UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
______________________________________________
FORM 10-K
______________________________________________
(Mark One)
| | | | | |
| x | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2024
OR
| | | | | |
| o | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 FOR THE TRANSITION PERIOD FROM TO |
Commission File Number 001-40097
______________________________________________
GINKGO BIOWORKS HOLDINGS, INC.
(Exact name of Registrant as specified in its Charter)
______________________________________________
| | | | | |
| Delaware | 87-2652913 |
(State or other jurisdiction of incorporation or organization) | (I.R.S. Employer Identification No.) |
| |
27 Drydock Avenue 8th Floor Boston, MA | 02210 |
| (Address of principal executive offices) | (Zip Code) |
Registrant’s telephone number, including area code: (877) 422-5362
______________________________________________
Securities registered pursuant to Section 12(b) of the Act:
| | | | | | | | | | | | | | |
| Title of each class | | Trading Symbol(s) | | Name of each exchange on which registered |
| Class A common stock, par value $0.0001 per share | | DNA | | NYSE |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the Registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes o No x
Indicate by check mark if the Registrant is not required to file reports pursuant to Section 13 or 15(d) of the Act. Yes o No x
Indicate by check mark whether the Registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the Registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No o
Indicate by check mark whether the Registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the Registrant was required to submit such files). Yes x No o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| | | | | | | | | | | | | | |
| Large accelerated filer | x | | Accelerated filer | o |
| Non-accelerated filer | o | | Smaller reporting company | o |
| | | Emerging growth company | o |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. o
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. x
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. o
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant's executive officers during the relevant recovery period pursuant to §240.10D-1(b). o
Indicate by check mark whether the Registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes o No x
As of June 28, 2024, the last business day of the registrant’s most recently completed second fiscal quarter, the aggregate market value of the voting and non-voting common equity held by our non-affiliates was approximately $617 million based upon the closing price reported for such date on the New York Stock Exchange.
As of February 17, 2025, there were 45,808,499 shares of Class A common stock, 9,225,101 shares of Class B common stock, and 3,000,000 shares of non-voting Class C common stock outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
The information required by Part III of this report is incorporated by reference from the registrant’s definitive proxy statement relating to its annual meeting of stockholders to be held in 2025, which definitive proxy statement will be filed with the Securities and Exchange Commission within 120 days after the end of the fiscal year to which this report relates.
Table of Contents
Cautionary Note Regarding Forward Looking Statements
This annual report on Form 10-K and our annual report to shareholders (the “Annual Report”) include forward-looking statements regarding, among other things, the plans, strategies and prospects, both business and financial, of Ginkgo Bioworks Holdings, Inc. (“Ginkgo”). These statements are based on the beliefs and assumptions of the management of Ginkgo. Although Ginkgo believes that its plans, intentions and expectations reflected in or suggested by these forward-looking statements are reasonable, Ginkgo cannot assure you that it will achieve or realize these plans, intentions or expectations. Forward-looking statements are inherently subject to risks, uncertainties and assumptions. Generally, statements that are not historical facts, including statements concerning possible or assumed future actions, business strategies, events or results of operations, are forward-looking statements. These statements may be preceded by, followed by or include the words “believes”, “estimates”, “expects”, “projects”, “forecasts”, “may”, “will”, “should”, “seeks”, “plans”, “scheduled”, “anticipates” or “intends” or similar expressions. Forward-looking statements contained in the Annual Report include, but are not limited to, statements about:
•Ginkgo’s ability to raise additional capital in the future and to comply with restrictive covenants related to long-term indebtedness;
•Ginkgo’s ability to retain or recruit, or adapt to changes required in, its founders, senior executives, key personnel or directors;
•factors relating to the business, operations and financial performance of Ginkgo, including:
◦the performance and output of Ginkgo’s cell engineering and biosecurity offerings;
◦Ginkgo’s ability to effectively manage its organizational changes, including its restructuring actions commenced in 2024, and related impacts on Ginkgo’s financial performance;
◦Ginkgo’s exposure to the volatility and liquidity risks inherent in holding equity interests in certain of its customers;
◦rapidly changing technology and extensive competition in the synthetic biology industry that could make the products and processes Ginkgo is developing obsolete or non-competitive unless it continues to collaborate on the development of new and improved products and processes and pursue new market opportunities;
◦Ginkgo’s ability to convert potential customers from “on prem” research and development (“R&D”) to outsourced services, Ginkgo’s reliance on its customers to develop, produce and manufacture products using the engineered cells and/or biomanufacturing processes that Ginkgo develops and Ginkgo’s ability to accurately predict customer demand, including with respect to the data we access and hold;
◦the anticipated growth of Ginkgo’s biomonitoring and bioinformatics services and the relative value of the services on Ginkgo’s future Biosecurity revenue;
◦the scope and timing of Ginkgo's partnerships around the world;
◦Ginkgo’s ability to comply with laws and regulations applicable to its business; and
◦market conditions and global and economic factors beyond Ginkgo’s control, including initiatives undertaken by the U.S. government in the biotechnology sector, the frequency and scale of biological risks and threats, and the future potential and commercial applications of artificial intelligence (“AI”) and the biotechnology sector.
Each forward-looking statement is subject to risks and uncertainties that could cause actual results to differ materially from those expressed or implied by such forward-looking statements. Applicable risks and uncertainties include, among others:
•intense competition and competitive pressures from other companies worldwide in the industries in which Ginkgo operates;
•litigation, including securities or shareholder litigation, and the ability to adequately protect Ginkgo’s intellectual property rights;
•the success of Ginkgo’s programs, including the growing efficiency and cost-advantage of Foundry cell engineering services, and their potential to contribute revenue, and the relative contribution of Ginkgo’s programs to its future revenue, including the potential for future revenue related to downstream value to be in the form of potential future milestone payments, royalties, and/or equity consideration; and
•other factors detailed under the section entitled “Risk Factors.”
These and other factors that could cause actual results to differ from those implied by the forward-looking statements in this Annual Report are more fully described under the heading “Risk Factors” and elsewhere in this report. The risks described under the heading “Risk Factors” are not exhaustive. Other sections of this Annual Report describe additional
factors that could adversely affect the business, financial condition or results of Ginkgo. New risk factors emerge from time to time and it is not possible to predict all such risk factors, nor can Ginkgo assess the impact of all such risk factors on the business of Ginkgo, or the extent to which any factor or combination of factors may cause actual results to differ materially from those contained in any forward-looking statements. Forward-looking statements are not guarantees of performance. You should not put undue reliance on these statements, which speak only as of the date hereof. All forward-looking statements attributable to Ginkgo or persons acting on its behalf are expressly qualified in their entirety by the foregoing cautionary statements. Ginkgo undertakes no obligation to update or revise publicly any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law.
Risk Factors Summary
Investing in our securities involves risks. You should carefully consider the risks described in “Risk Factors” beginning on page 22 before making a decision to invest in our Class A common stock. If any of these risks actually occur, our business, financial condition and results of operations would likely be materially adversely affected. Some of the risks related to Ginkgo’s business and industry are summarized below. References in the summary below to “we,” “us,” “our” and “the Company” generally refer to Ginkgo. •We have a history of net losses. We expect to continue to incur losses for the foreseeable future, and we may never achieve or maintain profitability.
•Only our employees and directors are entitled to hold shares of Class B common stock (including shares of Class B common stock issued in the future), which have ten votes per share. This limits or precludes other stockholders’ ability to influence the outcome of matters submitted to stockholders for approval, including the election of directors, the approval of certain employee compensation plans, the adoption of certain amendments to our organizational documents and the approval of any merger, consolidation, sale of all or substantially all of our assets, or other major corporate transactions requiring stockholder approval.
•We may need substantial additional capital in the future in order to fund our business.
•We have experienced periods of significant organizational change, and if we fail to effectively manage these changes, then our business, results of operations, and financial condition could be adversely affected.
•Our limited operating history and evolving business makes it difficult to evaluate our current business and future prospects.
•We have exposure to the volatility and liquidity risks inherent in holding equity interests in other operating companies and other non-cash consideration.
•We have in the past, and in the future may continue to pursue strategic acquisitions and investments that are dilutive to our stockholders and that could have an adverse impact on our business if they are unsuccessful.
•We must continue to secure and maintain sufficient and stable supplies of laboratory reagents, consumables, equipment, and laboratory services. We depend on a limited number of suppliers, some of which are single-source suppliers, and contract manufacturers for critical supplies, equipment, and services for research, development, and manufacturing of our products and processes. Our reliance on these third parties exposes us to risks relating to costs, contractual terms, supply, and logistics, and the loss of any one or more of these suppliers or contract manufacturers or their failure to supply us with the necessary supplies, equipment, or services on a timely basis, could cause delays in our research, development, or production capacity and adversely affect our business.
•We use biological, hazardous, flammable and/or regulated materials that require considerable training, expertise and expense for handling, storage and disposal and may result in claims against us.
•Third parties may use our engineered cells, materials, and organisms and accompanying production processes in ways that could damage our reputation.
•Our investments in and use of AI may result in reputational harm, liabilities, or other adverse consequences to our business operations.
•Our recent restructuring actions in connection with our plans to reduce operational expenditures may not result in anticipated savings, could result in total costs and expenses that are greater than expected and could disrupt our business.
•If our customers discontinue their development, production and manufacturing efforts using our engineered cells and/or biomanufacturing processes, our future financial position may be adversely impacted.
•Further, because our revenue is concentrated in a limited number of customers, some of which are related parties, our revenue, results of operations, cash flows and reputation in the marketplace may suffer upon the loss of a significant customer.
•We are or could become involved in securities or shareholder litigation and other related matters, which could be expensive and time-consuming. Such litigation and related matters could harm our business.
•In certain cases, our business partners may have discretion in determining when and whether to make announcements about the status of our collaborations, including about developments and timelines for advancing programs, and the price of our common stock may decline as a result of announcements of unexpected results or developments.
•Uncertainty regarding the demand for passive monitoring programs and biosecurity services could materially adversely affect our business.
•Rapidly changing technology and emerging competition in the synthetic biology industry could make the platform, programs, and products we and our customers are developing obsolete or non-competitive unless we continue to develop our platform and pursue new market opportunities.
•Ethical, legal and social concerns about genetically modified organisms (“GMOs”) and genetically modified plant or animal cells and genetically modified proteins and biomaterials (collectively, “Genetically Modified Materials”) and their resulting products could limit or prevent the use of products or processes using our technologies, limit public acceptance of such products or processes and limit our revenues.
•If we are unable to obtain, maintain and defend patents protecting our intellectual property, our competitive position will be harmed. If we are unable to protect the confidentiality of our trade secrets, our business and competitive position will be harmed. We may become involved in lawsuits or other enforcement proceedings to protect or enforce our patents or other intellectual property, which could be expensive, time consuming and potentially unsuccessful.
•We rely on our customers, joint venturers, equity investees and other third parties to deliver timely and accurate information in order to accurately report our financial results in the time frame and manner required by law.
•We had in the past identified a material weakness in our internal controls over financial reporting, and we may identify additional material weaknesses in the future. A failure to maintain an effective system of internal control over financial reporting may result in a failure to accurately report our financial results or prevent fraud. As a result, stockholders could lose confidence in our financial and other public reporting, which would harm our business and the trading price of our common stock.
•Failure to comply with federal, state, local and international laws and regulations could expose us to significant liabilities or penalties and adversely affect our business, our financial condition and results of operations and we may incur significant costs complying with such laws and regulations
•We and our laboratory partners are subject to a variety of laboratory testing standards, compliance with which is an expensive and time-consuming process, and any failure to comply could result in substantial penalties and disruptions to our business.
•Significant disruptions to our and our service providers’ information technology systems or data security incidents could result in significant financial, legal, regulatory, business and reputational harm to us.
PART I
Item 1. Business.
Unless the context otherwise requires, all references in this section to the “Company,” “Ginkgo,” “we,” “us,” or “our” refer to the business of Ginkgo Bioworks Holdings, Inc. and our subsidiaries.
Overview: Our Mission is to Make Biology Easier to Engineer
Our mission is to make biology easier to engineer. That has never changed. Every choice we’ve made with respect to our business model, our platform, our people, and our culture is grounded in whether it will advance our mission.
Why? Because:
1.Biology is programmable. All living things run on the same DNA code.
2.Biology matters. The ability to engineer biology has had and will have a profound impact on how we develop new medicines and vaccines, grow our food, and manufacture many of the things we use every day.
3.Biology is hard. Today, it is still too difficult and too costly to engineer biology, preventing critical innovations from reaching the market.
Ginkgo sells services in two business segments: cell engineering, where we provide biological R&D services for our customers across a range of industries, and biosecurity, where we provide services to government and commercial customers so they can work to identify, monitor, prevent, mitigate, and ultimately protect humanity from biological threats. An overview of these two business segments is provided below.
Cell engineering
Our cell engineering customers work with biology to discover and manufacture new products that have transformative potential across industries:
•in medicine, developing innovative new therapeutics and vaccines;
•in agriculture, advancing the sustainability and security of our food systems;
•in industrial biotechnology, advancing the way we manufacture a wide range of products for better performance and lower environmental impact; and
•in government, advancing new R&D priorities of strategic importance to the United States and its allies.
Because engineering biology is difficult and unpredictable, biotech R&D is traditionally performed by in-house labs filled with highly trained scientists running lab experiments by hand over several years in the hope of ultimately developing a working product. Many cell engineering projects fail in development due to scientific challenges, and many are terminated because they are taking too long or are over budget.
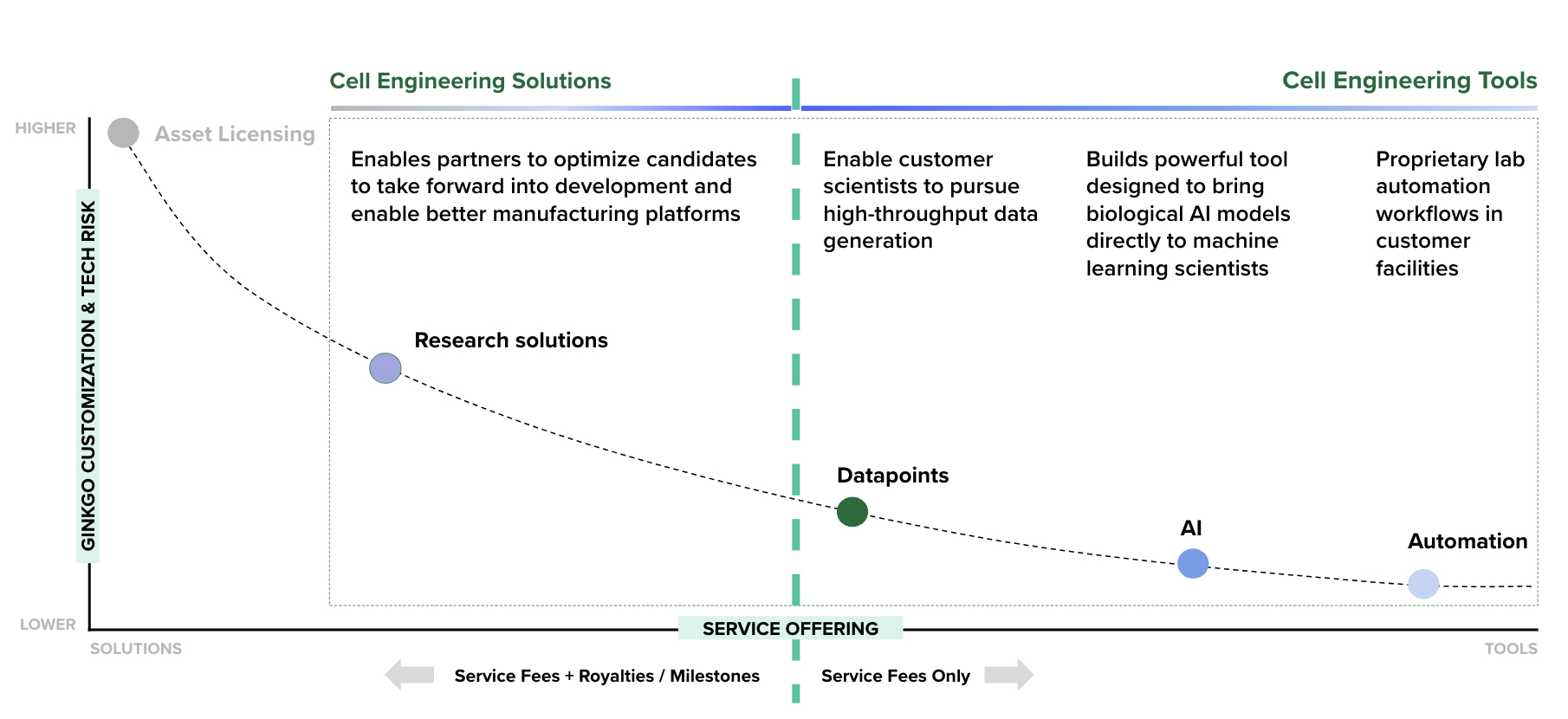
Ginkgo does not make end products; instead, we offer biological R&D services on our platform to enable our customers to bring their products to market. Historically, Ginkgo’s primary service offering has been end-to-end cell engineering R&D services (solutions). In 2024, Ginkgo expanded its service offering to include services that provide our customers cell engineering tools for biological R&D, which are intended to provide more targeted and bespoke resources to customers that continue to conduct in-house R&D. Our services are designed to offer customers better results on the dimensions of probability of success, speed, or cost – and ideally on all three.
The fundamental advantage of our cell engineering platform over traditional cell engineering done by hand at our customers’ labs is that our platform improves with scale while in-house cell engineering in our customers' labs largely does not. Compounding and mutually reinforcing improvements of our laboratory automation and software infrastructure—our Foundry—and our reusable data assets—our Codebase—enable us to improve our services with each successive project.
Our Foundry is a flexible capability for large scale data generation; it powers generative AI and machine learning (“ML”) tools that enable more successful biological R&D. We now offer services providing such data generation, AI and automation tools directly to Ginkgo customers. Our Codebase is a data asset comprising best practices for cell engineering, along with sequences and host cells that have been honed through dozens of programs and can be directly reusable for our end-to-end cell engineering solutions.
Cell engineering service offerings, depicted on a spectrum of customization and technical risk borne by Ginkgo on the vertical axis, and the mix of up-front service fees and downstream value we charge on the horizontal axis.
Biosecurity
In every technological revolution, reaping the benefits to the economy and society requires grappling with the corresponding risks. A critical part of making biology easier to engineer is creating robust biosecurity infrastructure to help manage the many accelerating and diversifying sources of biological risk, whether natural or engineered, accidental or malicious.
In the digital world, we’ve learned that we need to build comprehensive infrastructure to protect our digital systems —from financial markets to power grids—from harmful code. The modern cybersecurity industry offers tools to constantly identify, monitor, prevent, and mitigate cyber risk in near real-time. This is happening constantly, all around us. Our physical world demands the same type of widespread biosecurity infrastructure to detect, characterize, respond to, attribute, and prevent biological threats.
Building widespread biosecurity infrastructure is not easy, but the COVID-19 pandemic and subsequent biothreats (e.g., H5N1 and mpox) illustrate the growing risk and urgent need for a solution. During the COVID-19 pandemic, our healthcare infrastructure, the biomedical technology industry, and communities across the world mobilized in valiant and unprecedented ways, but millions of lives and trillions of dollars were still lost. Our current systems are overly reactive and remain insufficient to protect us from future biothreats, whether they come from Mother Nature, bioerror, or bioterror.
We need a fundamentally different approach to securing biology—one that starts with data. The genomic information that underlies the biological world is what allows us to program it like computers, and it’s what allows us to understand biology at a molecular level and learn to predict how it’s going to behave in the world. Our biosecurity platform is built on the premise that genetic information is a critical data asset that will form the foundation for next-generation biosecurity. By building services to help our customers monitor and analyze this data, we believe we are contributing to a step change in humanity's ability to rapidly and reliably identify, monitor, prevent, and mitigate biological threats.
Because biosecurity is a matter of national and global security, our primary biosecurity customers are governments. Our biosecurity offering has evolved over the past several years. We currently provide biosecurity services via two core offerings as introduced in early 2024:
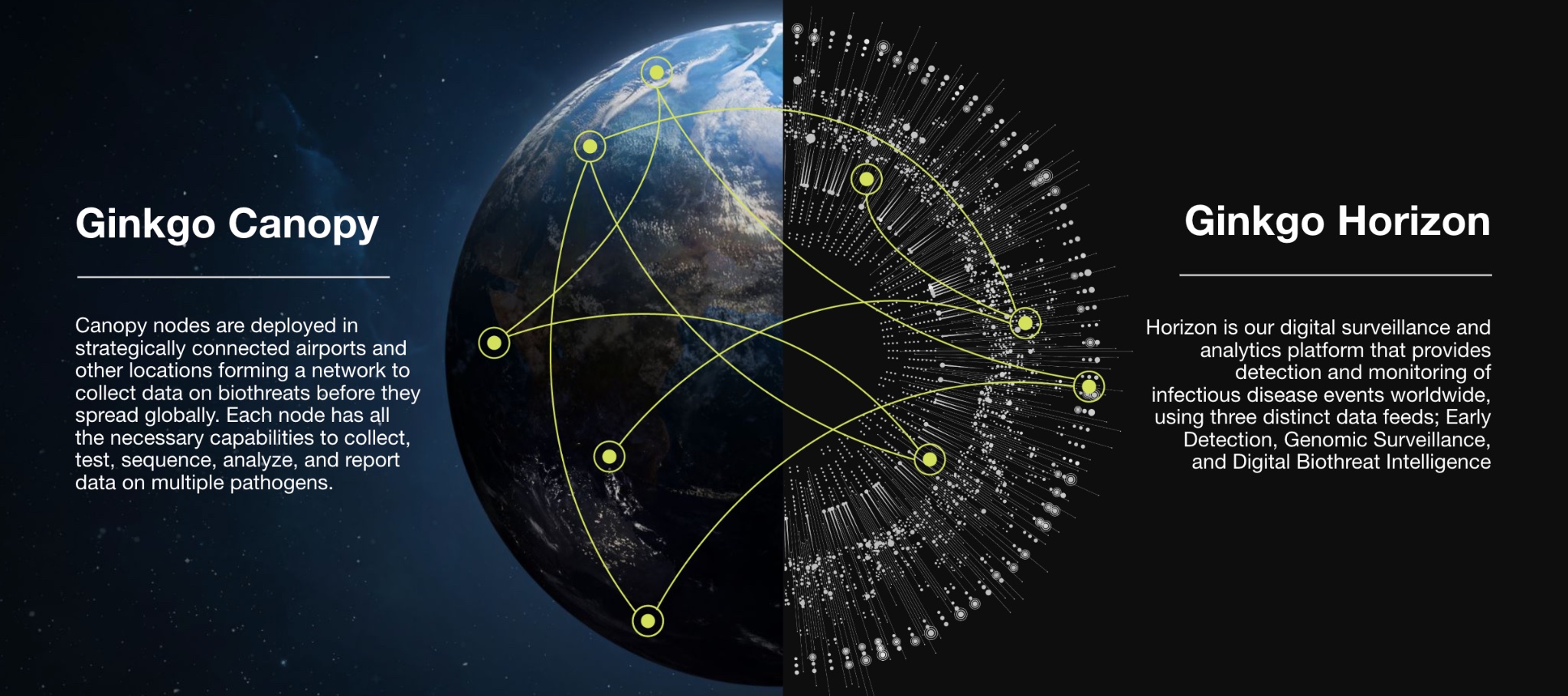
•Canopy, which helps our customers generate high value genomic data from strategically-positioned nodes (like airports and border checkpoints) via end-to-end biomonitoring programs; and
•Horizon, our digital surveillance, analytics and insights platform that detects and monitors biothreats worldwide.
Like our cell engineering platform, our biosecurity platform gets better with scale. As we deploy more detection nodes, we can achieve earlier detection and develop deeper insights about biothreats as they originate and travel across the globe. We invest in our platform to build out our operational (e.g., new node types), lab (e.g., new target detection), and digital (e.g., better prediction) capabilities. Increasing scale substantially strengthens the efficacy of our platform, as global data from multiple sources provides insights beyond what any single country’s data could yield alone.
Cell Engineering: enabling the discovery, functional optimization and efficient manufacturing of biotechnology products
Biology runs on a digital code. It’s just A’s, T’s, C’s, and G’s rather than 0’s and 1’s. There are sequences that code for programming logic—turning genes on when certain conditions are met—and there are sequences that encode functions and behaviors—the physical structures of proteins and enzymes that create biological structures and materials or catalyze chemical reactions. Synthetic biologists build cell programs by writing new sequences combining regulatory and functional elements into a synthesized strand of DNA and booting them up in cells to perform useful tasks, usually producing a particular bioproduct such as RNA, protein, enzyme, or chemical.
Biological code programs the world of atoms, not bits. This is what makes the potential impact of cell engineering so great, and inspires us to work to make biology easier to engineer and secure. But it also poses incredible challenges that make cell programming so hard today. Our code is a physical object with chemical properties. It folds and binds and interacts in many complex ways. It produces proteins that catalyze chemical reactions that interact in a complex web of connections. Even the simplest cell programs encounter incredible complexity, emerging from all of the interactions of chemicals, DNA, RNA, and proteins inside of a cell.
Because all organisms run on the same DNA code, general-purpose cell programming can be applied across many different markets to enable the design of new innovative products as well as improve manufacturing cost and sustainability of existing ones. Given the breadth of application areas and the potential of biology, we believe that the end markets for bioengineered products will be enormous. As we develop a greater ability to program biology and direct it towards novel and more challenging applications, the spectrum of possibilities will undoubtedly grow.
Today, our services span markets and modalities to enable a wide range of biotech products, including, but not limited to, systems for the discovery, optimization, and manufacturing of:
•DNA sequences delivered as vaccines and gene therapies,
•RNA sequences for vaccines and therapeutics including mRNA, circular RNA, and other approaches,
•Proteins used in biologic medicines and antibodies, adeno-associated virus (“AAV”) capsids and other delivery methods for gene therapies, vaccines, plant traits for crop protection, and food and alternative meat and dairy,
•Enzymes used in biocatalysis, diagnostics, therapeutics, and RNA vaccine production,
•Small molecules and natural products that can be produced via pathways of multiple enzymes in engineered cells for pharmaceutical ingredients and adjuvants, agricultural biochemicals, cosmetics and food ingredients, and specialty or commodity chemicals,
•Microbial cells that can provide crop nutrition or crop protection in agriculture, impact soil carbon sequestration to help address climate change, and microbiome therapeutics, and
•Mammalian cells for manufacturing of biologics, genomic medicines, and cell therapies.
Ginkgo provides these services using its platform for cell engineering. This platform brings together technology, data, biological assets and subject matter experts:
•proprietary automation technologies that enable flexibility and scale,
•in-house software, machine learning, and generative AI models for cell programming,
•massive databases of DNA sequences and labeled data on functional performance of engineered cells,
•reusable assets that enable faster and more predictable cell programming, and
•expert scientists that leverage platform tools and data to enable partners to achieve their desired results
Enabling customer success across markets
We sell end-to-end cell engineering solutions and cell engineering tools offerings to customers across markets. Our customers bring incredible depth and expertise in their unique technical domains and market areas. Whether it’s their understanding of underlying disease biology or plant physiology, their experience with the performance of regulatory trials in animal studies, in the clinic, or in the field, or their knowledge of product formulation and functional testing, they have specialized deeply to be able to develop, manufacture, distribute, and market a product. Our role is to enable: we provide our customers with R&D solutions and tools that help them access more biological design space in order to discover and optimize functionality and develop efficient manufacturing methods for their products.
Pharmaceutical and Biotechnology
There is an urgent and critical need for new, better, and more accessible therapeutics and vaccines worldwide. There is also widespread realization across the pharmaceutical industry that research productivity must be enhanced in order to meet this need. Yet, even with hundreds of billions of dollars spent annually on pharmaceutical R&D, the cost to bring a new drug to market is only increasing.
The pharmaceutical industry today relies heavily on outsourced R&D, both to specialized, innovative small biotech, as well as to contract research organizations (“CROs”) that can automate and scale specific common workflows at different stages of the R&D process for enhanced efficiency. These approaches enable access to both innovation and efficiency, but incur high switching costs both organizationally and technically.
At the same time, there is great promise in how AI tools may help uncover new disease biology and targets for therapeutics, as well as enable the programming of new medicines, in particular biologics and genomic medicines that are directly encoded in DNA and RNA sequences. Pharmaceutical R&D teams are looking for ways to generate and federate data to train better AI models, design and test more technical approaches, drive candidates at the preclinical stage to “fail fast” before costly clinical trials, and develop better leads by simultaneously optimizing along multiple dimensions important for therapeutic index as well as manufacturability and cost.
Our pharmaceutical and biotechnology customers use our services to develop new manufacturing methods for gene therapies, biologics, vaccines, and small molecule therapeutics and active pharmaceutical ingredients (“APIs”), and to discover new RNA therapeutics, natural products, and much more. They use our tools to generate high-quality data for training and validating AI models of cell and disease biology for use in target identification, target validation and drug discovery as well as for antibody developability.
Agriculture
Agriculture likewise faces an urgent need for innovation to address growing pressure on growers and food systems. Worldwide, agricultural innovation struggles due to long timelines, complex regulatory paths, and siloed data and capabilities.
Innovators in agricultural technology tap into biological diversity to develop new crop protection strategies, to combat resistance, and to provide safer, low residue options for growers that meet consumer expectations and regulatory
guidelines. These product developers need to understand mode of action and improve the performance and stability of innovative biologicals for crop nutrition and crop protection. And increasingly, they are also innovating in soil carbon sequestration and climate strategies.
Our customers in agriculture use our services to improve the performance and manufacturability of existing agricultural biologics, develop revolutionary new products for crop nutrition in nitrogen fixation, phosphate solubilization, or carbon sequestration, and design new insect control proteins and other crop protection products to protect food security. We also offer 3,000 liters of pilot plant capacity for efficacy trial material generation, as well as a network of growth chambers and an approximately 12,000 square foot greenhouse facility for screening engineered microbes and wild-type microbes in planta.
Industrial Biotechnology
There is an enormous breadth of products—chemicals, enzymes, and proteins—already produced via biotechnology today or being actively developed by companies across many markets, including food and nutritional ingredients, wellness, cosmetics, and personal care, industrial processes and chemicals, and materials innovation.
Our customers in industrial biotech use our services to improve the manufacturing efficiency and cost of goods sold for their new and existing biotechnological products, innovate materials with enhanced performance, develop enzymes for breaking down harmful pollutants or cells and proteins optimized for capturing rare earth elements, or valorize waste streams into feedstocks for more valuable products.
Government
Ginkgo is a trusted partner to government agencies worldwide as they work to protect people, supply chains, and critical resources, all by leveraging the power of biology. We regularly serve as a prime contractor on major research awards, bringing together partners and integrating their diverse biological capabilities. We also often support projects and research consortia as an R&D subcontractor. The government agencies we partner with are tasked with safeguarding the future of national agriculture and food security, public health and biosecurity, energy independence and environmental sustainability, as well as healthcare, wellbeing, and longevity.
The end-to-end cell engineering solutions that we sell
A selection of our end-to-end cell engineering solutions offerings are described below.
Protein engineering and production for biopharmaceutical applications
We provide a suite of microservices for product developers across industries that support bioengineers with protein engineering an enzyme variant library design service, access to our proprietary low-viscosity Aspergillus niger strains, the performance of proof-of-concept protein production studies to evaluate and optimize protein production in high-performance chassis, as well as access to our EncapS platform - a cutting-edge, ultra-high throughput screening service designed to rapidly identify strains with enhanced protein titers. These on-demand services balance flexibility and cost-effectiveness with access to powerful technologies that enhance R&D efficiency.
Genetic medicines
We provide comprehensive R&D services spanning the major genetic medicine modalities: gene therapy, cell therapy and gene editing, and RNA therapeutics. Our capabilities include developing precision delivery systems through viral and non-viral approaches, engineering payloads for targeted expression, and optimizing manufacturing processes at scale. For gene therapy, we provide AAV capsid design and optimization, payload engineering, and high-titer production solutions. In cell therapy and gene editing, we support CAR-T development, gene editor discovery, and immune cell engineering with our high-throughput screening platforms. For RNA therapeutics, our services are intended to help customers optimize mRNA and circular RNA sequences for improved stability and expression, alongside lipid nanoparticle delivery system development. These services are complemented by our suite of licensable assets including capsids, promoters, untranslated regions (“UTRs”), internal ribosome entry sites (“IRESs”), and chimeric antigen receptor (“CAR”) components, validated
through extensive in vitro and in vivo testing. Our integrated approach combines domain expertise in protein engineering, high-throughput experimentation, and advanced analytics to address key challenges in immunogenicity, off-target effects, and regulatory compliance.
Packaged solutions for biopharmaceutical applications
We provide standardized, ready-to-use solutions for accelerating biopharmaceutical R&D. We provide curated panels of enzymes for active pharmaceutical ingredient (API) manufacturing, including 192 Imine Reductase (IRED) enzymes and 384 2-Oxoglutarate Dependent Dioxygenases (2ODD) enzymes. Our EncapS platform for API production leverages ultra-high throughput screening technology to improve production strain productivity by 10-30%, screening libraries of over 1 million variants through nanoliter co-encapsulation. For genetic medicines, we provide licensable assets including promoters that surpass industry standards, optimized UTRs for enhanced RNA stability and protein expression, engineered AAV capsids with reduced antigenicity and improved tissue targeting, and synthetic immune receptors for cell therapies. These offerings are supported by easy-to-access fee-for-service programs, including our collaboration with Virica Biotech for AAV manufacturing optimization.
Small molecules & biologics for biopharmaceutical applications
We provide comprehensive R&D services spanning both small molecule therapeutics and biologics. For small molecules, we enable the discovery and production of diverse compounds including APIs, natural products, antibiotics, antifungals, antivirals, and antibody-drug conjugate (ADC) payloads through our strain engineering expertise and biosynthetic pathway discovery capabilities. Our biologics services support the development and optimization of therapeutic proteins, peptides, enzymes, and other biological products using AI-driven protein engineering and our proprietary production platforms, including high-output methanol-free Pichia pastoris strains. These complementary capabilities are supported by our integrated approach to strain design, high-throughput screening, fermentation optimization, and downstream processing, all focused on achieving economical and high-quality production outcomes for both chemical and biological therapeutics.
Crop nutrition
We provide comprehensive R&D services focused on advancing biological solutions for crop nutrition and plant health. Our services span three key areas: nutrient use efficiency, where we help develop products that optimize nutrient bioavailability; carbon fixation, where we work on biologicals that enhance soil health; and abiotic stress management, where we develop solutions to improve crop resilience against environmental stressors. These capabilities are supported by our extensive strain collection of over 315,000 agriculturally-relevant microorganisms, validated in vitro and in planta screening assays, and expertise in scaling up both whole-cell and biochemical active products. We offer both fee-for-service testing using our validated assay cascade from 384-well plates to greenhouse studies, as well as collaborative R&D partnerships spanning strain engineering through process development and formulation optimization. A flagship example of our capabilities is our partnership with Bayer Crop Sciences, where we are developing biological solutions for nitrogen fixation in non-legume crops to reduce reliance on industrial fertilizers.
Crop protection
We provide comprehensive R&D services to help partners develop and optimize biological crop protection products. Our capabilities include validated in vitro and in planta screening assays that enable confident selection of lead candidates, extensive expertise in scaling up active ingredients including both Cry proteins and novel bioactives, and access to our proprietary collection of over 315,000 strains isolated from agriculturally relevant environments. We work with partners through both fee-for-service arrangements focused on generating rigorous data packages to inform decision-making, as well as collaborative R&D projects aimed at developing market-ready products. Our services span the full product development cycle from strain selection and screening through process optimization, formulation development, and field trial support. Partners can access our platform to expand their product portfolios with novel biocontrols, optimize the performance of existing products, reduce manufacturing costs, or generate reliable data to support their development programs. Throughout the development process, we focus on optimizing manufacturing costs and process efficiency to ensure products can be produced at commercially viable price points that meet market requirements for growers and product developers that market to them.
Plant traits
We provide comprehensive R&D services for discovering and optimizing plant traits, leveraging our proprietary metagenomic library of over 2.7 billion genes (with less than 5% overlap with public databases). Our capabilities in this space combine machine learning-based protein engineering, informed by over 15 years of experimental data collection, with expertise in optimizing multi-gene-of-interest trait expression in complex eukaryotic systems. We provide services spanning the full development cycle, from early trait discovery using patented enrichment and amplification techniques, through lead optimization using iterative high-throughput screening approaches, to gene construct design with optimized codons and promoters for more reliable expression. Our pilot plant site allows us to leverage proprietary host strains optimized for protein and enzyme production to test out material generation capabilities through large-scale efficacy trials.
Protein engineering and production for industrial biotechnology
We provide R&D services that help our partners optimize production hosts for nutritional and functional protein manufacturing, and to develop effective enzymes and biocatalysts for industrial applications. Our industrial partners can access our enzyme discovery, enzyme optimization, assay development & high-throughput screening, and protein production expertise, taking advantage of our range of well-characterized and high-performance bacterial, fungal, and mammalian chassis strains, as well as our very wide range of experience designing and running scientific campaigns to engineer and improve these hosts. Our partners in the nutritional and functional protein space work with us to build research campaigns that leverage the above capabilities, as well as our investment into an in-house suite of host strains designed for glycoprotein, iron-bound, and structural protein production. Whether they are developing sweeteners, alternative dairy, alternative egg, thickeners, preservatives, or other exciting protein-based innovations, we can offer our partners in this space a mix of host evaluation and proof-of-concept studies, production host design and optimization campaigns, enzyme discovery and optimization research, and process development & scale-up (described further below) capabilities to help de-risk and accelerate their R&D and go-to-market timelines.
Small molecules for industrial biotechnology
We work with partners in the sustainable ingredients and chemical spaces, as well as across the flavors, fragrance, and colorant industries to support their R&D and manufacturing optimization efforts. Our particular expertise includes experience with microbial bio-production of many classes of natural products, including of terpenoids, esters, aromatics, organic acids, pigments, and bioactive alkaloids. We offer a range of custom R&D solutions that support our customers with molecule prototyping and pathway discovery, strain design and optimization for bioproduction, non-genetically modified strain improvement technologies, and our bioprocess development and scale-up capabilities (described below). In the flavors, fragrances, and colorants industries, customers also often access our proprietary database of microbial chassis and specialized tailoring enzymes (like terpene synthases, glycosyl transferases (UGTs), O-methyltransferases, and cytochrome P450s) which supports their efforts to improve pathway optimization, byproduct reduction, and enhanced product safety.
Fermentation and scale-up
Our Foundry includes a fleet of Sartorius Ambr® 250 bioreactors, as well as a suite of colocated analytical instruments that our customers can leverage to deeply and widely explore strain performance across multiple fermentation and media conditions. We can pair this service with customized ML-enabled analysis to obtain and provide to our customers deep physiological, genetic, and chemical insight into strain performance.
On the larger scale, we also offer in-house pilot fermentation capacities at 5, 10, 30, and all the way up to 3,000 liters of pilot plant capacity for bioprocess development and scale-up. We can support our partners in proving out production processes with iterative and statistical approaches to develop robust fermentation process conditions, informed by automated sampling and standard analyses of secreted metabolites.
The cell engineering tools that we sell
A selection of our cell engineering tools offerings are described below.
Datapoints
Recent advances in ML, molecular simulation, and other computational techniques hold great promise to improve our ability to program cells. We believe our Foundry is well-positioned to build the kind of large, well-structured datasets that such computational approaches need to succeed. In time, we believe computational approaches will reduce the need for certain kinds of experiments (for example, we already use ML to make protein and enzyme design projects more efficient).
To this end, we have introduced two new data generation services to provide high-quality data at the scale, price, and speed that AI-powered drug development demands:
●Our Functional Genomics Datapoints services generate large, high fidelity transcriptomic and phenotypic datasets in the disease context of our customers’ choice to power AI models of cell and disease biology for use in target identification, target validation, and drug discovery; and
●Our Antibody Developability Datapoints services generate biophysical antibody characterization developability datasets for our customers to use in AI model training and validation.
Reconfigurable Automation Cart (“RAC”) Systems
Ginkgo Automation’s capabilities build on years of internal expertise honed at Ginkgo and Zymergen, encompassing hardware design, software integration, and applications development, epitomized in our offering of RACs: our Reconfigurable Automation Cart systems. The modularity and flexibility of the RACs enables high walkway time, high uptime, and high throughput experimentation for high-mix biological workflows like the kinds performed in Ginkgo’s Foundry and in our partners’ labs. In addition to providing advanced automation hardware and software, Ginkgo Automation’s deployments to third party customers include access to Catalyst Flow, a fully remote, active error resolution and troubleshooting support service. Catalyst Flow’s proactive monitoring is expected to enable Ginkgo’s scientists and engineers to identify and resolve approximately 80-90% of system errors remotely, without the need for our customers to initiate tickets.
AI models
As Ginkgo drives scalability through our models, we have heavily invested in the use and creation of AI foundational and fine-tuned models, which we believe can provide significant benefits to our customers. Efficient use of AI is only possible with the use of massive amounts of data. Because of our access to large amounts of data, Ginkgo has the ability to build superior foundational models and from there, build fine-tuned models designed to cater to our customer needs, all powered by our partnership with Google Cloud. We are releasing a stream of these models on our Model API.
Biosecurity: scaling biological intelligence for securing lives and livelihoods
Addressing biosecurity starts with being clear-eyed about biological risks and threats. We hold at our core the tremendous positive potential of biology, and we know that we’re facing a biological landscape with more frequent, more severe, and more varied threats through time.
Our world is increasingly interconnected through travel and trade, giving pathogens and biological agents new opportunities to spread across the globe, impacting people’s health along with the complex global supply chains that our societies depend on to function. Climate change and habitat disruption are creating conditions for pathogens to emerge and spill over between animal populations and into humans more often and with more severe consequences. A global boom in investments into bio-laboratory capacity, designed to improve our tools to combat such pathogens, also comes with heightened risk of lab accidents—in spite of substantial efforts to improve biosafety. And unfortunately, there are those who seek to use biology for nefarious purposes, misusing its incredible potential to cause harm.
These trends are intertwined with geopolitical competition and destabilization, eroding buy-in and trust in institutions, and emerging technologies in both biotechnology and AI/ML, presenting a core security challenge for nations and the world. Our biosecurity platform is designed to help national and global leaders answer questions about and potentially protect from biological threats.
Governments around the world are currently our primary customers as they seek to protect their citizens, economies, and critical infrastructures from biothreats. We have worked extensively with the U.S. government across the federal, state, and regional levels, and maintain the lab, logistical, and local relationships necessary to work closely with partners across the country in the event of any large-scale biothreat response.
The biosecurity services that we sell
We serve these customers through two core offerings:
•Canopy helps our customers generate high value genomic data from strategically positioned nodes (e.g., airports and borders) via end-to-end biomonitoring programs including:
oProgram services such as program design, lab enablement, procurement, collection, and lab support;
oData services such as bioinformatics and epidemiological tools, data delivery and data visualization;
oInsights such as specialized reports and technical briefings specific to customers’ programs; and
oProducts such as standard of procedures (SOPs) and specialized lab assays.
•Horizon is our data, analytics and insights platform that detects and monitors biothreats worldwide. It seeks to answer questions like:
oWhat biothreats are occurring? Where in the world do we see emerging biothreats?
oWhat is this new biothreat and how bad is it? How might it spread and evolve? Who (or what infrastructure) will be affected? How likely is the biothreat to result in a major outbreak?
oWhere did a biothreat emerge and how? Is there evidence of misuse and if so, what can we learn about the perpetrators?
oWhat can I do about a biothreat? How effective will existing countermeasures be? Should we develop new countermeasures, and if so, what should they look like? What are my ideal response options given resource constraints and mitigation goals?
Ginkgo Biosecurity’s tailored Canopy product is a persistent, pervasive, locally-operated collection of environmental samples from strategic high-risk nodes—designed to detect and identify biothreats across a growing array of collection and sample types. The samples are analyzed through genomic sequencing of non-human DNA to turn the environment into data. Today, we are looking for a large and growing set of known threats, and we are developing methods that are threat agnostic and able to pick up on entirely novel genetic signatures.
Our Canopy technologies are expanding, and our goal is to be able to sample from a wide variety of nodes where pathogens emerge or spread, such as those pictured below. Our network scales to be more efficient as we add greater volume and diversity of nodes. Our Canopy product scales primarily by driving efficiency through increased volume by better physical and lab operations including program learnings, lower overhead requirements, and purchasing power.
(Note: nodes are illustrative and not necessarily indicative of current or past programs.)
Horizon is our data, analytics and insights platform that provides reporting of biothreat events worldwide, using three distinct data feeds: Early Detection, Genomic Surveillance, and Digital Biothreat Intelligence. The Canopy data from given programs or jurisdictions are integrated with other global data sources—from our monitoring network, open-source intelligence capability, and other sources—and analyzed using a suite of computational tools to help customers gain a more comprehensive picture of the biothreats they’re facing. Our Horizon business also scales as volume and node diversity increases as we have more information (e.g., via volumes) and more diverse data (e.g., via nodes), our insights across the system are further enhanced.
Our global network includes countries and several multilateral organizations who are actively building biosecurity infrastructure with our services.
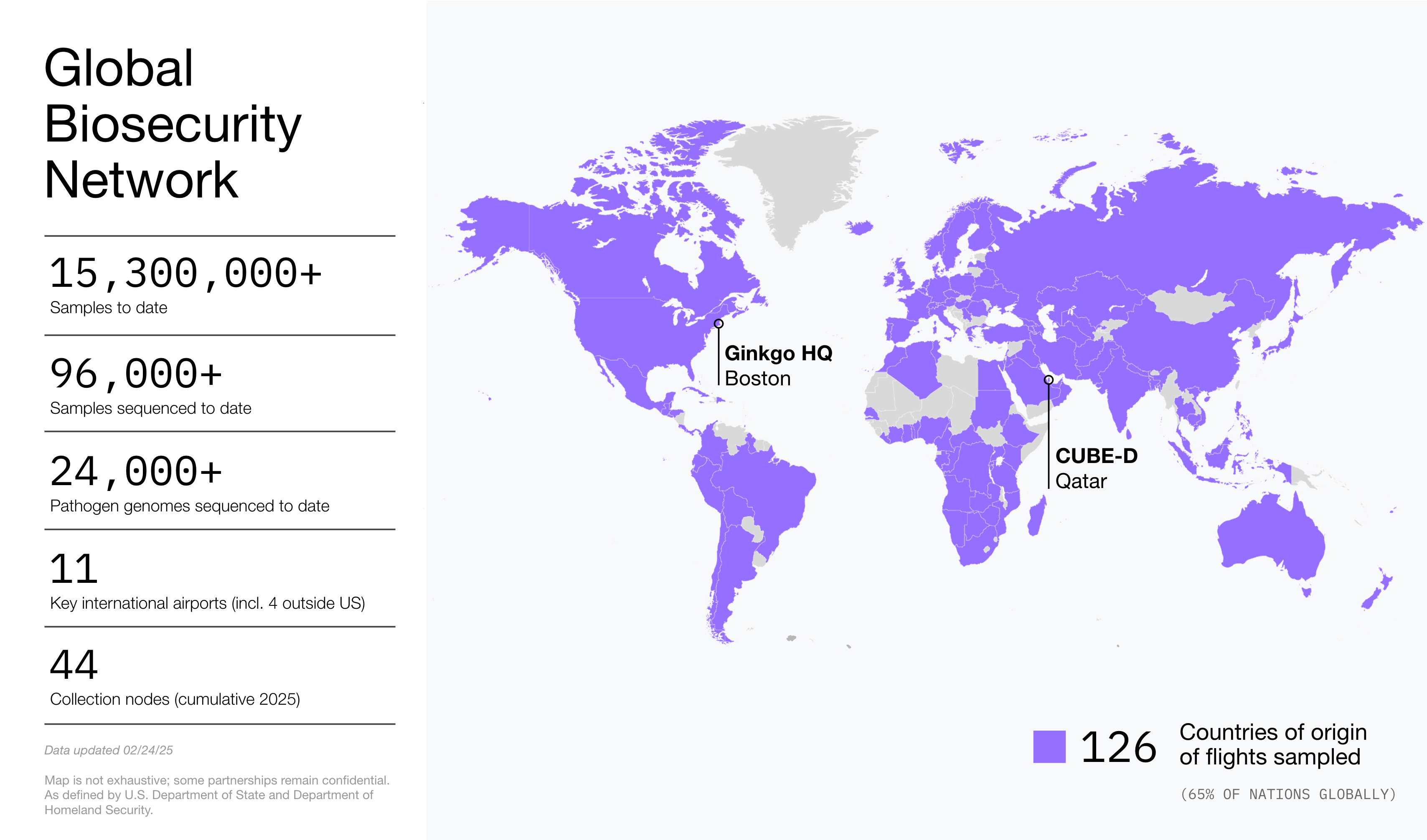
Together, these products are serving customers across the globe including nodes with over 126 countries of origin sampled as of February 19, 2025. Our operations are anchored by Ginkgo’s headquarters in Boston which serves as a hub for managing our programs and analyzing data from our global network. Our network is increasingly bolstered by our operational and laboratory partners throughout the world. We will continue to enhance this network in the future through both new partnerships and regional hubs, known as Centers for Unified Biosecurity Excellence (CUBEs). After initially planning for a location in Doha, Qatar, Ginkgo Biosecurity and its in-country partner decided to move the facility to another location in Doha. Negotiations are ongoing, and we anticipate completion of the facility in 2026. When completed, this facility will support analysis of data from bioradar nodes across the region to generate insights for our customers.
How we make money
Cell engineering revenue
End-to-end cell engineering solutions
Our end-to-end cell engineering solutions are typically scoped and delivered as a program ranging in duration from several months to several years. A typical deliverable for the program would comprise an engineered strain or cell line and an associated bioprocess. For each of these programs, we generate economic value in two primary ways.
First, we charge service fees for Foundry services, in much the same way that cloud computing companies charge usage fees for utilization of computing capacity or CROs charge for services. R&D is inherently risky and our customers recognize that this is a cost they will incur regardless of success and whether they are working on the program in-house or with a partner. Typically, service fees for a program include a fixed fee for a fixed scope of work and may also include payments contingent upon hitting certain technical milestones. If we are able to deliver program results with less work through the use of Codebase assets and/or generative AI tools, then we can achieve the same revenue with lower cost or in
a shorter duration. Service fees provide a strong foundation of revenue that is independent of any commercialization efforts by our partners.
Second, as the key enabling technology for our customers’ products, we have historically negotiated a value share with our customers (in the form of royalties, milestones, and/or equity interests) in order to align our economics with the success of the programs enabled by our platform. Because we typically do not incur material downstream costs (e.g., manufacturing or product development, which our customers manage), these value share payments flow through with approximately 100% contribution margin. We are quite flexible and have structured a variety of value sharing mechanisms, including royalties, lump-sum milestones, and equity payments. As Ginkgo has matured, we have shifted our downstream value towards milestone payments and commercial royalties rather than equity. In addition, commencing in the second quarter of 2024, we announced changes in prospective commercial terms, including the removal of downstream value share from certain program types.
This flexible business model allows for more predictable near-term revenue in up-front research fees and technical milestones without sacrificing our ability to create long-term value with asymmetric upside through downstream value share (typically in the form of a royalty stream, milestone, and/or equity share). As we add more programs to the platform over time, we expect downstream value share to contribute income, and therefore we believe our overall margins and cash flow profile for our end-to-end cell engineering solutions will grow significantly. The realization of potential revenue related to downstream value in the form of potential future milestone payments and royalties and/or equity consideration is dependent upon a number of factors, including our ability to successfully develop engineered cells, bioprocesses, data packages, or other deliverables, and the product development and commercialization success of our customers.
Cell engineering tools offerings
We charge customers fees for the services we provide in our cell engineering tools offerings. Typically, these fees are structured as a fixed fee for a fixed scope of work. Fees for our Datapoints services are typically earned over a shorter period of time (weeks to months) than for end-to-end cell engineering solutions, which may be multi-year programs. A typical deliverable for a Datapoints program is a data package. Fees for our automation solutions (RAC systems) are typically earned over a period that covers design, build, and deployment and range from six to twelve months. In addition, we offer support services for our RAC systems with fixed fees covering the support periods.
Biosecurity revenue
Since the end of the COVID-19 public health emergency in May 2023, Ginkgo has transitioned its Biosecurity business to focus on building out scalable biosecurity infrastructure through our two offerings, Canopy and Horizon. Through our partnerships, Ginkgo operates Canopy programs for collections, testing, sequencing, and insights delivery on pathogen samples in different countries. Ginkgo is also investing in building our Horizon product, in consultation with our existing network and additional public and private partners, as we think it has the potential to significantly drive revenue in the future. Our revenue flows are becoming more recurring as we increasingly incorporate longer-term contracts with recurring monthly fee models for Canopy program services, data, and analytics.
Our commitment to caring about how our platform is developed and used
Biotechnologies already touch nearly every part of society, and they will only grow in importance to our collective security and livelihoods in the future. Because of their far-reaching impacts on the world and because we are biological beings who are both dependent on and vulnerable to the capabilities we enable, we must take great care in the ways these technologies are developed and used.
We are cognizant that making biology easier to engineer won’t make the world better by default, but we believe these capabilities are essential to creating a better future where we can contend with both existing and emerging threats. To succeed in our long-term mission, we must avoid multiple failure modes. We must avoid creating capabilities that cause harm in ways that aren’t or can’t be mitigated. We must avoid reinforcing inequities in the uses of technologies and to whom their benefits accrue—thereby claiming to change the world but changing not much at all. We must avoid a loss in trust in biotechnologies and the motivations of their developers that limits our ability to bring solutions to global challenges
from protecting against pandemics to feeding the planet. And so, we must chase, every day, the development of capabilities and partnerships that can lead to value generation undergirded by sustained attention to the values they reflect.
As our platform grows, so too does our power to enable and shape many impacts we care about. While we are proud of our direct impacts on making biology easier to engineer, most of the world cares about the impacts on the world that we indirectly enable through helping our customers with the products they deliver. We grow as a platform precisely because we help create more value for our customers and the world than we capture. Our position serving customers across many industries provides strategic insights into what issues need collective attention to ensure future products can deliver meaningful solutions. But as a platform we cannot anticipate and control all future uses of our technologies by our customers and those they work with.
Far from abdicating responsibility, we realize our power is to inspire and help enable others to carefully steward technologies with attention to their impacts over time. This is directly in line with our long-term value proposition, as we need our customers and the ecosystem to succeed in avoiding the failure models outlined above and build the collective biotechnology-enabled future we all can wish for. We believe that stewardship starts with our platform and the people within it. Just as we must build and inspire trust in our partners to steward our technologies with care, we build and inspire trust in all of our bioworker-owners to build our platform with care.
We must also pay special attention to the governance of leading capabilities for we have outsized ability to shape.
At a macroscopic level, building biosecurity capabilities is an example of where we assessed the need for complementary efforts that could safeguard future biotechnologies–including those developed on our platform. But this same philosophy applies across our platform including as we work to harness powerful new capabilities in AI. We believe that our platform design is a foundation for architecting security and access that can both enable positive uses while better understanding and protecting against scenarios of misuse.
We see caring about how our platform is developed and used not as a net cost but as an enabler at multiple scales of impact aligned with long-term value. It builds trust and credibility not only in our capabilities but those of our customers. It motivates and enables our employee-owners to drive the platform towards the many diverse uses they co-envision with our platform. It also advances a framework to go beyond a reactive historical frame that has often positioned genetic engineering as a risk to the environment rather than a value.
We recognize that platforms across other industries have lessons—many negative—on how to steward their development and use. Our high-level commitment to care also comes with the expectation of needing to regularly revisit the approaches to realizing that commitment.
Our People & Culture
A company is made of people. We have sought to bring together a talented and multidisciplinary group of people who share our mission to make biology easier to engineer. Today, our extensive cross-functional team is collaborating to build our ecosystem, from organism designers to automation engineers, software developers to the people team, business development to facilities management, finance to molecular biology.
A culture built on care
We’ve strived to grow a culture based on care. As engineers, it is easy to fall into the trap of thinking of ourselves simply as tool builders. Tools can be used in many different ways, both good and bad, and engineers often discuss their tools as value neutral. But tools reflect the social beliefs and biases of the people who make them.
We are keenly aware of the need to care about how our platform is used. More significant than the impacts we have seen from digital platforms on our social world, biology is our health, our bodies, our food, and our environment. As we build the tools for programming biology, we must also care how those tools are used, and ensure that the risks and benefits are transparently and equitably shared.
A talented, world-class team
As of December 31, 2024 we had 834 employees. In addition to our employees, our success would not be possible without the collaboration and support of the network of partners, contractors, contingent workers and temporary staff who make up the Ginkgo team.
Technologies reflect the values of the people who build them. We believe that it is essential to build a talented team where people from different backgrounds are included and empowered to speak up and shape the growth of this technology. We are committed to growing a team reflecting a broad range of viewpoints, backgrounds and skills and continuing to empower an inclusive culture with strong employee ownership and engagement.
Laying the groundwork for strong employee engagement in the future
As a founder-led company we have been able to infuse the organization with long-term strategic thinking from the start. The long-term engagement and resilience of our employees can be seen in their response to a very challenging 2024: despite a significant restructuring and reduction of more than 40% of our workforce, we continued to execute on programs, achieve milestones, and launch new products. We have emerged from the restructuring with a strengthened focus on the long-term performance and sustainability of our business.
The individuals who work at Ginkgo and build our platform care deeply about how that platform is used and the impact our company will have in the world. We hope to maintain the long-term mentality we have benefited from as a founder-led public company. We believe a workforce with strong equity ownership will make the wise decisions needed to build long-term value for our company and build a company whose long-term impacts make them proud. That is why we have implemented a multi-class stock structure that permits all employees (current and future), not just founders, to hold high-vote (10 votes per share) common stock. We believe that our multi-class stock structure will help maintain this long-term mentality and encourage long-term equity ownership by our employees, thereby resulting in increasing employee ownership over time. For more information, see “Risk Factors—Risks Related to Ginkgo’s Business—Risks Related to Our Organizational Structure and Governance—Only our employees and directors are entitled to hold shares of Class B common stock (including shares of Class B common stock granted or otherwise issued to our employees and directors in the future), which shares have ten votes per share. This limits or precludes other stockholders’ ability to influence the outcome of matters submitted to stockholders for approval, including the election of directors, the approval of certain employee compensation plans, the adoption of certain amendments to our organizational documents and the approval of any merger, consolidation, sale of all or substantially all of our assets, or other major corporate transaction requiring stockholder approval.”
Competition
To our knowledge, there are currently no other cell engineering companies that serve the same breadth of industries that we do. The services and products offered by potential competitors vary in size, breadth, and scope, and given our broad set of application areas, we could face competition in many different forms. We face competition from customers’ internal R&D departments and other research solution providers that largely conduct genetic engineering by-hand. We also compete against companies that seek to utilize synthetic biology technologies to develop specific products or target certain end markets. Additionally, with our expansion into cell engineering tools offerings in 2024, we compete against life science tools companies and CROs as well as newer AI entrants in the emerging TechBio area. Finally, competing platforms may emerge from various sources, including from joint ventures and partnerships between well-capitalized technology and life sciences companies. We identify the following three groups as our principal set of competitors:
Cell Engineering competition
The Status Quo: “on prem” cell programming efforts
The main source of competition we encounter for our end-to-end cell engineering solutions offerings is from potential customers choosing to build or maintain in-house cell engineering teams and capabilities. This status quo includes building out laboratory space and then hiring a team of highly trained scientists to conduct research, largely “by-hand” and with
limited scale efficiencies. Some internal R&D operations maintain a full suite of capabilities and can design, build and test relatively complex pathways while others may have certain internal capabilities and need to outsource other elements to CROs. We believe this is far less efficient for the customer and likely to yield worse outcomes as customers get fewer shots on goal for a given program budget.
That said, it can still be very difficult for companies to choose to trust Ginkgo with their R&D efforts versus building more traditional “on prem” labs. Smaller companies may feel like they’re “betting the farm” on Ginkgo, while larger companies may be sensitive to displacing existing R&D teams. As such, a key focus area for us is reducing the barriers to adoption for the platform by decreasing the upfront investment for earlier-stage companies and by helping larger companies integrate their scientists closely into our workflows and empower their scientists to manage requests directly so we feel more like a resource and partner than a fully outsourced provider. Our recently launched Datapoints products in Functional Genomics and Antibody Developability are key examples of new services designed with our customers’ scientists in mind.
The vast majority of therapeutics companies that are leveraging genetic engineering have in-house capabilities, including Biogen, Novo Nordisk, Vertex, Regeneron, Bayer, and many others. These companies may be viewed as competitors to Ginkgo because they are creating products, using cell programming, that may compete with the products Ginkgo is enabling for our customers. However, as a platform company, we view these companies not as competitors but as potential customers and focus not on “beating” them but rather on demonstrating our value proposition.
Verticalized cell engineering platforms
Within certain end markets, Ginkgo may compete against vertically-focused biotechnology companies providing cell engineering R&D capabilities to customers within a narrow set of end markets. While we believe the siloed nature of these companies limits their long-term potential, in the near-term, we may have a harder time penetrating those end markets given the incumbent vertical specialists in that space. The vast majority of these companies exist within therapeutic end markets given the history of cell engineering in that market segment. In theory, the expertise and learnings they develop from work in one field could be leveraged into neighboring end markets if these companies decided to adopt (and invest in) a more horizontal strategy. Examples of these vertically-focused platforms include AbCellera (antibody discovery), Codexis (enzymes), Senti Bio (cell therapy for oncology applications) and WuXi biologics (therapeutics).
Contract research organizations (CROs) and life science tools companies
With our expansion into cell engineering tools offerings, we now offer data generation services and RAC laboratory automation to our customers. These offerings appeal to customers who want to retain either scientific control and/or experimental execution of their biological R&D. As such, we now more directly compete with CROs that offer research services, though usually such services are not purpose-built for generating high quality, large datasets to train and validate AI/ML models. Examples of these CROs include Evotec, WuXi Biologics, and Charles River Laboratories. Similarly, we compete with integrated automation companies like HighRes Biosolutions, Automata, and Thermo Fisher Scientific with our RAC laboratory automation products.
Other possible entrants
We may also face competition from new entrants in the market, including well-capitalized technology companies with possible strategic interests in synthetic biology and its capabilities. Such companies may emerge as competitors given their access to capital, capacity to create multi-disciplinary teams across biology, chemistry, computer science and engineering, and flexibility to enter strategic ventures with life sciences companies.
Biosecurity competition
We’re unique in the global biosecurity market because our approach is global and comprehensively covers end-to-end biosecurity needs. We face competition from a small number of companies who operate in focused biosecurity verticals, such as wastewater monitoring (e.g., Verily and Biobot, both primarily in the U.S.) and digital biosurveillance and modeling (e.g., BlueDot, Airfinity, and the Public Health Company), as well as internationally from BGI, China’s national champion for sequencing and diagnostics. As we partner with national governments, we also face competition from
homegrown public solutions to particular challenges, especially among high-income countries and large multilaterals with little history of engagement with the private sector.
We have several important attributes that contribute to our competitive advantage:
•A comprehensive offering that allows customers to come to a single platform for multimodal physical and digital surveillance and integrated global insights, rather than fragmented approaches;
•A suite of technical capabilities across epidemiological, bioinformatics, and scientific R&D;
•Unique technological tools, like our ENDAR platform for engineering detection;
•Our prominent role as a thought-leader and talent-attractor in this market;
•A foundation of partnerships with countries across the globe and key multilaterals such as Africa CDC, African Risk Capacity, and the International Livestock Research Institute; and
•Our global leadership in the airport-based pathogen monitoring space.
Intellectual Property
Overview: Foundry and Codebase
As discussed above, Ginkgo’s two core platform assets include:
•Ginkgo’s Foundry, which enables high-throughput cell programming; and
•Ginkgo’s Codebase, which includes reusable biological assets that can be used to accelerate cell programs.
Ginkgo protects each of these core assets—the Foundry and the Codebase—through a combination of patents and trade secret protections.
Patents
Our general policy has been to seek patent protection for those inventions likely to be incorporated into our offerings and for which patent protection will provide value or competitive advantages to Ginkgo.
Our worldwide patent portfolio includes patents acquired in transactions over time. We may decide that it is in our interest to abandon, sell, or otherwise dispose of certain patents or patent applications that we determine are no longer relevant to our business.
Patents generally have a term of twenty years from the date they are filed. As our patent portfolio has been built over time, the remaining terms of the individual patents across our patent portfolio vary. No single patent or patent family is essential to Ginkgo as a whole or to any of Ginkgo’s subsidiaries. In addition to developing our patent portfolio, we license patents from third parties.
We intend to pursue additional patent protection to the extent that we believe that it would be beneficial and cost-effective. We cannot provide any assurance that any of our current or future patent applications will result in the issuance of patents. We also cannot assure the scope of any of our future issued patents or warrant that any of our patents will prevent others from commercializing infringing products or technology.
Trade secrets
Ginkgo’s technology-related intellectual property that is not patent-protected is maintained as trade secrets. We employ a variety of safeguards to protect our information and trade secrets, including contractual arrangements with our employees, consultants, contractors and other advisors that impose obligations of confidentiality, assignment of inventions, and security; digital security measures; and physical security precautions.
We require confidentiality and material transfer agreements from third parties that receive our confidential data or materials, and we also incorporate confidentiality and material transfer precautions into our collaboration agreements.
Trademarks and domain names
Although our business is directed at sophisticated corporate customers rather than end consumers, we have trademark rights and registrations in our name, logo, and other brand indicia in the United States and other jurisdictions around the world. We also have registered domain names for websites that we use in our business, such as www.ginkgobioworks.com.
Intellectual property transaction structure
The intellectual property terms for our agreements with our customers vary based on deal type, customer type, economics, industry, business segment, customer market, and other factors. For example, in end-to-end cell engineering solutions programs, our collaboration agreements typically provide that Ginkgo will own Foundry IP and improvements to Ginkgo’s background IP developed in the collaboration. With respect to other Codebase IP developed in the collaboration, we provide customers rights commensurate with transaction economics, customer goals, and other factors. Many of our collaboration agreements also provide a limited non-exclusive license to our background IP and, to the extent applicable, rights to some of the new non-Foundry technologies developed in the collaboration.
Suppliers
Ginkgo’s suppliers for cell engineering operations comprise primarily manufacturers and distributors of life science tools, consumables and equipment as well as certain specific providers of contract research, development and manufacturing services. We will sometimes enter into long-term, strategic partnerships with innovative suppliers. Because of the scale of our operations, we believe we are often an early adopter and the largest customer at scale of certain new life science tools and technologies. We will also occasionally acquire technology or Codebase assets for strategic reasons, including integration into our platform.
We utilize various third party software and information technology service providers, including Google Cloud and Amazon Web Services, for data storage and processing. We also routinely engage a variety of third parties for professional services, contract employment services and consulting services.
Government Contracts
We have entered into agreements with governmental entities and contractors in the past to serve as a U.S. government contractor or subcontractor and may do so again in the future. See “Risk Factors—Risks Related to Governmental Regulation and Litigation—We have pursued in the past and may pursue additional U.S. Government contracting and subcontracting opportunities in the future and as a U.S. Government prime contractor and subcontractor, we are subject to a number of procurement rules and regulations.”
Government Regulations
Our business, or the business of our customers, may be regulated by the FDA and other federal authorities in the United States, including the U.S. Federal Trade Commission (“FTC”), U.S. Department of Agriculture (“USDA”), U.S. Drug Enforcement Administration (“DEA”) and U.S. Environmental Protection Agency (“EPA”), as well as comparable authorities in foreign jurisdictions and various state and local authorities in the United States. Failure to comply with applicable regulations may result in enforcement actions, civil or criminal sanctions, and adverse publicity.
FDA regulation
We provide cell engineering and product discovery services to customers engaged in the manufacture of pharmaceutical, food, and cosmetic products. The FDA regulates the research, development, testing, quality control, import, export, safety, effectiveness, storage, recordkeeping, premarket review, approval or licensure, processing, formulation, manufacturing, packaging, labeling, advertising, promotion, marketing, distribution, sale, post-market monitoring and reporting of our customers’ pharmaceutical, food and cosmetic products, and the FTC also regulates the advertising and promotion of these products.
We have acted as a systems integrator and authorized distributor of certain COVID-19 over-the counter diagnostic tests manufactured by independent third parties. We worked with laboratory partners that provide surveillance testing services as part of the COVID-19 and other pathogen surveillance testing services we offer, and these tests and test kits may be subject to regulation by the FDA. In particular, the tests and test kits used in our testing services may be subject to regulation by the FDA as medical devices, and may be required to comply with the requirement that such products have obtained clearance, approval, or other marketing authorizations, before they can be commercialized, as well as post-market requirements such as adverse event reporting and restrictions on labeling, marketing, and distribution.
Laboratories must seek FDA marketing authorization and otherwise comply with FDA device regulations when marketing COVID-19 Laboratory Developed Tests (“LDTs”). An LDT is an in vitro diagnostic test that is intended for clinical use and is designed, manufactured, and used within a single laboratory. LDTs are classified as medical devices, but the FDA has historically exercised enforcement discretion and has generally not enforced FDA requirements, including premarket review, with respect to laboratories that offer LDTs. However, FDA intends to phase out its enforcement discretion for LDTs. While HHS and FDA have announced their intention to require premarket review of COVID-19 LDTs, either agency may change its position in the future.
Medical products, including COVID-19 tests, that are granted a clearance, Emergency Use Authorization (“EUA”), or other marketing authorization must comply fully with the terms and conditions provided in the clearance, EUA, or other marketing authorization. For example, EUAs for COVID-19 tests may include conditions of authorization applicable to the EUA holder, authorized distributors and authorized laboratories. Noncompliance with applicable requirements could result in negative consequences, including adverse publicity, judicial or administrative enforcement, warning letters or untitled letters from the FDA, mandated corrective promotional materials, advertising or communications with doctors, and civil or criminal penalties, among others. The FDA can also withdraw marketing authorization for the applicable product, and in the case of a product subject to an EUA, the FDA may require EUA holders to transition to permanent marketing authorization which could impact some of the tests in our supply chain.
DEA regulation
We are engaged in the research, development, and export of certain products that may be regulated as controlled substances. The Controlled Substances Act of 1970, as amended from time to time, establishes registration, security, recordkeeping, reporting, storage, distribution and other requirements administered by the DEA. The DEA is concerned with the control of handlers of controlled substances, and with the equipment and raw materials used in their manufacture and packaging, in order to prevent loss and diversion into illicit channels of commerce. The DEA regulates controlled substances as Schedule I, II, III, IV or V substances. Schedule I substances by definition have no established medicinal use, and may not be marketed or sold in the United States. Schedule I substances are considered to present the highest risk of abuse, and Schedule V substances the lowest relative risk of abuse among controlled substances. Marijuana is classified as a Schedule I controlled substance. However, the term does not include “hemp,” which means the cannabis plant and any part of that plant, including the seeds and all derivatives, extracts, cannabinoids, isomers, acids, salts, and salts of isomers, whether growing or not, with a delta-9 THC concentration of not more than 0.3% on a dry weight basis.
Annual registration is required for any facility that manufactures, distributes, dispenses, imports or exports any controlled substance. The registration is specific to the particular location, business activity and controlled substance schedule. For example, separate registrations are needed for import and manufacturing, and each registration will specify which controlled substance schedule is authorized for that activity.
The DEA typically inspects a facility to review its security measures prior to issuing a registration. The DEA requires “effective controls and procedures” to guard against theft and diversion of controlled substances. Security requirements vary by controlled substance schedule (with the most stringent requirements applying to Schedule I and Schedule II substances), type of business activity conducted, quantity of substances handled, and a variety of other factors. Required security measures include background checks on employees and physical control of inventory. While the specific means by which effective controls and procedures are achieved may vary, security practices may include use of cages, surveillance cameras and inventory reconciliations. Records must be maintained for the handling of all controlled substances, and, in
certain scenarios, periodic reports made to the DEA. Reports must also be made for thefts or losses of any controlled substance, and disposal of controlled substances must adhere to various methods authorized by the regulations. In addition, special authorization and notification requirements apply to imports and exports.
Failure by registered establishments to maintain compliance with applicable requirements, particularly as manifested in loss or diversion, can result in enforcement action. The DEA may seek civil penalties, refuse to renew necessary registrations, or initiate proceedings to revoke those registrations. In certain circumstances, violations could eventuate in criminal proceedings. Individual states also regulate controlled substances.
Laboratory Licensing and Certification Requirements
The clinical laboratories we partnered with for our COVID-19 testing program are subject to federal oversight under the Clinical Laboratory Improvement Amendment of 1988 (“CLIA”), which requires all clinical laboratories to meet certain quality assurance, quality control and personnel standards. Laboratories also must undergo proficiency testing and are subject to inspections. Standards for testing under CLIA are based on the complexity of the tests performed by the laboratory, with tests classified as “high complexity,” “moderate complexity,” or “waived.” Laboratories performing high complexity testing are required to meet more stringent requirements than moderate complexity laboratories. Certain of our partner laboratories must undergo on-site surveys at least every two years, which may be conducted by the Centers for Medicare and Medicaid Services (“CMS”) under the CLIA program or by a private CMS-approved accrediting agency. The sanction for failure to comply with CLIA requirements may be suspension, revocation or limitation of a laboratory’s CLIA certificate, which is necessary to conduct business, as well as significant fines and criminal penalties.
The operations of our partner laboratories holding CLIA Certificates of Waiver are also subject to state and local laboratory regulation. CLIA provides that a state may adopt laboratory regulations different from or more stringent than those under federal law, and a number of states have implemented their own laboratory regulatory requirements. State laws may require that laboratory personnel meet certain qualifications, specify certain quality controls, or require maintenance of certain records. No assurances can be given that our partner laboratories will pass all future licensure or certification inspections.
Our facilities and laboratories hold local, state and federal permits, licenses and registrations necessary for compliance in specific work and operations, including from the Massachusetts Water Resource Authority, Boston Fire Department, Massachusetts Department of Environmental Protection, Boston Public Health Commission, Cambridge Biosafety Committee, Massachusetts Department of Public Health, USDA and DEA.
Federal Select Agent Regulations
Our research facilities that synthesize DNA sequences or perform other activities could become subject to the FSAP, which involves rules administered by the CDC and the USDA Animal and Plant Health Inspection Service (“APHIS”). The FSAP regulates the possession, use, and transfer of biological select agents and toxins that have the potential to pose a severe threat to public health, animal or plant health, or animal or plant products. FSAP regulatory requirements include: (i) registration with the CDC and/or APHIS for research facilities that deal with the select agents and toxins; (ii) submission to periodic biosafety and security inspections; and (iii) reporting of theft, loss or release of select agents. Federal agency enforcement actions for violations of FSAP regulations can include the initiation of corrective actions, complete or partial suspension or revocation of select agent registrations or civil or criminal liability.
Genetically Modified Materials Regulations
Our technologies and the technologies of our customers involve the use of genetically modified cells, organisms and biomaterials, including, without limitation, GMOs and genetically modified microorganisms (“GMMs”), and their respective products. In the United States, the FDA, the USDA through its APHIS, and the EPA are the primary agencies that regulate the use of GMOs, GMMs and potential products derived from GMOs or GMMs or Genetically Modified Materials, pursuant to the Coordinated Framework for the Regulation of Biotechnology.
The FDA reviews the safety of food consumed by humans and of feed consumed by animals under the Federal Food, Drug and Cosmetic Act (“FDCA”). Under the FDCA, food and feed manufacturers are responsible for ensuring that the products they market, including those developed through genetic engineering, are safe and properly labeled. In addition, the FDA must approve the use of any food additives, including GMOs, before marketing.
USDA's APHIS examines whether a plant itself presents a “plant pest” risk under the Plant Protection Act (“PPA”). Specifically, APHIS is responsible for regulating the introduction (i.e., importation, interstate movement or release into the environment) of certain GMOs and plants under the plant pest provisions in the PPA to ensure that they do not pose a plant pest risk. APHIS finalized changes to the PPA’s implementing regulations with respect to certain GMOs in May 2020. A person or organization may request a regulatory status review from APHIS to determine whether a GMO is unlikely to pose a plant pest risk and, therefore, is not regulated under the plant pest provisions of the PPA or the regulations codified at 7 C.F.R. Part 340; requesting a regulatory status review tends to assume the GMO at issue does not otherwise fall within a regulatory exemption. If the GMO does not qualify for an exemption or if the APHIS regulatory status review process finds that the plant poses a plausible plant pest risk, then the GMO may require an APHIS permit, i.e., be a regulated article under Part 340. A regulated article may be subject to APHIS for the environmental release, importation, or interstate movement of the GMO or its progeny.
EPA regulates, under the Federal Insecticide, Fungicide and Rodenticide Act (“FIFRA”), the pesticides (including plant incorporated protectants) that are used with crops, including GMO herbicide-tolerant crops. FIFRA generally requires all pesticides to be registered before distribution or sale, unless they are exempted. Under FIFRA, a pesticide registrant must demonstrate that the pesticide at issue, when used pursuant to its specifications, “will not generally cause unreasonable adverse effects on the environment” to secure a registration. EPA must approve each distinct pesticide product, each distinct use pattern, and each distinct use site. In addition to EPA’s FIFRA authority, EPA also regulates potential human health impacts from pesticides under the FDCA. EPA does so by establishing “tolerance levels” (i.e., “the amount of pesticide that may remain on food products”) under the FDCA.
Certain genetically modified microorganisms that are not otherwise regulated under FIFRA and FDCA may be subject to EPA regulation under the Toxic Substances Control Act (“TSCA”). New microorganisms that are formed by combining genetic material from organisms in different genera (known as intergeneric microorganisms) may be subject to reporting requirements prior to production or distribution in commerce (Microbial Activity Commercial Activity Notice), or use in research and development (TSCA Experimental Release Application), unless the entity can meet all required criteria to obtain an exemption under TSCA.
Federal and state data privacy and security regulations
Numerous state, federal and foreign laws, including consumer protection laws and regulations, govern the collection, dissemination, use, access to, confidentiality and security of personal information, including health-related information. In the United States, numerous federal and state laws and regulations, including data breach notification laws, health information privacy and security laws, including HIPAA, and federal and state consumer protection laws and regulations (e.g., Section 5 of the FTC Act), that govern the collection, use, disclosure, and protection of health-related and other personal information could apply to our operations or the operations of our partners. HIPAA, and its respective implementing regulations, imposes obligations on “covered entities,” including certain health care providers, health plans, and health care clearinghouses, and their respective “business associates” that create, receive, maintain or transmit individually identifiable health information for or on behalf of a covered entity, as well as their covered subcontractors with respect to safeguarding the privacy, security and transmission of individually identifiable health information. Violations of the HIPAA privacy and security regulations may result in civil and criminal penalties. HHS is required to conduct periodic compliance audits of covered entities and their business associates. HIPAA also authorizes state attorneys general to bring civil actions seeking either an injunction or damages in response to violations of HIPAA privacy and security regulations.
In addition, certain state laws, such as the California Confidentiality of Medical Information Act, govern the privacy and security of health-related information in certain circumstances, some of which are more stringent than HIPAA and many of which differ from each other in significant ways and may not have the same effect, thus complicating compliance efforts.
States including California, Virginia, Colorado, Connecticut and Utah have also enacted comprehensive privacy laws that are currently in effect, and similar laws have been passed or are being considered in several other states, as well as at the federal and local levels. Failure to comply with these laws, where applicable, can result in the imposition of significant civil and/or criminal penalties and private litigation. Privacy and security laws, regulations, and other obligations are constantly evolving, may conflict with each other (thus complicating compliance efforts), and can result in investigations, proceedings, or actions that lead to significant civil or criminal penalties and restrictions on data processing.
Ginkgo Corporate Information
Ginkgo’s principal executive office is located at 27 Drydock Avenue, Boston, Massachusetts 02210, and Ginkgo’s telephone number is (877) 422-5362. Ginkgo’s corporate website address is www.ginkgobioworks.com. We make available on the Investor Relations section of our website, free of charge, our annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, proxy statements, and Forms 3, 4 and 5, and amendments to those reports as soon as reasonably practicable after filing such documents with, or furnishing such documents to, the U.S. Securities and Exchange Commission (the “SEC”). The SEC maintains a website (www.sec.gov) that contains reports, proxy and information statements and other information regarding issuers that file electronically with the SEC.
The information contained on, or accessible through, our corporate website is not incorporated into this Annual Report and should not be considered part of this Annual Report. The inclusion of the corporate website address is an inactive textual reference only.
Item 1A. Risk Factors.
An investment in our securities involves a high degree of risk. You should carefully consider the following risk factors, together with all of the other information included in this Annual Report, before making an investment decision. Our business, prospects, financial condition or operating results could decline due to any of these risks and, as a result, you may lose all or part of your investment.
Unless the context otherwise requires, all references in this section to the “Company,” “we,” “us” or “our” refer to the business of Ginkgo and its subsidiaries.
Risks Related to Ginkgo’s Business
We have a history of net losses. We expect to continue to incur losses for the foreseeable future, and we may never achieve or maintain profitability.
We have incurred significant operating losses since our inception. Our net loss attributable to our stockholders was approximately $547.0 million, $892.9 million, and $2.1 billion for the fiscal years ended December 31, 2024, 2023 and 2022, respectively.
As of December 31, 2024, we had an accumulated deficit of approximately $5.8 billion. We may incur losses and negative cash flow from operating activities for the foreseeable future as we continue to invest significant additional funds toward further developing our platform and customer offerings, the cell engineering programs we perform on behalf of our customers and otherwise growing our business, including our Biosecurity and Automation business units. We expect that our operating expenses will either remain consistent or decline in 2025 as compared to 2024, reflecting a stabilization in our operational overhead and restructuring actions commenced in 2024. However, our operating expenses could increase in connection with employee incentive programs offered or from additional costs or expenses resulting from our restructuring actions. We have derived a significant portion of our revenues from fees and milestone payments from technical development services provided to customers to advance programs, as well as a significant portion of our revenues from Biosecurity. Historically, these fees have not been sufficient to cover the full cost of our operations. Additionally, if our customers terminate their agreements or development plans with us, our near-term revenues could be adversely affected. In addition, certain of our customer agreements provide for milestone payments, future royalties and other forms of contingent consideration, the payment of which are uncertain, as they are dependent on our ability to successfully develop engineered cells, bioprocesses, or other deliverables and our customers’ ability and willingness to successfully develop and commercialize products and processes.
Our expenses may continue to exceed revenues in the foreseeable future and we may not achieve profitability. If we fail to achieve profitability, or if the time required to achieve profitability is longer than we anticipate, we may not be able to expand or continue our business, and the value of our common stock could be negatively impacted. Our ability to achieve or sustain profitability is based on numerous factors, many of which are beyond our control, including the development of our platform, the initiation of new programs with new and existing customers, the commercial terms of our programs, the realization of any potential downstream value from our programs, our ability to advance cell engineering programs in a timely and cost-effective manner, our ability to extend new offerings to customers, our customers’ ability to scale up bioprocesses, the ability of our customers to produce and sell products, the impact of market acceptance of our customers’ products, and our customers’ market penetration and margins. Even if we do achieve profitability, we may not be able to sustain or increase profitability on a quarterly or annual basis.
We may need substantial additional capital in the future in order to fund our business.
We have consumed considerable amounts of capital to date, and we expect to incur continued net losses for the foreseeable future as we continue to develop our business, advance our programs, expand and enhance our platform and customer offerings, perform on behalf of our customers, make the capital investments necessary to scale up our Foundry operations and Codebase assets and as we continue our restructuring actions commenced in 2024 . We have used, and may continue to use, additional capital for Biosecurity, strategic investments and acquisitions. We believe that our cash and cash equivalents, short-term investments, and interest earnings will be sufficient to meet our projected operating requirements until we reach profitability. However, these assumptions may prove to be incorrect and we could exhaust our available capital resources sooner than we currently expect. Because of the numerous risks and uncertainties associated with our programs, including risks and uncertainties that could impact the rate of progress of our programs, we are unable to estimate with certainty the amounts of capital outlays and operating expenditures associated with these activities.
We do not currently have any commitments for future funding. We may receive fees, milestones, and royalty payments under our customer agreements, but these are not guaranteed. We have received non-cash consideration in the past and we may receive additional non-cash consideration in the future where we may not be able to realize the eventual cash proceeds expected or at all such that the amount of cash proceeds eventually realized may be less than the revenue recognized and the costs incurred to perform those services. Additionally, we may wish to sell our equity interests in certain subsidiaries or collaborations but most of these equity stakes are in private companies and we may not be able to find a buyer due to contractual restrictions or otherwise, or may incur significant impairment if we sell these positions for liquidity. We may not receive any further funds or future non-cash consideration under those agreements, the funds or non-cash consideration we receive may be lower than projected and/or disclosed as potential downstream value, or our program costs may be higher than projected. In addition, we may not be able to sign new customer agreements or enter into new development plans with existing customers with adequate funds to cover program development expenses. As a result of these and other factors, we do not know whether additional financing will be available when needed, or, if available, whether such financing would be on terms favorable to our stockholders or us.
If future financings involve the issuance of equity securities, our existing stockholders would suffer dilution. If we raise debt financing in the future, we may be subject to restrictive covenants that limit our ability to conduct our business. Our ability to raise funds may be adversely impacted by current or future economic conditions. If we fail to raise sufficient funds and continue to incur losses, our ability to fund our operations, take advantage of strategic opportunities, or otherwise respond to competitive pressures could be significantly limited. If adequate funds are not available, we may not be able to successfully execute our business plan or continue our business.
We have experienced periods of significant organizational change and if we fail to effectively manage these changes, then our business, results of operations, and financial condition could be adversely affected.
We have experienced both rapid growth and recent periods of decline in our business since inception, which has placed and may continue to place significant demands on our company culture, operational infrastructure, and management. We believe that our culture has been a critical component of our success. We have invested substantial time and resources in building our team and nurturing a culture of empowerment of, and active engagement by, our employees. In addition, in order to manage through these periods of organizational change, we will need to continue to adapt our operational, financial, and management controls and processes, as well as our reporting systems and procedures. As we manage our business and mature as a public company, we may find it difficult to maintain our culture and adapt effectively. Any failure to manage our organizational changes in a manner that preserves the key aspects of our culture and allows us to effectively adapt, could be detrimental to future success in pursuing our objectives, including our ability to recruit and retain personnel, maintain reliable service levels and offerings for our customers, and achieve the necessary level of capacity,
quality and efficiency in performing services and other development activities, or the necessary level of efficiency in our organizational structures. This, in turn, could adversely affect our business, results of operations, and financial condition.
Our limited operating history and evolving business makes it difficult to evaluate our current business and future prospects.
We have a portfolio of cell engineering programs which vary in start date, duration, complexity, and revenue potential. Additionally, our downstream economics in the form of royalties, milestones, and/or equity interests add an additional level of uncertainty to our possible future performance. Consequently, predictions about our future success or viability are highly uncertain and may not be as accurate as they could be if we had a longer company history of successfully developing, commercializing and generating revenue from our programs and/or downstream economic participation.
Our long-term objective is to generate free cash flow from the commercialization of programs for customers across a variety of industries, as well as from our biosecurity-focused offerings. Our estimated costs and timelines for the completion of programs are based on our experiences to date and our expectations for each stage of the program in development. Given the variety of types of programs we support and the continued growth of our platform, there is variability in timelines and costs for launching and executing programs, and completion dates can change over the course of a customer engagement. Our costs and timelines may be greater or subject to variability where regulatory requirements lead to longer timelines, such as in agriculture, food, and therapeutics. In addition, we have equity interests in certain companies and there is and will continue to be variability in the financial performance of these other companies or future companies in which we may have equity interests.
We have encountered in the past, and will encounter in the future, risks and uncertainties frequently experienced by growing companies with limited operating histories in emerging and rapidly changing industries. If our assumptions regarding these risks and uncertainties, which we use to plan and operate our business, are incorrect or change, or if we do not address these risks successfully, our results of operations could differ materially from our expectations, and our business, financial condition, and results of operations could be adversely affected.
Uncertainty regarding the demand for biosecurity services could materially adversely affect our business.
Our Biosecurity offering consists of pathogen testing, sequencing, and insights delivery which are subject to inherent risks of commercial viability, such as demand for services and price or market share erosion due to competition. For example, the White House and World Health Organization each announced the end of the public health emergency effective May 2023 and the revenue stream of our COVID-19 school testing services ended in the third quarter of 2023.
As a result, our Biosecurity business is now focusing on global surveillance programs and analytic services. However, creating the commercial and technical infrastructure to provide Biosecurity services globally is expensive. We may also be limited in our ability to scale up based on expense or unavailability of the required materials, equipment, personnel and infrastructure necessary to deliver biosecurity on a large, international scale. We may not be able to recover our investment expenses with sufficient revenue generated by our Biosecurity efforts.
Our ability to commercialize our Biosecurity offerings is also subject to available government, private, and multilateral funding. If governments decide that the Biosecurity offerings are not necessary or that they do not have the funds to support them, we may experience difficulty in expanding and growing the biosecurity business.
We are or could become involved in securities or shareholder litigation and other related matters, which could be expensive and time-consuming. Such litigation and related matters could harm our business.
We have been and may in the future be a target for securities and shareholder lawsuits. The outcome of such pending and potential litigation is uncertain. Such disputes, including any related governmental or regulatory investigations and the cost of defending such, could result in an adverse effect on our business, results of operations, financial condition, reputation and cash flows, and could adversely impact the market price of our common stock. Although the results of lawsuits and claims cannot be predicted with certainty, defending against such claims could be costly and could impose a significant burden on management and employees. Any litigation to which we become a party may result in an onerous or unfavorable judgment, or may be resolved with a monetary payment.
If we cannot maintain and expand current customer partnerships and enter into new customer partnerships, our cell engineering business could be adversely affected.
We do not generate substantial revenue from our own products, and instead generate revenue from selling end-to-end cell engineering solutions and tools service offerings. We receive fees for such solutions and tools, and for our end-to-end cell engineering solutions, we also have historically received downstream value in the form of royalties, milestone payments, and/or equity. As a result, our success depends on our ability to expand the number, size and scope of our customer programs. Our ability to win new business depends on many factors, including our reputation in the market, the quality of our service offerings relative to alternatives, the pricing and efficiency of our services relative to alternatives, our technical and operational capabilities, our sales team effectiveness, and the customer’s ability to fund new work. If we fail to maintain a position of strength in any of these factors, our ability to deliver on customer programs, sign new customer programs, and/or launch new programs with existing customers may suffer and this could adversely affect our prospects. Additionally, in the process of delivering programs, we generate Foundry know-how and accumulate meaningful biological and data assets, including optimized proteins and organisms, characterized genetic parts, enhanced understanding of metabolic pathways, biological, chemical, and genetic libraries, and other elements of biological data. Data and know-how generated from our programs provide the basis for expanded capabilities that we believe further supports our customer relationships. As a result, in addition to reducing our revenue or delaying the delivery of our programs, the loss of one or more of our customer relationships or the failure to add new customers or programs may hinder our accumulation of such information, thus hindering our efforts to advance our technological differentiation and improve our platform.
We engage in conversations with companies regarding potential customer relationships on an ongoing basis. We may spend considerable time and money engaging in these conversations and feasibility assessments, including understanding the technical approach, customer concerns and limitations, and legal or regulatory landscape of a potential program or offering, which may not result in a commercial agreement. Even if an agreement is reached, the resulting relationship is not always successful, which may be for many reasons, including our inability to complete a program to our customers’ specifications or within our customers’ time frames, or, in the case of end-to-end cell engineering solutions, unsuccessful development or commercialization of products or processes by our customers. In such circumstances, our revenues and downstream value potential from such a collaboration has been and might in the future be meaningfully reduced.
We have exposure to the volatility and liquidity risks inherent in holding equity interests in other operating companies and other non-cash consideration.
We currently own equity interests in several of our customers, and we may receive non-cash consideration for our services, which involves estimations of fair market value. The initial fair market value of the non-cash consideration we receive has decreased in the past with respect to certain of our holdings, and in the future may decrease after contract inception and the amount of cash proceeds eventually realized may be less than the revenue recognized, or may even be zero. In the future, we may also own equity interests in other companies. The process and timing by which we receive equity interests and the factors we consider in deciding whether to accept, hold or dispose of these equity positions may differ significantly from those that an independent investor would evaluate when considering equity interests in a company. Owning equity interests increases our exposure to the risks of the other company and, in the case of customers, beyond the products of our collaborations. Our equity ownership positions expose us to market volatility and the potential for negative returns. We may have restrictions on resale or limited markets to sell our equity ownership. In many cases, our equity position is a minority position which exposes us to further risk, as we are not able to exert control over the companies in which we hold securities.
In connection with future collaborations or joint ventures, we may, from time to time, receive warrants or options, all of which involve additional risks involving, for example, pricing differences between the market value of underlying securities and our exercise price for the warrants or options, a possible lack of liquidity, and the related inability to close a warrant or option position, all of which could ultimately have an adverse effect on our financial position.
We leverage our own resources and partner with strategic and financial investors in order to help early stage companies and innovators secure funding and benefit from our platform, which exposes us to a number of risks.
Since our founding, we have helped to launch new companies by bringing together strategic and financial investors to secure funding for early stage and small companies. Going forward, we may continue to leverage our own balance sheet and partner with investors to enable companies at all stages to benefit from our platform.
Partnering with and investing in early stage and small companies may expose us to a number of risks, including that early stage and small companies may have:
●shorter operating histories, narrower product lines and smaller market shares than larger businesses, which tend to render small companies more vulnerable to competitors’ actions and market conditions, as well as general economic downturns;
●more limited access to capital and higher funding costs, may be in a weaker financial position and may need more capital than originally anticipated to expand, compete and operate their business;
●the inability to obtain financing from the public capital markets or other traditional sources, such as commercial banks, in part because loans made to these types of companies entail higher risks than loans made to companies that have larger businesses, greater financial resources or are otherwise able to access traditional credit sources on more attractive terms;
●a higher likelihood of holding cash deposits or maintaining lines of credit with banks focused on providing banking services to early stage or venture-backed companies, such as Silicon Valley Bank (“SVB”), which failed in March 2023;
●a higher likelihood of depending on the management talents and efforts of a small group of persons; therefore, the death, disability, resignation or termination of one or more of these persons could have a material adverse impact on such company and, in turn, on us;
●less predictable operating results, may be engaged in rapidly changing businesses with products subject to a substantial risk of obsolescence, and may require substantial additional capital to support their operations, finance expansion or maintain their competitive position;
●particular vulnerabilities to changes in customer preferences and market conditions, depend on a limited number of customers, and face intense competition, including from companies with greater financial, technical, managerial and marketing resources; and
●fewer administrative resources, which can lead to greater uncertainty in their ability to generate accurate and reliable financial data, including their ability to deliver audited financial statements.
Any of these factors or changes thereto could impair an early stage or small company’s financial condition, results of operation, cash flow or result in other adverse events, such as bankruptcy. This, in turn, could result in losses in our investments and a change in our income (loss) on investments.
We may be unable to complete future strategic acquisitions or successfully integrate strategic acquisitions which could adversely affect our business and financial condition.
Our inability to complete any future strategic acquisitions or to successfully integrate any new strategic acquisitions could have a material adverse effect on our business. Our business strategy includes the acquisition of technologies and businesses that complement or augment our existing products and services. We may continue to seek attractive opportunities to acquire technologies or businesses, enter into joint ventures and make other investments that are complementary to our existing strengths. There are no assurances, however, that any strategic acquisition opportunities will arise or, if they do, that they will be consummated. Certain acquisitions may be difficult to complete for a number of reasons, including the need to satisfy customary closing conditions, the need for antitrust and/or other regulatory approvals, as well as disputes or litigation. In addition, any strategic acquisition we may complete may be made at a substantial premium over the fair value of the net identifiable assets in the acquisition and thus our realization of this value relies on successful integration and continued operations. We may not be able to successfully integrate acquired technologies, assets, products, operations or businesses , make acquired businesses profitable, retain key employees (or integrate employees) or realize anticipated revenues, cost savings, or synergies, if any, from these acquisitions, or do so in an effective, timely and non-disruptive manner, which could adversely affect our business and financial condition. Further, our ongoing business may be disrupted, and our management's attention may be diverted, by acquisitions, investments, transition and/or integration activities.
We have in the past, and in the future may continue to pursue strategic acquisitions and investments that are dilutive to our stockholders, and such strategic acquisitions or investments could have an adverse impact on our business if they are unsuccessful.
We have made acquisitions in the past and, as appropriate opportunities become available, we may acquire additional businesses, assets, technologies, or products to enhance our business or engage in other strategic transactions in the future, but our ability to do so successfully cannot be ensured. We have also made investments in companies that we view as
synergistic with our business. Although we conduct due diligence on these acquisitions and investments, such processes may underestimate or fail to reveal significant liabilities and we could incur losses resulting from liabilities of the acquired business that are not covered by indemnification we may obtain from the seller. Even if we identify suitable opportunities, including pending transactions, we may not be able to complete such acquisitions on favorable terms or at all, which could damage our business.
Additionally, pursuing acquisitions or other strategic transactions, whether successful or unsuccessful, could result in civil litigation and regulatory penalties. Any acquisitions we make may not strengthen our competitive position, and these transactions may be viewed negatively by customers or investors. We may decide to incur debt or spend cash in connection with a strategic acquisition, which may cause us to face liquidity concerns or be subject to restrictive covenants in the future. We have issued, and in the future may issue, common stock or other equity securities to the stockholders of the acquired company, which, if such issuances were unregistered, we may be contractually required to register on Form S-3 and may be subject to piggyback registration rights. Such issuances could constitute a material portion of our then-outstanding shares of common stock and may reduce the percentage ownership of our existing stockholders.
Acquisitions or other strategic transactions may also increase our expenses and reduce our cash available for operations and other uses. In addition, we may not be able to fully recover the costs of such acquisitions or be successful in leveraging any such strategic transactions into increased business, revenue, or profitability. We also cannot predict the number, timing, or size of any future acquisitions or the effect that any such transactions might have on our operating results.
Accordingly, although there can be no assurance that we will undertake or successfully complete any future acquisitions, any transaction that we have completed, or in the future do complete, may not yield the anticipated benefits and may be subject to the foregoing or other risks and have a material and adverse effect on our business, financial condition, results of operations, and prospects. Conversely, any failure to pursue or delay in completing any acquisition or other strategic transaction that would be beneficial to us, including those caused by competing parties, could impact our ability to sign new programs, delay the development of our platform, or slow the advancement of our programs and, thus, potential commercialization of our customer’s products.
Our programs may not achieve milestones, earn royalties or complete other anticipated key events on the expected timelines or at all, which could have an adverse impact on our business and could cause the price of our common stock to decline.
We may adopt various technical, manufacturing, regulatory, commercial, and other objectives for our programs. These milestones may include our or our customers’ expectations regarding the commencement or completion of technical development, the achievement of manufacturing targets, the submission of regulatory filings, or the realization of other development, regulatory, or commercialization objectives by us or our customers. The achievement of many of these milestones may be outside of our control. All of these milestones are based on a variety of assumptions, including assumptions regarding capital resources, constraints, and priorities, progress of and results from R&D activities, and other factors, any of which may cause the timing of achievement of the milestones to vary considerably. If we, our collaborators, or our customers fail to achieve milestones in the expected timeframes, the commercialization of our programs may be delayed, our credibility may be undermined, our expectations with respect to potential future downstream value may be inaccurate, our ability to earn royalties may be impacted, our business and results of operations may be harmed, and the trading price of our common stock may decline.
We must continue to secure and maintain sufficient and stable supplies of laboratory reagents, consumables, equipment, and laboratory services. We depend on a limited number of suppliers, some of which are single-source suppliers, and contract manufacturers for critical supplies, equipment, and services for research, development, and manufacturing of our products and processes. Our reliance on these third parties exposes us to risks relating to costs, contractual terms, supply, and logistics, and the loss of any one or more of these suppliers or contract manufacturers or their failure to supply us with the necessary supplies, equipment, or services on a timely basis, could cause delays in our research, development, or production capacity and adversely affect our business.
Widespread inflationary pressures and other adverse macroeconomic pressures, such as tariffs, increased duties and taxes, international trade disputes, political instability, supply or raw material shortages, and labor disruptions, exist across global economies, resulting in disruptions or higher costs for disposable lab equipment, raw materials and synthetic biology materials and services, and significant increases in the future could adversely affect our results of operations. We have experienced shortages in some of our key equipment and supplies, including those required in our labs, as well as disruptions in services provided by third parties, and may do so in the future as a result of supply chain issues tied to global
pandemics, conflicts, or otherwise. We may also experience price increases, quality issues and longer lead times due to unexpected material shortages, service disruptions, and other unanticipated events, which may adversely affect our supply of lab equipment, lab supplies, chemicals, reagents, supplies, and lab services. For some suppliers, we do not enter into long-term agreements and instead secure our materials and services on a purchase order basis. Our suppliers may reduce or cease their supply of materials or services to us at any time in the future. If the supply of materials or services is interrupted, our programs may be delayed.
We depend on a limited number of suppliers for critical items, including lab consumables and equipment, for the development of our programs. Some of these suppliers are single-source suppliers. We do not currently have the infrastructure or capability internally to manufacture these items at the necessary scale or at all. Although we have a reserve of supplies and although alternative suppliers exist for some of these critical products, services, and equipment, our existing processes used in our Foundry have been designed based on the functions, limitations, features, and specifications of the products, services, and equipment that we currently utilize. While we work with a variety of domestic and international suppliers, our suppliers may not be obligated to supply products or services or our arrangements may be terminated with relatively short notice periods. Additionally, we do not have any control over the process or timing of the acquisition or manufacture of materials by our manufacturers and cannot ensure that they will deliver to us the items we order on time, or at all.
In particular, we rely on Twist for custom DNA synthesis and Thermo Fisher Scientific Inc. and others for certain instruments and consumables. The price and availability of DNA, chemicals, reagents, equipment, consumables, and instruments have a material impact on our ability to provide Cell Engineering services.
The loss of the products, services, and equipment provided by one or more of our suppliers could require us to change the design of our research, development, and manufacturing processes based on the functions, limitations, features, and specifications of the replacement items or seek out a new supplier to provide these items. Additionally, as we grow, our existing suppliers may not be able to meet our increasing demand, and we may need to find additional suppliers. We may not be able to secure suppliers who provide lab supplies at, or equipment and services to, the specification, quantity, and quality levels that we demand (or at all) or be able to negotiate acceptable fees and terms of services with any such suppliers.
As described above, some lab equipment, lab consumables, and other services and materials that we purchase are purchased from single-source or preferred suppliers, which limits our negotiating leverage and our ability to rely on additional or alternative suppliers for these items. Our dependence on these single-source and preferred suppliers exposes us to certain risks, including the following:
●our suppliers may cease or reduce production or deliveries, raise prices, or renegotiate terms;
●we may be unable to locate a suitable replacement on acceptable terms or on a timely basis, if at all;
●if there is a disruption to our single-source or preferred suppliers’ operations, and if we are unable to enter into arrangements with alternative suppliers, we will have no other means of continuing the relevant research, development, or manufacturing operations until they restore the affected facilities or we or they procure alternative sources of supply;
●delays caused by supply issues may harm our reputation, frustrate our customers, and cause them to turn to our competitors for future programs; and
●our ability to progress the development of existing programs and the expansion of our capacity to begin future programs could be materially and adversely impacted if the single-source or preferred suppliers upon which we rely were to experience a significant business challenge, disruption, or failure due to issues such as financial difficulties or bankruptcy, issues relating to other customers such as regulatory or quality compliance issues, or other financial, legal, regulatory, or reputational issues.
Moreover, to meet anticipated market demand, our suppliers may need to increase manufacturing capacity, which could involve significant challenges. This may require us and our suppliers to invest substantial additional funds and hire and retain the technical personnel who have the necessary experience. Neither we nor our suppliers may successfully complete any required increase to existing research, development, or manufacturing capacity in a timely manner, or at all.
For the year ended December 31, 2024, our cost of lab equipment, lab supplies, and lab services accounted for a significant portion of our total R&D expenses. In the event of price increases by suppliers, whether as a result of inflationary pressures, tariffs or otherwise, we may attempt to pass the increased costs to our customers. However, we may not be able to raise the prices of our Cell Engineering services sufficiently to cover increased costs resulting from increases in the cost of our materials and services, or the interruption of a sufficient supply of materials or services. As a result, materials and services costs, including any price increase for our materials and services, may negatively impact our business, financial condition, and results of operations.
Some of our suppliers and contract manufacturers are foreign entities. We may face disruptions due to the inability to obtain customs clearances in a timely manner or restrictions on shipping or international travel. As a result of ongoing global supply chain challenges resulting in very long lead times for certain products and equipment, we may order in larger volumes in order to secure the supplies we require for our future operations, which may negatively impact our financial conditions, especially if we are unable to use the supplies ordered.
We use biological, hazardous, flammable and/or regulated materials that require considerable training, expertise and expense for handling, storage and disposal and may result in claims against us.
We work with biological and chemical materials that could be hazardous to human, animal, or plant health and safety or the environment. Our operations produce hazardous and biological waste products, and we largely contract with third parties for the disposal of these products. Federal, state, and local laws and regulations govern the use, generation, manufacture, storage, handling, and disposal of these materials and wastes. Compliance with applicable laws and regulations is expensive, and current or future laws and regulations may restrict our operations. If we do not comply with applicable laws and regulations, we may be subject to fines and penalties.
In addition, we cannot eliminate the risk of (a) accidental or intentional injury or (b) release, or contamination from these materials or wastes, which could expose us to liability. Furthermore, laws and regulations are complex, change frequently, and have tended to become more stringent. We cannot predict the impact of such changes and cannot be certain of our future compliance. Accordingly, in the event of release, contamination, or injury, we could be liable for the resulting harm or penalized with fines in an amount exceeding our resources and our operations could be suspended or otherwise adversely affected. These liabilities could also include regulatory actions, litigation, investigations, remediation obligations, damage to our reputation and brand, supplemental disclosure obligations, loss of customer, consumer, and partner confidence in the safety of our laboratory operations, impairment to our business, and corresponding fees, costs, expenses, loss of revenues, and other potential liabilities, as well as increased costs or loss of revenue or other harm to our business.
The release of GMOs or Genetically Modified Materials, whether inadvertent or purposeful, into uncontrolled environments could have unintended consequences, which may result in increased regulatory scrutiny and otherwise harm our business and financial condition.
The genetically engineered organisms and materials that we develop may have significantly altered characteristics compared to those found in the wild, and the full effects of deployment or release of our genetically engineered organisms and materials into uncontrolled environments may be unknown. In particular, such deployment or release, including an unauthorized release, could impact the environment or community generally or the health and safety of our employees, our customers’ employees, and the consumers of our customers’ products.
In addition, if a high profile biosecurity breach or unauthorized release of a biological agent occurs within our industry, our customers and potential customers may lose trust in the security of the laboratory environments in which we produce GMOs, Genetically Modified Microorganisms (“GMMs”) and Genetically Modified Materials, even if we are not directly affected. Any adverse effect resulting from such a release, by us or others, could have a material adverse effect on the public acceptance of products from engineered cells and our business and financial condition. Such a release could result in increased regulatory scrutiny of our facilities, platform, and programs, and could require us to implement additional costly measures to maintain our regulatory permits, licenses, authorizations and approvals. To the extent such regulatory scrutiny or changes impact our ability to execute on existing or new programs for our customers, or make doing so more costly or difficult, our business, financial condition, or results of operations may be adversely affected. In addition, we could have exposure to liability for any resulting harm, as well as to regulatory actions, litigation, investigations, remediation obligations, damage to our reputation and brand, supplemental disclosure obligations, loss of customer, consumer, and partner confidence in the safety of engineered cells materials and organisms, impairment to our business, and corresponding fees, costs, expenses, loss of revenues, and other potential liabilities, as well as increased costs or loss of revenue or other harm to our business.
We could synthesize DNA sequences or engage in other activity that inadvertently contravenes biosecurity requirements, or regulatory authorities could promulgate more far-reaching biosecurity requirements that our standard business practices cannot accommodate, which could give rise to substantial legal liability, impede our business, and damage our reputation.
The Federal Select Agent Program (“FSAP”) involves rules administered by the Centers for Disease Control and Prevention and the USDA's APHIS that regulate possession, use, and transfer of biological select agents and toxins that have the potential to pose a severe threat to public, animal, or plant health or to animal or plant products. In accordance with the International Gene Synthesis Consortium’s (“IGSC”) Harmonized Screening Protocol for screening of synthetic DNA sequence orders, we follow biosafety and biosecurity industry practices and avoid DNA synthesis activities that implicate FSAP rules by screening synthetic DNA sequence orders against the IGSC’s Regulated Pathogen Database; however, we could err in our observance of compliance program requirements in a manner that leaves us in noncompliance with FSAP or other biosecurity rules. In addition, authorities could promulgate new biosecurity requirements that restrict our operations. One or more resulting legal penalties, restraints on our business or reputational damage could have material adverse effects on our business, financial condition, or results of operations.
Third parties may use our engineered cells, materials, and organisms and accompanying production processes in ways that could damage our reputation.
After our customers have received our engineered cells, materials, and organisms and accompanying production processes, we do not have any control over their use and our customers may use them in ways that are harmful to our reputation. In addition, while we have established biosecurity offerings designed to comply with biosafety and biosecurity requirements and export control requirements in an effort to ensure that third parties do not obtain our engineered cells or other biomaterials for malevolent purposes, we cannot guarantee that these preventative measures will eliminate or reduce the risk of the domestic and global opportunities for the misuse or negligent use of our engineered cells materials, organisms and production processes. Accordingly, in the event of such misuse or negligent use, our reputation, future revenue, and operating results may suffer.
International expansion of our business exposes us to business, regulatory, political, operational, financial, and economic risks associated with doing business outside of the United States.
We currently market our services and deliver our programs, materials, and processes outside of the United States and may market future offerings outside of the United States. We, and our suppliers, collaborators, and customers, currently conduct business outside of the United States. From time to time, our services may include the hiring or secondment of our employees outside the United States at third party facilities or require the hiring or secondment of foreign persons within our facilities, including as a result of foreign acquisitions. Accordingly, we are subject to a variety of risks inherent in doing business internationally, and our exposure to these risks will increase as we continue to expand our operations and customer base. These risks include:
●political, social and economic instability;
●higher levels of credit risk, corruption, and payment fraud;
●enhanced difficulties of integrating any foreign acquisitions;
●increased expenses and diversion of our management’s attention from advancing programs;
●regulations that might add difficulties in repatriating cash earned outside the United States and otherwise prevent us from freely moving cash;
●import and export controls and restrictions and changes in trade regulations;
●compliance with the U.S. Foreign Corrupt Practices Act, the U.K. Bribery Act, and similar laws in other jurisdictions;
●multiple, conflicting and changing laws and regulations such as privacy, security and data use regulations, tax laws, tariffs, including tariffs announced by the Trump administration in 2025, trade regulations, economic sanctions and embargoes, employment laws, anti-corruption laws, regulatory requirements, reimbursement or payor regimes and other governmental approvals, permits and licenses;
●failure by us, our collaborators or our customers to obtain regulatory clearance, authorization or approval for the use of our services in various countries;
●additional potentially relevant third-party patent rights;
●complexities and difficulties in obtaining intellectual property protection and enforcing our intellectual property;
●difficulties in staffing and managing foreign operations, including difficulties related to the increased operations, travel, infrastructure and legal compliance costs associated with international locations;
●logistics and regulations associated with shipping chemicals, biomaterials and product samples, including infrastructure conditions and transportation delays;
●financial risks, such as longer payment cycles, difficulty collecting accounts receivable, widespread inflationary pressure, the impact of local and regional financial crises, on demand and payment for our products and exposure to foreign currency exchange rate fluctuations;
●natural disasters, political and economic instability, including wars, terrorism and political unrest, the outbreak of disease, or public health epidemics/pandemics, such as COVID-19, which could have an adverse impact on our employees, contractors, customers, partners, travel and the global economy;
●breakdowns in infrastructure, utilities and other services;
●boycotts, curtailment of trade and other business restrictions; and
●the other risks and uncertainties described in this Annual Report.
Additionally, as part of our growth strategy, we will continue to evaluate potential opportunities for international expansion. Operating in international markets requires significant resources and management attention and will subject us to regulatory, economic and political risks in addition to those we face in the United States. However, our international expansion efforts may not be successful, which could limit the size of our market or the ability to provide services or programs internationally.
In addition, due to potential costs from any international expansion efforts and potentially higher supplier costs outside of the United States, our international operations may operate with a lower margin profile. As a result, our margins may fluctuate as we expand our operations and customer base internationally.
Any of these factors could significantly harm our future international expansion and operations and, consequently, our revenue and results of operations.
Our investments in and use of AI may result in reputational harm, liabilities, or other adverse consequences to our business operations.
In August 2023, we entered into a strategic partnership with Google Cloud to develop and deploy AI tools for biology and biosecurity. Under the strategic partnership, Ginkgo has developed and will continue to develop new, state-of-the-art large language models (“LLMs”) running on Google Cloud's Vertex AI platform across genomics, protein function, and synthetic biology, helping Ginkgo's customers accelerate innovation and discovery in fields as diverse as drug discovery, agriculture, industrial manufacturing, and biosecurity.
In September 2024, we launched our first model API, a tool aimed at making biological AI models accessible to researchers, developers, and machine learning scientists. However, our development and use of AI technology in our products and operations remains in the early phases. While we aim to develop and use AI responsibly and attempt to mitigate ethical and legal issues presented by its use, we may ultimately be unsuccessful in identifying or resolving issues before they arise. There is no guarantee that Ginkgo will be successful in developing or successfully deploying the use of commercially available AI tools and as with many innovations, the use of AI presents many risks and challenges, including misuse, intellectual property infringement or trade secret misappropriation, flawed algorithms, inadequate data provenance for datasets used to train models, and insufficient, flawed, and/or biased datasets.
Additionally, AI technologies, including current tools available for business use in the marketplace, are complex and rapidly evolving. Uncertainty around new and emerging AI technologies may require additional investment to remain commercially relevant and/or to develop appropriate protections and safeguards. These investments may be costly and could increase our expenses as we contemplate expanding the use of AI in our platform and services. In addition, perceived or actual technical, legal, compliance, privacy, security, ethical or other issues relating to the use of AI may cause public confidence in AI to be undermined, which could slow our customers’ adoption of our products and services that use AI. We may also face significant potential disruption as a result of rapidly evolving domestic and international laws and regulations, which could impose significant costs and obligations on the company. For example, in 2023, an executive order on safe, secure and trustworthy AI was issued; and a White House memorandum released in 2024 as a result of this executive order made specific recommendations for enhanced biosecurity screening and software for biofoundries using AI. The EU has similarly introduced the AI Act to establish rules for providers and users. Emerging regulations may pertain to data privacy, data protection, and the ethical use of AI, as well as clarifying intellectual property considerations.
Challenges inherent to the use of AI generally or specific to Google's AI systems could adversely impact the reliability of our data and subject us to delays and competitive harm, result in new or enhanced governmental or regulatory scrutiny,
pose confidentiality or security risks, ethical concerns, or legal liability, as well as brand or reputational harm, and our business and results of operations may suffer. Our use of AI could result in cybersecurity incidents that implicate the personal data or confidential information of the Company or our customers, which could adversely affect our reputation and results of operations.
Our recent restructuring actions in connection with our plans to reduce operational expenditures may not result in anticipated savings, could result in total costs and expenses that are greater than expected and could disrupt our business.
In connection with our plans to reduce operational expenditures, we implemented a restructuring plan, including a reduction in workforce and a planned consolidation and subleasing of certain facilities. Initial workforce reductions commenced in June 2024 and continued throughout 2024, with further reductions expected in 2025. All reductions are expected to be substantially completed in 2025, subject to compliance with applicable laws. Our restructuring actions may result in other disruptions to our business, including customer program delivery issues, loss of historical customer or technical knowledge, our ability to comply with applicable laws and regulations, and our ability to retain key employees. Our efforts to reduce the size of our patent portfolio may result in inadequate protection of our intellectual property assets. The Company plans to consolidate certain facilities through various actions, including combining office and laboratory operations into fewer locations, subleasing unused or underutilized facilities, and has taken or plans to take other related measures. While the Company aims to complete the majority of its facility consolidation actions in 2025, the actual timing may vary, especially for subleasing unused or underutilized facilities, which may extend beyond 2025 or may not occur prior to termination of such leases, depending on market conditions. Additionally, restructuring expenses related to potential asset impairments, contract amendments or terminations for any facilities no longer in use or underutilized could be material. The Company currently estimates the costs for the reduction in force to range from $20.0 million to $23.0 million primarily in the Cell Engineering segment and to consist of cash severance and related costs.
Risks Related to Our Customers
We are dependent on our customers’ willingness and ability to develop, produce and manufacture products using the engineered cells, other biological assets and/or biomanufacturing processes that we develop and on the success of our customers’ development, production, and manufacturing efforts.
We sell end-to-end cell engineering solutions and cell engineering tools service offerings. For our solutions offerings, we rely on our customers to commercialize products that may be enabled by our engineered cells, other biological assets (e.g., enzyme DNA sequences) and/or biomanufacturing processes. A portion of the value in such customer collaborations has historically been earned through downstream value sharing in the form of royalty streams, milestone payments, and/or equity interests. If our customers are not successful in bringing these products to market, or if these products are not successful once on the market, the downstream portion of our value will be adversely impacted. Because we do not directly control manufacturing, product or downstream process development or commercialization, we have limited ability to impact the quality of our partners’ production processes and ultimate commercial success. Our ability to secure new business for our R&D services and tools is dependent on our customers’ willingness and ability to invest into R&D and continued outsourcing. Our business could be significantly impacted by any decline in R&D spending or outsourcing activities.
In addition, our customers have chosen, and may in the future choose, not to develop or commercialize a product we have enabled in which we are entitled to downstream value sharing. In our current relationships, we would have limited or no recourse to find alternative methods to monetize these products without the original customer. Because this industry is still nascent and the regulatory environment is evolving, we have limited historical information on the probability of commercial success for bioengineered products or biomanufacturing processes in the market and have limited ability to underwrite the likelihood that our customers will be able to create valuable products or processes in their market using the results of their programs with us. If we overestimate the probability or scale of commercial success, the price of our common stock may be adversely impacted as a result of lower expectations for future cash flows from customer collaborations.
Our revenue is concentrated in a limited number of customers, some of which are related parties, and our revenue, results of operations, cash flows and reputation may suffer upon the loss of a significant customer.
We have derived, and may continue to derive, a significant portion of our revenue from a limited number of large customers. During the year ended December 31, 2024, three customers each represented more than 10% of our total
revenue and cumulatively represented 49% of our total revenue. Due to the significant time required to acquire new customers, to plan and develop new programs for customers, and to satisfactorily execute on existing programs, the loss of any of these customers, or the loss of any other significant customer or a significant reduction in the amount of demand from a significant customer would adversely affect our revenue, results of operations, cash flows and reputation in the marketplace. There is always a risk that existing customers will not elect to do business with us in the future or will experience financial difficulties. If our customers experience financial difficulties or business reversals which reduce or eliminate the need for our services, they may be unable or unwilling to fulfill their contracts with us. There is also the risk that our customers will attempt to impose new or additional requirements on us that reduce the profitability of the services performed by us. Our customer concentration also increases the concentration of our accounts receivable and our exposure to payment defaults by key customers, which could expose us to substantial and potentially unrecoverable costs if we do not receive payment from key customers. Additionally, the loss of any significant customer could pose reputational harm to us and make it more challenging to acquire new customers.
In addition, while our customer collaborations are typically multi-year, we generally do not require our customers to generate a minimum amount of annual demand and without such contracts, our customers are not obligated to use our services beyond the amounts they choose to incur. Our customers may choose to use fewer of our services depending on program progress, their own technological capabilities, market demand for their products and/or their own internal budget cycles. As a result, we cannot accurately predict our customers’ decisions to reduce or cease utilizing our services. Even where we enter into long-term contracts with our customers, there is no guarantee that such agreements will be negotiated on terms that are commercially favorable to us in the long-term. In addition, existing customers may choose to perform some or all of the services they expect from us internally, with another third-party partner or by using capabilities from acquisitions of assets.
In certain cases, our business partners may have discretion in determining when and whether to make announcements about the status of our collaborations, including about developments and timelines for advancing programs, and the price of our common stock may decline as a result of announcements of unexpected results or developments.
Generally, we and our customers must mutually agree on determining when and whether to make announcements about the status of our collaborations, including developments in our programs and timelines for commercialization of or improvements to products using engineered cells developed using our platform. However, in some cases our customers may report or otherwise may be obligated to disclose certain matters without our consent. Our partners may also wish to report such information more or less frequently than we intend to or may not wish to report such information at all. We or our partners may announce a collaboration or partnership even if there is no guarantee that we will recognize program fees. The price of our common stock may decline as a result of a public announcement of unexpected results or developments in our partnerships, or as a result of our partners not consenting to an announcement or withholding information.
Risks Related to our Historic COVID-19 Testing Services
We may be subject to tort liability if the COVID-19 tests we utilized in our testing programs provided inaccurate results.
The Public Readiness and Emergency Preparedness Act (the “PREP Act”) provides immunity for manufacturers, distributors, program planners, qualified persons, and their officials, agents, and employees from certain claims under state or federal law for a “loss” arising out of the administration or use of a “covered countermeasure” in the United States. Distributors are certain persons or entities engaged in the distribution of drugs, biologics, or devices. Program planners include persons who supervise or administer a program with respect to the administration, distribution, provision, or use of a Covered Countermeasure (as defined in the PREP Act). Covered Countermeasures include security countermeasures and “qualified pandemic or epidemic products,” including products intended to diagnose or treat pandemic or epidemic disease, such as COVID-19 diagnostic tests, as well as treatments intended to address conditions caused by such products. Covered Countermeasures must also be approved, cleared, or authorized for emergency use, or otherwise authorized for investigational use, by the FDA in order to be considered Covered Countermeasures under the PREP Act.
For these immunities to apply, the Secretary of HHS must issue a declaration in cases of public health emergency or “credible risk” of a future public health emergency. On March 10, 2020, the Secretary of HHS issued a declaration under the PREP Act and has issued subsequent amendments thereto to provide liability immunity for activities related to certain countermeasures against the COVID-19 pandemic.
In the past, we were the authorized distributor of certain third-party COVID-19 tests and collection kits that received an EUA and supervised testing programs for COVID-19 testing customers. There can be no assurance that our test distribution
and program planning activities regarding these programs would be covered under the provisions of the PREP Act. Also, there can be no assurance that the U.S. Congress will not act in the future to reduce coverage under the PREP Act or to repeal it altogether.
Furthermore, some of the third-party tests that were used as part of our pooled testing program were not covered by an EUA and, at this time, we do not believe that such testing services, administration, or program planning related to our pooled testing program will qualify for PREP Act immunity. If product liability lawsuits are brought against us in connection with allegations of harm connected to our prior COVID-19 testing services, we may incur substantial liabilities. The PREP Act is a complex law with limited judicial precedent, and thus even for the third-party COVID-19 tests and collection kits used in our testing services that were subject to EUAs, we may have to expend significant time and legal resources to obtain dismissal of a lawsuit on the basis of PREP Act immunity.
If we cannot successfully defend ourselves against claims that our COVID-19 testing services caused injuries and if we are not entitled to immunity under the PREP Act, or the U.S. Congress limits or eliminates coverage under the PREP Act, or if the liability protections under the PREP Act are not adequate to cover all claims, we may incur substantial liabilities. Regardless of merit or eventual outcome, product liability claims may result in decreased demand for our services, injury to our reputation, costs to defend litigation, loss of revenue, and substantial money awards to customers.
Risks Related to the Synthetic Biology Industry
Rapidly changing technology and emerging competition in the synthetic biology industry could make the platform, programs, and products we and our customers are developing obsolete or non-competitive unless we continue to develop our platform and pursue new market opportunities.
The synthetic biology industry is still emerging and is characterized by rapid and significant technological changes, frequent new product introductions and enhancements, and evolving industry demands and standards. Our future success will depend on our ability to sign and initiate new programs that address the evolving needs of our customers on a timely and cost-effective basis, to advance existing programs and to pursue new market opportunities that develop as a result of technological and scientific advances. Additionally, our customers may face significant competition or other risks which may adversely impact our business and results of operations.
There are a number of companies in the broader synthetic biology industry, and our future success will depend on our ability to maintain a competitive position with respect to technological advances. Technological developments, including emerging AI technologies, may result in our platform becoming obsolete. Our ability to compete successfully will depend on our ability to develop proprietary technologies that enable our customers to develop products using our platform in a manner that is either less expensive, faster, superior or otherwise differentiated from what a competitor’s technologies and products might enable. If we are unable to continue to successfully advance our platform or the services it provides at scale, or if our customers are unable to commercialize the products or processes made or improved upon by using our platform, our business and results of operations will be adversely impacted.
Due to the significant lead time involved in launching a new program or developing a new product or process using our platform, our customers are required to make a number of assumptions and estimates regarding the commercial feasibility of a new product, including assumptions and estimates regarding the size of an emerging product category and demand for those end-products and processes which will use our technology, the ability to scale-up manufacturing processes to produce a product on a commercial scale, the ability to penetrate that emerging product category, customer adoption of a downstream product, the existence or non-existence of products being simultaneously developed by competitors, potential market penetration and obsolescence, planned or unplanned. As a result, it is possible that we may commence a new program with a customer who wishes to develop a product or process that has been displaced by the time of launch, addresses a market that no longer exists or is smaller than previously thought, that end-consumers do not like or otherwise is not competitive at the time of launch, in each case, after the incurrence of significant opportunity costs on our part to develop such product. The ultimate success of the products developed by our customers using our services may be dependent on the success of other markets in which we or our customers do not operate in or have knowledge or expertise or which, in each case, may not reach the size anticipated by us or our customers or may be replaced by another emerging product category or eliminated entirely.
The market, including customers and potential investors, may be skeptical of our ability to deliver on programs because they are based on a relatively novel and complex technology.
The market, including customers and potential investors, may be skeptical of the viability and benefits of bioengineered products as well as our enabling abilities, including our platform and programs, because they are based on a relatively novel approach and the adoption of complex technology and because we are still demonstrating to the market the value of our platform. There can be no assurance that our platform and programs will be understood, approved, or accepted by customers, regulators and potential investors or that we will be able to sell our services profitably at competitive prices and with features sufficient to establish demand.
In addition, in order for novel products from our programs to be successfully commercialized, support from the entire relevant supply chain is needed. Relationships with all parts of the supply chain are important in order to gain visibility into market trends and feature and specification requirements and in order to ensure customers are able to successfully manufacture their products, obtain regulatory approval and gain access to key distribution channels. If we are unable to convince these potential customers, their suppliers, or the consumers who purchase products containing or made or developed using engineered cells and/or biomanufacturing processes, of the utility and value of such products or that such products are superior to the products they currently use, we will not be successful in entering these markets and our business and results of operations will be adversely affected. If potential investors are skeptical of the success of our platform or cell programs, our ability to raise capital and the value of our common stock may be adversely affected.
Ethical, legal and social concerns about GMOs and Genetically Modified Materials and their resulting products could limit or prevent the use of products or processes using our technologies, limit public acceptance of such products or processes and limit our revenues.
Our technologies and the technologies of our customers involve the use of genetically modified cells, organisms and biomaterials, including, without limitation, GMOs, GMMs, Genetically Modified Materials and their respective products. The use, production and marketing of Genetically Modified Materials are subject to laws and regulations in many countries, some of which are new and some of which are still evolving. In the United States, the FDA, the EPA and the USDA are the primary agencies that regulate the use of GMOs, GMMs and potential products derived from GMOs or GMMs. If regulatory approval of the Genetically Modified Materials or resulting products is not secured, our business operations, financial condition and our ability to grow as a business could be adversely affected. We expect to encounter regulations regarding Genetically Modified Materials in most, if not all, of the countries in which our customers may seek to establish production capabilities or sell their products and the scope and nature of these regulations will likely be different from country to country. Governmental authorities could, for safety, social or other purposes, impose limits on, or implement regulation of, the use, production or marketing of Genetically Modified Materials. If our customers cannot meet the applicable requirements in other countries in which they intend to produce or sell their products, or if it takes longer than anticipated to obtain such approvals, our business could be adversely affected.
In addition, public perception regarding the safety and environmental hazards of, and ethical concerns over, Genetically Modified Materials or the processes used to create them, including gene editing or gene regulating technologies, could influence public acceptance of our and our customers’ technologies, products, and processes. For instance, certain advocacy groups engage in efforts that include regulatory legal challenges and labeling campaigns for genetically modified products, as well as application of pressure to consumer retail outlets seeking a commitment not to carry genetically modified foods. These groups in the past have pressured retail food outlets and grocery store chains to publicly state that they will not carry genetically modified foods and have pressured food brands to publicly state that they will not use ingredients produced by genetically modified microbes. In addition, certain labeling-related initiatives have heightened consumer awareness of GMOs, which may make consumers less likely to purchase products containing GMO ingredients, and could have a negative impact on the commercial success of our customers’ products and programs. These concerns could result in increased expenses, regulatory scrutiny, delays or other impediments to our programs. The subject of Genetically Modified Materials has received negative publicity, which has aroused public debate. This adverse publicity has led to, and could continue to lead to, greater regulation and trade restrictions on imports of Genetically Modified Materials or their resulting products. In addition, we have expanded into the European Union market, which has increased government regulation and scrutiny over genetically modified products. There is a risk that products produced using our technologies could cause adverse health effects or other adverse events, which could also lead to negative publicity, regulatory action or private litigation. If we are unable to overcome the ethical, legal and social concerns relating to genetic engineering, our programs could face increased expenses, regulatory scrutiny, delays or other impediments to deliver our programs or the commercialization of resulting products and processes.
Risks Related to Intellectual Property
If we are unable to obtain, maintain and defend patents protecting our intellectual property, our competitive position could be harmed.
Our success depends in part on our ability to obtain and maintain intellectual property protection for our proprietary technologies. We protect our proprietary technologies through patents and trade secrets, both of which entail risk. If we are unable to obtain, maintain or protect intellectual property rights related to our technology, or if our intellectual property rights are inadequate, our competitive position, business, financial conditions, results of operations and prospects may be harmed.
Because of the volume and nature of our inventions, patent protection may not be practicable, available, or appropriate for some aspects of our proprietary technologies. While we own patents and pending patent applications in the United States and in foreign jurisdictions, these applications do not ensure the protection of our intellectual property. There may be prior art of which we are not aware. Additionally, obtaining, maintaining, defending and enforcing patents is costly, time consuming and complex, and we may not be able to file and prosecute all necessary or desirable patent applications, or maintain and enforce any patents that may issue from such patent applications at a reasonable cost or in a timely manner. As a result of our restructuring and cost-reduction efforts, we have determined to reduce the size of our patent portfolio and, therefore, some of our intellectual property assets may be inadequate protected. It is also possible that we will fail to identify patentable aspects of our technologies before it is too late to obtain patent protection. Although we enter into confidentiality agreements with parties who have access to confidential or patentable aspects of our R&D output, such as our employees, collaborators, consultants, advisors and other third parties, any of these parties may breach the agreements and disclose such output before a patent application is filed, thereby jeopardizing our ability to seek patent protection.
Further, pending applications may not be issued or may be issued with claims significantly narrower than we currently seek. Patents for which claims have been allowed may be successfully challenged and invalidated. Unless and until our pending applications issue, their protective scope is impossible to determine and, even after issuance, their protective scope may be limited.
Recent changes in patent law may make patents covering life science inventions more difficult to obtain and enforce. Further legislative changes or changes in the interpretation of existing patent law could increase the uncertainty and cost surrounding the prosecution of our owned patent applications and the maintenance, enforcement or defense of our owned patents. The Leahy-Smith America Invents Act (“the Leahy-Smith Act”) included changes that affect the way patent applications are prosecuted; redefine prior art; enable third-party submission of prior art to the United States Patent and Trademark Office (“USPTO”) during patent prosecution; and provide cost-effective avenues for competitors and other third parties to challenge the validity of patents at USPTO-administered post-grant proceedings, including post-grant review, inter partes review, and derivation proceedings. Thus, the Leahy-Smith Act and its continued implementation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents, all of which could have a material adverse effect on our business, financial condition, results of operations, and prospects.
Other changes in the law may further detract from the value of life science patents and facilitate challenges to our patents. In some cases, we develop inventions with the assistance of machine learning and other computational tools that may be considered to be AI, and we expect to use such tools, and to use generative AI, in future development. Because the law is in flux with respect to AI-assisted inventions, there is uncertainty and risk associated with patenting such inventions. Depending on future actions by the U.S. Congress, the federal courts and the USPTO, the laws and regulations governing patents could change in unpredictable ways that could have a further material adverse effect on our patent rights and our ability to protect, defend and enforce our patent rights in the future. It is also possible that disclosure requirements with respect to use of AI tools may be imposed by the patent office, which could increase the cost of patent prosecution and cause uncertainty and delay in the enforcement of patent rights.
In some cases, we use genetic sequence information from naturally occurring organisms. U.S. Supreme Court rulings have narrowed the scope of patent protection for naturally occurring sequences and for inventions based on the observation and exploitation of natural phenomena. These decisions have weakened the rights of patent owners in certain situations. The Federal Circuit and the Supreme Court have also issued a series of rulings that create obstacles to the patenting of groups of genetic sequences that share functional characteristics, making it more difficult to obtain claims to certain genetic constructs, particularly antibodies. These changes in the law have created uncertainty with respect to the validity and enforceability of patents covering natural and engineered sequences.
Further, the issuance of a patent is not conclusive as to its inventorship, scope, validity or enforceability, and our patents may be challenged in the courts or patent offices in the United States and abroad. An adverse determination in any such challenge could result in loss of exclusivity, or patent claims being narrowed, invalidated or held unenforceable, in whole or in part. Any of these results could limit our ability to stop others from using or commercializing similar or identical technology to compete directly with us. In addition, if the breadth or strength of protection provided by our patents or patent applications is threatened, regardless of the outcome, it could dissuade companies from collaborating with us. Such proceedings also may result in substantial cost and require significant time from our scientists and management, even if the eventual outcome is favorable to us.
The laws of some countries do not protect intellectual property rights to the same extent as do the laws of the United States or may apply different rules concerning the assignment of intellectual property rights. Many companies have encountered significant problems in protecting and defending intellectual property rights in certain jurisdictions. We may encounter similar difficulties, particularly as we expand to work with foreign employees and contractors and expand our collaboration activities into foreign markets. The legal systems of certain countries, particularly certain developing countries, do not favor the enforcement of patents by foreign holders and, in some cases, do not favor the enforcement of patents at all, particularly patents in the life sciences. This could make it difficult for us to stop the infringement of our patents. Proceedings to enforce our patent rights in foreign jurisdictions could result in substantial costs and divert our efforts and attention from other aspects of our business and could be unsuccessful.
Reductions in the scope or enforceability of our patent protection may adversely affect our customers’ ability to commercialize their products and may thus reduce our downstream value from royalties, commercial milestone payments and/or equity.
If we are unable to protect the confidentiality of our trade secrets, our business and competitive position may be harmed.
Because patent protection may not be available or appropriate for significant aspects of the technology we are developing, our success may depend in large part on our proprietary information, including genetic and other chemical and biological data, processes, know-how, and other trade secrets developed over years of R&D, some of which are embodied in proprietary software. We rely heavily on trade secret protections, especially in cases where we believe patents or other forms of registered intellectual property protection may not be appropriate or obtainable. However, trade secrets are difficult to protect. The secrecy of the Company’s trade secrets must be maintained for them to retain their status and protection as trade secrets. While we strive to protect the secrecy of our trade secrets and other proprietary information, including by requiring our employees, customers, consultants, and contractors to enter into confidentiality agreements and instituting multilayered protections covering our digital environment and biomaterials, we may not be able to adequately protect our trade secrets or other proprietary information. We cannot guarantee that we have entered into such agreements with every party that may have or has had access to our trade secrets, biomaterials or proprietary technology and processes. Further, despite these efforts, any of these parties may breach the agreements and disclose our proprietary information, including our trade secrets, and we may not be able to obtain adequate remedies for such breaches.
We seek to preserve the integrity and confidentiality of our information by maintaining physical security of our premises and physical and electronic security of our information technology systems, but it is possible that these security measures could be breached. We also rely on systems provided by third parties, which may suffer security breaches or incidents. Such security breaches may be inadvertent or may come about due to intentional misconduct or other malfeasance or by human error or technical malfunctions, including those caused by hackers, employees, contractors, or vendors. It may be difficult or impossible to recover trade secrets or other confidential information once it is hacked, and hackers may operate from jurisdictions that will not cooperate with such efforts. Enforcing any claim that a third party unlawfully obtained and is using any of our trade secrets is expensive and time consuming, and the outcome is unpredictable. In addition, courts in some jurisdictions are less willing or unwilling to protect trade secrets even when a hacker or thief can be identified.
Our competitors may lawfully obtain or independently develop knowledge that is equivalent to one or more of our trade secrets. Were they to do so, we would be unable to prevent them from using that independently developed knowledge. Such a competitor could claim that we had learned the trade secret from them and bring an action against us on that basis. If any of our trade secrets were to be disclosed to or independently developed by a competitor or other third party, our competitive position could be materially and adversely harmed. Moreover, a competitor could file for patent protection covering intellectual property that we have chosen to protect as a trade secret. In such a case, we might be restricted or excluded from using that intellectual property even if we had developed it before our competitor did.
Our facilities hold large collections of microbial strains, cell lines and other biomaterials. Failure to implement adequate controls and protections, failure to implement adequate disposal procedures, unauthorized visitors in the labs, or customers’ failure to adequately protect biological materials can put us and our customers at risk of losing valuable assets through negligence or theft and enabling the use of those lost materials by our competitors. While we believe that we take reasonable measures to protect the security of biomaterials owned by us or our customers, it is possible that our security controls and practices may not prevent unauthorized or other improper access to such genetic material. Any unauthorized access, acquisition, use, destruction, or release of the GMOs we engineer could result in our having exposure to significant liability under our contracts, as well as to regulatory actions, litigation, investigations, remediation obligations, damage to our reputation and brand, supplemental disclosure obligations, loss of customer, consumer, or partner confidence in the security of our platform, impairment to our business, and corresponding fees, costs, expenses, loss of revenues, and other potential liabilities.
Our customers sometimes provide organisms, genetic material and/or data to us in connection with our collaborations. In the event that we fail to protect customer materials or data or inadvertently use such materials or data for unauthorized purposes, we could be liable to our customers under trade secret laws or contractual provisions.
We may be subject to claims challenging the inventorship or ownership of our patents, biomaterials and other intellectual property.
Certain of our employees, consultants and contractors were previously employed at universities or other software or biotechnology companies, including our competitors or potential competitors. Additionally, some of our consultants or contractors may have ongoing relationships with universities. Although we try to ensure that our employees, consultants and contractors do not use the intellectual property of others in their work for us, we may be subject to claims that these individuals or other contractors have used or disclosed intellectual property, including trade secrets or other proprietary information, of another. Litigation may result from these claims.
While it is our policy to require that our employees, consultants and contractors who may be involved in the development of intellectual property for us execute agreements assigning such intellectual property to us, we may be unsuccessful in executing such an agreement with each party who in fact develops intellectual property that we regard as our own. Our intellectual property assignment agreements with them may not be self-executing or may be breached, and we may be forced to bring claims against third parties, or defend claims they may bring against us, to determine the ownership of what we regard as our intellectual property. Such claims could have a material adverse effect on our business, financial condition, results of operations and prospects.
If we are unsuccessful in litigating any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel, which could have a material adverse effect on our competitive business position and prospects. Such intellectual property rights could be awarded to a third party, and we could be required to obtain a license from such third party to use or commercialize our technology or products, which license might not be available on commercially reasonable terms, or at all. Even if we are successful in prosecuting or defending against such claims, litigation could result in substantial costs and be a distraction to our management and employees.
The life science academic and research community has abided by norms of free exchange of biomaterials, but norms have begun to change so that parties may assert ownership and control over biomaterials that they permitted to be freely disseminated in the past. Thus, despite our best efforts to confirm our right to use biomaterials in our possession, we may use organisms that we believe to be free of encumbrance that are, in fact, subject to claims of title by others. In such a situation, litigation may be required to clear title, if it can be cleared at all. Similarly, we may be subject to claims that we have used biomaterials obtained from licensors or repositories for unauthorized purposes, or purposes not consistent with the licensing terms of the providing organization.
We may become involved in lawsuits or other enforcement proceedings to protect or enforce our patents or other intellectual property, which could be expensive, time consuming and potentially unsuccessful.
Competitors and other third parties may infringe or otherwise violate our issued patents or other intellectual property. In addition, our patents may become involved in inventorship, ownership, or priority disputes. We may also become subject to claims by collaboration partners that intellectual property or biomaterials that we believe to be owned by us are actually owned by them. Any litigation concerning any of these issues would be expensive, time consuming and uncertain. There can be no assurances that we would prevail in any suit brought by us or against us by third parties, or successfully settle or
otherwise resolve those claims. Significant litigation would have substantial costs, even if the eventual outcome were favorable to us, and would divert management’s attention from our business objectives.
Under certain circumstances, we may share or lose rights to intellectual property developed under U.S. federally funded research grants and contracts.
Some of our inventions, data, or other intellectual property have been or may be developed during the course of research funded by the U.S. government. The U.S. government may have the right to take title to government-funded inventions if we fail to disclose the inventions to the government in a timely manner or fail to file a patent for the intellectual property within specified time limits. Further, in consequence of our receiving government funding, the U.S. government may have certain rights to intellectual property that we use in our platform or programs pursuant to the Bayh-Dole Act of 1980, as amended (the “Bayh-Dole Act”). Under the Bayh-Dole Act, U.S. government rights in certain “subject inventions” developed under a government-funded program may include a non-exclusive, irrevocable worldwide license to use inventions for any governmental purpose. In some circumstances, the U.S. government may acquire unlimited rights in data we generate. In addition, the U.S. government has the right to require us, or an assignee or exclusive licensee to U.S. Government-funded inventions, to grant licenses to any of these inventions to the government or a third party if the government determines that: (i) adequate steps have not been taken to commercialize the invention; (ii) government action is necessary to meet public health or safety needs; (iii) government action is necessary to meet requirements for public use under federal regulations; or (iv) the right to use or sell such inventions is exclusively licensed to an entity within the United States and substantially manufactured outside the United States without the U.S. government’s prior approval. Additionally, we may be restricted from granting exclusive licenses for the right to use or sell such inventions unless the licensee agrees to comply with relevant Bayh-Dole Act restrictions (e.g., manufacturing substantially all of the invention in the United States) and reporting requirements. In addition, the U.S. government may acquire title in any country in which a patent application is not filed. Certain technology and inventions are also subject to transfer restrictions during the term of these agreements with the U.S. government and for a period thereafter. These restrictions may limit sales of products or components, transfers to foreign subsidiaries for the purpose of the relevant agreements, and transfers to certain foreign third parties. If any of our intellectual property becomes subject to any of the rights or remedies available to the U.S. government or third parties pursuant to the Bayh-Dole Act, this could impair the value of our intellectual property and could adversely affect our business.
The use of digital genetic sequence information may be subject to the Nagoya Protocol or other treaties or local legislation concerning biodiversity, which could increase our costs and adversely affect our business.
The Nagoya Protocol is a supplemental agreement to the Convention on Biological Diversity (“CBD”) that is designed to provide for equitable sharing of benefits arising from the utilization of genetic resources and traditional knowledge. There are other international treaties, as well as local legislation in many countries, with similar objectives. Under the Nagoya Protocol and many other treaties and laws, countries possessing genetic resources (“source countries”) are tasked with setting up procedures and institutional infrastructure for researchers to obtain prior informed consent, both from the source country and from any relevant indigenous or traditional communities, for biological research. Many have been slow to adopt workable institutions permitting the rational negotiation of benefit-sharing agreements. Many source countries are now asserting that the use of digital genetic sequence information is subject to the constraints of the Nagoya Protocol or similar national- or local-level benefit-sharing requirements. It is unclear whether this position will ultimately be adopted or what the implications of such adoption might be. It is unclear what a source country might assert if we used genetic sequences (i) extracted by a third party from a natural resource that was removed from its source country before that source country ratified the CBD or signed the Nagoya Protocol (ii) extracted by a third party and uploaded to public sequence databases after the source country ratified the CBD; (iii) in a heterologous host organism; or (iv) as a base for further engineering, so that the sequence we use no longer conforms to the natural sequence on which it was based.
We make extensive use of public and proprietary sequence databases to support our work. While we undertake efforts to identify and comply with laws and international protocols relating to the use of genetic resources, the uncertainty surrounding the use of digital sequence information and the lack of workable institutions in many source countries for the efficient negotiation of benefit-sharing agreements may limit our use or cause uncertainty in our use of certain sequences that we obtain from public access databases or natural sources. New financial obligations may arise regarding our use of sequence information. Customers that must certify their compliance with Nagoya Protocol obligations may be reluctant to do business with us unless we engage in expensive and time-consuming benefit-sharing negotiations with source countries of publicly available genetic sequences. Moreover, the World Intellectual Property Organization is considering requiring disclosures in patents of the origin of genetic resources, which may further increase uncertainty and the cost of patent
prosecution. These changes could increase our R&D costs and adversely affect our business, financial condition, and results.
Third party patents may limit our freedom to operate in certain areas, which may adversely affect our business.
There may be patents that affect our freedom to operate in certain areas, and we may as a result choose to design around or license such patents from third parties. If we must spend significant time and money designing around or licensing patents held by others, our business and financial prospects may be harmed. We may be restricted from carrying out certain operations in our Foundry, or we may be limited in our ability to design new products for our customers. We may become subject to claims by third parties alleging that we are infringing, misappropriating, or otherwise violating their intellectual property rights.
If we are sued for infringing, misappropriating, or otherwise violating intellectual property rights of third parties, such litigation could be costly and time consuming and could prevent or delay us from using our platform and technologies.
Litigation arising from disputes relating to the intellectual property of third parties is expensive, time-consuming, and uncertain. There can be no assurance that we will prevail in such disputes. Parties making claims against us might be able to obtain injunctive or other relief, which could block our or our customers’ ability to develop, commercialize and sell products or use our technologies, and could result in the award of substantial damages against us, including treble damages, attorney’s fees, costs and expenses if we were found to have willfully infringed. In the event of a successful claim against us, we or our customers might be required to pay damages and ongoing royalties, and obtain licenses from third parties, or be prohibited from selling certain products or using certain technologies. We may not be able to obtain these licenses on acceptable or commercially reasonable terms, if at all. In addition, we or our customers could encounter delays in product or service introductions while we attempt to develop alternative designs or redesign existing products or technologies to avoid or resolve these claims. Our loss in any lawsuit or failure to obtain a license could prevent us from using our platform and technologies. Such a loss or failure could materially affect our business and reputation. Any litigation pertaining to these issues would have substantial costs, even if the eventual outcome were favorable to us, and would divert management’s attention from our business objectives.
If our trademarks and trade names are not adequately protected, then we may not be able to build name recognition in our markets of interest and our business may be adversely affected.
Our registered or unregistered trademarks or trade names may be challenged, infringed, diluted, tarnished, circumvented or declared generic or determined to be infringing on other marks. We may not be able to protect our rights to these trademarks and trade names, which we need to build name recognition among potential collaborators or customers in our markets of interest. At times, competitors may adopt trade names or trademarks similar to ours, thereby impeding our ability to build brand identity and possibly leading to market confusion. In addition, there could be potential trade name or trademark infringement, dilution or tarnishment claims brought by owners of other trademarks or trademarks that incorporate variations of our registered or unregistered trademarks or trade names. Over the long term, if we are unable to establish name recognition based on our trademarks and trade names, then we may not be able to compete effectively and our business may be adversely affected. Our efforts to enforce or protect our proprietary rights related to trademarks, trade names, trade secrets, domain names, copyrights or other intellectual property may be ineffective and could result in substantial costs and diversion of resources and could adversely affect our business, financial condition, results of operations and prospects.
Any claims or lawsuits relating to infringement of, misappropriating, or otherwise violating intellectual property rights brought by or against us will be costly and time consuming and may adversely affect our business, financial condition and results of operations.
Any of the risks identified above could result in significant litigation. In addition to the specific litigation-related risks identified above, litigation of any kind carries certain inherent risks. Because of the substantial amount of discovery required in connection with litigation in U.S. courts, there is a risk that some of our confidential information could be compromised in the discovery process. There could be public announcements of the results of hearings, motions or other interim proceedings or developments. If securities analysts or investors perceive these results to be negative, it could have a substantial adverse effect on our share price.
Further, our agreements with some of our customers, suppliers or other entities require us to defend or indemnify these parties if they become involved in infringement claims that target our products, services or technologies, or in certain other
situations. If we must defend or indemnify third parties, we could incur significant costs and expenses that could adversely affect our business, operating results or financial condition.
Intellectual property rights do not necessarily address all potential threats to our business.
The degree of future protection afforded by our intellectual property rights is uncertain because intellectual property rights have limitations and may not adequately protect our business or permit us to maintain our competitive advantage. The following examples are illustrative:
●we may choose not to file a patent in order to maintain certain intellectual property as trade secrets or know-how, and a third party may subsequently file a patent covering such intellectual property;
●others may independently develop similar or alternative technologies or duplicate any of our technologies without infringing our owned or licensed intellectual property rights;
●the patents of others may harm our business;
●we might not have been the first to make the inventions covered by the issued patents or pending patent applications that we own;
●we might not have been the first to file patent applications covering certain of our inventions; and
●issued patents that we hold rights to may fail to provide us with any competitive advantage, or may be held invalid or unenforceable, including as a result of legal challenges by our competitors.
Should any of these events occur, they could harm our business, financial condition, results of operations and prospects.
Intellectual property disputes of third parties and customers could have a material adverse effect on our business, financial condition, and results.
We rely, and expect to continue to rely on, certain capital equipment, machinery, consumables, reagents, software, services and intellectual property that we purchase or license from third parties for use in our operations, platform, products, services and offerings. We cannot be certain that our vendors, suppliers, and licensors are not infringing upon the intellectual property rights of others or that they have sufficient rights to the third-party technology used in our business in all jurisdictions in which we may operate. Disputes with any of these third parties over uses or terms could result in the payment of additional royalties or penalties by us, cancellation or non-renewal of the underlying license, termination of supplies or rights to use, or litigation. In the event that we cannot resolve issues of this kind, we may be required to discontinue or limit our use of the operations, platform, products, services or offerings that include or incorporate the licensed intellectual property. Any such discontinuation or limitation could have a material and adverse impact on our business, financial condition and results of operation.
Our customers may become involved in intellectual property disputes with third parties that are related or unrelated to any products or services we have supplied or rendered to them. Such disputes could result in a customer being unable to market its products, thus depriving us of license, milestone, or other revenues. Such deprivation could have a material adverse impact on our financial condition and results.
Our use of “open-source” software could negatively affect our ability to market or provide our services and could subject us to possible litigation.
We have used “open-source” software in connection with the development and deployment of our software platform, and we expect to continue to use open-source software in the future. Open-source software is licensed by its authors or other third parties under open-source licenses, which in some instances may subject us to certain unfavorable conditions, including requirements that we offer our products that incorporate the open-source software for no cost, that we make publicly available all or part of the source code for any modifications or derivative works we create based upon, incorporating or using the open-source software, or that we license such modifications or derivative works under the terms of the particular open-source license.
Companies that incorporate open-source software into their products have, from time to time, faced claims challenging the use of open-source software and compliance with open-source license terms. We could be subject to similar suits by parties claiming ownership of what we believe to be open-source software or claiming noncompliance with open-source licensing terms. While we monitor our use of open-source software and try to ensure that none is used in a manner that would require us to disclose our proprietary source code or that would otherwise breach the terms of an open-source agreement, we
cannot guarantee that we will be successful, that all open-source software is reviewed prior to use in our platform, that our developers have not incorporated open- source software into our products that we are unaware of or that they will not do so in the future.
Furthermore, there are an increasing number of open-source software license types, almost none of which have been interpreted by U.S. or foreign courts, resulting in a dearth of guidance regarding the proper legal interpretation of such licenses. As a result, there is a risk that open-source software licenses could be construed in a manner that imposes unanticipated conditions or restrictions on our ability to market or provide our products and services. If we are held to have breached or failed to fully comply with all the terms and conditions of an open-source software license, we could face infringement claims or other liability, or be required to seek costly licenses from third parties to continue providing our offerings on terms that are not economically feasible, if at all, to re-engineer all or a portion of our platform, to discontinue or delay the provision of our offerings if re-engineering could not be accomplished on a timely basis or to make generally available, in source code form, our proprietary code. Further, in addition to risks related to license requirements, use of certain open-source software carries greater technical and legal risks than does the use of third-party commercial software. For example, open-source software is generally provided without any support or warranties or other contractual protections regarding infringement or the quality of the code, including the existence of security vulnerabilities. To the extent that our platform depends upon the successful operation of open-source software, any undetected errors or defects in open-source software that we use could prevent the deployment or impair the functionality of our systems and injure our reputation. In addition, the public availability of such software may make it easier for others to compromise our platform. Any of the foregoing risks could materially and adversely affect our business, financial condition and results of operations.
Risks Related to Personnel, IT and Physical Infrastructure
Loss of key personnel, including our founders and senior executives, and/or failure to attract, train and retain additional key personnel could delay our cell engineering programs, harm our platform development efforts, limit our biosecurity and public health offerings, and harm our ability to meet our business objectives, particularly given the substantial investment required to recruit, hire and train our employees.
Our business involves complex, global operations across a variety of markets and requires a management team and employee workforce that is knowledgeable in the many areas in which we operate. Our future success depends upon our ability to attract, train, retain and motivate highly qualified management, scientific, engineering, information technology, operations, business development and marketing personnel, among others. In addition, the market for qualified personnel is competitive because of (a) the limited number of people available who have the necessary technical skills and understanding of our technology and products and (b) the nature of our industry which requires certain of our technical personnel to be on-site in our facilities. We compete for qualified technical personnel with other life sciences and information technology companies, as well as academic institutions and research institutions in the markets in which we operate, including: Massachusetts, USA; California, USA; The Netherlands; and Switzerland. In addition, as we add international operations, we will increasingly need to recruit qualified personnel outside the United States. However, doing so may also require us to comply with laws to which we are not currently subject, which could cause us to allocate or divert capital, personnel and other resources from our organization, which could adversely affect our business, financial condition, results of operations, prospects and reputation. Establishing international operations and recruiting personnel has in the past been impacted by COVID-19 travel and operational restrictions. Our senior leadership team is critical to our vision, strategic direction, platform development, operations and commercial efforts. Our employees, including members of our leadership team, could leave our company with little or no prior notice and would be free to work for a competitor. We also do not maintain “key person” life insurance on any of our employees. The departure of one or more of our founders, senior leadership team members or other key employees could be disruptive to our business until we are able to hire qualified successors.
Our continued platform development, growth and commercial success depends, in part, on recruiting and retaining highly-trained personnel across our various target industries and markets with the necessary background and ability to develop and use our platform and to effectively identify and sell to current and new customers. New hires and employees onboarded as a result of any of our recent acquisitions may require significant training and, in most cases, take significant time before they achieve full productivity. Our failure to successfully hire and integrate these key personnel into our business could adversely affect our business. To attract top talent, we believe we will need to offer competitive compensation and benefits packages, including equity incentive programs, which may require significant investment. If we are unable to offer competitive compensation this may make it more difficult for us to attract and retain key employees. Moreover, if the perceived value of our equity awards declines, it may adversely affect our ability to attract and retain key employees. If we
do not maintain the necessary personnel to accomplish our business objectives, we may experience staffing constraints that adversely affect our ability to support our programs and operations.
In addition, some of our personnel are qualified foreign nationals whose ability to live and work in the U.S. is contingent upon the continued availability of appropriate visas and whose ability to work on some of our technologies may require the procurement of appropriate export licenses. Due to the competition for qualified personnel in the key markets in which we operate, we expect to continue to utilize foreign nationals to fill part of our recruiting needs. As a result, changes to United States immigration policies have restrained, and could further restrain, the flow of technical and professional talent into the United States and adversely affect our ability to hire and retain qualified personnel.
Our business and results of operations are dependent on adequate access to laboratory and office space and suitable physical infrastructure to conduct our operations. If we are unable to access enough space or we experience failures of our physical infrastructure, including due to natural disasters affecting us or our suppliers, our business and results of operations could be adversely affected.
Our business depends on providing customers with R&D services and tools. In order to properly conduct our business, we need access to sufficient laboratory space and equipment to perform the activities necessary to advance and complete our programs. Additionally, we need to ensure that our laboratories and corporate offices remain operational at all times, which includes maintaining suitable physical infrastructure, including electrical, plumbing and HVAC, logistics and transportation systems and network infrastructure. We own certain properties in California and lease most of our laboratories and office spaces. We rely on the landlords for basic maintenance of our leased laboratories and office buildings. If one of our landlords has not maintained a leased property sufficiently, we may be forced into an early exit from the facility, which could be disruptive to our business. Furthermore, we may continue to acquire laboratories not built by us in order to sufficiently scale and expand our output capacity. If we discover that these buildings and their infrastructure assets are not in the condition we expected when they were acquired, we may be required to incur substantial additional costs to repair or upgrade the laboratories.
Problems in and around one or more of our laboratories or corporate offices, whether or not within our control, could result in service interruptions or significant infrastructure or equipment damage. These could result from numerous factors, including:
●human error;
●equipment failure;
●physical, electronic and cybersecurity breaches;
●fire, earthquake, hurricane, flood, tornado and other natural disasters;
●extreme temperatures;
●flood and/or water damage;
●fiber cuts;
●power loss;
●terrorist acts, including acts of bioterrorism;
●sabotage, vandalism and cyberattacks; and
●local epidemics or global pandemics such as the COVID-19 pandemic.
Certain of our facilities are located in an active earthquake and tsunami zone, and certain of our suppliers conduct their operations in the same region or in other locations that are susceptible to natural disasters. The occurrence of a natural or other disaster, such as an earthquake, tsunami, hurricane, drought, flood, fire, wildfire or any potential effects of climate change or localized extended outages of critical utilities or transportation systems, or any critical resource shortages affecting us or our suppliers or manufacturers could cause a significant interruption in our business, damage or destroy our facilities, production equipment or inventory or those of our suppliers and cause us to incur significant costs or result in limitations on the availability of our raw materials, any of which could harm our business, financial condition and results of operations.
We have timeline obligations to certain customers with respect to their programs. As a result, service interruptions or significant equipment damage in our laboratories could result in difficulty maintaining program timelines for these customers and potential claims related to such failures. Because the services we provide in our laboratories are critical to many of our customers’ businesses, service interruptions or significant equipment damage in our laboratories could also
result in lost revenue or other indirect or consequential damages to our customers. We cannot guarantee that a court would enforce any contractual limitations on our liability in the event that one of our customers brings a lawsuit against us as a result of a problem at one of our laboratories and we may decide to reach settlements with affected customers irrespective of any such contractual limitations. In addition, any loss of service, equipment damage or inability to meet our service obligations could reduce the confidence of our customers and could consequently impair our ability to obtain and retain customers, which would adversely affect both our ability to generate revenues and our operating results.
Furthermore, we are dependent upon internet service providers, telecommunications carriers and other website operators, some of which have experienced significant system failures and electrical outages in the past.
Our customers may, in the future, experience difficulties due to system failures unrelated to our systems and offerings. If, for any reason, these providers fail to provide the required services, our business, financial condition and results of operations could be materially and adversely impacted.
Risks Related to Financial Reporting
We use estimates in determining the fair value of certain assets and liabilities. If our estimates prove to be incorrect, we may be required to write down the value of these assets or write up the value of these liabilities, which could adversely affect our financial position.
Our ability to measure and report our financial position and operating results is influenced by the need to estimate the fair value of an asset or liability. Fair value is estimated based on a hierarchy that maximizes the use of observable inputs and minimizes the use of unobservable inputs. Observable inputs are inputs that reflect the assumptions that market participants would use in pricing the asset or liability developed based on market data obtained from sources independent of the reporting entity. Unobservable inputs are inputs that reflect the reporting entity’s own assumptions about the assumptions market participants would use in pricing the asset or liability developed based on the best information available in the circumstances. We estimate the impact or outcome of future events on the basis of information available at the time of the financial statements. An accounting estimate is considered critical if it requires that management make assumptions about matters that were highly uncertain at the time the accounting estimate was made. If actual results differ from management’s judgments and assumptions, then they may have an adverse impact on our results of operations and cash flows.
Our ability to use our net operating loss carryforwards and certain other tax attributes may be limited.
We have incurred net losses since our inception and we may never achieve or sustain profitability. Generally, for U.S. federal income tax purposes, net operating losses incurred will carry forward. However, net operating loss carryforwards generated prior to January 1, 2018 are subject to expiration for U.S. federal income tax purposes. As of December 31, 2024, we had federal net operating loss carryforwards of approximately $1.2 billion, of which $139.2 million will begin to expire in 2029 and $1.1 billion can be carried forward indefinitely. As of December 31, 2024, we had state net operating loss carryforwards of approximately $1.2 billion, of which $991.7 million will begin to expire in 2030 and $162.3 million can be carried forward indefinitely. As of December 31, 2024, we had federal research and development tax credit carryforwards of approximately $37.7 million, which begin to expire in 2029. As of December 31, 2024, we also had state research and development and investment tax credit carryforwards of approximately $30.2 million, which begin to expire in 2030.
Under Sections 382 and 383 of the Internal Revenue Code of 1986, as amended, if a corporation undergoes an “ownership change,” generally defined as a greater than 50% change by value in its equity ownership by certain stockholders over a three-year period, the corporation’s ability to use its pre-ownership change net operating loss carryforwards and other pre-ownership change tax attributes, such as research tax credits, to offset its post-ownership change income or taxes may be limited. Similar provisions of state tax law may also apply to limit the use of our state net operating loss carryforwards and other state tax attributes. We have not performed an analysis to determine whether our past issuances of stock and other changes in our stock ownership may have resulted in one or more ownership changes. If it is determined that we have in the past experienced an ownership change, or if we undergo one or more ownership changes as a result of future transactions in our stock, which may be outside our control, then our ability to utilize our net operating loss carryforwards and other tax attributes may be materially limited. As a result, even if we earn taxable income, we may be unable to use a material portion of our net operating loss carryforwards and other tax attributes, which could adversely affect our future cash flows. There is also a risk that regulatory changes, such as suspensions on the use of net operating losses or other unforeseen reasons, may result in our existing net operating loss carryforwards expiring or otherwise becoming unavailable
to offset future taxable income. For these reasons, we may not be able to utilize a material portion of our net operating loss carryforwards and other tax attributes even if we attain profitability.
We had in the past identified a material weakness in our internal controls over financial reporting, and we may identify additional material weaknesses in the future. A failure to maintain an effective system of internal control over financial reporting, may result in failure to accurately report our financial results or prevent fraud. As a result, stockholders could lose confidence in our financial and other public reporting, which would harm our business and the trading price of our common stock.
SEC and New York Stock Exchange (“NYSE”) rules and regulations require, among other things, that we establish and periodically evaluate procedures with respect to our internal control over financial reporting. In addition, we are required to document and test our internal control over financial reporting pursuant to Section 404 of the Sarbanes-Oxley Act of 2002 so that our management can certify as to the effectiveness of our internal control over financial reporting. Likewise, our independent registered public accounting firm is required to attest to the effectiveness of our internal control over financial reporting.
As previously disclosed in Part II-Item 9A, “Controls and Procedures”, of our Annual Report on Form 10-K for the year ended December 31, 2023, in connection with the audit of our financial statements for the year ended December 31, 2023, we concluded that there was a material weakness in our internal controls over financial reporting. The material weakness identified did not result in any material misstatement of our financial statements. The material weakness identified for the year ended December 31, 2023 was remediated as of December 31, 2024. However, we may in the future discover other areas of our internal controls that require remediation.
We cannot provide assurances that there will not be material weaknesses or significant deficiencies in our internal control over financial reporting in the future. Any failure to maintain internal control over financial reporting could severely inhibit our ability to accurately report our financial condition, or results of operations. Any material weaknesses or significant deficiencies in our internal control over financial reporting could cause investors to lose confidence in the accuracy and completeness of our financial reports, the market price of shares of our common stock to decline, and result in sanctions or investigations by NYSE, the SEC or other regulatory authorities. Failure to remedy material weaknesses in our internal control over financial reporting or to implement or maintain other effective control systems could also restrict our future access to the capital markets.
Adverse developments affecting the financial services industry could adversely affect our business operations, financial condition and results of operations.
Actual or rumored events involving reduced or limited liquidity, defaults, non-performance or other adverse developments that affect financial institutions or other companies in the financial services industry or the financial services industry generally, have in the past and may in the future lead to market-wide liquidity problems. For example, the closures of SVB, Signature Bank and First Republic Bank in the spring of 2023 created bank-specific and broader financial institution liquidity risk and concerns. Future adverse developments with respect to specific financial institutions or the broader financial services industry may lead to market-wide liquidity shortages, impair the ability of companies to access working capital needs, and create additional market and economic uncertainty.
We regularly maintain cash balances at third-party financial institutions in excess of the Federal Deposit Insurance Corporation insurance limit. Any failure of a depository institution to return our deposits, or if a depository institution is subject to other adverse conditions in the financial or credit markets, could further impact access to our invested cash or cash equivalents and could adversely impact our operating liquidity and financial performance.
In addition, widespread investor concerns regarding the U.S. or international financial systems could result in less favorable commercial financing terms, including higher interest rates or costs and tighter financial and operating covenants, or systemic limitations on access to credit and liquidity sources, thereby making it more difficult for us to acquire financing on acceptable terms or at all. Any decline in available funding or access to our cash and liquidity resources could, among other risks, adversely impact our ability to meet our operating expenses, financial obligations or fulfill our other obligations, or result in breaches of our financial and/or contractual obligations. Any of these impacts, or any other impacts resulting from the factors described above or other related or similar factors not described above, could have material adverse impacts on our liquidity and our current and/or projected business operations and financial condition and results of operations.
Risks Related to Governmental Regulation and Litigation
Failure to comply with federal, state, local and international laws and regulations could adversely affect our business and our financial condition.
A variety of federal, state, local and international laws and regulations govern certain aspects of our business. For example, we maintain a registration from the DEA for the research of certain controlled substances and permits from the Boston Public Health Commission to conduct work with recombinant DNA. Some of our programs or products made or developed using our engineered cells and/or biomanufacturing processes are subject to regulations, including those promulgated by the FDA, DEA, EPA or USDA. In addition, we are subject to laws relating to, among other things, anti-bribery, insider trading, sourcing of biological materials and data privacy. The legal and regulatory requirements that apply to our business may be interpreted and applied in a manner that is inconsistent from one jurisdiction to another or may conflict with other rules or our practices. As a result, our practices may not comply, or may not comply in the future with all such laws, regulations, requirements and obligations. Any failure, or perceived failure, by us to comply with any federal, state, local or international laws, regulations, industry self-regulatory principles, industry standards or codes of conduct, regulatory guidance, orders to which we may be subject or other legal obligations could adversely affect our reputation, brand and business, and may result in claims, proceedings or actions against us by governmental entities or others or other liabilities or require us to change our operations. We may also be contractually required to indemnify and hold harmless third parties from the costs or consequences of non-compliance with any laws, regulations or other legal obligations.
We may also become subject to increasing regulation in the future as we expand our business. As we continue to expand our operations and offerings domestically and globally, we will have to expend significant management and financial resources to maintain compliant practices in those locations. Non-compliance could lead to litigation, which would require substantial management and financial resources.
We may incur significant costs complying with environmental, health and safety laws and regulations, and failure to comply with these laws and regulations could expose us to significant liabilities.
We use hazardous chemical and biological materials in our business and are subject to a variety of federal, state, local and international laws and regulations governing, among other matters, the use, generation, manufacture, transportation, storage, handling, disposal of, and human exposure to these materials, including regulation by governmental regulatory agencies, such as the Occupational Safety and Health Administration and the EPA. We have incurred, and will continue to incur, capital and operating expenditures and other costs in the ordinary course of our business in complying with these laws and regulations.
Although we have implemented safety procedures for storing, handling and disposing of these materials and waste products in an effort to comply with these laws and regulations, we cannot be sure that our safety measures will be compliant or capable of eliminating the risk of injury or contamination from the generation, manufacturing, use, storage, transportation, handling, disposal of and human exposure to hazardous materials and/or flammable chemicals. Failure to comply with environmental, health and safety laws could subject us to liability and resulting damages. There can be no assurance that violations of environmental, health and safety laws will not occur as a result of human error, accident, equipment failure, contamination, intentional misconduct or other causes. Compliance with applicable environmental laws and regulations may be expensive, and the failure to comply with past, present or future laws could result in the imposition of fines, regulatory oversight costs, third party property damage, product liability and personal injury claims, investigation and remediation costs, the suspension of production or a cessation of operations, and our liability may exceed our total assets. Liability under environmental laws can be imposed for the full amount of damages without regard to comparative fault for the investigation and cleanup of contamination and impacts to human health and for damages to natural resources. Contamination at properties we may own and operate and at properties to which we send hazardous materials, may result in liability for us under environmental laws and regulations.
Our business and operations may be affected by other new environmental, health and safety laws and regulations, which may require us to change our operations, or result in greater compliance costs and increasing risks and penalties associated with violations, which could impair our research, development or production efforts and harm our business.
If we fail to comply with healthcare and other governmental regulations, we could face substantial penalties and our business, financial condition and results of operations could be adversely affected.
Our business activities may be subject to regulation and enforcement by the FDA, U.S. Department of Justice, HHS, Office of Inspector General, and other federal and state governmental authorities. Although our offerings are not currently billed
to any third-party payor, including any commercial payor or government healthcare program, we may, in the future, submit claims for our services to third-party payors, including government healthcare programs. If we submit claims to third-party payors, such activity will expand the scope of federal and state healthcare laws applicable to us.
Federal and state healthcare laws and regulations that may affect our ability to conduct business include, without limitation:
●the federal Anti-Kickback Statute, which prohibits, among other things, persons or entities from knowingly and willfully soliciting, offering, receiving or paying any remuneration, directly or indirectly, overtly or covertly, in cash or in kind, to induce or reward either the referral of an individual for, or the purchase, lease, order, or arranging for or recommending the purchase, lease or order of, any item or service, for which payment may be made, in whole or in part, under federal healthcare programs such as Medicare and Medicaid. A person or entity does not need to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation;
●the federal physician self-referral prohibition, commonly known as the Stark Law, which prohibits a physician, in the absence of an applicable exception, from making a referral for certain designated health services covered by the Medicare or Medicaid program, including clinical laboratory services, if the physician or an immediate family member of the physician has a financial relationship with the entity providing the designated health services. The Stark Law also prohibits the entity furnishing the designated health services from billing, presenting or causing to be presented a claim for the designated health services furnished pursuant to the prohibited referral;
●the federal civil false claims laws, including without limitation the federal False Claims Act (which can be enforced through “qui tam,” or whistleblower actions, by private citizens on behalf of the federal government), and civil monetary penalties laws, which prohibit, among other things, individuals or entities from knowingly presenting, or causing to be presented, false or fraudulent claims for payment of government funds, or knowingly making, using or causing to be made or used, a false record or statement material to an obligation to pay money to the government or knowingly and improperly avoiding, decreasing or concealing an obligation to pay money to the federal government. In addition, the government may assert that a claim including items and services resulting from a violation of the federal Anti-Kickback Statute or Stark Law constitutes a false or fraudulent claim for purposes of the civil False Claims Act;
●the EKRA, which created a new federal crime for knowingly and willfully: (1) soliciting or receiving any remuneration in return for referring a patient to a recovery home, clinical treatment facility, or laboratory; or (2) paying or offering any remuneration to induce such a referral or in exchange for an individual using the services of a recovery home, clinical treatment facility, or laboratory. Unlike the Anti-Kickback Statute, EKRA is not limited to services reimbursable under a government health care program, but instead extends to all services reimbursed by “health care benefit programs”;
●the healthcare fraud statutes under HIPAA, which impose criminal and civil liability for, among other things, knowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit program, or knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false statement, in connection with the delivery of, or payment for healthcare benefits, items or services by a healthcare benefit program, which includes both government and privately funded benefits programs. Similar to the federal Anti-Kickback Statute, a person or entity does not need to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation;
●HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act (“HITECH”) enacted as part of the American Recovery and Reinvestment Act of 2009, and its implementing regulations, and as amended again by the Modifications to the HIPAA Privacy, Security, Enforcement and Breach Notification Rules Under HITECH and the Genetic Information Nondiscrimination Act; Other Modifications to the HIPAA Rules, commonly referred to as the Final HIPAA Omnibus Rule, published in January 2013, which imposes certain obligations, including mandatory contractual terms, on covered entities subject to HIPAA (i.e., health plans, healthcare clearinghouses and certain healthcare providers), as well as their business associates that perform certain services for or on their behalf involving the use or disclosure of individually identifiable health
information, to safeguard the privacy, security and transmission of individually identifiable health information from any unauthorized use or disclosure;
●the U.S. Food, Drug, and Cosmetic Act (“FDCA”) which imposes civil and criminal liability for engaging in any of a number of Prohibited acts, including distributing drugs, devices and foods that are adulterated or misbranded. To charge a criminal misdemeanor violation of the FDCA, no intent need be shown;
●federal consumer protection and unfair competition laws, which broadly regulate platform activities and activities that potentially harm consumers; and
●state law equivalents of each of the above federal laws, such as anti-kickback, self-referral, and fee-splitting, and false claims laws which may apply to items or services reimbursed by any third-party payor, including commercial insurers and self-pay patients.
Because of the breadth of these laws and the narrowness of available statutory and regulatory exemptions, exceptions, and safe harbors, it is possible that some of our activities could be subject to challenge under one or more of such laws. We may face claims and proceedings by private parties, and claims, investigations and other proceedings by governmental authorities, relating to allegations that our business practices do not comply with current or future laws or regulations involving applicable fraud and abuse or other healthcare laws and regulations, and it is possible that courts or governmental authorities may conclude that we or any of our partners have not complied with them, or that we may find it necessary or appropriate to settle any such claims or other proceedings. Any action brought against us for violation of these or other laws or regulations, even if we successfully defend against it, could cause us to incur significant legal expenses and divert our management’s attention from the operation of our business. If our operations are found to be in violation of any federal or state laws described above or any other current or future fraud and abuse or other healthcare laws and regulations that apply to us, we may be subject to claims and proceedings by private parties, investigations and other proceedings by governmental authorities, as well as penalties, including significant criminal, civil and administrative penalties, damages and fines, disgorgement, additional reporting requirements and oversight if we become subject to a corporate integrity agreement or similar agreement to resolve allegations of noncompliance with these laws or regulations, imprisonment for individuals and exclusion from participation in government programs, such as Medicare and Medicaid, as well as contractual damages and reputational harm. We could also be required to curtail or cease our operations. In addition, if any customers, healthcare professionals we engage, laboratory partners or other entities with whom we do business are found not to be in compliance with applicable laws, they may be subject to the same criminal, civil or administrative sanctions, including exclusion from government-funded healthcare programs. Any of the foregoing could seriously harm our business and financial results.
We and our laboratory partners are subject to a variety of laboratory testing standards, compliance with which is an expensive and time-consuming process, and any failure to comply could result in substantial penalties and disruptions to our business.
We and the third-party laboratories that we partner with are subject to the CLIA. CLIA is a federal law that regulates clinical laboratories that perform testing on specimens derived from humans for the purpose of providing information for the diagnosis, prevention or treatment of disease. CLIA requires certain clinical laboratories to be certified by the federal government and mandates compliance with various operational, personnel, facilities administration, quality and proficiency testing requirements depending on the level of complexity for which the laboratory is certified. CLIA certification is also a prerequisite to be eligible to bill state and federal healthcare programs, as well as many private third-party payors, for laboratory testing services. Our partner laboratories hold CLIA certifications for high complexity testing, which mandate compliance with various operational, personnel, facilities administration, quality and proficiency testing requirements depending on the level of complexity for which the laboratory is certified. Sanctions for failure to comply with CLIA requirements may include suspension, revocation, or limitation of a laboratory’s CLIA certificate, as well as the imposition of significant fines or criminal penalties. Any sanction imposed under CLIA, its implementing regulations, or state or foreign laws or regulations governing licensure, or our partner laboratories’ failure to renew a CLIA certificate, a state or foreign license, or accreditation, could have a material adverse effect on our business.
In addition, our partner laboratories are subject to state laws and regulations governing laboratory licensure. Some states have enacted state licensure laws that are more stringent than CLIA. In certain markets such as California, New York, and Pennsylvania, we or our partner laboratories may also need to obtain and maintain additional licensure from such states. It is uncertain that our partner laboratories will be granted such licensure and, in such cases, we cannot offer testing to patients located in those states, which could limit our ability to offer testing on a wide scale.
It is possible that additional states may enact laboratory licensure requirements in the future, which could further limit our ability to expand our services.
We rely on third-party laboratories in the conduct of our biosecurity and public health business offerings. If any of our partners cease working with us, or face supply chain disruptions or other difficulties, our business could be harmed.
The testing industry is subject to complex and costly regulation and if government regulations are interpreted or enforced in a manner adverse to us, we may be subject to enforcement actions, penalties, exclusion, and other material limitations on our operations.
In connection with our previous offering of COVID-19 testing services, we partnered with third-party laboratories, diagnostic test manufacturers and manufacturers of collection kits, which are subject to extensive and frequently changing federal, state and local laws and regulations governing various aspects of our business, including significant governmental certification and licensing regulations. Additionally, new laws, regulations and judicial decisions, or new interpretations of existing laws, regulations and decisions, may limit potential revenues, and we may need to revise our R&D or commercialization programs. The costs of defending claims associated with violations, as well as any sanctions imposed, could significantly adversely affect our financial performance.
Our testing does not identify nor sequence any individual human DNA or RNA nor can results be tied to any individual. As a result, we do not collect informed consents from any individual participating in our programs. However, our approach could be challenged in the future based on the claims or privacy considerations and searches governed by the 4th Amendment of the U.S. Constitution. Any legal challenges could consume our management and financial resources.
Current regulations governing the testing services we offer are shifting and in some cases unclear. In addition, our laboratory partners may be unsuccessful in validating, or obtaining or maintaining authorizations for, the tests we relied on to provide our prior COVID-19 testing services. If any third-party manufacturers or laboratories offering tests that we use in our testing services are deemed by the FDA or other regulatory authorities to have violated applicable law or if the tests or test components are marketed, processed or distributed in violation of applicable law, we may be subject to enforcement action or litigation, or we may be required to find alternative tests to support our testing services, which could increase our costs.
In addition, we are required to comply with applicable FDA regulations with respect to distribution of certain COVID-19 diagnostic test kits and collection kits, including, for certain kits, compliance with applicable terms and conditions of an EUA. Such conditions may include requirements related to collection of information on the performance of the product, reporting of adverse events, recordkeeping requirements, and labeling and promotional activities. To the extent that we marketed or promoted third-party tests or test kits outside of the uses authorized for these products or in a false or misleading manner, the tests or collection kits could be considered misbranded or adulterated and distributing them in interstate commerce could violate the FDCA. Violations of applicable FDA requirements could result in enforcement actions, such as warning or “untitled” letters, revocation of EUAs, seizures, injunctions, civil penalties and criminal prosecutions and fines, and violation of the FTC Act could result in injunctions and other associated remedies, all of which could have a material adverse effect on our business. Most states also have similar regulatory and enforcement authority for laboratory testing and distribution of related collection kits. For example, many state laws require us to hold a specific form of license to distribute COVID-19 diagnostic test kits and collection kits into such states. These requirements vary from one state to another and frequently change. Complying with state laws and regulations may subject us to similar risks and delays as those we could experience under federal regulation.
Our surveillance testing efforts do not collect identifying individual data and do not return a diagnostic result, but some surveillance methods, such as double collection, require samples from individuals. Regulatory authorities could take issue with our characterization of such testing as surveillance and/or impose additional requirements or restrictions.
Advertising for any of the tests or collection kits we distribute or the testing services we offer is also subject to regulation by the Federal Trade Commission (“FTC”), under the Federal Trade Commission Act (“FTC Act”). The FTC may take enforcement action for advertising claims that are not adequately substantiated or that are false or misleading.
We are subject to federal and state laws and regulations governing the protection, use, and disclosure of health information and other types of personal information, and our failure to comply with those laws and regulations or to adequately secure the information we hold could result in significant liability or reputational harm.
Numerous state and federal laws, regulations, standards and other legal obligations, including consumer protection laws and regulations, which govern the collection, dissemination, use, access to, confidentiality, security and processing of personal information, including health-related information, could apply to our operations or the operations of our partners. For example, HIPAA imposes privacy, security and breach notification obligations on certain healthcare providers, health plans, and healthcare clearinghouses, known as covered entities, as well as their business associates that perform certain services that involve creating, receiving, maintaining or transmitting individually identifiable health information for or on behalf of such covered entities, and their covered subcontractors. HIPAA requires covered entities and business associates to develop and maintain policies with respect to the protection of, use and disclosure of protected health information (“PHI”), including the adoption of administrative, physical and technical safeguards to protect such information, and certain notification requirements in the event of a breach of unsecured PHI. If in the future we engage in certain types of standard electronic transactions involving payors, including billing the Medicare or Medicaid programs or commercial health plans, we will be subject to HIPAA as a “covered entity.” We are currently subject to HIPAA as a “business associate” because we performed certain services involving the use or disclosure of PHI on behalf of covered entity customers with respect to our prior COVID-19 testing service offerings. Implementation of the infrastructure necessary to meet HIPAA standards requires substantial investment. Being subject to HIPAA as a covered entity or business associate exposes us to significant fines and penalties, including criminal fines and penalties.
Entities that are found to be in violation of HIPAA as the result of a breach of unsecured PHI, a complaint about privacy practices or an audit by HHS, may be subject to significant civil, criminal and administrative fines and penalties and/or additional reporting and oversight obligations if required to enter into a resolution agreement and corrective action plan with HHS to settle allegations of HIPAA non-compliance. HIPAA also authorizes state attorneys general to file suit on behalf of their residents. Courts may award damages, costs and attorneys’ fees related to violations of HIPAA in such cases. While HIPAA does not create a private right of action allowing individuals to sue us in civil court for violations of HIPAA, its standards have been used as the basis for duty of care in state civil suits such as those for negligence or recklessness in the misuse or breach of PHI.
Even when HIPAA or a state law does not apply, according to the FTC, violating consumers’ privacy rights or failing to take appropriate steps to keep consumers’ personal information secure may constitute unfair and/or deceptive acts or practices in violation of Section 5(a) of the FTC Act. The FTC expects a company’s data security measures to be reasonable and appropriate in light of the sensitivity and volume of consumer information it holds, the size and complexity of its business, and the cost of available tools to improve security and reduce vulnerabilities.
Several states have enacted privacy laws governing the use and disclosure of health information, such as the California Confidentiality of Medical Information Act; these laws are not preempted by HIPAA to the extent they are more stringent than HIPAA. Such laws and regulations will be subject to interpretation by various courts and other governmental authorities, thus creating potentially complex compliance issues for us and our partners. Further, in recent years, there have been a number of well-publicized data breaches involving the improper dissemination of personal information of individuals both within and outside of the healthcare industry. Laws in all 50 states require businesses to provide notice to individuals whose personally identifiable information has been disclosed as a result of a data breach. The laws are not consistent, and compliance in the event of a widespread data breach is costly. States are also constantly amending existing laws, and creating new data privacy and security laws, requiring attention to frequently changing regulatory requirements. For example, the California Consumer Privacy Act of 2018 (“CCPA”) went into effect on January 1, 2020. The CCPA creates new transparency requirements and grants California residents several new rights with respect to their personal information. Failure to comply with the CCPA may result in, among other things, significant civil penalties and injunctive relief, or potential statutory or actual damages. On November 3, 2020, California voters passed a ballot initiative for the California Privacy Rights Act (“CPRA”), which significantly expands the CCPA. The CPRA imposes additional data protection obligations on covered businesses, including additional consumer rights processes, limitations on data uses, new audit requirements for higher risk data, and opt outs for certain uses of sensitive data, such as the right to opt out of the sale of personal information or the sharing of personal information for purposes of cross-context behavioral advertising. The CPRA also provides for a private right of action for certain data breaches. It also creates a new California data protection agency authorized to issue substantive regulations and could result in increased privacy and information security enforcement. Additional compliance investment and potential business process changes may be required to remain compliant with similar laws that have been proposed or passed in other states. For example, comprehensive privacy laws akin to the CPRA have recently gone into effect in twelve other states, and several other states have passed similar laws that will go into effect in the next two years. It is possible that other states, federal agencies or local governments will follow suit. The data privacy laws under consideration by federal and state legislators also include sector-specific laws. The My Health My Data Act, which recently became effective in Washington, contains new notice and consent requirements
for the processing of “consumer health data” with the potential for large penalties enforceable through private lawsuits. The FTC and other authorities are likewise imposing standards for the collection, use, dissemination and security of personal information under consumer protection laws. Additionally, in the United States, laws in all 50 states require businesses to provide notice to individuals whose personally identifiable information has been disclosed as a result of a data breach. The laws are not consistent, and compliance in the event of a widespread data breach is costly. In addition, laws, regulations, and standards covering marketing and advertising activities conducted by telephone, email, mobile devices and the internet are applicable to our business, including the Telephone Consumer Protection Act (the “TCPA”) and the Controlling the Assault of Non‑Solicited Pornography and Marketing Act (“CAN-SPAM Act”). The TCPA places certain restrictions on making certain outbound calls, faxes, and text messages to consumers. The CAN-SPAM Act imposes penalties for the transmission of commercial emails that do not comply with certain requirements, such as providing an opt-out mechanism for stopping future emails from the sender. Further, state and federal auto-renewal laws continue to evolve, which may require us to make changes to our processes in order to comply with such laws. The evolving patchwork of differing state and federal privacy and data security laws increases the cost and complexity of operating our business and increases our exposure to liability, including from third-party litigation and regulatory investigations, enforcement, fines, and penalties.
Through our wholly owned subsidiaries with established offices in the European Union, parts of our business are subject to the European Union General Data Protection Regulation (“GDPR”), which went into effect in May 2018, and imposes strict requirements for processing the personal data of individuals within the European Economic Area. Companies that must comply with the GDPR face increased compliance obligations and risk, including more robust regulatory enforcement of data protection requirements and potential fines for noncompliance of up to €20 million or 4% of the annual global revenues of the noncompliant company, whichever is greater. Among other requirements, the GDPR regulates transfers of personal data subject to the GDPR to third countries that have not been found to provide adequate protection to such personal data, including the United States. Further, from January 1, 2021, companies that process the personal information of UK residents have to comply with the United Kingdom GDPR (the “UK GDPR”), which, together with the amended UK Data Protection Act 2018, retains the GDPR in UK national law. The UK GDPR mirrors the fines under the GDPR, i.e., fines up to the greater of €20 million (£17.5 million) or 4% of global turnover. Enforcement uncertainty and the costs associated with ensuring compliance may be onerous and adversely affect our business, operating results, prospects and financial condition.
Although we work to comply with applicable laws, regulations and standards, contractual obligations and other legal obligations relating to data privacy, protection and security, these requirements are evolving and may be modified, interpreted and applied in an inconsistent manner from one jurisdiction to another, and may conflict with one another or other legal obligations with which Ginkgo must comply. Monitoring, preparing for and complying with these obligations requires us to devote significant resources (including, without limitation, financial and time-related resources). And as our operations and business grow, we may become subject to or affected by new or additional data protection laws and regulations and face increased scrutiny or attention from regulatory authorities. There is also increased public awareness of privacy issues in the wake of revelations about the data-collection activities of various government agencies and in the number of private privacy-related lawsuits filed against companies. Any failure or perceived failure by us or our employees, representatives, contractors, consultants, collaborators, or other third parties to comply with such requirements or adequately address privacy and security concerns, even if unfounded, could result in fines, legal claims, or proceedings, including regulatory investigations and actions, or liability for failure to comply with privacy and information security laws, which could disrupt our operations, damage our reputation, and expose us to claims from impacted individuals, any of which could have a material adverse effect on our business, financial condition, and results of operations.
Our employees, agents, contractors, research partners, consultants or vendors may engage in misconduct or other improper activities, including noncompliance with regulatory standards and requirements.
We are exposed to the risk that our employees, agents, contractors, research partners, consultants or vendors may engage in fraudulent or other illegal activity or misconduct. Misconduct by these parties could include intentional, reckless and/or negligent conduct or disclosure of unauthorized activities to us that causes us to breach our contracts and/or violates applicable laws and regulations, including but not limited to laws:
●applicable to the provision of health care services;
●governing the storage and handling of controlled substances;
●requiring the reporting of true, complete and accurate information to the FDA, USDA, and other government agencies;
●specifying vendor qualification standards and recordkeeping requirements;
●international, federal and state fraud and abuse laws and regulations;
●protecting the privacy and security of personally identifiable information and requiring breach notification;
●relating to anti-corruption, anti-bribery, and anti-money laundering; and
●requiring the true, complete and accurate reporting of services, financial information, or data.
Specifically, the health care industry and government contractors are subject to extensive laws and regulations intended to prevent fraud, kickbacks, self-dealing and other abusive practices. These laws and regulations may restrict or prohibit a wide range of pricing, discounting, marketing and promotion, sales commission, and other business arrangements. Additionally, activities that involve the improper use or misrepresentation of information obtained in the course of research or creating fraudulent data could result in breach of contract, regulatory sanctions, and serious harm to our reputation. It is not always possible to identify and deter misconduct by our employees and other third parties, and the precautions we take to detect and prevent this kind of activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations, other actions, or lawsuits stemming from a failure to be in compliance with such laws or regulations. Additionally, we are subject to the risk that a person could allege such fraud or other misconduct, even if none occurred. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a significant impact on our business, including the imposition of civil, criminal and administrative penalties, damages, monetary fines, possible exclusion from participation in Medicare, Medicaid and other federal health care programs, debarment under 21 U.S.C. § 335a or a comparable foreign law, contractual damages, reputational harm, diminished potential profits and future earnings, and curtailment of our operations, any of which could adversely affect our business, financial condition, results of operations or prospects.
Distribution and use of screening and/or diagnostic tests marketed under an EUA from the FDA are subject to certain limitations, and the continued availability of such authorizations is subject to government discretion.
Screening and/or diagnostic tests used in the testing programs and services of our Biosecurity business are subject to EUAs granted by the FDA to the manufacturers or laboratories marketing such tests. Each EUA requires compliance with certain conditions, including specific workflow requirements, and imposes other limitations on the test’s marketing, distribution, and use. The FDA has signaled that, while there will be a grace period for EUA holders to transition their devices, not all of the EUA products we distribute may apply for or be approved by the FDA and may need to withdraw from the market. Sourcing and finding products that transition from EUA to FDA cleared status may increase our costs of sourcing these products and may impact our profitability.
We have pursued in the past and may pursue additional U.S. government contracting and subcontracting opportunities in the future and as a U.S. government prime contractor and subcontractor, we are subject to a number of procurement rules and regulations.
We have entered into agreements with governmental entities and contractors in the past to serve as a U.S. government prime contractor or subcontractor and may do so again in the future. U.S. government procurement contractors and subcontractors must comply with specific procurement regulations and other requirements. These requirements, although customary in U.S. government contracts, could impact our performance and compliance costs, including by limiting or delaying our ability to share information with business partners, customers and investors. The U.S. government has in the past and may in the future demand contract terms that are less favorable than standard arrangements with private sector customers and may have statutory, contractual, or other legal rights to terminate contracts with us for convenience or for other reasons. Generally, U.S. government contracts contain provisions permitting unilateral termination or modification, in whole or in part, at the government’s convenience. Under general principles of government contracting law, if the government terminates a contract for convenience, the government contractor may recover only its incurred or committed costs, settlement expenses and profit on work completed prior to the termination. If the government terminates a contract for default, the government contractor is entitled to recover costs incurred and associated profits on accepted items only and may be liable for excess costs incurred by the government in procuring undelivered items from another source. Any termination for default may also adversely affect our ability to contract with other government customers and agencies, as well as our reputation, business, financial condition and results of operations. In addition, changes in U.S. government administration and budgetary priorities, including with respect to funding allocated to government agencies, could affect the availability of U.S. government contracting, subcontracting, or funding opportunities, which could lead to modification, reduction, or termination of our U.S. government contracts or subcontracts. If and to the extent such changes occur, they could impact our results and potential growth opportunities, and there can be no assurance that we will be able to derive further revenue from the public sector or our government contracts.
Furthermore, our U.S. government contracts grant the government the right to use technologies developed by us under the government contract or the right to share data related to our technologies, for or on behalf of the government. Under our government contracts, we may not be able to limit third parties, including our competitors, from accessing certain of these technology or data rights, including intellectual property, in providing products and services to the government.
In addition, failure by us, our employees, representatives, contractors, partners, agents, intermediaries, other customers or other third parties to comply with these regulations and requirements could result in reductions of the value of contracts, contract modifications or termination, claims for damages, refund obligations, the assessment of civil or criminal penalties and fines, loss of rights in our intellectual property and temporary suspension or permanent debarment from government contracting, all of which could negatively impact our results of operations and financial condition. Any such damages, penalties, disruptions or limitations in our ability to do business with the public sector could result in reduced sales of our products, reputational damage, penalties and other sanctions, any of which could harm our business, reputation and results of operations.
We are engaged in certain research activities involving controlled substances which may be subject to significant regulation by the DEA, FDA, and other regulatory agencies.
We are engaged in certain research activities involving the development of microbes designed to generate precursors and other chemical intermediaries which may be regulated as controlled substances in the United States. Controlled substances are subject to state, federal, and foreign laws and regulations regarding their manufacture, use, sale, importation, exportation, and distribution. Among other laws, controlled substances are regulated under the federal Controlled Substances Act of 1970 and implementing regulations of the DEA. The DEA regulates controlled substances as Schedule I, II, III, IV or V substances. Schedule I substances by definition have no established medicinal use and may generally not be marketed or sold in the United States. Schedule I substances are subject to the most stringent controls and Schedule V the least controls of the five schedules, based on their relative risk of abuse.
Regulations associated with controlled substances govern manufacturing, labeling, packaging, testing, dispensing, production and procurement quotas, recordkeeping, reporting, handling, shipment and disposal. These regulations include required security measures, such as background checks on employees and physical control of inventory and increase the personnel needs and the expense associated with development and commercialization of products or product candidates including controlled substances. Regulators conduct periodic inspections of entities involved in handling, manufacturing, or otherwise distributing controlled substances, and have broad enforcement authorities. If we are found to be non-compliant with applicable controlled substance registrations and related requirements, we may need to modify its business activities and/or stop handling or producing the products regulated as controlled substances, and could be subject to enforcement action, significant fines or penalties, and/or adverse publicity, among other consequences.
Various states also independently regulate controlled substances. Though state-controlled substances laws often mirror federal law, because the states are separate jurisdictions, they may separately schedule substances, as well. The failure to comply with applicable regulatory requirements could lead to enforcement actions and sanctions from the states in addition to those from the DEA or otherwise arising under federal law.
Changes in government regulations may materially and adversely affect our sales and results of operations.
The markets where we provide our services are heavily influenced by foreign, federal, state and local government regulations and policies. The U.S. or foreign governments may take administrative, legislative, or regulatory action that could materially interfere with our customer’s ability to sell products derived from engineered cells in certain countries and/or to certain customers. The uncertainty regarding future standards and policies may also affect our ability to develop our programs or to license engineered cells to customers and to initiate new programs with our customers, which could have a material adverse effect on our business, financial condition and results of operations.
Changes in U.S. trade policy more generally could trigger retaliatory actions by affected countries, which could impose restrictions on our ability to do business in or with affected countries or prohibit, reduce or discourage purchases of our services by foreign customers, leading to increased program costs, increased costs of developing or manufacturing our customers’ products and higher prices for their products in foreign markets. Changes in, and responses to, U.S. trade policy could reduce the competitiveness of our services or our customers’ products, cause our services to be less in demand and our sales to decline and adversely impact our ability to compete, which could materially and adversely impact our business, financial condition and results of operations.
We are subject to certain U.S. and foreign anti-corruption, anti-bribery and anti-money laundering laws and regulations. We can face serious consequences for violations.
We are subject to the U.S. Foreign Corrupt Practices Act of 1977, as amended (the “FCPA”), the U.S. domestic bribery statute contained in 18 U.S.C. § 201, the U.S. Travel Act, the U.K. Bribery Act and possibly other anti-corruption, anti-bribery and anti-money laundering laws and regulations in the jurisdictions in which we do business, both domestic and abroad. Anti-corruption and anti-bribery laws have been enforced aggressively in recent years. The FCPA and other anti-corruption laws generally prohibit companies, their employees, agents, representatives, business partners and third-party intermediaries from corruptly promising, authorizing, offering, or providing, directly or indirectly, anything of value to government officials, political parties, or candidates for public office for the purpose of obtaining or retaining business or securing an improper business advantage. The UK Bribery Act and other anti-corruption laws also prohibit commercial bribery not involving government officials, and requesting or accepting bribes; and anti-money laundering laws prohibit engaging in certain transactions involving criminally-derived property or the proceeds of criminal activity.
We and our third-party business partners, representatives and agents may have direct or indirect interactions with officials and employees of government agencies or state-owned or -affiliated universities or other entities (for example, to obtain necessary permits, licenses, patent registrations and other regulatory approvals), which increases our risks under the FCPA and other anti-corruption laws. We also engage contractors, consultants and other third parties from time to time to conduct business development activities abroad. We may be held liable for the corrupt or other illegal activities of our employees or third parties even if we do not explicitly authorize such activities. We have increased and, in the future, expect our non-U.S. activities to increase over time, which may also increase our exposure under these laws.
The FCPA also requires that we keep accurate books and records and maintain a system of adequate internal controls. While we have controls to address compliance with such laws and will continue to review and enhance our compliance program, we cannot assure you that our employees, agents, representatives, business partners or third-party intermediaries will always comply with our policies and applicable law, for which we may be ultimately held responsible.
Any allegations or violation of the FCPA or other applicable anti-bribery, anti-corruption laws and anti-money laundering laws may result in whistleblower complaints, sanctions, settlements, investigations, prosecution, enforcement actions, substantial criminal fines and civil penalties, disgorgement of profits, imprisonment, debarment, tax reassessments, breach of contract and fraud litigation, loss of export privileges, suspension or debarment from U.S. government contracts, adverse media coverage, reputational harm and other consequences, all of which may have an adverse effect on our reputation, business, financial condition, results of operations and prospects. Responding to an investigation or action can also result in a materially significant diversion of management’s attention and resources and significant defense costs and other professional fees.
Significant disruptions to our and our service providers’ information technology systems or data security incidents could result in significant financial, legal, regulatory, business and reputational harm to us.
We are increasingly dependent on information technology systems and infrastructure, including services licensed, leased or purchased from third parties such as cloud computing infrastructure and operating systems, to operate our business. In the ordinary course of business, we collect, store, process and transmit large amounts of sensitive information, including intellectual property, proprietary business information, personal information and other confidential information. It is critical that we do so in a secure manner to maintain the confidentiality, integrity and availability of such sensitive information. We have also outsourced elements of our operations (including elements of our information technology infrastructure) to third parties, and as a result, we manage a number of third-party vendors who may have access to our networks or our confidential information. While we take measures to safeguard and protect this information, threats to network and data security are constantly evolving and growing in frequency and sophistication. We may also face increased cybersecurity risks due to our reliance on internet technology and the number of our employees working remotely, which may create additional opportunities for cybercriminals to exploit vulnerabilities. We are required to expend significant resources in an effort to protect against security incidents, and may be required or choose to spend additional resources or modify our business activities, particularly where required by applicable data privacy and security laws or regulations or industry standards. Security incidents result from the actions of a wide variety of actors with a wide range of motives and expertise, such as traditional hackers, personnel or the personnel of third parties, sophisticated nation-states and nation-state-supported actors. While we have developed systems and processes designed to protect the integrity, confidentiality and security of the confidential and personal information under our control, we cannot guarantee that any security measures that we or our third-party service providers implement will be effective in preventing security breaches and incidents, cyberattacks or similar events such as viruses and worms, phishing attacks and other forms of social engineering, denial-of-
service attacks, ransomware attacks, physical or electronic break-ins, third-party or employee theft or misuse, and other negligent actions, errors or malfeasance by employees or other third parties, and similar disruptions from unauthorized tampering with our servers and computer systems or those of third parties that we use in our operations. These incidents could lead to interruptions, delays, loss or corruption of critical data, and unauthorized access to or acquisition of health-related and other personal information. In addition, we may be the target of email scams and other social engineering attacks that attempt to acquire personal information or company assets or access to our systems. Additionally, future or past business transactions (such as acquisitions or integrations) could expose us to additional cybersecurity risks and vulnerabilities, as our systems could be negatively affected by vulnerabilities present in acquired or integrated entities’ systems and technologies. Despite our efforts to create security barriers to such threats, we may not be able to entirely mitigate these risks. Our third-party service providers face similar risks. Any cyberattack that attempts to obtain our data or assets, including data that we maintain on behalf of our customers, disrupt our service, or otherwise access our systems, or those of third parties we use, or any other security breach or incident, could adversely affect our business, financial condition and operating results, be expensive to remedy, and damage our reputation. We and our third-party service providers may be unable to anticipate or detect attempted security incidents or face difficulties or delays in identifying or otherwise responding to any attacks or actual or potential security breaches or security incidents. We may incur significant costs and operational consequences of investigating, remediating, eliminating and putting in place additional tools and devices designed to prevent actual or perceived security breaches and other security incidents, including in response to any actual or perceived incident we may suffer, and substantial costs to comply with any notification or other legal obligations resulting from any security breaches or other security incidents. In addition, any such breaches or incidents, or the perception that they have occurred, may result in substantial remediation costs and expose us to litigation (including class claims), regulatory enforcement action (for example, investigations, fines, penalties, audits, and inspections), liability under laws that protect the privacy of personal information, additional reporting requirements and/or oversight, indemnification obligations, negative publicity, reputational harm, and interruptions in our operations (including availability of data), any of which could have a material adverse effect on our business, financial condition, and operating results.
Although we maintain insurance coverage that may cover certain liabilities in connection with security breaches and other security incidents, we cannot be certain our insurance coverage will be adequate for liabilities actually incurred, that insurance will continue to be available to us on commercially reasonable terms (if at all) or that any insurer will not deny coverage as to any future claim.
Governmental trade controls, including export and import controls, sanctions, customs requirements and related regimes, could subject us to liability or loss of contracting privileges or limit our ability to compete in certain markets.
Our programs and technologies are subject to U.S. and non-U.S. export controls. Export authorizations may be required for biotechnology products, technologies, or services to be exported outside of the United States, to a foreign person, or outside of a foreign jurisdiction. Our current or future programs or technologies are, and may in the future, be subject to the Export Administration Regulations (“EAR”). If a program, technology, or service meets certain criteria for control under the EAR, then that engineered cell, production process, resulting product, technology, or service would be exportable outside the United States or to a foreign person or from one foreign jurisdiction to another foreign jurisdiction only if we obtain the applicable export license or other applicable authorization including qualifying for a license exception, if required. Compliance with the U.S. and foreign export laws and regulations and other applicable regulatory requirements regarding the sales, shipment and use of our engineering cells, bioprocesses and other technology may affect our ability to work with foreign partners, affect the speed at which we can introduce new products into non-U.S. markets, or limit our ability to sell programs or services or license technologies into some countries.
Additionally, certain materials that we use in our programs are subject to U.S. import controls. We currently have, and may in the course of business need to procure, certain import authorizations, for example, related to plant pests, chemicals, biological agents and other controlled materials, including from the USDA, EPA and CDC. Compliance with applicable regulatory requirements regarding the import of such materials may limit our access to materials critical to our development activities or affect the speed at which we can advance new programs.
Our activities are also subject to the economic sanctions, laws and regulations of the United States and other jurisdictions. Such controls prohibit certain transactions, potentially including financial transactions and the transfer of products, technologies and services, to sanctioned countries, governments and persons, without a license or other appropriate authorization. U.S. sanctions policy changes could affect our or our customers’ ability to interact, directly and indirectly, with targeted companies or companies in sanctioned countries.
While we take precautions to comply with U.S. and non-U.S. export control, import control and economic sanctions laws and regulations, we cannot guarantee that such precautions will prevent violations of such laws, including transfers to unauthorized persons or destinations, and including inadvertent violations as a result of a misclassification of a product, technology or service under export control laws. Violations could result in our business being subject to government investigations, denial of export or import privileges, significant fines or penalties, denial of government contracts and reputational harm. Any limitation on our ability to export our engineered cells, production processes, resulting products, technology, or services, or import materials critical to our programs would likely adversely affect our business and financial condition.
Changes in U.S. and foreign tax laws could have a material adverse effect on our business, cash flow, results of operations or financial condition.
We are subject to income and non-income based taxes in the U.S. and foreign jurisdictions. Changes in tax laws, regulations and policies, or their interpretation and application, in the jurisdictions where we are subject to tax, could have a material adverse effect on our business, cash flow, results of operations or financial condition. The U.S. Congress frequently debates changes to U.S. corporate income tax laws and the Group of Twenty (G20), the Organization for Economic Co-operation and Development (OECD), the European Commission (EC) and individual taxing jurisdictions have published proposals covering various international tax-related issues, including country-by-country reporting, permanent establishment rules, transfer pricing and tax treaties. It is possible that any future tax legislation which may be enacted could materially impact our effective tax rate and cash tax liability as well as tax credits and incentives.
We may become subject to lawsuits or indemnity claims in the ordinary course of business, which could materially and adversely affect our business and results of operations.
From time to time, we may in the ordinary course of business be named as a defendant in lawsuits, indemnity claims and other legal proceedings. These actions may seek, among other things, compensation for alleged product liability, personal injury, employment discrimination, breach of contract, property damage and other losses or injunctive or declaratory relief.
The marketing, sale and use of our services engineered cells, production processes and resulting products could lead to the filing of product liability claims were someone to allege that our services, engineered cells, production processes or resulting products failed to perform as designed or intended or caused injury or other harms. A product liability claim could result in substantial damages and be costly and time-consuming for us to defend.
Regardless of merit or eventual outcome, product liability claims may result in:
●decreased demand for programs and resulting products;
●loss of revenue;
●substantial monetary payments;
●significant time and costs to defend related litigation;
●the inability to commercialize any products from our programs; and
●injury to our reputation and significant negative media attention.
In the event that such actions, claims or proceedings are ultimately resolved unfavorably to us at amounts exceeding our accrued liability, or at material amounts, the outcome could materially and adversely affect our business and results of operations. In addition, payments of significant amounts, even if reserved, could adversely affect our liquidity position. We maintain product liability insurance, but this insurance may not fully protect us from the financial impact of defending against product liability claims. Any product liability claim brought against us, with or without merit, could increase our insurance rates or prevent us from securing insurance coverage in the future. Additionally, any product liability lawsuit could cause current collaborators to terminate existing agreements or potential collaborators to seek other companies, any of which could impact our business and results of operations.
Risks Related to our Common Stock, Organizational Structure and Governance
Only our employees and directors are entitled to hold shares of Class B common stock (including shares of Class B common stock granted or otherwise issued to our employees and directors in the future), which shares have ten votes per share. This limits or precludes other stockholders’ ability to influence the outcome of matters submitted to stockholders for approval, including the election of directors, the approval of certain employee compensation plans, the
adoption of certain amendments to our organizational documents and the approval of any merger, consolidation, sale of all or substantially all of our assets, or other major corporate transaction requiring stockholder approval.
Shares of our Class B common stock have ten votes per share, whereas shares of our Class A common stock have one vote per share and shares of our Class C common stock have no voting rights (except as otherwise expressly provided in our amended and restated certificate of incorporation (the “Charter”) or required by applicable law). As of December 31, 2024, our directors and executive officers hold in the aggregate almost half of the total voting power of our outstanding capital stock, and our directors, founders and executive officers hold in the aggregate more than half of the total voting power of our outstanding capital stock. Accordingly, holders of shares of Class B common stock are able to significantly influence the outcome of matters submitted to our stockholders for approval, including the election of directors, the approval of certain employee compensation plans, the adoption of amendments to our organizational documents and the approval of any merger, consolidation, sale of all or substantially all of our assets or other major corporate transaction requiring stockholder approval. This concentrated voting power limits or precludes other stockholders’ ability to influence the outcome of these matters. Holders of Class B common stock may have interests that differ from holders of Class A common stock and may vote in a way with which holders of Class A common stock disagree and which may be adverse to the interests of holders of Class A common stock. This concentrated voting power is likely to have the effect of limiting the likelihood of an unsolicited merger proposal, unsolicited tender offer or proxy contest for the removal of directors. As a result, our governance structure and Charter may have the effect of depriving our stockholders of an opportunity to sell their shares at a premium over prevailing market prices and make it more difficult to replace our directors and management. Furthermore, this concentrated voting power could discourage a potential investor from acquiring Class A common stock due to the limited voting power of such stock relative to Class B common stock, which could also adversely affect the trading price of Class A common stock.
Our multi-class stock structure is intended to preserve our existing founder-led governance structure, to promote employee retention and engagement, to facilitate continued innovation and the risk-taking that it requires, to permit us to continue to prioritize our long-term goals rather than short-term results, to enhance the likelihood of continued stability in the composition of our board of directors and its policies, and to discourage certain types of transactions that may involve an actual or threatened acquisition of the company, all of which we believe are essential to the long-term success of our company and to long-term stockholder value. We expect to maintain this concentrated voting power among our founders and employees for the foreseeable future, including by issuing additional shares of Class B common stock to our employees pursuant to our equity compensation plans and allowing our employees and directors to exchange shares of Class A common stock for shares of Class B common stock.
Future transfers of shares of Class B common stock to persons other than Ginkgo directors and employees, or trusts or legal entities through which the right to vote the shares of Class B common stock held thereby is exercised exclusively by one or more of Ginkgo’s directors or employees (any such director, employee, trust or legal entity, an “Eligible Holder”), or the holder of shares of Class B common stock ceasing to be an Eligible Holder, will generally result in those shares converting to shares of Class A common stock on a one-to-one basis, subject to certain exceptions and unless a majority of the independent directors of our board of directors determine that such transfer or event will not result in such automatic conversion. Each share of Class B common stock is also convertible at any time at the option of the holder into one share of Class A common stock. The conversion of Class B common stock to Class A common stock over time will have the effect of increasing the relative voting power of those holders of Class B common stock who retain their shares of Class B common stock in the long term. As a result, the relative voting power of holders of Class A common stock is expected to remain limited for a significant period of time, and it is possible that one or more of the persons or entities holding Class B common stock could gain significant voting control as other holders of Class B common stock sell or otherwise convert their shares into Class A common stock. In addition, the conversion of Class B common stock to Class A common stock would dilute holders of Class A common stock in terms of voting power within the Class A common stock. Because holders of Class C common stock have no voting rights (except as otherwise expressly provided in the Charter or required by applicable law), the holders of Class B common stock may be able to significantly influence the outcome of matters submitted to our stockholders for approval for a longer period of time than would be the case if we issued Class A common stock rather than Class C common stock in such transactions.
Our share price may change significantly over time, and you may not be able to resell our common stock at or above the price you paid or at all, and you could lose all or part of your investment as a result.
The trading price of our Class A common stock has been in the past and is likely to continue to be volatile. Such volatility may be, in part, attributable to:
●future sales of our common stock or other securities by us or our existing stockholders, or the perception of such future sales;
●results of operations of the company or our competitors that vary from the expectations of securities analysts and investors;
●changes in expectations as to our future financial performance and growth, including assessments of our business, prospects, financial estimates and investment recommendations by securities analysts, investors and short sellers;
●additions or departures of key management personnel or members of our board of directors;
●announcements by us or our competitors of significant contracts, new products, acquisitions, joint marketing relationships, joint ventures, other strategic relationships or capital commitments;
●announcements relating to actual or potential civil and non-civil litigation, as well as governmental or regulatory investigations or inquiries;
●guidance that we provide to the public, any changes in this guidance or our failure to meet this guidance;
●changes in the perception of our offerings or the synthetic biology industry more general including changes in regulatory conditions;
●the development and sustainability of an active trading market for our common stock;
●changes in accounting principles;
●changes in general economic or market conditions or trends in our industry or markets;
●other events or factors, including those resulting from natural disasters, pandemics, epidemics, war, acts of terrorism or responses to these events.
These factors among others may materially adversely affect the market price of our Class A common stock, regardless of our actual operating performance. In addition, price volatility may be greater if the public float and trading volume of our common stock are low.
In the past, following periods of market volatility, stockholders have instituted securities class action litigation. If we were involved in securities litigation, it could have a substantial cost and divert resources and the attention of executive management from our business regardless of the outcome of such litigation.
Future sales, or the perception of future sales, by us or our stockholders in the public market could cause the market price for our securities to decline.
The sale of our securities in the public market, including by entities to which we have issued shares in connection with transactions, or the perception that such sales could occur, could harm the prevailing market price of our securities. These sales, or the possibility that these sales may occur, also might make it more difficult for us to sell equity securities in the future at a time and at a price that we deem appropriate.
There are up to approximately 5 million shares of common stock that may be earned if the trading price is greater than or equal to certain earnout price thresholds ranging from $500 to $800 for any point in a trading day during 20 trading days in a 30 consecutive trading day period, of which approximately 1.3 million shares were earned as of December 31, 2024. The vast majority of the shares that are part of the earnout will not be subject to lock-up once the earnout conditions are met.
In connection with the SRNG Business Combination, in September 2021, Jason Kelly, Reshma Shetty, Austin Che and Bartholomew Canton were granted restricted stock units, which vested, along with certain related earnout shares that achieved the $500 price threshold, on October 1, 2022. Certain of such shares have been sold into the market, and any future sales could harm the prevailing market price of our securities.
We have also issued shares of our common stock in connection with certain of our acquisitions, which issuances dilute our existing shareholders. In addition, the shares of our common stock reserved for future issuance under our equity incentive plans will become eligible for sale in the public market once those shares are issued, subject to provisions relating to various vesting agreements and, in some cases, limitations on volume and manner of sale applicable to affiliates under Rule 144, as applicable. Our compensation committee of our board of directors may determine the exact number of shares to be reserved for future issuance under our equity incentive plans at its discretion. We have filed, and expect to file in the future, one or more registration statements on Form S-8 under the Securities Act of 1933, as amended (the “Securities Act”) to register shares of Class A common stock or securities convertible into or exchangeable for shares of Class A common stock
issued pursuant to our equity incentive plans. Any such Form S-8 registration statements automatically become effective upon filing. Accordingly, shares registered under such registration statements will be available for sale in the open market.
Short sellers may engage in manipulative activity intended to drive down the market price of our Class A common stock, which could also result in related regulatory and governmental scrutiny, among other effects.
Short selling is the practice of selling securities that the seller does not own but rather has borrowed or intends to borrow from a third party with the intention of later buying lower priced identical securities to return to the lender. Accordingly, it is in the interest of a short seller of our Class A common stock for the price to decline. At any time, short sellers may publish, or arrange for the publication of, opinions or characterizations that are intended to create negative market momentum. Issuers, like us, whose securities have historically had limited trading history or volumes and/or have been susceptible to relatively high volatility levels can be vulnerable to such short seller attacks. Short selling reports can cause increased volatility in an issuer’s stock price, and result in regulatory and governmental inquiries. On October 6, 2021, such a report was published about us. Shortly after, we received a preliminary and informal inquiry from the U.S. Department of Justice related to this report. Any related inquiry or formal investigation from a governmental organization or other regulatory body, including any inquiry from the SEC, is not within the control of the Company. Although we have received confirmation from the SEC that it concluded its inquiry into Ginkgo Bioworks Holdings, Inc. begun in October 2021 or soon after with no recommendation of enforcement action, any inquiry or formal investigation by any governmental organization or regulatory body could result in a material diversion of our management’s time and could have a material adverse effect on our business and results of operations.
Our Charter authorizes a large number of shares of Class B common stock for issuance in the future. The future issuance of shares of Class B common stock may have the effect of further concentrating voting power with our employees and other Class B stockholders, and could have an adverse effect on the trading price of Class A common stock.
Under our Charter, we are authorized to issue 4,500.0 million shares of Class B common stock, which are entitled to ten votes per share. We currently intend to issue additional shares of Class B common stock in the future to existing and newly hired employees pursuant to our equity compensation plans. Our authorized but unissued shares of Class B common stock are available for issuance to Eligible Holders with the approval of our board of directors without stockholder approval, except as may be required by the Listing Rules of the NYSE. In addition, our authorized but unissued shares of Class B common stock are available for issuance to persons other than Eligible Holders only with the approval of a majority of our directors elected by the holders of Class B common stock, voting separately as a class. If we issue additional shares of Class B common stock in the future, holders of shares of Class A common stock, which are entitled to one vote per share, will experience disproportionate voting power dilution relative to economic dilution, and the holders of Class B common stock may be able to significantly influence the outcome of matters submitted to our stockholders for approval for a longer period of time than would be the case if we issued shares of Class A common stock.
See “Risk Factors-Risks Related to Our Common Stock Organizational Structure and Governance-Only our employees and directors are entitled to hold shares of Class B common stock (including shares of Class B common stock granted or otherwise issued to our employees and directors in the future), which shares have ten votes per share. This limits or precludes other stockholders’ ability to influence the outcome of matters submitted to stockholders for approval, including the election of directors, the approval of certain employee compensation plans, the adoption of amendments to our organizational documents and the approval of any merger, consolidation, sale of all or substantially all of our assets or other major corporate transaction requiring stockholder approval.”
Under our Charter, we are authorized to issue 800.0 million shares of Class C common stock, which have no voting rights (except as otherwise expressly provided in the Charter or required by applicable law). Outstanding Class C common stock may have the effect of extending voting power in Class B common stock, and may discourage potential acquisitions of our business and could have an adverse effect on the trading price of Class A common stock.
Under our Charter, we are authorized to issue 800.0 million shares of Class C common stock, which have no voting rights (except as required by law). Class C common stock may be used for a variety of corporate purposes, including financings, acquisitions and investments. Our authorized but unissued shares of Class C common stock are available for issuance with the approval of our board of directors without stockholder approval, except as may be required by the Listing Rules of the NYSE. Because the Class C common stock carries no voting rights (except as otherwise expressly provided in the Charter or required by applicable law), is not convertible into any other capital stock, and is not listed for trading on an exchange or registered for sale with the SEC, shares of Class C common stock may be less liquid and less attractive to any future recipients of these shares than shares of Class A common stock, although we may seek to list the Class C common stock
for trading and register shares of Class C common stock for sale in the future. In addition, because our Class C common stock has no voting rights (except as otherwise expressly provided in the Charter or required by applicable law), the holders of Class B common stock may be able to significantly influence the outcome of matters submitted to our stockholders for approval for a longer period of time than would be the case if we issued Class A common stock rather than Class C common stock in such transactions. In addition, further issuances of Class C common stock would have a dilutive effect on the economic interests of Class A common stock and Class B common stock. Any such issuance could also cause the trading price of Class A common stock to decline.
We cannot predict the effect the multi-class structure of our common stock may have on the trading price of our Class A common stock.
The holding of low-voting stock, such as Class A common stock, may not be permitted by the investment policies of certain institutional investors or may be less attractive to the portfolio managers of certain institutional investors. In addition, certain index providers have announced restrictions on including companies with multiple-class share structures in certain of their indices. Because of our multi-class stock structure, our Class A common stock will likely continue to be excluded from certain indices, and we cannot assure you that other stock indices will not take similar actions. Given the sustained flow of investment funds into passive strategies that seek to track certain indices, exclusion from stock indices would likely preclude investment by many of these funds in our Class A common stock and could make shares of our Class A common stock less attractive to other investors. As a result, the trading price of shares of our Class A common stock could be adversely affected.
Our focus on the long-term best interests of our company and our consideration of all of our stakeholders, including our stockholders, workforce, customers, suppliers, academic researchers, governments, communities and other stakeholders that we may identify from time to time, may conflict with short-term or medium-term financial interests and business performance, which may adversely impact the value of our common stock.
We believe that focusing on the long-term best interests of our company and our consideration of all of our stakeholders, including our stockholders, workforce, customers, suppliers, academic researchers, governments, communities and other stakeholders we may identify from time to time, is essential to the long-term success of our company and to long-term stockholder value. Therefore, we have made decisions, and may in the future make decisions, that we believe are in the long-term best interests of our company and our stockholders, even if such decisions may negatively impact the short- or medium-term performance of our business, results of operations, and financial condition or the short- or medium-term performance of our Class A common stock. Our commitment to pursuing long-term value for the company and its stockholders, potentially at the expense of short- or medium-term performance, may materially adversely affect the trading price of our Class A common stock, including by making owning our Class A common stock less appealing to investors who are focused on returns over a shorter time horizon. Our decisions and actions in pursuit of long-term success and long-term stockholder value, which may include our multi-class stock structure, making investments in R&D and our employees, and investing in and introducing new products and services, may not result in the long-term benefits that we expect, in which case our business, results of operations and financial condition, as well as the trading price of our Class A common stock, could be materially adversely affected.
Item 1B. Unresolved Staff Comments.
None.
Item 1C. Cybersecurity.
Cybersecurity risk management and strategy
Ginkgo integrates risk management into its overall cybersecurity strategy, and has implemented processes designed to identify, assess, prioritize and manage risks to protect Ginkgo’s data, intellectual property and information assets. As part of our risk governance and management, Ginkgo has developed processes designed to: identify and assess risks, evaluate those risks against pre-defined criteria, develop and implement strategies to address identified risks, monitor and review those risks and communicate risks to relevant stakeholders. Identifying Ginkgo’s cybersecurity risks involves a multifaceted approach that encompasses both internal assessments and external information sources. For example, we use security audits conducted by internal and external auditors to assess compliance with security policies and industry frameworks; vulnerability assessments to discover vulnerabilities in networks, systems and applications; penetration testing using simulated cyberattacks to test the resilience of systems and identify weaknesses; and risk assessment processes to evaluate IT infrastructure, including using a risk register to identify risks, likelihood of their occurrence, potential impact, and remediation. We also oversee third-party service providers by conducting vendor diligence upon onboarding and additional monitoring. Vendors are assessed for risk based on the nature of their services, access to data and systems and supply chain risk.
Cybersecurity risk management is overseen by Ginkgo’s Chief Information Security Officer (“CISO”), who is supported by full-time information security staff. The CISO advises the executive team on the development and implementation of the information security program.
Ginkgo incorporates learning from its cybersecurity risk management process into its overall cybersecurity program. To date, Ginkgo has not experienced a cybersecurity incident that resulted in a material effect on our business strategy, results of operations, or financial condition. Despite our efforts, we cannot provide assurance that we will not be materially affected in the future by cybersecurity risks or any future material incidents. For more information, see Item 1A. Risk Factors, “Significant disruptions to our and our service providers’ information technology systems or data security incidents could result in significant financial, legal, regulatory, business and reputational harm to us.”
Cybersecurity governance
The Board provides regular oversight of the Company’s cybersecurity risk management program. The CISO presents to the Board and the audit committee of our Board (the “Audit Committee”) at least annually and quarterly updates via business review dashboards. The Board provides guidance to the CISO, including with respect to any changes to business priorities, risk tolerance, or security initiatives. These briefings are also augmented by ongoing and continuous interactions between the Board and the CISO, as needed.
Ginkgo's CISO has primary responsibility for assessing and managing Ginkgo’s risks from cybersecurity threats. The CISO has over 20 years of public and private-sector experience in information technology and has served as Ginkgo’s CISO since 2018. Executive leadership provides oversight and governance through monthly business reviews of the cybersecurity program.
Ginkgo also has a Disclosure Committee, which is composed of representatives from executive leadership from various departments across Ginkgo (e.g., legal, finance, accounting). Their role is to determine materiality of a cyber incident and provide guidance with respect to any disclosure obligations resulting from a cyber incident.
Item 2. Properties.
Ginkgo’s headquarters are located in the Seaport district of Boston, Massachusetts and comprise a set of non-cancellable operating leases within a facility totaling over 320,000 square feet of office and laboratory space. These lease agreements expire on dates ranging from 2030 to 2036 and each contain one option to extend the lease for a five-year period at then-market rates. Of this 320,000 square feet of leased space, 27,000 is currently subleased, with an additional 129,000 available for sublease in connection with our restructuring.
We also lease approximately 179,000 square feet of office and lab space in Cambridge, Massachusetts; Emeryville, California; Basel, Switzerland; and Zeist, Netherlands. This includes 32,000 square feet subleased in Emeryville, and 63,000 in Cambridge with leases ending in February 2026. We have exited the Cambridge spaces and they are now available for sublease in connection with our restructuring.
In April 2021, we entered into a lease, as amended, consisting of approximately 260,000 rentable square feet of new office and laboratory space being developed in Boston, Massachusetts near our headquarters. The lease commenced on April 11, 2024, with rent payments beginning in June 2024, and it will expire on the fifteenth anniversary of the rent commencement date. The lease includes an option to extend for an additional ten years at then-market rates, as well as an expansion option if the owner constructs an additional building on the property. We believe our footprint prior to this new facility is now sufficient to meet our needs, therefore, this facility is available for sublease.
We also own approximately 193,000 square feet of real property in West Sacramento, California, of which 3,000 is subleased and an additional 26,000 is available for sublease.
We intend to continue to evaluate our space needs and offer any excess space for subleasing.
Item 3. Legal Proceedings.
From time to time, the Company may in the ordinary course of business be named as a defendant in lawsuits, indemnity claims and other legal proceedings. The Company does not believe any pending litigation to be material, or that the outcome of any such pending litigation, in management’s judgment based on information currently available, would have a material adverse effect on the Company’s results of operations, cash flows or financial condition.
See Note 11, Commitments and Contingencies, to the consolidated financial statements included elsewhere in this Annual Report on Form 10-K. Item 4. Mine Safety Disclosures.
Not Applicable.
PART II
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities.
Market Information
Our Class A common stock began trading on the NYSE under the symbol “DNA”on September 17, 2021. Prior to that date, there was no public trading market for our Class A common stock.
Holders of Record
As of December 31, 2024, there were approximately 435 stockholders of record of our Class A common stock, 188 stockholders of record of our Class B common stock and 1 stockholder of record of our Class C common stock, which does not include persons whose stock is held in nominee or “street name” accounts through brokers, banks and intermediaries.
Securities Authorized for Issuance Under Equity Compensation Plans
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Number of securities to be issued upon exercise of outstanding options and vesting of outstanding restricted stock units (#) | | | | Weighted-average exercise price of outstanding options ($) | | Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in the first column) (#) | | |
| Equity compensation plans approved by security holders (1) | 3,886,076 | | | (2) | | $ | 73.92 | | | 4,868,329 | | | (3) |
| Equity compensation plans not approved by security holders (4) | 208,842 | | | | | — | | | 186,770 | | | |
| Total | 4,094,918 | | | | | $ | 73.92 | | | 5,055,099 | | | |
(1)Includes the Ginkgo Bioworks Holdings, Inc. 2021 Equity Incentive Plan.
(2)Includes 767,520 shares of common stock issuable upon the exercise of outstanding stock options and 3,118,556 shares of common stock issuable upon settlement of outstanding restricted stock units.
(3)The Plan provides that the number of shares of common stock reserved and available for issuance under the Plan shall be cumulatively increased on January 1 of each year. The number of shares of common stock increased each year will be equal to the lesser of: (i) 4% of the number of shares of common stock issued and outstanding on the immediately preceding December 31 or (ii) such lesser amount as determined by our board of directors.
(4)Includes the Ginkgo Bioworks Holdings, Inc. 2022 Inducement Plan.
Performance Graph
The following graph compares the cumulative total stockholder return on our Class A common stock relative to the cumulative total returns of the S&P 500 Index and the S&P Biotechnology Select Industry Index between September 17, 2021 (the date our common stock began trading on the NYSE after the SRNG Business Combination) through December 31, 2024. All values assume a $100 initial investment at market close on September 17, 2021 and data for the S&P 500 and the S&P Biotechnology Select indices assume reinvestment of all dividends.
Recent Sales of Unregistered Securities
On October 15, 2024, we issued a total of 293,578 shares of our Class A common stock to the sellers of Circularis, valued at approximately $2.5 million, as settlement for employee retention payments, in a private placement transaction exempt from the registration requirements of the Securities Act pursuant to Section 4(a)(2) of the Securities Act.
On October 17, 2024 and November 26, 2024, we issued a total of 622,026 shares of our Class A common stock to certain former equity holders of FGen AG, valued at approximately $4.8 million, in connection with the achievement of certain milestones, in a private placement transaction exempt from the registration requirements of the Securities Act, pursuant to Section 4(a)(2) of the Securities Act.
Issuer Purchases of Equity Securities
None.
Item 6. [Reserved]
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations.
The following discussion and analysis of our financial condition and results of operations should be read in conjunction with the consolidated financial statements and related notes thereto included elsewhere in this Annual Report on Form 10-K. Further, this section of this Form 10-K generally discusses 2024 and 2023 items and year-to-year comparisons between 2024 and 2023. For discussion related to 2022 items and year-to-year comparisons between 2023 and 2022 that are not included in this Form 10-K, please refer to Part II, Item 7. Management’s Discussion and Analysis of Financial Condition
and Results of Operations in our 2023 Form 10-K, filed with the United States Securities and Exchange Commission on February 29, 2024. This discussion contains forward-looking statements that reflect our plans, estimates and beliefs that involve risks and uncertainties. Actual results may differ materially from those anticipated in these forward-looking statements as a result of many factors, including those discussed in Item 1A. “Risk Factors” and “Cautionary Note Regarding Forward-Looking Statements” elsewhere in this Annual Report on Form 10-K.
Overview
Our mission is to make biology easier to engineer.
Ginkgo sells services in two business segments: cell engineering, where we provide biological research and development (“R&D”) services for our customers across a range of industries, and biosecurity, where we provide services to government and commercial customers so they can work to identify, monitor, prevent, mitigate, and ultimately protect humanity from biological threats.
Cell Engineering
Ginkgo does not make end products; instead, we offer biological R&D services on our platform to enable our customers to bring their products to market. Historically, Ginkgo’s primary service offering has been end-to-end cell engineering R&D services (solutions). In 2024, Ginkgo expanded its service offering to also include services that provide our customers cell engineering tools for biological R&D, which are intended to provide more targeted and bespoke resources to customers that continue to conduct in-house R&D.
Compounding and mutually reinforcing improvements of our laboratory automation and software infrastructure—our Foundry—and our reusable data assets—our Codebase—enable us to improve our services with each successive project.
•Our Foundry is a flexible capability for large scale data generation; it powers generative artificial intelligence (“AI”) and machine learning (“ML”) tools that enable more successful biological R&D. We now offer services providing such data generation, AI and automation tools directly to Ginkgo customers.
•Our Codebase is a data asset comprising best practices for cell engineering, along with sequences and host cells that have been honed through dozens of programs and can be directly reusable for our end-to-end cell engineering solutions.
Our end-to-end cell engineering solutions are typically scoped and delivered as a program ranging in duration from several months to several years. A typical deliverable for the program would comprise an engineered strain or cell line and an associated bioprocess. For each of these programs, we generate economic value in two primary ways. First, we charge usage fees for services, in much the same way that cloud computing companies charge usage fees for utilization of computing capacity or contract research organizations charge for services. Additionally, we have historically negotiated a value share with our customers (in the form of royalties, milestones, and/or equity interests) in order to align our economics with the success of the programs enabled by our platform. Commencing in the second quarter of 2024, we announced changes in prospective commercial terms, including the removal of downstream value share from certain program types.
We charge customers fees for the services we provide in our cell engineering tools offerings. Typically, these fees are structured as a fixed fee for a fixed scope of work. Fees for our Datapoints services are typically earned over a shorter period of time (weeks to months) than for end-to-end cell engineering solutions which may be multi-year programs. Fees for our automation solutions are typically earned over a period that covers design, build, and deployment and range from six to twelve months. In addition, we offer support services with fixed fees covering the support periods.
Biosecurity
With a mission to make biology easier to engineer, we have always recognized the need to invest in biosecurity as a key component of our platform. We are building the future bioeconomy with our customers and partners, and we envision the future of biosecurity as a global immune system equipped with the capabilities to rapidly and reliably identify, monitor, prevent, and mitigate biological threats. The first, critical step in realizing this future is to build a robust early warning system for biological threats—this is the primary focus of Ginkgo’s Biosecurity business.
Our primary biosecurity customers are governments. We currently provide biosecurity services via two core offerings as introduced in early 2024:
•Canopy, which helps our customers generate high value genomic data from strategically positioned nodes (like airports and border checkpoints) via end-to-end biomonitoring programs; and
•Horizon, our digital surveillance, analytics and insights platform that detects and monitors biothreats worldwide.
Our recent strategic business and asset acquisitions are described in detail in Note 4 of our audited consolidated financial statements included elsewhere in this Annual Report on Form 10-K. Generating Economic Value Through Cell Programs
Our cell engineering platform is a key enabling technology and source of intellectual property for our customers’ products. We earn Cell Engineering revenue for our R&D services as well as generally through a share of the value of products created using our platform.
We typically structure Cell Engineering revenue to include some combination of the following:
•service fees, which may comprise cash and/or non-cash consideration, in the form of:
◦upfront payments upon consummation of an agreement or other fixed payments that are generally recognized over our period of performance;
◦reimbursement for costs incurred for R&D services;
◦milestone payments upon the achievement of specified technical criteria;
plus, when applicable,
•downstream value share payments in the form of:
◦milestone payments, which may comprise cash and/or non-cash consideration, upon the achievement of specified commercial criteria;
◦royalties on sales of products from or comprising engineered organisms;
◦royalties related to cost of goods sold reductions realized by our customers;
or,
•downstream value share in the form of equity interests in our customer.
◦downstream value share in the form of equity interest appreciation is not recognized as revenue but is expected to contribute to future cash flows upon liquidation, the amount and timing of which is inherently unpredictable.
Customer arrangements which involve non-cash consideration generally fall into two categories: Platform Ventures and Structured Partnerships.
Platform Ventures
Platform Ventures enable Ginkgo to partner with leading multinationals and financial investors to form new ventures in identified market segments with potential to benefit from synthetic biology. In exchange for an equity position in the venture, we contribute license rights to our proprietary cell programming technology and intellectual property, while our partners contribute relevant industry expertise, other resources and venture funding. We also provide R&D services for which we receive cash consideration on a fixed-fee or cost-plus basis.
Structured Partnerships
Structured Partnerships allow Ginkgo to: (i) partner with early stage synthetic biology product companies to adopt our Foundry as their cell programming R&D platform, in which we offer flexible commercial terms on the service fees including the ability to pay a portion or all of such upfront fees in the form of non-cash consideration (convertible financial instruments and/or equity securities), in addition to downstream value share consideration (“Startup Structured Partnership”); and (ii) partner with existing entities with complementary assets for high potential synthetic biology applications in a large-scale, multi-program collaboration (“Legacy Structured Partnership”). In 2024, we did not enter into
any new Startup Structured Partnerships. In 2023, we entered into six Startup Structured Partnerships and received prepayments of service fees in the form of equity securities or convertible financial instruments in the amount of $18.9 million that is recognized as revenue over our period of performance.
See Notes 6 and 16 of our audited consolidated financial statements included elsewhere in this Annual Report on Form 10-K for further details of our investments in and the material terms of our agreements with our Platform Ventures and Structured Partnerships. Key Business Metrics
In the past, we reported New Programs, Current Active Programs and Cumulative Programs as our key business metrics. We have undertaken a strategic review of these metrics, including in light of our new service offerings and our restructuring plan announced and commenced in the second quarter of 2024, and, beginning with the three months ending December 31, 2024, we no longer rely on New Programs, Current Active Programs and Cumulative Programs as key business metrics. We may in the future report on key business metrics, which metrics may change or be substituted for additional or different metrics as our business develops.
Components of Results of Operations
Revenue
Cell Engineering Revenue
We generate Cell Engineering revenue primarily through license and collaboration agreements, under which customers obtain rights to our proprietary technology and intellectual property for use in the development and commercialization of engineered organisms and derived products. Under these agreements, we typically provide R&D services for cell programming with the goal of producing an engineered cell that meets a mutually agreed specification. Our customers obtain license rights to the output of our services, which are primarily the optimized strains or cell lines, in order to manufacture and commercialize products derived from that licensed strain or cell line. Generally, the terms of these agreements provide that we receive some combination of: (1) service fees in the form of (i) upfront payments upon consummation of the agreement or other fixed payments, (ii) reimbursement for costs incurred for R&D services and (iii) milestone payments upon the achievement of specified technical criteria, plus (2) downstream value share payments in the form of (i) milestone payments upon the achievement of specified commercial criteria, (ii) royalties on sales of products from or comprising engineered organisms arising from the collaboration or licensing agreement and/or (iii) royalties related to cost of goods sold reductions realized by our customers. Royalties did not comprise a material amount of our revenue during any of the periods presented.
Beginning in the second quarter of 2024, we announced changes to the commercial terms applicable to some new customer contracts, including revised intellectual property terms more favorable to customers and, in many cases, the removal of downstream value share from certain program types.
In the third quarter of 2024, we launched several new cell engineering tools offerings, including Datapoints, an AI model API, and lab automation solutions. Datapoints' data generation products provide large, biological datasets for customers to train their AI models, synthesizing and testing the output of customer existing models, and generating datasets for lead selection, hit selection, or a variety of other data science applications. Our model API provides users with access to both publicly available models and Ginkgo’s own protein sequence LLM trained on Ginkgo’s proprietary datasets. Our lab automation solutions combine modular hardware, control software and managed support to provide customers the ability to automate their own lab workflows in house.
There has been no material impact on our revenue recognition policies to date from the announced changes in our new commercial terms and Cell Engineering offerings.
Cell Engineering revenue includes transactions with Platform Ventures and Legacy Structured Partnerships where, as part of these transactions, we received an equity interest in such entities. Specifically related to the Platform Ventures, in these transactions, we received upfront non-cash consideration in the form of common equity interests in these entities, while the Platform Ventures each received cash equity investments from strategic partners and financial investors. We view the upfront non-cash consideration as prepayments for licenses which will be granted in the future as we complete mutually agreed upon technical development plans. In these instances, we also receive cash consideration for the R&D services
performed by us on a fixed fee or cost-plus basis. We are not compensated through additional milestone or royalty payments under these arrangements. Our transactions with Genomatica and Synlogic included the purchase of equity securities and the provision of R&D services. As we perform R&D services under the mutually agreed upon development plans, we recognize a reduction in the prefunded obligation on a cost-plus basis. These arrangements are further described in Notes 6, 7, 16 and 20 of our audited consolidated financial statements included elsewhere in this Annual Report on Form 10-K.Cell Engineering revenue also includes transactions with Startup Structured Partnerships where, as part of these transactions, we received upfront non-cash consideration in the form of current equity interests or financial instruments that are convertible into equity upon a triggering event. We issued the customer a prepaid Cell Engineering services credit in exchange for the upfront non-cash consideration, which can and has been drawn down as payment for R&D services performed under mutually agreed upon development plans. Downstream value share in the form of equity interest appreciation is not recognized as revenue but is expected to contribute to future cash flows upon liquidation, the amount and timing of which is inherently unpredictable. The initial fair market value of the equity interests received may also decrease after contract inception and the amount of cash proceeds eventually realized may be less than the revenue recognized. Equity investments are accounted for under the equity method, cost method or are carried at fair value.
Biosecurity Revenue
We offer biosecurity services through our two core offerings: Canopy and Horizon. We are currently offering biomonitoring and bioinformatics support services domestically through our partnerships with the CDC and XpresCheck, and internationally through our international programs, including those in Qatar and Ukraine. We are also engaged in a series of smaller partnerships that generate revenues through biosecurity services and R&D.
We generate service revenue through the sale of our end-to-end biomonitoring and bioinformatics support services. These service offerings generally consist of goods and services including, but not limited to, sample collection, sample storage and transportation, outsourced laboratory analysis, access to results reported through a web-based portal, analytical reporting of results, and overall program management. Prior to 2024, we generated product revenue by selling lateral flow assay (“LFA”) diagnostic test kits, polymerase chain reaction (“PCR”) sample collection kits, and pooled test kits associated with COVID-19 tests to customers on a standalone basis.
In general, these agreements stipulate that we are entitled to compensation for service revenue as services are performed, and for product revenue, prior to 2024, upon delivery of diagnostic test kits. The timing of revenue recognition depends on the identified performance obligations but is generally recognized over time or as results are reported to the customer.
Costs and Operating Expenses
Cost of Biosecurity Service Revenue
The cost of Biosecurity service revenue consists of costs related to our biomonitoring and bioinformatics support services. This includes costs incurred for sample collection equipment and materials, outsourced laboratory analysis, access to results reported through our proprietary web-based portal, and reporting of results to government and non-government customers. Additionally, the cost of Biosecurity service revenue includes direct labor cost associated with bioinformatics, lab network management, delivery logistics, and customer support.
Cost of Biosecurity Product Revenue
Prior to 2024, the cost of Biosecurity product revenue consisted of costs associated with the sale of diagnostic and sample collection test kits, which included costs incurred to purchase test kits from third parties.
Cost of Other Revenue
Cost of other revenue consists of costs related to our Cell Engineering tools offerings, including Datapoints and lab automation solutions. Such costs primarily include hardware, software, materials and labor.
Research and Development Expenses
The nature of our business, and primary focus of our activities, generates a significant amount of R&D expenses. R&D expenses represent costs incurred by us for the following:
•development, operation, expansion and enhancement of our Foundry and Codebase;
•costs incurred to deliver our end-to-end cell engineering solutions offering to customers; and
•development of new offerings, such as Biosecurity.
The activities above incur the following expenses:
•personnel compensation and benefits;
•rent, facilities, depreciation, software, professional fees and other direct and allocated overhead expenses; and
•laboratory supplies, consumables and related services provided under agreements with third parties and in-licensing arrangements.
We expense R&D expenses as incurred. We experienced lower R&D costs in 2024 compared to 2023 primarily due to our restructuring plan announced and commenced in the second quarter of 2024 as we rationalize our current development programs and prioritize our investments in our Foundry, Codebase, AI and new offerings. We expect that our R&D expenses will either remain consistent or decline in 2025 as compared to 2024, reflecting the stabilization of our operational overhead and the impact of our restructuring actions. However, our R&D expenses could increase in 2025 due to employee incentive programs offered or additional costs and expenses arising from these restructuring actions. The nature, timing, and estimated costs required to support our growth will be dependent on advances in technology, our ability to attract new customers, and the rate of market penetration within our existing customer industries.
General and Administrative Expenses
General and administrative (“G&A”) expenses consist primarily of costs for personnel in executive, business development, finance, human resources, legal and other corporate administrative functions. G&A expenses also include professional legal services fees and costs incurred relating to litigation, corporate, intellectual property and patent matters, professional fees incurred for accounting, auditing, tax and administrative consulting services, insurance costs, facility-related costs not otherwise included in R&D expenses, and asset impairments.
We experienced lower G&A costs in 2024 compared to 2023 primarily due to our restructuring plan announced and commenced in the second quarter of 2024, as we began reducing our operational overhead. We expect that our G&A expenses will either remain consistent or decline in 2025 as compared to 2024, reflecting the stabilization of our operational overhead and the impact of our restructuring actions. However, our G&A expenses could increase in 2025 due to employee incentive programs offered or additional costs and expenses arising from these restructuring actions. Conversely, we intend to maintain a strategic and opportunistic approach regarding inorganic G&A expenses arising from mergers, acquisitions, and other inorganic growth initiatives.
Impairment of Lease Assets
Impairment of lease assets relates to impairment losses recognized on a right-of-use asset and the related leasehold improvements associated with exited leased facilities.
Goodwill Impairment
In the second quarter of 2024, we fully impaired the goodwill attributable to our Cell Engineering reporting unit. Refer to further discussion within “Critical Accounting Estimates”.
Restructuring Charges
Restructuring charges are related to our restructuring plan, which was announced and commenced in the second quarter of 2024. These charges primarily include severance and other employee termination costs from a reduction in force that commenced in June 2024, as well as the impairment of a right-of-use asset due to the subleasing of a facility as part of real estate consolidation. Reductions in force are expected to be substantially completed in 2025, subject to compliance with applicable laws. While we aim to complete the majority of our facility consolidation actions in 2025, the actual timing may vary, especially for subleasing unused or underutilized facilities, which may extend beyond 2025 or may not occur prior to
termination of such lease, depending on market conditions. Additionally, restructuring expenses related to potential asset impairments or contract amendments or terminations for any facilities no longer in use or underutilized could be material.
Additional details are included in Note 3, Restructuring, of our consolidated financial statements included elsewhere in this Annual Report on Form 10-K. Interest Income
Interest income consists primarily of interest earned on our cash and cash equivalents.
Loss on Equity Method Investments
Loss on equity method investments includes our share of losses from certain of our equity method investments under the hypothetical liquidation at book value (“HLBV”) method.
Loss on Investments
Loss on investments includes the change in fair value of our marketable equity securities in publicly traded companies and impairment losses recognized on non-marketable equity securities in privately held companies.
Loss on Deconsolidation of Subsidiaries
Loss on deconsolidation of subsidiaries pertains to the deconsolidation of our former foreign subsidiary Altar SAS (“Altar”) in 2024 as a result of a sale and the deconsolidation of our former subsidiary Zymergen Inc. (“Zymergen”) in 2023.
Change in Fair Value of Warrant Liabilities
The change in fair value of warrant liabilities reflects adjustments to the fair value of private placement warrants (“Private Placement Warrants”) and warrants formerly publicly traded on the NYSE (“Public Warrants”). These warrants, classified as liabilities, were assumed as part of our merger with Soaring Eagle Acquisition Corp. (“SRNG”) on September 16, 2021, and were initially issued in connection with SRNG’s initial public offering. Warrant liabilities are remeasured at fair value at each balance sheet date and have substantially no value as of December 31, 2024.
Other Income, Net
Other income, net primarily consists of sublease rent income and changes in fair value of notes receivable that we elected to account for under the fair value option.
Provision for Income Taxes
Income taxes are recorded in accordance with ASC 740, Income Taxes, which provides for deferred taxes using an asset and liability approach. We recognize deferred tax assets and liabilities for the expected future tax consequences of events that have been included in our audited consolidated financial statements or tax returns. Deferred tax assets and liabilities are determined based on the difference between the financial statement and tax bases of assets and liabilities using enacted tax rates in effect for the year in which the differences are expected to reverse. A valuation allowance against deferred tax assets is recorded if, based on the weight of the available evidence, it is more likely than not that some or all the deferred tax assets will not be realized. For all periods presented, we have recorded a valuation allowance against the deferred tax assets that are not expected to be realized.
We account for uncertain tax positions using a more-likely-than-not threshold for recognizing and resolving uncertain tax positions. The evaluation of uncertain tax positions is based on factors, including, but not limited to, changes in the law, the measurement of tax positions taken or expected to be taken in tax returns, the effective settlement of matters subject to audit, new audit activity and changes in facts or circumstances related to a tax position.
As of December 31, 2024, we had federal net operating loss carryforwards of approximately $1.2 billion, of which $139.2 million will begin to expire in 2029 and $1.1 billion can be carried forward indefinitely. As of December 31, 2024, we had state net operating loss carryforwards of approximately $1.2 billion, of which $991.7 million will begin to expire in 2030 and $162.3 million can be carried forward indefinitely. As of December 31, 2024, we had federal research and development tax credit carryforwards of approximately $37.7 million, which will begin to expire in 2029. As of December 31, 2024, we also had state research and development and investment tax credit carryforwards of approximately $30.2 million, which will begin to expire in 2030.
Income taxes are determined at the applicable tax rates adjusted for non-deductible expenses, R&D tax credits and other permanent differences. Our income tax provision may be affected by changes to our estimates.
Results of Operations
Comparison of the Years Ended December 31, 2024 and 2023
The following table presents our result of operations for the periods indicated:
| | | | | | | | | | | | | | | | | |
| Year Ended December 31, | | |
| (in thousands) | 2024 | | 2023 | | Change |
| Cell Engineering revenue | $ | 173,972 | | | $ | 143,531 | | | $ | 30,441 | |
| Biosecurity revenue: | | | | | |
| Service | 53,071 | | | 78,975 | | | (25,904) | |
| Product | — | | | 28,949 | | | (28,949) | |
| Total revenue | 227,043 | | | 251,455 | | | (24,412) | |
| Costs and operating expenses: | | | | | |
| Cost of Biosecurity service revenue | 38,549 | | | 46,524 | | | (7,975) | |
| Cost of Biosecurity product revenue | — | | | 7,481 | | | (7,481) | |
| Cost of other revenue | 5,999 | | | — | | | 5,999 | |
Research and development (1) | 424,061 | | | 580,621 | | | (156,560) | |
General and administrative (1) | 246,161 | | | 385,025 | | | (138,864) | |
| Impairment of lease assets | — | | | 96,210 | | | (96,210) | |
| Goodwill impairment | 47,858 | | | — | | | 47,858 | |
| Restructuring charges | 24,172 | | | — | | | 24,172 | |
| Total operating expenses | 786,800 | | | 1,115,861 | | | (329,061) | |
| Loss from operations | (559,757) | | | (864,406) | | | 304,649 | |
| Other income (expense): | | | | | |
| Interest income | 38,612 | | | 57,217 | | | (18,605) | |
| Interest expense | (94) | | | (93) | | | (1) | |
| Loss on equity method investments | — | | | (2,635) | | | 2,635 | |
| Loss on investments | (28,827) | | | (54,827) | | | 26,000 | |
| Loss on deconsolidation of subsidiaries | (7,013) | | | (42,502) | | | 35,489 | |
| Change in fair value of warrant liabilities | 5,701 | | | 5,168 | | | 533 | |
| | | | | |
| Other income, net | 3,870 | | | 9,138 | | | (5,268) | |
| Total other income (expense) | 12,249 | | | (28,534) | | | 40,783 | |
| Loss before income taxes | (547,508) | | | (892,940) | | | 345,432 | |
| Income tax benefit | (479) | | | (71) | | | (408) | |
| Net loss | $ | (547,029) | | | $ | (892,869) | | | $ | 345,840 | |
(1)The following table presents the allocation of stock-based compensation expense, inclusive of employer payroll taxes. Stock-based compensation expense during the year ended December 31, 2024 was partially offset by a $12.6 million expense reversal resulting from the forfeiture of RSUs related to our restructuring plan. Of the total reversal, $9.2 million was recorded to research and development expenses and $3.4 million was recorded to general and administrative expenses.
| | | | | | | | | | | |
| Year Ended December 31, |
| (in thousands) | 2024 | | 2023 |
| Research and development | $ | 57,723 | | | $ | 148,861 | |
| General and administrative | 57,576 | | | 86,047 | |
| Total | $ | 115,299 | | | $ | 234,908 | |
Cell Engineering Revenue
Cell Engineering revenue was $174.0 million in 2024, compared to $143.5 million in 2023, an increase of $30.4 million. The increase was primarily due to the recognition of $45.4 million in non-cash revenue from the release of a deferred revenue balance associated with the terminated Motif FoodWorks, Inc. (“Motif”) contract in 2024 (see Note 16 of our audited consolidated financial statements included elsewhere in this Annual Report on Form 10-K) and an increase in revenue related to programs with large enterprise customers primarily in the pharmaceutical, biotechnology and U.S. government (healthcare and defense) industries, partially offset by decreases in revenue related to programs with early stage customers in the pharmaceutical, biotechnology and industrial biotechnology (food and nutrition, industrial and environmental, and consumer and technology) industries. As discussed above in Components of Results of Operations, Cell Engineering revenue comprises both cash and non-cash consideration. Cell Engineering revenue recognized relating to non-cash consideration increased from $48.5 million in 2023 to $61.4 million in 2024. The increase was primarily due to the recognition of $45.4 million in non-cash revenue from the release of the deferred revenue balance associated with the terminated Motif contract in 2024, partially offset by lower non-cash revenue from other customers.
Biosecurity Revenue
Biosecurity revenue was $53.1 million in 2024, compared to $107.9 million in 2023, a decrease of $54.9 million. This total decrease consisted of a $25.9 million decline in service revenue and a $28.9 million decline in product revenue.
The decrease in Biosecurity revenue is primarily due to the end of our COVID-19 testing in schools in 2023, partially offset by new expanded offerings of biomonitoring and bioinformatics support services in 2023 and 2024.
Since the end of the COVID-19 public health emergency in May 2023, we shifted our Biosecurity business focus to developing scalable biosecurity infrastructure and delivering global surveillance programs and analytics services. Biosecurity revenue in 2024 was comprised of our expanded offerings of biomonitoring and bioinformatics support services. Through our partnerships, we operate programs for collections, testing, sequencing, and insights delivery on pathogen samples in different countries.
Cost of Biosecurity Service and Product Revenue
Cost of Biosecurity service and product revenue was $38.5 million in 2024, compared to $54.0 million in 2023, a decrease of $15.5 million. This decrease was primarily due to the end of our COVID-19 testing in schools in 2023, partially offset by growth in our expanded offerings of biomonitoring and bioinformatics support services following the transition of our Biosecurity business to developing scalable biosecurity infrastructure and delivering global surveillance programs and analytics services.
Cost of Other Revenue
Cost of other revenue was $6.0 million in 2024 and zero in 2023. These costs relate to our new Cell Engineering customer offerings, Datapoints and lab automation solutions, which were launched in 2024. Costs related to our end-to-end cell engineering solutions offering are included in research and development expenses.
Research and Development Expenses
Our research and development expenses principally relate to the development of new offerings and the operation, expansion and enhancement of our existing service offerings utilizing our proprietary platform, which includes our Foundry and Codebase assets, to our cell engineering customers. Research personnel costs, including stock-based compensation, is our largest expense, aggregating to $184.9 million and $304.3 million for the years ended December 31, 2024 and 2023, respectively. We also acquired and expensed in-process research and development primarily through the issuance of our equity, aggregating to $19.8 million and $9.6 million for the years ended December 31, 2024 and 2023, respectively. Our remaining research and development costs are comprised primarily of rent and related facilities costs, information technology costs, depreciation pertaining to facilities and equipment, laboratory consumables, contract services and routine costs and fees.
Research and development expenses were $424.1 million in 2024, compared to $580.6 million in 2023, a decrease of $156.6 million. This decrease was primarily due to a reduction in stock-based compensation expense of $87.5 million (inclusive of employer payroll taxes) and research and development expenses of $50.5 million from the deconsolidation of Zymergen. Additionally, there were decreases in personnel-related compensation and benefits expense of $14.6 million, professional fees of $14.2 million, lab equipment impairment of $12.3 million, temporary labor and contractors of $3.1 million, allocated overhead expenses of $6.9 million from R&D to G&A, and other operating expenses of $8.6 million, primarily due to our restructuring plan announced and commenced in the second quarter of 2024. These decreases were partially offset by an increase in rent and related facilities costs of $19.4 million, acquired in-process research and development costs of $11.5 million, software and technology expense of $6.1 million, and depreciation of $4.2 million. Increases in research and development expenses supported the growth of cell engineering capabilities prior to the commencement of our restructuring plan.
General and Administrative Expenses
General and administrative expenses were $246.2 million in 2024, compared to $385.0 million in 2023, a decrease of $138.9 million. This decrease was primarily due to a reduction of $72.8 million in general and administrative expenses from the deconsolidation of Zymergen. Excluding this impact, the decrease was largely attributable to our restructuring plan announced and commenced in the second quarter of 2024, which resulted in reductions in professional fees of $49.1 million (including a $17.6 million decrease in litigation costs), stock-based compensation expense of $26.6 million (inclusive of employer payroll taxes), temporary labor and contractor fees of $7.5 million, the change in fair value of contingent consideration liabilities resulting from acquisitions of $6.0 million, and other operating expenses of $2.2 million. These decreases were partially offset by an increase in personnel-related compensation and benefits expense of $10.1 million, rent and related facilities costs of $9.4 million from a new facility lease that commenced in 2024, and the impairment of construction in progress assets of $5.8 million.
Impairment of Lease Assets
In 2023, we recognized an impairment loss of $96.2 million related to a right-of-use asset and the associated leasehold improvements for an exited Zymergen leased facility. During 2023, Zymergen permanently ceased use of and vacated the leased space, which triggered an impairment analysis and resulted in a write-down of the carrying value of the assets to their estimated fair value.
Goodwill Impairment
In 2024, we recorded goodwill impairment expense of $47.9 million related to our Cell Engineering reporting unit, further discussed within “Critical Accounting Estimates” below.
Restructuring Charges
In 2024, we incurred restructuring charges of $24.2 million in connection with our restructuring plan announced and commenced in the second quarter of 2024, primarily in the Cell Engineering segment. These charges primarily consisted of employee termination costs from the reduction in force commenced in June 2024 and the impairment of a right-of-use asset
relating to facilities consolidation. See Note 3 of our audited consolidated financial statements included elsewhere in this Annual Report on Form 10-K for further details. Interest Income
Interest income was $38.6 million in 2024, compared to $57.2 million in 2023, a decrease of $18.6 million. This decrease was primarily due to lower average cash balances in interest bearing accounts.
Loss on Equity Method Investments
Loss on equity method investments was zero in 2024, compared to $2.6 million in 2023. The 2023 loss represented our share of losses from certain equity method investees resulting from the application of the HLBV method. Under the HLBV method, as a common unit holder, we absorb losses before preferred unit holders due to a substantive profit-sharing agreement that grants preferred unit holders preferential distribution rights. Since we have no obligation to fund the losses of our equity method investees beyond our initial investment, no additional losses were recognized in 2024, as the investments had already been reduced to zero prior to that year.
Loss on Investments
Loss on investments was $28.8 million in 2024, compared to $54.8 million in 2023, a decrease of $26.0 million. The higher loss in 2023 was due to greater impairment losses on our non-marketable equity investments in privately held companies compared to the corresponding period in 2024. We assess our non-marketable equity investments quarterly for potential impairment and remeasure to fair value when events or changes in circumstances indicate that the carrying value may not be recoverable.
Loss on Deconsolidation of Subsidiaries
In 2024, we recorded a $7.0 million loss on the deconsolidation of our former foreign subsidiary Altar as a result of a sale of this business.
In 2023, we recorded a $42.5 million loss on the deconsolidation of Zymergen following Zymergen's bankruptcy filing in October 2023.
Change in Fair Value of Warrant Liabilities
The change in fair value of warrant liabilities was a gain of $5.7 million in 2024, compared to a gain of $5.2 million in 2023, an increase of $0.5 million. The change in fair value of warrant liabilities is primarily driven by fluctuations in the value of our common stock. Increases or decreases in the value of our common stock result in a loss or gain, respectively, in the fair value of warrant liabilities. There was substantially no value related to these warrant liabilities as of December 31, 2024.
Other Income, Net
Other income, net was $3.9 million in 2024, compared to $9.1 million in 2023, a decrease of $5.3 million. This decrease was primarily due to reduced sublease rent income following the deconsolidation of Zymergen.
Non-GAAP Information
In addition to our results determined in accordance with GAAP, we use earnings before interest, taxes, depreciation and amortization (“EBITDA”) and Adjusted EBITDA internally to evaluate our performance and make financial and operational decisions. We believe these non-GAAP measures, when viewed with our GAAP results, may be helpful to investors in assessing our operating performance.
We define EBITDA as net loss attributable to Ginkgo Bioworks Holdings, Inc. stockholders before the impact of interest income, interest expense, provision for income taxes and depreciation and amortization.
We define Adjusted EBITDA as EBITDA adjusted for stock-based compensation expense, gain or loss on equity method investments, gain or loss on investments, change in fair value of warrant liabilities, gain or loss on deconsolidation of subsidiaries, transaction and integration costs associated with planned, completed or terminated mergers and acquisitions, including related litigation costs, restructuring and impairment charges (inclusive of impairments of goodwill and long-lived assets), costs associated with the bankruptcy filing of our former subsidiary, Zymergen (the “Zymergen
Bankruptcy”), and certain other income and expenses. We believe that the use of EBITDA and Adjusted EBITDA provides an additional tool for investors to use in evaluating ongoing operating results and trends because it eliminates the effect of financing activities, investing activities, and certain non-cash charges and other items that are not related to our core operating performance or affect comparability period over period.
In 2024, we updated our definition of Adjusted EBITDA to no longer exclude the impact of acquired in-process research and development expenses. Accordingly, the comparable 2023 period has been recast to conform to the revised definition.
Our non-GAAP financial measures have limitations as analytical tools and should not be considered in isolation or as a substitute for GAAP performance measures. These measures exclude significant expenses and income required by GAAP, which impacts their alignment with consolidated financial statements. They also rely on management's judgment to determine which items are included or excluded, making them inherently subjective. Additionally, non-GAAP measures lack uniform definitions and may differ from those used by other companies, limiting comparability. A reconciliation of EBITDA and Adjusted EBITDA to net loss, the most directly comparable GAAP financial measure, is presented below:
| | | | | | | | | | | |
| Year Ended December 31, |
| (in thousands) | 2024 | | 2023 |
Net loss (1) | $ | (547,029) | | | $ | (892,869) | |
| Interest income | (38,612) | | | (57,217) | |
| Interest expense | 94 | | | 93 | |
| Income tax benefit | (479) | | | (71) | |
| Depreciation and amortization | 63,020 | | | 70,507 | |
| EBITDA | (523,006) | | | (879,557) | |
Stock-based compensation (2) | 115,299 | | | 234,908 | |
Impairment expense (3) | 53,654 | | | 121,404 | |
Restructuring charges (4) | 24,172 | | | — | |
Merger and acquisition related expenses (5) | 4,417 | | | 61,189 | |
| Loss on equity method investments | — | | | 2,635 | |
| Loss on investments | 28,827 | | | 54,827 | |
| Loss on deconsolidation of subsidiaries | 7,013 | | | 42,502 | |
| Change in fair value of warrant liabilities | (5,701) | | | (5,168) | |
| Change in fair value of convertible notes | 2,014 | | | 2,295 | |
| Adjusted EBITDA | $ | (293,311) | | | $ | (364,965) | |
(1)All periods include non-cash revenue when earned, including $45.4 million in the year ended December 31, 2024, recognized pursuant to the termination of revenue contracts with Motif.
(2)For the years ended December 31, 2024 and 2023, includes $3.0 million and $5.0 million, respectively, in related employer payroll taxes.
(3)For 2024, includes $47.9 million related to goodwill impairment and $5.8 million related to lab equipment. For 2023, includes a $25.2 million impairment loss on lab equipment and a $96.2 million impairment loss on lease assets associated with an exited Zymergen leased facility.
(4)Restructuring charges consist of employee termination costs from the reduction in force commenced in June 2024, as well as the impairment of a right-of-use asset relating to facilities consolidation.
(5)Represents transaction and integration costs directly related to mergers and acquisitions, including: (i) due diligence, legal, consulting and accounting fees associated with acquisitions, (ii) post-acquisition employee retention bonuses and severance payments, (iii) the fair value adjustments to contingent consideration liabilities resulting from acquisitions, and (iv) costs associated with the Zymergen Bankruptcy, as well as securities litigation costs, net of insurance recovery. Not included in this adjustment are acquired in-process research and development expenses, which totaled $19.8 million and $9.6 million for the years ended December 31, 2024 and 2023, respectively.
Liquidity and Capital Resources
On August 19, 2024, with the approval of our board of directors and shareholders, we effected a one-for-forty (1:40) reverse stock split for our common stock. Accordingly, all common shares presented herein have been retrospectively adjusted to reflect the reverse stock split.
Sources of Liquidity
Upon the closing of our merger with SRNG in September 2021, we received net proceeds totaling approximately $1.5 billion, inclusive of $760.0 million from investments from certain accredited investors for 1.9 million shares of our Class A common stock. As of December 31, 2024, we had cash and cash equivalents of $561.6 million, which we believe will be sufficient to enable us to fund our projected operations through at least the next 12 months from the date of filing of this Annual Report on Form 10-K.
Material Cash Requirements
We anticipate that our expenditures will exceed our revenue through at least the next 12 months from the date of filing of this Annual Report on Form 10-K, as we:
•continue our R&D activities under existing and new programs and further invest in our Foundry and Codebase;
•develop and expand our offerings, including Biosecurity;
•upgrade, expand or adapt our operational, financial and management systems and support our operations;
•potentially acquire and integrate companies, assets or intellectual property that advance our company objectives;
•maintain, expand, and protect our intellectual property; and
•continue our restructuring actions.
Leases
We have various noncancelable operating leases for office and laboratory space, with significant leases expiring between 2030 and 2036. As of December 31, 2024, we have minimum rental commitments under noncancellable operating leases of $61.2 million in 2025 and $662.5 million thereafter. See Note 9 to the consolidated financial statements in Part II, Item 8 of this Annual Report on Form 10-K for more information. Purchase Obligations
In August 2023, we entered into a five-year strategic cloud and AI partnership with Google Cloud, which includes minimum annual commitments to purchase cloud hosting services. As of December 31, 2024, the remaining aggregate commitment was $279.3 million, with approximately $44.3 million payable in 2025 and $235.0 million thereafter.
In March 2022, we entered into a four-year noncancelable supply agreement with Twist for the purchase of diverse products including synthetic DNA. Under this agreement, we are obligated to spend a minimum of $58.0 million over the four-year term, with approximately $24.8 million payable in 2025 and $4.8 million thereafter.
Cash Flows
The following table provides information regarding our cash flows for each period presented:
| | | | | | | | | | | |
| Year Ended December 31, |
| (in thousands) | 2024 | | 2023 |
| Net cash used in: | | | |
| Operating activities | $ | (319,585) | | | $ | (295,500) | |
| Investing activities | (62,236) | | | (80,693) | |
| Financing activities | (1,739) | | | (3,216) | |
| Effect of exchange rate changes | (281) | | | (588) | |
| Net decrease in cash, cash equivalents and restricted cash | $ | (383,841) | | | $ | (379,997) | |
Operating Activities
Net cash used in operating activities for the year ended December 31, 2024 consisted of a net loss of $547.0 million, adjusted for a net decrease in cash due to changes in operating assets and liabilities of $89.7 million and non-cash charges of $317.1 million. The net change in operating assets and liabilities was primarily driven by a $40.4 million decrease in accrued expenses and other current liabilities primarily due to the payment or release of restructuring-related accruals and litigation costs, a $68.6 million decrease in deferred revenue primarily from a one-time release of a deferred revenue balance associated with a terminated customer contract, and a $14.9 million decrease in operating lease liabilities from rent payments, partially offset by a $23.5 million decrease in operating lease right-of-use assets from lease incentives received and a $10.1 million decrease in prepaid expenses and other current assets, primarily driven by the derecognition of an insurance receivable and a reduction in contract renewals resulting from our restructuring actions. Non-cash adjustments primarily consisted of $63.0 million in depreciation and amortization, $112.3 million in stock-based compensation expense, $28.8 million loss on investments, $28.1 million non-cash lease expense, $19.8 million in acquired in-process research and development expense, and $58.5 million in various asset impairment charges.
Net cash used in operating activities for the year ended December 31, 2023 consisted of a net loss of $892.9 million, adjusted for a net increase in cash due to changes in operating assets and liabilities of $29.8 million and non-cash charges of $567.5 million. The net change in operating assets and liabilities was primarily driven by (i) a $50.1 million decrease in accounts receivable from collections of Biosecurity receivables and the end of COVID-19 testing in schools in 2023, (ii) a $10.5 million decrease in prepaid expenses and other current assets primarily from depletion of inventory coinciding with the reduction of Biosecurity product revenue plus the timing of directors and officers insurance payments in the prior year, (iii) a $9.3 million decrease in operating lease right-of-use assets from lease incentives received, (iv) a $16.9 million increase in accrued expenses and other current liabilities primarily from accrued litigation costs, partially offset by (v) a $35.9 million decrease in deferred revenue and (vi) a $22.8 million decrease in operating lease liabilities from rent payments. Non-cash adjustments primarily consisted of $70.5 million of depreciation and amortization, $229.9 million of stock-based compensation, $57.5 million loss on investments including equity method investments, $9.2 million loss on the change in fair value of contingent consideration liabilities, $28.3 million of non-cash lease expense, $121.4 million in impairments of long-lived assets, and $42.5 million loss on deconsolidation of Zymergen.
Investing Activities
Net cash used in investing activities for the year ended December 31, 2024 primarily consisted of $62.5 million in purchases of property and equipment related to a build out of new office and laboratory space being developed near our headquarters, $5.4 million paid for the acquisition of certain Zymergen assets, offset by $4.5 million in proceeds from the sale of marketable securities.
Net cash used in investing activities for the year ended December 31, 2023 primarily consisted of purchases of property and equipment of $40.8 million associated with Foundry capacity and capability investments, relinquishment of $43.0 million in cash upon the deconsolidation of Zymergen, offset by $4.4 million in proceeds from the sale of equipment.
Financing Activities
Net cash used in financing activities for the year ended December 31, 2024 primarily consisted of principal payments on finance leases and payments of contingent consideration related to business acquisitions.
Net cash used in financing activities for the year ended December 31, 2023 primarily consisted of principal payments on finance leases and payments of contingent consideration related to business acquisitions.
Critical Accounting Estimates
Our management’s discussion and analysis of our financial condition and results of operations is based on our consolidated financial statements, which have been prepared in accordance with GAAP. The preparation of these consolidated financial statements requires us to make judgments and estimates that affect the reported amounts of assets, liabilities, revenues and expenses and the disclosure of contingent assets and liabilities in our consolidated financial statements. We base our estimates on historical experience, known trends and events and various other factors that we believe to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions. On an ongoing basis, we evaluate our judgments and estimates in light of changes in circumstances, facts and experience. The effects of material revisions in estimates, if any, are reflected in our consolidated financial statements prospectively from the date of change in estimates.
While our significant accounting policies are described in more detail in Note 2 to our audited consolidated financial statements appearing elsewhere in this Annual Report on Form 10-K, we believe the following accounting policies used in the preparation of our consolidated financial statements require the most significant judgments and estimates. Revenue Recognition
Cell Engineering Revenue
For certain Cell Engineering revenue contracts, we recognize revenue over the period of performance using a measure of progress based on costs incurred to date relative to total expected costs (i.e., cost-to-cost method). A significant level of judgment is involved in estimating the total expected costs. When estimating total expected costs, we make assumptions and estimates regarding the contracted scope of work, tasks required to complete each project, technical and schedule risks associated with the science, the expected duration of each project, and the total amount of internal and external resources required.
Our collaboration and licensing agreements often include multiple promises, such as (i) licenses and assignments of intellectual property and materials and (ii) research and development services. We assess whether each promise constitutes a distinct performance obligation based on the specific terms of each agreement. Determining whether the promises within a customer contract should be accounted for separately as distinct performance obligations requires significant judgment. Therefore, we review customer contracts to identify all individual promises to transfer goods and services that qualify as performance obligations.
Options to acquire additional goods and services are evaluated to determine whether they provide a material right to the customer that would not otherwise be available without entering into the contract. Judgment is required to assess whether a customer option constitutes a material right. If a material right is identified, the option is treated as a separate performance obligation, and the revenue allocated to the option is deferred until the option is either exercised or expires.
We also evaluate contract modifications and amendments to determine whether any changes should be accounted for as a separate contract, prospectively or on a cumulative catch-up basis.
Certain customer contracts include payment in the form of equity securities or other financial instruments that convert into equity upon a triggering event. Any non-cash consideration is measured at its estimated fair value at contract inception. For equity securities and financial instruments that are not actively traded, we generally determine the estimated fair value by referencing a recent financing round or utilizing a scenario-based valuation model. Significant unobservable inputs are used in these valuations, including expectations regarding future financings of the customer, scenario dates and probabilities, expected volatility, discount rates, and recovery rates. Changes in these assumptions can materially affect the fair value of the non-cash consideration and, consequently, the total revenue recognized for the contract.
Impairment of Long-Lived Assets
We review our long-lived assets and asset groups for impairment whenever events or changes in circumstances indicate that the carrying value of the assets may not be recoverable. Recoverability is measured by comparing the carrying value of the long-lived assets to the future undiscounted cash flows expected to be generated by the assets. In determining the expected future cash flows, we use assumptions believed to be reasonable, but which are unpredictable and inherently uncertain. Actual future cash flows may differ from the estimates used in impairment testing. We recognize an impairment loss when and to the extent that the estimated fair value of the long-lived assets is less than their carrying value.
Goodwill
We assess goodwill for impairment at the reporting unit level on an annual basis during the fourth quarter or whenever events or changes in circumstances indicate that the carrying amount may not be recoverable. Goodwill impairment assessments require a significant amount of management judgment and the use of estimates and assumptions that could have a significant effect on whether or not an impairment charge is recorded and the magnitude of such a charge.
During the year ended December 31, 2024, due to a sustained decrease in the market price of our Class A common stock and market capitalization, we identified that a possible indicator of impairment was present as of June 30, 2024. As such, we completed a quantitative impairment test related to our Cell Engineering reporting unit. To conduct the impairment test of goodwill, the estimated fair value of the reporting unit was compared to its carrying value. The estimated fair value of the Cell Engineering reporting unit was determined using a weighted approach that considered a discounted cash flow (“DCF”) model under the income approach and the guideline public company (“GPC”) method under the market approach. Inputs used in the DCF model included the projected future operating results of the reporting unit and the applicable
discount rate, while inputs used in the GPC method consisted of a revenue multiple. The projected future operating results were based on historical experience and internal annual operating plans reviewed by management, extrapolated over the forecast period. The discount rate was determined using a weighted average cost of capital adjusted for risk factors specific to the reporting unit. The revenue multiple was based on the GPC method using comparable publicly traded company multiples of revenue for a group of benchmark companies. The DCF method was weighted 75% and the GPC 25%. We reconciled the resulting fair value of the reporting unit to our market capitalization to corroborate the fair value estimate used in the impairment test.
The interim impairment test indicated that the estimated fair value of the reporting unit was less than its carrying value. As a result, we fully impaired goodwill and recorded an impairment loss of $47.9 million in the second quarter of 2024 and for the year ended December 31, 2024.
Investments in Non-Marketable Equity Securities
We account for our non-marketable equity securities using the measurement alternative, where the carrying value is measured at cost, less any impairment, plus or minus changes resulting from observable price changes in orderly transactions for the identical or a similar investment of the same issuer. Determining whether an observed transaction is similar to the security owned by us requires judgment based on the rights and obligations of the investments.
We evaluate non-marketable equity investments for impairment on a quarterly basis, considering both qualitative and quantitative factors that may have a significant effect on the investment’s fair value. Qualitative factors considered include the companies’ financial and liquidity position, access to capital resources, adverse changes in the economic environment of the investee, and adverse changes in the business prospects of the investee, among others. When indicators of impairment exist, we prepare quantitative assessments of the fair value of our non-marketable equity securities using market and income approaches that require judgment and the use of estimates, including discount rates, assumptions around the investees' expected time to exit event, investee revenues and expenses, and comparable market data of guideline public companies, among others. When our assessment indicates that an impairment exists, we write down the investment to its fair value.
Recently Issued Accounting Pronouncements
See Note 2, “Summary of Significant Accounting Policies,” of our consolidated financial statements contained in Part II, Item 8 of this Annual Report on Form 10-K for a discussion of recently issued accounting pronouncements. Item 7A. Quantitative and Qualitative Disclosures About Market Risk.
Interest Rate Risk
We are exposed to market risk related to changes in interest rates. Our primary exposure to market risk is interest rate sensitivity, which is affected by changes in the general level of U.S. interest rates, particularly because our cash equivalents have historically been invested in short-term U.S. Treasury obligations. However, because of the short-term nature of the instruments in our portfolio as of December 31, 2024, an immediate change in market interest rates of 100 basis points would not have a material impact on the fair market value of our cash and cash equivalents or on our financial position or results of operations. In February 2025, we expanded our investment of excess cash and cash equivalents to include U.S. Treasuries, corporate papers and bonds, bank obligations and certificates, and other interest bearing securities, whereby no underlying security may have a term greater than two years and in which the entire portfolio would have weighted average maturity of six-months or less. An immediate change in market interest rates of 100 basis points on the new investments would not have a material impact on the fair market value of our cash and cash equivalents or on our financial position or results of operations.
Foreign Currency Exchange Rate Risk
We are subject to foreign currency exchange rate risk from the translation of the financial statements of our foreign subsidiaries, whose financial condition and results of operations are reported in their local currencies and then translated into U.S. dollars at the applicable currency exchange rate for inclusion in our consolidated financial statements. Foreign currency translation (loss) gain was $(4.8) million and $4.1 million for the years ended December 31, 2024 and 2023, respectively. Foreign currency translation adjustments are accounted for as a component of accumulated other comprehensive (loss) income within stockholders’ equity. Additionally, we have contracted with and may continue to contract with foreign customers, suppliers, and contractors. We do not believe that an immediate 10% increase or decrease in the relative value of the U.S. dollar to other currencies would have a material effect on operating results or financial condition.
Inflation Risk
Inflation generally affects us by increasing our cost of labor, laboratory supplies, consumables and equipment. We do not believe that inflation had a material effect on our business, financial condition or results of operations during the years ended December 31, 2024 and 2023.
Item 8. Financial Statements and Supplementary Data.
Item 9. Changes in and Disagreements With Accountants on Accounting and Financial Disclosure.
None.
Item 9A. Controls and Procedures.
Evaluation of Disclosure Controls and Procedures
Under the supervision and with the participation of our management, including our Chief Executive Officer and Chief Financial Officer, we evaluated the effectiveness of our disclosure controls and procedures (as that term is defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934, as amended (the “Exchange Act”)) as of December 31, 2024, which is the end of the period covered by this Annual Report. Based on this evaluation, our Chief Executive Officer and Chief Financial Officer concluded that the Company’s disclosure controls and procedures were effective as of December 31, 2024.
Management’s Annual Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting as such terms are defined in Exchange Act Rules 13a-15(f) and 15d-15(f). Our internal control over financial reporting is a framework designed under the supervision and with the participation of our management, including our Chief Executive Officer and Chief Financial Officer, and effected by our board of directors, management, and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of our financial statements for external purposes in accordance with GAAP. Our internal control over financial reporting includes those policies and procedures that: (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of our assets; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with GAAP, and that our receipts and expenditures are being made only in accordance with authorizations of management and our directors; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of our assets that could have a material effect on the financial statements.
As of December 31, 2024, our management conducted an evaluation of the effectiveness of our internal control over financial reporting based on the criteria established by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) in Internal Control – Integrated Framework (2013). Based on this evaluation, we concluded that the Company's system of internal control over financial reporting was effective as of December 31, 2024.
The effectiveness of our internal control over financial reporting as of December 31, 2024 has been audited by our independent registered public accounting firm, Deloitte & Touche LLP, as stated in their report which is included herein.
Remediation of Previously Reported Material Weakness in Internal Control Over Financial Reporting
In preparing our financial statements in connection with our Annual Report on Form 10-K for the year ended December 31, 2023 and continuing through the nine months ended September 30, 2024, we previously identified a material weakness in our internal control over financial reporting. The material weakness related to ineffective management review controls to address the risks of material misstatement of various significant accounts. Management’s evaluation of the completeness and accuracy of data used in the performance of its controls was insufficient, as was the precision of the review, identification and resolution of items requiring follow-up, and/or timeliness of the review.
Following the identification of the material weakness, and with the oversight of the Audit Committee, we commenced remediation efforts that continued during fiscal 2024 to address the material weakness and enhance our control environment, including our internal control over financial reporting. Our remediation efforts included:
•Employee training related to internal control over financial reporting specifically focused on data used in the operation of management review controls and the execution of management review controls with an appropriate level of precision and appropriate documentation of the identification and resolution of follow-up items;
•Implementation and enhancement of control activities, including automation of certain control processes; and,
•Development of other tools and enablers, including increasing the standardization of control support and documentation.
Based on these remediation actions, as well as testing the operating effectiveness of the applicable financial reporting controls over a sustained period of financial reporting cycles, we have concluded that the previously reported material weakness has been effectively remediated as of December 31, 2024.
Changes in Internal Control over Financial Reporting
Except as otherwise noted above under “Remediation of Previously Reported Material Weakness in Internal Control Over Financial Reporting”, there were no changes in our internal control over financial reporting (as such term is defined in Exchange Act Rule 13a-15(f) and 15d-15(f)) during the most recent fiscal quarter that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the stockholders and the Board of Directors of Ginkgo Bioworks Holdings, Inc.
Opinion on Internal Control over Financial Reporting
We have audited the internal control over financial reporting of Ginkgo Bioworks Holdings, Inc. (the “Company”) as of December 31, 2024, based on criteria established in Internal Control — Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). In our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2024, based on criteria established in Internal Control — Integrated Framework (2013) issued by COSO.
We have also audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the consolidated financial statements as of and for the year ended December 31, 2024, of the Company and our report dated February 25, 2025, expressed an unqualified opinion on those financial statements.
Basis for Opinion
The Company’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Management’s Annual Report on Internal Control over Financial Reporting. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
Definition and Limitations of Internal Control over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
/s/ Deloitte & Touche LLP
Boston, Massachusetts
February 25, 2025
Item 9B. Other Information.
None.
Item 9C. Disclosure Regarding Foreign Jurisdictions that Prevent Inspections.
Not applicable.
PART III
Item 10. Directors, Executive Officers and Corporate Governance.
The information required by this item is incorporated by reference to the relevant information from our definitive Proxy Statement for our 2025 Annual Meeting of Shareholders, which will be filed not later than 120 days after December 31, 2024.
Insider Trading Compliance Policy
The Company has adopted an Insider Trading Compliance Policy governing the purchase, sale and/or other disposition of its securities by directors, officers and employees, or the Company itself, that it believes is reasonably designed to promote compliance with insider trading laws, rules and regulations and applicable NYSE listing standards. A copy of the Company’s Insider Trading Compliance Policy is filed with this Annual Report on Form 10-K as Exhibit 19.1.
Item 11. Executive Compensation.
The information required by this item is incorporated by reference to the relevant information from our definitive Proxy Statement for our 2025 Annual Meeting of Shareholders, which will be filed not later than 120 days after December 31, 2024.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters.
The information required by this item is incorporated by reference to the relevant information from our definitive Proxy Statement for our 2025 Annual Meeting of Shareholders, which will be filed not later than 120 days after December 31, 2024.
Item 13. Certain Relationships and Related Transactions, and Director Independence.
The information required by this item is incorporated by reference to the relevant information from our definitive Proxy Statement for our 2025 Annual Meeting of Shareholders, which will be filed not later than 120 days after December 31, 2024.
Item 14. Principal Accounting Fees and Services.
The information required by this item is incorporated by reference to the relevant information from our definitive Proxy Statement for our 2025 Annual Meeting of Shareholders, which will be filed not later than 120 days after December 31, 2024.
PART IV
Item 15. Exhibits, Financial Statement Schedules.
(1)As part of this Annual Report on Form 10-K, the consolidated financial statements are listed in the accompanying index to financial statements on page F-1. (2)Financial statement schedules have been omitted because they are either not required or not applicable or the information is included in the consolidated financial statements or the notes thereto.
(3)Exhibits:
| | | | | |
| Exhibit Number | Description |
| 2.2 | |
| |
| 2.3 | |
| |
| 3.1 | |
| |
| 3.2 | |
| |
| 4.1 | |
| |
| 4.2* | |
| |
| 4.3 | |
| |
| 4.4 | |
| |
| 10.1+* | |
| |
| 10.2+* | |
| |
| 10.3+ | |
| |
| 10.4+* | |
| |
| 10.5+ | |
| |
| 10.6+* | |
| |
| 10.7+* | |
| |
| | | | | |
| 10.8+ | |
| |
| 10.9+* | |
| |
| 10.10+* | |
| |
| 10.11+ | |
| |
| 10.12+ | |
| |
| 10.13+ | |
| |
| 10.14+ | |
| |
| 10.15+ | |
| |
| 10.16†‡ | |
| |
| 10.17† | |
| |
| 10.18† | |
| |
| 10.19 | |
| |
| 10.20† | |
| |
| 10.21† | |
| |
| 10.22 | |
| |
| 10.23† | |
| |
| 10.24† | |
| |
| 10.25† | |
| |
| | | | | |
| 10.26† | |
| |
| 10.27† | |
| |
| 10.28† | |
| |
| 10.29† | |
| |
| 10.30 | |
| |
| 10.31 | |
| |
| 10.32 | |
| |
| 10.33†‡ | |
| |
| 10.34† | |
| |
| 10.35 | |
| |
| 10.36†‡ | |
| |
| 10.37†+ | |
| |
| 10.38+ | |
| |
| 10.39+ | |
| |
| 10.40 | |
| |
| 10.41 | |
| |
| 10.42+ | |
| |
| 19.1* | |
| | | | | |
| |
| 21.1* | |
| |
| 23.1* | |
| |
| 23.2* | |
| |
| 31.1* | |
| |
| 31.2* | |
| |
| 32.1* | |
| |
| 32.2* | |
| |
| 97 | |
| |
| 101.INS | Inline XBRL Instance Document – the instance document does not appear in the Interactive Data File because XBRL tags are embedded within the Inline XBRL document |
| |
| 101.SCH | Inline XBRL Taxonomy Extension Schema Document |
| |
| 101.CAL | Inline XBRL Taxonomy Extension Calculation Linkbase Document |
| |
| 101.DEF | Inline XBRL Taxonomy Extension Definition Linkbase Document |
| |
| 101.LAB | Inline XBRL Taxonomy Extension Label Linkbase Document |
| |
| 101.PRE | Inline XBRL Taxonomy Extension Presentation Linkbase Document |
| |
| 104 | Cover Page Interactive Data File (embedded within the Inline XBRL document) |
________________________________________________________
*Filed herewith.
†The annexes, schedules, and certain exhibits to this Exhibit have been omitted pursuant to Item 601(b)(2) of Regulation S-K. The Registrant hereby agrees to furnish supplementally a copy of any omitted annex, schedule or exhibit to the SEC upon request.
‡ Certain confidential information contained in this Exhibit has been omitted because it is (i) not material and (ii) of the type that the registrant treats as private or confidential.
+ Indicates a management contract or compensatory plan.
Item 16. Form 10-K Summary
None.
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, as amended, the Registrant has duly caused this Report to be signed on its behalf by the undersigned, thereunto duly authorized.
| | | | | | | | |
| GINKGO BIOWORKS HOLDINGS, INC. |
| Date: February 25, 2025 | By: | /s/ Jason Kelly |
| | Jason Kelly |
| | Chief Executive Officer |
Pursuant to the requirements of the Securities Exchange Act of 1934, as amended, this Report has been signed below by the following persons on behalf of the Registrant in the capacities and on the dates indicated.
| | | | | | | | | | | | | | |
| Name | | Title | | Date |
| | | | |
| /s/ Jason Kelly | | Chief Executive Officer and Director | | February 25, 2025 |
| Jason Kelly | | (Principal Executive Officer) | | |
| | | | |
| /s/ Mark Dmytruk | | Chief Financial Officer | | February 25, 2025 |
| Mark Dmytruk | | (Principal Financial Officer) | | |
| | | | |
| /s/ Steven Coen | | Chief Accounting Officer | | February 25, 2025 |
| Steven Coen | | (Principal Accounting Officer) | | |
| | | | |
| /s/ Shyam Sankar | | Director, Chair of the Board | | February 25, 2025 |
| Shyam Sankar | | | | |
| | | | |
| /s/ Ross Fubini | | Director | | February 25, 2025 |
| Ross Fubini | | | | |
| | | | |
/s/ Kathy Hopinkah Hannan | | Director | | February 25, 2025 |
| Kathy Hopinkah Hannan | | | | |
| | | | |
| /s/ Christian Henry | | Director | | February 25, 2025 |
| Christian Henry | | | | |
| | | | |
| /s/ Sri Kosuri | | Director | | February 25, 2025 |
| Sri Kosuri | | | | |
| | | | |
| /s/ Myrtle Potter | | Director | | February 25, 2025 |
| Myrtle Potter | | | | |
| | | | |
| /s/ Reshma Shetty | | President, Chief Operating Officer and Director | | February 25, 2025 |
| Reshma Shetty | | | | |
| | | | |
| /s/ Harry E. Sloan | | Director | | February 25, 2025 |
| Harry E. Sloan | | | | |
GINKGO BIOWORKS HOLDINGS, INC.
Index to Consolidated Financial Statements as of December 31, 2024 and 2023 and for the Years Ended December 31, 2024, 2023 and 2022
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the stockholders and the Board of Directors of Ginkgo Bioworks Holdings, Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheet of Ginkgo Bioworks Holdings, Inc. and subsidiaries (the “Company”) as of December 31, 2024, and the related consolidated statements of operations and comprehensive loss, stockholders' equity, and cash flows for the year ended December 31, 2024, and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2024, and the results of its operations and its cash flows for the year ended December 31, 2024, in conformity with accounting principles generally accepted in the United States of America.
We have also audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the Company’s internal control over financial reporting as of December 31, 2024, based on criteria established in Internal Control — Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission and our report dated February 25, 2025, expressed an unqualified opinion on the Company’s internal control over financial reporting.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audit. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. Our audit included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audit also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audit provides a reasonable basis for our opinion.
Critical Audit Matters
The critical audit matters communicated below are matters arising from the current-period audit of the financial statements that were communicated or required to be communicated to the audit committee and that (1) relate to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the financial statements, taken as a whole, and we are not, by communicating the critical audit matters below, providing separate opinions on the critical audit matters or on the accounts or disclosures to which they relate.
Cell Engineering Revenue Recognition — Refer to Notes 2 and 14 to the financial statements
Critical Audit Matter Description
The Company generates Cell Engineering revenue primarily through license and collaboration agreements, under which customers obtain rights to the Company’s proprietary technology and intellectual property for use in the research, development and commercialization of engineered organisms and derived products. Under these agreements, the Company typically provides research and development services, including granting a license to its intellectual property. Additionally, the customer may obtain license rights to the output of the Company’s services in order to commercialize the resulting output of such services. Cell Engineering revenue was $174 million for the year ended December 31, 2024.
The Company generally recognizes Cell Engineering revenue over time based on the cost incurred to date relative to total expected costs or for cost-plus contracts, as costs are incurred. The Company evaluates its measure of progress to recognize revenue at each reporting period and, as necessary, adjusts the measure of progress and related revenue recognition. The Company’s measure of progress and revenue recognition involves significant judgment and assumptions, including, but not limited to, evaluating contract terms and estimating the cost to complete the performance obligation.
Auditing the Cell Engineering revenue was challenging due to the extent of audit effort required to evaluate the volume and complexity of customer contracts, including estimates of the measure of progress.
How the Critical Audit Matter Was Addressed in the Audit
Our audit procedures related to Cell Engineering revenue included the following, among others:
•We selected a sample of Cell Engineering contracts and performed the following:
◦Evaluated whether the contracts were properly included in management’s calculation of Cell Engineering revenue based on the terms and conditions of each contract, including whether continuous transfer of control to the customer occurred as progress was made toward fulfilling the performance obligation(s).
◦Compared the transaction price to the consideration expected to be received based on current rights and obligations under the contracts and any modifications that were agreed upon with the customers.
◦Tested management’s identification of distinct performance obligations.
◦To the extent a contract did not represent a single distinct performance obligation, we tested the allocation of the transaction price to each distinct performance obligation by comparing the relative standalone selling prices to the selling prices of similar goods or services.
◦Tested the accuracy and completeness of the costs incurred to date for each performance obligation.
◦Evaluated the estimates of total cost incurred and total estimated cost, when applicable, for the performance obligation by:
▪Testing the costs incurred to date.
▪Evaluating management’s estimates of total cost by performing corroborating inquiries with those that are responsible for managing the contract execution, and comparing the estimates to management’s work plans.
◦Tested the mathematical accuracy of management’s calculation of revenue for the performance obligation.
•We evaluated management’s ability to estimate total costs accurately by comparing actual costs to management’s historical estimates for performance obligations that have been fulfilled.
Goodwill Impairment — Refer to Notes 2 and 8 to the financial statements
Critical Audit Matter Description
The Company’s assessment of goodwill for impairment involves the comparison of the fair value of the reporting unit to its carrying value. The Company used a discounted cash flow model and guideline public company method to estimate fair value of its Cell Engineering reporting unit, which requires management to make significant estimates and assumptions related to projected future operating results, discount rates, and peer company multiples. Changes in these assumptions could have a significant impact on either the fair value of the Cell Engineering reporting unit, the amount of any goodwill impairment charge, or both.
As a result of an indicator of impairment, the Company performed an interim goodwill impairment assessment as of June 30, 2024, and determined that the fair value of the Cell Engineering reporting unit was less than its carrying value and that the goodwill was fully impaired. As a result, a goodwill impairment charge of $47.9 million was recorded in the year ended December 31, 2024.
Given the Company’s determination of the fair value of its Cell Engineering reporting unit required management to make significant estimates and assumptions related to projected future operating results, discount rates, and peer company multiples, auditing the reasonableness of the assumptions used involved especially subjective judgment and an increased extent of effort, including the need to involve of our fair value specialists. We identified goodwill as a critical audit matter because of the significant judgments made by management to estimate the fair value of the Cell Engineering reporting unit.
How the Critical Audit Matter Was Addressed in the Audit
Our audit procedures related to the projected future operating results, discount rates, and peer company multiples used by management to estimate the fair value of the Cell Engineering reporting unit included the following, among others:
•We evaluated the reasonableness of management’s projected future operating results by comparing the projections to (1) historical operating results, (2) internal communications to management and the Board of Directors, (3) information included in analyst and industry reports as well as certain publicly available peer company information, and (4) comparing actual results to management’s historical forecasts.
•With the assistance of our fair value specialists, we evaluated the reasonableness of the valuation methodology, discount rates and peer company multiples by:
◦Testing the source information underlying the determination of the discount rates and peer company multiples, and the mathematical accuracy of the calculations.
◦Developing a range of independent estimates and comparing those to the discount rates and peer company multiples selected by management.
/s/ Deloitte & Touche LLP
Boston, Massachusetts
February 25, 2025
We have served as the Company’s auditor since 2024.
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Stockholders and the Board of Directors of Ginkgo Bioworks Holdings, Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheet of Ginkgo Bioworks Holdings, Inc. (the Company) as of December 31, 2023, the related consolidated statements of operations and comprehensive loss, stockholders’ equity and cash flows for each of the two years in the period ended December 31, 2023, and the related notes (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company at December 31, 2023, and the results of its operations and its cash flows for each of the two years in the period ended December 31, 2023, in conformity with U.S. generally accepted accounting principles.
Basis for Opinion
These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ Ernst & Young LLP
We served as the Company’s auditor from 2018 to 2024.
Boston, Massachusetts
February 29, 2024, except for note 15 and the effects of the reverse stock split described in note 2, as to which the date is February 25, 2025
Ginkgo Bioworks Holdings, Inc.
Consolidated Balance Sheets
(in thousands, except share data)
| | | | | | | | | | | |
| As of December 31, |
| 2024 | | 2023 |
| Assets | | | |
| Current assets: | | | |
| Cash and cash equivalents | $ | 561,572 | | | $ | 944,073 | |
| Accounts receivable, net | 21,857 | | | 17,157 | |
| Accounts receivable - related parties | 586 | | | 742 | |
| Prepaid expenses and other current assets | 18,729 | | | 39,777 | |
| Total current assets | 602,744 | | | 1,001,749 | |
| Property, plant and equipment, net | 203,720 | | | 188,193 | |
| Operating lease right-of-use assets | 394,435 | | | 206,801 | |
| Investments | 48,704 | | | 78,565 | |
| Intangible assets, net | 72,510 | | | 82,741 | |
| Goodwill | — | | | 49,238 | |
| Other non-current assets | 55,336 | | | 58,055 | |
| Total assets | $ | 1,377,449 | | | $ | 1,665,342 | |
| Liabilities and Stockholders’ Equity | | | |
| Current liabilities: | | | |
| Accounts payable | $ | 14,169 | | | $ | 9,323 | |
| Deferred revenue (includes $795 and $5,426 from related parties) | 27,710 | | | 44,486 | |
| Accrued expenses and other current liabilities | 65,387 | | | 110,051 | |
| Total current liabilities | 107,266 | | | 163,860 | |
| Non-current liabilities: | | | |
| Deferred revenue, net of current portion (includes $72,260 and $119,053 from related parties) | 98,783 | | | 158,062 | |
| Operating lease liabilities, non-current | 438,766 | | | 221,835 | |
| Other non-current liabilities | 16,576 | | | 24,433 | |
| Total liabilities | 661,391 | | | 568,190 | |
| Commitments and contingencies (Note 11) | | | |
| Stockholders’ equity: | | | |
| Preferred stock, $0.0001 par value; 200,000,000 shares authorized; none issued | — | | | — | |
| Common stock, $0.0001 par value (Note 12) | 5 | | | 5 | |
| Additional paid-in capital | 6,555,416 | | | 6,386,191 | |
| Accumulated deficit | (5,837,557) | | | (5,290,528) | |
| Accumulated other comprehensive (loss) income | (1,806) | | | 1,484 | |
| Total stockholders’ equity | 716,058 | | | 1,097,152 | |
| Total liabilities and stockholders’ equity | $ | 1,377,449 | | | $ | 1,665,342 | |
The accompanying notes are an integral part of these consolidated financial statements.
F-6
Ginkgo Bioworks Holdings, Inc.
Consolidated Statements of Operations and Comprehensive Loss
(in thousands, except share data)
| | | | | | | | | | | | | | | | | |
| Year Ended December 31, |
| 2024 | | 2023 | | 2022 |
Cell Engineering revenue (1) | $ | 173,972 | | | $ | 143,531 | | | $ | 143,666 | |
| Biosecurity revenue: | | | | | |
| Service | 53,071 | | | 78,975 | | | 298,585 | |
| Product | — | | | 28,949 | | | 35,455 | |
| Total revenue | 227,043 | | | 251,455 | | | 477,706 | |
| Costs and operating expenses: | | | | | |
| Cost of Biosecurity service revenue | 38,549 | | | 46,524 | | | 183,570 | |
| Cost of Biosecurity product revenue | — | | | 7,481 | | | 20,646 | |
| Cost of other revenue | 5,999 | | | — | | | — | |
| Research and development | 424,061 | | | 580,621 | | | 1,052,643 | |
| General and administrative | 246,161 | | | 385,025 | | | 1,429,799 | |
| Impairment of lease assets | — | | | 96,210 | | | — | |
| Goodwill impairment | 47,858 | | | — | | | — | |
| Restructuring charges | 24,172 | | | — | | | — | |
| Total operating expenses | 786,800 | | | 1,115,861 | | | 2,686,658 | |
| Loss from operations | (559,757) | | | (864,406) | | | (2,208,952) | |
| Other income (expense): | | | | | |
| Interest income | 38,612 | | | 57,217 | | | 20,262 | |
| Interest expense | (94) | | | (93) | | | (106) | |
| Loss on equity method investments | — | | | (2,635) | | | (43,761) | |
| Loss on investments | (28,827) | | | (54,827) | | | (53,335) | |
| (Loss) gain on deconsolidation of subsidiaries | (7,013) | | | (42,502) | | | 31,889 | |
| Change in fair value of warrant liabilities | 5,701 | | | 5,168 | | | 124,970 | |
| Other income, net | 3,870 | | | 9,138 | | | 7,634 | |
| Total other income (expense) | 12,249 | | | (28,534) | | | 87,553 | |
| Loss before income taxes | (547,508) | | | (892,940) | | | (2,121,399) | |
| Income tax benefit | (479) | | | (71) | | | (15,027) | |
| Net loss | (547,029) | | | (892,869) | | | (2,106,372) | |
| Loss attributable to non-controlling interest | — | | | — | | | (1,443) | |
| Net loss attributable to Ginkgo Bioworks Holdings, Inc. stockholders | $ | (547,029) | | | $ | (892,869) | | | $ | (2,104,929) | |
Net loss per share attributable to Ginkgo Bioworks Holdings, Inc. common stockholders: | | | | | |
| Basic | $ | (10.54) | | | $ | (18.37) | | | $ | (50.15) | |
| Diluted | $ | (10.54) | | | $ | (18.37) | | | $ | (50.20) | |
| Weighted average common shares outstanding: | | | | | |
| Basic | 51,894,639 | | 48,610,507 | | 41,976,537 |
| Diluted | 51,894,639 | | 48,610,507 | | 41,995,972 |
| Comprehensive loss: | | | | | |
| Net loss | $ | (547,029) | | | $ | (892,869) | | | $ | (2,106,372) | |
| Other comprehensive (loss) income: | | | | | |
| Foreign currency translation adjustment | (4,782) | | | 4,116 | | | (917) | |
| Reclassification of foreign currency translation adjustment realized upon sale of foreign subsidiary | 1,492 | | | — | | | — | |
The accompanying notes are an integral part of these consolidated financial statements.
F-7
Ginkgo Bioworks Holdings, Inc.
Consolidated Statements of Operations and Comprehensive Loss
(in thousands, except share data)
| | | | | | | | | | | | | | | | | |
| Total other comprehensive (loss) income | (3,290) | | | 4,116 | | | (917) | |
| Comprehensive loss | $ | (550,319) | | | $ | (888,753) | | | $ | (2,107,289) | |
(1)Includes related party revenue of $53,041, $22,222, and $38,813 for the years ended December 31, 2024, 2023, and 2022, respectively.
The accompanying notes are an integral part of these consolidated financial statements.
F-8
Ginkgo Bioworks Holdings, Inc.
Consolidated Statements of Stockholders’ Equity
(in thousands except share data)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Common Stock | | | | | | | | | | |
| Shares | | Amount | | Additional Paid-In Capital | | Accumulated Deficit | | Accumulated Other Comprehensive (Loss) Income | | Non- Controlling Interest | | Total Stockholders’ Equity |
| Balance as of December 31, 2021 | 40,284,804 | | $ | 4 | | | $ | 3,805,001 | | | $ | (2,297,925) | | | $ | (1,715) | | | $ | 62,014 | | | $ | 1,567,379 | |
| Issuance of common stock upon exercise or vesting of equity awards | 3,116,275 | | — | | 252 | | — | | — | | — | | 252 |
| Tax withholdings related to net share settlement of equity awards | (7,390) | | — | | (981) | | — | | — | | — | | (981) |
| Issuance of common stock for business and asset acquisitions, net of issuance costs | 2,862,931 | | — | | 279,745 | | — | | — | | — | | 279,745 |
| Issuance of common stock pursuant to public offering, net of issuance costs | 1,034,597 | | 1 | | 98,909 | | — | | — | | — | | 98,910 |
| Issuance of common stock in exchange for services | 8,182 | | — | | 1,000 | | — | | — | | — | | 1,000 |
| Deconsolidation of subsidiaries | — | | — | | — | | — | | — | | (55,408) | | (55,408) |
| Acquisition of non-controlling interests | — | | — | | 7,390 | | — | | — | | (7,390) | | — |
| Adoption of ASC 842 | — | | — | | — | | 5,195 | | — | | — | | 5,195 |
| Stock-based compensation expense | — | | — | | 1,945,247 | | — | | — | | 2,227 | | 1,947,474 |
| Foreign currency translation | — | | — | | — | | — | | (917) | | — | | (917) |
| Net loss | — | | — | | | — | | | (2,104,929) | | | — | | | (1,443) | | | (2,106,372) | |
| Balance as of December 31, 2022 | 47,299,399 | | 5 | | | 6,136,563 | | | (4,397,659) | | | (2,632) | | | — | | | 1,736,277 | |
| Issuance of common stock upon exercise or vesting of equity awards | 2,469,178 | | — | | 555 | | — | | — | | — | | 555 |
| Tax withholdings related to net share settlement of equity awards | (360) | | — | | (23) | | — | | — | | — | | (23) |
| Settlement of contingent consideration | 96,198 | | — | | 8,896 | | — | | — | | — | | 8,896 |
| Issuance of common stock for asset acquisitions | 119,278 | | — | | 6,820 | | — | | — | | — | | 6,820 |
| Issuance of common stock in exchange for services | 50,587 | | — | | 2,500 | | — | | — | | — | | 2,500 |
| Forfeiture of restricted stock | (1,407) | | — | | — | | — | | — | | — | | — |
| Stock-based compensation expense and other | — | | — | | 230,880 | | — | | — | | — | | 230,880 |
| Foreign currency translation | — | | — | | — | | — | | 4,116 | | — | | 4,116 |
| Net loss | — | | | — | | | — | | | (892,869) | | | — | | | — | | | (892,869) | |
| Balance as of December 31, 2023 | 50,032,873 | | 5 | | | 6,386,191 | | | (5,290,528) | | | 1,484 | | | — | | | 1,097,152 | |
| Issuance of common stock upon exercise or vesting of equity awards | 1,783,763 | | — | | | 543 | | | — | | | — | | | — | | | 543 | |
| Payment for fractional shares after reverse stock split | — | | — | | | (4) | | | — | | | — | | | — | | | (4) | |
| Settlement of contingent consideration | 1,385,532 | | — | | | 14,742 | | | — | | | — | | | — | | | 14,742 | |
The accompanying notes are an integral part of these consolidated financial statements.
F-9
Ginkgo Bioworks Holdings, Inc.
Consolidated Statements of Stockholders’ Equity
(in thousands except share data)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Common Stock | | | | | | | | | | |
| Shares | | Amount | | Additional Paid-In Capital | | Accumulated Deficit | | Accumulated Other Comprehensive (Loss) Income | | Non- Controlling Interest | | Total Stockholders’ Equity |
| Issuance of common stock for asset acquisitions | 802,038 | | — | | | 36,801 | | | — | | | — | | | — | | | 36,801 | |
| Issuance of common stock in exchange for services | 361,579 | | — | | | 4,799 | | | — | | | — | | | — | | | 4,799 | |
| Stock-based compensation expense | — | | — | | | 112,344 | | | — | | | — | | | — | | | 112,344 | |
| Reclassification of foreign currency translation adjustment realized upon sale of foreign subsidiary | — | | — | | | — | | | — | | | 1,492 | | | — | | | 1,492 | |
| Foreign currency translation | — | | — | | | — | | | — | | | (4,782) | | | — | | | (4,782) | |
| Net loss | — | | — | | | — | | | (547,029) | | | — | | | — | | | (547,029) | |
| Balance as of December 31, 2024 | 54,365,785 | | | $ | 5 | | | $ | 6,555,416 | | | $ | (5,837,557) | | | $ | (1,806) | | | $ | — | | | $ | 716,058 | |
The accompanying notes are an integral part of these consolidated financial statements.
F-10
Ginkgo Bioworks Holdings, Inc.
Consolidated Statements of Cash Flows
(in thousands)
| | | | | | | | | | | | | | | | | |
| Year Ended December 31, |
| 2024 | | 2023 | | 2022 |
| Cash flows from operating activities: | | | | | |
| Net loss | $ | (547,029) | | | $ | (892,869) | | | $ | (2,106,372) | |
| Adjustments to reconcile net loss to net cash used in operating activities: | | | | | |
| Depreciation and amortization | 63,020 | | | 70,507 | | | 42,552 | |
| Stock-based compensation | 112,344 | | | 229,884 | | | 1,930,641 | |
| Goodwill impairment | 47,858 | | | — | | | — | |
| Restructuring related impairment charges | 4,823 | | | — | | | — | |
| Non-cash customer consideration | (1,117) | | | (1,373) | | | (34,263) | |
| Loss on equity method investments | — | | | 2,635 | | | 43,761 | |
| Loss on investments | 28,827 | | | 54,827 | | | 53,335 | |
| Change in fair value of notes receivable | 2,014 | | | 2,416 | | | (3,757) | |
| Change in fair value of warrant liabilities | (5,701) | | | (5,168) | | | (124,970) | |
| Change in fair value of contingent consideration liability | 3,214 | | | 9,168 | | | (1,262) | |
| Loss (gain) on deconsolidation of subsidiaries | 7,013 | | | 42,502 | | | (31,889) | |
| Impairment of long-lived assets | 5,796 | | | 121,404 | | | — | |
| Deferred income tax benefit | (936) | | | (801) | | | (14,609) | |
| Loss on disposal of equipment | 844 | | | 842 | | | 3,091 | |
| Non-cash lease expense | 28,095 | | | 28,313 | | | 19,082 | |
| Non-cash in-process research and development | 19,796 | | | 9,182 | | | 1,162 | |
| Non-cash severance and retention bonus expense associated with an acquisition | — | | | — | | | 6,152 | |
| Other non-cash activity | 1,224 | | | 3,194 | | | 2,154 | |
| Changes in operating assets and liabilities: | | | | | |
| Accounts receivable ($156, $816 and $3,040 from related parties) | (4,725) | | | 50,068 | | | 55,024 | |
| Prepaid expenses and other current assets | 10,085 | | | 10,473 | | | (8,523) | |
| Operating lease right-of-use assets | 23,463 | | | 9,275 | | | 13,233 | |
| Other non-current assets | (1,394) | | | 2,570 | | | 921 | |
| Accounts payable | 4,771 | | | (1,183) | | | (10,844) | |
| Accrued expenses and other current liabilities | (40,438) | | | 16,899 | | | (39,639) | |
| Deferred revenue, current and non-current ($(51,422), $(17,018) and $(19,324) from related parties) | (68,645) | | | (35,917) | | | (36,417) | |
| Operating lease liabilities, current and non-current | (14,881) | | | (22,800) | | | (10,792) | |
| Other non-current liabilities | 2,094 | | | 452 | | | 31 | |
| Net cash used in operating activities | (319,585) | | | (295,500) | | | (252,198) | |
| Cash flows from investing activities: | | | | | |
| Purchases of property and equipment | (62,541) | | | (40,801) | | | (52,271) | |
| Deconsolidation of subsidiaries - cash | — | | | (42,980) | | | (55,721) | |
| Business acquisitions, net of cash acquired | (5,400) | | | — | | | 82,367 | |
| Asset acquisitions, net of cash acquired | — | | | — | | | (7,639) | |
| Purchases of notes receivable (2022: $10,000 from related party) | — | | | (350) | | | (40,000) | |
| Proceeds from notes receivable | — | | | — | | | 10,000 | |
| Purchase of investment in equity securities | — | | | — | | | (3,691) | |
| Proceeds from sales of marketable securities | 4,519 | | | — | | | — | |
| Proceeds from sale of equipment | 648 | | | 4,428 | | | — | |
| Other | 538 | | | (990) | | | (439) | |
| Net cash used in investing activities | (62,236) | | | (80,693) | | | (67,394) | |
| | | | | |
| Cash flows from financing activities: | | | | | |
The accompanying notes are an integral part of these consolidated financial statements.
F-11
Ginkgo Bioworks Holdings, Inc.
Consolidated Statements of Cash Flows
(in thousands)
| | | | | | | | | | | | | | | | | |
| Year Ended December 31, |
| 2024 | | 2023 | | 2022 |
| Proceeds from exercise of stock options | 84 | | | 93 | | | 240 | |
| Taxes paid related to net share settlement of equity awards | — | | | (23) | | | (981) | |
| Principal payments on finance leases | (897) | | | (1,295) | | | (1,237) | |
| Proceeds from public offering, net of issuance costs | — | | | — | | | 99,303 | |
| Contingent consideration payment | (922) | | | (1,411) | | | (521) | |
| Payment of equity issuance costs and other | (4) | | | (580) | | | (1,467) | |
| Net cash (used in) provided by financing activities | (1,739) | | | (3,216) | | | 95,337 | |
| Effect of foreign exchange rates on cash and cash equivalents | (281) | | | (588) | | | 908 | |
| Net decrease in cash, cash equivalents and restricted cash | (383,841) | | | (379,997) | | | (223,347) | |
| | | | | |
| Cash and cash equivalents, beginning of year | 944,073 | | | 1,315,792 | | | 1,550,004 | |
| Restricted cash, beginning of year | 45,511 | | | 53,789 | | | 42,924 | |
| Cash, cash equivalents and restricted cash, beginning of year | 989,584 | | | 1,369,581 | | | 1,592,928 | |
| | | | | |
| Cash and cash equivalents, end of year | 561,572 | | | 944,073 | | | 1,315,792 | |
| Restricted cash, end of year | 44,171 | | | 45,511 | | | 53,789 | |
| Cash, cash equivalents and restricted cash, end of year | $ | 605,743 | | | $ | 989,584 | | | $ | 1,369,581 | |
The accompanying notes are an integral part of these consolidated financial statements.
F-12
Ginkgo Bioworks Holdings, Inc.
Notes to Consolidated Financial Statements
1. Organization and Basis of Presentation
Business
The mission of Ginkgo Bioworks Holdings, Inc. (“Ginkgo” or the “Company”) is to make biology easier to engineer. The Company provides biological research and development services for customers across multiple markets and industries. Since inception, the Company has devoted its efforts to improving its platform for programming cells to enable customers to leverage biology to create impactful products across a range of industries. The Company’s platform comprises (i) equipment, robotic automation, software, data pipelines and tools, and standard operating procedures for high throughput cell engineering, fermentation, and analytics (referred to collectively as the “Foundry”), (ii) a library of proprietary biological assets and associated performance data (referred to collectively as “Codebase”), and (iii) the Company’s team of expert users, developers and operators of the Foundry and Codebase.
The Company’s Biosecurity business provides services to government and commercial customers working to identify, monitor, prevent, mitigate, and ultimately protect humanity from biological threats.
2. Summary of Significant Accounting Policies
Basis of Presentation
The accompanying consolidated financial statements have been prepared in conformity with the rules and regulations of the Securities and Exchange Commission (“SEC”) and generally accepted accounting principles in the United States of America (“GAAP”). Any reference in these notes to applicable guidance is meant to refer to the authoritative GAAP as found in the Accounting Standards Codification (“ASC”) of the Financial Accounting Standards Board (“FASB”).
Principles of Consolidation
The accompanying consolidated financial statements include the accounts of the Company, its wholly owned subsidiaries, majority owned subsidiaries and variable interest entities if the Company is the primary beneficiary. All intercompany accounts and transactions have been eliminated.
Reclassifications
Certain prior year amounts have been reclassified for consistency with the current year presentation. In the accompanying consolidated balance sheet, $5.7 million in warrant liabilities as of December 31, 2023 were reclassified from warrant liabilities to other non-current liabilities. Total liabilities as of December 31, 2023 is not changed as a result of this reclassification. In the accompanying consolidated statements of cash flows, $1.0 million and $1.9 million were reclassified from amortization of finance lease right-of-use assets to other non-cash activity for the years ended December 31, 2023 and 2022, respectively. The total cash used in operating activities for the years ended December 31, 2023 and 2022 is not changed as a result of these reclassifications.
Reverse Stock Split
On August 19, 2024 (the “Effective Date”), with the approval of the Company's board of directors and shareholders, the Company effected a one-for-forty (1:40) reverse stock split (the “Reverse Stock Split”) for the Company’s common stock (inclusive of Class A common stock, Class B common stock and Class C common stock, par value $0.0001 per share). Accordingly, all common shares, common stock equity awards and common stock per share amounts presented herein have been retrospectively adjusted to reflect the Reverse Stock Split.
On the Effective Date, every forty shares of common stock issued and outstanding immediately prior to the Effective Date were automatically combined into one share of such class of common stock without any change to the par value per share. The number of shares reserved under the Company’s equity plans and the number of shares underlying awards outstanding under the Company’s equity plans was reduced proportionately. No fractional shares were issued in connection with the Reverse Stock Split. Shareholders entitled to receive a fractional share as a result of the Reverse Stock Split received a cash payment in lieu of such fractional shares. The number of authorized shares of common stock was not reduced.
Ginkgo Bioworks Holdings, Inc.
Notes to Consolidated Financial Statements
Variable Interest Entities
The Company evaluates its variable interests in variable interest entities (“VIE”) and consolidates VIEs when the Company is the primary beneficiary. The Company determines whether it is the primary beneficiary of each VIE based on its assessment of whether the Company possesses both (i) the power to direct the activities that most significantly affect the VIE’s economic performance and (ii) the obligation to absorb losses that could be significant to the VIE or the right to receive benefits that could be significant to the VIE. The Company reevaluates the accounting for its VIEs upon the occurrence of events that could change the primary beneficiary conclusion. As of December 31, 2024 and 2023, the maximum risk of loss related to the Company’s VIEs was limited to the carrying value of its investment in such entities.
Use of Estimates
The preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, revenues and expenses, and the disclosure of contingent liabilities in the consolidated financial statements. The Company bases its estimates on historical experience and other market-specific or relevant assumptions that it believes to be reasonable under the circumstances. Reported amounts and disclosures reflect the overall economic conditions that management believes are most likely to occur, and the anticipated measures management intends to take. Actual results could differ materially from those estimates. All revisions to accounting estimates are recognized in the period in which the estimates are revised.
Concentrations of Credit Risk
Financial instruments that potentially subject the Company to concentrations of credit risk primarily consist of cash and cash equivalents, restricted cash, trade accounts receivable and notes receivable. The Company’s cash and cash equivalents and restricted cash are maintained in bank deposit accounts and money market funds that regularly exceed federally insured limits. The Company is exposed to credit risk on its cash, cash equivalents and restricted cash in the event of default by the financial institutions to the extent account balances exceed the amount insured by the Federal Deposit Insurance Corporation. The Company believes that it is not exposed to significant credit risk as its deposits are generally held in financial institutions that management believes to be of high credit quality. To date, the Company has not experienced any material write-offs related to its trade accounts receivable. A portion of the Company’s Biosecurity revenue is derived from sales of services to foreign government agencies in certain developing countries. The Company’s maximum credit risk exposure with respect to notes receivable is equivalent to the carrying value of the notes as of the balance sheet date. The Company mitigates this risk by requiring collateral for certain notes and monitoring the counterparty’s financial condition.
For the year ended December 31, 2024, two customers in the Cell Engineering segment accounted for 13% and 20% of the Company’s total revenue, while one customer in the Biosecurity segment accounted for 16% of the Company’s total revenue. For the year ended December 31, 2023, one customer in the Cell Engineering segment and one customer in the Biosecurity segment accounted for 12% and 11%, respectively, of the Company's total revenue. For the year ended December 31, 2022, two customers in the Biosecurity segment each accounted for 11% of the Company’s total revenue.
Cash and Cash Equivalents
The Company’s cash is comprised of bank deposits, overnight sweep accounts and money market funds. The Company considers all highly liquid investments with original maturities of three months or less at the date of purchase to be cash equivalents. The carrying value of the Company’s cash and cash equivalents approximate fair value due to their short-term maturities.
Restricted Cash
Restricted cash primarily includes cash balances collateralizing letters of credit associated with the Company’s facility leases and customer prepayments requiring segregation and restrictions in its use in accordance with the customer agreement. Restricted cash is included in prepaid expenses and other current assets and other non-current assets on the consolidated balance sheet.
Allowance for Credit Losses
The Company maintains an allowance for credit losses to provide for the estimated amounts of receivables that will not be collected over the estimated life of the assets. The allowance is calculated by considering previous loss history, delinquency of receivables balances, current economic conditions and anticipated future economic conditions in the geographies and industries in which the Company’s customers operate. To the extent an individual customer’s credit
Ginkgo Bioworks Holdings, Inc.
Notes to Consolidated Financial Statements
quality deteriorates, the Company measures an allowance based on the risk characteristics of the individual customer. Once a receivable is deemed to be uncollectible, such balance is charged against the allowance. The allowance is calculated at each reporting period with changes recorded to general and administrative expense in the consolidated statements of operations and comprehensive loss. Accounts receivable are net of an allowance for credit losses of $1.9 million and $1.0 million at December 31, 2024 and 2023, respectively.
Property, Plant and Equipment, net
Property, plant and equipment are stated at cost less accumulated depreciation. Land is stated at cost. Depreciation is computed using the straight-line method over the estimated useful lives of the assets or the remaining lease term for leasehold improvements. Estimated lives of property, plant and equipment are as follows:
| | | | | |
| Estimated Useful Life |
| Computer equipment and software | 2 to 5 years |
| Furniture and fixtures | 7 years |
| Lab equipment | 1 to 5 years |
| Buildings and facilities | 15 to 30 years |
| Vehicles | 5 years |
| Leasehold improvements | Shorter of useful life or remaining lease term |
Expenditures for maintenance and repairs are expensed as incurred. When assets are retired or otherwise disposed of, the related cost and accumulated depreciation is removed from the balance sheet and any resulting gain or loss is recorded in the consolidated statements of operations and comprehensive loss. Construction in progress relates to assets which have not been placed in service as of the period end.
Equity Method Investments
The Company utilizes the equity method to account for its investments in common stock, or in-substance common stock, when it possesses the ability to exercise significant influence, but not control, over the operating and financial policies of the investee. The Company uses judgment when determining the level of influence over the operating and financial policies of the investee considering key factors including, among others, the Company’s ownership interest, representation on the board of directors, participation in policy-making decisions and material contractual arrangements and obligations. Income and losses are allocated based upon relative ownership interest unless there is a substantive profit-sharing agreement in place.
For investments with a substantive profit-sharing agreement, the Company utilizes the hypothetical liquidation at book value (“HLBV”) method to allocate income and losses from the equity method investment. Under the HLBV method, the Company utilizes the capital account at the end of the period assuming the book value of the entity was liquidated or sold minus the same calculation at the beginning of the period. The difference is the share of earnings or losses attributable to the equity method investment.
Under the equity method, if there is a commitment for the Company to fund the losses of its equity method investees, the Company would continue to record its share of losses resulting in a negative equity method investment, which would be presented as a liability on the consolidated balance sheet. Commitments may be explicit and may include formal guarantees, legal obligations, or arrangements by contract. Implicit commitments may arise from reputational expectations, intercompany relationships, statements by the Company of its intention to provide support, a history of providing financial support or other facts and circumstances. When the Company has no commitment to fund the losses of its equity method investees, the carrying value of its equity method investments will not be reduced below zero. The Company had no commitment to fund additional losses of its equity method investments during the years ended December 31, 2024, 2023 and 2022, other than dissolution costs for Joyn Bio, LLC in 2022 (see Notes 4 and 7). The Company evaluates its equity method investments for impairment whenever events or circumstances indicate that the carrying value of the investment may not be recoverable. The Company considers the investee’s financial position, forecasts and economic outlook, and the estimated duration and extent of losses to determine whether a recovery is anticipated. An impairment that is other-than-temporary is recognized in the period identified. The Company has not
Ginkgo Bioworks Holdings, Inc.
Notes to Consolidated Financial Statements
recognized an impairment loss related to its equity method investments for the years ended December 31, 2024, 2023 and 2022.
The Company may elect the fair value option for its equity method investments on an investment-by-investment basis. For all equity method investments accounted for under the fair value option, the Company carries the equity method investment at fair value and records all subsequent changes in fair value as a component of loss on equity method investments in the consolidated statements of operations and comprehensive loss.
Investments
Investments include marketable equity securities in publicly-traded companies, non-marketable equity securities in privately-held companies, Simple Agreement for Future Equity (“SAFE”) and warrants, in each case, in which the Company does not possess the ability to exercise significant influence over the investee.
Investments in marketable equity securities or warrants of publicly-traded companies are measured at fair value with subsequent changes in fair value recorded in loss on investments in the consolidated statements of operations and comprehensive loss. Marketable equity securities are classified as non-current on the balance sheet as they are not currently available for sale.
Investments in non-marketable equity securities of privately-held companies and SAFEs, which do not have readily determinable fair values, are carried at cost, less any impairments, plus or minus changes resulting from observable price changes in orderly transactions for the identical or a similar investment of the same issuer. Each period the Company assesses relevant transactions to identify observable price changes, and the Company regularly monitors these investments to evaluate whether there is an indication of impairment. The Company evaluates whether an investment’s fair value is less than its carrying value using an estimate of fair value, if such an estimate is available. For periods in which there is no estimate of fair value, the Company evaluates whether an event or change in circumstances has occurred that may have a significant adverse effect on the value of the investment. See Notes 5 and 6 for additional information on Investments. Fair Value Measurements
The Company categorizes its assets and liabilities measured at fair value in accordance with the authoritative accounting guidance that establishes a consistent framework for measuring fair value and requires disclosures for each major asset and liability category measured at fair value on either a recurring or nonrecurring basis.
ASC 820, Fair Value Measurement (“ASC 820”), establishes a fair value hierarchy for instruments measured at fair value that distinguishes between assumptions based on market data (observable inputs) and the Company’s own assumptions (unobservable inputs). Observable inputs are inputs that market participants would use in pricing the asset or liability based on market data obtained from sources independent of the Company. Unobservable inputs are inputs that reflect the Company’s assumptions about the inputs that market participants would use in pricing the asset or liability and are developed based on the best information available in the circumstances.
ASC 820 identifies fair value as the exchange price, or exit price, representing the amount that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants. As a basis for considering market participant assumptions in fair value measurements, ASC 820 establishes a three-tier fair value hierarchy that distinguishes among the following:
•Level 1- Quoted prices (unadjusted) in active markets for identical assets or liabilities;
•Level 2- Inputs other than quoted prices included within Level 1 that are either directly or indirectly observable; and
•Level 3- Unobservable inputs in which little or no market activity exists, therefore requiring an entity to develop its own assumptions about the assumptions that market participants would use in pricing.
To the extent that the valuation is based on models or inputs that are either less observable or unobservable in the market, the determination of fair value requires more judgment. Accordingly, the degree of judgment exercised by the Company in determining fair value is greatest for instruments categorized in Level 3. A financial instrument’s level within the fair value hierarchy is based on the lowest level of any input that is significant to the fair value measurement.
Ginkgo Bioworks Holdings, Inc.
Notes to Consolidated Financial Statements
The Company valued its money market fund holdings, notes receivable, marketable equity securities, warrant liabilities and contingent consideration liabilities at fair value on a recurring basis. The carrying amounts of the Company’s other financial instruments, which include accounts receivable, certain prepaid expenses and other current assets, accounts payable and accrued expenses and other current liabilities approximate their fair values due to their short-term nature.
Impairment of Long-Lived Assets
The Company reviews its long-lived assets or asset groups for impairment whenever events or changes in circumstances indicate that the carrying value of the assets may not be recoverable. Recoverability is measured by comparing the carrying value of the long-lived assets to the future undiscounted cash flows expected to be generated by the assets. In determining the expected future cash flows, the Company uses assumptions believed to be reasonable, but which inherently uncertain. Actual future cash flows may differ from the estimates used in impairment testing. The Company recognizes an impairment loss when and to the extent that the estimated fair value of long-lived asset is less than the carrying value. See Notes 3, 9 and 10 for a description of impairment losses recorded on long-lived assets. Business Combinations
The Company accounts for business combinations using the acquisition method of accounting. The Company recognizes the identifiable assets acquired and liabilities assumed at their acquisition-date fair values and recognizes any excess of the total consideration paid over the fair value of the identifiable net assets as goodwill. Any purchase price that is considered contingent consideration is measured at its estimated fair value at the acquisition date and remeasured at each reporting period, with changes in estimated fair value recorded in general and administrative expenses in the consolidated statements of operations and comprehensive loss. Acquisition transaction costs are expensed when incurred. The operating results of an acquisition are included in the Company’s consolidated financial statements as of the acquisition date.
Intangible Assets, net
Intangible assets, net consist of certain definite-lived assets including patents, processes and know-how related to technology acquired through business combinations and asset acquisitions. The Company amortizes such intangible assets on a straight-line basis over their estimated useful life.
Goodwill
Goodwill represented the excess of the acquisition cost over the fair market value of the net assets acquired. All goodwill was allocated to the Cell Engineering reporting unit and segment identified in Note 15. The Company considers various qualitative factors that could indicate impairment of goodwill such as macroeconomic conditions, industry and market environment, overall financial performance of the Company, cash flow from operating activities and market capitalization. If the qualitative assessment indicates that it is more likely than not that the fair value of the reporting unit is less than its carrying amount, the Company performs a quantitative assessment to compare the fair value of the reporting unit to its carrying value, including goodwill. If the carrying value of the reporting unit exceeds the fair value, an impairment loss is recognized limited to the total amount of goodwill allocated to that reporting unit. A combination of the income approach and the market approach may be used to determine fair value of the reporting unit. The Company recorded a full goodwill impairment during the year ended December 31, 2024 (see Note 8). No impairment losses were recognized during the years ended December 31, 2023, and 2022. Leases
The Company adopted Accounting Standards Update (“ASU”) 2016-02, Leases (Topic 842), (“ASC 842”) on January 1, 2022 using the modified retrospective approach with a cumulative-effect adjustment to the opening balance of accumulated deficit in the period of adoption.
In accordance with ASC 842, the Company determines if an arrangement is or contains a lease at contract inception based on the terms and conditions in the contract. A contract contains a lease if there is an identified asset and the Company has the right to control the asset. For leases with terms greater than 12 months, the Company recognizes a right-of-use asset (“ROU asset”) and a lease liability as of the lease commencement date on the balance sheet. ROU assets represent the Company’s right to use an underlying asset over the lease term and lease liabilities represent the Company’s obligation to make lease payments arising from the lease. Lease ROU assets and liabilities are measured based on the present value of fixed lease payments that are unpaid as of the lease commencement date. The Company’s ROU assets balance is increased by any initial direct costs and reduced by lease incentives received or expected to be received. Some of the Company's
Ginkgo Bioworks Holdings, Inc.
Notes to Consolidated Financial Statements
leases include options to extend or terminate the lease; these options are included in the lease term for calculations of its ROU assets and liabilities when it is reasonably certain that the Company will exercise those options.
The Company’s leases are classified as either operating or finance, as determined at inception, with the classification affecting the pattern of expense recognition in the statement of operations. A lease is classified as a finance lease if risks and rewards are conveyed without the transfer of control. For operating leases, expense is generally recognized on a straight-line basis over the lease term. For finance leases, interest on the lease liability is recognized using the effective interest method, while the ROU asset is amortized on a straight-line basis from the commencement date to the earlier of the end of the useful life of the ROU asset or the end of the lease term. Leases with an initial term of 12 months or less which meet the definition of a short-term lease are not recorded on the balance sheet and the lease expense for these leases is recognized on a straight-line basis over the lease term. In limited instances, the Company acts as a lessor, primarily with certain real estate subleases. Finance leases, short-term leases and subleases are not a significant component of the Company's financial condition or results of operations. The current portion of the Company’s operating lease liabilities is included in accrued expenses and other current liabilities on the balance sheet.
The Company has lease agreements with both lease and non-lease components (such as real estate taxes, insurance and common area maintenance charges) and has elected the practical expedient to combine these lease and non-lease components for its real estate leases and non-lab equipment leases. The Company has not elected this practical expedient for lab equipment leases and the lease and non-lease components are accounted for separately for these leases. Non-lease components are typically variable in nature and are recognized as lease expense in the period in which they arise.
As most of the Company’s leases do not provide an implicit interest rate, the Company uses an incremental borrowing rate based on the information available at lease commencement date in determining the present value of lease payments and uses the implicit rate when it is readily determinable. The Company’s incremental borrowing rate is based on management’s estimate of the rate of interest the Company would have to pay to borrow on a fully collateralized basis over a similar term an amount equal to the lease payments in a similar economic environment.
Revenue Recognition
The Company accounts for revenue in accordance with ASC 606, Revenue from Contracts with Customers (“ASC 606”). Under ASC 606, the Company recognizes revenue when the customer obtains control of the promised goods or services at an amount that reflects the consideration the Company expects to receive in exchange for those goods or services. To determine revenue recognition for arrangements that are within the scope of ASC 606, the Company performs the following five steps: (i) identify the contract(s) with a customer, (ii) identify the promises and distinct performance obligations in the contract, (iii) determine the transaction price, (iv) allocate the transaction price to the performance obligations in the contract, and (v) recognize revenue when (or as) the Company satisfies the performance obligations.
Cell Engineering Revenue
The Company generates Cell Engineering service revenue by providing end-to-end cell engineering solutions and tools to customers.
Cell engineering solutions offerings consist of the Company's license and collaboration agreements, under which customers obtain rights to the Company’s proprietary technology and intellectual property for use in the research, development and commercialization of engineered organisms and derived products. Under these agreements, the Company typically provides research and development services, including granting a license to its intellectual property. Additionally, the customer obtains license rights to the output of the Company’s services in order to commercialize the resulting output of such services. Generally, the terms of these agreements provide that the Company receives some combination of: (1) service fees in the form of (i) upfront payments upon consummation of the contract or other fixed payments, (ii) reimbursement for costs incurred for research and development services and (iii) milestone payments upon the achievement of specified technical criteria, plus (2) downstream value share payments in the form of (i) milestone payments upon the achievement of specified commercial criteria, (ii) royalties on sales of products from or comprising engineered organisms arising from the collaboration or licensing contract and (iii) royalties related to cost of goods sold reductions realized by customers.
Cell engineering tools, launched in the third quarter of 2024, consist of several service offerings, including Datapoints, an artificial intelligence (AI) model application programming interface (API), and lab automation solutions. Datapoints' data generation products provide large, biological datasets for customers to train their AI models, synthesizing and testing the output of customer existing models, and generating datasets for lead selection, hit selection, or a variety of other data science applications. The Company's model API provides users with access to both publicly available models and Ginkgo’s
Ginkgo Bioworks Holdings, Inc.
Notes to Consolidated Financial Statements
own protein sequence large language model (LLM) trained on Ginkgo’s proprietary datasets. The Company's lab automation solutions combine modular hardware, control software and managed support to provide customers the ability to automate their own lab workflows in house. The amount of revenue recognized from these new Cell Engineering offerings is not significant for the year ended December 31, 2024.
The Company’s collaboration and licensing agreements often contain multiple promises, including (i) licenses and assignments of intellectual property and materials and (ii) research and development services, and the Company determines whether each of the promises is a distinct performance obligation based on the nature of each contract. As the Company is generally performing research and development services that are highly integrated and interrelated to the licenses and assignments of intellectual property and materials, the promises are generally inseparable and therefore not distinct. As such, the Company typically combines the research and development services, licenses, and assignments into a single performance obligation. However, for certain contracts, the Company only grants licenses or effects such transfers and assignments upon the successful completion of the research and development services or delivery of a developed product. For these contracts, the Company typically considers (i) the research and development services and (ii) the licenses, transfers, and assignments as distinct performance obligations, as each is transferred separately and has a separately identifiable benefit to the customer.
Options to acquire additional distinct goods and services are evaluated to determine if such options provide a material right to the customer that it would not have otherwise received without entering into the contract. If so, the option is accounted for as a separate performance obligation. If not, the option is considered a marketing offer which is accounted for as a separate contract upon the customer’s election of the option.
At contract inception, the Company determines the transaction price, including fixed consideration and any estimated amounts of variable consideration. Any upfront cash payment received upon consummation of the contract is fixed and generally nonrefundable. Variable consideration is subject to a constraint, and amounts are included in the transaction price to the extent that it is probable that a significant reversal in the amount of cumulative revenue recognized will not occur. This is attained when the uncertainty associated with the variable consideration is subsequently resolved. Variable consideration may include reimbursement for costs incurred for the Company’s research and development efforts, milestone payments upon the achievement of certain technical and/or commercial criteria, and royalties on sales of products from or comprising engineered organisms arising from the contract. With respect to the research and development reimbursements and milestone payments, the Company uses the most likely amount method to estimate variable consideration. Milestone payments are generally not included in the transaction price until the milestone is achieved.
Certain agreements include payment in the form of equity securities or other financial instruments that convert into equity upon a triggering event. Any non-cash consideration is measured at its estimated fair value at contract inception. For equity securities and financial instruments that are not actively traded, the estimated fair value is generally determined by referencing a recent financing round or utilizing a scenario-based valuation model. Significant unobservable inputs are used in these valuations, including expectations regarding future financings of the customer, scenario dates and probabilities, expected volatility, discount rates, and recovery rates. Changes in these assumptions can materially affect the fair value of the non-cash consideration and, consequently, the total revenue recognized for the contract. The Company did not have material non-cash consideration included in contracts entered into during 2024, but does continue to recognize non-cash revenue from contracts originating in prior periods.
For agreements with promises that are combined into a single performance obligation, the entire transaction price is allocated to the single performance obligation. For agreements with multiple performance obligations, the transaction price is allocated to the performance obligations using the relative standalone selling price methodology. For agreements featuring variable consideration, the Company allocates variable consideration to one or more, but not all, performance obligations if certain conditions are met. Specifically, the Company assesses whether the variable consideration relates solely to its efforts to satisfy the performance obligation and whether allocating such variable consideration entirely to the performance obligation is consistent with the overall allocation objective. If these conditions are not met, the Company allocates the variable consideration based on the relative standalone selling price methodology. The key assumptions utilized in determining the standalone selling price for each performance obligation include development timelines, estimated research and development costs, commercial markets, likelihood of exercise (in the case of options considered to be material rights), and probabilities of success.
For agreements where licenses or assignments are considered separate performance obligations or represent the only performance obligation, the Company recognizes revenue at the point in time when the license is effectively granted, as the
Ginkgo Bioworks Holdings, Inc.
Notes to Consolidated Financial Statements
licenses or assignments represent functional intellectual property. For agreements where licenses and research and development services are combined into a single performance obligation, the Company recognizes revenue over the performance period using the cost-to-cost method. This method measures progress based on the ratio of costs incurred to date to total estimated costs, as it best reflects the transfer of control to the customer for obligations satisfied over time.
The Company evaluates its measure of progress to recognize revenue at each reporting period and, as necessary, adjusts the measure of progress and related revenue recognition. The Company’s measure of progress and revenue recognition involves significant judgment and assumptions, including, but not limited to, estimated costs to complete its performance obligations. The Company evaluates contract modifications and amendments to determine whether any changes should be accounted for prospectively or on a cumulative catch-up basis. The Company utilizes the right to invoice practical expedient when it has a right to consideration in an amount that corresponds directly with the value of the Company’s performance to date.
Royalties are recognized as revenue when (or as) the later of the sales occurrence or the satisfaction (or partial satisfaction) of the related performance obligation. The Company has determined that applying this exception is appropriate when the license granted in the contract is the predominant item to which the royalties relate.
As the Company receives upfront payments for technical services under certain of its arrangements, the Company evaluates whether any significant financing components exist given the term over which the fees will be earned may exceed one year. Based on the nature of the Company’s agreements, there are no significant financing components as the purpose of the upfront payment is not to provide financing, but rather to secure technical services, exclusivity rights, and Foundry capacity, or the timing of transfer of those goods or services is at the discretion of the customer.
Deferred revenue represents consideration received by the Company in excess of revenue recognized and primarily results from transactions where the Company receives upfront cash payments or non-cash equity consideration. In instances where the Company has received consideration in advance for an undefined number of technical development plans (“TDPs”) under its customer agreements, the Company records the advance payments as deferred revenue, net of current portion on the consolidated balance sheet. Upon the execution of a specific TDP, the Company reclassifies the estimated consideration to be earned under that TDP within the next twelve months as current deferred revenue. The Company also classifies unexercised material rights related to future TDPs as deferred revenue, net of current portion on the consolidated balance sheet. When a TDP is executed, and the material right is exercised, the amount allocated to the material right, which will be earned within the next twelve months, is reclassified to current deferred revenue. All other deferred revenue is classified as current or non-current based on the timing of when the Company expects to earn the underlying revenue based upon the projected progress of activities under the TDP.
As of December 31, 2024 any costs to obtain contracts with customers were immaterial.
Biosecurity Revenue
The Company generates Biosecurity revenue through its biomonitoring and bioinformatics services provided to both government and non-government customers through the Company's two core offerings: Canopy and Horizon.
Prior to 2024, product revenue consisted of sales of lateral flow assay (“LFA”) diagnostic test kits, polymerase chain reaction (“PCR”) sample collection kits, and pooled test kits, which the Company sold to customers on a standalone basis. Product revenue was billed and recognized when the test kits were shipped, and risk of loss was transferred to the carrier. The Company’s test kits were generally not subject to a customer right of return except for product recalls under the rules and regulations of the U.S. Food and Drug Administration (“FDA”). The Company included shipping and handling fees billed to customers as a component of Biosecurity revenue.
Biosecurity service revenue generally consists of various biomonitoring and bioinformatics services including, but not limited to, sample collection, sample storage and transportation, outsourced laboratory analysis, access to results reported through a web-based portal, analytical reporting of results, and overall program management. The various services are generally combined into one performance obligation as they are either not distinct or have substantially the same pattern of transfer to the customer. Service revenue is generally recognized over time using the time elapsed method as the related services are performed, which best depicts the pattern of transfer to the customer.
Ginkgo Bioworks Holdings, Inc.
Notes to Consolidated Financial Statements
The Company’s contracts with customers are generally two years or less in length and contain a fixed amount of consideration. Under typical payment terms for testing services, amounts are billed monthly in arrears for services performed or in advance based on contractual billing terms.
Options to acquire additional services are evaluated to determine whether they provide the customer with a material right that it would not have otherwise received without entering into the contract. If so, the option is accounted for as a separate performance obligation. If not, the option is considered a marketing offer, and upon the customer's election of the option, it is accounted for as a separate contract.
Cost of Biosecurity Revenue
The cost of Biosecurity service revenue consists of costs related to the Company's biomonitoring and bioinformatics services. This includes costs incurred for sample collection equipment, services and materials, outsourced laboratory analysis, access to results reported through a proprietary web-based portal, and reporting of results to public authorities. Additionally, the cost of Biosecurity service revenue includes direct labor cost associated with bioinformatics, lab network management, delivery logistics, and customer support. Prior to 2024, cost of Biosecurity product revenue consisted of costs associated with the sale of diagnostic and sample collection test kits, which included costs incurred to purchase test kits from third parties.
Cost of Other Revenue
Cost of other revenue consists of costs related to the Company's Cell Engineering tools offerings, including Datapoints and lab automation solutions. Such costs primarily include hardware, software, materials and labor. Costs related to the Company’s end-to-end cell engineering solutions offering are included in research and development expenses.
Research and Development Costs
Research and development costs are expensed as incurred. Research and development costs consist of direct and indirect internal costs related to specific projects and initiatives, acquired intellectual property deemed to be in-process research and development, as well as fees paid to other entities that conduct certain research and development activities on the Company’s behalf.
Patent Costs
The Company expenses all costs as incurred in connection with the filing, prosecution, maintenance, defense, and enforcement of patent applications, including direct application fees and related legal and consulting expenses. Patent costs are included in general and administrative expenses within the consolidated statements of operations and comprehensive loss.
Stock-Based Compensation
The Company measures and recognizes compensation expense for all stock-based awards based on estimated grant-date fair values recognized over the requisite service period. For awards that vest solely based on a service condition, the Company recognizes compensation expense on a straight-line basis over the requisite service period. For awards that vest based on multiple conditions, the Company recognizes compensation expense using the accelerated attribution method on a tranche-by-tranche basis over the requisite service period such that the amount of compensation expense recognized at each reporting period is at least equal to the vested tranches at that date. For awards with a performance-based vesting condition, the Company recognizes stock-based compensation when achievement of the performance condition is deemed probable. Upon achieving a performance condition that was not previously considered as probable, the Company records a cumulative catch-up adjustment to reflect the portion of the grantee’s requisite service that has been provided to date. For awards with market conditions, the compensation expense recognized over the requisite service period is not reversed if the market condition is not satisfied. The Company recognizes forfeitures as they occur.
The Company estimates the grant date fair value of stock options using the Black-Scholes option-pricing model, inclusive of assumptions for expected term, expected volatility, risk-free interest rate and expected dividend yield. The expected term is determined using the “simplified” method, which estimates the expected term as the average of the vesting term plus the contractual term. The Company uses the “simplified” method as it does not have sufficient historical data regarding employee exercise behavior. Expected volatility is based on the historical volatility of the Company's Class A common stock. The risk-free interest rate is based on the yield available on U.S. Treasury zero-coupon issues similar in duration to
Ginkgo Bioworks Holdings, Inc.
Notes to Consolidated Financial Statements
the expected term of the stock options. The Company has not paid, and does not expect to pay, dividends in the foreseeable future.
For awards with market conditions, the Company recognizes stock-based compensation based on the estimated grant-date fair value of the awards, determined using a Monte Carlo simulation model. This model incorporates assumptions for expected stock price volatility, risk-free interest rates, expected term, and expected dividend yield. Volatility is estimated using either the historical volatility of the Company’s Class A common stock or a weighted average of its own historical volatility and the historical volatility of selected comparable publicly traded companies, particularly for awards granted when there was limited trading history for the Company’s Class A common stock. The risk-free interest rate is derived from the yield on U.S. Treasury zero-coupon securities with a duration similar to the expected term of the awards. The expected term equals the contractual term, and a dividend yield of zero is assumed.
Income Taxes
The Company accounts for income taxes using the asset and liability method, which requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been recognized in the consolidated financial statements or in the Company’s tax returns. Under this method, deferred tax assets and liabilities are determined based on differences between the financial statement carrying amounts and the tax bases of the assets and liabilities using the enacted tax rates in effect in the years in which the differences are expected to reverse. A valuation allowance against deferred tax assets is recorded if, based on the weight of the available evidence, it is more likely than not that some or all of the deferred tax assets will not be realized. Potential for recovery of deferred tax assets is evaluated by considering several factors, including estimating the future taxable profits expected, estimating future reversals of existing taxable temporary differences, considering taxable profits in carryback periods, and considering prudent and feasible tax planning strategies.
The Company accounts for uncertain tax positions using a more-likely-than-not threshold for recognizing and resolving uncertain tax positions. The evaluation of uncertain tax positions is based on factors including, but not limited to, changes in the law, the measurement of tax positions taken or expected to be taken in tax returns, the effective settlement of matters subject to audit, new audit activity, and changes in facts or circumstances related to a tax position. The Company evaluates uncertain tax positions on an annual basis and adjusts the level of the liability to reflect any subsequent changes in the relevant facts surrounding the uncertain positions. The Company accounts for interest and penalties related to uncertain tax positions as part of its provision for income taxes. As of December 31, 2024 and 2023, the Company did not have any uncertain tax positions. The Company does not expect a material change in unrecognized tax benefits in the next twelve months.
Comprehensive Loss
Comprehensive loss is defined as the change in equity during a period from transactions and other events and circumstances from non-owner sources. Other comprehensive loss consists of foreign currency translation adjustments.
Net Loss per Share
The Company computes basic net loss per share by dividing the net loss attributable to Ginkgo Bioworks Holdings, Inc. common stockholders by the weighted average number of common shares outstanding during the period. For the purposes of the net loss per share calculation, the Company has combined Class A common stock, Class B common stock, and Class C common stock, as all classes of common stock are legally entitled to equal per-share distributions, whether through dividends or liquidation.
Diluted net loss per share is computed using the weighted average number of common shares outstanding during the period, increased to include the effect of dilutive potential common shares, such as outstanding stock options, unvested restricted stock awards, unvested restricted stock units, warrants, and contingently issued shares. Dilutive securities are excluded from the calculation of diluted weighted average common shares outstanding if their effect would be anti-dilutive under the treasury stock method.
Recent Accounting Pronouncements
Recently Adopted Accounting Pronouncements
In November 2023, the FASB issued ASU No. 2023-07, Segment Reporting (Topic 280). This standard requires that a public entity disclose, on an annual and interim basis, significant segment expenses that are regularly provided to the chief
Ginkgo Bioworks Holdings, Inc.
Notes to Consolidated Financial Statements
operating decision maker (“CODM”) and included within each reported measure of segment profit or loss, as well as other segment items and a description of its components. Additionally, it requires disclosure of the title and position of the CODM and an explanation of how the CODM uses the reported measure(s) of segment profit or loss in assessing segment performance and deciding how to allocate resources. The ASU does not change how a public entity identifies its operating segments, aggregates those operating segments, or applies the quantitative thresholds to determine its reportable segments. The Company adopted the standard on January 1, 2024. The adoption did not have a material impact on the Company’s consolidated financial statements or related disclosures.
In June 2022, the FASB issued ASU No. 2022-03, Fair Value Measurement of Equity Securities Subject to Contractual Sale Restrictions. This standard clarifies that contractual restrictions on the sale of an equity security are not considered part of the unit of account for the equity security and, therefore, are not factored into the measurement of fair value. It also introduced new disclosure requirements for equity securities subject to such contractual sale restrictions. The Company adopted this standard on January 1, 2024. The adoption did not have a material impact on the Company’s consolidated financial statements or related disclosures.
Recently Issued Accounting Pronouncements
In November 2024, the FASB issued ASU No. 2024-03, Income Statement - Reporting Comprehensive Income - Expense Disaggregation Disclosures (Subtopic 220-40): Disaggregation of Income Statement Expenses. This ASU requires public business entities to disaggregate, on both an interim and annual basis, each relevant expense caption presented on the face of the income statement into specific expense categories. Additionally, entities are required to provide a qualitative description of amounts remaining in relevant expense captions that are not separately disaggregated quantitatively, as well as disclosure of the total amount of selling expenses and, in annual reporting periods, an entity's definition of selling expenses. The amendments in this ASU are effective for annual reporting periods beginning after December 15, 2026, and interim reporting periods beginning after December 15, 2027. Early adoption is permitted. The Company is currently evaluating the impact that this ASU will have on its disclosures in the consolidated financial statements.
In December 2023, the FASB issued ASU No. 2023-09, Income Taxes (Topic 740), which focuses on improvements to income tax disclosures primarily related to the rate reconciliation and income taxes paid information. The amendments in this ASU require that public business entities on an annual basis (1) disclose specific categories in the tabular rate reconciliation, using both percentages and reporting currency amounts, and (2) provide additional information for reconciling items that meet a quantitative threshold. In addition, all entities are required to disclose income taxes paid, net of refunds received disaggregated by federal, state/local, and foreign and by jurisdiction if the amount is at least 5% of total income tax payments, net of refunds received. The amendments in this ASU are effective for annual periods beginning after December 15, 2024, with early adoption permitted. The amendments should be applied on a prospective basis, with retrospective application permitted. The Company is currently evaluating the impact that this ASU will have on its disclosures in the consolidated financial statements.
3. Restructuring
In the second quarter of 2024, in connection with the Company’s plans to reduce operational expenditures, management, with the approval of the Board of Directors, approved and commenced a restructuring plan. This plan includes a reduction in labor expenses, primarily through a workforce reduction of more than 40%, and the consolidation and subleasing of certain facilities. Initial workforce reductions commenced in June 2024 and continued throughout 2024, with further reductions expected in 2025. All workforce reductions are expected to be substantially completed in 2025, subject to compliance with applicable laws. The Company plans to consolidate certain facilities through various actions, including combining office and laboratory operations into fewer locations, subleasing unused or underutilized facilities, and has taken or plans to take other related measures, such as the sale of its subsidiary, Altar, in the third quarter of 2024 (see Note 4). While the Company aims to complete the majority of its facility consolidation actions in 2025, the actual timing may vary, especially for subleasing unused or underutilized facilities, which may extend beyond 2025 or may not occur prior to termination of such lease, depending on market conditions. Additionally, restructuring expenses related to potential asset impairments or contract amendments or terminations for any facilities no longer in use or underutilized could be material.
The costs for the reduction in force are expected to range from $20.0 million to $23.0 million primarily in the Cell Engineering segment and consist of cash severance and related costs. The employee termination costs are recognized as of the communication date to employees, given (i) the Company instituted a one-time employee termination benefit related to its restructuring, and (ii) the employees will not be retained to render service beyond a minimum retention period. The Company is currently unable to estimate the costs associated with consolidating its facilities. These costs may include, but
Ginkgo Bioworks Holdings, Inc.
Notes to Consolidated Financial Statements
are not limited to, losses on subleases, contract terminations, asset impairments, sale or disposal of equipment or other long-lived assets, and related costs and fees pertaining to the consolidation, closure, or disposition of facilities. Additional charges may be incurred as the Company progresses its restructuring plan and such charges could be material.
The following table presents restructuring costs incurred during the year ended December 31, 2024, which are recorded as “Restructuring charges” in the consolidated statements of operations and comprehensive loss (in thousands):
| | | | | |
| Year Ended December 31, 2024 |
| Employee termination costs and other | $ | 19,349 | |
| Impairment of right-of-use asset (1) | 4,823 | |
| Total restructuring | $ | 24,172 | |
(1) Relates to the sublease of a facility in connection with the restructuring and reflects the excess of the right-of-use asset's carrying value over its fair value, which was determined based on estimates of future discounted cash flows and is classified as Level 3 in the fair value hierarchy.
Additionally, the Company recorded a $7.0 million loss on the sale and deconsolidation of Altar as a component of other income (expense) in the consolidated statements of operations and comprehensive loss for year ended December 31, 2024.
The following table presents the change in the accrued liability balance related to the restructuring activities, which is included in “Accounts payable” and “Accrued expenses and other current liabilities” in the accompanying consolidated balance sheet as of December 31, 2024 (in thousands):
| | | | | |
| Employee Termination Costs and Other |
| Expenses incurred | $ | 19,349 | |
| Cash payments | (16,495) | |
| Liability balance at December 31, 2024 | $ | 2,854 | |
4. Acquisitions and Divestitures
2024 AgBiome Acquisition
On April 10, 2024, the Company acquired certain platform assets, including fully sequenced and isolated strains, unique gene sequences, relevant functional data and metadata, and a development pipeline from AgBiome, Inc. (“AgBiome”), a biotechnology company in the agriculture industry. These assets expand the Company’s proprietary unified metagenomics database. The fair value of the consideration transferred totaled $18.2 million and was paid with the issuance of 407,240 shares of Ginkgo's Class A common stock. The Company accounted for the transaction as an asset acquisition since substantially all of the value received was concentrated in the acquired developed technology, which is being amortized over a useful life of three years.
2024 Other Asset Acquisitions
The Company completed three other asset acquisitions during the year ended December 31, 2024. The aggregate purchase price for the three acquisitions was $19.8 million and was paid with the issuance of 394,799 shares of Ginkgo's Class A common stock. Each transaction was accounted for as an asset acquisition as the acquired assets, consisting primarily of intellectual property rights, did not meet the definition of a business. The assets acquired represent in-process research and development with no alternative future use. Accordingly, the Company recorded $19.8 million as acquired in-process research and development expense in the accompanying consolidated statements of operations and comprehensive loss for the year ended December 31, 2024.
2023 StrideBio Acquisition
On April 5, 2023, the Company entered into an Asset Purchase Agreement (“APA”) with StrideBio, Inc. (“StrideBio”) to acquire StrideBio's adeno-associated virus capsid discovery and engineering platform assets, with a secondary closing contingent upon the transfer of certain additional in-license agreements to Ginkgo. The secondary closing was finalized in October 2023. The Company accounted for the transaction as an asset acquisition as substantially all of the fair value of the
Ginkgo Bioworks Holdings, Inc.
Notes to Consolidated Financial Statements
assets acquired was concentrated in a single identifiable asset. The fair value of the consideration transferred totaled $7.6 million and consisted of 119,278 shares of Ginkgo's Class A common stock valued at $6.8 million and a $0.8 million contingent holdback, all of which was expensed as in-process research and development during the year ended December 31, 2023. The APA, as amended, also provides for royalty payments of up to $21.3 million as described in Note 11. 2022 Zymergen Acquisition
On October 19, 2022 (the “Zymergen Closing Date”), the Company acquired all of the outstanding equity of Zymergen Inc. (“Zymergen”), a former company that specialized in integrating computational and manufacturing technologies to design, develop, and commercialize bio-based products across a broad range of industries (the “Zymergen Acquisition”). Under the merger agreement (“Agreement and Plan of Merger”), on the Zymergen Closing Date, each share of Zymergen common stock that was issued and outstanding as of immediately prior to the effective time was automatically cancelled, extinguished and converted into the right to receive 0.0229 shares of the Company’s Class A common stock and cash in lieu of any fractional shares.
The following table summarizes the acquisition date fair value of the purchase price consideration transferred for Zymergen (in thousands):
| | | | | |
Fair value of Class A common stock issued to Zymergen shareholders (1) | $ | 236,331 | |
Fair value of replacement Ginkgo RSUs and Ginkgo Class A common stock issued under Zymergen RIFs attributable to pre-combination services (2) | 1,571 | |
Less: Cash severance and retention bonuses incurred for the benefit of the combined company (3) | (6,152) | |
| Total Zymergen purchase price consideration | $ | 231,750 | |
(1)As consideration for the Zymergen Acquisition, the Company delivered to Zymergen stockholders 2,485,573 shares of its Class A common stock, of which approximately 2,421,490 represents consideration transferred for the Zymergen Acquisition under ASC 805. The fair value of the Company’s Class A common stock issued as consideration transferred was determined based on $97.60 per share, which was the closing price of the Company’s Class A common stock on the Zymergen Closing Date. An immaterial amount related to the incremental value received by the holders of Zymergen stock options was excluded from total consideration transferred and recognized as post-combination compensation expense.
(2)Represents the fair value of the replacement Ginkgo RSUs and Ginkgo Class A common stock issued under the Zymergen RIFs attributable to pre-combination services. The remaining portion of the fair value is associated with future service and was recognized as stock-based compensation expense in the period subsequent to the Zymergen Acquisition over the remaining service period.
(3)Represents cash bonuses payable to Zymergen employees in accordance with Zymergen severance and retention plans at the Zymergen Closing Date. These payments were determined to be for the benefit of the combined company, and accordingly, a portion of the fair value otherwise recognized as consideration transferred was allocated to post-combination compensation expense.
The Zymergen Acquisition was accounted for as a business combination in accordance with ASC 805, Business Combinations ("ASC 805"). The Company allocated the consideration transferred to the tangible and identifiable intangible assets acquired and liabilities assumed based on their respective estimated fair values on the acquisition date. During 2023, as a result of updated information about facts and circumstances that existed at the acquisition date regarding the collectability of an acquired accounts receivable balance and accrued expenses under a collaboration agreement, the Company recorded a measurement period adjustment to the estimated fair values initially recorded as of October 19, 2022, which resulted in a decrease to goodwill of $2.2 million, an increase to accounts receivable of $1.8 million, and a decrease to accrued expenses and other current liabilities of $0.4 million. Goodwill was primarily attributed to Zymergen’s assembled workforce and the expected synergies from combining operations and was assigned to the Cell Engineering segment. Goodwill is not tax deductible.
Ginkgo Bioworks Holdings, Inc.
Notes to Consolidated Financial Statements
The following table presents the final allocation of the purchase price to the assets acquired and liabilities assumed as of the acquisition date (in thousands):
| | | | | |
| Final Allocation |
| Cash and cash equivalents | $ | 150,553 | |
| Accounts receivable | 2,817 | |
| Inventory | 1,166 | |
| Prepaid expenses and other current assets | 11,592 | |
| Property and equipment | 97,194 | |
| Operating lease right-of-use assets | 205,349 | |
| Intangible assets | 18,600 | |
| Goodwill | 10,660 | |
| Other non-current assets | 11,898 | |
| Accounts payable | (13,907) | |
| Deferred revenue | (8,189) | |
| Accrued expenses and other current liabilities | (55,541) | |
| Operating lease liabilities | (194,582) | |
| Deferred tax liability | (5,690) | |
| Other non-current liabilities | (171) | |
| Net assets acquired | $ | 231,750 | |
The fair value of intangible assets was determined using the relief from royalty method of the income approach. The fair value measurements were primarily based on significant inputs not observable in the market and thus represent a Level 3 measurement. The significant inputs used included the estimated annual net cash flows (including projected revenues attributable to the asset, royalty rates and obsolescence rates), and the discount rate that reflects the risks inherent in the future cash flows. Property and equipment is mostly comprised of lab equipment, leasehold improvements and construction in progress. The fair value of property and equipment was primarily determined using the cost approach, which estimates fair value by determining the replacement or reproduction cost of an asset of comparable utility, adjusted for loss in value due to depreciation and economic obsolescence.
The following table presents the final purchase price allocation and remaining useful lives for identifiable intangible assets acquired as of the acquisition date (in thousands):
| | | | | | | | | | | |
| Estimated fair value | | Estimated useful life (in years) |
| Developed technology | $ | 14,900 | | | 10 |
| Database | 3,700 | | | 7 |
| Total | $ | 18,600 | | | |
In conjunction with the Agreement and Plan of Merger, Zymergen initiated a reduction-in-workforce implemented in stages (each a “RIF”) for the benefit of the combined company. Under the RIFs, employees received enhanced severance benefits consisting of cash bonuses and accelerated vesting of their outstanding Zymergen restricted stock units ("Zymergen RSU"). These benefits were triggered upon a change in control occurring within twelve months of the employee’s termination date. The Company recognized $11.1 million in cash-based severance and stock-based compensation costs in the consolidated statements of operations and comprehensive loss for the year ended December 31, 2022 related to the RIFs.
In August and September 2022, Zymergen also approved the grant of retention bonuses to certain employees denominated in cash and/or Zymergen RSUs designed to retain and reward key talent of Zymergen during the pendency of the proposed Zymergen Acquisition and thereafter. These retention bonuses were deemed for the benefit of the combined company. A portion of the retention bonuses vested and became payable upon the closing of the Zymergen Acquisition, with the remaining portion recognized as post-combination compensation expense over the requisite service period. The Company
Ginkgo Bioworks Holdings, Inc.
Notes to Consolidated Financial Statements
recognized $7.4 million in cash-based retention and stock-based compensation costs in the consolidated statements of operations and comprehensive loss for the year ended December 31, 2022.
The Company’s revenue and net loss for the year ended December 31, 2022 included $2.2 million and $26.0 million, respectively, from Zymergen since the Zymergen Closing Date.
The Company incurred transaction and integration costs of $11.9 million during fiscal year 2022, which were included in general and administrative expenses, inclusive of a success fee which was partly paid in 8,182 shares of Ginkgo Class A common stock. Additionally, the Company incurred $1.7 million of equity issuance costs during fiscal year 2022, which were included in additional paid-in capital in the consolidated balance sheet.
2023 Zymergen Bankruptcy and Deconsolidation
On October 3, 2023, Zymergen and certain of its subsidiaries filed voluntary petitions for relief under Chapter 11 of the United States Bankruptcy Code (the “Zymergen Bankruptcy”) in the U.S. Bankruptcy Court for the District of Delaware (“Bankruptcy Court”). Neither the Company nor any of its other subsidiaries filed for bankruptcy protection. Zymergen has been operated as a distinct legal entity, separate and apart from the Company, since it was acquired in October 2022. Shortly after its acquisition, the Company entered into a non-exclusive license with Zymergen with respect to Zymergen’s intellectual property, including its databases, automation, and software capabilities.
In connection with the Zymergen Bankruptcy, also on October 3, 2023, the Company entered into an asset purchase agreement with Zymergen (the “Zymergen APA”) as the stalking horse bidder under Section 363 of the U.S. Bankruptcy Code to acquire exclusive rights to substantially all of Zymergen’s intellectual property assets and certain other assets. The Company’s bid included a $6.2 million cash component, assumption of a facility lease (previously included in the Company's consolidated financial statements prior to Zymergen's deconsolidation discussed below), and acquiring Zymergen's workforce. On December 14, 2023, Zymergen concluded its auction. On December 21, 2023, the Bankruptcy Court approved the sale of substantially all of Zymergen’s assets to the Company through certain of the Company's affiliates as contemplated by the Zymergen APA.
While as of December 31, 2023, Zymergen remained a wholly-owned subsidiary of the Company, as a result of the bankruptcy proceedings, the Company no longer had a controlling financial interest over Zymergen as defined under ASC 810, Consolidation, and therefore deconsolidated Zymergen’s financial position as of October 2, 2023. The deconsolidation included the derecognition of the carrying amounts of Zymergen’s consolidated assets and liabilities that were previously included in the Company’s consolidated financial statements. Upon deconsolidation, the Company recorded a loss of $42.5 million, representing the remaining net book value of the Company’s investment that was reduced to a fair value of zero. Subsequent to the deconsolidation, the Company accounted for its investment in Zymergen using the cost method of accounting, which was recorded at zero in the Company’s consolidated balance sheet as of December 31, 2023. Zymergen’s results of operations were removed from the Company’s consolidated statements of operations and comprehensive loss beginning October 3, 2023. The historical financial results for Zymergen have not been classified as a discontinued operation because it does not represent a strategic shift with a major effect on the Company's operations and financial results.
The following table presents Zymergen’s consolidated assets and liabilities which have been deconsolidated from the Company's consolidated balance sheet as of October 2, 2023. The amounts presented are before the elimination of intercompany balances.
Ginkgo Bioworks Holdings, Inc.
Notes to Consolidated Financial Statements
| | | | | |
| October 2, 2023 |
| Assets | |
| Current assets: | |
| Cash and cash equivalents | $ | 34,321 | |
| Accounts receivable, net | 11,047 | |
| Prepaid expenses and other current assets | 11,190 | |
| Total current assets | 56,558 | |
| Property, plant and equipment, net | 8,938 | |
| Operating lease right-of-use assets | 135,800 | |
| Intangible assets, net | 16,679 | |
| Goodwill | 10,660 | |
| Other non-current assets | 19,486 | |
| Total assets | 248,121 | |
| Liabilities | |
| Current liabilities: | |
| Deferred revenue | 730 | |
| Accrued expenses and other current liabilities | 20,426 | |
| Total current liabilities | 21,156 | |
| Non-current liabilities: | |
| Operating lease liabilities, non-current | 184,301 | |
| Other non-current liabilities | 172 | |
| Total liabilities | 205,629 | |
| Net assets deconsolidated | $ | 42,492 | |
| |
| |
| |
| |
| |
The following table presents Zymergen’s results of operations for the periods presented, included in the Company's consolidated statements of operations and comprehensive loss prior to the elimination of intercompany balances.
| | | | | | | | | | | |
| Period from January 1, 2023 - October 2, 2023 | | Period from October 19, 2022 - December 31, 2022 |
| Total revenue | $ | 8,370 | | | $ | 2,249 | |
| Total operating expenses | 200,975 | | | 29,459 | |
| Loss from operations | (192,605) | | | (27,210) | |
| Total other income, net | 23,620 | | | 1,260 | |
| Loss before income taxes | (168,985) | | | (25,950) | |
| Income tax provision | 14 | | | 3 | |
| Net loss | $ | (168,999) | | | $ | (25,953) | |
Related Party Transactions
Prior to the deconsolidation, the Company had an existing employee leasing arrangement with Zymergen. The employee leasing charges were considered intercompany transactions and were eliminated in the Company's consolidated financial statements. As of the deconsolidation date, the employee leasing charges were considered related party transactions and have been recognized in the Company's consolidated financial statements. Employee lease expense totaled $4.9 million for the period from October 3, 2023 to December 31, 2023, and was immaterial during fiscal 2024. The Company had
Ginkgo Bioworks Holdings, Inc.
Notes to Consolidated Financial Statements
$1.7 million due to Zymergen as of December 31, 2023, included in accrued expenses and other current liabilities on the balance sheet. This amount was subsequently paid in fiscal 2024.
2024 Zymergen Acquisition
On January 18, 2024, the Company, through certain of its affiliates, completed its acquisition of substantially all of Zymergen’s assets under the Zymergen APA, including offering employment to 91 of Zymergen’s employees. On February 5, 2024, Zymergen’s plan of liquidation was confirmed by the Bankruptcy Court. All of the Company’s interests in the Zymergen entities were extinguished and terminated as of February 23, 2024. The acquisition under the Zymergen APA was accounted for as a business combination in accordance with ASC 805 and was not material to the Company's consolidated financial statements. The total cash purchase price was $6.2 million, with $5.4 million paid at closing and $0.8 million released from escrow. The allocation of the purchase price to the assets acquired and liabilities assumed as of the acquisition date primarily includes $19.9 million of operating lease right-of-use assets, $6.0 million of property and equipment, and $19.9 million of operating lease liabilities. No goodwill or intangible assets were recognized. Transaction costs associated with the Zymergen APA were not material for the year ended December 31, 2024.
2022 Bayer Acquisition and Joint Venture Dissolution
On October 17, 2022, the Company completed an asset purchase under the Asset Purchase Agreement (“APA”) with Bayer CropScience LP, a Delaware limited partnership (“Bayer”). Pursuant to the APA, the Company acquired certain assets and liabilities of Bayer, including Bayer’s 175,000-square-foot West Sacramento Biologics Research & Development site, team, and internal discovery and lead optimization platform.
Concurrently with the APA, Bayer and Ginkgo entered into the Joint Venture Termination Agreement (“JV Termination Agreement”) and the Technical Development Agreement (“Bayer TDA”). The JV Termination Agreement initiated the dissolution of Joyn Bio, LLC (“Joyn Bio”), the joint venture created by Ginkgo and Bayer in 2017, and provided for the disbursement of contributed intellectual property back to the respective owners, the disbursement of joint ownership of certain intellectual property rights created by Joyn Bio, including with respect to Joyn Bio’s nitrogen fixation technology to each party, the disbursement of property and equipment as agreed to by the parties, the assumption by Ginkgo of Joyn Bio's two real estate leases and the transfer of certain employees to Ginkgo. Under the Bayer TDA, (i) Ginkgo granted Bayer exclusive licenses to Ginkgo’s joint ownership right, title and interest to Joyn Bio’s nitrogen fixation intellectual property, (ii) for a three-year period, the parties will research, develop and produce microbial strains and related processes to enable the research, development, production, manufacturing and commercialization of Bayer products in agriculture as part of cell programs pursuant to TDPs agreed to by the parties, including one targeted to nitrogen fixation and (iii) for a three-year period, Ginkgo will provide certain non-cell-engineering services to Bayer related to product support as described in statements of work agreed to by the parties. In consideration for all programs, services and related licenses, Ginkgo will receive $90.0 million in equal quarterly installments over the three-year term plus royalties on worldwide net sales of certain Bayer products developed under the Bayer TDA.
The APA, JV Termination Agreement and Bayer TDA were accounted for as a single transaction as they were entered into at the same time and in contemplation of one another, the occurrence of each agreement was dependent on the occurrence of the other agreements, and the work performed under the Bayer TDA will utilize the tangible assets acquired from Bayer under the APA and the IP distributed to Ginkgo under the JV Termination Agreement.
The assets acquired under the APA and JV Termination Agreement meet the definition of a business and were accounted for under ASC 805. The Bayer TDA was accounted for under ASC 606. A summary of the purchase price relating to the business combination is as follows (in thousands):
| | | | | |
| Cash | $ | 79,825 | |
| Fair value of previously held equity interest in Joyn Bio | 14,000 |
| Fair value of notes receivable from Joyn Bio | 10,119 |
| Total purchase consideration | $ | 103,944 | |
Prior to the completion of the business combination, the Company, through its then majority-owned holding company Cooksonia, LLC (“Cooksonia”), held a 50% equity interest in Joyn Bio that was accounted for as an equity method investment. The Company remeasured its 50% equity interest in Joyn Bio at fair value as of the acquisition date and recorded a gain of $14.0 million equal to the difference between the carrying value of its equity method investment in Joyn
Ginkgo Bioworks Holdings, Inc.
Notes to Consolidated Financial Statements
Bio of zero and the fair value of $14.0 million on the acquisition date. The gain is included within loss on equity method investments in the consolidated statements of operations and comprehensive loss for the year ended December 31, 2022.
Additionally, prior to the completion of the business combination, Joyn Bio had issued to Ginkgo a series of convertible promissory notes in the aggregate principal amount of $10.0 million (see Note 20). The notes were effectively settled as part of the business combination and were included as part of the consideration transferred for the business combination. The carrying value of the notes prior to the acquisition was $4.8 million due to losses attributable to the equity method investment being allocated to the notes receivable as a result of the equity method investment being reduced to zero during the year ended December 31, 2022. The Company recorded a gain on the notes receivable of $5.3 million within other income, net in the consolidated statements of operations and comprehensive loss for the year ended December 31, 2022 for the excess of the $10.1 million outstanding principal and accrued interest over their carrying value of the notes. The following table presents the final allocation of the purchase price to the assets acquired and liabilities assumed as of the acquisition date (in thousands):
| | | | | |
| Property, plant, and equipment | $ | 83,951 | |
| Intangible assets | 11,500 | |
| Goodwill | 11,172 | |
| Deferred tax liability | (2,679) | |
| Net assets acquired | $ | 103,944 | |
The fair value of Ginkgo’s equity interest in Joyn Bio pre-dissolution was determined using a discounted cash flow method. The fair value of intangible assets, which consists of Joyn Bio's developed technology, was determined using the relief from royalty method of the income approach. Significant assumptions used in the valuations included the estimated annual net cash flows (including projected future revenues and costs, terminal growth rates, royalty rates and obsolescence rates), and a discount rate that reflects the risks inherent in the future cash flows. Property, plant, and equipment consists of land, buildings, site improvements and personal property. The fair value of land was determined using the sales comparison approach and the fair value of the buildings, site improvements and personal property was determined using the cost and sales comparison approaches. Under the cost approach, the Company estimated the cost to acquire or construct comparable assets and made adjustments for physical deterioration. Intangible assets consist of Joyn Bio's developed technology and have an estimated useful life of five years. Goodwill primarily reflects the value of future programs expected to arise after the acquisition and the assembled workforce. Goodwill is not tax deductible.
The Company incurred $0.2 million and $3.0 million in costs associated with the winding up and dissolution of Joyn Bio during the years ended December 31, 2023 and 2022, respectively, which were recorded within operating expenses. Dissolution costs are shared equally between Ginkgo and Bayer. The joint venture was fully dissolved in 2023. The Company incurred transaction and integration costs of $12.0 million during the year ended December 31, 2022 related to the business combination, which were included in general and administrative expenses in the consolidated statements of operations and comprehensive loss. The transaction does not represent a material business combination and, therefore, pro forma financial information is not provided. Operating results of the acquired business have been included in the consolidated statements of operations and comprehensive loss since the date of acquisition and were not material to the Company’s results of operations for the year ended December 31, 2022.
2022 Altar Acquisition and 2024 Divestiture
On October 3, 2022, the Company acquired all outstanding shares of capital stock of Altar SAS (“Altar”), a French biotechnology company with a proprietary adaptive evolution platform. Altar's fleet of automated adaptive laboratory evolution instruments was integrated into Ginkgo's Foundry to serve customers across various industries. The total purchase consideration was $12.0 million and consisted of $2.8 million in cash, $1.4 million in restricted shares of Ginkgo Class A common stock subject to forfeiture if certain vesting conditions are not met, $5.6 million in unrestricted shares of Ginkgo Class A common stock, $1.6 million in contingent consideration and $0.6 million in assumed liabilities. The Company accounted for the transaction as a business combination under ASC 805. The net assets acquired primarily consisted of $8.4 million of intangible assets related to Altar's developed technology and $4.7 million of goodwill, which is not deductible for tax purposes. The business is reported as part of the Company’s Cell Engineering reportable segment. The Company incurred $2.3 million in acquisition related costs during the year ended December 31, 2022, which were included in general and administrative expenses. Pro forma information has not been presented because it is not material to the financial statements. Altar's results of operations have been included in the consolidated statements of operations and
Ginkgo Bioworks Holdings, Inc.
Notes to Consolidated Financial Statements
comprehensive loss since the date of acquisition and were not material to the Company’s results of operations for the year ended December 31, 2022.
On September 30, 2024, the Company sold the equity interests of Altar for a nominal amount. As a result of the sale, the Company deconsolidated all of Altar's assets and liabilities from its consolidated financial statements effective September 30, 2024, and recognized a loss on deconsolidation of $7.0 million in the consolidated statements of operations and comprehensive loss for the year ended December 31, 2024. The loss on deconsolidation includes a $1.5 million reclassification of accumulated currency translation adjustments to earnings. The sale did not meet the criteria to be reported as a discontinued operation.
2022 FGen Acquisition
On April 1, 2022, the Company acquired all of the outstanding equity interests of FGen AG (“FGen”), a company organized under the laws of Switzerland that specializes in strain development and optimization. FGen has developed an ultra-high-throughput screening platform built on nanoliter reactor technology which the Company believed would enhance its cell screening capabilities and potentially increase the likelihood of finding enzymes, pathways, and strains or cell lines that perform to diverse cell program specifications.
The Company accounted for the transaction as a business combination under ASC 805. Accordingly, the assets and liabilities acquired were recorded at their estimated fair value on the date of acquisition. FGen's results of operations have been included in the consolidated statements of operations and comprehensive loss since the date of acquisition and were not material to the Company’s results of operations for the year ended December 31, 2022. The FGen acquisition does not represent a material business combination and, therefore, pro forma financial information is not provided.
The consideration paid was comprised of common stock and contingent consideration as follows (in thousands):
| | | | | |
| Fair value of Class A common stock | $ | 17,015 | |
| Fair value of contingent consideration - restricted stock | 3,842 | |
| Fair value of contingent consideration - milestones | 8,464 | |
| Total FGen consideration | $ | 29,321 | |
The Company issued 143,749 shares of its Class A common stock on the acquisition date comprised of 101,278 unrestricted shares valued at $17.0 million based on the closing market price of $168.00 per share and 42,471 restricted shares classified as contingent consideration and subject to vesting conditions. The contingent consideration in the form of restricted stock was valued at $3.8 million as of the acquisition date based on management’s estimate of the number of shares expected to vest and the closing market price of $168.00. The restricted shares were issued in three tranches with separate vesting conditions. Tranches 1 and 2 vested on April 4, 2022 when the Company filed its Form S-1 registration statement and a total of 11,530 shares vested and 14,606 shares were forfeited. The remaining 16,335 tranche 3 restricted shares vested on the 24-month anniversary of the closing.
As part of the acquisition, the Company is required to make milestone payments up to a maximum of $25.0 million, with $20.0 million payable based on the successful integration and deployment of the FGen technology across the Company's programs over a 36-month period and $5.0 million payable to certain employees based on continuing service. The milestones are payable in cash or Class A common stock at the election of the Company. The $5.0 million payable to employees is accounted for separately from the business combination as post combination compensation expense and recognized over the requisite service period. The fair value of the $20.0 million in contingent consideration on the acquisition date was determined using a scenario-based method. The significant assumptions used include the expected time of achievement and probability of success related to each milestone and a discount rate.
The Company allocated the purchase price to the tangible and identifiable intangible assets acquired and liabilities assumed based on their respective estimated fair values on the acquisition date. During the year ended December 31, 2022, the Company recorded measurement period adjustments which did not have a material impact on goodwill.
The intangible assets acquired consist of FGen's developed technology which was measured at fair value using the multi-period excess earnings method under the income approach. Under this method, an intangible asset's fair value is equal to the present value of the incremental after-tax cash flows attributable only to the intangible asset after deducting charges representing the contribution of other assets to those cash flows. The significant assumptions used include the estimated annual net cash flows (including revenue growth rates, EBITDA and EBIT margins, applicable tax rate, and contributory
Ginkgo Bioworks Holdings, Inc.
Notes to Consolidated Financial Statements
asset charges), a discount rate, and the tax amortization benefit. Goodwill represents the amount by which the purchase price exceeds the estimated fair value of the net assets acquired and primarily reflects the value of future programs expected to arise after the acquisition.
The Company incurred $1.7 million of acquisition-related costs during the year ended December 31, 2022, which were included in general and administrative expenses in the consolidated statements of operations and comprehensive loss.
The following table presents the final allocation of the purchase price to the assets acquired and liabilities assumed as of the acquisition date (in thousands):
| | | | | |
| Final Allocation |
| Cash and cash equivalents | $ | 1,430 | |
| Accounts receivable | 144 | |
| Other non-current assets | 10 | |
| Property and equipment | 34 | |
Intangible assets (1) | 21,100 | |
Goodwill (2) | 10,615 | |
| Accounts payable and accrued expenses | (29) | |
| Deferred revenue | (104) | |
| Deferred tax liability | (3,879) | |
| Net assets acquired | $ | 29,321 | |
(1) Estimated useful life of 15 years.
(2) Non-deductible for tax purposes.
2022 Asset Acquisitions
On October 3, 2022, the Company completed the acquisition of all of the outstanding equity interests in Circularis Biotechnologies, Inc., (“Circularis”), a biotechnology company with a proprietary circular RNA and promoter screening platform. The aggregate purchase consideration was $18.6 million, of which $4.3 million was paid in cash, $10.2 million was paid in Ginkgo Class A common stock, $3.7 million represented contingent consideration and $0.4 million represented direct transaction costs. The Company accounted for the transaction as an asset acquisition as substantially all of the value received was concentrated in the acquired developed technology. The Company allocated the purchase consideration primarily to the developed technology intangible asset, which is being amortized over a useful life of five years. Additionally, the purchase agreement included $2.5 million of employee retention payments, which was recognized as compensation expense over the requisite service period.
On August 17, 2022, the Company acquired certain epidemiological data infrastructure assets from Baktus, Inc., a Delaware-based public benefit corporation. The Company accounted for the transaction as an asset acquisition as the value acquired primarily related to a single identifiable intangible asset. The total purchase consideration was $11.1 million and consisted of $2.0 million in cash, $8.4 million in Ginkgo Class A common stock and $0.7 million of direct transaction costs. Of the shares issued, 6,470 were restricted shares that vested on the 18-month anniversary of the closing and were classified as contingent consideration liability in the consolidated balance sheet (see Note 5). Additionally, the purchase agreement included $1.0 million of employee retention payments, which was recognized as compensation expense over the requisite service period. As a result of the acquisition, the Company recognized $11.2 million in intangible assets consisting of developed technology, customer relationships and assembled workforce and $0.1 million in deferred revenue.
Ginkgo Bioworks Holdings, Inc.
Notes to Consolidated Financial Statements
5. Fair Value Measurements
The following tables present information about the Company’s financial assets and liabilities measured at fair value on a recurring basis (in thousands):
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | As of December 31, 2024 |
| | Classification | | Total | | Level 1 | | Level 2 | | Level 3 |
| Assets: | | | | | | | | | | |
| Money market funds | | Cash and cash equivalents | | $ | 521,457 | | | $ | 521,457 | | | $ | — | | | $ | — | |
Synlogic, Inc. warrants (1) | | Investments | | 238 | | | — | | | 238 | | | — | |
| Marketable equity securities | | Investments | | 17,559 | | | 17,559 | | | — | | | — | |
| Notes receivable | | Other non-current assets | | 14,170 | | | — | | | 12,327 | | | 1,843 | |
| Total assets | | | | $ | 553,424 | | | $ | 539,016 | | | $ | 12,565 | | | $ | 1,843 | |
| Liabilities: | | | | | | | | | | |
| Contingent consideration | | Accrued expenses and other current liabilities | | $ | 5,438 | | | $ | — | | | $ | — | | | $ | 5,438 | |
| Contingent consideration | | Other non-current liabilities | | 4,484 | | | — | | | — | | | 4,484 | |
| Total liabilities | | | | $ | 9,922 | | | $ | — | | | $ | — | | | $ | 9,922 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | As of December 31, 2023 |
| | Classification | | Total | | Level 1 | | Level 2 | | Level 3 |
| Assets: | | | | | | | | | | |
| Money market funds | | Cash and cash equivalents | | $ | 913,729 | | | $ | 913,729 | | | $ | — | | | $ | — | |
Synlogic, Inc. warrants (1) | | Investments | | 654 | | | — | | | 654 | | | — | |
Marketable equity securities (2) | | Investments | | 19,190 | | | 18,401 | | | 789 | | | — | |
| Notes receivable | | Prepaid expenses and other current assets | | 12,293 | | | — | | | — | | | 12,293 | |
| Notes receivable | | Other non-current assets | | 13,601 | | | — | | | 11,765 | | | 1,836 | |
| Total assets | | | | $ | 959,467 | | | $ | 932,130 | | | $ | 13,208 | | | $ | 14,129 | |
| Liabilities: | | | | | | | | | | |
| Public Warrants | | Other non-current liabilities | | $ | 3,794 | | | $ | 3,794 | | | $ | — | | | $ | — | |
| Private Placement Warrants | | Other non-current liabilities | | 1,906 | | | — | | | 60 | | | 1,846 | |
| Contingent consideration | | Accrued expenses and other current liabilities | | 18,468 | | | — | | | — | | | 18,468 | |
| Contingent consideration | | Other non-current liabilities | | 5,805 | | | — | | | — | | | 5,805 | |
| Total liabilities | | | | $ | 29,973 | | | $ | 3,794 | | | $ | 60 | | | $ | 26,119 | |
(1)The fair value of Synlogic, Inc. warrants is calculated as the quoted price of the underlying common stock, less the unpaid exercise price of the warrants.
(2)Marketable equity securities classified as Level 2 reflect a discount for lack of marketability due to regulatory sales restrictions.
Transfers to and from Levels 1, 2 and 3 are recognized at the end of the reporting period in which a change in valuation technique or methodology occurs. In 2024 and 2023, transfers from Level 2 to Level 1 occurred due to lapse of regulatory sales restrictions on marketable equity securities. Additionally, in 2024, a portion of the Private Placement Warrants' estimated fair value was transferred from Level 3 to Level 2 as a result of the Private Placement Warrants having substantially the same terms as the Public Warrants when transferred to anyone other than the initial purchasers or their permitted transferees, leading the Company to determine their fair value to be equivalent to that of the Public Warrants. There were no other transfers between Levels 1, 2, or 3 during 2024 or 2023.
Ginkgo Bioworks Holdings, Inc.
Notes to Consolidated Financial Statements
The table below provides a reconciliation of the beginning and ending balances for assets and liabilities measured at fair value using Level 3 significant unobservable inputs for the years ended December 31 (in thousands):
| | | | | | | | | | | | | |
| | Notes Receivable | | Private Placement Warrants | | Contingent Consideration |
| Balance at January 1, 2024 | $ | 14,129 | | | $ | 1,846 | | | $ | 24,273 | |
| Additions | 1,407 | | | — | | | — | |
| Change in fair value | (3,217) | | | (1,697) | | | 3,214 | |
| Settlements and payments | — | | | — | | | (17,565) | |
| Transfers to Level 2 | — | | | (149) | | | — | |
| Conversion to common stock | (10,476) | | | — | | | — | |
| Balance at December 31, 2024 | $ | 1,843 | | | $ | — | | | $ | 9,922 | |
| | | | | |
| Balance at January 1, 2023 | $ | 7,660 | | | $ | 3,860 | | | $ | 24,473 | |
| Additions | 2,653 | | | — | | | 1,397 | |
| Additions from note exchanges and amendments | 13,939 | | | — | | | — | |
| Change in fair value | (2,416) | | | (2,014) | | | 9,168 | |
| Settlements and payments | (7,707) | | | — | | | (10,765) | |
| Balance at December 31, 2023 | $ | 14,129 | | | $ | 1,846 | | | $ | 24,273 | |
Notes Receivable
For all of its notes receivable, the Company has elected the fair value option, for which changes in fair value are recorded in other income, net in the consolidated statements of operations and comprehensive loss.
As of December 31, 2024 and 2023, the Company's notes receivable includes a senior secured note in the original principal amount of $11.8 million, issued by Bolt Threads, Inc. (“Bolt Threads”), which bears interest at 12% per annum, is due December 31, 2027, and is included in other non-current assets at its estimated fair value. The Company previously held a convertible promissory note, also issued by Bolt Threads, in the principal amount of $10.0 million, which bore interest at 8% per annum, was convertible into equity securities of Bolt Threads upon a qualified financing, a non-qualified financing, or special purpose acquisition company transaction, at a conversion price based on certain conditions as defined in the note agreement, or was otherwise payable on demand any time after the maturity date of October 4, 2024. During the year ended December 31, 2024, $10.5 million in principal and accrued interest on the convertible promissory note was converted into 2.7 million shares of the issuer's common stock, which were classified as marketable equity securities until they were sold.
The Company used the yield method to value the senior secured note. Under this method, the estimated future cash flows, consisting of principal and interest payments, are discounted to present value using an applicable market yield or discount rate. Increases or decreases in the market yield or discount rate would result in a decrease or increase, respectively, in the fair value measurement. The market yield is determined using a corporate bond yield curve corresponding to the credit rating category of the issuer. The fair value of the senior secured note is based on observable market inputs, which represents a Level 2 measurement within the fair value hierarchy.
The Company also holds a series of convertible debt instruments issued by customers as payment for Cell Engineering services. The Company used a scenario-based method to value the convertible debt instruments issued by customers and by Bolt Threads prior to conversion. Under this method, future cash flows are evaluated under various payoff scenarios, probability-weighted, and discounted to present value. The significant unobservable (Level 3) inputs used in the fair value measurement as of December 31, 2024 included scenario probabilities ranging from 5% to 45%, a discount rate of 15.5% and estimated time to event date of up to two years. The significant unobservable (Level 3) inputs used in the fair value measurement as of December 31, 2023 included scenario probabilities ranging from 5% to 85%, a discount rate of 17% and estimated time to event date of one to two years. Significant changes in these inputs could have resulted in a significantly lower or higher fair value measurement. As of December 31, 2024, the convertible debt instruments had an unpaid principal balance of $13.2 million and a fair value of $1.8 million. As of December 31, 2023, the convertible debt instruments had an unpaid principal balance of $21.0 million and a fair value of $14.1 million.
Ginkgo Bioworks Holdings, Inc.
Notes to Consolidated Financial Statements
In December 2023, the Company entered into an amendment with a customer regarding two outstanding convertible promissory notes, with an aggregate principal amount of $10.3 million. The Company used a scenario-based method to value the convertible notes as of the amendment date. The significant unobservable (Level 3) inputs used in the fair value measurement as of the amendment date included scenario probabilities ranging from 10% to 75%, a discount rate of 15%, time to event date of up to one year, and an estimated fair value per share of the equity securities to which the Company would be entitled to upon conversion of the notes, obtained from a third-party valuation.
Warrant Liabilities
In connection with the Company's merger with Soaring Eagle Acquisition Corp. (“SRNG”) on September 16, 2021 (“SRNG Business Combination”), the Company assumed 34.5 million warrants (formerly traded on the New York Stock Exchange (the “NYSE,” and such warrants, the “Public Warrants”) and 17.3 million private placement warrants (the “Private Placement Warrants”, and together with the Public Warrants, the “Warrants”), which were initially issued in connection with SRNG’s initial public offering. The number of outstanding Warrants did not change as a result of the Reverse Stock Split. However, each Warrant equals one-fortieth (1/40) of one share of Class A common stock (a minimum of 40 Warrants must be exercised for one share of Class A common stock) following the Reverse Stock Split.
The fair value of the Public Warrants was based on their observable quoted price on the NYSE. However, the Public Warrants were delisted by the NYSE on September 4, 2024, due to abnormally low selling price levels, and subsequently began trading on the over-the-counter markets. As of December 31, 2024, the Company determined that the Public Warrants had no value.
The Private Placement Warrants are identical to the Public Warrants, except that they are exercisable on a cashless basis and are non-redeemable as long as they are held by the initial purchasers or their permitted transferees. As of December 31, 2024, the Company concluded that the difference between the fair values of the Public Warrants and Private Placement Warrants was de minimis. Therefore, the Private Placement Warrants were measured by reference to the value of the Public Warrants and had no value.
As of December 31, 2023, the fair value of the Private Placement Warrants was estimated using a Black-Scholes option pricing model, which was considered a Level 3 fair value measurement. The primary unobservable input used in the valuation of the Private Placement Warrants was expected stock-price volatility, estimated through a Monte-Carlo simulation of the redeemable Public Warrants that assumed optimal exercise of the Company's redemption option at the earliest possible date. A change in this input could have significantly affected the valuation.
Contingent Consideration
In connection with various business acquisitions, the Company is required to make contingent earnout payments payable upon the achievement of certain technical, commercial and/or performance milestones. The Company also issued restricted stock in connection with acquisitions, which is subject to vesting conditions and is classified as contingent consideration liability.
The Company can settle a majority of its contingent consideration liabilities in cash or shares of Class A common stock at the Company’s election with the remainder payable in cash. During the year ended December 31, 2024, the Company settled $17.6 million in contingent consideration liability through payment of $2.8 million in cash and vesting of 1,413,909 shares of restricted stock valued at $14.7 million. During the year ended December 31, 2023, the Company settled $10.8 million in contingent consideration liabilities through payment of $1.9 million in cash and vesting of 137,427 shares of restricted stock valued at $8.9 million. Of that amount, $1.4 million was recorded as an increase to the acquired intangible asset with an offset to additional paid-in-capital as the contingent consideration liability was deemed not probable of occurring.
The fair value of contingent consideration related to earnout payments from acquisitions was estimated using unobservable (Level 3) inputs as illustrated in the table below. The fair value of contingent consideration related to restricted stock was estimated using the quoted price of Ginkgo's Class A common stock, an estimate of the number of shares expected to vest, probability of vesting, and a discount rate. Material increases or decreases in these inputs could result in a higher or lower fair value measurement. Changes in the fair value of contingent consideration are recorded in general and administrative expense in the consolidated statements of operations and comprehensive loss.
The following table provides quantitative information regarding Level 3 inputs used in the fair value measurements of contingent consideration liabilities as of the periods presented:
Ginkgo Bioworks Holdings, Inc.
Notes to Consolidated Financial Statements
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Contingent Consideration Liability | | Valuation Technique | | Unobservable Input | | December 31, 2024
Range | | December 31, 2023
Range |
Earnout payments (FGen, Dutch DNA and Altar acquisitions) (1) | | Probability-weighted present value | | Probability of payment | | 5% - 50% | | 10% - 100% |
| | | | Discount rate | | 9.3 | % | | 13.4% |
Earnout payments (Dutch DNA acquisition) (1) | | Discounted cash flow | | Projected years of payments | | 2028-2031 | | 2025-2028 |
| | | | Discount rate | | 10.6 | % | | 10.3 | % |
(1) For FGen and Altar acquisitions, see Note 4. In July 2021, the Company acquired Dutch DNA Biotech B.V. (“Dutch DNA”) and is obligated to make contingent earnout payments up to a maximum of $20.0 million, payable upon the achievement of certain technical and commercial milestones by Dutch DNA. Nonrecurring Fair Value Measurements
The Company measures the fair value of certain assets, including investments in privately held companies without readily determinable fair values, on a nonrecurring basis when events or changes in circumstances indicate that the carrying amount of the assets may not be recoverable, or when observable price changes occur for identical or similar securities from the same issuer.
The fair value of non-marketable equity securities is classified as Level 3 within the fair value hierarchy when the Company estimates fair value using unobservable inputs to measure an impairment loss. It is classified as Level 2 when fair value is estimated using the observable transaction price paid by third-party investors for an identical or similar security of the same issuer.
Investment Impairments
During the years ended December 31, 2024, 2023 and 2022, the Company recorded impairment losses of $11.9 million, $33.0 million, and $10.1 million, respectively, related to its investment in Genomatica preferred stock. The fair value estimates used to determine the impairment charges in 2023 were derived using the guideline public company method under the market approach, while an enterprise value analysis was performed in 2022, with an equal weighting between discounted cash flow analyses and the guideline public company method. Significant unobservable (Level 3) inputs included estimated annual net cash flows (including revenue and expense growth rates and capitalization rates), the weighted-average cost of capital used to discount future cash flows, and the selection of guideline public company multiples for revenue and EBITDA. Material increases or decreases in these inputs could result in higher or lower fair value measurements. As of December 31, 2024, the Company determined that the investment had substantially no value.
During the years ended December 31, 2024 and 2023, the Company recorded impairment losses of $1.7 million and $8.3 million, respectively, related to an investment in the preferred stock of a privately held company. The fair value as of December 31, 2023 was determined by deriving the investee’s equity value from a 2021 financing transaction involving its own securities and applying an 87% downward market adjustment to the implied equity value. The equity value was then allocated to the different classes of the securities of the investee using the option-pricing model (“OPM”). The OPM involves making assumptions around the investees’ expected time to liquidity and volatility derived from selected guideline public companies. These assumptions are considered Level 3 inputs. As of December 31, 2024, the Company determined that the investment had substantially no value.
SAFEs
During the years ended December 31, 2023 and 2022, the Company received a total purchase amount of $11.0 million and $39.5 million, respectively, in SAFEs from customers as prepayment for Cell Engineering services. The Company used a scenario-based method to value the SAFEs as of each contract inception date, which resulted in total fair value of $4.5 million and $22.1 million for SAFEs received during the years ended December 31, 2023 and 2022, respectively. Under the scenario-based method, future cash flows were evaluated under qualified financing and dissolution scenarios with partial recovery and no recovery in dissolution. The cash flows under each scenario were probability-weighted and discounted to present value. The significant unobservable (Level 3) inputs used in the fair value measurement at contract inception during 2023 were scenario probabilities in the range of 20% to 60%, a discount rate of 14% and estimated time to event date of one to two years. The significant unobservable (Level 3) inputs used in the fair value measurement at contract inception during 2022 were scenario probabilities in the range of 18% and 65%, a discount rate of 13% and estimated time to event date of one to two years.
Ginkgo Bioworks Holdings, Inc.
Notes to Consolidated Financial Statements
During the years ended December 31, 2024 and 2023, the Company recorded impairment losses of $7.2 million and $2.7 million, respectively, related to SAFEs. The fair values were generally estimated using the scenario-based method, where various payout scenarios were probability-weighted and discounted to present value.
Additionally, during the years ended December 31, 2024 and 2023, the Company recorded impairments of lab equipment, construction in progress assets, and assets related to an operating lease. Refer to Note 10 for additional detail. 6. Investments and Equity Method Investments
The Company has partnered with other investors to form business ventures, including Motif FoodWorks, Inc. (“Motif”), Allonnia, LLC (“Allonnia”), Arcaea, LLC (“Arcaea”), Verb Biotics, LLC (“Verb Biotics”), BiomEdit, LLC (“BiomEdit”) and Ayana Bio, LLC (“Ayana Bio”) (collectively “Platform Ventures”). The Company has also partnered with existing entities, including Genomatica, Inc. (“Genomatica”) and Synlogic, Inc. (“Synlogic”) (collectively, “Legacy Structured Partnerships”) with complementary assets for synthetic biology applications. The Company holds equity interests in these Platform Ventures and Legacy Structured Partnerships. The Company also holds equity interests in other public and private companies as a result of entering into collaboration and license revenue arrangements with these entities.
The Company accounts for its investments in Platform Ventures under the equity method. The Company's marketable equity securities consist of Synlogic common stock, Synlogic warrants and the shares of common stock of other publicly traded companies. Marketable equity securities are measured at fair value with changes in fair value recorded in other income (expense) in the consolidated statements of operations and comprehensive loss. The Company’s non-marketable equity securities consist of preferred stock of Genomatica and preferred and common stock of other privately held companies without readily determinable fair values. Non-marketable equity securities are initially recorded using the measurement alternative at cost and subsequently adjusted for any impairment and observable price changes in orderly transactions for the identical or a similar security of the same issuer. Impairment losses and adjustments from observable price changes are recorded in loss on investments in the consolidated statements of operations and comprehensive loss.
The Company also holds investments in early-stage synthetic biology product companies via SAFEs. The Company entered into SAFE agreements in conjunction with a revenue contract with a customer under which the Company grants the customer a prepaid Cell Engineering services credit equal to the principal amount of the SAFE (the “Purchase Amount”), which may be used and drawn down as payment for the Company’s research and development services. The SAFEs will automatically convert into shares of preferred stock equal to the Purchase Amount divided by the discount price, which is calculated as the price per share sold in a qualified equity financing multiplied by a discount rate. The SAFEs also provide the Company with the right to future equity of the entity in a liquidation scenario or the cash-out amount in liquidation and dissolution scenarios or at the election of the SAFE issuer prior to an agreed outside date. The Company initially records SAFEs at fair value (see Note 5) and adjusts the carrying amount of the instrument at each reporting period for any impairments. Investments consisted of the following (in thousands):
| | | | | | | | | | | |
| As of December 31, |
| 2024 | | 2023 |
| Investments: | | | |
| SAFEs | $ | 16,689 | | | $ | 23,898 | |
| Non-marketable equity securities | 14,218 | | | 22,938 | |
| Marketable equity securities | 17,559 | | | 19,190 | |
| Genomatica preferred stock | — | | | 11,885 | |
| Synlogic warrants | 238 | | | 654 | |
| Total | $ | 48,704 | | | $ | 78,565 | |
Ginkgo Bioworks Holdings, Inc.
Notes to Consolidated Financial Statements
Loss on investments and equity method investments consisted of the following (in thousands):
| | | | | | | | | | | | | | | | | |
| Year Ended December 31, |
| 2024 | | 2023 | | 2022 |
| Loss on investments: | | | | | |
| Genomatica preferred stock | $ | (11,885) | | | $ | (33,000) | | | $ | (10,115) | |
| Non-marketable equity securities | (1,735) | | | (9,928) | | | (195) | |
| Marketable equity securities | (7,583) | | | (7,874) | | | (38,795) | |
| SAFEs | (7,208) | | | (2,742) | | | — | |
| Synlogic warrants | (416) | | | (1,283) | | | (4,230) | |
| Total | $ | (28,827) | | | $ | (54,827) | | | $ | (53,335) | |
| Loss on equity method investments: | | | | | |
| BiomEdit | $ | — | | | $ | (1,461) | | | $ | (8,503) | |
Joyn Bio (1) | — | | | — | | | (3,043) | |
| Verb Biotics | — | | | — | | | (15,900) | |
| Ayana Bio | — | | | — | | | (15,989) | |
| Other | — | | | (1,174) | | | (326) | |
| Total | $ | — | | | $ | (2,635) | | | $ | (43,761) | |
(1)The loss on equity method investment in Joyn Bio for the year ended December 31, 2022 is comprised of a $17.0 million loss offset by a $14.0 million gain on the remeasurement of the retained equity interest in Joyn Bio at fair value as of the acquisition date (see Note 4). The loss on equity method investment in excess over the carrying value of zero of the equity method investment in Joyn Bio during the year ended December 31, 2022 was recorded as a reduction in the convertible promissory notes receivable from Joyn Bio (see Note 20). The components of loss on investments for each period were as follows (in thousands):
| | | | | | | | | | | | | | | | | |
| Year Ended December 31, |
| 2024 | | 2023 | | 2022 |
| Impairment charges | $ | (20,828) | | | $ | (44,043) | | | $ | (10,310) | |
| Realized and unrealized losses recognized on marketable equity securities | (7,999) | | | (9,157) | | | (43,025) | |
| Downward adjustments from observable price changes | — | | | (1,627) | | | — | |
| Total loss on investments | $ | (28,827) | | | $ | (54,827) | | | $ | (53,335) | |
Total realized and unrealized losses associated with equity investments accounted for at fair value or the fair value measurement alternative consisted of the following (in thousands): | | | | | | | | | | | | | | | | | |
| Year Ended December 31, |
| 2024 | | 2023 | | 2022 |
Realized loss recognized on equity investments sold (1) | $ | (5,957) | | | $ | — | | | $ | — | |
| Net unrealized losses recognized on equity investments held as of the end of the period | (22,870) | | | (54,827) | | | (53,335) | |
| Total loss on investments | $ | (28,827) | | | $ | (54,827) | | | $ | (53,335) | |
(1) Reflects the difference between the sale proceeds and the carrying value of the equity investments at the beginning of the period or the acquisition date, if later.
The carrying value of non-marketable equity securities accounted for using the fair value measurement alternative and still held as of December 31, 2024, including cumulative unrealized losses, were as follows (in thousands):
Ginkgo Bioworks Holdings, Inc.
Notes to Consolidated Financial Statements
| | | | | |
| As of December 31, 2024 |
| Total initial cost | $ | 107,996 | |
| Impairment charges | (75,461) | |
| Downward adjustments from observable price changes | (1,628) | |
| Carrying value | $ | 30,907 | |
7. Variable Interest Entities
2022 Deconsolidations: Ayana Bio and Verb Biotics
The Company holds an interest in 9,000,000 common units (representing 100% of common units at inception) in each of Ayana Bio and Verb Biotics, two Platform Ventures formed in September 2021 by the Company and certain of its investors. The Company has agreed to provide Ayana Bio and Verb Biotics with certain licenses to intellectual property for use in the development or production of products that the parties agree to research and develop under technical development plans (“TDPs”). Additionally, in September 2021, Ayana Bio and Verb Biotics entered into a Series A Preferred Unit Purchase Agreement under which each entity sold 9,000,000 Series A preferred units to certain of the Company’s investors for aggregate proceeds of approximately $30.0 million each. During 2021, the Company concluded that it held a variable interest in and was the primary beneficiary of Ayana Bio and Verb Biotics as it controlled the most significant activities of these entities. These conclusions were reached because, as of the primary beneficiary assessment dates in 2021, for both Verb Biotics and Ayana Bio: (i) the Company had substantive control of the board of directors; (ii) all capital contributions were made by related parties of Ginkgo; and (iii) Ginkgo or its related parties comprised the entirety of the joint steering committee (“JSC”), the governing body which holds significant oversight with respect to the entities' research and development programs.
During 2022, Verb Biotics and Ayana Bio each hired a new chief executive officer who was not an affiliate, related party or agent of Ginkgo. The chief executive officer was also appointed to each entity's JSC and board of directors. As a result, the Company concluded it no longer had substantive control of each entity's JSC and board of directors. Accordingly, the Company concluded that it was no longer the primary beneficiary of Verb Biotics and Ayana Bio as it no longer controlled the most significant activities of the entities. As a result of this change in the primary beneficiary determination, the Company deconsolidated Verb Biotics and Ayana Bio and recorded a gain on deconsolidation of $31.9 million for the year ended December 31, 2022 in the consolidated statements of operations and comprehensive loss. The gain on deconsolidation was equal to the fair value of the retained interest in each entity as of the deconsolidation date and was calculated using the option pricing method. The option pricing method used a back-solve methodology to infer the total equity value based on the pricing of the Series A preferred unit financing, which is the most recent financing transaction to the deconsolidation event.
The JSC, with equal representation from each of Verb Biotics or Ayana Bio and Ginkgo, governs the TDPs under which the Company will perform agreed-upon research and development services in return for consideration on a cost-plus basis for all services provided. Ginkgo has agreed to provide Verb Biotics and Ayana Bio with licenses to certain of its intellectual property for use in the development, production and commercialization of each entity's products under the TDPs. The Company's common unit investment in Verb Biotics and Ayana Bio is accounted for as an equity method investment, and accordingly, Verb Biotics and Ayana Bio are related parties of Ginkgo. The initial carrying value of the equity method investment was equal to the fair value of the retained interest of $15.9 million for Verb Biotics and $16.0 million for Ayana Bio as of the applicable deconsolidation date. The Series A preferred units issued by Verb Biotics and Ayana Bio receive a liquidation preference prior to common units. As such, the Company concluded that this represents a substantive profit-sharing arrangement and the Company is recognizing earnings and losses on the equity method investment using the HLBV method. The Company recorded a loss on its equity method investment in Verb Biotics and Ayana Bio of $31.9 million in the year ended December 31, 2022, due to a basis difference associated with in-process research and development identified as part of the initial accounting for the equity method investment. This loss reduced the carrying value of the equity method investment in each of Verb Biotics and Ayana Bio to zero. There is no commitment for the Company to provide further financial support to Verb Biotics and Ayana Bio, and therefore the carrying value of the equity method investment will not be reduced below zero.
Ginkgo Bioworks Holdings, Inc.
Notes to Consolidated Financial Statements
Additional Variable Interest Entities
With respect to the Company’s investments in Motif, Allonnia, Genomatica, Arcaea, and BiomEdit, the Company has concluded these entities represent VIEs. While the Company has board representation on certain of these entities and is involved in the ongoing development activities of these entities via its participation on such entities' JSC, the Company has concluded that it is not the primary beneficiary of these entities because: (i) the Company does not control the board of directors of any of the VIEs, and no voting or consent agreements exist between the Company and other members of each respective board of directors or other investors, (ii) the holders of preferred security interests in the VIEs hold certain rights that require their consent prior to taking certain actions, which include certain significant operating and financing decisions, and (iii) the Company’s representation on the JSC of each respective entity does not give it control over the development activities of any of the VIEs, as all JSC decisions are made by consensus and there are no agreements in place that would require any of the entities to vote in alignment with the Company. As the Company’s involvement in the VIEs does not give it the power to control the decisions with respect to their development or other activities, which are their most significant activities, the Company has concluded that it is not the primary beneficiary of the VIEs.
With respect to Cooksonia’s investment in Joyn Bio prior to the joint venture’s termination on October 17, 2022 (see Note 4), as Cooksonia did not control Joyn Bio’s board of directors, it did not have the power to control the decisions related to the development activities of Joyn Bio, which were its most significant activities. Accordingly, the Company concluded that Cooksonia was not the primary beneficiary of Joyn Bio. The Company provided $10.0 million in financial support to Joyn Bio during the year ended December 31, 2022 in the form of convertible promissory notes (see Note 20), which were deemed necessary to fund Joyn Bio’s operations pre-dissolution. Joyn Bio was fully dissolved in 2023. Additionally, the Company holds equity interests in certain other privately-held companies that are not consolidated as the Company is not the primary beneficiary. As of December 31, 2024 and 2023, the maximum risk of loss related to the Company’s unconsolidated VIEs was limited to the carrying value of its investments in such entities.
Refer to Notes 6 and 16 for additional details on the Company’s investments and equity method investments. 8. Goodwill and Intangible Assets, net
During the year ended December 31, 2024, due to a sustained decrease in the market price of the Company's Class A common stock and market capitalization, the Company identified that an indicator of impairment was present during the second quarter of 2024. As such, the Company completed a quantitative impairment test related to its Cell Engineering reporting unit. To conduct the impairment test of goodwill, the estimated fair value of the reporting unit was compared to its carrying value. The estimated fair value of the reporting unit was determined using a weighted approach that considered a discounted cash flow (“DCF”) model under the income approach and the guideline public company (“GPC”) method under the market approach. Significant inputs used in the DCF model included the projected future operating results of the reporting unit and the applicable discount rate, while inputs used in the GPC method consisted of a revenue multiple. The projected future operating results were based on historical experience and internal annual operating plans reviewed by management, extrapolated over the forecast period. The discount rate was determined using a weighted average cost of capital adjusted for risk factors specific to the reporting unit. The revenue multiple was based on the GPC method using comparable publicly traded company multiples of revenue for a group of benchmark companies. The DCF method was weighted 75% and the GPC 25%. The fair value measurement of the reporting unit is classified as Level 3 in the fair value hierarchy because it involves significant unobservable inputs. The Company reconciled the resulting fair value of its reporting unit to the market capitalization of the Company to corroborate the fair value estimate used in the impairment test.
The result of the interim impairment test indicated that the estimated fair value of the reporting unit was less than its carrying value. As a result, the Company fully impaired goodwill and recorded an impairment loss of $47.9 million in the second quarter of 2024 and for the year ended December 31, 2024.
Ginkgo Bioworks Holdings, Inc.
Notes to Consolidated Financial Statements
Changes in the carrying amount of goodwill consisted of the following (in thousands):
| | | | | | | | | | | |
| As of December 31, |
| 2024 | | 2023 |
| Beginning balance | $ | 49,238 | | | $ | 60,210 | |
| Goodwill impairment (accumulated impairment loss) | (47,858) | | | — | |
| Deconsolidation of Zymergen | — | | | (10,660) | |
Measurement period adjustments (1) | — | | | (2,120) | |
| Impact of foreign currency translation | (1,380) | | | 1,808 | |
| Ending balance | $ | — | | | $ | 49,238 | |
(1)Primarily related to the Zymergen acquisition. See Note 4 for a description. Intangible assets, net consisted of the following (in thousands):
| | | | | | | | | | | | | | | | | | | | | | | |
| Gross Carrying Value (1) | | Accumulated Amortization (1) | | Net Carrying Value | | Weighted Average Amortization Period (in Years) |
| December 31, 2024: | | | | | | | |
| Developed technology | $ | 111,393 | | | $ | (38,883) | | | $ | 72,510 | | | 6.6 |
| December 31, 2023: | | | | | | | |
| Developed technology | $ | 105,279 | | | $ | (22,663) | | | $ | 82,616 | | | 8.8 |
| Customer relationships | 380 | | | (261) | | | 119 | | | 0.9 |
| Assembled workforce | 190 | | | (184) | | | 6 | | | 0.3 |
| Total | $ | 105,849 | | | $ | (23,108) | | | $ | 82,741 | | | |
(1)Gross carrying value and accumulated amortization include the impact of cumulative foreign currency translation adjustments.
During the year ended December 31, 2024, the increase in gross intangible assets was primarily attributable to the acquisition of $18.2 million in developed technology from AgBiome, partially offset by a $8.3 million decrease in developed technology intangible assets due to the deconsolidation of Altar (see Note 4). During the year ended December 31, 2023, gross intangible assets decreased $16.7 million due to the deconsolidation of Zymergen (see Note 4). Amortization expense was $18.0 million, $15.7 million and $5.6 million for the years ended December 31, 2024, 2023 and 2022, respectively. The estimated future amortization expense for intangible assets remaining as of December 31, 2024 is as follows (in thousands):
| | | | | |
| 2025 | $ | 18,896 | |
| 2026 | 18,896 | |
| 2027 | 11,260 | |
| 2028 | 2,633 | |
| 2029 | 2,633 | |
| Thereafter | 18,192 | |
| Total | $ | 72,510 | |
9. Leases
The Company leases real estate for office and lab space as well as equipment used in research and development activities under operating and finance leases.
Ginkgo Bioworks Holdings, Inc.
Notes to Consolidated Financial Statements
The Company’s real estate leases have initial lease terms ranging from 17 months to 15.3 years and are all classified as operating. Real estate leases may contain periods of free rent, tenant improvement incentives, expansion options, rent escalation clauses at pre-determined rates or at the prevailing market rates at the time of the increase, and options to extend or terminate the lease without cause at the option of either party during the lease term. The Company is not reasonably certain to exercise these options at the commencement of the lease. Equipment leases have initial lease terms ranging from 26 to 60 months and are classified as operating or finance if the lease contains bargain purchase options which the Company is reasonably certain to exercise.
Variable lease cost for real estate leases primarily consists of certain non-lease components such as real estate taxes, insurance and common area maintenance charges. These non-lease components are typically variable in nature and are recognized as lease expense in the period in which they arise. None of the Company's lease agreements contain material restrictive covenants or residual value guarantees.
The Company's headquarters are located in the Seaport district of Boston, Massachusetts and comprise a set of non-cancellable operating leases within a facility totaling over 320,000 square feet of office and laboratory space. These leases expire on dates ranging from 2030 to 2036 and each contain one option to extend the lease for a five-year period at then-market rates. Of this 320,000 square feet, 27,000 is currently subleased.
In April 2021, the Company entered into a lease, as amended, consisting of approximately 260,000 rentable square feet of new office and laboratory space being developed in Boston, Massachusetts near the Company's headquarters. The lease commenced on April 11, 2024, with rent payments beginning in June 2024, and it will expire on the fifteenth anniversary of the rent commencement date. The lease includes an option to extend for an additional ten years at then-market rates, as well as an expansion option if the owner constructs an additional building on the property.
The Company has a substantial amount of excess space and is seeking to sublease excess space consistent with its restructuring plan. The leased facilities continue to be included in the Cell Engineering asset group as they have not been abandoned and do not have separately identifiable cash flows. If the Company enters into subleases at rates that are below the existing lease minimum payments, terminates or amends existing leases, or abandons the leased facilities, the right-of-use lease assets and any associated leasehold improvements would be evaluated for potential impairment and impairment charges could be material.
In September 2023, the Company’s former subsidiary, Zymergen, ceased the use of and exited a leased facility consisting of approximately 300,000 square feet of office and laboratory space in Emeryville, California. The facility was used pursuant to an operating lease with a minimum term expiring in August 2033. Zymergen’s exit resulted in an impairment loss of $96.2 million, including $36.6 million for the right-of-use asset and $59.6 million for the related leasehold improvements. The impairment loss represents the amount by which the carrying value of the assets exceed their estimated fair values, as determined using a discounted cash flow model under the income approach. The fair value measurements are based on significant inputs not observable in the market and therefore represent Level 3 fair value measurements. The key inputs used in the valuation were estimated sublease rental income and a discount rate of 8.5%. The impairments are presented as impairment of lease assets in the consolidated statements of operations and comprehensive loss for the year ended December 31, 2023.
Ginkgo Bioworks Holdings, Inc.
Notes to Consolidated Financial Statements
The following table presents the components of total lease cost (in thousands):
| | | | | | | | | | | | | | | | | |
| Year Ended December 31, |
| 2024 | | 2023 | | 2022 |
| Operating lease cost | $ | 57,996 | | | $ | 59,588 | | | $ | 35,242 | |
| Finance lease cost: | | | | | |
| Amortization of ROU assets | 655 | | | 1,047 | | | 1,871 | |
| Interest on lease liabilities | 29 | | | 79 | | | 104 | |
| Finance lease cost | 684 | | | 1,126 | | | 1,975 | |
| Variable lease cost | 19,421 | | | 15,862 | | | 8,879 | |
| Sublease income | (5,177) | | | (11,170) | | | (5,190) | |
| Total lease cost | $ | 72,924 | | | $ | 65,406 | | | $ | 40,906 | |
Supplemental cash flow information related to the Company’s operating leases were as follows (in thousands):
| | | | | | | | | | | | | | | | | |
| Year Ended December 31, |
| 2024 | | 2023 | | 2022 |
| Cash paid for amounts included in the measurement of lease liabilities: | | | | | |
| Operating cash flows from operating leases | $ | 45,019 | | | $ | 44,051 | | | $ | 13,587 | |
| Operating cash flows from finance leases | 32 | | | 83 | | | 92 | |
| Financing cash flows from finance leases | 897 | | | 1,295 | | | 1,237 | |
Supplemental balance sheet information related to operating leases were as follows:
| | | | | | | | | | | |
| As of December 31, |
| 2024 | | 2023 |
| Weighted average remaining lease term - operating leases (in years) | 11.8 | | 10.1 |
| Weighted average remaining lease term - finance leases (in years) | 0.6 | | 1.5 |
| Weighted average discount rate - operating leases | 7.5 | % | | 7.1 | % |
| Weighted average discount rate - finance leases | 3.8 | % | | 3.7 | % |
The following table summarizes the maturity of the Company’s lease liabilities (in thousands):
| | | | | | | | | | | |
| Years Ending December 31, | Operating Leases | | Finance Leases |
| 2025 | $ | 61,225 | | | $ | 333 | |
| 2026 | 56,218 | | | 17 | |
| 2027 | 56,683 | | | — | |
| 2028 | 58,431 | | | — | |
| 2029 | 60,199 | | | — | |
| Thereafter | 430,919 | | | — | |
| Total undiscounted payments | 723,675 | | | 350 | |
| Less: imputed interest | (256,279) | | | 11 | |
| Total lease liability | 467,396 | | | 361 | |
| Less: current portion of lease liability | (28,630) | | | (335) | |
| Lease liabilities, non-current | $ | 438,766 | | | $ | 26 | |
The Company subleases a portion of its office and lab space to certain of its equity method investees, which are considered related parties. These lease agreements generally have lease terms of up to 5 years and may include renewal options. Related party sublease income for the years ended December 31, 2024, 2023 and 2022 was $2.0 million, $2.1 million and
Ginkgo Bioworks Holdings, Inc.
Notes to Consolidated Financial Statements
$3.5 million, respectively, included within other income, net in the consolidated statements of operations and comprehensive loss.
10. Supplemental Financial Information
Cash, Cash Equivalents and Restricted Cash
The reconciliation of cash, cash equivalents and restricted cash reported within the consolidated balance sheet to the totals shown within the consolidated statements of cash flows is as follows (in thousands):
| | | | | | | | | | | | | | | | | |
| As of December 31, |
| 2024 | | 2023 | | 2022 |
| Cash and cash equivalents | $ | 561,572 | | | $ | 944,073 | | | $ | 1,315,792 | |
Restricted cash included in prepaid expenses and other current assets (1) | 5,839 | | | 4,789 | | | 8,221 | |
Restricted cash included in other non-current assets (1) | 38,332 | | | 40,722 | | | 45,568 | |
| Total cash, cash equivalents and restricted cash | $ | 605,743 | | | $ | 989,584 | | | $ | 1,369,581 | |
(1)Includes cash balances collateralizing letters of credit associated with the Company’s facility leases and customer prepayments requiring segregation and restrictions in its use in accordance with the customer agreement.
Property, Plant and Equipment, net
Property, plant and equipment, net consisted of the following (in thousands):
| | | | | | | | | | | |
| As of December 31, |
| 2024 | | 2023 |
| Lab equipment | $ | 150,887 | | | $ | 147,185 | |
| Leasehold improvements | 135,964 | | | 71,564 | |
| Buildings and facilities | 48,255 | | | 47,034 | |
| Construction in progress | 1,984 | | | 15,830 | |
| Computer equipment and software | 14,897 | | | 14,780 | |
| Furniture and fixtures | 6,545 | | | 6,458 | |
| Land | 6,060 | | | 6,060 | |
| Total property, plant, and equipment | 364,592 | | | 308,911 | |
| Less: Accumulated depreciation | (160,872) | | | (120,718) | |
| Property, plant and equipment, net | $ | 203,720 | | | $ | 188,193 | |
Depreciation expense for the years ended December 31, 2024, 2023 and 2022 totaled $45.0 million, $54.8 million and $36.9 million, respectively.
During the year ended December 31, 2024, the Company determined that $5.8 million of construction in progress assets were impaired and this loss is included in general and administrative expense in the consolidated statements of operations and comprehensive loss.
During the year ended December 31, 2023, the Company identified excess lab equipment at two of its facilities whereby the assets were sold, classified as held for sale or otherwise impaired, resulting in aggregate impairment losses of $25.2 million, included in general and administrative expense in the consolidated statements of operations and comprehensive loss.
Ginkgo Bioworks Holdings, Inc.
Notes to Consolidated Financial Statements
Accrued Expenses and Other Current Liabilities
Accrued expenses and other current liabilities consisted of the following (in thousands):
| | | | | | | | | | | |
| As of December 31, |
| 2024 | | 2023 |
| Operating lease liabilities | $ | 28,630 | | | $ | 16,419 | |
| Employee compensation and benefits | 9,894 | | | 15,678 | |
| Contingent consideration liability | 5,438 | | | 18,468 | |
| Biosecurity costs | 4,032 | | | 3,564 | |
| Deferred other income | 2,889 | | | 4,009 | |
| Employee termination costs | 2,387 | | | — | |
| Professional fees and securities litigation costs | 2,167 | | | 27,884 | |
| External research and development expenses | 713 | | | 2,739 | |
| Finance lease liabilities | 335 | | | 1,055 | |
| Property and equipment | 138 | | | 2,667 | |
| Other current liabilities | 8,764 | | | 17,568 | |
| Accrued expenses and other current liabilities | $ | 65,387 | | | $ | 110,051 | |
Supplemental cash flow information
The following table presents supplemental cash flow information for each reporting period (in thousands):
| | | | | | | | | | | | | | | | | |
| Year Ended December 31, |
| 2024 | | 2023 | | 2022 |
| Cash paid for interest | $ | 32 | | | $ | 83 | | | $ | 92 | |
| Cash paid for income taxes | 894 | | | 670 | | | — | |
| Non-cash investing and financing activities: | | | | | |
| ROU assets obtained in exchange for new operating lease liabilities upon adoption of ASC 842 | $ | — | | | $ | — | | | $ | 147,744 | |
| ROU assets obtained in exchange for new finance lease liabilities upon adoption of ASC 842 | — | | | — | | | 3,397 | |
| ROU assets obtained in exchange for new operating lease liabilities | 223,853 | | | 27,668 | | | 79,984 | |
| ROU assets obtained in exchange for new finance lease liabilities | — | | | — | | | 1,729 | |
| Purchase of minority interest in Cooksonia | — | | | — | | | 7,390 | |
| Purchases of property and equipment included in accounts payable and accrued expenses | 2,565 | | | 2,915 | | | 12,881 | |
| Equity received in related parties | — | | | — | | | 8,873 | |
| Convertible financial instruments received for Cell Engineering services | — | | | 4,542 | | | 29,074 | |
| Equity securities and warrants received for Cell Engineering services | 55 | | | 17,450 | | | 3,423 | |
| Non-cash consideration paid for the acquisition of Zymergen | — | | | — | | | 231,750 | |
| Common stock issued for acquisitions | 18,245 | | | 6,820 | | | 40,382 | |
| Acquisition date fair value of contingent consideration liability | — | | | — | | | 19,912 | |
| Common stock issued as settlement of contingent consideration liabilities | 14,742 | | | 8,896 | | | — | |
| Common stock issued for retention payments related to business and asset acquisitions | 5,258 | | | — | | | — | |
| Return of investment in equity securities for reduction in deferred revenue | 6,760 | | | — | | | — | |
| Conversion of notes receivable for common stock | 10,476 | | | — | | | — | |
| Equity issuance costs in accounts payable and accrued expenses | — | | | — | | | 578 | |
Ginkgo Bioworks Holdings, Inc.
Notes to Consolidated Financial Statements
11. Commitments and Contingencies
Purchase Obligations
On August 29, 2023, the Company entered into a five-year strategic cloud and artificial intelligence (“AI”) partnership with Google Cloud, intended to enable the Company to develop and deploy AI tools for biology and biosecurity. The partnership includes minimum annual commitments over the contract year ending August 31 to purchase cloud hosting services in exchange for various discounts on such services. The minimum annual commitments are as follows: year 1, $8.0 million; year 2, $28.0 million; year 3, $54.0 million; year 4, $86.0 million; and year 5, $113.0 million. The Company purchased $11.6 million in year 1 of the contract and currently expects to have a shortfall in year 2. The minimum commitments may be terminated by the Company upon payment of a cancellation fee representing a percentage of the remaining purchase commitment. If the Company is unable to negotiate a modification to the annual commitments that align with its projected requirements, material losses could be incurred. As of December 31, 2024, the aggregate remaining purchase commitment was $279.3 million.
The Company also entered into an agreement pursuant to which Google Cloud will provide up to $56.3 million in cash funding upon the Company’s achievement of certain milestones by the target completion dates through September 2026. The costs of Google Cloud services are recorded as research and development expenses as incurred in the accompanying consolidated statements of operations and comprehensive loss. Milestone payments received are recognized as a reduction of the associated Google Cloud services costs within research and development expenses when achieved. The first two milestones are initially recognized as liabilities until they become non-refundable upon the Company's achievement of a certain milestone. As of December 31, 2024, the Company has received three milestone payments totaling $10.0 million, with $5.0 million recorded as a reduction to research and development expenses during the year ended December 31, 2024 and $5.0 million recorded as other non-current liability on the balance sheet.
On March 31, 2022, the Company entered into a four-year supply agreement with Twist for the purchase of diverse products including synthetic DNA. The agreement is effective as of April 1, 2022 and obligates the Company to spend a minimum of $58.0 million over the four-year term with the following minimum annual commitments (each annual year is defined as April 1 to March 31): year 1, $10.0 million; year 2, $13.0 million; year 3, $16.0 million; and year 4, $19.0 million. During the contract period ended March 31, 2024, the Company purchased $13.0 million and currently expects to have a shortfall for year 3 of the contract. If the Company is unable to negotiate a modification to the annual commitments that align with its expected requirements, losses could be incurred. As of December 31, 2024, the aggregate remaining purchase commitment was $29.6 million.
Contingent Consideration Related to Asset Acquisitions
In connection with the StrideBio acquisition (see Note 4), the Company is obligated to make royalty payments of up to $21.3 million payable in cash or shares of Class A common stock at the Company's election until the earlier of the tenth anniversary date of the initial closing and the date on which the aggregate amount of the royalty payments equals the amount cap. The royalties are calculated based on 10% of the net licensing revenue and 40% of all consideration received for a license or sale of a product incorporating the acquired platform assets. No amounts for the royalty payments have been recorded during the years ended December 31, 2024 and 2023. The Company routinely acquires rights to intellectual property that may provide for payment of future contingent consideration, including royalties, should revenue be generated from the use of such.
Legal Proceedings
From time to time, the Company may in the ordinary course of business be named as a defendant in lawsuits, indemnity claims and other legal proceedings. The Company accrues for a loss contingency when it concludes that the likelihood of a loss is probable and the amount of loss can be reasonably estimated. The Company adjusts its accruals from time to time as it receives additional information. The Company does not believe any pending litigation to be material, or that the outcome of any such pending litigation, in management’s judgment based on information currently available, would have a material adverse effect on the Company’s results of operations, cash flows or financial condition.
Indemnification Agreements
The Company enters into standard indemnification agreements and has agreements with indemnification clauses in the ordinary course of business. Under such arrangements, the Company indemnifies, holds harmless and agrees to reimburse the indemnified party for losses suffered or incurred by the indemnified party, who are generally the Company’s business partners. The terms of these indemnification arrangements are generally perpetual and effective any time after contract
Ginkgo Bioworks Holdings, Inc.
Notes to Consolidated Financial Statements
execution. The maximum potential liability resulting from these indemnification arrangements may be unlimited. The Company has never incurred costs to defend lawsuits or settle claims as a result of such indemnifications and the Company is not aware of any indemnification arrangements that could have a material effect on its financial position, results of operations or cash flows, and it has not accrued any liabilities related to such obligations as of December 31, 2024.
12. Stockholders' Equity
Capitalization
The following table presents the Company’s authorized, issued, and outstanding common stock as of the dates indicated:
| | | | | | | | | | | | | | | | | |
| Authorized | | Issued | | Outstanding |
| Common stock as of December 31, 2024 | | | | | |
| Class A | 10,500,000,000 | | 45,575,423 | | 42,696,585 |
| Class B | 4,500,000,000 | | 9,239,682 | | 8,669,200 |
| Class C | 800,000,000 | | 3,000,000 | | 3,000,000 |
| 15,800,000,000 | | 57,815,105 | | 54,365,785 |
| Common stock as of December 31, 2023 | | | | | |
| Class A | 10,500,000,000 | | 40,997,131 | | 38,126,447 |
| Class B | 4,500,000,000 | | 9,477,690 | | 8,906,426 |
| Class C | 800,000,000 | | 3,000,000 | | 3,000,000 |
| 15,800,000,000 | | 53,474,821 | | 50,032,873 |
Shelf Registration Statement
On October 4, 2022, the Company filed with the Securities and Exchange Commission (“SEC”) a shelf registration statement on Form S-3 (File No. 333-267743), which was declared effective on October 14, 2022. Under the shelf registration, the Company may offer and sell from time to time, in one or more series or issuances and on terms determined at the time of the offering, any combination of its Class A common stock, preferred stock, warrants and/or units up to an aggregate amount of $500 million. As of December 31, 2024, approximately $400 million remain available under the shelf registration.
Underwritten Public Offering
On November 15, 2022, the Company entered into an underwriting agreement (the “Underwriting Agreement”) with BTIG, LLC (the “Underwriter”), pursuant to which the Company agreed to issue and sell to the Underwriter an aggregate of 1,034,597 shares at a public offering price of $96.66 per share, representing an underwriting discount of 9%. Under the terms of the Underwriting Agreement, the Company granted the Underwriter an option exercisable for 30 days to purchase up to an additional 155,190 shares of its Class A common stock, which expired unexercised. The shares were sold pursuant to an effective shelf registration statement on Form S-3 (File No. 333-267743) and a related prospectus supplement filed with the SEC. The net proceeds to the Company from the offering was approximately $98.9 million, after deducting offering expenses. The net proceeds of this offering were used to offset the cash used to finance the acquisition of certain of the assets and liabilities of Bayer and for other general corporate purposes.
Preferred Stock
The Company is authorized to issue 200 million shares of preferred stock with a par value $0.0001 per share. The Company’s board of directors are authorized, without stockholder approval, to issue such shares of preferred stock in one or more series, to establish from time to time the number of shares to be included in each such series, and to fix the designations, powers, voting, and other rights, preferences and privileges of the shares. There were no issued and outstanding shares of preferred stock as of December 31, 2024.
Ginkgo Bioworks Holdings, Inc.
Notes to Consolidated Financial Statements
Common Stock
The Company is authorized to issue 15,800 million shares of common stock, including 10,500 million shares of Class A common stock, par value $0.0001 per share, 4,500 million shares of Class B common stock, par value $0.0001 per share, and 800 million shares of Class C common stock, par value $0.0001 per share.
Voting
Holders of Class A common stock are entitled to one vote per share and holders of Class B common stock are entitled to ten votes per share. Holders of Class C common stock are not entitled to vote except as otherwise expressly provided in the certificate of incorporation or required by applicable law.
Dividends
Common stockholders are entitled to receive dividends, as may be declared by the board of directors. Different classes of common stock are legally entitled to equal per share distributions whether through dividends or liquidation. No dividends have been declared to date.
Conversion
Each share of Class B common stock is convertible at any time at the option of the holder into one share of Class A common stock. Generally, shares of Class B common stock will convert automatically into Class A common stock upon the holder ceasing to be an Eligible Holder (i.e., director, employee, trust or legal entity of Ginkgo), unless otherwise determined by affirmative vote of a majority of independent directors of Ginkgo.
Common Stock Reserved for Future Issuances
The Company had the following common stock reserved for future issuance as of the date indicated:
| | | | | |
| December 31, 2024 |
| Shares available for grant under the 2021 Plan | 4,868,329 |
| Restricted stock units outstanding | 3,327,398 |
| Warrants to purchase Class A common stock | 1,295,622 |
| Shares available for grant under the ESPP | 1,965,466 |
| Shares available for grant under the 2022 Inducement Plan | 186,770 |
| Stock options issued and outstanding | 767,520 |
Total common stock reserved for future issuances (1) | 12,411,105 |
(1)Excludes unvested earnout shares, which are restricted shares issued to equity holders of legacy Ginkgo prior to the closing of the SRNG Business Combination and to SRNG. These earnout shares are recorded in equity as shares outstanding upon satisfying the vesting conditions.
13. Stock-Based Compensation
The following table summarizes stock-based compensation expense by financial statement line item in the Company’s consolidated statements of operations and comprehensive loss for the periods presented (in thousands):
| | | | | | | | | | | | | | | | | |
| Year Ended December 31, |
| 2024 | | 2023 | | 2022 |
| Research and development | $ | 56,020 | | | $ | 145,879 | | | $ | 731,996 | |
| General and administrative | 56,324 | | | 84,005 | | | 1,198,645 | |
| Total | $ | 112,344 | | | $ | 229,884 | | | $ | 1,930,641 | |
2022 Inducement Plan
On October 16, 2022, the Company's Board of Directors adopted the Ginkgo Bioworks Holdings, Inc. 2022 Inducement Plan (the “2022 Inducement Plan”), which is a non-shareholder approved equity incentive plan adopted pursuant to the
Ginkgo Bioworks Holdings, Inc.
Notes to Consolidated Financial Statements
“inducement exception” provided under NYSE Listed Company Manual Section 303A.08. Pursuant to the terms of the 2022 Inducement Plan, the Company may grant nonstatutory stock options, stock appreciation rights, restricted stock units, restricted stock and other stock-based awards as an inducement material to individuals being hired or rehired following a bona fide period of interruption of employment, as an employee of the Company or any of its subsidiaries, including in connection with a merger or acquisition. The terms of the 2022 Inducement Plan are substantially similar to the terms of the Company’s 2021 Incentive Award Plan. The Company has reserved 625,000 shares of the Company’s common stock (which may be shares of Class A common stock or Class B common stock) for issuance under the 2022 Inducement Plan. As of December 31, 2024, 186,770 shares are available for future issuance under the 2022 Inducement Plan.
2021 Incentive Award Plans
On September 16, 2021, the 2021 Incentive Award Plan (the “2021 Plan”) became effective. The 2021 Plan provides for the grant of stock options, including incentive stock options (“ISOs”) and nonqualified stock options, stock appreciation rights, restricted stock, dividend equivalents, RSUs and other stock or cash-based awards to employees, consultants and directors of Ginkgo and its subsidiaries.
The aggregate number of shares of common stock available for issuance under the 2021 Plan, which may be issued as Class A common stock and/or Class B common stock, was initially 5,011,024 shares. As of December 31, 2024, 4,868,329 shares are available for future issuance under the 2021 Plan. The number of shares of common stock reserved for issuance under the 2021 Plan will automatically increase for ten years on January 1 of each year in an amount equal to the lesser of (a) 4% of the aggregate number of shares of common stock outstanding on the final day of the immediately preceding calendar year and (b) such smaller number of shares as is determined by the Board. The maximum number of shares of common stock that may be issued pursuant to the exercise of incentive stock options granted under the 2021 Plan is 5,000,000 shares. Shares issued under the 2021 Plan may consist of authorized but unissued shares, shares purchased on the open market or treasury shares.
2021 Employee Stock Purchase Plan
On September 16, 2021, the 2021 Employee Stock Purchase Plan (the “ESPP”) became effective. The ESPP authorizes (i) the grant of options that are intended to qualify for favorable U.S. federal tax treatment under Section 423 of the Internal Revenue Code of 1986 (the “Section 423 Component”) and (ii) the grant of options that are not intended to be tax-qualified (the “Non-Section 423 Component”). All of the Company’s employees are expected to be eligible to participate in the ESPP. However, with respect to the Section 423 Component, an employee may not be granted rights to purchase stock under the ESPP if the employee, immediately after the grant, would own (directly or through attribution) stock possessing 5% or more of the total combined voting power or value of all classes of the Company’s common stock.
The ESPP initially permits the Company to deliver up to 500,000 shares of common stock pursuant to awards issued under the ESPP, which may be Class A common stock and/or Class B common stock. The number of shares of common stock reserved for issuance under the ESPP will automatically increase each January 1 by an amount equal to the lesser of (a) 1% of the aggregate number of shares of common stock outstanding on the final day of the immediately preceding calendar year and (b) such smaller number of shares as is determined by the Board, provided that no more than 2,500,000 shares may be issued under the Section 423 Component. Prior to or in connection with issuing any shares of common stock under the ESPP, the ESPP administrator may convert awards covering shares of Class B common stock to Class A common stock. As of December 31, 2024, no awards have been granted under the ESPP, and 1,965,466 shares remain available for future issuance.
2014 Stock Incentive Plan
The 2014 Stock Incentive Plan (the “2014 Plan”) provided for the Company to grant options, stock appreciation rights, restricted stock, restricted stock units (“RSUs”) and other stock-based awards. From and after the effective date of the 2021 Incentive Award Plan, the Company ceased granting awards under the 2014 Plan. However, the 2014 Plan continues to govern the terms and conditions of the outstanding awards previously granted thereunder. Shares of common stock underlying any awards that are forfeited, cancelled, repurchased, or otherwise terminated by the Company under the 2014 Plan will be added back to the shares available for issuance under the 2021 Incentive Award Plan.
Time-based Stock Options
All time-based options outstanding consist of awards granted to non-employee directors and are of two types: (i) initial awards granted to newly elected or appointed directors, which vest in three equal annual installments, and (ii) subsequent
Ginkgo Bioworks Holdings, Inc.
Notes to Consolidated Financial Statements
awards, which vest on the earlier of the first anniversary of the grant date or the day prior to the next annual shareholder meeting. These options expire no later than ten years from the grant date. The exercise price of each option is equal to the closing price of the Company’s common stock on the date of grant.
A summary of time-based stock options activity for the year ended December 31, 2024 is presented below:
| | | | | | | | | | | | | | | | | | | | | | | |
| Number of Shares | | Weighted Average Exercise Price per Share | | Weighted Average Remaining Contractual Term (in years) | | Aggregate Intrinsic Value (1) |
| Outstanding as of December 31, 2023 | 151,232 | | $ | 35.59 | | | | | |
| Granted | 246,700 | | 14.72 | | | | | |
| Exercised | (101,976) | | | 0.80 | | | | | |
| Forfeited | (28,436) | | | 77.36 | | | | | |
| Outstanding as of December 31, 2024 | 267,520 | | 25.17 | | | 9.21 | | $ | 138 | |
| Exercisable as of December 31, 2024 | 33,547 | | 94.75 | | | 6.53 | | — | |
(1)The aggregate intrinsic value is calculated as the difference between the Company's closing stock price on the last trading day of the year and the exercise prices, multiplied by the number of in-the-money stock options.
The total intrinsic value of options exercised during the years ended December 31, 2024, 2023 and 2022 was $0.9 million, $9.1 million and $21.5 million, respectively. The weighted-average fair value of options granted during the years ended December 31, 2024, 2023, and 2022 was $11.35, $57.20 and $76.80 per share, respectively, and was calculated using the following key assumptions in the Black-Scholes option-pricing model:
| | | | | | | | | | | | | | | | | |
| Year Ended December 31, |
| 2024 | | 2023 | | 2022 |
| Risk-free interest rate | 4.27 | % | | 3.94 | % | | 3.13 | % |
| Expected volatility | 97 | % | | 93 | % | | 77 | % |
| Expected term (in years) | 5.7 | | 5.5 | | 5.6 |
| Dividend yield | — | % | | — | % | | — | % |
As of December 31, 2024, there was $1.8 million of unrecognized compensation expense related to options recognizable over a weighted-average period of 1.7 years.
Market-based Stock Options
In April 2024, the Company granted to each of the Company's four founders an option to purchase in aggregate 125,000 shares of Ginkgo's Class A common stock with an exercise price of $100 per share, subject both to time-based and market-based vesting criteria (the “Founder Options”). The market-based vesting is tied to the achievement of four specified stock price hurdles within a five-year period, with 10% of the Founder Options vesting based on the achievement of a 90-calendar-day average stock price of $200, 10% of the Founder Options vesting based on the achievement of a 90-calendar-day average stock price of $300, 20% of the Founder Options vesting based on the achievement of a 90-calendar-day average stock price of $400 and the remaining 60% of the Founder Options vesting based on the achievement of a 90-calendar-day average stock price of $500. If the market-based criteria are achieved during the five-year period, the awards will vest on the five-year anniversary of the grant date.
Ginkgo Bioworks Holdings, Inc.
Notes to Consolidated Financial Statements
The weighted-average grant-date fair value of the options granted was $7.80 per share and was calculated using a Monte Carlo simulation model with the following assumptions:
| | | | | |
| Year Ended December 31, 2024 |
| Risk-free interest rate | 4.65 | % |
| Expected volatility | 72 | % |
| Suboptimal exercise multiple | 2.8 |
| Dividend yield | — | % |
As of December 31, 2024, there was $3.4 million of unrecognized compensation expense related to the market-based options recognizable over a weighted-average period of 4.3 years.
Restricted Stock Units
RSUs granted under the 2014 Plan are subject to two vesting conditions: (i) a service-based vesting condition, generally satisfied over four years with 25% of the shares vesting on the first anniversary of the grant date and monthly vesting thereafter, and (ii) a performance-based vesting condition, which was met in 2021 in connection with the Company's merger with SRNG. RSUs granted under the 2021 Plan are subject only to the service-based vesting condition.
A summary of RSU activity for the year ended December 31, 2024 is presented below:
| | | | | | | | | | | |
| Number of Shares | | Weighted Average Grant Date Fair Value |
| Nonvested as of December 31, 2023 | 3,805,093 | | $ | 125.89 | |
| Granted | 3,077,650 | | 43.81 | |
| Vested | (1,672,658) | | 139.26 | |
| Forfeited | (1,882,687) | | 80.77 | |
| Nonvested as of December 31, 2024 | 3,327,398 | | 69.00 | |
The weighted average grant date fair value of RSUs granted during the years ended December 31, 2024, 2023 and 2022 was $43.81, $55.60 and $127.60, respectively. The total fair value of the RSUs that vested during the years ended December 31, 2024, 2023 and 2022 was $232.9 million, $365.3 million and $1,783.8 million, respectively. The total amount of share-based liabilities settled was $9.8 million for the year ended December 31, 2022.
As of December 31, 2024, there was $189.8 million of unrecognized compensation expense related to RSUs recognizable over a weighted-average period of 2.6 years.
Earnouts
Earnout shares represent equity awards, primarily in the form of restricted stock, granted to existing employees of the Company as of the closing date of the Company's merger with SRNG on September 16, 2021 (the “Closing Date”). These earnout shares are subject to the same time-based vesting and performance conditions (change in control or an initial public offering) as the underlying awards, including provisions related to vesting and termination. Additionally, the earnout shares are subject to a market condition, which is satisfied when the trading price of the Company's common stock is greater than or equal to $500, $600, $700 and $800 per share for any 20 trading days within a 30 consecutive trading day period, on or before the fifth anniversary of the Closing Date (collectively, the “Earnout Targets”). The first Earnout Target of $500 per share was achieved on November 15, 2021.
Ginkgo Bioworks Holdings, Inc.
Notes to Consolidated Financial Statements
A summary of activity during the year ended December 31, 2024 for the earnout shares is presented below:
| | | | | | | | | | | |
| Number of Shares | | Weighted Average Grant Date Fair Value |
| Nonvested as of December 31, 2023 | 564,227 | | $ | 511.17 | |
| Vested | (8,466) | | 533.60 | |
| Forfeited | (3,304) | | 515.30 | |
| Nonvested as of December 31, 2024 | 552,457 | | 510.80 | |
The total fair value of the earnout shares that vested during the years ended December 31, 2024, 2023 and 2022 was $4.5 million, $7.6 million and $52.0 million, respectively.
As of December 31, 2024, there was $0.2 million of unrecognized compensation expense related to earnout shares recognizable over a weighted-average period of 0.5 years.
14. Revenue Recognition
Disaggregation of Revenue
The following table sets forth the percentage of Cell Engineering revenues by industry based on total Cell Engineering revenue:
| | | | | | | | | | | | | | | | | |
| Year Ended December 31, |
| 2024 | | 2023 | | 2022 |
| Food and nutrition | 32 | % | | 16 | % | | 9 | % |
| Pharmaceutical and biotechnology | 26 | | | 30 | | | 22 | |
| Agriculture | 19 | | | 24 | | | 8 | |
| Government and defense | 13 | | | 6 | | | 4 | |
| Industrial and environment | 6 | | | 16 | | | 12 | |
| Consumer and technology | 4 | | | 8 | | | 45 | |
| Total Cell Engineering revenue | 100 | % | | 100 | % | | 100 | % |
Cell Engineering revenue includes both cash and non-cash consideration. The non-cash consideration primarily consists of equity received from customers as partial or full payment in certain contracts, which is recognized as revenue as services are provided or upon contract termination. The Company did not receive equity as consideration for any customer contracts entered into during the year ended December 31, 2024, but continues to recognize revenue from prior contracts. Cell Engineering revenue recognized relating to non-cash consideration was $61.4 million, $48.5 million, and $75.8 million for the years ended December 31, 2024, 2023, and 2022, respectively.
The Company’s total revenue is derived from customers located primarily in the United States. For the years ended December 31, 2024, 2023, and 2022, the Company’s revenue from customers within the United States comprised 81%, 82% and 88%, respectively, of total revenue.
Contract Balances
The Company recognizes a contract asset when the Company transfers goods or services to a customer before the customer pays consideration or before payment is due, excluding any amounts presented as accounts receivable. The Company had no contract asset balances as of December 31, 2024 and 2023. The Company’s accounts receivable consists of both billed and unbilled amounts. Unbilled receivables arise when revenue is recognized in excess of invoiced amounts and represent the Company’s unconditional right to consideration for goods or services already transferred to the customer. The balance of unbilled accounts receivable, included in accounts receivable, net in the accompanying consolidated balance sheets, was $11.3 million and $10.1 million as of December 31, 2024 and 2023, respectively.
Ginkgo Bioworks Holdings, Inc.
Notes to Consolidated Financial Statements
Contract liabilities, or deferred revenue, primarily consist of payments received in advance of performance under the contract or when the Company has an unconditional right to consideration under the terms of the contract before it transfers goods or services to the customer. The Company’s collaborative arrangements with its investees and related parties typically include upfront payments consisting of cash or non-cash consideration for future research and development services and non-cash consideration in the form of convertible financial instruments and equity securities for licenses that will be transferred in the future. The Company records the upfront cash payments and fair value of the convertible financial instruments and equity securities as deferred revenue.
The Company also invoices customers based on contractual billing schedules, which results in the recording of deferred revenue to the extent payment is received prior to the Company’s performance of the related services. Contract liabilities are recognized as revenue as (or when) the Company performs under the contract.
During the year ended December 31, 2024, the Company recognized $88.1 million of revenue that was included in the contract liabilities balance of $202.5 million as of December 31, 2023. During the year ended December 31, 2023, the Company recognized $65.9 million of revenue that was included in the contract liabilities balance of $222.6 million as of December 31, 2022.
Performance Obligations
The aggregate amount of the transaction price that was allocated to performance obligations that have not yet been satisfied or are partially satisfied as of December 31, 2024 and 2023 was $85.8 million and $110.0 million, respectively. The Company has elected the practical expedient not to provide the remaining performance obligation disclosures related to contracts for which the Company recognizes revenue on a cost-plus basis in the amount to which it has the right to invoice. As of December 31, 2024, approximately $45.9 million of the unsatisfied or partially satisfied performance obligations is expected to be recognized as revenue in 2025, based on the projected customer program end date; $14.2 million between 2025 and 2026; and $25.7 million between 2025 and 2027.
When a milestone subject to the variable consideration constraint is achieved, the Company updates its estimate of the transaction price to include the milestone payment and records a cumulative catch-up in revenue. For the years ended December 31, 2024, 2023 and 2022, the Company recorded $7.2 million, $2.3 million and $10.0 million, respectively, as cumulative catch-up in revenue, primarily due to the recognition of previously constrained variable consideration related to milestones or a contract modification.
15. Segment Information
The Company operates in two operating and reportable segments: Cell Engineering and Biosecurity. This structure reflects the Company's internal management framework and the approach its CODM uses to evaluate operating results and allocate resources. The Company’s reportable segments are described as follows:
•Cell Engineering consists of end-to-end cell engineering solutions and cell engineering tools offerings for biological R&D. The Company’s cell engineering platform includes two core assets: the Foundry, a highly efficient biology laboratory powered by proprietary workflows, custom software, robotic automation, and data science and analytics, and the Codebase, a collection of biological “parts” and a database of biological data used to program cells. The Cell Engineering segment includes costs incurred for the development, operation, expansion and enhancement of the Foundry and Codebase. Cell Engineering revenue is generated primarily through service fees and downstream value share in the form of milestone payments, royalties or equity interests.
•Biosecurity consists of the Company’s biomonitoring and bioinformatics support services, offered to both government and non-government customers through the Company's two core offerings: Canopy and Horizon. Biosecurity revenue is generated from fees for data, analytics, and services. Prior to 2024, Biosecurity revenue also included sales of COVID-19 diagnostic and sample collection test kits.
The Company's reportable segments are those for which discrete financial information is available and whose results are regularly provided to the Company’s CODM, consisting of the Chief Executive Officer and the Chief Operating Officer, for the purpose of allocating resources and assessing financial performance. The CODM evaluates the financial performance of the Company’s segments based on segment operating income (loss). The CODM is primarily provided with the segment operating income (loss) on a quarterly basis, as well as during the annual budgeting and forecasting process, and uses this information to monitor the Company’s performance, including budget-to-actual results, and to make decisions about the allocation of operating and capital resources to each segment. For management reporting purposes, the
Ginkgo Bioworks Holdings, Inc.
Notes to Consolidated Financial Statements
Company’s measure of segment operating income (loss) excludes the impact of stock-based compensation expense, depreciation and amortization, asset impairment charges, restructuring charges, costs associated with excess space, transaction and integration costs associated with planned, completed or terminated mergers and acquisitions, and acquired in-process research and development expenses. The Company has determined its significant segment expenses are cost of revenue for Biosecurity, research and development expenses for Cell Engineering, and general and administrative expenses for both segments, which are regularly provided to the CODM.
The CODM is not provided with asset information by segment; therefore, such information is not presented. The accounting policies used to prepare the reportable segments financial information are the same as those used to prepare the Company’s consolidated financial statements.
The following table presents summary results of the Company’s reportable segments and a reconciliation of total segment operating loss to consolidated loss before income taxes (in thousands):
| | | | | | | | | | | | | | | | | |
| Year Ended December 31, |
| 2024 | | 2023 | | 2022 |
| Cell Engineering | | | | | |
| Revenue | $ | 173,972 | | | $ | 143,531 | | | $ | 143,666 | |
| Costs and operating expenses: | | | | | |
| Cost of other revenue | 5,999 | | | — | | | — | |
| Research and development | 271,512 | | | 335,943 | | | 259,607 | |
| General and administrative | 115,028 | | | 171,210 | | | 133,239 | |
| Cell Engineering operating loss | (218,567) | | | (363,622) | | | (249,180) | |
| Biosecurity | | | | | |
| Service revenue | 53,071 | | | 78,975 | | | 298,585 | |
| Product revenue | — | | | 28,949 | | | 35,455 | |
| Costs and operating expense: | | | | | |
| Cost of Biosecurity service revenue | 38,549 | | | 46,524 | | | 183,570 | |
| Cost of Biosecurity product revenue | — | | | 7,481 | | | 20,646 | |
| Research and development | 771 | | | 1,599 | | | 1,937 | |
| General and administrative | 44,370 | | | 55,514 | | | 56,353 | |
| Biosecurity operating (loss) income | (30,619) | | | (3,194) | | | 71,534 | |
| Total segment operating loss | (249,186) | | | (366,816) | | | (177,646) | |
| Reconciling items to reconcile total segment operating loss to loss before income taxes: |
Stock-based compensation (1) | 115,299 | | | 234,908 | | | 1,940,920 | |
Impairment expense (2) | 53,654 | | | 121,404 | | | — | |
| Depreciation and amortization | 63,020 | | | 70,507 | | | 42,552 | |
Restructuring charges (3) | 24,172 | | | — | | | — | |
Carrying cost of excess space (net of sublease income) (4) | 25,986 | | | — | | | — | |
Merger and acquisition related expenses (5) | 4,417 | | | 61,188 | | | 46,229 | |
| Acquired in-process research and development | 19,849 | | | 9,582 | | | 1,605 | |
Other (income) expense, net (6) | (8,075) | | | 28,535 | | | (87,553) | |
| Loss before income taxes | $ | (547,508) | | | $ | (892,940) | | | $ | (2,121,399) | |
(1)Includes $3.0 million, $5.0 million, and $10.3 million in related employer payroll taxes for the years ended December 31, 2024, 2023, and 2022, respectively.
(2)For 2024, includes $47.9 million related to goodwill impairment and $5.8 million related to lab equipment. For 2023, includes a $25.2 million impairment loss on lab equipment and a $96.2 million impairment loss on lease assets associated with an exited Zymergen leased facility.
(3)See Note 3, Restructuring, for composition of costs.
Ginkgo Bioworks Holdings, Inc.
Notes to Consolidated Financial Statements
(4)The carrying cost of excess space includes base rent, common area maintenance charges, and real estate taxes associated with facilities the Company is not occupying, net of any sublease income from these spaces.
(5)Represents transaction and integration costs directly related to mergers and acquisitions, including: (i) due diligence, legal, consulting and accounting fees associated with acquisitions, (ii) post-acquisition employee retention bonuses and severance payments, (iii) the fair value adjustments to contingent consideration liabilities resulting from acquisitions, and (iv) costs associated with the Zymergen Bankruptcy, as well as securities litigation costs, net of insurance recovery.
(6)Includes interest income, interest expense, loss on investments, losses/gains on deconsolidation of subsidiaries, changes in fair value of certain assets and liabilities, and other gains or losses.
16. Significant Collaboration Transactions with Related Parties
BiomEdit, LLC
In April 2022, the Company, along with one of its investors and third-party investors, including Elanco Animal Health Inc. (“Elanco”), launched BiomEdit, LLC (“BiomEdit”), a microbiome innovation company that intends to discover, design and develop novel probiotics, microbiome derived bioactives and engineered microbial medicines in the field of animal health. Concurrently with the launch, the Company entered into (i) an Intellectual Property Contribution Agreement (“BiomEdit IP Agreement”) that granted BiomEdit a license to certain of the Company’s intellectual property, (ii) a Technical Development Agreement (“BiomEdit TDA”) that establishes the terms under which the Company will provide technical research and development services, and (iii) a Common Unit Issuance Agreement (“BiomEdit CUIA”) which compensates the Company for its intellectual property contribution. Contemporaneous with these agreements, BiomEdit entered into a Series A Preferred Unit Purchase Agreement under which it sold 6.7 million Series A preferred units to one of the Company’s investors and a third-party investor, for aggregate proceeds of approximately $32.5 million. After the initial closing, BiomEdit may issue up to an additional 1.5 million Series A preferred units (the “Additional Units”) to one or more purchasers reasonably acceptable to the existing holders of Series A preferred units. In a subsequent closing in 2023, BiomEdit sold 0.8 million Additional Units for aggregate proceeds of $4.0 million and closed its Series A preferred unit financing.
Under the BiomEdit IP Agreement, the Company licensed certain intellectual property to BiomEdit for use in the development or production of BiomEdit’s products that the parties will subsequently agree to research and develop under technical development plans (“TDP”). The license rights provide BiomEdit with the ability to commercialize the specified products from the corresponding TDP under the BiomEdit TDA. In return for the license to the intellectual property, BiomEdit issued the Company 3.9 million common units upon execution of the BiomEdit CUIA, with 0.7 million of those units subject to forfeiture in the event BiomEdit does not sell all of the Additional Units. Under the BiomEdit TDA, the parties jointly agree on TDPs, through equal representation on a joint steering committee, under which the Company will perform agreed-upon research and development services in return for consideration on a fixed fee or cost-plus basis for all services provided.
Accounting Analysis
The common unit investment in BiomEdit is considered an equity method investment as a result of the Company’s ability to exercise significant influence over BiomEdit’s financial and operating policies through its ownership of common units. The initial carrying value of the equity method investment in BiomEdit is the fair value of the nonforfeitable common units of $8.9 million received in exchange for the BiomEdit IP Agreement which, as discussed below, is being accounted for as non-cash consideration under ASC 606. The Company determined that the 0.7 million common units held by Ginkgo subject to forfeiture are considered variable consideration that is fully constrained at contract inception until the contingencies related to the issuance of the additional shares are resolved. Upon the closing of BiomEdit's Series A preferred unit financing in 2023, Ginkgo forfeited 0.3 million common units and retained 0.4 million common units for total consideration of $1.1 million. The fair value of BiomEdit’s common units was determined at inception of the agreements using the option pricing method. The option pricing method used a back-solve methodology to infer the total equity value based on the pricing of the Series A preferred unit financing, which was contemporaneous with the BiomEdit IP Agreement.
The Series A preferred units issued by BiomEdit receive a liquidation preference prior to common units. As such, the Company concluded that this represents a substantive profit-sharing arrangement, and the Company is recognizing earnings and losses on the equity method investment using the HLBV method. The Company recorded a $1.5 million loss on its equity method investment in BiomEdit during the year ended December 31, 2023, which reduced the carrying value of the
Ginkgo Bioworks Holdings, Inc.
Notes to Consolidated Financial Statements
equity method investment in BiomEdit to zero. There is no commitment for the Company to provide further financial support to BiomEdit, and therefore the carrying value of the equity method investment will not be reduced below zero.
The relationship with BiomEdit is a vendor-customer relationship and is within the scope of ASC 606, as the provision of services and corresponding license rights are considered a part of the Company’s ordinary activities. The common units issued to the Company represent non-cash consideration. While the BiomEdit TDA has been executed by the parties and provides the payment terms for future services, the BiomEdit TDA does not provide for any transfer of goods or services between the parties. However, the Company will provide licenses and services upon execution of the contemplated TDPs. Accordingly, the Company concluded that the BiomEdit TDA, in combination with the BiomEdit CUIA, met the definition of a contract under ASC 606. Each TDP executed under the BiomEdit TDA will be accounted for in accordance with ASC 606.
The Company’s performance obligations under the BiomEdit TDA consist of four material rights to future technical research and development services and commercial licenses under individual TDPs that the Company expects to execute. The material rights represent an advance payment for the license rights, which will be granted upon the execution of future TDPs. As there is no additional payment for these license rights when future TDPs are executed, the Company has determined that there is a material right associated with each of the contemplated TDPs under the BiomEdit TDA. The Company has allocated approximately $2.2 million of the upfront non-cash consideration to each of the four material rights based on the estimated standalone selling price of the performance obligations. In 2023, the additional $1.1 million of non-cash consideration, which represents previously constrained variable consideration, was allocated to each of the four performance obligations under the arrangement with BiomEdit of $0.3 million each consistent with the initial relative selling price allocation.
Upon the execution of a TDP underlying a material right, the Company is obligated to provide technical research and development services under the TDP and a license to applicable patents and other intellectual property designed and developed under the TDP. The technical research and development services and license provided under a TDP are highly interdependent and interrelated with one another. Without the Company’s knowledge, expertise, and platform, there would not be a licensable strain or other commercializable product to transfer to BiomEdit. Further, BiomEdit has rights to intellectual property created as part of each TDP, irrespective of the result of the development. Therefore, each executed TDP underlying a material right consists of one combined performance obligation for the technical research and development services and license to be provided by the Company.
For each TDP underlying a material right, the transaction price consists of (i) either a fixed fee or, if a cost-plus arrangement, variable consideration for the most likely amount of estimated consideration to be received and (ii) non-cash consideration allocated to the material rights. As the services performed by the Company under a TDP create or enhance an asset that
BiomEdit controls as the asset is created or enhanced, the Company satisfies the performance obligation and recognizes revenue over time. The Company uses an input method that compares total costs incurred relative to total estimated cost to complete to estimate progress under the contract. Any revisions to the estimated total budgeted costs to complete, and the resulting impact on revenue recognition, are reflected in the period of the change through a cumulative catch-up adjustment.
As of December 31, 2024 and 2023, the Company had a deferred revenue balance of $7.6 million and $7.7 million, respectively, with BiomEdit. During the years ended December 31, 2024, 2023 and 2022, the Company recognized revenue of $0.2 million, $2.2 million and $1.0 million, respectively, from services provided to BiomEdit.
Arcaea, LLC
Summary of Arrangement
Arcaea was formed in March 2021 to focus on the application of synthetic biology in the personal care products industry. In March 2021, the Company entered into (i) an Intellectual Property Contribution Agreement (“Arcaea IP Agreement”) that granted Arcaea a license to certain of the Company’s intellectual property, (ii) a Technical Development Agreement (“Arcaea TDA”) that establishes the terms under which the Company will provide technical research and development services, and (iii) a Common Unit Issuance Agreement (“Arcaea CUIA”) which compensates the Company for its intellectual property contribution. Contemporaneous with these transactions, Arcaea entered into a Series A Preferred Unit Purchase Agreement under which it sold 1.8 million Series A preferred units to certain of the Company’s investors, for
Ginkgo Bioworks Holdings, Inc.
Notes to Consolidated Financial Statements
aggregate proceeds of approximately $19.5 million. The Series A Preferred Unit Purchase Agreement provided for the sale and issuance of up to an additional 7.2 million Series A preferred units subsequent to the initial closing. In subsequent closings during 2021, Arcaea issued an additional 5.1 million Series A preferred units to existing and third-party investors for aggregate proceeds of approximately $57.1 million and closed its Series A preferred unit financing. As a result, the Company received an additional 5.2 million common units in Arcaea for total consideration of $35.5 million.
Under the Arcaea IP Agreement, the Company licensed certain intellectual property to Arcaea for use in the development or production of Arcaea’s products that the parties will subsequently agree to research and develop under TDPs. The license rights provide Arcaea with the ability to commercialize the specified products from the corresponding TDP under the Arcaea TDA. In return for the license to the intellectual property, Arcaea has agreed to issue the Company up to 9.0 million common units in accordance with certain terms and conditions set forth within the agreements. The Company received 1.8 million common units upon execution of the Arcaea CUIA and an additional 5.2 million common units upon subsequent closings of the Series A preferred unit financing in 2021 (as discussed above). No additional common units are expected to be issued to the Company.
Under the Arcaea TDA, the parties jointly agree on TDPs, through equal representation on a joint steering committee, under which the Company will perform agreed-upon research and development services in return for consideration on a fixed fee or cost-plus basis for all services provided.
Accounting Analysis
The common unit investment in Arcaea is considered an equity method investment as a result of the Company’s ability to exercise significant influence over Arcaea’s financial and operating policies through its ownership of common units. The initial carrying value of the equity method investment in Arcaea is the fair value of the common units of $11.9 million received in exchange for the Arcaea IP Agreement which, as discussed below, was accounted for as deferred revenue at inception. The fair value of Arcaea’s common units was determined at inception of the agreements using the option pricing method. The option pricing method used a back-solve methodology to infer the total equity value based on the pricing of the Series A preferred unit financing, which was contemporaneous with the Arcaea IP Agreement. Further, the Company determined the rights to up to an additional 7.2 million common units did not meet the definition of a freestanding financial instrument and are not representative of a derivative. The right to the additional common units is considered variable consideration that is fully constrained at inception and until the contingencies related to the issuance of the additional shares are resolved.
The Series A preferred units issued by Arcaea receive a liquidation preference prior to common units. As such, the Company concluded that this represents a substantive profit-sharing arrangement, and the Company is recognizing earnings and losses on the equity method investment using the HLBV method. The Company recorded a $11.9 million loss on its equity method investment in Arcaea in 2021. The loss allocated to the Company primarily relates to Arcaea’s accounting for the non-cash consideration related to the Arcaea IP Agreement as in-process research and development, which resulted in the full value of the Company’s intellectual property contribution being expensed in 2021. As of December 31, 2021, the carrying value of the equity method investment in Arcaea has been reduced to zero. There is no commitment for the Company to provide further financial support to Arcaea, and therefore the carrying value of the equity method investment will not be reduced below zero.
The relationship with Arcaea is a vendor-customer relationship and is within the scope of ASC 606, as the provision of services and corresponding license rights are considered a part of the Company’s ordinary activities. The common units issued to the Company represent non-cash consideration. While the Arcaea TDA has been executed by the parties and provides the payment terms for future services, the Arcaea TDA does not provide for any transfer of goods or services between the parties. However, the Company will provide licenses and services upon execution of the contemplated TDPs. Accordingly, the Company concluded that the Arcaea TDA, in combination with the Arcaea CUIA, met the definition of a contract under ASC 606. Each TDP executed under the Arcaea TDA will be accounted for in accordance with ASC 606.
The Company’s performance obligations under the contract consist of ten material rights to future technical research and development services and commercial licenses under individual TDPs that the Company expects to execute under the Arcaea TDA. The material rights represent an advance payment for the license rights, which will be granted upon the execution of future TDPs. As there is no additional payment for these license rights when future TDPs are executed, the Company has determined that there is a material right associated with each of the contemplated additional TDPs under the Arcaea TDA. The Company has allocated approximately $1.2 million of the upfront non-cash consideration to each of the ten material rights based on the estimated standalone selling price of the performance obligations. During the year ended
Ginkgo Bioworks Holdings, Inc.
Notes to Consolidated Financial Statements
December 31, 2021, the additional $35.5 million of non-cash consideration, which represents previously constrained variable consideration, was allocated to each of the ten performance obligations under the arrangement with Arcaea of $3.6 million each consistent with the initial relative selling price allocation. Unexercised material rights are recorded as non-current deferred revenue until such time as the parties execute a TDP conveying a commercial license.
Upon the execution of a TDP underlying a material right, the Company is obligated to provide technical research and development services under the TDP and a license to applicable patents and other intellectual property designed and developed under the TDP. The technical research and development services and license provided under a TDP are highly interdependent and interrelated with one another. Without the Company’s knowledge, expertise, and platform, there would not be a licensable strain or other commercializable product to transfer to Arcaea. Further, Arcaea has rights to intellectual property created as part of each TDP, irrespective of the result of the development. Therefore, each executed TDP underlying a material right consists of one combined performance obligation for the technical research and development services and license to be provided by the Company.
For each TDP underlying a material right, the transaction price consists of variable consideration for the most likely amount of estimated consideration to be received under the cost-plus arrangement and non-cash consideration allocated to the material rights. As the services performed by the Company under a TDP create or enhance an asset that Arcaea controls as the asset is created or enhanced, the Company satisfies the performance obligation and recognizes revenue over time. The Company uses an input method that compares total costs incurred relative to total estimated cost to complete to estimate progress under the contract. Any revisions to the estimated total budgeted costs to complete, and the resulting impact to revenue recognition, are reflected in the period of the change through a cumulative catch-up adjustment.
As of December 31, 2024 and 2023, the Company had a deferred revenue balance of $28.4 million and $33.1 million, respectively, with Arcaea. During the years ended December 31, 2024, 2023 and 2022, the Company recognized revenue of $4.7 million, $6.0 million and $13.5 million, respectively, from services provided to Arcaea.
Allonnia, LLC
Summary of Arrangement
In December 2019, the Company entered into (i) an Intellectual Property Contribution Agreement (“Allonnia IP Agreement”) that granted Allonnia a license to certain of the Company’s intellectual property, (ii) a Technical Development Agreement (“Allonnia TDA”) that establishes the terms under which the Company is providing technical development services, and (iii) a Common Unit Issuance Agreement which provides for the issuance of common units of Allonnia to the Company in exchange for the license rights granted under the Allonnia IP Agreement. Contemporaneous with these agreements, Allonnia entered into a Series A Preferred Unit Purchase Agreement under which Allonnia sold 3.0 million Series A Preferred Units to certain of the Company’s investors, as well as a third-party investor, for aggregate proceeds of approximately $33.0 million. Allonnia also agreed to issue an additional 0.6 million Series A Preferred Units to a strategic partner as compensation for the delivery of future services to Allonnia. The Series A Preferred Unit Purchase Agreement also provided for the sale and issuance of up to an additional 5.4 million Series A Preferred Units subsequent to the initial closing. In 2020, Allonnia issued an additional 1.8 million Series A Preferred Units, 1.7 million of which were sold for aggregate proceeds of $18.5 million and 180,000 of which were issued in exchange for the rights to certain intellectual property which will vest based on the achievement of milestones associated with the development of the intellectual property received. In 2021, Allonnia issued an additional amount of less than 0.1 million Series A Preferred Units for aggregate proceeds of $0.2 million and closed its Series A Preferred Unit financing. In 2023, Allonnia raised an additional $30 million through a Series A extension.
Under the Allonnia IP Agreement, the Company licensed intellectual property to Allonnia for use in the development or production of its products that the parties will subsequently agree to develop under TDPs. The license rights provide Allonnia with the ability to commercialize the specified products from the corresponding strain or enzyme, which can only be developed by the Company under the Allonnia TDA. The Company received 3.6 million common units as consideration for the license upon execution of the Allonnia IP Agreement and an additional 1.9 million common units during the year ended December 31, 2021 in connection with the closing of the Series A preferred unit financing.
Under the Allonnia TDA, the parties jointly agree, through equal representation on a joint steering committee, on TDPs for specific strains and enzymes, in which the Company will perform agreed upon development services in return for consideration on a fixed fee or cost-plus basis for all services provided.
Ginkgo Bioworks Holdings, Inc.
Notes to Consolidated Financial Statements
Accounting Analysis
The common unit investment in Allonnia is considered an equity method investment as a result of the Company’s ability to exercise significant influence over Allonnia's financial and operating policies through its ownership of common units. The initial carrying value of the equity method investment in Allonnia is the fair value of the common units of $24.5 million received in exchange for the Allonnia IP Agreement which, as discussed below, was accounted for as deferred revenue at inception. The fair value of Allonnia’s common units was determined at inception of the agreements using the option pricing method. The option pricing method used a back-solve methodology to infer the total equity value based on the pricing of the Series A Preferred Unit financing, which was contemporaneous with the Allonnia IP Agreement. Further, the Company determined the rights to up to an additional 5.4 million common units did not meet the definition of a freestanding financial instrument and are not representative of a derivative. The right to the additional common units is considered variable consideration that is fully constrained at inception and until the contingencies related to the issuance of the additional shares are resolved. This contingency was resolved in 2021 when the Company received an additional 1.9 million common units in connection with the closing of the Series A preferred unit financing.
The Series A Preferred Units issued by Allonnia receive a liquidation preference prior to common units. As such, the Company concluded that this represents a substantive profit-sharing arrangement and the Company is recognizing earnings and losses on the equity method investment using the HLBV method. The Company recorded a loss on equity method investment of $24.5 million in 2019 and $12.7 million in 2021 as a result of the application of the HLBV method. The loss allocated to the Company primarily relates to Allonnia’s accounting for the non-cash consideration related to the Allonnia IP Agreement as in-process research and development, which resulted in the full value of the Company’s intellectual property contribution being expensed in the year that the shares were issued. As of December 31, 2021, the carrying value of the equity method investment in Allonnia has been reduced to 0. There is no commitment for the Company to provide further financial support to Allonnia and therefore the carrying value of the equity method investment will not be reduced below zero.
The relationship with Allonnia is a vendor-customer relationship and is within the scope of ASC 606 as the provision of services and corresponding license rights are considered a part of the Company’s ordinary activities and the common units represent non-cash consideration. While the Allonnia TDA has been executed by the parties and provides the payment terms for future services, the Allonnia TDA does not provide for any transfer of goods or services between the parties. However, the Company will provide licenses and services upon execution of the contemplated TDPs. Accordingly, the Company concluded that the Allonnia TDA met the definition of a contract under ASC 606 and each TDP executed under the Allonnia TDA will be accounted for in accordance with ASC 606.
The Company’s performance obligations under the contract consist of ten material rights related to the estimated number of TDPs the parties expect to execute under the Allonnia TDA. The material rights represent an advance payment for the license rights which will be granted upon the execution of each TDP. As there is no additional payment for these license rights upon execution of a TDP, the Company has determined that there is a material right associated with each of the contemplated future TDPs. The Company has allocated $2.5 million of the upfront non-cash consideration to each of the 10 performance obligations under the contract based on the estimated standalone selling price of the performance obligations. Unexercised material rights are recorded as non-current deferred revenue until such time as the parties execute a TDP.
Upon the execution of each TDP, the Company is obligated to provide development services under the TDP and a license to applicable patents and other intellectual property to the ingredient developed under the plan. The license and research and development services under a TDP are highly interdependent and interrelated with one another. Without the Company’s knowledge, expertise, and platform, there would not be a licensable strain or other commercializable product to transfer to Allonnia. Further, Allonnia has rights to all development intellectual property created as part of each TDP, irrespective of the result of the development. Therefore, each executed TDP consists of one combined performance obligation for the license and research and development services to be performed by the Company.
For each TDP, the transaction price consists of variable consideration for the most likely amount of estimated consideration to be received under the cost-plus arrangement and the $2.5 million allocation of the fixed non-cash consideration. As the services performed by the Company create or enhance an asset that Allonnia controls as the asset is created or enhanced, the Company satisfies the performance obligation and recognizes revenue over time. The Company uses an input method that compares total costs incurred relative to total estimated cost to complete to estimate progress under the contract. Any revisions to the estimated total budgeted costs to complete, and the resulting impact to revenue recognition, are reflected in the period of the change through a cumulative catch-up adjustment. In 2023, the additional non-cash consideration of $12.7 million, which represents previously constrained variable consideration, was allocated to all of the performance obligations
Ginkgo Bioworks Holdings, Inc.
Notes to Consolidated Financial Statements
consistent with the initial relative selling price allocation and a cumulative catch up was recognized for the TDPs in process.
As of December 31, 2024 and 2023, the Company had a deferred revenue balance of $36.5 million and $36.1 million, respectively, with Allonnia. During the years ended December 31, 2024, 2023 and 2022, the Company recognized revenue of $0.1 million, $0.5 million and $4.3 million, respectively, from services provided to Allonnia.
Motif FoodWorks, Inc.
Summary of Arrangement
In September 2018, the Company entered into (i) an Intellectual Property Contribution Agreement (“Motif IP Agreement”) with Motif that granted Motif a license to certain of the Company’s intellectual property and (ii) a Technical Development Agreement (“Motif TDA”) that establishes the terms under which the Company is providing technical development services.
Under the Motif IP Agreement, the Company licensed intellectual property to Motif for use in strain development to produce ingredients that the parties will subsequently agree to develop under TDPs. The license rights provide Motif with the ability to commercialize the specified ingredients from the corresponding strain, which can only be developed by the Company under the Motif TDA. In return for the license to the intellectual property, Motif granted the Company 9.0 million shares of common stock. Concurrent with the Motif IP Agreement, Motif also sold 8.1 million shares of Series A preferred stock to certain of the Company’s investors, as well as third-party investors, for aggregate proceeds of approximately $90.0 million.
The Motif TDA governs the procurement of the Company’s expertise and technical development services to collaborate in the research, development, and commercialization of specified ingredients. Under the Motif TDA, the parties jointly agree on TDPs for specific ingredients, in which the Company will perform agreed upon development services in return for consideration on a fixed fee or cost-plus fixed margin basis for all services provided. At inception, the Company estimated that it would execute ten TDPs with Motif.
Accounting Analysis
The investment in Motif common stock is considered an equity method investment as a result of the Company’s ability to exercise significant influence over the financial and operating policies through its common stock ownership. The initial carrying value of the equity method investment in Motif is the fair value of the common stock received in exchange for the Motif IP Agreement of $65.1 million which, as discussed below, is being accounted for as non-cash consideration under ASC 606. As Motif’s Series A preferred stockholders receive a liquidation preference prior to common stock, the Company concluded that this represents a substantive profit-sharing arrangement. Accordingly, the Company is recognizing earnings and losses on the equity method investment using the HLBV method. The Company recorded a loss on equity method investment of $65.1 million from inception through December 31, 2018 which reduced the carrying value to zero. The loss allocated to the Company primarily relates to Motif’s accounting for the non-cash consideration related to the Motif IP Agreement as in-process research and development, which resulted in the full value of Company’s intellectual property contribution being expensed in the period ended December 31, 2018, at which time the carrying value of the equity method investment in Motif had been reduced to zero. There is no commitment for the Company to provide further financial support to Motif and therefore the carrying value of the equity method investment will not be reduced below zero. As a result, no loss was recognized during the years ended December 31, 2024, 2023 and 2022 on the equity method investment.
The overall arrangement with Motif is a vendor-customer relationship and is within the scope of ASC 606 as the provision of development services and corresponding license rights are considered a part of the Company’s ordinary activities. The licenses contemplated under the Motif IP Agreement are contingent upon a TDP being agreed to by the parties under the Motif TDA and only relate to strains that are developed under a TDP. While the TDPs require approval by the parties, the parties initially estimated that ten TDPs would be negotiated under the arrangement.
The Company’s performance obligations under the Motif IP Agreement consist of ten material rights, related to the initial set of ingredients that the parties desired to develop in the first two years. The material rights represent an advance payment for the license rights which will be granted upon the execution of each TDP. As there is no additional payment for these license rights upon execution of a TDP, the Company has determined that there is a material right associated with each of the contemplated TDPs. The common stock received under the Motif IP Agreement is considered non-cash consideration and has been recognized at fair value. The Company determined the fair value of the common stock was $65.1 million at
Ginkgo Bioworks Holdings, Inc.
Notes to Consolidated Financial Statements
inception of the agreement with the assistance of a third-party valuation specialist, which was initially recorded as non-current deferred revenue. The option pricing model used a back-solve methodology to determine the total equity value based on the pricing of the Series A financing, which was contemporaneous with the Motif IP Agreement. The Company has allocated $6.5 million to each of the ten material rights. The Company allocated the transaction price based on the estimated standalone selling price of the material rights which is, in turn, based on the intrinsic value of the right and the probability of exercise.
Upon the execution of each TDP, the Company is obligated to provide development services under the TDP and a license to applicable patents and other intellectual property to the ingredient developed under the plan. The license and research and development services under a TDP are highly interdependent and interrelated with one another. Without the Company’s knowledge, expertise and platform, there would not be a licensable strain or other commercializable product to transfer to Motif. Further, Motif has rights to all development intellectual property created as part of each TDP, irrespective of the result of the development. Therefore, each executed TDP consists of one combined performance obligation for the license and research and development services to be performed by the Company.
For each TDP, the transaction price consists of variable consideration for the most likely amount of estimated consideration to be received under the fixed fee or cost-plus arrangement and the $6.5 million which was allocated to the associated material right under the Motif IP Agreement. As the services performed by the Company create or enhance an asset (i.e., the specified ingredient) that Motif controls as the asset is created or enhanced, the Company satisfies the performance obligation and recognizes revenue over time. The Company uses an input method that compares total costs incurred relative to total estimated cost to complete to estimate progress under the contract. Any revisions to the estimated total budgeted costs to complete, and the resulting impact to revenue recognition, are reflected in the period of the change through a cumulative catch-up adjustment.
As of December 31, 2023, the Company had a deferred revenue balance of $45.4 million with Motif. Effective in August 2024, the Motif IP Agreement and the Motif TDA were mutually terminated with no adjustment to the original consideration. As a result, the Company has no further obligation to perform services for Motif and, accordingly, the remaining $45.4 million in deferred revenue has been recognized in full as revenue during the year ended December 31, 2024. Revenue recognized during the years ended December 31, 2023 and 2022 was $6.7 million and $1.9 million, respectively.
Genomatica, Inc.
2016 Genomatica Agreement
In 2016, the Company purchased Series A preferred stock of Genomatica, Inc. (“Genomatica”), a biotechnology company specializing in the development and manufacturing of intermediate and specialty chemicals from both sugar and alternative feedstocks. The Company also entered into a Collaboration Agreement with Genomatica (“Genomatica Collaboration”) in connection with the financing. The Genomatica Collaboration was entered into to share expertise on biotechnology solutions. Specifically, Genomatica provided the Company with scale-up and process optimization functions, and the Company has provided Genomatica with certain technology development functions generally centered on high throughput strain engineering capabilities. The Genomatica Collaboration’s focus was on obtaining new customers for either party that could benefit from the combined expertise of both parties, and the agreement provides for profit-sharing allocations between Genomatica and the Company depending on the category of the potential product. Each party is responsible for their own costs incurred under an agreed upon TDP.
2018 Genomatica Agreement
In September 2018, the Company entered into a stock purchase agreement with Genomatica under which it received $40.0 million of Series B preferred stock from Genomatica. In lieu of cash consideration, the Company entered into a Foundry Terms of Service Agreement (“Genomatica FSA”) with Genomatica in which the Company would provide up to $40.0 million in services at no charge to Genomatica (“Initial Prepayment”). The Genomatica FSA terminated the Genomatica Collaboration and changed the pricing terms for work performed under TDPs to a cost-plus fixed margin agreement. Genomatica can apply a portion of the $40.0 million in prepaid services to outstanding invoices under the Genomatica FSA, subject to certain limitations that require cash payment for services over certain monthly thresholds. Further, while the Genomatica FSA replaced the Genomatica Collaboration, any fees that would have been paid to or by the Company under contracts previously governed by the Genomatica Collaboration continued to be shared between the parties. These amounts are either (i) added to, if payable to the Company, or (ii) reduced from, if payable to Genomatica, the balance of
Ginkgo Bioworks Holdings, Inc.
Notes to Consolidated Financial Statements
the prepaid services over the term of the arrangement, with certain restrictions. As of December 31, 2021 and 2020, the Company has received $8.3 million and $6.9 million, respectively, under the Genomatica FSA. All contracts previously governed by the Genomatica Collaboration have ended as of December 31, 2021, therefore, no additional payments are expected.
Accounting Analysis
The Company concluded the preferred stock investment was not in-substance common stock and therefore did not qualify for accounting as an equity method investment. Rather, the Company concluded the preferred stock investment should be accounted for as an equity security as it represents an ownership interest in Genomatica that is not mandatorily redeemable nor does the Company have the unilateral right to redeem the preferred stock. Genomatica’s preferred stock is not exchange-traded and does not have a readily determinable fair value. Therefore, the Company accounts for the Genomatica preferred stock under the measurement alternative for equity investments that do not have a readily determinable fair value, which in this case is at historical cost. As of December 31, 2024 and 2023, the cost of the investment in Genomatica preferred stock was zero and $11.9 million, respectively, and is included in investments on the consolidated balance sheet.
Under the Genomatica Collaboration, the Company was entitled to receive a portion of fees earned from third party customers of Genomatica that were within the scope of the agreement. The Company accounted for the collaboration under ASC 808, however the Company applied ASC 606 by analogy for measurement and recognition purposes. Under the Genomatica Collaboration, the Company’s promises consisted of (i) licenses to the Company’s intellectual property, related to the specified development work, and (ii) research and development services. The Company determined that there was a single, combined performance obligation consisting of research services and licenses to certain intellectual property. The Company recognized the revenue for the combined performance obligation using an over-time input method, as the Company’s performance under the contract created or enhanced the target product or strain as such product or strain was developed. The Company measured progress based on the cost incurred relative to total forecasted cost.
The Genomatica FSA represents a modification to the Genomatica Collaboration that resulted in a change in transaction price from milestones to a cost-plus fixed margin structure. The Genomatica FSA did not result in the addition of any distinct promised goods or services, and the Company’s remaining obligation post-modification was to finish the partially satisfied development work that had commenced under the Genomatica Collaboration. This performance obligation was satisfied during the year ended December 31, 2019 and the parties have entered into subsequent TDPs under the Genomatica FSA.
As of December 31, 2024 and 2023, the Company had a deferred revenue balance of $0.6 million and $2.0 million, respectively, with Genomatica. During the years ended December 31, 2024, 2023 and 2022, the Company recognized revenue of $1.5 million, $4.2 million and $10.9 million, respectively, from services provided to Genomatica.
Joyn Bio, LLC
Summary of Arrangement
In September 2017, the Company and certain other investors formed Cooksonia for the purposes of holding the Company’s investment in Joyn Bio. Concurrently, Cooksonia entered into a commitment agreement with Bayer CropScience LP (“Bayer”) to form Joyn Bio. The purpose of Joyn Bio was to research, develop, discover, and commercialize engineered microbes for use in agriculture. The initial program used advanced techniques in biology to study and engineer naturally occurring soil microbes and their nitrogen-fixing genes to enable crops to produce their own fixed nitrogen and reduce the nitrogen fertilizer required.
The Company contributed $5.0 million in cash and certain intellectual property to Cooksonia in exchange for a 70% equity interest in Cooksonia (“Class A Units”). Cooksonia received $20.0 million in cash from another investor, who is a related party of the Company, for a 20% equity interest in Cooksonia (“Class B Units”). Cooksonia also received certain intellectual property from Genomatica and issued Genomatica a 10% equity interest in Cooksonia (“Cooksonia Class C Units”) and paid Genomatica $5.0 million in cash. Subsequently, Cooksonia contributed $20.0 million and all intellectual property received from the Company and Genomatica in exchange for a 50% equity interest in Joyn Bio. Bayer contributed $20.0 million in cash funding plus specified intellectual property. In addition, Bayer committed to contribute up to an additional $60.0 million to be paid subject to certain funding procedures. In return, Bayer obtained a 50% equity interest in Joyn Bio. The agreements may be terminated by mutual agreement, following a change in control, and for breach.
Ginkgo Bioworks Holdings, Inc.
Notes to Consolidated Financial Statements
Joyn Bio was governed by a Board of Managers (“Joyn Bio Board”) comprised of equal representation of the Company and Bayer. The Joyn Bio Board had all the rights, powers, obligations, and authority to manage the business and affairs of Joyn Bio.
The Company also entered into a Foundry Services Agreement (“Joyn Bio FSA”) with Joyn Bio under which the Company will provide Joyn Bio with technical services and preferred access to the Company’s facilities. Joyn Bio paid the Company a nonrefundable $20.0 million prepayment for services to be provided under the Joyn Bio FSA (“Joyn Bio Prepaid Services”). The Joyn Bio Prepaid Services can be utilized for technical services performed by the Company, its subcontractors, and third parties involved in the performance of the overall technical services. Amounts due to the Company are applied to the balance of Joyn Bio Prepaid Services as earned. During the year ended December 31, 2019, Joyn Bio made an additional $15.0 million prepayment for services (“Joyn Bio Additional Prepaid Services”). Under certain Joyn Bio termination scenarios, any amount of unused Joyn Bio Additional Prepaid Services shall be repaid by the Company to Joyn Bio.
Accounting Analysis
From inception, the Company’s investment in Cooksonia has represented a controlling financial interest, resulting in consolidation of Cooksonia within the Company’s consolidated financial statements (see Note 7). The initial cash and in-kind contributions the Company made to Cooksonia have been recorded at carrying value as the transaction was with entities under common control. All assets of Cooksonia after the initial investments, net of the amounts paid to Genomatica, were contributed to Joyn Bio for a 50% equity interest in Joyn Bio. The initial carrying value of the Company’s equity interest in Cooksonia was $13.1 million, comprised of the initial $5.0 million cash investment and an $8.1 million adjustment for Cooksonia’s claim on net assets in accordance with ASC 810, Consolidation, recognized to reflect a certain investor’s liquidation preference in a termination event that represents a substantive profit-sharing agreement. The initial carrying value of the non-controlling interest was comprised of cash and intellectual property contributions from the other investors of $29.7 million, less the $8.1 million adjustment for the non-controlling interest holders’ claim on the net assets of Cooksonia. Cooksonia accounted for its 50% equity interest in Joyn Bio as an equity method investment based on the size of its equity interest and its influence on the board of directors. The equity method investment in Joyn Bio was recorded at an initial carrying value of $97.9 million, which was the fair value of Cooksonia’s interest in Joyn Bio. The fair value was determined by management with the assistance of a third-party valuation specialist. The option pricing model used a back-solve methodology to determine the total equity value based on the pricing of the Class B Units which were exchanged for cash. The license of intellectual property to Joyn Bio has been accounted for under ASC 606 as described below. Upon liquidation, the net assets of Joyn Bio are not distributed in accordance with each party’s respective ownership interest. Depending on the circumstances or type of liquidation event, Bayer or Cooksonia may receive certain preference payments or priority in the assets that are distributed. These preferences represent a substantive profit-sharing arrangement and, accordingly, Cooksonia recognized earnings and losses on its equity method investment using the HLBV method. Refer to Note 7 for additional details on Cooksonia's investment in Joyn Bio. The Company accounted separately under ASC 606 for Cooksonia’s contribution of its intellectual property and the services performed by the Company under technical project plans governed by the Joyn Bio FSA. The Company accounted for the intellectual property sale and the technical services separately as the two agreements were not negotiated with a single commercial objective, the consideration under each agreement was not interdependent, and the intellectual property contribution from Cooksonia was separate and distinct from the research and development services performed under the Joyn Bio FSA.
The Company considers the granting of licenses to the Company’s intellectual property as part of its ordinary business activities, and therefore Cooksonia’s contribution of intellectual property to Joyn Bio represented a contract with a customer. The intellectual property contained multiple licenses for which control transferred at inception and all revenue associated with the licenses was recognized during the year ended December 31, 2017.
The Joyn Bio FSA functioned as a master services agreement that provided a framework for the research and development services relationship between the Company and Joyn Bio. The Joyn Bio FSA did not create a contract under ASC 606 as it did not identify goods or services to be performed nor did it define consideration under the contract. Upon the execution of a technical project plan under the Joyn Bio FSA, the arrangement qualified as a contract under ASC 606.
Ginkgo Bioworks Holdings, Inc.
Notes to Consolidated Financial Statements
The Company accounted for each technical project separately. Each technical project plan provided for distinct services in the context of the contract, was separately negotiated with Joyn Bio, focused on different specified strains with separate scopes of work, and had its own budget. The sole performance obligation under each individual technical project plan consisted of the research and development services as the requisite licenses were transferred prior to the execution of the technical project plans. The transaction price for each technical project plan was determined at plan inception based on the consideration that the Company negotiated in exchange for the services to be provided. The Company’s performance under each technical project plan created or enhanced assets under Joyn Bio’s control. Joyn Bio received the benefits of the output of the research and development services which allowed Joyn Bio to make strategic business decisions on the direction of each product candidate. Therefore, the Company satisfied the respective performance obligations and recognized revenue over time.
On October 17, 2022, Bayer and Ginkgo entered into the JV Termination Agreement, which initiated the dissolution of Joyn Bio (see Note 4). Upon dissolution, the Company's deferred revenue balance with Joyn Bio was applied to Bayer’s Technical Development Agreement with the Company. During the year ended December 31, 2022, the Company recognized revenue of $2.9 million from services provided to Joyn Bio for which the balance was applied against deferred revenue.
17. Employee Benefit Plan
The Company maintains a 401(k) retirement savings plan for its employees who satisfy certain eligibility requirements. Under this plan, the Company makes a 5% non-elective contribution to all eligible employees equal to up to 5% of eligible compensation, which fully vests once such eligible participant has completed two years of continuous service. Effective January 1, 2024, the 5% non-elective contribution is capped for employees earning $100,000 or more in annual salary, resulting in a maximum employer contribution of $5,000. For the years ended December 31, 2024, 2023 and 2022, the Company contributed $5.8 million, $8.2 million and $6.1 million, respectively, to the plan.
18. Income Taxes
For the years ended December 31, 2024, 2023 and 2022, the loss before income taxes consisted of the following (in thousands):
| | | | | | | | | | | | | | | | | |
| Year Ended December 31, |
| 2024 | | 2023 | | 2022 |
| Domestic | $ | (514,354) | | | $ | (890,986) | | | $ | (2,118,095) | |
| Foreign | (33,154) | | | (1,954) | | | (3,304) | |
| Total | $ | (547,508) | | | $ | (892,940) | | | $ | (2,121,399) | |
For the years ended December 31, 2024, 2023 and 2022, the Company recorded the following income tax expense (benefit) (in thousands):
| | | | | | | | | | | | | | | | | |
| Year Ended December 31, |
| 2024 | | 2023 | | 2022 |
| Current: | | | | | |
| State | $ | 566 | | | $ | 690 | | | $ | 271 | |
| Foreign | (109) | | | 123 | | | 159 | |
| Total current | 457 | | | 813 | | | 430 | |
| Deferred: | | | | | |
| Federal | — | | | — | | | (10,500) | |
| State | — | | | — | | | (3,943) | |
| Foreign | (936) | | | (884) | | | (1,014) | |
| Total deferred | (936) | | | (884) | | | (15,457) | |
| Income tax benefit | $ | (479) | | | $ | (71) | | | $ | (15,027) | |
Ginkgo Bioworks Holdings, Inc.
Notes to Consolidated Financial Statements
A reconciliation of income tax benefit computed at the statutory corporate income tax rate to the effective income tax rate for the years ended December 31, 2024, 2023 and 2022 is as follows:
| | | | | | | | | | | | | | | | | |
| Year Ended December 31, |
| 2024 | | 2023 | | 2022 |
| Federal income tax at statutory rate | 21.0 | % | | 21.0 | % | | 21.0 | % |
| State income tax | 0.1 | | | 1.3 | | | — | |
| Change in valuation allowance | (10.4) | | | 13.6 | | | 0.8 | |
| Stock-based compensation | (7.7) | | | (14.2) | | | (16.7) | |
| Executive compensation | 0.2 | | | 8.1 | | | (5.3) | |
| Tax credits | 0.5 | | | 0.8 | | | 0.6 | |
| Investments in subsidiaries and other | (0.6) | | | (29.0) | | | — | |
| Other | (3.0) | | | (1.6) | | | 0.3 | |
| Effective tax rate | 0.1 | % | | — | % | | 0.7 | % |
The Company’s deferred tax assets and liabilities consist of the following (in thousands):
| | | | | | | | | | | |
| Year Ended December 31, |
| 2024 | | 2023 |
| Deferred tax assets: | | | |
| Net operating loss carryforwards | $ | 332,482 | | | $ | 277,559 | |
| Tax credit carryforwards | 61,497 | | | 64,157 | |
| Capitalized research and development costs | 214,208 | | | 185,462 | |
| Accrued expenses | 986 | | | 616 | |
| Deferred revenue | 28,549 | | | 36,225 | |
| Stock-based compensation | 52,388 | | | 83,037 | |
| Amortizable intangibles | 10,695 | | | 5,505 | |
| Lease liabilities | 116,118 | | | 60,197 | |
| Investments in subsidiaries | 57,534 | | | 58,447 | |
| Other | 1,232 | | | 952 | |
| Deferred tax assets before valuation allowance | 875,689 | | | 772,157 | |
| Valuation allowance | (771,852) | | | (711,778) | |
| Deferred tax assets, net of valuation allowance | 103,837 | | | 60,379 | |
| Deferred tax liabilities: | | | |
| Amortizable intangibles | (11,660) | | | (16,873) | |
| Property, plant and equipment | (545) | | | (410) | |
| Lease right-of-use assets | (98,184) | | | (52,409) | |
| Deferred tax liabilities | (110,389) | | | (69,692) | |
| Net deferred taxes | $ | (6,552) | | | $ | (9,313) | |
Activity in the deferred tax assets valuation allowance is summarized as follows (in thousands):
| | | | | | | | | | | | | | | | | |
| Beginning of Period | | Additions
(Subtractions) | | End of Period |
| Deferred tax assets valuation allowance: | | | | | |
| Year ended December 31, 2024 | $ | 711,778 | | | $ | 60,074 | | | $ | 771,852 | |
| Year ended December 31, 2023 | $ | 833,086 | | | $ | (121,308) | | | $ | 711,778 | |
Ginkgo Bioworks Holdings, Inc.
Notes to Consolidated Financial Statements
The Company has evaluated the positive and negative evidence bearing upon its ability to realize the deferred tax assets. The Company considered its history of cumulative net losses incurred since inception and has concluded that it is more likely than not that it will not realize the benefits of the deferred tax assets. Accordingly, a valuation allowance has been established against the deferred tax assets as of December 31, 2024 and 2023 that are not expected to be realized. The Company reevaluates the positive and negative evidence at each reporting period. The valuation allowance increased on a net basis by $60.1 million during the year ended December 31, 2024 primarily due to increases in the deferred tax assets related to net operating loss carryforwards, and capitalized research and development costs as required by the Tax Cuts and Jobs Act of 2017, and decreases in the deferred tax liabilities related to intangible assets due to the sale of Altar SAS, partially offset by decreases in the deferred tax asset related to equity compensation.
As of December 31, 2024, the Company had federal net operating loss carryforwards of approximately $1.2 billion, of which $139.2 million will begin to expire in 2029 and $1.1 billion can be carried forward indefinitely. As of December 31, 2024, the Company had state net operating loss carryforwards of approximately $1.2 billion, of which $991.7 million will begin to expire in 2030 and $162.3 million can be carried forward indefinitely.
As of December 31, 2024, the Company had federal research and development tax credit carryforwards of approximately $37.7 million, which will begin to expire in 2029. As of December 31, 2024, the Company also had state research and development and investment tax credit carryforwards of approximately $30.2 million, which will begin to expire in 2030.
Under Sections 382 and 383 of the U.S. Internal Revenue Code, if a corporation undergoes an ownership change, the corporation’s ability to use its pre-change net operating loss carryforwards and other pre-change tax attributes, such as research tax credits, to offset its post-change income and taxes may be limited. The Company may have experienced an ownership change in the past and may experience ownership changes in the future as a result of future transactions in its share capital, some of which may be outside of the Company’s control. As a result, if the Company earns net taxable income, the Company's ability to use its pre-change net operating loss carryforwards, or other pre-change tax attributes, to offset U.S. federal and state taxable income and taxes may be subject to significant limitations.
The Company evaluates the impact of various tax reform proposals and modifications to existing tax treaties in all jurisdictions where it operates to assess their potential effect on its business and assumptions regarding future taxable income. The Company cannot predict whether specific proposals will be enacted, the terms of such proposals, or their potential impact on its business if enacted. In 2024, no major tax legislation was enacted in the jurisdictions where the Company operates that materially impacted its consolidated financial statements.
The Company files tax returns as prescribed by the tax laws of the jurisdictions in which the Company operates. In the normal course of business, the Company is subject to examination by U.S. federal, state, local, and foreign taxing authorities, where applicable. There are currently no tax examinations in progress. As of December 31, 2024, with few exceptions, the Company is no longer subject to U.S. federal, state, local, or foreign examinations by tax authorities for tax years before 2015. To the extent the Company has tax attribute carryforwards, the tax years in which the attribute was generated may still be adjusted upon examination by the Internal Revenue Service or state taxing authorities to the extent utilized in a future period.
The Company accounts for uncertain tax positions using a more likely than not threshold for recognizing and resolving uncertain tax positions. The evaluation of uncertain tax positions is based on factors that include, but are not limited to, changes in tax law, the measurement of tax positions taken or expected to be taken in tax returns, the effective settlement of matters subject to audit, new audit activity and changes in facts or circumstances related to a tax position. The Company evaluates uncertain tax positions on an annual basis and adjusts the level of the liability to reflect any subsequent changes in the relevant facts surrounding the uncertain positions. The Company accounts for interest and penalties related to uncertain tax positions as part of its provision for income taxes. As of December 31, 2024 and 2023, the Company had no recorded liabilities for uncertain tax positions and had no accrued interest or penalties related to uncertain tax positions. The Company does not expect a material change in unrecognized tax benefits in the next twelve months.
Ginkgo Bioworks Holdings, Inc.
Notes to Consolidated Financial Statements
19. Net Loss per Share
The calculation of basic and diluted earnings per common share is as follows (in thousands, except share data):
| | | | | | | | | | | | | | | | | |
| Year Ended December 31, |
| 2024 | | 2023 | | 2022 |
| Numerator: | | | | | |
| Net loss attributable to Ginkgo Bioworks Holdings, Inc. stockholders, basic | $ | (547,029) | | | $ | (892,869) | | | $ | (2,104,929) | |
| Less: change in fair value of contingent consideration common shares liability | — | | | — | | | 3,143 | |
| Net loss attributable to Ginkgo Bioworks Holdings, Inc. stockholders, diluted | $ | (547,029) | | | $ | (892,869) | | | $ | (2,108,072) | |
| Denominator | | | | | |
| Weighted average common shares outstanding, basic | 51,894,639 | | 48,610,507 | | 41,976,537 |
| Effect of dilutive securities: | | | | | |
| Contingent consideration common shares | — | | — | | 19,435 |
| Weighted average common shares outstanding, diluted | 51,894,639 | | 48,610,507 | | 41,995,972 |
| Basic net loss per share | $ | (10.54) | | | $ | (18.37) | | | $ | (50.15) | |
| Diluted net loss per share | $ | (10.54) | | | $ | (18.37) | | | $ | (50.20) | |
The following potential common shares, presented based on amounts outstanding at each period end, were excluded from the calculation of diluted net loss per share attributable to Ginkgo Bioworks Holdings, Inc. common stockholders for the periods presented because including them would have been anti-dilutive:
| | | | | | | | | | | | | | | | | |
| As of December 31, |
| 2024 | | 2023 | | 2022 |
| Warrants to purchase Class A common stock | 1,295,622 | | 1,295,622 | | 1,295,622 |
| Unvested RSUs | 3,327,398 | | 3,804,189 | | 3,360,911 |
Earnout shares (1) | 3,794,243 | | 3,803,049 | | 3,919,517 |
| Outstanding stock options | 767,520 | | 192,208 | | 317,768 |
Escrow shares (2) | 20,332 | | | — | | | — | |
| 9,205,115 | | 9,095,068 | | 8,893,818 |
(1)Represents employee and non-employee earnout shares for which the service-based and/or market-based vesting conditions have not been satisfied.
(2)Represents restricted common stock issued in connection with asset acquisitions, held in escrow for indemnification purposes, and subject to forfeiture.
Ginkgo Bioworks Holdings, Inc.
Notes to Consolidated Financial Statements
20. Related Parties
The Company’s significant transactions with its related parties are primarily comprised of revenue generating activities under collaboration and license agreements.
Significant related party transactions included in the consolidated balance sheet, excluding the Company’s investments and equity method investments, are summarized below (in thousands):
| | | | | | | | | | | |
| As of December 31, |
| 2024 | | 2023 |
| Deferred revenue, current and non-current: | | | |
| Allonnia | $ | 36,495 | | | $ | 36,062 | |
| Arcaea | 28,413 | | | 33,066 | |
| BiomEdit | 7,583 | | | 7,712 | |
| Genomatica | 564 | | | 2,018 | |
| Motif FoodWorks | — | | | 45,426 | |
| Ayana Bio | — | | | 56 | |
| Other equity investees | — | | | 139 | |
| $ | 73,055 | | | $ | 124,479 | |
Significant related party transactions included in the consolidated statements of operations and comprehensive loss, excluding the losses on the Company’s investments and equity method investments, are summarized below (in thousands):
| | | | | | | | | | | | | | | | | |
| Year Ended December 31, |
| 2024 | | 2023 | | 2022 |
| Cell Engineering revenue: | | | | | |
| Motif FoodWorks | $ | 45,445 | | | $ | 6,660 | | | $ | 1,937 | |
| Arcaea | 4,653 | | | 6,024 | | | 13,490 | |
| Genomatica | 1,453 | | | 4,232 | | | 10,861 | |
| Ayana Bio | 1,028 | | | 1,323 | | | 1,266 | |
| BiomEdit | 197 | | | 2,171 | | | 1,016 | |
| Verb Biotics | — | | | 584 | | | 2,359 | |
| Allonnia | 126 | | | 523 | | | 4,332 | |
| Joyn Bio | — | | | — | | | 2,896 | |
| Other equity investees | 139 | | | 705 | | | 656 | |
| $ | 53,041 | | | $ | 22,222 | | | $ | 38,813 | |
Refer to Notes 6 and 16 for additional details on the Company’s investments and equity method investments held in its related parties. Beginning in April 2022, the Company purchased a series of convertible promissory notes from its then equity method investee, Joyn Bio, in the aggregate principal amount of $10.0 million for the purpose of financing Joyn Bio's working capital needs. Each convertible promissory note was unsecured, had a maturity date of March 31, 2023 and an interest rate of 4.5% per annum. The notes were automatically convertible into equity at a 20% discount upon a qualifying equity financing. Additionally, the Company could elect to convert the notes into equity at a 20% discount upon a non-qualifying equity financing, at maturity, or elect to be repaid in cash upon a change in control or initial public offering. The Company evaluated the notes’ conversion and redemption features for embedded derivatives and determined that there is no embedded derivative to record. The Company also determined that the convertible notes are not in-substance common stock and therefore are not considered an additional investment in the equity method investee. During the year ended December 31, 2022, the carrying amount of the notes was reduced by $5.3 million, which represents the excess loss on the equity method investment in Joyn Bio over the carrying value of the investment, which has been reduced to zero during the year ended December 31, 2022. The outstanding balance of the notes receivable was effectively settled as part of the
Ginkgo Bioworks Holdings, Inc.
Notes to Consolidated Financial Statements
business combination transaction with Bayer and Joyn Bio described in Note 4 and was included as part of the consideration paid for the business combination.