
PROVECTUS BIOPHARMACEUTICALS, INC. Committed to developing new treatments for cancer and inflammatory skin diseases Provectus Biopharmaceuticals, Inc. • 7327 Oak Ridge Highway, Knoxville, Tennessee USA 37931 • +1 (866) 594-5999 • www.provectusbio.com Exhibit 99.1

Forward-Looking Statements This presentation contains "forward-looking statements" as defined under U.S. federal securities laws. These statements reflect management’s current knowledge, assumptions, beliefs, estimates, and expectations and express management’s current views of future performance, results, and trends and may be identified by their use of terms such as "anticipate,” "believe," "could," "estimate,“ "expect," "intend," "may," "plan," "predict," "project," "will," and other similar words. Forward-looking statements are subject to a number of risks and uncertainties that could cause our actual results to materially differ from those described in the forward-looking statements. Readers should not place undue reliance on forward-looking statements. Such statements are made as of the date hereof, and we undertake no obligation to update such statements after this date. Risks and uncertainties that could cause our actual results to materially differ from those described in forward-looking statements include those discussed in our filings with the U.S. Securities and Exchange Commission (including those described in items 1A of our Annual Report on 10-K for the year ended December 31, 2015, as supplemented by those described in Part II, Item 1A of our Quarterly Report on Form 10-Q for the quarter ended September 30, 2016). Provectus Biopharmaceuticals, Inc. (“Provectus”) assumes no obligation to update any forward-looking statements or information that speaks as to their respective dates. No claims with respect to Provectus’ investigational drug PV-10 for solid tumor cancers and/or investigational drug PH-10 for inflammatory dermatoses are intended regarding safety or efficacy in the context of the forward-looking statements in this presentation. This investor presentation may be found at www.provectusbio.com/news. This investor presentation may be found at www.provectusbio.com/news.
Provectus Biopharmaceuticals Founded in 2002 by scientists from Oak Ridge National Laboratory U.S Dept. of Energy multi-program science and technology facility with rich history of discovery and innovation Focused on engineering of Rose Bengal-based drugs Oncology (PV-10): under development for solid tumor cancers, alone or in combination with other therapeutic agents and therapies Dermatology (PH-10): under development for psoriasis, atopic dermatitis and other inflammatory skin diseases Clinical data have demonstrated preliminary efficacy; side effects consistent with local therapy 1,2 Data for PV-10 and PH-10 encompasses clinical experience with over 500 hundred patients 1 Thompson et al., Mel Res 18, 405, 2008. 2 Thompson et al., Ann Surg Oncol 22, 2135, 2015.
Rose Bengal A Unique Compound with a Long History of Human Use A water-soluble dye created by Gnehm in Switzerland in 1882 1 More than a half century of clinical use as diagnostic marker An established safety profile - Intravenous hepatic diagnostic 2 (Robengatope®) - Topical ophthalmic diagnostic 3,4 (Rosettes® and Minims®) 3,835 medical literature citations, 246 related to cancer 5 1 Gnehm R.Ueber Tetrachlorphtalsäure. Justus Liebigs Annalen der Chemie 1887; 238:318–338. 2 Delprat GD. Arch Int Med 1923; 32(3): 401-401. 3 Feenstra RPG and Tseng CG. Arch Ophthalmol 1992; 110:984–993. 4 Norn MS. Acta Ophthalmol 1970;48(3):546-559. 5 PubMed search terms “rose bengal” and “rose bengal cancer,” respectively, through December 16, 2015. 6 Ito A et al., JNCI 1986; 77: 277. Therapeutic potential remained undiscovered in literature until Provectus began preclinical studies 6
Our Rose Bengal-Based Investigational Drugs PV-10 Selectively cytotoxic to cancer cells at high concentrations 1,2 10% Rose Bengal disodium solution Direct injection into solid tumors Rapid autolysis of tumor cells 3 Generates measurable systemic immune response PH-10 Efficient photosensitizer at low concentrations 0.005% Rose Bengal disodium hydrogel Topical application to skin (daily) Photoactivation by visible (green-yellow) light Potential anti-inflammatory activity in diseased tissue 1 Liu et al., Oncotarget 7, 37893, 2016. 2 Qin et al., Cell Death and Disease 8, e2584, 2017. 3 Wachter et al., SPIE 4620, 143, 2002.
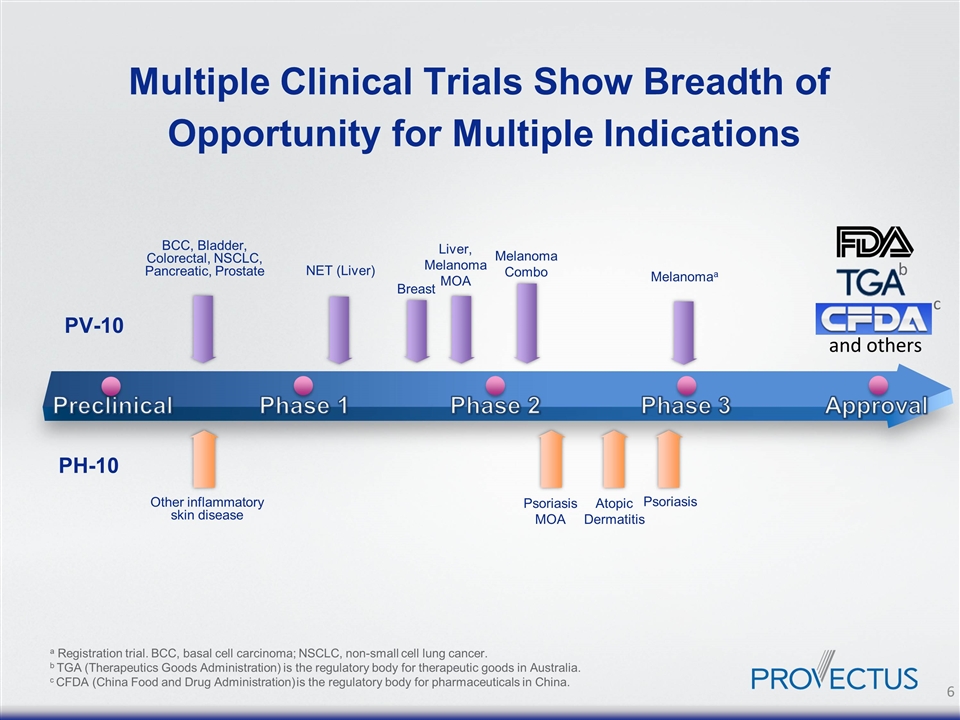
Multiple Clinical Trials Show Breadth of Opportunity for Multiple Indications Preclinical Phase 1 Phase 2 Phase 3 Approval and others b c a Registration trial. BCC, basal cell carcinoma; NSCLC, non-small cell lung cancer. b TGA (Therapeutics Goods Administration) is the regulatory body for therapeutic goods in Australia. c CFDA (China Food and Drug Administration) is the regulatory body for pharmaceuticals in China. Other inflammatory skin disease PH-10 Psoriasis Psoriasis MOA Atopic Dermatitis Liver, Melanoma MOA BCC, Bladder, Colorectal, NSCLC, Pancreatic, Prostate Melanoma Combo Melanomaa PV-10 Breast NET (Liver)
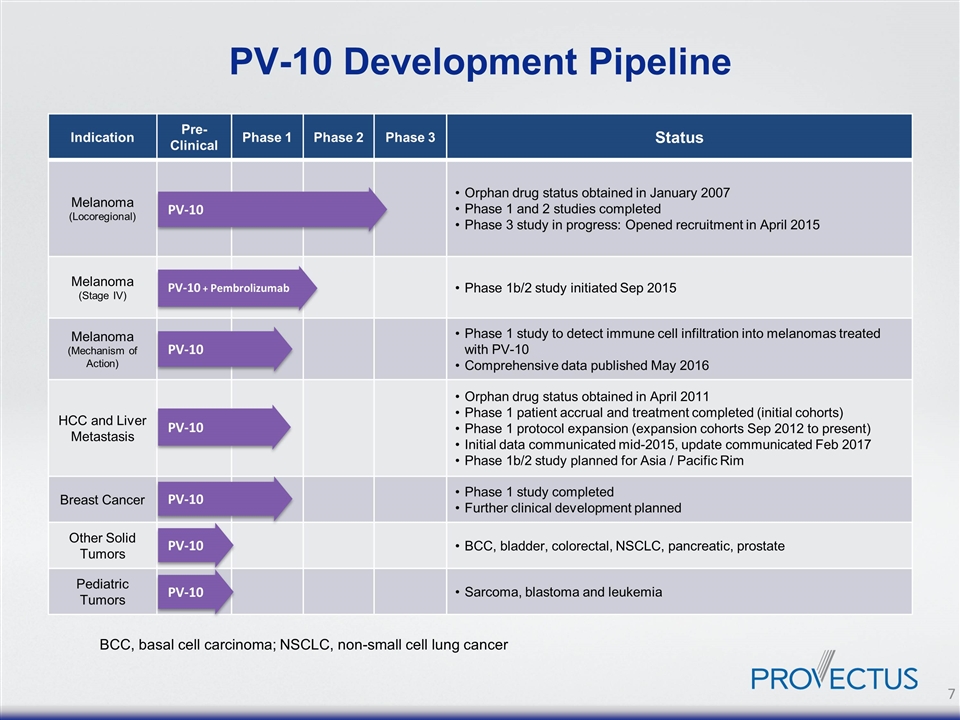
PV-10 PV-10 Indication Pre-Clinical Phase 1 Phase 2 Phase 3 Status Melanoma (Locoregional) Orphan drug status obtained in January 2007 Phase 1 and 2 studies completed Phase 3 study in progress: Opened recruitment in April 2015 Melanoma (Stage IV) Phase 1b/2 study initiated Sep 2015 Melanoma (Mechanism of Action) Phase 1 study to detect immune cell infiltration into melanomas treated with PV-10 Comprehensive data published May 2016 HCC and Liver Metastasis Orphan drug status obtained in April 2011 Phase 1 patient accrual and treatment completed (initial cohorts) Phase 1 protocol expansion (expansion cohorts Sep 2012 to present) Initial data communicated mid-2015, update communicated Feb 2017 Phase 1b/2 study planned for Asia / Pacific Rim Breast Cancer Phase 1 study completed Further clinical development planned Other Solid Tumors BCC, bladder, colorectal, NSCLC, pancreatic, prostate Pediatric Tumors Sarcoma, blastoma and leukemia PV-10 BCC, basal cell carcinoma; NSCLC, non-small cell lung cancer PV-10 + Pembrolizumab PV-10 PV-10 PV-10 PV-10 Development Pipeline PV-10 PV-10 7
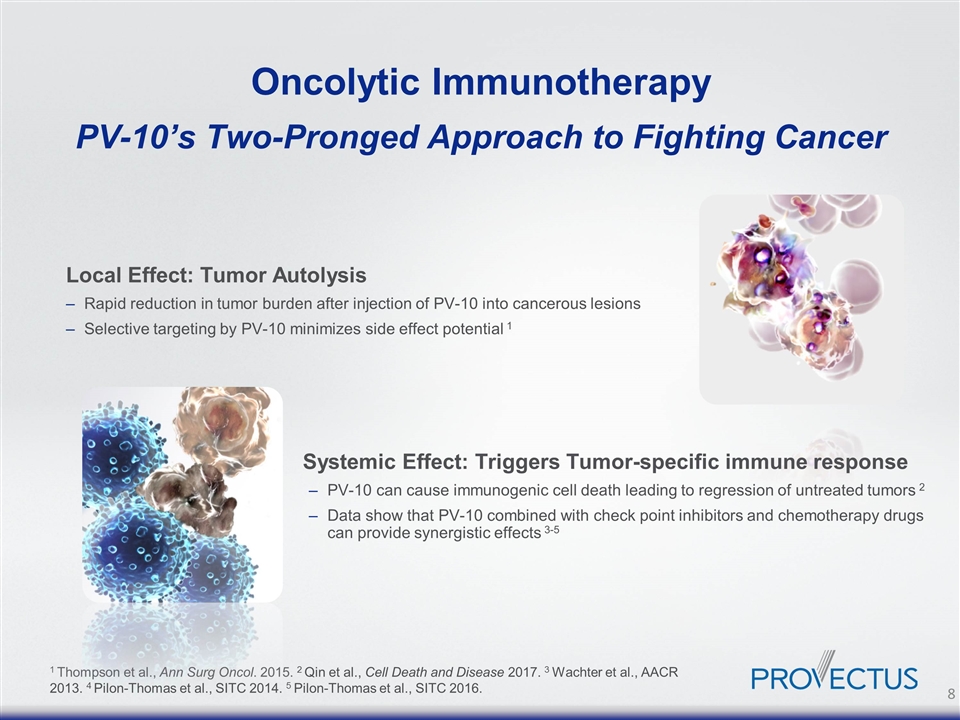
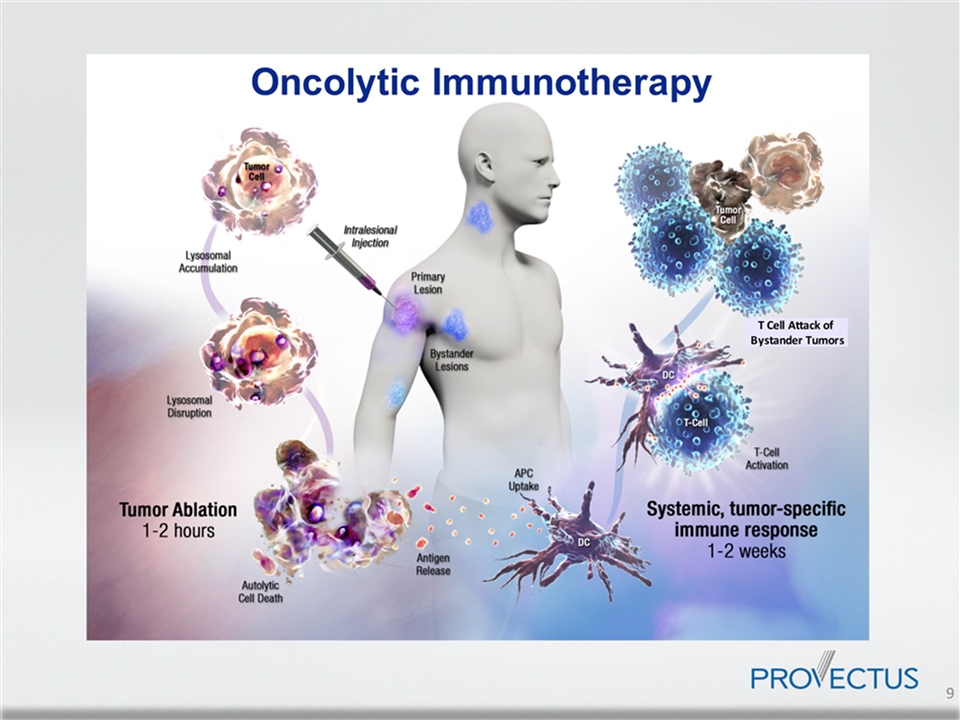
Oncolytic Immunotherapy PV-10’s Two-Pronged Approach to Fighting Cancer Local Effect: Tumor Autolysis Rapid reduction in tumor burden after injection of PV-10 into cancerous lesions Selective targeting by PV-10 minimizes side effect potential 1 Systemic Effect: Triggers Tumor-specific immune response PV-10 can cause immunogenic cell death leading to regression of untreated tumors 2 Data show that PV-10 combined with check point inhibitors and chemotherapy drugs can provide synergistic effects 3-5 1 Thompson et al., Ann Surg Oncol. 2015. 2 Qin et al., Cell Death and Disease 2017. 3 Wachter et al., AACR 2013. 4 Pilon-Thomas et al., SITC 2014. 5 Pilon-Thomas et al., SITC 2016.
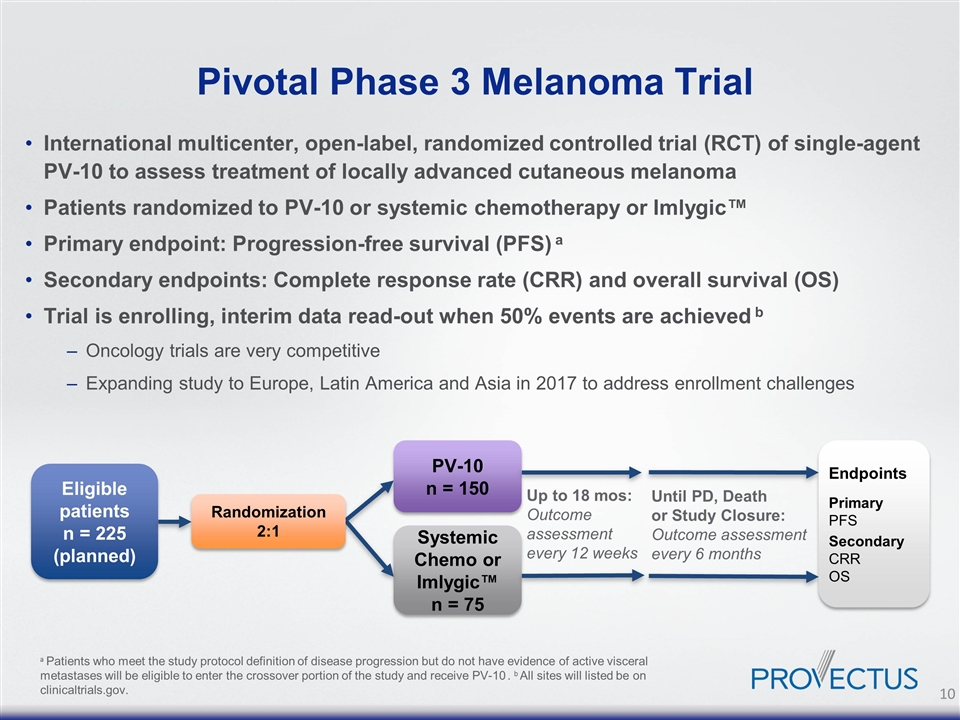
Pivotal Phase 3 Melanoma Trial International multicenter, open-label, randomized controlled trial (RCT) of single-agent PV-10 to assess treatment of locally advanced cutaneous melanoma Patients randomized to PV-10 or systemic chemotherapy or Imlygic™ Primary endpoint: Progression-free survival (PFS) a Secondary endpoints: Complete response rate (CRR) and overall survival (OS) Trial is enrolling, interim data read-out when 50% events are achieved b Oncology trials are very competitive Expanding study to Europe, Latin America and Asia in 2017 to address enrollment challenges a Patients who meet the study protocol definition of disease progression but do not have evidence of active visceral metastases will be eligible to enter the crossover portion of the study and receive PV-10 . b All sites will listed be on clinicaltrials.gov. PV-10 n = 150 Systemic Chemo or Imlygic™ n = 75 Up to 18 mos: Outcome assessment every 12 weeks Until PD, Death or Study Closure: Outcome assessment every 6 months Eligible patients n = 225 (planned) Randomization 2:1 Endpoints Primary PFS Secondary CRR OS
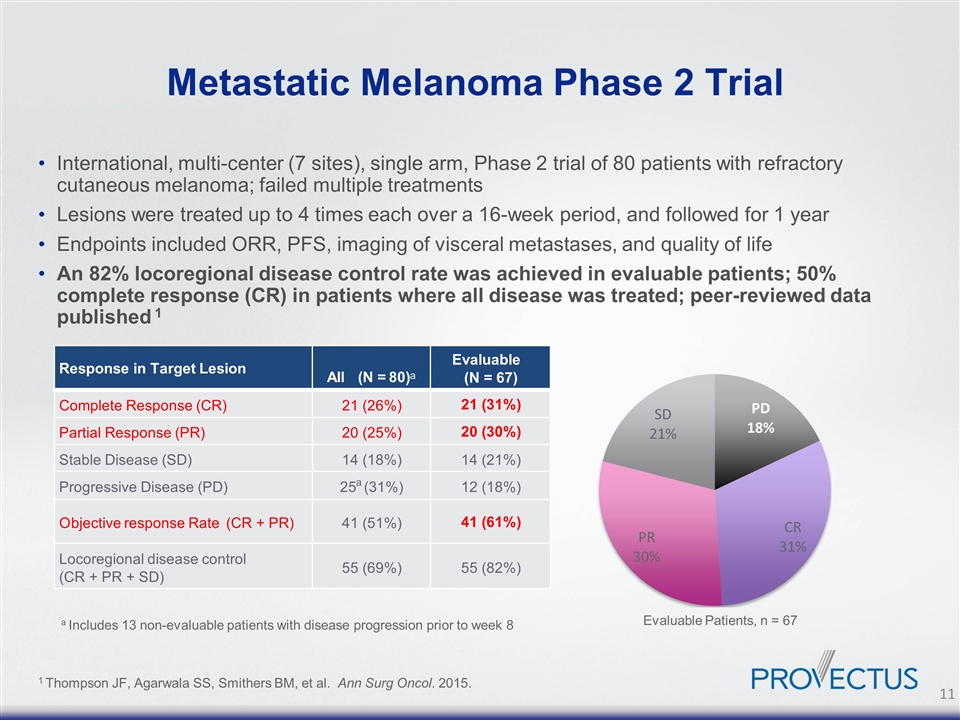
Metastatic Melanoma Phase 2 Trial International, multi-center (7 sites), single arm, Phase 2 trial of 80 patients with refractory cutaneous melanoma; failed multiple treatments Lesions were treated up to 4 times each over a 16-week period, and followed for 1 year Endpoints included ORR, PFS, imaging of visceral metastases, and quality of life An 82% locoregional disease control rate was achieved in evaluable patients; 50% complete response (CR) in patients where all disease was treated; peer-reviewed data published 1 a Includes 13 non-evaluable patients with disease progression prior to week 8 1 Thompson JF, Agarwala SS, Smithers BM, et al. Ann Surg Oncol. 2015. Evaluable Patients, n = 67 Response in Target Lesion All (N = 80) a Evaluable ( N = 67) Complete Response (CR) 21 (26%) 21 (31%) Partial Response (PR) 20 (25%) 20 (30%) Stable Disease (SD) 14 (18%) 14 (21%) Progressive Disease (PD) 25 a (31%) 12 (18%) O bjective response Rate (CR + PR) 41 (51%) 41 (61%) Locoregional disease control (CR + PR + SD) 55 (69%) 55 (82%)
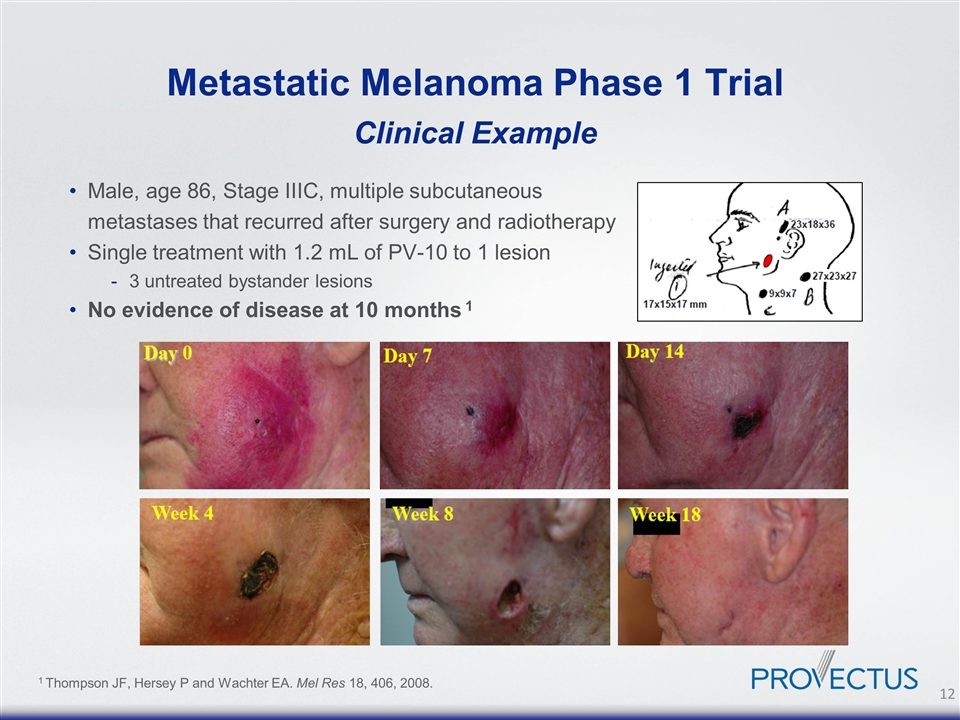
Male, age 86, Stage IIIC, multiple subcutaneous metastases that recurred after surgery and radiotherapy Single treatment with 1.2 mL of PV-10 to 1 lesion 3 untreated bystander lesions No evidence of disease at 10 months 1 Metastatic Melanoma Phase 1 Trial Clinical Example 1 Thompson JF, Hersey P and Wachter EA. Mel Res 18, 406, 2008.
Melanoma Combination Therapy Phase 1b/2 Trial Combination of intralesional PV-10 and immune checkpoint inhibition PV-10 administered every 3 weeks Pembrolizumab administered 2 mg/kg every 3 weeks, per prescribing information (label) Phase 1b/2 trial of Stage IV patients with advanced melanoma (Stage IV) Phase 1b: PV-10 and pembrolizumab Phase 2: PV-10 and pembrolizumab vs. pembrolizumab Primary endpoints: Safety and tolerability (Phase 1b), Progression-Free Survival (Phase 2) Secondary endpoints for both Phase 1b and 2: Progression-Free Survival (1b), Objective Response Rate, Change in Immune Biomarkers, Overall Survival Status: Study started in October 2015 and is enrolling See https://clinicaltrials.gov/ct2/show/NCT02557321 for more information

Peer-Reviewed Independent Results 2013 – PLOS⎮ONE Toomey et al.: “These studies establish that IL PV-10 therapy induces tumor-specific T cell-mediated immunity in multiple histologic subtypes and support the concept of combining IL PV10 with immunotherapy for advanced malignancies.” 2016 – Oncotarget Liu et al.: “IL injection of RB has been shown to induce regression of injected and uninjected tumors in murine models and clinical trials. These results support the role of IL RB to activate dendritic cells at the site of tumor necrosis for the induction of a systemic anti-tumor immune response.” 2017 – Cell Death and Disease Qin et al.: “Rose bengal (RB) is toxic at low concentrations to malignant cells and may induce damage-associated molecular patterns; therefore, we investigated its potential as an immunomodulator in colon cancer. In conclusion, RB serves as an inducer of ICD [immunogenic cell death] that contributes to enhanced specific antitumor immunity in colorectal cancer.”

PV-10: Commercial Plan Outpatient setting for cutaneous disease or short stay for visceral lesions Treatment decision: Medical or surgical oncologist Treatment delivery: Performed by physician or interventional radiologist (visceral lesions)
PH-10: Inflammatory Skin Disease PH-10 is a hydrogel formulation of Rose Bengal for topical application to the skin Photoactivated by ambient light Under development for psoriasis and atopic dermatitis (eczema) FPO
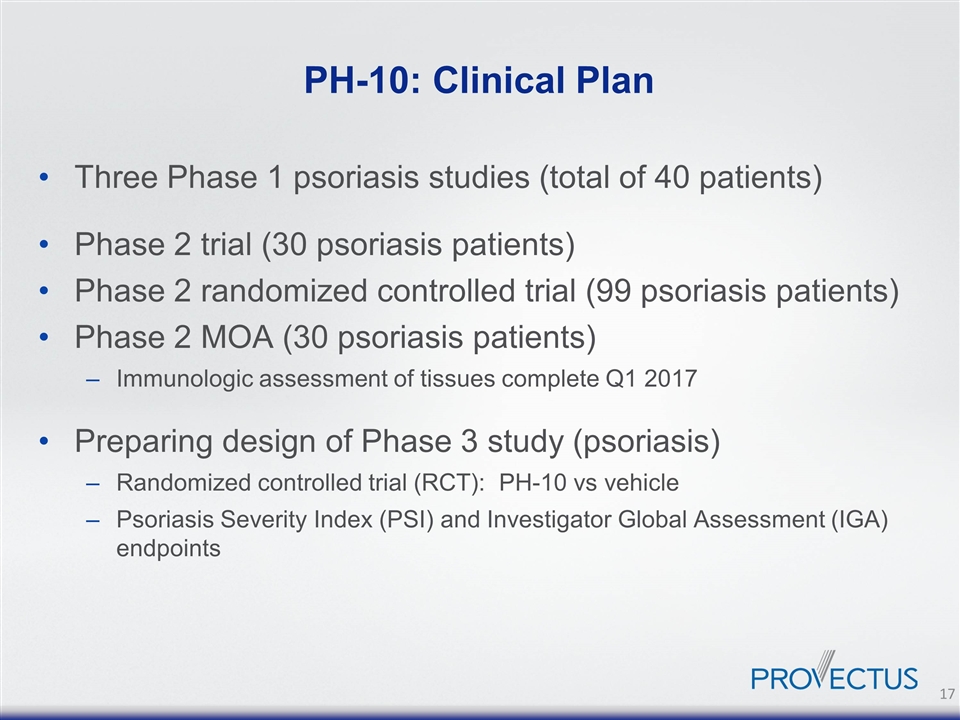
PH-10: Clinical Plan Three Phase 1 psoriasis studies (total of 40 patients) Phase 2 trial (30 psoriasis patients) Phase 2 randomized controlled trial (99 psoriasis patients) Phase 2 MOA (30 psoriasis patients) Immunologic assessment of tissues complete Q1 2017 Preparing design of Phase 3 study (psoriasis) Randomized controlled trial (RCT): PH-10 vs vehicle Psoriasis Severity Index (PSI) and Investigator Global Assessment (IGA) endpoints

PH-10: Commercial Plan Treatment delivered in an outpatient setting No pre-treatment or post-treatment care Topical gel activated by ambient light Treatment decision: Dermatologist Treatment delivery: Performed by Patient

Strong Intellectual Property Multiple foundational patents, patent applications, and trade secrets 32 issued U.S. patents Competitive protection: Second Medicinal Use, Method of Use, Formulation, Synthesis, and Combination Expiration: (combination and synthesis) 2030 to 2032 Combination therapy patent shared with Pfizer The treatment combination of PV-10 and immunomodulatory therapeutic agents (including checkpoint inhibitors); initial U.S. patent issued, divisional cases ongoing Drug substance manufacturing process patents Three U.S. patents issued on manufacturing of drug substance (active ingredient in PV-10 and PH-10) Extends the scope of protection of the manufacturing process conferred initially in 2013 to include coverage of the use of alternative raw material in manufacturing drug substance

Collaborations Letter of Intent with Boehringer Ingelheim (China) Investment Co. Ltd. to provide regulatory support and lay a foundation to collaborate in bringing PV-10 to market in mainland China, Hong Kong and Taiwan. Joint patent inventorship with Pfizer, Inc. The patent will protect use of PV-10 in combination with certain other types of drugs in the treatment of melanoma and cancers of the liver. Joint research agreement with POETIC focused on pediatric applications of PV-10 as a potential treatment for childhood cancers. The Pediatric Oncology Experimental Therapeutics Investigators' Consortium (POETIC) is composed of ten large academic medical centers in North America with a major emphasis on comprehensive cancer care and research that provide the collaborative and research strength needed to complete intensive phase I and II studies. POETIC's pediatric oncology studies focus on the biologic basis for anti-cancer therapy, and in particular, attempt to explore and evaluate new agents and novel combinations of therapies early in clinical development.
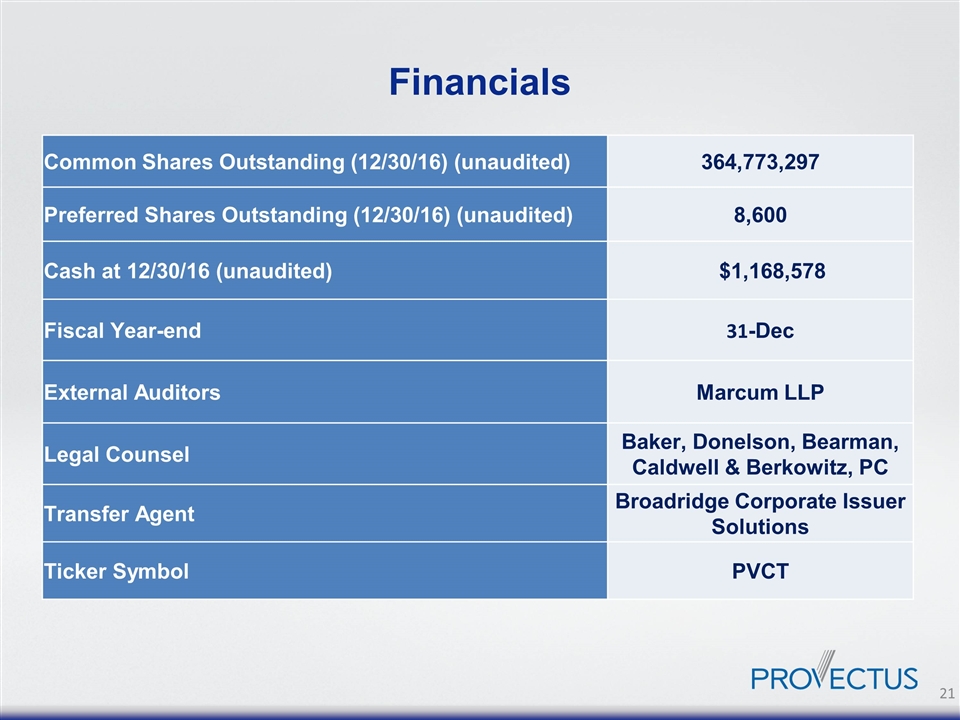
Financials Common Shares Outstanding (12/30/16) (unaudited) 364,773,297 Preferred Shares Outstanding (12/30/16) (unaudited) 8,600 Cash at 12/30/16 (unaudited) $1,168,578 Fiscal Year-end 31-Dec External Auditors Marcum LLP Legal Counsel Baker, Donelson, Bearman, Caldwell & Berkowitz, PC Transfer Agent Broadridge Corporate Issuer Solutions Ticker Symbol PVCT

The Provectus Leadership Team Lead innovator of PV-10 Co-founder of Provectus and President since 2002 Previously served in senior management positions at Photogen Technologies, Inc.; Genase LLC; and Oak Ridge National Laboratory Holder of 28 U.S. patents and Ph.D. in Chemical Engineering Experience researching, developing and testing potential pharma products Eric Wachter Chief Technology Officer Co-founder of Provectus in 2002 and Chief Technology Officer since 2012 Previously served in senior management positions at Photogen Technologies and Oak Ridge National Laboratory Holder of 30 U.S. patents and Ph.D. in Chemistry Responsibilities include pre-clinical development and clinical testing of photodynamic therapy pharmaceuticals and photoactivation systems Al Smith Chairman Founder of AE Smith Associates, LLC and serves as its Chief Executive Officer Senior Advisor for Kroll Bond Rating Agency; and K2 Global Consulting, N.A. Spent most of his career on Wall Street at Mitchell, Hutchins & Co.; CMJ Partners, LLC; Bear Wagner Specialists LLC ; and Hunter Specialists LLC Tim Scott, Ph.D. President John R Glass, Interim Chief Financial Officer President of J.R. Glass & Associates (financial, operating and marketing consulting firm) Controller for CytoCore, Inc. (OTCBB: CYOE) from January 2007-May 2014 Former Chief Financial Officer of U.S. Real Tec, Inc.; former Vice President and Chief Financial Officer of Heath Charage Corporation; former Vice President and Chief Financial Officer of Aluminum Distributors, Inc.
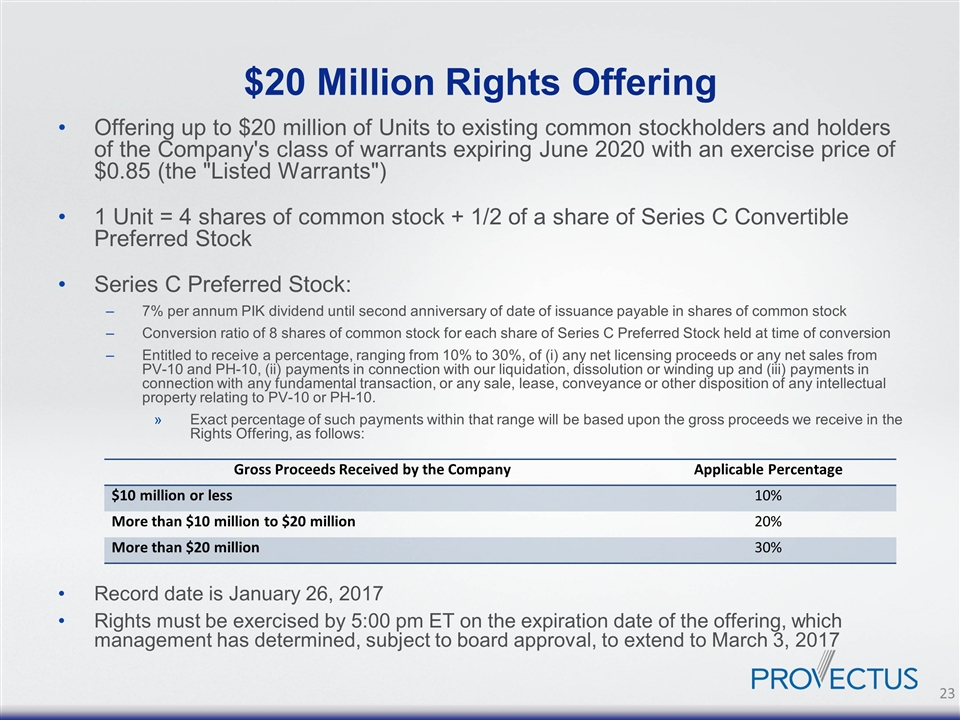
$20 Million Rights Offering Offering up to $20 million of Units to existing common stockholders and holders of the Company's class of warrants expiring June 2020 with an exercise price of $0.85 (the "Listed Warrants") 1 Unit = 4 shares of common stock + 1/2 of a share of Series C Convertible Preferred Stock Series C Preferred Stock: 7% per annum PIK dividend until second anniversary of date of issuance payable in shares of common stock Conversion ratio of 8 shares of common stock for each share of Series C Preferred Stock held at time of conversion Entitled to receive a percentage, ranging from 10% to 30%, of (i) any net licensing proceeds or any net sales from PV-10 and PH-10, (ii) payments in connection with our liquidation, dissolution or winding up and (iii) payments in connection with any fundamental transaction, or any sale, lease, conveyance or other disposition of any intellectual property relating to PV-10 or PH-10. Exact percentage of such payments within that range will be based upon the gross proceeds we receive in the Rights Offering, as follows: Record date is January 26, 2017 Rights must be exercised by 5:00 pm ET on the expiration date of the offering, which management has determined, subject to board approval, to extend to March 3, 2017 Gross Proceeds Received by the Company Applicable Percentage $10 million or less 10% More than $10 million to $20 million 20% More than $20 million 30%

$20 Million Rights Offering Use of Proceeds 80% of the proceeds for clinical development of PV-10 Balance for working capital and general corporate purposes

Investment Highlights Unique compound with long history of human use Large addressable global markets in oncology and dermatology Leadership team has deep experience with the drugs and technology Strong intellectual property Partnerships and collaborations

Provectus Biopharmaceuticals, Inc. Timothy C. Scott, PhD, President +1 (866) 594-5999, ext. 13 scott@pvct.com Investor and Media Relations: Allison & Partners Todd Aydelotte, Managing Director +1 (646) 428-0644 Provectus is grateful to both stockholders and colleagues for their role in improving treatment options for patients everywhere www.provectusbio.com

Questions

How to Read Your Rights Certificate Rights Certificate # Total Number of Rights that your are entitled to exercise Number of Rights = (Number of Shares or Warrants registered with our transfer agent) / 20

How to Read Your Rights Certificate Basic Subscription Right: Total Number of Units Purchased and Price (Up to Number of Rights listed on first page) Over-Subscription Privilege: Number of Additional Units (Optional, and only if you have exercised all of your Rights, listed on first page, in full) Complete based on your preference Select if desired (if your exercise of Rights would or may result in your ownership of common stock being in excess of 4.99% of the outstanding common stock post-Rights Offering)

How to Read Your Rights Certificate Specify form of payment Sign the form Enter broker name (if applicable) Questions should be directed To Maxim or Broadridge





























