UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 1-K
SEMIANNUAL REPORT PURSUANT TO
REGULATION A OF THE SECURITIES ACT OF 1933
For the annual period ended December 31, 2021
COLABS INT’L, CORP.
(Exact name of issuer as specified in its charter)
| Nevada | | 26-3233192 |
(State or other jurisdiction of incorporation) | | (IRS Employer Identification No.) |
| | | |
| 18593 Main St, Huntington Beach, CA | | 92648 |
| (Address of principal executive offices) | | (ZIP Code) |
(888) 878-5536
(Issuer’s telephone number, including area code)
Common Stock
(Title of each class of securities issued pursuant to Regulation A)
TABLE OF CONTENTS
ITEM 1. BUSINESS
Corporate Structure
CoLabs Int’l, Corp. (the “Company,” “CoLabs,” “we,” “our,” and “us”) was incorporated on August 5, 2008 under the laws of the State of Nevada as an OTC pharmaceutical company with multi-market applications for our revolutionary targeted epidermal-drug delivery system which is identified as: QuantaSphere® Technology (QS®).
We currently hold 28 issued patents and have an additional 16 patent applications pending.
Overview
Our technology places OTC drugs, pharmaceuticals, chemicals, and agrochemicals into micro-sized, electrostatically charged encapsulates as well as into other types of micro-encapsulates. These encapsulates are then suspended in uniquely designed formulations. This formulation of a highly advanced micro-technology can: target the delivery of topically applied drugs; limit unwanted absorption of chemicals, drugs, and cosmetics through the skin; and provide a designed release of active ingredients with a prescribed depth of skin penetration.
We have already developed commercially unique and innovative targeted skin delivery systems for topical pharmacology. Our technology limits unwanted side effects that can occur as a result of absorption through the skin. The penetration of chemicals through the skin can have serious impact on the body’s organs and systems. Our current applications include anti-bacterial/viral sanitizers/soaps, sunscreens, pest-repellents, and healing cosmetic lotions. For consumers, we provide elegant skin delivery of topically applied drugs and cosmetics. This combined end purpose is the unique foundation of our technology.
The Issue—Risk of Toxic Absorption of Drugs and Chemicals when Placed on Skin:
We believe that major issues exist for the direct topical application for pharmaceutical ingredients. When drugs are applied to our largest organ, the skin, toxicity from absorption may occur. The FDA recently released an alarming, highly publicized study that revealed that topically applied sunscreens were detected in blood after several reapplications (Journal of the American Medical Association, May 6, 2019).
The Toxic Substances Control Act defines a “toxic effect” as an adverse change in the structure or function of the test subject produced as a result of exposure to a chemical substance. This adverse change can be acute, subchronic, or from chronic exposure. Acute toxicity tests demonstrate the immediate effects of exposure which occur usually within eight hours of exposure. Subchronic toxicity results show toxicity effects that occur over a period of weeks. Longer term chronic effects tests measure long-term exposure effects which are revealed in months or even years. The skin is one route of exposure. This transdermal route, sometimes shown in combination with other routes, can produce toxic effects and is a frequently tested route. The testing of product ingredients from natural or manufactured substances is used to determine the safety of cosmetics, pharmaceuticals, food additives, pesticides, chemicals, additives, and consumer products. Toxic effects, when discovered, can result and produce a variety of symptoms that can be manifested acutely or in the long term and these testing requirements are designed to avoid potential toxicity exposure.
A scientific article published in the May 6, 2019, Journal of the American Medical Association, describes the results of an exploratory maximal usage trial (MUsT) evaluating the systemic absorption (through the skin and into the body) of sunscreen active ingredients using four commercially available sunscreen products applied under maximal use conditions. A MUsT study evaluates the systemic absorption of a topical drug (i.e., one applied to the skin) when used according to the maximum limits of the product’s directions for use. Because sunscreens are formulated to work on the surface of the skin, many assumed that sunscreens would not be absorbed in appreciable quantities and therefore that MUsT studies would be unnecessary. However, in this pilot study, all four active ingredients tested were absorbed from each formulation tested, showing that absorption of sunscreens is not just a theoretical concern.
While the fact that an ingredient is absorbed through the skin and into the body does not mean the ingredient is per se unsafe, the FDA expressed concern that further testing to determine the safety of that ingredient for repeated use is necessary. Such testing is part of the standard pre-market safety evaluation of most chronically administered drugs with appreciable systemic absorption. The FDA proposed updating the regulatory requirements for most sunscreen products in the United States, where sunscreens are regulated as drugs. Initial FDA studies (as described in the May 6, 2019 Journal of the American Medical Association article), which followed FDA application protocols, showed sunscreen active ingredients were absorbed into the bloodstream at a level of 0.5 ng/mL (nanograms per milliliter) or higher. Further testing is required to determine the risk for cancer, birth defects, or other adverse effects. Currently, as there are no toxicity studies on sunscreen absorption, the FDA does not know what levels of absorption can be considered safe.
The potential threat to users of topical skin medications provides the key significance for our unique science for medicine and vast consumer products. Notably, the FDA is in the process of developing new testing protocols (MUsT) for topical OTC drugs to evaluate their effects on the body when absorbed. This major issue is the target of our QS® Technology. (See “Description of Business” – “FDA: Maximum Usage Trials (MUsT) for Topical Active Ingredients.”)
QS® Technology is designed to improve effectiveness, durability, and tenaciousness as compared to other current competing application systems. Based on our own independent testing, we found that several of our current competitors’ skin medications offered uncontrolled release and systemic absorption, as compared to our products. We believe that these issues may be unacceptable to a growing number of knowledgeable consumers.
Our Innovative Solution:
We can introduce selected cosmetic drugs as well as FDA and EPA approved pharmaceutical and chemical active ingredients into proprietary Quantasphere® formulations. When these chemical active ingredients are adapted in our new delivery system, they are enhanced in terms of the targeted delivery of the active drug agent.
We believe that our delivery system is a market disruptive, generational step to improve topical drug delivery. We believe that our QS® formulations can reduce absorption of ingredients while still being effective. New formulations include slow or time-release systems for new pharmacology actives.
It is important to note that we are providing topical delivery for existing drug active ingredients. We are not currently developing and introducing new drugs for which a New Drug Application (“NDA”) is required. Thus, as new product formulations are developed, we may be able to avoid the long and costly FDA NDA process. We have also engaged a highly regarded FDA law firm to provide necessary FDA legal guidance and support for our products.
Regulatory Landscape
The regulatory landscape affecting our products is primarily controlled by the FDA, the EPA, and state regulatory agencies. Cosmetics, unless proven to be injurious, are not regulated by the FDA. Sunscreens are subject to guidelines outlined under 21 CFR 352 - Sunscreen Products for Over-the-Counter Human Use. We believe that our currently offered sunscreen products comply with the FDA Final Rule for sunscreen products under 21 CFR 352 Sunscreen products for Over-the-Counter Human Use. The FDA requires specific testing to ensure that all OTC products meet certain criteria. These tests have specific guidelines and protocols in order to determine the efficacy and safety of the products when used by consumers, all of which are delineated in the monograph. In addition, there are specific tests associated with OTC products and non-OTC products (i.e., cosmetics). The testing required for cosmetics is in place to ensure that the product is safe from microbes and pathogens through a stability criteria. Other tests may also be performed in order to meet any specific claims made for each product (e.g., comedogenicity testing in order to prove that the product is non-comedogenic).
Pest repellents are subject to EPA and state regulations. All ingredients in our pest repellent formulations comply with the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA) Minimum Risk Exemption regulations in 40 CFR 152.25(f) and our pest repellent products are thereby exempt from EPA registration. We only sell our products in states where our pest repellents are fully compliant with state law. Currently, our pest repellents are not compliant for sale only in the state of Indiana.
We have independent testing conducted for all of our products with regard to the regulatory testing protocols. We work with five different testing facilities that each perform different tests on our products. All of the testing facilities are GMP certified.
Intellectual Property
The current patent portfolio covering our QS® Technology and the products we currently commercialize are entirely owned by us. We were granted utility patents in France, Germany, Mexico, Japan, United Kingdom, and the United States, and have pending patent applications in Australia, Europe, and the United States. The series of products marketed under Klēnskin SPF 30 WashOn Sunscreens have patent protection in all of the aforementioned countries until November 6, 2033, except for Mexico, which expires on January 31, 2026. Klēnskin SPF 50 lotion and SPF 30 stick products have patent protection in France, Germany, Japan, United Kingdom, and the United States until November 6, 2033 and pending applications in Europe and the United States. Klēnskin SPF 20 SunBar products have patent protection in Japan and the United States until November 6, 2033. Klēnskin SPF 50 lip balm products have patent protection in Japan until November 6, 2033 and applications pending in Europe and the United States. Klēnskin insect repellent stick products have patent protection in the United States until November 6, 2033.
The current trademark portfolio is also entirely owned by us. KLĒNSKIN is registered in Canada, China, Europe, Japan, Singapore, and the United States in international class (IC) 003 for non-medicated skincare preparations and sunscreen preparations and 005 for medicated sunscreen preparations and insect repellents (China, Singapore, and United States only). A Malaysian trademark application for KLĒNSKIN is currently pending. The QS® logo is registered in Europe, Japan, and the United States in IC 001 for cellulose in capsule form and QUANTASPHERE® is registered in the United States in IC 001 for cellulose in capsule form. WASH ON is registered in Japan and the United States in IC 003 for sunscreen preparations and SHOWER ON is registered in the United States in IC 003 for sunscreen preparations.
Business Structure and Operations
We are a mid-stage biotech company, having developed patented, tested formulations, and commercialized products since 2008. We have four full time employees and seven independent contractors. We also have contracted laboratories for development and testing, specialized attorneys, and manufacturing facilities to support our operations.
We believe our unique and scientifically advanced technology will make us a global leader in targeted epidermal delivery systems for cosmeceuticals, fragrance delivery, antibacterial/anti-viral sanitizers/cleaners, and most importantly, topical pharmacology.
We directly market select products through the internet and in dermatologists’ offices. Additionally, we develop marketing relationships whereby we supply formulatory guidance and base formulations directly to our partners for distribution. As a supplier, we sell ingredients and/or finished products into this expanding distribution channel. We have also expanded sunscreen and sanitizers/soap distribution for our Klēnskin™ products internationally.
Our QuantaSphere® (QS®) Technology formulations provide advanced proprietary, epidermal therapeutic effects that are unique and market disruptive. As such, our products are consumer friendly. QS® Technology blends consumer needs for a healthy lifestyle with our targeted, eco-friendly, multi-tasking product. Our innovative QS® Technology reduces the penetration of active ingredients into the skin and can be applied in a variety of applications.
We have shown, in our tested and commercialized sun protection factor (“SPF”) and moisturizing formulations, that they provide anti-aging and cosmetically elegant protection. Importantly, this is being validated under the most gruelling sun-sport applications. Our Klēnskin™ SPF sunscreen, has been the official sunscreen of both the AVP Beach Volleyball Series and the International FIVB - World Series of Beach Volleyball, Auto Club Speedway, and Talladega Superspeedway.
Distribution
We have been focused on our Intellectual Property (“IP”) research and development and have not had a major shift of our attention to the marketing of our products, which is the focus of our use of proceeds of this Offering. (See “Use of Proceeds – Marketing.”)
Historically, a significant portion of our sales have come from two customers. However, during the year ended December 31, 2021 and 2020, a majority of sales were to Advanced Dermatology Care Center, a dermatology practice owned by Dr. Laura Cohen, our Chief Executive Officer, and which were $19,000 and $21,000, respectively, accounting for 24% and 20% of our revenue, respectively.
Domestically, we have participated in sports marketing and marketing to physicians to gain insight into the consumer acceptance of our products. As a result of these efforts, both distributors and retailers who have been exposed to our products have sought to engage in the sales of our products. Internationally, we have an agreement with Spero from which all of our international sales have been derived to date. With this Offering and deploying our marketing plan (see “Description of Business —Marketing Direction”), we plan to reduce our reliance on our current major customers/distributors.
We have a Distribution Agreement with Spero for the distribution of our Klēnskin™ products in the territory of Singapore, Malaysia, Vietnam, and Sri Lanka (as used in this paragraph, the “territory”). The Distribution Agreement provides Spero with a limited-exclusive right to market, sell, and distribute to retail distributors in the territory and the right to appoint any sub-distributors, retailers, or dealers for the Klēnskin™ products in the territory. The Distribution Agreement requires that Spero purchase minimum quantities of the Klēnskin™ products every year, 50% of the payment due at the time of order and the final payment due upon notification that the order is available for shipment to Spero. However, due to COVID-19 pandemic restrictions, we did not ship any products internationally during the year ended December 31, 2020. However, we resumed our international shipping to Spero in the latter half of 2021 and are continuing such shipments currently.
Our Facilities and Manufacturing
We are a Nevada corporation, with our administrative offices located in Huntington Beach, California. Our CEO, President, and CFO operate from Huntington Beach, and with its inventory space, we are able to fulfill our current product orders.
We operate a product development facility in Sarasota, Florida for our development, product design, and formulation. Daniel Traynor, our Vice-President of Product Development works in this Sarasota laboratory facility.
We also utilize certain manufacturing facilities that are compliant with FDA manufacturing guidelines that receive the specified raw materials for the manufacturing process of our commercialized products. Certain lotions and Wash-On™ products are manufactured in Florida. Our new solid SPF 30 Sticks and flavorful SPF 50 lip balms were introduced into the market in mid-2019. These are produced with a third-party manufacturer.
We also have partnerships in Asia which are expanding in several countries with a focus on the distribution of our Wash-On™ sunscreens and insect repellent products including pipeline soaps, with both natural and chemical repellents. A new ingredient-based bar-soap is in development and formulated for manufacture in the U.S. and our Aquea Wash On™ is bottled and packaged in Singapore.
Manufacturing Process
Our product development research starts in our facility in Sarasota, Florida. Testing and production design including labels and packaging is then completed. Once this process has been finalized, we then schedule production. We work with approximately 15 suppliers from which we order our ingredients. All of the ingredients come with a Certificate of Analysis and a Safety Data Sheet. All of the manufacturers that we work with are manufacturing facilities that are compliant with FDA guidelines which are GMP compliant.
Our Klenskin products go through several steps in the manufacturing process. First, since these products require an encapsulation, this step is conducted separately from the rest of the manufacturing process at another GMP certified facility. Once the actives are encapsulated, they are then sent to another manufacturing facility, along with the other ingredients, for production. Once produced, the bulk formulation is then bottled, labelled, and packaged. Throughout this process, we conduct testing to ensure specification standards are met and, if an OTC, we test for microbes and actives.
The entire manufacturing process can range from one to three months depending on the product, availability of the raw materials, packaging, and the schedule of the manufacturer.
CoLabs Int’l, Corp.— Scientific Revolution for Better Global Health and a Cleaner and Safer Environment.
Our Place in Biotechnology
Like other biotech companies, we exist because large pharmaceutical companies are not as focused, responsive, and creatively productive as the smaller, more innovative, and defined biotech companies. Our innovations have developed advanced encapsulate technology and our ownership of intellectual property provides a novel enhanced delivery system for the skin. QS® Technology is applicable for FDA approved, advanced pharmaceutical drugs and use with EPA pest repellents, pesticides, and herbicides. We designed QS® Technology, to potentially reduce potential toxic side-effects from unwanted drug absorption. Thus, we fit into the modern concept of biotech companies producing benefits that accrue to the benefit of consumers’ health and lifestyle and environmental sustainability.
Our Founder
Laura Cohen, MD, is a Board-Certified Dermatologist, Dermatologic Surgeon, and Academic Medical Clinician, in practice in Southern California. Dr. Cohen has been in private practice for over 30 years. She was previously part of the medical school clinical faculty at the University of South Florida; the Clinical Faculty at the University of California Irvine; and was the medical staff at the Veterans Hospitals in Tampa, Florida; Las Vegas, Nevada; and Long Beach, California. She has served on the Keck (USC) – Hoag Hospital Melanoma Advisory Board, St. Joseph Dermatology Specialty Advisory Group, and was a founding physician at Moffett Cancer Center in Tampa, Florida. Dr. Cohen is also a Fellow with the American Academy of Dermatology. (See “Directors, Executive Officers, And Significant Employees.”)
Dr. Cohen realized the need for precision targeting of a drug and/or its delivery system during her years of professionally treating patients with various stages and types of skin cancer. When queried to see if they used preventive sunscreen protection, the answers varied from usually, occasionally, and seldom or never. Her follow up question was, “Do you bathe?” which always resulted in an affirmative answer. Reasons for not wearing sunscreen are well documented in various marketing/research studies. Sunscreens are most typically perceived by consumers as sticky or tacky, inconvenient to use in their daily routine, irritating to the eyes, creating allergic reactions, collecting dust on the skin, and simply viewed as not needed, detrimental, or toxic. Dr. Cohen recognized the failure in the delivery system of sunscreens and sought to address this serious issue as well as the unintended drifting of topically applied chemicals and pharmaceuticals.
Unique Features of Klēnskin™ Sunscreen
Most consumers are unaware that the World Health Organization (WHO) places the sun’s UV radiation in the same category as smoking and plutonium in terms of dangerous cancer-causing sources. Sadly, most patients with skin cancers and severe skin aging, did not realize that regular use of sunscreen could have prevented or reduced the damage. Further, over 80% of sun damage is caused by daily incidental sun exposure. It was clear to Dr. Cohen and Lisa LeBlanc that a novel, new type of delivery system was needed. They concluded that a wet skin application sunscreen could become a major tool in preventing skin cancer caused by UV radiation exposure.
In 2008, Dr. Cohen and Ms. LeBlanc formed our company with a clear direction and scientific focus to develop a precise, user friendly, topical delivery system. Combining Dr. Cohen’s knowledge of skin pharmaceuticals and cosmetic chemistry with Ms. LeBlanc’s profound commitment to improved global health led to exhaustive research into polymer-based formulations that could be utilized for dermatologic applications. Together, they developed a Wash-On® SPF test of concept product which could be applied in a shower or used at the sink to provide sunscreen skin protection while performing routine daily hygiene as a wet skin application. The initial products were the Klēnskin™ Wash On® SPF 15 sunscreens, which were tested, commercialized, and dispensed through dermatologists’ offices.
The scientific significance of our advanced formulations is that the active ingredients are functionally designed to remain at the treatment site with only limited drifting and absorption. Importantly, this innovation reduces absorption systemically of drugs. In the case of sunscreens, this means that photoreaction takes place on the surface of the skin rather than in the deeper living layer of epidermis. CoLabs’ Klenskin formulations also have the active ingredients supplemented by antioxidants, which quench free radicals produced by the reaction of the chemical sunscreens and UV radiation, and also moisturizing agents, which further enhance the health of the skin.
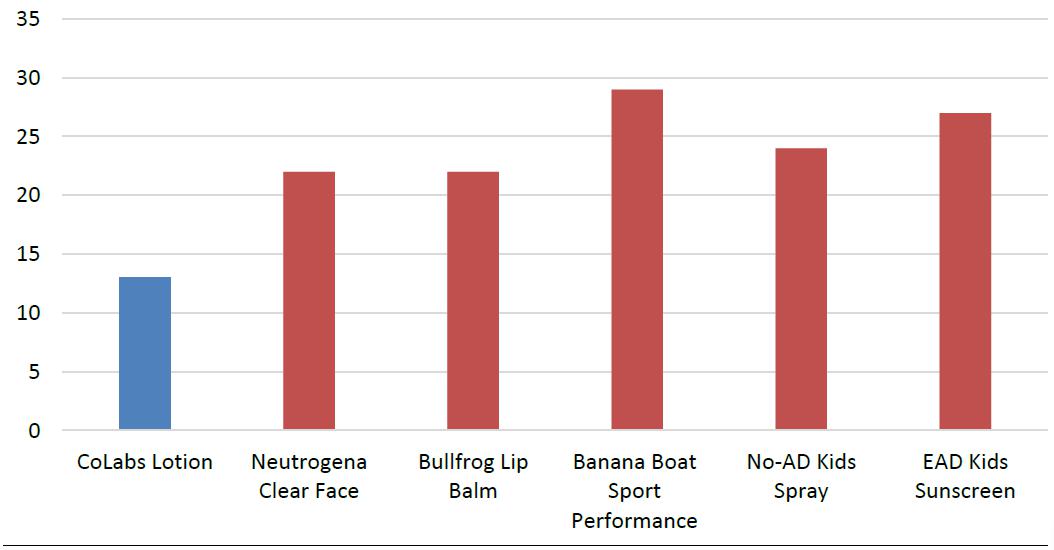
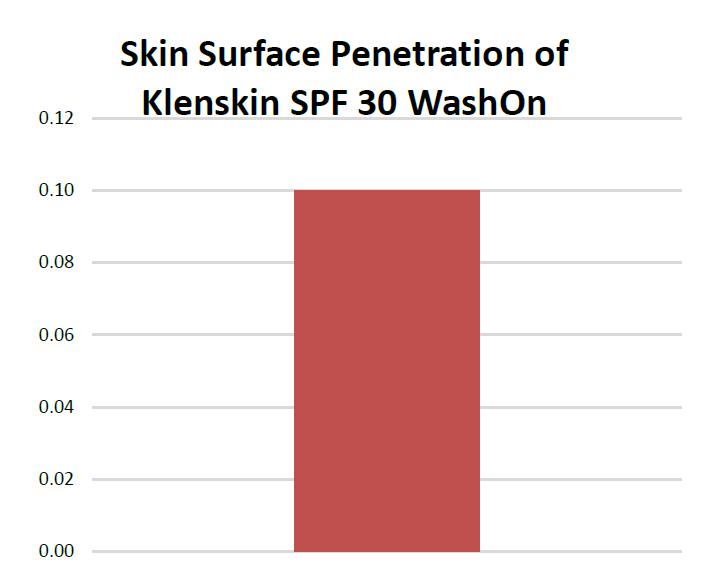
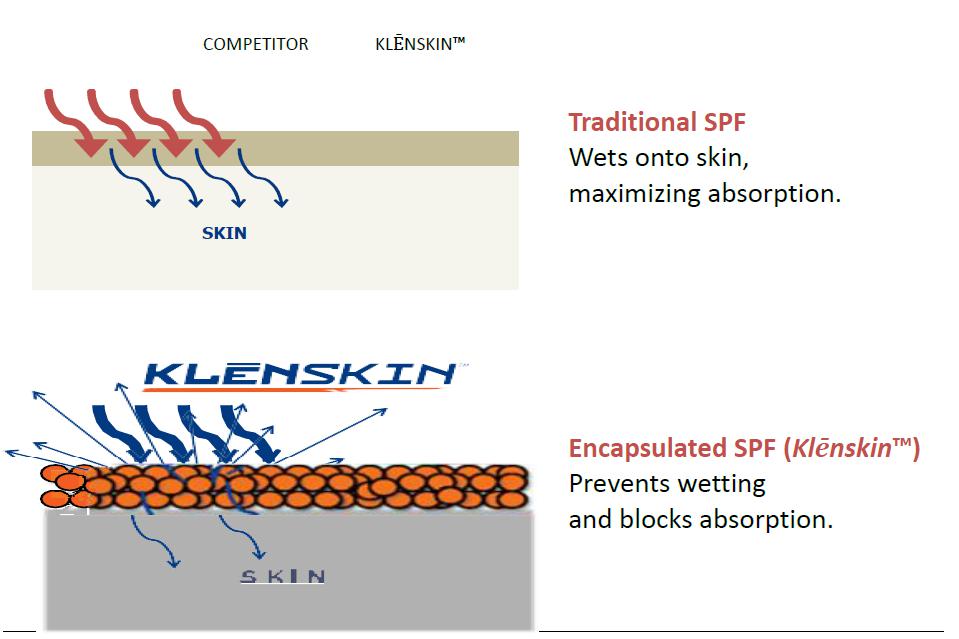
We intend to capture a strong position in the biotechnology sector with the ongoing development of our innovative formulations and potentially with licensing our technology to major pharmaceutical companies. QS® Technology is directly applicable to the commercialization for many approved products for skin cancer prevention and treatments, pest repellents, fragrances, sanitizing products, sun protection, and topical dermatologic treatments. By introducing these agents into our scientifically unique application and delivery system, we believe these products will become more efficacious and cosmetically elegant for consumers. (See “Description of Business – Targeted Biotech Industry Sectors.”)
Strategic Direction
Our innovative QS® Technology provides non-transdermal delivery and is technologically adaptive. We are taking advantage of our presence in this unique space, utilizing our existing reputation, existing IP, and design capacity in order to formulate specific versions of our QuantaSphere® Technology for use in major health and beauty market sectors. We are currently expanding our research to include the adaptation of our QS® to agrochemicals.
We have provided major pharmaceutical companies with sample formulations for testing and evaluation with the purpose of inclusion of our technology into their next generation of products. We believe that in the long-term, this strategy will produce marketing relationships with strong operating cash flows and expanding market share across multiple market sectors. We are also focused on applying our rapidly evolving biotech delivery technology to key global market sectors both directly and with marketing partners. Our marketing strategy seeks to use regionally defined marketing alliances thereby increasing the potential sales for each targeted market sector. As a result of these efforts, we are currently negotiating a licensing agreement with a pharmaceutical company to manufacture their formulations with our adapted QS® technology; provided however that there can be no assurance that we will ultimately enter into that agreement.
We are exploring new IP relating to the adaptation of encapsulates into a cross-linked mesh formation. Additional research is being conducted to develop new encapsulates that are application specific. We believe that our encapsulates can offer a better regulated and safer delivery system for both OTC and pharmaceutical drugs.
Our pest repellent and herbicide encapsulates are in the formulation and testing phase and based on the success of the tests, will be moved into a registration phase or be reformulated to improve performance. Initial test results have been encouraging for these natural, environmentally sustainable agricultural products.
Critical Success Factors
To understand what it will take to make us successful and accelerate us to a world-class leadership position in the biotechnology and pharmacology industrial sectors, we have examined our proposal from three perspectives:
| | 1. | Determine if our science and consumer acceptance intersect to the extent that growing demand will provide strong dynamics to fuel rapid sales and product expansion. |
| | 2. | Determine where in the product life cycle our products and technology reside vis-à-vis current and future competition. |
| | 3. | Determine whether funding will provide management the necessary components to propel our success in the global market. |
Our success is dictated by the benefits provided to the consumer in the form of advanced and properly targeted formulation designs and the sale and profitability accrued to those in our distribution chain.
Critical Success Factors: Creating Demand
There are important factors that dictate and drive the demand for our products and technologies. These factors, which are vital to our business plan, can be summarized as follows:
| | ● | growth of global consumer demand for competitively priced goods; |
| | ● | developing economies’ needs for scientifically advanced products; |
| | ● | growing economic strength throughout the global marketplace; |
| | ● | growth of regional trade agreements; |
| | ● | worldwide healthcare threats and concerns; |
| | ● | global need for enhancements to preventive healthcare products; |
| | ● | consumer focus on personal care and youthful appearance; |
| | ● | increased consumer awareness of advanced and innovative technologies; and |
| | ● | regulatory compliance. |
Critical Success Factors: Product Life Cycle
The product life cycle is divided into four segments: introduction, growth, maturity, and decline.
| | 1. | Introduction: Relatively slow growth and limited profits. |
| | 2. | Growth: Rapid expansion of sales and consumer acceptance with resulting high profitability. |
| | 3. | Maturity: No major sales growth with high profits but slowing profits. |
| | 4. | Decline: Eroding markets and declining sales volume with loss of profit margins. |
We believe that the ongoing expansion of our targeted market sectors are being fueled by worldwide economic growth, consumer demands, and complex health threats. As economies and healthful practices expand, we believe that consumers will focus on products that address the various risks to their own personal and family care needs and health. Pricing, distribution, and technology are driving the healthcare market expansion. Knowledge of advanced, powerful new technologies by both distributors and consumers provides the fuel for new product sales thereby replacing older products based on outdated technology.
As such, we consider our products to have biotechnology that is in the very early stages of the product growth cycle. We hope to achieve prominence in markets needing enhancements to products that our IP brings by introducing a new generation of products of Quantasphere Technology™ based derivative products. (See “Description of Business -- Product Pipeline.”)
Critical Success Factors: Components to Success
If we achieve high levels of satisfaction from our consumers and distributors with our formulations and products, we believe it will create an influx of new customers. This customer base, in turn, will fuel the expansion of distribution channels. We believe that the repeat consumer purchase cycle in combination with cost efficient distribution alliances are capable of creating overall profits combined with the valuable intrinsic satisfaction of the consumer base.
The following major factors will determine whether we will be successful:
| | ● | ability to develop effective distribution partnerships to present and market products to consumers in vast regional markets; |
| | ● | having the ability to continually satisfy and perpetuate a loyal consumer base; |
| | ● | continued growth of product sales to global markets and the expansion of the new product pipeline for new market sectors; |
| | ● | preemptive entry of advanced product technologies into the marketplace before competitors are able to scientifically compete; |
| | ● | having a focused and defined marketing plan, specifically designed for each targeted market, and having a capable marketing staff that is knowledgeable and capable to execute the plans; |
| | ● | concentrating and developing our expertise while keeping our team directed and focused; |
| | ● | rapidly developing new technologies and formulations with the related intellectual property, through efficiencies achieved by a focused staff and limited regulatory delays; and |
| | ● | adaptive use of new marketing technologies and mediums. |
Growth Strategy
Our growth strategy is to increase revenue and net income by expanding the worldwide demand for our products, formulations, and technology. The avenues to achieve success rest on the fundamentals of our patents and wide applications of our intellectual property. Further, as described in “Strategies for Advancing Market Penetration,” developing these various sales and funding channels are key to establishing a market presence with corresponding revenue growth.
Enhancement of Product Performance
We are positioned in several key large and expanding market sectors. For example, as reported in Zippia in December 19, 2021, the cosmetic market in 2021 was forecasted to have global value of $380 billion. This is our largest targeted sector for licensing agreements. Cosmetic products (skin care) inherently have numerous applications for our QS® formulations. Anti-aging and moisturization in combination with cosmetic elegance are key basic characteristics found in most cosmetic products. The actual active ingredients for these products do vary greatly. However, skin or revitalizing serums perform best when retained on the surface of the skin or time-released into the epidermis. We believe our formulations achieve these goals to the greatest extent available in today’s market. For example, after testing by a major cosmetics partner, our moisturizing formulation provided a high level of skin moisturization and the skin felt to the touch more moisturized when we compared it to competitive products. This result was because of the partial retention of the actives on the surface of the skin due to our formulation.
Leverage the Technology to Provide Value-Added Ingredient Base
We believe that current cosmetics, pest repellents, fragrances, sun care, and soap products ingredients move a generational level higher in performance when the QS® delivery system is employed. This is our competitive strength, which we believe is well suited for licensing agreement. We believe that our goal of enhancing value will be realized by using a combination of joint ventures, and licensing and distribution agreements.
Advanced Product Design
Our formulations and QS® provide innovative, targeted delivery for FDA designated over-the-counter (“OTC”) drugs and other highly effective skin products. Many medications exist that treat and/or ameliorate health symptoms.
Our QuantaSphere® delivery system for medications can be formulated to have characteristics to increase duration, have the benefits of fewer active ingredients, have less toxic risk from medications, and greater cosmetic elegance. We are looking to advance our pipeline with the appropriate related testing protocols, and the targeted outcome of producing finished commercializing and distributing products into appropriate markets. (See “Description of Business – Targeted Biotech Industry Sectors.”)
Current Products
Our current product portfolio is as follows:
| | ● | SPF 50 Lotion – Water Resistant to 80 Minutes |
| | ● | SPF 30 Wash On™ - Wet Skin Application (Three Variations) |
| | ● | SPF 50 Lip Balm - Reef Safe (Four Flavors) |
| | ● | SPF 30 Stick - Reef Safe |
| | ● | SPF 30 Aquea Wash On™ (International) |
| | ● | SPF 30 Gel Water Resistant 80 Minutes (Reef Safe) |
| | ● | Spray Hand Sanitizer |
| | ● | Gel Pump Sanitizer |
| | ● | Bug Stick Repellent |
Product Pipeline
We have an extensive pipeline of new products that are being developed for the Klēnskin™ brand of sunscreens and bug repellents as well as for private branding.
New Products in 2022 and 2023:
Naturally Absorbent Encapsulate (application for use to wick moisture from the skin):
| | ● | We have applied for a patent for a new technology for the safe absorption (wicking) of moisture from the skin. We plan to design the formulation and continue the testing process on this technology. |
| | ● | Successful commercialization of this “wicking” technology at this early stage of this development is undeterminable. |
Medical Encapsulated Cross-Linked Mesh
| | ● | In Development- Advanced encapsulation of drugs incorporating cross-linked mesh, potentially providing a safer and more regulated release of drugs for transdermal delivery applications. |
Agricultural and Veterinary Technology
Initial agrochemicals and formulations for veterinary use which include natural herbicides, pest repellents, and fertilizer prototypes, are expected in 2022 for testing with anticipated product launch in 2023.
Bug Repellents:
| | ● | Bug Repellent Roll-On and Spray Application (Ready for Production) Mosquito and Tick |
| | ● | Bed Bug Powder (In Testing) (Household) |
| | ● | Equine Fly Spray (In Testing) |
| | ● | Porch and Turf Cleaner and Fly Repellent (Testing) |
| | ● | Pet Products – Flea and Tick Line (In Development) |
Sunscreens:
| | ● | Reef Safe SPF 30 Gel |
| | ● | Reef Safe Whipped Zinc Face Cream SPF 35 (Finalizing Production Samples) |
| | ● | Reef Safe SPF 40 Body Oil |
Cosmetics:
| | ● | Phospholipid Complex Rejuvenating Cream (Ready for Production with Pending Licensing Agreement) |
Product Development
We currently meet our R&D needs internally with our existing staff combined with contracted laboratories to assist with additional testing and development. All critical scientific and product development, formulations, and design specifications are done internally and with our contracted labs for advanced development. Product development that provides needed and market accepted formulations is fundamental and inexplicably linked to positive consumer acceptance, brand recognition, and sales growth. We believe we are developing products that meet those criteria.
Unlike many other biotech companies, the time it takes for us to develop, formulate, test, and commercialize a product, is completed in a comparatively short span of time at a substantially lower cost. We have avoided debt and dilution by concentrating our focus on already approved OTC products, which have large markets, and require few regulatory filings.
Raw material components and advanced ingredients, regulatory advice, intellectual property registration, and legal support are obtained from outside sources.
Our management is engaged in all functions critical to our operations. We believe that our skilled management team provides a focused, high quality product, with cost controls, improved manufacturing capacity utilization, defined operational control, and tight product ingredient specifications which are confirmed by outside testing. This testing is done by independent FDA and EPA testing laboratories which follow approved guidelines for each targeted test.
Our internal R&D and Product Development groups and external partners are experienced and highly respected in the industry. Further, their unique focused knowledge in our realm of technology is a significant asset and a competitive advantage for us.
We have developed IP protected biotechnology designed for multiple new applications. This IP is technology driven, tested, proven, formidable, and commercialized. We currently hold 28 issued patents and have an additional 16 patents pending.
Our internal management and scientific team is augmented by partnerships which externally provide both material and development support. We are able to directly advance our research and manufacturing skills, which are then augmented by our partners’ scientific and production teams.
New formulations are repeatedly tested by independent labs that are compliant with FDA guidelines. Testing is done during the development, pre-production, and manufacturing stages. By maximizing our leverage with suppliers and manufacturing partnerships, we have held personnel costs to an efficient level while rapidly advancing our technological growth.
Our topical delivery system, IP, and internal efficiencies allow our focus to remain directed at the development of formulations for vast commercial applications.
FDA: Maximum Usage Trials (MUsT) for Topical Active Ingredients
Issue Background and a CoLabs Proposed Testing Protocol Standard for the FDA
We believe that the absorption and systemic effects of active ingredients for many topically applied OTC products is a major focus of concern. Currently, topically applied active ingredients can be absorbed readily into the body. QS® Technology formulations can be designed to be minimally absorbed over-time or have virtually no absorption through the stratum corneum. It is important to first identify the degree of surface absorption by focusing on the absorption characteristics of a particular active and/or its excipients within its delivery system. As with any test, a time stamped testing protocol must be followed to determine the rate at which the product enters the skin and what depth of penetration occurs.
We have sought to create the most user-friendly sunscreen possible by encapsulating the active ingredients and placing an electrostatic charge on the surface of the encapsulate. The cationic electrostatic charge creates an attraction to the anionic stratum corneum. This charged micro-encapsulated formulation is designed to create an attraction to the surface of the skin and inhibit absorption.
With this in mind, non-absorption was one of our key concerns for three main reasons:
| | 1. | Systemic absorption and accumulation of sunscreen chemicals in the body may have unwanted effects; |
| | 2. | Scientific concerns that absorbed chemical sunscreens produce a photoreaction within the skin which releases free radicals and could be damaging to living (DNA containing) epidermal cells; and |
| | 3. | Absorption of sunscreen chemicals is potentially systemically toxic. |
Our encapsulates are specifically manufactured to be approximately the size of a skin cell. We were confident that absorption through the epidermis is greatly inhibited by this specification. Additionally, we place an electrostatic charge on the surface of the encaps which causes the sunscreen encapsulate to attach to the stratum corneum. This unique design combination limits infiltration into the skin and possible systemic absorption. We sought to prove our scientific premise using the Confocal Stain Test (“CST”).
We proposed to the FDA that the CST be used as a methodology for a MUsT protocol for the following reasons:
| | ● | If a product is intended to stay on the surface of the skin, it is an easy testing protocol that could eliminate the need for blood or urine tests to detect unwanted chemicals introduced to the body via topical application; and |
| | ● | There are concerns regarding the accuracy of blood or urine tests because of the possibility that unwanted chemicals may be stored in the body, such as in adipose tissue or the liver. |
We have not received a direct response from the FDA. However, the FDA has conducted tests based upon the comments we previously supplied to them. We look forward to the opportunity to working with the FDA in the future to better control potential issues created by the absorption of topically applied drugs and chemicals.
Marketing Direction
We have developed a systematic long-term strategy to expand our technology, target product markets, and broaden our in-house capabilities.
We believe that we, in combination with our marketing partners, have the opportunity to become a significant force extending into a worldwide market that exceeds $380 billion as reported in Zippia (December 19, 2021). These expanding group of partnerships include pharmacy purchasing groups, retail stores, outdoor sports stores, medical practice marketers, and national and international sports organizations. We anticipate that these key market sectors, which includes the cosmetics, pest repellents, fragrances, sunscreens, and topical pharmacology markets to grow significantly over the coming years. More specifically, we have substantial scientific knowledge, expertise, and years of experience in these markets and applications.
We currently own issued and filed intellectual property addressing the next generation of product innovations that are directed at both consumers and the major industry suppliers for these global market sectors. We believe that the technology we have developed is both novel and scientifically advanced. Once adapted into products, our formulations arguably set an improved standard.
Strategies for Advancing Market Penetration
Direct Retail Distribution – We plan to expand our direct retail wholesale personnel in order to target distributers and retailers with our product lines. By servicing B2B accounts with internal and detail personnel, we can expand relationships and provide better hands-on support to retail outlets thereby encouraging their retail sales.
Distribution Partnerships – We will provide either Klēnskin™ branded products, base formulations, exclusive product formulations, or private label existing formulations to partners in order for them to market to their existing clientele.
Direct Consumer Marketing – We have been approached and have had discussions with television direct marketing groups. These discussions involved both informative commercials as well as shopping channel marketing. Typically, a celebrity or professional spokesperson is chosen to represent a company and its product lines. The cost of production, inventory, and the talent purchase are considerations weighed against the potential large volume of sales and rapid branding that occurs. We are currently considering expanding into this marketing channel.
Government Sales – Klēnskin™ products offer a generational advance in sanitizers, sunscreens, and bug repellents. These products have great potential to protect those government employees whose jobs require them performing their duties in contaminated environments and outdoors with exposure to the sun’s radiation. We intend for these products to be initially directed to military services and related job areas where these products may excel. We are in discussions with a proven government supplier who is assisting us in meeting the requirements to provide our products to agencies requesting bids for products whose specifications match Klēnskin™ products.
Global Sales – We have greatly expanded our presence in the Asian markets. We believe that targeting products designed for the tastes in this region will propel current relationships and can expand into other distribution companies. Increased marketing presence with experienced personnel, advertising, and new region-specific product offerings are key drivers to sales. We believe that the initial international market acceptance of our products has been exceptional. This is a targeted marketing area that is slated for expansion.
Internet Sales – One of our main goals is to expand sales to targeted consumers and affiliations with key online marketing sites. The priority of our internet sales and marketing staff will be to seek greater social media exposure linked to the activation of target marketing campaigns. The full engagement of social media marketing component can directly drive sales for company ecommerce, bloggers, social media exposure, lead generation, targeted promotions, and select marketing sites like Amazon. We expect this will be an important key element for new product launches.
Event Participation – As previously noted, our products are designed for active lifestyles. Branding thus far has associated Klēnskin™ with AVP Professional Beach Volleyball and NASCAR as well as prominent cancer fighting organizations and youth and college sports groups. These brands and their events and causes will be linked by our public relations firm, Brand Knew, to campaigns to develop name recognition for both Klenskin and CoLabs. One of our management principles is to support and be a part of the community to improve our health, environment, and quality of life. Our products are designed with safety as well as these societal benefits inherent in all of our formulations. We have successfully offset some of the marketing costs with sales at the event site. We have found that these fan sales are important since these purchasers have become repeat buyers. In sharing their satisfaction with our products, they become Klēnskin™ sales ambassadors to their social network.
SPIR/STTP Grants and SBA Programs – We anticipate submitting grant applications to various U.S. government agencies. These funding opportunities are designed for small businesses to provide assistance with needed product development and research prior to the products commercialization stage. SPIR/STTP Grants are not repayable and are offered in two phases. Phase 1 offers up to $225,000 in grants. Approximately 30 to 40% of applicants receive funding. Phase 2 provides up to an additional $1.5 million for product development. The SBIR/STTR programs are administered by the Small Business Administration (SBA) and processes through various government agencies. Additional SBA loan programs are being implemented to provide assistance during times of economic stress. We are hopeful that successful loan and grant applications for available SBA programs will assist with the development of several advanced products in the pipeline and provide funding for corporate operations and payroll, thereby helping assure our ongoing viability, however there can be no assurance that we will receive any funding through these programs.
Sales and Marketing Staff – We believe that it essential for our success to retain a qualified marketing team to advance sales. The development of partnerships, the expansion of internet presence, and the development of new domestic and global distribution channels, along with sufficient financial resources, are necessary for an effective campaign. Brand Ambassadors, supporting television and radio advertising linked to event sponsorship will be strategically activated to promote our participation and branding. We recognize that a well engaged and effective staff is vital because of their commitment to garnering sales and their experience to promote their existing relations for the benefit of our product lines. To that extent, we have retained retail sales brokers and consultants to assist with marketing development with retail chains, online stores, and other internet marketing sites.
Property
We currently do not own any real property. We lease office space and warehouse and product development spaces at:
18593 Main St.
Huntington Beach, CA 92648
7222 South Tamiami Trail
Sarasota, FL 34231
7251 W. Lake Mead Blvd., Ste 300
Las Vegas, NV 89128
On June 16, 2017, we entered into a First Amendment to Lease Agreement with Sarm Five Points Plaza, LLC for our Huntington Beach office for a term of five years beginning on November 1, 2016 and expiring on October 31, 2021, and we are currently on a month to month lease. Our current monthly rent is $2,956.73.
On December 1, 2017, we entered into a Commercial Lease with Branson Corp. for our Florida office for a one year term beginning on December 1, 2017, with an option to extend for subsequent one year periods. Our current monthly rent is $820.20.
We lease, on a month-to-month basis, a virtual office in Nevada in compliance with being incorporated in Nevada. The monthly rent amount is nominal.
ITEM 2. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion and analysis of our financial condition and results of operations should be read together with our financial statements and related notes appearing at the end of this Semiannual Report. This discussion and analysis contains forward-looking statements reflecting our current expectations that involve risks and uncertainties. Actual results and the timing of events may differ materially from those contained in these forward-looking statements due to a number of factors, including those discussed in the section entitled “Risk Factors” as disclosed in our Offering Circular, as amended or supplemented from time to time, and as may be updated from time to time by our future filings under Regulation A. The accompanying balance sheets and the related statements of operations, shareholders’ equity and cash flows as of December 31, 2021 and for the year ended December 31, 2021 and December 31, 2020 are audited.
Overview
CoLabs Int’l, Corp. (“we,” “us,” and “our”) was incorporated in Nevada in 2008 and is a closely held, majority woman-owned biotechnology company. Our corporate administrative office and warehouse facility are located in Huntington Beach, California.
We were founded upon our acquired and internally developed intellectual property on our belief that our intellectual property provides a commercial advantage in the global marketplace. We rely on patent, trade secret, copyright, and trademark law, as well as the contractual terms with our customers, to define, maintain, and enforce our intellectual property rights, technologies, and relationships.
We currently have 28 issued patents and 16 filed pending patents covering our technology. In addition, a significant amount of our intellectual property takes the form of trade secrets and copyrighted works of authorship. We currently have a portfolio of registered and pending trademarks in the United States and foreign jurisdictions, including registrations for the marks “Quantasphere®,” “QS®,” “KLĒNSKIN®,” “WASH ON®,” “KLĒN®,” AQUEA®, and “SHOWER ON®.”
Our patented Quantasphere® technology is a globally unique, widely adaptable delivery system providing improved effectiveness and consumer acceptance for a multitude of drugs, cosmetics, and chemical applications. Our technology places drugs into micro-sized encapsulates that have an electrostatic charge and then suspends these into unique formulations, limiting the unwanted absorption, or having a timed release. This one-of-a-kind unique delivery system significantly decreases the absorption of potentially dangerous chemicals into the body and organ systems, thereby avoiding potential toxic side-effects. This technology is also able to target the depth of penetration and the degree of dispersion or timed release of the active ingredients into the skin or onto the surface of the skin.
We are presently selling sunscreens, SPF lip balms, and bug repellent products, utilizing our patented Quantasphere® technology, to the consumer market under the KLĒNSKIN® brand name, with plans to expand our product lines to multiple additional markets, including dermatology, cosmetics, fragrances, and other therapeutic applications.
We amended our Articles of Incorporation on December 1, 2021, to conduct a forward stock split of 2:1 of our common stock (the “Forward Split).
Operating Results
Results of Operations for the Year Ended December 31, 2021 Compared to the Year Ended December 31, 2020
Refer to our Statements of Operations in our financial statements.
Revenue
Revenue decreased 24% to $80,000 during the year ended December 31, 2021, compared to $105,000 during the year ended December 31, 2020. The decrease in net sales of $25,000 was primarily due to the timing of orders from our international distributor.
Cost of Revenue
Cost of revenue primarily represents our material cost for manufacturing our products sold and changes in inventory reserves for slow-moving or potentially obsolete products. Our cost of revenue increased by $82,000 to $143,000 for the year ended December 31, 2021, compared to $61,000 for the year ended December 31, 2020. Our gross margin was a negative 79% for the year ended December 31, 2021, and 42% for the year ended December 31, 2020. The decrease in gross margin primarily due to inventory write downs, changes in inventory reserves, and change in product mix sold during the year ended December 31, 2021, compared to the year ended December 31, 2020.
Operating Expenses
Operating expenses include selling, general and administrative expenses, and research and development costs.
Our selling, general and administrative expenses increased approximately $284,000 to $1,022,000 during the year ended December 31, 2021, compared to $738,000 during the year ended December 31, 2020. The increase in selling, general and administrative expenses was from increased marketing related expenses of $146,000 and increased professional fees of $74,000. The remaining increase of $64,000 was from an increase in payroll related costs and routine changes in our selling, general and administrative expenses accounts to support our operations.
Research and development costs include employee, rent, advisors, consultants, product design, and development activity. Research and development expenses increased $54,000 to $181,000 during the year ended December 31, 2021 compared to $127,000 during the year ended December 31, 2020. The increase was primarily from increased payroll related costs.
Loss from Operations
Loss from operations increased $445,000 to $1,266,000 for the year ended December 31, 2022, compared to $821,000 for the year ended December 31, 2021. The increase in operating loss was due to our decreased gross profit and increased operating costs, as discussed above.
Gain on Forgiveness of Paycheck Protection Program Loans
During the year ended December 31, 2021, we received formal notice that our notes payable were forgiven. As a result, the gain from the forgiveness of the government assistance notes payable aggregating $110,000 was recognized in the statement of operations during the year ended December 31, 2021.
Interest Expense
Interest expense decreased to $1,000 for the year ended December 31, 2021 compared to $5,000 for the year ended December 31, 2020. The decrease in interest expense was due to the changes in our debt levels.
Net Loss
Net loss increased by $331,000 to $1,157,000 for the year ended December 31, 2021 compared to $826,000 for the year ended December 31, 2020. The increase in net loss was due to our decreased gross profit and increased operating costs, offset by a gain on forgiveness of Paycheck Protection Program loans, and decreased interest expense, as discussed above.
Liquidity and Capital Resources
Our financial statements have been prepared assuming that we will continue as a going concern, which contemplates continuity of operations, generation of sales revenue, realization of assets, and liquidation of liabilities in the normal course of business. As reflected in the financial statements, during the year ended December 31, 2021, we incurred a net loss of $1,157,000; used cash in operations of $1,198,000; and had a stockholders’ deficit of $12,000 as of December 31, 2021. These factors raise substantial doubt about our ability to continue as a going concern as a biotechnology company within one year after the date that the financial statements are issued without a further influx of equity capital, growth of sales, issuance of debt, or a combination of these capital inflows. The financial statements do not include any adjustments related to the recoverability and classification of recorded asset amounts or the amounts and classification of liabilities that might be necessary should we be unable to continue as a going concern.
In addition, our independent registered public accounting firm, in its report on our December 31, 2021 financial statements, has raised substantial doubt about our ability to continue as a going concern.
At December 31, 2021, we had cash on hand of $68,000. During the year ended December 31, 2021, we received $882,000 through the sales of our common stock, $105,000 in proceeds through the exercise of stock options, and proceeds of $49,000 from a loan. Our ability to continue as a going concern is and has been dependent on our ability to execute our strategy, raise additional funds, and expand revenue from sales. Management is currently seeking additional operating funds, primarily through the issuance of equity securities to expand marketing and overall business development. No assurance can be given that any future financing will be successful or the funding will be adequate to achieve our goals. Even if we can obtain additional financing, it may place restrictions on management. Substantial dilution for stockholders may occur from the structuring of either equity or debt financing.
Sources of Operating Revenues and Cash Flows
Refer to our Statements of Cash Flows in our financial statements and Note 2, Summary of Significant Accounting Policies, in our financial statements for further detail.
Cash Flows from Operating Assets
Net cash used in operating activities for the year ended December 31, 2021 totalled $1,198,000, compared to net cash used in operating activities for the year ended December 31, 2020 of $909,000. The increase in net cash used in operations for the year ended December 31, 2021 was primarily to fund our net loss, offset by $110,000 gain on forgiveness of Paycheck Protection Program loans.
Cash Flows from Financing Activities
Net cash provided by financing activities for the year ended December 31, 2021 was $1,036,000, which included proceeds of $105,000 from the exercise of stock options, proceeds of $882,000 received from a private placement offering of common stock, and proceeds of $49,000 received from a note payable. Net cash provided by financing activities for the year ended December 31, 2020 was $792,000, which included proceeds of $3,000 from the exercise of stock options, proceeds of $728,000 received from a private placement offering of common stock, and proceeds of $103,000 received from loans payable, offset by a $42,000 repayment of notes payable.
Outlook and Recent Trends
There are a number of trends potentially affecting our business. Both the macroeconomic and microeconomic outlook are being considered by our management team. These seemingly are impacted by public policy and the private sector response to economic packages. The COVID-19 impact of our domestic and global economies is still being addressed differently by various regulatory agencies and governments as well as the private sector.
Focusing on the U.S. economy, in the near-term, programs which will assist the country in building its infrastructure are expected to be introduced as well as further stimulus packages. The national debt has increased and may continue to increase, perhaps a catalyst for some inflation. Correspondingly, taxes are expected to increase to try to offset this deficit spending. Without an improved economy, credit risk and low credit demand will restrict expansion. Adding to this headwind is a likely decline in GDP growth. Offsetting this Keynesian solution is the pandemic and its related costs and undefinable course.
We are in the pharmaceutical/healthcare sector which due to the pandemic and the aging of baby boomers may continue to trend as a key growth area. We are focused on the delivery of drugs in multiple key sectors, including vector borne diseases, disinfectants, sun protections, anti-aging, pest repellents and anti-scabetic and anti-pediculosis drugs. We believe that our sanguine position addresses these massive focal point health issues. It is incumbent on our management to be proactive, intuitive, and farsighted to adjust to the quickly shifting sands that these uncertain economic events are presenting.
Critical Accounting Policies
See Note 2, Summary of Significant Accounting Policies, in our financial statements for further detail.
Off-Balance Sheet Arrangements
As of December 31, 2021 and December 31, 2020, we had no off-balance sheet arrangements.
Related Party Arrangements
For further information regarding Related Party Arrangements, please see Note 7 in the accompanying financial statements.
Recent Developments
In August 2021, we received notice from our SBA lender that our PPP loans in the aggregate amount of $110,000 were forgiven and no amounts were owed under the agreements (see Note 4 in our financial statements).
In August 2021, we began an offering under Rule 506(c) of Regulation D for the sale of our common stock at a price of $5.50 per share for a maximum offering of 3,000,000 shares for $16,500,000. After the Forward Split, the price per share of that offering decreased to $2.75. As of the date of this report, we have sold approximately 389,000 shares for $1,072,750.
On April 30, 2021, we began an offering pursuant to Regulation A of Section 3(6) of the Securities Act of 1933 for Tier 2 offerings, pursuant to which we began offering up to 3,000,000 shares of Common Stock at an offering price of $6.50 per share for gross proceeds of up to $19,500,000. After the Forward Split, the price per share of that offering decreased to $3.25. As of the date of this report, the Reg A+ offering was terminated. While marketing the Reg A+ we sold 5,500 shares for $18,875.
ITEM 3. DIRECTORS AND OFFICERS
| Name | | Position | | Age | | | Term of Office | |
| Executive Officers and Significant Employees: | | | | | | |
| Laura Cohen | | Chief Executive Officer | | 71 | | | 2008 - Present | |
| Lisa LeBlanc | | President | | 39 | | | 2008 - Present | |
| Daniel Traynor | | Vice President of Product Development | | 52 | | | 2017 - Present | |
| William Cohen | | Chief Financial Officer | | 76 | | | 2022- Present | |
| | | | | | | | | |
| Directors: | | | | | | | | |
| Laura Cohen, MD | | Chairman of the Board | | 71 | | | 2008 - Present | |
| Lisa LeBlanc | | Director | | 39 | | | 2008 - Present | |
| Charles Fritts, Esq. | | Independent Director | | 69 | | | 2017 - Present | |
| Dean Stathakis, Esq. | | Independent Director | | 59 | | | 2017 - Present | |
| Peter Barton Hutt, Esq. | | Independent Director | | 87 | | | 2017 - Present | |
There is no arrangement or understanding between the persons described above and any other person pursuant to which the person was selected to his or her office or position.
Family Relationships
Laura Cohen, our CEO, is the mother of Lisa LeBlanc, our President. Laura Cohen is married to William Cohen, our CFO. Aside from the aforementioned, there are no other family relationships between any other director, executive officer, person nominated or chosen by us to become a director or executive officer, or any other significant employee.
Certain Relationships
Except as set forth above and in our discussion below in “Interest of Management and Others in Certain Transactions,” none of our directors or executive officers have been involved in any transactions with us or any of our directors, executive officers, affiliates, or associates which are required to be disclosed pursuant to the rules and regulations of the SEC.
Business Experience:
Laura Cohen, MD, is a Board-Certified Dermatologist, Dermatologic Surgeon and Academic Medical Clinician, in practice in Southern California. Dr. Cohen is skilled, experienced, and knowledgeable concerning dermatological conditions, prevention, and formulation of treatment and prevention products. She received her medical degree from George Washington University School of Medicine and completed her residency in Dermatology at Indiana University School of Medicine. Dr. Cohen is a Fellow in the American Academy of Dermatology and was a Clinical Professor of Medicine at the University of California School of Medicine Irvine, Department of Dermatology from October 2004 to May 2020, and is on the Hoag-USC Melanoma Advisory Board. Dr. Cohen was the Director of the Nevada VA Hospital Department of Dermatology and was a Staff Physician at the Long Beach Veterans Administration Hospital from October 2004 to October 2017. Dr. Cohen is the owner of Advanced Dermatology Care Center, Inc. a Dermatology Practice in Newport Beach and Huntington Beach, California since April 2005 to the present. Previously, Dr. Cohen founded and sold her dermatology practice in Tampa, Florida. Dr. Cohen has authored and published a number of articles relating to skin disease. Dr. Cohen has been our founder and CEO from August 2008 to the present.
Lisa LeBlanc is a graduate of Arizona State University. She has been instrumental in the development of the formulation and marketing of the KlenSkin product and has been working with us since our founding in August 2008. Ms. LeBlanc has been a Manager of Advanced Dermatology Care Center, Inc. located in Southern California, a dermatology practice and skin care facility. Ms. LeBlanc has approximately 15 years of experience managing all aspects of a medical practice and spa.
Daniel Traynor is the Vice-President of Product Development. Mr. Traynor works from our product development facility in Sarasota, Florida. Mr. Traynor started working for us as an independent contractor in August 2010 and became an employee on August 2017. Mr. Traynor is a highly trained, skilled, and accomplished chemist, widely known and respected in the realm of cosmetic chemistry. He is responsible for the product development and formulation bases for use by us and our partners. Mr. Traynor works with corporate partners, who provide formulation and raw material support. He is also assisted by a part-time chemist, who shares the facilities in Sarasota with Mr. Traynor. He also supervises the ongoing independent testing of products both in early development and through the final manufacturing stages. He also conducts quality assurance audits at manufacturing sites.
William Cohen, M.B.A., J.D., is our Chief Financial Officer. Mr. Cohen graduated with a J.D. from the Antonin Scalia Law School at George Mason University, and received his M.B.A., majoring in International Business at George Washington University School of Business Administration,. Mr. Cohen completed his BS in Business at Indiana University in Gary, IN. Mr. Cohen has been in the securities industry since 1979 and was the President/CEO of Integrated Trading and Investments, Inc., a broker dealer, from 1999 through 2018. Mr. Cohen has assisted the founders and consulted with us since 2008.
Charles Fritts, Esq. Mr. Fritts is a member of our Board of Directors and is the Chairman of our Audit Committee. He graduated from the Antonin Scalia Law School at George Mason University. He is a retired senior director of Federal Government Relations for the Biotechnology Industry Organization in Washington D.C. where he worked from 2011 to 2020. Since 2020, Mr. Fritts acts as a Legal Consultant for this organization.
Dean Stathakis, Esq., Ph.D. is a member of our Board of Directors. He attended Whittier Law School and has a Doctorate Degree in Molecular Biology from the University of Virginia. He is experienced in health care, life sciences & biotechnology, intellectual property, and patent applications. Since 2017, Mr. Stathakis has been the President of UltimateEdge Law Group LLC in Irvine, California. Prior to that, from 2013 to 2017, he was the President and Chief Operations Officer at One3 IP Management, Irvine, California. Mr. Stathakis is known for patent work on Botox while previously employed at Allergan.
Peter Barton-Hutt, Esq. is a member of our Board of Directors. He received his LL.B. from Harvard Law and his LL.M from New York University Law School. He is currently Senior Counsel in the Washington, D.C. law firm of Covington & Burling LLP, where his has worked since 1960, specializing in Food and Drug Law. Mr. Barton-Hutt was formerly General Counsel at the FDA.
Involvement in Certain Legal Proceedings
To our knowledge, none of our current directors or executive officers has, during the past ten years:
| | ● | been convicted in a criminal proceeding or been subject to a pending criminal proceeding (excluding traffic violations and other minor offenses); |
| | ● | had any bankruptcy petition filed by or against the business or property of the person, or of any partnership, corporation, or business association of which he or she was a general partner or executive officer, either at the time of the bankruptcy filing or within two years prior to that time; |
| | ● | been subject to any order, judgment, or decree, not subsequently reversed, suspended, or vacated, of any court of competent jurisdiction or federal or state authority, permanently or temporarily enjoining, barring, suspending or otherwise limiting, his involvement in any type of business, securities, futures, commodities, investment, banking, savings and loan, or insurance activities, or to be associated with persons engaged in any such activity; |
| | ● | been found by a court of competent jurisdiction in a civil action or by the SEC or the Commodity Futures Trading Commission to have violated a federal or state securities or commodities law, and the judgment has not been reversed, suspended, or vacated; |
| | ● | been the subject of, or a party to, any federal or state judicial or administrative order, judgment, decree, or finding, not subsequently reversed, suspended or vacated (not including any settlement of a civil proceeding among private litigants), relating to an alleged violation of any federal or state securities or commodities law or regulation, any law or regulation respecting financial institutions or insurance companies including, but not limited to, a temporary or permanent injunction, order of disgorgement or restitution, civil money penalty or temporary or permanent cease-and-desist order, or removal or prohibition order, or any law or regulation prohibiting mail or wire fraud or fraud in connection with any business entity; or |
| | ● | been the subject of, or a party to, any sanction or order, not subsequently reversed, suspended, or vacated, of any self-regulatory organization (as defined in Section 3(a)(26) of the Exchange Act, any registered entity (as defined in Section 1(a)(29) of the Commodity Exchange Act), or any equivalent exchange, association, entity or organization that has disciplinary authority over its members or persons associated with a member. |
Except as disclosed above under “Involvement in Certain Legal Proceedings” in the “Description of Business” section, we are not currently a party to any legal proceedings, the adverse outcome of which, individually or in the aggregate, we believe will have a material adverse effect on our business, financial condition, or operating results.
Board Leadership Structure and Risk Oversight
Dr. Cohen is our Chief Executive Officer and Chairman of the Board. Our Board believes that it is in the best interest and the best interest of our stockholders for Dr. Cohen to serve in both roles at this time given her knowledge of our business the industry. We believe our board structure will provide appropriate leadership and oversight of our business and facilitates effective functioning of our management and our Board.
One of the key functions of our Board is informed oversight of our risk management process. We have formed supporting committees, including an Audit Committee and a Compensation Committee, each of which supports the Board by addressing risks specific to its respective areas of oversight.
Audit Committee
Our Audit Committee has the responsibility to consider and discuss our major financial risk exposures and the steps our management takes to monitor and control these exposures, including guidelines and policies to govern the process by which risk assessment and management is undertaken. The Audit Committee also monitors compliance with legal and regulatory requirements, in addition to oversight of the performance of our internal audit function. Currently, we have three members on our Audit Committee, one of whom is an independent director, as follows:
| | 1. | Charles Fritts - Chair (independent) |
| | 2. | Lisa LeBlanc |
| | 3. | William Cohen |
Compensation Committee
Our Compensation Committee assesses and monitors whether any of our compensation policies and programs has the potential to encourage excessive risk-taking. Currently, we have three members on our Compensation Committee, all of whom are independent directors, as follows:
| | 1. | Peter Barton-Hutt – Chair (independent) |
| | 2. | Dean Stathakis (independent) |
| | 3. | Charles Fritts (independent) |
Term of Office
Each of our officers holds office until his or her successor is elected and qualified. Directors are appointed to serve for one year until the meeting of the Board following the annual meeting of shareholders and until their successors have been elected and qualified.
Director Independence
We use the definition of “independence” of The NASDAQ Stock Market to make this determination. NASDAQ Listing Rule 5605(a)(2) provides that an “independent director” is a person other than an officer or employee of the company or any other individual having a relationship which, in the opinion of our Board, would interfere with the exercise of independent judgment in carrying out the responsibilities of a director. The NASDAQ listing rules provide that a director cannot be considered independent if:
| | ● | the director is, or at any time during the past three years was, an employee of the company; |
| | ● | the director or a family member of the director accepted any compensation from the company in excess of $120,000 during any period of twelve consecutive months within the three years preceding the independence determination (subject to certain exemptions, including, among other things, compensation for board or board committee service); |
| | ● | the director or a family member of the director is a partner in, controlling shareholder of, or an executive officer of an entity to which the company made, or from which the company received, payments in the current or any of the past three fiscal years that exceed 5% of the recipient’s consolidated gross revenue for that year or $200,000, whichever is greater (subject to certain exemptions); |
| | ● | the director or a family member of the director is employed as an executive officer of an entity where, at any time during the past three years, any of the executive officers of the company served on the compensation committee of such other entity; or |
| | ● | the director or a family member of the director is a current partner of the company’s outside auditor, or at any time during the past three years was a partner or employee of the company’s outside auditor, and who worked on the company’s audit. |
Under such definitions, Charles Fritts, Esq., Peter Barton-Hutt, Esq, and Dean Stathakis, Esq. are independent directors. However, our Shares are not currently quoted or listed on any national exchange or interdealer quotation system with a requirement that a majority of our Board be independent and, therefore, we are not subject to any director independence requirements.
Executive and Director Compensation
The following table represents information regarding the total compensation of our executive officers and directors during the fiscal year ended December 31, 2021:
Name and Capacity in which Compensation was Received | | Cash Compensation ($) | | | Other Compensation ($) | | | Total Compensation ($) | |
Laura Cohen, CEO and Director(1) | | $ | 21,000 | | | $ | 0 | | | $ | 21,000 | |
| Lisa LeBlanc, President, COO, and Director(1) | | $ | 188,250 | | | $ | 0 | | | $ | 188,250 | |
| Daniel Traynor, VP of Product Development | | $ | 157,134 | | | $ | 0 | | | $ | 157,134 | |
| William Cohen, Chief Financial Officer | | $ | 11,500 | | | $ | 0 | | | $ | 11,500 | |
| Charles Fritts, Director | | $ | 0 | | | $ | 0 | | | $ | 0 | |
| Dean Stathakis, Director | | $ | 0 | | | $ | 0 | | | $ | 0 | |
| Peter Barton-Hutt, Director | | $ | 0 | | | $ | 0 | | | $ | 0 | |
| (1) | Laura Cohen and Lisa LeBlanc did not receive any cash compensation in their capacities as directors. |
Employment Agreements, Arrangements, or Plans
The following describes the respective compensation arrangements entered into by us and our executive officers in place as of the date hereof.
Compensation for Laura Cohen
We have not entered into an employment agreement with Laura Cohen, MD. Dr. Cohen’s compensation is determined by the Board. Dr. Cohen’s compensation has been limited based on available working capital and below what we consider comparable to executives in comparable positions. Dr. Cohen has received and may continue to receive stock options as determined by the Board.
Compensation for Lisa LeBlanc
We have not entered into an employment agreement with Lisa LeBlanc. Ms. LeBlanc’s compensation is determined by the Board. Ms. LeBlanc’s compensation has been limited based on available working capital and below what we consider comparable to executives in comparable positions. Ms. LeBlanc has received and may continue to receive stock options as determined by the Board.
Employment Agreement with Daniel Traynor
Effective September 1, 2017, we entered into an Employment Agreement with Daniel Traynor, as our product development chemist and formulator. The agreement provided for an annualized salary of $120,000. We agreed to grant Mr. Traynor 600,000 stock options with ten-year terms, immediate vesting on the grant date, and both the issuance price and the market price was determined to be the share price offered in the private placement in effect at the date of the stock option grant. Under the Employment Agreement, additional stock options may be granted annually, up to maximum fair market value, as determined by us, of 50% of Mr. Traynor’s gross annual salary for the calendar year. Mr. Traynor’s salary was increased to $240,000 in 2021.
Compensation for William Cohen
Mr. Cohen received a minimum salary for his work in 2021. Mr. Cohen may continue to receive stock options or a combination of salary plus stock options as determined by the Board.
Director Compensation
We have five directors. We currently do not pay our directors any cash compensation for their services as board members.
Our directors are granted stock options by the Board annually, and decided and approved by the Board on a per Board meeting basis and as approved by the Board. During the year ended December 31, 2021, no stock options were granted by the Board for board director compensation.
ITEM 4. SECURITY OWNERSHIP OF MANAGEMENT AND CERTAIN SECURITYHOLDERS
The following table shows the beneficial ownership of our Shares as of the date of this filing held by (i) each person known to us to be the beneficial owner of more than 10% of any class of our voting securities; (ii) each director; (iii) each executive officer; and (iv) all directors and executive officers as a group. As of the date of this filing, there were 32,639,234 shares issued and outstanding.
Beneficial ownership is determined in accordance with the rules of the SEC, and generally includes voting power and/or investment power with respect to the securities held. Shares subject to options and warrants currently exercisable or which may become exercisable within 60 days of the date of this Memorandum, are deemed outstanding and beneficially owned by the person holding such options or warrants for purposes of computing the number of Shares and percentage beneficially owned by such person, but are not deemed outstanding for purposes of computing the percentage beneficially owned by any other person. Except as indicated in the footnotes to this table, the persons or entities named have sole voting and investment power with respect to all of our Common Stock shown as beneficially owned by them.
| Name, Title, and Address of Beneficial Owner | | Amount and Nature of Beneficial Ownership | | | Ownership Percentage | |
| Laura Cohen, Chief Executive Officer and Chairman of the Board | | | 20,480,000 | (1) | | | 47 | % |
| Lisa LeBlanc, President, Chief Operating Officer, and Director | | | 12,380,000 | (2) | | | 22 | % |
| William Cohen, Chief Financial Officer and Chief Development Officer | | | 1,700,000 | (3) | | | 4 | % |
| Daniel Traynor, VP of Product Development | | | 1,320,000 | (4) | | | 3 | % |
| Charles Fritts, Director | | | 250,000 | (5) | | | * | |
| Peter Barton-Hutt, Director | | | 240,000 | (6) | | | * | |
| Dean Stathakis, Director | | | 240,000 | (7) | | | * | |
| | | | | | | | | |
| All executive officers and directors as a whole | | | 33,810,000 | | | | 78 | % |
* = less than 1%
| (1) | Includes 4,690,894 options to purchase common stock (“Options”) at an exercise price of $0.50; 60,000 Options at an exercise price of $1.50; and 120,000 Options at an exercise price of $2.75. |
| (2) | Includes 4,890,000, Options at an exercise price of $0.50; 60,000 Options at an exercise price of $1.50; and 120,000 Options at an exercise price of $2.75. |
| (3) | Includes 400,000 Options at an exercise price of $1.50; and 400,000 Options at an exercise price of $2.75. |
| (4) | Consists of 1,320,000 Options at an exercise price of $0.50. |
| (5) | Includes 60,000 Options with an exercise price of $0.50; 60,000 Options with an exercise price of $1.50; and 120,000 Options with an exercise price of $2.75. |
| (6) | Consists of 60,000 Options with an exercise price of $0.50; 60,000 Options with an exercise price of $1.50; and 120,000 Options with an exercise price of $2.75. |
| (7) | Consists of 60,000 Options with an exercise price of $0.50; 60,000 Options with an exercise price of $1.50; and 120,000 Options with an exercise price of $2.75. |
ITEM 5. INTEREST OF MANAGEMENT AND CERTAIN SECURITY HOLDERS
Transactions with Related Persons
Advanced Dermatology Care Center, Inc. (“ADCC”) is a significant related wholesale purchaser of Klenskin products. These products are ordered and sold to patients of ADCC and our clients. ADCC is a general dermatology practice owned by our Chief Executive Officer, Laura Cohen, MD. The products sold there are to enhance skin protection from sun exposure to patients having sun damaged skin.
During the 12 months ended December 31, 2021 and 2020, sales to Advanced Dermatology Care Center, a dermatology practice owned by Dr. Laura Cohen, our Chief Executive Officer, were $19,000 and $21,000, respectively, accounting for 24% and 20% of our revenue, respectively.
Review, Approval, and Ratification of Related Party Transactions
We have formal policies and procedures for the review, approval, or ratification of transactions, such as those described above, with our executive officer(s), director(s), and significant shareholders. Our bylaws provide for the disqualification of certain directors or officers for voting on specific matters where that director or officer deals or contracts with us as either a vendor or purchaser. Additionally, in the employment agreements with our CEO and COO, we have conflict of interest provisions that require them to notify our board of directors before engaging in any activity that creates a potential conflict of interest, and a list of pre-approved activities.
ITEM 6. OTHER INFORMATION
On May 8, 2022, Gerald Birch, resigned as Chief Financial Officer of the Company and William Cohen was then appointed as our Chief Financial Officer. Mr. Birch remains engaged performing accounting duties and has indicated his willingness to return as CFO once the company has a full-time position available.
Since early 2022, we have been in negotiations to acquire additional patents and technology. Pricing and terms have been finalized by contract and the acquisition has been completed. The re-registration of already issued patents and those pending filings are now being addressed by management and this process should be completed in 2022. Full details of this acquisition will be announced once the updated ownership of the patents and any remaining filings have been completed.
ITEM 7. FINANCIAL STATEMENTS
COLABS INT’L, CORP.
FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2021 AND 2020
TABLE OF CONTENTS
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of
Colabs Int’l, Corp.
Huntington Beach, California
Opinion on the Financial Statements
We have audited the accompanying balance sheets of Colabs Int’l, Corp. (the “Company”) as of December 31, 2021 and 2020, the related statements of operations, stockholders’ equity (deficit), and cash flows for the years then ended, and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2021 and 2020, and the results of its operations and its cash flows for the years then ended, in conformity with accounting principles generally accepted in the United States of America.
Going Concern
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the financial statements, the Company has experienced recurring operating losses and negative operating cash flows since inception and has a stockholders’ deficit as of December 31, 2021. These conditions raise substantial doubt about the Company’s ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 1 to the financial statements. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provided a reasonable basis for our opinion.
We have served as the Company’s auditor since 2020.
Los Angeles, California
October 7, 2022
COLABS INT’L, CORP.
BALANCE SHEETS
| | | December 31, 2021 | | | December 31, 2020 | |
|
| Assets | | | | | | | | |
| Current assets: | | | | | | | | |
| Cash | | $ | 68,000 | | | $ | 230,000 | |
| Accounts Receivable | | | 1,000 | | | | 2,000 | |
| Inventory, net of reserves of $62,000, and $0, respectively | | | 8,000 | | | | 74,000 | |
| Prepaid and other current assets | | | 5,000 | | | | - | |
| Total current assets | | | 82,000 | | | | 306,000 | |
| | | | | | | | | |
| Right of use asset | | | 8,000 | | | | 20,000 | |
| | | | | | | | | |
| Other assets: | | | | | | | | |
| Other assets | | | 2,000 | | | | 2,000 | |
| Total assets | | $ | 92,000 | | | $ | 328,000 | |
| | | | | | | | | |
| Liabilities and Stockholders’ Equity | | | | | | | | |
| Current liabilities: | | | | | | | | |
| Accounts payable and accrued expenses | | $ | 39,000 | | | $ | 46,000 | |
| Deferred revenue | | | 14,000 | | | | - | |
| Lease liability, current portion | | | 9,000 | | | | 21,000 | |
| Loans payable, current portion | | | 3,000 | | | | 4,000 | |
| Total current liabilities | | | 65,000 | | | | 71,000 | |
| | | | | | | | | |
| Loans payable, net of current portion | | | 39,000 | | | | 99,000 | |
| Total liabilities | | | 104,000 | | | | 170,000 | |
| | | | | | | | | |
| Commitments and contingencies | | | | | | | | |
| | | | | | | | | |
| Stockholders’ equity (deficit): | | | | | | | | |
| Common stock, $.001 par value; 50,000,000 shares authorized; 31,747,500 and 31,390,000 shares issued and outstanding at December 31, 2021 and 2020, respectively | | | 32,000 | | | | 31,000 | |
| Additional paid-in capital | | | 11,160,000 | | | | 10,444,000 | |
| Common stock issuable (269,106 common shares) | | | 270,000 | | | | - | |
| Accumulated deficit | | | (11,474,000 | ) | | | (10,317,000 | ) |
| Total stockholders’ equity (deficit) | | | (12,000 | ) | | | 158,000 | |
| Total liabilities and stockholders’ equity (deficit) | | $ | 92,000 | | | $ | 328,000 | |
See accompanying notes to the financial statements.
COLABS INT’L, CORP.
STATEMENTS OF OPERATIONS
YEARS ENDED DECEMBER 31, 2021 AND 2020
| | | Years Ended December 31, | |
| | | 2021 | | | 2020 | |
| | | | | | | |
| Revenue (1) | | $ | 80,000 | | | $ | 105,000 | |
| Cost of revenue | | | 143,000 | | | | 61,000 | |
| Gross profit (loss) | | | (63,000 | ) | | | 44,000 | |
| Operating expenses: | | | | | | | | |
| Selling, general and administrative expenses | | | 1,022,000 | | | | 738,000 | |
| Research and development | | | 181,000 | | | | 127,000 | |
| Total operating expenses | | | 1,203,000 | | | | 865,000 | |
| | | | | | | | | |
| Loss from operations | | | (1,266,000 | ) | | | (821,000 | ) |
| | | | | | | | | |
| Gain on forgiveness of Paycheck Protection Program loans | | | 110,000 | | | | - | |
| Interest expense | | | (1,000 | ) | | | (5,000 | ) |
| Net loss | | $ | (1,157,000 | ) | | $ | (826,000 | ) |
| | | | | | | | | |
| Net loss per common share – basic and diluted | | $ | (0.04 | ) | | $ | (0.03 | ) |
| | | | | | | | | |
| Weighted average common shares outstanding – basic and diluted | | | 31,622,650 | | | | 27,209,868 | |
| | | | | | | | | |
| (1) Sales to related party | | $ | 19,000 | | | $ | 21,000 | |
See accompanying notes to the financial statements.
COLABS INT’L, CORP.
STATEMENTS OF CHANGES IN STOCKHOLDERS’ EQUITY (DEFICIT)
YEARS ENDED DECEMBER 31, 2021 AND 2020
| | | Common Stock | | | Common Shares | | | Additional | | | | | | Total Stockholders’ | |
| | | $.001 Par | | | Issuable | | | Paid- | | | Accumulated | | | Equity | |
| | | Shares | | | Amount | | | Shares | | | Amount | | | in Capital | | | Deficit | | | (Deficit) | |
| December 31, 2019 | | | 25,304,000 | | | $ | 25,000 | | | | - | | | $ | - | | | $ | 9,719,000 | | | $ | (9,491,000 | ) | | $ | 253,000 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Common shares issued for cash | | | 486,000 | | | | - | | | | - | | | | - | | | | 728,000 | | | | | | | | 728,000 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Common shares issued on exercise of stock options | | | 5,600,000 | | | | 6,000 | | | | - | | | | - | | | | (3,000 | ) | | | | | | | 3,000 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Net loss | | | | | | | | | | | | | | | | | | | | | | | (826,000 | ) | | | (826,000 | ) |
| December 31, 2020 | | | 31,390,000 | | | | 31,000 | | | | - | | | | - | | | $ | 10,444,000 | | | $ | (10,317,000 | ) | | $ | 158,000 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Common shares issued for cash | | | 357,500 | | | | 1,000 | | | | 60,000 | | | | 165,000 | | | | 716,000 | | | | | | | | 882,000 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Common shares issued on exercise of stock options | | | - | | | | - | | | | 209,106 | | | | 105,000 | | | | - | | | | | | | | 105,000 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Net loss | | | | | | | | | | | | | | | | | | | | | | | (1,157,000 | ) | | | (1,157,000 | ) |
| December 31, 2021 | | | 31,747,500 | | | $ | 32,000 | | | | 269,106 | | | $ | 270,000 | | | $ | 11,160,000 | | | $ | (11,474,000 | ) | | $ | (12,000 | ) |
See accompanying notes to the financial statements.
COLABS INT’L, CORP.
STATEMENTS OF CASH FLOWS
YEARS ENDED DECEMBER 31, 2021 AND 2020
| | | Years Ended December 31, | |
| | | 2021 | | | 2020 | |
| Cash flows from operating activities: | | | | | | | | |
| Net loss | | $ | (1,157,000 | ) | | $ | (826,000 | ) |
| Adjustments to reconcile net loss to cash used in operating activities: | | | | | | | | |
| Depreciation | | | - | | | | 2,000 | |
| Increase in inventory reserve | | | 62,000 | | | | - | |
| Gain on forgiveness of Paycheck Protection Program loans | | | (110,000 | ) | | | - | |
| Change in right of use asset | | | 29,000 | | | | 22,000 | |
| Change in lease liability | | | (29,000 | ) | | | (24,000 | ) |
| Change in operating assets and liabilities: | | | | | | | | |
| Accounts receivable | | | 1,000 | | | | 1,000 | |
| Inventory | | | 4,000 | | | | (74,000 | ) |
| Prepaids and other current assets | | | (5,000 | ) | | | - | |
| Accounts payable and accrued expenses | | | (7,000 | ) | | | (10,000 | ) |
| Deferred revenue | | | 14,000 | | | | - | |
| Net cash used in operating activities | | | (1,198,000 | ) | | | (909,000 | ) |
| Cash flows from financing activities: | | | | | | | | |
| Proceeds from the sale of common shares | | | 882,000 | | | | 728,000 | |
| Proceeds from the exercise of stock options | | | 105,000 | | | | 3,000 | |
| Proceeds from loans payable | | | 49,000 | | | | 103,000 | |
| Repayment of note payable | | | - | | | | (42,000 | ) |
| Net cash provided by financing activities | | | 1,036,000 | | | | 792,000 | |
| | | | | | | | | |
| Net decrease | | | (162,000 | ) | | | (117,000 | ) |
| Balance at the beginning of the period | | | 230,000 | | | | 347,000 | |
| Balance at the end of the period | | $ | 68,000 | | | $ | 230,000 | |
| Supplemental disclosures of cash flow information: | | | | | | | | |
| Income taxes paid | | $ | - | | | $ | - | |
| Interest paid | | $ | 1,000 | | | $ | 2,000 | |
| Supplemental non-cash disclosure: | | | | | | | | |
| Recognition of right-of-use asset and operating lease liability upon execution of new operating lease | | $ | 17,000 | | | $ | - | |
See accompanying notes to the financial statements.
COLABS INT’L, CORP.
NOTES TO FINANCIAL STATEMENTS
YEARS ENDED DECEMBER 31, 2021 AND 2020
| 1. | Organization and Basis of Presentation |
CoLabs Int’l, Corp. (the “Company,” “CoLabs,” “we,” “our,” and “us”) was incorporated on August 5, 2008 under the laws of the State of Nevada as an OTC pharmaceutical company with multi-market applications for our revolutionary targeted epidermal-drug delivery system which is identified as: QuantaSphere® Technology (QS®).
The Company has 27 issued patents and have an additional eight patent applications pending.
The Company places OTC drugs, pharmaceuticals, chemicals, and agrochemicals into micro-sized, electrostatically charged encapsulates as well as into other types of micro-encapsulates. These encapsulates are then suspended in uniquely designed formulations. This formulation of a highly advanced micro-technology can: target the delivery of topically applied drugs; limit unwanted absorption of chemicals, drugs, and cosmetics through the skin; and provide a designed release of active ingredients with a prescribed depth of skin penetration.
The Company has developed commercially unique and innovative targeted skin delivery systems for topical pharmacology. The Company’s technology limits unwanted side effects that can occur as a result of absorption through the skin. The penetration of chemicals through the skin can have serious impact on the body’s organs and systems. The Company’s current applications include anti-bacterial/viral sanitizers/soaps, sunscreens, pest-repellents, and healing cosmetic lotions. For consumers, we provide elegant skin delivery of topically applied drugs and cosmetics. This combined end purpose is the unique foundation of the Company’s technology.
The Company amended its Articles of Incorporation on January 12, 2021 to increase its authorized shares of common stock to 50,000,000.
On December 1, 2021, the Company effected a 2 for 1 split of its outstanding shares of common stock. All shares and per share amounts and information presented herein have been retroactively adjusted to reflect the stock split for all periods presented.
COVID-19 Considerations
Through the date these financial statements were issued, the COVID-19 pandemic has significantly impacted the Company’s operating results. Due to COVID-19, the Company experienced reduced demand for its products in its domestic and international markets. Marketing programs planned for 2020 were curtailed by the lockdowns related to the epidemic. These included: AVP Beach Volleyball, NASCAR and golf tournaments events were all cancelled and/or postponed until 2021. In many locations, beaches and outdoor activities were also restricted which again limited sunscreen sales. Since the company has only limited online presence, which is directly related to sport event marketing, sales were seriously affected.
The Company is however, recently experiencing an improving trend in customer orders from both its domestic and international markets. As lockdowns are gradually lifted, more outdoor activities are allowed resulting in increasing sales. In the future, the pandemic may cause reduced demand for the Company’s products. Should the pandemic result in a recessionary economic environment, consumers who purchase the Company’s products will be negatively affected. The Company has not observed any material impairments of its assets or a significant change in the fair value of its assets due to the COVID-19 pandemic.
The Company’s ability to operate without significant negative operational impact from the COVID-19 pandemic will in part depend on its ability to protect its employees and its supply chain. The Company has endeavored to follow the recommended actions of government and health authorities to protect its employees. However, the uncertainty resulting from the COVID-19 pandemic could result in an unforeseen disruption to its workforce and supply chain (for example, an inability of a key supplier or transportation supplier to source and transport materials) that could negatively impact operations.
Going Concern
The Company’s financial statements have been prepared assuming that it will continue as a going concern, which contemplates continuity of operations, the generation of sales revenue, the realization of assets, and liquidation of liabilities in the normal course of business. As reflected in the financial statements, during the year ended December 31, 2021, the Company incurred a net loss of $1,157,000, and used net cash in operating activities of $1,198,000, and had a stockholders’ deficit of $12,000 on December 31, 2021. These factors raise substantial doubt about the Company’s ability to continue as a going concern as a biotechnology company within one year after the date that the financial statements are issued without a further influx of equity capital, growth of sales or issuance of debt or a combination of these capital inflows. The financial statements do not include any adjustments related to the recoverability and classification of recorded asset amounts or the amounts and classification of liabilities that might be necessary should the Company be unable to continue as a going concern.
At December 31, 2021, the Company had cash on hand of $68,000. During the year ended December 31, 2021, the Company received proceeds of $882,000 from the sales of its common stock, and $105,000 of proceeds from the exercise of stock options. The ability of the Company to continue as a going concern is and has been dependent on the Company’s ability to execute its strategy and in its ability to raise additional funds and expand revenue from sales. Management is currently seeking additional operating funds, primarily through the issuance of equity securities to expand its marketing and overall business development. No assurance can be given that any future financing will be successful or, the funding will be adequate for the Company to achieve its goals. Even if the Company can obtain additional financing, it may place restrictions on management. Substantial dilution for stockholders may occur from the structuring of either equity or debt financing.
Reclassifications
Certain prior year amounts, consisting of patent and licensing legal and filing fees, were reclassified from research and development costs to selling, general and administrative costs. These reclassifications had no effect on the reported results of operations, total stockholders’ deficiency, or cash flows from operations.
| 2. | Summary of Significant Accounting Policies |
Revenue Recognition
The Company products are sold under the brand name KLĒNSKIN®, which consists of distinct sunscreen products titled SPF 30 Shampoo, Face and Body Wash, SunBar SPF 20 Bar Soap, and our Broad Spectrum SPF 50 Lotion, and a hand sanitizer and insect repellant. The products are offered for sale as finished goods only, and there are no performance obligations required post-shipment for customers to derive the expected value from them. Contracts with customers contain no incentives or discounts that could cause revenue to be allocated or adjusted over time. The Company does offer a general right of return, but historically the return rates have been insignificant.
The Company recognizes revenue in accordance with Financial Accounting Standards Board Accounting Standards Codification (ASC) 606, Revenue from Contracts with Customers. This standard provides authoritative guidance clarifying the principles for recognizing revenue and developing a common revenue standard for U.S. generally accepted accounting principles. The core principle of the guidance is that an entity should recognize revenue to depict the transfer of promised goods and services to customers in an amount that reflects the consideration to which the entity expects to be entitled in the exchange for those goods or services.
Under this guidance, revenue is recognized when control of promised goods or services is transferred to the Company’s customers, in an amount that reflects the consideration the Company expects to be entitled to in exchange for those goods or services. The Company reviews its sales transactions to identify contractual rights, performance obligations, and transaction prices, including the allocation of prices to separate performance obligations, if applicable. Revenue and costs of sales are recognized once products are delivered to the customer’s control and performance obligations are satisfied.
Revenue Concentrations
The Company performs a regular review of customer activity and associated credit risks and does not require collateral or other arrangements.
During the year ended December 31, 2021 and 2020, sales to Advanced Dermatology Care Center, a dermatology practice owned by Dr. Laura Cohen, the Company’s President and Chief Executive Officer, accounted for 24% and 20% of Company’s revenue, respectively. During the year ended December 31, 2021 and 2020, one international customer accounted for 14% and 44% of the Company’s revenue, respectively. No other customers accounted for more than 10% of our revenues during the years ended December 31, 2021 and 2020.
Stock-Based Compensation
The Company periodically issues stock options to employees. The Company accounts for stock options issued and vesting to employees based on the authoritative guidance provided by Accounting Standards Codification (“ASC”) Topic 718 – Stock Compensation, whereas the value of the award is measured on the date of grant and recognized over the vesting period.
The fair value of the Company’s common stock option grants is estimated using the Black-Scholes option-pricing model, which uses certain assumptions related to risk-free interest rates, expected volatility, expected life of the common stock options, and future dividends. Compensation expense is recorded based upon the value derived from the Black-Scholes option pricing model and based on actual experience. The assumptions used in the Black-Scholes option-pricing model could materially affect compensation expense recorded in future periods.
Fair Value Measurements
The Company uses various inputs in determining the fair value of its investments and measures these assets on a recurring basis. Financial assets recorded at fair value in the balance sheets are categorized by the level of objectivity associated with the inputs used to measure their fair value. Authoritative guidance provided by ASC Topic 820 - Fair Value Measurements and Disclosure defines the following levels directly related to the amount of subjectivity associated with the inputs to fair valuation of these financial assets:
Level 1—Quoted prices in active markets for identical assets or liabilities;
Level 2—Inputs, other than the quoted prices in active markets, that are observable either directly or indirectly; and
Level 3—Unobservable inputs based on the Company’s assumptions.
The Company is required to use observable market data if such data is available without undue cost and effort. At December 31, 2021 and 2020, the carrying amounts of financial assets and liabilities, such as cash, accounts receivable, inventory, other assets, and accounts payable and accrued expenses approximate their fair values due to their short-term nature. The carrying values of notes payable approximate their fair values due to the fact that the interest rates on these obligations are based on prevailing market interest rates.
Basic and Diluted Net Loss per Common Share
Basic net loss per share is computed by using the weighted-average number of common shares outstanding during the period. Diluted net loss per share is computed giving effect to all dilutive potential shares of Common Stock that were outstanding during the period. Dilutive potential shares of Common Stock consist of incremental shares of Common Stock issuable upon exercise of stock options. No dilutive potential shares of Common Stock were included in the computation of diluted net loss per share because their impact was anti-dilutive. As of December 31, 2021, and 2020, the Company had total outstanding options of 10,881,788 and 11,300,000, respectively, which were excluded from the computation of net loss per share because they are anti-dilutive.
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that may affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and reported amounts of revenues and expenses during the reporting period. Those estimates and assumptions include estimates for reserves of uncollectible accounts, reserves for inventory obsolescence, analysis of impairments of long-lived assets, accruals for potential liabilities, and valuation of deferred tax assets. Actual results could differ from those estimates.
Research and Development
Research and development costs are expensed as incurred. Research and development expenses for the years ended December 31, 2021 and 2020, were $181,000 and $127,000, respectively.
Advertising Costs
Advertising costs are charged to operations as part of selling, general and administrative expenses at the time the costs are incurred. Advertising costs for the years ended December 31, 2021 and 2020, were $278,000 and $132,000, respectively.
Patent and Licensing Legal and Filing Fees and Costs
Due to the significant uncertainty associated with the successful development of one or more commercially viable products based on the Company’s research efforts and related patent applications, all patent and licensing legal and filing fees and costs related to the development and protection of its intellectual property are charged to operations as incurred. Patent and licensing legal and filing fees and costs were $71,000 and $45,000 for the years ended December 31, 2021 and 2020, respectively. Patent and licensing legal and filing fees and costs are included in general and administrative costs in the Company’s statements of operations.
Concentration of Credit Risk
The Company maintains the majority of its cash balances with one financial institution, in the form of demand deposits. During the year ended December 31, 2021, the Company had cash deposits that exceeded the federally insured limit of $250,000. The Company believes that no significant concentration of credit risk exists with respect to these cash balances because of its assessment of the creditworthiness and financial viability of the financial institution.
Inventories
Inventories are stated at the lower of cost or net realizable value. Cost is computed on a first-in, first-out basis. The Company’s inventories consist almost entirely of finished goods as of December 31, 2021.
The Company provides inventory reserves based on excess and obsolete inventories determined primarily by future demand forecasts. The write down amount is measured as the difference between the cost of the inventory and net realizable value based upon assumptions about future demand and charged to the provision for inventory, which is a component of cost of sales. At the point of the loss recognition, a new, lower cost basis for that inventory is established, and subsequent changes in facts and circumstances do not result in the restoration or increase in that newly established cost basis. At December 31, 2021, the Company recorded a reserve of $62,000 for excess and obsolete inventory.
Recent Accounting Pronouncements
In June 2016, the Financial Accounting Standards Board (the “FASB”) issued Accounting Standards Update (“ASU”) 2016-13, Credit Losses (Topic 326) – Measurement of Credit Losses on Financial Instruments (“ASC 2016-13”). ASU 2016-13 requires entities to use a forward-looking approach based on current expected credit losses to estimate credit losses on certain types of financial instruments, including trade receivables, which may result in the earlier recognition of allowance for losses. ASU 2016-13 is effective beginning January 1, 2023 and early adoption is permitted. The adoption of ASU 2016-13 is not expected to have any impact on the Company’s financial statement presentation or disclosures.
In May 2021, the FASB issued ASU 2021-04, Earnings Per Share (Topic 260), Debt — Modifications and Extinguishments (Subtopic 470-50), Compensation — Stock Compensation (Topic 718), and Derivatives and Hedging — Contracts in Entity’s Own Equity (Subtopic 815-40): Issuer’s Accounting for Certain Modifications or Exchanges of Freestanding Equity-Classified Written Call Options (“ASU 2021-04”). ASU 2021-04 provides guidance as to how an issuer should account for a modification of the terms or conditions or an exchange of a freestanding equity-classified written call option (i.e., a warrant) that remains equity classified after modification or exchange as an exchange of the original instrument for a new instrument. An issuer should measure the effect of a modification or exchange as the difference between the fair value of the modified or exchanged warrant and the fair value of that warrant immediately before modification or exchange and then apply a recognition model that comprises four categories of transactions and the corresponding accounting treatment for each category (equity issuance, debt origination, debt modification, and modifications unrelated to equity issuance and debt origination or modification). ASU 2021-04 is effective for fiscal years beginning after December 15, 2021, including interim periods within those fiscal years. An entity should apply the guidance provided in ASU 2021-04 prospectively to modifications or exchanges occurring on or after the effective date. Early adoption is permitted, including adoption in an interim period. If an entity elects to early adopt ASU 2021-04 in an interim period, the guidance should be applied as of the beginning of the fiscal year that includes that interim period. The adoption of ASU 2021-04 is not expected to have any impact on the Company’s financial statement presentation or disclosures.
In October 2021, the FASB issued ASU 2021-08, Business Combinations (Topic 805) – Accounting for Contract Assets and Contract Liabilities from Contracts with Customers (“ASU 2021-08”). ASU 2021-08 requires that an entity recognize and measure contract assets and contract liabilities acquired in a business combination as if it had originated the contracts. This is a shift from existing guidance, which required the acquirer to recognize contract assets and contract liabilities at their fair value as of the acquisition date. ASU 2021-08 is effective for fiscal years beginning after December 15, 2022, including interim periods within those fiscal years. An entity should apply the guidance provided by ASU 2021-08 prospectively to business combinations occurring on or after January 1, 2023. Early adoption of ASU 2021-08 is permitted, including adoption in an interim period. An entity that early adopts the guidance in an interim period should apply the amendments (1) retrospectively to all business combinations for which the acquisition date occurs on or after the beginning of the fiscal year that includes the interim period of early application and (2) prospectively to all business combinations that occur on or after the date of initial application. The adoption of ASU 2021-08 is not expected to have any impact on the Company’s financial statement presentation or disclosure.
Management does not believe that any other recently issued, but not yet effective, authoritative guidance, if currently adopted, would have a material impact on the Company’s financial statement presentation or disclosures.
The Company determines whether a contract is, or contains, a lease at inception. Right-of-use assets represent the Company’s right to use an underlying asset during the lease term, and lease liabilities represent the Company’s obligation to make lease payments arising from the lease. Right-of-use assets and lease liabilities are recognized at lease commencement based upon the estimated present value of unpaid lease payments over the lease term. The Company leases its headquarters office, and certain office equipment and automobiles. Leases with an initial term of 12 months or less are not included on the balance sheets.
During the year ended December 31, 2021, the Company entered an office lease in Sarasota, Florida, for its research and development activities. The Sarasota lease is a 24-month lease expiring on November 30, 2022, with an initial monthly payment of $797. The Company also leases its corporate administrative office and warehouse facility located in Huntington Beach, California, on a month-to-month basis. Leases with an initial term of 12 months or less are not recorded on the balance sheet. The Company accounts for the lease and non-lease components of its leases as a single lease component. Rent expense is recognized on a straight-line basis over the lease term.
During the years ended December 31, 2021 and 2020, lease costs totaled $29,000 and $23,000, respectively.
As of December 31, 2020, lease liabilities totaled $21,000. During the year ended December 31, 2021, the Company added the Sarasota operating lease of $17,000 and made payments of $29,000 towards its operating lease liability. As of December 31, 2021, operating lease liabilities totaled $9,000.
As of December 31, 2021, the weighted average remaining lease terms for an operating lease are 0.92 years. As of December 31, 2021, the weighted average discount rate for operating lease is 10.00%.
Future minimum lease payments under the leases are as follows (in thousands):
| Years Ending December 31, | | Amounts | |
| 2022 | | $ | 10,000 | |
| 2023 | | | - | |
| Total payments | | | 10,000 | |
| Less: Amount representing interest | | | (1,000 | ) |
| Present value of net minimum lease payments | | | 9,000 | |
| Less: Current portion | | | (9,000 | ) |
| Non-current portion | | $ | - | |
The table below displays the Company’s notes payable balances as of December 31, 2021 and December 31, 2020, respectively.
| | | December 31, 2021 | | | December 31, 2020 | |
| Paycheck Protection Program (PPP) Loan (a) | | $ | - | | | $ | 61,000 | |
| Economic Injury Disaster Loan (EIDL) (b) | | | 42,000 | | | | 42,000 | |
| Total Loan Balance | | $ | 42,000 | | | $ | 103,000 | |
| Short Term Balance | | | 3,000 | | | | 4,000 | |
| Long Term Balance | | $ | 39,000 | | | $ | 99,000 | |
| | (a) | On May 6, 2020, the Company was granted a loan (the “PPP loan”) from Cross River Bank in the aggregate amount of $61,000, pursuant to the Paycheck Protection Program (the “PPP”) under the CARES Act. On February 1, 2021, the Company received notice it was granted a second loan from Cross River Bank in the aggregate amount of $49,000 pursuant to the Paycheck Protection Program (the “PPP”) under the CARES Act. The second PPP loan agreement matures in five years, bears interest at a rate of 1% per annum, is unsecured and guaranteed by the U.S. Small Business Administration (SBA). |
| | During the year ended December 31, 2021, the Company received formal notice that the notes payable was forgiven. As a result, the gain from the forgiveness of the government assistance notes payable aggregating $110,000 was recognized in the statement of operations during the year ended December 31, 2021. |
| | (b) | On June 2, 2020, the Company obtained an Economic Injury Disaster Loan in the amount of $42,000, pursuant to the Small Business Administration (SBA) authorized (under Section 7(b)) of the Small Business Act, as amended. Interest on the loan is at the rate of 3.75% per year, and all loan payments are deferred for twelve months, at which time monthly installment payments are payable over 30 years from the date of the promissory note. The promissory note is secured by all the assets of the Company. The Company will use all the proceeds of this Loan solely as working capital to alleviate economic injury caused by COVID-19. At December 31, 2021, the balance on the loan was $42,000, of which $3,000 were reflected as the current portion of note payable. |
Private Offerings
During the year ended December 31, 2021, the Company received aggregate proceeds of $882,000 from the sale of 417,500 shares of common stock. At December 31, 2021, 60,000 shares of common stock with proceeds of $165,000, were unissued, and recorded as a component of common shares issuable on the balance sheet at December 31, 2021.
During the year ended December 31, 2020, the Company received proceeds of $728,000 from the sale of 486,000 shares of common stock.
Stock Options
A summary of the stock options for the years ended December 31, 2021 and 2020, is as follows:
| | | | | | Weighted | |
| | | Number | | | Average | |
| | | Of | | | Exercise | |
| | | Options | | | Price | |
| Balance outstanding, December 31, 2019 | | | 16,900,000 | | | $ | 0.37 | |
| Options granted | | | - | | | | - | |
| Options exercised | | | (5,600,000 | ) | | | 0.0005 | |
| Options expired or forfeited | | | - | | | | - | |
| Balance outstanding, December 31, 2020 | | | 11,300,000 | | | | 0.55 | |
| Options granted | | | - | | | | - | |
| Options exercised | | | (209,106 | ) | | | 0.50 | |
| Options expired or forfeited | | | - | | | | - | |
| Balance outstanding, December 31, 2021 | | | 11,090,894 | | | $ | 0.56 | |
| Balance exercisable, December 31, 2021 | | | 11,090,894 | | | $ | 0.56 | |
Information relating to outstanding stock options at December 31, 2021, summarized by exercise price, is as follows:
| | | | Outstanding | | | Exercisable | |
| | | | | | | | | Weighted | | | | | | Weighted | |
| Exercise | | | | | | | | | Average | | | | | | Average | |
Price Per Share | | | Shares | | | Life
(Years) | | | Exercise Price | | | Shares | | | Exercise
Price | |
| $ | 0.50 | | | | 9,510,894 | | | | 8.23 | | | $ | 0.50 | | | | 9,510,894 | | | $ | 0.50 | |
| $ | 0.90 | | | | 1,580,000 | | | | 11.60 | | | $ | 0.90 | | | | 1,580,000 | | | $ | 0.90 | |
| | | | | | 11,090,894 | | | | 9.01 | | | $ | 0.56 | | | | 11,090,894 | | | $ | 0.56 | |
During the twelve months ended December 31, 2021, the Company received proceeds of $105,000 on the exercise of 209,106 stock options. As the common shares from the exercise of stock options were not issued at December 31, 2021, the Company reclassified the common shares as a component of commons shares issuable on the balance sheet.
During the twelve months ended December 31, 2020, the Company received proceeds of $3,000, on the exercise of 5,600,000 stock options.
As of December 31, 2021, the Company had no outstanding unvested options with future compensation costs. As of December 31, 2021, and December 31, 2020, both the outstanding options and exercisable options had an intrinsic value of $24,793,000 and $10,668,000, respectively.
Reconciliation between the expected federal income tax rate and the actual tax rate is as follows:
| | | Year Ended December 31, | |
| | | 2021 | | | 2020 | |
| | | | | | | |
| Federal statutory tax rate | | | 21 | % | | | 21 | % |
| State tax, net of federal benefit | | | 7 | % | | | 7 | % |
| Total tax rate | | | 28 | % | | | 28 | % |
| Allowance | | | (28 | )% | | | (28 | )% |
| Effective tax rate | | | - | % | | | - | % |
The following is a summary of the deferred tax assets:
| | | Year Ended December 31, | |
| | | 2021 | | | 2020 | |
| | | | | | | |
| Net operating loss carryforwards | | $ | 1,818,000 | | | $ | 1,494,000 | |
| Valuation allowance | | | (1,818,000 | ) | | | (1,494,000 | ) |
| Net deferred tax asset | | $ | - | | | $ | - | |
The Company has no tax provision for any period presented due to our history of operating losses. As of December 31, 2021, the Company had net operating loss carryforwards of approximately $6,492,000 that may be available to reduce future years’ taxable income through 2041. Future tax benefits which may arise because of these losses have not been recognized in these financial statements, as management has determined that their realization is not likely to occur and accordingly, the Company has recorded a valuation allowance for the full value of the deferred tax asset relating to these tax loss carry-forwards.
The Company adopted accounting rules which address the determination of whether tax benefits claimed or expected to be claimed on a tax return should be recorded in the financial statements. Under these rules, the Company may recognize the tax benefit from an uncertain tax position only if it is more likely than not that the tax position will be sustained on examination by the taxing authorities, based on the technical merits of the position. The tax benefits recognized in the financial statements from such a position are measured based on the largest benefit that has a greater than fifty percent likelihood of being realized upon ultimate settlement. These accounting rules also provide guidance on de-recognition, classification, interest, and penalties on income taxes, accounting in interim periods and requires increased disclosures. As of December 31, 2020, no liability for unrecognized tax benefits was required to be recorded.
As of December 31, 2021, there have been no allowances for the possibility of research and development tax credits, as the Company has not reach profitability.
During the year ended December 31, 2021 and 2020, sales to Advanced Dermatology Care Center, a dermatology practice owned by Dr. Laura Cohen, the Company’s President and Chief Executive Officer, accounted for 24% and 20% of Company’s revenue, respectively.
The Company has evaluated subsequent events occurring from January 1, 2022, through October 7, 2022, the date the financial statements were issued.
Subsequent to December 31, 2021, the Company received aggregate proceeds of $685,000 from the sale of 248,000 shares of common stock at $2.75 per share.
Subsequent to December 31, 2021, the Company received proceeds of $36,000 on the exercise of 71,734 stock options.
Subsequent to December 31, 2021, the Company granted an aggregate of 2,630,000 stock options to directors, executives and contractors.
Subsequent to December 31,2021, the Company was committed to pay a total of $175,000 under a sponsorship agreement.
ITEM 8. EXHIBITS
| Exhibit Number | | Description | | Form | | Exhibit | | Filing Date | | Filed
Herewith |
| 1.1 | | Articles of Incorporation of the Company, dated August 5, 2008 | | 1-A | | 2.1 | | 04/30/2021 | | |
| 3.1 | | Amended Articles of Incorporation, dated April 27, 2012 | | 1-A | | 2.2 | | 04/30/2021 | | |
| 3.2 | | Amended Articles of Incorporated, dated January 12, 2021 | | 1-A | | 2.3 | | 04/30/2021 | | |
| 3.3 | | Certificate of Change Pursuant to NRS 78.209, dated October 21, 2021 | | 1-U | | 3.1 | | 10/25/2021 | | |
| 2.4 | | Amended and Restated Bylaws of the Company | | 1-A | | 2.4 | | 04/30/2021 | | |
| 4.1 | | Form of Subscription Agreement | | 1-A | | 4.1 | | 04/30/2021 | | |
| 4.2 | | Distribution Agreement between CoLabs Int’l Corp and Spero Pte Ltd., dated March 23, 2021 | | 1-A | | 4.2 | | 04/30/2021 | | |
SIGNATURES
Pursuant to the requirements of Regulation A, the issuer has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| CoLabs Int’l, Corp. | |
| a Nevada corporation | |
| | |
| | /s/ Laura Cohen | |
| By: | Laura Cohen, Chief Executive Officer | |
| Date: | October 7, 2022 | |
Pursuant to the requirements of Regulation A, this report has been signed by the following persons on behalf of the issuer and in the capacities and on the dates indicated.
| | /s/ Laura Cohen | |
| By: | Laura Cohen | |
| Its: | Chief Executive Officer (Principal Executive Officer) | |
| Date: | October 7, 2022 | |
| | | |
| | /s/ William Cohen | |
| By: | William Cohen | |
| Its: | Chief Financial Officer (Principal Financial Officer and Principal Accounting Officer) | |
| Date: | October 7, 2022 | |


