
Catalyzing Precision Medicine with Integrated Rx/Dx in Oncology Ignyta Lilly Transaction, 3Q 2015 Company Highlights and Financial Results November 9, 2015 Exhibit 99.3

Safe Harbor Statement This document contains forward-looking statements, as that term is defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, about Ignyta, Inc. (“us” or the “Company”). Statements that are not purely historical are forward-looking statements. These include statements regarding, among other things: Ignyta’s corporate and scientific vision and goals, including our ability to reduce the size of tumors and to eradicate residual disease; the clinical and/or non-clinical data or plans underlying entrectinib, taladegib or any of our other development programs; our ability to design and conduct development activities for entrectinib, taladegib and our other development programs; our ability to develop or access companion diagnostics for our product candidates; our ability to obtain and maintain intellectual property protection for our product candidates; our ability to adequately fund our development programs; the Lilly transaction serving as a transformative event for us and the development and market potential of our pipeline; our ability to obtain regulatory approvals in order to market any of our product candidates; and our ability to successfully commercialize any approved products. Forward-looking statements involve known and unknown risks that relate to future events or the Company’s future financial performance, some of which may be beyond our control, and the actual results could differ materially from those discussed in this document. Accordingly, the Company cautions investors not to place undue reliance on the forward-looking statements contained in, or made in connection with, this document. Important factors that could cause actual results to differ materially from those indicated by such forward-looking statements, include, among others, the potential for results of past or ongoing clinical or non-clinical studies to differ from expectations or previous results; the interpretation of data from our clinical and non-clinical studies; our ability to initiate and complete clinical trials and non-clinical studies; regulatory developments; the potential advantages of our product candidates; the markets any approved products are intended to serve; and our capital needs; as well as those set forth under the headings “Special Note Regarding Forward-Looking Statements,” “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” contained in the Company’s Form 10-K filed with the Securities and Exchange Commission (“SEC”) on March 12, 2015, and similar disclosures made in the Company’s Form 10-Q filings and other SEC filings and press releases. The forward-looking statements contained in this document represent our estimates and assumptions only as of the date of this document, and we undertake no duty or obligation to update or revise publicly any forward-looking statements contained in this document as a result of new information, future events or changes in our expectations. Third-party information included herein has been obtained from sources believed to be reliable, but the accuracy or completeness of such information is not guaranteed by, and should not be construed as a representation by, the Company.

Agenda Ignyta’s BHAG* and scientific vision Lilly transaction and taladegib overview Taladegib opportunities Q3 2015 company highlights and financial results * Big Hairy Audacious Goal
Agenda Ignyta’s BHAG* and scientific vision Lilly transaction and taladegib overview Taladegib opportunities Q3 2015 company highlights and financial results * Big Hairy Audacious Goal
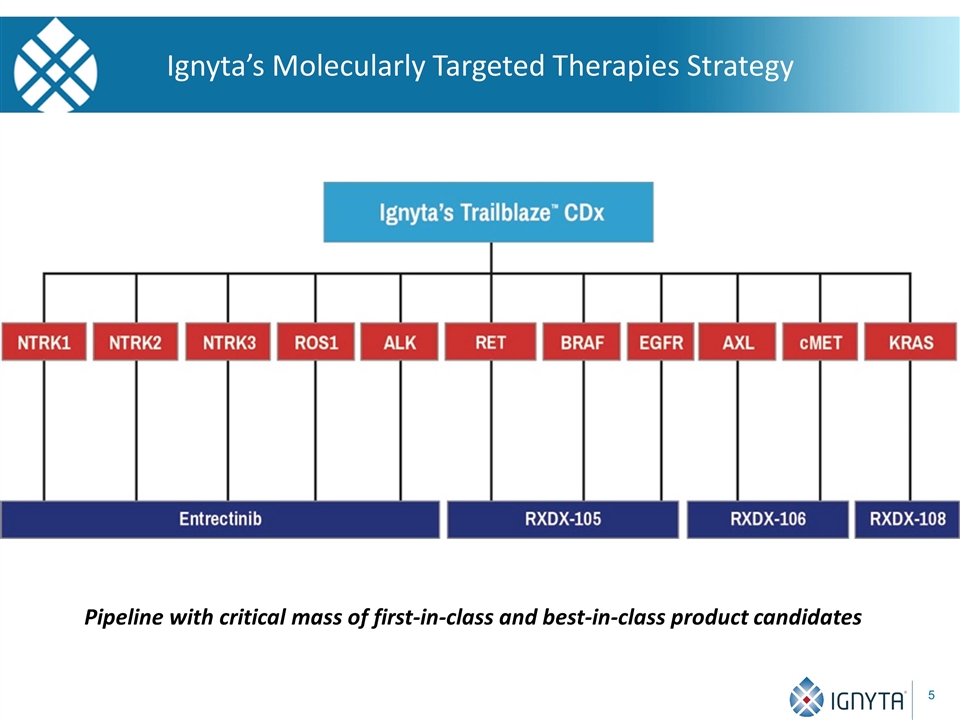
Ignyta’s Molecularly Targeted Therapies Strategy Pipeline with critical mass of first-in-class and best-in-class product candidates
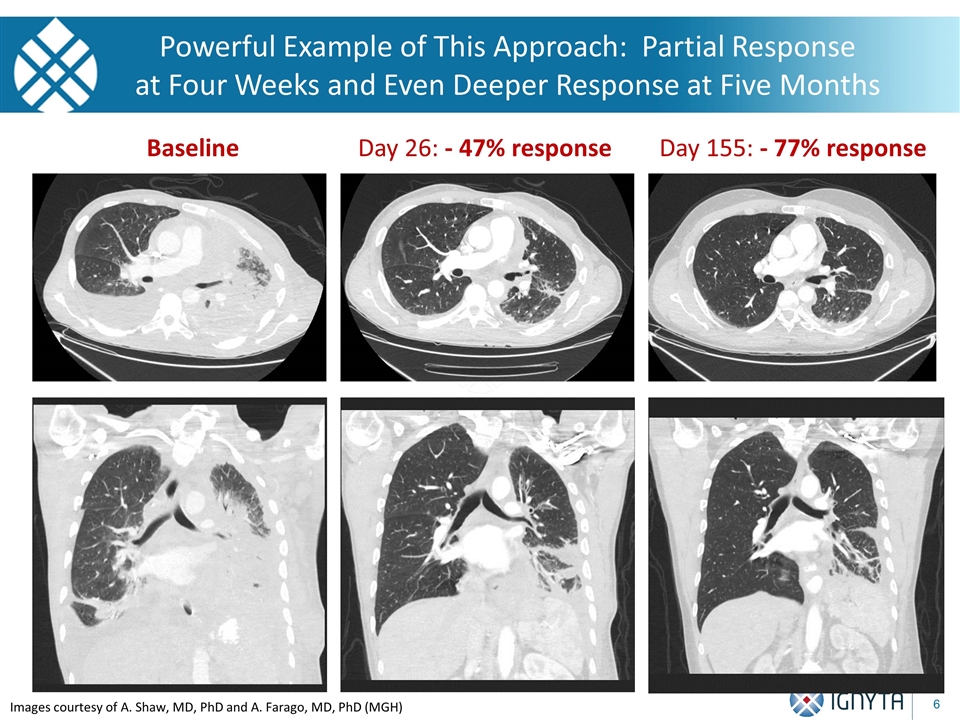
Images courtesy of A. Shaw, MD, PhD and A. Farago, MD, PhD (MGH) Images courtesy of A. Shaw, MD, PhD and A. Farago, MD, PhD (MGH) Baseline Day 26: - 47% response Day 155: - 77% response Powerful Example of This Approach: Partial Response at Four Weeks and Even Deeper Response at Five Months
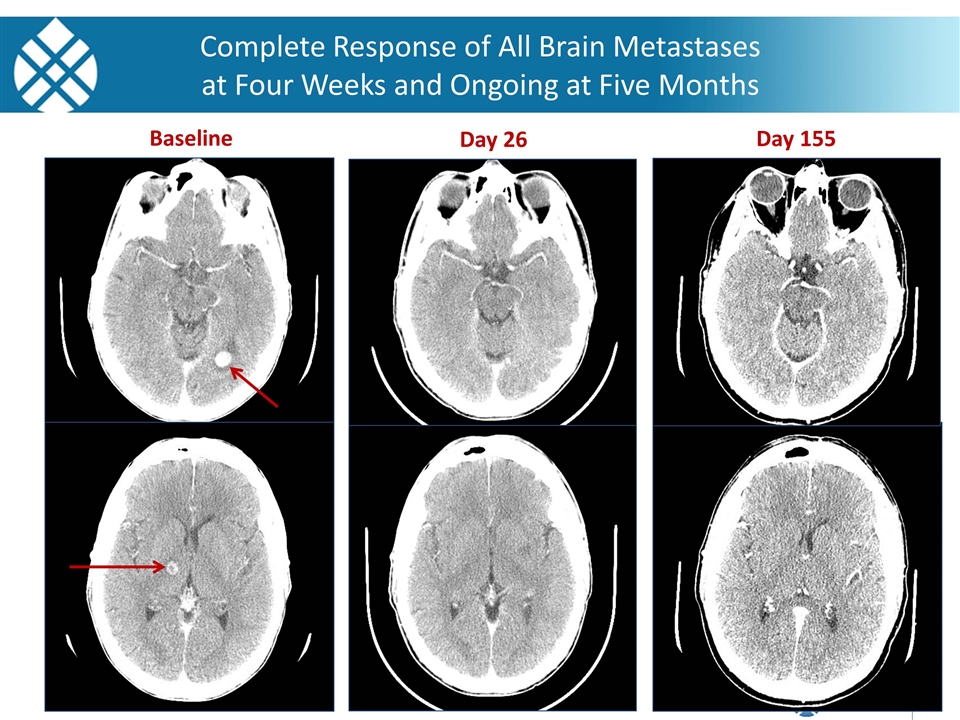
Images courtesy of A. Shaw, MD, PhD and A. Farago, MD, PhD (MGH) Baseline Day 26 Day 155 Complete Response of All Brain Metastases at Four Weeks and Ongoing at Five Months

Ignyta’s Big Hairy Audacious Goal (BHAG)
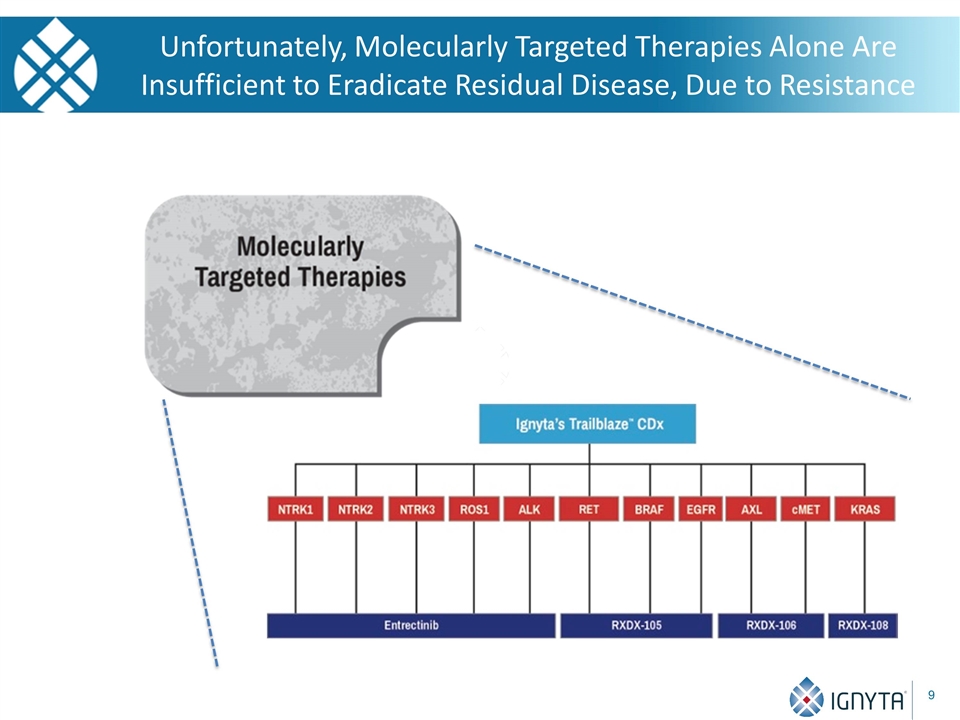
Unfortunately, Molecularly Targeted Therapies Alone Are Insufficient to Eradicate Residual Disease, Due to Resistance
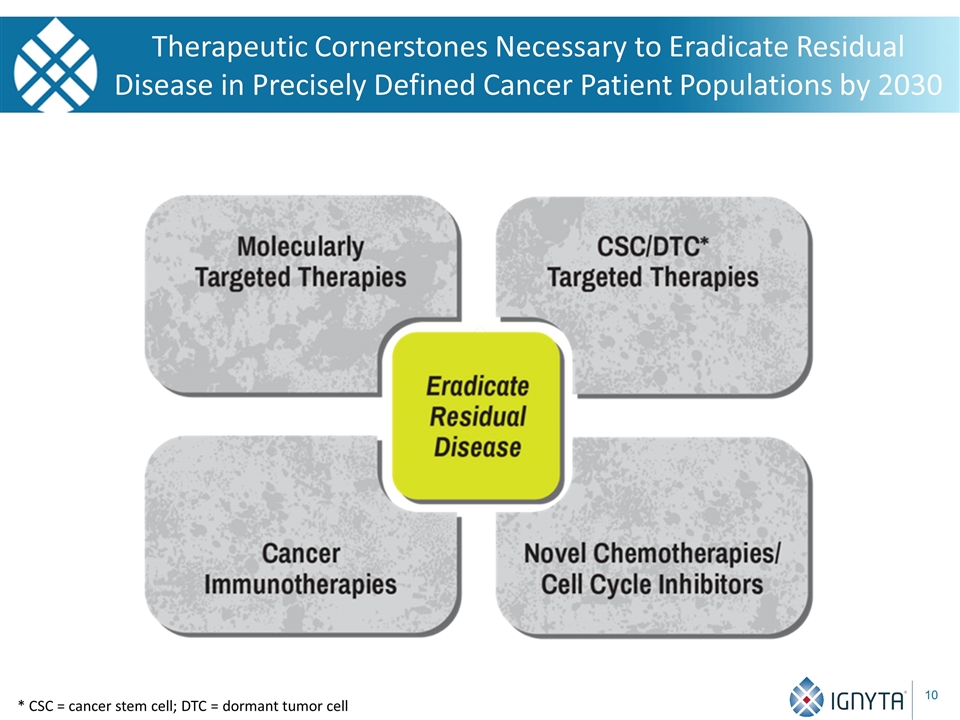
Therapeutic Cornerstones Necessary to Eradicate Residual Disease in Precisely Defined Cancer Patient Populations by 2030 * CSC = cancer stem cell; DTC = dormant tumor cell
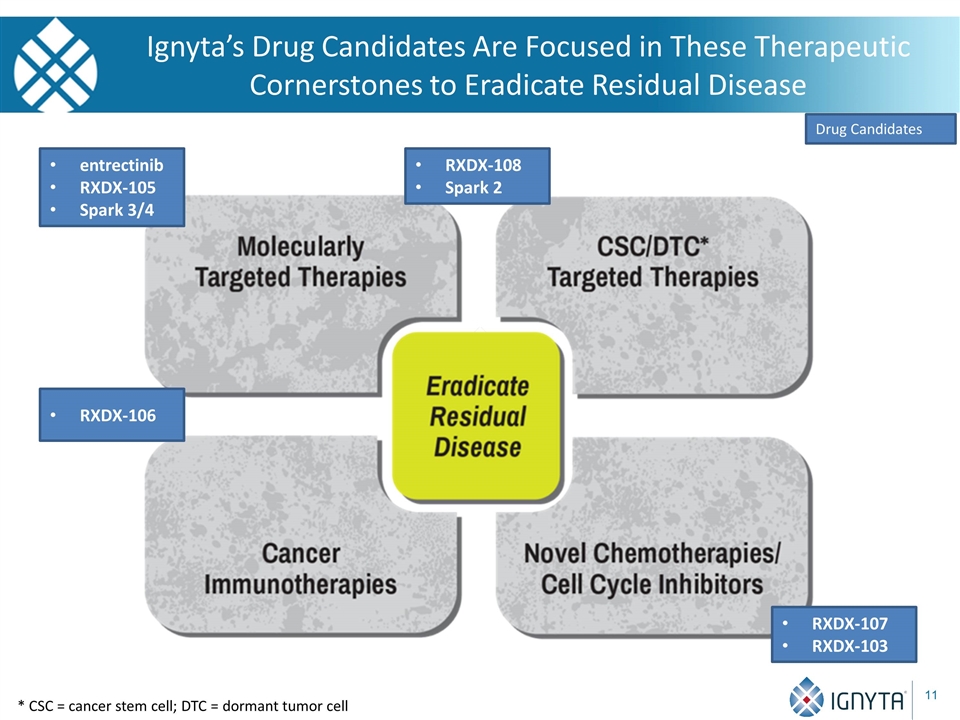
Ignyta’s Drug Candidates Are Focused in These Therapeutic Cornerstones to Eradicate Residual Disease RXDX-108 Spark 2 Drug Candidates entrectinib RXDX-105 Spark 3/4 RXDX-107 RXDX-103 * CSC = cancer stem cell; DTC = dormant tumor cell RXDX-106
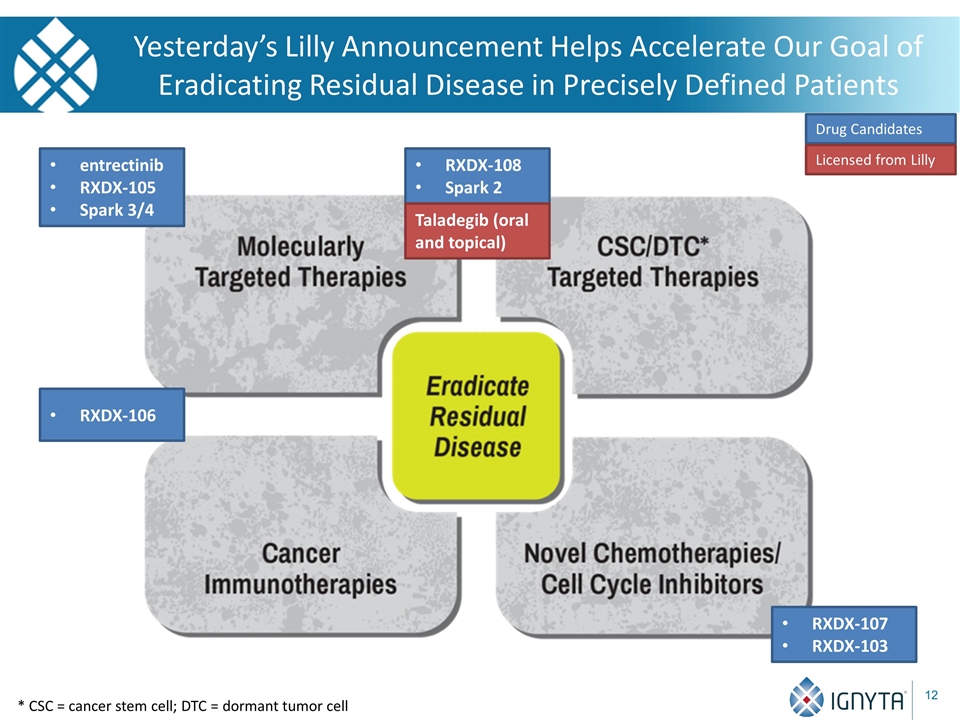
Yesterday’s Lilly Announcement Helps Accelerate Our Goal of Eradicating Residual Disease in Precisely Defined Patients RXDX-108 Spark 2 Drug Candidates entrectinib RXDX-105 Spark 3/4 RXDX-107 RXDX-103 * CSC = cancer stem cell; DTC = dormant tumor cell Licensed from Lilly Taladegib (oral and topical) RXDX-106

Agenda Ignyta’s BHAG* and scientific vision Lilly transaction and taladegib overview Taladegib opportunities Q3 2015 company highlights and financial results * Big Hairy Audacious Goal
Lilly Transaction: Ignyta Has Acquired Exclusive Rights to a Clinical Stage Oncology Program Lilly kicked off process in summer of 2015 to outlicense taladegib oral and topical programs Ignyta emerged as Lilly’s preferred option due to breadth and depth of precision oncology expertise Parties signed and announced Ignyta’s exclusive in-licensing of Lilly’s taladegib oral and topical programs on Sun., 11/8/15
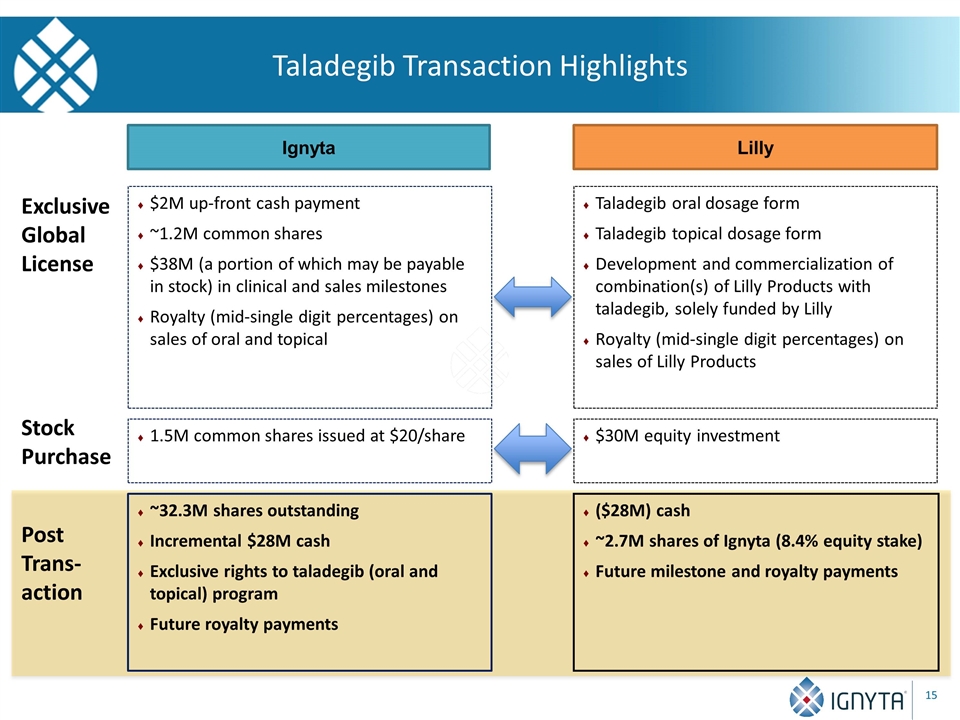
Taladegib Transaction Highlights $2M up-front cash payment ~1.2M common shares $38M (a portion of which may be payable in stock) in clinical and sales milestones Royalty (mid-single digit percentages) on sales of oral and topical Ignyta Lilly Taladegib oral dosage form Taladegib topical dosage form Development and commercialization of combination(s) of Lilly Products with taladegib, solely funded by Lilly Royalty (mid-single digit percentages) on sales of Lilly Products Exclusive Global License 1.5M common shares issued at $20/share $30M equity investment Stock Purchase Post Trans- action ~32.3M shares outstanding Incremental $28M cash Exclusive rights to taladegib (oral and topical) program Future royalty payments ($28M) cash ~2.7M shares of Ignyta (8.4% equity stake) Future milestone and royalty payments
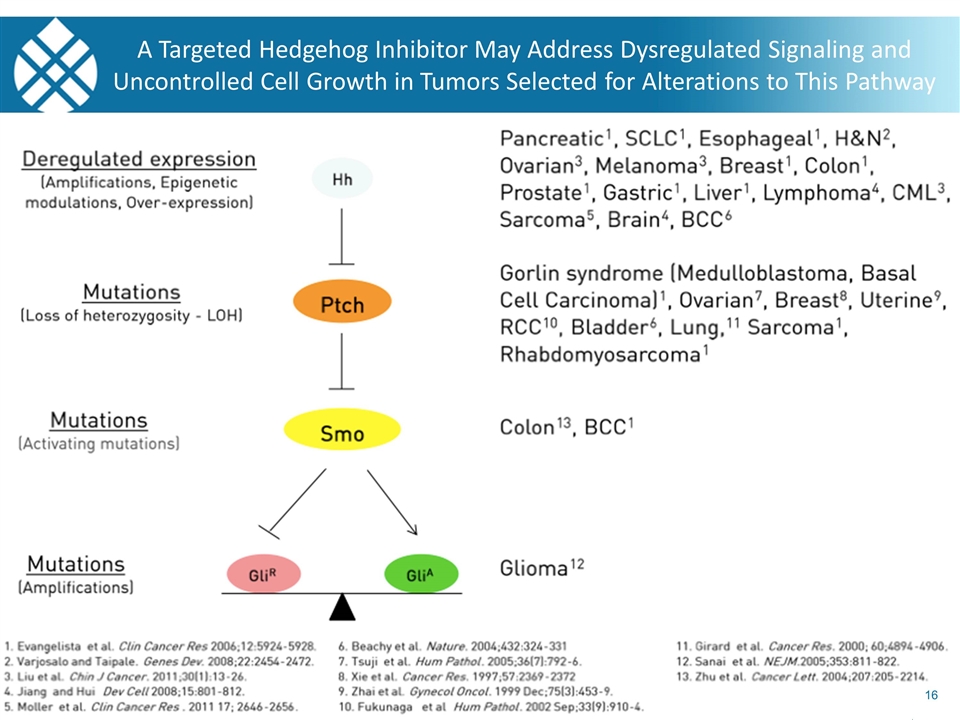
A Targeted Hedgehog Inhibitor May Address Dysregulated Signaling and Uncontrolled Cell Growth in Tumors Selected for Alterations to This Pathway
Key Attributes of Taladegib (LY2940680) Designed by LLY to be a potent & selective Hh/SMO antagonist Binds with high affinity to SMO (Ki 9 nM) and potently inhibits Hh/SMO pathway signaling in cell based assays (IC50 2.4 nM) Taladegib maintains activity in clinically relevant SMO mutants Good pharmaceutical properties including permeability and solubility Good plasma free fraction, brain penetration, and oral bioavailability Acceptable toxicology profile in multiple preclinical species Drug candidate has been studied in ~200 patients and healthy volunteers with good tolerability profile and highly promising signs of antitumor activity
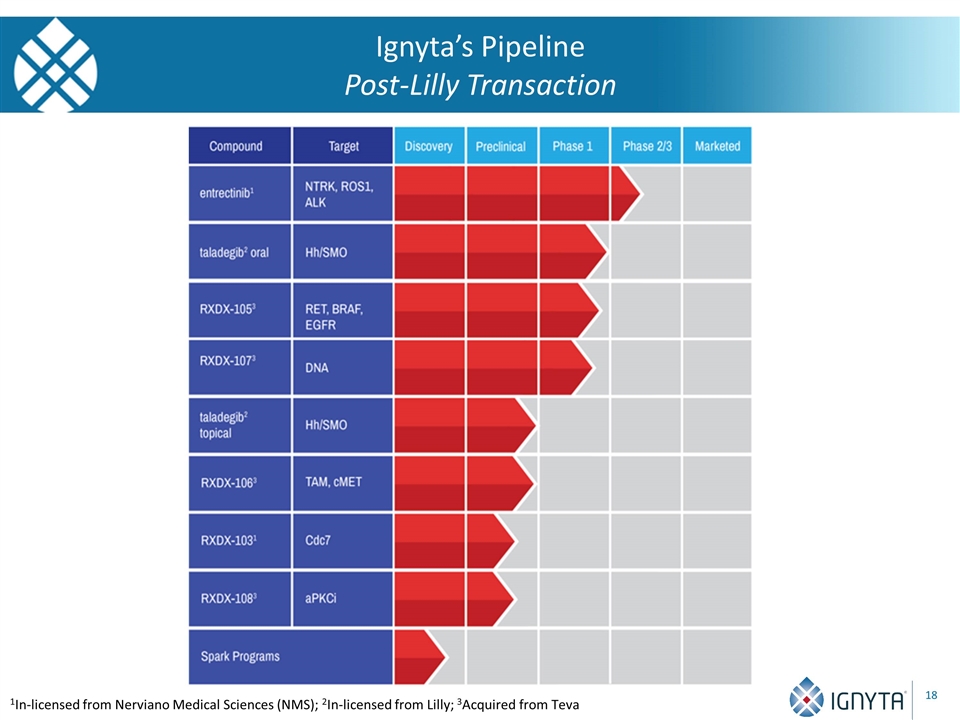
Ignyta’s Pipeline Post-Lilly Transaction 1In-licensed from Nerviano Medical Sciences (NMS); 2In-licensed from Lilly; 3Acquired from Teva
Agenda Ignyta’s BHAG* and scientific vision Lilly transaction and taladegib overview Taladegib opportunities Q3 2015 company highlights and financial results * Big Hairy Audacious Goal
Ignyta Plans to Target 4 Major Opportunities with Taladegib 1. Potential First-in-Class Hhi for 2L la/mBCC or Best-in-Class Hhi for 1L la/mBCC Potential fast to market opportunity in both 1L and 2L advanced BCC with two single-arm, potentially registrational Phase 2 studies BIC: best-in-class; FIC: first-in-class; 1L: 1st line; 2L: 2nd line; BCC: basal cell carcinoma; la/mBCC: locally advanced or metastatic BCC; SMO: smoothened; Hh: hedgehog; CNG: copy number gain; CSC: cancer stem cell; EMT: epithelial mesenchymal transition
Ignyta Plans to Target 4 Major Opportunities with Taladegib 1. Potential First-in-Class Hhi for 2L la/mBCC or Best-in-Class Hhi for 1L la/mBCC Potential fast to market opportunity in both 1L and 2L advanced BCC with two single-arm, potentially registrational Phase 2 studies 2. Leverage Rx/Dx expertise to assess efficacy in selected patients with Hh pathway activated tumors Potential significant upside opportunity in solid tumors beyond BCC based on targeted Rx/Dx strategy BIC: best-in-class; FIC: first-in-class; 1L: 1st line; 2L: 2nd line; BCC: basal cell carcinoma; la/mBCC: locally advanced or metastatic BCC; SMO: smoothened; Hh: hedgehog; CNG: copy number gain; CSC: cancer stem cell; EMT: epithelial mesenchymal transition
Ignyta Plans to Target 4 Major Opportunities with Taladegib 1. Potential First-in-Class Hhi for 2L la/mBCC or Best-in-Class Hhi for 1L la/mBCC Potential fast to market opportunity in both 1L and 2L advanced BCC with two single-arm, potentially registrational Phase 2 studies 2. Leverage Rx/Dx expertise to assess efficacy in selected patients with Hh pathway activated tumors Potential significant upside opportunity in solid tumors beyond BCC based on targeted Rx/Dx strategy 3. Combine with a) RXDX-108 aPKCi inhibitor and b) other agents to address residual disease and/or resistance Ignyta uniquely has a FIC aPKCiota and BIC Hh inhibitor combination to shut down the PKCi – SOX2 – Hh signaling axis of 3q26 amplifications, the most common CNG in solid tumors Taladegib could be a CSC/EMT-targeting linchpin for combining with Ignyta’s pipeline. Lilly will also be developing certain combinations, at its expense BIC: best-in-class; FIC: first-in-class; 1L: 1st line; 2L: 2nd line; BCC: basal cell carcinoma; la/mBCC: locally advanced or metastatic BCC; SMO: smoothened; Hh: hedgehog; CNG: copy number gain; CSC: cancer stem cell; EMT: epithelial mesenchymal transition
Ignyta Plans to Target 4 Major Opportunities with Taladegib 1. Potential First-in-Class Hhi for 2L la/mBCC or Best-in-Class Hhi for 1L la/mBCC Potential fast to market opportunity in both 1L and 2L advanced BCC with two single-arm, potentially registrational Phase 2 studies 2. Leverage Rx/Dx expertise to assess efficacy in selected patients with Hh pathway activated tumors Potential significant upside opportunity in solid tumors beyond BCC based on targeted Rx/Dx strategy 3. Combine with a) RXDX-108 aPKCi inhibitor and b) other agents to address residual disease and/or resistance Ignyta uniquely has a FIC aPKCiota and BIC Hh inhibitor combination to shut down the PKCi – SOX2 – Hh signaling axis of 3q26 amplifications, the most common CNG in solid tumors Taladegib could be a CSC/EMT-targeting linchpin for combining with Ignyta’s pipeline. Lilly will also be developing certain combinations, at its expense 4. Potential First-in-Class Hhi for superficial +/- nodular BCC Upside opportunity in early BCC with taladegib topical BIC: best-in-class; FIC: first-in-class; 1L: 1st line; 2L: 2nd line; BCC: basal cell carcinoma; la/mBCC: locally advanced or metastatic BCC; SMO: smoothened; Hh: hedgehog; CNG: copy number gain; CSC: cancer stem cell; EMT: epithelial mesenchymal transition
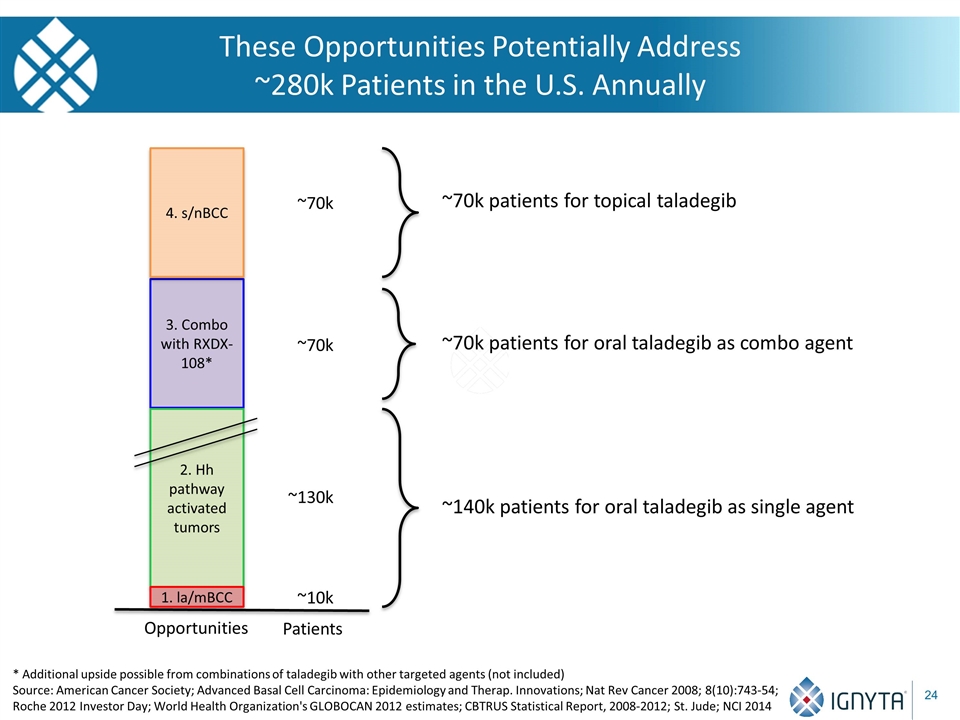
These Opportunities Potentially Address ~280k Patients in the U.S. Annually 3. Combo with RXDX-108* 4. s/nBCC ~10k ~130k ~70k ~70k ~140k patients for oral taladegib as single agent ~70k patients for oral taladegib as combo agent ~70k patients for topical taladegib Opportunities Patients 2. Hh pathway activated tumors 1. la/mBCC * Additional upside possible from combinations of taladegib with other targeted agents (not included) Source: American Cancer Society; Advanced Basal Cell Carcinoma: Epidemiology and Therap. Innovations; Nat Rev Cancer 2008; 8(10):743-54; Roche 2012 Investor Day; World Health Organization's GLOBOCAN 2012 estimates; CBTRUS Statistical Report, 2008-2012; St. Jude; NCI 2014
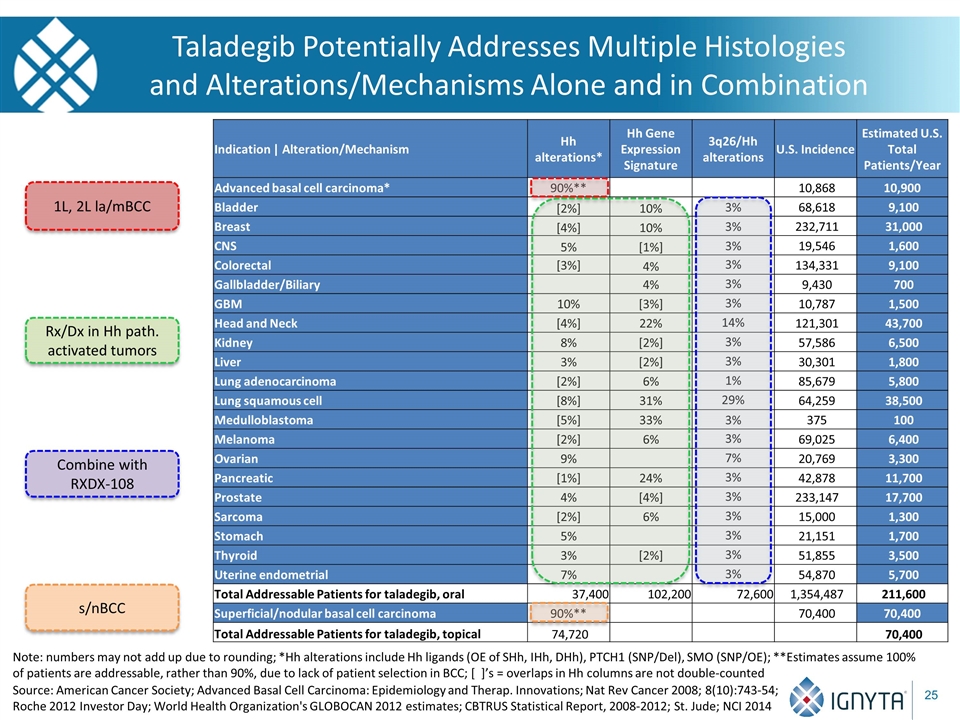
Indication | Alteration/Mechanism Hh alterations* Hh Gene Expression Signature 3q26/Hh alterations U.S. Incidence Estimated U.S. Total Patients/Year Advanced basal cell carcinoma* 90%** 10,868 10,900 Bladder [2%] 10% 3% 68,618 9,100 Breast [4%] 10% 3% 232,711 31,000 CNS 5% [1%] 3% 19,546 1,600 Colorectal [3%] 4% 3% 134,331 9,100 Gallbladder/Biliary 4% 3% 9,430 700 GBM 10% [3%] 3% 10,787 1,500 Head and Neck [4%] 22% 14% 121,301 43,700 Kidney 8% [2%] 3% 57,586 6,500 Liver 3% [2%] 3% 30,301 1,800 Lung adenocarcinoma [2%] 6% 1% 85,679 5,800 Lung squamous cell [8%] 31% 29% 64,259 38,500 Medulloblastoma [5%] 33% 3% 375 100 Melanoma [2%] 6% 3% 69,025 6,400 Ovarian 9% 7% 20,769 3,300 Pancreatic [1%] 24% 3% 42,878 11,700 Prostate 4% [4%] 3% 233,147 17,700 Sarcoma [2%] 6% 3% 15,000 1,300 Stomach 5% 3% 21,151 1,700 Thyroid 3% [2%] 3% 51,855 3,500 Uterine endometrial 7% 3% 54,870 5,700 Total Addressable Patients for taladegib, oral 37,400 102,200 72,600 1,354,487 211,600 Superficial/nodular basal cell carcinoma 90%** 70,400 70,400 Total Addressable Patients for taladegib, topical 74,720 70,400 Source: American Cancer Society; Advanced Basal Cell Carcinoma: Epidemiology and Therap. Innovations; Nat Rev Cancer 2008; 8(10):743-54; Roche 2012 Investor Day; World Health Organization's GLOBOCAN 2012 estimates; CBTRUS Statistical Report, 2008-2012; St. Jude; NCI 2014 Taladegib Potentially Addresses Multiple Histologies and Alterations/Mechanisms Alone and in Combination 1L, 2L la/mBCC Rx/Dx in Hh path. activated tumors Combine with RXDX-108 s/nBCC Note: numbers may not add up due to rounding; *Hh alterations include Hh ligands (OE of SHh, IHh, DHh), PTCH1 (SNP/Del), SMO (SNP/OE); **Estimates assume 100% of patients are addressable, rather than 90%, due to lack of patient selection in BCC; [ ]’s = overlaps in Hh columns are not double-counted
1. Potential First-in-Class Hedgehog Inhibitor (Hhi) for 2L la/mBCC and Best-in-Class Hhi for 1L la/mBCC Potential First-in-Class option for 2L la/mBCC and Best-in-Class profile for 1L la/mBCC with compelling early efficacy demonstrated in Phase 1 clinical study Compelling fast-to-market opportunity in advanced BCC with two single-arm Phase 2 studies potentially supportive of registration Ignyta estimates that of the 33,000 patients with advanced BCC in the U.S., taladegib oral could address ~10,000* of these cases Rapid registration in advanced BCC would provide Ignyta with potential optionality to expand into larger patient populations for single agent and combination agent use *2.2M non-melanoma skin cancers; BCC is 80% of this (1.76M); laBCC is 1% of BCC (9.5% not candidates for surgery/radiation); mBCC is 0.55% (95% not candidates for surgery/radiation), yielding 10,868 patients in the U.S. Source: American Cancer Society; Advanced Basal Cell Carcinoma: Epidemiology and Therapeutic Innovations
Study HHBB: Objectives And Methods of Phase 1 First-in-Human Clinical Study of Taladegib Primary Objective To determine a recommended phase 2 dose and regimen of taladegib that could be safely administered to patients with advanced cancer Secondary Objectives To evaluate pharmacokinetic (PK) parameters of taladegib and its major circulating and equipotent metabolite To evaluate antitumor activity To document the clinical benefit rate and duration of response in patients with advanced cancer Study Design Multicenter, nonrandomized, open-label, dose escalation phase 1 clinical study Patients were dosed once daily (QD) for 28 day cycles until discontinuation The study has three phases: Dose escalation phase in patients with advanced cancers Dose confirmation phase in patients with advanced cancers Disease expansion cohort with la/m BCC patients
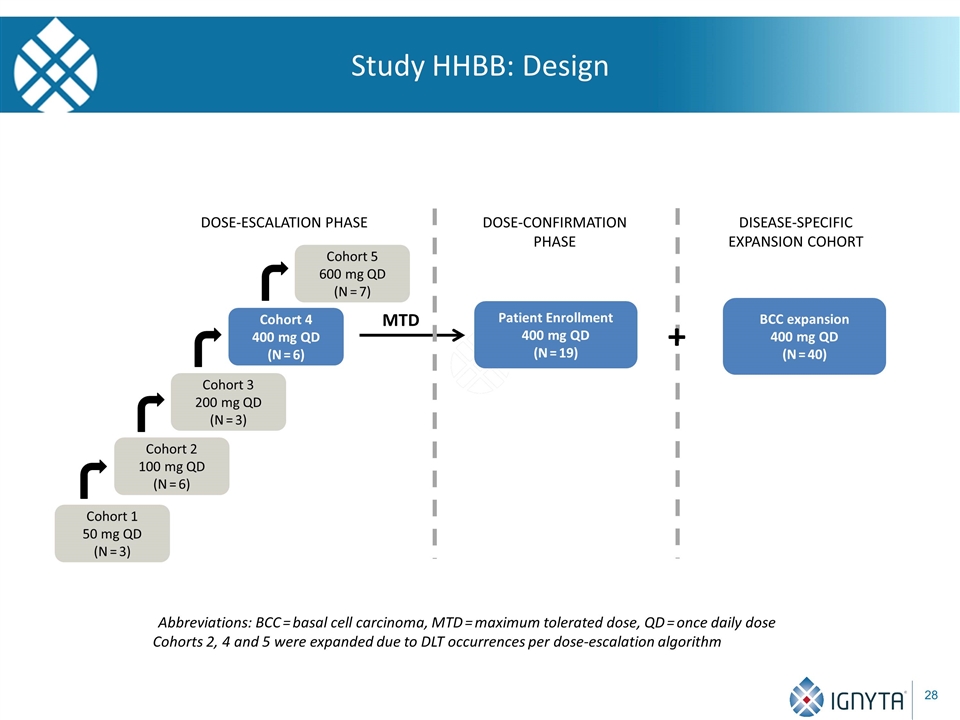
Study HHBB: Design Abbreviations: BCC = basal cell carcinoma, MTD = maximum tolerated dose, QD = once daily dose Cohorts 2, 4 and 5 were expanded due to DLT occurrences per dose-escalation algorithm Cohort 1 50 mg QD (N = 3) Cohort 2 100 mg QD (N = 6) Cohort 3 200 mg QD (N = 3) Cohort 4 400 mg QD (N = 6) Cohort 5 600 mg QD (N = 7) DOSE-ESCALATION PHASE MTD Patient Enrollment 400 mg QD (N = 19) DOSE-CONFIRMATION PHASE BCC expansion 400 mg QD (N = 40) DISEASE-SPECIFIC EXPANSION COHORT +
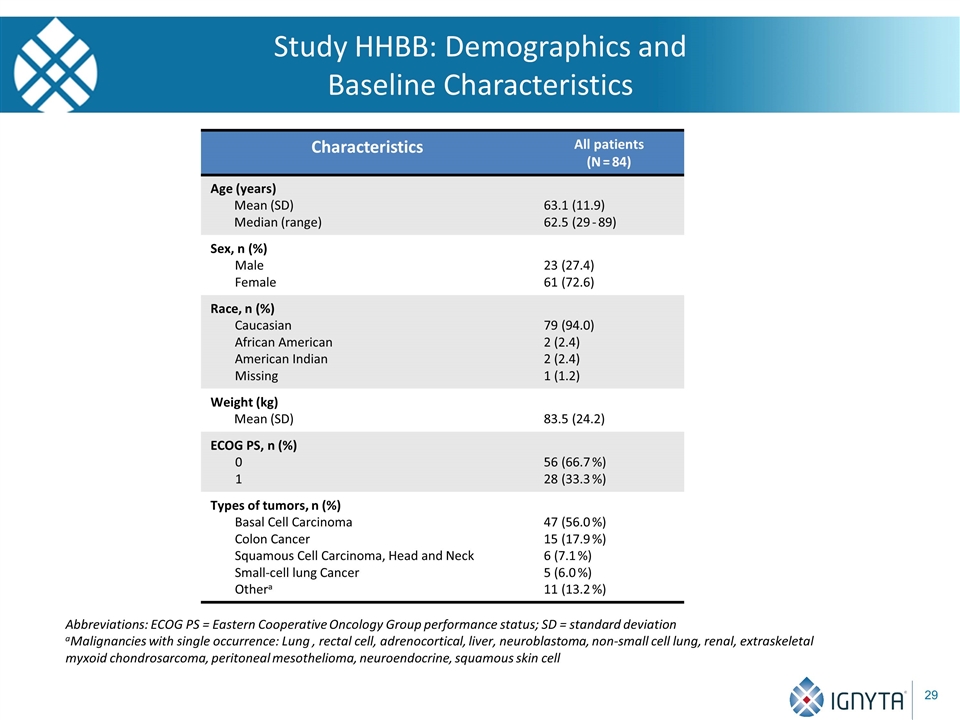
Study HHBB: Demographics and Baseline Characteristics Characteristics All patients (N = 84) Age (years) Mean (SD) Median (range) 63.1 (11.9) 62.5 (29 - 89) Sex, n (%) Male Female 23 (27.4) 61 (72.6) Race, n (%) Caucasian African American American Indian Missing 79 (94.0) 2 (2.4) 2 (2.4) 1 (1.2) Weight (kg) Mean (SD) 83.5 (24.2) ECOG PS, n (%) 0 1 56 (66.7 %) 28 (33.3 %) Types of tumors, n (%) Basal Cell Carcinoma Colon Cancer Squamous Cell Carcinoma, Head and Neck Small-cell lung Cancer Othera 47 (56.0 %) 15 (17.9 %) 6 (7.1 %) 5 (6.0 %) 11 (13.2 %) Abbreviations: ECOG PS = Eastern Cooperative Oncology Group performance status; SD = standard deviation aMalignancies with single occurrence: Lung , rectal cell, adrenocortical, liver, neuroblastoma, non-small cell lung, renal, extraskeletal myxoid chondrosarcoma, peritoneal mesothelioma, neuroendocrine, squamous skin cell
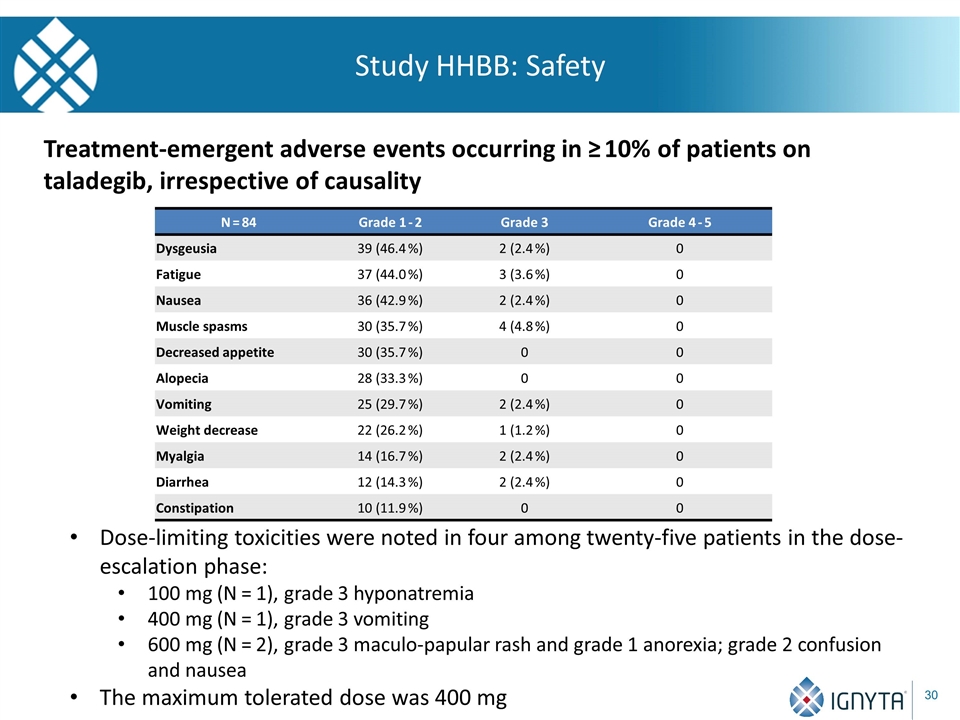
Study HHBB: Safety N = 84 Grade 1 - 2 Grade 3 Grade 4 - 5 Dysgeusia 39 (46.4 %) 2 (2.4 %) 0 Fatigue 37 (44.0 %) 3 (3.6 %) 0 Nausea 36 (42.9 %) 2 (2.4 %) 0 Muscle spasms 30 (35.7 %) 4 (4.8 %) 0 Decreased appetite 30 (35.7 %) 0 0 Alopecia 28 (33.3 %) 0 0 Vomiting 25 (29.7 %) 2 (2.4 %) 0 Weight decrease 22 (26.2 %) 1 (1.2 %) 0 Myalgia 14 (16.7 %) 2 (2.4 %) 0 Diarrhea 12 (14.3 %) 2 (2.4 %) 0 Constipation 10 (11.9 %) 0 0 Treatment-emergent adverse events occurring in ≥ 10% of patients on taladegib, irrespective of causality Dose-limiting toxicities were noted in four among twenty-five patients in the dose-escalation phase: 100 mg (N = 1), grade 3 hyponatremia 400 mg (N = 1), grade 3 vomiting 600 mg (N = 2), grade 3 maculo-papular rash and grade 1 anorexia; grade 2 confusion and nausea The maximum tolerated dose was 400 mg
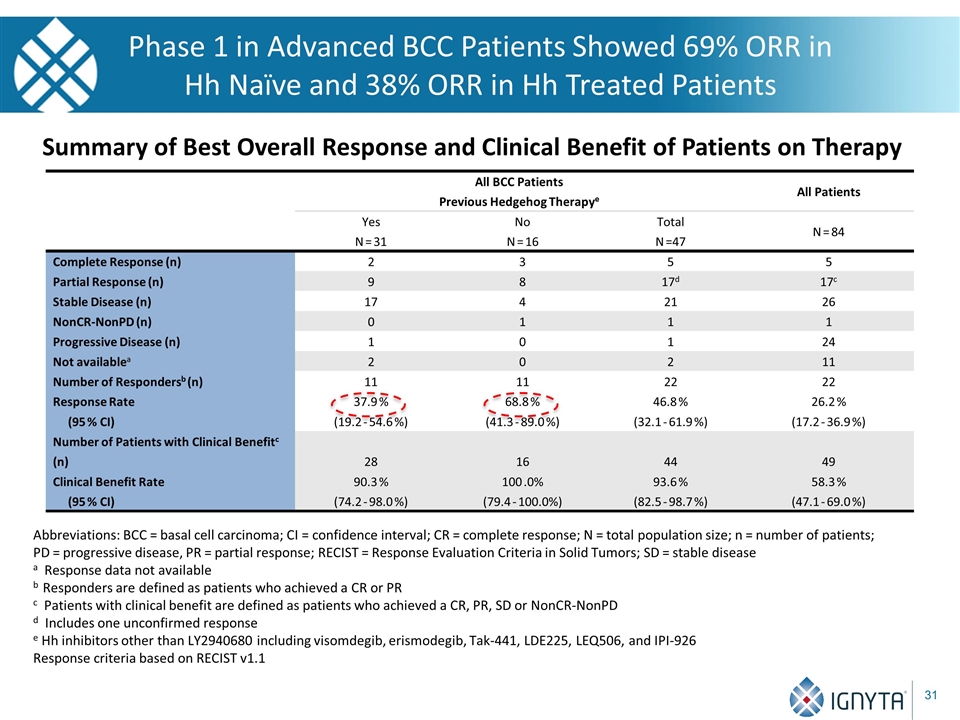
Phase 1 in Advanced BCC Patients Showed 69% ORR in Hh Naïve and 38% ORR in Hh Treated Patients All BCC Patients Previous Hedgehog Therapye All Patients Yes N = 31 No N = 16 Total N =47 N = 84 Complete Response (n) 2 3 5 5 Partial Response (n) 9 8 17d 17c Stable Disease (n) 17 4 21 26 NonCR-NonPD (n) 0 1 1 1 Progressive Disease (n) 1 0 1 24 Not availablea 2 0 2 11 Number of Respondersb (n) Response Rate (95 % CI) 11 37.9 % (19.2 - 54.6 %) 11 68.8 % (41.3 - 89.0 %) 22 46.8 % (32.1 - 61.9 %) 22 26.2 % (17.2 - 36.9 %) Number of Patients with Clinical Benefitc (n) Clinical Benefit Rate (95 % CI) 28 90.3 % (74.2 - 98.0 %) 16 100 .0% (79.4 - 100.0%) 44 93.6 % (82.5 - 98.7 %) 49 58.3 % (47.1 - 69.0 %) Abbreviations: BCC = basal cell carcinoma; CI = confidence interval; CR = complete response; N = total population size; n = number of patients; PD = progressive disease, PR = partial response; RECIST = Response Evaluation Criteria in Solid Tumors; SD = stable disease a Response data not available b Responders are defined as patients who achieved a CR or PR c Patients with clinical benefit are defined as patients who achieved a CR, PR, SD or NonCR-NonPD d Includes one unconfirmed response e Hh inhibitors other than LY2940680 including visomdegib, erismodegib, Tak-441, LDE225, LEQ506, and IPI-926 Response criteria based on RECIST v1.1 Summary of Best Overall Response and Clinical Benefit of Patients on Therapy
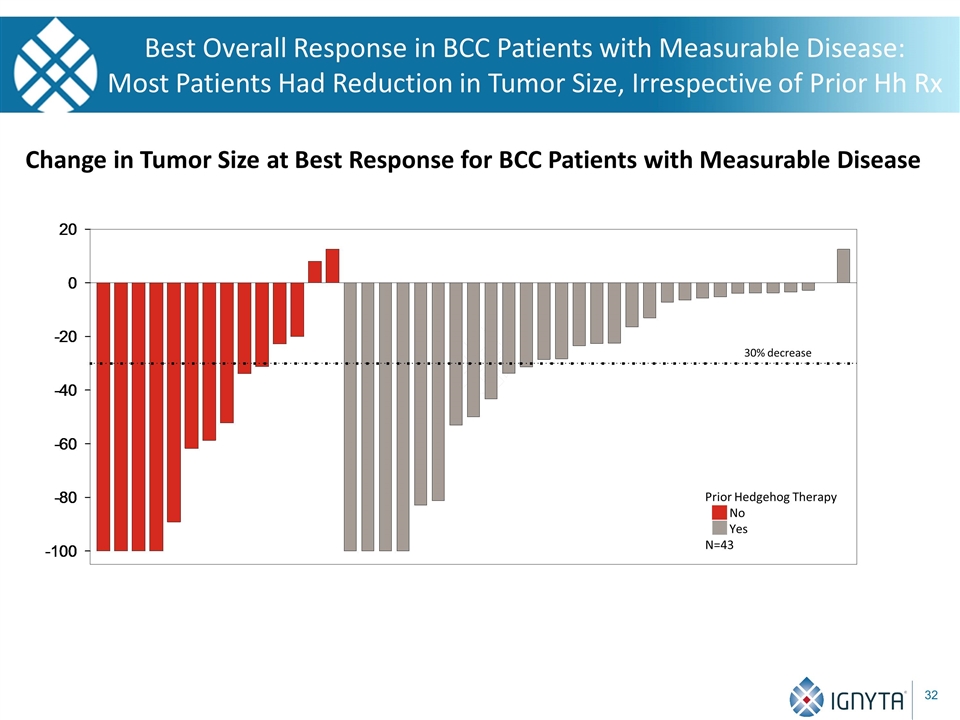
Best Overall Response in BCC Patients with Measurable Disease: Most Patients Had Reduction in Tumor Size, Irrespective of Prior Hh Rx Change in Tumor Size at Best Response for BCC Patients with Measurable Disease 30% decrease Prior Hedgehog Therapy No Yes N=43
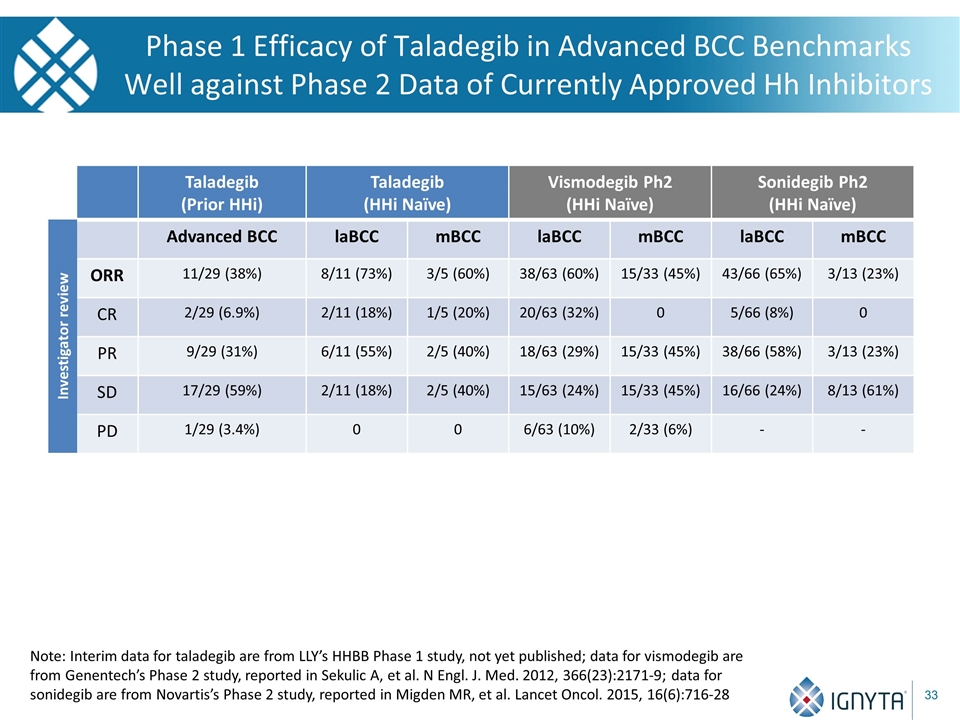
Phase 1 Efficacy of Taladegib in Advanced BCC Benchmarks Well against Phase 2 Data of Currently Approved Hh Inhibitors Taladegib (Prior HHi) Taladegib (HHi Naïve) Vismodegib Ph2 (HHi Naïve) Sonidegib Ph2 (HHi Naïve) Advanced BCC laBCC mBCC laBCC mBCC laBCC mBCC ORR 11/29 (38%) 8/11 (73%) 3/5 (60%) 38/63 (60%) 15/33 (45%) 43/66 (65%) 3/13 (23%) CR 2/29 (6.9%) 2/11 (18%) 1/5 (20%) 20/63 (32%) 0 5/66 (8%) 0 PR 9/29 (31%) 6/11 (55%) 2/5 (40%) 18/63 (29%) 15/33 (45%) 38/66 (58%) 3/13 (23%) SD 17/29 (59%) 2/11 (18%) 2/5 (40%) 15/63 (24%) 15/33 (45%) 16/66 (24%) 8/13 (61%) PD 1/29 (3.4%) 0 0 6/63 (10%) 2/33 (6%) - - Investigator review Note: Interim data for taladegib are from LLY’s HHBB Phase 1 study, not yet published; data for vismodegib are from Genentech’s Phase 2 study, reported in Sekulic A, et al. N Engl. J. Med. 2012, 366(23):2171-9; data for sonidegib are from Novartis’s Phase 2 study, reported in Migden MR, et al. Lancet Oncol. 2015, 16(6):716-28
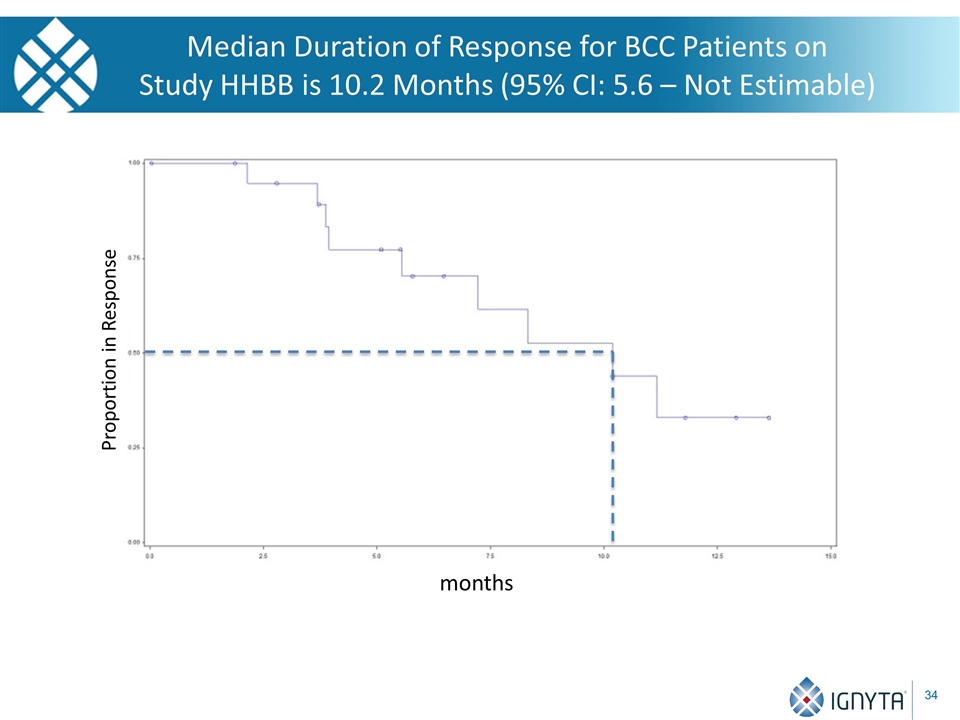
Median Duration of Response for BCC Patients on Study HHBB is 10.2 Months (95% CI: 5.6 – Not Estimable) Proportion in Response months
Study HHBB Preliminary Conclusions Taladegib treatment resulted in an acceptable safety profile in patients with advanced cancer, including la/m BCC Clinical responses were observed in la/m BCC patients naïve to Hh inhibitor treatment, with ORR comparing favorably to other 1L Hh inhibitor agents Clinical responses were also seen in those who had failed 1L treatment with other Hh inhibitor agents, with most patients experiencing some degree of tumor shrinkage Clinical responses were durable with current median estimate of 10.2 months with multiple patients censored with ongoing response This phase 1 study provides compelling support for further clinical development of taladegib in advanced BCC and potentially in other malignancies
Summary of Lilly’s Regulatory Interactions Based on feedback from U.S. and EU health authorities, a single-arm, pivotal trial (n~120 patients) may be acceptable to support registration in 2L advanced BCC (prior Hh therapy), assuming data are compelling An additional single-arm, pivotal trial (n~150 patients) in Hhi naïve patients may also be acceptable to support registration in 1L advanced BCC in the U.S., assuming data are compelling; a randomized study against a comparator is likely required in the EU Ignyta plans to meet with both agencies to confirm supportive trial design and registration plans for taladegib in advanced BCC
Commercial Considerations in BCC The Basal Cell Carcinoma (BCC) market is large and evolving 1.8 million patients in the U.S. alone are diagnosed annually with BCC ~33,000 U.S. patients are estimated to have advanced BCC (locally advanced or metastatic) The landscape in resistant BCC is still emerging, driven by two FDA-approved products available for first-line systemic treatment Taladegib is targeting a Total Addressable Market in advanced BCC of over $500 million in the U.S. Could be the sole Hhi indicated for second line use in patients refractory to first-line therapy Potential addressable market (1L and 2L) for taladegib is currently estimated to be ~10,000 patients Overall efficacy/safety profile could compare favorably to available Hhi’s Our path to commercial success is clear The BCC market has significant room for growth, as suggested by awareness among prescribers and payors of currently available systemic Hhi options As potentially the only systemic Hhi indicated in second line, taladegib would benefit from continued educational efforts for products currently available to patients in first line Demonstrated efficacy in first line advanced BCC could offer additional upside
2. Leverage Rx/Dx Expertise to Assess Efficacy in Selected Patients with Hh Pathway Activated Tumors Dysregulated Hh pathway signaling has been implicated in various malignancies: Type I: Ligand independent (e.g., BCC with > 90% Hh pathway alterations, Gorlin syndrome with PTCH1 LOF mutations); demonstrated clinical PoC Type II: Autocrine Ligand dependent (e.g., lung, breast, pancreatic cancer) Type III: Paracrine Ligand dependent (e.g., pancreatic cancer, lung squamous cell) Ignyta’s preliminary bioinformatic analysis of hedgehog pathway: TCGA database was queried for candidate activating SMO alterations or alterations upstream in the hedgehog pathway (Hh) Hh ligands (OE of SHh, IHh, DHh), PTCH1 (SNP/Del), SMO (SNP/OE) 53% of Hh alterations involve the overexpression of Hh ligands 25% are PTCH1 loss of function alterations (SNPs make up 39% of these) 22% are SMO activating alterations (SNPs make up 2.2% of these) Overall, Hh pathway alterations are rare in solid tumors (~2-10%), highlighting the need for a clear patient selection strategy (Dx) to select optimal patients for Hh targeted therapies There are ~130k patients in the U.S. who could potentially benefit from this approach SHh: Sonic Hh; IHh: Indian Hh; DHh: Desert Hh
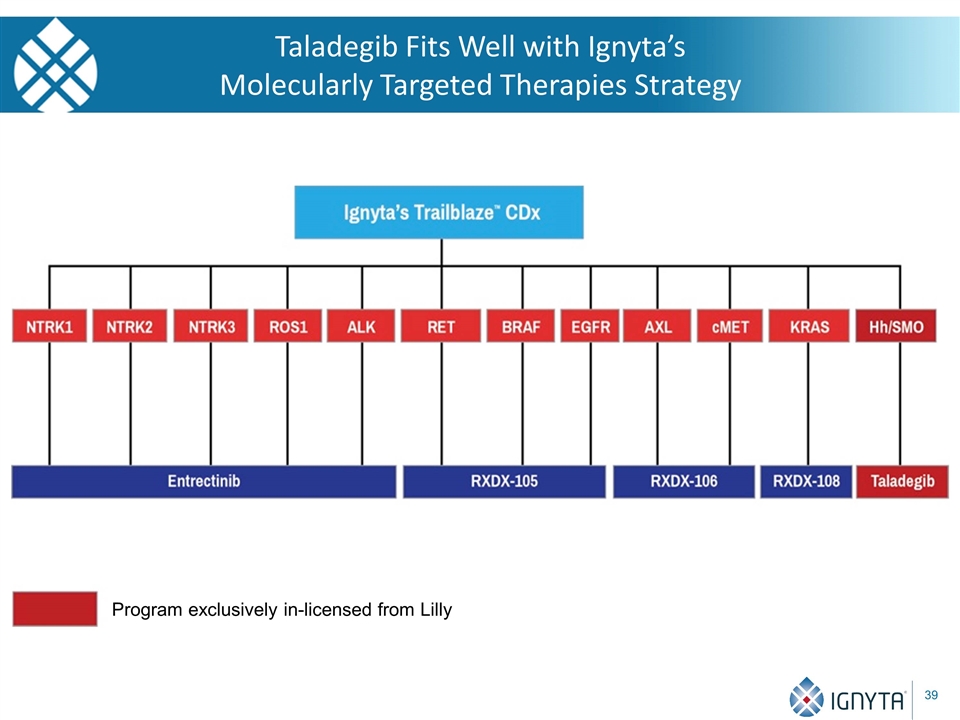
Taladegib Fits Well with Ignyta’s Molecularly Targeted Therapies Strategy Program exclusively in-licensed from Lilly
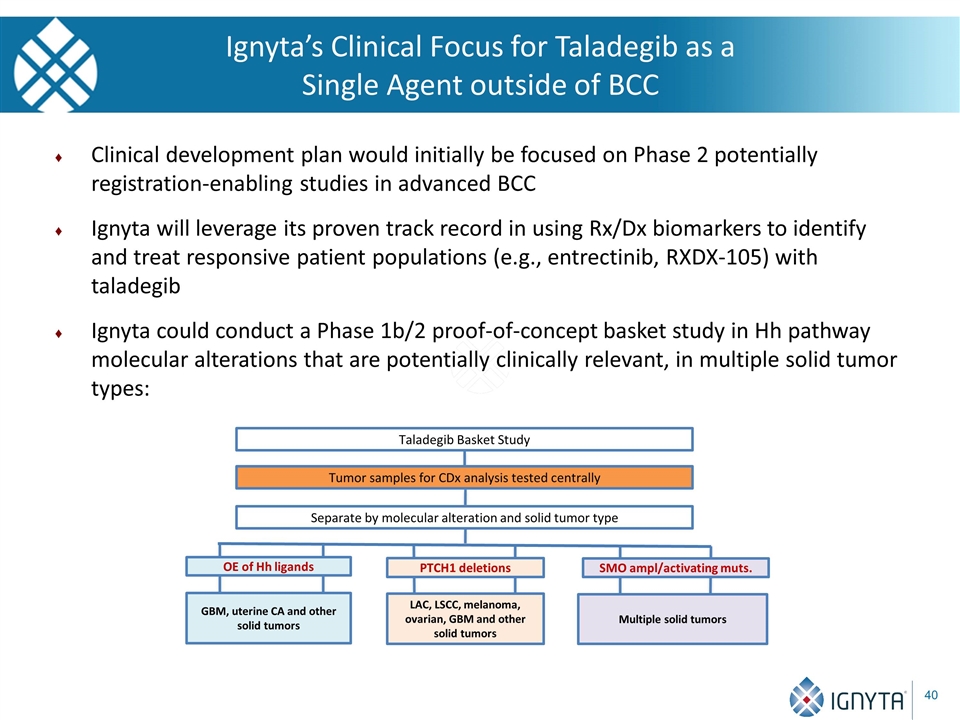
Ignyta’s Clinical Focus for Taladegib as a Single Agent outside of BCC Clinical development plan would initially be focused on Phase 2 potentially registration-enabling studies in advanced BCC Ignyta will leverage its proven track record in using Rx/Dx biomarkers to identify and treat responsive patient populations (e.g., entrectinib, RXDX-105) with taladegib Ignyta could conduct a Phase 1b/2 proof-of-concept basket study in Hh pathway molecular alterations that are potentially clinically relevant, in multiple solid tumor types: Tumor samples for CDx analysis tested centrally Separate by molecular alteration and solid tumor type LAC, LSCC, melanoma, ovarian, GBM and other solid tumors Taladegib Basket Study Multiple solid tumors PTCH1 deletions SMO ampl/activating muts. GBM, uterine CA and other solid tumors OE of Hh ligands
3a. Combine with RXDX-108 to Target 3q26 Amplifications, Most Common Copy Number Gain in Solid Tumors Atypical PKCiota (aPKCi) serine/threonine kinase is an emerging oncogenic target, essential for mutant RAS signaling and RAS/RAF/RAC mediated signaling downstream of oncogenic RTKs aPKCi is an important mediator of EMT, CSC expansion and self-renewal signals that drive oncogenic growth and drug resistance As a result of frequent 3q26 amplifications in cancer (reported to be as high as 15% of all solid tumors), a significant subset of patients harbor aPKCi and SOX2 co-amplification and co-overexpression, resulting in robust aPKCi-SOX2-Hh signaling, a stem cell-like phenotype and potential responsiveness to combined aPKCi/Hh inhibitor treatment Targeted aPKCi therapy, such as RXDX-108, a first-in-class, oral aPKCi inhibitor with favorable drug-like properties, could be a tractable precision medicine option for patients with high unmet medical need in genetically defined subsets of disease with either a KRAS mutation or 3q26 amplification Combining taladegib with RXDX-108 could help shut down the aPKCi – SOX2 – Hh signaling axis There are ~70k patients in the U.S. who could potentially benefit from this approach
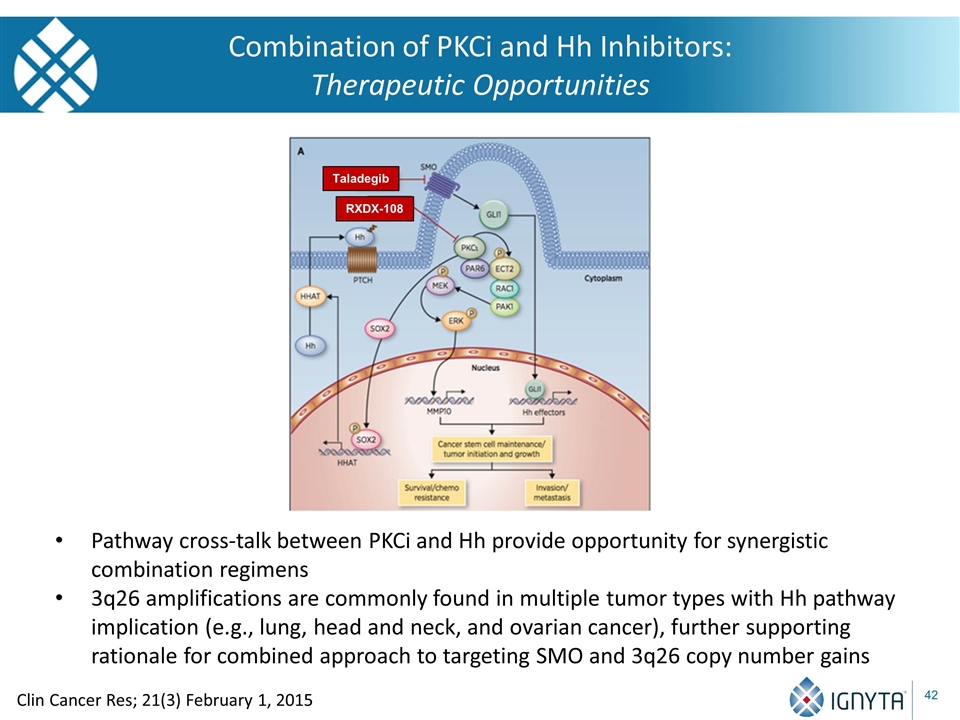
Combination of PKCi and Hh Inhibitors: Therapeutic Opportunities Clin Cancer Res; 21(3) February 1, 2015 Taladegib RXDX-108 Pathway cross-talk between PKCi and Hh provide opportunity for synergistic combination regimens 3q26 amplifications are commonly found in multiple tumor types with Hh pathway implication (e.g., lung, head and neck, and ovarian cancer), further supporting rationale for combined approach to targeting SMO and 3q26 copy number gains
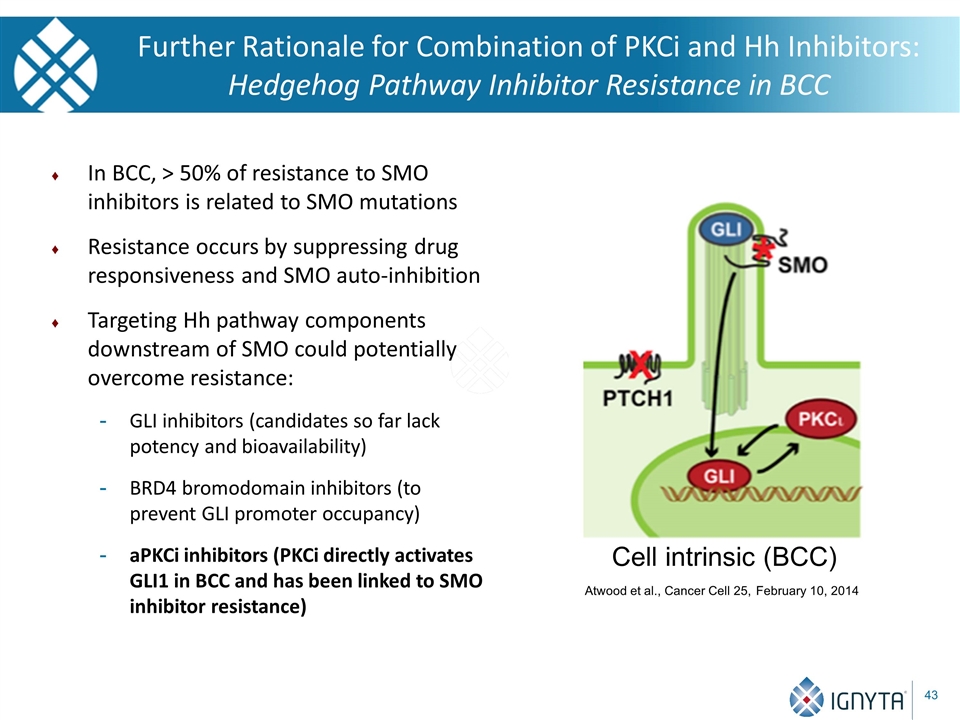
In BCC, > 50% of resistance to SMO inhibitors is related to SMO mutations Resistance occurs by suppressing drug responsiveness and SMO auto-inhibition Targeting Hh pathway components downstream of SMO could potentially overcome resistance: GLI inhibitors (candidates so far lack potency and bioavailability) BRD4 bromodomain inhibitors (to prevent GLI promoter occupancy) aPKCi inhibitors (PKCi directly activates GLI1 in BCC and has been linked to SMO inhibitor resistance) Atwood et al., Cancer Cell 25, February 10, 2014 Cell intrinsic (BCC) Further Rationale for Combination of PKCi and Hh Inhibitors: Hedgehog Pathway Inhibitor Resistance in BCC
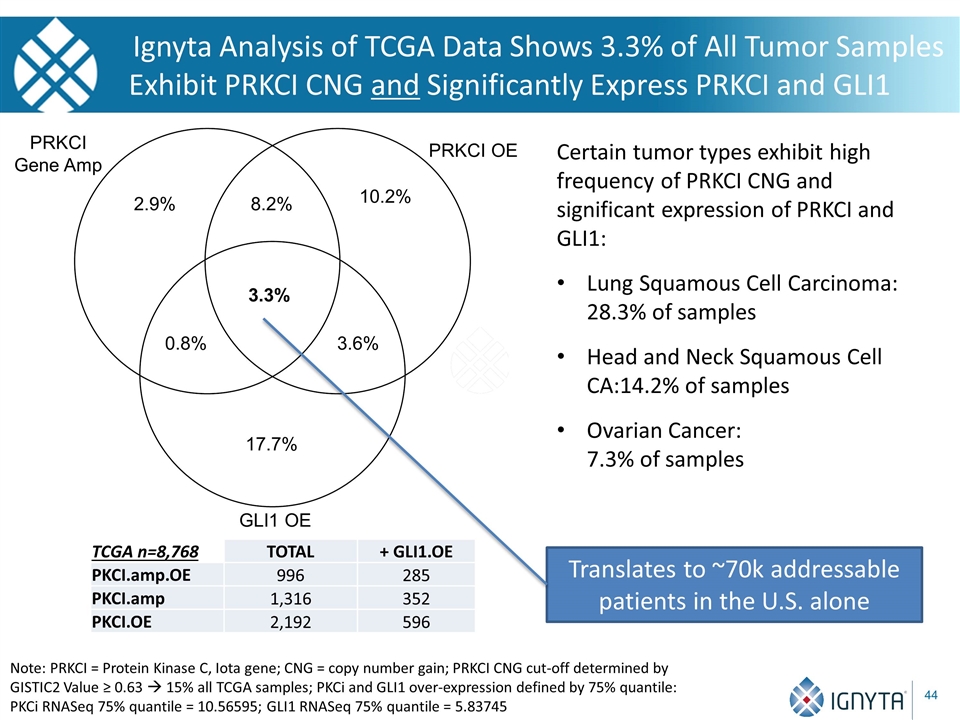
Ignyta Analysis of TCGA Data Shows 3.3% of All Tumor Samples Exhibit PRKCI CNG and Significantly Express PRKCI and GLI1 TCGA n=8,768 TOTAL + GLI1.OE PKCI.amp.OE 996 285 PKCI.amp 1,316 352 PKCI.OE 2,192 596 Certain tumor types exhibit high frequency of PRKCI CNG and significant expression of PRKCI and GLI1: Lung Squamous Cell Carcinoma: 28.3% of samples Head and Neck Squamous Cell CA:14.2% of samples Ovarian Cancer: 7.3% of samples GLI1 OE PRKCI OE PRKCI Gene Amp 2.9% 10.2% 8.2% 0.8% 17.7% 3.6% 3.3% Note: PRKCI = Protein Kinase C, Iota gene; CNG = copy number gain; PRKCI CNG cut-off determined by GISTIC2 Value ≥ 0.63 à 15% all TCGA samples; PKCi and GLI1 over-expression defined by 75% quantile: PKCi RNASeq 75% quantile = 10.56595; GLI1 RNASeq 75% quantile = 5.83745 Translates to ~70k addressable patients in the U.S. alone
3b. Combine with Other Targeted Agents to Potentially Address CSC or EMT Driven Adaptive and Acquired Resistance Limited historical success of Hh inhibitors in solid tumor indications Lack of patient selection Tumor heterogeneity and Hh Pathway crosstalk (EGFR, RAS/RAF/MAPK, PI3K/AKT, NOTCH, WNT, etc.) Role of Hh in CSC biology vs. bulk tumor cells Adaptive and acquired SMO inhibitor resistance Strong rationale for combinations of Hh inhibitors with targeted and non-targeted agents in selected patient populations with Hh pathway implications Taladegib could be explored in combination with Ignyta’s pipeline to target residual disease and/or resistance

Lilly has the right to develop and commercialize certain Lilly drug candidates (Lilly Products) in combination with taladegib These combination opportunities represent major indications targeting cancer stem cell pathways or other mechanisms that target residual disease and/or resistance Lilly will solely fund development and commercialization of Lilly Products In the event that Lilly successfully develops and commercializes any Lilly Product(s), then Ignyta will receive a royalty on net sales Lilly’s Pipeline Has Unique Candidates for Combination with Taladegib for Targeting Residual Disease and/or Resistance
4. Potential First-in-Class Hhi for superficial +/- nodular BCC Preclinical data indicate that Gli-1 inhibition in mini-pigs is greater with taladegib topical Hh inhibitor (55.1%) than that observed with sonidegib topical Hh inhibitor (17.9%) Topical toxicology study in mini-pigs completed and sufficient to support clinical development of topical formulation At Lilly’s Pre-IND meeting with FDA: FDA agreed that tox plan to support IND was sufficient FDA agreed with design of Phase 1b/2a study, except must exclude facial lesions for initial study Taladegib topical dosage form could be IND ready, pending technology transfer to Ignyta There are ~70k* patients in the U.S. who could potentially benefit from this approach *2.2M non-melanoma skin cancers; BCC is 80% of this (1.76M); sBCC is 15% of BCC; nBCC is 65% of BCC; assume 5% are inoperable, poor candidates, or given topical prior to surgery, yielding 70,400 patients in the U.S. Source: American Cancer Society; Advanced Basal Cell Carcinoma: Epidemiology and Therapeutic Innovations
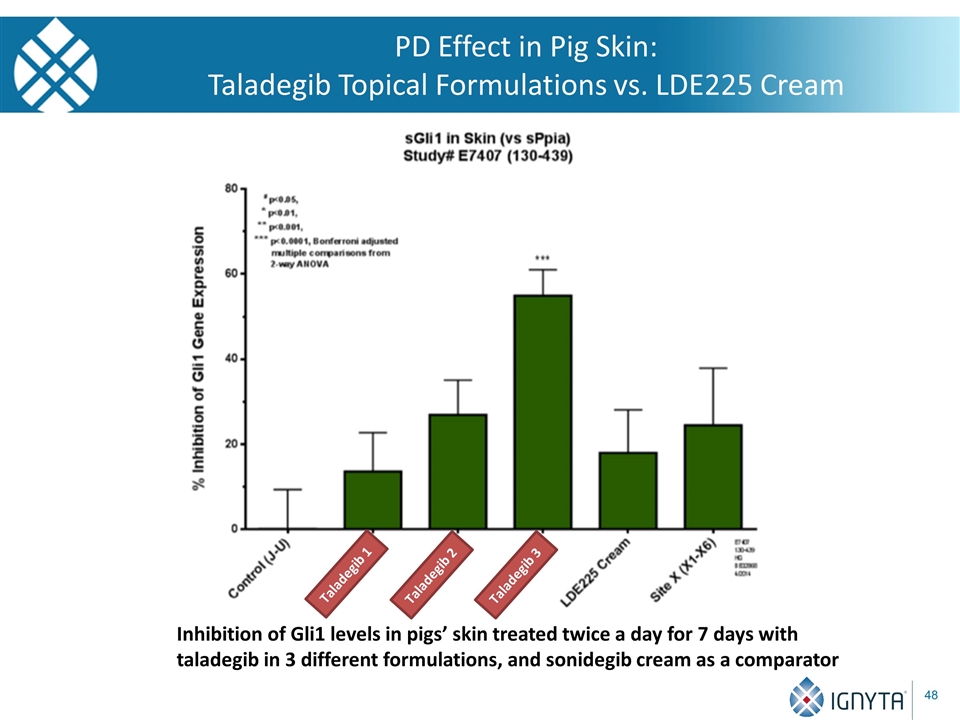
PD Effect in Pig Skin: Taladegib Topical Formulations vs. LDE225 Cream Inhibition of Gli1 levels in pigs’ skin treated twice a day for 7 days with taladegib in 3 different formulations, and sonidegib cream as a comparator Taladegib 1 Taladegib 2 Taladegib 3
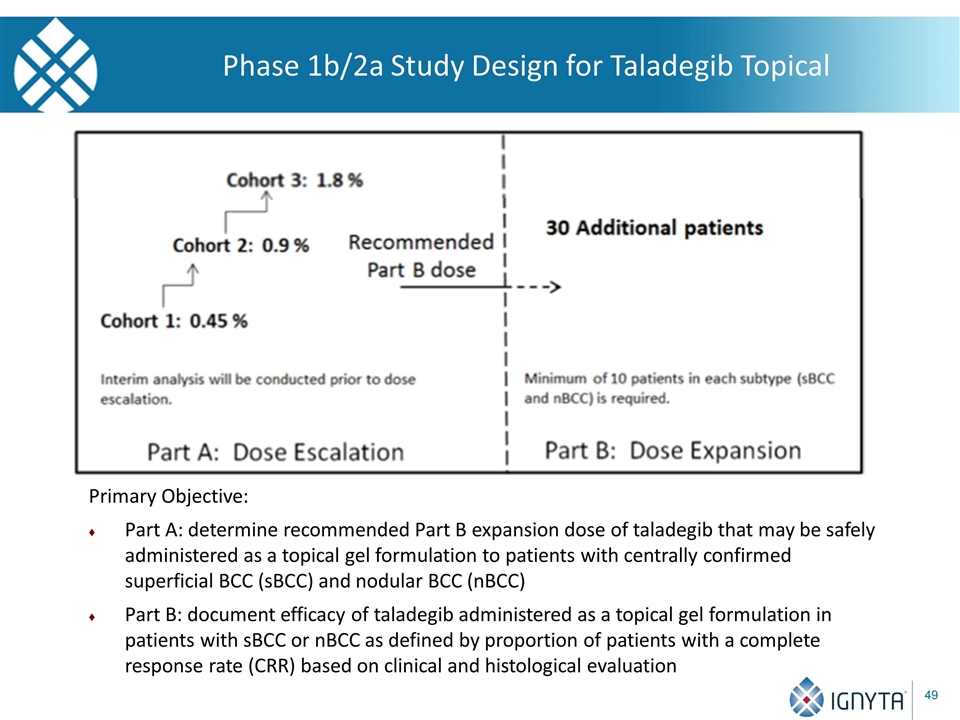
Phase 1b/2a Study Design for Taladegib Topical Primary Objective: Part A: determine recommended Part B expansion dose of taladegib that may be safely administered as a topical gel formulation to patients with centrally confirmed superficial BCC (sBCC) and nodular BCC (nBCC) Part B: document efficacy of taladegib administered as a topical gel formulation in patients with sBCC or nBCC as defined by proportion of patients with a complete response rate (CRR) based on clinical and histological evaluation
Summary of Ignyta’s Taladegib Opportunities Potential First-in-Class Hhi for 2L la/mBCC or Best-in-Class Hhi for 1L la/mBCC Potential fast to market opportunity in both 1L and 2L advanced BCC with two single-arm, potentially registrational Phase 2 studies Leverage Rx/Dx expertise to assess efficacy in selected patients with Hh pathway activated tumors Potential significant upside opportunity in solid tumors beyond BCC based on targeted Rx/Dx strategy Combine with RXDX-108 aPKCi inhibitor and other agents to address residual disease and/or resistance Ignyta uniquely has a FIC aPKCiota and BIC Hh inhibitor combination to shut down the PKCi – SOX2 – Hh signaling axis of 3q26 amplifications, the most common CNG in solid tumors Taladegib could be a CSC/EMT-targeting linchpin for combining with Ignyta’s pipeline. Lilly will also be developing certain combinations, at its expense Potential First-in-Class Hhi for superficial +/- nodular BCC Upside opportunity in early BCC with taladegib topical BIC: best-in-class; FIC: first-in-class; 1L: 1st line; 2L: 2nd line; BCC: basal cell carcinoma; la/mBCC: locally advanced or metastatic BCC; SMO: smoothened; Hh: hedgehog; CNG: copy number gain; CSC: cancer stem cell; EMT: epithelial mesenchymal transition

Agenda Ignyta’s BHAG* and scientific vision Lilly transaction and taladegib overview Taladegib opportunities Q3 2015 company highlights and financial results * Big Hairy Audacious Goal

STARTRK-1 Phase 1/2 dose escalation study of daily continuous dosing schedule in patients with NTRK1/2/3, ROS1 or ALK molecular alterations in US, EU and Asia Phase 1 initiated in July 2014 * RP2D = Recommended Phase 2 Dose ALKA-372-001 Phase 1 dose escalation study of intermittent and continuous dosing schedule in Italy: patients with TrkA, ROS1, or ALK alterations in Italy First-in-human study initiated by Nerviano Medical Sciences in October 2012 Ignyta assumed responsibility in November 2013 49 patients enrolled 43 patients enrolled Total experience: 92 patients enrolled as of 15 August 2015 RP2D*: 600 mg/day on a fed continuous daily dosing regimen Overview of Current Entrectinib Phase 1 Clinical Studies
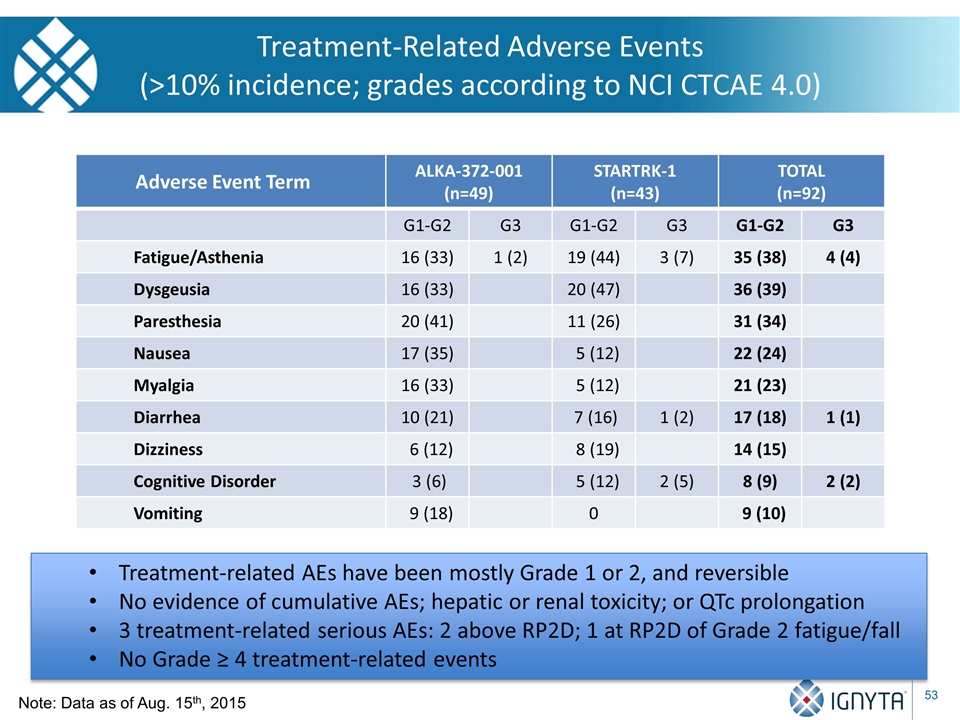
Adverse Event Term ALKA-372-001 (n=49) STARTRK-1 (n=43) TOTAL (n=92) G1-G2 G3 G1-G2 G3 G1-G2 G3 Fatigue/Asthenia 16 (33) 1 (2) 19 (44) 3 (7) 35 (38) 4 (4) Dysgeusia 16 (33) 20 (47) 36 (39) Paresthesia 20 (41) 11 (26) 31 (34) Nausea 17 (35) 5 (12) 22 (24) Myalgia 16 (33) 5 (12) 21 (23) Diarrhea 10 (21) 7 (16) 1 (2) 17 (18) 1 (1) Dizziness 6 (12) 8 (19) 14 (15) Cognitive Disorder 3 (6) 5 (12) 2 (5) 8 (9) 2 (2) Vomiting 9 (18) 0 9 (10) Treatment-Related Adverse Events (>10% incidence; grades according to NCI CTCAE 4.0) Treatment-related AEs have been mostly Grade 1 or 2, and reversible No evidence of cumulative AEs; hepatic or renal toxicity; or QTc prolongation 3 treatment-related serious AEs: 2 above RP2D; 1 at RP2D of Grade 2 fatigue/fall No Grade ≥ 4 treatment-related events Note: Data as of Aug. 15th, 2015
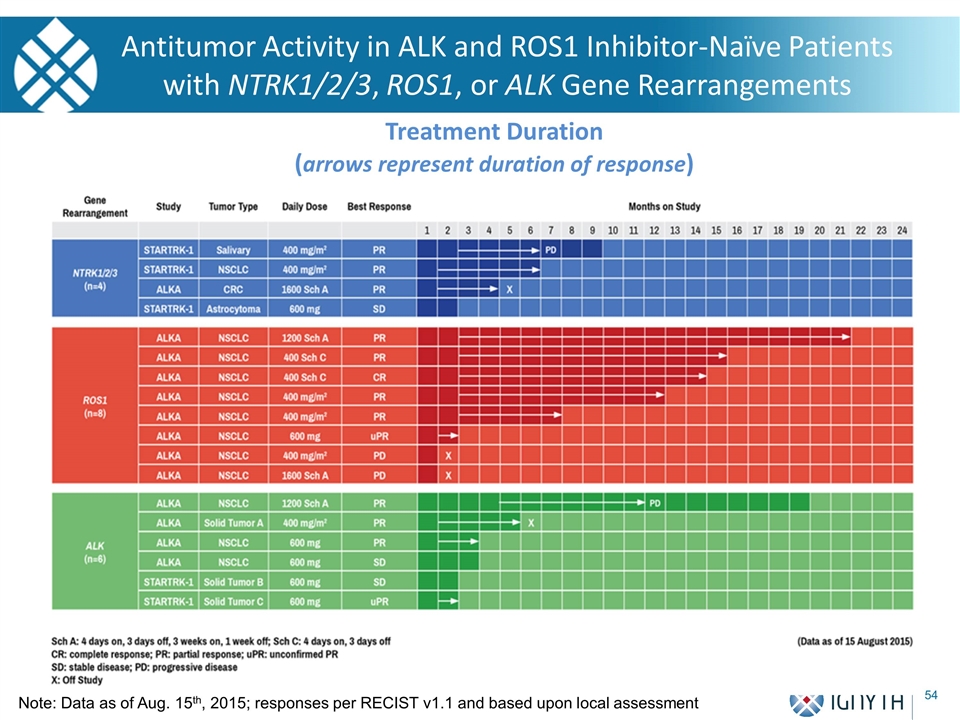
Treatment Duration (arrows represent duration of response) Antitumor Activity in ALK and ROS1 Inhibitor-Naïve Patients with NTRK1/2/3, ROS1, or ALK Gene Rearrangements Note: Data as of Aug. 15th, 2015; responses per RECIST v1.1 and based upon local assessment
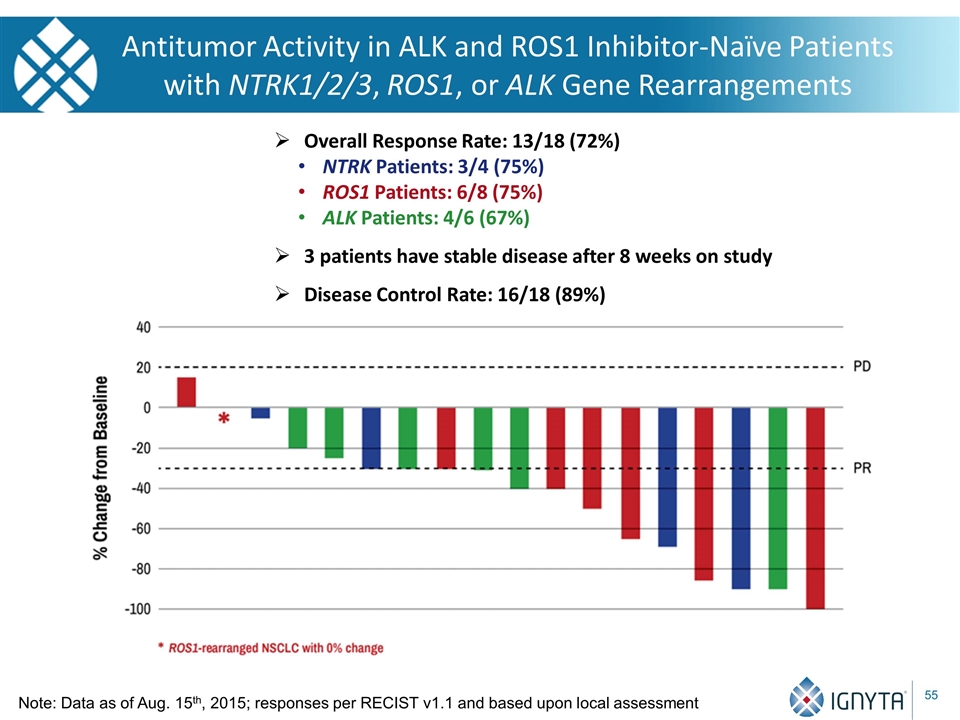
Overall Response Rate: 13/18 (72%) NTRK Patients: 3/4 (75%) ROS1 Patients: 6/8 (75%) ALK Patients: 4/6 (67%) 3 patients have stable disease after 8 weeks on study Disease Control Rate: 16/18 (89%) Antitumor Activity in ALK and ROS1 Inhibitor-Naïve Patients with NTRK1/2/3, ROS1, or ALK Gene Rearrangements Note: Data as of Aug. 15th, 2015; responses per RECIST v1.1 and based upon local assessment
Entrectinib Clinical Summary Status: Two Phase 1 clinical studies of entrectinib are ongoing; To date, 9 patients have been treated at or above the RP2D beyond 6 months and 1 patient beyond 1 year Safety: Entrectinib was well tolerated in patients with relapsed or refractory metastatic cancers harboring NTRK1/2/3, ROS1, or ALK molecular alterations RP2D: 600 mg/day on a fed continuous daily dosing regimen Preliminary efficacy: Among patients with NTRK1/2/3, ROS1, or ALK gene rearrangements who were ALK-inhibitor or ROS1-inhibitor-naïve, 13/18 (72%) patients treated at or above the RP2D exhibited objective responses as early as 4 weeks of treatment with durable responses for up to 21 months Entrectinib has demonstrated objective tumor response in the CNS Based on these promising data, STARTRK-2, a global potentially registration-enabling Phase 2 basket study, is investigating the activity of entrectinib in multiple tumor histologies Note: Data as of Aug. 15th, 2015
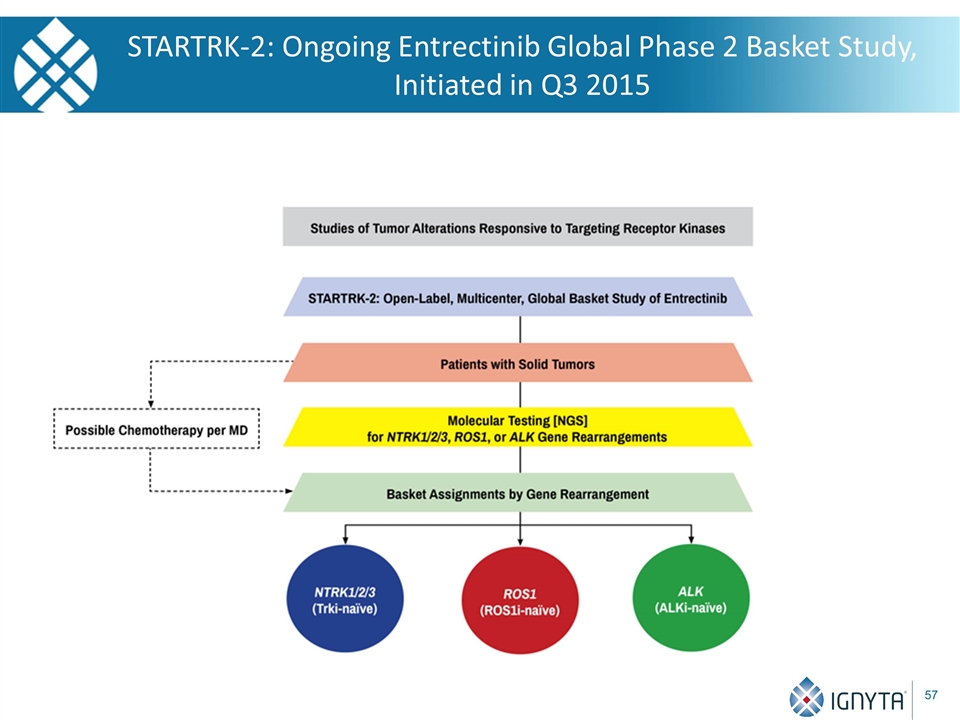
STARTRK-2: Ongoing Entrectinib Global Phase 2 Basket Study, Initiated in Q3 2015
Overview of Current RXDX-105 Phase 1 Clinical Study Study 1105 Phase 1/1b dose escalation clinical trial with continuous daily dosing regimen Phase 1 initiated under Teva, testing unselected patients with RXDX-105, an orally-available, small molecule multikinase inhibitor with potent activity against such key targets as RET and BRAF Total experience: 41 patients enrolled as of October 26th, 2015, in cohorts ranging from 20mg QD to 275mg QD 6 patients discontinued prior to first tumor assessment or were not evaluable for tumor response; 6 patients have not yet reached Cycle 2 tumor assessment; 29 patients have had post-baseline tumor evaluations and are evaluable for tumor regression Note: Data as of Oct. 26th, 2015
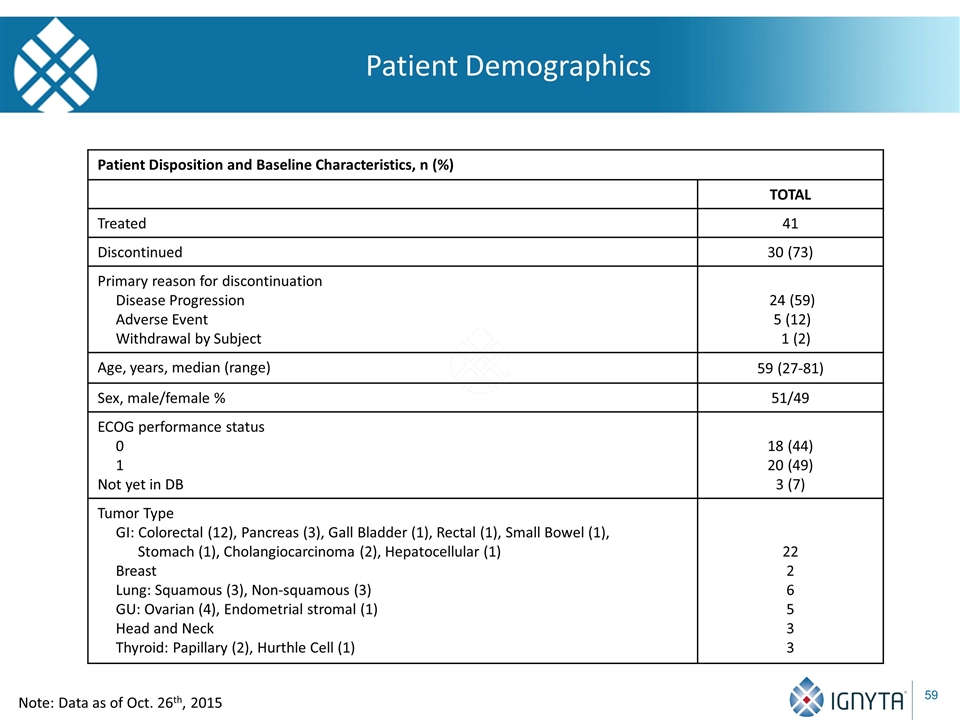
Patient Demographics Patient Disposition and Baseline Characteristics, n (%) TOTAL Treated 41 Discontinued 30 (73) Primary reason for discontinuation Disease Progression Adverse Event Withdrawal by Subject 24 (59) 5 (12) 1 (2) Age, years, median (range) 59 (27-81) Sex, male/female % 51/49 ECOG performance status 0 1 Not yet in DB 18 (44) 20 (49) 3 (7) Tumor Type GI: Colorectal (12), Pancreas (3), Gall Bladder (1), Rectal (1), Small Bowel (1), Stomach (1), Cholangiocarcinoma (2), Hepatocellular (1) Breast Lung: Squamous (3), Non-squamous (3) GU: Ovarian (4), Endometrial stromal (1) Head and Neck Thyroid: Papillary (2), Hurthle Cell (1) 22 2 6 5 3 3 Note: Data as of Oct. 26th, 2015
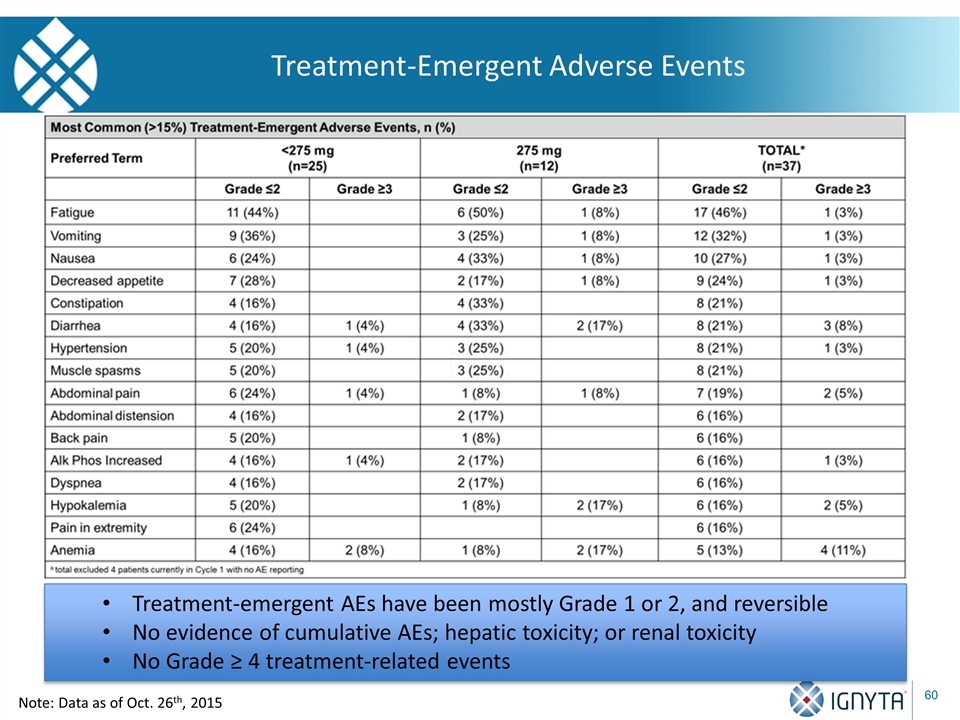
Treatment-Emergent Adverse Events Treatment-emergent AEs have been mostly Grade 1 or 2, and reversible No evidence of cumulative AEs; hepatic toxicity; or renal toxicity No Grade ≥ 4 treatment-related events Note: Data as of Oct. 26th, 2015
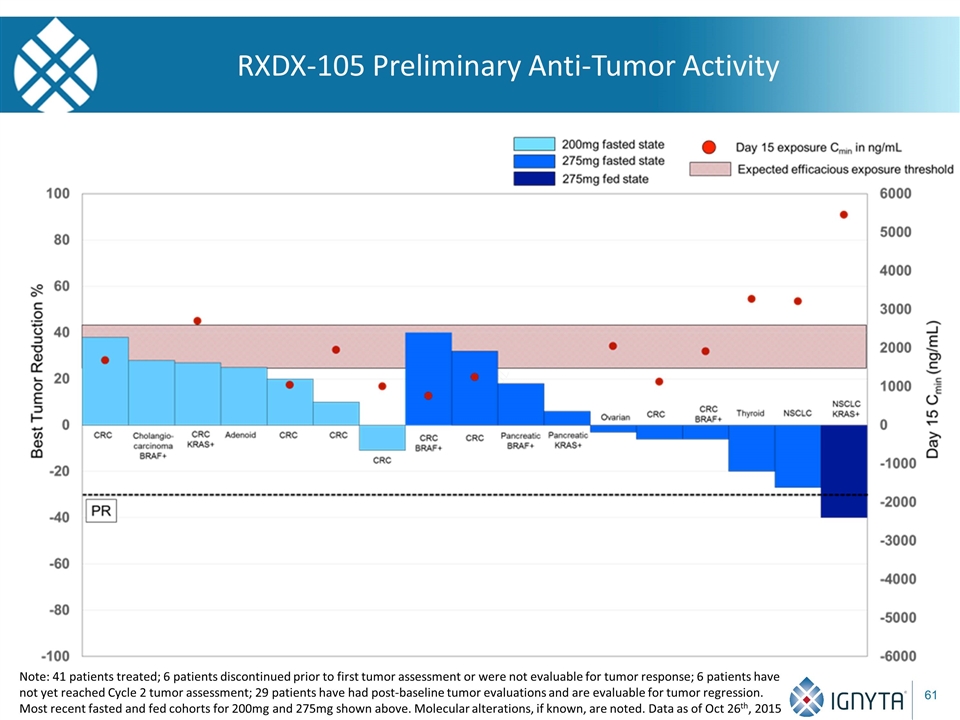
RXDX-105 Preliminary Anti-Tumor Activity Note: 41 patients treated; 6 patients discontinued prior to first tumor assessment or were not evaluable for tumor response; 6 patients have not yet reached Cycle 2 tumor assessment; 29 patients have had post-baseline tumor evaluations and are evaluable for tumor regression. Most recent fasted and fed cohorts for 200mg and 275mg shown above. Molecular alterations, if known, are noted. Data as of Oct 26th, 2015
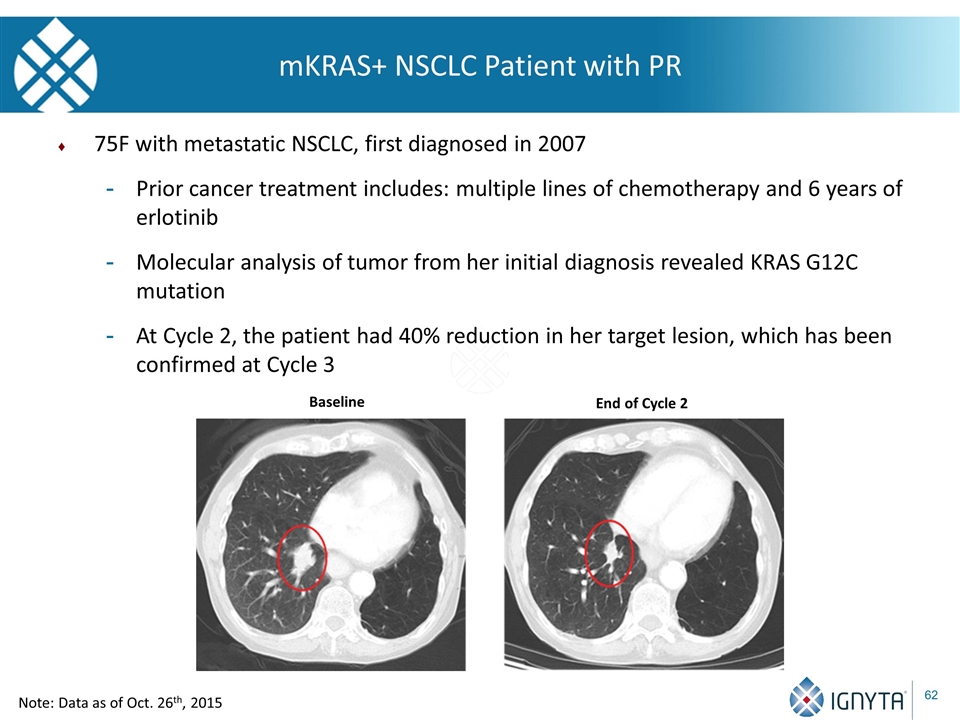
mKRAS+ NSCLC Patient with PR 75F with metastatic NSCLC, first diagnosed in 2007 Prior cancer treatment includes: multiple lines of chemotherapy and 6 years of erlotinib Molecular analysis of tumor from her initial diagnosis revealed KRAS G12C mutation At Cycle 2, the patient had 40% reduction in her target lesion, which has been confirmed at Cycle 3 Baseline End of Cycle 2 Note: Data as of Oct. 26th, 2015
RXDX-105 Clinical Summary as of October 26, 2015 Status: Phase 1 clinical study of RXDX-105 is ongoing; 41 patients have been treated Safety: RXDX-105 has been well-tolerated in patients with advanced or metastatic solid tumors Preliminary efficacy: Exposure is reaching efficacious levels, based on preclinical data from RET- and BRAF-driven PDX models Signals of anti-tumor activity have been noted, with a confirmed PR (40% reduction) observed in a patient with NSCLC positive for KRAS G12C who had the highest drug exposure to date In patients with tumor regression, there appears to be an exposure/response correlation Emerging hypotheses regarding mechanisms of action against oncogenic drivers and other targets are being evaluated in the preclinical setting and will inform the clinical plan These preliminary data support further development of RXDX-105
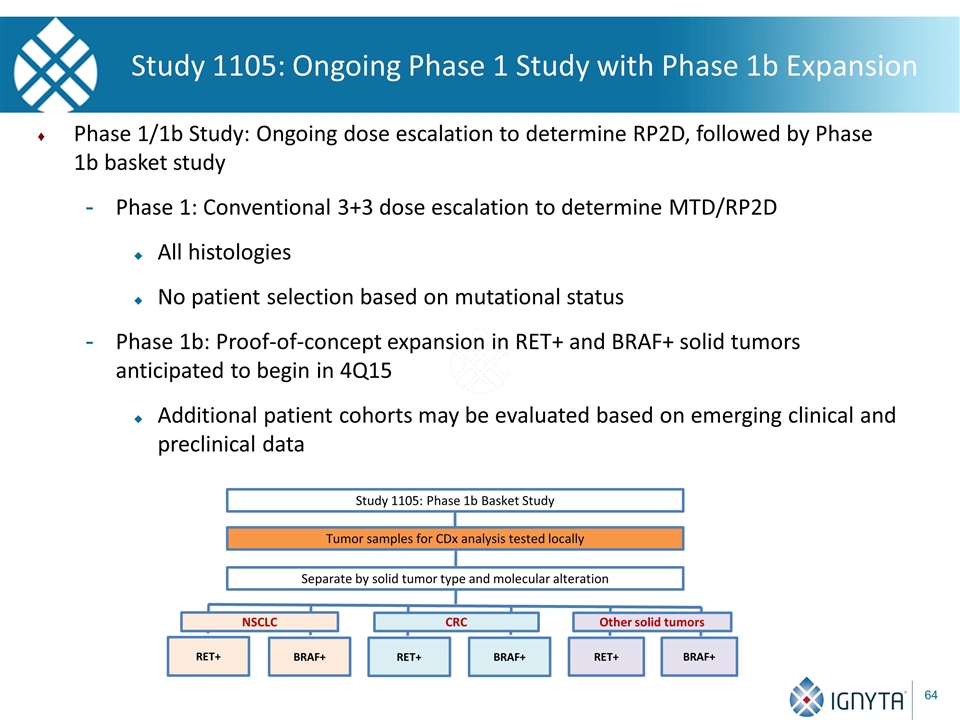
Study 1105: Ongoing Phase 1 Study with Phase 1b Expansion Phase 1/1b Study: Ongoing dose escalation to determine RP2D, followed by Phase 1b basket study Phase 1: Conventional 3+3 dose escalation to determine MTD/RP2D All histologies No patient selection based on mutational status Phase 1b: Proof-of-concept expansion in RET+ and BRAF+ solid tumors anticipated to begin in 4Q15 Additional patient cohorts may be evaluated based on emerging clinical and preclinical data Tumor samples for CDx analysis tested locally Separate by solid tumor type and molecular alteration RET+ Study 1105: Phase 1b Basket Study BRAF+ RET+ BRAF+ RET+ BRAF+ NSCLC CRC Other solid tumors
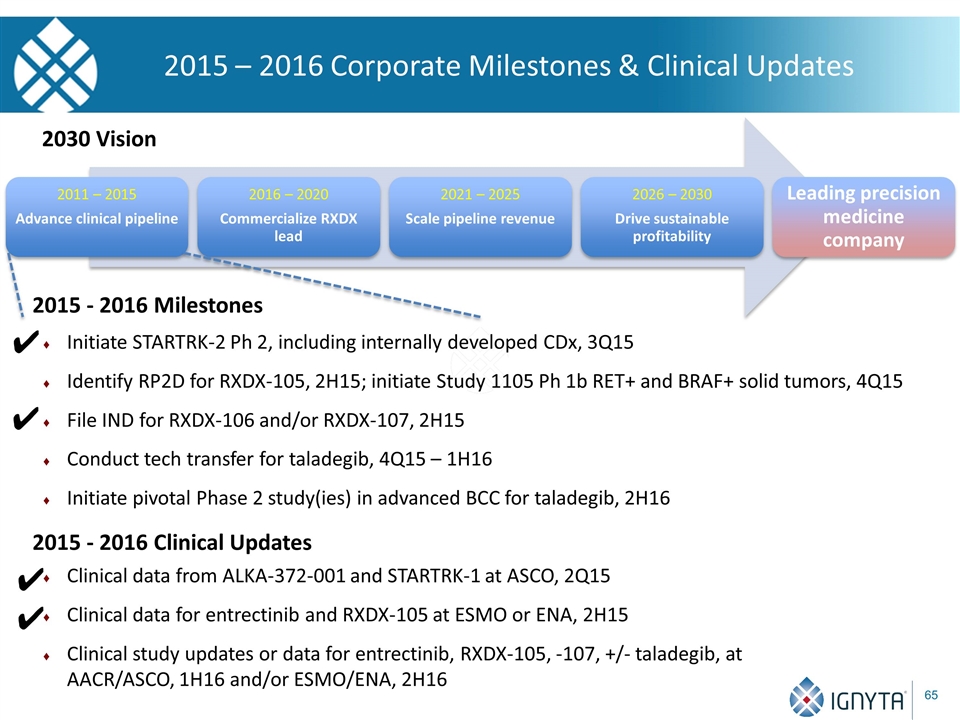
2015 – 2016 Corporate Milestones & Clinical Updates 2030 Vision 2015 - 2016 Milestones Initiate STARTRK-2 Ph 2, including internally developed CDx, 3Q15 Identify RP2D for RXDX-105, 2H15; initiate Study 1105 Ph 1b RET+ and BRAF+ solid tumors, 4Q15 File IND for RXDX-106 and/or RXDX-107, 2H15 Conduct tech transfer for taladegib, 4Q15 – 1H16 Initiate pivotal Phase 2 study(ies) in advanced BCC for taladegib, 2H16 Clinical data from ALKA-372-001 and STARTRK-1 at ASCO, 2Q15 Clinical data for entrectinib and RXDX-105 at ESMO or ENA, 2H15 Clinical study updates or data for entrectinib, RXDX-105, -107, +/- taladegib, at AACR/ASCO, 1H16 and/or ESMO/ENA, 2H16 ✔ ✔ 2015 - 2016 Clinical Updates ✔ ✔ 2011 – 2015 Advance clinical pipeline 2016 – 2020 Commercialize RXDX lead 2021 – 2025 Scale pipeline revenue 2026 – 2030 Drive sustainable profitability Leading precision medicine company

Company Highlights Leading precision oncology company with compelling vision to eradicate residual disease in precisely defined patient populations by 2030 Integrated approach to Rx/Dx development, with comprehensive diagnostic capabilities and biomarker strategies for patient screening and confirmation Experienced team with excellent track record in oncology Robust pipeline of targeted first-in-class and best-in-class product candidates under clinical development in four therapeutic cornerstones of oncology Two late stage product candidates with compelling Phase 1 clinical proof of concept, in or soon to be in, registration-enabling Phase 2 studies Strong IP and financial position

































































