
Investor Conference Call: Program Highlights at 2016 EORTC-NCI-AACR (ENA) Molecular Targets and Cancer Therapeutics Symposium December 1, 2016 Exhibit 99.2

Safe Harbor Statement This document contains forward-looking statements, as that term is defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, about Ignyta, Inc. (“us” or the “Company”). Statements that are not purely historical are forward-looking statements. These include statements regarding, among other things: Ignyta’s corporate and scientific vision and goals, including our ability to reduce the size of tumors and to eradicate residual disease; the clinical and/or non-clinical data or plans underlying RXDX-105, entrectinib or any of our other development programs; our ability to design and conduct development activities for RXDX-105, entrectinib and our other development programs; our ability to develop or access companion diagnostics for our product candidates; our ability to obtain and maintain intellectual property protection for our product candidates; our ability to adequately fund our development programs; our ability to obtain regulatory approvals in order to market any of our product candidates; and our ability to successfully commercialize any approved products. Forward-looking statements involve known and unknown risks that relate to future events or the Company’s future financial performance, some of which may be beyond our control, and the actual results could differ materially from those discussed in this document. Accordingly, the Company cautions investors not to place undue reliance on the forward-looking statements contained in, or made in connection with, this document. Important factors that could cause actual results to differ materially from those indicated by such forward-looking statements, include, among others, the potential for results of past or ongoing clinical or non-clinical studies to differ from expectations or previous results; the interpretation of data from our clinical and non-clinical studies; our ability to initiate and complete clinical trials and non-clinical studies; regulatory developments; the potential advantages of our product candidates; the markets any approved products are intended to serve; and our capital needs; as well as those set forth under the headings “Special Note Regarding Forward-Looking Statements,” “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” contained in the Company’s Form 10-K filed with the Securities and Exchange Commission (“SEC”) on March 14, 2016, and similar disclosures made in the Company’s Form 10-Q filings and other SEC filings and press releases. The forward-looking statements contained in this document represent our estimates and assumptions only as of the date of this document, and we undertake no duty or obligation to update or revise publicly any forward-looking statements contained in this document as a result of new information, future events or changes in our expectations. Third-party information included herein has been obtained from sources believed to be reliable, but the accuracy or completeness of such information is not guaranteed by, and should not be construed as a representation by, the Company.
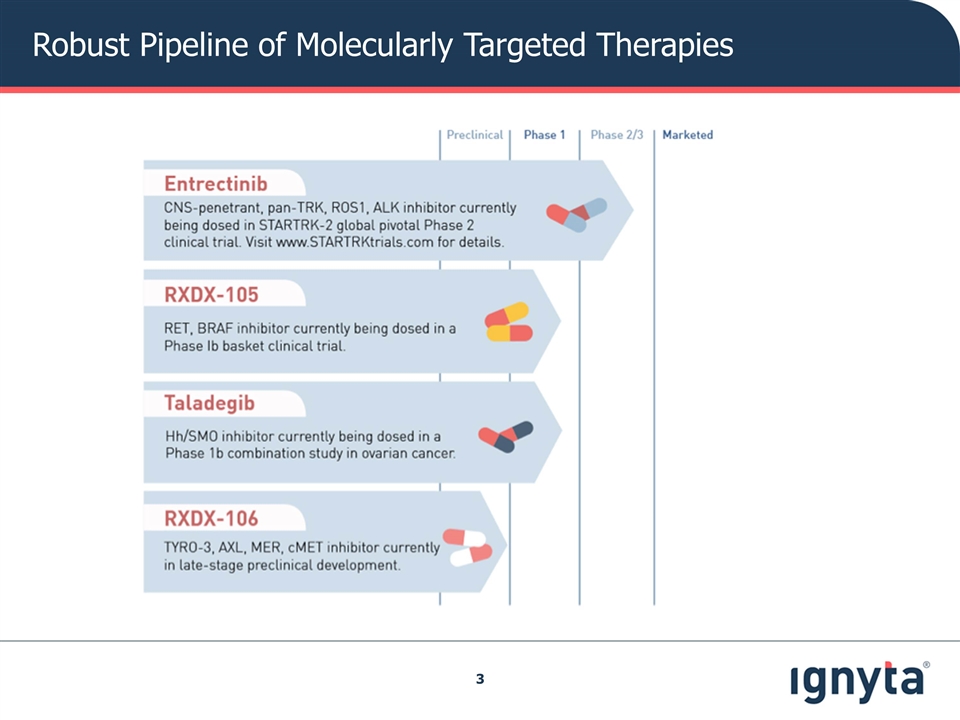
Robust Pipeline of Molecularly Targeted Therapies
Ignyta Presentations at ENA 2016 Program Title Format entrectinib Overcoming drug resistance to Trk inhibition by rational combination of entrectinib and trametinib: from bench to bedside Oral Plenary Session entrectinib Entrectinib, a highly potent pan-Trk, ROS1, and ALK inhibitor, has broad-spectrum, histology-agnostic anti-tumor activity in molecularly defined cancer Poster RXDX-105 A phase 1/1b study of RXDX-105, an oral RET and BRAF inhibitor, in patients with advanced solid tumors Poster RXDX-105 RXDX-105 demonstrates anti-tumor efficacy in multiple preclinical cancer models driven by molecular alterations in RET or BRAF oncogenes Poster RXDX-106 Immuno-oncologic efficacy of RXDX-106, a selective, TAM family small molecule kinase inhibitor Poster RXDX-106 RXDX-106 is an orally-available, potent and selective TAM/MET inhibitor demonstrating preclinical efficacy in MET-dependent human malignancies Poster 6 presentations, including oral plenary session, exemplify Ignyta’s strong progress across pipeline of investigational precision oncology therapeutics
Entrectinib Late-Breaking Oral Plenary Program Title Format entrectinib Overcoming drug resistance to Trk inhibition by rational combination of entrectinib and trametinib: from bench to bedside Oral Plenary Session entrectinib Entrectinib, a highly potent pan-Trk, ROS1, and ALK inhibitor, has broad-spectrum, histology-agnostic anti-tumor activity in molecularly defined cancer Poster RXDX-105 A phase 1/1b study of RXDX-105, an oral RET and BRAF inhibitor, in patients with advanced solid tumors Poster RXDX-105 RXDX-105 demonstrates anti-tumor efficacy in multiple preclinical cancer models driven by molecular alterations in RET or BRAF oncogenes Poster RXDX-106 Immuno-oncologic efficacy of RXDX-106, a selective, TAM family small molecule kinase inhibitor Poster RXDX-106 RXDX-106 is an orally-available, potent and selective TAM/c-MET inhibitor demonstrating preclinical efficacy in MET-dependent human malignancies Poster
Entrectinib Late-Breaking Oral Plenary: Overcoming Resistance – From Bench-to-Bedside Resistance to single agent tyrosine kinase inhibitor (TKI) therapy is frequently due to point mutations that interfere with TKI binding Resistance has been described for most, if not all, TKIs, including 2nd and 3rd generation TKIs against EGFR and ALK Ignyta has been proactively exploring therapeutic approaches to overcome TKI-resistance Rational TKI combinations that exploit inhibition of multiple targets within a signaling pathway represent one such approach Late-breaking oral plenary presentation will outline our translational data to support this combination approach, as well as a patient case report validating its clinical utility
RXDX-105 Ph 1/1b Study Update Program Title Format entrectinib Overcoming drug resistance to Trk inhibition by rational combination of entrectinib and trametinib: from bench to bedside Oral Plenary Session entrectinib Entrectinib, a highly potent pan-Trk, ROS1, and ALK inhibitor, has broad-spectrum, histology-agnostic anti-tumor activity in molecularly defined cancer Poster RXDX-105 A phase 1/1b study of RXDX-105, an oral RET and BRAF inhibitor, in patients with advanced solid tumors Poster RXDX-105 RXDX-105 demonstrates anti-tumor efficacy in multiple preclinical cancer models driven by molecular alterations in RET or BRAF oncogenes Poster RXDX-106 Immuno-oncologic efficacy of RXDX-106, a selective, TAM family small molecule kinase inhibitor Poster RXDX-106 RXDX-106 is an orally-available, potent and selective TAM/c-MET inhibitor demonstrating preclinical efficacy in MET-dependent human malignancies Poster
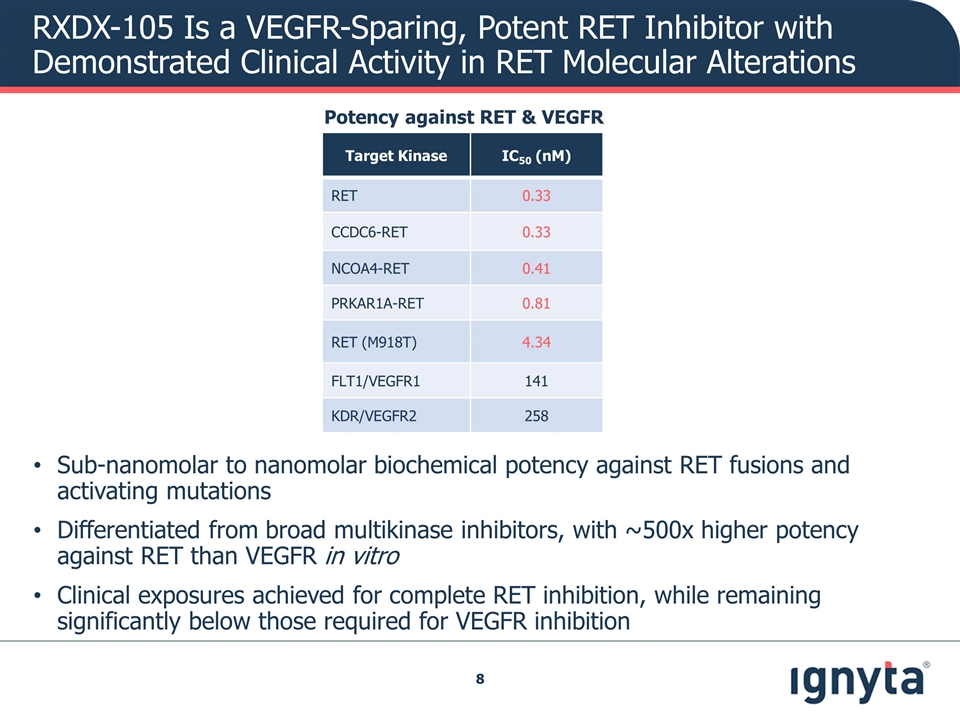
RXDX-105 Is a VEGFR-Sparing, Potent RET Inhibitor with Demonstrated Clinical Activity in RET Molecular Alterations Sub-nanomolar to nanomolar biochemical potency against RET fusions and activating mutations Differentiated from broad multikinase inhibitors, with ~500x higher potency against RET than VEGFR in vitro Clinical exposures achieved for complete RET inhibition, while remaining significantly below those required for VEGFR inhibition Potency against RET & VEGFR Target Kinase IC50 (nM) RET 0.33 CCDC6-RET 0.33 NCOA4-RET 0.41 PRKAR1A-RET 0.81 RET (M918T) 4.34 FLT1/VEGFR1 141 KDR/VEGFR2 258
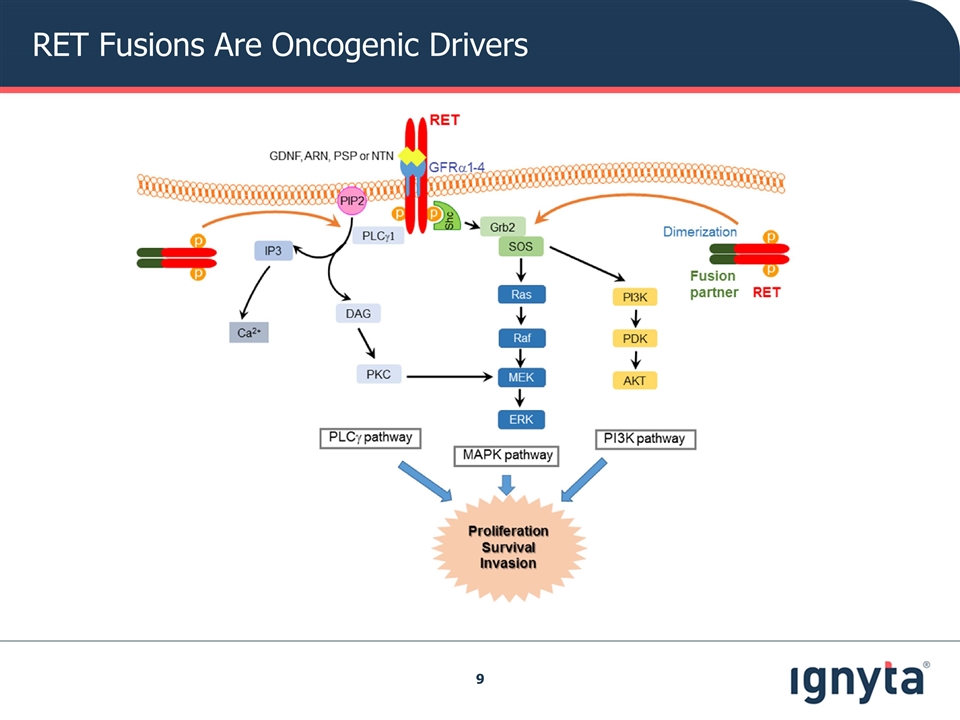
RET Fusions Are Oncogenic Drivers
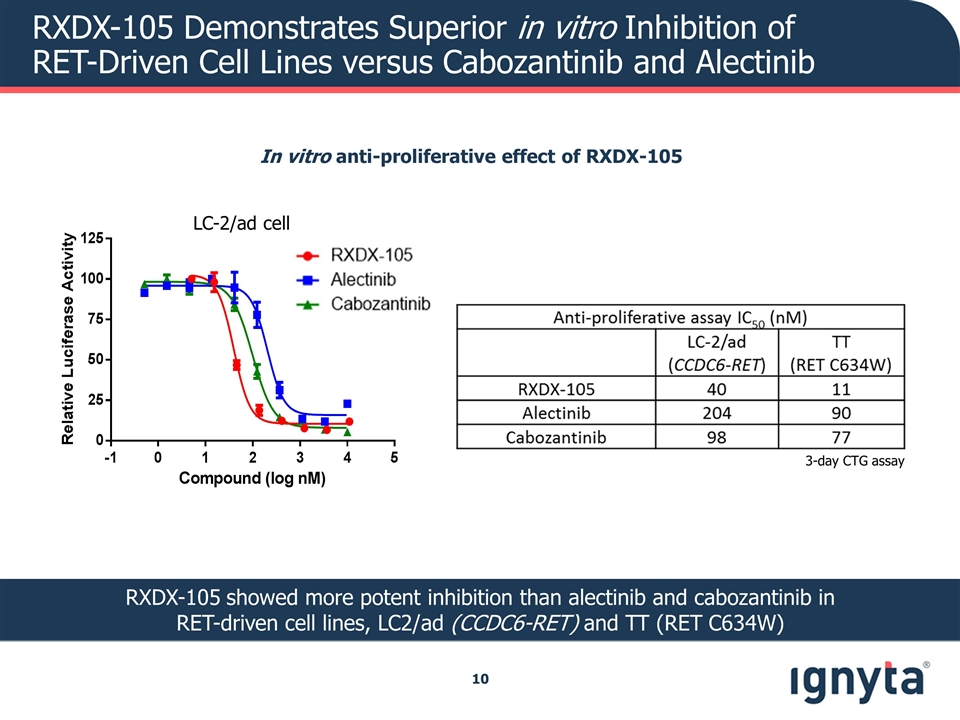
RXDX-105 Demonstrates Superior in vitro Inhibition of RET-Driven Cell Lines versus Cabozantinib and Alectinib In vitro anti-proliferative effect of RXDX-105 RXDX-105 showed more potent inhibition than alectinib and cabozantinib in RET-driven cell lines, LC2/ad (CCDC6-RET) and TT (RET C634W) 3-day CTG assay LC-2/ad cell
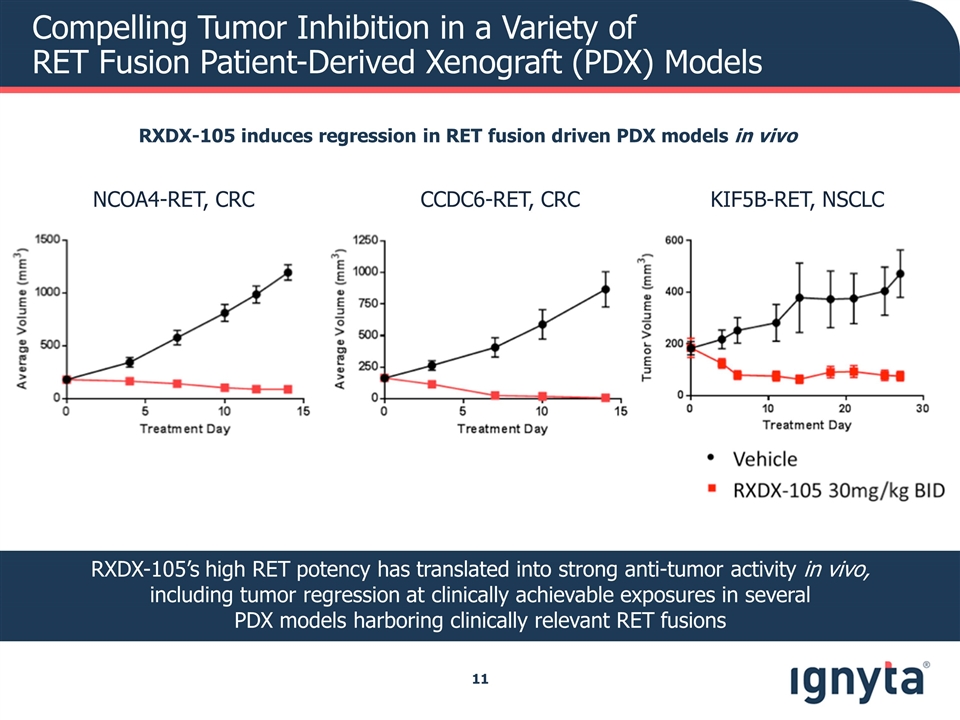
Compelling Tumor Inhibition in a Variety of RET Fusion Patient-Derived Xenograft (PDX) Models RXDX-105’s high RET potency has translated into strong anti-tumor activity in vivo, including tumor regression at clinically achievable exposures in several PDX models harboring clinically relevant RET fusions RXDX-105 induces regression in RET fusion driven PDX models in vivo NCOA4-RET, CRC CCDC6-RET, CRC KIF5B-RET, NSCLC
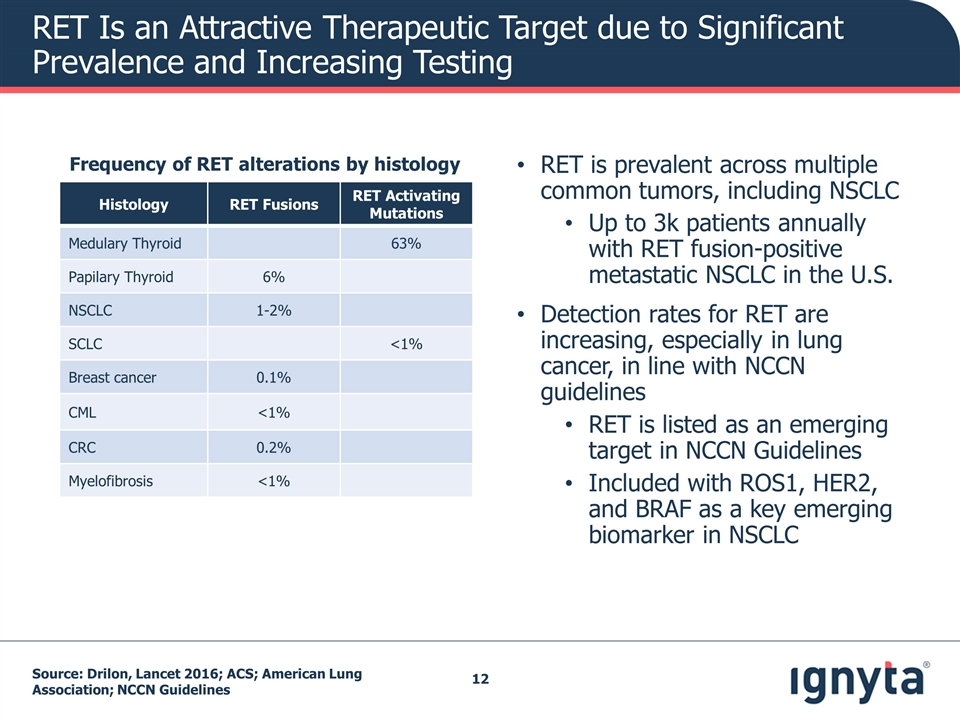
RET Is an Attractive Therapeutic Target due to Significant Prevalence and Increasing Testing RET is prevalent across multiple common tumors, including NSCLC Up to 3k patients annually with RET fusion-positive metastatic NSCLC in the U.S. Detection rates for RET are increasing, especially in lung cancer, in line with NCCN guidelines RET is listed as an emerging target in NCCN Guidelines Included with ROS1, HER2, and BRAF as a key emerging biomarker in NSCLC Source: Drilon, Lancet 2016; ACS; American Lung Association; NCCN Guidelines Histology RET Fusions RET Activating Mutations Medulary Thyroid 63% Papilary Thyroid 6% NSCLC 1-2% SCLC <1% Breast cancer 0.1% CML <1% CRC 0.2% Myelofibrosis <1% Frequency of RET alterations by histology
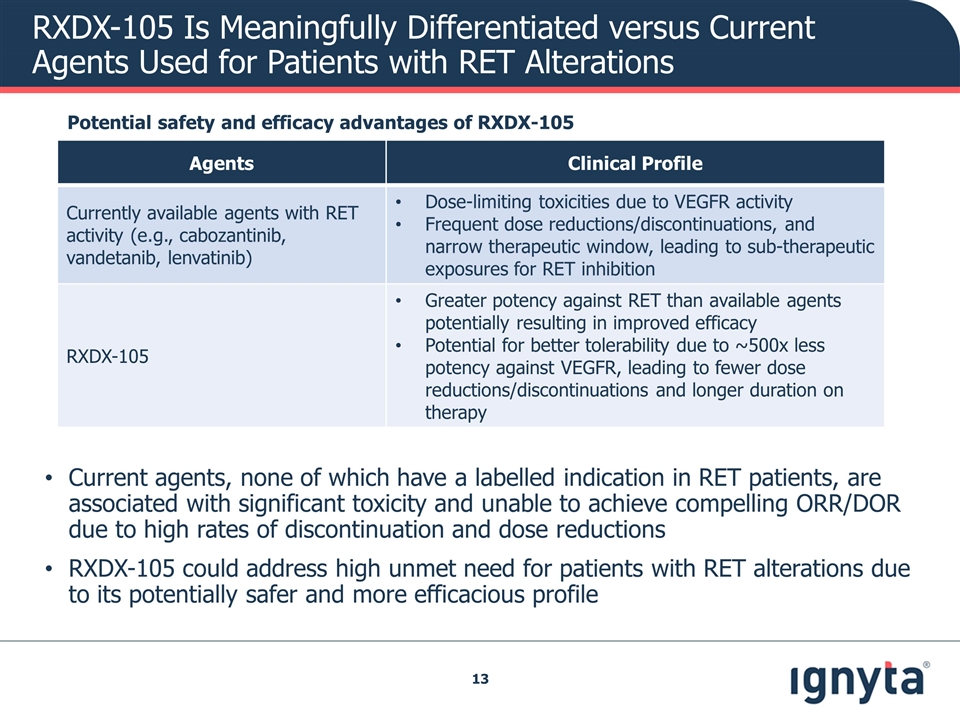
RXDX-105 Is Meaningfully Differentiated versus Current Agents Used for Patients with RET Alterations Agents Clinical Profile Currently available agents with RET activity (e.g., cabozantinib, vandetanib, lenvatinib) Dose-limiting toxicities due to VEGFR activity Frequent dose reductions/discontinuations, and narrow therapeutic window, leading to sub-therapeutic exposures for RET inhibition RXDX-105 Greater potency against RET than available agents potentially resulting in improved efficacy Potential for better tolerability due to ~500x less potency against VEGFR, leading to fewer dose reductions/discontinuations and longer duration on therapy Potential safety and efficacy advantages of RXDX-105 Current agents, none of which have a labelled indication in RET patients, are associated with significant toxicity and unable to achieve compelling ORR/DOR due to high rates of discontinuation and dose reductions RXDX-105 could address high unmet need for patients with RET alterations due to its potentially safer and more efficacious profile
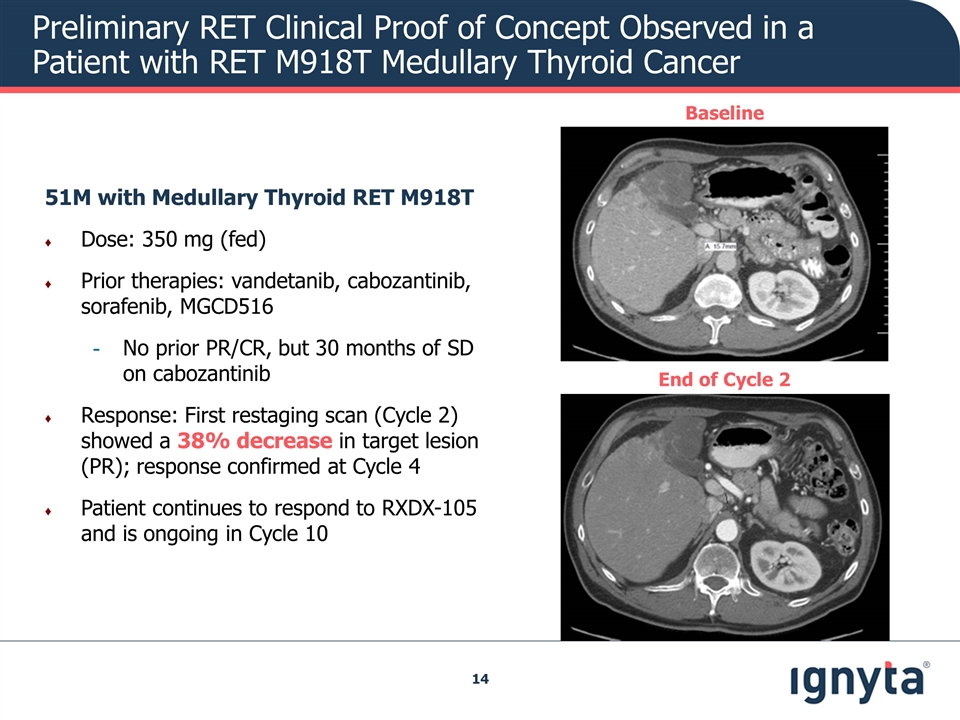
Preliminary RET Clinical Proof of Concept Observed in a Patient with RET M918T Medullary Thyroid Cancer 51M with Medullary Thyroid RET M918T Dose: 350 mg (fed) Prior therapies: vandetanib, cabozantinib, sorafenib, MGCD516 No prior PR/CR, but 30 months of SD on cabozantinib Response: First restaging scan (Cycle 2) showed a 38% decrease in target lesion (PR); response confirmed at Cycle 4 Patient continues to respond to RXDX-105 and is ongoing in Cycle 10 Baseline End of Cycle 2
RXDX-105 Phase 1b Basket Study RET Baskets Confirm / Re-establish RP2D Demonstrate preliminary efficacy Objectives Separate by solid tumor type and molecular alteration Other exploratory baskets (BRAF, NSCLC, SqCLC) RET+ Other histologies Medullary Thyroid NSCLC
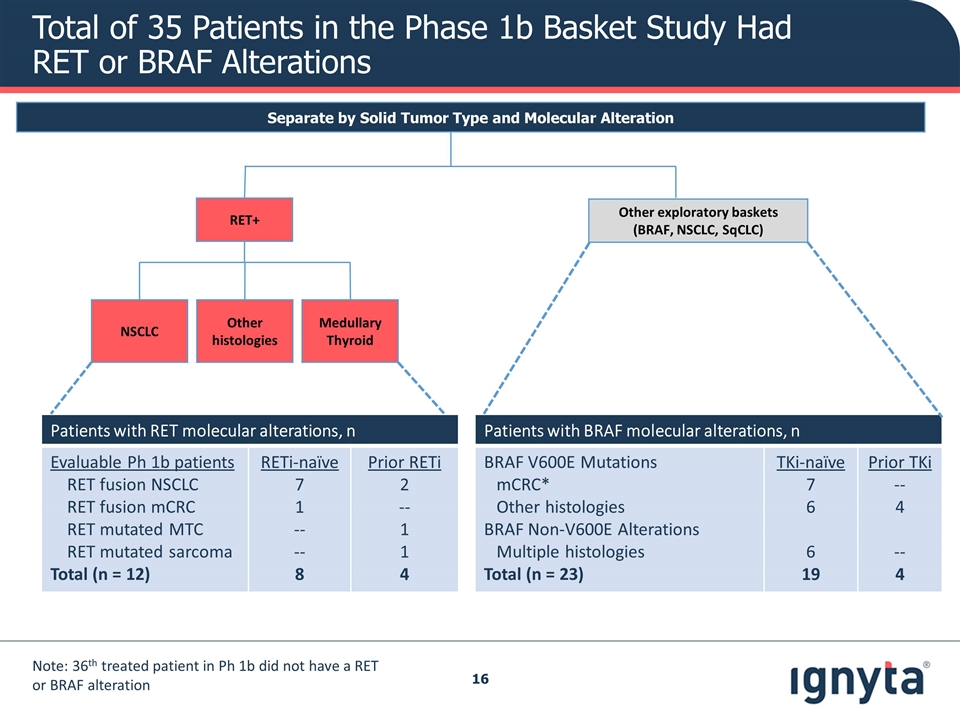
Patients with RET molecular alterations, n Evaluable Ph 1b patients RET fusion NSCLC RET fusion mCRC RET mutated MTC RET mutated sarcoma Total (n = 12) RETi-naïve 7 1 -- -- 8 Prior RETi 2 -- 1 1 4 Patients with BRAF molecular alterations, n BRAF V600E Mutations mCRC* Other histologies BRAF Non-V600E Alterations Multiple histologies Total (n = 23) TKi-naïve 7 6 6 19 Prior TKi -- 4 -- 4 Separate by Solid Tumor Type and Molecular Alteration Other exploratory baskets (BRAF, NSCLC, SqCLC) RET+ Other histologies Medullary Thyroid NSCLC Total of 35 Patients in the Phase 1b Basket Study Had RET or BRAF Alterations Note: 36th treated patient in Ph 1b did not have a RET or BRAF alteration
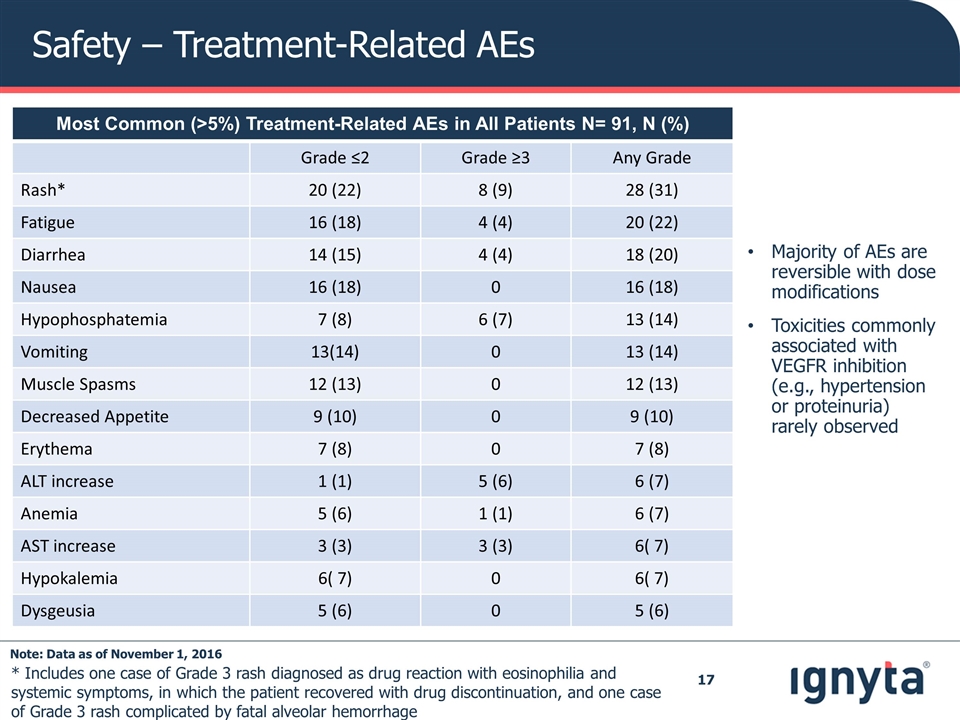
Safety – Treatment-Related AEs Majority of AEs are reversible with dose modifications Toxicities commonly associated with VEGFR inhibition (e.g., hypertension or proteinuria) rarely observed * Includes one case of Grade 3 rash diagnosed as drug reaction with eosinophilia and systemic symptoms, in which the patient recovered with drug discontinuation, and one case of Grade 3 rash complicated by fatal alveolar hemorrhage * Most Common (>5%) Treatment-Related AEs in All Patients N= 91, N (%) Grade ≤2 Grade ≥3 Any Grade Rash* 20 (22) 8 (9) 28 (31) Fatigue 16 (18) 4 (4) 20 (22) Diarrhea 14 (15) 4 (4) 18 (20) Nausea 16 (18) 0 16 (18) Hypophosphatemia 7 (8) 6 (7) 13 (14) Vomiting 13(14) 0 13 (14) Muscle Spasms 12 (13) 0 12 (13) Decreased Appetite 9 (10) 0 9 (10) Erythema 7 (8) 0 7 (8) ALT increase 1 (1) 5 (6) 6 (7) Anemia 5 (6) 1 (1) 6 (7) AST increase 3 (3) 3 (3) 6( 7) Hypokalemia 6( 7) 0 6( 7) Dysgeusia 5 (6) 0 5 (6) Note: Data as of November 1, 2016
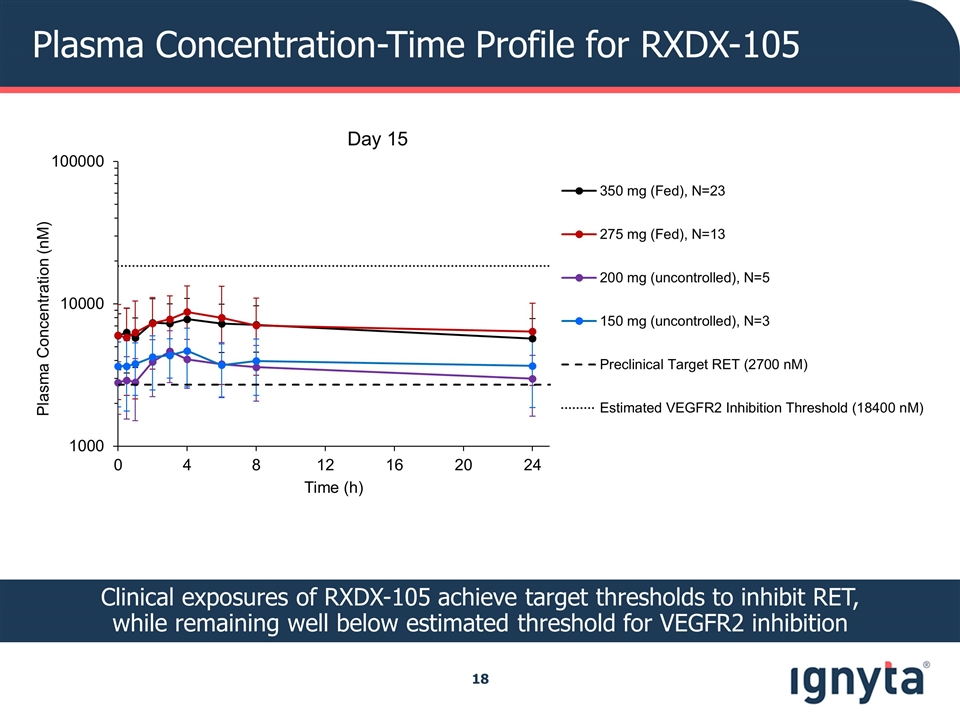
Plasma Concentration-Time Profile for RXDX-105 Clinical exposures of RXDX-105 achieve target thresholds to inhibit RET, while remaining well below estimated threshold for VEGFR2 inhibition
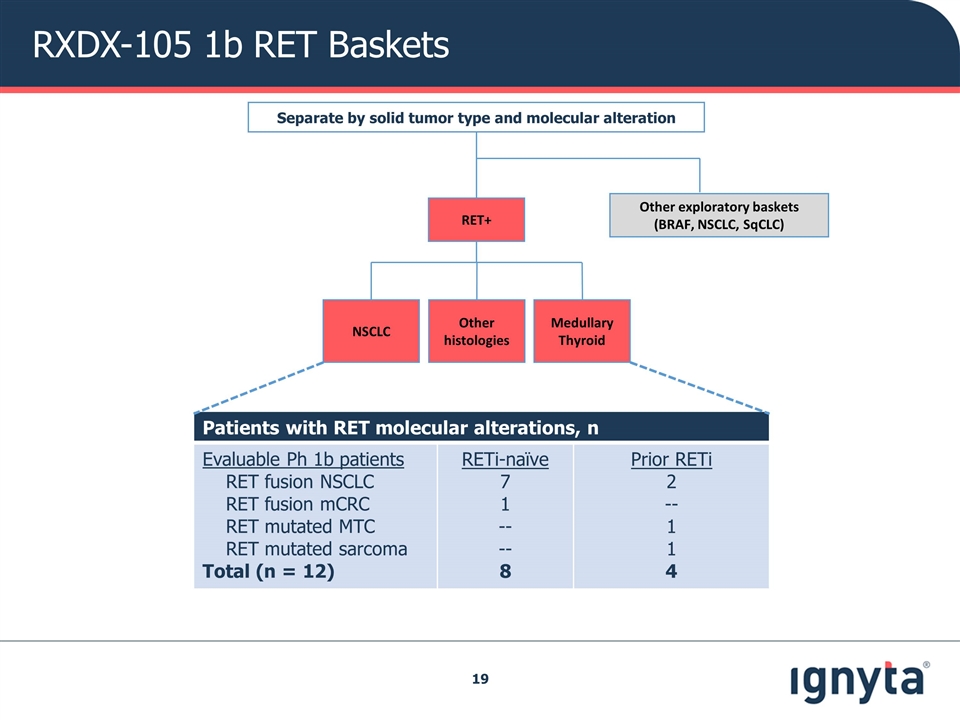
RXDX-105 1b RET Baskets Separate by solid tumor type and molecular alteration Other exploratory baskets (BRAF, NSCLC, SqCLC) RET+ Other histologies Medullary Thyroid NSCLC Patients with RET molecular alterations, n Evaluable Ph 1b patients RET fusion NSCLC RET fusion mCRC RET mutated MTC RET mutated sarcoma Total (n = 12) RETi-naïve 7 1 -- -- 8 Prior RETi 2 -- 1 1 4
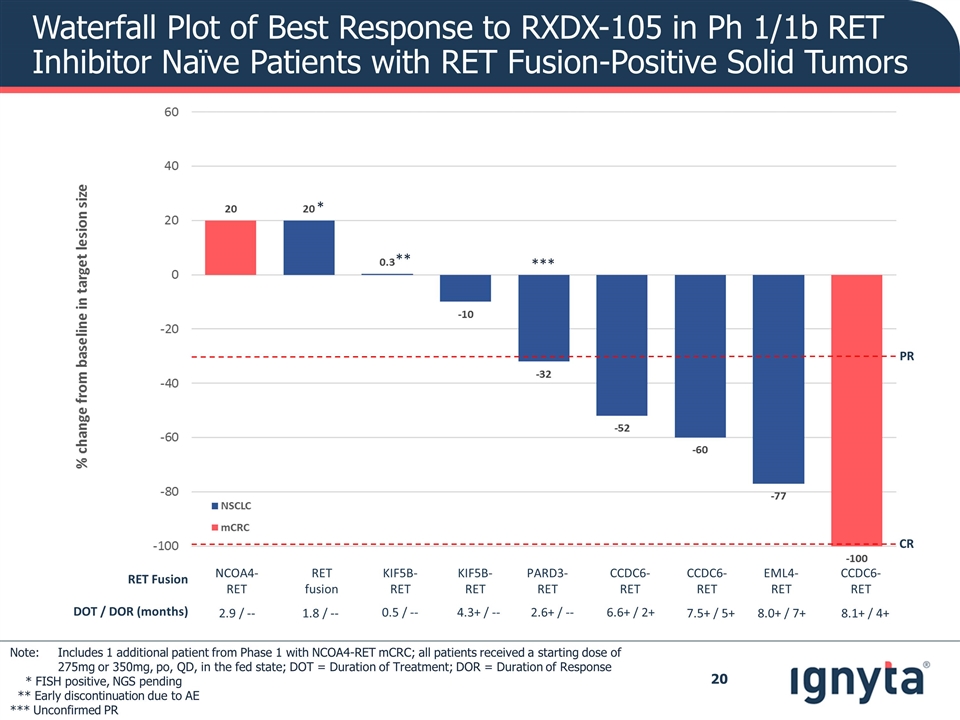
Waterfall Plot of Best Response to RXDX-105 in Ph 1/1b RET Inhibitor Naïve Patients with RET Fusion-Positive Solid Tumors * ** RET Fusion NCOA4-RET RET fusion KIF5B-RET KIF5B-RET PARD3-RET CCDC6-RET CCDC6-RET EML4-RET CCDC6-RET DOT / DOR (months) 2.9 / -- 1.8 / -- 0.5 / -- 4.3+ / -- 2.6+ / -- 6.6+ / 2+ 7.5+ / 5+ 8.0+ / 7+ 8.1+ / 4+ *** PR CR Note: Includes 1 additional patient from Phase 1 with NCOA4-RET mCRC; all patients received a starting dose of 275mg or 350mg, po, QD, in the fed state; DOT = Duration of Treatment; DOR = Duration of Response * FISH positive, NGS pending ** Early discontinuation due to AE *** Unconfirmed PR
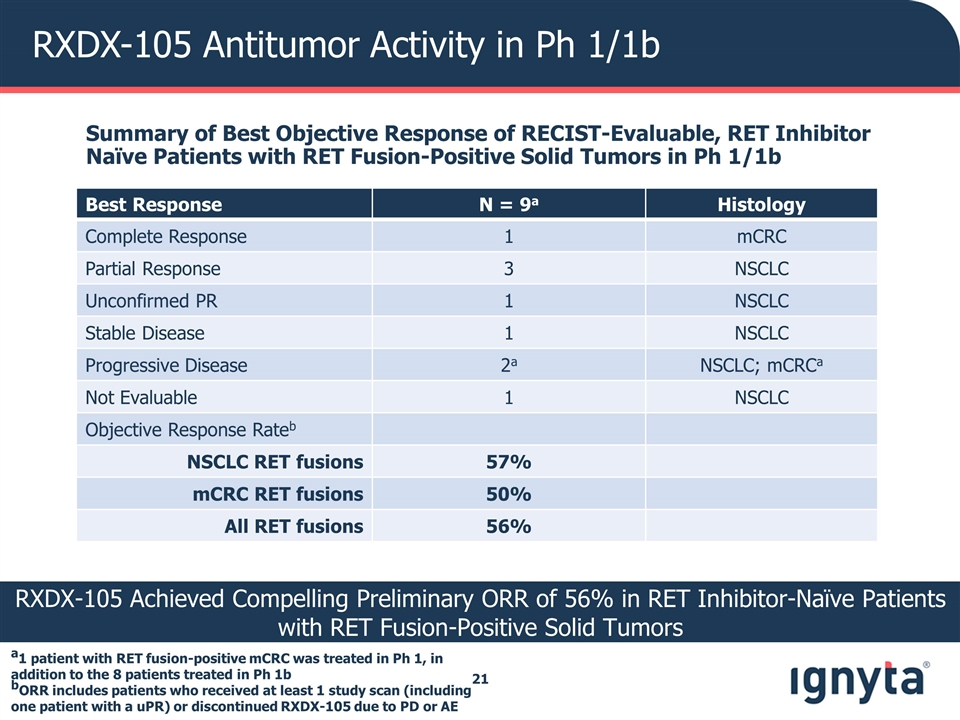
RXDX-105 Antitumor Activity in Ph 1/1b a1 patient with RET fusion-positive mCRC was treated in Ph 1, in addition to the 8 patients treated in Ph 1b bORR includes patients who received at least 1 study scan (including one patient with a uPR) or discontinued RXDX-105 due to PD or AE Best Response N = 9a Histology Complete Response 1 mCRC Partial Response 3 NSCLC Unconfirmed PR 1 NSCLC Stable Disease 1 NSCLC Progressive Disease 2a NSCLC; mCRCa Not Evaluable 1 NSCLC Objective Response Rateb NSCLC RET fusions 57% mCRC RET fusions 50% All RET fusions 56% RXDX-105 Achieved Compelling Preliminary ORR of 56% in RET Inhibitor-Naïve Patients with RET Fusion-Positive Solid Tumors Summary of Best Objective Response of RECIST-Evaluable, RET Inhibitor Naïve Patients with RET Fusion-Positive Solid Tumors in Ph 1/1b
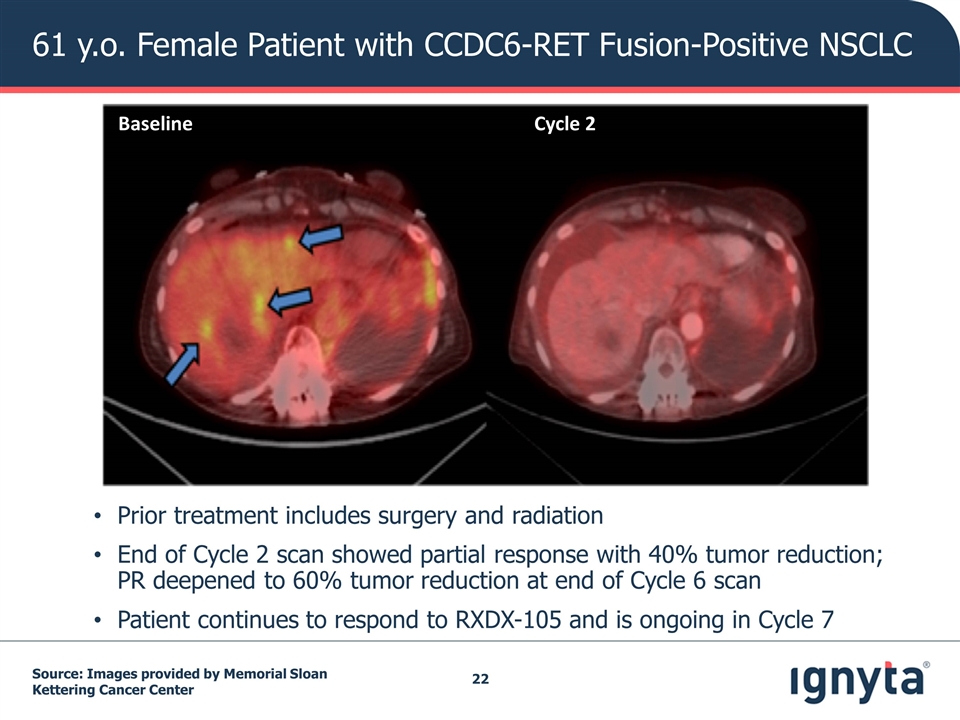
Source: Images provided by Memorial Sloan Kettering Cancer Center Prior treatment includes surgery and radiation End of Cycle 2 scan showed partial response with 40% tumor reduction; PR deepened to 60% tumor reduction at end of Cycle 6 scan Patient continues to respond to RXDX-105 and is ongoing in Cycle 7 61 y.o. Female Patient with CCDC6-RET Fusion-Positive NSCLC Baseline Cycle 2
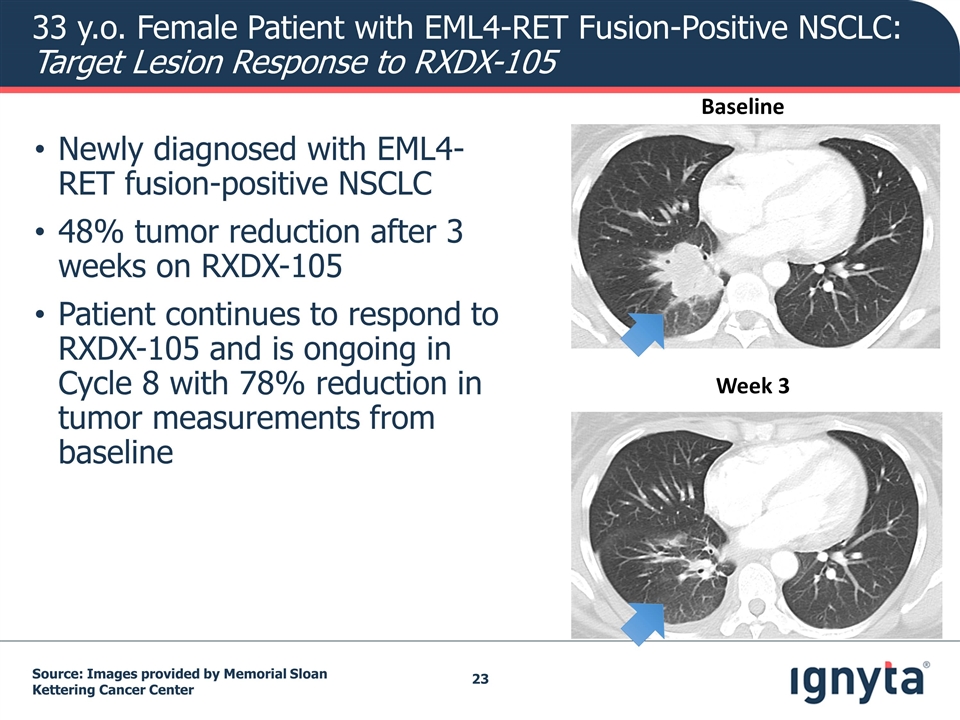
Baseline Week 3 Newly diagnosed with EML4-RET fusion-positive NSCLC 48% tumor reduction after 3 weeks on RXDX-105 Patient continues to respond to RXDX-105 and is ongoing in Cycle 8 with 78% reduction in tumor measurements from baseline Source: Images provided by Memorial Sloan Kettering Cancer Center 33 y.o. Female Patient with EML4-RET Fusion-Positive NSCLC: Target Lesion Response to RXDX-105
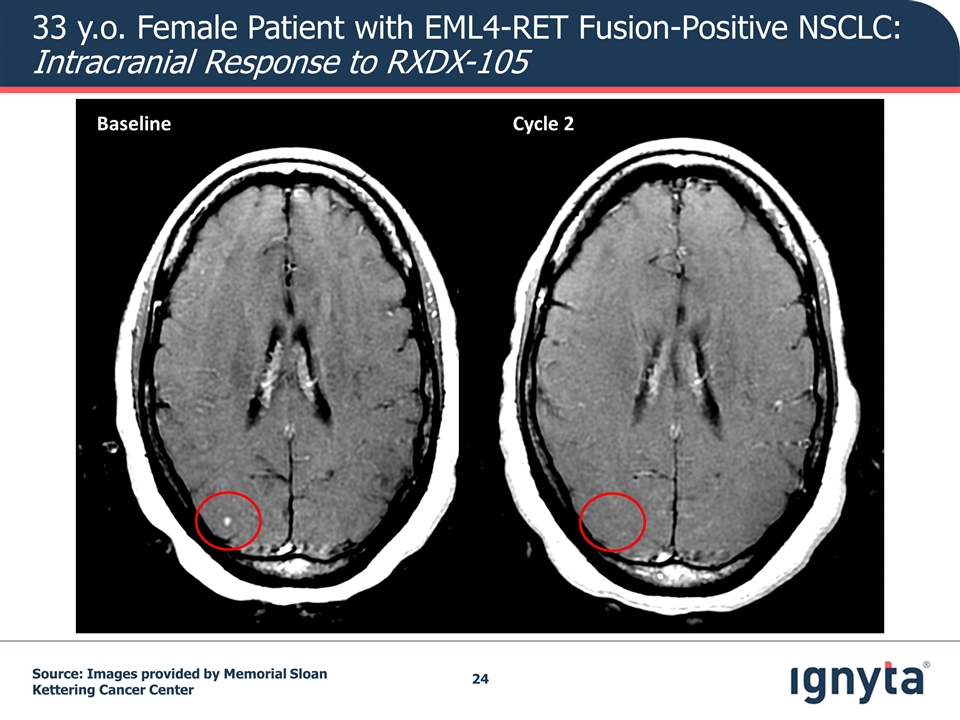
Baseline Cycle 2 33 y.o. Female Patient with EML4-RET Fusion-Positive NSCLC: Intracranial Response to RXDX-105 Source: Images provided by Memorial Sloan Kettering Cancer Center
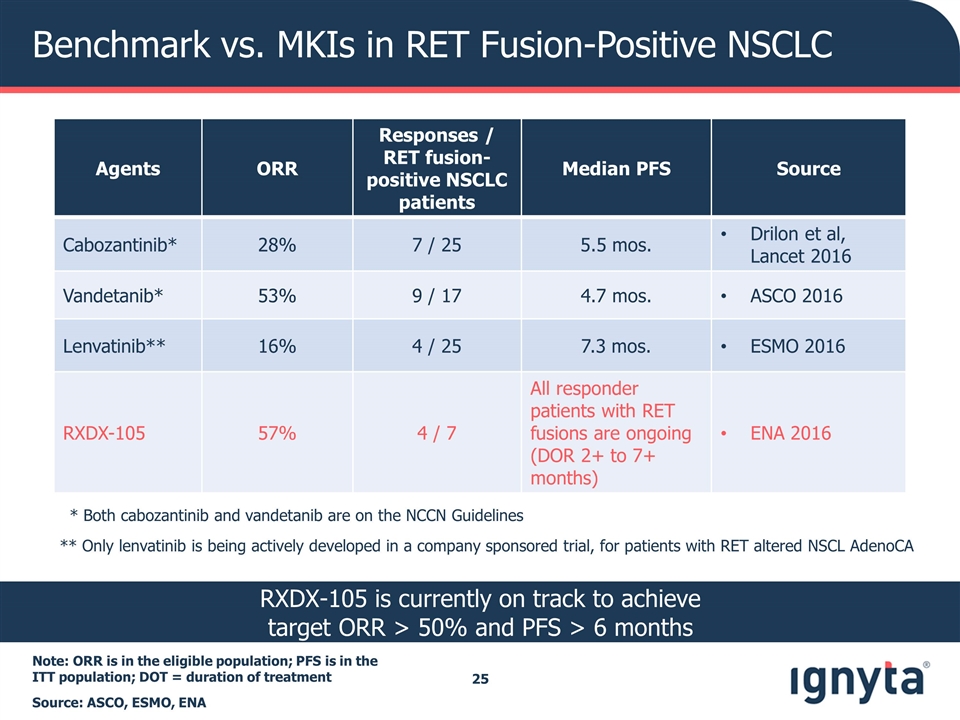
Benchmark vs. MKIs in RET Fusion-Positive NSCLC Note: ORR is in the eligible population; PFS is in the ITT population; DOT = duration of treatment Source: ASCO, ESMO, ENA Agents ORR Responses / RET fusion-positive NSCLC patients Median PFS Source Cabozantinib* 28% 7 / 25 5.5 mos. Drilon et al, Lancet 2016 Vandetanib* 53% 9 / 17 4.7 mos. ASCO 2016 Lenvatinib** 16% 4 / 25 7.3 mos. ESMO 2016 RXDX-105 57% 4 / 7 All responder patients with RET fusions are ongoing (DOR 2+ to 7+ months) ENA 2016 * Both cabozantinib and vandetanib are on the NCCN Guidelines ** Only lenvatinib is being actively developed in a company sponsored trial, for patients with RET altered NSCL AdenoCA RXDX-105 is currently on track to achieve target ORR > 50% and PFS > 6 months
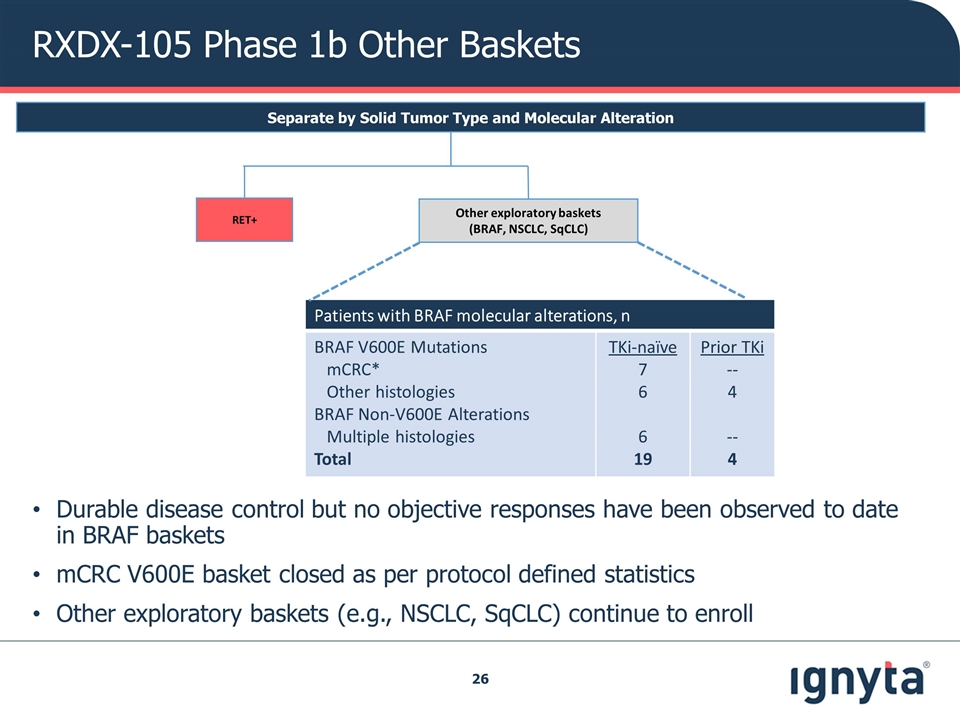
Patients with BRAF molecular alterations, n BRAF V600E Mutations mCRC* Other histologies BRAF Non-V600E Alterations Multiple histologies Total TKi-naïve 7 6 6 19 Prior TKi -- 4 -- 4 Separate by Solid Tumor Type and Molecular Alteration Other exploratory baskets (BRAF, NSCLC, SqCLC) RET+ RXDX-105 Phase 1b Other Baskets Durable disease control but no objective responses have been observed to date in BRAF baskets mCRC V600E basket closed as per protocol defined statistics Other exploratory baskets (e.g., NSCLC, SqCLC) continue to enroll
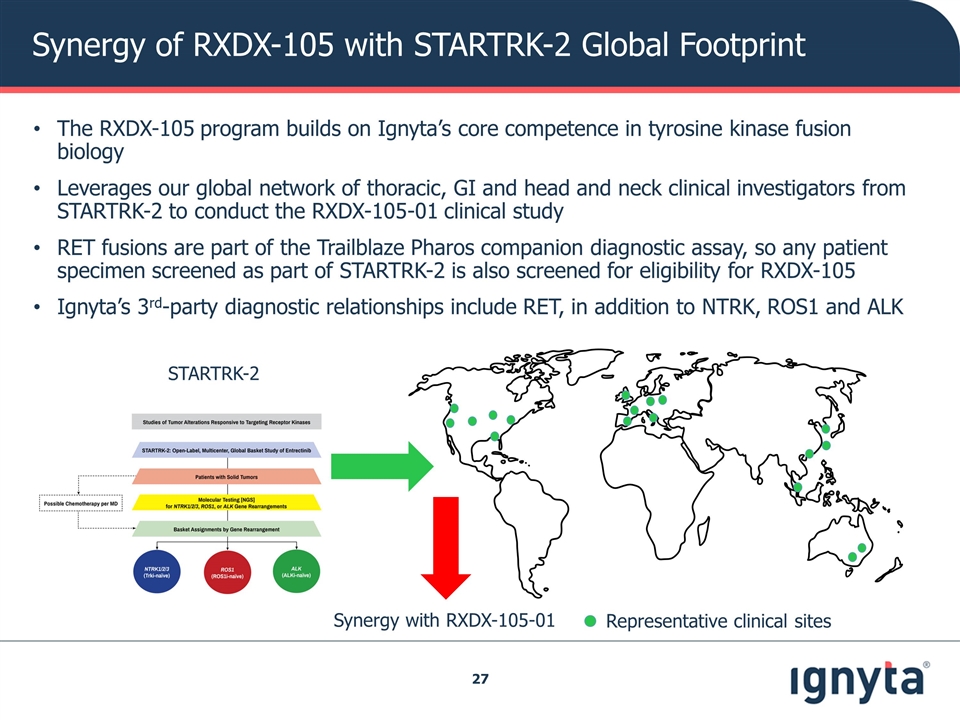
Synergy of RXDX-105 with STARTRK-2 Global Footprint The RXDX-105 program builds on Ignyta’s core competence in tyrosine kinase fusion biology Leverages our global network of thoracic, GI and head and neck clinical investigators from STARTRK-2 to conduct the RXDX-105-01 clinical study RET fusions are part of the Trailblaze Pharos companion diagnostic assay, so any patient specimen screened as part of STARTRK-2 is also screened for eligibility for RXDX-105 Ignyta’s 3rd-party diagnostic relationships include RET, in addition to NTRK, ROS1 and ALK Representative clinical sites STARTRK-2 Synergy with RXDX-105-01

RXDX-105 Key Takeaways RET activation is a compelling oncodriver target found in multiple histologies RET-altered addressable patient population of up to 3k NSCLC patients annually in U.S. alone; additional prevalence in other histologies (medullary thyroid, mCRC) Compelling clinical data indicate RXDX-105 may address critical unmet medical needs in RET positive patients, due to clinical utility of MKI VEGFR inhibitors with RET activity being constrained by significant safety liabilities and limited efficacy Potentially best-in-class reported ORR of 57% in RET fusion-positive NSCLC (56% in RET fusion-positive solid tumors, including mCRC) Promising durability: duration of response to RXDX-105 ranged from 2+ to 7+ months, with all responder patients currently continuing on treatment Activity across a range of different histologies: confirmed RECIST responses in thyroid cancer, NSCLC, and mCRC; across a range of RET alterations RXDX-105 benefits from substantial synergy with Ignyta’s global clinical, regulatory and diagnostic infrastructure RXDX-105 is the second asset that Ignyta has developed to clinical proof of concept and further highlights Ignyta’s precision oncology capabilities of developing targeted therapies for patients with genomically defined oncodrivers
RXDX-106 as an Immuno-Oncology Therapy Program Title Format entrectinib Overcoming drug resistance to Trk inhibition by rational combination of entrectinib and trametinib: from bench to bedside Oral Plenary Session entrectinib Entrectinib, a highly potent pan-Trk, ROS1, and ALK inhibitor, has broad-spectrum, histology-agnostic anti-tumor activity in molecularly defined cancer Poster RXDX-105 A phase 1/1b study of RXDX-105, an oral RET and BRAF inhibitor, in patients with advanced solid tumors Poster RXDX-105 RXDX-105 demonstrates anti-tumor efficacy in multiple preclinical cancer models driven by molecular alterations in RET or BRAF oncogenes Poster RXDX-106 Immuno-oncologic efficacy of RXDX-106, a selective, TAM family small molecule kinase inhibitor Poster RXDX-106 RXDX-106 is an orally-available, potent and selective TAM/c-MET inhibitor demonstrating preclinical efficacy in MET-dependent human malignancies Poster
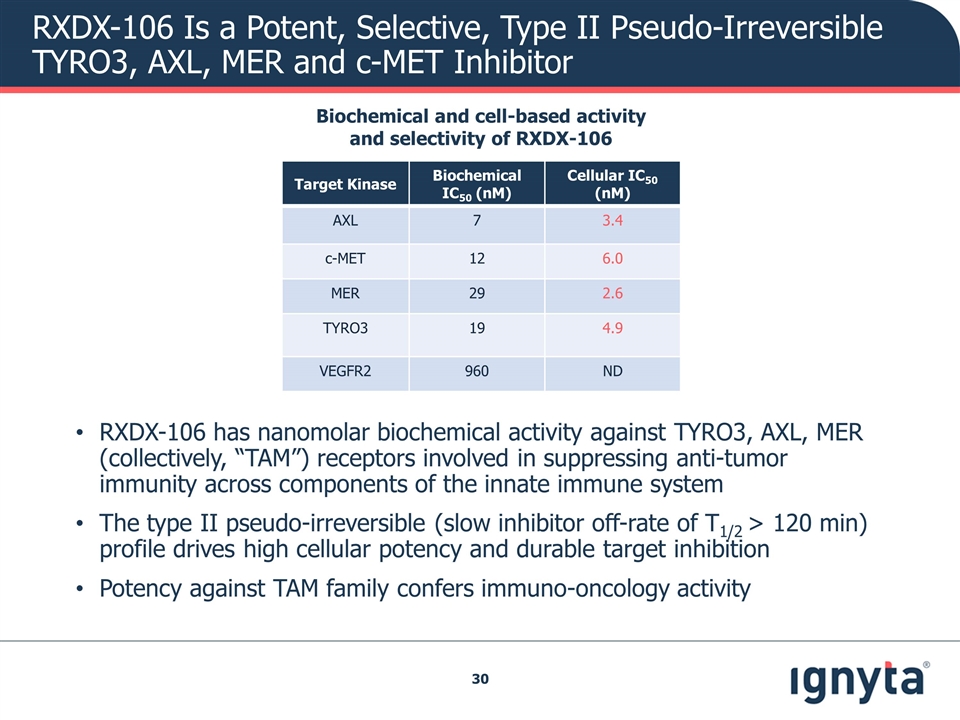
RXDX-106 Is a Potent, Selective, Type II Pseudo-Irreversible TYRO3, AXL, MER and c-MET Inhibitor Target Kinase Biochemical IC50 (nM) Cellular IC50 (nM) AXL 7 3.4 c-MET 12 6.0 MER 29 2.6 TYRO3 19 4.9 VEGFR2 960 ND RXDX-106 has nanomolar biochemical activity against TYRO3, AXL, MER (collectively, “TAM”) receptors involved in suppressing anti-tumor immunity across components of the innate immune system The type II pseudo-irreversible (slow inhibitor off-rate of T1/2 > 120 min) profile drives high cellular potency and durable target inhibition Potency against TAM family confers immuno-oncology activity Biochemical and cell-based activity and selectivity of RXDX-106
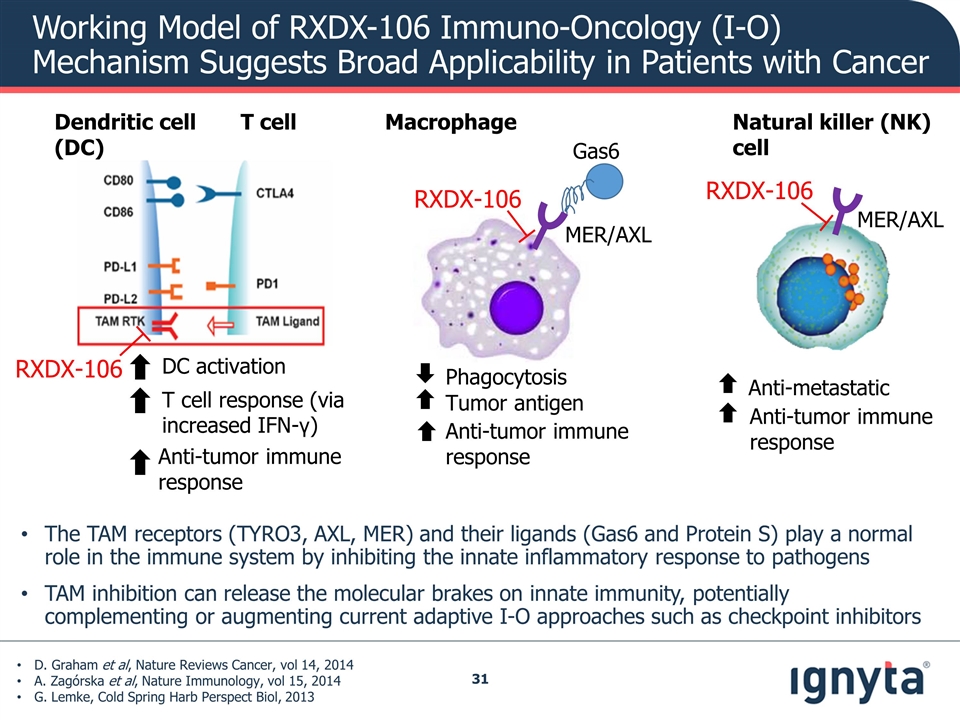
Working Model of RXDX-106 Immuno-Oncology (I-O) Mechanism Suggests Broad Applicability in Patients with Cancer Macrophage Natural killer (NK) cell Dendritic cell T cell (DC) T cell response (via increased IFN-γ) Anti-tumor immune response DC activation RXDX-106 Phagocytosis Tumor antigen Anti-tumor immune response Anti-tumor immune response Anti-metastatic Gas6 MER/AXL MER/AXL RXDX-106 RXDX-106 D. Graham et al, Nature Reviews Cancer, vol 14, 2014 A. Zagórska et al, Nature Immunology, vol 15, 2014 G. Lemke, Cold Spring Harb Perspect Biol, 2013 The TAM receptors (TYRO3, AXL, MER) and their ligands (Gas6 and Protein S) play a normal role in the immune system by inhibiting the innate inflammatory response to pathogens TAM inhibition can release the molecular brakes on innate immunity, potentially complementing or augmenting current adaptive I-O approaches such as checkpoint inhibitors
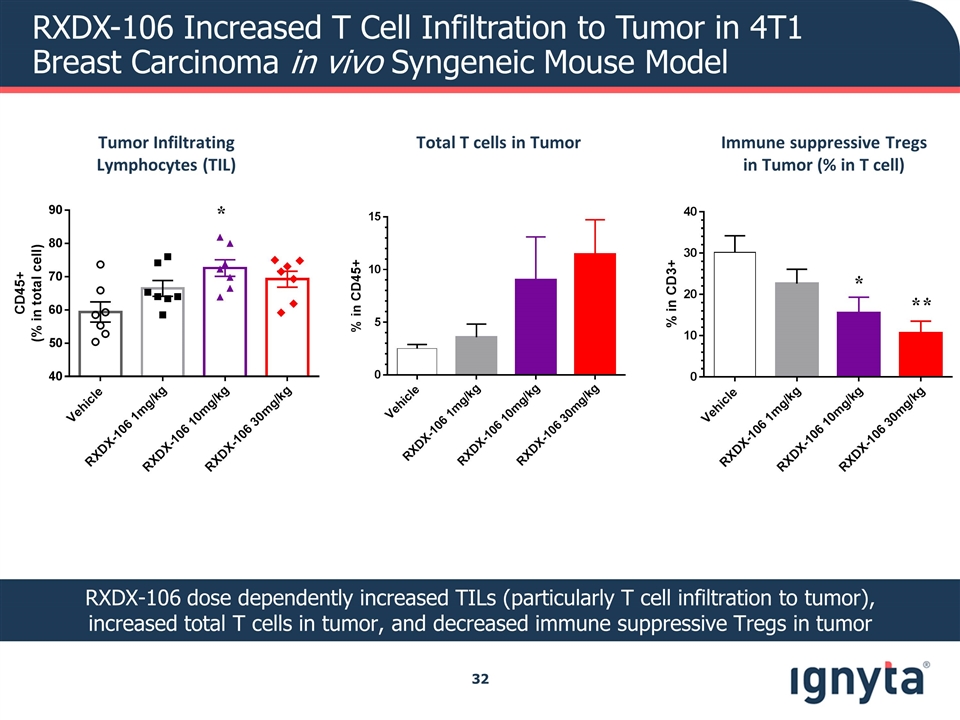
RXDX-106 Increased T Cell Infiltration to Tumor in 4T1 Breast Carcinoma in vivo Syngeneic Mouse Model RXDX-106 dose dependently increased TILs (particularly T cell infiltration to tumor), increased total T cells in tumor, and decreased immune suppressive Tregs in tumor Immune suppressive Tregs in Tumor (% in T cell) Tumor Infiltrating Lymphocytes (TIL) Total T cells in Tumor
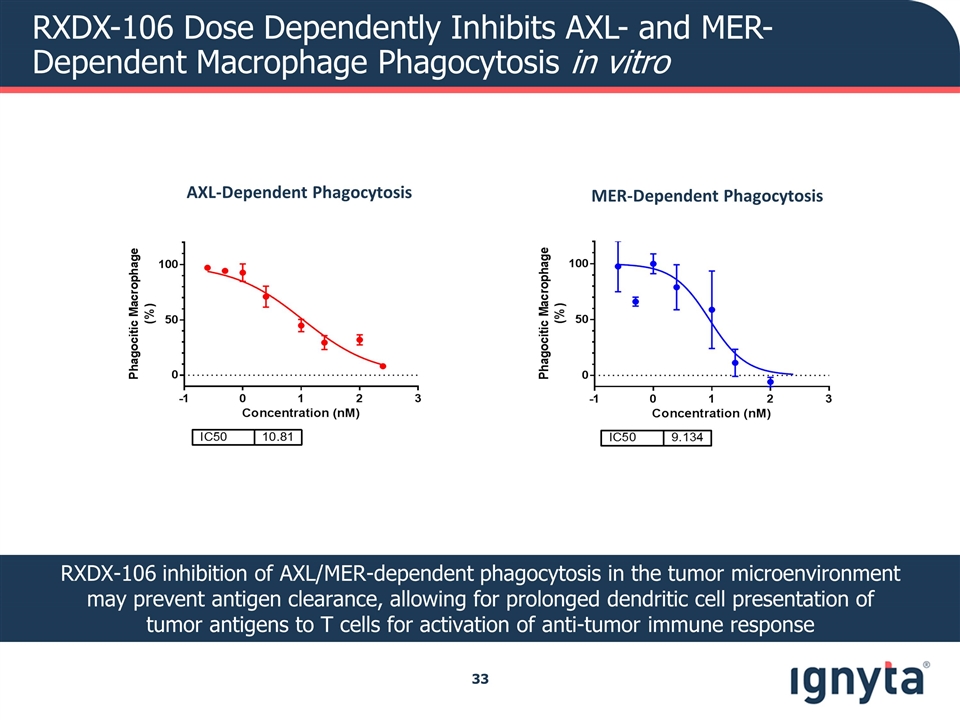
RXDX-106 Dose Dependently Inhibits AXL- and MER-Dependent Macrophage Phagocytosis in vitro RXDX-106 inhibition of AXL/MER-dependent phagocytosis in the tumor microenvironment may prevent antigen clearance, allowing for prolonged dendritic cell presentation of tumor antigens to T cells for activation of anti-tumor immune response AXL-Dependent Phagocytosis MER-Dependent Phagocytosis

RXDX-106 Recovered NK Function in Tumor in 4T1 Breast Carcinoma in vivo Syngeneic Mouse Model RXDX-106 recovered NK function in tumor microenvironment, which – by stimulating IFN-γ – could potentially turn “cold” tumors into “hot” tumors and therefore broaden clinical utility of current checkpoint inhibitors IFN-g in Splenic NK cells IFN-g in Tumor NK cells Note: NK cells = natural killer cells
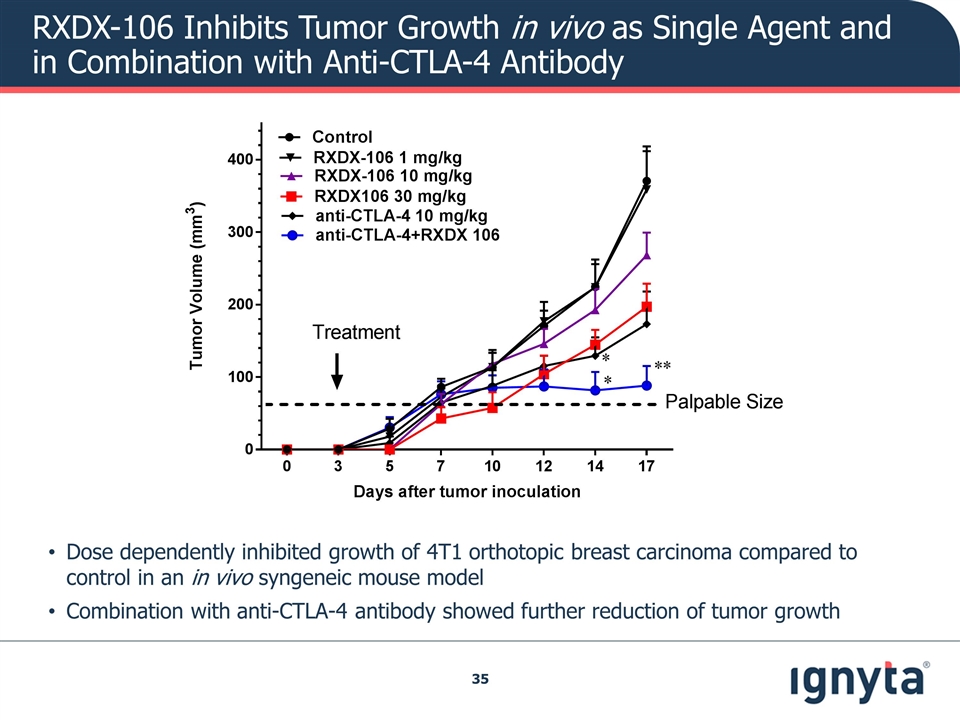
RXDX-106 Inhibits Tumor Growth in vivo as Single Agent and in Combination with Anti-CTLA-4 Antibody Dose dependently inhibited growth of 4T1 orthotopic breast carcinoma compared to control in an in vivo syngeneic mouse model Combination with anti-CTLA-4 antibody showed further reduction of tumor growth
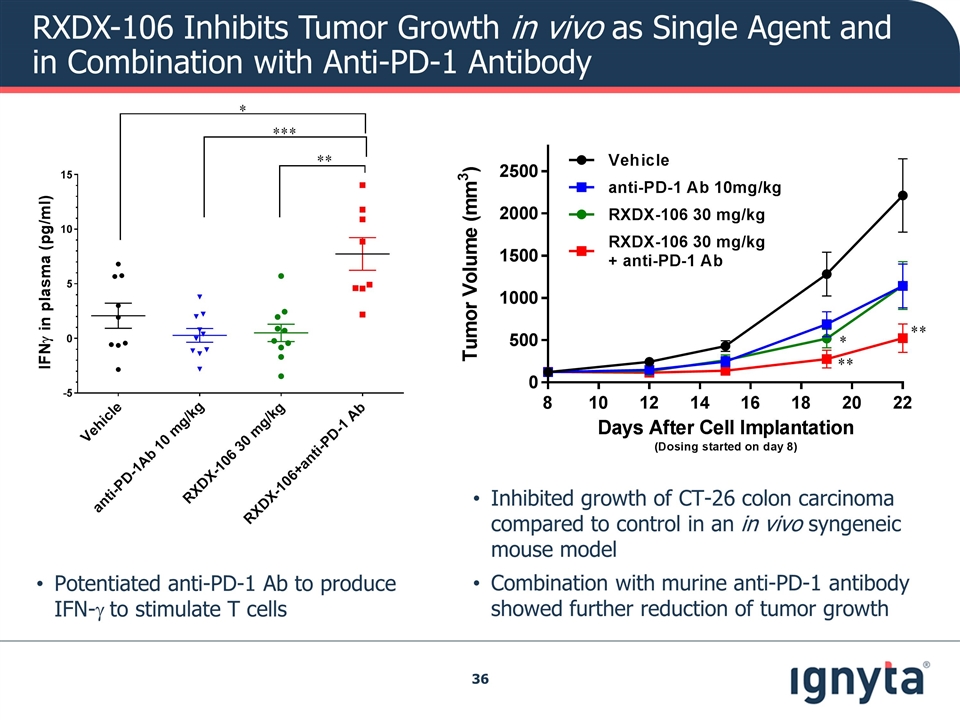
RXDX-106 Inhibits Tumor Growth in vivo as Single Agent and in Combination with Anti-PD-1 Antibody Inhibited growth of CT-26 colon carcinoma compared to control in an in vivo syngeneic mouse model Combination with murine anti-PD-1 antibody showed further reduction of tumor growth Potentiated anti-PD-1 Ab to produce IFN-g to stimulate T cells
RXDX-106 as a Targeted Oncology Therapy Program Title Format entrectinib Overcoming drug resistance to Trk inhibition by rational combination of entrectinib and trametinib: from bench to bedside Oral Plenary Session entrectinib Entrectinib, a highly potent pan-Trk, ROS1, and ALK inhibitor, has broad-spectrum, histology-agnostic anti-tumor activity in molecularly defined cancer Poster RXDX-105 A phase 1/1b study of RXDX-105, an oral RET and BRAF inhibitor, in patients with advanced solid tumors Poster RXDX-105 RXDX-105 demonstrates anti-tumor efficacy in multiple preclinical cancer models driven by molecular alterations in RET or BRAF oncogenes Poster RXDX-106 Immuno-oncologic efficacy of RXDX-106, a selective, TAM family small molecule kinase inhibitor Poster RXDX-106 RXDX-106 is an orally-available, potent and selective TAM/c-MET inhibitor demonstrating preclinical efficacy in MET-dependent human malignancies Poster
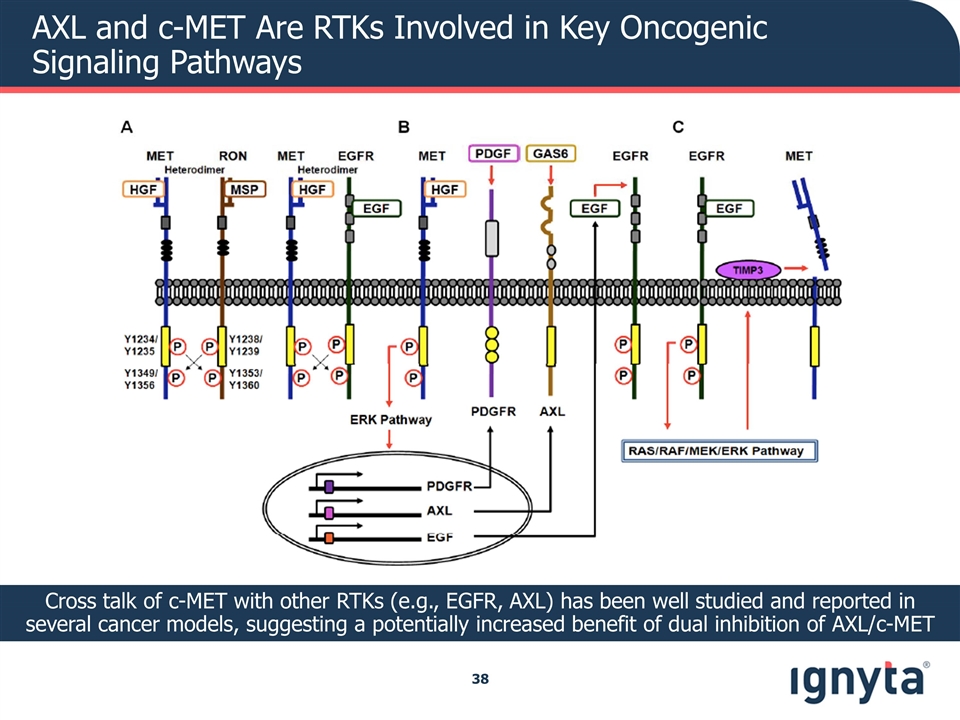
AXL and c-MET Are RTKs Involved in Key Oncogenic Signaling Pathways Cross talk of c-MET with other RTKs (e.g., EGFR, AXL) has been well studied and reported in several cancer models, suggesting a potentially increased benefit of dual inhibition of AXL/c-MET
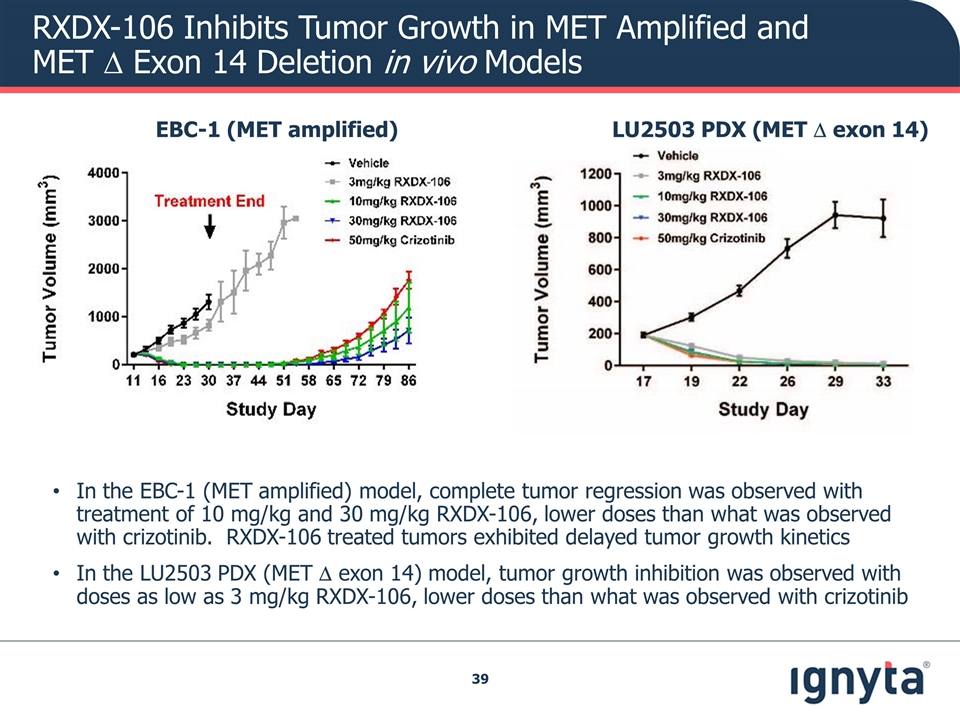
RXDX-106 Inhibits Tumor Growth in MET Amplified and MET D Exon 14 Deletion in vivo Models LU2503 PDX (MET D exon 14) EBC-1 (MET amplified) In the EBC-1 (MET amplified) model, complete tumor regression was observed with treatment of 10 mg/kg and 30 mg/kg RXDX-106, lower doses than what was observed with crizotinib. RXDX-106 treated tumors exhibited delayed tumor growth kinetics In the LU2503 PDX (MET D exon 14) model, tumor growth inhibition was observed with doses as low as 3 mg/kg RXDX-106, lower doses than what was observed with crizotinib
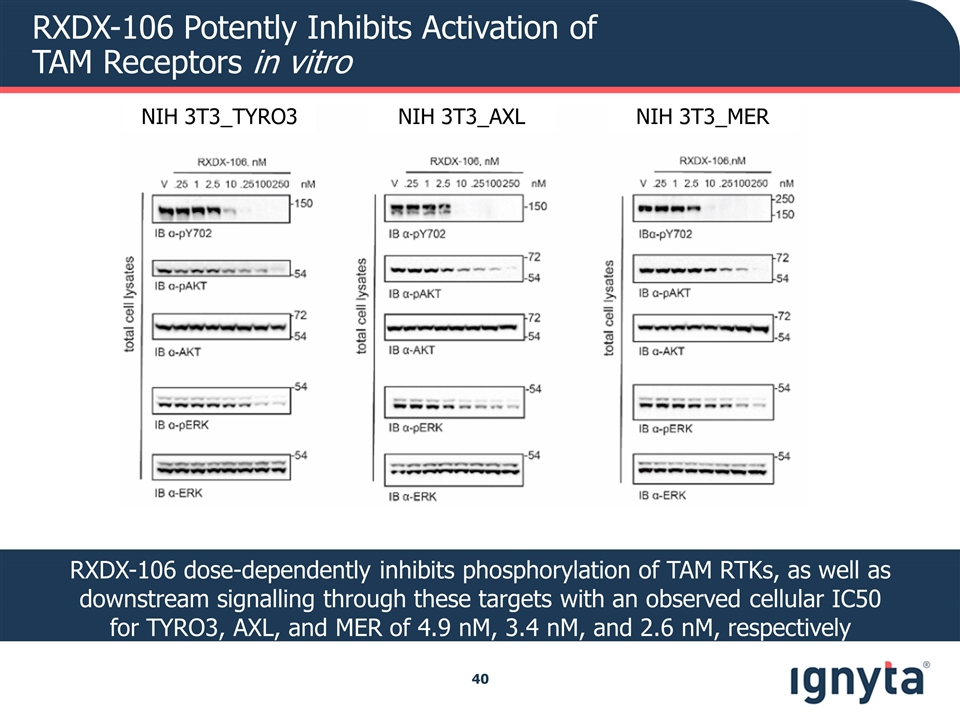
RXDX-106 Potently Inhibits Activation of TAM Receptors in vitro NIH 3T3_TYRO3 NIH 3T3_AXL NIH 3T3_MER RXDX-106 dose-dependently inhibits phosphorylation of TAM RTKs, as well as downstream signalling through these targets with an observed cellular IC50 for TYRO3, AXL, and MER of 4.9 nM, 3.4 nM, and 2.6 nM, respectively
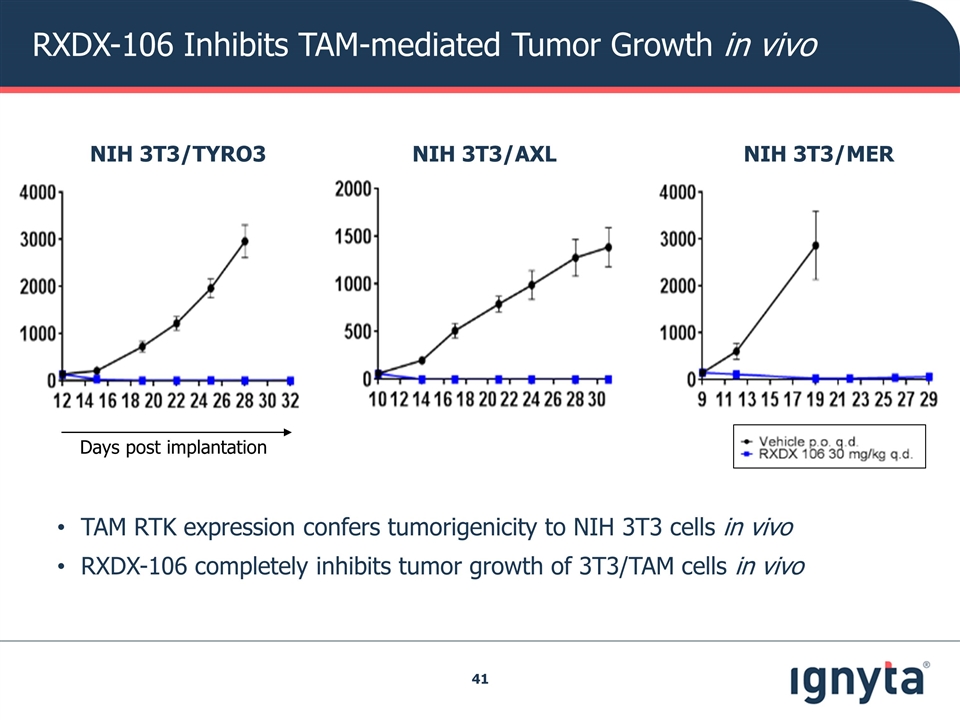
RXDX-106 Inhibits TAM-mediated Tumor Growth in vivo NIH 3T3/AXL NIH 3T3/MER NIH 3T3/TYRO3 Days post implantation TAM RTK expression confers tumorigenicity to NIH 3T3 cells in vivo RXDX-106 completely inhibits tumor growth of 3T3/TAM cells in vivo

RXDX-106 Key Takeaways Potent, selective, pseudo-irreversible type II inhibitor of TYRO3, AXL, MER (collectively, “TAM”), and c-MET RTKs with durable target inhibition Releases “molecular brakes” on immune activation in macrophages, NK cells, and T cells, resulting in repolarization of the immune response to create an anti-tumor environment Inhibited AXL- and MER-dependent macrophage phagocytosis Dose dependently increased tumor infiltrating leukocytes and total T cell infiltration to tumor, and decreased Tregs in tumor Recovered NK function and interferon gamma release in tumor environment Showed tumor growth inhibition as a single agent and potentiated antitumor effect of checkpoint inhibitors to potentially achieve efficacy in an expanded patient population Inhibited TAM-mediated and c-MET-mediated tumor growth in vivo We intend to explore this program in the clinic, which is anticipated in 2017

Key Program Highlights Robust pipeline of molecularly targeted therapies with strong proof-of-concept Entrectinib: Achieved ORR of 79% in patients with TRK, ROS1 or ALK fusion-positive extracranial disease (n = 24) and achieved both complete and durable responses in patients with CNS disease in Ph 1 studies RXDX-105: Now a 2nd targeted therapy program with strong clinical proof-of-concept, achieving a preliminary ORR of 56% (n = 9) in patients with RET fusion-positive solid tumors in Ph 1/1b study RXDX-106: Promising preclinical efficacy both as an immunomodulator and as a targeted therapy Ignyta’s precision medicine business model is bearing fruit In-licensing and re-positioning development stage programs into specific molecularly targeted patient populations is yielding impressive single agent efficacy and commercial opportunities Exploring combination therapies to address resistance and residual disease Multiple near-term catalysts to drive value Utilizing basket study designs for efficient clinical development plans to generate multiple clinical data readouts in next 12 months, while building long-term value for patients and stockholders










































