents & trademarks | | | | | | 31 | | | 29 | | | 88 | | | 85 | |
Amortization — property & equipment | | | | | | 113 | | | 101 | | | 337 | | | 310 | |
| | | | | | | | | | | | | |
| | | | | | 2,714 | | | 1,828 | | | 6,907 | | | 5,491 | |
| | | | | | | | | | | | | |
Operating loss before interest | | | | | | (2,705 | ) | | (1,822 | ) | | (6,876 | ) | | (5,470 | ) |
Interest Income | | | | | | 14 | | | 8 | | | 56 | | | 20 | |
Interest Expense | | | | | | — | | | 2 | | | — | | | (2 | ) |
| | | | | | | | | | | | | |
Net loss | | | | | | (2,691 | ) | | (1,812 | ) | | (6,820 | ) | | (5,452 | ) |
Deficit at beginning of period | | | | | | (37,614 | ) | | (29,052 | ) | | (33,485 | ) | | (25,412 | ) |
| | | | | | | | | | | | | |
Deficit at end of period | | | | | $ | (40,305 | ) | $ | (30,864 | ) | $ | (40,305 | ) | $ | (30,864 | ) |
| | | | | | | | | | | | | |
Weighted average number of shares | | | | | | 33,936 | | | 30,790 | | | 33,849 | | | 29,553 | |
| | | | | | | | | | | | | |
Basic and diluted loss per share | | | | | $ | (0.08 | ) | $ | (0.06 | ) | $ | (0.20 | ) | $ | (0.18 | ) |
SQI-F-3
SQI Diagnostics Inc.
INTERIM CONSOLIDATED STATEMENT OF CASH FLOWS
(Unaudited)
(Amounts are in thousands of dollars)
| | | | | | | | | | | | | |
| | Three Month
Period
Ended
June 30,
2011 | | Three Month
Period
Ended
June 30,
2010 | | Nine Month
Period
Ended
June 30,
2011 | | Nine Month
Period
Ended
June 30,
2010 | |
|---|
Cash flow from operating activities | | | | | | | | | | | | | |
Loss for the period | | $ | (2,691 | ) | $ | (1,812 | ) | $ | (6,820 | ) | $ | (5,452 | ) |
Add items not affecting cash | | | | | | | | | | | | | |
Amortization — patents & trademarks | | | 31 | | | 29 | | | 88 | | | 85 | |
— property and equipment | | | 113 | | | 101 | | | 337 | | | 310 | |
Stock-based compensation | | | 144 | | | 69 | | | 372 | | | 184 | |
Loss on sale of property and equipment | | | — | | | — | | | 43 | | | — | |
Interest accrual | | | — | | | — | | | (1 | ) | | — | |
| | | | | | | | | | |
| | | (2,403 | ) | | (1,613 | ) | | (5,981 | ) | | (4,873 | ) |
Changes in non-cash working capital items | | | | | | | | | | | | | |
Amounts receivable | | | (9 | ) | | (47 | ) | | (8 | ) | | (95 | ) |
Investment tax credit recoverable | | | 300 | | | (295 | ) | | — | | | (295 | ) |
Due from related party | | | 99 | | | — | | | 99 | | | 7 | |
Inventory | | | 1 | | | (153 | ) | | (252 | ) | | (458 | ) |
Prepaids and deposits | | | (122 | ) | | (75 | ) | | (260 | ) | | (95 | ) |
Accounts payable and accrued liabilities | | | 795 | | | 100 | | | 555 | | | 305 | |
| | | | | | | | | | |
| | | (1,339 | ) | | (2,083 | ) | | (5,847 | ) | | (5,504 | ) |
| | | | | | | | | | |
Cash flows used in investing activities | | | | | | | | | | | | | |
Purchase of property and equipment | | | (36 | ) | | (266 | ) | | (368 | ) | | (369 | ) |
Additions to patents and trademarks | | | (94 | ) | | (21 | ) | | (191 | ) | | (99 | ) |
Sale of property and equipment | | | — | | | — | | | 2 | | | — | |
| | | | | | | | | | |
| | | (130 | ) | | (287 | ) | | (557 | ) | | (468 | ) |
| | | | | | | | | | |
Cash flows from financing activities | | | | | | | | | | | | | |
Repayment of loans payable | | | — | | | (1 | ) | | — | | | (3 | ) |
Repayment of shareholder loan | | | 10 | | | — | | | 10 | | | — | |
Proceeds from private placement and exercise of warrants and options, net of share issuance costs | | | 40 | | | 2,093 | | | 236 | | | 8,561 | |
Deferred costs | | | (433 | ) | | — | | | (433 | ) | | — | |
| | | | | | | | | | |
| | | (383 | ) | | 2,092 | | | (187 | ) | | 8,558 | |
| | | | | | | | | | |
Increase (decrease) in cash during the period | | | (1,852 | ) | | (278 | ) | | (6,591 | ) | | 2,586 | |
Cash at beginning of period | | | 4,669 | | | 6,044 | | | 9,408 | | | 3,180 | |
| | | | | | | | | | |
Cash at end of period | | $ | 2,817 | | $ | 5,766 | | $ | 2,817 | | $ | 5,766 | |
| | | | | | | | | | |
Supplemental disclosure | | | | | | | | | | | | | |
Cash paid for interest | | | — | | $ | (2 | ) | | — | | $ | 2 | |
SQI-F-4
SQI Diagnostics Inc.
NOTES TO INTERIM CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
1. NATURE OF OPERATIONS
SQI Diagnostics Inc., (the "Company"), has its head office and development centre in Toronto, Ontario. The Company is a life sciences company that develops proprietary and commercializes proprietary technologies and products for advanced microarray diagnostics.. Our goal is to become a leader in the development and commercialization of microarray and multiplexed diagnostics by offering our customers a comprehensive "turnkey" solution that increases the efficiency and ease of diagnostic testing and test development.
During fiscal 2009 the Company obtained Health Canada licenses and self authorization to sell in the EU and during fiscal 2010 received United States Food & Drug Administration ("FDA") clearance of its SQiDworks and IgX PLEX Rheumatoid Arthritis (RA) system. During fiscal 2010 the Company obtained a Health Canada license for its IgX PLEX Celiac™ microarray test kits that run on the Company's automated SQiDworks™ platform. During the nine months ended June 30, 2011 the company obtained FDA clearance for its IgX PLEX Celiac™ panel and obtained a Health Canada license and self authorization to sell in the EU its second generation fully quantitative IgX PLEX Celiac™ panel. The Company has earned limited revenues from its IgX PLEX RA™ and IgX PLEX Celiac™ test kits run on installed SQiDworks™ platforms to date, and as such is considered to be a development stage company. The Company has a pipeline of additional autoimmune diagnostic products in various stages of development and commercialization. The Company expects to generate revenues from its IgXPLEX RA and IgXPLEX Celiac products as it grows its installed base of customers as well as from products to be launched as they complete commercialization. The continuation of the Company's research, development and commercialization activities is dependent upon the Company's ability to successfully generate product revenues, or to finance its cash requirements through further equity financings.
2. SIGNIFICANT ACCOUNTING POLICIES
These consolidated financial statements have been prepared by management in accordance with Canadian generally accepted accounting principles and follow the same accounting policies and methods of their application as the most recent audited consolidated financial statements for the years ended September 30, 2010 and 2009. These statements should be read in conjunction with the Company's above noted annual consolidated financial statements. The significant accounting policies are discussed below.
Basis of Consolidation
The consolidated financial statements include the accounts of the Company and its wholly owned subsidiary. Inter-company balances and transactions are eliminated upon consolidation.
Cash and Cash Equivalents
Cash and cash equivalents include bank deposits and highly liquid money market investments such as bankers acceptance notes, treasury bills, cashable money market funds, and cashable guaranteed investment certificates.
Inventory
Inventory is valued at the lower of cost and replacement cost, with cost determined on a first-in, first-out basis.
Property and Equipment
Property and equipment are recorded at cost and are amortized on the straight-line basis over their estimated useful lives as follows:
| | | | | | | | |
| | Computer hardware | | | — | | 3 years | | |
| | Computer software | | | — | | 3 years | | |
| | Laboratory fixtures and equipment | | | — | | 10 years | | |
| | Office equipment | | | — | | 10 years | | |
| | Leasehold improvements | | | — | | 10 years | | |
Patents and Trademarks
The costs relating to initial patent and trademark fees are deferred and amortized over 10 years on a straight-line basis. Patents and trademarks are recorded net of accumulated amortization of $715,000 (September 30, 2010 — $627,000).
SQI-F-5
SQI Diagnostics Inc.
NOTES TO INTERIM CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(Unaudited)
2. SIGNIFICANT ACCOUNTING POLICIES (Continued)
Research and Development Costs
Research costs are charged to operations in the period in which they are incurred. Development costs are expensed as incurred or deferred if they meet the criteria for deferral under Canadian generally accepted accounting principles and are expected to provide future benefits with reasonable certainty.
At June 30, 2011, the Company was developing IgXPLEX diagnostics assays for celiac, vasculitis, lupus (SLE), Crohn's (IBD), antiphospholipid syndrome and our second generation, fully quantitative IgXPLEX RA assay. The Company continued its work to complete scientific discovery and assay design work for a diagnostic assay to detect and measure infliximab (also referred to as anti-TNF) in the blood of autoimmune patients. Deferral criteria have not been met, and accordingly, all development costs have been expensed in the period.
Impairment of Long-Lived Assets
Long-lived assets comprise property and equipment and intangible assets with finite lives (patents and trademarks). The Company recognizes an impairment loss for a long-lived asset when events or changes in circumstances cause its carrying value to exceed the total undiscounted cash flows expected from its use and eventual disposition. An impairment loss is measured as the excess of the carrying value of the asset over its fair value.
Revenue Recognition
Product sales are recognized upon the shipment of products to customers, if a signed contract exists, the sales price is fixed and determinable, collection of the resulting receivables is reasonably assured and any uncertainties with regard to customer acceptance are insignificant. Sales are recorded net of discounts and sales returns.
The Company also provides consulting services from time to time. Consulting fee revenue is recognized when services are completed, amounts are invoiced to customers and collectability is reasonably assured.
Stock-Based Compensation and Other Stock-Based Payments
The Company applies a fair value based method of accounting for all stock-based payments. Accordingly, stock-based payments are measured at the fair value of the consideration received or the fair value of the equity instruments issued or liabilities incurred, whichever is more reliably measurable. Stock-based compensation is charged to operations over the vesting period and the offset is credited to contributed surplus. Consideration received upon the exercise of stock options is credited to share capital at which time the related contributed surplus is transferred to share capital.
Foreign Currency Translation
Monetary assets and liabilities denominated in foreign currencies are translated to Canadian dollars at exchange rates in effect at the balance sheet date. Non-monetary assets and liabilities are translated at rates of exchange in effect at each transaction date. Revenue and expenses are translated at the rate of exchange at each transaction date. Gains or losses on translation are included in operations.
Income Taxes
The Company follows the liability method of accounting for income taxes. Under this method, future income tax assets and liabilities are determined based on temporary differences between financial reporting and tax bases of assets and liabilities, as well as for the benefit of losses available to be carried forward to future years for tax purposes. Future income tax assets and liabilities are measured using enacted or substantively enacted tax rates and laws that will be in effect when the differences are expected to reverse. Future income tax assets are recorded in the financial statements if realization is considered more likely than not.
Investment Tax Credits
Investment tax credits are accrued when qualifying expenditures are incurred and there is reasonable assurance that the credits will be realized. Investment tax credits earned with respect to current expenditures for qualified research and development activities are included in the statements of operations as a reduction of research and development costs. Investment tax credits associated with capital expenditures are reflected as reductions in the carrying amounts of capital assets. During the three and nine months ended June 30, 2011 the company recorded $Nil and $300,000, respectively, as a reduction of research and development costs ($295,000 for the three and nine months ended June 30, 2010).
SQI-F-6
SQI Diagnostics Inc.
NOTES TO INTERIM CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(Unaudited)
2. SIGNIFICANT ACCOUNTING POLICIES (Continued)
Financial Instruments
Financial instruments are measured initially at fair value and thereafter based on their classification.
The Company has classified and measured its financial instruments as follows:
| | | | | |
| | Financial Instrument | | Classification | | Measurement Basis |
|---|
| | Cash and cash equivalents | | Held-for-trading | | Fair value |
| | Amounts receivable | | Loans and receivable | | Amortized cost |
| | Investment tax credits receivable | | Loans and receivable | | Amortized cost |
| | Due from related party | | Loans and receivable | | Amortized cost |
| | Accounts payable and accruals | | Other financial liabilities | | Amortized cost |
Comprehensive Income
The Company has not presented a statement of comprehensive income as it has no other comprehensive income.
Loss Per Share
Basic loss per share is computed using the weighted average number of common shares outstanding during the period. Diluted loss per share is computed using the weighted average number of common and potential common shares outstanding during the period. Potential common shares consist of the incremental common shares issuable upon the exercise of stock options and warrants determined using the treasury stock method.
Use of Estimates
The preparation of financial statements in conformity with Canadian generally accepted accounting principles requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amount of revenue and expenses during the period. Actual results could differ from those estimates.
Significant areas requiring the use of management estimates relate to the determination of the useful lives of property and equipment and patents and trademarks for amortization purposes, valuation of ITC's receivable, valuation of stock options and warrants and valuation allowance on future tax assets.
3. RECENT ACCOUNTING PRONOUNCEMENTS
- (i)
- Adoption of New Accounting Pronouncements
Business Combinations
In January 2009, the CICA issued Section 1582, Business Combinations, which replaces former guidance on business combinations. Section 1582 establishes principles and requirements of the acquisition method for business combinations and related disclosures. In addition, the CICA issued Sections 1601, Consolidated Financial Statements, and 1602, Non-Controlling Interests, which replaces the existing guidance. Section 1601 establishes standards for the preparation of consolidated financial statements, while section 1602 provides guidance on accounting for a non-controlling interest in a subsidiary in consolidated financial statements subsequent to a business combination.
These standards apply prospectively to business combinations for which the acquisition date is on or after the beginning of the first annual reporting period beginning on or after January 1, 2011 with earlier application permitted. The Company adopted the new standards on October 1, 2010.
- (ii)
- Recent Accounting Pronouncements Issued and Not Yet Applied
International Financial Reporting Standards
The CICA plans to converge Canadian Generally Accepted Accounting Principles with International Financial Reporting Standards ("IFRS") over a transition period expected to end in 2011, when IFRS will be fully adopted. The transition date of October 1, 2010 for the Company will require restatement for comparative purposes of amounts reported by the Company for the year ended September 30,
SQI-F-7
SQI Diagnostics Inc.
NOTES TO INTERIM CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(Unaudited)
3. RECENT ACCOUNTING PRONOUNCEMENTS (Continued)
2011. The Company continues to monitor, assess and quantify the impact of the convergence of Canadian GAAP and IFRS on its financial statements.
4. INVENTORY
Inventory consists of component parts that are to be used in the future production of SQiDworks™ Platform and IgXPLEX consumable assays.
5. DUE FROM RELATED PARTY
| | | | | | | | |
| |
| | June 30, 2011 | | September 30, 2010 | |
|---|
| |
| | (unaudited)
($000s)
| | (audited)
($000s)
| |
|---|
| | Amount due from an officer and director(i) and (ii) | | $ | — | | $ | 98 | |
| | Less: Current portion | | | — | | | (66 | ) |
| | | | | | | |
| | | | $ | — | | $ | 32 | |
| | | | | | | |
- (i)
- Amount due was secured, principal amount of $98,000 repayable in three equal payments on September 1, 2010, 2011 and 2012. Terms of the promissory note were amended on April 16, 2010 as follows: interest bearing at Canada Revenue Agency prescribed rate for taxable benefits to employees and shareholders on interest-free and low-interest loans, which was 1% per annum at June 30, 2011. Prior to amendment, the loan was bearing interest at 4.25% per annum during the year ended December 31, 2009 and non-interest bearing from October 1, 2009 to April 15, 2010.
- (ii)
- As at June 30, 2011, the loan was paid in full.
6. PROPERTY AND EQUIPMENT
| | | | | | | | | | | |
| | As at June 30, 2011 (unaudited): | | Cost | | Accumulated
Amortization | | Net | |
|---|
| |
| | ($000s)
| | ($000s)
| | ($000s)
| |
|---|
| | Computer hardware | | $ | 266 | | $ | 174 | | $ | 92 | |
| | Computer software | | | 179 | | | 143 | | | 36 | |
| | Laboratory fixtures and equipment | | | 4,343 | | | 1,888 | | | 2,455 | |
| | Office equipment | | | 176 | | | 135 | | | 41 | |
| | Leasehold improvements | | | 265 | | | 172 | | | 93 | |
| | | | | | | | | |
| | | | $ | 5,229 | | $ | 2,512 | | $ | 2,717 | |
| | | | | | | | | |
| | | | | | | | | | | |
| | As at September 30, 2010 (audited): | | Cost | | Accumulated
Amortization | | Net | |
|---|
| |
| | ($000s)
| | ($000s)
| | ($000s)
| |
|---|
| | Computer hardware | | $ | 193 | | $ | 141 | | $ | 52 | |
| | Computer software | | | 153 | | | 130 | | | 23 | |
| | Laboratory fixtures and equipment | | | 4,172 | | | 1,670 | | | 2,502 | |
| | Office equipment | | | 176 | | | 128 | | | 48 | |
| | Leasehold improvements | | | 263 | | | 157 | | | 106 | |
| | | | | | | | | |
| | | | $ | 4,957 | | $ | 2,226 | | $ | 2,731 | |
| | | | | | | | | |
7. RELATED PARTY TRANSACTIONS
Transactions with related parties occur in the normal course of business and are measured at the exchange amount. Related party transactions have been listed below, unless they have been disclosed elsewhere in the financial statements.
SQI-F-8
SQI Diagnostics Inc.
NOTES TO INTERIM CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(Unaudited)
7. RELATED PARTY TRANSACTIONS (Continued)
Included in general and administrative expense for the three month period ended June 30, 2011 is $13,000 (three month period ended June 30, 2010 — $12,000) compared to $38,000 for the nine month period ended June 30, 2011 (nine month period ended June 30, 2010 $37,000), related to recovery of occupancy costs from a corporation in which an officer of the Company is also an officer. Consulting fee revenue of NIL for the three month ended June 30, 2011 (three month ended June 30, 2010 — $6,000) was earned from this corporation compared to $9,000 for the nine month period ended June 30, 2011 (nine month period ended June 30, 2010 $21,000). At quarter-end, $1,000 (September 30, 2010 — $1,000) due from this corporation is included in amounts receivable.
8. CAPITAL STOCK
- (a)
- The Company has authorized an unlimited number of common shares. Issued common shares are detailed in the schedule below:
| | | | | | | | |
| |
| | Number | | Value | |
|---|
| |
| | (000s)
| | ($000s)
| |
|---|
| | Balance, September 30, 2010 (audited) | | | 33,758 | | $ | 35,026 | |
| | | | | | | |
| | Options exercised | | | 8 | | | 22 | |
| | | | | | | |
| | Balance, December 31, 2010 | | | 33,766 | | $ | 35,048 | |
| | | | | | | |
| | Options exercised | | | 40 | | | 104 | |
| | Warrants exercised (Note 9(i)) | | | 107 | | | 193 | |
| | Share issuance costs | | | — | | | (16 | ) |
| | | | | | | |
| | Balance, March 31, 2011 | | | 33,913 | | $ | 35,329 | |
| | | | | | | |
| | Options exercised | | | 33 | | | 48 | |
| | | | | | | |
| | Balance, June 30, 2011 | | | 33,946 | | $ | 35,377 | |
| | | | | | | |
- (b)
- During the period ended September 30, 2007, the Company made a non-interest bearing loan to an officer, which was used to acquire 100,000 common shares. The loan has been accounted for as a share purchase loan and, accordingly, the $10,000 loan balance has been deducted from share capital. The loan was paid in full during the three months ended June 30, 2011.
9. WARRANT CAPITAL
| | | | | | | | |
| |
| |
| | (audited)
| |
|---|
| |
| | Nine Months
Ended
June 30, 2011 | | Year Ended
September 30, 2010 | |
|---|
| |
| | ($000s)
| | ($000s)
| |
|---|
| | Balance, beginning of period | | $ | 1,799 | | $ | 461 | |
| | Issued on private placement | | | — | | | 1,424 | |
| | Finder warrants | | | — | | | 187 | |
| | Exercise of warrants(i) | | | (60 | ) | | (213 | ) |
| | Expiry of warrants(ii) | | | (125 | ) | | (60 | ) |
| | | | | | | |
| | Balance, end of period | | $ | 1,614 | | $ | 1,799 | |
| | | | | | | |
- (i)
- During the quarter-ended March 31, 2011, 106,520 warrants with an expiry of January 22, 2011 were exercised for proceeds of $133,000. Upon exercise $60,000 was transferred to share capital.
- (ii)
- During the quarter-ended December 31, 2010 143,886 warrants with an expiry of December 4, 2010 expired unexercised, and $125,000 was transferred to contributed surplus upon expiry.
SQI-F-9
SQI Diagnostics Inc.
NOTES TO INTERIM CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(Unaudited)
10. WARRANTS
The Company had the following warrants outstanding at June 30, 2011:
| | | | | | | | | |
| | Number of Warrants | | Purchase Price | | Expiry Date | |
|---|
| | (000s)
| |
| |
| |
|---|
| | | 1,199 | | $ | 4.00 | | | December 4, 2011 | |
| | | 237 | | $ | 1.90 | | | December 23, 2011 | |
| | | 1,140 | | $ | 5.00 | | | August 12, 2012 | |
| | | 57 | | $ | 2.50 | | | August 12, 2012 | |
| | | | | | | | | |
| | | 2,633 | | | | | | | |
| | | | | | | | | |
11. CONTRIBUTED SURPLUS
| | | | | | | | |
| |
| |
| | (audited)
| |
|---|
| |
| | Nine Months
Ended
June 30, 2011 | | Year Ended
September 30, 2010 | |
|---|
| |
| | ($000s)
| | ($000s)
| |
|---|
| | Balance, beginning of period | | $ | 8,832 | | $ | 8,431 | |
| | Stock-based compensation (Note 13) | | | 372 | | | 402 | |
| | Options exercised (Note 12 (i)) | | | (55 | ) | | (61 | ) |
| | Warrants expired (Note 9(ii)) | | | 125 | | | 60 | |
| | | | | | | |
| | Balance, end of period | | $ | 9,274 | | $ | 8,832 | |
| | | | | | | |
12. STOCK OPTIONS
The Company maintains a Stock Option Plan (the "Plan") for the benefit of directors, officers, employees and consultants. The maximum number of common shares reserved for issuance under the Plan, together with any other employee stock option plans, options for services and employee share purchase plans, will not exceed 10% of the issued and outstanding shares at the time of the option grant. Options granted pursuant to the Plan will have terms not to exceed five years, and are granted at an option price which will not be less than the fair market price at the time the options are granted. All options granted to individual optionees, other than consultants, generally vest in three equal installments over a period of 36 months.
The following summarizes the stock option activities under the Plan:
| | | | | | | | | | | | | | |
| |
| | Nine Month Period Ended
June 30, 2011 | | Nine Month Period Ended
June 30, 2010 | |
|---|
| |
| | Number of
Options | | Weighted Average
Exercise Price | | Number of
Options | | Weighted Average
Exercise Price | |
|---|
| |
| | (000s)
| |
| | (000s)
| |
| |
|---|
| | Beginning balance | | | 1,814 | | $ | 1.77 | | | 2,368 | | $ | 1.31 | |
| | Granted | | | 175 | | $ | 2.88 | | | 209 | | $ | 2.32 | |
| | Exercised(i) | | | (81 | ) | $ | 1.45 | | | (830 | ) | $ | 0.81 | |
| | Cancelled/Expired | | | | | | | | | (122 | ) | $ | 0.88 | |
| | Forfeited | | | (49 | ) | $ | 2.26 | | | (2 | ) | $ | 1.74 | |
| | | | | | | | | | | |
| | Ending balance | | | 1,859 | | $ | 1.85 | | | 1,623 | | $ | 1.67 | |
| | | | | | | | | | | |
| | Exercisable | | | 1,519 | | $ | 1.74 | | | 1,021 | | $ | 1.37 | |
| | | | | | | | | | | |
- (i)
- On exercise of stock options, $55,000 (nine months ended June 30, 2010 — $0) was transferred from contributed surplus to share capital.
SQI-F-10
SQI Diagnostics Inc.
NOTES TO INTERIM CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(Unaudited)
12. STOCK OPTIONS (Continued)
| | | | | | | | | |
| | Number of Options | | Exercise Price | | Expiry Date | |
|---|
| | (000s)
| |
| |
| |
|---|
| | | 58 | | $ | 1.20 | | | August 29, 2011 | |
| | | 143 | | $ | 1.74 | | | August 7, 2012 | |
| | | 50 | | $ | 1.50 | | | October 23, 2012 | |
| | | 758 | | $ | 1.60 | | | February 26, 2013 | |
| | | 255 | | $ | 1.75 | | | August 26, 2013 | |
| | | 78 | | $ | 1.30 | | | May 22, 2014 | |
| | | 25 | | $ | 3.26 | | | November 3, 2014 | |
| | | 107 | | $ | 2.25 | | | February 22, 2015 | |
| | | 50 | | $ | 2.10 | | | May 27, 2015 | |
| | | 175 | | $ | 2.50 | | | August 16, 2015 | |
| | | 100 | | $ | 2.90 | | | October 4, 2015 | |
| | | 60 | | $ | 2.85 | | | January 31, 2016 | |
| | | | | | | | | |
| | | 1,859 | | | | | | | |
| | | | | | | | | |
13. STOCK-BASED COMPENSATION
The fair value of the options granted during the nine month period ended June 30, 2011 was $327,000 (nine month period ended June 30, 2010 — $315,000), which will be recognized over the vesting period of 36 months. The total compensation expense for three and nine month period ended June 30, 2011 was $144,000 and $372,000 respectively (three and nine month period ended June 30, 2010 — $69,000 and $184,000 respectively). The total amount credited to contributed surplus for the nine month period ended June 30, 2011 was $144,000 and $372,000 respectively (three and nine month period ended June 30, 2010 — $69,000 and $184,000 respectively).
The fair value of each option granted has been estimated at the date of grant or the date when it became measurable using the Black-Scholes option pricing model with the following weighted average assumptions at the measurement date:
| | | | | | | | |
| |
| | Nine Months Ended
June 30, 2011 | | Nine Months Ended
June 30, 2010 | |
|---|
| | Dividend yield | | | 0 | % | | 0 | % |
| | Expected volatility | | | 80 | % | | 80 | % |
| | Risk-free interest rate | | | 2.31 | % | | 1.98 | % |
| | Expected life (years) | | | 5.00 | | | 5.00 | |
| | | | | | | |
| | Weighted average grant date fair value | | $ | 1.87 | | $ | 1.51 | |
| | | | | | | |
The Company has assumed no forfeiture rate as adjustments for actual forfeitures are made in the year they occur.
14. CONTINGENCIES
In the ordinary course of business, the Company may be contingently liable for litigation and claims with customers, suppliers, former employees or competitors. Management believes that adequate provisions have been recorded in accounts where required.
15. CAPITAL RISK MANAGEMENT
The Company's objective when managing capital is to safeguard the Company's ability to continue as a going concern so that it can complete its lead assay commercialization efforts and receive the required regulatory approvals to sell and market its products and provide returns for shareholders and benefits for other stakeholders.
The capital structure of the Company consists of shareholders' equity. The Company is not subject to externally imposed capital requirements.
SQI-F-11
SQI Diagnostics Inc.
NOTES TO INTERIM CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(Unaudited)
16. FINANCIAL RISK MANAGEMENT
Currency risk is the risk that the fair value or future cash flows of a financial instrument will fluctuate because of changes in foreign exchange rates. The Company is exposed to currency risk due to its purchases in US dollars. A 1% change in the foreign exchange rate would result in a change of approximately $13,000 in the reported profit and loss.
- d)
- Liquidity Risk
Liquidity risk is the risk that the Company will encounter difficulty in meeting obligations associated with financial liabilities. The Company's accounts payable and accrued liabilities are all current. The Company ensures that it has sufficient capital to meet short term financial obligations after taking into account its cash on hand.
17. SUBSEQUENT EVENTS
On July 4, 2011 the Company announced that it has entered into an agreement to acquire all of the share capital of Scienion AG, a German-based microarray manufacturing equipment and microarray print and development services company for a purchase price of $15,000,000 in cash and the issuance of 735,294 common shares in the capital of SQI. The closing of the transaction is subject to a number of customary closing conditions and regulatory approvals, and is subject to financing.
On July 7, 2011 the Company announced that it has filed a preliminary short form PREP based prospectus with the Ontario Securities Commission and a registration statement on form F-10 with the U.S. Securities and Exchange Commission in connection with a proposed offering of common shares. The anticipated amount of the offering is approximately $30,000,000 of which $15,000,000 will be used in payment of the cash portion of the purchase price of the Company's acquisition of Scienion. The completion of the public offering is subject to and conditional upon the receipt of all necessary approvals, including regulatory approval.
At June 30, 2011, the Company had incurred costs relating to the planned offering in the amount of $433,000 (September 30, 2010 — $Nil). These amounts have been reflected in deferred costs and will be offset against share capital upon completion of the offering. The Company also incurred cost relating to the acquisition as at June 30, 2011 in the amount of $324,000 (June 30, 2010 — $Nil). These amounts have been included in professional and consulting fees.
SQI-F-12
SQI Diagnostics Inc.
SUPPLEMENTAL NOTE REGARDING THE RECONCILIATION OF THE INTERIM
CONSOLIDATED FINANCIAL STATEMENTS FOR THE THREE AND NINE MONTH PERIODS
ENDED JUNE 30, 2011 AND 2010 WITH U.S. GAAP
(unaudited)
Dated September 1, 2011
The Company follows Canadian generally accepted accounting principles ("Canadian GAAP") which is different in some respects from the accounting principles applicable in the United States ("U.S. GAAP") and from practices prescribed by the United States Securities and Exchange Commission. These differences in Canadian GAAP and U.S. GAAP that would require restatement of any of the Company's reported financial statements and certain additional disclosures required under U.S. GAAP are detailed below:
- (i)
- Leasehold Improvements
The company amortizes leasehold improvements over their expected useful life. U.S. GAAP requires that leasehold improvements be amortized over the lesser of their useful life or the term of the lease. As the Company does not have a term lease in place for its premises, expenditures relating to leasehold improvements are booked to general and administrative expense in the statement of operations. Accordingly, the following adjustments would be required under U.S. GAAP:
(amounts in thousands of dollars)
| | | | | | | | |
| |
| | June 30,
2011 | | September 30,
2010 | |
|---|
| | Property and equipment — Canadian GAAP | | $ | 2,717 | | $ | 2,731 | |
| | Remove net book value of Leasehold Improvements | | | (93 | ) | | (106 | ) |
| | | | | | | |
| | Property and equipment — U.S. GAAP | | $ | 2,624 | | $ | 2,625 | |
| | | | | | | |
| | | | | | | | |
| |
| | June 30,
2011 | | September 30,
2010 | |
|---|
| | Deficit — Canadian GAAP | | $ | (40,305 | ) | $ | (33,485 | ) |
| | Net Adjustment | | | (93 | ) | | (106 | ) |
| | | | | | | |
| | Deficit — U.S. GAAP | | $ | (40,398 | ) | $ | (33,591 | ) |
| | | | | | | |
| | | | | | | | | | | | | | |
| |
| | Three Month
Period Ended
June 30, 2011 | | Three Month
Period Ended
June 30, 2010 | | Nine Month
Period Ended
June 30, 2011 | | Nine Month
Period Ended
June 30, 2010 | |
|---|
| | Net loss — Canadian GAAP | | $ | (2,691 | ) | $ | (1,812 | ) | $ | (6,820 | ) | $ | (5,452 | ) |
| | Net adjustment to expense Leasehold Improvements and remove amortization of Leasehold Improvements | | | 3 | | | (9 | ) | | 13 | | | (4 | ) |
| | | | | | | | | | | |
| | Net loss — U.S. GAAP | | $ | (2,688 | ) | $ | (1,821 | ) | $ | (6,807 | ) | $ | (5,456 | ) |
| | | | | | | | | | | |
| | | | | | | | | | | | | | |
| |
| | Three Month
Period Ended
June 30, 2011 | | Three Month
Period Ended
June 30, 2010 | | Nine Month
Period Ended
June 30, 2011 | | Nine Month
Period Ended
June 30, 2010 | |
|---|
| | Cash provided by (used in) operations — Canadian GAAP | | $ | (1,339 | ) | $ | (2,083 | ) | $ | (5,847 | ) | $ | (5,504 | ) |
| | Expense Leasehold Improvements | | | — | | | (16 | ) | | (2 | ) | | (23 | ) |
| | | | | | | | | | | |
| | Cash used in operations — U.S. GAAP | | $ | (1,339 | ) | $ | (2,099 | ) | $ | (5,849 | ) | $ | (5,527 | ) |
| | | | | | | | | | | |
| | Cash provided by (used in) investing — Canadian GAAP | | $ | (130 | ) | $ | (287 | ) | $ | (557 | ) | $ | (468 | ) |
| | Remove additions to Leasehold Improvements | | | — | | | 16 | | | 2 | | | 23 | |
| | | | | | | | | | | |
| | Cash provided by (used in) investing — U.S. GAAP | | $ | (130 | ) | $ | (271 | ) | $ | (555 | ) | $ | (445 | ) |
| | | | | | | | | | | |
SQI-F-13
SQI Diagnostics Inc.
SUPPLEMENTAL NOTE REGARDING THE RECONCILIATION OF THE INTERIM
CONSOLIDATED FINANCIAL STATEMENTS FOR THE THREE AND NINE MONTH PERIODS
ENDED JUNE 30, 2011 AND 2010 WITH U.S. GAAP (Continued)
(unaudited)
Dated September 1, 2011
- (ii)
- The Company is a development stage company. Accordingly, the following tables detailing the Company's Statements of Operations, Shareholders' Equity and Cash Flows from inception (December 1, 1999) to June 30, 2011 are required to be presented:
| | | | | | |
| |
| | From Inception to
June 30, 2011 | |
|---|
| | Revenue | | | | |
| | | Product Sales | | $ | 32 | |
| | | Consulting Fees | | | 1,269 | |
| | | | | |
| | | | | 1,301 | |
| | | | | |
| | Expenses | | | | |
| | | Salaries and wages | | | 4,462 | |
| | | General and administrative | | | 3,671 | |
| | | Professional and consulting fees | | | 3,680 | |
| | | Sales and marketing | | | 1,211 | |
| | | Stock-based compensation | | | 1,972 | |
| | | Research and development costs | | | 23,231 | |
| | | Amortization — patents and trademarks | | | 817 | |
| | | Amortization — property and equipment | | | 2,618 | |
| | | | | |
| | | | | 41,662 | |
| | | | | |
| | Loss before the undernoted items | | $ | (40,361 | ) |
| | Interest income | | |
437 | |
| | Interest expense | | | (87 | ) |
| | Settlement of litigation | | | (205 | ) |
| | Loss on disposal of property and equipment | | | (182 | ) |
| | | | | |
| | | | | (37 | ) |
| | | | | |
| | Net loss and deficit accumulated during the development stage, end of period | | $ | (40,398 | ) |
| | | | | |
SQI-F-14
SQI Diagnostics Inc.
SUPPLEMENTAL NOTE REGARDING THE RECONCILIATION OF THE INTERIM
CONSOLIDATED FINANCIAL STATEMENTS FOR THE THREE AND NINE MONTH PERIODS
ENDED JUNE 30, 2011 AND 2010 WITH U.S. GAAP (Continued)
(unaudited)
Dated September 1, 2011
Statement of Shareholders' Equity
(amounts in thousands of dollars, shares in thousands)
| | | | | | | | | | | | | | | | | |
| |
| | Share Capital | |
| |
| |
| |
|---|
| |
| |
| |
| | Deficit
Accumulated in
Development
Stage | |
|---|
| |
| | Number
of shares | | Amount | | Contributed
Surplus | | Warrant
Capital | |
|---|
| | Balance At Inception | | | 2 | | $ | 850 | | | | | | | | | — | |
| | Net loss | | | | | | | | | | | | | | $ | (4,631 | ) |
| | | | | | | | | | | | | |
| | Balance Dec 14, 2002, 2001 and 2000 | | | 2 | | $ | 850 | | | — | | | | | $ | (4,631 | ) |
| | | | | | | | | | | | | |
| | Net loss | | | | | | | | | | | | | | | (3,112 | ) |
| | | | | | | | | | | | | |
| | Balance December 14, 2003 | | | 2 | | $ | 850 | | | — | | | | | $ | (7,743 | ) |
| | | | | | | | | | | | | |
| | Issued in connection with a private placement | | | 10 | | | 1,516 | | | | | | | | | | |
| | Stock split | | | 5,977 | | | — | | | | | | | | | | |
| | Issued in connection with a private placement | | | 669 | | | 2,788 | | | | | | | | | | |
| | Forgiveness of indebtedness to parent on reorganization | | | | | | | | $ | 7,120 | | | | | | | |
| | Share issuance costs | | | | | | (342 | ) | | | | | | | | | |
| | Stock based compensation | | | | | | | | | 9 | | | | | | | |
| | Net loss | | | | | | | | | | | | | | | (1,559 | ) |
| | | | | | | | | | | | | |
| | Balance December 14, 2004 | | | 6,658 | | $ | 4,812 | | $ | 7,129 | | | — | | $ | (9,302 | ) |
| | | | | | | | | | | | | |
| | Issued on exercise of warrants | | | 100 | | | 10 | | | | | | | | | | |
| | Stock based compensation | | | | | | | | | 341 | | | | | | | |
| | Net loss | | | | | | | | | | | | | | | (1,804 | ) |
| | | | | | | | | | | | | |
| | Balance December 14, 2005 | | | 6,758 | | $ | 4,822 | | $ | 7,470 | | | — | | $ | (11,106 | ) |
| | | | | | | | | | | | | |
| | Conversion of convertible debentures | | | 2,073 | | | 4,147 | | | | | | | | | | |
| | Convertible debenture issuance costs | | | | | | (454 | ) | | | | | | | | | |
| | Stock based compensation | | | | | | | | | 268 | | | | | | | |
| | Net loss | | | | | | | | | | | | | | | (1,653 | ) |
| | | | | | | | | | | | | |
| | Balance December 14, 2006 | | | 8,831 | | $ | 8,515 | | $ | 7,738 | | | — | | $ | (12,759 | ) |
| | | | | | | | | | | | | |
| | Shares of Emblem Capital | | | 8,000 | | $ | 867 | | | | | | | | | | |
| | Share consolidation of Emblem Shares at 6 for 1 | | | (6,667 | ) | | | | | | | | | | | | |
| | Elimination of share capital due to reverse take over | | | | | | (867 | ) | | | | | | | | | |
| | Common shares of Umedik exchanged for shares SQI | | | (8,831 | ) | | | | | | | | | | | | |
| | Net asset value of SQI ascribed to issued shares | | | | | | 595 | | | | | | | | | | |
| | Issued on exercise of warrants | | | 78 | | | 8 | | | | | | | | | | |
| | Share issued to former shareholders of Umedik | | | 14,719 | | | | | | | | | | | | | |
| | Proceeds from private placement units | | | 3,568 | | | 5,212 | | | | | | | | | | |
| | Amount of private placement allocated to warrants | | | | | | (89 | ) | | | | | 89 | | | | |
| | Share issuance costs | | | | | | (128 | ) | | | | | | | | | |
| | Stock based compensation | | | | | | | | | 53 | | | | | | | |
| | Net loss | | | | | | | | | | | | | | | (3,098 | ) |
| | | | | | | | | | | | | |
| | Balance, September 30, 2007 | | | 19,698 | | $ | 14,113 | | $ | 7,791 | | $ | 89 | | $ | (15,857 | ) |
| | | | | | | | | | | | | |
| | Issued on exercise of warrants | | | 52 | | | 5 | | | | | | | | | | |
| | Issued in connection with private placement | | | 2,439 | | | 3,660 | | | | | | | | | | |
| | Issued on exercise of options | | | 28 | | | 39 | | | (6 | ) | | | | | | |
| | Share issuance costs, inluding broker warrants | | | | | | (494 | ) | | | | | 185 | | | | |
| | Stock based compensation | | | | | | | | | 283 | | | | | | | |
| | Net loss | | | | | | | | | | | | | | | (3,765 | ) |
| | | | | | | | | | | | | |
| | Balance, September 30, 2008 | | | 22,217 | | $ | 17,323 | | $ | 8,068 | | $ | 274 | | $ | (19,622 | ) |
| | | | | | | | | | | | | |
SQI-F-15
SQI Diagnostics Inc.
SUPPLEMENTAL NOTE REGARDING THE RECONCILIATION OF THE INTERIM
CONSOLIDATED FINANCIAL STATEMENTS FOR THE THREE AND NINE MONTH PERIODS
ENDED JUNE 30, 2011 AND 2010 WITH U.S. GAAP (Continued)
(unaudited)
Dated September 1, 2011
| | | | | | | | | | | | | | | | | |
| |
| | Share Capital | |
| |
| |
| |
|---|
| |
| |
| |
| | Deficit
Accumulated in
Development
Stage | |
|---|
| |
| | Number
of shares | | Amount | | Contributed
Surplus | | Warrant
Capital | |
|---|
| | Issued on exercise of warrants | | | 1,111 | | | 689 | | | | | | | | | | |
| | Issued in connection with private placement | | | 3,732 | | | 4,664 | | | | | | | | | | |
| | Issued on exercise of options | | | 133 | | | 35 | | | (17 | ) | | | | | | |
| | Share issuance costs, inluding broker warrants | | | | | | (345 | ) | | | | | 187 | | | | |
| | Stock based compensation | | | | | | | | | 380 | | | | | | | |
| | Net loss | | | | | | | | | | | | | | | (5,899 | ) |
| | | | | | | | | | | | | |
| | Balance, September 30, 2009 | | | 27,193 | | $ | 22,366 | | $ | 8,431 | | $ | 461 | | $ | (25,521 | ) |
| | | | | | | | | | | | | |
| | Issued on exercise of warrants | | | 970 | | | 2,008 | | | 60 | | | (213 | ) | | | |
| | Proceeds from private placement units | | | 4,678 | | | 12,295 | | | | | | | | | | |
| | Amount of private placement allocated to warrants | | | | | | (1,424 | ) | | | | | 1,424 | | | | |
| | Issued on exercise of options | | | 917 | | | 755 | | | (61 | ) | | | | | | |
| | Expiry of warrants | | | | | | | | | | | | (60 | ) | | | |
| | Share issuance | | | | | | (974 | ) | | | | | 187 | | | | |
| | Stock based compensation | | | | | | | | | 402 | | | | | | | |
| | Net loss | | | | | | | | | | | | | | | (8,070 | ) |
| | | | | | | | | | | | | |
| | Balance, September 30, 2010 | | | 33,758 | | $ | 35,026 | | $ | 8,832 | | $ | 1,799 | | $ | (33,591 | ) |
| | | | | | | | | | | | | |
| | Issued on exercise of warrants | | | 107 | | | 193 | | | | | | (60 | ) | | | |
| | Issued on exercise of options | | | 81 | | | 174 | | | (55 | ) | | (125 | ) | | | |
| | Expiry of warrants | | | | | | | | | 125 | | | | | | | |
| | Share issuance | | | | | | (16 | ) | | | | | | | | | |
| | Stock based compensation | | | | | | | | | 372 | | | | | | | |
| | Net loss | | | | | | | | | | | | | | | (6,807 | ) |
| | | | | | | | | | | | | |
| | Balance, June 30, 2011 | | | 33,946 | | $ | 35,377 | | $ | 9,274 | | $ | 1,614 | | $ | (40,398 | ) |
| | | | | | | | | | | | | |
SQI-F-16
SQI Diagnostics Inc.
SUPPLEMENTAL NOTE REGARDING THE RECONCILIATION OF THE INTERIM
CONSOLIDATED FINANCIAL STATEMENTS FOR THE THREE AND NINE MONTH PERIODS
ENDED JUNE 30, 2011 AND 2010 WITH U.S. GAAP (Continued)
(unaudited)
Dated September 1, 2011
| | | | | | |
| |
| | From Inception to
June 30, 2011 | |
|---|
| | Cash provided by (used in) | | | | |
| | Operations | | | | |
| | Net loss | | $ | (40,398 | ) |
| | Items not affecting cash | | | | |
| | | Amortization — patents and trademarks | | | 817 | |
| | | — property and equipment | | | 2,618 | |
| | | Patents written off | | | 97 | |
| | | Stock-based compensation | | | 2,108 | |
| | | Loss on sale of property and equipment | | | 225 | |
| | | Interest accrual | | | (11 | ) |
| | Changes in non-cash working capital items | | | | |
| | | Amounts receivable | | | (623 | ) |
| | | Investment tax credit recoverable | | | 610 | |
| | | Due from related party | | | 106 | |
| | | Inventory | | | (1,142 | ) |
| | | Prepaids and deposits | | | (423 | ) |
| | | Accounts payable and accrued liabilities | | | 1,668 | |
| | | | | |
| | | | | (34,348 | ) |
| | | | | |
| | Investing | | | | |
| | | Purchase of property and equipment | | | (4,832 | ) |
| | | Additions to patents and trademarks | | | (1,651 | ) |
| | | Sale of property and equipment | | | 2 | |
| | | Due from related party | | | (97 | ) |
| | | | | (6,578 | ) |
| | | | | |
| | Financing | | | | |
| | | Loans from related parties | | | 7,120 | |
| | | Advances (repayment) of loan/notes payable | | | 3 | |
| | | Proceeds from private placement and exercise of warrants and options, net of share issuance costs | | | 35,789 | |
| | | Deferred Share Issuance Costs | | | (433 | ) |
| | | Cash acquired on reverse takeover | | | 607 | |
| | | Share subscription proceeds received | | | 657 | |
| | | | | |
| | | | | 43,743 | |
| | | | | |
| | Net change in cash and cash equivalents during the period and cash at end of period | | $ | 2,817 | |
| | | | | |
| | Supplemental disclosure | | | | |
| | Equipment reallocated from inventory and segregated for use by the Company | | $ | 631 | |
| | Cash paid for interest | | $ | 109 | |
- (iii)
- Statements of Cash Flows
Canadian GAAP permits the disclosure of a subtotal of the amount of funds provided by operations before changes in non-cash operating working capital items in the consolidated statements of cash flows. United States GAAP does not permit this subtotal to be included.
SQI-F-17
SQI Diagnostics Inc.
SUPPLEMENTAL NOTE REGARDING THE RECONCILIATION OF THE INTERIM
CONSOLIDATED FINANCIAL STATEMENTS FOR THE THREE AND NINE MONTH PERIODS
ENDED JUNE 30, 2011 AND 2010 WITH U.S. GAAP (Continued)
(unaudited)
Dated September 1, 2011
- (iv)
- The Company grants stock options to directors, officers, employees and consultants as described in Note 12. The company has used the fair value method of accounting for stock options since the inception of the plan in accordance with Canadian GAAP and U.S GAAP. Other disclosures related to share-based compensation are as follows:
- a.
- The total intrinsic value of options exercised during the three and nine months ended June 30, 2011 was $61,000 and $128,000, respectively (three and nine months ended June 30, 2010 $452,000 and $1,364,000, respectively)
- b.
- The total number of options outstanding June 30, 2011 had a weighted average remaining term of 2.6 years (June 30, 2010 — 2.3 years), and a total intrinsic value of $3,029,000 (June 30, 2010 — $1,022,000)
- c.
- The total number of options vested and exercisable at June 30, 2011 had a weighted average remaining term of 2.5 years (June 30, 2010 — 1.7 years), and a total intrinsic value of $2,006,000 (June 30, 2010 — $556,00)
- d.
- As at June 30, 2011 compensation costs not yet recognized relating to stock option awards outstanding was $888,000 (June 30, 2010 — $864,000). Compensation costs will be recognized over the remaining weighted average period of approximately 1.6 years (June 30, 2010 — 2.4 years)
- e.
- Estimated forfeiture rate did not result in a material impact on the stock based compensation expense.
- (v)
- Breakdown of accounts payable and accrued liabilities are as follows (amounts in thousands of dollars):
| | | | | | | | |
| |
| | June 30,
2011 | | September 30,
2010 | |
|---|
| | Trade payables | | $ | 685 | | $ | 763 | |
| | Accruals | | | 777 | | | 200 | |
| | Payroll Liabilities | | | 66 | | | 9 | |
| | | | | | | |
| | | | $ | 1,528 | | $ | 972 | |
| | | | | | | |
- (vi)
- Patents and trademarks
The aggregate amortization expense related to patents and trademarks for the next five years as at June 30, 2011 is as follows (amounts in thousands of dollars):
| | | | | |
| | 2012 | | | 95 | |
| | 2013 | | | 69 | |
| | 2014 | | | 69 | |
| | 2015 | | | 69 | |
| | 2016 | | | 69 | |
- (vii)
- Recently adopted accounting pronouncements
In June 2009, the FASB launched the FASB Accounting Standards Codification, or the Codification, as the single source of authoritative U.S. GAAP recognized by the FASB. The Codification reorganizes various U.S. GAAP pronouncements into accounting topics and displays them using a consistent structure. Rules and interpretive releases of the SEC under authority of federal securities laws are also sources of authoritative U.S. GAAP for SEC registrants. The Codification is effective for interim and annual periods ending after September 15, 2009. The adoption of this standard had no impact on the Company's financial statements.
In February 2010, the FASB issued ASU No. 2010-09, "Subsequent Events (ASC Topic 855), Amendments to Certain Recognition and Disclosure Requirements." This Standard update requires a SEC Filer to (1) evaluate subsequent events through the date that the financial statements are issued or available to be issued, (2) defines "SEC Filer" as an entity that is required to file or furnish its financial statements with either the SEC or, with respect to an entity subject to Section 12(i) of the Securities Exchange Act of 1934, as amended, the appropriate agency under that Section, (3) not be bound to disclosing the date through which subsequent events have been evaluated, (4) note the definition ofpublic entity is not longer defined nor necessary for Topic 855, (5) note the scope of the reissuance disclosure requirements is refined to include revised financial statements only. These Updates are effective for interim or annual periods ending after June 15, 2010. ASU No. 2010-09 has no effect on the Company's financial position, statements of operations, or cash flows at this time.
SQI-F-18
SQI Diagnostics Inc.
SUPPLEMENTAL NOTE REGARDING THE RECONCILIATION OF THE INTERIM
CONSOLIDATED FINANCIAL STATEMENTS FOR THE THREE AND NINE MONTH PERIODS
ENDED JUNE 30, 2011 AND 2010 WITH U.S. GAAP (Continued)
(unaudited)
Dated September 1, 2011
In May 2009, the FASB issued new guidance for accounting for subsequent events. The new guidance, which is now part of ASC 855-10, Subsequent Events (formerly, SFAS No. 165, Subsequent Events) is consistent with existing auditing standards in defining subsequent events as events or transactions that occur after the balance sheet date but before the financial statements are issued or are available to be issued, but it also requires the disclosure of the date through which an entity has evaluated subsequent events and the basis for that date. The new guidance defines two types of subsequent events: "recognized subsequent events" and "non-recognized subsequent events." Recognized subsequent events provide additional evidence about conditions that existed at the balance sheet date and must be reflected in the Company's financial statements. Non-recognized subsequent events provide evidence about conditions that arose after the balance sheet date and are not reflected in the financial statements of a company. Certain non-recognized subsequent events may require disclosure to prevent the financial statements from being misleading. The new guidance was effective on a prospective basis for interim or annual periods ending after June 15, 2009. The Company adopted the provisions of ASC 855-10 as required.
SQI-F-19
AUDITORS' REPORT
To the Shareholders of
SQI Diagnostics Inc.
We have audited the consolidated balance sheets of SQI Diagnostics Inc. as at September 30, 2010 and 2009 and the consolidated statements of operations and deficit and cash flows for the years then ended. These financial statements are the responsibility of the company's management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with Canadian generally accepted auditing standards. Those standards require that we plan and perform an audit to obtain reasonable assurance whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation.
In our opinion, these consolidated financial statements present fairly, in all material respects, the financial position of the company as at September 30, 2010 and 2009 and the results of its operations and its cash flows for the years then ended, in accordance with Canadian generally accepted accounting principles.
| | |
| | | (Signed)COLLINS BARROW TORONTO LLP |
| Toronto, Ontario | | Licensed Public Accountants |
| November 19, 2010 | | Chartered Accountants |
SQI-F-20
SQI DIAGNOSTICS INC.
CONSOLIDATED BALANCE SHEETS
As at September 30, 2010 and 2009
(Amounts are in thousands of dollars)
| | | | | | | |
| | 2010 | | 2009 | |
|---|
ASSETS | | | | | | | |
Current | | | | | | | |
Cash and cash equivalents | | $ | 9,408 | | $ | 3,180 | |
Amounts receivable | | | 3 | | | 40 | |
Inventory (Note 4) | | | 260 | | | 322 | |
Prepaids and deposits | | | 165 | | | 67 | |
Due from related party (Note 5) | | | 66 | | | 40 | |
| | | | | | |
| | | 9,902 | | | 3,649 | |
Due from related party (Note 5) | | | 32 | | | 65 | |
Property and equipment (Note 6) | | | 2,731 | | | 2,097 | |
Patents and trademarks | | | 469 | | | 394 | |
| | | | | | |
| | $ | 13,134 | | $ | 6,205 | |
| | | | | | |
LIABILITIES | | | | | | | |
Current | | | | | | | |
Accounts payable and accrued liabilities | | $ | 972 | | $ | 369 | |
| | | | | | |
Shareholders' Equity | | | | | | | |
Capital stock (Note 8) | | | 35,026 | | | 22,366 | |
Warrants (Note 9) | | | 1,799 | | | 461 | |
Employee share purchase loan (Note 8) | | | (10 | ) | | (10 | ) |
Contributed surplus (Note 11) | | | 8,832 | | | 8,431 | |
Deficit | | | (33,485 | ) | | (25,412 | ) |
| | | | | | |
| | | 12,162 | | | 5,836 | |
| | | | | | |
| | $ | 13,134 | | $ | 6,205 | |
| | | | | | |
| | | | |
| Approved by the Board | | | | |
|
|
(Signed)PETER WINKLEY
Director |
|
(Signed)ERIC SCHNEIDER
Director |
See accompanying notes
SQI-F-21
SQI DIAGNOSTICS INC.
CONSOLIDATED STATEMENTS OF OPERATIONS AND DEFICIT
Years Ended September 30, 2010 and 2009
(Amounts are in thousands of dollars, except per share amounts)
| | | | | | | |
| | 2010 | | 2009 | |
|---|
Revenue | | | | | | | |
Product sales | | $ | 5 | | $ | 5 | |
Consulting fees (Note 7) | | | 30 | | | 27 | |
| | | | | | |
| | | 35 | | | 32 | |
| | | | | | |
Expenses | | | | | | | |
Salaries and wages | | | 556 | | | 469 | |
General and administrative (Note 7) | | | 471 | | | 447 | |
Professional and consulting fees | | | 659 | | | 436 | |
Sales and marketing | | | 474 | | | 409 | |
Stock based compensation (Note 13) | | | 402 | | | 380 | |
Research and development costs (Note 15) | | | 5,059 | | | 3,362 | |
Amortization — property and equipment | | | 415 | | | 417 | |
Amortization — patents and trademarks | | | 100 | | | 98 | |
| | | | | | |
| | | 8,136 | | | 6,018 | |
| | | | | | |
Loss before the undernoted items | | | (8,101 | ) | | (5,986 | ) |
| | | | | | |
Interest income | | | 32 | | | 121 | |
Interest expense | | | (4 | ) | | (45 | ) |
| | | | | | |
| | | 28 | | | 76 | |
| | | | | | |
Net loss | | | (8,073 | ) | | (5,910 | ) |
Deficit, beginning of year | | | (25,412 | ) | | (19,502 | ) |
| | | | | | |
Deficit, end of year | | $ | (33,485 | ) | $ | (25,412 | ) |
| | | | | | |
Loss per share | | | | | | | |
Basic and diluted | | $ | (0.27 | ) | $ | (0.23 | ) |
| | | | | | |
Weighted average number of common shares outstanding | | | | | | | |
Weighted average number of shares | | | 30,349 | | | 25,158 | |
| | | | | | |
See accompanying notes
SQI-F-22
SQI DIAGNOSTICS INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
Years Ended September 30, 2010 and 2009
(Amounts are in thousands of dollars)
| | | | | | | | | |
| | 2010 | | 2009 | |
|---|
Cash provided by (used in) | | | | | | | |
Operations | | | | | | | |
Net loss | | $ | (8,073 | ) | $ | (5,910 | ) |
Items not affecting cash | | | | | | | |
| | Amortization — patents and trademarks | | | 100 | | | 98 | |
| | | — property and equipment | | | 415 | | | 417 | |
| | Patents written off | | | — | | | 97 | |
| | Stock-based compensation | | | 402 | | | 380 | |
| | Interest accrual | | | (2 | ) | | (1 | ) |
| | | | | | |
Net changes in non-cash working capital | | | (7,158 | ) | | (4,919 | ) |
| | Amounts receivable | | | 37 | | | (15 | ) |
| | Inventory | | | (568 | ) | | (211 | ) |
| | Prepaids and deposits | | | (98 | ) | | (7 | ) |
| | Accounts payable and accrued liabilities | | | 604 | | | (16 | ) |
| | Investment tax credits recoverable | | | — | | | 1,075 | |
| | Due from related party | | | 7 | | | — | |
| | | | | | |
| | | (7,176 | ) | | (4,093 | ) |
| | | | | | |
Investing | | | | | | | |
Purchase of property and equipment | | | (418 | ) | | (113 | ) |
Additions to patents and trademarks | | | (175 | ) | | (186 | ) |
| | | | | | |
| | | (593 | ) | | (299 | ) |
| | | | | | |
Financing | | | | | | | |
Repayment of loans payable | | | — | | | (732 | ) |
Proceeds from private placement and exercise of warrants and stock options, net of share issuance costs | | | 13,997 | | | 5,213 | |
| | | | | | |
| | | 13,997 | | | 4,481 | |
| | | | | | |
Net change in cash and cash equivalents | | | 6,228 | | | 89 | |
Cash and cash equivalents, beginning of year | | | 3,180 | | | 3,091 | |
| | | | | | |
Cash and cash equivalents, end of year | | $ | 9,408 | | $ | 3,180 | |
| | | | | | |
Non-cash Investing Activity | | | | | | | |
Equipment reallocated from inventory and segregated for use by the Company | | $ | 631 | | $ | — | |
| | | | | | |
Supplemental Disclosure | | | | | | | |
Cash paid for interest | | $ | 4 | | $ | 44 | |
| | | | | | |
See accompanying notes
SQI-F-23
SQI Diagnostics Inc.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
September 30, 2010 and 2009
1. NATURE OF OPERATIONS
SQI Diagnostics Inc., (the "Company"), has its head office and development centre in Toronto, Ontario. The Company is a medical systems company that develops proprietary technology in multiplexing, miniaturization and automation. The Company provides laboratories the ability to simultaneously analyse multiple biomarkers, deliver accurate and quantitative patient results in less time, significantly reduce labour cost, and increase profits when compared with current diagnostic instrumentation.
During fiscal 2009 the Company obtained Health Canada licenses and self authorization to sell in the EU and during fiscal 2010 received United States Food & Drug Administration ("FDA") clearance of its SQiDworks and IgXPLEX Rheumatoid Arthritis (RA) system. During fiscal 2010 the Company obtained a Health Canada license for its IgXPLEX Celiac™ microarray test kits that run on the Company's automated SQiDworks™ platform. The Company has earned limited revenues from its IgXPLEX RA™ and IgXPLEX Celiac™ test kits run on installed SQiDworks™ platforms to date and as such is considered to be a development stage company. The Company has a pipeline of additional autoimmune diagnostic products in various stages of development and commercialization. The Company expects to generate revenues from its IgXPLEX RA and IgXPLEX Celiac products as it grows its installed base of customers as well as from products to be launched as they complete commercialization. The continuation of the Company's research, development and commercialization activities is dependent upon the Company's ability to successfully generate product revenues, or, to finance its cash requirements through further equity financings.
2. SIGNIFICANT ACCOUNTING POLICIES
These consolidated financial statements have been prepared by management in accordance with Canadian generally accepted accounting principles, within the framework of the significant accounting policies summarized below:
Basis of Consolidation
The consolidated financial statements include the accounts of the Company and its wholly owned subsidiary. Inter-company balances and transactions are eliminated upon consolidation.
Cash and Cash Equivalents
Cash and cash equivalents include bank deposits and highly liquid money market investments such as bankers acceptance notes, treasury bills, cashable money market funds, and cashable guaranteed investment certificates.
Inventory
Inventory is valued at the lower of cost and replacement cost, with cost determined on a first-in, first-out basis.
Property and Equipment
Property and equipment are recorded at cost and are amortized on the straight-line basis over their estimated useful lives as follows:
| | | | | | | | |
| | Computer hardware | | | — | | 3 years | | |
| | Computer software | | | — | | 3 years | | |
| | Laboratory fixtures and equipment | | | — | | 10 years | | |
| | Office equipment | | | — | | 10 years | | |
| | Leasehold improvements | | | — | | 10 years | | |
Patents and Trademarks
The costs relating to initial patent and trademark fees are deferred and amortized over 10 years on a straight-line basis. Patents and trademarks are recorded net of accumulated amortization of $627,000 (2009 — $527,000). At year end all patents and trademarks were reviewed and were related to the Company's current systems under development. Net capitalized costs of $97,000 related to three abandoned patents were written off in fiscal 2009, with the write off included in general and administrative expense.
Research and Development Costs
Research costs are charged to operations in the period in which they are incurred. Development costs are expensed as incurred or deferred if they meet the criteria for deferral under Canadian generally accepted accounting principles and are expected to provide future benefits with reasonable certainty.
SQI-F-24
SQI Diagnostics Inc.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
September 30, 2010 and 2009
2. SIGNIFICANT ACCOUNTING POLICIES (Continued)
At September 30, 2010, the Company was developing IgXPLEX diagnostics assays for celiac, vasculitis, lupus (SLE), Crohn's (IBD), antiphospholipid syndrome and our second generation, fully quantitative IgXPLEX RA assay. The Company continued its work to complete scientific discovery and assay design work for a diagnostic assay to detect and measure infliximab (also referred to as anti-TNF) in the blood of autoimmune patients. Deferral criteria have not been met, and accordingly, all development costs have been expensed in the period.
Impairment of Long-Lived Assets
Long-lived assets comprise property and equipment and intangible assets with finite lives (patents and trademarks). The Company recognizes an impairment loss for a long-lived asset when events or changes in circumstances cause its carrying value to exceed the total undiscounted cash flows expected from its use and eventual disposition. An impairment loss is measured as the excess of the carrying value of the asset over its fair value.
Revenue Recognition
Product sales are recognized upon the shipment of products to customers, if a signed contract exists, the sales price is fixed and determinable, collection of the resulting receivables is reasonably assured and any uncertainties with regard to customer acceptance are insignificant. Sales are recorded net of discounts and sales returns.
The Company also provides consulting services from time to time. Consulting fee revenue is recognized when services are completed, amounts are invoiced to customers and collectability is reasonably assured.
Stock-Based Compensation and Other Stock-Based Payments
The Company applies a fair value based method of accounting for all stock-based payments. Accordingly, stock-based payments are measured at the fair value of the consideration received or the fair value of the equity instruments issued or liabilities incurred, whichever is more reliably measurable. Stock-based compensation is charged to operations over the vesting period and the offset is credited to contributed surplus. Consideration received upon the exercise of stock options is credited to share capital at which time the related contributed surplus is transferred to share capital.
Foreign Currency Translation
Monetary assets and liabilities denominated in foreign currencies are translated to Canadian dollars at exchange rates in effect at the balance sheet date. Non-monetary assets and liabilities are translated at rates of exchange in effect at each transaction date. Revenue and expenses are translated at the rate of exchange at each transaction date. Gains or losses on translation are included in operations.
Income Taxes
The Company follows the liability method of accounting for income taxes. Under this method, future income tax assets and liabilities are determined based on temporary differences between financial reporting and tax bases of assets and liabilities, as well as for the benefit of losses available to be carried forward to future years for tax purposes. Future income tax assets and liabilities are measured using enacted or substantively enacted tax rates and laws that will be in effect when the differences are expected to reverse. Future income tax assets are recorded in the financial statements if realization is considered more likely than not.
Investment Tax Credits
Investment tax credits are accrued when qualifying expenditures are incurred and there is reasonable assurance that the credits will be realized. Investment tax credits earned with respect to current expenditures for qualified research and development activities are included in the statements of operations as a reduction of research and development costs. Investment tax credits associated with capital expenditures are reflected as reductions in the carrying amounts of capital assets.
During the year ended September 30, 2010, the Company recorded an amount of $295,000 (2009 — $87,000) as a reduction in research and development costs.
Financial Instruments
Financial instruments are measured initially at fair value and thereafter based on their classification.
SQI-F-25
SQI Diagnostics Inc.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
September 30, 2010 and 2009
2. SIGNIFICANT ACCOUNTING POLICIES (Continued)
| | | | | |
| | Financial Instrument | | Classification | | Measurement Basis |
|---|
| | Cash and cash equivalents | | Held-for-trading | | Fair value |
| | Amounts receivable | | Loans and receivable | | Amortized cost |
| | Due from related party | | Loans and receivable | | Amortized cost |
| | Accounts payable and accruals | | Other financial liabilities | | Amortized cost |
Comprehensive Income
The Company has not presented a statement of comprehensive income as it has no other comprehensive income.
Loss Per Share
Basic loss per share is computed using the weighted average number of common shares outstanding during the year. Diluted loss per share is computed using the weighted average number of common and potential common shares outstanding during the year. Potential common shares consist of the incremental common shares issuable upon the exercise of stock options and warrants determined using the treasury stock method.
Use of Estimates
The preparation of financial statements in conformity with Canadian generally accepted accounting principles requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amount of revenue and expenses during the year. Actual results could differ from those estimates.
Significant areas requiring the use of management estimates relate to the determination of the useful lives of property and equipment and patents and trademarks for amortization purposes, valuation of ITC's receivable, valuation of stock options and warrants and valuation allowance on future tax assets.
3. RECENT ACCOUNTING PRONOUNCEMENTS ISSUED AND NOT YET APPLIED
Business Combinations
In January 2009, the CICA issued Section 1582, Business Combinations, which replaces former guidance on business combinations. Section 1582 establishes principles and requirements of the acquisition method for business combinations and related disclosures. In addition, the CICA issued Sections 1601, Consolidated Financial Statements, and 1602, Non-Controlling Interests, which replaces the existing guidance. Section 1601 establishes standards for the preparation of consolidated financial statements, while section 1602 provides guidance on accounting for a non-controlling interest in a subsidiary in consolidated financial statements subsequent to a business combination.
These standards apply prospectively to business combinations for which the acquisition date is on or after the beginning of the first annual reporting period beginning on or after January 1, 2011 with earlier application permitted. The Company is currently evaluating the new sections to determine the potential impact of any future transactions on its consolidated financial statements.
International Financial Reporting Standards
The CICA plans to converge Canadian Generally Accepted Accounting Principles with International Financial Reporting Standards ("IFRS") over a transition period expected to end in 2011, when IFRS will be fully adopted. The transition date of October 1, 2010 for the Company will require restatement for comparative purposes of amounts reported by the Company for the year ended September 30, 2011. The Company continues to monitor and assess the impact of the convergence of Canadian GAAP and IFRS on its financial statements.
4. INVENTORY
Inventory consists of component parts that are to be used in the future production of SQiDworks™ Platform and IgXPLEX consumable assays.
SQI-F-26
SQI Diagnostics Inc.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
September 30, 2010 and 2009
5. DUE FROM RELATED PARTY
| | | | | | | | |
| |
| | 2010 | | 2009 | |
|---|
| |
| | ($000s)
| | ($000s)
| |
|---|
| | Amount due from an officer and director, secured, bearing interest as described in note(i), principal amount of $98,000 repayable in three equal payments on September 1, 2010, 2011 and 2012. Accrued interest is due on September 1, 2011 | | $ | 98 | | $ | 105 | |
| | Less: Current portion | | | (66 | ) | | (40 | ) |
| | | | | | | |
| | | | $ | 32 | | $ | 65 | |
| | | | | | | |
- (i)
- Terms of the promissory note were amended on April 16, 2010 as follows: interest bearing at Canada Revenue Agency prescribed rate for taxable benefits to employees and shareholders on interest-free and low-interest loans, which was 1% per annum at September 30, 2010. Prior to amendment, the loan was bearing interest at 4.25% per annum during the year ended December 31, 2009 and non-interest bearing from October 1, 2009 to April 15, 2010.
- (ii)
- As at September 30, 2010, the first payment of $33,000 is in arrears.
6. PROPERTY AND EQUIPMENT
| | | | | | | | | | | |
| | As at September 30, 2010: | | Cost | | Accumulated
Amortization | | Net | |
|---|
| |
| | ($000s)
| | ($000s)
| | ($000s)
| |
|---|
| | Computer hardware | | $ | 193 | | $ | 141 | | $ | 52 | |
| | Computer software | | | 153 | | | 130 | | | 23 | |
| | Laboratory fixtures and equipment | | | 4,172 | | | 1,670 | | | 2,502 | |
| | Office equipment | | | 176 | | | 128 | | | 48 | |
| | Leasehold improvements | | | 263 | | | 157 | | | 106 | |
| | | | | | | | | |
| | | | $ | 4,957 | | $ | 2,226 | | $ | 2,731 | |
| | | | | | | | | |
| | | | | | | | | | | |
| | As at September 30, 2009: | | Cost | | Accumulated
Amortization | | Net | |
|---|
| |
| | ($000s)
| | ($000s)
| | ($000s)
| |
|---|
| | Computer hardware | | $ | 141 | | $ | 114 | | $ | 27 | |
| | Computer software | | | 125 | | | 106 | | | 19 | |
| | Laboratory fixtures and equipment | | | 3,255 | | | 1,349 | | | 1,907 | |
| | Office equipment | | | 147 | | | 112 | | | 35 | |
| | Leasehold improvements | | | 240 | | | 131 | | | 109 | |
| | | | | | | | | |
| | | | $ | 3,908 | | $ | 1,812 | | $ | 2,097 | |
| | | | | | | | | |
7. RELATED PARTY TRANSACTIONS
Transactions with related parties occur in the normal course of business and are measured at the exchange amount. Related party transactions have been listed below, unless they have been disclosed elsewhere in the financial statements.
Included in general and administrative expense for the year ended September 30, 2010 is $49,000 (2009 — $50,000), related to recovery of occupancy costs, from a corporation in which an officer of the Company is also an officer. Consulting fee revenue of $30,000 for the year ended September 30, 2010 (2009 — $27,000) was earned from this corporation. At year end, $1,000 (2009 — $6,000) due from this corporation is included in amounts receivable.
SQI-F-27
SQI Diagnostics Inc.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
September 30, 2010 and 2009
8. CAPITAL STOCK
- (a)
- Authorized
unlimited common shares
Issued — common shares
| | | | | | | | |
| |
| | Number | | Value | |
|---|
| |
| | (000s)
| | ($000s)
| |
|---|
| | Balance, September 30, 2008 | | | 22,217 | | $ | 17,323 | |
| | Warrants exercised | | | 1,111 | | | 689 | |
| | Issued in connection with a private placement(i) | | | 3,732 | | | 4,664 | |
| | Option exercised | | | 133 | | | 35 | |
| | Share issuance cost(i) | | | — | | | (345 | ) |
| | | | | | | |
| | Balance, September 30, 2009 | | | 27,193 | | $ | 22,366 | |
| | Warrants exercised (Note 9(i)) | | | 970 | | | 2,008 | |
| | Issued in connection with a private placement(ii) | | | 4,678 | | | 12,295 | |
| | Allocated to warrants | | | — | | | (1,424 | ) |
| | Options exercised | | | 917 | | | 755 | |
| | Share issuance costs(ii) | | | — | | | (974 | ) |
| | | | | | | |
| | Balance, September 30, 2010 | | | 33,758 | | $ | 35,026 | |
| | | | | | | |
- (i)
- On December 23, 2008, pursuant to a private placement, the Company issued 2,400,000 shares at a price of $1.25 per share, resulting in gross proceeds of $3,000,000. The Company paid a finder's fee in relation to the private placement satisfied through the issuance of 236,800 warrants with an exercise price of $1.90 and expiring on December 23, 2011, valued at $127,000. Total share issuance costs were $127,000.
On January 21, 2009, pursuant to a private placement the Company issued 1,331,500 shares at a price of $1.25 per share, resulting in gross proceeds of $1,664,000. The Company paid a finder's fee in relation to the private placement satisfied through the issuance of 106,520 warrants with an exercise price of $1.25 and expiring on December 23, 2011, valued at $60,000 and a cash payment of $133,000. Total share issuance costs were $193,000.
Additional share issue costs amounted to $25,000.
- (ii)
- On December 4, 2009, pursuant to a private placement, the Company issued 2,398,104 units at a price of $2.75 per unit, each unit comprised one common share of the Company and one-half common share purchase warrant, resulting in gross proceeds of $6,595,000. Each whole warrant has an exercise price of $4.00 per share and expires on December 4, 2011. The value of capital stock includes value attributable to the warrants in the amount of $846,000, which has been included in warrant capital (see Note 9).
The Company paid a finder's fee in relation to the private placement satisfied through a cash payment of $453,000 and the issuance of 143,886 compensation unit warrants with an exercise price of $2.75 per unit and expiring on December 4, 2010, valued at $125,000. Each unit entitles the purchaser to purchase one common share and one-half common share purchase warrant with an exercise price of $4.00 per share, expiring on December 4, 2010. Total share issuance costs were $611,000.
On August 12, 2010, pursuant to a private placement the Company issued 2,280,000 units at a price of $2.50 per unit, each unit comprised one common share of the Company and one-half common share purchase warrant, resulting in gross proceeds of $5,700,000. Each whole warrant has an exercise price of $5.00 and expires on August 12, 2012. The value of capital stock includes value attributable to the warrants in the amount of $578,000, which has been included in warrant capital (see Note 9).
The Company paid a finder's fee in relation to the private placement satisfied through a cash payment of $260,000 and the issuance of 57,000 compensation unit warrants with an exercise price of $2.50 per unit and expiring on August 12, 2012, valued at $62,000. Each unit entitles the purchaser to purchase one common share at and one-half common share purchase warrant with an exercise price of $5.00 per share, expiring on August 12, 2012. The total share issuance costs were $363,000.
- (b)
- During the period ended September 30, 2007, the Company made a non-interest bearing loan to its officer, which was used to acquire 100,000 common shares. The loan has been accounted for as a share purchase loan and, accordingly, the $10,000 loan balance has been deducted from share capital.
SQI-F-28
SQI Diagnostics Inc.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
September 30, 2010 and 2009
9. WARRANT CAPITAL
| | | | | | | | |
| |
| | 2010 | | 2009 | |
|---|
| |
| | ($000s)
| | ($000s)
| |
|---|
| | Balance, beginning of period | | $ | 461 | | $ | 274 | |
| | Issued on private placement (Note 8(ii)) | | | 1,424 | | | — | |
| | Finder warrants (Note 8) | | | 187 | | | 187 | |
| | Exercise of warrants (Note 10 (i)) | | | (213 | ) | | — | |
| | Expiry of warrants (Note 10 (i)) | | | (60 | ) | | — | |
| | | | | | | |
| | Balance, end of period | | $ | 1,799 | | $ | 461 | |
| | | | | | | |
10. WARRANTS
The Company had the following warrants outstanding at September 30, 2010:
| | | | | | | | | |
| | Number of Warrants | | Purchase Price | | Expiry Date | |
|---|
| | (000s)
| |
| |
| |
|---|
| | | 144 | | $ | 2.750 | | | December 4, 2010 | (ii) |
| | | 107 | | $ | 1.250 | | | January 22, 2011 | |
| | | 1,199 | | $ | 4.000 | | | December 4, 2011 | |
| | | 237 | | $ | 1.900 | | | December 23, 2011 | |
| | | 1,140 | | $ | 5.000 | | | August 12, 2012 | |
| | | 57 | | $ | 2.500 | | | August 12, 2012 | |
| | | | | | | | | |
| | | 2,884 | | | | | | | |
| | | | | | | | | |
- (i)
- During the year ended September 30, 2010, 194,200 warrants with an expiry of June 3, 2010 were exercised resulting in the issuance of 194,200 shares and net proceeds of $291,000, 576,563 warrants with an expiry of June 29, 2010 were exercised resulting in the issuance of 576,563 shares and net proceeds of $1,384,000 and 199,493 warrants with an expiry of April 26, 2010 were exercised resulting in the issuance of 199,493 shares and net proceeds of $120,000. On exercise, $213,000 (2009 — $Nil) was transferred from warrant capital to share capital. The remaining 1,207,213 warrants with an expiry date of June 29, 2010 expired unexercised. On expiry, $60,000 (2009 — $Nil) was transferred from warrant capital to contributed surplus.
- (ii)
- Subsequent to the year ended September 30, 2010, these warrants expired unexercised.
11. CONTRIBUTED SURPLUS
| | | | | | | | |
| |
| | 2010 | | 2009 | |
|---|
| |
| | ($000s)
| | ($000s)
| |
|---|
| | Balance, beginning of period | | $ | 8,431 | | $ | 8,068 | |
| | Stock-based compensation (Note 13) | | | 402 | | | 380 | |
| | Options exercised (Note 12 (i)) | | | (61 | ) | | (17 | ) |
| | Warrants expired (Note 9(i)) | | | 60 | | | — | |
| | | | | | | |
| | Balance, end of period | | $ | 8,832 | | $ | 8,431 | |
| | | | | | | |
12. STOCK OPTIONS
The Company maintains a Stock Option Plan (the "Plan") for the benefit of directors, officers, employees and consultants. The aggregate number of common shares reserved for issuance under the Plan, together with any other employee stock option plans, options for services and employee share purchase plans, will not exceed 3,200,000. Options granted pursuant to the Plan will have terms not to
SQI-F-29
SQI Diagnostics Inc.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
September 30, 2010 and 2009
12. STOCK OPTIONS (Continued)
exceed five years, and are granted at an option price which will not be less than the fair market price at the time the options are granted. All options granted to individual optionees, other than consultants, generally vest in six equal installments over a period of 18 months.
The following summarizes the stock option activities under the Plan:
| | | | | | | | | | | | | | |
| |
| | September 30, 2010 | | September 30, 2009 | |
|---|
| |
| | Number of
Options | | Weighted Average
Exercise
Price | | Number of
Options | | Weighted Average
Exercise
Price | |
|---|
| |
| | (000s)
| |
| | (000s)
| |
| |
|---|
| | Beginning balance | | | 2,368 | | $ | 1.20 | | | 2,624 | | $ | 1.18 | |
| | Granted | | | 384 | | $ | 2.41 | | | 80 | | $ | 1.08 | |
| | Exercised(i) | | | (850 | ) | $ | 0.75 | | | (133 | ) | $ | 0.13 | |
| | Expired | | | — | | $ | — | | | (36 | ) | $ | 0.60 | |
| | Forfeited | | | (138 | ) | $ | 1.73 | | | (167 | ) | $ | 0.60 | |
| | | | | | | | | | | |
| | Ending balance | | | 1,764 | | $ | 1.75 | | | 2,368 | | $ | 1.20 | |
| | | | | | | | | | | |
| | Exercisable | | | 985 | | $ | 1.60 | | | 1,792 | | $ | 1.09 | |
| | | | | | | | | | | |
- (i)
- On exercise of stock options, $61,000 (2009 — $17,000) was transferred from contributed surplus to share capital.
In addition to the options exercised included in the table, there were 66,667 options that were not granted under the Plan that were exercised at a price of $0.90 per share during the year ended September 30, 2010.
There are no other options outstanding that are not part of the Plan.
The Company had the following stock options outstanding under the Plan at September 30, 2010:
| | | | | | | | | |
| | Number of Options | | Exercise Price | | Expiry Date | |
|---|
| | (000s)
| |
| |
| |
|---|
| | | 33 | | $ | 1.200 | | | June 29, 2011 | |
| | | 67 | | $ | 1.200 | | | August 29, 2011 | |
| | | 180 | | $ | 1.740 | | | August 7, 2012 | |
| | | 50 | | $ | 1.500 | | | October 23, 2012 | |
| | | 758 | | $ | 1.600 | | | February 26, 2013 | |
| | | 269 | | $ | 1.750 | | | August 26, 2013 | |
| | | 80 | | $ | 1.300 | | | May 22, 2014 | |
| | | 25 | | $ | 3.260 | | | November 3, 2014 | |
| | | 68 | | $ | 2.250 | | | February 22, 2015 | |
| | | 60 | | $ | 2.100 | | | May 27, 2015 | |
| | | 175 | | $ | 2.500 | | | August 16, 2015 | |
| | | | | | | | | |
| | | 1,764 | | | | | | | |
| | | | | | | | | |
13. STOCK-BASED COMPENSATION
The fair value of the options granted during the year ended September 30, 2010 was $591,000 (2009 — $67,000), which will be recognized over the vesting period of 18 to 36 months. The total compensation expense for the year ended September 30, 2010 was $402,000 (2009 — $380,000). The total amount credited to contributed surplus for the year ended September 30, 2010 was $402,000 (2009 — $380,000).
SQI-F-30
SQI Diagnostics Inc.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
September 30, 2010 and 2009
13. STOCK-BASED COMPENSATION (Continued)
The fair value of each option granted has been estimated at the date of grant or the date when it became measurable using the Black-Scholes option pricing model with the following weighted average assumptions at the measurement date:
| | | | | | | | |
| |
| | 2010 | | 2009 | |
|---|
| | Dividend yield | | | 0 | % | | 0 | % |
| | Expected volatility | | | 80 | % | | 80 | % |
| | Risk-free interest rate | | | 2.00 | % | | 1.98 | % |
| | Expected life (years) | | | 5.00 | | | 5.00 | |
| | | | | | | |
| | Weighted average grant date fair value | | $ | 1.540 | | $ | 0.842 | |
| | | | | | | |
The Company has assumed no forfeiture rate as adjustments for actual forfeitures are made in the year they occur.
14. INCOME TAXES
| | | | | | | | |
| |
| | 2010 | | 2009 | |
|---|
| |
| | ($000s)
| | ($000s)
| |
|---|
| | Loss before income taxes | | $ | (8,073 | ) | $ | (5,910 | ) |
| | Statutory rate | | | 31.75 | % | | 33.00 | % |
| | | | | | | |
| | Expected income tax recovery | | $ | (2,563 | ) | $ | (1,950 | ) |
| | Effect on income taxes of unrecognized future income tax assets relating to deductible temporary differences on: | | | | | | | |
| | Change in valuation allowance related to operations | | | 1,971 | | | 1,325 | |
| | Change in future tax rates | | | 1,150 | | | 121 | |
| | Impact of SR&ED filings | | | (689 | ) | | 302 | |
| | Non-deductible expenses and other items | | | 131 | | | 202 | |
| | | | | | | |
| | Income tax expense | | $ | — | | $ | — | |
| | | | | | | |
| | | | | | | | |
| |
| | 2010 | | 2009 | |
|---|
| |
| | ($000s)
| | ($000s)
| |
|---|
| | Amounts related to tax loss and other credits carry forwards | | $ | 8,646 | | $ | 6,448 | |
| | Property and equipment and intangibles | | | (279 | ) | | (276 | ) |
| | Share issue costs | | | 280 | | | 231 | |
| | | | | | | |
| | Net future tax asset | | | 8,647 | | | 6,403 | |
| | Less: Valuation allowance | | | (8,647 | ) | | (6,403 | ) |
| | | | | | | |
| | | | $ | — | | $ | — | |
| | | | | | | |
SQI-F-31
SQI Diagnostics Inc.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
September 30, 2010 and 2009
14. INCOME TAXES (Continued)
- (c)
- Loss and Tax Credit Carryforwards
As at September 30, 2010, the Company has non-capital losses of approximately $18,128,000 expiring as follows:
| | | | | |
| | 2013 | | $ | 879,000 | |
| | 2014 | | | 766,000 | |
| | 2025 | | | 1,146,000 | |
| | 2026 | | | 516,000 | |
| | 2027 | | | 1,154,000 | |
| | 2028 | | | 2,537,000 | |
| | 2029 | | | 3,287,000 | |
| | 2030 | | | 7,843,000 | |
| | | | | |
| | | | $ | 18,128,000 | |
| | | | | |
The Company has undeducted scientific research and experimental development costs of approximately $9,726,000 and investment tax credits relating to scientific research and development costs of approximately $2,046,000 available to apply against future taxable income.
The potential tax benefit relating to the non-capital losses and tax credit carryforwards has not been reflected in these financial statements.
15. RESEARCH AND DEVELOPMENT COSTS
The Company's autoimmune diagnostic research and development programs focus primarily on the following technology platforms:
- (a)
- Platform: The Company develops various platforms to enable its customers to run and analyze the Company's immunoassay IgXPLEX™ and QuantiSpot test kits. The Company's SQiDworks™ is a fully automated, multi plate system used to process up to three IgXPLEX™ or QuantiSpot test kits. SQiDworks™ is a fully load-and-go device that completes all sample handling, biochemistry, plate scanning and data analysis. SQiDman is a semi automated system used to process single IgXPLEX™ or QuantiSpot test kits. The Company's Platform research and development activities focus on continuous improvement to the Platforms, novel applications, required modifications for the Company's pipeline IgXPLEX™ and QuantiSpot content as well as the evolution of the Platform to meet customer needs and market demands. SQiDworksLITE™, in the concept stage in 2010, is designed to address customers' requirements for smaller, less costly, fully automated systems and is intended to address the IVD markets as well as the research and drug development markets.
- (b)
- IgXPLEX™: The Company develops multiplex microarrays used to aid in the diagnosis of a variety of autoimmune diseases and with a goal of expanding the use of this technology into infectious diseases and allergens. The IgXPLEX™ research and development program includes basic and advanced research and development in materials science, surface chemistry, biochemistry, ELISA chemistry, multiplexing, antibody kinetics, autofluorescing anti body marker biochemistry and detection, multiplexed calibration and normalization, qualitative and quantitative measurement and automation of the assay biochemistry. The development of the core IgXPLEX™ technology was completed in parallel with the development of the Company's first QuantiSpot RA and IgXPLEX™ test kits licensed in Canada, cleared in the US and authorized in the EU to aid in the diagnosis of rheumatoid arthritis.
- (c)
- Gastrointestinal ("GI"): The Company is developing a pipeline of IgXPLEX™ test kits targeted at autoimmune disorders of the digestive system. The first such test kit for Celiac disease has been licensed in Canada and is awaiting FDA approval in the United States. The IgXPLEX™ products currently in development include multiplex microarrays detecting and measuring serum antibodies to aid in the diagnosis of celiac disease and Crohn's/ulcerative colitis.
- (d)
- Vascular: The Company is developing a pipeline of IgXPLEX™ test kits targeted at autoimmune disorders of the vasculature. The IgXPLEX™ products currently in development include multiplex microarrays detecting and measuring serum antibodies to aid in the diagnosis of vasculitis and Antiphospholipid Syndrome (APS).
- (e)
- General Discovery Markers: The Company conducts discovery research as the first step in the development process. Discovery includes basic research to determine the suitability of candidate biomarkers that could be combined in marketable multiplex microarrays. The current research includes biomarkers used to aid in the diagnosis of autoimmune diseases and includes: lupus, thyroid disease, autoimmune hepatitis, autoimmune renal disease and food intolerance.
SQI-F-32
SQI Diagnostics Inc.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
September 30, 2010 and 2009
15. RESEARCH AND DEVELOPMENT COSTS (Continued)
- (f)
- Therapeutic Monitoring: The Company has initiated a program to develop monitoring tests used to aid in the therapeutic treatment of autoimmune diseases. These tests are used to measure biologic therapies in patient's blood to assist clinicians in their use of various clinical approaches. Therapeutic Monitoring may be used alone, or in combination with the Company's IgXPLEX™ products.
The following table summarizes the Company's research and development costs for the years ending September 30, 2010 and 2009:
| | | | | | | | |
| |
| | 2010 | | 2009 | |
|---|
| |
| | ($000s)
| | ($000s)
| |
|---|
| | Platform | | $ | 907 | | $ | 460 | |
| | IgXPLEX Technology | | | 1,431 | | | 2,050 | |
| | Gastrointestinal | | | 1,861 | | | 494 | |
| | Vascular | | | 345 | | | 217 | |
| | General Discovery Markets | | | 304 | | | 180 | |
| | Therapeutic Monitoring | | | 506 | | | 48 | |
| | | | | | | |
| | | | | 5,354 | | | 3,449 | |
| | ITC Refund | | | (295 | ) | | (87 | ) |
| | | | | | | |
| | | | $ | 5,059 | | $ | 3,362 | |
| | | | | | | |
16. CONTINGENCIES
In the ordinary course of business, the Company may be contingently liable for litigation and claims with customers, suppliers, former employees or competitors. Management believes that adequate provisions have been recorded in accounts where required.
17. CAPITAL RISK MANAGEMENT
The Company's objective when managing capital is to safeguard the Company's ability to continue as a going concern so that it can complete its lead assay commercialization efforts and receive the required regulatory approvals to sell and market its products and provide returns for shareholders and benefits for other stakeholders.
The capital structure of the Company consists of shareholders' equity. The Company is not subject to externally imposed capital requirements.
18. FINANCIAL RISK MANAGEMENT
Currency risk is the risk that the fair value or future cash flows of a financial instrument will fluctuate because of changes in foreign exchange rates. The Company is exposed to currency risk due to its purchases in US dollars. A 1% change in the foreign exchange rate would result in a change of approximately $16,000 in net loss.
SQI-F-33
SQI Diagnostics Inc.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
September 30, 2010 and 2009
18. FINANCIAL RISK MANAGEMENT (Continued)
19. COMPARATIVE FIGURES
Certain comparative figures have been reclassified to conform with the current year's financial statement presentation.
SQI-F-34
AUDITORS' REPORT ON THE SUPPLEMENTAL NOTE REGARDING THE
RECONCILIATION OF THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED SEPTEMBER 30, 2010 AND 2009 WITH U.S. GAAP
To the Shareholders of
SQI Diagnostics Inc.
We have audited the consolidated balance sheets of SQI Diagnostics Inc. as at September 30, 2010 and 2009 and the consolidated statements of operations and deficit and cash flows for the years then ended and have issued our report thereon dated November 19, 2010. We have also audited the reconciliation from Canadian Generally Accepted Accounting Principles ("GAAP") to United States GAAP of the Company contained herein. This reconciliation from Canadian GAAP to United States GAAP is the responsibility of the Company's management. Our responsibility is to express an opinion based on our audits. In our opinion, such reconciliation from Canadian GAAP to United States GAAP, when considered in relation to the consolidated financial statements taken as a whole, presents fairly, in all material respects, the information set forth herein.
| | |
Toronto, Ontario
July 4, 2011 | | (Signed)COLLINS BARROW TORONTO LLP
Licensed Public Accountants
Chartered Accountants |
SQI-F-35
SQI Diagnostics Inc.
SUPPLEMENTAL NOTE REGARDING THE
RECONCILIATION OF THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED SEPTEMBER 30, 2010 AND 2009 WITH U.S. GAAP
Dated July 4, 2011
The Company follows Canadian generally accepted accounting principles ("Canadian GAAP") which is different in some respects from the accounting principles applicable in the United States ("U.S. GAAP") and from practices prescribed by the United States Securities and Exchange Commission. These differences in Canadian GAAP and U.S. GAAP that would require restatement of any of the Company's reported financial statements and certain additional disclosures required under U.S. GAAP are detailed below:
- (i)
- Leasehold Improvements
The company amortizes leasehold improvements over their expected useful life. U.S. GAAP requires that leasehold improvements be amortized over the lesser of their useful life or the term of the lease. As the Company does not have a term lease in place for its premises, expenditures relating to leasehold improvements are booked to general and administrative expense in the statement of operations. Accordingly, the following adjustments would be required under U.S. GAAP:
| | | | | | | | |
| | (amounts in thousands of dollars) | | September 30,
2010 | | September 30,
2009 | |
|---|
| | Property and equipment — Canadian GAAP | | $ | 2,731 | | $ | 2,097 | |
| | Remove net book value of Leasehold Improvements | | | (106 | ) | | (109 | ) |
| | | | | | | |
| | Property and equipment — U.S. GAAP | | $ | 2,625 | | $ | 1,988 | |
| | | | | | | |
| | | | | | | | |
| |
| | September 30,
2010 | | September 30,
2009 | |
|---|
| | Net loss — Canadian GAAP | | $ | (8,073 | ) | $ | (5,910 | ) |
| | Net adjustment to expense Leasehold Improvements and remove amortization of Leasehold Improvements | | | 3 | | | 11 | |
| | | | | | | |
| | Net loss — U.S. GAAP | | $ | (8,070 | ) | $ | (5,899 | ) |
| | | | | | | |
| | | | | | | | |
| |
| | September 30,
2010 | | September 30,
2009 | |
|---|
| | Deficit — Canadian GAAP | | $ | (33,485 | ) | $ | (25,412 | ) |
| | Net Adjustment | | | (106 | ) | | (109 | ) |
| | | | | | | |
| | Deficit — U.S. GAAP | | $ | (33,591 | ) | $ | (25,521 | ) |
| | | | | | | |
| | | | | | | | |
| |
| | September 30,
2010 | | September 30,
2009 | |
|---|
| | Cash provided by (used in) operations — Canadian GAAP | | $ | (7,176 | ) | $ | (4,093 | ) |
| | Expense Leasehold Improvements | | | (23 | ) | | (13 | ) |
| | | | | | | |
| | Cash used in operations — U.S. GAAP | | $ | (7,199 | ) | $ | (4,106 | ) |
| | | | | | | |
| | Cash provided by (used in) investing — Canadian GAAP | | $ | (593 | ) | $ | (299 | ) |
| | Remove additions to Leasehold Improvements | | | 23 | | | 13 | |
| | | | | | | |
| | Cash provided by (used in) investing — U.S. GAAP | | $ | (570 | ) | $ | (286 | ) |
| | | | | | | |
SQI-F-36
SQI Diagnostics Inc.
SUPPLEMENTAL NOTE REGARDING THE
RECONCILIATION OF THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED SEPTEMBER 30, 2010 AND 2009 WITH U.S. GAAP (Continued)
Dated July 4, 2011
- (ii)
- The Company is a development stage company. Accordingly, the following tables detailing the Company's Statements of Operations, Shareholders' Equity and Cash Flows from inception (December 1, 1999) to September 30, 2010 and 2009 are required to be presented:
| | | | | | | | | |
| |
| | From
Inception to
September 30, 2010 | | From
Inception to
September 30, 2009 | |
|---|
| | Revenue | | | | | | | |
| | | Product Sales | | $ | 10 | | $ | 5 | |
| | | Consulting Fees | | | 1,260 | | | 1,230 | |
| | | | | | | |
| | | | | 1,270 | | | 1,235 | |
| | | | | | | |
| | Expenses | | | | | | | |
| | | Salaries and wages | | | 3,855 | | | 3,299 | |
| | | General and administrative | | | 3,220 | | | 2,727 | |
| | | Professional and consulting fees | | | 3,011 | | | 2,352 | |
| | | Sales and marketing | | | 883 | | | 409 | |
| | | Stock-based compensation | | | 1,600 | | | 1,198 | |
| | | Research and development costs | | | 19,175 | | | 14,116 | |
| | | Amortization — patents and trademarks | | | 729 | | | 629 | |
| | | Amortization — property and equipment | | | 2,295 | | | 1,905 | |
| | | | | | | |
| | | | $ | 34,768 | | $ | 26,635 | |
| | | | | | | |
| | | Loss before the undernoted items | | | (33,498 | ) | | (25,400 | ) |
| | | Interest income | | |
381 | | |
349 | |
| | | Interest expense | | | (87 | ) | | (83 | ) |
| | | Settlement of litigation | | | (205 | ) | | (205 | ) |
| | | Loss on disposal of property and equipment | | | (182 | ) | | (182 | ) |
| | | | | | | |
| | | | | (93 | ) | | (121 | ) |
| | | | | | | |
| | | Net loss and deficit accumulated during the development stage, end of period | | $ | (33,591 | ) | $ | (25,521 | ) |
| | | | | | | |
SQI-F-37
SQI Diagnostics Inc.
SUPPLEMENTAL NOTE REGARDING THE
RECONCILIATION OF THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED SEPTEMBER 30, 2010 AND 2009 WITH U.S. GAAP (Continued)
Dated July 4, 2011
Statement of Shareholders' Equity
(amounts in thousands of dollars, shares in thousands)
| | | | | | | | | | | | | | | | | |
| |
| | Share Capital | |
| |
| |
| |
|---|
| |
| |
| |
| | Deficit
Accumulated in
Development
Stage | |
|---|
| |
| | Number
of shares | | Amount | | Contributed
Surplus | | Warrant
Capital | |
|---|
| | Balance At Inception | | | 2 | | $ | 850 | | | | | | | | | — | |
| | Net loss | | | | | | | | | | | | | | $ | (4,631 | ) |
| | | | | | | | | | | | | |
| | Balance December 14, 2002, 2001 and 2000 | | | 2 | | $ | 850 | | | — | | | | | $ | (4,631 | ) |
| | | | | | | | | | | | | |
| | Net loss | | | | | | | | | | | | | | | (3,112 | ) |
| | | | | | | | | | | | | |
| | Balance December 14, 2003 | | | 2 | | $ | 850 | | | — | | | | | $ | (7,743 | ) |
| | | | | | | | | | | | | |
| | Issued in connection with a private placement | | | 10 | | | 1,516 | | | | | | | | | | |
| | Stock split | | | 5,977 | | | — | | | | | | | | | | |
| | Issued in connection with a private placement | | | 669 | | | 2,788 | | | | | | | | | | |
| | Forgiveness of indebtedness to parent on reorganization | | | | | | | | $ | 7,120 | | | | | | | |
| | Share issuance costs | | | | | | (342 | ) | | | | | | | | | |
| | Stock based compensation | | | | | | | | | 9 | | | | | | | |
| | Net loss | | | | | | | | | | | | | | | (1,559 | ) |
| | | | | | | | | | | | | |
| | Balance December 14, 2004 | | | 6,658 | | $ | 4,812 | | $ | 7,129 | | | — | | $ | (9,302 | ) |
| | | | | | | | | | | | | |
| | Issued on exercise of warrants | | | 100 | | | 10 | | | | | | | | | | |
| | Stock based compensation | | | | | | | | | 341 | | | | | | | |
| | Net loss | | | | | | | | | | | | | | | (1,804 | ) |
| | | | | | | | | | | | | |
| | Balance December 14, 2005 | | | 6,758 | | $ | 4,822 | | $ | 7,470 | | | — | | $ | (11,106 | ) |
| | | | | | | | | | | | | |
| | Conversion of convertible debentures | | | 2,073 | | | 4,147 | | | | | | | | | | |
| | Convertible debenture issuance costs | | | | | | (454 | ) | | | | | | | | | |
| | Stock based compensation | | | | | | | | | 268 | | | | | | | |
| | Net loss | | | | | | | | | | | | | | | (1,653 | ) |
| | | | | | | | | | | | | |
| | Balance December 14, 2006 | | | 8,831 | | $ | 8,515 | | $ | 7,738 | | | — | | $ | (12,759 | ) |
| | | | | | | | | | | | | |
| | Shares of Emblem Capital | | | 8,000 | | $ | 867 | | | | | | | | | | |
| | Share consolidation of Emblem Shares at 6 for 1 | | | (6,667 | ) | | | | | | | | | | | | |
| | Elimination of share capital due to reverse take over | | | | | | (867 | ) | | | | | | | | | |
| | Common shares of Umedik exchanged for shares SQI | | | (8,831 | ) | | | | | | | | | | | | |
| | Net asset value of SQI ascribed to issued shares | | | | | | 595 | | | | | | | | | | |
| | Issued on exercise of warrants | | | 78 | | | 8 | | | | | | | | | | |
| | Share issued to former shareholders of Umedik | | | 14,719 | | | | | | | | | | | | | |
| | Proceeds from private placement units | | | 3,568 | | | 5,212 | | | | | | | | | | |
| | Amount of private placement allocated to warrants | | | | | | (89 | ) | | | | | 89 | | | | |
| | Share issuance costs | | | | | | (128 | ) | | | | | | | | | |
| | Stock based compensation | | | | | | | | | 53 | | | | | | | |
| | Net loss | | | | | | | | | | | | | | | (3,098 | ) |
| | | | | | | | | | | | | |
| | Balance, September 30, 2007 | | | 19,698 | | $ | 14,113 | | $ | 7,791 | | $ | 89 | | $ | (15,857 | ) |
| | | | | | | | | | | | | |
| | Issued on exercise of warrants | | | 52 | | | 5 | | | | | | | | | | |
| | Issued in connection with private placement | | | 2,439 | | | 3,660 | | | | | | | | | | |
| | Issued on exercise of options | | | 28 | | | 39 | | | (6 | ) | | | | | | |
| | Share issuance costs, including broker warrants | | | | | | (494 | ) | | | | | 185 | | | | |
| | Stock based compensation | | | | | | | | | 283 | | | | | | | |
| | Net loss | | | | | | | | | | | | | | | (3,765 | ) |
| | | | | | | | | | | | | |
| | Balance, September 30, 2008 | | | 22,217 | | $ | 17,323 | | $ | 8,068 | | $ | 274 | | $ | (19,622 | ) |
| | | | | | | | | | | | | |
SQI-F-38
SQI Diagnostics Inc.
SUPPLEMENTAL NOTE REGARDING THE
RECONCILIATION OF THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED SEPTEMBER 30, 2010 AND 2009 WITH U.S. GAAP (Continued)
Dated July 4, 2011
| | | | | | | | | | | | | | | | | |
| |
| | Share Capital | |
| |
| |
| |
|---|
| |
| |
| |
| | Deficit
Accumulated in
Development
Stage | |
|---|
| |
| | Number
of shares | | Amount | | Contributed
Surplus | | Warrant
Capital | |
|---|
| | Issued on exercise of warrants | | | 1,111 | | | 689 | | | | | | | | | | |
| | Issued in connection with private placement | | | 3,732 | | | 4,664 | | | | | | | | | | |
| | Issued on exercise of options | | | 133 | | | 35 | | | (17 | ) | | | | | | |
| | Share issuance costs, including broker warrants | | | | | | (345 | ) | | | | | 187 | | | | |
| | Stock based compensation | | | | | | | | | 380 | | | | | | | |
| | Net loss | | | | | | | | | | | | | | | (5,899 | ) |
| | | | | | | | | | | | | |
| | Balance, September 30, 2009 | | | 27,193 | | $ | 22,366 | | $ | 8,431 | | $ | 461 | | $ | (25,521 | ) |
| | | | | | | | | | | | | |
| | Issued on exercise of warrants | | | 970 | | | 2,008 | | | 60 | | | (213 | ) | | | |
| | Proceeds from private placement units | | | 4,678 | | | 12,295 | | | | | | | | | | |
| | Amount of private placement allocated to warrants | | | | | | (1,424 | ) | | | | | 1,424 | | | | |
| | Issued on exercise of options | | | 917 | | | 755 | | | (61 | ) | | | | | | |
| | Expiry of warrants | | | | | | | | | | | | (60 | ) | | | |
| | Share issuance | | | | | | (974 | ) | | | | | 187 | | | | |
| | Stock based compensation | | | | | | | | | 402 | | | | | | | |
| | Net loss | | | | | | | | | | | | | | | (8,070 | ) |
| | | | | | | | | | | | | |
| | Balance, September 30, 2010 | | | 33,758 | | $ | 35,026 | | $ | 8,832 | | $ | 1,799 | | $ | (33,591 | ) |
| | | | | | | | | | | | | |
SQI-F-39
SQI Diagnostics Inc.
SUPPLEMENTAL NOTE REGARDING THE
RECONCILIATION OF THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED SEPTEMBER 30, 2010 AND 2009 WITH U.S. GAAP (Continued)
Dated July 4, 2011
| | | | | | | | | |
| |
| | From
Inception to
September 30,
2010 | | From
Inception to
September 30,
2009 | |
|---|
| | Cash provided by (used in) Operations | | | | | | | |
| | Net loss | | $ | (33,591 | ) | $ | (25,521 | ) |
| | Items not affecting cash | | | | | | | |
| | | Amortization | | | | | | | |
| | | — patents and trademarks | | | 729 | | | 629 | |
| | | — property and equipment | | | 2,295 | | | 1,905 | |
| | | Patents written off | | | 97 | | | 97 | |
| | | Stock-based compensation | | | 1,736 | | | 1,334 | |
| | | Loss on sale of property and equipment | | | 182 | | | 182 | |
| | | Interest accrual | | | (10 | ) | | (8 | ) |
| | Changes in non-cash working capital items | | | | | | | |
| | | Amounts receivable | | | (615 | ) | | (652 | ) |
| | | Investment tax credit recoverable | | | 610 | | | 610 | |
| | | Due from related party | | | 7 | | | | |
| | | Inventory | | | (890 | ) | | (322 | ) |
| | | Prepaids and deposits | | | (163 | ) | | (65 | ) |
| | | Accounts payable and accrued liabilities | | | 1,114 | | | 510 | |
| | | | | | | |
| | | | | (28,499 | ) | | (21,301 | ) |
| | | | | | | |
| | Investing | | | | | | | |
| | Purchase of property and equipment | | | (4,466 | ) | | (4,070 | ) |
| | Additions to patents and trademarks | | | (1,460 | ) | | (1,285 | ) |
| | Due from related party | | | (97 | ) | | (97 | ) |
| | | | | | | |
| | | | | (6,023 | ) | | (5,452 | ) |
| | | | | | | |
| | Financing | | | | | | | |
| | | Loans from related parties | | | 7,120 | | | 7,120 | |
| | | Advances (repayment) of loan/notes payable | | | 3 | | | 3 | |
| | | Proceeds from private placement and exercise of warrants and options, net of share issuance costs | | | 35,553 | | | 21,556 | |
| | | Employee share purchase loan | | | (10 | ) | | (10 | ) |
| | | Cash acquired on reverse takeover | | | 607 | | | 607 | |
| | | Share subscription proceeds received | | | 657 | | | 657 | |
| | | | | | | |
| | | | | 43,930 | | | 29,933 | |
| | | | | | | |
| | Net change in cash and cash equivalents during the period, and cash and cash equivalents at end of period | | $ | 9,408 | | $ | 3,180 | |
| | Supplemental disclosure | | | | | | | |
| | | Equipment reallocated from inventory and segregated for use by the Company | | $ | 631 | | $ | — | |
| | | Cash paid for interest | | $ | 109 | | $ | 105 | |
- (iii)
- Statements of Cash Flows
Canadian GAAP permits the disclosure of a subtotal of the amount of funds provided by operations before changes in non-cash operating working capital items in the consolidated statements of cash flows. United States GAAP does not permit this subtotal to be included.
SQI-F-40
SQI Diagnostics Inc.
SUPPLEMENTAL NOTE REGARDING THE
RECONCILIATION OF THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED SEPTEMBER 30, 2010 AND 2009 WITH U.S. GAAP (Continued)
Dated July 4, 2011
- (iv)
- The Company grants stock options to directors, officers, employees and consultants as described in Note 12. The company has used the fair value method of accounting for stock options since the inception of the plan in accordance with Canadian GAAP and U.S. GAAP. Other disclosures related to share-based compensation are as follows:
- a.
- The total intrinsic value of options exercised during the year ended September 30, 2010 was $1,418,000 (September 30, 2009 — $149,000)
- b.
- The total number of options outstanding at September 30, 2010 had a weighted average remaining term of 2.8 years (September 30, 2009 — 2.4 years), and a total intrinsic value of $2,064,000 (September 30, 2009 — $2,179,000)
- c.
- The total number of options vested and exercisable at September 30, 2010 had a weighted average remaining term of 2.3 years (September 30, 2009 — 1.7 years), and a total intrinsic value of $1,300,000 (September 30, 2009 — $1,846,000)
- d.
- As at September 30, 2010, compensation costs not yet recognized relating to stock option awards outstanding was $989,000 (September 30, 2009 — $804,000). Compensation costs will be recognized over the remaining weighted average period of approximately 2 years (September 30, 2009 — 2.9 years)
- e.
- Estimated forfeiture rate did not result in a material impact on the stock based compensation expense.
- (v)
- Breakdown of accounts payable and accrued liabilities is as follows (amounts in thousands of dollars):
| | | | | | | | |
| |
| | September 30,
2010 | | September 30,
2009 | |
|---|
| | Trade payables | | $ | 763 | | $ | 187 | |
| | Accruals | | | 200 | | | 174 | |
| | Payroll liabilities | | | 9 | | | 5 | |
| | Note payable to supplier, non-interest bearing | | | — | | | 3 | |
| | | | | | | |
| | | | $ | 972 | | $ | 369 | |
| | | | | | | |
- (vi)
- Patents and trademarks
The aggregate amortization expense related to patents and trademarks for the next five years as at September 30, 2010 is as follows (amounts in thousands of dollars):
| | | | | |
| | 2011 | | $ | 116 | |
| | 2012 | | | 74 | |
| | 2013 | | | 59 | |
| | 2014 | | | 59 | |
| | 2015 | | | 59 | |
- (vii)
- Recently adopted accounting pronouncements
In June 2009, the FASB launched the FASB Accounting Standards Codification, or the Codification, as the single source of authoritative U.S. GAAP recognized by the FASB. The Codification reorganizes various U.S. GAAP pronouncements into accounting topics and displays them using a consistent structure. Rules and interpretive releases of the SEC under authority of federal securities laws are also sources of authoritative U.S. GAAP for SEC registrants. The Codification is effective for interim and annual periods ending after September 15, 2009. The adoption of this standard had no impact on the Company's financial statements.
In February 2010, the FASB issued ASU No. 2010-09, "Subsequent Events (ASC Topic 855), Amendments to Certain Recognition and Disclosure Requirements." This Standard update requires a SEC Filer to (1) evaluate subsequent events through the date that the financial statements are issued or available to be issued, (2) defines "SEC Filer" as an entity that is required to file or furnish its financial statements with either the SEC or, with respect to an entity subject to Section 12(i) of the Securities Exchange Act of 1934, as amended, the appropriate agency under that Section, (3) not be bound to disclosing the date through which subsequent events have been evaluated, (4) note the definition of public entity is no longer defined nor necessary for Topic 855, (5) note the scope of the reissuance disclosure requirements is refined to include revised financial statements only. These Updates are effective for interim or annual periods ending after June 15, 2010. ASU No. 2010-09 has no effect on the Company's financial position, statements of operations, or cash flows at this time.
SQI-F-41
SQI Diagnostics Inc.
SUPPLEMENTAL NOTE REGARDING THE
RECONCILIATION OF THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED SEPTEMBER 30, 2010 AND 2009 WITH U.S. GAAP (Continued)
Dated July 4, 2011
In May 2009, the FASB issued new guidance for accounting for subsequent events. The new guidance, which is now part of ASC 855-10, Subsequent Events (formerly, SFAS No. 165, Subsequent Events) is consistent with existing auditing standards in defining subsequent events as events or transactions that occur after the balance sheet date but before the financial statements are issued or are available to be issued, but it also requires the disclosure of the date through which an entity has evaluated subsequent events and the basis for that date. The new guidance defines two types of subsequent events: "recognized subsequent events" and "non-recognized subsequent events." Recognized subsequent events provide additional evidence about conditions that existed at the balance sheet date and must be reflected in the Company's financial statements. Non-recognized subsequent events provide evidence about conditions that arose after the balance sheet date and are not reflected in the financial statements of a company. Certain non-recognized subsequent events may require disclosure to prevent the financial statements from being misleading. The new guidance was effective on a prospective basis for interim or annual periods ending after June 15, 2009. The Company adopted the provisions of ASC 855-10 as required.
SQI-F-42
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS OF SCIENION AG
| | | | |
| 1. | | Unaudited interim financial statements for Scienion for the six month period ended June 30, 2011 | | SC-F-2 |
2. |
|
Audited financial statements for Scienion for the years ended December 31, 2010 and 2009 |
|
SC-F-11 |
3. |
|
Auditors' Report |
|
SC-F-36 |
SC-F-1
Scienion AG,
Dortmund
INTERIM STATEMENT OF COMPREHENSIVE INCOME FOR THE PERIODS
FROM JANUARY 1ST TO JUNE 30TH, 2011
AND APRIL 1ST TO JUNE 30TH, 2011
(Unaudited)
| | | | | | | | | | | | | |
| |
| | Note | | Jan. 1st to
Jun. 30th
2011 | | Apr. 1st to
Jun. 30th
2011 | |
|---|
| |
| |
| | EUR thou.
| | EUR thou.
| |
|---|
| | 1. | | Revenue | | | (2 | ) | | 3,145 | | | 2,217 | |
| | 2. | | Change in inventories of work in process | | | | | | (166 | ) | | (112 | ) |
| | | | | | | | | | | | |
| | | | | | | | | | 2,979 | | | 2,105 | |
| | 4. | | Material expenses | | | | | | 783 | | | 500 | |
| | 5. | | Personnel expenses | | | | | | 983 | | | 455 | |
| | 6. | | Depreciation, amortization, impairments
on intangible assets and property, plant and equipment | | | | | | 117 | | | 58 | |
| | 7. | | Other operating expenses | | | | | | 614 | | | 365 | |
| | 8. | | Other operating income | | | | | | 142 | | | 79 | |
| | | | | | | | | | | | |
| | 9. | | Operating income | | | | | | 625 | | | 806 | |
| | 10. | | Interest expense | | | | | | 140 | | | 70 | |
| | 11. | | Interest income | | | | | | 1 | | | 1 | |
| | | | | | | | | | | | |
| | 12. | | Income before taxes | | | | | | 485 | | | 737 | |
| | 13. | | Taxes on income | | | (3 | ) | | 125 | | | 156 | |
| | 14. | | Other taxes | | | | | | 2 | | | 2 | |
| | | | | | | | | | | | |
| | 15. | | Total comprehensive income | | | | | | 359 | | | 579 | |
| | | | | | | | | | | | |
| | | | earnings per ordinary and preference share | | | | | | 1,92 EUR | | | 3,10 EUR | |
SC-F-2
Scienion AG,
Dortmund
INTERIM STATEMENT OF FINANCIAL POSITION AS OF 30TH JUNE, 2011
(Unaudited)
| | | | | | | | | | | | | |
| |
| | Note | | 6/30/2011 | | 12/31/2010 | |
|---|
| |
| |
| | EUR thou.
| | EUR thou.
| |
|---|
| | Assets | | | | | | | | | | |
|
A. |
|
Non-current assets |
|
|
|
|
|
|
|
|
|
|
| | I. | | Intangible assets | | | (4) | | | 232 | | | 253 | |
| | II. | | Property, plant and equipment | | | (4) | | | 491 | | | 561 | |
| | III. | | Deferred tax assets | | | | | | 107 | | | 14 | |
| | | | | | | | | | | | |
| | | | | | | | | | 830 | | | 828 | |
| | | | | | | | | | | | |
| | B. | | Current assets | | | | | | | | | | |
| | I. | | Inventories | | | (5) | | | 904 | | | 933 | |
| | | | | | | | | | | | |
| | II. | | Receivables and other assets | | | | | | | | | | |
| | | | 1. Trade receivables | | | (6)(7) | | | 2,194 | | | 643 | |
| | | | 2. Other receivables and assets | | | | | | 271 | | | 256 | |
| | | | | | | | | | | | |
| | | | | | | | | | 2,465 | | | 899 | |
| | | | | | | | | | | | |
| | III. | | Cash and cash equivalents | | | (8) | | | 379 | | | 392 | |
| | | | | | | | | | | | |
| | | | | | | | | | 4,578 | | | 3,052 | |
| | | | | | | | | | | | |
| | Liabilities | | | | | | | | | | |
|
A. |
|
Equity |
|
|
|
|
|
|
|
|
|
|
| | I. | | Subscribed capital | | | | | | 187 | | | 187 | |
| | II. | | Contributed Surplus | | | | | | 6,760 | | | 6,760 | |
| | III. | | Retained earnings | | | | | | (9,549 | ) | | (9,908 | ) |
| | | | | | | | | | | | |
| | | | | | | | | | (2,602 | ) | | (2,961 | ) |
| | | | | | | | | | | | |
| | B. | | Liabilities | | | | | | | | | | |
| | I. | | Non-current liabilities | | | | | | | | | | |
| | | | 1. non-current financial liabilities | | | (9) | | | 150 | | | 200 | |
| | | | 2. Deferred tax liabilities | | | | | | 308 | | | 89 | |
| | | | | | | | | | | | |
| | | | | | | | | | 458 | | | 289 | |
| | | | | | | | | | | | |
| | II. | | Current liabilities | | | | | | | | | | |
| | | | 1. Current financial liabilities | | | (9) | | | 5,329 | | | 5,177 | |
| | | | 2. Trade payables | | | (10) | | | 706 | | | 108 | |
| | | | 3. Prepayments received | | | | | | 2 | | | 0 | |
| | | | 4. Provisions | | | | | | 469 | | | 222 | |
| | | | 5. Other accrued liabilities | | | | | | 0 | | | 122 | |
| | | | 6. Other accounts payables | | | | | | 210 | | | 91 | |
| | | | 7. Deferred income | | | | | | 6 | | | 4 | |
| | | | | | | | | | | | |
| | | | | | | | | | 6,722 | | | 5,724 | |
| | | | | | | | | | | | |
| | | | | | | | | | 4,578 | | | 3,052 | |
| | | | | | | | | | | | |
SC-F-3
Scienion AG, Dortmund
INTERIM STATEMENT OF CHANGES IN EQUITY
(Unaudited)
| | | | | | | | | | | | | |
| | Subscribed
Capital | | Contributed
Surplus | | Retained earnings
(retained profits or
losses brought
forward) | | Total | |
|---|
| | EUR thou.
| | EUR thou.
| | EUR thou.
| | EUR thou.
| |
|---|
31st December, 2009 | | | 187 | | | 6,760 | | | (9,744 | ) | | (2,797 | ) |
| | | | | | | | | | |
Net loss for the year 2010 | | | 0 | | | 0 | | | (164 | ) | | (164 | ) |
| | | | | | | | | | |
Total comprehensive loss for the Period | | | 0 | | | 0 | | | (164 | ) | | (164 | ) |
| | | | | | | | | | |
31st December, 2010 | | | 187 | | | 6,760 | | | (9,908 | ) | | (2,961 | ) |
| | | | | | | | | | |
Net income for the half year period 2011 | | | 0 | | | 0 | | | 359 | | | 359 | |
| | | | | | | | | | |
Total comprehensive income for the Period | | | 0 | | | 0 | | | 359 | | | 359 | |
| | | | | | | | | | |
30th June, 2011 | | | 187 | | | 6,760 | | | (9,549 | ) | | (2,602 | ) |
| | | | | | | | | | |
SC-F-4
Scienion AG, Dortmund
INTERIM STATEMENT OF CASH FLOWS
(unaudited)
| | | | | | | |
| | Jan. 1st to
Jun. 30th
2011 | | Jan. 1st to
Dec. 31th
2010 | |
|---|
| | EUR thou.
| | EUR thou.
| |
|---|
Profit/loss before taxes | | | 485 | | | (154 | ) |
Depreciation of property, plant & equipment | | | 74 | | | 151 | |
Amortization of intangible assets | | | 42 | | | 76 | |
Gains/Losses from disposal of property, plant & equipment | | | 0 | | | (26 | ) |
Interest income | | | (1 | ) | | (2 | ) |
Interest expense | | | 140 | | | 284 | |
Movement in provisions | | | 247 | | | 177 | |
Gains on research and developments grants | | | (102 | ) | | (572 | ) |
| | | | | | |
Gross cash flow | | | 885 | | | (66 | ) |
| | | | | | |
Increase/decrease in inventories | | | 29 | | | (66 | ) |
Increase/decrease in trade and other receivables | | | (1,631 | ) | | 75 | |
Increase/decrease in trade and other payables | | | 597 | | | (323 | ) |
| | | | | | |
| | | (1,005 | ) | | (314 | ) |
| | | | | | |
Interest received | | | 1 | | | 2 | |
| | | | | | |
Income tax paid | | | 0 | | | 0 | |
| | | | | | |
Net cash from operating activities | | | (119 | ) | | (378 | ) |
| | | | | | |
Purchase of intangible assets | | | 0 | | | (84 | ) |
Purchase of property, plant & equipment | | | (21 | ) | | (90 | ) |
Cash receipts from disposal of assets | | | 4 | | | 51 | |
| | | | | | |
Net cash used in investing activities | | | (17 | ) | | (123 | ) |
| | | | | | |
Credit repayments | | | (25 | ) | | (100 | ) |
Interest paid | | | (9 | ) | | (25 | ) |
Payment of finance lease liabilities | | | (2 | ) | | (3 | ) |
Cash receipts from research and developments grants | | | 159 | | | 537 | |
| | | | | | |
Net cash used/from financing activities | | | 123 | | | 409 | |
| | | | | | |
Net decrease/increase in cash and cash equivalents | | | (13 | ) | | (92 | ) |
| | | | | | |
Cash and cash equivalents at beginning of year | | | 392 | | | 484 | |
Cash and cash equivalents at end of year | | | 379 | | | 392 | |
SC-F-5
Scienion AG, Dortmund
NOTES TO THE INTERIM FINANCIAL STATEMENTS
(unaudited)
REPORTING ENTITY
Scienion AG (the "Company") is an incorporated company (Aktiengesellschaft) domiciled in Germany. The address of the Company's registered office is Dortmund. The Company is primarily involved in the markets of ultra-low volume liquid handling systems and microarray technologies.
The interim financial statements for the six months ended 30 June 2011 were authorised for issuance by the Board of Directors on 25 August 2011.
The company is formally overindebted as of 30 June 2011. Most of the liabilities in connection with the silent participation, for which a declaration of subordination has been issued for up to € 3,506 thousand, have a remaining term until the end of 2011. The management board assumes that the contract signed with the investor SQI Diagnostics Inc., Toronto, Ontario, Canada, on 4 July 2011 which provides for the investor to acquire all shares and to redeem the silent participations as well as to equip Scienion AG with the necessary capital resources will take effect after certain contractually agreed conditions precedent have been satisfied. If the transaction fails, the company's ability to continue as a going concern hinges on the liabilities from silent participations, including interest and costs that will be due on 31 December 2011 not falling due after that date but being made available to the company as substitute equity to cover the capital deficit prevailing at the time. Subject to the final approval of their corporate bodies the silent shareholders have agreed to take appropriate measures ranging from a prolongation of the silent participation to a partial or even full waiver. The Company is therefore acting the assumption that it is more likely than not that Scienion AG will be able to continue as a going concern.
BASIS OF PREPARATION AND CHANGES TO THE COMPANY'S ACCOUNTING POLICIES
Basis of preparation
The interim financial statements for the six months ended 30 June 2011 have been prepared in accordance with IAS 34Interim Financial Reporting except for the figures of the comparative periods in the Interim Statement of Comprehensive Income, the Interim Statement of Changes in Equity and the Interim Statement of Cash Flows. No comparative figures have been disclosed as no financial data for the prior year interim periods could be reliably compiled.
The interim financial statements for the six months ended 30 June 2011 do not include all the information and disclosures required in the annual financial statements, and should be read in conjunction with the Company's annual financial statements as of 31 December 2010.
New standards, interpretations and amendments thereof, adopted by the Company
The accounting policies adopted for the preparation of the interim financial statements for the six months ended 30 June 2011 are consistent with those followed in the preparation of Company's annual financial statements for the year ended 31 December 2010 except for the following pronoucements which are mandatory as of fiscal years beginning on or after 1 January 2011:
- •
- IAS 24 Related party disclosures (Revised)
- •
- IAS 32 Classification of Rights (Amendment)
- •
- IFRIC 14 Prepayments of a Minimum Funding Requirement
- •
- IFRIC 19 Extinguishing Financial Liabilities with Equity Instruments
In addition, various IFRS were amended as part of the annual "improvement" project for 2010.
The application of these new or amended standards and interpretations did not have any effect on the interim financial statements for the six months ended 30 June 2011.
1. SEASONALITY OF OPERATIONS
As the Company is involved in markets for special technologies and therefore supplies a selected customer base, no substantial seasonal effects concerning its operations arise.
SC-F-6
Scienion AG, Dortmund
NOTES TO THE INTERIM FINANCIAL STATEMENTS (Continued)
(unaudited)
2. REVENUE
| | | | | | | | |
| |
| | Jan. 1st to Jun. 30th
2011 | | Apr. 1st to Jun. 30th
2011 | |
|---|
| |
| | EUR thou.
| | EUR thou.
| |
|---|
| | Delivery of Hardware | | | 2,973 | | | 2,202 | |
| | Consulting services | | | 169 | | | 14 | |
| | Other | | | 3 | | | 1 | |
| | | | | | | |
| | Total Revenue | | | 3,145 | | | 2,217 | |
| | | | | | | |
3. INCOME TAX
| | | | | | | | |
| |
| | Jan. 1st to Jun. 30th
2011 | | Apr. 1st to Jun. 30th
2011 | |
|---|
| |
| | EUR thou.
| | EUR thou.
| |
|---|
| | Current tax expense | | | 0 | | | 0 | |
| | Deferred tax expense | | | 125 | | | 156 | |
| | From temporary differences | | | 125 | | | 156 | |
| | From loss carryforwards | | | 0 | | | 0 | |
| | Total deferred taxes | | | 125 | | | 156 | |
| | | | | | | |
| | Total income tax expense | | | 125 | | | 156 | |
| | | | | | | |
As of 30 June 2011, the Company has tax losses carried forward of € 10,279 thousand (CIT) and of € 9,283 thousand (TT) that are not more likely than not available for offset against future taxable profits, because of the planned transfer of all shares. According to German tax law there is a high likelihood that the existing loss carry forwards will be forfeited resulting from the transfer of all shares of Scienion AG in 2011. Thus, the management did not capitalize any deferred taxes on the existing loss carry forwards.
Deferred tax assets have not been recognised in respect of the transfer of Scienion shares. The Company evaluated and concluded that it is not probable that deferred tax assets on existing tax losses will be recovered. The Company has neither taxable temporary differences nor any tax planning opportunities available that could partly support the recognition of these losses as deferred tax assets.
The following reconciliation of income tax expense for the 2011 interim period is based on an overall tax rate of 31.6% (previous year: 31.6%).
| | | | | | | | |
| |
| | Jan. 1st to Jun. 30th
2011 | | Apr. 1st to Jun. 30th
2011 | |
|---|
| |
| | EUR thou.
| | EUR thou.
| |
|---|
| | Net profit before income taxes | | | 485 | | | 796 | |
| | Expected tax expense 31.6% | | | 153 | | | 252 | |
| | Effect of non-deductible expenses and tax-exempt income | | | 3 | | | 1 | |
| | Recognition of previously unrecognised deferred tax assets related to tax loss carryforward or temporary differences | | | (27 | ) | | (95 | ) |
| | Other | | | (4 | ) | | (2 | ) |
| | | | | | | |
| | Total income tax expense | | | 125 | | | 156 | |
| | | | | | | |
4. PROPERTY, PLANT AND EQUIPMENT
During the interim financial period ending 30 June 2011, the Company acquired property, plant and equipment with a cost of € 4 thousand (2010: € 43 thousand).
Furthermore, no unscheduled write-downs were necessary in the interim financial period.
SC-F-7
Scienion AG, Dortmund
NOTES TO THE INTERIM FINANCIAL STATEMENTS (Continued)
(unaudited)
5. INVENTORIES
No write-downs of inventories to the net realisable value take place in the period from 1 January to 30 June 2011 as they were not necessary (previous year: € 17 thousand).
6. TRADE RECEIVABLES
| | | | | | | | |
| |
| | 30. Jun. 2011 | | 31. Dec. 2010 | |
|---|
| |
| | EUR thou.
| | EUR thou.
| |
|---|
| | Trade receivables | | | 2,083 | | | 704 | |
| | Allowances for doubtful accounts | | | (71 | ) | | (79 | ) |
| | Gross amounts due from customer for construction contracts/contract work | | | 182 | | | 0 | |
| | Foreign currency exchange | | | 0 | | | 18 | |
| | | | | | | |
| | Total trade receivables | | | 2,194 | | | 643 | |
| | | | | | | |
Due to the attainment of certain milestones in construction contracts, the amount of trade receivables as of 30 June 2011 rose substantially comparing to 31 December 2010.
7. GROSS AMOUNTS DUE FROM CUSTOMERS FOR CONSTRUCTION CONTRACTS/CONTRACT WORK
| | | | | | | | |
| |
| | 30. Jun. 2011 | | 31. Dec. 2010 | |
|---|
| |
| | EUR thou.
| | EUR thou.
| |
|---|
| | Contract revenue | | | 820 | | | 0 | |
| | Contract costs | | | 350 | | | 0 | |
| | Aggregate recognized revenues | | | 820 | | | 0 | |
| | Aggregate prepayments received | | | 638 | | | 0 | |
| | | | | | | |
| | Gross amounts due from customers for construction contracts/contract work | | | 182 | | | 0 | |
| | | | | | | |
Gross amounts due from customers for construction contracts/contract work as of reporting date 30 June 2011 relate to three major contracts.
8. CASH AND CASH EQUIVALENTS
Cash comprise of cash on hand, cash in bank and restricted cash due to rental deposits and a warranty guarantee. Cash in hand and bank balances at the reporting date were calculated as follows:
| | | | | | | | |
| |
| | 30. Jun. 2011 | | 31. Dec. 2010 | |
|---|
| |
| | EUR thou.
| | EUR thou.
| |
|---|
| | Cash on hand and bank balances | | | 342 | | | 359 | |
| | Restricted cash | | | 37 | | | 33 | |
| | | | | | | |
| | Total cash and cash equivalents | | | 379 | | | 392 | |
| | | | | | | |
9. FINANCIAL LIABILITIES
| | | | | | | | |
| |
| | 30. Jun. 2011 | | 31. Dec. 2010 | |
|---|
| |
| | EUR thou.
| | EUR thou.
| |
|---|
| | Silent partnerships organised under German law | | | 3,573 | | | 3,598 | |
| | Accumulated interest payable | | | 1,906 | | | 1,779 | |
| | | | | | | |
| | Total Financial Liabilities | | | 5,479 | | | 5,377 | |
| | | | | | | |
SC-F-8
Scienion AG, Dortmund
NOTES TO THE INTERIM FINANCIAL STATEMENTS (Continued)
(unaudited)
9. FINANCIAL LIABILITIES (Continued)
Contractual terms have not changed during the interim financial period and can be found in the financial statements as of 31 December 2010.
10. TRADE PAYABLES
At the balance sheet date 30 June 2011, the Company had the following trade payables:
| | | | | | | | |
| |
| | 30. Jun. 2011 | | 31. Dec. 2010 | |
|---|
| |
| | EUR thou.
| | EUR thou.
| |
|---|
| | Total trade payables | | | 706 | | | 108 | |
The amount of trade payables as of 30 June 2011 rose significantly compared to 31 December 2010 due to increased purchases of materials resulting from construction contracts.
11. FINANCE LEASES
As of June 30, 2011 and December 31, 2010 the Company had entered into one financial leasing contract regarding technical equipment:
As of June 30, 2011 the net book value of the leased asset amounted to € 37 thou. (December 31, 2010: € 45 thousand).
| | | | | | | | | | | | | | |
| |
| | 2011 | | 2012 | | 2013-2016 | | 2017 | |
|---|
| |
| | EUR thou.
| | EUR thou.
| | EUR thou.
| | EUR thou.
| |
|---|
| | Notional amount of future minimum lease payments | | | 17 | | | 20 | | | 14 | | | 0 | |
12. OTHER FINANCIAL COMMITMENTS
At 30 June 2011, the Company has not entered into any new financial commitment compared with 31 December 2010.
13. RELATED PARTY TRANSACTIONS
- a.
- Transaction with shareholder
The shareholders Peppermint Venture Capital GmbH & Co. KG, Berlin and NRW.Bank. Venture Fonds GmbH & Co. KG, Düsseldorf have significant influence over the Company. Additional they are silent partners. Therefore these shareholders have to be classified as related parties in accordance with IAS 24. In the past, the Company received financial support through silent partnerships. Due to subordinate agreements concerning prolongation of the partnerships signed by the parties no repayment has taken place in the financial periods.
The following table provides the total amount of transactions that have been entered into with Peppermint Venture Capital GmbH & Co. KG, Berlin and NRW.Bank. Venture Fonds GmbH & Co. KG, Düsseldorf for the relevant financial years:
| | | | | | | | | | | | | | | | | |
| |
| |
| |
| |
| | Amounts owed to related parties | |
|---|
| | EUR thou. | |
| | Interest
paid | | Repayments | | Due to
interests | | Due to
participation | |
|---|
| | Peppermint Venture Capital GmbH & Co. KG, Berlin | | | 30.06.2011 | | | — | | | — | | | 550 | | | 865 | |
| | | | | 31.12.2010 | | | | | | | | | 515 | | | 865 | |
| | NRW.Bank. Venture Fonds GmbH & Co. KG, Düsseldorf | | | 30.06.2011 | | | — | | | — | | | 295 | | | 462 | |
| | | | | | | | | | | | | | |
| | | | | 31.12.2010 | | | — | | | — | | | 275 | | | 462 | |
| | | | | | | | | | | | | | |
SC-F-9
Scienion AG, Dortmund
NOTES TO THE INTERIM FINANCIAL STATEMENTS (Continued)
(unaudited)
13. RELATED PARTY TRANSACTIONS (Continued)
- b.
- Transactions with key management personnel
Key management includes members of the management board as well as members of the supervisory board, which are composed as follows:
Management board
Dr. Holger Eickhoff, CEO
Dr. Günter Bauer, COO
Supervisory board
Dr. Joachim Rautter, (chairman)
Ute Mercker, (deputy chairman)
Dr. Jörn Erselius, Dr. Peter Güllmann,
Dr. Timm-H. Jessen, Dr. Richard Reinhardt,
The compensation paid or payable to key management is shown below:
The compensation paid to key management is shown below:
| | | | | | | | |
| |
| | Jan-July
2011 | | 2010 | |
|---|
| |
| | EUR thou.
| | EUR thou.
| |
|---|
| | Salaries of members of the management board | | | 138 | | | 307 | |
| | Attendance fee for members of the supervisory board | | | 6 | | | 22 | |
| | | | | | | | |
| |
| | Jan-July
2011 | | 2010 | |
|---|
| |
| | EUR thou.
| | EUR thou.
| |
|---|
| | Salaries of members of the management board | | | 68 | | | 135 | |
| | Attendance fee for members of the supervisory board | | | 7 | | | 2 | |
14. EVENTS AFTER THE BALANCE SHEET DATE
Dated July 4 2011 the shareholders of Scienion AG have concluded a share purchase agreement with SQI Diagnostics Inc., Toronto, Ontario, Canada. Based on the share purchase agreement all shares of Scionion AG are purchased by and will transferred to SQI Diagnostics. The completation of the sale/purchase of all Scienion AG shares is subject to several conditions as set out in the share purchase agreement. Among others the completation of the transaction is contingent upon the closing of the purchaser`s (SQI) financing of at least C$30 Mio.
Until the date of authorisation of these financial statements, the contract signed with SQI has had no significant influence to the results of operations, net assets and financial position of the Company with the exception of not capitalizing any deferred tax assets on the existing tax loss carry forwards.
No other events or developments of particular significance to the results of operations, net assets and financial position of the Scienion AG have occurred since the balance sheet date of 30 June 2011.
SC-F-10
Scienion Ag, Dortmund
STATEMENT OF COMPREHENSIVE INCOME FOR THE FISCAL YEAR 2010 AND 2009
| | | | | | | | | | | |
| |
| | Note | | 2010 | | 2009 | |
|---|
| |
| |
| | EUR thou.
| | EUR thou.
| |
|---|
| 1. | | Revenue | | (16) | | | 3,731 | | | 2,085 | |
| 2. | | Change in inventories of work in process | | (6) | | | 15 | | | 373 | |
| 3. | | Own work capitalized | | | | | 13 | | | 52 | |
| | | | | | | | | | |
| | | | | | | | 3,759 | | | 2,510 | |
| 4. | | Material expenses | | (18) | | | 1,140 | | | 799 | |
| 5. | | Personnel expenses | | (20) | | | 1,920 | | | 1,845 | |
| 6. | | Depreciation, amortization, impairments on intangible assets and property, plant and equipment | | (3),(4) | | | 227 | | | 206 | |
| 7. | | Other operating expenses | | (19) | | | 1,047 | | | 979 | |
| 8. | | Other operating income | | (17) | | | 703 | | | 620 | |
| | | | | | | | | | |
| 9. | | Operating income | | | | | 128 | | | (699 | ) |
| 10. | | Interest expense | | (21) | | | 284 | | | 288 | |
| 11. | | Interest income | | (21) | | | 2 | | | 18 | |
| | | | | | | | | | |
| 12. | | Loss before taxes | | | | | (154 | ) | | (969 | ) |
| 13. | | Taxes on income | | (22) | | | 9 | | | (8 | ) |
| 14. | | Other taxes | | | | | 1 | | | 1 | |
| | | | | | | | | | |
| 15. | | Net loss/ total comprehensive loss | | | | | (164 | ) | | (962 | ) |
| | | | | | | | | | |
|
|
earnings per ordinary and preference share |
|
(9) |
|
|
(0,88) EUR |
|
|
(5,15) EUR |
|
SC-F-11
Scienion AG, Dortmund
STATEMENT OF FINANCIAL POSITION AS OF 31st DECEMBER 2010 AND
AS OF 31st DECEMBER 2009
Assets
| | | | | | | | | | | | | | | |
| |
| | Note | | 12/31/2010 | | 12/31/2009 | | 1/1/2009 | |
|---|
| |
| |
| | EUR thou.
| | EUR thou.
| | EUR thou.
| |
|---|
A. | | Non-current assets | | | | | | | | | | | | | |
I. | | Intangible assets | | | (3 | ) | | 253 | | | 244 | | | 204 | |
II. | | Property, plant and equipment | | | (4 | ) | | 561 | | | 648 | | | 717 | |
III. | | Deferred tax assets | | | (5 | ) | | 14 | | | 45 | | | 16 | |
| | | | | | | | | | | | | |
| | | | | | | | 828 | | | 937 | | | 937 | |
| | | | | | | | | | | | | |
B. | | Current assets | | | | | | | | | | | | | |
I. | | Inventories | | | (6 | ) | | 933 | | | 867 | | | 485 | |
| | | | | | | | | | | | | |
II. | | Receivables and other assets | | | | | | | | | | | | | |
| | 1. Trade receivables | | | (7 | ) | | 643 | | | 671 | | | 815 | |
| | 2. Other receivables and assets | | | (7 | ) | | 256 | | | 267 | | | 355 | |
| | | | | | | | | | | | | |
| | | | | | | | 899 | | | 938 | | | 1.170 | |
| | | | | | | | | | | | | |
III. | | Cash and cash equivalents | | | (8 | ) | | 392 | | | 484 | | | 1,263 | |
| | | | | | | | | | | | | |
| | | | | | | | 3,052 | | | 3,226 | | | 3,855 | |
| | | | | | | | | | | | | |
SC-F-12
Scienion AG, Dortmund
STATEMENT OF FINANCIAL POSITION AS OF 31st DECEMBER 2010 AND
AS OF 31st DECEMBER 2009
Liabilities
| | | | | | | | | | | | | | | |
| |
| | Note | | 12/31/2010 | | 12/31/2009 | | 1/1/2009 | |
|---|
| |
| |
| | EUR thou.
| | EUR thou.
| | EUR thou.
| |
|---|
A. | | Equity | | | | | | | | | | | | | |
I. | | Subscribed capital | | | (9 | ) | | 187 | | | 187 | | | 187 | |
II. | | Contributed Surplus | | | (9 | ) | | 6,760 | | | 6,760 | | | 6,760 | |
III. | | Retained earnings | | | (9 | ) | | (9,908 | ) | | (9.744 | ) | | (8,782 | ) |
| | | | | | | | | | | | | |
| | | | | | | | (2,961 | ) | | (2,797 | ) | | (1,835 | ) |
| | | | | | | | | | | | | |
B. | | Liabilities | | | | | | | | | | | | | |
I. | | Non-current liabilities | | | | | | | | | | | | | |
| | 1. Non-current financial liabilities | | | (12 | ) | | 200 | | | 5,119 | | | 4,959 | |
| | 2. Deferred tax liabilities | | | (10 | ) | | 89 | | | 111 | | | 90 | |
| | | | | | | | | | | | | |
| | | | | | | | 289 | | | 5,230 | | | 5,049 | |
| | | | | | | | | | | | | |
II. | | Current liabilities | | | | | | | | | | | | | |
| | 1. Current financial liabilities | | | (12 | ) | | 5,177 | | | 100 | | | 62 | |
| | 2. Trade payables | | | (13 | ) | | 108 | | | 302 | | | 288 | |
| | 3. Prepayments received | | | (13 | ) | | 0 | | | 125 | | | 0 | |
| | 4. Provisions | | | (11 | ) | | 222 | | | 45 | | | 78 | |
| | 5. Other accrued liabilities | | | (13 | ) | | 122 | | | 112 | | | 101 | |
| | 6. Other accounts payables | | | (13 | ) | | 91 | | | 103 | | | 110 | |
| | 7. Deferred income | | | | | | 4 | | | 6 | | | 2 | |
| | | | | | | | | | | | | |
| | | | | | | | 5,724 | | | 793 | | | 641 | |
| | | | | | | | | | | | | |
| | | | | | | | 3,052 | | | 3,226 | | | 3,855 | |
| | | | | | | | | | | | | |
SC-F-13
Scienion AG, Dortmund
STATEMENT OF CHANGES IN EQUITY
| | | | | | | | | | | | | |
| | Subscribed
Capital | | Contributed
Surplus | | Retained earnings
(retained profits or losses brought forward) | | Total | |
|---|
| | EUR thou.
| | EUR thou.
| | EUR thou.
| | EUR thou.
| |
|---|
31st December, 2008 | | | 187 | | | 6,760 | | | (8,782 | ) | | (1,835 | ) |
| | | | | | | | | | |
Net loss for the year 2009 | | | 0 | | | 0 | | | (962 | ) | | (962 | ) |
| | | | | | | | | | |
Total comprehensive loss for the Period | | | 0 | | | 0 | | | (962 | ) | | (962 | ) |
| | | | | | | | | | |
31st December, 2009 | | | 187 | | | 6,760 | | | (9,744 | ) | | (2,797 | ) |
| | | | | | | | | | |
Net loss for the year 2010 | | | 0 | | | 0 | | | (164 | ) | | (164 | ) |
| | | | | | | | | | |
Total comprehensive loss for the Period | | | 0 | | | 0 | | | (164 | ) | | (164 | ) |
| | | | | | | | | | |
31st December, 2010 | | | 187 | | | 6,760 | | | (9,908 | ) | | (2,961 | ) |
| | | | | | | | | | |
SC-F-14
Scienion AG, Dortmund
STATEMENT OF CASH FLOWS
| | | | | | | |
| | 2010 | | 2009 | |
|---|
| | EUR thou.
| | EUR thou.
| |
|---|
Loss before tax | | | (154 | ) | | (969 | ) |
Depreciation of property plants & equipment | | | 151 | | | 146 | |
Amortisation of intangible assets | | | 76 | | | 61 | |
Gains from disposal of property plants & equipment | | | (26 | ) | | (13 | ) |
Interest income | | | (2 | ) | | (18 | ) |
Interest expense | | | 284 | | | 288 | |
Movement in provisions | | | 177 | | | (33 | ) |
Gains on research and developments grants | | | (572 | ) | | (464 | ) |
| | | | | | |
Gross cash flow | | | (66 | ) | | (1,003 | ) |
| | | | | | |
Increase/decrease in inventories | | | (66 | ) | | (382 | ) |
Increase/decrease in trade and other receivables | | | 75 | | | 232 | |
Increase/decrease in trade and other payables | | | (323 | ) | | 150 | |
| | | | | | |
| | | (314 | ) | | (109 | ) |
| | | | | | |
Interest received | | | 2 | | | 18 | |
| | | | | | |
Income tax paid | | | 0 | | | 0 | |
| | | | | | |
Net cash from operating activities | | | (378 | ) | | (1,094 | ) |
| | | | | | |
Purchase of intangible assets | | | (84 | ) | | (101 | ) |
Purchase of property, plant and equipment | | | (90 | ) | | (77 | ) |
Cash receipts from disposal of assets | | | 51 | | | 13 | |
| | | | | | |
Net cash used in investing activities | | | (123 | ) | | (165 | ) |
| | | | | | |
Credit repayments | | | (100 | ) | | (60 | ) |
Interest paid | | | (25 | ) | | (30 | ) |
Payments of finance lease liabilities | | | (3 | ) | | (3 | ) |
Cash receipts for research and developments grants | | | 537 | | | 537 | |
| | | | | | |
Net cash used/from financing activities | | | 409 | | | 480 | |
| | | | | | |
Net decrease/increase in cash and cash equivalents | | | (92 | ) | | (779 | ) |
| | | | | | |
Cash and cash equivalents at beginning of year | | | 484 | | | 1,263 | |
Cash and cash equivalents at end of year | | | 392 | | | 484 | |
SC-F-15
Scienion AG, Dortmund
NOTES TO THE FINANCIAL STATEMENTS 2010 AND 2009
1. CORPORATE INFORMATION
Scienion AG (the "Company") is an incorporated company (Aktiengesellschaft) domiciled in Germany. The address of the Company's registered office is Dortmund. The Company is primarily involved in the markets of ultra-low volume liquid handling systems and microarray technologies.
The financial statements were authorised for issue by the Board of Directors on 25. August 2011.
The Company is formally overindebted as of 31 December 2010. Most of the liabilities in connection with the silent participation, for which a declaration of subordination has been issued for up to € 3,437 thousand, have a remaining term until the end of 2011. The management board assumes that the contract signed with the investor SQI Diagnostics Inc., Toronto, Ontario, Canada, on 4 July 2011 which provides for the investor to acquire all shares and to redeem the silent participations as well as to equip Scienion AG with the necessary capital resources will take effect after certain contractually agreed conditions precedent have been satisfied. If the transaction fails, the Company's ability to continue as a going concern hinges on the liabilities from silent participations, including interest and costs that will be due on 31 December 2011 not falling due after that date but being made available to the Company as substitute equity to cover the capital deficit prevailing at the time. Subject to the final approval of their corporate bodies, the silent shareholders have agreed to take appropriate measures ranging from a prolongation of the silent participation to a partial or even full waiver. The Company is therefore acting the assumption that it is more likely than not that Scienion AG will be able to continue as a going concern.
2. BASIS OF PREPARATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
The financial statements for the year ended 31 December 2010 and 31 December 2009 are the first the Company has prepared in accordance with IFRS. An explanation of how the transition to IFRSs has affected the reported financial positions, financial performances and cash flows of the Company is provided in note 29.
The financial statements have been prepared in accordance with International Financial Reporting Standards and the interpretations issued by the International Accounting Standards Board (IASB). These comprise the obligatory IASs, IFRSs and the relevant interpretations (SIC/IFRIC) as at the balance sheet date 31 December 2010. The prior-period amounts were calculated in accordance with the same principles.
The financial statements have been prepared on the historical cost basis.
The following accounting standards and interpretations and amendments to existing standards were published as of the reporting date but were not yet required to be applied in preparing the IFRS financial statements for the year ended 31 December 2010:
| | | |
| | • AS 1 | | Presentation of Financial Statements (Amendment) |
| | • IAS 12 | | Deferred tax: Recovery of Underlying Assets (Amendment) |
| | • IAS 19 | | Employee benefits (Amendment) |
| | • IAS 24 | | Related party disclosures (Revised) |
| | • IAS 27R | | Separate Financial Statements |
| | • IAS 28R | | Investments in Associates and Joint Ventures |
| | • IAS 32 | | Classification of Rights (Amendment) |
| | • IFRIC 14 | | Prepayments of a Minimum Funding Requirement |
| | • IFRIC 19 | | Extinguishing Financial Liabilities with Equity Instruments |
| | • IFRS 1 | | Severe Hyperinflation and Removal of Fixed Dates for First-Time Adopters (Amendment) |
| | • IFRS 7 | | Amendments to improve disclosures during transfers of financial assets |
| | • IFRS 9 | | Financial Instruments: Classification and Measurement |
| | • IFRS 10 | | Consolidated Financial Statements |
| | • IFRS 11 | | Joint arrangements |
| | • IFRS 12 | | Disclosure of Interests in Other Entities |
| | • IFRS 13 | | Fair Value Measurement |
As part of the annual "improvement" project for 2010, various IFRS were amended. The future application of new or revised standards and interpretations is not expected to have a material impact on the financial statements.
The financial statements consist of the statements of financial position, the statement of comprehensive income, the statements of changes in equity and the statement of cash flows. The financial statements are prepared in euro (€). All amounts, including prior-period amounts, are stated in thousands of euro (€ thousand). All amounts are rounded in accordance with standard commercial practice. In some cases, this can result in negligible differences between the sum of the individual amounts and the stated total.
SC-F-16
Scienion AG, Dortmund
NOTES TO THE FINANCIAL STATEMENTS 2010 AND 2009 (Continued)
2. BASIS OF PREPARATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
For clarity, some individual items in the statements of financial position and in the statements of comprehensive income have been combined; these items are discussed in detail in the notes to the financial statements. Assets and liabilities are broken down into current and non-current items. The statements of comprehensive income are prepared in accordance with the nature of expense method. The statements of cash flows are prepared in accordance with the indirect method for cash flow from operating activities and the direct method for cash flow from investing and financing activities
- (b)
- Summary of significant accounting policies
Foreign currency translation
The Company's financial statements are presented in euros. Transactions in foreign currencies are initially recorded by the Company at the functional currency rates prevailing at the date of the transaction. Monetary assets and liabilities denominated in foreign currencies are retranslated at the functional currency spot rate of exchange ruling at the reporting date. All differences are taken to the statement of comprehensive income.
Scienion AG records trade payables and trade receivables traded in US Dollar which are not significant.
Property, plant and equipment
Property, plant and equipment encompasses all tangible assets that are held for use in the production or supply of goods or services, for rental to others or for administrative purposes and that are expected to be used during more than one period.
Property, plant and equipment is stated at cost less accumulated depreciation and impairments, plus any reversals of impairments. When significant parts of property, plant and equipment are required to be replaced at intervals, the Company derecognises the replaced part, and recognises the new part with its own associated useful life and depreciation. All repair and maintenance costs are recognised in the income statement as incurred.
Items of property, plant and equipment held under finance leases are carried at the lower of fair value and the present value of the minimum lease payments and are depreciated on a straight line basis over their expected useful life.
The Company assesses at each reporting date whether there is an indication that an asset may be impaired. If any indication exists, or when annual impairment testing for an asset is required, the Company estimates the asset's recoverable amount. An asset's recoverable amount is the higher of an asset's fair value less costs to sell and its value in use and is determined for an individual asset, unless the asset does not generate cash inflows that are largely independent of those from other assets or groups of assets. Where the carrying amount of an asset exceeds its recoverable amount, the asset is considered impaired and is written down to its recoverable amount.
For assets, an assessment is made at each reporting date as to whether there is any indication that previously recognised impairment losses may no longer exist or may have decreased. If such indication exists, the Company estimates the asset's recoverable amount. A previously recognised impairment loss is reversed only if there has been a change in the assumptions used to determine the asset's recoverable amount since the last impairment loss was recognised. The reversal is limited so that the carrying amount of the asset does not exceed its recoverable amount, nor exceed the carrying amount that would have been determined, net of depreciation, had no impairment loss been recognised for the asset in prior years. Such reversal is recognised in the income statement unless the asset is carried at a revalued amount, in which case the reversal is treated as a revaluation increase.
Intangible assets
Intangible assets include both internally generated and acquired assets.
Internally generated intangible assets are capitalised if the following criteria's are met:
- •
- Technical feasibility of completing the asset
- •
- Intention to complete the asset
- •
- Ability to use or sell the asset
- •
- Generate future economic benefits with the asset
- •
- Availability of adequate technical, financial and other resources to complete the asset
- •
- Ability to measure reliably the expenditure attributable to the intangible asset in the development phase.
SC-F-17
Scienion AG, Dortmund
NOTES TO THE FINANCIAL STATEMENTS 2010 AND 2009 (Continued)
2. BASIS OF PREPARATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
If an intangible asset fulfils the criteria it is initially carried at the cost incurred between the date on which their technological and economic reliability was established and the date on which they were completed. All other research and development costs are expensed as occurred.
Acquired intangible assets include concessions, licences, industrial and similar rights and assets, as well as technologies. Acquired intangible assets are initially carried at cost. Intangible assets whose useful lives can be determined are amortised on a straight-line basis over this period. The carrying amounts of intangible assets are reviewed if there is evidence to suggest that they may be impaired as a result of events or changes in circumstances.
Impairment testing is performed in the same way as for property, plant and equipment. If the reasons for impairment no longer apply, the corresponding write-down must be reversed up to a maximum of the amortised cost of the respective asset.
Useful lives
The depreciation of property, plant and equipment and the amortisation of intangible assets is based on the following useful lives:
| | | | | |
| |
| | Useful life | | Method of amortisation
and depreciation |
|---|
| | Software | | 3 to 5 years | | straight-line method |
| | Licenses and similar rights | | Over the term of the respective agreement | | straight-line method |
| | leasehold improvement | | 50 years | | straight-line method |
| | Technical equipment and machinery | | 3-13 years | | straight-line method |
| | Other equipment, operating and office equipment | | 3-13 years | | straight-line method |
The assets' residual values, useful lives and methods of amortisation and depreciation are reviewed at each financial year end and adjusted prospectively, if appropriate.
Lease
Finance leases which transfer to the Company substantially all the risks and benefits incidental to ownership of the leased item, are capitalized at the commencement of the lease at the fair value of the leased property or, if lower, at the present value of the minimum lease payments. Lease payments are apportioned between finance charges and reduction of the lease liability so as to achieve a constant rate of interest on the remaining balance of the liability. Finance charges are recognised in finance costs in the income statement.
A leased asset is depreciated over the useful life of the asset. However, if there is no reasonable certainty that the Company will obtain ownership by the end of the lease term, the asset is depreciated over the shorter of the estimated useful life of the asset and the lease term.
Operating lease payments are recognised as an operating expense in the income statement on a straight-line basis over the lease term.
Current income tax
Current income tax assets and liabilities for the current period are measured at the amount expected to be recovered from or paid to the taxation authorities. The tax rates and tax laws used to compute the amount are those that are enacted or substantively enacted. Current income tax relating to items recognized directly in equity is recognized in equity and not in the income statement. Management periodically evaluates positions taken in the tax returns with respect to situations in which applicable tax regulations are subject to interpretation and establishes provisions where appropriate.
Deferred taxes
Deferred tax is provided using the liability method on temporary differences at the reporting date between the tax bases of assets and liabilities and their carrying amounts for financial reporting purposes. Deferred tax liabilities are recognised for all taxable temporary differences, except:
- •
- Where the deferred tax liability arises from the initial recognition of goodwill or of an asset or liability in a transaction that is not a business combination and, at the time of the transaction, affects neither the accounting profit nor taxable profit or loss
SC-F-18
Scienion AG, Dortmund
NOTES TO THE FINANCIAL STATEMENTS 2010 AND 2009 (Continued)
2. BASIS OF PREPARATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
- •
- In respect of taxable temporary differences associated with investments in subsidiaries, associates and interests in joint ventures, where the timing of the reversal of the temporary differences can be controlled and it is probable that the temporary differences will not reverse in the foreseeable future
Deferred tax assets are recognised for all deductible temporary differences, carry forward of unused tax credits and unused tax losses, to the extent that it is probable that taxable profit will be available against which the deductible temporary differences, and the carry forward of unused tax credits and unused tax losses can be utilised, except:
- •
- Where the deferred tax asset relating to the deductible temporary difference arises from the initial recognition of an asset or liability in a transaction that is not a business combination and, at the time of the transaction, affects neither the accounting profit nor taxable profit or loss
- •
- In respect of deductible temporary differences associated with investments in subsidiaries, associates and interests in joint ventures, deferred tax assets are recognised only to the extent that it is probable that the temporary differences will reverse in the foreseeable future and taxable profit will be available against which the temporary differences can be utilised.
The carrying amount of deferred tax assets is reviewed at each reporting date and reduced to the extent that it is no longer probable that sufficient taxable profit will be available to allow all or part of the deferred tax asset to be utilised. Unrecognised deferred tax assets are reassessed at each reporting date and are recognised to the extent that it has become probable that future taxable profits will allow the deferred tax asset to be recovered. Deferred tax assets and liabilities are measured at the tax rates that are expected to apply in the year when the asset is realised or the liability is settled, based on tax rates (and tax laws) that have been enacted or substantively enacted at the reporting date.
Inventories
Inventories are carried at the lower of cost and net realisable value. The majority of the Company's inventories are measured using weighted average cost method. The cost of inventories includes direct costs, indirect materials and indirect labour costs relating to production and depreciation, as well as production related administrative expenses, but not borrowing costs. Net realisable value is the estimated selling price in the ordinary course of business less the estimated costs of completion and the estimated costs necessary to make the sale.
Financial instruments — initial recognition and subsequent measurement
- i)
- Financial assets
Initial recognition and measurement
Financial assets recognized on Scienion AG's statements of Financial Position include cash and trade receivables.
Trade receivables are recognized as receivables.
The Company determines the classification of its financial assets at initial recognition.
All financial assets are recognised initially at fair value plus, in the case of assets not at fair value through profit or loss, directly attributable transaction costs.
Purchases or sales of financial assets that require delivery of assets within a time frame established by regulation or convention in the marketplace (regular way trades) are recognised on the trade date, i.e., the date that the Company commits to purchase or sell the asset.
Subsequent measurement
Receivables are non-derivative financial assets with fixed or determinable payments that are not quoted in an active market. After initial measurement, such financial assets are subsequently measured at amortised cost using the effective interest rate method (EIR), less impairment. Amortised cost is calculated by taking into account any discount or premium on acquisition and fees or costs that are an integral part of the EIR. The EIR amortisation is included in finance income in the Statements of Comprehensive Income. The losses arising from impairment are recognised in the Statements of Comprehensive Income in other operating expenses.
SC-F-19
Scienion AG, Dortmund
NOTES TO THE FINANCIAL STATEMENTS 2010 AND 2009 (Continued)
2. BASIS OF PREPARATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Liabilities due to silent partners are reported as financial liabilities and carried at their settlement or repayment amount in accordance with the effective interest method. Finance lease liabilities are recognised in the amount of the present value of the lease instalments.
Initial recognition and measurement
Financial liabilities within the scope of IAS 39 are classified as financial liabilities at fair value through profit or loss, loans and borrowings, or as derivatives designated as hedging instruments in an effective hedge, as appropriate. The Company determines the classification of its financial liabilities at initial recognition. All financial liabilities are recognised initially at fair value and, in the case of loans and borrowings, carried at amortised cost. This includes directly attributable transaction costs.
The Company's financial liabilities include trade payables and liabilities due to silent partners.
Subsequent measurement
After initial recognition, trade payables, are subsequently measured at amortised cost using the effective interest rate method. Gains and losses are recognized in the income statement when the liabilities are derecognized as well as through the effective interest rate method (EIR) amortization process. Amortised cost is calculated by taking into account any discount or premium on acquisition and fees or costs that are an integral part of the EIR. The EIR amortization is included in finance costs in the Statements of Comprehensive Income.
Derecognition
A financial liability is derecognised when the obligation under the liability is discharged or cancelled or expires. When an existing financial liability is replaced by another from the same lender on substantially different terms, or the terms of an existing liability are substantially modified, such an exchange or modification is treated as a derecognition of the original liability and the recognition of a new liability, and the difference in the respective carrying amounts is recognised in the Statement of comprehensive Income
Cash and cash equivalents
Cash and cash equivalents in the Statement of Financial Position comprise cash at banks, cash on hand and restricted cash for rental deposits and a warranty guarantee and are carried at their principal amount. Cash and cash equivalents denominated in foreign currencies are translated at the exchange rate at the balance sheet date.
Subscribed capital
Ordinary shares are classified as shareholders' equity.
Provisions
Provisions are recognised when the Company has a current (legal or constructive) obligation from a past event that it is expected to be required to settle, and the amount of this obligation can be reliably estimated. The amount recognised as a provision is the best estimate of the consideration required to settle the current obligation as of the reporting date, taking into account the risks and uncertainties underlying the obligation.
Provisions for warranty-related costs are recognised when the product is sold or service provided. Initial recognition is based on historical experience. The initial estimate of warranty-related costs is revised annually.
SC-F-20
Scienion AG, Dortmund
NOTES TO THE FINANCIAL STATEMENTS 2010 AND 2009 (Continued)
Revenue recognition
Except for medium-term construction contracts, revenue from the sale of products is recognised when the significant risks and rewards of ownership have passed to the buyer, usually when products are delivered and transferred to the buyer. Revenue is measured at the fair value of the consideration received or receivable. Customer bonuses, trade discounts and volume rebates serve to reduce revenue.
Revenue relating to medium-term contracts is recognized using the percentage of completion method.
Under the percentage of completion method, contract revenue is recognised as revenue in profit or loss in the accounting periods in which the work is performed. Contract costs are recognised as an expense in profit or loss in the accounting periods in which the work to which they relate is performed (IAS 11.26).
The stage of completion of a contract is determined by the proportion that contract costs incurred for work performed to date bear to the estimated total contract costs (IAS 30a). Contract costs that relate to future activity on the contract are not included as the contract cost have not incurred as of the balance sheet date (IAS 31a).
Interest income is recognised on a time proportion basis over the remaining term of the respective asset on the basis of the effective interest rate and the amount of the outstanding receivable.
Borrowing costs
Borrowing costs are expensed in the period in which they are incurred as they are not directly attributable to the acquisition, construction or production of an asset.
Government grants
Government grants are recognised where there is reasonable assurance that the grant will be received and all attached conditions will be complied with. When the grant relates to an expense item (Research and Development grants), it is recognised as income over the period necessary to match the grant on a systematic basis to the costs that it is intended to compensate. Where the grant relates to an asset, it is recognised as deferred income and released to income in equal amounts over the expected useful life of the related asset.
Estimates
The preparation of the financial statements requires the use of estimates and assumptions affecting the Company's assets, liabilities, provisions, deferred tax assets and liabilities, income and expenses. Although the Company ensures that such estimates and assumptions are made in a careful and conscientious manner, the possibility that the actual amounts will deviate from the estimated amounts cannot be excluded.
Factors that could cause a negative deviation include deterioration in the global economy, exchange rate and interest rate developments, significant legal proceedings and amendments to the provisions of environmental law or other statutory provisions. Manufacturing defects, the loss of key customers and rising finance charges could also adversely affect the Company's future success. The following section presents the potential effects of changes in estimates on the recognition and measurement of assets and liabilities:
- a.
- Deferred taxes
When estimating the realisability of deferred tax assets, the Company's management assesses the extent to which the factors in favour of realisation outweigh those against it. The actual realisability of a deferred tax asset depends on the availability of future taxable income that can be offset against the tax loss carry-forwards. To this end, the Company's management examines the dates at which the deferred tax liabilities are expected to reverse and the future taxable income is expected to be received.
- b.
- Provisions and contingent liabilities
Initial capitalisation of costs is based on management's judgment that technological and economical feasibility is confirmed, usually when a product development project has reached a defined milestone according to an established project management model. In determining the amounts to be capitalised, management makes assumptions regarding the expected future cash generation of the project, discount rates to be applied and the expected period of benefits.
In addition, assumptions and estimates are required for allowances on doubtful debts as well as other provisions.
SC-F-21
Scienion AG, Dortmund
NOTES TO THE FINANCIAL STATEMENTS 2010 AND 2009 (Continued)
In some cases, actual values can deviate from the assumptions and estimates made, meaning that it is necessary to adjust significantly the carrying amount of the assets or liabilities concerned. Changes to estimates are recognised in income in accordance with IAS 8 at the time they become known. Values from the previous year did not need to be adjusted and can be taken as a comparison.
Notes to the statement of financial position:
Assets
Non-current assets
3. Intangible assets
All intangible assets are purchased, with the exception of developments by Scienion AG.
The cost of, additions to and disposals from intangible assets are composed as follows:
| | | | | | | | | | | |
| |
| | Internally generated intangible assets | | Acquired intangible assets | | Total | |
|---|
| |
| | EUR thou.
| | EUR thou.
| | EUR thou.
| |
|---|
| | Balance at 1 Jan. 2009 | | | 154 | | | 203 | | | 357 | |
| | Additions | | | 96 | | | 5 | | | 101 | |
| | Balance at 1 Jan. 2010 | | |
250 | | |
208 | | |
458 | |
| | Additions | | | 81 | | | 3 | | | 84 | |
| | Balance at 31 Dec. 2010 | | |
331 | | |
211 | | |
542 | |
| | | | | | | | | | | |
| |
| | Internally generated intangible assets | | Acquired intangible assets | | Total | |
|---|
| |
| | EUR thou.
| | EUR thou.
| | EUR thou.
| |
|---|
| | Balance at 1 Jan. 2009 | | | 19 | | | 134 | | | 153 | |
| | Amortisation in the year under review | | | 31 | | | 30 | | | 61 | |
| | Balance at 1 Jan. 2010 | | |
50 | | |
164 | | |
214 | |
| | Amortisation in the year under review | | | 50 | | | 25 | | | 76 | |
| | Balance at 31 Dec. 2010 | | |
100 | | |
189 | | |
289 | |
| | | | | | | | | | | |
| |
| | Internally generated intangible assets | | Acquired intangible assets | | Total | |
|---|
| |
| | EUR thou.
| | EUR thou.
| | EUR thou.
| |
|---|
| | Balance at 1 Jan. 2009 | | | 135 | | | 69 | | | 204 | |
| | Balance at 31 Dec. 2009 | | |
200 | | |
44 | | |
244 | |
| | Balance at 31 Dec. 2010 | | |
231 | | |
22 | | |
253 | |
Research costs and non-capitalisable development costs are expensed as incurred. In the financial year 2010, research and development costs totalled € 231 thousand (previous year: € 200 thousand).
4. Property, plant and equipment
Property, plant and equipment also include equipment held under a finance lease.
SC-F-22
Scienion AG, Dortmund
NOTES TO THE FINANCIAL STATEMENTS 2010 AND 2009 (Continued)
4. Property, plant and equipment (Continued)
The cost of, additions to and disposals from property, plant and equipment are composed as follows:
| | | | | | | | | | | | | | |
| |
| | Building | | Technical
equipment
and machinery | | Operating and
office equipment | | Total | |
|---|
| |
| | EUR thou.
| | EUR thou.
| | EUR thou.
| | EUR thou.
| |
|---|
| | Balance at 1 Jan. 2009 | | | 16 | | | 1,719 | | | 1,189 | | | 2,924 | |
| | Additions | | | 0 | | | 53 | | | 24 | | | 77 | |
| | Disposals | | | 0 | | | 93 | | | 0 | | | 93 | |
| | Balance at 1 Jan. 2010 | | |
16 | | |
1,679 | | |
1,213 | | |
2,908 | |
| | Additions | | | 0 | | | 73 | | | 17 | | | 90 | |
| | Disposals | | | 0 | | | 50 | | | 14 | | | 64 | |
| | Balance at 31 Dec. 2010 | | |
16 | | |
1,702 | | |
1,217 | | |
2,934 | |
| | | | | | | | | | | | | | |
| |
| | Building | | Technical
equipment
and machinery | | Operating and
office equipment | | Total | |
|---|
| |
| | EUR thou.
| | EUR thou.
| | EUR thou.
| | EUR thou.
| |
|---|
| | Balance at 1 Jan. 2009 | | | 2 | | | 1,274 | | | 931 | | | 2,207 | |
| | Amortisation in the year under review | | | (0.3 | ) | | 80 | | | 66 | | | 146 | |
| | Disposals | | | 0 | | | 93 | | | 0 | | | 93 | |
| | Balance at 1 Jan. 2010 | | |
2 | | |
1,261 | | |
997 | | |
2,260 | |
| | Amortisation in the year under review | | | (0.3 | ) | | 97 | | | 54 | | | 151 | |
| | Disposals | | | 0 | | | 29 | | | 10 | | | 39 | |
| | Balance at 31 Dec. 2010 | | |
3 | | |
1,329 | | |
1,042 | | |
2,373 | |
| | | | | | | | | | | | | | |
| |
| | Building | | Technical equipment
and machinery | | Operating and
office equipment | | Total | |
|---|
| |
| | EUR thou.
| | EUR thou.
| | EUR thou.
| | EUR thou.
| |
|---|
| | Balance at 1 Jan. 2009 | | | 13 | | | 445 | | | 258 | | | 716 | |
| | Balance at 31 Dec. 2009 | | |
13 | | |
418 | | |
217 | | |
648 | |
| | Balance at 31 Dec. 2010 | | |
13 | | |
373 | | |
175 | | |
561 | |
5. Deferred tax assets
| | | | | | | | | | | | |
| |
| | 31. Dec. 2010 | | 31. Dec. 2009 | | 1. Jan. 2009 | |
|---|
| |
| | EUR thou.
| | EUR thou.
| | EUR thou.
| |
|---|
| | From temporary differences | | | | | | | | | | |
| | | Inventories | | | 0 | | | 0 | | | 6 | |
| | | Prepayments received | | | 0 | | | 40 | | | 0 | |
| | | Other accounts payable | | | 14 | | | 5 | | | 10 | |
| | | | | | | | | |
| | Total | | | 14 | | | 45 | | | 16 | |
| | | | | | | | | |
SC-F-23
Scienion AG, Dortmund
NOTES TO THE FINANCIAL STATEMENTS 2010 AND 2009 (Continued)
6. Inventories
| | | | | | | | | | | |
| |
| | 31. Dec. 2010 | | 31. Dec. 2009 | | 1. Jan. 2009 | |
|---|
| |
| | EUR thou.
| | EUR thou.
| | EUR thou.
| |
|---|
| | Raw materials and consumables used | | | 224 | | | 202 | | | 203 | |
| | Work in progress | | | 654 | | | 639 | | | 266 | |
| | Advance payments | | | 55 | | | 26 | | | 16 | |
| | | | | | | | | |
| | Total | | | 933 | | | 867 | | | 485 | |
| | | | | | | | | |
Write downs of inventories to the net realisable value amounted to € 17 thousand (previous year: € 12 thousand).
Change in inventories of work in progress amounted to € 15 thousand (previous year: € 373 thousand).
€ 1,155 (previous year 1,172) of inventories recognised as an expense during the fiscal year 2010 (2009).
7. RECEIVABLES AND OTHER ASSETS
| | | | | | | | | | | |
| |
| | 31. Dec. 2010 | | 31. Dec. 2009 | | 1. Jan. 2009 | |
|---|
| |
| | EUR thou.
| | EUR thou.
| | EUR thou.
| |
|---|
| | Trade receivables | | | 643 | | | 671 | | | 815 | |
| | Other receivables and assets | | | 256 | | | 267 | | | 355 | |
| | | | | | | | | |
| | Total receivables and other assets | | | 899 | | | 938 | | | 1,170 | |
| | | | | | | | | |
| | | | | | | | | | | |
| |
| | 31. Dec. 2010 | | 31. Dec. 2009 | | 1. Jan. 2009 | |
|---|
| |
| | EUR thou.
| | EUR thou.
| | EUR thou.
| |
|---|
| | Trade receivables | | | 722 | | | 682 | | | 922 | |
| | Amounts due from customers for construction contracts/contract work | | | 0 | | | 113 | | | 28 | |
| | | | | | | | | |
| | Carrying amount (without Allowances for doubtful accounts) | | | 722 | | | 795 | | | 950 | |
| | Allowances for doubtful accounts | | | (79 | ) | | (124 | ) | | (135 | ) |
| | | | | | | | | |
| | Total trade receivables | | | 643 | | | 671 | | | 815 | |
| | | | | | | | | |
The carrying amounts of trade receivables correspond to their fair values.
Individual risks were taken into account and result in allowances totalling € 79 thousand (previous year: € 124 thousand).
All receivables disclosed in the balance sheet fall due within one year.
| | | | | | | | | | | |
| |
| | 31.12.2010 | | 31.12.2009 | | 1.1.2009 | |
|---|
| |
| | EUR thou.
| | EUR thou.
| | EUR thou.
| |
|---|
| | Carrying amount (without Allowances for doubtful accounts) | | | 722 | | | 795 | | | 950 | |
| | Thereof (partially) impaired | | | 84 | | | 138 | | | 232 | |
| | Neither past due nor impaired | | | 59 | | | 508 | | | 562 | |
| | Past due but not impaired | | | 579 | | | 149 | | | 156 | |
| | less than 30 days | | | 361 | | | 34 | | | 5 | |
| | 30 to 59 days | | | 96 | | | 6 | | | 3 | |
| | 60 to 89 days | | | 45 | | | 7 | | | 0 | |
| | 90 to 119 days | | | 12 | | | 1 | | | 25 | |
| | 120 days or more | | | 65 | | | 101 | | | 123 | |
SC-F-24
Scienion AG, Dortmund
NOTES TO THE FINANCIAL STATEMENTS 2010 AND 2009 (Continued)
7. RECEIVABLES AND OTHER ASSETS (Continued)
Gross Amounts due from customers for construction contracts/contract work
Gross Amounts due from customers for construction contracts/contract work are composed as follows:
| | | | | | | | | | | |
| |
| | 31. Dec. 2010 | | 31. Dec. 2009 | | 1. Jan. 2009 | |
|---|
| |
| | EUR thou.
| | EUR thou.
| | EUR thou.
| |
|---|
| | Contract revenue | | | 0 | | | 238 | | | 114 | |
| | Contract costs | | | 0 | | | 101 | | | 73 | |
| | Aggregate recognised revenues | | | 0 | | | 238 | | | 114 | |
| | Aggregate prepayments received | | | 0 | | | 125 | | | 86 | |
| | | | | | | | | |
| | Gross Amounts due from customers for construction contracts/contract work | | | 0 | | | 113 | | | 28 | |
| | | | | | | | | |
In the fiscal year 2010, all construction contracts were finalized. The corresponding revenue recognized due to these contracts amounted to € 916 thousand while expenses accrued to € 411 thousand.
Furthermore, at the balance sheet date, no contingent liabilities from construction contracts according to IAS 11.45 arose.
Other receivables and assets
Other receivables and assets are composed as follows:
| | | | | | | | | | | |
| |
| | 31. Dec. 2010 | | 31. Dec. 2009 | | 1. Jan. 2009 | |
|---|
| |
| | EUR thou.
| | EUR thou.
| | EUR thou.
| |
|---|
| | Receivables from tax authorities | | | 40 | | | 50 | | | 46 | |
| | Subsidies for research projects | | | 200 | | | 165 | | | 274 | |
| | Corporate tax receivables | | | 1 | | | 16 | | | 10 | |
| | Other | | | 15 | | | 36 | | | 25 | |
| | | | | | | | | |
| | Total other assets | | | 256 | | | 267 | | | 355 | |
| | | | | | | | | |
Receivables from tax authorities primarily relate to VAT receivables.
Income tax assets relate to corporate tax receivables and amounted to € 1 thousand at the balance sheet date (previous year: € 16 thousand, 1st Jan. 2009: € 10 thousand). All income tax assets are due within one year.
8. CASH AND CASH EQUIVALENTS
Cash comprise of cash on hand, cash in bank and restricted cash due to rental deposits and a warranty guarantee. Cash and cash equivalents at the balance sheet date were calculated as follows:
| | | | | | | | | | | |
| |
| | 31. Dec. 2010 | | 31. Dec. 2009 | | 1. Jan. 2009 | |
|---|
| |
| | EUR thou.
| | EUR thou.
| | EUR thou.
| |
|---|
| | Cash on hands and bank balances | | | 359 | | | 445 | | | 1,224 | |
| | Restricted cash | | | 33 | | | 39 | | | 39 | |
| | | | | | | | | |
| | Total cash and cash equivalents | | | 392 | | | 484 | | | 1,263 | |
| | | | | | | | | |
SC-F-25
Scienion AG, Dortmund
NOTES TO THE FINANCIAL STATEMENTS 2010 AND 2009 (Continued)
8. CASH AND CASH EQUIVALENTS (Continued)
NOTES TO THE STATEMENT OF FINANCIAL POSITION:
Equity and liabilities
9. EQUITY
Subscribed capital
The subscribed capital of Scienion AG remained constant in the financial year with € 187 thousand. Share capital contains of 50,000 common shares 57,701 preferred shares (A), 21,330 preferred share (B) and 57,634 preferred shares (C). The different types of shares have differing rights with respect to proceeds from the sale of the shares to another investor but not with respect to the distribution of annual profits. The Company's share capital is fully paid in.
On reporting date shares were hold by
| | | | | |
| |
| | % | |
|---|
| | Peppermint Venture Capital GmbH & Co. KG, Berlin | | | 27,59 | |
| | NRW.Bank. Venture Fonds GmbH & Co. KG, Düsseldorf | | | 23,50 | |
| | Dr. Holger Eickhoff | | | 11,89 | |
| | IBB Beteiligungsgesellschaft mbh, Berlin | | | 7,95 | |
| | Other | | | 29,07 | |
| | | | | |
| | | | | 100,00 | |
| | | | | |
Earnings per share
Basic earnings per share amounts are calculated by dividing net profit for the year attributable to equity holders by the weighted average number of shares outstanding during the year. The Company does not have any instruments with dilutive effects. Thus, basic and diluted earnings per share are identical.
The following reflects the income and share data used in the basic and diluted earnings per share computations:
| | | | | | | | |
| |
| | 31. Dec. 2010 | | 31. Dec. 2009 | |
|---|
| |
| | EUR thou.
| | EUR thou.
| |
|---|
| | Net loss attributable to equity holders | | | (164 | ) | | (962 | ) |
| | Net loss attributable to equity holders for basic and dilutive earnings | | | (164 | ) | | (962 | ) |
| | Weigted average number of ordinary shares in circulation | | | 50,000 | | | 50,000 | |
| | Earnings per share of ordinary shares | | | (0,88 | ) | | (5,15 | ) |
| | Weigted average number of preference shares in circulation | | | 136,665 | | | 136,665 | |
| | Earnings per share of preference shares | | | (0,88 | ) | | (5,15 | ) |
Capital management
As already mentioned in note 1, the Company is formally over indebted as of 31 December 2010 (EUR -2,961 thou.). Because the silent partnerships (EUR 3,698 thou.) are considered as being equity substitute, the management board assumes that the equity is factual positive.
Caused by the loss history the Company has no access to additional debt capital. As outlined in note 23 the stringent cash-management ensures short and medium-term liquidity.
The Scienion AG's strategic orientation sets the framework for the optimisation of capital management. The Company intends to generate a sustainable increase in enterprise value in the interests of its shareholders, customers and employees by way of a continuous increase in earnings driven by growth and the improved efficiency of its business processes. This requires a balance between the business and financial risks and the financial flexibility of the Company, which is achieved through intensive communications with the financial markets and banks in particular.
The Articles of Association of Scienion AG and the agreements for silent partnerships do not prescribe any specific capital requirements.
The development of shareholders' equity can be seen in the Statements of Changes in Equity.
SC-F-26
Scienion AG, Dortmund
NOTES TO THE FINANCIAL STATEMENTS 2010 AND 2009 (Continued)
10. DEFERRED TAX LIABILITIES
| | | | | | | | | | | | |
| |
| | 31. Dec. 2010 | | 31. Dec. 2009 | | 1. Jan. 2009 | |
|---|
| |
| | EUR thou.
| | EUR thou.
| | EUR thou.
| |
|---|
| | From temporary differences | | | | | | | | | | |
| | | Intangible assets | | | 73 | | | 63 | | | 43 | |
| | | Property, plant and equipment | | | 14 | | | 5 | | | 9 | |
| | | Inventories | | | 0 | | | 5 | | | 0 | |
| | | Receivables | | | 2 | | | 38 | | | 38 | |
| | | | | | | | | |
| | Total | | | 89 | | | 111 | | | 90 | |
| | | | | | | | | |
11. PROVISIONS
| | | | | | | | | | | | | | | | | | | | |
| |
| | Balance at
1. Jan. 2009 | | Utilisation | | Reversal | | Reclass* | | Addition | | Balance at
31. Dec. 2009 | |
|---|
| |
| | EUR thou.
| | EUR thou.
| | EUR thou.
| | EUR thou.
| | EUR thou.
| | EUR thou.
| |
|---|
| | Current Provisions | | | | | | | | | | | | | | | | | | | |
| | Bonuses | | | 30 | | | 10 | | | 0 | | | 20 | | | 0 | | | 0 | |
| | Warranties | | | 48 | | | 0 | | | 30 | | | 0 | | | 27 | | | 45 | |
| | | | | | | | | | | | | | | |
| | Total | | | 78 | | | 10 | | | 30 | | | 20 | | | 27 | | | 45 | |
| | | | | | | | | | | | | | | |
- *
- Reclassification provision for bonus payments from provisions to accrued liabilities as of 31st Dec 2009
| | | | | | | | | | | | | | | | | | | | |
| |
| | Balance at
1. Jan. 2010 | | Utilisation
| | Reversal | | Reclass | | Addition | | Balance at
31. Dec. 2010 | |
|---|
| |
| | EUR thou.
| | EUR thou.
| | EUR thou.
| | EUR thou.
| | EUR thou.
| | EUR thou.
| |
|---|
| | Current Provisions | | | | | | | | | | | | | | | | | | | |
| | Bonuses | | | 0 | | | 0 | | | 0 | | | 0 | | | 152 | | | 152 | |
| | Warranties | | | 45 | | | 0 | | | 0 | | | 0 | | | 25 | | | 70 | |
| | | | | | | | | | | | | | | |
| | Total | | | 45 | | | 0 | | | 0 | | | 0 | | | 177 | | | 222 | |
| | | | | | | | | | | | | | | |
Current provisions
Provisions for bonuses are based on the respective contractual agreements and the corresponding annual revenue figures.
Provisions for warranties are recognised for warranty payments and are expected to be utilised in the next fiscal year.
12. FINANCIAL LIABILITIES
| | | | | | | | | | | | |
| |
| | 31. Dec. 2010 | | 31. Dec. 2009 | | 1. Jan. 2009 | |
|---|
| |
| | EUR thou.
| | EUR thou.
| | EUR thou.
| |
|---|
| | Silent partnerships organised under German law | | | 3,598 | | | 3,698 | | | 3,758 | |
| | Accumulated interest payable | | | 1,779 | | | 1,521 | | | 1,263 | |
| | | | | | | | | |
| | Total Financial Liabilities | | | 5,377 | | | 5,219 | | | 5,021 | |
| | | | | | | | | |
| | | Thereof non-current financial liabilities | | | 200 | | | 5,119 | | | 4,959 | |
| | | Thereof current financial liabilities | | | 5,177 | | | 100 | | | 62 | |
In fiscal years 2001 to 2003 five silent partners have provided financing to the Company. Some of these silent partnership agreements were limited until December 31, 2005; however, for these contracts a retroactive prolongation was agreed in 2006. Besides, in the course
SC-F-27
Scienion AG, Dortmund
NOTES TO THE FINANCIAL STATEMENTS 2010 AND 2009 (Continued)
12. FINANCIAL LIABILITIES (Continued)
of the prolongation of the silent partnership agreements, one silent partner transferred his interest to one existing and respectively two new silent partners; thus increasing the number of silent partners to six.
The annual compensation due to the silent partners consists of a fixed amount (6% to 8.5% of borrowing amounts) and different variable amounts based on the respective net income of the Company.
Formally the silent partnership agreements require the repayment of the provided amounts as of December 31, 2011. Subject to the final approval of their corporate bodies, the silent shareholders have agreed to take appropriate measures together with the other silent shareholders ranging from a prolongation of the silent participation to a partial or even full waiver. Therefore a significant repayment of the borrowing amounts as of this date is unlikely.
13. OTHER CURRENT LIABILITIES
| | | | | | | | | | | |
| |
| | 31. Dec. 2010 | | 31. Dec. 2009 | | 1. Jan. 2009 | |
|---|
| |
| | EUR thou.
| | EUR thou.
| | EUR thou.
| |
|---|
| | Trade payables | | | 108 | | | 302 | | | 288 | |
| | Other accrued liabilities | | | 122 | | | 112 | | | 101 | |
| | Other accounts payables | | | 91 | | | 103 | | | 110 | |
| | Prepayments received | | | 0 | | | 125 | | | 0 | |
| | | | | | | | | |
| | Total other current liabilities | | | 321 | | | 642 | | | 499 | |
| | | | | | | | | |
Trade payables
At the balance sheet date 31 December 2010 and 2009, the Company had the following trade payables:
| | | | | | | | | | | |
| |
| | 31. Dec. 2010 | | 31. Dec. 2009 | | 1. Jan. 2009 | |
|---|
| |
| | EUR thou.
| | EUR thou.
| | EUR thou.
| |
|---|
| | Total trade payables | | | 108 | | | 302 | | | 288 | |
The fair values of trade payables are the same as their carrying amounts. Unchanged to the prior year all trade payables are due within one year.
Other accrued liabilities
Other accrued liabilities are composed as follows:
| | | | | | | | | | | |
| |
| | 31. Dec. 2010 | | 31. Dec. 2009 | | 1. Jan. 2009 | |
|---|
| |
| | EUR thou.
| | EUR thou.
| | EUR thou.
| |
|---|
| | Vacation | | | 48 | | | 41 | | | 41 | |
| | Year end closing costs | | | 18 | | | 20 | | | 23 | |
| | Outstanding invoices | | | 50 | | | 17 | | | 30 | |
| | Bonus | | | 0 | | | 20 | | | 0 | |
| | Other | | | 6 | | | 14 | | | 7 | |
| | | | | | | | | |
| | Total other accrued liabilities | | | 122 | | | 112 | | | 101 | |
| | | | | | | | | |
Accruals for year-end closing costs relate to external costs for the preparation and audit of the annual financial statements.
Accruals for vacation are calculated on the basis of the outstanding vacation entitlement and the individual salary paid to the individual employees.
The accruals for outstanding invoices include primarily invoices for legal and consultancy fees.
SC-F-28
Scienion AG, Dortmund
NOTES TO THE FINANCIAL STATEMENTS 2010 AND 2009 (Continued)
13. OTHER CURRENT LIABILITIES (Continued)
| | | | | | | | | | | |
| |
| | 31. Dec. 2010 | | 31. Dec. 2009 | | 1. Jan. 2009 | |
|---|
| |
| | EUR thou.
| | EUR thou.
| | EUR thou.
| |
|---|
| | Tax liabilities | | | 26 | | | 30 | | | 34 | |
| | Other | | | 65 | | | 73 | | | 76 | |
| | | | | | | | | |
| | Total other liabilities | | | 91 | | | 103 | | | 110 | |
| | | | | | | | | |
14. FINANCE LEASES
As of December 31, 2010 and resp. 2009 the Company had entered into one financial leasing contract regarding technical equipment which is part of a sale and lease back transaction. The original purchase price amounts to € 50 thousand (2009: € 45 thousand).
As of December 31, 2010 the net book value of the leased assets amounted to € 45 thousand (2009: € 15 thousand).
| | | | | |
| | Notional amount of future minimum
lease payments | | EUR thou. | |
|---|
| | 2011 | | | 20 | |
| | 2012 | | | 20 | |
| | 2013 | | | 14 | |
| | 2014 | | | — | |
| | Later | | | — | |
| | | | | |
| | Minimum lease payments aggregated | | | 53 | |
| | | | | |
| | Less interest portion | | | 8 | |
| | Present value of minimum lease payments | | | 45 | |
| | Less short term portion of the lease payment obligation | | | 20 | |
| | Long term portion of the lease payment obligation | | | 25 | |
15. OPERATING LEASES
As of December 31, 2010 and resp. 2009 the Company had entered into two operating leasing contracts regarding two buildings at the Company's locations in Dortmund and Berlin as well as technical equipment.
| | | | | |
| | Notional amount of future minimum
lease payments | | EUR thou. | |
|---|
| | 2011 | | | 123 | |
| | 2012 | | | 112 | |
| | 2013 | | | 112 | |
| | 2014 | | | 37 | |
| | Later | | | — | |
| | | | | |
| | Minimum lease payments aggregated | | | 384 | |
| | | | | |
Notes to the statement of comprehensive income:
SC-F-29
Scienion AG, Dortmund
NOTES TO THE FINANCIAL STATEMENTS 2010 AND 2009 (Continued)
16. REVENUE
| | | | | | | | |
| |
| | 2010 | | 2009 | |
|---|
| |
| | EUR thou.
| | EUR thou.
| |
|---|
| | Delivery of Hardware | | | 3,319 | | | 1,763 | |
| | Consulting services | | | 373 | | | 261 | |
| | Other | | | 39 | | | 61 | |
| | | | | | | |
| | Total Revenue | | | 3,731 | | | 2,085 | |
| | | | | | | |
17. OTHER OPERATING INCOME
| | | | | | | | |
| |
| | 2010 | | 2009 | |
|---|
| |
| | EUR thou.
| | EUR thou.
| |
|---|
| | Research and development grants | | | 572 | | | 464 | |
| | Payments received on written-off receivables | | | 46 | | | 28 | |
| | Currency translation gains | | | 22 | | | 7 | |
| | Gains from disposals of property, plant & equipment | | | 26 | | | 13 | |
| | Release of other accrued liabilities and provision | | | 2 | | | 46 | |
| | Other | | | 35 | | | 62 | |
| | | | | | | |
| | Total Revenue | | | 703 | | | 620 | |
| | | | | | | |
18. MATERIAL EXPENSES
| | | | | | | | |
| |
| | 2010 | | 2009 | |
|---|
| |
| | EUR thou.
| | EUR thou.
| |
|---|
| | Raw materials and precursors | | | 1,038 | | | 773 | |
| | Production-related services | | | 102 | | | 26 | |
| | | | | | | |
| | Total Material expenses | | | 1,140 | | | 799 | |
| | | | | | | |
19. OTHER OPERATING EXPENSES
| | | | | | | | |
| |
| | 2010 | | 2009 | |
|---|
| |
| | EUR thou.
| | EUR thou.
| |
|---|
| | Incidental premises expenses | | | 272 | | | 281 | |
| | Marketing and travel expenses | | | 239 | | | 178 | |
| | Freight, Packaging | | | 167 | | | 109 | |
| | Legal and consulting fees | | | 65 | | | 67 | |
| | Rental of equipment | | | 61 | | | 74 | |
| | Insurances | | | 35 | | | 35 | |
| | Communication | | | 32 | | | 28 | |
| | Warranties | | | 29 | | | 45 | |
| | Other | | | 147 | | | 162 | |
| | | | | | | |
| | Total Expenses | | | 1,047 | | | 979 | |
| | | | | | | |
SC-F-30
Scienion AG, Dortmund
NOTES TO THE FINANCIAL STATEMENTS 2010 AND 2009 (Continued)
19. OTHER OPERATING EXPENSES (Continued)
| | | | | | | | | | | | | | | | |
| | 2010 | | Foreign
Currency | | Payments
received on
written-of
allowances | | 2009 | | Foreign
Currency | | Payments
received on
written-of
allowances | |
|---|
| |
| | EUR thou.
| |
| |
| | EUR thou.
| |
| |
|---|
| | Trade Receivables | | | 18 | | | 46 | | Trade Receivables | | | 6 | | | 28 | |
| | | | | | | | | | | | | |
| | Trade Payables | | | (15 | ) | | 0 | | Trade Payables | | | 0 | | | 0 | |
| | | | | | | | | | | | | |
20. PERSONNEL EXPENSES
In 2010, personnel expenses increased by € 75 thousand to € 1,920 thousand (previous year: € 1,845 thousand). Staff costs include wages and salaries in the amount of € 1,687 thousand (previous year: € 1,600 thousand) and social security and other employee benefit costs totalling € 233 thousand (previous year: € 245 thousand).
21. NET FINANCE COSTS
| | | | | | | | |
| |
| | 2010 | | 2009 | |
|---|
| |
| | EUR thou.
| | EUR thou.
| |
|---|
| | Interest due to silent partners | | | 281 | | | 285 | |
| | Interest expenses resulting from finance lease | | | 3 | | | 3 | |
| | Current interest income | | | (2 | ) | | (18 | ) |
| | | | | | | |
| | Total net finance cost | | | 282 | | | 270 | |
| | | | | | | |
22. INCOME TAX EXPENSE
| | | | | | | | |
| |
| | 2010 | | 2009 | |
|---|
| |
| | EUR thou.
| | EUR thou.
| |
|---|
| | Income tax expense | | | 0 | | | 0 | |
| | Deferred taxes | | | 9 | | | (8 | ) |
| | From time differences | | | 9 | | | (8 | ) |
| | From loss carryforwards | | | 0 | | | 0 | |
| | Total deferred taxes | | | 9 | | | (8 | ) |
| | | | | | | |
| | Total income tax income/expense | | | 9 | | | (8 | ) |
| | | | | | | |
The Company has tax losses carried forward of € 10,366 thousand (CIT) and of € 9,418thousand (TT) (2009: € 10,207 thousand (CIT); € 9,367 thousand (TT)) that are not more likely than not available for offset against future taxable income, because of the planned transfer of all shares. According to German tax law there is a high likelihood that the existing loss carry forwards will be forfeited resulting from the transfer of all shares of Scienion AG in 2011. Thus, the management did not capitalize any deferred taxes on the existing loss carry forwards.
Deferred tax assets have not been recognised in respect of the transfer of Scienion shares. The Company evaluated and concluded that it is not probable that deferred tax assets on existing tax losses will be recovered. The Company has neither taxable temporary differences nor any tax planning opportunities available that could partly support the recognition of these losses as deferred tax assets.
SC-F-31
Scienion AG, Dortmund
NOTES TO THE FINANCIAL STATEMENTS 2010 AND 2009 (Continued)
22. INCOME TAX EXPENSE (Continued)
The following reconciliation of income tax expense for the 2010 financial year is based on an overall tax rate of 31.6% (previous year: 31.6%), thus conforming to the effective tax rate.
| | | | | | | | |
| |
| | 2010 | | 2009 | |
|---|
| |
| | EUR thou.
| | EUR thou.
| |
|---|
| | Net profit before income taxes | | | (154 | ) | | (969 | ) |
| | Expected tax income 31.6% | | | (49 | ) | | (306 | ) |
| | Effect of non-deductible expenses and tax-exempt income | | | 3 | | | (2 | ) |
| | tax losses of current year for which no deferred tax assets have been recognised | | | 53 | | | 301 | |
| | Other (deferred taxes) | | | 2 | | | (1 | ) |
| | | | | | | |
| | Total income tax expense | | | 9 | | | (8 | ) |
| | | | | | | |
23. FINANCIAL RISK MANAGEMENT
In addition to the identification, evaluation and monitoring of risks in its operating activities and, in particular, the resulting financial transactions, the Executive Board manages risk with a particular focus on identifying and hedging specific risks, such as price, counterparty default and liquidity risks.
Counterparty default risk management
At Scienion AG, risks relating to receivables from customers are monitored and assessed on a regular basis.
At the balance sheet date, the Company had trade receivables from various customers. The resulting counterparty default risk was negligible.
The maximum default risk is calculated as the sum of the carrying amounts of the financial receivables recognised on the face of the Statement of Financial Position.
Liquidity risk management
The management board implemented a stringent cash-management. The key components of this cash-management are continuous monitoring of the cash account movements and the liquidity requirements. As the Company has no access to the additional debt capital, it applies instruments like advance payments and prepayments.
24. OTHER FINANCIAL COMMITMENTS
At 31 December 2010, no other financial commitments apart from those related to operating lease obligations (note 15) as of balance sheet date exist.
25. STATEMENT OF CASH FLOWS
The Statements of Cash Flows is prepared in accordance with IAS 7 (Cash Flow Statements). A distinction is made between cash flows from operating, investing and financing activities. The cash and cash equivalents reported in the Statements of Cash Flows correspond to the cash in hand and bank balances reported on the face of the balance sheet.
Interest expenses paid to silent partners in the fiscal years 2010 (2009) amounted to € 25 thousand (€ 30 thousand).
26. GOVERNMENT GRANTS
The Company receives subsidies in connection with development projects. These subsidies are granted by various public or resp. non-public institutions. The grants of research & development amounted to € 572 thousand in 2010 and to € 464 thousand in 2009.
SC-F-32
Scienion AG, Dortmund
NOTES TO THE FINANCIAL STATEMENTS 2010 AND 2009 (Continued)
27. RELATED PARTY TRANSACTIONS
- a.
- Transaction with shareholder
Shareholders Peppermint Venture Capital GmbH & Co. KG, Berlin and NRW.Bank. Venture Fonds GmbH & Co. KG, Düsseldorf, have significant influence over the Company. Additional they are silent partners. Therefore these shareholders have to be classified as related parties in accordance with IAS 24. In the past, the Company received financial support through silent partnerships (note 12). Due to subordinate agreements concerning prolongation of the partnerships signed by the parties no repayment has taken place in the financial periods.
The following table provides the total amount of transactions that have been entered into with Peppermint Venture Capital GmbH & Co. KG, Berlin and NRW.Bank. Venture Fonds GmbH & Co. KG, Düsseldorf for the relevant financial years:
| | | | | | | | | | | | | | | | | |
| |
| |
| |
| |
| | Amounts owed to related parties | |
|---|
| |
| |
| | Interest
paid | | Repayments | | Due to
interests | | Due to
participation | |
|---|
| |
| | EUR thou.
| |
|---|
| | Peppermint Venture Capital GmbH & Co. KG, Berlin | | | 2010 | | | — | | | — | | | 515 | | | 865 | |
| | | | | 2009 | | | | | | | | | 441 | | | 865 | |
| | NRW.Bank. Venture Fonds GmbH & Co. KG, Düsseldorf | | | 2010 | | | — | | | — | | | 275 | | | 462 | |
| | | | | 2009 | | | — | | | — | | | 236 | | | 462 | |
- b.
- Transactions with key management personnel
Key management includes members of the management board as well as members of the supervisory board, which are composed as follows:
Management board
Dr. Holger Eickhoff, CEO
Dr. Günter Bauer, COO
Supervisory board
Dr. Joachim Rautter, (chairman)
Ute Mercker, (deputy chairman)
Dr. Jörn Erselius,
Dr. Peter Güllmann,
Dr. Timm-H. Jessen,
Dr. Richard Reinhardt
The compensation paid to key management is shown below:
| | | | | | | | |
| |
| | 2010 | | 2009 | |
|---|
| |
| | EUR thou.
| | EUR thou.
| |
|---|
| | Salaries of members of the management board | | | 307 | | | 259 | |
| | Attendance fee for members of the supervisory board | | | 22 | | | 24 | |
| | | | | | | | |
| |
| | 2010 | | 2009 | |
|---|
| |
| | EUR thou.
| | EUR thou.
| |
|---|
| | Salaries of members of the management board | | | 135 | | | 20 | |
| | Attendance fee for members of the supervisory board | | | 2 | | | 2 | |
SC-F-33
Scienion AG, Dortmund
NOTES TO THE FINANCIAL STATEMENTS 2010 AND 2009 (Continued)
28. EVENTS AFTER THE BALANCE SHEET DATE
Dated July 4 2011 the shareholders of Scienion AG have concluded a share purchase agreement with SQI Diagnostics Inc., Toronto, Ontario, Canada. Based on the share purchase agreement all shares of Scionion AG are purchased by and will transferred to SQI Diagnostics. The completation of the sale/purchase of all Scienion AG shares is subject to several conditions as set out in the share purchase agreement. Among others the completation of the transaction is contingent upon the closing of the purchaser`s (SQI) financing of at least C$30 Mio.
Until the date of authorisation of these financial statements, the contract signed with SQI has had no significant influence to the results of operations, net assets and financial position of the Company with the exception of not capitalizing any deferred tax assets on the existing tax loss carry forwards.
No other events or developments of particular significance to the results of operations, net assets and financial position of the Scienion AG have occurred since the balance sheet date of 31 December 2010.
29. EXPLANATION OF TRANSITION TO IFRSs
As stated in note 2(a), these are the Company's first financial statements prepared in accordance with IFRSs. The accounting policies set out in note 2b have been applied in preparing the financial statements for the year ended 31 December 2010, the comparative information presented in these financial statements for the year ended 31 December 2009 and in the preparation of an opening IFRS statement of financial position at 1 January 2009 (the Company's date of transition).
In preparing its opening IFRS statement of financial position, the Company has adjusted amounts reported previously in financial statements prepared in accordance with German GAAP (previous GAAP). Exemptions or elections as allowed in IFRS 1 were not take into account. An explanation of how the transition from previous GAAP to IFRSs has affected the Company's financial position, financial performance and cash flows is set out in the following tables and the notes that accompany the tables.
Reconciliation of Equity
| | | | | | | | | | | | | | | | | | | | | | | | |
| |
| | 1 January 2009 | | 31 December 2010 | |
|---|
| |
| | Note | | Previous
GAAP | | Effect of
Transition
to IFRSs | | IFRSs | | Previous
GAAP | | Effect of
transition
to IFRSs | | IFRSs | |
|---|
| |
| | In thousands of euro
| |
|---|
| | Assets | | | | | | | | | | | | | | | | | | | | | | |
| | | Intangible assets | | | a) | | | 69 | | | 135 | | | 204 | | | 21 | | | 232 | | | 253 | |
| | | Property, plant and equipment | | | b) | | | 736 | | | (19 | ) | | 717 | | | 546 | | | 15 | | | 561 | |
| | | Deferred Tax assets | | | e) | | | 0 | | | 16 | | | 16 | | | 0 | | | 14 | | | 14 | |
| | | | | | | | | | | | | | | | | | |
| | Total non-current assets | | | | | | 805 | | | 132 | | | 937 | | | 567 | | | 261 | | | 828 | |
| | | | | | | | | | | | | | | | | | |
| | | Inventories | | | c) | | | 504 | | | (19 | ) | | 485 | | | 933 | | | — | | | 933 | |
| | | Receivables | | | c)d) | | | 1,107 | | | 35 | | | 1,142 | | | 886 | | | (5 | ) | | 881 | |
| | | | | | | | | | | | | | | | | | |
| | | Cash and cash equivalents | | | | | | 1,263 | | | — | | | 1,263 | | | 392 | | | — | | | 392 | |
| | | | | | | | | | | | | | | | | | |
| | | Prepaid expenses and deferred charges | | | | | | 28 | | | — | | | 28 | | | 18 | | | — | | | 18 | |
| | | Deficit not covered by equity | | | f) | | | 1,997 | | | (1,997 | ) | | 0 | | | 3,116 | | | (3,116 | ) | | 0 | |
| | | | | | | | | | | | | | | | | | |
| | Total current assets | | | | | | 4,899 | | | (1,981 | ) | | 2,918 | | | 5,345 | | | (3,121 | ) | | 2,224 | |
| | | | | | | | | | | | | | | | | | |
| | Total assets | | | | | | 5,704 | | | (1,849 | ) | | 3,855 | | | 5,912 | | | (2,860 | ) | | 3,052 | |
| | | | | | | | | | | | | | | | | | |
| | Equity & liabilities | | | | | | | | | | | | | | | | | | | | | | |
| | | Members equity | | | | | | (1,997 | ) | | 162 | | | (1,835 | ) | | (3,116 | ) | | 155 | | | (2,961 | ) |
| | | Deficit not covered by equity | | | f) | | | 1,997 | | | (1,997 | ) | | 0 | | | 3,116 | | | (3,116 | ) | | 0 | |
| | | Special untaxed reserve | | | b) | | | 49 | | | (49 | ) | | 0 | | | 29 | | | (29 | ) | | 0 | |
| | | | | | | | | | | | | | | | | | |
| | | Non-current financial liabilities | | | | | | 4,959 | | | — | | | 4,959 | | | 200 | | | — | | | 200 | |
| | | | | | | | | | | | | | | | | | |
| | | Deferred tax liabilities | | | e) | | | 0 | | | 90 | | | 90 | | | 0 | | | 89 | | | 89 | |
| | | | | | | | | | | | | | | | | | |
| | Total equity & non-current liabilities | | | | | | 5,008 | | | (1,794 | ) | | 3,214 | | | 229 | | | (2,901 | ) | | (2,672 | ) |
| | | | | | | | | | | | | | | | | | |
| | | Provisions | | | g) | | | 179 | | | (101 | ) | | 78 | | | 348 | | | (126 | ) | | 222 | |
| | | Payables | | | g) | | | 515 | | | 46 | | | 561 | | | 5,331 | | | 167 | | | 5,498 | |
| | | | | | | | | | | | | | | | | | |
| | | Prepayments received | | | | | | 0 | | | — | | | 0 | | | 0 | | | — | | | 0 | |
| | | | | | | | | | | | | | | | | | |
| | | Deferred income | | | | | | 2 | | | — | | | 2 | | | 4 | | | — | | | 4 | |
| | | | | | | | | | | | | | | | | | |
| | Total current liabilities | | | | | | 696 | | | (55 | ) | | 641 | | | 5,683 | | | 41 | | | 5,724 | |
| | | | | | | | | | | | | | | | | | |
| | Total equity & liabilities | | | | | | 5,704 | | | (1,849 | ) | | 3,855 | | | 5,912 | | | (2,860 | ) | | 3,052 | |
| | | | | | | | | | | | | | | | | | |
SC-F-34
Scienion AG, Dortmund
NOTES TO THE FINANCIAL STATEMENTS 2010 AND 2009 (Continued)
29. EXPLANATION OF TRANSITION TO IFRSs (Continued)
| | | | | | | | | | | |
| |
| | Previous
GAAP | | Effect of
transition
to IFRSs | | IFRSs | |
|---|
| |
| | In thousands of euro
| |
|---|
| | Revenue | | | 3,732 | | | (1 | ) | | 3,731 | |
| | Change in inventories of work in progress | | | 30 | | | (15 | ) | | 15 | |
| | Own work capitalized | | | 13 | | | — | | | 13 | |
| | Other operating income | | | 719 | | | (16 | ) | | 703 | |
| | Material expenses | | | 1,140 | | | — | | | 1,140 | |
| | Personnel expenses | | | 1,992 | | | 72 | | | 1,920 | |
| | Deprecation and amortization | | | 167 | | | (60 | ) | | 227 | |
| | Other operating expenses | | | 1,083 | | | 36 | | | 1,047 | |
| | | | | | | | | |
| | Operating income | | | 112 | | | 16 | | | 128 | |
| | | | | | | | | |
| | Other interest and similar income | | | 2 | | | — | | | 2 | |
| | Interest and similar expenses | | | 281 | | | (3 | ) | | 284 | |
| | | | | | | | | |
| | Financial result | | | (279 | ) | | (3 | ) | | (282 | ) |
| | | | | | | | | |
| | Loss before taxes | | | (167 | ) | | (13 | ) | | (154 | ) |
| | | | | | | | | |
| | Taxes on income | | | 0 | | | 9 | | | 9 | |
| | Other taxes | | | 1 | | | — | | | 1 | |
| | Net loss/total comprehensive income for the year | | | 168 | | | 4 | | | 164 | |
| | | | | | | | | |
Comments on transition items
- a)
- Contrary to the financial statements prepared previous GAAP the Company recognized anintangible asset arising from development. The asset relates to software development and complies with all qualifications defined in IAS 38.57.
- b)
- Certain minor government grants provided in prior years amounting to € 39 thousands as of January 1, 2009, were deducted from the fundedtangible assets under IFRS.
For the capitalization of semi-finished and resp. finished goods under previous GAAP certain indirect costs of conversion have not been considered. At the date of the transition to IFRS these costs have been capitalized, leading to an increase in inventory amounting to € 54 thousands.
- d)
- In the course of setting-up financial statements under IFRS for the first time, a valuation allowance to tradereceivables amounting to € 7 thousands has been released.
- e)
- The various transitional adjustments lead to different temporary differences. According to the applicable accounting policies the Company has to account for such differences.Deferred tax adjustments are recognised in correlation to the underlying transaction either in retained earnings or a separate component of equity.
- f)
- Deficit not covered by equity is disclosed as part ofequity and liabilities in the balance sheet prepared under IFRS.
- g)
- In the financial statements prepared under previous GAAPprovisions and accruals (disclosed aspayables) are not disclosed separately. At the date of transition to IFRS the respective differentiated disclosure was set up.
Material adjustments to the Statements of Cash Flows
There are no material differences between the Statements of Cash Flows presented under IFRSs and the Statements of Cash Flows presented under previous GAAP.
SC-F-35
INDEPENDENT AUDITORS' REPORT
To the Scienion AG, Dortmund
We have audited the accompanying financial statements of Scienion AG, Dortmund, (the "Company") which comprise the statements of financial position as at December 31, 2010 and 2009 and January 1, 2009 and the statements of comprehensive income, statements of changes in equity and cash flow statements for the years ended December 31, 2010 and 2009 and the notes to the financial statements.
Management's Responsibility for the Financial Statements
Management is responsible for the preparation of these financial statements that give a true and fair view in accordance with International Financial Reporting Standards, and for such internal control as management determines is necessary to enable the preparation of financial statements that are free from material misstatement, whether due to fraud or error.
Auditors' Responsibility
Our responsibility is to express an opinion on these financial statements based on our audit. We conducted our audit in accordance with International Standards on Auditing. Those standards require that we comply with ethical requirements and plan and perform the audit to obtain reasonable assurance about whether the financial statements are free from material misstatement.
An audit involves performing procedures to obtain audit evidence about the amounts and disclosures in the financial statements. The procedures selected depend on the auditor's judgment, including the assessment of the risks of material misstatement of the financial statements, whether due to fraud or error. In making those risk assessments, the auditor considers internal control relevant to the entity's preparation of financial statements that give a true and fair view in order to design audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the entity's internal control. An audit also includes evaluating the appropriateness of accounting policies used and the reasonableness of accounting estimates made by management, as well as evaluating the overall presentation of the financial statements.
We believe that the audit evidence we have obtained in our audit is sufficient and appropriate to provide a basis for our audit opinion.
Opinion
In our opinion, the financial statements give a true and fair view of the financial position of Scienion AG as at December 31, 2010 and 2009 and January 1, 2009 and of its financial performance and cash flows for the years ended December 31, 2010 and 2009 in accordance with International Financial Reporting Standards.
Emphasis of Matter
We draw attention to Note 1 to the financial statements which states that "the Company is formally overindebted as of 31 December 2010. Most of the liabilities in connection with the silent participation, for which a declaration of subordination has been issued for up to EUR 3,437k, have a remaining term until the end of 2011. The management board assumes that the contract signed with the investor SQI Diagnostics Inc., Toronto, Ontario, Canada, on 4 July 2011 which provides for the investor to acquire all shares and to redeem the silent participations as well as to equip Scienion AG with the necessary capital resources will take effect after certain contractually agreed conditions precedent have been satisfied. If the transaction fails, the company's ability to continue as a going concern hinges on the liabilities from silent participations, including interest and costs that will be due on 31 December 2011 not falling due after that date but being made available to the company as substitute equity to cover the capital deficit prevailing at the time. Subject to the final approval of their corporate bodies, the silent shareholders have agreed to take appropriate measures ranging from a prolongation of the silent participation to a partial or even full waiver. The Company is therefore acting the
SC-F-36
assumption that it is more likely than not that Scienion AG will be able to continue as a going concern." Our opinion is not qualified in respect of this matter.
| | |
| Berlin, August 31, 2011 | | |
ERNST & YOUNG GmbH
Wirtschaftsprüfungsgesellschaft |
(Signed)
Seidel
Wirtschaftsprüfer
[German Public Auditor] |
|
(Signed)
Pfeiffer
Wirtschaftsprüferin
[German Public Auditor] |
SC-F-37
INDEX TO PRO FORMA FINANCIAL STATEMENTS
| | | | |
| | | Unaudited pro forma financial statements of SQI Diagnostics Inc. assuming the completion
of the acquisition of Scienion AG | | PF-2 |
PF-1
SQI Diagnostics Inc.
UNAUDITED PRO FORMA CONSOLIDATED BALANCE SHEET
(amounts are in thousands)
As at June 30, 2011
| | | | | | | | | | | | | | | | | | | | | | |
| | SQI Diagnostics Inc. | | Scienion | | Scienion | | Pro Forma
Adjustments | | Notes | | Pro Forma | |
|---|
| |
| | Euro
| | Canadian Dollar
| | Debit
| | Credit
| |
| |
| |
|---|
ASSETS | | | | | | | | | | | | | | | | | | | | | |
Current | | | | | | | | | | | | | | | | | | | | | |
| | Cash and cash equivalents | | | 2,817 | | | 379 | | | 531 | | | | | | 15,000 | | 2(a) | | | 15,216 | |
| | | | | | | | | | | | | | 26,868 | | | | | 2(b) | | | | |
| | Amounts receivable and other assets | | | 437 | | | 2,465 | | | 3,452 | | | | | | | | | | | 3,889 | |
| | Inventory | | | 512 | | | 904 | | | 1,266 | | | | | | | | | | | 1,778 | |
| | | | | | | | | | | | | | | | |
| | | 3,766 | | | 3,748 | | | 5,249 | | | 26,868 | | | 15,000 | | | | | 20,883 | |
| | | | | | | | | | | | | | | | |
| | Property and equipment | | | 2,717 | | | 491 | | | 688 | | | | | | | | | | | 3,405 | |
| | Patents | | | 572 | | | 232 | | | 325 | | | | | | | | | | | 897 | |
| | Deferred tax assets | | | | | | 107 | | | 150 | | | | | | | | 2(d) | | | 150 | |
| | Goodwill, other intangibles and unallocated purchase price | | | | | | | | | | | | 13,754 | | | | | 2(a) | | | 13,754 | |
| | Deferred Costs | | | 433 | | | | | | | | | | | | 433 | | 2(b) | | | — | |
| | | | | | | | | | | | | | | | |
| | | 7,488 | | | 4,578 | | | 6,412 | | | 40,622 | | | 15,433 | | | | | 39,089 | |
| | | | | | | | | | | | | | | | |
LIABILITIES | | | | | | | | | | | | | | | | | | | | | |
Current | | | | | | | | | | | | | | | | | | | | | |
| | Accounts payable and accrued liabilities | | | 1,529 | | | 1,393 | | | 1,951 | | | | | | | | | | | 3,480 | |
| | Short term financial liability | | | | | | 5,329 | | | 7,463 | | | 7,463 | | | | | 2(a) | | | | |
| | | | | | | | | | | | | | | | |
| | | 1,529 | | | 6,722 | | | 9,414 | | | 7,463 | | | | | | | | 3,480 | |
| | | | | | | | | | | | | | | | |
Long-term | | | | | | | | | | | | | | | | | | | | | |
| | Long-Term financial liability | | | | | | 150 | | | 210 | | | | | | | | | | | 210 | |
| | Deferred tax liabilities | | | | | | 308 | | | 431 | | | | | | | | 2(d) | | | 431 | |
| | | | | | | | | | | | | | | | |
| | | | | | 458 | | | 641 | | | | | | | | | | | 641 | |
| | | | | | | | | | | | | | | | |
| | | 1,529 | | | 7,180 | | | 10,055 | | | 7,463 | | | — | | | | | 4,121 | |
| | | | | | | | | | | | | | | | |
SHAREHOLDERS' EQUITY | | | | | | | | | | | | | | | | | | | | | |
| | Capital Stock | | | 35,377 | | | 187 | | | 262 | | | | | | 2,574 | | 2(a) | | | 64,386 | |
| | | | | | | | | | | | 262 | | | | | 2(a) | | | | |
| | | | | | | | | | | | | | | 28,935 | | 2(b) | | | | |
| | | | | | | | | | | | 2,500 | | | | | 2(b) | | | | |
| | Warrants | | | 1,614 | | | | | | | | | | | | | | | | | 1,614 | |
| | Contributed Surplus | | | 9,273 | | | 6,760 | | | 9,467 | | | 9,467 | | | | | 2(a) | | | 9,273 | |
| | Deficit | | | (40,305 | ) | | (9,549 | ) | | (13,373 | ) | | | | | 13,373 | | 2(a) | | | (40,305 | ) |
| | | | | | | | | | | | | | | | |
| | | 5,959 | | | (2,602 | ) | | (3,644 | ) | | 12,229 | | | 44,882 | | | | | 34,968 | |
| | | | | | | | | | | | | | | | |
| | | 7,488 | | | 4,578 | | | 6,411 | | | 19,692 | | | 44,882 | | | | | 39,089 | |
| | | | | | | | | | | | | | | | |
PF-2
SQI Diagnostics Inc.
UNAUDITED PRO FORMA CONSOLIDATED STATEMENT OF OPERATIONS
(amounts are in thousands except per share amounts)
As at September 30, 2010
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | SQI Diagnostics Inc. | | Scienion | | Scienion | |
| |
| |
| |
| |
| |
|---|
| | Year ended
September 30, 2010 | | Year ended
December 31, 2010 | | Year ended
December 31, 2010 | | IFRS
Adjustments | | Pro Forma
Adjustments | | Note | | Pro Forma | |
|---|
| |
| | Euro
| | Canadian Dollar
| |
| | Debit
| | Credit
| |
| |
| |
|---|
Revenue | | | | | | | | | | | | | | | | | | | | | | | | |
| | Product sales | | | 30 | | | 3,731 | | | 5,097 | | | | | | | | | | | | | | 5,127 | |
| | Consulting fees | | | 5 | | | | | | | | | | | | | | | | | | | | 5 | |
| | | | | | | | | | | | | | | | | | |
| | | 35 | | | 3,731 | | | 5,097 | | | | | | | | | | | | | | 5,132 | |
| | | | | | | | | | | | | | | | | | |
Expenses | | | | | | | | | | | | | | | | | | | | | | | | |
| | Cost of materials | | | | | | 1,112 | | | 1,519 | | | | | | | | | | | | | | 1,519 | |
| | Salaries and wages | | | 556 | | | 1,920 | | | 2,623 | | | | | | | | | | | | | | 3,179 | |
| | General and administrative | | | 471 | | | 916 | | | 1,251 | | | | | | | | | | | | | | 1,722 | |
| | Professional and consulting fees | | | 659 | | | | | | | | | | | | 600 | | | | | 2(c) | | | 1,259 | |
| | Sales and marketing | | | 474 | | | | | | | | | | | | | | | | | | | | 474 | |
| | Stock-based compensation | | | 402 | | | | | | | | | | | | | | | | | | | | 402 | |
| | Research and development costs | | | 5,059 | | | | | | | | | | | | | | | | | | | | 5,059 | |
| | Amortization | | | 515 | | | 227 | | | 310 | | | | | | | | | | | | | | 825 | |
| | Taxes | | | | | | 10 | | | 14 | | | | | | | | | | | | | | 14 | |
| | | | | | | | | | | | | | | | | | |
| | | 8,136 | | | 4,185 | | | 5,717 | | | | | | 600 | | | | | | | | 14,453 | |
| | | | | | | | | | | | | | | | | | |
Operating loss before undernoted items | | |
(8,101 |
) | |
(454 |
) | |
(620 |
) | | | | | | | | | | | | |
(9,321 |
) |
Other operating income | | | | | | 572 | | | 781 | | | | | | | | | | | | | | 781 | |
Interest Income | | | 32 | | | 2 | | | 3 | | | | | | | | | | | | | | 35 | |
Interest Expense | | | 4 | | | 284 | | | 388 | | | | | | | | | 384 | | 2(c) | | | 8 | |
| | | | | | | | | | | | | | | | | | |
| | | 28 | | | 290 | | | 396 | | | | | | | | | 384 | | | | | 808 | |
Net loss and comprehensive loss | | |
(8,073 |
) | |
(164 |
) | |
(224 |
) | | | | |
600 | | |
384 | | | | |
(8,513 |
) |
Weighted average number of shares outstanding | | | 30,349 | | | | | | | | | | | | | | | 745 | | 2(a) | | | 39,492 | |
| | | | | | | | | | | | | | | | | | 8,398 | | 2(b) | | | | |
| | | | | | | | | | | | | | | | | | |
Basic and diluted loss per share | | | | | | | | | | | | | | | | | | | | | | | (0.22 | ) |
| | | | | | | | | | | | | | | | | | |
PF-3
SQI Diagnostics Inc.
UNAUDITED PRO FORMA CONSOLIDATED STATEMENT OF OPERATIONS
(amounts are in thousands except per share amounts)
As at June 30, 2011
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | SQI Diagnostics
Inc. | | Scienion | | Scienion | |
| |
| |
| |
| |
| |
|---|
| | 9 months
June 30, 2011 | | 6 months ended
June 30, 2011 | | 6 months ended
June 30, 2011 | | IFRS
Adjustments | | Pro Forma
Adjustments | | Notes | | Pro Forma | |
|---|
| |
| | Euro
| | Canadian Dollar
| |
| | Debit
| | Credit
| |
| |
| |
|---|
Revenue | | | | | | | | | | | | | | | | | | | | | | | | |
| | Product sales | | | 22 | | | 3,145 | | | 4,314 | | | | | | | | | | | | | | 4,336 | |
| | Consulting fees | | | 9 | | | | | | | | | | | | | | | | | | | | 9 | |
| | | | | | | | | | | | | | | | | | |
| | | 31 | | | 3,145 | | | 4,314 | | | | | | | | | | | | | | 4,345 | |
| | | | | | | | | | | | | | | | | | |
Expenses | | | | | | | | | | | | | | | | | | | | | | | | |
| | Cost of materials | | | | | | 949 | | | 1,302 | | | | | | | | | | | | | | 1,302 | |
| | Salaries and wages | | | 607 | | | 983 | | | 1,348 | | | | | | | | | | | | | | 1,955 | |
| | General and administrative | | | 449 | | | 614 | | | 842 | | | | | | | | | | | | | | 1,291 | |
| | Professional and consulting fees | | | 669 | | | | | | | | | | | | | | | 324 | | 2(c) | | | 345 | |
| | Sales and marketing | | | 329 | | | | | | | | | | | | | | | | | | | | 329 | |
| | Stock-based compensation | | | 372 | | | | | | | | | | | | | | | | | | | | 372 | |
| | Research and development costs | | | 4,056 | | | | | | | | | | | | | | | | | | | | 4,056 | |
| | Amortization | | | 425 | | | 117 | | | 160 | | | | | | | | | | | | | | 585 | |
| | Taxes | | | | | | 127 | | | 174 | | | | | | | | | | | | | | 174 | |
| | | | | | | | | | | | | | | | | | |
| | | 6,907 | | | 2,790 | | | 3,826 | | | | | | | | | 324 | | | | | 10,409 | |
| | | | | | | | | | | | | | | | | | |
Operating loss before undernoted items | | |
(6,876 |
) | |
355 | | |
488 | | | | | | | | | | | | | |
(6,064 |
) |
Other operating income | | | | | | 142 | | | 195 | | | | | | | | | | | | | | 195 | |
Interest Income | | | 56 | | | 1 | | | 1 | | | | | | | | | | | | | | 57 | |
Interest Expense | | | | | | 140 | | | 192 | | | | | | | | | 174 | | 2(c) | | | 18 | |
| | | | | | | | | | | | | | | | | | |
| | | 56 | | | 3 | | | 4 | | | | | | | | | 174 | | | | | 234 | |
Net loss and comprehensive loss | | |
(6,820 |
) | |
359 | | |
492 | | | | | | | | |
498 | | | | |
(5,830 |
) |
Weighted average number of shares outstanding | | | 33,849 | | | | | | | | | | | | | | | 735 | | 2(a) | | | 42,982 | |
| | | | | | | | | | | | | | | | | | 8,398 | | 2(b) | | | | |
| | | | | | | | | | | | | | | | | | |
Basic and diluted loss per share | | | | | | | | | | | | | | | | | | | | | | | (0.14 | ) |
| | | | | | | | | | | | | | | | | | |
PF-4
SQI Diagnostics Inc.
NOTES TO PRO FORMA CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
1. BASIS OF PRESENTATION
The accompanying unaudited pro forma consolidated financial statements have been prepared by management for inclusion in the prospectus of SQI Diagnostics Inc. (SQI) dated September 6, 2011. These unaudited pro forma consolidated statements have been prepared to reflect the following transactions (the "Transaction"):
- •
- The acquisition of all of the outstanding common shares of Scienion AG (Scienion) by SQI for an estimated purchase price of $17,574,000 comprised of $15,000,000 on and the issuance of 735,294 common shares of SQI.
- •
- The issuance of an additional US$30,000,000 of common shares on the offering.
The unaudited pro forma consolidated balance sheet has been prepared as if the Transaction had closed on June 30, 2011 and has been derived from the unaudited balance sheets of SQI and Scienion as at June 30, 2011. The unaudited pro forma statements of operations have been prepared as if the Transaction had closed on October 1, 2009. The unaudited pro forma statements of operations for the year ended September 30, 2010 has been derived using the audited statement of operations of SQI for the year ended September 30, 2010 and audited statement of operations of Scienion for the year ended December 31, 2010. The unaudited pro forma statements of operations for the nine months ended June 30, 2011 has been derived using the unaudited statement of operations of SQI for the nine months ended June 30, 2011 and Scienion's unaudited statement of operation for the six months ended June 30, 2011.
The unaudited pro forma consolidated financial statements may not be indicative either of the results that actually would have occurred if the events reflected herein had taken place on the dates indicated or of the operating results that may be obtained in the future.
The unaudited pro forma consolidated financial statements should be read in conjunction with the unaudited interim financial statements noted above and the September 30, 2010 audited financial statements of SQI which are incorporated by reference and included in the prospectus and the December 31, 2010 audited financial statements of Scienion which are included in the prospectus.
The financial statements of Scienion were reported in accordance with International Financial Reporting Standards (IFRS) as issued by the International Accounting Standards Board (IASB). The financial statements of SQI Diagnostics Inc. have been prepared in accordance with Canadian generally accepted accounting principles. Accounting policy differences between Canadian GAAP and IFRS that would potentially have a material impact and which could be reasonably estimated are described in the notes to the pro forma statements. The financial statements of Scienion were reported in European euros using the current rate method. As such, the balance sheet of Scienion used in this pro forma was translated to Canadian dollars using the June 30, 2011 noon rate of Canadian $1.4005 for every European euro. The statement of operations for June 30, 2011 and December 31, 2010 were converted to Canadian dollars using the average rate for the six months ended June 30, 2011 and the twelve months ended December 31, 2010 of $1.3717 and $1.3661 for every European euro respectively.
Certain line items in the income statements and the balance sheet of Scienion, included in the pro forma statements and prepared in accordance with IFRS, have been reclassified for presentation purposes.
2. NOTES TO THE UNAUDITED PRO FORMA CONSOLIDATED FINANCIAL STATEMENTS:
- (a)
- Acquistion of Scienion AG
The unaudited pro forma consolidated balance sheet reflects the following acquistion of Scienion as if it had occurred on June 30, 2011. Upon closing the Company will acquire all the shares of Scienion. The acquisition is being accounted for as an acquisition of a business. The total estimated purchase consideration is $17,574,000 comprised of the following:
| | | | | | |
| | Cash | | $ | 15,000,000 | | |
| | 735,294 common shares | | | 2,574,000 | | |
| | | | | | |
| | | | $ | 17,574,000 | | |
| | | | | | |
The share consideration has been valued using the June 30, 2011 price of $3.50. The purchase price has been allocated to Scienion's assets and liabilites assumed under the purchase agreement based on their estimated fair values as at June 30, 2011 as described in the table below. Acquisition costs are estimated at $600,000. These estimates are preliminary and based on management's best estimate but are subject to change upon completion of the valuation of the Scienion assets following closing.
PF-5
SQI Diagnostics Inc.
NOTES TO PRO FORMA CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(Unaudited)
2. NOTES TO THE UNAUDITED PRO FORMA CONSOLIDATED FINANCIAL STATEMENTS: (Continued)
| | | | | |
| |
| | Estimated
Fair Value | |
|---|
| | Assets | | | | |
| | Cash | | | 531 | |
| | Receivables and other assets | | | 3,452 | |
| | Inventories | | | 1,266 | |
| | | | | |
| | | | | 5,249 | |
| | Patents | | |
325 | |
| | Property and equipment | | | 688 | |
| | Deferred tax assets | | | 150 | |
| | Goodwill, other intangibles and unallocated purchase price | | | 13,755 | |
| | | | | |
| | | | | 20,167 | |
| | | | | |
| | Liabilities | | | | |
| | Current | | | | |
| | Accounts payable and accrued liabilities | | | 1,952 | |
| | Short term financial liabilities | | | — | |
| | | | | |
| | | | | 1,952 | |
| | Long Term | | | | |
| | Deferred tax liabilities | | | 431 | |
| | Long-Term liabilities | | | 210 | |
| | | | | |
| | Net assets acquired | | | 17,574 | |
| | | | | |
The short term financial liability of Scienion will not be assumed under the acquisition agreement. As part of the share purchase agreement a debt settlement amount will be deducted from $15,000,000 cash purchase price. This debt settlement amount will be paid to Scienion to repay the outstanding loans prior to the closing of the acquisition.
- (b)
- Concurrent Financing
Concurrent with the completion of the proposed acquisition of Scienion, SQI will complete an issuance of common shares.
The Company expects to raise $28,935,000 (US $30,000,000) converted at the June 30th exchange rate of $.9645 for each US dollar net of estimated share issuance cost of $2,500,000. Upon the completion of the transaction costs previously deferred were netted against the proceeds.
- (c)
- Pro forma Statement of Operations Adjustments
The unaudited pro forma consolidated of operations statements reflects the acquistion of Scienion as if it had occurred on October 1, 2009.
Interest related to shareholder loans not assumed under the purchase agreement has been eliminated for the purposes of the pro forma statements.
Acquisition costs are estimated to be $600,000 and are reflected in the pro forma statement of operations for September 30, 2010. Actual acquisition costs expensed as of June 30, 2011 have been removed from the pro forma statement of operations.
- (d)
- Adjustments from IFRS to Canadian GAAP and accounting policies used by SQI
The treatement of deferred tax assets and liabilities under IFRS by Scienion have been identified as a potential Canadian GAAP difference. Under IFRS all deferred tax balances are classified as non-current, regardless of the classification of the underlying assets or liabilities, or the expected reversal date of the temporary difference. We have assumed for the purposes of the pro forma statements that all deferred tax assets and liabilites are long term.
PF-6
SQI Diagnostics Inc.
NOTES TO PRO FORMA CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(Unaudited)
2. NOTES TO THE UNAUDITED PRO FORMA CONSOLIDATED FINANCIAL STATEMENTS: (Continued)
The income statements of Scienion included in the pro forma statements have been prepared in accordance with IFRS. Certain differences exist between Canadian GAAP and IFRS; however, there are no material differences related to Scienion's financial statements.
The income statements of Scienion included in the pro forma statements have been adjusted to conform to the accounting policies of SQI. This has resulted in the reclassification of certain items.
- (e)
- Continuity of pro forma share capital
The following is a schedule of the pro forma share capital of SQI Diagnostics reflecting the transactions referred to in note 2(a) and 2(b)
| | | | | | | | |
| | Issued and outstanding common shares as at June 30, 2011 | | | 33,946 | | $ | 35,377 | |
| | Issued to acquire Scienion* | | | 735 | | | 2,574 | |
| | Issued for cash, net of issue costs of $2,500* | | | 8,398 | | | 26,435 | |
| | | | | | | |
| | Total Pro forma issued and outstanding common shares as at June 30, 2011 | | | 43,079 | | $ | 64,386 | |
- *
- Valued at June 30, 2011 closing price of $3.50
PF-7
• common shares

SQI DIAGNOSTICS INC.
Common Shares
Leerink Swann
Rodman & Renshaw, LLC
Kingsdale Capital Markets Inc.
Until • , 2011 (25 days after the commencement of this offering), all dealers that buy, sell or trade our common shares, whether or not participating in this offering, may be required to deliver a prospectus. This delivery requirement is in addition to the obligation of dealers to deliver a prospectus when acting as underwriters and with respect to their unsold allotments or subscriptions.
PART II
INFORMATION NOT REQUIRED TO BE DELIVERED TO
OFFEREES OR PURCHASERS
Indemnification of Directors and Officers
Under theCanada Business Corporations Act (the "CBCA"), the Registrant may indemnify its current or former directors or officers or another individual who acts or acted at the Registrant's request as a director or officer, or an individual acting in a similar capacity, of another entity, against all costs, charges and expenses, including an amount paid to settle an action or satisfy a judgment, reasonably incurred by the individual in respect of any civil, criminal, administrative, investigative or other proceeding in which the individual is involved because of his or her association with the Registrant or another entity, and the individual seeking indemnity shall have a right to such indemnity if such individual was not judged by the court or other competent authority to have committed any fault or omitted to do anything that such individual ought to have done. The CBCA also provides that the Registrant may advance moneys to such an individual for the costs, charges and expenses of such a proceeding.
The CBCA also provides that the Registrant may with the approval of a court, indemnify such an individual or advance moneys against all costs, charges and expenses reasonably incurred by the individual in connection with an action by or on behalf of the Registrant or other entity to procure a judgment in its favour, to which the individual is made a party because of the individual's association with the Registrant or other entity at the Registrant's request.
However, indemnification under any of the foregoing circumstances is prohibited under the CBCA unless the individual:
- •
- acted honestly and in good faith with a view to the Registrant's best interests, or the best interests of the other entity for which the individual acted as director or officer or in a similar capacity at the Registrant's request; and
- •
- in the case of a criminal or administrative action or proceeding that is enforced by a monetary penalty, the individual had reasonable grounds for believing that his or her conduct was lawful.
The Registrant's by-laws provide that the Registrant will indemnify a director or officer, a former director or officer or another individual who acts or acted at the Registrant's request as a director or officer (or an individual acting in a similar capacity) of another entity, against all costs, charges and expenses, including, without limitation, an amount paid to settle an action or satisfy a judgment, reasonably incurred by the individual in respect of any civil, criminal, administrative, investigative or other proceeding in which the individual is involved because of that association with the Registrant or other entity.
The Registrant's by-laws provide that the Registrant may advance monies to a director, officer or other individual for the costs, charges and expenses of a proceeding referred to above. That person must repay the monies if the person does not fulfill the conditions in the following paragraph.
The Registrant's by-laws provide that the Registrant will not indemnify a person referred to above unless the person: (a) acted honestly and in good faith with a view to the best interests of the Registrant or, as the case may be, to the best interests of the other entity for which the person acted as a director or officer or in a similar capacity at the Registrant's request; and (b) in the case of a criminal or administrative action or proceeding that is enforced by a monetary penalty, the person had reasonable grounds for believing that the person's conduct was lawful.
The Registrant's by-laws provide that the Registrant also has the right to indemnify a person referred to above in such other circumstances as the CBCA or law permits or requires. The Registrant's by-laws do not limit the right of any person entitled to indemnity to claim indemnity apart from the provisions of the Registrant's by-laws.
Subject to the CBCA, the Registrant may purchase and maintain insurance for the benefit of any individual referred to above as the board of directors of the Registrant may from time to time determine. The Registrant has purchased and maintains such an insurance policy.
II-1
* * *
Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers or persons controlling the Registrant pursuant to the foregoing provisions, the Registrant has been informed that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable.
II-2
EXHIBITS
See the Exhibit Index hereto.
II-3
PART III
UNDERTAKING AND CONSENT TO SERVICE OF PROCESS
Item 1. Undertaking.
The Registrant undertakes to make available, in person or by telephone, representatives to respond to inquiries made by the Securities and Exchange Commission staff, and to furnish promptly, when requested to do so by the Securities and Exchange Commission staff, information relating to the securities registered pursuant to this Form F-10 or to transactions in said securities.
Item 2. Consent to Service of Process.
The Registrant has previously filed with the Securities and Exchange Commission a written Appointment of Agent for Service of Process and Undertaking on Form F-X.
Any change to the name or address of the agent for service of the Registrant shall be communicated promptly to the Securities and Exchange Commission by an amendment to Form F-X referencing the file number of this registration statement.
III-1
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the Registrant certifies that it has reasonable grounds to believe that it meets all of the requirements for filing on Form F-10 and has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in Toronto, Ontario, Canada on this 8th day of September, 2011.
| | | | |
| | | SQI DIAGNOSTICS INC. |
|
|
By: |
|
/s/ ANDREW MORRIS
Name: Andrew Morris
Title: Chief Financial Officer
|
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement has been signed by the following persons in the capacities indicated on September 8, 2011.
| | |
Signature | | Title |
|---|
|
|
|
/s/ CLAUDE RICKS
Claude Ricks | | President, Chief Executive Officer and Director
(Principal Executive Officer) |
/s/ ANDREW MORRIS
Andrew Morris |
|
Chief Financial Officer
(Principal Financial and Accounting Officer) |
/s/ ERIC SCHNEIDER
Eric Schneider |
|
Chairman of the Board of Directors |
/s/ DAVID A. WILLIAMS
David A. Williams |
|
Director |
/s/ PAUL MOUNTAIN
Paul Mountain |
|
Director |
/s/ PETER WINKLEY
Peter Winkley |
|
Director |
/s/ SAIED NADJAFI
Saied Nadjafi |
|
Director |
/s/ PETER LEA
Peter Lea |
|
Director |
III-2
AUTHORIZED REPRESENTATIVE
Pursuant to the requirements of Section 6(a) of the Securities Act of 1933, the undersigned has signed this Registration Statement, solely in its capacity as the duly authorized representative of SQI Diagnostics Inc. in the United States, on September 8, 2011.
| | | | |
| | | PUGLISI & ASSOCIATES |
|
|
/s/ DONALD J. PUGLISI
|
| | | Name: | | Donald J. Puglisi |
| | | Title: | | Managing Director |
III-3
EXHIBIT INDEX
| | |
Exhibit | | Description |
|---|
| 3.1* | | Form of Underwriting Agreement. |
4.1† |
|
Annual Information Form, dated June 15, 2011. |
4.2† |
|
Management Proxy Circular, dated June 8, 2011, prepared in connection with the annual and special meeting of shareholders held on July 7, 2011. |
4.3 |
|
Audited consolidated financial statements for the years ended September 30, 2010 and 2009, together with the auditor's report thereon (included in the prospectus forming a part of this Registration Statement). |
4.4 |
|
Supplemental Note Regarding the Reconciliation of the Consolidated Financial Statements for the Years Ended September 30, 2010 and 2009 with U.S. GAAP (included in the prospectus forming a part of this Registration Statement). |
4.5† |
|
Management's discussion and analysis of consolidated results of operations and financial condition for the fiscal year ended September 30, 2010. |
4.6 |
|
Unaudited interim consolidated financial statements for the three and nine months ended June 30, 2011. |
4.7 |
|
Supplemental Note Regarding the Reconciliation of the Interim Consolidated Financial Statements for the Three and Nine Month Periods Ended June 30, 2011 and 2010 with U.S. GAAP. |
4.8 |
|
Management's discussion and analysis of consolidated results of operations and financial condition for the three and nine months ended June 30, 2011. |
4.9 |
|
Material Change Report, as amended, dated July 4, 2011. |
5.1 |
|
Consent of Collins Barrow Toronto LLP. |
5.2 |
|
Consent of Ernst & Young GmbH. |
6.1† |
|
Powers of Attorney. |
- *
- To be filed by amendment.
- †
- Previously filed.
QuickLinks
PART I INFORMATION REQUIRED TO BE DELIVERED TO OFFEREES OR PURCHASERSGENERAL MATTERSFORWARD-LOOKING STATEMENTSCURRENCY PRESENTATION AND EXCHANGE RATE INFORMATIONPROSPECTUS SUMMARYRISK FACTORSCAPITALIZATIONUSE OF PROCEEDSBUSINESS OF THE COMPANYLEGAL PROCEEDINGS AND REGULATORY ACTIONSACQUISITION OF SCIENION AGSELECTED CONSOLIDATED FINANCIAL INFORMATIONMANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONSMANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONSDIRECTORS AND OFFICERSDESCRIPTION OF CAPITAL STRUCTUREDIVIDEND POLICYMARKET FOR SECURITIESPRINCIPAL SHAREHOLDERSPLAN OF DISTRIBUTIONDOCUMENTS INCORPORATED BY REFERENCEWHERE YOU CAN FIND MORE INFORMATIONAUDITORS, TRANSFER AGENTS AND REGISTRARSCERTAIN CANADIAN FEDERAL INCOME TAX CONSIDERATIONSCERTAIN MATERIAL UNITED STATES FEDERAL INCOME TAX CONSIDERATIONSDOCUMENTS FILED AS PART OF THE U.S. REGISTRATION STATEMENTENFORCEABILITY OF CIVIL LIABILITIESEXPERTSLEGAL MATTERSMATERIAL CONTRACTSEXEMPTIONS FROM THE INSTRUMENTINTEREST OF EXPERTSINDEX TO CONSOLIDATED FINANCIAL STATEMENTS OF SQI DIAGNOSTICS INC.INTERIM CONSOLIDATED BALANCE SHEETSINTERIM CONSOLIDATED STATEMENT OF OPERATIONS AND DEFICITINTERIM CONSOLIDATED STATEMENT OF CASH FLOWSNOTES TO INTERIM CONSOLIDATED FINANCIAL STATEMENTSSUPPLEMENTAL NOTEAUDITORS' REPORTCONSOLIDATED BALANCE SHEETSCONSOLIDATED STATEMENTS OF OPERATIONS AND DEFICITCONSOLIDATED STATEMENTS OF CASH FLOWSNOTES TO CONSOLIDATED FINANCIAL STATEMENTSAUDITORS' REPORT ON THE SUPPLEMENTAL NOTESUPPLEMENTAL NOTEINDEX TO CONSOLIDATED FINANCIAL STATEMENTS OF SCIENION AGScienion AG, Dortmund INTERIM STATEMENT OF COMPREHENSIVE INCOME FOR THE PERIODS FROM JANUARY 1ST TO JUNE 30TH, 2011 AND APRIL 1ST TO JUNE 30TH, 2011 (Unaudited)Scienion AG, Dortmund INTERIM STATEMENT OF FINANCIAL POSITION AS OF 30TH JUNE, 2011 (Unaudited)Scienion AG, Dortmund INTERIM STATEMENT OF CHANGES IN EQUITY (Unaudited)Scienion AG, Dortmund INTERIM STATEMENT OF CASH FLOWS (unaudited)NOTES TO THE INTERIM FINANCIAL STATEMENTSScienion Ag, Dortmund STATEMENT OF COMPREHENSIVE INCOME FOR THE FISCAL YEAR 2010 AND 2009Scienion AG, Dortmund STATEMENT OF FINANCIAL POSITION AS OF 31st DECEMBER 2010 AND AS OF 31st DECEMBER 2009Scienion AG, Dortmund STATEMENT OF FINANCIAL POSITION AS OF 31st DECEMBER 2010 AND AS OF 31st DECEMBER 2009Scienion AG, Dortmund STATEMENT OF CHANGES IN EQUITYScienion AG, Dortmund STATEMENT OF CASH FLOWSNOTES TO THE FINANCIAL STATEMENTS 2010 AND 2009INDEPENDENT AUDITORS' REPORTINDEX TO PRO FORMA FINANCIAL STATEMENTSSQI Diagnostics Inc. UNAUDITED PRO FORMA CONSOLIDATED BALANCE SHEET (amounts are in thousands) As at June 30, 2011SQI Diagnostics Inc. UNAUDITED PRO FORMA CONSOLIDATED STATEMENT OF OPERATIONS (amounts are in thousands except per share amounts) As at September 30, 2010SQI Diagnostics Inc. UNAUDITED PRO FORMA CONSOLIDATED STATEMENT OF OPERATIONS (amounts are in thousands except per share amounts) As at June 30, 2011NOTES TO PRO FORMA CONSOLIDATED FINANCIAL STATEMENTSPART II INFORMATION NOT REQUIRED TO BE DELIVERED TO OFFEREES OR PURCHASERSEXHIBITSPART III UNDERTAKING AND CONSENT TO SERVICE OF PROCESSSIGNATURESAUTHORIZED REPRESENTATIVEEXHIBIT INDEX






