
ClearLight Biotechnologies, Inc.
428 Oakmead Parkway
Sunnyvale, CA 94085-4708.
TEL 800-251-8905
Up to 20,000 shares of Common Stock, par value $0.0001 (the "Offering")
SEE "DESCRIPTION OF CAPITAL STOCK" AT PAGE 44
| Price to Public | Underwriting discount and commissions (1) | Proceeds to issuer |
Per share of Common Stock | $12.00 | 0 | $240,000 |
Total Maximum | | | $240,000(2) |
(1) The issuer does not currently intend to use commissioned sales agents or underwriters. In the event it uses commissioned sales agents or underwriters, it will file an amendment to the Offering Statement of which the Offering Circular forms a part.
(2) Before deducting expenses, estimated to be approximately $50,000 including legal fees, blue sky filing fees and Edgar filing costs. For more details, please see the section of this offering circular captioned "Plan of Distribution." This is a "best efforts" offering. There is no minimum number of shares that must be distributed in this offering.
The offering will commence within two calendar days after the offering statement in which this offering circular is included has been qualified by the US Securities and Exchange Commission (the "Commission" or the "SEC") and includes an amount of shares that we reasonably expect to be offered and sold within two years from the date of initial qualification, unless subsequently amended. The offering will terminate at the earlier of: (1) the date at which the maximum offering amount has been sold, (2) the date which is one year from this offering being qualified by the Commission, or (3) the date at which the offering is earlier terminated by us in our sole discretion.
There is no minimum number of shares that we must sell in order to conduct a closing in the Offering. We have made no arrangements to place subscription funds in an escrow, trust or similar account. Shares issued under this offering will be issued on a continuous basis under Rule 251(d)(3) under the Securities Act, and the Company will have access to such funds from the first dollar invested, even if those proceeds do not cover the costs of this offering. For further details please see the section of this offering circular captioned "Plan of Distribution."
THE UNITED STATES SECURITIES AND EXCHANGE COMMISSION DOES NOT PASS UPON THE MERITS OF OR GIVE ITS APPROVAL TO ANY SECURITIES OFFERED OR THE TERMS OF THE OFFERING, NOR DOES IT PASS UPON THE ACCURACY OR COMPLETENESS OF ANY OFFERING CIRCULAR OR OTHER SELLING LITERATURE. ANY REPRESENTATION TO THE CONTRARY IS UNLAWFUL.
THE SECURITIES HAVE NOT BEEN REGISTERED UNDER THE SECURITIES ACT OF 1933, AS AMENDED (THE "SECURITIES ACT") AND THESE SECURITIES ARE OFFERED PURSUANT TO AN EXEMPTION FROM REGISTRATION WITH THE COMMISSION. HOWEVER, THE COMMISSION HAS NOT MADE AN INDEPENDENT DETERMINATION THAT THE SECURITIES OFFERED HEREUNDER ARE EXEMPT FROM REGISTRATION.
THIS OFFERING CIRCULAR CONTAINS ALL OF THE REPRESENTATIONS BY THE ISSUER CONCERNING THIS OFFERING, AND NO PERSON SHALL MAKE DIFFERENT OR BROADER STATEMENTS THAN THOSE CONTAINED HEREIN. INVESTORS ARE CAUTIONED NOT TO RELY UPON ANY INFORMATION NOT EXPRESSLY SET FORTH IN THIS OFFERING CIRCULAR.
GENERALLY, NO SALE MAY BE MADE TO YOU IN THIS OFFERING IF THE AGGREGATE PURCHASE PRICE YOU PAY IS MORE THAN 10% OF THE GREATER OF YOUR ANNUAL INCOME OR NET WORTH. DIFFERENT RULES APPLY TO ACCREDITED INVESTORS AND NON-NATURAL PERSONS. BEFORE MAKING ANY REPRESENTATION THAT YOUR INVESTMENT DOES NOT EXCEED APPLICABLE THRESHOLDS, WE ENCOURAGE YOU TO REVIEW RULE 251(d)(2)(i)(C) OF REGULATION A. FOR GENERAL INFORMATION ON INVESTING, WE ENCOURAGE YOU TO REFER TO www.investor.gov.
This offering is inherently risky. See "Risk Factors" on page 14.
Sales of these securities will commence on approximately [to be determined].
The issuer is following the Form S-1 format of disclosure under Regulation A.
In the Offering Circular, the terms "ClearLight", "CLB", the "issuer", the "Company", "us" or "we" refers to ClearLight Biotechnologies, Inc.
Statement Regarding Forward-Looking Statements
THIS OFFERING CIRCULAR MAY CONTAIN FORWARD-LOOKING STATEMENTS AND INFORMATION RELATING TO, AMONG OTHER THINGS, THE COMPANY, ITS BUSINESS PLAN AND STRATEGY, AND ITS INDUSTRY. THESE FORWARD-LOOKING STATEMENTS ARE BASED ON THE BELIEFS OF, ASSUMPTIONS MADE BY, AND INFORMATION CURRENTLY AVAILABLE TO THE COMPANY'S MANAGEMENT. WHEN USED IN THE OFFERING MATERIALS, THE WORDS "ESTIMATE," "PROJECT," "BELIEVE," "ANTICIPATE," "INTEND," "EXPECT" AND SIMILAR EXPRESSIONS ARE INTENDED TO IDENTIFY FORWARD-LOOKING STATEMENTS AND WHICH CONSTITUTE FORWARD-LOOKING STATEMENTS. THESE STATEMENTS REFLECT MANAGEMENT'S CURRENT VIEWS WITH RESPECT TO FUTURE EVENTS AND ARE SUBJECT TO RISKS AND UNCERTAINTIES THAT COULD CAUSE THE COMPANY'S ACTUAL RESULTS TO DIFFER MATERIALLY FROM THOSE CONTAINED IN THE FORWARD-LOOKING STATEMENTS. INVESTORS ARE CAUTIONED NOT TO PLACE UNDUE RELIANCE ON THESE FORWARD-LOOKING STATEMENTS, WHICH SPEAK ONLY AS OF THE DATE ON WHICH THEY ARE MADE. THE COMPANY DOES NOT UNDERTAKE ANY OBLIGATION TO REVISE OR UPDATE THESE FORWARD-LOOKING STATEMENTS TO REFLECT EVENTS OR CIRCUMSTANCES AFTER SUCH DATE OR TO REFLECT THE OCCURRENCE OF UNANTICIPATED EVENTS.
Industry and Market Data
Although we are responsible for all disclosure contained in the Offering Circular, in some cases we have relied on certain market and industry data obtained from third-party sources that we believe to be reliable. Market estimates are calculated by using independent industry publications in conjunction with our assumptions regarding the machine vision for the manufacturing industry and market. While we are not aware of any misstatements regarding any market, industry or similar data presented herein, such data involves risks and uncertainties and is subject to change based on various factors, including those discussed under the headings "Statement Regarding Forward-Looking Statements" and "Risk Factors" in the Offering Circular.
Balance Sheet Information |
| |
Cash and Cash Equivalents: | $718,333 |
| | |
Investment Securities: | 0 |
| | |
Accounts and Notes Receivable: | $17,595 |
| | |
Property, Plant and Equipment (PP&E): | $818,271 |
| | |
Total Assets: | $2,072,588 |
| | |
Accounts Payable and Accrued Liabilities: | $500,080 |
| | |
Long Term Debt: | 0 |
| | |
Total Liabilities: | $500,080 |
| | |
Total Stockholders' Equity: | $1,572,508 |
| | |
Total Liabilities and Equity: | $2,072,588 |
| | |
|
| |
Statement of Comprehensive Income Information |
| |
Total Revenues: | $29,874 |
| | |
Costs and Expenses Applicable to Revenues: | $157,760 |
Depreciation and Amortization: | $31,620 |
| | |
Net Income: | ($680,461) |
| | |
Earnings Per Share - Basic: | ($0.73) |
| | |
Earnings Per Share - Diluted: | ($0.73) |
| | |
| |
Name of Auditor (if any): | None |
OFFERING CIRCULAR SUMMARY
This summary highlights selected information contained elsewhere in the Offering Circular. This summary is not complete and does not contain all the information that you should consider before deciding whether to invest in our Common Stock. You should carefully read the entire Offering Circular, including the risks associated with an investment in the Company discussed in the "Risk Factors" section of the Offering Circular, before making an investment decision. Some of the statements in the Offering Circular are forward-looking statements. See the section entitled "Statement Regarding Forward-Looking Statements."
Company Information
ClearLight is an early-stage life sciences tools company. CLB provides tissue processing, imaging, and image analysis services to pharmaceutical, biotechnology and academic researchers. CLB utilizes the patent-protected CLARITY technology, licensed from Stanford University, Palo Alto, CA. The CLARITY technology allows clearing of animal and human tissue so that thick tissue samples can be imaged in three dimensions (3D) with a confocal or light-sheet microscope and then analyzed by a lab scientist in a 3D dataset. The Company is currently developing automated analysis software enabled by artificial intelligence. This software is intended to analyze the large datasets associated with 3D imaging and provide researchers with quantitative spatial data. The images and analysis may allow researchers to See More Biology™ facilitating better understanding of the tissue and tumor microenvironments. We believe this will facilitate discovery of new methods and drugs to diagnose and treat disease.
The Offering
The Company is offering up to 20,000 shares of Common Stock for purchase at $12.00 per share.
Securities offered: | Shares of Common Stock |
Number of outstanding shares of Common Stock before the offering: | 1,134,331 |
Number of shares of Common Stock to be outstanding after the offering: | 1,154,331 |
Price per share: | $12.00 |
Maximum offering amount: | $240,000 |
Use of proceeds: | Working capital for operating expenses and research and development. |
Risk Factors
Our business is subject to a number of risks and uncertainties, including those highlighted in the section titled "Risk Factors" immediately following this summary.
RISK FACTORS
The SEC requires the Company to identify risks that are specific to its business and its financial condition. The Company is still subject to all the same risks that all companies in its business, and all companies in the economy, are exposed to. These include risks relating to economic downturns, political and economic events and technological developments (such as hacking and the Company's ability to detect and prevent such hacking). Additionally, early-stage companies are inherently riskier than more developed companies. You should consider general risks as well as specific risks when deciding whether to invest.
RISKS RELATED TO BUSINESS STRATEGY
We have an evolving business model.
We are developing a technology based on CLARITY, which is an acronym for Clear Lipid-exchanged Acrylamide-hybridized Rigid Imaging/Immunostaining/In situ hybridization-compatible Tissue-hYdrogel. CLARITY technology entails the formation of a hydrogel matrix by cross-linking biological molecules to a 3-D (three-dimensional) network of hydrophilic polymers, followed by lipid removal to generate a transparent and structurally intact tissue.
We currently are offering services to researchers in both academic and commercial labs using our Tru3D® technology platform. We also offer reagents that have been optimized to facilitate the wider use and adoption of CLARITY tissue clearing in research labs. We are also developing software to analyze large 3D datasets that are generated by confocal and light sheet microscopes. We may try to offer additional types of products and services, although we cannot offer any assurance that any of the current of future service and product offerings will be successful. From time to time, we may also modify aspects of our business model relating to our product and service offering mix. We cannot offer any assurance that these or any other modifications will be successful or will not result in harm to the business. Among the risks associated with the introduction of new products and services are related research and development costs, costs associated with launching new products and services, difficulty predicting customer demand and effectively managing inventory levels to ensure adequate supply of a new product and avoid excess supply of a legacy product. We may not be able to manage growth effectively, which could damage our reputation, limit our growth, and negatively affect our operating results.
We may strategically enter into non-cancelable commitments with vendors to purchase materials for our products and services in advance of demand to take advantage of favorable pricing or address concerns about the availability of future supplies or long lead times. This practice may expose us to an increased risk of excess or obsolete inventory and resulting charges if actual demand is lower than anticipated. Our failure to effectively manage product transitions or accurately forecast customer demand may lead to an increased risk of excess or obsolete inventory and resulting charges.
We may use our financial and human resources to pursue a particular product candidate or service offering and fail to capitalize on product or service candidates that may be more profitable or for which there is a greater likelihood of success.
Allocation decisions may cause us to fail to capitalize on viable commercial products and services or other profitable market opportunities. Our spending on existing and future product and service candidates for specific indications may not yield any commercially viable products and services. If we do not accurately evaluate the commercial potential or target market for a particular product or service candidate, we may relinquish valuable rights to that product or service candidate through strategic alliance, licensing or other royalty arrangements in cases in which it would have been more advantageous for us to retain sole development and commercialization rights to such product or service candidate, or we may allocate internal resources to a product or service candidate in which it would have been more advantageous to enter into a partnering arrangement.
There may be implications of being an Emerging Growth company.
As an issuer with less than $1 billion total gross revenues for 2020 and all preceding years, and in fact only $32,000 in total gross revenues for 2020, we will qualify as an "emerging growth company" under the Jumpstart Our Business Startups Act of 2012 (the "JOBS Act"). An emerging growth company may take advantage of certain reduced reporting requirements and is relieved of certain other significant requirements that are otherwise generally applicable to public companies. In particular, as an emerging growth company, even if and when we become registered with the SEC, we:
| ● | Would not be required to obtain audited financial statements; |
| ● | Would not be required to obtain an auditor attestation on our internal control over financial reporting pursuant to the Sarbanes-Oxley Act of 2002; |
| ● | Would not be required to provide a detailed narrative disclosure discussing our compensation principles, objectives and elements and analyzing how those elements fit with our principles and objectives (commonly referred to as "compensation discussion and analysis"); |
| ● | Would not be required to obtain a non-binding advisory vote from our stockholders on executive compensation or golden parachute arrangements (commonly referred to as the "say-on-pay," "say-on-frequency" and "say-on-golden-parachute" votes); |
| ● | Would be exempt from certain executive compensation disclosure provisions requiring a pay-for-performance graph and CEO pay ratio disclosure; |
| ● | Can present only two years of unaudited financial statements and only two years of related Management's Discussion and Analysis of Financial Condition and Results of Operations; |
| ● | Would be eligible to claim longer phase-in periods for the adoption of new or revised financial accounting standards under §107 of the JOBS Act. |
We intend to take advantage of all of these reduced reporting requirements and exemptions, including the longer phase-in periods for the adoption of new or revised financial accounting standards under §107 of the JOBS Act. Our election to use the phase-in periods may make it difficult to compare our financial statements to those of non-emerging growth companies and other emerging growth companies that have opted out of the phase-in periods under §107 of the JOBS Act.
Under the JOBS Act, we may take advantage of the above-described reduced reporting requirements and exemptions for up to five years after our initial sale of common equity pursuant to a registration statement declared effective under the Securities Act, or such earlier time that we no longer meet the definition of an emerging growth Company. Note that this offering, while a public offering, is not a sale of common equity pursuant to a registration statement since the offering is conducted pursuant to an exemption from the registration requirements. In this regard, the JOBS Act provides that we would cease to be an "emerging growth Company" if we have more than $1 billion in annual revenues, have more than $700 million in market value of our Common Stock held by non-affiliates, or issue more than $1 billion in principal amount of non-convertible debt over a three-year period.
Our success depends on the success of CLARITY and Tru3D technology platform and market acceptance. CLARITY or Tru3D may or may not achieve or maintain significant commercial market acceptance.
Our commercial success is dependent upon our ability to continue to successfully market and sell our Tru3D technology platform, of which CLARITY is a significant part. Our ability to achieve and maintain commercial market acceptance of our Tru3D technology platform will depend on a number of factors, including:
| ● | our ability to increase awareness of the capabilities of our technology and solutions; |
| ● | our customers' willingness to adopt new technologies and workflows; |
| ● | whether our platform reliably provides advantages over legacy and other alternative technologies and is perceived by customers to be cost effective; |
| ● | our ability to execute on our strategy to provide multiple channels to access our Tru3D technology platform; |
| ● | the rate of adoption of our platform and solutions by biopharmaceutical companies, academic institutions, and others; |
| ● | prices we charge for a direct purchase of, or other access to, our platform; |
| ● | the relative reliability and robustness of our platform as a whole and the components of our platform, including, for example, CLARITY, immunostaining, imaging, and software (under development); |
| ● | our ability to develop new workflows and solutions for customers; |
| ● | competitors that have a platform that provides services or technology that competes with ours; |
| ● | the timing and scope of any approval that may be required by the U.S. Food and Drug Administration, or FDA, for our next generation products and services, and/or solutions; |
| ● | the impact of our investments in product innovation and commercial growth; |
| ● | negative publicity regarding our or our competitors' and services resulting from defects or errors; and |
| ● | our ability to further validate our technology through research and accompanying publications. |
We cannot assure you that we will be successful in addressing each of these criteria or other criteria that might affect the market acceptance of our products and services. If we are unsuccessful in achieving and maintaining market acceptance of our products and services, our business, financial condition, results of operations and prospects could be adversely affected.
The life sciences technology market is highly competitive, and if we cannot compete successfully in that market, we may be unable to increase or sustain our revenue, or we may be unable to achieve and sustain profitability.
We face significant competition in the life sciences technology market. We currently compete with both established and early-stage life sciences technology companies that design, manufacture and market systems, consumables, reagent kits and software for, among other applications, genomics, single-cell analysis, spatial analysis, and immunology, and/or provide services related to the same. Growing understanding of the importance of single-cell information is leading to more companies offering services related to collecting such information. There are many large and small competitors within our space. In addition, our customers may also elect to develop their workflows on legacy systems rather than new platforms such as ours.
Our competitors and potential competitors may enjoy a number of competitive advantages over us, including:
| ● | longer operating histories; |
| ● | larger customer bases; |
| ● | greater brand recognition and market penetration; |
| ● | greater financial resources; |
| ● | greater technological and research and development resources; |
| ● | better system reliability and robustness; |
| ● | greater selling and marketing capabilities; and |
| ● | better established, larger scale and lower cost manufacturing capabilities. |
As a result, our competitors and potential competitors may be able to respond more quickly to changes in customer requirements, devote greater resources to the development, promotion and sale of their platforms or products than we can or sell their platforms or products, or offer services competitive with our platform and services at prices designed to win significant levels of market share. We may not be able to compete effectively against these organizations.
In addition, competitors may be acquired by, receive investments from, or enter into other commercial relationships with larger, more well-established, and well-financed companies. Certain of our competitors may be able to secure key inputs from vendors on more favorable terms, devote greater resources to marketing and promotional campaigns, adopt more aggressive pricing policies and devote substantially more resources to product development than we can. If we are unable to compete successfully against current and future competitors, we may be unable to increase market adoption and sales of our platform, which could prevent us from increasing our revenue or achieving profitability.
We are currently limited to "research use only" with respect to many of the materials and components used in our services.
Our instruments, consumable products and assays are purchased from suppliers with a restriction that they be used for research use only, or "RUO." While we have focused initially on the life sciences research market and RUO products and services only, part of our business strategy is to expand our service offerings to encompass products and services that are intended to be used for the diagnosis of disease and precision healthcare, either alone or in collaboration with third parties. The use of our products or services for any such diagnostic purposes would require that we obtain regulatory clearance or approval to market our products or services for those purposes and also that we acquire the materials and components used in such products and services from suppliers without an RUO restriction. There can be no assurance that we will be able to acquire these materials and components for use in diagnostic products on acceptable terms, if at all. If we are unable to do so, we would not be able to expand our product offerings beyond RUO, and our business and prospects would suffer.
We may never develop our software.
Our Tru3D technology platform involves the production of large 3D image datasets of customer tissue specimens. We believe this may be useful to researchers trying to understand the biology within the tissue since we believe that researchers increasingly seek quantitative and spatial data that cannot be obtained through traditional 2D fixed formalin paraffin-embedded ("FFPE") analysis [which produces a much lower volume of datasets?]. We are currently developing software that can analyze these large 3D datasets and provide quantitative and spatial data. The software remains under development and there is no assurance that we can develop such software successfully. If we cannot develop the software, we may not generate sufficient revenues to continue our business operations or achieve profitability.
RISKS RELATED TO OUR FINANCIAL CONDITION, LACK OF REVENUES AND OUR CONTINUING NEED FOR CAPITAL
There is substantial doubt about the Company's ability to continue as a going concern.
The Company has been unprofitable since its inception. The financial statements have been prepared assuming that the Company will continue as a going concern. The Company's ability to continue as a going concern is dependent on our ability to raise capital to fund operating losses, which may continue over the next several years. The financial statements provided herein do not include any adjustments to reflect the possible future effects on the recoverability and classification of assets, or the amounts and classification of liabilities that may result if we do not continue as a going concern. Therefore, you should not rely on our balance sheet as an indication of the amount of proceeds that would be available to satisfy claims of creditors, and potentially be available for distribution to stockholders, in the event of liquidation.
We will be required to raise additional funds to finance our operations and remain a going concern; however, we may not be able to do so when necessary, and/or the terms of any financings may not be advantageous to us.
Our operations to date have consumed substantially all of the funds we have been able to raise. Negative cash flows from our operations are expected to continue over at least the next several years. Our cash utilization amount is highly dependent on the success of our service offering and future product and service offerings. Our current unrestricted cash and cash equivalents will not be sufficient to fund future losses or the development of our products and services utilizing our technologies.
Our financial summary expresses substantial doubt about our ability to continue as a going concern, which may hinder our ability to obtain future financing.
Our financial summary will be prepared assuming that we would continue as a going concern. Our ability to continue as a going concern is an issue raised as the Company has limited operations and no established source of significant revenue. Our ability to continue as a going concern is subject to our ability to obtain necessary funding from outside sources, including obtaining additional funding from the sale of our securities, obtaining loans and grants from various institutions where possible. Our continued net operating losses increase the difficulty in meeting such goals and there can be no assurances that such methods will prove successful. We are in the process of applying for Small Business Innovation Research grants with the National Institute of Health and the National Science Foundation. Such grants are very difficult to obtain and there can be no assurance that any application will be approved.
Significant revenues from service sales are not in the foreseeable future, if at all.
To obtain significant revenues from sales of our services, we must succeed, either alone or with third parties, in promoting our current service offering and developing our software. The pathology market is very slow to adopt new technology. We may never succeed in these activities, and we may not generate sufficient revenues to continue our business operations or achieve profitability.
We may need to implement additional finance and accounting systems, procedures, and controls as we grow our business and organization and to satisfy reporting requirements.
We are required to comply with a variety of reporting, accounting, and other rules and regulations and those rules and requirements will become more extensive if we become a public Company. Compliance with existing requirements is expensive. Further requirements may increase our costs and require additional management time and resources. We may need to implement additional finance and accounting systems, procedures, and controls to satisfy our reporting requirements. If our internal controls over financial reporting are determined to be ineffective, such failure could cause investors to lose confidence in our reported financial information, negatively affect the market price of our Common Stock, subject us to regulatory investigations and penalties, and adversely impact our business and financial condition.
We have not conducted an evaluation of the effectiveness of our internal control over financial reporting and will not be required to do so until well after we are a public company. If we are unable to implement and maintain effective internal control over financial reporting investors may lose confidence in the accuracy and completeness of our financial reports and the market price of our Common Stock may be negatively affected.
In the future, we hope to list as a public company or apply to trade it in the over-the-counter market. At that time various accounting rules applicable to public companies may apply to us. We can make no assurances that we have the procedures in place to make sure we meet those requirements, and compliance will be a burden. If and when required, an independent registered public accounting firm is unable to express an opinion as to the effectiveness of our internal control over financial reporting, at that time, investors may lose confidence in the accuracy and completeness of our financial reports and the market price of our Common Stock could be negatively affected, and we could become subject to investigations by the stock exchange on which our securities are listed, the SEC, or other regulatory authorities, which could require additional financial and management resources.
RISKS RELATED TO OWNERSHIP OF OUR COMMON STOCK
We have not paid cash dividends on Common Stock in the past and do not expect to pay dividends in the future. Any return on investment or return of investment may be limited to the value of our Common Stock, if any.
We have never paid cash dividends on our Common Stock and do not anticipate doing so in the foreseeable future. The payment of dividends on our Common Stock will depend on earnings, financial condition and other business and economic factors affecting us at such time as our board of directors may consider relevant. If we do not pay dividends, our Common Stock may be less valuable because a return on your investment will only occur if our stock price appreciates.
There is currently no trading market for our Common Stock, and we cannot ensure that one will ever develop or be sustained.
There is no current market for any of our shares of stock and a market may not develop. We hope to eventually apply to trade our Common Stock on the over-the-counter market to the extent any demand exists. Even if quoted on the OTCQB, OTCQX or the Pink Sheets, a liquid trading market may not develop. Investors should assume that they may not be able to liquidate their investment for some time, or they may not be able to pledge their shares as collateral. Even if traded, we may be subject to the penny stock rules and may not be able to have our stock accepted by many brokerage firms as a result. The Company intends to apply to trade its shares on the Pink Sheets following the Offering, but there is no assurance that it will accomplish this goal.
You will likely experience future dilution as a result of future equity offerings.
We may in the future offer additional shares of our Common Stock or other securities convertible into or exchangeable for our Common Stock. Although no assurances can be given that we will consummate a financing, in the event we do, or in the event we sell shares of Common Stock or other securities convertible into shares of our Common Stock in the future, additional and substantial dilution may occur. In addition, investors purchasing shares of Common Stock or other securities in the future could have rights superior to investors in the Offering. Subsequent offerings at a lower price (a "down round") could result in additional dilution.
Our stock price may experience significant volatility.
In recent years, stock markets in general, and the market for life sciences technology companies in particular (including companies in the genomics, biotechnology, diagnostics, and related sectors), have experienced significant price and volume fluctuations that have often been unrelated or disproportionate to changes in the operating performance of the companies whose stock is experiencing those price and volume fluctuations. Broad market and industry factors may seriously affect the market price of our common stock, regardless of our actual operating performance. These fluctuations may be even more pronounced in the trading markets for our stock shortly following this offering. Following periods of such volatility in the market price of a Company's securities, securities class action litigation has often been brought against that Company. Because of the potential volatility of our Common Stock price, we may become the target of securities litigation in the future. Securities litigation could result in substantial costs and divert management's attention and resources from our business.
Raising additional capital may cause dilution to our existing stockholders or restrict our operations.
We anticipate that we will seek additional capital through a combination of public and private equity offerings, debt financings, strategic partnerships and alliances and licensing arrangements in the future to fund our operations. We, and indirectly, our stockholders, will bear the cost of issuing and servicing such securities. Because our decision to issue debt or equity securities in any future offering will depend on market conditions and other factors beyond our control, we cannot predict or estimate the amount, timing, or nature of any future offerings. Our decision to issue debt or equity securities will also depend on contractual, legal, and other restrictions that may limit our ability to raise additional capital. To the extent that we raise additional capital through the sale of equity or debt securities, your ownership interest will be diluted, and the terms may include liquidation or other preferences that adversely affect your rights as a stockholder. The incurrence of indebtedness in the future could result in fixed payment obligations and could involve restrictive covenants, such as limitations on our ability to incur additional debt, limitations on our ability to acquire, sell or license intellectual property rights and other operating restrictions that could adversely impact our ability to conduct our business. Certain of the foregoing transactions may require us to obtain stockholder approval, which we may not be able to obtain.
If securities or industry analysts do not publish research or reports about our business or publish negative reports about our business, our share price and trading volume could decline.
The trading market for our Common Stock may depend in the future on the research and reports that securities or industry analysts publish about us or our business. We do not and will not have any control over these analysts. If one or more of the analysts who may cover us downgrade our shares or change their opinion of our Common Stock, our share price would likely decline. If one or more of these analysts cease coverage of our Company or fail to regularly publish reports on us, we could lose visibility in the financial markets, which could cause our Common Stock price or trading volume to decline.
RISKS RELATED TO MANUFACTURING AND SUPPLY
Our technology requires specific chemicals and other materials and use of specialized laboratory equipment and the inability to obtain those materials and to gain access to such equipment may have a material adverse effect on our ability to develop Tru3D technology platform.
Reagents, chemicals and biological materials needed for development of our Tru3D technology platform tool may be difficult to procure, requiring in-house production or preventing development of our platform and our ability to offer our services to our customers. Inability to obtain these materials and may slow down or negatively impact the development of our Tru3D technology platform, which will limit or prevent our ability to develop a useful technology for 3-D tissue analysis.
RISKS RELATED TO INTELLECTUAL PROPERTY RIGHTS AND LITIGATION
If we fail to successfully protect our intellectual property, our competitive position and operating results could suffer.
We rely on our Tru3D technology platform (including the CLARITY technology licensed from Stanford University), as well as the technical expertise, creativity, and knowledge of our personnel to maintain our position as a leading technology provider of 3D tissue analysis technology to facilitate pre-clinical and clinical research applications. Technology piracy and reverse engineering, specifically from companies in Russia and Asia, may result in counterfeit products and services that are misrepresented in the market as Tru3D products and services. To protect our intellectual property, we keep product development in-house, we limit the amount of reagents provided to clients, partners, and collaborators, and we include other preventative measures against technology piracy. We also rely on patent, trademark, copyright, and trade secret protection, as well as non-disclosure agreements with customers, suppliers, employees, and consultants as well as restricting access to our proprietary information by a combination of technical and internal security measures. These measures, however, may not be adequate to:
| ● | Protect our proprietary technology, |
| ● | Protect our patents from challenge, invalidation, or circumvention, or |
| ● | Ensure that our intellectual property will provide us with competitive advantages. |
From time to time, we may be subject to various claims and lawsuits by competitors, customers, or other parties arising in the ordinary course of business, including lawsuits charging patent infringement, or claims and lawsuits instituted by us to protect our intellectual property or for other reasons. These matters can be time-consuming, divert management's attention and resources, and cause us to incur significant expenses, regardless of the merit of a particular lawsuit or the eventual outcome. Furthermore, the results of any of these actions may have a material adverse effect on our operating results.
If we fail to successfully maintain our licensed technology, the license for our technology relating to CLARITY could be terminated by Stanford University. As a result, our competitive position and operating results could suffer.
Any of the following adverse circumstances could have a material adverse effect on our operating results:
Stanford University could terminate our license for CLARITY:
(1) if the Company is delinquent on any report or payment;
(2) if the Company is not diligently developing and commercializing the licensed technology;
(3) if the Company misses a milestone;
(4) if the Company is in breach of any provision of the License Agreement; or
(5) if the Company provides any false report
The Company may be subject to milestones which we may not be able to meet under the Stanford University License Agreement.
If we are unable to obtain and maintain sufficient intellectual property protection for our products and product candidates that we may identify, or if the scope of the intellectual property protection obtained is not sufficiently broad, our competitors could develop and commercialize products similar or identical to ours, and our ability to successfully commercialize our products and product candidates that we may pursue may be impaired.
As is the case with other companies in the biopharmaceutical sector, our success depends in large part on our ability to obtain and maintain protection of the intellectual property we may own or license from others, particularly patents, in the United States and other countries with respect to our product candidates and technology.
Obtaining and enforcing patents is costly, time consuming and complex, and we may not be able to file and prosecute all necessary or desirable patent applications, or maintain, enforce, and license any patents that may issue from such patent applications, at a reasonable cost or in a timely manner. It is also possible that we will fail to identify patentable aspects of our research and development output before it is too late to obtain patent protection. We may not have the right to control the preparation, filing and prosecution of patent applications, or to maintain the rights to patents licensed from third parties.
The patent position of companies in the biotechnology and pharmaceutical sector generally is highly uncertain, involves complex legal, technological, and factual questions and has in recent years been the subject of much litigation. In addition, the laws of foreign countries may not protect our rights to the same extent as the laws of the United States, or vice versa. Further, we may not be aware of all third-party intellectual property rights potentially relating to our product candidates. We cannot know with certainty whether we were the first to make the inventions claimed in our patents or pending patent applications, or that we were the first to file for patent protection of such inventions. As a result, the issuance, scope, validity, enforceability and commercial value of our patent rights are highly uncertain. Our pending and future patent applications may not result in patents being issued that protect our products and product candidates, in whole or in part, or which effectively prevent others from commercializing competitive products. Even if our patent applications issue as patents, they may not issue in a form that will provide us with any meaningful protection, prevent competitors from competing with us or otherwise provide us with any competitive advantage. Our competitors may be able to circumvent our patents by developing similar or alternative product candidates in a non-infringing manner.
Moreover, we may be subject to a third-party pre-issuance submission of prior art to the United States Patent and Trademark Office, or the USPTO, or become involved in opposition, derivation, reexamination, inter partes review, post-grant review or interference proceedings challenging our patent rights or the patent rights of others. An adverse determination in any such submission, proceeding or litigation could reduce the scope of, or invalidate, our patent rights, allow third parties to commercialize our product candidates and compete directly with us, without payment to us, or result in our inability to manufacture or commercialize drugs without infringing third-party patent rights. In addition, if the breadth or strength of protection provided by our patents and patent applications is threatened, regardless of the outcome, it could dissuade companies from collaborating with us to license, develop or commercialize current or future product candidates.
In addition, the issuance of a patent is not conclusive as to its inventorship, scope, validity or enforceability, and our patents may be challenged in the courts or patent offices in the United States and abroad. Such challenges may result in loss of exclusivity or freedom to operate or in patent claims being narrowed, invalidated, or held unenforceable, in whole or in part, which could limit our ability to stop others from using or commercializing similar or identical product candidates, or limit the duration of the patent protection of our product candidates. Given the amount of time required for the development, testing and regulatory review of new product candidates, patents protecting such candidates might expire before or shortly after such candidates are commercialized. As a result, our patent portfolio may not provide us with sufficient rights to exclude others from commercializing drugs similar or identical to ours.
If we fail to comply with our obligations in the agreements under which we license intellectual property rights from third parties or otherwise experience disruptions to our business relationships with our licensors, we could lose intellectual property rights that are important to our business.
We are a party to an exclusive license agreement with Stanford and may need to obtain additional licenses from others to advance our research and development activities or allow the commercialization of our products and product candidates. Our license agreement with Stanford imposes, and we expect that future license agreements will impose, various development, diligence, commercialization, and other obligations on us. For example, under our license agreement with Stanford we are required to use commercially reasonable efforts to engage in various development and commercialization activities with respect to licensed products, and to satisfy specified milestone and royalty payment obligations. In spite of our efforts, our licensors might conclude that we have materially breached our obligations under such license agreements and might therefore terminate the license agreements, thereby removing or limiting our ability to develop and commercialize products and technology covered by these license agreements. If these licenses are terminated, or if the underlying patents fail to provide the intended exclusivity, competitors or other third parties would have the freedom to market products identical to ours, and we may be required to cease development and commercialization of our products and product candidates. Any of the foregoing could have a material adverse effect on our competitive position, business, financial conditions, results of operations, and prospects.
Moreover, disputes may arise regarding intellectual property subject to a licensing agreement, including:
● | the scope of rights granted under the license agreement and other interpretation-related issues; |
● | the extent to which our product candidates, technology and processes infringe on intellectual property of the licensor that is not subject to the licensing agreement; |
● | the sublicensing of patent and other rights under our collaborative development relationships; |
● | our diligence obligations under the license agreement and what activities satisfy those diligence obligations; |
● | the inventorship and ownership of inventions and know-how resulting from the joint creation or use of intellectual property by our licensors and us and our partners; and |
● | the priority of invention of patented technology. |
In addition, the agreements under which we currently license intellectual property or technology from third parties are complex, and certain provisions in such agreements may be susceptible to multiple interpretations. The resolution of any contract interpretation disagreement that may arise could narrow what we believe to be the scope of our rights to the relevant intellectual property or technology, or increase what we believe to be our financial or other obligations under the relevant agreement, either of which could have a material adverse effect on our business, financial condition, results of operations, and prospects. Moreover, if disputes over intellectual property that we have licensed prevent or impair our ability to maintain our current licensing arrangements on commercially acceptable terms, we may be unable to successfully develop and commercialize the affected product candidates, which could have a material adverse effect on our business, financial conditions, results of operations, and prospects.
Third-party claims of intellectual property infringement may prevent or delay our development and commercialization efforts.
Our commercial success depends in part on our avoiding infringement of the patents and proprietary rights of third parties. However, our research, development and commercialization activities may be subject to claims that we infringe or otherwise violate patents or other intellectual property rights owned or controlled by third parties. There is a substantial amount of litigation, both within and outside the United States, involving patent and other intellectual property rights in the biotechnology industry, including patent infringement lawsuits, interferences, oppositions and inter partes reexamination proceedings before the USPTO, and corresponding foreign patent offices. Numerous U.S. and foreign issued patents and pending patent applications, which are owned by third parties, exist in the fields in which we are pursuing development candidates, and we may be subject to claims of infringement of the patent rights of third parties.
Third parties may assert that we are employing their proprietary technology without authorization. There may be third-party patents or patent applications with claims to materials, methods, or methods of manufacture related to the use or manufacture of our products and product candidates. Because patent applications can take many years to issue, there may be currently pending patent applications which may later result in issued patents that our products and product candidates may infringe. In addition, third parties may obtain patents in the future and claim that use of our technologies infringes upon these patents. If any third-party patents were held by a court of competent jurisdiction to cover our products, their use, or their manufacture, the holders of any such patents may be able to block our ability to commercialize such product candidate unless we obtained a license under the applicable patents, or until such patents expire. Such a license may not be available on commercially reasonable terms or at all, or it may be non-exclusive, which could result in our competitors gaining access to the same intellectual property.
Parties making claims against us may obtain injunctive or other equitable relief, which could effectively block our ability to further develop and commercialize our products and product candidates. Defense of these claims, regardless of their merit, would involve substantial litigation expense and would be a substantial diversion of employee resources from our business. In the event of a successful claim of infringement against us, we may have to pay substantial damages, including treble damages and attorneys' fees for willful infringement, pay royalties, redesign our infringing products or obtain one or more licenses from third parties, which may be impossible or require substantial time and monetary expenditure.
Parties making claims against us may be able to sustain the costs of complex patent litigation more effectively than we can because they have substantially greater resources. Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation or administrative proceedings, there is a risk that some of our confidential information could be compromised by disclosure. In addition, any uncertainties resulting from the initiation and continuation of any litigation could have material adverse effect on our ability to raise additional funds or otherwise have a material adverse effect on our business, results of operations, financial condition, and prospects.
Patent terms may be inadequate to protect our competitive position on our product candidates for an adequate amount of time.
Patents have a limited lifespan. In the United States, if all maintenance fees are timely paid, the natural expiration of a patent is generally 20 years from its earliest U.S. non-provisional filing date. Various extensions may be available, but the life of a patent, and the protection it affords, is limited. Even if patents covering our products and product candidates are obtained, once the patent life has expired, we may be open to competition from competitive products. As a result, our patent portfolio may not provide us with sufficient rights to exclude others from commercializing products similar or identical to ours.
If we are unable to protect the confidentiality of our trade secrets, the value of our technology could be materially adversely affected, and our business would be harmed.
We seek to protect our confidential proprietary information, in part, by confidentiality agreements and invention assignment agreements with our employees, consultants, scientific advisors, contractors and collaborators. These agreements are designed to protect our proprietary information. However, we cannot be certain that such agreements have been entered into with all relevant parties, and we cannot be certain that our trade secrets and other confidential proprietary information will not be disclosed or that competitors will not otherwise gain access to our trade secrets or independently develop substantially equivalent information and techniques. For example, any of these parties may breach the agreements and disclose our proprietary information, including our trade secrets, and we may not be able to obtain adequate remedies for such breaches. We also seek to preserve the integrity and confidentiality of our confidential proprietary information by maintaining physical security of our premises and physical and electronic security of our information technology systems, but it is possible that these security measures could be breached. If any of our confidential proprietary information were to be lawfully obtained or independently developed by a competitor, we would have no right to prevent such competitor from using that technology or information to compete with us, which could harm our competitive position.
Although we are not currently involved in any litigation, we may become involved in lawsuits to protect or enforce our patents or other intellectual property, which could be expensive, time consuming and unsuccessful.
Competitors may infringe our patents or other intellectual property. If we or one of our licensing partners initiated legal proceedings against a third party to enforce a patent covering one of our products or product candidates, the defendant could counterclaim that the patent covering our product or product candidate is invalid and/or unenforceable. In patent litigation in the United States, defendant counterclaims alleging invalidity and/or unenforceability are commonplace. Grounds for a validity challenge could be an alleged failure to meet any of several statutory requirements, including lack of novelty, obviousness, or non-enablement. Grounds for an unenforceability assertion could be an allegation that someone connected with prosecution of the patent withheld relevant information from the USPTO, or made a misleading statement, during prosecution. Third parties may also raise similar claims before administrative bodies in the United States or abroad, even outside the context of litigation. Such mechanisms include re-examination, post grant review, and equivalent proceedings in foreign jurisdictions (e.g., opposition proceedings). Such proceedings could result in revocation or amendment to our patents in such a way that they no longer cover our product candidates. The outcome following legal assertions of invalidity and unenforceability is unpredictable. With respect to the validity question, for example, we cannot be certain that there is no invalidating prior art, of which we and the patent examiner were unaware during prosecution. If a defendant were to prevail on a legal assertion of invalidity and/or unenforceability, we would lose at least part, and perhaps all, of the patent protection on our product candidates. Such a loss of patent protection would have a material adverse impact on our business.
Interference or derivation proceedings provoked by third parties or brought by us or declared by the USPTO may be necessary to determine the priority of inventions with respect to our patents or patent applications. An unfavorable outcome could require us to cease using the related technology or to attempt to license rights to it from the prevailing party. Our business could be harmed if the prevailing party does not offer us a license on commercially reasonable terms or at all, or if a non-exclusive license is offered and our competitors gain access to the same technology. Our defense of litigation or interference or derivation proceedings may fail and, even if successful, may result in substantial costs and distract our management and other employees. In addition, the uncertainties associated with litigation could have a material adverse effect on our ability to raise the funds necessary to continue our clinical trials, continue our research programs, license necessary technology from third parties, or enter into development partnerships that would help us bring our product candidates to market.
Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation. There could also be public announcements of the results of hearings, motions, or other interim proceedings or developments. If securities analysts or investors perceive these results to be negative, it could have a material adverse effect on the price of our common stock.
We may be subject to claims challenging the inventorship of our patents and other intellectual property.
We or our licensors may be subject to claims that former employees, collaborators or other third parties have an interest in our owned or in-licensed patents, trade secrets, or other intellectual property as an inventor or co-inventor. For example, we or our licensors may have inventorship disputes arise from conflicting obligations of employees, consultants or others who are involved in developing our product candidates. Litigation may be necessary to defend against these and other claims challenging inventorship or our or our licensors' ownership of our owned or in-licensed patents, trade secrets or other intellectual property. If we or our licensors fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights, such as exclusive ownership of, or right to use, intellectual property that is important to our product candidates. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management and other employees. Any of the foregoing could have a material adverse effect on our business, financial condition, results of operations and prospects.
We may be subject to claims that our employees, consultants, or independent contractors have wrongfully used or disclosed confidential information of third parties or that our employees have wrongfully used or disclosed alleged trade secrets of their former employers.
As is common in the biotechnology industry, we employ individuals who were previously employed at universities or other biotechnology companies. Although we try to ensure that our employees, consultants, and independent contractors do not use the proprietary information or know-how of others in their work for us, we may be subject to claims that we or our employees, consultants or independent contractors have inadvertently or otherwise used or disclosed intellectual property, including trade secrets or other proprietary information, of any of our employee's former employer or other third parties. Litigation may be necessary to defend against these claims. If we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel, which could adversely impact our business. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management and other employees.
Obtaining and maintaining our patent protection depends on compliance with various procedural, document submission, fee payment and other requirements imposed by governmental patent agencies, and our patent protection could be reduced or eliminated for non-compliance with these requirements.
Periodic maintenance fees, renewal fees, annuity fees and various other governmental fees on patents and/or applications will be due to be paid to the USPTO and various governmental patent agencies outside of the United States in several stages over the lifetime of the patents and/or applications. We have systems in place to remind us to pay these fees, and we employ an outside firm and rely on our licensor to pay these fees due to non-U.S. patent agencies. The USPTO and various non-U.S. governmental patent agencies require compliance with a number of procedural, documentary, fee payment and other similar provisions during the patent application process. We employ reputable law firms and other professionals to help us comply, and in many cases, an inadvertent lapse can be cured by payment of a late fee or by other means in accordance with the applicable rules. However, there are situations in which non-compliance can result in abandonment or lapse of the patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. In such an event, our competitors might be able to enter the market and this circumstance would have a material adverse effect on our business.
We may not be able to protect our intellectual property rights throughout the world.
Filing, prosecuting, and defending patents on our product candidates in all countries throughout the world would be prohibitively expensive, and our intellectual property rights in some countries outside the United States can be less extensive than those in the United States or non-existent. In addition, the laws of some foreign countries do not protect intellectual property rights to the same extent as federal and state laws in the United States. Consequently, we may not be able to prevent third parties from practicing our inventions in all countries outside the United States, or from selling or importing products made using our inventions in and into the United States or other jurisdictions. Competitors may use our technologies in jurisdictions where we have not obtained patent protection to develop their own products and may also export infringing products to territories where we have patent protection, but enforcement is not as strong as that in the United States. These products may compete with our products, and our patents or other intellectual property rights may not be effective or sufficient to prevent them from competing.
Changes in U.S. patent law could diminish the value of patents in general, thereby impairing our ability to protect our products.
Changes in either the patent laws or interpretation of the patent laws in the United States could increase the uncertainties and costs surrounding the prosecution of patent applications and the enforcement or defense of issued patents. Assuming that other requirements for patentability are met, prior to March 2013, in the United States, the first to invent the claimed invention was entitled to the patent, while outside the United States, the first to file a patent application was entitled to the patent. After March 2013, under the Leahy-Smith America Invents Act, or the America Invents Act, enacted in September 2011, the United States transitioned to a first inventor to file system in which, assuming that other requirements for patentability are met, the first inventor to file a patent application will be entitled to the patent on an invention regardless of whether a third party was the first to invent the claimed invention. A third party that files a patent application in the USPTO after March 2013, but before us could therefore be awarded a patent covering an invention of ours even if we had made the invention before it was made by such third party. This will require us to be cognizant of the time from invention to filing of a patent application. Since patent applications in the United States and most other countries are confidential for a period of time after filing or until issuance, we cannot be certain that we or our licensors were the first to either (i) file any patent application related to our product candidates or (ii) invent any of the inventions claimed in our or our licensor's patents or patent applications.
The America Invents Act also includes a number of significant changes that affect the way patent applications will be prosecuted and also may affect patent litigation. These include allowing third party submission of prior art to the USPTO during patent prosecution and additional procedures to attack the validity of a patent by USPTO administered post-grant proceedings, including post-grant review, inter partes review, and derivation proceedings. Because of a lower evidentiary standard in USPTO proceedings compared to the evidentiary standard in United States federal courts necessary to invalidate a patent claim, a third party could potentially provide evidence in a USPTO proceeding sufficient for the USPTO to hold a claim invalid even though the same evidence would be insufficient to invalidate the claim if first presented in a district court action. Accordingly, a third party may attempt to use the USPTO procedures to invalidate our patent claims that would not have been invalidated if first challenged by the third party as a defendant in a district court action. Therefore, the America Invents Act and its implementation could increase the uncertainties and costs surrounding the prosecution of our owned or in-licensed patent applications and the enforcement or defense of our owned or in-licensed issued patents, all of which could have a material adverse effect on our business, financial condition, results of operations, and prospects.
In addition, the patent positions of companies in the development and commercialization of biotechnology products are particularly uncertain. Recent U.S. Supreme Court rulings have narrowed the scope of patent protection available in certain circumstances and weakened the rights of patent owners in certain situations. This combination of events has created uncertainty with respect to the validity and enforceability of patents, once obtained. Depending on future actions by the U.S. Congress, the federal courts, and the USPTO, the laws and regulations governing patents could change in unpredictable ways that could have a material adverse effect on our existing patent portfolio and our ability to protect and enforce our intellectual property in the future.
Some intellectual property that we have in-licensed may have been discovered through government funded programs and thus may be subject to federal regulations such as "march-in" rights, certain reporting requirements and a preference for U.S.-based companies. Compliance with such regulations may limit our exclusive rights, and limit our ability to contract with non-U.S. manufacturers.
Many of the intellectual property rights we have licensed are generated through the use of U.S. government funding and are therefore subject to certain federal regulations. As a result, the U.S. government may have certain rights to intellectual property embodied in our current or future product candidates pursuant to the Bayh-Dole Act of 1980, or Bayh-Dole Act, and implementing regulations. These U.S. government rights in certain inventions developed under a government-funded program include a non-exclusive, non-transferable, irrevocable worldwide license to use inventions for any governmental purpose. In addition, the U.S. government has the right to require us or our licensors to grant exclusive, partially exclusive, or non-exclusive licenses to any of these inventions to a third party if it determines that: (i) adequate steps have not been taken to commercialize the invention; (ii) government action is necessary to meet public health or safety needs; or (iii) government action is necessary to meet requirements for public use under federal regulations (also referred to as "march-in rights"). The U.S. government also has the right to take title to these inventions if we, or the applicable licensor, fail to disclose the invention to the government and fail to file an application to register the intellectual property within specified time limits. These time limits have recently been changed by regulation, and may change in the future. Intellectual property generated under a government funded program is also subject to certain reporting requirements, compliance with which may require us or the applicable licensor to expend substantial resources. In addition, the U.S. government requires that any products embodying the subject invention or produced through the use of the subject invention be manufactured substantially in the United States. The manufacturing preference requirement can be waived if the owner of the intellectual property can show that reasonable but unsuccessful efforts have been made to grant licenses on similar terms to potential licensees that would be likely to manufacture substantially in the United States or that under the circumstances domestic manufacture is not commercially feasible. This preference for U.S. manufacturers may limit our ability to contract with non-U.S. product manufacturers for products covered by such intellectual property. To the extent any of our current or future intellectual property is generated through the use of U.S. government funding, the provisions of the Bayh-Dole Act may similarly apply. We may be exposed to product liability claims and may not be able to obtain or maintain adequate product liability insurance.
RISKS RELATED TO GOVERNMENT REGULATION
If the FDA determines that our products are medical devices or if we seek to market our products or services for clinical, diagnostic or health screening use, we will be required to obtain regulatory clearance(s) or approval(s), and may be required to cease or limit sales of our then marketed products, which could materially and adversely affect our business, financial condition, and results of operations. Any such regulatory process would be expensive, time-consuming, and uncertain, both in timing and in outcome.
We have focused initially on the life sciences research market. This includes laboratories associated with academic and governmental research institutions, as well as pharmaceutical, biotechnology and contract research companies. Accordingly, our products are labeled as "Research Use Only," or RUO, and are not intended for diagnostic use. While we have focused initially on the life sciences research market and RUO products only, our strategy is to expand our product line to encompass products that are intended to be used for the diagnosis of disease, either alone or in collaboration with third parties. Such in-vitro diagnostic, or IVD, products will be subject to regulation by the FDA as medical devices, or comparable international agencies, including requirements for regulatory clearance or approval of such products before they can be marketed. If the FDA were to determine that our products are intended for clinical use or if we decided to market our products for such use, we would be required to obtain FDA 510(k) clearance or premarket approval in order to sell our products in a manner consistent with FDA laws and regulations. Such regulatory approval processes or clearances are expensive, time-consuming, and uncertain; our efforts may never result in any approved premarket approval application, or PMA, or 510(k) clearance for our products; and failure by us or a collaborator to obtain or comply with such approvals and clearances could have an adverse effect on our business, financial condition, or operating results.
IVD products may be regulated as medical devices by the FDA and comparable international agencies and may require either clearance from the FDA following the 510(k) pre-market notification process or PMA from the FDA, in each case prior to marketing. If we or our collaborators are required to obtain a PMA or 510(k) clearance for products based on our technology, we or they would be subject to a substantial number of additional requirements for medical devices, including establishment registration, device listing, Quality Systems Regulations which cover the design, testing, production, control, quality assurance, labeling, packaging, servicing, sterilization (if required), and storage and shipping of medical devices (among other activities), product labeling, advertising, recordkeeping, post-market surveillance, post-approval studies, adverse event reporting, and correction and removal (recall) regulations. One or more of the products we or a collaborator may develop using our technology may also require clinical trials in order to generate the data required for PMA approval. Complying with these requirements will be time-consuming and expensive. We or our collaborators would be required to expend significant resources to ensure ongoing compliance with the FDA regulations and/or take satisfactory corrective action in response to enforcement action, which may have a material adverse effect on the ability to design, develop, and commercialize products using our technology as planned. Failure to comply with these requirements may subject us or a collaborator to a range of enforcement actions, such as warning letters, injunctions, civil monetary penalties, criminal prosecution, recall and/or seizure of products, and revocation of marketing authorization, as well as significant adverse publicity. If we or our collaborators fail to obtain, or experience significant delays in obtaining, regulatory approvals for IVD products, such products may not be able to be launched or successfully commercialized in a timely manner, or at all.
Laboratory developed tests, or LDTs, are a subset of IVD tests that are designed, manufactured, and used within a single laboratory. The FDA maintains that LDTs are medical devices and has for the most part exercised enforcement discretion for most LDTs. A significant change in the way that the FDA regulates any LDTs that we, our collaborators, or our customers develop using our technology could affect our business. If the FDA requires laboratories to undergo premarket review and comply with other applicable FDA requirements in the future, the cost and time required to commercialize an LDT will increase substantially and may reduce the financial incentive for laboratories to develop LDTs, which could reduce demand for our instruments and our other products. In addition, if the FDA were to change the way that it regulates LDTs to require that we undergo pre-market review or comply with other applicable FDA requirements before we can sell our instruments or our other products to clinical laboratories, our ability to sell our instruments and other products to this addressable market would be delayed, thereby impeding our ability to penetrate this market and generate revenue from sales of our instruments and our other products.
Failure to comply with applicable FDA requirements could subject us to misbranding or adulteration allegations under the Federal Food, Drug, and Cosmetic Act. We could be subject to a range of enforcement actions, including warning letters, injunctions, civil monetary penalties, criminal prosecution, and recall and/or seizure of products, as well as significant adverse publicity. In addition, changes to the current regulatory framework, including the imposition of additional or new regulations, could arise at any time during the development or marketing of our products, which may negatively affect our ability to obtain or maintain FDA or comparable regulatory approval of our products, if required.
Foreign jurisdictions have laws and regulations similar to those described above, which may adversely affect our ability to market our products as planned in such countries. The number and scope of these requirements are increasing. As in the U.S., the cost and time required to comply with regulatory requirements may be substantial, and there is no guarantee that we will obtain the necessary authorization(s) required to make our products commercially viable. As a result, the imposition of foreign requirements may also have a material adverse effect on the commercial viability of our operations.
Our biotech tool candidates are unproven and may not be approved by the FDA. We are committing a majority of our resources to the development of biotech tools to disrupt the diagnostic, prognostic, and predictive treatment of disease through technology that will non-destructively and digitally analyze diseased and normal tissue in 3D. Currently, our focus is on the life sciences research use only (RUO) market, where FDA clearance or approval is not required. However, in the future, we may develop products or services for clinical use. If so, these products and services may require FDA clearance or approval. There can be no assurance that any product or service candidate will meet FDA's standards for commercial distribution. Further, there can be no assurance that other product candidates that may be developed will achieve the targeted end points in the required clinical studies or perform as intended in other pre-clinical and clinical studies or lead to a submission or filing acceptance. Our failure to successfully develop and achieve final FDA approval of our product candidates may then have a material adverse effect on our financial condition.
Our operations are subject to environmental, health and safety, and other laws and regulations, with which compliance is costly and which exposes us to penalties for non-compliance.
Our business, properties and product candidates are subject to federal, state and local laws and regulations relating to the protection of the environment, natural resources and worker health and safety and the use, management, storage and disposal of hazardous substances, waste and other regulated materials. Various environmental laws also may impose liability on us for the costs of cleaning up and responding to hazardous substances that may have been released on our property, including releases unknown to us. These environmental laws and regulations also could require us to pay for environmental remediation and response costs at third-party locations where we dispose of or recycle hazardous substances. The costs of complying with these various environmental requirements, as they now exist or may be altered in the future, could adversely affect our financial condition and results of operations.
Future legislation may increase the difficulty and cost for us, to obtain, or prevent us from obtaining, marketing approval of and commercialize our product and service candidates and affect the prices we may obtain.
In the United States and some foreign jurisdictions, there have been a number of legislative and regulatory changes and proposed changes regarding the healthcare system that could prevent or delay marketing approval for our product and service candidates, restrict or regulate post-approval activities and affect our ability to profitably sell our product and service candidates. Legislative and regulatory proposals have been made to expand post-approval requirements and restrict sales and promotional activities for pharmaceutical-related products and services. We do not know whether additional legislative changes will be enacted, or whether the FDA regulations, guidance or interpretations will be changed, or what the impact of such changes on the marketing approvals of our product candidates, if any, may be. In addition, increased scrutiny by the U.S. Congress of the FDA's approval process may significantly delay or prevent marketing approval, as well as subject us to more stringent product labeling and post-marketing testing and other requirements.
Public health crises such as COVID-19, and similar pandemics or outbreaks, have caused and could cause disruptions of the development of our platform technologies and products, and adversely impact our business, financial condition, and results of operations.
In March 2020, the World Health Organization declared the novel coronavirus disease, or COVID-19, a global pandemic. The COVID-19 pandemic continues to evolve, and to date has led to the implementation of various responses, including government-imposed shelter-in-place orders, quarantines, travel restrictions and other public health safety measures, as well as reported adverse impacts on healthcare resources, facilities and providers across the United States and in other countries, all of which may become additionally heightened concerns upon a subsequent wave of infection. In response to the spread ofCOVID-19, and in accordance with direction from state and local government authorities, our facilities have restricted access to personnel and third parties who must perform critical activities that must be completed on-site, have limited the number of such personnel that can be present at our facilities at any one time, and have requested that most of our personnel work remotely. In the event that government authorities were to further modify current restrictions, our employees conducting research and development or manufacturing activities may not be able to access our laboratory or manufacturing space, and our core activities may be significantly limited or curtailed, possibly for an extended period of time. The COVID-19 pandemic has also created many negative headwinds that present risks to our business and results of operations. For example, it has generally disrupted the operations of our customers and prospective customers, and may continue to disrupt their operations, as a result of laboratory closures, travel restrictions and/or business shutdowns, uncertainty in the financial markets or other harm to their business and financial results. These disruptions have caused reduced capital spending by our existing customers and potential new customers, which has negatively impacted our instrument and consumables sales. These disruptions could result in further reductions to capital expenditure budgets, delayed purchasing decisions, longer sales cycles, extended payment terms or missed payments, and postponed or canceled projects, any of which would negatively impact our business and operating results, including sales and cash flows. We do not yet know the net impact that the COVID-19 pandemic may have on our business and cannot guarantee that it will not be materially negative. Although we continue to monitor the situation and may adjust our current policies as more information and public health guidance become available, the ongoing effects of the COVID-19 pandemic and/or the precautionary measures that we have adopted may create operational and other challenges, any of which could harm our business and results of operations. The uncertain development of the COVID-19 pandemic may also exacerbate the severity of the other risks disclosed herein.
We are currently subject to, and may in the future become subject to additional, U.S. state and federal, and non-U.S. laws and regulations, industry guidelines, and contracts, imposing obligations on how we collect, store, use and process personal information. Our actual or perceived failure to comply with such obligations could harm our business. Ensuring compliance with such laws could also impair our efforts to maintain and expand our customer base, and thereby decrease our revenue.
We are, and may increasingly become, subject to various laws and regulations, as well as contractual obligations and mandatory industry standards relating to data privacy and security in the jurisdictions in which we operate. The regulatory environment related to data privacy and security is increasingly rigorous, with new and constantly changing requirements applicable to our business, and enforcement practices are likely to remain uncertain for the foreseeable future. These laws and regulations may be interpreted and applied differently over time and from jurisdiction to jurisdiction, and it is possible that they will be interpreted and applied in ways that may have a material adverse effect on our business, financial condition, results of operations and prospects.
We do not have, and may never obtain, the regulatory approvals we need to market certain of our product candidates.
Following completion of clinical trials or other required testing, the results are evaluated and, depending on the outcome, submitted to the FDA in order to obtain FDA approval or clearance of the product or service and authorization to commence commercial marketing. The FDA may require additional testing or information, may require that the product labeling be modified, may impose post-approval study or reporting requirements or other restrictions on product distribution, or may deny the application. The timing of final FDA review and action varies greatly, but it can take years. In some cases, it may involve the input of an FDA advisory committee of outside experts. Product sales in the United States may commence only when FDA approval or clearance is granted.
To date, we have not applied for or received regulatory approvals or clearances required for the commercial sale of any of our products in the United States or in any foreign jurisdiction.
It is possible that none of our product candidates will be approved for marketing. Failure to obtain regulatory approvals, or delays in obtaining regulatory approvals, may adversely affect the successful commercialization of any products or services that we or our partners develop, may impose additional costs on us or our collaborators, may diminish any competitive advantages that we or our partners may attain, and/or may adversely affect our receipt of revenues or royalties.
Our products are and will be subject to extensive post-approval regulation.
The holder of an FDA approval or clearance is subject to periodic and other FDA monitoring and reporting obligations, including obligations to monitor and report adverse events and instances of the failure of a product to meet requirements. Application holders may be required to submit new or supplemental applications and obtain FDA approval for certain changes to the approved product, product labeling, or manufacturing process. Application holders must also submit advertising and other promotional material to the FDA and report on ongoing clinical trials.
Depending on the circumstances, failure to meet these post-approval requirements can result in criminal prosecution, fines, injunctions, recall or seizure of products, total or partial suspension of production, denial or withdrawal of pre-marketing product approvals, or refusal to allow us to enter into supply contracts, including government contracts. In addition, even if we comply with FDA and other requirements, new information regarding the safety or effectiveness of a product could lead the FDA to modify or withdraw product approval.
Even if we obtain regulatory approval to market our product candidates, our product candidates may not be accepted by the market.
Even if the FDA approves one or more of our product candidates, physicians and patients may not accept it or use it. Even if physicians and patients would like to use our products and services, our products and services may not gain market acceptance among healthcare payors such as managed care formularies, insurance companies or government programs such as Medicare or Medicaid. Acceptance and use of our products and services will depend upon a number of factors including: perceptions by members of the health care community, including physicians, about the safety and effectiveness of our product; cost-effectiveness of our product relative to competing products and services; availability of reimbursement for our product from government or other healthcare payers; and effectiveness of marketing and distribution efforts by us and our licensees and distributors, if any.
We face the risk of product liability claims and may not be able to obtain insurance.
Our business exposes us to the risk of product liability claims that are inherent in the testing, manufacturing, marketing and sale of pharmaceutical-related products and services. If the use of one or more of our or our products or services harms people, we may be subject to costly and damaging product liability claims brought against us by clinical trial participants, consumers, health care providers, pharmaceutical companies or others. We are currently covered by general and property liability insurance. This coverage may not be adequate to cover any product liability claims and may not be adequate to cover other liabilities we might incur. Specific product liability coverage is expensive and we currently do not have the resources to purchase it. Additionally, in the future, we may not be able to maintain such product liability insurance at a reasonable cost or in sufficient amounts to protect us against losses due to product liability claims. Our inability to obtain sufficient product liability insurance at an acceptable cost to protect against potential product liability claims could prevent or inhibit the commercialization of products and services that we develop, alone or with collaborators. We intend to expand our insurance coverage to include the sale of products and services, but we may be unable to obtain commercially reasonable product liability insurance for any products or services. If we are unable to obtain insurance at an acceptable cost or otherwise protect against potential product liability claims, we may be exposed to significant liabilities, which may materially and adversely affect our business and financial position. If we are sued for any injury allegedly caused by our products and services, our liability could exceed our total assets and our ability to pay the liability. A product liability claim, or series of claims, brought against us would decrease our cash and could cause our stock price to fall or be worthless. Any claims that are not covered by product liability insurance could have a material adverse effect on our business, financial condition and results of operations. The pharmaceutical industry is characterized by frequent product liability litigation. Those companies with significant financial resources will be better able to defend any such litigation. No assurance can be given that we would not become involved in such future litigation.
GENERAL RISKS RELATED TO TECHNOLOGY AND PRODUCT DEVELOPMENT
We are developing a new technology and our technology may not achieve commercial success.
Tru3D technology platform is designed to expedite non-destructive 3-D tissue analysis through our lab processes and software under development. Although our technological approach is based upon existing empirical data from tested science, our Tru3D technology platform may not provide enough scientific insight for researchers to alter current workflow and techniques used to analyze the biology of tissue specimens. If our Tru3D technology platform does not provide sufficient scientific insight into tissue biology, our intellectual property may be worthless.
We face significant competition in the markets targeted by our Tru3D technology platform. Many of our competitors have substantially greater resources than we do, and we expect that our product candidates under development will face intense competition from existing or future life science tools in the life sciences technology market.
We expect that the Tru3D technology platform will face significant competition from existing and future technologies marketed by larger companies. These competitors may successfully market competing products and services or may develop products and services that are more effective than our products and services.
Even if our products experience some initial success, these competitive factors could require us to conduct substantial new research and development activities to establish new product targets, which would be costly and time consuming. These activities would adversely affect our ability to commercialize products and services and to achieve revenue and profits.
We will need to expand our operations and increase the size of the Company, and we may experience difficulties in managing growth.
As we advance our product candidates and develop new product candidates, we will need to increase our product development, our headcount, our physical facilities and equipment to manage these programs. Our management, personnel, and systems currently in place may not be adequate to support this future growth. Our need to effectively manage our operations, growth and various projects requires that we successfully attract and recruit new employees with the expertise and experience we will require, expand our physical facilities and equipment, manage our regulatory compliance effectively, grow our nascent marketing and sales infrastructure and continue to improve our operational, manufacturing, financial and management controls, reporting systems and procedures.
If we are unable to successfully manage this growth and increased complexity of operations, our business may be adversely affected.
Corporate and academic collaborators may take actions to delay, prevent, or undermine the success of our products and services.
Our operating and financial strategy for the development of our Tru3D technology platform is dependent on our entering into successful collaborations with corporations, academic institutions, licensors, licensees, and other parties. Our current strategy assumes that we will successfully establish these collaborations, or similar relationships; however, there can be no assurance that we will be successful establishing such collaborations. Some of our existing collaborations are, and future collaborations may be, terminable at the sole discretion of the collaborator. Replacement collaborators might not be available on attractive terms, or at all. The activities of any collaborator will not be within our control and may not be within our power to influence. There can be no assurance that any collaborator will perform its obligations to our satisfaction or at all, that we will derive any revenue or profits from such collaborations, or that any collaborator will not compete with us. If any collaboration is not pursued, we may require substantially greater capital to undertake development and marketing of our product candidates and may not be able to develop and market such products and services effectively, if at all. In addition, a lack of development and marketing collaborations may lead to significant delays in introducing product candidates into certain markets and/or reduced sales of product candidates in such markets.
We are increasingly dependent on information technology and our systems and infrastructure face certain risks, including cybersecurity and data storage risks.
Significant disruptions to our information technology systems or breaches of information security would adversely affect our business. In the ordinary course of business, we collect, store, and transmit confidential information and large datasets. It is critical that we do so in a secure manner in order to maintain the confidentiality and integrity of such information. Our information technology systems are potentially vulnerable to service interruptions and security breaches from inadvertent or intentional actions by our employees, partners, vendors, or from attacks by malicious third parties. We are also vulnerable to third party attacks that would install malware on our systems commonly referred to as "ransomware". Maintaining the secrecy of this confidential, proprietary, and/or trade secret information is important to our competitive business position. While we have taken steps to protect such information and invested in information technology, there can be no assurance that our efforts will prevent service interruptions or security breaches in our systems or the unauthorized or inadvertent wrongful access or disclosure of confidential or other information that could adversely affect our business operations or result in the loss, dissemination, or misuse of critical or sensitive information. A breach of our security measures or the accidental loss, inadvertent disclosure, unapproved dissemination or misappropriation or misuse of trade secrets, proprietary information, or other confidential information, whether as a result of theft, hacking, or other forms of deception, or for any other cause, could enable others to produce competing products and services, use our proprietary technology and/or adversely affect our business position. Further, any such interruption, security breach, loss or disclosure of confidential information could result in financial, legal, business, and reputational harm to us and could have a material effect on our business, financial position, results of operations and/or cash flow.
Product and service development risks and uncertainties may delay development of our lead candidate or render its development too expensive or risky to pursue.
The process of developing products and services may require clinical, development and laboratory testing and clinical trials. In addition, commercialization of our product and service candidates will require that we obtain necessary regulatory approvals and that we establish sales, marketing, and manufacturing capabilities, either through internal hiring or through contractual relationships with others.
Our ability to generate revenues and achieve profitability will depend on numerous factors, including success in:
| ● | developing and testing product candidates; |
| ● | receiving regulatory approvals; |
| ● | commercializing our products and services; and |
| ● | establishing a favorable competitive position. |
Many of these factors will depend on circumstances beyond our control. We cannot assure you that we will secure all necessary FDA approvals for our business plan and products, and that we will bring any additional products and services to market or, if we are successful in doing so, that we will ever become profitable.
We expect to incur substantial additional operating expenses over the next several years as our development, regulatory and commercialization activities increase. The amount of future losses, and when, if ever, we will achieve profitability, are uncertain. Our ability to generate revenue and achieve profitability will depend on, among other things, successful completion of the development of our product candidates; obtaining necessary regulatory approvals from the FDA; establishing manufacturing, sales, and marketing arrangements with third parties; and raising sufficient funds to finance our activities. We might not succeed at any of these undertakings. If we are unsuccessful at some or all of these undertakings, our business, prospects, and results of operations will be materially adversely affected.
USE OF PROCEEDS
The Company will use the proceeds of the Offering for working capital, particularly for operating expenses and research and development.
DILUTION
During the 12 months preceding the Offering, a total of $2,310,012 in working capital was raised from the three controlling Common Stockholders of the Company, one of which is controlled by Albert Hansen. Mr. Hansen is the sole Director, Chief Executive Officer, President, Chief Financial Officer, Secretary and Treasurer of the Company. 162,501 shares of Common Stock were issued at $4 per share in January 2021, 125,001 shares of Common Stock were issued at $8 per share in June 2021 and 55,000 shares of Common Stock were issued at $12 per share in November 2021. The total of 342,502 shares of Common Stock were sold/issued at an average price of $6.74 per share.
MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
You should read the following discussion and analysis of financial condition and results of operations together with the financial statements and related notes included elsewhere in this offering circular. This discussion and other parts of this offering circular contain forward-looking statements that involve risks and uncertainties. Our actual results could differ materially from those discussed in or implied by these forward-looking statements. Factors that could cause or contribute to such differences include, but are not limited to, those discussed in the section titled "Risk Factors."
Overview
ClearLight is an early-stage life sciences tools company. CLB provides tissue processing, imaging, and image analysis services to pharmaceutical, biotechnology and academic researchers. CLB utilizes the patent-protected CLARITY technology, licensed from Stanford University, Palo Alto, CA. The CLARITY technology allows clearing of animal and human tissue so that thick tissue samples can be imaged in three dimensions ("3D") with a confocal or light-sheet microscope and then analyzed by a lab scientist as a 3D dataset. The Company is currently developing separate software enabled by artificial intelligence. This software is intended to analyze the large datasets associated with 3D imaging and provide researchers with quantitative spatial data. We believe the images and analysis will allow researchers to See More Biology™ enabling better understanding of the tissue and the tumor microenvironments to facilitate discovery of new methods and drugs to diagnose and treat disease.
We are an innovative life sciences tools company delivering 3D spatial biology services focused on transforming discovery and clinical research. Our mission is to continue to develop our 3D technology tools with life sciences researchers to See More Biology™ and better understand disease. If successful, our technology could empower clinicians to improve patient outcomes. Spatial biology refers to an evolving technology that enables academic and biopharma scientists to detect and map the distribution of cell types and biomarkers across whole tissue samples at single cell resolution, enabling researchers to See More Biology and advance their understanding of the tissue and tumor microenvironments. We believe this will eventually lead to better clinical outcomes due to increased knowledge of disease progression and patient response to therapies, both new and old.
Our current service provides customers with 3D images of tissue stained to illuminate biomarkers in spatial context while preserving tissue integrity. We are also developing machine learning software that will provide 3D quantitative spatial data of this tissue to our customers. Biomarkers are objective measures that capture what is happening in a cell or tissue at a given moment. Current genomic and proteomic methods, such as next-generation sequencing (NGS), single-cell analysis, flow cytometry and mass spectrometry, provide meaningful data but require the destruction of the tissue sample for analysis. While valuable and broadly adopted, these approaches allow scientists to analyze the biomarkers and cells that comprise the tissue but do not provide the fundamental information about tissue structure, cellular interactions and the localized measurements of key biomarkers. Furthermore, currently available spatial tissue analysis and histological methods provide some spatial information but rely on destructive 2D sectioning of tissue specimens. Expert pathologist interpretation is often required. Our Tru3D service provides an end-to-end solution that, once fully developed, should enable researchers to See More Biology leading to a better understanding of tissue and tumor microenvironments.
All of our revenue to date was generated in the United States, except for one small invoice from a Japanese customer.
As of October 31, 2021, we employed four scientists and one software engineer, augmented by eight consultants with expertise in management, strategy, e-marketing, sales, software development and finance.
We focus a significant portion of our resources on research and development, as well as on business development and sales and marketing. Our research and development efforts are focused on continued development of our Tru3D technology platform, including our proprietary Tru3D software (under development). We incurred research and development expenses of $184,021 and $75,781 for the six months ended June 30, 2021 and 2020, respectively, and $219,848 and $733,727 for the years ended December 31, 2020 and 2019, respectively. In 2019, we invested heavily in our software development with a third-party software developer located in Europe. In late 2019, in part due to time zone and other issues, we decided to transition software development to a United States-based team. After a period of review to try to identify a third-party developer, we decided to move software development to an internal team. It took significant time to identify and recruit a qualified team. In addition, this transition was adversely impacted by COVID-19. Our expenditures declined markedly in 2020 due to this transition period. We intend to continue making significant investments in this area for the near future. We also intend to continue to make investments in our scientific team, our infrastructure, and building our sales and marketing efforts. We incurred aggregate general, administrative, and sales and marketing expenses of $430,994 and $453,595 for the six months ended June 30, 2021 and 2020, respectively, and $896,218 and $1,238,195 for the years ended December 31, 2020 and 2019, respectively.
In October 2020, we began to offer reagent kits for sale. Reagent kits are comprised of optimized formulations of substances used to process tissue based on the CLARITY method. No sales were recorded in 2020. 18 kits were sold through October 15, 2021. We believe these reagent kits will help customers who are exploring the use of the CLARITY method to clear tissue specimens in their internal labs. We generally obtain our lab supplies from large distributors. These supplies are generally available as needed although there are shortages of certain supplies on occasion. In these situations, we are often able to substitute or source from another supplier. As of the date of the Offering Circular, we have financed our operations primarily from the issuance and sale of convertible preferred units (when we were a limited liability company), common stock and loans from our shareholders. We have incurred net losses in each year since our inception in 2014. Our net losses were $(680,461) and $(676,304) for the six months ended June 30, 2021 and 2020, respectively, $(1,428,020) and $(2,174,261) for the years ended December 31, 2020 and 2019, respectively. We expect to continue to incur significant expenses and operating losses for the foreseeable future. We expect our expenses will increase meaningfully as we attract, hire, and retain qualified personnel; market and sell new and existing services; invest in infrastructure to scale our business; support research and development to introduce new software and services; and expand and protect our intellectual property.
There are a number of factors that have impacted, and we believe will continue to impact, our business, results of operations and growth. Our ability to successfully address these factors is subject to various risks and uncertainties, including those described under the heading "Risk Factors."
Expanding our customer base
We are focused on increasing sales of our services to new and existing customers. Our financial performance will continue to be determined by our success in growing sales from new and existing customers.
We believe our market is relatively new and small at present. As spatial biology is further validated through additional peer-reviewed publications and growing adoption by the life sciences research market, we believe we have an opportunity to significantly increase our customer base. In order to capitalize on this opportunity to drive adoption of our technology across the entire market, we intend to expand our sales and marketing efforts.
Recurring revenue
We believe that expanding our services to existing customers is important to our future growth. Since our technology is still considered novel, many customers will initially try our service with one or two tissue specimens. A few customers have returned with larger numbers of samples if they are able to See More Biology. Our current service provides 3D images of customer tissue samples that we have stained with customer-designated biomarker panels. As a result, we offer researchers better visual understanding of the cellular interactions in their tissue samples. We are unable to provide quantitative data (such as biomarker and cell counts with spatial data) for these tissue samples at present. We are developing software to provide quantitative and spatial data.
Revenue mix and gross margin
Our revenue is primarily derived from sales of our tissue imaging services. In the future, we expect licensing of our proprietary software and to a lesser extent, reagents, to become significant revenue generators. Our costs are largely fixed so our gross margins are heavily impacted by the volume of samples processed in the lab. Our gross margins have been negative due to the small volume of samples that we have processed to date. We expect volumes will continue to increase at a moderate pace in the near future. Our gross margins are also impacted by our pricing. We believe our gross margins will be positive in the future if we are able to increase our volume, increase our price/sample and improve our pricing structure to include charging for customer changes to work in process. We believe our current price/sample is significantly less than what competitors charge for analogous service.
Future instrument and consumable selling prices and gross margins may fluctuate due to a variety of factors, including the introduction by others of competing products and solutions. We aim to mitigate any downward pressure on our average selling prices by increasing the value proposition offered by our services, primarily by offering quantitative and spatial data with our software once it is developed and, in the longer term, decreasing the turnaround time of our services.
COVID-19 Impact
In March 2020, COVID-19 was declared a global pandemic by the World Health Organization. In the following weeks, many states and counties across the United States responded by implementing a number of measures designed to prevent its spread, including stay-at-home or shelter-in-place orders, quarantines and closure of all non-essential businesses. The impact of this pandemic has been and will likely continue to be extensive in many aspects of society, which has resulted in and will likely continue to result in significant disruptions to the global economy, as well as businesses and capital markets around the world.
Impacts through June 30, 2021 to our business as a result of COVID-19 include disruptions to our operations, the operations of prospective customers, and the operations of our supply chain caused by facility closures, reductions in operating hours, staggered shifts and other social distancing efforts, decreased productivity and unavailability of materials or components, limitations on our employees' and customers' ability to travel, and delays in shipments to and from affected countries and within the United States. In light of the uncertain and rapidly evolving situation relating to the spread of COVID-19, we have taken precautionary measures intended to minimize the risk of the virus to our employees, our customers and the communities in which we operate, including temporarily closing our offices to visitors and limiting the number of employees in our offices to those that are deemed essential for laboratory operations, as well as virtualizing, postponing or canceling customer, employee and industry events.
Disruptions in our customers' operations have impacted and may continue to impact our business. For example, laboratory shutdowns and reduced capital spending by our customers have negatively impacted our ability to maintain and increase current customer revenues and attract new customers. We are focused on navigating the challenges presented by COVID-19, with a primary focus on preserving our liquidity and managing our cash flows by taking preemptive action to enhance our ability to meet our short-term liquidity needs. To address actual and expected reductions in revenue and cash flows, we reduced our spending.
We do not yet know the net impact that the COVID-19 pandemic may have on our business but we believe it has had a materially negative impact that will continue for the near future. Although we continue to monitor the situation and may adjust our current policies as more information and public health guidance become available, the ongoing effects of the COVID-19 pandemic and/or the precautionary measures that we have adopted may create operational and other challenges, any of which could harm our business and results of operations. While we maintain an inventory of finished reagent kits and raw materials, a worsening pandemic could lead to shortages in the raw materials necessary to provide our services. If we experience further disruption in our manufacturing, supply chains or commercial operations, or if demand for our services is significantly reduced as a result of the COVID-19 pandemic, we would expect to experience a material adverse impact on our business, financial condition, results of operations and prospects.
For businesses like ours, a significant portion of initial customer interactions have been conducted in person, and the rollout of new services has historically been supported by participation at industry conferences. Currently, as a result of the work and travel restrictions related to the COVID-19 pandemic, and the precautionary measures that we have adopted, substantially all of our sales activities are being conducted remotely, which has adversely impacted new customer acquisition. As of the date of this offering circular, we do not yet know the extent of the negative impact of such restrictions and precautionary measures on our ability to attract new customers or retain and expand our relationships with existing customers over the near and long term. The length of time and full extent to which the COVID-19 pandemic may directly or indirectly impact our business, results of operations and financial condition will depend on future developments that are highly uncertain, subject to change and difficult to predict. In October and November 2021, there were two industry conferences which allowed in-person attendance. We attended both and we intend to participate in selected future conferences that allow in-person attendance.
License Agreements
Effective September 1, 2015, the Company licensed certain technology from Stanford University covering inventions relating to methods to image brain and other tissues for use both in research and in the clinic (the "Stanford Agreement"). The technology is owned and patented by Stanford and includes US Patent 10,545,075 issued on January 28, 2020.
As part of the consideration for the Stanford Agreement, Stanford and co-inventors received equity interests which currently constitute less than 1% of outstanding shares. The Agreement states that Stanford University is entitled to receive yearly maintenance fees, milestone payments, and royalties. The amounts paid in 2020 and 2019 were $100,556 and $42,399, respectively.
Stanford University is entitled to the earned royalties as a percentage of net sales ranging from 1.5% - 4%, depending on the product or service sold.
Components of results of operations
Service and Other Revenue
We primarily generate service and other revenue from our laboratory services operation, where we provide 3D imaging services for customers' tissue specimens. Customers generate these specimens typically from mouse studies used in pre-clinical work for drug discovery. We charge a fee for each tissue sample processed. We typically offer a significant discount to academic labs. In October 2020, we also started offering reagent kits that were optimized for the CLARITY tissue clearing process. Reagent kits are manufactured internally. There were no sales of kits in 2020 and 18 sales of kits in 2021 as of the date of this circular. The Company records shipping and handling billed to customers as service and other revenue and the related costs in cost of service and other revenue in the statement of operations. All sales were to customers in the United States, except for one small sale to a Japanese customer.
Service and other revenue was $29,874 and $9,000 for the six months ended June 30, 2021 and June 30, 2020, respectively and $33,797 compared to $7,650 for the years ended December 31, 2020 and 2019, respectively.
Cost of Revenue, Gross Profit and Gross Margin
Cost of revenue for services and other primarily consists of salaries, other personnel costs, lab supplies and related costs used to process tissue samples.
We expect that our cost of revenue will increase if our revenue increases. Since a large part of our costs are salaries which are fixed, decreases in revenue will not result in corresponding decreases in cost of revenue. Also, since we are still developing our technology and the market for our services is new, we also often need to perform additional processing in the lab for our customers due to the fact that we are still developing our technology platform. This can result in significantly higher cost of revenue per sample processed.
Gross profit is calculated as revenue less cost of revenue. Our gross profit has been negative to date and we expect this to continue for some time in the future until our technology is more fully developed and our pricing better reflects our costs. Gross margin is gross profit expressed as a percentage of revenue. Our gross profit in future periods will depend on a variety of factors, including: market conditions that may impact our pricing, sales mix among services and reagents, changes in costs of lab supplies, excess and obsolete inventories and our cost structure for lab service operations relative to volume.
Gross profit was $(127,886) and $(128,009) for the six months ended June 30, 2021 and June 30, 2020, respectively and $(265,583) compared to $(157,097) for the years ended December 31, 2020 and 2019, respectively.
Operating expenses
Research and development. Research and development costs primarily consist of salaries, benefits, consulting fees, engineering/design costs, laboratory supplies, and materials expenses for employees and third parties engaged in research and product development. We expense all research and development costs in the period in which they are incurred.
We plan to continue to invest in our research and development efforts, including hiring additional employees, to enhance existing products and develop new products. As a result, we expect that our research and development expenses will continue to increase in absolute dollars in future periods. We expect these expenses to vary from period to period as a percentage of revenue.
Our research and development efforts are focused on continued development of our Tru3D technology platform including our proprietary software (under development). We incurred research and development expenses of $184,021 and $75,781 for the six months ended June 30, 2021 and June 30, 2020, respectively and $219,848 and $733,727 for the years ended December 31, 2020 and 2019, respectively. In 2019, we invested heavily in our software development with a third-party software developer located in Europe. In late 2019, in part due to time zone and other issues, we decided to transition software development to a United States-based team. After a period of review to try to identify a third-party developer, we decided to move software development to an internal team. It took significant time to identify and recruit a qualified team. In addition, this transition was adversely impacted by COVID-19. Our expenditures declined markedly in 2020 due to this transition period. We intend to continue making significant investments in this area for the foreseeable future.
Selling, general and administrative. Our selling, general and administrative expenses include the salaries and benefits for employees on our science team. Also included are expenses for dedicated consultants who are responsible for our executive, accounting and finance, sales and marketing, software development and business development expenses. Also included are professional services fees, such as miscellaneous consulting, audit, tax and legal fees, general corporate costs, travel expenses, shared laboratory facility costs, equipment and IT. We incurred selling, general and administrative expenses of $430,994 and $453,595 for the six months ended June 30, 2021 and June 30, 2020, respectively and $896,218 and $1,238,195 for the years ended December 31, 2020 and 2019, respectively. We expect that our sales, general and administrative expenses will continue to increase in absolute dollars after this offering, primarily due to increased headcount and facilities costs to support anticipated growth in the business and due to incremental costs associated with operating as a public company. Additionally, we expect an increase in absolute dollars as we expand our commercial sales, marketing, and business development teams; increase our market presence; expand our facilities and equipment; and increase marketing activities to drive awareness and adoption of our Tru3D technology platform. We expect these expenses to vary from period to period as a percentage of revenue.
Depreciation and amortization. Depreciation and amortization expenses primarily consist of depreciation of property and equipment and amortization of licensed technology. We expect that depreciation and amortization expenses will decrease as a percentage of revenue.
Other income (expense)
Interest expense. Interest expense consists primarily of interest related to borrowings under our debt obligations.
Other income (expense), net. Other income (expense), net consists primarily of miscellaneous items not related to operations.
Since our inception, we have experienced losses and negative cash flows from operations, and our consolidated net loss was $(680,461) and $(676,304) for the six months ended June 30, 2021 and June 30, 2020, respectively and $(1,428,020) compared to $(2,174,261) for the years ended December 31, 2020 and 2019, respectively.
We expect to incur additional operating losses in the foreseeable future as we continue to invest in the research and development of our technology, commercialize and launch new services, and expand into new markets. Based on our current business plan, we believe the net proceeds from this Offering, together with our existing cash and cash equivalents and anticipated cash flows from operations will be sufficient to meet our working capital and capital expenditure needs over at least the next six months following the date of the Offering Circular. We also believe our current shareholders will continue to provide liquidity as necessary, although there are no assurances that this will occur.
Our future capital requirements will depend on many factors, including, but not limited to our ability to successfully commercialize and launch products, and to achieve a level of sales adequate to support our cost structure. If we are unable to execute on our business plan and adequately fund operations, or if the business plan requires a level of spending in excess of cash resources, we will have to seek additional equity or debt financing. We anticipate that significant additional financings will be required from outside sources. We may not be able to raise financing on terms acceptable to us or at all. If we are unable to raise additional capital when desired, our business, financial condition, results of operations and prospects would be adversely affected and could result in liquidation with a total loss of investor capital.
Sources of Liquidity
Since our inception, we have financed our operations primarily from the issuance and sale of convertible preferred units, common stock and loans from our shareholders.
Financings
Through June 30, 2021, we have raised a total of $13.4 million from the issuance and sale of convertible preferred units and common stock.
Payroll Protection Program loan
During April 2020, we received a business loan of $84,042 under the Payroll Protection Program, part of the Coronavirus Aid, Relief and Economic Security Act, the CARES Act. This loan was forgiven under the provisions of the program in May 2021.
Cash flows
The following table summarizes our cash flows for the periods presented:
| | | Six months ended June 30, | | | Year ended December 31, | |
| ($ in thousands) | | 2021 | | | 2020 | | | 2020 | | | 2019 | |
| Net cash (used in) provided by: | | | | | | | | | | | | |
| Operating activities | $ | (966 | ) | $ | (726 | ) | $ | (763 | ) | $ | (1,805 | ) |
| Investing activities | | - | | | - | | | (664 | ) | | 71 | |
| Financing activities | | 1,359 | | | 813 | | | 1,735 | | | 381 | |
Net increase (decrease) in cash, cash equivalents, and restricted cash | $ | 393 | | $ | 88 | | $ | 308 | | $ | (1,352 | ) |
Operating activities
In the six months ended June 30, 2021, net cash used in operating activities increased by $239,723 to $(965,605) compared to $(725,881) used in the six months ended June 30, 2021. This increase is attributable to an increased net loss of $(4,157), a net increase in our net operating assets and liabilities of $(236,757) and decreased stock compensation expense of $(5,683) partially offset by an increase in depreciation of $6,873.
In the year ended December 31, 2020, net cash used in operating activities decreased by $1,041,939 to $(763,147) compared to $(1,805,086) in the year ended December 31, 2019. This decrease is attributable to a decreased net loss of $746,241 and a net decrease in our net operating assets and liabilities of $473,978 partially offset by decreased stock compensation expense of $(176,270) and a decrease in depreciation of $(2,010). The net decrease in our net operating assets and liabilities of $473,978 was primarily due to an increase in accounts payable of 478,596.
Investing activities
There were no investing activities in the six months ended June 30, 2021 nor in the six month ended June 30, 2020.
Net cash used in investing activities was $(663,546) in the year ended December 31, 2020 due to the purchase of a lightsheet microscope. Net cash provided in the year ended December 31, 2019 was $71,195 due to the sale of excess equipment.
Financing activities
Net cash provided by financing activities was $1,358,889 for the six months ended June 30, 2021 compared with $813,465 for the six months ended June 30, 2020. Net cash provided by financing activities during the six months ended June 30, 2021 resulted from $1,650,012 issuance in common stock proceeds offset by repayment of $(291,123) of notes. Net cash provided by financing activities for the six months ended June 30, 2020 resulted primarily from net cash proceeds of $750,000 issuance in common unit proceeds and the issuance of $63,465 of notes.
Net cash provided by financing activities was $1,358,889 for the six months ended June 30, 2021. This resulted from the issuance of $1,650,012 offset by repayment of notes of $(291,123). Net cash provided by financing activities was $1,735,111 for the year ended December 31, 2020 compared with $381,876 for the year ended December 31, 2019.Net cash provided by financing activities during the year ended December 31, 2020 resulted from $1,499,999 issuance in common unit and common stock proceeds. In addition, $285,111 of notes payable were issued. Net cash provided by financing activities for the year ended December 31, 2019 resulted primarily from net cash proceeds of $1,500,000 issuance of bridge notes that were exchanged for convertible preferred units offset by repayment of notes payable of $(1,118,124).
Customer concentration
For the six months ended June 30, 2021, the four largest customers accounted for 39%, 13%, 13% and 12% of our revenue, respectively. For the year ended December 31, 2020, the four largest customers accounted for 33%, 30%, 13% and 12% of our revenue, respectively. For the year ended December 31, 2019, one customer accounted for 78% of our revenue and another customer accounted for 16% of our revenue. No other customers exceeded 10% of revenue for the years ended December 31, 2020 and December 31, 2019, respectively.
Qualitative and Quantitative Disclosures About Market Risk
Interest rate risk
Customer financing exposure. We do not provide financing to our customers and our customers do not require financing for their purchases.
Fixed rate debt. We have periodically borrowed funds from our shareholders and may continue to do so in the future. However, there can be no assurance that such financing will be available in the future. A hypothetical 100 basis point change in interest rates would have resulted in a $4,518 increase in interest expense for the year ended December 31, 2020.
Bank deposit, money market and note receivable exposure. As of June 30, 2021, we had cash and cash equivalents, including restricted cash, of $718,333 which consisted primarily of bank deposits.
The primary objective of our investment is to preserve principal and provide liquidity. These bank deposits generate interest income at variable rates below 1%. A hypothetical 100 basis point decrease in interest rates would have had a negligible effect on our income.
Foreign currency risk
All of our revenue has been generated in U.S. dollars. As we expand to international markets in the future, to the extent we are required to enter into agreements denominated in a currency other than the U.S. dollar, our results of operations and cash flows may increasingly be subject to fluctuations due to changes in foreign currency exchange rates and may be adversely affected in the future due to changes in foreign exchange rates.
Critical accounting policies and estimates
We have prepared our consolidated financial statements in accordance with GAAP. Our preparation of these consolidated financial statements requires us to make estimates, assumptions and judgments that affect the reported amounts of assets, liabilities, revenue, expenses and related disclosures. We evaluate our estimates and judgments on an ongoing basis. We base our estimates on historical experience and on various other factors that we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not readily apparent from other sources. Actual results could therefore differ materially from these estimates under different assumptions or conditions.
While our significant accounting policies are described in more detail in Note 2 to our audited consolidated financial statements, we believe the following accounting policies to be critical to the judgments and estimates used in the preparation of our consolidated financial statements.
Impairment of long-lived assets and goodwill
The Company evaluates its long-lived assets for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. If indications of impairment exist, projected future undiscounted cash flows associated with the asset or asset group are compared to the carrying amount to determine whether the asset's value is recoverable. During this analysis, the Company reevaluates the significant assumptions used in determining the original cost and estimated lives of long-lived assets. Although the assumptions may vary from asset to asset, they generally include operating results, changes in the use of the asset, cash flows and other indicators of value. The Company then determines whether the remaining useful life continues to be appropriate or whether there has been an impairment of long-lived assets based primarily upon whether expected future undiscounted cash flows are sufficient to support the assets' recovery. If impairment exists, the Company would adjust the carrying value of the asset to fair value, generally determined by a discounted cash flow analysis. If the carrying value of the asset exceeds such projected undiscounted cash flows, the asset will be written down to its estimated fair value.
The Company tests intangible assets for impairment whenever events or changes in circumstances indicate that the carrying value of the asset may not be recoverable (i.e., upon occurrence of a triggering event).
Revenue recognition
The Company follows ASC 606, Revenue from Contracts with Customers.
We derive revenue mainly from service revenue, which is comprised mainly of laboratory services revenue. Revenue is recognized after service has been completed and images with a report has been issued to the customer.
We recognize revenue when we satisfy the performance obligations under the terms of a contract and a report and when any other deliverables have been provided to the customer. We recognize revenue in an amount that reflects the consideration we expect to receive from our customers in exchange for those services. This process involves identifying the contract with a customer, determining the performance obligations in the contract, determining the contract price, and recognizing revenue when the performance obligations have been satisfied. A performance obligation is considered distinct from other obligations in a contract when it provides a benefit to the customer either on its own or together with other resources that are readily available to the customer and is separately identified in the contract. We consider a performance obligation satisfied once we have transferred control of a good or service to the customer, meaning the customer has the ability to use and obtain the benefit of the good or service.
In order to determine the stand-alone selling price, we initially set a low price per sample to attract new customers. We believe our current selling price does not reflect the value we provide to customers and thus have raised the price on three occasions to date. Our current selling price is over 50% higher than our initial selling price in 2019. We expect further significant increases to our selling price in the future as we develop our technology and can offer greater value to customers. Our process for determining selling price requires judgment and considers multiple factors.
Taxes, such as sales, value-added and other taxes, collected from customers concurrent with revenue generating activities and remitted to governmental authorities are not included in revenue. Shipping and handling costs associated with outbound freight are accounted for as a fulfillment cost and are included in cost of sales.
The following describes the nature of our primary types of revenue and the revenue recognition policies and significant payment terms as they pertain to the types of transactions we enter into with our customers.
Service and other revenue
Service and other revenue is comprised almost completely of lab service revenue. Revenue was $29,874 for the six months ended June 30, 2021. Revenue was $33,797 and $7,650 for the years ended December 31, 2020 and December 31, 2019. There were no reagent kit sales in 2020 and eight kit sales in the six months ended June 30, 2021. Our standard arrangement with our customers is generally a purchase order or an executed contract. Revenue is recognized when a lab services report has been issued and discussed with the customer. Payment terms are generally thirty days from the date of invoicing.
The Company records shipping and handling billed to customers as service and other revenue and the related costs in cost of other revenue in the consolidated statement of operations.
Costs to obtain or fulfill a contract
Under ASC 606, the Company is required to capitalize certain costs to obtain customer contracts and costs to fulfill customer contracts. These costs are required to be amortized to expense on a systemic basis that is consistent with the transfer to the customer of the goods or services to which the asset relates, compared to previously being expensed as incurred. As a practical expedient, the Company recognizes any incremental costs to obtain a contract as an expense when incurred if the amortization period of the asset is one year or less.
Equity based compensation
In the past, the Company adopted an incentive compensation plan under which options and profit interests were granted primarily to employees and non-employee consultants. Equity-based compensation cost for profit interests was treated as deferred compensation and was not recognized under ASC 710 as the payments were not probable and the amounts could not be estimated. Equity- based compensation cost for options was measured at the grant date, based on the fair value of the award, and was recognized as expense over the requisite service period. The fair value of option awards to employees was estimated using the Black- Scholes option pricing model. We record forfeitures when they occurred.
Equity-based compensation expense for non-employee stock options was measured at the grant date based on fair market value using the Black-Scholes option pricing model and was recorded as the options vest. The grant date fair market value of non-employee stock options was recognized in the consolidated statement of operations on a straight-line basis over the requisite service period and forfeitures were recognized as they occurred.
The Company no longer issues options and profit interests. This plan was replaced by a restricted stock plan in December 2020. Restricted stock is expensed at fair market value at the grant date, as vesting is immediate. However, restricted stock can be repurchased by the Company at the sole discretion of the Company for two years following the date of grant at a price of $0.01/restricted share. A total of 69,239 restricted shares have been issued as of the date of this Offering Circular.
Common Stock Valuations Prior to our IPO
There has been no public market for our common stock to date. As such, the estimated fair value of our common stock has been determined at each issue or grant date by our management, based on the information known to us on the grant date and upon a review of any recent events and their potential impact on the estimated per share fair value of our common stock. Our management used assumptions based on various objective and subjective factors, combined with management judgment, to determine the fair value of our common stock as of each grant date, including:
● | the prices at which we previously sold convertible preferred units, common units and Common Stock; |
● | external market conditions affecting the life sciences research and development industry and trends within the industry; |
● | our stage of development and business strategy; |
● | our financial condition and operating results, including our levels of available capital resources, and forecasted results; |
● | developments in our business; |
● | the progress of our research and development efforts; |
● | equity market conditions affecting comparable public companies; and |
● | general United States market conditions and the lack of marketability of our common stock. |
Application of these approaches involves the use of estimates, judgment and assumptions that are subjective, such as those regarding our expected future revenue, expenses and future cash flows, discount rates, market multiples, the selection of comparable companies and the probability of possible future events. Changes in any or all of these estimates and assumptions or the relationships between those assumptions impact our valuations as of each valuation date and may have a material impact on the valuation of our common stock. For valuations after the completion of this initial public offering, our board of directors will determine the fair value of each share of unrestricted common stock based on the closing price of our common stock if a market develops for our stock. We intend to continue the issuance of restricted stock rather than options for the near future. The value of restricted shares will be determined by our board of directors with the assistance of a third-party valuation firm.
Recent accounting pronouncements
For information on recently issued accounting pronouncements, see Note 2 to our consolidated financial statements in this offering circular.
JOBS Act accounting election
We are an "emerging growth company," as defined in the Jumpstart Our Business Startups Act of 2012, or the JOBS Act. Under the JOBS Act, emerging growth companies can delay adopting new or revised accounting standards issued subsequent to the enactment of the JOBS Act until such time as those standards apply to private companies. We have elected to avail ourselves of this extended transition period, and, as a result, we will not be required to adopt new or revised accounting standards on the relevant dates on which adoption of such standards is required for other public companies. We intend to rely on other exemptions provided by the JOBS Act, including without limitation, not being required to comply with the auditor attestation requirements of Section 404(b) of the Sarbanes-Oxley Act of 2002.
Smaller reporting company status
We are also a "smaller reporting company," meaning that the market value of our stock held by non- affiliates plus the proposed aggregate amount of gross proceeds to us as a result of this offering is less than $700 million and our annual revenue is less than $100 million during the most recently completed fiscal year. We will continue to be a smaller reporting company after this offering if either (i) the market value of our stock held by non-affiliates is less than $250 million or (ii) our annual revenue is less than $100 million during the most recently completed fiscal year and the market value of our stock held by non-affiliates is less than $700 million. If we are a smaller reporting company at the time we cease to be an emerging growth company, we may continue to rely on exemptions from certain disclosure requirements that are available to smaller reporting companies. Specifically, as a smaller reporting company we may choose to present only the two most recent fiscal years of audited financial statements in our Annual Report and, similar to emerging growth companies, smaller reporting companies have reduced disclosure obligations regarding executive compensation.
OUR BUSINESS
Company Information
ClearLight is an early-stage life sciences tools company. CLB provides tissue processing, imaging, and image analysis services to pharmaceutical, biotechnology and academic researchers. CLB utilizes the patent-protected CLARITY technology, licensed from Stanford University, Palo Alto, CA. The CLARITY technology allows clearing of animal and human tissue so that thick tissue samples can be imaged in three dimensions ("3D") with a confocal or light-sheet microscope and then analyzed by a lab scientist as a 3D dataset. The Company is currently developing separate software enabled by artificial intelligence. This software is intended to analyze the large datasets associated with 3D imaging and provide researchers with quantitative spatial data. We believe the images and analysis will allow researchers to See More Biology™ enabling better understanding of the tissue and the tumor microenvironments to facilitate discovery of new methods and drugs to diagnose and treat disease.
The Company was incorporated as a limited liability company in Delaware on December 1, 2014. It converted to a Delaware corporation on October 12, 2020. Our executive office and laboratory facilities are located at ClearLight Biotechnologies, Inc., 428 Oakmead Parkway, Sunnyvale, CA 94085-4708.
Our website address is www.clearlightbiotech.com. We do not incorporate the information on or accessible through our website into the Offering Circular, and you should not consider any information on our website, or any information that can be accessed through our website, as part of the Offering Circular.
Summary of Our Business and our Technology
We are an innovative life sciences tools company delivering 3D spatial biology services focused on transforming discovery, pre-clinical, and clinical research. We use the patent-protected CLARITY1 technology, licensed from Stanford University, Palo Alto, CA. The CLARITY technology allows clearing of animal and human tissue so that thick tissue samples can be imaged in 3D with a confocal or light-sheet microscope and later analyzed by lab scientist. Our mission is to continue to develop our 3D technology tools with life sciences researchers to See More Biology™ and better understand models of disease. If successful, our technology could empower clinicians to improve patient outcomes. Spatial biology refers to an evolving technology that enables academic and biopharma scientists to detect and map the 3D distribution of various cell types, identified and classified using biomarkers, across whole tissue samples at single cell resolution, thereby enabling researchers to See More Biology and advance their understanding of the tissue and tumor microenvironments. We believe this will eventually lead to better clinical outcomes due to increased knowledge of disease progression and patient response to therapies, both new and old.
Our current service provides customers with 3D images of tissue stained to illuminate biomarkers in spatial context while preserving tissue integrity. We are also developing machine learning software that will provide 3D quantitative spatial data of this tissue to our customers. Biomarkers are objective measures that identify the present cell types and biological processes and capture what is happening within a cell or tissue microenvironment at a given moment. Current genomic and proteomic methods, such as next-generation sequencing (NGS), single-cell analysis, flow cytometry and mass spectrometry, provide meaningful data but require the destruction of the tissue sample for analysis. While valuable and broadly adopted, these approaches allow scientists to analyze the biomarkers and cells that comprise the tissue but do not provide the fundamental information about tissue structure, cellular interactions and the spatial orientation which are key factors in understanding disease progression and treatment outcomes. Furthermore, currently available spatial tissue analysis and histological methods provide some spatial information but rely on destructive 2D sectioning of tissue specimens. Expert pathologist interpretation is often required. Our Tru3D service provides an end-to-end solution that, once fully developed, should enable researchers to See More Biology leading to a better understanding of tissue and tumor microenvironments.
Our long-term vision is to disrupt the diagnostic, prognostic, and predictive treatment of disease by automating significant parts of traditional pathology workflow. We also believe our platform allows researchers to See More Biology™, potentially providing researchers a discovery platform for new drugs. Longer term, one possibility is to accomplish this with a combination of instrumentation and AI-enabled software. Although some initial work has been done on the instrumentation, preliminary estimates are that development of an automated instrument platform could exceed $25 million and take five years. No further development is currently planned for 2021 or 2022 due to lack of funding.
1 CLB utilizes CLARITY technology as a foundation of its Tru3D® tissue processing platform. CLARITY is an acronym for Clear Lipid-exchanged Acrylamide-hybridized Rigid Imaging/Immunostaining/In situ hybridization-compatible Tissue-hYdrogel. CLARITY technology entails the formation of a hydrogel matrix by cross-linking biological molecules to a 3D network of hydrophilic polymers, followed by lipid removal to generate a transparent and structurally intact tissue. CLARITY technology allows clearing of animal and human tissue so that it can be imaged in 3D with a powerful confocal or light-sheet microscope and later analyzed by a lab scientist.
Figure 1 below provides a visual overview of CLB's Tru3D technology platform:
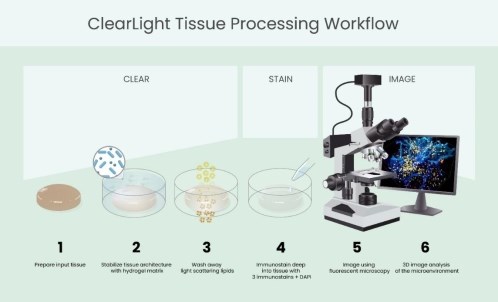
FIGURE 1
New technology has disrupted methodology for researchers in the past, although this often takes many years to gain acceptance by scientists. An example of this is the emergence of next generation sequencing (NGS) into clinical decision making. It took about 20 years for NGS to gain market acceptance. This was in part due to the natural skepticism of scientists who had many questions about the validity and usefulness of the technology. Over time, these valid scientific questions and concerns were answered. As a result, NGS is now an important tool for researchers and has become a multi-billion-dollar industry. We believe this time frame provides a useful business model to inform the development of 3D tissue analysis technology. As a result, CLB has decided that its initial focus will be on the research use only (RUO) market. The RUO market will enable the introduction of the technology to research scientists. These scientists will in turn guide development of the technology so that CLB can provide answers to important scientific questions about the technology platform and can improve the technology to meet customer needs. If successful, our long-term plan is to enter the clinical market as adoption of our technology increases and clinical utility is established. The RUO market does not require FDA clearance or approval, but any clinical application would require FDA clearance or approval.
We have used our initial funding to further develop our technology. We have obtained a US Patent (Patent No. 10,545,075 issued on January 28, 2020) for the CLARITY technology licensed from Stanford University. This patent includes the basic technology for lipid clearing from tissue samples. In addition to CLARITY, we have developed customized methods for immuno-staining and 3D imaging for both confocal and light-sheet microscopes. We are also developing software to analyze the voluminous datasets produced by 3D microscopes. This software will initially be used only by CLB with customers receiving the results of the software analysis.
We operate at our shared laboratory space in Sunnyvale, CA. We are part of a consortium of companies that share the facilities of the Molecular Medicine Research Institute.
In order to keep costs to a minimum, CLB operates with both employees who are scientists and with external consultants and advisors. The internal team is comprised of a vice president of research, an application scientist, and two research associate scientists. In addition, a deep learning software engineer has been hired to assist with software development efforts. The external consultants and advisors are responsible for CEO and CFO functions, software development, sales and marketing, human resources, IT, accounting/taxes, hardware engineering, website support and legal (corporate and IP). In addition, CLB has a President and sole Director experienced in the growth and funding of early-stage companies in the life science sector.
Description of Lab Market Strategy
CLB seeks to disrupt the research and pathology lab markets through its Tru3D® technology platform. Current research and pathology labs still rely on proven but often manually intensive techniques, some of which are more than 100 years old. CLB's long-term vision is to automate a significant portion of current research and pathology lab workflow, thus increasing throughput and efficiency of these labs. CLB's initial focus is on the research use only (RUO) market. This market is comprised of both academic and commercial scientific or drug researchers. A critical component of this technology is the development of cloud-based machine learning software to analyze 3D images of animal and human tissue specimens. Once developed, this software will be able to provide quantitative data for a 3D tissue specimen such as cell count, type of cell, presence or absence of up to three biomarkers, spatial x-, y-, and z-coordinates for each cell, and spatial distances between specific cell types. CLB is in a unique position to develop such software. Our nascent contract research service entails applying the patented CLARITY method to the diverse tissues provided by our customers and imaging their samples in 3D. This service model informs the development of our technology by solving real-world problems faced by our customers. This enables CLB to gain valuable and relevant insights into further development of our technology for our target market.
CLARITY Technology and its use in Pathology and Pre-Clinical Research
CLB was founded by Karl Deisseroth M.D., Ph.D. in December 2014 to commercialize CLARITY tissue clearing, as developed by Dr. Deisseroth and his colleagues in the Deisseroth Lab at Stanford University. CLB provides pharmaceutical, biotechnology, and academic researchers with an optimized CLARITY Tissue Clearing, 3D immunostaining, and Tru3D image analysis services. This technology, paired with proprietary 3D image analysis software in development, empowers researchers to See More Biology within the tissue micro-environment. Dr. Deisseroth's CLARITY method is the foundation of CLB's tissue processing workflow.
CLB seeks to disrupt the current gold-standard pathology approach through its patented CLARITY technology and ML software. As previously mentioned, some parts of the current gold-standard method for tissue pathology are over 100 years old. Current practice entails imaging formalin-fixed paraffin-embedded (FFPE) 2D thin section slides with a bright-field or confocal microscope. Clinical pathologists use FFPE slides to diagnose patients. Some academic researchers have begun to use the FFPE method to develop a spatial understanding of tissue microenvironment structure. To perform microscopy on FFPE tissues, the tissue specimen (e.g., a biopsy) must be first sectioned into thin slices (often 2-10 μm thick). This sectioning destroys the 3D structure of the tissue. The pathologist then chooses a number of slides (3-10+) to analyze. Preparing the thin tissue sections is time consuming and requires precision. In addition, it can be mind-numbing for the technicians, which may lead to costly mistakes.
CLB's long term vision is to develop an automated platform that will use CLARITY technology to process, image, and analyze tissue samples, thus automating many of the labor-intensive, tedious processes. By using the CLARITY tissue clearing technique, the need for destructive thin-sectioning of tissue will be eliminated. Moreover, tissue morphology and its natural 3D structure will be preserved. Ultimately, the Company's goal is to develop the technology so that pathologists can use the technology in a clinical lab setting to detect disease from patient tissue biopsies, including cancers such as micrometastases, that may be missed using traditional methods. Imaging tissue in 3D, immunostained with antibodies, may reveal novel tissue irregularities that cannot be obtained with current technology. Further, developing 3D image analysis software will enable the analysis of large datasets (up to a terabyte or more) generated by light-sheet microscopes. This should also increase the accuracy and consistency in the analysis and interpretation of results, enabling further adoption of 3D imaging of intact tissues for research and diagnostic purposes. We believe it will also be an important tool for researchers to "See More Biology" leading to discovery of new drugs, medical devices, and therapies.
Figure 2 below provides a pictorial overview of CLARITY technology:
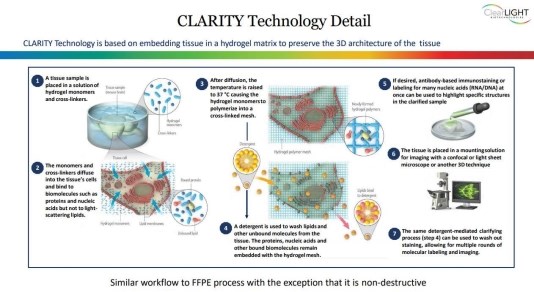
FIGURE 2. Adapted from Deisseroth, K. Scientific American, October 2016, Volume 315, number 4.
An initial area of interest of the Company is oncology prognostic and predictive applications. Predictive biomarker applications for immuno-oncology T-cell based drugs as well as standard-of-care biomarkers are an area of special interest. Another area of interest is in neuroscience with an emphasis on models of Alzheimer's disease. However, the technology can also be applied to any biological area that currently relies on 2D thin-section analysis of tissue or beyond. Besides oncology, particularly immuno-oncology, customers have provided tissue samples targeting skin and eye disease and implantable medical devices for 3D imaging and analysis.
FDA
Our instruments, consumable products and assays are purchased from suppliers with a restriction that they be used for research use only, or RUO. While we have focused initially on the life sciences RUO market only, part of our long-term business strategy is to expand our product line to encompass products that are intended to be used for the diagnosis of disease and precision healthcare, either alone or in collaboration with third parties. The use of our products for any such diagnostic purposes would require that we obtain regulatory clearance or approval to market our products for those purposes and also that we acquire the materials and components used in such products from suppliers without an RUO restriction. There can be no assurance that we will be able to acquire these materials and components for use in diagnostic products on acceptable terms, if at all. If we are unable to do so, we would not be able to expand our product offerings beyond RUO, and our business and prospects would be limited. However, we believe the RUO market is substantial.
ClearLight Objectives
Mission. Our mission is to disrupt the diagnostic, prognostic and predictive treatment of disease by developing our Tru3D next generation technology platform. It will be designed to analyze, non-destructively and digitally, diseased and normal tissue in 3D. We believe the past development of next generation sequencing technology disrupted tissue analysis over the last 20 years. We believe a similar steady trajectory is possible with our Tru3D technology platform for processing and imaging of tissue. Initially this will be adopted by the RUO market and then, eventually, we believe the clinical market may develop as clinical utility for patient care is tested and becomes well-established.
Software development. Currently, CLB is developing an alpha version of its software. CLB is not aware of any existing software service provider that can provide the quantitative spatial data in 3D for select biomarkers that we believe researchers will increasingly seek to better understand the biology of tissue and tumor microenvironments. CLB believes its Tru3D technology platform will enable researchers to analyze 3D tissue samples to See More Biology. To date, CLB has developed a preliminary pre-alpha software that can perform limited 3D cell segmentation, cell classification, and spatial analysis for select biomarkers. However, this software can only be used on small confocal datasets, for a narrow set of biomarkers, in a single tissue type and requires successful cell segmentation for subsequent applications. It also currently does not allow for annotation of biomarkers beyond the limited initial set of biomarkers.
CLB's initial pre-alpha software was developed by a third party. CLB determined that further development would best be done internally, so the software has been transferred to an internal development team. As part of this team, CLB has hired consultants experienced in software development to develop the software further. This development will involve transfer to a CLB-maintained environment on Microsoft's Azure cloud platform, modification of the general design, enhancement of the front-end graphical user interface, additional algorithm development, and training on diverse tissue datasets. Furthermore, the software must be scaled to handle the enormous file sizes associated with 3D tile scan datasets, particularly those generated by light sheet microscopy. These datasets can exceed one terabyte of data per tissue sample.
Competition and Industry Summary
CLB currently provides research services for life science customers, often referred to as contract research organizations (CROs)., CLB is also part of the life science research tools market through its nascent reagents business. Life science research tools is a relatively new market and it is not well-defined. A related market is the tissue diagnostics market. According to ReportLinker.com, the global tissue diagnostics market is expected to increase from $3.4 billion in 2020 to $4.7 billion by 2025 at a CAGR of 6.5%. Major factors driving this growth include rising healthcare spending, increasing incidence of cancer, a growing elderly population and adoption of automated systems. Research laboratories, pharmaceutical companies, and CROs all use tissue diagnostic tools.
Key industry market drivers include the need for anatomic pathology with the growing focus on personalized medicines and the need for tissue diagnostics, such as those for biopsy specimens. Underlying both of these market drivers is the high incidence of cancer, which necessitates the development of infrastructure for cancer diagnosis, increased cancer screening, and the availability of reimbursement for all of these. Other diseases, such as Alzheimer's, are also expected to experience substantial growth due to aging populations in the US and other developed countries.
CLB's competitors mainly consist of other companies that enable spatial visualization of tissue samples and/or provide quantitative data relating to these samples. Quantitative data regarding tissue samples is increasingly important for scientists to advance their understanding of the biology of the tissue and tumor microenvironments. There are a number of life sciences tools companies that represent competition for CLB, including Nanostring, Miltenyi Biotec, 10x Genomics, Akoya, Visikol, Life Canvas and Paige.AI. With the exception of Life Canvas, all of these companies are much larger and have access to much greater resources than does CLB. The research and efficacy of the various life sciences research tools are still in their nascent stages. Only Visikol and Life Canvas combine tissue-clearing services with 3D imaging in a manner similar to CLB's Tru3D technology platform. As mentioned above, CLB is not aware of anyone that provides software services capable of providing quantitative spatial data for selected biomarkers within large 3D datasets. We believe that the furthest advanced competitor in 2D spatial tissue analysis is Akoya. Akoya offers Phenoptics™ - a suite of technologies associated with their IHC (immunohistochemistry) platform, including staining reagents, imaging instruments, and software platforms for deconvolving signals. As demand for tissue diagnostics increases, existing and new competitors may try to offer technology solutions similar to CLB's proposed Tru3D technology platform. However, through active collaborations with researchers and customers, CLB has already expanded CLARITY applications with a variety of tissues and biomarkers. In doing so, we believe we are a CLARITY and 3D imaging leader in the nascent market for 3D tissue imaging. CLB expects this to be a key advantage to the development of clinically relevant technology.
Recently, the FDA cleared the first AI software technology for diagnosis of cancer (prostrate) based on artificial intelligence analysis of 2D slides. We believe this is an important milestone for the development of a clinical market for AI pathology software and supports our plan to develop 3D AI pathology software as a next generation technology.
CLB's future tissue processing platform could penetrate a variety of RUO markets. The biomarker segment is the largest and most relevant RUO market which covers preclinical research, clinical research, drug development, tumor profiling, implanted medical devices, individualized therapy, and therapy monitoring. The biomarker market in the US was estimated at $12 billion in 2020. The total spending by pharma on pre-clinical studies in oncology is not available, but it is estimated at billions of dollars per year. In the area of solid tissue oncology alone, the Total Available Market (TAM) is approximately $8 billion.
Human Therapeutics and Diagnostics Target Strategy
CLB's current business model is that of a contract research organization (CRO). CLB believes this is the best strategy to develop the market for 3D tissue analysis, currently in its infancy. In particular, the Company performs 3D tissue imaging and analysis, thus providing support to the pharmaceutical, biotechnology, and academic research sectors. The CRO market for pre-clinical services has grown as many small and large drug innovators look to outsource non-core functions. CLB provides research services to those customers seeking to outsource on a contract basis. As services are rendered, CLB develops and improves its Tru3D® technology platform for application to a broader set of tissue and disease types. Currently, our lab services are strictly RUO, providing a useful tool for pre-clinical development of new diagnostics, therapeutics, and medical devices. However, our long-term vision is to develop an automated platform to process and analyze tissue. Eventually, as the platform is more fully developed, CLB plans to obtain FDA clearance or approval for this platform so that it can be used for diagnosis of cancer and/or other disease. CLB also believes its Tru3D technology platform could be used to See More Biology of tissue and tumor microenvironments, providing a discovery platform for researchers seeking to discover new drugs and therapies.
License Agreement with Stanford University
In September 2015, we entered into a license agreement with the Board of Trustees of the Leland Stanford Junior University, or Stanford (the "Stanford Agreement"). We amended the Stanford Agreement in January and May 2018 and in August 2020. Under the Stanford Agreement, Stanford granted us a royalty-bearing, sublicensable, worldwide license under Stanford's patent rights related to CLARITY and Stanford's interest in certain materials, to exploit products covered by the licensed patent rights, or licensed products. The license under the licensed patent rights is exclusive for all uses, except that it is non-exclusive with respect to certain of the patent rights in the fields of research reagent and instrument sales, and services, and with respect to the license to materials. Also, the license is subject to Stanford's rights, on behalf of itself and other non-profit research institutions, to practice the licensed patent rights for non-profit uses. The Howard Hughes Medical Institute also has a fully-paid, non-exclusive license to use the licensed patent rights and materials for internal research purposes.
Pursuant to the Stanford Agreement, we must use commercially reasonable efforts to develop, manufacture and sell licensed products, to develop markets for licensed products, and to achieve certain specified milestones by certain dates.
In consideration for the rights granted to us under the Stanford Agreement, we paid Stanford a one-time issue royalty of $25,000 and issued Stanford 175 of our common units (as adjusted). We are required to pay Stanford an annual license maintenance fee of $25,000, which is creditable against earned royalties. We are also obligated to pay Stanford certain one-time milestone payments upon achievement of specified milestones, and royalties on net sales of each licensed product by us and our sublicensees. Further, Stanford is entitled to receive a percentage of the amounts-excluding royalties and certain payments-we receive as a result of the grant of a sublicense under the licensed patent rights. We are also obligated to pay for certain patent expenses with respect to the licensed patent rights. If there is a change of control, we will owe Stanford a change of control fee.
The Stanford Agreement will expire on the expiration of all licensed patent rights. Licensed patent rights are currently expected to expire in 2033, absent any patent term extension or adjustment. We may terminate the Stanford Agreement by providing 30 days written notice to Stanford. Stanford may terminate the Stanford Agreement if (i) we are delinquent on any report or payment, (ii) we are not diligently developing or commercializing licensed products, (iii) we miss one of the specified milestones, (iv) we are in breach of any provision of the Stanford Agreement, or (v) we provide any false report, in each case (i)-(v), if we do not remedy the problem within 30 days after written notice from Stanford.
The patent describes methods and compositions to prepare a biological specimen for 3D microscopic analysis without sectioning into thin 2D slices, maintaining the spatial morphology and microenvironment of the specimen. Coupled with immunohistochemistry (IHC) and in situ hybridization (ISH), the CLARITY methods can enhance many applications in medicine and preclinical research, such as in immuno-oncology, personalized medicine, monitoring disease or graft transplantation, anatomic pathology, and in preclinical drug development. Researchers involved in preclinical drug development can leverage these methods for specific research studies, narrowing studies, combination studies and toxicology and safety studies to advance drug candidates for clinical trial. Also included in the patent are reagents, devices, kits, and systems relating to the CLARITY technology. The patent contains 33 claims and expires in 2033.
This patent, owned by Stanford University in Palo Alto, California, is exclusively licensed to CLB for clinical use.
Legal Matters
The Company is not involved in any litigation, and its management is not aware of any pending or threatened legal actions relating to its intellectual property, conduct of its business activities, or otherwise. Certain legal matters with respect to the shares of Common Stock offered hereby will be passed upon by Karen Muller, Esq.
Employees
At the date of the Offering Circular, CLB has five full-time employees and eight part time independent contractors. CLB has a contract with TriNet Group, Inc. for its employees. TriNet is a professional employer organization that provides CLB with human resource capabilities, including payroll, access to benefits, tax filing, workers' compensation and online access to employees for benefits and payroll information. CLB retains full control of hiring and firing, compensation and benefits offered.
Company's Property
We share lab space at Molecular Medicine Research Institute ("MMRI") in Sunnyvale, CA at 428 Oakmead Parkway, Sunnyvale, CA 94085-4708. The agreement with MMRI automatically renews for the following three-month period on the first day of each month. The rent is variable based on the number of employees. Current rent is $10,500 per month.
DIRECTOR, EXECUTIVE OFFICERS AND SIGNIFICANT EMPLOYEES
Founder and Scientific Advisor
Karl Deisseroth, M.D. Ph.D., Founder and Scientific Advisor, is the D.H. Chen Professor of Bioengineering and of Psychiatry and Behavioral Sciences at Stanford University, and Investigator of the Howard Hughes Medical Institute. He received his undergraduate degree from Harvard, his PhD from Stanford, and his MD from Stanford; he also completed postdoctoral training, medical internship, and adult psychiatry residency at Stanford, and he is board-certified by the American Board of Psychiatry and Neurology. He continues as a practicing psychiatrist at Stanford with specialization in affective disorders and autism-spectrum disease, employing medications along with neural stimulation. Over a period of twelve years, his laboratory created and developed optogenetics, hydrogel- tissue chemistry (beginning with CLARITY), and a broad range of supportive and enabling methods. He also has employed his technologies to discover the neural cell types and connections that cause adaptive and maladaptive behaviors, and he has disseminated the technologies to thousands of laboratories around the world. For his discoveries, Deisseroth has received the NIH Director's Pioneer Award (2005), the Zuelch Prize (2012), the PerlPrize (2012), the BRAIN prize (2013), the Pasarow Prize (2013), the Breakthrough Prize (2015) the BBVA Award (2016), the Massry Prize (2016) and the Harvey Prize from the Technion/Israel (2017), and among other honors, was the sole recipient for optogenetics of the 2010 Koetser Prize, the 2010 Nakasone Prize, the 2011 Alden Spencer Prize, the 2013 Richard Lounsbery Prize, the 2014 Dickson Prize in Science, the 2015 Keio Prize, the 2015 Lurie Prize, the 2015 Albany Prize, the 2015 Dickson Prize in Medicine, the 2017 Redelsheimer Prize, the 2017 Fresenius Prize, the 2017 NOMIS Distinguished Scientist Award, the 2018 Eisenberg Prize, and the 2018 Kyoto Prize. He was selected a Howard Hughes Medical Institute Investigator in 2013, and he was elected to the US National Academy of Medicine in 2010 and to the US National Academy of Sciences in 2012. Dr. Deisseroth remains an active advisor to CLB and participates in weekly Board calls. His insights into the needs of scientists in research labs, such as the Deisseroth Lab, are invaluable to CLB's strategy.
Sole Director and Executive Officer
Albert Hansen. Albert Hansen is the Chief Executive Officer, President, Chief Financial Officer, Secretary and Treasurer of the Company and the sole member of the Board of Directors of the Company. Mr. Hansen is an experienced small company investor and executive with over 30 years' experience in small company investments, management, and investment banking, particularly in pharmaceuticals. He is currently CEO/founder of KESA Partners Inc. which provides seed financing and strategic management services to startups in small drug and medical device-related companies. He was Chairman and CEO of Bioserv Corporation, a KESA portfolio company. He is a director of three other KESA portfolio companies, one of which, Island Pharmaceuticals recently went public in Australia on the ASX. Formerly, as a partner with Signet Healthcare Partners, he was Chairman/Interim CEO of Questcor Pharmaceuticals (eventually sold to Mallinkrodt for $5 billion), Executive Chairman of Cedarburg Pharmaceuticals (later sold to Albany Molecular for over $40 million) and Chairman of Molecular Medicine Bioservices (sold to SAFC for over $20 million.). He was formerly a principal with Darby Overseas (since acquired by Franklin Templeton). He was also Director of Corporate Finance at the U.S. Treasury. He was an investment banker with Dillon Read and E.F. Hutton. He also served as a Special Forces officer in the U.S. Army. He holds an M.B.A. (with distinction) from the Wharton School and an A.B. from Princeton University.
Board of Directors' Compensation
Mr. Hansen serves as a member of the Board with no compensation, and there are no Board committees. Mr. Hansen has received no equity or cash compensation for his services as Chief Executive Officer, President, Chief Financial Officer, Treasurer and Secretary since January 2020. He is a significant shareholder in the Company through his wholly owned company, KESA Partners, Inc. ("KESA"). KESA has participated in several equity financings and has paid the same price/share as other shareholders in these financings.
Significant Employees
Sharla White, Ph.D., Head of Research and Development, leads and oversees Lab Services and the internal R&D evaluation of the automated platform development, image analysis software development, and CLARITY optimization efforts. She earned her B.S. in Chemical Engineering from Washington University, and Ph.D., Pharmacognosy, University of Illinois at Chicago. She completed her postdoctoral training at Stanford University, and previously worked in the Cancer Immunology Department at Genentech.
Amy Lam, Ph.D., Application Scientist, works with the Lab Services customers to address their research and development needs through CLARITY tissue clearing and 3D imaging. Dr. Lam also suggests R&D directions based on customer feedback and projects. She earned her B.S. in Applied Physics from Caltech, and her M.S. and Ph.D. in Biomedical Engineering from Columbia University. She completed her postdoctoral training at Stanford University.
Ahmed Alsinan, Ph.D., Senior Software DL Engineer, works with our software team to develop the deep learning portion of our software currently under development. He earned his B.S. and M.S. in Electrical Engineering from Michigan State University, and his Ph.D. in Electrical and Computer Engineering from Rutgers University. He completed his postdoctoral training in cancer detection, deep learning and medical imaging at the School of Medicine at Stanford University.
EXECUTIVE COMPENSATION
We are an emerging growth Company for purposes of the Commission's executive compensation disclosure rules. In accordance with such rules, we would be required to provide a summary compensation table, as well as limited narrative disclosures regarding executive compensation for our last completed fiscal year. Further, our reporting obligations would extend only to our "named executive officers," who are those individuals serving as our CEO and CFO who would be serving as executive officers at the end of the last completed fiscal year, after our first year of operations. We have no such named executive officers, other than our CEO, and to date, he has received no compensation, so no table is provided.
RESTRICTED STOCK PLAN
The Company has adopted a Restricted Common Stock Award Plan for directors, officers, employees and shareholder and selected consultants and vendors and allocated a total of 200,000 shares of Common Stock for awards under this plan. To date, a total of 69,239 shares have been awarded to employees, consultants and the founder shareholder, Mr. Deisseroth, and none to Mr. Hansen or directors.
RELATED PARTY TRANSACTIONS
Statement of Policy
Our board of directors recognizes the fact that transactions with related parties present a heightened risk of conflicts of interests and/or improper valuation (or the perception thereof). We currently do not anticipate that CLB will qualify for a listing in the over-the-counter markets or an exchange in the near future. In the event our shares are so approved for listing, our board of directors will adopt a written policy on transactions with related parties in conformity with the requirements for issuers having publicly held common stock quoted in the relevant over-the-counter market or exchange.
SECURITY OWNERSHIP OF MANAGEMENT AND CERTAIN SECURITYHOLDERS
The following table sets forth certain information known to us regarding beneficial ownership of our capital stock as of the date of this Offering Circular, for (i) all executive officers and directors as a group and (ii) each person, or group of affiliated persons, known by us to be the beneficial owner of more than five percent (5%) of our capital stock. The percentage of beneficial ownership in the table below is based on 1,134,331 shares of Common Stock deemed to be outstanding as of the date of this Offering Circular.
Name and Address of Beneficial Owner | Title of Class | Amount and Nature of Beneficial Ownership | Amount and Nature of Beneficial Ownership that is Acquirable | Percent of Class |
Wiegers & Co., LLC | Common Stock | 360,120 | 0 | 31.8% |
James Grosfeld Trust | Common Stock | 358,449 | 0 | 31.6% |
KESA Partners, Inc. Albert Hansen, a director of the Company and the sole Executive Officer, owns 100% of total common stock issued to KESA Partners, Inc. | Common Stock | 328,871 | 0 | 29.0% |
DESCRIPTION OF CAPITAL STOCK
Summary of Securities
The following description summarizes certain terms of our capital stock, as in effect upon the completion of this offering. Because it is only a summary, it does not contain all the information that may be important to you. For a complete description of the matters set forth in this section you should refer to our amended and restated certificate of incorporation and bylaws, which are included as exhibits to the Offering Statement of which the Offering Circular is a part, and to the applicable provisions of Delaware law.
Our authorized capital stock consists of 3,000,000 shares of capital stock, with 2,000,000 shares designated as Common Stock, $.0001 par value per share and 1,000,000 designated as Preferred Stock, $.0001 par value per share. There were 1,134,331 shares of Common Stock issued and outstanding and no shares of Preferred Stock issued and outstanding as of the date of this Offering Circular.
Common Stock
Dividend Rights
Since our inception, as a Delaware limited liability company on December 1, 2014, and subsequently, when we converted into a corporation on October 12, 2021, we have not paid dividends on our membership interests in the limited liability company nor the currently issued Common Stock; and we currently expect that, for the foreseeable future, all earnings (if any) will be retained for the development of our business. No dividends are expected to be declared or paid for the foreseeable future. In the longer-term future, the Board of Directors may decide, in their discretion, whether dividends may be declared and paid, taking into consideration, among other things, our earnings (if any), operating results, financial conditions and capital requirements, general business conditions and other pertinent facts.
Voting Rights
Holders of our Common Stock are entitled to one vote per share on any matter to be voted upon by stockholders, however, except as otherwise required by law. We have not provided for cumulative voting for the election of directors in our amended and restated certificate of incorporation. Our bylaws establish a board of directors whose term is for one year, to be elected at the annual meeting of stockholders, by a plurality of the vote.
No Preemptive or Similar Rights
None of our shareholders has preemptive or similar rights with regard to our common stock.
Board Composition and Filling Vacancies
The current number of directors of the Board is one (1). The Board or the shareholders may increase or decrease the size of the Board. A vacancy, however caused, occurring in the Board and any newly created directorship, may be filled by the Board. There is one current member of the Board, Mr. Hansen.
No Cumulative Voting
The Delaware General Corporation Law provides that stockholders are not entitled to the right to cumulate votes in the election of directors unless the certificate of incorporation provides otherwise. Neither our certificate of incorporation nor our bylaws provide for cumulative voting.
Preferred Stock
The 1,000,000 shares of Preferred Stock ("Blank Check Preferred Stock") may be issued from time to time and in one or more series. The Board is authorized to determine or alter the powers, preferences and rights, and the qualifications, limitations and restrictions granted to or imposed upon any wholly unissued series of Blank Check Preferred Stock, and within the limitations or restrictions stated in any resolution or resolutions of the Board originally fixing the number of shares constituting any series of Blank Check Preferred Stock, to increase or decrease (but not below the number of shares of any such series of Preferred Stock then outstanding) the number of shares of any such series of Blank Check Preferred Stock, and to fix the number of shares of any series of Blank Check Preferred Stock. In the event that the number of shares of any series of Blank Check Preferred Stock shall be so decreased, the shares constituting such decrease shall resume the status which such shares had prior to the adoption of the resolution originally fixing the number of shares of such series of Blank Check Preferred Stock subject to the requirements of applicable law.
Director Liability and Indemnification of Directors and Officers
Our bylaws provide that every person who was or is a party to, or is threatened to be made a party to, or is involved in any action, hearing or suit, of any kind whether civil, administrative or criminal, by reason of the fact that he/she or a person of whom he/she is the legal representative is or was a director or officer of the Company or is or was serving at the request of the Company or for its benefit as a director or officer of another corporation, or as a representative in an enterprise of any kind, shall be indemnified and held harmless to the fullest extent legally permissible under the General Corporation Law of the State of Delaware. This indemnification shall include all expenses, liability, and loss (including attorneys' fees, judgments, fines, and amounts paid or to be paid in settlement) reasonably incurred or suffered by him in connection therewith. The expenses of our officers and directors incurred in defending a civil or criminal action, suit, or proceeding must be paid by the Company as they are incurred and in advance of the final disposition of the action, suit, or proceeding upon receipt of an undertaking by or on behalf of the director or officer to repay the amount if it is ultimately determined by a court of competent jurisdiction that he or she is not entitled to be indemnified by the Company. Such right of indemnification shall be a contract right which may be enforced in any manner desired by such person. Such right of indemnification shall not be exclusive of any other right which our directors, officers, or representatives may have or hereafter acquire.
The Board may direct the Company to purchase and maintain insurance on behalf of any person who is or was a director or officer of the Company. Or on behalf of any person who is or was serving at the request of the Company as a director or officer of the Company, or is or was serving at the request of the Company as a director or officer of another corporation, or as its representative in a partnership, joint venture, trust, or other enterprise against any liability asserted against such person and incurred in any such capacity or arising out of such status, whether or not the Company would have the power to indemnify such person. We intend to purchase general liability insurance that covers certain liabilities of our directors and officers arising out of claims based on acts or omissions in their capacities as directors or officers, including liabilities under the Securities Act.
These provisions may discourage stockholders from bringing a lawsuit against our directors for breach of their fiduciary duty. These provisions may also have the effect of reducing the likelihood of derivative litigation against directors and officers, even though such an action, if successful, might otherwise benefit us and our stockholders. Furthermore, a stockholder's investment may be adversely affected to the extent we pay the costs of settlement and damage awards against directors and officers pursuant to these indemnification provisions. We believe that these indemnification provisions, and the insurance are necessary to attract and retain talented and experienced directors and officers. At present, there is no pending litigation or proceeding involving any of our directors or officers where indemnification will be required or permitted. We are not aware of any threatened litigation or proceeding that might result in a claim for such indemnification.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers, or persons who control us, we have been informed that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable.
Exchange or Over-the-Counter Listing
There is no current market for any of our shares of stock and a market may not develop. We hope to eventually apply to trade our Common Stock on the over-the-counter market to the extent any demand exists. Even if quoted on the OTCQB, OTCQX or the Pink Sheets, a liquid trading market may not develop. Investors should assume that they may not be able to liquidate their investment for some time, or they may not be able to pledge their shares as collateral. Even if traded, we may be subject to the penny stock rules and may not be able to have our stock accepted by many brokerage firms as a result. The Company intends to apply to trade its shares on the Pink Sheets following the Offering, but there is no assurance that it will be able to accomplish this goal.
Transfer Agent and Registrar
The Company has engaged CLEARTRUST, LLC, a limited liability company registered in the State of Florida and a registered transfer agent with the SEC, to act as transfer agent and registrar for the Common Stock. All shares of common stock will be uncertificated and maintained on a ledger by the transfer agent.
DIVIDEND POLICY
Since our inception, as a Delaware limited liability company on December 1, 2014, and subsequently, when we converted into a corporation on October 12, 2021, we have not paid dividends on our membership interests in the limited liability company nor the currently issued Common Stock; and we currently expect that, for the foreseeable future, all earnings (if any) will be retained for the development of our business. No dividends are expected to be declared or paid for the foreseeable future. In the longer-term future, the Board of Directors may decide, in their discretion, whether dividends may be declared and paid, taking into consideration, among other things, our earnings (if any), operating results, financial conditions and capital requirements, general business conditions and other pertinent facts.
SHARES ELIGIBLE FOR FUTURE SALE
Prior to this offering, there has been no market for our Common Stock. A market for our Common Stock may not develop. Future sales of substantial amounts of our Common Stock, or securities or instruments convertible into our Common Stock, in the public market, or the perception that such sales may occur, could adversely affect the market price of our Common Stock if our stock is publicly traded.. Furthermore, because there will be limits on the number of shares available for resale shortly after this offering concludes, due to contractual and legal restrictions described below, there may be resale of substantial amounts of our Common Stock in the public market after those restrictions lapse. This could adversely affect the market price of our Common Stock prevailing at that time.
Upon completion of this offering, assuming the maximum number of shares of Common Stock offered in this offering are sold, there will be 1,154,331 shares of our Common Stock outstanding. We have no warrants, options or profit interests outstanding.
20,000 shares of our Common Stock will be freely tradable in the public market, except to the extent they are acquired by an "affiliate" of ours; as such term is defined in Rule 405 under the Securities Act. Note, however that there is no current market for any of our shares of stock and a market may not develop. We hope to eventually apply to trade our Common Stock on the over-the-counter market to the extent any demand exists, but there is no assurance that the Company will accomplish this goal.
Under Rule 405, an affiliate of a specified person is a person that directly, or indirectly through one or more intermediaries, controls or is controlled by, or is under common control with, the specified person. Any affiliate of ours that acquires our shares can only further transact in such shares in compliance with Rule 144 under the Securities Act, which imposes sales volume limitations and other restrictions on such further transactions. See "Rule 144," below.
In addition to the foregoing, shares of our Common Stock not sold in this offering will be restricted securities within the meaning of Rule 144, and would be tradable only if they are sold pursuant to a registration statement under the Securities Act or if they qualify for an exemption from registration, including under Rule 144. See "Rule 144," below.
Rule 144
In general, a person who has beneficially owned restricted shares of our Common Stock for at least one year, in the event we are a reporting Company under Regulation A, or at least six months, in the event we have been a reporting Company under the Exchange Act for at least 90 days, would be entitled to sell such securities, provided that such person is not deemed to be an affiliate of ours at the time of sale or to have been an affiliate of ours at any time during the three months preceding the sale. A person who is an affiliate of ours at such time would be subject to additional restrictions, by which such person would be entitled to sell within any three month period only a number of shares that does not exceed the greater of the following:
● | 1% of the number of shares of our Common Stock then outstanding; or |
| | |
● | The average weekly trading volume of our Common Stock during the four calendar weeks preceding the filing by such person with the SEC of a notice on Form 144 with respect to the sale; provided that, in each case, we have been subject to the periodic reporting requirements of the Exchange Act for at least 90 days before the sale. Persons relying on Rule 144 to transact in our Common Stock must also comply with the manner of sale, notice and other provisions of Rule 144, to the extent applicable. In general, Rule 701 allows a stockholder who purchased shares of our capital stock pursuant to a written compensatory plan or contract and who is not deemed to have been an affiliate of ours during the immediately preceding 90 days to sell those shares in reliance upon Rule 144, but without being required to comply with the public information, holding period, volume limitation or notice provisions of Rule 144. Persons relying on Rule 701 to transact in our Common Stock, however, are required to wait until 90 days after the date of the Offering Circular before selling shares pursuant to Rule 701. |
PLAN OF DISTRIBUTION
The Company is offering up to 20,000 shares of Common Stock without a broker-dealer to assist in the placement of its securities. KESA Partners, Inc. has agreed to purchase any shares (up to 20,000) that are not sold to third parties.
Investment Fee on Sales of Securities
There are no investment fees in connection with purchases of securities under this offering.
Termination of Offering
The offering will terminate at the earlier of (1) the date at which the maximum offering amount has been sold, (2) the date that is twelve months from the date of the Offering Statement being qualified by the Commission, or (3) the date at which the Offering is earlier terminated by the Company in its sole discretion, which may happen at any time (the "Offering Termination Date").
Selling Security holders
No securities are being sold for the account of security holders; all net proceeds of this offering will go to the Company.
Investors' Tender of Funds
After the Offering Statement has been qualified by the Commission, the Company will accept tenders of funds to purchase the Common Stock. The Company may close on investments on a "rolling" basis (so not all investors will receive their shares on the same date). The funds tendered by potential investors will go directly to the Company, and the Company will issue shares thereafter, in its discretion. However, the offering will remain open and investor subscriptions will be accepted throughout the entire offering and will not be halted when funds are transferred to the Company or the Company closes on investments during the offering. Upon closing, funds tendered by investors will be made available to the Company for its use. The offering will terminate (and subscriptions will no longer be accepted) at the earlier of: (1) the date at which the maximum offering amount has been sold, (2) the date which is one year from this offering being qualified by the Commission, or (3) the date at which the offering is earlier terminated by us in our sole discretion.
Investors will be required to subscribe to offering by agreeing to the terms of the subscription agreement.
Offering Expenses
We are responsible for all offering fees and expenses, including the following: (i) fees and disbursements of our legal counsel, accountants, and other professionals we engage; (ii) fees and expenses incurred in the production of offering documents, including design, printing, photograph, and written material procurement costs; (iii) all filing fees, including blue sky notice filing fees; and (iv) all of the legal fees related to the filing of notice filings under state securities laws.
Investment Limitations
The Offering is limited to accredited investors, as defined under Rule 501 of Regulation D under the Securities Act (an "Accredited Investor"). If you meet one of the following tests you should qualify as an Accredited Investor:
● | You are a natural person who had individual income in excess of $200,000 in each of the two most recent years, or joint income with your spouse or spousal equivalent in excess of $300,000 in each of those years, and have a reasonable expectation of reaching the same income level in the current year; | |
| | | |
● | You are a natural person and your individual net worth, or joint net worth with your spouse or spousal equivalent, exceeds $1,000,000 at the time you purchase offered shares (please see below on how to calculate your net worth); |
| | |
● | You are a director, executive officer, or general partner of the issuer of the securities being offered or sold, or you are a director, executive officer, or general partner of a general partner of that issuer; |
| | |
● | You are an organization described in Section 501(c)(3) of the Internal Revenue Code of 1986, as amended, a corporation, a Massachusetts or similar business trust, partnership, or limited liability company, not formed for the specific purpose of acquiring the offered shares, with total assets in excess of $5,000,000; |
| | |
● | You are a bank or a savings and loan association or other institution as defined in the Securities Exchange Act of 1934 (the "Securities Act") whether acting in its individual or fiduciary capacity; a broker or dealer registered pursuant to Section 15 of the Securities Act; an investment adviser registered pursuant to Section 203 of the Investment Advisers Act of 1940 (the "Investment Advisers Act") or registered pursuant to the laws of the state, or an investment adviser relying on the exemption provisions of the Investment Advisers Act; an insurance Company as defined by the Securities Act; an investment Company registered under the Investment Advisers Act, or a business development company as defined in that act; a Small Business Investment Company licensed by the Small Business Investment Act of 1958; a Rural Business Investment Company under the Consolidated Farm and Rural Development Act; a plan established and maintained by a state, its political subdivisions, or any agency or instrumentality of a state or its political subdivisions, for the benefit of its employees, if such plan has total assets in excess of $5,000,000; an employee benefit plan within the meaning of the Employee Retirement Income Security Act of 1974 if the investment decision is made by a plan fiduciary, as defined in section 3(21) of such act, which is either a bank, savings and loan association, insurance company, or registered investment adviser, or if the employee benefit plan has total assets in excess of $5,000,000 or, if a self-directed plan, with investment decisions made solely by persons that are accredited investors; |
| | |
● | You are a private business development Company as defined in the Investment Advisers Act; |
| | |
● | You are an entity (including an Individual Retirement Account trust) in which each equity owner is an accredited investor; |
| | |
● | You are a trust with total assets in excess of $5,000,000, and your purchase of offered shares is directed by a person who either alone or with his purchaser representative(s) (as defined in Regulation D promulgated under the Securities Act) has such knowledge and experience in financial and business matters that he is capable of evaluating the merits and risks of the prospective investment, and you were not formed for the specific purpose of investing in the offered shares; |
| | |
● | You are an entity, of a type not listed above, which is not formed for the specific purpose of acquiring the securities offered, owning investments in excess of $5,000,000; |
| | |
● | You are a natural person holding in good standing one or more professional certifications or designations or credentials from an accredited educational institution that the Commission has designated as qualifying an individual for accredited investor status; |
| | |
● | You are a natural person who is a "knowledgeable employee," as defined under the Investment Advisers Act, of the issuer of the securities being offered or sold where the issuer would be an investment company, as defined in section 3 of such act, but for the exclusion provided by either section 3(c)(1) or section 3(c)(7) of such act; |
● | You are a "family office," as defined under the Investment Advisers Act with assets under management in excess of $5,000,000, that is not formed for the specific purpose of acquiring the securities offered, and whose prospective investment is directed by a person who has such knowledge and experience in financial and business matters that such family office is capable of evaluating the merits and risks of the prospective investment; or |
| | |
● | You are a "family client," as defined under the Investment Advisers Act, of a family office meeting the requirements set forth above, and whose prospective investment in the issuer is directed by such family office who has such knowledge and experience in financial and business matters that such family office is capable of evaluating the merits and risks of the prospective investment. |
In addition, investments will only be accepted from residents of the following states: California, Colorado, Michigan, Nevada, New York, Florida, and Texas.
Procedures for Subscribing
If you decide to subscribe for Shares, you should, contact Albert Hansen, the Chief Executive Officer and Chief Financial Officer, at CEO@clearlightdx.com. Mr. Hansen will coordinate the delivery and execution of a subscription agreement electronically and provide the wire or electronic funds transfer information for the payment of the subscription. Any potential investor will have ample time to review the subscription agreement, along with their counsel, prior to making any final investment decision. We shall only deliver such subscription agreement upon request after a potential investor has had ample opportunity to review the Offering Circular.
Right to Reject Subscriptions
After we receive your complete, executed subscription agreement and the funds required under the subscription agreement have been transferred to the Company's account, we have the right to review and accept or reject your subscription in whole or in part, for any reason or for no reason. We will return all monies from rejected subscriptions immediately to you, without interest or deduction.
Acceptance of Subscriptions
Upon our acceptance of a subscription agreement, we will countersign the subscription agreement and issue the uncertificated shares subscribed at closing. Once you submit the subscription agreement and it is accepted, you may not revoke or change your subscription or request your subscription funds. All accepted subscription agreements are irrevocable.
For the purposes of calculating your net worth, it is defined as the difference between total assets and total liabilities. This calculation must exclude the value of your primary residence and may exclude any indebtedness secured by your primary residence (up to an amount equal to the value of your primary residence). In the case of fiduciary accounts, net worth and/or income suitability requirements may be satisfied by the beneficiary of the account or by the fiduciary, if the fiduciary directly or indirectly provides funds for the purchase of the shares.
WHERE YOU CAN FIND ADDITIONAL INFORMATION
We have filed with the SEC a Regulation A Offering Statement on Form 1-A under the Securities Act with respect to the shares of Common Stock offered hereby. This Offering Circular, which constitutes a part of the Offering Statement, does not contain all of the information set forth in the Offering Statement or the exhibits and schedules filed therewith. For further information about us and the Common Stock offered hereby, we refer you to the Offering Statement and the exhibits and schedules filed therewith. Statements contained in the Offering Circular regarding the contents of any contract or other document that is filed as an exhibit to the Offering Statement are not necessarily complete, and each such statement is qualified in all respects by reference to the full text of such contract or other document filed as an exhibit to the Offering Statement. Upon the completion of this offering, we will be required to file ongoing reports with the SEC pursuant to Regulation A. You may read and copy this information at the SEC's Public Reference Room, 100 F Street, N.E., Room 1580, Washington, D.C. 20549. You may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330. The SEC also
maintains an Internet website that contains reports, proxy statements and other information about issuers, including us, that file electronically with the SEC. The address of this site is www.sec.gov.
INDEX TO FINANCIAL STATEMENTS (UNAUDITED)
CLEARLIGHT BIOTECHNOLOGIES, INC.
CONSOLIDATED BALANCE SHEETS
(unaudited)
| | | June 30, | | | December 31, | | | December 31, | |
| | | 2021 | | | 2020 | | | 2019 | |
| Assets | | | | | | | | | |
| Current assets: | | | | | | | | | |
| Cash and cash equivalents | $ | 718,333 | | $ | 325,049 | | $ | 16,631 | |
| Accounts receivable, net | | 17,595 | | | 1,595 | | | 2,550 | |
| Inventory | | 4,392 | | | 5,924 | | | - | |
| Prepaid expenses and other current assets | | 37,295 | | | 28,509 | | | 27,338 | |
| Total current assets | | 777,615 | | | 361,077 | | | 46,519 | |
| Property and equipment, net | | 818,271 | | | 842,627 | | | 228,932 | |
| Intangible assets, net | | - | | | - | | | 2,691 | |
| Other assets | | 476,702 | | | 483,967 | | | 498,203 | |
| Total assets | $ | 2,072,588 | | $ | 1,687,671 | | $ | 776,345 | |
| Liabilities and Stockholders' Equity | | | | | | | | | |
| Current liabilities: | | | | | | | | | |
| Accounts payable | $ | 438,009 | | $ | 744,124 | | $ | 158,646 | |
| Notes and other debt payable | | 22,382 | | | 313,624 | | | 27,900 | |
| Accrued expenses and other current liabilities | | 39,690 | | | 27,658 | | | 44,303 | |
| Total current liabilities | | 500,080 | | | 1,085,406 | | | 230,849 | |
| Stockholders' equity: | | | | | | | | | |
| Additional paid in capital | | 13,993,363 | | | 12,342,659 | | | 10,857,870 | |
| Accumulated deficit | | (12,420,855 | ) | | (11,740,394 | ) | | (10,312,374 | ) |
| Total stockholders' equity | | 1,572,508 | | | 602,265 | | | 545,496 | |
| Total liabilities and stockholders' equity | $ | 2,072,588 | | $ | 1,687,671 | | $ | 776,345 | |
See accompanying notes to the unaudited historical financial statements.
CLEARLIGHT BIOTECHNOLOGIES, INC.
CONSOLIDATED STATEMENTS OF OPERATIONS
(unaudited)
| | | Six months ended | | | | Twelve months ended | |
| | | June 30, | | | | December 31, | |
| | | 2021 | | | | 2020 | | | | 2020 | | | | 2019 | |
| Income | | | | | | | | | | | | | | | |
| Service and other revenue | $ | 29,874 | | | $ | 9,000 | | | $ | 33,797 | | | $ | 7,650 | |
| Total income | | 29,874 | | | | 9,000 | | | | 33,797 | | | | 7,650 | |
| Cost of Revenue | | 157,760 | | | | 137,009 | | | | 299,381 | | | | 164,747 | |
| Gross Profit | | (127,886 | ) | | | (128,009 | ) | | | (265,583 | ) | | | (157,097 | ) |
| Expenses | | | | | | | | | | | | | | | |
| Selling, general and administrative | | 430,994 | | | | 453,595 | | | | 896,218 | | | | 1,238,195 | |
| Research and development | | 184,021 | | | | 75,781 | | | | 219,848 | | | | 733,727 | |
| Depreciation and amortization | | 19,442 | | | | 16,582 | | | | 41,853 | | | | 44,173 | |
| Total expenses | | 634,457 | | | | 545,958 | | | | 1,157,918 | | | | 2,016,095 | |
| Net Operating Loss | | (762,343 | ) | | | (673,967 | ) | | | (1,423,502 | ) | | | (2,173,191 | ) |
| Interest expense, net | | 2,160 | | | | 2,337 | | | | 4,518 | | | | 1,070 | |
| Other Income | | 84,042 | | | | - | | | | - | | | | - | |
| Net Loss | $ | (680,461 | ) | | $ | (676,304 | ) | | $ | (1,428,020 | ) | | $ | (2,174,261 | ) |
| Net Loss per share attributable to common stockholders: | | | | | | | | | | | | | | | |
| Basic and Diluted EPS | $ | (0.73 | ) | | $ | (39.44 | ) | | $ | (9.48 | ) | | $ | (429.87 | ) |
| Weighted average shares | | | | | | | | | | | | | | | |
| Basic | | 931,426 | | | | 17,147 | | | | 150,593 | | | | 5,058 | |
| Diluted | | 931,435 | | | | 17,550 | | | | 150,856 | | | | 5,947 | |
See accompanying notes to the unaudited historical financial statements.
CLEARLIGHT BIOTECHNOLOGIES, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
(unaudited)
| | | Six Months Ended | | | Twelve Months ended | |
| | | June 30, | | | December 31, | |
| | | 2021 | | | 2020 | | | 2020 | | | 2019 | |
| | | | | | | | | | | | | |
| CASH FLOWS FROM OPERATING ACTIVITIES: | | | | | | | | | | | | |
| Net loss | $ | (680,461 | ) | $ | (676,304 | ) | $ | (1,428,020 | ) | $ | (2,174,261 | ) |
| Adjustments to reconcile net loss to net cash | | | | | | | | | | | | |
| used in operating activities: | | | | | | | | | | | | |
| Depreciation and amortization | | 31,620 | | | 24,747 | | | 66,778 | | | 68,788 | |
| Stock-based compensation expense | | 692 | | | 6,375 | | | 34,790 | | | 211,060 | |
| Changes in assets and liabilities | | | | | | | | | | | | |
| Accounts receivable | | (16,000 | ) | | (5,950 | ) | | 955 | | | (2,550 | ) |
| Inventory | | 1,532 | | | - | | | (5,924 | ) | | - | |
| Prepaid expenses and other current assets | | (8,786 | ) | | (11,635 | ) | | (1,171 | ) | | (8,254 | ) |
| Accounts payable | | (306,115 | ) | | (47,958 | ) | | 585,478 | | | 106,882 | |
| Accrued expenses and other liabilities | | 11,913 | | | (15,156 | ) | | (16,033 | ) | | (6,751 | ) |
| Net cash used in operating activities | | (965,605 | ) | | (725,881 | ) | | (763,147 | ) | | (1,805,086 | ) |
| CASH FLOWS FROM INVESTING ACTIVITIES: | | | | | | | | | | | | |
| Purchases of property and equipment | | - | | | - | | | (663,546 | ) | | - | |
| Other investing activities | | - | | | - | | | - | | | 71,195 | |
| Net cash used in investing activities | | - | | | - | | | (663,546 | ) | | 71,195 | |
| CASH FLOWS FROM FINANCING ACTIVITIES: | | | | | | | | | | | | |
| Notes Payable | | (291,123 | ) | | 63,465 | | | 285,111 | | | (1,118,124 | ) |
| Paid-In-Capital | | 1,650,012 | | | 750,000 | | | 1,449,999 | | | 1,500,000 | |
| Net cash provided by financing activities | | 1,358,889 | | | 813,465 | | | 1,735,111 | | | 381,876 | |
| Net increase in cash and cash equivalents | | 393,284 | | | 87,583 | | | 308,418 | | | (1,352,015 | ) |
| Cash and cash equivalents, beginning of period | | 325,049 | | | - | | | 16,631 | | | 1,368,646 | |
| Cash and cash equivalents, end of period | $ | 718,333 | | $ | 87,583 | | $ | 325,049 | | $ | 16,631 | |
See accompanying notes to the unaudited historical financial statements.
CLEARLIGHT BIOTECHNOLOGIES, INC.
CONSOLIDATED STATEMENTS OF STOCKHOLDER'S DEFICIT
THROUGH June 30, 2021
(unaudited)
| ($ in thousands, except per share amounts) | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | Total | |
| | | Preferred Units | | Common Units | | | Common Stock | | | Accumulated | | | Shareholders | |
| | | Units | | | $/Unit | | | Amount | | Units | | | $/Unit | | | Amount | | | Units | | | $/Unit | | | Amount | | | Deficit | | | Equity | |
| Outstanding at December 31, 2018 | | 3,594 | | | | | | 9,092 | | 1,464 | | | | | | 0 | | | | | | | | | | | | (8,084 | ) | | 1,009 | |
| Issuance of preferred units | | 500 | | | 1.50 | | | 1,500 | | | | | | | | | | | | | | | | | | | | | | | 1,500 | |
| Net Loss | | | | | | | | | | | | | | | | | | | | | | | | | | | | (2,174 | ) | | (2,174 | ) |
| Stock-based compensation | | | | | | | | | | | | | | | | | | | | | | | | | | | | 211 | | | 211 | |
| Outstanding at December 31, 2019 | | 4,094 | | | | | | 10,592 | | 1,464 | | | | | | 0 | | | - | | | | | | - | | | (10,047 | ) | | 545 | |
| Issuance of common units | | | | | | | | | | 18,750 | | | 40.00 | | | 750 | | | | | | | | | | | | | | | 750 | |
Issuance of additional Preferred Units to Preferred Unitholders as result of anti-dilution provision triggered by January 2020 common issuance | | 12,008 | | | - | | | - | | | | | | | | | | | | | | | | | | | | | | | - | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Conversion of preferred units to common units | | (16,102 | ) | | 637.63 | | | (10,267 | | 16,102 | | | 637.63 | | | 10,267 | | | | | | | | | | | | | | | - | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Conversion of common units in ClearLight Biotechnologies LLC to common stock in ClearLight Biotechnologies Inc. | | | | | | | | | | (36,316 | ) | | (677.63 | ) | | (11,018 | ) | | 36,316 | | | 1.02 | | | 11,018 | | | | | | - | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Issuance of common stock | | | | | | | | | | | | | | | | | | | 686,274 | | | 1.02 | | | 700 | | | | | | 700 | |
| Net Loss | | | | | | | | | | | | | | | | | | | | | | | | | | | | (1,428 | ) | | (1,428 | ) |
| Stock-based compensation | | | | | | | | | | | | | | | | | | | | | | | | | | | | 35 | | | 35 | |
| Outstanding at December 31, 2020 | | - | | | | | | 325 | | - | | | | | | - | | | 722,590 | | | | | | 11,718 | | | (11,440 | ) | | 602 | |
| Issuance of restricted stock | | | | | | | | | | | | | | | | | | | 69,239 | | | 0.01 | | | 1 | | | | | | 1 | |
| Issuance of common stock | | | | | | | | | | | | | | | | | | | 162,501 | | | 4.00 | | | 650 | | | | | | 650 | |
| Issuance of common stock | | | | | | | | | | | | | | | | | | | 125,001 | | | 8.00 | | | 1,000 | | | | | | 1,000 | |
| Net Loss | | | | | | | | | | | | | | | | | | | | | | | | | | | | (680 | ) | | (680 | ) |
| Outstanding at June 30, 2021 | | - | | | | | | 325 | | - | | | | | | - | | | 1,079,331 | | | | | | 13,368 | | | (12,121 | ) | | 1,573 | |
See accompanying notes to the unaudited historical financial statements.
NOTE 1 - ORGANIZATION AND GOING CONCERN
ClearLight Biotechnologies, LLC ("CLB" or the "Company") was established as a Delaware limited liability company in December 2014 under the original name Clarity Solutions, LLC. It changed its name to ClearLight Diagnostics LLC in July 2015 and changed to its current name in October 2018. The Company converted from a Delaware limited liability company to a Delaware corporation on October 12, 2020. The financial statements presented reflect partnership accounting for the period ended December 31, 2019, and corporation accounting for the period ended December 31, 2020. Management believes the income statements for 2019 and 2020 would not be materially different under partnership or corporation accounting.
CLB is an early-stage life sciences tools company. CLB provides tissue processing, imaging, and image analysis services to pharmaceutical, biotechnology and academic researchers. CLB utilizes the patent-protected CLARITY technology, licensed from Stanford University, Palo Alto, CA. The CLARITY technology allows clearing of animal and human tissue so that thick tissue samples can be imaged in three dimensions ("3D") with a confocal or light-sheet microscope and then analyzed by a lab scientist in a 3D dataset. The Company is currently developing separate software enabled by artificial intelligence. This software is intended to analyze the large datasets associated with 3D imaging and provide researchers quantitative spatial data. We believe the images and analysis will allow researchers to See More Biology™ enabling better understanding of the tissue and the tumor microenvironments to facilitate discovery of new methods and drugs to diagnose and treat disease.
Continuing losses and going concern
The Company has incurred significant losses since its inception. The Company expects to continue to incur losses in the next few years. The Company will require significant additional financing to continue its operations. There can be no assurance that such financing can be arranged on acceptable terms, if at all.
The accompanying financial statements have been prepared assuming the Company will continue as a going concern, which contemplates the realization of assets and satisfaction of liabilities in the ordinary course of business. The financial statements do not include any adjustments relating to the recoverability and classification of recorded asset amounts or the amounts and classification of liabilities that would be required if the Company is unable to continue as a going concern.
NOTE 2 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of Accounting
The accounting and reporting policies of the Company conform to accounting principles generally accepted in the United States of America (GAAP). The combined financial statements have been retroactively adjusted to apply the conversion from a limited liability company to a corporation effective at the beginning of the period presented. Therefore, the combined statement of stockholders' equity reflects the activity and balances as though the Company was a corporation for the periods presented.
CLB has adopted the calendar year as its basis of reporting.
Cash and Cash Equivalents
For purposes of the balance sheet and statement of cash flows, the Company considers all highly liquid instruments with maturity of three months or less at the time of issuance to be cash equivalents.
Accounts Receivable and Allowance for Doubtful Accounts
Accounts receivable are carried at their estimated collectible amounts and are periodically evaluated for collectability based on past credit history with clients and other factors. Provisions for losses on accounts receivable are determined on the basis of loss experience, known and inherent risk in the account balance, and current economic conditions. As of June 30, 2021, December 31, 2020 and 2019, accounts receivable amounted to $17,595, $1,595 and $2,550, respectively. There were no associated allowances for doubtful accounts established as of June 30, 2021, December 31, 2020 or 2019.
Property and Equipment
Property and equipment are recorded at cost. Depreciation is recorded for property and equipment using the straight-line method over the estimated useful lives of assets. The Company reviews the recoverability of all long-lived assets, including the related useful lives, whenever events or changes in circumstances indicate that the carrying amount of a long-lived asset might not be recoverable. As of June 30, 2021, December 31, 2020 and 2019, net property and equipment amounted to $818,271, $842,627 and $228,932, respectively.
Fair Value of Financial Instruments
Financial Accounting Standards Board ("FASB") guidance specifies a hierarchy of valuation techniques based on whether the inputs to those valuation techniques are observable or unobservable. Observable inputs reflect market data obtained from independent sources, while unobservable inputs reflect market assumptions. The hierarchy gives the highest priority to unadjusted quoted prices in active markets for identical assets or liabilities (Level 1 measurement) and the lowest priority to unobservable inputs (Level 3 measurement). The three levels of the fair value hierarchy are as follows:
Level 1 - Unadjusted quoted prices in active markets for identical assets or liabilities that the reporting entity has the ability to access at the measurement date. Level 1 primarily consists of financial instruments whose value is based on quoted market prices such as exchange-traded instruments and listed equities.
Level 2 - Inputs other than quoted prices included within Level 1 that are observable for the asset or liability, either directly or indirectly (e.g., quoted prices of similar assets or liabilities in active markets, or quoted prices for identical or similar assets or liabilities in markets that are not active).
Level 3 - Unobservable inputs for the asset or liability. Financial instruments are considered Level 3 when their fair values are determined using pricing models, discounted cash flows or similar techniques and at least one significant model assumption or input is unobservable.
The carrying amounts reported in the balance sheets approximate their fair value.
Convertible Instruments
U.S. GAAP requires companies to bifurcate conversion options from their host instruments and account for them as free standing derivative financial instruments according to certain criteria. The criteria include circumstances in which (a) the economic characteristics and risks of the embedded derivative instrument are not clearly and closely related to the economic characteristics and risks of the host contract, (b) the hybrid instrument that embodies both the embedded derivative instrument and the host contract is not re-measured at fair value under otherwise applicable generally accepted accounting principles with changes in fair value reported in earnings as they occur and (c) a separate instrument with the same terms as the embedded derivative instrument would be considered a derivative instrument.
When the Company has determined that the embedded conversion options should not be bifurcated from their host instruments, the Company records, when necessary, discounts to convertible instruments for the intrinsic value of conversion options embedded in their host instruments based upon the differences between the fair value of the underlying common stock at the commitment date of the instrument transaction and the effective conversion price embedded in the host instrument. Discounts under these arrangements are amortized over the term of the related instrument to their stated date of redemption.
Revenue Recognition
The Company recognizes revenue when: (1) persuasive evidence exists of an arrangement with the customer reflecting the terms and conditions under which products or services will be provided; (2) delivery has occurred, or services have been provided; (3) the fee is fixed or determinable; and (4) collection is reasonably assured. Revenues recognized for the six months ended June 30, 2021 and the years ended December 31, 2020 and 2019 amounted to $29,874, $33,797 and $7,650, respectively.
Research and Development Expenditures
Research and development costs are expensed in the period incurred. Research and development costs primarily consist of salaries and related expenses for personnel, research and development supplies and equipment, consulting, legal fees related to intellectual property, and outsourced engineering.
Software
Costs associated with maintaining software program are recognized as an expense as incurred. Development costs that are directly attributable to the design and testing of identifiable and unique software products are recognized as intangible assets when the following criteria are met: (a) it is technically feasible to complete the software so that it will be available for use, (b) management intends to complete the software and use or sell it, (c) there is an ability to use or sell the software, (d) it can be demonstrated how the software will generate probable future economic benefits, (e) adequate technical, financial and other resources to complete the development and to use or sell the software are available, and (f) the expenditure attributable to the software during its development can be reliably measured. Directly attributable costs that are capitalized as part of the software may include direct costs for software engineering and an appropriate portion of relevant overheads. Capitalized development costs are recorded as intangible assets and amortized from the point at which the asset is ready for use. Since software under development at the Company is still subject to significant technical and commercial uncertainties, management has elected to expense all software development costs in 2019, 2020 and 2021.
Other Income (Expense)
Included in other income (expense) is interest income which consists of interest earned on cash and cash equivalents.
Income Taxes
The Company was treated as a partnership for income tax purposes in 2019. For 2020, the Company was treated as a partnership for tax purposes up to October 12, 2020. All the Company's income or losses were passed through to its members in accordance with the operating agreement then in effect. Therefore, no provision or liability for income taxes has been included in the financial statements.
The Company converted to a (subchapter) C corporation on October 12, 2020. Losses were incurred for the reporting period from October 12, 2020 to June 30, 2021, therefore no income tax was due.
The Company complies with ASC 740 for accounting for uncertainty in income taxes recognized in a company's financial statements, which prescribes a recognition threshold and measurement process for financial statement recognition and measurement of a tax position taken or expected to be taken in a tax return. For those benefits to be recognized, a tax position must be more-likely-than-not to be sustained upon examination by taxing authorities. ASC 740 also provides guidance on derecognition, classification, interest and penalties, accounting in interim periods, disclosure and transition. Based on the Company's evaluation, it has been concluded that there are no significant uncertain tax positions requiring recognition in the Company's financial statements. The Company believes that its income tax positions would be sustained on audit and does not anticipate any adjustments that would result in a material change to its financial position.
The Company may in the future become subject to federal, state and local income taxation though it has not been since its inception. The Company is not presently subject to any income tax audit in any taxing jurisdiction.
NOTE 3 - LICENSED TECHNOLOGY
Effective September 1, 2015, the Company licensed certain technology from Stanford University covering inventions relating to methods to image brain and other tissues for use both in research and in the clinic (the "Stanford Agreement"). The technology is owned by Stanford and includes US Patent 10,545,075 issued on January 28, 2020.
As part of the consideration for the Stanford Agreement, Stanford and co-inventors received equity interests which currently constitute less than 1% of outstanding shares. The Agreement states that Stanford University is entitled to receive yearly maintenance fees, milestone payments, and royalties. The amounts paid in 2020 and 2019 were $100,556 and $42,399, respectively.
Stanford University is entitled to the earned royalties as a percentage of net sales ranging from 1.5% - 4%, depending on the product or service sold.
Amortization
Amortization of this technology is provided using the straight-line method for financial reporting purposes. Licensed technology, net, consisted of the following as of:
| | | June 30, | | | December 31 | |
| | | 2021 | | | 2020 | | | 2019 | |
| Licensed technology | $ | 456,344 | | $ | 456,344 | | $ | 456,344 | |
| Less: accumulated amortization | | (51,079 | ) | | (46,700 | ) | | (37,944 | ) |
| Licensed technology, net | $ | 405,265 | | $ | 409,644 | | $ | 418,400 | |
Term of the Agreement
The term of the Stanford Agreement commenced on September 1, 2015 and continues until the last expiry of any patents that issue from the licensed technology in 2033.
NOTE 4 - PROPERTY AND EQUIPMENT
Property and equipment are recorded at cost. Depreciation and amortization of property and equipment is provided using the straight-line method for financial reporting purposes and is deemed sufficient to recover the cost over the estimated useful lives of the assets.
Property and equipment, net, consisted of the following as of December 31:
| | | June 30, | | | December 31 | |
| | | 2021 | | | 2020 | | | 2019 | |
| Furniture and equipment | $ | 1,043,330 | | $ | 1,043,330 | | $ | 379,785 | |
| Software | | 33,508 | | | 33,508 | | | 33,508 | |
| | | 1,076,838 | | | 1,076,838 | | | 413,293 | |
| Less: accumulated depreciation and amortization | | (258,567 | ) | | (234,212 | ) | | (181,670 | ) |
| Furniture and equipment, net | $ | 818,271 | | $ | 842,627 | | $ | 231,623 | |
NOTE 5 - COMMITMENTS AND CONTINGENCIES
Lease Commitment
The Company subleases its office and laboratory space under a Sponsored Research Agreement from Molecular Medicine Research Institute ("MMRI") which commenced on July 1, 2015. The Agreement automatically renews for the following three-month period on the first day of each month ("Record Date") unless the terminating party sends written notice to the non-terminating party on or prior to the applicable Record Date. The rent is variable based on the number of employees. Rent expense for the six months ended June 30, 2021 and the years ended December 31, 2020, and 2019 was $52,500, $115,700 and $129,000, respectively.
Legal Proceedings
From time to time, the Company may be subject to legal proceedings and claims in the ordinary course of business. The Company is not aware of any such proceedings or claims that it believes will have, individually or in aggregate, a material adverse effect on its business, financial condition, or results of operations.
NOTE 6 - DEBT
Convertible Bridge Promissory Note
In December 2018, the Company's majority unit holder made a non-interest-bearing advance to CLB in the amount of $1,129,883.44. On January 1, 2019, this advance became a one-year, mandatorily convertible note. On February 20, 2019, the same investor entered into an additional mandatorily convertible note in the amount of $370,116.56 to bring the total investment to $1,500,000. The notes were converted to equity in accordance with the terms of the notes on December 31, 2019.
Demand Promissory Notes
The following promissory notes were issued in 2020 as a bridge to subsequent equity financings. The notes were promissory notes that were unsecured and subject to repayment upon demand.
Lender | Date of Issue in 2020 | Amount | Interest
Rate | Amount Outstanding
on June 30, 2021 |
| | | | | |
CS Investors, LLC | January 7 | $150,000 | 2% | - |
| | | | | |
Wiegers & Co., LLC | August 14 | $50,000 | 1% | - |
| | | | | |
Wiegers & Co., LLC | August 27 | $50,000 | 1% | - |
| | | | | |
KESA Partners, Inc. | February 14 | $75,000 | 2% | - |
| | | | | |
KESA Partners, Inc. | July 28 | $50,000 | 1% | - |
| | | | | |
KESA Partners, Inc. | August 14 | $50,000 | 1% | - |
| | | | | |
KESA Partners, Inc. | August 27 | $50,000 | 1% | - |
| | | | | |
KESA Partners, Inc. | September 25 | $35,000 | 1% | - |
| | | | | |
KESA Partners, Inc. | October 16 | $31,000 | 1% | - |
Paycheck Protection Program Loan
In May 2020, the Company received a $84,042 small business loan under the Payroll Protection Program, part of the Coronavirus Aid, Relief and Economic Security Act ("CARES ACT"). In March 2021, the full loan amount was forgiven. Forgiveness of indebtedness, in accordance with the CARES Act, does not give rise to federal taxable income.
NOTE 7 - SHARE-BASED COMPENSATION
In September 2015, the Company adopted an Employee Incentive Plan, that provided for both options and profit interests for employees and non-employee consultants. Equity-based compensation cost for profit interests was treated as deferred compensation and was not recognized under ASC 710 as the payments were not probable and the amounts could not be estimated. Equity- based compensation cost for options was measured at the grant date, based on the fair value of the award, and was recognized as expense over the requisite service period. The fair value of option awards to employees was estimated using the Black- Scholes option pricing model. We record forfeitures and expirations when they occurred.
The Company no longer issues options and profit interests. This plan was replaced by a restricted stock plan in December 2020 described below.
As of December 31, 2020, there were 251 total options outstanding as a result of the 2,000 to one reverse split on October 8, 2020. These options were exchanged for 16,000 shares of restricted stock in April 2021. As a result, no options were outstanding on June 30, 2021. All remaining profit interests were surrendered in November 2021.
NOTE 8 - STOCKHOLDER'S EQUITY
The Company was converted from a Delaware limited liability company to a Delaware corporation on October 12, 2020. The corporation is authorized to issue 2,000,000 shares of common stock ($0.0001 par value/common share) and 1,000,000 shares of preferred stock (par value $0.0001/preferred share). The Board can determine the terms of the preferred stock at issuance.
All share amounts have been adjusted for the 2000 to one reverse unit split that was effective October 8, 2020.
In December 2018, the Company's majority unit holder made a non-interest-bearing advance to CLB in the amount of $1,129,883.44. On January 1, 2019, this advance became a one-year, mandatorily convertible note. On February 20, 2019, the same investor entered into an additional mandatorily convertible note in the amount of 370,116.56 to bring the total investment to $1,500,000. The notes were converted to equity in accordance with the terms of the notes on December 31, 2019.
In March 2020, the Company issued 18,750 common units for gross proceeds of $750,000.
In August 2020, certain significant unitholders declined to participate in a planned financing. As a result, three existing unitholders agreed to increase their purchase of equity at a price of $1.02/common share (as adjusted for the 2000 to one reverse stock split) if all preferred units were converted to common and the form of the Company was converted from a limited liability company to a corporation. These conditions were accepted and completed by the time of the conversion from a Delaware limited liability company to a Delaware corporation on October 12, 2020.
In November 2020 the Company issued 686,274 common shares for gross proceeds of $700,000.
In January 2021, the Company issued 162,501 common shares for gross proceeds of $650,004.
In May/June 2021, the Company issued 125,001 common shares for gross proceeds of $1,000,008.
Restricted Stock Plan
On December 26, 2020, the Company adopted a Restricted Stock Plan under which the Company may grant restricted stock to individuals who are employees, managers, eligible consultants, and other service providers of the Company. The stock can be repurchased by the Company at the Company's option at a price of $0.01/share for the 24 months following the date of grant. After the expiration of 24 months, the shares are not restricted. A total of 100,000 common shares were initially reserved for issuance under the plan and an additional 100,000 common shares were added in November 2021. Currently, 69,239 shares have been awarded to employees, consultants and the founder shareholder, Dr. Deisseroth, and none to Mr. Hansen. The grant date of such awards is the date on which the board of directors makes its determination to issue awards.
NOTE 9 - RELATED PARTY TRANSACTIONS
For the year ended December 31, 2020 and 2019, the Company recognized $100,556 and $42,399 in selling, general and administrative expenses respectively in license fees to Stanford University. Stanford University currently holds less than 1% of our total outstanding shares but held more than 5% prior to October 2020.
During the years ended December 31, 2020 and 2019, the Company had loans outstanding to related parties as detailed above in Note 6.
As detailed in Note 8 above, a financing was initiated in August 2020 in which only three shareholders participated. The financing terms were offered to all shareholders. When completed in November 2020, the financing resulted in a high degree of dilution to shareholders that chose not to participate.
Effective September 1, 2015, the Company licensed certain technology from Stanford University covering inventions relating to methods to image brain and other tissues for use both in research and in the clinic (the "Stanford Agreement"). The technology is owned by Stanford and includes US Patent 10,545,075 issued on January 28, 2020.
As part of the consideration for the Stanford Agreement, Stanford and co-inventors received equity interests which currently constitute less than 1% of outstanding shares. The Agreement states that Stanford University is entitled to receive yearly maintenance fees, milestone payments, and royalties. The amounts paid in 2020 and 2019 were $100,556 and $42,399, respectively.
Stanford University is entitled to the earned royalties as a percentage of net sales ranging from 1.5% - 4%, depending on the product or service sold.
The following table sets forth certain information known to us regarding beneficial ownership of our capital stock as of the date of this Offering Circular, for (i) all executive officers and directors as a group and (ii) each person, or group of affiliated persons, known by us to be the beneficial owner of more than five percent (5%) of our capital stock. The percentage of beneficial ownership in the table below is based on 1,134,331 shares of Common Stock deemed to be outstanding as of the date of this Offering Circular.
Name and Address of Beneficial Owner | Title of Class | Amount and Nature of Beneficial Ownership | Amount and Nature of Beneficial Ownership that is Acquirable | Percent of Class |
Wiegers & Co., LLC | Common Stock | 360,120 | 0 | 31.7% |
James Grosfeld Trust | Common Stock | 358,499 | 0 | 31.6% |
KESA Partners, Inc. Albert Hansen, a director of the Company, and the sole Executive Officer, owns 100% of total common stock issued by KESA Partners, Inc. | Common Stock | 328,871 | 0 | 29.0% |
NOTE 10 - SUBSEQUENT EVENTS
Increase in Common Equity
In November 2021, the Company sold 55,000 shares of common stock at a price of $12.00 per share for gross proceeds of $660,000.
PART III - INDEX TO EXHIBITS
ITEM | DESCRIPTION | EXHIBIT |
2.1 | Certificate of Incorporation of ClearLight Biotechnologies, Inc. filed with the State of Delaware dated October 8, 2020 and filed on October 12, 2020 | 1A-2.1 |
2.2 | Statement by Sole Incorporator of ClearLight Biotechnologies, Inc., dated October 17, 2020 | 1A-2.2 |
2.3 | Amended and Restated Bylaws of ClearLight Biotechnologies, Inc. dated September, 2021 | 1A-2.3 |
4.1 | Form of Common Stock Subscription Agreement | 1A-4.1 |
6.1 | Stock Transfer Agent Agreement between ClearTrust, LLC and ClearLight Biotechnologies, Inc., dated November 7, 2021 | 1A-6.1 |
6.2 | ClearLight Biotechnologies, Inc. Restricted Common Stock Award Plan, dated December 26, 2020 | 1A-6.2 |
6.3 | Exclusive License (Equity) Agreement between The Board of Trustees of the Leland Stanford Junior University and Clear Light Diagnostics, LLC, effective September 1, 2015, as amended on January 23, 2018, as amended on May 10, 2018, as amended on August 28, 2020 [REDACTED COPY - portions of this Exhibit have been redacted pursuant to Instructions to Item 17] | 1A-6.3 |
6.4 | Sponsored Research Agreement (SRA) between Molecular Medicine Research Institute and Clarity Solutions, dated June 10, 2015 and effective July 1, 2015, as amended on July 15, 2015, as amended on December 9, 2015, as amended on May 3, 2017, as amended on October 1, 2018, as amended on February 9, 2021 | 1A-6.4 |
6.5 | TriNet Life Sciences Requisition Order and Agreement with ClearLight Diagnostics, LLC, dated August 25, 2016 | 1A-6.5 |
12.1 | Form of Legal Opinion of Karen Muller, Esq. | 1A-12.1 |
SIGNATURES
Pursuant to the requirements of Regulation A, the issuer certifies that it has reasonable grounds to believe that it meets all of the requirements for filing on Form 1-A and has duly caused this offering statement to be signed on its behalf by the undersigned, thereunto duly authorized in the City of Sunnyvale, State of California, on November 18, 2021.
| CLEARLIGHT BIOTECHNOLOGIES, INC. |
| |
| /s/ Albert Hansen |
| Albert Hansen, Chief Executive Officer |
The offering statement has been signed by the following persons in the capacities and on the dates indicated.
Signature | | Title | | Date |
| | | | |
/s/Albert Hansen | | Chief Executive Officer, President, Chief Financial Officer, Secretary Treasurer | | November 18, 2021 |
Albert Hansen | | | | |
| | | | |
| | | | |
/s/ Albert Hansen | | Director | | November 18, 2021 |
Albert Hansen | | | | |


