As confidentially submitted to the Securities and Exchange Commission on May 15, 2015
Registration No. 333-
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM F-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
GC Aesthetics plc*
(Exact name of registrant as specified in its charter)
| | | | |
| Ireland | | 3842 | | Not applicable |
(State or other jurisdiction of incorporation or organization) | | (Primary Standard Industrial Classification Code Number) | | (I.R.S. Employer Identification Number) |
Suite 601, Q House, Furze Road
Sandyford, Dublin 18, Ireland
Telephone: +353 1293 3836
(Address, including zip code, and telephone number, including area code, of registrant’s principal executive offices)
Ayse Kocak
Chief Executive Officer
GC Aesthetics plc
Suite 601, Q House, Furze Road
Sandyford, Dublin 18, Ireland
Telephone: +353 1293 3836
(Name, address, including zip code, and telephone number, including area code, of agent for service)
Copies to:
| | | | |
Scott D. Elliott Michael D. Beauvais Ropes & Gray LLP Prudential Tower 800 Boylston Street Boston, MA 02199 Telephone: (617) 951-7000 | | Ian Crosbie Chief Financial Officer GC Aesthetics plc Suite 601, Q House, Furze Road Sandyford, Dublin 18, Ireland Telephone: +353 1293 3836 | | Michael W. Benjamin Ilir Mujalovic Shearman & Sterling LLP 599 Lexington Avenue New York, NY 10022 Telephone: (212) 848-4000 |
Approximate date of commencement of proposed sale to public: As soon as practicable after this Registration Statement is declared effective.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box. ¨
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
CALCULATION OF REGISTRATION FEE
| | | | |
|
Title of each class of securities to be registered | | Proposed maximum aggregate offering price(1)(2) | | Amount of registration fee |
Ordinary Shares, par value | | $ | | $ |
|
|
| (1) | Estimated solely for the purpose of calculating the registration fee in accordance with Rule 457(o) of the Securities Act of 1933, as amended. |
| (2) | Includes shares that may be sold upon exercise of the underwriters’ option to purchase additional shares. See “Underwriting.” |
The Registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment which specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933 or until the Registration Statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to said Section 8(a), may determine.
| * | Until immediately prior to the completion of the initial public offering described in this registration statement, GC Aesthetics plc will be a shell company. GC Aesthetics plc will not have conducted any operations (other than activities incidental to its formation, the share-for-share exchange described in the accompanying prospectus and the initial public offering described herein). The separate entity through which we currently operate our business is Global Consolidated Aesthetics Limited, a private limited company organized under the laws of Ireland. Global Consolidated Aesthetics Limited and its subsidiaries will become wholly owned by GC Aesthetics plc pursuant to the share-for-share exchange by which the existing shareholders of Global Consolidated Aesthetics Limited will exchange their shares in Global Consolidated Aesthetics Limited for shares having substantially the same rights in GC Aesthetics plc. Unless otherwise indicated or the context otherwise requires, all references in this registration statement to the “Company,” “GCA,” “we,” “us” and “our” refer to (i) Global Consolidated Aesthetics Limited together with its consolidated subsidiaries, prior to the completion of the share-for-share exchange described above, and (ii) GC Aesthetics plc and its consolidated subsidiaries following such share-for-share exchange, which is expected to occur immediately prior to the completion of this offering. See “Prospectus Summary—Corporate Information.” |
The information in this prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and it is not soliciting an offer to buy these securities in any jurisdiction where the offer or sale is not permitted.
Subject to Completion
Preliminary Prospectus dated , 2015
PROSPECTUS
Ordinary Shares
GC Aesthetics plc
Ordinary Shares
This is GC Aesthetics plc’s initial public offering. We are selling ordinary shares.
We expect the public offering price to be between $ and $ per ordinary share. Currently, no public market exists for the ordinary shares. After pricing of the offering, we expect that the ordinary shares will trade on the under the symbol “ .”
We are an “emerging growth company” under the federal securities laws and will be subject to reduced public company reporting requirements. See “Prospectus Summary—Implications of Being an Emerging Growth Company.”
Investing in the ordinary shares involves risks that are described in the “Risk Factors” section beginning on page 15 of this prospectus.
| | | | | | | | |
| | | Per Share | | | Total | |
Public offering price | | $ | | | | $ | | |
Underwriting discount(1) | | $ | | | | $ | | |
Proceeds, before expenses, to us | | $ | | | | $ | | |
| | (1) | The underwriters will receive compensation in addition to the underwriting discount. See “Underwriting.” |
The underwriters may also exercise their option to purchase up to an additional ordinary shares from us, at the public offering price, less the underwriting discount, for 30 days after the date of this prospectus.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
The ordinary shares will be ready for delivery on or about , 2015.
Joint Book-Running Managers
| | | | |
| BofA Merrill Lynch | | Deutsche Bank Securities | | Cowen and Company |
William Blair
The date of this prospectus is , 2015.
TABLE OF CONTENTS
We have not, and the underwriters have not, authorized anyone to provide any information or to make any representations other than those contained in this prospectus or in any free writing prospectus prepared by or on behalf of us or to which we have referred you. We take no responsibility for, and can provide no assurance as to the reliability of, any other information that others may give you. This prospectus is an offer to sell only the ordinary shares offered hereby, and only under circumstances and in jurisdictions where it is lawful to do so. The information contained in this prospectus or in any applicable free writing prospectus is current only as of its date, regardless of its time of delivery or any sale of ordinary shares. Our business, financial condition, results of operations and prospects may have changed since that date.
i
PROSPECTUS SUMMARY
This summary highlights information contained in other parts of this prospectus. Because it is only a summary, it does not contain all of the information that you should consider before investing in our ordinary shares and it is qualified in its entirety by, and should be read in conjunction with, the more detailed information appearing elsewhere in this prospectus. You should read the entire prospectus carefully, especially “Risk Factors” and our financial statements and the related notes included elsewhere in this prospectus, before deciding to buy our ordinary shares. Unless otherwise indicated or the context otherwise requires, all references in this prospectus to the “Company,” “we,” “us” and “our” refer to (i) Global Consolidated Aesthetics Limited together with its consolidated subsidiaries, prior to the completion of the share-for-share exchange by which the existing shareholders of Global Consolidated Aesthetics Limited exchange their shares in Global Consolidated Aesthetics Limited for shares having substantially the same rights in GC Aesthetics plc, which is expected to occur immediately prior to the completion of this offering (the “Share-for-Share Exchange”), and (ii) GC Aesthetics plc and its consolidated subsidiaries following the Share-for-Share Exchange.
Overview
We are a leading pure-play female aesthetics company committed to becoming the trusted brand and partner for women seeking to look healthy, youthful, vibrant and beautiful, and to feel confident about themselves throughout their lifetime. To date, we have generated significant growth by focusing on the development, manufacturing and commercialization of one of the broadest ranges of implant products, principally silicone breast implants. We believe that through our two brands Nagôr and Eurosilicone, which have a combined 60 years of market presence, we have (i) a strong reputation for safety, quality and reliability, as evidenced by the five-year data from our ongoing Eurosilicone safety study, (ii) strong competitive advantages based on our innovative product design features—the result of our focused approach to research and development, which integrates feedback from women and surgeons, and (iii) a differentiated marketing and customer service approach, focusing on the needs of women.
We have developed and marketed our comprehensive range of products, comprising over 1,100 stock keeping units (“SKUs”), to address the different needs and preferences of women and surgeons. All of our implants are manufactured with medical-grade silicone supplied from either NuSil Technology LLC (“NuSil”) or Applied Silicone Corporation (“ASC”), both of which are U.S.-based, International Organization for Standardization (“ISO”) 9001-certified sources. We have manufacturing facilities in the United Kingdom and France and sell our products in 75 countries, with direct sales activities in six countries, including Brazil, the world’s largest medical aesthetics market by total number of plastic surgery procedures.
The global aesthetics products market in 2014 was estimated at $6.5 billion in total sales and is expected to grow at a compound annual growth rate (“CAGR”) of nearly 12%, reaching almost $10.2 billion in total sales by 2018, according to Medical Insight’s “The Global Aesthetic Market Study: Version XII” report (“Medical Insight”). We believe the overall market growth is driven by multiple factors, including a growing middle class in emerging markets, an increase in women’s disposable income, increasing awareness and acceptance of aesthetics procedures, as well as continuous innovation and improved accessibility to aesthetics procedures. While the United States has historically been the largest market for aesthetics products in terms of total revenues, a significant number of markets outside of the United States have been growing faster in terms of revenues and plastic surgeon numbers. For example, Brazil, Mexico and China have experienced faster revenue growth than the aesthetics products market in the United States, providing attractive growth opportunities. The global breast implant market is forecasted to reach nearly $1.3 billion by 2018, with some estimates forecasting the market will reach nearly $1.5 billion by 2019, growing at a CAGR ranging from approximately 4% to 6% from 2014 to 2018.
1
We have a diversified revenue base, with approximately 47% of our 2014 revenues derived from sales in Europe, the Middle East and Africa (“EMEA”) (inclusive of the United Kingdom, Switzerland, Bosnia, the European Economic Area (the “EEA”), the Commonwealth of Independent States (“CIS”) countries, the Middle East and Africa), approximately 42% in Latin America (inclusive of Mexico, the Dominican Republic, Panama and South America) and approximately 11% in Asia-Pacific. Our revenues were approximately $52.8 million for the year ended December 31, 2014, as compared to approximately $44.6 million for the year ended December 31, 2013, an increase of 18.4% at Constant Currency Revenue. For additional information regarding Constant Currency Revenue, which is a non-International Financial Reporting Standards (“IFRS”) measure, including a reconciliation of Constant Currency Revenue to revenue, see “—Summary Consolidated Financial Data” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Key Financial Metrics.”
The Global Aesthetics Products Market
The global aesthetics products market in 2014 was estimated at $6.5 billion in total sales and is expected to grow at a CAGR of nearly 12%, reaching almost $10.2 billion in total sales by 2018, according to Medical Insight. We believe the overall market growth is driven by multiple factors, including a growing middle class, increasing female disposable income and increasing awareness and acceptance of aesthetics procedures, as well as continuous innovation and improved accessibility to aesthetics procedures, through an increase in the number of surgeons. The number of middle- and upper-income women globally is forecasted to grow at a CAGR of approximately 5% from 2014 to 2019 to 346 million, according to industry sources.
While the United States has historically been the largest market for aesthetics products in terms of total revenues, a significant number of markets outside the United States have been growing faster in terms of revenues and plastic surgeon numbers. We believe that this growth in markets outside of the United States will continue based on increasing penetration rates in many of those markets, increases in plastic surgeon numbers and favorable demographic and economic trends. For example, in 2013, Brazil surpassed the United States in total number of plastic surgery procedures, accounting for 12.9% of global plastic surgery procedures as compared to 12.5% for the United States, according to the International Society of Aesthetic Plastic Surgeons (“ISAPS”). The international market has also seen significant growth in the number of plastic surgeons. While the number of plastic surgeons in the United States grew at a CAGR of approximately 1% from 2010 to 2013, the number of plastic surgeons in countries outside the United States included in the ISAPS Report grew at an estimated average CAGR of 8% for the same period. For example, China and South Korea grew their plastic surgeon populations at CAGRs of approximately 12% and 18%, respectively, from 2010 to 2013. Brazil’s plastic surgeon population of 5,473 in 2013 is close to that of the United States, at 6,133. We believe the increase in the plastic surgeon population is representative of improved accessibility and a growing infrastructure supportive of further expansion of the overall market.
Overview of the Global Breast Implant Market
Breast augmentation surgery represented the leading aesthetic surgical procedure, with approximately 15% of total aesthetic surgical procedures performed by plastic surgeons in 2013, based on ISAPS. The global breast implant market is forecasted to reach nearly $1.3 billion by 2018, with some estimates forecasting the market will reach nearly $1.5 billion by 2019, growing at a CAGR ranging from approximately 4% to 6% from 2014 to 2018. Breast implant surgery is used to enhance aesthetic appearance and comprises two general categories: (i) breast augmentation procedures for cosmetic purposes and (ii) breast reconstruction procedures to replace breast tissue that has been removed, mainly due to cancer or trauma.
A number of international breast implant markets have recently shown significantly faster growth than the United States and many of the non-U.S. markets are still highly underpenetrated, based on the number of
2
women undergoing breast implant procedures, compared to the penetration of global brands in the United States. We believe that current trends in non-U.S. markets will continue to increase penetration rates in those markets, creating a significant growth opportunity for us, given our global focus.
Favorable Demographics and Economic Trends for the Global Aesthetics Market
The primary market for aesthetics procedures is comprised of middle- and upper-class women, which BCC Research’s “Medical Aesthetic Devices: Technologies and Global Markets” report (“BCC Research”) defines as women with access to sufficient income to afford such aesthetics procedures. From 2014 to 2019, the global aesthetics products market is estimated to grow at a CAGR of .5% in the United States, in contrast to 11.0%, 8.3% and 8.2% in China, Mexico and Brazil, respectively, according to BCC Research. Moreover, the Company estimates that the global aesthetics products market is projected to grow at a higher CAGR than the world gross domestic product (“GDP”), and, in China, Brazil and Mexico, the aesthetics products market is projected to grow at a higher CAGR than their respective GDPs. In contrast, the U.S. aesthetics products market is projected to grow more slowly than the GDP of the United States. In contrast, the U.S. aesthetics products market is projected to grow more slowly than the GDP of the United States.
Euromonitor projects the global annual disposable income per capita for women will grow at a CAGR of approximately 6% over the next five years. We believe this growth will help drive the expansion of the global aesthetics market. In addition, over the next 20 years, it is estimated that there will be a significant increase in the world’s middle class population, which is expected to account for a significant increase in purchasing power and discretionary spending. We believe much of this growth in purchasing power will come from emerging markets, due to increasing annual disposable incomes in those markets. For example, the Chinese middle- and upper-income female population is expected to reach 78 million by 2019, matching that of the United States. For example, in Brazil, the number of breast implant units sold grew by 11.5% in 2012, according to industry sources, while the country’s GDP growth slowed to only 1.8% over that same period. In Europe, the number of breast implant units sold grew by 12.8% in 2012, according to the same source; in contrast, overall GDP grew by 0.3% over that same period.
Our Opportunity
We believe we have a significant opportunity to capitalize on the favorable trends in the aesthetics market and benefit from the underlying growth in women’s disposable income, particularly in emerging markets, given the strength of our brands, quality and breadth of our product portfolio, differentiated customer service, strong customer relationships, expanding global infrastructure and new product development. Currently, we focus on the breast implant market outside of the United States, as well as other pre- and post-breast implant surgery products globally. We have a significant presence in Europe, Latin America and Asia-Pacific and we intend to continue to further expand in these regions as well as in Russia and the Middle East and into emerging markets, including China, Thailand and additional CIS countries. Beyond breast implant products, we intend to expand our presence in other selected female aesthetics products, including those associated with implant surgery as well as other procedures, such as body sculpting treatments and dermal fillers.
Our Competitive Strengths
We believe our pure-play focus has allowed us to become a leading player in the female aesthetics market. The following are our key competitive strengths:
Highly regarded and trusted brands with a strong safety profile. We have two prominent brands, Nagôr and Eurosilicone, which have a combined 60 years of market presence. Based on a company-sponsored, Eurosilicone breast implant study in Europe with 534 subjects, the peer-reviewed five-year clinical safety data published in the Plastic and Reconstructive Surgery journal of the American Society of Plastic Surgeons (“Plastic and Reconstructive Surgery”) supported the strong safety profile of our breast implant products, with low incidence of rupture and rates of capsular contracture comparable to rates experienced by others in our industry for primary augmentation surgery.
3
Broad product range recognized for quality. Breast implant products and pre- and post-breast implant surgery products are our core competency. Unlike our primary competitors, who generate a very small portion of their total revenue from breast implant products, we generate substantially all of our revenue from our breast implant products and are focused on providing one of the most comprehensive selections of breast implant and other products, including over 1,100 SKUs, for our customers as well as consistently delivering high levels of customer satisfaction.
Global sales and marketing platform. We sell our products in 75 countries and are one of the leading breast implant manufacturers globally by units sold. We believe our strong sales and marketing platform will help us to successfully launch product line extensions and thereby drive additional revenue growth, particularly in rapidly growing emerging markets.
Extensive in-house development and manufacturing capabilities. All of our products have been developed in-house. Our manufacturing operations allow us to rapidly develop new products based on our close relationships with women and surgeons and our extensive industry experience. We have manufacturing facilities in the United Kingdom and France, all of which are ISO certified and are regularly inspected by their respective regulatory bodies.
Focus on integrated marketing and delivering high-quality customer service. We believe that our customer-focused approach to product design, marketing and customer care is a key differentiator. Our integrated marketing approach consists of woman-centric initiatives and surgeon-centric support programs.
Experienced management team. We are led by a proven management team with a successful track record in healthcare and aesthetics. Ayse Kocak is the only female chief executive officer of a breast implant company and has over 20 years of experience in the healthcare industry.
Our Growth Strategies
Our goal is to become the leading pure-play female aesthetics company. We are committed to becoming the trusted brand and partner for women seeking to look healthy, youthful, vibrant and beautiful, and to feel confident about themselves throughout their lifetime. To achieve these objectives, we are pursuing the following growth strategies:
Outperform the competition in existing markets. Our focus on female aesthetics and delivering high-quality service has distinguished our brands and has enabled us to gain market share from our major competitors in key markets, including Brazil and Mexico, the second and third largest markets by number of breast implant units sold. Based on internal estimates, from 2013 to 2014, our global market share outside of North America increased from 18% to 20%, based on breast implant units sold. We believe that as our brand awareness increases and our reputation for quality and service grows, we will be able to achieve additional growth in our existing markets.
Penetrate new geographical markets with a focus on direct operations and commercialize new products. We currently sell our products directly in six countries and through distributors in an additional 69 countries. We intend to increase the number of markets where we sell direct, which we believe will drive gross margin expansion, as our gross margin is higher in markets where we sell direct.
Leverage our surgeon relationships and strong brand recognition to introduce innovative products. By leveraging the established position of our Nagôr and Eurosilicone brands and strong surgeon relationships, we plan to expand our product range in female aesthetics. We plan to continue innovating and introducing new products like IMPLEO to expand our product portfolio to offer the best solutions for our customers.
4
Continue our customer-centric approach and launch additional novel initiatives. Unlike our larger competitors, we are a pure-play female aesthetics company. We intend to continue to effectively engage women and surgeons through an integrated approach that includes direct marketing, distribution partnerships and social media campaigns to help promote the acceptance of breast implants and help patients take action to change the way they feel about themselves.
Selectively pursue strategic acquisitions. We may selectively pursue complementary acquisitions that would allow us to enlarge our female aesthetics product portfolio, go direct in new or existing markets or expand further in existing markets.
Our Products
We have developed and marketed our comprehensive range of products, comprising over 1,100 SKUs, to address the different needs and preferences of women and surgeons. Through integrating our experience of developing, manufacturing and securing regulatory approval for our breast implant products and our close relationship with women and surgeons, we believe that we are well positioned to continue our track record of developing a continuing stream of innovative products.
Breast Augmentation and Breast Reconstruction Products
Breast Implant Products.Our product portfolio addresses a comprehensive range of options:
| | |
Shape | | Round and anatomical (also known as teardrop) |
Texture | | Smooth and three different textures |
Volume | | 100ml to 800ml |
Projection | | Low to extra-high |
Gel cohesivity | | Five different cohesivities |
We recently commenced active promotion of the IMPLEO breast implant product line, which we believe are the first round implants to be soft to the touch, deliver shape maintenance and a range of texturing as well as permitting a small surgical incision due to their unbreakable gel. We believe that through this combination of features, a wider range of surgeons is able to deliver an outcome to women that was previously achievable only by the highest quality surgeons.
The commercial impact of IMPLEO was significant in certain countries in 2014, its first year of active promotion in these markets; it accounted for approximately 20% of our sales in France and for approximately 56% of our sales in the United Kingdom. IMPLEO is currently available in 44 of the 75 countries in which we sell our products, and we are planning to launch it in almost all of our other markets in 2015, subject to receiving required regulatory approvals.
Breast Sizers.We produce a range of these temporary, single-use products that can be used by surgeons during surgery to help identify the desired style and size of implant for each woman.
Breast Tissue Expanders. These are used in breast reconstruction following cancer and other trauma injuries, and are temporary devices intended to aid in the process of recreating tissue coverage to allow for the placement of an implant to reconstruct the breast.
Other Implants
We offer a range of other implant products, including gluteal, testicular and calf implants, all made from medical-grade silicone. In 2014, these represented 5.5% of our sales.
5
Pre- and Post-Breast Implant Surgery Products
In the second half of 2015, we are planning to launch a range of consumer products to offer a broader solution and reinforce our brand loyalty among women considering breast augmentation and those who have had the procedure, including the following products:
The Enhance Breast Boost Pack. This non-invasive product, comprising a specialized bra and silicone breast forms, allows women to experience the look and feel of fuller breasts instantly, and we believe this will lead to an increase in the number of women considering surgical augmentation and consulting surgeons.
Silgel scar management specialty products. These gel and sheet products minimize the appearance of post-surgery scars, and help us build upon our existing relationships with surgeons and access other market channels.
Our Five-Year Clinical Data
In June 2014, Plastic and Reconstructive Surgery published the peer-reviewed five-year results from an ongoing study to evaluate the safety profile of our Eurosilicone breast implant products. This multi-center, surgeon-led study, sponsored by us, monitors 1,010 Eurosilicone breast implants in 534 subjects who have undergone either augmentation or reconstructive surgery. Each patient had a three-month post-surgery follow-up evaluation and annual follow-ups thereafter. The study’s five-year results demonstrate a low rupture rate and a strong safety profile. For primary augmentation, (i) the total risk of rupture was assessed at 0.8% per patient (only one of the 1,010 implants was found to be ruptured on examination) and (ii) the incidence of severe capsular contracture was 10.7%, which we believe is consistent with rates experienced by others in our industry.
Summary Risk Factors
An investment in our ordinary shares involves a high degree of risk. Any of the factors set forth under “Risk Factors” in this prospectus may limit our ability to successfully execute our business strategy. You should carefully consider all of the information set forth in this prospectus and, in particular, you should evaluate the specific factors set forth under “Risk Factors” in deciding whether to invest in our ordinary shares. Some of the principal risks relating to our business and our ability to execute our business strategy include:
| | • | | We have incurred significant net operating losses and cannot assure you that we will ever achieve or maintain profitability. |
| | • | | Our future profitability depends on the success of our breast implant products. |
| | • | | Any negative publicity concerning our products or our competitors’ products could harm our business and reputation and negatively impact our financial results. |
| | • | | Our success depends on our ability to continue to enhance our existing products and develop or commercialize new products that respond to customer needs and preferences. |
| | • | | The size of the addressable global aesthetics products market, and the breast implant products market in particular, has not been established with precision and may be smaller than we estimate, possibly materially. If our estimates and projections overestimate the size of these markets and the markets for our other products, our revenue and results of operations will be adversely affected. |
| | • | | Each of our brands relies on a single-source supplier for medical-grade silicone, which is the primary constitutive ingredient of our products. These two suppliers are under common ownership, which could result in increased prices when we seek to renew our contracts with these suppliers. |
6
| | • | | Recent studies have called into question the long-term safety of breast implants, which could have a material adverse effect on our business, results of operations and financial condition. |
| | • | | If we acquire or attempt to acquire new businesses or products, we may be unable to complete these acquisitions or to successfully integrate them in a cost-effective and non-disruptive manner, and we may not realize the benefits we anticipate from such acquisitions. |
| | • | | We will need to significantly increase the size of our organization, and we may experience difficulties in managing growth. |
| | • | | Our business could suffer if we lose the services of our senior management or other key personnel or are unable to attract and retain additional qualified personnel. |
| | • | | If we fail to maintain and further develop our direct sales forces and distributor networks, our business could suffer. Additionally, our third-party distributors may not effectively sell our products or may engage in activities that could harm our reputation and sales of our products. |
| | • | | Our results of operations could be materially adversely affected by fluctuations in foreign currency exchange rates. |
| | • | | Any disruption at our manufacturing facilities could adversely affect our business and results of operations. |
| | • | | If we fail to compete effectively against our competitors, many of whom have greater resources than we have, our revenues and results of operations may be negatively affected. |
| | • | | Our medical device products and operations, including manufacturing, are subject to extensive governmental regulation, including health, environmental, safety and anti-corruption and other laws, and our failure to comply with applicable requirements could cause our business to suffer. |
Implications of Being an Emerging Growth Company
As a company with less than $1.0 billion in revenue during our most recently completed fiscal year as of the initial filing date of the registration statement of which this prospectus forms a part, we qualify as an “emerging growth company” as defined in the Jumpstart our Business Startups Act of 2012 (the “JOBS Act”). As an emerging growth company, we may take advantage of specified reduced disclosure and other requirements that are otherwise applicable generally to public companies that are not emerging growth companies. These provisions include:
| | • | | the ability to present more limited financial data, including presenting only two years of audited financial statements and only two years of selected financial data in the registration statement on Form F-1 of which this prospectus is a part; |
| | • | | an exemption from certain disclosure obligations regarding executive compensation in the registration statement on Form F-1 of which this prospectus is a part and in our future periodic reports; |
| | • | | an exception from compliance with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act of 2002 (the “Sarbanes-Oxley Act”); and |
| | • | | exemptions from the requirements of holding a non-binding advisory vote on executive compensation and obtaining shareholder approval of any golden parachute payments. |
7
We may take advantage of these exemptions for up to five years or such time that we are no longer an emerging growth company. We would cease to be an emerging growth company if we have more than $1.0 billion in annual revenues as of the end of our fiscal year, we have more than $700 million in market value of our stock held by non-affiliates as of the end of our second fiscal quarter, or we issue more than $1.0 billion of non-convertible debt over a three-year period. We may choose to take advantage of some but not all of these reduced disclosure obligations. We have taken advantage of reduced disclosure obligations regarding financial statements and executive compensation arrangements in this prospectus, and we may choose to take advantage of some but not all of these obligations in future filings. If we do, you may receive different and less information than you might get from other public companies.
We will not take advantage of the extended transition period provided under Section 7(a)(2)(B) of the Securities Act of 1933, as amended (the “Securities Act”), for complying with new or revised accounting standards. Since IFRS as issued by the International Accounting Standards Board (“IASB”) (“IFRS as issued by the IASB”) makes no distinction between public and private companies for purposes of compliance with new or revised accounting standards, the requirements for our compliance as a private company and as a public company are the same.
Implications of Being a Foreign Private Issuer
We are also considered a “foreign private issuer.” In our capacity as a foreign private issuer, we are exempt from certain rules under the U.S. Securities Exchange Act of 1934, as amended (the “Exchange Act”), that impose certain disclosure obligations and procedural requirements for proxy solicitations under Section 14 of the Exchange Act. In addition, our officers, directors and principal shareholders are exempt from the reporting and short-swing profit recovery provisions of Section 16 of the Exchange Act and the rules under the Exchange Act with respect to their purchases and sales of our ordinary shares. Moreover, while we must file an annual report on Form 20-F, we are not required to file periodic reports and financial statements with the Securities and Exchange Commission (“SEC”) as frequently or as promptly as U.S. companies whose securities are registered under the Exchange Act, although we do intend to furnish quarterly reports on Form 6-K. In addition, although we plan to comply with Regulation FD, which restricts the selective disclosure of material non-public information, we are not required to comply with Regulation FD. As a foreign private issuer, we also may elect, and have elected, to follow Irish corporate governance rules to the extent permitted.
We may take advantage of these exemptions until such time as we are no longer a foreign private issuer. We would cease to be a foreign private issuer at such time as more than 50% of our outstanding voting securities are held by U.S. residents and any of the following three circumstances applies: (i) the majority of our executive officers or directors are U.S. citizens or residents, (ii) more than 50% of our assets are located in the United States or (iii) our business is administered principally in the United States.
We have taken advantage of certain reduced reporting and other requirements in this prospectus. Accordingly, the information contained herein may be different than the information you receive from other public companies.
Corporate Information
Global Consolidated Aesthetics Limited was incorporated in December 2007 under the laws of Ireland and is the holding company of Eurosilicone SAS and Nagôr Limited, the main operating entities within our group, which it acquired from Medicor Ltd. (which was in Chapter 11 bankruptcy proceedings) in May 2008. Eurosilicone SAS was incorporated in 1988 under the laws of France. Nagôr Limited was incorporated in 1979 under the laws of the Isle of Man, and operates as the registered branch of an overseas company in the United Kingdom. Global Consolidated Aesthetics Limited’s acquisition of Nagôr Limited and Eurosilicone SAS in
8
May 2008 was funded in part by an equity investment in our ordinary shares led by Oyster Technology Investments Limited (“Oyster”). Montreux Equity Partners IV, L.P. (“Montreux”) first became a shareholder in Global Consolidated Aesthetics Limited pursuant to an investment in October 2010. Both Montreux and Oyster have made significant further equity investments in Global Consolidated Aesthetics Limited since their initial investments. See “Description of Share Capital—History of Security Issuances.”
GC Aesthetics plc was incorporated in April 2015 under the laws of Ireland. Upon the Share-for-Share Exchange, GC Aesthetics plc will become the holding company of Global Consolidated Aesthetics Limited and its subsidiaries. Until immediately prior to the completion of the initial public offering described in this prospectus, GC Aesthetics plc will be a shell company. GC Aesthetics plc will not have conducted any operations (other than activities incidental to its formation, the Share-for-Share Exchange described below and the initial public offering described herein). Global Consolidated Aesthetics Limited is the entity through which the Company currently operates its business. Upon the Share-for-Share Exchange, the historical consolidated financial statements of Global Consolidated Aesthetics Limited included in this prospectus will become the historical consolidated financial statements of GC Aesthetics plc.
Our principal executive offices are located at Suite 601, Q House, Furze Road, Sandyford, Dublin 18, Ireland. Our telephone number at that address is +353 1293 3836. Our website address is www.gcaesthetics.com. Our website and the information contained on our website do not constitute a part of this prospectus.
9
The Offering
Ordinary shares offered by us | ordinary shares. |
Ordinary shares to be outstanding immediately after completion of this offering | ordinary shares ( ordinary shares if the option to purchase additional shares is exercised in full). |
Option to purchase additional ordinary shares | The underwriters have an option for a period of 30 days after the date of this prospectus to purchase up to an additional ordinary shares. |
Use of proceeds | We estimate that the net proceeds from this offering will be approximately $ million, or approximately $ million if the underwriters exercise their option to purchase additional ordinary shares in full, at an assumed initial public offering price of $ per ordinary share, the midpoint of the price range set forth on the cover of this prospectus, after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. We intend to use the net proceeds of this offering to expand our (i) operations into new countries, (ii) product portfolio, (iii) existing commercial infrastructure and (iv) manufacturing capabilities. In addition, we intend to use the net proceeds of this offering to fund potential future acquisitions. We will use any remaining proceeds for working capital and other general corporate purposes. See “Use of Proceeds.” |
Dividend policy | We do not currently expect to pay any dividends on our ordinary shares in the foreseeable future. Additionally, our ability to pay dividends on our ordinary shares is limited by restrictions on our and our subsidiaries’ ability to pay dividends or make distributions, including restrictions under the terms of the agreements governing our indebtedness and under Irish law. |
Risk factors | You should read the “Risk Factors” section of this prospectus for a discussion of factors to consider carefully before deciding to invest in our ordinary shares. |
Listing | We intend to apply to list our ordinary shares on the under the symbol “ .” |
The number of ordinary shares to be outstanding after this offering is based on ordinary shares of Global Consolidated Aesthetics Limited outstanding as of , giving effect to the Share-for-Share Exchange, and excludes ordinary shares of GC Aesthetics plc reserved for future issuance under its equity incentive plan to be adopted in connection with this offering.
10
Unless context otherwise requires, this prospectus reflects and assumes the following:
| | • | | the completion, prior to or simultaneously with the completion of this offering, of the Share-for-Share Exchange; |
| | • | | an initial public offering price of $ per ordinary share (the midpoint of the price range set forth on the front cover of this prospectus); |
| | • | | the adoption of our amended and restated memorandum and articles of association, to be effective upon the completion of this offering; |
| | • | | the exercise of all warrants and the conversion of all of our outstanding A Ordinary Shares, B Preference Shares and C Preference Shares into ordinary shares; and |
| | • | | no exercise by the underwriters of their option to purchase up to additional ordinary shares in this offering. |
11
Summary Consolidated Financial Data
The following summary consolidated financial data has been derived from the audited consolidated statements of operations data of Global Consolidated Aesthetics Limited for the years ended December 31, 2014 and December 31, 2013 and the audited consolidated balance sheet data of Global Consolidated Aesthetics Limited as of December 31, 2014 and December 31, 2013 appearing elsewhere in this prospectus. Our historical results for any prior period are not necessarily indicative of results to be expected in any future period. Our consolidated financial statements are presented in US dollars. However, the Euro is our measurement and functional currency and also that of the majority of our subsidiaries. The presentation currency of the Company is the US dollar. The following information should be read in conjunction with “Risk Factors,” “Capitalization,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our consolidated financial statements and the related notes included elsewhere in this prospectus.
| | | | | | | | |
| | | Year Ended | |
| | | December 31, 2014 | | | December 31,
2013 | |
| | | (in thousands) | |
Statements of operations and comprehensive loss: | | | | | | | | |
Revenue | | $ | 52,804 | | | $ | 44,630 | |
Cost of sales | | | (26,038 | ) | | | (22,548 | ) |
Operating expenses | | | (41,835 | ) | | | (30,858 | ) |
| | | | | | | | |
Operating loss | | | (15,069 | ) | | | (8,776 | ) |
Other (expense)(1) | | | (74,958 | ) | | | (9,711 | ) |
Income tax credit | | | 600 | | | | 1,195 | |
| | | | | | | | |
Net loss | | $ | (89,427 | ) | | $ | (17,292 | ) |
| | | | | | | | |
Basic and diluted loss per ordinary share | | $ | (18.74 | ) | | $ | (3.91 | ) |
| | |
Balance sheet data (at period end): | | | | | | | | |
Cash and cash equivalents | | $ | 10,616 | | | $ | 4,519 | |
Total current assets | | | 46,988 | | | | 30,576 | |
Total assets | | | 79,150 | | | | 69,726 | |
Total current liabilities | | | 19,592 | | | | 42,885 | |
Loans and borrowings | | | 51,457 | | | | 19,759 | |
Total non-current liabilities | | | 196,538 | | | | 92,243 | |
Total liabilities | | | 216,130 | | | | 135,128 | |
Accumulated deficit | | | (183,489 | ) | | | (94,062 | ) |
Total equity / (deficiency) | | | (136,980 | ) | | | (65,402 | ) |
| | |
Other financial data (unaudited): | | | | | | | | |
Constant Currency Revenue(2) | | $ | 52,804 | | | $ | 44,607 | |
Adjusted Gross Profit(3) | | | 27,788 | | | | 23,134 | |
Adjusted EBITDA(4) | | | (8,112 | ) | | | (3,385 | ) |
Adjusted Interest Expense(5) | | | (6,202 | ) | | | (3,564 | ) |
| (1) | Other (expense) includes the following expenses: |
| | | | | | | | |
| | | Year Ended December 31, | |
| | | 2014 | | | 2013 | |
| | | (in thousands) | |
Net interest (Expense) income | | $ | (33,746 | ) | | $ | (6,877 | ) |
Loss on conversion of shareholder loans | | | (27,737 | ) | | | — | |
Gain (Loss) on equity derivatives | | | 5,182 | | | | (4,828 | ) |
(Loss) Gain on foreign exchange | | | (18,657 | ) | | | 1,994 | |
12
| (2) | Constant Currency Revenue is defined as revenue calculated by translating revenues for both 2014 and 2013 using the average exchange rates for 2014 of the Euro, Sterling and the Brazilian Real, respectively, to one US dollar, our financial reporting currency. We believe that Constant Currency Revenue is a useful measure, indicating the actual growth of our operations, excluding the impact of foreign currency exchange rates changes. Constant Currency Revenue is not calculated in accordance with IFRS. This calculation may differ from similarly titled measures used by others and, accordingly, Constant Currency Revenue is not meant to be a substitution for revenues presented in conformity with IFRS nor should such non-IFRS financial measure be considered in isolation. Moreover, the presentation of Constant Currency Revenue is not necessarily indicative of historical or future results of operations. Currency fluctuations affect general economic and business conditions, including, for example, a country’s inflation and international trade competitiveness and, as a result, a company’s performance cannot be evaluated solely on the basis of a constant currency presentation. |
The following table reconciles Constant Currency Revenues to revenues, the most comparable IFRS measure, for each of the periods indicated:
| | | | | | | | |
| | | Year Ended December 31, | |
| | | 2014 | | | 2013 | |
| | | (in thousands) | |
Reconciliation of Constant Currency Revenue to Revenue: | | | | | | | | |
Revenue | | $ | 52,804 | | | $ | 44,630 | |
Effect of movement in exchange rates(1) | | | — | | | | (23 | ) |
| | | | | | | | |
Constant Currency Revenue | | $ | 52,804 | | | $ | 44,607 | |
| | | | | | | | |
| | (1) | Constant Currency Revenue is calculated by converting revenues for both 2014 and 2013 using the average exchange rates for 2014 of the Euro, to one US dollar. | |
| (3) | Adjusted Gross Profit is defined as gross profit excluding depreciation that is included in the cost of sales. We believe that Adjusted Gross Profit is a useful measure, indicating the actual profitability of our production excluding these non-cash charges. Adjusted Gross Profit is not calculated in accordance with IFRS. |
The following table reflects the reconciliation of Adjusted Gross Profit to gross profit, the most comparable IFRS measure, for each of the periods indicated:
| | | | | | | | |
| | | Year Ended December 31, | |
| | | 2014 | | | 2013 | |
| | | (in thousands) | |
Reconciliation of Adjusted Gross Profit to Gross Profit: | | | | | | | | |
Gross profit | | $ | 26,766 | | | $ | 22,082 | |
Depreciation included in cost of sales | | | 1,022 | | | | 1,052 | |
| | | | | | | | |
Adjusted Gross Profit | | $ | 27,788 | | | $ | 23,134 | |
| | | | | | | | |
| (4) | Adjusted EBITDA is defined as operating loss, adjusted for other expenses, depreciation, amortization and share-based and other equity-related compensation. We believe that Adjusted EBITDA is a useful metric for investors to understand and evaluate our operating results and ongoing profitability because it permits investors to evaluate our recurring profitability from our ongoing operating activities. Adjusted EBITDA is not calculated in accordance with IFRS. In addition, our calculation of Adjusted EBITDA may not be comparable to similar measures that other companies report because other companies may not calculate Adjusted EBITDA in the same manner as we do. |
By providing this non-IFRS financial measure, together with a reconciliation to IFRS results, we believe we are enhancing investors’ understanding of our business and our results of operations, as well as assisting investors in evaluating how we are executing strategic initiatives. We believe Adjusted EBITDA is widely used by investors and securities analysts as a supplemental measure to evaluate the overall operating
13
performance of companies in our industry without regard to items, such as interest expense, provision for income taxes and depreciation and amortization expense, that can vary substantially from company to company depending upon their financing and accounting methods, the book value of their assets, their capital structures and the method by which their assets were acquired.
Management uses Adjusted EBITDA:
| | • | | as a measurement used in comparing our operating performance on a consistent basis; |
| | • | | for planning purposes, including the preparation of our internal annual operating budget and financial projections; and |
| | • | | to evaluate the performance and effectiveness of our operational strategies. |
Although we use Adjusted EBITDA as a measure to assess the operating performance of our business, Adjusted EBITDA has limitations as an analytical tool, and you should not consider it in isolation, or as a substitute for analysis of our results as reported under IFRS. Because of these limitations, management does not view Adjusted EBITDA in isolation or as a primary performance measure.
The following table reconciles Adjusted EBITDA to operating loss, the most comparable IFRS measure, for each of the periods indicated:
| | | | | | | | |
| | | Year Ended December 31, | |
| | | 2014 | | | 2013 | |
| | | (in thousands) | |
Reconciliation of Adjusted EBITDA to Operating Loss: | | | | | | | | |
Operating loss | | $ | (15,069 | ) | | $ | (8,776 | ) |
Add: | | | | | | | | |
Other expenses | | | — | | | | 183 | |
Depreciation | | | 1,123 | | | | 1,175 | |
Amortization | | | 3,990 | | | | 4,033 | |
Share-based and other equity-related compensation | | | 1,844 | | | | — | |
| | | | | | | | |
Adjusted EBITDA | | $ | (8,112 | ) | | $ | (3,385 | ) |
| | | | | | | | |
| (5) | Adjusted Interest Expense is defined as interest expense adjusted for non-cash charges related to effective interest on our B Preference Shares and C Preference Shares. The B Preference Shares and C Preference Shares will convert into ordinary shares in connection with this offering; as a result, no further charges in respect thereof will be incurred in future periods. However, we believe Adjusted Interest Expense is a useful metric for investors to understand and evaluate our results of operations as it permits investors to evaluate the cash costs of servicing our debt in prior periods. Adjusted Interest Expense is not calculated in accordance with IFRS. |
The following table reconciles Adjusted Interest Expense to interest expense, the most comparable IFRS measure, for each of the periods indicated:
| | | | | | | | |
| | | Year Ended December 31, | |
| | | 2014 | | | 2013 | |
| | | (in thousands) | |
Reconciliation of Adjusted Interest Expense to Interest Expense: | | | | | | | | |
Interest expense | | $ | (33,746 | ) | | $ | (6,877 | ) |
Non-cash charges related to effective interest on B Preference Shares and C Preference Shares | | | 27,544 | | | | 3,313 | |
| | | | | | | | |
Adjusted Interest Expense | | $ | (6,202 | ) | | $ | (3,564 | ) |
| | | | | | | | |
14
RISK FACTORS
Investing in our ordinary shares involves a high degree of risk. You should carefully consider the risks and uncertainties described below together with all of the other information contained in this prospectus, including our consolidated financial statements and the related notes appearing at the end of this prospectus, before deciding to invest in our ordinary shares. If any of the following risks actually occurs, our business, prospects, results of operations and financial condition could suffer materially, the trading price of our ordinary shares could decline and you could lose all or part of your investment. The risks and uncertainties described below are not the only ones we face. Additional risks and uncertainties not presently known to us or that we currently believe to be immaterial may also adversely affect our business.
Risks Relating to Our Business and Our Industry
We have incurred significant net operating losses and cannot assure you that we will ever achieve or maintain profitability.
We have incurred significant net operating losses. As of December 31, 2014, we had an accumulated deficit of $183.5 million. To date, we have financed our operations primarily through sales of our products, cash flow from operations, issuances of ordinary shares, preferred stock and convertible loan notes and borrowings under shareholder and bank loans and under an Amended and Restated Credit Agreement (the “Credit Facility”) with Royalty Opportunities S.À R.L. (“OrbiMed”), an affiliate of OrbiMed Advisors LLC. We have devoted substantial resources to developing, manufacturing and obtaining regulatory approval for our products, the commercial launch of our products, the development of a sales force, marketing team and management team for our business.
For the year ended December 31, 2014, our gross profit was $26.8 million. However, although we have achieved a positive gross profit, we still operate at a substantial net loss. Following this offering, we expect that our operating expenses will continue to increase as we continue to build our commercial infrastructure, develop, enhance and commercialize new products and incur additional operational costs associated with being a public company. The extent of our future net operating losses and the timing of profitability are uncertain. We will need to increase our sales significantly to achieve profitability, and we might not be able to do so. Even if we do significantly increase sales, we might not be able to achieve, sustain or increase profitability on a quarterly or annual basis in the future. If we do not achieve profitability, it will be more difficult for us to finance our business, accomplish our strategic objectives and our financial performance and results of operations may be materially adversely affected.
Our future profitability depends on the success of our breast implant products.
Sales of our breast implant products accounted for 91.3% and 90.9% of our revenues for the years ended December 31, 2014 and December 31, 2013, respectively. We expect our revenues to continue to be substantially based on sales of our breast implant products. Any product liability lawsuits, introduction of competitive products by our competitors and other third parties, the loss of market acceptance or regulatory approval of our breast implant products, adverse rulings by regulatory authorities, adverse publicity or other adverse events relating to us or our breast implant products may significantly impact our sales and profitability, which would adversely affect our business, financial condition and results of operations.
Our business depends on maintaining and strengthening our brand and generating and maintaining ongoing, profitable customer demand for our products, and a significant reduction in such demand could materially affect our results of operations.
Our success depends on the reputation of our brands, which, in turn, depends on factors such as the safety and quality of our products, our communication activities, including marketing and education efforts, and our management of the customer experience, including through our Experience Centres and GCA Comfort Guarantee. Maintaining, promoting and positioning our brands are important to expanding our customer base,
15
and will depend largely on the success of our education and marketing efforts and our ability to provide a consistent, high-quality customer experience. We may need to make substantial investments in these areas in order to maintain and enhance our brand, and such investments may not be successful. Ineffective marketing, negative publicity, significant discounts by our competitors, product defects, counterfeit products, unfair labor practices and failure to protect the intellectual property rights in our brands are some of the potential threats to the strength of our business. To protect our brands’ status, we may need to make substantial expenditures to mitigate the impact of such threats. We believe that maintaining and enhancing our brands in the countries in which we currently sell our products and in new countries where we have limited brand recognition is important to expanding our customer base. If we are unable to maintain or enhance the strength of our brands in the countries in which we currently sell our products or new countries, then our growth strategy could be adversely affected.
Any negative publicity concerning our products or our competitors’ products could harm our business and reputation and negatively impact our financial results.
The responses of potential patients, physicians, the news media, legislative, regulatory and physician bodies and others to information about quality or complications or alleged complications of our products or our competitors’ products could result in negative publicity and could materially reduce market acceptance of our products. These responses or any investigations and potential resulting negative publicity may have a material adverse effect on our business and reputation and negatively impact our financial condition, results of operations or the market price of our ordinary shares. In addition, significant negative publicity could result in an increased number of product liability claims against us, which could materially adversely affect our business, financial condition and results of operations.
Our success depends on our ability to continue to enhance our existing products and develop or commercialize new products that respond to customer needs and preferences.
We may not be able to compete effectively with our competitors, and ultimately satisfy the needs and preferences of our customers, unless we can continue to enhance existing products and develop or acquire new innovative products. Product development requires the investment of significant financial, technological and other resources. Product improvements and new product introductions also require significant planning, design, development and testing at the technological, product and manufacturing process levels and we may not be able to timely or effectively develop product improvements or new products. Likewise, we may not be able to acquire new products on terms that are acceptable to us, or at all. Further, in most countries, we need to obtain regulatory approval in order to market and sell our products, which may limit our ability to act quickly in scaling commercialization in those countries. Our competitors’ new products may beat our products to market, be more effective with new features, obtain better market acceptance or render our products obsolete. Any new or modified products that we develop may not receive regulatory clearance or approval, or achieve market acceptance or otherwise generate any meaningful sales or profits for us relative to our expectations. Similarly, quality issues may arise with our products and although we have established internal procedures to minimize risks that may arise from quality issues, there can be no assurance that we will be able to eliminate or mitigate occurrences of these issues and associated liabilities. If we are unable to continue to enhance our existing products, develop or acquire new products, maintain the quality of our products or achieve market acceptance for our products, then our sales and profitability could be negatively impacted, which would adversely affect our business, financial condition and results of operations.
The size of the addressable global aesthetics products market, and the breast implant products market in particular, has not been established with precision and may be smaller than we estimate, possibly materially. If our estimates and projections overestimate the size of these markets and the markets for our other products, our revenue and results of operations will be adversely affected.
Our estimates of the addressable global aesthetics products market and the global breast implant market are based on a number of internal and third-party studies, reports and estimates, including, without limitation, the number of implants sold, the number of women and surgeons in the global market, third-party estimates regarding the number of certain types of aesthetics procedures performed annually, growth trends in the global
16
aesthetics products industry, regulatory trends, the increase in women’s purchasing power and the number of middle- and upper- income women in the global market. In addition, different industry sources define the global aesthetics products market differently. Our definition of the global aesthetics products market includes the following segments: breast implant products, skin tightening and body shaping, energy devices, neuromodulators, dermal fillers, chemical peels, liposuctions, self- and physician-dispensed topicals and injectables, removal of localized fat deposits, device-based alternatives to toxins for wrinkle reduction and therapy treatments for hair loss, excessive sweating (hyperhidrosis), malfunctioning veins (sclerotherapy) and scars. While we do not currently compete in all of these segments in the global aesthetics products market, we believe these studies and estimates have historically provided and may continue to provide us with effective tools in estimating the total global aesthetics products market and the global breast implant products market. Nevertheless, these estimates may not be correct and the conditions supporting our estimates may change at any time, thereby reducing the predictive accuracy of these underlying factors. As a result, our estimates of the global aesthetics products market and the global breast implant products market may prove to be incorrect. If the actual number of customers who would elect to purchase our products and the annual total addressable market for our products is smaller than we have estimated, it may impair our projected revenue growth and have an adverse impact on our business.
Each of our brands relies on a single-source supplier for medical-grade silicone, which is the primary constitutive ingredient of our products. These two suppliers are under common ownership, which could result in increased prices when we seek to renew our contracts with these suppliers.
We rely on NuSil and ASC as the sole suppliers of the medical-grade silicone used in each of our two brands’ products. NuSil and ASC are separate legal entities under the common ownership of NuSil Investments LLC. If NuSil or ASC becomes unable or unwilling to supply medical-grade silicone for our products, we will not be able to replace NuSil or ASC quickly, and any replacement supplier would have to be qualified with the relevant regulatory authorities, which is an expensive and time-consuming process during which we may experience a supply interruption. We may also be unsuccessful in negotiating favorable terms with such a supplier. As a result, our financial position and results of operations may be adversely affected. There is also no guarantee that NuSil or ASC will be able to meet our demand to produce sufficient quantities of medical-grade silicone in a timely manner.
Our current agreements with NuSil and ASC expire in April 2018 and December 2016, respectively. There can be no assurance that NuSil and/or ASC will agree to continue to supply us with medical-grade silicone following the expiration of our contracts on terms that are acceptable to us, or at all, which would have a material adverse effect on our business, financial condition and results of operations for the reasons set forth above.
In addition, our reliance on NuSil and ASC involves a number of other risks, including, among others, that:
| | • | | our medical-grade silicone may not be manufactured in accordance with agreed upon specifications or in compliance with regulatory requirements, or our suppliers’ manufacturing facilities may not be able to maintain compliance with regulatory requirements; |
| | • | | we may not be able to timely respond to unanticipated changes in customer orders, and if orders do not match forecasts, we may have excess or inadequate inventory of materials and components; |
| | • | | we may be subject to price fluctuations when a supply agreement is renegotiated or if our existing contract is not renewed; |
| | • | | NuSil or ASC may lose access to critical services and components, resulting in an interruption in the manufacture or shipment of medical-grade silicone or a shutdown of their facilities; |
| | • | | we may be required to obtain regulatory approvals related to any change in our supply chain; |
17
| | • | | NuSil or ASC may wish to discontinue supplying products to us; and |
| | • | | NuSil, ASC or their parent entity may encounter financial or other hardships unrelated to our demand for products, which could inhibit their ability to fulfill our orders and meet our requirements. |
If any of these risks materializes, it could significantly increase our costs, disrupt our manufacturing process, our ability to generate revenues would be impaired, market acceptance of our products could be adversely affected and customers may instead purchase or use our competitors’ products, which could materially adversely affect our business, financial condition and results of operations.
Recent studies have called into question the long-term safety of breast implants, which could have a material adverse effect on our business, results of operations and financial condition.
Although silicone breast implants have received regulatory approval, including from the U.S. Food and Drug Administration (the “FDA”), in recent years, silicone breast implants have been linked to a rare type of cancer, anaplastic large-cell lymphoma (“ALCL”). In January 2011, the FDA indicated that there was a possible association between saline and silicone gel-filled breast implants and ALCL. The review indicated that those with implants may have a very small but increased risk of ALCL. In March 2015, France’s national cancer institute (“NCI”) noted that there is a clearly established link between ALCL and breast implants, but NCI did not recommend suspension of marketing approval of the implants. In their statement, NCI stated that implants with macrotexture were used in most women found with ALCL. We do not produce macrotexture implants. An article published in March 2015, Plastic and Reconstructive Surgery reported the results of a study of 173 cases of ALCL occurring in women with breast implants. Of these 173 cases, the manufacturer of the implant was known in 112 cases. Nagôr was the manufacturer in three cases reported in this study; Eurosilicone was not the manufacturer in any case reported in this study. We are separately aware of two cases of ALCL involving Eurosilicone implants; there may be additional cases of ALCL involving our products of which we are not yet aware. Future studies or clinical experience may indicate that breast implants, including our products, expose individuals to a more substantial risk of developing ALCL or other unexpected complications. As a result, we may be exposed to potential class actions and other lawsuits from any individual who may develop ALCL after using our products, which would have a significant negative impact our revenues and could prevent us from achieving our forecasted revenue targets or achieving or sustaining profitability. Moreover, if long-term results and clinical experience indicate that our products cause unexpected or serious complications, we could be subject to mandatory product recalls, suspension or withdrawal of regulatory clearances and approvals and significant legal liability.
If we acquire or attempt to acquire new businesses or products, we may be unable to complete these acquisitions or to successfully integrate them in a cost-effective and non-disruptive manner, and we may not realize the benefits we anticipate from such acquisitions.
Our success depends on our ability to continually enhance and broaden our product offerings in response to changing customer demands, competitive pressures and innovation. We may consider opportunities to partner with or acquire other businesses, products or technologies that may enhance our product platform or technology, expand the breadth of our operations or customer base or advance our business strategies. We do not know if we will be able to identify acquisitions or joint ventures we deem suitable or to successfully complete any future acquisitions or joint ventures, or whether we will be able to successfully integrate any acquired business, product or technology or retain any key employees related thereto. Integrating any business, product or technology we acquire could be expensive and time-consuming, disrupt our ongoing business, impact our liquidity and distract our management. If we are unable to integrate any acquired businesses, products or technologies effectively, our business may suffer. Whether as a result of unsuccessful integration, unanticipated costs, including those associated with assumed liabilities and indemnification obligations, or other factors, we may not realize the economic benefits we anticipate from acquisitions. In addition, any amortization or charges resulting from the costs of acquisitions could increase our expenses.
18
We will need to significantly increase the size of our organization, and we may experience difficulties in managing growth.
As of March 31, 2015, we had 437 employees. Effectively executing our growth strategy requires that we increase revenues through expanded sales and marketing activities, recruit and retain additional employees, increase production and continue to improve our operational, financial and management controls, reporting systems and procedures. Such growth could place a strain on our administrative and operational infrastructure and we may not be able to make improvements to our management information and control systems in an efficient or timely manner and may discover deficiencies in existing systems and controls. Our management, personnel, systems and facilities currently in place may not be adequate to support this future growth. If we are not able to effectively expand our organization to manage and support our growth strategy, we may not be able to successfully execute our growth strategy, and our business, financial condition and results of operations may suffer and we may be exposed or subject to increased unforeseen or undisclosed liabilities as well as increased levels of indebtedness.
Our business could suffer if we lose the services of our senior management or other key personnel or are unable to attract and retain additional qualified personnel.
We are dependent upon the continued services of key personnel, including the members of our executive management team, who have extensive experience in our industry. The loss of any of these individuals could disrupt our operations or our strategic plans. Additionally, our future success will depend on, among other things, our ability to continue to hire and retain the necessary qualified sales, marketing, manufacturing, regulatory, development and managerial personnel, for whom we compete with numerous other companies, academic institutions and organizations. Competition for qualified management in our industry is intense and many of the companies with which we compete for management personnel have greater financial and other resources to dedicate to attracting and retaining personnel. The loss of key personnel or our inability to attract or retain other qualified personnel could have a material adverse effect on our business, results of operations and financial condition.
Although it will be subject to the underwriters’ lock-up arrangements and other restrictions on trading, a portion of the equity of our management team will not contain other contractual transfer restrictions at the time of this offering and is expected to become tradable after the expiration of the 180-day lock-up agreement with the underwriters. This liquidity will in many cases represent material wealth of such individuals that may impact retention and focus of existing key members of management.
If we fail to maintain and further develop our direct sales forces and distributor networks, our business could suffer. Additionally, our third-party distributors may not effectively sell our products or may engage in activities that could harm our reputation and sales of our products.
We have direct sales forces for our products in Brazil, France, Germany, Italy, Spain and the United Kingdom. We utilize a network of third-party distributors to sell our products in 69 additional countries and we also use agents and sub-distributors to supplement our direct sales forces in most of the countries where we sell directly. As we continue to expand into different countries and increase our marketing efforts, we will need to grow our distributor, sub-distributor and agent network and increase the size of our direct sales force. There is significant competition for sales personnel experienced in relevant medical device sales. If we are unable to attract, motivate, develop and retain qualified sales personnel, and thereby grow our direct sales forces in Europe, Latin America and Asia, or to expand our distributor, sub-distributor and agent network, we may not be able to maintain or increase our revenues. In addition, if our third-party distributors do not effectively sell our products, we may not be able to maintain or increase our revenues or expand into new countries. If a third-party distributor engages in certain activities, we may lose the ability to sell our products in certain countries or expand into new marks, which could adversely affect our reputation and our revenues.
19
We lack direct sales and marketing capabilities in many countries, and are wholly dependent on our distributors for the commercialization of our products in these countries. If we are unable to maintain or establish sales capabilities on our own or through third parties, we may not be able to commercialize any of our products in those countries.
We have no direct sales or marketing capabilities in many of the countries in which our products are sold. We have entered into distribution agreements with third parties to market and sell our products in those countries in which we do not have a direct sales force. If we are unable to maintain or enter into such distribution arrangements on acceptable terms, or at all, we may not be able to successfully commercialize our products in certain countries. Moreover, to the extent that we enter into distribution arrangements with other companies, our revenues, if any, will depend on the terms of any such arrangements and the efforts of others. These efforts may turn out not to be sufficient and our third-party distributors may not effectively sell our products. In addition, although our contract terms require our distributors to comply with all applicable laws regarding the sale of our products, including anti-competition, anti-money laundering and sanctions laws, we may not be able to ensure proper compliance. If our distributors fail to effectively market and sell our products in full compliance with applicable laws, our results of operations and business may suffer.
Risks associated with our global operations, including seeking, obtaining and maintaining regulatory approval to commercialize our products in the jurisdictions where our products are sold, could harm our business.
We have extensive global operations, which include seeking various regulatory approvals for most of our products in the jurisdictions where our products are sold. We expect that we are or will be subject to risks related to entering into these various countries, including:
| | • | | different regulatory requirements for product approvals; |
| | • | | reduced protection for intellectual property rights; |
| | • | | unexpected changes in tariffs, trade barriers and regulatory requirements; |
| | • | | economic weakness, including inflation, currency controls and political instability in particular global economies and markets; |
| | • | | compliance with tax, employment, immigration and labor laws for employees living or traveling in these countries; |
| | • | | different regulatory anti-corruption regimes; |
| | • | | taxes, including withholding of payroll taxes; and |
| | • | | business interruptions resulting from geopolitical actions, including war and terrorism, or natural disasters. |
If any of these disruptions in the countries in which our products are sold materializes, our financial condition and results of operations may be adversely affected.
Our results of operations could be materially adversely affected by fluctuations in foreign currency exchange rates.
Although we present our results of operations in US dollars, our functional currency is the Euro and a majority of our revenue is denominated in currencies other than the US dollar. In particular, we have significant revenue and expenses in the Euro, Sterling, US dollar and Brazilian Real. As such, unfavorable fluctuations in foreign currency exchange rates could have a material adverse effect on our results of operations.
20
Because our combined consolidated financial statements are presented in US dollars, we must translate revenues, expenses and income, as well as assets and liabilities, into US dollars at exchange rates in effect during or at the end of each reporting period. Therefore, changes in the value of the US dollar and Euro against other currencies will affect our revenues, operating income and the value of balance sheet items originally denominated in other currencies. These changes cause our growth in consolidated earnings stated in US dollars to be higher or lower than our growth in local currency when compared against other periods. Given our lack of currency hedging arrangements to protect us from fluctuations in the exchange rates of the Euro and other foreign currencies in relation to the US dollar (and/or from inflation of such foreign currencies), we may be exposed to material adverse effects from such movements. We cannot predict any future trends in rates of inflation or exchange rates of foreign currencies against the US dollar, and there can be no assurance that any contractual provisions will offset their impact, or that any future currency hedging activities will be successful.
Developments in emerging market countries may adversely affect our business.
Many of the countries in which our products are sold are emerging markets. Our global growth strategy contemplates the expansion of our existing sales activities in Latin America, EMEA and Asia-Pacific, and the potential addition of a manufacturing facility in Latin America. Our exposure to other emerging markets has increased in recent years, as have the number and importance of our distributor arrangements. Economic and political developments in Brazil and other emerging markets, including economic crises or political instability, have had in the past and may have in the future a material adverse effect on our financial condition and results of operations. Maintaining and strengthening our position in these emerging markets is a key component of our global growth strategy. Moreover, as these markets continue to grow, competitors may seek to enter these markets and existing market participants will likely try to aggressively protect or increase their market shares. Increased competition may result in price reductions, reduced margins and our inability to gain or hold market share, which could have a material adverse effect on our financial condition and results of operations.
If general economic conditions or changes in consumer spending, preferences or other trends reduce consumer demand for our products in the markets in which we sell our products, our revenues and profitability would suffer.
We are subject to the risks arising from adverse changes in general economic and market conditions. Elective procedures, such as breast augmentation, are typically not covered by insurance. Adverse changes in the economy may cause consumers to reassess their spending choices and reduce the demand for these surgeries and could have an adverse effect on consumer spending on discretionary procedures. Factors affecting the level of consumer spending for such discretionary items include general economic conditions, wages and unemployment, housing prices, consumer debt, interest rates, fuel and energy costs, taxation, political conditions, the availability of consumer credit and consumer confidence in future economic conditions. While trends in consumer discretionary spending remain unpredictable, consumer purchases of discretionary items tend to decline during recessionary periods when disposable income is lower or during other periods of economic instability or uncertainty. This shift could have an adverse effect on our revenues. Furthermore, in addition to worsening economic conditions, consumer preferences and trends may shift due to a variety of factors, including changes in demographic and social trends, public health initiatives and product innovations, which may reduce consumer demand for our products and result in a material adverse effect on our business, financial condition and results of operations.
Our results of operations could be materially harmed if we are unable to accurately forecast customer demand for our products and manage our inventory.
To ensure adequate inventory supply, we must forecast inventory needs and place orders with our suppliers based on our estimates of future demand for particular products. Our ability to accurately forecast demand for our breast implant and other products could be negatively affected by many factors, including our failure to accurately manage our expansion strategy, product introductions by competitors, an increase or decrease in customer demand for our products or for products of our competitors, our failure to accurately forecast customer
21
acceptance of new products, unanticipated changes in general market conditions or regulatory matters and weakening of economic conditions or consumer confidence in future economic conditions. Inventory levels in excess of customer demand may result in inventory write-downs or write-offs, which would cause our gross margin to suffer and could impair the strength of our brand. Conversely, if we underestimate customer demand for our products, our manufacturing facilities may not be able to deliver products to meet our requirements, and this could result in damage to our reputation and customer relationships. In addition, if we experience a significant increase in demand, additional supplies of raw materials or additional manufacturing capacity may not be available when required on terms that are acceptable to us, or at all, or suppliers or our manufacturing facilities may not be able to allocate sufficient capacity in order to meet our increased requirements, which could have an adverse effect on our ability to meet consumer demand for our products and our results of operations.
We and our distributors are required to maintain high levels of inventory, which could consume a significant amount of our resources and reduce our cash flows.
We and our distributors need to maintain substantial levels of inventory where the surgeons who use our breast implant products are located in order to ensure access to a wide range of our breast implant products and to protect ourselves and our distributors from supply interruptions, such as those caused by transportation- or importation-related delays. As a result of our and our distributors’ substantial inventory levels, we are subject to the risk that a substantial portion of our inventory becomes obsolete, which could have a material adverse effect on our earnings and cash flows due to the resulting costs associated with the inventory impairment charges and costs required to replace such inventory.
Various factors outside our direct control may adversely affect manufacturing, sterilization and distribution of our breast implant products and other products.
The manufacturing, sterilization and distribution of our breast implant products and other products are technically challenging. Changes that our suppliers may make outside the purview of our direct control can have an impact on our processes, on quality and the successful delivery of products to our customers. Mistakes and mishandling are not uncommon and can affect supply and delivery. Some of these risks include:
| | • | | failure to complete sterilization on time or in compliance with the required regulatory standards; |
| | • | | transportation and import and export risk, particularly given the global nature of our supply and distribution chains; |
| | • | | delays in analytical results or failure of analytical techniques that we depend on for quality control and release of products; |
| | • | | natural disasters, labor disputes, financial distress, lack of raw material supply, issues with facilities and equipment or other forms of disruption to business operations affecting our manufacturer or its suppliers; and |
| | • | | latent defects that may become apparent after products have been released and that may result in a recall of such products. |
If any of these risks were to materialize, our ability to provide our products to customers on a timely basis would be adversely impacted.
Any disruption at our manufacturing facilities could adversely affect our business and results of operations.
Our principal offices are located in Dublin, Ireland. All of our implant manufacturing is conducted at our facilities in Ashby de la Zouche, United Kingdom, Cumbernauld, United Kingdom, and Apt, France. Our inventory of finished goods is held at these facilities, as well as at our facilities in Brazil and Spain. Our agents in Germany and Italy hold additional inventory.
22
Despite our efforts to maintain and safeguard our manufacturing facilities, including acquiring insurance and adopting maintenance and health and safety protocols, vandalism, terrorism or a natural or other disaster, such as fire or flood, could damage or destroy our inventory of finished goods, cause substantial delays in our operations and manufacturing, result in the loss of key information and cause us to incur additional expenses. Our insurance may not cover our losses in any particular case. In addition, regardless of the level of insurance coverage, damage to our facilities may have a material adverse effect on our business, financial condition and results of operations.
If we fail to compete effectively against our competitors, many of whom have greater resources than we have, our revenues and results of operations may be negatively affected.
Our industry is intensely competitive and subject to rapid change from the introduction of new products, technologies and other activities of industry participants. Our primary competitors include Mentor Worldwide, LLC, a division of Johnson & Johnson, and Allergan, Inc. These companies are well-capitalized pharmaceutical companies that have been the market leaders for many years and control a majority share of the breast implant products market. These competitors also enjoy several competitive advantages over us, including:
| | • | | greater financial and human resources for sales, marketing and product development; |
| | • | | an established base of long-time customers; |
| | • | | larger and more established distribution networks; |
| | • | | greater ability to cross-sell products; and |
| | • | | more experience in conducting research and development, manufacturing and obtaining regulatory approvals or clearances. |
In addition, in Brazil, we compete with Silimed Ltda (“Silimed”), a leading Brazilian supplier of breast implant products. In certain of the countries in which we operate, we also compete with several other breast implant manufacturers, including Groupe Sebbin SAS and Establishment Labs S.A., which sell their breast implant products at significantly lower prices than those of our Nagôr and Eurosilicone breast implant products.
We may in the future also face increased competition from our primary competitors if they choose to focus more efforts or resources on the breast implant market and increasing their market share. If we fail to compete effectively against our competitors, our revenues and results of operations may be negatively affected.
We have been and may in the future be subject to product liability or warranty claims or other litigation in the ordinary course of business that may be costly, divert management’s attention, harm our reputation and adversely affect our business, financial condition and results of operations. We may not be able to maintain adequate product liability insurance.
As a supplier of medical devices, we have been and may in the future be subject to product liability or warranty claims alleging that the use of our products has resulted in adverse health effects or other litigation in the ordinary course of business that may require us to make significant expenditures to defend these claims or pay damage awards. The breast implant industry has a particularly significant history of product liability litigation. The risks of litigation exist even with respect to products that have received or in the future may receive regulatory approval for commercial sale. If we cannot successfully defend ourselves against product liability claims, we will incur substantial liabilities and reputational harm. In addition, regardless of merit or eventual outcome, product liability claims are costly, distract management’s attention from our primary business, diminish our ability to commercialize our existing or new products, decrease demand for our products, damage our business reputation and result in loss of revenue. In addition, our silicone breast implant products are sold
23
with our GCA Comfort Guarantee, a warranty providing for no-charge replacement implants throughout the life of the patient in the event of either (i) internal rupture of the implant due to the loss of shell integrity or (ii) severe capsular contracture.
We maintain product liability insurance, but this insurance is limited in amount and subject to significant deductibles. There is no guarantee that insurance will be available or adequate to protect against all claims. Our insurance policies are subject to annual renewal and we may not be able to obtain liability insurance in the future on acceptable terms or at all. In addition, our insurance premiums could be subject to increases in the future, which may be material. If the coverage limits are inadequate to cover our liabilities or our insurance costs continue to increase as a result of warranty or product liability claims or other litigation, then our business, financial condition and results of operations may be adversely affected.
If there are significant disruptions in our information technology systems, our business, financial condition and results of operations could be adversely affected.
The efficient operation of our business depends on our information technology systems. We rely on our information technology systems to effectively manage sales and marketing data, accounting and financial functions, inventory, product development tasks and customer service and technical support functions. We are currently implementing an enterprise resource planning system (“ERP system”). ERP system implementations are complex, long-term projects that require significant investment of capital and human resources, the re-engineering of many business processes and the attention of many employees who would otherwise be focused on other aspects of our business. Any disruptions, delays or deficiencies in the design and implementation of the improvements to our ERP system could result in potentially much higher costs than anticipated and could adversely affect our ability to develop and launch solutions, fulfill contractual obligations, file reports with the SEC in a timely manner or otherwise operate our business and our controls environment. Moreover, despite our security measures, our information technology systems, including the ERP system, are vulnerable to damage or interruption from fires, floods and other natural disasters, terrorist attacks, computer viruses or hackers, power losses and computer system or data network failures, which could result in significant data losses or theft of sensitive or proprietary information. Any of these consequences could have an adverse effect on our business.
In addition, a variety of our software systems are hosted by third-party service providers whose security and information technology systems are subject to similar risks. The failure of our or our service providers’ information technology could disrupt our entire operation or result in decreased sales, increased overhead costs and product shortages, all of which could have a material adverse effect on our reputation, business, financial condition and results of operations.
Fluctuations in insurance cost and availability could adversely affect our profitability and our risk management profile.
We hold a number of insurance policies, including product liability insurance, directors’ and officers’ liability insurance, public liability insurance and property insurance. If the costs of maintaining adequate insurance coverage increase significantly in the future, our results of operations could be materially adversely affected. Likewise, if any of our current insurance coverage should become unavailable to us or become economically impractical, we would be required to operate our business without indemnity from commercial insurance providers. If we operate our business without insurance, we could be responsible for paying claims or judgments against us and would be exposed to significant liabilities that would have otherwise been covered by insurance. A product liability claim or other claim with respect to uninsured liabilities or for amounts in excess of insured liabilities could have a material adverse impact on our results of operations or financial condition.
Labor issues could adversely affect our business.
As of December 31, 2014, approximately 65% of our employees were covered by collective bargaining agreements or represented by labor unions. We are required to consult with our employee representatives, such as
24
works councils, on certain matters, including restructurings, acquisitions and divestitures. Although we believe that our relations with our employees are good, there can be no assurance that new agreements will be reached or consultations will be completed without discord with these labor unions or works councils or on terms satisfactory to us. A strike, work slowdown or other labor unrest could impair our ability to supply our products to customers, which could result in reduced revenue and customer claims, and may distract our management from focusing on other aspects of our business and strategic priorities. In addition, as a result of our global operations, we are subject to differing labor relations laws and regulations, and our failure to comply with any of these laws could subject to us to monetary or other type of penalties, which could adversely affect our revenues and results of operations.
We are subject to credit risks related to our accounts receivable and failure to collect our accounts receivable could adversely affect our results of operations and financial condition.
The failure to collect outstanding receivables could have an adverse impact on our business, prospects, results of operations and financial condition. If the financial condition of our customers were to deteriorate, resulting in an impairment of their ability to make payments, or if our customers cannot pay us as a result of currency exchange controls limiting the amount of US dollars that may be exported from certain countries in which we sell our products, then we might be required to make additional allowances, which would adversely affect our results of operations in the period in which the determination or allowance was made. The current economic climate and our significant operations in emerging markets could increase the likelihood of customer defaults and bankruptcies. If a material portion of our customers or distributors were to default, become insolvent or otherwise were not able to satisfy their obligations to us, we would be materially harmed. As of December 31, 2014, of our $13.9 million of trade receivables, $6.4 million were past due and $1.1 million were impaired, and, after giving effect to this allowance for doubtful accounts, we had exposure of $5.3 million.
Risks Related to Our Financial Results and Need for Financing
Our quarterly revenues and results of operations are unpredictable and may fluctuate significantly from quarter to quarter due to many factors, including factors outside our control, which could adversely affect our business, results of operations and the trading price of our ordinary shares.
Our revenues and results of operations may vary significantly from quarter to quarter and year to year due to a number of factors, many of which are outside of our control and any of which may cause the price of our ordinary shares to fluctuate. Our revenues and results of operations will be affected by numerous factors, including:
| | • | | the impact of the buying patterns of patients and seasonal cycles in consumer spending, which may cause patients to delay elective procedures; |
| | • | | our ability to drive increased sales of breast implant and other products; |
| | • | | our ability to establish and maintain an effective and dedicated sales organization and distributor network; |
| | • | | reimbursements related to reconstruction procedures; |
| | • | | pricing pressure applicable to our products; |
| | • | | timing of our development activities and initiatives and inspections by regulatory or governmental bodies; |
| | • | | the mix of our products sold due to different profit margins among our products, direct versus distributor sales and geographic mix of our sales. |
25
| | • | | foreign exchange rate changes; |
| | • | | timing of new product offerings, acquisitions, licenses or other significant events by us or our competitors; |
| | • | | the ability of our suppliers to timely provide us with an adequate quality supply of medical-grade silicone; |
| | • | | the evolving product offerings of our competitors; |
| | • | | loss of existing regulatory approvals and legislative changes affecting the products we offer or those of our competitors; |
| | • | | adverse publicity or government actions or legal costs related to various lawsuits; |
| | • | | increased labor and related costs; |
| | • | | interruption in the manufacturing or distribution of our products or loss of any of our manufacturing facilities; |
| | • | | the effect of competing technological, industry and market developments; and |
| | • | | our ability to expand the geographic reach of our sales and marketing efforts. |
Many of the products we may seek to develop and introduce in the future will require regulatory approval or clearance and import licenses before we can sell such products in the countries in which we sell our products. Given that the timing of such approvals, clearances or licenses may be uncertain, it will be difficult for us to forecast sales projections for these products with any degree of certainty before such approvals, clearances or licenses are obtained. In addition, we will be increasing our operating expenses as we expand our commercial capabilities. Accordingly, we may experience significant, unanticipated quarterly losses. If our quarterly or annual results of operations fall below the expectations of investors or securities analysts, the price of our ordinary shares could decline substantially.
We may need to raise substantial additional funds in the future to achieve our goals, these funds may not be available on acceptable terms or at all, and a failure to obtain this necessary capital when needed could force us to delay, limit, scale back or cease our some or all operations.
As of December 31, 2014, we had $10.6 million in cash and cash equivalents. We believe that our available cash on hand and proceeds from this offering will be sufficient to satisfy our liquidity requirements for at least the next twelve months. However, the continued growth of our business, including the expansion of our sales force and marketing programs, and development activities, will significantly increase our expenses. In addition, the amount of our future product sales is difficult to predict and actual sales may not be in line with our forecasts. As a result, we may need to raise substantial additional funds in the future. Our need to raise additional funds will depend on many factors, including:
| | • | | the revenues generated by our breast implant products and other products, and any future products that we may develop and commercialize; |
| | • | | the costs associated with product guarantees; |
| | • | | the cost associated with expanding our sales force and marketing programs; |
26
| | • | | the cost associated with developing and commercializing our proposed products or technologies; |
| | • | | the cost of obtaining and maintaining regulatory clearances and approvals for our current or future products; |
| | • | | the cost of ongoing compliance with regulatory requirements; |
| | • | | the cost of ongoing compliance and debt service obligations under the Credit Facility; |
| | • | | expenses we incur in connection with potential litigation or governmental investigations; |
| | • | | anticipated or unanticipated capital expenditures; and |
| | • | | unanticipated administrative expenses and other costs. |
As a result of these and other factors, we do not know whether or to what extent we may be required to raise additional funds. We may in the future seek additional funds from public or private offerings of our ordinary shares and other equity instruments, debt offerings, borrowings under term loans or other sources, subject to the restrictions under the Credit Facility. If we issue equity or debt securities to raise additional funds, our existing shareholders may experience dilution, and the new equity or debt securities may have rights, preferences and privileges senior to those of our existing shareholders. In addition, if we raise additional funds through collaborations, licensing, joint ventures, strategic alliances, partnership arrangements or other similar arrangements, it may be necessary to relinquish valuable rights to our current or potential future products or proprietary technologies, or grant licenses on terms that are not favorable to us.
If we are unable to raise additional funds on a timely basis or on terms that are acceptable to us, or at all, we may not be able to expand our sales force and marketing programs, enhance our current products or develop new products, take advantage of future opportunities, or respond to competitive pressures, changes in supplier relationships, or unanticipated changes in customer demand. We may also default on the principal and interest due under the Credit Facility. Any of these events could adversely affect our ability to achieve our strategic objectives, which could have a material adverse effect on our business, financial condition and results of operations.
Our substantial indebtedness may limit cash flow available to invest in the ongoing needs of our business and restrict our operating flexibility.
We have a significant amount of indebtedness. As of April 2015, we have borrowed an aggregate principal amount of $59.0 million under the Credit Facility, which amount is guaranteed by substantially all of our subsidiaries and collateralized by substantially all of our assets. Beginning in February 2019, and quarterly thereafter, we are required to make principal payments equal to one-quarter of the aggregate principal amount outstanding under the Credit Facility as of January 22, 2019, until the outstanding principal has been repaid in full. We are also required to make cash interest payments on certain dates, including (i) the last day of each fiscal quarter, (ii) the maturity date and (iii) the date of any payment or prepayment of principal outstanding. This repayment structure will require a significant cash commitment.
If we decide or are required to repay the loans under the Credit Facility before their maturity dates, we are obligated to pay a prepayment penalty equal to 10% of the principal amount prepaid plus an additional fee based on the date of prepayment. We also would be required to make cash interest payments on any portion of such an accelerated repayment. The Credit Facility is subject to acceleration upon certain defaults and in the event any person or group, excluding Montreux, Oyster and their respective affiliates, acquires 35% or more of our share capital.
27
Our obligations under the Credit Facility coupled with our other financial obligations and contractual commitments could have significant adverse consequences for our business, including:
| | • | | requiring us to dedicate a substantial portion of cash and cash equivalents to the payment of interest on, and principal of, our debt, which will reduce the amounts available to fund working capital, capital expenditures, acquisitions, product development efforts and other general corporate purposes; |
| | • | | causing us to incur substantial fees from time to time in connection with debt amendments, refinancings or prepayments; |
| | • | | increasing our exposure to rising interest rates, because our borrowings are at variable interest rates; |
| | • | | increasing our vulnerability to adverse economic and industry conditions, which could place us at a competitive disadvantage compared to our competitors that have relatively less indebtedness; |
| | • | | limiting our flexibility in planning for, or reacting to, changes in our business and our industry; and |
| | • | | placing us at a competitive disadvantage compared to our competitors that have less debt or better debt servicing options. |
We intend to satisfy our current and future debt service obligations with our existing cash and cash equivalents and revenues from product sales. However, we may not have sufficient funds or may be unable to arrange for additional financing to pay the amounts due under the Credit Facility.
Failure to comply with the conditions of the Credit Facility could result in an event of default under the facility, which could result in an acceleration of amounts due under the Credit Facility. We may not have sufficient funds or may be unable to arrange for additional financing to repay our indebtedness or to make any accelerated payments, and OrbiMed could seek to enforce security interests in the collateral securing such indebtedness, which would have a material adverse effect on our business.
Changes in our effective tax rate may reduce our net income in future periods.
We cannot give any assurance as to what our effective tax rate will be because of, among other things, uncertainty regarding the tax policies of the jurisdictions where we operate. In general, under current Irish legislation, a company is regarded as resident for tax purposes in Ireland if it is centrally managed and controlled in Ireland, or if it is incorporated in Ireland. Trading income of an Irish resident company is generally taxable at the Irish corporation tax rate of 12.5%. Non-trading income of an Irish resident company (e.g., interest income, rental income, dividends or other passive income) is taxable at a rate of 25%. It is possible that in the future, whether as a result of a change in law or the practice of any relevant tax authority or as a result of any change in the conduct of our affairs, we could become, or be regarded as having become tax resident in a jurisdiction other than Ireland. Should we cease to be an Irish tax resident, we may be subject to a charge of Irish capital gains tax as a result of a deemed disposal of our assets. Our actual effective tax rate may vary from our expectation and that variance may be material. Additionally, the tax laws of Ireland and other jurisdictions could change in the future, and such changes could cause a material adverse change in our effective tax rate.
A number of factors may increase our future effective tax rates, including: the jurisdictions in which profits are determined to be earned and taxed; the resolution of issues arising from tax audits that may be undertaken by various tax authorities; changes in the valuation of our deferred tax assets and liabilities; increases in expense not deductible for tax purposes, including transaction costs and impairments of goodwill in connection with acquisitions; changes in available tax credits; changes in share-based compensation; changes in tax laws or the interpretation of such tax laws, and changes in generally accepted accounting principles; and
28
challenges to the transfer pricing policies related to our structure. Currently, the Organization for Economic Co-Operation and Development is reviewing the tax rules relating to base erosion and profit shifting. Our effective tax rate could be affected to the extent that countries adopt proposals arising from this review.
If our tax rates were to increase as described above, such increases could cause a material and adverse change in our worldwide effective tax rate and we may have to take action, at potentially significant expense, to seek to mitigate the effect of such changes. In addition, any amendments to the current double taxation treaties between Ireland and other jurisdictions could subject us to increased taxation. In the normal course of business, we are subject to examination by various taxing authorities, including Ireland, France, Italy, Spain, the United Kingdom, Germany, Brazil and Mexico. Any such amendments to double taxation treaties or increases in taxation based on examinations by taxing authorities (if such increases are ultimately sustained) could result in increased charges, financial loss, including penalties, and reputational damage and materially and adversely affect our results, financial condition and prospects.
Future changes in financial accounting standards may cause adverse unexpected revenue or expense fluctuations and affect our reported results of operations.
A change in accounting standards could have a significant effect on our reported results and may even affect our reporting of transactions completed before the change is effective. New pronouncements and varying interpretations of existing pronouncements have occurred and may occur in the future. Changes to existing rules or current practices may adversely affect our reported financial results of our business.
Risks Related to Our Legal and Regulatory Environment
Our medical device products and operations, including manufacturing, are subject to extensive governmental regulation, including health, environmental, safety and anti-corruption and other laws, and our failure to comply with applicable requirements could cause our business to suffer.
Our medical device products and operations, including manufacturing, are subject to regulation by the agencies governing medical device products in the various countries in which we operate, manufacture and sell our products. These agencies enforce laws and regulations that are meant to assure product safety and effectiveness, including the regulation of, among other things:
| | • | | design, development and manufacturing; |
| | • | | testing, labeling, content and language of instructions for use and storage; |
| | • | | marketing, sales and distribution; |
| | • | | regulatory clearances and approvals, including pre-market clearance and approval; |
| | • | | conformity assessment procedures; |
| | • | | product traceability and record keeping procedures; |
| | • | | advertising and promotion; |
| | • | | product complaints, complaint reporting, recalls and field safety corrective actions; |
29
| | • | | post-market surveillance, including reporting of deaths or serious injuries and malfunctions that, if they were to recur, could lead to death or serious injury; |
| | • | | post-market studies; and |
| | • | | product import and export. |
The regulations to which we are subject are complex and have tended to become more stringent over time. Regulatory changes could result in restrictions on our ability to carry on or expand our operations, higher than anticipated costs or lower than anticipated revenues.
Failure to comply with applicable laws and regulations could jeopardize our ability to manufacture or sell our products and result in enforcement actions such as warning letters, fines, injunctions and civil penalties, termination of distribution, recalls or seizures of products, delays in the introduction of products into the market, total or partial suspension of production, refusal of the regulating agency to grant future clearances or approvals, withdrawals or suspensions of current clearances or approvals, resulting in prohibitions on sales of our products and/or, in the most serious cases, criminal penalties. Any of these sanctions could result in higher than anticipated costs or lower than anticipated revenues and have a material adverse effect on our reputation, business, results of operations and financial condition.
We are subject to various laws protecting the confidentiality of certain patient health information, and our failure to comply could result in penalties and reputational damage.
Numerous countries in which we operate, manufacture and sell our products have, or are developing, laws protecting the confidentiality of certain patient health information. European Union (“EU”) member states and other jurisdictions have adopted data protection laws and regulations, which impose significant compliance obligations. For example, the EU Data Protection Directive, as implemented into national laws by the EU member states, imposes strict obligations and restrictions on the ability to collect, analyze and transfer personal data, including health data from clinical trials and adverse event reporting. Data protection authorities from different EU member states may interpret the EU Data Protection Directive and national laws differently, which adds to the complexity of processing personal data in the EU, and guidance on implementation and compliance practices are often updated or otherwise revised. The EU Data Protection Directive prohibits the transfer of personal data to countries outside of the EU member states that are not considered by the European Commission to provide an adequate level of data protection.
We have policies and practices that we believe make us compliant with applicable privacy regulations. Nevertheless, any failure to comply with the rules arising from the EU Data Protection Directive and related national laws of EU member states, as well as privacy laws in other countries in which we operate, could lead to government enforcement actions and significant sanctions or penalties against us, adversely impact our results of operations and subject us to negative publicity.
A proposal for an EU Data Protection Regulation, intended to replace the current EU Data Protection Directive, is currently under consideration. The EU Data Protection Regulation is expected to introduce new data protection requirements in the EU and substantial fines for breaches of the data protection rules. If the draft EU Data Protection Regulation is adopted in its current form, it may increase our responsibility and liability in relation to personal data that we process and we may be required to put in place additional mechanisms ensuring compliance with the new EU data protection rules.
We are required to comply with medical device reporting and vigilance requirements, and must report certain malfunctions, deaths and serious injuries associated with our products, which can result in voluntary corrective actions or agency enforcement actions.
All manufacturers placing medical devices on the market in the EEA are legally bound to report any incidents involving devices they produce or sell to the competent authority in whose jurisdiction the incident
30
occurred if the incident led or might have led to the death of or serious injury to a patient, user or other person. Manufacturers are also required to report incidents that occur outside the EEA if they result in corrective action being taken within the EEA. Were we to submit an incident report, the relevant competent authority would file an initial report, and there would then be a further inspection or assessment if there are particular issues. Similar reporting and vigilance requirements exist in many of the other countries in which we operate. Failure to comply with such reporting and vigilance requirements may result in various penalties, including criminal or civil sanctions or revocation of our product clearances.
Malfunction of our products could result in future voluntary corrective actions, such as recalls, including corrections, or customer notifications, or agency action, such as inspection or enforcement actions. If malfunctions do occur, we may be unable to correct the malfunctions adequately or prevent further malfunctions, in which case we may need to cease manufacture and distribution of the affected products, initiate voluntary recalls and redesign the products. Any corrective action, whether voluntary or involuntary, will require the dedication of our time and capital, distract management from operating our business and may harm our reputation and financial results. In addition, we may become subject to costly litigation by our surgeons or their patients.
If we fail to comply with our reporting obligations or corrective actions, regulatory authorities may also take actions against us, such as ordering recalls, imposing fines, seizing the affected products, issuing warning letters or untitled letters, instituting administrative actions or criminal prosecution, imposing civil monetary penalties, revoking our device clearances or delaying the clearance of future products.
We may be subject to regulatory or enforcement actions if we engage in improper marketing or promotion of our products.
Our educational and promotional activities and training methods must comply with the applicable regulatory laws of the jurisdictions in which we operate, including, but not limited to, the prohibition of the promotion of a medical device for a use that has not been cleared or approved by the applicable regulator. Use of a device outside of its cleared or approved indications is known as “off-label” use. If a governing regulatory agency determines that our educational and promotional activities or training constitutes promotion of an off-label use, it could request that we modify our training or promotional materials or subject us to regulatory or enforcement actions, including the issuance of warning letters, fines, penalties, injunctions or seizures, which could have an adverse impact on our reputation and financial results. Although our policy is to refrain from statements that could be considered off-label promotion of our products, regulatory agencies could disagree and conclude that we have engaged in off-label promotion. In addition, the off-label use of our products may increase the risk of product liability claims.
The regulatory approval process is costly and lengthy and we may not be able to obtain the clinical data require to successfully obtain approval to market and sell our products in the jurisdictions in which we intend to commercialize such products.
To market a medical device, in most jurisdictions, we must obtain approval or clearance from the relevant governing regulatory body. In some countries, this requires that we conduct controlled clinical trials designed to test the safety, and, depending on the device, the efficacy of the product for which we are seeking approval or clearance. Clinical testing is expensive, and typically takes many years, without any certainty of outcome. The data obtained from clinical trials may be inadequate to support approval or clearance. In addition, the occurrence of unexpected findings in connection with clinical trials may prevent or delay obtaining approval or clearance, and could affect approvals or clearances we have obtained for the product in other jurisdictions. The initiation and completion of any clinical trials may be prevented, delayed or halted for numerous reasons, including, but not limited to, the following reasons:
| | • | | regulatory authorities do not approve a clinical study protocol, force us to modify a previously approved protocol or place a clinical study on hold; |
31
| | • | | subjects do not enroll in, or enroll at the expected rate, or complete a clinical study; |
| | • | | subjects or investigators do not comply with study protocols; |
| | • | | subjects do not return for post-treatment follow-up at the expected rate; |
| | • | | subjects experience serious or unexpected adverse side effects for a variety of reasons that may or may not be related to our products, causing a clinical study to be put on hold; |
| | • | | sites participating in an ongoing clinical study may withdraw, requiring us to engage new sites; |
| | • | | difficulties or delays associated with establishing additional clinical sites; |
| | • | | third-party clinical investigators decline to participate in our clinical studies, do not perform the clinical studies on the anticipated schedule or act in a manner that is inconsistent with the investigator agreement, clinical study protocol, good clinical practices or other regulatory requirements; |
| | • | | third-party organizations do not perform data collection and analysis in a timely or accurate manner; |
| | • | | regulatory inspections of our clinical studies require us to undertake corrective action or suspend or terminate our clinical studies; |
| | • | | changes in applicable statutes, regulations or policies; |
| | • | | interim results are inconclusive or unfavorable as to immediate and long-term safety; |
| | • | | the study design is inadequate to demonstrate safety; or |
| | • | | the clinical trials do not meet the study endpoints. |
Failure can occur at any stage of clinical testing. Our clinical trials may produce negative or inconclusive results, and we may decide, or regulators may require us, to conduct additional clinical and/or non-clinical testing in addition to that which we have planned. Our failure to adequately demonstrate the safety and, if required, efficacy of any of our devices would prevent receipt of regulatory clearance or approval and, ultimately, the commercialization of that device in the target jurisdiction.
We are subject to the U.K. Bribery Act, the U.S. Foreign Corrupt Practices Act, the Irish Criminal Justice (Money Laundering and Terrorist Financing) Act and other anti-corruption and anti-money-laundering laws, as well as export control laws, customs laws, sanctions laws and other laws governing our operations. If we fail to comply with these laws, we could be subject to civil or criminal penalties, other remedial measures and legal expenses, which could adversely affect our business, results of operations and financial condition.
Our global operations expose us to trade and economic sanctions and other restrictions imposed by the United States, the European Union and other governments and organizations. The U.S. Departments of Justice, Commerce, State and Treasury and other federal agencies and authorities have a broad range of civil and criminal penalties they may seek to impose against corporations and individuals for violations of economic sanctions laws, export control laws, the Foreign Corrupt Practices Act (the “FCPA”) and other federal statutes and regulations, including those established by the Office of Foreign Assets Control (“OFAC”). In addition, the U.K. Bribery Act of 2010 (the “Bribery Act”) prohibits both domestic and international bribery, as well as bribery across both private and public sectors. An organization that “fails to prevent bribery” by anyone associated with
32
the organization can be charged under the Bribery Act unless the organization can establish the defense of having implemented “adequate procedures” to prevent bribery. The Irish Criminal Justice (Money Laundering and Terrorist Financing) Act 2010, as amended (the “Irish Money Laundering Act”), provides for criminal sanctions for engaging in “money laundering offences,” which are offenses committed where a person knows or believes that (or is reckless as to whether or not) the property represents the proceeds of criminal conduct and the party is involved in concealing or disguising the true nature, source, location, disposition, movement or ownership of property; converting, transferring, handling acquiring possession or using the property; or removing the property from, or bringing the property into, Ireland. Under these laws and regulations, as well as other anti-corruption laws, anti-money-laundering laws, export control laws, customs laws, sanctions laws and other laws governing our operations, various government agencies may require export licenses, may seek to impose modifications to business practices, including cessation of business activities in sanctioned countries or with sanctioned persons or entities and modifications to compliance programs, which may increase compliance costs, and may subject us to fines, penalties and other sanctions. A violation of these laws or regulations could adversely impact our business, results of operations and financial condition.
Recent events in Ukraine and Crimea have resulted in the European Union and the United States imposing and escalating sanctions on Russia and certain businesses, sectors and individuals in Russia. The European Union and the United States have also suspended the granting of certain types of export licenses to Russia. Russia has imposed its own sanctions on certain individuals in the United States and may impose other sanctions on the United States and the European Union and/or certain businesses or individuals from these regions. Although our products are not currently subject to such sanctions, we cannot assure you that the current sanctions or any further sanctions imposed by the European Union, the United States or other international interests will not materially adversely affect our operations.
We have implemented and maintain policies and procedures designed to ensure compliance by us, our subsidiaries and our directors, officers, employees, representatives, distributors, consultants and agents with the FCPA, OFAC restrictions, the Bribery Act, the Irish Money Laundering Act and other export control, anti-corruption, anti-money-laundering and anti-terrorism laws and regulations. We cannot assure you, however, that our policies and procedures are sufficient or that directors, officers, employees, representatives, distributors, consultants and agents have not engaged and will not engage in conduct for which we may be held responsible, nor can we assure you that our business partners have not engaged and will not engage in conduct that could materially affect their ability to perform their contractual obligations to us or even result in our being held liable for such conduct. Violations of the FCPA, OFAC restrictions, the Bribery Act, the Irish Money Laundering Act or other export control, anti-corruption, anti-money-laundering and anti-terrorism laws or regulations may result in severe criminal or civil sanctions, and we may be subject to other liabilities, which could have a material adverse effect on our business, financial condition, cash flows and results of operations.
Our relationships with customers, healthcare providers and professionals is subject to applicable anti-kickback, fraud and abuse and other healthcare laws and regulations, which could expose us to criminal sanctions, civil penalties, contractual damages, reputational harm and diminished profits and future earnings.
The medical device industry and related businesses have been the subject of various corruption investigations in connection with marketing to healthcare providers and hospitals in certain of the countries in which we operate. Surgeons and other health professionals play a primary role in the recommendation of our products. Our future arrangements with customers may expose us to broadly applicable fraud and abuse and other healthcare laws and regulations that may constrain the business or financial arrangements and relationships through which we market, sell and distribute our products. Efforts to ensure that our business arrangements with third parties comply with applicable healthcare laws and regulations involves substantial costs. It is possible that governmental authorities conclude that our business practices do not comply with current or future statutes, regulations or case law involving applicable fraud and abuse or other healthcare laws and regulations. If our operations are found to be in violation of any of these laws or any other governmental regulations that may apply to us, we may be subject to significant civil, criminal and administrative penalties, damages, fines and the curtailment or restructuring of our operations.
33
Our operations involve hazardous materials and we and third parties with whom we contract must comply with environmental laws and regulations, which can be expensive and restrict how we do business.
Our business activities involve the controlled storage and use of hazardous materials. We and suppliers with whom we may contract are subject to environmental, health and safety laws and regulations, including those governing the use, handling, storage, disposal and human exposure to hazardous and toxic materials. In some cases, these hazardous and toxic materials and various wastes resulting from their use are stored at our facilities pending their use and disposal. We cannot eliminate the risk of accidental contamination or injury from these materials, which could cause an interruption of our commercialization efforts, research and development efforts and business operations and environmental damage resulting in costly clean-up and liabilities under applicable laws and regulations governing the use, storage, handling and disposal of these materials and waste products. We cannot guarantee that the safety procedures utilized by the suppliers with whom we contract will comply with the standards prescribed by laws and regulations or will eliminate the risk of accidental contamination or injury from these materials. We may be held liable for any resulting damages and such liability could exceed our resources and European or other applicable authorities may curtail our use of certain materials and/or interrupt our manufacturing and business operations. Furthermore, liability under environmental laws and regulations can be joint and several and without regard to comparative fault, and environmental laws and regulations could become more stringent over time, imposing greater compliance costs and increasing risks and penalties associated with violations, which could have an adverse impact on our business. We cannot predict the impact of such changes and cannot be certain of our future compliance. Although we believe that our activities conform in all material respects with environmental, health and safety laws, there can be no assurance that violations of environmental, health and safety laws and regulations will not occur in the future as a result of human error, accident, equipment failure or other causes. The failure to comply with past, present or future laws and regulations could result in the imposition of fines, third-party property damage and personal injury claims, investigation and remediation costs, the suspension of production or a cessation of operations. We also expect that our operations will be affected by other new environmental, health and safety laws and regulations on an ongoing basis. Although we cannot predict the ultimate impact of any such new laws and regulations, they will likely result in additional costs, and may require us to change how we manufacture our products, which could have an adverse impact on our business.
We do not currently carry comprehensive hazardous waste insurance coverage. In the event of an accident or environmental discharge, we may be held liable for any consequential damage and any resulting claims for damages, which may exceed our financial resources and may materially adversely affect our business, results of operations and prospects and the value of our ordinary shares.
Risks Related to Our Intellectual Property
If our intellectual property rights do not adequately protect our products or technologies, others could compete against us more directly, which would hurt our profitability.
Our success depends in part on our ability to protect our intellectual property rights. Our intellectual property portfolio consists of one patent, and we also rely on trade secrets, proprietary know-how and regulatory barriers to protect our products and technologies and seek protection of our rights, in part, through confidentiality and proprietary information agreements. Although we generally require all of our employees, consultants, advisors and any third parties who have access to our proprietary know-how, information or technology to assign or grant similar rights to their inventions to us, and endeavor to execute confidentiality agreements with all such parties, we cannot be certain that we have executed such agreements with all parties who may have contributed to our intellectual property or who had access to our proprietary information, nor can we be certain that our agreements with such parties will not be breached. In addition, these agreements may not provide adequate remedies for violation of our rights in the event of unauthorized use or disclosure of confidential and proprietary information. Such unauthorized use or disclosure may enable competitors to duplicate or surpass our technological achievements. We cannot guarantee that our trade secrets and other confidential proprietary
34
information will not be publicly disclosed or that competitors will not otherwise gain access to our trade secrets or independently develop substantially equivalent information and techniques. Failure to protect our proprietary rights could seriously impair our competitive position.
Our patent may be challenged, invalidated, circumvented or rendered unenforceable. We cannot assure you that we will be successful should our patent be challenged for any reason. If our patent claims are rendered invalid or unenforceable, or narrowed in scope, it could impair our competitive position and have an adverse effect on our business. We cannot assure you that any future patent applications held by us will result in an issued patent, or that if patents are issued to us, that such patents will provide meaningful protection against competitors or against competitive technologies. The issuance of a patent is not conclusive as to its validity or its enforceability. Courts or patent offices may invalidate our patents or find them unenforceable. Competitors may also be able to design around our patents or obtain patent protection for more effective technologies, designs or methods. If any of these developments were to occur, it could impair our competitive position and have an adverse effect on our business.
If our trademarks, service marks or trade names are not adequately protected, then we may not be able to build name recognition in our markets of interest and our business may be adversely affected.
We rely on trademark protection to protect our business and our products and services. Our registered or unregistered trademarks, service marks or trade names may be challenged, infringed, circumvented or declared generic or determined to be infringing on other marks. We may not be able to protect our rights to these trademarks, service marks and trade names, any of which we may need to build name recognition among potential partners or customers in our markets of interest. Over the long-term, if we are unable to establish name recognition based on our trademarks, service marks or trade names, then we may not be able to compete effectively and our business may be adversely affected.
The medical device industry is characterized by intellectual property litigation and we could become subject to litigation that could be costly, result in the diversion of management’s time and efforts, require us to pay damages or prevent us from marketing our existing or future products.
Our commercial success will depend in part on not infringing or violating the intellectual property rights of others. Significant litigation regarding intellectual property occurs in our industry. Our competitors, some of which have substantially greater resources than we do and have made substantial intellectual property investments in competing technologies, may have applied for or obtained, or may in the future apply for and obtain, patent rights and other intellectual property that will prevent, limit or otherwise interfere with our ability to make, use and sell our products. We may not be aware of whether our products do or will infringe existing or future patents or the intellectual property rights of others. In addition, patent applications can be pending for many years, and may be confidential for a number of months after filing, and because pending patent claims can be revised before issuance, there may be applications of others now pending of which we are unaware that may later result in issued patents that will prevent, limit or otherwise interfere with our ability to make, use or sell our products. Any lawsuits resulting from allegations that the operation of our business infringes the intellectual property rights of third parties could subject us to significant liability for damages and invalidate our proprietary rights, even if they lack merit. Any potential intellectual property litigation also could force us to do one or more of the following:
| | • | | stop making, selling or using products or technologies that allegedly infringe the asserted intellectual property; |
| | • | | lose the opportunity to license our technology to others or to collect royalty payments based upon successful protection and assertion of our intellectual property rights against others; |
| | • | | incur significant legal expenses; |
35
| | • | | pay substantial damages or royalties to the party whose intellectual property rights we may be found to be infringing; |
| | • | | pay the attorney fees and costs of litigation to the party whose intellectual property rights we may be found to be infringing; |
| | • | | redesign or rename, in the case of trademark claims, those products that contain the allegedly infringing intellectual property, which could be costly, disruptive and/or infeasible; or |
| | • | | attempt to obtain a license to the relevant intellectual property from third parties, which may not be available on reasonable terms or at all. |
Any litigation or claim against us, even those without merit, may cause us to incur substantial costs, and could place a significant strain on our financial resources, divert the attention of management from our core business and harm our reputation. If we are found to infringe the intellectual property rights of third parties, we could be required to pay substantial damages and/or substantial royalties and could be prevented from selling our products unless we obtain a license or are able to redesign our products to avoid infringement. Any such license may not be available on reasonable terms, if at all, and there can be no assurance that we would be able to redesign our products in a way that would not infringe the intellectual property rights of others. If we fail to obtain any required licenses or make any necessary changes to our products or technologies, we may have to withdraw existing products from the market or may be unable to commercialize one or more of our products, all of which could have a material adverse effect on our business, results of operations and financial condition.
We may be subject to damages resulting from claims that we or our employees have wrongfully used or disclosed alleged know-how or trade secrets of our competitors or are in breach of non-competition or non-solicitation agreements with our competitors.
Some of our employees were previously employed at other medical device companies, including our competitors or potential competitors, in some cases until recently. We may be subject to claims that we or our employees have inadvertently or otherwise used or disclosed alleged trade secrets or other proprietary information of these former employers or competitors. In addition, we may be subject to claims that we caused an employee to breach the terms of his or her non-competition or non-solicitation agreement. Litigation may be necessary to defend against these claims. Even if we are successful in defending against these claims, litigation could result in substantial costs and could be a distraction to management. If our defense to those claims fails, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel. Any litigation or the threat thereof may adversely affect our ability to hire employees. A loss of key personnel or their work product could hamper or prevent our ability to commercialize our products, which could have an adverse effect on our business, results of operations and financial condition.
Our distributors may face intellectual property infringement claims and our distribution agreements could require us to indemnify our distributors against any such claims, which could be time-consuming and costly to defend or settle and, if adversely adjudicated, could result in the loss of significant rights.
Third parties may assert claims against our distributors that our products infringe their intellectual property rights. Any such claims, regardless of their merit or resolution, could be costly to defend or settle and could divert the efforts and attention of our management and technical personnel. Many of our customer and distributor agreements require us to indemnify and defend our distributors, as applicable, from third-party infringement claims and pay damages in the case of adverse rulings. Claims of this sort also could harm our relationships with our distributors and might deter future distributors from doing business with us. If any such proceedings result in an adverse outcome, we could be required to replace infringing technology or refund the distributor the amount paid for any infringing products. Any of the foregoing results could have a material adverse effect on our business, financial condition and results of operations.
36
We may not be able to enforce our intellectual property rights throughout the world.
Filing, prosecuting and defending our intellectual property in all countries throughout the world would be prohibitively expensive. Moreover, the laws of some countries outside of Europe and the United States do not afford intellectual property protection to the same extent as the laws of Europe and the United States, and many companies have encountered significant problems in protecting and defending intellectual property rights in certain foreign jurisdictions. If we face similar challenges in respect of material intellectual property matters, this could make it difficult for us to stop the misappropriation of our intellectual property rights, which could have an adverse effect on our business.
Risks Related to this Offering and Ownership of Our Ordinary shares
No public market for our ordinary shares currently exists and an active trading market may not develop or be sustained following this offering.
Prior to this initial public offering, there has been no public market for our ordinary shares. Although our ordinary shares have been approved for listing on , an active trading market may not develop or be sustained following the completion of this offering. The lack of an active market may impair your ability to sell your ordinary shares at the time you wish to sell them or at a price that you consider reasonable. The lack of an active market may also reduce the fair market value or the trading price of your ordinary shares. An inactive market may also impair our ability to raise capital by selling ordinary shares and may impair our ability to acquire other companies or technologies by using our ordinary shares as consideration.
The price of our ordinary shares may be volatile, and you may not be able to resell our ordinary shares at or above the initial public offering price.
The initial public offering price for our ordinary shares has been determined through our negotiations with the underwriters and may not be representative of the price that will prevail in the open market following the offering. Our ordinary share price after the completion of this offering may be highly volatile and could be subject to wide fluctuations in response to various factors, some of which are beyond our control. These factors include those discussed in this “Risk Factors” section of this prospectus and others, such as:
| | • | | a slowdown in the medical device industry, the aesthetics industry or the countries in which we operate; |
| | • | | actual or anticipated quarterly or annual variations in our results of operations or those of our competitors; |
| | • | | changes in foreign currency exchange rates; |
| | • | | changes in accounting principles or changes in interpretations of existing principles, which could affect our financial results; |
| | • | | actual or anticipated changes in our growth rate relative to our competitors; |
| | • | | changes in earnings estimates or recommendations by securities analysts; |
| | • | | fluctuations in the values of companies perceived by investors to be comparable to us; |
| | • | | announcements by us or our competitors of new products or services, significant contracts, commercial relationships, capital commitments or acquisitions; |
| | • | | our ability to continue obtaining raw material from our suppliers; |
| | • | | competition from existing technologies and products or new technologies and products that may emerge; |
37
| | • | | the entry into, modification of or termination of agreements with our sales representatives or distributors; |
| | • | | developments with respect to intellectual property rights; |
| | • | | sales, or the anticipation of sales, of our ordinary shares by us, our insiders or our other shareholders, including upon the expiration of contractual lock-up agreements; |
| | • | | our ability to develop and market new and enhanced products on a timely basis; |
| | • | | our commencement of, or involvement in, litigation; |
| | • | | additions or departures of key management or technical personnel; and |
| | • | | changes in laws or governmental regulations applicable to us. |
In recent years, the stock markets generally have experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of those companies. Broad market and industry factors may significantly affect the market price of our ordinary shares, regardless of our actual operating performance. These fluctuations may be even more pronounced in the trading market for our ordinary shares shortly following this offering. If the market price of our ordinary shares after this offering does not ever exceed the initial public offering price, you may not realize any return on your investment in us and may lose some or all of your investment.
We are an “emerging growth company” and intend to take advantage of reduced disclosure requirements applicable to emerging growth companies, which could make our ordinary shares less attractive to investors.
We are an “emerging growth company,” as defined in the JOBS Act, and we intend to take advantage of certain exemptions from various reporting requirements that apply to other public companies that are not “emerging growth companies.” We may take advantage of these exemptions until we are no longer an “emerging growth company.” We will remain an “emerging growth company” until the earliest of the following occurs: (i) the first fiscal year following the fifth anniversary of this offering, (ii) the first fiscal year after our annual gross revenue is $1 billion or more, (iii) the date on which we have, during the previous three-year period, issued more than $1 billion in non-convertible debt securities and (iv) the end of any fiscal year in which the market value of our ordinary shares held by non-affiliates exceeds $700 million as of the end of the second quarter of that fiscal year. Most of the exemptions from reporting requirements relate to disclosures that we would only be required to make if we also cease being a foreign private issuer in the future. As a foreign private issuer that is also an emerging growth company:
| | • | | we are permitted to present only two years of audited financial statements and only two years of related “Management’s Discussion and Analysis of Financial Condition and Results of Operations”; |
| | • | | we are exempt from complying with the auditor attestation requirements of Section 404 of the Sarbanes Oxley Act; |
| | • | | we are also exempt from the requirements of holding a non-binding advisory vote on executive compensation and shareholder approval of any golden parachute payments not previously approved; |
| | • | | we are permitted to present reduced disclosures regarding executive compensation in our periodic reports; and |
| | • | | we may take advantage of an extended transition period for complying with new or revised accounting standards. |
38
Following the completion of this offering, a small number of our shareholders will have a controlling influence over matters requiring shareholder approval, which could delay or prevent a change of control.
Following the completion of this offering, the largest beneficial owners of our ordinary shares, entities affiliated with Montreux, Oyster and OrbiMed, will own %, in the aggregate, of our ordinary shares, % if the underwriters exercise their option to purchase additional ordinary shares in full. In addition, these shareholders currently hold two director positions. As a result, these shareholders, should they choose to act together, or even if they act individually, will exert significant influence over our operations and business strategy and would together have sufficient voting power to control the outcome of matters requiring shareholder approval. These matters may include:
| | • | | the composition of the Board of Directors (the “Board”), which has the authority to direct our business and to appoint and remove our officers; |
| | • | | approving or rejecting a merger, consolidation or other business combination; |
| | • | | raising future capital; and |
| | • | | amending our articles of association, which govern the rights attached to our ordinary shares. |
This concentration of ownership of our ordinary shares could delay or prevent mergers, tender offers, open-market purchase programs or other purchases of our ordinary shares that might otherwise give you the opportunity to realize a premium over the then-prevailing market price of our ordinary shares. This concentration of ownership may also adversely affect the price of our ordinary shares.
Purchasers in this offering will experience immediate dilution in the book value of their investment.
The initial public offering price of our ordinary shares is higher than the net tangible book value per share of our ordinary shares immediately prior to this offering. Therefore, if you purchase our ordinary shares in this offering, you will incur an immediate dilution of $ in pro forma as adjusted net tangible book value per share as of , 2015 from the initial public offering price. New investors who purchase ordinary shares in this offering will contribute approximately % of the total amount of equity capital raised by us through the date of this offering, and will own approximately % of the outstanding share capital and approximately % of the voting rights. We have also issued options and warrants to acquire ordinary shares at prices below the initial public offering price. To the extent outstanding options and warrants are ultimately exercised, there will be further dilution to investors who purchase ordinary shares in this offering. In addition, if the underwriters exercise their option to purchase additional ordinary shares or if we issue additional equity securities, investors purchasing ordinary shares in this offering will experience additional dilution. As a result of the dilution to investors purchasing ordinary shares in this offering, investors may receive significantly less than the purchase price paid in this offering, if anything, in the event of our liquidation. For a further description of the dilution that you will experience immediately after this offering, see “Dilution.”
We do not anticipate paying any cash dividends in the foreseeable future, and accordingly, shareholders must rely on stock appreciation for any return on their investment.
After the completion of this offering, we do not anticipate declaring any cash dividends to holders of our ordinary shares in the foreseeable future. In addition, our ability to pay cash dividends is currently prohibited by Irish law and the terms of our existing loan agreement and may be prohibited by future loan agreements. As a result, capital appreciation, if any, of our ordinary shares will be your sole source of gain for the foreseeable future.
39
We will incur increased costs as a result of operating as a public company and our management will be required to devote substantial time to new compliance initiatives and corporate governance practices.
As a public company, and increasingly after we are no longer an “emerging growth company,” or if we lose our foreign private issuer status, we will incur significant legal, accounting and other expenses that we did not incur as a private company. In addition, U.S. securities laws and regulations and rules impose numerous requirements on public companies. Also, the Exchange Act requires, among other things, that we file annual and current reports with respect to our business and results of operations. Our management and other personnel will need to devote a substantial amount of time to compliance with these laws and regulations. These requirements have increased and will continue to increase our legal, accounting and financial compliance costs and have made and will continue to make some activities more time consuming and costly. For example, we expect these rules and regulations to make it more difficult and more expensive for us to obtain director and officer liability insurance, and we may be required to incur substantial costs to maintain the same or similar coverage. These rules and regulations could also make it more difficult for us to attract and retain qualified persons to serve on the Board and its committees or as executive officers.
Overall, we estimate that our incremental costs resulting from operating as a public company, including compliance with these rules and regulations, may be between $ million and $ million per year. However, these rules and regulations are often subject to varying interpretations, in many cases due to their lack of specificity, and, as a result, their application in practice may evolve over time as new guidance is provided by regulatory and governing bodies. This could result in continuing uncertainty regarding compliance matters and higher costs necessitated by ongoing revisions to disclosure and governance practices.
Our registered public accounting firm has included an explanatory paragraph relating to our ability to continue as a going concern in its report on our audited financial statements included in this prospectus.
Based on our recurring losses from operations and net capital deficiency, and not taking into consideration any proceeds that we will receive in connection with this offering, our independent registered public accounting firm included an explanatory paragraph in its report on our financial statements included elsewhere in this prospectus expressing doubt about our ability to continue as a going concern. While we believe that after receipt of the proceeds from this offering we will continue as a going concern for the foreseeable future, we may require additional funding to continue operations and realize our business objectives in the future. If we are unable to continue as a going concern in the future, we may be unable to meet our obligations under the Credit Facility, which could result in an acceleration of our obligation to repay all amounts owed thereunder, and we may be forced to liquidate our assets. In such a scenario, the values we receive for our assets in liquidation or dissolution could be significantly lower than the values reflected in our financial statements.
In the course of preparing our consolidated financial statements, we have identified deficiencies in our internal control over financial reporting that could result in a finding of a material weakness. As of the date of this prospectus, these deficiencies have not been remediated. If we fail to achieve effective internal control over financial reporting, our ability to produce accurate and timely financial statements could be impaired, which could materially and adversely affect our results of operations, investors’ views of us and, as a result, the value of our ordinary shares.
Historically, as a private company operating in Ireland, we were not required to prepare financial statements in accordance with IFRS as issued by IASB or to comply with the internal control requirements of the Sarbanes-Oxley Act. As a U.S. public company, our management will be required to report on the effectiveness of our internal control over financial reporting beginning with our Annual Report on Form 20-F for the year ended December 31, 2016. The rules governing the standards that must be met for management to assess our internal control over financial reporting are complex and require significant documentation, testing and possible remediation.
In the course of preparing our consolidated financial statements, we have identified deficiencies in the design and operating effectiveness of our internal control over financial reporting, including deficiencies relating
40
to: (i) revenue recognition in one of the countries in which we sell our products; (ii) calculation of tax; and (iii) valuation of the equity derivatives associated with our B Preference Shares and C Preference Shares. These deficiencies, or other deficiencies that we may discover in the future, could result in one or more findings of a material weakness. We plan to take various measures to remediate these deficiencies, including upgrading our information technology systems, hiring additional finance and accounting personnel with appropriate training, building our financial management and reporting infrastructure, hiring external advisors and further developing and documenting our accounting policies and financial reporting procedures. These actions that we are taking are subject to ongoing management review, as well as audit committee oversight. Although we plan to complete this remediation process as quickly as possible, we cannot at this time estimate how long it will take and whether our efforts will be successful. Even if successful, remediation of the deficiencies we have identified, and of any additional deficiencies or material weaknesses we may identify, in our control over financial reporting may cause us to incur higher than anticipated operating expenses. Additionally, testing of internal controls, and any remediation undertaken in connection with control deficiencies identified as a result thereof, will require substantial attention from management. Moreover, when we cease to be an “emerging growth company” under the JOBS Act, our auditors may be required to express an opinion on the effectiveness of our internal controls. We can make no assurances that our internal controls will be effective. If our internal control over financial reporting is not effective, we may not be able to accurately report our financial results or file our periodic reports in a timely manner, which could materially and adversely harm our results of operations, may cause investors to lose confidence in our reported financial information and may lead to a decline in the value of our ordinary shares. We also could be subject to, among other things, regulatory or enforcement actions by the SEC and and could be subject to securities litigation.
Future sales of our ordinary shares by existing shareholders could cause our stock price to decline.
Sales of a substantial number of our ordinary shares in the public market could occur at any time. These sales, or the perception in the market that our officers, directors or the holders of a large number of ordinary shares intend to sell shares, could reduce the market price of our ordinary shares. Based on ordinary shares outstanding as of , 2015, upon completion of this offering, we will have outstanding a total of ordinary shares, assuming no exercise of the underwriters’ option to purchase additional ordinary shares. Of these ordinary shares, only the ordinary shares sold in this offering by us, plus any ordinary shares sold upon exercise of the underwriters’ option to purchase additional ordinary shares, will be freely tradable, without restriction, in the public market immediately after the offering. Each of our directors and officers and all of our shareholders have entered into lock-up agreements with the underwriters that restrict their ability to sell or transfer their ordinary shares. The lock-up agreements pertaining to this offering will expire 180 days from the date of this prospectus; however, Merrill Lynch, Pierce, Fenner & Smith Incorporated may, in its sole discretion, permit our directors, officers and shareholders to sell ordinary shares prior to expiration of the lock-up agreements.
After the lock-up agreements expire, based on ordinary shares outstanding as of , 2015, up to an additional ordinary shares will be eligible for sale in the public market, approximately of which are held by our directors and officers. In addition, of our ordinary shares that are subject to outstanding options as of , 2015 will become eligible for sale in the public market to the extent permitted by the provisions of various vesting agreements and applicable securities laws.
In addition, after this offering, holders of an aggregate of approximately ordinary shares will have rights, subject to some conditions, to require us to file registration statements covering their shares or to include their shares in registration statements that we may file for ourselves or other shareholders.
We cannot predict what effect, if any, sales of our ordinary shares in the public market or the availability of shares for sale will have on the market price of our ordinary shares. Future sales of substantial amounts of our ordinary shares in the public market, including ordinary shares issued upon exercise of outstanding options, or the perception that such sales may occur, however, could adversely affect the market price of our ordinary shares and also could adversely affect our future ability to raise capital through the sale of our ordinary shares or other equity-related securities of ours at times and prices we believe appropriate.
41
Our management team may invest or spend the proceeds of this offering in ways with which you may not agree or in ways that may not yield a return.
Our management will have considerable discretion in the application of the net proceeds that we receive from this offering, including for any of the purposes described in the section entitled “Use of Proceeds,” and you will not have the opportunity as part of your investment decision to assess whether the net proceeds are being used appropriately. Because of the number and variability of factors that will determine our use of the net proceeds from this offering, their ultimate use may vary substantially from their currently intended use. Our management might not apply our net proceeds in ways that ultimately increase the value of your investment.
We expect to use the net proceeds from this offering primarily for commercialization of our products in additional geographic regions, the continued expansion of our sales force, marketing programs, product portfolio and manufacturing capabilities as well as potential future acquisitions. We intend to use the remaining proceeds for working capital and general corporate purposes. The failure by our management to apply these funds effectively could harm our business. Pending their use, we may invest the net proceeds from this offering in short-term, investment-grade, interest-bearing securities. These investments may not yield a favorable return to our shareholders. If we do not invest or apply the net proceeds from this offering in ways that enhance shareholder value, we may fail to achieve expected financial results, which could cause our stock price to decline.
As a holding company, our only material assets will be our equity interests in our operating subsidiaries, and our principal source of revenue and cash flow will be distributions from such subsidiaries, which may be limited by law and/or contract in making such distributions.
As a holding company, our principal source of revenue and cash flow will be distributions from our subsidiaries. Therefore, our ability to carry out our business plan, to fund and conduct our business, service our debt and pay dividends (if any) in the future will depend on the ability of our subsidiaries to generate sufficient net income and cash flow to make upstream cash distributions to us. Our subsidiaries are separate legal entities, and although they may be wholly owned or controlled by us, they have no obligation to make any funds available to us, whether in the form of loans, dividends or otherwise. The ability of our subsidiaries to distribute cash to us will also be subject to, among other things, restrictions that may be contained in our subsidiaries’ agreements (as entered into from time to time), availability of sufficient funds in such subsidiaries and applicable laws and regulatory restrictions. Claims of any creditors of our subsidiaries generally will have priority as to the assets of such subsidiaries over our claims and claims of our creditors and shareholders. To the extent the ability of our subsidiaries to distribute dividends or other payments to us could be limited in any way, this could materially limit our ability to fund and conduct our business, service our debt and pay dividends (if any).
If we are a passive foreign investment company, U.S. investors in our ordinary shares could be subject to adverse U.S. federal income tax consequences.
The rules governing passive foreign investment companies (“PFICs”) can have adverse effects for U.S. investors for U.S. federal income tax purposes. The tests for determining PFIC status for a taxable year depend upon the relative values of certain categories of assets and the relative amounts of certain kinds of income. As discussed in “Material Tax Considerations—Material U.S. Federal Income Tax Considerations,” we do not believe that we are currently a PFIC, and we do not anticipate becoming a PFIC in the foreseeable future. Notwithstanding the foregoing, the determination of whether we are a PFIC depends on the particular facts and circumstances (such as the valuation of our assets, including goodwill and other intangible assets) and may also be affected by the application of the PFIC rules, which are subject to differing interpretations. The fair market value of our assets is expected to depend, in part, upon (i) the market price of our ordinary shares and (ii) the composition of our income and assets, which will be affected by how, and how quickly, we spend any cash that is raised in any financing transaction, including this offering. In light of the foregoing, no assurance can be provided that we are not currently a PFIC or that we will not become a PFIC in any future taxable year.
42
If we are or become a PFIC, U.S. holders of our ordinary shares would be subject to adverse U.S. federal income tax consequences, such as ineligibility for any preferential tax rates on capital gains or on actual or deemed dividends, interest charges on certain taxes treated as deferred, and additional reporting requirements under U.S. federal income tax laws and regulations. Whether U.S. holders of our ordinary shares make a timely qualified electing fund (“QEF”), election or mark-to-market election may affect the U.S. federal income tax consequences to U.S. holders with respect to the acquisition, ownership and disposition of our ordinary shares and any distributions such U.S. holders may receive. We do not, however, expect to provide the information regarding our income that would be necessary in order for a U.S. Holder to make a QEF election with respect to ordinary shares if we are classified as a PFIC. Investors should consult their own tax advisors regarding all aspects of the application of the PFIC rules to our ordinary shares.
U.S. holders of 10% or more of the voting power of our ordinary shares may be subject to U.S. federal income taxation at ordinary income tax rates on undistributed earnings and profits.
There is a risk that we will be classified as a controlled foreign corporation (“CFC”) for U.S. federal income tax purposes. We will generally be classified as a CFC if more than 50% of our outstanding shares, measured by reference to voting power or value, are owned (directly, indirectly or by attribution) by “U.S. Shareholders.” For this purpose, a “U.S. Shareholder” is any U.S. person that owns directly, indirectly or by attribution, 10% or more of the voting power of our outstanding shares. If we are classified as a CFC, a U.S. Shareholder may be subject to U.S. income taxation at ordinary income tax rates on all or a portion of our undistributed earnings and profits attributable to “subpart F income” and may also be subject to tax at ordinary income tax rates on any gain realized on a sale of ordinary shares, to the extent of our current and accumulated earnings and profits attributable to such shares. The CFC rules are complex and U.S. Shareholders of the ordinary shares are urged to consult their own tax advisors regarding the possible application of the CFC rules to them in their particular circumstances.
If securities or industry analysts do not publish research or reports about our business, or if they issue an adverse or misleading opinion regarding our stock, our stock price and trading volume could decline.
The trading market for our ordinary shares will be influenced by the research and reports that industry or securities analysts publish about us or our business. We do not currently have and may never obtain research coverage by securities and industry analysts. If no or few securities or industry analysts commence coverage of us, the trading price for our ordinary shares would be negatively impacted. In the event we obtain securities or industry analyst coverage, if any of the analysts who cover us issue an adverse or misleading opinion regarding us, our business model or the performance of our ordinary shares and results of operations fail to meet the expectations of analysts, the price of our ordinary shares would likely decline. If one or more of these analysts cease coverage of us or fail to publish reports on us regularly, we could lose visibility in the financial markets, which in turn could cause the price of our ordinary shares or trading volume to decline.
We could be subject to securities class action litigation.
In the past, securities class action litigation has often been instituted against companies whose securities have experienced periods of volatility in market price. Securities litigation brought against us following volatility in the price of our ordinary shares, regardless of the merit or ultimate results of such litigation, could result in substantial costs, which would hurt our financial condition and results of operations and divert management’s attention and resources from our business.
43
Risks Related to Being a Foreign Private Issuer
As a foreign private issuer, we will not be subject to U.S. proxy rules and will be exempt from filing certain Exchange Act reports.
As a foreign private issuer, we will be exempt from a number of requirements under U.S. securities laws that apply to public companies that are not foreign private issuers. In particular, we will be exempt from the rules and regulations under the Exchange Act related to the furnishing and content of proxy statements, and our officers, directors and principal shareholders will be exempt from the reporting and short-swing profit recovery provisions contained in Section 16 of the Exchange Act. In addition, we will not be required under the Exchange Act to file annual and current reports and financial statements with the SEC as frequently or as promptly as U.S. domestic companies whose securities are registered under the Exchange Act and we will generally be exempt from filing quarterly reports with the SEC under the Exchange Act.
We will also be exempt from the provisions of Regulation FD, which prohibits the selective disclosure of material nonpublic information to, among others, broker-dealers and holders of a company’s securities under circumstances in which it is reasonably foreseeable that the holder will trade in the company’s securities on the basis of the information. These exemptions and leniencies will reduce the protections and the frequency and scope of information to which you would be entitled if we were not a foreign private issuer.
We would lose our foreign private issuer status if a majority of our directors or executive officers are U.S. citizens or residents and we fail to meet additional requirements necessary to avoid loss of foreign private issuer status. Although we have elected to comply with certain U.S. regulatory provisions, our loss of foreign private issuer status would make such provisions mandatory. We would also be required to follow U.S. proxy disclosure requirements with regard to the extent of disclosure of annual compensation of our five most highly compensated senior officers. The regulatory and compliance costs to us under U.S. securities laws as a U.S. domestic issuer may be significantly higher. If we are not a foreign private issuer, we will be required to file periodic reports and registration statements on U.S. domestic issuer forms with the SEC, which are more detailed and extensive than the forms available to a foreign private issuer. We may also be required to modify certain of our policies to comply with good governance practices associated with U.S. domestic issuers. Such conversion and modifications will involve additional costs. In addition, we may lose our ability to rely upon exemptions from certain corporate governance requirements on U.S. exchanges that are available to foreign private issuers.
Provisions contained in our articles of association, as well as provisions of Irish law, could impair a takeover attempt.
Our articles of association that will come into effect immediately prior to the completion of this offering, together with certain provisions of the Companies Act 2014 of Ireland (the “Irish Companies Act”), could have the effect of delaying or preventing changes in control or changes in our management without the consent of the Board.
There are a number of approaches for acquiring an Irish public limited company, including a court-approved scheme of arrangement under the Irish Companies Act, through a tender offer by a third party, by way of a merger with a company incorporated in the EEA under the European Communities (Cross-Border Mergers) Regulations 2008 (as amended) and by way of a merger with a company incorporated in Ireland under the Irish Companies Act. Each method requires shareholder approval or acceptance and different thresholds apply.
The Irish Takeover Panel Act, 1997 and the Irish Takeover Rules 2013 made thereunder (the “Irish Takeover Rules”) will govern a takeover or attempted takeover of our company by means of a court-approved scheme of arrangement or a tender offer. The Irish Takeover Rules contain detailed provisions for takeovers, including as to disclosure, process, dealing and timetable. The Irish Takeover Rules could discourage an investor from acquiring 30% or more of the outstanding ordinary shares of the Company unless such investor was prepared to make a bid to acquire all outstanding ordinary shares.
44
The Board may be limited by the Irish Takeover Rules in its ability to defend an unsolicited takeover attempt.
Under the Irish Takeover Rules, we will not be permitted to take certain actions that might “frustrate” an offer for our ordinary shares once the Board has received an offer, or has reason to believe an offer is or may be imminent, without the approval of more than 50% of shareholders entitled to vote at a general meeting of our shareholders and/or the consent of the Irish Takeover Panel. This could limit the ability of the Board to take defensive actions even if it believes that such defensive actions would be in the best interests of our company and shareholders.
Irish law differs from the laws in effect in the United States and U.S. investors may have difficulty enforcing civil liabilities against us, our directors or members of senior management named in this prospectus.
The majority of our directors and all of our executive officers named in this prospectus are non-residents of the United States, and all or a substantial portion of their assets are located outside the United States. As a result, it may not be possible to serve process on our officers and these directors, or us, in the United States or to enforce court judgments obtained in the United States against these individuals or us in Ireland based on the civil liability provisions of the U.S. federal or state securities laws. In addition, there is some uncertainty as to whether the courts of Ireland would recognize or enforce judgments of U.S. courts obtained against us or our directors or executive officers based on the civil liabilities provisions of the U.S. federal or state securities laws or hear actions against us or those persons based on those laws. We have been advised that the United States currently does not have a treaty with Ireland providing for the reciprocal recognition and enforcement of judgments in civil and commercial matters. Therefore, a final judgment for the payment of money rendered by any U.S. federal or state court based on civil liability, whether or not based solely on U.S. federal or state securities laws, would not automatically be enforceable in Ireland.
As an Irish company, we are principally governed by Irish law, which differs in some material respects from laws generally applicable to U.S. corporations and shareholders, including, among others, differences relating to interested director and officer transactions and shareholder lawsuits. Likewise, the duties of directors and officers of an Irish company generally are owed to the company only. Shareholders of Irish companies generally do not have a personal right of action against directors or other officers of the company and may exercise such rights of action on behalf of the company only in limited circumstances. Accordingly, holders of our ordinary shares may have more difficulty protecting their interests than would holders of shares of a corporation incorporated in a jurisdiction of the United States.
The rights of our shareholders may differ from the rights typically offered to shareholders of a U.S. corporation and these differences may make our ordinary shares less attractive to investors.
We are incorporated under Irish law and, therefore, certain of the rights of holders of our shares are governed by Irish law, including the provisions of the Irish Companies Act, and by our articles of association. These rights differ in certain respects from the rights of shareholders in typical U.S. corporations and these differences may make our ordinary shares less attractive to investors. The principal differences include the following:
| | • | | under Irish law, dividends may only be declared if we have, on an individual entity basis, profits available for distribution, within the meaning of the Irish Companies Act; |
| | • | | under Irish law, each shareholder generally has preemptive rights to subscribe on a proportionate basis to any issuance of shares. Preemption rights may be disapplied under Irish law for renewable five-year periods by Irish companies by way of a provision in such companies’ articles of association or a special resolution of their shareholders, which is an option we will avail ourselves of prior to the completion of this offering; |
| | • | | under Irish law, certain matters require the approval of holders of 75% of the votes cast at a general meeting of our shareholders, including amendments to our articles of association. This may make it |
45
| | more difficult for us to complete certain types of corporate transactions deemed advisable by the Board; |
| | • | | under Irish law, a bidder seeking to acquire us would need, on a tender offer, to receive shareholder acceptance in respect of 80% of our outstanding shares. If this 80% threshold is not achieved in the offer, under Irish law, the bidder cannot complete a “second step merger” to obtain 100% control of us. Accordingly, tender of 80% of our outstanding shares will likely be a condition in a tender offer to acquire us, not 50% as is more common in tender offers for corporations organized under U.S. law; and |
| | • | | under Irish law, shareholders may be required to disclose information regarding their equity interests upon our request, and the failure to provide the required information could result in the loss or restriction of rights attaching to the shares, including prohibitions on the transfer of the shares, as well as restrictions on voting, dividends and other payments. |
Our status as a “foreign private issuer” allows us to adopt IFRS, accounting principles, which are different than accounting principles under U.S. Generally Accepted Accounting Principles (“U.S. GAAP”).
We have adopted and presented our combined consolidated financial statements in accordance with IFRS. IFRS is an internationally recognized body of accounting principles that are used by many companies outside of the United States to prepare their financial statements, and the SEC recently permitted foreign private issuers such as our company to prepare and file their financial statements in accordance with IFRS rather than U.S. GAAP. IFRS accounting principles are different from those of U.S. GAAP, and SEC rules do not require us to provide a reconciliation of IFRS accounting principles to those of U.S. GAAP. Investors who are not familiar with IFRS may misunderstand certain information presented in our consolidated financial statements. Accordingly, we suggest that readers of our combined consolidated financial statements familiarize themselves with the provisions of IFRS accounting principles in order to better understand the differences between these two sets of principles.
A future transfer of your ordinary shares, other than one effected by means of the transfer of book entry interests in DTC, may be subject to Irish stamp duty.
Transfers of ordinary shares effected by means of the transfer of book entry interests in the Depository Trust Company (“DTC”) should not be subject to Irish stamp duty where ordinary shares are traded through DTC, either directly or through brokers who hold such shares on behalf of customers through DTC. However, if you hold your ordinary shares as of record rather than beneficially through DTC or through a broker that holds your ordinary shares through DTC, any transfer of your ordinary shares could be subject to Irish stamp duty (currently at the rate of 1% of the higher of the price paid or the market value of the shares acquired). Payment of Irish stamp duty is generally a legal obligation of the transferee. The potential for stamp duty to arise could adversely affect the price of our ordinary shares.
46
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus contains forward-looking statements. Forward-looking statements are neither historical facts nor assurances of future performance. Instead, they are based on our current beliefs, expectations and assumptions regarding the future of our business, future plans and strategies, our operational results and other future conditions. Forward-looking statements can be identified by words such as “anticipate,” “believe,” “estimate,” “expect,” “intend,” “may,” “plan,” “predict,” “project,” “seek,” “target,” “potential,” “will,” “would,” “could,” “should,” “continue,” “contemplate,” “might” and other similar expressions, although not all forward-looking statements contain these identifying words. These forward-looking statements include all matters that are not historical facts. They appear in a number of places throughout this prospectus and include statements regarding our intentions, beliefs or current expectation concerning, among other things, our results of operations, financial condition, liquidity, prospects, growth, strategies and the industry in which we operate.
By their nature, forward-looking statements involve risks and uncertainties because they relate to events and depend on circumstances that may or may not occur in the future. We believe that these risks and uncertainties include, but are not limited to, those described in the “Risk Factors” section of this prospectus, which include, but are not limited to, the following:
| | • | | We have incurred significant net operating losses and cannot assure you that we will ever achieve or maintain profitability. |
| | • | | Our future profitability depends on the success of our breast implant products. |
| | • | | Any negative publicity concerning our products or our competitors’ products could harm our business and reputation and negatively impact our financial results. |
| | • | | Our success depends on our ability to continue to enhance our existing products and develop or commercialize new products that respond to customer needs and preferences. |
| | • | | The size of the addressable global aesthetics products market, and the breast implant products market in particular, has not been established with precision and may be smaller than we estimate, possibly materially. If our estimates and projections overestimate the size of these markets and the markets for our other products, our revenue and results of operations will be adversely affected. |
| | • | | Each of our brands relies on a single-source supplier for medical-grade silicone, which is the primary constitutive ingredient of our products. These two suppliers are under common ownership, which could result in increased prices when we seek to renew our contracts with these suppliers. |
| | • | | Recent studies have called into question the long-term safety of breast implants, which could have a material adverse effect on our business, results of operations and financial condition. |
| | • | | If we acquire or attempt to acquire new businesses or products, we may be unable to complete these acquisitions or to successfully integrate them in a cost-effective and non-disruptive manner, and we may not realize the benefits we anticipate from such acquisitions. |
| | • | | We will need to significantly increase the size of our organization, and we may experience difficulties in managing growth. |
| | • | | Our business could suffer if we lose the services of our senior management or other key personnel or are unable to attract and retain additional qualified personnel. |
| | • | | If we fail to maintain and further develop our direct sales forces and distributor networks, our business could suffer. Additionally, our third-party distributors may not effectively sell our products or may engage in activities that could harm our reputation and sales of our products. |
47
| | • | | Risks associated with our global operations, including seeking, obtaining and maintaining approval to commercialize our products in the jurisdictions where our products are sold, could harm our business. |
| | • | | Our results of operations could be materially adversely affected by fluctuations in foreign currency exchange rates. |
| | • | | Any disruption at our manufacturing facilities could adversely affect our business and results of operations. |
| | • | | If we fail to compete effectively against our competitors, many of whom have greater resources than we have, our revenues and results of operations may be negatively affected. |
| | • | | Our quarterly revenues and results of operations are unpredictable and may fluctuate significantly from quarter to quarter due to factors outside our control, which could adversely affect our business, results of operations and the trading price of our ordinary shares. |
| | • | | Our medical device products and operations, including manufacturing, are subject to extensive governmental regulation, including health, environmental, safety and anti-corruption and other laws, and our failure to comply with applicable requirements could cause our business to suffer. |
These factors should not be construed as exhaustive and should be read with the other cautionary statements in this prospectus.
Although we base these forward-looking statements on assumptions that we believe are reasonable when made, we caution you that forward-looking statements are not guarantees of future performance and that our actual results of operations, financial condition and liquidity, and the development of the industry in which we operate, may differ materially from that referenced in or suggested by the forward-looking statements contained in this prospectus. In addition, even if our results of operations, financial condition and liquidity, and the development of the industry in which we operate, are consistent with the forward-looking statements contained in this prospectus, those results or developments may not be indicative of results or developments in subsequent periods.
Given these risks and uncertainties, you are cautioned not to place undue reliance on these forward-looking statements. Any forward-looking statement that we make in this prospectus speaks only as of the date of such statement, and we undertake no obligation to update any forward-looking statements or to publicly announce the results of any revisions to any of those statements to reflect future events or developments. Comparisons of results for current and any prior periods are not intended to express any future trends or indications of future performance, unless specifically expressed as such, and should only be viewed as historical data.
48
PRESENTATION OF FINANCIAL INFORMATION
Historical Financial Information
We prepare our consolidated financial statements in accordance with IFRS as issued by the IASB. Although we maintain our books and records in Euros, we present our financial statements in US dollars. Unless otherwise indicated, all references to currency amounts in this prospectus are in US dollars. All references to “€” are to the Euro.
The financial information contained in this prospectus includes our combined consolidated financial statements as of December 31, 2014 and 2013 and for the years ended December 31, 2014 and 2013, which have been audited by our independent registered public accounting firm, KPMG, as stated in their report included in this prospectus.
We have historically conducted our business through Global Consolidated Aesthetics Limited and its consolidated subsidiaries, and therefore our historical financial statements present the results of operations of such entities, presented on the assumption that the Share-for-Share Exchange, which is not expected to materially affect our historical results of operations, has occurred and is accounted for as a combination of businesses under common control of GC Aesthetics plc. Following the corporate reorganization and this offering, our financial statements will present the results of operations of GC Aesthetics plc and its consolidated subsidiaries.
This financial information should be read in conjunction with “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our combined consolidated financial statements, including the notes thereto, included elsewhere in this prospectus.
We have made rounding adjustments to some of the figures included in this prospectus. Accordingly, numerical figures shown as totals in some tables may not be an arithmetic aggregation of the figures that precede them.
Non-IFRS Financial Measures
In this prospectus, we use Constant Currency Revenue, Adjusted Gross Profit, Adjusted EBITDA and Adjusted Interest Expense. These measures are not calculated in accordance with IFRS, and we refer to these as non-IFRS financial measures. We use these non-IFRS financial measures when planning, monitoring, and evaluating our performance. We consider these non-IFRS financial measures to be useful metrics for management and investors to facilitate operating performance comparisons from period to period by excluding potential differences caused by variations in foreign exchange rates, depreciation, amortization, non-cash charges and certain other expenses that we believe are not representative of our core business. Constant Currency Revenues, Adjusted Gross Profit, Adjusted EBITDA and Adjusted Interest Expense have important limitations as analytical tools, should not be viewed in isolation, and do not purport to be alternatives to net income as indicators of operating performance or cash flows from operating activities as measures of liquidity. Constant Currency Revenue, Adjusted Gross Profit, Adjusted EBITDA and Adjusted Interest Expense exclude some, but not all, items that affect net income or cash flows from operating activities and these measures may vary among companies. For more information regarding these non-IFRS financial measures and a reconciliation of such measures to comparable IFRS financial measures, please see the footnotes to the financial statements presented in “Prospectus Summary—Summary Consolidated Financial Data” and “Management’s Discussion and Analysis of Financial Condition—Key Financial Metrics.”
49
TRADEMARKS AND SERVICE MARKS
We own or have rights to trademarks and service marks for use in connection with the operation of our business, including, but not limited to, COGEL, DREAMXCELL, EUROSILICONE, GC AESTHETICS, GCA, GCA BUSINESS BOOST, NAGOR and SILGEL. All other trademarks or service marks appearing in this prospectus that are not identified as marks owned by us are the property of their respective owners.
Solely for convenience, the registered trademarks, service marks and trade names referred to in this prospectus are listed without the®, ™ andSM symbols, but we assert, to the fullest extent under applicable law, our applicable rights in these registered trademarks, service marks and trade names.
50
MARKET AND INDUSTRY DATA
Although we are responsible for all of the disclosure contained in this prospectus, this prospectus includes estimates, projections and other information concerning our industry and market data that we obtained from periodic industry publications, third-party studies and surveys (certain of which we have sponsored) and internal company proprietary research and analysis. Industry publications and surveys generally state that the information contained therein has been obtained from sources believed to be reliable. Although we believe the industry and market data included in this prospectus is reliable as of the date hereof, this information could prove to be inaccurate, and should not be relied upon in making, or refraining from making, any investment decision. Industry and market data could be wrong because of the method by which sources obtained and analyzed the data they collected or because such data cannot always be verified with complete certainty due to limitations on the availability and reliability of raw data, the survey subject selection process and other limitations and uncertainties. In addition, we do not know all of the assumptions regarding general economic conditions or growth that were used by the sources in preparing the forecasts relied upon or cited herein. Our estimates involve risks and uncertainties and are subject to change based on various factors, including those discussed under the heading “Risk Factors” in this prospectus. Unless otherwise indicated, all market share information contained in this prospectus is based upon data prepared by one or more of the following sources: ISAPS, TechNavio Insights, Medical Insight, Inc., Euromonitor International, Ltd. and BCC Research LLC.
51
USE OF PROCEEDS
We estimate that the net proceeds to us from our issuance and sale of ordinary shares in this offering will be approximately $ million, after deducting underwriting discounts and commissions and estimated offering expenses payable by us (or approximately $ million if the underwriters exercise their option to purchase additional ordinary shares in full). This estimate assumes an initial public offering price of $ per ordinary share, the midpoint of the price range set forth on the cover page of this prospectus.
A $1.00 increase (decrease) in the assumed initial public offering price of $ per ordinary share would increase (decrease) the net proceeds to us from this offering by $ million, assuming the number of ordinary shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting the estimated underwriting discounts and commissions and estimated expenses payable by us.
We intend to use the net proceeds of this offering to expand our (i) operations into new countries, (ii) product portfolio, (iii) existing commercial infrastructure and (iv) manufacturing capabilities. In addition, we intend to use the net proceeds of this offering to fund potential future acquisitions. We will use any remaining proceeds for working capital and other general corporate purposes.
52
DIVIDEND POLICY
We have not declared or paid regular cash dividends on our ordinary shares. We currently expect to retain all future earnings for use in the operation and expansion of our business and do not anticipate paying cash dividends in the foreseeable future. Additionally, because we have an accumulated deficit, under Irish law, we may not declare or pay dividends and dividends may only be declared and paid if we have profits available for distribution. Our ability to pay dividends on our ordinary shares is further limited by restrictions on our and our subsidiaries’ ability to pay dividends or make distributions under the terms of the agreements governing our indebtedness. The declaration and payment of any dividends in the future will be determined by the Board, in its discretion, and will depend on a number of factors, including our earnings, capital requirements, overall financial condition and contractual restrictions.
It is anticipated that cash dividends on our ordinary shares, if any, will be paid in US dollars. As we are an Irish company, Irish dividend withholding tax (“DWT”), currently at a rate of 20%, will arise in respect of dividends or other distributions to our shareholders unless an exemption applies. There are exemptions that may be available to U.S. Holders (as defined in “Material U.S. Federal Income Tax Considerations”); such shareholders should consult their respective tax advisors. Where DWT arises, we are responsible for deducting DWT at source and accounting for the relevant amount to the Irish Revenue Commissioners. See “Material Irish Tax Considerations—Dividend Withholding Tax” and “Description of Share Capital—Dividends.”
53
CAPITALIZATION
The following table sets forth the cash and cash equivalents and capitalization of Global Consolidated Aesthetics Limited as of December 31, 2014:
| | • | | on a pro forma basis to reflect (i) the exercise of all warrants and the conversion of all of Global Consolidated Aesthetics Limited’s A Ordinary Shares, B Preference Shares and C Preference Shares into ordinary shares upon the completion of this offering and (ii) the effectiveness of our amended and restated memorandum and articles of association; and |
| | • | | on a pro forma basis as adjusted to reflect the issuance and sale by GC Aesthetics plc of ordinary shares in this offering and the use of proceeds as indicated under “Use of Proceeds.” |
The information below is illustrative only, and assumes an initial public offering price at the midpoint of the price range set forth on the cover page of this prospectus, after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us. Our capitalization following this offering will be adjusted based on the actual initial public offering price and other terms of this offering determined at pricing, including the amount by which actual offering expenses are higher or lower than estimated. The table should be read in conjunction with the information contained in “Use of Proceeds,” “Selected and Pro Forma Consolidated Financial Data” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” as well as our consolidated financial statements and the related notes included elsewhere in this prospectus.
| | | | | | | | | | | | | | | | | | | | |
| | | As of December 31, 2014 | |
| | | Actual | | | Pro forma
adjustments | | | Pro Forma | | | Offering pro
forma
adjustments(2) | | | Pro Forma,
as adjusted | |
Cash and cash equivalents at end of year | | $ | 10,616 | | | | | | | $ | — | | | | | | | $ | — | |
| | | | | | | | | | | | | | | | | | | | |
Long-term debt, including current portion: | | | | | | | | | | | | | | | | | | | | |
B Preference shares | | $ | 110,433 | | | | | | | | | | | | | | | | | |
C Preference shares | | | 5,777 | | | | | | | | | | | | | | | | | |
Equity derivative | | | 22,284 | | | | | | | | | | | | | | | | | |
Shareholder loans—interest bearing(1) | | | 50,412 | | | | | | | | | | | | | | | | | |
Other loans and borrowings | | | 1,839 | | | | | | | | | | | | | | | | | |
Provisions and other liabilities | | | 25,385 | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
Total long-term debt, including current portion | | | 216,130 | | | | — | | | | — | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | | | | |
Shareholders’ equity / (deficiency): | | | | | | | | | | | | | | | | | | | | |
Share capital | | | 70 | | | | | | | | | | | | | | | | | |
Additional paid-in capital | | | 34,287 | | | | | | | | | | | | | | | | | |
Accumulated deficit | | | (183,489 | ) | | | | | | | | | | | | | | | | |
Accumulated other comprehensive income | | | 12,152 | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
Total equity / (deficiency) | | | (136,980 | ) | | | — | | | | — | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | | | | |
Total equity and liabilities | | $ | 79,150 | | | $ | — | | | $ | — | | | $ | — | | | $ | — | |
| | | | | | | | | | | | | | | | | | | | |
54
Except as otherwise indicated, the table above excludes:
| | • | | 897,669 ordinary shares issuable upon the exercise of share options outstanding as of December 31, 2014 with a per ordinary share weighted-average exercise price of €0.01, issued under our 2010 Employee Share Option Plan; and |
| | • | | an additional ordinary shares reserved for future issuance under our equity incentive plans. |
| (1) | Includes the Credit Facility. In April 2015, we drew down an additional $10.0 million under the Credit Facility. At the same time, we issued 592,042 ordinary shares to OrbiMed for an aggregate subscription price of €5,920.42. |
| (2) | Each $1.00 increase (decrease) in the assumed initial public offering price of $ per ordinary share, the midpoint of the price range set forth on the cover page of this prospectus, would increase (decrease) each of additional paid-in capital, total shareholders’ equity, total capitalization and cash by approximately $ , assuming that the number of ordinary shares offered by us, as set forth on the cover page of this prospectus, remains the same, and after deducting underwriting discounts and commissions and estimated offering expenses payable by us. We may also increase or decrease the number of ordinary shares we are offering. Each increase (decrease) of 1,000,000 shares in the number of shares offered by us would increase (decrease) each of additional paid-in capital, total shareholders’ equity, total capitalization and cash by approximately $ , assuming that the assumed initial price to the public remains the same, and after deducting the underwriting discounts and commissions and estimated offering expenses payable by us. The pro forma as adjusted information discussed above is illustrative only and will adjust based on the actual initial public offering price and other terms of this offering determined at pricing. |
55
DILUTION
If you invest in our ordinary shares in this offering, your interest will be diluted to the extent of the difference between the initial public offering price per ordinary share of our ordinary shares in this offering and the pro forma as adjusted net tangible book value per share of our ordinary shares after this offering.
As of , 2015, on a pro forma basis giving effect to the Share-for-Share Exchange, we would have had a net tangible book value of $ million, or $ per ordinary share. We calculate net tangible book value per ordinary share by dividing the net tangible book value by the number of outstanding our ordinary shares.
After giving effect to the receipt of the estimated net proceeds from our sale of ordinary shares in this offering, assuming an initial public offering price of $ per ordinary share (the midpoint of the offering range shown on the cover of this prospectus), and the application of the estimated net proceeds therefrom as described under “Use of Proceeds,” our pro forma as adjusted net tangible book value at , 2015 would have been approximately $ , or $ per ordinary share. This represents an immediate increase in net tangible book value per ordinary share of $ to existing shareholders and an immediate dilution in net tangible book value per ordinary share of $ to you, or %. The following table illustrates this dilution per ordinary share.
| | | | | | |
Assumed initial public offering price per ordinary share | | | | $ | | |
Pro forma net tangible book value per ordinary share as of , 2015 | | | | | | |
Increase in net tangible book value per ordinary share attributable to new investors | | | | | | |
Pro forma as adjusted net tangible book value per ordinary share after this offering | | | | | | |
Dilution per ordinary share to new investors | | | | $ | | |
If the underwriters exercise their option to purchase additional ordinary shares in full, the pro forma as adjusted net tangible book value per ordinary share after giving effect to this offering would be $ per ordinary share. This represents an increase in pro forma as adjusted net tangible book value of $ per ordinary share to existing shareholders and dilution in pro forma as adjusted net tangible book value of $ per ordinary share to you, or %.
A $1.00 increase (decrease) in the assumed initial public offering price of $ per ordinary share would decrease (increase) our pro forma net tangible book value, after giving effect to the offering, by $ , assuming no change to the number of our ordinary shares offered by us as set forth on the cover page of this prospectus, and after deducting estimated underwriting discounts and commissions and estimated expenses payable by us.
The following table sets forth, as of , 2015, on the pro forma as adjusted basis described above, the number of ordinary shares purchased from us, the total consideration paid to us and the average price per share (i) paid by existing shareholders and (ii) to be paid by new investors purchasing ordinary shares in this offering, before deducting underwriting discounts and commissions and estimated offering expenses payable by us.
| | | | | | | | | | | | | | | | | | | | |
| | | Shares purchased | | | Total consideration | | | Average price
per share | |
| | | Number | | | Percent | | | Amount | | | Percent | | |
Existing shareholders | | $ | | | | | | % | | $ | | | | | | % | | $ | | |
New investors | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
Total | | $ | | | | | 100 | % | | $ | | | | | 100 | % | | $ | | |
| | | | | | | | | | | | | | | | | | | | |
If the underwriters were to fully exercise their option to purchase additional ordinary shares from us, the percentage of our ordinary shares held by existing shareholders would be %, and the percentage of our ordinary shares held by new investors would be %.
56
Each $1.00 increase (decrease) in the assumed initial public offering price of $ per ordinary share would increase (decrease) total consideration paid by new investors by $ million, assuming the number of ordinary shares we are offering, as set forth on the cover page of this prospectus, remains the same, after deducting underwriting discounts and commissions and estimated offering expenses payable by us. We may also increase or decrease the number of shares we are offering. An increase (decrease) of 1,000,000 in the number of shares offered by us would increase (decrease) total consideration paid by new investors by $ million, assuming that the assumed initial price to the public remains the same, and after deducting the underwriting discounts and commissions and estimated offering expenses payable by us.
The number of ordinary shares reflected in the discussion and tables above is based on ordinary shares outstanding as of , 2015, and excludes ordinary shares issuable upon the exercise of outstanding options as of , 2015, having a weighted average exercise price of $ per ordinary share, issued under our 2010 Employee Share Option Plan.
Effective as of the date of the effectiveness of the registration statement of which this prospectus forms a part, an aggregate of ordinary shares will be reserved for issuance under the GC Aesthetics Equity Incentive Plan 2015. Furthermore, we may choose to raise additional capital through the sale of equity or convertible debt securities due to market conditions or strategic considerations even if we believe we have sufficient funds for our current or future operating plans. To the extent that any of our options are exercised, new options are issued under our equity incentive plans or we issue additional ordinary shares or other equity or convertible debt securities in the future, there will be further dilution to investors participating in this offering.
57
CORPORATE STRUCTURE
Global Consolidated Aesthetics Limited was incorporated in December 2007 under the laws of Ireland and is the holding company of Eurosilicone SAS and Nagôr Limited, the main operating entities within our group, which it acquired from Medicor Ltd. (which was in Chapter 11 bankruptcy proceedings) in May 2008. Eurosilicone SAS was incorporated in 1988 under the laws of France. Nagôr Limited was incorporated in 1979 under the laws of the Isle of Man, and operates as the registered branch of an overseas company in the United Kingdom. Global Consolidated Aesthetics Limited’s acquisition of Nagôr Limited and Eurosilicone SAS in May 2008 was funded in part by an equity investment in our ordinary shares led by Oyster. Montreux first became a shareholder in Global Consolidated Aesthetics Limited pursuant to an investment in October 2010. Both Montreux and Oyster have made significant further equity investments in Global Consolidated Aesthetics Limited since their initial investments. See “Description of Share Capital—History of Security Issuances.”
GC Aesthetics plc was incorporated in April 2015 under the laws of Ireland. Upon the Share-for-Share Exchange, GC Aesthetics plc will become the holding company of Global Consolidated Aesthetics Limited and its subsidiaries. Until immediately prior to the completion of the initial public offering described in this prospectus, GC Aesthetics plc will be a shell company. GC Aesthetics plc will not have conducted any operations (other than activities incidental to its formation, the Share-for-Share Exchange described below and the initial public offering described herein). Global Consolidated Aesthetics Limited is the entity through which the Company currently operates its business. Upon the Share-for-Share Exchange, the historical consolidated financial statements of Global Consolidated Aesthetics Limited included in this prospectus will become the historical consolidated financial statements of GC Aesthetics plc.
All of our operations are conducted through Eurosilicone SAS, Nagôr Limited and various other subsidiaries, which are organized and operated according to the laws of their countries of organization.
The following chart shows our corporate structure after giving effect to the Share-for-Share Exchange described above:

58
SELECTED CONSOLIDATED FINANCIAL DATA
The following table sets forth our selected historical consolidated financial data as of the dates and for the periods indicated. The selected historical financial data as of December 31, 2014, December 31, 2013 and for the two years in the period ended December 31, 2014 presented in this table have been derived from the audited consolidated financial statements of Global Consolidated Aesthetics Limited included elsewhere in this prospectus. Historical results are not necessarily indicative of the results to be expected for future periods. Our financial statements have been prepared in accordance with IFRS, as issued by the IASB.
This selected historical consolidated financial data should be read in conjunction with the disclosures set forth under “Capitalization” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and the consolidated financial statements and the related notes thereto appearing elsewhere in this prospectus.
Consolidated Statements of Operations—twelve months ended December 31, 2014 and 2013
| | | | | | | | |
| | | Year ended December 31, | |
| | | 2014 | | | 2013 | |
Revenue | | $ | 52,804 | | | $ | 44,630 | |
Cost of sales | | | (26,038 | ) | | | (22,548 | ) |
| | | | | | | | |
Gross profit | | | 26,766 | | | | 22,082 | |
Operating expenses: | | | | | | | | |
Distribution and selling expenses | | | (18,187 | ) | | | (10,113 | ) |
Administrative expenses | | | (18,151 | ) | | | (15,042 | ) |
Research and development expenses | | | (1,507 | ) | | | (1,487 | ) |
Amortization of intangible assets | | | (3,990 | ) | | | (4,033 | ) |
Other expenses | | | — | | | | (183 | ) |
| | | | | | | | |
Operating expenses | | | (41,835 | ) | | | (30,858 | ) |
| | | | | | | | |
Operating loss | | | (15,069 | ) | | | (8,776 | ) |
| | |
Other income (expense): | | | | | | | | |
Net interest (expense) income | | | (33,746 | ) | | | (6,877 | ) |
Loss on conversion of shareholder loans | | | (27,737 | ) | | | — | |
Gain (Loss) on equity derivatives | | | 5,182 | | | | (4,828 | ) |
(Loss) Gain on foreign exchange | | | (18,657 | ) | | | 1,994 | |
| | | | | | | | |
Other income (expense) | | | (74,958 | ) | | | (9,711 | ) |
| | | | | | | | |
Loss before income tax credit | | | (90,027 | ) | | | (18,487 | ) |
Income tax credit | | | 600 | | | | 1,195 | |
| | | | | | | | |
Net loss | | $ | (89,427 | ) | | $ | (17,292 | ) |
| | | | | | | | |
Basic and diluted loss per ordinary share | | $ | (18.74 | ) | | $ | (3.91 | ) |
| | | | | | | | |
Weighted average number of ordinary shares outstanding: | | | | | | | | |
Basic and diluted | | | 4,772,101 | | | | 4,425,800 | |
| | | | | | | | |
Net loss | | $ | (89,427 | ) | | $ | (17,292 | ) |
Other comprehensive income (loss): | | | | | | | | |
Items that may be reclassified to profit or loss in subsequent periods: | | | | | | | | |
Foreign currency translation differences – foreign operations | | | 15,004 | | | | (2,192 | ) |
Items that will not be reclassified to profit or loss in subsequent years: | | | | | | | | |
Net actuarial (loss) gain on retirement obligation, net of tax | | | (36 | ) | | | 30 | |
| | | | | | | | |
Total comprehensive loss | | $ | (74,459 | ) | | $ | (19,454 | ) |
| | | | | | | | |
59
Other Financial Data—twelve months ended December 31, 2014 and 2013
| | | | | | | | |
| | | December 31, | |
| | | 2014 | | | 2013 | |
| | | (in thousands) | |
Other financial data (unaudited): | | | | | | | | |
Constant Currency Revenue (1) | | $ | 52,804 | | | $ | 44,607 | |
Adjusted Gross Profit (2) | | | 27,788 | | | | 23,134 | |
Adjusted EBITDA(3) | | | (8,112 | ) | | | (3,385 | ) |
Adjusted Interest Expense(4) | | | (6,202 | ) | | | (3,564 | ) |
| (1) | Constant Currency Revenue is defined as revenue calculated by translating revenues for both 2014 and 2013 using the average exchange rates for 2014 of the Euro, Sterling and the Brazilian Real, respectively, to one US dollar, our financial reporting currency. We believe that Constant Currency Revenue is a useful measure, indicating the actual growth of our operations, excluding the impact of foreign currency exchange rates changes. Constant Currency Revenue is not calculated in accordance with IFRS. This calculation may differ from similarly titled measures used by others and, accordingly, Constant Currency Revenue is not meant to be a substitution for revenues presented in conformity with IFRS nor should such non-IFRS financial measure be considered in isolation. Moreover, the presentation of Constant Currency Revenue is not necessarily indicative of historical or future results of operations. Currency fluctuations affect general economic and business conditions, including, for example, a country’s inflation and international trade competitiveness and, as a result, a company’s performance cannot be evaluated solely on the basis of a constant currency presentation. |
The following table reconciles Constant Currency Revenues to revenues, the most comparable IFRS measure, for each of the periods indicated:
| | | | | | | | |
| | | Year Ended December 31, | |
| | | 2014 | | | 2013 | |
| | | (in thousands) | |
Reconciliation of Constant Currency Revenue to Revenue: | | | | | | | | |
Revenue | | $ | 52,804 | | | $ | 44,630 | |
Effect of movement in exchange rates | | | — | | | | (23 | ) |
| | | | | | | | |
Constant Currency Revenue | | $ | 52,804 | | | $ | 44,607 | |
| | | | | | | | |
| (2) | Adjusted Gross Profit is defined as gross profit excluding depreciation that is included in the cost of sales. We believe that Adjusted Gross Profit is a useful measure, indicating the actual profitability of our production excluding these non-cash charges. Adjusted Gross Profit is not calculated in accordance with IFRS. |
The following table reflects the reconciliation of Adjusted Gross Profit to gross profit, the most comparable IFRS measure, for each of the periods indicated:
| | | | | | | | |
| | | Year Ended December 31, | |
| | | 2014 | | | 2013 | |
| | | (in thousands) | |
Reconciliation of Adjusted Gross Profit to Gross Profit: | | | | | | | | |
Gross profit | | $ | 26,766 | | | $ | 22,082 | |
Depreciation included in cost of sales | | | 1,022 | | | | 1,052 | |
| | | | | | | | |
Adjusted Gross Profit | | $ | 27,788 | | | $ | 23,134 | |
| | | | | | | | |
| (3) | Adjusted EBITDA is defined as operating loss, adjusted for other expenses, depreciation, amortization and share-based and other equity-related compensation. We believe that Adjusted EBITDA is a useful metric for investors to understand and evaluate our operating results and ongoing profitability because it permits investors to evaluate our recurring profitability from our ongoing operating activities. Adjusted EBITDA is not calculated in accordance with IFRS. In addition, our calculation of Adjusted EBITDA may not be comparable to similar measures that other companies report because other companies may not calculate Adjusted EBITDA in the same manner as we do. |
60
By providing this non-IFRS financial measure, together with a reconciliation to IFRS results, we believe we are enhancing investors’ understanding of our business and our results of operations, as well as assisting investors in evaluating how we are executing strategic initiatives. We believe Adjusted EBITDA is widely used by investors and securities analysts as a supplemental measure to evaluate the overall operating performance of companies in our industry without regard to items, such as interest expense, provision for income taxes and depreciation and amortization expense, that can vary substantially from company to company depending upon their financing and accounting methods, the book value of their assets, their capital structures and the method by which their assets were acquired.
Management uses Adjusted EBITDA:
| | • | | as a measurement used in comparing our operating performance on a consistent basis; |
| | • | | for planning purposes, including the preparation of our internal annual operating budget and financial projections; and |
| | • | | to evaluate the performance and effectiveness of our operational strategies. |
Although we use Adjusted EBITDA as a measure to assess the operating performance of our business, Adjusted EBITDA has limitations as an analytical tool, and you should not consider it in isolation, or as a substitute for analysis of our results as reported under IFRS. Because of these limitations, management does not view Adjusted EBITDA in isolation or as a primary performance measure.
The following table reconciles Adjusted EBITDA to operating loss, the most comparable IFRS measure, for each of the periods indicated:
| | | | | | | | |
| | | Year Ended December 31, | |
| | | 2014 | | | 2013 | |
| | | (in thousands) | |
Reconciliation of Adjusted EBITDA to Operating Loss: | | | | | | | | |
Operating loss | | $ | (15,069 | ) | | $ | (8,776 | ) |
Add: | | | | | | | | |
Other expenses | | | — | | | | 183 | |
Depreciation | | | 1,123 | | | | 1,175 | |
Amortization | | | 3,990 | | | | 4,033 | |
Share-based and other equity-related compensation | | | 1,844 | | | | — | |
| | | | | | | | |
Adjusted EBITDA | | $ | (8,112 | ) | | $ | (3,385 | ) |
| | | | | | | | |
| (4) | Adjusted Interest Expense is defined as interest expense adjusted for non-cash charges related to effective interest on our B Preference Shares and C Preference Shares. The B Preference Shares and C Preference Shares will convert into ordinary shares in connection with this offering; as a result, no further charges in respect thereof will be incurred in future periods. However, we believe Adjusted Interest Expense is a useful metric for investors to understand and evaluate our results of operations as it permits investors to evaluate the cash costs of servicing our debt in prior periods. Adjusted Interest Expense is not calculated in accordance with IFRS. |
The following table reconciles Adjusted Interest Expense to interest expense, the most comparable IFRS measure, for each of the periods indicated:
| | | | | | | | |
| | | Year Ended December 31, | |
| | | 2014 | | | 2013 | |
| | | (in thousands) | |
Reconciliation of Adjusted Interest Expense to Interest Expense: | | | | | | | | |
Interest expense | | $ | (33,746 | ) | | $ | (6,877 | ) |
Adjusted for non-cash charges related to effective interest on B Preference Shares and C Preference Shares | | | 27,544 | | | | 3,313 | |
| | | | | | | | |
Adjusted Interest Expense | | $ | (6,202 | ) | | $ | (3,564 | ) |
| | | | | | | | |
61
Consolidated Statements of Financial Position Data – as at December 31, 2014 and 2013
| | | | | | | | |
| | | December 31, | |
| | | 2014 | | | 2013 | |
| | | (in thousands) | |
Assets | | | | |
| |
Current | | | | |
Cash and cash equivalents | | $ | 10,616 | | | $ | 4,519 | |
Restricted cash | | | 3,642 | | | | — | |
Trade receivables, net | | | 12,840 | | | | 10,006 | |
Inventories, net | | | 14,929 | | | | 11,477 | |
Prepayments and other receivables | | | 4,539 | | | | 4,574 | |
Tax receivables | | | 422 | | | | — | |
| | | | | | | | |
Total current assets | | | 46,988 | | | | 30,576 | |
| | | | | | | | |
| |
Non-current | | | | |
Goodwill and other intangible assets, net of amortization | | | 25,941 | | | | 32,222 | |
Property, plant and equipment, net | | | 4,674 | | | | 4,976 | |
Deferred tax assets | | | 1,113 | | | | 1,334 | |
Other non-current assets | | | 434 | | | | 618 | |
| | | | | | | | |
Total non-current assets | | | 32,162 | | | | 39,150 | |
| | | | | | | | |
Total assets | | $ | 79,150 | | | $ | 69,726 | |
| | | | | | | | |
| |
Liabilities | | | | |
Current | | | | |
Trade payables | | $ | 7,206 | | | $ | 6,206 | |
Accruals and other creditors | | | 9,027 | | | | 6,887 | |
Other liabilities | | | 996 | | | | 1,395 | |
Tax liabilities | | | 1,569 | | | | 1,325 | |
Loans and borrowings | | | 794 | | | | 27,072 | |
| | | | | | | | |
Total current liabilities | | | 19,592 | | | | 42,885 | |
| | | | | | | | |
| |
Non-current | | | | |
B Preference Shares and C Preference Shares | | | 116,210 | | | | 46,518 | |
Equity derivative | | | 22,284 | | | | 16,741 | |
Deferred tax liabilities | | | 4,926 | | | | 6,485 | |
Provisions | | | 1,661 | | | | 1,951 | |
Loans and borrowings | | | 51,457 | | | | 19,759 | |
Other liabilities | | | — | | | | 789 | |
| | | | | | | | |
Total non-current liabilities | | | 196,538 | | | | 92,243 | |
| | | | | | | | |
Total liabilities | | | 216,130 | | | | 135,128 | |
| | | | | | | | |
Equity / (deficiency) | | | | |
Share capital | | | 70 | | | | 65 | |
Additional paid-in capital | | | 34,287 | | | | 31,411 | |
Accumulated deficit | | | (183,489 | ) | | | (94,062 | ) |
Accumulated other comprehensive income (loss) | | | 12,152 | | | | (2,816 | ) |
| | | | | | | | |
Total equity / (deficiency) | | | (136,980 | ) | | | (65,402 | ) |
| | | | | | | | |
Total equity and liabilities | | $ | 79,150 | �� | | $ | 69,726 | |
| | | | | | | | |
62
MANAGEMENT’S DISCUSSION AND ANALYSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The consolidated financial statements included elsewhere in this prospectus, which are the subject of the following discussion and analysis, are those of Global Consolidated Aesthetics Limited and its consolidated subsidiaries. We have historically conducted our business through Global Consolidated Aesthetics Limited and its subsidiaries, and upon completion of the Share-for-Share Exchange, Global Consolidated Aesthetics Limited will become our wholly-owned subsidiary, whereby the historical consolidated financial statements of Global Consolidated Aesthetics Limited will become the historical consolidated financial statements of GC Aesthetics plc. See “Corporate Structure.”
You should read the following discussion and analysis of our financial condition and results of our operations in conjunction with the consolidated financial statements and related notes included elsewhere in this prospectus for each of the years ended December 31, 2014 and 2013. Our financial statements are prepared in accordance with IFRS as issued by the IASB. Our historical results are not necessarily indicative of the results that should be expected in the future. Some of the information contained in this discussion and analysis or set forth elsewhere in this prospectus, including information with respect to our plans and strategy for our business, includes forward-looking statements that involve risks and uncertainties. You should read the “Risk Factors” and “Cautionary Note Regarding Forward-Looking Statements” sections of this prospectus for a discussion of important factors that could cause actual results to differ materially from the results described in or implied by the forward-looking statements contained in the following discussion and analysis. The terms “Company,” “GC Aesthetics,” “we,” “our” or “us” as used herein refer to GC Aesthetics plc and its consolidated subsidiaries unless otherwise stated or indicated by context.
Overview
We are a leading pure-play female aesthetics company committed to becoming the trusted brand and partner for women seeking to look healthy, youthful, vibrant and beautiful, and to feel confident about themselves throughout their lifetime. To date, we have generated significant growth by focusing on the development, manufacturing and commercialization of one of the broadest ranges of implant products, principally breast implants. We believe that through our two brands, Nagôr and Eurosilicone, which have a combined 60 years of market presence, we have (i) a strong reputation for safety, quality and reliability, as evidenced by the five-year data from our ongoing Eurosilicone safety study, (ii) strong competitive advantages based on our innovative product design features—the result of our focused approach to research and development, which integrates feedback from women and surgeons, and (iii) a differentiated marketing and customer service approach, focusing on the needs of women.
We have developed and marketed our comprehensive range of products, comprising over 1,100 SKUs, to address the different needs and preferences of women and surgeons. All of our implants are manufactured with medical grade silicone supplied from either NuSil or ASC, both of which are U.S.-based, ISO 9001-certified sources. We have manufacturing plants in the United Kingdom and France and sell our products in 75 countries, with direct sales activities in six countries, including Brazil, the world’s largest medical aesthetics market by total number of plastic surgery procedures.
We intend to expand our business by gaining market share in the countries in which we sell our products, entering new countries and expanding our product portfolio through product development, in-licensing and selective acquisitions. We have incurred significant net operating losses since inception. We anticipate that our operating losses will continue in the immediate- to near-term, as we continue to invest in our sales and marketing efforts, as well as product development, to expand our customer base. Our revenue for the year ended December 31, 2014 was $52.8 million, as compared to $44.6 million for the year ended December 31, 2013.
63
Key Financial Metrics
We use the following key financial metrics to evaluate and manage our business on an ongoing basis, which we believe are useful for investors to compare key financial data both within and across reporting periods:
| | • | | Constant Currency Revenue; |
| | • | | Adjusted Interest Expense. |
Constant Currency Revenue. We define Constant Currency Revenues as revenues calculated by translating revenues into US dollars, our financial reporting currency, using the same foreign currency exchange rate for the previous period as we used to translate revenues for the current period. We believe that Constant Currency Revenue is a useful measure, indicating the actual growth of our operations, excluding the impact of foreign currency exchange rates changes. Constant Currency Revenue is not calculated in accordance with IFRS.
The following table reflects the reconciliation of Constant Currency Revenue to revenue, the most comparable IFRS measure, for each of the periods indicated:
| | | | | | | | |
| | | Year Ended
December 31, | |
| | | 2014 | | | 2013 | |
| | | (in thousands) | |
Reconciliation of Constant Currency Revenue to Revenue: | | | | | | | | |
Revenue | | $ | 52,804 | | | $ | 44,630 | |
Effect of movement in exchange rates(1) | | | — | | | | (23 | ) |
| | | | | | | | |
Constant Currency Revenue | | $ | 52,804 | | | $ | 44,607 | |
| | | | | | | | |
| (1) | Constant Currency Revenue is calculated by converting revenues for both 2014 and 2013 using the average exchange rates for 2014 of the Euro, Sterling and the Brazilian Real, respectively, to one US dollar. |
Adjusted Gross Profit. We define Adjusted Gross Profit as gross profit excluding depreciation that is included in the cost of sales. We believe that Adjusted Gross Profit is a useful measure, indicating the actual profitability of our production excluding this non-cash charge. Adjusted Gross Profit is not calculated in accordance with IFRS.
The following table reflects the reconciliation of Adjusted Gross Profit to gross profit, the most comparable IFRS measure, for each of the periods indicated:
| | | | | | | | |
| | | Year Ended
December 31, | |
| | | 2014 | | | 2013 | |
| | | (in thousands) | |
Reconciliation of Adjusted Gross Profit to Gross Profit: | | | | | | | | |
Gross profit | | $ | 26,766 | | | $ | 22,082 | |
Depreciation included in cost of sales | | | 1,022 | | | | 1,052 | |
| | | | | | | | |
Adjusted Gross Profit | | $ | 27,788 | | | $ | 23,134 | |
| | | | | | | | |
Adjusted EBITDA. We define Adjusted EBITDA as operating loss, adjusted for other expenses, depreciation, amortization and share-based and other equity-related compensation. We believe that Adjusted EBITDA is a useful metric for investors to understand and evaluate our results of operations and ongoing profitability because it permits investors to evaluate our recurring profitability from our ongoing operating activities. Adjusted EBITDA is not calculated in accordance with IFRS. See “Summary—Summary Consolidated Financial Data.”
64
The following table reflects the reconciliation of Adjusted EBITDA to operating loss, the most comparable IFRS measure, for each of the periods indicated:
| | | | | | | | |
| | | Year Ended
December 31, | |
| | | 2014 | | | 2013 | |
| | | (in thousands) | |
Reconciliation of Adjusted EBITDA to operating loss: | | | | | | | | |
Operating loss | | $ | (15,069 | ) | | $ | (8,776 | ) |
Add: | | | | | | | | |
Other expenses | | | — | | | | 183 | |
Depreciation | | | 1,123 | | | | 1,175 | |
Amortization | | | 3,990 | | | | 4,033 | |
Share-based and other equity-related compensation | | | 1,844 | | | | — | |
| | | | | | | | |
Adjusted EBITDA | | $ | (8,112 | ) | | $ | (3,385 | ) |
| | | | | | | | |
Adjusted Interest Expense. We define Adjusted Interest Expense as interest expense adjusted for non-cash charges related to effective interest on our B Preference Shares and C Preference Shares. The B Preference Shares and C Preference Shares will convert into ordinary shares in connection with this offering; as a result, no further charges in respect thereof will be incurred in future periods. However, we believe that Adjusted Interest Expense is a useful metric for investors to understand and evaluate our results of operations, because it permits investors to evaluate the cash costs of servicing our debt. Adjusted Interest Expense is not calculated in accordance with IFRS. See Note 7 to our consolidated financial statements included elsewhere in this prospectus for more information on our B Preference Shares and C Preference Shares and the effective interest with respect to such shares.
The following table reflects the reconciliation of Adjusted Interest Expense to interest expense, the most comparable IFRS measure, for each of the periods indicated:
| | | | | | | | |
| | | Year Ended
December 31, | |
| | | 2014 | | | 2013 | |
| | | (in thousands) | |
Reconciliation of Adjusted Interest Expense to Interest Expense: | | | | | | | | |
Interest expense | | $ | (33,746 | ) | | $ | (6,877 | ) |
Non-cash charges related to effective interest on B Preference Shares and C Preference Shares | | | 27,544 | | | | 3,313 | |
| | | | | | | | |
Adjusted Interest Expense | | $ | (6,202 | ) | | $ | (3,564 | ) |
| | | | | | | | |
Certain Factors Affecting Our Performance and Financial Condition
We conduct our business in 75 countries, a large majority of which are emerging markets. As a result, our performance is affected by a number of factors and risks relating to economic, social and political conditions, laws, practices and local customs in the countries in which we sell our products. For example, we may fail to collect outstanding receivables if customers default on payments owed to us or cannot pay us on account of foreign currency controls. In addition, inflation and foreign exchange rate movements may make our products more expensive in the countries in which our products are sold and increase the credit risks to which we are exposed. These risks are more prevalent in the emerging markets where we sell our products.
We currently have direct sales operations in six countries and sell through distributors in an additional 69 countries. We also use agents and sub-distributors to supplement our direct sales forces in most of the countries where we sell directly. In the future we intend to increase the number of countries in which we have our own direct sales operations. In the countries where we currently have direct sales operations, we have higher
65
average selling prices and gross margins as compared to those in which we sell our products through distributors. We also have higher distribution, selling and administrative expenses as a result of our own sales operations in those countries. If our distributors do not sell our products effectively or if one or more of our distributors becomes insolvent or fails to comply with local or other laws, our results of operations will be negatively affected. In addition, changes in product mix sold and foreign currency exchange rates also impact our gross margin.
Our future profitability depends on several factors, including our ability to increase revenues through sales and marketing activities, the continued market and regulatory acceptance of our products and our ability to continue to enhance our existing products and develop or acquire new products that meet our customers’ needs and preferences.
Product development and improvements require investments of significant financial, technological and other resources in existing and new products. In addition, they require significant planning, design, development and testing at the technological, product and manufacturing process levels, and we may not be able to timely or cost-effectively develop new products or improve existing products. Likewise, we may not be able to acquire new products on terms that are acceptable to us, or at all.
Our success also depends on our ability to manage manufacturing costs, including raw materials costs, and manufacture products that meet applicable regulatory and commercial requirements and, where applicable, maintain regulatory approvals for our existing products and secure regulatory approvals for new products. The need to obtain and maintain regulatory approvals in order to market and sell our products may limit our ability to act quickly in scaling commercialization in those countries.
We also have and will continue to incur share compensation expenses. Prior to this offering, we have granted employee compensation in the form of equity awards to certain of our employees, pursuant to the terms of their respective employment arrangements. In addition, in connection with this offering, we expect to implement an equity compensation incentive plan that provides for future grants of equity and other incentive compensation awards to certain of our employees. The implementation of this plan will result in increased compensation expense in future periods. We will record the associated share compensation expense in the period in which we grant such awards.
Further, the activities associated with this initial public offering process, as well as any future public offerings, may have a significant impact on our results of operations and cash flows. We expect to incur substantial costs in connection with becoming a publicly traded company, such as a material increase in incremental administrative expenses and other related costs. These costs include expenses associated with our financial and operational reporting, investor relations, stock exchange fees, registrar and transfer agent fees, incremental insurance costs and accounting and legal services. See “Risk Factors—Risks Related to this Offering and Ownership of Our Ordinary Shares.”
Components of Our Results of Operations
Revenue
We generate our revenues through the sales of our breast implants and other products and freight services. We have direct operations in six countries and sales in 69 additional countries through distributors. The countries in which we currently sell our products directly are Brazil, the United Kingdom, France, Spain, Italy and Germany; we also use agents and sub-distributors in these countries. When we refer to sales in direct countries, we refer to sales by our own sales force and sales by our agents and sub-distributors in these countries (“direct countries”). Sales in direct countries contributed $20.6 million, or 39% of revenues, in 2014, with the remainder from countries in which we sell our products solely through distributors (“distributor countries”).
Our revenue can be significantly impacted by fluctuations in foreign currency exchange rates, as the majority of our sales are denominated in currencies other than the US dollar.
66
Cost of Sales
We manufacture our implant products at our manufacturing facilities in the United Kingdom and France. Third parties manufacture our Enhance and Silgel products. Cost of sales primarily includes costs for raw materials and components, labor, sterilization, packaging, freight, manufacturing overhead and depreciation charges. Manufacturing overhead includes expenses for regulatory affairs, quality assurance, manufacturing engineering, material procurement, inventory control, facilities, equipment, operations supervision and management. Cost of sales also includes the cost of replacement breast implants under our GCA Comfort Guarantee in the event of rupture or severe capsular contracture.
Gross Profit
We expect our gross profit, which is calculated as revenues less cost of sales for a given period, to fluctuate in future periods as a result of several factors, including the changing mix of products sold with different gross margins, changing percentage of products sold to distributors versus directly to individual customers, changes in foreign exchange rates, changes in our manufacturing processes or costs and increased manufacturing output. Any new products that we sell in the future may change our gross profit. The gross profit margin of our direct operations is higher than that of our distributor operations due to the higher prices per implant and other products that we have been able to achieve when we sell direct.
Distribution and Selling Expenses
Our distribution and selling expenses primarily consist of compensation for personnel, including base salaries, share-based compensation and incentive compensation associated with sales results, benefits and travel for our sales, marketing and customer support personnel. Other significant expenses include the cost of agents, sales and marketing support for our distributors, if applicable, as well as expenses for trade shows, product demonstration samples, our surgeon-centric support programs and women-centric initiatives, including our Experience Centres. We expect our distribution and selling expenses to increase as our business expands.
Administrative Expenses
Our administrative expenses primarily consist of compensation for personnel, including base salaries, bonuses, benefits and all share-based compensation for our employees (except those in Distribution and Selling Expenses) and directors. Other administrative expenses include outside legal counsel, independent auditors and other outside consultants, insurance, facilities and information technology expenses. We expect our administrative expenses to increase in connection with becoming a public company, which may increase further when we no longer qualify for the “emerging growth company” exemptions we are afforded under the JOBS Act or if we lose our “foreign private issuer” status.
Research and Development Expenses
Our research and development (“R&D”) expenses primarily consist of regulatory expenses, clinical expenses, product development, consulting services, quality control and other costs associated with the development of our products. R&D expenses also include related personnel and consultant compensation costs. We either expense R&D costs as they are incurred or capitalize them. R&D costs are capitalized only if such costs can be measured reliably, the product or process is technically and commercially feasible, future economic benefits are probable and we have sufficient resources to complete development and use or sell the asset. If capitalized, the expenditure capitalized includes the cost of materials, direct labor, overhead costs directly attributable to preparing the asset for its intended use and capitalized borrowing costs. We expect our R&D expenses to increase as we continue to expand our product development and regulatory development programs.
67
Amortization of Intangible Assets
Charges for amortization are related to trademarks, brands, our customer base, technology, registrations and R&D. Intangible assets, other than goodwill, are recognized where they are separable or arising from contractual or other legal rights and can be reliably measured. They are stated at cost (cost being the fair value at the date of acquisition where they relate to a business combination) less accumulated amortization and impairment losses. Amortization is charged to profit and loss account in the statement of comprehensive income on a straight-line basis.
Other Expenses
Other expenses consisted of exceptional costs in 2013, which were non-recurring in 2014.
Interest Expense
Interest expense includes interest expense net of interest income, arrangement and other fees incurred to secure the Credit Facility and non-cash charges with respect to our B Preference Shares and C Preference Shares relating to effective interest thereon. The B Preference Shares and C Preference Shares will convert to ordinary shares in connection with this offering; as a result, no further charges in respect thereof will be incurred in future periods. See Note 14 to our consolidated financial statements included elsewhere in this prospectus.
Gain (loss) on Equity Derivatives
Gain (loss) on equity derivatives relates to non-cash charges in respect of the equity derivatives associated with our B Preference Shares and C Preference Shares. The B Preference Shares and C Preference Shares will convert to ordinary shares in connection with this offering; as a result, no further charges in respect thereof will be incurred in future periods. See Note 14 to our consolidated financial statements included elsewhere in this prospectus.
Loss (gain) on Foreign Exchange
Loss (gain) on foreign exchange relates to non-cash charges in respect of foreign currency exchange rate changes for our foreign currency denominated loans and our B Preference and C Preference Shares. The B Preference Shares and C Preference Shares will convert to ordinary shares in connection with this offering; as a result, no further charges in respect thereof will be incurred in future periods. See Note 14 to our consolidated financial statements included elsewhere in this prospectus.
Income Tax Credit
Income tax expense on the profit or loss for the year comprises current and deferred tax. Taxation is recognized in profit or loss in the statement of operations, except to the extent that it relates to items recognized in other comprehensive income or directly in equity, in which case the related tax is recognized in other comprehensive income or directly in equity, respectively. Current tax is the expected tax payable on the taxable income for the relevant period, using tax rates and laws that have been enacted at the reporting period end date, and any adjustment to tax payable in respect of previous periods.
Deferred tax is provided using the balance sheet liability method, providing for temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for taxation purposes. If the deferred tax arises from initial recognition of an asset or liability in a transaction other than a business combination that at the time of the transaction does not affect accounting or taxable profit or loss, it is not recognized. Deferred tax is provided on temporary differences arising on investments in subsidiaries and
68
joint ventures, except where the timing of the reversal of the temporary difference is controlled by the Company and it is probable that the temporary difference will not reverse in the foreseeable future. The amount of deferred tax provided is based on the expected manner of realization or settlement of the carrying amount of assets and liabilities, using tax rates enacted at the reporting year end date.
A deferred tax asset is recognized only to the extent that it is probable that future taxable profits will be available against which the asset can be utilized.
Results of Operations
The following table sets forth our results of operations for the fiscal years ended December 31, 2014 and 2013. This data should be read together with our financial statements and related notes included elsewhere in this prospectus, and is qualified in its entirety by reference to such financial statements and related notes.
| | | | | | | | | | | | | | | | |
| | | Year Ended December 31, | | | Change from Prior Period | |
| | | 2014 | | | 2013 | | | $ | | | % | |
| | | (in thousands, except percentages) | |
Revenue | | $ | 52,804 | | | $ | 44,630 | | | $ | 8,174 | | | | 18.3% | |
Cost of sales | | | (26,038 | ) | | | (22,548 | ) | | | (3,490 | ) | | | 15.5% | |
| | | | | | | | | | | | | | | | |
Gross profit | | | 26,766 | | | | 22,082 | | | | 4,684 | | | | 21.2% | |
Operating expenses: | | | | | | | | | | | | | | | | |
Distribution and selling expenses | | | (18,187 | ) | | | (10,113 | ) | | | (8,074 | ) | | | 79.8% | |
Administrative expenses | | | (18,151 | ) | | | (15,042 | ) | | | (3,109 | ) | | | 20.7% | |
Research and development expenses | | | (1,507 | ) | | | (1,487 | ) | | | (20 | ) | | | 1.3% | |
Amortization of intangible assets | | | (3,990 | ) | | | (4,033 | ) | | | 43 | | | | (1.1)% | |
Other expenses | | | — | | | | (183 | ) | | | 183 | | | | * | |
| | | | | | | | | | | | | | | | |
Operating expenses | | | (41,835 | ) | | | (30,858 | ) | | | (10,977 | ) | | | 35.6% | |
| | | | | | | | | | | | | | | | |
Operating loss | | | (15,069 | ) | | | (8,776 | ) | | | (6,293 | ) | | | 71.7% | |
Other income (expense): | | | | | | | | | | | | | | | | |
Net interest (expense) income | | | (33,746 | ) | | | (6,877 | ) | | | (26,869 | ) | | | 390.7% | |
Loss on conversion of shareholder loans | | | (27,737 | ) | | | — | | | | (27,737 | ) | | | * | |
Gain (Loss) on equity derivatives | | | 5,182 | | | | (4,828 | ) | | | 10,010 | | | | (207.3)% | |
(Loss) Gain on foreign exchange | | | (18,657 | ) | | | 1,994 | | | | (20,651 | ) | | | * | |
| | | | | | | | | | | | | | | | |
Other (expense) | | | (74,958 | ) | | | (9,711 | ) | | | (65,247 | ) | | | * | |
| | | | | | | | | | | | | | | | |
Loss before income tax credit | | | (90,027 | ) | | | (18,487 | ) | | | (71,540 | ) | | | 387.0% | |
Income tax credit | | | 600 | | | | 1,195 | | | | (595 | ) | | | (49.8)% | |
| | | | | | | | | | | | | | | | |
Net loss | | | (89,427 | ) | | | (17,292 | ) | | | (72,135 | ) | | | 417.2% | |
| | | | |
Other comprehensive income (loss): | | | | | | | | | | | | | | | | |
Items that may be reclassified to profit or loss in subsequent periods: | | | | | | | | | | | | | | | | |
Foreign currency translation differences—foreign operations | | | 15,004 | | | | (2,192 | ) | | | 17,196 | | | | * | |
Items that will not be reclassified to profit or loss in subsequent years: | | | | | | | | | | | | | | | | |
Net actuarial (loss) gain on retirement obligation, net of tax | | | (36 | ) | | | 30 | | | | (66 | ) | | | (220.0)% | |
| | | | | | | | | | | | | | | | |
Total comprehensive loss | | $ | (74,459 | ) | | $ | (19,454 | ) | | $ | (55,005 | ) | | | 282.7% | |
| | | | | | | | | | | | | | | | |
69
The following table sets forth our revenue by geographic region for the fiscal years ended December 31, 2014 and 2013.
| | | | | | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2014 | | | 2013 | |
| | | $ | | | % | | | $ | | | % | |
| | | (in thousands, except for percentages) | |
Revenue | | | | | | | | | | | | | | | | |
Europe, Middle East and Africa (“EMEA”) | | $ | 24,824 | | | | 47.0 | % | | $ | 24,182 | | | | 54.2 | % |
Latin America | | | 22,171 | | | | 42.0 | % | | | 13,880 | | | | 31.1 | % |
Asia-Pacific | | | 5,809 | | | | 11.0 | % | | | 6,568 | | | | 14.7 | % |
| | | | | | | | | | | | | | | | |
Total revenue | | $ | 52,804 | | | | 100.0 | % | | $ | 44,630 | | | | 100.0 | % |
| | | | | | | | | | | | | | | | |
Revenue
Revenues increased $8.2 million, or 18.3%, to $52.8 million for the year ended December 31, 2014, as compared to $44.6 million for the year ended December 31, 2013. This increase was primarily due to growth in sales volumes, driven by sales of our breast implant products in Latin America resulting from increased commercialization activities, including the expansion of our sales organization, increased marketing activities and greater familiarity with our products and customer service offerings by plastic surgeons. In particular, sales in Brazil increased by $3.3 million, or 48.9%, to $10.0 million, sales in Mexico increased as a result of the addition of a new distributor from $0 to $4.7 million and sales in France increased by $0.7 million, or 48.4%, to $2.0 million.
The number of implant products sold for the year ended December 31, 2014 increased by approximately 50,000 units (each unit representing one implant), or approximately 20%, to approximately 280,000 units, as compared to approximately 235,000 units for the year ended December 31, 2013. Revenues from sales of breast implants, and other implantable products and other revenue for the year ended December 31, 2014 were $48.2 million, $2.9 million and $1.7 million, respectively, as compared to $40.6 million, $2.7 million and $1.4 million, respectively, for the year ended December 31, 2013.
The number of implant products sold in our distributor countries for the year ended December 31, 2014 increased by approximately 25,000 units, or approximately 15%, to approximately 190,000 units, as compared to approximately 165,000 units sold for the year ended December 31, 2013. The number of implant products sold in our direct countries for the year ended December 31, 2014 increased by approximately 20,000 units, or approximately 29%, to approximately 90,000 units, as compared to approximately 70,000 units sold for the year ended December 31, 2013. Revenue from direct countries for the year ended December 31, 2014, increased 30%, or $4.7 million, to $20.3 million, as compared to the year ended December 31, 2013, while revenue from distributor countries for the year ended December 31, 2014 increased by $3.2 million, or 11.8%, to $30.8 million, as compared to the year ended December 31, 2013.
For the year ended December 31, 2014, the number of implant products sold in EMEA decreased by approximately 5,000 units, or approximately 4%, to approximately 105,000 units, as compared to approximately 110,000 units for the year ended December 31, 2013 and the number of implant products sold in Asia-Pacific decreased by approximately 10,000 units, or approximately 20%, to approximately 35,000 units, for the year ended December 31, 2014. During the same period, the number of implants sold in Latin America increased by approximately 60,000 units, or approximately 72%, to approximately 140,000 units as compared to approximately 80,000 units for the year ended December 31, 2013. Revenue from sales of implant products in EMEA, Latin America and Asia-Pacific for the year ended December 31, 2014 increased (decreased) by $0.2 million, $8.5 million and $(0.8) million, respectively, or 1.4%, 63% and (12)%, respectively, to $23.5 million, $22.0 million and $5.6 million, respectively, as compared to the year ended December 31, 2013. Implantable product revenue in EMEA increased from $23.3 million in 2013 to $23.5 million in 2014, despite the decrease in units sold for the year ended December 31, 2014 as a result of our shift to direct sales in certain European countries.
70
Cost of Sales
Cost of sales increased $3.5 million, or 15.5%, to $26.0 million for the year ended December 31, 2014, as compared to $22.5 million for the year ended December 31, 2013. This increase was primarily due to an increase in sales volume.
Gross Profit
Gross profit increased $4.7 million, or 21.2%, to $26.8 million for the year ended December 31, 2014, as compared to $22.1 million for the year ended December 31, 2013. This increase was primarily due to an increase in sales volume and a shift to direct sales in certain countries. Excluding the impact of depreciation, gross profit increased $4.7 million, or 20.1%, to $27.8 million for the year ended December 31, 2014, as compared to $23.1 million for the year ended December 31, 2013. Our gross margin increased 121 basis points to 50.7%, as compared to 49.5% in 2013. The increase in gross margin was driven by additional sales volume, and moving to direct sales in two countries, offset by the significant increase in sales to our Mexican distributor where the average selling price was lower than the Company average.
Distribution and Selling Expenses
Distribution and selling expenses increased $8.1 million, or 79.8%, to $18.2 million for the year ended December 31, 2014, as compared to $10.1 million for the year ended December 31, 2013. This increase was primarily due to increased compensation and other costs as a result of an expansion of our direct sales operations in Brazil, the United Kingdom, France and Spain, and the establishment of our direct sales operations in Italy and Germany.
Administrative Expenses
Administrative expenses increased $3.1 million, or 20.7%, to $18.2 million for the year ended December 31, 2014, as compared to $15.0 million for the year ended December 31, 2013. This increase was primarily due to compensation, including share-based compensation, and other employee related expenses due to the expansion of our senior management team.
Research and Development Expenses
R&D expenses, which are related predominately to our breast implant products, remained constant at $1.5 million for the years ended December 31, 2014 and for the year ended December 31, 2013.
Other Expenses
Other expenses of $0.2 million for the year ended December 31, 2013 was associated with professional fees related to bank debt restructuring. There were no such expenses for the year ended December 31, 2014.
Amortization
Amortization expenses remained constant at $4.0 million for the years ended December 31, 2014 and December 31, 2013.
Other Income (Expense)
Other income (expense) increased $65.3 million, to an expense of $75.0 million for the year ended December 31, 2014, as compared to an expense of $9.7 million for the year ended December 31, 2013. This increase in expense was due to (i) loss on conversion of shareholder loans of $27.7 million for the year ended December 31, 2014, (ii) a non-cash gain on the equity derivatives associated with our B Preference Shares and C
71
Preference Shares of $5.2 million for the year ended December 31, 2014, an increase of $10.0 million, compared to the loss of $4.8 million for the year ended December 31, 2013; (iii) an increase in interest expense of $26.9 million, or 390.7%, to $33.7 million for the year ended December 31, 2014, as compared to $6.9 million for the year ended December 31, 2013, which was primarily due to increased borrowings on shareholder loans and non-cash charges in respect of the effective interest on our B Preference Shares and C Preference Shares, and (iv) a non-cash loss on foreign exchange movements related to our foreign currency denominated loans and our B Preference Shares and C Preference Shares of $18.7 million for the year ended December 31, 2014, an increase of $20.7 million compared to the gain of $2.0 million for the year ended December 31, 2013. The B Preference Shares and C Preference Shares will convert to ordinary shares in connection with this offering; as a result, no further charges in respect thereof will be incurred in future periods. Excluding the impact of non-cash charges, our interest expense increased $2.6 million, or 74.0%, to $6.2 million for the year ended December 31, 2014, as compared to $3.6 million for the year ended December 31, 2013.
Income Tax Credit
Income tax credit decreased $0.6 million, or 49.8%, to $0.6 million for the year ended December 31, 2014, as compared to $1.2 million for the year ended December 31, 2013. This decrease was primarily due to an increased corporation tax charge and a reduction in the origination and reversal of temporary differences in certain deferred tax assets. See Note 17 to our consolidated financial statements included elsewhere in this prospectus.
Liquidity and Capital Resources
Overview
Since our inception, we have incurred significant net operating losses and anticipate that our losses will continue in the immediate- to near-term. Based on our recurring losses from operations and net capital deficiency, and not taking into consideration any proceeds that we will receive in connection with this offering, our independent registered public accounting firm included an explanatory paragraph in its report on our consolidated financial statements for the year ended December 31, 2014 expressing doubt about our ability to continue as a going concern. To date, we have financed our operations primarily through sales of our products, cash flow from operations, issuances of ordinary shares, preferred shares and convertible loan notes and borrowings under shareholder and bank loans and the Credit Facility. We have devoted substantially all of our resources to developing, manufacturing and obtaining regulatory approvals for our products, commercialization of our products and the development of a sales force, marketing team and management team for our business.
Our liquidity position and capital requirements are subject to a number of factors. For example, our cash inflow and outflow may be impacted by the following:
| | • | | the revenues generated by our breast implant and other products and any other future products that we may develop and commercialize; |
| | • | | the costs associated with expanding our sales force and marketing programs; |
| | • | | the cost associated with developing and commercializing our proposed products or technologies; |
| | • | | the cost of obtaining and maintaining regulatory clearances and approvals for our current or future products; |
| | • | | the cost of ongoing compliance with regulatory requirements; |
| | • | | expenses in connection with potential litigation or governmental investigations; |
| | • | | capital expenditures; and |
| | • | | unanticipated administrative expenses and other costs. |
72
Our primary short-term capital needs, which are subject to change, include expenditures related to:
| | • | | support of our commercialization efforts related to current and future products; |
| | • | | new product development, acquisitions, improvements to existing products and R&D efforts; |
| | • | | payment of interest due under the Credit Facility and other loans; |
| | • | | potential facilities expansion needs; and |
| | • | | entry into new direct countries. |
At December 31, 2014, we had $10.6 million in cash and cash equivalents. Although we cannot predict with certainty all of our particular short-term cash uses or the timing or amount of cash used, we believe that our available cash on hand and proceeds from this offering will be sufficient to satisfy our liquidity requirements for at least the next twelve months. However, the continued growth of our business will significantly increase our expenses and cash requirements. In addition, the amount of our future product sales is difficult to predict, and actual sales may not be in line with our forecasts. As a result, we may be required to seek additional funds in the future from issuances of equity or debt securities, obtaining additional credit facilities or loans or from other sources, subject to the restrictions under the Credit Facility or other agreements. For example, the Credit Facility currently restricts our ability to incur additionalpari passu debt. Moreover, additional capital, if needed, may not be available to us on satisfactory terms, if at all. Any additional equity financing may be dilutive to shareholders, and debt financing, if available, may include additional restrictive covenants. For a further discussion of going concern and other factors that may impact our future liquidity and capital funding requirements, see “Risk Factors—Risks Related to Our Financial Results and Need for Financing.” For a description of our Credit Facility, see “Indebtedness—Credit Facility” below.
Cash Flows
Our historical cash outflows have primarily been associated with activities relating to commercialization and increases in working capital and repayment of loans.
The following table shows a summary of our cash flows provided by (used in) operating, investing and financing activities for the periods indicated:
| | | | | | | | |
| | | Year Ended
December 31, | |
| | | 2014 | | | 2013 | |
| | | (in thousands) | |
Net cash used in operating activities | | $ | (12,940 | ) | | $ | (1,299 | ) |
Net cash used in investing activities | | | (2,669 | ) | | | (705 | ) |
Net cash provided by financing activities | | | 22,248 | | | | 4,026 | |
Effect of exchange rate fluctuations on cash and cash equivalents | | | (542 | ) | | | 63 | |
| | | | | | | | |
Net increase in cash and cash equivalents | | $ | 6,097 | | | $ | 2,085 | |
| | | | | | | | |
Operating Activities
Net cash used in operating activities for the year ended December 31, 2014 was $12.9 million, as compared to net cash used in operating activities of $1.3 million for the year ended December 31, 2013. The change in cash used was primarily associated with the increase in net loss of $72.1 million and an increase in cash outflows from operating assets and liabilities resulting from an increase in inventories and accounts receivable as a result of our strong growth in sales, partially offset by an increase in accounts payable. The net loss for the year ended December 31, 2014 was significantly impacted by non-cash charges associated with (i) our B Preference Shares and C Preference Shares relating to (A) $27.5 million in effective interest, as
73
compared to $3.3 million for the year ended December 31, 2013, (B) a $5.2 million gain on the associated equity derivative, as compared to a loss of $4.8 million for the year ended December 31, 2013, (C) a $15.4 million foreign exchange loss on B Preference Shares and C Preference Shares, as compared to a gain of $1.8 million for year ended December 31, 2013 and (D) a $27.7 million loss on conversion of shareholder loans during the year ended December 31, 2014, and (ii) a $3.3 million foreign exchange loss on foreign currency denominated loans, as compared to a $0.2 million gain for the year ended December 31, 2013. The B Preference Shares and C Preference Shares will convert to ordinary shares in connection with this offering; as a result, no further charges in respect thereof will be incurred in future periods.
Investing Activities
The net cash used in investing activities for the year ended December 31, 2014 was $2.7 million, as compared to net cash used in investing activities of $0.7 million for the year ended December 31, 2013. The change in net cash used was primarily due to an increase of $1.0 million in our capital expenditure program to support the maintenance of and expansion of our manufacturing facilities and $1.0 million due to an investment in product registrations.
Financing Activities
Net cash provided by financing activities for the year ended December 31, 2014 was $22.2 million, as compared to net cash provided by financing activities of $4.0 million for the year ended December 31, 2013. The change in net cash provided was primarily due to the issuance of preferred shares and funds borrowed under the Credit Facility, offset by higher financing costs and repayment of our bank loans.
Indebtedness
Credit Facility
On January 22, 2014, our wholly-owned subsidiary, GC Aesthetics Finance Limited, entered into a $34 million senior term loan facility with OrbiMed. This term loan facility was amended on November 26, 2014 to provide for an additional senior term loan facility of $15 million and was further amended on April 27, 2015 to provide for an additional senior term loan facility of $10 million (together, these term loan facilities are referred to herein as the “Credit Facility”). All three tranches of loans under the Credit Facility have been fully drawn down.
Interest is payable at a rate equal to 8.50% plus the higher of (i) the three-month London Interbank Offered Rate and (ii) 1% in the relevant period. In addition, during the term of the Credit Facility, we must pay an amount of additional interest for each fiscal year equal to the sum of (A) 1.80% of the aggregate revenues of our products for such fiscal year, up to €50 million of such revenues, plus (B) 2.15% of the aggregate revenues of our products during such fiscal year in excess of €50 million but not exceeding €75 million (“Additional Interest”).
The maturity date of the Credit Facility is January 22, 2020. Commencing on February 22, 2019, and quarterly thereafter, unless otherwise waived by OrbiMed, we are obligated to repay one-quarter of the outstanding aggregate principal amount under the Credit Facility. A 10% prepayment premium will be payable if the Credit Facility is repaid prior to the maturity date. In addition, on any repayment of the loans under the Credit Facility (whether on or before the maturity date), we must pay OrbiMed an additional premium, calculated on a variable scale depending on the date of repayment, up to a maximum amount of $16.2 million, less the aggregate amount of all payment of Additional Interest we have previously paid.
The Credit Facility is secured by first-priority security interests over the shares or other equity interests of our significant subsidiaries and over the most significant assets of such subsidiaries, including their bank accounts, receivables and intellectual property.
74
The Credit Facility contains restrictive covenants that require us to maintain certain cash reserves and a specified minimum revenue base, and that limit our ability to transfer or dispose of certain assets, engage in new lines of business, change certain members of our management team, merge with or acquire other companies, incur additional debt, create new liens and encumbrances, pay dividends or subordinated debt and enter into certain transactions with affiliates, among other things. In addition, the Credit Facility will become repayable in full immediately in the event that any “person” or “group” (within the meaning of Rule 13d-5 of the Exchange Act) other than Montreux or Oyster obtains ownership, directly or indirectly, of more than 35% of our voting shares. We are currently in compliance with all covenants under the Credit Facility.
Other Indebtedness
€80,951 of our interest bearing loan notes issued to certain of our shareholders on May 30, 2008 and December 24, 2009 (the “PIK Loan Notes”) remain outstanding. Interest accrues at 15% per annum and as at December 31, 2014 had a balance of €438,985 and will continue to accrue until settled. In addition, as at December 31, 2014, €1.8 million accrued interest on the portion of the PIK Loan Notes that was previously converted to B Preference Shares remains outstanding and continues to accrue interest at a rate of 8% per annum. Unless we repay these amounts, they will remain outstanding until the earlier of (i) May 30, 2016 and (ii) the completion of a sale transaction (defined as a direct or indirect sale of all or substantially all of the Company’s business and/or assets or transfer or disposal of the legal or beneficial ownership of the ordinary shares of the Company resulting in a change in control of the Company, “Sale Transaction”). Notwithstanding the provisions concerning repayment on a Sale Transaction, the outstanding principal and accruing interest in respect of the PIK Loan Notes are all subject to a subordination agreement with OrbiMed dated January 22, 2014, and pursuant to the terms of such agreement will not, without the consent of OrbiMed, be repaid until six months after the maturity date of the Credit Facility. See “Related Party Transactions.”
€70,734 remains outstanding to Dreamxcell Holdings Limited and we agreed to repay such balance in the event of a Sale Transaction.
We also have other loans with banks and regional development authorities in certain countries outstanding in an aggregate amount of $458,065 as of December 31, 2014. A portion of these loans are interest free and unsecured and the remainder accrues interest at a rate of 27.57% per annum. For a complete discussion of our bank loans, see Note 8 to our audited financial statements.
Contractual Obligations
The following table sets forth our contractual obligations as of December 31, 2014. Amounts we pay in future periods may vary from those reflected in the table.
| | | | | | | | | | | | | | | | | | | | |
| | | Total | | | Less
than 1
Year | | | 1 to 3
Years | | | 3 to 5
Years | | | More than
5 Years | |
| | | (in thousands) | |
Preference Shares(1) | | $ | 172,616 | | | $ | — | | | $ | — | | | $ | — | | | $ | 172,616 | |
Debt Obligations(2) | | | 85,097 | | | | 6,803 | | | | 13,396 | | | | 59,746 | | | | 5,152 | |
Operating Lease Obligations | | | 1,687 | | | | 748 | | | | 642 | | | | 271 | | | | 26 | |
| | | | | | | | | | | | | | | | | | | | |
Total | | $ | 259,400 | | | $ | 7,551 | | | $ | 14,038 | | | $ | 60,017 | | | $ | 177,794 | |
| | | | | | | | | | | | | | | | | | | | |
| (1) | The B Preference Shares and C Preference Shares will convert into ordinary shares in connection with this offering. |
| (2) | In April 2015, we drew down an additional $10.0 million under the Credit Facility, as described in “—Indebtedness—Credit Facility” above. |
75
Off-Balance Sheet Arrangements
As of December 31, 2014, we did not have any relationships with unconsolidated entities or financial partnerships, such as entities often referred to as structured finance or special purpose entities, which would have been established for the purpose of facilitating off-balance sheet arrangements or other contractually narrow or limited purposes.
Seasonality
We do not believe that seasonality has a material impact on our revenues or results of operations.
Critical Accounting Policies
The preparation of financial statements in accordance with IFRS requires our Board and management to make critical accounting estimates and judgments that affect the amounts reported in the financial statements and accompanying notes. These estimates and judgments are continually evaluated and are based on historical experience and other factors, including the expectation of future events that are believed to be reasonable under the circumstances at any particular point in time. The resulting accounting estimates will, by definition, seldom equate to the related actual results. The estimates and assumptions that are material to our financial reporting are discussed further below and are subject to a degree of subjectivity and complexity.
The impact and any associated risks related to these policies on our business operations are discussed throughout this section where such policies affect our reported and expected financial results. Note that the preparation of the financial statements included in this prospectus requires us to make estimates and assumptions that affect the reported amount of assets and liabilities, disclosure of contingent assets and liabilities at the date of our financial statements and the reported amounts of revenue and expenses during the reporting period. There can be no assurance that actual results will not differ from those estimates.
While our significant accounting policies are more fully described in Note 2 to our financial statements included in this prospectus, we believe the following accounting policies to be most critical to the judgments and estimates used in the preparation of our financial statements.
Revenue Recognition
Significant management judgments and estimates must be made and used in connection with the recognition of revenue in each accounting period. Material differences in the amount of revenue in any given period may result if these judgments or estimates prove to be incorrect or if management’s estimates change on the basis of development of the business or market conditions. To date there have been no material differences arising from these judgments and estimates.
Revenue from products is generally recorded as of the date of shipment, consistent with our typical shipment terms. Where the shipment terms do not permit revenue to be recognized as of the date of shipment, revenue is recognized when we have satisfied all of our obligations to the customer in accordance with the shipping terms.
Revenue is recognized to the extent that it is probable that economic benefit will flow to the Company, that the risks and rewards of ownership have passed to the buyer and that the revenue can be measured. This normally occurs on dispatch in relation to goods sold to end users and distributors. No revenue is recognized if there is uncertainty regarding recovery of the consideration due at the outset of the transaction or the possible return of goods. Revenue is disclosed net of value added tax, trade discounts, rebates and other allowances.
76
Allowance for Slow-Moving and Obsolete Inventory
We evaluate the realizability of our inventory on a case-by-case basis and make adjustments to the inventory provision based on our estimates of expected losses. We write off any inventory that is approaching its “use-by” date and for which no further re-processing can be performed. We also consider recent trends in revenues for various inventory items and instances where the realizable value of inventory is likely to be less than its carrying value. There were no material changes in estimates made during the years ended December 31, 2014 or 2013 that would have had a material impact on the carrying values of inventory during those periods.
Trade Receivables
We evaluate customer accounts with past-due outstanding balances or specific accounts for which we have information that the customer may be unable to meet its financial obligations. Based upon a review of these accounts and management’s analysis and judgment, we estimate the future cash flows expected to be recovered from these receivables. The amount of the impairment on doubtful receivables is measured individually and recorded as a specific allowance against that customer’s receivable balance to the amount expected to be recovered. The allowance is reevaluated and adjusted periodically as additional information is received.
Impairment Testing
For impairment assets purposes, certain of our assets, comprising trademarks, brands, registrations, goodwill, research and development and customer base purchased on acquisition, are grouped at the lowest levels for which there are largely independent cash inflows (cash-generating units). As a result, some assets are tested individually for impairment and some are tested at a cash-generating unit level.
Goodwill is allocated to those cash-generating units that are expected to benefit from synergies of a related business combination and represent the lowest level within the Company at which management monitors goodwill. Cash generating units to which goodwill has been allocated are tested for impairment at least annually. All other individual assets or cash-generating units are tested for impairment whenever events or changes in circumstances indicate that the carrying amount may not be recoverable.
Our corporate assets do not generate separate cash inflows. If there is an indication that a corporate asset may be impaired, then the recoverable amount is determined for the Company’s cash-generating unit to which the corporate asset belongs.
An impairment loss is recognized for the amount by which the asset’s (or the cash-generating unit’s) carrying amount exceeds its recoverable amount, which is the higher of fair value less costs of disposal and value-in-use. To determine value-in-use, management estimates expected future cash flows from each cash-generating unit and determines a suitable discount rate in order to calculate the present value of those cash flows. The data used for impairment testing procedures are directly linked to our latest Board approved budget, adjusted as necessary to exclude the effects of future reorganizations and asset enhancement where relevant. Discount factors are determined individually for each cash-generating unit and reflect current market assessments of the time value of money and cash-generating unit asset-specific risk factors.
An impairment loss is recognized if the carrying amount of an asset or its cash-generating unit exceeds its estimated recoverable amount. Impairment losses are recognized in the statement of operations. Impairment losses recognized in respect of cash-generating units are allocated first to reduce the carrying amount of any goodwill allocated to the units, and then to reduce the carrying amounts of the other assets in the cash-generating unit on a pro rata basis.
With the exception of goodwill, all assets are subsequently reassessed for indications that an impairment loss previously recognized may no longer exist. An impairment loss is reversed if the asset’s or cash-generating unit’s recoverable amount exceeds its carrying amount.
77
Ordinary Shares Valuation and Share-Based Compensation
We operate an equity-settled, share-based payment plan that includes the issuance of equity options to our employees in accordance with the provisions set out in the 2010 Employee Share Option Plan (the “2010 Plan”).
Options granted under this plan have a maximum exercise period of seven years from the grant date. The awards vest on an installment basis with a portion of the award vesting immediately and the remaining portion vesting over a number of months on a pro-rata basis. The number of option shares in respect of which an option may be exercised is cumulative, so that once an option is exercisable as to any option shares it continues to be exercisable as to such option shares until the option is fully exercised or lapses as provided in the plan. There are no market conditions attached to the options granted and outstanding.
As of December 31, 2014, the following equity options, as granted to our employees, were outstanding under the plan:
| | | | | | | | |
| | | Number of
Options | | | Weighted average
exercise price (€) | |
Outstanding at the beginning of 2014 | | | — | | | | — | |
Granted during the year | | | 1,179,791 | | | | 0.01 | |
Forfeited during the year | | | — | | | | — | |
Expired during the year | | | — | | | | — | |
Exercised during the year | | | — | | | | — | |
| | | | | | | | |
Outstanding at the end of 2014 | | | 1,179,791 | | | | 0.01 | |
| | | | | | | | |
Exercisable at the end of 2014 | | | 368,885 | | | | 0.01 | |
| | | | | | | | |
The exercise price for equity options outstanding at December 31, 2014 was €0.01 (US$0.013). The weighted average remaining contractual life of the outstanding equity options, which have a seven-year term, is 6.97 years. There were no equity options outstanding at December 31, 2013.
The total expense of equity options for fiscal year 2014 was $1.8 million.
The fair value of equity options granted is estimated using the Black-Scholes-Merton option valuation model, which incorporates various assumptions including expected life, volatility, risk-free interest rates and dividend yield.
The following tables list the inputs to the models used for the equity option plan for the year ended December 31, 2014:
| | |
| | | December 31, 2014 |
Dividend yield (%) | | 0% |
Expected volatility (%) | | 50% |
Risk–free interest rate (%) | | 0.17% |
Expected life of equity options (years) | | 7 years |
Weighted average share price (€) | | €3.47 |
Model used | | Black-Scholes-Merton |
The expected life of equity options represents the estimated duration between grant date and the expected exercise or cancellation date. We estimated the expected life of equity options granted based on the terms and conditions of the equity awards granted to our employees.
We estimated the volatility of our outstanding equity options based on peer company analysis.
78
We obtain the risk-free interest rate figures based on a U.S. government security of similar term to the equity option at the grant date. U.S. securities were chosen for reference on the basis that we intend to list our ordinary shares in the United States.
We have never paid or declared any cash dividends on our ordinary shares and we do not have present plans to pay cash dividends on our ordinary shares. Consequently, we used an expected dividend yield of zero in the Black-Scholes-Merton option valuation model.
Our Board has historically estimated the fair value of our ordinary shares on each grant date, including consideration of the conclusions of the contemporaneous valuations performed by an independent valuation specialist. The contemporaneous valuations were performed in accordance with applicable methodologies, approaches and assumptions of the technical practice-aid issued by American Institute of Certified Public Accountants and considered many objective and subjective factors to determine the fair market value of the ordinary shares for each valuation date. The following factors, among others, were considered:
| | • | | our stage of development and business strategy; |
| | • | | the financial condition and operating results of publicly owned companies with similar lines of business and their historical volatility; |
| | • | | external market conditions that could affect companies in the aesthetics products and medical device sectors; |
| | • | | the prices of our preference shares sold to outside investors and the rights, preferences and privileges of our preference shares as compared to those of our ordinary shares, including the liquidation preference of our preference shares; |
| | • | | the likelihood of a liquidity event such as an initial public offering, a merger or the sale of the Company; |
| | • | | the lack of marketability of our ordinary shares as a private company; and |
| | • | | recent relevant transactions. |
Following the closing of this offering, our Board will determine the fair value of our ordinary shares based on its closing price as reported on on the date of grant.
Income Taxes
Given the global nature of our business and the multiple tax jurisdictions in which we operate, the determination of our provision for income taxes requires significant judgments and estimates, the ultimate tax outcome of which may be uncertain. Although we believe our estimates are reasonable, the final outcome of these matters may be different than those reflected in the historical income tax provisions and accruals. Such differences could have a material effect on the income tax provision and results of operations in the period during which such determination is made.
Deferred tax assets and liabilities are determined using enacted tax rates for the effects of net operating losses and temporary differences between the book and tax bases of assets and liabilities. In assessing the reliability of deferred tax assets, management considers whether it is more likely than not that some portion or all of the deferred tax assets will not be realized. The ultimate realization of deferred tax assets is dependent upon the generation of future taxable income during the periods in which those temporary differences become deductible. While management considers the scheduled reversal of deferred tax liabilities, and projected future taxable income, in making this assessment, there can be no assurance that these deferred tax assets may be realizable.
79
In addition, we may also be subject to audits in the multiple taxing jurisdictions in which we operate. These audits can involve complex issues that may require an extended period of time for resolution. Management believes that adequate provisions for income taxes have been made in the financial statements.
Fair Value of Equity Derivatives
We have an equity derivative and, as required under IFRS 13, this equity derivative is required to be revalued annually. We employed third-party experts to provide a Company valuation.
The valuation was undertaken with regard to comparable market transactions between informed market participants. The transaction values are derived from data publicly available; however, due to the limited activity levels for transactions for peer companies, a degree of professional judgment was used by the consultants with regard to the final valuation. For this reason, the valuation of the equity derivative is classified as level three (as defined by IFRS 13) and presented in the Fair Value Table in our financial statements.
The individual derivatives share value is based on using a Black-Scholes-Merton valuation model based on the enterprise value of the Company.
Functional and Presentation Currency
The Company financial statements are presented in US dollars. While the Company’s consolidated financial statements are presented in US dollars, the functional currency of the Company and most of its subsidiaries is the Euro. Items included in the individual financial statements of each of our subsidiaries are measured using the currency of the primary economic environment in which the entity operates, primarily the Euro, Sterling, Mexican Peso and Brazilian Real.
Foreign Currency Transactions
Transactions in foreign currencies are re-measured at the foreign exchange rate in effect at the date of the transaction. Monetary assets are translated at the rate in effect at the balance sheet date. Non-monetary assets carried at historical cost are translated at their historical rates.
For financial statement presentation, monetary and non-monetary assets are translated at the rate in effect at the balance sheet date. Monetary assets and liabilities denominated in foreign currencies at the end of the reporting year are translated to functional currencies at the foreign exchange rate in effect at that date. Foreign exchange differences arising on translation are recognized in the statement of comprehensive income.
Qualitative and Quantitative Disclosures about Market Risk
The following discussion provides forward-looking quantitative and qualitative information about our potential exposure to market risk. Market risk represents the potential loss arising from adverse changes in the value of financial instruments. The risk of loss is assessed based on the likelihood of adverse changes in fair values, cash flows or future earnings. We are exposed to market risk for collection of receivables from our customers and distributors (credit risk), the impact of interest rate changes (interest rate risk), foreign currency exchange rate changes (foreign currency exchange risk) and inflation.
Credit Risk
Our sales are to a broad range of direct-users and distributors, most of which are in emerging markets. As a result of our operations in emerging markets, we are exposed to a greater possibility of loss arising from customer default. We seek to address this risk by performing credit checks before commencing new client relationships, maintaining customer credit limits and regularly monitoring our commercial relationships with our customers. No single customer represented more than 10% of our sales in 2014. Trade receivables net of
80
impairment losses were $12.8 million for the year ended December 31, 2014 as compared to $10.0 million in 2013, an increase of 28.3% mainly due to increase in sales volumes. As of December 31, 2014, of our $13.9 million of gross trade receivables, $6.4 million were past due and $1.1 million were impaired, and, after giving effect to our allowance for doubtful accounts, we had exposure of $5.3 million. For more information on our trade receivables, see Notes 4 and 20 to our consolidated financial statements included elsewhere in this prospectus.
Foreign Currency Exchange Risk
Although our financial statements are presented in US dollars, our functional currency and the functional currency for the majority of our subsidiaries is the Euro, and as a result, we may incur foreign exchange gains or losses resulting from movements in the exchange rate of the Euro, particularly versus the US dollar, Sterling, Mexican Peso and Brazilian Real.
Our products are available in 75 countries and our sales and costs are incurred in multiple currencies, including the US dollar, the Euro, Sterling, Mexican Peso and Brazilian Real. Accordingly, we are exposed to significant currency risk. We may not be able to change our prices to reflect the impact of foreign exchange rate changes. We manage this foreign currency risk, in part, through operational means, including managing foreign currency revenues in relation to same currency costs as well as managing foreign currency assets in relation to same currency liabilities; however, we are not able to fully match our foreign currency revenues to our costs. We are also exposed to potential earnings effects from the revaluation of our assets and liabilities. Other methodologies to limit our foreign exchange risks are being evaluated, which may include foreign exchange forward contracts or options.
In an appreciating US dollar environment, we expect our sales to fall and our profitability to increase, and vice versa in a depreciating US dollar environment.
Commodities Price Risk
The Company is dependent on the ability of our suppliers to provide raw manufacturing products on a timely basis at favorable pricing terms. The loss of certain principal suppliers, significant increases in costs of raw materials or a significant reduction in raw material availability could have a material adverse effect on our operations, cash flows and financial position.
The Company has two key suppliers of raw materials for manufacturing, one for each of our brands, Nagôr and Eurosilicone. These two suppliers are under common ownership. Purchases from these two suppliers comprised 43% and 31% of all purchases made in 2014 and 2013, respectively. The largest supplier for the years ended December 31, 2014 and 2013 accounted for 59% and 55%, respectively, of all raw materials purchases. Any significant interruption by the suppliers could have a material adverse impact on our operations. See Note 13 to our consolidated financial statements included elsewhere in this prospectus.
Interest Rate Risk
Our exposure to interest rate risk relates primarily to the floating interest rate of the Credit Facility. Interest on the $59.0 million principal is payable at a rate equal to 8.50% plus the higher of (i) the three-month London Interbank Offered Rate and (ii) 1% in the relevant period. We have no current intention to hedge our interest rate risk.
Inflation
Although at reduced levels in recent years, inflation continues to apply upward pressure on the cost of goods and services that we use. The competitive environments in many countries substantially limit our ability to fully recover these higher costs through increased selling prices. We continually seek to mitigate the adverse effects of inflation through cost containment and improved productivity and manufacturing processes.
81
Recently Issued Accounting Standards
The following new standards impacted the Company:
| | • | | Amendments to IAS 27, Separate Financial Statements; |
| | • | | Amendments to IAS 32, Financial Instruments: Presentation re: Offsetting Financial Assets and Financial Liabilities; |
| | • | | Amendments to IAS 36, Impairment of Assets: Recoverable Amount Disclosures for Non-Financial Assets; |
| | • | | Amendments to IFRS 10, Consolidated Financial Statements; |
| | • | | Amendments to IFRS 12, Disclosure of Interests in Other Entities; |
| | • | | Amendment to IAS 19, Employee Benefits; |
| | • | | Amendments to IAS 39, Financial instruments: Recognition and Measurement re: Novation of Derivatives and Continuation of Hedge Accounting; |
| | • | | Annual Improvements (2010-2012 Cycle); and |
| | • | | Annual Improvements (2011-2013 Cycle). |
The amendments to the foregoing standards did not have a significant impact on our financial statements. We reviewed the other new standards and International Financial Reporting Interpretations Committee’s and related guidance issued by the IASB during the year, and considered their applicability to the Company, and only those items listed above were considered applicable.
Emerging Growth Company Status
As a company with less than $1.0 billion in revenue during our most recently completed fiscal year as of the initial filing date of the registration statement of which this prospectus forms a part, we qualify as an “emerging growth company” under the JOBS Act. As an emerging growth company, we may take advantage of specified reduced disclosure and other requirements that are otherwise applicable generally to public companies that are not emerging growth companies. These provisions include:
| | • | | the ability to present more limited financial data, including presenting only two years of audited financial statements and only two years of selected financial data in the registration statement on Form F-1 of which this prospectus is a part; |
| | • | | an exemption from certain disclosure obligations regarding executive compensation in the registration statement on Form F-1 of which this prospectus is a part and in our future periodic reports; |
| | • | | an exception from compliance with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act; and |
| | • | | exemptions from the requirements of holding a non-binding advisory vote on executive compensation and obtaining shareholder approval of any golden parachute payments. |
82
We may take advantage of these exemptions for up to five years or such time that we are no longer an emerging growth company. We would cease to be an emerging growth company if we have more than $1.0 billion in annual revenues as of the end of any fiscal year, we have more than $700 million in market value of our shares held by non-affiliates as of the end of our second fiscal quarter or we issue more than $1.0 billion of non-convertible debt over a three-year period. We may choose to take advantage of some but not all of these reduced disclosure obligations. We have taken advantage of reduced disclosure obligations regarding financial statements and executive compensation arrangements in this prospectus, and we may choose to take advantage of some but not all of these obligations in future filings. If we do, you may receive different and less information than you might get from other public companies.
We will not take advantage of the extended transition period provided under Section 7(a)(2)(B) of the Securities Act for complying with new or revised accounting standards. Since IFRS makes no distinction between public and private companies for purposes of compliance with new or revised accounting standards, the requirements for our compliance as a private company and as a public company are the same.
Foreign Private Issuer Status
We are also considered a “foreign private issuer.” In our capacity as a foreign private issuer, we are exempt from certain rules under the Exchange Act that impose certain disclosure obligations and procedural requirements for proxy solicitations under Section 14 of the Exchange Act. In addition, our officers, directors and principal shareholders are exempt from the reporting and short-swing profit recovery provisions of Section 16 of the Exchange Act and the rules under the Exchange Act with respect to their purchases and sales of our ordinary shares. Moreover, while we must file an annual report on Form 20-F, we are not required to file periodic reports and financial statements with the SEC as frequently or as promptly as U.S. companies whose securities are registered under the Exchange Act, although we do intend to furnish quarterly reports on Form 6-K. In addition, although we plan to comply with Regulation FD, which restricts the selective disclosure of material non-public information, we are not required to comply with Regulation FD. As a foreign private issuer, we also may elect, and have elected, to follow Irish corporate governance rules to the extent permitted.
We may take advantage of these exemptions until such time as we are no longer a foreign private issuer. We would cease to be a foreign private issuer at such time as more than 50% of our outstanding voting securities are held by U.S. residents and any of the following three circumstances applies: (i) the majority of our executive officers or directors are U.S. citizens or residents, (ii) more than 50% of our assets are located in the United States or (iii) our business is administered principally in the United States.
We have taken advantage of certain reduced reporting and other requirements in this prospectus. Accordingly, the information contained herein may be different than the information you receive from other public companies.
Controls and Procedures
Internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements in accordance with IFRS. We have not performed an evaluation of our internal control over financial reporting, such as required by Section 404 of the Sarbanes-Oxley Act, nor have we engaged an independent registered public accounting firm to perform an audit of our internal control over financial reporting as of any balance sheet date or for any period reported in our financial statements. In the course of preparing our consolidated financial statements, we have identified deficiencies in the design and operating effectiveness of our internal control over financial reporting, including deficiencies relating to (i) revenue recognition in one of the countries in which we sell our products; (ii) calculation of tax; and (iii) valuation of the equity derivatives associated with our B Preference Shares and C Preference Shares. These deficiencies, or other deficiencies that may arise in the future, could result in one or more findings of a material weakness. We plan to take various measures to remediate these deficiencies, including upgrading our information
83
technology systems, hiring additional finance and accounting personnel with appropriate training, building our financial management and reporting infrastructure, hiring external advisors and further developing and documenting our accounting policies and financial reporting procedures. Although we plan to complete this remediation process as quickly as possible, we cannot at this time estimate how long it will take and whether our efforts will be successful.
Presently, our management is not required to perform an annual assessment of the effectiveness of our internal control over financial reporting. This requirement will first apply to our Annual Report on Form 20-F for the year ended December 31, 2016. Our independent registered public accounting firm will first be required to attest to the effectiveness of our internal control over financial reporting for our Annual Report on Form 20-F for the first year in which we are an accelerated filer and we are no longer an “emerging growth company” under the JOBS Act. See “Risk Factors—Risks Related to this Offering and Ownership of Our Ordinary Shares.”
84
BUSINESS
Overview
We are a leading pure-play female aesthetics company committed to becoming the trusted brand and partner for women seeking to look healthy, youthful, vibrant and beautiful, and to feel confident about themselves throughout their lifetime. To date, we have generated significant growth by focusing on the development, manufacturing and commercialization of one of the broadest ranges of implant products, principally silicone breast implants. We believe that through our two brands Nagôr and Eurosilicone, which have a combined 60 years of market presence, we have (i) a strong reputation for safety, quality and reliability, as evidenced by the five-year data from our ongoing Eurosilicone safety study, (ii) strong competitive advantages based on our innovative product design features—the result of our focused approach to research and development, which integrates feedback from women and surgeons, and (iii) a differentiated marketing and customer service approach, focusing on the needs of women.
We have developed and marketed our comprehensive range of products, comprising over 1,100 SKUs, to address the different needs and preferences of women and surgeons. All of our implants are manufactured with medical-grade silicone supplied from either NuSil or ASC, both of which are U.S.-based, ISO 9001-certified sources. We have manufacturing facilities in the United Kingdom and France and sell our products in 75 countries, with direct sales activities in six countries, including Brazil, the world’s largest medical aesthetics market by total number of plastic surgery procedures.
The global aesthetics products market in 2014 was estimated at $6.5 billion in total sales and is expected to grow at a CAGR of nearly 12%, reaching almost $10.2 billion in total sales by 2018, according to Medical Insight. We believe the overall market growth is driven by multiple factors, including a growing middle class in emerging markets, an increase in women’s disposable income, increasing awareness and acceptance of aesthetics procedures, as well as continuous innovation and improved accessibility to aesthetics procedures. While the United States has historically been the largest market for aesthetics products in terms of total revenues, a significant number of markets outside of the United States have been growing faster in terms of revenues and plastic surgeon numbers, For example, Brazil, Mexico and China have experienced faster revenue growth than the aesthetics products market in the United States, providing attractive growth opportunities. The global breast implant market is forecasted to reach nearly $1.3 billion by 2018, with some estimates forecasting the market will reach nearly $1.5 billion by 2019, growing at a CAGR ranging from approximately 4% to 6% from 2014 to 2018.
We have a diversified revenue base, with approximately 47% of our 2014 revenues derived from sales in EMEA, approximately 42% in Latin America and approximately 11% in Asia-Pacific. Our revenues were approximately $52.8 million for the year ended December 31, 2014, as compared to approximately $44.6 million for the year ended December 31, 2013, an increase of 18.4% at Constant Currency Revenue. For additional information regarding Constant Currency Revenue, which is a non-IFRS measure, including a reconciliation of Constant Currency Revenue to revenue, see “Prospectus Summary—Summary Consolidated Financial Data” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Key Financial Metrics.”
We seek to become the trusted brand and partner for women and surgeons. We believe we have an opportunity to expand the current female aesthetics market by making aesthetics procedures more acceptable and accessible by dispelling misconceptions, offering a platform that provides women with support to make informed decisions about their bodies and, ultimately, providing innovative, high-quality products to meet their needs. Our strategy to support long-term growth is to (i) continue to outperform our competitors and gain market share through our singular focus on female aesthetics and delivery of high-quality service, (ii) expand our direct sales efforts in existing markets and new high-growth markets, such as South Korea, Thailand and the CIS countries, and commercialize new female aesthetics products in existing markets, (iii) continue developing and maintaining our surgeon relationships and strong brand recognition to introduce innovative products, (iv) leverage our customer-centric approach and launch additional novel initiatives, and (v) selectively pursue strategic acquisitions to enlarge our product portfolio, go direct in new markets or expand further in existing markets.
85
The Global Aesthetics Products Market
The global aesthetics products market in 2014 was estimated at $6.5 billion in total sales and is expected to grow at a CAGR of nearly 12%, reaching almost $10.2 billion in total sales by 2018, according to Medical Insight. We believe the overall market growth is driven by multiple factors, including a growing middle class, increasing female disposable income and increasing awareness and acceptance of aesthetics procedures, as well as continuous innovation and improved accessibility to aesthetics procedures, through an increase in the number of surgeons. The number of middle- and upper-income women globally is forecasted to grow at a CAGR of approximately 5% from 2014 to 2019 to 346 million, according to industry sources.
While the United States has historically been the largest market for aesthetics products in terms of total revenues, a significant number of markets outside the United States have been growing faster in terms of revenues and plastic surgeon numbers. We believe that this growth in markets outside the United States will continue based on increasing penetration rates in many of those markets, increases in plastic surgeon numbers and favorable demographic and economic trends. For example, in 2013, Brazil surpassed the United States in total number of plastic surgery procedures, accounting for 12.9% of global plastic surgery procedures, as compared to 12.5% for the United States, according to ISAPS. The international market has also seen significant growth in the number of plastic surgeons. While the number of plastic surgeons in the United States grew at a CAGR of approximately 1% from 2010 to 2013, the number of plastic surgeons in certain countries outside the United States grew at an estimated average CAGR of 8% for the same period. For example, China and South Korea grew their plastic surgeon populations at CAGRs of approximately 12% and 18%, respectively, from 2010 to 2013. Brazil’s plastic surgeon population of 5,473 in 2013 is close to that of the United States, at 6,133. We believe the increase in the plastic surgeon population is representative of improved accessibility and a growing infrastructure supportive of further expansion of the overall market.
Overview of the Global Breast Implant Market
Breast augmentation surgery represented the leading aesthetic surgical procedure, with approximately 15% of total aesthetic surgical procedures performed by plastic surgeons in 2013, based on ISAPS. The global breast implant market is forecasted to reach nearly $1.3 billion by 2018, with some estimates forecasting the market will reach nearly $1.5 billion by 2019, growing at a CAGR ranging from approximately 4% to 6% from 2014 to 2018. Breast implant surgery is used to enhance aesthetic appearance and comprises two general categories: (i) breast augmentation procedures for cosmetic purposes and (ii) breast reconstruction procedures to replace breast tissue that has been removed, mainly due to cancer or trauma.
Non-U.S. Market Penetration Rates Compared to U.S. Penetration Rate
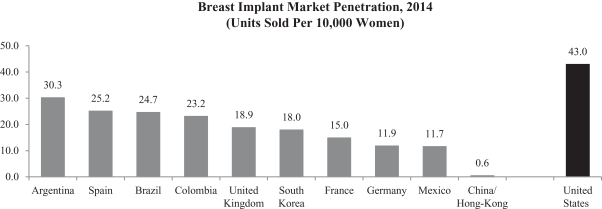
As illustrated above, many of the non-U.S. markets are still highly underpenetrated, based on the number of women undergoing breast implant procedures, compared to the penetration of global brands in the United States. We believe that current trends in non-U.S. markets will continue to increase penetration rates in those markets, creating a significant growth opportunity for us, given our global focus.
86
Favorable Demographics and Economic Trends for the Global Aesthetics Market
The primary market for aesthetics procedures is comprised of middle- and upper-class women, which BCC Research defines as women with access to sufficient income to afford such aesthetics procedures. From 2014 to 2019, the global aesthetics products market is estimated to grow at a CAGR of 1.5% in the United States, in contrast to 11.0%, 8.3% and 8.2% in China, Mexico and Brazil, respectively, according to BCC Research. Moreover, the Company estimates that the global aesthetics products market is projected to grow at a higher CAGR than the world GDP, and, in China, Brazil and Mexico, the aesthetics products market is projected to grow at a higher CAGR than their respective GDPs. In contrast, the U.S. aesthetics products market is projected to grow more slowly than the GDP of the United States. In contrast, the U.S. aesthetics products market is projected to grow more slowly than the GDP of the United States.
In addition, the breast implant market has grown despite economic slowdowns in various geographies. For example, in Brazil, the number of breast implant units sold grew by 11.5% in 2012, according to industry sources, while the country’s GDP growth slowed to only 1.8% over that same period. In Europe, the number of breast implant units sold grew by 12.8% in 2012, according to the same source; in contrast, overall GDP grew by 0.3% over that same period. We believe these statistics are indicative of the stability and growth of the breast implant market throughout economic cycles.
Euromonitor projects the global annual disposable income per capita for women will grow at a CAGR of approximately 6% over the next five years. We believe this growth will help drive the expansion of the global aesthetics market. In addition, over the next 20 years, it is estimated that there will be a significant increase in the world’s middle-class population, which is expected to account for a significant increase in purchasing power and discretionary spending. We believe much of this growth in purchasing power will come from emerging markets, due to increasing annual disposable incomes in those markets. For example, the Chinese middle- and upper-income female population is expected to reach 78 million by 2019, matching that of the United States. For example, according to Euromonitor, over the next five years, the annual disposable incomes in Asia-Pacific and Latin America are forecasted to expand at a CAGR of approximately 9% and 20%, respectively, outpacing the 4% growth in the United States.
Our Opportunity
We believe we have a significant opportunity to capitalize on the favorable trends in the aesthetics market and benefit from the underlying growth in women’s disposable income, particularly in emerging markets, given the strength of our brands, quality and breadth of product portfolio, differentiated customer service, strong customer relationships, expanding global infrastructure and new product development. Currently, we focus on the breast implant products market outside of the United States, as well as on other pre- and post-breast implant surgery products globally. We have a significant presence in Europe, Latin America and Asia-Pacific and we intend to continue to further expand in these regions as well as in Russia and the Middle East and into emerging markets, including China, Thailand and additional CIS countries. Beyond breast implant products, we intend to expand our presence in other selected female aesthetics products, including those associated with implant surgery as well as other procedures, such as body sculpting treatments and dermal fillers.
We seek to become the trusted brand and partner for women and surgeons. We believe we have an opportunity to expand the current female aesthetics market by making aesthetics procedures more acceptable and accessible by dispelling misconceptions, offering a platform that provides women with support to make informed decisions about their bodies and, ultimately, providing innovative, high-quality products to meet their needs.
87
Competitive Strengths
We believe our pure-play focus has allowed us to become a leading player in the female aesthetics market. The following are our key competitive strengths:
Highly regarded and trusted brands with a strong safety profile. We have two prominent brands, Nagôr and Eurosilicone, which have a combined 60 years of market presence, and a well-established track record for safety, quality and reliability. Millions of women worldwide have used our products. Based on a company-sponsored, Eurosilicone breast implant study in Europe with 534 subjects, the peer-reviewed five-year clinical safety data published in Plastic and Reconstructive Surgery supported the strong safety profile of our breast implant products, with low incidence of rupture and rates of capsular contracture comparable to rates experienced by others in our industry for primary augmentation surgery.
Broad product range recognized for quality. Breast implant products and pre- and post-breast implant surgery products are our core competency. Unlike our primary competitors, who generate a very small portion of their total revenue from breast implant products, we generate substantially all of our revenue from our breast implant products, and are focused on providing one of the most comprehensive selections of breast implant and other products for our customers. Our product portfolio, including our breast implant products, includes over 1,100 SKUs. Our breast implant products include round and anatomical implants with different projections, textures and gel cohesivities. Through this breadth of product offerings, we believe our breast implant products allow surgeons and women to achieve their desired look and feel. We believe they recognize that our breast implant products offer an attractive profile of shape retention, durability and natural feel, and we are focused on consistently delivering high levels of customer satisfaction.
Global sales and marketing platform. We sell our products in 75 countries and are one of the leading breast implant manufacturers globally by units sold. Through our strong sales and marketing platform, we promote the Nagôr and Eurosilicone brands globally through our direct sales activities in Brazil, Spain, the United Kingdom, France, Germany and Italy and an additional 69 countries through distributors. We believe our strong sales and marketing platform will help us to successfully launch product line extensions and thereby drive additional revenue growth, particularly in rapidly growing emerging markets.
Extensive in-house development and manufacturing capabilities. All of our products have been developed in-house. Our manufacturing operations allow us to rapidly develop new products based on our close relationships with women and surgeons and our extensive industry experience. We have manufacturing facilities in the United Kingdom and France, all of which are ISO certified and are regularly inspected by their respective regulatory bodies. We remain focused on innovation to produce differentiated, best-in-class products for our customers. An example of our commitment to innovation is the development and recent well-received launch of our IMPLEO product line, which we believe is the first round implant that offers shape maintenance, an unbreakable gel and a natural feel—important qualities for women and surgeons in choosing a breast implant.
Focus on integrated marketing and delivering high-quality customer service.We believe that our customer-focused approach to product design, marketing and customer care is a key differentiator. Our integrated marketing approach consists of woman-centric initiatives, including our Experience Centres, which allow women to consider a variety of implants and gain familiarity with aesthetics procedures in a comfortable and supportive setting. In addition, we offer surgeon-centric support programs, including Business Boost, our program that assists surgeons in building their businesses. We believe our GCA Comfort Guarantee is distinctive in our industry and we are not aware of any other implant company that offers an equivalent lifetime guarantee for implant replacement in the event of rupture or severe capsular contracture. Our customer service teams provide 24-hour telephonic and email customer support to our sales representatives and customers. We believe our commitment to delivering customer satisfaction will help expand our addressable market and create the opportunity to gain additional market share.
88
Experienced management team. We are led by a proven management team, with a successful track record in healthcare and aesthetics. Ayse Kocak is the only female chief executive officer of a breast implant company and has over 20 years of experience in the healthcare industry. Our management team has over 135 years of combined experience across diverse backgrounds, including significant experience with pharmaceutical and healthcare product companies, and has been the team responsible for developing, implementing and executing our strategy for continued growth.
Our Growth Strategies
Our goal is to become the leading pure-play female aesthetics company. We are committed to becoming the trusted brand and partner for women seeking to look healthy, youthful, vibrant and beautiful, and to feel confident about themselves throughout their lifetime. To achieve these objectives, we are pursuing the following growth strategies:
Outperform the competition in existing markets. Our focus on female aesthetics and delivering high-quality service has distinguished our brands and has enabled us to gain market share from our major competitors in key markets, including Brazil and Mexico, the second and third largest markets by number of breast implant units sold, as shown in the market share gain data depicted below. Based on internal estimates, from 2013 to 2014, our global market share outside of North America increased from 18% to 20%, based on breast implant units sold. We believe that as our brand awareness increases and our reputation for quality and service grows, we will be able to achieve additional growth in our existing markets.
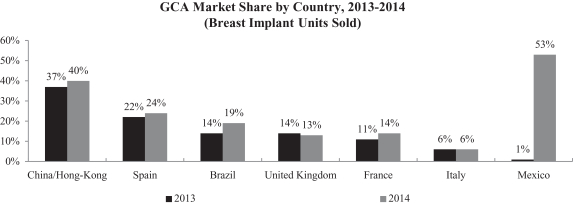
Penetrate new geographical markets with a focus on direct operations and commercialize new products. We currently sell our products directly in six countries and through distributors in an additional 69 countries. We intend to increase the number of markets where we sell direct, which we believe will drive gross margin expansion, as our gross margin is higher in markets where we sell direct. In particular, we are focused on establishing a direct sales presence in key additional markets, such as South Korea and Thailand. We also plan to expand our product portfolio by commercializing additional female aesthetics products in the markets where we currently operate.
Leverage our surgeon relationships and strong brand recognition to introduce innovative products. By leveraging the established position of our Nagôr and Eurosilicone brands and strong surgeon relationships, we plan to expand our product range in female aesthetics. We recently launched our new breast implant product, IMPLEO, which has been well received by women and surgeons and has already achieved strong sales performance in several key markets. We plan to continue innovating and introducing new products like IMPLEO to expand our product portfolio to offer the best solutions for our customers. We also intend to leverage our brands and market position to help drive revenues as we introduce new pre- and post-implant surgery products into our markets.
89
Continue our customer-centric approach and launch additional novel initiatives. Unlike our larger competitors, we are a pure-play female aesthetics company, and we are focused on supporting our surgeons through programs such as Business Boost, streamlining their ordering and shipping processes, familiarizing them with our commitment to quality through factory tours and offering highly-responsive customer service. We intend to continue to effectively engage women and surgeons through an integrated approach that includes direct marketing, distribution partnerships and social media campaigns to help promote the acceptance of breast implants and help patients take action to change the way they feel about themselves. For example, we have launched Experience Centres that allow women to choose the implant that is right for them in a relaxed and supportive environment, helping women get comfortable with making the decision to have an aesthetic procedure. Our goal is to help women enhance their lifestyles, increase their self-confidence by finding their desired fit and be 100% satisfied with our products, with peace of mind from our GCA Comfort Guarantee lifetime warranty. We believe these efforts will help expand our addressable market by encouraging more women to consider aesthetics procedures, including breast implant surgery.
Selectively pursue strategic acquisitions. We may selectively pursue complementary acquisitions that would allow us to enlarge our female aesthetics product portfolio, go direct in new or existing markets or expand further in existing markets. We expect to expand our product portfolio by adding products, such as garments, anti-scar treatments, fillers, toxins and body sculpting treatments. We may go direct in more markets by acquiring certain of our existing distributors, which we believe will contribute to increased gross margins and allow us to control our messaging to more effectively market our products with key decision makers. In addition, we may pursue acquisitions of other aesthetic implant players where it will provide complementary product lines, technology or market access.
Our Products
We have developed and marketed our comprehensive range of products, comprising over 1,100 SKUs, to address the different needs and preferences of women and surgeons.
We have two strong and established brands, Nagôr and Eurosilicone, which have a combined 60 years of market experience in breast implant products. Nagôr products are manufactured at our facilities in the United Kingdom and Eurosilicone products are manufactured at our facility in France. All of our implants are manufactured with medical-grade silicone supplied from either NuSil or ASC, both of which are U.S.-based, ISO 9001-certified sources.
Breast Augmentation and Breast Reconstruction Products
Breast Implant Products.Our product portfolio addresses a comprehensive range of options:
| | |
Shape | | Round and anatomical (also known as teardrop) |
Texture | | Smooth and three different textures |
Volume | | 100ml to 800ml |
Projection | | Low to extra-high |
Gel cohesivity | | Five different cohesivities |
We recently commenced active promotion of the IMPLEO breast implant product line, which we believe are the first round implants to be soft to the touch, deliver shape maintenance and a range of texturing as well as permitting a small surgical incision due to their unbreakable gel. We believe that through this combination of features, a wider range of surgeons is able to deliver an outcome to women that was previously achievable only by the highest quality surgeons.
90
The commercial impact of IMPLEO was significant in certain countries in 2014, its first year of active promotion in such markets; it accounted for approximately 20% of our sales in France and for approximately 56% of our sales in the United Kingdom in 2014. IMPLEO is currently available in 44 of the 75 countries in which we sell our products, and we are planning to launch it in almost all of our other markets in 2015, subject to receiving required regulatory approvals.
Breast Sizers. We produce a range of these temporary, single-use products that can be used by surgeons during surgery to help identify the desired style and size of implant for each woman.
Breast Tissue Expanders.These are used in breast reconstruction following cancer and other trauma injuries, and are temporary devices intended to aid in the process of recreating tissue coverage to allow for the placement of an implant to reconstruct the breast.
Other Implants
We offer a range of other implant products, including gluteal, testicular and calf implants, all made from medical-grade silicone. In 2014, these represented 5.5% of our sales.
Pre- and Post-Breast Implant Surgery Products
In 2015, we are planning to launch a range of consumer products to offer a broader solution and reinforce our brand loyalty among women considering breast augmentation and those who have had the procedure, including the following products:
The Enhance Breast Boost Pack. Based upon our extensive experience and understanding of the female breast form, this non-invasive product, comprising a specialized bra and silicone breast forms, allows women to experience the look and feel of fuller breasts instantly, and we believe this will lead to an increase in the number of women considering surgical augmentation and consulting surgeons.
Silgel scar management specialty products. These gel and sheet products leverage our experience of working with medical-grade silicone in products that minimize the appearance of post-surgery scars, and help us build upon our existing relationships with surgeons and access other market channels, such as pharmacies and internet sales.
Our Five-Year Clinical Data
In June 2014, Plastic and Reconstructive Surgery published the peer-reviewed five-year results from an ongoing study to evaluate the safety profile of our Eurosilicone breast implant products. This multi-center, surgeon-led study, sponsored by us, monitors 1,010 Eurosilicone breast implants in 534 subjects who have undergone either augmentation or reconstructive surgery. Each patient had a three-month post-surgery follow-up evaluation and annual follow-ups thereafter. The study’s five-year results demonstrate a low rupture rate and a strong safety profile for primary augmentation surgery. The primary augmentation total risk of rupture was assessed at 0.8% per patient; only one of the 1,010 implants was found to be ruptured on examination. The incidence of primary augmentation severe capsular contracture was 10.7%, which we believe is consistent with rates experienced by others in our industry.
91
Product Development
All of our products have been developed in-house. Through integrating our significant experience developing, manufacturing and securing regulatory approval for our breast implant products and our close relationships with women and surgeons, we believe that we are well positioned to continue our track record of developing innovative products based on customer needs. We have a team of 5 people involved in product development as of March 31, 2015. We intend to continue to invest into our current product portfolio to help us maintain one of the broadest and safest ranges of implants.
Sales and Marketing
As of December 31, 2014, we had a sales organization of 63 employees. We currently sell our products directly in six countries and through distributors in an additional 69 countries. The countries in which we sell our products directly are Brazil, the United Kingdom, France, Spain, Italy and Germany. In certain of the countries where we sell direct, we also use agents and sub-distributors. We believe the expansion of our sales force provides us with significant opportunities for future growth as we continue to penetrate existing markets and enter new markets. In addition, we believe that as we expand our female aesthetics product portfolio, we can leverage this platform to sell these additional products.
We believe one of the keys to our commercial success has been our ability to partner with the plastic surgeon community through medical and education initiatives, including publications, conferences, workshops and key opinion leader (KOL) sponsorship programs, as well as surgeon-specific initiatives, including providing consultation kits and arranging factory tours. In addition, our sales team is supported by customer service teams, which provide telephonic and email customer support to our customers and sales representatives.
Our marketing team leads our efforts in brand development, advertising, tradeshow attendance, educational forums, product messaging and website development. They also educate surgeons on the advantages of our products through a variety of methods and custom training tools. We intend to continue to promote broader acceptance of aesthetics procedures and to drive adoption of our products by engaging women and surgeons through our integrated, customer-centric approach that includes our Experience Centres, GCA Comfort Guarantee, Business Boost and ongoing communication through social media. We believe these efforts will expand our addressable market by encouraging additional women to consider aesthetics procedures and additional surgeons to use our products.
We sell our products through distributors in the 69 countries in which we do not have direct operations. We also use agents and sub-distributors in countries where we have direct operations. We have developed long-standing relationships with a number of our distributors. We require that all of our distributors adhere to confidentiality obligations and comply with health, environmental and labor regulatory standards as well as anti-corruption, sanctions and similar regulatory regimes to which we are subject, including those of the United Kingdom, Ireland, France and the United States.
Manufacturing and Supply
We manufacture our products in ISO-certified manufacturing facilities located in the United Kingdom and France, with raw materials and components sourced by external suppliers. We perform inspections of these materials before use in our manufacturing operations. Throughout the manufacturing process, we maintain rigorous quality control testing. We believe our facilities comply with international current Good Manufacturing Practice (“cGMP”) regulations for medical devices, and we are subject to periodic inspections and audits by various foreign regulatory bodies. As a result, we must follow stringent design, testing, production, control, documentation and other quality assurance procedures during all aspects of the manufacturing process. Our in-house manufacturing team includes over 260 employees. We believe our manufacturing experience, proprietary know-how and trade secrets represent a barrier to entry for potential competitors. We increased our unit production by 60% in 2014 and are evaluating the construction of a new manufacturing facility in Latin America.
92
We obtain our medical-grade silicone from NuSil and ASC, each of which is the sole-source supplier for our respective brands. We maintain long-term contracts with these suppliers and they have generally met our demand for their products on a timely basis in the past. NuSil and ASC have recently come under common ownership.
Competition
Our industry is intensely competitive and subject to rapid change from the introduction of new products and technologies and other activities of industry participants. While we believe that our technology, knowledge, experience, customer service and brand reputation provide us with competitive advantages, we face significant competition from companies selling similar products.
Our primary competitors include Mentor Worldwide, LLC, a division of Johnson & Johnson, and Allergan, Inc. These companies are well-capitalized and control a majority share of the breast implant products market. These competitors also enjoy several competitive advantages over us, including greater financial and human resources for sales, marketing and product development, larger and more established distribution networks, greater ability to cross-sell products and more experience in conducting research and development, manufacturing and obtaining regulatory approvals and clearances. We also compete with these companies for personnel, licensing and development partners, complementary businesses and technologies we may seek to acquire and exclusive distribution arrangements. In addition, we compete with Silimed, a leading Brazilian supplier of breast implant products. In certain of the countries in which we operate, we also compete with several other breast implant manufacturers, including Groupe Sebbin SAS and Establishment Labs S.A., which sell their breast implant products at significantly lower prices than those of our Nagôr and Eurosilicone breast implant products.
The key competitive factors affecting the success of our products are safety, brand reputation, product quality, product innovation and customer service. For our products to be successful, we must be able to manufacture and effectively market them to a sufficient number of women, surgeons, clinics and hospitals, such that they purchase our current products and the new products we may introduce.
Government Regulation
Medical Devices
Our breast implant products are characterized as Class III medical devices in many of the countries in which we operate, while others of our products are characterized as Class I, Class IIa or Class IIb medical devices in many of the countries in which we operate. Sales of medical devices are subject to regulatory requirements that vary widely from country to country, ranging from simple product registration requirements in some countries to more complex and stringent clearance and post-market controls in others.
For example, in order to be placed on the market within the EEA, medical devices must meet the essential requirements set out in the relevant medical device legislation. The principal legislation regulating general medical devices in the EEA is Directive 93/42/EEC (and subsequent amending directives), referred to herein as the EU Medical Devices Directive. In the case of low-risk (Class I) medical devices, such as Silgel, the manufacturer may self-certify conformity with the EU Medical Devices Directive by issuing a declaration of conformity. In the case of medium- to high-risk (Class IIa, IIb and III) medical devices, such as our breast implant products and tissue expanders, an EC Certificate issues from a notified body. Where a medical device meets the essential requirements set out in the EU Medical Devices Directive and complies with the appropriate conformity assessment procedure, based on the classification of the medical device, an EC Certificate will be issued and a CE Mark may then be affixed to the product. Once a CE Mark has been affixed to the medical device, it may then be placed on the market in any country within the EEA, Switzerland and Turkey (subject to certain localized registration and language requirements).
93
We have received an EC Certificate from the notified body for all of our breast implant products and our breast tissue expanders, allowing us to affix the CE mark to each such product. In order to maintain our EC Certificate and CE Mark, we must continue to comply with the EU Medical Devices Directive and pass annual facilities audit inspections by an inspection agency of the EEA. In addition, a notified body or other competent authority in an EEA country may perform post-marketing audits on our products and premises from time to time. Failure to comply with such requests in a timely manner, and any adverse findings in any such audit, could result in the withdrawal of our EC Certificate and our CE Mark, and the recall or withdrawal of our products from the EEA market. Each EC Certificate is generally valid for a maximum of five years. Our existing EC Certificates for Eurosilicone breast implant medical device products are valid until November 2018 (EC Design Certificate for breast implants) and August 2015 (EC Full Quality Assurance Certificate for breast implant and other implant products, including breast tissue expanders) and our existing EC Certificates for Nagôr breast implant medical device products are valid until March 2019 (EC Design Certificates for each of saline and silicone breast implants), May 2017 (EC Full Quality Assurance Certificate covering round breast tissue expanders) and April 2019 (EC Full Quality Assurance Certificate covering anatomical breast tissue expanders). At the end of each period of validity, we are required to apply to the notified body for a renewal of our certificate of conformity. There may be delays in the renewal of our certificate of conformity and the notified body may require modifications to our products or to the related technical files before it agrees to issue a new certificate of conformity.
In Brazil, marketing authorization requires submission of an application to the Brazilian Health Surveillance Agency (ANVISA), which will approve products deemed safe and effective. The manufacturing facilities in which the products are produced also must be operated in compliance with Brazilian cGMP regulations. An import license may be obtained following approval of a product. Maintenance of a product registration in Brazil requires annual inspection of the manufacturing facility. Our existing marketing approvals in Brazil covering Eurosilicone breast implant products are valid until October 2019 (breast implants) and December 2018 (tissue expanders).
In Mexico, marketing authorization requires submission of an application to the Federal Commission for the Protection against Sanitary Risk (“COFEPRIS”). COFEPRIS will approve products deemed safe and effective. The manufacturing facilities in which products are produced are not required to be inspected. Following approval, an import license for a product may be obtained. Our existing marketing approvals in Mexico covering Eurosilicone breast implant products are valid until May 2018 (Matrix range), January 2019 (Round Textured range), and February 2019 (Round Smooth range) and January 2019 (for breast tissue expanders) and for Nagôr Breast implant products are valid until February 2018 (IMPLEO and Cogel ranges) and May 2018 (GFX and RGI ranges).
We have also obtained an ISO 13485 quality system certification, which confirms that our medical device manufacturing quality management system is compliant with globally recognized standards set forth by the ISO. We are required to keep up-to-date and remain compliant with the most recently issued standards.
Approval in one jurisdiction does not assure that a product will be approved in another jurisdiction. The processes and time periods required to obtain marketing approvals vary widely from jurisdiction to jurisdiction, and in some instances we may not receive marketing approval for a product in a particular jurisdiction within the timeframe we anticipate. In order to maintain our marketing authorizations, we must comply with the laws and regulations of each jurisdiction in which we sell our products. In addition, we will be subject to the laws and regulations of any additional jurisdiction in which we obtain marketing approval for a product. Failure to comply with applicable laws and regulations could jeopardize our ability to sell our products and result in a variety of enforcement actions.
94
Other Regulations
Our global activities are subject to the Bribery Act, the FCPA and other countries’ anti-bribery laws. These laws generally prohibit companies and their intermediaries from offering, promising, authorizing or providing payments or anything of value to any foreign government official, government staff member, political party or political candidate for the purpose of obtaining or retaining business or securing any other improper advantage. The Bribery Act, in addition, prohibits commercial bribery and makes it a crime for companies to fail to prevent bribery. Companies have the burden of proving that they have adequate procedures in place to prevent bribery. The enforcement of such laws has increased dramatically in recent years, and authorities have indicated that the medical device industry will be a significant focus for enforcement efforts. Although we have procedures in place to ensure that we, our employees and our agents comply with the Bribery Act, the FCPA and related laws, there is no assurance that such procedures will protect us against liability under the Bribery Act, the FCPA or related laws for actions taken by our agents, including our distributors, employees and intermediaries with respect to our business.
We also are subject to anti-money-laundering laws and regulations and economic sanctions and other restrictions imposed by the European Union and other governments and organizations. Under these laws and regulations, as well as other export control laws, customs laws, sanctions laws and other laws governing our operations, various government agencies may, among other things, require export licenses, may seek to impose modifications to business practices, including cessation of business activities in sanctioned countries or with sanctioned persons or entities, and modifications to compliance programs. If we or our employees or agents violate these laws or regulations, it could adversely impact our business, results of operations and financial condition.
In addition, our business activities involve the use of hazardous materials. We and certain of our suppliers are subject to environmental, health and safety laws and regulations, including those governing the use, handling, storage, disposal and human exposure to hazardous and toxic materials. Many of our operations require permits and controls to monitor or prevent pollution. We have incurred and will continue to incur substantial ongoing capital and operating expenditures to ensure compliance with these laws and regulations and we expect that our operations will be affected by increasingly stringent and new environmental, health and safety laws and regulations on an ongoing basis, which likely will result in additional costs, and may require us to change how we manufacture our products.
For a discussion of the risks relating to the failure to comply with these laws and regulations, see “Risk Factors.”
Trademarks and Other Intellectual Property
We regard intellectual property and other proprietary rights as important to our success. We own trademarks and service marks that have been registered with various jurisdictions in which we operate, including, but not limited to, COGEL, DREAMXCELL, EUROSILICONE, GC AESTHETICS, GCA, GCA BUSINESS BOOST, NAGOR and SILGEL.
We also own domain names, including gcaesthetics.com. In addition, we have made application to register various trademarks and service marks in a number of additional countries and expect to continue to do so in the future. However, there can be no assurance that we can obtain the registration for the marks in every jurisdiction where registration has been sought or that we can maintain registrations in the jurisdictions where we currently have them.
We own one patent related to a textured breast implant and its method of manufacture. Our patented process provides texturing on the exterior surface of the implant in order to reduce the possibility of capsular contracture of the implant once it is in a patient’s body.
95
We also rely upon trade secrets and know-how to develop and maintain our competitive position. We protect our intellectual property rights through a variety of methods, including trademark and trade secret laws, as well as confidentiality agreements with vendors, employees, consultants and others who have access to our proprietary information.
Employees
As of December 31, 2014, 2013 and 2012, we had 420, 330 and 311 employees, respectively. As of December 31, 2014, we employed a total of 401 full-time employees and 19 part-time employees, with 63 in our sales organization, including sales and marketing and customer service employees, 267 in manufacturing, three in product development, 21 in quality and regulatory and 66 in administrative and other functions. Our employees in Brazil, France and Spain are represented by labor unions or collective bargaining arrangements. We believe we have good relationships with these labor unions and our employees.
Properties
Our principal executive offices are located at Suite 601, Q House, Furze Road, Sandyford, Dublin 18, Ireland. We lease approximately 10,000 square feet in Ashby de la Zouch, United Kingdom under leases that expire in February 2018 and June 2019. We also lease approximately 31,000 square feet in the Cumbernauld, United Kingdom under leases that expire from April 2017 through May 2018. We use the facilities in Ashby de la Zouch and Cumbernauld primarily for manufacturing for our Nagôr products. We manufacture our Eurosilicone products in Apt, France under leases for approximately 35,000 square feet that expire in January 2017, February 2027 and April 2025. We believe that our existing facilities are sufficient to meet our needs through at least 2016.
Legal Proceedings
We are involved from time to time in various lawsuits, including product liability claims, arising out of or incident to the ordinary conduct of our business. Although no assurances can be given, we do not expect that any of these matters will have a material adverse effect on our business or financial condition.
96
MANAGEMENT
Our Executive Officers and Directors
Below is a list of the names and ages of our directors and officers as of April 15, 2015, and a brief description of the business experience of each of them. The business address for our directors and officers is c/o GC Aesthetics plc, Suite 601, Q House, Furze Road, Sandyford, Dublin 18, Ireland.
| | | | |
Name | | Age | | Position |
Ayse Kocak | | 43 | | Chief Executive Officer, Director (Class I) |
Ian Crosbie | | 47 | | Chief Financial Officer |
Barry Hatt | | 59 | | Chief Technical Operations Officer |
Vikash Agarwal | | 40 | | VP Corporate Business Development & Strategic Planning |
Julia Marsan | | 53 | | Global Human Resources Director |
Bernardo Garduño | | 46 | | VP Sales, Latin America |
Ayhan Aslan | | 48 | | VP Sales, Emerging Markets |
Ignasi Salla | | 43 | | Director Sales, Europe |
Daniel K. Turner III | | 53 | | Chairman (Class II) |
William McCabe | | 59 | | Director (Class II) |
Declan McKeon | | 63 | | Director (Class III) |
Michel Rouif, M.D. | | 54 | | Director (Class III) |
Executive Officers
Ayse Kocak has served as our Chief Executive Officer since February 2013 and has been a member of the Board since February 2012. Prior to joining the Company as Chief Executive Officer, from February 2009 to February 2013, Ms. Kocak started and served as a general manager at moksha8 Pharmaceuticals, Inc. in Mexico, a specialty pharmaceutical company that focuses on therapeutics to treat the central nervous system and mental health disorders. Previously, Ms. Kocak served in various commercial roles with subsidiaries of Pfizer Inc. around the world, including in the United States, Europe, Latin America, Africa and the Middle East, and was involved in the launch and commercialization of Lipitor®, Viagra®, Norvasc® and Geodon®. Ms. Kocak holds a bachelor’s degree from Bosphorous University.
Ian Crosbie has served as our Chief Financial Officer since January 2015. Prior to joining the Company, from November 2013 to December 2014, Mr. Crosbie served as a senior vice president of corporate development at Circassia Pharmaceuticals plc, a specialty biopharmaceutical company that develops a range of immunotherapies for the treatment of allergies. Previously, Mr. Crosbie was an independent corporate consultant from May 2013 to October 2013 and a managing director at Jefferies International Ltd. from March 2008 to April 2013. Mr. Crosbie holds a master’s degree in Engineering, Economics and Management from Oxford University.
Barry Hatt has served as our Chief Technical Operations Officer since April 2015. Previously, from May 2011 to March 2015 and May 2008 to May 2011, Mr. Hatt served as our Chief Operating Officer and Vice President of Manufacturing and Operations, respectively. Previously, Mr. Hatt has held various management positions in both pharmaceutical and medical devices sectors with Bristol-Myers Squibb Company, Warner-Lambert Co. and Inamed Corp. At Inamed, he ran the international breast implant manufacturing operations. Mr. Hatt holds a Ph.D. in microbiology and a bachelor’s degree from University College, Dublin.
Vikash Agarwal has served as our Vice President of Corporate Business Development & Strategic Planning since August 2014. Prior to joining the Company, from January 2013 to August 2014, Mr. Agarwal served as head of the mergers and acquisitions group for Sun Pharmaceuticals Industries Ltd., a global specialty pharmaceutical company. Previously, Mr. Agarwal was an independent advisor in the health care industry from
97
December 2011 to December 2012 and held various positions in the investment banking department of HSBC Bank plc from April 2006 to November 2011. Mr. Agarwal holds a master’s degree in Business and Entrepreneurship from INSEAD and a bachelor’s degree from Delhi University.
Julia Marsanhas served as our Global Human Resources Director since December 2013. Prior to joining the Company, from 2006 to 2012, Ms. Marsan was the human resources director for DX Network Services, an independent mail, parcels and logistics end-to-end network operator in the United Kingdom and Ireland. Ms. Marsan holds a bachelor’s degree from the University of Leeds.
Bernardo Garduño has served as our Vice President of Sales—Latin America since January 2014. He served as Mexico Country Manager / Regional Manager of Hispanic Latin America from October 2012 to December 2013. Prior to joining the Company, from July 2005 to August 2012, Mr. Garduño held a variety of positions, most recently regional commercial director—Latin America, at Hospira Inc., a pharmaceutical and medical device company that develops and manufactures injectable pharmaceutical drugs and infusion technologies. Mr. Garduño holds a master’s degree in Regional Studies/International Political Economy from the University of Tsukuba, a post-graduate diploma in Globalization/Economic Development from Sophia University, Japan and a bachelor’s degree from Universidad de las Americas—Puebla.
Ayhan Aslan has served as our Vice President of Sales—Emerging Markets since February 2013. Prior to joining the Company, from March 2004 to March 2013, Mr. Aslan served as the international commercial director of MN Pharmaceuticals, a manufacturer of active pharmaceutical ingredients and finished dosage forms, until it was acquired by Amgen. Mr. Aslan also served on the board of directors of MN Pharmaceuticals. Mr. Aslan holds a bachelor’s degree from the University of Marmara.
Ignasi Salla has served as our Director of Sales—Europe since January 2015. Previously, from March 2011 to January 2015, Mr. Salla served as our Country Manager for Spain. Prior to joining the Company, from November 2005 to March 2011, Mr. Salla served as a sales manager for Mediform Group, an aesthetics distributor in Spain. Mr. Salla holds a bachelor’s degree from Escuela de Administración de Empresas, Barcelona.
Board of Directors
Daniel K. Turner III has been a member of the Board and its Chairman since October 2010. Mr. Turner is the managing director and a general partner at Montreux Equity Partners, a private investment company he founded in 1993. Mr. Turner currently serves on the boards of directors of moksha8 Pharmaceuticals, Inc. and has served on the boards of directors of multiple companies, including Epirus Biopharmaceuticals, Inc., Glaukos Corporation, Orexigen Therapeutics, Inc., Renal CarePartners, Inc., Transcept Pharmaceuticals, Inc. and Tobira Therapeutics, Inc. Mr. Turner holds a bachelor’s degree from California State University, Sacramento.
William McCabe has been a member of the Board since May 2008. Mr. McCabe is the executive chairman of Oyster Capital Partners, a private investment company he founded in September 2000. Mr. McCabe is also the chief executive officer of Novaerus Limited, a position he has held since September 2013. Mr. McCabe currently serves on the board of directors of Fieldaware Group Limited, Novaerus Group Limited, Novaerus UK Limited and Oyster Technologies Limited and has served on the boards of directors of Datahug Limited and Fleetmatics Group Limited. Mr. McCabe holds a bachelor’s degree from Queens University of Belfast.
Declan McKeon has been a member of the Board since April 2015. Mr. McKeon has been a consultant to PricewaterhouseCoopers (“PwC”) since 2007. From 1986 to 2007, Mr. McKeon served as a partner at PwC and held leadership positions on the audit and business advisory teams in Ireland and was an executive of the PwC Europe audit and business advisory services group. Mr. McKeon currently serves on the boards of directors of Icon plc and Ryanair plc. Mr. McKeon holds both a master’s degree in Business and Accounting and a bachelor’s degree from University College Dublin.
98
Michel Rouif, M.D. has been a member of the Board since April 2015. Dr. Rouif is an aesthetic plastic surgeon in France and has been in private practice since 1994. Dr. Rouif is also a professor at François Rabelais University in Tours and René Descartes University, in Paris, France. Dr. Rouif is Board Certified in France in Plastic, Reconstructive and Aesthetic Surgery and Microsurgery, and has a Diploma of Legal Compensation of Bodily Injuries. Dr. Rouif received a medical degree from René Descartes University, in Paris, France.
There are no family relationships among any of our directors or executive officers.
Composition of the Board
Immediately following the completion of this offering, the Board will consist of five directors, including four non-executive directors and one executive director, to serve terms that expire in three separate years in a manner similar to a “staggered” board of directors under Delaware law. Directors are elected to serve three-year terms, except that the current term of will expire at the annual general meeting in 2016, the current terms of will expire at the annual general meeting in 2017 and the current terms of will expire at the annual general meeting in 2018. A director may be re-elected to serve for an additional term an unlimited number of times. As a result of the staggered terms, not all of our directors will be elected in any given year.
Messrs. McCabe and Turner were appointed to the Board pursuant to a shareholders’ agreement, which will terminate in connection with the listing of our ordinary shares on . For a further discussion of this agreement, see “Related Party Transactions—Shareholders’ Agreement.”
The directors are appointed by the general meeting of shareholders. A director may, subject to compliance with certain Irish statutory procedures, be removed with or without cause by a resolution passed by a majority of the votes cast by those present in person or by proxy at a meeting and who are entitled to vote. The Board may also in certain circumstances appoint additional directors.
Foreign Private Issuer Status
The listing rules of , which we also refer to as the Rules, include certain accommodations in the corporate governance requirements that allow foreign private issuers, such as us, to follow “home country” corporate governance practices in lieu of the otherwise applicable corporate governance standards of . The application of such exceptions requires that we disclose each exchange requirement that we do not follow and describe the Irish corporate governance practices we do follow in lieu thereof. When our ordinary shares are listed on , we intend to continue to follow Irish corporate governance practices in lieu of the corporate governance requirements of in respect of the following:
Board Structure and Committee Composition
The Board has established, or will establish prior to the completion of this offering, an audit committee; a compensation committee; and a nominating and corporate governance committee.
Audit Committee
Upon completion of this offering, our audit committee will consist of and , who will serve as chairman of the committee, and will have at least one independent member. We anticipate that, prior to the completion of this offering, the Board will determine that meets the independence requirements under the Rules and under Rule 10A-3 under the Exchange Act. The Board has determined that is an “audit committee financial expert” within the meaning of Item 407 of Regulation S-K. The audit committee’s primary responsibilities and duties upon completion of this offering will include:
| | • | | appointing, approving the compensation of, and assessing the qualifications, performance and independence of our independent registered public accounting firm; |
99
| | • | | pre-approving audit and permissible non-audit services, and the terms of such services, to be provided by our independent registered public accounting firm; |
| | • | | reviewing the internal audit plan with the independent registered public accounting firm and members of management responsible for preparing our financial statements; |
| | • | | reviewing and discussing with management and the independent registered public accounting firm our annual and quarterly financial statements and related disclosures as well as critical accounting policies and practices used by us; |
| | • | | reviewing the adequacy of our internal control over financial reporting; |
| | • | | establishing policies and procedures for the receipt and retention of accounting-related complaints and concerns; |
| | • | | recommending, based upon the audit committee’s review and discussions with management and the advice of the independent registered public accounting firm, whether our audited financial statements shall be included in our Annual Report on Form 20-F; |
| | • | | monitoring our compliance with legal and regulatory requirements as they relate to our financial statements and accounting matters; |
| | • | | annually reviewing and reassessing the adequacy of the committee charter with regard to compliance with the listing requirements of ; |
| | • | | reviewing all related party transactions for potential conflict of interest situations and approving all permissible related party transactions; and |
| | • | | reviewing and discussing with management and our independent registered public accounting firm our earnings releases and scripts. |
Compensation Committee
Upon completion of this offering, our compensation committee will consist of and , who will serve as chairman of the committee. The Rules applicable to foreign private issuers do not require our compensation committee to be comprised entirely of independent directors. The compensation committee’s responsibilities upon completion of this offering will include reviewing and approving the compensation of our executive officers, overseeing and administering our compensation plans and reviewing and making recommendations to the Board with respect to director compensation.
Nominating and Corporate Governance Committee
In addition to the audit committee and the compensation committee, the Company will establish a nominating and corporate governance committee. The responsibilities of the Nominating and Corporate Governance Committee upon completion of this offering will include identifying individuals qualified to become members of the Board and recommending such persons to the Board to be nominated for election as directors, making recommendations to the Board regarding the composition and function of each Board committee and developing and recommending to the Board a set of corporate governance principles.
Compensation
Non-Executive Director Compensation
We will pay the reasonable costs and expenses incurred by our directors in connection with attending meetings of the Board and its committees. The aggregate compensation, including benefits in kind, we have paid or accrued for payments to our non-executive directors for board service for the year ended December 31, 2014 was €36,000.
100
Executive Officer Compensation
For the year ended December 31, 2014, the aggregate amount of the compensation and benefits paid or provided to our executive officers totaled approximately $3.6 million. We pay our executive officers in local currencies and have applied the applicable exchange rates as of December 31, 2014 to determine the aggregate amount set forth in the preceding sentence. This amount includes salaries, cash bonuses, health insurance and life insurance benefits, car, housing and certain fuel and meal allowances and fees paid to our chief executive officer in her capacity as a member of our Board.
For the year ended December 31, 2014, each of our executive officers was eligible for and received an annual bonus based 50% on the attainment of specified company targets and 50% on the attainment of specified personal goals.
For the year ended December 31, 2014, we granted options exercisable for an aggregate of 897,669 A ordinary shares to our executive officers, which have a weighted-average exercise price of €0.01 per share. These options have a variety of vesting schedules, under which a portion of the options vest on the date of the grant and the remaining options vest in equal monthly installments over the vesting term. These options all terminate on December 18, 2021. We have employment agreements with our executive officers that provide for benefits upon termination. See “—Employment Agreements.” No director, executive officer or any relative of such persons has been advanced any loans, credits or guarantees by us.
Pension Contributions
We operate pension plans for our employees in the United Kingdom and France, and we will match each employee’s contributions up to 8% and 10%, respectively, of the employee’s base salary. As of December 31, 2014, we have set aside $450,000 to fund retirement obligations with respect to our operations in France.
Share Incentive Arrangements
2010 Employee Share Option Plan
Global Consolidated Aesthetics Limited currently operates the 2010 Plan pursuant to which 2,846,200 A Ordinary Shares are reserved for issuance pursuant to the exercise of options granted under the plan.
Unless decided otherwise by the Board, any options granted under the 2010 Plan are subject to four-year vesting such that 25% of such options shall vest upon the first anniversary of the date of grant and the remaining options are subject to vesting in equal monthly installments over the following three years. Options are granted at an exercise price equal to the fair market value of the shares underlying the option, as determined by the Board.
As part of the Share-for-Share Exchange, the 2010 Plan will terminate and all existing participants will exchange the options granted to them thereunder for options of equivalent value and with equivalent exercise price and terms to be granted under the equity incentive plan to be adopted by GC Aesthetics plc as described below.
GC Aesthetics Equity Incentive Plan 2015
GC Aesthetics plc intends to adopt the GC Aesthetics Equity Incentive Plan 2015 (the “Incentive Plan”) upon the completion of the Share-for-Share Exchange. The following is a summary of certain features of the Incentive Plan.
Purpose. The purpose of the Incentive Plan is to provide long-term incentive compensation opportunities tied to our performance and the performance of our ordinary shares to our directors, consultants and employees.
101
Reservation of Shares. Subject to adjustments as described below, the maximum aggregate number of ordinary shares that may be issued pursuant to awards granted under the Incentive Plan will be . Any ordinary shares delivered under the Incentive Plan will consist of authorized and unissued shares or issued shares held by any share trust established by us for the purposes of administering the Incentive Plan.
In the event of any alteration taking place in our capital structure that does not involve receipt of consideration by us, whether by way of capitalization of profits or reserves or any consolidation or subdivision or reduction of the capital of GC Aesthetics plc or otherwise, the number of ordinary shares subject to any award, the exercise price or base price payable for each of those ordinary shares, the total number of ordinary shares over which awards may be granted and any other terms of an award that are affected by the event may be adjusted in such manner as the compensation committee determines to be fair and reasonable.
Share Counting. Awards that are required to be paid in cash pursuant to their terms will not reduce the share reserve. To the extent that an award granted under the Incentive Plan is cancelled, expired, forfeited, surrendered, repurchased, settled by delivery of fewer ordinary shares than the number underlying the award or otherwise terminated without delivery of all relevant ordinary shares to the participant, the ordinary shares retained by or returned to us or repurchased by us will become available for future awards under the Incentive Plan. Notwithstanding the foregoing, ordinary shares that are withheld or separately surrendered in payment of the exercise or purchase price or taxes relating to such an award or are not issued or delivered as a result of the net settlement of an outstanding share option or share appreciation right will not be available for future awards under the Incentive Plan and will be deducted from the share reserve accordingly.
Administration. The Incentive Plan will be administered by the compensation committee. Subject to the limitations set forth in the Incentive Plan, the compensation committee has the authority to determine the persons to whom awards are to be granted, prescribe the restrictions, terms and conditions of all awards, accelerate the vesting or exercisability of awards at any time, interpret the Incentive Plan, adopt rules for the administration, interpretation and application of the Incentive Plan and adopt such procedures and sub-plans as are necessary or appropriate to permit or facilitate participation in the plan by eligible persons employed or engaged outside Ireland.
Eligibility. Awards under the Incentive Plan may be granted to any employees, directors, consultants or professional advisers of or to us or our subsidiaries.
Share Options. The compensation committee will determine the exercise price of each option (which must, for each ordinary share, be not less than nominal value, or in the case of U.S. taxpayers, the fair market value of an ordinary share on the date of grant), the vesting and/or exercisability requirements and the term of exercise of each option, including the effect of termination of service of a participant or a change in control. The vesting requirements may be based on the continued employment or service of the participant for a specified time period or on the attainment of specified business performance goals established by the compensation committee. The maximum term of an option will be seven years from the date of grant.
To exercise an option, the participant must pay the exercise price, subject to specified conditions, (i) in cash, (ii) by cash equivalent acceptable to the compensation committee, or (iii) to the extent permitted by the compensation committee in its sole discretion:
| | • | | in ordinary shares valued at the fair market value of such shares on the date of exercise, |
| | • | | through an open-market, broker-assisted sales transaction pursuant to which we are promptly delivered the amount of proceeds necessary to satisfy the exercise price, |
| | • | | by reducing the number of ordinary shares otherwise deliverable upon the exercise of the option by the number of ordinary shares having a fair market value on the date of exercise equal to the |
102
| | exercise price (provided that the participant shall pay an amount equal to the nominal value of the shares allotted to or transferred to him or her following such reduction) in consideration for such allotment or transfer, |
| | • | | by a combination of the methods described above or |
| | • | | by such other method as may be approved by the compensation committee. |
An award agreement may provide that ordinary shares delivered upon exercise of an option may be subject to a restricted share award.
Share Appreciation Rights. A share appreciation right may be granted either in tandem with an option or without a related option. A share appreciation right entitles the participant, upon settlement or exercise, to receive a payment based on the excess of the fair market value of an ordinary share on the date of settlement or exercise over the base price of the right, multiplied by the number of ordinary shares as to which the right is being settled or exercised. The base price of a share appreciation right may not be less than the nominal value of an ordinary share, or in the case of a US taxpayer, the fair market value of an ordinary share on the date of grant. The compensation committee will determine the vesting requirements and the term of exercise of each share appreciation right, including the effect of termination of service of a participant or a change in control. The maximum term of a share appreciation right will be seven years from the date of grant. Share appreciation rights may be payable in cash or in ordinary shares valued at their fair market value on the date of exercise or payment or in a combination of both. Except as otherwise determined by the compensation committee or set forth in an award agreement, dividends may not be paid and dividend equivalent rights may not be granted with respect to the ordinary shares subject to share appreciation rights.
Restricted Share Awards. A restricted share award represents ordinary shares that are issued subject to restrictions on transfer and vesting requirements. The vesting requirements may be based on the continued service of the participant for a specified time period or on the attainment of specified performance goals or such other terms and conditions as approved by the compensation committee. Shares granted under any restricted share award may not be transferred, assigned or subject to any encumbrance, pledge or charge until all applicable restrictions are removed or have expired. Failure to satisfy any applicable restrictions shall result in the subject shares of the restricted share award being forfeited and repurchased by us or transferred to a share trust nominated by us in such manner as is set out in the award agreement. Subject to the foregoing restrictions, a participant shall have all rights of a shareholder with respect to the ordinary shares granted to the participant under a restricted share award, including the right to vote the shares and receive all dividends and other distributions paid or made with respect thereto, unless the compensation committee determines otherwise at the time the restricted share award is granted.
Restricted Share Units. An award of restricted share units (“RSUs”) provides the participant the right to receive a payment based on the value of an ordinary share. The requirements for vesting of an RSU may be based on the continued service of the participant with us for a specified time period (or periods), or on such other terms and conditions as approved by the compensation committee (including performance goal(s)) in its discretion.
RSU awards are payable in cash or in ordinary shares or in a combination of both provided that any ordinary shares issued to a participant upon vesting is paid up as to its nominal value. RSUs may be granted together with a dividend equivalent right with respect to the ordinary shares subject to the award. Dividend equivalent rights may be subject to forfeiture conditions that apply to the underlying RSUs.
Cash Performance Awards. A performance award is denominated in a cash amount (rather than in shares) and is payable based on the attainment of pre-established business and/or individual performance goals. The requirements for vesting may be also based upon the continued service of the participant during the performance period, and vesting may be accelerated in certain circumstances, as determined by the compensation committee.
103
Cash performance awards may be paid, at the discretion of the compensation committee, in any combination of cash or ordinary shares, based on the fair market value of the ordinary shares at the time of payment, provided that any ordinary shares issued in payment of an award are issued at nominal value.
Share Awards. A share award represents ordinary shares that are issued free of restrictions on transfer and free of forfeiture conditions and to which the participant is entitled all incidents of ownership. A share award may be granted for past services, in lieu of bonus or other cash compensation, directors’ fees or for any other valid purpose as determined by the compensation committee. The compensation committee will determine the terms and conditions of share awards and the issue price of the ordinary shares (being not less than nominal value) and such share awards may be made without vesting requirements. Upon the issuance of ordinary shares under a share award, the participant will have all rights of a shareholder with respect to such ordinary shares, including the right to vote the ordinary shares and receive all dividends and other distributions on the ordinary shares.
Effect of Change in Control. In the event of a “change in control” (as defined in the Incentive Plan), or if the compensation committee considers any such event may occur, the compensation committee shall be entitled (without a participant’s consent unless otherwise specified in an award agreement) at its discretion and notwithstanding any other provision contained in the Incentive Plan:
| | • | | to request any participants to exercise all outstanding awards held in relation to the whole or a specified portion of the ordinary shares to which such awards relate and in respect of which such awards have vested and are exercisable and within such time or times and subject to any other conditions or limitations as the compensation committee may at its discretion determine (including conditions that such exercise be conditional on the change in control being effected). If a participant does not comply with any such request such award shall lapse and cease to be exercisable upon the expiry of the time specified for exercise by the compensation committee; |
| | • | | to accelerate payment of any cash awards; |
| | • | | to agree that outstanding awards will be substituted by the surviving company or its parent (or the acquiring company or its parent where a takeover or other acquisition occurs) for awards that are at the date of the change in control equivalent in value and on substantially the same terms as to the awards originally granted under the Incentive Plan (as determined by the compensation committee); |
| | • | | to arrange for the continuation by us of outstanding awards (if we are the surviving company or an acquiring company in a takeover or acquisition); |
| | • | | to make payment of a cash settlement to any participant of an amount per ordinary share to which the relevant participant’s award refers equal to or in excess of the difference between the amount to be paid for one ordinary share under the terms of the change in control and the exercise price applicable to such participant’s option (less any taxes, duties, levies or other charges required to be withheld); |
| | • | | to agree to accelerate the vesting and exercisability of any outstanding awards; |
| | • | | to cancel (whether selectively or otherwise) any awards which have not vested at the time of the change in control; and/or |
| | • | | to otherwise vary the exercise of outstanding awards on such conditions as the compensation committee may decide. |
The compensation committee may determine that one or any combination of the above shall apply.
104
Forfeiture. The compensation committee may specify in an award agreement that an award will be subject to reduction, cancellation, forfeiture or recoupment upon the occurrence of certain specified events, including termination of service for “cause” (as defined in the Incentive Plan), violation of material Company policies, breach of noncompetition, confidentiality or other restrictive covenants that may apply to the participant, or other conduct by the participant that is detrimental to our business or reputation. Unless otherwise provided by the compensation committee and set forth in an award agreement, if (i) a participant’s service is terminated for “cause” or (ii) after termination of service for any other reason, the compensation committee determines in its discretion either that, (A) during the participant’s period of service, the participant engaged in an act which would have warranted termination from service for “cause” or (B) after termination, the participant engaged in conduct that violates any continuing obligation or duty of the participant in respect of us or any of our subsidiaries, such participant’s rights, payments and benefits with respect to such award may be subject to cancellation, forfeiture and/or recoupment.
Right of Recapture. If a participant receives compensation pursuant to an award based on financial statements that are subsequently required to be restated in a way that would decrease the value of such compensation, the participant will, upon our written request, forfeit and repay to us the difference between what the participant received and what the participant should have received based on the accounting restatement, in accordance with (i) any compensation recovery, “clawback” or similar policy, as may be in effect from time to time and (ii) any compensation recovery, “clawback” or similar policy made applicable by law.
Tax Withholding. Participants in the Incentive Plan are responsible for the payment of any taxes or similar charges required by law to be paid or withheld from an award or an amount paid in satisfaction of an award.
Term, Amendment and Termination. The Board may amend, modify, suspend or terminate the Incentive Plan at any time. On termination we shall not grant any further awards, but no such termination shall affect or modify any subsisting rights or obligations of the participants in respect of any existing awards and, notwithstanding such termination, we shall continue to do and perform such acts in accordance with the provisions the Incentive Plan as are necessary for or incidental to the administration and management of outstanding rights and obligations that arose under or by virtue of the Incentive Plan. The Board may at any time vary, amend or revoke any of the provisions of the Incentive Plan in such manner as the Board may consider necessary, provided that:
| | • | | the purpose of the Incentive Plan shall not be altered; |
| | • | | no such variation amendment or revocation shall increase the amount payable by any participant or otherwise impose obligations which are materially more onerous on any participant in respect of an award that has already been granted without the consent in writing of such participant; and |
| | • | | no such variation amendment or revocation shall be made without shareholder approval if (i) such approval is necessary to comply with any tax or regulatory requirement applicable to the Incentive Plan, (ii) such amendment increases the number of ordinary shares available under the Incentive Plan (other than increases permitted in respect of certain alteration of our capital structure as set out above), or (iii) such amendment results in a change in eligibility requirements under the Incentive Plan. |
Employment Agreements
Each of our executive officers has entered into written service or employment agreements with certain of our operating subsidiaries. Each of these agreements includes terms related to each executive officer’s title, salary, bonus eligibility and benefits, confidentiality of information and term of employment. Our employment agreements with these officers are filed as exhibits to the registration statement of which this prospectus forms a part.
105
RELATED PARTY TRANSACTIONS
Arrangements with Our Investors
Shareholders’ Agreement
We entered into a shareholders’ agreement with certain investors in October 2010, which was amended in February 2014 in connection with the issuance of our C Preference Shares. The shareholders’ agreement contains provisions that allow each of Montreux and Oyster to appoint two members to the Board. Messrs. McCabe, and Turner were appointed to the Board under the terms of this agreement. In connection with the listing of our ordinary shares on , this shareholders’ agreement will terminate in accordance with its terms.
Registration Rights Agreement
We have granted Montreux and OrbiMed registration rights pursuant to a registration rights agreement entered into in October 2010 and amended and restated in February 2014 in connection with the issuance of our C Preferences Shares. Six months following this offering Montreux and OrbiMed will have demand registration rights, including shelf registration rights, in respect of any ordinary shares or securities convertible into ordinary shares held by them, subject to certain conditions. In addition, in the event that we register additional ordinary shares for sale to the public following the completion of this offering, we will be required to give notice of such registration to Montreux and OrbiMed, and, subject to certain limitations, include ordinary shares held by them in such registration. The agreement includes customary indemnification provisions in favor of Montreux and OrbiMed, any person who controls Montreux or OrbiMed (within the meaning of the Exchange Act) and related parties against certain losses and liabilities (including reasonable legal expenses).
Management Agreement with Montreux
We entered into a Management Rights Agreement (the “Management Agreement”) with Montreux in October 2010 to provide us with management, consulting and financial and other advisory services. Pursuant to the Management Agreement, we have been required to pay Montreux an annual fee of $500,000, payable in advance on a quarterly basis. During 2014, 2013 and 2012, we paid Montreux $500,000 each year in management fees. In addition, we reimburse Montreux for expenses incurred by it in connection with services provided to us. Upon the listing of our ordinary shares on , the Management Agreement will terminate in accordance with its terms.
Service Agreements with Oyster
In December 2011, we entered into Service Agreements with Oyster under which Oyster agreed to provide the services of William McCabe and Martin Scully to act as non-executive consultants to GC Aesthetics Management Limited, an indirect, wholly-owned subsidiary of Global Aesthetics Consolidated Limited, and its affiliated companies. Pursuant to the Service Agreement appointing Mr. McCabe, we paid Oyster consultancy fees of €233,000 in 23 monthly installments paid between January 2012 and December 2013. The agreement terminated upon payment of the final installment. Pursuant to the Service Agreement appointing Mr. Scully, we have paid Oyster €10,000 in consultancy fees each month since January 2012. Following the completion of this offering, we may terminate the agreement at any time.
Credit Facility
The Company is party to the Credit Facility with OrbiMed. OrbiMed holds 592,042 ordinary shares and 1,451,762 C Preference Shares, representing approximately 9.3% of the Company’s outstanding equity as of April 30, 2015. See “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Indebtedness—Credit Facility” and see “Note 23 to our consolidated financial statements.”
106
Subscription Agreements
Since January 1, 2012, Global Consolidated Aesthetics Limited has entered into the following subscription agreements with shareholders:
| | • | | On July 31, 2012, a note and warrant subscription agreement pursuant to which Montreux, Oyster and certain other of its existing shareholders agreed to subscribe for interest bearing loan notes in the aggregate amount of €7,500,000 and warrants to purchase an aggregate of 40,002 B Preference Shares and 750,000 ordinary shares. The notes and warrants were issued in two tranches, on July 31, 2012 and December 14, 2012. |
| | • | | On January 29, 2013, a note and warrant subscription agreement pursuant to which Montreux and Oyster subscribed for interest bearing loan notes in the aggregate amount of €1,000,000 and warrants to purchase an aggregate of 5,333 B Preference Shares and 100,000 ordinary shares, exercisable at nominal value. |
| | • | | On April 16, 2013, a note and warrant subscription agreement pursuant to which Montreux and Oyster subscribed for interest bearing loan notes in the aggregate amount of €500,000 and warrants to purchase an aggregate of 2,667 B Preference Shares and 50,000 ordinary shares, exercisable at nominal value. |
| | • | | On May 10, 2013, a note and warrant subscription agreement pursuant to which Montreux, Oyster and certain other existing shareholders subscribed for interest bearing loan notes in the aggregate amount of €1,665,884 and warrants to purchase an aggregate of 8,884 B Preference Shares and 166,589 ordinary shares, exercisable at nominal value. The notes and warrants were issued in two tranches, on May 10, 2013 and October 24, 2013. |
| | • | | On January 20, 2014, as amended on February 19, 2014 and December 19, 2014, a subscription agreement with Montreux, Oyster and certain other shareholders pursuant to which all outstanding loan notes issued by it, together with interest thereon, were converted into B Preference Shares and additional B Preference Shares were subscribed for in cash. A total of 8,612,983 B Preference Shares were issued pursuant to this subscription agreement at a subscription price of $2.22181 per share. |
| | • | | On February 13, 2014, a subscription agreement pursuant to which 1,451,762 C Preference Shares were issued to OrbiMed at an aggregate subscription price of $6,000,000. |
| | • | | On April 27, 2015, a subscription agreement pursuant to which 592,042 ordinary shares were issued to OrbiMed at an aggregate subscription price of €5,920.42. |
Arrangements with Our Employees
Employment Agreements
See “Management—Employment Agreements.”
107
PRINCIPAL SHAREHOLDERS
As of April 30, 2015, giving effect to the Share-for-Share Exchange and the exercise of all warrants and the conversion of all our A Ordinary Shares, B Preference Shares and C Preference Shares to ordinary shares, we would have had ordinary shares outstanding. Giving effect to the Share-for-Share Exchange and the exercise of all warrants and the conversion of all our A Ordinary Shares, B Preference Shares and C Preference Shares to ordinary shares, the following table and accompanying footnotes set forth pro forma information relating to the beneficial ownership of our ordinary shares as of April 30, 2015 by:
| | • | | each person, or group of affiliated persons, known by us to beneficially own more than 5% of our outstanding ordinary shares; |
| | • | | each of our named executive officers; and |
| | • | | all directors and executive officers as a group. |
Following the Share-for-Share Exchange and the exercise of all warrants and the conversion of all our A Ordinary Shares, B Preference Shares and C Preference Shares to ordinary shares, our major shareholders will not have voting rights that are different from our shareholders in general.
108
Beneficial ownership is determined in accordance with SEC rules. In general, under these rules a beneficial owner of a security includes any person who, directly or indirectly, through any contract, arrangement, understanding, relationship or otherwise has or shares voting power or investment power with respect to such security. A person is also deemed to be a beneficial owner of a security if that person has the right to acquire beneficial ownership of such security within 60 days. Except as otherwise indicated, and subject to applicable community property laws, the persons named in the table have sole voting and investment power with respect to all ordinary shares held by that person. Ordinary shares that a person has the right to acquire within 60 days of April 30, 2015 are deemed outstanding for purposes of computing the percentage ownership of such person, but are not deemed outstanding for purposes of computing the percentage ownership of any other person, except with respect to the percentage ownership of all directors and executive officers as a group. Unless otherwise indicated below, the address for each beneficial owner listed is c/o GC Aesthetics plc, Suite 601, Q House, Furze Road, Sandyford, Dublin 18, Ireland.
| | | | | | | | | | | | | | | | |
| | | Ordinary Shares Beneficially
Owned Before the Offering | | | Ordinary Shares Beneficially
Owned After the Offering |
| | | | | | | | | Excluding Exercise of the
Underwriters’ Option to
Purchase Additional
Ordinary Shares | | Including Exercise of the
Underwriters’ Option to
Purchase Additional
Ordinary Shares |
Name of beneficial owner | | Number of
ordinary shares | | | Percentage of
ordinary
shares | | | Number of
ordinary
shares | | Percentage of
ordinary
shares | | Number of
ordinary
shares | | Percentage of
ordinary
shares |
Beneficial Owners of 5% or More of Our Ordinary Shares: | | | | | | | | | | | | | | | | |
Montreux(1) | | | 11,737,298 | | | | 49.0 | % | | | | | | | | |
Oyster Technology Investments Limited | | | 5,464,754 | | | | 22.8 | % | | | | | | | | |
Barry’s Tea Holdings | | | 1,502,723 | | | | 6.3 | % | | | | | | | | |
OrbiMed | | | 2,043,804 | | | | 8.5 | % | | | | | | | | |
Named Executive Officers and Directors: | | | | | | | | | | | | | | | | |
Ayse Kocak | | | — | | | | * | | | | | | | | | |
Ian Crosbie | | | — | | | | * | | | | | | | | | |
Barry Hatt | | | 90,836 | | | | * | | | | | | | | | |
Julia Marsan | | | 24,045 | | | | * | | | | | | | | | |
Vikash Agarwal | | | — | | | | * | | | | | | | | | |
Bernardo Garduno | | | 143,787 | | | | * | | | | | | | | | |
Ayhan Aslan | | | 139,336 | | | | * | | | | | | | | | |
Ignasi Salla | | | 35,947 | | | | * | | | | | | | | | |
Daniel K. Turner III(2) | | | 11,737,298 | | | | 49.0 | % | | | | | | | | |
William McCabe(3) | | | 5,464,754 | | | | 22.8 | % | | | | | | | | |
Declan McKeon | | | — | | | | * | | | | | | | | | |
Michel Rouif, MD | | | — | | | | * | | | | | | | | | |
All executive officers and directors as a group (12 persons) | | | 17,636,003 | | | | 73.7 | % | | | | | | | | |
| * | Represents beneficial ownership of less than one percent of our outstanding ordinary shares |
| (1) | Consists of (i) 10,594,535 ordinary shares held by Montreux Equity Partners IV, L.P. and (ii) 217,542 shares held by Montreux IV Associates, LLC. |
| (2) | Daniel K. Turner III, a director of the Company, is the managing director of Montreux Equity Partners, and by virtue of such status may be deemed to be the beneficial owner of the ordinary shares held by Montreux Equity Partners IV, L.P. and its affiliated entities. |
| (3) | William McCabe, a director of the Company, is, together with family members, the ultimate owner of Oyster Technology Investments Limited, and by virtue of such status may be deemed to be the beneficial owner of the ordinary shares held by Oyster Technology Investments Limited. |
As of the date of this prospectus, giving effect to the Share-for-Share Exchange and the exercise of all warrants and the conversion of all our A Ordinary Shares, B Preference Shares and C Preference Shares to ordinary shares, of our outstanding ordinary shares are held by record holders in the United States.
109
DESCRIPTION OF SHARE CAPITAL
The following is a summary of some of the terms of our ordinary shares, based on our memorandum and articles of association, as they will become effective upon their amendment prior to the completion of this offering, and the Irish Companies Act. In this section and the section entitled “Differences in Corporate Law,” we refer to our memorandum and articles of association as amended and in effect upon the completion of this offering as our “articles of association.”
The following summary is subject to, and is qualified in its entirety by reference to, the provisions of the memorandum and articles of association, the form of which is filed as an exhibit to the registration statement of which this prospectus is a part.
Except as otherwise specified below, references to voting by our shareholders contained in this Description of Share Capital are references to voting by holders of ordinary shares entitled to attend and vote generally at general meetings of our shareholders.
Organization
We are an Irish public company with limited liability. We were organized in Ireland on April 8, 2015 under the name GC Aesthetics Limited with registered number 559895, and we have subsequently re-registered as a public limited company with the name GC Aesthetics plc. Our affairs are governed by our memorandum and articles of association that will come into effect immediately prior to the completion of this offering and Irish law.
Objective
As provided by and described in our memorandum of association, our principal objective is to carry on the business of a holding company and all associated related activities and to carry on various activities associated with that objective.
Share Capital
Immediately after the completion of this offering, our authorized share capital will be € , divided into ordinary shares with a nominal value of $ per ordinary share. Upon the completion of this offering and the use of proceeds therefrom, we expect to have of our ordinary shares outstanding and no outstanding shares of any other class.
Transfer and Registration of Shares
Our share register is maintained by our transfer agent. Registration in this share register will be determinative of membership in us. Any of our shareholders who hold ordinary shares beneficially will not be the holder of record of such ordinary shares. Instead, the depository or other nominee will be the holder of record of such shares. Accordingly, a transfer of ordinary shares from a person who holds such ordinary shares beneficially to a person who will also hold such ordinary shares beneficially through the same depository or other nominee will not be registered in our official share register, as the depository or other nominee will remain the holder of record of such ordinary shares.
A written instrument of transfer will be required under Irish law in order to register on our official share register any transfer of ordinary shares (i) from a person who holds such ordinary shares directly to any other person or (ii) from a person who holds such ordinary shares beneficially to another person who also will hold such ordinary shares beneficially where the transfer involves a change in the depository or other nominee that is the record owner of the transferred ordinary shares. An instrument of transfer will be required for a shareholder who directly holds ordinary shares to transfer those ordinary shares into his or her own broker account (or vice versa). Such instruments of transfer may give rise to Irish stamp duty, which must be paid prior to registration of the transfer on our official Irish share register. However, a shareholder who directly holds ordinary shares may
110
transfer those ordinary shares into his or her own broker account (or vice versa) without giving rise to Irish stamp duty, provided that there is no change in the ultimate beneficial ownership of the ordinary shares as a result of the transfer or the transfer is not made in contemplation of a sale of the ordinary shares.
Accordingly, we strongly recommend that shareholders hold their shares through DTC (or through a broker who holds such shares through DTC).
Any transfer of our ordinary shares that is subject to Irish stamp duty will not be registered in the name of the buyer unless such stamp duty is paid and details of the transfer are provided to our transfer agent. We do not expect to pay any stamp duty on behalf of any acquirer of ordinary shares in our capital. See “Taxation—Material Irish Tax Considerations.” The Company may, in its absolute discretion, pay (or cause one of its affiliates to pay) any stamp duty.
Our articles of association provide that, in the event of any such payment, we (i) may seek reimbursement from the transferor or transferee (at our discretion), (ii) may set-off the amount of the stamp duty against future dividends payable to the transferor or transferee (at our discretion) and (iii) will have a lien against the Company’s shares in respect of which we have paid stamp duty. Our articles of association grant the Board general discretion to decline to register an instrument of transfer without giving a reason. In addition, the Board may decline to register a transfer of shares unless a registration statement under the Securities Act is in effect with respect to the transfer or the transfer is exempt from registration. See “—Anti-Takeover Provisions—Shareholder Rights Plans and Share Issuances.”
The registration of transfers may be suspended at such times and for such periods, not exceeding 30 days in any year, as the Board may from time to time determine (except as may be required by law).
Issuance of Shares
We have the authority, pursuant to our articles of association, to increase our authorized but unissued share capital by ordinary resolution by creating additional shares of any class or series. An ordinary resolution of our company requires more than 50% of the votes cast at an annual general meeting by shareholders entitled to vote at that meeting. As a matter of Irish law, the board of directors of a company may issue authorized but unissued new shares without shareholder approval once authorized to do so by the articles of association of the company or by an ordinary resolution adopted by the shareholders at a general meeting. The authority conferred can be granted for a maximum period of five years, at which point it must be renewed by the shareholders by an ordinary resolution. Because of this requirement of Irish law, our articles of association authorize the Board to issue new shares up to the amount of our authorized but unissued share capital without shareholder approval for a period of five years from the date our articles of association were adopted in substantially the form attached as an exhibit to the registration statement of which this prospectus forms a part. We expect that we will seek to renew such general authority at an annual general meeting before the end of that five-year period. Our articles of association authorize our Board, without shareholder approval, to determine the terms of the undesignated shares issued by us and, subject to the relevant provisions of our articles of association, the terms of any class of preferred shares or deferred shares. The Board is authorized, without obtaining any shareholder vote or consent, to provide from time to time for the issuance of ordinary shares or other classes or series of shares and to establish the characteristics of each such other class or series, including the number of shares and their preferred or deferred or other special rights and privileges or limitations, conditions and restrictions, whether in regard to dividend, voting, return of capital, conversion, redemption or otherwise.
No Share Certificates
We do not intend to issue share certificates unless (i) certificates are required by law, any stock exchange, a recognized depository, any operator of any clearance or settlement system or the terms of issue of any class or series of our shares or (ii) a holder of our ordinary shares applies for share certificates evidencing ownership of our shares.
111
Under our articles of association, holders of our ordinary shares will have no right to certificates for their ordinary shares, except on request and on such terms as the Board, at its sole discretion, determines.
Holders’ rights to request certificates for ordinary shares are subject to any resolution of the Board determining otherwise.
No Sinking Fund
Our ordinary shares will have no sinking fund provisions.
No Liability for Further Calls or Assessments
The ordinary shares to be sold in this offering are duly and validly issued, will be credited as fully paid up and will be non-assessable.
Pre-emption Rights, Share Warrants and Share Options
Under Irish law, certain statutory pre-emption rights apply automatically in favor of our ordinary shareholders when our ordinary shares are issued for cash. However, we have opted out of these pre-emption rights in our articles of association as permitted under Irish law. This opt-out may be renewed every five years under Irish law by a special resolution of the shareholders. A special resolution requires not less than 75% of the votes cast by our shareholders at a meeting of shareholders. We expect that we will seek renewal of the opt-out at an annual general meeting within five years from the date on which our articles of association were adopted in substantially the form attached as an exhibit to the registration statement of which this prospectus forms a part. If the opt-out expires and is not renewed, ordinary shares issued for cash must be offered to our pre-existing ordinary shareholders pro rata based on their existing shareholding before the ordinary shares can be issued to any new shareholders or pre-existing shareholders in an amount greater than their pro rata entitlements. The statutory pre-emption rights:
| | • | | generally do not apply where shares are issued for non-cash consideration; |
| | • | | do not apply to the issuance of non-equity shares (that is, shares that have the right to participate only up to a specified amount in any dividend and capital distribution, which are sometimes referred to as non-participating shares); and |
| | • | | do not apply to the issuance of shares pursuant to certain employee compensation plans, including the GC Aesthetics Equity Incentive Plan 2015. |
The Irish Companies Act provides that directors may issue share warrants or options without shareholder approval once authorized to do so by the articles of association or an ordinary resolution of shareholders. This authority can be granted for a maximum period of five years, after which it must be renewed by the shareholders by an ordinary resolution. Our articles of association provide that the Board is authorized to grant, upon such terms as the Board deems advisable, options to purchase (or commitments to issue at a future date) our shares of any class or series, and to cause warrants or other appropriate instruments evidencing such options or commitments to be issued. This authority under the articles will lapse after five years from the date our articles of association were adopted in substantially the form attached as an exhibit to the registration statement of which this prospectus forms a part. We expect that we will seek renewal of this authority at an annual general meeting before the end of that five-year period. Under the same authority, the Board may issue ordinary shares upon exercise of warrants or options or other commitments without shareholder approval or authorization (up to the relevant authorized but unissued share capital). Statutory pre-emption rights will apply to the issuance of warrants and options issued by us unless an opt-out applies or shareholder approval for an opt-out is obtained in the same manner described directly above for our ordinary shares. We are subject to rules requiring shareholder approval of certain ordinary share issuances. The Irish Takeover Rules may be applicable in certain circumstances and can impact on our ability to issue ordinary shares. See “Risk Factors—Risks Related to this Offering and Ownership of our Ordinary Shares.”
112
Share Repurchases and Redemptions
Overview
Our articles of association provide that any ordinary share the Company has agreed to acquire shall be deemed to be a redeemable share. Accordingly, for Irish law purposes, the repurchase of ordinary shares by the Company may technically be effected as a redemption of those ordinary shares as described below under “Repurchases and Redemptions.” If our articles of association did not contain such provisions, repurchases by the Company would be subject to many of the same rules that apply to purchases of our ordinary shares by subsidiaries described below under “Purchases by Subsidiaries,” including the shareholder approval requirements described below. Except where otherwise noted, when we refer elsewhere in this prospectus to repurchasing or buying back our ordinary shares, we are referring to the redemption of ordinary shares by the Company pursuant to the articles of association or the purchase of our ordinary shares by a subsidiary of the Company, in each case in accordance with our articles of association and Irish law as described below.
Repurchases and Redemptions
Under Irish law, a company can issue redeemable shares and redeem them out of distributable reserves (which are described below under “Dividends”) or the proceeds of a new issue of shares for that purpose. The redemption of redeemable shares may only be made by a public limited company where the nominal value of the issued share capital that is not redeemable is not less than 10% of the nominal value of the total issued share capital of the company. All redeemable shares must also be fully paid and the terms of redemption of the shares must provide for payment on redemption. Redeemable shares may, upon redemption, be cancelled or held in treasury. Shareholder approval will not be required to redeem the Company’s shares.
The Board will also be entitled to issue other classes or series of shares that may be redeemed at the option of either the Company or the shareholder, depending on the terms of such shares. See “Capital Structure—Authorized Share Capital.” Repurchased and redeemed shares may be cancelled or held as treasury shares. The nominal value of treasury shares held by the Company at any time must not exceed 10% of the nominal value of the issued share capital of the Company. While the Company holds shares as treasury shares, it cannot exercise any voting rights in respect of those shares. Treasury shares may be cancelled by the Company or re-issued subject to certain conditions.
Purchases by Subsidiaries
Under Irish law, it may be permissible for an Irish or non-Irish subsidiary to purchase shares of the company. A general authority of the shareholders of the company is required to allow a subsidiary of the company to make on-market purchases of the company’s shares; however, as long as this general authority has been granted, no specific shareholder authority for a particular on-market purchase by a subsidiary of the company’s shares is required. A company may elect to seek such general authority, which must expire no later than 18 months after the date on which it was granted, at the first annual general meeting of a company and at subsequent annual general meetings. For an off-market purchase by a subsidiary of a company, the proposed purchase contract must be authorized by special resolution of the shareholders of the company before the contract is entered into. The person whose shares are to be bought back cannot vote in favor of the special resolution and, for at least 21 days prior to the special resolution, the purchase contract must be on display or must be available for inspection by shareholders at the registered office of the company.
The number of shares held by the subsidiaries of the company at any time will count as treasury shares and will be included in any calculation of the permitted treasury share threshold of 10% of the nominal value of the issued share capital of the company. While a subsidiary holds shares of the company, it cannot exercise any voting rights in respect of those shares. The acquisition of the shares of the company by a subsidiary must be funded out of distributable reserves of the subsidiary.
113
Dividends
Under Irish law, dividends and distributions may only be made from distributable reserves. Distributable reserves, broadly, means the accumulated realized profits of a company, less accumulated realized losses of the company on a standalone basis. In addition, no dividend or distribution may be made unless the net assets of a company are not less than the aggregate of the company’s called up share capital plus undistributable reserves and the distribution does not reduce the company’s net assets below such aggregate. Undistributable reserves include a company’s undenominated capital (effectively its share premium and capital redemption reserve) and the amount by which the company’s accumulated unrealized profits, so far as not previously utilized by any capitalization, exceed the company’s accumulated unrealized losses, so far as not previously written off in a reduction or reorganization of capital. The determination as to whether or not the company has sufficient distributable reserves to fund a dividend must be made by reference to “relevant accounts” of the company. The “relevant accounts” are either the last set of unconsolidated annual audited financial statements or unaudited financial statements prepared in accordance with the Irish Companies Act, which give a “true and fair view” of the company’s unconsolidated financial position in accordance with accepted accounting practice in Ireland. These “relevant accounts” must be filed in the Companies Registration Office (the official public registry for companies in Ireland). The Company’s articles of association authorize the Board to declare such dividends as appear justified from the profits of the company without the approval of the shareholders. The dividends can be declared and paid in the form of cash or non-cash assets, subject to applicable law. The Company may pay dividends in any currency but, if it elects to pay dividends, intends to do so in US dollars. The Board may deduct from any dividend or other moneys payable to any shareholder all sums of money, if any, due from the shareholder to the company in respect of ordinary shares of the Company. The Board is also authorized to issue shares in the future with preferred rights to participate in dividends declared by the Company. The holders of such preference shares may, depending on their terms, rank senior to the holders of the ordinary shares of the company with respect to dividends.
For information about the Irish tax considerations relating to dividend payments, see “Taxation—Material Irish Tax Considerations.”
Bonus Shares
Under our articles of association, upon the recommendation of the Board, the shareholders by ordinary resolution may authorize the Board to capitalize any amount credited to our undenominated capital, any of our profits available for distribution or any amount representing unrealized revaluation reserves, and use such amount for the issuance to shareholders of shares as fully paid bonus shares.
Consolidation and Division; Subdivision
Under our articles of association, we may, by ordinary resolution, divide any or all of our share capital into shares of smaller nominal value than its existing shares (often referred to as a stock split) or consolidate any or all of our share capital into shares of larger nominal value than its existing shares (often referred to as a reverse stock split).
Reduction of Share Capital
We may, by ordinary resolution, reduce our authorized but unissued share capital. We also may, by special resolution and subject to confirmation by the Irish High Court, reduce our issued share capital, and any undenominated share capital.
General Meetings of Shareholders
We are required under Irish law to hold an annual general meeting at intervals of no more than 15 months, provided that an annual general meeting is held in each calendar year and no more than nine months after our fiscal year-end. Any annual general meeting may be held outside Ireland, provided that technological
114
means are provided to enable shareholders to participate in the meeting without leaving Ireland. Our articles of association include a provision requiring annual general meetings to be held within such time periods as required by Irish law.
The only matters that must, as a matter of Irish law, be transacted at an annual general meeting are the presentation of the annual profit and loss account, balance sheet and reports of the directors and auditors, the appointment of auditors and the fixing of the auditor’s fees (or delegation of same). If no resolution is made in respect of the reappointment of an auditor at an annual general meeting, the previous auditor will be deemed to have continued in office, subject to certain limited exceptions. Our articles of association provide that, at each annual general meeting, directors will be elected to fill the board seats of those directors whose terms expire at that annual general meeting. At any annual general meeting, only such business may be conducted as has been brought before the meeting (i) by or at the direction of the Board, (ii) in certain circumstances, at the direction of the Irish High Court, (iii) as required by law or (iv) such business that the chairman of the meeting determines is properly within the scope of the meeting. In addition, shareholders entitled to vote at an annual general meeting may make nominations of candidates for election to the Board.
Our extraordinary general meetings may be convened (i) by the Board, (ii) on requisition of the shareholders holding the number of our shares prescribed by the Irish Companies Act (currently 10% of the paid-up share capital of the Company carrying voting rights), or (iii) in certain circumstances, on requisition of our auditors.
Extraordinary general meetings are generally held for the purposes of approving such of our shareholder resolutions as may be required from time to time. The business to be conducted at any extraordinary general meeting must be set forth in the notice of the meeting.
In the case of an extraordinary general meeting requisitioned by our shareholders, the proposed purpose of the meeting must be set out in the requisition notice of the meeting. The requisition notice can propose any business to be considered at the meeting. Under Irish law, upon receipt of this requisition notice, the Board has 21 days to convene the extraordinary general meeting of our shareholders to vote on the matters set out in the requisition notice. This meeting must be held within two months of receipt of the requisition notice. If the Board does not proceed to convene the meeting within such 21-day period, the requisitioning shareholders, or any of them representing more than one-half of the total voting rights of all of them, may themselves convene a meeting, which meeting must be held within three months of the receipt of the requisition notice by the Board.
If the Board becomes aware that our net assets are half or less of the amount of our called up share capital, the Board must, not later than 28 days from the date that it learns of this fact, convene an extraordinary general meeting of our shareholders to be held not later than 56 days from such date.
This meeting must be convened for the purposes of considering whether any, and if so what, measures should be taken to address the situation.
At least 21 days’ notice of any annual general meeting or general meeting at which a special resolution is proposed and 14 days in all other circumstances must be given to shareholders, each director and our auditors, under our articles of association.
Quorum for Shareholder Meetings
Under our articles of association, the presence, in person or by proxy, of one or more shareholders holding at least 50% of the voting power of our issued shares that carry the right to vote at the meeting constitutes a quorum for the conduct of any business at a general meeting.
In the case of a meeting to vary the rights of any class or series of shares, discussed below under “—Voting—Variation of Rights Attaching to a Class or Series of Shares,” Irish law provides that the necessary
115
quorum is the presence, in person or by proxy, of at least two shareholders representing 1/3 in nominal value (or, at an adjourned meeting, at least one shareholder representing any amount of nominal value) of the relevant class.
Voting
Generally
Holders of our ordinary shares vote on all matters submitted to a vote of shareholders and are entitled to one vote per share.
All votes at a general meeting will be decided by way of a poll. Voting rights on a poll may be exercised by shareholders registered in our share register as of the record date for the meeting or by a duly appointed proxy of such a registered shareholder, which proxy need not be a shareholder. All proxies must be appointed in accordance with our articles of association. Our articles of association provide that the Board may permit the appointment of proxies by the shareholders to be notified to us electronically.
In accordance with our articles of association, the Board may, from time to time, cause us to issue preference or any other class or series of shares. These shares may have such voting rights, if any, as may be specified in the terms of such shares (e.g., they may carry more votes per share than ordinary shares or may entitle their holders to a class vote on such matters as may be specified in the terms of the shares).
Treasury shares (i.e., shares held by us) and our shares held by our subsidiaries will not entitle their holders to vote at general meetings of shareholders.
Except where a greater majority is required by Irish law or our articles of association, any question proposed for consideration at any of our general meetings or of any class of shareholders will be decided by an ordinary resolution passed by a simple majority of the votes cast by shareholders entitled to vote at such meeting.
Irish law requires special resolutions of the shareholders at a general meeting to approve certain matters. A special resolution requires not less than 75% of the votes cast by shareholders at a meeting of shareholders.
Examples of matters requiring special resolutions include:
| | • | | amending our objects as contained in our memorandum of association; |
| | • | | amending our articles of association; |
| | • | | approving a change of name; |
| | • | | authorizing the entry into a guarantee or the granting of security in connection with a loan, quasi loan or credit transaction in favor of a director or connected person of a director (which generally includes a family member or business partner of the director and any entity controlled by the director); |
| | • | | opting out of pre-emption rights on the issuance of new shares; |
| | • | | re-registering from a public limited company to a private company; |
| | • | | purchasing of our own shares off-market; |
| | • | | reducing issued share capital; |
116
| | • | | resolving that we be wound up by the Irish courts; |
| | • | | resolving in favor of a shareholders’ voluntary winding-up; |
| | • | | re-designating shares into different share classes; |
| | • | | setting the re-issue price of treasury shares; and |
| | • | | merging with other Irish companies or with companies incorporated in the EEA, as described below under “—Acquisitions.” |
Action by Written Consent
Our articles of association provide that anything that may be done by resolution at a general meeting may be done by resolution in writing, but only if it is signed by or on behalf of all of the shareholders who would be entitled to attend the relevant meeting and vote on the relevant resolution.
Variation of Rights Attaching to a Class or Series of Shares
Variation of any rights attached to any class or series of our issued shares (including our ordinary shares) must, in accordance with our articles of association, be approved by (i) a resolution of the shareholders of the class or series affected, passed by the affirmative vote of the holders of 2/3 of the shares of that class or series voted at a meeting of that class or series or (ii) the written consent of all of the shareholders of that class or series. In the case of a meeting to vary the rights of any class or series of shares, Irish law provides that the necessary quorum is the presence, in person or by proxy, of at least two shareholders representing 1/3 in nominal value (or, at an adjourned meeting, at least one shareholder representing any amount of nominal value) of the relevant class. Every shareholder of the affected class or series will have one vote for each share of such class or series that he or she holds as of the record date for the meeting.
Record Dates
Our articles of association provide that the Board may set the record date for the purposes of determining which shareholders are entitled to notice of, or to vote at, a general meeting and the record date must not occur before the date on which the board resolution fixing such record date is adopted. If no record date is fixed by the Board, the record date will be the day on which the notice of the meeting is mailed.
Shareholder Proposals
Under Irish law, there is no general right for a shareholder to put items on the agenda of an annual general meeting other than as set out in the articles of association of a company. Our articles of association provide that shareholders may nominate persons to be elected as directors both at an annual general meeting or an extraordinary general meeting requisitioned by shareholders.
Shareholders’ Suits
In Ireland, the decision to institute proceedings on behalf of a company is generally taken by the company’s board of directors. In certain limited circumstances, a shareholder may be entitled to bring a derivative action on our behalf. The central question at issue in deciding whether a minority shareholder may be permitted to bring a derivative action is whether, unless the action is brought, a wrong committed against us would otherwise go unredressed. The cause of action may be against a director, another person or both.
A shareholder may also bring proceedings against us in his or her own name where the shareholder’s rights as such have been infringed or where our affairs are being conducted, or the powers of the Board are being
117
exercised, in a manner oppressive to any shareholder or shareholders or in disregard of their interests as shareholders. Oppression connotes conduct that is burdensome, harsh or wrong. This is an Irish statutory remedy under Section 212 of the Irish Companies Act and the court can grant any order it sees fit, including providing for the purchase or transfer of the shares of any shareholder.
Inspection of Books and Records
Holders of ordinary shares carrying voting rights have certain rights under the Irish Companies Act to inspect books and records, including the right to: (i) receive a copy of our memorandum and articles of association and any act of the Irish Parliament that alters our memorandum and articles of association; (ii) inspect and obtain copies of the minutes of general meetings of shareholders (including resolutions adopted at such meetings); (iii) inspect and receive a copy of the register of shareholders, register of directors and secretaries, register of directors’ interests and other statutory registers maintained by us; (iv) receive copies of the most recent balance sheets and directors’ and auditors’ reports which have previously been sent to shareholders prior to an annual general meeting; and (v) receive balance sheets of any of our subsidiary companies that have previously been sent to shareholders prior to an annual general meeting for the preceding ten years. Our auditors also have the right to inspect all of our books and records. The auditors’ report must be circulated to the shareholders with our Financial Statements (as defined below) at least 21 days before the annual general meeting, and such report must be read to the shareholders at our annual general meeting. The Financial Statements referenced above mean our balance sheet, profit and loss account and, so far as they are not incorporated in the balance sheet or profit and loss account, any group accounts and the directors’ and auditors’ reports, together with any other document required by law to be annexed to the balance sheet.
Acquisitions
There are a number of mechanisms for acquiring an Irish public limited company, including:
| | • | | a court-approved scheme of arrangement under the Irish Companies Act. A scheme of arrangement with one or more classes of shareholders requires a court order from the Irish High Court and the approval of: (i) more than 50% in number of the shareholders of each participating class or series voting on the scheme of arrangement, or (ii) representing 75% or more by value of the shares of such participating class or series held by the shareholders voting on the scheme of arrangement, in each case at the relevant meeting or meetings. A scheme of arrangement, if authorized by the shareholders of each participating class or series and the court, is binding on all of the shareholders of each participating class or series. Shares held by the acquiring party are not excluded from the tally of a vote on the scheme, but such shares may be considered to belong to a separate class for the purposes of approving the scheme, in which case the acquiring party’s shares would not be voted for the purposes of the separate class approval required from the remaining, non-acquiring shareholders; |
| | • | | through a tender offer by a third party pursuant to the Irish Takeover Rules. Where the holders of 80% or more in value of a class of our shares (excluding any shares already beneficially owned by the offeror) have accepted an offer for their shares, the remaining shareholders in that class may be statutorily required to also transfer their shares, unless, within one month, the non-tendering shareholders can obtain an Irish court order otherwise providing. If the offeror has acquired acceptances of 80% of all of our shares but does not exercise this “squeeze out” right, the non-accepting shareholders also have a statutory right to require the offeror to acquire their shares on the same terms as the original offer, or such other terms as the offeror and the non-tendering shareholders may agree or on such terms as an Irish court, on application of the offeror or non-tendering shareholder, may order. If our shares were listed on the Irish Stock Exchange or another regulated stock exchange in the European Union, this 80% threshold would be increased to 90%; and |
118
| | • | | by way of a merger with a company incorporated in the EEA under the European Communities (Cross-Border Mergers) Regulations 2008, which implement the EU Cross Border Merger Directive 2005/56 in Ireland or with another Irish company under the Irish Companies Act. Such a merger must be approved by a special resolution. Shareholders also may be entitled to have their shares acquired for cash. See “—Appraisal Rights.” |
The approval of the Board, but not shareholder approval, is required for a sale, lease or exchange of all or substantially all of our assets, except that such a transaction between us and one of our directors or a person or entity connected to such a director may require shareholder approval.
Appraisal Rights
Generally, under Irish law, shareholders of an Irish company do not have statutory appraisal rights. If we are being merged as the transferor company with another EEA company under the European Communities (Cross-Border Mergers) Regulations 2008 or if we are being merged with another Irish company under the Irish Companies Act, (i) any of our shareholders who voted against the special resolution approving the merger or (ii) if 90% of our shares are held by the successor company, any other of our shareholder, may be entitled to require that the successor company acquire its shares for cash.
Disclosure of Interests in Shares
Under the Irish Companies Act, our shareholders must notify us if, as a result of a transaction, (i) the shareholder will be interested in our ordinary shares that carry voting rights or (ii) the shareholder will cease to be interested in our ordinary shares that carry voting rights. In addition, where a shareholder is interested in our relevant ordinary shares, the shareholder must notify us of any alteration of its interest that brings its total holding, whether an increase or a reduction. All such disclosures must be notified to us within two days of the event that gave rise to the requirement to notify. Where a person fails to comply with the notification requirements described above, no right or interest of any kind whatsoever in respect of any of our ordinary shares held by such person will be enforceable by such person, whether directly or indirectly, by action or legal proceeding. However, such person may apply to the Irish High Court to have the rights attaching to its ordinary shares reinstated. In addition to the disclosure requirement described above, under the Irish Companies Act, we may, by notice in writing, and must, on the requisition of shareholders holding 10% or more of the paid up capital of the Company carrying voting rights, require a person whom we know or have reasonable cause to believe is, or at any time during the three years immediately preceding the date on which such notice is issued was, interested in shares comprised in our relevant share capital to: (i) indicate whether or not it is the case and (ii) where such person holds or has during that time held an interest in our ordinary shares, to give certain further information as may be required by us including particulars of such person or beneficial owner’s past or present interests in our ordinary shares.
Any information given in response to the notice is required to be given in writing within such reasonable time as may be specified in the notice.
Where such a notice is served by us on a person who is or was interested in our ordinary shares and that person fails to give us any information required within the reasonable time specified, we may apply to court for an order directing that the affected ordinary shares be subject to certain restrictions. Under the Irish Companies Act, the restrictions that may be placed on the ordinary shares by the court are as follows:
| | • | | any transfer of those ordinary shares or, in the case of unissued shares, any transfer of the right to be issued with ordinary shares and any issue of such ordinary shares, shall be void; |
| | • | | no voting rights shall be exercisable in respect of those ordinary shares; |
| | • | | no further shares shall be issued in respect of those ordinary shares or in pursuance of any offer made to the holder of those ordinary shares; and |
119
| | • | | no payment shall be made of any sums due from us on those ordinary shares, whether in respect of capital or otherwise. |
Where our ordinary shares are subject to these restrictions, the court may order the ordinary shares to be sold and may also direct that the ordinary shares shall cease to be subject to these restrictions.
In addition, persons or groups (within the meaning of the Exchange Act) beneficially owning 5% or more of our ordinary shares must comply with the reporting requirements under Section 13 of the Exchange Act.
Anti-Takeover Provisions
Shareholder Rights Plans and Share Issuances
Irish law does not expressly prohibit companies from issuing share purchase rights or adopting a shareholder rights plan as an anti-takeover measure. However, there is no directly relevant case law on the validity of such plans under Irish law.
Our articles of association allow the Board to adopt any shareholder rights plan upon such terms and conditions as the Board deems expedient and in our best interest, subject to applicable law, including the Irish Takeover Rules and Substantial Acquisition Rules described below and the requirement for shareholder authorization for the issue of shares described above.
Subject to the Irish Takeover Rules described below and the Irish Companies Act, the Board also has the power to issue any of our authorized and unissued shares on such terms and conditions as it may determine to be in our best interest. It is possible that the terms and conditions of any issue of shares could discourage a takeover or other transaction that holders of some or a majority of our ordinary shares might believe to be in their best interest or in which holders of our ordinary shares might receive a premium for their shares over the then-market price of the shares.
Irish Takeover Rules and Substantial Acquisition Rules
A tender offer by which a third party makes an offer generally to our shareholders or a class of shareholders to acquire shares of any class conferring voting rights will be governed by the Irish Takeover Panel Act 1997 and the Irish Takeover Rules made thereunder and will be regulated by the Irish Takeover Panel (as well as being governed by the Exchange Act and the regulations promulgated thereunder). The “General Principles” of the Irish Takeover Rules and certain important aspects of the Irish Takeover Rules are described below. Takeovers by means of a scheme of arrangement are also generally subject to these regulations.
General Principles. The Irish Takeover Rules are based on the following General Principles that will apply to any transaction regulated by the Irish Takeover Panel:
| | • | | in the event of an offer, all classes of shareholders of the target company should be afforded equivalent treatment and, if a person acquires control of a company, the other holders of securities must be protected; |
| | • | | the holders of securities in the target company must have sufficient time and information to allow them to make an informed decision regarding the offer. If the board of directors of the target company advises the holders of the securities with respect to the offer, it must advise on the effects of the implementation of the offer on employment, employment conditions and the locations of the target company’s places of business; |
| | • | | the board of a target company must act in the interests of the company as a whole and must not deny the holders of securities the opportunity to decide on the merits of the offer; |
120
| | • | | false markets must not be created in the securities of the target company or any other company concerned by the offer in such a way that the rise or fall of the prices of the securities becomes artificial and the normal functioning of the markets is distorted; |
| | • | | an offeror can only announce an offer after ensuring that it can fulfill in full any cash consideration offered, and after taking all reasonable measures to secure the implementation of any other type of consideration; |
| | • | | a target company may not be hindered in the conduct of its affairs for longer than is reasonable by an offer for its securities. This is a recognition that an offer will disrupt the day-to-day running of a target company, particularly if the offer is hostile and the board of the target company must divert its attention to resist the offer; and |
| | • | | a “substantial acquisition” of securities (whether such acquisition is to be effected by one transaction or a series of transactions) will only be allowed to take place at an acceptable speed and shall be subject to adequate and timely disclosure. |
Mandatory Offer. If an acquisition of shares were to increase the aggregate holding of an acquirer and its concert parties (which generally mean persons acting in concert with the acquirer) to shares carrying 30% or more of the voting rights in our shares, the acquirer and, depending on the circumstances, its concert parties would be mandatorily required (except with the consent of the Irish Takeover Panel) to make a cash tender offer for the remaining outstanding shares at a price not less than the highest price paid for the shares by the acquirer or its concert parties during the previous twelve months.
This requirement would also be triggered by an acquisition of shares by a person holding (together with its concert parties) shares carrying between 30% and 50% of the voting rights in us if the effect of such acquisition were to increase the percentage of the voting rights held by that person (together with its concert parties) by 0.05% within a twelve month period.
Voluntary Offer; Requirements to Make a Cash Offer and Minimum Price Requirements. A voluntary offer is a tender offer that is not a mandatory offer. If an offeror or any of its concert parties acquires any of our shares of the same class as the shares that are the subject of the voluntary offer within the period of three months prior to the commencement of the offer period, the offer price must be not less than the highest price paid for our shares of that class by the offeror or its concert parties during that period. The Irish Takeover Panel has the power to extend the “look back” period to twelve months if the Panel, having regard to the General Principles, believes it is appropriate to do so.
If the offeror or any of its concert parties has acquired our shares of the same class as the shares that are the subject of the voluntary offer (i) during the period of twelve months prior to the commencement of the offer period which represent 10% or more of the nominal value of the issued shares of that class or (ii) at any time after the commencement of the offer period, the offer shall be in cash (or accompanied by a full cash alternative) and the price per share shall be not less than the highest price paid by the offeror or its concert parties for shares (of that class) during, in the case of (i), the period of twelve months prior to the commencement of the offer period and, in the case of (ii), the offer period. The Irish Takeover Panel may apply this rule to an offeror who, together with its concert parties, has acquired less than 10% of the nominal value of the issued shares of the class of shares that is the subject of the offer in the twelve-month period prior to the commencement of the offer period if the Panel, having regard to the General Principles, considers it just and proper to do so.
An offer period will generally commence from the date of the first announcement of an offer or proposed offer.
Substantial Acquisition Rules. The Irish Takeover Rules also contain rules governing substantial acquisitions of shares which restrict the speed at which a person may increase his or her holding of shares and rights over shares to an aggregate of between 15% and 30% of the voting rights in our shares. Except in certain
121
circumstances, an acquisition or series of acquisitions of shares or rights over shares representing 10% or more of the voting rights in our shares is prohibited, if such acquisition(s), when aggregated with shares or rights already held, would result in the acquirer holding 15% or more but less than 30% of the voting rights in our shares and such acquisitions are made within a period of seven days. These rules also require accelerated disclosure of certain other acquisitions of shares or rights over shares relating to such holdings.
Frustrating Action. Under the Irish Takeover Rules, the Board is not permitted to take any action that might frustrate an offer for our shares during the course of an offer or at any earlier time at which the Board has reason to believe an offer is or may be imminent, except as noted below. Potentially frustrating actions such as (i) the issue of shares, options or convertible securities, (ii) material disposals, (iii) entering into contracts other than in the ordinary course of business or (iv) any action, other than seeking alternative offers, which may result in the frustration of an offer, are prohibited during the course of an offer or at any time during which the Board has reason to believe that an offer is or may be imminent. Exceptions to this prohibition are available where:
| | • | | the action is approved by our shareholders at a general meeting; or |
| | • | | with the consent of the Irish Takeover Panel, where: |
| | • | | the Irish Takeover Panel is satisfied that the action would not constitute a frustrating action; |
| | • | | the holders of at least 50% of the voting rights state in writing that they approve the proposed action and would vote in favor of it at a general meeting; |
| | • | | the action is in accordance with a contract entered into prior to the announcement of the offer (or prior to a time at which the Board has reason to believe that an offer is or may be imminent); or |
| | • | | the decision to take such action was made before the announcement of the offer (or prior to a time at which the Board has reason to believe that an offer is or may be imminent) and has been either at least partially implemented or is in the ordinary course of business. |
Insider Dealing. The Irish Takeover Rules also provide that no person, other than the offeror, who is privy to confidential price-sensitive information concerning an offer made in respect the acquisition of the company (or a class of its securities) or a contemplated offer shall deal in relevant securities of the offeree during the period from the time at which such person first has reason to suppose that such an offer, or an approach with a view to such an offer being made, is contemplated to the time of (i) the announcement of such offer or approach or (ii) the termination of discussions relating to such offer, whichever is earlier.
For other provisions that could be considered to have an anti-takeover effect, see “—Transfer and Registration of Shares,” “—Pre-emption Rights, Share Warrants and Share Options,” “—Voting—Generally,” “—Voting—Variation of Rights Attaching to a Class or Series of Shares,” “—Disclosure of Interests in Shares” and “—Corporate Governance.”
Corporate Governance
Generally
Our articles of association allocate authority over the management of the Company to the Board. The Board may then delegate management of the Company to committees of the Board or such other persons as it thinks fit. Regardless of any delegation, the Board will remain responsible, as a matter of Irish law, for the proper management of the affairs of our Company. The Board may create new committees or change the responsibilities of existing committees from time to time.
See “Management—Committees of the Board of Directors.”
122
Directors: Term and Appointment
Directors are elected or appointed at the annual general meeting or at any extraordinary general meeting called for that purpose. Each director is elected by the affirmative vote of a majority of the votes cast with respect to such director.
Our articles of association provide that the Board is divided into three classes serving staggered three-year terms. Shareholders do not have cumulative voting rights. Accordingly, the holders of a majority of the voting rights attaching to our ordinary shares will, as a practical matter, be entitled to control the election of all directors. At each annual general meeting, directors will be elected for a full term of three years to succeed those directors of the relevant class whose terms are expiring. Any nominee for director who does not receive a majority of the votes cast is not elected to the Board.
Under our articles of association, the Board has the authority to appoint directors to the Board, either to fill a vacancy or as an additional director. A vacancy on the Board created by the removal of a director may be filled by an ordinary resolution of the shareholders at the meeting at which such director is removed and, in the absence of such election or appointment, the remaining directors may fill the vacancy. The Board may fill a vacancy by an affirmative vote of a majority of the directors constituting a quorum. If there is an insufficient number of directors to constitute a quorum, the Board may nonetheless act to fill such vacancies or call a general meeting of the shareholders. Under our articles of association, if the Board fills a vacancy, the director’s term expires at the same time as the term of the other directors of the class of directors to which the new director is appointed. If there is an appointment to fill a casual vacancy or an addition to the Board, the total number of directors shall not at any time exceed the number of directors from time to time fixed by the Board in accordance with the articles of association.
Removal of Directors
The Irish Companies Act provides that, notwithstanding anything contained in the articles of association of a company or in any agreement between that company and a director, the shareholders may, by an ordinary resolution, remove a director from office before the expiration of his or her term, provided that notice of any such resolution be given to the shareholders not less than 28 days before the meeting at which the director is to be removed, and the director will be entitled to be heard at such meeting. The power of removal is without prejudice to any claim for damages for breach of contract (e.g., employment agreement) that the director may have against us in respect of his or her removal.
Directors’ Duties
Our directors have certain statutory and fiduciary duties. All of the directors have equal and overall responsibility for the management of the Company (although directors who also serve as employees will have additional responsibilities and duties arising under their employment agreements and will be expected to exercise a greater degree of skill and diligence than non-executive directors). The principal fiduciary duties include the statutory and common law fiduciary duties of acting in good faith in the interests of the company and exercising due care and skill. Other statutory duties include ensuring the maintenance of proper books of account, having annual accounts prepared, having an annual audit performed, maintaining certain registers and making certain filings as well as the disclosure of personal interests. Particular duties also apply to directors of insolvent companies (for example, the directors could be liable to sanctions where they are deemed by the court to have carried on our business while insolvent, without due regard to the interests of creditors). For public limited companies like us, directors are under a specific duty to ensure that the corporate secretary is a person with the requisite knowledge and experience to discharge the role.
Conflicts of Interest
As a matter of Irish law, a director is under a fiduciary duty to avoid conflicts of interest. Irish law and our articles of association provide that: (i) a director may be a director of or otherwise interested in a company
123
relating to us and will not be accountable to us for any remuneration or other benefits received as a result, unless we otherwise direct; (ii) a director or a director’s firm may act for us in a professional capacity other than as auditor; and (iii) a director may hold an office or place of profit in us and will not be disqualified from contracting with us. If a director has a personal interest in an actual or proposed contract with us, the director must declare the nature of his or her interest and we are required to maintain a register of such declared interests that must be available for inspection by the shareholders. Such a director may vote on any resolution of the Board in respect of such a contract, and such a contract will not be voidable solely as a result.
Indemnification of Directors and Officers; Insurance
To the fullest extent permitted by Irish law, our articles of association confer an indemnity on our directors and officers. However, this indemnity is limited by the Irish Companies Act, which prescribes that an advance commitment to indemnify only permits a company to pay the costs or discharge the liability of a director or corporate secretary where judgment is given in favor of the director or corporate secretary in any civil or criminal action in respect of such costs or liability, or where an Irish court grants relief because the director or corporate secretary acted honestly and reasonably and ought fairly to be excused. Any provision whereby an Irish company seeks to commit in advance to indemnify its directors or corporate secretary over and above the limitations imposed by the Irish Companies Act will be void under Irish law, whether contained in its articles of association or any contract between the company and the director or corporate secretary. This restriction does not apply to our executives who are not directors, the corporate secretary or other persons who would be considered “officers” within the meaning of that term under the Irish Companies Act.
Our articles of association also contain indemnification and expense advancement provisions for persons who are not directors or our corporate secretary.
We are permitted under our articles of association and the Irish Companies Act to take out directors’ and officers’ liability insurance, as well as other types of insurance, for our directors, officers, employees and agents.
Additionally, we and certain of our subsidiaries intend to enter into agreements to indemnify our directors to the maximum extent allowed under applicable law before the completion of the offering. These agreements, among other things, will provide that we will indemnify our directors for certain expenses (including attorneys’ fees), judgments, fines and settlement amounts reasonably incurred by such person in any action or proceeding, including any action by or in our right, on account of any services undertaken by such person on our behalf or that person’s status as our director.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to our directors, officers or persons controlling the registrant pursuant to the foregoing provisions, we have been informed that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable.
Duration; Dissolution; Rights upon Liquidation
Our duration will be unlimited. We may be dissolved at any time by way of either a shareholder’s voluntary winding up or a creditors’ winding up. In the case of a shareholder’s voluntary winding up, the Company must be solvent and a special resolution of the shareholders is required. We may also be dissolved by way of court order on the application of a creditor, or by the Director of Corporate Enforcement in Ireland where the affairs of the Company have been investigated by an inspector and it appears from the report or any information obtained by the Director of Corporate Enforcement that the Company should be wound up.
The rights of the shareholders to a return of our assets on dissolution or winding up, following the settlement of all claims of creditors, may be prescribed in our articles of association or the terms of any shares issued by the Board from time to time. If the articles of association and terms of issue of the shares of the Company contain no specific provisions in respect of a dissolution or winding up then, subject to the shareholder
124
priorities and the rights of any creditors, the assets will be distributed to shareholders in proportion to the paid-up nominal value of the shares held. Our articles of association provide that our ordinary shareholders may be entitled to participate in a winding up, and the method by which the property will be divided shall be determined by the liquidator, subject to a special resolution of the shareholders, but such rights of ordinary shareholders to participate may be subject to the rights of any preference shareholders to participate under the terms of any series or class of preference shares.
Transfer Agent and Registrar
The transfer agent and registrar for our ordinary shares is Computershare Trust Company, N.A. (“Computershare”).
Exchange Controls
There is no limitation imposed by Irish law or by our articles of association on the right of a non-resident to hold or vote our ordinary shares.
Listing
We intend to apply for listing of our ordinary shares on under the symbol “ .”
Differences in Corporate Law
We, and our relationships with our shareholders, are governed by Irish corporate law and not by the corporate law of any U.S. state. As a result, our directors and shareholders are subject to different responsibilities, rights and privileges than are available to directors and shareholders of U.S. corporations. To help you understand these differences, we have prepared the following summary comparing certain important provisions of Irish corporate law (as modified by our articles of association) with those of Delaware corporate law. Before investing, you should consult your legal advisor regarding the impact of Irish corporate law on your specific circumstances and reasons for investing.
Duties of Directors
Our business is managed by the Board. Members of the board of directors of an Irish company owe a fiduciary duty to the company to act in good faith in their dealings with or on behalf of the company and to exercise their powers and fulfill the duties of their offices on the same basis. This duty includes the following essential elements:
| | • | | a duty to act in good faith and what the director considers to be in the interests of the company; |
| | • | | a duty not to make a personal profit from opportunities that arise from the office of director; |
| | • | | a duty to exercise the care, diligence and skill that a reasonably prudent person would exercise in carrying out their duties as a director; |
| | • | | to act honestly and responsibly in relation to the affairs of a company; |
| | • | | to act in accordance with the company’s constitution; |
| | • | | not to use the company’s property unless permitted by the constitution or approved by a shareholders’ resolution; |
| | • | | generally not to agree to a restraint on the exercise of directors’ powers unless permitted by the constitution or approved by a resolution of the company in a general meeting; |
125
| | • | | to avoid conflicts of interest; and |
| | • | | to have regard to interests of company’s employees and its members. |
Under Irish law, the fiduciary duty of the directors is to the company, and not to the company’s individual shareholders. Our shareholders may not generally sue our directors directly for a breach of fiduciary duty.
The business of a Delaware corporation is also managed by or under the direction of its board of directors. In exercising their powers, directors are charged with a fiduciary duty of care to protect the interests of the corporation and a fiduciary duty of loyalty to act in the best interests of its shareholders. The duty of care requires that directors act in an informed and deliberative manner and inform themselves, prior to making a business decision, of all material information reasonably available to them. The duty of care also requires that directors exercise care in overseeing and investigating the conduct of corporate employees. The duty of loyalty may be summarized as the duty to act in good faith, not out of self-interest, and in a manner which the director reasonably believes to be in the best interests of the shareholders. These duties are similar to those imposed on the directors by the Irish Companies Act.
Under Irish law, the question of whether a director has acted properly will typically be assessed on a case-by-case basis, with regard to the circumstances surrounding the director’s action. In contrast, Delaware law presumes that directors act on an informed basis and in the best interests of the company and its shareholders.
Unless this presumption is rebutted, the decision of the board of a Delaware company will be upheld unless the action had no rational business purpose or constituted corporate waste. If the presumption is rebutted, the directors must demonstrate that the challenged action was entirely fair to the company.
Interested Directors
Under Irish law, directors who have an interest in a transaction or proposed transaction with us must disclose that interest to the Board when the proposed transaction is first considered (unless such interest has previously been disclosed). Not disclosing such an interest is a criminal offense, punishable by a fine.
Our articles of association provide that an interested director may vote on a resolution concerning a matter in which he or she has declared an interest.
Delaware law does not allow for criminal penalties but does specify that if a director has an interest in a transaction, that transaction would be voidable by a court unless either (i) the material facts about the interested director’s relationship or interests are disclosed or are known to the board of directors and a majority of the disinterested directors authorize the transaction, (ii) the material facts about the interested director’s relationship or interests are disclosed or are known to the shareholders entitled to vote and the transaction is specifically approved in good faith by such shareholders or (iii) the transaction was fair to the company when it was authorized, approved or ratified. In addition, the interested director could be held liable for a transaction in which he or she derived an improper personal benefit. Under Irish law, directors also have a general duty to avoid conflicts of interest. A director may be required to account to the company for any personal profit he or she has made in breach of this duty unless he or she has been specifically released from the duty by shareholder vote.
Voting Rights and Quorum Requirements
Under Irish law, the voting rights of our shareholders are regulated by our articles of association and the Irish Companies Act. Under our articles of association, two or more shareholders (or if there is only one shareholder of the relevant class or series of shareholders, then one shareholder) present in person or by proxy and holding shares representing at least 50% of the issued shares carrying the right to vote at such meeting will constitute a quorum. Most shareholder actions or resolutions may be passed by a simple majority of votes cast.
126
Certain actions (including the amendment of our articles of association) require approval by 75% of the votes cast at a meeting of shareholders. For a Delaware corporation, the presence, either in person or by proxy, of as few as one third of the shares eligible to vote may constitute a quorum. Except for certain extraordinary transactions, such as approving a merger, shareholders of a Delaware corporation may act by the majority vote of the shares present, either in person or by proxy.
Under Irish law and our articles of association, the election of directors at a general meeting of shareholders will require a majority of votes cast at such meeting. In contrast, the election of directors for a Delaware corporation requires only a plurality vote.
Any individual who is a shareholder of our company and who is present at a meeting may vote in person, as may any corporate shareholder that is represented by a duly authorized representative at a meeting of shareholders. Our articles of association also permit attendance at general meetings by proxy, provided the instrument appointing the proxy is in common form or such other form as the directors may determine. Under our articles of association, each holder of ordinary shares is entitled to one vote per ordinary share held.
Amalgamations, Mergers and Similar Arrangements
Under Irish law, the disposal of or acquisition of assets by a company requires the approval of its board of directors. However, certain acquisitions and disposal of assets may also require shareholder approval. Under Delaware law, with certain exceptions, a merger, consolidation or sale of all or substantially all the assets of a corporation must be approved by the board of directors and the shareholders. Under Delaware law, a shareholder of a corporation participating in a major corporate transaction may, under certain circumstances, be entitled to appraisal rights which would allow him or her to receive the fair value of his or her shares (as determined by a court) in cash instead of the consideration he or she would otherwise receive in the transaction. Irish public companies may be acquired by way of a merger with a company incorporated in the EEA under the European Communities (Cross-Border Mergers) Regulations 2008, which implement the EU Cross-Border Merger Directive 2005/56 in Ireland or by way of a merger with another Irish company under the Irish Companies Act. Such a merger must be approved by a special resolution. Shareholders also may be entitled to have their shares acquired for cash. While, generally, under Irish law, shareholders of an Irish company do not have statutory appraisal rights, if we are being merged as the transferor company with another EEA company under these Regulations or another Irish company under the Irish Companies Act (i) any of our shareholders who vote against the special resolution approving the merger or (ii) if 90% of our shares are held by the successor company, any other of our shareholders may be entitled to require that the successor company acquire its shares for cash.
Takeovers
Takeover of certain Irish public companies, including us, are regulated by statutory takeover rules, which are administered by the Irish Takeover Panel.
In addition to the merger mechanisms under the European Communities (Cross-Border Mergers) Regulations 2008 and the Irish Companies Act referred to above, Irish law provides two principal ways for the control of a public company to change. The first method involves a public offer for the shares of that company. The number of shares required to vote in favor of a proposal to force minority shareholders of a public company, such as us, is 80% under the Irish Companies Act.
The second method of acquiring control of an Irish public company is by a scheme of arrangement. A company proposes the scheme of arrangement to its shareholders, which, if accepted, would result in the company being acquired by a third party. A scheme of arrangement must be approved by a majority in number of shareholders representing 75% in value of the shares of each relevant class actually voting at a general meeting.
If the scheme is approved, and subsequently confirmed by the Irish High Court, it becomes binding on all of the target shareholders, regardless of whether they voted on the scheme.
127
A general principle of Irish takeover law is that the directors of a company that is the target of an offer (or of a company which the directors believe will soon be the target of an offer) must refrain from frustrating that offer or depriving shareholders of the opportunity to consider the merits of the offer, unless the shareholders approve of such actions in a general meeting.
Under Delaware law, the board of directors may take defensive actions against a takeover if the directors believe in good faith that the takeover is a threat to the company’s interests and if the response is reasonable in light of the threat posed by the takeover. However, the board may not use such measures for its own personal interests. For example, a board may institute defensive measures to allow it to negotiate a higher price with the acquirer or prevent shareholders from being coerced into selling at a price that is clearly too low.
However, the board may not use such measures just to keep itself in control of the company. In contrast, Irish takeover law only allows the directors to advise shareholders (by way of a publicly available announcement) on the merits and drawbacks of any particular offer and to recommend shareholders to accept or reject such offer.
Our directors may not enact “poison pills” or other defensive measures.
Shareholders’ Suits
Under Irish law, our shareholders generally may not sue for wrongs suffered by the Company.
In Ireland, the decision to institute proceedings on behalf of a company is generally taken by the company’s board of directors. In certain limited circumstances, a shareholder may be entitled to bring a derivative action on our behalf. The central question at issue in deciding whether a minority shareholder may be permitted to bring a derivative action is whether, unless the action is brought, a wrong committed against the company would otherwise go un-redressed. The cause of action may be against the director, another person, or both.
In contrast to a derivative action, Irish law permits an action by a shareholder in his or her own right on the basis of the infringement of his or her personal rights as a shareholder. A shareholder may commence a suit in a representative capacity for him or herself as well as other similarly affected consenting shareholders. Additionally, under Irish law, any shareholder who claims that our affairs are being conducted, or that the powers of our directors are being exercised, in a manner oppressive to his or her interests as a shareholder, may apply to the Irish courts for an appropriate order.
Delaware law generally allows a shareholder to sue for wrongs suffered by the company if he first demands that the company sue on its own behalf and the company declines to do so, but allows the shareholder to. In certain situations, such as when there are specific reasons to believe that the directors are protecting their personal interests, the shareholder may sue directly without first making the demand.
Indemnification of Directors and Officers
In general, the Irish Companies Act prohibits us from indemnifying any director against liability due to his or her negligence, default, breach of duty or breach of trust due to us. We may, however, indemnify our officers if they are acquitted in a criminal proceeding or are successful in a civil proceeding. To the fullest extent permitted by Irish law, our articles of association confer an indemnity on our directors and officers.
Under Delaware law, a corporation may indemnify a director or officer against expenses (including attorneys’ fees), judgments, fines and settlement amounts that he or she reasonably incurred in defending him or her self in a lawsuit. The director or officer must have acted in good faith and, if being charged with a crime, must not have had a reasonable cause to believe that he or she was breaking the law.
128
Inspection of Corporate Records
Members of the general public have the right to inspect our public documents available at the Irish Companies Registration Office. Our shareholders have the additional right to inspect our register of directors and secretaries and minutes of general meetings. Our audited financial statements must be presented to our shareholders at an annual general meeting (and made available to our shareholders in advance of an annual general meeting).
The register of members of a company is also open to inspection by shareholders without charge, and by members of the general public on payment of a fee. A company is required to maintain its share register in Ireland. A company is required to keep at its registered office a register of directors and officers that is also open for inspection. Irish law does not, however, provide a general right for shareholders to inspect or obtain copies of any other corporate records.
Delaware law permits a shareholder to inspect or obtain copies of a corporation’s shareholder list and its other books and records for any purpose reasonably related to his or her interest as a shareholder.
Calling of Special Shareholders’ Meetings
Under Irish law, an extraordinary general meeting may be convened (i) by the Board, (ii) on requisition of the shareholders holding the number of shares prescribed by the Irish Companies Act (currently 10% of the paid-up share capital of the Company carrying voting rights) or (iii) in certain circumstances, on requisition of our auditors.
Under Delaware law, a special meeting of the shareholders may be called by the board of directors or by any person who is authorized by the corporation’s certificate of incorporation or bylaws.
Amendment of Organizational Documents
Irish law provides that the memorandum and/or articles of association of a company may be amended by a resolution of shareholders, either by written resolution (which requires the signature of all shareholders) or by resolution passed at a general meeting of shareholders of which due notice has been given. A 75% majority of votes cast at a general meeting is required to pass such a resolution.
Under Delaware law, a company’s certificate of incorporation may be amended if the amendment is approved by both the board of directors and the shareholders. Unless a different percentage is provided for in the certificate of incorporation, a majority of the voting power of the shareholders of the corporation is required to approve an amendment. Under Irish law, the certificate of incorporation of a company (which simply evidences the date of the company’s incorporation and its registered number and the fact that it has been incorporated) may not be amended. Under Delaware law, the certificate of incorporation may limit or remove the voting power of a class of the company’s stock. However, if the amendment would alter the number of authorized shares or par value or otherwise adversely affect the rights or preference of a class of stock, the holders of shares of that class are entitled to vote, as a class, upon the proposed amendment, without regard to the restriction in the certificate of incorporation.
Delaware law allows the bylaws of the corporation to be amended either by the shareholders or, if allowed in the certificate of incorporation, by the board of directors by a majority of voting power. Under Irish law, a 75% majority of votes cast at a general meeting of shareholders is required to amend either a company’s memorandum or its articles of association.
129
History of Security Issuances
In the last three years, GC Aesthetics plc has not issued securities.
In the last three years, Global Consolidated Aesthetics Limited has issued the following securities:
| | • | | On July 31, 2012, to Montreux, Oyster and certain other of its existing shareholders, interest bearing loan notes in the aggregate amount of €7,500,000 and warrants to purchase an aggregate of 40,002 B Preference Shares and 750,000 ordinary shares. The notes and warrants were issued in two tranches, on July 31, 2012 and December 14, 2012. |
| | • | | On January 29, 2013, to Montreux and Oyster, interest bearing loan notes in the aggregate amount of €1,000,000 and warrants to purchase an aggregate of 5,333 B Preference Shares and 100,000 ordinary shares, exercisable at nominal value. |
| | • | | On April 16, 2013, to Montreux and Oyster, interest bearing loan notes in the aggregate amount of €500,000 and warrants to purchase an aggregate of 2,667 B Preference Shares and 50,000 ordinary shares, exercisable at nominal value. |
| | • | | On May 10, 2013, to Montreux, Oyster and certain other existing shareholders, interest bearing loan notes in the aggregate amount of €1,665,884 and warrants to purchase an aggregate of 8,884 B Preference Shares and 166,589 ordinary shares, exercisable at nominal value. The notes and warrants were issued in two tranches, on May 10, 2013 and October 24, 2013. |
| | • | | On January 20, 2014, as amended on February 19, 2014 and December 19, 2014, to Montreux, Oyster and certain other shareholders, all outstanding loan notes issued by Global Consolidated Aesthetics Limited, together with interest thereon, were converted into B Preference Shares and additional B Preference Shares were subscribed for in cash. A total of 8,612,983 B Preference Shares were issued pursuant to this subscription agreement at a subscription price of $2.22181 per share. |
| | • | | On February 13, 2014, to Oyster, 400,000 ordinary shares pursuant to an exercise of warrants previously issued to Oyster. |
| | • | | On February 19, 2014, to OrbiMed, 1,451,762 C Preference Shares at an aggregate subscription price of $6,000,000. |
| | • | | On April 27, 2015, to OrbiMed, 592,042 ordinary shares at an aggregate subscription price of €5,920.42. |
130
SHARES ELIGIBLE FOR FUTURE SALE
Prior to the completion of this offering, there has been no public market for our ordinary shares. Future sales of our ordinary shares in the public market, or the availability of such ordinary shares for sale in the public market, could adversely affect market prices prevailing from time to time. As described below, only a limited number of ordinary shares will be available for sale shortly following the completion of this offering due to contractual and legal restrictions on resale. Nevertheless, sales of our ordinary shares in the public market after such restrictions lapse, or the perception that those sales may occur, could adversely affect the prevailing market price at such time and our ability to raise equity capital in the future.
Based on the number of ordinary shares outstanding as of , upon the completion of this offering, ordinary shares will be outstanding. The total number of ordinary shares to be outstanding also assumes no exercise of the underwriters’ option to purchase additional ordinary shares and no other exercises of outstanding options. Of the outstanding ordinary shares, (i) all of the ordinary shares sold in this offering will be freely tradable upon completion of the offering, (ii) ordinary shares held by non-affiliates will be freely tradable in the public market under Rule 144 of the Securities Act (“Rule 144”) or Regulation S of the Securities Act (“Regulation S”) and (iii) the ordinary shares held by affiliates will be freely tradable by affiliates, if in accordance with the lock-up arrangements described below, under Rule 144, subject to the volume and manner of sale limitations described below, Rule 701 of the Securities Act (“Rule 701”) or under Regulation S, except that some shares issued under our existing share incentive arrangements may not be sold until the certain vesting requirements are met.
Rule 144
In general, under Rule 144, beginning 90 days after the date of this prospectus, any person who is not our affiliate and has held their ordinary shares for at least six months, including the holding period of any prior owner other than one of our affiliates, may sell ordinary shares without restriction, subject to the availability of current public information about us. In addition, under Rule 144, any person who is not our affiliate and has not been our affiliate at any time during the preceding three months and has held their ordinary shares for at least one year, including the holding period of any prior owner other than one of our affiliates, would be entitled to sell an unlimited number of ordinary shares immediately upon the completion of this offering without regard to whether current public information about us is available.
Beginning 90 days after we become subject to the public company reporting requirements of the Exchange Act, a person who is our affiliate or who was our affiliate at any time during the preceding three months and who has beneficially owned restricted securities for at least six months, including the holding period of any prior owner other than one of our affiliates, is entitled to sell a number of ordinary shares within any three-month period that does not exceed the greater of: (i) 1% of the number of our ordinary shares outstanding, which will equal approximately ordinary shares immediately after this offering; and (ii) the average weekly trading volume of our ordinary shares on during the four calendar weeks preceding the filing of a notice on Form 144 with respect to the sale.
Sales under Rule 144 by our affiliates are also subject to certain manner of sale provisions, notice requirements and to the availability of current public information about us.
Rule 701
In general, under Rule 701, as in effect on the date of this prospectus, beginning 90 days after we become subject to the public company reporting requirements of the Exchange Act, any of our employees, directors or officers who acquired ordinary shares from us in connection with a written compensatory stock or option plan or other written agreement in compliance with Rule 701 is entitled to sell such ordinary shares in reliance on Rule 144 but without compliance with certain of the requirements contained in Rule 144. Accordingly, beginning 90 days after we become subject to the public company reporting requirements of the
131
Exchange Act, under Rule 701, persons who are not our affiliates may resell those ordinary shares without complying with the minimum holding period or public information requirements of Rule 144, and persons who are our affiliates may resell those ordinary shares without compliance with Rule 144’s minimum holding period requirements. However, all Rule 701 ordinary shares are subject to lock-up agreements as described below and will become eligible for sale upon the expiration of the restrictions set forth in those agreements.
Regulation S
Regulation S provides generally that sales made in offshore transactions are not subject to the registration or prospectus-delivery requirements of the Securities Act.
Lock-up Agreements
We, each of our directors and executive officers and other shareholders have agreed that, without the prior written consent of Merrill Lynch, Pierce, Fenner & Smith Incorporated, on behalf of the underwriters, we and they will not, subject to limited exceptions, directly or indirectly sell or dispose of any ordinary shares or any securities convertible into or exchangeable or exercisable for ordinary shares for a period of 180 days (subject to extension pursuant to the terms of the underwriting agreement) after the date of this prospectus. The lock-up restrictions and specified exceptions are described in more detail under “Underwriting.”
Registration Statements on Form S-8
Following this offering, we intend to file with the SEC a registration statement on Form S-8 under the Securities Act covering the ordinary shares that are subject to outstanding options and other awards issuable pursuant to our Plan dated . Ordinary shares covered by such registration statement will be available for sale in the open market following its effective date, subject to certain Rule 144 limitations applicable to affiliates and the terms of lock-up agreements applicable to those ordinary shares.
Registration Rights
The Registration Rights Agreement grants certain registration rights with respect to our ordinary shares. See “Related Party Transactions—Arrangements with Our Investors—Registration Rights Agreement.”
132
MATERIAL TAX CONSIDERATIONS
Material U.S. Federal Income Tax Considerations
The following is a description of material U.S. federal income tax considerations of the acquisition, ownership and disposition of ordinary shares acquired pursuant to this offering by a U.S. Holder, as defined below. This description only applies to ordinary shares held as “capital assets” (generally, property held for investment) and does not address, except as explicitly set forth below, aspects of U.S. federal income taxation that may be applicable to U.S. Holders that are subject to special tax rules, such as:
| | • | | banks or other financial institutions; |
| | • | | real estate investment trusts; |
| | • | | regulated investment companies; |
| | • | | tax-exempt organizations; |
| | • | | persons that will own ordinary shares through partnerships or other pass-through entities; |
| | • | | dealers or traders in securities or currencies; |
| | • | | U.S. Holders that have a functional currency other than the US dollar; |
| | • | | certain former citizens and former long-term residents of the United States; |
| | • | | U.S. Holders that will hold ordinary shares as part of a position in a straddle or as part of a hedging, conversion or integrated transaction for U.S. federal income tax purposes; |
| | • | | U.S. Holders that will hold or acquire ordinary shares in connection with a trade or business carried on by such U.S. Holders through a branch or agency in Ireland; or |
| | • | | direct, indirect or constructive owners of 10% or more of our total combined voting power. |
Moreover, this description does not address the 3.8% Medicare contribution tax on net investment income, the U.S. federal estate and gift tax or the alternative minimum tax consequences of the acquisition, ownership, and disposition of ordinary shares. We have not received nor do we expect to seek a ruling from the Internal Revenue Service (“IRS”) regarding any matter discussed herein. No assurance can be given that the IRS would not assert, or that a court would not sustain, a position contrary to any of those set forth below. Each prospective investor should consult its own tax advisors with respect to the U.S. federal, state, local and non-U.S. tax consequences of acquiring, owning and disposing of ordinary shares.
This description is based on the Internal Revenue Code, U.S. Treasury Regulations promulgated thereunder and administrative and judicial interpretations thereof, each as available and in effect on the date hereof, all of which are subject to change or differing interpretations, possibly with retroactive effect, which could affect the tax considerations described herein.
133
For purposes of this description, a “U.S. Holder” is a beneficial owner of ordinary shares who for U.S. federal income tax purposes is:
| | • | | a citizen or individual resident of the United States; |
| | • | | a corporation (or any other entity treated as a corporation for U.S. federal income tax purposes) organized in or under the laws of the United States or any State thereof, or the District of Columbia; |
| | • | | an estate the income of which is subject to U.S. federal income taxation regardless of its source; or |
| | • | | a trust (i) that has a valid election in effect to be treated as a U.S. person for U.S. federal income tax purposes or (ii)(A) the administration over which a U.S. court can exercise primary supervision and (B) all of the substantial decisions of which one or more U.S. persons have the authority to control. |
If an entity or arrangement treated as a partnership for U.S. federal income tax purposes holds ordinary shares, the tax treatment of the partnership and a partner in such partnership generally will depend on the status of the partner and the activities of the partnership. Such partner or partnership should consult its own tax advisors as to the U.S. federal income tax consequences of acquiring, owning and disposing of the ordinary shares.
PROSPECTIVE INVESTORS SHOULD CONSULT THEIR OWN TAX ADVISORS WITH REGARD TO THE PARTICULAR TAX CONSEQUENCES APPLICABLE TO THEIR SITUATIONS AS WELL AS THE APPLICATION OF ANY STATE, LOCAL, NON-U.S. OR OTHER TAX LAWS, INCLUDING GIFT AND ESTATE TAX LAWS.
Distributions on Ordinary Shares
As described in “Dividend Policy,” above, we do not currently anticipate paying any distributions on our ordinary shares in the foreseeable future. However, to the extent there are any distributions made with respect to our ordinary shares, subject to the considerations regarding passive foreign investment companies (“PFICs”) discussed below in “—Passive Foreign Investment Company Considerations,” a U.S. Holder of ordinary shares generally will be required to treat distributions received with respect to such ordinary shares (including the amount of Irish taxes withheld, if any) as dividend income to the extent of our current or accumulated earnings and profits (computed using U.S. federal income tax principles), with the excess treated as a non-taxable return of capital to the extent of the holder’s adjusted tax basis in the ordinary shares and, thereafter, as capital gain recognized on a sale or exchange on the day actually or constructively received by you. We do not maintain calculations of our earnings and profits in accordance with U.S. federal income tax accounting principles. U.S. Holders should therefore assume that any distribution with respect to our ordinary shares will constitute ordinary dividend income. Dividends paid on the ordinary shares will not be eligible for the dividends received deduction allowed to U.S. corporations.
Dividends paid to a non-corporate U.S. Holder by a “qualified foreign corporation” may be subject to reduced rates of taxation if certain holding period and other requirements are met. A qualified foreign corporation generally includes a foreign corporation (other than a PFIC) if (i) its ordinary shares are readily tradable on an established securities market in the United States or (ii) it is eligible for benefits under a comprehensive U.S. income tax treaty that includes an exchange of information program and which the U.S. Treasury Department has determined is satisfactory for these purposes. Our ordinary shares are expected to be readily tradable on an established securities market, . U.S. Holders should consult their own tax advisors regarding the availability of the reduced tax rate on dividends in light of their particular circumstances.
Non-corporate U.S. Holders will not be eligible for reduced rates of taxation on any dividends received from us if we are a PFIC in the taxable year in which such dividends are paid or in the preceding taxable year.
Distributions paid in a currency other than US dollars will be included in a U.S. Holder’s gross income in a US dollar amount based on the spot exchange rate in effect on the date of actual or constructive receipt,
134
whether or not the payment is converted into US dollars at that time. The U.S. Holder will have a tax basis in such currency equal to such US dollar amount, and any gain or loss recognized upon a subsequent sale or conversion of the foreign currency for a different US dollar amount will be U.S. source ordinary income or loss. If the dividend is converted into US dollars on the date of receipt, a U.S. Holder generally should not be required to recognize foreign currency gain or loss in respect of the dividend income.
Under current Irish law (see “—Material Irish Tax Considerations—Dividend Withholding Tax”), dividends paid by an Irish corporation to a U.S. Holder may be subject to Irish dividend withholding tax unless an exemption applies. A U.S. Holder may be entitled, subject to certain limitations, to a credit against its U.S. federal income tax liability for Irish taxes withheld from dividends. Application of the U.S. foreign tax credit rules are complex. U.S. Holders should consult their own tax advisors concerning the foreign tax credit rules in light of their particular circumstances.
Sale, Exchange or Other Taxable Disposition of Ordinary Shares
Subject to the discussion below in “—Controlled Foreign Corporation Considerations” and “—Passive Foreign Investment Company Considerations,” upon the sale, exchange, or other taxable disposition of ordinary shares, a U.S. Holder generally will recognize gain or loss on the taxable sale or exchange of the ordinary shares in an amount equal to the difference between the US dollar amount realized on such sale or exchange (determined in the case of shares sold or exchanged for currencies other than US dollars by reference to the spot exchange rate in effect on the date of the sale or exchange or, if the ordinary shares sold or exchanged are traded on an established securities market and the U.S. Holder is a cash basis taxpayer or an electing accrual basis taxpayer, the spot exchange rate in effect on the settlement date) and the U.S. Holder’s adjusted tax basis in the ordinary shares determined in US dollars. The initial tax basis of the ordinary shares to a U.S. Holder will be the U.S. Holder’s US dollar purchase price for the shares (determined by reference to the spot exchange rate in effect on the date of the purchase, or if the shares purchased are traded on an established securities market and the U.S. Holder is a cash basis taxpayer or an electing accrual basis taxpayer, the spot exchange rate in effect on the settlement date).
Subject to the discussion below regarding potential CFC (as defined below) status and assuming we are not a PFIC and have not been treated as a PFIC during your holding period for our ordinary shares, such gain or loss will be capital gain or loss and will be long-term gain or loss if the ordinary shares have been held for more than one year. Under current law, long-term capital gains of non-corporate U.S. Holders generally are eligible for reduced rates of taxation. The deductibility of capital losses is subject to limitations. Capital gain or loss, if any, recognized by a U.S. Holder generally will be treated as U.S. source income or loss for U.S. foreign tax credit purposes. U.S. Holders are encouraged to consult their own tax advisors regarding the availability of the U.S. foreign tax credit in their particular circumstances.
Controlled Foreign Corporation Considerations
Generally, a non-U.S. company, such as us, will be classified as a controlled foreign corporation (“CFC”) if more than 50% (by vote or value) of the shares of the company are held, directly, indirectly or constructively, by “U.S. Shareholders.” For this purpose, a U.S. Shareholder is generally any U.S. Holder that possesses, directly, indirectly or constructively, 10% or more of the combined voting power of all classes of shares of the company. If we were classified as a CFC, any of our U.S. Holders that are U.S. Shareholders generally would be required to include in gross income (as ordinary income) at the end of each of our taxable years an amount equal to the U.S. Shareholder’s pro rata share of our “subpart F income.” Subpart F income generally includes dividends, interest, rents and royalties, gains from the sale of securities and income from certain transactions with related parties. In addition, if we are a CFC, a U.S. Shareholder that realizes gain from the sale or exchange of our ordinary shares may be required to classify a portion of such gain as dividend income rather than capital gain (see discussion above in “—Distributions on Ordinary Shares” regarding the tax treatment of dividend income).
135
Based on our current ownership structure, we do not believe that we are currently a CFC. It is possible, however, that following this offering, a shareholder treated as a U.S. person for U.S. federal income tax purposes will acquire, directly or indirectly, enough shares to be treated as a U.S. Shareholder after application of the constructive ownership rules and, together with any other U.S. Shareholders of the Company, cause the Company to be treated as a CFC for U.S. federal income tax purposes. We believe that certain of our shareholders are U.S. Shareholders for U.S. federal income tax purposes. U.S. Holders should consult their own tax advisors with respect to the potential adverse U.S. tax consequences of becoming a U.S. Shareholder in a CFC.
If we are classified as both a CFC and a PFIC, we generally will not be treated as a PFIC with respect to those U.S. Holders that meet the definition of a U.S. Shareholder during the period in which we are a CFC.
Passive Foreign Investment Company Considerations
Status as a PFIC
The rules governing PFICs can have adverse tax effects on U.S. Holders. We generally will be classified as a PFIC for U.S. federal income tax purposes if, for any taxable year, either: (i) 75% or more of our gross income consists of certain types of passive income, or (ii) the average value (determined on a quarterly basis) of our assets that produce, or are held for the production of, passive income is 50% or more of the value of all of our assets.
Passive income generally includes dividends, interest, rents and royalties (other than certain rents and royalties derived in the active conduct of a trade or business), annuities and gains from assets that produce passive income. If a non-U.S. corporation owns at least 25% by value of the stock of another corporation, the non-U.S. corporation is treated for purposes of the PFIC tests as owning its proportionate share of the assets of the other corporation and as receiving directly its proportionate share of the other corporation’s income.
Additionally, if we are classified as a PFIC in any taxable year with respect to which a U.S. Holder owns ordinary shares, we generally will continue to be treated as a PFIC with respect to such U.S. Holder in all succeeding taxable years, regardless of whether we continue to meet the tests described above, unless the U.S. Holder makes the “deemed sale election” described below.
We do not believe that we are currently a PFIC, and we do not anticipate becoming a PFIC in the foreseeable future. Notwithstanding the foregoing, the determination of whether we are a PFIC is made annually and depends on the particular facts and circumstances (such as the valuation of our assets, including goodwill and other intangible assets) and also may be affected by the application of the PFIC rules, which are subject to differing interpretations. The fair market value of our assets is expected to depend, in part, upon (i) the market price of our ordinary shares, which is likely to fluctuate, and (ii) the composition of our income and assets, which will be affected by how, and how quickly, we spend any cash that is raised in any financing transaction, including this offering. In light of the foregoing, no assurance can be provided that we are not currently a PFIC or that we will not become a PFIC in any future taxable year. Prospective investors should consult their own tax advisors regarding our PFIC status.
U.S. Federal Income Tax Treatment of a Shareholder of a PFIC
If we are classified as a PFIC for any taxable year during which a U.S. Holder owns ordinary shares, the U.S. Holder, absent certain elections (including the mark-to-market and QEF elections described below), generally will be subject to adverse rules (regardless of whether we continue to be classified as a PFIC) with respect to (i) any “excess distributions” (generally, any distributions received by the U.S. Holder on its ordinary shares in a taxable year that are greater than 125% of the average annual distributions received by the U.S. Holder in the three preceding taxable years or, if shorter, the U.S. Holder’s holding period for its ordinary shares) and (ii) any gain realized on the sale or other disposition of its ordinary shares.
136
Under these adverse rules (i) the excess distribution or gain will be allocated ratably over the U.S. Holder’s holding period, (ii) the amount allocated to the current taxable year and any taxable year prior to the first taxable year in which we are classified as a PFIC will be taxed as ordinary income and (iii) the amount allocated to each other taxable year during the U.S. Holder’s holding period in which we were classified as a PFIC (A) will be subject to tax at the highest rate of tax in effect for the applicable category of taxpayer for that year and (B) will be subject to an interest charge at a statutory rate with respect to the resulting tax attributable to each such other taxable year.
If we are classified as a PFIC, a U.S. Holder will generally be treated as owning a proportionate amount (by value) of stock or shares owned by us in any direct or indirect subsidiaries that are also PFICs and will be subject to similar adverse rules with respect to any distributions we receive from, and dispositions we make of, the stock or shares of such subsidiaries.
If we are classified as a PFIC and then cease to be so classified, a U.S. Holder may make an election (a “deemed sale election”) to be treated for U.S. federal income tax purposes as having sold such U.S. Holder’s ordinary shares on the last day our taxable year during which we were a PFIC. A U.S. Holder that makes a deemed sale election would then cease to be treated as owning stock in a PFIC by reason of ownership of our ordinary shares. However, gain recognized as a result of making the deemed sale election would be subject to the adverse rules described above and loss would not be recognized.
PFIC “Mark-to-Market” Election
In certain circumstances, a U.S. Holder can avoid certain of the adverse rules described above by making a mark-to-market election with respect to its ordinary shares, provided that the ordinary shares are “marketable.” Ordinary shares will be marketable if they are “regularly traded” on a “qualified exchange” or other market within the meaning of applicable U.S. Treasury Regulations. The ordinary shares will be treated as “regularly traded” in any calendar year in which more than ade minimis quantity of the ordinary shares are traded on a qualified exchange on at least 15 days during each calendar quarter. A “qualified exchange” includes a national securities exchange that is registered with the SEC.
A U.S. Holder that makes a mark-to-market election must include in gross income, as ordinary income, for each taxable year that we are a PFIC an amount equal to the excess, if any, of the fair market value of the U.S. Holder’s ordinary shares at the close of the taxable year over the U.S. Holder’s adjusted tax basis in its ordinary shares. An electing U.S. Holder may also claim an ordinary loss deduction for the excess, if any, of the U.S. Holder’s adjusted tax basis in its ordinary shares over the fair market value of its ordinary shares at the close of the taxable year, but this deduction is allowable only to the extent of any net mark-to-market gains previously included in income. A U.S. Holder that makes a mark-to-market election generally will adjust such U.S. Holder’s tax basis in its ordinary shares to reflect the amount included in gross income or allowed as a deduction because of such mark-to-market election. Gains from an actual sale or other disposition of ordinary shares in a year in which we are a PFIC will be treated as ordinary income, and any losses incurred on a sale or other disposition of ordinary shares will be treated as ordinary losses to the extent of any net mark-to-market gains previously included in income.
If we are classified as a PFIC for any taxable year in which a U.S. Holder owns ordinary shares but before a mark-to-market election is made, the adverse PFIC rules described above will apply to any mark-to-market gain recognized in the year the election is made. Otherwise, a mark-to-market election will be effective for the taxable year for which the election is made and all subsequent taxable years. The election cannot be revoked without the consent of the IRS unless the ordinary shares cease to be marketable, in which case the election is automatically terminated.
A mark-to-market election is not permitted for the shares of any of our subsidiaries that are also classified as PFICs. Prospective investors should consult their own tax advisors regarding the availability of, and the procedure for making, a mark-to-market election.
137
PFIC “QEF” Election
In some cases, a shareholder of a PFIC can avoid the interest charge and the other adverse PFIC consequences described above by obtaining certain information from such PFIC and by making a QEF election to be taxed currently on its share of the PFIC’s undistributed income. We do not, however, expect to provide the information regarding our income that would be necessary in order for a U.S. Holder to make a QEF election with respect to ordinary shares if we are classified as a PFIC.
PFIC Information Reporting Requirements
If we are a PFIC in any year, a U.S. Holder of ordinary shares in such year will be required to file an annual information return on IRS Form 8621 regarding their ownership of ordinary shares, any distributions received on such ordinary shares, and any gain realized on disposition of such ordinary shares. You are urged to consult your own tax advisor regarding the application of the PFIC rules to your investment in our ordinary shares.
NO ASSURANCE CAN BE GIVEN THAT WE ARE NOT CURRENTLY A PFIC OR THAT WE WILL NOT BECOME A PFIC IN THE FUTURE. U.S. HOLDERS SHOULD CONSULT THEIR OWN TAX ADVISORS WITH RESPECT TO THE OPERATION OF THE PFIC RULES AND RELATED REPORTING REQUIREMENTS IN LIGHT OF THEIR PARTICULAR CIRCUMSTANCES, INCLUDING THE ADVISABILITY OF MAKING ANY ELECTION THAT MAY BE AVAILABLE.
U.S. Backup Withholding Tax and Information Reporting
Backup withholding and information reporting requirements may apply to distributions on, and to proceeds from the sale or disposition of, ordinary shares that are held by U.S. Holders. The payor will be required to backup withhold tax on payments made within the United States, or by a U.S. payor or U.S. middleman (and certain subsidiaries thereof), on an ordinary share to a U.S. Holder, other than an exempt recipient, if the U.S. Holder fails to furnish its correct taxpayer identification number or otherwise fails to comply with, or establish an exemption from, the backup withholding requirements.
Backup withholding is not an additional tax. Amounts withheld as backup withholding may be credited against a U.S. Holder’s U.S. federal income tax liability. A U.S. Holder may obtain a refund of any excess amounts withheld under the backup withholding rules by filing the appropriate claim for a refund with the IRS and furnishing any required information in a timely manner.
Certain non-corporate U.S. Holders are required to report information with respect to investments in ordinary shares not held through an account with certain financial institutions. U.S. Holders that fail to report required information could become subject to substantial penalties. U.S. Holders are urged to consult their own tax advisors regarding the effect, if any, of these requirements on their acquisition, ownership and disposition of ordinary shares.
Material Irish Tax Considerations
Scope of Discussion
The following is a general summary of the material Irish tax considerations applicable to certain investors who are the owners of our shares. It is based on existing Irish law and our understanding of the practices of the Irish Revenue Commissioners on the date of this document. Legislative, administrative or judicial changes may modify the tax consequences described below, possibly with retroactive effect. Furthermore, we can provide no assurances that the consequences contained in this summary will not be challenged by the Irish Revenue Commissioners or will be sustained by a court if challenged.
138
The statements do not constitute tax advice and are intended only as a general guide. Furthermore, this information applies only to our shares that are held as capital assets and does not apply to all categories of shareholders, such as dealers in securities, trustees, insurance companies, collective investment schemes or shareholders who have, or who are deemed to have, acquired their shares by virtue of an Irish office or employment.
This summary is not exhaustive and shareholders should consult their own tax advisors as to the tax consequences in Ireland, or other relevant jurisdictions of this offering, including the acquisition, ownership and disposition of our shares.
Irish tax on Chargeable Gains
A disposal of our shares by a shareholder who is neither resident nor ordinarily resident for tax purposes in Ireland should not give rise to Irish tax on any chargeable gain realized on such disposal unless such shares are used, held or acquired for the purposes of a trade or business carried on by such shareholder through a branch or agency in Ireland.
A disposal of our shares by an Irish resident or ordinarily resident shareholder may, depending on the circumstances (including the availability of exemptions and reliefs), give rise to a chargeable gain or allowable loss for that shareholder. The rate of capital gains tax in Ireland is currently 33%.
A holder of our shares who is an individual and who is temporarily non-resident in Ireland may, under Irish anti-avoidance legislation, be liable to Irish tax on any chargeable gain realized on a disposal during the period in which such individual is non-resident.
Dividend Withholding Tax
Dividend withholding tax (“DWT”) (currently at a rate of 20%) may arise in respect of dividends or distributions from an Irish resident company unless an exemption applies. Where DWT does arise in respect of dividends, the company is responsible for deducting DWT at source and forwarding the relevant payment to the Irish Revenue Commissioners.
Certain shareholders are entitled to an exemption from DWT. In particular, dividends paid to a non-Irish resident shareholder, who does not hold shares in connection with a trade or business carried on through a branch or agency in Ireland, should not be subject to DWT if the shareholder is:
| | • | | an individual shareholder resident for tax purposes in a “relevant territory” and the individual is neither resident nor ordinarily resident in Ireland; |
| | • | | a corporate shareholder resident for tax purposes in a “relevant territory” provided that the corporate shareholder is not under the control, whether directly or indirectly, of a person or persons who is or are resident in Ireland; |
| | • | | a corporate shareholder that is not resident for tax purposes in Ireland and which is ultimately controlled, directly or indirectly, by persons resident in a “relevant territory;” |
| | • | | a corporate shareholder that is not resident for tax purposes in Ireland and whose principal class of shares (or those of its 75% parent) is substantially and regularly traded on a recognized stock exchange either in a “relevant territory” or on such other stock exchange approved by the Irish Minister for Finance; or |
139
| | • | | a corporate shareholder that is not resident for tax purposes in Ireland and is wholly owned, directly or indirectly, by two or more companies where the principal class of shares of each of such companies is substantially and regularly traded on a recognized stock exchange in a “relevant territory” or on such other stock exchange approved by the Irish Minister for Finance; |
and provided that, in all cases noted above (but subject to the exception in the paragraph below regarding “U.S. Resident Shareholders”), the shareholder has provided a relevant Irish DWT declaration form to his or her broker before the record date for the dividend (in the case of shares held through DTC), and the relevant information is further transmitted to the Company (in the case of shares held through DTC) or to our Transfer Agent (in the case of shares held outside of DTC).
A list of “relevant territories” for the purposes of DWT is set forth below.
| | | | | | | | | | |
Albania | | China | | Greece | | Luxembourg | | Panama | | Spain |
Armenia | | Croatia | | Hong Kong | | Macedonia | | Poland | | Sweden |
Australia | | Cyprus | | Hungary | | Malaysia | | Portugal | | Switzerland |
Austria | | Czech Republic | | Iceland | | Malta | | Qatar | | Thailand |
Bahrain | | Denmark | | India | | Mexico | | Romania | | Turkey |
Belarus | | Egypt | | Israel | | Moldova | | Russia | | Ukraine |
Belgium | | Estonia | | Italy | | Montenegro | | Saudi Arabia | | United Arab Emirates |
Bosnia & Herzegovina | | Ethiopia | | Japan | | Morocco | | Serbia | | United Kingdom |
Botswana | | Finland | | Republic of Korea | | Netherlands | | Singapore | | United States of America |
Bulgaria | | France | | Kuwait | | New Zealand | | Slovak Republic | | Uzbekistan |
Canada | | Georgia | | Latvia | | Norway | | Slovenia | | Vietnam |
Chile | | Germany | | Lithuania | | Pakistan | | South Africa | | Zambia |
Prior to paying any dividend, the Company will put in place an agreement with an entity that is recognized by the Irish Revenue Commissioners as a “qualifying intermediary” which satisfies one of the Irish requirements for dividends to be paid free of DWT to certain shareholders who hold their shares through DTC.
U.S. Resident Shareholders
Dividends paid in respect of shares in an Irish resident company that are owned by residents of the United States and held through DTC should not be subject to DWT provided that the address of the beneficial owner of the shares in the records of the broker is in the United States. We strongly recommend that such shareholders ensure that their information has been properly recorded by their brokers (so that such brokers can provide the relevant information to a qualifying intermediary appointed by us).
Dividends paid in respect of shares in an Irish resident company that are owned by residents of the United States and held outside of DTC will not be subject to DWT provided that the shareholder has completed the relevant Irish DWT declaration form and this declaration form remains valid. Such shareholders must provide the relevant Irish DWT declaration form to our Transfer Agent at least seven business days before the record date for the first dividend payment to which they are entitled.
If a U.S resident shareholder receives a dividend subject to DWT, that shareholder should generally be able to make an application for a refund of DWT from the Irish Revenue Commissioners subject to certain time limits.
Residents of “Relevant Territories” other than the United States
Shareholders who are residents of “relevant territories” other than the United States (regardless of when such shareholders acquired their shares) must complete the appropriate Irish DWT declaration form in order to receive dividends without DWT.
140
Shareholders must provide the appropriate Irish DWT declaration form to their brokers (so that such brokers can provide the relevant information to a qualifying intermediary appointed by us) before the record date for the first dividend to which they are entitled (in the case of shares held through DTC), or to our Transfer Agent at least seven business days before such record date (in the case of shares held outside of DTC). We strongly recommend that such shareholders complete the appropriate Irish DWT declaration form and provide them to their brokers or our Transfer Agent as soon as possible.
If a shareholder who is resident in a “relevant territory” receives a dividend subject to DWT, that shareholder should generally be able to make an application for a refund of DWT from the Irish Revenue Commissioners subject to certain time limits.
Irish Resident Shareholders
Irish tax resident or ordinarily resident shareholders will generally be subject to DWT in respect of dividends or distributions received from an Irish resident company.
Irish tax resident or ordinarily resident shareholders that are entitled to receive dividends without DWT must complete the relevant Irish DWT declaration form and provide the declaration form to their brokers (so that such brokers can provide the relevant information to a qualifying intermediary appointed by us) before the record date for the first dividend to which they are entitled (in the case of shares held through DTC), or to our Transfer Agent at least seven business days before such record date (in the case of shares held outside of DTC).
Irish tax resident or ordinarily resident shareholders who are not entitled to an exemption from DWT and who are subject to Irish tax should consult their own tax advisor.
Other Persons
Shareholders that do not fall within one of the categories mentioned above may fall within other exemptions from DWT. Such shareholders should consult their respective tax advisors.
If a shareholder is exempt from DWT but receives a dividend subject to DWT, that shareholder may be able to claim a refund of DWT from the Irish Revenue Commissioners subject to certain time limits.
Income Tax on Dividends
Non-Irish Resident Shareholders
A shareholder who is neither resident nor ordinarily resident for tax purposes in Ireland and who is entitled to an exemption from DWT generally has no liability to Irish income tax or related charges on a dividend from an Irish resident company unless that shareholder holds the shares through a branch or agency which carries on a trade in Ireland.
A shareholder who is neither resident nor ordinarily resident for tax purposes in Ireland and who is not entitled to an exemption from DWT generally has no additional liability to Irish income tax or related charges unless that shareholder holds the shares through a branch or agency which carries on a trade in Ireland. The shareholder’s liability to tax is effectively limited to the amount of DWT already deducted by the company.
Irish Resident Shareholders
Irish resident or ordinarily resident shareholders may be subject to Irish income tax and related charges on dividends received from us. Such shareholders should consult their respective tax advisors.
141
Capital Acquisitions Tax
Irish capital acquisitions tax (“CAT”), comprises gift tax and inheritance tax. A gift or inheritance of our shares (including where such shares are held in DTC) could attract a charge to CAT regardless of the place of residence, ordinary residence or domicile of the transferor or transferee of the shares. This is because a charge to CAT can arise on a gift or inheritance which comprises property situated in Ireland. Our shares are regarded as property situated in Ireland because our share register must be held in Ireland. The person who receives the gift or inheritance is the person who is primarily liable to pay any CAT that arises.
The rate of CAT is currently 33% and is payable if the taxable value of the gift or inheritance exceeds certain thresholds, referred to as “group thresholds.” CAT is applied on the excess over the threshold amount.
The appropriate threshold amount depends upon the relationship between the transferor and the transferee of the shares and also the aggregation of the values of previous gifts and inheritances received by the transferee from persons within the same group threshold. A gift or inheritance received from a spouse is exempt from CAT.
Stamp Duty
Irish stamp duty typically arises on the transfer of shares in an Irish incorporated company.
Shares Held Through DTC
A transfer of our shares effected by means of the transfer of book entry interests in DTC should not be subject to Irish stamp duty.
Shares Transferred Into or Out of DTC
A shareholder may transfer our shares into (or out of) DTC without giving rise to Irish stamp duty so long as:
| | (i) | there is no change in the ultimate beneficial ownership of the shares as a result of the transfer; and |
| | (ii) | the transfer into (or out of) DTC is not in contemplation of a sale of the shares by the beneficial owner to a third party. |
Shares Held Outside of DTC
A transfer of our shares where any of the parties to the transfer hold the shares outside of DTC may be subject to Irish stamp duty (currently at the rate of 1% of the higher of the price paid or the market value of the shares acquired). The transferee of the shares is typically the person that is liable to pay stamp duty.
Due to the potential Irish stamp duty on transfers of our shares outside of DTC, we strongly recommend that shareholders hold their shares through DTC (or through a broker who holds such shares through DTC).
DTC Requirement
In order for DTC, Cede & Co. and National Securities Clearing Corporation (“NSCC”), which provide clearing services for securities that are eligible for the depository and book-entry transfer services provided by DTC and registered in the name of Cede & Co., which entities are referred to collectively as the DTC Parties, to agree to provide services with respect to our ordinary shares, the Company contemplates concluding a composition agreement with the Revenue Commissioners of Ireland under which we have assumed any
142
obligation to discharge any Irish stamp duty or similar Irish transfer or documentary tax with respect to our ordinary shares on (i) transfers to which any of the DTC Parties is a party, or (ii) which may be processed through the services of any of the DTC Parties and the DTC Parties have received confirmation from the Revenue Commissioners of Ireland that while such composition agreement remains in force, the DTC Parties shall not be liable for any Irish stamp duty with respect to our ordinary shares.
In addition, to assure the DTC Parties that they will not be liable for any Irish stamp duty or similar Irish transfer or documentary tax with respect to our ordinary shares under any circumstances (including as a result of a change in applicable law), and to make other provisions with respect to our ordinary shares required by the DTC Parties, we and Computershare will enter into a Special Eligibility Agreement for Securities, with DTC, Cede & Co. and NSCC (the “DTC Eligibility Agreement”).
The DTC Eligibility Agreement will provide for certain indemnities of the DTC Parties by us and Computershare (as to which we have agreed to indemnify Computershare) and also will provide that DTC may impose a global lock on our ordinary shares or otherwise limit transactions in the shares, or cause the shares to be withdrawn, and NSCC may, in its sole discretion, exclude our ordinary shares from its Continuous Net Settlement service or any other service, and any of the DTC Parties may take other restrictive measures with respect to our ordinary shares as it may deem necessary and appropriate, without any liability on the part of any of the DTC Parties, (i) at any time that it may appear to any of the DTC Parties, in any such party’s sole discretion, that to continue to hold or process transactions in our ordinary shares will give rise to any Irish stamp duty or similar Irish transfer or documentary tax liability with respect to our ordinary shares on the part of any of the DTC Parties or (ii) otherwise as the DTC’s rules or the NSCC’s rules provide.
143
UNDERWRITING
Merrill Lynch, Pierce, Fenner & Smith Incorporated and Deutsche Bank Securities Inc. are acting as the representatives of the underwriters named below. Subject to the terms and conditions set forth in an underwriting agreement among us and the underwriters, we have agreed to sell to the underwriters, and each of the underwriters has agreed, severally and not jointly, to purchase from us, the number of ordinary shares set forth opposite its name below.
| | |
| Underwriter | | Number of Ordinary
Shares |
Merrill Lynch, Pierce, Fenner & Smith
Incorporated | | |
Deutsche Bank Securities Inc. | | |
Cowen and Company, LLC | | |
William Blair & Company, L.L.C. | | |
Total | | |
Subject to the terms and conditions set forth in the underwriting agreement, the underwriters have agreed, severally and not jointly, to purchase all of the ordinary shares sold under the underwriting agreement if any of these ordinary shares are purchased. If an underwriter defaults, the underwriting agreement provides that the purchase commitments of the non-defaulting underwriters may be increased or the underwriting agreement may be terminated.
We have agreed to indemnify the underwriters against certain liabilities, including liabilities under the Securities Act, or to contribute to payments the underwriters may be required to make in respect of those liabilities.
The underwriters are offering the ordinary shares, subject to prior sale, when, as and if issued to and accepted by them, subject to approval of legal matters by their counsel, including the validity of the ordinary shares, and other conditions contained in the underwriting agreement, such as the receipt by the underwriters of officer’s certificates and legal opinions. The underwriters reserve the right to withdraw, cancel or modify offers to the public and to reject orders in whole or in part.
The address of Merrill Lynch, Pierce, Fenner & Smith Incorporated is One Bryant Park, New York, New York 10036. The address of Deutsche Bank Securities Inc. is 60 Wall Street, New York, New York 10005.
Commissions and Discounts
The representatives have advised us that the underwriters propose initially to offer the ordinary shares to the public at the public offering price set forth on the cover page of this prospectus and to dealers at that price less a concession not in excess of $ per ordinary share. After the initial offering, the public offering price, concession or any other term of the offering may be changed.
The following table shows the public offering price, underwriting discount and proceeds before expenses to us. The information assumes either no exercise or full exercise by the underwriters of their option to purchase additional ordinary shares.
| | | | | | | | | | | | |
| | | Per Ordinary
Share | | | Without Option | | | With Option | |
Public offering price | | $ | | | | $ | | | | $ | | |
Underwriting discount | | $ | | | | $ | | | | $ | | |
Proceeds, before expenses, to GC Aesthetics plc | | $ | | | | $ | | | | $ | | |
144
The expenses of the offering, not including the underwriting discount, are estimated at $ and are payable by us. We have also agreed to reimburse the underwriters for certain of their expenses, in an amount of up to $ , as set forth in the underwriting agreement.
Option to Purchase Additional Ordinary Shares
We have granted an option to the underwriters, exercisable for 30 days after the date of this prospectus, to purchase up to additional ordinary shares at the public offering price, less the underwriting discount. If the underwriters exercise this option, each will be obligated, subject to conditions contained in the underwriting agreement, to purchase a number of additional ordinary shares proportionate to that underwriter’s initial amount reflected in the above table.
No Sales of Similar Securities
We, our executive officers and directors and our other existing security holders have agreed not to sell or transfer any ordinary shares or securities convertible into, exchangeable for, exercisable for or repayable with ordinary shares for 180 days after the date of this prospectus without first obtaining the written consent of Merrill Lynch, Pierce, Fenner & Smith Incorporated. Specifically, we and these other persons have agreed, with certain limited exceptions, not to directly or indirectly,
| | • | | offer, pledge, sell or contract to sell any ordinary shares, |
| | • | | sell any option or contract to purchase any ordinary shares, |
| | • | | purchase any option or contract to sell any ordinary shares, |
| | • | | grant any option, right or warrant for the sale of any ordinary shares, |
| | • | | lend or otherwise dispose of or transfer any ordinary shares, |
| | • | | request or demand that we file a registration statement related to the ordinary shares, or |
| | • | | enter into any swap or other agreement that transfers, in whole or in part, the economic consequence of ownership of any ordinary shares, whether any such swap or transaction is to be settled by delivery of ordinary shares or other securities, in cash or otherwise. |
This lock-up provision applies to ordinary shares and to securities convertible into or exchangeable or exercisable for or repayable with ordinary shares. It also applies to ordinary shares owned now or acquired later by the person executing the agreement or for which the person executing the agreement later acquires the power of disposition.
Listing
We expect the ordinary shares to be approved for listing on , subject to notice of issuance, under the symbol “ .”
Before this offering, there has been no public market for our ordinary shares. The initial public offering price will be determined through negotiations between us and the representatives. In addition to prevailing market conditions, the factors to be considered in determining the initial public offering price are
| | • | | the valuation multiples of publicly traded companies that the representatives believes to be comparable to us, |
| | • | | our financial information, |
145
| | • | | the history of, and the prospects for, our company and the industry in which we compete, |
| | • | | an assessment of our management, its past and present operations, and the prospects for, and timing of, our future revenues, |
| | • | | the present state of our development, and |
| | • | | the above factors in relation to market values and various valuation measures of other companies engaged in activities similar to ours. |
An active trading market for the ordinary shares may not develop. It is also possible that after the offering the ordinary shares will not trade in the public market at or above the initial public offering price.
The underwriters do not expect to sell more than 5% of the ordinary shares in the aggregate to accounts over which they exercise discretionary authority.
Price Stabilization, Short Positions and Penalty Bids
Until the distribution of the ordinary shares is completed, SEC rules may limit underwriters and selling group members from bidding for and purchasing our ordinary shares. However, the representatives may engage in transactions that stabilize the price of the ordinary shares, such as bids or purchases to peg, fix or maintain that price.
In connection with the offering, the underwriters may purchase and sell our ordinary shares in the open market. These transactions may include short sales, purchases on the open market to cover positions created by short sales and stabilizing transactions. Short sales involve the sale by the underwriters of a greater number of ordinary shares than they are required to purchase in the offering. “Covered” short sales are sales made in an amount not greater than the underwriters’ option to purchase additional ordinary shares described above. The underwriters may close out any covered short position by either exercising their option to purchase additional ordinary shares or purchasing ordinary shares in the open market. In determining the source of ordinary shares to close out the covered short position, the underwriters will consider, among other things, the price of ordinary shares available for purchase in the open market as compared to the price at which they may purchase ordinary shares through the option granted to them. “Naked” short sales are sales in excess of such option. The underwriters must close out any naked short position by purchasing ordinary shares in the open market. A naked short position is more likely to be created if the underwriters are concerned that there may be downward pressure on the price of our ordinary shares in the open market after pricing that could adversely affect investors who purchase in the offering. Stabilizing transactions consist of various bids for or purchases of ordinary shares made by the underwriters in the open market prior to the completion of the offering.
The underwriters may also impose a penalty bid. This occurs when a particular underwriter repays to the underwriters a portion of the underwriting discount received by it because the representative has repurchased ordinary shares sold by or for the account of such underwriter in stabilizing or short covering transactions.
Similar to other purchase transactions, the underwriters’ purchases to cover the syndicate short sales may have the effect of raising or maintaining the market price of our ordinary shares or preventing or retarding a decline in the market price of our ordinary shares. As a result, the price of our ordinary shares may be higher than the price that might otherwise exist in the open market. The underwriters may conduct these transactions on the , in the over-the-counter market or otherwise.
Neither we nor any of the underwriters make any representation or prediction as to the direction or magnitude of any effect that the transactions described above may have on the price of our ordinary shares. In addition, neither we nor any of the underwriters make any representation that the representatives will engage in these transactions or that these transactions, once commenced, will not be discontinued without notice.
146
Electronic Distribution
In connection with the offering, certain of the underwriters or securities dealers may distribute prospectuses by electronic means, such as email.
Other Relationships
Some of the underwriters and their affiliates have engaged in, and may in the future engage in, investment banking and other commercial dealings in the ordinary course of business with us or our affiliates. They have received, or may in the future receive, customary fees and commissions for these transactions.
In addition, in the ordinary course of their business activities, the underwriters and their affiliates may make or hold a broad array of investments and actively trade debt and equity securities (or related derivative securities) and financial instruments (including bank loans) for their own account and for the accounts of their customers. Such investments and securities activities may involve securities and/or instruments of ours or our affiliates. The underwriters and their affiliates may also make investment recommendations and/or publish or express independent research views in respect of such securities or financial instruments and may hold, or recommend to clients that they acquire, long and/or short positions in such securities and instruments.
Notice to Prospective Investors in the European Economic Area
In relation to each Member State of the EEA (each, a “Relevant Member State”), no offer of ordinary shares may be made to the public in that Relevant Member State other than:
| | • | | to any legal entity which is a qualified investor as defined in the Prospectus Directive; |
| | • | | to fewer than 100 or, if the Relevant Member State has implemented the relevant provision of the 2010 PD Amending Directive, 150, natural or legal persons (other than qualified investors as defined in the Prospectus Directive), as permitted under the Prospectus Directive, subject to obtaining the prior consent of the representative; or |
| | • | | in any other circumstances falling within Article 3(2) of the Prospectus Directive, |
provided that no such offer of ordinary shares shall require the Company or the representatives to publish a prospectus pursuant to Article 3 of the Prospectus Directive or supplement a prospectus pursuant to Article 16 of the Prospectus Directive.
Each person in a Relevant Member State who initially acquires any ordinary shares or to whom any offer is made will be deemed to have represented, acknowledged and agreed that it is a “qualified investor” within the meaning of the law in that Relevant Member State implementing Article 2(1)(e) of the Prospectus Directive. In the case of any ordinary shares being offered to a financial intermediary as that term is used in Article 3(2) of the Prospectus Directive, each such financial intermediary will be deemed to have represented, acknowledged and agreed that the ordinary shares acquired by it in the offer have not been acquired on a non-discretionary basis on behalf of, nor have they been acquired with a view to their offer or resale to, persons in circumstances which may give rise to an offer of any ordinary shares to the public other than their offer or resale in a Relevant Member State to qualified investors as so defined or in circumstances in which the prior consent of the representatives has been obtained to each such proposed offer or resale.
The Company, the representatives and their affiliates will rely upon the truth and accuracy of the foregoing representations, acknowledgements and agreements.
147
This prospectus has been prepared on the basis that any offer of ordinary shares in any Relevant Member State will be made pursuant to an exemption under the Prospectus Directive from the requirement to publish a prospectus for offers of ordinary shares. Accordingly any person making or intending to make an offer in that Relevant Member State of ordinary shares that are the subject of the offering contemplated in this prospectus may only do so in circumstances in which no obligation arises for the Company or any of the underwriters to publish a prospectus pursuant to Article 3 of the Prospectus Directive in relation to such offer. Neither the Company nor the underwriters have authorized, nor do they authorize, the making of any offer of ordinary shares in circumstances in which an obligation arises for the Company or the underwriters to publish a prospectus for such offer.
For the purpose of the above provisions, the expression “an offer to the public” in relation to any ordinary shares in any Relevant Member State means the communication in any form and by any means of sufficient information on the terms of the offer and the ordinary shares to be offered so as to enable an investor to decide to purchase or subscribe the ordinary shares, as the same may be varied in the Relevant Member State by any measure implementing the Prospectus Directive in the Relevant Member State and the expression “Prospectus Directive” means Directive 2003/71/EC (including the 2010 PD Amending Directive, to the extent implemented in the Relevant Member States) and includes any relevant implementing measure in the Relevant Member State and the expression “2010 PD Amending Directive” means Directive 2010/73/EU.
Notice to Prospective Investors in the United Kingdom
In addition, in the United Kingdom, this document is being distributed only to, and is directed only at, and any offer subsequently made may only be directed at persons who are “qualified investors” (as defined in the Prospectus Directive) (i) who have professional experience in matters relating to investments falling within Article 19 (5) of the Financial Services and Markets Act 2000 (Financial Promotion) Order 2005, as amended (the “Order”) and/or (ii) who are high net worth companies (or persons to whom it may otherwise be lawfully communicated) falling within Article 49(2)(a) to (d) of the Order (all such persons together being referred to as “relevant persons”). This document must not be acted on or relied on in the United Kingdom by persons who are not relevant persons. In the United Kingdom, any investment or investment activity to which this document relates is only available to, and will be engaged in with, relevant persons.
Notice to Prospective Investors in Switzerland
The ordinary shares may not be publicly offered in Switzerland and will not be listed on the SIX Swiss Exchange (“SIX”) or on any other stock exchange or regulated trading facility in Switzerland. This document has been prepared without regard to the disclosure standards for issuance prospectuses under art. 652a or art. 1156 of the Swiss Code of Obligations or the disclosure standards for listing prospectuses under art. 27 ff. of the SIX Listing Rules or the listing rules of any other stock exchange or regulated trading facility in Switzerland. Neither this document nor any other offering or marketing material relating to the ordinary shares or the offering may be publicly distributed or otherwise made publicly available in Switzerland.
Neither this document nor any other offering or marketing material relating to the offering, the Company, the ordinary shares have been or will be filed with or approved by any Swiss regulatory authority. In particular, this document will not be filed with, and the offer of ordinary shares will not be supervised by, the Swiss Financial Market Supervisory Authority FINMA (FINMA), and the offer of ordinary shares has not been and will not be authorized under the Swiss Federal Act on Collective Investment Schemes (“CISA”). The investor protection afforded to acquirers of interests in collective investment schemes under the CISA does not extend to acquirers of ordinary shares.
Notice to Prospective Investors in the Dubai International Financial Centre
This prospectus relates to an Exempt Offer in accordance with the Offered Securities Rules of the Dubai Financial Services Authority (“DFSA”). This prospectus is intended for distribution only to persons of a type
148
specified in the Offered Securities Rules of the DFSA. It must not be delivered to, or relied on by, any other person. The DFSA has no responsibility for reviewing or verifying any documents in connection with Exempt Offers. The DFSA has not approved this prospectus nor taken steps to verify the information set forth herein and has no responsibility for the prospectus. The ordinary shares to which this prospectus relates may be illiquid and/or subject to restrictions on their resale. Prospective purchasers of the ordinary shares offered should conduct their own due diligence on the ordinary shares. If you do not understand the contents of this prospectus you should consult an authorized financial advisor.
Notice to Prospective Investors in Australia
No placement document, prospectus, product disclosure statement or other disclosure document has been lodged with the Australian Securities and Investments Commission (“ASIC”), in relation to the offering. This prospectus does not constitute a prospectus, product disclosure statement or other disclosure document under the Corporations Act 2001 (the “Corporations Act”), and does not purport to include the information required for a prospectus, product disclosure statement or other disclosure document under the Corporations Act.
Any offer in Australia of the ordinary shares may only be made to persons (the “Exempt Investors”) who are “sophisticated investors” (within the meaning of section 708(8) of the Corporations Act), “professional investors” (within the meaning of section 708(11) of the Corporations Act) or otherwise pursuant to one or more exemptions contained in section 708 of the Corporations Act so that it is lawful to offer the ordinary shares without disclosure to investors under Chapter 6D of the Corporations Act.
The ordinary shares applied for by Exempt Investors in Australia must not be offered for sale in Australia in the period of twelve months after the date of allotment under the offering, except in circumstances where disclosure to investors under Chapter 6D of the Corporations Act would not be required pursuant to an exemption under section 708 of the Corporations Act or otherwise or where the offer is pursuant to a disclosure document which complies with Chapter 6D of the Corporations Act. Any person acquiring ordinary shares must observe such Australian on-sale restrictions.
This prospectus contains general information only and does not take account of the investment objectives, financial situation or particular needs of any particular person. It does not contain any securities recommendations or financial product advice. Before making an investment decision, investors need to consider whether the information in this prospectus is appropriate to their needs, objectives and circumstances, and, if necessary, seek expert advice on those matters.
Notice to Prospective Investors in Hong Kong
The ordinary shares have not been offered or sold and will not be offered or sold in Hong Kong, by means of any document, other than (a) to “professional investors” as defined in the Securities and Futures Ordinance (Cap. 571) of Hong Kong and any rules made under that Ordinance; or (b) in other circumstances which do not result in the document being a “prospectus” as defined in the Companies Ordinance (Cap. 32) of Hong Kong or which do not constitute an offer to the public within the meaning of that Ordinance. No advertisement, invitation or document relating to the ordinary shares has been or may be issued or has been or may be in the possession of any person for the purposes of issue, whether in Hong Kong or elsewhere, which is directed at, or the contents of which are likely to be accessed or read by, the public of Hong Kong (except if permitted to do so under the securities laws of Hong Kong) other than with respect to ordinary shares which are or are intended to be disposed of only to persons outside Hong Kong or only to “professional investors” as defined in the Securities and Futures Ordinance and any rules made under that Ordinance.
Notice to Prospective Investors in Japan
The ordinary shares have not been and will not be registered under the Financial Instruments and Exchange Law of Japan (Law No. 25 of 1948, as amended) and, accordingly, will not be offered or sold, directly
149
or indirectly, in Japan, or for the benefit of any Japanese Person or to others for re-offering or resale, directly or indirectly, in Japan or to any Japanese Person, except in compliance with all applicable laws, regulations and ministerial guidelines promulgated by relevant Japanese governmental or regulatory authorities in effect at the relevant time. For the purposes of this paragraph, “Japanese Person” shall mean any person resident in Japan, including any corporation or other entity organized under the laws of Japan.
Notice to Prospective Investors in Singapore
This prospectus has not been registered as a prospectus with the Monetary Authority of Singapore. Accordingly, this prospectus and any other document or material in connection with the offer or sale, or invitation for subscription or purchase, of ordinary shares may not be circulated or distributed, nor may the ordinary shares be offered or sold, or be made the subject of an invitation for subscription or purchase, whether directly or indirectly, to persons in Singapore other than (i) to an institutional investor under Section 274 of the Securities and Futures Act, Chapter 289 of Singapore (the “SFA”), (ii) to a relevant person pursuant to Section 275(1), or any person pursuant to Section 275(1A), and in accordance with the conditions specified in Section 275, of the SFA, or (iii) otherwise pursuant to, and in accordance with the conditions of, any other applicable provision of the SFA.
Where the ordinary shares are subscribed or purchased under Section 275 of the SFA by a relevant person which is:
| | • | | a corporation (which is not an accredited investor (as defined in Section 4A of the SFA)) the sole business of which is to hold investments and the entire share capital of which is owned by one or more individuals, each of whom is an accredited investor; or |
| | • | | a trust (where the trustee is not an accredited investor) whose sole purpose is to hold investments and each beneficiary of the trust is an individual who is an accredited investor, |
securities (as defined in Section 239(1) of the SFA) of that corporation or the beneficiaries’ rights and interest (howsoever described) in that trust shall not be transferred within six months after that corporation or that trust has acquired the ordinary shares pursuant to an offer made under Section 275 of the SFA except:
| | • | | to an institutional investor or to a relevant person defined in Section 275(2) of the SFA, or to any person arising from an offer referred to in Section 275(1A) or Section 276(4)(i)(B) of the SFA; |
| | • | | where no consideration is or will be given for the transfer; |
| | • | | where the transfer is by operation of law; |
| | • | | as specified in Section 276(7) of the SFA; or |
| | • | | as specified in Regulation 32 of the Securities and Futures (Offers of Investments) (Shares and Debentures) Regulations 2005 of Singapore. |
150
LEGAL MATTERS
Certain legal matters in connection with this offering will be passed upon for us by Ropes & Gray LLP, Boston, Massachusetts, United States. The validity of the issuance of our ordinary shares offered in this prospectus will be passed upon for us by Maples & Calder, Dublin, Ireland. The underwriters are being represented by Shearman & Sterling LLP, New York, New York, United States.
EXPERTS
The statement of financial position of GC Aesthetics plc as of May 5, 2015 has been included herein in reliance upon the report of KPMG, Ireland, independent registered public accounting firm, appearing elsewhere herein, and upon the authority of said firm as experts in accounting and auditing. Our consolidated financial statements, prepared in accordance with IFRS, as of December 31, 2014 and 2013, and for each of the years in the two year period ended December 31, 2014, appearing in this confidential submission have been audited by KPMG, independent registered public accounting firm, as set forth in its report included in this confidential submission, and are included upon KPMG’s authority as experts in accounting and auditing. The audit report contains an explanatory paragraph that states that the Company’s recurring losses and net capital deficiency raise substantial doubt about the Company’s ability to continue as a going concern. The consolidated financial statements do not include any adjustments that might result from the outcome of that uncertainty.
Prior to the engagement of KPMG as our independent registered public accounting firm to perform audits in accordance with the independence rules of the SEC and the standards of the United States Public Company Accounting Oversight Board (“PCAOB”), the Spanish member firm of KPMG provided legal and company secretarial services to a wholly-owned subsidiary of Global Consolidated Aesthetics Limited. The legal services were provided in the context of a transaction whereby existing bank debt was extinguished and new funds were made available by a third party. The legal services were limited to assisting the subsidiary of the Global Consolidated Aesthetics Limited with the cancellation of the charges that had been previously in place and reviewing new charge documents, in favor of a new lender and assisting with the signing logistics related to the same. The corporate secretarial services were ministerial, not requiring judgment and at the direction of an officer of the subsidiary.
While these services were permissible under our home country independence rules, they are not permissible under the independence rules of the SEC.
Notwithstanding the inconsistencies noted above with respect to the auditor independence rules in the United States, KPMG informed us that, after considering all the facts and circumstances and the impact that these matters may have had on independence with respect to us, it believes it was and is capable of exercising objective and impartial judgment on all issues encompassed within the conduct of its audits of our consolidated financial statements.
The Board also reviewed and considered the impact that these matters had on KPMG’s independence with respect to us under the applicable SEC and PCAOB independence rules. After considering all of the facts and circumstances, the Board determined that a reasonable investor with knowledge of all relevant facts and circumstances would conclude that KPMG was and is capable of exercising objective and impartial judgment on all issues encompassed within its audit engagement.
The offices of KPMG are located at 1 Stokes Place, St. Stephen’s Green, Dublin 2, Ireland.
151
ENFORCEMENT OF CIVIL LIABILITIES
We are incorporated under the laws of Ireland. Many of our directors and officers, and some of the experts named in this prospectus, are residents of Ireland or otherwise reside outside of the United States, and all or a substantial portion of their assets, and all or a substantial portion of our assets, are located outside of the United States. We have appointed an agent for service of process in the United States, but it may be difficult for shareholders who reside in the United States to effect service within the United States upon those directors, officers and experts who are not residents of the United States.
In addition, it may not be possible to enforce court judgments obtained in the United States against us in Ireland based on the civil liability provisions of the U.S. federal or state securities laws. We have been advised by Maples & Calder, our Irish counsel, that the United States currently does not have a treaty with Ireland providing for the reciprocal recognition and enforcement of judgments in civil and commercial matters.
The following requirements must be met before a judgment of a U.S. court will be deemed to be enforceable in Ireland:
| | • | | the judgment must be for a definite sum; |
| | • | | the judgment must be final and conclusive; and |
| | • | | the judgment must be provided by a court of competent jurisdiction. |
An Irish court will also exercise its right to refuse enforcement if the U.S. judgment was obtained by fraud, if the judgment violates Irish public policy, if the judgment is in breach of natural justice or if it is irreconcilable with an earlier foreign judgment. There is some uncertainty as to whether the courts of Ireland would recognize or enforce judgments of U.S. courts obtained against us or our directors or officers based on the civil liabilities provisions of the U.S. federal or state securities laws or hear actions against us or those persons based on those laws. Therefore, a final judgment for the payment of money rendered by any U.S. federal or state court based on civil liability, whether or not based solely on U.S. federal or state securities laws, would not automatically be enforceable in Ireland.
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the SEC a registration statement on Form F-1 under the Securities Act with respect to the ordinary shares offered hereby. This prospectus, which constitutes a part of the registration statement, does not contain all of the information set forth in the registration statement or the exhibits and schedules filed therewith. For further information with respect to us and the ordinary shares offered hereby, please refer to the registration statement and the exhibits and schedules filed therewith. Statements contained in this prospectus regarding the contents of any contract or any other document that is filed as an exhibit to the registration statement are not necessarily complete, and each such statement is qualified in all respects by reference to the full text of such contract or other document filed as an exhibit to the registration statement. A copy of the registration statement and the exhibits and schedules filed therewith may be inspected without charge at the public reference room maintained by the SEC, located at 100 F Street N.E., Washington, D.C. 20549, and copies of all or any part of the registration statement may be obtained from such offices upon the payment of the fees prescribed by the SEC. Please call the SEC at 1-800-SEC-0330 for further information about the public reference room. The SEC also maintains a website that contains reports, proxy and information statements and other information regarding registrants that file electronically with the SEC. The address is www.sec.gov.
152
We are subject to periodic reporting and other informational requirements of the Exchange Act as applicable to foreign private issuers. Accordingly, even though we must file an annual report on Form 20-F, we are not required to file periodic reports and financial statements with the SEC as frequently or as promptly as U.S. companies whose securities are registered under the Exchange Act, although we do intend to furnish quarterly reports on Form 6-K. Moreover, as a foreign private issuer, we are exempt from the rules of the Exchange Act prescribing the furnishing and content of proxy statements to shareholders and Section 16 short-swing profit reporting for our officer, directors and holders of more than 10% of our ordinary shares.
153
EXPENSES RELATED TO THIS OFFERING
The following table sets forth the costs and expenses, other than the underwriting discounts and commissions, payable by the registrant in connection with the sale of ordinary shares being registered. All amounts are estimates except for the SEC, registration fee, the Financial Industry Regulatory Authority filing fee and the listing fee.
| | | | |
Item | | Amount to be
paid | |
SEC registration fee | | $ | * | |
FINRA filing fee | | $ | * | |
Stock exchange listing fee | | $ | * | |
Blue sky fees and expenses | | $ | * | |
Printing and engraving expenses | | $ | * | |
Legal fees and expenses | | $ | * | |
Accounting fees and expenses | | $ | * | |
Transfer Agent fees and expenses | | $ | * | |
Miscellaneous expenses | | $ | * | |
Total | | $ | * | |
| | | | |
| * | To be completed by amendment. |
154
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
F-1
Report of KPMG, Independent Registered Public Accounting Firm
The Board of Directors and Shareholders
GC Aesthetics PLC:
We have audited the accompanying statement of financial position of GC Aesthetics plc (the Company) as of May 5, 2015. This financial statement is the responsibility of the Company’s management. Our responsibility is to express an opinion on this financial statement based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the statement of financial position is free of material misstatement. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the statement of financial position, assessing the accounting principles used and significant estimates made by management, and evaluating the overall presentation of the statement of financial position. We believe that our audit of the statement of financial position provides a reasonable basis for our opinion.
In our opinion, the statement of financial position referred to above presents fairly, in all material respects, the financial position of GC Aesthetics plc as of May 5, 2015, in conformity with International Financial Reporting Standards, as issued by the International Accounting Standards Board.
/s/ KPMG
Dublin, Ireland
May 15, 2015
F-2
GC AESTHETICS PLC
Statement of Financial Position
| | | | | | | | |
| | | Notes | | | May 5, 2015 | |
Current | | | | | | | | |
Other receivables | | | 3 | | | $ | 42,231 | |
| | | | | | | | |
Total current assets | | | | | | | 42,231 | |
| | | | | | | | |
Total assets | | | | | | $ | 42,231 | |
| | | | | | | | |
Equity | | | | | | | | |
Share capital, 4,000,000 authorized, 3,809,242 issued and outstanding, €0.01 par value ($0.0112) | | | | | | $ | 41,088 | |
Accumulated other comprehensive income | | | | | | | 1,143 | |
| | | | | | | | |
Total equity | | | | | | $ | 42,231 | |
| | | | | | | | |
The accompanying notes are an integral part of the financial statements
F-3
GC AESTHETICS PLC
Notes to Financial Statements
May 5, 2015
1. General
Business description
GC Aesthetics plc (“the Company”) is a holding company formed on April 8, 2015 to own Global Consolidated Aesthetics Limited. It is registered and domiciled in Ireland. Its assets currently consist only of other receivables (see Note 3). The intended holding company has incurred no operating expenses to date. Accordingly, statements of operations, comprehensive income, changes in equity and cash flows have been omitted.
Upon the Share-for-Share Exchange, GC Aesthetics plc will become the holding company of Global Consolidated Aesthetics Limited and its subsidiaries. Until immediately prior to the completion of an anticipated initial public offering, GC Aesthetics plc will be a shell company. GC Aesthetics plc will not have conducted any operations (other than activities incidental to its formation, the Share-for-Share Exchange described below and the initial public offering). Global Consolidated Aesthetics Limited is the entity through which the Company currently operates its business.
The Company financial statement of position was approved by the Board of Directors and authorized for issue by the directors on May 15, 2015.
The accounting policies applied in the preparation of the Company’s statements of financial position as of May 5, 2015 are set out in Note 2—Significant Accounting Policies. These have been applied consistently.
The address of the Company’s registered office is Suite 601, Q House, Furze Road, Sandyford, Dublin 18, Ireland.
Statement of compliance
The financial statements have been prepared in accordance with IFRS as issued by the International Accounting Standards Board (“IASB”) (“IFRS as issued by the IASB”).
2. Significant accounting policies
Use of estimates and judgments
The preparation of financial statements requires management to make judgments, estimates and assumptions that affect the application of accounting policies and the reported amounts of assets, liabilities, income and expenses. The estimates and associated assumptions are based on historical experience and various other factors that are believed to be reasonable under the circumstances. Actual results may differ from these estimates.
Estimates and underlying assumptions are reviewed on an ongoing basis. Revisions to accounting estimates are recognized in the year in which the estimate is revised and in any future periods affected.
F-4
GC AESTHETICS PLC
Basis of presentation
The condensed statement of financial position includes only the assets and share capital of GC Aesthetics plc. The Company’s financial year end is December 31.
Basis of measurement and presentation currency
Euro is the functional currency of the Company. The presentation currency of the Company is the US dollar. Accordingly, the condensed statement of financial position of the Company is presented in US dollars and has been rounded to the nearest US dollar, as of the date of the statement of financial position.
Basis of restructuring
After the restructuring, GC Aesthetics plc will become the parent company of Global Consolidated Aesthetics Limited. For financial information regarding Global Consolidated Aesthetics Limited and its subsidiaries, please refer to the F-pages above.
3. Other receivables
The carrying value of other receivables approximates its fair value. Other receivables represents cash held in a client account with the Company’s outside legal counsel, rather than in the Company’s bank account.
4. Share capital
The Company has authorized 4,000,000 Ordinary Shares of €0.01 ($0.010786 at April 23, 2015), of which 3,809,242 was issued and outstanding.
The Ordinary Shares have the following rights attached:
| | • | | Entitled to receive notice of, to attend and to vote at general meetings of the Company; and |
| | • | | Entitled to receive such dividend as declared by the Company on a pari passu basis apportioned proportionately between the holders of the Ordinary Shares. |
5. Related parties
There were no related party transactions during the period, other than the issuance of shares and the transaction described in Note 6.
6. Guarantees provided by the Company
GC Aesthetics plc and substantially all of its subsidiaries are obligors and bound by the various facility agreements in respect of the loan payable to Royalty Opportunities S.Á R.L., the investment vehicle of OrbiMed Advisors, LLC, of $59.0 million as at May 5, 2015.
7. Subsequent events
There were no significant events post-period end.
F-5
Report of KPMG, Independent Registered Public Accounting Firm
The Board of Directors and Shareholders
Global Consolidated Aesthetics Limited
We have audited the accompanying consolidated statements of financial position of Global Consolidated Aesthetics Limited and subsidiaries (the Company) as of as of December 31, 2014 and 2013, and the related consolidated statements of operations, consolidated statements of comprehensive loss, changes in shareholders’ equity and cash flows for each of the years then ended. These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audit included consideration of the internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of Global Consolidated Aesthetics Limited and subsidiaries as of December 31, 2014 and 2013, and the results of their operations and their cash flows for each of the years then ended, in conformity with International Financial Reporting Standards (IFRS) as issued by the International Accounting Standards Board.
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 2 to the financial statements, the Company has suffered recurring losses from operations and has a net capital deficiency. These conditions along with other matters described in Note 2 raise substantial doubt about its ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 2. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
/s/ KPMG
Dublin, Ireland
May 15, 2015
F-6
GLOBAL CONSOLIDATED AESTHETICS LIMITED
Consolidated Statements of Financial Position
| | | | | | | | | | | | |
| | | | | | December 31, | |
| | | Notes | | | 2014 | | | 2013 | |
| | | | | | (in thousands) | |
Assets | | | | | | | | | | | | |
Current | | | | | | | | | | | | |
Cash and cash equivalents | | | 10 | | | $ | 10,616 | | | $ | 4,519 | |
Restricted cash | | | 10 | | | | 3,642 | | | | — | |
Trade receivables, net | | | 4 | | | | 12,840 | | | | 10,006 | |
Inventories, net | | | 3 | | | | 14,929 | | | | 11,477 | |
Prepayments and other receivables | | | 4 | | | | 4,539 | | | | 4,574 | |
Tax receivables | | | | | | | 422 | | | | — | |
| | | | | | | | | | | | |
Total current assets | | | | | | | 46,988 | | | | 30,576 | |
| | | | | | | | | | | | |
Non-current | | | | | | | | | | | | |
Goodwill and other intangible assets, net of amortization | | | 6 | | | | 25,941 | | | | 32,222 | |
Property, plant and equipment, net | | | 5 | | | | 4,674 | | | | 4,976 | |
Deferred tax assets | | | 16 | | | | 1,113 | | | | 1,334 | |
Other non-current assets | | | | | | | 434 | | | | 618 | |
| | | | | | | | | | | | |
Total non-current assets | | | | | | | 32,162 | | | | 39,150 | |
| | | | | | | | | | | | |
Total assets | | | | | | $ | 79,150 | | | $ | 69,726 | |
| | | | | | | | | | | | |
Liabilities | | | | | | | | | | | | |
Current | | | | | | | | | | | | |
Trade payables | | | 9 | | | $ | 7,206 | | | $ | 6,206 | |
Accruals and other creditors | | | 9 | | | | 9,027 | | | | 6,887 | |
Other liabilities | | | 9 | | | | 996 | | | | 1,395 | |
Tax liabilities | | | 9 | | | | 1,569 | | | | 1,325 | |
Loans and borrowings | | | 8 | | | | 794 | | | | 27,072 | |
| | | | | | | | | | | | |
Total current liabilities | | | | | | | 19,592 | | | | 42,885 | |
| | | | | | | | | | | | |
Non-current | | | | | | | | | | | | |
B Preference Shares and C Preference Shares | | | 7 | | | | 116,210 | | | | 46,518 | |
Equity derivative | | | 7 | | | | 22,284 | | | | 16,741 | |
Deferred tax liabilities | | | 16 | | | | 4,926 | | | | 6,485 | |
Provisions | | | 11 | | | | 1,661 | | | | 1,951 | |
Loans and borrowings | | | 8 | | | | 51,457 | | | | 19,759 | |
Other liabilities | | | 9 | | | | — | | | | 789 | |
| | | | | | | | | | | | |
Total non-current liabilities | | | | | | | 196,538 | | | | 92,243 | |
| | | | | | | | | | | | |
Total liabilities | | | | | | | 216,130 | | | | 135,128 | |
| | | | | | | | | | | | |
Commitments and contingencies | | | 13 | | | | | | | | | |
Equity / (deficiency) | | | | | | | | | | | | |
Share capital | | | 7 | | | $ | 70 | | | $ | 65 | |
Additional paid-in capital | | | | | | | 34,287 | | | | 31,411 | |
Accumulated deficit | | | | | | | (183,489 | ) | | | (94,062 | ) |
Accumulated other comprehensive income (loss) | | | | | | | 12,152 | | | | (2,816 | ) |
| | | | | | | | | | | | |
Total equity / (deficiency) | | | | | | | (136,980 | ) | | | (65,402 | ) |
| | | | | | | | | | | | |
Total equity and liabilities | | | | | | $ | 79,150 | | | $ | 69,726 | |
| | | | | | | | | | | | |
The accompanying notes are an integral part of the financial information.
F-7
GLOBAL CONSOLIDATED AESTHETICS LIMITED
Consolidated Statements of Operations
| | | | | | | | | | | | |
| | | Notes | | | Year Ended December 31, | |
| | | 2014 | | | 2013 | |
| | | | | | (in thousands, except
share data) | |
Revenue | | | 22 | | | $ | 52,804 | | | $ | 44,630 | |
Cost of sales | | | | | | | (26,038 | ) | | | (22,548 | ) |
| | | | | | | | | | | | |
Gross profit | | | | | | | 26,766 | | | | 22,082 | |
Operating expenses: | | | | | | | | | | | | |
Distribution and selling expenses | | | | | | | (18,187 | ) | | | (10,113 | ) |
Administrative expenses | | | | | | | (18,151 | ) | | | (15,042 | ) |
Research and development expenses | | | | | | | (1,507 | ) | | | (1,487 | ) |
Amortization of intangible assets | | | 6 | | | | (3,990 | ) | | | (4,033 | ) |
Other expenses | | | | | | | — | | | | (183 | ) |
| | | | | | | | | | | | |
Operating expenses | | | | | | | (41,835 | ) | | | (30,858 | ) |
| | | | | | | | | | | | |
Operating loss | | | | | | | (15,069 | ) | | | (8,776 | ) |
Other income (expense): | | | | | | | | | | | | |
Net interest (expense) income | | | 14 | | | | (33,746 | ) | | | (6,877 | ) |
Loss on conversion of shareholder loans | | | 12 | | | | (27,737 | ) | | | — | |
Gain (Loss) on equity derivatives | | | 14 | | | | 5,182 | | | | (4,828 | ) |
(Loss) Gain on foreign exchange | | | 14 | | | | (18,657 | ) | | | 1,994 | |
| | | | | | | | | | | | |
Other (expense) | | | | | | | (74,958 | ) | | | (9,711 | ) |
| | | | | | | | | | | | |
Loss before income tax credit | | | | | | | (90,027 | ) | | | (18,487 | ) |
Income tax credit | | | 17 | | | | 600 | | | | 1,195 | |
| | | | | | | | | | | | |
Net loss | | | | | | $ | (89,427 | ) | | $ | (17,292 | ) |
| | | | | | | | | | | | |
Basic and diluted loss per ordinary share | | | 19 | | | $ | (18.74 | ) | | $ | (3.91 | ) |
| | | | | | | | | | | | |
Weighted average number of ordinary shares outstanding: | | | | | | | | | | | | |
Basic and diluted | | | | | | | 4,772,101 | | | | 4,425,800 | |
| | | | | | | | | | | | |
The accompanying notes are an integral part of the financial information.
F-8
GLOBAL CONSOLIDATED AESTHETICS LIMITED
Consolidated Statements of Comprehensive Loss
| | | | | | | | |
| | | Year Ended December 31, | |
| | 2014 | | | 2013 | |
| | | (in thousands) | |
Net loss | | $ | (89,427 | ) | | $ | (17,292 | ) |
Other comprehensive income (loss): | | | | | | | | |
Items that may be reclassified to profit or loss in subsequent periods: | | | | | | | | |
Foreign currency translation differences—foreign operations | | | 15,004 | | | | (2,192 | ) |
Items that will not be reclassified to profit or loss in subsequent years: | | | | | | | | |
Net actuarial (loss) gain on retirement obligation, net of tax | | | (36 | ) | | | 30 | |
| | | | | | | | |
Total comprehensive loss | | $ | (74,459 | ) | | $ | (19,454 | ) |
| | | | | | | | |
The accompanying notes are an integral part of the financial information.
F-9
GLOBAL CONSOLIDATED AESTHETICS LIMITED
Consolidated Statements of Changes in Shareholders’ Equity
(in thousands)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Notes | | | Share
capital | | | Additional Paid-in Capital | | | Accumulated
deficit | | | Accumulated Other
Comprehensive Income | | | Total
equity | |
| | | | Share
premium | | | Share-
based
payment
reserve | | | Capital
contribution
reserve | | | Other
reserves | | | | Foreign
currency translation
reserve | | | Retirement
obligation
reserve | | |
Balance at January 1, 2013 | | | | | | $ | 65 | | | $ | 21,299 | | | $ | — | | | $ | 10,112 | | | $ | 3,295 | | | $ | (77,165 | ) | | $ | (654 | ) | | $ | — | | | $ | (43,048 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Movements during the year | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Net loss | | | | | | | — | | | | — | | | | — | | | | — | | | | — | | | | (17,292 | ) | | | — | | | | — | | | | (17,292 | ) |
Other comprehensive losses: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Actuarial gain on retirement obligation, net of tax | | | 11 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 30 | | | | 30 | |
Exchange differences on foreign operations | | | 7 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | (2,192 | ) | | | — | | | | (2,192 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Total comprehensive loss | | | | | | | — | | | | — | | | | — | | | | — | | | | — | | | | (17,292 | ) | | | (2,192 | ) | | | 30 | | | | (19, 454 | ) |
Settlement in lieu of issuing ordinary shares | | | 7 | | | | — | | | | — | | | | — | | | | — | | | | (2,900 | ) | | | — | | | | — | | | | — | | | | (2,900 | ) |
Reclassification of gain on settlement in lieu of issuing ordinary shares | | | 7 | | | | — | | | | — | | | | — | | | | — | | | | (395 | ) | | | 395 | | | | — | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Total | | | | | | | — | | | | — | | | | — | | | | — | | | | (3,295 | ) | | | (16,897 | ) | | | (2,192 | ) | | | 30 | | | | (22,354 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance at December 31, 2013 | | | | | | | 65 | | | | 21,299 | | | | — | | | | 10,112 | | | | — | | | | (94,062 | ) | | | (2,846 | ) | | | 30 | | | | (65,402 | ) |
Movements during the year | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Net loss | | | | | | | — | | | | — | | | | — | | | | — | | | | — | | | | (89,427 | ) | | | — | | | | — | | | | (89,427 | ) |
Other comprehensive losses: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Actuarial loss on retirement obligation, net of tax | | | 11 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | (36 | ) | | | (36 | ) |
Exchange differences on foreign operations | | | 7 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 15,004 | | | | — | | | | 15,004 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Total comprehensive (loss) income | | | | | | | — | | | | — | | | | — | | | | — | | | | — | | | | (89,427 | ) | | | 15,004 | | | | (36 | ) | | | (74,459 | ) |
Capital contribution on subordinated loans | | | 7 | | | | — | | | | — | | | | — | | | | 1,032 | | | | — | | | | — | | | | — | | | | — | | | | 1,032 | |
Share-based payment compensation | | | 15 | | | | — | | | | — | | | | 1,844 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 1,844 | |
Exercise of warrants on ordinary shares | | | 7 | | | | 5 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 5 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Total | | | | | | | 5 | | | | — | | | | 1,844 | | | | 1,032 | | | | — | | | | (89,427 | ) | | | 15,004 | | | | (36 | ) | | | (71,578 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance at December 31, 2014 | | | | | | $ | 70 | | | $ | 21,299 | | | $ | 1,844 | | | $ | 11,144 | | | $ | — | | | $ | (183,489 | ) | | $ | 12,158 | | | $ | (6 | ) | | $ | (136,980 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
The accompanying notes are an integral part of the financial information.
F-10
GLOBAL CONSOLIDATED AESTHETICS LIMITED
Consolidated Statements of Cash Flows
| | | | | | | | | | | | |
| | | Notes | | | Year ended
December 31, | |
| | | 2014 | | | 2013 | |
| | | | | | (in thousands) | |
Cash flows from operating activities | | | | | | | | | | | | |
Net loss | | | | | | $ | (89,427 | ) | | $ | (17,292 | ) |
Adjustments to reconcile net loss to net cash used in operating activities: | | | | | | | | | | | | |
Amortization of intangible assets | | | 6 | | | | 3,990 | | | | 4,033 | |
Depreciation of property, plant and equipment | | | 5 | | | | 1,123 | | | | 1,175 | |
Share-based compensation | | | 15 | | | | 1,844 | | | | — | |
(Gain) Loss on equity derivatives | | | 14 | | | | 5,182 | | | | 4,828 | |
Interest expense | | | 14 | | | | 33,746 | | | | 6,877 | |
Loss on conversion of shareholder loans | | | 12 | | | | 27,737 | | | | — | |
Loss (Gain) on foreign exchange | | | 14 | | | | 18,657 | | | | (1,994 | ) |
Net loss on sale of property, plant and equipment | | | | | | | 19 | | | | 15 | |
Income tax credit | | | 17 | | | | (600 | ) | | | (1,195 | ) |
Change in net operating assets and liabilities: | | | | | | | | | | | | |
(Increase) in trade and other receivables | | | | | | | (4,413 | ) | | | (1,303 | ) |
(Increase) decrease in inventories | | | | | | | (5,313 | ) | | | 2,872 | |
Increase in trade and other payables | | | | | | | 5,263 | | | | 35 | |
Increase in provisions | | | | | | | 53 | | | | 785 | |
Taxation paid | | | | | | | (437 | ) | | | (135 | ) |
| | | | | | | | | | | | |
Net cash used in operating activities | | | | | | | (12,940 | ) | | | (1,299 | ) |
| | | | | | | | | | | | |
Cash flows from investing activities | | | | | | | | | | | | |
Purchase of property, plant and equipment | | | | | | | (1,380 | ) | | | (368 | ) |
Purchase of intangible assets | | | | | | | (1,289 | ) | | | (337 | ) |
| | | | | | | | | | | | |
Net cash used in investing activities | | | | | | | (2,669 | ) | | | (705 | ) |
| | | | | | | | | | | | |
Cash flows from financing activities | | | | | | | | | | | | |
Payment of interest costs | | | | | | | (6,246 | ) | | | (915 | ) |
Proceeds from the issue of C Preference Shares | | | | | | | 6,000 | | | | — | |
Proceeds from the issue of ordinary shares | | | | | | | 5 | | | | — | |
Repayment of finance lease liabilities | | | | | | | (340 | ) | | | (409 | ) |
Proceeds from borrowings | | | | | | | 49,000 | | | | 7,351 | |
Repayment of borrowings | | | | | | | (21,329 | ) | | | (1,089 | ) |
Transfer to restricted cash | | | 10 | | | | (3,642 | ) | | | — | |
Settlement in lieu of issuing ordinary shares | | | | | | | (1,200 | ) | | | (912 | ) |
| | | | | | | | | | | | |
Net cash provided by financing activities | | | | | | | 22,248 | | | | 4,026 | |
| | | | | | | | | | | | |
Net increase in cash and cash equivalents | | | | | | | 6,639 | | | | 2,022 | |
Cash and cash equivalents at beginning of year | | | | | | | 4,519 | | | | 2,434 | |
Effect of exchange rate fluctuations on cash and cash equivalents | | | | | | | (542 | ) | | | 63 | |
| | | | | | | | | | | | |
Cash and cash equivalents at end of year | | | 10 | | | $ | 10,616 | | | $ | 4,519 | |
| | | | | | | | | | | | |
Supplemental disclosure of cash flow information—cash paid for: | | | | | | | | | | | | |
Interest and finance costs | | | | | | $ | 6,246 | | | $ | 915 | |
| | | | | | | | | | | | |
Income taxes | | | | | | $ | 437 | | | $ | 135 | |
| | | | | | | | | | | | |
The accompanying notes are an integral part of the financial information.
F-11
GLOBAL CONSOLIDATED AESTHETICS LIMITED
Notes to Consolidated Financial Statements
1. General
Business description
Global Consolidated Aesthetics Limited (“GCAL” or the “Company”) is a company registered, operating and domiciled in Ireland. The Company is an industry leading supplier of implantable medical devices for medical and aesthetic uses. The Company’s products are sold, either directly or through its distributor network, to hospitals, clinics and physicians in 75 countries.
The consolidated financial statements for the years ended December 31, 2014 and 2013 consolidate the individual financial statements of the Company and its subsidiaries.
The Company financial statements were approved by the Board of Directors and authorized for issue by the directors on May 15, 2015.
The accounting policies applied in the preparation of the Company’s consolidated financial statements for the years ended December 31, 2014 and 2013 are set out in Note 2—Significant Accounting Policies. These have been applied consistently.
The address of the Company’s registered office is Suite 601, Q House, Furze Road, Sandyford, Dublin 18, Ireland.
Statement of compliance
In accordance with International Accounting Standards (“IAS”) the consolidated financial statements have been prepared in accordance with IFRS as issued by the International Accounting Standards Board (“IASB”) (“IFRS as issued by the IASB”).
Details of new accounting standards or amendments to accounting standards, which are not yet effective and have not been early adopted in these consolidated financial statements, and the likely impact on future financial statements are set forth in the New Standards and Interpretations section.
2. Significant accounting policies
Use of estimates and judgments
The preparation of financial statements requires management to make judgments, estimates and assumptions that affect the application of accounting policies and the reported amounts of assets, liabilities, income and expenses. The estimates and associated assumptions are based on historical experience and various other factors that are believed to be reasonable under the circumstances. Actual results may differ from these estimates.
Estimates and underlying assumptions are reviewed on an ongoing basis. Revisions to accounting estimates are recognized in the year in which the estimate is revised and in any future periods affected.
In particular, information about significant areas of estimation uncertainty and critical judgments in applying accounting policies that have the most significant effect on the amounts recognized in the financial statements is included in the following notes:
| | • | | Note 6—measurement of the recoverable amounts of cash-generating units (“CGUs”) containing goodwill |
| | • | | Note 11—measurement of provisions and retirement obligations |
| | • | | Note 12—loss on conversion of shareholder loans |
F-12
GLOBAL CONSOLIDATED AESTHETICS LIMITED
Notes to Consolidated Financial Statements—Continued
| | • | | Note 15— measurement of share based payment compensation |
| | • | | Note 21— measurement of equity derivative |
Basis of consolidation
The consolidated financial statements include the financial statements of Global Consolidated Aesthetics Limited and all of its subsidiaries. The financial year end of all entities in the Company’s consolidated financial statements is December 31.
The financial statements of subsidiaries are included in the consolidated financial statements from the date on which control is obtained and cease to be consolidated from the date on which control is lost. Control exists when the Company has the power to direct the relevant activities of the entities and is therefore exposed to the risks and rewards of the business.
All intra-Company balances and transactions, including unrealized gains arising from intra-Company transactions, have been eliminated in full. Unrealized losses are eliminated in the same manner as unrealized gains except to the extent that they provide evidence of impairment.
Basis of measurement
The consolidated financial statements of the Company are prepared on the historical cost basis except for the following items:
| | • | | the measurement of provisions |
| | • | | the measurement of retirement obligations |
| | • | | the measurement of certain financial instruments classified as fair value through the consolidated statements of operations |
| | • | | the measurement of conversion of shareholders’ notes to equity instruments classified as fair value through the consolidated statements of operations |
The methods used to measure fair values are discussed in Notes 20 and 21.
Foreign currency
Functional and presentation currency
The Company financial statements are presented in US dollars. While the Company’s consolidated financial statements are presented in US dollars, the Company’s functional currency is the Euro, as are the functional currencies for the majority of its subsidiaries. Items included in the individual financial statements of each of the Company’s subsidiaries are measured using the currency of the primary economic environment in which the entity operates, which is primarily the Euro, Sterling, Mexican Peso and Brazilian Real.
The Company used the following exchange rates in translating balances from Euro to US dollars at December 31, 2012, December 31, 2013 and December 31 2014, and for the years ended December 31, 2013 and 2014:
| | | | | | | | | | | | |
| | | 2014 | | | 2013 | | | 2012 | |
Average exchange rate | | | 1.3272 | | | | 1.3279 | | | | | |
Closing exchange rate | | | 1.2142 | | | | 1.3766 | | | | 1.3184 | |
F-13
GLOBAL CONSOLIDATED AESTHETICS LIMITED
Notes to Consolidated Financial Statements—Continued
Foreign currency transactions
Transactions in foreign currencies are remeasured at the foreign exchange rate in effect at the date of the transaction. Monetary assets are translated at the rate in effect at the balance sheet date. Non-monetary assets carried at historical cost are translated at their historical rate.
For financial statement presentation, monetary and non-monetary assets are translated at the rate in effect at the balance sheet date. Monetary assets and liabilities denominated in foreign currencies at the end of the reporting year are translated to functional currencies at the foreign exchange rate in effect at that date. Foreign exchange differences arising on translation are recognized in the statement of comprehensive income.
Financial statements of foreign operations
To the extent that the Company’s foreign operations have functional currencies which are different from the Company’s presentation currency, the assets and liabilities of foreign operations, including goodwill and fair value adjustments arising on consolidation, and long term intra-Company loans that are part of the net investment because repayment is not planned or foreseen, are translated to US dollar at foreign exchange rates in effect at the reporting date. The revenues and expenses of these foreign operations are translated to US dollars at rates approximating the foreign exchange rates in effect at the dates of the transactions. Foreign exchange differences arising on translation are recognized in other comprehensive income and recognized on sale or other disposal of foreign operations.
Accounting standards and interpretations, adopted or assessed
Newly effective accounting standards
The following new standards were adopted by the Company for the first time in the current financial reporting period.
| | |
| | | Effective Date |
Amendments to IAS 27,Separate financial statements | | January 1, 2014 |
Amendments to IAS 32,Financial Instruments: Presentation re: Offsetting Financial Assets and Financial Liabilities | | January 1, 2014 |
Amendments to IAS 36,Impairment of Assets: Recoverable Amount Disclosures forNon-Financial Assets | | January 1, 2014 |
IFRIC 21,Levies | | January 1, 2014 |
Amendments to IFRS 10,Consolidated Financial Statements | | January 1, 2014 |
Amendments to IFRS 12,Disclosure of Interests in Other Entities | | January 1, 2014 |
Amendment to IAS 19, Employee Benefits | | July 1, 2014 |
Amendments to IAS 39,Financial instruments: Recognition and measurement re: Novation of derivatives and Continuation of Hedge Accounting | | July 1, 2014 |
Annual Improvements (2010-2012 Cycle) | | July 1, 2014 |
Annual Improvements (2011-2013 Cycle) | | July 1, 2014 |
The foregoing standards and amendments to the foregoing standards did not have a significant impact on the Company financial statements.
F-14
GLOBAL CONSOLIDATED AESTHETICS LIMITED
Notes to Consolidated Financial Statements—Continued
New standards and interpretations, not yet adopted
The following new standards, amendments or interpretations are either not expected to have a material impact on the consolidated financial statements once applied or are still under assessment by the Company as to whether or not they will have a material effect.
| | |
| | | Effective date |
Amendment to IFRS 7,Financial Instruments: Disclosures Mandatory Effective Date and Transition Disclosures | | January 1, 2015 |
Amendment to IAS 1,Presentation of Financial Statements | | January 1, 2016 |
Amendment to IAS 16,Property, Plant and Equipment | | January 1, 2016 |
Amendment to IAS 27,Separate financial statements: Equity Method in Separate Financial Statements | | January 1, 2016 |
Amendment to IAS 38, Intangible Assets,Clarification of Acceptable Methods of Depreciation and Amortization | | January 1, 2016 |
Amendment to IAS 41,Agriculture: Bearer Plants | | January 1, 2016 |
Amendment to IFRS 10 and IAS 28,Sale or Contribution of Assets Between an Investor and its Associate or Joint Venture | | January 1, 2016 |
Amendment to IFRS 10,Consolidated Financial Statements, IFRS 12,Disclosure of Interests in Other Entities, and IAS 28,Investments in Associates and Joint Ventures | | January 1, 2016 |
Amendment to IFRS 11,Accounting for Acquisitions of Interests in Joint Operations joint operations | | January 1, 2016 |
IFRS 14,Regulatory Deferral Accounts | | January 1, 2016 |
Annual improvements (2012-2014 Cycle) | | January 1, 2016 |
IFRS 15,Revenue from contracts with customers | | January 1, 2017 |
IFRS 9,Financial Instruments | | January 1, 2018 |
Amendment to IFRS 9,Financial instruments | | January 1, 2018 |
Amendment to IFRS 7,Financial instruments: Disclosures | | January 1, 2018 |
Revenue
Revenue comprises the fair value of goods supplied to external customers and exclusive of inter-company sales and value added tax, after allowing for trade discounts, rebates and other allowances. Revenue is recognized to the extent that it is probable that the economic benefits will flow to the Company, that recovery of consideration is probable, that it can be reliably measured, and that the significant risks and rewards of ownership of the goods have passed to the buyer. This occurs on dispatch in relation to sale of non-consignment goods, and upon the consumption of goods on consignment.
The Company analyses current economic conditions, specific facts and circumstances related to its customers, news and trends, historical bad debts, customer concentrations, customer credit-worthiness and changes in customer payment terms when evaluating revenue recognition and the adequacy of the provision for uncollectible trade receivables. If we determine that collection of a payment is not reasonably assured, we defer revenue recognition until collection becomes reasonably assured, which is generally upon receipt of cash or when the uncertainty is resolved.
The Company does not recognize taxes within gross revenues. VAT, excise, customs duties and other taxes collected are excluded from revenues and recorded as a liability. The liability is discharged at the time the Company remits payment to the respective taxing authorities.
F-15
GLOBAL CONSOLIDATED AESTHETICS LIMITED
Notes to Consolidated Financial Statements—Continued
Inventories
Inventories are valued on the first in, first out basis, at the lower of cost or estimated net realizable value. Cost includes all expenditures which have been incurred in the normal course of business in bringing the products to their present location and condition.
In the case of finished goods, cost includes direct materials, direct labor and a proportion of manufacturing overhead based on normal operating capacity but excluding borrowing costs. Net realizable value is the estimated selling price of inventory on hand, less all further costs to bring the product to completion, and all costs expected to be incurred in marketing, distribution and selling.
The Company delivers finished goods to certain customers on a consignment basis. This inventory is retained in the Company’s consolidated statements of financial position as inventory until consumed by (sold to) the ultimate consumer. At December 31, 2014, the Company’s net inventory balance reflected on its balance sheet included $3.7 million (2013: $1.7 million) of consignment inventory.
Property, plant and equipment
Property, plant and equipment are stated at cost less accumulated depreciation, with the exception of land which is not depreciated. Subsequent costs are included in an asset’s carrying amount or recognized as a separate asset, as appropriate, only when it is probable that future economic benefits associated with the item will flow to the Company. Property, plant and equipment is depreciated over its expected useful economic life on a straight-line basis over the following periods:
| | |
Buildings | | 20 years |
Fixtures, plant and equipment | | 2 - 20 years |
Motor vehicles | | 4 - 5 years |
The residual value and useful lives of property, plant and equipment are reviewed annually and adjusted, if appropriate, at each end of reporting period.
On disposal of property, plant and equipment, the cost and related accumulated depreciation and impairments are removed from the financial statements and the net amount, less any proceeds, is recognized through profit or loss in the consolidated statements of operations.
The carrying amounts of the property, plant and equipment are reviewed at each year end date to determine whether there is any indication of impairment. If any such indication exists, the asset’s recoverable amount is estimated and an impairment provision is recorded through profit or loss in the consolidated statements of operations.
Intangible assets
Intangible assets, other than goodwill, are recognized where they are separable or arising from contractual or other legal rights and can be reliably measured. They are stated at cost (cost being the fair value at the date of acquisition where they relate to a business combination) less accumulated amortization and impairment losses.
F-16
GLOBAL CONSOLIDATED AESTHETICS LIMITED
Notes to Consolidated Financial Statements—Continued
Amortization is charged to profit and loss accounts in the consolidated statements of operations on a straight-line basis over the following useful economic lives:
| | |
Category | | Useful Economic Life |
Brands | | 15 years |
Trademarks | | 10 years |
Customer relationships | | 15 years |
Technology | | 5 - 10 years |
Registration | | Period of registration |
Research and development expenditures (subject to the criteria below) | | 5 years |
Goodwill
Goodwill on acquisitions is initially measured at cost, representing the excess of the fair value of the consideration paid over the acquirer’s interest in the net fair value of the identifiable asset, liabilities and contingent liabilities. Goodwill is reviewed for impairment annually or more frequently if events or changes in circumstances indicate that the carrying value may be impaired, using a recovery method based on expected cash flows.
Impairment
For impairment testing purposes, assets are grouped at the lowest levels for which there are largely independent cash inflows (either individual cash-generating assets or grouped as CGUs. As a result, some assets are tested individually for impairment and some are tested at the CGU level.
Goodwill is allocated to those CGUs that are expected to benefit from synergies of a related business combination and represent the lowest level within the Company at which management monitors goodwill. All CGUs are tested for impairment at least annually.
The Company’s corporate assets do not generate separate cash inflows. If there is an indication that a corporate asset may be impaired, then the recoverable amount is determined for the Company’s CGU to which the corporate asset belongs.
An impairment loss is recognized for the amount by which the asset’s (or the CGUs) carrying amount exceeds its recoverable amount, which is the higher of fair value less costs of disposal and value-in-use. To determine value-in-use, management estimates expected future cash flows from each CGU and determines a suitable discount rate in order to calculate the present value of those cash flows. The data used for impairment testing procedures are directly linked to the Company’s latest approved budget, adjusted as necessary to exclude the effects of future reorganizations and asset enhancement where relevant. Discount factors are determined individually for each CGU and reflect current market assessments of the time value of money and CGU asset-specific risk factors.
An impairment loss is recognized if the carrying amount of an asset or its CGU exceeds its estimated recoverable amount. Impairment losses are recognized in the statement of operations. Impairment losses recognized in respect of CGUs are allocated first to reduce the carrying amount of any goodwill allocated to the units, and then to reduce the carrying amounts of the other assets in the CGU on a pro rata basis.
With the exception of goodwill, all assets are subsequently reassessed for indications that an impairment loss previously recognized may no longer exist. An impairment loss is reversed if the asset’s or CGU’s recoverable amount exceeds its carrying amount.
F-17
GLOBAL CONSOLIDATED AESTHETICS LIMITED
Notes to Consolidated Financial Statements—Continued
Share capital
Ordinary shares
Ordinary shares are classified as equity. Incremental costs directly attributable to the issue of ordinary shares and share options are recognized as a reduction from equity, net of any income tax effects.
Preference share capital
Preference share capital is classified as a financial liability if it is redeemable on a specific date or at the option of the shareholders, or if dividend payments are not discretionary. Legally cumulative dividends thereon are recognized as interest expense in profit or loss as accrued. The B Preference Shares and C Preference Shares are classified as a debt instrument due to their redemption features.
Conversion options attaching to shares
If a conversion option attaching to shares will be settled by the entity delivering a fixed number of shares for a fixed amount of cash or another financial asset in the functional currency of the entity, then the conversion option is an equity instrument of the entity. However, if a conversion option attaching to shares will be settled by the entity delivering a variable number of shares for a fixed amount of cash in the functional currency of the entity, or a fixed number of shares for a variable amount of cash in the functional currency of the entity, then the conversion option is a derivative financial asset or liability of the entity. The Company’s conversion options are settled in Euro-denominated shares.
Dividends
Dividend distributions to the Company’s shareholders are recognized in the financial statements as they are paid or if they have been approved by the shareholders before the end of the financial year. Dividends approved but unpaid before the end of the financial year are recognized as a liability in the Company’s financial statements. Legally cumulative dividends on cumulative B Preference Shares and C Preference Shares are recognized as deductions from income from continuing operations attributable to ordinary shareholders if the cumulative B Preference Shares and C Preference Shares are classified as permanent or temporary equity. Legally cumulative dividends on B Preference Shares and C Preference Shares that are classified as a liability are recognized as interest expense in profit or loss as accrued.
Leased assets
Finance (Capital) leases
Items of property, plant and equipment acquired under finance leases are included in the statement of financial position at the lower of their equivalent capital value or the present value of the minimum lease payments, and are depreciated over the shorter of the lease term or their useful lives. The corresponding liabilities are recorded as interest-bearing loans and borrowings and the interest element of the finance lease rentals are charged through profit or loss in the consolidated statements of operations on an annuity basis.
Operating leases
Rental payments under operating leases are charged through profit or loss in the consolidated statements of operations on a straight-line basis over the lease period, taking into account any lease incentives or escalators.
F-18
GLOBAL CONSOLIDATED AESTHETICS LIMITED
Notes to Consolidated Financial Statements—Continued
Other income and expense
Other income includes interest income on short or long-term investments, including bank deposits, which is recognized in the consolidated statements of operations as it accrues, using the effective interest rate method.
Other expenses include interest expense on borrowings, interest expense on finance leases, unwinding of discounts on provisions which are recognized in the consolidated statements of operations using the effective interest rate method where applicable, fair value movement on derivative financial instruments and fees/charges levied by its bankers. Finance expenses also include currency gains and losses on the retranslation of foreign currency denominated financial instruments and imputed interest charges in relation to zero interest free loans.
Also included within other income and expenses is the finance cost on pension liabilities which is recognized in the consolidated statements of operations in accordance with IAS 19.
Advertising and marketing
Advertising and marketing costs are expensed as incurred, and classified in distribution and selling expenses, which are contained within operating expenses in the consolidated statements of operations.
Share-based payments
The Company operates an equity-settled share-based payment plan which includes the issuance of share options to certain Directors and Senior Managers of the Company and its subsidiaries and any other person nominated by the Board in accordance with the provisions set out in the Share Option Plan (see Note 15).
Equity-settled transactions
The cost of equity-settled transactions is determined by the fair value at the date when the grant is made, using an appropriate valuation model.
That cost is recognized, together with a corresponding increase in share-based payment reserves in equity, over the vesting period (see Note 15). The cumulative expense recognized for equity-settled transactions at each reporting date, until the vesting date, reflects the extent to which the vesting period has expired and the Company’s best estimate of the number of equity instruments that will ultimately vest. The expenses or credit for a period in the consolidated statements of operations represents the movement in cumulative expense recognized as at the beginning and end of that period and is recognized in employee benefits expense.
No expense is recognized for awards that do not ultimately vest, except for equity-settled transactions for which vesting is conditional upon a market or non-vesting condition. These are treated as vesting, irrespective of whether or not the market or non-vesting condition is satisfied, provided that all other performance and/or service conditions are satisfied.
When the terms of an equity-settled award are modified, the minimum expense recognized is the expense had the terms not been modified, if the original terms of the award are met. An additional expense is recognized for any modification that increases the total fair value of the share-based payment transaction, or is otherwise beneficial to the employee as measured at the date of modification. Share-based payment compensation expense is classified within the consolidated statements of operations, according to the employees’ functional classification.
F-19
GLOBAL CONSOLIDATED AESTHETICS LIMITED
Notes to Consolidated Financial Statements—Continued
Income tax
Income tax expense (benefit) on the profit or loss for the year comprises both current and deferred taxes. Taxation is recognized in profit or loss in the consolidated statements of operations except to the extent that it relates to items recognized in other comprehensive income or directly in equity, in which case the related tax is recognized in other comprehensive income or directly in equity, respectively. Current tax is the expected tax payable on the taxable income for the year, using tax rates and laws that have been enacted or substantially enacted at the reporting period end date, and any adjustment to tax payable in respect of previous periods.
Deferred tax is provided using the balance sheet liability method, providing for temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for taxation purposes. If the deferred tax arises from initial recognition of an asset or liability in a transaction other than a business combination that, at the time of the transaction does not affect accounting or taxable profit or loss, it is not recognized. Deferred tax is provided on temporary differences arising on investments in subsidiaries and joint ventures, except where the timing of the reversal of the temporary difference is controlled by the Company and it is probable that the temporary difference will not reverse in the foreseeable future. The amount of deferred tax provided is based on the expected manner of realization or settlement of the carrying amount of assets and liabilities, using tax rates enacted or substantively enacted at the reporting year end date.
A deferred tax asset is recognized only to the extent that it is probable that future taxable profits will be available against which the asset can be utilized. Deferred tax assets are reduced to the extent that it is no longer probable that the related tax benefit will be realized.
Earnings per share
The Company calculates basic earnings per share (“EPS”) using the Two-Class Method for purposes of SEC presentation. For SEC presentation purposes, the Company is required to comply with the requirements of U.S. GAAP Accounting Standards Codification 260,Earnings Per Share. This method requires the income (loss) per share for ordinary shares to be calculated assuming 100% of the Company’s earnings are distributed as dividends to ordinary shareholders and participating security shareholders, based on their respective dividend rights, regardless of whether the Company actually distributes its earnings as dividends. Under Irish law, cash dividends (distributions) are not allowed when the Company has an accumulated deficit. Cumulative dividends on the B Preference Shares and C Preference Shares, which are classified as liabilities, are recognized as interest expense using the effective interest rate method (see Note 7).
For the basic earnings per share calculation, net income available to shareholders is allocated among our ordinary shares and participating securities. The allocation is based on the Two-Class Method on a one-for-one per share basis, as ordinary shares and convertible preference shares share pro rata in earnings. Only ordinary shares share in losses since there is no legal obligation for participating securities to fund losses. Net income or loss available to shareholders is allocated using this method.
For periods in which the Company has income from continuing operations, basic earnings per share applicable to ordinary shareholders is computed by dividing allocated earnings applicable to ordinary shareholders by the weighted-average number of ordinary shares outstanding. Income applicable to ordinary shareholders is net of the following: dividend interest expense, foreign exchange fluctuations recognized on the B Preference Shares and C Preference Shares, and fair value changes in the equity derivative.
For periods in which the Company has a loss from continuing operations, basic earnings per share applicable to ordinary shareholders is computed by dividing the loss applicable to ordinary shareholders by the weighted-average number of ordinary shares outstanding. Losses are not allocable to participating securities (preference shares).
F-20
GLOBAL CONSOLIDATED AESTHETICS LIMITED
Notes to Consolidated Financial Statements—Continued
Diluted net earnings per share assumes the conversion of all convertible securities that are convertible into ordinary shares, if dilutive, using the If-Converted Method, and includes any dilutive effect of share options and warrants using the treasury stock method. When income from continuing operations is a loss, basic earnings per share equals fully diluted earnings per share.
The following summarizes the weighted-average number of ordinary shares outstanding during the year:
| | | | | | | | |
| | | Year Ended December 31, | |
| | | 2014 | | | 2013 | |
Weighted average ordinary shares outstanding for basic earnings per share | | | 4,772,101 | | | | 4,425,800 | |
| | | | | | | | |
Weighted average ordinary shares outstanding for fully diluted earnings per share | | | 4,772,101 | | | | 4,425,800 | |
| | | | | | | | |
Weighted average anti-dilutive shares resulting from: | | | | | | | | |
Share Options | | | 41,950 | | | | — | |
Ordinary Share Warrants | | | 1,218,242 | | | | 1,482,208 | |
B Preference Share Warrants | | | 182,027 | | | | 177,603 | |
B Preference Shares | | | 14,459,209 | | | | 6,527,774 | |
C Preference Shares | | | 1,256,868 | | | | — | |
| | | | | | | | |
Weighted average anti-dilutive shares | | | 17,158,296 | | | | 8,187,585 | |
| | | | | | | | |
Options to purchase 1,179,791 and –0– ordinary shares at December 31, 2014 and 2013, respectively, at price per option of €0.01 ($0.0121 and $0.0138 at December 31, 2014 and 2013) were outstanding but were not included in the computation of diluted EPS because they were anti-dilutive. The options, which expire on December 18, 2021, were still outstanding at the end of 2014.
Ordinary share warrants to purchase 1,166,589 and 1,566,589 ordinary shares at December 31, 2014 and 2013, respectively, at a price per ordinary share warrant of €0.01 ($0.0121 and $0.0138 at December 31, 2014 and 2013, respectively) were outstanding but were not included in the computation of diluted EPS because they were also anti-dilutive. During 2014, 400,000 warrants to purchase ordinary shares were exercised.
B Preference Share warrants to purchase 182,335 and 182,335 B Preference Shares, at a price per B Preference Share warrant of €0.01 ($0.0121 and $0.0138 at December 31, 2014 and 2013, respectively) were outstanding at December 31, 2014 and 2013, respectively, but were not included in the computation of diluted EPS because they were also anti-dilutive.
B Preference Shares, convertible into 15,140,757 and 6,527,774 ordinary shares at December 31, 2014 and 2013, respectively, at a one–for–one share exchange, were issued and outstanding at December 31, 2014 and 2013 but were not included in the computation of diluted EPS because they were anti-dilutive.
C Preference Shares, convertible into 1,451,762 and –0– ordinary shares at December 31, 2014 and 2013, respectively, at a one–for–one share exchange, were issued and outstanding at December 31, 2014 and 2013 but were not included in the computation of diluted EPS because they were anti-dilutive.
Financial instruments
Non-derivative financial instruments
Non-derivative financial instruments comprise trade receivables, prepayments and other receivables, cash and cash equivalents, restricted cash, loans and borrowings, and trade payables, accruals and other creditors, and deferred considerations.
F-21
GLOBAL CONSOLIDATED AESTHETICS LIMITED
Notes to Consolidated Financial Statements—Continued
Derivative financial instruments
Derivative financial instruments are financial instruments where their value changes in response to an underlying variable, requires little or no initial investment and is settled at a future date. They are recognized initially at fair value and thereafter are stated at fair value.
Fair value is the amount which an asset could be exchanged, or a liability settled, between knowledgeable willing parties in an arms-length transaction under current market conditions. Where derivatives do not fulfil the criteria for hedge accounting, changes in fair values are reported through profit or loss in the consolidated statements of operations. For the years presented, hedge accounting has not been applied, therefore any resultant gains or losses have been recognized in profit or loss in the consolidated statements of operations (see Note 21).
Trade and other receivables and payables
Trade and other receivables and payables are initially recorded at fair value, and thereafter net of provisions for uncollectible accounts, which approximates their fair value given the short-term nature of these assets and liabilities. The provision for impairment of trade receivables is established when there is objective evidence that the Company will not be able to collect all amounts due according to the original term of the receivables.
The Company’s charge-off policy is to charge-off uncollectible amounts when the amount has been provided for at least a year, and the Company determines there is a low probability of recovery.
Cash and cash equivalents
Cash and cash equivalents includes cash at the bank and in hand, deposits held with banks and other short-term highly liquid investments with original maturities of three months or less.
Restricted cash
Restricted cash reflects cash balances that are not available to the Company for use in operations.
Loans and borrowings
Loans and borrowings are recognized initially at fair value, net of transaction costs incurred. Borrowings are subsequently stated at amortized cost; any difference between the proceeds (net of transaction costs) and the redemption value is recognized through profit or loss in the consolidated statements of operations over the period of the borrowings using the effective interest rate method.
Determination of fair values
Certain of the Company’s accounting policies and disclosures require the determination of fair value, for both financial and non-financial assets and liabilities. Fair values have been determined for measurement and/or disclosure purposes based on the following methods. When applicable, further information about the assumptions made in determining fair values is disclosed in the notes specific to that asset or liability.
Loans and borrowings and other financial instruments
The fair value of loans and borrowings and other financial instruments is estimated as the present value of future cash flows, discounted at the market rate of interest at the reporting date.
F-22
GLOBAL CONSOLIDATED AESTHETICS LIMITED
Notes to Consolidated Financial Statements—Continued
Derivatives
Equity derivatives have been valued using an option pricing model, where the main inputs are the share price, expected volatility of the share price, anticipated term to a liquidity event, expected exercise price and probability attached to a liquidity event.
Retirement benefits
Defined contribution pension plans
Obligations for contributions to defined contribution pension plans are recognized as an expense in the consolidated statements of operations, as incurred.
Post-retirement benefits
In accordance with statutory requirements in France, one of the Company’s principal subsidiaries provides post-retirement benefits to certain employees. This post retirement benefit is accounted for as a defined benefit plan in accordance with IAS 19,Employee Benefits. This plan is unfunded.
The calculation of the obligations accruing is performed using the projected unit credit method. The costs relating to this plan primarily consist of the increase in the actuarial present value of the obligation for projected benefits based on employee service during the year and the interest on this obligation in respect of employee service in previous periods. The obligation is discounted using a rate that represents the yield at the reporting date on a high quality corporate bond that have maturity dates approximating the terms of the Company’s post-retirement benefit obligations. Any actuarial gains or losses arising are recognized in the Company’s consolidated statements of other comprehensive income (loss). Current service cost and interest income (expense) are recognized in the consolidated statements of operations.
Provisions
A provision is recognized in the statement of financial position when the Company has a legal or constructive present obligation as a result of a past event, it is probable that an outflow of economic benefits will be required to settle the obligation and the amount of the obligation can be measured reliably. Where the effect of the time value of money is material, provisions are determined by discounting the expected future cash flows at a pre-tax rate that reflects current market assessments of the time value of money and the risk specific to the liability.
Liquidity
The Company has historically generated net losses and negative cash flows from operations. During the year ended December 31, 2014, the Company used $12.9 million of cash in operations. At December 31, 2014 the Company had cash and cash equivalents of $10.6 million, restricted cash of $3.6 million and an accumulated deficit of $183.5 million. These conditions indicate the existence of substantial doubt over the Company’s ability to continue as a going concern.
Management assessed the operations of the Company, and considered the following significant factors, among others, to conclude that the Company is a going concern and will meet its liabilities as and when they become due for a period of at least twelve months from the date the consolidated financial statements were authorized for issue:
| | • | | Sales in fiscal year 2014 increased by 18% compared to fiscal year 2013 and are expected to continue to increase in fiscal years 2015 and 2016. |
F-23
GLOBAL CONSOLIDATED AESTHETICS LIMITED
Notes to Consolidated Financial Statements—Continued
| | • | | In 2015, the Company obtained an additional loan from Royalty Opportunities S.À R.L. amounting to $10.0 million. |
| | • | | The Company has no material debt obligation maturing in the period to December 31, 2018. |
| | • | | The Company plans to further expand its direct sales and marketing activities which management believes will increase sales and generate higher margins. |
| | • | | Further, the Company plans to expand its sales and marketing activities in other female aesthetics products, including those associated with implant surgery as well as other procedures, such as body sculpting treatments and dermal fillers. |
| | • | | Management plans to capitalize on the favorable trends in the aesthetics market and benefit from the underlying growth in women’s disposable income, particularly in emerging geographies. |
| | • | | The Company is currently exploring additional financing options including the possibility of an initial public offering. |
Pursuant to this plan the Company expects to require additional capital to finance the anticipated growth. The Company’s ability to meet its obligations is dependent on meeting managements plan. There can be no assurance the Company will be successful and failure to generate revenue and margin growth or raise additional capital may adversely impact on the Company’s ability to achieve its intended business objectives.
After carefully considering the above factors, the Directors have concluded that the going concern basis of financial statement presentation is appropriate.
Concentration of risks and uncertainties
Financial instruments that potentially subject us to concentrations of credit risk consist primarily of trade accounts receivable. The Company monitors the creditworthiness of our customers to which we grant credit terms in the normal course of our business, as well as the general economic and political conditions in the countries where they operate. Concentrations of credit risk associated with these receivables are monitored on an ongoing basis.
The Company does not normally require collateral or other security to support normal credit sales (see Note 4).
Trade accounts receivable are generated from direct sales and sales to distributors, in Europe, Latin America, Asia and other parts of the world. The general economy and competition in the marketplace, regulatory changes, litigation and other considerations may impact our sales volume and, consequently, an adverse change in either of these factors could negatively affect our consolidated net sales. We operate throughout Latin America, which we believe is subject to greater political, monetary, economic and regulatory risks than our operations in Europe and other parts of the world.
The Company does not have a significant customer concentration risk.
The Company is dependent on the ability of our suppliers to provide raw manufacturing products on a timely basis at favorable pricing terms. The loss of certain principal suppliers, significant increased acquisition costs of raw materials or a significant reduction in raw material availability could have a material adverse effect on our operations, cash flows and financial position.
F-24
GLOBAL CONSOLIDATED AESTHETICS LIMITED
Notes to Consolidated Financial Statements—Continued
Accounting for business combinations
From January 1, 2010, the Company has applied IFRS 3Business Combinations (2008). This change of accounting policy has been applied prospectively.
The Company’s policy is that business combinations are accounted for using the acquisition method as at the acquisition date, which is the date on which control is transferred to the Company. There is control when the Company is exposed to, or has rights to, variable returns from its involvement with the entity and has the ability to affect these returns through its power over the entity.
Acquisitions on or after January 1, 2010
The Company’s policy for accounting for acquisitions after January 1, 2010 is that goodwill is measured at the acquisition date as:
| | • | | the fair value of the consideration transferred; plus |
| | • | | the recognized amount of any non-controlling interests in the acquisition; plus |
| | • | | if the business combination is achieved in stages, the fair value of the existing equity interest acquisition; less |
| | • | | the net recognized amount (generally fair value) of the identifiable assets acquired and liabilities assumed, including indefinite-lived and definite-lived intangible assets |
When the sum of these components is positive, goodwill is recognized in that amount. When the sum of these components is negative, a bargain purchase on acquisition is recognized immediately in the Company’s consolidated statements of operations.
The consideration transferred does not include amounts related to settlement of pre-existing relationships. Such amounts are generally recognized the Company’s statement of operations as satisfaction of previously recognized assets or liabilities.
Costs related to the acquisition that the Company incurs in connection with a business combination, other than those associated with the issue of debt or equity securities, are expensed as incurred.
Deferred purchase consideration
To the extent that deferred purchase consideration is payable after more than one year from the date of acquisition, it is discounted at an appropriate loan interest rate and, accordingly, is carried at net present value on the consolidated statements of financial position. An appropriate interest charge, at a constant rate on the carrying amount adjusted to reflect market conditions, is reflected in the consolidated statements of operations over the period, increasing the value of the provision so that the obligation will reflect its settlement value at the time of maturity.
F-25
GLOBAL CONSOLIDATED AESTHETICS LIMITED
Notes to Consolidated Financial Statements—Continued
3. Inventories
| | | | | | | | |
| | | December 31, | |
| | | 2014 | | | 2013 | |
| | | (in thousands) | |
Raw materials | | $ | 2,847 | | | $ | 2,190 | |
Work in progress | | | 1,504 | | | | 1,442 | |
Finished goods, net | | | 6,907 | | | | 6,100 | |
Consignment | | | 3,671 | | | | 1,745 | |
| | | | | | | | |
Total inventories | | $ | 14,929 | | | $ | 11,477 | |
| | | | | | | | |
The cost of inventories recognized as an expense in cost of sales during the year ended December 31, 2014 amounted to $24.1 million(2013: $22.0 million). The provision for obsolete stock at December 31, 2014 was $0.9 million(2013: $0.7 million).
4. Trade receivables, prepayments and other receivables
| | | | | | | | |
| | | December 31, | |
| | | 2014 | | | 2013 | |
| | | (in thousands) | |
Trade receivables | | $ | 13,912 | | | $ | 12,237 | |
Less, provision for impairment losses | | | (1,072 | ) | | | (2,231 | ) |
| | | | | | | | |
Trade receivables, net of provision | | | 12,840 | | | | 10,006 | |
Prepayments and other receivables | | | 4,539 | | | | 4,574 | |
| | | | | | | | |
Trade receivables, prepayments and other receivables | | $ | 17,379 | | | $ | 14,580 | |
| | | | | | | | |
The fair value of trade and other receivables is considered to equal the net carrying values above. Further details are set out in Note 20.
The Company’s exposure to credit and currency risks, and impairment losses related to trade receivables, is disclosed in Note 20.
F-26
GLOBAL CONSOLIDATED AESTHETICS LIMITED
Notes to Consolidated Financial Statements—Continued
5. Property, plant and equipment
| | | | | | | | | | | | | | | | |
| | | Land
and
buildings | | | Fixtures,
plant and
equipment | | | Motor
vehicles | | | Total | |
| | | (in thousands) | |
Cost | | | | | | | | |
Balance, January 1, 2013 | | $ | 3,333 | | | $ | 6,769 | | | $ | 406 | | | $ | 10,508 | |
Additions | | | — | | | | 312 | | | | 56 | | | | 368 | |
Disposals | | | — | | | | (100 | ) | | | — | | | | (100 | ) |
Foreign exchange | | | 147 | | | | 268 | | | | 24 | | | | 439 | |
| | | | | | | | | | | | | | | | |
Balance, December 31, 2013 | | | 3,480 | | | | 7,249 | | | | 486 | | | | 11,215 | |
| | | | | | | | | | | | | | | | |
Additions | | | — | | | | 1,360 | | | | 54 | | | | 1,414 | |
Disposals | | | — | | | �� | (76 | ) | | | (86 | ) | | | (162 | ) |
Foreign exchange | | | (409 | ) | | | (862 | ) | | | (54 | ) | | | (1,325 | ) |
| | | | | | | | | | | | | | | | |
Balance, December 31, 2014 | | $ | 3,071 | | | $ | 7,671 | | | $ | 400 | | | $ | 11,142 | |
| | | | | | | | | | | | | | | | |
Depreciation | | | | | | | | | | | | | | | | |
Balance, January 1, 2013 | | $ | 1,082 | | | $ | 3,560 | | | $ | 243 | | | $ | 4,885 | |
Charge | | | 234 | | | | 833 | | | | 108 | | | | 1,175 | |
Disposals | | | — | | | | (84 | ) | | | — | | | | (84 | ) |
Foreign exchange | | | 56 | | | | 194 | | | | 13 | | | | 263 | |
| | | | | | | | | | | | | | | | |
Balance, December 31, 2013 | | | 1,372 | | | | 4,503 | | | | 364 | | | | 6,239 | |
| | | | | | | | | | | | | | | | |
Charge | | | 233 | | | | 816 | | | | 74 | | | | 1,123 | |
Disposals | | | — | | | | (61 | ) | | | (82 | ) | | | (143 | ) |
Foreign exchange | | | (181 | ) | | | (529 | ) | | | (41 | ) | | | (751 | ) |
| | | | | | | | | | | | | | | | |
Balance, December 31, 2014 | | $ | 1,424 | | | $ | 4,729 | | | $ | 315 | | | $ | 6,468 | |
| | | | | | | | | | | | | | | | |
Net book value: | | | | | | | | |
At December 31, 2014 | | $ | 1,647 | | | $ | 2,942 | | | $ | 85 | | | $ | 4,674 | |
| | | | | | | | | | | | | | | | |
At December 31, 2013 | | $ | 2,108 | | | $ | 2,746 | | | $ | 122 | | | $ | 4,976 | |
| | | | | | | | | | | | | | | | |
Included in the net book value of property, plant and equipment at December 31, 2014 is $1.8 million (2013: $2.3 million) of assets held under finance leases. Depreciation on these assets during the year ended December 31, 2014 amounted to $0.3 million (2013: $0.3 million).
The depreciable component of land and buildings at December 31, 2014 was $2.9 million (2013: $3.3 million).
Security
At December 31, 2014, Royalty Opportunities S.À R.L. held a security interest via a fixed and floating charge over land and buildings, fixtures, plant and equipment, motor vehicles, intangible assets, inventory and receivables in the subsidiaries of the Company.
F-27
GLOBAL CONSOLIDATED AESTHETICS LIMITED
Notes to Consolidated Financial Statements—Continued
6. Goodwill and other intangible assets
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Trademarks | | | Brands | | | Customer
base | | | Technology | | | Registrations | | | Goodwill | | | Research and development | | | Total | |
| | | (in thousands) | |
Cost | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance, January 1, 2013 | | $ | 3,637 | | | $ | 20,368 | | | $ | 19,700 | | | $ | 20,194 | | | $ | — | | | $ | 2,442 | | | $ | 1,202 | | | $ | 67,543 | |
Additions | | | — | | | | — | | | | — | | | | — | | | | 228 | | | | — | | | | 109 | | | | 337 | |
Foreign exchange | | | 161 | | | | 899 | | | | 870 | | | | 869 | | | | 9 | | | | 108 | | | | 58 | | | | 2,974 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance, December 31, 2013 | | | 3,798 | | | | 21,267 | | | | 20,570 | | | | 21,063 | | | | 237 | | | | 2,550 | | | | 1,369 | | | | 70,854 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Additions | | | — | | | | — | | | | — | | | | 1,398 | | | | 80 | | | | — | | | | — | | | | 1,478 | |
Foreign exchange | | | (449 | ) | | | (2,509 | ) | | | (2,427 | ) | | | (2,615 | ) | | | (40 | ) | | | (300 | ) | | | (162 | ) | | | (8,502 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance, December 31, 2014 | | $ | 3,349 | | | $ | 18,758 | | | $ | 18,143 | | | $ | 19,846 | | | $ | 277 | | | $ | 2,250 | | | $ | 1,207 | | | $ | 63,830 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Trademarks | | | Brands | | | Customer
base | | | Technology | | | Registrations | | | Goodwill | | | Research
and
development | | | Total | |
| | | (in thousands) | |
Amortization and impairment | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance, January 1, 2013 | | $ | 3,637 | | | $ | 13,088 | | | $ | 6,018 | | | $ | 9,855 | | | $ | — | | | $ | — | | | $ | 374 | | | $ | 32,972 | |
Amortised | | | — | | | | 704 | | | | 1,323 | | | | 1,836 | | | | — | | | | — | | | | 170 | | | | 4,033 | |
Foreign exchange | | | 161 | | | | 604 | | | | 314 | | | | 503 | | | | — | | | | — | | | | 45 | | | | 1,627 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance, December 31, 2013 | | | 3,798 | | | | 14,396 | | | | 7,655 | | | | 12,194 | | | | — | | | | — | | | | 589 | | | | 38,632 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Amortised | | | — | | | | 704 | | | | 1,322 | | | | 1,682 | | | | 57 | | | | — | | | | 225 | | | | 3,990 | |
Foreign exchange | | | (449 | ) | | | (1,759 | ) | | | (1,016 | ) | | | (1,414 | ) | | | (6 | ) | | | — | | | | (89 | ) | | | (4,733 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance, December 31, 2014 | | $ | 3,349 | | | $ | 13,341 | | | $ | 7,961 | | | $ | 12,462 | | | $ | 51 | | | $ | — | | | $ | 725 | | | $ | 37,889 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Trademarks | | | Brands | | | Customer
base | | | Technology | | | Registrations | | | Goodwill | | | Research
and
development | | | Total | |
| | | (in thousands) | |
Net carrying values | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
At December 31, 2014 | | $ | — | | | $ | 5,417 | | | $ | 10,182 | | | $ | 7,384 | | | $ | 226 | | | $ | 2,250 | | | $ | 482 | | | $ | 25,941 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
At December 31, 2013 | | $ | — | | | $ | 6,871 | | | $ | 12,915 | | | $ | 8,869 | | | $ | 237 | | | $ | 2,550 | | | $ | 780 | | | $ | 32,222 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Amortization period | | | 10 years | | | | 15 years | | | | 15 years | | | | 5-10 years | | |
| Period of
registration |
| | | N/A | | | | 5 years | | | | | |
Average remaining life | | | — | | | | 7.69 | | | | 7.70 | | | | 4.39 | | | | 3.96 | | | | Indefinite | | | | 2.14 | | | | | |
Amortization charges are included within operating expenses in the consolidated statements of operations. The loans payable to Royalty Opportunities S.À R.L. are secured by the assets of the Company which includes the Company’s intangible assets.
The Company does not renew or extend the term of intangible assets, with the exception of Registrations, which give the Company the right to sell its implantable medical device products within a specific country.
F-28
GLOBAL CONSOLIDATED AESTHETICS LIMITED
Notes to Consolidated Financial Statements—Continued
Goodwill acquired through business combinations was allocated at acquisition to the Company’s CGUs identified according to specific geographical region of operation. A summary of goodwill at December 31, 2014 and December 31, 2013 is presented below:
| | | | | | | | | | | | | | | | | | | | |
| | | December 31,
2012 | | | Foreign
exchange
effect | | | December 31,
2013 | | | Foreign
exchange
effect | | | December 31,
2014 | |
| | | (in thousands) | |
France | | $ | 2,112 | | | $ | 93 | | | $ | 2,205 | | | $ | (259 | ) | | $ | 1,946 | |
UK | | | 330 | | | | 15 | | | | 345 | | | | (41 | ) | | | 304 | |
| | | | | | | | | | | | | | | | | | | | |
Total | | $ | 2,442 | | | $ | 108 | | | $ | 2,550 | | | $ | (300 | ) | | $ | 2,250 | |
| | | | | | | | | | | | | | | | | | | | |
The Company’s weighted average amortization period for definite-lived intangible assets is 5.94 years. The estimated amortization expense associated with intangible assets over the next five years is as follows:
| | | | |
Year | | Estimated
aggregate
amortization | |
| | | (in thousands) | |
2015 | | $ | 3,990 | |
2016 | | | 3,990 | |
2017 | | | 3,797 | |
2018 | | | 3,763 | |
2019 | | | 2,026 | |
| | | | |
Total | | $ | 17,566 | |
| | | | |
Impairment test on goodwill and intangible assets
The Company performs annual testing for impairment of goodwill, and for other definite-lived intangible assets, whether or not there is any indication that those assets may be impaired. The Company calculates the asset’s recoverable amount in accordance with IAS 36.
An impairment loss is recognized for the amount, if any, by which an asset’s carrying amount exceeds its recoverable amount. The recoverable amount is based on the discounted present values of the future cash flows (on avalue in use basis) expected to arise from the CGU to which the asset relates. A CGU is generally based on a specific geographical region of operations within the Company.
The cash flow forecasts employed in determining value in use are extracted from the budget and cash flows for the subsequent five years, and are projected based on a current assessment of anticipated market conditions and future trends over that period. The key assumptions in the cash flow forecasts include management’s estimates of future profitability, growth rates ranging from 5 to 25%, replacement capital expenditure requirements, trade working capital investment, and tax considerations. Cash flows beyond this five-year period are estimated, in accordance with IFRS, into perpetuity using a terminal growth rate of 5%. The growth rate is based on the historic pattern of the Company operations. The recoverable amount resulting from this exercise represents the present value of future cash flows, including terminal value, discounted at rates between 17% and 18%, with reference to the Company’s weighted average cost of capital of 15%. The discount rates also incorporate risk factors relating to market circumstances and, where appropriate, specific CGUs.
F-29
GLOBAL CONSOLIDATED AESTHETICS LIMITED
Notes to Consolidated Financial Statements—Continued
No impairment charge has arisen from the impairment review in the current year and the Company is satisfied that, based on its sensitivity analysis review, any reasonable changes to the inputs would not give rise to material impairment losses. The Company also considered fair value less cost to sell, based on valuations of the Company, approved by the Board, which further supported this assessment.
The most recent tests were performed in the last quarter of 2014.
7. Share capital
Authorized:
26,000,000 (2013: 20,000,000) ordinary shares of €0.01 ($0.0121) each
2,846,200 (2013: 1,050,200) A Ordinary Shares of €0.01 ($0.0121) each
–0– (2013: 525,100) B Ordinary Shares of €0.01 ($0.0121) each
16,000,000 (2013: 6,693,267) 8% B Preference Shares of €0.01 ($0.0121) each
1,500,000 (2013: –0–) 8% C Preference Shares of €0.01 ($0.0121) each
Warrants issued but not exercised:
1,166,589 (2013: 1,566,589) ordinary share warrants of €0.01 ($0.0121) each
182,335 (2013: 182,335) B Preference Share warrants of €0.01 ($0.0121) each
| | | | | | | | |
| | | December 31, | |
| | | 2014 | | | 2013 | |
| | | (in thousands) | |
Allotted, called up and fully paid: | | | | | | | | |
4,825,800 (2013: 4,425,800) ordinary shares of €0.01($0.0121) each | | $ | 70,164 | | | $ | 64,682 | |
15,140,757 (2013: 6,527,774) B Preference Shares of €0.01 ($0.0121) each | | | 206,557 | | | | 91,141 | |
1,451,762 (2013: –0–) C Preference Shares of €0.01 ($0.0121) each | | | 19,454 | | | | — | |
| | | | | | | | |
| | $ | 296,175 | | | $ | 155,823 | |
| | | | | | | | |
There were 400,000 ordinary share warrants exercised during the year. There were no additional ordinary share warrants and B Preference Share warrants issued during the year.
Share premium
The share premium reserve arises from premiums paid over the par value of issued ordinary share capital. The share premium is reduced by the issue of bonus shares where relevant and, upon conversion of zero coupon loan notes, includes any premium over the par value of the issued shares in exchange for the present value of the liability converted.
F-30
GLOBAL CONSOLIDATED AESTHETICS LIMITED
Notes to Consolidated Financial Statements—Continued
The movements during the year in relation to the share capital and share premium accounts recognized in equity, in accordance with IFRS, are as follows:
| | | | | | | | | | | | |
| | | Included in Shareholders’ Equity | |
| | | Share capital | | | Share premium | | | Total | |
| | | (in thousands) | |
Balance, January 1, 2014 | | $ | 65 | | | $ | 21,299 | | | $ | 21,364 | |
Exercise of warrants on ordinary shares | | | 5 | | | | — | | | | 5 | |
| | | | | | | | | | | | |
Balance, December 31, 2014 | | $ | 70 | | | $ | 21,299 | | | $ | 21,369 | |
| | | | | | | | | | | | |
The movements during the year in relation to the share capital and share premium accounts recognized as liabilities, in accordance with IFRS, are as follows:
| | | | | | | | | | | | |
| | | Included in Liabilities | |
| | | Share capital | | | Share premium | | | Total | |
| | | (in thousands) | |
Balance, January 1, 2014 | | $ | 91 | | | $ | 43,419 | | | $ | 43,510 | |
B Preference Shares issued during the year | | | 116 | | | | 19,021 | | | | 19,137 | |
C Preference Shares issued during the year | | | 19 | | | | 5,981 | | | | 6,000 | |
| | | | | | | | | | | | |
Balance, December 31, 2014 | | $ | 226 | | | $ | 68,421 | | | $ | 68,647 | |
| | | | | | | | | | | | |
The holders of the B Preference Shares and C Preference Shares have a right to receive a repayment of $100.9 million and $6.0 million, respectively, plus accrued dividends on the occurrence of a liquidity event, which is defined as a liquidation, winding up or dissolution of the Company, whether voluntary or compulsory, an asset sale or a sale (“Liquidity Event”). They also have a conversion option that gives them the right to additional payments, on an as-converted basis, denominated in US dollars, the value of which is based on the value of the underlying equity shares in the Company. Consequently, the B Preference Shares and C Preference Shares and the embedded equity derivative have been classified as non-current liabilities in accordance with IAS 32. The change in carrying value of the B Preference Shares and C Preference Shares and equity derivative is set out below:
| | | | | | | | | | | | |
| | | Preference share | | | Equity derivative | | | Total | |
| | | (in thousands) | |
Opening balance, January 1, 2014 | | $ | 46,518 | | | $ | 16,741 | | | $ | 63,259 | |
B Preference Shares issued during the year | | | 10,601 | | | | 8,535 | | | | 19,136 | |
C Preference Shares issued during the year | | | 3,810 | | | | 2,190 | | | | 6,000 | |
Fair value movement on equity derivative | | | — | | | | (5,182 | ) | | | (5,182 | ) |
Interest on B Preference Shares | | | 25,577 | | | | — | | | | 25,577 | |
Loss on conversion of shareholder loans | | | 27,737 | | | | — | | | | 27,737 | |
Interest on C Preference Shares | | | 1,967 | | | | — | | | | 1,967 | |
| | | | | | | | | | | | |
Closing balance, December 31, 2014 | | $ | 116,210 | | | $ | 22,284 | | | $ | 138,494 | |
| | | | | | | | | | | | |
Foreign currency translation reserve
The foreign currency translation reserve comprises all foreign currency exchange differences arising from the translation of the net assets of the Company’s non-US dollar denominated operations, including the translation of the profits or losses of such operations for the period.
F-31
GLOBAL CONSOLIDATED AESTHETICS LIMITED
Notes to Consolidated Financial Statements—Continued
Capital contribution reserve
The capital contribution reserve arose initially as a result of the imputed interest arising on zero-coupon loan notes received by the Company from its shareholders on initial recognition amounting to $12.8 million. In 2011, as a result of the conversion of the zero coupon loan notes to equity, an amount of $2.7 million, equivalent to the amount of imputed interest charged to profit or loss in the statement of comprehensive income up to the date of conversion, has been reclassified from the capital contribution reserve to accumulated deficit. In 2014, as a result of a subordination agreement of the existing shareholders and PIK loans, the Company has recognized the difference between the carrying value of the loans and the discounted value of the loan based on the amended maturity date in the capital contribution reserve amounting to $1.0 million.
Other reserves
The amount of $3.3 million included in the shares to be issued reserve at December 31, 2012 related to the fair value of shares to be issued as part of consideration for the Company’s purchase of various brands in 2010. During the year ended December 31, 2013 the Company entered into an agreement to settle this obligation to issue these shares by paying $100,000 per month for a period of 30 months. An amount of $2.9 million, representing the fair value of the remaining monthly payments due under the agreement, has been reclassified from shares to be issued reserve to settlement in lieu of issuing shares included in other payables. The remaining amount of $0.4 million which was included within the shares to be issued reserve has been reclassified to accumulated deficit during the year ended December 31, 2013.
The gain on the settlement in lieu of issuing ordinary shares within accumulated deficit of $0.4 million at December 31, 2014 (2013: $0.4 million) is not distributable.
Classification of shares as equity and debt instruments
The proceeds from the issuance of B Preference Shares of $62.6 million (2013: $43.5 million) and C Preference Shares of $6.0 million (2013: $0) on various dates is repayable upon a Liquidity Event at $100.9 million and $6.0 million, respectively, plus accrued dividends, and has been classified as non-current liabilities.
The holders of the B Preference Shares and C Preference Shares have a conversion option which gives them the right to additional payments, on an as-converted basis, denominated in US dollars, the value of which is based on the value of the underlying equity shares in the Company. This conversion feature entitles the holder to participate in the net assets of the Company on a Liquidity Event subject to the conversion conditions set out in the Articles of Association of the Company. This is classified as an embedded equity derivative liability.
In accordance with IAS 32, the B Preference Shares and C Preference Shares and related share premium are classified as non-current financial liabilities, comprising the financial liability (debt) element and the embedded equity derivative liability. The equity derivative liability portion is initially calculated based on fair value at inception. This derivative liability is stated at fair value at each reporting date with changes in the fair value being recognized through profit or loss in the consolidated statements of operations.
Reference to liquidation event means a liquidation, winding up or dissolution of the Company whether voluntary or compulsory, an asset sale or a direct or indirect transfer or other disposal of legal and/or beneficial interest or title to 50% or more of the shares in issue or a merger or consolidation of or by the Company with or into any other entity in which the shareholders of the Company prior to the merger or consolidation do not own a majority of the surviving entity.
F-32
GLOBAL CONSOLIDATED AESTHETICS LIMITED
Notes to Consolidated Financial Statements—Continued
The ordinary shares and shareholders have the following rights attached:
| | • | | Entitled to receive notice of, to attend and to vote at general meetings of the Company; |
| | • | | Entitled to receive such dividend as declared subject to the preferential dividend rights of the B Preference Shares and C Preference Shares; and, |
| | • | | Upon a Liquidity Event, entitled to share in the remaining assets or proceeds available for distribution to the shareholders pro rata to the number of ordinary shares held, subject to the entitlement of the B Preference Shares and C Preference Shareholders. |
The ordinary shares warrants and shareholders have the following rights attached:
There are outstanding warrants issued by the Company to certain shareholders which give those shareholders the right to subscribe for up to 1,166,589 (2013: 1,566,589) ordinary shares at an exercise price of €0.01 per share. These warrants are exercisable for a period up to the earlier of (i) 30 June 2019 and (ii) the date of completion of a specified liquidity event.
The A Ordinary Shares and shareholders have the following rights attached:
| | • | | Not entitled to receive notice of, to attend or to vote at general meetings of the Company; |
| | • | | Entitled to receive such dividend as declared subject only to the preferential dividend rights of the B Preference Shares and C Preference Shares; |
| | • | | No rights to participate in the profits or capital of the Company except in respect of dividends as outlined above and upon a Liquidity Event, where they are entitled to share in the remaining assets or proceeds available for distribution, pro rata to the number of ordinary shares held, subject to the entitlement of the B Preference Shareholders and C Preference Shareholders; and |
| | • | | All A ordinary shares automatically converted into fully paid ordinary shares on a one for one basis on the date of, and immediately prior to an exchange listing. |
The B Ordinary Shares and shareholders have the following rights attached:
| | • | | Not entitled to receive notice of, to attend or to vote at any general meetings of the Company; |
| | • | | Entitled to receive such dividend as declared subject only to the preferential dividend rights of the B Preference Shares and C Preference Shares; |
| | • | | No rights to participate in the profits or capital of the Company except in respect of dividends as outlined above and upon a Liquidity Event, where they are entitled to share in the remaining assets or proceeds available for distribution, pro rata to the number of ordinary shares held, subject to the entitlement of the B Preference Shareholders and C Preference Shareholders; and, |
| | • | | All B ordinary shares automatically converted into fully paid ordinary shares on a one for one basis on the date of, and immediately prior to an exchange listing. |
The total authorized B ordinary shares were cancelled during the year.
F-33
GLOBAL CONSOLIDATED AESTHETICS LIMITED
Notes to Consolidated Financial Statements—Continued
The B Preference Shares and C Preference Shares and shareholders have the following rights attached:
| | • | | B Preference Shareholders and C Preference Shareholders are entitled to receive notice of, to attend and to vote at general meetings of the Company; |
| | • | | Entitled to appoint up to two board directors under certain conditions; |
| | • | | Consent required for all decisions requiring shareholder approval; |
| | • | | Right to receive a cumulative preference dividend at a rate of 8 per cent per annum based on an issue price of $6.665445 per B Preference Share and $4.13 per C Preference Share, in priority to any distribution to the holders of any other class of share in the capital of the Company. If there are insufficient profits available for distribution or if the Company fails to declare the preference dividend, the unpaid preference dividend shall accrue. No dividend or other distribution accrued on the B Preference Shares shall be paid during the period prior to the C Preference Shares issue date other than with the required C majority consent. All dividends to preference shares issued subsequent to C Preference Shares issue date shall be paid on apari passu basis to the holder of B Preference Shares and C Preference Shares; |
| | • | | In addition to the right to receive the preference dividend noted above, the right to receive and participate on a pro rata basis in any dividends paid on the ordinary shares on an as converted basis; |
| | • | | Upon a Liquidity Event, entitled to receive in preference to the holders of any other class of shares the greater of; |
| | (a) | the aggregate of the price paid per B Preference Shares and C Preference Share plus any unpaid dividends whether declared or not based on an issue price of $6.665445 per share for the B Preference Shares and $4.13 per share for the C Preference Shares; and |
| | (b) | the amount per ordinary share (on an as-converted basis) that would have been payable if all of the B Preference Shares and C Preference Shares had been converted into ordinary shares immediately prior to the occurrence of the relevant Liquidity Event; |
| | • | | Elect to have redeemed by the Company all (or a specified proportion) of the Preference Shares at any time after the later of the fifth anniversary of the date of issue of the Preference Shares and 6 months after the maturity date or date of repayment in full of the loan payable to Royalty Opportunities S.À R.L. (see Note 8) at a price equal to $6.665445 per B Preference Share and $4.13 per C Preference Share together with all dividends unpaid thereon; |
| | • | | Elect to have all, or any number, of the B Preference Shares or C Preference Shares held by it converted into ordinary shares at any time after the date of issue of the Preference Shares based on a specified conversion formula which results in a variable number of shares. |
The B Preference Shares warrants and shareholders have the following rights attached:
There are outstanding warrants issued by the Company to certain shareholders which give those shareholders the right to subscribe for up to 125,448 (2013: 125,448) B Preference Shares at an exercise price of US$6.665445 per share, and to subscribe for up to 56,887 (2013: 56,887) B Preference Shares at an exercise price of €0.01 per share. These warrants are exercisable for a period up to the earlier of (i) 30 June 2019 and (ii) the date of completion of a specified liquidity event.
F-34
GLOBAL CONSOLIDATED AESTHETICS LIMITED
Notes to Consolidated Financial Statements—Continued
8. Loans and borrowings
The analysis of current and non-current loans and borrowings are set out below:
| | | | | | | | | | | | |
| | | | | | December 31, | |
| | | | | | 2014 | | | 2013 | |
| | | Notes | | | (in thousands) | |
Current | | | | | | | | | | | | |
Shareholder loans – interest bearing | | | | | | $ | 263 | | | $ | 19,736 | |
Finance lease liabilities | | | 13 | | | | 194 | | | | 394 | |
Bank loans | | | | | | | 264 | | | | 6,860 | |
Other loans | | | | | | | 73 | | | | 82 | |
| | | | | | | | | | | | |
Total current loans and borrowings | | | | | | $ | 794 | | | $ | 27,072 | |
| | | | | | | | | | | | |
Non-current | | | | | | | | | | | | |
Shareholder loans – interest bearing | | | | | | $ | 50,149 | | | $ | 3,155 | |
Payment in kind loans | | | | | | | 633 | | | | 937 | |
Finance lease liabilities | | | 13 | | | | 554 | | | | 806 | |
Bank loans | | | | | | | — | | | | 14,640 | |
Other loans | | | | | | | 121 | | | | 221 | |
| | | | | | | | | | | | |
Total non-current loans and borrowings | | | | | | $ | 51,457 | | | $ | 19,759 | |
| | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | 1 year or Greater | | | | | | | |
| | | Less than
1 year | | | 1-2 years | | | 2–5 years | | | Greater
than 5 years | | | Total 1 year or
greater | | | Total | |
| | | (in thousands) | |
December 31, 2013 | | | | | | | | | | | | | | | | | | | | | | | | |
Shareholder loans – interest bearing | | $ | 19,736 | | | $ | — | | | $ | 3,155 | | | $ | — | | | $ | 3,155 | | | $ | 22,891 | |
Payment in kind loans | | | — | | | | — | | | | 937 | | | | — | | | | 937 | | | | 937 | |
Finance lease liabilities | | | 394 | | | | 201 | | | | 372 | | | | 233 | | | | 806 | | | | 1,200 | |
Bank loans | | | 6,860 | | | | 14,640 | | | | — | | | | — | | | | 14,640 | | | | 21,500 | |
Other loans | | | 82 | | | | 83 | | | | 138 | | | | — | | | | 221 | | | | 303 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Total, December 31, 2013 | | $ | 27,072 | | | $ | 14,924 | | | $ | 4,602 | | | $ | 233 | | | $ | 19,759 | | | $ | 46,831 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
December 31, 2014 | | | | | | | | | | | | | | | | | | | | | | | | |
Shareholder loans – interest bearing | | $ | 263 | | | $ | — | | | $ | 48,388 | | | $ | 1,761 | | | $ | 50,149 | | | $ | 50,412 | |
Payment in kind loans | | | — | | | | — | | | | — | | | | 633 | | | | 633 | | | | 633 | |
Finance lease liabilities | | | 194 | | | | 230 | | | | 154 | | | | 170 | | | | 554 | | | | 748 | |
Bank loan | | | 264 | | | | — | | | | — | | | | — | | | | — | | | | 264 | |
Other loans | | | 73 | | | | 121 | | | | — | | | | — | | | | 121 | | | | 194 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Total, December 31, 2014 | | $ | 794 | | | $ | 351 | | | $ | 48,542 | | | $ | 2,564 | | | $ | 51,457 | | | $ | 52,251 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
F-35
GLOBAL CONSOLIDATED AESTHETICS LIMITED
Notes to Consolidated Financial Statements—Continued
Bank loan facilities
The Company’s bank loan facilities with Ulster Bank Ireland Limited and debt facilities with Credit Agricole SA were fully settled during 2014. The details of the facilities in 2014 and 2013 are as follows:
Ulster Bank Ireland Limited
These facilities comprised two loan facilities and a revolving credit facility as follows:
| | • | | The first loan facility comprised three loans denominated in Euros, Sterling and US dollars with varying rates of interest under the original facility documentation. These loans were repayable by installments every six months on June 30 and December 31, up to the maturity in May 2015. Interest was payable at the end of each calendar quarter. At December 31, 2013 the Company owed $5.5 million (€4.0 million) on the Euro loan, $2.1 million (£1.3 million) on the Sterling loan and $3.8 million on the US dollar loan. This loan facility was fully settled in 2014. |
| | • | | The second loan facility, which was denominated in Euros, was a mezzanine loan facility and bore interest at a variable rate, a portion of which was rolled up over the life of the loan and was repayable at maturity. The balance of the interest was repayable at the end of each calendar quarter. The loan principal was repayable in one amount together with rolled up interest at maturity in November 2015. At December 31, 2013, the Company owed $7.0 million on the second loan facility. This loan was fully settled in 2014. |
| | • | | The revolving credit facility of $2.8 million could be continuously redrawn for a period of up to six months following repayment to the bank. The facility bore interest at a variable rate and was repayable at the end of each calendar quarter. Of this amount, $1.4 million was payable in May 2014 and $1.4 million was payable in May 2015. This loan facility was fully settled in 2014. |
The facilities were secured with the assets of the Company, which comprised a security interest via a fixed and floating charge over the majority of assets in the Company.
Credit Agricole SA
At December 31, 2014 the Company owed $0 (2013: $0.1 million) on a term loan facility. The loan bore interest at market rates and was unsecured.
Other loans
During 2010, the Company received two term loans from Regional Development Authorities in France of €0.2 million and €0.1 million. Both loans have seven-year terms with a moratorium on principal repayments in years one and two, and are repayable over years three to seven. The loans are non-interest bearing and are unsecured. The principal condition of the loans is that employment levels must remain above a certain threshold during the loan terms. The balance of the loan at December 31, 2014 amounted to $0.2 million (2013: $0.2 million).
The Company received a bank loan with a 90-day rolling maturity through our Brazilian subsidiary for BRL700,000, secured against receivables for that business. Under the terms of the loan, we have agreed that our Brazilian subsidiary will maintain accounts receivable at a level that is at least equal to the amount outstanding on the loan at any given time. We make monthly cash interest payments, and we may draw down or repay principal at our convenience. As of December 31, 2014, we owed $263,792 under this facility.
The Company accessed its 90-day revolver facility in Brazil in 2014. The outstanding balance at December 31, 2014 amounted to $0.3 million(2013: $0).
F-36
GLOBAL CONSOLIDATED AESTHETICS LIMITED
Notes to Consolidated Financial Statements—Continued
Loans from shareholders
Shareholder loans—interest bearing
Royalty Opportunities S.Á R.L.
In 2014, the Company obtained two senior secured term loans and royalty interest with Royalty Opportunities S.Á R.L. as follows:
| | • | | the first senior secured term loan and royalty interest amounted to $34.0 million. The loan is repayable quarterly from February 22, 2019 in four equal installments. Interest will accrue at 8.5% plus the higher between 1.0% and LIBOR for the interest period. The interest on the debt will increase if LIBOR exceeds 1.0%. Royalty interest is payable based on net sales at 1.25% of revenue up to €50.0 million and 1.5% of revenue in excess of €50.0 million up to €75.0 million. Both interest and royalty interest are payable on a quarterly basis. |
| | • | | the second senior secured term loan and royalty interest amounted to $15.0 million. The loan is repayable quarterly from February 22, 2019 in four equal installments. Interest will accrue at 8.5% plus the higher between 1% and LIBOR for the interest period. The interest on the debt will increase if LIBOR exceeds 1.0%. Royalty interest is payable based on net sales at 0.55% of revenue up to €50.0 million and 0.65% of revenue in excess of €50.0 million up to €75.0 million. Both interest and royalty interest are payable on a quarterly basis. |
Interest and royalty interest payments are recognized in the consolidated financial statements using the effective interest rate for the two loans above.
The loans are secured over the assets of the Company, which comprises a fixed and floating charge over the majority of assets in the Company.
As part of the loan with Royalty Opportunities S.Á R.L., the Company is subject to specific financial covenants and non-financial covenants on a monthly, quarterly and annual basis. In addition, the existing Payment In Kind (“PIK”) and shareholders’ loan were subordinated together with the intercompany loans between the Company and its subsidiaries.
Payment In Kind loans
PIK loans have been received from certain of the Company’s shareholders, and are unsecured and guaranteed by the Company. Interest is charged at 15.0% per annum on a straight line basis on the remaining accumulated interest.
In January 2014, the loans were subordinated to the loans payable to Royalty Opportunities S.Á R.L. As a result, the maturity date was amended to the later of i) May 30, 2016, or ii) six months after the maturity date of loans payable to Royalty Opportunities S.Á R.L, or repayment in full of loans payable to Royalty Opportunities S.Á R.L. At December 31, 2014 the outstanding PIK loans amounted to $0.633 million(2013: $0.937 million).
Unsecured loan
In 2010, the Company received an unsecured loan from certain of the Company’s shareholders. Interest is charged at 8.0% per annum on a straight line basis on the remaining accumulated interest.
F-37
GLOBAL CONSOLIDATED AESTHETICS LIMITED
Notes to Consolidated Financial Statements—Continued
In January 2014, the loans were subordinated by the loans payable to Royalty Opportunities S.Á R.L. As a result, the maturity date was amended to the later of (i) May 30, 2016, or (ii) six months after the maturity date of loans payable to Royalty Opportunities S.Á R.L., or repayment in full of loans payable to Royalty Opportunities S.Á R.L.
In 2012 and 2013 the Company received an unsecured loan from certain of the Company’s shareholders. The loan is unsecured, is not guaranteed, and bears interest at a rate of 10.0%. Each 6 month period from date of receipt, the accrued interest is capitalized into principal and rolled forward. The total principal and accrued interest outstanding as of December 31, 2013 together with the interest that accrued during 2014 up to the date of conversion, amounted to $19.1 million all of which was converted to B Preference Shares by way of the issue of 8,612,983 B Preference Shares.
Following is a summary of the Company’s loans and borrowings:
| | | | | | | | | | | | | | | | |
| | | Agreement Currency | | | Effective
interest
rate | | | Latest
year of
maturity | | | Carrying
amount | |
| | | (in thousands) | |
December 31, 2013 | | | | | | | | | | | | | | | | |
Shareholders’ loans | | | Euro | | | | 8-10 | % | | | 2016 | | | $ | 22,891 | |
Finance lease liabilities | | | Euro | | | | 3-11 | % | | | 2025 | | | | 1,199 | |
Payment In Kind loan | | | Euro | | | | 15 | % | | | 2016 | | | | 937 | |
Bank and other loans | | | Euro | | | | 2.92 | % | | | 2015 | | | | 64 | |
Bank term loan | | | Euro | | | | 11 | % | | | 2015 | | | | 7,030 | |
Bank term loan | | | Euro | | | | 3.47 | % | | | 2015 | | | | 5,528 | |
Bank term loan | | | USD | | | | 3.50 | % | | | 2015 | | | | 3,782 | |
Bank term loan | | | GBP | | | | 3.77 | % | | | 2015 | | | | 2,148 | |
Bank revolving loan facility | | | Euro | | | | 3.48 | % | | | 2014 | | | | 2,949 | |
| | | | | | | | | | | | | | | | |
Total interest-bearing liabilities | | | | | | | | | | | | | | $ | 46,528 | |
| | | | | | | | | | | | | | | | |
December 31, 2014 | | | | | | | | | | | | | | | | |
Shareholders’ loans | | | Euro | | | | 12.7 | % | | | 2020 | | | $ | 1,847 | |
Shareholders’ loans | | | USD | | | | 15.5-15.8 | % | | | 2019 | | | | 48,565 | |
Finance lease liabilities | | | Euro | | | | 3-11 | % | | | 2025 | | | | 748 | |
Payment In Kind loan | | | Euro | | | | 13.9 | % | | | 2020 | | | | 633 | |
Bank and other loans | | | BRL | | | | 27.6 | % | | | 2015 | | | | 264 | |
| | | | | | | | | | | | | | | | |
Total interest-bearing liabilities | | | | | | | | | | | | | | $ | 52,057 | |
| | | | | | | | | | | | | | | | |
All of the Company’s assets are pledged to Royalty Opportunities S.Á R.L. with the exception of trade accounts receivable in Brazil, which are pledged to Banco Itau under the 90-day rolling maturity noted described above.
F-38
GLOBAL CONSOLIDATED AESTHETICS LIMITED
Notes to Consolidated Financial Statements—Continued
9. Trade payables, accruals and other creditors and liabilities
The analysis of current and non-current trade and other payables are set out below:
| | | | | | | | | | | | |
| | | | | | December 31, | |
| | | | | | 2014 | | | 2013 | |
| | | Notes | | | (in thousands) | |
Current: | | | | | | | | | | | | |
Trade payable | | | | | | $ | 7,206 | | | $ | 6,206 | |
Other taxes | | | 18 | | | | 1,569 | | | | 1,325 | |
Accruals and other creditors | | | | | | | 9,027 | | | | 6,887 | |
Deferred consideration | | | | | | | 100 | | | | 102 | |
Settlement in lieu of issuing shares | | | | | | | 896 | | | | 1,293 | |
| | | | | | | | | | | | |
| | | | | | $ | 18,798 | | | $ | 15,813 | |
| | | | | | | | | | | | |
Non-Current: | | | | | | | | | | | | |
Settlement in Lieu of issuing shares | | | | | | $ | — | | | $ | 789 | |
| | | | | | | | | | | | |
The carrying value of trade and other payables above approximate to their fair values. Deferred consideration represents a payable on the settlement for certain trademarks, trade names and domain names, due within one year.
10. Analysis of total funds and debt
| | | | | | | | |
| | | December 31, | |
| | | 2014 | | | 2013 | |
| | | (in thousands) | |
Cash at bank and in hand | | $ | 14,258 | | | $ | 4,519 | |
Less, restricted cash under financing agreements | | | (3,642 | ) | | | — | |
| | | | | | | | |
Cash and cash equivalents | | $ | 10,616 | | | $ | 4,519 | |
| | | | | | | | |
Loans and borrowings: | | | | | | | | |
Current | | $ | 794 | | | $ | 27,072 | |
Non-current | | | 51,457 | | | | 19,759 | |
| | | | | | | | |
Total loans and borrowings | | | 52,251 | | | | 46,831 | |
| | |
Add: non-current private equity funding: | | | | | | | | |
B Preference Shares and C Preference Shares | | | 116,210 | | | | 46,518 | |
Equity derivative | | | 22,284 | | | | 16,741 | |
| | | | | | | | |
Total non-current private equity funding | | | 138,494 | | | | 63,259 | |
| | |
Less: cash and cash equivalents | | | (10,616 | ) | | | (4,519 | ) |
| | | | | | | | |
Net debt | | $ | 180,129 | | | $ | 105,571 | |
| | | | | | | | |
Total deficit | | $ | (136,980 | ) | | $ | (65,402 | ) |
| | | | | | | | |
As a result of the Credit Facility, the Company is required to maintain a minimum cash balance of €3.0 million ($3.6 million at December 31, 2014). This amount is reflected as Restricted Cash in the consolidated statements of position and in financing activities-transfers to restricted cash in the consolidated statements of cash flows. In May 2015, the agreements were amended to reduce the required minimum cash balance from €3.0 million ($3.6 million at December 31, 2014) to €2.0 million ($2.4 million).
F-39
GLOBAL CONSOLIDATED AESTHETICS LIMITED
Notes to Consolidated Financial Statements—Continued
11. Provisions and retirement obligation
Changes in the provision during the year and preceding period are set out below:
| | | | | | | | | | | | |
| | | Provision | | | Retirement
obligation | | | Total | |
| | | (in thousands) | |
Balance, January 1, 2013 | | $ | 762 | | | $ | 372 | | | $ | 1,134 | |
Included within administrative expenses | | | | | | | | | | | | |
Additional provision | | | 946 | | | | — | | | | 946 | |
Write-down of provision during the year | | | (193 | ) | | | — | | | | (193 | ) |
Interest cost | | | — | | | | 11 | | | | 11 | |
Current service cost | | | — | | | | 44 | | | | 44 | |
| | | | | | | | | | | | |
| | | 753 | | | | 55 | | | | 808 | |
| | | | | | | | | | | | |
Included within other comprehensive income | | | | | | | | | | | | |
Actuarial loss | | | — | | | | (45 | ) | | | (45 | ) |
Foreign exchange movements | | | 38 | | | | 16 | | | | 54 | |
| | | | | | | | | | | | |
| | | 38 | | | | (29 | ) | | | 9 | |
| | | | | | | | | | | | |
Balance, December 31, 2013 | | $ | 1,553 | | | $ | 398 | | | $ | 1,951 | |
| | | | | | | | | | | | |
Included within administrative expenses | | | | | | | | | | | | |
Additional provision | | | 60 | | | | — | | | | 60 | |
Write down of provision during the year | | | (296 | ) | | | — | | | | (296 | ) |
Interest cost | | | — | | | | 11 | | | | 11 | |
Current service cost | | | — | | | | 48 | | | | 48 | |
Benefits paid | | | — | | | | (5 | ) | | | (5 | ) |
| | | | | | | | | | | | |
| | | (236 | ) | | | 54 | | | | (182 | ) |
| | | | | | | | | | | | |
Included within other comprehensive income | | | | | | | | | | | | |
Actuarial loss | | | — | | | | 55 | | | | 55 | |
Foreign exchange movements | | | (106 | ) | | | (57 | ) | | | (163 | ) |
| | | | | | | | | | | | |
| | | (106 | ) | | | (2 | ) | | | (108 | ) |
| | | | | | | | | | | | |
Balance, December 31, 2014 | | $ | 1,211 | | | $ | 450 | | | $ | 1,661 | |
| | | | | | | | | | | | |
Provisions
From time to time, the Company is involved in claims and legal actions, which arise in the normal course of business. Based on information currently available to the Company, the directors believe that the provision is adequate to deal with the outcome of such litigations.
Retirement obligation
The Company maintains a retirement indemnity provision in its French subsidiary in relation to lump sum retirement indemnities payable by that Company to employees at retirement date. At December 31, 2014 this provision amounted to $0.4 million (2013: $0.4 million).
The indemnities are calculated based on the employee agreement applicable to the Company activity sector and typically represent a number of months’ salary depending on the length of service of each employee. The method used by the Company to calculate the provision is the projected unit credit method, performed to comply with IAS 19.
F-40
GLOBAL CONSOLIDATED AESTHETICS LIMITED
Notes to Consolidated Financial Statements—Continued
The financial assumptions used for the calculation are set out below:
| | | | | | | | |
| | | December 31, | |
| | | 2014 % p.a. | | | 2013 % p.a. | |
Salary growth rate | | | 1.1 | % | | | 2.0 | % |
Discount rate | | | 1.3 | % | | | 3.0 | % |
Inflation rate | | | 1.1 | % | | | 2.0 | % |
Assumptions regarding future mortality are based on published statistics and mortality tables. Members are assumed to retire at the age of 67 (2013: 67).
The most significant actuarial assumptions used in calculating the retirement obligation above are the discount rate and the salary growth rate. A 0.7% increase in the discount rate would lead to no change in the liability. A 0.5% increase in the salary growth rate would lead to no change in the liability.
The retirement obligation is unfunded. The weighted average duration of the provision is 19.0 years (2013: 20.8 years).
12. Loss on conversion of shareholder loans
An amount of $27.7 million was charged as a loss on the conversion of shareholder loans in other income (expense) on the consolidated statements of operations for the year ended December 31, 2014. At various dates in 2014, shareholder loans were converted into 8% B Preference Shares. The loss on conversion results from the difference between the carrying value of the loans converted into the B Preference Shares and the fair value of the B Preference Shares which were exchanged.
| | | | | | | | | | | | |
| | | B Preference
Shares | | | Fair Value | | | Total | |
Shareholder interest bearing loans, January 1, 2014 | | | | | | | | | | $ | 19,736 | |
Accrued interest from January 1, 2014 to the date of conversion | | | | | | | | | | | 85 | |
Withholding tax on the converted accrued interest | | | | | | | | | | | (161 | ) |
Foreign currency movement | | | | | | | | | | | (524 | ) |
B Preference Shares issued for loan conversion, January 20, 2014, at fair value | | | 7,881,156 | | | $ | 33,922 | | | | | |
Related equity derivative | | | | | | | 7,703 | | | | | |
B Preference Shares issued for loan conversion, February 19, 2014, at fair value | | | 13,864 | | | | 60 | | | | | |
Related equity derivative | | | | | | | 14 | | | | | |
B Preference Shares issued for loan conversion, April, 28, 2014, at fair value | | | 152,597 | | | | 657 | | | | | |
Related equity derivative | | | | | | | 149 | | | | | |
B Preference Shares issued for loan conversion, December 19, 2014, at fair value | | | 565,366 | | | | 3,698 | | | | | |
Related equity derivative | | | | | | | 670 | | | | | |
| | | | | | | | | | | | |
Total B Preference Shares | | | 8,612,983 | | | | | | | | | |
| | | | | | | | | | | | |
Fair value of B Preference Shares and related equity derivatives | | | | | | | | | | | (46,873 | ) |
| | | | | | | | | | | | |
Loss on conversion | | | | | | | | | | $ | (27,737 | ) |
| | | | | | | | | | | | |
F-41
GLOBAL CONSOLIDATED AESTHETICS LIMITED
Notes to Consolidated Financial Statements—Continued
13. Commitments and contingencies
Litigation, claims and assessments
The Company, from time to time, is subject to various inquiries or audits by taxing authorities (income taxes or other) originating from our Irish or foreign operations, covering a wide range of matters that arise in the ordinary course of business. Each of these matters is subject to various uncertainties, and it is possible that some of these matters may not be resolved in our favor. We have established accruals for matters that we believe are probable and reasonably estimable. The Company believes that any liability that may ultimately result from the resolution of these matters in excess of amounts provided will not have a material adverse effect on our financial position or results of operations.
Contingencies
From time to time, the Company is involved in claims and legal actions, which arise in the normal course of business. Based on information currently available to the Company, and legal advice, the Company believes such litigation will not individually, or in aggregate, have a material adverse effect on the financial statements and that the Company is adequately positioned to deal with the outcome of any such litigation. The Company has recognized a provision for claims and legal actions in which payment is probable and amount can be estimated reliably (see Note 11).
As of December 31, 2014, the redemption value of the B Preference Shares and C Preference Shares is $116.2 million(2013: $46.5 million), which includes $18.0 million(2013: $10.0 million) in accrued and unpaid dividends.
While the Royalty Opportunities S.À R.L. loan agreement is in existence, the Company is prohibited from paying dividends.
Under Irish law, the Company is prohibited from paying cash dividends while the Company has an accumulated deficit.
Operating leases
Total commitments payable under non-cancellable operating leases are as follows:
| | | | | | | | | | | | |
| | | Land and
buildings | | | Others | | | Total | |
| | | (in thousands) | |
Within one year | | $ | 535 | | | $ | 213 | | | $ | 748 | |
Between one and two years | | | 274 | | | | 103 | | | | 377 | |
Between two and five years | | | 431 | | | | 105 | | | | 536 | |
More than five years | | | 26 | | | | — | | | | 26 | |
| | | | | | | | | | | | |
| | $ | 1,266 | | | $ | 421 | | | $ | 1,687 | |
| | | | | | | | | | | | |
The Company leases motor vehicles, plant and equipment and property under operating leases. The leases typically run for a period of three to ten years. Property leases are generally reviewed every five years.
During the year ended December 31, 2014, $0.9 million (2013: $0.6 million) was recognized as an expense in the statement of operations in respect of operating leases.
F-42
GLOBAL CONSOLIDATED AESTHETICS LIMITED
Notes to Consolidated Financial Statements—Continued
Finance (Capital) lease obligations
Finance lease liabilities are payable as follows:
| | | | | | | | | | | | |
| | | Minimum
lease
payments | | | Interest | | | Principal | |
| | | (in thousands) | |
December 31, 2014 | | | | | | | | | | | | |
Less than one year | | $ | 227 | | | $ | 33 | | | $ | 194 | |
Between one and two years | | | 254 | | | | 24 | | | | 230 | |
Between two and five years | | | 204 | | | | 50 | | | | 154 | |
After five years | | | 191 | | | | 21 | | | | 170 | |
| | | | | | | | | | | | |
| | $ | 876 | | | $ | 128 | | | $ | 748 | |
| | | | | | | | | | | | |
| | | | | | | | | | | | |
| | | Minimum
lease
payments | | | Interest | | | Principal | |
| | | (in thousands) | |
December 31, 2013 | | | | | | | | | | | | |
Less than one year | | $ | 452 | | | $ | 58 | | | $ | 394 | |
Between one and two years | | | 238 | | | | 37 | | | | 201 | |
Between two and five years | | | 443 | | | | 72 | | | | 371 | |
After five years | | | 267 | | | | 33 | | | | 234 | |
| | | | | | | | | | | | |
| | $ | 1,400 | | | $ | 200 | | | $ | 1,200 | |
| | | | | | | | | | | | |
Depreciation of finance leases was included within administrative expense in the consolidated statements of operations. Depreciation expense related to finance leases was $0.3 million for the year ended December 31, 2014 (2013: $0.3 million).
Guarantees
Pursuant to the provisions of Section 17 of the Companies (Amendment) Act, 1986, the Company has guaranteed the liabilities of all its subsidiary companies incorporated in Ireland for the financial year to December 31, 2014 and as a result such subsidiaries are exempt from the filing provisions of Section 17, Companies (Amendment) Act, 1986.
Where the Company enters into financial guarantee contracts to guarantee the indebtedness of wholly-owned subsidiaries, the Company considers these to be insurance arrangements and accounts for them as such. The Company treats the guarantee contract as a contingent liability until such time as it becomes probable that the Company will be required to make a payment under the guarantee.
The Company has guaranteed the liabilities of PIK loans of $0.6 million at December 31, 2014 details of which are set out in Note 8. In addition, the Company has provided guarantees in respect of rental payments to the lessors of certain properties leased by subsidiaries.
Each company in the Company is an obligor and bound by the various facility agreements in respect of the loan payable to Royalty Opportunities S.À R.L., the investment vehicle of OrbiMed Advisors, LLC.
The Company’s policy is to provide financial guarantees only to wholly-owned subsidiaries.
F-43
GLOBAL CONSOLIDATED AESTHETICS LIMITED
Notes to Consolidated Financial Statements—Continued
Relationship with suppliers
To a certain extent, we depend on two raw material suppliers to provide us with the products that we use in manufacturing. The Company’s contracts with those suppliers are generally non-exclusive and may be terminated with proper notice.
Any termination of one or more of the Company’s purchase agreements with its raw material suppliers or any significant deterioration in our relationship with our major suppliers could reduce the availability of the products that the Company manufactures and offers for sale. The Company’s agreements offer the ability to purchase raw materials for its manufacturing plants on open account with a credit limit determined by the supplier based on its own credit criteria. The supplier may reduce the amount of credit that it extends to us under this open account at any time if it determines that our condition, including our financial condition, is unacceptable to it. If the supplier makes such a determination, it could cancel purchase orders, require us to pay cash and/or delay shipment of raw materials.
Collective bargaining agreements
The Company has collective bargaining agreements in France, Brazil and Spain encompassing a total of approximately 270 employees (approximately 65% of total employees). There are no collective bargaining agreements expiring within the next twelve months.
Other commitments
The Company does not have any long-term, non-cancellable obligations other than those described above.
14. Other expense (income)
| | | | | | | | |
| | | Year ended December 31, | |
| | | 2014 | | | 2013 | |
| | | (in thousands) | |
Interest expense: | | | | | | | | |
Interest on B Preference Shares and C Preference Shares | | $ | 27,544 | | | $ | 3,313 | |
Interest on shareholder loans | | | 5,969 | | | | 1,707 | |
Interest on bank loans | | | 176 | | | | 1,772 | |
Finance lease interest | | | 57 | | | | 85 | |
| | | | | | | | |
Total interest expense | | | 33,746 | | | | 6,877 | |
| | | | | | | | |
Gain (Loss) on foreign exchange: | | | | | | | | |
Foreign exchange loss (gain) on B Preference Shares and C Preference Shares | | | 15,399 | | | | (1,789 | ) |
Foreign exchange loss (gain) on bank loans | | | 3,258 | | | | (205 | ) |
| | | | | | | | |
Total loss (gain) on foreign exchange | | | 18,657 | | | | (1,994 | ) |
| | | | | | | | |
Loss on conversion of shareholder loans | | | 27,737 | | | | — | |
Fair value (gain) loss on equity derivative | | | (5,182 | ) | | | 4,828 | |
| | | | | | | | |
| | $ | 74,958 | | | $ | 9,711 | |
| | | | | | | | |
Included in interest on bank loans is $0 million(2013: $0.7 million) paid during the year. At December 31, 2014, the total amount of such interest accrued but not paid on the loans amounted to $0(2013: $2.3 million).
F-44
GLOBAL CONSOLIDATED AESTHETICS LIMITED
Notes to Consolidated Financial Statements—Continued
The interest on shareholder loans comprises interest of $0.1 million (2013: $0.3 million) on PIK loans, interest of $0.2 million (2013: $1.4 million) on an unsecured loan and interest of $5.7 million (2013: $0) on loans payable to Royalty Opportunities S.À R.L., as more fully explained in Note 8. Interest on PIK and unsecured shareholder loans are payable on maturity date.
During 2014, foreign exchange losses rose on the translation of Euro and Sterling loans to US dollar loans at period end rates which are different from the rate at the prior period end together with gains or losses arising on amounts repaid during the year.
On an annual basis the fair value of the equity derivative associated with the B Preference Shares and C Preference Shares are reviewed. The changes in value of this equity related component amounted to a gain of $5.2 million at December 31, 2014 (2013: $4.8 million loss) which is charged through the consolidated statements of operations.
15. Share-based payment compensation
The Company operates an equity settled share based payment plan which includes the issuance of share options to certain directors and senior Managers of the Company and its subsidiaries and any other person nominated by the Board in accordance with the provisions set out in the Share Option Plan 2010.
Options have a maximum exercise period of seven years from the grant date. The awards vest on an installment basis with a portion of the award vesting immediately and the remaining portion vesting over a number of months on a pro-rata basis. The number of ordinary shares in respect of which an option may be exercised shall be cumulative, so that once an option shall become exercisable as to any ordinary shares, it shall continue to be exercisable as to such ordinary shares, until the option is fully exercised or lapses as provided in this Plan. There are no market conditions attached to the option granted during the year.
The following options, as granted to the Company’s employees, were outstanding under the share option plans during the year:
| | | | | | | | |
| | | Number of
Options | | | Weighted
average
exercise price | |
Outstanding at January 1, 2014 | | | — | | | € | — | |
Granted during the year | | | 1,179,791 | | | | 0.01 | |
Forfeited during the year | | | — | | | | — | |
Expired during the year | | | — | | | | — | |
Exercised during the year | | | — | | | | — | |
| | | | | | | | |
Outstanding at December 31, 2014 | | | 1,179,791 | | | € | 0.01 | |
| | | | | | | | |
Exercisable at December 31, 2014 | | | 368,885 | | | € | 0.01 | |
| | | | | | | | |
The exercise price for options outstanding at the end of 2014 was €0.01 ($0.0133). The weighted average remaining contractual life is 6.97 years. There were no options outstanding at the end of the year in 2013.
The total expense of options for 2014 was $1.8 million.
The fair value of options granted is estimated using the Black-Scholes-Merton option valuation model, which incorporates various assumptions including expected life, volatility, risk-free interest rates, and dividend yield.
F-45
GLOBAL CONSOLIDATED AESTHETICS LIMITED
Notes to Consolidated Financial Statements—Continued
The following tables list the inputs to the models used for the option plan for the year ended December 31, 2014:
| | | | |
| | | December 31,
2014 | |
Dividend yield (%) | | | 0 | % |
Expected volatility (%) | | | 50 | % |
Risk-free interest rate (%) | | | 0.17 | % |
Expected life of share options (years) | | | 7 years | |
Weighted average share price (€) | | | 3.47 | |
Model used | | | Black-Scholes-Merton | |
The expected life of share options represents the estimated duration between grant date and the expected exercise or cancellation date. The Company estimated the expected life of share options granted based on the terms and conditions of the share awards granted to employees.
The Company has estimated the volatility of its options based on peer-company analysis.
The Company obtains the risk-free interest rates based on a U.S. government security of similar term to the option at the grant date. U.S. securities were chosen on the basis that the Company intends to list in the United States in 2015.
The Company has never paid or declared any cash dividends on its ordinary shares and does not have present plans to pay cash dividends on its ordinary shares. Consequently, the Company used an expected dividend yield of zero in the Black-Scholes-Merton option valuation model.
16. Deferred taxation
The value of deferred tax assets which have not been recognized in respect of unused tax losses and other timing differences at December 31, 2014 is $10.0 million (2013: $6.8 million). The Company has recorded a full valuation allowance against all unrecognized deferred tax assets because the time period in which the benefit of these losses can be utilized against future taxable profits in the relevant tax jurisdictions is uncertain.
No deferred tax is recognized on the unremitted earnings of overseas subsidiaries, branches and joint ventures where the Company does not anticipate additional tax on any ultimate remittance.
Recognized deferred tax assets and liabilities
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | December 31, 2014 | | | December 31, 2013 | |
| | | Assets | | | Liabilities | | | Net assets/
(liabilities) | | | Assets | | | Liabilities | | | Net assets/
(liabilities) | |
| | | (in thousands) | |
Intangible assets | | $ | — | | | $ | (4,927 | ) | | $ | (4,927 | ) | | $ | — | | | $ | (6,484 | ) | | $ | (6,484 | ) |
Property, plant & equipment | | | — | | | | (645 | ) | | | (645 | ) | | | 8 | | | | (794 | ) | | | (786 | ) |
Retirement obligation | | | 149 | | | | — | | | | 149 | | | | 482 | | | | — | | | | 482 | |
Losses | | | 1,391 | | | | — | | | | 1,391 | | | | 1,535 | | | | — | | | | 1,535 | |
Other items | | | 219 | | | | — | | | | 219 | | | | 102 | | | | — | | | | 102 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Tax asset/(liability) | | | 1,759 | | | | (5,572 | ) | | | (3,813 | ) | | | 2,127 | | | | (7,278 | ) | | | (5,151 | ) |
Set off of tax | | | (646 | ) | | | 646 | | | | — | | | | (793 | ) | | | 793 | | | | — | |
| | | | | | | | | | | | | | | | | | | | | | | | |
| | $ | 1,113 | | | $ | (4,926 | ) | | $ | (3,813 | ) | | $ | 1,334 | | | $ | (6,485 | ) | | $ | (5,151 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | |
F-46
GLOBAL CONSOLIDATED AESTHETICS LIMITED
Notes to Consolidated Financial Statements—Continued
Analysis of deferred tax (asset)/liability
| | | | | | | | | | | | | | | | | | | | |
| | | Balance at January 1, | | | Recognized in profit or loss | | | Recognized in equity | | | Foreign exchange transaction | | | Balance at December 31, | |
| | | (in thousands) | |
2013 | | | | | | | | | | | | | | | | | | | | |
Intangible assets | | $ | 7,156 | | | $ | (954 | ) | | $ | — | | | $ | 282 | | | $ | 6,484 | |
Property, plant & equipment | | | 348 | | | | 408 | | | | — | | | | 30 | | | | 786 | |
Retirement obligation | | | — | | | | (480 | ) | | | 15 | | | | (17 | ) | | | (482 | ) |
Losses | | | (11 | ) | | | (1,470 | ) | | | — | | | | (54 | ) | | | (1,535 | ) |
Other items | | | (1,163 | ) | | | 1,073 | | | | — | | | | (12 | ) | | | (102 | ) |
| | | | | | | | | | | | | | | | | | | | |
Tax (asset)/liability | | $ | 6,330 | | | $ | (1,423 | ) | | $ | 15 | | | $ | 229 | | | $ | 5,151 | |
| | | | | | | | | | | | | | | | | | | | |
2014 | | | | | | | | | | | | | | | | | | | | |
Intangible assets | | $ | 6,484 | | | $ | (865 | ) | | $ | — | | | $ | (692 | ) | | $ | 4,927 | |
Property, plant & equipment | | | 786 | | | | (54 | ) | | | — | | | | (87 | ) | | | 645 | |
Retirement obligation | | | (482 | ) | | | 320 | | | | (19 | ) | | | 32 | | | | (149 | ) |
Losses | | | (1,535 | ) | | | (41 | ) | | | — | | | | 185 | | | | (1,391 | ) |
Other items | | | (102 | ) | | | (140 | ) | | | — | | | | 23 | | | | (219 | ) |
| | | | | | | | | | | | | | | | | | | | |
Tax (asset)/liability | | $ | 5,151 | | | $ | (780 | ) | | $ | (19 | ) | | $ | (539 | ) | | $ | 3,813 | |
| | | | | | | | | | | | | | | | | | | | |
17. Income tax
(a) Analysis of income tax expense in year
| | | | | | | | |
| | | Year ended December 31, | |
| | | 2014 | | | 2013 | |
| | | (in thousands) | |
Current tax: | | | | | | | | |
Ireland corporation tax | | | | | | | | |
Corporation tax charge | | $ | — | | | $ | — | |
| | |
Overseas tax | | | | | | | | |
Corporation tax charge | | | — | | | | 43 | |
Others | | | 180 | | | | 185 | |
| | | | | | | | |
Total current tax charge | | | 180 | | | | 228 | |
| | | | | | | | |
Deferred tax: | | | | | | | | |
Ireland deferred tax | | | | | | | | |
Origination and reversal of temporary differences | | | — | | | | — | |
| | |
Overseas deferred tax | | | | | | | | |
Origination and reversal of temporary differences | | | (780 | ) | | | (1,392 | ) |
Effect of change in tax rates | | | — | | | | (31 | ) |
| | | | | | | | |
Total deferred tax credit | | | (780 | ) | | | (1,423 | ) |
| | | | | | | | |
Total credit | | $ | (600 | ) | | $ | (1,195 | ) |
| | | | | | | | |
F-47
GLOBAL CONSOLIDATED AESTHETICS LIMITED
Notes to Consolidated Financial Statements—Continued
(b) Factors affecting tax charge for year
The tax assessed for the year is lower than the standard rate of corporation tax in Ireland. The differences are explained below:
| | | | | | | | |
| | | Year ended
December 31, | |
| | | (in thousands) | |
| | | 2014 | | | 2013 | |
Loss on ordinary activities before tax | | $ | (90,027 | ) | | $ | (18,487 | ) |
| | | | | | | | |
Loss on ordinary activities multiplied by the standard rate of tax of 12.5% | | | (11,253 | ) | | | (2,311 | ) |
| | |
Effects of: | | | | | | | | |
Expenses not deductible for corporation tax purposes and other items | | | 8,926 | | | | 902 | |
Deferred tax movement not previously recognized | | | (233 | ) | | | — | |
Effect of tax rates in foreign jurisdictions | | | (2,977 | ) | | | (736 | ) |
Unrecognized deferred tax asset | | | 4,665 | | | | 765 | |
Others | | | 272 | | | | 185 | |
| | | | | | | | |
Total tax credit | | $ | (600 | ) | | $ | (1,195 | ) |
| | | | | | | | |
18. Other taxes
| | | | | | | | |
| | | December 31, | |
| | | 2014 | | | 2013 | |
| | | (in thousands) | |
Value Added Tax | | $ | 742 | | | $ | 346 | |
Payroll taxes and related social welfare contributions | | | 540 | | | | 979 | |
Other taxes | | | 287 | | | | — | |
| | | | | | | | |
| | $ | 1,569 | | | $ | 1,325 | |
| | | | | | | | |
19. Loss per ordinary share
| | | | | | | | |
| | | December 31, | |
| | | 2014 | | | 2013 | |
Basic loss per ordinary share | | $ | (18.74 | ) | | $ | (3.91 | ) |
Diluted loss per ordinary share | | | (18.74 | ) | | | (3.91 | ) |
Basic loss per ordinary share
Basic loss per ordinary share amounts is calculated by dividing the loss for the year attributable to ordinary shareholders by the weighted average number of ordinary shares outstanding during the year.
| | | | | | | | |
| | | December 31, | |
| | | 2014 | | | 2013 | |
| | | (in thousands) | |
Loss attributable to shareholders | | $ | (89,427 | ) | | $ | (17,292 | ) |
Less: Dividend requirements on B Preference Shares and C Preference Shares | | | — | | | | — | |
| | | | | | | | |
Loss attributable to ordinary shareholders | | $ | (89,427 | ) | | $ | (17,292 | ) |
| | | | | | | | |
F-48
GLOBAL CONSOLIDATED AESTHETICS LIMITED
Notes to Consolidated Financial Statements—Continued
Reconciliation of ordinary shares to weighted average loss per share denominator:
| | | | | | | | |
| | | December 31, | |
| | | 2014 | | | 2013 | |
| | | (in thousands) | |
Number of ordinary shares on January 1 | | | 4,425,800 | | | | 4,425,800 | |
Weighted average number of ordinary shares issued during the year | | | 346,301 | | | | — | |
| | | | | | | | |
Basic weighted average number of ordinary shares | | | 4,772,101 | | | | 4,425,800 | |
| | | | | | | | |
Diluted earnings per ordinary share
The profit attributable to shareholders must be adjusted for any items relating to potential ordinary shares.
| | | | | | | | |
| | | December 31, | |
| | | 2014 | | | 2013 | |
| | | (in thousands) | |
Loss attributable to shareholders | | $ | (89,427 | ) | | $ | (17,292 | ) |
| | | | | | | | |
Loss attributable to ordinary shareholders | | $ | (89,427 | ) | | $ | (17,292 | ) |
| | | | | | | | |
For the diluted earnings per share calculation, the weighted average number of ordinary shares outstanding during the year is not adjusted for the effect of ordinary shares that are potentially issuable in connection with employee share-based payment plans, exercise of warrants and conversion of B Preference Shares and C Preference Shares because they were anti-dilutive.
20. Financial instruments
Financial risk management
Overview
The Company is exposed to various risks from its use of financial instruments, including
This note presents information about the Company’s exposure to each of the above risks, the Company’s objectives, policies and processes for measuring and managing risk, and the Company’s management of capital. Further quantitative disclosures are included throughout these consolidated financial statements.
The financial risks with which the Company is faced are managed by the Board and the Company’s Chief Financial Officer. The Company does not engage in speculative activity. Financial instruments are used to raise capital and to manage the financial risks resulting from the Company’s operations. The main financial risks that the Company is exposed to include, credit risk, liquidity risk and market risk (including changes to foreign exchange rates). These risks are summarized below.
F-49
GLOBAL CONSOLIDATED AESTHETICS LIMITED
Notes to Consolidated Financial Statements—Continued
The Company has risk management procedures to identify and analyze the risks faced by the Company, set appropriate risk limits and controls and monitor risks and adherence to limits. The Company has structures and controls in place to manage its risks but has limited formally documented risk management policies and procedures. The Company intends to put such policies and procedures in place during 2015.
Company management oversees how local management monitors compliance and manages risk.
The Board oversees how senior management monitor compliance and manage risks through regular formal Board meetings with management. In addition, the Board has established an Audit Committee and a Remuneration Committee to manage and review risks and compliance and oversee executive and director compensation.
Credit risk
Credit risk is the risk of financial loss to the Company if a customer or counterparty to a financial instrument fails to meet its contractual obligations, and arises principally from the Company’s receivables from customers.
Trade and other receivables
The Company’s exposure to credit risk is influenced mainly by the individual characteristics of each customer. The demographics of the Company’s customer base, including the default risk of the industry and the country in which our customers operate, has less of an influence on credit risk.
Credit risk arises due to the Company’s decisions to extend credit terms to its customers. All customers’ balances are reviewed on a regular basis by the Company’s credit control function. Where management believes that amounts may not be recoverable, the amount estimated to be not recoverable is provided for in full.
The maximum exposure to credit risk for trade receivables (net of provision for impairment losses already recognized) at the reporting date by geographic region are as follows:
| | | | | | | | |
| | | December 31, | |
| | | 2014 | | | 2013 | |
| | | (in thousands) | |
Latin America (LA) | | $ | 6,214 | | | $ | 4,933 | |
Europe, Middle-East and Africa (EMEA) | | | 2,634 | | | | 3,679 | |
Asia-Pacific (AP) | | | 3,992 | | | | 1,394 | |
| | | | | | | | |
Total | | $ | 12,840 | | | $ | 10,006 | |
| | | | | | | | |
The maximum exposure to credit for trade receivables by customer type (net of provision for impairment losses already recognized) are as follows:
| | | | | | | | |
| | | December 31, | |
| | | 2014 | | | 2013 | |
| | | (in thousands) | |
Distributors | | $ | 5,350 | | | $ | 7,495 | |
Direct sales | | | 7,490 | | | | 2,511 | |
| | | | | | | | |
Total | | $ | 12,840 | | | $ | 10,006 | |
| | | | | | | | |
F-50
GLOBAL CONSOLIDATED AESTHETICS LIMITED
Notes to Consolidated Financial Statements—Continued
The maximum exposure to credit risk for all financial assets is considered to be their carrying amount.
The aging of trade receivables at the reporting date was:
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | Gross | | | Impairment | | | Net | |
| | | December 31, | | | December 31, | | | December 31, | |
| | | 2014 | | | 2013 | | | 2014 | | | 2013 | | | 2014 | | | 2013 | |
| | | (in thousands) | |
Not past due | | $ | 7,477 | | | $ | 8,043 | | | $ | — | | | $ | 115 | | | $ | 7,477 | | | $ | 7,928 | |
Past due 0 – 30 days | | | 1,768 | | | | 1,235 | | | | — | | | | 22 | | | | 1,768 | | | | 1,213 | |
Past due 31 – 90 days | | | 2,518 | | | | 681 | | | | 493 | | | | — | | | | 2,025 | | | | 681 | |
More than 90 days | | | 2,149 | | | | 2,278 | | | | 579 | | | | 2,094 | | | | 1,570 | | | | 184 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Total | | $ | 13,912 | | | $ | 12,237 | | | $ | 1,072 | | | $ | 2,231 | | | $ | 12,840 | | | $ | 10,006 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
The movement in the allowance for impairment in respect of trade receivables during the year was as follows:
| | | | | | | | |
| | | December 31, | |
| | | 2014 | | | 2013 | |
| | | (in thousands) | |
Balance, January 1 | | $ | 2,231 | | | $ | 2,394 | |
Provision utilized during the year | | | (1,632 | ) | | | (1,863 | ) |
Impairment loss recognized in the statement of comprehensive income | | | 653 | | | | 1,604 | |
Foreign exchange (gain) loss | | | (180 | ) | | | 96 | |
| | | | | | | | |
Balance, December 31, | | $ | 1,072 | | | $ | 2,231 | |
| | | | | | | | |
A provision for impairment of trade receivables is established when there is evidence that the Company will not be able to collect all monies due according to the original term of the receivables.
Liquidity risk
Liquidity risk is the risk that the Company will not be able to meet its financial obligations as they fall due. The Company’s approach to managing liquidity is to ensure as far as possible that it will have sufficient liquidity to meet its liabilities when due, under both normal and stressed conditions without incurring unacceptable losses or risking damage to the Company’s reputation.
The Company operates a prudent approach to liquidity management using a combination of long and short term debt and cash and cash equivalents to meet its liabilities when due.
At December 31, 2014, the Company had cash balances of $10.6 million (2013: $4.5 million), loans and borrowings of $52.3 million (2013: $46.8 million) and unused loan facilities of $0 (2013: $0).
During the year, the Company raised additional funds of $55.0 million in debt and equity from Royalty Opportunities S.Á R.L to fund working capital requirements and to settle the Company’s bank debt obligations. In April 2015, the Company obtained an additional loan from Royal Opportunities S.Á R.L. in the amount of $10.0 million.
The Company considers the private equity funding in the form of the B Preference Shares and C Preference Shares, including the equity derivative, to be part of the capital base of the Company.
The Board together with the Chief Financial Officer determines the financial and liquidity requirements of the Company, put in place plans to meet these requirements and execute appropriate financial arrangements.
F-51
GLOBAL CONSOLIDATED AESTHETICS LIMITED
Notes to Consolidated Financial Statements—Continued
The following are the contractual maturities of financial liabilities, including interest payments.
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | December 31, 2014 | |
| | | Carrying
amount | | | Contractual
cash flows | | | Less than
1 year | | | Due in
1 – 2
years | | | 2 – 5
years | | | Due
after 5
years | |
| | | (in thousands) | |
Non-derivative financial liabilities | | | | | | | | | | | | | | | | | | | | | | | | |
Private equity funding: | | | | | | | | | | | | | | | | | | | | | | | | |
B Preference Shares and C Preference Shares | | $ | 116,210 | | | $ | 172,616 | | | $ | — | | | $ | — | | | $ | — | | | $ | 172,616 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Shareholder loan liabilities: | | | | | | | | | | | | | | | | | | | | | | | | |
Payment In Kind loans | | | 633 | | | | 1,376 | | | | — | | | | — | | | | — | | | | 1,376 | |
Shareholders’ loans | | | 50,412 | | | | 82,387 | | | | 6,239 | | | | 6,439 | | | | 66,124 | | | | 3,585 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | 51,045 | | | | 83,763 | | | | 6,239 | | | | 6,439 | | | | 66,124 | | | | 4,961 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Private equity and shareholder loan liabilities | | | 167,255 | | | | 256,379 | | | | 6,239 | | | | 6,439 | | | | 66,124 | | | | 177,577 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Other non-derivative financial liabilities: | | | | | | | | | | | | | | | | | | | | | | | | |
Trade payables | | | 7,206 | | | | 7,206 | | | | 7,206 | | | | — | | | | — | | | | — | |
Finance lease liabilities | | | 748 | | | | 876 | | | | 227 | | | | 254 | | | | 204 | | | | 191 | |
Bank and other loans | | | 458 | | | | 458 | | | | 337 | | | | 121 | | | | — | | | | — | |
Deferred consideration | | | 100 | | | | 100 | | | | 100 | | | | — | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | 8,512 | | | | 8,640 | | | | 7,870 | | | | 375 | | | | 204 | | | | 191 | |
Other derivative financial liabilities: | | | | | | | | | | | | | | | | | | | | | | | | |
Equity derivative* | | | 22,284 | | | | — | | | | — | | | | — | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | 30,796 | | | | 8,640 | | | | 7,870 | | | | 375 | | | | 204 | | | | 191 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
| | $ | 198,051 | | | $ | 265,019 | | | $ | 14,109 | | | $ | 6,814 | | | $ | 66,328 | | | $ | 177,768 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
| * | The equity derivative has no contractual settlement date as it is contingent upon the occurrence of certain events. |
F-52
GLOBAL CONSOLIDATED AESTHETICS LIMITED
Notes to Consolidated Financial Statements—Continued
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | December 31, 2013 | |
| | | Carrying
amount | | | Contractual
cash flows | | | Less than
1 year | | | Due in
1 – 2
years | | | 2 – 5
years | | | Due
after 5
years | |
| | | (in thousands) | |
Non-derivative financial liabilities | | | | | | | | | | | | | | | | | | | | | | | | |
Private equity funding: | | | | | | | | | | | | | | | | | | | | | | | | |
B Preference Shares and C Preference Shares | | $ | 46,518 | | | $ | 60,915 | | | $ | — | | | $ | 42,000 | | | $ | 18,915 | | | $ | — | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Shareholder loan liabilities: | | | | | | | | | | | | | | | | | | | | | | | | |
Payment In Kind loans | | | 937 | | | | 1,316 | | | | — | | | | — | | | | 1,316 | | | | — | |
Shareholders’ loans | | | 22,891 | | | | 26,387 | | | | 22,629 | | | | — | | | | — | | | | 3,758 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | 23,828 | | | | 27,703 | | | | 22,629 | | | | — | | | | 1,316 | | | | 3,758 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Private equity and shareholder loan liabilities | | | 70,346 | | | | 88,618 | | | | 22,629 | | | | 42,000 | | | | 20,231 | | | | 3,758 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Other non-derivative financial liabilities: | | | | | | | | | | | | | | | | | | | | | | | | |
Trade payables | | | 6,206 | | | | 6,206 | | | | 6,206 | | | | — | | | | — | | | | — | |
Finance lease liabilities | | | 1,200 | | | | 1,400 | | | | 452 | | | | 238 | | | | 443 | | | | 267 | |
Bank and other loans | | | 21,803 | | | | 22,914 | | | | 7,730 | | | | 15,046 | | | | 138 | | | | — | |
Deferred consideration | | | 102 | | | | 102 | | | | 102 | | | | — | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | 29,311 | | | | 30,622 | | | | 14,490 | | | | 15,284 | | | | 581 | | | | 267 | |
Other derivative financial liabilities: | | | | | | | | | | | | | | | | | | | | | | | | |
Equity derivative* | | | 16,741 | | | | — | | | | — | | | | — | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | 46,052 | | | | 30,622 | | | | 14,490 | | | | 15,284 | | | | 581 | | | | 267 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
| | $ | 116,398 | | | $ | 119,240 | | | $ | 37,119 | | | $ | 57,284 | | | $ | 20,812 | | | $ | 4,025 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
| * | The equity derivative has no contractual settlement date as it is contingent upon the occurrence of certain events. |
Market risk
Market risk is the risk that changes in market prices, such as foreign exchange rates, interest rates and equity prices will affect the Company’s income or the value of its holdings of financial instruments. The objective of market risk management is to manage and control market risk exposures within acceptable parameters, while optimizing the return.
Transaction risk
The Company is exposed to currency risk on sales, purchases and borrowings that are denominated in a currency other than that of the respective functional currency of each individual Company entity. Where possible, the Company tries to match its foreign currency denominated assets and liabilities. The Company does not have a formal policy in relation to transactional exposures, but is planning to put such a policy in place and, where necessary, engage the services of specialized personnel or consultants to manage such risks.
The Company has a Brazilian subsidiary, Eurosilicone Brazil Importaçao e Exportaçao LTDA, and a Mexican subsidiary, GC Aesthetics Mexico S. de R.L. de C.V. The Brazilian subsidiary has revenues and running/importation costs in Brazilian Reals, but the cost of the product and related liability (ultimately to a fellow Company undertakings) is in Euro. The Mexican subsidiary’s revenues and importation costs are in US dollars while operating costs are in Mexican Pesos.
F-53
GLOBAL CONSOLIDATED AESTHETICS LIMITED
Notes to Consolidated Financial Statements—Continued
The following table sets out the Company’s exposure to foreign currency risk at the end of the reporting period:
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | December 31, 2014 | |
| | | US Dollar | | | Euro | | | Sterling | | | African Rand | | | Mexican Peso | | | Total | |
| | | (in thousands) | |
Trade and other receivables | | $ | 5,799 | | | $ | 103 | | | $ | — | | | $ | 315 | | | $ | — | | | $ | 6,217 | |
Intercompany receivables | | | 4,117 | | | | 96,832 | | | | 5,305 | | | | — | | | | — | | | | 106,254 | |
Cash and cash equivalents | | | 3,263 | | | | 270 | | | | — | | | | — | | | | — | | | | 3,533 | |
Trade and other payables | | | (3,849 | ) | | | 29 | | | | — | | | | — | | | | 8 | | | | (3,812 | ) |
Intercompany payables | | | (4,111 | ) | | | (74,444 | ) | | | (2,016 | ) | | | — | | | | — | | | | (80,571 | ) |
Bank and other loans | | | (13 | ) | | | (4,262 | ) | | | — | | | | — | | | | — | | | | (4,275 | ) |
Deferred consideration | | | (100 | ) | | | — | | | | — | | | | — | | | | — | | | | (100 | ) |
B Preference Shares and C Preference Shares | | | (116,210 | ) | | | — | | | | — | | | | — | | | | — | | | | (116,210 | ) |
Equity derivative | | | (22,284 | ) | | | — | | | | — | | | | — | | | | — | | | | (22,284 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | |
Total | | $ | (133,388 | ) | | $ | 18,528 | | | $ | 3,289 | | | $ | 315 | | | $ | 8 | | | $ | (111,248 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | December 31, 2013 | |
| | | US Dollar | | | Euro | | | Sterling | | | African Rand | | | Mexican Peso | | | Total | |
| | | (in thousands) | |
Trade and other receivables | | $ | 3,571 | | | $ | 1 | | | $ | — | | | $ | 453 | | | $ | — | | | $ | 4,025 | |
Intercompany receivables | | | 505 | | | | 4,757 | | | | 2,233 | | | | — | | | | — | | | | 7,495 | |
Cash and cash equivalents | | | 1,075 | | | | — | | | | 4 | | | | — | | | | — | | | | 1,079 | |
Trade and other payables | | | (3,236 | ) | | | — | | | | — | | | | — | | | | — | | | | (3,236 | ) |
Intercompany payables | | | (488 | ) | | | (10,069 | ) | | | (3,645 | ) | | | — | | | | — | | | | (14,202 | ) |
Bank and other loans | | | (3,790 | ) | | | — | | | | (2,147 | ) | | | — | | | | — | | | | (5,937 | ) |
Deferred consideration | | | (102 | ) | | | — | | | | — | | | | — | | | | — | | | | (102 | ) |
B Preference Shares and C Preference Shares | | | (46,518 | ) | | | — | | | | — | | | | — | | | | — | | | | (46,518 | ) |
Equity derivative | | | (16,741 | ) | | | — | | | | — | | | | — | | | | — | | | | (16,741 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | |
Total | | $ | (65,724 | ) | | $ | (5,311 | ) | | $ | (3,555 | ) | | $ | 453 | | | $ | — | | | $ | (74,137 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | |
F-54
GLOBAL CONSOLIDATED AESTHETICS LIMITED
Notes to Consolidated Financial Statements—Continued
Sensitivity analysis
The following table demonstrates the sensitivity of the Company’s income before income tax and the Company’s equity to a reasonably possible change in value of the US dollar, Euro and Sterling, with all other variables held constant. The impact on the Company’s profit before income tax is due to changes in the fair value of monetary assets and liabilities in relation to the functional currency of the Company subsidiaries. The impact on the Company’s equity is due to changes in value resulting from translating the Company’s subsidiaries to presentation currency. The Company’s exposure to foreign currency changes for all other currencies is not material.
| | | | | | | | | | | | | | | | |
| | | As of and for the year ended December 31, | |
| | | 2014 | | | 2013 | |
| | | Impact on income | | | Impact on equity | | | Impact on income | | | Impact on equity | |
| | | (in thousands) | |
10% strengthening of US dollar | | $ | 13,329 | | | $ | (11,811 | ) | | $ | 6,562 | | | $ | (5,931 | ) |
10% weakening of US dollar | | | (13,329 | ) | | | 11,811 | | | | (6,562 | ) | �� | | 5,931 | |
10% strengthening of Euro | | | (1,853 | ) | | | — | | | | 531 | | | | — | |
10% weakening of Euro | | | 1,853 | | | | — | | | | (531 | ) | | | — | |
10% strengthening of Sterling | | | (329 | ) | | | — | | | | 356 | | | | — | |
10% weakening of Sterling | | | 329 | | | | — | | | | (356 | ) | | | — | |
Interest rate risk
Profile
At the reporting date, the interest rate profile of the Company’s interest-bearing financial instruments was:
| | | | | | | | |
| | | December 31, | |
| | | 2014 | | | 2013 | |
| | | (in thousands) | |
Fixed rate instruments | | | | |
B Preference Shares and C Preference Shares | | $ | 116,210 | | | $ | 46,518 | |
Financial liabilities | | | 3,406 | | | | 25,093 | |
| | | | | | | | |
Variable rate instruments | | | | | | | | |
Financial liabilities | | $ | 48,302 | | | $ | 21,240 | |
| | | | | | | | |
The majority of the Company’s ongoing operations are financed from a mixture of cash generated from operations, equity and preference share capital and borrowings. The Company considers the B Preference Share and C Preference Share capital to be part of its capital base.
Fair values
Fair value of financial instruments
Effective January 1, 2013, the Company adopted IFRS 13, Fair Value Measurements, which defines fair value, establishes a framework for measuring fair value and expands required disclosures about fair value measurements. Under the standard, fair value refers to the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants in the market in which the reporting entity transacts. IFRS 13 clarifies the principle that fair value should be based on the assumptions market participants would use when pricing the asset or liability.
F-55
GLOBAL CONSOLIDATED AESTHETICS LIMITED
Notes to Consolidated Financial Statements—Continued
IFRS 13 establishes a fair value hierarchy which requires the Company to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value. We primarily apply the market approach for recurring fair value measurements. The standard describes three levels of inputs that may be used to measure fair value:
Level 1: Quoted prices in active markets for identical assets or liabilities.
Level 2: Observable inputs other than Level 1 prices, such as quoted prices for similar assets or liabilities; quoted prices in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities.
Level 3: Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities.
As of December 31, 2014 and December 31, 2013, those assets and liabilities that are measured at fair value on a recurring basis consist of the Company’s short-term and long-term marketable equity securities it classifies as available-for-sale, foreign currency exchange contracts, and interest rate swap instruments. The Company believes that the carrying amounts of its other financial instruments, including cash and cash equivalents, short-term investments, accounts receivable, prepaid expenses and other current assets, accounts payable, accrued expenses and other current liabilities, and amounts drawn on our revolving credit facilities consist primarily of instruments without extended maturities, the fair value of which, based on management’s estimates, approximates their carrying value due to the short-term maturities of these instruments.
The equity derivative has been valued using an option valuation technique (Level 3 hierarchy) where the main inputs are the share price, expected volatility of the share price, anticipated term to a Liquidity Event, expected exercise price and probability attached to a Liquidity Event.
The following table presents information about our assets and liabilities measured at fair value on a recurring basis as of December 31, 2014 and December 31, 2013, and indicates the fair value hierarchy of the valuation techniques utilized to determine such fair value.
F-56
GLOBAL CONSOLIDATED AESTHETICS LIMITED
Notes to Consolidated Financial Statements—Continued
Fair values versus carrying amounts
The fair values of financial assets and liabilities, together with the carrying amounts shown in the statement of financial position, are as follows:
| | | | | | | | | | | | | | | | | | | | |
| | | December 31, 2014 | |
| | | Carrying
value | | | Fair value measurements using: | |
| | | | | | Quoted prices
in active
markets for
identical
items
(Level 1) | | | Significant other
observable
inputs (Level 2) | | | Significant other
unobservable
inputs
(Level 3) | | | Total | |
| | | (in thousands) | |
Assets at fair value: | | | | | | | | | | | | |
Cash and cash equivalents | | $ | 10,616 | | | $ | 10,616 | | | $ | — | | | $ | — | | | $ | 10,616 | |
Restricted cash | | | 3,642 | | | | 3,642 | | | | — | | | | — | | | | 3,642 | |
Trade receivables | | | 12,840 | | | | 12,840 | | | | — | | | | — | | | | 12,840 | |
Intangible assets | | | 23,691 | | | | — | | | | — | | | | 23,691 | | | | 23,691 | |
Prepayments and other receivables | | | 4,539 | | | | 4,539 | | | | — | | | | — | | | | 4,539 | |
| | | | | | | | | | | | | | | | | | | | |
Total assets at fair value | | $ | 55,328 | | | $ | 31,637 | | | $ | — | | | $ | 23,691 | | | $ | 55,328 | |
| | | | | | | | | | | | | | | | | | | | |
Liabilities at fair value: | | | | | | | | | | | | |
Bank and other loans | | $ | (458 | ) | | $ | (428 | ) | | $ | — | | | $ | — | | | $ | (428 | ) |
Payment In Kind loans | | | (633 | ) | | | (633 | ) | | | — | | | | — | | | | (633 | ) |
Finance lease liabilities | | | (748 | ) | | | (604 | ) | | | — | | | | — | | | | (604 | ) |
Trade payables | | | (7,206 | ) | | | (7,206 | ) | | | — | | | | — | | | | (7,206 | ) |
Accruals and other creditors | | | (9,923 | ) | | | (9,923 | ) | | | — | | | | — | | | | (9,923 | ) |
Deferred considerations | | | (100 | ) | | | (100 | ) | | | — | | | | — | | | | (100 | ) |
Shareholder loans | | | (50,412 | ) | | | — | | | | (51,250 | ) | | | — | | | | (51,250 | ) |
B Preference Shares and C Preference Shares | | | (116,210 | ) | | | — | | | | — | | | | (124,376 | ) | | | (124,376 | ) |
Equity derivatives | | | (22,284 | ) | | | — | | | | — | | | | (22,348 | ) | | | (22,348 | ) |
| | | | | | | | | | | | | | | | | | | | |
Total liabilities at fair value | | $ | (207,974 | ) | | $ | (18,894 | ) | | $ | (51,250 | ) | | $ | (146,724 | ) | | $ | (216,868 | ) |
| | | | | | | | | | | | | | | | | | | | |
F-57
GLOBAL CONSOLIDATED AESTHETICS LIMITED
Notes to Consolidated Financial Statements—Continued
| | | | | | | | | | | | | | | | | | | | |
| | | December 31, 2013 | |
| | | Carrying
value | | | Fair value measurements using: | |
| | | | | | Quoted prices
in active
markets for
identical
items
(Level 1) | | | Significant other
observable
inputs (Level 2) | | | Significant other
unobservable
inputs (Level 3) | | | Total | |
| | | (in thousands) | |
Assets at fair value: | | | | | | | | | | | | | | | | | | | | |
Cash and cash equivalents | | $ | 4,519 | | | $ | 4,519 | | | $ | — | | | $ | — | | | $ | 4,519 | |
Restricted cash | | | — | | | | — | | | | — | | | | — | | | | — | |
Trade receivables | | | 10,006 | | | | 10,006 | | | | — | | | | — | | | | 10,006 | |
Intangible assets | | | 29,672 | | | | — | | | | — | | | | 29,672 | | | | 29,672 | |
Prepayments and other receivables | | | 4,574 | | | | 4,574 | | | | — | | | | — | | | | 4,574 | |
| | | | | | | | | | | | | | | | | | | | |
Total assets at fair value | | $ | 48,771 | | | $ | 19,099 | | | $ | — | | | $ | 29,672 | | | $ | 48,771 | |
| | | | | | | | | | | | | | | | | | | | |
Liabilities at fair value: | | | | | | | | | | | | | | | | | | | | |
Bank and other loans | | $ | (21,803 | ) | | $ | (18,189 | ) | | $ | — | | | $ | — | | | $ | (18,189 | ) |
Payment In Kind loans | | | (937 | ) | | | (937 | ) | | | — | | | | — | | | | (937 | ) |
Finance lease liabilities | | | (1,200 | ) | | | (1,074 | ) | | | — | | | | — | | | | (1,074 | ) |
Trade payables | | | (6,206 | ) | | | (6,206 | ) | | | — | | | | — | | | | (6,206 | ) |
Accruals and other creditors | | | (8,969 | ) | | | (8,969 | ) | | | — | | | | — | | | | (8,969 | ) |
Deferred considerations | | | (102 | ) | | | (102 | ) | | | — | | | | — | | | | (102 | ) |
Shareholder loans | | | (22,891 | ) | | | — | | | | (15,803 | ) | | | — | | | | (15,803 | ) |
B Preference Shares and C Preference Shares | | | (46,518 | ) | | | — | | | | — | | | | (45,980 | ) | | | (45,980 | ) |
Equity derivatives | | | (16,741 | ) | | | — | | | | — | | | | (16,741 | ) | | | (16,741 | ) |
| | | | | | | | | | | | | | | | | | | | |
Total liabilities at fair value | | $ | (125,367 | ) | | $ | (35,477 | ) | | $ | (15,803 | ) | | $ | (62,721 | ) | | $ | (114,001 | ) |
| | | | | | | | | | | | | | | | | | | | |
During the years ended December 31, 2014 and December 31, 2013, the Company did not transfer financial instruments between fair value measurement levels.
21. Fair value of equity derivatives
The Company has an equity derivative and, as required under IFRS 13, this equity derivative is required to be revalued annually. The Company employed third-party experts to provide a valuation.
The valuation was undertaken with regard to comparable market transactions between informed market participants. The transaction values were derived from data publicly available, however, due to the limited activity levels for transactions for companies of similar nature, a degree of professional judgment was used by the consultants with regards to the final valuation. For this reason, the valuation of the equity derivative is classified as level 3 as defined by IFRS 13 and presented in the Fair Value table.
The following is a summary of valuation methods used in relation to the equity derivative valuation.
F-58
GLOBAL CONSOLIDATED AESTHETICS LIMITED
Notes to Consolidated Financial Statements—Continued
The individual derivatives share value is based on using a Black-Scholes-Merton valuation model based on the enterprise value of the company.
| | | | |
Valuation technique | | Significant unobservable inputs | | Inter-relationship between key
unobservable inputs and fair value
measurement |
Comparable market transactions | | | | The estimated fair value would
increase/(decrease) if: |
| | |
The value is based on comparable market transactions with the following characteristics: 1) Involvement in the medical Aesthetic sector, medical devices 2) High growth companies active in the medical sector | | Recent comparable market transactions with a multiple of 3-5 last twelve months (“LTM”) revenues. | | —the revenue multiple if LTM
revenue were to be higher/
(lower) |
| | |
| Comparable quoted market companies | | | | The estimated fair value would
increase/(decrease) if: |
| | |
The value is based on comparable quoted companies with the following characteristics: 1) Involvement in the medical Aesthetic sector, medical devices 2) High growth companies active in the medical implant sector | | Recent comparable market transactions with a multiple of 1.4-6.3x LTM revenues. | | —the revenue multiple if LTM
revenue were to be higher/
(lower) |
22. Segment information
The Company operates in a single business segment, which encompasses the design, manufacture and distribution of implantable medical devices. The Company’s business is homogenous with respect to its production process, customer base and products. Accordingly, as the Company functions as a single operating segment, that operating segment also represents its sole reporting structure. The chief operating decision maker (“CODM”) is the team of the Company’s Chief Executive Officer, Chief Financial Officer and Chief Technical Operations Officer, as these roles act as a single management unit with respect to overall management of the Company. Management does not segregate its business for internal reporting. The CODM relies upon consolidated financial information for assessing performance and supporting resource allocation decisions. The Board of Directors are presented with consolidated financial information to monitor performance of the Company. The Company’s operations are directed from their corporate headquarters in Dublin, Ireland. Therefore, the disclosure requirements of IFRS 8:Operating segments in relation to segment profit or loss, segment assets and segment liabilities are not shown below as these are the consolidated results as presented in the Consolidated Statements of Comprehensive Income and Consolidated Statements of Financial Position.
F-59
GLOBAL CONSOLIDATED AESTHETICS LIMITED
Notes to Consolidated Financial Statements—Continued
Revenue, which excludes value added tax, sales between Company entities and trade discounts, represents the fair value of goods supplied, which comprise medical and surgical devices. The Company’s products are sold, either directly or through its distributor network, in 75 countries. Sales during 2014 and 2013 to the Company’s country of domicile and foreign countries representing more than 10% of 2014 revenues are as follows:
| | | | | | | | |
| | | Year ended
December 31, | |
| | | 2014 | | | 2013 | |
| | | (in thousands) | |
Ireland | | $ | 164 | | | $ | 180 | |
Brazil | | | 9,980 | | | | 6,705 | |
All other countries | | | 42,660 | | | | 37,745 | |
| | | | | | | | |
Total revenue | | $ | 52,804 | | | $ | 44,630 | |
| | | | | | | | |
The analysis of non-current assets (other than financial instruments or deferred tax assets) by country is as follows:
| | | | | | | | |
| | | Year ended
December 31, | |
| | | 2014 | | | 2013 | |
| | | (in thousands) | |
Goodwill and intangible assets | | | | | | | | |
Ireland | | $ | 22,949 | | | $ | 29,176 | |
Others | | | 2,992 | | | | 3,046 | |
| | | | | | | | |
| | $ | 25,941 | | | $ | 32,222 | |
| | | | | | | | |
Property, plant and equipment | | | | | | | | |
Ireland | | $ | 12 | | | $ | 8 | |
France | | | 3,360 | | | | 4,147 | |
United Kingdom | | | 1,133 | | | | 682 | |
Others | | | 169 | | | | 139 | |
| | | | | | | | |
| | $ | 4,674 | | | $ | 4,976 | |
| | | | | | | | |
The distribution of revenue by major product was as follows:
| | | | | | | | |
| | | Year ended
December 31, | |
| | | 2014 | | | 2013 | |
| | | (in thousands) | |
Breast implants | | $ | 48,216 | | | $ | 40,582 | |
Other implantable products | | | 2,889 | | | | 2,680 | |
Others | | | 1,699 | | | | 1,368 | |
| | | | | | | | |
Total revenue | | $ | 52,804 | | | $ | 44,630 | |
| | | | | | | | |
There were no revenues with a single external customer that amount to 10% or more of the Company’s revenues.
F-60
GLOBAL CONSOLIDATED AESTHETICS LIMITED
Notes to Consolidated Financial Statements—Continued
23. Related parties
Under IAS 24Related party disclosures, the Company has a related-party relationship with its key management. The Company has defined its key management as its directors and senior managers. Details of the compensation of key management are set out below.
Key management remuneration
| | | | | | | | |
| | | Number | |
| | | December 31, | |
| | | 2014 | | | 2013 | |
Number of individuals | | | 8 | | | | 8 | |
| | | | | | | | |
Comprising the following at year end | | | | | | | | |
Directors (including non-executive directors) | | | 5 | | | | 5 | |
Other key management personnel | | | 3 | | | | 3 | |
| | | | | | | | |
| | | 8 | | | | 8 | |
| | | | | | | | |
| | | | | | | | |
| | | Year ended
December 31, | |
| | | 2014 | | | 2013 | |
| | | (in thousands) | |
Salaries and other short term employee benefits | | $ | 2,979 | | | $ | 931 | |
Post-employment benefits | | | 103 | | | | 113 | |
Termination payments | | | 51 | | | | — | |
Directors’ fees | | | 60 | | | | 60 | |
| | | | | | | | |
Charged to the statement of comprehensive income | | $ | 3,193 | | | $ | 1,104 | |
| | | | | | | | |
Directors’ fees include fees to non-executive directors.
Related party transactions between the Company and its related parties
Related party transactions and balances to the extent not disclosed elsewhere in these financial statements are set out below.
During the year, the loan payable to Oyster Technology Investments Limited amounting to $4.0 million was converted to 1,797,202 B Preference Shares and 400,000 warrants on ordinary shares were exercised. At December 31, 2014, Oyster Technology Investments Limited owned 2,917,300(2013: 2,517,300) ordinary shares and 2,526,329(2013: 729,147) B Preference Shares of the Company and the carrying value of loans payable by the Company to Oyster Technology Investments Limited was $0.6 million(2013: $5.2 million). During the year management fees to Oyster Technology Investments Limited amounted to $0.3 million(2013: $0.3 million).
The Company has granted Nil warrants(2013: 400,000) over ordinary shares and Nil(2013: 21,105)warrants over B Preference Shares to Oyster Technology Investments Limited.
DBL Capital Partners limited (DBL) owns 195,100(2013: 195,100) ordinary shares.
DBL Capital Nominees Limited (DBL Nominees) owned 307,000 ordinary shares(2013: 307,000) and 165,021 (2013: 165,021) B Preference Shares of the Company and at year end, the carrying value of loans payable by the Company to DBL Capital Partners Limited (which is considered a related party of DBL Nominees) was $0.9 million(2013: $1.6 million).
F-61
GLOBAL CONSOLIDATED AESTHETICS LIMITED
Notes to Consolidated Financial Statements—Continued
During the year, the loan payable to Barry’s Tea Holdings (“Barry’s”) amounting to $1.8 million was converted to 812,438 B Preference Shares. At December 31, 2014, Barry’s owned 343,600(2013: 343,600) ordinary shares and 1,043,491(2013: 231,053) B Preference Shares in the Company and the carrying value of loans payable by the Company to Barry’s was $0.6 million(2013: $2.8 million). The Company has granted Nil(2013: 24,286) warrants over B Preference Shares to Barry’s. The Company has granted Nil(2013: 91,347)warrants over ordinary shares to Barry’s.
During the year, the loan payable to Tony Barry amounting to $0.7 million was converted to 320,916 B Preference Shares. At December 31, 2014, Tony Barry owned 176,800(2013: 176,800) ordinary shares and 436,989(2013: 116,073) B Preference Shares in the Company and the carrying value of loans payable by the Company to Tony Barry was $0.3 million(2013: $1.2 million). During the year, the Company has granted Nil(2013: 11,417) warrants over B Preference Shares to Tony Barry. The Company has granted Nil(2013: 32,053)warrants over ordinary shares to Tony Barry.
During the year, the loan payable to Montreux Equity Partners IV, L.P. (Montreux Equity Partners) amounting to $12.2 million was converted to 5,495,938 B Preference Shares. At December 31, 2014, Montreux Equity Partners owned 10,594,535 B Preference Shares(2013: 5,098,597) of the Company. During the year, management fees to Montreux Equity Partners amounted to $0.5 million or €0.4 million(2013: $0.5 million or €0.4 million). The carrying value of loans payable by the Company to Montreux Equity Partners at year-end was $0(2013: $12.8 million). During the year, the Company has granted Montreux Equity Partners Nil(2013: 1,034,217) warrants over ordinary shares and Nil(2013: 108,546) warrants over B Preference Shares.
During the year, the loan payable to Montreux IV Associates, LLC (Montreux Associates) amounting to $0.2 million was converted to 97,520 B Preference Shares. Montreux Associates owns 217,542 B Preference Shares in the Company at year end(2013: 120,022).
Daniel K. Turner III and Howard D. Palefsky are members and have an ownership interest in Montreux Equity Management IV, L.L.C. (Montreux Equity Management) which is the general partner of Montreux Equity Partners and the manager of Montreux Associates. Montreux Equity Management is a limited partner in Montreux Equity Partners and as such through their ownership interests in Montreux Equity Management have a limited partnership interest in Montreux Equity Partners. They do not have an economic interest in Montreux Associates.
Royalty Opportunities S.À R.L. owns 1,451,762 C Preference Shares(2013: Nil) of the Company at year end. The carrying value of loans payable by the Company to Royalty Opportunities S.À R.L. at year-end is disclosed in Note 18 in loans from shareholders section.
Dr. Michel Rouif was appointed to the Company’s Board of Directors on April 10, 2015. Previously, Dr. Rouif was a member of the Company’s professional expert group, and was compensated for his participation as an external expert advisor.
24. Subsequent events
In April 2015, the Company obtained an additional loan from Royalty Opportunities S.À R.L. amounting to $10.0 million and an equity subscription for 592,042 ordinary shares.
Dr. Michel Rouif was appointed to the Company’s Board of Directors on April 10, 2015.
Declan McKeon was appointed to the Company’s Board of Directors on April 10, 2015.
F-62
GLOBAL CONSOLIDATED AESTHETICS LIMITED
Notes to Consolidated Financial Statements—Continued
25. Company undertakings
The following are the Company undertakings of Global Consolidated Aesthetics Limited, all of which are included in the consolidated financial statements, and which are incorporated and operating in Ireland unless otherwise indicated.
| | | | | | | | | | |
Name and registered office | | Principal activity | | Percentage held by: | |
| | | | | Company | | | Company
undertaking | |
GC Aesthetics Holdings Limited Suite 601, Q House Furze Road, Sandyford Dublin 18 | | Holding company | | | 100 | % | | | — | |
| | | |
GC Aesthetics Finance Limited Suite 601, Q House Furze Road, Sandyford Dublin 18 | | Finance Company | | | — | | | | 100 | % |
| | | |
Nagôr Limited 8 Mount Pleasant Douglas, Isle of Man | | Distribution of medical devices | | | — | | | | 100 | % |
| | | |
BioSil Limited 8 Mount Pleasant Douglas, Isle of Man | | Distribution and manufacture of medical devices | | | — | | | | 100 | % |
| | | |
Eurosilicone SAS Zone Industrial la Peyroliere 84402 APT, France | | Distribution and manufacture of medical devices | | | — | | | | 100 | % |
| | | |
Global Consolidated Aesthetics(UK) Limited Unit 2 Ivanhoe Industrial Estate Tournament Way Ashby-de-la-Zouche LE65 2UU | | Holding company | | | — | | | | 100 | % |
| | | |
GC Aesthetics (IP) Limited Suite 601, Q House Furze Road, Sandyford Dublin 18 | | Intellectual property company | | | — | | | | 100 | % |
| | | |
GC Aesthetics (Manufacturing) Limited Suite 601 Q House Furze Road, Sandyford Dublin 18 | | Trading and intellectual property company | | | — | | | | 100 | % |
| | | |
GC Aesthetics France SAS ZI la Peyroliere 84402 APT France | | Holding company | | | — | | | | 100 | % |
| | | |
GC Aesthetics Management Limited Suite 601, Q House Furze Road, Sandyford Dublin 18 | | Management services company | | | — | | | | 100 | % |
F-63
GLOBAL CONSOLIDATED AESTHETICS LIMITED
Notes to Consolidated Financial Statements—Continued
| | | | | | | | | | |
Name and registered office | | Principal activity | | Percentage held by: | |
| | | | | Company | | | Company
undertaking | |
| | | |
Dreamxcel IP Limited 2nd Floor, Block B, Medine Mews Chaussee Street, Port Louis, Mauritius | | Dormant | | | — | | | | 100 | % |
| | | |
Dreamxcell International Limited 2nd Floor, Block B, Medine Mews Chaussee Street, Port Louis, Mauritius | | Dormant | | | — | | | | 100 | % |
| | | |
Dreamxcell GmbH Weidenweg, 8 D-40822 Metmann, Germany | | Management services company | | | — | | | | 100 | % |
| | | |
Global Consolidated Aesthetics (US) Corp 4213 State Street Suite 205, Santa Barbara, CA 93110 | | Distribution of medical devices | | | — | | | | 100 | % |
| | | |
GC Aesthetics Holdings (Brazil) Limited Suite 601, Q House Furze Road, Sandyford Dublin 18 | | Holding company | | | — | | | | 100 | % |
| | | |
Eurosilicone Brazil Importçao eExportaçao LTDA Alameda Araguai 230 Alphaville Industrial 06455—000 Sao Paulo Brazil | | Import, export and trading company | | | — | | | | 100 | % |
| | | |
GC Aesthetics do (Brazil) ParticipaçõesLtda Av. Brasil 471 Sao Paulo | | Dormant | | | — | | | | 100 | % |
| | | |
GC Aesthetics Spain, S.L.U. Plaza Eguilaz, 3 Planta Baja Local 6 08017, Barcelona Spain | | Import and trading company | | | — | | | | 100 | % |
| | | |
GC Aesthetics Mexico S. de R.L.deC.V. Pasco de la Reforma 505, piso 31 Col Cuauhtémoc CP06500 Mexico D.F. | | Import and trading company | | | — | | | | 100 | % |
| | | |
GC Aesthetics GmbH c/o TAP Dr Schlumburger und Partner mbB Prinz-Ludwig-Str.7 80333 Munchen Deutschland | | Import and trading company | | | — | | | | 100 | % |
| | | |
GCA China Consulting Shanghai Co.,Ltd. Rm. B5, 24th Floor, Purple Mountain Hotel 528 Laoshan Road, Shanghai, China | | Import and trading company | | | — | | | | 100 | % |
F-64
GLOBAL CONSOLIDATED AESTHETICS LIMITED
Schedule II – Valuation and Qualifying Accounts
| | | | | | | | | | | | | | | | | | | | |
Allowance for doubtful accounts receivable | | Balance at
beginning of
period | | | Charged to
costs and
expenses | | | Deductions(1) | | | Foreign
exchange
(gain) loss(2) | | | Balance at end
of period | |
| | | (in thousands) | |
2014 | | $ | 2,231 | | | $ | 653 | | | $ | (1,632 | ) | | $ | (180 | ) | | $ | 1,072 | |
2013 | | $ | 2,394 | | | | 1,604 | | | | (1,863 | ) | | | 96 | | | | 2,231 | |
| (1) | Deductions include receivables written-off netted against recoveries from prior charge-offs. |
| (2) | Foreign currency translation adjustments. |
F-65
Through and including , 2015 (the 25th day after the date of this prospectus), all dealers effecting transactions in the Ordinary Shares, whether or not participating in this offering, may be required to deliver a prospectus. This delivery requirement is in addition to a dealer’s obligation to deliver a prospectus when acting as an underwriter and with respect to an unsold allotment or subscription.
Ordinary Shares
GC Aesthetics plc
Ordinary Shares
PROSPECTUS
BofA Merrill Lynch
Deutsche Bank Securities
Cowen and Company
William Blair
, 2015
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 6. Indemnification of directors and officers
To the fullest extent permitted by Irish law, our articles of association (which are substantially in the form attached as Exhibit 3.1 to this registration statement) will confer an indemnity on our directors and officers. However, this indemnity is limited by the Irish Companies Act, which prescribes that an advance commitment to indemnify only permits a company to pay the costs or discharge the liability of a director or corporate secretary where judgment is given in favor of the director or corporate secretary in any civil or criminal action in respect of such costs or liability, or where an Irish court grants relief because the director or corporate secretary acted honestly and reasonably and ought fairly to be excused. Any provision whereby an Irish company seeks to commit in advance to indemnify its directors or corporate secretary over and above the limitations imposed by the Irish Companies Act will be void under Irish law, whether contained in its articles of association or any contract between the company and the director or corporate secretary. This restriction does not apply to our executives who are not directors, the corporate secretary or other persons who would be considered “officers” within the meaning of that term under the Irish Companies Act.
Our articles of association will also contain indemnification and expense advancement provisions for persons who are not directors or our corporate secretary.
We plan to purchase directors’ and officers’ liability insurance, as well as other types of insurance, for our directors, officers, employees and agents, which is permitted under our articles of association and the Irish Companies Acts.
We and certain of our subsidiaries intend to enter into agreements to indemnify our directors to the maximum extent allowed under applicable law.
Item 7. Recent Issuances of Unregistered Securities
In the three years preceding the filing of this registration statement, GC Aesthetics plc has not issued securities that were not issued under the Securities Act.
In the three years preceding the filing of this registration statement, Global Consolidated Aesthetics Limited has issued the following securities that were not registered under the Securities Act:
| | • | | On July 31, 2012, to Montreux, Oyster and certain other of its existing shareholders, interest bearing loan notes in the aggregate amount of €7,500,000 and warrants to purchase an aggregate of 40,002 B Preference Shares and 750,000 ordinary shares. The notes and warrants were issued in two tranches, on July 31, 2012 and December 14, 2012. |
| | • | | On January 29, 2013, to Montreux and Oyster, interest bearing loan notes in the aggregate amount of €1,000,000 and warrants to purchase an aggregate of 5,333 B Preference Shares and 100,000 ordinary shares, exercisable at nominal value. |
| | • | | On April 16, 2013, to Montreux and Oyster, interest bearing loan notes in the aggregate amount of €500,000 and warrants to purchase an aggregate of 2,667 B Preference Shares and 50,000 ordinary shares, exercisable at nominal value. |
| | • | | On May 10, 2013, to Montreux, Oyster and certain other existing shareholders, interest bearing loan notes in the aggregate amount of €1,665,884 and warrants to purchase an aggregate of 8,884 B Preference Shares and 166,589 ordinary shares, exercisable at nominal value. The notes and warrants were issued in two tranches, on May 10, 2013 and October 24, 2013. |
II-1
| | • | | On January 20, 2014, as amended on February 19, 2014 and December 19, 2014, to Montreux, Oyster and certain other shareholders, all outstanding loan notes issued by Global Consolidated Aesthetics Limited, together with interest thereon, were converted into B Preference Shares and additional B Preference Shares were subscribed for in cash. A total of 8,612,983 B Preference Shares were issued pursuant to this subscription agreement at a subscription price of $2.22181 per share. |
| | • | | On February 13, 2014, to Oyster, 400,000 ordinary shares pursuant to an exercise of warrants previously issued to Oyster. |
| | • | | On February 19, 2014, to OrbiMed, 1,451,762 C Preference Shares at an aggregate subscription price of $6,000,000. |
| | • | | On April 27, 2015, to OrbiMed, 592,042 ordinary shares at an aggregate subscription price of €5,920.42. |
Item 8. Exhibits and financial statement schedules
(a) Exhibits
| | |
Exhibit
number | | Exhibit Title |
| |
| 1.1* | | Form of Underwriting Agreement |
| |
| 3.1* | | Memorandum and Articles of Association of GC Aesthetics plc |
| |
| 4.1* | | Amended and Restated Registration Rights Agreement dated February 19, 2014 |
| |
| 5.1* | | Opinion of Maples and Calder |
| |
| 10.1* | | Amended and Restated Credit Agreement by and between GC Aesthetics Finance Limited and Royalty Opportunities S.À R.L., dated November 26, 2014 |
| |
| 10.2* | | First Amendment to Amended and Restated Credit Agreement by and between GC Aesthetics Finance Limited and Royalty Opportunities S.À R.L., dated as of April 27, 2015 |
| |
| 10.3* | | Supply Agreement between NuSil Technologies LLC and Eurosilicone SAS, dated April 12, 2015. |
| |
| 10.4* | | Supply, Mutual Confidentiality & Non-Disclosure Agreement between Biosil Ltd. and Applied Silicone Corporation, dated December 15, 2008 |
| |
| 10.5* | | Amendment to Supply, Mutual Confidentiality & Non-Disclosure Agreement between Biosil Ltd. and Applied Silicone Corporation, dated January 15, 2010 |
| |
| 10.6* | | Amendment to Supply, Mutual Confidentiality & Non-Disclosure Agreement between Biosil Ltd. and Applied Silicone Corporation, dated January 10, 2012 |
| |
| 10.7* | | Amendment to Supply, Mutual Confidentiality & Non-Disclosure Agreement between Biosil Ltd. and Applied Silicone Corporation, dated January 10, 2014 |
| |
| 10.8* | | Service Agreement between Nagôr Limited and Ayse Kocak, dated February 15, 2013 |
| |
| 10.9* | | Contract of Employment between Nagôr Ltd. and Ian Crosbie, dated October 23, 2014 |
| |
| 10.10* | | Service Agreement between GC Aesthetics Management Limited and Barry Hatt, dated May 26, 2011 |
| |
| 10.11* | | Contract of Employment between Nagôr Ltd. and Vikash Agarwal, dated June 25, 2014 |
| |
| 10.12* | | Individual Employment Agreement between Global Consolidated Aesthetics Medico, S. De R.L. DE C.V. and Bernardo Garduno, dated October 15, 2012 |
II-2
| | |
Exhibit
number | | Exhibit Title |
| |
| 10.13* | | Service Agreement between GC Aesthetics Management Limited and Ayhan Aslan, dated February 26, 2013 |
| |
| 10.14* | | Employment Contract between GC Aesthetics Spain S.L.U. and Ignasi Salla, dated February 11, 2011 |
| |
| 10.15* | | Contract of Employment between Nagôr Ltd. and Julia Marsan, dated November 22, 2013 |
| |
| 10.16* | | Global Consolidated Aesthetics Limited Amended and Restated Share Option Plan 2010 |
| |
| 10.17* | | GC Aesthetics Equity Incentive Plan 2015 |
| |
| 10.18* | | Occupational Lease between Robert McCarthy, Michael McCarthy, Stephen McCarthy, Mark O’Brien, John Lombard and David Arnold, Furze Road Management Company Limited, GC Aesthetics Management Limited and Global Consolidated Aesthetics Limited, dated September 10, 2008 |
| |
| 10.19* | | Lease between Calspring Ltd and Biosil Ltd., dated July 1, 2009 |
| |
| 10.20* | | Lease between Leicestershire County Council and Biosil Ltd., dated January 25, 2005 |
| |
| 10.21* | | Renewal of lease between Leicestershire County Council and Biosil Ltd., dated May 10, 2010 |
| |
| 10.22* | | Sub-lease between Photo-me International PLC and Biosil Ltd., dated April 2, 2009 |
| |
| 10.23* | | Sub-lease between Scottish Enterprise, Biosil Limited, Nagôr Limited and Highcross (Cumbernauld) Limited, dated April 3, 1995 |
| |
| 10.24* | | Lease between Cumbernauld Limited Partnership and Biosil Ltd, dated April 29, 2002 |
| |
| 10.25* | | Lease between Natiocredimurs Societe en nom Collectif and Laboratoires Eurosilicone, dated March 8, 2002 |
| |
| 10.26* | | Lease between Natiocredibail and Eurosilicone SAS, dated April 2, 2010 |
| |
| 21.1* | | Subsidiaries of the registrant |
| |
| 23.1* | | Consent of Independent Registered Public Accountant |
| |
| 23.2* | | Consent of Maples and Calder (included in Exhibit 5.1) |
| |
| 24.1* | | Power of Attorney (included on signature page) |
| * | To be filed by amendment. |
(b) Financial statement schedules
All schedules have been omitted because the information required to be set forth therein is not applicable or is shown in the financial statements or notes thereto.
Item 9. Undertakings
| | (a) | The undersigned Registrant hereby undertakes to provide to the underwriters at the closing specified in the underwriting agreement certificates in such denominations and registered in such names as required by the underwriters to permit prompt delivery to each purchaser. |
| | (b) | Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the Registrant pursuant to the foregoing provisions, or otherwise, the Registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act of 1933 and is, therefore, unenforceable. In the event that a claim for indemnification against such |
II-3
| | liabilities (other than the payment by the Registrant of expenses incurred or paid by a director, officer or controlling person of the Registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the Registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act of 1933 and will be governed by the final adjudication of such issue. |
| | (c) | The undersigned registrant hereby undertakes that: |
| | (1) | That for purposes of determining any liability under the Securities Act of 1933, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the Registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act of 1933 shall be deemed to be part of this registration statement as of the time it was declared effective. |
| | (2) | That for the purpose of determining any liability under the Securities Act of 1933, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
II-4
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the city of Dublin, Ireland on , 2015.
| | |
| GC Aesthetics plc |
| |
| By: | | |
| Name: | | |
| Title: | | |
***
POWER OF ATTORNEY
The undersigned directors and officers of GC Aesthetics plc hereby appoint each of , as attorney-in-fact for the undersigned, with full power of substitution and resubstitution, for and in the name, place and stead of the undersigned, to sign and file with the Securities and Exchange Commission under the Securities Act of 1933 any and all amendments (including post-effective amendments) and exhibits to this registration statement onForm F-1 (or any other registration statement for the same offering that is to be effective upon filing pursuant to Rule 462(b) under the Securities Act of 1933) and any and all applications and other documents to be filed with the Securities and Exchange Commission pertaining to the registration of the securities covered hereby, with full power and authority to do and perform any and all acts and things whatsoever requisite and necessary or desirable, hereby ratifying and confirming all that said attorney-in-fact, or his or her substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities and on the dates indicated.
| | | | |
Signature | | Title | | Date |
| | |
Ayse Kocak | | Chief Executive Officer, Director (Principal Executive Officer) | | , 2015 |
| | |
Ian Crosbie | | Chief Financial Officer, (Principal Financial Officer) | | , 2015 |
| | |
Daniel K. Turner III | | Chairman | | , 2015 |
| | |
William McCabe | | Director | | , 2015 |
| | |
Declan McKeon | | Director | | , 2015 |
| | |
Michel Rouif, MD | | Director | | , 2015 |
| | |
| | Authorized Representative in the United States | | , 2015 |
II-5
| | |
Exhibit
number | | Exhibit Title |
| |
| 1.1* | | Form of Underwriting Agreement |
| |
| 3.1* | | Memorandum and Articles of Association of GC Aesthetics plc |
| |
| 4.1* | | Amended and Restated Registration Rights Agreement dated February 19, 2014 |
| |
| 5.1* | | Opinion of Maples and Calder |
| |
| 10.1* | | Amended and Restated Credit Agreement by and between GC Aesthetics Finance Limited and Royalty Opportunities S.À R.L., dated November 26, 2014 |
| |
| 10.2* | | First Amendment to Amended and Restated Credit Agreement by and between GC Aesthetics Finance Limited and Royalty Opportunities S.À R.L., dated as of April 27, 2015 |
| |
| 10.3* | | Supply Agreement between NuSil Technologies LLC and Eurosilicone SAS, dated April 12, 2015. |
| |
| 10.4* | | Supply, Mutual Confidentiality & Non-Disclosure Agreement between Biosil Ltd. and Applied Silicone Corporation, dated December 15, 2008 |
| |
| 10.5* | | Amendment to Supply, Mutual Confidentiality & Non-Disclosure Agreement between Biosil Ltd. and Applied Silicone Corporation, dated January 15, 2010 |
| |
| 10.6* | | Amendment to Supply, Mutual Confidentiality & Non-Disclosure Agreement between Biosil Ltd. and Applied Silicone Corporation, dated January 10, 2012 |
| |
| 10.7* | | Amendment to Supply, Mutual Confidentiality & Non-Disclosure Agreement between Biosil Ltd. and Applied Silicone Corporation, dated January 10, 2014 |
| |
| 10.8* | | Service Agreement between Nagôr Limited and Ayse Kocak, dated February 15, 2013 |
| |
| 10.9* | | Contract of Employment between Nagôr Ltd. and Ian Crosbie, dated October 23, 2014 |
| |
| 10.10* | | Service Agreement between GC Aesthetics Management Limited and Barry Hatt, dated May 26, 2011 |
| |
| 10.11* | | Contract of Employment between Nagôr Ltd. and Vikash Agarwal, dated June 25, 2014 |
| |
| 10.12* | | Individual Employment Agreement between Global Consolidated Aesthetics Medico, S. De R.L. DE C.V. and Bernardo Garduno, dated October 15, 2012 |
| |
| 10.13* | | Service Agreement between GC Aesthetics Management Limited and Ayhan Aslan, dated February 26, 2013 |
| |
| 10.14* | | Employment Contract between GC Aesthetics Spain S.L.U. and Ignasi Salla, dated February 11, 2011 |
| |
| 10.15* | | Contract of Employment between Nagôr Ltd. and Julia Marsan, dated November 22, 2013 |
| |
| 10.16* | | Global Consolidated Aesthetics Limited Amended and Restated Share Option Plan 2010 |
| |
| 10.17* | | GC Aesthetics Equity Incentive Plan 2015 |
| |
| 10.18* | | Occupational Lease between Robert McCarthy, Michael McCarthy, Stephen McCarthy, Mark O’Brien, John Lombard and David Arnold, Furze Road Management Company Limited, GC Aesthetics Management Limited and Global Consolidated Aesthetics Limited, dated September 10, 2008 |
| |
| 10.19* | | Lease between Calspring Ltd and Biosil Ltd., dated July 1, 2009 |
| |
| 10.20* | | Lease between Leicestershire County Council and Biosil Ltd., dated January 25, 2005 |
| |
| 10.21* | | Renewal of lease between Leicestershire County Council and Biosil Ltd., dated May 10, 2010 |
| |
| 10.22* | | Sub-lease between Photo-me International PLC and Biosil Ltd., dated April 2, 2009 |
| |
| 10.23* | | Sub-lease between Scottish Enterprise, Biosil Limited, Nagôr Limited and Highcross (Cumbernauld) Limited, dated April 3, 1995 |
| | |
Exhibit
number | | Exhibit Title |
| |
| 10.24* | | Lease between Cumbernauld Limited Partnership and Biosil Ltd, dated April 29, 2002 |
| |
| 10.25* | | Lease between Natiocredimurs Societe en nom Collectif and Laboratoires Eurosilicone, dated March 8, 2002 |
| |
| 10.26* | | Lease between Natiocredibail and Eurosilicone SAS, dated April 2, 2010 |
| |
| 21.1* | | Subsidiaries of the registrant |
| |
| 23.1* | | Consent of Independent Registered Public Accountant |
| |
| 23.2* | | Consent of Maples and Calder (included in Exhibit 5.1) |
| |
| 24.1* | | Power of Attorney (included on signature page) |
| * | To be filed by amendment. |


