
Corporate update and financial results March 30, 2021 Fourth Quarter and Full Year 2020 Harnessing the full potential of the immune system to solve global health problems Exhibit 99.2

This slide presentation includes forward-looking statements This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, as amended, including BioNTech’s expected revenues and net profit related to sales of BioNTech and Pfizer’s COVID-19 vaccine, referred to as COMIRNATY® in the European Union as authorized for use under conditional marketing approval, in territories controlled by BioNTech’s collaboration partners, particularly those such figures that are derived from preliminary estimates provided by BioNTech’s partners; the extent to which a COVID-19 vaccine continues to be necessary in the future; competition from other COVID-19 vaccines or related to BioNTech’s other product candidates, including those with different mechanisms of action and different manufacturing and distribution constraints, on the basis of, among other things, efficacy, cost, convenience of storage and distribution, breadth of approved use, side-effect profile and durability of immune response; the pricing and reimbursement of BioNTech and Pfizer’s COVID-19 vaccine and BioNTech’s investigational medicines, if approved; the rate and degree of market acceptance of BioNTech and Pfizer’s COVID-19 vaccine and BioNTech’s investigational medicines, if approved; the initiation, timing, progress, results, and cost of BioNTech’s research and development programs and BioNTech’s current and future preclinical studies and clinical trials, including statements regarding the timing of initiation and completion of studies or trials and related preparatory work, the period during which the results of the trials will become available and BioNTech’s research and development programs; the timing of and BioNTech’s ability to obtain and maintain regulatory approval for BioNTech’s product candidates; the ability and willingness of BioNTech’s third-party collaborators to continue research and development activities relating to BioNTech’s development candidates and investigational medicines; the impact of the COVID-19 pandemic on BioNTech’s development programs, supply chain, collaborators and financial performance; unforeseen safety issues and claims for personal injury or death arising from the use of BioNTech and Pfizer’s COVID-19 vaccine, and other products and product candidates developed or manufactured by BioNTech; BioNTech’s estimates of its expenses, ongoing losses, future revenue and capital requirements and BioNTech’s needs for or ability to obtain additional financing; the development of and projections relating to BioNTech’s competitors or its industry; BioNTech’s ability to effectively scale its production capabilities and manufacture its products, including BioNTech and Pfizer’s COVID-19 vaccine, and BioNTech’s product candidates; BioNTech’s projected net sales for the COVID-19 vaccine in 2021; BioNTech’s projected gross margins, expenses and expenditures and tax rate for 2021; BioNTech’s target vaccine production for 2021; and BioNTech’s COVID-19 vaccine revenues and net sales, which are subject to numerous estimates as more fully described in our Annual Report on Form 20-F. In some cases, forward-looking statements can be identified by terminology such as “will,” “may,” “should,” “expects,” “intends,” “plans,” “aims,” “anticipates,” “believes,” “estimates,” “predicts,” “potential,” “continue,” or the negative of these terms or other comparable terminology, although not all forward-looking statements contain these words. The forward-looking statements in this press release are neither promises nor guarantees, and you should not place undue reliance on these forward-looking statements because they involve known and unknown risks, uncertainties, and other factors, many of which are beyond BioNTech’s control and which could cause actual results to differ materially from those expressed or implied by these forward-looking statements. You should review the risks and uncertainties described under the heading “Risk Factors” in BioNTech’s Annual Report on Form 20-F filed with the US Securities and Exchange Commission (SEC) on March 31, 2020 and in subsequent filings made by BioNTech with the SEC, including the third quarter report, which are available on the SEC’s website at www.sec.gov. Except as required by law, BioNTech disclaims any intention or responsibility for updating or revising any forward-looking statements contained in this press release in the event of new information, future developments or otherwise. These forward-looking statements are based on BioNTech’s current expectations and speak only as of the date hereof. 2

Safety Information 3 AUTHORIZED USE IN THE U.S.: The Pfizer-BioNTech COVID19 Vaccine is authorized for use under an Emergency Use Authorization (EUA) for active immunization to prevent coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in individuals 16 years of age and older. IMPORTANT SAFETY INFORMATION FROM U.S. FDA EMERGENCY USE AUTHORIZATION PRESCRIBING INFORMATION: Do not administer Pfizer-BioNTech COVID-19 Vaccine to individuals with known history of a severe allergic reaction (e.g., anaphylaxis) to any component of the Pfizer-BioNTech COVID-19 Vaccine. Appropriate medical treatment used to manage immediate allergic reactions must be immediately available in the event an acute anaphylactic reaction occurs following administration of Pfizer- BioNTech COVID-19 Vaccine. Monitor Pfizer-BioNTech COVID-19 Vaccine recipients for the occurrence of immediate adverse reactions according to the Centers for Disease Control and Prevention guidelines (https://www.cdc.gov/vaccines/covid-19/). Immunocompromised persons, including individuals receiving immunosuppressant therapy, may have a diminished immune response to the Pfizer-BioNTech COVID-19 Vaccine. The Pfizer-BioNTech COVID-19 Vaccine may not protect all vaccine recipients. In clinical studies, adverse reactions in participants 16 years of age and older included pain at the injection site (84.1%), fatigue (62.9%), headache (55.1%), muscle pain (38.3%), chills (31.9%), joint pain (23.6%), fever (14.2%), injection site swelling (10.5%), injection site redness (9.5%), nausea (1.1%), malaise (0.5%), and lymphadenopathy (0.3%). Severe allergic reactions, including anaphylaxis, have been reported following the Pfizer-BioNTech COVID-19 Vaccine during mass vaccination outside of clinical trials. Additional adverse reactions, some of which may be serious, may become apparent with more widespread use of the Pfizer-BioNTech COVID-19 Vaccine. Available data on Pfizer-BioNTech COVID-19 Vaccine administered to pregnant women are insufficient to inform vaccine-associated risks in pregnancy. Data are not available to assess the effects of Pfizer-BioNTech COVID-19 Vaccine on the breastfed infant or on milk production/excretion. There are no data available on the interchangeability of the Pfizer-BioNTech COVID-19 Vaccine with other COVID-19 vaccines to complete the vaccination series. Individuals who have received one dose of Pfizer-BioNTech COVID-19 Vaccine should receive a second dose of Pfizer-BioNTech COVID-19 Vaccine to complete the vaccination series. Vaccination providers must report Adverse Events in accordance with the Fact Sheet to VAERS at https://vaers.hhs.gov/reportevent.html or by calling 1-800-822-7967. The reports should include the words "Pfizer-BioNTech COVID-19 Vaccine EUA" in the description section of the report. Vaccination providers should review the Fact Sheet for Information to Provide to Vaccine Recipients/Caregivers and Mandatory Requirements for Pfizer-BioNTech COVID-19 Vaccine Administration Under Emergency Use Authorization. Please see Emergency Use Authorization (EUA) Fact Sheet for Healthcare Providers Administering Vaccine (Vaccination Providers) including Full EUA Prescribing Information available at www.cvdvaccine-us.com.

Agenda 4 Full Year 2020 Highlights Oncology Pipeline Update Financial Results COVID-19 Vaccine Update Strategic Outlook
2020: A momentous year for BioNTech First commercial product 5
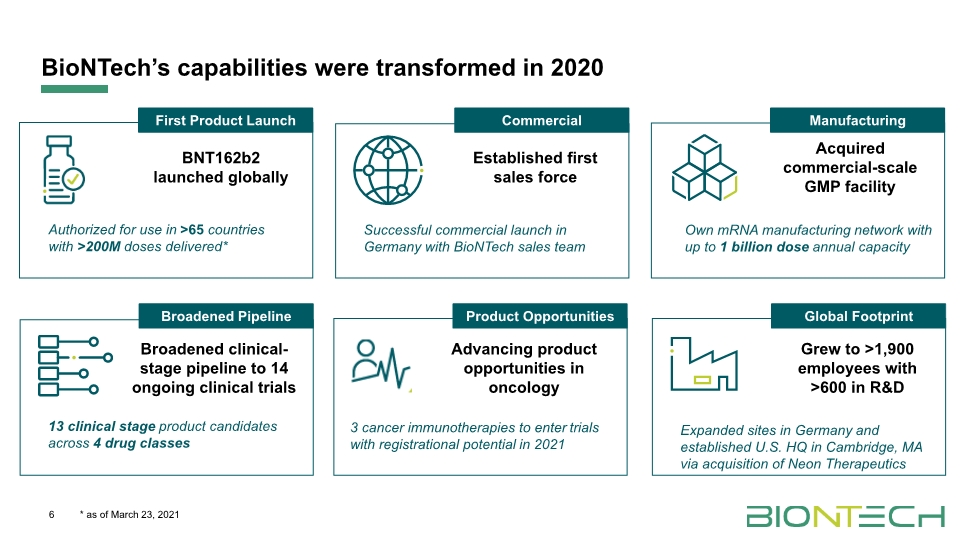
BioNTech’s capabilities were transformed in 2020 6 * as of March 23, 2021
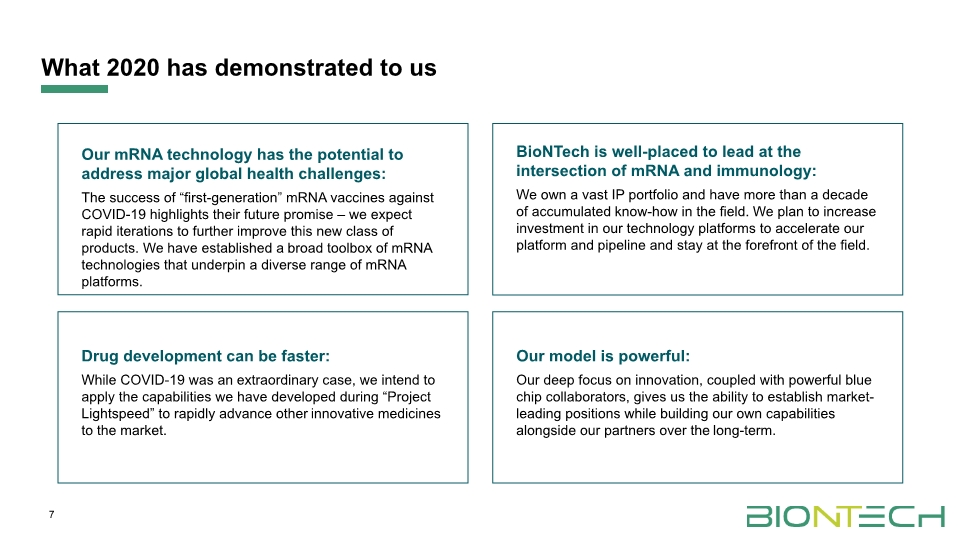
What 2020 has demonstrated to us 7
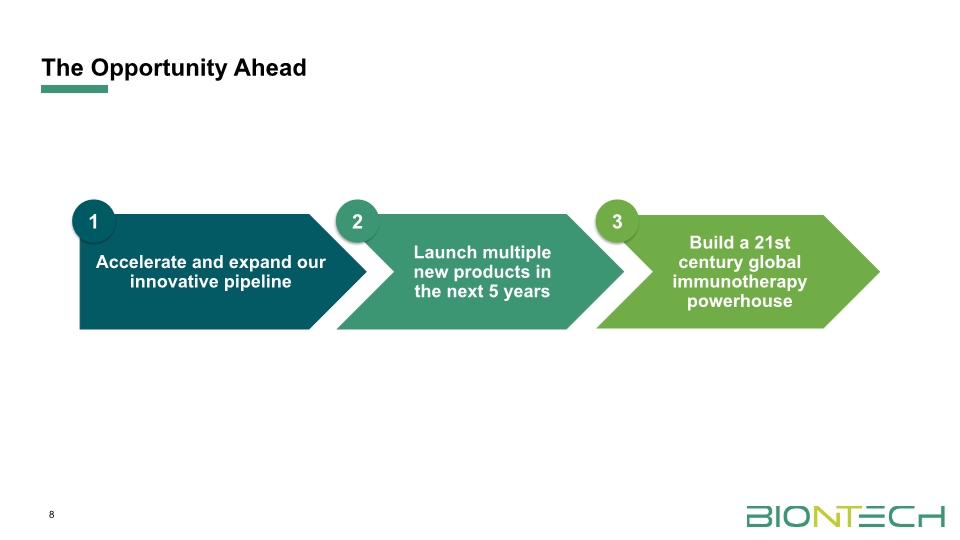
The Opportunity Ahead 1 2 3 8
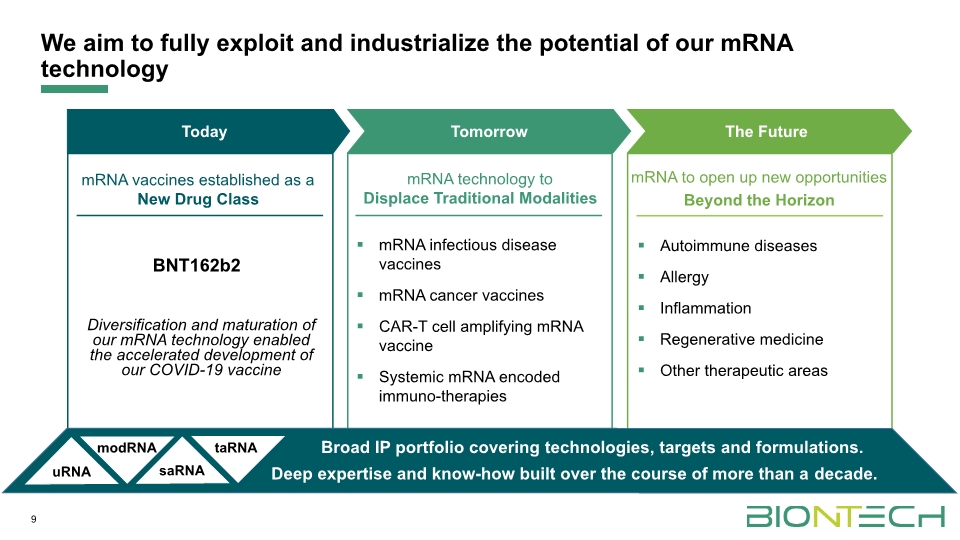
9 Today Tomorrow mRNA vaccines established as a New Drug Class Diversification and maturation of our mRNA technology enabled the accelerated development of our COVID-19 vaccine mRNA to open up new opportunities Beyond the Horizon Autoimmune diseases Allergy Inflammation Regenerative medicine Other therapeutic areas mRNA technology to Displace Traditional Modalities mRNA infectious disease vaccines mRNA cancer vaccines CAR-T cell amplifying mRNA vaccine Systemic mRNA encoded immuno-therapies Broad IP portfolio covering technologies, targets and formulations. Deep expertise and know-how built over the course of more than a decade. The Future We aim to fully exploit and industrialize the potential of our mRNA technology uRNA modRNA saRNA taRNA BNT162b2

Agenda 10 COVID-19 Vaccine Update Full Year 2020 Highlights Oncology Pipeline Update Financial Results Strategic Outlook

Strong clinical results 95% effective against symptomatic COVID-19 infections1 94% efficacy in participants >65 years Well tolerated safety profile High titers of neutralizing antibodies Robust and poly-epitopic CD8+ and Th1 CD4+ T-cell responses2 1Polack FP, et al. NEJM 2020, 383:2603-2615 2Sahin U, et al. preprint 2020 (https://www.medrxiv.org/content/10.1101/2020.12.09.20245175v1) 11

Compelling real-world evidence Two weeks post-dose 2 About 97% effective in preventing symptomatic COVID-19 severe/critical COVID 19 Hospitalizations Deaths 94% effective against asymptomatic infection Protective against B.1.1.7 variant Real-World-Data announced by The Israel Ministry of Health (MoH) on March 11, 2021: https://www.businesswire.com/news/home/20210311005482/en/ Haas EJ, et al. preprint 2021; https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3811387 12
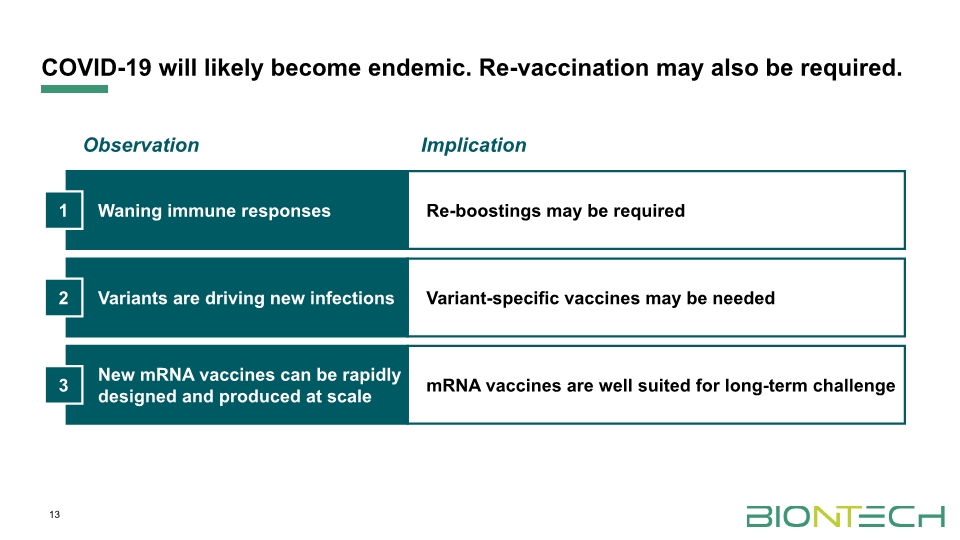
Re-boostings may be required Variant-specific vaccines may be needed mRNA vaccines are well suited for long-term challenge COVID-19 will likely become endemic. Re-vaccination may also be required. 13 Waning immune responses Variants are driving new infections New mRNA vaccines can be rapidly designed and produced at scale 1 2 3 Observation Implication
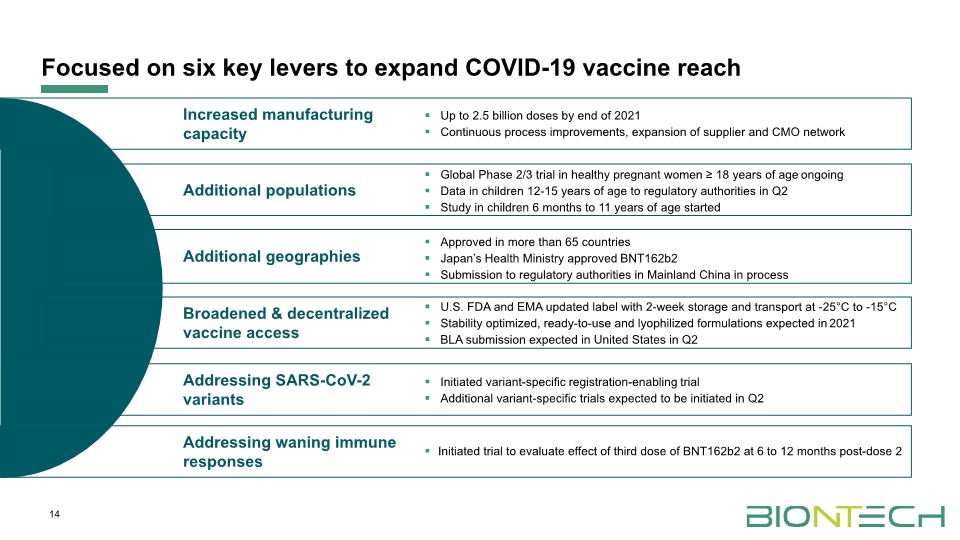
14 Focused on six key levers to expand COVID-19 vaccine reach
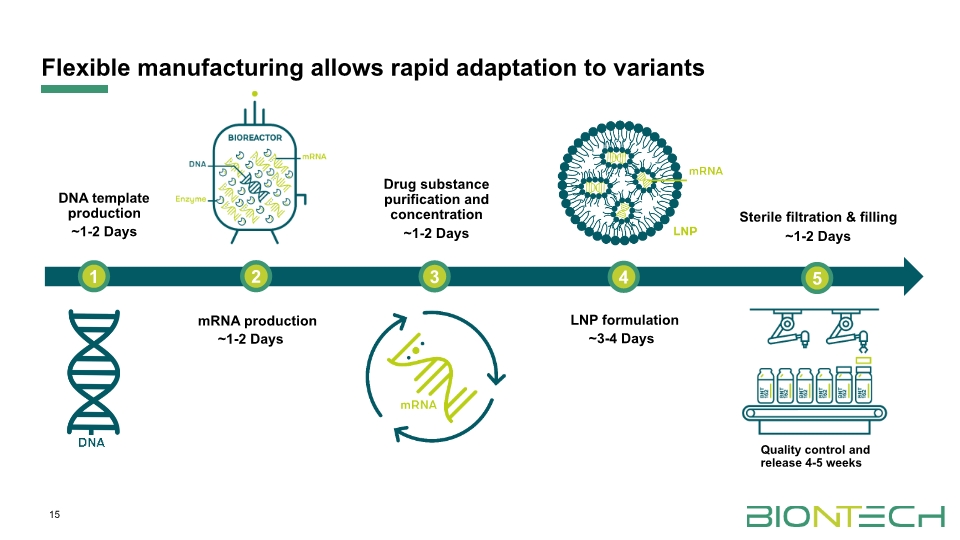
Flexible manufacturing allows rapid adaptation to variants 15 mRNA production Drug substance purification and concentration LNP formulation Sterile filtration & filling 1 2 3 4 ~1-2 Days ~1-2 Days ~3-4 Days ~1-2 Days Quality control and release 4-5 weeks ~1-2 Days DNA template production 5
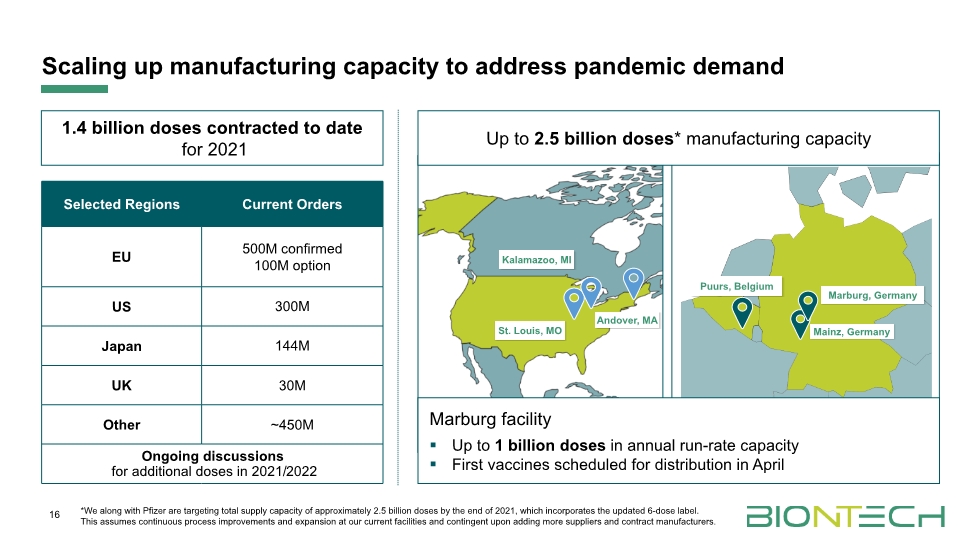
16 1.4 billion doses contracted to date for 2021 Scaling up manufacturing capacity to address pandemic demand Puurs, Belgium Marburg facility Up to 1 billion doses in annual run-rate capacity First vaccines scheduled for distribution in April Up to 2.5 billion doses* manufacturing capacity *We along with Pfizer are targeting total supply capacity of approximately 2.5 billion doses by the end of 2021, which incorporates the updated 6-dose label. This assumes continuous process improvements and expansion at our current facilities and contingent upon adding more suppliers and contract manufacturers.
Agenda 17 Full Year 2020 Highlights Oncology Pipeline Update Financial Results COVID-19 Vaccine Update Strategic Outlook
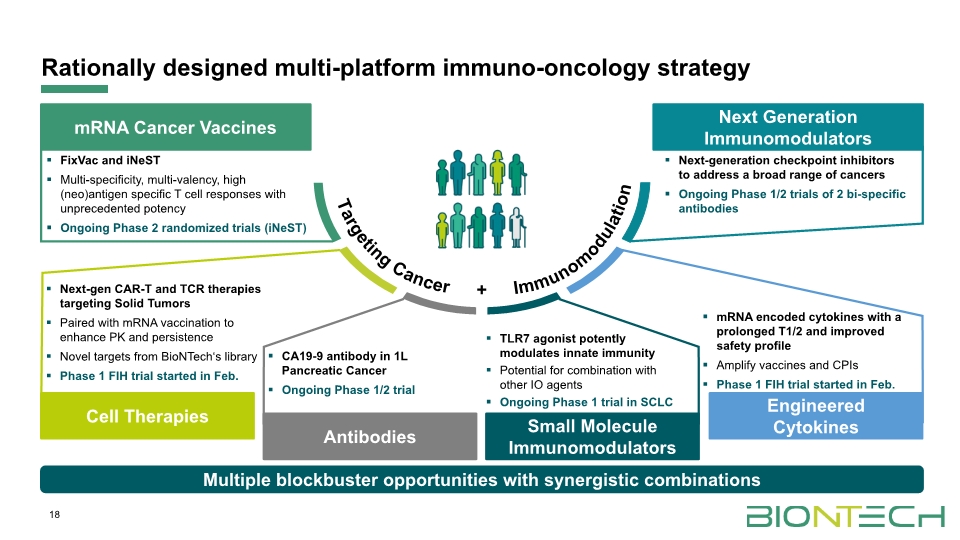
18 Rationally designed multi-platform immuno-oncology strategy Multiple blockbuster opportunities with synergistic combinations mRNA Cancer Vaccines Antibodies Small Molecule Immunomodulators + FixVac and iNeST Multi-specificity, multi-valency, high (neo)antigen specific T cell responses with unprecedented potency Ongoing Phase 2 randomized trials (iNeST) Next-gen CAR-T and TCR therapies targeting Solid Tumors Paired with mRNA vaccination to enhance PK and persistence Novel targets from BioNTech‘s library Phase 1 FIH trial started in Feb. Next-generation checkpoint inhibitors to address a broad range of cancers Ongoing Phase 1/2 trials of 2 bi-specific antibodies CA19-9 antibody in 1L Pancreatic Cancer Ongoing Phase 1/2 trial mRNA encoded cytokines with a prolonged T1/2 and improved safety profile Amplify vaccines and CPIs Phase 1 FIH trial started in Feb. TLR7 agonist potently modulates innate immunity Potential for combination with other IO agents Ongoing Phase 1 trial in SCLC Cell Therapies Next Generation Immunomodulators Engineered Cytokines
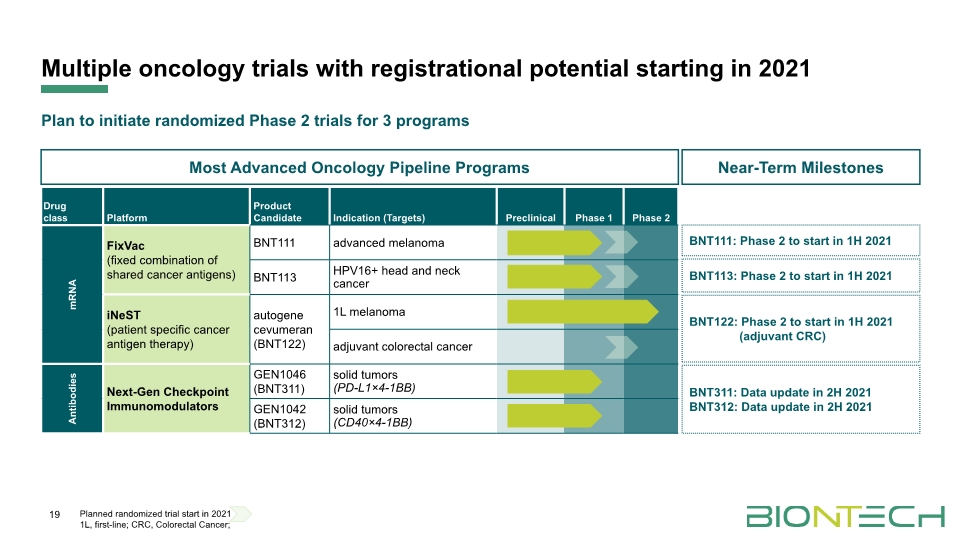
Multiple oncology trials with registrational potential starting in 2021 19 Planned randomized trial start in 2021 1L, first-line; CRC, Colorectal Cancer; BNT111: Phase 2 to start in 1H 2021 BNT311: Data update in 2H 2021 BNT312: Data update in 2H 2021 BNT113: Phase 2 to start in 1H 2021 BNT122: Phase 2 to start in 1H 2021 (adjuvant CRC) Near-Term Milestones Most Advanced Oncology Pipeline Programs Plan to initiate randomized Phase 2 trials for 3 programs
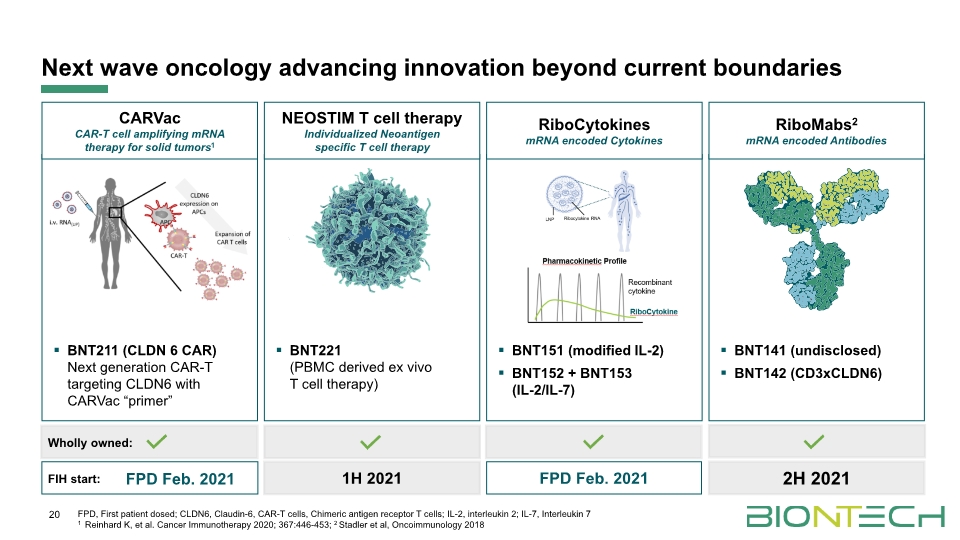
Next wave oncology advancing innovation beyond current boundaries 20 BNT211 (CLDN 6 CAR) Next generation CAR-T targeting CLDN6 with CARVac “primer” CARVac CAR-T cell amplifying mRNA therapy for solid tumors1 BNT221 (PBMC derived ex vivo T cell therapy) NEOSTIM T cell therapy Individualized Neoantigen specific T cell therapy BNT151 (modified IL-2) BNT152 + BNT153 (IL-2/IL-7) RiboCytokines mRNA encoded Cytokines BNT141 (undisclosed) BNT142 (CD3xCLDN6) RiboMabs2 mRNA encoded Antibodies Wholly owned: FIH start: FPD Feb. 2021 FPD, First patient dosed; CLDN6, Claudin-6, CAR-T cells, Chimeric antigen receptor T cells; IL-2, interleukin 2; IL-7, Interleukin 7 1 Reinhard K, et al. Cancer Immunotherapy 2020; 367:446-453; 2 Stadler et al, Oncoimmunology 2018 2H 2021 FPD Feb. 2021 1H 2021
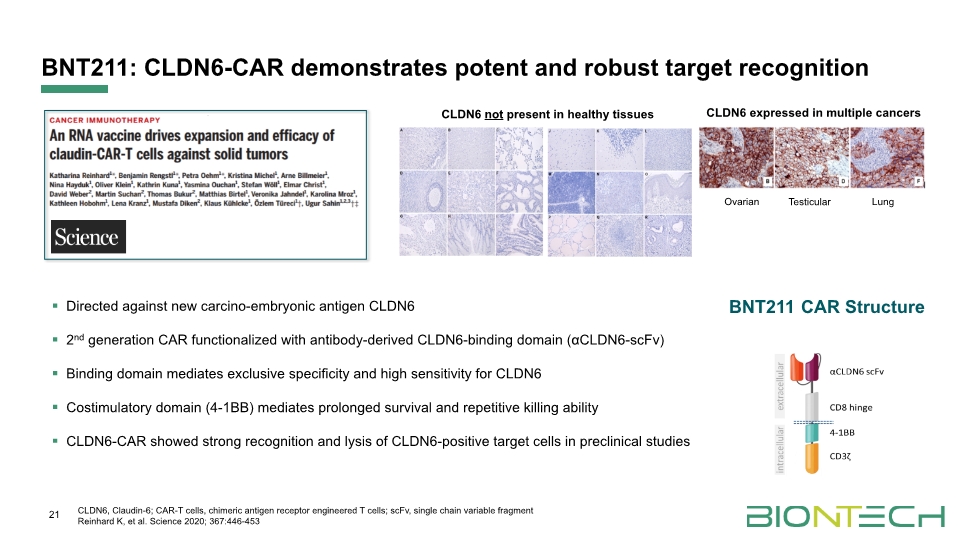
BNT211: CLDN6-CAR demonstrates potent and robust target recognition Directed against new carcino-embryonic antigen CLDN6 2nd generation CAR functionalized with antibody-derived CLDN6-binding domain (αCLDN6-scFv) Binding domain mediates exclusive specificity and high sensitivity for CLDN6 Costimulatory domain (4-1BB) mediates prolonged survival and repetitive killing ability CLDN6-CAR showed strong recognition and lysis of CLDN6-positive target cells in preclinical studies 21 CLDN6, Claudin-6; CAR-T cells, chimeric antigen receptor engineered T cells; scFv, single chain variable fragment Reinhard K, et al. Science 2020; 367:446-453 CLDN6 not present in healthy tissues CLDN6 expressed in multiple cancers Ovarian Testicular Lung
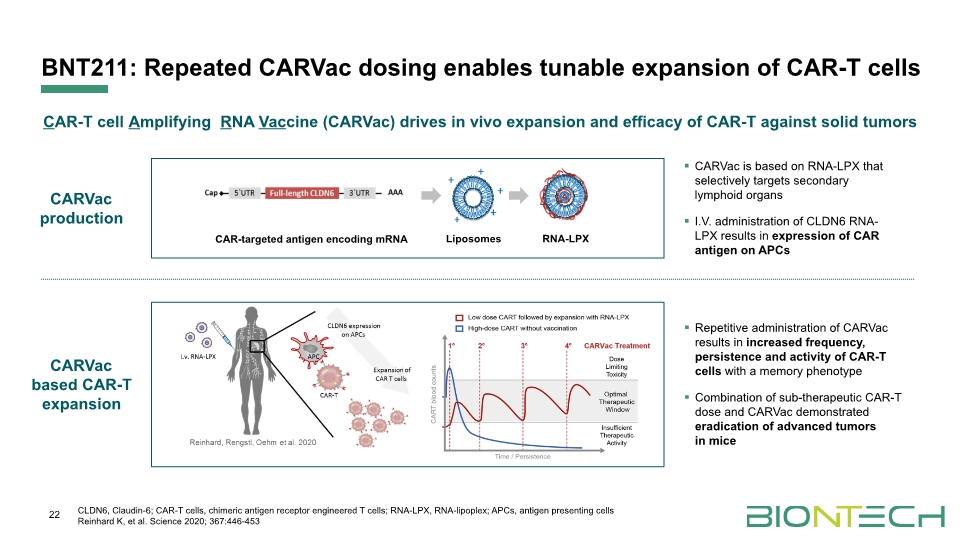
BNT211: Repeated CARVac dosing enables tunable expansion of CAR-T cells 22 CAR-T cell Amplifying RNA Vaccine (CARVac) drives in vivo expansion and efficacy of CAR-T against solid tumors CLDN6, Claudin-6; CAR-T cells, chimeric antigen receptor engineered T cells; RNA-LPX, RNA-lipoplex; APCs, antigen presenting cells Reinhard K, et al. Science 2020; 367:446-453
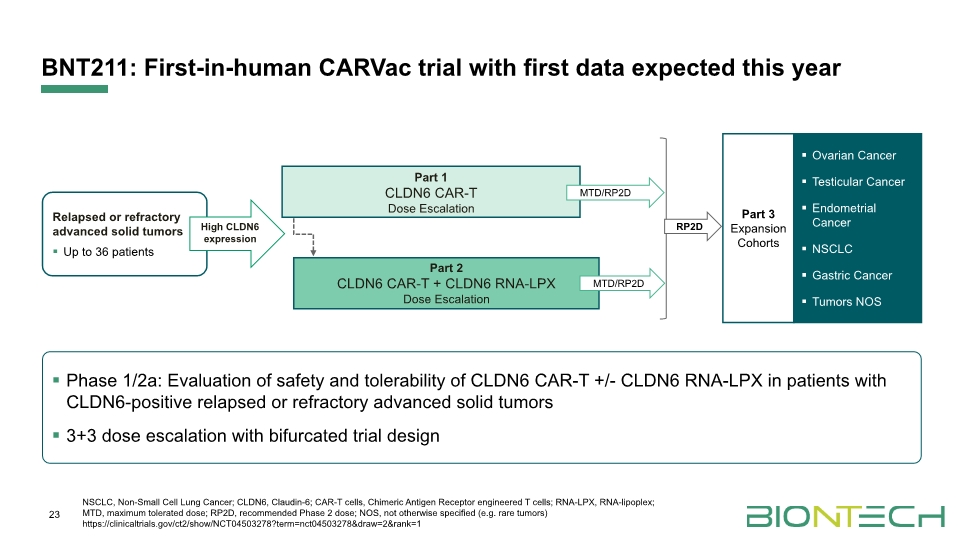
BNT211: First-in-human CARVac trial with first data expected this year 23 NSCLC, Non-Small Cell Lung Cancer; CLDN6, Claudin-6; CAR-T cells, Chimeric Antigen Receptor engineered T cells; RNA-LPX, RNA-lipoplex; MTD, maximum tolerated dose; RP2D, recommended Phase 2 dose; NOS, not otherwise specified (e.g. rare tumors) https://clinicaltrials.gov/ct2/show/NCT04503278?term=nct04503278&draw=2&rank=1 Phase 1/2a: Evaluation of safety and tolerability of CLDN6 CAR-T +/- CLDN6 RNA-LPX in patients with CLDN6-positive relapsed or refractory advanced solid tumors 3+3 dose escalation with bifurcated trial design MTD/RP2D MTD/RP2D
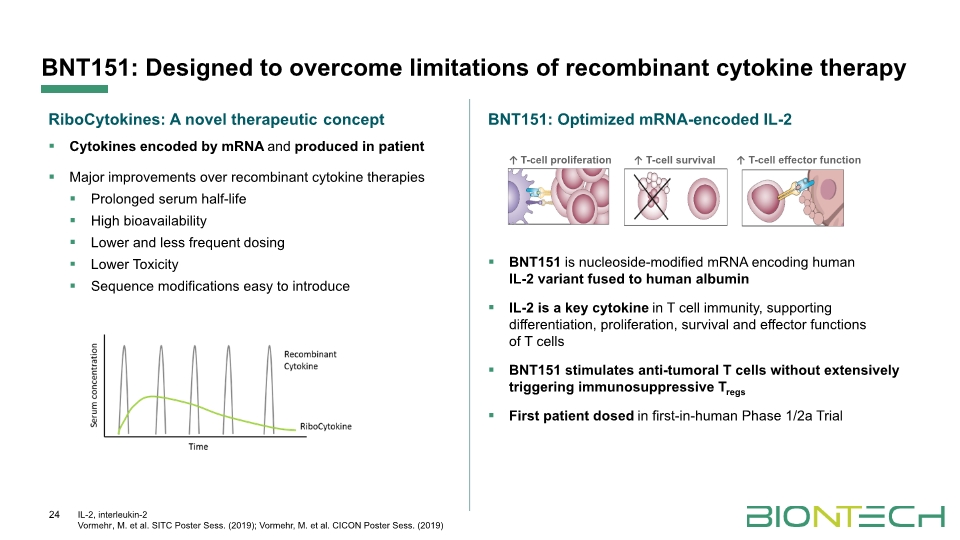
BNT151: Optimized mRNA-encoded IL-2 BNT151 is nucleoside-modified mRNA encoding human IL-2 variant fused to human albumin IL-2 is a key cytokine in T cell immunity, supporting differentiation, proliferation, survival and effector functions of T cells BNT151 stimulates anti-tumoral T cells without extensively triggering immunosuppressive Tregs First patient dosed in first-in-human Phase 1/2a Trial RiboCytokines: A novel therapeutic concept Cytokines encoded by mRNA and produced in patient Major improvements over recombinant cytokine therapies Prolonged serum half-life High bioavailability Lower and less frequent dosing Lower Toxicity Sequence modifications easy to introduce BNT151: Designed to overcome limitations of recombinant cytokine therapy 24 IL-2, interleukin-2 Vormehr, M. et al. SITC Poster Sess. (2019); Vormehr, M. et al. CICON Poster Sess. (2019)
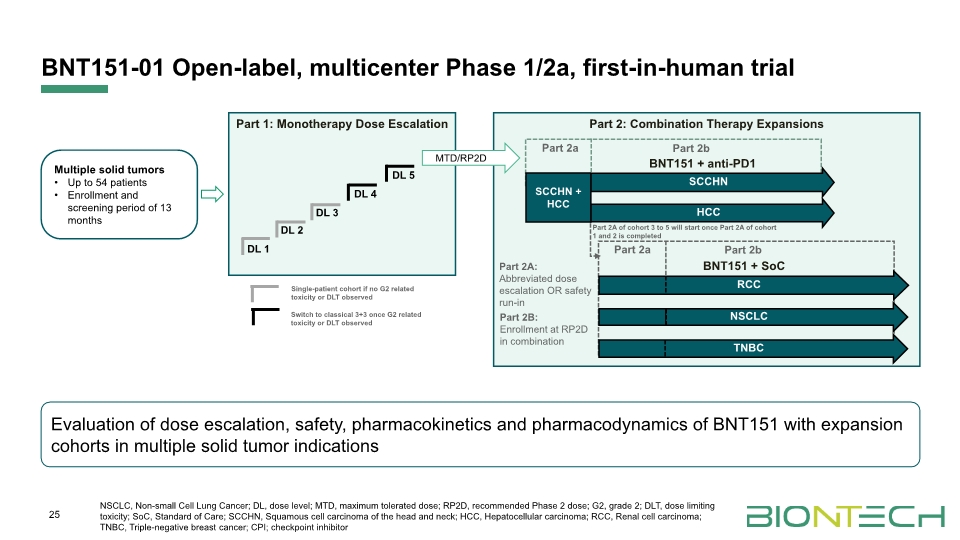
BNT151-01 Open-label, multicenter Phase 1/2a, first-in-human trial 25 Evaluation of dose escalation, safety, pharmacokinetics and pharmacodynamics of BNT151 with expansion cohorts in multiple solid tumor indications Part 2: Combination Therapy Expansions Part 2a Part 2b SCCHN HCC SCCHN + HCC MTD/RP2D DL 1 DL 2 DL 3 DL 4 DL 5 Part 2A of cohort 3 to 5 will start once Part 2A of cohort 1 and 2 is completed Part 2A: Abbreviated dose escalation OR safety run-in Part 2B: Enrollment at RP2D in combination BNT151 + anti-PD1 Part 2a Part 2b BNT151 + SoC RCC NSCLC TNBC Single-patient cohort if no G2 related toxicity or DLT observed Switch to classical 3+3 once G2 related toxicity or DLT observed NSCLC, Non-small Cell Lung Cancer; DL, dose level; MTD, maximum tolerated dose; RP2D, recommended Phase 2 dose; G2, grade 2; DLT, dose limiting toxicity; SoC, Standard of Care; SCCHN, Squamous cell carcinoma of the head and neck; HCC, Hepatocellular carcinoma; RCC, Renal cell carcinoma; TNBC, Triple-negative breast cancer; CPI; checkpoint inhibitor

Agenda 26 Full Year 2020 Highlights Oncology Pipeline Update Financial Results COVID-19 Vaccine Update Strategic Outlook
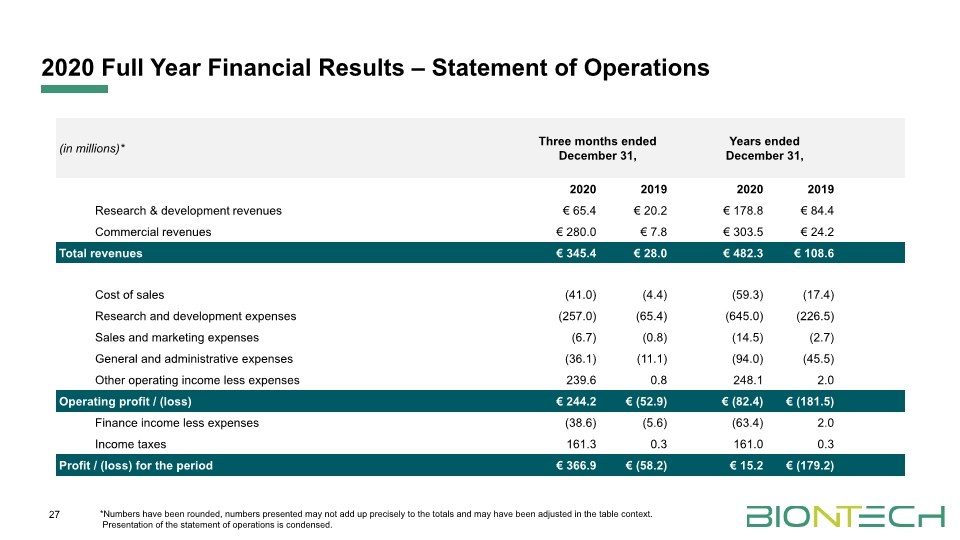
2020 Full Year Financial Results – Statement of Operations 27 *Numbers have been rounded, numbers presented may not add up precisely to the totals and may have been adjusted in the table context. Presentation of the statement of operations is condensed.
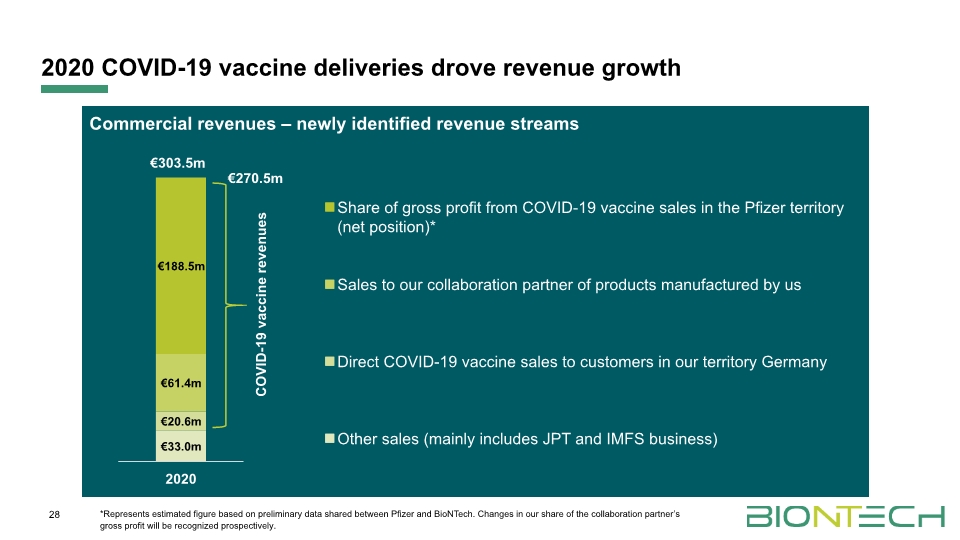
28 *Represents estimated figure based on preliminary data shared between Pfizer and BioNTech. Changes in our share of the collaboration partner’s gross profit will be recognized prospectively. 2020 COVID-19 vaccine deliveries drove revenue growth
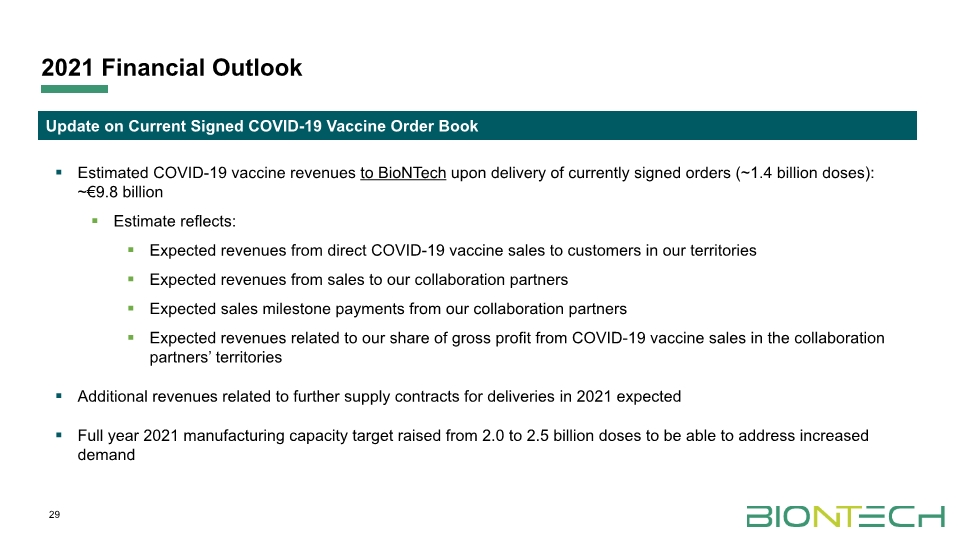
2021 Financial Outlook 29 Estimated COVID-19 vaccine revenues to BioNTech upon delivery of currently signed orders (~1.4 billion doses): ~€9.8 billion Estimate reflects: Expected revenues from direct COVID-19 vaccine sales to customers in our territories Expected revenues from sales to our collaboration partners Expected sales milestone payments from our collaboration partners Expected revenues related to our share of gross profit from COVID-19 vaccine sales in the collaboration partners’ territories Additional revenues related to further supply contracts for deliveries in 2021 expected Full year 2021 manufacturing capacity target raised from 2.0 to 2.5 billion doses to be able to address increased demand Update on Current Signed COVID-19 Vaccine Order Book
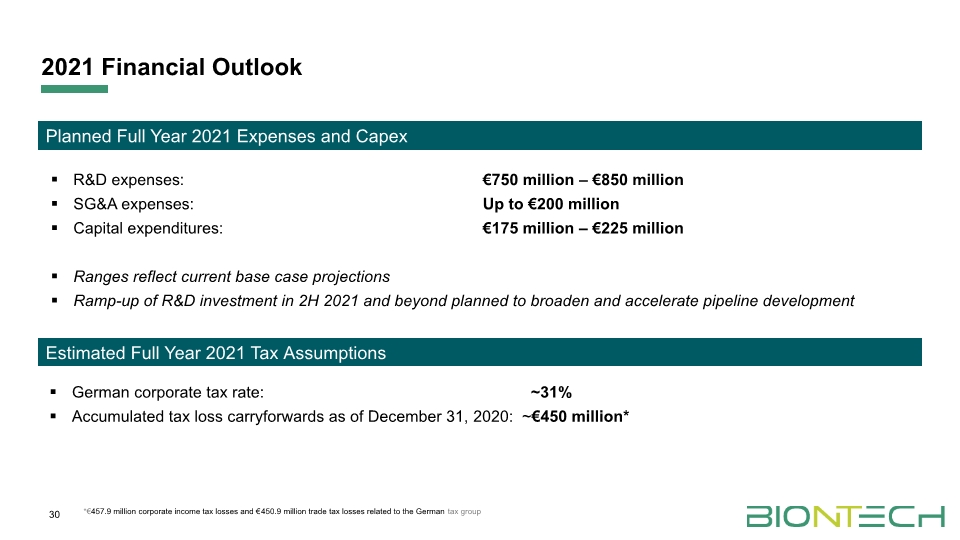
30 2021 Financial Outlook R&D expenses: €750 million – €850 million SG&A expenses: Up to €200 million Capital expenditures: €175 million – €225 million Ranges reflect current base case projections Ramp-up of R&D investment in 2H 2021 and beyond planned to broaden and accelerate pipeline development German corporate tax rate: ~31% Accumulated tax loss carryforwards as of December 31, 2020: ~€450 million* Estimated Full Year 2021 Tax Assumptions Planned Full Year 2021 Expenses and Capex *€457.9 million corporate income tax losses and €450.9 million trade tax losses related to the German tax group

Agenda 31 Full Year 2020 Highlights Oncology Pipeline Update Financial Results COVID-19 Vaccine Update Strategic Outlook
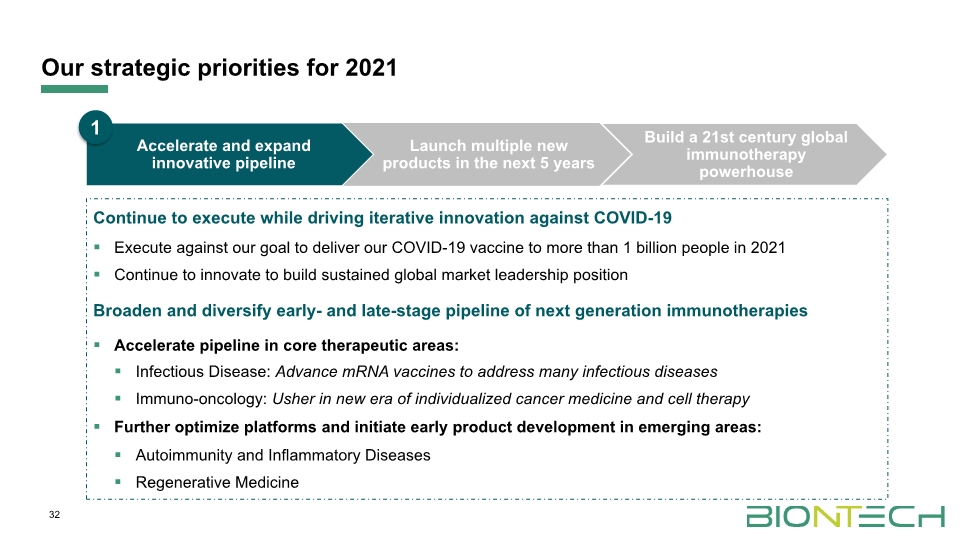
Our strategic priorities for 2021 1 32 Continue to execute while driving iterative innovation against COVID-19 Execute against our goal to deliver our COVID-19 vaccine to more than 1 billion people in 2021 Continue to innovate to build sustained global market leadership position Broaden and diversify early- and late-stage pipeline of next generation immunotherapies Accelerate pipeline in core therapeutic areas: Infectious Disease: Advance mRNA vaccines to address many infectious diseases Immuno-oncology: Usher in new era of individualized cancer medicine and cell therapy Further optimize platforms and initiate early product development in emerging areas: Autoimmunity and Inflammatory Diseases Regenerative Medicine
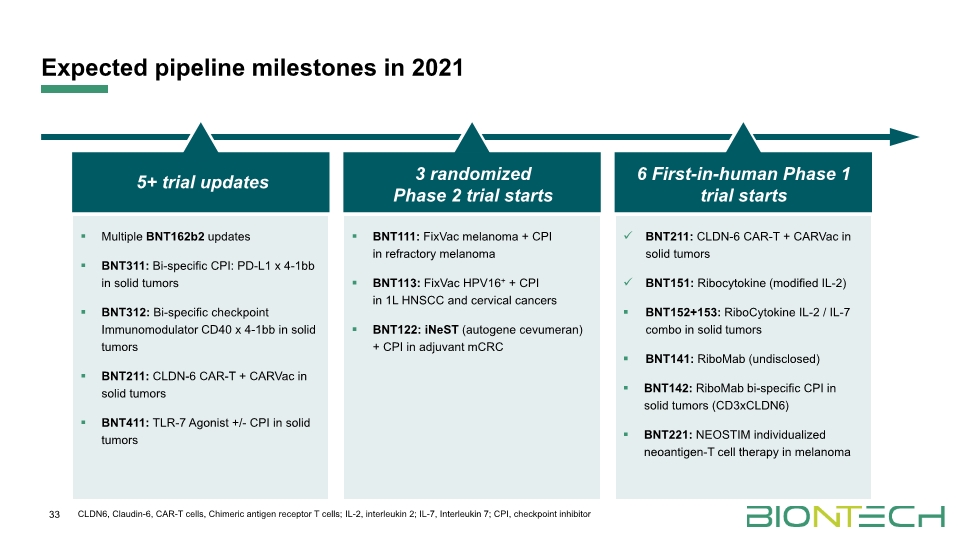
Expected pipeline milestones in 2021 33 Multiple BNT162b2 updates BNT311: Bi-specific CPI: PD-L1 x 4-1bb in solid tumors BNT312: Bi-specific checkpoint Immunomodulator CD40 x 4-1bb in solid tumors BNT211: CLDN-6 CAR-T + CARVac in solid tumors BNT411: TLR-7 Agonist +/- CPI in solid tumors BNT111: FixVac melanoma + CPI in refractory melanoma BNT113: FixVac HPV16+ + CPI in 1L HNSCC and cervical cancers BNT122: iNeST (autogene cevumeran) + CPI in adjuvant mCRC BNT211: CLDN-6 CAR-T + CARVac in solid tumors BNT151: Ribocytokine (modified IL-2) BNT152+153: RiboCytokine IL-2 / IL-7 combo in solid tumors BNT141: RiboMab (undisclosed) BNT142: RiboMab bi-specific CPI in solid tumors (CD3xCLDN6) BNT221: NEOSTIM individualized neoantigen-T cell therapy in melanoma 5+ trial updates 3 randomized Phase 2 trial starts 6 First-in-human Phase 1 trial starts CLDN6, Claudin-6, CAR-T cells, Chimeric antigen receptor T cells; IL-2, interleukin 2; IL-7, Interleukin 7; CPI, checkpoint inhibitor

COMING SOON 34 BioNTech Capital Markets Day SECOND HALF 2021 34
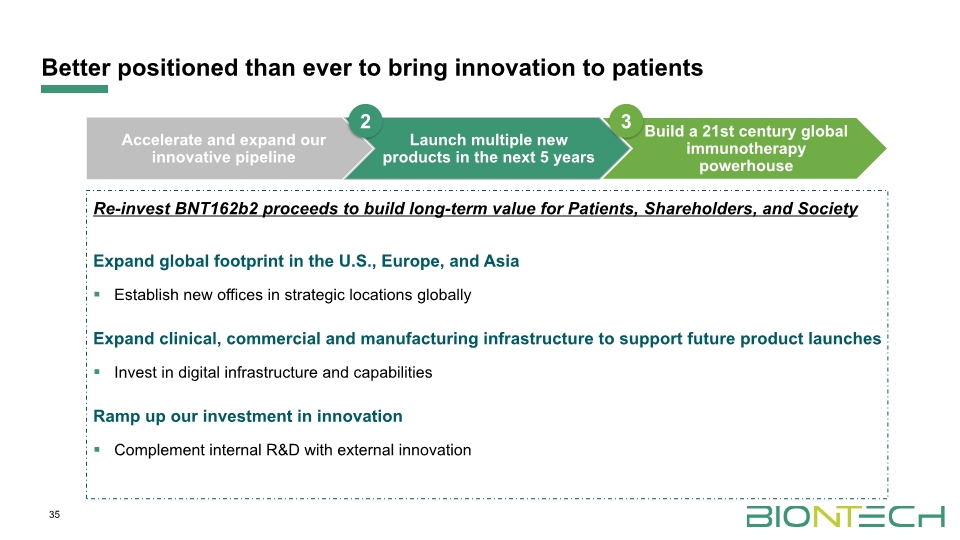
Better positioned than ever to bring innovation to patients 35 Re-invest BNT162b2 proceeds to build long-term value for Patients, Shareholders, and Society Expand global footprint in the U.S., Europe, and Asia Establish new offices in strategic locations globally Expand clinical, commercial and manufacturing infrastructure to support future product launches Invest in digital infrastructure and capabilities Ramp up our investment in innovation Complement internal R&D with external innovation 2 3

© Copyright BioNTech SE 2021. All Rights Reserved. An der Goldgrube 12 55131 Mainz Germany M: investors@biontech.de



































