0000310158us-gaap:FairValueInputsLevel3Memberus-gaap:ForeignPlanMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:OtherInvestmentsMember2023-01-012023-12-31
As filed with the Securities and Exchange Commission on February 25, 2025
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D. C. 20549
_________________________________
FORM 10-K
| | | | | | | | |
| | ☒ | Annual Report Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 |
For the Fiscal Year Ended December 31, 2024
OR
| | | | | | | | |
| ☐ | Transition Report Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 |
For the transition period from to
Commission File No. 1-6571
Merck & Co., Inc.
| | | | | | | | | | | | | | |
| 126 East Lincoln Avenue |
| Rahway | New Jersey | 07065 |
(908) 740-4000
| | | | | | | | |
| New Jersey | 22-1918501 |
| (State or other jurisdiction of incorporation) | (I.R.S. Employer Identification No.) |
| | | | | | | | |
| Securities Registered pursuant to Section 12(b) of the Act: |
| Title of Each Class | Trading Symbol(s) | Name of Each Exchange on which Registered |
| Common Stock ($0.50 par value) | MRK | New York Stock Exchange |
| 1.875% Notes due 2026 | MRK/26 | New York Stock Exchange |
| 3.250% Notes due 2032 | MRK/32 | New York Stock Exchange |
| 2.500% Notes due 2034 | MRK/34 | New York Stock Exchange |
| 1.375% Notes due 2036 | MRK 36A | New York Stock Exchange |
| 3.500% Notes due 2037 | MRK/37 | New York Stock Exchange |
| 3.700% Notes due 2044 | MRK/44 | New York Stock Exchange |
| 3.750% Notes due 2054 | MRK/54 | New York Stock Exchange |
| | |
| Securities Registered pursuant to Section 12(g) of the Act: None |
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☒ No ☐
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐ No ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Large accelerated filer | ☒ | Accelerated filer | ☐ | Non-accelerated filer | ☐ | Smaller reporting company | ☐ | Emerging growth company | ☐ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ☒
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. ☐
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
Number of shares of Common Stock ($0.50 par value) outstanding as of January 31, 2025: 2,526,036,240.
Aggregate market value of Common Stock ($0.50 par value) held by non-affiliates on June 28, 2024 based on the closing price on June 28, 2024, the last business day of the registrant’s most recently completed second fiscal quarter: approximately $313,799,000,000.
| | | | | | | | |
| | |
| Documents Incorporated by Reference: | | |
| Document | | Part of Form 10-K |
Proxy Statement for the Annual Meeting of Shareholders to be held May 27, 2025, to be filed with the Securities and Exchange Commission within 120 days after the close of the fiscal year covered by this report | | Part III |
Table of Contents
| | | | | | | | | | | |
| | | | Page |
|
| Item 1. | | |
| Item 1A. | | |
| | |
| Item 1B. | | |
| Item 1C. | | |
| Item 2. | | |
| Item 3. | | |
| Item 4. | | |
| | |
|
| Item 5. | | |
| Item 6. | | |
| Item 7. | | |
| Item 7A. | | |
| Item 8. | | |
| (a) | | |
| | | |
| | | |
| Item 9. | | |
| Item 9A. | | |
| | |
| Item 9B. | | |
| Item 9C. | | |
|
| Item 10. | | |
| Item 11. | | |
| Item 12. | | |
| Item 13. | | |
| Item 14. | | |
|
| Item 15. | | |
| | |
| Item 16. | | |
| | |
PART I
Item 1.Business.
Merck & Co., Inc. (Merck or the Company) is a global health care company that delivers innovative health solutions through its prescription medicines, including biologic therapies, vaccines and animal health products. The Company’s operations are principally managed on a product basis and include two operating segments, Pharmaceutical and Animal Health, both of which are reportable segments.
The Pharmaceutical segment includes human health pharmaceutical and vaccine products. Human health pharmaceutical products consist of therapeutic and preventive agents, generally sold by prescription, for the treatment of human disorders. The Company sells these human health pharmaceutical products primarily to drug wholesalers and retailers, hospitals, government agencies and managed health care providers such as health maintenance organizations, pharmacy benefit managers and other institutions. Human health vaccine products consist of preventive pediatric, adolescent and adult vaccines. The Company sells these human health vaccines primarily to physicians, wholesalers, distributors and government entities.
The Animal Health segment discovers, develops, manufactures and markets a wide range of veterinary pharmaceutical and vaccine products, as well as health management solutions and services, for the prevention, treatment and control of disease in all major livestock and companion animal species. The Company also offers an extensive suite of digitally connected identification, traceability and monitoring products. The Company sells its products to veterinarians, distributors, animal producers, farmers and pet owners.
All product or service marks appearing in type form different from that of the surrounding text are trademarks or service marks owned, licensed to, promoted or distributed by Merck, its subsidiaries or affiliates, except as noted. All other trademarks or service marks are those of their respective owners.
Product Sales
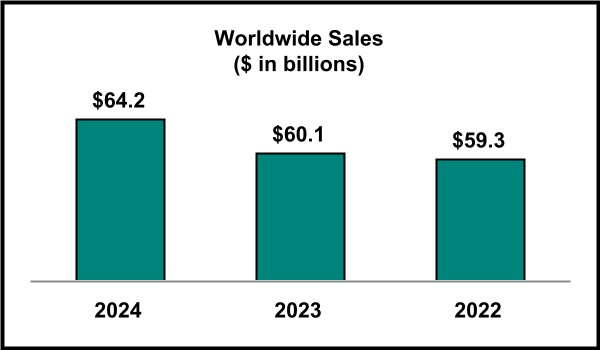
Total Company sales, including sales of the Company’s top pharmaceutical products, as well as sales of animal health products, were as follows:
| | | | | | | | | | | | | | | | | |
| ($ in millions) | 2024 | | 2023 | | 2022 |
| Total Sales | $ | 64,168 | | | $ | 60,115 | | | $ | 59,283 | |
| Pharmaceutical | 57,400 | | | 53,583 | | | 52,005 | |
| Keytruda | 29,482 | | | 25,011 | | | 20,937 | |
Gardasil/Gardasil 9 | 8,583 | | | 8,886 | | | 6,897 | |
ProQuad/M-M-R II/Varivax | 2,485 | | | 2,368 | | | 2,241 | |
| Januvia/Janumet | 2,268 | | | 3,366 | | | 4,513 | |
| Bridion | 1,764 | | | 1,842 | | | 1,685 | |
Alliance revenue - Lynparza(1) | 1,311 | | | 1,199 | | | 1,116 | |
Alliance revenue - Lenvima(1) | 1,010 | | | 960 | | | 876 | |
| Lagevrio | 964 | | | 1,428 | | | 5,684 | |
| Vaxneuvance | 808 | | | 665 | | | 170 | |
| Prevymis | 785 | | | 605 | | | 428 | |
| RotaTeq | 711 | | | 769 | | | 783 | |
| Animal Health | 5,877 | | | 5,625 | | | 5,550 | |
| Livestock | 3,462 | | | 3,337 | | | 3,300 | |
| Companion Animal | 2,415 | | | 2,288 | | | 2,250 | |
Other Revenues(2) | 891 | | | 907 | | | 1,728 | |
(1)Alliance revenue represents Merck’s share of profits, which are product sales net of cost of sales and commercialization costs.
(2)Other revenues are primarily comprised of miscellaneous corporate revenues, including revenue hedging activities, as well as revenue from third-party manufacturing arrangements.
Pharmaceutical
The Pharmaceutical segment includes human health pharmaceutical and vaccine products. Human health pharmaceutical products consist of therapeutic and preventive agents, generally sold by prescription, for the treatment of human disorders. Human health vaccine products consist of preventive pediatric, adolescent and adult vaccines. Certain of the products within the Company’s franchises are as follows:
Oncology
Keytruda is an anti-PD-1 (programmed death receptor-1) therapy that has been approved as monotherapy for the treatment of certain patients with cervical cancer, classical Hodgkin lymphoma (cHL), cutaneous squamous cell carcinoma, esophageal or gastroesophageal junction (GEJ) carcinoma, head and neck squamous cell carcinoma (HNSCC), hepatocellular carcinoma (HCC), melanoma, Merkel cell carcinoma, microsatellite instability-high (MSI-H) or mismatch repair deficient (dMMR) solid tumors (including MSI-H/dMMR colorectal cancer and endometrial carcinoma), non-small-cell lung cancer (NSCLC), primary mediastinal large B-cell lymphoma (PMBCL), tumor mutational burden-high (TMB-H) solid tumors, and urothelial cancer including non-muscle invasive bladder cancer. Keytruda is also approved as monotherapy for the adjuvant treatment of certain patients with melanoma, and for certain patients with renal cell carcinoma (RCC) post-surgery. Keytruda is approved for adjuvant treatment following resection and platinum-based chemotherapy for certain patients with NSCLC. Additionally, Keytruda is approved for patients with certain types of resectable NSCLC in combination with chemotherapy as neoadjuvant treatment, and then continued as a single agent as adjuvant treatment after surgery. Keytruda is also approved for certain patients with high-risk early stage triple-negative breast cancer (TNBC) in combination with chemotherapy as neoadjuvant treatment, and then continued as a single agent as adjuvant treatment after surgery. In addition, Keytruda is approved in combination with chemotherapy for the treatment of certain patients with advanced NSCLC, advanced malignant pleural mesothelioma, HNSCC, advanced biliary tract cancer, advanced esophageal cancer, advanced TNBC, and advanced or recurrent endometrial carcinoma; in combination with chemotherapy with or without bevacizumab, and in combination with chemoradiotherapy, for the treatment of certain patients with advanced cervical cancer; in combination with trastuzumab and chemotherapy for the treatment of certain patients with advanced human epidermal growth factor receptor 2 (HER2)-positive gastric or GEJ adenocarcinoma with programmed death-ligand 1 (PD-L1) (CPS ≥1), and in combination with chemotherapy for the treatment of certain patients with advanced HER2-negative gastric or GEJ adenocarcinoma; in combination with axitinib for the treatment of certain patients with advanced RCC; in combination with Lenvima (lenvatinib) for the treatment of certain patients with advanced RCC or advanced endometrial carcinoma; and in combination with enfortumab vedotin for certain patients with locally advanced or metastatic urothelial cancer. Welireg (belzutifan) is a medication for the treatment of adult patients with certain von Hippel-Lindau (VHL) disease-associated tumors not requiring immediate surgery, and for the treatment of adult patients with advanced RCC following a PD-1 or PD-L1 inhibitor and a vascular endothelial growth factor tyrosine kinase inhibitor. In addition, the Company recognizes alliance revenue related to sales of Lynparza (olaparib), an oral poly (ADP-ribose) polymerase (PARP) inhibitor, for certain types of advanced or recurrent ovarian, early or metastatic breast, metastatic pancreatic, and metastatic castration-resistant prostate cancers; alliance revenue related to sales of Lenvima, an oral receptor tyrosine kinase inhibitor, for certain types of thyroid cancer, RCC, HCC, in combination with everolimus for certain patients with advanced RCC, and in combination with Keytruda for certain patients with advanced endometrial carcinoma or advanced RCC; and alliance revenue related to Reblozyl (luspatercept-aamt) for the treatment of certain types of anemia.
Vaccines
Gardasil (Human Papillomavirus Quadrivalent [Types 6, 11, 16 and 18] Vaccine, Recombinant)/Gardasil 9 (Human Papillomavirus 9-valent Vaccine, Recombinant), vaccines to help prevent certain cancers and diseases caused by certain types of human papillomavirus (HPV); ProQuad (Measles, Mumps, Rubella and Varicella Virus Vaccine Live), a pediatric combination vaccine to help protect against measles, mumps, rubella and varicella; M−M−R II (Measles, Mumps and Rubella Virus Vaccine Live), a vaccine to help prevent measles, mumps and rubella; Varivax (Varicella Virus Vaccine Live), a vaccine to help prevent chickenpox (varicella); Vaxneuvance (Pneumococcal 15-valent Conjugate Vaccine), a vaccine to help prevent invasive pneumococcal disease in individuals 6 weeks of age and older; RotaTeq (Rotavirus Vaccine, Live Oral, Pentavalent), a vaccine to help protect against rotavirus gastroenteritis in infants and children; and Pneumovax 23 (pneumococcal vaccine polyvalent), a vaccine to help prevent pneumococcal disease.
Hospital Acute Care
Bridion (sugammadex), a medication for the reversal of two types of neuromuscular blocking agents used during surgery; Prevymis (letermovir) for the prophylaxis of cytomegalovirus (CMV) infection and disease, or of CMV disease, in certain high risk adult and pediatric recipients of an allogeneic hematopoietic stem cell transplant or of a kidney transplant, respectively; Dificid (fidaxomicin) for the treatment of C. difficile-associated diarrhea; Zerbaxa (ceftolozane and tazobactam) for injection, a combination antibacterial and beta-lactamase inhibitor for the treatment of certain bacterial infections; and Noxafil (posaconazole), an antifungal agent for the prevention of certain invasive fungal infections.
Cardiovascular
Winrevair (sotatercept-csrk), an activin signaling inhibitor indicated for the treatment of adults with pulmonary arterial hypertension (PAH) (World Health Organization [WHO] Group 1) to increase exercise capacity,
improve WHO functional class and reduce the risk of clinical worsening events; Adempas (riociguat), a cardiovascular drug for the treatment of chronic thromboembolic pulmonary hypertension or pulmonary arterial hypertension in certain patients; and Verquvo (vericiguat), a medicine to reduce the risk of cardiovascular death and heart failure hospitalization following a hospitalization for heart failure or need for outpatient intravenous diuretics in certain adults with symptomatic chronic heart failure and reduced ejection fraction.
Virology
Lagevrio (molnupiravir), an investigational oral antiviral COVID-19 medicine available in the U.S. under Emergency Use Authorization (EUA); Isentress/Isentress HD (raltegravir), an HIV integrase inhibitor for use in combination with other antiretroviral agents for the treatment of HIV-1 infection; Delstrigo (doravirine/lamivudine/tenofovir disoproxil fumarate), a complete regimen for the treatment of HIV-1 infection in adult patients with no prior antiretroviral treatment history or to replace the current antiretroviral regime in certain patients who are virologically suppressed on a stable antiretroviral regimen; and Pifeltro (doravirine), a non-nucleoside reverse transcriptase inhibitor for use in combination with other antiretroviral agents for the treatment of HIV-1 infection in adult patients with no prior antiretroviral treatment history or to replace the current antiretroviral regime in certain patients who are virologically suppressed on a stable antiretroviral regimen.
Neuroscience
Belsomra (suvorexant), an orexin receptor antagonist, indicated for the treatment of insomnia, characterized by difficulties with sleep onset and/or sleep maintenance.
Diabetes
Januvia (sitagliptin) and Janumet (sitagliptin/metformin HCl) for the treatment of type 2 diabetes.
Animal Health
The Animal Health segment discovers, develops, manufactures and markets a wide range of veterinary pharmaceuticals, vaccines and health management solutions and services, as well as an extensive suite of digitally connected identification, traceability and monitoring products. Principal products in this segment include:
Livestock Products
Nuflor (Florfenicol) antibiotic range for use in cattle and swine; Bovilis/Vista vaccine lines for infectious diseases in cattle, including Bovilis Cryptium for protection against Cryptosporidium parvum; Banamine (Flunixin meglumine) bovine and swine anti-inflammatory; Estrumate (cloprostenol sodium) for the treatment of fertility disorders in cattle; Matrix (altrenogest) fertility management for swine; Resflor (florfenicol and flunixin meglumine), a combination broad-spectrum antibiotic and non-steroidal anti-inflammatory drug for bovine respiratory disease; Zuprevo (tildipirosin) for bovine respiratory disease; Revalor (trenbolone acetate and estradiol) to improve production efficiencies in beef cattle; Safe-Guard (fenbendazole) de-wormer for cattle; M+Pac (Mycoplasma Hyopneumoniae Bacterin) swine pneumonia vaccine; Porcilis (Lawsonia intracellularis baterin) and Circumvent (Porcine Circovirus Vaccine, Type 2, Killed Baculovirus Vector) vaccine lines for infectious diseases in swine; Nobilis/Innovax (Live Marek’s Disease Vector), vaccine lines for poultry; Paracox and Coccivac coccidiosis vaccines; Exzolt, a systemic treatment for poultry red mite infestations; Slice (emamectin benzoate) parasiticide and Imvixa (lufenuron) for sea lice control in salmon; Clynav vaccine for protection against pancreas disease in salmon; Aquavac (Avirulent Live Culture)/Norvax vaccines against bacterial and viral disease in fish; Aquaflor (florfenicol) antibiotic for farm-raised fish; Flexolt (fluralaner) against lice in sheep; and Allflex Livestock Intelligence solutions for animal identification, monitoring and traceability.
Companion Animal Products
Bravecto, a line of oral, topical and injectable parasitic control products, including the original Bravecto (fluralaner) products for dogs and cats that last up to 12 weeks; Bravecto (fluralaner) One-Month, a monthly product for dogs, Bravecto (fluralaner) Injectable/Quantum, an injectable product for dogs that lasts up to one-year, and Bravecto Plus (fluralaner/moxidectin), a two-month product for cats; Sentinel, a line of oral parasitic products for dogs including Sentinel Spectrum (milbemycin oxime, lufenuron, and praziquantel) and Sentinel Flavor Tabs (milbemycin oxime, lufenuron); Optimmune (cyclosporine), an ophthalmic ointment; Nobivac vaccine lines for flexible dog and cat vaccination, including Nobivac NXT for canine flu and feline leukemia virus; GilvetMab, an immune checkpoint inhibitor monoclonal antibody conditionally licensed for melanoma and mastocytoma tumors; Otomax (gentamicin sulfate, USP; Betamethasone valerate USP; and Clotrimazole USP ointment)/Mometamax (gentamicin sulfate, USP, Mometasone Furoate Monohydrate and Clotrimazole, USP, Otic Suspension)/Mometamax Ultra (gentamicin sulfate, mometasone furoate monohydrate and posaconazole suspension)/Posatex (orbifloxacin, mometasone furoate monohydrate and posaconazole, suspension) ear ointments for acute and chronic otitis; Caninsulin/Vetsulin (porcine insulin zinc suspension) diabetes mellitus treatment for dogs and cats; Panacur (fenbendazole)/Safeguard (fenbendazole) broad-spectrum anthelmintic (de-wormer) for use in many animals; Regumate (altrenogest) fertility management for horses; Prestige vaccine line for horses; Scalibor (Deltamethrin)/Exspot for protecting against bites
from fleas, ticks, mosquitoes and sandflies; and Sure Petcare products for companion animal identification and well-being, including the microchip and pet recovery system Home Again.
For a further discussion of sales of the Company’s products, see Item 7. “Management’s Discussion and Analysis of Financial Condition and Results of Operations” below.
Product Approvals
Set forth below is a summary of significant product approvals received by the Company in 2024 and, to date, in 2025.
| | | | | | | | |
| Product | Date | Approval |
| Keytruda | January 2024 | U.S. Food and Drug Administration (FDA) approval in combination with chemoradiotherapy for the treatment of patients with FIGO (International Federation of Gynecology and Obstetrics) 2014 Stage III-IVA cervical cancer, based on the KEYNOTE-A18 trial. |
| January 2024 | FDA full approval for the treatment of patients with HCC secondary to hepatitis B who have received prior systemic therapy other than a PD-1/PD-L1 containing regimen. The conversion from an accelerated to full (regular) approval is based on the KEYNOTE-394 trial. |
| February 2024 | China’s National Medical Products Administration (NMPA) approval in combination with gemcitabine and cisplatin for the first-line treatment of patients with locally advanced or metastatic biliary tract carcinoma, based on the KEYNOTE-966 trial. |
| March 2024 | European Commission (EC) approval in combination with platinum-containing chemotherapy as neoadjuvant treatment, and then continued as monotherapy as adjuvant treatment, for resectable NSCLC at high risk of recurrence in adults, based on the KEYNOTE-671 trial. |
| May 2024 | Japan’s Ministry of Health, Labor and Welfare (MHLW) approval in combination with fluoropyrimidine and platinum-containing chemotherapy for the first-line treatment of patients with locally advanced unresectable or metastatic gastric or GEJ adenocarcinoma, based on the KEYNOTE-859 trial. |
| May 2024 | Japan’s MHLW approval in combination with standard of care chemotherapy (gemcitabine and cisplatin) for the treatment of patients with locally advanced unresectable or metastatic biliary tract cancer, based on the KEYNOTE-966 trial. |
| June 2024 | FDA approval in combination with carboplatin and paclitaxel, followed by Keytruda as a single agent, for the treatment of adult patients with primary advanced or recurrent endometrial carcinoma, based on the KEYNOTE-868 trial. |
| June 2024 | China’s NMPA approval in combination with trastuzumab, fluoropyrimidine and platinum-containing chemotherapy for the first-line treatment of patients with locally advanced unresectable or metastatic HER2 positive gastric or GEJ adenocarcinoma whose tumors express PD-L1 as determined by a fully validated test, based on the KEYNOTE-811 trial. |
| September 2024 | EC approval in combination with Padcev (enfortumab vedotin-ejfv), an antibody-drug conjugate, for the first-line treatment of unresectable or metastatic urothelial carcinoma in adults, based on the KEYNOTE-A39 trial that was conducted in collaboration with Seagen (now Pfizer Inc.) and Astellas. |
| September 2024 | FDA approval in combination with pemetrexed and platinum chemotherapy for the first-line treatment of adult patients with unresectable advanced or metastatic malignant pleural mesothelioma, based on the IND.227/KEYNOTE-483 trial. |
| September 2024 | Japan’s MHLW approval in combination with chemotherapy as a neoadjuvant treatment, then continued as monotherapy as an adjuvant treatment for patients with NSCLC, based on the KEYNOTE-671 trial. |
| | | | | | | | |
| Keytruda | September 2024 | Japan’s MHLW approval in combination with Padcev for the first-line treatment of patients with radically unresectable urothelial carcinoma, based on the KEYNOTE-A39 trial. |
| September 2024 | Japan’s MHLW approval as monotherapy in patients with radically unresectable urothelial carcinoma who are not eligible for any platinum-containing chemotherapy, based on the KEYNOTE-052 trial. |
| September 2024 | China’s NMPA approval for the first-line treatment of adult patients with unresectable or metastatic melanoma, and conversion from conditional to full approval for the second-line treatment of adult patients with unresectable or metastatic melanoma following failure of one prior line of therapy, based on the LEAP-003 trial. |
| October 2024 | EC approval in combination with chemoradiotherapy for the treatment of FIGO 2014 Stage III-IVA locally advanced cervical cancer in adults who have not received prior definitive therapy, based on the KEYNOTE-A18 trial. |
| October 2024 | EC approval in combination with carboplatin and paclitaxel followed by Keytruda as a single agent for the first-line treatment of primary advanced or recurrent endometrial carcinoma in adults who are candidates for systemic therapy, based on the KEYNOTE-868 trial. |
| November 2024 | Japan’s MHLW approval in combination with chemoradiotherapy as treatment for patients with locally advanced cervical cancer, based on the KEYNOTE-A18 trial. |
| December 2024 | Japan’s MHLW approval in combination with carboplatin and paclitaxel as treatment for adult patients with advanced or recurrent endometrial carcinoma, based on the KEYNOTE-868 trial. |
| December 2024 | China’s NMPA approval in combination with platinum-containing chemotherapy as neoadjuvant treatment and then continued as monotherapy as adjuvant treatment after surgery for patients with resectable stage II, IIIA, or IIIB NSCLC, based on the KEYNOTE-671 trial. |
| December 2024 | China’s NMPA approval in combination with chemoradiotherapy for the treatment of patients with FIGO 2014 Stage III-IVA cervical cancer, based on the KEYNOTE-A18 trial. |
| January 2025 | China’s NMPA approval in combination with Padcev for adult patients with locally advanced or metastatic urothelial cancer, based on the KEYNOTE-A39 trial. |
| Bravecto | January 2024 | EC approval of injectable formulation for dogs for the persistent killing of fleas and ticks for 12 months after treatment. |
| Capvaxive | June 2024 | FDA approval for the prevention of invasive pneumococcal disease and pneumococcal pneumonia caused by certain serotypes in individuals 18 years of age and older. |
| Gardasil | January 2025 | China’s NMPA approval for use in males 9-26 years of age to help prevent certain HPV-related cancers and diseases. |
Lynparza(1) | January 2025 | China’s NMPA approval for the adjuvant treatment of adult patients with deleterious or suspected deleterious germline BRCA-mutated, HER2-negative high-risk early breast cancer who have been previously treated with neoadjuvant or adjuvant chemotherapy, based on the OlympiA trial. |
| | | | | | | | |
| Welireg | November 2024 | China’s NMPA approval for the treatment of adult patients with VHL disease who require therapy for associated RCC, central nervous system hemangioblastomas or pancreatic neuroendocrine tumors. |
| February 2025 | EC conditional approval as monotherapy both for the treatment of adult patients with VHL disease who require therapy for associated, localized RCC, central nervous system hemangioblastomas, or pancreatic neuroendocrine tumors, and for whom localized procedures are unsuitable, and for the treatment of adult patients with advanced clear cell RCC that progressed following two or more lines of therapy that included a PD-1 or PD-L1 inhibitor and at least two VEGF targeted therapies. The EC approval of these two indications is based on results from the LITESPARK-004 and LITESPARK-005 trials. |
| Winrevair | March 2024 | FDA approval for the treatment of adults with PAH (WHO Group 1) to increase exercise capacity, improve WHO functional class (FC), and reduce the risk of clinical worsening events. |
| August 2024 | EC approval in combination with other PAH therapies, for the treatment of PAH in adult patients with WHO FC II to III, to improve exercise capacity. |
(1) Being jointly developed and commercialized in a worldwide collaboration with AstraZeneca.
Competition and the Health Care Environment
Competition
The markets in which the Company conducts its business and the pharmaceutical industry in general are highly competitive and highly regulated. The Company’s competitors include other worldwide research-based pharmaceutical companies, smaller research companies with more limited therapeutic focus, generic drug manufacturers, and animal health care companies. The Company’s operations may be adversely affected by generic and biosimilar competition as the Company’s products mature, as well as technological advances of competitors, industry consolidation, patents granted to competitors, competitive combination products, new products of competitors, the generic availability of competitors’ branded products, and new information from clinical trials of marketed products or post-marketing surveillance. In addition, patent rights are increasingly being challenged by competitors, and the outcome can be highly uncertain. An adverse result in a patent dispute can preclude commercialization of products or negatively affect sales of existing products and could result in the payment of royalties or in the recognition of an impairment charge with respect to intangible assets associated with certain products.
Pharmaceutical competition involves a rigorous search for technological innovations and the ability to market these innovations effectively. With its long-standing emphasis on research and development, the Company is well-positioned to compete in the search for technological innovations. The Company is active in acquiring and marketing products through external alliances, such as licensing arrangements and collaborations, and has been refining its sales and marketing efforts to address changing industry conditions. However, the introduction of new products and processes by competitors may result in price reductions and product displacements, even for products protected by patents. For example, the number of compounds available to treat a particular disease typically increases over time and can result in slowed sales growth or reduced sales of the Company’s products in that therapeutic category.
The highly competitive animal health business is affected by several factors including regulatory and legislative issues, scientific and technological advances, product innovation, the quality and price of the Company’s products as well as competitors’ products, effective promotional efforts and the frequent introduction of generic products by competitors.
Health Care Environment and Government Regulation
Global efforts toward health care cost containment continue to exert pressure on product pricing and market access. Changes to the U.S. health care system as part of health care reform, as well as increased purchasing power of entities that negotiate on behalf of Medicare, Medicaid, and private sector beneficiaries, have contributed to pricing pressure. In several international markets, government-mandated pricing actions have reduced prices of generic and patented drugs. In addition, the Company’s sales performance in 2024 was negatively affected by other cost-reduction measures taken by governments and other third parties to lower health care costs. In the U.S., the Executive Branch and Congress continue to discuss legislation designed to control health care costs, including the cost of drugs. The Company anticipates all of these actions and additional actions in the future will continue to negatively affect sales and profits.
In addressing global cost containment pressures, the Company engages in public policy advocacy with policymakers and continues to work to demonstrate that its medicines provide value to patients and to those who pay for health care. The Company advocates with government policymakers to encourage a long-term approach to sustainable health care financing that ensures access to innovative medicines and does not disproportionately target pharmaceuticals as a source of budget savings. In markets with historically low rates of health care spending, the Company encourages those governments to increase their investments and adopt market reforms in order to improve their citizens’ access to appropriate health care, including medicines.
Operating conditions have become more challenging under the global pressures of competition, industry regulation and cost containment efforts. Although no one can predict the effect of these and other factors on the Company’s business, the Company continually takes measures to evaluate, adapt and improve the organization and its business practices to better meet customer needs and believes that it is well-positioned to respond to the evolving health care environment and market forces.
United States
The Company faces increasing pricing pressure from managed care organizations, government agencies and programs that could negatively affect the Company’s sales and profit margins, including, through (i) practices of managed care organizations, federal and state exchanges, and institutional and governmental purchasers, and (ii) federal laws and regulations related to Medicare and Medicaid, including the Medicare Prescription Drug, Improvement, and Modernization Act of 2003, the Patient Protection and Affordable Care Act of 2010 (ACA), the American Rescue Plan Act of 2021 (American Rescue Plan Act), and the Inflation Reduction Act of 2022 (IRA). Additionally, increased utilization of the 340B Federal Drug Discount Program and restrictions on the Company’s ability to identify inappropriate discounts are having a negative impact on Company performance.
In the U.S., federal and state governments for many years have pursued methods to reduce the cost of drugs and vaccines for which they pay. For example, federal and state laws require the Company to pay specified rebates for medicines reimbursed by Medicaid and to provide discounts for medicines purchased by certain state and federal entities such as the Department of Defense, Veterans Affairs, Public Health Service entities and hospitals serving a disproportionate share of low income or uninsured patients.
Additionally in the U.S., consolidation and integration among health care entities is a major factor in the competitive marketplace for pharmaceutical products. Health plans and pharmacy benefit managers have been consolidating into fewer, larger entities, thus enhancing their purchasing strength and importance. Private third-party insurers, as well as governments, employ formularies to control costs by negotiating discounted prices in exchange for formulary inclusion. Failure to obtain timely or adequate pricing or formulary placement for Merck’s products or obtaining such placement at unfavorable pricing could adversely affect revenue. In addition to formulary tier co-pay differentials, private health insurance companies and self-insured employers have been increasing the cost-sharing required from beneficiaries, particularly for branded pharmaceuticals and biotechnology products. Private health insurance companies, as well as governments, also are increasingly imposing utilization management tools, such as clinical protocols, requiring prior authorization for a branded product or requiring the patient to first fail on one or more generic products before permitting access to a branded medicine. These same management tools are also used in treatment areas in which the payor has taken the position that multiple branded products are therapeutically comparable. As the U.S. payor market concentrates further, the Company may face greater pricing pressure from private third-party payors.
Legislative Changes
In 2022, Congress passed the IRA, which makes significant changes to how drugs are covered and paid for under the Medicare program, including the creation of financial penalties for drugs whose prices rise faster than the rate of inflation, redesign of the Medicare Part D program to require manufacturers to bear more of the liability for certain drug benefits, which has taken effect in 2025, and government price setting for certain Medicare Part D drugs, starting in 2026, and Medicare Part B drugs starting in 2028. Government price setting may also impact pricing in the private market negatively affecting the Company’s performance. In August 2023, the U.S. Department of Health and Human Services (HHS), through the Centers for Medicare & Medicaid Services (CMS), selected Januvia for the first year of the IRA’s “Drug Price Negotiation Program” (Program). Pursuant to the IRA’s Program, a government price was set for Januvia, which will become effective on January 1, 2026. In January 2025, HHS announced that Janumet and Janumet XR have been selected for government price setting, which will become effective on January 1, 2027. In addition, the Company expects that Keytruda will be selected in 2026 for government price setting, which would become effective on January 1, 2028 and the Company expects that, as a result, U.S. sales of Keytruda will decline after that time. The Company has sued the U.S. government regarding the IRA’s Program (see Item 8 “Financial Statements and Supplementary Data,” Note 10. “Contingencies and Environmental Liabilities” below). Furthermore,
the Executive Branch and Congress continue to discuss legislation designed to control health care costs, including the cost of drugs.
The long-term implications of the IRA remain uncertain and subject to various factors, including the manner in which HHS decides to implement the statute. Many experts and analysts, both within the industry and outside, have predicted that the law will harm innovation in the pharmaceutical industry and result in fewer new treatments being developed and approved over time. Merck is working to mitigate the potentially harmful effects that the law could have, which could include a detrimental impact on innovation.
In addition, in 2021, Congress passed the American Rescue Plan Act, which included a provision that eliminates the statutory cap on rebates drug manufacturers pay to Medicaid beginning in January 2024. These rebates act as a discount off the list price and eliminating the cap means that manufacturer discounts paid to Medicaid can increase. Prior to this change, manufacturers have not been required to pay more than 100% of the Average Manufacturer Price (AMP) in rebates to state Medicaid programs for Medicaid-covered drugs. As a result of this provision, manufacturers may have to pay state Medicaid programs more in rebates than they received on sales of particular products. This change presents a risk to Merck for drugs that have high Medicaid utilization and rebate exposure that is more than 100% of the AMP.
The Company also faces increasing pricing pressure in the states, which are looking to exert greater influence over the price of prescription drugs. A number of states have passed pharmaceutical price and cost transparency laws. These laws typically require manufacturers to report certain product price information or other financial data to the state. Some laws also require manufacturers to provide advance notification of price increases. The Company expects that states will continue their focus on pharmaceutical pricing and will increasingly shift to more aggressive price control tools such as Prescription Drug Affordability Boards that have the authority to conduct affordability reviews and establish upper payment limits and that Company products may be selected for such reviews. In addition, in 2024, the FDA authorized, for a two-year period, Florida’s application to import prescription drugs from Canada.
Regulatory Changes
The pharmaceutical industry also could be considered a potential source of savings via other legislative and administrative proposals that have been debated but not enacted. These types of revenue generating or cost saving proposals include additional direct price controls.
European Union
Efforts toward health care cost containment remain intense in the European Union (EU). The Company faces competitive pricing pressure resulting from generic and biosimilar drugs. In addition, a majority of countries in the EU attempt to contain drug costs by engaging in reference pricing in which authorities examine pre-determined markets for published prices of drugs. Reference pricing may either compare a product’s prices in other markets (external reference pricing), or compare a product’s price with those of other products in a national class (internal reference pricing). The authorities then use the price data to set new local prices for brand-name drugs, including the Company’s drugs. Reference pricing mechanisms are usually set at the national level and can be changed pursuant to local regulations or guidance.
Some EU Member States have established free-pricing systems, but regulate the pricing for drugs through profit control plans. Others seek to negotiate or set prices based on the cost-effectiveness of a product or an assessment of whether it offers a therapeutic benefit over other products in the relevant class.
The downward pressure on health care costs in general, particularly prescription drugs, has become intense. As a result, increasingly high barriers are being erected to the entry of new products. In some EU Member States, cross-border imports from low-priced markets also exert competitive pressure that may reduce pricing within an EU Member State.
Additionally, EU Member States have the power to restrict the range of pharmaceutical products for which their national health insurance systems provide reimbursement. In the EU, pricing and reimbursement plans vary widely from Member State to Member State. Some EU Member States provide that drug products may be marketed only after a reimbursement price has been agreed. Some EU Member States may require the completion of additional studies that compare the cost-effectiveness of a particular product candidate to already available therapies or a so-called health technology assessment (HTA), in order to obtain reimbursement or pricing approval. The HTA of pharmaceutical products is becoming an increasingly common part of the pricing and reimbursement procedures in most EU Member States. The HTA process, which is currently governed by the national laws of these countries, involves the assessment of the cost-effectiveness, public health impact, therapeutic impact and/or the economic and social impact of use of a given pharmaceutical product in the national health care system of the individual country in
which it is conducted. Ultimately, an HTA measures the added value of a new health technology compared to existing ones.
The EU Health Technology Assessment Regulation 2021/2282 (HTAR) applies in 2025. This provides for the conduct of an EU level comparative Joint Clinical Assessment (JCA) of a new product versus relevant comparators identified by the EU Member States. JCAs will be carried out in parallel with the review of a marketing authorization application, so that a JCA report is available shortly after the product is authorized. The HTAR applies to all new active substance oncology products and advanced therapy medicinal products, including cell and gene therapies, beginning January 1, 2025; to new active substance orphan medicinal products beginning January 1, 2028; and to all products approved via the centralized procedure beginning in 2030.
EU Member States remain responsible for pricing and reimbursement decisions but must take “due consideration” of JCA reports when making national market access decisions. This means that EU Member State pricing and reimbursement processes are likely to evolve and more EU Member States may use HTAs as part of their decision-making.
The outcome of HTAs regarding specific pharmaceutical products will increasingly influence the pricing and reimbursement status granted to these pharmaceutical products by the market access authorities of individual EU Member States. A negative HTA of one of the Company’s products may mean that the product is not reimbursable or may force the Company to reduce its reimbursement price or offer discounts or rebates.
A negative HTA by a leading and recognized HTA body could also undermine the Company’s ability to obtain reimbursement for the relevant product outside a jurisdiction. For example, EU Member States that have not yet developed HTA mechanisms may rely to some extent on JCAs under the HTAR or an HTA performed in other countries with a developed HTA framework, to inform their pricing and reimbursement decisions. HTA procedures require additional data, reviews and administrative processes, all of which increase the complexity, timing and costs of obtaining product reimbursement and exert downward pressure on available reimbursement.
To obtain reimbursement or pricing approval in some EU Member States, the Company may be required to conduct studies that compare the cost-effectiveness of the Company’s product candidates to other therapies that are considered the local standard of care. There can be no assurance that any EU Member State will allow favorable pricing, reimbursement and market access conditions for any of the Company’s products, or that it will be feasible to conduct additional cost-effectiveness studies, if required.
Japan
In Japan, the pharmaceutical industry is subject to government-mandated annual price reductions of pharmaceutical products and certain vaccines. Furthermore, the government can order re-pricings for specific products if it determines that use of such product will exceed certain thresholds defined under applicable re-pricing rules. In addition, if a Merck product has the same medical action or composition of another product that is subject to market expansion re-pricing, the Merck product could also be subject to re-pricing unless it meets exception criteria. The next government-mandated price reduction is scheduled to occur in April 2025.
China
The Company’s business in China has grown in the past few years, and the importance of China to the Company’s overall pharmaceutical and vaccines business has increased accordingly. Continued growth of the Company’s business in China is dependent upon ongoing development of a favorable environment for innovative pharmaceutical products and vaccines, sustained access for the Company’s currently marketed products, and the absence of trade impediments or adverse pricing controls. In recent years, the Chinese government has introduced and implemented a number of structural reforms to accelerate the shift to innovative products and reduce costs. There have been multiple new policies introduced by the government to improve access to new innovation, reduce the complexity of regulatory filings, and accelerate the review and approval process. This has led to a significant increase in the number of new products being approved each year. While the mechanism for drugs being added to the government’s National Reimbursement Drug List (NRDL) evolves, inclusion may require a price negotiation which could impact the outlook in the market for selected brands. A new NRDL was recently completed in which new entries averaged 63% price reductions. While pricing pressure has always existed in China, health care reform has increased this pressure in part due to the acceleration of generic substitution through volume-based procurement (VBP). In 2019, the government implemented the VBP program through a tendering process for mature products which have generic substitutes with a Generic Quality Consistency Evaluation approval. Mature products that have entered into the last five rounds of VBP had, on average, a price reduction of more than 50%. The Company expects VBP to be a semi-annual process that will have a significant impact on mature products moving forward.
Emerging Markets
The Company’s focus on emerging markets, in addition to China, has continued. Governments in many emerging markets are also focused on constraining health care costs and have enacted price controls and measures impacting intellectual property, including in exceptional cases, threats of compulsory licenses, that aim to put pressure on the price of innovative pharmaceuticals or result in constrained market access to innovative medicine. The Company anticipates that pricing pressures and market access challenges will continue in the future to varying degrees in the emerging markets.
Beyond pricing and market access challenges, other conditions in emerging market countries can affect the Company’s efforts to continue to grow in these markets, including potential political instability, changes in trade sanctions and embargoes, significant currency fluctuation and controls, financial crises, limited or changing availability of funding for health care, credit worthiness of health care partners, such as hospitals, and other developments that may adversely impact the business environment for the Company. Further, the Company may engage third-party agents to assist in operating in emerging market countries, which may affect its ability to realize continued growth and may also increase the Company’s risk exposure.
Regulation
The pharmaceutical industry is also subject to regulation by regional, country, state and local agencies around the world focused on standards and processes for determining drug safety and effectiveness, as well as conditions for sale or reimbursement.
Of particular importance is the FDA in the U.S., which administers requirements covering the testing, approval, safety, effectiveness, manufacturing, labeling, and marketing of prescription pharmaceuticals. In some cases, the FDA requirements and practices have increased the amount of time and resources necessary to develop new products and bring them to market in the U.S. At the same time, the FDA has committed to expediting the development and review of products bearing the “breakthrough therapy” designation, which has accelerated the regulatory review process for medicines with this designation. The FDA has also undertaken efforts to bring generic competition to market more efficiently and in a more timely manner.
The EU has adopted directives and other legislation concerning the classification, approval for marketing, labeling, advertising, manufacturing, wholesale distribution, integrity of the supply chain, pharmacovigilance and safety monitoring of medicinal products for human use. These provide mandatory standards throughout the EU, which may be supplemented or implemented with additional regulations by the EU Member States. In particular, EU regulators may approve products subject to a number of post-authorization conditions. Examples of typical post-authorization commitments include additional pharmacovigilance, the conduct of clinical trials, the establishment of patient registries, physician or patient education and controlled distribution and prescribing arrangements. Non-compliance with post-authorization conditions, pharmacovigilance and other obligations can lead to regulatory action, including the variation, suspension or withdrawal of the marketing authorizations, or other enforcement or regulatory actions, including the imposition of financial penalties. The Company’s policies and procedures are already consistent with the substance of these directives; consequently, it is believed that they will not have any material effect on the Company’s business.
The Company believes that it will continue to be able to conduct its operations, including launching new drugs, in this regulatory environment. (See “Research and Development” below for a discussion of the regulatory approval process.)
Access to Medicines
As a global health care company, Merck’s primary role is to discover and develop innovative medicines and vaccines. The Company also recognizes that, in collaboration with key stakeholders, it has a role to play in helping to ensure that its science advances health care, and its products are accessible and affordable globally. The Company is committed to ensuring a high-quality, safe, reliable, supply of its medicines and vaccines, and to implementing innovative solutions that address barriers to sustainable access to its products.
Merck’s approach is designed to enable it to serve the greatest number of patients today, while meeting the needs of patients in the future. The Company's wide-ranging efforts to expand access to health encompass a set of principles embedded in its business strategies and operations. These principles guide its global approach to addressing significant public health burdens and unmet medical needs. The Company systematically evaluates its pipeline candidates to assess their potential in low and middle-income countries and underserved health care settings. Throughout the life cycle of its products, Merck seeks to evaluate their potential and adapt to changes in the external environment. Collaborating with various stakeholders, including private, governmental, multilateral, and non-profit organizations, the Company seeks to design and deliver sustainable access solutions at the payer, provider,
and patient levels. Furthermore, the Company incorporates access to health metrics in its scorecard, making it a component of calculating annual incentive pay for the majority of its global employees.
In addition, through social investments, including philanthropic programs and impact investing, Merck is helping to strengthen health systems and build capacity, particularly in communities underserved by health care. The Merck Patient Assistance Program provides certain medicines and adult vaccines for free to people in the U.S. and U.S. territories who do not have prescription drug or health insurance coverage and who, without the Company’s assistance, cannot afford their Merck medicines and vaccines. Globally, Merck has made substantial contributions to access to health through key initiatives, including product donations for humanitarian assistance in low-income countries through the Medical Outreach Program. The Mectizan Donation Program, the longest running disease-specific drug donation program of its kind, supports the elimination of two neglected tropical diseases – onchocerciasis and lymphatic filariasis. Additionally, through Merck for Mothers, the Company provides funding, and scientific and business acumen to help global health partners strengthen health systems, expand access to critical maternal health services, and end preventable deaths from complications of pregnancy and childbirth. Merck also supports the Merck Foundation, an independent grantmaking organization helping to address systemic barriers to access to health care.
Privacy and Data Protection
The Company is subject to a significant number of privacy and data protection laws and regulations globally, many of which place restrictions on the Company’s ability to collect, transfer, access and use personal data across its business. The legislative and regulatory landscape for privacy and data protection continues to evolve. There has been increased attention to privacy and data protection issues in both developed and emerging markets with the potential to affect directly the Company’s business, including the EU General Data Protection Regulation (GDPR), which imposes penalties of up to 4% of global revenue.
The GDPR and related implementing laws in individual EU Member States govern the collection and use of personal health data and other personal data in the EU. The GDPR increased responsibility and liability in relation to personal data that the Company processes. It also imposes a number of strict obligations and restrictions on the ability to process (which includes collection, analysis and transfer of) personal data, including health data from clinical trials and adverse event reporting. The GDPR also includes requirements relating to the consent of the individuals to whom the personal data relates, the information provided to the individuals prior to processing their personal data or personal health data, notification of data processing obligations to the national data protection authorities, and the security and confidentiality of the personal data. Further, the GDPR prohibits the transfer of personal data to countries outside of the EU that are not considered by the EC to provide an adequate level of data protection, including to the U.S., except if the data controller meets very specific requirements. Following the Schrems II decision of the Court of Justice of the EU in 2020, uncertainty has existed as to the permissibility of international data transfers under the GDPR. In light of the implications of this decision, the Company may face difficulties regarding the transfer of personal data from the EU to third countries. Since then, the Company entered into the EU-approved Standard Contractual Clauses with its vendors, suppliers, collaboration partners and clinical trial sites in order to facilitate the lawful transfer of personal data from the EU to the U.S. In addition, former President Biden issued Executive Order 14086 in October 2022 to address the data privacy concerns raised in the Schrems II decision through introducing, among other measures, further safeguards and oversight of personal data collection by U.S. signals intelligence activities and providing individuals with a redress mechanism in the U.S. for their data protection concerns. Further certainty for the international transfer of personal data from the EU via the EU-U.S. Data Privacy Framework (successor to the invalidated EU-U.S. Privacy Shield) came about by way of a new EU Adequacy Decision, issued by the EC in July 2023. However, the new Adequacy Decision has already been contested by privacy advocates and is subject to legal review.
Failure to comply with the requirements of the GDPR and the related national data protection laws of the EU Member States may result in significant monetary fines and other administrative penalties as well as civil liability claims from individuals whose personal data was processed. Data protection authorities from the different EU Member States may still implement certain variations, enforce the GDPR and national data protection laws differently, and introduce additional national regulations and guidelines, which adds to the complexity of processing personal data in the EU. Guidance developed at both the EU level and at the national level in individual EU Member States concerning implementation and compliance practices is often updated or otherwise revised.
There is, moreover, a growing trend towards required public disclosure of clinical trial data in the EU which adds to the complexity of obligations relating to processing health data from clinical trials. Failing to comply with these obligations could lead to government enforcement actions and significant penalties against the Company, harm to its reputation, and adversely impact its business and operating results. The uncertainty regarding the interplay between
different regulatory frameworks further adds to the complexity that the Company faces with regard to data protection regulation.
In 2021, China passed the Personal Information Protection Law (PIPL) that aims to standardize the handling of personal information in China. The PIPL currently applies to the processing of personal information of natural persons in China, the processing of personal information outside China where the purpose is to provide products and services in China, and to analyze the activities of individuals in China. While similar to the GDPR, the PIPL contains unique requirements not found in the GDPR. The Company has developed and implemented comprehensive plans to ensure compliance with the PIPL, including plans relating to data localization and cross-border transfers.
Additional laws and regulations enacted in Canada, Europe, Asia, Latin America, the Middle East and 19 states in the U.S. have increased enforcement and litigation activity in the U.S. and other developed markets, as well as increased regulatory cooperation among privacy authorities globally. The Company has adopted a comprehensive global privacy program to manage these evolving requirements and risks and to facilitate the transfer of personal information across international borders.
Distribution
The Company sells its human health pharmaceutical products primarily to drug wholesalers and retailers, hospitals, government agencies and managed health care providers, such as health maintenance organizations, pharmacy benefit managers and other institutions. Human health vaccines are sold primarily to physicians, wholesalers, distributors and government entities. The Company’s professional representatives communicate the effectiveness, safety and value of the Company’s pharmaceutical and vaccine products to health care professionals in private practice, group practices, hospitals and managed care organizations. The Company sells its animal health products to veterinarians, distributors, animal producers, farmers and pet owners.
Raw Materials
The Company obtains raw materials essential to its business from numerous suppliers worldwide. Most of the principal materials the Company uses in its manufacturing operations are available from more than one source. However, the Company obtains certain raw or intermediate materials primarily from only one source. The Company attempts, if possible, to mitigate the potential risk associated with raw materials, components and supplies through inventory and appropriate supplier management.
Patents, Trademarks and Licenses
Patent protection is considered, in the aggregate, to be of material importance to the Company’s marketing of its products in the U.S. and in most major foreign markets. Patents may not only cover a product per se, but also pharmaceutical formulations of a product, processes for making a product, including intermediates useful in those processes, and methods of treatment or other uses of a product. Patent protection for individual products extends for varying periods in accordance with the legal life of patents in individual countries. The protection afforded, which may also vary from country to country, depends upon the type of patent and its scope of coverage.
Patent portfolios developed for products introduced by the Company normally provide varying degrees of market exclusivity. Key patents, which generally cover the product per se, may be subject to a patent term restoration (also known as patent term extension or PTE) of up to five years in the U.S., Japan, and certain other jurisdictions. In Europe, up to five years of extended term may be available in the form of a Supplementary Protection Certificate (SPC). PTEs and SPCs are awarded to offset a portion of the patent term lost during the clinical testing and regulatory review process of a product prior to approval. The Food and Drug Administration Modernization Act includes a Pediatric Exclusivity Provision that may provide an additional six months of market exclusivity (added to the patent term for all Orange Book-listed patents, and to the regulatory data exclusivity term for small molecule and biologic products) in the U.S. for indications of new or currently marketed drugs if certain agreed upon pediatric studies are completed by the applicant. The EU also provides an additional six months of pediatric market exclusivity attached to a product’s SPC term for both small molecule and biologic products. Japan attaches the additional term for pediatric studies to market exclusivity and this extension is unrelated to patent term. In some countries, one or more regulatory exclusivities, including data exclusivity, may provide parallel market protection that is complementary to patent protection and, in some cases, may provide more effective or longer lasting marketing exclusivity than a product’s patent portfolio. In the U.S., the regulatory data/marketing protection term generally runs five years from first marketing approval of a new chemical entity, extended to seven years for an orphan drug indication, and twelve years from first marketing approval of a biological product.
The table below provides a list of expiration dates, which include any pending PTE and SPC periods where indicated, for the key patent protection in the U.S., the EU, Japan and China for the following marketed products:
| | | | | | | | | | | | | | | | | | | | | | | |
| Product | Year of Expiration (U.S.) | | Year of Expiration (EU)(1) | | Year of Expiration (Japan)(2) | | Year of Expiration (China) |
| Januvia | 2026(3) | | Expired | | 2025-2026 | | Expired |
| Janumet | 2026(3) | | Expired | | N/A | | Expired |
Janumet XR | 2026(3) | | N/A | | N/A | | Expired |
| Isentress | Expired(4) | | Expired | | 2026(5) | | Expired |
Lenvima(6) | 2026 | | 2026(7) | | 2026 | | Expired |
| Bridion | 2026 | | Expired | | Expired | | Expired |
| Bravecto | 2027 | | 2029 | | 2029 | | 2025 |
| Gardasil | 2028 | | Expired | | Expired | | Expired |
Gardasil 9 | 2028 | | 2030(7) | | 2030 | | 2025 |
| Keytruda | 2028(8) | | 2031 | | 2032-2033 | | 2028 |
Lynparza(9) | 2027(7) (with pending PTE) | | 2029(7) | | 2028-2029 | | Expired |
| Winrevair | 2027(10) | | 2026(10) | | N/A | | N/A |
Adempas(11) | N/A(12) | | 2028(7) | | 2027-2028 | | Expired |
| Belsomra | 2029 | | N/A | | 2031 | | N/A |
| Prevymis | 2029 | | 2029(13) | | 2029 | | Expired |
| Vaxneuvance | 2031(14) | | No Patent(15) | | No Patent(15) | | N/A |
| Welireg | 2035 (with pending PTE) | | N/A | | N/A | | N/A |
| Capvaxive | 2038 | | N/A | | N/A | | N/A |
Note: Compound patent unless otherwise noted. Certain of the products listed may be the subject of patent litigation. See Item 8. “Financial Statements and Supplementary Data,” Note 10. “Contingencies and Environmental Liabilities” below.
N/A: Currently no marketing approval.
(1)The EU date represents the expiration date for the following four countries: France, Germany, Italy, and Spain (Major EU Markets). If SPC applications have been filed but have not been granted in all Major EU Markets, both the patent expiry date and the SPC expiry date are listed.
(2)The PTE system in Japan allows for a patent to be extended more than once provided the later approval is directed to a different indication from that of the previous approval. This may result in multiple PTE approvals for a given patent, each with its own expiration date.
(3)As a result of settlement agreements related to a patent directed to the specific sitagliptin salt form of the products, exclusivity will extend through May 2026 for Januvia and Janumet, and through July 2026 for Janumet XR.
(4)Generic entry is not anticipated in 2025.
(5)Expiry date reflects granted PTE for the 600 mg tablet in Japan.
(6)Part of a global strategic oncology collaboration with Eisai Co., Ltd.
(7)Eligible for six months pediatric market exclusivity.
(8)The compound patent family contains two additional patents that expire in 2029 due to patent term adjustment resulting from patent office delay. These patents are based on the initial discovery of the active ingredient in Keytruda. While these patents may provide additional protection, the Company expects that they will be the subject of litigation in the future.
(9)Part of a global strategic oncology collaboration with AstraZeneca.
(10)Eligible for 12 years of data exclusivity in the U.S. and 10 years in the EU, which will expire in 2036 and 2034, respectively. Granted patents covering methods of treating pulmonary arterial hypertension with Winrevair, which will expire in 2037 (absent PTE or SPC), may provide additional exclusivity.
(11)Commercialized under a worldwide collaboration with Bayer AG.
(12)The Company has no marketing rights in the U.S.
(13)Data exclusivity has also been granted in the EU and expires in January 2028; eligible for two additional years of market exclusivity based on pediatric studies for an orphan product.
(14)PTE pending but is not included in the listed patent expiry date. Data exclusivity has been granted in the U.S. and expires in July 2033.
(15)Data exclusivity has been granted in the EU and Japan, and expires in December 2031 and September 2030, respectively.
The Company has the following key U.S. patent protection for drug candidates under review in the U.S. by the FDA:
| | | | | |
| Under Review in the U.S. | Currently Anticipated
Year of Expiration (in the U.S.) |
MK-1022 (patritumab deruxtecan)(1) | 2035 |
| MK-1654 (clesrovimab) | 2036 |
(1) Being developed in a collaboration with Daiichi Sankyo. The FDA issued a Complete Response Letter for the application in June 2024.
The Company also has the following key U.S. patent protection for drug candidates in Phase 3 development:
| | | | | |
| Phase 3 Drug Candidate | Currently Anticipated
Year of Expiration (in the U.S.) |
MK-8591A (doravirine + islatravir)(1) | 2032 |
MK-2400 (ifinatamab deruxtecan)(2) | 2034 |
| MK-1308A (quavonlimab + pembrolizumab) | 2035 |
| MK-1026 (nemtabrutinib) | 2035 |
V940(2) | 2036 |
| MK-3543 (bomedemstat) | 2036 |
| MK-5684 (opevesostat) | 2037 |
MK-8591D (islatravir + lenacapavir)(1)(2) | 2037 (with pending PTE for lenacapavir patent) |
| MK-2140 (zilovertamab vedotin) | 2038 |
MK-4482 Lagevrio(2)(3) | 2038 |
MK-2870 (sacituzumab tirumotecan)(2) | 2040 |
| MK-3475A (pembrolizumab + hyaluronidase subcutaneous) | 2039 |
| MK-0616 (enlicitide decanoate) | 2040 |
| MK-1084 | 2040 |
| MK-7240 (tulisokibart) | 2040 |
MK-3000(4) | 2041 |
(1)On partial clinical hold for higher doses of islatravir than those used in current clinical trials.
(2)Being developed in a collaboration.
(3)Available in the U.S. under Emergency Use Authorization.
(4)Program is in a Phase 2/3 study.
Unless otherwise noted, the patents in the above tables cover the product per se (also known as compound patents). For those drug candidates under review or in development, the key U.S. patents may be subject to a future PTE of up to five years and/or six months of pediatric market exclusivity. In addition, depending on the circumstances surrounding any final regulatory approval of the product, there may be other granted patents or pending patent applications that could have relevance to the product as finally approved.
While the expiration of the compound patent generally results in loss of market exclusivity for the covered pharmaceutical product, other patents may provide additional market exclusivity associated with certain aspects of the product that extends beyond the compound patent expiration, including those derived from the initial discovery of the product’s active ingredient(s) or from product-related innovation that occurs after this initial discovery. These include later-expiring patents directed to (i) processes and intermediates related to methods of manufacture of the active ingredient(s), (ii) use(s) of the product, and (iii) novel compositions and formulations of the product. The effect of product patent expiration on pharmaceutical product sales may also depend upon many other factors such as the nature of the market and the position of the product in it, the growth of the market, the complexities and economics of the process for manufacture of the active ingredient(s) of the product and the requirements of new drug provisions of the Federal Food, Drug and Cosmetic Act or similar laws and regulations in other countries. In addition, in the U.S. and certain other countries, a variety of different regulatory exclusivities that impact market exclusivity may be available under relevant law.
For further information with respect to the Company’s patents, see Item 1A. “Risk Factors” and Item 8. “Financial Statements and Supplementary Data,” Note 10. “Contingencies and Environmental Liabilities” below.
Worldwide, all of the Company’s important products are sold under trademarks that are considered in the aggregate to be of material importance. Trademark protection continues in some countries as long as used; in other countries, as long as registered. Registration is for fixed terms and can be renewed indefinitely.
Royalty income in 2024 on patent and know-how licenses and other rights amounted to $1.1 billion. Merck also incurred royalty expenses amounting to $1.9 billion in 2024 under patent and know-how licenses it holds.
Research and Development
The Company’s business is characterized by the introduction of new products or new uses for existing products through a strong research and development program. At December 31, 2024, approximately 23,500 people were employed in the Company’s research activities. The Company prioritizes its research and development efforts and focuses on candidates that it believes represent breakthrough science for unmet medical needs that will make a difference for patients and payers.
The Company maintains a number of long-term exploratory and fundamental research programs in biology and chemistry as well as research programs directed toward product development. The Company’s research and development model is designed to increase productivity and improve the probability of success by prioritizing the Company’s research and development resources on candidates the Company believes are capable of providing unambiguous, promotable advantages to patients and payers and delivering the maximum value of its approved medicines and vaccines through new indications and new formulations. Merck is pursuing emerging product opportunities independent of therapeutic area or modality. The Company is committed to ensuring that externally sourced programs remain an important component of its pipeline strategy, with a focus on supplementing its internal research through acquisitions as well as a licensing and external alliance strategy focused on the entire spectrum of collaborations from early research to late-stage compounds, as well as access to new technologies.
The Company’s clinical pipeline includes candidates in multiple disease areas, including cancer, cardiovascular diseases, metabolic diseases, infectious diseases, neurosciences, immunology, ophthalmology, respiratory diseases, and vaccines.
In the development of human health products, industry practice and government regulations in the U.S. and most foreign countries provide for the determination of effectiveness and safety of new chemical compounds through preclinical tests and controlled clinical evaluation. Before a new drug or vaccine may be marketed in the U.S., recorded data on preclinical and clinical experience are included in the New Drug Application (NDA) for a drug or the Biologics License Application (BLA) for a vaccine or biologic submitted to the FDA for the required approval.
Once the Company’s scientists discover a new small molecule compound or biologic that they believe has promise to treat a medical condition, the Company commences preclinical testing with that compound. Preclinical testing includes laboratory testing and animal safety studies to gather data on chemistry, pharmacology, immunogenicity and toxicology. Pending acceptable preclinical data, the Company will initiate clinical testing in accordance with established regulatory requirements. The clinical testing begins with Phase 1 studies, which are designed to assess safety, tolerability, pharmacokinetics, and preliminary pharmacodynamic activity of the compound in humans. If favorable, additional, larger Phase 2 studies are initiated to determine the efficacy of the compound in the affected population, define appropriate dosing for the compound, as well as identify any adverse effects that could limit the compound’s usefulness. In some situations, the clinical program incorporates adaptive design methodology to use accumulating data to decide how to modify aspects of the ongoing clinical study as it continues, without undermining the validity and integrity of the trial. One type of adaptive clinical trial is an adaptive Phase 2a/2b trial design, a two-stage trial design consisting of a Phase 2a proof-of-concept stage and a Phase 2b dose-optimization finding stage. If data from the Phase 2 trials are satisfactory, the Company commences large-scale Phase 3 trials to confirm the compound’s efficacy and safety. Another type of adaptive clinical trial is an adaptive Phase 2/3 trial design, a study that includes an interim analysis and an adaptation that changes the trial from having features common in a Phase 2 study (e.g., multiple dose groups) to a design similar to a Phase 3 trial. An adaptive Phase 2/3 trial design reduces timelines by eliminating activities which would be required to start a separate study. Upon completion of Phase 3 trials, if satisfactory, the Company submits regulatory filings with the appropriate regulatory agencies around the world to have the product candidate approved for marketing. There can be no assurance that a compound that is the result of any particular program will obtain the regulatory approvals necessary for it to be marketed.
Vaccine development follows the same general pathway as for drugs. Preclinical testing focuses on the vaccine’s safety and ability to elicit a protective immune response (immunogenicity). Pre-marketing vaccine clinical trials are typically done in three phases. Initial Phase 1 clinical studies are conducted in normal subjects to evaluate the safety, tolerability and immunogenicity of the vaccine candidate. Phase 2 studies are dose-ranging studies and provide additional data on safety, immunogenicity and/or effectiveness. Finally, Phase 3 trials are conducted in the
intended population for licensure and provide data on immunogenicity and/or effectiveness, as well as safety, to support applications for regulatory approvals. If successful, the Company submits regulatory filings with the appropriate regulatory agencies.
United States
In the U.S., the FDA review process begins once a complete NDA or BLA is submitted, received and accepted for review by the agency. Within 60 days after receipt, the FDA determines if the application is sufficiently complete to permit a substantive review. The FDA also assesses, at that time, whether the application will be granted a priority review or standard review. Pursuant to the Prescription Drug User Fee Act VII (PDUFA), the FDA review period target for NDAs or original BLAs is either six months, for priority review, or ten months, for a standard review, from the time the application is deemed sufficiently complete. For original efficacy supplements to an NDA or BLA, the FDA review period target is six months, for priority review, or ten months, for a standard review, from the time the supplemental application is received. Once the review timelines are determined, the FDA will generally act upon the application within those timeline goals, unless a major amendment has been submitted (either at the Company’s own initiative or the FDA’s request) to the pending application. If this occurs, the FDA may extend the review period to allow for review of the new information, but by no more than three months. Extensions to the review period are communicated to the Company. The FDA can act on an application either by issuing an approval letter or by issuing a Complete Response Letter (CRL) stating that the application will not be approved in its present form and describing all deficiencies that the FDA has identified. Should the Company wish to pursue an application after receiving a CRL, it can resubmit the application with information that addresses the questions or issues identified by the FDA in order to support approval. Resubmissions are subject to review period targets, which vary depending on the underlying submission type and the content of the resubmission.
The FDA has four program designations — Fast Track, Breakthrough Therapy, Accelerated Approval, and Priority Review — to facilitate and expedite development and review of new drugs to address unmet medical needs in the treatment of serious or life-threatening conditions. The Fast Track designation provides pharmaceutical manufacturers with opportunities for frequent interactions with FDA reviewers during the product’s development and the ability for the manufacturer to do a rolling submission of the NDA/BLA. A rolling submission allows completed portions of the application to be submitted and reviewed by the FDA on an ongoing basis. The Breakthrough Therapy designation provides manufacturers with all of the features of the Fast Track designation as well as intensive guidance on implementing an efficient development program for the product and a commitment by the FDA to involve senior managers and experienced staff in the review. The Accelerated Approval designation allows the FDA to approve a product based on an effect on a surrogate or intermediate endpoint that is reasonably likely to predict a product’s clinical benefit and generally requires the manufacturer to conduct required post-approval confirmatory trials to verify the clinical benefit. The Priority Review designation means that the FDA’s goal is to take action on the NDA/BLA within six months, compared to ten months under standard review. More than one of these special designations can be granted for a given application (i.e., a product designated as a Breakthrough Therapy may also be eligible for Priority Review).
Due to the COVID-19 public health crisis, in 2020, the U.S. Secretary of Health and Human Services (Secretary) exercised statutory authority to determine that a public health emergency existed, and declared those circumstances justified the emergency use of drugs and biological products as authorized by the FDA. In 2023, the Secretary issued an amended determination that a public health emergency or a significant potential for a public health emergency existed, and declared that circumstances continued to justify authorization of emergency use of these products. While in effect, this declaration (as amended) enables the FDA to issue Emergency Use Authorizations (EUAs) permitting distribution and use of specific medical products absent NDA/BLA submission or approval, including products to treat or prevent diseases or conditions caused by the SARS-CoV-2 virus, subject to the terms of any such EUAs. The Company is currently marketing Lagevrio in the U.S. pursuant to an EUA. The FDA must make certain findings to grant an EUA, including that it is reasonable to believe based on the totality of evidence that the drug or biologic may be effective, and that known or potential benefits when used under the terms of the EUA outweigh known or potential risks. Additionally, the FDA must find that there is no adequate, approved and available alternative to the emergency use of the authorized drug or biologic. The FDA may revise or revoke an EUA if the circumstances justifying its issuance no longer exist, the criteria for its issuance are no longer met, or other circumstances make a revision or revocation appropriate to protect the public health or safety.
European Union
The primary method the Company uses to obtain marketing authorization of pharmaceutical products in the EU is through the “centralized procedure.” This procedure is compulsory for certain pharmaceutical products, in particular those using biotechnological processes, and is also available for certain new chemical compounds and products. A company seeking to market an innovative pharmaceutical product through the centralized procedure must file a complete set of safety data and efficacy data as part of a Marketing Authorization Application (MAA) with the
European Medicines Agency (EMA). After the EMA evaluates the MAA, it provides a recommendation to the EC and the EC then approves or denies the MAA. It is also possible for new chemical products to obtain marketing authorization in the EU through a “mutual recognition procedure” in which an application is made to a single member state and, if the member state approves the pharmaceutical product under a national procedure, the applicant may submit that approval to the mutual recognition procedure of some or all other EU Member States.
Japan
In Japan, the Company submits new drug applications to the PMDA for its pharmaceutical regulatory review. The PMDA is an independent administrative agency which is under the jurisdiction of the Ministry of Health, Labor and Welfare (MHLW). The PMDA considers multiple factors in its review process, including the drug’s safety, efficacy, quality, and manufacturing process in accordance with the Act on Securing Quality, Efficacy and Safety of Products Including Pharmaceuticals and Medical Devices. In addition, there are various other regulations and guidelines issued by the MHLW or the PMDA that must also be complied with in order to secure approval. The length of the PMDA review process can vary, but it typically takes around one year for a new drug to be approved in Japan. The review period may be shortened if the drug candidate is designated by the MHLW as an innovative drug satisfying certain conditions.
China
In China, the Company submits marketing applications to the NMPA for an independent review. The NMPA considers multiple factors in its review process, including the drug‘s safety, efficacy, quality, and manufacturing process. Moreover, the NMPA implements strict regulations to ensure that all drugs meet the same standards as those set by the WHO. The agency establishes stringent safety and efficacy requirements for drug approval. The length of the NMPA review process can vary, but it typically takes around one to two years for a new drug to be approved in China.
Other Markets
Outside of the U.S., the EU, Japan and China, the Company submits marketing applications to national regulatory authorities. Examples of such are Health Canada, Agência Nacional de Vigilância Sanatária in Brazil, Korea Food and Drug Administration in South Korea, and the Therapeutic Goods Administration in Australia. Each country has a separate and independent review process and timeline. In many markets, approval times can be longer as the regulatory authority requires approval in a major market, such as the U.S. or the EU, and issuance of a Certificate of Pharmaceutical Product from that market before initiating their local review process.
Research and Development Update
The Company currently has several candidates under regulatory review in the U.S. and internationally or in late-stage clinical development.
MK-1022, patritumab deruxtecan, is a potential first-in-class HER3 directed DXd antibody drug conjugate (ADC), under review by the FDA for the treatment of adult patients with locally advanced or metastatic EGFR-mutated NSCLC previously treated with two or more systemic therapies. The BLA is based on the primary results from the HERTHENA-Lung01 pivotal Phase 2 trial and data results presented at the IASLC 2023 World Conference on Lung Cancer, which were simultaneously published in the Journal of Clinical Oncology. In June 2024, the FDA issued a CRL for the BLA due to findings pertaining to an inspection of a third-party manufacturing facility. The CRL did not identify any issues with the efficacy or safety data submitted. Patritumab deruxtecan (HER3-DXd) was discovered by Daiichi Sankyo and is being jointly developed by Daiichi Sankyo and Merck. Merck is working with Daiichi Sankyo to address FDA feedback.
MK-6482, Welireg, is under review in Japan both for the treatment of adults with VHL disease based on the LITESPARK-004 clinical trial and for the treatment of certain adults with previously treated advanced RCC based on the LITESPARK-005 clinical trial. Additionally, in January 2025, the FDA accepted for priority review a supplemental NDA seeking approval of Welireg for the treatment of adult and pediatric patients (12 years and older) with advanced, unresectable, or metastatic pheochromocytoma and paraganglioma, based on the LITESPARK-015 trial. The FDA set a PDUFA, or target action, date of May 26, 2025.
V116, Capvaxive, a 21-valent pneumococcal conjugate vaccine designed to help prevent invasive pneumococcal disease and pneumococcal pneumonia caused by certain serotypes in adults, is under review in the EU and Japan. The applications are supported by results from multiple Phase 3 clinical studies evaluating V116 in both vaccine-naïve and vaccine-experienced adult patient populations. In January 2025, the Committee for Medicinal Products for Human Use (CHMP) of the EMA recommended the approval of Capvaxive for active immunization for the prevention of invasive disease and pneumonia caused by certain types of Streptococcus pneumoniae in individuals 18 years of age and older. The CHMP’s recommendation will now be reviewed by the EC for marketing authorization in the EU, and a final decision is expected by the second quarter of 2025.
MK-7962, Winrevair, Merck’s novel activin signaling inhibitor, is under review in Japan for the treatment of adult patients with PAH based on the Phase 3 STELLAR trial. In November 2024, Merck announced positive topline results from the Phase 3 ZENITH study, evaluating Winrevair in adults with PAH with WHO Group 1 FC III or IV at high risk of mortality. Based on the positive results of an interim analysis, an independent data monitoring committee recommended that the study be stopped early due to overwhelming efficacy. In addition, in January 2025, Merck announced the Phase 3 HYPERION study evaluating Winrevair in newly diagnosed adults with PAH FC II or III at intermediate or high risk of disease progression was also stopped early based on the positive results from the interim analysis of the ZENITH trial and a review of the totality of data from the Winrevair clinical program to date. All participants in both the ZENITH and HYPERION studies will be offered the opportunity to receive Winrevair as part of the open-label, long-term extension study, SOTERIA.
MK-1654, clesrovimab, is an investigational prophylactic long-acting monoclonal antibody designed to protect infants from respiratory syncytial virus (RSV) disease during their first RSV season. In December 2024, the FDA accepted the BLA for clesrovimab and set a PDUFA date of June 10, 2025. Clesrovimab is also under review in the EU.
MK-3475, Keytruda, is an anti-PD-1 therapy approved for the treatment of many cancers that is in clinical development for expanded indications. These studies encompass more than 30 cancer types including: biliary, estrogen receptor positive breast, triple-negative breast, cervical, colorectal, endometrial, esophageal, gastric, glioblastoma, head and neck, hepatocellular, Hodgkin lymphoma, non-Hodgkin lymphoma, non-small-cell lung, small-cell lung, melanoma, malignant pleural mesothelioma, ovarian, prostate, renal, and urothelial, several of which are currently in Phase 3 clinical development. Further trials are being planned for other cancers.
Keytruda is under review in the EU and Japan for the first-line treatment of adult patients with unresectable advanced or metastatic malignant pleural mesothelioma, based on the Phase 2/3 IND.227/KEYNOTE-483 trial. In November 2024, the EMA’s CHMP adopted a positive opinion recommending approval of Keytruda in combination with pemetrexed and platinum chemotherapy for the first-line treatment of adult patients with unresectable non-epithelioid malignant pleural mesothelioma. In December 2024, the Company requested a re-examination from the EMA’s CHMP for an extension of the indication to include approval in combination with pemetrexed and platinum chemotherapy for the first-line treatment of adults and adolescents aged 12 years and older with unresectable advanced or metastatic malignant pleural mesothelioma, based on final results from the KEYNOTE-483 trial.
The Company is diversifying its oncology portfolio and executing on its strategy which is broadly based on three strategic pillars: immuno-oncology, precision molecular targeting and tissue targeting. Merck has numerous Phase 3 oncology programs within these pillars.
Immuno-oncology
•MK-1308A is the coformulation of quavonlimab, Merck’s novel investigational anti-CTLA-4 antibody, in combination with pembrolizumab, being evaluated for the treatment of RCC.
•MK-3475, Keytruda, is being evaluated in the therapeutic areas of hepatocellular, ovarian and small-cell lung cancers.
•MK-3475A is the subcutaneous coformulation of pembrolizumab in combination with hyaluronidase being evaluated for comparability with intravenous pembrolizumab in metastatic NSCLC.
•V940 (mRNA-4157) is an investigational individualized neoantigen therapy being evaluated in combination with Keytruda as an adjuvant treatment in patients with certain types of melanoma. The FDA and EMA granted Breakthrough Therapy designation and Priority Medicines (PRIME) scheme designation, respectively, for V940 (mRNA-4157) in combination with Keytruda for the adjuvant treatment of patients with certain stages of high-risk melanoma following complete resection. V940 (mRNA-4157) is also being evaluated as adjuvant and perioperative treatment for certain patients with NSCLC. V940 is being developed as part of a collaboration with Moderna, Inc.
Precision molecular targeting
•MK-1026, nemtabrutinib, is an oral, reversible, non-covalent Bruton’s tyrosine kinase (BTK) inhibitor, being evaluated for the treatment of hematological malignancies, including chronic lymphocytic leukemia and small lymphocytic lymphoma.
•MK-1084 is an investigational oral selective KRAS G12C inhibitor being evaluated in combination with Keytruda for the first-line treatment of certain patients with metastatic NSCLC.
•MK-3543, bomedemstat, is an investigational orally available lysine-specific demethylase 1 inhibitor, being evaluated for the treatment of certain patients with essential thrombocythemia. Bomedemstat has FDA
Orphan Drug and Fast Track Designation for the treatment of essential thrombocythemia and myelofibrosis, Orphan Drug Designation for the treatment of acute myeloid leukemia and PRIME scheme designation by the EMA for the treatment of myelofibrosis.
•MK-5684, opevesostat, is an investigational cytochrome P450 11A1 (CYP11A1) inhibitor being evaluated for the treatment of certain patients with metastatic castration-resistant prostate cancer.
•MK-7339, Lynparza, is an oral PARP inhibitor being evaluated in combination with Keytruda for expanded indications in the therapeutic areas of NSCLC and SCLC. Lynparza is being developed as part of a collaboration with AstraZeneca PLC.
•MK-7902, Lenvima, is an oral receptor tyrosine kinase inhibitor being evaluated in combination with Keytruda for expanded indications in the therapeutic area of esophageal cancer. Lenvima is being developed as part of a collaboration with Eisai Co., Ltd.
Tissue targeting
•MK-1022, patritumab deruxtecan, is being evaluated in the therapeutic area of NSCLC as noted above.
•MK-2140, zilovertamab vedotin, is an ADC targeting receptor tyrosine kinase-like orphan receptor 1 (ROR1) being evaluated for the treatment of hematological malignancies, including diffuse large B cell lymphoma.
•MK-2400, ifinatamab deruxtecan, is an ADC being evaluated in patients with relapsed SCLC versus chemotherapy. MK-2400 is being developed as part of a collaboration with Daiichi Sankyo.
•MK-2870, sacituzumab tirumotecan, is an investigational trophoblast cell-surface antigen 2 (TROP2)-directed ADC being evaluated for certain patients with breast, cervical, endometrial, gastric and non-small-cell lung cancers. The FDA granted Breakthrough Therapy designation to sacituzumab tirumotecan for the treatment of patients with advanced or metastatic nonsquamous NSCLC with EGFR mutations whose disease progressed on or after tyrosine kinase inhibitor and platinum-based chemotherapy. Sacituzumab tirumotecan is being developed as part of a collaboration with Kelun-Biotech.
Additionally, the Company currently has candidates in Phase 3 clinical development in several other therapeutic areas.
•MK-3000 is an investigational, potentially first-in-class tetravalent, tri-specific antibody that acts as an agonist of the Wingless-related integration site signaling pathway, which is in clinical development for the treatment of diabetic macular edema and neovascular age-related macular degeneration. MK-3000 was obtained in connection with the July 2024 acquisition of Eyebiotech Limited.
•MK-8591A is a once-daily oral combination of doravirine and islatravir, an investigational nucleoside reverse transcriptase translocation inhibitor (NRTTI), being evaluated for the treatment of HIV infection in previously untreated adults and as a switch in antiretroviral therapy in virologically suppressed adults. MK-8591D is an oral once-weekly combination of islatravir and Gilead Sciences Inc.’s lenacapavir being evaluated for the treatment of HIV infection in virologically suppressed adults. In 2021, the FDA placed clinical holds on the islatravir investigational NDAs based on observations of decreases in total lymphocyte and CD4+ T-cell counts in some participants receiving islatravir in clinical studies. The investigational NDAs for the doravirine/islatravir and the islatravir/lenacapavir once-weekly treatment regimens remain under a partial clinical hold for any studies that would use islatravir doses higher than the doses considered for the revised clinical programs.
•MK-0616, enlicitide decanoate, is an investigational, oral proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor being evaluated for hypercholesterolemia, including in studies evaluating low-density lipoprotein cholesterol reduction and a cardiovascular outcomes study.
•MK-7240, tulisokibart, is a humanized monoclonal antibody directed to tumor necrosis factor-like ligand 1A, a target associated with both intestinal inflammation and fibrosis, being evaluated for the treatment of Crohn’s disease and ulcerative colitis.
•MK-4482, Lagevrio, is an investigational oral antiviral medicine for the treatment of mild to moderate COVID-19 in adults who are at risk for progressing to severe disease. Merck is developing Lagevrio in collaboration with Ridgeback Biotherapeutics LP (Ridgeback). The FDA granted Emergency Use Authorization for Lagevrio in December 2021, which was last reissued in November 2023. Lagevrio is authorized for the treatment of adults with a current diagnosis of mild to moderate COVID-19, and who are at high risk for progression to severe COVID-19, including hospitalization or death, and for whom alternative COVID-19 treatment options approved or authorized by the FDA are not accessible or clinically appropriate. Lagevrio is not approved for any use in the U.S. and is authorized only for the duration of the declaration that circumstances exist justifying the authorization of its emergency use under the Food, Drug and Cosmetic Act, unless the authorization is terminated or revoked sooner. In 2024, an additional Phase 3 clinical trial
(MOVe-NOW) was initiated to evaluate Lagevrio for the treatment of adults with COVID-19 at high risk for disease progression. MOVe-NOW will build on existing Lagevrio data to assess efficacy in the current COVID-19 environment and support applications for licensure.
The Company also terminated certain of its development programs in 2024.
•MK-7264, gefapixant, is a non-narcotic, oral selective P2X3 receptor antagonist for the treatment of refractory or unexplained chronic cough in adults. In December 2023, the FDA issued a second CRL regarding the resubmission of Merck’s NDA for gefapixant. In the CRL, the FDA concluded that Merck’s application did not meet substantial evidence of effectiveness for treating refractory or unexplained chronic cough. The CRL was not related to the safety of gefapixant. Merck has withdrawn its application for gefapixant from the FDA and does not plan to refile.
•In December 2024, Merck announced the discontinuation of the Phase 3 KeyVibe-003 and KeyVibe-007 trials, which were evaluating MK-7684A, the fixed-dose combination of vibostolimab, an anti-TIGIT antibody, and pembrolizumab, in certain patients with NSCLC, based on the recommendation of an independent data monitoring committee. In a pre-planned analysis, both trials met the pre-specified futility criteria for the primary endpoint of overall survival. Considering the totality of data from the Phase 3 KeyVibe studies, including the efficacy outcomes from KeyVibe-003 and KeyVibe-007, the Company decided to discontinue the Phase 3 KeyVibe-006 trial and other vibostolimab studies.
•Also in December 2024, Merck announced the discontinuation of the clinical development program for favezelimab, an anti-LAG-3 antibody, and will stop enrollment in the Phase 3 KEYFORM-008 trial evaluating the fixed-dose combination of favezelimab and pembrolizumab (MK-4280A) in patients with relapsed or refractory cHL whose disease has progressed following prior anti-PD-1 therapy. The Company made this decision after a thorough evaluation of data from the favezelimab clinical program. Data analyses for the Phase 3 trials are ongoing, and the results will be shared with the scientific community.
•Based on the topline results of the MK-2060 Phase 2 study, Merck will not proceed to Phase 3 clinical development. The Phase 2 study results will be presented at a scientific meeting later in 2025.
•The Phase 2b clinical trial for MK-8189 as a monotherapy for acute schizophrenia did not meet its primary efficacy endpoint and further development in schizophrenia, bipolar, and dementia indications has stopped. Potential alternative indications for MK-8189 are being explored.
The chart below reflects the Company’s research pipeline as of February 21, 2025. Candidates shown in Phase 3 include the date such candidate entered into Phase 3 development. Candidates shown in Phase 2 include the most advanced compound with a specific mechanism or, if listed compounds have the same mechanism, they are each currently intended for commercialization in a given therapeutic area. Small molecules and biologics are given MK-number designations and vaccine candidates are given V-number designations. Except as otherwise noted, candidates in Phase 1, additional indications in the same therapeutic area (other than with respect to cancer and immunology) and additional claims, line extensions or formulations for in-line products are not shown.
| | | | | | | | |
| Phase 2 |
Alzheimer’s MK-1167 Cancer MK-1022 (patritumab deruxtecan)(1) Biliary Bladder Cervical Colorectal Endometrial Esophageal Gastric Head and Neck Hepatocellular Melanoma Ovarian Pancreatic Prostate MK-1308 (quavonlimab)(2) Non-Small-Cell Lung MK-1308A (quavonlimab+pembrolizumab) Colorectal MK-2400 (ifinatamab deruxtecan)(1) Biliary Bladder Breast Cervical Colorectal Endometrial Esophageal Head and Neck Hepatocellular Melanoma Ovarian Pancreatic MK-2870 (sacituzumab tirumotecan)(1)(3) Biliary Colorectal Neoplasm Malignant Pancreatic MK-3475 Keytruda Advanced Solid Tumors Prostate MK-3475A (pembrolizumab+hyaluronidase subcutaneous) Cutaneous Squamous Cell Hematological Malignancies
| Cancer MK-5890 (boserolimab)(2) Neoplasm Malignant MK-5909 (raludotatug deruxtecan)(1) Bladder Cervical Endometrial Ovarian Renal Cell MK-6482 Welireg(3) Breast Endometrial Esophageal Hepatocellular MK-7339 Lynparza(1)(3) Advanced Solid Tumors
| Cancer V940 (1)(2) Bladder Renal Cell Dengue Fever Virus Vaccine V181 HIV-1 Infection MK-8591B (islatravir+MK-8507)(4) HIV-1 Pre-Exposure Prophylaxis MK-8527 Immunology MK-6194 Lupus Vitiligo MK-7240 (tulisokibart) Systemic Sclerosis Metabolic Dysfunction-Associated Steatohepatitis (MASH) MK-6024 (efinopegdutide) Pulmonary Hypertension-Chronic Obstructive Pulmonary Disease MK-5475 Pulmonary Hypertension Due To Left Heart Disease MK-7962 Winrevair
|
| | | | | | | | |
| Phase 3 (Phase 3 entry date) | Under Review |
Antiviral COVID-19 MK-4482 Lagevrio (U.S.) (May 2021)(1)(6) Cancer MK-1022 (patritumab deruxtecan)(1) Non-Small-Cell Lung (May 2022) (EU) MK-1026 (nemtabrutinib) Hematological Malignancies (March 2023) MK-1084 (2) Non-Small-Cell Lung (May 2024) MK-1308A (quavonlimab+pembrolizumab) Renal Cell (April 2021) MK-2140 (zilovertamab vedotin) Hematological Malignancies (September 2024) MK-2400 (ifinatamab deruxtecan)(1) Small-Cell Lung (July 2024) MK-2870 (sacituzumab tirumotecan)(1)(3) Breast (April 2024) Cervical (July 2024) Endometrial (December 2023) Gastric (May 2024) Non-Small-Cell Lung (November 2023) MK-3475 Keytruda Hepatocellular (May 2016) (EU) Ovarian (December 2018) Small-Cell Lung (May 2017) MK-3475A (pembrolizumab+hyaluronidase subcutaneous) Non-Small-Cell Lung (February 2023) MK-3543 (bomedemstat) Myeloproliferative Disorders (December 2023) MK-5684 (opevesostat) Prostate (December 2023) MK-7339 Lynparza(1)(2) Non-Small-Cell Lung (June 2019) Small-Cell Lung (December 2020) MK-7902 Lenvima(1)(2) Esophageal (July 2021) V940(1)(2) Melanoma (July 2023) Non-Small-Cell Lung (December 2023) Diabetic Macular Edema MK-3000(7) HIV-1 Infection MK-8591A (doravirine+islatravir) (February 2020)(5) MK-8591D (islatravir+lenacapavir) (October 2024)(1)(5) Hypercholesterolemia MK-0616 (enlicitide decanoate) (August 2023) Immunology MK-7240 (tulisokibart) Crohn’s Disease (June 2024) Ulcerative Colitis (October 2023)
| New Molecular Entities Cancer MK-1022 (patritumab deruxtecan)(1)(8) Non-Small-Cell Lung (U.S.) MK-6482 Welireg Renal Cell (JPN) Von Hippel-Lindau (VHL) Disease (JPN) Pneumococcal Vaccine Adult V116 Capvaxive (EU) (JPN) Pulmonary Arterial Hypertension MK-7962 Winrevair (JPN) Respiratory Syncytial Virus MK-1654 (clesrovimab) (U.S.) (EU)
| Certain Supplemental Filings Cancer MK-3475 Keytruda • First-Line Unresectable Advanced or Metastatic Malignant Pleural Mesothelioma (KEYNOTE-483) (EU) (JPN)
MK-6482 Welireg • Advanced, Unresectable, or Metastatic Pheochromocytoma and Paraganglioma (LITESPARK-015) (U.S.) |
Footnotes: (1) Being developed in a collaboration. (2) Being developed in combination with Keytruda. (3) Being developed as monotherapy and/or in combination with Keytruda. (4) FDA lifted clinical hold on December 4, 2024. (5) On FDA partial clinical hold for higher doses of islatravir than those used in current clinical trials. (6) Available in the U.S. under Emergency Use Authorization. (7) Program is in a Phase 2/3 study that commenced in August 2024. (8) In June 2024, the FDA issued a CRL for the BLA for patritumab deruxtecan. Merck is working with Daiichi Sankyo to address FDA feedback.
|
Human Capital
As of December 31, 2024, the Company had approximately 75,000 employees worldwide, with approximately 31,000 employed in the U.S., including Puerto Rico, and approximately 15,000 third-party contractors globally. Third-party contractors include the Company’s temporary workers, independent contractors, and freelancers who are viewed as full-time equivalent employees; they exclude outsourced service providers. Approximately 73,000 of the Company’s employees are full-time employees. Globally, women comprise 52% of employees, and in the U.S. individuals from underrepresented ethnic groups comprise 37% of its workforce (the Company defines workforce as its employees). Women comprise 46% of the members of the Board of Directors. Additionally, the Company’s senior management team is made up of 39% women. Approximately 21% of the Company’s employees are represented by various collective bargaining groups. The Company’s voluntary turnover rate was approximately 4.6% and 5.6%, in 2024 and 2023, respectively.
The Company recognizes that its employees are critical to meet the needs of its patients and customers and that its ability to excel depends on the integrity, skill, and collaboration of its employees.
Talent Acquisition
The Company uses a comprehensive approach to ensure recruiting, retention and leadership development goals are systematically executed throughout the Company and that it hires talented leaders with a wide
range of knowledge, skills, backgrounds and perspectives. In addition, the Company utilizes a comprehensive communications strategy, employee branding and marketing outreach, social media and strategic alliance partnerships to reach a broad pool of talent in its critical business areas. In 2024, the Company hired approximately 7,300 employees across the globe through various channels including the Company’s external career site, direct passive candidate sourcing, employee referrals, universities and other external sources.
Enabling a Collaborative Work Environment
Fostering a collaborative environment is fundamental to the Company’s success and core to future innovation. The Company strives to create an environment of acceptance, engagement and empowerment. The Company seeks to hire and develop the best talent by providing equal opportunity to all people. The Company creates competitive advantages by leveraging practices which help to meet the needs of all our patients worldwide. This includes evaluating social determinants of health when developing commercialization strategies and leveraging employee insights to improve performance.
Compensation and Benefits
The Company provides a valuable suite of compensation and benefits programs that reflect its commitment to attract, retain and motivate its talent, and support its employees and their families in every stage of life. The Company continuously monitors and adjusts its compensation and benefit programs to ensure they are competitive, contemporary, helpful and engaging, and that they support strategic imperatives such as fairness, flexibility, quality, security and affordability. For example, the Company regularly monitors and evaluates its pay practices and policies to ensure that it is paying employees fairly. The Company offers a personal health care concierge service to assist U.S. employees participating in the Company medical plan with their health care needs. Aligned with its business and in support of its cancer care strategy, the Company provides enhanced cancer screening benefits with cash incentives, immediate access to two leading cancer centers of excellence for U.S. employees and high value cancer support resources (e.g., caregiving and mental health) for employees and their families. Globally, the Company implemented a minimum standard of 12 weeks of paid parental leave. In the U.S., the Company’s benefits rank in the top quartile of Fortune 100 companies under the Aon 2024 Benefits Index. The Company has been included in the Seramount (previously the Working Mother) 100 Best Companies ranking for 38 consecutive years and was named a top ten Best Company for Moms in 2024.
Employee Well-being
The Company is committed to helping its employees and their families improve their own health and well-being, whether physical, mental, financial, or social. The Company’s programs ensure quality, competitive value, protection from significant financial hardship and access to tools and resources to support employees and their families in all stages of their career and their lives, earning the Company accolades such as the Business Group on Health’s Best Employers Excellence in Health & Well-being and the CEO Roundtable on Cancer’s Global Gold Standard Employer accreditation in 2024. As part of the Company’s overall culture of well-being, the Company fosters an array of flexible work arrangements and offers onsite services so employees can thrive. For example, in the U.S., these include onsite health care professionals at many major sites, cafeterias committed to healthy menu offerings, onsite childcare, onsite gyms, and the convenient option to bank through an employee credit union.
Engaging Employees
The Company strengthens employee engagement by fostering a safe, positive, and supportive workplace. Merck encourages candid employee feedback through global employee surveys and peer feedback processes. The Company encourages professional networking and collaboration, enabling employees to connect and grow. Additionally, Merck provides community volunteering opportunities, reflecting its commitment to social responsibility. By building strong relationships with its employees, the Company strives to ensure that every voice is heard, fostering an engaging employee experience that drives Merck forward.
Talent Management and Development
As the Company pursues its goal of becoming the world’s premier research-based biopharmaceutical company, there is a consistent focus on the importance of continuously developing its motivated and talented people. The Company is committed to talent growth for all, allowing its employees to move more fluidly across the organization, unlocking an environment that allows them to shape their career pathways via non-linear and wide-ranging opportunities and experience. Merck’s current talent management system supports company-wide performance management, leadership development, talent reviews and succession planning. Annual performance reviews help further the professional development of the Company’s employees and ensure that the Company’s workforce is aligned with the Company’s objectives. The Company seeks to continuously build the skills and capabilities of its workforce to accelerate talent, improve performance and mitigate risk through relevant continuous learning experiences. This includes, but is not limited to, building leadership and management skills, as well as providing technical and functional training to all employees.
Environmental Matters
Environmental Sustainability
The Company is committed to enabling a safe, sustainable and healthy future and strives to be a strong environmental steward, evolving its efforts in the face of a changing world. The Company’s environmental sustainability strategy has three focus areas:
•Driving operational efficiency;
•Designing new products to minimize environmental impact; and
•Reducing any impacts in the Company’s upstream and downstream value chain.
The Company ensures its ongoing commitment to these areas through thoughtful governance. Its Environmental, Health and Safety Council (EHS Council) is a cross-functional body with leadership representation from each area of the Company’s business and is responsible for overseeing its environmental sustainability strategy, policy, and risk mitigation controls. The EHS Council monitors performance against the Company’s goals and increases transparency on environmental issues within the Company, senior management, and the Board of Directors (the Board). The Global Safety and Environment (GSE) vice president communicates progress on environmental sustainability goals, objectives and other important issues to the Board, senior management and the EHS Council. Additionally, the head of the Environmental Sustainability Center of Excellence is a member of the Environmental, Social and Governance Strategy Management Team, a group of functional experts that advises, shapes, and drives the Company’s long-term sustainability strategy with guidance from an internal cross divisional forum of senior leaders. The Company’s cross-functional Environmental Sustainability Implementation Steering Committee was designated by the EHS Council to oversee the progress of initiatives that support the achievement of the Company’s public goals and provide guidance on resourcing of the Company’s environmental sustainability strategy.
Merck believes that climate change could present risks to its business, as discussed in further detail in Item 1A. “Risk Factors” below under the headings “Climate change or legal, regulatory or market measures to address climate change may negatively affect the Company’s business, results of operations, cash flows and prospects” and “Environmental, social and governance matters may impact the Company’s business and reputation.” Some of the potential impacts of climate change to the Company’s business include increased operating costs due to additional regulatory requirements, physical risks to the Company’s facilities, water limitations and disruptions to its supply chain. These potential risks are integrated into the Company’s business planning, including investment in reducing energy usage, water use and greenhouse gas (GHG) emissions.
The Company has adopted a set of climate goals to help position it to succeed in an increasingly resource-constrained world. These goals address the rising expectations of the Company’s customers, investors, external stakeholders and employees regarding the environmental impact of its operations and supply chain. The Company’s climate goals include reducing Scope 1 and 2 operational GHG emissions 46% by 2030 (from a 2019 baseline), sourcing 100% of its purchased electricity from renewable sources by 2025, and reducing Scope 3 GHG emissions 30% by 2030 (from a 2019 baseline). In 2024, the Company committed to a net-zero target for its GHG emissions across its global operations (Scopes 1, 2 and 3) by 2045, aligned with the guidelines of the Science Based Targets initiative (SBTi). Other environmental sustainability initiatives of the Company include:
•Partnering for progress across the Company’s value chain. The Company is working to reduce its Scope 3 emissions through a robust supplier engagement approach to drive collaboration upstream and downstream in its value chain. By engaging with its suppliers, the Company can identify key ways to reduce its GHG emissions and pinpoint additional tangible benefits for the business.
•Playbooks for a sustainable environment. To help direct and track projects in support of its goals, the Company has developed a series of guidance documents for its global sites. In 2021, the Company launched its Low Carbon Transition Playbook (LCTP), a common platform that includes a gap assessment to help the Company’s global sites evaluate the maturity of their energy programs and help create short- and long-term plans to reduce sites’ carbon intensity and build toward a low-carbon future. Based on learnings from use, the Company issued LCTP 2.0 in 2022 with a capability to facilitate knowledge sharing across sites. In 2022, the Company also created the Waste Diversion Playbook, which takes a similar approach to guide sites on developing a roadmap to their and the Company’s shared 2025 goals on waste diversion, including local waste-diversion strategies and environmentally responsible procurement practices. In 2024, the Company expanded this list with the addition of a Water Conservation Playbook. This approach guides projects consistently across the Company’s global network of sites and enables continuous improvement toward meetings its goals.
•Realizing the benefits of green and sustainable science. The Company believes that meeting its environmental sustainability goals is intrinsically linked to the creation of innovative, cost-efficient manufacturing processes with low environmental impact. The Company aims to develop efficient and sustainable processes at product launch, with the goal of minimizing material use and waste from its commercial manufacturing. The Company utilizes an innovative “green-by-design” development strategy with a goal to progress from an initial early clinical supply route to a fully optimized and sustainable commercial manufacturing process. In 2024, for the fifth year in a row, the Company received the Peter J. Dunn Award for Green Chemistry and Engineering Impact, an award given by the American Chemical Society in recognition of outstanding implementation of novel green chemistry in the pharmaceutical industry.
•Waste diversion. The Company continuously evaluates its sites’ waste disposal methods to gain a better understanding of its network and changes therein, as well as to identify risks and opportunities in its value chain. Based on its evaluation, the Company implemented programs to divert non-hazardous landfill waste from its two highest landfill-generating sites. The Company remains committed to its 2025 public waste diversion goals of no more than 20% of the Company’s global operational waste sent to landfills or incinerators (without energy recovery) and that 50% of its sites will send zero waste to landfills by 2025.
•Water as a shared resource. As water is a key input to the Company’s manufacturing operations, the Company assesses water risk throughout its network as a standard business practice. Both of the Company’s priority water-stress risk sites have conservation plans in place, and site staff are actively working on water use reduction and recycling improvement projects. These projects are consistent with the Company’s ongoing commitment to achieving its stated goal of maintaining global water use at or below 2015 levels by 2025. The Company’s sites are employing various technologies and techniques aimed at reducing its water footprint and improving operational performance. The Company’s endorsement of the United Nations CEO Water Mandate enables alignment of the Company’s water program with the mandate’s principles. The Company has continued to identify partnerships to help it advance its water stewardship priorities in the areas in which it operates.
The Company continues to review and explore other opportunities to further its environmental strategy and will evaluate potential impacts and commitments.
Management does not believe that expenditures related to these initiatives should have a material adverse effect on the Company’s financial condition, results of operations, liquidity or capital resources for any year.
Environmental Regulation and Remediation
The Company believes that there are no compliance issues associated with applicable environmental laws and regulations that would have a material adverse effect on the Company. The Company is also remediating environmental contamination resulting from past industrial activity at certain of its sites. Expenditures for remediation and environmental liabilities were $4 million in 2024 and are estimated to be $26 million in the aggregate for the years 2025 through 2029. These amounts do not consider potential recoveries from other parties. The Company has taken an active role in identifying and accruing for these costs and, in management’s opinion, the liabilities for all environmental matters that are probable and reasonably estimable have been accrued and totaled $41 million and $42 million at December 31, 2024 and 2023, respectively. Although it is not possible to predict with certainty the outcome of these matters, or the ultimate costs of remediation, management does not believe that any reasonably possible expenditures that may be incurred in excess of the liabilities accrued should exceed approximately $46 million in the aggregate. Management also does not believe that these expenditures should result in a material adverse effect on the Company’s financial condition, results of operations or liquidity for any year.
Geographic Area Information
The Company’s operations outside the U.S. are conducted primarily through subsidiaries. Sales worldwide by subsidiaries outside the U.S. as a percentage of total Company sales was 50% in 2024, 53% in 2023 and 54% in 2022.
The Company’s worldwide business is subject to risks of currency fluctuations, governmental actions and other governmental proceedings abroad. The Company does not regard these risks as a deterrent to further expansion of its operations abroad. However, the Company closely reviews its methods of operations and adopts strategies responsive to changing economic and political conditions.
Merck has operations in countries located in Latin America, the Middle East, Africa, Eastern Europe and Asia Pacific. Business in these developing areas, while sometimes less stable, offers important opportunities for growth over time.
Available Information
The Company’s Internet website address is merck.com. The Company will make available, free of charge at the “Investors” portion of its website, its Annual Report on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K, and all amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934, as amended, as soon as reasonably practicable after such reports are electronically filed with, or furnished to, the U.S. Securities and Exchange Commission (SEC). The address of that website is sec.gov. In addition, the Company will provide without charge a copy of its Annual Report on Form 10-K, including financial statements and schedules, upon the written request of any shareholder to the Office of the Secretary, Merck & Co., Inc., 126 East Lincoln Avenue, Rahway, NJ 07065 U.S.A.
The Company’s corporate governance guidelines and the charters of the Board of Directors’ four standing committees are available on the Company’s website at www.merck.com/company-overview/leadership/board-of-directors/ and all such information is available in print to any shareholder who requests it from the Company.
The Company’s 2023/2024 Impact Report, which provides enhanced sustainability disclosures, is available in the Sustainability section of the Company’s website at www.merck.com. Information in the Company’s Impact Report is not incorporated by reference into this Form 10-K.
Item 1A.Risk Factors.
Summary Risk Factors
The Company is subject to a number of risks that if realized could materially adversely affect its business, results of operations, cash flows, financial condition or prospects. The following is a summary of the principal risk factors facing the Company:
•The Company is dependent on its patent rights, and if its patent rights are invalidated or circumvented, its business could be materially adversely affected.
•As the Company’s products lose market exclusivity, the Company generally experiences a significant and rapid loss of sales from those products.
•Key products generate a significant amount of the Company’s profits and cash flows, and any events that adversely affect the markets for its leading products could have a material adverse effect on the Company’s results of operations and financial condition.
•The Company’s research and development efforts may not succeed in developing commercially successful products and the Company may not be able to acquire commercially successful products in other ways; consequently, the Company may not be able to replace sales of successful products that lose patent protection.
•The Company’s success is dependent on the successful development and marketing of new products, which are subject to substantial risks.
•The Company faces continued pricing pressure with respect to its products.
•Unfavorable or uncertain economic conditions, together with cost-reduction measures being taken by certain governments, could negatively affect the Company’s operating results.
•The Company faces intense competition from both lower cost generic and biosimilar products and competitors’ products.
•The Company has significant global operations, which expose it to additional risks, and any adverse event could have a material adverse effect on the Company’s results of operations and financial condition.
•Climate change or legal, regulatory or market measures to address climate change may negatively affect the Company’s business, results of operations, cash flows and prospects.
•Environmental, social and governance matters may impact the Company’s business and reputation.
•Failure to attract and retain highly qualified personnel could affect the Company’s ability to successfully develop and commercialize products.
•The Company may experience difficulties and delays in manufacturing certain of its products, including vaccines.
•The Company’s business in China has grown in the past few years, and the importance of China to the Company’s overall pharmaceutical and vaccines business has increased accordingly. In 2024, the Company experienced lower sales of Gardasil/Gardasil 9 in China and expects that sales of Gardasil/Gardasil 9 in China will decline significantly in 2025.
•The Company may not be able to realize the expected benefits of its investments in emerging markets.
•The Company is exposed to market risk from fluctuations in currency exchange rates and interest rates.
•Pharmaceutical products can develop unexpected safety or efficacy concerns.
•Reliance on third-party relationships and outsourcing arrangements could materially adversely affect the Company’s business.
•Negative events in the animal health industry could have a material adverse effect on future results of operations and financial condition of the Company or its Animal Health business.
•Biologics and vaccines carry unique risks and uncertainties, which could have a material adverse effect on the Company’s future results of operations and financial condition.
•The health care industry in the U.S. has been, and will continue to be, subject to increasing regulation and political action.
•The Company’s products, including products in development, cannot be marketed unless the Company obtains and maintains regulatory approval.
•Developments following regulatory approval may adversely affect sales of the Company’s products.
•The Company is subject to a variety of U.S. and international laws and regulations.
•The Company is subject to evolving and complex tax laws, which may result in additional liabilities that may affect results of operations and financial condition.
•Adverse outcomes in current or future legal matters could negatively affect Merck’s business.
•Product liability insurance for products may be limited, cost prohibitive or unavailable.
•The Company is increasingly dependent on sophisticated software applications and computing infrastructure, including the use of cloud-based applications and environments. The Company continues to be a target of cyber-attacks that could lead to a disruption of its worldwide operations, including manufacturing, research and sales operations.
•The Company is increasing its use of artificial intelligence (AI) systems to automate processes, analyze data, and support decision-making which poses inherent risks.
•Social media and mobile messaging platforms present risks and challenges.
The above list is not exhaustive, and the Company faces additional challenges and risks. Investors should carefully consider all of the information set forth in this Form 10-K, including the following risk factors, before deciding to invest in any of the Company’s securities.
Risk Factors
The risks below are not the only ones the Company faces. Additional risks not currently known to the Company or that the Company presently deems immaterial may also impair its business operations. The Company’s business, financial condition, results of operations, cash flows or prospects could be materially adversely affected by any of these risks. This Form 10-K also contains forward-looking statements that involve risks and uncertainties. The Company’s results could materially differ from those anticipated in these forward-looking statements as a result of certain factors, including the risks it faces described below and elsewhere. See “Cautionary Factors that May Affect Future Results” below.
Risks Related to the Company’s Business
The Company is dependent on its patent rights, and if its patent rights are invalidated or circumvented, its business could be materially adversely affected.
Patent protection is considered, in the aggregate, to be of material importance to the Company’s marketing of human health and animal health products in the U.S. and in most major foreign markets. Patents covering products that it has introduced normally provide market exclusivity, which is important for the successful marketing and sale of its products. The Company seeks patents covering each of its products in each of the markets where it intends to sell the products and where meaningful patent protection is available.
Even if the Company succeeds in obtaining patents covering its products, third parties or government authorities may challenge or seek to invalidate or circumvent its patents and patent applications. It is important for the Company’s business to successfully assert and defend the patent rights that provide market exclusivity for its products. The Company is often involved in patent disputes relating to challenges to its patents or claims by third parties of infringement against the Company. The Company asserts and defends its patents both within and outside the U.S., including by filing claims of infringement against other parties. See Item 8. “Financial Statements and Supplementary Data,” Note 10. “Contingencies and Environmental Liabilities” below. In particular, manufacturers of generic or biosimilar pharmaceutical products from time to time file abbreviated NDAs or BLAs with the FDA seeking to market generic/biosimilar forms of the Company’s products prior to the expiration of relevant patents owned or licensed by the Company. The Company normally responds by asserting one or more of its patents with a lawsuit alleging patent infringement. Patent litigation and other challenges to the Company’s patents are costly and unpredictable and may deprive the Company of market exclusivity for a patented product or, in some cases, third-party patents may prevent the Company from marketing and selling a product in a particular geographic area.
Additionally, certain foreign governments have indicated that compulsory licenses to patents may be granted in the case of national emergencies or in other circumstances, which could diminish or eliminate sales and profits from those regions and negatively affect the Company’s results of operations. Further, court decisions relating to other companies’ patents, potential legislation in both the U.S. and certain foreign markets relating to patents, as well as regulatory initiatives may result in a more general weakening of intellectual property protection.
If one or more important products lose patent protection in profitable markets, sales of those products are likely to decline significantly as a result of generic versions of those products becoming available. The Company’s results of operations may be adversely affected by the lost sales unless and until the Company has launched commercially successful products that replace the lost sales. In addition, if products that were measured at fair value and capitalized in connection with acquisitions experience difficulties in the market that negatively affect product cash flows, the Company may recognize material non-cash impairment charges with respect to the value of those products.
A chart listing the key patent protection for certain of the Company’s marketed products, and U.S. patent protection for candidates in Phase 3 clinical development is set forth above in Item 1. “Business — Patents, Trademarks and Licenses.”
As the Company’s products lose market exclusivity, the Company generally experiences a significant and rapid loss of sales from those products.
The Company depends upon patents to provide it with exclusive marketing rights for its products for some period of time. Loss of patent protection for one of the Company’s products typically leads to a significant and rapid loss of sales for that product as lower priced generic versions of that drug become available. In the case of products that contribute significantly to the Company’s sales, the loss of market exclusivity can have a material adverse effect on the Company’s business, cash flows, results of operations, financial condition and prospects. The Company lost market exclusivity for Bridion in Europe and Japan in 2023 and 2024, respectively, and the Company has experienced a substantial decline in Bridion sales in those markets. Bridion will lose market exclusivity in the U.S. in 2026 (subject to patent litigation discussed below) and the Company expects that sales of Bridion in the U.S. will decline substantially thereafter. In addition, the Company expects U.S. sales of Keytruda to decline beginning in January 2028 upon implementation of government pricing under the IRA, and to further decline upon loss of market exclusivity following expiration of the U.S. compound patent in December 2028. The Company expects to lose market exclusivity in Europe for Keytruda in 2031 following compound patent expiration. There may, however, be attempts by one or more companies to challenge the patent or launch a biosimilar product despite the patent in some European jurisdictions following the expiration of data exclusivity in Europe in July 2026.
Key products generate a significant amount of the Company’s profits and cash flows, and any events that adversely affect the markets for its leading products could have a material adverse effect on the Company’s results of operations and financial condition.
The Company’s ability to generate profits and operating cash flows depends largely upon the continued profitability of the Company’s key products, such as Keytruda, Gardasil/Gardasil 9, Lynparza, Bravecto, and Bridion. In 2024, the Company’s oncology portfolio, led by Keytruda, represented substantially all of the Company’s revenue growth. In particular, in the aggregate, in 2024, sales of Keytruda represented 46% of the Company’s total sales. As a result of the Company’s dependence on key products, any event that adversely affects any of these products or the markets for any of these products, such as the slowing demand for Gardasil/Gardasil 9 in China which the Company has experienced, could have a significant adverse impact on results of operations and financial condition. Other events could include loss of patent protection, increased costs associated with manufacturing, generic or over-the-counter availability of the Company’s product or a competitive product, the discovery of previously unknown side effects, results of post-approval trials, increased competition from the introduction of new, more effective treatments and discontinuation or removal from the market of the product for any reason. Such events could have a material adverse effect on the sales of any such products.
The Company’s research and development efforts may not succeed in developing commercially successful products and the Company may not be able to acquire commercially successful products in other ways; consequently, the Company may not be able to replace sales of successful products that lose patent protection.
In order to remain competitive, the Company, like other major pharmaceutical companies, must continue to launch new products. Expected declines in sales of products after the loss of market exclusivity mean that the Company’s future success is dependent on its pipeline of new products, including new products that it may develop through collaborations and joint ventures and products that it is able to obtain through license or acquisition. To accomplish this, the Company commits substantial effort, funds and other resources to research and development, both through its own dedicated resources and through various collaborations with third parties. There is a high rate of failure inherent in the research and development process for new drugs and vaccines. As a result, there is a high risk that funds invested by the Company in research programs will not generate financial returns. This risk profile is compounded by the fact that this research has a long investment cycle. To bring a pharmaceutical compound from the discovery phase to market may take a decade or more and failure can occur at any point in the process, including later in the process after significant funds have been invested.
For a description of the research and development process, see Item 1. “Business — Research and Development” above. Each phase of testing is highly regulated and during each phase there is a substantial risk that the Company will encounter serious obstacles or will not achieve its goals. Therefore, the Company may abandon a product in which it has invested substantial amounts of time and resources. Some of the risks encountered in the research and development process include the following: preclinical testing of a new compound may yield disappointing results; competing products from other manufacturers may reach the market first; clinical trials of a new drug may not be successful; a new drug may not be effective or may have harmful side effects; a new drug may not be approved by the regulators for its intended use; it may not be possible to obtain a patent for a new drug; payers may refuse to cover or reimburse the new product; or sales of a new product may be disappointing.
The Company cannot state with certainty when or whether any of its products now under development will be approved or launched; whether it will be able to develop, license or otherwise acquire compounds, product candidates or products; or whether any products, once launched, will be commercially successful. The Company must maintain a continuous flow of successful new products and successful new indications for existing products sufficient both to cover its substantial research and development costs and to replace sales that are lost as profitable products lose market exclusivity or are displaced by competing products or therapies. Failure to do so in the short term or long term would have a material adverse effect on the Company’s business, results of operations, cash flows, financial condition and prospects.
The Company’s success is dependent on the successful development and marketing of new products, which are subject to substantial risks.
Products that appear promising in development may fail to reach the market or fail to succeed for numerous reasons, including the following:
•findings of ineffectiveness, superior safety or efficacy of competing products, or harmful side effects in clinical or preclinical testing;
•failure to receive the necessary regulatory approvals, including delays in the approval of new products and new indications, or the anticipated labeling, and uncertainties about the time required to obtain regulatory approvals and the benefit/risk standards applied by regulatory agencies in determining whether to grant approvals;
•failure in certain markets to obtain reimbursement commensurate with the level of innovation and clinical benefit presented by the product;
•lack of economic feasibility due to manufacturing costs or other factors; and
•preclusion from commercialization by the proprietary rights of others.
In the future, if certain pipeline programs are cancelled or if the Company believes that their commercial prospects have been reduced, the Company may recognize material non-cash impairment charges for those programs that were measured at fair value and capitalized in connection with acquisitions or certain collaborations.
Failure to successfully develop and market new products in the short term or long term would have a material adverse effect on the Company’s business, results of operations, cash flows, financial condition and prospects.
The Company faces continued pricing pressure with respect to its products.
The Company faces continued pricing pressure globally and, particularly in mature markets, from managed care organizations, government agencies and programs that could negatively affect the Company’s sales and profit margins. In the U.S., these include (i) U.S. federal laws and regulations related to Medicare and Medicaid, including the Medicare Prescription Drug Improvement and Modernization Act of 2003, the ACA, and the IRA, (ii) practices of managed care groups and institutional and governmental purchasers, and (iii) state activities aimed at increasing price transparency, including new laws as noted above in Item 1. “Competition and the Health Care Environment.” Changes to the health care system enacted as part of health care reform in the U.S., as well as increased purchasing power of entities that negotiate on behalf of Medicare, Medicaid, and private sector beneficiaries, could result in further pricing pressures. As noted in Item 1. “Competition and the Health Care Environment,” in 2023, HHS selected Januvia for the first year of the IRA’s price setting program, which will result in a government set price becoming effective on January 1, 2026. Government price setting may also impact pricing in the private market, negatively affecting the Company’s performance. In January 2025, HHS announced that Janumet and Janumet XR have been selected for government price setting, which will become effective on January 1, 2027. Furthermore, the Company expects that in 2026 HHS will include Keytruda in a subsequent selection of products to undergo IRA price setting, with such price to become effective on January 1, 2028 and the Company expects that, as a result, U.S. sales of Keytruda will decline after that time. In addition, in the U.S., larger customers have received higher rebates on drugs in certain highly competitive categories. The Company must also compete to be placed on formularies of managed care organizations. Exclusion of a product from a formulary can lead to reduced usage in the managed care organization. The Company is also facing pricing pressure from purchasers of certain vaccines in highly competitive categories.
Outside the U.S., numerous major markets, including the EU, Japan and China have pervasive government involvement in funding health care and, in that regard, fix the pricing and reimbursement of pharmaceutical and vaccine products. Consequently, in those markets, the Company is subject to government decision making and budgetary actions with respect to its products. In Japan, the pharmaceutical industry is subject to government-mandated annual price reductions of pharmaceutical products and certain vaccines. Furthermore, the Japanese government can order re-pricing for specific products if it determines that use of such product will exceed certain thresholds defined under applicable re-pricing rules.
The Company expects pricing pressures to continue in the future.
Unfavorable or uncertain economic conditions, together with cost-reduction measures being taken by certain governments, could negatively affect the Company’s operating results.
The Company’s business may be adversely affected by local and global economic conditions, including with respect to inflation, interest rates, and costs of raw materials and packaging. Uncertainty in global economic and geopolitical conditions may result in a slowdown to the global economy that could affect the Company’s business by reducing the prices that drug wholesalers and retailers, hospitals, government agencies and managed health care providers may be able or willing to pay for the Company’s products or by reducing the demand for the Company’s products, which could in turn negatively impact the Company’s sales and result in a material adverse effect on the Company’s business, cash flows, results of operations, financial condition and prospects.
As discussed above in Item 1. “Competition and the Health Care Environment,” global efforts toward health care cost containment continue to exert pressure on product pricing and market access worldwide. Changes to the U.S. health care system as part of health care reform, as well as increased purchasing power of entities that negotiate on behalf of Medicare, Medicaid, and private sector beneficiaries, have contributed to pricing pressure. In several international markets, government-mandated pricing actions have reduced prices of generic and patented drugs. In addition, the Company’s sales performance in 2024 was negatively affected by other cost-reduction measures taken by governments and other third parties to lower health care costs, including in the U.S., the expansion of the Federal 340B Drug Discount Program. The Company anticipates all of these actions, and additional actions in the future, will continue to negatively affect sales and profits.
If credit and economic conditions worsen, the resulting economic and currency impacts in the affected markets and globally could have a material adverse effect on the Company’s results.
The Company faces intense competition from both lower cost generic and biosimilar products and competitors’ products.
In general, the Company faces increasing competition from lower-cost generic and biosimilar products. The patent rights that protect its products are of varying strengths and durations. In addition, in some countries, patent protection is significantly weaker than in the U.S. or in the EU. In the U.S. and the EU, political pressure to reduce spending on prescription drugs has led to legislation and other measures that encourage the use of generic and biosimilar products. Although it is the Company’s policy to actively protect its patent rights, generic challenges to the Company’s products can arise at any time, and the Company’s patents may not prevent the emergence of generic competition for its products.
Loss of patent protection for a product typically is followed promptly by generic or biosimilar substitutes, reducing the Company’s sales of that product. Availability of generic substitutes for the Company’s drugs may adversely affect its results of operations and cash flows. In addition, proposals emerge from time to time in the U.S. and other countries for legislation to further encourage the early and rapid approval of generic drugs. Any such proposal that is enacted into law could worsen this substantial negative effect on the Company’s sales, business, cash flows, results of operations, financial condition and prospects.
Also, the Company’s products face intense competition from competitors’ products. This competition may increase as new products enter the market. In such an event, the competitors’ products may be safer or more effective, more convenient to use, have better insurance coverage or reimbursement levels or be more effectively marketed and sold than the Company’s products. Alternatively, in the case of generic competition, including the generic availability of competitors’ branded products, they may be equally safe and effective products that are sold at a substantially lower price than the Company’s products. As a result, if the Company fails to maintain its competitive position, this could have a material adverse effect on its business, cash flows, results of operations, financial condition and prospects. In addition, if products that were measured at fair value and capitalized in connection with acquisitions experience difficulties in the market that negatively impact product cash flows, the Company may recognize material non-cash impairment charges with respect to the value of those products.
The Company has significant global operations, which expose it to additional risks, and any adverse event could have a material adverse effect on the Company’s results of operations and financial condition.
The extent of the Company’s operations outside the U.S. is significant. Risks inherent in conducting a global business include:
•changes in medical reimbursement policies and programs and pricing restrictions in key markets;
•multiple regulatory requirements that could restrict the Company’s ability to manufacture and sell its products in key markets;
•trade protection measures and import or export licensing requirements, including the imposition of trade sanctions or similar restrictions by the U.S. or other governments;
•foreign exchange fluctuations;
•diminished protection of intellectual property in some countries; and
•possible nationalization and expropriation.
The U.S. government has announced plans to significantly increase tariffs on foreign imports into the U.S., particularly from Canada and Mexico and has already increased tariffs on imports from China. It is too early for the
Company to assess if, or to what extent, such policies will be implemented or continue to be implemented, and the extent of any measures that have been or will be taken by any impacted countries. In addition, there may be changes to the Company’s business if there is instability, disruption or destruction in a significant geographic region, regardless of cause, including war, terrorism, riot, civil insurrection or social unrest; and natural or man-made disasters, including famine, flood, fire, earthquake, storm or disease. Events like these, such as the ongoing war between Russia and Ukraine, and the conflict in the Middle East, and/or policy changes with respect to international trade protection measures, could result in material adverse effects on macroeconomic conditions, currency exchange rates and financial markets, and may adversely affect the Company’s business, results of operations, cash flows and financial condition.
Climate change or legal, regulatory or market measures to address climate change may negatively affect the Company’s business, results of operations, cash flows and prospects.
The Company believes that climate change has the potential to negatively affect its business, results of operations, cash flows and prospects. The Company is exposed to physical risks (such as extreme weather conditions, inland flooding or rising sea levels), risks in transitioning to a low-carbon economy (such as additional legal or regulatory requirements, changes in technology, market risk and reputational risk) and social and human effects (such as population dislocations and harm to health and well-being) associated with climate change. These risks can be either acute (short-term) or chronic (long-term).
The adverse impacts of climate change include increased frequency and severity of natural disasters and extreme weather events such as hurricanes, tornados, wildfires (exacerbated by drought), flooding, and extreme heat. Extreme weather, inland flooding and sea-level rise pose physical risks to the Company’s facilities as well as those of its suppliers. Such risks include losses incurred as a result of physical damage to facilities, loss or spoilage of inventory, and business interruption caused by such natural disasters and extreme weather events. Other potential physical impacts due to climate change include reduced access to high-quality water in certain regions and the loss of biodiversity, which could impact future product development. These risks could disrupt the Company’s operations and its supply chain, which may result in increased costs.
New legal and regulatory requirements are being enacted to prevent, mitigate, or adapt to the implications of a changing climate and its effects on the environment. These regulations, which may differ across jurisdictions, could result in the Company being subject to new or expanded carbon pricing or taxes, increased compliance costs, restrictions on GHG emissions, investment in new technologies, increased GHG emission disclosure (including costs resulting from mandatory or voluntary reporting, diligence or disclosure) and transparency, recurring investments in data gathering and reporting systems, upgrades of facilities to meet new building codes, and the redesign of utility systems, which could increase the Company’s operating costs, including the cost of electricity and energy used by the Company. The Company’s supply chain would likely be subject to these same transitional risks and would likely pass along any increased costs to the Company, which may affect the Company’s ability to procure raw materials or other supplies required for the operation of the Company’s business at the quantities and levels required.
Environmental, social and governance matters may impact the Company’s business and reputation.
Governmental authorities, non-governmental organizations, customers, investors, external stakeholders and employees are sensitive to environmental, social and governance concerns, such as human capital, climate change, water use, recyclability or recoverability of packaging, and plastic waste. The focus on these concerns may lead to new requirements that could result in increased costs associated with developing, manufacturing and distributing the Company’s products, and related reporting obligations. The Company’s ability to compete could also be affected by changing customer preferences and requirements, such as growing demand for validated net zero GHG emission targets and more environmentally friendly products, packaging or supplier practices, or by failure to meet such customer expectations or demand. The Company risks negative shareholder reaction, including from proxy advisory services, as well as damage to its brand and reputation and inability to attract and retain employee talent, if the Company fails to act responsibly, or if the Company is perceived to not be acting responsibly, in key areas, including equitable access to medicines and vaccines, product quality and safety, environmental stewardship, reduction of GHG emissions, support for local communities, corporate governance and transparency, and addressing human capital factors in the Company’s operations. Responding to these considerations as well as any applicable regulatory requirements and implementation of the Company’s goals and initiatives involves risks and uncertainties, requires investments, and depends in part on third-party performance or data that is outside of the Company’s control. In addition, some governmental authorities, non-governmental organizations, and stakeholders may disagree with the Company’s goals and initiatives. If the Company does not meet the evolving and varied regulatory requirements and expectations of its investors, customers and other stakeholders, the Company could experience negative impacts to the Company’s business and results of operations. In addition, the Company is subject to expanding mandatory and voluntary reporting, diligence and disclosure requirements, including the EU’s Corporate
Sustainability Reporting Directive (CSRD) and potentially the SEC’s climate-related reporting requirements (which are currently stayed), the legislation in California requiring reporting of GHG emissions and climate risk, and similar regulatory requirements in other jurisdictions outside the U.S. These evolving regulatory requirements are likely to result in increased costs and complexities of compliance in order to collect, measure and report on the relevant information.
Failure to attract and retain highly qualified personnel could affect the Company’s ability to successfully develop and commercialize products.
The Company’s success is largely dependent on its continued ability to attract and retain highly qualified scientific, technical and management personnel, as well as personnel with expertise in clinical research and development, governmental regulation and commercialization. Competition for qualified personnel in the pharmaceutical industry, both in the U.S. and internationally, is intense. The Company cannot be sure that it will be able to attract and retain qualified personnel or that the costs of doing so will not materially increase.
The Company may experience difficulties and delays in manufacturing certain of its products, including vaccines.
Merck from time to time experiences difficulties in manufacturing certain of its products, including vaccines. For example, the Company is currently experiencing manufacturing delays related to Varivax and ProQuad which will result in supply constraints in 2025. The Company may, in the future, experience other difficulties and delays in manufacturing its products, such as (i) failure of the Company or any of its vendors or suppliers to comply with Current Good Manufacturing Practices and other applicable regulations and quality assurance guidelines that could lead to manufacturing shutdowns, product shortages and delays in product manufacturing; (ii) delays related to the construction of new facilities or the expansion of existing facilities, including those intended to support future demand for the Company’s products; and (iii) other manufacturing or distribution problems including supply chain delays, shortages in raw materials, changes in manufacturing production sites and limits to manufacturing capacity due to regulatory requirements, changes in types of products produced, or physical limitations that could impact continuous supply. In addition, the Company could experience difficulties or delays in manufacturing its products caused by natural disasters, such as hurricanes. Manufacturing difficulties can result in product shortages, leading to lost sales and reputational harm to the Company.
The Company’s business in China has grown in the past few years, and the importance of China to the Company’s overall pharmaceutical and vaccines business has increased accordingly. In 2024, the Company experienced lower sales of Gardasil/Gardasil 9 in China and expects that sales of Gardasil/Gardasil 9 in China will decline significantly in 2025.
The Company’s business in China has grown in the past few years, and the importance of China to the Company’s overall pharmaceutical and vaccines business has increased accordingly. Beginning in mid-2024, the Company observed a significant decline in shipments from its distributor and commercialization partner in China, Chongqing Zhifei Biological Products Co., Ltd. (Zhifei), to disease and control prevention institutions and correspondingly into the points of vaccination, resulting in above normal inventory levels at Zhifei. Accordingly, the Company shipped less than its contracted doses to Zhifei in the latter part of 2024. Lower demand in China persisted and, at the end of 2024, overall channel inventory levels in China remained elevated at above normal levels. Therefore, the Company made a decision to temporarily pause shipments to China beginning in February 2025 through at least the middle of the year and as a result, combined sales of Gardasil/Gardasil 9 will decline significantly in 2025 compared with 2024. Furthermore, the government's anti-corruption campaign, particularly the increased number of inspections and audits, could substantially increase the administrative burden on health care institutions and health care professionals throughout the whole industry in China and potentially have a negative impact on the Company's sales. In addition to its commercial operations, the Company has significant research and manufacturing operations in China, including working with Chinese entities such as Wuxi Apptech Co., Ltd. If geopolitical tensions were to increase and disrupt the Company’s operations in China, such disruption could result in a material adverse effect on the Company’s product development, sales, business, cash flows, results of operations, financial condition and prospects.
Also, continued growth of the Company’s business in China is dependent upon ongoing development of a favorable environment for innovative pharmaceutical products and vaccines, sustained access for the Company’s currently marketed products, and the absence of trade impediments or adverse pricing controls. As noted above in Item 1. “Competition and the Health Care Environment,” pricing pressure in China has increased as the Chinese government has been taking steps to reduce costs, including implementing health care reform that has led to the acceleration of generic substitution, where available. While the mechanism for drugs being added to the NRDL evolves, inclusion may require a price negotiation which could impact the outlook in the market for selected brands. A
new NRDL was recently completed in which new entries averaged 63% price reductions. While pricing pressure has always existed in China, health care reform has increased this pressure in part due to the acceleration of generic substitution through the government’s VBP program. In 2019, the government implemented the VBP program through a tendering process for mature products which have generic substitutes with a Generic Quality Consistency Evaluation approval. Mature products that have entered into the last five rounds of VBP had, on average, a price reduction of more than 50%. The Company expects VBP to be a semi-annual process that will have a significant impact on mature products moving forward.
The Company may not be able to realize the expected benefits of its investments in emerging markets.
The Company has been taking steps to increase its sales in emerging markets. However, there is no guarantee that the Company’s efforts to expand sales in these markets will succeed. Some countries within emerging markets may be especially vulnerable to periods of global financial instability or may have very limited resources to spend on health care. In order for the Company to operate successfully in emerging markets, it must attract and retain qualified personnel. The Company may also be required to increase its reliance on third-party agents within less developed markets, which may affect its ability to realize continued growth and may also increase the Company’s risk exposure. In addition, many of these countries have currencies that fluctuate substantially and, if such currencies devalue and the Company cannot offset the devaluations, the Company’s financial performance within such countries could be adversely affected.
For all these reasons, sales within emerging markets carry significant risks. However, at the same time, macro-economic growth of selected emerging markets is expected to lead to significant increased health care spending in those countries and access to innovative medicines for patients. A failure to maintain the Company’s presence in emerging markets could therefore have a material adverse effect on the Company’s business, cash flows, results of operations, financial condition and prospects.
The Company is exposed to market risk from fluctuations in currency exchange rates and interest rates.
The Company operates in multiple jurisdictions and virtually all sales are denominated in currencies of the local jurisdiction. Additionally, the Company has entered and will enter into business development transactions, borrowings or other financial transactions that may give rise to currency and interest rate exposure.
Since the Company cannot, with certainty, foresee and mitigate against such adverse changes, fluctuations in currency exchange rates, interest rates and inflation could negatively affect the Company’s business, cash flows, results of operations, financial condition and prospects. For example, Argentina is currently experiencing hyperinflation, which is affecting the Company’s operations in that market.
In order to mitigate against the adverse impact of these market fluctuations, the Company will from time to time enter into hedging agreements. While hedging agreements, such as currency options and forwards, and interest rate swaps, may limit some of the exposure to exchange rate and interest rate fluctuations, such attempts to mitigate these risks may be costly and not always successful.
Pharmaceutical products can develop unexpected safety or efficacy concerns.
Unexpected safety or efficacy concerns can arise with respect to marketed products, whether or not scientifically justified, leading to product recalls, withdrawals, or declining sales, as well as product liability, consumer fraud and/or other claims, including potential civil or criminal governmental actions.
Reliance on third-party relationships and outsourcing arrangements could materially adversely affect the Company’s business.
The Company depends on third parties, including suppliers, distributors, alliances with other pharmaceutical and biotechnology companies, and third-party service providers, for key aspects of its business including development, manufacture and commercialization of its products and support for its information technology (IT) systems. Failure of these third parties to meet their contractual, regulatory and other obligations to the Company or the development of factors that materially disrupt the relationships between the Company and these third parties could have a material adverse effect on the Company’s business.
Negative events in the animal health industry could have a material adverse effect on future results of operations and financial condition of the Company or its Animal Health business.
Future sales of key animal health products could be adversely affected by a number of risk factors including certain risks that are specific to the animal health business. For example, the outbreak of disease carried by animals, such as Avian Influenza or African Swine Fever, could lead to their widespread death and precautionary destruction as well as the reduced consumption and demand for animals, which could adversely affect the Company’s results of operations. Also, the outbreak of any highly contagious diseases near the Company’s main production sites could require the Company to immediately halt the manufacture of its animal health products at such sites or force the Company to incur substantial expenses in procuring raw materials or products elsewhere. Other risks specific to animal health include epidemics and pandemics affecting livestock, government procurement and pricing practices, weather and global agribusiness economic events. In addition, in 2024, sales of Bravecto were $1.1 billion, which represented 19% of the Company’s Animal Health segment sales. Any negative event with respect to Bravecto could have a material adverse effect on the Company’s Animal Health sales. If the Animal Health segment of the Company’s business becomes more significant, the impact of any such events on future results of operations could also become more significant.
Biologics and vaccines carry unique risks and uncertainties, which could have a material adverse effect on the Company’s future results of operations and financial condition.
The successful development, testing, manufacturing and commercialization of biologics and vaccines, particularly human and animal health vaccines, is a long, complex, expensive and uncertain process. There are unique risks and uncertainties related to biologics and vaccines, including:
•There may be limited access to, and supply of, normal and diseased tissue samples, cell lines, pathogens, bacteria, viral strains and other biological materials. In addition, government regulations in multiple jurisdictions, such as the U.S. and the EU, could result in restricted access to, or transport or use of, such materials. If the Company loses access to sufficient sources of such materials, or if tighter restrictions are imposed on the use of such materials, the Company may not be able to conduct research activities as planned and may incur additional development costs.
•The development, manufacturing and marketing of biologics and vaccines are subject to regulation by the FDA, the EMA and other regulatory bodies. These regulations are often more complex and extensive than the regulations applicable to other pharmaceutical products. For example, in the U.S., a BLA, including both preclinical and clinical trial data and extensive data regarding the manufacturing procedures, is required for human vaccine candidates, and FDA approval is generally required for the release of each manufactured commercial human vaccine lot.
•Manufacturing biologics and vaccines, especially in large quantities, is complex and may require the use of innovative technologies to handle living micro-organisms. Each lot of an approved biologic and vaccine must undergo thorough testing for identity, strength, quality, purity and potency. Manufacturing biologics requires facilities specifically designed for and validated for this purpose, and sophisticated quality assurance and quality control procedures are necessary. Slight deviations anywhere in the manufacturing process, including filling, labeling, packaging, storage and shipping, and quality control and testing, may result in lot failures, product recalls or spoilage. When changes are made to the manufacturing process, the Company may be required to provide preclinical and clinical data showing the comparable identity, strength, quality, purity or potency of the biologics and vaccines before and after such changes.
•Biologics and vaccines are costly to manufacture because production ingredients are derived from living animal or plant material, and most biologics and vaccines cannot be made synthetically. In particular, keeping up with the demand for vaccines may be difficult due to the complexity of producing vaccines.
•The use of biologically derived ingredients can lead to variability in the manufacturing process and could lead to allegations of harm, including infections or allergic reactions, which allegations would be reviewed through a standard investigation process that could lead to closure of product facilities due to possible contamination. Any of these events could result in substantial costs.
Risks Relating to Government Regulation and Legal Proceedings
The health care industry in the U.S. has been, and will continue to be, subject to increasing regulation and political action.
As discussed above in Item 1. “Competition and the Health Care Environment,” the Company believes that the health care industry will continue to be subject to increasing regulation as well as political and legal action, as future proposals to reform the health care system are considered by the Executive Branch, Congress and state legislatures.
In 2022, Congress passed the IRA, which makes significant changes to how drugs are covered and paid for under the Medicare program, including the creation of financial penalties for drugs whose prices rise faster than the rate of inflation, redesign of the Medicare Part D program to require manufacturers to bear more of the liability for certain drug benefits, which has taken effect in 2025, and government price setting for certain Medicare Part D drugs, starting in 2026, and Medicare Part B drugs starting in 2028. Furthermore, government price setting may also impact pricing in the private market, negatively affecting the Company’s performance. As noted in Item 1. “Competition and the Health Care Environment,” in 2023, HHS selected Januvia for the first year of the IRA’s price setting program, which will result in a government set price becoming effective on January 1, 2026. On January 17, 2025, HHS announced that Janumet and Janumet XR have been selected for government price setting, which will become effective on January 1, 2027. Furthermore, the Company expects that in 2026 HHS will include Keytruda in a subsequent selection of products to undergo IRA price setting, with such price to become effective on January 1, 2028 and the Company expects that, as a result, U.S. sales of Keytruda will decline after that time.
In addition, in 2021, Congress passed the American Rescue Plan Act, which included a provision that eliminates the statutory cap on rebates drug manufacturers pay to Medicaid beginning in January 2024. These rebates act as a discount off the list price and eliminating the cap means that manufacturer discounts paid to Medicaid can increase. Prior to this change, manufacturers have not been required to pay more than 100% of the Average Manufacturer Price (AMP) in rebates to state Medicaid programs for Medicaid-covered drugs. As a result of this provision, manufacturers may have to pay state Medicaid programs more in rebates than they received on sales of particular products. This change presents a risk to Merck for drugs that have high Medicaid utilization and rebate exposure that is more than 100% of the AMP. Additionally, increased utilization of the 340B Federal Drug Discount Program and restrictions on the Company’s ability to identify inappropriate discounts are having a negative impact on the Company’s performance. Also, the Company expects that states will continue their focus on pharmaceutical pricing and will increasingly shift to more aggressive price control tools such as Prescription Drug Affordability Boards that have the authority to conduct affordability reviews and establish upper payment limits and that Company products may be selected for such reviews.
In the U.S., members of the government have made public statements in favor of, and may take steps to implement, various regulatory changes that could negatively impact the pharmaceutical industry, including the Company. Those potential changes include some related to vaccines and vaccine development, as well as personnel and policy changes at the FDA and other government agencies and programs. For example, HHS could undergo changes that could make it more difficult for the FDA to grant regulatory approvals for drugs and vaccines and the U.S. Centers for Disease Control and Prevention (CDC) to issue or maintain recommendations for vaccines. Additionally, if the FDA drug user fee programs were eliminated, that could cause significant delays to facility inspections and approvals of new products. It is too early for the Company to assess which, if any, of the policy changes that have been publicly referenced would be implemented, and the Company cannot predict what additional future changes in the health care industry in general, or the pharmaceutical industry in particular, will occur; however, any changes could have a material adverse effect on the Company’s business, cash flows, results of operations, financial condition and prospects.
The Company’s products, including products in development, cannot be marketed unless the Company obtains and maintains regulatory approval.
The Company’s activities, including research, preclinical testing, clinical trials and the manufacturing and marketing of its products, are subject to extensive regulation by numerous federal, state and local governmental authorities in the U.S., including the FDA, and by foreign regulatory authorities, including in the EU, Japan and China. In the U.S., the FDA administers requirements covering the testing, approval, safety, effectiveness, manufacturing, labeling and marketing of prescription pharmaceuticals and vaccines. In some cases, the FDA requirements have increased the amount of time and resources necessary to develop new products and bring them to market in the U.S. Regulation outside the U.S. also is primarily focused on drug safety and effectiveness and, in many cases, reduction in the cost of drugs. The FDA and foreign regulatory authorities, including in the EU, Japan and China, have substantial discretion to require additional testing, to delay or withhold registration and marketing approval and to otherwise preclude distribution and sale of a product.
Even if the Company is successful in developing new products, it will not be able to market any of those products unless and until it has obtained all required regulatory approvals (which in limited circumstances may include authorizations for emergency use) in each jurisdiction where it proposes to market the new products. Once obtained, the Company must maintain approval as long as it plans to market its new products in each jurisdiction where approval is required. The Company’s failure to obtain approval, significant delays in the approval process, or its failure to maintain approval in any jurisdiction will prevent it from selling the products in that jurisdiction and realizing sales.
Developments following regulatory approval may adversely affect sales of the Company’s products.
Even after a product reaches the market, certain developments following regulatory approval may decrease demand for the Company’s products, including the following:
•results in post-approval Phase 4 trials or other studies;
•the re-review of products that are already marketed;
•the recall or loss of marketing approval of products that are already marketed;
•changing government standards or public expectations regarding safety, efficacy, quality or labeling changes;
•scrutiny of advertising and promotion; and
•the withdrawal of indications granted pursuant to accelerated approvals.
In the past, clinical trials and post-marketing surveillance of certain marketed drugs of the Company and of competitors within the industry have raised concerns that have led to recalls, withdrawals or adverse labeling of marketed products. Clinical trials and post-marketing surveillance of certain marketed drugs also have raised concerns among some prescribers and patients relating to the safety or efficacy of pharmaceutical products in general that have negatively affected the sales of such products. In addition, increased scrutiny of the outcomes of clinical trials has led to increased volatility in market reaction. Further, these matters often attract litigation and, even where the basis for the litigation is groundless, considerable resources may be needed to respond.
In addition, following in the wake of product withdrawals and other significant safety issues, health authorities such as the FDA, the EMA, Japan’s PMDA and China’s NMPA have increased their focus on safety when assessing the benefit/risk balance of drugs. Some health authorities appear to have become more cautious when making decisions about approvability of new products or indications.
If previously unknown side effects are discovered or if there is an increase in negative publicity regarding known side effects of any of the Company’s products, it could significantly reduce demand for the product or require the Company to take actions that could negatively affect sales, including removing the product from the market, restricting its distribution or applying for labeling changes. Further, in the environment in which all pharmaceutical companies operate, the Company is at risk for product liability and consumer protection claims and civil and criminal governmental actions related to its products, research and/or marketing activities. In addition, dissemination of promotional materials through evolving digital channels serves to increase visibility and scrutiny in the marketplace.
The Company is subject to a variety of U.S. and international laws and regulations.
The Company is currently subject to a number of government laws and regulations and, in the future, could become subject to new government laws and regulations. The costs of compliance with such laws and regulations, or the negative results of non-compliance, could adversely affect the business, cash flows, results of operations, financial condition and prospects of the Company; these laws and regulations include (i) additional health care reform initiatives in the U.S. or in other countries, including additional mandatory discounts or fees; (ii) the U.S. Foreign Corrupt Practices Act (FCPA) or other anti-bribery and corruption laws; (iii) new laws, regulations and judicial or other governmental decisions affecting pricing, drug reimbursement, and access or marketing within or across jurisdictions; (iv) changes in intellectual property laws; (v) changes in accounting standards; (vi) new and increasing data privacy regulations and enforcement, particularly in the EU, the U.S., and China; (vii) legislative mandates or preferences for local manufacturing of pharmaceutical or vaccine products; (viii) emerging and new global regulatory requirements for reporting payments and other value transfers to health care professionals; (ix) sustainability regulations, such as the EU’s CSRD; and (x) the potential impact of importation restrictions, embargoes, trade sanctions and legislative and/or other regulatory changes.
The Company is subject to evolving and complex tax laws, which may result in additional liabilities that may affect results of operations and financial condition.
The Company is subject to evolving and complex tax laws in the jurisdictions in which it operates. Significant judgment is required for determining the Company’s tax liabilities, and the Company’s tax returns are routinely examined by various tax authorities. The Internal Revenue Service (IRS) is currently conducting examinations of the Company’s tax returns for the years 2017 and 2018, including the one-time transition tax enacted under the Tax Cuts and Jobs Act of 2017 (TCJA). If the IRS disagrees with the Company’s transition tax position, it may result in a significant tax liability. The IRS is also currently conducting examinations of the Company’s tax returns for the years 2021 and 2022. In addition, various state and foreign tax examinations are in progress. In connection with the Organization for Economic Cooperation and Development (OECD) Base Erosion and Profit Shifting project, companies are required to disclose more information to tax authorities on operations around the world, which may lead to greater audit scrutiny of profits earned in other countries. The Company believes that its accrual for tax contingencies is adequate for all open years based on past experience, interpretations of tax law, and judgments about potential actions by tax authorities; however, due to the complexity of tax contingencies, the ultimate resolution of any tax matters may result in payments greater or less than amounts accrued. In addition, the Company may be negatively affected by changes in tax laws, or new tax laws, affecting, for example, tax rates, and/or revised tax law interpretations in domestic or foreign jurisdictions, including, among others, any potential changes to the existing U.S. tax law by the Executive Branch and Congress, as well as any changes in tax law resulting from the implementation of the OECD’s two-pillar solution to reform the international tax landscape.
The Company has taken the position, based on the opinions of tax counsel, that its distribution of Organon & Co. (Organon) common stock in connection with the 2021 spin-off (Spin-Off) qualifies as a transaction that is tax-free for U.S. federal income tax purposes. If any facts, assumptions, representations, and undertakings from the Company and Organon regarding the past and future conduct of their respective businesses and other matters are incorrect or not otherwise satisfied, the Spin-Off may not qualify for tax-free treatment, which could result in significant U.S. federal income tax liabilities for the Company and its shareholders.
Adverse outcomes in current or future legal matters could negatively affect Merck’s business.
Current or future litigation, claims, proceedings and government investigations could preclude or delay the commercialization of Merck’s products or could adversely affect Merck’s business, results of operations, cash flows, financial condition and prospects. Such legal matters may include, but are not limited to: (i) intellectual property disputes; (ii) adverse decisions in litigation, including product safety and liability matters, such as the litigation involving Gardasil, consumer protection and commercial cases; (iii) anti-bribery regulations, such as the FCPA, including compliance with ongoing reporting obligations to the government resulting from any settlements; (iv) recalls or withdrawals of pharmaceutical products or forced closings of manufacturing plants; (v) product pricing and promotional matters; (vi) lawsuits, claims and administrative proceedings asserting, or investigations into, violations of securities, antitrust, federal and state pricing, consumer protection, data privacy and other laws and regulations; (vii) environmental, health, safety and sustainability matters, including regulatory actions in response to climate change; and (viii) tax liabilities resulting from assessments from tax authorities.
See Item 8. “Financial Statements and Supplementary Data,” Note 10, “Contingencies and Environmental Liabilities” for more information on the Company’s legal matters.
Product liability insurance for products may be limited, cost prohibitive or unavailable.
As a result of a number of factors, product liability insurance has become less available while the cost of such insurance has increased significantly. The Company is subject to a substantial number of product liability claims. See Item 8. “Financial Statements and Supplementary Data,” Note 10. “Contingencies and Environmental Liabilities” below for more information on the Company’s current product liability litigation. With respect to product liability, the Company self-insures substantially all of its risk, as the availability of commercial insurance has become more restrictive. The Company has evaluated its risks and has determined that the cost of obtaining product liability insurance outweighs the likely benefits of the coverage that is available and, as such, has no insurance for most product liabilities. The Company will continually assess the most efficient means to address its risk; however, there can be no guarantee that insurance coverage will be obtained or, if obtained, will be sufficient to fully cover product liabilities that may arise.
Risks Related to Technology
The Company is increasingly dependent on sophisticated software applications and computing infrastructure, including the use of cloud-based applications and environments. The Company continues to be a target of cyber-attacks that could lead to a disruption of its worldwide operations, including manufacturing, research and sales operations.
The Company is increasingly dependent on sophisticated software applications, complex information technology systems, computing infrastructure, and cloud service providers (collectively, IT systems) to conduct critical operations and financial reporting. Certain of these systems are managed, hosted, provided or used by third parties to assist in conducting the Company’s business. Disruption, degradation, or manipulation of these IT systems through intentional or accidental means by the Company’s employees, third parties with authorized access or unauthorized third parties could adversely affect key business processes. Cyber-attacks against the Company’s IT systems or third-party providers’ IT systems, such as cloud-based systems, could result in exposure of confidential information, the modification of critical data, and/or the failure of critical operations. Misuse of any of these IT systems could result in the disclosure of sensitive personal information or the theft of trade secrets, intellectual property, or other confidential business information. The Company continues to leverage new and innovative technologies across the enterprise to replace outmoded technology and improve the efficacy and efficiency of its business processes, including data acquisition, the use of which can create new risks. In addition, the Company’s Animal Health business sells technology products that, when deployed, could potentially be compromised by a third party and cause disruption both internally and externally.
Although the aggregate impact of cyber-attacks and network disruptions on the Company’s operations and financial condition has not been material to date, the Company continues to be a target of events of this nature and expects them to continue. The Company monitors its data, information technology and personnel usage of Company IT systems to identify and attempt to reduce these risks and continues to do so on an ongoing basis for any current or potential threats. There can be no assurance that the Company’s efforts to protect its data and IT systems or the efforts of third-party providers to protect their IT systems will be successful in preventing disruptions to the Company’s operations, including its manufacturing, research, and sales operations. Such disruptions have in the past and could in the future result in loss of revenue, or the loss of critical or sensitive information from the Company’s or the Company’s third-party providers’ databases or IT systems and have in the past and could in the future also result in financial, legal, business or reputational harm to the Company and substantial remediation costs.
The Company is increasing its use of artificial intelligence (AI) systems to automate processes, analyze data, and support decision-making which poses inherent risks.
The Company’s growing use of artificial intelligence (AI) systems to automate processes, analyze data, and support decision-making poses inherent risks. Flaws, biases, or malfunctions in these systems could lead to operational disruptions, data loss, or erroneous decision-making, impacting the Company’s business operations, financial condition, and reputation. Ethical and legal challenges may arise, including biases or discrimination in AI outcomes, non-compliance with data protection regulations and laws specifically governing the use of AI systems and tools, and lack of transparency. Furthermore, the deployment of AI systems could expose the Company to increased cybersecurity threats, such as data breaches and unauthorized access leading to financial losses, legal liabilities, and reputational damage. The Company also faces competitive risks if it fails to adopt AI or other machine learning technologies in a timely fashion.
Social media and mobile messaging platforms present risks and challenges.
The inappropriate and/or unauthorized use of certain social media and mobile messaging channels could cause brand damage or information leakage or could lead to legal implications, including from the improper collection and/or dissemination of personally identifiable information. In addition, negative or inaccurate posts or comments about the Company or its products on any social networking platforms could damage the Company’s reputation, brand image and goodwill. Further, the disclosure of non-public Company-sensitive information by the Company’s workforce or others through external media channels could lead to information loss. Although there are internal Company Social Media and Mobile Messaging Policies that guide employees on appropriate personal and professional use of these platforms for communication about the Company, the processes in place may not completely secure and protect information. Identifying potential new points of unauthorized entry as new communication tools expand also presents new challenges.
Cautionary Factors that May Affect Future Results
(Cautionary Statements Under the Private Securities Litigation Reform Act of 1995)
This report and other written reports and oral statements made from time to time by the Company may contain so-called “forward-looking statements,” all of which are based on management’s current expectations and are subject to risks and uncertainties which may cause results to differ materially from those set forth in the statements. One can identify these forward-looking statements by their use of words such as “anticipates,” “expects,” “plans,” “will,” “estimates,” “forecasts,” “projects” and other words of similar meaning, or negative variations of any of the foregoing. One can also identify them by the fact that they do not relate strictly to historical or current facts. These statements are likely to address the Company’s growth strategy, financial results, product approvals, product potential, development programs, environmental or other sustainability initiatives. One must carefully consider any such statement and should understand that many factors could cause actual results to differ materially from the Company’s forward-looking statements. These factors include inaccurate assumptions and a broad variety of other risks and uncertainties, including some that are known and some that are not. No forward-looking statement can be guaranteed and actual future results may vary materially. The Company does not assume the obligation to update any forward-looking statement. The Company cautions you not to place undue reliance on these forward-looking statements. Although it is not possible to predict or identify all such factors, they may include the following:
•Competition from generic and/or biosimilar products as the Company’s products lose patent protection.
•Increased “brand” competition in therapeutic areas important to the Company’s long-term business performance.
•The difficulties and uncertainties inherent in new product development. The outcome of the lengthy and complex process of new product development is inherently uncertain. A drug candidate can fail at any stage of the process and one or more late-stage product candidates could fail to receive regulatory approval. New product candidates may appear promising in development but fail to reach the market because of efficacy or safety concerns, the inability to obtain necessary regulatory approvals, the difficulty or excessive cost to manufacture and/or the infringement of patents or intellectual property rights of others. Furthermore, the sales of new products may prove to be disappointing and fail to reach anticipated levels.
•Pricing pressures, both in the U.S. and abroad, including rules and practices of managed care groups, judicial decisions and governmental laws and regulations related to Medicare, Medicaid and health care reform, pharmaceutical reimbursement and pricing in general.
•Changes in government laws and regulations, including laws governing intellectual property, and the enforcement thereof affecting the Company’s business.
•Efficacy or safety concerns with respect to marketed products, whether or not scientifically justified, leading to product recalls, withdrawals or declining sales.
•Significant changes in customer relationships or changes in the behavior and spending patterns of purchasers of health care products and services, including delaying medical procedures, rationing prescription medications, reducing the frequency of physician visits and foregoing health care insurance coverage.
•Legal factors, including product liability claims, antitrust litigation and governmental investigations, including tax disputes, environmental concerns and patent disputes with branded and generic competitors, any of which could preclude commercialization of products or negatively affect the profitability of existing products.
•Cyber-attacks on the Company’s or third-party providers’ IT systems, which could disrupt the Company’s operations.
•Lost market opportunity resulting from delays and uncertainties in the approval process of the FDA and/or foreign regulatory authorities.
•Increased focus on privacy issues in countries around the world, including the U.S., the EU, and China. The legislative and regulatory landscape for privacy and data protection continues to evolve, and there has been an increasing amount of focus on privacy and data protection issues with the potential to affect directly the Company’s business, including laws in a majority of states in the U.S. requiring security breach notification.
•Changes in tax laws including changes related to the taxation of foreign earnings.
•Changes in accounting pronouncements promulgated by standard-setting or regulatory bodies, including the Financial Accounting Standards Board and the SEC, that are adverse to the Company.
•Economic factors over which the Company has no control, including changes in inflation, interest rates and foreign currency exchange rates.
This list should not be considered an exhaustive statement of all potential risks and uncertainties. See “Risk Factors” above.
Item 1B.Unresolved Staff Comments.
None.
Item 1C. Cybersecurity.
The Company’s cybersecurity measures are primarily focused on ensuring the security and protection of its IT systems and data. The Company’s information security program is managed by a dedicated Chief Information Security Officer (CISO), whose group is responsible for leading enterprise-wide cybersecurity risk management, strategy, policy, standards, architecture, and processes. The CISO has worked in the cybersecurity and national security fields for more than 30 years. He has a Master of Science in Telecommunications and Computers. He has served as a board member of the Health Information Sharing and Analysis Center for 10 years. Oversight of the information security program has been integrated into the Company’s overall enterprise risk management program.
The CISO provides periodic reports to the Audit Committee (Audit Committee) of the Board of Directors (Board), the full Board, as well as to the Company’s Chief Executive Officer and other members of senior management, as appropriate. These reports include updates on the Company’s cybersecurity risks and threats, the status of projects intended to strengthen its information security systems, assessments of the information security program (including remediation, mitigation, and management of identified vulnerabilities), and the emerging threat landscape. The information security program is regularly evaluated by internal and external consultants and auditors with the results of those reviews reported to senior management and the Audit Committee, which is comprised entirely of independent directors and has oversight responsibility for these risks.
The Company’s information security group monitors the Company’s information systems to prevent, detect, mitigate, and remediate cybersecurity incidents. The Company uses tools and techniques to continually assess and monitor, manage and mitigate cybersecurity threats to its IT systems in a manner consistent with industry practice. The Company engages with key vendors, industry participants, and intelligence and law enforcement communities as part of its continuing efforts to obtain current threat intelligence, collaborate on security enhancements, and evaluate and improve the effectiveness of its information security program. As part of this program, the Company conducts periodic tabletop and red-teaming exercises to assess its cybersecurity incident response processes and defenses. The Company also maintains vendor management diligence and oversight processes to identify and monitor potential risks from cybersecurity threats attendant to its use of third-party service providers. Additionally, the Company monitors cybersecurity threat intelligence received from key third-party service providers associated with the Company.
In the event of a cybersecurity incident, the Company has a process in place whereby members of the information security group will alert the CISO and the CISO will alert the appropriate levels of management, including an incident assessment team, as well as the legal and finance departments so that the materiality of any such event can be assessed in furtherance of fulfilling any reporting requirements. If warranted, senior management will notify the Audit Committee or the full Board, as appropriate.
The Company has been and continues to be the target of cyber-attacks and network disruptions. To date, the risks posed by such cybersecurity threats have not materially affected the Company and its business strategy, results of operations and financial condition, and as of the date of this report, the Company is not aware of any material risks from cybersecurity threats that are reasonably likely to do so, but there can be no assurance that the Company will not be materially affected by such risks in the future. For further information, see Item 1A. “Risk Factors — The Company is increasingly dependent on sophisticated software applications and computing infrastructure. The Company continues to be a target of cyber-attacks that could lead to a disruption of its worldwide operations, including manufacturing, research and sales operations.”
Item 2.Properties.
The Company’s corporate headquarters are located in Rahway, New Jersey. The Company also maintains divisional headquarters in Upper Gwynedd, Pennsylvania. Principal U.S. research facilities are located in Rahway, New Jersey; West Point, Pennsylvania; Boston and Cambridge, Massachusetts; South San Francisco, California; and Elkhorn, Nebraska (Animal Health). Principal research facilities outside the U.S. are located in the United Kingdom, Switzerland and China. Merck’s manufacturing operations are currently headquartered in Rahway, New Jersey. The Company also has production facilities for human health products at six locations in the U.S. and Puerto Rico.
Outside the U.S., through subsidiaries, the Company owns or has an interest in manufacturing plants or other properties in Western Europe, Africa and Asia.
The Company and its subsidiaries own their principal facilities and manufacturing plants under titles that they consider to be satisfactory. The Company believes that its properties are in good operating condition and that its machinery and equipment have been well maintained. The Company believes that its plants for the manufacture of products are suitable for their intended purposes and have capacities and projected capacities, including previously disclosed capital expansion projects, that will be adequate for current and projected needs for existing Company products. Some capacity of the plants is being converted, with any needed modification, to the requirements of newly introduced and future products.
Item 3.Legal Proceedings.
The information called for by this Item is incorporated herein by reference to Item 8. “Financial Statements and Supplementary Data,” Note 10. “Contingencies and Environmental Liabilities.”
Item 4.Mine Safety Disclosures.
Not Applicable.
Executive Officers of the Registrant (ages as of February 1, 2025)
All officers listed below serve at the pleasure of the Board of Directors. None of these officers was elected pursuant to any arrangement or understanding between the officer and any other person(s).
| | | | | | | | |
| Name | Age | Offices and Business Experience |
| Robert M. Davis | 58 | Chairman, Chief Executive Officer and President (since December 2022); Chief Executive Officer and President (July 2021-December 2022); Executive Vice President, Global Services, and Chief Financial Officer (April 2016-July 2021) |
| Sanat Chattopadhyay | 65 | Executive Vice President and President, Merck Manufacturing Division (since March 2016) |
| Richard R. DeLuca, Jr. | 62 | Executive Vice President and President, Merck Animal Health (since September 2011) |
| Cristal Downing | 56 | Executive Vice President and Chief Communications & Public Affairs Officer (since August 2021); Prior to that, Vice President Medical Devices, Global Communications and Public Affairs Johnson & Johnson (December 2020-August 2021); Vice President Financial Communication, Johnson & Johnson (January 2018-December 2020) |
| Chirfi Guindo | 59 | Senior Vice President, Chief Marketing Officer, Human Health (since July 2022); Prior to that, Executive Vice President, Head of Global Product Strategy and Commercialization, Biogen Inc. (July 2018-July 2022) |
| Betty D. Larson | 49 | Executive Vice President and Chief Human Resources Officer (since April 2024); Prior to that, Chief People Officer, GE HealthCare (February 2022-April 2024); Executive Vice President and Chief Human Resources Officer, Becton Dickinson (June 2018-February 2022) |
| Dean Li | 62 | Executive Vice President, President, Merck Research Laboratories (since January 2021); Senior Vice President, Discovery Sciences and Translational Medicine, Merck Research Laboratories (November 2017-January 2021) |
| Caroline Litchfield | 56 | Executive Vice President and Chief Financial Officer (since April 2021); Senior Vice President, Corporate Treasurer (January 2018-March 2021) |
| Johannes J. Oosthuizen | 57 | Senior Vice President and President Merck U.S. Human Health (since January 2022); Senior Vice President and Head of Global Oncology Commercial (January 2021-December 2021); Senior Vice President and President of MSD K.K. (July 2016-December 2020) |
| Joseph Romanelli | 51 | Senior Vice President and President MSD International Human Health (since July 2022); Prior to that, Chief Executive Officer JiXing Pharmaceuticals (July 2021-July 2022); President MSD China (December 2016-July 2021) |
| Dalton Smart | 58 | Senior Vice President Finance – Global Controller (since December 2023); Vice President, Assistant Controller (September 2023-December 2023); Vice President, Internal Audit (March 2015-September 2023) |
| David M. Williams | 56 | Executive Vice President, Chief Information and Digital Officer (since August 2020); Acting Chief Information and Digital Officer (December 2019-August 2020) |
| Jennifer Zachary | 47 | Executive Vice President and General Counsel (since April 2018) |
PART II
Item 5.Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities.
The principal market for trading of the Company’s Common Stock is the New York Stock Exchange (NYSE) under the symbol MRK.
As of January 31, 2025, there were approximately 85,700 shareholders of record of the Company’s Common Stock.
Issuer purchases of equity securities for the three months ended December 31, 2024 were as follows:
Issuer Purchases of Equity Securities
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | Total Number of Shares Purchased as Part of Publicly Announced Plans or Programs | | ($ in millions) |
| Period | | Total Number of Shares Purchased(1) | | Average Price
Paid Per
Share | | | Approximate Dollar Value of Shares That May Yet Be Purchased Under the Plans or Programs(1) |
| October 1 — October 31 | | 2,428,680 | | | $108.45 | | 2,428,680 | | | $2,620 |
| November 1 — November 30 | | 993,250 | | $99.29 | | 993,250 | | $2,522 |
| December 1 — December 31 | | 1,215,000 | | $99.51 | | 1,215,000 | | $2,401 |
| Total | | 4,636,930 | | | $104.15 | | 4,636,930 | | | |
(1) All shares purchased during the period were made as part of a plan approved by the Board of Directors in October 2018 to purchase up to $10 billion in Merck shares for its treasury. In January 2025, the Board of Directors approved a plan to purchase up to an additional $10 billion in Merck shares for its treasury.
Performance Graph
The following graph assumes a $100 investment on December 31, 2019, and reinvestment of all dividends, in each of the Company’s Common Stock, the S&P 500 Index, and a composite peer group of major U.S. and European-based pharmaceutical companies, which are: AbbVie Inc., Amgen Inc., AstraZeneca plc, Bristol-Myers Squibb Company, Johnson & Johnson, Eli Lilly and Company, Gilead Sciences Inc., GlaxoSmithKline plc, Novartis AG, Pfizer Inc., Roche Holding AG, and Sanofi SA.
Comparison of Five-Year Cumulative Total Return
Merck & Co., Inc., Composite Peer Group and S&P 500 Index
| | | | | | | | | | | |
| End of
Period Value | | 2024/2019 CAGR* |
| MERCK | $134 | | 6% |
| PEER GROUP** | 154 | | 9% |
| S&P 500 | 197 | | 15% |
| | | | | | | | | | | | | | | | | | | | |
| 2019 | 2020 | 2021 | 2022 | 2023 | 2024 |
| MERCK | $ | 100.0 | | $ | 92.8 | | $ | 94.5 | | $ | 141.1 | | $ | 142.5 | | $ | 133.6 | |
| PEER GROUP | 100.0 | | 106.5 | | 130.4 | | 138.1 | | 142.0 | | 153.7 | |
| S&P 500 | 100.0 | | 118.4 | | 152.3 | | 124.7 | | 157.5 | | 196.8 | |
* Compound Annual Growth Rate
** Peer group average was calculated on a market cap weighted basis as of December 31, 2019.
This Performance Graph will not be deemed to be incorporated by reference into any filing under the Securities Act of 1933 or the Securities Exchange Act of 1934, except to the extent that the Company specifically incorporates it by reference. In addition, the Performance Graph will not be deemed to be “soliciting material” or to be “filed” with the SEC or subject to Regulation 14A or 14C, other than as provided in Regulation S-K, or to the liabilities of section 18 of the Securities Exchange Act of 1934, except to the extent that the Company specifically requests that such information be treated as soliciting material or specifically incorporates it by reference into a filing under the Securities Act or the Exchange Act.
Item 6. [Reserved]
Item 7.Management’s Discussion and Analysis of Financial Condition and Results of Operations.
The following section of this Form 10-K generally discusses 2024 and 2023 results and year-to-year comparisons between 2024 and 2023. Discussion of 2022 results and year-to-year comparisons between 2023 and 2022 that are not included in this Form 10-K can be found in Part II, Item 7. “Management’s Discussion and Analysis of Financial Condition and Results of Operations” in the Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2023 filed on February 26, 2024.
Description of Merck’s Business
Merck & Co., Inc. (Merck or the Company) is a global health care company that delivers innovative health solutions through its prescription medicines, including biologic therapies, vaccines and animal health products. The Company’s operations are principally managed on a product basis and include two operating segments, Pharmaceutical and Animal Health, both of which are reportable segments.
The Pharmaceutical segment includes human health pharmaceutical and vaccine products. Human health pharmaceutical products consist of therapeutic and preventive agents, generally sold by prescription, for the treatment of human disorders. The Company sells these human health pharmaceutical products primarily to drug wholesalers and retailers, hospitals, government agencies and managed health care providers such as health maintenance organizations, pharmacy benefit managers and other institutions. Human health vaccine products consist of preventive pediatric, adolescent and adult vaccines. The Company sells these human health vaccines primarily to physicians, wholesalers, distributors and government entities.
The Animal Health segment discovers, develops, manufactures and markets a wide range of veterinary pharmaceutical and vaccine products, as well as health management solutions and services, for the prevention, treatment and control of disease in all major livestock and companion animal species. The Company also offers an extensive suite of digitally connected identification, traceability and monitoring products. The Company sells its products to veterinarians, distributors, animal producers, farmers and pet owners.
Overview
Financial Highlights
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| ($ in millions except per share amounts) | 2024 | | % Change | | % Change
Excluding Foreign
Exchange | | 2023 | | % Change | | % Change
Excluding Foreign
Exchange | | 2022 |
| Sales | $ | 64,168 | | | 7 | % | | 10 | % | | $ | 60,115 | | | 1 | % | | 4 | % | | $ | 59,283 | |
| | | | | | | | | | | | | |
| Net Income Attributable to Merck & Co., Inc.: | | | | | | | | | | | | | |
| GAAP | $ | 17,117 | | | * | | * | | $ | 365 | | | (97) | % | | (95) | % | | $ | 14,519 | |
Non-GAAP (1) | $ | 19,444 | | | * | | * | | $ | 3,837 | | | (80) | % | | (75) | % | | $ | 19,005 | |
| | | | | | | | | | | | | |
| Earnings per Common Share Assuming Dilution Attributable to Merck & Co., Inc. Common Shareholders: | | | | | | | | | | | | | |
| GAAP | $ | 6.74 | | | * | | * | | $ | 0.14 | | | (98) | % | | (95) | % | | $ | 5.71 | |
Non-GAAP (1) | $ | 7.65 | | | * | | * | | $ | 1.51 | | | (80) | % | | (75) | % | | $ | 7.48 | |
* > 100%
(1) Non-GAAP net income and non-GAAP earnings per share (EPS) exclude acquisition- and divestiture-related costs, restructuring costs, income and losses from investments in equity securities, and certain other items from Merck’s results prepared in accordance with generally accepted accounting principles in the U.S. (GAAP). For further discussion and a reconciliation of GAAP to non-GAAP net income and EPS, see “Non-GAAP Income and Non-GAAP EPS” below.
Executive Summary
Merck’s performance during 2024 was driven by continued demand across its innovative portfolio, including for recently launched products, enabled by the operational and commercial execution of its science-led strategy. The Company maintained its focus on the pursuit of breakthrough science and innovation, making disciplined investments in compelling science to drive long-term value for patients, customers, and shareholders. Merck advanced its robust early- and late-phase pipeline which includes growing diversity across new therapeutic areas and modalities and completed several promising business development transactions. The Company continued to return capital to shareholders, primarily through dividends.
Worldwide sales were $64.2 billion in 2024, an increase of 7% compared with 2023, or 10% excluding the unfavorable effect of foreign exchange. The sales increase was primarily due to growth in oncology, cardiovascular and animal health, partially offset by declines in diabetes, virology (driven largely by lower sales of COVID-19 medication Lagevrio), immunology (as Merck’s marketing rights to these products ended in 2024) and vaccines.
Merck continues to execute value creating business development opportunities focused on innovation to augment its robust internal pipeline with compelling external science. Highlights of 2024 activity include the following:
•Closed an exclusive global license to develop, manufacture and commercialize MK-2010 (LM-299), a novel investigational programmed death receptor-1 (PD-1)/vascular endothelial growth factor (VEGF) bispecific antibody from LaNova Medicines Ltd (LaNova).
•Closed an exclusive global license to develop, manufacture and commercialize MK-4082 (HS-10535), an investigational preclinical oral small molecule GLP-1 receptor agonist from Hansoh Pharma (Hansoh).
•Acquired global rights to MK-1045 (formerly CN201), a novel investigational clinical-stage bispecific antibody for the treatment of B-cell associated diseases from Curon Pharmaceutical (Curon).
•Acquired Eyebiotech Limited (EyeBio), a privately held ophthalmology-focused biotechnology company developing candidates for the prevention and treatment of vision loss.
•Acquired Harpoon Therapeutics, Inc. (Harpoon), a clinical-stage immunotherapy company developing a novel class of T-cell engagers designed to harness the power of the body’s immune system to treat patients suffering from cancer and other diseases.
During 2024, Merck continued its efforts to address unmet medical needs by launching new products with significant patient benefit, including the U.S. launches of Winrevair, for the treatment of certain adults with pulmonary arterial hypertension (PAH), and Capvaxive, for the prevention of invasive pneumococcal disease and pneumococcal pneumonia in adults. Winrevair was also approved in the EU.
The Company received more than 25 regulatory approvals in major markets in 2024, including the Winrevair and Capvaxive approvals noted above, along with numerous approvals in oncology. Keytruda received approval for additional indications in the U.S. and/or internationally as monotherapy in the therapeutic areas of hepatocellular carcinoma (HCC), melanoma and urothelial carcinoma, in combination with chemotherapy in the therapeutic areas of biliary tract cancer, cervical cancer, endometrial carcinoma, gastric or gastroesophageal junction (GEJ) adenocarcinoma, malignant pleural mesothelioma and non-small-cell lung cancer (NSCLC), as well as in combination with Padcev (enfortunab vedotin-ejfv) for advanced urothelial carcinoma. Also in 2024, Welireg was approved in China for the treatment of adult patients with certain von Hippel-Lindau (VHL) disease-associated tumors not requiring immediate surgery. Lynparza, which is being developed in collaboration with AstraZeneca PLC (AstraZeneca), received approval in China for the treatment of certain adult patients with germline BRCA-mutated, human epidermal growth factor receptor 2 (HER2)-negative high-risk early breast cancer.
In addition to the regulatory approvals discussed above, the Company advanced its late-stage pipeline with several regulatory submissions.
•MK-1022, patritumab deruxtecan, is a potential first-in-class HER3 directed DXd antibody drug conjugate (ADC), under review by the U.S. Food and Drug Administration (FDA) for the treatment of adult patients with locally advanced or metastatic epidermal growth factor receptor (EGFR)-mutated NSCLC previously treated with two or more systemic therapies. In June 2024, the FDA issued a complete response letter (CRL) for the Biologics License Application (BLA) due to findings pertaining to an inspection of a third-party manufacturing facility. The CRL did not identify any issues with the efficacy or safety data submitted. Patritumab deruxtecan (HER3-DXd) was discovered by Daiichi Sankyo and is
being jointly developed by Daiichi Sankyo and Merck. Merck is working with Daiichi Sankyo to address FDA feedback.
•MK-6482, Welireg, is under review in Japan both for the treatment of adults with VHL disease and for the treatment of certain adults with previously treated advanced renal cell carcinoma (RCC). Welireg is also under priority review in the U.S. for the treatment of certain patients with advanced, unresectable or metastatic pheochromocytoma and paraganglioma.
•V116, Capvaxive, a 21-valent pneumococcal conjugate vaccine designed to help prevent invasive pneumococcal disease and pneumococcal pneumonia caused by certain serotypes in adults, is under review in the EU and Japan.
•MK-7962, Winrevair, Merck’s novel activin signaling inhibitor, is under review in Japan for the treatment of adult patients with PAH.
•MK-1654, clesrovimab, is an investigational prophylactic long-acting monoclonal antibody designed to protect infants from respiratory syncytial virus (RSV) disease during their first RSV season under review by the FDA. Clesrovimab is also under review in the EU.
•Additionally, Keytruda is under review in the EU and Japan for a supplemental indication for the treatment of certain patients with malignant pleural mesothelioma.
During 2024, the Company initiated more than 20 Phase 3 studies spanning cardiometabolic, immunology, infectious diseases, oncology, ophthalmology and vaccines.
The Company is diversifying its oncology portfolio and executing on its strategy which is broadly based on three strategic pillars: immuno-oncology, precision molecular targeting and tissue targeting. Merck’s Phase 3 oncology programs within these pillars are as follows:
Immuno-oncology
•MK-1308A, the coformulation of quavonlimab, Merck’s novel investigational anti-CTLA-4 antibody, in combination with pembrolizumab for RCC;
•MK-3475, Keytruda, in the therapeutic areas of hepatocellular, ovarian and small-cell lung cancers;
•MK-3475A, the subcutaneous coformulation of pembrolizumab in combination with hyaluronidase, being evaluated for comparability with intravenous pembrolizumab in metastatic NSCLC; and
•V940 (mRNA-4157), an investigational individualized neoantigen therapy, in combination with Keytruda, as an adjuvant treatment in patients with certain types of melanoma and NSCLC, being developed as part of a collaboration with Moderna, Inc.
Precision molecular targeting
•MK-1026, nemtabrutinib, an oral, reversible, non-covalent Bruton’s tyrosine kinase (BTK) inhibitor, for hematological malignancies, including chronic lymphocytic leukemia and small lymphocytic lymphoma;
•MK-1084, an investigational oral selective KRAS G12C inhibitor, in combination with Keytruda, for metastatic NSCLC;
•MK-3543, bomedemstat, an investigational orally available lysine-specific demethylase 1 inhibitor for myeloproliferative disorders;
•MK-5684, opevesostat, an investigational cytochrome P450 11A1 (CYP11A1) inhibitor for metastatic castration-resistant prostate cancer;
•MK-7339, Lynparza, in combination with Keytruda, for non-small-cell lung and small-cell lung cancers; and
•MK-7902, Lenvima, being developed as part of a collaboration with Eisai Co., Ltd. (Eisai), in combination with Keytruda, for esophageal cancer.
Tissue targeting
•MK-1022, patritumab deruxtecan, being developed in collaboration wtih Daiichi Sankyo, for NSCLC as noted above;
•MK-2140, zilovertamab vedotin, an ADC targeting receptor tyrosine kinase-like orphan receptor 1 (ROR1) for hematological malignancies, including diffuse large B cell lymphoma;
•MK-2400, ifinatamab deruxtecan, an ADC being evaluated in patients with relapsed SCLC versus chemotherapy, being developed as part of a collaboration with Daiichi Sankyo; and
•MK-2870, sacituzumab tirumotecan, an investigational trophoblast cell-surface antigen 2 (TROP2)-directed ADC, being developed as part of a collaboration with Kelun-Biotech for breast, cervical, endometrial, gastric and non-small-cell lung cancers.
Additionally, the Company currently has candidates in Phase 3 clinical development in several other therapeutic areas:
•MK-3000, an investigational, potentially first-in-class tetravalent, tri-specific antibody that acts as an agonist of the Wingless-related integration site signaling pathway, for the treatment of diabetic macular edema and neovascular age-related macular degeneration;
•MK-8591A, a once-daily oral combination of doravirine and islatravir, an investigational nucleoside reverse transcriptase translocation inhibitor, for the treatment of HIV-1 infection (which is on partial clinical hold for higher doses of islatravir than those used in current clinical trials);
•MK-8591D, islatravir in combination with lenacapavir for the treatment of HIV-1 infection (which is on partial clinical hold for higher doses of islatravir than those used in current clinical trials), being developed in collaboration with Gilead Sciences Inc.;
•MK-0616, enlicitide decanoate, an investigational, oral proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor for hypercholesterolemia, including in studies evaluating low-density lipoprotein cholesterol reduction and a cardiovascular outcomes study;
•MK-7240, tulisokibart, a humanized monoclonal antibody directed to tumor necrosis factor-like ligand 1A, a target associated with both intestinal inflammation and fibrosis, for Crohn’s disease and ulcerative colitis; and
•MK-4482, Lagevrio, which is reflected in Phase 3 development in the U.S. as it remains investigational following Emergency Use Authorization (EUA) in 2021.
Merck’s capital allocation strategy continues to prioritize investments in its business to drive near- and long-term growth, including investing in the Company’s key growth drivers and expansive pipeline of novel candidates, each of which has potential to address important unmet medical needs. Research and development expenses in 2024 reflect increased development spending particularly in the therapeutic areas of oncology, immunology and cardiometabolic. In addition, Merck remains committed to its dividend and will continue to pursue the most compelling external science and technologies through value-enhancing business development transactions.
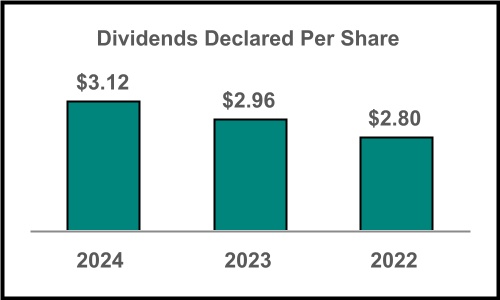
In November 2024, Merck’s Board of Directors approved an increase to the Company’s quarterly dividend, raising it to $0.81 per share from $0.77 per share on the Company’s outstanding common stock. During 2024, the Company returned $9.1 billion to shareholders through dividends of $7.8 billion and share repurchases of $1.3 billion. In January 2025, Merck’s Board of Directors authorized a new share repurchase program of up to an additional $10 billion of Merck’s common stock for its treasury.
GAAP and non-GAAP EPS were negatively affected in 2024, 2023 and 2022 by $1.28, $6.21, and $0.22, respectively, of charges for certain upfront and pre-approval milestone payments related to collaborations and licensing agreements, as well as charges related to pre-approval assets obtained in transactions accounted for as asset acquisitions.
Pricing
Global efforts toward health care cost containment continue to exert pressure on product pricing and market access worldwide. Changes to the U.S. health care system as part of health care reform, as well as increased purchasing power of entities that negotiate on behalf of Medicare, Medicaid, and private sector beneficiaries, have contributed to pricing pressure. In 2021, the U.S. Congress passed the American Rescue Plan Act, which included a provision that eliminated the statutory cap on rebates drug manufacturers pay to Medicaid beginning in January 2024. As a result of this provision, the Company paid state Medicaid programs more in rebates than it received on Medicaid sales of Januvia, Janumet and Janumet XR in 2024. In 2022, the U.S. Congress passed the Inflation Reduction Act (IRA), which made significant changes to how drugs are covered and paid for under the Medicare program, including the creation of financial penalties for drugs whose prices rise faster than the rate of inflation, redesign of the Medicare Part D program to require manufacturers to bear more of the liability for certain drug benefits (which has taken effect in 2025), and government price setting for certain Medicare Part D drugs (starting in 2026) and Medicare Part B drugs (starting in 2028). Government price setting may also impact pricing in the private market negatively affecting the Company’s performance. In 2023, the U.S. Department of Health and Human Services (HHS), through the Centers for Medicare & Medicaid Services (CMS), selected Januvia for the first year of the IRA’s “Drug Price Negotiation Program” (Program). Pursuant to the IRA’s Program, a government price was set for Januvia, which will become effective on January 1, 2026. In January 2025, the U.S. Department of HHS, through the CMS, announced that Janumet and Janumet XR would be in included in the second year of the IRA’s Program, with government price setting to become effective on January 1, 2027. The Company has sued the U.S. government regarding the IRA’s Program (see Note 10 to the consolidated financial statements). Additionally, increased utilization of the 340B Federal Drug Discount Program and restrictions on the Company’s ability to identify inappropriate discounts are having a negative impact on Company performance. Furthermore, the Executive Branch and Congress continue to discuss legislation designed to control health care costs, including the cost of drugs. In several international markets, government-mandated pricing actions have reduced prices of generic and patented drugs. In addition, the Company’s sales performance in 2024 was negatively affected by other cost-reduction measures taken by governments and other third parties to lower health care costs. The Company anticipates all of these actions and additional actions in the future will continue to negatively affect sales and profits.
Operating Results
Sales
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| ($ in millions) | 2024 | | % Change | | % Change
Excluding Foreign
Exchange | | 2023 | | % Change | | % Change
Excluding Foreign
Exchange | | 2022 |
| United States | $ | 32,277 | | | 13 | % | | 13 | % | | $ | 28,480 | | | 5 | % | | 5 | % | | $ | 27,206 | |
| International | 31,891 | | | 1 | % | | 6 | % | | 31,635 | | | (1) | % | | 4 | % | | 32,077 | |
| Total | $ | 64,168 | | | 7 | % | | 10 | % | | $ | 60,115 | | | 1 | % | | 4 | % | | $ | 59,283 | |
Worldwide sales were $64.2 billion in 2024, representing growth of 7% compared with 2023, or 10% excluding the unfavorable effect of foreign exchange. The devaluation of the Argentine peso contributed approximately 2 percentage points of the negative impact of foreign exchange, which was largely offset by inflation-related price increases consistent with practice in that market. Global sales growth was primarily due to higher sales in the oncology franchise, largely due to strong growth of Keytruda and Welireg, as well as increased alliance revenue from Reblozyl and Lynparza. Also contributing to revenue growth were higher sales in the cardiovascular
franchise, largely attributable to the launch of Winrevair, higher sales of certain hospital acute care products, particularly Prevymis, as well as higher sales of animal health products. Sales growth in 2024 was partially offset by lower sales in the diabetes franchise, due to Januvia and Janumet, and lower sales in the virology franchise largely attributable to Lagevrio. Lower sales in the immunology franchise due to the return of the marketing rights for Remicade and Simponi in former Merck territories to Johnson & Johnson on October 1, 2024, and lower sales in the vaccines franchise primarily due to Gardasil/Gardasil 9 also offset sales growth in 2024.
Sales in the U.S. grew 13% to $32.3 billion in 2024 primarily driven by higher sales of Keytruda, Winrevair, Gardasil 9, Welireg, Bridion, Lagevrio, and Prevymis, as well as higher alliance revenue from Reblozyl, partially offset by lower sales of Januvia and Vaxneuvance.
International sales grew 1% in 2024, or 6% excluding the unfavorable effect of foreign exchange. The devaluation of the Argentine peso contributed approximately 3 percentage points of the negative impact of foreign exchange, which was largely offset by inflation-related price increases consistent with practice in that market. International sales growth was primarily due to higher sales of Keytruda, Vaxneuvance, Prevymis, as well as higher sales of animal health products, partially offset by lower sales of Gardasil/Gardasil 9, Lagevrio, Bridion, Janumet, Januvia, and Simponi. International sales represented 50% and 53% of total sales in 2024 and 2023, respectively.
See Note 18 to the consolidated financial statements for details on sales of the Company’s products. A discussion of performance for select products in the franchises follows.
Pharmaceutical Segment
Oncology
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| ($ in millions) | 2024 | | % Change | | % Change
Excluding Foreign
Exchange | | 2023 | | % Change | | % Change
Excluding Foreign
Exchange | | 2022 |
| Keytruda | $ | 29,482 | | | 18 | % | | 22 | % | | $ | 25,011 | | | 19 | % | | 21 | % | | $ | 20,937 | |
Alliance Revenue - Lynparza (1) | 1,311 | | | 9 | % | | 11 | % | | 1,199 | | | 7 | % | | 9 | % | | 1,116 | |
Alliance Revenue - Lenvima (1) | 1,010 | | | 5 | % | | 6 | % | | 960 | | | 10 | % | | 11 | % | | 876 | |
| Welireg | 509 | | | * | | * | | 218 | | | 77 | % | | 77 | % | | 123 | |
Alliance Revenue - Reblozyl (2) | 371 | | | 75 | % | | 75 | % | | 212 | | | 28 | % | | 28 | % | | 166 | |
* > 100%
(1) Alliance revenue for Lynparza and Lenvima represents Merck’s share of profits, which are product sales net of cost of sales and commercialization costs (see Note 4 to the consolidated financial statements).
(2) Alliance revenue for Reblozyl represents royalties and, for 2022, also includes a payment received related to the achievement of a regulatory approval milestone (see Note 4 to the consolidated financial statements).
Keytruda is an anti-PD-1 therapy that has been approved in over 40 indications in the U.S., including 18 tumor types and 2 tumor-agnostic indications, and has similarly been approved in markets worldwide for many of these indications. The Keytruda clinical development program includes studies across a broad range of cancer types.
Global sales of Keytruda grew 18% in 2024, or 22% excluding the unfavorable effect of foreign exchange. The negative impact of foreign exchange was primarily due to the devaluation of the Argentine peso, which was largely offset by inflation-related price increases consistent with practice in that market. Keytruda sales growth in the U.S. reflects higher demand across the multiple approved metastatic indications, in particular for the treatment of certain types of bladder, endometrial, microsatellite instability-high (MSI-H) and renal cell cancers, as well as increased uptake across earlier-stage indications, including in certain types of high-risk early-stage triple-negative breast cancer (TNBC), NSCLC and RCC, and higher pricing. Keytruda sales growth in international markets reflects higher demand predominately for the TNBC, melanoma and RCC earlier-stage indications, as well as uptake in cervical, gastric and renal cell cancer metastatic indications. The Company expects that the 2025 launch and reimbursement of new indications for Keytruda in the EU will have a negative impact on pricing in those markets.
Summarized below are the Keytruda regulatory approvals received in 2024 and, to date, in 2025.
| | | | | |
| Date | Approval |
| January 2024 | FDA approval in combination with chemoradiotherapy for the treatment of patients with FIGO (International Federation of Gynecology and Obstetrics) 2014 Stage III-IVA cervical cancer, based on the KEYNOTE-A18 trial. |
| | | | | |
| January 2024 | FDA full approval for the treatment of patients with HCC secondary to hepatitis B who have received prior systemic therapy other than a PD-1/programmed death-ligand 1 (PD-L1) containing regimen. The conversion from an accelerated to full (regular) approval is based on the KEYNOTE-394 trial. |
| February 2024 | China’s National Medical Products Administration (NMPA) approval in combination with gemcitabine and cisplatin for the first-line treatment of patients with locally advanced or metastatic biliary tract carcinoma, based on the KEYNOTE-966 trial. |
| March 2024 | European Commission (EC) approval in combination with platinum-containing chemotherapy as neoadjuvant treatment, and then continued as monotherapy as adjuvant treatment, for resectable NSCLC at high risk of recurrence in adults, based on the KEYNOTE-671 trial. |
| May 2024 | Japan’s Ministry of Health, Labor and Welfare (MHLW) approval in combination with fluoropyrimidine- and platinum-containing chemotherapy for the first-line treatment of patients with locally advanced unresectable or metastatic gastric or GEJ adenocarcinoma, based on the KEYNOTE-859 trial. |
| May 2024 | Japan’s MHLW approval in combination with standard of care chemotherapy (gemcitabine and cisplatin) for the treatment of patients with locally advanced unresectable or metastatic biliary tract cancer, based on the KEYNOTE-966 trial. |
| June 2024 | FDA approval in combination with carboplatin and paclitaxel, followed by Keytruda as a single agent, for the treatment of adult patients with primary advanced or recurrent endometrial carcinoma, based on the KEYNOTE-868 trial. |
| June 2024 | China’s NMPA approval in combination with trastuzumab, fluoropyrimidine- and platinum-containing chemotherapy for the first-line treatment of patients with locally advanced unresectable or metastatic HER2 positive gastric or GEJ adenocarcinoma whose tumors express PD-L1 as determined by a fully validated test, based on the KEYNOTE-811 trial. |
| September2024 | EC approval in combination with Padcev, an ADC, for the first-line treatment of unresectable or metastatic urothelial carcinoma in adults, based on the KEYNOTE-A39 trial that was conducted in collaboration with Seagen (now Pfizer Inc.) and Astellas. |
| September 2024 | FDA approval in combination with pemetrexed and platinum chemotherapy for the first-line treatment of adult patients with unresectable advanced or metastatic malignant pleural mesothelioma, based on the IND.227/KEYNOTE-483 trial. |
| September 2024 | Japan’s MHLW approval in combination with chemotherapy as a neoadjuvant treatment, then continued as monotherapy as an adjuvant treatment, for patients with NSCLC, based on the KEYNOTE-671 trial. |
| September 2024 | Japan’s MHLW approval in combination with Padcev for the first-line treatment of patients with radically unresectable urothelial carcinoma, based on the KEYNOTE-A39 trial. |
| September 2024 | Japan’s MHLW approval as monotherapy in patients with radically unresectable urothelial carcinoma who are not eligible for any platinum-containing chemotherapy, based on the KEYNOTE-052 trial. |
| September 2024 | China’s NMPA approval for the first-line treatment of adult patients with unresectable or metastatic melanoma, and conversion from conditional to full approval for the second-line treatment of adult patients with unresectable or metastatic melanoma following failure of one prior line of therapy, based on the LEAP-003 trial. |
| October 2024 | EC approval in combination with chemoradiotherapy for the treatment of FIGO 2014 Stage III-IVA locally advanced cervical cancer in adults who have not received prior definitive therapy, based on the KEYNOTE-A18 trial. |
| October 2024 | EC approval in combination with carboplatin and paclitaxel followed by Keytruda as a single agent for the first-line treatment of primary advanced or recurrent endometrial carcinoma in adults who are candidates for systemic therapy, based on the KEYNOTE-868 trial. |
| November 2024 | Japan’s MHLW approval in combination with chemoradiotherapy as treatment for patients with locally advanced cervical cancer, based on the KEYNOTE-A18 trial. |
| December 2024 | China’s NMPA approval in combination with platinum-containing chemotherapy as neoadjuvant treatment and then continued as monotherapy as adjuvant treatment after surgery for patients with resectable stage II, IIIA, or IIIB NSCLC, based on the KEYNOTE-671 trial. |
| December 2024 | Japan’s MHLW approval in combination with carboplatin and paclitaxel as a treatment for adult patients with advanced or recurrent endometrial carcinoma, based on the KEYNOTE-868 trial. |
| | | | | |
| December 2024 | China’s NMPA approval in combination with chemoradiotherapy for the treatment of patients with FIGO 2014 Stage III-IVA cervical cancer, based on the KEYNOTE-A18 trial. |
| January 2025 | China’s NMPA approval in combination with Padcev for adult patients with locally advanced or metastatic urothelial cancer, based on the KEYNOTE-A39 trial. |
The Company is a party to license agreements pursuant to which the Company pays royalties on sales of Keytruda. Under the terms of the more significant of these agreements, Merck paid a royalty of 6.5% on worldwide sales of Keytruda through December 2023 to one third party; this royalty declined to 2.5% in 2024 and will continue through 2026 terminating thereafter. The Company pays an additional 2% royalty on worldwide sales of Keytruda to another third party, the termination date of which varies by country; this royalty expired in the U.S. in September 2024 and will expire on varying dates in major European markets in the second half of 2025. The royalty expenses are included in Cost of sales.
Lynparza is an oral poly (ADP-ribose) polymerase (PARP) inhibitor being developed and commercialized as part of a collaboration with AstraZeneca (see Note 4 to the consolidated financial statements). Lynparza is approved for the treatment of certain types of advanced or recurrent ovarian, early or metastatic breast, metastatic pancreatic and metastatic castration-resistant prostate cancers. Alliance revenue related to Lynparza grew 9% in 2024 largely due to higher demand in most international markets. In January 2025, China’s NMPA approved Lynparza as adjuvant treatment for adult patients with germline BRCA-mutated, HER2-negative high-risk early breast cancer, based on the OlympiA trial.
Lenvima is an oral receptor tyrosine kinase inhibitor being developed and commercialized as part of a collaboration with Eisai (see Note 4 to the consolidated financial statements). Lenvima is approved for the treatment of certain types of thyroid cancer, RCC, HCC, in combination with everolimus for certain patients with advanced RCC, and in combination with Keytruda for certain patients with advanced endometrial carcinoma or advanced RCC. Alliance revenue related to Lenvima grew 5% in 2024 primarily reflecting higher demand and pricing in the U.S.
Sales of Welireg, for the treatment of adult patients with certain VHL disease-associated tumors and certain adult patients with previously treated advanced RCC, more than doubled in 2024 primarily due to higher demand in the U.S. reflecting in part continued uptake of the RCC indication following approval by the FDA in December 2023. In November 2024, Welireg was approved in China for the treatment of adult patients with certain VHL disease-associated tumors not requiring immediate surgery based on the LITESPARK-004 clinical trial. In February 2025, the EC conditionally approved Welireg as monotherapy both for the treatment of adult patients with VHL disease who require therapy for associated, localized RCC, central nervous system hemangioblastomas, or pancreatic neuroendocrine tumors, and for whom localized procedures are unsuitable, and for the treatment of adult patients with advanced clear cell RCC that progressed following two or more lines of therapy that included a PD-1 or PD-L1 inhibitor and at least two VEGF targeted therapies. The EC approval of these two indications is based on results from the LITESPARK-004 and LITESPARK-005 trials. The conditional approval of Welireg will be valid for one year, subject to yearly renewal, pending certain additional clinical data. Timing for commercial availability of Welireg in individual EU countries will depend on multiple factors, including the completion of national reimbursement procedures.
Reblozyl is a first-in-class erythroid maturation recombinant fusion protein that is being commercialized through a global collaboration with Bristol Myers Squibb Company (BMS) (see Note 4 to the consolidated financial statements). Reblozyl is approved for the treatment of anemia in certain rare blood disorders. Alliance revenue related to this collaboration (consisting of royalties) increased 75% in 2024 due to strong underlying sales performance.
Vaccines
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| ($ in millions) | 2024 | | % Change | | % Change
Excluding Foreign
Exchange | | 2023 | | % Change | | % Change
Excluding Foreign
Exchange | | 2022 |
Gardasil/Gardasil 9 | $ | 8,583 | | | (3) | % | | (2) | % | | $ | 8,886 | | | 29 | % | | 33 | % | | $ | 6,897 | |
| ProQuad | 920 | | | 6 | % | | 6 | % | | 870 | | | 4 | % | | 4 | % | | 839 | |
M-M-R II | 464 | | | 8 | % | | 9 | % | | 430 | | | 5 | % | | 4 | % | | 411 | |
| Varivax | 1,102 | | | 3 | % | | 4 | % | | 1,068 | | | 8 | % | | 8 | % | | 991 | |
| Vaxneuvance | 808 | | | 22 | % | | 23 | % | | 665 | | | * | | * | | 170 | |
Pneumovax 23 | 263 | | | (36) | % | | (34) | % | | 412 | | | (32) | % | | (31) | % | | 602 | |
* > 100%
Combined worldwide sales of Gardasil and Gardasil 9, vaccines to help prevent certain cancers and other diseases caused by certain types of human papillomavirus (HPV), declined 3% in 2024 primarily driven by lower demand in China. Outside of China, Gardasil/Gardasil 9 achieved strong growth in most other international markets due to higher demand, particularly in Japan due to a national catch-up immunization program, and in the U.S. due to public sector buying patterns, higher pricing and demand. Beginning in mid-2024, the Company observed a significant decline in shipments from its distributor and commercialization partner in China, Chongqing Zhifei Biological Products Co., Ltd. (Zhifei), to disease and control prevention institutions and correspondingly into the points of vaccination, resulting in above normal inventory levels at Zhifei. Accordingly, the Company shipped less than its contracted doses to Zhifei in the latter part of 2024. Lower demand in China persisted and, at the end of 2024, overall channel inventory levels in China remained elevated at above normal levels. Therefore, the Company made a decision to temporarily pause shipments to China beginning in February 2025 through at least the middle of the year and, as a result, Gardasil/Gardasil 9 sales will decline significantly in 2025 compared with 2024. In January 2025, China’s NMPA approved Gardasil for use in males 9-26 years of age to help prevent certain HPV-related cancers and diseases.
The Company is a party to license agreements pursuant to which the Company pays royalties on sales of Gardasil/Gardasil 9. Under the terms of the more significant of these agreements, Merck pays a 7% royalty on sales of Gardasil/Gardasil 9 in the U.S. to one third party (this royalty expires in December 2028). Merck paid an additional 7% royalty on worldwide sales of Gardasil/Gardasil 9 to another third party; this royalty expired in December 2023. The royalty expenses are included in Cost of sales.
Global sales of ProQuad, a pediatric combination vaccine to help protect against measles, mumps, rubella and varicella, grew 6% in 2024 primarily due to higher pricing in the U.S. Worldwide sales of M-M-R II, a vaccine to help protect against measles, mumps and rubella, grew 8% in 2024 primarily due to higher demand in certain international markets, partially offset by lower demand in the U.S. Global sales of Varivax, a vaccine to help prevent chickenpox (varicella), grew 3% in 2024 primarily attributable to higher pricing in the U.S., partially offset by lower sales in Latin America due to supply constraints. The Company is experiencing manufacturing delays related to ProQuad and Varivax. As a result, the Company anticipates that some international markets will experience supply constraints during 2025. In order to ensure consistent supply in the U.S., in January 2025, the Company borrowed doses of ProQuad from the U.S. Centers for Disease Control and Prevention (CDC) Pediatric Vaccine Stockpile. The borrowing will reduce sales of ProQuad in the first quarter of 2025 by approximately $70 million. These doses will be used to support routine vaccination in the U.S.
Worldwide sales of Vaxneuvance, a vaccine to help protect against invasive pneumococcal disease caused by certain serotypes, rose 22% in 2024 primarily due to continued uptake following launches in the pediatric indication in Europe, Japan, and other countries in the Asia Pacific region, partially offset by lower demand in the U.S. due to competition. Merck is a party to license agreements pursuant to which the Company pays royalties on sales of Vaxneuvance. Under the most significant of these agreements, Merck pays a royalty of 7.25% on net sales of Vaxneuvance through 2026; this royalty will decline to 2.5% on net sales from 2027 through 2035. The royalty expenses are included in Cost of sales.
Worldwide sales of Pneumovax 23, a vaccine to help prevent pneumococcal disease, declined 36% in 2024 due to lower global demand, particularly in the U.S. as the market has shifted toward newer adult pneumococcal conjugate vaccines.
In June 2024, the FDA approved Capvaxive (Pneumococcal 21-valent Conjugate Vaccine) for the prevention of invasive pneumococcal disease and pneumococcal pneumonia caused by certain serotypes in individuals 18 years of age and older. The approval was supported by results from multiple Phase 3 clinical studies evaluating Capvaxive in both vaccine-naïve and vaccine-experienced adult patient populations, including STRIDE-3, STRIDE-4, STRIDE-5 and STRIDE-6. Sales of Capvaxive were $97 million in 2024. Merck is a party to license agreements pursuant to which the Company pays royalties on sales of Capvaxive. Under the most significant of these agreements, Merck pays a royalty of 7.25% on net sales of Capvaxive through 2026; this royalty will decline to 2.5% on net sales from 2027 through 2035. The royalty expenses are included in Cost of sales.
Hospital Acute Care
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| ($ in millions) | 2024 | | % Change | | % Change
Excluding Foreign
Exchange | | 2023 | | % Change | | % Change
Excluding Foreign
Exchange | | 2022 |
| Bridion | $ | 1,764 | | | (4) | % | | (3) | % | | $ | 1,842 | | | 9 | % | | 11 | % | | $ | 1,685 | |
| Prevymis | 785 | | | 30 | % | | 33 | % | | 605 | | | 41 | % | | 43 | % | | 428 | |
Global sales of Bridion, for the reversal of two types of neuromuscular blocking agents used during surgery, declined 4% in 2024 primarily driven by lower demand in certain international markets due to generic competition, particularly in the EU and the Asia Pacific region, including in Japan. The Bridion sales decline was partially offset by higher demand and pricing in the U.S. The patents that provided market exclusivity for Bridion in the EU and Japan expired in July 2023 and January 2024, respectively. Accordingly, the Company is experiencing sales declines of Bridion in these markets and expects the declines to continue.
Worldwide sales of Prevymis, a medicine for prophylaxis (prevention) of cytomegalovirus (CMV) infection and disease in certain high risk adult and pediatric recipients of an allogenic hematopoietic stem cell transplant and for prophylaxis of CMV disease in certain high risk adult and pediatric recipients of a kidney transplant, grew 30% in 2024 largely due to higher global demand, particularly in the U.S.
Cardiovascular
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| ($ in millions) | 2024 | | % Change | | % Change
Excluding Foreign
Exchange | | 2023 | | % Change | | % Change
Excluding Foreign
Exchange | | 2022 |
| Winrevair | $ | 419 | | | — | % | | — | % | | $ | — | | | — | % | | — | % | | $ | — | |
Alliance Revenue - Adempas/Verquvo (1) | 415 | | | 13 | % | | 13 | % | | 367 | | | 8 | % | | 8 | % | | 341 | |
| Adempas | 287 | | | 12 | % | | 14 | % | | 255 | | | 7 | % | | 8 | % | | 238 | |
(1) Alliance revenue for Adempas and Verquvo represents Merck’s share of profits from sales in Bayer’s marketing territories, which are product sales net of cost of sales and commercialization costs (see Note 4 to the consolidated financial statements).
In March 2024, the FDA approved Winrevair for the treatment of adults with pulmonary arterial hypertension (PAH) (World Health Organization [WHO] Group 1) to increase exercise capacity, improve WHO functional class (FC), and reduce the risk of clinical worsening events. In August 2024, the EC approved Winrevair, in combination with other PAH therapies, for the treatment of PAH in adult patients with WHO FC II to III, to improve exercise capacity. The FDA and EC approvals were based on the STELLAR trial. Winrevair has since launched in Germany. Timing for commercial availability of Winrevair in the remaining EU countries will depend on multiple factors, including the completion of national reimbursement procedures, which is expected to occur in most other major EU markets in the second half of 2025. Winrevair is the subject of a licensing agreement pursuant to which Merck pays a 22% royalty on sales of Winrevair to BMS. The royalty expenses are included in Cost of sales.
Adempas and Verquvo are part of a worldwide collaboration with Bayer AG (Bayer) to market and develop soluble guanylate cyclase (sGC) modulators (see Note 4 to the consolidated financial statements). Adempas is approved for the treatment of certain types of PAH and chronic pulmonary hypertension (PH). Verquvo is approved to reduce the risk of cardiovascular death and heart failure hospitalization following a hospitalization for heart failure or need for outpatient intravenous diuretics in adults with symptomatic chronic heart failure and reduced ejection fraction. Alliance revenue from the collaboration grew 13% in 2024 reflecting higher demand in Bayer’s marketing territories. Revenue also includes sales of Adempas and Verquvo in Merck’s marketing territories. Sales of Adempas in Merck’s marketing territories grew 12% in 2024 primarily due to higher demand.
Virology
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| ($ in millions) | 2024 | | % Change | | % Change
Excluding Foreign
Exchange | | 2023 | | % Change | | % Change
Excluding Foreign
Exchange | | 2022 |
| Lagevrio | 964 | | | (33) | % | | (28) | % | | 1,428 | | | (75) | % | | (74) | % | | 5,684 | |
Lagevrio is an investigational oral antiviral COVID-19 medicine being developed in a collaboration with Ridgeback Biotherapeutics LP (Ridgeback) (see Note 4 to the consolidated financial statements). Sales of Lagevrio declined 33% in 2024 primarily due to lower demand and pricing in several markets in the Asia Pacific region, particularly in Japan, partially offset by uptake from commercial distribution in the U.S. under Emergency Use Authorization.
Immunology
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| ($ in millions) | 2024 | | % Change | | % Change
Excluding Foreign
Exchange | | 2023 | | % Change | | % Change
Excluding Foreign
Exchange | | 2022 |
| Simponi | $ | 543 | | | (24) | % | | (23) | % | | $ | 710 | | | 1 | % | | — | % | | $ | 706 | |
| Remicade | 114 | | | (39) | % | | (36) | % | | 187 | | | (9) | % | | (8) | % | | 207 | |
Simponi and Remicade are treatments for certain inflammatory diseases that the Company marketed in Europe, Russia and Türkiye. The Company’s marketing rights with respect to these products reverted to Johnson & Johnson on October 1, 2024 resulting in sales declines for these products versus prior year.
Diabetes
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| ($ in millions) | 2024 | | % Change | | % Change
Excluding Foreign
Exchange | | 2023 | | % Change | | % Change
Excluding Foreign
Exchange | | 2022 |
| Januvia/Janumet | $ | 2,268 | | | (33) | % | | (29) | % | | $ | 3,366 | | | (25) | % | | (23) | % | | $ | 4,513 | |
Worldwide combined sales of Januvia and Janumet, medicines that help lower blood sugar levels in adults with type 2 diabetes, declined 33% in 2024 primarily due to lower sales in the U.S., largely reflecting lower pricing and lower demand due to competitive pressures, as well as the ongoing impact of the loss of exclusivity in most markets in Europe, the Asia Pacific region, and in Canada.
The American Rescue Plan Act enacted in the U.S. in 2021 included a provision that eliminated the statutory cap on rebates drug manufacturers pay to Medicaid beginning in January 2024. As a result of this provision, the Company paid state Medicaid programs more in rebates than it received on Medicaid sales of Januvia, Janumet and Janumet XR in 2024.
In early 2025, Merck lowered the list price of the Januvia family of products to more closely align them with net prices. The lower list price will reduce the rebate amount Merck pays to Medicaid, resulting in higher realized net pricing, which will be partially offset by continuing volume declines. The Company expects higher U.S. net sales of these products in 2025 compared with 2024.
While the key U.S. patent for Januvia, Janumet and Janumet XR claiming the sitagliptin compound expired in January 2023, as a result of favorable court rulings and settlement agreements related to a later expiring patent directed to the specific sitagliptin salt form of the products (see Note 10 to the consolidated financial statements), the Company expects that Januvia and Janumet will not lose market exclusivity in the U.S. until May 2026 and Janumet XR will not lose market exclusivity in the U.S. until July 2026, although a non-automatically substitutable form of sitagliptin that differs from the form in the Company’s sitagliptin products has been approved by the FDA. Additionally, in 2023, the U.S. Department of HHS, through the CMS, announced that Januvia would be included in the first year of the IRA’s Program. Pursuant to the IRA’s Program, a government price was set for Januvia, which will become effective on January 1, 2026. Also, in January 2025, the U.S. Department of HHS, through the CMS, announced that Janumet and Janumet XR would be in included in the second year of the IRA’s Program, with government price setting to become effective on January 1, 2027. The Company has sued the U.S. government regarding the IRA’s Program (see Note 10 to the consolidated financial statements). As a result of the anticipated patent expiries in 2026, the government price setting in 2026 and 2027 noted above, as well as ongoing competitive pressures, the Company anticipates significant sales declines for Januvia, Janumet and Janumet XR in the U.S. in 2026 and thereafter.
The Company lost market exclusivity for Januvia in all of the EU and for Janumet in some European countries in September 2022. Exclusivity for Janumet was lost in other European countries in April 2023. Accordingly, the Company is experiencing sales declines in these markets and expects the declines to continue. Generic equivalents of Januvia and Janumet have also launched in China.
Animal Health Segment
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| ($ in millions) | 2024 | | % Change | | % Change
Excluding Foreign
Exchange | | 2023 | | % Change | | % Change
Excluding Foreign
Exchange | | 2022 |
| Livestock | $ | 3,462 | | | 4 | % | | 9 | % | | $ | 3,337 | | | 1 | % | | 4 | % | | $ | 3,300 | |
| Companion Animal | 2,415 | | | 6 | % | | 7 | % | | 2,288 | | | 2 | % | | 3 | % | | 2,250 | |
| $ | 5,877 | | | 4 | % | | 8 | % | | $ | 5,625 | | | 1 | % | | 3 | % | | $ | 5,550 | |
Animal Health sales grew 4% in 2024, or 8% excluding the unfavorable effect of foreign exchange. The devaluation of the Argentine peso contributed approximately 2 percentage points of the negative impact of foreign exchange, which was largely offset by inflation-related price increases consistent with practice in that market.
Sales of livestock products grew 4% in 2024 primarily due to higher pricing, increased demand for poultry and swine products, as well as the inclusion of sales from the July 2024 acquisition of the aqua business of Elanco Animal Health Incorporated (Elanco aqua business). See Note 3 to the consolidated financial statements for additional information related to the acquisition of the Elanco aqua business.
Sales of companion animal products grew 6% in 2024 reflecting higher pricing. Sales of the Bravecto line of products were $1.1 billion in 2024, an increase of 6% compared with 2023, or 8% excluding the impact of foreign exchange.
Costs, Expenses and Other
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| ($ in millions) | 2024 | | % Change | | 2023 | | % Change | | 2022 |
| Cost of sales | $ | 15,193 | | | (6) | % | | $ | 16,126 | | | (7) | % | | $ | 17,411 | |
| Selling, general and administrative | 10,816 | | | 3 | % | | 10,504 | | | 5 | % | | 10,042 | |
| Research and development | 17,938 | | | (41) | % | | 30,531 | | | * | | 13,548 | |
| Restructuring costs | 309 | | | (48) | % | | 599 | | | 78 | % | | 337 | |
| Other (income) expense, net | (24) | | | * | | 466 | | | (69) | % | | 1,501 | |
| | $ | 44,232 | | | (24) | % | | $ | 58,226 | | | 36 | % | | $ | 42,839 | |
* >100%
Cost of Sales
Cost of sales was $15.2 billion in 2024 and $16.1 billion in 2023. Cost of sales includes the amortization of intangible assets recorded in connection with acquisitions, collaborations, and licensing arrangements, which totaled $2.4 billion in 2024 and $2.0 billion in 2023. Amortization expense in 2024 and 2023 includes $48 million and $154 million, respectively, of cumulative catch-up amortization related to Merck’s collaborations with AstraZeneca and Eisai, respectively. (See Note 4 to the consolidated financial statements for more information on Merck’s collaborative arrangements). Also included in cost of sales are expenses associated with restructuring activities, which amounted to $495 million in 2024 and $211 million in 2023, primarily reflecting accelerated depreciation and asset impairment charges related to the planned sale or closure of manufacturing facilities. Separation costs associated with manufacturing-related headcount reductions have been incurred and are reflected in Restructuring costs as discussed below.
Gross margin was 76.3% in 2024 compared with 73.2% in 2023. The gross margin improvement was primarily due to the favorable effects of product mix (including lower royalty rates related to Keytruda and Gardasil/Gardasil 9 sales) and foreign exchange, partially offset by increased amortization of intangible assets, higher restructuring costs (primarily reflecting asset impairment charges), and increased manufacturing-related costs (including inventory write-offs).
Selling, General and Administrative
Selling, general and administrative (SG&A) expenses were $10.8 billion in 2024, an increase of 3% compared with 2023. The increase was primarily due to higher administrative costs (including compensation and benefits), and increased promotional costs (reflecting prioritization in support of key growth drivers including new product launches), as well as higher selling and acquisition-related costs, partially offset by the favorable effect of foreign exchange and lower restructuring costs.
Research and Development
Research and development (R&D) expenses were $17.9 billion in 2024, a decline of 41% compared with 2023. The decline was primarily due to lower charges for business development activity and the favorable effect of foreign exchange.
Significant business development transactions in 2024 include charges of:
•$1.35 billion for the acquisition of EyeBio and $100 million for a related developmental milestone
•$750 million for the acquisition of MK-1045 (formerly CN201) from Curon
•$656 million for the acquisition of Harpoon
•$588 million for a global license agreement with LaNova
•$112 million for a global license agreement with Hansoh
Significant business development transactions in 2023 include charges of:
•$10.2 billion for the acquisition of Prometheus
•$5.5 billion related to the formation of a collaboration with Daiichi Sankyo
•$1.2 billion for the acquisition of Imago
•$175 million for a license and collaboration agreement with Kelun-Biotech
The decline in R&D expenses was partially offset by higher compensation and benefit costs (reflecting in part increased headcount) and increased clinical development spending, including for recently acquired programs.
R&D expenses are comprised of the costs directly incurred by Merck Research Laboratories (MRL), the Company’s research and development division that focuses on human health-related activities, which were $10.1 billion in 2024 and $9.0 billion in 2023. Also included in R&D expenses are Animal Health research costs, upfront payments for collaboration and licensing agreements (including charges for the transactions with LaNova, Hansoh, Daiichi Sankyo and Kelun-Biotech noted above), charges for transactions accounted for as asset acquisitions (including charges for the acquisitions of EyeBio, MK-1045, Harpoon, Prometheus and Imago noted above) and costs incurred by other divisions in support of R&D activities, including depreciation, production and general and administrative, which in the aggregate were $7.7 billion in 2024 and $20.7 billion in 2023. R&D expenses also include an impairment charge of $779 million in 2023 (related to gefapixant). See Note 8 to the consolidated financial statements for additional information related to this impairment charge. The Company may recognize additional impairment charges in the future related to the cancellation or delay of other pipeline programs that were measured at fair value and capitalized in connection with business combinations and such charges could be material.
Restructuring Costs
In January 2024, the Company approved a new restructuring program (2024 Restructuring Program) intended to continue the optimization of the Company’s Human Health global manufacturing network as the future pipeline shifts to new modalities and also optimize the Animal Health global manufacturing network to improve supply reliability and increase efficiency. The actions contemplated under the 2024 Restructuring Program are expected to be substantially completed by the end of 2031, with the cumulative pretax costs to be incurred by the Company to implement the program estimated to be approximately $4.0 billion. Approximately 60% of the cumulative pretax costs will be non-cash, relating primarily to the accelerated depreciation of facilities to be closed or divested. The remainder of the costs will result in cash outlays, relating primarily to facility shut-down costs. The Company expects to record charges of approximately $550 million in 2025 related to the 2024 Restructuring Program. The Company anticipates the actions under the 2024 Restructuring Program will result in cumulative annual net cost savings of approximately $750 million by the end of 2031.
In 2019, Merck approved a global restructuring program (2019 Restructuring Program) as part of a worldwide initiative focused on optimizing the Company’s manufacturing and supply network, as well as reducing its global real estate footprint. The actions under the 2019 Restructuring Program were substantially complete at the end of 2023 and, as of January 1, 2024, any remaining activities are being accounted for as part of the 2024 Restructuring Program.
Restructuring costs of $309 million in 2024 and $599 million in 2023 include separation and other costs associated with these restructuring activities. Separation costs incurred were associated with actual headcount reductions, as well as estimated expenses under existing severance programs for involuntary headcount reductions that were probable and could be reasonably estimated. Other expenses in Restructuring costs include facility shut-down and other related costs, as well as employee-related costs such as curtailment, settlement and termination
charges associated with pension and other postretirement benefit plans and share-based compensation plan costs. For segment reporting, restructuring costs are unallocated expenses.
Additional costs associated with the Company’s restructuring activities are included in Cost of sales, Selling, general and administrative expenses and Research and development costs. The Company recorded aggregate pretax costs related to restructuring program activities of $888 million in 2024 and $933 million in 2023 (of which $190 million related to the 2024 Restructuring Program). See Note 5 to the consolidated financial statements for additional details.
Other (Income) Expense, Net
Other (income) expense, net, was $24 million of income in 2024 compared with $466 million of expense in 2023 primarily reflecting a $572.5 million charge in 2023 related to settlements with certain plaintiffs in the Zetia antitrust litigation. The favorability was also due to $170 million of income in 2024 related to the expansion of an existing development and commercialization agreement with Daiichi Sankyo, as well as lower foreign exchange losses in 2024. Other (income) expense, net, was unfavorably affected in 2024 by lower income from investments in equity securities and higher net interest expense compared with 2023.
For details on the components of Other (income) expense, net, see Note 14 to the consolidated financial statements.
| | | | | | | | | | | | | | | | | |
| Segment Profits | | | | | |
| | | | | |
| ($ in millions) | 2024 | | 2023 | | 2022 |
| Pharmaceutical segment profits | $ | 44,533 | | | $ | 38,880 | | | $ | 36,852 | |
| Animal Health segment profits | 1,938 | | | 1,737 | | | 1,963 | |
| Non-segment activity | (26,535) | | | (38,728) | | | (22,371) | |
| Income Before Taxes | $ | 19,936 | | | $ | 1,889 | | | $ | 16,444 | |
Pharmaceutical segment profits are comprised of segment sales less standard costs, as well as SG&A expenses directly incurred by the segment. Animal Health segment profits are comprised of segment sales, less all cost of sales, as well as SG&A and R&D expenses directly incurred by the segment. For internal management reporting presented to the chief operating decision maker, Merck does not allocate the remaining cost of sales not included in segment profits as described above, R&D expenses incurred by MRL, or general and administrative expenses not directly incurred by the segments, nor the cost of financing these activities. Separate divisions maintain responsibility for monitoring and managing these costs, including depreciation related to fixed assets utilized by these divisions and, therefore, they are not included in segment profits. Also excluded from the determination of segment profits are costs related to restructuring activities and acquisition- and divestiture-related costs, including the amortization of intangible assets and amortization of purchase accounting adjustments, intangible asset impairment charges, and expense or income related to changes in the estimated fair value measurement of liabilities for contingent consideration. Additionally, segment profits do not reflect other expenses from corporate and manufacturing cost centers and other miscellaneous income or expense. These unallocated items are reflected in “Non-segment activity” in the above table. Also included in “Non-segment activity” are miscellaneous corporate profits (losses), as well as operating profits (losses) related to third-party manufacturing arrangements.
Pharmaceutical segment profits grew 15% in 2024 primarily due to higher sales, partially offset by higher administrative and promotional costs, as well as the unfavorable effect of foreign exchange. Animal Health segment profits increased 12% in 2024 primarily due to higher sales and lower manufacturing-related costs, partially offset by increased administrative costs, as well as the unfavorable effect of foreign exchange.
Taxes on Income
The effective income tax rate of 14.1% in 2024 reflects a favorable mix of income and expense, as well as a 2.6 percentage point favorable impact due to a $519 million reduction in reserves for unrecognized income tax benefits resulting from the expiration in 2024 of the statute of limitations for assessments related to the 2019 and 2020 federal tax return years. The effective income tax rate in 2024 also reflects a 1.5 percentage point combined unfavorable impact of charges for the acquisition of Harpoon, for which no tax benefit was recognized, and the acquisitions of EyeBio and MK-1045 for which minimal tax benefits were realized.
While many jurisdictions in which Merck operates have adopted the global minimum tax provision of the Organization for Economic Cooperation and Development (OECD) Pillar 2, effective for tax years beginning in January 2024, it resulted in a minimal impact to the Company’s 2024 effective income tax rate due to the accounting
for the tax effects of intercompany transactions. The Company expects the impact of the global minimum tax will increase its effective income tax rate by approximately 2% in 2025. In addition, beginning in 2026, the tax rates on foreign earnings and export income are scheduled to increase under existing provisions of the Tax Cuts and Jobs Act of 2017 (TCJA) and may result in an increase to the Company’s effective income tax rate. Also, in the event that the provision of the TCJA requiring capitalization and amortization of R&D expenses for tax purposes is repealed along the lines proposed in the Tax Relief for American Families and Workers Act of 2024, the Company will again be able to realize the benefit of U.S. R&D expenses as incurred, but expects no material impact to its effective income tax rate.
The effective income tax rate of 80.0% in 2023 includes a 65.6 percentage point combined unfavorable impact of charges for the acquisitions of Prometheus and Imago (for which no tax benefits were recognized) and the Daiichi Sankyo collaboration. These charges reduced domestic pretax income by approximately $16.9 billion in 2023. In addition, the effective income tax rate in 2023 reflects higher foreign taxes and the impact of the R&D capitalization provision of the TCJA on the Company’s U.S. global intangible low-taxed income inclusion, partially offset by a favorable mix of income and expense, as well as higher foreign tax credits.
The Internal Revenue Service (IRS) is currently conducting examinations of the Company’s tax returns for the years 2017 and 2018, including the one-time transition tax enacted under the TCJA. If the IRS disagrees with the Company’s transition tax position, it may result in a significant tax liability. The IRS is also currently conducting examinations of the Company’s tax returns for the years 2021 and 2022. In addition, various state and foreign tax examinations are in progress.
Non-GAAP Income and Non-GAAP EPS
Non-GAAP income and non-GAAP EPS are alternative views of the Company’s performance that Merck is providing because management believes this information enhances investors’ understanding of the Company’s results since management uses non-GAAP measures to assess performance. Non-GAAP income and non-GAAP EPS exclude certain items because of the nature of these items and the impact that they have on the analysis of underlying business performance and trends. The excluded items (which should not be considered non-recurring) consist of acquisition- and divestiture-related costs, restructuring costs, income and losses from investments in equity securities, and certain other items. These excluded items are significant components in understanding and assessing financial performance.
Non-GAAP income and non-GAAP EPS are important internal measures for the Company. Senior management receives a monthly analysis of operating results that includes a non-GAAP EPS metric. Management uses non-GAAP measures internally for planning and forecasting purposes and to measure the performance of the Company along with other metrics. In addition, annual employee compensation, including senior management’s compensation, is derived in part using a non-GAAP pretax income metric. Since non-GAAP income and non-GAAP EPS are not measures determined in accordance with GAAP, they have no standardized meaning prescribed by GAAP and, therefore, may not be comparable to the calculation of similar measures of other companies. The information on non-GAAP income and non-GAAP EPS should be considered in addition to, but not as a substitute for or superior to, net income and EPS prepared in accordance with GAAP.
A reconciliation between GAAP financial measures and non-GAAP financial measures is as follows:
| | | | | | | | | | | | | | | | | |
| ($ in millions except per share amounts) | 2024 | | 2023 | | 2022 |
| Income before taxes as reported under GAAP | $ | 19,936 | | | $ | 1,889 | | | $ | 16,444 | |
| Increase (decrease) for excluded items: | | | | | |
Acquisition- and divestiture-related costs (1) | 2,519 | | | 2,876 | | | 3,704 | |
| Restructuring costs | 888 | | | 933 | | | 666 | |
| Loss (income) from investments in equity securities, net | 45 | | | (279) | | | 1,348 | |
| Other items: | | | | | |
| Charge for Zetia antitrust litigation settlements | — | | | 573 | | | — | |
| Non-GAAP income before taxes | 23,388 | | | 5,992 | | | 22,162 | |
| Taxes on income as reported under GAAP | 2,803 | | | 1,512 | | | 1,918 | |
Estimated tax benefit on excluded items (2) | 606 | | | 631 | | | 1,232 | |
| Tax benefit resulting from the expiration of the statute of limitations for assessments related to the 2019 and 2020 federal tax return years | 519 | | | — | | | — | |
| Non-GAAP taxes on income | 3,928 | | | 2,143 | | | 3,150 | |
| Non-GAAP net income | 19,460 | | | 3,849 | | | 19,012 | |
| Less: Net income attributable to noncontrolling interests as reported under GAAP | 16 | | | 12 | | | 7 | |
| Non-GAAP net income attributable to Merck & Co., Inc. | $ | 19,444 | | | $ | 3,837 | | | $ | 19,005 | |
EPS assuming dilution as reported under GAAP (3) | $ | 6.74 | | | $ | 0.14 | | | $ | 5.71 | |
| EPS difference | 0.91 | | | 1.37 | | | 1.77 | |
Non-GAAP EPS assuming dilution (3) | $ | 7.65 | | | $ | 1.51 | | | $ | 7.48 | |
(1)Amounts in 2024, 2023 and 2022 include $39 million, $792 million and $1.7 billion, respectively, of intangible asset impairment charges.
(2) The estimated tax impact on the excluded items is determined by applying the statutory rate of the originating territory of the non-GAAP adjustments.
(3) GAAP and non-GAAP EPS were negatively affected in 2024, 2023 and 2022 by $1.28, $6.21, and $0.22, respectively, of charges for certain upfront and pre-approval milestone payments related to collaborations and licensing agreements, as well as charges related to pre-approval assets obtained in transactions accounted for as asset acquisitions.
Acquisition- and Divestiture-Related Costs
Non-GAAP income and non-GAAP EPS exclude the impact of certain amounts recorded in connection with acquisitions and divestitures of businesses. These amounts include the amortization of intangible assets and amortization of purchase accounting adjustments to inventories, as well as intangible asset impairment charges, and expense or income related to changes in the estimated fair value measurement of liabilities for contingent consideration. Also excluded are integration, transaction, and certain other costs associated with acquisitions and divestitures. Non-GAAP income and non-GAAP EPS also exclude amortization of intangible assets related to collaborations and licensing arrangements.
Restructuring Costs
Non-GAAP income and non-GAAP EPS exclude costs related to restructuring actions (see Note 5 to the consolidated financial statements). These amounts include employee separation costs and accelerated depreciation associated with facilities to be closed or divested. Accelerated depreciation costs represent the difference between the depreciation expense to be recognized over the revised useful life of the asset, based upon the anticipated date the site will be closed or divested or the equipment disposed of, and depreciation expense as determined utilizing the useful life prior to the restructuring actions. Restructuring costs also include other exits costs, such as asset impairment, facility shut-down and other related costs, as well as employee-related costs such as curtailment, settlement and termination charges associated with pension and other postretirement benefit plans and share-based compensation costs.
Income and Losses from Investments in Equity Securities
Non-GAAP income and non-GAAP EPS exclude realized and unrealized gains and losses from investments in equity securities either owned directly or through ownership interests in investment funds.
Certain Other Items
Non-GAAP income and non-GAAP EPS exclude certain other items. These items are adjusted for after evaluating them on an individual basis, considering their quantitative and qualitative aspects. Typically, these items
are unusual in nature, significant to the results of a particular period or not indicative of future operating results. Excluded from non-GAAP income and non-GAAP EPS in 2024 is a benefit due to reductions in reserves for unrecognized income tax benefits resulting from the expiration of the statute of limitations for assessments related to the 2019 and 2020 federal tax return years (see Note 15 to the consolidated financial statements). Excluded from non-GAAP income and non-GAAP EPS in 2023 is a charge related to settlements with certain plaintiffs in the Zetia antitrust litigation (see Note 10 to the consolidated financial statements).
Research and Development
Research Pipeline
The Company currently has several candidates under regulatory review in the U.S. and internationally, as well as in late-stage clinical development. A chart reflecting the Company’s current research pipeline as of February 21, 2025 and related discussion is set forth in Item 1. “Business — Research and Development” above.
Acquisitions, Research Collaborations and Licensing Agreements
Merck continues to remain focused on pursuing opportunities that have the potential to drive both near- and long-term growth. Certain recent transactions are summarized below; additional details are included in Note 3 to the consolidated financial statements. Merck actively monitors the landscape for growth opportunities that meet the Company’s strategic criteria.
In December 2024, Merck closed an exclusive global license to develop, manufacture and commercialize MK-2010 (LM-299), a novel investigational PD-1/VEGF bispecific antibody from LaNova. Merck recorded a charge of $588 million to Research and development expenses in 2024, or $0.18 per share, for the upfront payment, which was made in January 2025. LaNova is also eligible to receive milestone payments associated with the technology transfer, development, regulatory approval and commercialization of MK-2010 (LM-299) across multiple indications.
Also in December 2024, Merck closed an exclusive global license to develop, manufacture and commercialize MK-4082 (HS-10535), an investigational preclinical oral small molecule GLP-1 receptor agonist from Hansoh. Merck recorded a charge of $112 million to Research and development expenses in 2024, or $0.04 per share, for the upfront payment, which was made in February 2025. Hansoh is also eligible to receive future contingent milestone payments associated with the development, regulatory approval and commercialization of MK-4082 (HS-10535) as well as tiered royalties on future net sales of MK-4082 (HS-10535), if approved. Under the agreement, Hansoh may co-promote or solely commercialize MK-4082 (HS-10535) in Chinese mainland, Hong Kong and Macau, subject to certain conditions.
In September 2024, Merck acquired MK-1045 (formally CN201), a novel investigational clinical-stage bispecific antibody for the treatment of B-cell associated diseases, from Curon Biopharmaceutical (Curon) for an upfront payment of $700 million. In addition, Curon is eligible to receive future contingent developmental and regulatory milestone payments. The transaction was accounted for as an asset acquisition. Merck recorded a charge of $750 million (reflecting the upfront payment and other related costs) to Research and development expenses, or $0.29 per share, in 2024 related to the execution of the transaction. In connection with the agreement, Merck is also obligated to pay a third party future contingent developmental, regulatory and sales-based milestone payments, as well as tiered royalties ranging from a mid-single-digit rate to a low-double-digit rate on future net sales of MK-1045, if approved.
In July 2024, Merck acquired EyeBio, a privately held ophthalmology-focused biotechnology company, for $1.2 billion (including payments to settle share-based equity awards) and also incurred $207 million of transaction costs. The acquisition agreement also provides for former EyeBio shareholders to receive future contingent developmental, regulatory and sales-based milestone payments. EyeBio’s lead candidate, MK-3000 (formerly EYE103), is an investigational, potentially first-in-class tetravalent, tri-specific antibody that acts as an agonist of the Wingless-related integration site signaling pathway, which is in clinical development for the treatment of diabetic macular edema and neovascular age-related macular degeneration. The transaction was accounted for as an asset acquisition. Merck recorded net assets of $21 million, as well as a charge of $1.35 billion to Research and development expenses, or $0.52 per share, in 2024 related to the acquisition. Additionally, a $100 million developmental milestone was triggered and paid in 2024 upon initiation of a Phase 2/3 clinical trial evaluating MK-3000 for the treatment of diabetic macular edema, which was also recorded as a charge to Research and development expenses ($0.04 per share).
In March 2024, Merck acquired Harpoon, a clinical-stage immunotherapy company developing a novel class of T-cell engagers designed to harness the power of the body’s immune system to treat patients suffering from cancer and other diseases for $765 million and also incurred $56 million of transaction costs. Harpoon’s lead candidate, MK-6070 (formerly HPN328), is a T-cell engager targeting delta-like ligand 3 (DLL3), an inhibitory
canonical Notch ligand that is expressed at high levels in small-cell lung cancer and neuroendocrine tumors. The transaction was accounted for as an asset acquisition. The Company recorded net assets of $165 million, as well as a charge of $656 million to Research and development expenses, or $0.26 per share, in 2024 related to the transaction. There are no future contingent payments associated with the acquisition. In August 2024, Merck and Daiichi Sankyo expanded their existing global co-development and co-commercialization agreement to include MK-6070. Merck recognized income (recorded within Other (income) expense, net) of $170 million, or $0.05 per share, due to the receipt of an upfront cash payment from Daiichi Sankyo and has also satisfied a contingent quid obligation from the original collaboration agreement.
Acquired In-Process Research and Development
In connection with business combinations, the Company records the fair value of in-process research projects which, at the time of acquisition, had not yet reached technological feasibility. At December 31, 2024, the balance of in-process research and development (IPR&D) was $430 million, primarily consisting of MK-1026 (nemtabrutinib), $418 million, which is in Phase 3 clinical development.
The IPR&D projects that remain in development are subject to the inherent risks and uncertainties in drug development and it is possible that the Company will not be able to successfully develop and complete the IPR&D programs and profitably commercialize the underlying product candidates. The time periods to receive approvals from the FDA and other regulatory agencies are subject to uncertainty. Significant delays in the approval process, or the Company’s failure to obtain approval at all, would delay or prevent the Company from realizing revenues from these products. Additionally, if the IPR&D programs require additional clinical trial data than previously anticipated, or if the programs fail or are abandoned during development, then the Company will not recover the fair value of the IPR&D recorded as an asset as of the acquisition date. If such circumstances were to occur, the Company’s future operating results could be adversely affected and the Company may recognize impairment charges, which could be material.
In 2023 and 2022, the Company recorded IPR&D impairment charges within Research and development expenses of $779 million and $1.6 billion, respectively (see Note 8 to the consolidated financial statements).
Additional research and development will be required before any of the remaining programs reach technological feasibility. The costs to complete the research projects will depend on whether the projects are brought to their final stages of development and are ultimately submitted to the FDA or other regulatory agencies for approval.
Capital Expenditures
Capital expenditures were $3.4 billion in 2024, $3.9 billion in 2023 and $4.4 billion in 2022. Expenditures in the U.S. were $2.4 billion in 2024, $2.5 billion in 2023 and $2.7 billion in 2022. The Company plans to invest approximately $20 billion in capital projects from 2024-2028, more than $11 billion of which relates to investments in the U.S., including expanding manufacturing capacity for oncology, vaccine and animal health products.
Depreciation expense was $2.1 billion in 2024, $1.8 billion in 2023 and $1.8 billion in 2022, of which $1.4 billion in 2024, $1.2 billion in 2023 and $1.3 billion in 2022, related to locations in the U.S. Total depreciation expense in 2024, 2023 and 2022 included accelerated depreciation of $254 million, $140 million and $120 million, respectively, associated with restructuring activities (see Note 5 to the consolidated financial statements).
Analysis of Liquidity and Capital Resources
Merck’s strong financial profile enables it to fund research and development, finance acquisitions and external alliances, support in-line products and maximize upcoming launches while providing significant cash returns to shareholders.
| | | | | | | | | | | | | | | | | |
| Selected Data | | | | | |
| ($ in millions) | 2024 | | 2023 | | 2022 |
| Working capital | $ | 10,362 | | | $ | 6,474 | | | $ | 11,483 | |
| Total debt to total liabilities and equity | 31.7 | % | | 32.9 | % | | 28.1 | % |
| Cash provided by operating activities to total debt | 0.6:1 | | 0.4:1 | | 0.6:1 |
Cash provided by operating activities was $21.5 billion in 2024 compared with $13.0 billion in 2023 reflecting stronger operating performance. Cash provided by operating activities was reduced by upfront, milestone, option and continuation payments related to certain collaborations of $1.1 billion in 2024 compared with $4.2 billion in 2023 (including payments related to the formation of a collaboration with Daiichi Sankyo). Cash provided by operating activities in 2023 was also reduced by a payment of $572.5 million for the previously disclosed Zetia antitrust settlement. Cash provided by operating activities continues to be the Company’s primary source of funds to finance
operating needs, with excess cash serving as the primary source of funds to finance business development transactions, capital expenditures, dividends paid to shareholders and treasury stock purchases.
Cash used in investing activities was $7.7 billion in 2024 compared with $14.1 billion in 2023. The lower use of cash in investing activities was primarily due to lower cash used for acquisitions, lower capital expenditures, as well as lower purchases of securities and other investments, partially offset by lower proceeds from sales of securities and other investments.
Cash used in financing activities was $7.0 billion in 2024 compared with $4.8 billion in 2023. The higher use of cash in financing activities was primarily due to lower proceeds from the issuance debt (see below) and higher dividends paid to shareholders, partially offset by lower payments on long-term debt (see below), higher proceeds from the exercise of stock options and lower purchases of treasury stock.
In May 2024, MSD Netherlands Capital B.V., a wholly owned finance subsidiary of Merck, completed a registered public offering of €3.4 billion in aggregate principal amount of euro-dominated senior notes. The net cash proceeds from the offering were used for general corporate purposes. In May 2023, the Company issued $6.0 billion in aggregate principal amount of senior unsecured notes. The Company used a portion of the $5.9 billion net proceeds from this offering to fund a portion of the cash consideration paid for the acquisition of Prometheus, including related fees and expenses, and used the remaining net proceeds for general corporate purposes including to repay commercial paper borrowings and other indebtedness with upcoming maturities.
In 2024, the Company’s $750 million, 2.90% notes and the Company’s €500 million, 0.50% euro-denominated notes matured in accordance with their terms and were repaid. In 2023, the Company’s $1.75 billion, 2.80% notes matured in accordance with their terms and were repaid. In 2022, the Company’s $1.25 billion, 2.35% notes and the Company’s $1.0 billion, 2.40% notes matured in accordance with their terms and were repaid.
The Company has a $6.0 billion credit facility that matures in May 2028. The facility provides backup liquidity for the Company’s commercial paper borrowing facility and is to be used for general corporate purposes. The Company has not drawn funding from this facility.
In March 2024, the Company filed a securities registration statement with the U.S. Securities and Exchange Commission (SEC) under the automatic shelf registration process available to “well-known seasoned issuers” which is effective for three years.
Effective as of November 3, 2009, the Company executed a full and unconditional guarantee of the then existing debt of its subsidiary Merck Sharp & Dohme Corp. (MSD) and MSD executed a full and unconditional guarantee of the then existing debt of the Company (excluding commercial paper), including for payments of principal and interest. These guarantees do not extend to debt issued subsequent to that date.
In November 2024, Merck’s Board of Directors increased the quarterly dividend, declaring a quarterly dividend of $0.81 per share on the Company’s outstanding common stock for the first quarter of 2025 that was paid in January 2025. In January 2025, the Board of Directors declared a quarterly dividend of $0.81 per share on the Company’s outstanding common stock for the second quarter of 2025 payable in April 2025.
In 2018, Merck’s Board of Directors authorized purchases of up to $10 billion of Merck’s common stock for its treasury. The treasury stock purchase authorization has no time limit and will be made over time in open-market transactions, block transactions on or off an exchange, or in privately negotiated transactions. In 2024, the Company purchased $1.3 billion (approximately 11 million shares) of its common stock for its treasury under this program. As of December 31, 2024, the Company’s remaining share repurchase authorization was $2.4 billion. The Company purchased $1.3 billion of its common stock during 2023 under the authorized share repurchase program. The Company did not purchase any shares of its common stock under this program in 2022. In January 2025, Merck’s Board of Directors authorized purchases of up to an additional $10 billion of Merck’s common stock for its treasury.
The Company believes it maintains a conservative financial profile. The Company places its cash and investments in instruments that meet high credit quality standards, as specified in its investment policy guidelines. These guidelines also limit the amount of credit exposure to any one issuer. The Company does not participate in any off-balance sheet arrangements involving unconsolidated subsidiaries that provide financing or potentially expose the Company to unrecorded financial obligations.
The Company expects foreseeable liquidity and capital resource requirements to be met through existing cash and cash equivalents and anticipated cash flows from operations, as well as commercial paper borrowings and long-term borrowings if needed. Merck believes that its sources of financing will be adequate to meet its future requirements. The Company’s material cash requirements arising in the normal course of business primarily include:
Debt Obligations and Interest Payments — See Note 9 to the consolidated financial statements for further detail of the Company’s debt obligations and the timing of expected future principal and interest payments.
Tax Liabilities — In connection with the enactment of the TCJA, the Company is required to pay a one-time transition tax, which the Company has elected to pay over a period of eight years through 2025 as permitted under the TCJA. Additionally, the Company has liabilities for unrecognized tax benefits, including interest and penalties. See Note 15 to the consolidated financial statements for further information pertaining to the transition tax and liabilities for unrecognized tax benefits.
Operating Leases — See Note 9 to consolidated financial statements for further details of the Company’s lease obligations and the timing of expected future lease payments.
Collaboration-Related Payments — At December 31, 2024, the Company has accrued liabilities for contingent sales-based milestone payments related to a collaboration with AstraZeneca where the related sales-based milestones were achieved, but payment was not yet due according to the payment terms. These sales-based milestones were subsequently paid in January 2025. Additionally, the Company has an accrued liability for a future continuation payment related to a collaboration with Daiichi Sankyo. See Note 4 to the consolidated financial statements for additional information related to these payments.
Purchase Obligations — Purchase obligations are enforceable and legally binding obligations for purchases of goods and services including minimum inventory contracts, research and development and advertising. Purchase obligations also include future inventory purchases the Company has committed to in connection with certain divestitures. As of December 31, 2024, the Company had total purchase obligations of $7.2 billion, of which $2.8 billion is estimated to be payable in 2025.
Financial Instruments Market Risk Disclosures
The Company manages the impact of foreign exchange rate movements and interest rate movements on its earnings, cash flows and fair values of assets and liabilities through operational means and through the use of various financial instruments, including derivative instruments.
A significant portion of the Company’s revenues and earnings in foreign affiliates is exposed to changes in foreign exchange rates. The objectives of and accounting related to the Company’s foreign currency risk management program, as well as its interest rate risk management activities are discussed below.
Foreign Currency Risk Management
The Company has established revenue hedging, balance sheet risk management, and net investment hedging programs to protect against volatility of future foreign currency cash flows and changes in fair value caused by changes in foreign exchange rates.
The objective of the revenue hedging program is to reduce the variability caused by changes in foreign exchange rates that would affect the U.S. dollar value of future cash flows derived from foreign currency denominated sales, primarily the euro, Japanese yen and Chinese renminbi. To achieve this objective, the Company will hedge a portion of its forecasted foreign currency denominated third-party and intercompany distributor entity sales (forecasted sales) that are expected to occur over its planning cycle, typically no more than two years into the future. The Company will layer in hedges over time, increasing the portion of forecasted sales hedged as it gets closer to the expected date of the forecasted sales. The portion of forecasted sales hedged is based on assessments of cost-benefit profiles that consider natural offsetting exposures, revenue and foreign exchange rate volatilities and correlations, and the cost of hedging instruments. The Company manages its anticipated transaction exposure principally with purchased local currency put options, forward contracts, and purchased collar options.
The fair values of these derivative contracts are recorded as either assets (gain positions) or liabilities (loss positions) in the Consolidated Balance Sheet. Changes in the fair value of derivative contracts are recorded each period in either current earnings or Other Comprehensive Income (Loss) (OCI), depending on whether the derivative is designated as part of a hedge transaction and, if so, the type of hedge transaction. For derivatives that are designated as cash flow hedges, the unrealized gains or losses on these contracts are recorded in Accumulated Other Comprehensive Loss (AOCL) and reclassified into Sales when the hedged anticipated revenue is recognized. The amount reclassified into earnings as a result of the discontinuation of cash flow hedges because it was no longer deemed probable the forecasted hedged transactions would occur was not material for the years ended December 31, 2024, 2023 or 2022. For those derivatives which are not designated as cash flow hedges, but serve as economic hedges of forecasted sales, unrealized gains or losses are recorded in Sales each period. The cash flows from both designated and non-designated contracts are reported as operating activities in the Consolidated Statement of Cash Flows. The Company does not enter into derivatives for trading or speculative purposes.
Because Merck principally sells foreign currency in its revenue hedging program, a uniform weakening of the U.S. dollar would yield the largest overall potential loss in the market value of these hedge instruments. The market value of Merck’s hedges would have declined by an estimated $569 million and $754 million at December 31, 2024 and 2023, respectively, from a uniform 10% weakening of the U.S. dollar. The market value was determined using a foreign exchange option pricing model and holding all factors except exchange rates constant. Although not predictive in nature, the Company believes that a 10% threshold reflects reasonably possible near-term changes in Merck’s major foreign currency exposures relative to the U.S. dollar.
The Company manages operating activities and net asset positions at each local subsidiary in order to mitigate the effects of foreign exchange on monetary assets and liabilities. Monetary assets and liabilities denominated in a currency other than the functional currency of a given subsidiary are remeasured at spot rates in effect on the balance sheet date with the effects of changes in spot rates reported in Other (income) expense, net. The Company also uses a balance sheet risk management program to mitigate the exposure of such assets and liabilities from the effects of volatility in foreign exchange. Merck principally utilizes forward exchange contracts to offset the effects of foreign exchange on exposures when it is deemed economical to do so based on a cost-benefit analysis that considers the magnitude of the exposure, the volatility of the foreign exchange rate and the cost of the hedging instrument (primarily the euro, Swiss franc, Japanese yen, and Chinese renminbi). The forward contracts are not designated as hedges and are marked to market through Other (income) expense, net. Accordingly, fair value changes in the forward contracts help mitigate the changes in the value of the remeasured assets and liabilities attributable to changes in foreign currency exchange rates, except to the extent of the spot-forward differences. These differences are not significant due to the short-term nature of the contracts, which typically have average maturities at inception of less than six months. The cash flows from these contracts are reported as operating activities in the Consolidated Statement of Cash Flows.
A sensitivity analysis to changes in the value of the U.S. dollar on foreign currency denominated derivatives, investments and monetary assets and liabilities indicated that if the U.S. dollar uniformly weakened by 10% against all currency exposures of the Company at December 31, 2024 and 2023, Income Before Taxes would have declined by approximately $239 million and $221 million in 2024 and 2023, respectively. Because the Company was in a net short (payable) position relative to its major foreign currencies after consideration of forward contracts, a uniform weakening of the U.S. dollar will yield the largest overall potential net loss in earnings due to exchange. This measurement assumes that a change in one foreign currency relative to the U.S. dollar would not affect other foreign currencies relative to the U.S. dollar. Although not predictive in nature, the Company believes that a 10% threshold reflects reasonably possible near-term changes in Merck’s major foreign currency exposures relative to the U.S. dollar. The cash flows from these contracts are reported as operating activities in the Consolidated Statement of Cash Flows.
The Company also uses forward exchange contracts to hedge a portion of its net investment in foreign operations against movements in foreign exchange rates. The forward contracts are designated as hedges of the net investment in a foreign operation. The unrealized gains or losses on these contracts are recorded in foreign currency translation adjustment within OCI and remain in AOCL until either the sale or complete or substantially complete liquidation of the subsidiary. The Company excludes certain portions of the change in fair value of its derivative instruments from the assessment of hedge effectiveness (excluded components). Changes in fair value of the excluded components are recognized in OCI. The Company recognizes in earnings the initial value of the excluded components on a straight-line basis over the life of the derivative instrument, rather than using the mark-to-market approach. The cash flows from these contracts are reported as investing activities in the Consolidated Statement of Cash Flows.
Foreign exchange risk is also managed through the use of foreign currency debt. Certain of the Company’s senior unsecured euro-denominated notes have been designated as, and are effective as, economic hedges of the net investment in a foreign operation. Accordingly, foreign currency transaction gains or losses due to spot rate fluctuations on the euro-denominated debt instruments are included in foreign currency translation adjustment within OCI.
Interest Rate Risk Management
The Company may use interest rate swap contracts on certain investing and borrowing transactions to manage its net exposure to interest rate changes and to reduce its overall cost of borrowing. The Company does not use leveraged swaps and, in general, does not leverage any of its investment activities that would put principal at risk.
At December 31, 2024, the Company was a party to six pay-floating, receive-fixed interest rate swap contracts designated as fair value hedges of the fixed-rate notes as detailed in the table below.
| | | | | | | | | | | | | | | | | |
| ($ in millions) | 2024 |
| Debt Instrument | Par Value of Debt | | Number of Interest Rate Swaps Held | | Total Swap Notional Amount |
| 4.50% notes due 2033 | $ | 1,500 | | | 6 | | | $ | 1,500 | |
The interest rate swap contracts are designated hedges of the fair value changes in the notes attributable to changes in the benchmark Secured Overnight Financing Rate (SOFR) swap rate. The fair value changes in the notes attributable to changes in the SOFR swap rate are recorded in interest expense along with the offsetting fair value changes in the swap contracts. In January 2025, the Company entered into an additional interest rate swap with a notional amount of $250 million related to its 5.00% notes due 2053. The cash flows from these contracts are reported as operating activities in the Consolidated Statement of Cash Flows.
The Company’s investment portfolio includes cash equivalents and short-term investments, the market values of which are not significantly affected by changes in interest rates. The market value of the Company’s medium- to long-term fixed-rate investments is modestly affected by changes in U.S. interest rates. Changes in medium- to long-term U.S. interest rates have a more significant impact on the market value of the Company’s fixed-rate borrowings, which generally have longer maturities. A sensitivity analysis to measure potential changes in the market value of Merck’s investments and debt from a change in interest rates indicated that a one percentage point increase in interest rates at December 31, 2024 and 2023 would have positively affected the net aggregate market value of these instruments by $2.4 billion and $2.5 billion, respectively. A one percentage point decrease at December 31, 2024 and 2023 would have negatively affected the net aggregate market value by $2.9 billion and $3.0 billion, respectively. The fair value of Merck’s debt was determined using pricing models reflecting one percentage point shifts in the appropriate yield curves. The fair values of Merck’s investments were determined using a combination of pricing and duration models.
Critical Accounting Estimates
The Company’s consolidated financial statements are prepared in conformity with GAAP and, accordingly, include certain amounts that are based on management’s best estimates and judgments. Estimates are used when accounting for amounts recorded in connection with acquisitions, including initial fair value determinations of assets and liabilities in a business combination (primarily IPR&D, other intangible assets and contingent consideration), as well as subsequent fair value measurements. Additionally, estimates are used in determining such items as provisions for sales discounts, rebates and returns, depreciable and amortizable lives, recoverability of inventories, including those produced in preparation for product launches, amounts recorded for contingencies, environmental liabilities, accruals for contingent sales-based milestone payments and other reserves, pension and other postretirement benefit plan assumptions, share-based compensation assumptions, restructuring costs, impairments of long-lived assets (including intangible assets and goodwill) and investments, and taxes on income. Because of the uncertainty inherent in such estimates, actual results may differ from these estimates. Application of the following accounting policies result in accounting estimates having the potential for the most significant impact on the financial statements.
Acquisitions and Dispositions
To determine whether transactions should be accounted for as acquisitions (or disposals) of assets or businesses, the Company makes certain judgments, which include assessment of the inputs, processes, and outputs associated with the acquired set of activities. If the Company determines that substantially all of the fair value of gross assets included in a transaction is concentrated in a single asset (or a group of similar assets), the assets would not represent a business. To be considered a business, the assets in a transaction need to include an input and a substantive process that together significantly contribute to the ability to create outputs.
In a business combination, the acquisition method of accounting requires that the assets acquired and liabilities assumed be recorded as of the date of the acquisition at their respective fair values with limited exceptions. The fair values of intangible assets are determined utilizing information available near the acquisition date based on expectations and assumptions that are deemed reasonable by management. Given the considerable judgment involved in determining fair values, the Company typically obtains assistance from third-party valuation specialists for significant items. Assets acquired and liabilities assumed in a business combination that arise from contingencies are generally recognized at fair value. If fair value cannot be determined, the asset or liability is recognized if probable and reasonably estimable; if these criteria are not met, no asset or liability is recognized. Fair value is defined as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. Accordingly, the Company may be required to value assets at fair value measures that do not reflect the Company’s intended use of those assets. Any excess of the purchase price (consideration transferred) over the estimated fair values of net assets acquired is recorded as goodwill. Transaction costs and costs to
restructure the acquired company are expensed as incurred. The operating results of the acquired business are reflected in the Company’s consolidated financial statements after the date of the acquisition.
The judgments made in determining estimated fair values assigned to assets acquired and liabilities assumed in a business combination, as well as asset lives, can materially affect the Company’s results of operations.
The fair values of identifiable intangible assets related to currently marketed products are primarily determined by using an income approach through which fair value is estimated based on each asset’s discounted projected net cash flows. The Company’s estimates of market participant net cash flows consider historical and projected pricing, margins and expense levels; the performance of competing products where applicable; relevant industry and therapeutic area growth drivers and factors; current and expected trends in technology and product life cycles; the time and investment that will be required to develop products and technologies; the ability to obtain additional marketing and regulatory approvals; the ability to manufacture and commercialize the products; the extent and timing of potential new product introductions by the Company’s competitors; and the life of each asset’s underlying patent and related patent term extension, if any. The net cash flows are then probability-adjusted where appropriate to consider the uncertainties associated with the underlying assumptions, as well as the risk profile of the net cash flows utilized in the valuation. The probability-adjusted future net cash flows of each product are then discounted to present value utilizing an appropriate discount rate.
The fair values of identifiable intangible assets related to IPR&D are also determined using an income approach, through which fair value is estimated based on each asset’s probability-adjusted future net cash flows, which reflect the different stages of development of each product and the associated probability of successful completion. The net cash flows are then discounted to present value using an appropriate discount rate. Amounts allocated to acquired IPR&D are capitalized and accounted for as indefinite-lived intangible assets, subject to impairment testing until completion or abandonment of the projects. Upon successful completion of each IPR&D project, Merck will make a determination as to the then-useful life of the intangible asset, generally determined by the period in which the substantial majority of the cash flows are expected to be generated, and begin amortization.
Certain of the Company’s business combinations involve the potential for future payment of consideration that is contingent upon the achievement of performance milestones, including product development milestones and royalty payments on future product sales. The fair value of contingent consideration liabilities is determined at the acquisition date using unobservable inputs. These inputs include the estimated amount and timing of projected cash flows, the probability of success (achievement of the contingent event) and the risk-adjusted discount rate used to present value the probability-weighted cash flows. Subsequent to the acquisition date, at each reporting period until the contingency is resolved, the contingent consideration liability is remeasured at current fair value with changes (either expense or income) recorded in earnings. Changes in any of the inputs may result in a significantly different fair value adjustment.
If the Company determines the transaction will not be accounted for as an acquisition of a business, the transaction will be accounted for as an asset acquisition rather than a business combination and, therefore, no goodwill will be recorded. In an asset acquisition, acquired IPR&D with no alternative future use is charged to expense and contingent consideration is not recognized at the acquisition date.
Contingent Sales-Based Milestones
The terms of certain business development transactions, including collaborative arrangements, licensing agreements and asset acquisitions, require the Company to make payments contingent upon the achievement of sales-based milestones. Sales-based milestones payable by Merck are accrued and capitalized, subject to cumulative amortization catch-up, when determined by the Company to be probable of being achieved based on future sales forecasts. The amortization catch-up is calculated either from the time of the first regulatory approval for products that were unapproved at the time the transaction was completed or, for new indications of products that were approved prior to the transaction, from the time the transaction was completed. The related intangible asset that is recognized is amortized over its remaining useful life, subject to impairment testing.
Revenue Recognition
Recognition of revenue requires evidence of a contract, probable collection of sales proceeds and completion of substantially all performance obligations. Merck acts as the principal in substantially all of its customer arrangements and therefore records revenue on a gross basis. The majority of the Company’s contracts related to the Pharmaceutical and Animal Health segments have a single performance obligation - the promise to transfer goods. Shipping is considered immaterial in the context of the overall customer arrangement and damages or loss of goods in transit are rare. Therefore, shipping is not deemed a separately recognized performance obligation.
The vast majority of revenues from sales of products are recognized at a point in time when control of the goods is transferred to the customer, which the Company has determined is when title and risks and rewards of ownership transfer to the customer and the Company is entitled to payment. For certain services in the Animal Health segment, revenue is recognized over time, generally ratably over the contract term as services are provided. These service revenues are not material.
The nature of the Company’s business gives rise to several types of variable consideration including discounts and returns, which are estimated at the time of sale generally using the expected value method, although the most likely amount method is used for prompt pay discounts.
In the U.S., sales discounts are issued to customers at the point-of-sale, through an intermediary wholesaler (known as chargebacks), or in the form of rebates. Additionally, sales are generally made with a limited right of return under certain conditions. Revenues are recorded net of provisions for sales discounts and returns, which are established at the time of sale. In addition, if collection of accounts receivable is expected to be in excess of one year, sales are recorded net of time value of money discounts, which have not been material.
The U.S. provision for aggregate customer discounts covers chargebacks and rebates. Chargebacks are discounts that occur when a contracted customer purchases through an intermediary wholesaler. The wholesaler then charges the Company back for the difference between the price initially paid by the wholesaler and the contract price agreed to between Merck and the customer. The provision for chargebacks is based on expected sell-through levels by the Company’s wholesale customers to contracted customers, as well as estimated wholesaler inventory levels. Rebates are amounts owed based upon definitive contractual agreements or legal requirements with private sector and public sector (Medicaid and Medicare Part D) benefit providers after the final dispensing of the product to a benefit plan participant. The provision for rebates is based on expected patient usage, as well as inventory levels in the distribution channel to determine the contractual obligation to the benefit providers. The Company uses historical customer segment utilization mix, sales forecasts, changes to product mix and price, inventory levels in the distribution channel, government pricing calculations and prior payment history in order to estimate the expected provision. Amounts accrued for aggregate customer discounts are evaluated on a quarterly basis through comparison of information provided by the wholesalers, health maintenance organizations, pharmacy benefit managers, federal and state agencies, and other customers to the amounts accrued. Merck remains committed to the 340B Program and to providing 340B discounts to eligible covered entities. See Note 10 to the consolidated financial statements for information regarding 340B legal proceedings.
Summarized information about changes in the aggregate customer discount accrual related to U.S. sales is as follows:
| | | | | | | | | | | |
| ($ in millions) | 2024 | | 2023 |
| Balance January 1 | $ | 2,486 | | | $ | 2,918 | |
| Current provision | 13,450 | | | 12,540 | |
| Adjustments to prior years | (139) | | | (70) | |
| Payments | (13,334) | | | (12,902) | |
| Balance December 31 | $ | 2,463 | | | $ | 2,486 | |
Accruals for chargebacks are reflected as a direct reduction to accounts receivable and accruals for rebates as current liabilities. The accrued balances relative to these provisions included in Accounts receivable and Accrued and other current liabilities were $293 million and $2.2 billion, respectively, at December 31, 2024 and were $188 million and $2.3 billion, respectively, at December 31, 2023.
Outside of the U.S., variable consideration in the form of discounts and rebates are a combination of commercially-driven discounts in highly competitive product classes, discounts required to gain or maintain reimbursement, or legislatively mandated rebates. In certain European countries, legislatively mandated rebates are calculated based on an estimate of the government’s total unbudgeted spending and the Company’s specific payback obligation. Rebates may also be required based on specific product sales thresholds. The Company applies an estimated factor against its actual invoiced sales to represent the expected level of future discount or rebate obligations associated with the sale.
The Company maintains a returns policy that allows its U.S. pharmaceutical customers to return product within a specified period prior to and subsequent to the expiration date (generally, three to six months before and 12 months after product expiration). The estimate of the provision for returns is based upon historical experience with actual returns. Additionally, the Company considers factors such as levels of inventory in the distribution channel, product dating and expiration period, whether products have been discontinued, entrance in the market of generic or other competition, changes in formularies or launch of over-the-counter products, among others. The product returns
provision for U.S. pharmaceutical sales as a percentage of U.S. net pharmaceutical sales was 0.8% in 2024, 1.0% in 2023 and 1.1% in 2022. Outside of the U.S., returns are only allowed in certain countries on a limited basis.
Merck’s payment terms for U.S. pharmaceutical customers are typically 36 days from receipt of invoice and for U.S. animal health customers are typically 30 days from receipt of invoice; however, certain products have longer payment terms, including Keytruda, which has payment terms of 90 days. Payment terms for vaccine sales in the U.S. typically range from 30 days to 60 days. Outside of the U.S., payment terms are typically 30 days to 90 days, although certain markets have longer payment terms.
Through its distribution programs with U.S. wholesalers, the Company encourages wholesalers to align purchases with underlying demand and maintain inventories below specified levels. The terms of the programs allow the wholesalers to earn fees upon providing visibility into their inventory levels, as well as by achieving certain performance parameters such as inventory management, customer service levels, reducing shortage claims and reducing product returns. Information provided through the wholesaler distribution programs includes items such as sales trends, inventory on-hand, on-order quantity and product returns.
Inventories Produced in Preparation for Product Launches
The Company capitalizes inventories produced in preparation for product launches sufficient to support estimated initial market demand. Capitalization of such inventory does not begin until regulatory approval is considered by the Company to be probable. The Company monitors the status of each respective product during the research and regulatory approval process. If the Company is aware of any specific risks or contingencies other than the normal regulatory approval process or if there are any specific issues identified during the research process relating to safety, efficacy, manufacturing, marketing or labeling, the related inventory would generally not be capitalized. Expiry dates of the inventory are affected by the stage of completion. The Company manages the levels of inventory at each stage to optimize the shelf life of the inventory in relation to anticipated market demand in order to avoid product expiry issues. For inventories that are capitalized, anticipated future sales and shelf lives support the realization of the inventory value as the inventory shelf life is sufficient to meet initial product launch requirements. Inventories produced in preparation for product launches capitalized at December 31, 2024 and 2023 were $412 million and $790 million, respectively.
Contingencies and Environmental Liabilities
The Company is involved in various claims and legal proceedings of a nature considered normal to its business, including product liability, intellectual property, commercial litigation and securities litigation, as well as certain additional matters, including governmental and environmental matters (see Note 10 to the consolidated financial statements). The Company records accruals for contingencies when it is probable that a liability has been incurred and the amount can be reasonably estimated. These accruals are adjusted periodically as assessments change or additional information becomes available. Generally, for product liability claims, a portion of the overall accrual is actuarially determined and considers such factors as past experience, number of claims reported and estimates of claims incurred but not yet reported. Individually significant contingent losses are accrued when probable and reasonably estimable.
Legal defense costs expected to be incurred in connection with a loss contingency are accrued when probable and reasonably estimable. Some of the significant factors considered in the review of these legal defense reserves are as follows: the actual costs incurred by the Company; the development of the Company’s legal defense strategy and structure in light of the scope of its litigation; the number of cases being brought against the Company; the costs and outcomes of completed trials and the most current information regarding anticipated timing, progression, and related costs of pre-trial activities and trials in the associated litigation. The amount of legal defense reserves as of December 31, 2024 and 2023 of approximately $225 million and $210 million, respectively, represents the Company’s best estimate of the minimum amount of defense costs to be incurred in connection with its outstanding litigation; however, events such as additional trials and other events that could arise in the course of its litigation could affect the ultimate amount of legal defense costs to be incurred by the Company. The Company will continue to monitor its legal defense costs and review the adequacy of the associated reserves and may determine to increase the reserves at any time in the future if, based upon the factors set forth, it believes it would be appropriate to do so.
The Company and its subsidiaries are parties to a number of proceedings brought under the Comprehensive Environmental Response, Compensation and Liability Act, commonly known as Superfund, and other federal and state equivalents. When a legitimate claim for contribution is asserted, a liability is initially accrued based upon the estimated transaction costs to manage the site. Accruals are adjusted as site investigations, feasibility studies and related cost assessments of remedial techniques are completed, and as the extent to which other potentially responsible parties who may be jointly and severally liable can be expected to contribute is determined.
The Company is also remediating environmental contamination resulting from past industrial activity at certain of its sites and takes an active role in identifying and accruing for these costs. In the past, Merck performed a worldwide survey to assess all sites for potential contamination resulting from past industrial activities. Where assessment indicated that physical investigation was warranted, such investigation was performed, providing a better evaluation of the need for remedial action. Where such need was identified, remedial action was then initiated. As definitive information became available during the course of investigations and/or remedial efforts at each site, estimates were refined and accruals were established or adjusted accordingly. These estimates and related accruals continue to be refined annually.
The Company believes that there are no compliance issues associated with applicable environmental laws and regulations that would have a material adverse effect on the Company. Expenditures for remediation and environmental liabilities were $4 million in 2024 and are estimated to be $26 million in the aggregate for the years 2025 through 2029. In management’s opinion, the liabilities for all environmental matters that are probable and reasonably estimable have been accrued and totaled $41 million and $42 million at December 31, 2024 and 2023, respectively. These liabilities are undiscounted, do not consider potential recoveries from other parties and will be paid out over the periods of remediation for the applicable sites, which are expected to occur primarily over the next 15 years. Although it is not possible to predict with certainty the outcome of these matters, or the ultimate costs of remediation, management does not believe that any reasonably possible expenditures that may be incurred in excess of the liabilities accrued should exceed approximately $46 million in the aggregate. Management also does not believe that these expenditures should result in a material adverse effect on the Company’s financial condition, results of operations or liquidity for any year.
Share-Based Compensation
The Company expenses all share-based payment awards to employees, including grants of stock options, over the requisite service period based on the grant date fair value of the awards. The Company determines the fair value of certain share-based awards using the Black-Scholes option-pricing model which uses both historical and current market data to estimate the fair value. This method incorporates various assumptions such as the risk-free interest rate, expected volatility, expected dividend yield and expected life of the options. Total pretax share-based compensation expense was $761 million in 2024, $645 million in 2023 and $541 million in 2022. At December 31, 2024, there was $1.1 billion of total pretax unrecognized compensation expense related to nonvested stock option, restricted stock unit and performance share unit awards which will be recognized over a weighted-average period of 1.9 years. For segment reporting, share-based compensation costs are unallocated expenses.
Pensions and Other Postretirement Benefit Plans
Net periodic benefit cost for pension plans totaled $107 million in 2024, $126 million in 2023 and $554 million in 2022. Net periodic benefit credit for other postretirement benefit plans was $84 million in 2024, $61 million in 2023 and $93 million in 2022. Pension and other postretirement benefit plan information for financial reporting purposes is calculated using actuarial assumptions including a discount rate for plan benefit obligations and an expected rate of return on plan assets. The changes in net periodic benefit cost year over year for pension plans are primarily attributable to lower settlement charges incurred by certain plans in 2024 and 2023 compared with 2022, as well as changes in expected returns and the discount rates.
The Company reassesses its benefit plan assumptions on a regular basis. For both the pension and other postretirement benefit plans, the discount rate is evaluated on measurement dates and modified to reflect the prevailing market rate of a portfolio of high-quality fixed-income debt instruments that would provide the future cash flows needed to pay the benefits included in the benefit obligation as they come due. The discount rates for the Company’s U.S. pension and other postretirement benefit plans ranged from 5.50% to 5.70% at December 31, 2024, compared with a range of 5.25% to 5.45% at December 31, 2023.
The expected rate of return for both the pension and other postretirement benefit plans represents the average rate of return to be earned on plan assets over the period the benefits included in the benefit obligation are to be paid. In developing the expected rate of return, the Company considers long-term compound annualized returns of historical market data, current market conditions and actual returns on the Company’s plan assets. Using this reference information, the Company develops forward-looking return expectations for each asset category and a weighted-average expected long-term rate of return for a target portfolio allocated across these investment categories. The expected portfolio performance reflects the contribution of active management as appropriate. For 2025, the expected rate of return for the Company’s U.S. pension and other postretirement benefit plans will be 7.70% compared with 7.75% in 2024.
The Company has established investment guidelines for its U.S. pension and other postretirement plans to create an asset allocation that is expected to deliver a rate of return sufficient to meet the long-term obligation of each plan, given an acceptable level of risk. The target investment portfolio of the Company’s U.S. pension and other
postretirement benefit plans is allocated 25% to 40% in U.S. equities, 15% to 30% in international equities, 40% to 50% in fixed-income investments, and up to 8% in cash and other investments. The portfolio’s equity weighting is consistent with the long-term nature of the plans’ benefit obligations. The expected annual standard deviation of returns of the target portfolio, which approximates 12%, reflects both the equity allocation and the diversification benefits among the asset classes in which the portfolio invests. For international pension plans, the targeted investment portfolio varies based on the duration of pension liabilities and local government rules and regulations. Although a significant percentage of plan assets are invested in U.S. equities, concentration risk is mitigated through the use of strategies that are diversified within management guidelines.
Actuarial assumptions are based upon management’s best estimates and judgment. A reasonably possible change of plus (minus) 25 basis points in the discount rate assumption, with other assumptions held constant, would have had an estimated $45 million favorable (unfavorable) impact on the Company’s net periodic benefit cost in 2024. A reasonably possible change of plus (minus) 25 basis points in the expected rate of return assumption, with other assumptions held constant, would have had an estimated $57 million favorable (unfavorable) impact on Merck’s net periodic benefit cost in 2024. Required funding obligations for 2025 relating to the Company’s pension and other postretirement benefit plans are not expected to be material. The preceding hypothetical changes in the discount rate and expected rate of return assumptions would not impact the Company’s funding requirements.
Net gain/loss amounts, which primarily reflect differences between expected and actual returns on plan assets as well as the effects of changes in actuarial assumptions, are recorded as a component of AOCL. Expected returns for pension plans are based on a calculated market-related value of assets. Net gain/loss amounts in AOCL in excess of certain thresholds are amortized into net periodic benefit cost over the average remaining service life of employees.
Restructuring Costs
Restructuring costs have been recorded in connection with restructuring program activities. As a result, the Company has made estimates and judgments regarding its future plans, including future employee termination costs to be incurred in conjunction with involuntary separations when such separations are probable and estimable. When accruing termination costs, the Company will recognize the amount within a range of costs that is the best estimate within the range. When no amount within the range is a better estimate than any other amount, the Company recognizes the minimum amount within the range. In connection with these actions, management also assesses the recoverability of long-lived assets employed in the business. In certain instances, asset lives have been shortened based on changes in the expected useful lives of the affected assets. Severance and employee-related costs, as well as other costs, such as facility shut-down costs, are reflected within Restructuring costs. Asset-related charges are reflected within Cost of sales, Selling, general and administrative expenses and Research and development expenses depending upon the nature of the asset.
Impairments of Long-Lived Assets
The Company assesses changes in economic, regulatory and legal conditions and makes assumptions regarding estimated future cash flows in evaluating the value of the Company’s property, plant and equipment, goodwill and other intangible assets.
The Company periodically evaluates whether current facts or circumstances indicate that the carrying values of its long-lived assets to be held and used may not be recoverable. If such circumstances are determined to exist, an estimate of the undiscounted future cash flows of these assets, or appropriate asset groupings, is compared to the carrying value to determine whether an impairment exists. If the asset is determined to be impaired, the loss is measured based on the difference between the asset’s fair value and its carrying value. If quoted market prices are not available, the Company will estimate fair value using a discounted value of estimated future cash flows approach.
Goodwill represents the excess of the consideration transferred over the fair value of net assets acquired in a business combination. Goodwill is assigned to reporting units and evaluated for impairment at least annually, or more frequently if impairment indicators exist, by first assessing qualitative factors to determine whether it is more likely than not that the fair value of a reporting unit is less than its carrying amount. Some of the factors considered in the assessment include general macroeconomic conditions, conditions specific to the industry and market, cost factors which could have a significant effect on earnings or cash flows, the overall financial performance of the reporting unit, and whether there have been sustained declines in the Company’s share price. If the Company concludes it is more likely than not that the fair value of a reporting unit is less than its carrying amount, a quantitative fair value test is performed. If the carrying value of a reporting unit is greater than its fair value, a goodwill impairment charge will be recorded for the difference (up to the carrying value of goodwill).
Other acquired intangible assets (excluding IPR&D) are initially recorded at fair value, assigned an estimated useful life, and amortized primarily on a straight-line basis over their estimated useful lives. When events or
circumstances warrant a review, the Company will assess recoverability from future operations using pretax undiscounted cash flows derived from the lowest appropriate asset groupings. Impairments are recognized in operating results to the extent that the carrying value of the intangible asset exceeds its fair value, which is determined based on the net present value of estimated future cash flows.
IPR&D that the Company acquires in conjunction with a business combination represents the fair value assigned to incomplete research projects which, at the time of acquisition, have not reached technological feasibility. The amounts are capitalized and accounted for as indefinite-lived intangible assets, subject to impairment testing until completion or abandonment of the projects. The Company evaluates IPR&D for impairment at least annually, or more frequently if impairment indicators exist (such as unfavorable clinical trial data, changes in the commercial landscape or delays in the clinical development program and related regulatory filing and approval timelines), by performing a quantitative test that compares the fair value of the IPR&D intangible asset with its carrying value. For impairment testing purposes, the Company may combine separately recorded IPR&D intangible assets into one unit of account based on the relevant facts and circumstances. Generally, the Company will combine IPR&D intangible assets for testing purposes if they operate as a single asset and are essentially inseparable. If the fair value is less than the carrying amount, an impairment loss is recognized in operating results.
The judgments made in evaluating impairment of long-lived intangibles can materially affect the Company’s results of operations.
Taxes on Income
The Company’s effective tax rate is based on pretax income, statutory tax rates and tax planning opportunities available in the various jurisdictions in which the Company operates. An estimated effective tax rate for a year is applied to the Company’s quarterly operating results. In the event that there is a significant unusual or one-time item recognized, or expected to be recognized, in the Company’s quarterly operating results, the tax attributable to that item would be separately calculated and recorded at the same time as the unusual or one-time item. The Company considers the resolution of prior year tax matters to be such items. Significant judgment is required in determining the Company’s tax provision and in evaluating its tax positions. The recognition and measurement of a tax position is based on management’s best judgment given the facts, circumstances and information available at the reporting date. The Company evaluates tax positions to determine whether the benefits of tax positions are more likely than not of being sustained upon audit based on the technical merits of the tax position. For tax positions that are more likely than not of being sustained upon audit, the Company recognizes the amount of the benefit that is greater than 50% likely of being realized upon ultimate settlement in the financial statements. For tax positions that are not more likely than not of being sustained upon audit, the Company does not recognize any portion of the benefit in the financial statements. If the more likely than not threshold is not met in the period for which a tax position is taken, the Company may subsequently recognize the benefit of that tax position if the tax matter is effectively settled, the statute of limitations expires, or if the more likely than not threshold is met in a subsequent period (see Note 15 to the consolidated financial statements).
Tax regulations require items to be included in the tax return at different times than the items are reflected in the financial statements. Timing differences create deferred tax assets and liabilities. Deferred tax assets generally represent items that can be used as a tax deduction or credit in the tax return in future years for which the Company has already recorded the tax benefit in the financial statements. The Company establishes valuation allowances for its deferred tax assets when the amount of expected future taxable income is not likely to support the use of the deduction or credit. Deferred tax liabilities generally represent tax expense recognized in the financial statements for which payment has been deferred or expense for which the Company has already taken a deduction on the tax return, but has not yet recognized as expense in the financial statements.
Recently Issued Accounting Standards
For a discussion of recently issued accounting standards, see Note 2 to the consolidated financial statements.
Cautionary Factors That May Affect Future Results
This report and other written reports and oral statements made from time to time by the Company may contain so-called “forward-looking statements,” all of which are based on management’s current expectations and are subject to risks and uncertainties which may cause results to differ materially from those set forth in the statements. One can identify these forward-looking statements by their use of words such as “anticipates,” “expects,” “plans,” “will,” “estimates,” “forecasts,” “projects” and other words of similar meaning, or negative variations of any of the foregoing. One can also identify them by the fact that they do not relate strictly to historical or current facts. These statements are likely to address the Company’s growth strategy, financial results, product approvals, product potential, development programs, environmental or other sustainability initiatives. One must carefully consider any
such statement and should understand that many factors could cause actual results to differ materially from the Company’s forward-looking statements. These factors include inaccurate assumptions and a broad variety of other risks and uncertainties, including some that are known and some that are not. No forward-looking statement can be guaranteed and actual future results may vary materially.
The Company does not assume the obligation to update any forward-looking statement. One should carefully evaluate such statements in light of factors, including risk factors, described in the Company’s filings with the Securities and Exchange Commission, especially on this Form 10-K and Forms 10-Q and 8-K. In Item 1A. “Risk Factors” of this annual report on Form 10-K the Company discusses in more detail various important risk factors that could cause actual results to differ from expected or historic results. The Company notes these factors for investors as permitted by the Private Securities Litigation Reform Act of 1995. One should understand that it is not possible to predict or identify all such factors. Consequently, the reader should not consider any such list to be a complete statement of all potential risks or uncertainties.
Item 7A.Quantitative and Qualitative Disclosures about Market Risk.
The information required by this Item is incorporated by reference to the discussion under “Financial Instruments Market Risk Disclosures” in Item 7. “Management’s Discussion and Analysis of Financial Condition and Results of Operations.”
Item 8.Financial Statements and Supplementary Data.
(a)Financial Statements
The consolidated balance sheet of Merck & Co., Inc. and subsidiaries as of December 31, 2024 and 2023, and the related consolidated statements of income, of comprehensive income (loss), of equity and of cash flows for each of the three years in the period ended December 31, 2024, the notes to consolidated financial statements, and the report dated February 25, 2025 of PricewaterhouseCoopers LLP, independent registered public accounting firm, are as follows:
Consolidated Statement of Income
Merck & Co., Inc. and Subsidiaries
Years Ended December 31
($ in millions except per share amounts)
| | | | | | | | | | | | | | | | | |
| 2024 | | 2023 | | 2022 |
| Sales | $ | 64,168 | | | $ | 60,115 | | | $ | 59,283 | |
| Costs, Expenses and Other | | | | | |
| Cost of sales | 15,193 | | | 16,126 | | | 17,411 | |
| Selling, general and administrative | 10,816 | | | 10,504 | | | 10,042 | |
| Research and development | 17,938 | | | 30,531 | | | 13,548 | |
| Restructuring costs | 309 | | | 599 | | | 337 | |
| Other (income) expense, net | (24) | | | 466 | | | 1,501 | |
| | 44,232 | | | 58,226 | | | 42,839 | |
| Income Before Taxes | 19,936 | | | 1,889 | | | 16,444 | |
| Taxes on Income | 2,803 | | | 1,512 | | | 1,918 | |
| Net Income | 17,133 | | | 377 | | | 14,526 | |
| Less: Net Income Attributable to Noncontrolling Interests | 16 | | | 12 | | | 7 | |
| Net Income Attributable to Merck & Co., Inc. | $ | 17,117 | | | $ | 365 | | | $ | 14,519 | |
| Basic Earnings per Common Share Attributable to Merck & Co., Inc. Common Shareholders | $ | 6.76 | | | $ | 0.14 | | | $ | 5.73 | |
| Earnings per Common Share Assuming Dilution Attributable to Merck & Co., Inc. Common Shareholders | $ | 6.74 | | | $ | 0.14 | | | $ | 5.71 | |
Consolidated Statement of Comprehensive Income (Loss)
Merck & Co., Inc. and Subsidiaries
Years Ended December 31
($ in millions)
| | | | | | | | | | | | | | | | | |
| 2024 | | 2023 | | 2022 |
| Net Income Attributable to Merck & Co., Inc. | $ | 17,117 | | | $ | 365 | | | $ | 14,519 | |
| Other Comprehensive Income (Loss) Net of Taxes: | | | | | |
| Net unrealized income (loss) on derivatives, net of reclassifications | 266 | | | (97) | | | (71) | |
| Benefit plan net gain (loss) and prior service credit (cost), net of amortization | 466 | | | (385) | | | 335 | |
| Cumulative translation adjustment | (516) | | | 89 | | | (603) | |
| | 216 | | | (393) | | | (339) | |
| Comprehensive Income (Loss) Attributable to Merck & Co., Inc. | $ | 17,333 | | | $ | (28) | | | $ | 14,180 | |
The accompanying notes are an integral part of these consolidated financial statements.
Consolidated Balance Sheet
Merck & Co., Inc. and Subsidiaries
December 31
($ in millions except per share amounts)
| | | | | | | | | | | |
| 2024 | | 2023 |
| Assets | | | |
| Current Assets | | | |
| Cash and cash equivalents | $ | 13,242 | | | $ | 6,841 | |
| Short-term investments | 447 | | | 252 | |
Accounts receivable (net of allowance for doubtful accounts of $89 in 2024 and $88 in 2023) | 10,278 | | | 10,349 | |
Inventories (excludes inventories of $4,193 in 2024 and $3,348 in 2023 classified in Other assets - see Note 7) | 6,109 | | | 6,358 | |
| Other current assets | 8,706 | | | 8,368 | |
| Total current assets | 38,782 | | | 32,168 | |
| Investments | 463 | | | 252 | |
| Property, Plant and Equipment (at cost) | | | |
| Land | 307 | | | 326 | |
| Buildings | 16,360 | | | 14,966 | |
| Machinery, equipment and office furnishings | 18,283 | | | 17,763 | |
| Construction in progress | 7,984 | | | 8,262 | |
| 42,934 | | | 41,317 | |
| Less: accumulated depreciation | 19,155 | | | 18,266 | |
| | 23,779 | | | 23,051 | |
| Goodwill | 21,668 | | | 21,197 | |
| Other Intangibles, Net | 16,370 | | | 18,011 | |
| Other Assets | 16,044 | | | 11,996 | |
| | $ | 117,106 | | | $ | 106,675 | |
| Liabilities and Equity | | | |
| Current Liabilities | | | |
| Loans payable and current portion of long-term debt | $ | 2,649 | | | $ | 1,372 | |
| Trade accounts payable | 4,079 | | | 3,922 | |
| Accrued and other current liabilities | 15,694 | | | 15,766 | |
| Income taxes payable | 3,914 | | | 2,649 | |
| Dividends payable | 2,084 | | | 1,985 | |
| Total current liabilities | 28,420 | | | 25,694 | |
| Long-Term Debt | 34,462 | | | 33,683 | |
| Deferred Income Taxes | 1,387 | | | 871 | |
| Other Noncurrent Liabilities | 6,465 | | | 8,792 | |
| Merck & Co., Inc. Stockholders’ Equity | | | |
Common stock, $0.50 par value Authorized - 6,500,000,000 shares Issued - 3,577,103,522 shares in 2024 and 2023 | 1,788 | | | 1,788 | |
| Other paid-in capital | 44,704 | | | 44,509 | |
| Retained earnings | 63,069 | | | 53,895 | |
| Accumulated other comprehensive loss | (4,945) | | | (5,161) | |
| 104,616 | | | 95,031 | |
| Less treasury stock, at cost: 1,049,466,187 shares in 2024 and 1,045,470,249 shares in 2023 | 58,303 | | | 57,450 | |
| Total Merck & Co., Inc. stockholders’ equity | 46,313 | | | 37,581 | |
| Noncontrolling Interests | 59 | | | 54 | |
| Total equity | 46,372 | | | 37,635 | |
| | $ | 117,106 | | | $ | 106,675 | |
The accompanying notes are an integral part of this consolidated financial statement.
Consolidated Statement of Equity
Merck & Co., Inc. and Subsidiaries
Years Ended December 31
($ in millions except per share amounts)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Common
Stock | | Other
Paid-In
Capital | | Retained
Earnings | | Accumulated
Other
Comprehensive
Loss | | Treasury
Stock | | Non-
controlling
Interests | | Total |
| Balance January 1, 2022 | $ | 1,788 | | | $ | 44,238 | | | $ | 53,696 | | | $ | (4,429) | | | $ | (57,109) | | | $ | 73 | | | $ | 38,257 | |
| Net income attributable to Merck & Co., Inc. | — | | | — | | | 14,519 | | | — | | | — | | | — | | | 14,519 | |
| Other comprehensive loss, net of taxes | — | | | — | | | — | | | (339) | | | — | | | — | | | (339) | |
| Cash dividends declared on common stock ($2.80 per share) | — | | | — | | | (7,134) | | | — | | | — | | | — | | | (7,134) | |
| | | | | | | | | | | | | |
| Net income attributable to noncontrolling interests | — | | | — | | | — | | | — | | | — | | | 7 | | | 7 | |
| Distributions attributable to noncontrolling interests | — | | | — | | | — | | | — | | | — | | | (13) | | | (13) | |
| Share-based compensation plans and other | — | | | 141 | | | — | | | — | | | 620 | | | — | | | 761 | |
| Balance December 31, 2022 | 1,788 | | | 44,379 | | | 61,081 | | | (4,768) | | | (56,489) | | | 67 | | | 46,058 | |
| Net income attributable to Merck & Co., Inc. | — | | | — | | | 365 | | | — | | | — | | | — | | | 365 | |
| Other comprehensive loss, net of taxes | — | | | — | | | — | | | (393) | | | — | | | — | | | (393) | |
| Cash dividends declared on common stock ($2.96 per share) | — | | | — | | | (7,551) | | | — | | | — | | | — | | | (7,551) | |
| Treasury stock shares purchased | — | | | — | | | — | | | — | | | (1,346) | | | — | | | (1,346) | |
| Net income attributable to noncontrolling interests | — | | | — | | | — | | | — | | | — | | | 12 | | | 12 | |
| Distributions attributable to noncontrolling interests | — | | | — | | | — | | | — | | | — | | | (25) | | | (25) | |
| Share-based compensation plans and other | — | | | 130 | | | — | | | — | | | 385 | | | — | | | 515 | |
| Balance December 31, 2023 | 1,788 | | | 44,509 | | | 53,895 | | | (5,161) | | | (57,450) | | | 54 | | | 37,635 | |
| Net income attributable to Merck & Co., Inc. | — | | | — | | | 17,117 | | | — | | | — | | | — | | | 17,117 | |
| Other comprehensive income, net of taxes | — | | | — | | | — | | | 216 | | | — | | | — | | | 216 | |
| Cash dividends declared on common stock ($3.12 per share) | — | | | — | | | (7,943) | | | — | | | — | | | — | | | (7,943) | |
| Treasury stock shares purchased | — | | | — | | | — | | | — | | | (1,306) | | | — | | | (1,306) | |
| Net income attributable to noncontrolling interests | — | | | — | | | — | | | — | | | — | | | 16 | | | 16 | |
| Distributions attributable to noncontrolling interests | — | | | — | | | — | | | — | | | — | | | (12) | | | (12) | |
| Share-based compensation plans and other | — | | | 195 | | | — | | | — | | | 453 | | | 1 | | | 649 | |
| Balance December 31, 2024 | $ | 1,788 | | | $ | 44,704 | | | $ | 63,069 | | | $ | (4,945) | | | $ | (58,303) | | | $ | 59 | | | $ | 46,372 | |
The accompanying notes are an integral part of this consolidated financial statement.
Consolidated Statement of Cash Flows
Merck & Co., Inc. and Subsidiaries
Years Ended December 31
($ in millions)
| | | | | | | | | | | | | | | | | |
| 2024 | | 2023 | | 2022 |
| Cash Flows from Operating Activities | | | | | |
| Net income | $ | 17,133 | | | $ | 377 | | | $ | 14,526 | |
| Adjustments to reconcile net income to net cash provided by operating activities: | | | | | |
| Amortization | 2,395 | | | 2,044 | | | 2,085 | |
| Depreciation | 2,104 | | | 1,828 | | | 1,824 | |
| Intangible asset impairment charges | 39 | | | 792 | | | 1,749 | |
| (Income) loss from investments in equity securities, net | (14) | | | (340) | | | 1,419 | |
| Charges for certain research and development asset acquisitions | 3,456 | | | 11,409 | | | — | |
| Deferred income taxes | (1,249) | | | (1,899) | | | (1,568) | |
| Share-based compensation | 761 | | | 645 | | | 541 | |
| Other | 510 | | | 355 | | | 1,301 | |
| Net changes in assets and liabilities: | | | | | |
| Accounts receivable | (244) | | | (1,148) | | | (644) | |
| Inventories | (835) | | | (816) | | | (161) | |
| Trade accounts payable | 182 | | | (380) | | | (289) | |
| Accrued and other current liabilities | (2,328) | | | 1,783 | | | (50) | |
| Income taxes payable | 1,023 | | | 214 | | | 380 | |
| Noncurrent liabilities | (49) | | | 456 | | | (545) | |
| Other | (1,416) | | | (2,314) | | | (1,473) | |
| Net Cash Provided by Operating Activities | 21,468 | | | 13,006 | | | 19,095 | |
| Cash Flows from Investing Activities | | | | | |
| Capital expenditures | (3,372) | | | (3,863) | | | (4,388) | |
| Purchases of securities and other investments | (519) | | | (955) | | | (1,204) | |
| Proceeds from sale of Seagen Inc. common stock | — | | | 1,145 | | | — | |
| Proceeds from sales of securities and other investments | 377 | | | 1,658 | | | 721 | |
| Acquisition of Eyebiotech Limited, net of cash acquired | (1,344) | | | — | | | — | |
| Acquisition of Elanco Animal Health Incorporated aqua business | (1,303) | | | — | | | — | |
| Acquisition of Harpoon Therapeutics, Inc., net of cash acquired | (746) | | | — | | | — | |
| Acquisition of MK-1045 from Curon Pharmaceutical | (700) | | | — | | | — | |
| Acquisition of Prometheus Biosciences, Inc., net of cash acquired | — | | | (10,705) | | | — | |
| Acquisition of Imago BioSciences Inc., net of cash acquired | — | | | (1,327) | | | — | |
| Other | (127) | | | (36) | | | (89) | |
| Net Cash Used in Investing Activities | (7,734) | | | (14,083) | | | (4,960) | |
| Cash Flows from Financing Activities | | | | | |
| Payments on debt | (1,290) | | | (1,755) | | | (2,251) | |
| Proceeds from issuance of debt | 3,599 | | | 5,939 | | | — | |
| Purchases of treasury stock | (1,306) | | | (1,346) | | | — | |
| Dividends paid to stockholders | (7,840) | | | (7,445) | | | (7,012) | |
| Proceeds from exercise of stock options | 177 | | | 125 | | | 384 | |
| Other | (372) | | | (328) | | | (240) | |
| Net Cash Used in Financing Activities | (7,032) | | | (4,810) | | | (9,119) | |
| Effect of Exchange Rate Changes on Cash, Cash Equivalents and Restricted Cash | (293) | | | 23 | | | (410) | |
| Net Increase (Decrease) in Cash, Cash Equivalents and Restricted Cash | 6,409 | | | (5,864) | | | 4,606 | |
Cash, Cash Equivalents and Restricted Cash at Beginning of Year (includes $68, $79 and $71 of restricted cash at January 1, 2024, 2023 and 2022, respectively, included in Other current assets) | 6,909 | | | 12,773 | | | 8,167 | |
Cash, Cash Equivalents and Restricted Cash at End of Year (includes $76, $68 and $79 of restricted cash at December 31, 2024, 2023 and 2022, respectively, included in Other current assets) | $ | 13,318 | | | $ | 6,909 | | | $ | 12,773 | |
The accompanying notes are an integral part of this consolidated financial statement.
Notes to Consolidated Financial Statements
Merck & Co., Inc. and Subsidiaries
($ in millions except per share amounts)
1. Nature of Operations
Merck & Co., Inc. (Merck or the Company) is a global health care company that delivers innovative health solutions through its prescription medicines, including biologic therapies, vaccines and animal health products. The Company’s operations are principally managed on a product basis and include two operating segments, Pharmaceutical and Animal Health, both of which are reportable segments.
The Pharmaceutical segment includes human health pharmaceutical and vaccine products. Human health pharmaceutical products consist of therapeutic and preventive agents, generally sold by prescription, for the treatment of human disorders. The Company sells these human health pharmaceutical products primarily to drug wholesalers and retailers, hospitals, government agencies and managed health care providers such as health maintenance organizations, pharmacy benefit managers and other institutions. Human health vaccine products consist of preventive pediatric, adolescent and adult vaccines. The Company sells these human health vaccines primarily to physicians, wholesalers, distributors and government entities.
The Animal Health segment discovers, develops, manufactures and markets a wide range of veterinary pharmaceutical and vaccine products, as well as health management solutions and services, for the prevention, treatment and control of disease in all major livestock and companion animal species. The Company also offers an extensive suite of digitally connected identification, traceability and monitoring products. The Company sells its products to veterinarians, distributors, animal producers, farmers and pet owners.
2. Summary of Accounting Policies
Principles of Consolidation — The consolidated financial statements include the accounts of the Company and all of its subsidiaries in which a controlling interest is maintained. Intercompany balances and transactions are eliminated. Controlling interest is determined by majority ownership interest and the absence of substantive third-party participating rights or, in the case of variable interest entities, by majority exposure to expected losses, residual returns or both. For those consolidated subsidiaries where Merck ownership is less than 100%, the outside shareholders’ interests are shown as Noncontrolling interests in equity. Investments in affiliates over which the Company has significant influence but not a controlling interest, such as interests in entities owned equally by the Company and a third party that are under shared control, are carried on the equity method basis.
Acquisitions — In a business combination, the acquisition method of accounting requires that the assets acquired and liabilities assumed be recorded as of the date of the acquisition at their respective fair values with limited exceptions. Assets acquired and liabilities assumed in a business combination that arise from contingencies are generally recognized at fair value. If fair value cannot be determined, the asset or liability is recognized if probable and reasonably estimable; if these criteria are not met, no asset or liability is recognized. Fair value is defined as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. Accordingly, the Company may be required to value assets at fair value measures that do not reflect the Company’s intended use of those assets. Any excess of the purchase price (consideration transferred) over the estimated fair values of net assets acquired is recorded as goodwill. Transaction costs and costs to restructure the acquired company are expensed as incurred. The operating results of the acquired business are reflected in the Company’s consolidated financial statements after the date of the acquisition.
If the Company determines the assets acquired do not meet the definition of a business under the acquisition method of accounting, the transaction will be accounted for as an acquisition of assets rather than a business combination and, therefore, no goodwill will be recorded. In an asset acquisition, acquired in-process research and development (IPR&D) with no alternative future use is charged to expense and contingent consideration is not recognized at the acquisition date.
Foreign Currency Translation — The net assets of international subsidiaries where the local currencies have been determined to be the functional currencies are translated into U.S. dollars using current exchange rates and results of operations are translated at average exchange rates. The U.S. dollar effects that arise from translating the net assets of these subsidiaries at changing rates are recorded in Other Comprehensive Income (OCI) and remain in Accumulated other comprehensive loss (AOCL) until either the sale or complete or substantially complete liquidation of the subsidiary. For those subsidiaries that operate in highly inflationary economies and for those subsidiaries where the U.S. dollar has been determined to be the functional currency, non-monetary foreign currency
assets and liabilities are translated using historical rates, while monetary assets and liabilities are translated at current rates, with the U.S. dollar effects of rate changes included in Other (income) expense, net.
Cash Equivalents — Cash equivalents are comprised of certain highly liquid investments with original maturities of less than three months.
Inventories — Inventories are valued at the lower of cost or net realizable value. The cost of a substantial majority of U.S. human health inventories is determined using the last-in, first-out (LIFO) method for both financial reporting and tax purposes. The cost of all other inventories is determined using the first-in, first-out (FIFO) method. Inventories consist of currently marketed products, as well as certain inventories produced in preparation for product launches that are considered by the Company to be probable of obtaining regulatory approval. In evaluating the recoverability of inventories produced in preparation for product launches, the Company considers the likelihood that revenue will be obtained from the future sale of the related inventory together with the status of the product during the research and regulatory approval process.
Investments — Investments in marketable debt securities classified as available-for-sale are reported at fair value. Fair values of the Company’s investments in marketable debt securities are determined using quoted market prices in active markets for identical assets or quoted prices for similar assets or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities. Changes in fair value that are not impairment related are reported net of taxes in OCI. The Company considers available evidence in evaluating potential impairments of its investments in marketable debt securities, including the extent to which fair value is less than cost, whether an allowance for credit loss is required, as well as adverse factors that could affect the value of the securities. An impairment has occurred if the Company does not expect to recover the entire amortized cost basis of the marketable debt security. If the Company does not intend to sell the impaired debt security, and it is not more likely than not it will be required to sell the debt security before the recovery of its amortized cost basis, the amount of the impairment recognized in earnings, recorded in Other (income) expense, net, is limited to the portion attributed to credit loss. The remaining portion of the impairment related to other factors is recognized in OCI. Realized gains and losses for debt securities are included in Other (income) expense, net.
Investments in publicly traded equity securities are reported at fair value as determined using quoted market prices in active markets for identical assets or quoted prices for similar assets or other inputs that are observable or can be corroborated by observable market data. Changes in fair value are included in Other (income) expense, net. Unrealized gains and losses from investments that are directly owned are determined at the end of the reporting period. Gains and losses from ownership interests in investment funds, which are accounted for as equity method investments, are reported on a one quarter lag. Investments in equity securities without readily determinable fair values are recorded at cost, plus or minus subsequent observable price changes in orderly transactions for identical or similar investments, minus impairments. Such adjustments are recognized in Other (income) expense, net. Realized gains and losses for equity securities are included in Other (income) expense, net.
Revenue Recognition — Recognition of revenue requires evidence of a contract, probable collection of sales proceeds and completion of substantially all performance obligations. Merck acts as the principal in substantially all of its customer arrangements and therefore records revenue on a gross basis. The majority of the Company’s contracts related to the Pharmaceutical and Animal Health segments have a single performance obligation - the promise to transfer goods. Shipping is considered immaterial in the context of the overall customer arrangement and damages or loss of goods in transit are rare. Therefore, shipping is not deemed a separately recognized performance obligation.
The vast majority of revenues from sales of products are recognized at a point in time when control of the goods is transferred to the customer, which the Company has determined is when title and risks and rewards of ownership transfer to the customer and the Company is entitled to payment. The Company recognizes revenue from the sales of vaccines to the U.S. federal government for placement into vaccine stockpiles in accordance with Securities and Exchange Commission (SEC) Interpretation, Commission Guidance Regarding Accounting for Sales of Vaccines and BioTerror Countermeasures to the Federal Government for Placement into the Pediatric Vaccine Stockpile or the Strategic National Stockpile. This interpretation allows companies to recognize revenue for sales of vaccines into U.S. government stockpiles even though these sales might not meet the criteria for revenue recognition under other accounting guidance. For certain services in the Animal Health segment, revenue is recognized over time, generally ratably over the contract term as services are provided. These service revenues are not material.
The nature of the Company’s business gives rise to several types of variable consideration including discounts and returns, which are estimated at the time of sale generally using the expected value method, although the most likely amount method is used for prompt pay discounts.
In the U.S., sales discounts are issued to customers at the point-of-sale, through an intermediary wholesaler (known as chargebacks), or in the form of rebates. Additionally, sales are generally made with a limited right of return under certain conditions. Revenues are recorded net of provisions for sales discounts and returns, which are established at the time of sale. In addition, if collection of accounts receivable is expected to be in excess of one year, sales are recorded net of time value of money discounts, which have not been material.
The U.S. provision for aggregate customer discounts covering chargebacks and rebates was $13.3 billion in 2024, $12.5 billion in 2023 and $12.3 billion in 2022. Chargebacks are discounts that occur when a contracted customer purchases through an intermediary wholesaler. The wholesaler then charges the Company back for the difference between the price initially paid by the wholesaler and the contract price agreed to between Merck and the customer. The provision for chargebacks is based on expected sell-through levels by the Company’s wholesale customers to contracted customers, as well as estimated wholesaler inventory levels. Rebates are amounts owed based upon definitive contractual agreements or legal requirements with private sector and public sector (Medicaid and Medicare Part D) benefit providers after the final dispensing of the product to a benefit plan participant. The provision for rebates is based on expected patient usage, as well as inventory levels in the distribution channel to determine the contractual obligation to the benefit providers. The Company uses historical customer segment utilization mix, sales forecasts, changes to product mix and price, inventory levels in the distribution channel, government pricing calculations and prior payment history in order to estimate the expected provision. Amounts accrued for aggregate customer discounts are evaluated on a quarterly basis through comparison of information provided by the wholesalers, health maintenance organizations, pharmacy benefit managers, federal and state agencies, and other customers to the amounts accrued. The accrued balances relative to the provisions for chargebacks and rebates included in Accounts receivable and Accrued and other current liabilities were $293 million and $2.2 billion, respectively, at December 31, 2024 and were $188 million and $2.3 billion, respectively, at December 31, 2023.
Outside of the U.S., variable consideration in the form of discounts and rebates are a combination of commercially-driven discounts in highly competitive product classes, discounts required to gain or maintain reimbursement, or legislatively mandated rebates. In certain European countries, legislatively mandated rebates are calculated based on an estimate of the government’s total unbudgeted spending and the Company’s specific payback obligation. Rebates may also be required based on specific product sales thresholds. The Company applies an estimated factor against its actual invoiced sales to represent the expected level of future discount or rebate obligations associated with the sale.
The Company maintains a returns policy that allows its U.S. pharmaceutical customers to return product within a specified period prior to and subsequent to the expiration date (generally, three to six months before and 12 months after product expiration). The estimate of the provision for returns is based upon historical experience with actual returns. Additionally, the Company considers factors such as levels of inventory in the distribution channel, product dating and expiration period, whether products have been discontinued, entrance in the market of generic or other competition, changes in formularies or launch of over-the-counter products, among others. Outside of the U.S., returns are only allowed in certain countries on a limited basis.
Merck’s payment terms for U.S. pharmaceutical customers are typically 36 days from receipt of invoice and for U.S. animal health customers are typically 30 days from receipt of invoice; however, certain products have longer payment terms, including Keytruda (pembrolizumab), which has payment terms of 90 days. Payment terms for vaccine sales in the U.S. typically range from 30 days to 60 days. Outside of the U.S., payment terms are typically 30 days to 90 days, although certain markets have longer payment terms.
See Note 18 for disaggregated revenue disclosures.
Depreciation — Depreciation is provided over the estimated useful lives of the assets, principally using the straight-line method. For tax purposes, accelerated tax methods are used. The estimated useful lives primarily range from 25 to 45 years for Buildings, and from 3 to 15 years for Machinery, equipment and office furnishings. Depreciation expense was $2.1 billion in 2024, $1.8 billion in 2023 and $1.8 billion in 2022.
Advertising and Promotion Costs — Advertising and promotion costs are expensed as incurred. The Company recorded advertising and promotion expenses of $2.4 billion in 2024, $2.3 billion in 2023 and $2.2 billion in 2022.
Software Capitalization — The Company capitalizes certain costs incurred in connection with obtaining or developing internal-use software including external direct costs of material and services, and payroll costs for employees directly involved with the software development. These costs are included in Property, plant and equipment. In addition, the Company capitalizes certain costs incurred to implement a cloud computing arrangement
that is considered a service agreement, which are included in Other Assets. Capitalized software costs are being amortized over periods ranging from 2 to 10 years, with the longer lives generally associated with enterprise-wide projects implemented over multiple years. Costs incurred during the preliminary project stage and post-implementation stage, as well as maintenance and training costs, are expensed as incurred.
Goodwill — Goodwill represents the excess of the consideration transferred over the fair value of net assets acquired in a business combination. Goodwill is assigned to reporting units and evaluated for impairment at least annually, or more frequently if impairment indicators exist, by first assessing qualitative factors to determine whether it is more likely than not that the fair value of a reporting unit is less than its carrying amount. If the Company concludes it is more likely than not that the fair value of a reporting unit is less than its carrying amount, a quantitative fair value test is performed. If the carrying value of a reporting unit is greater than its fair value, a goodwill impairment charge will be recorded for the difference (up to the carrying value of goodwill).
Acquired Intangibles — Intangibles acquired in a business combination include product rights, trade names and patents, licenses and other, which are initially recorded at fair value, assigned an estimated useful life, and amortized primarily on a straight-line basis over their estimated useful lives ranging from 2 to 24 years. The Company periodically evaluates whether current facts or circumstances indicate that the carrying values of its acquired intangibles may not be recoverable. If such circumstances are determined to exist, an estimate of the undiscounted future cash flows of these assets, or appropriate asset groupings, is compared to the carrying value to determine whether an impairment exists. If the asset is determined to be impaired, the loss is measured based on the difference between the carrying value of the intangible asset and its fair value, which is determined based on the net present value of estimated future cash flows.
Acquired In-Process Research and Development — IPR&D that the Company acquires in conjunction with a business combination represents the fair value assigned to incomplete research projects which, at the time of acquisition, have not reached technological feasibility. The amounts are capitalized and are accounted for as indefinite-lived intangible assets, subject to impairment testing until completion or abandonment of the projects. Upon successful completion of each IPR&D project, Merck will make a determination as to the then-useful life of the intangible asset, generally determined by the period in which the substantial majority of the cash flows are expected to be generated, and begin amortization. The Company evaluates IPR&D for impairment at least annually, or more frequently if impairment indicators exist, by performing a quantitative test that compares the fair value of the IPR&D intangible asset with its carrying value. If the fair value is less than the carrying amount, an impairment loss is recognized in operating results.
Contingent Consideration for Business Combinations — Certain of the Company’s acquisitions involve the potential for future payment of consideration that is contingent upon the achievement of performance milestones, including product development milestones and royalty payments on future product sales. If the transaction is accounted for as a business combination, the fair value of contingent consideration liabilities is determined at the acquisition date using unobservable inputs. These inputs include the estimated amount and timing of projected cash flows, the probability of success (achievement of the contingent event) and the risk-adjusted discount rate used to present value the probability-weighted cash flows. Subsequent to the acquisition date, at each reporting period until the contingency is resolved, the contingent consideration liability is remeasured at current fair value with changes (either expense or income) recorded in earnings. Significant events that increase or decrease the probability of achieving development and regulatory milestones or that increase or decrease projected cash flows will result in corresponding increases or decreases in the fair values of the related contingent consideration obligations.
Research and Development — Research and development is expensed as incurred. Nonrefundable advance payments for goods and services that will be used in future research and development activities are expensed when the activity has been performed or when the goods have been received rather than when the payment is made. Research and development expenses include restructuring costs and IPR&D impairment charges. In addition, research and development expenses include expense or income related to changes in the estimated fair value measurement of liabilities for contingent consideration associated with IPR&D assets. Research and development expenses also include upfront and milestone payments related to asset acquisitions and licensing transactions involving clinical development programs that have not yet received regulatory approval.
Collaborative Arrangements — Merck has entered into collaborative arrangements that provide the Company with varying rights to develop, produce and market products together with its collaborative partners. When Merck is the principal on sales transactions with third parties, the Company recognizes sales, cost of sales and selling, general and administrative expenses on a gross basis. Profit sharing amounts it pays to its collaborative partners are recorded within Cost of sales. When the collaborative partner is the principal on sales transactions with third parties, the Company records profit sharing amounts received from its collaborative partners as alliance revenue
(within Sales). Alliance revenue is recorded net of cost of sales and includes an adjustment to share commercialization costs between the partners in accordance with the collaboration agreement. The adjustment is determined by comparing the commercialization costs Merck has incurred directly and reported within Selling, general and administrative expenses with the costs the collaborative partner has incurred. Research and development costs Merck incurs related to collaborations are recorded within Research and development expenses. Cost reimbursements to the collaborative partner or payments received from the collaborative partner to share these costs pursuant to the terms of the collaboration agreements are recorded as increases or decreases to Research and development expenses.
In addition, the terms of the collaboration agreements may require the Company to make payments based upon the achievement of certain developmental, regulatory approval or commercial milestones. Upfront and milestone payments payable by Merck to collaborative partners prior to regulatory approval are expensed as incurred and included in Research and development expenses. Payments due to collaborative partners upon or subsequent to regulatory approval are capitalized and amortized to Cost of sales over the estimated useful life of the corresponding intangible asset, provided that future cash flows support the amounts capitalized. Sales-based milestones payable by Merck to collaborative partners are accrued and capitalized, subject to cumulative amortization catch-up, when determined by the Company to be probable of being achieved based on future sales forecasts. The amortization catch-up is calculated either from the time of the first regulatory approval for products that were unapproved at the time the collaboration was formed or, for new indications of approved products, from the time of the formation of the collaboration. The related intangible asset that is recognized is amortized to Cost of sales over its remaining useful life, subject to impairment testing.
Share-Based Compensation — The Company expenses all share-based payments to employees over the requisite service period based on the grant-date fair value of the awards.
Restructuring Costs — The Company records liabilities for costs associated with exit or disposal activities in the period in which the liability is incurred. In accordance with existing benefit arrangements, future employee termination costs to be incurred in conjunction with involuntary separations are accrued when such separations are probable and estimable. When accruing these costs, the Company will recognize the amount within a range of costs that is the best estimate within the range. When no amount within the range is a better estimate than any other amount, the Company recognizes the minimum amount within the range. Costs for one-time termination benefits in which the employee is required to render service until termination in order to receive the benefits are recognized ratably over the future service period.
Contingencies and Legal Defense Costs — The Company records accruals for contingencies and legal defense costs expected to be incurred in connection with a loss contingency when it is probable that a liability has been incurred and the amount can be reasonably estimated.
Taxes on Income — Deferred taxes are recognized for the future tax effects of temporary differences between financial and income tax reporting based on enacted tax laws and rates. The Company evaluates tax positions to determine whether the benefits of tax positions are more likely than not of being sustained upon audit based on the technical merits of the tax position. For tax positions that are more likely than not of being sustained upon audit, the Company recognizes the amount of the benefit that is greater than 50% likely of being realized upon ultimate settlement in the financial statements. For tax positions that are not more likely than not of being sustained upon audit, the Company does not recognize any portion of the benefit in the financial statements. The Company recognizes interest and penalties associated with uncertain tax positions as a component of Taxes on Income. The Company accounts for the tax effects of the tax on global intangible low-taxed income (GILTI) of certain foreign subsidiaries in the income tax provision in the period the tax arises. The Company’s policy for releasing disproportionate income tax effects from AOCL is to utilize the item-by-item approach.
Reclassifications — Certain reclassifications have been made to prior year amounts to conform to the current year presentation.
Use of Estimates — The consolidated financial statements are prepared in conformity with accounting principles generally accepted in the U.S. (GAAP) and, accordingly, include certain amounts that are based on management’s best estimates and judgments. Estimates are used when accounting for amounts recorded in connection with acquisitions, including initial fair value determinations of assets and liabilities in a business combination (primarily IPR&D, other intangible assets and contingent consideration), as well as subsequent fair value measurements. Additionally, estimates are used in determining such items as provisions for sales discounts, rebates and returns, depreciable and amortizable lives, recoverability of inventories, including those produced in preparation for product launches, amounts recorded for contingencies, environmental liabilities, accruals for contingent sales-
based milestone payments and other reserves, pension and other postretirement benefit plan assumptions, share-based compensation assumptions, restructuring costs, impairments of long-lived assets (including intangible assets and goodwill) and investments, and taxes on income. Because of the uncertainty inherent in such estimates, actual results may differ from these estimates.
Recently Adopted Accounting Standards — In August 2023, the Financial Accounting Standards Board (FASB) issued amended guidance that requires a newly formed joint venture to recognize and initially measure its assets and liabilities at fair value upon formation. The amended guidance includes exceptions to fair value measurement that are consistent with the accounting for business combinations guidance. The Company adopted the guidance effective July 1, 2024 on a prospective basis. There was no impact to the Company’s consolidated financial statements upon adoption.
In November 2023, the FASB issued guidance intended to improve reportable segment disclosure requirements, primarily through expanded disclosures for significant segment expenses. The Company adopted the guidance effective for the 2024 annual period. The guidance resulted in incremental disclosures to the Company’s segment reporting disclosures. See Note 18 for further details.
Recently Issued Accounting Standards Not Yet Adopted — In December 2023, the FASB issued guidance intended to improve the transparency of income tax disclosures by requiring consistent categories and disaggregation of information in the effective income tax rate reconciliation and income taxes paid disclosures by jurisdiction. The guidance also includes other amendments to improve the effectiveness of income tax disclosures by removing certain previously required disclosures. The guidance is effective for 2025 annual reporting. The guidance will result in incremental disclosures within the footnotes to the Company’s financial statements.
In November 2024, the FASB issued guidance intended to improve financial reporting by requiring entities to disclose additional information about specific expense categories at interim and annual reporting periods. The guidance is effective for 2027 annual reporting and 2028 interim reporting. Early adoption is permitted. The guidance, which can be applied on a prospective or retrospective basis, will result in incremental disclosures within the footnotes to the Company’s financial statements.
3. Acquisitions, Divestitures, Research Collaborations and Licensing Agreements
The Company continues to pursue acquisitions and the establishment of external alliances such as research collaborations and licensing agreements to complement its internal research capabilities. These arrangements often include upfront payments; expense reimbursements or payments to the third party; milestone, royalty or profit share arrangements contingent upon the occurrence of certain future events linked to the success of the asset in development; and can also include option and continuation payments. The Company also reviews its marketed products and pipeline to examine candidates which may provide more value through out-licensing and, as part of its portfolio assessment process, may also divest certain assets. Pro forma financial information for acquired businesses is not presented if the historical financial results of the acquired entity are not significant when compared with the Company’s financial results.
Recent Transactions
In January 2025, Merck and WuXi Vaccines, a wholly owned subsidiary of WuXi Biologics, entered into a definitive agreement pursuant to which Merck will acquire WuXi Vaccines’ facility in Dundalk, Ireland for a payment of approximately $440 million at closing. The transaction is expected to close in the first quarter of 2025, subject to the satisfaction of customary closing conditions. There are no future contingent payments associated with the acquisition.
2024 Transactions
In December 2024, Merck closed an exclusive global license to develop, manufacture and commercialize MK-2010 (LM-299), a novel investigational PD-1/vascular endothelial growth factor (VEGF) bispecific antibody from LaNova Medicines Ltd (LaNova). Merck recorded a charge of $588 million to Research and development expenses in 2024 for the upfront payment, which was made in January 2025. LaNova is also eligible to receive $300 million upon technology transfer, which is anticipated to be completed in 2025, as well as future contingent developmental milestone payments of up to $140 million, regulatory milestone payments of up to $860 million and sales-based milestone payments of up to $1.4 billion.
Also in December 2024, Merck closed an exclusive global license to develop, manufacture and commercialize MK-4082 (HS-10535), an investigational preclinical oral small molecule GLP-1 receptor agonist from Hansoh Pharma (Hansoh). Merck recorded a charge of $112 million to Research and development expenses in 2024 for the upfront payment, which was made in February 2025. Hansoh is also eligible to receive future contingent
development-related milestone payments of up to $115 million, regulatory milestone payments of up to $315 million and sales-based milestone payments of up to $1.47 billion, as well as tiered royalties ranging from a high-single-digit rate to a low-double-digit rate on future net sales of MK-4082 (HS-10535), if approved. Under the agreement, Hansoh may co-promote or solely commercialize MK-4082 (HS-10535) in Chinese mainland, Hong Kong and Macau, subject to certain conditions.
In September 2024, Merck acquired MK-1045 (formally CN201), a novel investigational clinical-stage bispecific antibody for the treatment of B-cell associated diseases, from Curon Biopharmaceutical (Curon) for an upfront payment of $700 million. In addition, Curon is eligible to receive future contingent developmental milestone payments of up to $300 million and regulatory milestone payments of up to $300 million. The transaction was accounted for as an asset acquisition. Merck recorded a charge of $750 million (reflecting the upfront payment and other related costs) to Research and development expenses in 2024 related to the execution of the transaction. In connection with the agreement, Merck is also obligated to pay a third party future contingent developmental, regulatory and sales-based milestone payments of up to $128 million in the aggregate, as well as tiered royalties ranging from a mid-single-digit rate to a low-double-digit rate on future net sales of MK-1045, if approved.
In July 2024, Merck acquired the aqua business of Elanco Animal Health Incorporated (Elanco aqua business) for total consideration of $1.3 billion. The Elanco aqua business consists of an innovative portfolio of medicines and vaccines, nutritionals and supplements for aquatic species; two related aqua manufacturing facilities in Canada and Vietnam; as well as a research facility in Chile. The acquisition broadens Animal Health’s aqua portfolio with products, such as Clynav, a new generation DNA-based vaccine that protects Atlantic salmon against pancreas disease, and Imvixa, an anti-parasitic sea lice treatment. This acquisition also brings a portfolio of water treatment products for warm water production, complementing Animal Health’s warm water vaccine portfolio. In addition to these products, the DNA-based vaccine technology that is a part of the business has the potential to accelerate the development of novel vaccines to address the unmet needs of the aqua industry. There are no contingent payments associated with the acquisition, which was accounted for as a business combination.
The estimated fair values of assets acquired and liabilities assumed from the Elanco aqua business are as follows:
| | | | | | | | |
| | July 9, 2024 |
| Inventories | | $ | 65 | |
| Property, plant and equipment | | 66 |
Product rights - Clynav (useful life 15 years) (1) | | 340 |
Other product rights (useful lives 15 years) (1) | | 291 |
| Other assets and liabilities, net | | 23 | |
| Total identifiable net assets | | 785 | |
Goodwill (2) | | 518 |
| Consideration transferred | | $ | 1,303 | |
(1) The estimated fair values of Clynav and other product rights were determined using an income approach, specifically the multi-period excess earnings method. The future probability-weighted net cash flows were discounted to present value utilizing a discount rate of 8.5%. Actual cash flows are likely to be different than those assumed.
(2) The goodwill recognized is largely attributable to anticipated synergies expected to arise after the acquisition and was allocated to the Animal Health segment. The goodwill is expected to be deductible for tax purposes.
Also in July 2024, Merck acquired Eyebiotech Limited (EyeBio), a privately held ophthalmology-focused biotechnology company, for $1.2 billion (including payments to settle share-based equity awards) and also incurred $207 million of transaction costs. The acquisition agreement also provides for former EyeBio shareholders to receive future contingent developmental milestone payments of up to $200 million (of which $100 million was triggered and paid in 2024 as noted below), regulatory milestone payments of up to $1.0 billion and sales-based milestone payments of up to $500 million. EyeBio’s development work focused on candidates for the prevention and treatment of vision loss associated with retinal vascular leakage, a known risk factor for retinal diseases. EyeBio’s lead candidate, MK-3000 (formerly EYE103), is an investigational, potentially first-in-class tetravalent, tri-specific antibody that acts as an agonist of the Wingless-related integration site signaling pathway, which is in clinical development for the treatment of diabetic macular edema and neovascular age-related macular degeneration. The transaction was accounted for as an asset acquisition since MK-3000 accounted for substantially all of the fair value of the gross assets acquired (excluding cash and deferred income taxes). Merck recorded net assets of $21 million, as well as a charge of $1.35 billion to Research and development expenses in 2024 related to the acquisition. Additionally, a $100 million developmental milestone was triggered and paid in 2024 upon initiation of a Phase 2/3 clinical trial
evaluating MK-3000 for the treatment of diabetic macular edema, which was also recorded as a charge to Research and development expenses.
Additionally in July 2024, Merck and Orion Corporation (Orion) announced the mutual exercise of an option to convert the companies’ ongoing co-development and co-commercialization agreement for opevesostat (MK-5684/ODM-208), an investigational cytochrome P450 11A1 (CYP11A1) inhibitor in Phase 3 clinical development, and other candidates targeting CYP11A1, into an exclusive global license for Merck. With the exercise of the option, Merck assumed full responsibility for all past and future development and commercialization expenses associated with the candidates covered by the original agreement entered into in 2022 as discussed below. In addition, Orion became eligible to receive developmental milestone payments of up to $30 million, regulatory milestone payments of up to $625 million and sales-based milestone payments of up to $975 million, as well as annually tiered royalties ranging from a low double-digit rate up to a rate in the low twenties on net sales for any commercialized licensed product. Orion retained responsibility for the manufacture of clinical and commercial supply for Merck. No payment was associated with the exercise of the option, which became effective in September 2024.
Also in July 2024, Merck notified Kelun-Biotech (a holding subsidiary of Sichuan Kelun Pharmaceutical Co., Ltd.) it was terminating the license and collaboration agreement entered into in July 2022 in which Merck gained exclusive worldwide rights for the development, manufacture and commercialization of an investigational antibody drug conjugate (ADC) MK-1200 (SKB315) for the treatment of solid tumors. As a result of this termination, which became effective in September 2024, all rights to SKB315 have reverted to Kelun-Biotech.
In March 2024, Merck acquired Harpoon Therapeutics, Inc. (Harpoon), a clinical-stage immunotherapy company developing a novel class of T-cell engagers designed to harness the power of the body’s immune system to treat patients suffering from cancer and other diseases for $765 million and also incurred $56 million of transaction costs. Harpoon’s lead candidate, MK-6070 (formerly HPN328), is a T-cell engager targeting delta-like ligand 3 (DLL3), an inhibitory canonical Notch ligand that is expressed at high levels in small-cell lung cancer and neuroendocrine tumors. The transaction was accounted for as an asset acquisition since MK-6070 represented substantially all of the fair value of the gross assets acquired (excluding cash and deferred income taxes). Merck recorded net assets of $165 million, as well as a charge of $656 million to Research and development expenses in 2024 related to the transaction. There are no future contingent payments associated with the acquisition. In August 2024, Merck and Daiichi Sankyo expanded their existing global co-development and co-commercialization agreement to include MK-6070. See Note 4 for more information on Merck’s collaboration with Daiichi Sankyo.
2023 Transactions
In October 2023, Merck and Daiichi Sankyo entered into a global development and commercialization agreement for three of Daiichi Sankyo’s deruxtecan (DXd) ADC candidates: patritumab deruxtecan (HER3-DXd) (MK-1022), ifinatamab deruxtecan (I-DXd) (MK-2400) and raludotatug deruxtecan (R-DXd) (MK-5909). See Note 4 for additional information related to this collaboration.
In June 2023, Merck acquired Prometheus Biosciences, Inc. (Prometheus), a clinical-stage biotechnology company pioneering a precision medicine approach for the discovery, development, and commercialization of novel therapeutic and companion diagnostic products for the treatment of immune-mediated diseases. Total consideration paid of $11.0 billion included $1.2 billion of costs to settle share-based equity awards (including $700 million to settle unvested equity awards). Prometheus’ lead candidate, tulisokibart (MK-7240, formerly PRA023), is a humanized monoclonal antibody directed to tumor necrosis factor-like ligand 1A, a target associated with both intestinal inflammation and fibrosis. Tulisokibart is being developed for the treatment of immune-mediated diseases including ulcerative colitis, Crohn’s disease, and other autoimmune conditions. Phase 3 clinical trials evaluating tulisokibart for Crohn’s disease and ulcerative colitis are underway. The transaction was accounted for as an asset acquisition since tulisokibart accounted for substantially all of the fair value of the gross assets acquired (excluding cash and deferred income taxes). Merck recorded net assets of $877 million, including cash of $368 million, investments of $296 million, deferred tax assets of $218 million and other net liabilities of $5 million, as well as a charge of $10.2 billion to Research and development expenses in 2023 related to the transaction. There are no future contingent payments associated with the acquisition.
In February 2023, Merck and Kelun-Biotech closed a license and collaboration agreement expanding their relationship in which Merck gained exclusive rights for the research, development, manufacture and commercialization of up to seven investigational preclinical ADCs for the treatment of cancer. Kelun-Biotech retained the right to research, develop, manufacture and commercialize certain licensed and option ADCs for Chinese mainland, Hong Kong and Macau. Merck made an upfront payment of $175 million, which was recorded as a charge to Research and development expenses in 2023. In October 2023, Merck notified Kelun-Biotech it was terminating two of the seven candidates under the agreement. Subsequently, in April 2024, Merck notified Kelun-Biotech it was terminating one additional candidate under the agreement. In July 2024, Merck notified Kelun-Biotech that it was
exercising an existing license option for one of the candidates under the agreement, granting Merck a license for the development, manufacture and commercialization worldwide excluding China. There are now three candidates licensed under the original agreement and one candidate for which the license option remains unexercised. Merck paid Kelun-Biotech $38 million in connection with the July option exercise, following which Kelun-Biotech remains eligible to receive future contingent payments aggregating up to $540 million in development-related payments, $1.5 billion in regulatory milestones, and $3.1 billion in sales-based milestones, if Kelun-Biotech does not retain Chinese mainland, Hong Kong and Macau rights for the remaining option ADC and all remaining candidates achieve regulatory approval. In addition, Kelun-Biotech is eligible to receive tiered royalties ranging from a mid-single-digit rate to a low-double-digit rate on future net sales for any commercialized ADC product. Also, in connection with the agreement, Merck invested $100 million in Kelun-Biotech shares in January 2023.
In January 2023, Merck acquired Imago BioSciences, Inc. (Imago), a clinical-stage biopharmaceutical company developing new medicines for the treatment of myeloproliferative neoplasms and other bone marrow diseases, for $1.35 billion (including payments to settle share-based equity awards) and also incurred approximately $60 million of transaction costs. Imago’s lead candidate, bomedemstat (MK-3543, formerly IMG-7289), which is in Phase 3 clinical development, is an investigational orally available lysine-specific demethylase 1 inhibitor currently being evaluated in multiple clinical trials for the treatment of essential thrombocythemia, myelofibrosis, and polycythemia vera, in addition to other indications. The transaction was accounted for as an asset acquisition since bomedemstat represented substantially all of the fair value of the gross assets acquired (excluding cash and deferred income taxes). Merck recorded net assets of $219 million, as well as a charge of $1.2 billion to Research and development expenses in 2023 related to the transaction. There are no future contingent payments associated with the acquisition.
2022 Transactions
In October 2022, Merck and Royalty Pharma plc (Royalty Pharma) entered into a funding arrangement under which Royalty Pharma paid Merck $50 million to co-fund Merck’s development costs for a Phase 2b trial of MK-8189, an investigational oral phosphodiesterase 10A (PDE10A) inhibitor, which was being evaluated for the treatment of schizophrenia. As Royalty Pharma was sharing the risk of technical and regulatory success with Merck, the development funding was recognized by Merck as an obligation to perform contractual services. Accordingly, the payment received was recognized by Merck as a reduction to Research and development expenses ratably over the estimated Phase 2b research period. In 2024, it was determined the Phase 2b clinical trial for MK-8189 as a monotherapy for acute schizophrenia did not meet its primary efficacy endpoint; therefore, further development in schizophrenia, bipolar, and dementia indications has stopped, and the funding arrangement was terminated.
In September 2022, Merck exercised its option to jointly develop and commercialize V940 (mRNA-4157), an investigational individualized neoantigen therapy, pursuant to the terms of an existing collaboration and license agreement with Moderna, Inc. (Moderna). See Note 4 for additional information related to this collaboration.
In August 2022, Merck and Orna Therapeutics (Orna), a biotechnology company pioneering a new investigational class of engineered circular RNA (oRNA) therapies, entered into a collaboration agreement to discover, develop, and commercialize multiple programs, including vaccines and therapeutics in the areas of infectious disease and oncology. Under the terms of the agreement, Merck made an upfront payment to Orna of $150 million, which was recorded as a charge to Research and development expenses in 2022. In addition, Orna is eligible to receive future contingent payments aggregating up to $440 million in development-related payments, $675 million in regulatory milestones, and $2.4 billion in sales-based milestones associated with the progress of the multiple vaccine and therapeutic programs, as well as royalties ranging from a high-single-digit rate to a low-double-digit rate on any approved products derived from the collaboration. Merck also invested $100 million in Orna’s Series B preferred shares in 2022.
In July 2022, Merck and Orion Corporation (Orion) announced a global co-development and co-commercialization agreement for Orion’s investigational candidate opevesostat (MK-5684/ODM-208) and other drugs targeting cytochrome P450 11A1 (CYP11A1), an enzyme important in steroid production. Merck made an upfront payment to Orion of $290 million, which was recorded as a charge to Research and development expenses in 2022. Orion is responsible for the manufacture of clinical and commercial supply of opevesostat. In addition, the contract provided both parties with an option to convert the initial co-development and co-commercialization agreement into a global exclusive license to Merck, which was mutually exercised in July 2024 (as noted above).
In May 2022, in connection with an existing arrangement, Merck exercised its option to obtain an exclusive license outside of Chinese mainland, Hong Kong, Macau and Taiwan for the development, manufacture and commercialization of Kelun-Biotech’s trophoblast antigen 2 (TROP2)-targeting ADC programs, including its lead compound, sacituzumab tirumotecan (MK-2870/SKB-264), which is currently in Phase 3 clinical development. Under the terms of the agreement, Merck and Kelun-Biotech are collaborating on certain early clinical development plans,
including evaluating the potential of sacituzumab tirumotecan as a monotherapy and in combination with Keytruda for advanced solid tumors. Upon option exercise, Merck made a payment of $30 million, which was recorded as a charge to Research and development expenses in 2022. Additionally, Merck made an additional payment of $25 million upon technology transfer in 2023. Merck has also made all contingent developmental milestone payments under the agreement, which aggregated $90 million, nearly all of which were paid in 2024 and were recorded to Research and development expenses. In addition, Kelun-Biotech is eligible to receive future contingent milestone payments (which include all program compounds) aggregating up to $290 million in first commercial sale milestones, and $780 million in sales-based milestones. The agreement also provides for Merck to pay tiered royalties ranging from a mid-single-digit rate to a low-double-digit rate on future net sales.
Spin-Off of Organon & Co.
In connection with the 2021 spin-off of Organon & Co. (Organon), Merck and Organon entered into a series of interim operating agreements pursuant to which in various jurisdictions where Merck held licenses, permits and other rights in connection with marketing, import and/or distribution of Organon products prior to the separation, Merck continued to market, import and distribute such products on behalf of Organon until such time as the relevant licenses and permits transferred to Organon, with Organon receiving all of the economic benefits and burdens of such activities. As of December 31, 2024, only one jurisdiction remains under an interim operating agreement. Additionally, Merck and Organon entered into a number of manufacturing and supply agreements (MSAs) with terms ranging from four years to ten years. The amounts included in the consolidated statement of income for the above MSAs include sales of $392 million, $394 million and $383 million in 2024, 2023 and 2022, respectively, and related cost of sales of $390 million, $422 million and $404 million in 2024, 2023 and 2022, respectively. The amounts due from Organon under all spin-off related agreements were $330 million and $632 million at December 31, 2024 and 2023, respectively, and are reflected in Other current assets. The amounts due to Organon under these agreements were $113 million and $598 million at December 31, 2024 and 2023, respectively, and are included in Accrued and other current liabilities.
4. Collaborative Arrangements
Merck has entered into collaborative arrangements that provide the Company with varying rights to develop, produce and market products together with its collaborative partners. Both parties in these arrangements are active participants and exposed to significant risks and rewards dependent on the commercial success of the activities of the collaboration. Merck’s more significant collaborative arrangements are discussed below.
AstraZeneca PLC
In 2017, Merck and AstraZeneca PLC (AstraZeneca) entered into a global strategic oncology collaboration to co-develop and co-commercialize AstraZeneca’s Lynparza (olaparib) for multiple cancer types. Independently, Merck and AstraZeneca are developing and commercializing Lynparza in combinations with their respective PD-1 and PD-L1 medicines, Keytruda and Imfinzi. The companies are also jointly developing and commercializing AstraZeneca’s Koselugo (selumetinib) for multiple indications. Under the terms of the agreement, AstraZeneca and Merck share the development and commercialization costs for Lynparza and Koselugo monotherapy and non-PD-1/PD-L1 combination therapy opportunities.
Profits from Lynparza and Koselugo product sales generated through monotherapies or combination therapies are shared equally. AstraZeneca is the principal on Lynparza and Koselugo sales transactions. Merck records its share of Lynparza and Koselugo product sales, net of cost of sales and commercialization costs, as alliance revenue, and its share of development costs associated with the collaboration as part of Research and development expenses. Reimbursements received from AstraZeneca for research and development expenses are recognized as reductions to Research and development costs.
As part of the agreement, Merck made an upfront payment to AstraZeneca and also made payments over a multi-year period for certain license options. In addition, the agreement provides for contingent payments from Merck to AstraZeneca related to the successful achievement of sales-based and regulatory milestones.
In 2024, sales of Koselugo triggered a $100 million sales-based milestone payment from Merck to AstraZeneca. Accordingly, Merck recorded a $100 million liability (which remained accrued at December 31, 2024 and was subsequently paid in January 2025) and a corresponding increase to the intangible asset related to Koselugo. Merck also recognized $48 million of cumulative amortization catch-up expense related to the recognition of this milestone in 2024. Merck made a sales-based milestone payment to AstraZeneca of $400 million in 2022 (which had been previously accrued for). Additionally, in 2022, Merck determined it was probable that sales of Lynparza in the future would trigger a $600 million sales-based milestone payment from Merck to AstraZeneca. Accordingly, Merck recorded a $600 million liability (which remained accrued at December 31, 2024 and was
subsequently paid in January 2025) and a corresponding increase to the intangible asset related to Lynparza. Merck also recognized $250 million of cumulative amortization catch-up expense related to the recognition of this milestone in 2022. Potential future sales-based milestone payments of $2.0 billion have not yet been accrued as they are not deemed by the Company to be probable at this time.
Lynparza received regulatory approvals triggering capitalized milestone payments from Merck to AstraZeneca of $245 million, $105 million and $250 million in 2024, 2023 and 2022, respectively (each of which had been previously accrued for). In 2024, the partners agreed that no future regulatory milestone payments from Merck to AstraZeneca are likely under the agreement.
The intangible asset balances related to Lynparza (which includes capitalized sales-based and regulatory milestone payments) and Koselugo (which reflects the 2024 capitalized sales-based milestone payment) were $1.2 billion and $49 million, respectively, at December 31, 2024 and are included in Other Intangibles, Net. The assets are being amortized over their estimated useful lives (through 2028 for Lynparza and through 2029 for Koselugo) as supported by projected future cash flows, subject to impairment testing.
Summarized financial information related to this collaboration is as follows:
| | | | | | | | | | | | | | | | | |
| Years Ended December 31 | 2024 | | 2023 | | 2022 |
| Alliance revenue - Lynparza | $ | 1,311 | | | $ | 1,199 | | | $ | 1,116 | |
| Alliance revenue - Koselugo | 170 | | | 97 | | | 54 | |
| Total alliance revenue | $ | 1,481 | | | $ | 1,296 | | | $ | 1,170 | |
| | | | | |
Cost of sales (1) | 378 | | | 311 | | | 492 | |
| Selling, general and administrative | 165 | | | 192 | | | 185 | |
| Research and development | 77 | | | 79 | | | 106 | |
| | | | | |
| December 31 | 2024 | | 2023 | | |
Receivables from AstraZeneca included in Other current assets | $ | 424 | | | $ | 341 | | | |
Payables to AstraZeneca included in Accrued and other current liabilities (2) | 713 | | | 256 | | | |
Payables to AstraZeneca included in Other Noncurrent Liabilities (2) | — | | | 600 | | | |
(1) Represents amortization of capitalized milestone payments. Amounts in 2024 and 2022 include $48 million and $250 million, respectively, of cumulative amortization catch-up expense as noted above.
(2) Includes accrued milestone payments.
Eisai Co., Ltd.
In 2018, Merck and Eisai Co., Ltd. (Eisai) announced a strategic collaboration for the worldwide co-development and co-commercialization of Lenvima (lenvatinib), an orally available tyrosine kinase inhibitor discovered by Eisai. Under the agreement, Merck and Eisai are developing and commercializing Lenvima jointly, both as monotherapy and in combination with Keytruda. Eisai records Lenvima product sales globally (Eisai is the principal on Lenvima sales transactions) and Merck and Eisai share applicable profits equally. Merck records its share of Lenvima product sales, net of cost of sales and commercialization costs, as alliance revenue. Expenses incurred during co-development are shared by the two companies in accordance with the collaboration agreement and reflected in Research and development expenses. Certain expenses incurred solely by Merck or Eisai are not shareable under the collaboration agreement, including costs incurred in excess of agreed upon caps and costs related to certain combination studies of Keytruda and Lenvima.
Under the agreement, Merck made an upfront payment to Eisai and also made payments over a multi-year period for certain option rights. In addition, the agreement provides for contingent payments from Merck to Eisai related to the successful achievement of sales-based and regulatory milestones.
Merck made sales-based milestone payments to Eisai aggregating $125 million, $125 million and $600 million in 2024, 2023 and 2022, respectively. In 2023, Merck determined it was probable that sales of Lenvima in the future would trigger $250 million of sales-based milestone payments from Merck to Eisai. Accordingly, Merck recorded $250 million of liabilities (of which $125 million was subsequently paid in each of 2024 and 2023 as noted above) and corresponding increases to the intangible asset related to Lenvima. Merck also recognized $154 million of cumulative amortization catch-up expense related to the recognition of these milestones in 2023. Potential future sales-based milestone payments of $2.3 billion have not yet been accrued as they are not deemed by the Company to be probable at this time.
In 2022, Lenvima received regulatory approvals triggering capitalized milestone payments of $50 million from Merck to Eisai. There are no regulatory milestone payments remaining under the agreement.
The intangible asset balance related to Lenvima (which includes capitalized sales-based and regulatory milestone payments) was $442 million at December 31, 2024 and is included in Other Intangibles, Net. The amount is being amortized over its estimated useful life through 2026 as supported by projected future cash flows, subject to impairment testing.
Summarized financial information related to this collaboration is as follows:
| | | | | | | | | | | | | | | | | |
| Years Ended December 31 | 2024 | | 2023 | | 2022 |
| Alliance revenue - Lenvima | $ | 1,010 | | | $ | 960 | | | $ | 876 | |
| | | | | |
Cost of sales (1) | 241 | | | 381 | | | 212 | |
| Selling, general and administrative | 159 | | | 189 | | | 158 | |
| Research and development | 21 | | | 66 | | | 136 | |
| | | | | |
| December 31 | 2024 | | 2023 | | |
Receivables from Eisai included in Other current assets | $ | 257 | | | $ | 226 | | | |
Payables to Eisai included in Accrued and other current liabilities (2) | — | | | 125 | | | |
(1) Represents amortization of capitalized milestone payments. Amount in 2023 includes $154 million of cumulative amortization catch-up expense as noted above.
(2) Represents an accrued milestone payment.
Bayer AG
In 2014, the Company entered into a worldwide clinical development collaboration with Bayer AG (Bayer) to market and develop soluble guanylate cyclase (sGC) modulators including Bayer’s Adempas (riociguat) and Verquvo (vericiguat). The two companies have implemented a joint development and commercialization strategy. Under the agreement, Bayer commercializes Adempas in the Americas, while Merck commercializes in the rest of the world. For Verquvo, Merck commercializes in the U.S. and Bayer commercializes in the rest of the world. Both companies share in development costs and profits on sales. Merck records sales of Adempas and Verquvo in its marketing territories, as well as alliance revenue. Alliance revenue represents Merck’s share of profits from sales of Adempas and Verquvo in Bayer’s marketing territories, which are product sales net of cost of sales and commercialization costs. Cost of sales includes Bayer’s share of profits from sales in Merck’s marketing territories.
In addition, the agreement provided for contingent payments from Merck to Bayer related to the successful achievement of sales-based milestones. In 2022, Merck made the final $400 million sales-based milestone payment under this collaboration to Bayer.
The intangible asset balances related to Adempas (which includes the acquired intangible asset balance, as well as capitalized sales-based milestone payments attributed to Adempas) and Verquvo (which reflects the portion of the final sales-based milestone payment that was attributed to Verquvo) were $372 million and $42 million, respectively, at December 31, 2024 and are included in Other Intangibles, Net. The assets are being amortized over their estimated useful lives (through 2027 for Adempas and through 2031 for Verquvo) as supported by projected future cash flows, subject to impairment testing.
Summarized financial information related to this collaboration is as follows:
| | | | | | | | | | | | | | | | | |
| Years Ended December 31 | 2024 | | 2023 | | 2022 |
| Alliance revenue - Adempas/Verquvo | $ | 415 | | | $ | 367 | | | $ | 341 | |
| Net sales of Adempas recorded by Merck | 287 | | | 255 | | | 238 | |
| Net sales of Verquvo recorded by Merck | 37 | | | 36 | | | 22 | |
| Total sales | $ | 739 | | | $ | 658 | | | $ | 601 | |
| | | | | |
Cost of sales (1) | 244 | | | 224 | | | 210 | |
| Selling, general and administrative | 111 | | | 131 | | | 153 | |
| Research and development | 102 | | | 90 | | | 75 | |
| | | | | |
| December 31 | 2024 | | 2023 | | |
Receivables from Bayer included in Other current assets | $ | 160 | | | $ | 156 | | | |
Payables to Bayer included in Accrued and other current liabilities | 82 | | | 80 | | | |
(1) Includes amortization of intangible assets, cost of products sold by Merck, as well as Bayer’s share of profits from sales in Merck’s marketing territories.
Ridgeback Biotherapeutics LP
In 2020, Merck and Ridgeback Biotherapeutics LP (Ridgeback), a closely held biotechnology company, entered into a collaboration agreement to develop Lagevrio (molnupiravir), an investigational orally available antiviral candidate for the treatment of patients with COVID-19. Merck gained exclusive worldwide rights to develop and commercialize Lagevrio and related molecules. Following initial authorizations in certain markets in 2021, Lagevrio has since received multiple additional authorizations.
Under the terms of the agreement, Ridgeback received an upfront payment and is eligible to receive future contingent payments dependent upon the achievement of certain developmental and regulatory approval milestones. The agreement also provides for Merck to reimburse Ridgeback for a portion of certain third-party contingent milestone payments and royalties on net sales, which is part of the profit-sharing calculation. Merck is the principal on sales transactions, recognizing sales and related costs, with profit-sharing amounts recorded within Cost of sales. Profits from the collaboration are split equally between the partners. Reimbursements from Ridgeback for its share of research and development costs (deducted from Ridgeback’s share of profits) are reflected as decreases to Research and development expenses.
Summarized financial information related to this collaboration is as follows:
| | | | | | | | | | | | | | | | | |
| Years Ended December 31 | 2024 | | 2023 | | 2022 |
Net sales of Lagevrio recorded by Merck | $ | 964 | | | $ | 1,428 | | | $ | 5,684 | |
| | | | | |
Cost of sales (1) | 554 | | | 852 | | | 3,038 | |
| Selling, general and administrative | 57 | | | 97 | | | 147 | |
| Research and development | 13 | | | 60 | | | 88 | |
| | | | | |
| December 31 | 2024 | | 2023 | | |
Payables to Ridgeback included in Accrued and other current liabilities (2) | $ | 68 | | | $ | 113 | | | |
(1) Includes cost of products sold by Merck, Ridgeback’s share of profits, royalty expense, amortization of capitalized milestone payments and inventory reserves.
(2) Includes accrued royalties.
Daiichi Sankyo
In October 2023, Merck and Daiichi Sankyo entered into a global development and commercialization agreement for three of Daiichi Sankyo’s DXd ADC candidates: patritumab deruxtecan (HER3-DXd) (MK-1022), ifinatamab deruxtecan (I-DXd) (MK-2400) and raludotatug deruxtecan (R-DXd) (MK-5909). All three potentially first-in-class DXd ADCs are in various stages of clinical development for the treatment of multiple solid tumors both as monotherapy and/or in combination with other treatments. The companies will jointly develop and potentially commercialize these ADC candidates worldwide, except in Japan where Daiichi Sankyo will maintain exclusive rights. Daiichi Sankyo will be solely responsible for manufacturing and supply.
Under the terms of the agreement, Merck made payments to Daiichi Sankyo totaling $4.0 billion in 2023. These payments included $1.0 billion ($500 million each for patritumab deruxtecan and ifinatamab deruxtecan) which may be refundable on a pro-rated basis in the event of early termination of development with respect to either program. In addition, the agreement provided for a continuation payment of $750 million related to patritumab deruxtecan, which Merck paid in October 2024, and a continuation payment of $750 million related to raludotatug deruxtecan due from Merck in October 2025. If Merck does not make the remaining continuation payment for raludotatug deruxtecan, the rights for that program will revert to Daiichi Sankyo and the non-refundable upfront payments already paid will be retained by Daiichi Sankyo. The agreement also provides for contingent payments from Merck to Daiichi Sankyo of up to an additional $5.5 billion for each DXd ADC upon the successful achievement of certain sales-based milestones.
In conjunction with this transaction, Merck recorded an aggregate pretax charge of $5.5 billion to Research and development expenses in 2023 for the $4.0 billion of upfront payments and the $1.5 billion of continuation payments. Merck determined it was appropriate to expense the $1.0 billion refundable portion of the consideration in 2023 because of the significant number of clinical studies that were underway and planned in the near future, as well as certain studies in advanced stages, making it highly likely that the programs would continue to progress and incur substantial expenses, and therefore the likelihood of the programs terminating before the end of the refundable period was deemed remote. Merck also determined that it was appropriate to expense the continuation payments upon execution of the agreement because such payments do not result in the Company gaining any additional intellectual property rights. In addition, the significant number of ongoing and planned clinical studies and the short-term nature of the option period makes the likelihood of Merck not making these payments remote.
Merck and Daiichi Sankyo equally share research and development costs, except for raludotatug deruxtecan, where Merck is responsible for 75% of the first $2.0 billion of research and development expenses. Merck includes its share of development costs associated with the collaboration as part of Research and development expenses. Following regulatory approval, Daiichi Sankyo will generally record sales worldwide (Daiichi Sankyo will be the principal on sales transactions) and the companies will equally share expenses as well as profits worldwide except for Japan where Daiichi Sankyo retains exclusive rights and Merck will receive a 5% sales-based royalty. Merck will record its share of product sales, net of cost of sales and commercialization costs, as alliance revenue.
In August 2024, Merck and Daiichi Sankyo expanded their agreement to include MK-6070, an investigational delta-like ligand 3 (DLL3) targeting T-cell engager, which Merck obtained through its acquisition of Harpoon (see Note 3). The companies are planning to evaluate MK-6070 in combination with ifinatamab deruxtecan in certain patients with SCLC, as well as other potential combinations. Merck received an upfront cash payment of $170 million from Daiichi Sankyo (recorded within Other (income) expense, net) and has also satisfied a contingent quid obligation from the original collaboration agreement. The companies will jointly develop and commercialize MK-6070 worldwide and share research and development and commercialization expenses. Research and development expenses related to MK-6070 in combination with ifinatamab deruxtecan will be shared in a manner consistent with the original agreement for ifinatamab deruxtecan. Merck will be solely responsible for manufacturing and supply of MK-6070. If approved, Merck will generally record sales for MK-6070 worldwide (Merck will be the principal on sales transactions) and the companies will equally share expenses as well as profits worldwide, except for Japan where Merck retains exclusive rights and Daiichi Sankyo will receive a 5% sales-based royalty.
Summarized financial information related to this collaboration is as follows:
| | | | | | | | | | | | | |
| Years Ended December 31 | 2024 | | 2023 | | |
| Selling, general and administrative | $ | 26 | | | $ | 3 | | | |
Research and development (1) | 351 | | | 5,549 | | | |
| | | | | |
| December 31 | 2024 | | 2023 | | |
Receivables from Daiichi Sankyo included in Other current assets | $ | 8 | | | $ | — | | | |
Payables to Daiichi Sankyo included in Accrued and other current liabilities (2) | 817 | | | 800 | | | |
Payables to Daiichi Sankyo included in Other Noncurrent Liabilities (2) | — | | | 750 | | | |
(1) Expenses in 2023 include the $5.5 billion charge for the upfront and continuing option payments noted above.
(2) Includes accrued continuation payment.
Moderna, Inc.
In 2022, Merck exercised its option to jointly develop and commercialize V940 (mRNA-4157), an investigational individualized neoantigen therapy, pursuant to the terms of an existing collaboration and license agreement with Moderna, Inc. (Moderna), which resulted in a $250 million payment that was charged to Research and development expenses in 2022. V940 (mRNA-4157) is currently being evaluated in combination with Keytruda in multiple Phase 3 clinical trials. Merck and Moderna share costs and will share any profits equally under this worldwide collaboration. Merck records its share of development costs associated with the collaboration as part of Research and development expenses. Any reimbursements received from Moderna for research and development expenses are recognized as reductions to Research and development costs. Merck has also capitalized certain of the shared costs, mainly related to facility costs, which aggregated $198 million at December 31, 2024 and will be amortized over the assets’ estimated useful lives.
Summarized financial information related to this collaboration is as follows:
| | | | | | | | | | | | | | | | | |
| Years Ended December 31 | 2024 | | 2023 | | 2022 |
| Selling, general and administrative | $ | 16 | | | $ | 5 | | | $ | — | |
Research and development (1) | 358 | | | 218 | | | 288 | |
| | | | | |
| December 31 | 2024 | | 2023 | | |
Payables to Moderna included in Accrued and other current liabilities | $ | 57 | | | $ | 63 | | | |
(1) Expenses in 2022 include the $250 million option exercise payment noted above.
Bristol-Myers Squibb Company
Reblozyl (luspatercept-aamt) is a first-in-class erythroid maturation recombinant fusion protein that is being commercialized through a global collaboration with Bristol-Myers Squibb Company (BMS). Reblozyl is approved in the U.S., Europe, and certain other markets for the treatment of anemia in certain rare blood disorders and is also being evaluated for additional indications for hematology therapies. BMS is the principal on sales transactions for Reblozyl; however, Merck co-promotes Reblozyl (and may co-promote any future products approved under this collaboration) in North America, which is reimbursed by BMS. Merck receives tiered royalties ranging from 20% to 24% based on sales levels. This royalty will be reduced by 50% upon the earlier of patent expiry or generic entry on an indication-by-indication basis in each market. Additionally, Merck is eligible to receive future contingent sales-based milestone payments of up to $80 million. Alliance revenue related to this collaboration (recorded within Sales) consists of royalties and, for 2022, also includes the receipt of a regulatory approval milestone payment of $20 million. Merck recorded alliance revenue related to this collaboration of $371 million in 2024, $212 million in 2023 and $166 million in 2022.
5. Restructuring
In January 2024, the Company approved a new restructuring program (2024 Restructuring Program) intended to continue the optimization of the Company’s Human Health global manufacturing network as the future pipeline shifts to new modalities and also optimize the Animal Health global manufacturing network to improve supply reliability and increase efficiency. The actions contemplated under the 2024 Restructuring Program are expected to be substantially completed by the end of 2031, with the cumulative pretax costs to be incurred by the Company to implement the program estimated to be approximately $4.0 billion. Approximately 60% of the cumulative pretax costs will be non-cash, relating primarily to the accelerated depreciation of facilities to be closed or divested. The remainder of the costs will result in cash outlays, relating primarily to facility shut-down costs. The Company recorded total pretax costs of $888 million and $190 million in 2024 and 2023, respectively, related to the 2024 Restructuring Program, bringing total cumulative pretax costs incurred through December 31, 2024 to $1.1 billion.
In 2019, Merck approved a global restructuring program (2019 Restructuring Program) as part of a worldwide initiative focused on optimizing the Company’s manufacturing and supply network, as well as reducing its global real estate footprint. The Company recorded total pretax costs of $743 million in 2023 and $666 million in 2022 related to the 2019 Restructuring Program. The actions under the 2019 Restructuring Program were substantially complete at the end of 2023 and, as of January 1, 2024, any remaining activities are being accounted for as part of the 2024 Restructuring Program.
For segment reporting, restructuring charges are unallocated expenses.
The following table summarizes the charges related to the restructuring programs by type of cost:
| | | | | | | | | | | | | | | | | | | | | | | |
| Accelerated
Depreciation | | Separation
Costs | | Other Exit Costs | | Total |
| Year Ended December 31, 2024 | | | | | | | |
| 2024 Restructuring Program | | | | | | | |
| Cost of sales | $ | 254 | | | $ | — | | | $ | 241 | | | $ | 495 | |
| Selling, general and administrative | — | | | — | | | 83 | | | 83 | |
| Research and development | — | | | — | | | 1 | | | 1 | |
| Restructuring costs | — | | | 122 | | | 187 | | | 309 | |
| $ | 254 | | | $ | 122 | | | $ | 512 | | | $ | 888 | |
| Year Ended December 31, 2023 | | | | | | | |
| 2024 Restructuring Program | | | | | | | |
| Cost of sales | $ | — | | | $ | — | | | $ | 62 | | | $ | 62 | |
| Restructuring costs | — | | | 115 | | | 13 | | | 128 | |
| — | | | 115 | | | 75 | | | 190 | |
| 2019 Restructuring Program | | | | | | | |
| Cost of sales | 131 | | | — | | | 18 | | | 149 | |
| Selling, general and administrative | 9 | | | — | | | 113 | | | 122 | |
| Research and development | — | | | — | | | 1 | | | 1 | |
| Restructuring costs | — | | | 339 | | | 132 | | | 471 | |
| | 140 | | | 339 | | | 264 | | | 743 | |
| $ | 140 | | | $ | 454 | | | $ | 339 | | | $ | 933 | |
| Year Ended December 31, 2022 | | | | | | | |
| 2019 Restructuring Program | | | | | | | |
| Cost of sales | $ | 72 | | | $ | — | | | $ | 133 | | | $ | 205 | |
| Selling, general and administrative | 19 | | | — | | | 75 | | | 94 | |
| Research and development | 29 | | | — | | | 1 | | | 30 | |
| Restructuring costs | — | | | 212 | | | 125 | | | 337 | |
| | $ | 120 | | | $ | 212 | | | $ | 334 | | | $ | 666 | |
Accelerated depreciation costs primarily relate to manufacturing, research and administrative facilities and equipment to be sold or closed as part of the programs. Accelerated depreciation costs represent the difference between the depreciation expense to be recognized over the revised useful life of the asset, based upon the anticipated date the site will be closed or divested or the equipment disposed of, and depreciation expense as determined utilizing the useful life prior to the restructuring actions. All the sites will continue to operate up through the respective closure dates and, since future undiscounted cash flows are sufficient to recover the respective book values, Merck is recording accelerated depreciation over the revised useful life of the site assets. Anticipated site closure dates, particularly related to manufacturing locations, have been and may continue to be adjusted to reflect changes resulting from regulatory or other factors.
Separation costs are associated with actual headcount reductions, as well as involuntary headcount reductions which were probable and could be reasonably estimated.
Other exit costs in 2024, 2023 and 2022 include asset impairment, facility shut-down and other related costs, as well as pretax gains and losses resulting from the sales of facilities and related assets. Additionally, other activity includes certain employee-related costs associated with pension and other postretirement benefit plans (see Note 13) and share-based compensation.
The following table summarizes the charges and spending relating to restructuring program activities:
| | | | | | | | | | | | | | | | | | | | | | | |
| Accelerated
Depreciation | | Separation
Costs | | Other Exit Costs | | Total |
| Restructuring reserves January 1, 2023 | $ | — | | | $ | 479 | | | $ | 34 | | | $ | 513 | |
| Expenses | 140 | | | 454 | | | 339 | | | 933 | |
| (Payments) receipts, net | — | | | (252) | | | (158) | | | (410) | |
| Non-cash activity | (140) | | | — | | | (184) | | | (324) | |
| Restructuring reserves December 31, 2023 | — | | | 681 | | | 31 | | | 712 | |
| Expenses | 254 | | | 122 | | | 512 | | | 888 | |
| (Payments) receipts, net | — | | | (239) | | | (206) | | | (445) | |
| Non-cash activity | (254) | | | — | | | (337) | | | (591) | |
| Restructuring reserves December 31, 2024 | $ | — | | | $ | 564 | | | $ | — | | | $ | 564 | |
6. Financial Instruments
Derivative Instruments and Hedging Activities
The Company manages the impact of foreign exchange rate movements and interest rate movements on its earnings, cash flows and fair values of assets and liabilities through operational means and through the use of various financial instruments, including derivative instruments.
A significant portion of the Company’s revenues and earnings in foreign affiliates is exposed to changes in foreign exchange rates. The objectives of and accounting related to the Company’s foreign currency risk management program, as well as its interest rate risk management activities are discussed below.
Foreign Currency Risk Management
The Company has established revenue hedging, balance sheet risk management and net investment hedging programs to protect against volatility of future foreign currency cash flows and changes in fair value caused by changes in foreign exchange rates.
The objective of the revenue hedging program is to reduce the variability caused by changes in foreign exchange rates that would affect the U.S. dollar value of future cash flows derived from foreign currency denominated sales, primarily the euro, Japanese yen and Chinese renminbi. To achieve this objective, the Company will hedge a portion of its forecasted foreign currency denominated third-party and intercompany distributor entity sales (forecasted sales) that are expected to occur over its planning cycle, typically no more than two years into the future. The Company will layer in hedges over time, increasing the portion of forecasted sales hedged as it gets closer to the expected date of the forecasted sales. The portion of forecasted sales hedged is based on assessments of cost-benefit profiles that consider natural offsetting exposures, revenue and foreign exchange rate volatilities and correlations, and the cost of hedging instruments. The Company manages its anticipated transaction exposure principally with purchased local currency put options, forward contracts, and purchased collar options.
The fair values of these derivative contracts are recorded as either assets (gain positions) or liabilities (loss positions) in the Consolidated Balance Sheet. Changes in the fair value of derivative contracts are recorded each period in either current earnings or OCI depending on whether the derivative is designated as part of a hedge transaction and, if so, the type of hedge transaction. For derivatives that are designated as cash flow hedges, the unrealized gains or losses on these contracts are recorded in AOCL and reclassified into Sales when the hedged anticipated revenue is recognized. The amount reclassified into earnings as a result of the discontinuation of cash flow hedges because it was no longer deemed probable the forecasted hedged transactions would occur was not material for the years ended December 31, 2024, 2023 or 2022. For those derivatives which are not designated as cash flow hedges, but serve as economic hedges of forecasted sales, unrealized gains or losses are recorded in Sales each period. The cash flows from both designated and non-designated contracts are reported as operating activities in the Consolidated Statement of Cash Flows. The Company does not enter into derivatives for trading or speculative purposes.
The Company manages operating activities and net asset positions at each local subsidiary in order to mitigate the effects of foreign exchange on monetary assets and liabilities. Monetary assets and liabilities denominated in a currency other than the functional currency of a given subsidiary are remeasured at spot rates in effect on the balance sheet date with the effects of changes in spot rates reported in Other (income) expense, net. The Company also uses a balance sheet risk management program to mitigate the exposure of such assets and liabilities from the effects of volatility in foreign exchange. Merck principally utilizes forward exchange contracts to
offset the effects of foreign exchange on exposures when it is deemed economical to do so based on a cost-benefit analysis that considers the magnitude of the exposure, the volatility of the foreign exchange rate and the cost of the hedging instrument (primarily the euro, Swiss franc, Japanese yen, and Chinese renminbi). The forward contracts are not designated as hedges and are marked to market through Other (income) expense, net. Accordingly, fair value changes in the forward contracts help mitigate the changes in the value of the remeasured assets and liabilities attributable to changes in foreign currency exchange rates, except to the extent of the spot-forward differences. These differences are not significant due to the short-term nature of the contracts, which typically have average maturities at inception of less than six months. The cash flows from these contracts are reported as operating activities in the Consolidated Statement of Cash Flows.
The Company also uses forward exchange contracts to hedge a portion of its net investment in foreign operations against movements in foreign exchange rates. The forward contracts are designated as hedges of the net investment in a foreign operation. The unrealized gains or losses on these contracts are recorded in foreign currency translation adjustment within OCI, and remain in AOCL until either the sale or complete or substantially complete liquidation of the subsidiary. The Company excludes certain portions of the change in fair value of its derivative instruments from the assessment of hedge effectiveness (excluded components). Changes in fair value of the excluded components are recognized in OCI. The Company recognizes in earnings the initial value of the excluded components on a straight-line basis over the life of the derivative instrument, rather than using the mark-to-market approach. The cash flows from these contracts are reported as investing activities in the Consolidated Statement of Cash Flows.
Foreign exchange risk is also managed through the use of foreign currency debt. Certain of the Company’s senior unsecured euro-denominated notes have been designated as, and are effective as, economic hedges of the net investment in a foreign operation. Accordingly, foreign currency transaction gains or losses due to spot rate fluctuations on the euro-denominated debt instruments are included in foreign currency translation adjustment within OCI.
The effects of the Company’s net investment hedges on OCI and the Consolidated Statement of Income are shown below:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Amount of Pretax (Gain) Loss Recognized in Other Comprehensive Income (1) | | Amount of Pretax (Gain) Loss Recognized in Other (income) expense, net for Amounts Excluded from Effectiveness Testing |
| Years Ended December 31 | 2024 | | 2023 | | 2022 | | 2024 | | 2023 | | 2022 |
| Net Investment Hedging Relationships | | | | | | | | | | | |
| Foreign exchange contracts | $ | (30) | | | $ | — | | | $ | (48) | | | $ | (4) | | | $ | 1 | | | $ | (1) | |
| Euro-denominated notes | (192) | | | 105 | | | (162) | | | — | | | — | | | — | |
(1) No amounts were reclassified from AOCL into income related to the sale of a subsidiary.
Interest Rate Risk Management
The Company may use interest rate swap contracts on certain investing and borrowing transactions to manage its net exposure to interest rate changes and to reduce its overall cost of borrowing. The Company does not use leveraged swaps and, in general, does not leverage any of its investment activities that would put principal at risk.
At December 31, 2024, the Company was a party to six pay-floating, receive-fixed interest rate swap contracts designated as fair value hedges of the fixed-rate notes as detailed in the table below.
| | | | | | | | | | | | | | | | | |
| Par Value of Debt | | Number of Interest Rate Swaps Held | | Total Swap Notional Amount |
| 4.50% notes due 2033 | $ | 1,500 | | | 6 | | | $ | 1,500 | |
The interest rate swap contracts are designated hedges of the fair value changes in the notes attributable to changes in the benchmark Secured Overnight Financing Rate (SOFR) swap rate. The fair value changes in the notes attributable to changes in the SOFR swap rate are recorded in interest expense along with the offsetting fair value changes in the swap contracts. In January 2025, the Company entered into an additional interest rate swap contract with a notional amount of $250 million related to its 5.00% notes due 2053. The cash flows from these contracts are reported as operating activities in the Consolidated Statement of Cash Flows.
The table below presents the location of amounts recorded in the Consolidated Balance Sheet related to cumulative basis adjustments for fair value hedges as of December 31:
| | | | | | | | | | | | | | | | | | | | | | | |
| Carrying Amount of Hedged Liabilities | | Cumulative Amount of Fair Value Hedging Adjustment Increase Included in the Carrying Amount |
| 2024 | | 2023 | | 2024 | | 2023 |
| Balance Sheet Caption | | | | | | | |
| Long-Term Debt | $ | 1,509 | | | $ | 1,056 | | | $ | 17 | | | $ | 56 | |
Presented in the table below is the fair value of derivatives on a gross basis segregated between those derivatives that are designated as hedging instruments and those that are not designated as hedging instruments as of December 31:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | 2024 | | 2023 |
| | | | Fair Value of
Derivative | | U.S. Dollar
Notional | | Fair Value of
Derivative | | U.S. Dollar
Notional |
| | | | Asset | | Liability | | Asset | | Liability | |
| Derivatives Designated as Hedging Instruments | Balance Sheet Caption | | | | | | | | | | | | |
| Interest rate swap contracts | Other Assets | | $ | 17 | | | $ | — | | | $ | 1,500 | | | $ | 57 | | | $ | — | | | $ | 1,000 | |
| Foreign exchange contracts | Other current assets | | 323 | | | — | | | 8,662 | | | 106 | | | — | | | 6,138 | |
| Foreign exchange contracts | Other Assets | | 66 | | | — | | | 2,125 | | | 26 | | | — | | | 1,929 | |
| Foreign exchange contracts | Accrued and other current liabilities | | — | | | 1 | | | 162 | | | — | | | 76 | | | 3,680 | |
| Foreign exchange contracts | Other Noncurrent Liabilities | | — | | | 1 | | | 16 | | | — | | | 1 | | | 7 | |
| | | | $ | 406 | | | $ | 2 | | | $ | 12,465 | | | $ | 189 | | | $ | 77 | | | $ | 12,754 | |
| Derivatives Not Designated as Hedging Instruments | Balance Sheet Caption | | | | | | | | | | | | |
| Foreign exchange contracts | Other current assets | | $ | 323 | | | $ | — | | | $ | 12,544 | | | $ | 153 | | | $ | — | | | $ | 9,693 | |
| Foreign exchange contracts | Accrued and other current liabilities | | — | | | 343 | | | 13,551 | | | — | | | 162 | | | 8,104 | |
| | | | $ | 323 | | | $ | 343 | | | $ | 26,095 | | | $ | 153 | | | $ | 162 | | | $ | 17,797 | |
| | | | $ | 729 | | | $ | 345 | | | $ | 38,560 | | | $ | 342 | | | $ | 239 | | | $ | 30,551 | |
As noted above, the Company records its derivatives on a gross basis in the Consolidated Balance Sheet. The Company has master netting agreements with several of its financial institution counterparties (see Concentrations of Credit Risk below). The following table provides information on the Company’s derivative positions subject to these master netting arrangements as if they were presented on a net basis, allowing for the right of offset by counterparty and cash collateral exchanged per the master agreements and related credit support annexes as of December 31:
| | | | | | | | | | | | | | | | | | | | | | | |
| 2024 | | 2023 |
| Asset | | Liability | | Asset | | Liability |
| Gross amounts recognized in the consolidated balance sheet | $ | 729 | | | $ | 345 | | | $ | 342 | | | $ | 239 | |
| Gross amounts subject to offset in master netting arrangements not offset in the consolidated balance sheet | (299) | | | (299) | | | (215) | | | (215) | |
| Cash collateral received | (165) | | | — | | | (3) | | | — | |
| Net amounts | $ | 265 | | | $ | 46 | | | $ | 124 | | | $ | 24 | |
The table below provides information regarding the location and amount of pretax gains and losses of derivatives designated in fair value or cash flow hedging relationships:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Years Ended December 31 | 2024 | | 2023 | | 2022 | | 2024 | | 2023 | | 2022 | | 2024 | | 2023 | | 2022 |
| Financial Statement Caption in which Effects of Fair Value or Cash Flow Hedges are Recorded | Sales | | Other (income) expense, net (1) | | Other comprehensive income (loss) |
| $ | 64,168 | | | $ | 60,115 | | | $ | 59,283 | | | $ | (24) | | | $ | 466 | | | $ | 1,501 | | | $ | 216 | | | $ | (393) | | | $ | (339) | |
| (Gain) loss on fair value hedging relationships: | | | | | | | | | | | | | | | | | |
| Interest rate swap contracts | | | | | | | | | | | | | | | | | |
| Hedged items | — | | | — | | | — | | | (39) | | | 56 | | | (13) | | | — | | | — | | | — | |
| Derivatives designated as hedging instruments | — | | | — | | | — | | | 39 | | | (57) | | | 4 | | | — | | | — | | | — | |
| Impact of cash flow hedging relationships: | | | | | | | | | | | | | | | | | |
| Foreign exchange contracts | | | | | | | | | | | | | | | | | |
Amount of gain recognized in OCI on derivatives | — | | | — | | | — | | | — | | | — | | | — | | | 508 | | | 114 | | | 684 | |
Increase in Sales as a result of AOCL reclassifications | 167 | | | 249 | | | 773 | | | — | | | — | | | — | | | (167) | | | (249) | | | (773) | |
| Interest rate contracts | | | | | | | | | | | | | | | | | |
Amount of gain recognized in Other (income) expense, net on derivatives | — | | | — | | | — | | | (1) | | | (1) | | | (2) | | | — | | | — | | | — | |
Amount of (loss) gain recognized in OCI on derivatives | — | | | — | | | — | | | — | | | — | | | — | | | (1) | | | 13 | | | (2) | |
(1) Interest expense is a component of Other (income) expense, net.
The table below provides information regarding the income statement effects of derivatives not designated as hedging instruments:
| | | | | | | | | | | | | | | | | | | | | | | |
| | | Amount of Derivative Pretax Loss (Gain) Recognized in Income |
| Years Ended December 31 | | | 2024 | | 2023 | | 2022 |
| Derivatives Not Designated as Hedging Instruments | Income Statement Caption | | | | | | |
Foreign exchange contracts (1) | Other (income) expense, net | | $ | 251 | | | $ | (6) | | | $ | (49) | |
Foreign exchange contracts (2) | Sales | | (28) | | | 5 | | | (37) | |
(1) These derivative contracts primarily mitigate changes in the value of remeasured foreign currency denominated monetary assets and liabilities attributable to changes in foreign currency exchange rates.
(2) These derivative contracts serve as economic hedges of forecasted transactions.
At December 31, 2024, the Company estimates $262 million of pretax net unrealized gains on derivatives maturing within the next 12 months that hedge foreign currency denominated sales over that same period will be reclassified from AOCL to Sales. The amount ultimately reclassified to Sales may differ as foreign exchange rates change. Realized gains and losses are ultimately determined by actual foreign exchange rates at maturity.
Investments in Debt and Equity Securities
Information on investments in debt and equity securities at December 31 is as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | 2024 | | 2023 |
| | Amortized
Cost | | Gross Unrealized | | Fair
Value | | Amortized
Cost | | Gross Unrealized | | Fair
Value |
| Gains | | Losses | | Gains | | Losses | |
| Commercial paper | $ | 348 | | | $ | — | | | $ | — | | | $ | 348 | | | $ | 252 | | | $ | — | | | $ | — | | | $ | 252 | |
| U.S. government and agency securities | 188 | | | — | | | — | | | 188 | | | 72 | | — | | | — | | | 72 | |
| Corporate notes and bonds | — | | | — | | | — | | | — | | | 13 | | | — | | | — | | | 13 | |
| Total debt securities | $ | 536 | | | $ | — | | | $ | — | | | $ | 536 | | | $ | 337 | | | $ | — | | | $ | — | | | $ | 337 | |
Publicly traded equity securities (1) | | | | | | | 920 | | | | | | | | | 764 | |
| Total debt and publicly traded equity securities | | | | | | | $ | 1,456 | | | | | | | | | $ | 1,101 | |
(1) Unrealized net losses of $30 million were recorded in Other (income) expense, net in 2024 on equity securities still held at December 31, 2024. Unrealized net gains of $411 million were recorded in Other (income) expense, net in 2023 on equity securities still held at December 31, 2023.
At December 31, 2024 and 2023, the Company also had $863 million and $832 million, respectively, of equity investments without readily determinable fair values included in Other Assets. The Company records unrealized gains on these equity investments based on favorable observable price changes from transactions involving similar investments of the same investee and records unrealized losses based on unfavorable observable price changes, which are included in Other (income) expense, net. During 2024, the Company recorded unrealized gains of $19 million and unrealized losses of $51 million related to certain of these equity investments still held at December 31, 2024. During 2023, the Company recorded unrealized gains of $10 million and unrealized losses of $61 million related to certain of these equity investments still held at December 31, 2023. Cumulative unrealized gains and cumulative unrealized losses based on observable price changes for investments in equity investments without readily determinable fair values still held at December 31, 2024 were $309 million and $107 million, respectively.
At December 31, 2024, 2023 and 2022, the Company also had $267 million, $417 million and $598 million, respectively, recorded in Other Assets for equity securities held through ownership interests in investment funds. Losses recorded in Other (income) expense, net relating to these investment funds were $29 million, $106 million and $1.0 billion for the years ended December 31, 2024, 2023 and 2022, respectively.
Fair Value Measurements
Fair value is defined as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. The Company uses a fair value hierarchy which maximizes the use of observable inputs and minimizes the use of unobservable inputs when measuring fair value. There are three levels of inputs used to measure fair value with Level 1 having the highest priority and Level 3 having the lowest:
Level 1 — Quoted prices (unadjusted) in active markets for identical assets or liabilities.
Level 2 — Observable inputs other than Level 1 prices, such as quoted prices for similar assets or liabilities, or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities.
Level 3 — Unobservable inputs that are supported by little or no market activity. Level 3 assets or liabilities are those whose values are determined using pricing models, discounted cash flow methodologies, or similar techniques with significant unobservable inputs, as well as assets or liabilities for which the determination of fair value requires significant judgment or estimation.
If the inputs used to measure the financial assets and liabilities fall within more than one level described above, the categorization is based on the lowest level input that is significant to the fair value measurement of the instrument.
Financial Assets and Liabilities Measured at Fair Value on a Recurring Basis
Financial assets and liabilities measured at fair value on a recurring basis at December 31 are summarized below:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Fair Value Measurements Using | | Fair Value Measurements Using |
| Level 1 | | Level 2 | | Level 3 | | Total | | Level 1 | | Level 2 | | Level 3 | | Total |
| | 2024 | | 2023 |
| Assets | | | | | | | | | | | | | | | |
| Investments | | | | | | | | | | | | | | | |
| Commercial paper | $ | — | | | $ | 348 | | | $ | — | | | $ | 348 | | | $ | — | | | $ | 252 | | | $ | — | | | $ | 252 | |
| U.S. government and agency securities | — | | | 99 | | | — | | | 99 | | | — | | | — | | | — | | | — | |
| Publicly traded equity securities | 463 | | | — | | | — | | | 463 | | | 252 | | | — | | | — | | | 252 | |
| | 463 | | | 447 | | | — | | | 910 | | | 252 | | | 252 | | | — | | | 504 | |
Other assets (1) | | | | | | | | | | | | | | | |
| U.S. government and agency securities | 89 | | | — | | | — | | | 89 | | | 72 | | | — | | | — | | | 72 | |
| Corporate notes and bonds | — | | | — | | | — | | | — | | | 13 | | | — | | | — | | | 13 | |
Publicly traded equity securities (2) | 457 | | | — | | | — | | | 457 | | | 512 | | | — | | | — | | | 512 | |
| 546 | | | — | | | — | | | 546 | | | 597 | | | — | | | — | | | 597 | |
Derivative assets (3) | | | | | | | | | | | | | | | |
| Forward exchange contracts | — | | | 499 | | | — | | | 499 | | | — | | | 202 | | | — | | | 202 | |
| Purchased currency options | — | | | 213 | | | — | | | 213 | | | — | | | 83 | | | — | | | 83 | |
| Interest rate swaps | — | | | 17 | | | — | | | 17 | | | — | | | 57 | | | — | | | 57 | |
| | — | | | 729 | | | — | | | 729 | | | — | | | 342 | | | — | | | 342 | |
| Total assets | $ | 1,009 | | | $ | 1,176 | | | $ | — | | | $ | 2,185 | | | $ | 849 | | | $ | 594 | | | $ | — | | | $ | 1,443 | |
| Liabilities | | | | | | | | | | | | | | | |
| Other liabilities | | | | | | | | | | | | | | | |
| Contingent consideration | $ | — | | | $ | — | | | $ | 193 | | | $ | 193 | | | $ | — | | | $ | — | | | $ | 354 | | | $ | 354 | |
Derivative liabilities (3) | | | | | | | | | | | | | | | |
| Forward exchange contracts | — | | | 338 | | | — | | | 338 | | | — | | | 239 | | | — | | | 239 | |
| Written currency options | — | | | 7 | | | — | | | 7 | | | — | | | — | | | — | | | — | |
| — | | | 345 | | | — | | | 345 | | | — | | | 239 | | | — | | | 239 | |
| Total liabilities | $ | — | | | $ | 345 | | | $ | 193 | | | $ | 538 | | | $ | — | | | $ | 239 | | | $ | 354 | | | $ | 593 | |
(1) Investments included in other assets are restricted as to use, including for the payment of benefits under employee benefit plans.
(2) Balance at December 31, 2024 includes securities with a fair value of $81 million, which are subject to a contractual sale restriction that expires in March 2025. Balance at December 31, 2023 includes securities with a fair value of $177 million, which were subject to a contractual sale restriction that expired in July 2024.
(3) The fair value determination of derivatives includes the impact of the credit risk of counterparties to the derivatives and the Company’s own credit risk, the effects of which were not significant.
As of December 31, 2024 and 2023, Cash and cash equivalents included $12.3 billion and $6.0 billion of cash equivalents, respectively (which would be considered Level 2 in the fair value hierarchy).
Contingent Consideration
Summarized information about the changes in the fair value of liabilities for contingent consideration associated with business combinations is as follows:
| | | | | | | | | | | |
| 2024 | | 2023 |
| Fair value January 1 | $ | 354 | | | $ | 456 | |
Changes in estimated fair value (1) | (10) | | | 15 | |
| Payments | (151) | | | (117) | |
Fair value December 31 (2) | $ | 193 | | | $ | 354 | |
(1) Recorded in Cost of sales, Research and development expenses, and Other (income) expense, net. Includes cumulative translation adjustments.
(2) Balance at December 31, 2024 includes $148 million of current liabilities, of which $123 million relates to the termination of the Sanofi Pasteur MSD joint venture in 2016. As part of the termination, Merck recorded a liability for contingent future royalty payments of 11.5% on net sales of all Merck products that were previously sold by the joint venture through December 31, 2024. The fair value of this liability is determined utilizing the estimated amount and timing of projected cash flows using a risk-adjusted discount rate to present value the cash flows.
The payments of contingent consideration in 2024 include $126 million related to the Sanofi Pasteur MSD liabilities described above and $25 million related to the first commercial sale of Lyfnua (gefapixant) in the European Union (EU). The payments of contingent consideration in 2023 relate to the Sanofi Pasteur MSD liabilities.
Other Fair Value Measurements
Some of the Company’s financial instruments, such as cash and cash equivalents, receivables and payables, are reflected in the balance sheet at carrying value, which approximates fair value due to their short-term nature.
The estimated fair value of loans payable and long-term debt (including current portion) at December 31, 2024, was $32.6 billion compared with a carrying value of $37.1 billion and at December 31, 2023, was $32.0 billion compared with a carrying value of $35.1 billion. Fair value was estimated using recent observable market prices and would be considered Level 2 in the fair value hierarchy.
Concentrations of Credit Risk
On an ongoing basis, the Company monitors concentrations of credit risk associated with corporate and government issuers of securities and financial institutions with which it conducts business. Credit exposure limits are established to limit a concentration with any single issuer or institution. Cash and investments are placed in instruments that meet high credit quality standards, as specified in the Company’s investment policy guidelines.
The majority of the Company’s accounts receivable arise from product sales in the U.S., Europe and China and are primarily due from drug wholesalers, distributors and retailers, hospitals and government agencies. The Company monitors the financial performance and creditworthiness of its customers so that it can properly assess and respond to changes in their credit profile. The Company also continues to monitor global economic conditions, including the volatility associated with international sovereign economies, and associated impacts on the financial markets and its business.
The Company’s customers with the largest accounts receivable balances are: McKesson Corporation, Cencora, Inc. and Cardinal Health, Inc., which represented approximately 21%, 21% and 13%, respectively, of total accounts receivable at December 31, 2024. Vaccines distributed by Chongqing Zhifei Biological Products Co., Ltd. (Zhifei) represent a substantial portion of total sales in China; however, nearly all of the accounts receivable for Zhifei were factored as of December 31, 2024, as part of the Company’s factoring program discussed below. The Company monitors the creditworthiness of its customers to which it grants credit terms in the normal course of business. Bad debts have been minimal. The Company does not normally require collateral or other security to support credit sales.
The Company has accounts receivable factoring agreements with financial institutions in certain countries to sell accounts receivable. The Company factored $2.1 billion and $3.0 billion of accounts receivable as of December 31, 2024 and 2023, respectively, under these factoring arrangements, which reduced outstanding accounts receivable. The cash received from the financial institutions is reported within operating activities in the Consolidated Statement of Cash Flows. In certain of these factoring arrangements, for ease of administration, the Company will collect customer payments related to the factored receivables, which it then remits to the financial institutions, generally within thirty days after receipt. At December 31, 2024 and 2023, the Company had collected $55 million and $44 million, respectively, on behalf of the financial institutions, which is reflected as restricted cash in Other current assets, and the related obligation to remit the cash is recorded in Accrued and other current liabilities. The net cash flows related to these collections are reported as financing activities in the Consolidated Statement of Cash Flows. The cost of factoring such accounts receivable was de minimis.
Derivative financial instruments are executed under International Swaps and Derivatives Association master agreements. The master agreements with several of the Company’s financial institution counterparties also include credit support annexes. These annexes contain provisions that require collateral to be exchanged depending on the value of the derivative assets and liabilities, the Company’s credit rating, and the credit rating of the counterparty. Cash collateral received by the Company from various counterparties was $165 million and $3 million at December 31, 2024 and 2023, respectively. The obligation to return such collateral is recorded in Accrued and other current liabilities.
7. Inventories
Inventories at December 31 consisted of:
| | | | | | | | | | | |
| 2024 | | 2023 |
| Finished goods | $ | 2,022 | | | $ | 1,954 | |
| Raw materials and work in process | 8,831 | | | 8,037 | |
| Supplies | 289 | | | 277 | |
| 11,142 | | | 10,268 | |
| Decrease to LIFO cost | (840) | | | (562) | |
| | $ | 10,302 | | | $ | 9,706 | |
| Recognized as: | | | |
| Inventories | $ | 6,109 | | | $ | 6,358 | |
| Other Assets | 4,193 | | | 3,348 | |
Inventories valued under the LIFO method comprised approximately $3.4 billion and $3.1 billion at December 31, 2024 and 2023, respectively, after reflecting the decrease to LIFO cost. Amounts recognized as Other Assets are comprised almost entirely of raw materials and work in process inventories. At December 31, 2024 and 2023, these amounts included $3.8 billion and $2.6 billion, respectively, of inventories not expected to be sold within one year. In addition, these amounts included $412 million and $790 million at December 31, 2024 and 2023, respectively, of inventories produced in preparation for product launches.
8. Goodwill and Other Intangibles
The following table summarizes goodwill activity by segment:
| | | | | | | | | | | | | | | | | |
| Pharmaceutical | | Animal Health | | Total |
| Balance January 1, 2023 | $ | 17,936 | | | $ | 3,268 | | | $ | 21,204 | |
| | | | | |
Other (1) | (14) | | | 7 | | | (7) | |
Balance December 31, 2023 (2) | 17,922 | | | 3,275 | | | 21,197 | |
| Acquisitions | — | | | 518 | | | 518 | |
Other (1) | (19) | | | (28) | | | (47) | |
Balance December 31, 2024 (2) | $ | 17,903 | | | $ | 3,765 | | | $ | 21,668 | |
(1) Includes cumulative translation adjustments on goodwill balances.
(2) Accumulated goodwill impairment losses were $531 million at both December 31, 2024 and 2023.
Other acquired intangibles at December 31 consisted of:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | 2024 | | 2023 |
| Gross
Carrying
Amount | | Accumulated
Amortization | | Net | | Gross
Carrying
Amount | | Accumulated
Amortization | | Net |
| Product rights | $ | 29,988 | | | $ | 19,066 | | | $ | 10,922 | | | $ | 23,643 | | | $ | 17,765 | | | $ | 5,878 | |
| IPR&D | 430 | | | — | | | 430 | | | 6,816 | | | — | | | 6,816 | |
| Trade names | 2,881 | | | 954 | | | 1,927 | | | 2,881 | | | 776 | | | 2,105 | |
| Licenses and other | 8,863 | | | 5,772 | | | 3,091 | | | 8,263 | | | 5,051 | | | 3,212 | |
| | $ | 42,162 | | | $ | 25,792 | | | $ | 16,370 | | | $ | 41,603 | | | $ | 23,592 | | | $ | 18,011 | |
Some of the more significant acquired intangibles included in product rights, on a net basis, related to human health marketed products at December 31, 2024 were Winrevair, $5.9 billion; Reblozyl, $2.8 billion; and Zerbaxa, $260 million. Additionally, the Company had $4.3 billion of net acquired intangibles related to animal health at December 31, 2024, of which $1.7 billion related to product rights and $1.9 billion was attributable to trade names, primarily related to Allflex. At December 31, 2024, IPR&D primarily relates to MK-1026 (nemtabrutinib), obtained through the acquisition of ArQule, Inc. (ArQule), which had a balance of $418 million. Some of the more significant net intangible assets included in licenses and other above at December 31, 2024 include Lynparza, $1.2 billion, related to a collaboration with AstraZeneca; Lenvima, $442 million, related to a collaboration with Eisai; and Adempas, $372 million, related to a collaboration with Bayer. See Note 4 for additional information related to the intangible assets associated with these collaborations.
IPR&D that the Company acquires through business combinations represents the fair value assigned to incomplete research projects which, at the time of acquisition, have not reached technological feasibility. Amounts capitalized as IPR&D are accounted for as indefinite-lived intangible assets, subject to impairment testing until completion or abandonment of the projects. Upon successful completion of each IPR&D project, the Company will make a separate determination as to the then-useful life of the asset and begin amortization.
In 2023, the Company recorded a $779 million IPR&D impairment charge within Research and development expenses related to MK-7264, gefapixant, a non-narcotic, oral selective P2X3 receptor antagonist, that was in development for the treatment of refractory or unexplained chronic cough in adults. In December 2023, the FDA issued a Complete Response Letter (CRL) regarding the resubmission of Merck’s New Drug Application (NDA) for gefapixant. In the CRL, the FDA concluded that Merck’s application did not meet substantial evidence of effectiveness for treating refractory chronic cough and unexplained chronic cough. The CRL was not related to the safety of gefapixant. The marketing application for gefapixant was based on results from the COUGH-1 and COUGH-2 clinical trials. In January 2022, the FDA issued a CRL regarding Merck’s original NDA for gefapixant. In that CRL, the FDA requested additional information related to the cough counting system that was used to assess efficacy. Receipt of the second CRL from the FDA constituted a triggering event that required the evaluation of the gefapixant intangible asset for impairment. The Company estimated the current fair value of gefapixant utilizing an income approach, which calculates the present value of projected future cash flows. The market participant assumptions used to derive the forecasted cash flows were updated to reflect revised market launch plans, resulting in a reduction in the estimated fair value. The revised estimated fair value of gefapixant when compared with its related carrying value resulted in the impairment charge noted above. The remaining intangible asset balance related to Lyfnua (gefapixant) at December 31, 2024 of $21 million is included in product rights in the table above and is being amortized over its expected useful life as supported by projected future cash flows in the markets where it is approved including Japan and the EU.
In 2022, the Company recorded $1.7 billion of intangible asset impairment charges within Research and development expenses, of which $1.6 billion represents IPR&D impairment charges related to nemtabrutinib (MK-1026), an oral, reversible, non-covalent Bruton’s tyrosine kinase (BTK) inhibitor currently being evaluated for the treatment of hematological malignancies that was obtained through the 2020 acquisition of ArQule. Following discussions with regulatory authorities in the third quarter of 2022, the development period for nemtabrutinib was extended, which constituted a triggering event that required the evaluation of the nemtabrutinib intangible asset for impairment. The Company estimated the current fair value of nemtabrutinib utilizing an income approach which calculates the present value of projected future cash flows. The market participant assumptions used to derive the forecasted cash flows were updated to reflect a delay in the anticipated launch date for nemtabrutinib, which resulted in lower cumulative revenue forecasts and a reduction in the estimated fair value. The revised estimated fair value of nemtabrutinib when compared with its related carrying value resulted in a $807 million impairment charge recorded in the third quarter of 2022. In December 2022, regulatory authorities provided additional feedback with respect to clinical study design that led to a further reassessment of the development plan for nemtabrutinib, which was expected to result in changes to the clinical study design, and corresponding delays in the anticipated approval and launch timelines, which constituted a triggering event. Utilizing an income approach, the forecasted cash flows were updated to reflect a decline in forecasted revenue coupled with an increase in development cost forecasts, which reduced projected cash flows lowering the estimated fair value of nemtabrutinib. The revised estimated fair value of nemtabrutinib when compared with its then-related carrying value resulted in a $780 million impairment charge. The remaining IPR&D intangible asset related to nemtabrutinib is $418 million. If the assumptions used to estimate the fair value of nemtabrutinib prove to be incorrect and the development of nemtabrutinib does not progress as anticipated thereby adversely affecting projected future cash flows, the Company may record an additional impairment charge in the future and such charge could be material. The Company also recorded an $80 million intangible asset impairment charge in 2022 related to derazantinib resulting from the termination of the out-licensing agreement and the decision by Merck not to pursue development of derazantinib.
The IPR&D projects that remain in development are subject to the inherent risks and uncertainties in drug development and it is possible that the Company will not be able to successfully develop and complete the IPR&D programs and profitably commercialize the underlying product candidates.
The Company may recognize additional non-cash impairment charges in the future related to marketed products or pipeline programs and such charges could be material.
Aggregate amortization expense primarily recorded within Cost of sales was $2.4 billion in 2024, $2.0 billion in 2023 and $2.1 billion in 2022. The estimated aggregate amortization expense for each of the next five years is as follows: 2025, $2.4 billion; 2026, $2.3 billion; 2027, $2.1 billion; 2028, $1.8 billion; 2029, $1.5 billion.
9. Loans Payable, Long-Term Debt and Leases
Loans Payable
Loans payable at December 31, 2024 included $2.5 billion of notes due in 2025 and $149 million of long-dated notes that are subject to repayment at the option of the holders. Loans payable at December 31, 2023 included $1.3 billion of notes due in 2024 and $69 million of long-dated notes that are subject to repayment at the option of the holders. The weighted-average interest rate of commercial paper borrowings was 5.18% and 5.14% for the years ended December 31, 2024 and 2023, respectively. There were no commercial paper borrowings outstanding at December 31, 2024 or 2023.
Long-Term Debt
Long-term debt at December 31 consisted of:
| | | | | | | | | | | |
| 2024 | | 2023 |
| 2.15% notes due 2031 | $ | 1,989 | | | $ | 1,988 | |
| 2.75% notes due 2051 | 1,980 | | | 1,980 | |
| 3.70% notes due 2045 | 1,980 | | | 1,979 | |
| 3.40% notes due 2029 | 1,742 | | | 1,740 | |
| 4.50% notes due 2033 | 1,509 | | | 1,547 | |
| 1.70% notes due 2027 | 1,497 | | | 1,495 | |
| 2.90% notes due 2061 | 1,484 | | | 1,484 | |
| 5.00% notes due 2053 | 1,482 | | | 1,481 | |
| 4.00% notes due 2049 | 1,474 | | | 1,473 | |
| 4.15% notes due 2043 | 1,240 | | | 1,240 | |
| 1.45% notes due 2030 | 1,240 | | | 1,238 | |
| 2.45% notes due 2050 | 1,216 | | | 1,214 | |
| 1.875% euro-denominated notes due 2026 | 1,041 | | | 1,103 | |
| 0.75% notes due 2026 | 998 | | | 996 | |
| 1.90% notes due 2028 | 996 | | | 995 | |
| 5.15% notes due 2063 | 987 | | | 987 | |
| 3.90% notes due 2039 | 987 | | | 986 | |
| 2.35% notes due 2040 | 986 | | | 985 | |
| 3.25% euro-denominated notes due 2032 | 880 | | | — | |
| 3.50% euro-denominated notes due 2037 | 877 | | | — | |
| 3.70% euro-denominated notes due 2044 | 876 | | | — | |
| 3.75% euro-denominated notes due 2054 | 873 | | | — | |
| 4.30% notes due 2030 | 746 | | | 745 | |
| 4.90% notes due 2044 | 740 | | | 740 | |
| 6.50% notes due 2033 | 702 | | | 707 | |
| 1.375% euro-denominated notes due 2036 | 517 | | | 548 | |
| 2.50% euro-denominated notes due 2034 | 517 | | | 548 | |
| 4.05% notes due 2028 | 498 | | | 497 | |
| 3.60% notes due 2042 | 492 | | | 492 | |
| 6.55% notes due 2037 | 404 | | | 406 | |
| 5.75% notes due 2036 | 339 | | | 339 | |
| 5.95% debentures due 2028 | 307 | | | 307 | |
| 5.85% notes due 2039 | 271 | | | 271 | |
| 6.40% debentures due 2028 | 251 | | | 250 | |
| 6.30% debentures due 2026 | 135 | | | 135 | |
| 2.75% notes due 2025 | — | | | 2,498 | |
| Other | 209 | | | 289 | |
| $ | 34,462 | | | $ | 33,683 | |
Other (as presented in the table above) includes borrowings at variable rates that resulted in effective interest rates of 5.02% and 4.82% for 2024 and 2023, respectively.
With the exception of the 6.30% debentures due 2026, the notes listed in the table above are redeemable in whole or in part, at Merck’s option at any time, at varying redemption prices. Effective as of November 3, 2009, the Company executed a full and unconditional guarantee of the then existing debt of its subsidiary Merck Sharp & Dohme LLC. (MSD) and MSD executed a full and unconditional guarantee of the then existing debt of the Company
(excluding commercial paper), including for payments of principal and interest. These guarantees do not extend to debt issued subsequent to that date.
In May 2024, MSD Netherlands Capital B.V., a wholly owned finance subsidiary of Merck, completed a registered public offering of €3.4 billion in aggregate principal amount of euro-dominated senior notes comprised of €850 million of 3.25% senior notes due 2032, €850 million of 3.50% senior notes due 2037, €850 million of 3.70% senior notes due 2044 and €850 million of 3.75% senior notes due 2054 (collectively, the Euronotes). The Company has fully and unconditionally guaranteed all of MSD Netherlands Capital B.V.’s obligations under the Euronotes and no other subsidiary of the Company will guarantee these obligations. MSD Netherlands Capital B.V. is a “finance subsidiary” as defined in Rule 13-01(a)(4)(vi) of Regulation S-X of the Exchange Act, with no assets or operations other than those related to the issuance, administration and repayment of the Euronotes. The financial condition, results of operations and cash flows of MSD Netherlands Capital B.V. are consolidated in the financial statements of the Company. The net cash proceeds from the offering were used for general corporate purposes.
Certain of the Company’s borrowings require that Merck comply with covenants and, at December 31, 2024, the Company was in compliance with these covenants.
The aggregate maturities of long-term debt for each of the next five years are as follows: 2025, $2.6 billion; 2026, $2.2 billion; 2027, $1.5 billion; 2028, $2.1 billion; 2029, $1.7 billion. Interest payments related to these debt obligations are as follows: 2025, $1.2 billion; 2026, $1.2 billion; 2027, $1.1 billion; 2028, $1.1 billion; 2029, $1.0 billion.
The Company has a $6.0 billion credit facility that matures in May 2028. The facility provides backup liquidity for the Company’s commercial paper borrowing facility and is to be used for general corporate purposes. The Company has not drawn funding from this facility.
Leases
The Company has operating leases primarily for manufacturing facilities, research and development facilities, corporate offices, employee housing, vehicles and certain equipment. The Company determines if an arrangement is a lease at inception. When evaluating contracts for embedded leases, the Company exercises judgment to determine if there is an explicit or implicit identified asset in the contract and if Merck controls the use of that asset. Embedded leases, primarily associated with contract manufacturing organizations, are immaterial. The lease term includes options to extend or terminate the lease when it is reasonably certain that Merck will exercise that option. Real estate leases for facilities have an average remaining lease term of approximately six years, which include options to extend the leases for up to five years where applicable. Vehicle leases are generally in effect for four years. The Company elected to exclude short-term leases (leases with an initial term of 12 months or less) from the lease assets and liabilities on the balance sheet.
Lease expense for operating lease payments is recognized on a straight-line basis over the term of the lease. Operating lease assets and liabilities are recognized based on the present value of lease payments over the lease term. Since the Company’s leases do not have a readily determinable implicit discount rate, the Company uses its incremental borrowing rate to calculate the present value of lease payments by asset class. On a quarterly basis, an updated incremental borrowing rate is determined based on the average remaining lease term of each asset class and the Company’s pretax cost of debt for that same term. The updated rates for each asset class are applied prospectively to new leases. The Company does not separate lease components (e.g., payments for rent, real estate taxes and insurance costs) from non-lease components (e.g. common-area maintenance costs) in the event that the agreement contains both. Merck includes both the lease and non-lease components for purposes of calculating the right-of-use asset and related lease liability (if the non-lease components are fixed). For vehicle leases and employee housing, the Company applies a portfolio approach to account for the operating lease assets and liabilities.
Certain of the Company’s lease agreements contain variable lease payments that are adjusted periodically for inflation or for actual operating expense true-ups compared with estimated amounts; however, these amounts are immaterial. Sublease income was immaterial and there were no sale and leaseback transactions in 2024. Merck’s lease agreements do not contain any material residual value guarantees or material restrictive covenants.
Operating lease cost was $348 million in 2024, $339 million in 2023 and $334 million in 2022. Cash paid for amounts included in the measurement of operating lease liabilities was $357 million in 2024, $347 million in 2023 and $335 million in 2022. Operating lease assets obtained in exchange for lease obligations were $47 million in 2024, $122 million in 2023 and $57 million in 2022.
Supplemental balance sheet information related to operating leases is as follows:
| | | | | | | | | | | | | |
| December 31 | 2024 | | 2023 | | |
| Assets | | | | | |
Other Assets (1) | $ | 1,370 | | | $ | 1,437 | | | |
| Liabilities | | | | | |
| Accrued and other current liabilities | 282 | | | 285 | | | |
| Other Noncurrent Liabilities | 877 | | | 928 | | | |
| $ | 1,159 | | | $ | 1,213 | | | |
| Weighted-average remaining lease term (years) | 6.0 | | 7.0 | | |
| Weighted-average discount rate | 3.2 | % | | 3.3 | % | | |
(1) Includes prepaid leases that have no related lease liability.
Maturities of operating leases liabilities are as follows:
| | | | | |
| 2025 | $ | 329 | |
| 2026 | 292 | |
| 2027 | 235 | |
| 2028 | 146 | |
| 2029 | 116 | |
| Thereafter | 403 | |
| Total lease payments | 1,521 | |
| Less: Imputed interest | 362 | |
| $ | 1,159 | |
At December 31, 2024, the Company had entered into additional real estate operating leases that had not yet commenced; the obligations associated with these leases total $183 million.
10. Contingencies and Environmental Liabilities
The Company is involved in various claims and legal proceedings of a nature considered normal to its business, including product liability, intellectual property, commercial litigation, and securities litigation, as well as certain additional matters including governmental and environmental matters. In the opinion of the Company, it is unlikely that the resolution of these matters will be material to the Company’s financial condition, results of operations or cash flows.
Given the nature of the litigation discussed below and the complexities involved in these matters, the Company is unable to reasonably estimate a possible loss or range of possible loss for such matters until the Company knows, among other factors, (i) what claims, if any, will survive dispositive motion practice, (ii) the extent of the claims, including the size of any potential class, particularly when damages are not specified or are indeterminate, (iii) how the discovery process will affect the litigation, (iv) the settlement posture of the other parties to the litigation and (v) any other factors that may have a material effect on the litigation.
The Company records accruals for contingencies when it is probable that a liability has been incurred and the amount can be reasonably estimated. These accruals are adjusted periodically as assessments change or additional information becomes available. Generally, for product liability claims, a portion of the overall accrual is actuarially determined and considers such factors as past experience, number of claims reported and estimates of claims incurred but not yet reported. Individually significant contingent losses are accrued when probable and reasonably estimable. Legal defense costs expected to be incurred in connection with a loss contingency are accrued when probable and reasonably estimable.
The Company’s decision to obtain insurance coverage is dependent on market conditions, including cost and availability, existing at the time such decisions are made. The Company has evaluated its risks and has determined that the cost of obtaining product liability insurance outweighs the likely benefits of the coverage that is available and, as such, has no insurance for most product liabilities.
Product Liability Litigation
Dr Scholl’s Foot Powder
As previously disclosed, Merck is a defendant in product liability lawsuits in the U.S. arising from consumers’ alleged exposure to talc in Dr. Scholl’s foot powder, which Merck acquired through its merger with Schering-Plough Corporation and sold as part of the divestiture of Merck’s consumer care business to Bayer in 2014. In these actions, plaintiffs allege that they were exposed to asbestos-contaminated talc and developed mesothelioma as a result. As of December 31, 2024, approximately 415 cases were pending against Merck in various state courts.
Gardasil/Gardasil 9
As previously disclosed, Merck is a defendant in product liability lawsuits in the U.S. involving Gardasil (Human Papillomavirus Quadrivalent [Types 6, 11, 16 and 18] Vaccine, Recombinant) and Gardasil 9 (Human Papillomavirus 9-valent Vaccine, Recombinant). As of December 31, 2024, approximately 225 cases were filed and pending against Merck in either federal or state court. In these actions, plaintiffs allege, among other things, that they suffered various personal injuries after vaccination with Gardasil or Gardasil 9, with postural orthostatic tachycardia syndrome (POTS) as a predominate alleged injury. In August 2022, the U.S. Judicial Panel on Multidistrict Litigation ordered that Gardasil/Gardasil 9 product liability cases pending in federal courts nationwide be transferred to Judge Robert J. Conrad in the Western District of North Carolina for coordinated pre-trial proceedings. In February 2024, the multidistrict litigation was reassigned to Judge Kenneth D. Bell. As previously disclosed, there are fewer than 15 product liability cases pending outside the U.S.
On January 28, 2025, a trial commenced in California state court. Plaintiff claims that she suffers from POTS and fibromyalgia as a result of her Gardasil vaccinations. On February 14, 2025, after four weeks of trial and an opportunity to litigate plaintiff’s claims before a jury, plaintiff’s counsel approached Merck and proposed that the jury be discharged and the case adjourned. Merck agreed, subject to an explicit stipulation that Merck would provide no financial or other consideration in exchange for the agreement to adjourn. The case has thus been adjourned until a new trial date of September 15, 2025. Merck is vigorously defending this case and believes that evidence presented in court will show that Gardasil had no role in causing any of plaintiff’s conditions.
Governmental Proceedings
Civil Investigative Demands
As previously disclosed, in June 2024, Merck received a Civil Investigative Demand (CID) from the U.S. Department of Justice, pursuant to a False Claims Act investigation, seeking documents and materials related to Steglatro, Januvia and certain related drugs. The CID states that it is investigating Merck’s price reporting under the Medicaid Drug Rebate Program as well as compliance with anti-kickback requirements in connection with patient assistance programs. The Company is cooperating with the investigation.
As previously disclosed, in June 2020, Merck received a CID from the U.S. Department of Justice. The CID requests answers to interrogatories, as well as various documents, regarding temperature excursions at a third-party storage facility containing certain Merck products. Merck is cooperating with the government’s investigation and intends to produce information and/or documents as necessary in response to the CID.
Inflation Reduction Act
As previously disclosed, in June 2023, Merck filed a complaint in the U.S. District Court for the District of Columbia against the U.S. government regarding the Inflation Reduction Act’s “Drug Price Negotiation Program” for Medicare (the Program). This litigation seeks relief from the Program by challenging its constitutionality as violative of the First and Fifth Amendments to the U.S. Constitution.
Other Matters
As previously disclosed, in April 2019, Merck received a set of investigative interrogatories from the California Attorney General’s Office pursuant to its investigation of conduct and agreements that allegedly affected or delayed competition to Lantus in the insulin market. The interrogatories seek information concerning Merck’s development of an insulin glargine product, and its subsequent termination, as well as Merck’s patent litigation against Sanofi S.A. concerning Lantus and the resolution of that litigation. Merck is cooperating with the California Attorney General’s investigation.
As previously disclosed, from time to time, the Company’s subsidiaries in China receive inquiries regarding their operations from various Chinese governmental agencies. Some of these inquiries may be related to matters involving other multinational pharmaceutical companies, as well as Chinese entities doing business with such companies. The Company’s policy is to cooperate with these authorities and to provide responses as appropriate.
As previously disclosed, from time to time, the Company receives inquiries and is the subject of preliminary investigation activities from competition and other governmental authorities in markets outside the U.S. These authorities may include regulators, administrative authorities, and law enforcement and other similar officials, and these preliminary investigation activities may include site visits, formal or informal requests or demands for documents or materials, inquiries or interviews and similar matters. Certain of these preliminary inquiries or activities may lead to the commencement of formal proceedings. Should those proceedings be determined adversely to the Company, monetary fines and/or remedial undertakings may be required.
Securities Litigation
In February 2025, a putative class action was filed against Merck and certain of its officers in the U.S. District Court for the District of New Jersey purportedly on behalf of all purchasers of Merck common stock between February 2022 and February 2025. Plaintiff alleges that Merck violated federal securities laws by making materially false and misleading statements and material omissions regarding demand for Gardasil/Gardasil 9 in China. Plaintiff seeks unspecified monetary damages, pre-judgment and post-judgment interest, and fees and costs.
Commercial and Other Litigation
Zetia Antitrust Litigation
As previously disclosed, Merck, MSD, Schering Corporation, Schering-Plough Corporation, and MSP Singapore Company LLC (collectively, the Merck Defendants) were defendants in a number of lawsuits filed in 2018 on behalf of direct and indirect purchasers of Zetia (ezetimibe) alleging violations of federal and state antitrust laws, as well as other state statutory and common law causes of action. The cases were consolidated in a federal multidistrict litigation (Zetia MDL) before Judge Rebecca Beach Smith in the Eastern District of Virginia. In April 2023, the Merck Defendants reached settlements with the direct purchaser and retailer plaintiffs and a settlement with the indirect purchaser class that the court approved in October 2023.
As previously disclosed, in 2020 and 2021, United Healthcare Services, Inc. (United Healthcare), Humana Inc. (Humana), Centene Corporation and others (Centene), and Kaiser Foundation Health Plan, Inc. (Kaiser) (collectively, the Insurer Plaintiffs), each filed a lawsuit in a jurisdiction outside of the Eastern District of Virginia against the Merck Defendants and others, making similar allegations as those made in the Zetia MDL, as well as additional allegations about Vytorin. These cases were transferred to the Eastern District of Virginia to proceed with the Zetia MDL.
In December 2023, the U.S. Judicial Panel on Multidistrict Litigation remanded the four Insurer Plaintiff cases to the transferor courts in the Northern District of California (Kaiser), the District of Minnesota (United Healthcare), and the District of New Jersey (Humana and Centene). The Merck Defendants filed motions to dismiss in each of the Insurer Plaintiff cases. On December 30, 2024, the court granted in part and denied in part the motions to dismiss in the Humana and Centene cases, and on January 29, 2025, Humana and Centene filed amended complaints.
RotaTeq Antitrust Litigation
As previously disclosed, in March 2023, the Mayor and City Council of Baltimore filed a putative class action against MSD in the Eastern District of Pennsylvania on behalf of all third-party payors in 35 states that indirectly purchased, paid, and/or provided reimbursement for some or all of the purchase price of RotaTeq (Rotavirus Vaccine, Live Oral, Pentavalent), other than for resale, from March 3, 2019 to the present. Plaintiff alleges that MSD violated federal and state antitrust laws and state consumer protection laws. Plaintiff alleges that MSD has implemented an anticompetitive vaccine bundling scheme whereby MSD leverages its alleged monopoly power in certain pediatric vaccine markets to maintain its alleged monopoly power in the U.S. market for rotavirus vaccines in order to charge supracompetitive prices for RotaTeq. Plaintiff seeks permanent injunctive relief and unspecified monetary damages on purchases of RotaTeq, trebled, and fees and costs. In May 2023, MSD moved to dismiss the complaint. In November 2023, the court granted in part and denied in part the motion to dismiss, dismissing plaintiff’s Idaho and Utah consumer law claims and allowing all other claims to proceed.
Bravecto Litigation
As previously disclosed, in January 2020, the Company was served with a complaint in the U.S. District Court for the District of New Jersey. Following motion practice, the plaintiffs filed a third amended complaint in August 2024, seeking to certify a nationwide class action of purchasers or users of Bravecto (fluralaner) products in the U.S. or its territories between May 1, 2014 and July 1, 2021. Plaintiffs contend Bravecto causes neurological events in dogs and cats and alleges violations of the New Jersey Consumer Fraud Act, Breach of Warranty, Product Liability, and related theories. The Company moved to dismiss or, alternatively, to strike the class allegations from the third amended complaint, and that motion is pending. A similar case was filed in Quebec, Canada in May 2019. The
Superior Court certified a class of dog owners in Quebec who gave Bravecto Chew to their dogs between February 16, 2017 and November 2, 2018 whose dogs experienced one of the conditions in the post-marketing adverse reactions section of the labeling approved on November 2, 2018. The Company and plaintiffs each appealed the class certification decision. The Court of Appeal of Quebec amended the class period to start July 2, 2014, allowed the second plaintiff to serve as a class representative, and modified the list of conditions in the class definition. The Company sought leave to appeal to the Supreme Court of Canada, which was denied. The case is proceeding in the Superior Court.
340B Program Litigation
As previously disclosed, Merck filed a complaint in the U.S. District Court for the District of Columbia to challenge the letter Merck received from the U.S. Health Resources and Services Administration (HRSA) in May 2022 regarding Merck’s 340B Program integrity initiative. On September 17, 2024, the court entered a consent judgment granting Merck the relief it had sought in the litigation, including declarations that HRSA’s May 2022 letter was unlawful and that the version of Merck’s 340B Program integrity initiative at issue in the litigation did not violate Section 340B on its face.
Qui Tam Litigation
As previously disclosed, in June 2012, the U.S. District Court for the Eastern District of Pennsylvania unsealed a complaint that had been filed against the Company under the federal False Claims Act by two former employees alleging, among other things, that the Company defrauded the U.S. government by falsifying data in connection with a clinical study conducted on the mumps component of the Company’s M-M-R II vaccine. The complaint alleges the fraud took place between 1999 and 2001. The U.S. government had the right to participate in and take over the prosecution of this lawsuit but notified the court that it declined to exercise that right. The two former employees pursued the lawsuit without the involvement of the U.S. government. In July 2023, the court denied relators’ motion for summary judgment, granted two of the Company’s motions for summary judgment, and denied the Company’s remaining motions for summary judgment as moot. The court entered judgment in favor of the Company and dismissed relators’ amended complaint in full with prejudice. Relators appealed that decision, and in August 2024, the Third Circuit affirmed the district court’s decision.
In addition, as previously disclosed, two putative class action lawsuits on behalf of direct purchasers of the M‑M‑R II vaccine, which charge that the Company misrepresented the efficacy of the M-M-R II vaccine in violation of federal antitrust laws and various state consumer protection laws, are pending in the Eastern District of Pennsylvania. The court granted the Company’s motion for summary judgment as to plaintiffs’ state law claims and denied the motion as to plaintiffs’ antitrust claim. The Company appealed, and on October 7, 2024, the Third Circuit reversed-in-part the district court’s order and remanded the case with instructions to enter summary judgment for the Company. On November 20, 2024, plaintiffs-appellees filed a petition for rehearing and rehearing en banc, and on February 10, 2025, the court denied the petition.
Merck KGaA Litigation
As previously disclosed, in January 2016, to protect its long-established brand rights in the U.S., the Company filed a lawsuit against Merck KGaA, Darmstadt, Germany (KGaA), historically operating as the EMD Group in the U.S., alleging it improperly uses the name “Merck” in the U.S. KGaA has filed suit against the Company in a number of jurisdictions outside of the U.S. alleging, among other things, unfair competition, trademark infringement and/or corporate name infringement. In certain of those jurisdictions, KGaA also alleges breach of the parties’ coexistence agreement. The litigation is ongoing in the U.S. with no trial date set, and also ongoing in jurisdictions outside of the U.S.
Patent Litigation
From time to time, generic manufacturers of pharmaceutical products file abbreviated New Drug Applications (ANDAs) with the U.S. Food and Drug Administration (FDA) seeking to market generic forms of the Company’s products prior to the expiration of relevant patents owned by the Company. To protect its patent rights, the Company may file patent infringement lawsuits against such generic companies. Similar lawsuits defending the Company’s patent rights may exist in other countries. The Company intends to vigorously defend its patents, which it believes are valid, against infringement by companies attempting to market products prior to the expiration of such patents. As with any litigation, there can be no assurance of the outcomes, which, if adverse, could result in significantly shortened periods of exclusivity for these products and, with respect to products acquired through acquisitions accounted for as business combinations, potentially significant intangible asset impairment charges.
Bridion — As previously disclosed, between January and November 2020, the Company received multiple Paragraph IV Certification Letters under the Hatch-Waxman Act notifying the Company that generic drug companies had filed applications to the FDA seeking pre-patent expiry approval to sell generic versions of Bridion (sugammadex)
Injection. In March, April and December 2020, the Company filed patent infringement lawsuits in the U.S. District Courts for the District of New Jersey and the Northern District of West Virginia against those generic companies. All actions in the District of New Jersey were consolidated. The West Virginia case was jointly dismissed with prejudice in August 2022 in favor of proceeding in New Jersey. The remaining defendants in the New Jersey action have stipulated to infringement of the asserted claims and withdrew all remaining claims and defenses other than a defense seeking to shorten the patent term extension (PTE) of the sugammadex patent to December 2022. The U.S. District Court for the District of New Jersey held a one-day trial in December 2022 on this remaining PTE calculation defense. The court ordered a post-trial briefing on this defense and held closing arguments in February 2023.
As previously disclosed, in June 2023, the U.S. District Court for the District of New Jersey ruled in Merck’s favor. The court held that Merck’s calculation of PTE for the sugammadex patent covering the compound is not invalid and that the U.S. Patent & Trademark Office correctly granted a full five-year extension. This ruling affirms and validates Merck’s U.S. patent protection for Bridion through at least January 2026. Also in June 2023, the U.S. District Court for the District of New Jersey issued a final judgment prohibiting the FDA from approving any of the pending or tentatively approved generic applications until January 27, 2026, except for any subsequent agreements between defendants and Merck or further order by the court.
In July 2023, defendants filed a notice of appeal with the U.S. Court of Appeals for the Federal Circuit. The appeal is currently pending. Oral argument took place on February 4, 2025.
While the New Jersey action was pending, the Company settled with five generic companies providing that these generic companies can bring their generic versions of Bridion to the market in January 2026 (which may be delayed by any applicable pediatric exclusivity) or earlier under certain circumstances. The Company agreed to stay the lawsuit filed against two generic companies, which in exchange agreed to be bound by a judgment on the merits of the consolidated action in the District of New Jersey. One of the generic companies in the consolidated action requested dismissal of the action against it and the Company did not oppose this request, which was subsequently granted by the court. The Company does not expect this company to bring its generic version of Bridion to the market before January 2026 or later, depending on any applicable pediatric exclusivity.
In February 2024, the Company received another Paragraph IV Certification Letter under the Hatch-Waxman Act notifying the Company that Hikma Pharmaceuticals USA Inc. (Hikma) had filed an application to the FDA seeking pre-patent expiry approval to sell a generic version of Bridion Injection. In March 2024, the Company filed a patent infringement lawsuit in the U.S. District Court for the District of New Jersey against Hikma, postponing FDA approval of the Hikma generic drug for 30 months or until expiration of the sugammadex patent (January 27, 2026) and any potentially applicable pediatric exclusivity or an adverse court decision, if any, whichever may occur earlier. Expiration of the patent, and any potentially applicable pediatric exclusivity, will occur earlier than expiry of the 30-month stay. On April 16, 2024, the district court stayed the case during the pendency of the Federal Circuit appeal noted above.
Januvia, Janumet, Janumet XR — As previously disclosed, the FDA granted pediatric exclusivity with respect to Januvia (sitagliptin), Janumet (sitagliptin/metformin HCl), and Janumet XR (sitagliptin and metformin HCl extended-release), which provides a further six months of exclusivity in the U.S. beyond the expiration of all patents listed in the FDA’s Orange Book. Adding this exclusivity to the term of the key patent protection extended exclusivity on these products to January 2023. However, Januvia, Janumet, and Janumet XR contain sitagliptin phosphate monohydrate and the Company has another patent covering certain phosphate salt and polymorphic forms of sitagliptin that expires in May 2027, including pediatric exclusivity (salt/polymorph patent).
As previously disclosed, beginning in 2019, a number of generic drug companies filed ANDAs seeking approval of generic forms of Januvia and Janumet along with paragraph IV certifications challenging the validity of the salt/polymorph patent. The Company responded by filing infringement suits which have all been settled. The Company has settled with a total 26 generic companies providing that these generic companies can bring their generic versions of Januvia and Janumet to the market in the U.S. in May 2026 or earlier under certain circumstances, and their generic versions of Janumet XR to the market in July 2026 or earlier under certain circumstances.
In March 2021, the Company filed a patent infringement lawsuit in the U.S. District Court for the District of Delaware against Zydus Worldwide DMCC, Zydus Pharmaceuticals (USA) Inc., and Cadila Healthcare Ltd. (collectively, Zydus). In that lawsuit, the Company alleged infringement of the salt/polymorph patent based on the filing of Zydus’s NDA seeking approval of a form of sitagliptin that is a different from than that used in Januvia. In December 2022, the parties reached settlement that included dismissal of the case without prejudice enabling Zydus to seek final approval of a non-automatically substitutable product.
In January 2023, the Company received a Paragraph IV Certification Letter under the Hatch-Waxman Act notifying the Company that Zydus filed an ANDA seeking approval of sitagliptin/metformin HCl tablets and certifying that no valid or enforceable claim of any of the patents listed in FDA’s Orange Book for Janumet will be infringed by the proposed Zydus product. In March 2023, the parties reached settlement enabling Zydus to seek final approval of a non-automatically substitutable product containing a different form of sitagliptin than that used in Janumet. In November 2023, the Company received a Paragraph IV Certification Letter under the Hatch-Waxman Act notifying the Company that Zydus filed an ANDA seeking approval of sitagliptin/metformin HCl Extended Release tablets. In January 2024, the parties reached settlement enabling Zydus to seek final approval of a non-automatically substitutable version containing a different form of sitagliptin than that used in Janumet XR.
As a result of these settlement agreements related to the later expiring salt/polymorph patent directed to the specific sitagliptin salt form of the products, the Company expects that Januvia and Janumet will not lose market exclusivity in the U.S. until May 2026 and Janumet XR will not lose market exclusivity in the U.S. until July 2026, although Zydus has received FDA approval for a non-automatically substitutable form of sitagliptin that differs from the form in the Company’s sitagliptin products.
In March 2024, the Company received another Paragraph IV Certification Letter under the Hatch-Waxman Act from Azurity Pharmaceuticals, Inc. (Azurity) asserting that a different sitagliptin product subject to its ANDA does not infringe the salt/polymorph patent. In May 2024, Merck filed a civil action in the U.S. District Court of Delaware alleging infringement. The case was dismissed without prejudice in July 2024. Following the dismissal, the Company granted Azurity a covenant not to assert the salt/polymorph patent against the Azurity product that is the subject of such ANDA.
Supplementary Protection Certificates (SPCs) for Janumet expired in April 2023 for the majority of European countries. Prior to expiration, generic companies sought revocation of the Janumet SPCs in a number of European countries. In February 2022, a Finnish court referred certain questions to the Court of Justice of the European Union that could impact the validity of the Janumet SPCs in Europe. A decision was rendered on December 19, 2024. The decision provides guidance on points of law and does not directly apply these to the Janumet SPCs. Thus, additional proceedings in certain countries where generic companies were prevented from launching products during the SPC period may be necessary to determine whether the SPCs are valid and if not, whether damages are appropriate. Those countries include Belgium, Czech Republic, Ireland, Finland, France, Slovakia and Switzerland. If the Janumet SPCs are ultimately upheld, the Company has reserved its rights related to the pursuit of damages for those countries where a generic launched prior to expiry of the Janumet SPC.
In October 2023, the Company filed a patent infringement lawsuit against Sawai Pharmaceuticals Co., Ltd. and Medisa Shinyaku Co., Ltd (collectively, Defendants) in the Tokyo District Court seeking an injunction to stop the manufacture, sale and offer for sale of the Defendants’ sitagliptin dihydrogen phosphate product, while the Company’s patents and patent term extensions are in force. The lawsuit is in response to the Defendants’ application for marketing authorization to sell a generic sitagliptin dihydrogen phosphate product, in the anhydrate form, which was approved in August 2023. Merck asserts that the Defendants’ activity infringes a patent term extension associated with Merck’s patent directed to the sitagliptin compound patent.
Keytruda — As previously disclosed, in November 2022, the Company filed a complaint against The Johns Hopkins University (JHU) in the U.S. District Court of Maryland. This action concerns patents emerging from a joint research collaboration between Merck and JHU regarding the use of pembrolizumab, which Merck sells under the trade name Keytruda. Merck and JHU partnered to design and conduct a clinical study administering Keytruda to cancer patients having tumors that had the genetic biomarker known as microsatellite instability-high (MSI-H). After the conclusion of the study, JHU secured U.S. patents citing the joint research study. Merck alleges that JHU has breached the collaboration agreement by filing and obtaining these patents without informing or involving Merck and then licensing the patents to others. Merck therefore brought this action for breach of contract, declaratory judgment of noninfringement, and promissory estoppel. JHU answered the complaint in April and May 2023, denying Merck’s claims, and counterclaiming for willful infringement of nine issued U.S. patents, including a demand for damages. Between November 30, 2023 and March 13, 2024, the Company filed inter partes review petitions with the United States Patent & Trademark Office Patent Trial and Appeal Board (PTAB), challenging the validity of all nine patents asserted in the case. Between June 2024 and October 2024, the PTAB instituted a review of all nine asserted patents. In July 2024, the district court granted Merck’s motion to stay the case in its entirety pending the outcome of the PTAB proceeding instituted in June 2024.
Lynparza — As previously disclosed, in December 2022, AstraZeneca Pharmaceuticals LP received a Paragraph IV Certification Letter under the Hatch-Waxman Act notifying AstraZeneca that Natco Pharma Limited (Natco) has filed an application to the FDA seeking pre-patent expiry approval to sell generic versions of Lynparza (olaparib) tablet. In February 2023, AstraZeneca and the Company filed a patent infringement lawsuit in the U.S.
District Court for the District of New Jersey against Natco. This lawsuit, which asserts one or more patents covering olaparib, automatically stays FDA approval of the generic application until June 2025 or until an adverse court decision, if any, whichever may occur earlier. In May, June, July, and November 2024, AstraZeneca and the Company filed additional patent infringement lawsuits in the U.S. District Court for the District of New Jersey against Natco asserting additional patents covering olaparib.
In December 2023, AstraZeneca Pharmaceuticals LP received a second Paragraph IV Certification Letter under the Hatch-Waxman Act notifying AstraZeneca that Sandoz Inc. has filed an application to the FDA seeking pre-patent expiry approval to sell generic versions of Lynparza (olaparib) tablet. In February 2024, AstraZeneca and the Company filed a patent infringement lawsuit in the U.S. District Court for the District of New Jersey against Sandoz. This lawsuit, which asserts one or more patents covering olaparib, automatically stays FDA approval of the generic application until June 2026 or until an adverse court decision, if any, whichever may occur earlier. In May, July, and November 2024, AstraZeneca and the Company filed additional patent infringement lawsuits in the U.S. District Court for the District of New Jersey against Sandoz asserting additional patents covering olaparib.
In May 2024, AstraZeneca Pharmaceuticals LP received a third Paragraph IV Certification Letter under the Hatch-Waxman Act notifying AstraZeneca that Cipla USA, Inc. and Cipla Limited (collectively, Cipla) filed an application to the FDA seeking pre-patent expiry approval to sell generic versions of Lynparza (olaparib) tablet. In June 2024, AstraZeneca and the Company filed a patent infringement lawsuit in the U.S. District Court for the District of New Jersey against Cipla. This lawsuit, which asserts one or more patents covering olaparib, automatically stays FDA approval of the generic application until November 2026 or until an adverse court decision, if any, whichever may occur earlier. In June, July, and November 2024, AstraZeneca and the Company filed additional patent infringement lawsuits in the U.S. District Court for the District of New Jersey against Cipla asserting additional patents covering olaparib.
In November 2024, AstraZeneca Pharmaceuticals LP received another Paragraph IV Certification Letter under the Hatch-Waxman Act notifying AstraZeneca that Zydus Pharmaceuticals (USA) Inc. filed an application to the FDA seeking pre-patent expiry approval to sell generic versions of Lynparza (olaparib) tablet. In November 2024, AstraZeneca and the Company filed a patent infringement lawsuit in the U.S. District Court for the District of New Jersey against Zydus. This lawsuit, which asserts one or more patents covering olaparib, automatically stays FDA approval of the generic application until May 2027 or until an adverse court decision, if any, whichever may occur earlier. In November 2024, AstraZeneca and the Company filed an additional patent infringement lawsuit in the U.S. District Court for the District of New Jersey against Zydus asserting an additional patent covering olaparib.
Other Litigation
There are various other pending legal proceedings involving the Company, principally product liability and intellectual property lawsuits. While it is not feasible to predict the outcome of such proceedings, in the opinion of the Company, either the likelihood of loss is remote or any reasonably possible loss associated with the resolution of such proceedings is not expected to be material to the Company’s financial condition, results of operations or cash flows either individually or in the aggregate.
Legal Defense Reserves
Legal defense costs expected to be incurred in connection with a loss contingency are accrued when probable and reasonably estimable. Some of the significant factors considered in the review of these legal defense reserves are as follows: the actual costs incurred by the Company; the development of the Company’s legal defense strategy and structure in light of the scope of its litigation; the number of cases being brought against the Company; the costs and outcomes of completed trials and the most current information regarding anticipated timing, progression, and related costs of pre-trial activities and trials in the associated litigation. The amount of legal defense reserves as of December 31, 2024 and 2023 of approximately $225 million and $210 million, respectively, represents the Company’s best estimate of the minimum amount of defense costs to be incurred in connection with its outstanding litigation; however, events such as additional trials and other events that could arise in the course of its litigation could affect the ultimate amount of legal defense costs to be incurred by the Company. The Company will continue to monitor its legal defense costs and review the adequacy of the associated reserves and may determine to increase the reserves at any time in the future if, based upon the factors set forth, it believes it would be appropriate to do so.
Environmental Matters
The Company and its subsidiaries are parties to a number of proceedings brought under the Comprehensive Environmental Response, Compensation and Liability Act, commonly known as Superfund, and other federal and state equivalents. These proceedings seek to require the operators of hazardous waste disposal facilities, transporters of waste to the sites and generators of hazardous waste disposed of at the sites to clean up the sites or
to reimburse the government for cleanup costs. The Company has been made a party to these proceedings as an alleged generator of waste disposed of at the sites. In each case, the government alleges that the defendants are jointly and severally liable for the cleanup costs. Although joint and several liability is alleged, these proceedings are frequently resolved so that the allocation of cleanup costs among the parties more nearly reflects the relative contributions of the parties to the site situation. The Company’s potential liability varies greatly from site to site. For some sites the potential liability is de minimis and for others the final costs of cleanup have not yet been determined. While it is not feasible to predict the outcome of many of these proceedings brought by federal or state agencies or private litigants, in the opinion of the Company, such proceedings should not ultimately result in any liability which would have a material adverse effect on the financial condition, results of operations or liquidity of the Company. The Company has taken an active role in identifying and accruing for these costs and such amounts do not include any reduction for anticipated recoveries of cleanup costs from former site owners or operators or other recalcitrant potentially responsible parties.
In management’s opinion, the liabilities for all environmental matters that are probable and reasonably estimable have been accrued and totaled $41 million and $42 million at December 31, 2024 and 2023, respectively. These liabilities are undiscounted, do not consider potential recoveries from other parties and will be paid out over the periods of remediation for the applicable sites, which are expected to occur primarily over the next 15 years. Although it is not possible to predict with certainty the outcome of these matters, or the ultimate costs of remediation, management does not believe that any reasonably possible expenditures that may be incurred in excess of the liabilities accrued should exceed approximately $46 million in the aggregate. Management also does not believe that these expenditures should result in a material adverse effect on the Company’s financial condition, results of operations or liquidity for any year.
11. Equity
The Merck certificate of incorporation authorizes 6,500,000,000 shares of common stock and 20,000,000 shares of preferred stock.
Capital Stock
A summary of common stock and treasury stock transactions (shares in millions) is as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | 2024 | | 2023 | | 2022 |
| | Common
Stock | | Treasury
Stock | | Common
Stock | | Treasury
Stock | | Common
Stock | | Treasury
Stock |
| Balance January 1 | 3,577 | | | 1,045 | | | 3,577 | | | 1,039 | | | 3,577 | | | 1,049 | |
| Purchases of treasury stock | — | | | 11 | | | — | | | 13 | | | — | | | — | |
Issuances (1) | — | | | (7) | | | — | | | (7) | | | — | | | (10) | |
| Balance December 31 | 3,577 | | | 1,049 | | | 3,577 | | | 1,045 | | | 3,577 | | | 1,039 | |
(1) Issuances primarily reflect activity under share-based compensation plans.
12. Share-Based Compensation Plans
The Company has share-based compensation plans under which the Company grants restricted stock units (RSUs) and performance share units (PSUs) to certain management level employees. In addition, employees and non-employee directors may be granted options to purchase shares of Company common stock at the fair market value at the time of grant. These plans were approved by the Company’s shareholders.
At December 31, 2024, 75 million shares collectively were authorized for future grants under the Company’s share-based compensation plans. These awards are settled with treasury shares.
Employee stock options are granted to purchase shares of Company stock at the fair market value at the time of grant. These awards generally vest one-third each year over a three-year period, with a contractual term of 7-10 years. RSUs are stock awards that are granted to employees and entitle the holder to shares of common stock as the awards vest. The fair value of the stock option and RSU awards is determined and fixed on the grant date based on the Company’s stock price. PSUs are stock awards where the ultimate number of shares issued will be contingent on the Company’s performance against a pre-set objective or set of objectives. The fair value of each PSU is determined on the date of grant based on the Company’s stock price. For RSUs and PSUs, dividends declared during the vesting period are payable to the employees only upon vesting. Over the PSU performance period, the number of shares of stock that are expected to be issued will be adjusted based on the probability of achievement of a performance target and final compensation expense will be recognized based on the ultimate number of shares issued. RSU and PSU distributions will be in shares of Company stock after the end of the vesting or performance
period, subject to the terms applicable to such awards. PSU awards generally vest after three years. RSU awards generally vest one-third each year over a three-year period.
Total pretax share-based compensation cost recorded in 2024, 2023 and 2022 was $761 million, $645 million and $541 million, respectively. Income tax benefits for share-based compensation expense recognized in 2024, 2023 and 2022 were $117 million, $96 million and $78 million, respectively.
The Company uses the Black-Scholes option pricing model for determining the fair value of option grants. In applying this model, the Company uses both historical data and current market data to estimate the fair value of its options. The Black-Scholes model requires several assumptions including expected dividend yield, risk-free interest rate, volatility, and term of the options. The expected dividend yield is based on historical patterns of dividend payments. The risk-free interest rate is based on the rate at grant date of zero-coupon U.S. Treasury Notes with a term equal to the expected term of the option. Expected volatility is estimated using a blend of historical and implied volatility. The historical component is based on historical monthly price changes. The implied volatility is obtained from market data on the Company’s traded options. The expected life represents the amount of time that options granted are expected to be outstanding, based on historical and forecasted exercise behavior.
The weighted average exercise price of options granted in 2024, 2023 and 2022 was $129.22, $117.89 and $87.10 per option, respectively. The weighted average fair value of options granted in 2024, 2023 and 2022 was $25.60, $21.69 and $15.45 per option, respectively, and were determined using the following assumptions:
| | | | | | | | | | | | | | | | | |
| Years Ended December 31 | 2024 | | 2023 | | 2022 |
| Expected dividend yield | 3.0 | % | | 3.1 | % | | 3.1 | % |
| Risk-free interest rate | 4.7 | % | | 3.4 | % | | 3.0 | % |
| Expected volatility | 20.5 | % | | 22.4 | % | | 22.5 | % |
| Expected life (years) | 5.8 | | 5.8 | | 5.9 |
Summarized information relative to stock option plan activity (options in thousands) is as follows:
| | | | | | | | | | | | | | | | | | | | | | | |
| Number
of Options | | Weighted
Average
Exercise
Price | | Weighted
Average
Remaining
Contractual
Term (Years) | | Aggregate
Intrinsic
Value |
| Outstanding January 1, 2024 | 13,527 | | | $ | 77.54 | | | | | |
| Granted | 1,753 | | | 129.22 | | | | | |
| Exercised | (2,581) | | | 68.90 | | | | | |
| Forfeited | (199) | | | 111.48 | | | | | |
| Outstanding December 31, 2024 | 12,500 | | | $ | 86.04 | | | 5.9 | | $ | 249 | |
| Vested and expected to vest December 31, 2024 | 12,201 | | | $ | 85.10 | | | 5.9 | | $ | 249 | |
| Exercisable December 31, 2024 | 9,084 | | | $ | 74.00 | | | 4.9 | | $ | 241 | |
Additional information pertaining to stock option plans is provided in the table below:
| | | | | | | | | | | | | | | | | |
| Years Ended December 31 | 2024 | | 2023 | | 2022 |
| Total intrinsic value of stock options exercised | $ | 144 | | | $ | 95 | | | $ | 225 | |
| Fair value of stock options vested | 32 | | | 30 | | | 30 | |
| Cash received from the exercise of stock options | 177 | | | 125 | | | 384 | |
A summary of nonvested RSU and PSU activity (shares in thousands) is as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | RSUs | | PSUs |
| | | Number
of Shares | | Weighted
Average
Grant Date
Fair Value | | Number
of Shares | | Weighted
Average
Grant Date
Fair Value |
| Nonvested January 1, 2024 | | 12,542 | | | $ | 100.10 | | | 1,966 | | | $ | 90.80 | |
| Granted | | 6,356 | | | 128.79 | | | 968 | | | 121.91 | |
| Vested | | (6,091) | | | 92.97 | | | (1,109) | | | 73.50 | |
| Forfeited | | (575) | | | 113.18 | | | (59) | | | 121.12 | |
| Nonvested December 31, 2024 | | 12,232 | | | $ | 117.94 | | | 1,766 | | | $ | 117.57 | |
| Expected to vest December 31, 2024 | | 10,976 | | | $ | 117.25 | | | 1,669 | | | $ | 116.26 | |
At December 31, 2024, there was $1.1 billion of total pretax unrecognized compensation expense related to nonvested stock options, RSU and PSU awards which will be recognized over a weighted average period of 1.9 years. For segment reporting, share-based compensation costs are unallocated expenses.
13. Pension and Other Postretirement Benefit Plans
The Company has defined benefit pension plans covering eligible employees in the U.S. and in certain of its international subsidiaries. In addition, the Company provides medical benefits, principally to its eligible U.S. retirees and their dependents, through its other postretirement benefit plans. The Company uses December 31 as the year-end measurement date for all of its pension plans and other postretirement benefit plans.
Net Periodic Benefit Cost
The net periodic benefit cost (credit) for pension and other postretirement benefit plans consisted of the following components:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Pension Benefits | | | | | | |
| U.S. | | International | | Other Postretirement Benefits |
| Years Ended December 31 | 2024 | | 2023 | | 2022 | | 2024 | | 2023 | | 2022 | | 2024 | | 2023 | | 2022 |
| Service cost | $ | 373 | | | $ | 326 | | | $ | 372 | | | $ | 243 | | | $ | 196 | | | $ | 283 | | | $ | 30 | | | $ | 32 | | | $ | 48 | |
| Interest cost | 537 | | | 526 | | | 457 | | | 294 | | | 299 | | | 145 | | | 56 | | | 63 | | | 46 | |
| Expected return on plan assets | (826) | | | (735) | | | (753) | | | (554) | | | (517) | | | (383) | | | (80) | | | (64) | | | (86) | |
| Amortization of unrecognized prior service (credit) cost | — | | | (1) | | | (32) | | | (13) | | | 2 | | | (14) | | | (43) | | | (49) | | | (57) | |
| Net loss (gain) amortization | 43 | | | — | | | 128 | | | 5 | | | (3) | | | 96 | | | (51) | | | (42) | | | (43) | |
| Termination benefits | 5 | | | 3 | | | 2 | | | 1 | | | — | | | 1 | | | 4 | | | — | | | — | |
| Curtailments | — | | | 8 | | | 12 | | | — | | | (1) | | | — | | | — | | | (1) | | | (1) | |
| Settlements | — | | | 28 | | | 239 | | | (1) | | | (5) | | | 1 | | | — | | | — | | | — | |
| Net periodic benefit cost (credit) | $ | 132 | | | $ | 155 | | | $ | 425 | | | $ | (25) | | | $ | (29) | | | $ | 129 | | | $ | (84) | | | $ | (61) | | | $ | (93) | |
In connection with restructuring actions (see Note 5), termination charges were recorded in 2024, 2023 and 2022 on pension and other postretirement benefit plans related to expanded eligibility for certain employees exiting Merck. Also, in connection with these restructuring activities, curtailments and settlements were recorded on certain pension plans. Lump sum payments to U.S. pension plan participants also contributed to the settlements recorded during 2023 and 2022.
The components of net periodic benefit cost (credit) other than the service cost component are included in Other (income) expense, net (see Note 14), with the exception of certain amounts for termination benefits, curtailments and settlements, which are recorded in Restructuring costs if the event giving rise to the termination benefits, curtailment or settlement is related to restructuring actions.
Obligations and Funded Status
Summarized information about the changes in plan assets and benefit obligations, the funded status and the amounts recorded at December 31 is as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Pension Benefits | | Other
Postretirement
Benefits |
| U.S. | | International | |
| 2024 | | 2023 | | 2024 | | 2023 | | 2024 | | 2023 |
| Fair value of plan assets January 1 | $ | 9,804 | | | $ | 9,094 | | | $ | 9,562 | | | $ | 8,473 | | | $ | 1,045 | | | $ | 947 | |
| Actual return on plan assets | 266 | | | 1,077 | | | 637 | | | 832 | | | 35 | | | 115 | |
| Company contributions | 262 | | | 307 | | | 198 | | | 249 | | | 46 | | | 74 | |
| Effects of exchange rate changes | — | | | — | | | (522) | | | 283 | | | — | | | — | |
| Benefits paid | (615) | | | (497) | | | (250) | | | (256) | | | (89) | | | (95) | |
| Settlements | — | | | (177) | | | (14) | | | (53) | | | — | | | (2) | |
| Other | — | | | — | | | 36 | | | 34 | | | 3 | | | 6 | |
| Fair value of plan assets December 31 | $ | 9,717 | | | $ | 9,804 | | | $ | 9,647 | | | $ | 9,562 | | | $ | 1,040 | | | $ | 1,045 | |
| Benefit obligation January 1 | $ | 10,446 | | | $ | 9,854 | | | $ | 9,042 | | | $ | 7,755 | | | $ | 1,104 | | | $ | 1,157 | |
| Service cost | 373 | | | 326 | | | 243 | | | 196 | | | 30 | | | 32 | |
| Interest cost | 537 | | | 526 | | | 294 | | | 299 | | | 56 | | | 63 | |
Actuarial (gains) losses (1) | (595) | | | 403 | | | (549) | | | 766 | | | 32 | | | (58) | |
| Benefits paid | (615) | | | (497) | | | (250) | | | (256) | | | (89) | | | (95) | |
| Effects of exchange rate changes | — | | | — | | | (473) | | | 288 | | | (4) | | | 1 | |
| Plan amendments | — | | | — | | | (56) | | | 14 | | | — | | | — | |
| Curtailments | — | | | 8 | | | — | | | (1) | | | — | | | — | |
| Termination benefits | 5 | | | 3 | | | 1 | | | — | | | 4 | | | — | |
| Settlements | — | | | (177) | | | (14) | | | (53) | | | — | | | (2) | |
| Other | — | | | — | | | 36 | | | 34 | | | 3 | | | 6 | |
| Benefit obligation December 31 | $ | 10,151 | | | $ | 10,446 | | | $ | 8,274 | | | $ | 9,042 | | | $ | 1,136 | | | $ | 1,104 | |
| Funded status December 31 | $ | (434) | | | $ | (642) | | | $ | 1,373 | | | $ | 520 | | | $ | (96) | | | $ | (59) | |
| Recognized as: | | | | | | | | | | | |
| Other Assets | $ | 26 | | | $ | — | | | $ | 1,785 | | | $ | 1,019 | | | $ | 51 | | | $ | 107 | |
| Accrued and other current liabilities | (55) | | | (49) | | | (18) | | | (19) | | | (7) | | | (8) | |
| Other Noncurrent Liabilities | (405) | | | (593) | | | (394) | | | (480) | | | (140) | | | (158) | |
(1) Actuarial (gains) losses primarily reflect changes in discount rates.
At December 31, 2024 and 2023, the accumulated benefit obligation was $18.1 billion and $19.1 billion, respectively, for all pension plans, of which $10.0 billion and $10.3 billion, respectively, related to U.S. pension plans.
Information related to the funded status of selected pension plans at December 31 is as follows:
| | | | | | | | | | | | | | | | | | | | | | | |
| U.S. | | International |
| 2024 | | 2023 | | 2024 | | 2023 |
| Pension plans with a projected benefit obligation in excess of plan assets | | | | | | | |
| Projected benefit obligation | $ | 9,517 | | | $ | 10,446 | | | $ | 1,847 | | | $ | 2,961 | |
| Fair value of plan assets | 9,057 | | | 9,804 | | | 1,435 | | | 2,462 | |
| Pension plans with an accumulated benefit obligation in excess of plan assets | | | | | | | |
| Accumulated benefit obligation | $ | 442 | | | $ | 9,700 | | | $ | 1,768 | | | $ | 1,791 | |
| Fair value of plan assets | — | | | 9,186 | | | 1,385 | | | 1,336 | |
Plan Assets
Entities are required to use a fair value hierarchy which maximizes the use of observable inputs and minimizes the use of unobservable inputs when measuring fair value. There are three levels of inputs used to measure fair value with Level 1 having the highest priority and Level 3 having the lowest:
Level 1 — Quoted prices (unadjusted) in active markets for identical assets or liabilities.
Level 2 — Observable inputs other than Level 1 prices, such as quoted prices for similar assets or liabilities, or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities.
Level 3 — Unobservable inputs that are supported by little or no market activity. The Level 3 assets are those whose values are determined using pricing models, discounted cash flow methodologies, or similar techniques with significant unobservable inputs, as well as instruments for which the determination of fair value requires significant judgment or estimation. At December 31, 2024 and 2023, $700 million and $788 million, respectively, or approximately 4% of the Company’s pension investments were categorized as Level 3 assets.
If the inputs used to measure the financial assets fall within more than one level described above, the categorization is based on the lowest level input that is significant to the fair value measurement of the instrument.
The fair values of the Company’s pension plan assets at December 31 by asset category are as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Fair Value Measurements Using | | | | | | Fair Value Measurements Using | | | | |
| Level 1 | | Level 2 | | Level 3 | | NAV (1) | | Total | | Level 1 | | Level 2 | | Level 3 | | NAV (1) | | Total |
| 2024 | | | | | | 2023 | | | | |
| U.S. Pension Plans | | | | | | | | | | | | | | | | | | | |
| Cash and cash equivalents | $ | 43 | | | $ | — | | | $ | — | | | $ | 121 | | | $ | 164 | | | $ | 34 | | | $ | — | | | $ | — | | | $ | 124 | | | $ | 158 | |
| Investment funds | | | | | | | | | | | | | | | | | | | |
| Developed markets equities | 170 | | | — | | | — | | | 2,385 | | | 2,555 | | | 224 | | | — | | | — | | | 2,573 | | | 2,797 | |
| Emerging markets equities | — | | | — | | | — | | | 1,265 | | | 1,265 | | | — | | | — | | | — | | | 740 | | | 740 | |
| Real estate | — | | | — | | | — | | | 174 | | | 174 | | | — | | | — | | | — | | | 113 | | | 113 | |
| Equity securities | | | | | | | | | | | | | | | | | | | |
| Developed markets | 2,171 | | | — | | | — | | | — | | | 2,171 | | | 2,071 | | | — | | | — | | | — | | | 2,071 | |
| Fixed income securities | | | | | | | | | | | | | | | | | | | |
| Government and agency obligations | — | | | 2,101 | | | — | | | — | | | 2,101 | | | — | | | 2,307 | | | — | | | — | | | 2,307 | |
| Corporate obligations | — | | | 1,293 | | | — | | | — | | | 1,293 | | | — | | | 1,485 | | | — | | | — | | | 1,485 | |
| Mortgage and asset-backed securities | — | | | 21 | | | — | | | — | | | 21 | | | — | | | 21 | | | — | | | — | | | 21 | |
| Other investments (liabilities) | | | | | | | | | | | | | | | | | | | |
| Derivatives | (29) | | | — | | | — | | | — | | | (29) | | | 109 | | | — | | | — | | | — | | | 109 | |
| Other | — | | | — | | | 2 | | | — | | | 2 | | | — | | | — | | | 3 | | | — | | | 3 | |
| Plan assets at fair value | $ | 2,355 | | | $ | 3,415 | | | $ | 2 | | | $ | 3,945 | | | $ | 9,717 | | | $ | 2,438 | | | $ | 3,813 | | | $ | 3 | | | $ | 3,550 | | | $ | 9,804 | |
| International Pension Plans | | | | | | | | | | | | | | | | | | | |
| Cash and cash equivalents | $ | 112 | | | $ | — | | | $ | — | | | $ | 11 | | | $ | 123 | | | $ | 98 | | | $ | — | | | $ | — | | | $ | 20 | | | $ | 118 | |
| Investment funds | | | | | | | | | | | | | | | | | | | |
| Developed markets equities | 599 | | | 3,537 | | | — | | | 96 | | | 4,232 | | | 507 | | | 3,257 | | | — | | | 106 | | | 3,870 | |
| Government and agency obligations | 262 | | | 2,974 | | | — | | | 149 | | | 3,385 | | | 234 | | | 3,123 | | | — | | | 166 | | | 3,523 | |
| Corporate obligations | 23 | | | 8 | | | — | | | 149 | | | 180 | | | 23 | | | 8 | | | — | | | 166 | | | 197 | |
| Emerging markets equities | 54 | | | — | | | — | | | 91 | | | 145 | | | 44 | | | — | | | — | | | 66 | | | 110 | |
| Other fixed income obligations | 8 | | | 7 | | | — | | | 4 | | | 19 | | | 9 | | | 8 | | | — | | | 3 | | | 20 | |
| Real estate | — | | | — | | | — | | | 12 | | | 12 | | | — | | | — | | | — | | | 10 | | | 10 | |
| Equity securities | | | | | | | | | | | | | | | | | | | |
| Developed markets | 287 | | | — | | | — | | | — | | | 287 | | | 278 | | | — | | | — | | | — | | | 278 | |
| Fixed income securities | | | | | | | | | | | | | | | | | | | |
| Government and agency obligations | — | | | 368 | | | — | | | — | | | 368 | | | — | | | 423 | | | — | | | — | | | 423 | |
| Corporate obligations | — | | | 141 | | | — | | | — | | | 141 | | | — | | | 160 | | | — | | | — | | | 160 | |
| Mortgage and asset-backed securities | — | | | 54 | | | — | | | — | | | 54 | | | — | | | 61 | | | — | | | — | | | 61 | |
| Other investments | | | | | | | | | | | | | | | | | | | |
Insurance contracts (2) | — | | | 1 | | | 698 | | | 2 | | | 701 | | | — | | | 1 | | | 785 | | | 2 | | | 788 | |
| Other | — | | | — | | | — | | | — | | | — | | | 4 | | | — | | | — | | | — | | | 4 | |
| Plan assets at fair value | $ | 1,345 | | | $ | 7,090 | | | $ | 698 | | | $ | 514 | | | $ | 9,647 | | | $ | 1,197 | | | $ | 7,041 | | | $ | 785 | | | $ | 539 | | | $ | 9,562 | |
(1) Certain investments that were measured at net asset value (NAV) per share or its equivalent have not been classified in the fair value hierarchy. The NAV amounts presented in this table are intended to permit reconciliation of the fair value hierarchy to the fair value of plan assets at December 31, 2024 and 2023.
(2) The plans’ Level 3 investments in insurance contracts are generally valued using a crediting rate that approximates market returns and invest in underlying securities whose market values are unobservable and determined using pricing models, discounted cash flow methodologies, or similar techniques.
The table below provides a summary of the changes in fair value, including transfers in and/or out, of all financial assets measured at fair value using significant unobservable inputs (Level 3) for the Company’s pension plan assets:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | 2024 | | 2023 |
| Insurance
Contracts | | Other | | Total | | Insurance
Contracts | | Other | | Total |
| U.S. Pension Plans | | | | | | | | | | | |
| Balance January 1 | $ | — | | | $ | 3 | | | $ | 3 | | | $ | — | | | $ | 4 | | | $ | 4 | |
| Actual return on plan assets: | | | | | | | | | | | |
| Relating to assets still held at December 31 | — | | | (2) | | | (2) | | | — | | | (2) | | | (2) | |
| Relating to assets sold during the year | — | | | 2 | | | 2 | | | — | | | 2 | | | 2 | |
| Purchases and sales, net | — | | | (1) | | | (1) | | | — | | | (1) | | | (1) | |
| Balance December 31 | $ | — | | | $ | 2 | | | $ | 2 | | | $ | — | | | $ | 3 | | | $ | 3 | |
| International Pension Plans | | | | | | | | | | | |
| Balance January 1 | $ | 785 | | | $ | — | | | $ | 785 | | | $ | 761 | | | $ | — | | | $ | 761 | |
| Actual return on plan assets: | | | | | | | | | | | |
| Relating to assets still held at December 31 | (26) | | | — | | | (26) | | | 77 | | | — | | | 77 | |
| Purchases and sales, net | (61) | | | — | | | (61) | | | (53) | | | — | | | (53) | |
| Balance December 31 | $ | 698 | | | $ | — | | | $ | 698 | | | $ | 785 | | | $ | — | | | $ | 785 | |
The fair values of the Company’s other postretirement benefit plan assets at December 31 by asset category are as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Fair Value Measurements Using | | | | | | Fair Value Measurements Using | | | | |
| Level 1 | | Level 2 | | Level 3 | | NAV (1) | | Total | | Level 1 | | Level 2 | | Level 3 | | NAV (1) | | Total |
| 2024 | | | | | | 2023 | | | | |
| Cash and cash equivalents | $ | — | | | $ | — | | | $ | — | | | $ | 5 | | | $ | 5 | | | $ | — | | | $ | — | | | $ | — | | | $ | 13 | | | $ | 13 | |
| Investment funds | | | | | | | | | | | | | | | | | | | |
| Developed markets equities | 3 | | | — | | | — | | | 46 | | | 49 | | | 24 | | | — | | | — | | | 277 | | | 301 | |
| Emerging markets equities | — | | | — | | | — | | | 24 | | | 24 | | | — | | | — | | | — | | | 80 | | | 80 | |
| Real estate | — | | | — | | | — | | | 3 | | | 3 | | | — | | | — | | | — | | | 12 | | | 12 | |
| Equity securities | | | | | | | | | | | | | | | | | | | |
| Developed markets | 41 | | | — | | | — | | | | | 41 | | | 223 | | | — | | | — | | | — | | | 223 | |
| Fixed income securities | | | | | | | | | | | | | | | | | | | |
| Corporate obligations | — | | | 598 | | | — | | | | | 598 | | | — | | | 157 | | | — | | | — | | | 157 | |
| Government and agency obligations | — | | | 266 | | | — | | | | | 266 | | | — | | | 245 | | | — | | | — | | | 245 | |
| Mortgage and asset-backed securities | — | | | 54 | | | — | | | — | | | 54 | | | — | | | 2 | | | — | | | — | | | 2 | |
| Other Investments (liabilities) | | | | | | | | | | | | | | | | | | | |
| Derivatives | — | | | — | | | — | | | — | | | — | | | 12 | | | — | | | — | | | — | | | 12 | |
| Plan assets at fair value | $ | 44 | | | $ | 918 | | | $ | — | | | $ | 78 | | | $ | 1,040 | | | $ | 259 | | | $ | 404 | | | $ | — | | | $ | 382 | | | $ | 1,045 | |
(1) Certain investments that were measured at net asset value (NAV) per share or its equivalent have not been classified in the fair value hierarchy. The NAV amounts presented in this table are intended to permit reconciliation of the fair value hierarchy to the fair value of plan assets at December 31, 2024 and 2023.
The Company has established investment guidelines for its U.S. pension and other postretirement plans to create an asset allocation that is expected to deliver a rate of return sufficient to meet the long-term obligation of each plan, given an acceptable level of risk. The target investment portfolio of the Company’s U.S. pension and other postretirement benefit plans is allocated 25% to 40% in U.S. equities, 15% to 30% in international equities, 40% to 50% in fixed-income investments, and up to 8% in cash and other investments. The portfolio’s equity weighting is consistent with the long-term nature of the plans’ benefit obligations. The expected annual standard deviation of returns of the target portfolio, which approximates 12%, reflects both the equity allocation and the diversification benefits among the asset classes in which the portfolio invests. For international pension plans, the targeted investment portfolio varies based on the duration of pension liabilities and local government rules and regulations.
Although a significant percentage of plan assets are invested in U.S. equities, concentration risk is mitigated through the use of strategies that are diversified within management guidelines.
Expected Contributions
Contributions during 2025 are expected to be approximately $270 million for U.S. pension plans, approximately $180 million for international pension plans and approximately $70 million for other postretirement benefit plans.
Expected Benefit Payments
Expected benefit payments are as follows:
| | | | | | | | | | | | | | | | | |
| U.S. Pension Benefits | | International Pension
Benefits | | Other
Postretirement
Benefits |
| 2025 | $ | 771 | | | $ | 291 | | | $ | 86 | |
| 2026 | 775 | | | 275 | | | 87 | |
| 2027 | 789 | | | 286 | | | 89 | |
| 2028 | 799 | | | 300 | | | 91 | |
| 2029 | 822 | | | 314 | | | 96 | |
| 2030 — 2034 | 4,386 | | | 1,792 | | | 515 | |
Expected benefit payments are based on the same assumptions used to measure the benefit obligations and include estimated future employee service.
Amounts Recognized in Other Comprehensive Income (Loss)
Net gain/loss amounts reflect differences between expected and actual returns on plan assets as well as the effects of changes in actuarial assumptions. Net gain/loss amounts in excess of certain thresholds are amortized into net periodic benefit cost over the average remaining service life of employees. The following amounts were reflected as components of OCI:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Pension Plans | | Other Postretirement
Benefit Plans |
| U.S. | | International | |
| Years Ended December 31 | 2024 | | 2023 | | 2022 | | 2024 | | 2023 | | 2022 | | 2024 | | 2023 | | 2022 |
| Net gain (loss) arising during the period | $ | 35 | | | $ | (69) | | | $ | (42) | | | $ | 634 | | | $ | (438) | | | $ | 116 | | | $ | (78) | | | $ | 110 | | | $ | — | |
| Prior service credit (cost) arising during the period | — | | | — | | | — | | | 56 | | | (16) | | | (4) | | | — | | | — | | | — | |
| | $ | 35 | | | $ | (69) | | | $ | (42) | | | $ | 690 | | | $ | (454) | | | $ | 112 | | | $ | (78) | | | $ | 110 | | | $ | — | |
| Net loss (gain) amortization included in benefit cost | $ | 43 | | | $ | — | | | $ | 128 | | | $ | 5 | | | $ | (3) | | | $ | 96 | | | $ | (51) | | | $ | (42) | | | $ | (43) | |
| Prior service (credit) cost amortization included in benefit cost | — | | | (1) | | | (32) | | | (13) | | | 2 | | | (14) | | | (43) | | | (49) | | | (57) | |
| Settlements and curtailments | — | | | 36 | | | 251 | | | (1) | | | (6) | | | 1 | | | — | | | (1) | | | (1) | |
| | $ | 43 | | | $ | 35 | | | $ | 347 | | | $ | (9) | | | $ | (7) | | | $ | 83 | | | $ | (94) | | | $ | (92) | | | $ | (101) | |
Actuarial Assumptions
The Company reassesses its benefit plan assumptions on a regular basis. The weighted average assumptions used in determining U.S. pension and other postretirement benefit plan and international pension plan information are as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | U.S. Pension and Other
Postretirement Benefit Plans | | International Pension Plans |
| December 31 | 2024 | | 2023 | | 2022 | | 2024 | | 2023 | | 2022 |
| Net periodic benefit cost | | | | | | | | | | | |
| Discount rate | 5.30 | % | | 5.50 | % | | 3.00 | % | | 3.40 | % | | 3.90 | % | | 1.50 | % |
| Expected rate of return on plan assets | 7.75 | % | | 7.00 | % | | 6.70 | % | | 5.20 | % | | 5.00 | % | | 3.70 | % |
| Salary growth rate | 4.60 | % | | 4.60 | % | | 4.60 | % | | 3.20 | % | | 3.20 | % | | 2.90 | % |
| Interest crediting rate | 5.30 | % | | 5.30 | % | | 5.00 | % | | 3.40 | % | | 3.30 | % | | 3.00 | % |
| Benefit obligation | | | | | | | | | | | |
| Discount rate | 5.70 | % | | 5.30 | % | | 5.50 | % | | 3.70 | % | | 3.40 | % | | 3.90 | % |
| Salary growth rate | 4.80 | % | | 4.60 | % | | 4.60 | % | | 3.10 | % | | 3.20 | % | | 3.20 | % |
| Interest crediting rate | 5.40 | % | | 5.30 | % | | 5.30 | % | | 3.50 | % | | 3.40 | % | | 3.30 | % |
For both the pension and other postretirement benefit plans, the discount rate is evaluated on measurement dates and modified to reflect the prevailing market rate of a portfolio of high-quality fixed-income debt instruments that would provide the future cash flows needed to pay the benefits included in the benefit obligation as they come due. The expected rate of return for both the pension and other postretirement benefit plans represents the average rate of return to be earned on plan assets over the period the benefits included in the benefit obligation are to be paid and is determined on a plan basis. The expected rate of return for each plan is developed considering long-term historical returns data, current market conditions, and actual returns on the plan assets. Using this reference information, the long-term return expectations for each asset category and a weighted-average expected return for each plan’s target portfolio is developed according to the allocation among those investment categories. The expected portfolio performance reflects the contribution of active management as appropriate. For 2025, the expected rate of return for the Company’s U.S. pension and other postretirement benefit plans will be 7.70%, as compared to 7.75% in 2024.
The health care cost trend rate assumptions for other postretirement benefit plans are as follows:
| | | | | | | | | | | |
| December 31 | 2024 | | 2023 |
| Health care cost trend rate assumed for next year | 7.90 | % | | 7.80 | % |
| Rate to which the cost trend rate is assumed to decline | 4.50 | % | | 4.50 | % |
| Year that the trend rate reaches the ultimate trend rate | 2040 | | 2038 |
Savings Plans
The Company also maintains defined contribution savings plans in the U.S. The Company matches a percentage of each employee’s contributions consistent with the provisions of the plan for which the employee is eligible. Total employer contributions to these plans in 2024, 2023 and 2022 were $215 million, $199 million and $175 million, respectively.
14. Other (Income) Expense, Net
Other (income) expense, net, consisted of:
| | | | | | | | | | | | | | | | | |
| Years Ended December 31 | 2024 | | 2023 | | 2022 |
| Interest income | $ | (415) | | | $ | (365) | | | $ | (157) | |
| Interest expense | 1,271 | | | 1,146 | | | 962 | |
| Exchange losses | 227 | | | 370 | | | 237 | |
(Income) loss from investments in equity securities, net (1) | (14) | | | (340) | | | 1,419 | |
| Net periodic defined benefit plan (credit) cost other than service cost | (633) | | | (498) | | | (279) | |
| Other, net | (460) | | | 153 | | | (681) | |
| | $ | (24) | | | $ | 466 | | | $ | 1,501 | |
(1) Includes net realized and unrealized gains and losses from investments in equity securities either owned directly or through ownership interests in investment funds. Unrealized gains and losses from investments that are directly owned are determined at the end of the reporting period, while gains and losses from ownership interests in investment funds are accounted for on a one quarter lag.
Other, net (as reflected in the table above) in 2024 includes $170 million of income related to the expansion of a collaboration agreement with Daiichi Sankyo (see Note 4). Other, net, in 2023 includes a $572.5 million charge related to settlements with certain plaintiffs in the Zetia antitrust litigation (see Note 10).
Interest paid was $1.3 billion in 2024, $1.1 billion in 2023 and $937 million in 2022.
15. Taxes on Income
A reconciliation between the effective tax rate and the U.S. statutory rate is as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | 2024 | | 2023 | | 2022 |
| Amount | | Tax Rate | | Amount | | Tax Rate | | Amount | | Tax Rate |
| U.S. statutory rate applied to income before taxes | $ | 4,186 | | | 21.0 | % | | $ | 397 | | | 21.0 | % | | $ | 3,453 | | | 21.0 | % |
| Differential arising from: | | | | | | | | | | | |
| Foreign earnings | (1,301) | | | (6.5) | | | (941) | | | (49.8) | | | (1,821) | | | (11.1) | |
| Tax settlements and statute lapses | (557) | | | (2.8) | | | — | | | — | | | (10) | | | (0.1) | |
| R&D tax credit | (202) | | | (1.0) | | | (214) | | | (11.3) | | | (117) | | | (0.7) | |
| Inventory donations | (71) | | | (0.4) | | | (65) | | | (3.5) | | | (52) | | | (0.3) | |
| State taxes | (39) | | | (0.2) | | | (117) | | | (6.2) | | | (110) | | | (0.7) | |
| Charges for certain research and development asset acquisitions | 554 | | | 2.8 | | | 253 | | | 13.4 | | | — | | | — | |
| Valuation allowances | 54 | | | 0.3 | | | 70 | | | 3.7 | | | 108 | | | 0.7 | |
| Restructuring | 52 | | | 0.3 | | | 41 | | | 2.2 | | | 11 | | | 0.1 | |
| GILTI and the foreign-derived intangible income deduction | 29 | | | 0.1 | | | (80) | | | (4.3) | | | 462 | | | 2.8 | |
| Acquisition-related costs, including amortization | 18 | | | 0.1 | | | 42 | | | 2.2 | | | (3) | | | — | |
| Acquisition of Prometheus | — | | | — | | | 2,139 | | | 113.3 | | | — | | | — | |
| Other | 80 | | | 0.4 | | | (13) | | | (0.7) | | | (3) | | | — | |
| | $ | 2,803 | | | 14.1 | % | | $ | 1,512 | | | 80.0 | % | | $ | 1,918 | | | 11.7 | % |
Where applicable, the impact of changes in uncertain tax positions is reflected in the reconciling items above.
The Company’s remaining transition tax liability under the Tax Cuts and Jobs Act (TCJA) of 2017, which has been reduced by payments and the expected utilization of foreign tax credits, was a net liability of $518 million at December 31, 2024, which is comprised of a $1.2 billion tax liability included in Income taxes payable, offset by $702 million of foreign tax credits included in Other Assets that Merck expects to be applied upon the completion of the IRS’s examination of the Company’s tax returns for the 2017 and 2018 federal tax years. As a result of the transition tax under the TCJA, the Company is no longer indefinitely reinvested with respect to its undistributed earnings from foreign subsidiaries and has provided a deferred tax liability for foreign withholding taxes that would apply. The
Company remains indefinitely reinvested with respect to its financial statement basis in excess of tax basis of its foreign subsidiaries. A determination of the net deferred tax liability with respect to this basis difference is not practicable.
The foreign earnings tax rate differentials in the tax rate reconciliation above primarily reflect the impacts of operations in jurisdictions with different effective tax rates than the U.S., particularly Ireland, the Netherlands and Switzerland, as well as Singapore and Puerto Rico which operate under tax incentive grants (which begin to expire in 2025), thereby yielding a favorable impact on the effective tax rate compared with the U.S. statutory rate of 21%. The Company has an additional Cantonal tax holiday in Switzerland that provides for a tax rate reduction and is effective through 2032. The Company’s income that is subject to tax incentive grants and the Cantonal tax holiday in Switzerland is subject to the global minimum tax provision of the Organization for Economic Cooperation and Development (OECD) Pillar 2, effective in 2024.
Income before taxes consisted of:
| | | | | | | | | | | | | | | | | |
| Years Ended December 31 | 2024 | | 2023 | | 2022 |
| Domestic | $ | (1,849) | | | $ | (15,622) | | | $ | 1,011 | |
| Foreign | 21,785 | | | 17,511 | | | 15,433 | |
| | $ | 19,936 | | | $ | 1,889 | | | $ | 16,444 | |
Taxes on income consisted of:
| | | | | | | | | | | | | | | | | |
| Years Ended December 31 | 2024 | | 2023 | | 2022 |
| Current provision | | | | | |
| Federal | $ | 944 | | | $ | 928 | | | $ | 2,265 | |
| Foreign | 3,123 | | | 2,435 | | | 1,164 | |
| State | (15) | | | 48 | | | 57 | |
| | 4,052 | | | 3,411 | | | 3,486 | |
| Deferred provision | | | | | |
| Federal | (1,475) | | | (1,559) | | | (1,510) | |
| Foreign | 212 | | | (233) | | | 71 | |
| State | 14 | | | (107) | | | (129) | |
| | (1,249) | | | (1,899) | | | (1,568) | |
| | $ | 2,803 | | | $ | 1,512 | | | $ | 1,918 | |
Deferred income taxes at December 31 consisted of:
| | | | | | | | | | | | | | | | | | | | | | | |
| | 2024 | | 2023 |
| Assets | | Liabilities | | Assets | | Liabilities |
| Product intangibles and licenses | $ | 71 | | | $ | 978 | | | $ | — | | | $ | 1,308 | |
| R&D capitalization | 3,062 | | | — | | | 2,099 | | | — | |
| Inventory related | 84 | | | 413 | | | 86 | | | 370 | |
| Accelerated depreciation | — | | | 645 | | | — | | | 626 | |
| Undistributed foreign earnings | 275 | | | 371 | | | 76 | | | 118 | |
| Equity investments | — | | | 90 | | | — | | | 73 | |
| Pensions and other postretirement benefits | 224 | | | 400 | | | 323 | | | 249 | |
| Compensation related | 400 | | | — | | | 357 | | | — | |
| Unrecognized tax benefits | 152 | | | — | | | 147 | | | — | |
| Net operating losses and other tax credit carryforwards | 910 | | | — | | | 868 | | | — | |
| Other | 802 | | | 159 | | | 755 | | | 214 | |
| Subtotal | 5,980 | | | 3,056 | | | 4,711 | | | 2,958 | |
| Valuation allowance | (710) | | | | | (656) | | | |
| Total deferred taxes | $ | 5,270 | | | $ | 3,056 | | | $ | 4,055 | | | $ | 2,958 | |
| Net deferred income taxes | $ | 2,214 | | | | | $ | 1,097 | | | |
| Recognized as: | | | | | | | |
| Other Assets | $ | 3,601 | | | | | $ | 1,968 | | | |
| Deferred Income Taxes | | | $ | 1,387 | | | | | $ | 871 | |
The Company has net operating loss (NOL) carryforwards in several jurisdictions. As of December 31, 2024, $324 million of deferred tax assets on NOL carryforwards relate to foreign jurisdictions. Valuation allowances of $264 million have been established on these foreign NOL carryforwards and other foreign deferred tax assets. In addition, the Company has $586 million of deferred tax assets relating to various U.S. tax credit carryforwards and NOL carryforwards. Valuation allowances of $446 million have been established on these U.S. tax credit carryforwards and NOL carryforwards.
Income taxes paid in 2024, 2023 and 2022 consisted of:
| | | | | | | | | | | | | | | | | |
| Years Ended December 31 | 2024 | | 2023 | | 2022 |
Domestic (1) | $ | 974 | | | $ | 2,258 | | | $ | 1,891 | |
| Foreign | 2,954 | | | 2,080 | | | 1,348 | |
| | $ | 3,928 | | | $ | 4,338 | | | $ | 3,239 | |
(1) Includes TCJA transition tax payments.
Tax benefits relating to stock option exercises were $26 million in 2024, $12 million in 2023 and $45 million in 2022.
A reconciliation of the beginning and ending amount of unrecognized tax benefits is as follows:
| | | | | | | | | | | | | | | | | |
| 2024 | | 2023 | | 2022 |
| Balance January 1 | $ | 2,384 | | | $ | 1,835 | | | $ | 1,529 | |
| Additions related to current year positions | 421 | | | 553 | | | 344 | |
| Additions related to prior year positions | 35 | | | 91 | | | 48 | |
| Reductions for tax positions of prior years | (33) | | | (20) | | | (40) | |
| Settlements | (18) | | | (23) | | | (6) | |
Lapse of statute of limitations (1) | (528) | | | (52) | | | (40) | |
| Balance December 31 | $ | 2,261 | | | $ | 2,384 | | | $ | 1,835 | |
(1) Amount in 2024 reflects a reduction of $451 million resulting from the expiration of the statute of limitations related to the 2019 and 2020 federal tax return years.
If the Company were to recognize the unrecognized tax benefits of $2.3 billion at December 31, 2024, the income tax provision would reflect a favorable net impact of $2.2 billion.
The Company is under examination by numerous tax authorities in various jurisdictions globally. The Company believes that it is reasonably possible that the total amount of unrecognized tax benefits as of December 31, 2024 could decrease by up to approximately $22 million in the next 12 months as a result of various audit closures, settlements or the expiration of the statute of limitations. The ultimate finalization of the Company’s examinations with relevant taxing authorities can include formal administrative and legal proceedings, which could have a significant impact on the timing of the reversal of unrecognized tax benefits. The Company believes that its reserves for uncertain tax positions are adequate to cover existing risks or exposures.
Interest and penalties associated with uncertain tax positions amounted to an expense of $51 million in 2024, $131 million in 2023 and $54 million in 2022. These amounts reflect the beneficial impacts of various tax settlements. Liabilities for accrued interest and penalties were $437 million and $388 million as of December 31, 2024 and 2023, respectively.
In 2024, the Company recorded a benefit of $519 million due to a reduction in reserves for unrecognized income tax benefits resulting from the expiration in 2024 of the statute of limitations for assessments related to the 2019 and 2020 federal tax return years. The Internal Revenue Service (IRS) is currently conducting examinations of the Company’s tax returns for the years 2017 and 2018, including the one-time transition tax enacted under the TCJA. If the IRS disagrees with the Company’s transition tax position, it may result in a significant tax liability. The IRS is also currently conducting examinations of the Company’s tax returns for the years 2021 and 2022. In addition, various state and foreign tax examinations are in progress and for these jurisdictions, the Company’s income tax returns are open for examination for the period 2009 through 2024.
16. Earnings per Share
The calculations of earnings per share (shares in millions) are as follows:
| | | | | | | | | | | | | | | | | |
| Years Ended December 31 | 2024 | | 2023 | | 2022 |
| Net Income Attributable to Merck & Co., Inc. | $ | 17,117 | | | $ | 365 | | | $ | 14,519 | |
| Average common shares outstanding | 2,532 | | | 2,537 | | | 2,532 | |
Common shares issuable (1) | 9 | | | 10 | | | 10 | |
| Average common shares outstanding assuming dilution | 2,541 | | | 2,547 | | | 2,542 | |
| Basic Earnings per Common Share Attributable to Merck & Co., Inc. Common Shareholders | $ | 6.76 | | | $ | 0.14 | | | $ | 5.73 | |
| Earnings per Common Share Assuming Dilution Attributable to Merck & Co., Inc. Common Shareholders | $ | 6.74 | | | $ | 0.14 | | | $ | 5.71 | |
(1) Issuable primarily under share-based compensation plans.
In 2024, 2023 and 2022, 6 million, 5 million and 2 million, respectively, of common shares issuable under share-based compensation plans were excluded from the computation of earnings per common share assuming dilution because the effect would have been antidilutive.
17. Other Comprehensive Income (Loss)
Changes in each component of other comprehensive income (loss) are as follows:
| | | | | | | | | | | | | | | | | | | | | | | |
| Derivatives | | Employee
Benefit
Plans | | Foreign Currency
Translation
Adjustment | | Accumulated Other
Comprehensive Loss |
| Balance at January 1, 2022, net of taxes | $ | 144 | | | $ | (2,743) | | | $ | (1,830) | | | $ | (4,429) | |
| Other comprehensive income (loss) before reclassification adjustments, pretax | 684 | | | 70 | | | (584) | | | 170 | |
| Tax | (143) | | | 12 | | | (19) | | | (150) | |
| Other comprehensive income (loss) before reclassification adjustments, net of taxes | 541 | | | 82 | | | (603) | | | 20 | |
| Reclassification adjustments, pretax | (775) | | (1) | 329 | | (2) | — | | | (446) | |
| Tax | 163 | | | (76) | | | — | | | 87 | |
| Reclassification adjustments, net of taxes | (612) | | | 253 | | | — | | | (359) | |
| Other comprehensive income (loss), net of taxes | (71) | | | 335 | | | (603) | | | (339) | |
| Balance at December 31, 2022, net of taxes | 73 | | | (2,408) | | | (2,433) | | | (4,768) | |
| Other comprehensive income (loss) before reclassification adjustments, pretax | 114 | | | (413) | | | 17 | | | (282) | |
| Tax | (24) | | | 86 | | | 63 | | | 125 | |
| Other comprehensive income (loss) before reclassification adjustments, net of taxes | 90 | | | (327) | | | 80 | | | (157) | |
| Reclassification adjustments, pretax | (237) | | (1) | (64) | | (2) | 9 | | | (292) | |
| Tax | 50 | | | 6 | | | — | | | 56 | |
| Reclassification adjustments, net of taxes | (187) | | | (58) | | | 9 | | | (236) | |
| Other comprehensive income (loss), net of taxes | (97) | | | (385) | | | 89 | | | (393) | |
| Balance at December 31, 2023, net of taxes | (24) | | | (2,793) | | (3) | (2,344) | | | (5,161) | |
| Other comprehensive income (loss) before reclassification adjustments, pretax | 508 | | | 647 | | | (559) | | | 596 | |
| Tax | (109) | | | (138) | | | 23 | | | (224) | |
| Other comprehensive income (loss) before reclassification adjustments, net of taxes | 399 | | | 509 | | | (536) | | | 372 | |
| Reclassification adjustments, pretax | (168) | | (1) | (60) | | (2) | 20 | | | (208) | |
| Tax | 35 | | | 17 | | | — | | | 52 | |
| Reclassification adjustments, net of taxes | (133) | | | (43) | | | 20 | | | (156) | |
| Other comprehensive income (loss), net of taxes | 266 | | | 466 | | | (516) | | | 216 | |
| Balance at December 31, 2024, net of taxes | $ | 242 | | | $ | (2,327) | | (3) | $ | (2,860) | | | $ | (4,945) | |
(1) Primarily relates to foreign currency cash flow hedges that were reclassified from AOCL to Sales (see Note 6).
(2) Includes net amortization of prior service cost, actuarial gains and losses, settlements and curtailments included in net periodic benefit cost (see Note 13).
(3) Includes pension plan net loss of $3.0 billion and $3.5 billion at December 31, 2024 and 2023, respectively, and other postretirement benefit plan net gain of $400 million and $500 million at December 31, 2024 and 2023, respectively, as well as pension plan prior service credit of $174 million and $141 million at December 31, 2024 and 2023, respectively, and other postretirement benefit plan prior service credit of $61 million and $95 million at December 31, 2024 and 2023, respectively.
18. Segment Reporting
The Company’s operations are principally managed on a product basis and include two operating segments, Pharmaceutical and Animal Health, both of which are reportable segments.
The Pharmaceutical segment includes human health pharmaceutical and vaccine products. Human health pharmaceutical products consist of therapeutic and preventive agents, generally sold by prescription, for the treatment of human disorders. The Company sells these human health pharmaceutical products primarily to drug wholesalers and retailers, hospitals, government agencies and managed health care providers such as health maintenance organizations, pharmacy benefit managers and other institutions. Human health vaccine products consist of preventive pediatric, adolescent and adult vaccines. The Company sells these human health vaccines primarily to physicians, wholesalers, distributors and government entities. A large component of pediatric and adolescent vaccine sales are made to the U.S. Centers for Disease Control and Prevention Vaccines for Children program, which is funded by the U.S. government. Additionally, the Company sells vaccines to the Federal government for placement into vaccine stockpiles.
The Animal Health segment discovers, develops, manufactures and markets a wide range of veterinary pharmaceutical and vaccine products, as well as health management solutions and services, for the prevention, treatment and control of disease in all major livestock and companion animal species. The Company also offers an extensive suite of digitally connected identification, traceability and monitoring products. The Company sells its products to veterinarians, distributors, animal producers, farmers and pet owners.
Sales of the Company’s products were as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Years Ended December 31 | 2024 | | 2023 | | 2022 |
| U.S. | | Int’l | | Total | | U.S. | | Int’l | | Total | | U.S. | | Int’l | | Total |
| Pharmaceutical: | | | | | | | | | | | | | | | | | |
| Oncology | | | | | | | | | | | | | | | | | |
| Keytruda | $ | 17,872 | | | $ | 11,610 | | | $ | 29,482 | | | $ | 15,114 | | | $ | 9,897 | | | $ | 25,011 | | | $ | 12,686 | | | $ | 8,251 | | | $ | 20,937 | |
Alliance revenue - Lynparza (1) | 626 | | | 685 | | | 1,311 | | | 607 | | | 592 | | | 1,199 | | | 584 | | | 532 | | | 1,116 | |
Alliance revenue - Lenvima (1) | 705 | | | 305 | | | 1,010 | | | 657 | | | 303 | | | 960 | | | 579 | | | 297 | | | 876 | |
| Welireg | 466 | | | 43 | | | 509 | | | 209 | | | 10 | | | 218 | | | 123 | | | — | | | 123 | |
Alliance revenue - Reblozyl (2) | 303 | | | 68 | | | 371 | | | 168 | | | 43 | | | 212 | | | 123 | | | 43 | | | 166 | |
| Vaccines | | | | | | | | | | | | | | | | | |
Gardasil/Gardasil 9 | 2,425 | | | 6,158 | | | 8,583 | | | 2,083 | | | 6,803 | | | 8,886 | | | 2,065 | | | 4,832 | | | 6,897 | |
| ProQuad/M-M-R II/Varivax | 1,919 | | | 566 | | | 2,485 | | | 1,837 | | | 531 | | | 2,368 | | | 1,724 | | | 518 | | | 2,241 | |
| Vaxneuvance | 461 | | | 347 | | | 808 | | | 561 | | | 103 | | | 665 | | | 163 | | | 7 | | | 170 | |
| RotaTeq | 472 | | | 239 | | | 711 | | | 493 | | | 276 | | | 769 | | | 508 | | | 275 | | | 783 | |
Pneumovax 23 | 56 | | | 207 | | | 263 | | | 127 | | | 285 | | | 412 | | | 346 | | | 256 | | | 602 | |
| Hospital Acute Care | | | | | | | | | | | | | | | | | |
| Bridion | 1,401 | | | 363 | | | 1,764 | | | 1,156 | | | 686 | | | 1,842 | | | 922 | | | 762 | | | 1,685 | |
| Prevymis | 371 | | | 414 | | | 785 | | | 264 | | | 341 | | | 605 | | | 188 | | | 240 | | | 428 | |
| Dificid | 303 | | | 37 | | | 340 | | | 274 | | | 28 | | | 302 | | | 241 | | | 22 | | | 263 | |
| Zerbaxa | 146 | | | 106 | | | 252 | | | 119 | | | 100 | | | 218 | | | 89 | | | 79 | | | 169 | |
| Noxafil | 7 | | | 170 | | | 177 | | | 32 | | | 181 | | | 213 | | | 51 | | | 187 | | | 238 | |
| Cardiovascular | | | | | | | | | | | | | | | | | |
| Winrevair | 408 | | | 11 | | | 419 | | | — | | | — | | | — | | | — | | | — | | | — | |
Alliance revenue - Adempas/Verquvo (3) | 388 | | | 27 | | | 415 | | | 350 | | | 16 | | | 367 | | | 329 | | | 12 | | | 341 | |
| Adempas | — | | | 287 | | | 287 | | | — | | | 255 | | | 255 | | | — | | | 238 | | | 238 | |
| Virology | | | | | | | | | | | | | | | | | |
| Lagevrio | 176 | | | 787 | | | 964 | | | 10 | | | 1,418 | | | 1,428 | | | 1,523 | | | 4,161 | | | 5,684 | |
| Isentress/Isentress HD | 185 | | | 209 | | | 394 | | | 215 | | | 268 | | | 483 | | | 274 | | | 359 | | | 633 | |
| Delstrigo | 56 | | | 193 | | | 249 | | | 49 | | | 152 | | | 201 | | | 39 | | | 111 | | | 151 | |
| Pifeltro | 113 | | | 50 | | | 163 | | | 101 | | | 41 | | | 142 | | | 87 | | | 30 | | | 118 | |
| Neuroscience | | | | | | | | | | | | | | | | | |
| Belsomra | 72 | | | 150 | | | 222 | | | 81 | | | 150 | | | 231 | | | 79 | | | 179 | | | 258 | |
| Immunology | | | | | | | | | | | | | | | | | |
| Simponi | — | | | 543 | | | 543 | | | — | | | 710 | | | 710 | | | — | | | 706 | | | 706 | |
| Remicade | — | | | 114 | | | 114 | | | — | | | 187 | | | 187 | | | — | | | 207 | | | 207 | |
| Diabetes | | | | | | | | | | | | | | | | | |
| Januvia | 469 | | | 865 | | | 1,334 | | | 1,151 | | | 1,039 | | | 2,189 | | | 1,248 | | | 1,565 | | | 2,813 | |
| Janumet | 161 | | | 774 | | | 935 | | | 223 | | | 954 | | | 1,177 | | | 355 | | | 1,344 | | | 1,700 | |
Other pharmaceutical (4) | 729 | | | 1,782 | | | 2,510 | | | 658 | | | 1,675 | | | 2,333 | | | 663 | | | 1,803 | | | 2,462 | |
| Total Pharmaceutical segment sales | 30,290 | | | 27,110 | | | 57,400 | | | 26,539 | | | 27,044 | | | 53,583 | | | 24,989 | | | 27,016 | | | 52,005 | |
| Animal Health: | | | | | | | | | | | | | | | | | |
| Livestock | 732 | | | 2,729 | | | 3,462 | | | 700 | | | 2,637 | | | 3,337 | | | 710 | | | 2,590 | | | 3,300 | |
| Companion Animal | 1,129 | | | 1,287 | | | 2,415 | | | 1,104 | | | 1,184 | | | 2,288 | | | 1,112 | | | 1,138 | | | 2,250 | |
| Total Animal Health segment sales | 1,861 | | | 4,016 | | | 5,877 | | | 1,804 | | | 3,821 | | | 5,625 | | | 1,822 | | | 3,728 | | | 5,550 | |
| Total segment sales | 32,151 | | | 31,126 | | | 63,277 | | | 28,343 | | | 30,865 | | | 59,208 | | | 26,811 | | | 30,744 | | | 57,555 | |
Other (5) | 126 | | | 765 | | | 891 | | | 137 | | | 770 | | | 907 | | | 395 | | | 1,333 | | | 1,728 | |
| | $ | 32,277 | | | $ | 31,891 | | | $ | 64,168 | | | $ | 28,480 | | | $ | 31,635 | | | $ | 60,115 | | | $ | 27,206 | | | $ | 32,077 | | | $ | 59,283 | |
U.S. plus international may not equal total due to rounding.
(1) Alliance revenue for Lynparza and Lenvima represents Merck’s share of profits, which are product sales net of cost of sales and commercialization costs (see Note 4).
(2) Alliance revenue for Reblozyl represents royalties and, for 2022, also includes a payment received related to the achievement of a regulatory approval milestone (see Note 4).
(3) Alliance revenue for Adempas/Verquvo represents Merck’s share of profits from sales in Bayer’s marketing territories, which are product sales net of cost of sales and commercialization costs (see Note 4).
(4) Other pharmaceutical primarily reflects sales of other human health pharmaceutical products, including products within the franchises not listed separately.
(5) Other is primarily comprised of miscellaneous corporate revenue, including revenue hedging activities which increased sales by $195 million, $244 million and $810 million in 2024, 2023 and 2022, respectively, as well as revenue from third-party manufacturing arrangements (including sales to Organon). Other for 2024, 2023 and 2022 also includes $106 million, $118 million and $165 million, respectively, related to upfront and milestone payments received by Merck for out-licensing arrangements.
Consolidated sales by geographic area where derived are as follows:
| | | | | | | | | | | | | | | | | |
| Years Ended December 31 | 2024 | | 2023 | | 2022 |
| United States | $ | 32,277 | | | $ | 28,480 | | | $ | 27,206 | |
| Europe, Middle East and Africa | 14,041 | | | 13,254 | | | 14,493 | |
| China | 5,494 | | | 6,802 | | | 5,191 | |
| Latin America | 3,459 | | | 3,086 | | | 2,582 | |
| Japan | 3,280 | | | 3,164 | | | 3,629 | |
| Asia Pacific (other than China and Japan) | 3,058 | | | 3,225 | | | 3,614 | |
| Other | 2,559 | | | 2,104 | | | 2,568 | |
| | $ | 64,168 | | | $ | 60,115 | | | $ | 59,283 | |
A reconciliation of segment profits to Income Before Taxes is as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Years Ended December 31 | 2024 | | 2023 | | 2022 |
| Pharma-ceutical | | Animal Health | | Total | | Pharma-ceutical | | Animal Health | | Total | | Pharma-ceutical | | Animal Health | | Total |
| Segment sales | $ | 57,400 | | | $ | 5,877 | | | $ | 63,277 | | | $ | 53,583 | | | $ | 5,625 | | | $ | 59,208 | | | $ | 52,005 | | | $ | 5,550 | | | $ | 57,555 | |
Less segment costs: (1) | | | | | | | | | | | | | | | | | |
| Cost of sales | 6,828 | | | 2,469 | | | | | 8,849 | | | 2,498 | | | | | 9,678 | | | 2,259 | | | |
| Selling, general and administrative | 6,128 | | | 1,084 | | | | | 5,903 | | | 1,038 | | | | | 5,474 | | | 999 | | | |
Research and development (2) | — | | | 385 | | | | | — | | | 353 | | | | | — | | | 329 | | | |
Other segment items (3) | (89) | | | 1 | | | | | (49) | | | (1) | | | | | 1 | | | — | | | |
| Total segment profits | 44,533 | | | 1,938 | | | 46,471 | | | 38,880 | | | 1,737 | | | 40,617 | | | 36,852 | | | 1,963 | | | 38,815 | |
| Other profits | | | | | 492 | | | | | | | 474 | | | | | | | 1,160 | |
| Unallocated: | | | | | | | | | | | | | | | | | |
| Interest income | | | | | 415 | | | | | | | 365 | | | | | | | 157 | |
| Interest expense | | | | | (1,271) | | | | | | | (1,146) | | | | | | | (962) | |
| Amortization | | | | | (2,395) | | | | | | | (2,044) | | | | | | | (2,085) | |
| Depreciation | | | | | (1,843) | | | | | | | (1,625) | | | | | | | (1,642) | |
| Research and development | | | | | (17,350) | | | | | | | (30,008) | | | | | | | (13,011) | |
| Restructuring costs | | | | | (309) | | | | | | | (599) | | | | | | | (337) | |
| Charge for Zetia antitrust litigation settlements | | | | | — | | | | | | | (573) | | | | | | | — | |
| Other unallocated, net | | | | | (4,274) | | | | | | | (3,572) | | | | | | | (5,651) | |
| | | | | $ | 19,936 | | | | | | | $ | 1,889 | | | | | | | $ | 16,444 | |
(1) The significant expense categories and amounts align with the segment level information that is regularly provided to the chief operating decision maker.
(2) Human health-related research and development expenses incurred by Merck Research Laboratories are not allocated to segment profits as noted below.
(3) Includes equity (income) loss from affiliates and other miscellaneous non-operating expenses.
Pharmaceutical segment profits are comprised of segment sales less standard costs, as well as selling, general and administrative expenses directly incurred by the segment. Animal Health segment profits are comprised of segment sales, less all cost of sales, as well as selling, general and administrative expenses and research and development costs directly incurred by the segment. The chief operating decision maker (Merck’s Chief Executive Officer) uses segment profit to allocate resources predominately during the planning and forecasting process. For internal management reporting presented to the chief operating decision maker, Merck does not allocate the remaining cost of sales not included in segment profits as described above, research and development expenses incurred by Merck Research Laboratories, the Company’s research and development division that focuses on human health-related activities, or general and administrative expenses not directly incurred by the segments, nor the cost of financing these activities. Separate divisions maintain responsibility for monitoring and managing these costs, including depreciation related to fixed assets utilized by these divisions and, therefore, they are not included in segment profits. In addition, costs related to restructuring activities, as well as the amortization of intangible assets and amortization of purchase accounting adjustments are not allocated to segments.
Other profits are primarily comprised of miscellaneous corporate profits, as well as operating profits (losses) related to third-party manufacturing arrangements.
Other unallocated, net, includes expenses from corporate and manufacturing cost centers, intangible asset impairment charges, gains or losses on sales of businesses, expense or income related to changes in the estimated fair value measurement of liabilities for contingent consideration, and other miscellaneous income or expense items.
Equity income from affiliates and depreciation included in segment profits is as follows:
| | | | | | | | | | | | | | | | | |
| Pharmaceutical | | Animal Health | | Total |
| Year Ended December 31, 2024 | | | | | |
| Equity income from affiliates | $ | 144 | | | $ | — | | | $ | 144 | |
| Depreciation | 5 | | | 256 | | | 261 | |
| Year Ended December 31, 2023 | | | | | |
| Equity income from affiliates | $ | 111 | | | $ | — | | | $ | 111 | |
| Depreciation | 5 | | | 198 | | | 203 | |
| Year Ended December 31, 2022 | | | | | |
| Equity income from affiliates | $ | 39 | | | $ | — | | | $ | 39 | |
| Depreciation | 5 | | | 177 | | | 182 | |
Property, plant and equipment, net, by geographic area where located is as follows:
| | | | | | | | | | | | | | | | | |
| December 31 | 2024 | | 2023 | | 2022 |
| United States | $ | 14,724 | | | $ | 13,915 | | | $ | 12,891 | |
| Europe, Middle East and Africa | 7,548 | | | 7,562 | | | 6,993 | |
| Asia Pacific (other than China and Japan) | 982 | | | 1,022 | | | 966 | |
| China | 202 | | | 193 | | | 207 | |
| Japan | 143 | | | 133 | | | 135 | |
| Latin America | 133 | | | 222 | | | 225 | |
| Other | 47 | | | 4 | | | 5 | |
| | $ | 23,779 | | | $ | 23,051 | | | $ | 21,422 | |
The Company does not disaggregate assets on a products and services basis for internal management reporting and, therefore, such information is not presented.
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Stockholders of Merck & Co., Inc.
Opinions on the Financial Statements and Internal Control over Financial Reporting
We have audited the accompanying consolidated balance sheet of Merck & Co., Inc. and its subsidiaries (the “Company”) as of December 31, 2024 and 2023, and the related consolidated statements of income, of comprehensive income (loss), of equity and of cash flows for each of the three years in the period ended December 31, 2024, including the related notes (collectively referred to as the “consolidated financial statements”). We also have audited the Company’s internal control over financial reporting as of December 31, 2024, based on criteria established in Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO).
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of the Company as of December 31, 2024 and 2023, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2024 in conformity with accounting principles generally accepted in the United States of America. Also in our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2024, based on criteria established in Internal Control - Integrated Framework (2013) issued by the COSO.
Basis for Opinions
The Company’s management is responsible for these consolidated financial statements, for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting, included in Management’s Report on Internal Control Over Financial Reporting appearing under Item 9A. Our responsibility is to express opinions on the Company’s consolidated financial statements and on the Company’s internal control over financial reporting based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud, and whether effective internal control over financial reporting was maintained in all material respects.
Our audits of the consolidated financial statements included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audits also included performing such other procedures as we considered necessary in the circumstances. We believe that our audits provide a reasonable basis for our opinions.
Definition and Limitations of Internal Control over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Critical Audit Matters
The critical audit matter communicated below is a matter arising from the current period audit of the consolidated financial statements that was communicated or required to be communicated to the audit committee and that (i) relates to accounts or disclosures that are material to the consolidated financial statements and (ii) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit matter below, providing a separate opinion on the critical audit matter or on the accounts or disclosures to which it relates.
U.S. Rebate Accruals - Medicaid, Managed Care and Medicare Part D
As described in Note 2 to the consolidated financial statements, the Company records certain variable consideration including discounts, which are estimated at the time of sale generally using the expected value method. Amounts accrued for aggregate customer discounts are evaluated on a quarterly basis through comparison of information provided by the wholesalers, health maintenance organizations, pharmacy benefit managers, federal and state agencies, and other customers to the amounts accrued. Certain of these discounts representing a portion of the accrual take the form of rebates, which are amounts owed based upon definitive contractual agreements or legal requirements with private sector (Managed Care) and public sector (Medicaid and Medicare Part D) benefit providers, after the final dispensing of the product to a benefit plan participant. The provision for rebates is based on expected patient usage, as well as inventory levels in the distribution channel to determine the contractual obligation to the benefit providers. Management uses historical customer segment utilization mix, sales forecasts, changes to product mix and price, inventory levels in the distribution channel, government pricing calculations and prior payment history in order to estimate the expected provision. The accrued balance relative to the provision for rebates included in accrued and other current liabilities was $2.2 billion as of December 31, 2024, of which the majority relates to U.S. rebate accruals – Medicaid, Managed Care and Medicare Part D.
The principal considerations for our determination that performing procedures relating to U.S. rebate accruals - Medicaid, Managed Care and Medicare Part D is a critical audit matter are the significant judgment by management due to the significant measurement uncertainty involved in developing the rebate accruals, as the accruals are based on assumptions developed using pricing information and historical customer segment utilization mix, and a high degree of auditor judgment, subjectivity and effort in performing procedures and evaluating evidence related to these assumptions.
Addressing the matter involved performing procedures and evaluating audit evidence in connection with forming our overall opinion on the consolidated financial statements. These procedures included testing the effectiveness of controls relating to U.S. rebate accruals - Medicaid, Managed Care and Medicare Part D, including management’s controls over the assumptions used to estimate the corresponding rebate accruals. These procedures also included, among others (i) developing an independent estimate of the rebate accruals by utilizing third party data on historical customer segment utilization mix in the U.S., pricing information, the terms of the specific rebate programs, and the historical trend of actual rebate claims paid, (ii) comparing the independent estimate to the rebate accruals recorded by management, and (iii) testing rebate claims paid, including evaluating those claims for consistency with the contractual terms of the Company’s rebate agreements.
PricewaterhouseCoopers LLP
Florham Park, New Jersey
February 25, 2025
We have served as the Company’s auditor since 2002.
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure.
Not applicable.
Item 9A. Controls and Procedures.
Management of the Company, with the participation of its Chief Executive Officer and Chief Financial Officer, has evaluated the effectiveness of the Company’s disclosure controls and procedures. Based on their evaluation, as of the end of the period covered by this Form 10-K, the Company’s Chief Executive Officer and Chief Financial Officer have concluded that the Company’s disclosure controls and procedures (as defined in Rules 13a-15(e) or 15d-15(e) under the Securities Exchange Act of 1934, as amended (the Act)) are effective. For the fourth quarter of 2024, there have been no changes in internal control over financial reporting that materially affected, or are reasonably likely to materially affect, the Company’s internal control over financial reporting.
Management is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Rule 13a-15(f) of the Act. Management conducted an evaluation of the effectiveness of internal control over financial reporting based on the framework in Internal Control — Integrated Framework issued in 2013 by the Committee of Sponsoring Organizations of the Treadway Commission. Based on this evaluation, management concluded that internal control over financial reporting was effective as of December 31, 2024. PricewaterhouseCoopers LLP, an independent registered public accounting firm, has performed its own assessment of the effectiveness of the Company’s internal control over financial reporting and its attestation report is included in this Form 10-K filing.
Management’s Report
Management’s Responsibility for Financial Statements
Responsibility for the integrity and objectivity of the Company’s financial statements rests with management. The financial statements report on management’s stewardship of Company assets. These statements are prepared in conformity with generally accepted accounting principles and, accordingly, include amounts that are based on management’s best estimates and judgments. Nonfinancial information included in the Annual Report on Form 10-K has also been prepared by management and is consistent with the financial statements.
To assure that financial information is reliable and assets are safeguarded, management maintains an effective system of internal controls and procedures, important elements of which include: careful selection, training and development of operating and financial managers; an organization that provides appropriate division of responsibility; and communications aimed at assuring that Company policies and procedures are understood throughout the organization. A staff of internal auditors regularly monitors the adequacy and application of internal controls on a worldwide basis.
To ensure that personnel continue to understand the system of internal controls and procedures, and policies concerning good and prudent business practices, annually all employees of the Company are required to complete Code of Conduct training. This training reinforces the importance and understanding of internal controls by reviewing key corporate policies, procedures and systems. In addition, the Company has compliance programs, including an ethical business practices program to reinforce the Company’s long-standing commitment to high ethical standards in the conduct of its business.
The financial statements and other financial information included in the Annual Report on Form 10-K fairly present, in all material respects, the Company’s financial condition, results of operations and cash flows. Our formal certification to the Securities and Exchange Commission is included in this Form 10-K filing.
Management’s Report on Internal Control Over Financial Reporting
Management is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Rule 13a-15(f) under the Securities Exchange Act of 1934. The Company’s internal control over financial reporting is designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles in the United States of America. Management conducted an evaluation of the effectiveness of internal control over financial reporting based on the framework in Internal Control — Integrated Framework issued in 2013 by the Committee of Sponsoring Organizations of the Treadway Commission. Based on this evaluation, management concluded that internal control over financial reporting was effective as of December 31, 2024.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Projections of any evaluation of effectiveness to future periods are subject to the risk that controls
may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
The effectiveness of the Company’s internal control over financial reporting as of December 31, 2024, has been audited by PricewaterhouseCoopers LLP, an independent registered public accounting firm, as stated in their report which appears herein.
| | | | | |
| Robert M. Davis | Caroline Litchfield |
| Chairman, Chief Executive Officer and President | Executive Vice President and Chief Financial Officer |
Item 9B. Other Information.
Insider Trading Arrangements
During the three months ended December 31, 2024, none of the Company’s directors or executive officers adopted or terminated any Rule 10b5-1 trading arrangements or non-Rule 10b5-1 trading arrangements.
Item 9C. Disclosure Regarding Foreign Jurisdictions that Prevent Inspections.
Not Applicable.
PART III
Item 10.Directors, Executive Officers and Corporate Governance.
The required information on directors and nominees is incorporated by reference from the discussion under Proposal 1. Election of Directors of the Company’s Proxy Statement for the Annual Meeting of Shareholders to be held on May 27, 2025. Information on executive officers is set forth in Part I of this document on page 43. The required information on compliance with Section 16(a) of the Securities Exchange Act of 1934, if applicable, is incorporated by reference from the discussion under the heading “Stock Ownership Information” of the Company’s Proxy Statement for the Annual Meeting of Shareholders to be held on May 27, 2025.
The Company has a Code of Conduct — Our Values and Standards applicable to all employees, including the principal executive officer, principal financial officer, and principal accounting officer. The Code of Conduct is available on the Company’s website at www.merck.com/company-overview/culture-and-values/code-of-conduct/values-and-standards/. The Company intends to disclose future amendments to certain provisions of the Code of Conduct, and waivers of the Code of Conduct granted to executive officers and directors, if any, on the website within four business days following the date of any amendment or waiver. Every Merck employee is responsible for adhering to business practices that are in accordance with the law and with ethical principles that reflect the highest standards of corporate and individual behavior.
The required information on the identification of the audit committee and the audit committee financial expert is incorporated by reference from the discussion under the heading “Board Meetings and Committees” of the Company’s Proxy Statement for the Annual Meeting of Shareholders to be held on May 27, 2025.
The required information about the Company’s insider trading policy is incorporated by reference from the discussion under the heading “Insider Trading Policy” of the Company's Proxy Statement for the Annual Meeting of Shareholders to be held on May 27, 2025.
Item 11.Executive Compensation.
The information required on executive compensation is incorporated by reference from the discussion under the headings “Compensation Discussion and Analysis,” “Summary Compensation Table,” “All Other Compensation” table, “CEO Pay Ratio,” “Pay versus Performance” table, “Grants of Plan-Based Awards” table, “Outstanding Equity Awards” table, “Option Exercises and Stock Vested” table, “Pension Benefits” table, “Nonqualified Deferred Compensation” table, and “Potential Payments Upon Termination or a Change in Control”, as well as all footnote information to the various tables, of the Company’s Proxy Statement for the Annual Meeting of Shareholders to be held on May 27, 2025.
The required information on director compensation is incorporated by reference from the discussion under the heading “Director Compensation” and related “2024 Schedule of Director Fees” table and “2024 Director Compensation” table of the Company’s Proxy Statement for the Annual Meeting of Shareholders to be held on May 27, 2025.
The required information under the headings “Compensation and Management Development Committee Interlocks and Insider Participation” and “Compensation and Management Development Committee Report” is incorporated by reference from the Company’s Proxy Statement for the Annual Meeting of Shareholders to be held on May 27, 2025.
Item 12.Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters.
Information with respect to security ownership of certain beneficial owners and management is incorporated by reference from the discussion under the heading “Stock Ownership Information” of the Company’s Proxy Statement for the Annual Meeting of Shareholders to be held on May 27, 2025.
Equity Compensation Plan Information
The following table summarizes information about the options, warrants and rights and other equity compensation under the Company’s equity compensation plans as of the close of business on December 31, 2024. The table does not include information about tax qualified plans such as the Merck U.S. Savings Plan.
| | | | | | | | | | | | | | | | | | | | |
| Plan Category | | Number of
securities to be
issued upon
exercise of
outstanding
options, warrants
and rights
(a) | | Weighted-average
exercise price of
outstanding
options, warrants
and rights
(b) | | Number of
securities remaining
available for future
issuance under equity
compensation plans
(excluding
securities
reflected in column (a))
(c) |
Equity compensation plans approved by security holders(1) | | 12,500,010(2) | | $ | 86.04 | | | 74,988,831 | |
| Equity compensation plans not approved by security holders | | — | | | — | | | — | |
| Total | | 12,500,010 | | | $ | 86.04 | | | 74,988,831 | |
(1)Includes options to purchase shares of Company Common Stock and other rights under the following shareholder-approved plans: the Merck & Co., Inc. 2010 and 2019 Incentive Stock Plans, and the Merck & Co., Inc. 2010 Non-Employee Directors Stock Option Plan.
(2)Excludes approximately 12,232,051 shares of restricted stock units and 3,531,246 performance share units (assuming maximum payouts) under the Merck Sharp & Dohme 2010 and 2019 Incentive Stock Plans. Also excludes 153,540 shares of phantom stock deferred under the MSD Employee Deferral Program and 518,423 shares of phantom stock deferred under the Merck & Co., Inc. Plan for Deferred Payment of Directors’ Compensation.
Item 13.Certain Relationships and Related Transactions, and Director Independence.
The required information on transactions with related persons is incorporated by reference from the discussion under the heading “Related Person Transactions” of the Company’s Proxy Statement for the Annual Meeting of Shareholders to be held on May 27, 2025.
The required information on director independence is incorporated by reference from the discussion under the heading “Independence of Directors” of the Company’s Proxy Statement for the Annual Meeting of Shareholders to be held on May 27, 2025.
Item 14.Principal Accountant Fees and Services.
The information required for this item is incorporated by reference from the discussion under Proposal 3. Ratification of Appointment of Independent Registered Public Accounting Firm for 2025 beginning with the caption “Pre-Approval Policy for Services of Independent Registered Public Accounting Firm” through “Fees for Services Provided by the Independent Registered Public Accounting Firm” of the Company’s Proxy Statement for the Annual Meeting of Shareholders to be held on May 27, 2025.
PART IV
Item 15.Exhibits and Financial Statement Schedules.
(a) The following documents are filed as part of this Form 10-K
1. Financial Statements
Consolidated statement of income for the years ended December 31, 2024, 2023 and 2022
Consolidated statement of comprehensive income (loss) for the years ended December 31, 2024, 2023 and 2022
Consolidated balance sheet as of December 31, 2024 and 2023
Consolidated statement of equity for the years ended December 31, 2024, 2023 and 2022
Consolidated statement of cash flows for the years ended December 31, 2024, 2023 and 2022
Notes to consolidated financial statements
Report of PricewaterhouseCoopers LLP, independent registered public accounting firm (PCAOB ID 238)
2. Financial Statement Schedules
Schedules are omitted because they are either not required or not applicable.
Financial statements of affiliates carried on the equity basis have been omitted because, considered individually or in the aggregate, such affiliates do not constitute a significant subsidiary.
3. Exhibits
| | | | | | | | | | | | | | |
Exhibit Number | | | | Description |
| 3.1 | | — | | |
| 3.2 | | — | | |
| 4.1 | | — | | Indenture, dated as of April 1, 1991, between Merck Sharp & Dohme Corp. (f/k/a Schering Corporation) and U.S. Bank Trust National Association (as successor to Morgan Guaranty Trust Company of New York), as Trustee (the 1991 Indenture) — Incorporated by reference to Exhibit 4 to MSD’s Registration Statement on Form S-3 (No. 33-39349) |
| 4.2 | | — | | |
| 4.3 | | — | | |
| 4.4 | | — | | |
| 4.5 | | — | | |
| 4.6 | | — | | |
| 4.7 | | — | | |
| 4.8 | | — | | |
| 4.9 | | — | | |
| 4.10 | | — | | |
| 4.11 | | — | | |
| *10.1 | | — | | |
| *10.2 | | — | | |
| *10.3 | | — | | |
| *10.4 | | — | | |
| *10.5 | | — | | |
| | | | | | | | | | | | | | |
| *10.6 | | — | | |
| *10.7 | | — | | |
| *10.8 | | — | | |
| *10.9 | | — | | |
| *10.10 | | — | | |
| *10.11 | | — | | |
| *10.12 | | — | | |
| *10.13 | | — | | Merck & Co., Inc. U.S. Separation Benefits Plan (amended and restated as of January 1, 2019) as further amended by Amendments 2019-1 (as of December 19, 2019), 2020-1 (as of February 25, 2020), 2020-2 (as of December 10, 2020), 2021-1 (as of March 31, 2021), 2021-2 (as of December 16, 2021), 2022-1 (as of December 14, 2022), 2022-2 (as of December 13, 2021), 2023-1 (as of December 15, 2023) and 2024-1 (as of October 22, 2024) |
| *10.14 | | — | | |
| *10.15 | | — | | |
| *10.16 | | — | | |
| *10.17 | | — | | |
| *10.18 | | — | | |
| *10.19 | | — | | |
| *10.20 | | — | | |
| *10.21 | | — | | |
| | | | | | | | | | | | | | |
| *10.22 | | — | | |
| *10.23 | | — | | |
| *10.24 | | — | | |
| *10.25 | | — | | |
| *10.26 | | — | | |
| *10.27 | | — | | |
| *10.28 | | — | | |
| *10.29 | | — | | |
| *10.30 | | — | | |
| *10.31 | | — | | |
| *10.32 | | — | | |
| *10.33 | | — | | |
| *10.34 | | — | | |
| *10.35 | | — | | |
| *10.36 | | — | | |
| 19 | | — | | |
| 21 | | — | | |
| 23 | | — | | |
| 24.1 | | — | | |
| 24.2 | | — | | |
| 31.1 | | — | | |
| 31.2 | | — | | |
| 32.1 | | — | | |
| 32.2 | | — | | |
| 97 | | — | | |
| | | | | | | | | | | | | | |
| Exhibit 101: | | | | |
| 101.INS | | — | | XBRL Instance Document - The instance document does not appear in the interactive data file because its XBRL tags are embedded within the Inline XBRL document. |
| 101.SCH | | — | | XBRL Taxonomy Extension Schema Document. |
| 101.CAL | | — | | XBRL Taxonomy Extension Calculation Linkbase Document. |
| 101.DEF | | — | | XBRL Taxonomy Extension Definition Linkbase Document. |
| 101.LAB | | — | | XBRL Taxonomy Extension Label Linkbase Document. |
| 101.PRE | | — | | XBRL Taxonomy Extension Presentation Linkbase Document. |
| 104 | | — | | Cover Page Interactive Data File (formatted as Inline XBRL and contained in Exhibit 101). |
| | | | | |
| * | Management contract or compensatory plan or arrangement. |
| Long-term debt instruments under which the total amount of securities authorized does not exceed 10% of Merck & Co., Inc.’s total consolidated assets are not filed as exhibits to this report. Merck & Co., Inc. will furnish a copy of these agreements to the Securities and Exchange Commission on request. |
Item 16. Form 10-K Summary
Not applicable.
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
Dated: February 25, 2025
| | | | | | | | |
| MERCK & CO., INC. |
| |
| By: | ROBERT M. DAVIS |
| (Chairman, Chief Executive Officer and President) |
| | |
| By: | /s/ JENNIFER ZACHARY |
| | Jennifer Zachary |
| | (Attorney-in-Fact) |
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the Registrant and in the capacities and on the dates indicated.
| | | | | | | | | | | | | | |
| Signatures | | Title | | Date |
| | | | |
| ROBERT M. DAVIS | | Chairman, Chief Executive Officer and President; Principal Executive Officer | | February 25, 2025 |
| CAROLINE LITCHFIELD | | Executive Vice President and Chief Financial Officer; Principal Financial Officer | | February 25, 2025 |
| DALTON SMART | | Senior Vice President Finance-Global Controller; Principal Accounting Officer | | February 25, 2025 |
| DOUGLAS M. BAKER, JR. | | Director | | February 25, 2025 |
| MARY ELLEN COE | | Director | | February 25, 2025 |
| PAMELA J. CRAIG | | Director | | February 25, 2025 |
| THOMAS H. GLOCER | | Director | | February 25, 2025 |
| SURENDRALAL L. KARSANBHAI | | Director | | February 25, 2025 |
| RISA J. LAVIZZO-MOUREY | | Director | | February 25, 2025 |
| STEPHEN L. MAYO | | Director | | February 25, 2025 |
| PAUL B. ROTHMAN | | Director | | February 25, 2025 |
| PATRICIA F. RUSSO | | Director | | February 25, 2025 |
| CHRISTINE E. SEIDMAN | | Director | | February 25, 2025 |
| INGE G. THULIN | | Director | | February 25, 2025 |
| KATHY J. WARDEN | | Director | | February 25, 2025 |
Jennifer Zachary, by signing her name hereto, does hereby sign this document pursuant to powers of attorney duly executed by the persons named, filed with the Securities and Exchange Commission as an exhibit to this document, on behalf of such persons, all in the capacities and on the date stated, such persons including a majority of the directors of the Company.
| | | | | | | | |
| By: | | /S/ JENNIFER ZACHARY |
| | Jennifer Zachary |
| | (Attorney-in-Fact) |