AS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION ON AUGUST 1, 2008
REGISTRATION STATEMENT NO. 333-144351
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
AMENDMENT 6 TO FORM S-1
REGISTRATION STATEMENT UNDER THE SECURITIES ACT OF 1933
BENDA PHARMACEUTICAL, INC.
(Name of Small Business Issuer in Its Charter)
Delaware | | 2834 | | 41-2185030 |
(State or other jurisdiction of incorporation or organization) | | (Primary Standard Industrial Classification Code Number) | | (IRS Employee Identification No.) |
Room 13, Floor 25, Sunny New World Tower,
No. 231 Xin Hua Road, Jianghan District,
Wuhan, Hubei, PRC. Post Code: 430015
+86 (27) 8537-5532
(Address and telephone number of principal executive
offices and principal place of business)
Yiqing Wan, Chief Executive Officer
Room 13-16, Floor 25, Yangguang Xin Tandi Building,
No. 231 Xin Hua Road, Jianghan District,
Wuhan, Hubei, PRC. Post Code: 430015
+86 (27) 8537-5532
(Name, address and telephone number of Agent for Service)
COPY TO:
Richard I. Anslow, Esq.
Anslow & Jaclin, LLP
195 Route 9 South, Suite 204
Manalapan, New Jersey 07726
APPROXIMATE DATE OF PROPOSED SALE TO THE PUBLIC: FROM TIME TO TIME
AFTER THE
EFFECTIVE DATE OF THIS REGISTRATION STATEMENT.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, other than securities offered only in connection with dividend or interest reinvestment plans, check the following box. x
If this form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
If this form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
If delivery of the prospectus is expected to be made pursuant to Rule 434, please check the following box. o
CALCULATION OF REGISTRATION FEE
| Title of securities to be registered | | Amount to be registered | | Proposed maximum offering price per share | | Proposed maximum aggregate offering price | | Amount of registration fee | |
| Common Stock, $.001 par value, to be issued upon exercise of fixed-priced warrants | | | 15,774,375 | | $ | 0.555 | (2) | $ | 8,754,779 | (2) | $ | 344 | |
| Common Stock, $.001 par value | | | 2,106,561 | | $ | 0.31 | (1) | $ | 653,034 | (1) | $ | 26 | |
| Total | | | 17,880,936 | | | | | $ | 9,407,813 | | $ | 370 | |
(1) Estimated solely for the purpose of calculating the amount of the registration fee pursuant to Rule 457(c) of the Securities Act of 1933, the price per share and aggregate offering price are based upon the average of the high and low prices of the common stock of the Registrant as traded in the Over-The-Counter Market and reported in the Electronic Bulletin Board of the National Association of Securities Dealers on April 24, 2008.
(2) Calculated in accordance with Rule 457(g)(1) under the Securities Act.
THE REGISTRANT HEREBY AMENDS THIS REGISTRATION STATEMENT ON SUCH DATE OR DATES AS MAY BE NECESSARY TO DELAY ITS EFFECTIVE DATE UNTIL THE REGISTRANT SHALL FILE A FURTHER AMENDMENT WHICH SPECIFICALLY STATES THAT THIS REGISTRATION STATEMENT SHALL THEREAFTER BECOME EFFECTIVE IN ACCORDANCE WITH SECTION 8(A) OF THE SECURITIES ACT OR UNTIL THE
REGISTRATION STATEMENT SHALL BECOME EFFECTIVE ON SUCH DATE AS THE SECURITIES AND EXCHANGE COMMISSION, ACTING PURSUANT TO SECTION 8(A), MAY DETERMINE.
SUBJECT TO COMPLETION, DATED AUGUST 1, 2008
PROSPECTUS
2,106,561 SHARES COMMON STOCK
WARRANTS TO PURCHASE 15,774,375 SHARES OF COMMON STOCK
This prospectus covers the resale by selling stockholders named on page 15 of up to 17,880,936 shares of our common stock, $.001 par value, which include:
| | 1. | 2,106,561 shares of common stock, of which 29,968 shares were issued in January 2006 to two shareholders for services rendered; 295,378 were purchased in January and March of 2006 and are now held by two shareholders; 169,241 were issued on November 15, 2006 for services rendered in connection with the Financing and Acquisition of Ever Leader; and 1,611,974 were issued to eleven shareholders in exchange for their Ever Leader shares; and |
| | 2. | 15,774,375 shares of common stock based on 150% of the common stock issuable upon exercise of outstanding warrants we issued in connection with our issuance of the Units, at an exercise price of $0.555 per share in conjunction with our private placement completed on November 15, 2006; 150% of the common stock issuable upon exercise of outstanding warrants we issued in connection with our issuance of the Additional Units, at an exercise price of $0.555 per share on April 5, 2007; and 150% of the common stock issuable upon exercise of outstanding warrants we issued to the Placement Agent, at an exercise price of $0.555 per share in conjunction with our private placement completed on November 15, 2006. |
This offering is not being underwritten. These securities will be offered for sale by the selling stockholders identified in this prospectus in accordance with the methods and terms described in the section of this prospectus entitled "Plan of Distribution." We will not receive any of the proceeds from the sale of these shares. We will pay all expenses, except for the brokerage expenses, fees, discounts and commissions, which will all be paid by the selling stockholders, incurred in connection with the offering described in this prospectus. Our common stock and warrants are more fully described in the section of this prospectus entitled "Description of Securities."
The prices at which the selling stockholders may sell the shares of common stock that are part of this offering will be determined by the prevailing market price for the shares at the time the shares are sold, a price related to the prevailing market price, at negotiated prices or prices determined, from time to time by the selling stockholders. See "Plan of Distribution."
Our common stock is currently listed on the Over the Counter Bulletin Board under the symbol “BPMA.” On July 30, 2008, the closing price of the shares was $.25 per share.
Our agent for service in the state of Delaware is Corporation Service Company located at 2711 Centerville Road, Suite 400, Wilmington, DE 19808.
AN INVESTMENT IN OUR COMMON STOCK INVOLVES A HIGH DEGREE OF RISK. SEE "RISK FACTORS" BEGINNING AT PAGE 4.
NEITHER THE SECURITIES AND EXCHANGE COMMISSION NOR ANY STATE SECURITIES COMMISSION HAS APPROVED OR DISAPPROVED THESE SECURITIES OR PASSED UPON THE ADEQUACY OR ACCURACY OF THIS PROSPECTUS. ANY REPRESENTATION TO THE CONTRARY IS A CRIMINAL OFFENSE.
The date of this prospectus is __________, 2008.
TABLE OF CONTENTS
| Prospectus Summary | | | 7 | |
| Risk Factors | | | 17 | |
| Use of Proceeds | | | 33 | |
| Selling Security Holders | | | 33 | |
| Plan of Distribution | | | 39 | |
| Legal Proceedings | | | 40 | |
| Officers and Directors | | | 41 | |
| Security Ownership of Certain Beneficial Owners and Management | | | 44 | |
| Description of Securities | | | 48 | |
| Legal Matters | | | 55 | |
| Experts | | | 55 | |
| Disclosure of Commission Position of Indemnification for Securities Act Liabilities Description of Business | | | 55 | |
| Selected Consolidated Financial Data | | | | |
| Management's Discussion and Analysis of Financial Condition and Results of Operations | | | 87 | |
| Description of Property | | | 104 | |
| Certain Relationships and Related Transactions | | | 107 | |
| Market For Common Equity and Related Stockholder Matters | | | 111 | |
| Dividend Policy | | | | |
| Executive Compensation | | | 111 | |
| Changes in and Disagreements with Accountants on Accounting and Financial Disclosure | | | 113 | |
| Where You Can Find More Information | | | 114 | |
| Financial Statements | | | 115 | |
CAUTIONARY NOTICE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus contains forward-looking statements. Such forward-looking statements are not covered by the safe harbor provisions. Such forward-looking statements include statements regarding, among other things, (a) our projected sales and profitability, (b) our growth strategies, (c) anticipated trends in our industry, (d) our future financing plans, and (e) our anticipated needs for working capital. Forward-looking statements, which involve assumptions and describe our future plans, strategies, and expectations, are generally identifiable by use of the words "may," "will," "should," "expect," "anticipate," "estimate," "believe," "intend," or "project" or the negative of these words or other variations on these words or comparable terminology. This information may involve known and unknown risks, uncertainties, and other factors that may cause our actual results, performance, or achievements to be materially different from the future results, performance, or achievements expressed or implied by any forward-looking statements. These statements may be found under “Prospectus Summary”, "Management's Discussion and Analysis of Financial Condition and Results of Operations" and "Description of Business," as well as in this prospectus generally. Actual events or results may differ materially from those discussed in forward-looking statements as a result of various factors, including, without limitation, the risks outlined under "Risk Factors" and matters described in this prospectus generally. This prospectus may contain market data related to our business, which may have been included in articles published by independent industry sources. Although we believe these sources are reliable, we have not independently verified this market data. This market data includes projections that are based on a number of assumptions. If any one or more of these assumptions turns out to be incorrect, actual results may differ materially from the projections based on these assumptions. In light of these risks and uncertainties, there can be no assurance that the forward-looking statements contained in this filing will in fact occur. In addition to the information expressly required to be included in this filing, we will provide such further material information, if any, as may be necessary to make the required statements, in light of the circumstances under which they are made, not misleading.
Each forward-looking statement should be read in context with, and with an understanding of, the various other disclosures concerning our company and our business made elsewhere in this prospectus as well as other pubic reports which may be filed with the United States Securities and Exchange Commission (the "SEC"). You should not place undue reliance on any forward-looking statement as a prediction of actual results or developments. We are not obligated to update or revise any forward-looking statement contained in this prospectus to reflect new events or circumstances, unless and to the extent required by applicable law. Neither the Private Securities Litigation Reform Act of 1995 nor Section 27A of the Securities Act of 1933 provides any protection for statements made in this prospectus.
PROSPECTUS SUMMARY
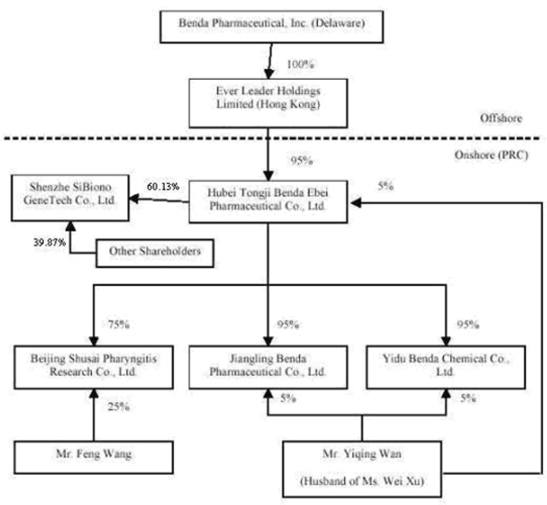
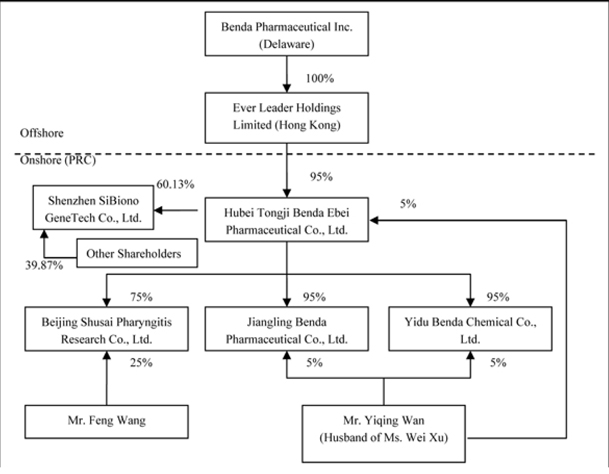
This summary highlights information contained elsewhere in this prospectus. It does not contain all of the information that you should consider before investing in our common stock. You should read the entire prospectus carefully, including the section entitled "Risk Factors" and our consolidated financial statements and the related notes. In this prospectus, we refer to Benda Pharmaceutical, Inc. and our wholly owned subsidiary, Ever Leader Holdings Limited, and Ever Leader’s subsidiary Hubei Tongji Benda Ebei Pharmaceutical Co., Ltd., and indirect subsidiaries Jingling Benda Pharmaceutical Co., Ltd., Yidu Benda Chemical Co., Ltd., Beijing Shusai Pharyngitis Research Co., Ltd, and Shenzhen SiBiono Gene Technology Co., Ltd. as “Benda”, "our company," "we," "us" and "our."
OUR COMPANY
Through our wholly owned subsidiary Ever Leader Holdings Limited (“Ever Leader”), we are a pharmaceutical company that identifies, discovers, develops and manufactures both conventional medications and Traditional Chinese Medicines (“TCMs”) for the treatment of some of the largest common ailments and diseases (e.g., common cold, diabetes, cancer). We are also dedicated to the development, manufacturing and commercialization of gene therapy products.
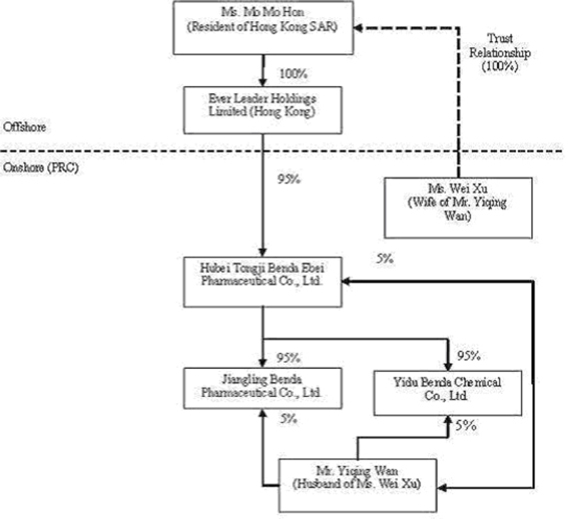
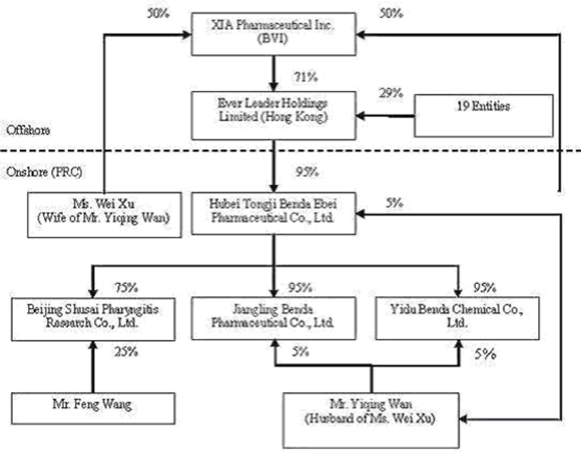
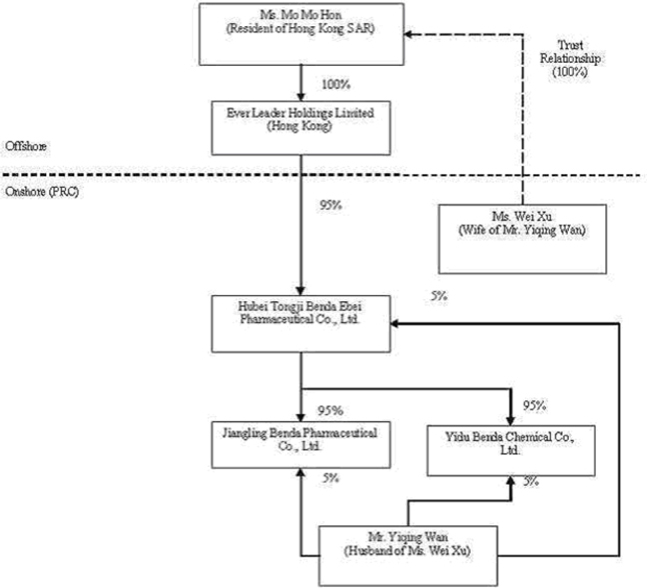
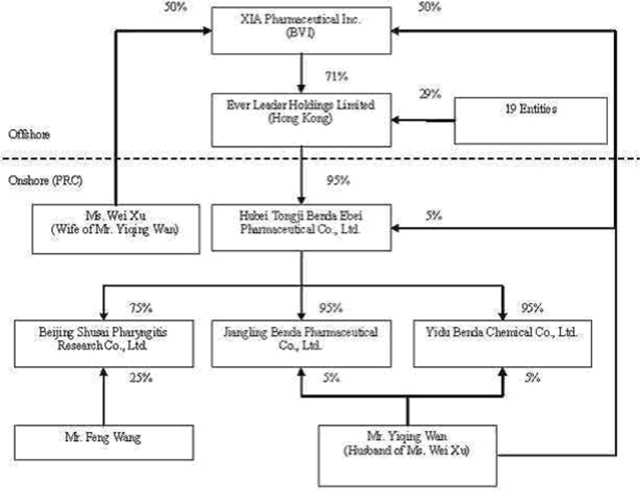
We own all of the capital stock of Ever Leader Holdings Limited, a holding company incorporated under the laws of Hong Kong SAR on October 29, 2005. Ever Leader owns 95% of the issued and outstanding capital stock of Hubei Tongji Benda Ebei Pharmaceutical Co., Ltd., a Sino-Foreign Equity Joint Venture company incorporated in April 2001 under the laws of the PRC. Benda Ebei owns: (i) 95% of the issued and outstanding capital stock of Jiangling Benda Pharmaceutical Co., Ltd., a company formed in October 2001 under the laws of the PRC; (ii) 95% of the issued and outstanding capital stock of Yidu Benda Chemical Co., Ltd., a company incorporated in March 2002 under the laws of the PRC; (iii) 75% of the issued and outstanding capital stock of Beijing Shusai Pharyngitis Research Co., Ltd., a company incorporated in June 2006 under the laws of the PRC; and (iv) 60.13% of the issued and outstanding capital stock of Shenzhen SiBiono Gene Technology Co., Ltd. (“SiBiono”), a company incorporated in March 1998 under the laws of the PRC.
We distribute our medicines, through agents who sell them to hospitals that administer them to patients. We sell generics to medical wholesalers for resale to hospitals. The company sells its Over the Counter (“OTC”) medicines to wholesalers specializing in selling to retail chain drug stores. Our “Active Pharmaceutical Ingredients” (“APIs”) are typically sold to large drug manufacturers under long-term supply contracts. The bulk chemicals are purchased by other Chinese drug companies.
In fiscal 2007, our revenues were $26,384,608 and our restated net income was ($7,362,825). In fiscal 2006, our revenues were $15,932,075 and our restated net income was $2,785,529. All prices, revenues, and income referred to herein are stated in United States dollars.
Share Exchange Transaction
We did not become engaged in the pharmaceutical business until November of 2006. Before closing our recent share exchange transaction in November 2006, we were a shell company with nominal assets and operations, whose sole business was to identify, evaluate and investigate various companies with the intent that, if such investigation warrants, a business combination be negotiated and completed pursuant to which we (formerly known as Applied Spectrum Technologies, Inc.) would acquire a target company with an operating business with the intent of continuing the acquired company's business as a publicly held entity. We entered in an Exchange Agreement dated September 7, 2006 (the “Exchange Agreement”) with KI Equity Partners II, LLC (“KI Equity”), Ever Leader, a company incorporated under the laws of Hong Kong, and the owners of 100% of the capital shares of Ever Leader. The closing of the Exchange Agreement occurred on November 15, 2006. At the closing of the Exchange Agreement, we acquired all of Ever Leader's capital shares (the “Ever Leader Shares”) from the Ever Leader Shareholders, and the Ever Leader Shareholders transferred and contributed all of their Ever Leader Shares to us. In exchange, we issued 64,942,360 shares of our Common Stock to the Ever Leader Shareholders.
In connection with the share exchange transaction, we engaged Keating Investments, LLC to act as a financial advisor in connection with the Exchange transaction. At the closing the Exchange Agreement, Keating Investments, LLC was paid an advisory fee of $395,000.
Recent Financing
The closing of the Exchange Agreement described above was contingent on a minimum of $10,000,000 (or such lesser amount as mutually agreed to by Ever Leader and the placement agent) being subscribed for, and funded into escrow, by certain accredited and institutional investors ("Investors") in a private placement offering for the purchase of Units, each Unit consisting of 54,087 shares of our Common Stock ("Common Stock") and 54,087 common stock purchase warrants promptly after the closing of the Exchange transaction under terms and conditions approved by our board of directors immediately following the Exchange (the “Financing”). The closing of the Financing was contingent on the closing of the Exchange transaction, and the Exchange transaction was contingent on the closing of the Financing. On November 15, 2006, we completed this private placement offering. We received gross proceeds of approximately $12 million in connection with the Financing from the Investors. Pursuant to Subscription Agreements entered into with these Investors, we sold 480 Units for a total of 25,961,760 shares of its Common Stock and warrants to purchase an additional 25,961,760 shares of our common stock to the Investors. The price per Unit in the Financing was $25,000.
Keating Securities, LLC (“Placement Agent”), an affiliate of Keating Investments, LLC, acted as placement agent in connection with the Financing. For their services, the Placement Agents received a commission equal to 7.5% of the gross proceeds from the offering and a non-accountable expense allowance equal to 1.5% of the gross proceeds. In addition, the Placement Agents received, for nominal consideration, warrants to purchase 10% of the number of shares of common stock sold in connection with the Financing, which in the aggregate totaled 2,596,176 shares of our common stock at an exercise price of $0.555 per share. The warrants are fully vested and have a term of five years. The Placement Agent warrants will have registration rights similar to the registration rights afforded to the holders of Common Stock and Warrants subscribed for in the Financing. We also paid for the out-of-pocket expenses incurred by the Placement Agent and all purchasers in the amount of $100,000.
In order to finance the acquisition of a majority of the shares of Shenzhen SiBiono GeneTech Co., Ltd. (“SiBiono”), on April 5, 2007, we entered into an Investment Agreement (“April Financing”) with certain accredited and institutional investors (“Investors”) who had also participated in the subscription for $12,000,000 of our common stock pursuant to certain Securities Purchase Agreements dated November 15, 2006 (“November Financing”). Pursuant to the Investment Agreement, the Investors purchased a total of 252 Units for $7,560,000 with each Unit consisting of (i) a convertible promissory note in the principal amount of Thirty Thousand Dollars ($30,000) which shall be convertible into 54,087 shares of the Company's common stock, par value $0.001 per share, and (ii) a warrant to acquire 54,087 shares of Common Stock at an exercise price of $0.555 per share. The Notes bear an interest rate of four percent per annum until the Buyer elects to exercise the right to convert, and mature on March 28, 2009. The Warrants issued in both the November Financing and the April Financing have full ratchet anti-dilution protection, as more fully described in the section entitled “Description of Securities” set forth herein.
In March 2007 the Company and the Investors entered into a Modification Agreement amending the November Financing Documents to allow for certain issuances of the Company’s securities, including additional purchases of the Company’s equity securities pursuant to the Investment Agreement; shares issuances required under the Equity Transfer Agreements; and issuances of options pursuant to an approved Qualified Employment Stock Option Plan. All of the investors in the November Financing had the right to participate in the purchase of additional units under the Investment Agreement and all of such investors either participated in the Investment Agreement or have waived their right to participate in such. In addition, those investors that did not participate in the Investment Agreement also waived their right to object to the changes to the Warrants, Registration Rights Agreement and Make Good Agreement which were set forth in the Modification Agreement.
On or prior to forty five (45) days from the Closing Date of the Investment Agreement, we are required to deliver to the Buyers our financial statements for the years ending December 31, 2005 and December 31, 2006, audited by Kempisty & Company Certified Public Accountants, P.C., prepared in accordance with GAAP, during each year involved and fairly presenting in all material respects our financial position as of the dates thereof and the results of our operations and cash flows for each such year then ended. Such financial statements for the years ending December 31, 2005 and December 31, 2006 were filed with our Form 10KSB for the year ending December 31, 2006 filed with the Securities and Exchange Commission on May 4, 2007. In addition, on or prior to seventy five (75) days from the Closing Date, we are also required to deliver to the Buyers audited financial statements for SiBiono for the required time periods for the Form 8-K filing required by the Securities and Exchange Commission. Such financial statements were filed with our Amendment No. 1 to Form 8K filed June 15, 2007.
Shenzhen SiBiono GeneTech Co., Ltd.
On April 5, 2007, Hubei Tongji Benda Ebei Pharmaceutical Co., Ltd., a Sino-Foreign Equity Joint Venture company incorporated under the laws of the PRC (“Benda Ebei”), of which Ever Leader Holdings Limited, a company incorporated under the laws of Hong Kong SAR ("Ever Leader") and a wholly owned subsidiary of Benda Pharmaceutical, Inc. (the “Company”), owns 95% of the outstanding common stock, has entered into Equity Transfer Agreements with certain shareholders of Shenzhen SiBiono Gene Technology Co., Ltd. (“SiBiono”), a corporation established and validly existing under the law of the PRC, to purchase a total of approximately 57.57% of the shares of SiBiono’s common stock for total consideration of RMB60,000,000 (or $7.88 million) due and payable on or before April 30, 2007. As of March 31, 2008, an amount of $6.49 million for the purchase of the SiBiono shares had been paid, leaving a balance of approximately $1.39 million. The Company has entered into an oral agreement with the Sibiono shareholders to extend the due date through the year ended 2008. There are no penalty payments to be made in connection with the extended due date.
In connection with the Equity Transfer Agreements, we entered into a Financial Consultancy Agreement with Super Pioneer International Limited (“Super Pioneer”) for financial consultancy services rendered by Super Pioneer. Pursuant to the Financial Consultancy Agreement, we agreed to issue 2,100,000 shares of our common stock to Super Pioneer within three months from the date of the agreement. The shares were issued to Super Pioneer in September, 2007. The public trading price of our common stock on April 5, 2007 and July 5, 2007 was $1.55 and $2.50. The 2,100,000 shares are valued at $7,560,000 based on a price of $3.60. The valuation price was based on the redemption price, as well as the parties expectations at the execution of the agreement. Super Pioneer agreed to lock up the shares for a period of twelve months from the date of the issuance of the shares (the “Lock-up Period”). The Lock-up Period expired on April 5, 2008. Within three months from the Lock-up Period, in the event that the public trading price of our shares did not reach $3.60 per share and we are not listed in the capital market of NASDAQ or AMEX, Super Pioneer shall have the option to require us to redeem 1,960,000 shares of the stock owned by Super Pioneer at a price of $3.60 per share. Such option shall expire within one month from the last date of the three month period. As of June 24, 2008, we are not listed in the capital market of NASDAQ or AMEX, and the current trading price of our common stock as of June 23, 2008 is $.32. Therefore, on July 5, 2008, Super Pioneer shall have the option of requiring us to redeem 1,960,000 shares of our common stock at $3.60 per share. Such option will expire on August 5, 2008.
On June 11, 2007, Benda Ebei entered into Equity Transfer Agreements with Yaojin Wang and Huimin Zhang, shareholders of SiBiono, for the purchase of an additional 2.56% of the shares of SiBiono’s common stock for total consideration of RMB2,560,000 (or $0.34 million) due and payable on or before June 30, 2007. As of September 30, 2007, the full amount of $0.34 million, for the purchase of the SiBiono shares had been paid.
In connection with the Equity Transfer Agreements, we entered into Technical Consultancy Agreements with Yaojin Wang and Huimin Zhang for technical consultancy services rendered by Yaojin Wang and Huimin Zhang. Pursuant to the Technical Consultancy Agreements, we agreed to issue 33,585 shares of our common stock to Yaojin Wang and 55,975 shares of our common stock to Huimin Zhang within three months from the date of the agreement. The shares were issued to Yaojin Wang and Huimin Zhang in September, 2007. The public trading price of our common stock on June 11, 2007 and September 11, 2007 was $2.00 and $2.25. The 33,585 shares are valued at $120,906 and the 55,975 shares are valued at $201,510 based on a price of $3.60. The valuation price was based on the redemption price, as well as the parties expectations at the execution of the agreement. Yaojin Wang and Huimin Zhang agreed to lock up their shares for a period of twelve months from the date of the issuance of the shares (the “Lock-up Period”). The Lock-up Period will expired on June 11, 2008. Within three months from the Lock-up Period or September 11, 20008, in the event that the public trading price of our shares did not reach $3.60 per share and we are not listed in the capital market of NASDAQ or AMEX, Yaojin Wang and Huimin Zhang shall have the option to require us to redeem the shares of the stock owned by Yaojin Wang and Huimin Zhang at a price of $3.60 per share. Such option shall expire within one month from the last date of the three month period. We are not currently listed in the capital market of NASDAQ or AMEX, and the current trading price of our common stock as of June 23, 2008 is $.32. Therefore, if we do not meet these requirements by September 11, 2008, Yaojin Wang and Huimin Zhang shall have the option of requiring us to redeem 33,585 and 55,975 shares of our common stock at $3.60 per share. Such option will expire on October 11, 2008. The total consideration for 60.13% of the outstanding shares of SiBiono’s common stock was Rmb62.56 million or $8.22 million. As of March 31, 2008, an accumulated amount, approximately Rmb52.9 million or $6.83 million was paid and leaving a balance approximately $1.39 million.
Contact Information
We are a Delaware corporation. Our principal executive offices are located at Room 13, Floor 25, Sunny New World Tower, No. 231 Xin Hua Road, Jianghan District, Wuhan, Hubei, PRC. Post Code: 430015.
Our telephone number is +86 (27) 8537-5532.
Our agent for service in the state of Delaware is Corporation Service Company located at 2711 Centerville Road, Suite 400, Wilmington, DE 19808.
THE OFFERING
The shares issued and outstanding consist of 100,803,509 shares of common stock. We are registering shares of our common stock for sale by the selling stockholders identified in the section of this prospectus entitled "Selling Security Holders." The shares included in the table identifying the selling stockholders consist of:
| | 1. | 2,106,561 shares of common stock, of which 29,968 shares were issued in January 2006 to two shareholders for services rendered; 295,378 were purchased in January and March of 2006 and are now held by two shareholders; 169,241 were issued on November 15, 2006 for services rendered in connection with the Financing and Acquisition of Ever Leader; and 1,611,974 were issued to eleven shareholders in exchange for their Ever Leader shares; and |
| | 2. | 15,774,375 shares of common stock based on 150% of the common stock issuable upon exercise of outstanding warrants we issued in connection with our issuance of the Units, at an exercise price of $0.555 per share in conjunction with our private placement completed on November 15, 2006; 150% of the common stock issuable upon exercise of outstanding warrants we issued in connection with our issuance of the Additional Units, at an exercise price of $0.555 per share on April 5, 2007; and 150% of the common stock issuable upon exercise of outstanding warrants we issued to the Placement Agent, at an exercise price of $0.555 per share in conjunction with our private placement completed on November 15, 2006. |
The shares of common stock offered under this prospectus may be sold by the selling security holders on the public market, in negotiated transactions with a broker-dealer or market maker as principal or agent, or in privately negotiated transactions not involving a broker or dealer. Information regarding the selling shareholders, the common shares they are offering to sell under this prospectus, and the times and manner in which they may offer and sell those shares is provided in the sections of this prospectus captioned "Selling Security Holders," "Registration Rights" and "Plan of Distribution," respectively. We will not receive any of the proceeds from those sales. Should the selling security holders, in their discretion, exercise any of the common share purchase warrants underlying the common shares offered under this prospectus, we would, however, receive the exercise price for those warrants. The registration of common shares pursuant to this prospectus does not necessarily mean that any of those shares will ultimately be offered or sold by the selling stockholders, or that any of the common share purchase warrants underlying the common shares offered under this prospectus will be exercised.
Value of Shares Underlying Notes
The total dollar value of the 13,629,924 shares of common stock underlying the Notes is $22,489,375. This number is based on the market price per share of $1.65 for those securities on the April 5, 2007 sale of the Notes.
Fees and Payments Associated with Transaction
The following table discloses the dollar amount of each payment (including the dollar value of any payments to be made in common stock) in connection with the November Financing and April Financing (collectively, the “Financings”) that the Company has paid, or may be required to pay to any Selling Stockholder, any affiliate of a Selling Stockholder, or any person with whom any Selling Stockholder has a contractual relationship regarding the Financings. The table also reflects the potential net proceeds to the Company from the $19,560,000 Financings and the total possible payments to all selling shareholders and any of their affiliates in the first year following the sale of convertible notes.
There are no other persons with whom any Selling Stockholder has a contractual relationship with regarding the transaction.
| | Placement Agent Fee(1) | | Structuring, Legal, and Misc. Fees(2) | | Maximum Possible Interest Payments(3) | | Maximum Possible Liquidated Damages(4) | | Maximum First Year Payments(5) | | Maximum Possible Payments(6) | | Net Proceeds to Company(7) | | Net Proceeds to Company After Maximum First Year Payments(8) | |
| | | | | | | | | | | | | | | | | |
| $ | 2,001,998 | | $ | 260,254 | | $ | 628,561 | | $ | 1,956,000 | | $ | 4,526,258 | | $ | 4,846,813 | | $ | 14,713,187 | | $ | 15,033,742 | |
(1) | A total of $2,001,998 was paid to Keating Securities., LLC as placement agent for the Financings. The fee was allocated as follows: |
| November 2006 7.5% placement fee | | $ | 897,798 | |
| November 2006 non-accountable 1.5% expense fee | | $ | 180,000 | |
| November 2006 reverse merger advisory fee | | $ | 395,000 | |
| April 2007 7% placement fee | | $ | 529,200 | |
(2) | The Company paid $260,254 in structuring, legal and miscellaneous fees to the following parties in connection with the Financings: |
| Computershare Trust - escrow fee & make good escrow agreement | | $ | 5,000 | |
| John B. Lowy, P.C. - legal fee | | | 10,000 | |
| RR Donnelley - printing fee | | | 21,229 | |
| H. Rivkin & Co. - consulting Fee | | | 50,000 | |
| Anslow & Jaclin LLP - legal fees | | | 174,025 | |
(3) | Maximum amount of interest that can accrue at a rate of 4% per annum assuming all Notes aggregating $7,560,000 issued on April 5, 2007 and remain outstanding until the maturity date on April 5, 2009. The Company, at its option, may pay accrued interest in either cash or, in shares of its common stock. |
(4) | Under certain circumstances we may be assessed liquidated damages prior to the maturity date equal to 1% of the aggregate subscription amount of the Financings if the registration statement was not filed within 60 days from the closing of the Financing, if the registration statement was not declared effective within 180 days from the closing of the Financing or, among other things, we fail to cure any defects in a request for acceleration of this registration statement, or fail to file a pre-effective amendment of this registration statement for every 30 day period (or part) thereafter, in each case until cured, provided that the total liquidated damages shall not exceed 10% of the purchase price of the Financings. This represents the maximum liquidated damages the Company would pay assuming to total liquidated damages equaled the maximum of 10% of the purchase price of the Financings. |
| | |
(5) | Total maximum payments that the Company may be required to pay to the Selling Stockholders for the twelve (12) months following the sale of all Notes, which is comprised of placement agent fee of $2,001,998, structuring, legal and miscellaneous fees of $260,254, first year interest of $308,006, and liquidated damages of $1,956,000. |
| | |
(6) | Total maximum payments payable by Company, includes placement agent fee of $2,001,998, structuring, legal and miscellaneous fees of $260,254, maximum possible interest of $628,561 and maximum possible liquidated damages of $1,956,000. |
| | |
(7) | Total net proceeds to the Company assuming that the Company was required to make all possible payments as described in footnote 6. |
| | |
(8) | Total net proceeds to the Company assuming that the Company was required to make all possible payments in the next twelve months as described in footnote 5. |
Total Possible Profit Selling Stockholders Could Realize
Notes
The following table discloses the total possible profit Selling Stockholders could realize as a result of the conversion discount for the securities underlying the $7,560,000 in Notes.
| | Market Price(1) | | Conversion Price(2) | | Shares Underlying Notes(3) | | Combined Market Price of Shares(4) | | Total Conversion Price(5) | | Total Possible Discount to Market Price(6) | |
| | | | | | | | | | | | | |
| $ | 1.55 | | $ | 0.555 | | | 13,621,622 | | $ | 21,113,514 | | $ | 7,560,000 | | $ | 13,553,514 | |
(1) | Market price per share of our common stock on the Issuance Date (April 5, 2007). |
(2) | The original fixed conversion price is $0.555, except that the Notes contain anti-dilution protections which in certain circumstances may result in a reduction to the conversion price. |
| | |
(3) | Total number of shares of common stock underlying the Notes assuming full conversion of the aggregate principal amount as of the Issuance Date. |
| | |
(4) | Total market value of shares of common stock underlying the Notes assuming full conversion of the aggregate principal amount as of the Issuance Date based on the market price of the common stock on the Issuance Date. |
| | |
(5) | Total value of shares of common stock underlying the Notes assuming full conversion as of the aggregate principal amount as of the Issuance Date based on the conversion price. |
| | |
(6) | Discount to market price calculated by subtracting the total conversion price (result in footnote (5)) from the combined market price (result in footnote (4)). |
Warrants
In the Financings, we also issued to Selling Stockholders five year Warrants to purchase an aggregate of 39,591,684 shares of our common stock, exercisable at a price per share of $0.555. The following table discloses the total possible profit Selling Stockholders could realize as a result of the exercise of the Warrants.
| | Market Price(1) | | Exercise Price(2) | | Shares Underlying Warrants(3) | | Combined Market Price(4) | | Total Conversion Price(5) | | Total Possible Discount to Market Price(6) | |
| | | | | | | | | | | | | |
| $ | 1.55 | | $ | 0.555 | | | 39,591,684 | | $ | 61,367,110 | | $ | 21,973,385 | | $ | 39,393,725 | |
(1) | Market price per share of our common stock on the Issuance Date (April 5, 2007). |
| | |
(2) | The exercise price per share of our common stock underlying the Warrants is fixed at $0.555 except that the Warrants contain anti-dilution protections which in certain circumstances may result in a reduction to the exercise price. |
(3) | Total number of shares of common stock underlying the Warrants assuming full exercise as of the Issuance Date. Upon certain adjustments of the exercise price of the warrants, the number of shares underlying the Warrants may also be adjusted such that the proceeds to be received by us would remain constant. |
| | |
(4) | Total market value of shares of common stock underlying the Warrants assuming full exercise as of the Issuance Date based on the market price of the common stock on the Issuance Date. |
| | |
(5) | Total value of shares of common stock underlying the Warrants assuming full exercise as of the Issuance Date based on the exercise price. |
| | |
(6) | Discount to market price calculated by subtracting the total conversion price (result in footnote (5)) from the combined market price (result in footnote (4)). The result of an exercise of the Warrants at the exercise price and a sale at the market price would be a gain to the Selling Stockholder. Since the market price of our common stock is more than the Warrants’ exercise price, the Warrants are “in the money” and a profit would be realized as of April 5, 2007. |
Net Proceeds Payable to the Company and Combined Total Possible Profit Selling Stockholders Could Realize
The following table summarizes the potential proceeds available to the Company pursuant to the financing with the Investors and the Investors’ return on investment. For purposes of this table, we assumed that the Investors exercise all of the in-the-money Warrants, if any.
| Gross Proceeds Payable to Company(1) | | Maximum Possible Payments by Company(2) | | Net Proceeds to Company(3) | | Combined Total Possible Profit to Investors(4) | | All Payments + Possible Profit / Net Proceeds(5) | | All Payments + Possible Profit / Net Proceeds Averaged Over 2 Years(6) | |
| | | | | | | | | | | | | |
| | $ 19,560,000 | | $ | 4,846,813 | | $ | 14,713,187 | | $ | 52,947,239 | | | 295 | % | | 148 | % |
(1) | Total amount of the proceeds of the November Financing ($12,000,000) and the April Financing ($7,560,000). |
| | |
(2) | Total maximum payments payable by Company, includes placement agent fee of $2,001,998, structuring, legal and miscellaneous fees of $260,254, maximum possible interest of $628,561 and maximum possible liquidated damages of $1,956,000. |
| | |
(3) | Total net proceeds calculated by subtracting the Maximum Possible Payments by the Company ($4,846,813) from the Gross Proceeds Payable to the Company ($19,560,000). |
| | |
(4) | Total possible profit to the Investors is based on the aggregate discount to market price of the conversion of the Notes and exercise of Warrants. The Notes’ conversion price is based on a fixed price of $0.555. The exercise price per share of our common stock underlying the Warrants is fixed at $0.555 except that the Warrants contain anti-dilution protections which in certain circumstances may result in a reduction to the exercise price. |
(5) | Percentage equal to the maximum possible payments by us in the transaction ($4,846,813) plus total possible discount to the market price of the shares underlying the Notes ($13,553,514), plus profit from 39,591,684 warrants in the money ($39,393,725), divided by the net proceeds to the Company resulting from the Financings ($19,560,000). |
| | |
(6) | Calculated by dividing 295% (footnote 5) by the term of the Notes, two years. |
Prior Securities Transactions with Selling Stockholders
We have not engaged in any prior securities transactions with the Selling Stockholders, any affiliates of the Selling Stockholders, or any person with whom any Selling Stockholder has a contractual relationship regarding the transaction (or any predecessors of those persons).
Shares Outstanding Prior to the Transaction
The following table discloses certain information comparing the number of shares outstanding prior to the transaction, number of shares registered by the Selling Stockholders, or their affiliates, in prior registration statements (along with that number still held and number sold pursuant to such prior registration statement) and the number of shares registered for resale in this Registration Statement relating to the financing transaction.
| Number of shares outstanding held by persons other than affiliates of the Company. | | | 53,642,807 | |
| Number of shares registered for resale by Selling Stockholders or affiliates in prior registration statements. | | | 0 | |
| Number of shares registered for resale by Selling Stockholders or affiliates of Selling Stockholders that continue to be held by Selling Stockholders or affiliates of Selling Stockholders. | | | 0 | |
| Number of shares sold in registered resale by Selling Stockholders or affiliates of Selling Stockholders. | | | 0 | |
| Number of shares registered for resale on behalf of Selling Stockholders or affiliates of Selling Stockholders in current transaction. | | | 17,880,936 | |
Repayment, Shorting and Prior Transactions with Selling Stockholders
The Company intends to repay the overlying securities and believes that it will have the financial ability to make all payments on the Notes when they become due and payable. To the best of our knowledge, and based on information obtained from the Selling Stockholders, none of the selling shareholders have an existing short position in the Company’s common stock.
Other than in the November Financing and April Financing, the Company has not in the past three (3) years engaged in any securities transaction with any of the Selling Stockholders, any affiliates of the Selling Stockholders, or, after due inquiry and investigation, to the knowledge of the management of the Company, any person with whom any Selling Stockholder has a contractual relationship regarding the transaction (or any predecessors of those persons). In addition, other than in connection with the contractual obligations set forth in the transaction documents filed as Exhibits to our Form 8-Ks filed November 17, 2006 and April 6, 2007, the Company does not have any agreements or arrangements with the Selling Stockholders with respect to the performance of any current or future obligations.
RISK FACTORS
You should carefully consider the risks described below together with all of the other information included in this report before making an investment decision with regard to our securities. The statements contained in or incorporated into this offering that are not historic facts are forward-looking statements that are subject to risks and uncertainties that could cause actual results to differ materially from those set forth in or implied by forward-looking statements. If any of the following risks actually occurs, our business, financial condition or results of operations could be harmed. In that case, the trading price of our common stock could decline, and you may lose all or part of your investment.
Risks Relating to Our Business
| WE NEED TO MANAGE GROWTH IN OPERATIONS TO MAXIMIZE OUR POTENTIAL GROWTH AND ACHIEVE OUR EXPECTED REVENUES AND OUR FAILURE TO MANAGE GROWTH WILL CAUSE A DISRUPTION OF OUR OPERATIONS RESULTING IN THE FAILURE TO GENERATE REVENUE. |
In order to maximize potential growth in our current and potential markets, we believe that we must expand our manufacturing and marketing operations. This expansion will place a significant strain on our management and our operational, accounting, and information systems. We expect that we will need to continue to improve our financial controls, operating procedures, and management information systems. We will also need to effectively train, motivate, and manage our employees. Our failure to manage our growth could disrupt our operations and ultimately prevent us from generating the revenues we expect.
In order to achieve the above mentioned targets, the general strategies of our company are to maintain and search for hard-working employees who have innovative initiatives; on the other hands, our company will also keep a close eye on expanding opportunities, for example, acquisition of state-owned enterprises.
| WE CANNOT ASSURE YOU THAT OUR ORGANIC GROWTH STRATEGY WILL BE SUCCESSFUL WHICH MAY RESULT IN A NEGATIVE IMPACT ON OUR GROWTH, FINANCIAL CONDITION, RESULTS OF OPERATIONS AND CASH FLOW. |
One of our strategies is to grow organically through increasing the distribution and sales of our products by penetrating existing markets in PRC and entering new geographic markets in PRC as well as other parts of Asia and the United States. However, many obstacles to entering such new markets exist, including, but not limited to, international trade and tariff barriers, shipping and delivery costs, costs associated with marketing efforts abroad and maintaining attractive foreign exchange ratios. We cannot, therefore, assure you that we will be able to successfully overcome such obstacles and establish our products in any additional markets. Our inability to implement this organic growth strategy successfully may have a negative impact on our growth, future financial condition, results of operations or cash flows.
| WE CANNOT ASSURE YOU THAT OUR ACQUISITION GROWTH STRATEGY WILL BE SUCCESSFUL RESULTING IN OUR FAILURE TO MEET GROWTH AND REVENUE EXPECTATIONS. |
In addition to our organic growth strategy, we also expect to grow through strategic acquisitions. We intend to pursue opportunities to acquire businesses in PRC that are complementary or related in product lines and business structure to us. We may not be able to locate suitable acquisition candidates at prices that we consider appropriate or to finance acquisitions on terms that are satisfactory to us. If we do identify an appropriate acquisition candidate, we may not be able to negotiate successfully the terms of an acquisition, or, if the acquisition occurs, integrate the acquired business into our existing business. Acquisitions of businesses or other material operations may require debt financing or additional equity financing, resulting in leverage or dilution of ownership. Integration of acquired business operations could disrupt our business by diverting management away from day-to-day operations. The difficulties of integration may be increased by the necessity of coordinating geographically dispersed organizations, integrating personnel with disparate business backgrounds and combining different corporate cultures. We also may not be able to maintain key employees or customers of an acquired business or realize cost efficiencies or synergies or other benefits we anticipated when selecting our acquisition candidates. In addition, we may need to record write-downs from future impairments of intangible assets, which could reduce our future reported earnings. At times, acquisition candidates may have liabilities or adverse operating issues that we fail to discover through due diligence prior to the acquisition. In addition to the above, acquisitions in PRC, including of state owned businesses, will be required to comply with laws of the People's Republic of China ("PRC"), to the extent applicable. There can be no assurance that any given proposed acquisition will be able to comply with PRC requirements, rules and/or regulations, or that we will successfully obtain governmental approvals which are necessary to consummate such acquisitions, to the extent required. If our acquisition strategy is unsuccessful, we will not grow our operations and revenues at the rate that we anticipate. If our acquisition strategy is not successful, our revenues and profit will be solely dependent upon our organic growth.
| WE HAVE PREVIOUSLY HAD AN EXPLOSION AT OUR YIDU PLANT THAT RESULTED IN TWO DEATHS AND ANY SUCH OCURRENCE IN THE FUTURE CAN EXPOSE US TO LIABILITY FOR SUCH AN EVENT |
On November 10, 2005, there was a small explosion in the Yidu plant resulting in the deaths of two of our workers. This tragic accident resulted from the violation of our operating procedures by one of the workers killed in the explosion. We paid RMB 260,000 (or $32,500) to each of the victims’ families in settlement of any compensation issues. On November 11, 2005, there was another explosion in the same factory resulting from a chemical reaction triggered by the prior explosion. Fortunately management had anticipated this incident and the plant had already been temporarily sealed so that no further injury occurred.
Following the events of November 10 and 11, 2005, Yidu plant was temporarily closed for approximately one month; during the one month plant shut down, we put in place very strict work safety procedures and revised the design of the relevant production process in order to avoid similar incidents in the future. We believe Benda is not liable for any further material liabilities arising from these explosions. Notwithstanding this fact, there can be a future explosion that may result in death or serious injury. If this occurs and in light of the fact that it previously occurred, may result in exposure to extensive liability to us.
Currently, we do not have any insurance policy for Yidu Benda due to the fact that it has been closed since January 2007 for improvement of our waste water treatment systems. However, once Yidu Benda resumes to its production, we will deploy appropriate insurance policy for the workers.
| IF WE NEED ADDITIONAL CAPITAL TO FUND OUR GROWING OPERATIONS, WE MAY NOT BE ABLE TO OBTAIN SUFFICIENT CAPITAL AND MAY BE FORCED TO LIMIT THE SCOPE OF OUR OPERATIONS. |
If adequate additional financing is not available on reasonable terms, we may not be able to undertake plant expansion, purchase additional machinery and purchase equipment for our operations and we would have to modify our business plans accordingly. There is no assurance that additional financing will be available to us.
In connection with our growth strategies, we may experience increased capital needs and accordingly, we may not have sufficient capital to fund our future operations without additional capital investments. Our capital needs will depend on numerous factors, including (i) our profitability; (ii) the release of competitive products by our competition; (iii) the level of our investment in research and development; and (iv) the amount of our capital expenditures, including acquisitions. We cannot assure you that we will be able to obtain capital in the future to meet our needs.
In recent years, the securities markets in the United States have experienced a high level of price and volume volatility, and the market price of securities of many companies have experienced wide fluctuations that have not necessarily been related to the operations, performances, underlying asset values or prospects of such companies. For these reasons, our shares of common stock can also be expected to be subject to volatility resulting from purely market forces over which we will have no control. If we need additional funding, the market fluctuations affect on our stock price could limit our ability to obtain equity financing.
If we cannot obtain additional funding, we may be required to: (i) limit our investments in research and development; (ii) limit our marketing efforts; and (iii) decrease or eliminate capital expenditures.
Such reductions could materially adversely affect our business and our ability to compete.
Since Jiangling Benda was resumed its production since August 10, 2007, we estimate that an additional working capital approximately $4.6 MM is need in order to fully utilize its production capacity. Furthermore, when Yidu Benda resumes its production, the operation cost to maintain the water waste treatment system is estimated at approximately $0.3 MM per year.
Even if we do find a source of additional capital, we may not be able to negotiate terms and conditions for receiving the additional capital that are acceptable to us. Any future capital investments could dilute or otherwise materially and adversely affect the holdings or rights of our existing shareholders. In addition, new equity or convertible debt securities issued by us to obtain financing could have rights, preferences and privileges senior to our common stock. We cannot give you any assurance that any additional financing will be available to us, or if available, will be on terms favorable to us.
| WE MAY BE REQUIRED TO REPURCHASE SHARES OF OUR COMMON STOCK IN CONNECTION WITH THE FINANCIAL CONSULTANCY AGREEMENT AND TECHNICAL CONSULTANCY AGREEMENTS. THE REPURCHASE OF THESE SHARES MAY HAVE A NEGATIVE IMPACT ON OUR PROFITABILITY. |
In connection with the Equity Transfer Agreements, we entered into a Financial Consultancy Agreement on April 5, 2007 with Super Pioneer for financial consultancy services rendered by Super Pioneer, and Technical Consultancy Agreements on June 11, 2007 with Yaojin Wang and Huimin Zhang for technical consultancy services rendered. Pursuant to these Agreements, we agreed to issue 2,100,000 shares of our common stock to Super Pioneer, 33,585 shares of our common stock to Yaojin Wang, and 55,975 shares of our common stock to Huimin Zhang within three months from the date of the agreements. The parties to the agreements agreed to lock up the shares for a period of twelve months from the date of the issuance of the shares (the “Lock-up Period”). Within three months from the Lock-up Period, in the event that the public trading price of our shares did not reach $3.60 per share and we are not listed in the capital market of NASDAQ or AMEX, Super Pioneer shall have the option to require us to redeem 1,960,000 shares of the stock owned by Super Pioneer and Yaojin Wang and Huimin Zhang shall have the option to require us to redeem the shares of the stock owned by Yaojin Wang and Huimin Zhang at a price of $3.60 per share. Such option shall expire within one month from the last date of the three month period. Therefore, as of July 5, 2008, we may be required to redeem 1,960,000 shares of common stock held by Super Pioneer at a price of $3.60 per share for a total of $7,560,000. As of September 11, 2008, we may be required to redeem the 33,585 shares of our common stock held by Yaojin Wang at $3.60 per share for a total of $120,906 and the 55,975 shares held by Huimin Zhang at $3.60 per share for a total of $201,510. As of April 24, 2008, the last closing bid price of our common stock was $.31. If we would be required to redeem the shares of our common stock a price of $3.60 per share, we will not have these funds to devote to our operations and could have a negative impact on our profitability.
| JIANGLING BENDA AND YIDU BENDA CURRENTLY ARE ENTITLED TO A BENEFICIAL TAX EXEMPTION FOR A FIVE YEAR PERIOD; HOWEVER, SUCH TAX EXEMPTION MAY BE INTERPRETED TO BE NOT IN COMPLIANCE WITH PRC TAX LAWS IN THE FUTURE CAUSING US TO SET ASIDE CERTAIN CONTINGENCY FUNDS FOR DEALING WITH POTENTIAL RETROSPECTIVE TAX LIABILITIES. |
Jiangling Benda and Yidu Benda are entitled to enjoy the “Two exemption Three half” tax holiday based on the local government’s policy to encourage outside investment into the locality. However, the definition of the “Two exemption Three half” policy defined by Yidu City and Jiangling County’s governments is different from the usual understanding of the term. According to PRC tax laws, “Two exemption Three half” policy means foreign investment enterprises including Benda Ebei may enjoy an exemption from corporate income tax for 2 years starting from its first profitable year, followed by 3 years at a rate that is one half of the regular rate for corporate income tax. However, in the documents issued by Yidu City and Jiangling County’s governments, the term means that an outside enterprise can enjoy the “two exemption three half” privilege treatment only with respect to the part allotted to the local government. According to our Chinese legal counsel, under current PRC tax laws, most enterprises are required to pay corporate income tax at a rate of 33% of its income before tax, of which, about 90.91% (30% of the corporate income) is paid over to the Central government, only about 9.09% (3% of the corporate incomes) is reserved for the local government. Accordingly, there is a risk that the local government may only be able to dispose of the “9.09% (3%)” reserved for the local government and Jiangling Benda and Yidu Benda actually would receive much less preferential treatment than a foreign investment enterprise could enjoy.
The beneficial tax exemption of Jiangling Benda and Yidu Benda was started on November 4, 2005, and they would subject to corporate income tax rate 16.5% starting from November 4, 2007 and they would subject to corporate income tax at 25% according to the new PRC corporate income tax with the effective date January 1, 2008, starting from November 4, 2010.
If Jiangling Benda and Yidu Benda could not have any beneficial tax exemption, the tax contingency, at full rate 25%, would be as follows:
| | a) | For the year of 2005: Jiangling Benda: Nil; Yidu Benda: approximately $360,000; |
| | b) | For the year of 2006: Jiangling Benda: Nil; Yidu Benda: approximately $580,000. |
As to the tax treatment promised by local governments to purely domestic enterprises, i.e., Jiangling Benda and Yidu Benda, invested by non-local (but not foreign) investors under the so called preferential policy announced by local governments, our consultation with PRC certified public accountants and lawyers, is that the above policy is not compliant with the PRC laws. Even though such practice exists in many areas across the country, the policy faces the risk of being ruled illegal at any time for non-compliance with relevant laws. In this event, there is a risk that we might be assessed retrospective tax liabilities.
| WE MAY HAVE DIFFICULTY DEFENDING OUR INTELLECTUAL PROPERTY RIGHTS FROM INFRINGEMENT RESULTING IN LAWSUITS REQUIRING US TO DEVOTE FINANCIAL AND MANAGEMENT RESOURCES THAT WOULD HAVE A NEGATIVE IMPACT ON OUR OPERATING RESULTS. |
We regard our service marks, trademarks, trade secrets, patents and similar intellectual property as critical to our success. We rely on trademark, patent and trade secret law, as well as confidentiality and license agreements with certain of our employees, customers and others to protect our proprietary rights. We have received trademark and patent protection for certain of our products in the People's Republic of China. No assurance can be given that our patents and licenses will not be challenged, invalidated, infringed or circumvented, or that our intellectual property rights will provide competitive advantages to us. There can be no assurance that we will be able to obtain a license from a third-party technology that we may need to conduct our business or that such technology can be licensed at a reasonable cost.
Presently, all of our products are sold to clients in PRC. To date, no trademark or patent filings have been made other than in PRC. To the extent that we market our products in other countries, we may have to take additional action to protect our intellectual property. The measures we take to protect our proprietary rights may be inadequate and we cannot give you any assurance that our competitors will not independently develop formulations and processes that are substantially equivalent or superior to our own or copy our products.
Currently, the State Food and Drug Administration (“SFDA”) does not automatically stay drug registration approval upon initiation of an infringement lawsuit by a third party. At present, we must wait until a copycat manufacturer has received marketing approval from SFDA before we can bring an infringement lawsuit. Furthermore, Chinese courts have been hesitant to issue preliminary injunctions to suspend sales until a final judgment is issued in the lawsuit. Our sales could be lowered were a competitor to infringe our intellectual property rights by marketing one or more versions of SFDA-approved drugs proprietary to us, such as the Qiweiben capsule, until we can curtail such infringement through legal action. Pursuing infringement lawsuits would require us to devote financial and management resources that could impact the results of our operations.
| WE DEPEND ON THE SUPPLY OF RAW MATERIALS, AND ANY ADVERSE CHANGES IN SUCH INTENSE COMPETITION FROM EXISTING AND NEW ENTITIES MAY ADVERSELY AFFECT OUR REVENUES AND PROFITABILITY. |
We compete with other companies, many of whom are developing or can be expected to develop products similar to ours. Our markets are large with many competitors. The market for pharmaceutical raw materials manufacturing is particularly competitive. In this market, our competitors include a number of contract manufacturers and, from time to time, demand for particular products may be greatly exceeded by production capacity. Many of our competitors are more established than we are, and have significantly greater financial, technical, marketing and other resources than us. Some of our competitors may have greater name recognition and a larger customer base. These competitors may be able to respond more quickly to new or changing opportunities and customer requirements and may be able to undertake more extensive promotional activities, offer more attractive terms to customers, and adopt more aggressive pricing policies. We intend to create greater brand awareness for our brand name so that we can successfully compete with our competitors. We cannot assure you that we will be able to compete effectively with current or future competitors or that the competitive pressures we face will not harm our business.
Our company does not depend on any sole source suppliers, since the raw materials market is opened in the sense that we could have plenty of choices of the suppliers. We only choose those suppliers who could offer better terms to us and having a long term co-operation relationship.
| OUR PRODUCTS AND THE PROCESSES COULD EXPOSE US TO SUBSTANTIAL PRODUCT LIABILITY CLAIMS WHICH WILL NEGATIVELY IMPACT OUR PROFITABILITY. |
We face an inherent business risk of exposure to product liability claims in the event that the use of our products is alleged to have resulted in adverse side effects. Side effects or marketing or manufacturing problems pertaining to any of our products could result in product liability claims or adverse publicity. These risks will exist for those products in clinical development and with respect to those products that have received regulatory approval for commercial sale. We do not have insurance to cover any potential product liability claims. To date, we have not experienced any product liability claims. However, that does not mean that we will not have any such claims with respect to our products in the future which will negatively impact our profitability.
| WE DEPEND ON OUR KEY MANAGEMENT PERSONNEL AND THE LOSS OF THEIR SERVICES COULD ADVERSELY AFFECT OUR BUSINESS. |
We place substantial reliance upon the efforts and abilities of our executive officers, Yiqing Wan, our Chairman and Chief Executive Officer, Wei Xu, our Vice President of Operations, Hui Long, our Vice President of Technology. We have entered into a five year employment contract with Mr. Wan and three year contracts with Ms. Xu. As per his contract, Mr. Wan is to receive salary of $160,000 per year, Ms. Xu is to receive $100,000, with the possibility of a discretionary bonus as determined by the Board of Directors. Each employment contract immediately terminates upon death or disability, and may be terminated by the Company either with or without cause after 30 days notice, or terminated by the officer for good reason with 60 days notice. We are not currently aware of the plans of any key employees to retire or leave the Company. However, the loss of the services of any of our executive officers could have a material adverse effect on our business, operations, revenues or prospects. We do not maintain key man life insurance on the lives of these individuals.
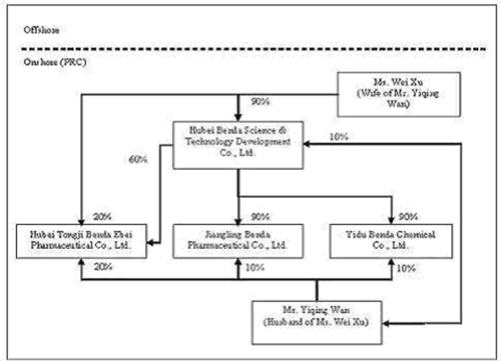
| MR. YIQING WAN AND MS. WEI XU ARE HUSBAND AND WIFE. THE SEPARATION OR DIVORCE OF THE COUPLE IN THE FUTURE COULD ADVERSELY AFFECT OUR BUSINESS. |
Mr. Yiqing Wan, our Chairman and Chief Executive Officer, and Ms. Wei Xu, Vice President of Operations, are married. They are two of our principal executives and are a vital part of our operations. If they were to become separated or divorced and could not amicably work with each other, one of them may decide to cease his or her employment with us. Alternatively, their work performance may not be satisfactory if they become preoccupied with such negative situation. In both cases, our operations could be negatively affected by our production resulting in a decrease in revenues.
| MANAGEMENT EXERCISES SIGNIFICANT CONTROL OVER MATTERS REQUIRING SHAREHOLDER APPROVAL WHICH MAY RESULT IN THE DELAY OR PREVENTION OF A CHANGE IN OUR CONTROL. |
Mr. Yiqing Wan, our Chairman and Chief Executive Officer, and Ms. Wei Xu, our Vice President of Operations, each have a 50% equity ownership in XIA Pharmaceutical Inc. Through their joint ownership in XIA Pharmaceutical, Inc., Mr. Wan and Ms. Xu currently share voting power equal to approximately 29.25% of our voting securities. When combined with the common stock ownership of our other officers and directors, management has combined voting power in our Company equal to approximately 31% of our voting securities. As a result, management through such stock ownership exercises significant control over all matters requiring shareholder approval, including the election of directors and approval of significant corporate transactions. This concentration of ownership in management may also have the effect of delaying or preventing a change in control of us that may be otherwise viewed as beneficial by shareholders other than management.
| INTERNATIONAL OPERATIONS REQUIRE US TO COMPLY WITH A NUMBER OF UNITED STATES AND INTERNATIONAL REGULATIONS WHICH MAY HAVE A NEGATIVE IMPACT ON OUR GROWTH. |
We are required to comply with a number of international regulations in countries outside of the United States. In addition, we must comply with the Foreign Corrupt Practices Act, or FCPA, which prohibits U.S. companies or their agents and employees from providing anything of value to a foreign official for the purposes of influencing any act or decision of these individuals in their official capacity to help obtain or retain business, direct business to any person or corporate entity or obtain any unfair advantage. Any failure by us to adopt appropriate compliance procedures and ensure that our employees and agents comply with the FCPA and applicable laws and regulations in foreign jurisdictions could result in substantial penalties and/or restrictions in our ability to conduct business in certain foreign jurisdictions. We believe we are currently in compliance with such regulations. The U.S. Department of The Treasury's Office of Foreign Asset Control, or OFAC, administers and enforces economic and trade sanctions against targeted foreign countries, entities and individuals based on U.S. foreign policy and national security goals. As a result, we are restricted from entering into transactions with certain targeted foreign countries, entities and individuals except as permitted by OFAC which may reduce our future growth.
| WE MAY INCUR SIGNIFICANT COSTS TO ENSURE COMPLIANCE WITH UNITED STATES CORPORATE GOVERNANCE AND ACCOUNTING REQUIREMENTS. |
We may incur significant costs associated with our public company reporting requirements, costs associated with newly applicable corporate governance requirements, including requirements under the Sarbanes-Oxley Act of 2002 and other rules implemented by the Securities and Exchange Commission. We expect all of these applicable rules and regulations to significantly increase our legal and financial compliance costs and to make some activities more time consuming and costly. We also expect that these applicable rules and regulations may make it more difficult and more expensive for us to obtain director and officer liability insurance and we may be required to accept reduced policy limits and coverage or incur substantially higher costs to obtain the same or similar coverage. As a result, it may be more difficult for us to attract and retain qualified individuals to serve on our board of directors or as executive officers. We are currently evaluating and monitoring developments with respect to these newly applicable rules, and we cannot predict or estimate the amount of additional costs we may incur or the timing of such costs.
| WE MAY NOT BE ABLE TO MEET THE ACCELERATED FILING AND INTERNAL CONTROL REPORTING REQUIREMENTS IMPOSED BY THE SECURITIES AND EXCHANGE COMMISSION RESULTING IN A POSSIBLE DECLINE IN THE PRICE OF OUR COMMON STOCK AND OUR INABILITY TO OBTAIN FUTURE FINANCING. |
As directed by Section 404 of the Sarbanes-Oxley Act, the Securities and Exchange Commission adopted rules requiring each public company to include a report of management on the company's internal controls over financial reporting in its annual reports. In addition, the independent registered public accounting firm auditing a company's financial statements must also attest to and report on management's assessment of the effectiveness of the company's internal controls over financial reporting as well as the operating effectiveness of the company's internal controls. We will not be subject to these requirements for the fiscal year ended December 31, 2006.
While we expect to expend significant resources in developing the necessary documentation and testing procedures required by Section 404 of the Sarbanes-Oxley Act, there is a risk that we may not be able to comply timely with all of the requirements imposed by this rule. In the event that we are unable to receive a positive attestation from our independent registered public accounting firm with respect to our internal controls, investors and others may lose confidence in the reliability of our financial statements and our stock price and ability to obtain equity or debt financing as needed could suffer.
In addition, in the event that our independent registered public accounting firm is unable to rely on our internal controls in connection with its audit of our financial statements, and in the further event that it is unable to devise alternative procedures in order to satisfy itself as to the material accuracy of our financial statements and related disclosures, it is possible that we would be unable to file our Annual Report on Form 10-K with the Securities and Exchange Commission, which could also adversely affect the market price of our common stock and our ability to secure additional financing as needed.
Risks Relating to the People's Republic of China
| THE SALES PRICES OF SOME MEDICINES ARE CURRENTLY CONTROLLED BY THE CHINESE GOVERNMENT AND THAT MAY ADVERSELY AFFECT OUR BUSINESS. |
Prices paid by end consumers for many of our medicines are regulated by PRC's State Development and Reform Commission. PRC justifies its need to control the drug prices on the basis that, at present, only workers at state or private companies have health insurance. About 900 million rural Chinese people and 35 million urban unemployed Chinese people lack insurance coverage and cannot afford expensive drugs. Our future profitability might suffer if a significant portion of our revenues were to be derived from products whose final selling prices were state-controlled and if those prices were held at levels close to or below our cost of sales.
| SALES OF OUR PRODUCTS COULD BE HARMED BY THE WIDESPREAD PRESENCE OF COUNTERFEIT MEDICATION IN PRC NEGATIVELY IMPACTING OUR PROFITABILITY. |
Chinese counterfeiting of pharmaceuticals and other products affecting public health has grown in tandem with counterfeiting and piracy of goods such as brand-name clothing, compact discs and computer software. Exact data are impossible to collect, but the FBI believes that more than half of the pharmaceuticals sold in PRC are counterfeit. Examples of the seriousness of the problem include: six months after Viagra was introduced in 2002, state media reported that some 90 percent of little blue pills sold in Shanghai were counterfeit; and 192,000 Chinese patients were reported to have died in 2001 from fake drugs. Counterfeit products shrink markets for legitimate goods. This situation affects Benda and other major domestic and foreign drug manufacturers in PRC, especially for products marketed through the OTC rather than hospital channel. However, we believe the Chinese authorities are becoming increasingly vigilant against counterfeiting because in 2001 the authorities closed 1,300 factories while investigating 480,000 cases of counterfeit drugs. Currently, active pharmaceutical ingredients are governed only by chemical regulations. We believe that a major step towards controlling the problem would be taken should the SFDA be given oversight over PRC’s bulk chemicals producers. However, our ability to increase sales as rapidly as we would like, and our profitability, could be affected if this problem persists or worsens.
| THERE COULD BE CHANGES IN GOVERNMENT REGULATIONS TOWARDS THE PHARMACEUTICAL AND HEALTH SUPPLEMENT INDUSTRIES THAT MAY ADVERSELY AFFECT OUR GROWTH AND PROFITABILITY. |
The manufacture and sale of APIs in the PRC is heavily regulated by many state, provincial and local authorities. These regulations have significantly increased the difficulty and costs involved in obtaining and maintaining regulatory approvals for marketing new and existing products. Our future growth and profitability depend to a large extent on our ability to obtain regulatory approvals.
The SFDA of PRC recently implemented new guidelines for licensing of APIs. All existing manufacturers with licenses were required to apply for GMP certifications by June 30, 2004, and to receive approvals by December 31, 2004. We have received certification for our Benda Ebei injection vial production facilities and expect to receive certifications for the remaining plant that require such certification before the end of 2006. However, should we fail to receive or maintain the GMP certifications under the new guidelines in the future; our businesses would be materially and adversely affected.
Moreover, the laws and regulations regarding acquisitions of the pharmaceutical industry in the PRC may also change and may significantly impact our ability to grow through acquisitions.
| CERTAIN POLITICAL AND ECONOMIC CONSIDERATIONS RELATING TO THE PRC COULD ADVERSELY AFFECT OUR COMPANY. |
The PRC is transitioning from a planned economy to a market economy. While the PRC government has pursued economic reforms since its adoption of the open-door policy in 1978, a large portion of the PRC economy is still operating under five-year plans and annual state plans. Through these plans and other economic measures, such as control on foreign exchange, taxation and restrictions on foreign participation in the domestic market of various industries, the PRC government exerts considerable direct and indirect influence on the economy. Many of the economic reforms carried out by the PRC government are unprecedented or experimental, and are expected to be refined and improved. Other political, economic and social factors can also lead to further readjustment of such reforms. This refining and readjustment process may not necessarily have a positive effect on our operations or future business development. Our operating results may be adversely affected by changes in the PRC's economic and social conditions as well as by changes in the policies of the PRC government, such as changes in laws and regulations (or the official interpretation thereof), measures which may be introduced to control inflation, changes in the interest rate or method of taxation, and the imposition of additional restrictions on currency conversion.
| THE RECENT NATURE AND UNCERTAIN APPLICATION OF MANY PRC LAWS APPLICABLE TO US CREATE AN UNCERTAIN ENVIRONMENT FOR BUSINESS OPERATIONS AND THEY COULD HAVE A NEGATIVE EFFECT ON US. |
The PRC legal system is a civil law system. Unlike the common law system, the civil law system is based on written statutes in which decided legal cases have little value as precedents. In 1979, the PRC began to promulgate a comprehensive system of laws and has since introduced many laws and regulations to provide general guidance on economic and business practices in the PRC and to regulate foreign investment. Progress has been made in the promulgation of laws and regulations dealing with economic matters such as corporate organization and governance, foreign investment, commerce, taxation and trade. The promulgation of new laws, changes of existing laws and the abrogation of local regulations by national laws could have a negative impact on our business and business prospects.
Since all the Company’s operation plants are all located in China, therefore we have to comply with those relevant PRC laws and regulations. The changes of those relevant PRC laws and regulations do affect our operation, for example, due to the changes of the relevant regulations of SFDA in PRC, it may take more time to approve the application of new drug certificate so that the forecasted operation plan for such particular drug product would be delayed and thus the potential earning of revenue and net profit would be affectee. In this aspect, the new drug certificate application progresses of Qiweiben Capsule and Yan Long Anti-cancer Oral Liquid are delayed. Furthermore, due to the changes of the requirements of the Environmental PRC agencies, Yidu Benda’s operation was ceased since January 2007, therefore the company needs to re-design the production facilities so the process of re-opening is also delayed. As these laws, regulations and legal requirements are relatively recent, their interpretation and enforcement involve significant uncertainty.
| THE APPROVAL OF THE CHINESE SECURITIES REGULATORY COMMISSION (“CRSC”) MAY BE REQUIRED IN CONNECTION WITH THIS OFFERING UNDER A RECENTLY ADOPTED PRC REGULATION; SINCE THIS OFFERING DID NOT COMMENCE PRIOR TO THE EFFECTIVE DATE OF THE REGULATION, WE MAY BE REQUIRED TO OBTAIN CRSC APPROVAL FOR THIS OFFERING AND WE CAN NOT CURRENTLY PREDICT THE CONSEQUENCES OF ANY FAILURE TO OBTAIN SUCH APPROVAL. |
On August 8, 2006, six PRC regulatory agencies, including the Chinese Securities Regulatory Commission, or CSRC, promulgated a regulation that became effective on September 8, 2006. This regulation, among other things, purports to require offshore special purpose vehicles, or SPVs, formed for listing purposes through acquisitions of PRC domestic companies and controlled by PRC individuals, such as our company, to obtain the approval of the CSRC prior to publicly listing their securities on an overseas stock exchange. While the application of this new regulation is not yet clear, we believe, based on the advice of our PRC counsel, that CSRC approval is not required if trading of our shares of common stock commenced prior to the effective date of the regulation. Although the CSRC is expected to promulgate formal implementing rules and/or regulations and possibly other clarifications, the procedures, criteria and timing for obtaining any required CSRC approval have not been established and it is unclear when these will be established. Therefore, since this offering did not commence prior to the effective date of the regulation and our shares of common stock did not commence trading prior to the effective date of the regulation, we may be required to obtain CSRC approval for this offering and we cannot currently predict the criteria, timing or procedures for obtaining the CSRC approval or the consequences of any failure to obtain such approval.
| RECENT PRC REGULATIONS RELATING TO THE ESTABLISHMENT OF OFFSHORE SPECIAL PURPOSE COMPANIES BY PRC RESIDENTS MAY SUBJECT OUR PRC RESIDENT SHAREHOLDERS TO PERSONAL LIABILITY AND LIMIT OUR ABILITY TO INJECT CAPITAL INTO OUR PRC SUBSIDIARIES, LIMIT OUR PRC SUBSIDIARIES’ ABILITY TO DISTRIBUTE PROFITS TO US, OR OTHERWISE ADVERSELY AFFECT US. |
The State Administration of Foreign Exchange (“SAFE”) was one of six PRC regulatory agencies promulgating a regulation that became effective on September 8, 2006 requiring PRC residents to register with the local SAFE branch before establishing or controlling any company outside of China for the purpose of capital financing with assets or equities of PRC companies, referred to in the notice as an “offshore special purpose company.” PRC residents that are shareholders of offshore special purpose companies established before November 1, 2005 were required to register with the local SAFE branch before March 31, 2006. Our current beneficial owners who are PRC residents have registered with the local SAFE branch as required under the SAFE notice. The failure of these beneficial owners to timely amend their SAFE registrations pursuant to the SAFE notice or the failure of future beneficial owners of our company who are PRC residents to comply with the registration procedures set forth in the SAFE notice may subject such beneficial owners to fines and legal sanctions and may also limit our ability to contribute additional capital into our PRC subsidiaries, limit our PRC subsidiaries’ ability to distribute dividends to our company or otherwise adversely affect our business.
Other Risks
| CURRENCY CONVERSION AND EXCHANGE RATE VOLATILITY COULD ADVERSELY AFFECT OUR FINANCIAL CONDITION. |
The PRC government imposes control over the conversion of Renminbi into foreign currencies. Under the current unified floating exchange rate system, the People's Bank of China publishes an exchange rate, which we refer to as the PBOC exchange rate, based on the previous day's dealings in the inter-bank foreign exchange market. Financial institutions authorized to deal in foreign currency may enter into foreign exchange transactions at exchange rates within an authorized range above or below the PBOC exchange rate according to market conditions.
Pursuant to the Foreign Exchange Control Regulations of the PRC issued by the State Council which came into effect on April 1, 1996, and the Regulations on the Administration of Foreign Exchange Settlement, Sale and Payment of the PRC which came into effect on July 1, 1996, regarding foreign exchange control, conversion of Renminbi into foreign exchange by Foreign Investment Enterprises, or FIEs, for use on current account items, including the distribution of dividends and profits to foreign investors, is permissible. FIEs are permitted to convert their after-tax dividends and profits to foreign exchange and remit such foreign exchange to their foreign exchange bank accounts in the PRC. Conversion of Renminbi into foreign currencies for capital account items, including direct investment, loans, and security investment, is still under certain restrictions. On January 14, 1997, the State Council amended the Foreign Exchange Control Regulations and added, among other things, an important provision, which provides that the PRC government shall not impose restrictions on recurring international payments and transfers under current account items.
Enterprises in the PRC (including FIEs) which require foreign exchange for transactions relating to current account items, may, without approval of the State Administration of Foreign Exchange, or SAFE, effect payment from their foreign exchange account or convert and pay at the designated foreign exchange banks by providing valid receipts and proofs.
Convertibility of foreign exchange in respect of capital account items, such as direct investment and capital contribution, is still subject to certain restrictions, and prior approval from the SAFE or its relevant branches must be sought.
Furthermore, the Renminbi is not freely convertible into foreign currencies nor can freely remitted abroad. Under the PRC’s Foreign Exchange Control Regulations and the Administration of Settlement, Sales and Payment of Foreign Exchange Regulations, foreign invested enterprises are permitted either to repatriate or distribute its profits or dividends in foreign currencies out of its foreign exchange accounts, or exchange Renminbi for foreign currencies through banks authorized to conduct foreign exchange business. The conversion of Renminbi into foreign exchange by foreign invested enterprises for recurring items, including the distribution of dividends to foreign investors, is permissible. The conversion of Reminbi into foreign currencies for capital items, such as direct investment, loans and security investment, is subject, however, to more stringent controls.
Since 1994, the exchange rate for Renminbi against the United States dollar has remained relatively stable, most of the time in the region of approximately RMB8.28 to $1.00. However, in 2005, the Chinese government announced that it would begin pegging the exchange rate of the Chinese Renminbi against a number of currencies, rather than just the U.S. dollar and, the exchange rate for the Renminbi against the U.S. dollar became RMB8.02 to $1.00. As our operations are primarily in PRC, any significant revaluation or devaluation of the Chinese Renminbi may materially and adversely affect our cash flows, revenues and financial condition. We may not be able to hedge effectively against in any such case. For example, to the extent that we need to convert United States dollars into Chinese Renminbi for our operations, appreciation of this currency against the United States dollar could have a material adverse effect on our business, financial condition and results of operations. Conversely, if we decide to convert Chinese Renminbi into United States dollars for other business purposes and the United States dollar appreciates against this currency, the United States dollar equivalent of the Chinese Renminbi we convert would be reduced. There can be no assurance that future movements in the exchange rate of Renminbi and other currencies will not have an adverse effect on our financial condition. Benda’s operating companies are FIEs to which the Foreign Exchange Control Regulations are applicable. There can be no assurance that we will be able to obtain sufficient foreign exchange to pay dividends or satisfy other foreign exchange requirements in the future.
| IT MAY BE DIFFICULT TO AFFECT SERVICE OF PROCESS AND ENFORCEMENT OF LEGAL JUDGMENTS UPON OUR COMPANY AND OUR OFFICERS AND DIRECTORS BECAUSE THEY RESIDE OUTSIDE THE UNITED STATES. |
As our operations are presently based in PRC and a majority of our directors and all of our officers reside in PRC, service of process on our company and such directors and officers may be difficult to effect within the United States. Also, our main assets are located in PRC and any judgment obtained in the United States against us may not be enforceable outside the United States.
| ANY FUTURE OUTBREAK OF AVIAN INFLUENZA, OR THE ASIAN BIRD FLU, OR ANY OTHER EPIDEMIC IN PRC COULD HAVE A MATERIAL ADVERSE EFFECT ON OUR BUSINESS OPERATIONS, FINANCIAL CONDITION AND RESULTS OF OPERATIONS. |
Since mid-December 2003, a number of Asian countries have reported outbreaks of highly pathogenic avian influenza in chickens and ducks. Since all of our operations are in PRC, an outbreak of the Asian Bird Flu in PRC in the future may disrupt our business operations and have a material adverse effect on our financial condition and results of operations. For example, a new outbreak of Asian Bird Flu, or any other epidemic, may reduce the level of economic activity in affected areas, which may lead to a reduction in our revenue if our clients cancel existing contracts or defer future expenditures. In addition, health or other government regulations may require temporary closure of our offices, or the offices of our customers or partners, which will severely disrupt our business operations and have a material adverse effect on our financial condition and results of operations.
| OUR BUSINESS MAY BE AFFECTED BY UNEXPECTED CHANGES IN REGULATORY REQUIREMENTS IN THE JURISDICTIONS IN WHICH WE OPERATE. |
We are subject to many general regulations governing business entities and their behavior in PRC and in other jurisdictions in which we have, or plan to have, operations and market our products. In particular, we are subject to laws and regulations covering food, dietary supplements and APIs. Such regulations typically deal with licensing, approvals and permits. Any change in product licensing may make our products more or less available on the market. Such changes may have a positive or negative impact on the sale of our products and may directly impact the associated costs in compliance and our operational and financial viability. Such regulatory environment also covers any existing or potential trade barriers in the form of import tariff and taxes that may make it difficult for us to import our products to certain countries and regions, such as Hong Kong, which would limit our international expansion.
| WE MAY EXPERIENCE CURRENCY FLUCTUATION AND LONGER EXCHANGE RATE PAYMENT CYCLES WHICH WILL NEGATIVELY AFFECT THE COSTS OF OUR PRODUCTS SOLD AND THE VALUE OF OUR LOCAL CURRENCY PROFITS. |
The local currencies in the countries in which we sell our products may fluctuate in value in relation to other currencies. Such fluctuations may affect the costs of our products sold and the value of our local currency profits. While we are not conducting any meaningful operations in countries other than PRC at the present time, we may expand to other countries and may then have an increased risk of exposure of our business to currency fluctuation.
| SINCE MOST OF OUR ASSETS ARE LOCATED IN PRC, ANY DIVIDENDS OF PROCEEDS FROM LIQUIDATION IS SUBJECT TO THE APPROVAL OF THE RELEVANT CHINESE GOVERNMENT AGENCIES. |
Our assets are predominantly located inside PRC. Under the laws governing foreign invested enterprises in PRC, dividend distribution and liquidation are allowed but subject to special procedures under the relevant laws and rules. Any dividend payment will be subject to the decision of the board of directors and subject to foreign exchange rules governing such repatriation. Any liquidation is subject to the relevant government agency's approval and supervision as well as the foreign exchange control. This may generate additional risk for our investors in case of dividend payment and liquidation.
Risks Associated with Our Shares of Common Stock
| OUR SHARES OF COMMON STOCK ARE VERY THINLY TRADED, AND THE PRICE MAY NOT REFLECT OUR VALUE AND THERE CAN BE NO ASSURANCE THAT THERE WILL BE AN ACTIVE MARKET FOR OUR SHARES OF COMMON STOCK EITHER NOW OR IN THE FUTURE. |
Our shares of common stock are very thinly traded, and the price if traded may not reflect our value. There can be no assurance that there will be an active market for our shares of common stock either now or in the future. The market liquidity will be dependent on the perception of our operating business and any steps that our management might take to bring us to the awareness of investors. There can be no assurance given that there will be any awareness generated. Consequently, investors may not be able to liquidate their investment or liquidate it at a price that reflects the value of the business. If a more active market should develop, the price may be highly volatile. Because there may be a low price for our shares of common stock, many brokerage firms may not be willing to effect transactions in the securities. Even if an investor finds a broker willing to effect a transaction in the shares of our common stock, the combination of brokerage commissions, transfer fees, taxes, if any, and any other selling costs may exceed the selling price. Further, many lending institutions will not permit the use of such shares of common stock as collateral for any loans.
Certain of our stockholders have been granted registration rights which entitle them to require us to register their shares of our common stock at the same time that the shares of our common stock are being registered. The inclusion of this additional stock in the registration may negatively affect the resale price that may be obtained for the shares of our common stock when they are registered.
| WE ARE SUBJECT TO THE PENNY STOCK RULES WHICH WILL MAKE THE SHARES OF OUR COMMON STOCK MORE DIFFICULT TO SELL. |
| SALES OF OUR CURRENTLY ISSUED AND OUTSTANDING STOCK MAY BECOME FREELY TRADABLE PURSUANT TO RULE 144 AND MAY DILUTE THE MARKET FOR YOUR SHARES AND HAVE A DEPRESSIVE EFFECT ON THE PRICE OF THE SHARES OF OUR COMMON STOCK. |
A substantial majority of our outstanding shares of common stock are "restricted securities" within the meaning of Rule 144 under the Securities Act. As restricted shares, these shares may be resold only pursuant to an effective registration statement or under the requirements of Rule 144 or other applicable exemptions from registration under the Act and as required under applicable state securities laws. Rule 144 provides in essence that a person who has held restricted securities for a period of at least one year may, under certain conditions, sell every three months, in brokerage transactions, a number of shares that does not exceed the greater of 1% of a company's outstanding shares of common stock or the average weekly trading volume during the four calendar weeks prior to the sale (the four calendar week rule does not apply to companies quoted on the OTC Bulletin Board). There is no limit on the amount of restricted securities that may be sold by a non-affiliate after the restricted securities have been held by the owner for a period of two years or more and such owner has not been an affiliate for the 90 day period prior to sale. A sale under Rule 144 or under any other exemption from the Act, if available, or pursuant to subsequent registrations of our shares of common stock, may have a depressive effect upon the price of our shares of common stock in any active market that may develop.
| THERE CAN BE NO ASSURANCE THAT THE PRICE OF OUR SHARES OF COMMON STOCK WILL MEET OR EXCEED THE EXERCISE PRICE OF THE WARRANTS DURING THE EXERCISE PERIOD OR AT ANY TIME THEREAFTER. |
Unless the price of our shares of Common Stock equals or exceeds the exercise price of the Warrants at the time of such exercise, an Investor may not be able to exercise his Warrants profitably. There can be no assurance that the price of our shares of Common Stock will meet or exceed the exercise price of the Warrants during the exercise period or at any time thereafter. Accordingly, should an Investor choose to exercise the Warrants, the value of our shares of Common Stock purchased upon such exercise may be less than the Warrant exercise price the Investor pays. The Warrant may be worthless and expire unexercised if the price of our shares of Common Stock does not exceed the Warrant exercise price. Please see “Description of Securities - Warrants” for additional information as to the terms of the Warrants.
| MANAGEMENT EXERCISES SIGNIFICANT CONTROL OVER MATTERS REQUIRING SHAREHOLDER APPROVAL AND INVESTORS WILL HAVE CONTROL OVER COMPANY ACTIONS. |
As a practical matter, our officers and directors will have control of us and will be able to assert significant influence over the election of directors and other matters presented for a vote of stockholders. Even after the Offering is complete, a majority of our shares of Common Stock will be owned by Mr. Yiqing Wan and Ms. Wei Xu, husband and wife. Through their concentration of voting power, they could delay, deter or prevent a change in our control or other business combinations that might otherwise be beneficial to the other stockholders. In deciding how to vote on such matters, Mr. Yiqing Wan and Ms. Wei Xu may be influenced by interests that conflict with other stockholders’ interests. Investors will not have a voice in management decisions and will exercise very little control. In the event that the requisite approval of stockholders is obtained, dissenting or non-participating stockholders generally would be bound by such vote. Accordingly, Offerees should not subscribe for Units in this Offering unless they are willing to entrust all aspects of operational control to our current management team. Further, Investors in the Offering will rely on our management team to use the proceeds as they determine to be in our best interest. Although management has indicated its current intended uses, those may be changed upon their decision.
In addition, our existing officers, directors, affiliates and other insiders are permitted to purchase Units in the Offering. If they do so, their interest in us after the acquisition may be even greater.
| INVESTORS MAY EXPERIENCE IMMEDIATE AND SUBSTANTIAL DILUTION IN NET TANGIBLE BOOK VALUE PER SHARE OF OUR COMMON STOCK IF THEY ELECT TO EXERCISE THE WARRANTS. |
Investors may experience immediate and substantial dilution in net tangible book value per share of our common stock if they elect to exercise the Warrants. The exercise price of the Warrants may be substantially higher than the net tangible book value per share. Accordingly, if Investors exercise the Warrants, they will likely experience immediate and substantial dilution in the net tangible book value per share and further dilution if we issue shares of our common stock in the future. As a result of this dilution, in the event of our subsequent liquidation, Investors exercising their Warrants may receive significantly less than the full exercise price that they paid for the shares of our Common Stock.
| INVESTORS MAY EXPERIENCE IMMEDIATE AND SUBSTANTIAL DILUTION IN NET TANGIBLE BOOK VALUE PER SHARE OF OUR COMMON STOCK IN THE EVENT WE ISSUE SHARES OF OUR COMMON STOCK IN THE FUTURE. |
There are additional authorized but unissued shares of our Common Stock that may be later issued by our management for any purpose without the consent or vote of the stockholders. Investors purchasing in this Offering may be further diluted in their percentage ownership on an as-converted basis in the event additional shares are issued by us in the future.
| OUR ARTICLES OF INCORPORATION AUTHORIZE THE ISSUANCE OF SHARES OF PREFERRED STOCK WHICH, IF ISSUED, THE RIGHTS, PREFERENCES, DESIGNATIONS AND LIMITATIONS OF SUCH PREFERRED STOCK COULD OPERATE TO THE DISADVANTAGE OF THE SHARES OF OUR OUTSTANDING COMMON STOCK. |
Our articles of incorporation authorize the issuance of shares of preferred stock, the rights, preferences, designations and limitations of which may be set by the board of directors. While no preferred stock is currently outstanding or subject to be issued, the articles of incorporation have authorized issuance of up to 5,000,000 shares of preferred stock (“Preferred Stock”) in the discretion of the board of directors. Such Preferred Stock may be issued upon filing of amended Articles of Incorporation and the payment of required fees; no further shareholder action is required. If issued, the rights, preferences, designations and limitations of such Preferred Stock would be set by our board of directors and could operate to the disadvantage of the shares of our outstanding Common Stock. Such terms could include, among others, preferences as to dividends and distributions on liquidation.
| MOEN AND COMPANY, THE AUDITORS THAT AUDITED EVER LEADER’S FINANCIAL STATEMENTS AND NOTES FOR THE YEARS ENDING DECEMBER 31, 2005 LOST ITS STATUS AS A PARTICIPATING AUDIT FIRM IN THE CANADIAN PUBLIC ACCOUNTABILITY BOARD’S (“CPAB”) OVERSIGHT PROGRAM FOR FAILING TO MEET ELIGIBILITY REQUIREMENTS FOR PARTICPATING IN THE CPAB’S OVERSIGHT PROGRAM WHICH CAN RESULT IN THE PUBLIC PERCEPTION THAT OUR FINANCIAL STATEMENTS FOR THE YEARS AUDITED BY SUCH AUDITOR ARE NOT RELIABLE. ACCORDINGLY, WE HAVE RE-AUDITED OUR FINANCIAL STATEMENTS FOR THE YEAR ENDING DECEMBER 31, 2005. |
On September 22, 2006, Moen & Company LLP (“Moen”), the auditors that audited Ever Leader’s financial statements and notes for the year ending December 31, 2005, lost its status as a participating audit firm in the Canadian Public Accountability Board’s (“CPAB”) oversight program for failing to implement the CPAB’s oversight program set out in paragraph 11.1 of the CPAB’s By-law No. 1. As a result of this, Moen is no longer eligible to audit the financial statements of entities that are reporting issuers under provincial securities legislation in Canada. In addition, the CPAB carried out a quality inspection of Moen in 2005 and issued its final inspection report on August 25, 2005. The report made numerous recommendations to Moen to address weaknesses in the firm’s system of quality control of deficiencies in specific audit engagements. The CPAB conducted follow up visits to Moen in late November 2005, early March 2006 and early May 2006 and concluded that the recommendations in the CPAB report to Moen had not been implemented. Moen's loss of its status as a participating audit firm in the CPAB oversight program has been publicly disclosed, including, without limitation, through CPAB's assessment of Moen available on the CPAB web site. Management has determined that Moen's loss of its status as a participating audit firm in the CPAB oversight program may result in a negative perception of Ever Leader's financial statements for such years. Accordingly, we had our financial statements for the year ending December 31, 2005 audited by Kempisty & Company Certified Public Accountants, P.C.
USE OF PROCEEDS
We will not receive any proceeds from the sale of the shares by the selling stockholders. All proceeds from the sale of the shares offered hereby will be for the account of the selling stockholders, as described below in the sections entitled "Selling Stockholders" and "Plan of Distribution." With the exception of any brokerage fees and commission which are the obligation of the selling stockholders, we are responsible for the fees, costs and expenses of this offering which are estimated to be $100,000, inclusive of our legal and accounting fees, printing costs and filing and other miscellaneous fees and expenses.
A portion of the shares covered by this prospectus are, prior to their sale under this prospectus, issuable upon exercise of common stock purchase warrants. In the event all of the common stock purchase warrants are exercised for cash, assuming no adjustments to the exercise price for anti-dilution protection, we estimate that we would receive approximately $23,414,942 in gross proceeds. Any proceeds received from the exercise of the warrants will be used for general corporate purposes.
There can be no assurance that any warrants will be exercised or that we will receive any proceeds therefrom. It is common that such warrants are never exercised because the price of the common stock does not justify the exercise or the warrant expires by its terms.
SELLING SECURITY HOLDERS
We are registering 15,774,375 of the 38,942,653 shares equal to 150% of the common shares underlying the related warrants to purchase 25,961,760 shares at $0.555 per share held by certain of the Investors and warrants issued to the placement agent, its employees and other persons acting on behalf of the placement agent to purchase 3,296,105 shares at $0.555 per shares (collectively, "Warrants"). The Warrants were issued to the Selling Security Holders in a private placement offering which closed on November 15, 2006. The Warrants were issued in transactions exempt from the registration requirements of the 1933 Act under Section 4(2) of the 1933 Act to persons reasonably believed to be "accredited investors" as defined in Regulation D under the 1933 Act. Pursuant to the terms of the subscription agreement under which the Common Stock and related Warrants were issued, we agreed to file this registration statement in order to permit those investors to sell the shares underlying the Warrants. We are also registering a further 2,106,561 shares of common stock, of which 29,968 shares were issued in January 2006 to two shareholders for services rendered; 295,378 were purchased in January and March of 2006 and are now held by two shareholders; 169,241 were issued on November 15, 2006 for services rendered in connection with the Financing and Acquisition of Ever Leader; and 1,611,974 were issued to eleven shareholders in exchange for their Ever Leader shares.. The securities issued to such shareholders who received securities prior to the private placement were also issued in transactions exempt from the registration requirements of the 1933 Act under Section 4(2) thereof either (a) "accredited investors" within the meaning of Rule 501 of Regulation D promulgated under the Securities Act, or (b) not a "U.S. person" as that term is defined in Rule 902(k) of Regulation S under the Act, and that such shareholders were acquiring our common stock, for investment purposes for their own respective accounts and not as nominees or agents, and not with a view to the resale or distribution thereof, and that the Investors understood that the shares of our common stock may not be sold or otherwise disposed of without registration under the Securities Act or an applicable exemption therefrom.
The table below lists the Selling Security Holders and other information regarding the beneficial ownership of the shares of common stock by each of the Selling Security Holders. The second column lists the number of shares of common stock beneficially owned by each Selling Security Holder as of July 5, 2007, assuming the exercise of all of the Warrants held by the Selling Security Holders on that date. The third column lists the shares of common stock being offered pursuant to this prospectus by each of the Selling Security Holders. The fourth column lists the number of shares that will be beneficially owned by the Selling Security Holders assuming all of the shares offered pursuant to this prospectus are sold and that shares beneficially owned by them, as of July 5, 2007, but not offered hereby are not sold.
The inclusion of any securities in the following table does not constitute an admission of beneficial ownership by the persons named below. Except as indicated in the footnotes to the table, no Selling Security Holder has had any material relationship with us or our predecessors or affiliates during the last three years.
BENDA PHARMACEUTICAL, INC.
NAME OF SELLING STOCKHOLDER | | NUMBER OF SHARES OWNED BEFORE OFFERING | | NUMBER OF SHARES BEING OFFERED | | NUMBER OF SHARES OWNED AFTER OFFERING | | PERCENTAGE OF SHARES OWNED AFTER OFFERING | |
Timothy J. Keating (1) (34) | | | 450,000 | | | 101,141 | | | 450,000 | | | * | |
Luca Toscani (1) (34) | | | 315,370 | | | 70,882 | | | 315,370 | | | * | |
Kyle L. Rogers (1) (34) | | | 121,158 | | | 27,231 | | | 121,158 | | | * | |
Margie L. Blackwell (1) (34) | | | 69,228 | | | 15,559 | | | 69,228 | | | * | |
Justin Davis (1) (34) | | | 60,000 | | | 13,486 | | | 60,000 | | | * | |
Pamela A. Solly (1) (34) | | | 30,000 | | | 6,743 | | | 30,000 | | | * | |
Ranjit P. Mankekar (1) (34) | | | 100,000 | | | 22,476 | | | 100,000 | | | * | |
Michael J. Keating (1) (34) | | | 80,000 | | | 17,981 | | | 80,000 | | | * | |
Melissa D. Salinas (1) (34) | | | 20,000 | | | 4,495 | | | 20,000 | | | * | |
Song He (1) (34) | | | 50,000 | | | 11,238 | | | 50,000 | | | * | |
Jeff L. Andrews (1) (34) | | | 137,900 | | | 30,994 | | | 137,900 | | | * | |
Steven J. Hendricks (1) (34) | | | 252,381 | | | 56,725 | | | 252,381 | | | * | |
Nohora Patricia Londono Gonzalez (1) | | | 297,619 | | | 66,892 | | | 297,619 | | | * | |
Randy B. Fields (1) (34) | | | 32,278 | | | 7,255 | | | 32,278 | | | * | |
Professional Trader Fund, LLC (1) | | | 75,000 | | | 16,857 | | | 75,000 | | | * | |
Westrock Advisors, Inc. (1) (34) | | | 57,765 | | | 12,983 | | | 57,765 | | | * | |
Robert Maloney (1) (34) | | | 23,701 | | | 5,327 | | | 23,701 | | | * | |
Denis Culverwell (1) (34) | | | 8,117 | | | 1,824 | | | 8,117 | | | * | |
Vincent Sbano (1) (34) | | | 325 | | | 73 | | | 325 | | | * | |
Alfred Barnes McNevin (1) (34) | | | 325 | | | 73 | | | 325 | | | * | |
Tricon Ventures (1) | | | 16,234 | | | 3,649 | | | 16,234 | | | * | |
Accelera Ventures Limited (8) (32) | | | 649,044 | | | 121,565 | | | 527,479 | | | * | |
Lars B. Ahlstrom (32) | | | 108,174 | | | 20,261 | | | 87,913 | | | * | |
Anima S.G.R.p.A. Rubrica - Anima Asia (9) (32) | | | 2,163,480 | | | 405,218 | | | 1,758,262 | | | 1.73 | % |
Anima S.G.R.p.A. Rubrica - Anima Emerging Markets (10) (32) | | | 1,298,088 | | | 243,130 | | | 1,054,958 | | | 1.04 | % |
Joseph F. Barletta (32) | | | 108,174 | | | 20,261 | | | 87,913 | | | * | |
BH Capital Investments LP (11) (32) (33) | | | 1,298,088 | | | 243,130 | | | 1,054,958 | | | 1.04 | % |
David L. Dowler (32) (33) | | | 216,348 | | | 40,522 | | | 175,826 | | | * | |
Victor J. Dowling Jr. & Jody C. Dowling (32) | | | 649,044 | | | 121,565 | | | 527,479 | | | * | |
Excalibur Limited Partnership (12) (32) (33) | | | 4,002,438 | | | 749,653 | | | 3,252,785 | | | 3.14 | % |
Excalibur Limited Partnership II (13) (32) (33) | | | 2,055,306 | | | 384,957 | | | 1,670,349 | | | 1.63 | % |
F Berdon Co LP (14) (32) (34) | | | 324,522 | | | 60,783 | | | 263,739 | | | * | |
Elaine P. Fields (32) (33) | | | 432,696 | | | 81,044 | | | 351,652 | | | * | |
James W. Fuller (32) (34) | | | 108,174 | | | 20,261 | | | 87,913 | | | * | |
Banca Gesfid (32) (33) | | | 5,733,222 | | | 1,073,827 | | | 4,659,395 | | | 4.48 | % |
Joseph W. Grealish (32) | | | 108,174 | | | 20,261 | | | 87,913 | | | * | |
David Austin Grose (32) | | | 108,174 | | | 20,261 | | | 87,913 | | | * | |
Halter Pope USX China Fund (15) (32) | | | 432,696 | | | 81,044 | | | 351,652 | | | * | |
Linda Hechter (32) | | | 324,522 | | | 60,783 | | | 263,739 | | | * | |
Hedge Capital Partners LLC (16) (32) (33) | | | 649,044 | | | 121,565 | | | 527,479 | | | * | |
Mark and Stacia Hollmann as Tenants by the Entirety (32) | | | 108,174 | | | 20,261 | | | 87,913 | | | * | |
Scot C. Hollmann (32) (33) | | | 216,348 | | | 40,522 | | | 175,826 | | | * | |
Jayhawk Private Equity Fund, L.P. (17) (32) (33) | | | 3,830,188 | | | 717,390 | | | 3,112,798 | | | 3.02 | % |
Jayhawk Private Equity Co-Invest Fund, LP (32) (33) | | | 64,076 | | | 12,001 | | | 52,075 | | | * | |
John K. Kopra (32) (33) | | | 216,348 | | | 40,522 | | | 175,826 | | | * | |
Peter Levy (32) | | | 108,174 | | | 20,261 | | | 87,913 | | | * | |
LKCM Private Discipline Master Fund, SPC (18) (32) (33) | | | 865,392 | | | 162,087 | | | 703,305 | | | * | |
Suresh Madan & Sarita Madan (32) | | | 216,348 | | | 40,522 | | | 175,826 | | | * | |
Paul Masters IRA (32) (34) | | | 108,174 | | | 20,261 | | | 87,913 | | | * | |
Christopher McCarty & Jennifer Grey McCarty (32) | | | 108,174 | | | 20,261 | | | 87,913 | | | * | |
MCF Navigator Master Fund, Ltd. (19) (32) (34) | | | 432,696 | | | 81,044 | | | 351,652 | | | * | |
Edmund H. Melhado (32) | | | 216,348 | | | 40,522 | | | 175,826 | | | * | |
Gabriel Micek (32) | | | 108,174 | | | 20,261 | | | 87,913 | | | * | |
John Micek (20) (32) | | | 216,348 | | | 40,522 | | | 175,826 | | | * | |
Jordan Micek (32) | | | 108,174 | | | 20,261 | | | 87,913 | | | * | |
Maurice & Jennifer Micek JTWROS (32) | | | 108,174 | | | 20,261 | | | 87,913 | | | * | |
Peter Micek (32) | | | 108,174 | | | 20,261 | | | 87,913 | | | * | |
MidSouth Investor Fund LP (21) (32) (33) | | | 2,379,828 | | | 445,739 | | | 1,934,089 | | | 1.89 | % |
Nite Capital LP (22) (32) | | | 1,081,740 | | | 202,609 | | | 879,131 | | | * | |
Michael J. O'Halloran (32) | | | 108,174 | | | 20,261 | | | 87,913 | | | * | |
Stephen B. Olore (32) (34) | | | 108,174 | | | 20,261 | | | 87,913 | | | * | |
Jerry W. Peterson (32) | | | 216,348 | | | 40,522 | | | 175,826 | | | * | |
Pope Investments LLC (23) (32) (33) | | | 42,836,904 | | | 7,540,827 | | | 35,296,077 | | | 27.22 | % |
Professional Offshore Opportunity Fund, Ltd. (24) (32) | | | 1,514,436 | | | 283,652 | | | 1,230,784 | | | 1.21 | % |
Steven R. Purvis (32) (33) | | | 216,348 | | | 40,522 | | | 175,826 | | | * | |
RFJM Partners LLC (25) (32) (33) | | | 432,696 | | | 81,044 | | | 351,652 | | | * | |
Rock Associates (26) (32) | | | 216,348 | | | 40,522 | | | 175,826 | | | * | |
Marvin Rosenfield (32) | | | 108,174 | | | 20,261 | | | 87,913 | | | * | |
Steven Rothstein (32) (33) | | | 216,348 | | | 40,522 | | | 175,826 | | | * | |
Don Russell (32) | | | 108,174 | | | 20,261 | | | 87,913 | | | * | |
Silver Rock I, Ltd. (27) (32) | | | 1,298,088 | | | 243,131 | | | 1,054,957 | | | 1.04 | % |
Silicon Prairie Partners (28) (32) | | | 432,696 | | | 81,044 | | | 351,652 | | | * | |
Simgest SpA (29) (32) | | | 3,245,220 | | | 607,827 | | | 2,637,393 | | | 2.57 | % |
Surya LP (32) (33) | | | 216,348 | | | 40,522 | | | 175,826 | | | * | |
Richard Todd Truitt (32) (33) | | | 216,348 | | | 40,522 | | | 175,826 | | | * | |
Jonathan Ungar (32) | | | 432,696 | | | 81,044 | | | 351,652 | | | * | |
White Sand Investor Group, LP (30) (32) | | | 649,044 | | | 121,565 | | | 527,479 | | | * | |
Steven Zelinger & Lisa Gordon JTWROS (32) | | | 216,348 | | | 40,522 | | | 175,826 | | | * | |
Richard Anslow (3) | | | 247,976 | | | 37,157 | | | 210,819 | | | * | |
Gregg Jaclin (3) | | | 165,316 | | | 24,771 | | | 140,545 | | | * | |
Kristina Trauger (3) | | | 10,000 | | | 1,498 | | | 8,502 | | | * | |
KI Equity Partners III, LLC (4) | | | 1,971,302 | | | 295,378 | | | 1,675,924 | | | 1.66 | % |
Olympic Capital Group (5) | | | 76,835 | | | 11,513 | | | 65,322 | | | * | |
John B. Lowy (5) | | | 450,000 | | | 67,428 | | | 382,572 | | | * | |
Andrew McGough (5) | | | 111,750 | | | 16,744 | | | 95,006 | | | * | |
H. Rivkin & Co., Inc. (5) | | | 33,610 | | | 5,036 | | | 28,574 | | | * | |
Fiona Huang (5) | | | 25,000 | | | 3,746 | | | 21,254 | | | * | |
Zhicheng Ma (5) | | | 6,000 | | | 899 | | | 5,101 | | | * | |
Jia You (5) | | | 3,000 | | | 449 | | | 2,551 | | | * | |
Kevin Keating (6) | | | 100,000 | | | 14,984 | | | 85,016 | | | * | |
Youliang Tang (31) | | | 705,534 | | | 105,717 | | | 599,817 | | | * | |
Ming Guo (31) | | | 846,654 | | | 126,862 | | | 719,792 | | | * | |
Hua Xu (31) | | | 1,881,445 | | | 281,914 | | | 1,599,531 | | | 1.59 | % |
Hui Xu (31) | | | 1,699,801 | | | 254,697 | | | 1,445,104 | | | 1.43 | % |
| | | | | | | | | | | | | | |
Ping Xu (31) | | | 1,411,068 | | | 211,433 | | | 1,199,635 | | | 1.19 | % |
Su Wang (31) | | | 74,554 | | | 11,171 | | | 63,383 | | | * | |
Kai Wang (31) | | | 282,175 | | | 42,281 | | | 239,894 | | | * | |
Xiangzhi Zhou (31) | | | 188,138 | | | 28,190 | | | 159,948 | | | * | |
Hanfen Zhang (31) | | | 2,822,135 | | | 422,866 | | | 2,399,269 | | | 2.38 | % |
Lim Sin Hiok (31) | | | 282,175 | | | 42,281 | | | 239,894 | | | * | |
Tong Siew Chung (31) | | | 564,349 | | | 84,562 | | | 479,787 | | | * | |
Garisch Financial, Inc. (7) | | | 100,000 | | | 14,984 | | | 85,016 | | | * | |
* Represents less than one percent.
| | (1) | We are registering a portion of the shares of our common stock underlying Warrants issued to the placement agent, its employees and other persons acting on behalf of the placement agent to purchase 2,597,401 shares at $0.555 per share. |
| | (3) | We are registering a portion of the 423,292 shares of our common stock in connection with legal services rendered by Anslow & Jaclin, LLP. Richard Anslow and Gregg Jaclin are the principals of Anslow & Jaclin, LLP. |
| | (4) | KI Equity Partners III, LLC purchased 2,281,302 shares of our common stock in a private transaction on December 14, 2005. KI Equity Partners III, LLC purchased an additional 1,500,000 shares from us on January 4, 2006 for $75,000, and an additional 700,000 shares from us on March 10, 2006 for $35,000. Keating Investments, LLC is the managing member of KI Equity Partners III, LLC. Timothy J. Keating is the principal member of Keating Investments, LLC. Keating Investments, LLC is also the managing member and 100% owner of Keating Securities, LLC, a registered broker-dealer. KI Equity Partners III, LLC purchased these shares in the ordinary course of business and they do not have plans to distribute the securities. |
| | | |
| | (5) | We are registering a portion of the 706,195 shares of our common stock in connection with consulting services rendered in connection with the Exchange and Financing. |
| | | |
| | (6) | We are registering a portion of the 100,000 shares of our common stock issued to Kevin R. Keating on January 4, 2006 for services rendered as the sole officer and director of the Company at that time. |
| | | |
| | (7) | We are registering a portion of the 100,000 shares of our common stock issued to Garisch Financial, Inc. on January 4, 2006 for financial consulting services rendered to the Company. |
| | | |
| | (8) | Accelera Ventures Limited is controlled by Dennis Kam Thai Leong. |
| | | |
| | (9) | Anima S.G.R.p.A. Rubrica -Anima Asia is controlled by Giovanni Brambilla. |
| | | |
| | (10) | Anima S.G.R.p.A. Rubrica -Anima Emerging Markets is controlled by Giovanni Brambilla. |
| | | |
| | (11) | BH Capital Investments LP is controlled by Henry Brachfeld. |
| | | |
| | (12) | Excalibur Limited Partnership is controlled by William Hechter. |
| | | |
| | (13) | Excalibur Limited Partnership II is controlled by William Hechter. |
| | | |
| | (14) | F Berdon Co. LP is controlled by Frederick Berdon. |
| | | |
| | (15) | Halter Pope USX China Fund is controlled by Stephen L. Parr. |
| | (16) | Hedge Capital Partners LLC is controlled by Allan Rothstein. |
| | | |
| | (17) | Jayhawk Private Equity Fund, L.P. is controlled by Michael D. Schmitz. |
| | | |
| | (18) | LKCM Private Discipline Master Fund, SPC is indirectly controlled by J. Luther King, Jr. and J. Bryan King |
| | | |
| | (19) | MCF Navigator Master Fund, Ltd. is controlled by Gregory S. Curhan. |
| | | |
| | (20) | John Micek is a member of our Board of Directors. |
| | | |
| | (21) | MidSouth Investor Fund LP is controlled by Lyman O. Heidtke. |
| | | |
| | (22) | Nite Capital LP is controlled by Keith Goodman. |
| | | |
| | (23) | Pope Investments LLC is controlled by William P. Wells. |
| | (24) | Professional Offshore Opportunity Fund, Ltd. is controlled by Howard Berger. |
| | (25) | RFJM Partners LLC is controlled by Jeffrey Markowitz. |
| | (26) | Rock Associates is controlled by Stuart Schapiro. |
| | (27) | Silver Rock I, Ltd. is controlled by Rima Salam. |
| | (28) | Silicon Prairie Partners is controlled by John Micek, a member of our Board of Directors. |
| | (29) | Simgest SpA is controlled by Oscar Guidetti. |
| | (30) | White Sand Investor Group, LP is controlled by Elliott Donnelley II. |
| | | |
| | (31) | These shareholders received shares of our common stock on November 15, 2006 pursuant to an Exchange Agreement with KI Equity Partners II, LLC (“KI Equity”), Ever Leader, and the owners of 100% of the capital shares of Ever Leader, in exchange for their shares of Ever Leader. |
| | (32) | We are registering a portion of the 38,942,653 shares of common stock based on 150% of the common stock issuable upon exercise of outstanding warrants we issued in connection with our issuance of the Units, at an exercise price of $0.555 per share in conjunction with our private placement completed on November 15, 2006. The price per Unit was $25,000, and each Unit consisted of each Unit consisting of 54,087 shares of our Common Stock and 54,087 common stock purchase warrants. |
| | (33) | We are registering a portion of the 20,444,891 shares of common stock based on 150% of the common stock issuable upon exercise of outstanding warrants we issued in connection with our issuance of the Additional Units, at an exercise price of $0.555 per share on April 5, 2007. |
| | (34) | These shareholders are affiliates of a broker dealer. Such shareholders purchased the shares in the ordinary course of business and do not have plans to distribute the securities. |
We are registering shares of our common stock for resale by the selling stockholders identified in the section above entitled "Selling Stockholders." We will receive none of the proceeds from the sale of these shares by the selling stockholders. The common stock may be sold from time to time to purchasers:
| | | through the OTC Bulletin Board at prevailing market prices; or |
| | | through underwriters, broker-dealers or agents who may receive compensation in the form of discounts, concessions or commissions from the selling stockholders or the purchasers of the common stock. |
The selling stockholders may use any one or more of the following methods when selling shares:
| | | ordinary brokerage transactions and transactions in which the broker-dealer solicits purchasers; |
| | | |
| | | a block trade in which the broker-dealer so engaged will attempt to sell such shares as agent, but may position and resell a portion of the block as principal to facilitate the transaction; |
| | | |
| | | purchases by a broker-dealer as principal and resale by such broker-dealer for its own account pursuant to this prospectus; |
| | | |
| | | an exchange distribution in accordance with the rules of the applicable exchange; |
| | | |
| | | privately negotiated transactions; |
| | | |
| | | settlement of short sales; |
| | | |
| | | broker-dealers may agree with the selling stockholders to sell a specified number of such shares at a stipulated price per share; |
| | | |
| | | a combination of any such methods of sale; |
| | | |
| | | through the writing or settlement of options or other hedging transactions, whether through an options exchange or otherwise; or |
| | | |
| | | any other method permitted pursuant to applicable law. |
Neither the selling stockholders nor Benda can presently estimate the amount of compensation in the form of discounts, concessions or commissions that underwriters, broker-dealers or agents may receive from the selling stockholders or the purchasers of the common stock. We know of no existing arrangements between the selling stockholders, broker-dealers, underwriters or agents relating to the sale or distribution of the shares.
The selling stockholders may also enter into hedging transactions, and persons with whom they effect such transactions, including broker-dealers, may engage in short sales of our common shares. Our selling stockholders may also engage in short sales and short sales against the box, and in options, swaps, derivatives and other transactions in our securities, and may sell and deliver the shares covered by this prospectus in connection with such transactions or in settlement of securities loans. These transactions may be entered into with broker-dealers or other financial institutions that may resell those shares pursuant to this prospectus (as supplemented or amended to reflect such transaction).
The selling stockholders and any broker-dealers or agents that are involved in selling the shares may be deemed to be "underwriters" within the meaning of section 2(11) of the Securities Act of 1933, as amended, in connection with the sales and distributions contemplated under this prospectus, and may have civil liability under Sections 11 and 12 of the Securities Act for any omissions or misstatements in this prospectus and the registration statement of which it is a part. Additionally, any profits which our selling stockholders may receive might be deemed to be underwriting compensation under the Securities Act. Because the selling stockholders may be deemed to be an underwriter under Section 2(11) of the Securities Act, the selling stockholders will be subject to the prospectus delivery requirements of the Securities Act.
The resale shares will be sold only through registered or licensed broker-dealers if required under applicable state securities laws. In addition, in certain states, the resale shares may not be sold unless they have been registered or qualified for sale in the applicable state or an exemption from the registration or qualification requirement is available and is complied with.
We will bear all expenses relating to the sale of our common shares under this prospectus, except that the selling stockholders will pay any applicable underwriting commissions and expenses, brokerage fees and transfer taxes, as well as the fees and disbursements of counsel to and experts for the selling stockholders. We have agreed to indemnify some of the selling stockholders against certain losses, claims, obligations, damages and liabilities, including liabilities under the Securities Act.
Any common shares offered under this prospectus that qualify for sale pursuant to Rule 144 of the Securities Act may also be sold under Rule 144 rather than pursuant to this prospectus.
We have agreed to keep this prospectus effective at least for a period ending with the first to occur of (i) the date that all of the shares covered by this prospectus have been sold; (ii) the date that all shares covered by this prospectus may be sold without restrictions pursuant to Rule 144(k), provided that a legal opinion with respect to the availability of Rule 144 for the resale of such shares of Common Stock has been rendered by a law firm acceptable to both Applied Spectrum and the holder of such shares as evidence that Rule 144 is available for such securities; or (iii) the date one year after this registration statement is declared effective by the Commission, provided, however, that if at the end of such one year period, any holder of shares covered by this prospectus is not able to immediately, freely resell all of the shares covered by this prospectus that it owns, then we shall continue to keep this prospectus effective until terminated pursuant to clause (i) or (ii) .
Under applicable rules and regulations under the Exchange Act of 1934, any person engaged in the distribution of the resale shares may not simultaneously engage in market making activities with respect to our common stock for a period of two business days prior to the commencement of the distribution. In addition, the selling stockholders will be subject to applicable provisions of the Exchange Act and the rules and regulations thereunder, including Regulation M, which may limit the timing of purchases and sales of shares of our common stock by the selling stockholders or any other person. We will make copies of this prospectus available to the selling stockholders and have informed them of the need to deliver a copy of this prospectus to each purchaser at or prior to the time of the sale.
LEGAL PROCEEDINGS
On November 23, 2006, Benda Ebei entered into an Equity Transfer Agreement with Xiaozhi Zhang (“Zhang”), to purchase approximately 6.24% of SiBiono’s common stock for a total consideration of Rmb12.48 million (Rmb6.24 million in cash and shares of our common stock equal to Rmb6.24 million) (or $1.71 million) which was due and payable on or before March 31, 2007.
Due to the fact that the signed agreement on November 23, 2006 was not practically executable according to the PRC regulations, Benda Ebei asked Zhang to terminate the signed agreement and sign a new agreement that was feasible under PRC regulations with essentially the same terms.
However, Zhang refused to sign the new agreement and applied to the Shenzhen Arbitration Commission (the “Commission”) in April 2007 for enforcement of the original agreement. Zhang requested the Commission to require Benda Ebei to pay for the total consideration, penalty for late payment and the related legal and arbitration expenses.
On November 27, 2007, Shenzhen Arbitration Commission determined that:
| 1. | Benda Ebei should pay for the consideration of Rmb 6.24 million, equal to 50% of the total consideration set forth in the Equity Transfer Agreement. For the other 50% of the total consideration which was supposed to be settled in the form of issuing common stock, since Zhang did not make an arbitration request on how to execute the arrangement, the Arbitration Commission did not make an award on this particular part. |
| 2. | Benda Ebei should pay for the penalty of Rmb 46,800; |
| 3. | Benda Eebi should pay for legal and arbitration expenses of Rmb 268,971. |
On May 22, 2008, Benda Ebei submitted a “Termination Execution Application” to the Peoples’ Court of Shenzhen Nan Shan District in order to terminate the arbitration award concluded by Shenzhen Arbitration Commission dated November 27, 2007 based on the fact that Xiaozhi Zhang only holds 3.28% equity interest of SiBiono, not 6.24% as previously disclosed to the Company. The application has been accepted by the Commission, and we are currently waiting the determination.
Except as disclosed above, we are not aware of any pending or threatened legal proceedings in which we are involved.
OFFICERS AND DIRECTORS
The following table identifies our current executive officers and directors, their respective offices and positions, and their respective dates of election or appointment:
Name | | Age | | Position | | Date of Appointment |
| Yiqing Wan | | 45 | | Chief Executive Officer and Chairman | | November 10, 2006 |
| Wei Xu | | 44 | | Vice President of Operations | | November 15, 2006 |
| Hui Long | | 46 | | Vice President of Technology | | November 15, 2006 |
| Jingbo Wu | | 45 | | Vice President | | November 15, 2006 |
| Dr. Q.Y. Ma | | 50 | | Chairman of Compensation Committee and Director | | March 20, 2007 |
| Eric Yu | | 39 | | Chief Financial Officer and Director | | February 9, 2007 |
| Jun Tang | | 46 | | Director | | April 25, 2008 |
Business Experience Descriptions:
Mr. Yiqing Wan, Chairman, and Chief Executive Officer
Mr. Wan was appointed as Chairman and Chief Executive Officer on November 10, 2006. Mr. Wan founded Benda Ebei in April 2001and has served as its president and general manager overseeing the day to day operations of the Company since such time. Before founding Benda Ebei, Mr. Wan was Chairman of Zhanjiang Jinhui Pharmaceutical Corp. (from 1995 to 1998), Chairman of Shandong Jinhai Real Estate Development Co. (from 1992 to 1995), Manager of Hainan Pharmaceutical Co. Ltd Guangzhou Division (from 1990 to 1992), and Director of Yichang No.4 Drug Plant (from 1982 to 1990). Mr. Wan has held a range of operational and executive positions in a number of pharmaceutical enterprises for more than two decades and has developed significant management experience in production planning and implementation and in product marketing. Mr. Wan earned B.S. degree in Biological Engineering from Sanxia University in 1982.
Ms. Wei Xu, Vice President of Operations
Ms. Xu was appointed as Vice President of Operations on November 15, 2006. In April, 2007, she was appointed as the General Manger of Shenzhen SiBiono Gene Tech Co., Ltd., a subsidiary of the company. From 2002 to present, she serves as Chairperson of a related company, Hubei Benda Science and Technology Co., Ltd. Prior to such time, she was the Chairman at Hubei Tongji Benda Pharmaceutical Co., Ltd. (from 1999 to 2004), General Manager at the Zhanjiang Jinhui Pharmaceutical Co., Ltd (from 1995 to 1998) and Manager at the Hainan Pharmaceutical Co., Ltd., Guangzhou Division (from 1991 to 1995).
Mr. Hui Long, Vice President of Technology
Mr. Long was appointed as Vice President of Technology on November 15, 2006. Mr. Long has held senior technical and production management positions with pharmaceutical enterprises for approximately 20 years. From 2001 to present, he is the General Manager of Jiangling Benda Pharmaceutical Co., Ltd. Prior to such time, he was he deputy general manager of Zhanjiang Jinhui Pharmaceuticals Co. Ltd (from 1995 to 2000), the Chief of Technical Division of Sanxia Pharmaceutical Co., Ltd (from 1993 to 1995) and t. Mr. Long earned a B.S. in Biological Engineering from Sanxia University in 1982 and an M.S. in Biological Engineering from Sanxia University in 1988.
Mr. Jingbo Wu, Vice President
Mr. Wu was appointed as Vice President on November 15, 2006. Since 2002, Mr. Wu has been the general manager of North Hubei Tongji Benda Pharmaceutical Company Ltd., overseeing the company’s daily activities and GMP authentication. Prior to this position, he was the deputy general manager of Ebei Pharmaceutical Corp. (from 2000 to 2001) and the deputy general manager of Zhejiang Jinhui Pharmaceutical Co. Ltd. (from 1999 to 2000). Mr. Wu received his certification of graduation in Business Administration from Wuhan University in 1995.
Dr. Q.Y. Ma, Director
Dr. Ma was appointed as a Director on March 20, 2007. Dr. Ma has over 20 years of R&D and managerial experience in US. Dr. Ma has been the managing director of Time Innovation Ventures, a venture capital firm focused on funding technology start-ups and joint ventures in China, since 2000. He has also been a director of ComTech, a Chinese semiconductor company, since 2004. He served as an Associate Professor of electrical engineering at the University of Hong Kong from 1998 to 2005. Dr. Ma also served as an Associate Professor at the Department of Electrical Engineering at Columbia University and in the Department of Radiology at Harvard Medical School from 1994 to 2005. He has served as a consultant to IBM, General Electric, TRW Inc. and DuPont. Dr. Ma is a co-founder of and advisor to Semiconductor Manufacturing International Corp., and the Chairman of TiMed. He has served as an adviser to the Ministry of Information Industry, Beijing Government, and as senior advisor to Zhangjiang Hi-Tech Park in Shanghai. He has published over 160 papers and obtained 7 patents. Dr. Ma received his PhD from Columbia University, and attended the Executive Program of Stanford University's School of Business.
Eric Yu Tak Shing, Chief Financial Officer and Director
Mr. Yu is a Certified Public Accountant with thirteen years of experience in public and corporate accounting and finance. In February of 2007, Mr. Yu was appointed as the Chief Financial Officer of the Company. Mr. Yu has served as the Financial Controller for Beijing Teletron Telecom Engineering Co. Ltd. from March of 2006 to January of 2007 and was in charge of the company’s issuance of private placements and Pre-IPO issues. From March of 2005 to March of 2006, Mr. Yu served as the Financial Controller for Payease Inc., where he was in charge of merger and acquisition fund raising and arranging bridge loans. From June of 2001 to February of 2005, Mr. Yu served as the Financial Controller for several companies including Dynegy Network Technology Services (BJ) Ltd., an internet data center, China Gas Holdings, Ltd. and Tianjin East Ocean Gas Co. Ltd., manufacturers in natural gas. From May of 2000 to February of 2005, Mr. Yu also served as the Financial Controller of the project companies of AsiaVest Investment Advisory Limited. His responsibilities included managing the accounting and listing department, and planning for an IPO with a fund raising target of HK$150 million.
Mr. Yu received a Bachelor of Commerce degree with a Accountancy and Legal Studies major in 1993 from University of Wollongong, Australia. Mr. Yu has worked closely with investment bankers for Pre-IPO and Private Placements, and in constructing financial and accounting systems and practice according to the high standards required for public listing.
Mr. Yu has served as a professional accountant to a widespread range of clients in China, Hong Kong, and the United States, and has worked in several different industries including Natural Gas, Telecom, Manufacturing, Infrastructure Construction, Hospitality, and Auditing.
Jun Tang, Director
Prior to joining Xin Hua Du Industrial Group Co. this April of 2008, Mr. Tang served as the president and director of Shanda Interactive Entrainment Limited, a company listed in NASDAQ, and now remain as a member of Shanda's board of directors and serve as an advisor to the CEO of Shanda.
Prior to joining Shanda in 2004, Mr. Tang served as the president of Microsoft China Co., Ltd. from March 2002 to January 2004 and the general manager of Microsoft Asia product support and service and Microsoft Global Technical Engineering Center from January 1998 to March 2002. He received the Microsoft Chairman Bill Gates Award in 1998 and the Microsoft Top Honor Award in 2002 from Microsoft Corporation, and remains the honorary president of Microsoft China Co. Ltd. Prior to joining Microsoft, Dr. Tang founded Intertex Company, a software and entertainment company, in Los Angeles, California in 1993.
Mr. Tang received his doctorate degree, master’s degree and bachelor’s degree in the U.S., Japan and China, respectively. Mr. Tang was honoured as PRC Top 10 Economic Person, PRC Top 10 IT Person, PRC Top 10 Valuable CEO etc.
Employment Agreements
On November 15, 2006, we executed employment agreements with each of our executive officers, specifically, Yiqing Wan, our Chief Executive Officer; Wei Xu, our Vice President of Operations; Hui Long, our Vice President of Technology; Daping Gu, our Vice President of Marketing; and Jingbo Wu, our Vice President. The employment agreements are for terms of three years, except for Yiqing Wan, whose term is for five years. The employment agreements provide for annual salaries and annual bonuses in amounts as set forth in the table herein entitled “Executive Compensation Summary.”
Involvement in Certain Legal Proceedings
To the best of Applied Spectrum's knowledge, none of the officers or directors appointed following the closing of the Exchange transaction, including any of their affiliates, currently beneficially own any equity securities or rights to acquire any of our securities, and no such persons have been involved in any transaction with us or any of our directors, executive officers or affiliates that is required to be disclosed pursuant to the rules and regulations of the SEC, other than with respect to the transactions that have been described herein. To the best of our knowledge, none of the officers and directors appointed following the closing of the Exchange transaction have been convicted in a criminal proceeding, excluding traffic violations or similar misdemeanors, nor have they been a party to any judicial or administrative proceeding during the past five years, except for matters that were dismissed without sanction or settlement, that resulted in a judgment, decree or final order enjoining the person from future violations of, or prohibiting activities subject to, federal or state securities laws, or a finding of any violation of federal or state securities laws.
Audit Committee and Audit Committee Financial Expert
The Audit Committee assists the Board of Directors in its oversight of the quality and integrity of the accounting, auditing and reporting practices of the Company. The Audit Committee has the authority and responsibility to hire one or more independent auditors to audit the Company’s books, records and financial statements and to review the Company’s systems of accounting (including its systems of internal control), to discuss with such independent auditors the results of such audit and review, to conduct periodic independent reviews of the systems of accounting (including systems of internal control), and to make reports periodically to the Board of Directors with respect to its findings. The Board of Directors has determined that each Audit Committee member has sufficient knowledge in financial and auditing matters to serve on the Committee. The committee is also responsible for making recommendations to the Board of Directors or otherwise acting with respect to corporate governance matters, including board size and membership qualifications, new director orientation, committee structure and membership, communications with shareholders, and board and committee self-evaluations. The Audit Committee operates under a written charter adopted by our Board of Directors.
The members of the Audit Committee are independent of the Company (as defined under Rule 4200(a)(15) of the NASDAQ Marketplace Rules). We are currently looking for a “financial expert” as defined under Item 407 of Regulation S-B to replace Mr. Mo who resigned on June 15, 2008.
Code of Ethics
A code of ethics relates to written standards that are reasonably designed to deter wrongdoing and to promote:
| Honest and ethical conduct, including the ethical handling of actual or apparent conflicts of interest between personal and professional relationships; |
| Full, fair, accurate, timely and understandable disclosure in reports and documents that are filed with, or submitted to, the SEC and in other public communications made by an issuer; |
| Compliance with applicable governmental laws, rules and regulations; |
| The prompt internal reporting of violations of the code to an appropriate person or persons identified in the code; and |
| Accountability for adherence to the code. |
Due to the limited scope of our current operations, we have not adopted a corporate code of ethics that applies to its principal executive officer, principal accounting officer, or persons performing similar functions.
Indemnification
Under Delaware law and pursuant to our articles of incorporation and bylaws, we may indemnify our officers and directors for various expenses and damages resulting from their acting in these capacities. Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to our officers or directors pursuant to those provisions, our counsel has informed us that, in the opinion of the SEC, this indemnification is against public policy as expressed in the Securities Act, and is therefore unenforceable.
Section 16(a) Beneficial Ownership Reporting
Section 16(a) of the Securities Exchange Act of 1934, as amended, requires that directors, executive officers and persons who own more than 10% of the outstanding common stock of certain reporting companies file initial reports of ownership and reports of changes in ownership in such common stock with the Securities and Exchange Commission ("SEC"). Officers, directors and stockholders who own more than 10% of the outstanding common stock of certain reporting companies are required by the SEC to furnish such companies with copies of all Section 16(a) reports they file. We are required to comply with Section 16(a). Accordingly, stock ownership information contained in this report is based on what is known to us.
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
The following table sets forth information as of June 24, 2008 with respect to the beneficial ownership of the outstanding shares of the Company’s capital stock by (i) each person known by the Company who will beneficially own five percent (5%) or more of the outstanding shares; (ii) the officers and directors of the Company; and (iii) all the aforementioned officers and directors as a group.
Beneficial ownership is determined in accordance with the rules of the Securities and Exchange Commission and generally includes voting or investment power with respect to securities. Shares of common stock subject to options, warrants or convertible securities exercisable or convertible within 60 days of June 24, 2008 are deemed outstanding for computing the percentage of the person or entity holding such options, warrants or convertible securities but are not deemed outstanding for computing the percentage of any other person, and is based on 155,773,711 common shares issued and outstanding on a fully converted basis as of June 24, 2008.
Name of Beneficial Owner | | Amount of Beneficial Ownership | | Percent of Beneficial Ownership (5) | |
| XIA Pharmaceutical Inc. (1) | | | 44,687,136 | (2) | | 28.69 | % |
| Hui Long (1) | | | 0 | | | 0 | |
| Yiqing Wan (1) | | | 44,687,136 | (2) | | 28.69 | % |
| Wei Xu (1) | | | 44,687,136 | (2) | | 28.69 | % |
| John Micek, III (1) | | | 973,566 | (4) | | 0.62 | % |
| Dr. Q.Y. Ma (1) | | | 0 | | | 0 | |
| Eric Yu (1) | | | 1,500,000 | (6) | | 1 | % |
| Daping Gu (1) | | | 0 | | | 0 | |
| Ruilu Song (1) | | | 0 | | | 0 | |
| Jingbo Wu (1) | | | 0 | | | 0 | |
| Pope Investments, LLC (3) 5100 Poplar Ave., Suite 805 Memphis, TN 38137 | | | 42,836,904 | | | 27.50 | % |
| All Executive Officers and Directors as a group (7 persons) | | | 47,160,702 | | | 30.28 | % |
| (1) | Address is c/o Room 13, Floor 25, Sunny New World Tower, No. 231 Xin Hua Road, Jianghan District, Wuhan, Hubei, PRC. |
| (2) | Yiqing Wan and Wei Xu each have a 50% equity ownership in XIA Pharmaceutical Inc. They are both our executive officers and Yiqing Wan is a director. In addition, they are husband and wife. |
| (3) | William P. Wells is the manager of Pope Investments, LLC (“Pope”) and exercises sole voting and investment control over such shares. In addition to the 11,466,444 shares of common stock, Pope also holds warrants for the right to purchase a total of 21,418,452 additional shares, and a Convertible Promissory Note convertible into 9,952,008 additional shares. |
| (4) | John Micek is a member of our Board of Directors. He is the beneficial owner of 216,348 shares of common stock held in the name of Silicon Prairie Partners LLC, of which he is the Managing Member; 54,087 shares of common stock held in the name of Mr. Micek’s son Jordan Micek; 54,087 shares of common stock held in the name of Mr. Micek’s son Peter Micek; and 54,087 shares of common stock held in the name of Mr. Micek’s daughter Gabriel Micek. He is also the beneficial owner of warrants to purchase 216,348 shares of common stock held in the name of Silicon Prairie Partners LLC, of which he is the Managing Member; 54,087 shares of common stock held in the name of Mr. Micek’s son Jordan Micek; 54,087 shares of common stock held in the name of Mr. Micek’s son Peter Micek; and 54,087 shares of common stock held in the name of Mr. Micek’s daughter Gabriel Micek. |
| (5) | Applicable percentage of ownership is based on 100,803,509 shares of common stock outstanding as of June 24, 2008 together with securities exercisable or convertible into shares of common stock within sixty (60) days of June 24, 2008 for each stockholder. Beneficial ownership is determined in accordance with the rules of the SEC and generally includes voting or investment power with respect to securities. Shares of common stock subject to securities exercisable or convertible into shares of common stock that are currently exercisable or exercisable within 60 days of June 24, 2008 are deemed to be beneficially owned by the person holding such options for the purpose of computing the percentage of ownership of such person, but are not treated as outstanding for the purpose of computing the percentage ownership of any other person. |
| (6) | Eric Yu is a member or our Board of Directors and our Chief Financial Officer. XIA Pharmaceutical, Inc. transferred 1,500,000 shares to Mr. Yu. |
CHANGE OF CONTROL
The Company entered into a Share Exchange Agreement dated September 7, 2006 (“Exchange Agreement”) with KI Equity Partners III, LLC, a Delaware limited liability company (“KI Equity”), Ever Leader Holdings Limited, a company incorporated under the laws of Hong Kong SAR ("Ever Leader"), and each of the equity owners of Ever Leader (the “Ever Leader Shareholders”), whereby the Company acquired all of the equity interest of Ever Leader in exchange for issuing 64,942,360 shares of our common stock to the Ever Leader shareholders. The Exchange Agreement was contingent upon the Company receiving a minimum of $10,000,000 in signed subscriptions (the “Subscription Agreements”) to purchase Units in a private placement offering (the “Financing”) exempt from registration under the Securities Act. On November 15, 2006, the Company received the requisite amount of Subscription Agreements and the transactions contemplated by the Exchange Agreement closed.
In connection with the Exchange Agreement and Financing: (i) effective November 15, 2006, Mr. Kevin R. Keating resigned as member of the board of directors of the Company. There were no disagreements between or among Mr. Kevin R. Keating and any officer or director of the Company; (ii) effective November 15, 2006, Mr. Kevin R. Keating resigned as the Chief Executive Officer, President, Chief Financial Officer, Secretary and Treasurer of the Company; (iv) effective November 15, 2006, Yiqing Wan, Ruilu Song, Jingbo Wu, Hulian Song, and John Micek, III were appointed as members of the Company’s Board of Directors (the “New Board”); and (v) effective November 15, 2006, the New Board appointed Yiqing Wan as the Chief Executive Officer and President, Wei Xu as the Vice President of Operations, Hui Long as the Vice President of Technology, Daping Gu as the Vice President of Marketing, Ruilu Song as Vice President, and Jingbo Wu as Vice President of the Company.
The consummation of the Exchange was contingent on a minimum of $10,000,000 (or such lesser amount as mutually agreed to by Ever Leader and the placement agent) being subscribed for, and funded into escrow, by certain accredited and institutional investors for the purchase of shares of the Common Shares promptly after the closing of the Exchange under terms and conditions approved by our board of directors immediately following the Exchange.
Upon Closing, we received gross proceeds of $12,000,000 in connection with the Financing from the Investors. Pursuant to Subscription Agreements entered into with these Investors, we sold 480 Units, with each Unit consisting of 54,087 shares of our Common Stock, and Warrants to purchase 54,087 shares of our Common Stock at an exercise price of $0.555 per share. The price of each Unit was $25,000. After commissions and expenses, we received net proceeds of approximately $10,470,000 from the Financing. Upon completion of the Financing, the Investors in the aggregate received 25,961,760 shares of our Common Stock. The Investors also received Warrants to purchase an additional 25,961,760 shares of Applied Spectrum’s common stock in connection with the financing.
DESCRIPTION OF SECURITIES
As of June 24, 2008, our authorized capital stock consists of 150,000,000 shares of common stock, par value $0.001 per share, and 5,000,000 shares of preferred stock, par value $0.001 per share. As of June 24, 2008, an aggregate of 100,803,509 shares of Common Stock were outstanding.
There are no shares of preferred stock outstanding.
The following descriptions of our capital stock are only summaries and do not purport to be complete and is subject to and qualified by our Articles of Incorporation, as amended, its By-laws, the Certificates of Determination, copies of which will be provided by us upon request, and by the provisions of applicable corporate laws of the State of Delaware. The descriptions of the common stock and preferred stock, as well as warrants to purchase our common stock, reflect changes to our capital structure that occurred immediately prior to or upon the closing of this Exchange transaction and the Financing:
Common Stock
Subject to preferences that may apply to shares of preferred stock outstanding at the time, the holders of outstanding shares of Common Stock are entitled to receive dividends out of assets legally available therefore at times and in amounts as our board of directors may determine. Each stockholder is entitled to one vote for each share of Common Stock held on all matters submitted to a vote of the stockholders. Cumulative voting is not provided for in our amended articles of incorporation, which means that the majority of the shares voted can elect all of the directors then standing for election. The Common Stock is not entitled to preemptive rights and is not subject to conversion or redemption. Upon the occurrence of a liquidation, dissolution or winding-up, the holders of shares of Common Stock are entitled to share ratably in all assets remaining after payment of liabilities and satisfaction of preferential rights of any outstanding preferred stock. There are no sinking fund provisions applicable to the Common Stock. The outstanding shares of Common Stock are, and the shares of Common Stock to be issued upon conversion of the Warrants will be, fully paid and non-assessable.
Participation Rights
From the closing of the Offering until one hundred and eighty (180) trading days following the Effective Date of the Registration Rights Agreement, we have agreed to refrain from selling or offering to sell any of our equity or convertible securities. From the Trigger Date until the second anniversary of the Closing Date, if we intend to undertake an offering of its equity or convertible securities, the Investors shall have the right to participate in such offering based on the Investor’s pro rata portion of the number of Common Shares purchased in the Offering.
Buy-In Damages
If we fail to deliver to the Investor unlegended certificates within three (3) business days of receipt of all documents necessary for removal of the legend (the “Deadline Date”), then if the Investor purchases shares to deliver in satisfaction of a sale of shares that the Investor anticipated receiving from us, then we shall within three (3) business days of Investor’s request, either (1) pay cash to the Investor in the amount equal to the Investor’s total purchase price for the shares so purchased, at which point the our obligation to deliver such certificate shall terminate, or (2) promptly honor its obligation to deliver to the Investor the certificate and pay cash to the Investor in the amount equal to the excess, if any, of such purchase price over the product of (a) such number of shares of Common Stock, times (b) the closing bid price on the Deadline Date.
Preferred Stock
Our board of directors has the authority, within the limitations and restrictions in our amended articles of incorporation, to issue 5,000,000 shares of preferred stock in one or more series and to fix the rights, preferences, privileges and restrictions thereof, including dividend rights, dividend rates, conversion rights, voting rights, terms of redemption, redemption prices, liquidation preferences and the number of shares constituting any series or the designation of any series, without further vote or action by the stockholders. The issuance of preferred stock may have the effect of delaying, deferring or preventing a change in our control without further action by the stockholders. The issuance of preferred stock with voting and conversion rights may adversely affect the voting power of the holders of Common Stock, including voting rights, of the holders of Common Stock. In some circumstances, this issuance could have the effect of decreasing the market price of the Common Stock. We currently have no plans to issue any shares of preferred stock.
Make Good Agreement
In connection with the November Financing, we, along with Keating Securities, LLC, as the authorized agent of the Investors (the "Investor Agent"), Mr. Yiqing Wan and Ms. Wei Xu, as individuals (collectively, the "XIA Shareholders") and Moveup Investments Limited, a company organized under the laws of the British Virgin Islands ("Moveup" and together with the XIA Shareholders, the "Depositors") entered into a Make Good Agreement (the “Make Good Agreement”).
Pursuant to the Make Good Agreement, we have presented financial projections indicating that we will report net income of at least $9 million, with an allowable grace margin of $1 million, equating to net income of $8 million for the fiscal year ending December 31, 2007 (the “Performance Threshold”), based upon an audit conducted in conformity with United States (“U.S.”) Generally Accepted Accounting Principles (“GAAP”) and U.S. based auditing standards. As an inducement to the Investors in this Offering, we along with the Investor Agent, the Depositors, and Computershare Trust Company, Inc., the transfer agent for Applied Spectrum (the “Shares Escrow Agent”), have entered into a Make Good Escrow Agreement (the “Make Good Escrow Agreement”) whereby the Depositors have agreed that they will place a total of 15 million shares (to be equitably adjusted for stock splits, stock dividends, and similar adjustments) of our common stock into escrow (the “Escrow Shares”) at the Closing for the benefit of the Investors in the event that we fail to satisfy the Performance Threshold (“Make Good Provision”).
On or prior to sixty (60) days from the Closing Date of the Securities Purchase Agreement, as defined therein, (the "Audited Financial Statement Delivery Deadline"), we are required under the Securities Purchase Agreement to deliver to the Investors its financial statements for the years ending December 31, 2004 and December 31, 2005, audited by Rotenberg & Company, LP (the "New Audit Financial Statements"), prepared in accordance with GAAP, during each year involved and fairly presenting in all material respects our financial position as of the dates thereof and the results of our operations and cash flows for each such year then ended.
If either the new audited revenue (“New Audited Revenue”) or the new audited cash flow from operations (“New Audited Cash Flows from Operations”) from the financial statements audited by Rotenberg & Company, LP are more than 10% less than the old audited revenue (“Old Audited Revenue”) or the old audited cash flow from operations (“Old Audited Cash Flows from Operations”) audited by Moen and Company LLP, then we and the Investor Agent shall promptly provide a joint written instruction to the Shares Escrow Agent to deliver as promptly as practicable to each Investor such number of Escrow Shares equal to the product of (x) the New Financial Statement Escrow Shares (as defined below) and (y) the quotient of (A) the number of shares of Common Stock acquired by such Investor in the Offering and (B) the number of shares of Common Stock acquired by all Investors in the Offering. For purposes of this Agreement, "New Financial Statement Escrow Shares" means such number of Escrow Shares equal to the product of (x) one million and (y) the greater of the number of percentage points in excess of 10% in which (A) the Old Audited Revenues exceeds the New Audited Revenues and (B) the Old Audited Cash Flow from Operations exceeds the New Cash Flow From Operations; provided, that such number of New Financial Statement Escrow Shares shall be capped at the total number of Escrow Shares deposited with the Shares Escrow Agent.
We will adopt the calendar year end as our fiscal year end. We shall provide to the Placement Agent our audited fiscal year 2007 financial statements (“FY2007 Financial Statements”), prepared in accordance with U.S. GAAP on or before March 31, 2008 so as to allow the Placement Agent the opportunity to evaluate whether our actual reported net income for 2007 (“FY07 ARNI”) meets the Performance Threshold. For the purpose of the Make Good Provision, in calculation of the FY07 ARNI, any non-cash charges incurred as a result of the Offering (due to possible non-cash amortization on warrants charged to the Company’s results of operation) will be added back to FY07 ARNI.
In the event the Performance Threshold is not attained, we shall provide written instruction to the escrow agent to deliver as promptly as practicable to the Investors holding at least 100 shares of Common Stock as of April 10, 2008 (the “Eligible Investors”), an amount of Escrow Shares based from the following formula (the “Released Escrow Shares”):
(($8 million - FY07 Net Income) / $8 million) X Escrow Shares
The Released Escrow Shares shall be capped at the number of Escrow Shares remaining in escrow after the distribution of the New Financial Statement Escrow Shares, if any.
The stock certificates evidencing the Released Escrow Shares shall be registered in the name of each Investor according to their pro rata portion of the Released Escrow Shares. Each Investor’s pro rata portion of the Released Escrow Shares shall be equal to such Investor’s pro rata portion of the Escrow Shares. The investors’ pro rata portion of the Escrow Shares shall be based on the respective number of shares of our capital stock acquired by each Investor during the Offering pursuant to its Subscription Agreement. Only those investors who remain as our shareholders at the time that any Escrow Shares become deliverable shall be entitled to their pro rata portion of the Escrow Shares. The Shares Escrow Agent shall release the Escrow Shares to the Investor Agent, who shall thereafter promptly deliver to the Investors such stock certificates.
We will ensure that the Released Escrow Shares will be registered under Section 5 of the Securities Act of 1933 for purposes of resale, which registration statement shall be filed with the SEC within 30 days of the delivery of the Released Escrow Shares. The registration statement shall remain effective and the prospectus constituting a part thereof available for delivery in connection with the resale of the Released Escrow Shares for a period of 12 months commencing on the delivery date of the Released Escrow Shares.
In March 2007, the Company and the Investors entered into a Modification Agreement amending the November Financing Documents to allow for certain issuances of the Company’s securities, including additional purchases of the Company’s equity securities pursuant to the Investment Agreement; shares issuances required under the Equity Transfer Agreements; and issuances of options pursuant to an approved Qualified Employment Stock Option Plan. All of the investors in the November Financing had the right to participate in the purchase of additional units under the Investment Agreement and all of such investors either participated in the Investment Agreement or have waived their right to participate in such. In addition, those investors that did not participate in the Investment Agreement also waived their right to object to the changes to the Warrants, Registration Rights Agreement and Make Good Agreement which were set forth in the Modification Agreement.
Pursuant to the Modification Agreement, on or prior to forty five (45) days from the Closing Date of the Investment Agreement, we are required to deliver to the Buyers our financial statements for the years ending December 31, 2005 and December 31, 2006, audited by Kempisty & Company Certified Public Accountants, P.C., prepared in accordance with GAAP, during each year involved and fairly presenting in all material respects our financial position as of the dates thereof and the results of our operations and cash flows for each such year then ended. In addition, on or prior to seventy five (75) days from the Closing Date, we are also required to deliver to the Buyers audited financial statements for SiBiono for the required time periods for the Form 8-K filing required by the Securities and Exchange Commission.
The securities underlying the Notes and Warrants issued to the Buyers pursuant to the terms of the Investment Agreement shall be subject to the terms of the Make Good Agreement entered into in connection with the November Financing (the “Make Good Agreement”). We further represented to the Buyers that the targeted net income of the Company for fiscal year end 2007 (“FY07 Net Income”) will be greater than or equal to $10.0 million (adjusted for any non-cash charges associated with this Agreement and the SPA) (the "Performance Threshold"). In the event the Performance Threshold is not attained, then we shall promptly issue, or cause to be issued to the Buyers or their designee, a pro rata portion of One Million (1,000,000) shares of Common Stock for every one (1) cent by which the Company’s earnings per share, determined on a fully diluted basis (“Earnings Per Share”) is less than $0.065. In addition, we are also required to issue or cause to be issued to each Buyer or its designee Two Thousand (2,000) shares of Common Stock per Unit held for every percentage point in excess of 10% in which (A) the Old Audited Revenues exceeds the New Audited Revenues and (B) the Old Audited Cash Flow from Operations exceeds the New Cash Flow From Operations. The maximum amount of shares of Common Stock that can be issued to Buyer shall be capped based on a maximum excess of fifteen (15) percentage points.
The Company failed to meet the Performance Threshold for FY07 Net Income. Accordingly, the Company will be required to make a distribution from the Make Good Shares to the Buyers.
Convertible Promissory Notes
On April 5, 2007 (the “Closing Date”), we entered into an Investment Agreement with certain accredited and institutional investors (“Investors”) who had also participated in the subscription for $12,000,000 of our common stock pursuant to certain Securities Purchase Agreements (“SPA”) dated November 15, 2006 (“November Financing”). Pursuant to the Investment Agreement, the Investors purchased a total of 252 Units for $7,560,000 with each Unit consisting of (1) a convertible promissory note (the “Note”) in the principal amount of Thirty Thousand Dollars ($30,000) which shall be convertible into 54,087 shares of the Company's common stock, par value $0.001 per share (the "Common Stock"), and (ii) a warrant (a “Warrant”) to acquire 54,087 shares of Common Stock at an exercise price of $0.555 per share. The Notes shall bear an interest rate of four percent per annum until the Buyer elects to exercise the right to convert, and shall mature on March 28, 2009. We have agreed to register for resale, the shares of Common Stock underlying the Notes.
The Notes shall bear an interest rate of fifteen percent per annum upon the occurrence of an event of default. An event of default shall occur upon the nonpayment of any obligation, the breach of any covenants of the Notes, or bankruptcy. Additionally, the Notes have an anti-dilution provision that requires that any adjustment to the Warrant exercise price shall simultaneously trigger an adjustment to the conversion price of the Notes.
The securities underlying the Notes and Warrants issued to the Buyers pursuant to the terms of the Investment Agreement shall be subject to the terms of the Make Good Agreement entered into in connection with the November Financing (the “Make Good Agreement”). We further represented to the Buyers that the targeted net income of the Company for fiscal year end 2007 (“FY07 Net Income”) will be greater than or equal to $10.0 million (adjusted for any non-cash charges associated with this Agreement and the SPA) (the "Performance Threshold"). In the event the Performance Threshold is not attained, then we shall promptly issue, or cause to be issued to the Buyers or their designee, a pro rata portion of One Million (1,000,000) shares of Common Stock for every one (1) cent by which the Company’s earnings per share, determined on a fully diluted basis (“Earnings Per Share”) is less than $0.065. In addition, we are also required to issue or cause to be issued to each Buyer or its designee Two Thousand (2,000) shares of Common Stock per Unit held for every percentage point in excess of 10% in which (A) the Old Audited Revenues exceeds the New Audited Revenues and (B) the Old Audited Cash Flow from Operations exceeds the New Cash Flow From Operations. The maximum amount of shares of Common Stock that can be issued to Buyer shall be capped based on a maximum excess of fifteen (15) percentage points.
Warrants
The Warrants to purchase Common Stock are a component of the Units. Each Unit in both the November 2006 and April 2007 Financings includes Warrants to purchase 54,087 shares of our common stock. Each Warrant entitles the holder to purchase one share of our Common Stock. The Warrants will be exercisable, in whole or in part, at an exercise price equal to $0.555 per share (“Exercise Price”). The Warrants may be exercised at any time upon the election of the Investor, beginning on the date of issuance and ending of the fifth anniversary of the final closing of this Offering. If the Company should fail to issue the shares to the Investor within three business days of receipt of an exercise notice, the Investor shall be entitled to cash in an amount equal to 2.0% of the product of (A) the sum of the number of shares of common stock not issued in a timely basis and (B) the last closing trade price for such security as reported by Bloomberg on the immediately preceding trading day upon which we could have issued such shares without violating the terms of the Warrant. Additionally, if we fail to deliver to the Warrant Shares within three (3) business days of the exercise notice, then if the Investor purchases shares to deliver in satisfaction of a sale of shares that the Investor anticipated receiving from us, then we shall within three (3) business days of Investor’s request, either (1) pay cash to the Investor in the amount equal to the Investor’s total purchase price for the shares so purchased, at which point the Company’s obligation to deliver such Warrant Shares shall terminate, or (2) promptly honor its obligation to deliver to the Investor the Warrant Shares and pay cash to the Investor in the amount equal to the excess, if any, of such purchase price over the product of (a) such number of shares of Common Stock, times (b) the closing bid price on the date of exercise.
We shall not be permitted to effect the exercise of the Warrant if such exercise would cause the Investor to beneficially own in excess of 4.99% of the shares of the Company’s outstanding common stock. However, by written notice to us, the Investor may from time to time (x) increase or decrease such maximum percentage to any other percentage not in excess of 9.99% specified in such notice or (y) waive the beneficial ownership limitation altogether; provided that (i) any such increase or waiver will not be effective until the sixty-first (61st) day after such notice is delivered to us, and (ii) any such increase or decrease or waiver will apply only to the holder of the Warrant and not to any other holder of the Warrants.
The exercise price of the Warrants shall have “full ratchet” anti-dilution protection for issuances of our Common Stock, or securities exercisable for or convertible into Common Stock, at an issuance price, exercise price or conversion price of less than $0.555 per share of Common Stock, except with respect to: (i) the issuance of shares of Common Stock upon exercise of the Warrants; or (ii) the issuance of Common Stock to employees or directors pursuant to an equity incentive plan approved by the Company’s stockholders.
The Company will adjust the exercise price to reflect any stock splits, stock dividends or other combinations or distributions to holders of Benda's securities where the consideration is less than the current exercise price. Benda will not make this adjustment with respect to shares issued (2) for the issuance of common stock upon the exercise of warrants (including any warrants issued to the Placement Agent) pursuant to the terms of such warrants as of the date hereof issued to the Investors.
Any grant by Benda (whether directly or by assumption in a merger or otherwise, in any manner) of any warrants, rights to subscribe for, or options to purchase any common stock (collectively, the “ Options ”) or any issuance or sale by Benda (whether directly or by assumption in a merger or otherwise, in any manner) of any security convertible into or exchangeable for shares of common stock (“ Convertible Securities ”) shall be deemed to be an issuance of common stock for anti-dilution purposes, whether or not immediately exercisable or convertible. The number of shares of common stock deemed to be issued shall be equal to the maximum number of shares of common stock issuable upon the exercise of such Options or conversion of such Convertible Securities. The price per share of common stock in such deemed issuance or sale of common stock shall be determined by dividing: (A) the sum of (a) the total amount, if any, received or receivable by Benda as consideration for the granting of such Options or issuance of such Convertible Securities, plus (b) the minimum aggregate amount of additional consideration payable to Benda upon the full exercise of all such Options or the full conversion of all such Convertible Securities (“ Total Consideration ”); by (B) the total maximum number of shares of common stock issuable upon the exercise of such Options or the conversion of such Convertible Securities.
The Warrants will be detachable and separately transferable only during the Warrant exercise period; upon the expiration of the Warrant exercise period, the Warrants will expire and become void.
In order to exercise the Warrants, the Warrant must be surrendered at the office of the Warrant Agent prior to the expiration of the Warrant exercise period, with the form of exercise appearing with the Warrant completed and executed as indicated, accompanied by payment of the full exercise price for the number of Warrants being exercised. Payment shall be by certified funds or cashier's check payable to “Benda Pharmaceutical, Inc.” In the case of partial exercise, the Warrant Agent will issue a new warrant to the exercising warrant holder, or assigns, evidencing the Warrants which remain unexercised. In our discretion, the Warrant Agent may designate a location other than our office for surrender of Warrants in the case of transfer or exercise.
The exercise price and number of shares of Common Stock to be received upon the exercise of Warrants are subject to adjustment upon the occurrence of certain events, such as stock splits, stock dividends or our recapitalization. In the event of our liquidation, dissolution or winding up, the holders of Warrants will not be entitled to participate in the distribution of our assets.
If we shall make any dividend or other distribution of assets to holders of its common stock (a “Distribution”), then the Exercise Price shall be reduced by multiplying such Exercise Price by a fraction of which (i) the numerator shall be the closing bid price of the shares of common stock on the trading day immediately preceding such record date minus the value of the Distribution applicable to one share of common stock, and (ii) the denominator shall be the closing bid price of the shares of common stock on the trading day immediately preceding such record date. Additionally, the number of shares underlying the Warrant shall be increased to a number of shares of common stock obtainable immediately prior to the close of business on the record date fixed for determining holders of shares entitled to receive the Distribution multiplied by the reciprocal of the fraction set forth above. Holders of Warrants will have no voting, pre-emptive, subscription or other rights of shareholders in respect of the Warrants, nor shall the Holders be entitled to receive dividends.
If we grant, issue or sell any options, convertible securities, or other rights to purchase stock to the record holders of our common stock, then the Holder will be entitled to purchase such securities, as if the Holder had held the number of shares of common stock acquirable upon complete exercise of the Warrants.
As additional compensation for the Placement Agent's services, we also paid the Placement Agent a Warrant Solicitation Fee with respect to the exercise, in whole or in part, of any Warrant equal to 3.0% of the total exercise price of the Common Stock issued in such exercise of such Warrant. Such cash Warrant Solicitation Fees shall be paid to the Placement Agent, in immediately available funds, within three (3) business days following receipt, directly or indirectly, by us, of any cash or other proceeds from the exercise of such Warrant.
Registration Rights
We have agreed to register an amount of stock equal to the common stock underlying the Notes and 150% of the shares of Common Stock issuable in connection with the Warrants (“Underlying Common Stock”), on a registration statement to be filed by us. Such Registration Statement shall be declared effective on or prior to August 15, 2007 (the “Effectiveness Deadline”). If the Registration Statement does not become effective by the Effectiveness Deadline or if we fail to maintain the effectiveness of the Registration Statement, for any reason, we will be required to pay Investors in cash an amount equal to 1% of the purchase price of each Unit held by Investors on such Effectiveness Deadline or the first day of such failure to maintain the Registration Period, as applicable, and for every 30 day period (or part) thereafter, in each case until cured, provided that the Registration Delay Payments shall not exceed 10% of the purchase price of the Offering. In the event that the Registration Delay Payments are not made in a timely manner, such Registration Delay Payments shall bear interest at a rate of 1.5% per month until paid in full. We shall pay the usual costs of such registration.
Except for the shares underlying our Notes and as follows, no holder of any of our currently outstanding securities has any registration rights with respect to the securities held by them: (i) 706,195 shares of our Common Stock issued for consulting services, (ii) 1,971,302 shares of our Common Stock held by KI Equity; (iii) 2,400,000 shares of our Common Stock held by Pope Investments LLC; (iv) 423,292 shares of our Common Stock held by the principals and employees of Anslow & Jaclin, LL; (v) 200,000 shares of our Common Stock held by two shareholders for services rendered; and (v) 2,189,560 total shares of our Common Stock held by Super Pioneer International Limited, Yaojin Wang, and Huimin Zhang in connection with the Financial Consultancy Agreements. We shall not file any other registration statement for any of our securities (other than the shares of Common Stock sold in this offering, the Underlying Common Stock and the Common Stock underlying the Agent Warrants) until such time as the Registration Statement has been filed and declared effective; provided, however, we may, subject to stockholder approval, establish an equity performance or stock option plan for the benefit of our employees and directors for up to 5% of the outstanding shares of our Common Stock and file a registration statement to register such shares on Form S-8 or a comparable form for such purpose.
LEGAL MATTERS
The validity of the common stock to be sold by the selling stockholders under this prospectus will be passed upon for us by Anslow & Jaclin, LLP. As of June 24, 2008, the principals of Anslow & Jaclin, LLP are shareholders of record of 423,292 shares of our common stock.
EXPERTS
The financial statements included in this prospectus have been audited by Kempisty & Company Certified Public Accountants, P.C., independent certified public accountants to the extent and for the periods set forth in their report appearing elsewhere herein and are included in reliance upon such report given upon the authority of that firm as experts in auditing and accounting.
DESCRIPTION OF BUSINESS
OVERVIEW
Summary
Benda Pharmaceutical, Inc. (“we”, “us”, “our”, “Benda” or the “Company”), through our wholly owned subsidiary Ever Leader Holdings Limited (“Ever Leader”), is a pharmaceutical company that identifies, discovers, develops and manufactures both conventional medications and Traditional Chinese Medicines (“TCMs”) for the treatment of some of the largest common ailments and diseases (e.g., common cold, diabetes, cancer). We are also dedicated to the development, manufacturing and commercialization of gene therapy products.
Benda owns all of the capital stock of Ever Leader Holdings Limited, a holding company incorporated under the laws of Hong Kong SAR on October 29, 2005. Ever Leader owns 95% of the issued and outstanding capital stock of Hubei Tongji Benda Ebei Pharmaceutical Co., Ltd., a Sino-Foreign Equity Joint Venture company incorporated under the laws of the PRC. Benda Ebei owns: (i) 95% of the issued and outstanding capital stock of Jiangling Benda Pharmaceutical Co., Ltd., a company formed under the laws of the PRC; (ii) 95% of the issued and outstanding capital stock of Yidu Benda Chemical Co., Ltd., a company incorporated under the laws of the PRC; (iii) 75% of the issued and outstanding capital stock of Beijing Shusai Pharyngitis Research Co., Ltd., a company incorporated under the laws of the PRC; and (iv) 60.13% of the issued and outstanding capital stock of Shenzhen SiBiono Gene Technology Co., Ltd. (“SiBiono”), a company incorporated under the laws of the PRC.
We distribute our high value, branded medicines, through agents who sell them to hospitals that administer them to patients. We sell generics to medical wholesalers for resale to hospitals. The company sells its Over the Counter (“OTC”) medicines to wholesalers specializing in selling to retail chain drug stores. Our “Active Pharmaceutical Ingredients” (“APIs”) are typically sold to large drug manufacturers under long-term supply contracts. The bulk chemicals are purchased by other Chinese drug companies.
History and Recent Developments
We were organized as a Minnesota corporation on February 17, 1982. The technology on which our original products were based, including Spread Spectrum Technology, permit data and telemetry to be transmitted simultaneously over telephone wire without interfering with normal voice service. Our products were known as data/voice multiplexing (“DVM”) equipment and were aimed at operating telephone companies in the telecommunications market. Our lack of financial resources caused us to pursue a plan of dissolution as approved by our Board of Directors and approved by our shareholders on November 30, 1993.
During fiscal 1994, we began implementing a plan of voluntary dissolution pursuant to Minnesota law that was approved by our shareholders at a Special Shareholders’ Meeting held on November 30, 1993. Under our plan of dissolution, most of our assets were sold during 1994 with some payments deferred into 1995 and beyond. The recovery period ran through 1997. During fiscal 1995, most of the tangible asset sales were collected and only technology licenses remained to be collected. During fiscal 1996, we continued to collect license fees and payments on one equipment lease. The results of the plan of dissolution were successful and all liabilities and expenses were either paid or were covered in reserves.
On November 17, 2000, a Special Meeting of the shareholders of the Company was held at which time the plan of dissolution was revoked. Pursuant to the proposal for revocation, a liquidating dividend of approximately $212,000 was paid pro-rata to our shareholders in August 2001. We have been inactive since 1994.
On October 7, 2005, we and Applied Spectrum Technologies, Inc., a Delaware corporation (“Applied - Delaware”) entered into a certain Agreement and Plan of Merger (“Plan of Merger”). Pursuant to the Plan of Merger, subject to stockholder approval, we were to merge with and into Applied - Delaware for the purposes of the redomestication from the State of Minnesota to the State of Delaware (the “Merger”). On October 24, 2005, we filed a Definitive Proxy Statement pursuant to Section 14(a) of the Securities Exchange Act of 1934, as amended, with the Securities and Exchange Commission for the purpose of voting on the Merger.
On November 14, 2005, the holders of a majority of our outstanding shares of common stock approved the Merger. The Merger was completed on November 17, 2005, and the Articles of Merger were filed with the States of Minnesota and Delaware on November 17, 2005.
On December 14, 2005, Norwood Venture Corp. (“Norwood”), a former shareholder of the Company and KI Equity Partners III, LLC (“KI Equity”) entered into a certain securities purchase agreement, as amended, under which KI Equity agreed to purchase and Norwood agreed to sell an aggregate of 2,281,302 shares of common stock of the Company, representing approximately 77.2% of the Company’s outstanding shares of common stock, to KI Equity at a price of $175,000. The closing of the transactions under the Purchase Agreement occurred on December 29, 2005.
On October 7, 2005, we and Applied Spectrum Technologies, Inc., a Delaware corporation (“Applied - Delaware”) entered into a certain Agreement and Plan of Merger (“Plan of Merger”). Pursuant to the Plan of Merger, subject to stockholder approval, we were to merge with and into Applied - Delaware for the purposes of the redomestication from the State of Minnesota to the State of Delaware (the “Merger”). On October 24, 2005, we filed a Definitive Proxy Statement pursuant to Section 14(a) of the Securities Exchange Act of 1934, as amended, with the Securities and Exchange Commission for the purpose of voting on the Merger.
On November 14, 2005, the holders of a majority of our outstanding shares of common stock approved the Merger. The Merger was completed on November 17, 2005, and the Articles of Merger were filed with the States of Minnesota and Delaware on November 17, 2005.
On December 14, 2005, Norwood Venture Corp. (“Norwood”), a former shareholder of the Company and KI Equity Partners III, LLC (“KI Equity”) entered into a certain securities purchase agreement, as amended, under which KI Equity agreed to purchase and Norwood agreed to sell an aggregate of 2,281,302 shares of common stock of the Company, representing approximately 77.2% of the Company’s outstanding shares of common stock, to KI Equity at a price of $175,000. The closing of the transactions under the Purchase Agreement occurred on December 29, 2005.
Kevin R. Keating, our former President and sole officer and director who resigned on November 15, 2006, is the father of Timothy J. Keating, the majority member of Keating Investments, LLC. Keating Investments, LLC is the managing member of KI Equity Partners III, LLC, which is the party acquiring the controlling interest in us pursuant to the Purchase Agreement. Keating Investments, LLC is also the managing member and 90% owner of Keating Securities, LLC, a registered broker-dealer. Kevin R. Keating is not affiliated with and has no equity interest in Keating Investments, LLC, KI Equity Partners III, LLC or Keating Securities, LLC and disclaims any beneficial interest in the shares of Applied Spectrum’s common stock to be acquired by KI Equity Partners III, LLC.
We did not become engaged in the pharmaceutical business until November of 2006. Before closing our recent share exchange transaction in November 2006, we were a shell company with nominal assets and operations, whose sole business was to identify, evaluate and investigate various companies with the intent that, if such investigation warrants, a business combination be negotiated and completed pursuant to which we (formerly known as Applied Spectrum Technologies, Inc.) would acquire a target company with an operating business with the intent of continuing the acquired company's business as a publicly held entity. We entered in an Exchange Agreement dated September 7, 2006 (the “Exchange Agreement”) with KI Equity Partners II, LLC (“KI Equity”), Ever Leader, a company incorporated under the laws of Hong Kong, and the owners of 100% of the capital shares of Ever Leader. The closing of the Exchange Agreement occurred on November 15, 2006. At the closing of the Exchange Agreement, we acquired all of Ever Leader's capital shares (the “Ever Leader Shares”) from the Ever Leader Shareholders, and the Ever Leader Shareholders transferred and contributed all of their Ever Leader Shares to us. In exchange, we issued 64,942,360 shares of our Common Stock to the Ever Leader Shareholders.
As a result of the closing of the Exchange Agreement, Ever Leader became our wholly owned subsidiary and we adopted Ever Leader’s main operational business. The Exchange transaction, for accounting and financial reporting purposes, is deemed to be a reverse acquisition, where we (the legal acquirer) are considered the accounting acquiree and Ever Leader (the legal acquiree) is considered the accounting acquirer, and thus the historical financial statements of Ever Leader are the financial statements of Benda.
Business
Our operations are headquartered in Wuhan, Hubei Province, China. We are a profitable, mid-sized Chinese pharmaceutical company that identifies, discovers, develops and manufactures both conventional medications and Traditional Chinese Medicines (“TCMs”) for the treatment of some of the largest common ailments and diseases (e.g., common cold, diabetes, cancer).
We currently have three core operating companies:
| Yidu Benda develops, manufactures and sells bulk chemicals (or pharmaceutical intermediates), which are the raw materials used to make “Active Pharmaceutical Ingredients” (“APIs”). About 11.8 per cent of the bulk chemicals that we produce in 2004 were used for our own medicines. The remainder was sold in the market to unrelated parties. We did not sell to related Benda companies in 2005 or 2006. |
| Jiangling Benda develops, manufactures and sells APIs, which are one of the two components of any capsules, tablets and fluids that are pharmaceutically active. An API is the substance in a drug that produces the desired medicinal effect. The “excipient” is the inert material that holds the API (such as gelatin or water). About 3.7 per cent of the APIs that we produce in 2004 were used to produce some of our finished medicines. The remainder was sold in the market to unrelated parties. Jiangling Benda did not sell to related Benda companies in 2005 or 2006. |
| Benda Ebei develops, manufactures and sells (a) conventional finished medicines, which are non-patented, branded, proprietary small volume injection solutions (vials) used for a variety of treatments including hepatitis; and (b) Traditional Chinese Medicines (“TCMs”), which are herb-based and natural medicines used in TCM therapies (via our newly formed subsidiary Beijing Shusai). Some of the medicines we produce are of our own origination and protected from competition by certificates issued by China’s State Food and Drug Administration (“SFDA”). There are no differences between the regulatory processes for conventional medicines and Traditional Chinese Medicines. Traditional Chinese Medicines are ready-make medicines, which are produced according certain curing principles and prescriptions and can be used immediately, such as pills, medicinal granules and capsules. |
The Chinese government agency, SFDA, provides approval for drugs released into the market in China.. Unlike the Food and Drug Administration (“FDA”) in the United States, however, the SFDA provides intellectual property and competitive protection to certain classes of approved drugs.
Each core Benda operating company has its own manufacturing facility located near Wuhan, in Hubei Province. Good Manufacturing Practices (“GMP”) certification was issued to Benda Ebei on November 11, 2003 for the production of injection vials. Benda Ebei was designated a High and New Technology Enterprise by the Science and Technology Bureau of Hubei Province on July 6, 2005 for a period of two years. This designation represents formal recognition by the provincial government that a company has developed or acquired new technology of significance, and triggers a number of government support and incentive policies, including availability of land for expansion, research grants and discounts on bank loan interest. The designation expired on July 6, 2007; however, we have passed the re-examination and the new designation was received on August 2007. Although we have not received any support from the government to date, such a designation may be useful in obtaining incentives in the future. Our Yidu Benda facility produces bulk chemical intermediates for raw material medicine and does not require GMP certification. Our Jiangling Benda facility was closed for renovation in July 2004 to comply with GMP standards. The Jiangling plant reopened on August 10, 2007, and has been producing Ribose, the only product that does not require GMP approval. We are currently working on obtaining GMP certification so that we can use the Jiangling plant for our other products. We currently have all of the production equipment in place and the new facilities have been installed and tested. All workshops at the Jiangliing Benda facility need to pass GMP certification except for the Tetraacetylribofuranose shop. It is estimated that the Ribavirin workshop will pass GMP certification in February 2008; and the comprehensive workshop, which produces Mesylate Levoflaxacin and Asarone, applied for GMP certification at the end of December. If we are unable to obtain GMP certification, further delays and expenses will result, and we will be unable to operate out of such workshops until the GMP certification has been obtained.
Good Manufacturing Practices (“GMP”) is an internationally-recognized standard for pharmaceutical plant design and construction. GMP has been defined as “that part of quality assurance which ensures that products are consistently produced and controlled to the quality standards appropriate for their intended use and as required by the marketing authorization” (World Health Organization). GMP covers all aspects of the manufacturing process: defined manufacturing process; validated critical manufacturing steps; suitable premises, storage, transport; qualified and trained production and quality control personnel; adequate laboratory facilities; approved written procedures and instructions; records to show all steps of defined procedures taken; full traceability of a product through batch processing records and distribution records; and systems for recall and investigation of complaints.
We distribute our high value, branded medicines, through agents who sell them to hospitals that administer them to patients. We sell generics to medical wholesalers for resale to hospitals. We sell our Over the Counter (“OTC”) medicines to wholesalers specializing in selling to retail chain drug stores. Our APIs are typically sold to large drug manufacturers under long-term supply contracts. Our bulk chemicals are purchased by other Chinese drug companies.
History and Corporate Organization
Ever Leader was incorporated in Hong Kong on October 29, 2005 for the purpose of functioning as an off-shore holding company to obtain ownership interests in various entities (collectively “Benda”) that were previously owned, either directly or indirectly, by Mr. Yiqing Wan (“Wan”) and his wife, Ms. Wei Xu (“Xu”).
The following paragraphs summarize the original ownership structure of various entities owned by Wan and Xu and the subsequent reorganization and transfer of ownership interests in these entities, either directly or indirectly, to Ever Leader.
Ownership Structure Prior to Reorganization
Hubei Benda Science and Technology Development Co., Ltd. (“Benda Science”) was incorporated in the Province of Hubei, PRC in October of 2002, primarily functioning as a holding company with ownership interests in various entities operated by Wan and Xu. Wan and Xu are the sole owners of Benda Science, with ownership interests of 10% and 90%, respectively.
Benda Ebei was incorporated in the Province of Hubei, PRC in April of 2001. Benda Ebei has registered capital of $2,419,404 which is fully paid up. Prior to the reorganization of Benda as further described in the paragraphs below, Benda Science, Wan, and Xu were the sole owners of Benda Ebei, with ownership interests of 60%, 20%, and 20%, respectively. Benda Ebei develops, manufactures, and sells small volume injection solutions (vials) and other conventional medicines.
Jiangling Benda was incorporated in the Province of Hubei, PRC in October of 2001. Jiangling Benda has registered capital of $967,738 which is fully paid. Prior to the reorganization of Benda, Benda Science and Wan were the sole owners of Jiangling Benda, with ownership interests of 90% and 10%, respectively. Jiangling Benda develops, manufactures and sells active pharmaceutical ingredients (“APIs”). Jiangling Benda’s primary production facility was closed for upgrades and renovations in July 2004 in order to secure a GMP certification from the Chinese SFDA. This facility is expected to reopen and resume production in August of 2007.
Yidu Benda was incorporated in the Province of Hubei, PRC in March of 2002. Yidu Benda has registered capital of $4,233,854 which is fully paid. Prior to the reorganization of Benda, Benda Science and Wan were the sole owners of Yidu Benda, with ownership interests of 90% and 10%, respectively. Yidu Benda develops, manufactures and sells bulk chemicals (or pharmaceutical intermediates) for use in the production of APIs. The organization and ownership structure of Benda prior to reorganization is as follows:
Reorganization and Revised Ownership Structure
As previously stated in the paragraphs above, Ever Leader was incorporated in Hong Kong on October 29, 2005 for the purpose of functioning as an off-shore holding company to obtain ownership interests in various Benda entities that were previously owned, either directly or indirectly, by Wan and Xu. Ms. Mo Mo Hon (“Hon”), a Hong Kong SAR resident, was the sole registered shareholder of Ever Leader, holding the single issued and outstanding share of Ever Leader in trust for Xu.
Pursuant to three separate Equity Transfer Agreements entered into in November of 2005 among Ever Leader, Benda Science, Xu, and Wan, Ever Leader obtained a 95% ownership interest in Benda Ebei in exchange for a commitment to pay $2,298,434 in aggregate consideration to Benda Science, Wan, and Xu. The $2,298,434 acquisition price represented 95% of the $2,419,404 of registered capital of Benda Ebei, but was not representative of the fair value of the assets acquired or liabilities assumed. Specifically, as transfers of ownership interests in PRC entities to offshore holding companies for zero or nominal consideration is prohibited by the Chinese Government (regardless of whether these PRC entities and offshore holding companies are directly or indirectly owned and controlled by the same individual or individuals), an amount equal to 95% of the value of the registered capital of Benda Ebei was established for purposes of the transfer of the 95% ownership interest in Benda Ebei (directly and indirectly 100% owned and controlled by Wan and Xu) to Ever Leader (beneficially 100% owned and controlled by Xu). As a result of each of these entities being 100% directly and indirectly controlled by Wan and Xu, this transaction has been accounted for as a combination of entities under common control (see additional discussion of accounting treatment in the paragraphs that follow), with Ever Leader’s commitment to pay $2,298,434 in aggregate consideration to Benda Science, Wan and Xu being reflected as a current liability at both December 31,2005 and 2004, with corresponding reductions to paid-in capital.
Pursuant to an Equity Transfer Agreement entered into on December 3, 2005 among Benda Ebei, Benda Science, and Wan, Benda Science transferred and assigned its 90% ownership interest in Jiangling Benda to Benda Ebei and Wan transferred and assigned a 5% ownership interest in Jiangling Benda to Benda Ebei (for zero consideration as Benda Ebei and Jiangling Benda were both directly and indirectly 100% owned and controlled by Wan and Xu).
Pursuant to a second Equity Transfer Agreement entered into on December 4, 2005 among Benda Ebei, Benda Science, and Wan, Benda Science transferred and assigned its 90% ownership interest in Yidu Benda to Benda Ebei and Wan transferred and assigned a 5% ownership interest in Yidu Benda to Benda Ebei (for zero consideration as Benda Ebei and Yidu Benda were both directly and indirectly 100% owned and controlled by Wan and Xu).
The organization and ownership structure of the Company subsequent to the consummation of the reorganization as summarized in the paragraphs above is as follows:
In July of 2006, Benda Ebei invested approximately $112,500 for a 75% ownership interest in Beijing Shusai, with the remaining 25% owned by an unrelated PRC individual. Beijing Shusai, a PRC limited liability company, was incorporated on June 15, 2006 and commenced primary operations in July 2006. Benda Ebei is setting up self-operated and franchised Pharyngitis Clinics in leading hospitals throughout major cities in China. It is currently operating two clinics for the Pharyngitis Killer therapy in Beijing, PRC.
On September 5, 2006, Ever Leader increased its number of authorized shares of common stock from 10,000 to 1,000,000 and effected a 100 to 1 stock split, resulting in Hon (the original sole registered shareholder of Ever Leader holding one share in trust for Xu) receiving 99 additional shares in the Company.
On September 5, 2006, Ever Leader transferred and assigned 711,202 shares of common stock to Xia Pharmaceutical, Inc. (“XIA”), an offshore holding company incorporated in the British Virgin Islands (“BVI”) that is 100% owned and controlled by Wan and Xu.
On September 5, 2006, Ever Leader issued 288,698 shares of common stock to 19 entities (some of whom are considered related parties) at par value. Additionally, Hon transferred and assigned her ownership interest in her 100 shares of Ever Leader to one of these entities.
The organization and ownership structure of the Company subsequent to the consummation of the reorganization as summarized in the paragraphs above is as follows:
The organizational chart after the acquisition of SiBiono would be stated as follows:
PRINCIPAL PRODUCTS
In 2006, our revenues were principally derived from sales of products listed in Figure 1. We have SFDA approval for all medicines and active pharmaceutical ingredients that we market. Sales of herbal TCM’s and bulk chemicals do not require SFDA approval. Our medicines have undergone pharmacological experiments in order to research the medicines’ effect and mechanism on organisms. On the other side, the experiments also research the organisms’ effect on the medicine. It includes pharmacodynamics and pharmacokinetics. Pharmacological experiments and clinical trials have similarities. Clinical trials are part of pharmacological experiments. However, pharmacological experiments mainly use animals as research subjects, while clinical trials generally utilize patients as research subjects. Pharmacological experiments are carried out by regulations of non-clinical medicine research and quality control issued by SFDA.
Main Products
Manufacturer | | Product | | Type | | Function |
| Benda Ebei | | Jixuening injection vial | | Generic | | Haemostatic (stops bleeding) |
| Benda Ebei | | Xujing injection vial | | Generic | | Haemostatic |
| Benda Ebei | | Nokeqing injection vial | | Generic | | Used to treat hepatitis |
| Benda Ebei | | Yidingshu injection vial | | Generic | | Vitamin to treat lack of Riboflavin |
| Benda Ebei | | Shusai-A injection vial | | Generic | | Anti-inflammatory analgesic |
| Benda Ebei | | Suzheng-B injection vial | | Generic | | Vitamin; complementary medicine used to treat hepatitis |
| Benda Ebei | | Ribavirin injection vial | | Generic | | Anti-virus, to treat acute upper respiratory tract infection |
| Benda Ebei | | Gentamycin Sulfate Injection vial | | Generic | | Broad spectrum antibiotic |
| Benda Ebei | | Vitamin B6 injection vial | | Generic | | Vitamin; complementary medicine used to treat hepatitis |
| Benda Ebei | | Inosine injection vial | | Generic | | Nutrition, complementary medicine used to treat hepatitis |
| Benda Ebei | | Vitamin C injection vial | | Generic | | To treat deficiency of vitamin C |
| Jiangling Benda | | Ribavirin API (1) | | API | | Ribavirin drug manufacture. |
| Jiangling Benda | | Asarin API (1) | | API | | Asarin manufacture to treat acute upper respiratory system infection |
| Jiangling Benda | | Levofloxacin Mesylate API (1) | | API | | Broad spectrum antibiotic drug manufacture |
| Yidu Benda | | Triazol carboxylic acid methyl ester (“TCA”) | | Bulk chemical | | Ribavirin manufacture, anti-virus |
| Yidu Benda | | L-methionine | | Bulk chemical Nutrition | | An essential amino acid for humans |
| Yidu Benda | | Tricabroxylic acid amide (“TAA”) | | Bulk chemical | | Ribavirin manufacture, anti-virus drug manufacture |
| Yidu Benda | | 1,2,3,5-Tetraacetyl-ß-D-Ribose | | Bulk chemical | | Ribavirin manufacture, anti-virus drug manufacture |
| (1) | Ribavirin API, Asarin API and Levofloxacin mesylate API are not in production during the Jiangling Benda facilities GMP renovation period. Production is expected to begin in February 2008. |
SFDA Compared to the FDA
The SFDA approval process is similar to the FDA approval process. They both require three phases of clinical trials.
| | | Phase I: Test the safety of drugs. 20-80 cases are required by FDA, while 20-30 cases are required by SFDA. |
| | | Phase II: Test the efficacy of drugs. Several hundred cases are required by FDA, while 100 cases are required by SFDA. |
| | | Phase III: Expand the sample group and further test the safety and effectiveness of drugs. Several hundreds, even thousands are required by FDA, while 300 hundred cases are required by SFDA. |
Before a drug can be sold in China, the drug needs to undergo the above three phases, namely pre-clinical trial, clinical trial and finally, GMP approval. The approval of a drug by SFDA does not guarantee the approval by FDA. However, drugs approved by SFDA can be exempted from certain steps in US clinical trials before applying for FDA.
Benda Ebei Products
Of our branded medicines, the Shusai-A Nefopam Hydrochloride solution, sold in injection vials, is particularly noteworthy. According to a pharmacological experiment, Nefopam Hydrochloride has an analgesic effect 10.4 times greater than that of aspirin. Furthermore, it is not addictive and causes no known side effects.
Pharmacological experiments research the medicines’ effect and mechanism on organisms. On the other side, the experiments also research the organisms’ effect on the medicine. It includes pharmacodynamics and pharmacokinetics. Pharmacological experiments and clinical trials have similarities. Clinical trials are part of pharmacological experiments. However, pharmacological experiments mainly use animals as research subjects, while clinical trials generally utilize patients as research subjects. Pharmacological experiments are carried out by regulations of non-clinical medicine research and quality control issued by SFDA.
The chemical name of this product is Nefopam hydrochloride. It mainly contains: 5-Methyl-1-Phenyl-3,4,5,6-Tetrahydrocannabinol-1H-2,5-benzoxazocine, fenazoxine hydrochloride. It is a new type of Non-narcotic analgesics which has the function of low-grade Antipyretic and muscle relaxants. Its chemical structure belongs to O-methyl benzene ring of diphenhydramine, so it does not have the attribute of Non-steroidal anti-inflammatory drugs, nor Opioid receptor agonist. It is effective for middle-grade and heavy-grade pain. Intramuscular injecting 20mg of Nefopam hydrochloride equals to intramuscular injecting 12mg morphine. It has light effectiveness on respiration inhibition. It has no inhibition on circulatory system. It has no tolerance or dependence. It can be rapidly oral absorbed, Tmax 1-3 hours, and it has obvious effect while first pass. T1/2 4-8 hours, the binding rate of plasma protein is 71%-76%. It is metabolized by liver to lose its pharmacological activities. Most of it will be excreted through kidney. The prototype drug will be less than 5% and only a little will be excreted along with excretion. It is used for pain-killing after operation, cancer pain, and acute pain. It is also used for visceral smooth muscle cramps such as acute gastritis, in-biliary ascariasis, ureterolithiasis.
The clinical trials were conducted in 1993, instructed by Doctor Guozhong, Peng. Ebei plant paid for all clinical trial expenses. It passed the clinical trials in 6 clinical institutes including the provincial hospital of Hebei, the second and the forth hospital attached Hebei School of Medicine, Bethune international peace hospital and etc. The efficacy rate is around 80% for all 374 cases examined. There is no follow up results. Shusai-A Nefopam Hydrochloride, has been used for more than 30 years in China and proven a safety, effective, mature and stable drug; and because of that it is recorded in the State Promulgated Pharmacopoeia of PRC. Thus, there is no need for us to perform duplicated experiment anymore. The drug registered number in SFDA of Shusai-A Nefopam Hydrochloride is H42021041.
Generics are common, low-cost, medicines used by doctors in hospitals nationwide. Our generics have been marketed for more than 10 years and are generally used by low-income patients in rural and country districts. The profit margins for our generics, which constitute about 3 per cent of Benda Ebei’s current sales volume, are lower than those of our branded products. However, our generic products are an effective means for promoting our corporate name, image and brands nationwide.
Jiangling Benda Products
Jiangling Benda plans to produce four types of active pharmaceutical ingredients and they are Ribavirin, Asarin, Levofloxacin and Ribose whereas the production of Ribose does not require the GMP certificate, but the production of the other three products do require the GMP certificate.
| Ribavirin has been used to produce antivirus medicine to treat SARS and SARS-like illnesses. Ribavirin is also used to treat severe virus pneumonia in infants and young children and a viral liver infection known as hepatitis C. It can be used in patients who have hepatitis C or human immunodeficiency virus (“HIV”) infection. Alliance Pharm, Inc. is advising us on modifications to our production processes in our effort to achieve U.S. FDA certification. Currently, there is only one other pharmaceutical company in PRC that has received U.S. FDA certification to produce Ribavirin API. On April 9, 2008, Jiangling Benda received the approved GMP Certificate from the SFDA which authorizing the production of Ribavrin. |
| Asarin is used to treat infections of the upper respiratory system. Our Asarin API is synthesized chemically rather than being extracted from natural raw materials, making it a cost effective and price competitive product. Benda’s Asarin received SFDA approval as a new API on December 27, 2005. We plan to extend our reach further down the value chain and manufacture consumer-ready Asarin medicines, in injection, vial and pill form, from our Asarin API. We have already filed for SFDA approval for these three types of finished Asarin products. |
| Levofloxacin Mesylate is a synthetic broad spectrum antibacterial agent for oral and intravenous administration. Benda’s Levofloxacin received SFDA approval as new drug ingredient on March 5, 2006. |
Yidu Benda Products
Yidu produces three types of bulk chemicals:
| Triazol carboxylic acid methyl ester (“TCA”). This is our main bulk chemical product. We have an installed capacity of 500 metric tons of TCA per year, which we believe is the highest in PRC. We expect to increase capacity from 500 to at least 700 metric tons per year by the end of 2006. |
| 1,2,3,5-Tetraacetyl-ß-D-Ribose is used mainly for Ribavirin production. |
| Tricabroxylic acid amide (“TAA”), used mainly for Ribavirin production. Since 2004, we have sold tricabroxylic acid amide to Shenzhen Saikane Chemical Engineering Pharmaceutical Company, who owns the right to export this product to its customers. |
In addition to bulk chemicals, Yidu Benda also produces L-methionine, an amino acid nutrition ingredient. L-methionine is a precursor in protein synthesis and also participates in a wide range of biochemical reactions.
While products produced by Yidu Benda do not represent a high-growth area for us, we derive significant cash flow from them. High barriers to entry exist in the intermediates sector, providing us with a relatively secure and stable market position. These barriers derive from: high capital equipment costs; economies of scale; high installed capacity of incumbents; reciprocal supply agreements; consumer-related advantages in established brands and reputation; proprietary production processes; long-term relationships with customers; and extensive distribution channels.
Due to a government order issued by the local government on January 10, 2007, our Yidu Benda plant has been shut down since the middle of January 2007 for improvement of our waste water treatment systems. The order requires us to finish the improvement and be compliant by June 30, 2007. On September 25, and October 1, 2007, Yidu Benda had passed the environmental assessment and safety assessment by the Yichang Environmental Protection Bureau and Yichang Safety Supervision Bureau These two bureaus have issued “Environmental Influence Report” and “Safety Assessment Report” in November and December of 2007, respectively. Yichang Environmental Protection Bureau issued an approval document, (Document Number: Yichang Environmental Audit [2007] No. 111) and permitted the trial production of Yidu Benda on December 28, 2007, at which time Yidu Benda resumed full production. The related government agencies through trial production physically inspect the products produced by the newly installed production facilities. The main purpose for doing so is to ensure that the quality of the products is such that there will be no harm to the environment. In addition, Yidu Benda had also passed the examinations conducted by Yichang Public Security Bureau, Yichang Lightning Protection Institute and Yichang Special Equipment Inspection and Test Institute, in terms of the fire apparatus and facilities, lightning protection and static proof facilities etc. Therefore, the Company has fulfilled the requirements for full production.
MARKETING AND DISTRIBUTION METHODS OF PRODUCTS AND SERVICES
Prescription Medicines
Two types of distribution channels exist in the Chinese medicine industry.
For high value branded medicines: Pharmaceutical Manufacturers → Agents → Sub Agents → Medicine Representatives → Hospitals or Pharmacies → Patients
For low value generics: Pharmaceutical Manufacturers → Wholesalers → Secondary Wholesalers → Hospitals or Pharmacies → Patients
The major difference between an agent and a wholesaler is that the agent has exclusive product sales rights from each manufacturer in each region, which is generally a province. Sometimes manufacturers have several wholesalers in a region.
The table below illustrates price markups along the distribution channel for a typical Benda Ebei branded drug, Shusai-A.
Shusai-A Price Markup Pattern
| | | Purchase Price per piece in RMB | | Price Markup | |
| Patients | | | 40.00 | | | 54 | % |
| Retail/Hospitals | | | 26.00 | | | 622 | % |
| Medicine Reps | | | 3.60 | | | 29 | % |
| Sub-agents | | | 2.80 | | | 56 | % |
| Agents | | | 1.80 | | | | |
| Benda Ebei | | | n/a | | | | |
The highest price markup along the distribution channel is on sales by medicine reps to hospitals because such markups finance kick-backs paid by the reps to doctors. This unfortunate, but common, practice is condemned by PRC’s patients and medical industry regulators, but no effective method has been found to stamp it out. In reality, there are “kick-backs” phenomena which are paid by the reps to doctors, not by our company to doctors. However, in the normal course of business, sales commission would be incurred between our company and clients, such as agents or wholesalers etc., in order to have a sense of incentive to them. This sales commission scheme is fully disclosed in the sales contracts and is also allowed by the relevant PRC regulations.
Benda Ebei sells its products to agents or wholesalers. This method minimizes the need for a direct sales force and distances Benda Ebei from questionable kick-backs and potential legal consequences.
Active Pharmaceutical Ingredients (“APIs”)
Our APIs are purchased by other Chinese drug companies on an order-by-order basis. The domestic industry is tight-knit and API marketing still relies on word-of-mouth, reputation, and personal contacts. Although we have temporarily closed the Jiangling plant to complete renovation and obtain GMP certification, we have maintained relationships with all our former clients and expect to bring on other drug companies as new customers.
Jiangling Plant is primarily engaged in producing Ribose, a bulk chemical which does not require GMP approval and Active Pharmaceutical Ingredients (API) which need GMP approval.
Jiangling Benda was re-opened in August 2007. The products that are planned to be produced in Jiangling Benda are as follows:
| | a) | Ribavirin API (Anti-virus) |
| | b) | Asarin API (Antibiotic) |
| | c) | Levofloxacin (Antibiotic) |
| | d) | Ribose |
These four products are classified as API whereas the production of Ribavirin, Asarin and Levofloxacin need to obtain GMP approval, however the production of Ribose does not need the GMP approval. Currently, Jiangling Benda only produces Ribose and which is a kind of API.
Bulk chemicals
We market our bulk chemicals by cultivating strong, long-term relationships with loyal customers. We usually supply customers pursuant to annual renewable contracts. Customers usually start by buying small quantities and gradually increasing order sizes. We also enjoy long-standing relationships with a number of important exporters. These sales contracts are signed annually.
Beijing Shusai Pharyngitis Research Co., Ltd.
Beijing Shusai Pharyngitis Research Co., Ltd, a recently established subsidiary of Benda Ebei, handles our Pharyngitis Killer therapy operation, promotion, and distribution. Key functional departments are as follows:
| Training. Trains doctors, doctor assistants, and medical workers. |
| Advisory. Advises each clinic on how best to apply the Pharyngitis Killer treatment. |
| Business Development. Extend the footprint of Pharyngitis clinics and implement patient outreach programs. |
| Marketing. Formulate and execute marketing plans. |
| Finance. Provide internal financial services to support business operations. |
| Logistics. Ensure no bottlenecks or shortages in product supply to the clinics. |
Special Marketing Initiatives
(1) | Qiweiben Capsule Initiative. Benda Ebei intends to develop a series of products based on the Qiweiben Capsule and designed to treat diabetes. They will be sold through diabetes recovery centers and regional distributors. |
(2) | Jixuening Initiative. We plan to develop a group of haemostatic medicines based on our core Jixuening brand. Benda Ebei’s Jixuening has been listed in the Catalog of Basic Medicines Covered by Social Medical Security. |
(3) | Analgesic Initiative. The treatment of pain attracts more attention from PRC’s medical community and hospitals around the country that are setting up pain clinics. Our Shusai-A and Lappaconitine Hydrobromide products are uniquely powerful pain killers and are not addictive. We plan to leverage their popularity to promote our other pain killers and thereby build a series of pain killer medicines. |
(4) | Asarin Initiative. We intend to form a group of medicines, based on Asarin, which will be designed to cure upper respiratory tract infection. |
STATUS OF PUBLICLY ANNOUNCED NEW PRODUCTS/SERVICES
We expect that the following products in our development pipeline will generate growth in our revenues in the next few years. We expect to begin mass production for products for which we recently received SFDA approval as soon as possible.
The registration of a medicine must first be undertaken at the provincial level. First, you need to make an application in the provincial Food and Drug administration, then you must pass the on-the-spot examination of the provincial registration office and the initial inspection of information experts. After that, you can apply for the experts’ technological evaluation of National Medicine Analysis and Judgment center. Only after you received the technological evaluation, your registration can be transferred to SFDA to have administrative ratification. After the ratification, you will be issued new license and ratification number. In most situations, after the application and record of provincial Food and Drug administration, the company’s products will not be examined again by National Medicine Analysis and Judgment center, which is similar to the first step of technological check.
Due to the restructuring of SFDA, the process of approval new drug has been on hold since the beginning of 2007. We do not know how long it will take to resume the approval process.
The Company can only obtain the license for the new medicine and the production license after the completion of the clinical experiments. The procedures of obtaining new drug certificate with the SFDA are as follows:
1. Submit the application to the Province SFDA;
2. Then the Province SFDA will perform the physical inspection;
3. If we pass the physical inspection, the related application would be transferred to the State SFDA;
4. After ratification, the new drug license and ratification number would be issued.
Development Status of Key Products in Our Pipeline
Name of Product | | Type | | Main Function | | Status |
| Pharyngitis Killer | | Herbal TCM Oral Liquid and Treatment | | Anti-respiratory tract infections | | Market launch underway; SFDA Certificate not necessary |
| | | | | | | |
| Qiweiben Capsule | | Branded TCM | | Diabetes treatment | | New Medicine Application is accepted by SFDA; awaiting for SFDA approval and production permit |
| | | | | | | |
| Yan Long Anti-cancer Oral Liquid | | Branded TCM | | Treatment of cancers of the digestive tract | | Awaiting for SFDA approval (1) |
| | | | | | | |
| 500mg:5ml Tranexamic Acid Injection vial | | Generic | | Haemostatic | | SFDA production approval H20044601 received; Put in production line. |
| | | | | | | |
| 200mg:2ml Ribavirin Injection vial | | Generic | | Antibiotic | | SFDA production approval H42021048 received; Put in production line. |
| | | | | | | |
| 1000mg:2.5mlVitamin C Injection vial | | Generic | | Vitamin | | Achieved State acceptance and hearing Y0405945; SFDA production approval H20067577 received; Put in production line. |
| | | | | | | |
| 0.1g:2ml Lomefloxacin Aspartate Injection vial | | Generic | | Antibiotic | | SFDA production approval H20056701 received; Put in production line. |
| | | | | | | |
| 0.2g:5ml Lomefloxacin Aspartate Injection vial | | Generic | | Antibiotic | | SFDA production approval H20056702 received; Put in production line. |
| | | | | | | |
| Lappaconitine Hydrobromide Injection vial | | Branded Medicine | | Analgesic | | SFDA production approval H20055966 received; Put in production line. |
| | | | | | | |
| Asarin Injection vial | | Generic | | Treatment of upper respiratory infection | | Filing completed at provincial bureau level; filed with SFDA in May 2006 |
| | | | | | | |
| Asarin pill | | Generic | | Treatment of upper respiratory infection | | Filing completed at provincial bureau level; filed with SFDA in August 2006; Need further bio-clinical trial. |
| | | | | | | |
| Asarin granular medicine | | Generic | | Treatment of upper respiratory infection | | Filing completed at provincial bureau level; filing with SFDA in September 2006 |
| | | | | | | |
| Asarin oral liquid | | Generic | | Treatment of upper respiratory infection | | Filing completed at provincial bureau level; filing with SFDA in September 2006; Need further bio-clinical trial. |
| Lysine Hydrochloride Injection vial | | Generic | | Amino acid | | Filing completed at provincial bureau level; filed with SFDA in July 2006; Need further bio-clinical trial. |
| | | | | | | |
| Arginine Monohydrochloride Injection vial | | Generic | | Amino acid | | Filing completed at provincial bureau level; filed with SFDA in July 2006; Need further bio-clinical trial. |
| | | | | | | |
| 100mg:5ml Levofloxacin Hydrochloride Injection vial | | Generic | | Antibiotic | | Filing completed at provincial bureau level; filed with SFDA in July 2006; Need further bio-clinical trial. |
| | | | | | | |
| 500mg:5ml Levofloxacin Hydrochloride Injection vial | | Generic | | Antibiotic | | Filing completed at provincial bureau level; filed with SFDA in July 2006; Need further bio-clinical trial. |
| | | | | | | |
| α-Asarin raw medicines | | API | | Upper respiratory infection | | SFDA production approval H20059540 received; prepare for production. |
| | | | | | | |
| Levofloxacin mesylate API | | API | | Antivirus | | Filing completed at provincial bureau level; SFDA approval received; prepare for production. |
| | | | | | | |
| GCLE | | Bulk chemical | | Production of Antibiotics | | As it is a chemical product, it does not need SFDA approval. |
(1) Yanlong oral solution has finished its earlier stage of research, and the Company is in the process of applying for SFDA’s permission of clinical trials. The new drug certificate will only be issued after the clinical trails.
New Branded Medicines
Our upcoming branded medicines include Qiweiben capsule and Yanlong anti-cancer oral liquid, which are proprietary traditional Chinese medicines, and Pharyngitis Killer Therapy, which is a combination of herb-based traditional Chinese medicine and treatments. We expect these products to have a significant positive impact on our future operational results.
Our New Branded Traditional Chinese Medicines
Features | | Pharyngitis Killer Therapy | | Qiweiben Capsule | | Yanlong Anti- cancer oral liquid |
| Targeted IP/ Formula Protection Period (1) | | Not Applicable (2) | | 7+7 years (7) | | 7+7 years (7) |
| Our Ownership | | 75% (3) | | 100% | | 100% with reservation (4) |
| Completion Date Of Clinical Tests | | Not Applicable | | Pening (6) | | Pending (6) |
| New Medicine Certification Date | | Not Applicable | | Pening (6) | | Pending (6) |
| Expected installation of GMP quality production line (5) | | Not Applicable | | Pending (6) | | Pending (6) |
| Expected SFDA Production Certification | | Not Applicable | | Pending (6) | | Pending (6) |
| Expected Commencement of Production | | Launched June 2006 | | Pending (6) | | Pending (6) |
(1) The first protection period will commence when the proprietary TCM protection application is approved. The second protection period can be applied for when the first protection period is expired.
(2) TCM therapies do not require SFDA approval. We chose not to apply for patent protection for this product’s formula due to our concerns about disclosures required in the patent application process.
(3) Benda owns 75% of Beijing Shusai Pharyngitis Research Co., Ltd., a company that owns all product and market exploitation rights to Pharyngitis Killer Therapy.
(4) The inventor of Yanlong Anti-cancer oral liquid, Mr. Yan Li, has reserved the right to sell the product to one hospital in Hong Kong, one hospital in Taiwan and one hospital in Shenzhen province.
(5) GMP certification is expected once the facility installation for the products below referenced products and examination by the related local government agency is completed.
In order to produce Qiwweiben, the following table shows the production facilities are purchased and installed, and they are all located in Benda Ebei and waiting for the government agency for inspection:
No. | | Equipment | | Model No. | | Quantity |
| 1 | | Muller | | 300A | | 2 |
| 2 | | powder shifter | | ZS-350 | | 2 |
| 3 | | powder shifter | | XS-650 | | 1 |
| 4 | | 3D mixer blender | | HD-1000 | | 1 |
| 5 | | Ultrasonic spray drier | | FL-120 | | 1 |
| 6 | | Wet mixer granulator | | GHL-250 | | 1 |
| 7 | | Slot shape Mixer | | CHL-150 | | 2 |
| 8 | | granule drier | | TC-Z-Ⅱ | | 1 |
| 9 | | Capsule filling machine | | NJP-1200 | | 1 |
| 10 | | Capsule polishing machine | | YPJ-Ⅱ | | 1 |
| 11 | | fast packing machine | | DPH-250D | | 1 |
In order to produce Yanlong Anti-cancer Oral Liquid, the following table shows the production facilities are purchased and installed, and they are all located in Benda Ebei and waiting for the government agency for inspection:
No. | | Equipment | | Model No. | | Category |
| 1 | | Solution Preparation Reservoir | | PLG-1.0 | | |
| 2 | | Fluid Storage Reservoir | | ZYG-1.0 | | |
| 3 | | High Level Reservoir | | N/A, general machinery | | Liquefy |
| 4 | | Micro-filters | | N/A, general machinery | | System |
| 5 | | Filters for Sugar Syrup | | N/A, general machinery | | |
| 6 | | Sugar Dissolving Reservoir | | HTG-0.5 | | |
| 7 | | Bottle Filling Machine | | QCX60 | | |
| 8 | | Sterilization System | | AQS1.2 | | Bottling |
| 9 | | Labeling Machine | | TWJA | | System |
| 10 | | Light Examine Workstations | | N/A | | |
(6) Due to recent restructuring within SFDA, the approval process for new medicines has been ceased since November 2006, except for the first class new medicines which are drugs that have never been sold or appeared in the market in China.. The management expects that such situation would still last for an indefinite period.
(7) According to the relevant Chinese patent drug policies of SFDA, all national protective Chinese patent drugs can apply for a seven-year protective period. During the period, other pharmaceutical enterprises cannot produce imitation products. After the first seven years, the company can apply for another seven years’ protective period.
Pharyngitis Killer Therapy (Anti-Respiratory Tract Infections)
Product Description: This is an entirely natural Chinese medicine and treatment containing no hormones or antibiotics, which cures a wide range of upper respiratory tract infections, ranging from the common cough to more advanced respiratory illnesses, such as acute and chronic pharyngitis, tracheitis and bronchitis which has already been proven by clinical trials which are conducted by the company together with the co-operated hospitals.
The clinical trials were conducted by the company together with co-operated hospitals which was based on the sample size 50 chronic respiratory patients who either had the symptom pharyngoxerosis throat itch, chest distress and feeling suffocated or oppressed, throat swelling and sore or vocal cord weary and hoarseness. The medical products used as a comparison were Pharyngitis Killer Therapy (in form of herb drink) alone and chronic comprehensive therapy.
We believe the oral liquid taken alone has a success rate of up to 80% in treating chronic pharyngitis; however, if accompanied by further treatment, success rates of up to 98% can be achieved.
This medicine, in the form of herbal drink, was developed and kept secret over many generations by the Wang family of Beijing. The formula and treatment was initially invented in 682 A.D. by Wang Zhaojing, a famous doctor in Chinese medical history. We believe the oral liquid taken alone has a success rate of up to 80% in treating chronic pharyngitis; however, if accompanied by further treatment, success rates of up to 98% can be achieved.
However, the above data was only conducted by the company together with co-operated hospitals which was based on the sample size 50 chronic respiratory patients. In order words, such clinical trials were not performed by the formal requirement of SFDA.
Our Pharyngitis Killer therapy:
| Swiftly smoothes away throat itching and coughing |
| Controls the development of disease in the mucus of the mouth |
| Rebuilds the immune function of the throat area |
| Provides a natural therapy, which is without side effects and free of antibiotics |
Market Outlook: With the recent urbanization and industrialization of PRC, pollution has become a big problem. Air pollution in particular causes several diseases of the pharynx. Researchers believe that approximately 10% of the people who live in cities suffer from pharynx disease, while in some rural areas, the ratio is a lower, though still significant, 5.5%. Until now treatments for upper respiratory tract infections have relied on antibiotics to control acute pharyngitis temporarily. However, no medicine in the market has thus far been effective at treating chronic pharyngitis. Pharyngitis Killer therapy overcomes the deficiencies of other treatments and eradicates the disease.
Purchase: The operating company for the Pharyngitis Killer therapy is Beijing Shusai. The total registered capital is 1.2 million RMB. As part of the joint venture, Mr. Wang contributed the Pharyngitis Killer formula and treatment as intangible assets, valued at 300,000 RMB, in return for 25% of Benda Shusai’s equity. Benda Ebei invested 900,000 RMB in cash for 75% equity.
IP protection/ technology confidentiality: Pharyngitis Killer is a traditional Chinese medicine and as such does not require SFDA approval. Benda Ebei has chosen not to file for patent protection on the formula in order to avoid disclosing the product formula. It believes that the formula and treatment are effectively protected by: (a) the separation of auxiliary ingredients with a proprietary catalyst powder called Yao Yin; (b) a proprietary production method for the oral liquid; and (3) by proprietary treatment techniques. We plan to apply for patent protection for both the processing method used to make the oral liquid and the treatment method applied on patients.
Promotion: We plan to market Pharyngitis Killer as a stand alone oral liquid medicine that can be combined with surgery-like treatment to achieve results. In August 2006, the PRC State Administration of Traditional Chinese Medicine (“SATCM”) issued an official promotional document to provincial SATCM’s, in which Pharyngitis Killer therapy is categorized as a recommended TCM and treatment. This official promotional document was issued on August 18, 2006 based on an expert panel review of Pharyngitis Killer conducted in August 2006.
Marketing and Distribution: We are setting up self-operated and franchised Pharyngitis Clinics. Benda Ebei currently has two clinics in full operation in Beijing. Benda Ebei has prepared advertising and marketing campaigns to support the rapid expansion of the franchise, which will begin after the closing of this offering. Our self-operated clinics will be located inside hospitals. A proportion of the gross revenues will be paid to the host hospitals in lieu of rent and as compensation for any marketing efforts made on our behalf. Franchised Pharyngitis Killer clinics will be operated by franchisees, which will buy the medicine from us and pay us a franchising fee. We plan to open franchising clinics only at the beginning of the roll-out period in order to accelerate the revenue growth and minimize start-up costs. At a later stage, we plan to open self operated clinics only because we expect that they will be comparatively more profitable and easier to manage.
Status of Planned Pharyngitis Clinics
Due to the restructuring of SFDA, the related policies of SFDA have changed such that the local hospitals are not allowed to co-operate with third parties. These changes have caused us to discontinue our plans of setting of Pharyngitis Clinics. The management of the company is seeking and considering other alternatives to launch this particular drug, however up to now there is no definite plan yet.
Qiweiben Capsule (alleviates symptoms of diabetes)
Product Description: Benda Ebei has the exclusive right to produce a new, effective, herbal medicine against diabetes, which was developed in cooperation with Shandong Haiyang Biotech Co., Ltd. (“Haiyang”). Qiweiben capsule is a medicine used to reduce symptoms of patients suffering from type II diabetes and improve their sexual ability. It contains 7 active pharmaceutical ingredients (“APIs”) extracted from animals and plants; its major API is an extract from virgin male silk moths. This medicine has the following key benefits:
| (1) | Reduces blood glucose. |
| (2) | Cures erectile dysfunction and reduces sexual dysfunction caused by diabetes. The Qiweiben capsule is unique amongst diabetic medicines in this regard. |
| (3) | As it is a complementary medicine based on natural ingredients, the patient reduces intake of chemical medicines. |
Market Outlook: Diabetes has become a common and frequently occurring disease that threatens the health of an increasing portion of the population. According to the 5th International Diabetes Union conference held in Beijing in 2002, there are 130 million type II diabetes patients around the world, over 40 million of which are in PRC. In the 21st century, type II diabetes is expected to be epidemic in developing countries such as PRC and India. Such a large patient group provides a significant market opportunity for Qiweiben Capsule. Artificial insulin is currently the most widely used medicine for the treatment of diabetes. Patients using artificial insulin can become addicted to it and also suffer the discomfort of needle injections. These disadvantages can be reduced by using our Qiweiben capsule as a complement to artificial insulin. Pilot studies conducted by Haiyang in 2001 have shown that the Qiweiben capsule significantly reduces one or more symptoms of diabetes in 88% of cases.
Rights Purchase: Qiweiben is the brand name of this medicine. The registered drug name is Qiwei Xiaoke capsule. The SFDA production permit (SFDA Z200110150) was held by Shandong Leaf Pharmaceutical Co., Ltd. On March 14, 2004, Benda Ebei agreed to pay Haiyang RMB 5 million ($625,000) for the technology required for the extraction of virgin male silk moth essence, the SFDA production permit (SFDA Z200110150) and the New Medicine Certificate (SFDA Z20010134). The purchase agreement provided for the immediate transfer of the production permit and the deferred payment of the RMB 5 million ($625,000) within three years after Benda Ebei begins to sell the product.
IP protection/ technology confidentiality: Benda Ebei will apply for TCM protection for Qiweiben and expects a reply from the SFDA no later than 6 months thereafter. According to new regulations, protected TCM status is granted for 7 years from the registration date, during which period no other party may produce it. Thereafter, a further 7 year protection may be granted to the right holder depending on factors such as the effectiveness, production standards, quality and safety of the product. Benda Ebei also has pending patents for the protection of technological and manufacturing processes used to produce this medicine.
Production: We have been building Qiweiben production facilities. In order to produce qiwweiben, the following table shows the production facilities or equipment which have been purchased and installed. The equipment are all located in Benda Ebei and waiting for the government agency for inspection:
No. | | Equipment | | Model No. | | Quantity |
| 1 | | Muller | | 300A | | 2 |
| 2 | | powder shifter | | ZS-350 | | 2 |
| 3 | | powder shifter | | XS-650 | | 1 |
| 4 | | 3D mixer blender | | HD-1000 | | 1 |
| 5 | | Ultrasonic spray drier | | FL-120 | | 1 |
| 6 | | Wet mixer granulator | | GHL-250 | | 1 |
| 7 | | Slot shape Mixer | | CHL-150 | | 2 |
| 8 | | granule drier | | TC-Z-Ⅱ | | 1 |
| 9 | | Capsule filling machine | | NJP-1200 | | 1 |
| 10 | | Capsule polishing machine | | YPJ-Ⅱ | | 1 |
| 11 | | fast packing machine | | DPH-250D | | 1 |
We intend to start manufacturing and marketing the product immediately after receiving GMP certification. However, due to recent restructuring within SFDA, the approval process for new medicines has been ceased since November 2006, except for the first class new medicines. The management expects that such situation would still last for an indefinite period.
Clinical Experiments:
Qi Wei Xiao Ke capsule is a Chinese traditional medicine capsule whose main raw material is male Sangcan moth of no copulation. Biochemistry engineering technique is used to extract its useful components, which are produced to traditional Chinese medicine capsule preparation by adding six additional kinds of traditional Chinese medicinal materials.
Experiments with animals have shown that it can lower the blood glucose of streptozotocin diabetes big mouse and ALX diabetes little mouse and can increase the big mouse’s glucose tolerance content of ALX and prolong the submerged weight swimming time of ALX diabetes little mouse.
The male Sangcan moth is rich in brain hormone, ecdysone, male hormone, etc. and contains many amino acid, vitamins and microelement that are needed by the human body. The biological extracts of the male Sangcan moth which are added with milk veteh, the extracts of root of yellow kudzuvine and sealwort, and the extracts of medlar, and then mixed with trichosanthes and rhubarb micro mist, are adequate for traditional medicine’s curing for deficiency of both yin and yang, II diabetes of Qi deficiency and blood silt. It is tested for 420 clinical researches in Beijing Guang An Men Hospital-a subordinate hospital of China Academy of Chinese Medical Sciences and Shandong traditional Chinese medicine hospital, Liaoning traditional Chinese medicine hospital, and Jiangsu traditional Chinese medicine hospital which are four clinical experiment base of new drug. The total effective rate is 88.67% regardless of whether the disease history is long or short, and it has no poisonous side effect to human body.
The medicine was conferred patent of invention by National Patent Bureau in 1996 (patent No. Z96105259·7). Qi Wei Xiao Ke capsule received the new drug license (SFDA No.: Z20010134) and production approval license (SFDA No.: Z200110150) issued by SFDA in October, 2001.
Up to date, we have already submitted the related documents and awaiting for the approval of SFDA.
Yan Long Anti-cancer Oral Liquid
Product Description: The Yan Long Anti-Cancer Oral Liquid (“Yan Long”) is a conventional Chinese patent drugeffective against cancers of the digestive tract such as anal, bile duct, colon, esophageal, gallbladder, liver, pancreatic, rectal, and gastric cancers. It is especially effective in helping patients withstand chemotherapy treatment. Yan Long has successfully completed first and second phase clinical trials and Benda Ebei plans to begin the third phase.
Yanlong Anti-cancer Oral Liquid is a kind of pure mixture contains Chinese traditional medicine such as: Baiying, Morel, Salvia, Gentiana, Angelica, etc.
Starting in 1989, Yanlong anti-cancer oral liquid was given to 30 patients with primary liver cancer for the clinical trails. The number of the survivals for the year of 1990, 1991, 1992 and 1993 were 23, 17, 11 and 8 respectively. By March 2004, among the eight surviving patients, two do not have any signs of recurrence, three take care of themselves but are still physically weak, three regressed and returned to the hospital for treatment. I n this clinical research, the survival rate of the patients observed for over 14 years reached 26.67%.
Inventor: The development of Yan Long has been the life work of Dr. Yan Li, one of the nation’s most well-recognized experts in cancer studies. Dr. Li has been an oncology specialist for over 40 years and has held various prestigious positions in the cancer research field in PRC. He was born in 1931. In 1956, he went to study in Beijing College of TCM and graduated in 1962. After that, he worked in the Beijing TCM Hospital Oncology Department for 12 years. In 1970, he was promoted to physician in charge. In 1974, he transferred to the institute of oncology of Beijing Medical University. In 1984, he was appointed as vice president of Beijing Sino-Japan Friendship Hospital. He developed an anti-cancer oral liquid based on his decades of research and experience and named it Yan Long Anti-Cancer Oral Liquid.
Rights Purchase: Benda Ebei and Dr. Yan Li (“Li”) signed an agreement regarding the production, marketing, and sales of Yan Long. Benda Ebei signed agreement to buy all rights to Yan Long from Mr. Li for RMB 5 million ($625,000). Once sales of Yan Long commence a monthly payment of RMB 150,000 ($18,750) per month for the first six months and RMB 300,000 ($37,500) thereafter will be paid. Benda Ebei will cease payment when the accumulated monthly payments reach the RMB 5 million ($625,000) agreed purchase price. Li and Benda Ebei are obliged to keep the Yan Long formula secret. Li retains the right to produce and sell Yan Long in his own clinic. Benda Ebei management believes that this will not significantly impact Benda Ebei’s revenue from Yan Long.
IP protection/ technology confidentiality: Benda Ebei has applied for patent protection for Yan Long and expects to receive a 10 year patent when the registration process is completed
Production: The production facilities and equipment for Yan Long were installed and implemented in July 2007. We intend to start manufacturing and marketing the product immediately after receiving GMP certification. Due to recent restructuring within SFDA, the approval process for new medicines has been ceased since November 2006, except for the first class new medicines. The management expects that such situation would still last for an indefinite period. The process of third phase clinic trail of Yang Long, application for IP protection and the application of certificate are pending.
New Bulk Chemicals
We are in the process of developing and bringing to market 7-Phenylacetamido-3-chloromethyl cephalosporanic acid P-Metoxy Benzyl Ester (“GCLE”), which is a medicine intermediate, chemical name which is used for cephalotin antibiotics. Japan Otsuka Chemical Industry Company originally developed the process to produce GCLE and held the worldwide patent on the GCLE production process. The PRC patent protection period expired in 2005. Since then Benda’s research center and the Wuhan Institute of Chemical Technology have jointly mastered this process of GCLE production. We have completed small-scale and mid-scale trial productions, and expect to be able to produce 200 tons of GCLE in 2007 and 500 tons in 2008. Domestic demand for this product is expected to increase by 28% in 2006 to 1,200 metric tons3 . This demand is currently met by imports.
This new product will be produced in our Yidu plant. Due to an government order issued by the local government on January 10, 2007, our Yidu Benda plant has been shut down since the mid of January 2007 for improvement of our waste water treatment systems. The order requires us to finish the improvement and be compliant by June 30, 2007. We expect our Yidu Benda plant to resume production in the first quarter of 2008.
Up to date, Yidu Benda has received an oral consent from the local government agencies that the re-designed production facilities have passed the examinations and the related feasibility studies of the product that are going to produced in Yidu Benda has also been submitted. It is estimated that the final approval from the local government agencies would be obtained in the first quarter of 2008.
3 | Source: China National Medical Information Center Southern Sub center |
| 3 | Source: China National Medical Information Center Southern Sub center |
INDUSTRY AND COMPETITIVE FACTORS
There is certain industry and competitive factors which we believe will be critical to achieving our growth:
| Rapidly growing Chinese pharmaceutical market. In 2005, China was the 9th largest and fastest growing pharmaceutical market in the world. The Chinese currently spend about $12 per capita on pharmaceuticals compared to $340 per capital in the U.S. As the Chinese population ages and becomes wealthier, the already large Chinese pharmaceutical market is poised for continued explosive growth. According to IMS Health, Inc., a research firm, the Chinese pharmaceutical market grew by over 28% and 20% in 2004 and 2005, respectively. According to Boston Consulting Group, China’s pharmaceutical market will become the 5th largest in the world by 2010. Further, a recent report by McKinsey & Co. reported that Chinese healthcare spending will grow from $21 billion in 2000 to approximately $323 billion by 2025, or at a compounded growth rate of 11.6%. (Farrell D., Gersch U., Stephenson E., (2006) The Value of China’s Emerging Middle Class, McKinsey Quarterly) |
| Benda is uniquely positioned to capture market share. Our growth potential will be largely driven by our launch of three innovative and proprietary Chinese medicines, recently added to our product line: Pharyngitis Killer Therapy (anti-respiratory infection treatment), Qiweiben Capsule (diabetes) and Yanlong Anti-Cancer Oral Liquid. Benda also currently produces and sell 81 types of medicines certified by the SFDA, three types of APIs and five types of bulk chemicals. Several additional products are in development, at various stages. Our wide range of certified medicines, APIs and bulk chemicals have historically, and will continue to provide us with steady growth in revenues and profits. |
| Low cost producer. Our continuing success in optimizing our manufacturing processes and minimizing our production costs provides us with a competitive advantage. For example, we have recently developed innovative processes that achieve Ribavirin (an API in common anti-viral injections) yield rates 4.5 per cent higher than the competition, while at the same time reducing Ribavirin manufacturing costs by 9 per cent. SFDA data reveals that we are the only Chinese company that currently synthesizes Asarin (an API in common treatments of the upper respiratory system) chemically; as a result, our Asarin production costs are 16 per cent below our competitors. |
| Government sponsored industry consolidation presents opportunities for acquisitions. According to Business China magazine, China’s thousands of domestic companies account for 70 percent of the pharmaceutical market. Anticipating the effects of WTO entry and in an effort to compete with foreign firms, the Chinese government has decided to nurture its own large pharmaceutical companies, by encouraging the consolidation of its government-owned companies. To this end, the Chinese State Economic and Trade Commission (SETC) announced plans to consolidate the industry and support the development of 10 to 15 largest pharmaceutical firms. According to government statistics China currently has about 3,500 drug companies, down from over 5,000 in 2004. The number is expected to drop further. As the industry undergoes further consolidation, Benda will have the opportunity to grow by acquisition. |
| Benda’s GMP compliant manufacturing processes provide us with a strong competitive advantage in the Chinese healthcare market. The government has formulated an Action Plan for the Modernization of Chinese Medicine to boost the quality of Chinese medicine and enhance China's ability to compete in world markets. As such, all domestic producers of APIs were required to be GMP compliant by the end of 2004. Since the end of June 2004, the SFDA has been closing down manufacturers that do not meet the new GMP standards. In contrast to many of its competitors, Benda has made significant investments in refitting our manufacturing systems to comply with GMP standards. Our Benda Ebei plant received GMP certificate from SFDA on November 11, 2003. We have completed the installation of our Jiangling Benda manufacturing facilities (for the production APIs) and applied for GMP authentification in December 2007. Our Yidu Benda facilities, which produce bulk chemicals, are not subject to GMP certification requirement. |
| Potential for API export sales. We have demonstrated some initial success to date in exporting indirectly our products and increasing our international exposure. Star Lake Bioscience Co., Ltd. (“Star Lake”), one of our customers which is located in Zaoqing City, Guangdong province, is the only drug manufacturer in China to receive U.S. FDA approval to export its medicine to the U.S. We have an established business relationship with Star Lake since 2003. We are presently undergoing the FDA certification process for one of our APIs, which is used to manufacture drugs to alleviate SARS and SARS-like diseases. During the SARS period in PRC, one of the API products, Ribavirin, was used as a basic element and combined together with hormones in order to make an effective drug for the treatment of SARS. The Company will begin exporting the product once it has obtained FDA approval. |
RAW MATERIALS AND PRINCIPAL SUPPLIERS
Benda Ebei Suppliers
Benda Ebei plant manufactures injection vials from APIs. Benda Ebei’s largest supplier of APIs is Hunan Dongting Pharmaceutical Co., Ltd. In 2005, Benda Ebei purchased approximately $600,000 worth of Tranexamic Acid from this supplier, equivalent to 11.9 per cent of Benda Ebei’s raw materials purchases by value. In 2006, Benda Ebei’s largest supplier of APIs is Nan Yang Pung Gong Co. Ltd and Benda Ebei purchased approximately $994,000 worth of Tranexamic Acid from this supplier, equivalent to 16 per cent of Benda Ebei’s raw materials purchases by value.
Jiangling Benda Suppliers
Our Jiangling Benda plant manufactures APIs from bulk chemicals. Jiangling Benda’s largest supplier of bulk chemicals is Wuhan Zhongnan Supplying Co., Ltd. In 2004, Jiangling Benda purchased $400,000 worth of Acetic Anhydride from this supplier, equivalent to 26.2 per cent of Jiangling Benda’s raw materials purchases by value. The Jiangling Benda plant has made no further raw materials purchases since its closure for renovation.
Yidu Benda Suppliers
Our Yidu Benda plant manufactures bulk chemicals from other bulk chemicals. Yidu Benda’s largest supplier of bulk chemicals is Zaoqing Star Lake Bioscience Co., Ltd., which is based in Guangdong province. In 2005, Yidu Benda purchased $1,600,000 worth of bulk chemicals from this supplier, equivalent to 27.4 per cent of Yidu’s raw materials purchases by value. In 2006, Yidu Benda’s largest supplier of bulk chemicals is Shenzhen Xiang Hua Chemistry Co. Ltd., and it purchased approximately $,1270,000 worth of bulk chemicals from this supplier, equivalent to 36.92 per cent of Yidu’s raw materials purchases by value.
Packaging Material Suppliers
Our packaging materials are purchased from two main suppliers. In 2005, we purchased $300,000 worth of plastic packaging material from each of Anlu ZhongYa Plastics Package Factory and Anlu Zhong’Ao Printing Factory, equivalent in each case to 33.1 per cent of our packaging supplies by value. In 2006, we purchased approximately $1,229,000 worth of plastic packaging material from each of Anlu ZhongYa Plastics Package Factory, equivalent to 19 per cent of our packaging supplies by value.
The standardized purchasing contract, which is designed by the PRC government agency, is being adopted. The major terms (or components) of the purchasing contracts are stated are as follows:
| 1. | payment schedule which is based on the negotiation; |
| 2. | the goods are inspected and received according to the PRC regulations for materials of producing drug; |
| 3. | breach contract, then parties have the right to sue in the PRC local civil courts; |
As there were many transaction contracts during the years, since the company formed purchasing contract with the suppliers for lots of material purchased, therefore we just listed three of those and shown as a sample for reference.
However, our company does not depend on any sole source suppliers, since the raw materials market is opened in the sense that we could have plenty of choices of the suppliers (each category of materials purchased may have three to five suppliers in the market). We only choose those suppliers who could offer better terms to us and having a long term co-operation relationship.
OUR INTELLECTUAL PROPERTY
We regard our service marks, trademarks, trade secrets, patents and similar intellectual property as critical factors to our success. We rely on patent, trademark and trade secret law, as well as confidentiality and license agreements with certain of our employees, customers and others to protect our proprietary rights.
Pursuant to the PRC TCM Protection Regulation, certain ready made TCM products which have received SFDA approval have automatic protected intellectual property rights for a seven-year period from the date of grant of such approval. An application can subsequently be made to extend such protection for up to three consecutive seven-year periods. Once this protection period has expired, a company may apply for patent protection.
To a large extent, we rely on such protection regulation to protect our intellectual property rights with respect to such products. In addition, as of December 31, 2005, we filed four patents for manufacturing technologies, primarily relating to our medicine products and manufacturing techniques and the applications ate still in the approval process
Our Patents
Two types of medicine-related patents exist in PRC: the medicine production technique patent and medicine invention formula patent. In PRC, illegal infringements of production technique patents are widespread. A tiny modification to a filed medicine production technique might be construed as a new one and argued as not violating the patent laws. Therefore, Benda Ebei has historically tried to avoid applying for this kind of patent in order to protect its technological secrets.
Invention formula patents are as easily imitated as production technique patents. However, since only one SFDA production certificate can be issued on new branded medicine based on the major pharmaceutical ingredients used, a minor modification to the formula would not warrant a new SFDA production certificate. This means that, even if another manufacturer gets to know a patented formula, it won’t be able to produce it, at least in PRC, without the proper SFDA production certificate.
Our company has already applied for 7 patents, in which the national patent licences “A Way of Producing Recombinant Adenoviruses” (Patent No.: ZL98123346.5), and “Virus Carrier and Recombinant Human Tumor Suppressor Gene and Application” (Patent No.: ZL02115228.4) were received in the year of 2002 and 2004 respectively. The product that produced from these patents is “Gendicine”.
In the year of 2007, two other patents are also received they are “The Recombinant Human Ad-p53 Ddrug Ffor Ccuring Hhyperplastic Disease” and “The New Use of The Recombinant Human Ad-p53 Products in Tumor Therapy”.
The application of other three patents are under the process of examination by the related government agencies.
The validity of the patent is 20 years.
Our Trademarks
Benda currently owns four trademarks: Jixuening, Benda, Suzheng-B, and Shusai-A. Benda has filed trademark applications, the approvals of which are pending, for other 13 medicine names or general trademarks and the applications ate still in the approval process.
WORK SAFETY ISSUES
On November 10, 2005, there was a small explosion in the Yidu plant resulting in the deaths of two of our workers. This tragic accident resulted from the violation of our operating procedures by one of the workers killed in the explosion. We paid RMB 260,000 ($32,000) to each of the victims’ families in settlement of any compensation issues. On November 11, 2005, there was another explosion in the same factory resulting from a chemical reaction triggered by the prior explosion. Fortunately management had anticipated this incident and the plant had already been temporally sealed so nobody was killed.
Following the events of November 10 and 11, 2005 we have put in place very strict work safety procedures and revised the design of the relevant production process in order to avoid similar incidents in the future. We believe Benda is not liable for any further material liabilities arising from these explosions.
RESEARCH AND DEVELOPMENT ACTIVITIES DURING THE PRIOR TWO FISCAL YEARS
We spent $30,821 and $16,604 on direct research and development (“R&D”) efforts in 2006 and 2005, respectively. Rather than spend considerable sums internally on R&D in a market where we can not easily protect our results of development, we prefer to leverage our management team’s extensive industrial network and knowledge in finding new drugs and treatments that may be potentially very successful but which have not yet been brought to market. We carefully evaluate each of these drugs before making a purchase decision. We believe each of our new products will enjoy considerable success in the market.
We also leverage our network of leading research institutions and universities to optimize the effectiveness of our investment in production R&D. We allocate and fund research projects to these institutions. Our in-house scientists coordinate and supervise the outsourced R&D processes. We have the rights to all results of the R&D efforts including IP rights. In order to protect our interests we usually divide each project into several sections and assign each section to a different research institution. Each research ally therefore only works on a limited part of each project. Some universities and institutes that work with us are:
| Life Science College of Wuhan University. |
| Biochemical College of Sanxia University |
| Shanghai Institute of Pharmacy of Chinese Academy of Sciences. |
| | |
| Wuhan University of Chemical Technology. |
The science and technology cooperation agreement between College of Chemistry and Life Science of China Three Gorges University and Yidu Benda:
Party A: China Three Gorges University (Party A for short in the following)
Party B: Yidu Benda Chemical Co.Ltd. (Party B for short in the following)
To increase the science and technology progress of Yidu Benda Chemical Engineering, Inc. (“Yidu Benda”), to improve the quality of its products and its production efficiency, and to fully make use of scientific intelligence, technologies and experimental equipments of College of Chemistry and Life Science of China Three Gorges University (“University”), after negotiation, the two parties decided to cooperatively build a “drug raw material research and development group”. The concrete agreements are as follows:
| 1. | The “drug raw material research and development group” is consist of members of both parties, including one group leader, two assistant group leaders and five science and technology workers. The group leader is assigned by University, while the two assistant leaders are assigned by the two parties, one for each party. The remaining five workers are decided by negotiation according to the demands of project research. |
| 2. | The research and development projects are put forward by Yidu Benda, and are decided after the demonstration of the two parties. |
| 3. | The research funds are supported by Yidu Benda, and University shall supply all the experimental equipment and the related condition according to needs, in which including: University provide certain number of chemistry and chemical engineering apparatus and equipment for common usage and help Yidu Benda to build a basic laboratory and assist Yidu Benda to build a pilot laboratory. |
| 4. | The expense of the research and development projects includes: drug reagent, low priced and easily worn detecting fees, the transportation fees between school and company and allowance of research workers. |
| 5. | University can directly send relative researchers to Yidu Benda to organize and guide project task research according to needs. |
| 6. | The property rights and interests of the project achievements will be appointed by Project Contract. |
COMPLIANCE WITH ENVIRONMENTAL LAW
We comply with the Environmental Protection Law of PRC as well as applicable local regulations. In addition to statutory and regulatory compliance, we actively ensure the environmental sustainability of our operations. Penalties would be levied upon us if we fail to adhere to and maintain certain standards. Such failure has not occurred in the past, and we generally do not anticipate that it will occur in the future, but no assurance can be given in this regard.
Due to an government order issued by the local government on January 10, 2007, our Yidu Benda plant has been shut down since the mid of January 2007 for improvement of our waste water treatment systems. The order requires us to finish the improvement and be compliant by June 30, 2007. Up to date, the company had spent approximately $0.2 MM to re-design and improve the existing system. Nowadays, Yidu Benda has received an oral consent from the local government agencies that the re-designed production facilities have passed the examinations and the related feasibility studies of the product that are going to produced in Yidu Benda has also been submitted. It is estimated that the final approval from the local government agencies would be obtained in October 2007.
SHENZHEN SIBIONO GENE TECHNOLOGY CO., LTD.
On April 5, 2007, Hubei Tongji Benda Ebei Pharmaceutical Co., Ltd., a Sino-Foreign Equity Joint Venture company incorporated under the laws of the PRC (“Benda Ebei”), of which Ever Leader Holdings Limited, a company incorporated under the laws of Hong Kong SAR ("Ever Leader") and a wholly owned subsidiary of Benda Pharmaceutical, Inc. (the “Company”), owns 95% of the outstanding common stock, has entered into Equity Transfer Agreements with certain shareholders of Shenzhen SiBiono Gene Technology Co., Ltd. (“SiBiono”), a corporation established and validly existing under the law of the PRC, to purchase a total of approximately 57.57% of the shares of SiBiono’s common stock for total consideration of RMB60,000,000 due and payable on or before April 30, 2007.
In connection with the Equity Transfer Agreements, we entered into a Financial Consultancy Agreement with Super Pioneer International Limited (“Super Pioneer”) for financial consultancy services rendered by Super Pioneer. Pursuant to the Financial Consultancy Agreement, we agreed to issue 2,100,000 shares of our common stock to Super Pioneer within three months from the date of the agreement. Super Pioneer agreed to lock up the shares for a period of twelve months from the date of the issuance of the shares (the “Lock-up Period”). Within three months from the Lock-up Period, in the event that the public trading price of our shares did not reach $3.6 per share and we are not listed in the capital market of NASDAQ or AMEX, Super Pioneer shall have the option to require us to redeem 1,960,000 shares of the stock owned by Super Pioneer at a price of $3.6 per share. Such option shall expire within one month from the last date of the three month period.
On June 11, 2007, Benda Ebei entered into Equity Transfer Agreements with Yaojin Wang and Huimin Zhang, shareholders of SiBiono, for the purchase of an additional 2.56% of the shares of SiBiono’s common stock for total consideration of RMB2,560,000 due and payable on or before June 30, 2007. Accordingly, Benda Ebei holds a total of 60.13% of the shares of SiBiono’s common stock.
In connection with the Equity Transfer Agreements, we entered into Technical Consultancy Agreements with Yaojin Wang and Huimin Zhang for technical consultancy services rendered by Yaojin Wang and Huimin Zhang. Pursuant to the Technical Consultancy Agreements, we agreed to issue 33,585 shares of our common stock to Yaojin Wang and 55,975 shares of our common stock to Huimin Zhang within three months from the date of the agreement. Yaojin Wang and Huimin Zhang agreed to lock up their shares for a period of twelve months from the date of the issuance of the shares (the “Lock-up Period”). Within three months from the Lock-up Period, in the event that the public trading price of our shares did not reach $3.6 per share and we are not listed in the capital market of NASDAQ or AMEX, Yaojin Wang and Huimin Zhang shall have the option to require us to redeem the shares of the stock owned by Yaojin Wang and Huimin Zhang at a price of $3.6 per share. Such option shall expire within one month from the last date of the three month period.
Business of SiBiono
Shenzhen SiBiono GeneTech Co., Ltd. (hereinafter referred to as SiBiono) is a gene therapy company dedicated to the development, manufacturing and commercialization of gene therapy products. The Company was founded in early 1998 and is located in Shenzhen Hi-Tech Industrial Park, Shenzhen, China. As a pioneer in gene therapy in China, SiBiono’s mission is to develop innovative gene therapy products for the improvement of human health and life quality. The Company has developed two core technology platforms: Viral Vector Gene Delivery System and Non-Viral Vector Gene Delivery System focusing on development of gene therapy product for cancer and cardiovascular diseases.
On October 16, 2003, SiBiono successfully obtained a New Drug License from the State Food & Drug Administration of China (SFDA), and then, in April 2004, SiBiono obtained “Manufacture Certificate” and “Certificate of GMP for Pharmaceutical Product”, so far being fully qualified for the market launch of Recombinant Human Ad-p53 Injection, trademarked as Gendicine® in China. Gendicine® is the first ever commercialized gene therapy product approved in the world by a government agency. It is only approved and commercialized in PRC alone; however there is no other gene therapy product like Gendicine has been approved and commercialized in other countries. One of the factor causing this situation may be due to the fact that China’s lack of regulatory obstacles in conducting gene therapy trails.
SiBiono has established the validated GMP manufacturing plant for the production of gene therapy drugs. A complete set of quality-control assays and large-scale production processes were implemented in SiBiono in accordance with international regulations and standards for consistent manufacture of high quality gene therapy products. Based on SiBiono’s QC procedures and standards, the SFDA constituted and issued the national technological guideline - “Points to Consider for Human Gene Therapy and Product Quality Control” in May 2004. This document was also published in the magazine of Biopharm International for reference and peer review.
SiBiono has undertaken a number of national and provincial research and development projects, including biotechnology projects of “National 863 Plan”, projects of “National 973 Plan”, key research project of “National Tenth Five-Year-Plan”, projects funded by “National Innovation Fund”, projects of “National Key Scientific Development Plan”, National Hi-tech Industrialization Projects, Key Platform Technology Development Projects in Guangdong Province, as well as Hi-Tech Industrialization Projects of Shenzhen Municipality. By participating in those national projects, the reputation of SiBiono could be raised in China and also it allows us to apply for the funding from the local government agencies to support our gene therapy research activities.
The Company has 60.19 million RMB as its registered capital and currently has about 80 full-time employees.
Milestones of SiBiono
Since its establishment in March of 1998, SiBiono has evolved from a small start-up company to an internationally recognized gene therapy company with its successful launch of the world’s first approved gene therapy product “Gendicine”. The Company has achieved the following major milestones in the past (Table 1):
Table 1. Milestones of SiBiono’s Development
Time | | Events |
| October 2006 | | SiBiono was awarded with “Global Entrepolis @ Singapore” Innovation Award. The GES Award, honoring the “Technopreneur of the Year” in the Asia-Pacific region, was presented by the President of Singapore, SR Nathan at the Opening Ceremony of Global Entrepolis@Singapore 2006. A record-breaking 224 entries from applicants in 14 countries and territories in the Asia-Pacific region submitted the application for competition. Wall Street Journal Asia presented the story of SiBiono in 2 separate issues. |
| | | |
| December 2005 | | Dr. Zhaohui Peng was awarded a Special Recognition Award by ISCGT (International Society for Cell and Gene Therapy of Cancer) in recognition for SiBiono GeneTech, Co., Ltd.'s great contribution to the gene therapy field. |
| | | |
| June 2005 | | Recombinant Human Ad-p53 Injection (Gendicine) was granted the “State Key-New product Certificate” issued jointly by the Ministry of Science and Technology, the Ministry of Commerce, the General Administration of Quality Supervision, Inspection and Quarantine and the State Environmental Protection Administration of the People’s Republic of China. |
| | | |
| April 2004 | | Gendicine was launched into market |
| | | |
| March 2004 | | SiBiono’s Gendicine manufacturing facility is granted with “Certificate of GMP for Pharmaceutical Product” by SFDA. |
| | | |
| January 20, 2004 | | SiBiono was granted “Manufacture Certificate” for Gendicine by the SFDA |
| | | |
| October 16, 2003 | | Gendicine was granted “New Drug License” by the SFDA, and became the first gene therapy product ever approved by a government agency in the world. |
| | | |
| September 2003 | | Mr. Zeng Qinghong, Vice President of P.R.China, visited SiBiono GeneTech and gave the Chinese Brand name (今又生) for Gendicine. |
| | | |
| September 2003 | | Completion of the clinical trials, defense and assessment of “recombinant human p53 adenoviral injection” product |
| | | |
| July 2003 | | Corporate restructuring was finished, the registration capital increased to be 48.19 million RMB |
| | | |
| November 2002 | | SiBiono was granted the Pharmaceutical Manufacture Permission by the Guangdong Drug Administration. |
| | | |
| September 1998 | | SiBiono obtained SFDA’s permission to initiate Gendicine clinical trials. |
| | | |
| March 1998 | | Shenzhen SiBiono GeneTech Co., Ltd. was established. |
Dr. Zhaohui Peng is the founder and Chairman of SiBiono. He graduated from a medical university in China, served as director of a research institute at the South Medical University in Guangzhou and as a visiting professor at both the University of Chiba in Japan and the University of California. He also conducted research at two US biotech companies. Dr. Peng has devoted more than fifteen years to gene therapy research, development and commercialization. He led the research, development, industrialization and commercialization of Gendicine. He is also the main inventor of all patents in SiBiono.
Attribute to his great contribution to the gene therapy field by developing and launching the world's first gene medicine product - Gendicine®, Dr. Peng was honored with the “Gene Therapy Achievement Award” at the 2005 ISCGT Annual Conference in December 2005, the person of cover story on Forbes (Chinese Edition) entitled with the “Technology Pioneer in China” in October 2006, the “Certificate of Recognition” for outstanding achievement and innovative contribution to bioscience research and development by California State Assembly in November 2006, and the nominee of “CCTV 2006 People of the Year in China’s Economy” in November 2006.
On September 9, 2007, Benda achieved a great success in the national forum on "Gene Therapy for Tumors" sponsored by the Chinese Academy of Medical Sciences and the China Pharmaceutical Biotechnology Association. The forum is China’s large annual medical biotechnology conference run from September 7 - 10 in Qing Dao, Shandong Province. In conjunction with the national conference, SiBiono held a national forum discussing “Gene Therapy for Tumors”. Approximately 600 leading Chinese doctors attended SiBiono’s forum, representing 26 different provinces and over 200 Tier I Chinese hospitals (Tier I hospitals offer the best medical services in China; there are currently 775 Tier I hospitals in China).
The main purpose of the forum was to allow 15 national renowned medical experts to share their experiences in the effective application of Gendicine® to maximize its treatment efficacy.
As of December 31, 2007, SiBiono shipped 10,652 of the 16,000 vials ordered. From January 1 to February 25, 2008, another 733 vials out of the 16,000 vials order had shipped. From February 26 to July 10, 2008, an additional 2,412 vials shipped. Accordingly, we have shipped and received payment for a total of 13,797 vials of the 16,000 vial order. The 16,000 vial order for Gendicine was ordered by various selling agents with average selling price RMB2,500 per vial.
In January 2008, our quality control department recognized that during the process of purification, one of the components of Gendicine did not fulfill the requirements of the internal quality control; therefore further purification was necessary. Gendicine is a gene therapy product which needs to undergo a series of internal quality control processes. Once the problem was located, our quality control and production department solved the issue immediately. Accordingly, the sales volume was held up for a short period of time during the mentioned period while the inventory was retained at high level.
Commercial Bank Note
On August 14, 2007, Benda Ebei entered into a three year non-interest bearing commercial bank note issuable agreement with Shanghai Pudong Development Bank. The total commercial note issuable limit is Rmb 60 million; however 50% of the deposit has to be made to the bank in order to secure the issuance of the commercial bank note, thus the net available amount is Rmb 30 million. The repayment period of each commercial note payable is six months. If the net amount of each commercial bank note payable is not settled on the due date, the penalty will be the penalty rate of the PRC bank loan on a daily and compound basis.
The credit facility was originally guaranteed by SiBiono and secured by the buildings, machinery and equipment of Benda Ebei. The Sibiono guarantee may have been a violation of the terms of the convertible promissory notes entered into with certain accredited and institutional investors on April 5, 2007. However, on December 15, 2007, Benda Ebei received a consent letter from Pudong Bank that Pudong Bank agreed to cancel SiBiono’s guarantee toward this credit facility.
As of December 31, 2007, Benda Ebei and Jiangling Benda deposited $2,615,254 in Shanghai Pudong Development Bank as deposit for the issuance of commercial bank notes. Such deposits will be released when the commercial bank notes are cleared. As of December 31, 2007, the balance if the commercial bank notes payable was $5,118,758. Thus the net commercial bank notes payable was $2,503,504 as of December 31, 2007.
On January 21, 2008, Benda Ebei entered into a supplementary agreement with Shanghai Pudong Development Bank, to supplement the commercial bank note issuance agreement dated on August 14, 2007. According to this supplementary agreement, the credit facility is further secured by the buildings, machinery and equipment of Jiangling Benda. As of December 31, 2007, the net book value of secured property and equipment was approximately Rmb67.33 million (or $9.2 million).
EMPLOYEES
As of December 31, 2007, we had approximately 489 full-time employees, including 48 sales people, 19 technology and R&D staff, and 408 production staff and no part-time employees. Among our current employees are two Ph.D.s and 17 holders of master’s degrees. All executives have received master-degree level executive training and all members of our staff have undertaken GMP training.
We have a sales office in each of three cities that promote our branded medicines. As of December 31, 2007, approximately 18 people in our Central China Sales Office were located in Wuhan, and 16 people were in our Southern China Sales Office located in Shenzhen.
MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
Forward-Looking Statements
The following discussion may contain certain forward-looking statements. Such statements are not covered by the safe harbor provisions. These statements include the plans and objectives of management for future growth of the Company, including plans and objectives related to the consummation of acquisitions and future private and public issuances of the Company's equity and debt securities. The forward-looking statements included herein are based on current expectations that involve numerous risks and uncertainties. Assumptions relating to the foregoing involve judgments with respect to, among other things, future economic, competitive and market conditions and future business decisions, all of which are difficult or impossible to predict accurately and many of which are beyond the control of the Company. Although the Company believes that the assumptions underlying the forward-looking statements are reasonable, any of the assumptions could be inaccurate and, therefore, there can be no assurance that the forward-looking statements included in this report will prove to be accurate. In light of the significant uncertainties inherent in the forward-looking statements included herein, the inclusion of such information should not be regarded as a representation by the Company or any other person that the objectives and plans of the Company will be achieved.
The words “we,” “us” and “our” refer to the Company. The words or phrases “would be,” “will allow,” “intends to,” “will likely result,” “are expected to,” “will continue,” “is anticipated,” “estimate,” “project,” or similar expressions are intended to identify “forward-looking statements.” Actual results could differ materially from those projected in the forward looking statements as a result of a number of risks and uncertainties, including but not limited to: (a) limited amount of resources devoted to achieving our business plan; (b) our failure to implement our business plan within the time period we originally planned to accomplish; (c) because we are seeking to merge with an operating business which has not yet been identified, you will be unable to determine whether we will ever become profitable; and (d) other risks that are discussed in this report or included in our previous filings with the Securities and Exchange Commission.
Quarter Ended March 31, 2008
Critical Accounting Policies
Accounting policies discussed in this section are those that we consider to be most critical to an understanding of our financial statements because they inherently involve significant judgment and uncertainties. For all of these estimates, we caution that future events rarely develop exactly as forecast, and the best estimates routinely require adjustment. Also see note 4, Summary of Significant Accounting Policies.
Revenue Recognition
Among the most important accounting policies affecting our consolidated financial statements is our policy of recognizing revenue in accordance with the SEC's Staff Accounting Bulletin ("SAB") No. 104. Under this policy, all of the following criteria must be met in order for us to recognize revenue:
1. Persuasive evidence of an arrangement exists;
2. Delivery has occurred or services have been rendered;
3. The seller's price to the buyer is fixed or determinable; and
4. Collectibility is reasonably assured.
The majority of the Company's revenue results from sales contracts with distributors and revenue is recorded upon the shipment of goods. Management conducts credit background checks for new customers as a means to reduce the subjectivity of assuring collectibility. Based on these factors, the Company believes that it can apply the provisions of SAB 104 with minimal subjectivity.
Estimates Affecting Accounts Receivable and Inventories
The preparation of our consolidated financial statements requires management to make estimates and assumptions that affect our reporting of assets and liabilities (and contingent assets and liabilities). However, it is explicated that the changes in estimation were not material in the preparation of our consolidation financial statements.
As of March 31, 2008 and 2007, the Company provided a $1,464,538 and $400,380 respectively for the allowance of doubtful accounts against trade receivables, other receivables and prepaid and deposits. Management's estimate of the appropriate allowance on those accounts receivable for the reporting periods was based on the aged nature of these accounts. In making its judgment, management assessed its customers' ability to continue to pay their outstanding invoices and the collectibility of those accounts on a timely basis, and whether their financial position might deteriorate significantly in the future, which would result in their inability to pay their debts to the Company.
Inventories, which are primarily comprised of raw materials, packaging materials, and finished goods, are stated at the lower of cost or net realizable value. Cost being determined on the basis of a moving average. The Group evaluates the need for reserves associated with obsolete, slow-moving and non-salable inventory by reviewing net realizable values on a periodic basis.
For the three months ended March 31, 2008 and 2007, the Company provided for a reserve against its work-in-progress amounting to $4,300,015 and none, respectively.
The provision of reserve was resulted from the manufacturing process of Gendicine, SiBiono’s sole product and SiBiono was acquired by the company in April 2007.
The following chart shows the manufacturing process of Gendicine (Ad-p53):
In the production process of finished goods, Gendicine, several working steps are needed: (i) large-scale culturing of adenovirus from master adenovirus bank; (ii) culturing of cell from master cell bank; (iii) purification. The whole process including step (i) to step (iii) takes approximately twenty-four days to make reagent (“original liquid”). This particular liquid can only be stored for approximately five years. It takes approximately another seven days for mixing and bottling original liquid to finished goods which is known as Gendicine.
Therefore, up to the stage of reagent, all the related production costs are treated as work-in-progress. The major components of those production costs are: (i) direct labor; (ii) direct materials; (iii) power; (iv) supplies and other materials; and (v) manufacturing overheads.
Before acquisition, as of March 31, 2007, the accumulated units of original liquid produced was 198,075 and which could be converted to approximately 226,736 vials of Gendicine. However, the accumulated vials of Gendicine sold throughout the years 2004 to three-month period ended March 31, 2007 were only approximately 18,424 vials. Thus the accumulated production costs of $4,080,644 were remained as work-in-process as of March 31, 2007.
Furthermore, due to the special feature of the original liquid which can only be stored for five years, and most of the original liquid was produced in the year of 2004, and the provision of reserve on work- in-progress was $3,696,083 as of March 31, 2007.
After the acquisition with the effective date April 1, 2007, the same accounting treatment was adopted for the treatment of the provision of reserve on work-in-progress. As of March 31, 2008, the provision of reserve on work-in-progress was $4,300,015.
However, no reserve for obsolete, slow-moving or non-salable inventory was required for the reporting periods. Management determination of this allowance was based on potential impairments to the current carrying value of the inventories due to potential obsolescence of aged inventories. In making its estimate, management considered the probable demand for our products in the future and historical trends in the turnover of our inventories.
While the Company currently believes that there is little likelihood that actual results will differ materially from these current estimates.
Operational Results
Three months ended March 31, 2008 Compared to Three months ended March 31, 2007
The following table provides key components of our operational results for the three months ended March 31, 2008 and 2007 for Benda Pharmaceutical, Inc.
| | | March 31, | | March 31, | |
| | | 2008 | | 2007 | |
| | | (Unaudited) | | (Unaudited) | |
| | | (Restated) | | (Restated) | |
| Revenue | | $ | 5,947,370 | | $ | 3,029,035 | |
| Cost of goods sold | | | (3,817,246 | ) | | (1,847,212 | ) |
| Gross profit | | | 2,130,124 | | | 1,181,823 | |
| | | | | | | | |
| Selling expenses | | | (436,371 | ) | | (88,183 | ) |
| | | | | | | | |
| General and administravive expenses | | | (2,642,918 | ) | | (485,330 | ) |
| Gains / (losses) on disposals of fixed assets | | | - | | | (12,025 | ) |
| Research and development expenses | | | (22,064 | ) | | - | |
| Total operating expenses | | | (3,101,353 | ) | | (585,538 | ) |
| Operating income / (loss) | | | (971,229 | ) | | 596,285 | |
| | | | | | | | |
| Interest expenses | | | (1,212,282 | ) | | (17,585 | ) |
| Other income (expenses) | | | 301,693 | | | 937 | |
| | | | | | | | |
| Income / (loss) before minority interest and income taxes | | | (1,881,818 | ) | | 579,637 | |
| Income taxes | | | (133,197 | ) | | - | |
| Minority interest | | | (22,398 | ) | | (46,906 | ) |
| | | | | | | | |
| Net Income / (loss) | | $ | (2,037,413 | ) | $ | 532,731 | |
| | | | | | | | |
| Earnings / (loss) per share - basic | | $ | (0.02 | ) | $ | 0.01 | |
| | | | | | | | |
| Weighted average shares outstanding - basic | | | 100,312,873 | | | 96,281,951 | |
| | | | | | | | |
| Earnings / (loss) per share - diluted | | $ | (0.02 | ) | $ | 0.00 | |
| | | | | | | | |
| Weighted average shares outstanding - diluted | | | 100,312,873 | | | 124,099,914 | |
Restatement
The Company restated its previously recognized Depreciation expense by ($559,131) and ($205,757) for the three months ended March 31, 2008 and 2007 due to incorrect calculation of depreciation expense previously reported.
The Company restated its previously recognized Amortization expense by ($201,054) and ($67,406) for the three months ended March 31, 2008 and 2007 due to incorrect calculation of amortization expense previously reported.
Net Revenue:
The Company has five core operating segments: Benda Ebei, Jiangling Benda, Yidu Benda, Beijing Shusai and SiBiono. Benda Ebei manufactures branded/generic medicines; Jiangling Benda manufactures active pharmaceutical ingredients (API); Yidu Benda manufactures bulk chemicals; Beijing Shusai operates and distributes Pharyngitis Killer Therapy; and SiBiono is a gene therapy company dedicated to the development, manufacturing and commercialization of gene therapy product, Gedicine.
Net revenue increased by $2.92 million (or 96%) to $5.95 million for the three months ended March 31, 2008, from $3.03 million for the three months ended March 31, 2007. Increase in revenue is principally attributed by the following factors:
| 1. | One of the Benda’s subsidiary, Benda Ebei’s net revenue increased $3.18 million (or 136%) to $5.52 million for the three months ended March 31, 2008 from $2.34 million for the three months ended March 31, 2007. It was mainly due to the increase sales of the existing major products and the introduction of new products and they are shown in the table below: |
| | | Three months ended March 31, | | Variance | | Variance | |
| Products (Million) | | 2008 | | 2007 | | + / (-) | | + / (-) % | |
| | | (Unaudited) | | (Unaudited) | | | | | |
| Suzheng-B | | $ | 0.88 | | $ | 0.50 | | $ | 0.37 | | | 75 | % |
| Shusai-A | | | 0.80 | | | 0.15 | | | 0.65 | | | 445 | % |
| Xujing | | | 0.71 | | | 0.48 | | | 0.23 | | | 48 | % |
| Yidingshu | | | 0.73 | | | 0.27 | | | 0.45 | | | 166 | % |
| Jixuening | | | 0.35 | | | 0.12 | | | 0.23 | | | 196 | % |
| Gentamycin 80 Thousand | | | 0.11 | | | 0.02 | | | 0.09 | | | 366 | % |
| Anagin | | | 0.09 | | | - | | | 0.09 | | | 100 | % |
| Lincomycin | | | 0.31 | | | - | | | 0.31 | | | 100 | % |
| Kanamycin | | | 0.14 | | | - | | | 0.14 | | | 100 | % |
| Troxerutin | | $ | 0.05 | | $ | - | | $ | 0.05 | | | 100 | % |
| 2. | One of the Benda’s subsidiary, Jiangling Benda, there was no reported net revenue for the three months ended March 31, 2007 due to the fact that it only resumed its production in October 2007. For the three months ended March 31, 2008, since it was still under the stage of production planning, therefore there was net revenue generated. |
Jiangling Benda plans to produce four types of active pharmaceutical ingredients and they are Ribavirin, Asarin, Levofloxacin and Ribose whereas the production of Ribose does not require the GMP certificate, but the production of the other three products do require the GMP certificate.
On April 9, 2008, Jiangling Benda received the approved GMP Certificate from the Chinese State Food and Drug Administration ("SFDA") which authorizing the production of Ribavrin. The other two products, Asarin and Levolfozacin, are still under the stage of GMP certificate approving process. The management could not estimate the exact timing for obtaining those certificates.
| 3. | One of the Benda’s subsidiary, Yidu Benda’s net revenue dropped $0.68 million (or 100%) to zero for the three month ended March 31, 2008 from $0.68 million for the three month ended March 31, 2007 due to the fact that the plant was temporarily closed since mid January to upgrade its waster water treatment system to comply with new environmental standards enforced by PRC local government. |
Yidu Benda has completed its upgrading of the waster water system and passed the government’s verification and testing of equipments in October 2007. It is now permitted for the testing on actual production process. Once the actual products are produced, then the environmental government bodies will re-test the production results. The management could not estimate the exact timing for obtaining the final approval on the actual production process.
| 4. | One of the Benda’s subsidiary, Beijing Shusai which incorporated on July 15, 2006. China’s State Food and Drug Administration (SFDA) recently experienced an overhaul in its policies and regulatory systems in an effort to fight against corruption in Chinese pharmaceutical industry. Beijing Shusai’s operation has been adversely affected by this recent policy changes which prohibits some state-owned hospitals from forming alliances with private companies. The management could not estimate that such situation could be resolved in the coming future. |
| 5. | One of the Benda’s subsidiary, SiBiono, acquired and effective since April 1, 2007, realized net revenue of $0.42 million for the three month ended March 31, 2008. SiBiono’s flagship product, Gendicine is the commercialized gene therapy product for the treatment of cancer and about 1,087 vials of Gendicine were sold in the first quarter in 2008. |
Cost of Goods Sold
Cost of goods sold increased $1.97 million (or 100%) to 3.82 million for the three month ended March 31, 2008 from $1.85 million for the three month ended March 31, 2007, primarily due to the corresponding increase in net revenue.
Gross Profit
Gross profit increased $0.95 million (or 90%) to $2.13 million for the three month ended March 31, 2008 from $1.18 million for the three month ended March 31, 2007, which were mainly due to: (1) the contribution of the gross profit of SiBiono, at about $0.4 million and (2) increase the sales volume of Benda Ebei as the net revenue of it increased sharply.
Selling Expenses:
Selling expenses increased $348K (or 395%) to $436K million for the three month ended March 31, 2008 from $88K for the three month ended March 31, 2007, primarily due to the promotional fees for Gendicine in SiBiono and the introductory new products in Benda Ebei.
General and Administrative Expenses:
General and administrative expenses increased $2.16 million (or 445%) to $2.64 million for the three month ended March 31, 2008 from $485K for the three month ended March 31, 2007. The following table shows the major expenses that significantly increase the G&A expenses during the reporting periods:
| | | Three Months Ended March 31, | | | |
| Major general & administrative expenses | | 2007 | | 2006 | | Variance | |
| | | (Unaudited) | | (Unaudited) | | | |
| Amortization on intangible assets | | $ | 51,575 | | $ | 26,284 | | $ | 25,291 | |
| Amortization on debt issue cost | | | 65,879 | | | 8,743 | | | 57,136 | |
| Audit and accounting | | | 129,334 | | | 3,500 | | | 125,834 | |
| Office expenses | | | 138,971 | | | 67,199 | | | 71,772 | |
| Repair and maintenance | | | 76,654 | | | 3,472 | | | 73,182 | |
| Salaries and wages | | | 349,270 | | | 131,362 | | | 217,908 | |
| Consultant and Professional Fees | | | 232,914 | | | - | | | 232,914 | |
| Training & professional development | | | 5,793 | | | 1,156 | | | 4,637 | |
| Conference | | | 12,877 | | | 9,600 | | | 3,277 | |
| Rent & Utilities | | | 35,540 | | | 4,490 | | | 31,050 | |
| Penalty to Investor for late filings on 10KSB | | | 722,205 | | | 120,000 | | | 602,205 | |
| Investor relation, Transfer agent and filing fees | | | 18,846 | | | 35,930 | | | -17,084 | |
| Director Renumeration | | | 110,873 | | | - | | | 110,873 | |
| Travel and transportation | | | 70,563 | | | 12,045 | | | 58,518 | |
| | | $ | 2,021,294 | | $ | 423,781 | | $ | 1,597,513 | |
Operating Income/ (Loss):
The Company experienced an operating loss of $0.97 million for the three month ended March 31, 2008 slipping $1.57 million from comparative period for 2007, which was mainly due to significant increase in G&A expenses.
Interest Expenses:
Interest expenses increased to $1.21 million for the three months ended March 31, 2008 from $18K for the three month ended March 31, 2007 primarily because of the financing cost associated with the issuance of convertible promissory note. In which $75,393 was incurred for the interest of the notes and $941,122 in interest expense related to the amortization of the debt discount associated with the Warrants and the debt discount associated with the beneficial conversion feature. (please refer to Note 24 of the Notes to Consolidation Financial Statements for details).
Income Taxes:
Benda is subject to Delaware, United State of America tax, but no provision for income taxes were made for the three months ended March 31, 2008 and 2007 as Benda did not have reportable taxable income for the period.
Ever Leader, a wholly owned subsidiary of Benda, is subject to Hong Kong tax, but no provisions for income taxes were made for the three months ended March 31, 2008 and 2007 as Ever Leader did not have reportable taxable income for the periods.
Benda Ebei was registered as a Sino-Foreign Equity Joint Venture on May 26, 2004 and is subject to the tax laws applicable to Sino-Foreign Equity Joint Ventures in the PRC. Benda Ebei, starting from 2005, is fully exempt from PRC enterprise income tax for two years starting from the first profit-making year, followed by a 50% reduction in the state income taxes, for the following three years, commencing from the first profitable year.
Jiangling Benda and Yidu Benda are cross-municipal investment entities and enjoy the same tax treatment as Sino-Foreign Joint Ventures, starting from 2005, and were therefore exempt from PRC enterprise income tax for two years starting from the first profit-making year, followed by a 50% reduction in the state income taxes, for the following three years, commencing from the first profitable year. Cross-municipal investments entities refer to entities that are incorporated in one municipal region but have investments in another municipal region.
The exemption periods for Benda Ebei, Jiangling Benda and Yidu Benda expired in the year of 2006, after which they are subject to a 50% reduction in state income taxes, at 18%; whereas the full income tax rate is 33%. The remaining tax holidays will be expired in 2010.
However, starting and effective from January 1, 2008, the full income tax rate would be changed from 33% to 25% according to the new PRC taxation regulations. Therefore these subsidiaries will be subject to the regular full income tax rate at 25% after the tax holidays expire in 2010.
According to the new taxation regulations starting and effective from January 1, 2008, Beijing Shusai is subject to the full income tax rate of 25%.
According to the new taxation regulations starting and effective from January 1, 2008, SiBiono, which is located in Shenzhen, a Special Economic District of PRC, is subject to the full income tax rate of 25% gradually in five years as following:
| Year | | Tax rate | |
| 2008 | | | 18 | % |
| 2009 | | | 20 | % |
| 2010 | | | 22 | % |
| 2011 | | | 24 | % |
| 2012 and thereafter | | | 25 | % |
For the three months ended March 31, 2008, only Ebei Benda had taxable income and incurred income tax of Rmb 0.95 million or $0.13 million. There was no income tax of the Group for the three months ended March 31, 2007.
LIQUIDITY AND CAPITAL RESOURCES
Net cash provided by the operating activities was negative $1.64 million for the three months ended March 31, 2008 while for the three months ended March 31, 2007 was negative $215K.
| a) | Non-cash operating activities, reconciliation items to the net income |
For the three months ended March 31, 2007, an amount about 163K of non-cash operating activities was reconciled back to the net income and which mainly included bad debt provision, amortization of intangible assets, depreciation, minority interest and amortization of debt discount and debt issue cost.
However, for the three months ended March 31, 2008, a significant amount, about $2.44 million of non-cash operating activities was reconciled back to the net income and summarized as follows:
| | 1. | $941K that incurred as interest expenses related to the amortization of discount on long-term debt and issuance cost associated with the warrants and the beneficial conversion features (please refer to Note 24 of the Notes to Consolidated Financial Statements for details); |
| | 2. | $230K that incurred as the penalty payment made in form of shares issuance to PIPE investors due to the late effective of the registered statement; and |
| | 3. | Other factors: $471K incurred on bad debt provision; $453K incurred on depreciation; $184K incurred on amortization of intangible assets; $65K million incurred on amortization of debt issue cost; and $22K incurred on minority interest. |
The net changes of trade receivable was increased by $1.33 million as of three months ended March 31, 2008 resulting from the increment in net revenue in the first quarter of 2008. The management also noticed that the net balance of the trade receivable, as of March 31, 2008, was a significant asset to the company. However, the management believes that the above situation is temporarily due to the following reasons:
Trade receivables
| | a) | Customers whom have sales relationship with our company are all relatively big business wholesale enterprises and they have all passed the examination of GMP Certificate so that the collectibility from those is out of question; |
| | b) | Due to the fact that there was a long Chinese New Year holiday and a big snowing in the first quarter of 2008, it did affect the collection of trade receivables to certain extend; and |
| | c) | The management realized that it did affect the cash flow situation of the company, therefore the company will put more efforts to reduce the balance of trade receivables starting from the second quarter of 2008. |
Inventories
During the reporting period three month ended March 31, 2008, the net changes of inventories was about $760K which was due to the increase of the purchase of raw materials in SiBiono and Benda Ebei which was incurred under the normal course of production.
Tax Payable
During the reporting period three months ended March 31, 2008, an amount $481K was paid for income tax which incurred in Benda Ebei.
Investing cash outflow was remained the same level for the reporting periods, three month ended March 31, 2008 and 2007 and the amounts were $214K and $267K respectively. The funding was mainly used in the purchase of property and equipment and the payment of construction-in-progress.
Financing cash inflow was $5.3 million for the reporting period three month ended March 31, 2007. The major event of it was an amount $5.52 million was received from the issuance of convertible promissory notes. For the reporting period three month ended March 31, 2007, the financing cash inflow was $1.6 million. The major of it was a net amount $1.5 million was received from the commercial bank notes; please refer to the Note 11 of the Notes to Consolidated Financial Statements for the details. Year Ended December 31, 2007
Critical Accounting Policies
Accounting policies discussed in this section are those that we consider to be most critical to an understanding of our financial statements because they inherently involve significant judgment and uncertainties. For all of these estimates, we caution that future events rarely develop exactly as forecast, and the best estimates routinely require adjustment. Also see note 4, Summary of Significant Accounting Policies.
Revenue Recognition
Among the most important accounting policies affecting our consolidated financial statements is our policy of recognizing revenue in accordance with the SEC's Staff Accounting Bulletin ("SAB") No. 104. Under this policy, all of the following criteria must be met in order for us to recognize revenue:
1. Persuasive evidence of an arrangement exists;
2. Delivery has occurred or services have been rendered;
3. The seller's price to the buyer is fixed or determinable; and
4. Collectibility is reasonably assured.
The majority of the Company's revenue results from sales contracts with distributors and revenue is recorded upon the shipment of goods. Management conducts credit background checks for new customers as a means to reduce the subjectivity of assuring collectibility. Based on these factors, the Company believes that it can apply the provisions of SAB 104 with minimal subjectivity.
Estimates Affecting Accounts Receivable and Inventories
The preparation of our consolidated financial statements requires management to make estimates and assumptions that affect our reporting of assets and liabilities (and contingent assets and liabilities). However, it is explicated that the changes in estimation were not material in the preparation of our consolidation financial statements.
For the year ended December 31, 2007 and 2006, the Company provided for the allowance for doubtful accounts of $943,667 and $467,015, respectively, against accounts receivable. Management's estimate of the appropriate allowance on accounts receivable for the reporting periods was based on the aged nature of these accounts receivable. In making its judgment, management assessed its customers' ability to continue to pay their outstanding invoices on a timely basis, and whether their financial position might deteriorate significantly in the future, which would result in their inability to pay their debts to the Company.
Inventories, which are primarily comprised of raw materials, packaging materials, and finished goods, are stated at the lower of cost or net realizable value. Cost being determined on the basis of a moving average. The Group evaluates the need for reserves associated with obsolete, slow-moving and non-salable inventory by reviewing net realizable values on a periodic basis.
For the year ended December 31, 2007 and 2006, the Company provided for a reserve against its work-in-progress amounting to $4,422,014 and $0, respectively.
The provision of reserve was resulted from the manufacturing process of Gendicine, SiBiono’s sole product and SiBiono was acquired by the company in April 2007.
The following chart shows the manufacturing process of Gendicine (Ad-p53):
In the production process of finished goods, Gendicine, several working steps are needed: (i) large-scale culturing of adenovirus from master adenovirus bank; (ii) culturing of cell from master cell bank; (iii) purification. The whole process including step (i) to step (iii) takes approximately twenty-four days to make reagent (“original liquid”). This particular liquid can only be stored for approximately five years. It takes approximately another seven days for mixing and bottling original liquid to finished goods which is known as Gendicine.
Therefore, up to the stage of reagent, all the related production costs are treated as work-in-progress. The major components of those production costs are: (i) direct labor; (ii) direct materials; (iii) power; (iv) supplies and other materials; and (v) manufacturing overheads.
Before acquisition, as of March 31, 2007, the accumulated units of original liquid produced was 198,075 and which could be converted to approximately 226,736 vials of Gendicine. However, the accumulated vials of Gendicine sold throughout the years 2004 to three-month period ended March 31, 2007 were only approximately 18,424 vials. Thus the accumulated production costs of $4,080,644 were remained as work-in-process as of three-month period ended March 31, 2007.
Furthermore, due to the special feature of the original liquid which can only be stored for five years, and most of the original liquid was produced in the year of 2004, and the provision of reserve on work- in-progress was $3,696,083 as of three-month period ended March 31, 2007.
After the acquisition with the effective date April 1, 2007, the same accounting treatment was adopted for the treatment of the provision of reserve on work-in-progress. As of December 31, 2007, the provision of reserve on work-in-progress was $4,422,014.
However, no reserve for obsolete, slow-moving or non-salable inventory was required for the reporting periods. Management determination of this allowance was based on potential impairments to the current carrying value of the inventories due to potential obsolescence of aged inventories. In making its estimate, management considered the probable demand for our products in the future and historical trends in the turnover of our inventories.
While the Company currently believes that there is little likelihood that actual results will differ materially from these current estimates.
Operational Results
Year ended December 31, 2007 Compared to Year ended December 31, 2006
The following table provides key components of our operational results for the year ended December 31 for Benda Pharmaceutical, Inc.
| | | December 31, | | December 31, | |
| | | 2007 | | 2006 | |
| | | (Restated) | | (Restated) | |
| Revenue | | $ | 26,384,608 | | $ | 15,932,075 | |
| Other Sales | | | - | | | 2,937 | |
| Cost of goods sold | | | (13,421,697 | ) | | (9,103,515 | ) |
| Gross profit | | | 12,962,911 | | | 6,831,497 | |
| | | | | | | | |
| Selling expenses | | | (1,581,655 | ) | | (599,571 | ) |
| | | | | | | | |
| General and administravive expenses | | | (13,901,577 | ) | | (2,453,604 | ) |
| Gains / (losses) on disposals of fixed assets | | | (30,027 | ) | | (249,381 | ) |
| Research and development expenses | | | (553,962 | ) | | (30,821 | ) |
| Total operating expenses | | | (16,067,221 | ) | | (3,333,377 | ) |
| Operating income / (loss) | | | (3,104,310 | ) | | 3,498,120 | |
| | | | | | | | |
| Interest expenses | | | (3,389,676 | ) | | (108,811 | ) |
| Other income (expenses) | | | 55,964 | | | (281,190 | ) |
| Government subsidies / grants | | | 2,276,574 | | | - | |
| | | | | | | | |
| Income / (loss) before minority interest and income taxes | | | (4,161,448 | ) | | 3,108,119 | |
| Income taxes | | | (965,183 | ) | | - | |
| Minority interest | | | (2,236,194 | ) | | (322,590 | ) |
| | | | | | | | |
| Net Income / (loss) | | $ | (7,362,825 | ) | $ | 2,785,529 | |
| | | | | | | | |
| Earnings / (loss) per share - basic | | $ | (0.07 | ) | $ | 0.04 | |
| | | | | | | | |
| Weighted average shares outstanding - basic | | | 98,181,685 | | | 73,414,057 | |
| | | | | | | | |
| Earnings / (loss) per share - diluted | | $ | (0.06 | ) | $ | 0.04 | |
| | | | | | | | |
| Weighted average shares outstanding - diluted | | | 127,534,003 | | | 74,864,283 | |
Restatement
The Company restated its previously recognized Depreciation expense by($1,446,185) and ($718,387) for the year ended December 31, 2007 and 2006 due to incorrect calculation of depreciation expense previously reported.
The Company restated its previously recognized Amortization expense by ($572,491) and ($254,990) for the year ended December 31, 2007 and 2006 due to incorrect calculation of amortization expense previously reported.
Net Revenue:
The Company has five core operating segments: Benda Ebei, Jiangling Benda, Yidu Benda, Beijing Shusai and SiBiono. Benda Ebei manufactures branded/generic medicines; Jiangling Benda manufactures active pharmaceutical ingredients (API); Yidu Benda manufactures bulk chemicals; Beijing Shusai operates and distributes Pharyngitis Killer Therapy; and SiBiono is a gene therapy company dedicated to the development, manufacturing and commercialization of gene therapy product, Gedicine.
Net revenue increased by $10.46 million (or 66%) to $26.38 million for the year ended December 31, 2007, from $15.93 million for the year ended December 31, 2006. Increase in revenue is principally attributed by the following factors:
| 5. | One of the Benda’s subsidiary, Benda Ebei’s net revenue increased $9.7 million (or 101%) to $19.3 million for the year ended December 31, 2007 from $9.6 million for the year ended December 31, 2006. It was mainly due to the increase sales of the existing major products and the introduction of new products and they are shown in the table below: |
| | | December 31, | | December 31, | | Variance | | Variance | |
| Products (Million) | | 2007 | | 2006 | | + / (-) | | + / (-) % | |
| Suzheng-B | | $ | 3.38 | | $ | 1.93 | | $ | 1.45 | | | 75 | % |
| Shusai-A | | | 2.13 | | | 0.95 | | | 1.18 | | | 125 | % |
| Xujing | | | 2.90 | | | 2.45 | | | 0.45 | | | 19 | % |
| Vitamin B6 and B1 | | | 0.79 | | | 0.21 | | | 0.58 | | | 284 | % |
| Gentamycin 80 Thousand | | | 0.59 | | | - | | | 0.59 | | | 100 | % |
| Anagin | | | 0.56 | | | - | | | 0.56 | | | 100 | % |
| Lincomycin | | | 1.33 | | | - | | | 1.33 | | | 100 | % |
| Kanamycin | | $ | 0.33 | | $ | - | | $ | 0.33 | | | 100 | % |
| 6. | One of the Benda’s subsidiary, Jiangling Benda’s net revenue increased $0.51 million (or 3,315%) to $0.53 million for the year ended December 31, 2007 from $0.02 million for the year ended December 31, 2006. It was mainly due to Jiangling Benda resumed its production in October 2007. The sole product that being sold in the reporting period of the year 2007 was Ribose which does not required the GMP certificate. Furthermore, the upgrade process of Jiangling Benda had been completed and is now waiting for the China’s State Food and Drug Administration (SFDA)’s approval for GMP certificate. However, due to the overhaul and restructuring of SFDA, the management could not estimate the exact timing for obtaining GMP certificate. |
Jiangling Benda plans to produce four types of active pharmaceutical ingredients and they are Ribavirin, Asarin, Levofloxacin and Ribose whereas the production of Ribose does not require the GMP certificate, but the production of the other three products do require the GMP certificate.
| 7. | One of the Benda’s subsidiary, Yidu Benda’s net revenue dropped $5.54 million (or 89%) to about $700,000 for the year ended December 31, 2007 from $6.2 million for the year ended December 31, 2006 due to the fact that the plant was temporarily closed since mid January to upgrade its waster water treatment system to comply with new environmental standards enforced by PRC local government. |
Yidu Benda has completed its upgrading of the waster water system and passed the government’s verification and testing of equipments in October 2007. It is now permitted for the testing on actual production process. Once the actual products are produced, then the environmental government bodies will re-test the production results. The management could not estimate the exact timing for obtaining the final approval on the actual production process.
| 8. | One of the Benda’s subsidiary, Beijing Shusai which incorporated on July 15, 2006 realized net revenue of about $44,000 for the year ended December 31, 2007. China’s State Food and Drug Administration (SFDA) recently experienced an overhaul in its policies and regulatory systems in an effort to fight against corruption in Chinese pharmaceutical industry. Beijing Shusai’s operation has been adversely affected by this recent policy changes which prohibits some state-owned hospitals from forming alliances with private companies. The management could not estimate that such situation could be resolved in the coming future. |
| 5. | Benda’s newly acquired entity SiBiono (acquired and effective on April 1, 2007) realized net revenue of $5.76 million for the year ended December 31, 2007. SiBiono’s flagship product, Gendicine is the commercialized gene therapy product for the treatment of cancer. While only 1,869 vials of Gendicine were sold in the first quarter in 2007 by SiBiono, sales ramped to 21,020 vials in the nine months ended December 31, 2007 after Benda took over SiBiono in April 2007. |
Cost of Goods Sold
Cost of goods sold increased $4.32 million (or 45%) to 13.42 million for the year ended December 31, 2007 from $9.1 million for the year ended December 31, 2006, primarily due to the corresponding increase in net revenue.
Gross Profit
Gross profit increased $6.13 million (or 96%) to $12.96 million for the year ended December 31, 2007 from $6.83 million for the year ended December 31, 2006, which was mainly due to the contribution of the gross profit of SiBiono, at about $5.27 million, which was acquired and effective on April 1, 2007,.
Selling Expenses:
Selling expenses increased $982K (or 164%) to $1.58 million for the year ended December 31, 2007 from $600K for the year ended December 31, 2006, primarily due to the promotional fees for Gendicine.
General and Administrative Expenses:
General and administrative expenses increased $11.4 million (or 456%) to $13.9 million for the year ended December 31, 2007 from $2.5 million for the year ended December 31, 2006. The following table shows the major expenses that significantly increase the G&A expenses during the reporting periods:
| | | December 31, | | December 31, | | | |
| Note Major general & administrative expenses | | 2007 | | 2006 | | Variance | |
| 1 | | | Amortization on debt issue cost | | $ | 201,255 | | $ | - | | $ | 201,255 | |
| 2 | | | Finder fees to China Hi-Tech Funds Co. Ltd. | | | 243,590 | | | - | | | 243,590 | |
| 3 | | | Consulting and professional fees | | | 8,353,615 | | | 326,403 | | | 8,027,212 | |
| 4 | | | Penalty to investors | | | 1,022,275 | | | - | | | 1,022,275 | |
| 5 | | | Advertising | | | 93,747 | | | 3,942 | | | 89,805 | |
| 6 | | | Legal fees | | | 304,649 | | | 114,993 | | | 189,656 | |
| 7 | | | Office expenses | | | 478,383 | | | 210,696 | | | 267,687 | |
| 8 | | | Salaries and wages | | | 961,797 | | | 221,174 | | | 740,623 | |
| 9 | | | Conference | | | 79,754 | | | 18,495 | | | 61,259 | |
| 10 | | | Consulting | | | 221,044 | | | 191,248 | | | 29,796 | |
| 11 | | | Investor relation, transfer agent and filing fees | | | 109,540 | | | 9,828 | | | 99,712 | |
| 12 | | | Director Remuneration | | | 94,247 | | | - | | | 94,247 | |
| 13 | | | Travel and transportation | | | 239,316 | | | 87,264 | | | 152,052 | |
| | | | | | $ | 12,403,212 | | $ | 1,184,043 | | $ | 11,219,169 | |
Note:
| 1. | See Note 23 of Notes to Consolidated Financial Statements for details; |
| 2. | Finder fee to China Hi-Tech Funds Co. Ltd; |
| 3. | See Note 18 of Notes to Consolidated Financial Statements for details; |
| 4. | Being the penalty for late filing 10K-SB and registration statement, see Note 19 of Notes to Consolidated Financial Statements for details; |
| 5. | The increment mainly incurred for the investor relationship activities; |
| 6. | The increment mainly incurred in SiBiono and the other subsidiaries of the Group; |
| 7. | The increment mainly incurred for the re-opening of Jiangling Benda and the expenses incurred in the newly acquired subsidiary, SiBiono; |
| 8. | The increment mainly due to the involvement of CEO, COO and CFO salaries starting in the Year of 2007 and the salary expenses incurred in the newly acquired subsidiary, SiBiono. |
| 9. | The increment mainly incurred for the events including road shows and gene therapy conferences; |
| 10. | The increment mainly due to the expenses incurred in the newly acquired subsidiary, SiBiono; |
| 11. | The increment mainly incurred for the investor relationship activities; |
| 12. | It is only incurred starting from the year of 2007; |
| 13. | The increment mainly incurred for the investor relationship activities. |
Operating Income/ (Loss):
The Company experienced an operating loss of $3.1 million for the year ended December 31, 2007, slipping $6.6 million from comparative period for 2006, which was mainly due to significant increase in G&A expenses.
Interest Expenses:
Interest expenses increased to $3.4 million for the year ended December 31, 2007 from $109,000 for the year ended December 31, 2006 primarily because of the financing cost associated with the issuance of convertible promissory note (please refer to Note 24 of the Notes to Consolidation Financial Statements for details).
Government Subsidies / Grants:
The Company recognized $2.3 million as government subsidies / grant for the year ended December 31, 2007 (please refer to Note 22 of the Note to Consolidated Financial Statements for details).
Income Taxes:
Benda is subject to Delaware, United State of America tax, but no provision for income taxes were made for the years ended December 31, 2007 and 2006 as Benda did not have reportable taxable income for the period in the United States of America.
Ever Leader, a wholly owned subsidiary of Benda, is subject to Hong Kong tax, but no provisions for income taxes were made for the years ended December 31, 2007 and 2006 as Ever Leader did not have reportable taxable income for the periods.
Benda Ebei was registered as a Sino-Foreign Equity Joint Venture on May 26, 2004 and is subject to the tax laws applicable to Sino-Foreign Equity Joint Ventures in the PRC. Benda Ebei, starting from 2005, is fully exempt from PRC enterprise income tax for two years starting from the first profit-making year, followed by a 50% reduction in income taxes, at 18%, for the following three years, commencing from the first profitable year. In 2007, Ebei Benda reported corporate income taxes of $965,183 with a corporate taxes payable of $246,460 as of December 31, 2007.
Jiangling Benda and Yidu Benda are cross-municipal investment entities and enjoy the same tax treatment as Sino-Foreign Joint Ventures, starting from 2005, and were therefore exempt from PRC enterprise income tax for two years starting from the first profit-making year, followed by a 50% reduction in income taxes, at 18%, for the following three years, commencing from the first profitable year. Cross-municipal investments entities refer to entities that are incorporated in one municipal region but have investments in another municipal region.
The exemption periods for Benda Ebei, Jiangling Benda and Yidu Benda expired in the year of 2006, after which they are subject to 50% reduction in income taxes; whereas the full income tax rate is 33%. The remaining tax holidays will be expired in 2010.
However, starting and effective from January 1, 2008, the full income tax rate is changed from 33% to 25% according to the new PRC Taxation Regulations. Thereafter these subsidiaries will be subject to the regular 25%.
Beijing Shusai did not have taxable income for the years ended December 31, 2007 and 2006.
According to the taxation regulations of Shenzhen, a Special Economic District of PRC, SiBiono is subject to the full income tax rate of 15% on taxable income. The net losses for the previous year can be carried forward for a maximum period of five years. If the company is approved and recognized as high-tech company, the company can enjoy three years of 50% of the full tax rate with an extension for the coming next three years. Therefore, there was no provision for income taxes made for the year ended December 31, 2007. SiBiono also plans to apply for the PRC enterprise tax exemption for two years starting from the first profit-making year, followed by a 50% reduction in income taxes for the following three years.
As a result, Benda reported corporate income tax expense of $965,183 and $0, as well as corporate income taxes payable of $246,460 and $0, for the years ended December 31, 2007 and 2006, respectively.
LIQUIDITY AND CAPITAL RESOURCES
Operating cash flow decreased by $0.26 million to $2.21 million for the year ended December 31, 2007 from $2.47 million for the year ended December 31, 2006 primarily due to the following factors:
| c) | Non-cash operating activities, reconciliation items to the net income |
In the year ended December 31, 2006, an amount $1.74 million of non-cash operating activities was reconciled back to the net income and which mainly included bad debt provision, amortization of intangible assets, depreciation, minority interest and consulting and professional fees which was paid in form of common shares issuance.
However, in the year ended December 31, 2007, a significant amount, about $13.8 million of non-cash operating activities was reconciled back to the net income and summarized as follows:
| | 4. | $8.4 million that incurred for the issuance of common shares related to the acquisition of SiBiono and the exercise of warrant of placement agent through the cashless arrangements (please refer to Note 19 of the Notes to Consolidated Financial Statements for details); |
| | 5. | $2.9 million that incurred as interest expenses related to the amortization of discount on long-term debt and issuance cost associated with the warrants and the beneficial conversion features (please refer to Note 24 of the Notes to Consolidated Financial Statements for details); |
| | 6. | During the year of 2007, SiBiono recognized an amount $2.3 million as government subsidies/grants as other income. This amount was obtained from the partial discharge of the long term government debt payable as the government agencies had examined the corresponding research and development projects. However, this amount should not be included in the operating activities as there was no actual cash inflow impact (please refer to Note 22 of the Notes to Consolidated Financial Statements for details); and |
| | 7. | Other factors: $0.3 million incurred on bad debt provision; $1 million incurred on depreciation; $0.5 million incurred on amortization of intangible assets; $0.2 million incurred on amortization of debt issue cost; and $2.2 million incurred on minority interest. |
In the year ended December 31, 2006, the net changes of trade receivable was increased by $2.2 million, and the changes of trade receivable was increased by $4.4 million in the year ended December 31, 2007 resulting from the significant increase in net revenue in 2007. According to the aging analysis of trade receivable as of December 31, 2007, about 90% of the balances were incurred less than 90 days, thus management expects that there would be no material bad debt provision would be incurred in the following year.
Investing cash outflow was $11.1 million for the year ended December 31, 2007 due to the following events:
| | a) | $2.67 million as the partial payment of the total consideration for SiBiono acquisition; |
| | b) | $5.3 million for fixed asset additions, mainly in equipment and construction in progress; and |
| | c) | $3.1 million for the purchase of new drug license and land use right. |
Financing cash inflow was $8.2 million in the year ended December 31, 2007 caused by:
| 1. | The proceeds from issuance of convertible promissory note, the net proceeds was $7.03 million. |
| 2. | The net proceeds from the issuance of commercial bank note. As of December 31, 2007, Benda Ebei and Jiangling Benda deposited an amount $2,615,254 in Shanghai Pudong Development Bank as deposit for the issuance of commercial bank notes. Such deposits will be released when the commercial bank notes are cleared. As of December 31, 2007, the balance if the commercial bank notes payable was $5,118,758. Thus the net commercial bank notes payable was $2,503,504 as of December 31, 2007 (please refer to Note 11 of Notes to Consolidated Financial Statements for details). |
DESCRIPTION OF PROPERTY
Production Facilities and Equipment
Locations
Our head office is located in Wuhan, the capital city of Hubei Province. Wuhan is the biggest hub city in Central China. Divided by the Yangtze River, Wuhan has come to be known as the Three Towns of Wuhan, with Hankou and Hanyang on the west bank, and Wuchang on the east. Hubei borders with Henan to the north, Anhui to the east, Jiangxi to the southeast, Hunan to the south, Chongqing to the west, and Shanxi to the northwest. The high-profile Three Gorges Dam is located in Yichang, in western Hubei. Hubei's economy ranks 10th in the country and its GDP in 2004, adjusted for purchasing power parity, was approximately 330 billion RMB ($4,600 per capita).
Addresses of Benda Offices and Plants
Address | | Function | | Size |
Hubei Tongji Benda Ebei Pharmaceutical Co. Ltd., Sunny New World Tower, 25th Floor, 231 Xinhua Road, Wuhan, Hubei Province, PRC | | Headquarters/ administration office | | 800 sq feet (Leased) |
| Hubei Tongji Benda Ebei Pharmaceutical Co. Ltd. Shanlihe,Yingshan District, Guangshui City, Hubei Province, PRC | | GMP certified injection vial production plant | | 121,110 sq feet |
| Jiangling Benda Pharmaceuticals Co., Ltd., 84 South Street, Tanqiao Town, Jiangling County, Hubei Province, PRC | | API production plant under renovation/ awaiting GMP certification | | 182,750 sq feet |
| Yidu Chemical Industry Co., Ltd., Chayuansi Village, Yidu City, Hubei Province, PRC | | Bulk chemicals production plant | | 172,000 sq feet |
| Beijing Shusai Pharyngitis Research Co., Ltd., 6-3-601, Yangguang Xinganxian, Yiyuan, Anhuibeili, Chaoyang District, Beijing, PRC | | Operating, promoting, and distributing Pharyngitis Killer | | 2,000 sq feet (leased) office space in Beijing |
Finished Medicines - The Benda Ebei Plant
The Benda Ebei plant produces and packages Benda’s finished medicines. The plant employs about 200 personnel and has six world-class production lines and 11 stand-alone machinery for injection vials, with an annual aggregate production capacity of approximately 900 million units. In PRC, based on management estimate, there are 197 factories with similar manufacturing facilities for injection vials. Benda Ebei’s capacity is sixth in PRC and first in Hubei province. In 2006 and 2007, Benda Ebei produced approximately 250 million and 418 million units of injection vials respectively, equivalent to 27% capacity and 90% capacity respectively. The production processes and equipment at Benda Ebei meets international quality and control standards and have been GMP certified by the SFDA since November 2003.
Benda Ebei’s plant will produce lozenges, capsules, granules, oral liquid and pills. Benda Ebei has invested approximately $5 million on its oral medicine production facilities. To date, we have already completed the equipment and facilities installation We expect the production of oral liquid medicines in second quarter of 2008. The Yanlong Anti-cancer Oral Liquid, Qiweiben Capsule will be produced in this section of the plant. (Please refer to the section of New Branded Medicines for the status of progress of these two new drugs.)
Active Pharmaceutical Ingredients - The Jiangling Benda Plant
We manufacture APIs at our Jiangling Benda plant. We closed the plant in July 2004 in order to renovate the buildings, equipment and processes and secure GMP certification by the SFDA. Upon completion, the new plant will be comprised of three large facilities on a total land area of 68 acres. The total expense of these renovations will be approximately $3 million. All the equipment has been ordered.
The upgrade process of Jiangling Benda had been completed and re-opened in August 2007 and is now waiting for the China’s State Food and Drug Administration (SFDA)’s approval for GMP certificate. However, due to the overhaul and restructuring of SFDA, the management could not estimate the exact timing for obtaining GMP certificate.
The sole product that being sold in the reporting period of the year 2007 was Ribose which does not required the GMP certificate but the production of the other three products do require the GMP certificate.
Bulk chemicals - The Yidu Benda Plant
The Yidu Benda plant has four chemical production facilities which produce Benda’s two bulk chemicals.
Yidu Benda was temporarily closed since mid January 2007 to upgrade its waster water treatment system to comply with new environmental standards enforced by PRC local government.
Yidu Benda has completed its upgrading of the waster water system and passed the government’s verification and testing of equipments in October 2007. It is now permitted for the testing on actual production process. Once the actual products are produced, then the environmental government bodies will re-test the production results. The management could not estimate the exact timing for obtaining the final approval on the actual production process.
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS
Described below are certain transactions or series of transactions since inception between Applied Spectrum and our subsidiaries and our executive officers, directors and the beneficial owners of 5% or more of our common stock, on an as converted basis, and certain persons affiliated with or related to these persons, including family members, in which they had or will have a direct or indirect material interest in an amount that exceeds $60,000 other than compensation arrangements that are otherwise required to be described under "Executive Compensation."
Kevin R. Keating
Keating Securities, LLC (the “Placement Agent”) acted as placement agent in connection with the Financing. For their services, the Placement Agent received a commission equal to 7.5% of the gross proceeds or approximately $900,000 from the offering and a non-accountable expense allowance equal to 1.5% of the gross proceeds or approximately $180,000. In addition, the Placement Agent received, for nominal consideration, five-year warrants to purchase 2,596,176 shares of our common stock, or 10% of the number of shares of Common Stock sold in the offering, at an exercise price of $0.555 (“Placement Agent Warrants”). We also paid for the out-of-pocket expenses incurred by the Placement Agent and all purchasers in the amount of approximately $100,000. As additional compensation for the Placement Agent's services, we will also pay the Placement Agent the Warrant Solicitation Fee with respect to the exercise, in whole or in part, of any Warrant equal to 3.0% of the total exercise price of the Common Stock issued in such exercise of such Warrant. Such cash Warrant Solicitation Fees shall be paid to the Placement Agent, in immediately available funds, within three (3) business days following receipt, directly or indirectly, by us, of any cash or other proceeds from the exercise of such Warrant.
At or prior to the Closing, pursuant to the terms of the Exchange Agreement, we will enter into a certain financial advisory agreement with Keating Securities, LLC ("Keating Securities"), a registered broker-dealer, under which Keating Securities will be compensated by us for its advisory services rendered to us in connection with the Exchange Agreement. The transaction advisory fee will be $395,000. This fee shall be paid upon the Closing of the Exchange Agreement.
Kevin R. Keating, our former President and sole officer and director who resigned on November 15, 2006, is the father of Timothy J. Keating, the principal member of Keating Investments, LLC. Keating Investments, LLC is the managing member of KI Equity, which is our majority stockholder. Keating Investments, LLC is also the managing member and 100% owner of Keating Securities, LLC, a registered broker-dealer. Kevin R. Keating is not affiliated with and has no equity interest in Keating Investments, LLC, KI Equity, or Keating Securities, LLC and disclaims any beneficial interest in the shares of our common stock owned by KI Equity. Similarly, Keating Investments, LLC, KI Equity and Keating Securities, LLC disclaim any beneficial interest in the shares of our common stock currently owned by Kevin R. Keating.
Effective January 1, 2006, we entered into a contract with Vero Management, L.L.C. ("Vero") for managerial and administrative services. Vero has not been engaged to provide, and Vero does not render, legal, accounting, auditing, investment banking or capital formation services. Kevin R. Keating, our former officer and director, is the manager of Vero. The term of the contract is for one year, but the contract may be terminated at any time. In consideration of the services provided, Vero is paid $2,500 for each month in which services are rendered.
Mark R. Littell and Norwood
On December 14, 2005, KI Equity entered into a Purchase Agreement with Norwood Venture Corp. (“Norwood”) for 2,281,302 shares of our common stock, representing 77.2% of the common shares then outstanding. Mark R. Little, our former Chairman, Chief Executive Officer, President and Chief Financial Officer who resigned on December 30, 2005, is the President and controlling shareholder of Norwood. In connection with the Purchase Agreement, Mark R. Littell and Norwood entered into an agreement releasing Applied from any and all claims they have against us.
During 2006, Norwood paid an accrued legal expense on behalf of Applied in the amount of $1,568, which was recorded as additional paid-in capital.
In connection with the Purchase Agreement, we paid Norwood approximately $18,936 for consulting services rendered by it to Applied Spectrum.
Related Party Transactions and Long Term Loan Receivable
Due from related parties at December 31, 2007 and 2006 were comprised as follows:
| | | December 31, | | December 31, | |
| | | 2007 | | 2006 | |
| Yiqing, Wan | | | | | | | |
| Due to Ever Leader Holdings Co. Ltd. | | $ | 646,429 | | $ | 455,275 | |
| Due to Hubei Tongji Benda Ebei Phamacetucial Co. Ltd. | | | 72,949 | | | - | |
| Due to Shenzhen SiBiono Gene Tech Co. Ltd. | | | 3,608 | | | - | |
| Hubei Benda Science and Technology Co. Ltd | | | | | | | |
| Due to Yidu Benda Chemicals Co. Ltd. | | | 1,502,118 | | | 1,299,479 | |
| Due to Ever Leader Holdings Co. Ltd. | | | 230,160 | | | 210,518 | |
| Feng Wang | | | | | | | |
| Due to Beijing Shusai Pharyngitis Research Co. Ltd. | | | 29,318 | | | 11,543 | |
| Hua Shen | | | | | | | |
| Due to Shenzhen SiBiono Gene Tech Co. Ltd. | | | 137,097 | | | - | |
| Pong Tsaiohuei | | | | | | | |
| Due to Shenzhen SiBiono Gene Tech Co. Ltd. | | | 3,257 | | | - | |
| Xiaozhi Zhang | | | | | | | |
| Due to Shenzhen SiBiono Gene Tech Co. Ltd. | | | 5,083 | | | - | |
| Total due from related parties | | $ | 2,630,019 | | $ | 1,976,815 | |
Due to related parties at December 31, 2007 and, 2006 were comprised as follows:
| | | December 31, | | December 31, | |
| | | 2007 | | 2006 | |
| Hubei Benda Science and Technology Co. Ltd | | | | | | | |
| Due from Hubei Tongji Benda Ebei Phamacetucial Co. Ltd. | | $ | 49,056 | | $ | 236,205 | |
| Due from Jiangliang Benda Pharamaceutical Co. Ltd. | | | 1,872,374 | | | 1,833,358 | |
| Due from Beijing Shusai Pharyngitis Research Co. Ltd. | | | 6,846 | | | - | |
| Wei Xu | | | | | | | |
| Due from Hubei Tongji Benda Ebei Phamacetucial Co. Ltd. | | | 1,009,792 | | | 943,865 | |
| Due from Beijing Shusai Pharyngitis Research Co. Ltd. | | | 61,491 | | | 20,937 | |
| Yiqing, Wan | | | | | | | |
| Due from Shenzhen SiBiono Gene Tech Co. Ltd. | | | 137,687 | | | - | |
| Hui Xu | | | | | | | |
| Due from Hubei Tongji Benda Ebei Phamacetucial Co. Ltd. | | | 26,622.00 | | | - | |
| Due from Beijing Shusai Pharyngitis Research Co. Ltd. | | | 3,153.00 | | | - | |
| Hua Shen | | | | | | | |
| Due from Shenzhen SiBiono Gene Tech Co. Ltd. | | | 26,597.00 | | | - | |
| Total due to related parties | | $ | 3,193,618 | | $ | 3,034,365 | |
The above advances bear no interest and the above loans due to related parties are unsecured, non-interest bearing and are not convertible into equity. Proceeds from the above loans were used primarily for general working capital purposes and are also long-term debts in nature, due on December 31, 2012.
Reorganization Related Transactions
Ever Leader was incorporated in Hong Kong on October 29, 2005 for the purpose of functioning as an off-shore holding company to obtain ownership interests in various Benda entities that were previously owned, either directly or indirectly, by Wan and Xu. Ms. Mo Mo Hon (“Hon”), a Hong Kong SAR resident, is the sole registered shareholder of Ever Leader, holding the single issued and outstanding share of Ever Leader in trust for Xu.
Pursuant to three separate Equity Transfer Agreements entered into in November of 2005 among Ever Leader, Benda Science, Xu, and Wan, Ever Leader obtained a 95% ownership interest in Benda Ebei in exchange for a commitment to pay $2,298,434 in aggregate consideration to Benda Science, Wan, and Xu. The $2,298,434 acquisition price represented 95% of the $2,419,404 of registered capital of Benda Ebei, but was not representative of the fair value of the assets acquired or liabilities assumed. Specifically, as transfers of ownership interests in PRC entities to offshore holding companies for zero or nominal consideration is prohibited by the Chinese Government (regardless of whether these PRC entities and offshore holding companies are directly or indirectly owned and controlled by the same individual or individuals), an amount equal to 95% of the value of the registered capital of Benda Ebei was established for purposes of the transfer of the 95% ownership interest in Benda Ebei (directly and indirectly 100% owned and controlled by Wan and Xu) to Ever Leader (beneficially 100% owned and controlled by Xu).
Pursuant to an Equity Transfer Agreement entered into on December 3, 2005 among Benda Ebei, Benda Science, and Wan, Benda Science transferred and assigned its 90% ownership interest in Jiangling Benda to Benda Ebei and Wan transferred and assigned a 5% ownership interest in Jiangling Benda to Benda Ebei (for zero consideration as Benda Ebei and Jiangling Benda were both directly and indirectly 100% owned and controlled by Wan and Xu).
Pursuant to a second Equity Transfer Agreement entered into on December 4, 2005 among Benda Ebei, Benda Science, and Wan, Benda Science transferred and assigned its 90% ownership interest in Yidu Benda to Benda Ebei and Wan transferred and assigned a 5% ownership interest in Yidu Benda to Benda Ebei (for zero consideration as Benda Ebei and Yidu Benda were both directly and indirectly 100% owned and controlled by Wan and Xu).
The organization and ownership structure of the Company subsequent to the consummation of the reorganization as summarized in the paragraphs above is as follows:
In July of 2006, Benda Ebei invested approximately $112,500 for a 75% ownership interest in Beijing Shusai, with the remaining 25% owned by an unrelated PRC individual. Beijing Shusai, a PRC limited liability company, was incorporated on June 15, 2006 and commenced primary operations in July 2006, operating two clinics in Beijing, PRC.
On September 5, 2006, Ever Leader increased its number of authorized shares of common stock from 10,000 to 1,000,000 and effected a 100 to 1 stock split, resulting in Hon (the original sole registered shareholder of Ever Leader holding one share in trust for Xu) receiving 99 additional shares in the Company.
On September 5, 2006, Ever Leader transferred and assigned 711,202 shares of common stock to Xia Pharmaceutical, Inc. (“XIA”), an offshore holding company incorporated in the British Virgin Islands (“BVI”) that is 100% owned and controlled by Wan and Xu.
On September 5, 2006, Ever Leader issued 288,698 shares of common stock to 19 entities (some of whom are considered related parties) at par value. Additionally, Hon transferred and assigned her ownership interest in her 100 shares of Ever Leader to one of these entities.
The organization and ownership structure of the Company subsequent to the consummation of the reorganization as summarized in the paragraphs above is as follows:
MARKET FOR COMMON EQUITY AND RELATED STOCKHOLDER MATTERS
Our common stock was traded on the national, over-the-counter market under the symbol ASTI after our initial public offering in January 1988. However, we were notified by NASD that, due to low trading volume, it would not report transactions in our common stock after October 13, 1989.
On September 16, 2005, our common stock commenced quotation on the Over-the-Counter Bulletin Board (“OTC BB”) maintained by the NASD. Our common stock, having $.001 par value per share ("Common Stock"), is traded on the Over-The-Counter Bulletin Board ("OTCBB") under the symbol "BPMA". As of March 27, 2008, there were approximately 841 holders of record of our common stock.
The following table sets forth, for the periods indicated, the reported high and low closing bid quotations for our common stock as reported on the OTCBB. The bid prices reflect inter-dealer quotations, do not include retail markups, markdowns or commissions and do not necessarily reflect actual transactions.
Quarter Ended | | High Bid | | Low Bid | |
| December 31, 2007 | | $ | 2.69 | | $ | .65 | |
| September 30, 2007 | | $ | 2.60 | | $ | 1.90 | |
| June 30, 2007 | | $ | 3.00 | | $ | 1.15 | |
| March 31, 2007 | | $ | 1.65 | | $ | .85 | |
| December 29, 2006 | | $ | 1.05 | | $ | .76 | |
| September 30, 2006 | | $ | .55 | | $ | .40 | |
| June 30, 2006 | | $ | .55 | | $ | .40 | |
| March 31, 2006 | | $ | .75 | | $ | .40 | |
| December 31, 2005 | | $ | .50 | | $ | .15 | |
| September 30, 2005 | | $ | .10 | | $ | .05 | |
Transfer Agent and Registrar
Computershare Trust Company, Inc. is currently the transfer agent and registrar for our Common Stock. Its address is 350 Indiana Street, Suite 800, Golden, Colorado 80401. Its phone number is (303) 262-0600.
Dividend Policy
Any future determination as to the declaration and payment of dividends on shares of our Common Stock will be made at the discretion of our board of directors out of funds legally available for such purpose. We are under no contractual obligations or restrictions to declare or pay dividends on our shares of Common Stock. In addition, we currently have no plans to pay such dividends. However, even if we wish to pay dividends, because our cash flow is dependent on dividend distributions from our affiliated entities in PRC, we may be restricted from distributing dividends to our holders of shares of our common stock in the future if at the time we are unable to obtain sufficient dividend distributions from Ebei, Jiangling, Yudi or Shusai. Our board of directors currently intends to retain all earnings for use in the business for the foreseeable future. See “Risk Factors.”
EXECUTIVE COMPENSATION
Summary of cash and other compensation
The following table shows the compensation paid over the past three fiscal years with respect to: (i) the Company’s President as of the end of the 2007 and 2006 fiscal year; (ii) the two other most highly compensated executive officers (in terms of salary and bonus) serving at the end of the 2007 and 2006 fiscal year whose annual salary and bonus exceeded $100,000; and (iii) up to two additional individuals who would be in category (ii) but for the fact that the individual was not serving as an executive officer of the Company at the end of the last completed fiscal year (the “named executive officers”):
SUMMARY COMPENSATION TABLE |
Name and principal position | | Year | | Salary ($) | | Bonus ($) | | Stock Awards ($) | | Option Awards ($) | | Non-Equity Incentive Plan Compensation ($) | | Nonqualified Deferred Compensation Earnings ($) | | All Other Compensation ($) | | Total ($) | |
| Yiqing Wan, CEO and President | | | 2007 | | $ | 160,000 | | | | | | | | | | | | | | | | | | | | $ | 160,000 | |
| | | | 2006 | | $ | 20,000 | | | — | | | — | | | — | | | — | | | — | | | — | | $ | 20,000 | |
| Wei Xu, Vice President | | | 2007 | | $ | 100,000 | | | | | | | | | | | | | | | | | | | | $ | 100,000 | |
| | | | 2006 | | $ | 17,500 | | | — | | | — | | | — | | | — | | | — | | | — | | $ | 17,500 | |
| Hui Long, Vice President | | | 2007 | | $ | 6,170 | | | | | | | | | | | | | | | | | | | | | 6,170 | |
| | | | 2006 | | $ | 6,170 | | | — | | | — | | | — | | | — | | | — | | | — | | $ | 6,170 | |
| Daping Gu, Vice President | | | 2007 | | $ | 6,000 | | | | | | | | | | | | | | | | | | | | | 6,000 | |
| | | | 2006 | | $ | 5,553 | | | — | | | — | | | — | | | — | | | — | | | — | | $ | 5,553 | |
| Jingbo Wu, Vice President | | | 2007 | | $ | 6,000 | | | | | | | | | | | | | | | | | | | | | 6,000 | |
| | | | 2006 | | $ | 3,085 | | | — | | | — | | | — | | | — | | | — | | | — | | $ | 3,085 | |
| YEric Yu, | | | 2007 | | $ | 89,000 | | | | | | | | | | | | | | | | | | | | $ | 89,000 | |
| CFO | | | 2006 | | $ | 0 | | | — | | | — | | | — | | | — | | | — | | | — | | $ | 0 | |
No stock options were granted or exercised by any executive officer during the fiscal year ended December 31, 2007.
For the year ended 31, 2007, we paid $94,247 as the director remuneration to our independent directors.
We have entered into a five year employment contract with Mr. Wan and three year contracts with Ms. Xu, Mr. Yu, and Mr. Long. Pursuant to the Employment Agreements entered into with the executive officers, starting from January 1, 2007, the compensation of CEO, CFO and Vice President, Wei Xu would be as follows:
| | 1. | CEO, Yiqing Wan’s yearly salary would be $160,000; |
| | 2. | CFO, Eric Yu’s yearly salary would be $100,000; |
| | 3. | Vice President, Wei Xu’s salary would be $100,000. |
| | 4. | The other Vice Presidents’ yearly salary would remain the same as the fiscal year of 2006. |
There would be no specific salary increment and bonus scheme for the above mentioned key managements for the next three years; it all depends on the profitability of the company and subject to the Board of Directors’ approval.
Each employment contract immediately terminates upon death or disability, and may be terminated by the Company either with or without cause after 30 days notice, or terminated by the officer for good reason with 60 days notice. We are not currently aware of the plans of any key employees to retire or leave the Company.
Director Compensation
The following table shows the compensation paid to all directors over the fiscal year ended December 31, 2007:
| Name | | Fees Earned or Paid in Cash ($) | | Stock Awards ($) | | Option Awards ($) | | Non-Equity Incentive Plan Compensation ($) | | Change in Pension Value and Nonqualified Deferred Compensation Earnings ($) | | All Other Compensation ($) | | Total ($) | |
| John Micek | | $ | 40,000 | | | — | | | — | | | — | | | — | | | — | | $ | 40,000 | |
| Q.Y. Ma | | $ | 39,315 | | | — | | | — | | | — | | | — | | | — | | $ | 39,315 | |
| Charles Mo | | $ | 14,932 | | | — | | | — | | | — | | | — | | | — | | $ | 14,932 | |
CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND
FINANCIAL DISCLOSURE
On November 14, 2006, we dismissed De Joya Griffith & Company, LLC (“De Joya”), as our independent registered public accounting firm.
The report of De Joya on the Company’s financial statement for the fiscal year ended September 30, 2005 did not contain an adverse opinion or disclaimer of opinion. During the fiscal year ended September 30, 2005, our financial statements did not contain any adverse opinions or disclaimers of opinion, and were not qualified or modified as to uncertainty, audit scope, or accounting principles. The Company and De Joya did not have any disagreements with regard to any matter of accounting principles or practices, financial statement disclosure, or auditing scope or procedure for the audited financials for the fiscal year ended September 30, 2005 and subsequent interim period from October 1, 2005 through the date of dismissal. During the Company’s fiscal year ended September 30, 2005 and subsequent interim period from October 1, 2005 through the date of dismissal, the Company did not experience any reportable events.
On November 14, 2006, Rotenberg & Co. LLP (“Rotenberg”) was engaged as our new independent registered public accounting firm. Prior to engaging Rotenberg, the Company had not consulted Rotenberg regarding the application of accounting principles to a specified transaction, completed or proposed, the type of audit opinion that might be rendered on the Company’s financial statements or a reportable event, nor did the Company consult with Rotenberg regarding any disagreements with its prior auditor on any matter of accounting principles or practices, financial statement disclosure, or auditing scope or procedure, which disagreements, if not resolved to the satisfaction of the prior auditor, would have caused it to make a reference to the subject matter of the disagreements in connection with its report.
Commencing January 2007, Rotenberg has decided to no longer undertake audits of Chinese based companies. Therefore, Schwartz Levitsky Feldman LLP, as Rotenberg's sub-contractor on the Company's audit shall be the Company's independent registered public accounting firm.
On February 8, 2007, we dismissed Schwartz Levitsky Feldman LLP (“SLF”) as our independent registered public accounting firm. SLF was previously appointed on January 9, 2007 as our independent auditors for the fiscal year ended September 30, 2006 on whose financial statements they rendered an unqualified opinion on January 11, 2007. On November 17, 2006, SLF was also engaged to audit the financial statements of Everleader for the fiscal years ended December 31, 2005 and 2004. Benda and SLF did not have any disagreements with regard to any matter of accounting principles or practices, financial statement disclosure, or auditing scope or procedure during the year ended September 30, 2006 for which SLF issued an audit report, and the subsequent interim period through February 8, 2007. As required by applicable Securities legislation, Benda engaged SLF to audit the financial statements of Everleader for the fiscal years ended December 31, 2005 and 2004. During the period of the engagement with SLF, neither Benda nor Everleader had retained a qualified Chief Financial Officer. Accordingly, neither Benda nor Everleader had the technical expertise and as a result was unable to provide sufficient audit documentation and/or information to allow SLF to complete the audit of Everleader for the years ended December 31, 2005 and 2004.
On March 7, 2007, we engaged Kempisty and Company Certified Public Accountants, P.C. (“Kempisty”) to be our new independent registered public accounting firm. Prior to engaging Kempisty, the Company had not consulted Kempisty regarding the application of accounting principles to a specified transaction, completed or proposed, the type of audit opinion that might be rendered on the Company’s financial statements or a reportable event, nor did the Company consult with Kempisty regarding any disagreements with its prior auditor on any matter of accounting principles or practices, financial statement disclosure, or auditing scope or procedure, which disagreements, if not resolved to the satisfaction of the prior auditor, would have caused it to make a reference to the subject matter of the disagreements in connection with its report.
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the SEC a registration statement on Form S-1 under the Securities Act with respect to the common stock being offered in this offering. This prospectus does not contain all of the information set forth in the registration statement and the exhibits and schedules filed as part of the registration statement. For further information with respect to us and our common stock, we refer you to the registration statement and the exhibits and schedules filed as a part of the registration statement. Statements contained in this prospectus concerning the contents of any contract or any other document are not necessarily complete. If a contract or document has been filed as an exhibit to the registration statement, we refer you to the copy of the contract or document that has been filed. Each statement in this prospectus relating to a contract or document filed as an exhibit is qualified in all respects by the filed exhibit. The reports and other information we file with the SEC can be read and copied at the SEC's Public Reference Room at 450 Fifth Street, N.W., Washington D.C. 20549. Copies of these materials can be obtained at prescribed rates from the Public Reference Section of the SEC at the principal offices of the SEC, 450 Fifth Street, N.W., Washington D.C. 20549. You may obtain information regarding the operation of the public reference room by calling 1(800) SEC-0330. The SEC also maintains a website (http://www.sec.gov) that contains reports, proxy and information statements, and other information regarding issuers that file electronically with the SEC.
After this offering, we will be subject to the information and periodic reporting requirements of the Securities Exchange Act of 1934, and we intend to file periodic reports, proxy statements and other information with the SEC.
BENDA PHARMACEUTICAL, INC.
(an exploration stage company)
FINANCIAL STATEMENTS
AS OF MARCH 31, 2008
CONTENTS
| PAGE | F1 | CONDENSED BALANCE SHEETS AS OF MARCH 31, 2008 (UNAUDITED) AND AS OF DECEMBER 31, 2007 (AUDITED). |
| | | |
| PAGE | F2 | CONDENSED STATEMENTS OF OPERATIONS FOR THE THREE MONTHS ENDED MARCH 31, 2008 AND 2007 (UNAUDITED). |
| | | |
| PAGE | F3 | CONDENSED STATEMENTS OF CASH FLOWS FOR THE THREE MONTHS ENDED MARCH 31, 2008 AND 2007 (UNAUDITED). |
| | | |
| PAGES | F4 - F32 | NOTES TO CONDENSED FINANCIAL STATEMENTS (UNAUDITED). |
Benda Pharmaceutical, Inc.
Consolidated Balance Sheets
(Amounts expressed in U.S. Dollars)
| | | March 31 | | | |
| | | 2008 | | December 31 | |
| | | Restated | | 2007 | |
| | | (Unaudited) | | Restated | |
Assets | | | | | | | |
| Current Assets | | | | | | | |
| Cash and cash equivalents | | $ | 1,401,178 | | $ | 1,266,240 | |
| Trade receivables, net (Note 5) | | | 11,215,913 | | | 10,472,233 | |
| Other receivables (Note 5) | | | 716,743 | | | 453,595 | |
| Refundable purchase price paid (Note 6) | | | - | | | 1,200,000 | |
| Inventories (Note 7) | | | 2,712,963 | | | 1,952,348 | |
| Prepaid expenses and deposits (Note 5) | | | 1,176,692 | | | 933,299 | |
| Total current assets | | | 17,223,489 | | | 16,277,715 | |
| Due from related parties (Note 18) | | | 2,785,347 | | | 2,630,019 | |
| Property and equipments, net (Note 8) | | | 28,297,036 | | | 27,123,035 | |
| Intangible assets, net (Note 9) | | | 6,616,622 | | | 6,494,510 | |
| Goodwill (Note 10) | | | 7,704,058 | | | 7,395,752 | |
| Restricted cash (Note 11) | | | 5,454,150 | | | 3,957,624 | |
| Refundable purchase price paid (Note 6) | | | 1,200,000 | | | | |
| Other assets (Note 12) | | | 1,782,297 | | | 1,710,972 | |
| Debt issue costs (Note 24) | | | 262,066 | | | 327,945 | |
| Total Assets | | $ | 71,325,065 | | $ | 65,917,572 | |
| | | | | | | | |
Liabilities & Shareholders' Equity | | | | | | | |
| Current Liabilities | | | | | | | |
| Bank indebtedness (Note 11) | | $ | 1,160,976 | | $ | 874,490 | |
| Bank loans payable (current portion) (Note 13) | | | 2,934,657 | | | 2,867,004 | |
| Long term debt payable (current portion) (Note 14) | | | 1,732,498 | | | 1,787,239 | |
| Accounts payable and accrued liabilities (Note 15) | | | 5,519,316 | | | 4,665,984 | |
| Commercial notes payable (Note 11) | | | 8,187,194 | | | 5,118,758 | |
| Taxes payable | | | 797,940 | | | 1,279,385 | |
| Acquisition price payable (Note 10) | | | 1,388,825 | | | 1,333,246 | |
| Wages payable | | | 848,922 | | | 664,785 | |
| Total current liabilities | | | 22,570,328 | | | 18,590,891 | |
| Long term debt payable (long term portion) (Note 14) | | | 442,718 | | | 425,001 | |
| Long-term convertible promissory notes (Note 24) | | | 3,816,197 | | | 2,875,075 | |
| Due to related parties (Long-term) (Note 18) | | | 3,284,540 | | | 3,193,618 | |
| Total liabilities | | | 30,113,783 | | | 25,084,585 | |
| | | | | | | | |
| Minority interest | | | 5,752,933 | | | 5,502,755 | |
| | | | | | | | |
| Redeemable common stock, 2,049,560 shares at $3.6 per share (Note 19) | | | 7,376,366 | | | 7,376,366 | |
| | | | | | | | |
| Shareholders' Equity | | | | | | | |
| Preferred stock, $0.001 par value; 5,000,000 shares authorized; None issued and outstanding (Note 25) | | | - | | | - | |
| Common stock, $0.001 par value; 150,000,000 shares authorized; 100,803,509 shares issued and outstanding as of 3/31/2008; 100,170,071 shares issued and outstanding as of 12/31/2007 (Note 25) | | | 100,803 | | | 100,170 | |
| Additional paid in capital (Note 25) | | | 21,853,508 | | | 21,547,929 | |
| Retained earnings (unrestricted) | | | (1,936,960 | ) | | 100,452 | |
| Statutory surplus reserve fund (Note 17) | | | 2,310,681 | | | 2,310,681 | |
| Accumulative other comprehensive income | | | 5,250,091 | | | 3,390,774 | |
| Shares issuable for acquisition and services | | | 503,860 | | | 503,860 | |
| Total Shareholders' Equity | | | 28,081,983 | | | 27,953,866 | |
| Total Liabilities & Shareholders' Equity | | $ | 71,325,065 | | $ | 65,917,572 | |
The accompanying notes are an integral part of these consolidated financial statements.
Benda Pharmaceutical, Inc.
Consolidated Statements of Operations
(Amounts expressed in U.S. Dollars)
| | | THREE MONTHS ENDED MARCH 31 | |
| | | | | | |
| | | 2008 | | 2007 | |
| | | Restated | | Restated | |
| | | (Unaudited) | | (Unaudited) | |
| Revenue | | $ | 5,947,370 | | $ | 3,029,035 | |
| Cost of goods sold | | | (3,817,246 | ) | | (1,847,212 | ) |
| Gross profit | | | 2,130,124 | | | 1,181,823 | |
| | | | | | | | |
| Selling expenses | | | (436,371 | ) | | (88,183 | ) |
| | | | | | | | |
| General and administrative expenses | | | | | | | |
| Amortization of intangible assets | | | (51,575 | ) | | (26,284 | ) |
| Amortization of debt issue costs (Note 24) | | | (65,879 | ) | | (8,743 | ) |
| Depreciation | | | (123,220 | ) | | (76,576 | ) |
| Bad debts | | | (471,297 | ) | | 70,986 | |
| Director remuneration | | | (110,873 | ) | | - | |
| Penalty to investors (Note 20) | | | (722,205 | ) | | (120,000 | ) |
| Other general and administrative expenses (Note 21) | | | (1,097,869 | ) | | (324,713 | ) |
| Total general and administrative expenses | | | (2,642,918 | ) | | (485,330 | ) |
| Gains / (losses) on disposals of fixed assets | | | - | | | (12,025 | ) |
| Research and development expenses | | | (22,064 | ) | | - | |
| Total operating expenses | | | (3,101,353 | ) | | (585,538 | ) |
| Operating income / (loss) | | | (971,229 | ) | | 596,285 | |
| | | | | | | | |
| Interest income / (expenses) (Note 24) | | | (1,212,282 | ) | | (17,585 | ) |
| Other income (expenses) | | | 301,693 | | | 937 | |
| Government subsidies / grants (Note 22) | | | - | | | - | |
| | | | | | | | |
| Income / (loss) before minority interest and income taxes | | | (1,881,818 | ) | | 579,637 | |
| Income taxes (Note 23) | | | (133,197 | ) | | - | |
| Minority interest (Note 28) | | | (22,398 | ) | | (46,906 | ) |
| | | | | | | | |
| Net income / (loss) | | $ | (2,037,413 | ) | $ | 532,731 | |
| | | | | | | | |
| Earnings / (loss) per share - basic | | $ | (0.02 | ) | $ | 0.01 | |
| | | | | | | | |
| Weighted average shares outstanding - basic | | | 100,312,873 | | | 96,281,951 | |
| | | | | | | | |
| Earnings / (loss) per share - diluted | | $ | (0.02 | ) | $ | 0.00 | |
| | | | | | | | |
| Weighted average shares outstanding - diluted (Note 26) | | | 100,312,873 | | | 124,099,914 | |
The accompanying notes are an integral part of these consolidated financial statements.
Benda Pharmaceutical, Inc.
Consolidated Statements of Cash Flows
(Amounts expressed in U.S. Dollars)
| | | THREE MONTHS ENDED MARCH 31 | |
| | | 2008 | | 2007 | |
| | | Restated | | Restated | |
| | | (Unaudited) | | (Unaudited) | |
Cash Flows From Operating Activities | | | | | | | |
| Net income / (loss) | | $ | (2,037,413 | ) | $ | 532,731 | |
| Adjustments to reconcile net income / (loss) to net cash provided by operating activities: | | | | | | | |
| Bad Debt provision | | | 471,297 | | | (70,986 | ) |
| Minority interest | | | 22,398 | | | 46,906 | |
| Loss on disposals of fixed assets | | | - | | | 12,025 | |
| Depreciation | | | 453,235 | | | 99,861 | |
| Amortization of intangible assets | | | 184,115 | | | 50,467 | |
| Amortization of debt issue costs (Note 24) | | | 65,879 | | | 8,743 | |
| Interest expense (amortization of debt discount) (Note 24) | | | 941,122 | | | 15,497 | |
| Penalty to investors settled by issuance of common stock | | | 230,312 | | | - | |
| Directors remuneration settled by issuance of common stock | | | 75,900 | | | - | |
| Changes in operating assets and liabilities: | | | | | | | |
| Trade receivables | | | (1,327,952 | ) | | (920,421 | ) |
| Other receivables | | | (138,461 | ) | | 35 | |
| Prepaid expenses and deposits | | | (304,699 | ) | | (4,686 | ) |
| Inventories | | | (760,615 | ) | | 165,295 | |
| Accounts payable and accrued liabilities | | | 966,144 | | | (204,275 | ) |
| Others payable | | | - | | | 84,693 | |
| Taxes payable | | | (481,445 | ) | | (31,208 | ) |
Net cash provided by operating activities | | | (1,640,183 | ) | | (215,323 | ) |
| | | | | | | | |
Cash Flows From Investing Activities | | | | | | | |
| Purchases of property and equipment and construction-in-progress | | | (214,454 | ) | | (100,973 | ) |
| Loans to related parties, net | | | - | | | (166,612 | ) |
Net cash used in investing activities | | | (214,454 | ) | | (267,585 | ) |
| | | | | | | | |
Cash Flows From Financing Actives | | | | | | | |
| Proceeds from issuance of convertible promissory note (Note 24) | | | - | | | 5,520,000 | |
| Proceeds and repayments of borrowings under related parties, net | | | (64,406 | ) | | 77,442 | |
| Proceeds and repayments of borrowings under government debts payable, net | | | 230,527 | | | - | |
| Proceeds and repayments of borrowings under commercial bank notes, net (Note 11) | | | 1,498,624 | | | - | |
| Proceeds and repayments of borrowings under bank loans, net | | | (51,863 | ) | | (256,492 | ) |
Net cash provided by (used in) financing activities | | | 1,612,882 | | | 5,340,950 | |
| Effect of exchange rate changes on cash | | | 376,693 | | | 98,441 | |
Net increase in cash and cash equivalents | | | 134,938 | | | 4,956,483 | |
| | | | | | | | |
Cash and cash equivalents, beginning of period | | | 1,266,240 | | | 1,676,119 | |
| | | | | | | | |
Cash and cash equivalents, end of period | | $ | 1,401,178 | | $ | 6,632,601 | |
| | | | | | | | |
Supplemental Disclosure of Cash Flow Information | | | | | | | |
Cash paid for interest | | $ | 211,407 | | $ | 2,565 | |
Cash paid for income taxes | | $ | 487,318 | | $ | - | |
The accompanying notes are an integral part of these consolidated financial statements.
Benda Pharmaceutical, Inc.
Notes to Consolidated Financial Statements
(Amounts expressed in U.S. Dollars)
1. | Restatement of Previously Issued Financial Statements |
Benda’s previously issued financial statements are being restated as a result of an internal review of its previously issued financial statements, which review was prompted by an SEC comment during its review of the Company’s Form S-1 filing. After a review of the comment and further analysis, Benda determined that it incorrectly calculated its depreciation and amortization expense. Benda also believes such restatements reflect the correction of any errors and omissions of material disclosures in the financial statements in accordance with SFAS 154: Accounting changes and error corrections (as amended).
The following are explanations of the restatement adjustments and presentation of affected accounts in the consolidated balance sheet and statement of operations as previously reported and restated.
Statements of Operations for the three months ended March 31, 2008 and 2007
The Company restated its previously recognized Depreciation expense by ($559,131) and ($205,757) for the three months ended March 31, 2008 and 2007 due to incorrect calculation of depreciation expense previously reported.
The Company restated its previously recognized Amortization expense by ($201,054) and ($67,406) for the three months ended March 31, 2008 and 2007 due to incorrect calculation of amortization expense previously reported.
Balance Sheets March 31, 2008 and 2007
Adjustments were made to Property and Equipment, net and Intangible assets, net and Retained (deficit) earnings unrestricted to reflect the restatement of the statements of operations.
The consolidated financial statements as of March 31, 2008 and 2007 and for the ended March 31, 2008 and 2007, and the notes thereto, have been restated to include the items identified above. The following financial statement line items were impacted:
Consolidated Balance Sheet
| | | As Previously | | | |
| | | Reported | | Restated | |
| | | Three Month Ended | | Three Month Ended | |
| | | March 31, | | March 31, | |
| | | 2008 | | 2008 | |
| Property and equipments, net | | $ | 27,343,976 | | $ | 28,297,036 | |
| Intangible assets, net | | | 6,464,173 | | | 6,616,622 | |
| Retained (deficit) earnings unrestricted | | $ | (2,987,195 | ) | $ | (1,936,960 | ) |
Consolidated Statement of Operations
| | | As Previously | | | |
| | | Reported | | Restated | |
| | | Three Month Ended | | Three Month Ended | |
| | | March 31, | | March 31, | |
| | | 2008 | | 2008 | |
| Cost of goods sold | | $ | (3,940,080 | ) | $ | (3,817,246 | ) |
| As included in General and administrative expenses | | | | | | | |
| Depreciation expense | | $ | (123,220 | ) | $ | (123,220 | ) |
| Amortization expense | | | (51,575 | ) | | (51,575 | ) |
| Total operating expense | | | (3,101,353 | ) | | (3,101,353 | ) |
| Operating income / (loss) | | | (1,094,063 | ) | | (971,229 | ) |
| Income / (loss) before minority interest and income taxes | | | (2,004,652 | ) | | (1,881,818 | ) |
| Net income / (loss) | | | (2,154,106 | ) | | (2,037,413 | ) |
| Earnings / (loss) per share - basic | | | (0.02 | ) | | (0.02 | ) |
| Earnings / (loss) per share - diluted | | $ | (0.02 | ) | $ | (0.02 | ) |
Consolidated Balance Sheet
| | | As Previously | | | |
| | | Reported | | Restated | |
| | | Three Month Ended | | Three Month Ended | |
| | | March 31, | | March 31, | |
| | | 2007 | | 2007 | |
| Property and equipments, net | | $ | 13,766,129 | | $ | 14,295,607 | |
| Intangible assets, net | | | 1,459,487 | | | 1,544,181 | |
| Retained (deficit) earnings unrestricted | | $ | 7,853,700 | | $ | 8,437,165 | |
Consolidated Statement of Operations
| | | As Previously | | | |
| | | Reported | | Restated | |
| | | Three Month Ended | | Three Month Ended | |
| | | March 31, | | March 31, | |
| | | 2007 | | 2007 | |
| Cost of goods sold | | $ | (1,970,047 | ) | $ | (1,847,212 | ) |
| As included in General and administrative expenses | | | | | | | |
| Depreciation expense | | $ | (76,576 | ) | $ | (76,576 | ) |
| Amortization expense | | | (26,284 | ) | | (26,284 | ) |
| Total operating expense | | | (585,538 | ) | | (585,538 | ) |
| Operating income / (loss) | | | 473,450 | | | 596,285 | |
| Income / (loss) before minority interest and income taxes | | | 456,802 | | | 579,637 | |
| Net income / (loss) | | | 416,037 | | | 532,731 | |
| Earnings / (loss) per share - basic | | | 0.00 | | | 0.01 | |
| Earnings / (loss) per share - diluted | | $ | 0.00 | | $ | 0.00 | |
Benda Pharmaceutical, Inc. (“Benda”) is a corporation organized under the Florida Laws and headquartered in Hubei Province, the People’s Republic of China (“PRC”).
Ever Leader Holdings Limited (“Ever Leader”), a wholly owned subsidiary of Benda, is a company incorporated under the laws of Hong Kong SAR.
Ever Leader owns 95% of the issued and outstanding capital of Hubei Tongi Benda Ebei Pharmaceutical Co. Ltd. (“Benda Ebei”), a Sino-Foreign Equity Joint Venture company incorporated under the laws of PRC. Mr. Yiqing Wan owns 5% of the issued and outstanding capital stock of Benda Ebei. Benda Ebei owns: (i) 95% of the issued and outstanding capital stock of Jiangling Benda Pharmaceutical Co. Ltd., (“Jiangling Benda”) a company formed under the laws of PRC; (ii) 95% of the issued and outstanding capital stock of Yidu Benda Chemical Co. Ltd., (“Yidu Benda”) a company incorporated under the laws of PRC; and (iii) 75% of the issued and outstanding capital stock of Beijing Shusai Pharyngitis Research Co. Ltd., (“Beijing Shusai”) a company incorporated under the laws of PRC. Mr. Yiqing Wan owns: (i) 5% of the issued and outstanding capital stock of Jingling Benda; and (ii) 5% of the issued and outstanding capital stock of Yidu Benda. Mr. Feng Wang owns 25% of the issued and outstanding capital stock of Beijing Shusai.
On April 5, 2007, Benda Ebei entered into an Equity Transfer Agreements with Shenzhen Yuanzheng Investment Development Co., Ltd. and Shenzhen Yuanxing Gene City Development Co., Ltd., the then shareholders of Shenzhen SiBiono GeneTech Co., Ltd (“SiBiono”), to purchase 27.57% and 30% respectively of the shares of SiBiono’s common stock for total consideration of Rmb 60 million (or $7.88 million) due and payable on or before April 30, 2007. On June 11, 2007, Benda Ebei entered into an Equity Transfer Agreements with Huimin Zhang and Yaojin Wang, the individual shareholders of SiBiono, and to purchase 1.6% and 0.96% respectively of the shares of SiBiono’s common stock for total consideration of Rmb 2.56 million (or $0.34 million) due and payable on or before June 30, 2007. Altogether, the total consideration for 60.13% shares of SiBiono’s common stock was Rmb 62.56 million or $8.22 million. As of March 31, 2008, an accumulated amount, approximately Rmb 52.9 million or $6.83 million was paid and leaving a balance approximately $1.39 million (please refer to Note 10 for the details).
Benda, Ever Leader, Benda Ebei, Jingling Benda, Yidu Benda, Beijing Shusai and SiBiono shall be referred to herein collectively as the “Group”. The Group is engaged principally in the business of identifying, discovering, developing, and manufacturing conventional medicines, active pharmaceuticals, bulk chemicals (or pharmaceutical immediates), and Traditional Chinese Medicines (“TCM”) for the treatment of some of the most widespread common ailments and diseases (e.g. common cold, diabetes, and cancer).
As of March 31, 2008, the organization and ownership structure of the Group is as follows:
3. | Significant Accounting Policies |
Cash and Cash Equivalents
Cash and cash equivalents include cash on hand, cash on deposit with various financial institutions, and all highly-liquid investments with original maturities of three months or less at the time of purchase.
Estimates Affecting Trade Receivables, Other Receivables, Prepaid and Deposits and Inventories
The preparation of our consolidated financial statements requires management to make estimates and assumptions that affect our reporting of assets and liabilities (and contingent assets and liabilities). However, it is explicated that the changes in estimation were not material in the preparation of our consolidation financial statements.
As of March 31, 2008 and 2007, the Company provided a $1,464,538 and $400,380 respectively for the allowance of doubtful accounts against trade receivables, other receivables and prepaid and deposits (please refer to Note 5 for details). Management's estimate of the appropriate allowance on those accounts receivable for the reporting periods was based on the aged nature of these accounts. In making its judgment, management assessed its customers' ability to continue to pay their outstanding invoices and the collectibility of those accounts on a timely basis, and whether their financial position might deteriorate significantly in the future, which would result in their inability to pay their debts to the Company.
Inventories, which are primarily comprised of raw materials, packaging materials, and finished goods, are stated at the lower of cost or net realizable value. Cost being determined on the basis of a moving average. The Group evaluates the need for reserves associated with obsolete, slow-moving and non-salable inventory by reviewing net realizable values on a periodic basis.
For the three month ended March 31, 2008 and 2007, the Company provided a reserve against its work-in-progress amounting to $4,300,015 and none respectively. The reserve was raised due to the fact that most of the original liquid, a liquid element o produce Gendicine, was produced in the year of 2004 and this particular liquid can only be stored for approximately five years, therefore a reserve was provide against the work-in-progress (see Note 7). However, no reserve for obsolete, slow-moving or non-salable inventory was required for the reporting periods.
Property and Equipment
Property and equipment are recorded at cost and depreciated using the straight-line method, with an estimated 5% salvage value of original cost, over the estimated useful lives of the assets as follows:
| Buildings | | | 20-30years | |
| Machinery and equipment | | | 10-15 years | |
| Motor Vehicles | | | 5 years | |
| Electronics and office equipment | | | 5 years | |
Expenditures for repairs and maintenance, which do not improve or extend the expected useful lives of the assets, are expensed as incurred while major replacements and improvements are capitalized.
When property or equipment is retired or disposed of, the cost and accumulated depreciation are removed from the accounts, with any resulting gains or losses being included in net income or loss in the year of disposition.
Impairment of Long-Lived Assets
The Group evaluates potential impairment of long-lived assets, in accordance with Statement of Financial Accounting Standards (“SFAS”) No. 144, Accounting for the Impairment or Disposal of Long-Lived Assets, which requires the Group to (a) recognize an impairment loss only if the carrying amount of a long-lived asset is not recoverable from its undiscounted cash flows and (b) measure an impairment loss as the difference between the carrying amount and fair value of the asset. The Group believes that long-lived assets in the accompanying consolidated balance sheets are appropriately valued at three month ended March 31, 2008 and 2007.
Intangible Assets
The Group’s intangible assets are stated at cost less accumulated amortization and are comprised of land-use rights, drug permits and licenses, patent and technology formulas know-how. Land-use rights are related to land the Group occupies in Hubei and Guangdong Province, PRC and are being amortized on a straight-line basis over a period of 40 years. Other intangible assets are being amortized on a straight-line basis over a period of 10 years.
Revenue Recognition
Among the most important accounting policies affecting our consolidated financial statements is our policy of recognizing revenue in accordance with the SEC's Staff Accounting Bulletin ("SAB") No. 104. Under this policy, all of the following criteria must be met in order for us to recognize revenue:
1. Persuasive evidence of an arrangement exists;
2. Delivery has occurred or services have been rendered;
3. The seller's price to the buyer is fixed or determinable; and
4. Collectibility is reasonably assured.
The majority of the Group's revenue results from sales contracts with distributors and revenue is recorded upon the shipment of goods. Management conducts credit background checks for new customers as a means to reduce the subjectivity of assuring collectibility. Based on these factors, the Group believes that it can apply the provisions of SAB 104 with minimal subjectivity.
Research and Development
Research and development costs are expensed as incurred and consist primarily of salaries and related expenses of personnel engaged in research and development activities. The Company spent $22,064 and none on direct research and development (“R&D”) efforts for the three month ended March 31, 2008 and 2007, respectively.
Income Taxes
The Group accounts for income taxes under the liability method in accordance with SFAS No. 109, Accounting for Income Taxes. Deferred tax assets and liabilities are recorded for the estimated future tax effects of temporary differences between the tax basis of assets and liabilities and amounts reported in the accompanying consolidated balance sheets. Deferred tax assets are reduced by a valuation allowance if current evidence indicates that it is considered more likely than not that these benefits will not be realized.
Comprehensive Income
The Group has adopted SFAS No. 130, Reporting Comprehensive Income, which establishes standards for reporting and displaying comprehensive income, its components, and accumulated balances in a full-set of general-purpose financial statements. Accumulated other comprehensive income represents the accumulated balance of foreign currency translation adjustments.
Concentration of Credit Risk
A significant portion of the Group's cash at three month ended March 31, 2008 and 2007 is maintained at various financial institutions in the PRC which do not provide insurance for amounts on deposit.
The Group has not experienced any losses in such accounts and believes it is not exposed to significant credit risk in this area.
The Group operates principally in the PRC and grants credit to its customers in this geographic region. Although the PRC is economically stable, it is always possible that unanticipated events in foreign countries could disrupt the Group’s operations.
The following table shows the individual customer’s revenue and account receivable balance which was higher than 5% of total revenue and total account receivables for the three month ended March 31, 2008 and 2007:
| | | | | THREE MONTH ENDED MARCH 31, | |
| | | | | 2008 | | 2007 | |
| | | | | (Unaudited) | | (Unaudited) | |
| | | Revenue | | $ | 5,947,370 | | | % | | $ | 3,029,035 | | | % | |
| | | | | | | | | | | | | | | | |
| | | Individual customer's revenue | | | | | | | | | | | | | |
| 1 | | Zhuhai Gongbei Pharmaceutical Co, Ltd. | | | 1,527,292 | | | 26 | % | | 684,290 | | | 23 | % |
| 2 | | Hubei Hengchuan Health Products Co.,Ltd. | | | 970,148 | | | 16 | % | | - | | | 0 | % |
| 3 | | Shenzhen Huihua Pharmaceutical Co. Ltd. | | | 875,175 | | | 15 | % | | 535,528 | | | 18 | % |
| 4 | | Shenyang Pharmaceutical Co. Ltd. | | | 712,182 | | | 12 | % | | 444,736 | | | 15 | % |
| 5 | | Jiangxi Huiren Pharmaceutical Co. Ltd. | | | 352,842 | | | 6 | % | | 252,573 | | | 8 | % |
| | | | | | | | | | | | | | | | |
| | | Account receivable, gross | | $ | 12,237,873 | | | | % | $ | 7,555,831 | | | | % |
| | | | | | | | | | | | | | | | |
| | | Individual customer's account receivable gross balance | | | | | | | | | | | | | |
| 1 | | Zhuhai Gongbei Pharmaceutical Co, Ltd. | | | 2,196,438 | | | 18 | % | | 1,130,530 | | | 15 | % |
| 2 | | Hubei Hengchuan Health Products Co.,Ltd. | | | 1,577,335 | | | 13 | % | | - | | | 0 | % |
| 3 | | Shenzhen Huihua Pharmaceutical Co. Ltd. | | | 1,347,251 | | | 11 | % | | 1,444,580 | | | 19 | % |
| 4 | | Shenyang Pharmaceutical Co. Ltd. | | | 1,078,182 | | | 9 | % | | 910,440 | | | 12 | % |
| 5 | | Jiangxi Huiren Pharmaceutical Co. Ltd. | | | 571,918 | | | 5 | % | | 838,128 | | | 11 | % |
Basic and Diluted Earnings Per Share
The Group adopted Statement of Financial Accounting Standards No. 128, “Earnings Per Share” (SFAS128). SFAS 128 requires the presentation of earnings per share (EPS) as Basic and Diluted EPS. Basic earnings per share are calculated by taking net income divided by the weighted average shares of common stock outstanding during the period. Diluted earnings per share is calculated by taking basic weighted average shares of common stock and increasing it for dilutive common stock equivalents such as warrants that are in the money.
Foreign Currency Translation
The functional currency of the Group is the Renminbi (“RMB”), the PRC’s currency. The Group maintains its financial statements using the functional currency. Monetary assets and liabilities denominated in currencies other than the functional currency are translated into the functional currency at rates of exchange prevailing at the balance sheet dates. Transactions denominated in currencies other than the functional currency are translated into the functional currency at the exchange rates prevailing at the dates of the transaction. Exchange gains or losses arising from foreign currency transactions are included in the determination of net income (loss) for the respective periods.
For financial reporting purposes, the financial statements of the Group, which are prepared using the RMB, are translated into the Group’s reporting currency, United States Dollars. Balance sheet accounts are translated using the closing exchange rate in effect at the balance sheet date and income and expense accounts are translated using the average exchange rate prevailing during the reporting period. Adjustments resulting from the translation, if any, are included in accumulated other comprehensive income (loss) in stockholder’s equity.
The exchange rates in effect at March 31, 2008 and 2007 were stated as follows: (for RMB 1.00):
| | | March 31, | | March 31, | |
| | | 2008 | | 2007 | |
| Fixed rate | | $ | 0.1428 | | $ | 0.1295 | |
| Average rate | | $ | 0.1398 | | $ | 0.1290 | |
For the three month ended 2008, the foreign exchange loss was $3,196; and for the three month ended March 2007, the foreign exchange gain was $9,860.
Fair Value of Financial Instruments
The Group's financial instruments include cash equivalents, accounts receivable, other receivables, accounts payable, accrued expenses, value-added taxes, short-term and long-term bank loans, and loans payable to related parties. The carrying amounts of financial instruments other than long-term obligations approximate fair value due to their short maturities. Long-term obligations approximate fair value based upon rates currently available for similar instruments.
Recent Accounting Pronouncements
On December 4, 2007, the Financial Accounting Standards Board (FASB) issued SFAS No. 160, Noncontrolling interest in Consolidated Financial Statements (SFAS No. 160). SFAS No. 160 requires all entities to report noncontrolling (minority) interests in subsidiaries as equity in the consolidated financial statements. The statement establishes a single method of accounting for changes in a parent’s ownership interest in a subsidiary that do not result in deconsolidation and expands disclosures in the consolidated financial statements. SFAS No. 160 is effective for fiscal years beginning after December 15, 2008 and interim periods within those fiscal years. We have not yet determined the impact of the adoption of SFAS No. 160 on our consolidated financial statements and footnote disclosures.
On December 4, 2007, the FASB issued SFAS No.141R, Business Combinations (SFAS No. 141R). SFAS No. 141R requires the acquiring entity in a business combination to recognize all the assets acquired and liabilities assumed, establishes the acquisition date fair value as the measurement objective for all assets acquired and liabilities assumed, and requires the acquirer to expand disclosures about the nature and financial effect of the business combination. SFAS No. 141R is effective for business combinations for which the acquisition date is on or after the beginning of the first annual reporting period beginning on or after December 15, 2008. We have not yet determined the impact of the adoption of SFAS No. 141R on our consolidated financial statements and footnote disclosures.
Recently Adopted Accounting Pronouncements
SFAS No. 123R, Share-Based Payment, an Amendment of SFAS No. 123, was issued in December 2004 and was effective as of the beginning of the Group’s 2006 fiscal year. SFAS No. 123R requires all share-based payments to qualified individuals, including grants of employee stock options, to be recognized as compensation expense in the financial statements based on their grant date fair values.
In September 2006, the FASB issued SFAS No. 157, "Fair Value Measurements" ("SFAS 157"). SFAS 157 defines fair value, establishes a framework and gives guidance regarding the methods used for measuring fair value, and expands disclosures about fair value measurements. In February 2008, the FASB issued FASB Staff Position 157-1, "Application of FASB Statement No. 157 to FASB Statement No. 13 and Other Accounting Pronouncements That Address Fair Value Measurements for Purposes of Lease Classification or Measurement under Statement 13" ("FSP 157-1") and FASB Staff Position 157-2, "Effective Date of FASB Statement No. 157" ("FSP 157-2"). FSP 157-1 amends SFAS 157 to remove certain leasing transactions from its scope. FSP 157-2 delays the effective date of SFAS 157 for all non-financial assets and non-financial liabilities, except for items that are recognized or disclosed at fair value in the financial statements on a recurring basis (at least annually), until fiscal years beginning after November 15, 2008. SFAS 157 is effective for financial statements issued for fiscal years beginning after November 15, 2007, and interim periods within those fiscal years. The Company adopted SFAS 157 effective January 1, 2008 for all financial assets and liabilities as required. The adoption of SFAS 157 was not material to the Company's financial statements or results of operations.
In February 2007, the FASB issued SFAS No. 159, “The Fair Value Option for Financial Assets and Financial Liabilities — Including an Amendment of FASB Statement No. 115,” (“SFAS 159”) which is effective for fiscal years beginning after November 15, 2007. SFAS 159 is an elective standard which permits an entity to choose to measure many financial instruments and certain other items at fair value at specified election dates. Subsequent unrealized gains and losses on items for which the fair value option has been elected will be reported in earnings. The Company has not elected the fair value option for any assets or liabilities under SFAS 159.
The unaudited consolidated financial statements of Benda and its subsidiaries have been prepared in accordance with U.S. generally accepted accounting principles for interim financial information and pursuant to the requirements for reporting on Form 10-Q. Accordingly, they do not include all the information and footnotes required by accounting principles generally accepted in the United States of America for annual financial statements. However, the information included in these interim financial statements reflects all adjustments (consisting solely of normal recurring adjustments) which are, in the opinion of management, necessary for the fair presentation of the consolidated financial position and the consolidated results of operations. Results shown for interim periods are not necessarily indicative of the results to be obtained for a full year. The consolidated balance sheet information as of December 31, 2007 was derived from the audited consolidated financial statements included in the Company's Annual Report on Form 10-KSB. These interim financial statements should be read in conjunction with that report. Certain prior-year amounts have been reclassified to conform to the current-year presentation. These reclassifications had no effect on the results of operations or shareholder’s equity as previously reported.
Use of Estimates
The preparation of financial statements in conformity with US GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements, and the reported amounts of revenue and expenses during the reporting period. Actual results when ultimately realized could differ from those estimates.
5. | Trade Receivables, Other Receivables and Prepaid and Deposits - Allowance for Doubtful Debts |
As mentioned in Note 4 that the company estimates of the appropriate allowance on those accounts receivable for the reporting periods was based on the aged nature of these accounts. The table below shows the allowance for doubtful debts of the Group’s trade receivables, other receivables and prepaid and deposit as of March 31, 2008 and December 31, 2007:
| | | March 31, 2008 (Unaudited) | | December 31, 2007 | |
| Trade receivables, gross | | $ | 12,237,873 | | $ | 10,909,921 | |
| Allowance for doubtful debts | | | (1,021,960 | ) | | (437,688 | ) |
| Trade receivables, net | | $ | 11,215,913 | | $ | 10,472,233 | |
| | | | | | | | |
| | | | March 31, 2008 (Unaudited) | | | December 31, 2007 | |
| Other receivables, gross | | $ | 753,432 | | $ | 614,971 | |
| Allowance for doubtful debts | | | (36,689 | ) | | (161,376 | ) |
| Other receivables, net | | $ | 716,743 | | $ | 453,595 | |
| | | | | | | | |
| | | | March 31, 2008 (Unaudited) | | | December 31, 2007 | |
| Prepaid and deposits, gross | | $ | 1,582,581 | | $ | 1,277,882 | |
| Allowance for doubtful debts | | | (405,889 | ) | | (344,583 | ) |
| Prepaid and deposits, net | | $ | 1,176,692 | | $ | 933,299 | |
The change of the allowance for doubtful debts between the reporting periods, as of March 31, 2008 and 2007, is displayed as follows:
| | | THREE MONTHS ENDED MARCH 31 | |
| | | 2008 | | 2007 | |
| | | (Unaudited) | | (Unaudited) | |
| Balance at beginning of period | | $ | (943,647 | ) | $ | (467,015 | ) |
| Provision/Reversal during the period | | | (471,297 | ) | | 70,986 | |
| Foreign exchange difference | | | (49,594 | ) | | (4,351 | ) |
| Balance at end of period | | $ | (1,464,538 | ) | $ | (400,380 | ) |
In general, the company set full allowance for trade receivables aged over 120 days which is the general credit term granted to the customers. After deducting those allowances from the gross trade receivable, based on the management’s past experience, there would be no collectability issue on the net amount.
For the three months ended March 31, 2008, the management assessed certain balances aged over 120 days and reviewed customer by customer and specified which did not have collectability issue; then a 100% provision was made against all remaining balances outstanding over 120 days.
For the three months ended March 31, 2008, the net trade receivable turnover day was 140 days which was slightly above the company’s general principle for the allowance which was mainly due to the fact that there was a long Chinese New Year holiday and a big snowing in the first quarter of 2008, it did affect the collection of trade receivables to a certain extent. On the whole, the allowance made during the reporting period still fell within the normal range.
6. | Refundable Purchase Price Paid |
On, December 7, 2006, Benda Ebei paid $1.2 million to SECO (Shenzhen) Biotech Co., Ltd. (“SECO”) pursuant to a purchase agreement signed between SECO and Benda Ebei on December 3, 2006 to acquire a technology know-how and drug specifications / technical parameters in producing a Gastropathy drug owned by SECO. As at March 31, 2008, the deal has not been closed as the product certificate still not received from FDA of the United States of America and the amount paid is refundable per agreement if such approval is not obtained. As the date when the certificate can be received from FDA of the United States of America cannot be determined, the amount paid is reclassified as non-current assets as of March 31, 2008.
The Group’s inventories at March 31, 2008 and December 31, 2007 were comprised as follows:
| | | March 31, | | | |
| | | 2008 | | December 31, | |
| | | (Unaudited) | | 2007 | |
| Raw materials | | $ | 1,011,499 | | $ | 1,078,438 | |
| Packing materials | | | 163,316 | | | 155,101 | |
| Other materials / supplies | | | 76,719 | | | 93,206 | |
| Finished goods | | | 763,224 | | | 415,627 | |
| Work-in-progress | | | 4,998,220 | | | 4,631,990 | |
| Total inventories at cost | | | 7,012,978 | | | 6,374,362 | |
| | | | | | | | |
| Less: Reserves on work-in-progress | | | (4,300,015 | ) | | (4,422,014 | ) |
| | | | | | | | |
| Total inventories, net | | $ | 2,712,963 | | $ | 1,952,348 | |
A reserve for obsolete, slow-moving or non-salable inventory was made on work-in-progress at the amount of $4,300,015 and $4,422,014 as of March 31, 2008 and December 31, 2007, respectively.
The provision of reserve was resulted from the manufacturing process of Gendicine, SiBiono’s sole product and SiBiono was acquired by the company in April 2007.
The following chart shows the manufacturing process of Gendicine (Ad-p53):
In the production process of finished goods, Gendicine, several working steps are needed: (i) large-scale culturing of adenovirus from master adenovirus bank; (ii) culturing of cell from master cell bank; (iii) purification. The whole process including step (i) to step (iii) takes approximately twenty-four days to make reagent (“original liquid”). This particular liquid can only be stored for approximately five years. It takes approximately another seven days for mixing and bottling original liquid to finished goods which is known as Gendicine.
Therefore, up to the stage of reagent, all the related production costs are treated as work-in-progress. The major components of those production costs are: (i) direct labor; (ii) direct materials; (iii) power; (iv) supplies and other materials and (v) manufacturing overheads.
Before acquisition, as of March 31, 2007, the accumulated units of original liquid produced was 198,075 and which could be converted to approximately 226,736 vials of Gendicine. However, the accumulated vials of Gendicine sold throughout the years 2004 to three-months period ended March 31, 2007 were only approximately 18,424 vials. The accumulated production costs of $4,080,644 were remained as work-in-process as of March 31, 2007.
Furthermore, due to the special feature of the original liquid which can only be stored for five years, and most of the original liquid was produced in the year of 2004, and the provision of reserve on work in progress was $3,696,083 as of March 31, 2007.
After the acquisition with the effective date April 1, 2007, the same accounting treatment was adopted for the treatment of the provision of reserve on work-in-progress. As of March 31, 2008, the provision of reserve on work in progress was $4,300,015.
8. | Property and Equipment (Restated) |
The Group’s property and equipment as of March 31, 2008 and December 31, 2007 were comprised as follows:
| | | December 31, 2007 | | Addition | | Foreign Currency Translation Difference | | March 31, 2008 (Unaudited) | |
| Buildings | | $ | 8,858,126 | | | 2,691 | | | 469,315 | | $ | 9,330,132 | |
| Machinery and equipment | | | 15,260,167 | | | 33,983 | | | 815,481 | | | 16,109,631 | |
| Office equipment | | | 28,762 | | | 5,605 | | | 5,336 | | | 39,703 | |
| Motor Vehicles | | | 206,252 | | | 9,924 | | | 11,226 | | | 227,402 | |
| Cost | | | 24,353,307 | | | 52,203 | | | 1,301,358 | | | 25,706,868 | |
| | | | | | | | | | | | | | |
| Less: Accumulated Depreciation | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| Buildings | | $ | (981,116 | ) | | (106,983 | ) | | (51,854 | ) | $ | (1,139,953 | ) |
| Machinery and equipment | | | (2,423,002 | ) | | (337,115 | ) | | (98,985 | ) | | (2,859,102 | ) |
| Office equipment | | | (4,281 | ) | | (474 | ) | | 1,992 | | | (2,763 | ) |
| Motor Vehicles | | | (46,447 | ) | | (8,663 | ) | | (2,744 | ) | | (57,854 | ) |
| Accumulated Depreciation | | | (3,454,846 | ) | | (453,235 | ) | | (151,591 | ) | | (4,059,672 | ) |
| | | | | | | | | | | | | | |
| Construction in progress | | $ | 6,224,573 | | | 162,252 | | | 263,015 | | $ | 6,649,840 | |
| | | | | | | | | | | | | | |
| Total property and equipment, net | | $ | 27,123,035 | | | (238,780 | ) | | 1,412,782 | | $ | 28,297,036 | |
As mentioned in Note 11, Benda Ebei entered into a commercial bank note issuance agreement with the Shanghai Pudong Development Bank (“Pudong Bank’) on August 14, 2007 and a supplementary agreement on January 21, 2008. Under the agreement this credit facility is secured by the buildings, machinery and equipment of Benda Ebei and Jiangling Benda.
As of March 31, 2008, the net book value of secured property and equipment was approximately Rmb141.69 million or $20.2 million.
The depreciation expense for the three months ended March 31, 2008 was calculated as follows:
| | | Original Cost | | | | | | | | | | | |
| | | December 31, | | March 31, | | | | Salvage | | Estimate | | Depreciation | | Deprecation | | | |
| | | 2007 | | 2008 | | Average | | Value | | Useful Lives | | Calculated | | Reported | | Difference | |
| Building | | $ | 8,858,126 | | | 9,330,132 | | | 9,094,129 | | | 5 | % | | 25 | | | 86,394 | | | 106,983 | | | (20,589 | ) |
| Property and equipment | | | 15,260,167 | | | 16,109,631 | | | 15,684,899 | | | 5 | % | | 13 | | | 298,013 | | | 337,115 | | | (39,102 | ) |
| Office equipment | | | 28,762 | | | 39,703 | | | 34,233 | | | 5 | % | | 5 | | | 1,626 | | | 474 | | | 1,152 | |
| Motor vehicle | | | 206,252 | | | 227,402 | | | 216,827 | | | 5 | % | | 5 | | | 10,299 | | | 8,663 | | | 1,636 | |
| Total property and equipments | | $ | 24,353,307 | | | 25,706,868 | | | 25,030,088 | | | | | | | | | 396,332 | | | 453,235 | | | (56,903 | ) |
The above table shows the calculation of depreciation expenses for the three months ended March 31, 2008. The difference between the depreciation calculated and depreciation reported was due to the changes of foreign exchange translation.
The weighted average useful lives are used as the base for the calculation of depreciation as the estimated useful lives for buildings and property and equipments are varies from 20 to 30 years and 10 to 15 years, respectively.
The total depreciation expense was $453,235 and $99,861 for the three months ended March 31, 2008 and 2007, respectively, and is broken down as follows:
| | | THREE MONTHS ENDED MARCH 31 | |
| | | 2008 | | 2007 | |
| | | (Unaudited) | | (Unaudited) | |
| Cost of sales | | $ | 330,015 | | $ | 23,285 | |
| Operating expenses | | | 123,220 | | | 76,576 | |
| Balance at end of period | | $ | 453,235 | | $ | 99,861 | |
9. | Intangible Assets (Restated) |
The Group’s intangible assets as of March 31, 2008 and December 31, 2007 were comprised as follows:
| | | December 31, 2007 | | Addition | | Foreign Currency Translation Difference | | March 31, 2008 (Unaudited) | |
| Land-use rights | | $ | 2,875,796 | | | - | | | 143,851 | | $ | 3,019,647 | |
| Drugs permits and licenses | | | 2,564,996 | | | - | | | 128,305 | | | 2,693,301 | |
| Technology formulas | | | 1,220,164 | | | - | | | 50,865 | | | 1,271,029 | |
| Patent | | | 1,685,919 | | | - | | | 70,280 | | | 1,756,199 | |
| Cost | | | 8,346,875 | | | - | | | 393,301 | | | 8,740,176 | |
| | | | | | | | | | | | | | |
| Land-use rights | | $ | (201,030 | ) | | (18,247 | ) | | (9,993 | ) | $ | (229,270 | ) |
| Drugs permits and licenses | | | (1,291,425 | ) | | (64,927 | ) | | (60,050 | ) | | (1,416,402 | ) |
| Technology formulas | | | (154,863 | ) | | (31,098 | ) | | (7,133 | ) | | (193,094 | ) |
| Patent | | | (205,047 | ) | | (69,843 | ) | | (9,899 | ) | | (284,789 | ) |
| Accumulated amortization | | | (1,852,364 | ) | | (184,115 | ) | | (87,075 | ) | | (2,123,554 | ) |
| | | | | | | | | | | | | | |
| Total intangible assets, net | | $ | 6,494,510 | | | (184,115 | ) | | 306,226 | | $ | 6,616,622 | |
The amortization expense for the three months ended March 31, 2008 was calculated as follows:
| | | Original Cost | | | | | | | | | |
| | | December 31, | | March 31, | | | | Estimated | | Amortization | | Amortization | | | |
| | | 2007 | | 2008 | | Average | | Useful lives | | Calculated | | Reported | | Difference | |
| Land-use rights | | $ | 2,875,796 | | | 3,019,647 | | | 2,947,722 | | | 40 | | $ | 18,423 | | | 18,247 | | | 176 | |
| Drugs permits and licenses | | | 2,564,996 | | | 2,693,301 | | | 2,629,149 | | | 10 | | | 65,729 | | | 64,927 | | | 802 | |
| Technology formulas | | | 1,220,164 | | | 1,271,029 | | | 1,245,597 | | | 10 | | | 31,140 | | | 31,098 | | | 42 | |
| Patent | | | 1,685,919 | | | 1,756,199 | | | 1,756,199 | | | 6 | | | 73,175 | | | 69,843 | | | 3,332 | |
| Total Intangible assets | | $ | 8,346,875 | | | 8,740,176 | | | 8,578,666 | | | | | $ | 188,467 | | | 184,115 | | | 4,352 | |
The above table shows the calculation of amortization expenses for the three months ended March 31, 2008. The difference between the amortization calculated and amortization reported was due to the changes of foreign exchange translation.
From the above calculation table, the patent, $1.68 million, was acquired in during the acquisition of SiBiono. It is amortized over the remaining useful lives, 6 years, whereas the original useful lives are 10 years.
The total amortization expense was $184,115 and $50,467 for the three months ended March 31, 2008, and 2007, respectively, and is broken down as follows:
| | | THREE MONTHS ENDED MARCH 31 | |
| | | 2008 | | 2007 | |
| | | (Unaudited) | | (Unaudited) | |
| Cost of sales | | $ | 132,540 | | $ | 24,183 | |
| Operating expenses | | | 51,575 | | | 26,284 | |
| Balance at end of period | | $ | 184,115 | | $ | 50,467 | |
10. | Goodwill and Acquisition Cost Payable |
FASB 141 requires all acquisitions must be accounted for by allocating the acquisition consideration to the assets acquired based upon the fair market value of those assets. Consideration value that cannot be allocated to the acquired assets must be assigned to goodwill. In addition, it also requires eliminating the pooling treatment and eliminating the amortization of goodwill. FASB 142 requires that a company carrying goodwill on its books must revalue the assets acquired in a business combination. If there is an overall decline in the value of the acquired assets, then earlier booked goodwill is deemed “impaired” and must be written down. FASB 142 requires a two step impairment test. The fair value of a reporting unit is first compared to its carrying value, including goodwill. Then the implied fair value of the goodwill is compared to the carrying value of the goodwill. If the fair value is lower, it is considered to be impaired.
As of March 31, 2008, there was an amount approximately $7.7 million was recorded as goodwill. Since SiBiono was turned to be profitable from the year of 2007, no impairment would be provided.
As of March 31, 2008, the total purchase price was Rmb62.56 million (or $8.93 million). Out of which, Rmb53.95 million (or $7.7 million) was recognized as goodwill and the remaining Rmb8.61 million (or $1.23 million) was allocated to identifiable assets and assumed liabilities of SiBiono as following:
| | In '000 | |
Current Assets | | | | |
| Cash and cash equivalents | | $ | 590 | |
| Receivables, prepaid expenses and deposits | | | 969 | |
| Inventories | | | 688 | |
Total current assets | | | 2,246 | |
Non-current Assets | | | | |
| Property and equipments, net | | | 6,789 | |
| Intangible assets, net | | | 1,939 | |
Total non-current assets | | | 8,728 | |
Total Assets | | $ | 10,975 | |
| | | | | |
Current Liabilities | | | | |
| Trade payables and miscellaneous payables | | $ | 1,175 | |
| Current portion of long term loans payable | | | 1,813 | |
| Current portion of long term government debts payable | | | 2,710 | |
Total current liabilities | | | 5,699 | |
Non-current Liabilities | | | | |
| Long term loans payable | | | 1,360 | |
| Long term government debts payable | | | 1,723 | |
| Due to related parties (long term) | | | 338 | |
Total non-current liabilities | | | 3,420 | |
Total liabilities | | $ | 9,119 | |
Net assets | | $ | 1,855 | |
| | | 60.13 | % |
Net assets acquired | | $ | 1,116 | Note a |
Note a. From the above table, the net assets of SiBiono as of March 31, 2007 was Rmb8.61 million, and when translating into US currency upon the time acquired, such net assets amount was translated into $1.12 million (whereas the average exchange rate prevailing on that date was $0.12592). However, when we subtract the total acquisition price against the goodwill, the net assets would be $1.18 million. The slightly difference was occurred, $0.11 million and which was solely due to the foreign currency translation.
As of March 31, 2008, out of the total acquisition cost Rmb62.56 million (or $8.93 million), the following payments were made:
In the year of 2006, Benda, through its subsidiaries Everleader and Benda Ebei, paid Rmb19.52 million and Rmb13.03 million or totaling Rmb32.55 million to the selling shareholders of SiBiono and reported in the consolidated balance sheet and cash flow statement as “refundable purchase price paid”. It was recorded as refundable assets due to the fact that the deal was not concluded as of December 31, 2006. The acquisition was closed on April 5, 2007, and thus the total refundable amount of $4.17 million was reclassified as investment cost.
In the year of 2007, an additional amount Rmb20.28 million was paid. The remaining balance was reported as “acquisition cost payable” on the balance sheet. As of March 31, 2008, the total amount paid was Rmb52.83 million (or $7.54 million) and the outstanding balance was Rmb9.73 million (or $1.39 million).
The Group has already obtained the oral consent from the selling shareholders of SiBiono that the remaining balance could be settled within the year 2008.
11. | Restricted Cash, Bank Indebtedness and Commercial Notes Payable |
The Group’s restricted cash at March 31, 2008 and December 31, 2007 was comprised as follows:
| | | March 31, | | | |
| | | 2008 | | December 31, | |
| | | (Unaudited) | | 2007 | |
| Deposits for issuance of commercial notes | | $ | 4,185,066 | | $ | 2,615,254 | |
| Funds from government technolgy agencies | | | 1,269,084 | | | 1,342,370 | |
| Total restricted cash | | $ | 5,454,150 | | $ | 3,957,624 | |
| A) | On August 14, 2007, Benda Ebei entered into a commercial bank note issuable agreement with Shanghai Pudong Development Bank. Pursuant to this agreement, the following terms are included: |
| | a) | Duration of the agreement is three years; |
| | b) | It is non-interest bearing; |
| | c) | The repayment period of each commercial note payable is six months; |
| | d) | The total commercial note issuable limit is Rmb 60 million; however 50% of deposit should be made into the bank in order to secure the issuance of commercial bank note, thus the net available amount is Rmb 30 million; |
| | e) | If the net amount of each commercial bank note payable is not settled on the due date, the penalty will be the penalty rate of the PRC bank loan on daily and compound basis. |
| | f) | As mentioned in Note 8, this credit facility is guaranteed by SiBiono and secured by the buildings, machinery and equipment of Benda Ebei. On December 15, 2007, Benda Ebei received a consent letter from Pudong Bank that Pudong Bank agreed to cancel SiBiono’s guarantee toward this credit facility. |
On January 21, 2008, Benda Ebei entered into a supplementary agreement with Shanghai Pudong Development Bank, to supplement the commercial bank note issuance agreement dated on August 14, 2007 (see Note 8). According to this supplementary agreement, the credit facility is further secured by the buildings, machinery and equipment of Jiangling Benda..
As of March 31, 2008, Benda Ebei and Jiangling Benda deposited an amount $4,185,066 in Shanghai Pudong Development Bank as deposit for the issuance of commercial bank notes. Such deposits will be released when the commercial bank notes are cleared. As of March 31, 2008, the balance of the commercial bank notes payable was $8,187,194. Thus the net commercial bank notes payable was $4,002,128 as of March 31, 2008.
| B) | The bank indebtedness was resulted from the acquisition of SiBiono with the effective date April 1, 2007. The reasons for causing bank indebtedness were stated as follows: |
| | a) | Among the cash and cash equivalents balances of SiBiono were composed of two parts; (i) unrestricted cash, which were generated from either operations, or loans from bank and financial institutions, or invested capital; (ii) restricted cash, which were obtained from the various government technology agencies as long term debt payable (see note 13 for the related details). |
| | b) | The cash obtained from the various government technology agencies as long term debt payable could only be dedicated to the related project’s research and development activities and purchase of fixed assets and construction in progress, therefore the cash balances for that part will be classified as restricted cash. |
| | c) | Due to the above reasons, SiBiono relocated the balances of restricted cash from the cash and cash equivalents balances for the reporting periods. |
| | d) | However, since the balance of the restricted cash was larger than the balance of cash and cash equivalents balances, thus bank indebtedness were resulted for the reporting periods. |
Due to the above reasons, SiBiono relocated the balances of restricted cash from the cash and cash equivalents balances with an amount $1,269,084 as of March 31, 2008. However, since the balances of the restricted cash were larger than the balance of cash and cash equivalents balances, bank indebtedness were resulted with an amount $1,160,976 as of March 31, 2008.
On November 23, 2006, Benda Ebei entered into an Equity Transfer Agreement with Xiaozhi Zhang (“Zhang”), to purchase approximately 6.24% of SiBiono’s common stock for a total consideration of Rmb12.48 million (Rmb6.24 million in cash and shares of our common stock equal to Rmb6.24 million) (or $1.71 million) which was due and payable on or before March 31, 2007.
Due to the fact that the signed agreement on November 23, 2006 was not practically executable according to the PRC regulations, Benda Ebei asked Zhang to terminate the signed agreement and sign a new agreement that was feasible under PRC regulations with essentially the same terms.
However, Zhang refused to sign the new agreement and applied to the Shenzhen Arbitration Commission (the “Commission”) in April 2007 for enforcement of the original agreement. Zhang requested the Commission to require Benda Ebei to pay for the total consideration, penalty for late payment and the related legal and arbitration expenses.
On November 27, 2007, Shenzhen Arbitration Commission determined that:
| 1. | Benda Ebei should pay for the consideration of Rmb 6.24 million, equal to 50% of the total consideration set forth in the Equity Transfer Agreement. For the other 50% of the total consideration which was supposed to be settled in the form of issuing common stock, since Zhang did not make an arbitration request on how to execute the arrangement, the Arbitration Commission did not make an award on this particular part. |
| 2. | Benda Ebei should pay for the penalty of Rmb 46,800; |
| 3. | Benda Eebi should pay for legal and arbitration expenses of Rmb 268,971. |
Following this arbitration decision, Benda Ebei recognized the liability as total acquisition cost payable of Rmb12.48 million, plus the penalty and related legal and arbitration expenses, totaling approximately Rmb12.80 million or $1.83 million (Note 15). Accordingly Benda Eebi recognized the right to purchase the 6.24% equity shares in SiBiono and recorded as other assets at Rmb 12.48 million or $1.78 million.
The Group’s bank loans as of March 31, 2008 and December 31, 2007 were comprised as follows:
| | | March 31, | | | |
| | | 2008 | | December 31, | |
| | | (Unaudited) | | 2007 | |
| Bank loans due within one year | | $ | 2,934,657 | | $ | 2,867,004 | |
| Bank loans due after one year | | | - | | | - | |
| Total bank loans | | $ | 2,934,657 | | $ | 2,867,004 | |
As of March 31, 2008 and December 31, 2007, Sibiono, had one outstanding bank loan with an amount $2,934,657 and $2,867,004, respectively, which was used primarily to fund construction in progress projects and for general working capital purposes. This loan carries annual interest rate of 6.34% and payable monthly on the 20th day of each month with maturity in 3 years, starting from April 30, 2005 and matures on April 29, 2008. This loan is personal guaranteed by Zhaohui Peng, the Chairman and a shareholder of SiBiono.
Total interest expense paid related to the Group’s outstanding bank loans was $48,853 and none for the three months ended March 31, 2008 and 2007, respectively.
14. | Long Term Debt Payable |
As of March 31, 2008, long term debt payable was raised due to the fact that various technology funds were obtained from various government technology agencies to support its gene therapy research and development activities during the past years and recorded as long term debt payable.
The long term debt payable as of March 31, 2008, and December 31, 2007 were comprised as follows:
| | | March 31, | | | |
| | | 2008 | | December 31, | |
| | | (Unaudited) | | 2007 | |
| Long-term debt payable due within one year | | $ | 1,732,498 | | $ | 1,787,239 | |
| Long-term debt payable due after one year | | | 442,718 | | | 425,001 | |
| Total long-term debt payable | | $ | 2,175,216 | | $ | 2,212,240 | |
Even though there were $1,732,498 long term debt payable due within one year as of March 31, 2008, because of its non-repayable feature, the obligations will be discharged once the examination by the various government technology agencies is conducted and most of the examination will be carried out and completed.
During the three months ended March 31, 2008, there was no long term debt payable discharged and recorded as government subsidies (see Note 22 for the related details).
15. | Accounts Payable and Accrued Liabilities |
The Group’s accounts payable and accrued liabilities as of March 31, 2008 and December 31, 2007were comprised as follows:
| | | March 31, | | | |
| | | 2008 | | December 31, | |
| | | (Unaudited) | | 2007 | |
| Trade payable | | $ | 407,863 | | $ | 305,443 | |
| Deposits paid by customer | | | 14,451 | | | 160,818 | |
| Acquistion cost payable following the arbitration (Note 12) | | | 1,827,393 | | | 1,754,263 | |
| Accrued liabilities | | | 2,934,776 | | | 2,162,455 | |
| Miscellaneous payables | | | 334,832 | | | 283,005 | |
| Total account payables and accurred liabilities | | $ | 5,519,316 | | $ | 4,665,984 | |
16. | Welfare and Employment Liabilities |
As stipulated by the relevant laws and regulations for enterprises operating in the PRC, the Group’s PRC entities are required to maintain a welfare plan for all of its employees who are residents of the PRC. Based on the wages payable and according to the labor law of the PRC, the Group accrued 14% on a monthly basis, for employees’ welfare, labor union fees, and education and training programs, respectively. As of March 31, 2008 and December 31, 2007, the Group accrued approximately $462,010 and $345,000 for the employees’ welfare respectively.
As stipulated by the relevant laws and regulations for enterprises operating in the PRC, the Group is required to make annual appropriations to a statutory surplus reserve fund for each of its PRC subsidiaries. Specifically, the Group is required to allocate 15% its profits after taxes at the fiscal year end, as determined in accordance with the PRC accounting standards applicable to the Group’s PRC subsidiaries, to a statutory surplus reserve until such reserve reaches 50% of the registered capital of the Group’s PRC subsidiaries. As of March 31, 2008 and December 31, 2007, the registered capital of the Group’s PRC subsidiaries was $20,026,617.
18. | Related Party Transactions |
Due from related parties as of March 31, 2008 and December 31, 2007 were comprised as follows:
| | | March 31, | | | |
| | | 2008 | | December 31, | |
| | | (Unaudited) | | 2007 | |
| Yiqing Wan | | | | | | | |
| Due to Ever Leader Holdings Co. Ltd. | | $ | 648,278 | | $ | 646,429 | |
| Due to Hubei Tongji Benda Ebei Phamacetucial Co. Ltd. | | | 155,613 | | | 72,949 | |
| Due to Shenzhen SiBiono Gene Tech Co. Ltd. | | | - | | | 3,608 | |
| Hubei Benda Science and Technology Co. Ltd | | | | | | | |
| Due to Yidu Benda Chemicals Co. Ltd. | | | 1,564,737 | | | 1,502,118 | |
| Due to Ever Leader Holdings Co. Ltd. | | | 230,818 | | | 230,160 | |
| Feng Wang | | | | | | | |
| Due to Beijing Shusai Pharyngitis Research Co. Ltd. | | | 30,541 | | | 29,318 | |
| Qin Yu | | | | | | | |
| Due to Shenzhen SiBiono Gene Tech Co. Ltd. | | | 5,427 | | | - | |
| Hua Shen | | | | | | | |
| Due to Shenzhen SiBiono Gene Tech Co. Ltd. | | | 142,797 | | | 137,097 | |
| Pong Tsaiohuei | | | | | | | |
| Due to Shenzhen SiBiono Gene Tech Co. Ltd. | | | 1,273 | | | 3,257 | |
| Xiaozhi Zhang | | | | | | | |
| Due to Shenzhen SiBiono Gene Tech Co. Ltd. | | | 5,295 | | | 5,083 | |
| Xiaojing Wang | | | | | | | |
| Due to Shenzhen SiBiono Gene Tech Co. Ltd. | | | 282 | | | - | |
| Rong He | | | | | | | |
| Due to Shenzhen SiBiono Gene Tech Co. Ltd. | | | 286 | | | - | |
| Total due from related parties | | $ | 2,785,347 | | $ | 2,630,019 | |
Due to related parties as of March 31, 2008 and December 31, 2007 were comprised as follows:
| | | March 31, | | | |
| | | 2008 | | December 31, | |
| | | (Unaudited) | | 2007 | |
| Hubei Benda Science and Technology Co. Ltd | | | | | | | |
| Due from Hubei Tongji Benda Ebei Phamacetucial Co. Ltd. | | $ | 27,848 | | $ | 49,056 | |
| Due from Jiangliang Benda Pharamaceutical Co. Ltd. | | | 1,932,149 | | | 1,872,374 | |
| Due from Beijing Shusai Pharyngitis Research Co. Ltd. | | | 13,879 | | | 6,846 | |
| Wei Xu | | | | | | | |
| Due from Shenzhen SiBiono Gene Tech Co. Ltd. | | | 1,574 | | | - | |
| Hua Xu | | | | | | | |
| Due from Shenzhen SiBiono Gene Tech Co. Ltd. | | | 24,849 | | | - | |
| Due from Hubei Tongji Benda Ebei Phamacetucial Co. Ltd. | | | 1,051,887 | | | 1,009,792 | |
| Due from Beijing Shusai Pharyngitis Research Co. Ltd. | | | 64,054 | | | 61,491 | |
| Yiqing, Wan | | | | | | | |
| Due from Yidu Benda Chemicals Co. Ltd. | | | 545 | | | - | |
| Due from Shenzhen SiBiono Gene Tech Co. Ltd. | | | 139,668 | | | 137,687 | |
| Hui Xu | | | | | | | |
| Due from Hubei Tongji Benda Ebei Phamacetucial Co. Ltd. | | | 27,732 | | | 26,622 | |
| Due from Beijing Shusai Pharyngitis Research Co. Ltd. | | | - | | | 3,153 | |
| Hua Shen | | | | | | | |
| Due from Shenzhen SiBiono Gene Tech Co. Ltd. | | | 355 | | | 26,597 | |
| Total due to related parties | | | | | | | |
| | | $ | 3,284,540 | | $ | 3,193,618 | |
The above advances bear no interest and the above loans due to related parties are unsecured, non-interest bearing and are not convertible into equity. Proceeds from the above loans were used primarily for general working capital purposes and are also long-term debts in nature, due on December 31, 2012.
19. | Redeemable Common Stock Issuable For Services |
On April 5, 2007, Benda Ebei entered into Equity Transfer Agreements with certain shareholders of SiBiono to purchase a total of approximately 57.57% of the shares of SiBiono’s common stock for total consideration of RMB 60,000,000 due and payable on or before April 30, 2007.
On June 11, 2007, Benda Ebei entered into additional Equity Transfer Agreements with Yaojin Wang (“Wang”) and Huimin Zhang “(Zhang”), also shareholders of SiBiono, for the purchase of an additional 2.56% of the shares of SiBiono’s common stock for total consideration of RMB 2,560,000 due and payable on or before June 30, 2007.
In connection with the above Equity Transfer Agreements, Benda entered into a Financial Consultancy Agreement with Super Pioneer International Limited (“Super Pioneer”) and Technical Consultancy Agreements with Wang and Zhang for financial and technical consultancy services to be rendered. Pursuant to the Financial and Technical Consultancy Agreements (the “Agreements”), Benda agreed to issue an aggregate of 2,189,560 shares of its common stock to Super Pioneer (2.1 million shares, out of which 1.9 million shares is redeemable), Wang (33,585 shares, redeemable) and Zhang (55,975 share, redeemable) within three months from the date of the Agreements. Super Pioneer, Wang and Zang also agreed to refrain from selling shares of Benda’s common stock for a period of twelve months from the date of the issuance of the shares (the “Lock-up Period”). Within three months from expiration of the Lock-up Period, in the event that the public trading price of Benda’s common stock has not reach $3.60 per share and Benda’s common stock has not been listed on either the NASDAQ or AMEX stock exchanges, Super Pioneer, Wang, and Zang will have the option to require Benda to redeem an aggregate 2,049,560 shares of Benda’s common stock owned by Super Pioneer, Wang, and Zhang at a price of $3.60 per share.
In accordance EITF Topic D-98, “Classification and Measurement of Redeemable Securities” (“EITF Topic D-98”), as the Agreements governing the issuance of the 2,189,560 shares of common stock to Super Pioneer, Wang, and Zang contain provisions requiring Benda to repurchase 2,049,560 of these shares at a redemption price of $3.60 per share at the option of the holders (if certain events outside of control of the Group do not occur), these 2,049,560 shares have been classified as redeemable common stock, at their redemption price of $3.60 per share or totaling $7,376,366, outside of permanent equity at March 31, 2008.
In accordance with FASB staff position No. EITF00-19-2 the Group recorded $722,205 in Registration Delay Expense, for the three month ended March 31, 2008. Out of the total penalty, $230,312 was settled by issuance of 523,438 shares of common stock.
21. | Other general and administrative expenses |
For the three months ended March 31, 2008 and 2007, the amount of other general and administrative expenses mainly composed of the following events:
| | | THREE MONTHS ENDED MARCH 31 | |
| | | 2008 | | 2007 | |
| | | (Unaudited) | | (Unaudited) | |
| Audit and accounting | | $ | 129,334 | | $ | 3,500 | |
| Legal fees | | | 8,053 | | | 62,316 | |
| Office expenses | | | 138,971 | | | 67,199 | |
| Salaries and wages | | | 349,270 | | | 131,362 | |
| Consultancy and professional fees | | | 232,914 | | | - | |
| Rent & Utilities | | | 35,540 | | | 4,490 | |
| Investor relation, Transfer agent and filing fees | | | 18,846 | | | 35,930 | |
| Travel and transportation | | | 70,563 | | | 12,045 | |
| Repair and maintanence | | | 76,654 | | | 3,472 | |
| Miscellaneous | | | 37,724 | | | 4,399 | |
| Total other general & administrative | | $ | 1,097,869 | | $ | 324,713 | |
22. | Government Subsidies / Grants |
As mentioned in the Note 14, long term debt payable, various technology funds were obtained from various government technology agencies to support its gene therapy research and development activities during the past years and recorded as long term debt payable. According to the technology fund agreements, the various government technology agencies will examine the results of research and development according to the status of the projects. Once the examination is taken place, the obligation of a particular debt payable is discharge accordingly.
According to US GAAP, once the obligation of a particular debt payable is discharged, the amount of this particular debt payable should be treated as government subsidies / grants. During the three months ended March 31, 2008, there were no government subsidies / grants recognized as revenue.
Benda is subject to Delaware, United State of America tax, but no provision for income taxes were made for the three months ended March 31, 2008 and 2007 as Benda did not have reportable taxable income for the period.
Ever Leader, a wholly owned subsidiary of Benda, is subject to Hong Kong tax, but no provisions for income taxes were made for the three months ended March 31, 2008 and 2007 as Ever Leader did not have reportable taxable income for the periods.
Benda Ebei was registered as a Sino-Foreign Equity Joint Venture on May 26, 2004 and is subject to the tax laws applicable to Sino-Foreign Equity Joint Ventures in the PRC. Benda Ebei, starting from 2005, is fully exempt from PRC enterprise income tax for two years starting from the first profit-making year, followed by a 50% reduction in the state income taxes, for the following three years, commencing from the first profitable year.
Jiangling Benda and Yidu Benda are cross-municipal investment entities and enjoy the same tax treatment as Sino-Foreign Joint Ventures, starting from 2005, and were therefore exempt from PRC enterprise income tax for two years starting from the first profit-making year, followed by a 50% reduction in the state income taxes, for the following three years, commencing from the first profitable year. Cross-municipal investments entities refer to entities that are incorporated in one municipal region but have investments in another municipal region.
The exemption periods for Benda Ebei, Jiangling Benda and Yidu Benda expired in the year of 2006, after which they are subject to a 50% reduction in state income taxes, at 18%; whereas the full income tax rate is 33%. The remaining tax holidays will be expired in 2010.
However, starting and effective from January 1, 2008, the full income tax rate would be changed from 33% to 25% according to the new PRC taxation regulations. Therefore these subsidiaries will be subject to the regular full income tax rate at 25% after the tax holidays expire in 2010.
According to the new taxation regulations starting and effective from January 1, 2008, Beijing Shusai is subject to the full income tax rate of 25%.
According to the new taxation regulations starting and effective from January 1, 2008, SiBiono, which is located in Shenzhen, a Special Economic District of PRC, is subject to the full income tax rate of 25% gradually in five years as following:
| Year | | Tax rate | |
| 2008 | | | 18 | % |
| 2009 | | | 20 | % |
| 2010 | | | 22 | % |
| 2011 | | | 24 | % |
| 2012 and thereafter | | | 25 | % |
For the three months ended March 31, 2008, only Ebei Benda had taxable income and incurred income tax of Rmb 0.95 million or $0.13 million. There was no income tax of the Group for the three months ended March 31, 2007.
24. | Long Term Convertible Promissory Note |
Our financial statements include enhanced note disclosure addressing the fair value of the convertible promissory notes and warrants and the appropriate accounting treatment for the overall transaction.
Additionally, the enhanced note disclosure includes a detailed discussion of the accounting for the debt discount resulting from the relative fair value of the warrants issued in conjunction with the convertible promissory notes (calculated in accordance with APB 14) and the debt discount resulting from the beneficial conversion feature / conversion discount associated with the convertible promissory notes (calculated in accordance with EITF 98-5 and EITF 00-27) as follows:
In March and April of 2007, the Group entered into Investment Agreements (“Agreements”) with certain accredited and institutional investors (“Investors”) pursuant to which the Investors purchased from the Group a total of 252 Units (“Units”), resulting in aggregate gross proceeds to the Group $7,560,000, with each Unit consisting of: (i) a Convertible Promissory Note (“Note”) in the principal amount of $30,000 and convertible into 54,087 shares of the Group's common stock; and (ii) a Common Stock Warrant (“Warrant”) to acquire 54,087 shares of the Group’s common stock at an exercise price of $0.555 per share and expiring on November 14, 2011. The Notes are immediately convertible at the option of each Investor, bear interest at a rate of 4% per annum, and mature on March 28, 2009. As of March 31, 2007, an amount $5,520,000 was received; while as of March 31, 2008, the aggregate $7,560,000 principal balance of the notes remained outstanding and for the three months ended March 31, 2008, the Group recorded $75,393 of interest expense related to the Notes.
The Group has accounted for the Warrants issued in conjunction with the Notes in accordance with the provisions of APB No. 14, “Accounting for Convertible Debt and Debt Issued with Stock Purchase Warrants” (“APB 14”). Accordingly, the Warrants were valued using a Black-Scholes option pricing model with the following assumptions: (i) a risk free interest rate of 4.63%, (ii) a contractual life of 4.6 years, (iii) an expected volatility of 25%, and (iv) a dividend yield of zero. The relative fair value of the Warrants, based on an allocation of the value of the Notes and the value of the Warrants issued in conjunction with the Notes, was recorded as a debt discount (with a corresponding increase to additional paid-in capital) in the amount of $5,174,911, and is being amortized to interest expense over the expected term of the Notes.
Additionally, the difference between the effective conversion price of the Notes into shares of the Group’s common stock, and the fair value of the Group’s common stock on the date of issuance of the Notes, resulted in a beneficial conversion feature of $2,385,089 (capped at the $7,560,000 of gross proceeds raised less the previously calculated $5,174,911 debt discount associated with Warrants issued in conjunction with the Notes) and was calculated in accordance with EITF 98-5, “Accounting for Convertible Securities with Beneficial Conversion Features or Contingently Adjustable Conversion Ratios” and EITF 00-27, “Application of Issue No. 98-5 to Certain Convertible Instruments”. This beneficial conversion feature was recorded as an additional debt discount (with a corresponding increase to additional paid-in capital) and is being amortized to interest expense over the expected term of the Notes.
During the three months ended March 31, 2008, the Group recorded $941,122 in interest expense related to the amortization of the debt discount associated with the Warrants and the debt discount associated with the beneficial conversion feature.
The Group also incurred $529,200 in placement agent commissions related to the issuance of the Notes and Warrants. This amount was recorded by the Group as debt issue costs and is being amortized over expected term of the Notes. For the three months ended March 31, 2008, the Group recorded $65,879 in amortization expense related to debt issue costs.
The securities underlying the Notes and Warrants issued to the Investors are also subject to the terms of a Make Good Agreement entered into in connection with a financing the Group executed in November of 2006 (the “Make Good Agreement”). The Group further represented to the Investors that its target net income for fiscal year 2007 (“FY07 Net Income”) will be greater than or equal to $10.0 million (adjusted for a variety of non-cash charges) (the “Performance Threshold”). In the event the Performance Threshold is not attained, the Group is required to issue to the Investors a pro rata portion of 1,000,000 shares of the Group’s common stock for every one (1) cent by which the Group’s earnings per share, determined on a fully diluted basis, is less than $0.065.
In the accounting for the Long Term Converible Promissory Note, the Group’s analysis of the Performance Threshold had no effect because of the cap of the debt discounted and beneficial conversion feature as calculated in accordance with EITF 98-5.
The Group is also required to register for resale: (i) the shares of common stock underlying the Notes; and (ii) 150% of the shares of common stock underlying the Warrants, on a registration statement to be filed with the Securities and Exchange Commission (“Registration Statement”) and such Registration Statement is required to be declared effective by August 15, 2007 (the “Effectiveness Deadline”). If the Registration Statement does not become effective by the Effectiveness Deadline, or if the Group fails to maintain the effectiveness of the Registration Statement, for any reason, the Group is required to pay the Investors in cash an amount equal to 1% of the purchase price of each Unit held by the Investors on such Effectiveness Deadline or the first day of such failure to maintain the Registration Period, as applicable, and for every 30 day period (or part) thereafter, in each case until cured (“Registration Delay Payments”), provided that the Registration Delay Payments will not exceed 10% of the purchase price of the Units. In the event that the Registration Delay Payments are not made in a timely manner, such Registration Delay Payments will bear interest at a rate of 1.5% per month until paid in full.
The calculated fair value of the warrants (issued in conjunction with the convertible promissory notes) was recorded as a debt discount (reduction to the carrying value of the convertible promissory notes) with a corresponding increase (credit) to additional paid in capital.
In accordance with the registration rights granted to investors who purchased the convertible promissory notes and warrants, we are required to file a registration statement with the Securities and Exchange Commission (“Registration Statement”) attempting to register the potentially issuable shares of common stock underlying the convertible promissory notes and warrants. There is no cash settlement requirement, in the event the Company cannot deliver Registered Shares.
In the event the Registration Statement is never declared effective, the Registration Delay Payments are capped at 10% of the purchase price paid by each investor for the convertible promissory notes and warrants. As a result, the initial requirement for the Company to deliver registered shares upon conversion of the convertible promissory notes or exercise of the warrants is negated and paragraphs 14 - 18 of EITF 00-19 are not applicable (for purposes of classifying the fair value of the warrants issued in conjunction with the convertible promissory notes as a liability with changes in fair value recorded in earnings).
In accordance with FASB staff position No. EITF00-19-2, during the three months ended March 31, 2008 the Group recorded $722,205 for Registration Delay Expense or penalty to investors. EITF 00-19-2 requires the Group to record the expense when it is probable that the liability has been incurred and the amount can be reasonably estimated.
25. | Common Stock, Preferred Stock, Additional Paid-in Capital, Warrants and Options |
As of March 31,2008 and December 31, 2007, the Group had an aggregate of:
| | (a) | None shares of preferred stock issued and outstanding; |
| | (b) | As of December 31, 2007, 100,170,071 shares of common stock were issued and outstanding, par value $0.001 each; During the three months ended March 31, 2008:; (i) 523,438 shares were issued to PIPE investors for being late submission of 10K-SB (see Note 20); and (ii) 110,000 shares were issued to the independent directors in lieu of their reumeration. Thus, the share of common stock issued and outstanding was 100,803,509 as of March 31, 2008. |
| | (c) | The following tables shows the events occurred in additional paid-in capital: |
| Events | | December 31, 2007 | | Events occurred during the reporting period | | March 31, 2008 (Unaudited) | |
| To record the changes of par value from $0.01 to $0.001 of the outstanding common stock as of 11/17/2005 | | $ | 584,481 | | | - | | $ | 584,481 | |
| | | | | | | | | | | |
| To adjust the par value of outstanding common stock as of 3/31/2006 | | | (5,354 | ) | | - | | | (5,354 | ) |
| | | | | | | | | | | |
| Waiver of shareholder loan on 9/5/2006 | | | 2,298,434 | | | - | | | 2,298,434 | |
| | | | | | | | | | | |
| To eliminate the common stock and additional paid-in capital of the former shell company "Applied Spectrum Technologies, Inc." on 11/15/2006 | | | 16,215,770 | | | - | | | 16,215,770 | |
| | | | | | | | | | | |
| To eliminate the accumulated deficit of the former shell company "Applied Spectrum Technologies, Inc." on 11/15/2006 | | | (16,209,962 | ) | | - | | | (16,209,962 | ) |
| | | | | | | | | | | |
| Issuance of common stock, 25,961,760 shares at $0.4622 per share, on 11/15/2006 | | | 11,974,038 | | | - | | | 11,974,038 | |
| | | | | | | | | | | |
| Issuance of common stock, 64,942,369 shares at $0.001 per share on 11/15/2006 | | | (64,942 | ) | | - | | | (64,942 | ) |
| | | | | | | | | | | |
| To relocate the original common stock of Ever Leader on 11/15/2006 | | | 1,285 | | | - | | | 1,285 | |
| | | | | | | | | | | |
| To record the placement agent commission and transaction related fee of reverse merger on 11/15/2006 | | | (1,694,326 | ) | | - | | | (1,694,326 | ) |
| | | | | | | | | | | |
| Issuance of common stock 706,195 at $0.4622 per share | | | 325,697.00 | | | - | | | 325,697 | |
| | | | | | | | | | | |
| Debt discount on beneficial conversion feature on convertible promissory notes | | | 2,385,089.00 | | | - | | | 2,385,089 | |
| | | | | | | | | | | |
| Debt discount on warrants issued with convertible promissory notes | | | 5,174,911.00 | | | - | | | 5,174,911 | |
| | | | | | | | | | | |
| Placement agent exercised 849,007 warrants at strike price through cashless arrangement | | | 470,347.00 | | | - | | | 470,347 | |
| | | | | | | | | | | |
| Issuance of common stock to PIPE investors as penalty for late submission of 10KSB | | | 92,461.00 | | | - | | | 92,461 | |
| | | | | | | | | | | |
| Issuance of common stock to directors in lieu of their remuneration | | | - | | | 75,790 | | | 75,790 | |
| | | | | | | | | | | |
| Issuance of common stock to PIPE investors as penalty for late submission of 10KSB | | | - | | | 229,789 | | | 229,789 | |
| | | | | | | | | | | |
| Additional paid-in capital, balance for the period ended | | $ | 21,547,929 | | | 305,579 | | $ | 21,853,508 | |
| | (d) | As of December 31, 2007, 27,708,929 Warrants, each convertible into one (1) share of the Group’s Common Stock, issued and outstanding. During the three months ended March 31, 2008, 1,597,075 warrants were issuable to CRT Capital Group, LLC and 956,245 warrants were issuable to EastGate Financial, Inc. in lieu of their consultancy service. Thus the remaining balance as March 31, 2008 was 30,262,249 |
| | (e) | .None options issued and outstanding. |
26. | Weighted average shares outstanding - diluted |
Basic net income (loss) per share is computed by dividing net income (loss) by the weighted-average number of shares outstanding during the period.
Diluted net income (loss) per share is computed by using the weighted-average number of shares of common stock outstanding and, when dilutive, potential shares from stock options and warrants to purchase common stock, using the treasury stock method.
The following table illustrates the computation of basic and dilutive net income (loss) per share and provides a reconciliation of the number of weighted-average basic and diluted shares outstanding:.
| | | THREE MONTHS ENDED MARCH 31 | |
| | | 2008 | | 2007 | |
| | | (Unaudited) | | (Unaudited) | |
| Numerator: | | | | | | | |
| Net income (loss) | | $ | (2,037,413 | ) | $ | 532,731 | |
| | | | | | | | |
| Denominator: | | | | | | | |
| Basic weighted-average shares outstanding | | | 100,312,873 | | | 96,281,951 | |
| Effect of dilutive stock options & warrants | | | - | | | 27,817,963 | |
| Diluted weighted-average shares outstanding | | | 100,312,873 | | | 124,099,914 | |
| | | | | | | | |
| Net income (loss) per share: | | | | | | | |
| Basic | | $ | (0.02 | ) | $ | 0.01 | |
| Diluted | | $ | (0.02 | ) | $ | 0.00 | |
27. | Commitments and Contingencies |
As of March 31, 2008, there was an operating lease commitment and the amounts are stated as follows;
| | | March 31, | |
| | | 2008 | |
| | | (Unaudited) | |
| Rental and Property Management Fee | | | | |
| Within one year | | $ | 91,715 | |
| One to two year | | | 76,763 | |
| Total commitments payable | | $ | 168,478 | |
The company states the segment information according to the requirement stated in paragraph 37 of SFAS 131. The company produces five different categories of products and each category of product is produced in different subsidiaries or operation plants. The details are stated as follows:
| | 1. | Benda Ebei produces branded and generic medicines; |
| | 2. | Jiangling Benda produces active pharmaceutical ingredients, APIs; |
| | 3. | Yidu Benda produces bulk chemicals; |
| | 4. | Beijing Shusai produces pharyngitis killer therapy; and |
| | 5. | SiBiono produces gene therapy medicines, Gendicine. |
Since each subsidiary produces the corresponding products by using the same production facilities of each subsidiary, therefore according to the requirement stated in paragraph of SFAS 131, the Group reports the segment information according to the un-identical products that produced in each subsidiary.
Selected financial information for each of these segments for the three months ended March 31, 2008 and 2007 were as follows:
| | | THREE MONTHS ENDED MARCH 31 | |
| | | 2008 | | 2007 | |
| | | (Unaudited) | | (Unaudited) | |
| | | | | | |
Branded/Generic medicine segment | | 2008 | | 2007 | |
| Revenue from external customers | | $ | 5,521,109 | | $ | 2,339,463 | |
| Cost of sales | | | (3,781,154 | ) | | (1,456,232 | ) |
| Gross profit | | | 1,739,955 | | | 883,231 | |
| Gross margin | | | 32 | % | | 38 | % |
| Research and development | | | - | | | - | |
| Selling expense | | | (214,651 | ) | | (55,803 | ) |
| General and administrative expense | | | (441,833 | ) | | (134,147 | ) |
| Segment contribution | | $ | 1,083,471 | | $ | 693,281 | |
| Contribution margin | | | 20 | % | | 30 | % |
| | | | | | | | |
| Total assets, segment | | $ | 37,198,711 | | $ | 15,565,853 | |
Active pharmaceutical ingredients segment | | 2008 | | 2007 | |
| Revenue from external customers | | $ | 348 | | $ | - | |
| Cost of sales | | | - | | | (5,805 | ) |
| Gross profit | | | 348 | | | (5,805 | ) |
| Gross margin | | | 100 | % | | - | |
| Research and development | | | - | | | - | |
| Selling expense | | | - | | | (3,011 | ) |
| General and administrative expense | | | (243,569 | ) | | 28,767 | |
| Segment contribution | | $ | (243,221 | ) | $ | 19,951 | |
| Contribution margin | | | -69891 | % | | - | |
| | | | | | | | |
| Total assets, segment | | $ | 8,909,348 | | $ | 4,004,119 | |
Bulk chemicals segment | | 2008 | | 2007 | |
| Revenue from external customers | | $ | - | | $ | 684,465 | |
| Cost of sales | | | - | | | (384,868 | ) |
| Gross profit | | | - | | | 299,597 | |
| Gross margin | | | - | | | 44 | % |
| Research and development | | | - | | | - | |
| Selling expense | | | - | | | (26,409 | ) |
| General and administrative expense | | | (297,977 | ) | | (90,564 | ) |
| Segment contribution | | $ | (297,977 | ) | $ | 182,624 | |
| Contribution margin | | | - | | | 27 | % |
| | | | | | | | |
| Total assets, segment | | $ | 7,536,881 | | $ | 8,692,511 | |
Pharynigitis killer therapy segment | | 2008 | | 2007 | |
| Revenue from external customers | | $ | 2,097 | | $ | 5,107 | |
| Cost of sales | | | (115 | ) | | (307 | ) |
| Gross profit | | | 1,982 | | | 4,800 | |
| Gross margin | | | 95 | % | | 94 | % |
| Research and development | | | - | | | - | |
| Selling expense | | | (699 | ) | | (2,960 | ) |
| General and administrative expense | | | (12,317 | ) | | (24,081 | ) |
| Segment contribution | | $ | (11,034 | ) | $ | (22,241 | ) |
| Contribution margin | | | -526 | % | | -436 | % |
| | | | | | | | |
| Total assets, segment | | $ | 101,173 | | $ | 97,785 | |
Gendicine (Ad-p53) segment | | 2008 | | 2007 | |
| Revenue from external customers | | $ | 423,816 | | $ | - | |
| Cost of sales | | | (35,977 | ) | | - | |
| Gross profit | | | 387,839 | | | - | |
| Gross margin | | | 92 | % | | - | |
| Research and development | | | (22,064 | ) | | - | |
| Selling expense | | | (221,021 | ) | | - | |
| General and administrative expense | | | (310,157 | ) | | - | |
| Segment contribution | | $ | -165,403 | | $ | - | |
| Contribution margin | | | -39 | % | | - | |
| | | | | | | | |
| Total assets, segment | | $ | 14,031,627 | | $ | - | |
| | | TOTAL | |
| | | 2008 | | 2007 | |
| Total revenue from external customers | | $ | 5,947,370 | | $ | 3,029,035 | |
| Cost of sales | | | (3,817,246 | ) | | (1,847,212 | ) |
| Gross profit | | | 2,130,124 | | | 1,181,823 | |
| Gross margin | | | 36 | % | | 39 | % |
| Research and development | | | (22,064 | ) | | 0 | |
| Selling expense | | | (436,371 | ) | | (88,183 | ) |
| General and administrative expense | | | (1,305,853 | ) | | (220,025 | ) |
| Segment contribution | | $ | 365,836 | | $ | 873,615 | |
| Contribution margin | | | 6 | % | | 29 | % |
| | | | | | | | |
| Total assets, segment | | $ | 67,777,739 | | $ | 28,360,268 | |
The results of the total consolidated net profit before income taxes for the reporting periods are as follows:
| | | THREE MONTHS ENDED MARCH 31 | |
| | | 2008 | | 2007 | |
| | | (Unaudited) | | (Unaudited) | |
| Total segment contribution | | $ | 365,836 | | $ | 873,615 | |
| Unallocated amounts: | | | | | | | |
| Other income | | | 301,693 | | | 937 | |
| Other corporate expenses | | | (2,549,347 | ) | | (294,915 | ) |
| | | | | | | | |
| Total income / (loss) before minority interest and income taxes | | $ | (1,881,818 | ) | $ | 579,637 | |
The other corporate expenses for the three months ended March 31, 2008 and 2007 composed of the following events:
| | | 2007 | | 2006 | |
| Penalty to investor | | $ | 722,205 | | $ | 120,000 | |
| Wages and salaries | | | 151,765 | | | 60,000 | |
| Audit and accounting | | | 45,306 | | | 3,500 | |
| Amortization of debt issue cost | | | 65,879 | | | 8,743 | |
| Consulting fee | | | 180,500 | | | - | |
| Investor relation, transfer agent and filing fees | | | 18,846 | | | 35,930 | |
| Director renumeration | | | 110,873 | | | - | |
| Legal fee | | | 31,624 | | | 35,095 | |
| Brokerage fees | | | | | | | |
| Travel and transportation | | | 4,842 | | | - | |
| Office expense | | | 1,120 | | | 2,297 | |
| Interest expense | | | 1,212,282 | | | 17,585 | |
| Miscellaneous | | | 4,105 | | | 11,765 | |
| | | | | | | | |
| Total | | $ | 2,549,347 | | $ | 294,915 | |
For the details of information of this particular, it should be read in conjunction with the management discussion and analysis section.
The following table shows the reconciliation between the segments assets and the total assets as of March 31, 2008 and December 31, 2007:
| | | March 31, | | March 31, | |
| | | 2008 | | 2007 | |
| | | (Unaudited) | | (Unaudited) | |
| Total assets, segment | | $ | 67,777,739 | | $ | 28,360,268 | |
| | | | | | | | |
| Total assets of corporate: | | | | | | | |
| Cash and cash equivalent | | | 44,958 | | | 5,845,699 | |
| Bank indebtedness | | | 1,160,976 | | | - | |
| Prepaid expesnes and deposit | | | 230 | | | 2,288 | |
| Refundable purchase price paid | | | 1,200,000 | | | 3,680,492 | |
| Due from related parties | | | 879,096 | | | 263,296 | |
| Debit issue costs: | | | | | | | |
| placement agent commission | | | 262,066 | | | 377,657 | |
| | | | | | | | |
| Total assets | | $ | 71,325,065 | | $ | 38,529,700 | |
The following table shows how the minority interest for the three months ended March 31, 2008 and 2007 was derived:
| | | Three Months Ended March 31, 2008 | |
| | | Benda | | Jiangling | | Yidu | | Beijing | | | | | |
| | | Ebei | | Benda | | Benda | | Shusai | | SiBiono | | Total | |
Segment operating profit / (loss) | | $ | 1,083,472 | | | (243,221 | ) | | (297,977 | ) | | (11,034 | ) | | (165,403 | ) | $ | 365,837 | |
| Interest income/ (expenses) | | | (147,734 | ) | | 1,065 | | | (18 | ) | | (2 | ) | | (48,853 | ) | | (195,542 | ) |
| Other income / (expenses) | | | (5,647 | ) | | 493 | | | 992 | | | 7,278 | | | 298,577 | | | 301,693 | |
| Income taxes | | | (133,197 | ) | | - | | | - | | | - | | | - | | | (133,197 | ) |
Income / (loss) before minority interest | | $ | 796,894 | | | (241,663 | ) | | (297,003 | ) | | (3,758 | ) | | 84,321 | | $ | 338,791 | |
| | | | | | | | | | | | | | | | | | | | |
| MI percentage | | | 5.00 | % | | 9.75 | % | | 9.75 | % | | 28.75 | % | | 42.88 | % | | | |
| MI interest | | $ | 39,845 | | | (23,562 | ) | | (28,958 | ) | | (1,080 | ) | | 36,154 | | $ | 22,398 | |
| | | Three Months Ended March 31, 2007 | |
| | | Benda | | Jiangling | | Yidu | | Beijing | | | | | |
| | | Ebei | | Benda | | Benda | | Shusai | | SiBiono | | Total | |
Segment operating profit / (loss) | | $ | 693,280 | | | 19,951 | | | 182,624 | | | (22,241 | ) | | - | | $ | 873,614 | |
| Loss on disposal of assets | | | - | | | (6,955 | ) | | (5,070 | ) | | - | | | - | | | (12,025 | ) |
| Interest income/ (expenses) | | | 636 | | | (3 | ) | | (2,597 | ) | | (19 | ) | | - | | | (1,983 | ) |
| Other income / (expenses) | | | - | | | 65 | | | 872 | | | - | | | - | | | 937 | |
Income / (loss) before minority interest | | $ | 693,916 | | | 13,058 | | | 175,829 | | | (22,260 | ) | | - | | $ | 860,543 | |
| | | | | | | | | | | | | | | | | | | | |
| MI percentage | | | 5.00 | % | | 9.75 | % | | 9.75 | % | | 28.75 | % | | - | | | | |
| MI interest | | $ | 34,696 | | | 1,273 | | | 17,143 | | | (6,400 | ) | | - | | $ | 46,906 | |
Further, set forth below is a breakdown of the various product segments:
Manufacturer | | Product | | Type | | Function |
| Benda Ebei | | Jixuening injection vial | | Generic | | Haemostatic (stops bleeding) |
| Benda Ebei | | Xujing injection vial | | Generic | | Haemostatic |
| Benda Ebei | | Nokeqing injection vial | | Generic | | Used to treat hepatitis |
| Benda Ebei | | Yidingshu injection vial | | Generic | | Vitamin to treat lack of Riboflavin |
| Benda Ebei | | Shusai-A injection vial | | Generic | | Anti-inflammatory analgesic |
| Benda Ebei | | Suzheng-B injection vial | | Generic | | Vitamin; complementary medicine used to treat hepatitis |
| Benda Ebei | | Ribavirin injection vial | | Generic | | Anti-virus, to treat acute upper respiratory tract infection |
| Benda Ebei | | Gentamycin Sulfate Injection vial | | Generic | | Broad spectrum antibiotic |
| Benda Ebei | | Vitamin B6 injection vial | | Generic | | Vitamin; complementary medicine used to treat hepatitis |
| Benda Ebei | | Inosine injection vial | | Generic | | Nutrition, complementary medicine used to treat hepatitis |
| Benda Ebei | | Vitamin C injection vial | | Generic | | To treat deficiency of vitamin C |
| Jiangling Benda | | Ribavirin API (1) | | API | | Ribavirin drug manufacture. |
| Jiangling Benda | | Asarin API (1) | | API | | Asarin manufacture to treat acute upper respiratory system infection |
| Jiangling Benda | | Levofloxacin Mesylate API (1) | | API | | Broad spectrum antibiotic drug manufacture |
| Yidu Benda | | Triazol carboxylic acid methyl ester (“TCA”) | | Bulk chemical | | Ribavirin manufacture, anti-virus |
| Yidu Benda | | L-methionine | | Bulk chemical Nutrition | | An essential amino acid for humans |
| Yidu Benda | | Tricabroxylic acid amide (“TAA”) | | Bulk chemical | | Ribavirin manufacture, anti-virus drug manufacture |
| Yidu Benda | | 1,2,3,5-Tetraacetyl-ß-D-Ribose | | Bulk chemical | | Ribavirin manufacture, anti-virus drug manufacture |
| (a) | SiBiono patents - on January 29, 2007, SiBiono entrusted Grandall Legal Group Shenzhen Law Firm to issue a legal letter to Zhaohui Peng, one of the shareholders of Sibiono and the inventor of Gendicine, requesting him to transfer all the title of patents to SiBiono. |
On June 18, 1999, during the formation of SiBiono, Zhaohui Peng transferred the rights to the patent “A new method for manufacturing recombinant adenovirus” and related research results to SiBiono as a payment for the registered capital. In return, Zhaohui Peng was granted 32.03% of the common stock of SiBiono.
From 1999 to 2007, SiBiono successfully obtained various technology funds from various government technology agencies to support the further research and development activities of Gendicine. Due to this significant funding obtained by SiBiono, Sibiono developed five additional patents which are summarized as follows:
Item | | Patent name | | Countries / Date | | Application Number (1) | | Publication Number (2) | | Approved Patent Number (3) | | Name of Patent Inventor (6) | | Name of Applicant (6) | | Patent Assignees |
| 1 | | A new method for manufacturing recombinant adenovirus | | | | | | | | | | | | | | |
| | | A | | China | | 98123346.5 | | CN1228474A | | ZL98123346.5 | | Peng | | Peng | | SiBiono |
| | | | | Date | | 12/14/1998 | | 9/15/1999 | | 7/3/2002 | | | | | | |
| 2 | | A recombinant constructed by a virus vector and a | | | | | | | | | | | | | | |
| | | human tumor suppressor gene and its use | | | | | | | | | | | | | | |
| | | A | | China | | 02115228.4 | | CN1401778A | | ZL02115228.4 | | Peng / Zhang | | Peng / Zhang | | Peng / Zhang |
| | | | | Date | | 5/8/2002 | | 3/12/2003 | | 11/24/2004 | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | B | | PCT (4) | | PCT/CN/2004/000465 | | WO2004/078987A1 | | Not Approved | | Peng / Zhang | | Peng / Zhang | | N/A |
| | | | | Date | | 3/8/2004 | | 9/16/2004 | | N/A | | | | | | |
| 3 | | Recombinant gene medicine of adenovirus vector and | | | | | | | | | | | | | | |
| | | and gene p54 for treating proloferative diseases | | | | | | | | | | | | | | |
| | | A | | China | | 03125129.3 | | CN1471977A | | ZL03125129.3 | | Peng / Zhang | | Peng / Zhang | | Peng / Zhang |
| | | | | Date | | 5/10/2003 | | 2/4/2004 | | 7/25/2007 | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | B | | PCT (4) | | PCT/CN/2004/000458 | | WO2004/104204A1 | | Not Approved | | Peng / Zhang | | Peng / Zhang | | N/A |
| | | | | Date | | 5/9/2004 | | 12/2/2004 | | N/A | | | | | | |
| 4 | | The application of recombinant adenoviral p53 | | | | | | | | | | | | | | |
| | | as cancer vaccine (tentative title) | | | | | | | | | | | | | | |
| | | A | | China | | 200510002779.1 | | CN1679641A | | ZL200510002779.1 | | Peng / Zhang | | Peng / Zhang | | Peng / Zhang |
| | | | | Date | | 1/26/2005 | | 10/12/2005 | | 8/29/2007 | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | B | | PCT (4) | | PCT/CN/2005/000111 | | WO2006/079244A1 | | Not Approved | | Peng / Zhang | | Peng / Zhang | | N/A |
| | | | | Date | | 1/26/2005 | | 8/3/2006 | | N/A | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | C | | US (5) | | 11/075035 | | 2005/0281785A1 | | Not Approved | | Peng / Zhang | | Unidentified Yet | | N/A |
| | | | | Date | | 3/7/2005 | | 12/22/2005 | | N/A | | | | | | |
| 5 | | Human Embryonic Kidney (HEK) sub-clone cell line | | | | | | | | | | | | | | |
| | | A | | China | | 03126889.7 | | CN1513985A | | Not Approved | | Peng / Zhang | | Peng / Zhang | | N/A |
| | | | | Date | | 6/13/2003 | | 7/21/2004 | | N/A | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | B | | PCT (4) | | PCT/CN/2004/000457 | | WO2004/111239 | | Not Approved | | Peng / Zhang | | Peng / Zhang | | N/A |
| | | | | Date | | 5/9/2004 | | 12/23/2004 | | N/A | | | | | | |
| 6 | | The complex of polypeptide liposome and human VGEF | | | | | | | | | | | | | | |
| | | gene, and its use and human VGEF gene, and its use | | | | | | | | | | | | | | |
| | | A | | China | | 02134321.7 | | CN1389269A | | Not Approved | | Peng / Zhang / Zhu | | Peng / Zhang / Zhu | | N/A |
| | | | | Date | | 7/4/2002 | | 1/8/2003 | | N/A | | | | | | |
Note:
(1)Application number is obtained when application is submitted;
(2)Publication number is obtained after the first phase examination;
(3) Approved patent number is obtained after the final examination;
(4) PCT is referred to an International Patent Organization in Paris;
(5) US is referred to the application is made in United States of America alone;
(6) Peng is referred to Zhaohui Peng; Zhang is referred to Xiaozhi Zhang; Zhu is referred to Jinya Zhu.
As indicated in the above table, Item 1, the patent “A new method for manufacturing recombinant adenovirus” had been assigned to SiBiono; however, the other approved patents (item 2 through item 4) in PRC still have not been assigned to SiBiono. The Group believes that all the above mentioned patents should be rightfully transferred to SiBiono, a subsidiary of the Group. Accordingly, the above mentioned legal letter was issued on this ground.
The Group and Zhaohui Peng are currently in negotiations regarding the patent transfers. However, if Zhaozhui Peng refuses to transfer all the patents’s title to SiBiono, the Group will take a legal action against him.
| (b) | As mentioned in Note 12, following this arbitration decision, Benda Ebei has the obligation to pay the total acquisition cost payable of Rmb 12.48 million, plus the penalty and related legal and arbitration expenses, totaling approximately Rmb 12.80 million (or $1.75 million). Up to now, since both parties are still in the process of negotiation about the date of the payment, no actual equity transfer is made yet. |
(an exploration stage company)
FINANCIAL STATEMENTS
AS OF DECEMBER 31, 2007
CONTENTS
| | Page |
| Report of Independent Registered Public Accounting Firm | F-1 |
| Consolidated Balance Sheet - As of December 31, 2007 and 2006 | F-3 |
| Consolidated Statements of Operations - For the years ended December 31, 2007 and 2006 | F-4 |
| Consolidated Statement of Stockholders’ Equity - For the years ended December 31, 2007 and 2006 | F-5 |
| Consolidated Statements of Cash Flows - For the years ended December 31, 2007 and 2006 | F-6 |
| Notes to Consolidated Financial Statements | F-7 to F-34 |
KEMPISTY & COMPANY |
CERTIFIED PUBLIC ACCOUNTANTS, P.C. |
15 MAIDEN LANE - SUITE 1003 - NEW YORK, NY 10038 - TEL (212) 406-7272 - FAX (212) 513-1930 |
| |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
Board of Directors
Benda Pharmaceutical, Inc.
We have audited the accompanying consolidated balance sheets of Benda Pharmaceutical, Inc. and subsidiaries (the "Company") as of December 31, 2007 and 2006 and the related consolidated statements of operations, changes in stockholders’ equity and cash flows for each of the years in the two year period ended December 31, 2007. These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company is not required at this time, to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company's internal control over financial reporting. Accordingly, we express no such opinion. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audit provides a reasonable basis for our opinion.
As discussed in Note 1 to the consolidated financial statements, the accompanying consolidated balance sheets as of December 31, 2007 and 2006, and the related consolidated statements of operations, changes in stockholders equity and cash flows for the years then ended have been restated.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of Benda Pharmaceutical, Inc. and subsidiaries at December 31, 2007 and 2006 and the results of its operations and its cash flows for each of the years in the two year period ended December 31, 2007 in conformity with accounting principles generally accepted in the in the United States of America.
|
| Kempisty & Company |
| Certified Public Accountants PC |
| New York, New York |
| March 18, 2008 (Except for Note 1, June 18, 2008) |
Benda Pharmaceutical, Inc.
Consolidated Balance Sheets
(Amounts expressed in U.S. Dollars)
| | | December 31 | | December 31 | |
| | | 2007 | | 2006 | |
| | | (Restated) | | (Restated) | |
Assets | | | | | | | |
| Current Assets | | | | | | | |
| Cash and cash equivalents | | $ | 1,266,240 | | $ | 1,676,119 | |
| Trade receivables, net (Note 5) | | | 10,472,233 | | | 6,193,585 | |
| Other receivables (Note 5) | | | 453,595 | | | 99,733 | |
| Refundable purchase price paid (Note 6) | | | 1,200,000 | | | 5,367,801 | |
| Inventories (Note 7) | | | 1,952,348 | | | 701,339 | |
| Prepaid expenses and deposits (Note 5) | | | 933,299 | | | 372,548 | |
| Total current assets | | | 16,277,715 | | | 14,411,125 | |
| Due from related parties (Note 18) | | | 2,630,019 | | | 1,976,815 | |
| Property and equipments, net (Note 8) | | | 27,123,035 | | | 14,096,649 | |
| Intangible assets, net (Note 9) | | | 6,494,510 | | | 1,569,238 | |
| Goodwill (Note 10) | | | 7,395,752 | | | - | |
| Restricted cash (Note 11) | | | 3,957,624 | | | - | |
| Other assets (Note 12) | | | 1,710,972 | | | - | |
| Debt issue costs (Note 24) | | | 327,945 | | | - | |
| Total Assets | | $ | 65,917,572 | | $ | 32,053,827 | |
| | | | | | | | |
Liabilities & Shareholders' Equity | | | | | | | |
| Current Liabilities | | | | | | | |
| Bank indebtedness (Note 11) | | $ | 874,490 | | $ | - | |
| Bank loans payable (current portion) (Note 13) | | | 2,867,004 | | | 256,492 | |
| Long term debt payable (current portion) (Note 14) | | | 1,787,239 | | | - | |
| Accounts payable and accrued liabilities (Note 15) | | | 4,665,984 | | | 1,823,030 | |
| Commercial notes payable (Note 11) | | | 5,118,758 | | | - | |
| Taxes payable | | | 1,279,385 | | | 226,931 | |
| Acquisition price payable (Note 10) | | | 1,333,246 | | | - | |
| Wages payable | | | 664,785 | | | 145,904 | |
| Deferred revenues | | | - | | | 1,732 | |
| Total current liabilities | | | 18,590,891 | | | 2,454,089 | |
| Long term debt payable (long term portion) (Note 14) | | | 425,001 | | | - | |
| Long-term convertible promissory notes (Note 24) | | | 2,875,075 | | | - | |
| Due to related parties (Long-term) (Note 18) | | | 3,193,618 | | | 3,034,365 | |
| Total liabilities | | | 25,084,585 | | | 5,488,454 | |
| | | | | | | | |
| Commitments and contingencies (Note 26) | | | - | | | - | |
| Minority interest | | | 5,502,755 | | | 2,178,922 | |
| | | | | | | | |
| Redeemable common stock, 2,049,560 shares at $3.6 per share (Note 19) | | | 7,376,366 | | | - | |
| | | | | | | | |
| Shareholders' Equity | | | | | | | |
| Preferred stock, $0.001 par value; 5,000,000 shares authorized; | | | | | | | |
| None issued and outstanding (Note 25) | | | - | | | - | |
| Common stock, $0.001 par value; 150,000,000 shares authorized; | | | | | | | |
| 100,170,071 shares issued and outstanding as of 12/31/2007; | | | | | | | |
| 96,258,411 shares issued and outstanding as of 12/31/2006 (Note 25) | | | 100,170 | | | 96,258 | |
| Additional paid in capital (Note 25) | | | 21,547,929 | | | 13,099,424 | |
| Retained earnings (unrestricted) | | | 100,452 | | | 7,904,434 | |
| Statutory surplus reserve fund (Note 17) | | | 2,310,681 | | | 1,869,523 | |
| Accumulative other comprehensive income | | | 3,390,774 | | | 1,090,409 | |
| Shares issuable for acquisition and services | | | 503,860 | | | 326,403 | |
| Total Shareholders' Equity | | | 27,953,866 | | | 24,386,451 | |
| Total Liabilities & Shareholders' Equity | | $ | 65,917,572 | | $ | 32,053,827 | |
The accompanying notes are an integral part of these consolidated financial statements.
Benda Pharmaceutical, Inc.
Consolidated Statements of Operations
(Amounts expressed in U.S. Dollars)
| | | December 31, | | December 31, | |
| | | 2007 | | 2006 | |
| | | (Restated) | | (Restated) | |
| Revenue | | $ | 26,384,608 | | $ | 15,932,075 | |
| Other Sales | | | - | | | 2,937 | |
| Cost of goods sold | | | (13,421,697 | ) | | (9,103,515 | ) |
| Gross profit | | | 12,962,911 | | | 6,831,497 | |
| | | | | | | | |
| Selling expenses | | | (1,581,655 | ) | | (599,571 | ) |
| | | | | | | | |
| General and administrative expenses | | | | | | | |
| Amortization of intangible assets | | | (141,681 | ) | | (96,615 | ) |
| Amortization of debt issue costs (Note 24) | | | (201,255 | ) | | - | |
| Depreciation | | | (333,964 | ) | | (132,747 | ) |
| Bad debts | | | (287,482 | ) | | (364,064 | ) |
| Director remuneration | | | (94,247 | ) | | - | |
| Penalty to investors (Note 20) | | | (1,022,275 | ) | | - | |
| Finder fee | | | (293,751 | ) | | - | |
| Consulting and professional fees (Note 19) | | | (8,353,615 | ) | | (326,403 | ) |
| Other general and administrative expenses (Note 21) | | | (3,173,307 | ) | | (1,533,775 | ) |
| Total general and administrative expenses | | | (13,901,577 | ) | | (2,453,604 | ) |
| Gains / (losses) on disposals of fixed assets | | | (30,027 | ) | | (249,381 | ) |
| Research and development expenses | | | (553,962 | ) | | (30,821 | ) |
| Total operating expenses | | | (16,067,221 | ) | | (3,333,377 | ) |
| Operating income / (loss) | | | (3,104,310 | ) | | 3,498,120 | |
| | | | | | | | |
| Interest income / (expenses) (Note 24) | | | (3,389,676 | ) | | (108,811 | ) |
| Other income (expenses) | | | 55,964 | | | (281,190 | ) |
| Government subsidies / grants (Note 22) | | | 2,276,574 | | | - | |
| | | | | | | | |
| Income / (loss) before minority interest and income taxes | | | (4,161,448 | ) | | 3,108,119 | |
| Income taxes (Note 23) | | | (965,183 | ) | | - | |
| Minority interest (Note 27) | | | (2,236,194 | ) | | (322,590 | ) |
| | | | | | | | |
| Net income / (loss) | | $ | (7,362,825 | ) | $ | 2,785,529 | |
| | | | | | | | |
| Earnings / (loss) per share - basic | | $ | (0.07 | ) | $ | 0.04 | |
| | | | | | | | |
| Weighted average shares outstanding - basic | | | 98,181,685 | | | 73,414,057 | |
| | | | | | | | |
| Earnings / (loss) per share - diluted | | $ | (0.06 | ) | $ | 0.04 | |
| | | | | | | | |
| Weighted average shares outstanding - diluted | | | 127,534,003 | | | 74,864,283 | |
The accompanying notes are an integral part of these consolidated financial statements.
Benda Pharmaceutical, Inc.
Consolidated Statements of Changes in Stockholders’ Equity
(Amounts expressed in U.S. Dollars)
| | | | | | | Additional | | (Restated) Retained | | Statutory Surplus | | Accumulated Other | | Shares Issuable for | | Total | |
| | | Common Stock | | Paid-in | | Earnings | | Reserve | | Comprehensive | | Acquisition | | Stockholder's | |
| | | Shares | | Amount | | Capital | | (Unrestricted) | | Fund | | Income | | and Services | | Equity | |
| | | | | | | | | | | | | | | | | | |
Balance at December 31, 2005 | | | 64,942,360 | | $ | 64,942 | | $ | 584,481 | | $ | 5,751,566 | | $ | 1,231,427 | | $ | 271,555 | | $ | - | | $ | 7,903,972 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Issuance of common stock for cash: | | | | | | | | | | | | | | | | | | | | | | | | | |
| to Original Shareholders of Applied Spectrum Technologies, Inc. | | | 2,954,141 | | | 2,954 | | | - | | | - | | | - | | | - | | | - | | | 2,954 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Issuance of common stock for cash: | | | | | | | | | | | | | | | | | | | | | | | | | |
| 1/4/06, 1,500,000 shares at $0.05 per share | | | 1,500,000 | | | 1,500 | | | - | | | - | | | - | | | - | | | - | | | 1,500 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Issuance of common stock for services: | | | | | | | | | | | | | | | | | | | | | | | | | |
| 1/4/06, 200,000 shares at $0.05 per share | | | 200,000 | | | 200 | | | - | | | - | | | - | | | - | | | - | | | 200 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Issuance of common stock for cash: | | | | | | | | | | | | | | | | | | | | | | | | | |
| 3/10/06, 700,000 shares at $0.05 per share | | | 700,000 | | | 700 | | | - | | | - | | | - | | | - | | | - | | | 700 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| To adjust par value of common stock outstanding as of 3/31/06 for Applied Spectrum Co. Ltd. | | | - | | | - | | | (5,354 | ) | | - | | | - | | | - | | | - | | | (5,354 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Collective issuance of common stock on 7/21/06 | | | 50 | | | - | | | - | | | - | | | - | | | - | | | - | | | - | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Collective issuance of common stock on 8/30/06 | | | 100 | | | - | | | - | | | - | | | - | | | - | | | - | | | - | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Waiver of the shareholder loan from Ever Leader on 9/5/06 | | | - | | | - | | | 2,298,434 | | | - | | | - | | | - | | | - | | | 2,298,434 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Issuance of common stock for cash: | | | | | | | | | | | | | | | | | | | | | | | | | |
| 11/15/06, 25,961,760 shares at $0.4622 per share | | | 25,961,760 | | | 25,962 | | | 11,974,038 | | | - | | | - | | | - | | | - | | | 12,000,000 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Eliminate the common stock and additional paid-in capital of Applied Spectrum Technologies, Inc. on 11/15/06 | | | - | | | - | | | 16,215,770 | | | - | | | - | | | - | | | - | | | 16,215,770 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Eliminate the accumulated deficit of Applied Spectrum Technologies, Inc., according to reverse merger on 11/15/06 | | | - | | | - | | | (16,209,962 | ) | | - | | | - | | | - | | | - | | | (16,209,962 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Reallocate original common stock of Ever Leader | | | | | | | | | | | | | | | | | | | | | | | | | |
| to paid-in capital, on 11/15/06 | | | - | | | - | | | 1,285 | | | - | | | - | | | - | | | - | | | 1,285 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Issuance of common stock to Ever Leader: | | | | | | | | | | | | | | | | | | | | | | | | | |
| 11/15/06, 64,942,369 at par value $0.001 | | | - | | | - | | | (64,942 | ) | | - | | | - | | | - | | | - | | | (64,942 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Reverse merger on 11/15/06, placement agent commission and transaction related fee | | | - | | | - | | | (1,694,326 | ) | | - | | | - | | | - | | | - | | | (1,694,326 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Allocation of retained earnings to statutory reserve fund | | | - | | | - | | | - | | | (638,096 | ) | | 638,096 | | | - | | | - | | | - | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Foreign currency translation adjustment | | | - | | | - | | | - | | | - | | | - | | | 818,854 | | | - | | | 818,854 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Issuance of common stock to be issued for services: | | | | | | | | | | | | | | | | | | | | | | | | | |
| 11/15/06, 706,195 shares | | | | | | | | | | | | | | | | | | | | | 326,403 | | | 326,403 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Eliminate the net deficit of Applied Spectrum Technologies, Inc. 11/15/06 | | | - | | | - | | | - | | | 5,435 | | | - | | | - | | | - | | | 5,435 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Net income for the year, 12/31/06 | | | - | | | - | | | - | | | 2,785,529 | | | - | | | - | | | - | | | 2,785,529 | |
Balance at December 31, 2006 | | | 96,258,411 | | | 96,258 | | | 13,099,424 | | | 7,904,434 | | | 1,869,523 | | | 1,090,409 | | | 326,403 | | | 24,386,451 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Issuance of common stock to various consultants, $0.001 par value; | | | | | | | | | | | | | | | | | | | | | | | | | |
| 706,195 shares, services valued at $0.4622 per share; | | | 706,195 | | | 706 | | | 325,697 | | | - | | | - | | | - | | | (326,403 | ) | | - | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Debt discount - beneficial conversion feature on convertible promissory notes (Note 24) | | | - | | | - | | | 2,385,089 | | | - | | | - | | | - | | | - | | | 2,385,089 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Debt discount - warrants issued with convertible promissory notes (Note 24) | | | - | | | - | | | 5,174,911 | | | - | | | - | | | - | | | - | | | 5,174,911 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Issuance of common stock for financial and technical services rendered | | | | | | | | | | | | | | | | | | | | | | | | | |
| 7/12/07, 2,189,560 shares at $3.6 per share | | | 2,189,560 | | | 2,190 | | | - | | | | | | | | | | | | 503,860 | | | 506,050 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Issuance of common stock being placement agent exercised warrants | | | | | | | | | | | | | | | | | | | | | | | | | |
| 7/31/07, 546,994 shares at $0.555 per share | | | 546,994 | | | 547 | | | 303,032 | | | - | | | - | | | - | | | - | | | 303,579 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Issuance of common stock being placement agent exercised warrants | | | | | | | | | | | | | | | | | | | | | | | | | |
| 8/16/07, 65,200 shares at $0.555 per share | | | 65,200 | | | 65 | | | 36,121 | | | - | | | - | | | - | | | - | | | 36,186 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Issuance of common stock being placement agent exercised warrants | | | | | | | | | | | | | | | | | | | | | | | | | |
| 10/04/07,236,813 shares @ $0.555 per share | | | 236,813 | | | 237 | | | 131,194 | | | - | | | - | | | - | | | - | | | 131,431 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Issuance of common stock for pernalty - late effective of registration registration | | | | | | | | | | | | | | | | | | | | | | | | | |
| 10/12/07, 166,898 shares @ $0.555 each | | | 166,898 | | | 167 | | | 92,461 | | | - | | | - | | | - | | | - | | | 92,628 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Allocation of retained earnings to statutory reserve fund | | | - | | | - | | | - | | | (441,158 | ) | | 441,158 | | | - | | | - | | | 0 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Net loss for the year, 12/31/07 | | | - | | | - | | | - | | | (7,362,825 | ) | | | | | - | | | - | | | (7,362,825 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Foreign currency translation adjustment | | | - | | | - | | | - | | | - | | | | | | 2,300,365 | | | - | | | 2,300,365 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance at December 31, 2007 | | | 100,170,071 | | $ | 100,170 | | $ | 21,547,929 | | $ | 100,452 | | $ | 2,310,681 | | $ | 3,390,774 | | $ | 503,860 | | $ | 27,953,866 | |
The accompanying notes are an integral part of these consolidated financial statements.
Benda Pharmaceutical, Inc.
Consolidated Statements of Cash Flows
(Amounts expressed in U.S. Dollars)
| | | December 31, | | December 31, | |
| | | 2007 | | 2006 | |
| | | (Restated) | | (Restated) | |
Cash Flows From Operating Activities | | | | | | | |
| Net income / (loss) | | $ | (7,362,825 | ) | $ | 2,785,529 | |
| Adjustments to reconcile net income / (loss) to net cash provided by operating activities: | | | | | | | |
| Consulting and professional fees (Note 19) | | | 8,353,615 | | | 326,403 | |
| Bad Debt provision | | | 287,482 | | | 364,064 | |
| Minority interest | | | 2,236,194 | | | 322,590 | |
| Loss on disposals of fixed assets | | | 30,027 | | | 249,381 | |
| Depreciation | | | 1,022,603 | | | 294,805 | |
| Amortization of intangible assets | | | 504,736 | | | 187,235 | |
| Amortization of debt issue costs (Note 24) | | | 201,255 | | | - | |
| Interest expense (amortization of debt discount) (Note 24) | | | 2,875,075 | | | - | |
| Penalty to investors settled by issuance of common stock | | | 92,628 | | | - | |
| Government subsidies/grants, (Note 22) | | | (2,250,240 | ) | | - | |
| Changes in operating assets and liabilities: | | | | | | | |
| Trade receivables | | | (4,351,652 | ) | | (2,210,832 | ) |
| Other receivables | | | 196,755 | | | 12,787 | |
| Prepaid expenses and deposits | | | (560,751 | ) | | (20,236 | ) |
| Inventories | | | (523,131 | ) | | (254,257 | ) |
| Accounts payable and accrued liabilities | | | 597,600 | | | 526,557 | |
| Others payable | | | - | | | 22,787 | |
| Taxes payable | | | 862,074 | | | (131,454 | ) |
Net cash provided by operating activities | | | 2,211,445 | | | 2,475,359 | |
| | | | | | | | |
Cash Flows From Investing Activities | | | | | | | |
| Acquisition cost paid | | | (2,664,244 | ) | | - | |
| Purchases of property and equipment and construction-in-progress | | | (5,319,037 | ) | | (3,294,590 | ) |
| Purchases of intangible assets | | | (3,094,245 | ) | | (787,087 | ) |
| Refundable purchase price paid (Note 6) | | | - | | | (5,367,801 | ) |
| Loans to related parties, net | | | - | | | (532,026 | ) |
Net cash used in investing activities | | | (11,077,526 | ) | | (9,981,503 | ) |
| | | | | | | | |
Cash Flows From Financing Actives | | | | | | | |
| Proceeds of sales unit of common stock, net of financing cost | | | - | | | 10,305,674 | |
| Proceeds from issuance of convertible promissory note (Note 24) | | | 7,030,800 | | | - | |
| Proceeds of shareholder loan from Ever Leader | | | - | | | 2,298,434 | |
| Proceeds and repayments of borrowings under related parties, net | | | (592,893 | ) | | (2,438,555 | ) |
| Proceeds and repayments of borrowings under government debts payable, net | | | 19,210 | | | - | |
| Proceeds and repayments of borrowings under commercial bank notes, net (Note 11) | | | 2,503,504 | | | | |
| Proceeds and repayments of borrowings under bank loans, net | | | (748,367 | ) | | (1,714,492 | ) |
Net cash provided by (used in) financing activities | | | 8,212,254 | | | 8,451,061 | |
| Effect of exchange rate changes on cash | | | 243,948 | | | 423,119 | |
Net increase in cash and cash equivalents | | | (409,879 | ) | | 1,368,037 | |
| | | | | | | | |
Cash and cash equivalents, beginning of period | | | 1,676,119 | | | 308,082 | |
| | | | | | | | |
Cash and cash equivalents, end of period | | $ | 1,266,240 | | $ | 1,676,119 | |
| | | | | | | | |
Supplemental Disclosure of Cash Flow Information | | | | | | | |
Cash paid for interest | | $ | 213,113 | | $ | 113,458 | |
Cash paid for income taxes | | $ | 718,723 | | $ | - | |
The accompanying notes are an integral part of these consolidated financial statements.
Benda Pharmaceutical, Inc.
Notes to Consolidated Financial Statements
(Amounts expressed in U.S. Dollars)
1. | Restatement of Previously Issued Financial Statements |
Benda’s previously issued financial statements are being restated as a result of an internal review of its previously issued financial statements, which review was prompted by an SEC comment during its review of the Company’s Form S-1 filing. After a review of the comment and further analysis, Benda determined that it incorrectly calculated its depreciation and amortization expense. Benda also believes such restatements reflect the correction of any errors and omissions of material disclosures in the financial statements in accordance with SFAS 154: Accounting changes and error corrections (as amended).
The following are explanations of the restatement adjustments and presentation of affected accounts in the consolidated balance sheet and statement of operations as previously reported and restated.
Statements of Operations for the Year Ended December 31, 2007 and 2006
The Company restated its previously recognized Depreciation expense by($1,446,185) and ($718,387) for the year ended December 31, 2007 and 2006 due to incorrect calculation of depreciation expense previously reported.
The Company restated its previously recognized Amortization expense by ($572,491) and ($254,990) for the year ended December 31, 2007 and 2006 due to incorrect calculation of amortization expense previously reported.
Balance Sheets December 31, 2007 and 2006
Adjustments were made to Property and Equipment, net and Intangible assets, net and Retained (deficit) earnings unrestricted to reflect the restatement of the statements of operations.
The consolidated financial statements as of December 31, 2007 and 2006 and for the ended December 31, 2007 and 2006, and the notes thereto, have been restated to include the items identified above. The following financial statement line items were impacted:
Consolidated Balance Sheet
| | | As Previously | | | |
| | | Reported | | Restated | |
| | | Year Ended | | Year Ended | |
| | | December 31, | | December 31, | |
| | | 2007 | | 2007 | |
| Property and equipments, net | | $ | 26,275,871 | | $ | 27,123,035 | |
| Intangible assets, net | | | 6,359,000 | | | 6,494,510 | |
| Retained (deficit) earnings unrestricted | | $ | (833,090 | ) | $ | 100,452 | |
Consolidated Statement of Operations
| | | As Previously | | | |
| | | Reported | | Restated | |
| | | Year Ended | | Year Ended | |
| | | December 31, | | December 31, | |
| | | 2007 | | 2007 | |
| Cost of goods sold | | $ | (13,913,034 | ) | $ | (13,421,697 | ) |
| As included in General and administrative expenses | | | | | | | |
| Depreciation expense | | $ | (333,964 | ) | $ | (333,964 | ) |
| Amortization expense | | | (141,681 | ) | | (141,681 | ) |
| Total operating expense | | | (16,067,221 | ) | | (16,067,221 | ) |
| Operating income / (loss) | | | (3,595,647 | ) | | (3,104,310 | ) |
| Income / (loss) before minority interest and income taxes | | | (4,652,785 | ) | | (4,161,448 | ) |
| Net income / (loss) | | | (7,829,595 | ) | | (7,362,825 | ) |
| Earnings / (loss) per share - basic | | | (0.08 | ) | | (0.07 | ) |
| Earnings / (loss) per share - diluted | | $ | (0.06 | ) | $ | (0.06 | ) |
Consolidated Balance Sheet
| | | As Previously | | | |
| | | Reported | | Restated | |
| | | Year Ended | | Year Ended | |
| | | December 31, | | December 31, | |
| | | 2006 | | 2006 | |
| Property and equipments, net | | $ | 13,673,067 | | $ | 14,096,649 | |
| Intangible assets, net | | | 1,501,483 | | | 1,569,238 | |
| Retained (deficit) earnings unrestricted | | $ | 7,437,663 | | $ | 7,904,434 | |
Consolidated Statement of Operations
| | | As Previously | | | |
| | | Reported | | Restated | |
| | | Year Ended | | Year Ended | |
| | | December 31, | | December 31, | |
| | | 2006 | | 2006 | |
| Cost of goods sold | | $ | (9,594,852 | ) | $ | (9,103,515 | ) |
| As included in General and administrative expenses | | | | | | | |
| Depreciation expense | | $ | (132,747 | ) | $ | (132,747 | ) |
| Amortization expense | | | (96,615 | ) | | (96,615 | ) |
| Total operating expense | | | (3,333,377 | ) | | (3,333,377 | ) |
| Operating income / (loss) | | | 3,006,783 | | | 3,498,120 | |
| Income / (loss) before minority interest and income taxes | | | 2,616,782 | | | 3,108,119 | |
| Net income / (loss) | | | 2,318,758 | | | 2,785,529 | |
| Earnings / (loss) per share - basic | | | 0.03 | | | 0.04 | |
| Earnings / (loss) per share - diluted | | $ | 0.03 | | $ | 0.04 | |
Benda Pharmaceutical, Inc. (“Benda”) is a corporation organized under the Florida Laws and headquartered in Hubei Province, the People’s Republic of China (“PRC”).
Ever Leader Holdings Limited (“Ever Leader”), a wholly owned subsidiary of Benda, is a company incorporated under the laws of Hong Kong SAR.
Ever Leader owns 95% of the issued and outstanding capital of Hubei Tongi Benda Ebei Pharmaceutical Co. Ltd. (“Benda Ebei”), a Sino-Foreign Equity Joint Venture company incorporated under the laws of PRC. Mr. Yiqing Wan owns 5% of the issued and outstanding capital stock of Benda Ebei. Benda Ebei owns: (i) 95% of the issued and outstanding capital stock of Jiangling Benda Pharmaceutical Co. Ltd., (“Jiangling Benda”) a company formed under the laws of PRC; (ii) 95% of the issued and outstanding capital stock of Yidu Benda Chemical Co. Ltd., (“Yidu Benda”) a company incorporated under the laws of PRC; and (iii) 75% of the issued and outstanding capital stock of Beijing Shusai Pharyngitis Research Co. Ltd., (“Beijing Shusai”) a company incorporated under the laws of PRC. Mr. Yiqing Wan owns: (i) 5% of the issued and outstanding capital stock of Jingling Benda; and (ii) 5% of the issued and outstanding capital stock of Yidu Benda. Mr. Feng Wang owns 25% of the issued and outstanding capital stock of Beijing Shusai.
On April 5, 2007, Benda Ebei entered into an Equity Transfer Agreements with Shenzhen Yuanzheng Investment Development Co., Ltd. and Shenzhen Yuanxing Gene City Development Co., Ltd., the then shareholders of Shenzhen SiBiono GeneTech Co., Ltd (“SiBiono”), to purchase 27.57% and 30% respectively of the shares of SiBiono’s common stock for total consideration of Rmb 60 million (or $7.88 million) due and payable on or before April 30, 2007. On June 11, 2007, Benda Ebei entered into an Equity Transfer Agreements with Huimin Zhang and Yaojin Wang, the individual shareholders of SiBiono, and to purchase 1.6% and 0.96% respectively of the shares of SiBiono’s common stock for total consideration of Rmb 2.56 million (or $0.34 million) due and payable on or before June 30, 2007. Altogether, the total consideration for 60.13% shares of SiBiono’s common stock was Rmb62.56 million or $8.22 million. As of December 31, 2007, an accumulated amount, approximately Rmb52.9 million or $6.89 million was paid and leaving a balance approximately $1.33 million (please refer to Note 10 for the details).
Benda, Ever Leader, Benda Ebei, Jingling Benda, Yidu Benda, Beijing Shusai and SiBiono shall be referred to herein collectively as the “Group”. The Group is engaged principally in the business of identifying, discovering, developing, and manufacturing conventional medicines, active pharmaceuticals, bulk chemicals (or pharmaceutical immediates), and Traditional Chinese Medicines (“TCM”) for the treatment of some of the most widespread common ailments and diseases (e.g. common cold, diabetes, and cancer).
As of December 31, 2007, the organization and ownership structure of the Group is as follows:
3. | Significant Accounting Policies |
Cash and Cash Equivalents
Cash and cash equivalents include cash on hand, cash on deposit with various financial institutions, and all highly-liquid investments with original maturities of three months or less at the time of purchase.
Estimates Affecting Trade Receivables, Other Receivables, Prepaid and Deposits and Inventories
The preparation of our consolidated financial statements requires management to make estimates and assumptions that affect our reporting of assets and liabilities (and contingent assets and liabilities). However, it is explicated that the changes in estimation were not material in the preparation of our consolidation financial statements.
As of December 31, 2007 and 2006, the Company provided a $943,667 and $467,015 respectively for the allowance of doubtful accounts against trade receivables, other receivables and prepaid and deposits (please refer to Note 5 for details). Management's estimate of the appropriate allowance on those accounts receivable for the reporting periods was based on the aged nature of these accounts. In making its judgment, management assessed its customers' ability to continue to pay their outstanding invoices and the collectibility of those accounts on a timely basis, and whether their financial position might deteriorate significantly in the future, which would result in their inability to pay their debts to the Company.
Inventories, which are primarily comprised of raw materials, packaging materials, and finished goods, are stated at the lower of cost or net realizable value. Cost being determined on the basis of a moving average. The Group evaluates the need for reserves associated with obsolete, slow-moving and non-salable inventory by reviewing net realizable values on a periodic basis.
For the year ended December 31, 2007 and 2006, the Company provided a reserve against its work-in-progress amounting to $4,422,014 and none respectively. The reserve was raised due to the fact that most of the original liquid, a liquid element o produce Gendicine, was produced in the year of 2004 and this particular liquid can only be stored for approximately five years, therefore a reserve was provide against the work-in-progress (see Note 7). However, no reserve for obsolete, slow-moving or non-salable inventory was required for the reporting periods.
Property and Equipment
Property and equipment are recorded at cost and depreciated using the straight-line method, with an estimated 5% salvage value of original cost, over the estimated useful lives of the assets as follows:
| Buildings | | | 20-30years | |
| Machinery and equipment | | | 10-15 years | |
| Motor Vehicles | | | 5 years | |
| Electronics and office equipment | | | 5 years | |
Expenditures for repairs and maintenance, which do not improve or extend the expected useful lives of the assets, are expensed as incurred while major replacements and improvements are capitalized.
When property or equipment is retired or disposed of, the cost and accumulated depreciation are removed from the accounts, with any resulting gains or losses being included in net income or loss in the year of disposition.
Impairment of Long-Lived Assets
The Group evaluates potential impairment of long-lived assets, in accordance with Statement of Financial Accounting Standards (“SFAS”) No. 144, Accounting for the Impairment or Disposal of Long-Lived Assets, which requires the Group to (a) recognize an impairment loss only if the carrying amount of a long-lived asset is not recoverable from its undiscounted cash flows and (b) measure an impairment loss as the difference between the carrying amount and fair value of the asset. The Group believes that long-lived assets in the accompanying consolidated balance sheets are appropriately valued at December 31, 2007 and 2006.
Intangible Assets
The Group’s intangible assets are stated at cost less accumulated amortization and are comprised of land-use rights, drug permits and licenses, patent and technology formulas know-how. Land-use rights are related to land the Group occupies in Hubei and Guangdong Province, PRC and are being amortized on a straight-line basis over a period of 40 years. Other intangible assets are being amortized on a straight-line basis over a period of 10 years.
Revenue Recognition
Among the most important accounting policies affecting our consolidated financial statements is our policy of recognizing revenue in accordance with the SEC's Staff Accounting Bulletin ("SAB") No. 104. Under this policy, all of the following criteria must be met in order for us to recognize revenue:
| 1. | Persuasive evidence of an arrangement exists; |
| 2. | Delivery has occurred or services have been rendered; |
| 3. | The seller's price to the buyer is fixed or determinable; and |
| 4. | Collectibility is reasonably assured. |
The majority of the Group's revenue results from sales contracts with distributors and revenue is recorded upon the shipment of goods. Management conducts credit background checks for new customers as a means to reduce the subjectivity of assuring collectibility. Based on these factors, the Group believes that it can apply the provisions of SAB 104 with minimal subjectivity.
Research and Development
Research and development costs are expensed as incurred and consist primarily of salaries and related expenses of personnel engaged in research and development activities. The Company spent $553,962 and $30,821 on direct research and development (“R&D”) efforts in 2007 and 2006, respectively.
Income Taxes
The Group accounts for income taxes under the liability method in accordance with SFAS No. 109, Accounting for Income Taxes. Deferred tax assets and liabilities are recorded for the estimated future tax effects of temporary differences between the tax basis of assets and liabilities and amounts reported in the accompanying consolidated balance sheets. Deferred tax assets are reduced by a valuation allowance if current evidence indicates that it is considered more likely than not that these benefits will not be realized.
Comprehensive Income
The Group has adopted SFAS No. 130, Reporting Comprehensive Income, which establishes standards for reporting and displaying comprehensive income, its components, and accumulated balances in a full-set of general-purpose financial statements. Accumulated other comprehensive income represents the accumulated balance of foreign currency translation adjustments.
Concentration of Credit Risk
A significant portion of the Group's cash at December 31, 2007 and 2006 is maintained at various financial institutions in the PRC which do not provide insurance for amounts on deposit.
The Group has not experienced any losses in such accounts and believes it is not exposed to significant credit risk in this area.
The Group operates principally in the PRC and grants credit to its customers in this geographic region. Although the PRC is economically stable, it is always possible that unanticipated events in foreign countries could disrupt the Group’s operations.
The following table shows the individual customer’s revenue and account receivable balance which was higher than 5% of total revenue and total account receivables for the years ended December 31, 2007 and 2006:
| | | December 31, | | December 31, | |
| | | 2007 | | 2006 | |
| Revenue | | $ | 26,384,608 | | | % | | $ | 15,935,012 | | | % | |
| | | | | | | | | | | | | | |
Individual customer's revenue | | | | | | | | | | | | | |
| Zhuhai Gongbei Pharmaceutical Co, Ltd. | | | 5,034,917 | | | 10 | % | | 2,691,168 | | | 17 | % |
| Shenzhen Huihua Pharmaceutical Co. Ltd. | | | 3,264,033 | | | 8 | % | | 2,126,246 | | | 13 | % |
| Shenyang Pharmaceutical Co. Ltd. | | | 2,784,912 | | | 8 | % | | 2,054,462 | | | 13 | % |
| Jiangxi Huiren Pharmaceutical Co. Ltd. | | | 2,360,264 | | | 7 | % | | 1,962,436 | | | 12 | % |
| Hubei Jiuzhoutong Pharmaceutical Co. Ltd | | | 1,314,444 | | | 5 | % | | 128,869 | | | 1 | % |
| Zhongxin Pharmaceutical Co.,Ltd | | | 1,263,442 | | | 5 | % | | - | | | 0 | % |
| | | | | | | | | | | | | | |
| Account receivable, gross | | $ | 10,909,921 | | | | | $ | 6,650,854 | | | % | |
| | | | | | | | | | | | | | |
Individual customer's account receivable gross balance | | | | | | | | | | | | | |
| Zhuhai Gongbei Pharmaceutical Co, Ltd. | | | 1,658,203 | | | 15 | % | | 2,691,168 | | | 40 | % |
| Hubei Hengzhou Health Products Ltd. | | | 1,154,881 | | | 11 | % | | - | | | 0 | % |
| Shenzhen Huihua Pharmaceutical Co. Ltd. | | | 1,111,545 | | | 10 | % | | 2,126,246 | | | 32 | % |
| Shenyang Pharmaceutical Co. Ltd. | | | 903,202 | | | 8 | % | | 2,054,462 | | | 31 | % |
| Zhongxin Pharmaceutical Co.,Ltd | | | 694,637 | | | 6 | % | | - | | | 0 | % |
| Jiangxi Huiren Pharmaceutical Co. Ltd. | | | 486,843 | | | 4 | % | | 1,962,436 | | | 30 | % |
Basic and Diluted Earnings Per Share
The Group adopted Statement of Financial Accounting Standards No. 128, “Earnings Per Share” (SFAS128). SFAS 128 requires the presentation of earnings per share (EPS) as Basic and Diluted EPS. Basic earnings per share are calculated by taking net income divided by the weighted average shares of common stock outstanding during the period. Diluted earnings per share is calculated by taking basic weighted average shares of common stock and increasing it for dilutive common stock equivalents such as warrants that are in the money.
Foreign Currency Translation
The functional currency of the Group is the Renminbi (“RMB”), the PRC’s currency. The Group maintains its financial statements using the functional currency. Monetary assets and liabilities denominated in currencies other than the functional currency are translated into the functional currency at rates of exchange prevailing at the balance sheet dates. Transactions denominated in currencies other than the functional currency are translated into the functional currency at the exchange rates prevailing at the dates of the transaction. Exchange gains or losses arising from foreign currency transactions are included in the determination of net income (loss) for the respective periods.
For financial reporting purposes, the financial statements of the Group, which are prepared using the RMB, are translated into the Group’s reporting currency, United States Dollars. Balance sheet accounts are translated using the closing exchange rate in effect at the balance sheet date and income and expense accounts are translated using the average exchange rate prevailing during the reporting period. Adjustments resulting from the translation, if any, are included in accumulated other comprehensive income (loss) in stockholder’s equity.
The exchange rates in effect at December 31, 2007 and 2006 were stated as follows: (for RMB 1.00):
| | | December 31, | | December 31, | |
| | | 2007 | | 2006 | |
| Fixed rate | | $ | 0.1371 | | $ | 0.1283 | |
| Average rate | | $ | 0.1317 | | $ | 0.1256 | |
For the year ended December 31, 2007, the foreign exchange gain was $2,302; and for the year ended December 31, 2006, the foreign exchange loss was $39,377.
Fair Value of Financial Instruments
The Group's financial instruments include cash equivalents, accounts receivable, other receivables, accounts payable, accrued expenses, value-added taxes, short-term and long-term bank loans, and loans payable to related parties. The carrying amounts of financial instruments other than long-term obligations approximate fair value due to their short maturities. Long-term obligations approximate fair value based upon rates currently available for similar instruments.
Recent Accounting Pronouncements
In September 2006, the FASB issued Statement of Financial Accounting Standards ("SFAS") No. 157, "Fair Value Measurements", which establishes a framework for reporting fair value and expands disclosures about fair value measurements. SFAS No. 157 is effective for financial statements issued for fiscal years beginning after November 15, 2007 and interim periods within those fiscal years. The Group is currently assessing the impact of the adoption of this standard on its financial statements.
In February 2007, the Financial Accounting Standards Board ("FASB") issued FASB 159 "The Fair Value Option for Financial Assets and Financial Liabilities" ("FASB 159"). FASB 159 permits entities to measure financial instruments and certain other items at fair value that are not currently measured at fair value. The Group is currently evaluating the impact of the adoption of SFAS 159 will have, if any, on our financial statements.
Recently Adopted Accounting Pronouncements
SFAS No. 123R, Share-Based Payment, an Amendment of SFAS No. 123, was issued in December 2004 and was effective as of the beginning of the Group’s 2006 fiscal year. SFAS No. 123R requires all share-based payments to qualified individuals, including grants of employee stock options, to be recognized as compensation expense in the financial statements based on their grant date fair values.
On December 4, 2007, the Financial Accounting Standards Board (FASB) issued SFAS No. 160, Noncontrolling interest in Consolidated Financial Statements (SFAS No. 160). SFAS No. 160 requires all entities to report noncontrolling (minority) interests in subsidiaries as equity in the consolidated financial statements. The statement establishes a single method of accounting for changes in a parent’s ownership interest in a subsidiary that do not result in deconsolidation and expands disclosures in the consolidated financial statements. SFAS No. 160 is effective for fiscal years beginning after December 15, 2008 and interim periods within those fiscal years. We have not yet determined the impact of the adoption of SFAS No. 160 on our consolidated financial statements and footnote disclosures.
On December 4, 2007, the FASB issued SFAS No.141R, Business Combinations (SFAS No. 141R). SFAS No. 141R requires the acquiring entity in a business combination to recognize all the assets acquired and liabilities assumed, establishes the acquisition date fair value as the measurement objective for all assets acquired and liabilities assumed, and requires the acquirer to expand disclosures about the nature and financial effect of the business combination. SFAS No. 141R is effective for business combinations for which the acquisition date is on or after the beginning of the first annual reporting period beginning on or after December 15, 2008. We have not yet determined the impact of the adoption of SFAS No. 141R on our consolidated financial statements and footnote disclosures.
The accompanying consolidated financial statements of Benda and its subsidiaries have been prepared in accordance with the accounting principles generally accepted in the United States of America (“U.S. GAAP”) for consolidated financial information
These consolidated financial statements include the accounts of Benda, Ever Leader, Benda Ebei, Jiangling Benda, Yidu Benda and Beijing Shusai for the full year and Sibiono from April 1, 2007 (date of acquisition) All significant inter-company balances and transactions have been eliminated in consolidation.
Use of Estimates
The preparation of financial statements in conformity with US GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements, and the reported amounts of revenue and expenses during the reporting period. Actual results when ultimately realized could differ from those estimates.
5. | Trade Receivables, Other Receivables and Prepaid and Deposits - Allowance for Doubtful Debts |
As mentioned in Note 4 that the company estimates of the appropriate allowance on those accounts receivable for the reporting periods was based on the aged nature of these accounts. The table below shows the allowance for doubtful debts of the Group’s trade receivables, other receivables and prepaid and deposit as of December 31, 2007 and 2006:
| | | December 31, | | December 31, | |
| | | 2007 | | 2006 | |
| Trade receivables, gross | | $ | 10,909,921 | | $ | 6,660,600 | |
| Allowance for doubtful debts | | | (437,688 | ) | | (467,015 | ) |
| Trade receivables, net | | $ | 10,472,233 | | $ | 6,193,585 | |
| | | December 31, | | December 31, | |
| | | 2007 | | 2006 | |
| Other receivables, gross | | $ | 614,971 | | $ | 99,733 | |
| Allowance for doubtful debts | | | (161,376 | ) | | - | |
| Other receivables, net | | $ | 453,595 | | $ | 99,733 | |
| | | December 31, | | December 31, | |
| | | 2007 | | 2006 | |
| Prepaid and deposits, gross | | $ | 1,277,882 | | $ | 372,548 | |
| Allowance for doubtful debts | | | (344,583 | ) | | - | |
| Prepaid and deposits, net | | $ | 933,299 | | $ | 372,548 | |
The change of the allowance for doubtful debts between the reporting periods, as of December 31, 2007 and 2006, is displayed as follows:
| | | December 31, | | December 31, | |
| | | 2007 | | 2006 | |
| Balance at beginning of year | | $ | (467,015 | ) | $ | (92,014 | ) |
| Provision during the year | | | (287,482 | ) | | (364,064 | ) |
| Addition in lieu of acquisition of SiBiono | | | (121,912 | ) | | - | |
| Foreign exchange difference | | | (67,238 | ) | | (10,937 | ) |
| Balance at end of year | | $ | (943,647 | ) | $ | (467,015 | ) |
In general, the company set full allowance for trade receivables aged over 120 days which is general credit term granted to the customers. After deducting those allowances from the gross trade receivable, based on the management’s past experience, there would be no collectability issue on the net amount.
For the year ended December 31, 2007, the management assessed certain balances aged over 120 days and reviewed customer by customer and specified which did not have collectibility issue; then a 100% provision was made against all remaining balances outstanding over 120 days.
For the year ended December 31, 2007, the net trade receivable turnover day was 99 days, therefore the allowance made during the reporting period still fall in the normal situation. For the year ended December 31, 2006, the net trade receivable turnover day was 99 days.
| 6. | Refundable Purchase Price Paid |
The Group’s refundable purchase price at December 31, 2007 and 2006 are comprised as follows:
| | | December 31, | | December 31, | |
| | | 2007 | | 2006 | |
| Advance for acquire intangible assets | | $ | 1,200,000 | | $ | 1,200,000 | |
| Advance for acquire Sibiono | | | - | | | 4,167,801 | |
| Total | | $ | 1,200,000 | | $ | 5,367,801 | |
| a) | On, December 7, 2006, Benda Ebei paid $1.2 million to SECO (Shenzhen) Biotech Co., Ltd. (“SECO”) pursuant to a purchase agreement signed between SECO and Benda Ebei on December 3, 2006 to acquire a technology know-how and drug specifications / technical parameters in producing a Gastropathy drug owned by SECO. As at December 31, 2007, the deal has not been closed as the product certificate still not received from FDA of the United States of America and the amount paid is refundable per agreement if such approval is not obtained. |
| b) | In December 2006, Benda, through its 95% owned subsidiary Benda Ebei, plans to enter into an agreement with the two controlling shareholders of Shenzhen Sibiono GeneTech Co. Ltd. (“SiBiono”) to purchase a total of approximately 58% of the ownership of Sibiono for total cash consideration of approximately Rmb 60 million (or $7.7 million). Due to this possible acquisition, Benda, through its subsidiaries Everleader and Benda Ebei, as of December 31, 2006, advanced approximately RMB 13.03 million (or $1.67 million) to Shenzhen Yuanxing Gene City Development Co., Ltd. and approximately HK$19.42 million (or $2.5 million) to Shenzhen Yuanzheng Investment Development Co., Ltd. as deposits for the pending acquisition of their shares in SiBiono. The acquisition was closed on April 5, 2007, and thus the total advanced amount of $4,167,801 was relcassified as investment cost. |
The Group’s inventories at December 31, 2007 and December 31, 2006 were comprised as follows:
| | | December 31, | | December 31, | |
| | | 2007 | | 2006 | |
| Raw materials | | $ | 1,078,438 | | $ | 311,064 | |
| Packing materials | | | 155,101 | | | 80,639 | |
| Other materials / supplies | | | 93,206 | | | - | |
| Finished goods | | | 415,627 | | | 309,637 | |
| Work-in-progress | | | 4,631,990 | | | - | |
| Total inventories at cost | | | 6,374,362 | | | 701,339 | |
| | | | | | | | |
| Less: Reserves on work-in-progress | | | (4,422,014 | ) | | - | |
| | | | | | | | |
| Total inventories, net | | $ | 1,952,348 | | $ | 701,339 | |
No reserve for obsolete, slow-moving or non-salable inventory were required at the year ended December 31, 2006, and an amount $4,422,014 was made a reserve on work-in-progress at the year ended December 31, 2007.
The provision of reserve was resulted from the manufacturing process of Gendicine, SiBiono’s sole product and SiBiono was acquired by the company in April 2007.
The following chart shows the manufacturing process of Gendicine (Ad-p53):
In the production process of finished goods, Gendicine, several working steps are needed: (i) large-scale culturing of adenovirus from master adenovirus bank; (ii) culturing of cell from master cell bank; (iii) purification. The whole process including step (i) to step (iii) takes approximately twenty-four days to make reagent (“original liquid”). This particular liquid can only be stored for approximately five years. It takes approximately another seven days for mixing and bottling original liquid to finished goods which is known as Gendicine.
Therefore, up to the stage of reagent, all the related production costs are treated as work-in-progress. The major components of those production costs are: (i) direct labor; (ii) direct materials; (iii) power; (iv) supplies and other materials and (v) manufacturing overheads.
Before acquisition, as of March 31, 2007, the accumulated units of original liquid produced was 198,075 and which could be converted to approximately 226,736 vials of Gendicine. However, the accumulated vials of Gendicine sold throughout the years 2004 to three-months period ended March 31, 2007 were only approximately 18,424 vials. The accumulated production costs of $4,080,644 were remained as work-in-process as of three-month period ended March 31, 2007.
Furthermore, due to the special feature of the original liquid which can only be stored for five years, and most of the original liquid was produced in the year of 2004, and the provision of reserve on work in progress was $3,696,083 as of three-month period ended March 31, 2007.
After the acquisition with the effective date April 1, 2007, the same accounting treatment was adopted for the treatment of the provision of reserve on work-in-progress. As of December 31, 2007, the provision of reserve on work in progress was $4,422,014.
8. | Property and Equipment (Restated) |
The Group’s property and equipment at December 31, 2007 and December 31, 2006 were comprised as follows:
| | | December 31, 2006 | | Addition | | Disposal | | Foreign Currency Translation Difference | | December 31, 2007 | |
| Buildings | | $ | 2,227,710 | | | 6,108,113 | | | (19,835 | ) | | 542,138 | | $ | 8,858,126 | |
| Machinery and equipment | | | 3,941,187 | | | 10,571,781 | | | (302,952 | ) | | 1,050,151 | | | 15,260,167 | |
| Office equipment | | | 10,672 | | | 18,766 | | | (491 | ) | | (185 | ) | | 28,762 | |
| Motor Vehicles | | | 32,971 | | | 182,597 | | | - | | | (9,316 | ) | | 206,252 | |
| Cost | | | 6,212,540 | | | 16,881,257 | | | (323,278 | ) | | 1,582,788 | | | 24,353,307 | |
| | | | | | | | | | | | | | | | | |
| Less: Accumulated Depreciation | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| Buildings | | $ | (778,238 | ) | | (137,645 | ) | | 5,098 | | | (70,331 | ) | $ | (981,116 | ) |
| Machinery and equipment | | | (1,512,562 | ) | | (845,147 | ) | | 106,028 | | | (171,321 | ) | | (2,423,002 | ) |
| Office equipment | | | (2,748 | ) | | (2,520 | ) | | 61 | | | 926 | | | (4,281 | ) |
| Motor Vehicles | | | (7,129 | ) | | (37,291 | ) | | - | | | (2,027 | ) | | (46,447 | ) |
| Accumulated Depreciation | | | (2,300,678 | ) | | (1,022,603 | ) | | 111,187 | | | (242,753 | ) | | (3,454,846 | ) |
| | | | | | | | | | | | | | | | | |
| Construction in progress | | $ | 10,184,787 | | | (4,478,528 | ) | | - | | | 518,316 | | $ | 6,224,575 | |
| | | | | | | | | | | | | | | | | |
| Total property and equipment, net | | $ | 14,096,649 | | | 11,380,126 | | | (212,091 | ) | | 1,858,351 | | $ | 27,123,035 | |
The table above shows the fixed assets movement during the year of 2007. In which, the net book value of SiBiono’s fixed assets was recorded as the addition in the year of 2007 when the acquisition was taken place and effective on April 1, 2007. The accumulated depreciation from SiBiono as of March 31, 2007 was not carried forward. As of March 31, 2007, the net book value of SiBiono’s fixed assets was approximately $6.9 million, in which $183K was accounted for buildings; $1.6 million was accounted for machinery and equipment; $5.7K was accounted for office equipment; $18.5K was accounted for motor vehicles; and $5.1 million was accounted for construction in progress at original cost.
As mentioned in Note 11 and Note 28, Benda Ebei entered into a commercial bank note issuance agreement with Shanghai Pudong Development Bank on August 14, 2007 and this credit facility is guaranteed by SiBiono and secured by the buildings, machinery and equipment of Benda Ebei. As of December 31, 2007, the net book value of secured property and equipment was approximately Rmb 67.33 million (or $9.2 million).
The depreciation expense for the year ended December 31, 2007 was calculated as follows:
| | | Cost | | Salvage | | Estimated | | Depreciation | | Deprecation | | Difference | |
| | | December 31, | | December 31, | | Average | | Value | | Useful lives | | Calculated | | Reported | | | |
| | | 2006 | | 2007 | | | | | | | | | | | | | |
| Building | | $ | 2,227,710 | | | 8,858,126 | | | 5,542,918 | | | 5 | % | | 25 | | | 210,625 | | | 137,645 | | | 72,980 | |
| Property and equipment | | | 3,941,187 | | | 15,260,167 | | | 9,600,677 | | | 5 | % | | 12.5 | | | 729,650 | | | 845,147 | | | (115,497 | ) |
| Office equipment | | | 10,672 | | | 28,762 | | | 19,717 | | | 5 | % | | 5 | | | 3,746 | | | 2,520 | | | 1,226 | |
| Motor vehicle | | | 32,971 | | | 206,252 | | | 119,611 | | | 5 | % | | 5 | | | 22,726 | | | 37,291 | | | (14,565 | ) |
| Total | | $ | 6,212,540 | | | 24,353,307 | | | 15,282,924 | | | | | | | | | 966,747 | | | 1,022,603 | | | (55,856 | ) |
The above table shows the calculation of depreciation expenses for the year ended December 31, 2007. The difference between the depreciation calculated and depreciation reported was due to the changes of foreign exchange translation.
The weighted average useful lives are used as the base for the calculation of depreciation as the estimated useful lives for buildings and property and equipments are varies from 20 to 30 years and 10 to 15 years, respectively.
The total depreciation expense was $1,022,603and $294,805 for the years ended December 31, 2007 and 2006, respectively, and is broken down as follows:
| | | December 31, | | December 31, | |
| | | 2007 | | 2006 | |
| Cost of sales | | $ | 688,639 | | $ | 162,058 | |
| Operating expenses | | | 333,964 | | | 132,747 | |
| Balance at end of period | | $ | 1,022,603 | | $ | 294,805 | |
9. | Intangible Assets (Restated) |
The Group’s intangible assets at December 31, 2007 and December 31, 2006 were comprised as follows:
| | | December 31, 2006 | | Addition | | Foreign Currency Translation Difference | | December 31, 2007 | |
| Land-use rights | | $ | 1,068,036 | | | 1,656,038 | | | 151,722 | | $ | 2,875,796 | |
| Drugs permits and licenses | | | 1,055,893 | | | 1,316,700 | | | 192,403 | | | 2,564,996 | |
| Technology formulas | | | 679,700 | | | 474,012 | | | 66,452 | | | 1,220,164 | |
| Patent | | | - | | | 1,619,180 | | | 66,739 | | | 1,685,919 | |
| Cost | | | 2,803,630 | | | 5,065,930 | | | 477,316 | | | 8,346,875 | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| Land-use rights | | $ | (136,790 | ) | | (51,202 | ) | | (13,038 | ) | $ | (201,030 | ) |
| Drugs permits and licenses | | | (1,031,392 | ) | | (175,685 | ) | | (84,348 | ) | | (1,291,425 | ) |
| Technology formulas | | | (66,210 | ) | | (80,757 | ) | | (7,896 | ) | | (154,863 | ) |
| Patent | | | - | | | (197,092 | ) | | (7,955 | ) | | (205,047 | ) |
| Accumulated amortization | | | (1,234,391 | ) | | (504,736 | ) | | (113,237 | ) | | (1,852,364 | ) |
| | | | | | | | | | | | | | |
| Total intangible assets, net | | $ | 1,569,238 | | | 4,561,194 | | | 364,079 | | $ | 6,494,510 | |
The table above shows the intangible assets movement during the year of 2007. In which, the net book value of SiBiono’s intangible assets was recorded as the addition in the year of 2007 when the acquisition was taken place and effective on April 1, 2007. In order words, the accumulated amortization from SiBiono, as of March 31, 2007 was not carried forward. As of March 31, 2007, the net book value of SiBiono’s intangible assets was approximately $1.97 million, in which $353K was accounted for land use rights; $1.62 million was accounted for patents.
As of December 31, 2007, the cost of intangible assets was increased by $5,065,930 which was mainly composed of the followings:
| | a) | Due to the acquisition of SiBiono, two items were included: (1) patent, the innovation and research results of Genedicine, with cost $1,619,180; (2) land use right with a total area 20,574 square meters, with cost $352,505 |
| | b) | Benda Ebei purchased a land use right with total purchase cost Rmb 9.9 million (or $1.3 million). During the year ended December 31, 2007, an amount Rmb 6.71 million (or $0.92 million) was paid. |
| | c) | Benda Ebei purchased a license for a new drug, which is a kind of anti-virus product in nature with total purchase cost Rmb 10 million (or $1.30 million). During the year ended December 31, 2007, an amount Rmb 9 million (or $1.20 million) was paid. |
| | d) | In May 2007, SiBiono enter into a co-operation agreement with DNAVEC, a Japanese gene therapy research institute. Under the terms of the agreement, DNAVEC will leverage SiBiono’s proven gene therapy manufacturing platform and will transfer the exclusive development and distribution rights of SeV-Gag in China to SiBiono. The total purchase cost Rmb 2 million and the full amount was paid during year ended December 31, 2007 for obtaining such exclusive right. |
| | e) | In July 2004, Yidu Benda entered into a co-operation agreement with Sanxia University Chemical Science Research Institute. Under the terms of the agreement, Sanxia University Chemical Science Research Institute will improve the existing product L-Methionine and transfer the exclusive development and distribution rights of L-Methionine to Yidu Benda. The total purchase cost Rmb1.6 million and $0.15 million was paid in the Year of 2006 for obtaining such exclusive right and recognized as intangible assets as of December 31, 2007. |
The amortization expense for the year ended December 31, 2007 was calculated as follows:
| | | Cost | | | | | | | | | |
| | | December 31, | | December 31, | | | | Estimated | | Amortization | | Amortization | | | |
| | | 2006 | | 2007 | | Average | | Useful lives | | Calculated | | Reported | | Difference | |
| Land-use rights | | $ | 1,068,036 | | | 2,875,796 | | | 1,971,916 | | | 40 | | | 49,298 | | | 51,202 | | | (1,904 | ) |
| Drugs permits and licenses | | | 1,055,893 | | | 2,564,996 | | | 1,810,444 | | | 10 | | | 181,044 | | | 175,685 | | | 5,359 | |
| Technology formulas | | | 679,700 | | | 1,220,164 | | | 949,932 | | | 10 | | | 94,993 | | | 80,757 | | | 14,236 | |
| Patent | | | - | | | 1,685,919 | | | 1,685,919 | | | 6 | | | 210,740 | | | 197,092 | | | 13,648 | |
| Total | | $ | 2,803,629 | | | 8,346,875 | | | 6,418,211 | | | | | | 536,075 | | | 504,736 | | | 31,339 | |
The above table shows the calculation of amortization expenses for the year ended December 31, 2007. The difference between the amortization calculated and amortization reported was due to the changes of foreign exchange translation.
From the above calculation table, the patent, $1.68 million, was acquired in during the acquisition of SiBiono. It is amortized over the remaining useful lives, 6 years, whereas the original useful lives are 10 years.
The total depreciation expense was $1,022,603and $294,805 for the years ended December 31, 2007 and 2006, respectively, and is broken down as follows:
| | | December 31, | | December 31, | |
| | | 2007 | | 2006 | |
| Cost of sales | | $ | 363,055 | | $ | 90,620 | |
| Operating expenses | | | 141,681 | | | 96,615 | |
| Balance at end of period | | $ | 504,736 | | $ | 187,235 | |
10. | Goodwill and Acquisition Cost Payable |
FASB 141 requires all acquisitions must be accounted for by allocating the acquisition consideration to the assets acquired based upon the fair market value of those assets. Consideration value that cannot be allocated to the acquired assets must be assigned to goodwill. In addition, it also requires eliminating the pooling treatment and eliminating the amortization of goodwill. FASB 142 requires that a company carrying goodwill on its books must revalue the assets acquired in a business combination. If there is an overall decline in the value of the acquired assets, then earlier booked goodwill is deemed “impaired” and must be written down. FASB 142 requires a two step impairment test. The fair value of a reporting unit is first compared to its carrying value, including goodwill. Then the implied fair value of the goodwill is compared to the carrying value of the goodwill. If the fair value is lower, it is considered to be impaired.
As of December 31, 2007, the total purchase price was Rmb62.56 million (or $8.58 million). Out of which, Rmb53.95 million (or $7.4 million) was recognized as goodwill and the remaining Rmb8.61 million (or $1.18 million) was allocated to identifiable assets and assumed liabilities of SiBiono as following:
| Condensed Balance Sheet of SiBiono, as of March 31, 2007 | | In '000 | |
Current Assets | | | | |
| Cash and cash equivalents | | $ | 590 | |
| Receivables, prepaid expenses and deposits | | | 969 | |
| Inventories | | | 688 | |
Total current assets | | | 2,246 | |
Non-current Assets | | | | |
| Property and equipments, net | | | 6,789 | |
| Intangible assets, net | | | 1,939 | |
Total non-current assets | | | 8,728 | |
Total Assets | | $ | 10,975 | |
Current Liabilities | | | | |
| Trade payables and miscellaneous payables | | $ | 1,175 | |
| Current portion of long term loans payable | | | 1,813 | |
| Current portion of long term government debts payable | | | 2,710 | |
Total current liabilities | | | 5,699 | |
Non-current Liabilities | | | | |
| Long term loans payable | | | 1,360 | |
| Long term government debts payable | | | 1,723 | |
| Due to related parties (long term) | | | 338 | |
Total non-current liabilities | | | 3,420 | |
Total liabilities | | $ | 9,119 | |
Net assets | | $ | 1,855 | |
| % of equity interest acquired | | | 60.13 | % |
| Net assets acquired | | $ | 1,116 | Note a |
Note a. From the above table, the net assets of SiBiono as of March 31, 2007 was Rmb8.61 million, and when translating into US currency upon the time acquired, such net assets amount was translated into $1.12 million (whereas the average exchange rate prevailing on that date was $0.12592). However, when we subtract the total acquisition price against the goodwill, the net assets would be $1.18 million. The slightly difference was occurred, $0.06 million and which was solely due to the foreign currency translation.
As of December 31, 2007, out of the total acquisition cost Rmb62.56 million (or $8.58 million), the following payments were made:
In the year of 2006, Benda, through its subsidiaries Everleader and Benda Ebei, paid Rmb19.52 million (or $2.5 million or HK$19.42 million) and Rmb13.03 million (or $1.67 million) or totaling Rmb32.55 million (or $4.17million) to the selling shareholders of SiBiono and reported in the consolidated balance sheet and cash flow statement as “refundable purchase price paid”. It was recorded as refundable assets due to the fact that the deal was not concluded as of December 31, 2006. The acquisition was closed on April 5, 2007, and thus the total refundable amount of $4.17 million was reclassified as investment cost.
In the year of 2007, an additional amount Rmb20.28 million (or $2.64 million) was paid. The remaining balance was reported as “acquisition cost payable” on the balance sheet. As of December 31, 2007, the total amount paid was Rmb52.83 million and the outstanding balance was Rmb9.73 million (or $1.33million).
The Group has already obtained the oral consent from the selling shareholders of SiBiono that the remaining balance could be settled within the year 2008.
11. | Restricted Cash, Bank Indebtedness and Commercial Notes Payable |
The Group’s restricted cash at December 31, 2007 and December 31, 2006 was comprised as follows:
| | | December 31, | | December 31, | |
| | | 2007 | | 2006 | |
| Deposits for issuance of commercial notes | | $ | 2,615,254 | | $ | - | |
| Funds from government technolgy agencies | | | 1,342,370 | | | - | |
| Total restricted cash | | $ | 3,957,624 | | $ | - | |
| C) | On August 14, 2007, Benda Ebei entered into a commercial bank note issuable agreement with Shanghai Pudong Development Bank. Pursuant to this agreement, the following terms are included: |
| | a) | Duration of the agreement is three years; |
| | b) | It is non-interest bearing; |
| | c) | The repayment period of each commercial note payable is six months; |
| | d) | The total commercial note issuable limit is Rmb 60 million; however 50% of deposit should be made into the bank in order to secure the issuance of commercial bank note, thus the net available amount is Rmb 30 million; |
| | e) | If the net amount of each commercial bank note payable is not settled on the due date, the penalty will be the penalty rate of the PRC bank loan on daily and compound basis. |
As of December 31, 2007, Benda Ebei and Jiangling Benda deposited an amount $2,615,254 in Shanghai Pudong Development Bank as deposit for the issuance of commercial bank notes. Such deposits will be released when the commercial bank notes are cleared. As of December 31, 2007, the balance if the commercial bank notes payable was $5,118,758. Thus the net commercial bank notes payable was $2,503,504 as of December 31, 2007.
| D) | The bank indebtedness was resulted from the acquisition of SiBiono with the effective date April 1, 2007. The reasons for causing bank indebtedness were stated as follows: |
| | a) | Among the cash and cash equivalents balances of SiBiono were composed of two parts; (i) unrestricted cash, which were generated from either operations, or loans from bank and financial institutions, or invested capital; (ii) restricted cash, which were obtained from the various government technology agencies as long term debt payable (see note 13 for the related details). |
| | b) | The cash obtained from the various government technology agencies as long term debt payable could only be dedicated to the related project’s research and development activities and purchase of fixed assets and construction in progress, therefore the cash balances for that part will be classified as restricted cash. |
| | c) | Due to the above reasons, SiBiono relocated the balances of restricted cash from the cash and cash equivalents balances for the reporting periods. |
| | d) | However, since the balance of the restricted cash was larger than the balance of cash and cash equivalents balances, thus bank indebtedness were resulted for the reporting periods. |
Due to the above reasons, SiBiono relocated the balances of restricted cash from the cash and cash equivalents balances with an amount $1,342,370 as of December 31, 2007. However, since the balances of the restricted cash were larger than the balance of cash and cash equivalents balances, bank indebtedness were resulted with an amount $874,490 as of December 31, 2007.
On November 23, 2006, Benda Ebei entered into an Equity Transfer Agreement with Xiaozhi Zhang (“Zhang”), to purchase approximately 6.24% of SiBiono’s common stock for a total consideration of Rmb12.48 million (Rmb6.24 million in cash and shares of our common stock equal to Rmb6.24 million) (or $1.71 million) which was due and payable on or before March 31, 2007.
Due to the fact that the signed agreement on November 23, 2006 was not practically executable according to the PRC regulations, Benda Ebei asked Zhang to terminate the signed agreement and sign a new agreement that was feasible under PRC regulations with essentially the same terms.
However, Zhang refused to sign the new agreement and applied to the Shenzhen Arbitration Commission (the “Commission”) in April 2007 for enforcement of the original agreement. Zhang requested the Commission to require Benda Ebei to pay for the total consideration, penalty for late payment and the related legal and arbitration expenses.
On November 27, 2007, Shenzhen Arbitration Commission determined that:
| 4. | Benda Ebei should pay for the consideration of Rmb 6.24 million, equal to 50% of the total consideration set forth in the Equity Transfer Agreement. For the other 50% of the total consideration which was supposed to be settled in the form of issuing common stock, since Zhang did not make an arbitration request on how to execute the arrangement, the Arbitration Commission did not make an award on this particular part. |
| 5. | Benda Ebei should pay for the penalty of Rmb 46,800; |
| 6. | Benda Eebi should pay for legal and arbitration expenses of Rmb 268,971. |
Following this arbitration decision, Benda Ebei recognized the liability as total acquisition cost payable of Rmb 12.48 million, plus the penalty and related legal and arbitration expenses, totaling approximately Rmb 12.80 million or ($1.75 million) (Note 15). Accordingly Benda Eebi recognized the right to purchase the 6.24% equity shares in SiBiono and recorded as other assets at Rmb 12.48 million or $1.71 million.
The Group’s bank loans as of December 31, 2007 and December 31, 2006 were comprised as follows:
| | | December 31, | | December 31, | |
| | | 2007 | | 2006 | |
| Bank loans due within one year | | $ | 2,867,004 | | $ | 256,492 | |
| Bank loans due after one year | | | - | | | - | |
| Total bank loans | | $ | 2,867,004 | | $ | 256,492 | |
As of December 31, 2006, the Group had one outstanding bank loans with an amount $256,492 which was used primarily to fund construction in progress projects and for general working capital purposes and this loan had been settled as of December 31, 2007.
As of December 31, 2007, Sibiono, had one outstanding bank loan with an amount $2,867,004 which was used primarily to fund construction in progress projects and for general working capital purposes. This loan carries annual interest rate of 6.34% and payable monthly on the 20th day of each month with maturity in 3 years, starting from April 30, 2005 and matures on April 29, 2008. This loan is considered as a term loan and is not revolving or renewable. This loan is personal guaranteed by Zhaohui Peng, the Chairman and a shareholder of SiBiono.
Total interest expense paid related to the Group’s outstanding bank loans was $214,113 and $113,458 for the years ended December 31, 2007 and 2006, respectively.
14. | Long Term Debt Payable |
As of December 31, 2007, long term debt payable was raised due to the fact that various technology funds were obtained from various government technology agencies to support its gene therapy research and development activities during the past years and recorded as long term debt payable.
The long term debt payable at December 31, 2007 and December 31, 2006 were comprised as follows:
| | | December 31, | | December 31, | |
| | | 2007 | | 2006 | |
| Long-term debt payable due within one year | | $ | 1,787,239 | | $ | - | |
| Long-term debt payable due after one year | | | 425,001 | | | - | |
| Total long-term debt payable | | $ | 2,212,240 | | $ | - | |
Even though there were $1,787,239 long term debt payable due within one year as of December 31, 2007, because of its non-repayable feature, the obligations will be discharged once the examination by the various government technology agencies is conducted and most of the examination will be carried out and completed.
During the year ended December 31, 2007, an amount of Rmb 17,090,000 (or $2,250,240) of long term debt payable was discharged and recorded as government subsidies, (see Note 22 for the related details).
15. | Accounts Payable and Accrued Liabilities |
The Group’s accounts payable and accrued liabilities as of December 31, 2007 and December 31, 2006 were comprised as follows:
| | | December 31, | | December 31, | |
| | | 2007 | | 2006 | |
| Trade payable | | $ | 305,443 | | $ | 387,073 | |
| Deposits paid by customer | | | 160,818 | | | 2,488 | |
| Acquistion cost payable following the arbitration (Note 12) | | | 1,754,263 | | | | |
| Accrued liabilities | | | 2,162,455 | | | 1,165,300 | |
| Miscellaneous payables | | | 283,005 | | | 268,169 | |
| Total account apyables and accurred liabilities | | $ | 4,665,984 | | $ | 1,823,030 | |
16. | Welfare and Employment Liabilities |
As stipulated by the relevant laws and regulations for enterprises operating in the PRC, the Group’s PRC entities are required to maintain a welfare plan for all of its employees who are residents of the PRC. Based on the wages payable and according to the labor law of the PRC, the Group accrued 14% on a monthly basis, for employees’ welfare, labor union fees, and education and training programs, respectively. As of December 31, 2007 and 2006, the Group accrued approximately $345,000 and $146,000 for the employees’ welfare respectively.
As stipulated by the relevant laws and regulations for enterprises operating in the PRC, the Group is required to make annual appropriations to a statutory surplus reserve fund for each of its PRC subsidiaries. Specifically, the Group is required to allocate 15% its profits after taxes at the fiscal year end, as determined in accordance with the PRC accounting standards applicable to the Group’s PRC subsidiaries, to a statutory surplus reserve until such reserve reaches 50% of the registered capital of the Group’s PRC subsidiaries. As of December 31, 2007 and 2006, the registered capital of the Group’s PRC subsidiaries was $20,026,617 and $12,017,575, respectively.
18. | Related Party Transactions |
Due from related parties at December 31, 2007 and 2006 were comprised as follows:
| | | December 31, | | December 31, | |
| | | 2007 | | 2006 | |
| Yiqing, Wan | | | | | | | |
| Due to Ever Leader Holdings Co. Ltd. | | $ | 646,429 | | $ | 455,275 | |
| Due to Hubei Tongji Benda Ebei Phamacetucial Co. Ltd. | | | 72,949 | | | - | |
| Due to Shenzhen SiBiono Gene Tech Co. Ltd. | | | 3,608 | | | - | |
| Hubei Benda Science and Technology Co. Ltd | | | | | | | |
| Due to Yidu Benda Chemicals Co. Ltd. | | | 1,502,118 | | | 1,299,479 | |
| Due to Ever Leader Holdings Co. Ltd. | | | 230,160 | | | 210,518 | |
| Feng Wang | | | | | | | |
| Due to Beijing Shusai Pharyngitis Research Co. Ltd. | | | 29,318 | | | 11,543 | |
| Hua Shen | | | | | | | |
| Due to Shenzhen SiBiono Gene Tech Co. Ltd. | | | 137,097 | | | - | |
| Pong Tsaiohuei | | | | | | | |
| Due to Shenzhen SiBiono Gene Tech Co. Ltd. | | | 3,257 | | | - | |
| Xiaozhi Zhang | | | | | | | |
| Due to Shenzhen SiBiono Gene Tech Co. Ltd. | | | 5,083 | | | - | |
| Total due from related parties | | $ | 2,630,019 | | $ | 1,976,815 | |
Due to related parties at December 31, 2007 and, 2006 were comprised as follows:
| | | December 31, | | December 31, | |
| | | 2007 | | 2006 | |
| Hubei Benda Science and Technology Co. Ltd | | | | | | | |
| Due from Hubei Tongji Benda Ebei Phamacetucial Co. Ltd. | | $ | 49,056 | | $ | 236,205 | |
| Due from Jiangliang Benda Pharamaceutical Co. Ltd. | | | 1,872,374 | | | 1,833,358 | |
| Due from Beijing Shusai Pharyngitis Research Co. Ltd. | | | 6,846 | | | - | |
| Wei Xu | | | | | | | |
| Due from Hubei Tongji Benda Ebei Phamacetucial Co. Ltd. | | | 1,009,792 | | | 943,865 | |
| Due from Beijing Shusai Pharyngitis Research Co. Ltd. | | | 61,491 | | | 20,937 | |
| Yiqing, Wan | | | | | | | |
| Due from Shenzhen SiBiono Gene Tech Co. Ltd. | | | 137,687 | | | - | |
| Hui Xu | | | | | | | |
| Due from Hubei Tongji Benda Ebei Phamacetucial Co. Ltd. | | | 26,622.00 | | | - | |
| Due from Beijing Shusai Pharyngitis Research Co. Ltd. | | | 3,153.00 | | | - | |
| Hua Shen | | | | | | | |
| Due from Shenzhen SiBiono Gene Tech Co. Ltd. | | | 26,597.00 | | | - | |
| Total due to related parties | | $ | 3,193,618 | | $ | 3,034,365 | |
The above advances bear no interest and the above loans due to related parties are unsecured, non-interest bearing and are not convertible into equity. Proceeds from the above loans were used primarily for general working capital purposes and are also long-term debts in nature, due on December 31, 2012.
19. | Redeemable Common Stock Issuable For Services |
On April 5, 2007, Benda Ebei entered into Equity Transfer Agreements with certain shareholders of SiBiono to purchase a total of approximately 57.57% of the shares of SiBiono’s common stock for total consideration of RMB 60,000,000 due and payable on or before April 30, 2007.
On June 11, 2007, Benda Ebei entered into additional Equity Transfer Agreements with Yaojin Wang (“Wang”) and Huimin Zhang “(Zhang”), also shareholders of SiBiono, for the purchase of an additional 2.56% of the shares of SiBiono’s common stock for total consideration of RMB 2,560,000 due and payable on or before June 30, 2007.
In connection with the above Equity Transfer Agreements, Benda entered into a Financial Consultancy Agreement with Super Pioneer International Limited (“Super Pioneer”) and Technical Consultancy Agreements with Wang and Zhang for financial and technical consultancy services to be rendered. Pursuant to the Financial and Technical Consultancy Agreements (the “Agreements”), Benda agreed to issue an aggregate of 2,189,560 shares of its common stock to Super Pioneer (2.1 million shares, out of which 1.9 million shares is redeemable), Wang (33,585 shares, redeemable) and Zhang (55,975 share, redeemable) within three months from the date of the Agreements. Super Pioneer, Wang and Zang also agreed to refrain from selling shares of Benda’s common stock for a period of twelve months from the date of the issuance of the shares (the “Lock-up Period”). Within three months from expiration of the Lock-up Period, in the event that the public trading price of Benda’s common stock has not reach $3.60 per share and Benda’s common stock has not been listed on either the NASDAQ or AMEX stock exchanges, Super Pioneer, Wang, and Zang will have the option to require Benda to redeem an aggregate 2,049,560 shares of Benda’s common stock owned by Super Pioneer, Wang, and Zhang at a price of $3.60 per share.
In accordance EITF Topic D-98, “Classification and Measurement of Redeemable Securities” (“EITF Topic D-98”), as the Agreements governing the issuance of the 2,189,560 shares of common stock to Super Pioneer, Wang, and Zang contain provisions requiring Benda to repurchase 2,049,560 of these shares at a redemption price of $3.60 per share at the option of the holders (if certain events outside of control of the Group do not occur), these 2,049,560 shares have been classified as redeemable common stock, at their redemption price of $3.60 per share or totaling $7,376,366, outside of permanent equity at December 31, 2007.
The total consulting and professional fees was $8,353,615 and which was composed of:
| | a) | Since the issuance of common shares to Super Pioneer, Wang and Zhang was in the form of financial and technical consultancy services to be rendered, thus the corresponding amount $7,882,416 was recorded as consulting and professional fees. |
| | b) | As of December 31, 2007, the placement agent, Keating Investment, exercised it warrants at a strike price $0.555 per share through the cashless arrangement. The amount of warrants being exercised was 849,007, thus the corresponding amount $471,199 was recorded as consulting and professional fees. |
In accordance with FASB staff position No. EITF00-19-2 the Group recorded $1,022,275 in Registration Delay Expense, starting from August 15, 2007 till December 31, 2007. Out of the total penalty, $143, 225 was paid in cash during the year ended December 31, 2007; $92,628 was settled by issuance of 166,898 shares of common stock at strike price of $0.555 per share.
21. | Other general and administrative |
For the years ended December 31, 2007 and 2006, the amount of other general and administrative expenses mainly composed of the following events:
| | | December 31, | | December 31, | |
| | | 2007 | | 2006 | |
| Audit and accounting | | $ | 405,364 | | $ | 521,977 | |
| Legal fees | | | 304,649 | | | 114,993 | |
| Office expenses | | | 428,219 | | | 210,696 | |
| Salaries and wages | | | 961,797 | | | 221,174 | |
| Consulting | | | 221,044 | | | 191,248 | |
| Rent & Utilities | | | 77,132 | | | 21,313 | |
| Investor relation, Transfer agent and filing fees | | | 109,540 | | | 9,828 | |
| Travel and transportation | | | 239,316 | | | 87,264 | |
| Miscellaneous | | | 426,246 | | | 155,282 | |
| Total other general & administrative | | $ | 3,173,307 | | $ | 1,533,775 | |
22. | Government Subsidies / Grants |
As mentioned in the Note 14, long term debt payable, various technology funds were obtained from various government technology agencies to support its gene therapy research and development activities during the past years and recorded as long term debt payable. According to the technology fund agreements, the various government technology agencies will examine the results of research and development according to the status of the projects. Once the examination is taken place, the obligation of a particular debt payable is discharge accordingly.
According to US GAAP, once the obligation of a particular debt payable is discharged, the amount of this particular debt payable should be treated as government subsidies / grants. During the reporting period ended December 31, 2007, an amount of Rmb 17,090,000 (or $2,250,240) was recognized as government subsidies / grants and the corresponding debt payable was discharged. In addition, SiBiono obtained government rewards to enterprise holding superior intellectual property rights of Rmb 200,000 (or $26,334), which was also recognized as subsidy income.
Benda is subject to Delaware, United State of America tax, but no provision for income taxes were made for the years ended December 31, 2007 and 2006 as Benda did not have reportable taxable income for the period.
Ever Leader, a wholly owned subsidiary of Benda, is subject to Hong Kong tax, but no provisions for income taxes were made for the the years ended December 31, 2007 and 2006 as Ever Leader did not have reportable taxable income for the periods.
Benda Ebei was registered as a Sino-Foreign Equity Joint Venture on May 26, 2004 and is subject to the tax laws applicable to Sino-Foreign Equity Joint Ventures in the PRC. Benda Ebei, starting from 2005, is fully exempt from PRC enterprise income tax for two years starting from the first profit-making year, followed by a 50% reduction in income taxes, at 18%, for the following three years, commencing from the first profitable year.
Jiangling Benda and Yidu Benda are cross-municipal investment entities and enjoy the same tax treatment as Sino-Foreign Joint Ventures, starting from 2005, and were therefore exempt from PRC enterprise income tax for two years starting from the first profit-making year, followed by a 50% reduction in income taxes, at 18%, for the following three years, commencing from the first profitable year. Cross-municipal investments entities refer to entities that are incorporated in one municipal region but have investments in another municipal region.
The exemption periods for Benda Ebei, Jiangling Benda and Yidu Benda expired in the year of 2006, after which they are subject to 50% reduction in income taxes; whereas the full income tax rate is 33%. The remaining tax holidays will be expired in 2010.
However, starting and effective from January 1, 2008, the full income tax rate would be changed from 33% to 25% according to the new PRC Taxation Regulations. Thereafter these subsidiaries will be subject to the regular full income tax rate at 25%.
Beijing Shusai did not have taxable income for the years ended December 31, 2007 and 2006.
According to the taxation regulations of Shenzhen, a Special Economic District of PRC, SiBiono is subject to the full income tax rate of 15% on taxable income. The net losses for the previous year can be carried forward for a maximum period of five years. If the company is approved and recognized as high-tech company, the company can enjoy three years of 50% of the full tax rate with an extension for the coming next three years. Therefore, there was no provision for income taxes made for the year ended December 31, 2007. SiBiono also plans to apply for the PRC enterprise tax exemption for two years starting from the first profit-making year, followed by a 50% reduction in income taxes for the following three years.
As a result, only Benda Ebei incurred income tax for the year ended December 31, 2007 of Rmb 7.3 million (or $0.9 million). There was no income tax of the Group for the years ended December 31, 2006.
24. | Long Term Convertible Promissory Note |
Our financial statements include enhanced note disclosure addressing the fair value of the convertible promissory notes and warrants and the appropriate accounting treatment for the overall transaction.
Additionally, the enhanced note disclosure includes a detailed discussion of the accounting for the debt discount resulting from the relative fair value of the warrants issued in conjunction with the convertible promissory notes (calculated in accordance with APB 14) and the debt discount resulting from the beneficial conversion feature / conversion discount associated with the convertible promissory notes (calculated in accordance with EITF 98-5 and EITF 00-27) as follows:
In March and April of 2007, the Group entered into Investment Agreements (“Agreements”) with certain accredited and institutional investors (“Investors”) pursuant to which the Investors purchased from the Group a total of 252 Units (“Units”), resulting in aggregate gross proceeds to the Group $7,560,000, with each Unit consisting of: (i) a Convertible Promissory Note (“Note”) in the principal amount of $30,000 and convertible into 54,087 shares of the Group's common stock; and (ii) a Common Stock Warrant (“Warrant”) to acquire 54,087 shares of the Group’s common stock at an exercise price of $0.555 per share and expiring on November 14, 2011. The Notes are immediately convertible at the option of each Investor, bear interest at a rate of 4% per annum, and mature on March 28, 2009. At December 31, 2007, the aggregate $7,560,000 principal balance of the notes remained outstanding and during the year ended December 31, 2007, the Group recorded $224,522 of interest expense related to the Notes.
The Group has accounted for the Warrants issued in conjunction with the Notes in accordance with the provisions of APB No. 14, “Accounting for Convertible Debt and Debt Issued with Stock Purchase Warrants” (“APB 14”). Accordingly, the Warrants were valued using a Black-Scholes option pricing model with the following assumptions: (i) a risk free interest rate of 4.63%, (ii) a contractual life of 4.6 years, (iii) an expected volatility of 25%, and (iv) a dividend yield of zero. The relative fair value of the Warrants, based on an allocation of the value of the Notes and the value of the Warrants issued in conjunction with the Notes, was recorded as a debt discount (with a corresponding increase to additional paid-in capital) in the amount of $5,174,911, and is being amortized to interest expense over the expected term of the Notes.
Additionally, the difference between the effective conversion price of the Notes into shares of the Group’s common stock, and the fair value of the Group’s common stock on the date of issuance of the Notes, resulted in a beneficial conversion feature of $2,385,089 (capped at the $7,560,000 of gross proceeds raised less the previously calculated $5,174,911 debt discount associated with Warrants issued in conjunction with the Notes) and was calculated in accordance with EITF 98-5, “Accounting for Convertible Securities with Beneficial Conversion Features or Contingently Adjustable Conversion Ratios” and EITF 00-27, “Application of Issue No. 98-5 to Certain Convertible Instruments”. This beneficial conversion feature was recorded as an additional debt discount (with a corresponding increase to additional paid-in capital) and is being amortized to interest expense over the expected term of the Notes.
During the year ended December 31, 2007, the Group recorded $2,875,075 in interest expense related to the amortization of the debt discount associated with the Warrants and the debt discount associated with the beneficial conversion feature.
The Group also incurred $529,200 in placement agent commissions related to the issuance of the Notes and Warrants. This amount was recorded by the Group as debt issue costs and is being amortized over expected term of the Notes. For the year ended December 31, 2007, the Group recorded $201,255 in amortization expense related to debt issue costs.
The securities underlying the Notes and Warrants issued to the Investors are also subject to the terms of a Make Good Agreement entered into in connection with a financing the Group executed in November of 2006 (the “Make Good Agreement”). The Group further represented to the Investors that its target net income for fiscal year 2007 (“FY07 Net Income”) will be greater than or equal to $10.0 million (adjusted for a variety of non-cash charges) (the “Performance Threshold”). In the event the Performance Threshold is not attained, the Group is required to issue to the Investors a pro rata portion of 1,000,000 shares of the Group’s common stock for every one (1) cent by which the Group’s earnings per share, determined on a fully diluted basis, is less than $0.065.
In the accounting for the Long Term Converible Promissory Note, the Group’s analysis of the Performance Threshold had no effect because of the cap of the debt discounted and beneficial conversion feature as calculated in accordance with EITF 98-5.
The Group is also required to register for resale: (i) the shares of common stock underlying the Notes; and (ii) 150% of the shares of common stock underlying the Warrants, on a registration statement to be filed with the Securities and Exchange Commission (“Registration Statement”) and such Registration Statement is required to be declared effective by August 15, 2007 (the “Effectiveness Deadline”). If the Registration Statement does not become effective by the Effectiveness Deadline, or if the Group fails to maintain the effectiveness of the Registration Statement, for any reason, the Group is required to pay the Investors in cash an amount equal to 1% of the purchase price of each Unit held by the Investors on such Effectiveness Deadline or the first day of such failure to maintain the Registration Period, as applicable, and for every 30 day period (or part) thereafter, in each case until cured (“Registration Delay Payments”), provided that the Registration Delay Payments will not exceed 10% of the purchase price of the Units. In the event that the Registration Delay Payments are not made in a timely manner, such Registration Delay Payments will bear interest at a rate of 1.5% per month until paid in full.
The calculated fair value of the warrants (issued in conjunction with the convertible promissory notes) was recorded as a debt discount (reduction to the carrying value of the convertible promissory notes) with a corresponding increase (credit) to additional paid in capital.
In accordance with the registration rights granted to investors who purchased the convertible promissory notes and warrants, we are required to file a registration statement with the Securities and Exchange Commission (“Registration Statement”) attempting to register the potentially issuable shares of common stock underlying the convertible promissory notes and warrants. There is no cash settlement requirement, in the event the Company cannot deliver Registered Shares.
Specifically, in the event the Registration Statement is not declared effective by August 15, 2007 (“Effectiveness Deadline”), we are required to pay to the investors in cash an amount equal to 1% of the purchase price paid by each investor for the convertible promissory notes and warrants (“Registration Delay Payments”). Additionally, for every 30 day period (or part) thereafter that the Registration Statement is not declared effective, we are required to continue to make Registration Delay Payments, provided that such Registration Delay Payments shall not exceed 10% of the purchase price paid by each investor for the convertible promissory notes and warrants.
In the event the Registration Statement is never declared effective, the Registration Delay Payments are capped at 10% (of the purchase price paid by each investor for the convertible promissory notes and warrants). As a result, the initial requirement for the Company to deliver registered shares upon conversion of the convertible promissory notes or exercise of the warrants is negated and paragraphs 14 - 18 of EITF 00-19 are not applicable (for purposes of classifying the fair value of the warrants issued in conjunction with the convertible promissory notes as a liability with changes in fair value recorded in earnings).
In accordance with FASB staff position No. EITF00-19-2, as of December 31, 2007 the Group recorded $1,022,275 for Registration Delay Expense or penalty to investors. EITF 00-19-2 requires the Group to record the expense when it is probable that the liability has been incurred and the amount can be reasonably estimated.
25. | Common Stock, Preferred Stock, Additional Paid-in Capital, Warrants and Options |
As of December 31, 2007, and 2006, the Group had an aggregate of:
| | (f) | None shares of preferred stock issued and outstanding; |
| | (g) | As of December 31, 2006, 96,258,411 shares of common stock were issued and outstanding, par value $0.001 each; During the reporting period of the year 2007: (i) 2,189,560 common shares were issued to Super Pioneer, Yaojin Wang and Huimin Zhang (see Note 19); (ii) 849,007 common shares were issued to placement agent for being exercised their warrants (see Note 19); (iii) 166,898 shares were issued to PIPE investors for being late submission of 10K-SB (see Note 20); and (iv) 706,195 shares were issued to the various consultants who rendered services related to the reverse merger and were valued at the private placement offering price at $0.4622 per share on November 15, 2006. Thus, the share of common stock issued and outstanding was 100,170,071 as of December 31, 2007. |
| | (h) | The following tables shows the events occurred in additional paid-in capital: |
| | | December 31, | | Events occurred | | December 31, | |
| Events | | 2006 | | during the year | | 2007 | |
| To record the changes of par value from $0.01 to $0.001 of the outstanding common stock as of 11/17/2005 | | $ | 584,481 | | | - | | $ | 584,481 | |
| | | | | | | | | | | |
| To adjust the par value of outstanding common stock as of 3/31/2006 | | | (5,354 | ) | | - | | | (5,354 | ) |
| | | | | | | | | | | |
| Waiver of shareholder loan on 9/5/2006 | | | 2,298,434 | | | - | | | 2,298,434 | |
| | | | | | | | | | | |
| To eliminate the common stock and additional paid-in capital of the former shell company "Applied Spectrum Technologies, Inc." on 11/15/2006 | | | 16,215,770 | | | - | | | 16,215,770 | |
| | | | | | | | | | | |
| To eliminate the accumulated deficit of the former shell company "Applied Spectrum Technologies, Inc." on 11/15/2006 | | | (16,209,962 | ) | | - | | | (16,209,962 | ) |
| | | | | | | | | | | |
| Issuance of common stock, 25,961,760 shares at $0.4622 per share, on 11/15/2006 | | | 11,974,038 | | | - | | | 11,974,038 | |
| | | | | | | | | | | |
| Issuance of common stock, 64,942,369 shares at $0.001 per share on 11/15/2006 | | | (64,942 | ) | | - | | | (64,942 | ) |
| | | | | | | | | | | |
| To relocate the original common stock of Ever Leader on 11/15/2006 | | | 1,285 | | | - | | | 1,285 | |
| | | | | | | | | | | |
| To record the placement agent commission and transaction related fee of reverse merger on 11/15/2006 | | | (1,694,326 | ) | | - | | | (1,694,326 | ) |
| | | | | | | | | | | |
| Issuance of common stock 706,195 at $0.4622 per share | | | - | | | 325,697 | | | 325,697 | |
| | | | | | | | | | | |
| Debt discount on beneficial conversion feature on convertible promissory notes | | | - | | | 2,385,089 | | | 2,385,089 | |
| | | | | | | | | | | |
| Debt discount on warrants issued with convertible promissory notes | | | - | | | 5,174,911 | | | 5,174,911 | |
| | | | | | | | | | | |
| Placement agent exercised 849,007 warrants at strike price through cashless arrangement | | | - | | | 470,347 | | | 470,347 | |
| | | | | | | | | | | |
| Issuance of common stock to PIPE investors as penalty for late submission of 10KSB | | | - | | | 92,461 | | | 92,461 | |
| | | | | | | | | | | |
| Additional paid-in capital, balance for the period ended | | $ | 13,099,424 | | | 8,448,505 | | $ | 21,547,929 | |
| (i) | As of December 31, 2006, 28,557,936 Warrants, each convertible into one (1) share of the Group’s Common Stock, issued and outstanding, and as of December 31, 2007, since the placement agent exercised 849,007 warrants through the cashless arrangement, thus the remaining balance was 27,708,929; |
| (j) | None options issued and outstanding. |
26. | Commitments and Contingencies |
As of December 31 2007, there was an operating lease commitment and the amounts are stated as follows;
| | | Decemebr 31, | |
| | | 2007 | |
| Rental and Property Management Fee | | | | |
| Within one year | | $ | 107,600 | |
| One to two year | | | 73,691 | |
| Total commitments payable | | $ | 181,291 | |
The company states the segment information according to the requirement stated in paragraph 37 of SFAS 131. The company produces five different categories of products and each category of product is produced in different subsidiaries or operation plants. The details are stated as follows:
| | 6. | Benda Ebei produces including branded and generic medicines; |
| | 7. | Jiangling Benda produces active pharmaceutical ingredients, APIs; |
| | 8. | Yidu Benda produces bulk chemicals; |
| | 9. | Beijing Shusai produces pharyngitis killer therapy; and |
| | 10. | SiBiono produces gene therapy medicines, Gendicine. |
Since each subsidiary produces the corresponding products by using the same production facilities of each subsidiary, therefore according to the requirement stated in paragraph of SFAS 131, the Group reports the segment information according to the un-identical products that produced in each subsidiary.
Selected financial information for each of these segments for the years ended December 31, 2007 and 2006 were as follows:
Branded/generic medicine segment | | December 31, 2007 | | December 31, 2006 | |
| Revenue from external customers | | $ | 19,353,654 | | $ | 9,635,938 | |
| Cost of sales | | | (12,046,687 | ) | | (5,775,634 | ) |
| Gross profit | | | 7,306,967 | | | 3,860,304 | |
| Gross margin | | | 38 | % | | 40 | % |
| Research and development | | | (2,963 | ) | | - | |
| Selling expense | | | (715,588 | ) | | (359,504 | ) |
| General and administrative expense | | | (628,426 | ) | | (524,762 | ) |
| Segment contribution | | $ | 5,959,990 | | $ | 2,976,038 | |
| Contribution margin | | | 31 | % | | 31 | % |
| | | | | | | | |
| Total assets, segment | | $ | 32,817,426 | | $ | 12,506,547 | |
Active pharmaceutical ingredients segment | | December 31, 2007 | | December 31, 2006 | |
| Revenue from external customers | | $ | 531,531 | | $ | 15,564 | |
| Cost of sales | | | (528,473 | ) | | (15,703 | ) |
| Gross profit | | | 3,058 | | | (139 | ) |
| Gross margin | | | 1 | % | | -1 | % |
| Research and development | | | (359 | ) | | - | |
| Selling expense | | | (505 | ) | | (10,356 | ) |
| General and administrative expense | | | (224,754 | ) | | (159,484 | ) |
| Segment contribution | | $ | (222,560 | ) | $ | (169,979 | ) |
| Contribution margin | | | -42 | % | | -1092 | % |
| | | | | | | | |
| Total assets, segment | | $ | 8,515,278 | | $ | 4,079,747 | |
Bulk chemicals segment | | December 31, 2007 | | December 31, 2006 | |
| Revenue from external customers | | $ | 698,578 | | $ | 6,237,621 | |
| Cost of sales | | | (425,529 | ) | | (3,309,654 | ) |
| Gross profit | | | 273,049 | | | 2,927,967 | |
| Gross margin | | | 39 | % | | 47 | % |
| Research and development | | | - | | | (30,821 | ) |
| Selling expense | | | (27,309 | ) | | (221,955 | ) |
| General and administrative expense | | | (590,759 | ) | | (332,110 | ) |
| Segment contribution | | $ | (345,019 | ) | $ | 2,343,081 | |
| Contribution margin | | | -49 | % | | 38 | % |
| | | | | | | | |
| Total assets, segment | | $ | 7,425,241 | | $ | 8,389,477 | |
Pharynigitis killer therapy segment | | December 31, 2007 | | December 31, 2006 | |
| Revenue from external customers | | $ | 43,890 | | $ | 42,952 | |
| Other sales | | | | | | 2,937 | |
| Cost of sales | | | (2,341 | ) | | (2,524 | ) |
| Gross profit | | | 41,549 | | | 43,365 | |
| Gross margin | | | 95 | % | | 101 | % |
| Research and development | | | - | | | - | |
| Selling expense | | | (43,680 | ) | | (7,756 | ) |
| General and administrative expense | | | (116,825 | ) | | (74,946 | ) |
| Segment contribution | | $ | (118,956 | ) | $ | (39,337 | ) |
| Contribution margin | | | -271 | % | | -92 | % |
| | | | | | | | |
| Total assets, segment | | $ | 125,882 | | $ | 130,171 | |
Gendicine (Ad-p53) segment | | December 31, 2007 | | December 31, 2006 | |
| Revenue from external customers | | $ | 5,756,955 | | $ | - | |
| Cost of sales | | | (418,667 | ) | | - | |
| Gross profit | | | 5,338,288 | | | - | |
| Gross margin | | | 93 | % | | - | |
| Research and development | | | (550,640 | ) | | - | |
| Selling expense | | | (794,573 | ) | | - | |
| General and administrative expense | | | (1,295,906 | ) | | - | |
| Segment contribution | | $ | 2,697,169 | | $ | - | |
| Contribution margin | | | 47 | % | | - | |
| | | | | | | | |
| Total assets, segment | | $ | 13,673,580 | | $ | - | |
| | | TOTAL | |
| | | December 31, 2007 | | December 31, 2006 | |
| Total revenue from external customers | | $ | 26,384,608 | | $ | 15,932,075 | |
| Other sales | | | - | | | 2,937 | |
| Cost of sales | | | (13,421,697 | ) | | (9,103,515 | ) |
| Gross profit | | | 12,962,911 | | | 6,831,497 | |
| Gross margin | | | 49 | % | | 43 | % |
| Research and development | | | (553,962 | ) | | (30,821 | ) |
| Selling expense | | | (1,581,655 | ) | | (599,571 | ) |
| General and administrative expense | | | (2,856,670 | ) | | (1,091,302 | ) |
| Segment contribution | | $ | 7,970,624 | | $ | 5,109,803 | |
| Contribution margin | | | 30 | % | | 32 | % |
| | | | | | | | |
| Total assets, segment | | $ | 62,557,407 | | $ | 25,105,941 | |
The results of the total consolidated net profit before income taxes for the reporting periods are as follows:
| | | December 31, 2007 | | December 31, 2006 | |
| Total segment contribution | | $ | 7,970,624 | | $ | 5,109,803 | |
| Unallocated amounts: | | | | | | | |
| Government subsidies | | | 2,276,574 | | | - | |
| Loss on disposal of assets | | | (30,027 | ) | | (249,381 | ) |
| Other corporate expense | | | (14,378,619 | ) | | (1,752,303 | ) |
| Total income / (loss) before minority interest and income taxes | | $ | (4,161,448 | ) | $ | 3,108,119 | |
The other corporate expenses for the year ended December 31, 2007 and 2006 composed of the following events:
| | | 2007 | | 2006 | |
| Consulting and professional fees (Note 19) | | $ | 8,353,615 | | $ | 326,403 | |
| Penalty to investor | | | 1,022,275 | | | - | |
| Wages and salaries | | | 347,949 | | | 37,500 | |
| Audit and accounting | | | 334,106 | | | 509,839 | |
| Amortization of debt issue cost | | | 201,255 | | | - | |
| Consulting fee | | | 157,619 | | | 191,248 | |
| Investor relation, transfer agent and filing fees | | | 109,540 | | | - | |
| Director renumeration | | | 94,247 | | | - | |
| Brokerage fees | | | 50,161 | | | - | |
| Travel and transportation | | | 51,071 | | | - | |
| Office expense | | | 53,594 | | | - | |
| Foreign exchange | | | (7,914 | ) | | 108,811 | |
| Interest expense | | | 3,090,952 | | | (5,490 | ) |
| Miscellaneous | | | 520,149 | | | 583,992 | |
| Total | | $ | 14,378,619 | | $ | 1,752,303 | |
For the details of information of this particular, it should be read in conjunction with the management discussion and analysis section.
The following table shows the reconciliation between the segments assets and the total assets for the years ended December 31, 2007 and 2006:
| | | December 31, 2007 | | December 31, 2006 | |
| Total assets, segment | | $ | 62,557,407 | | $ | 25,105,941 | |
| | | | | | | | |
| Total assets of corporate: | | | | | | | |
| Cash and cash equivalent | | | 80,291 | | | 914,261 | |
| Bank indebtedness | | | 874,490 | | | - | |
| Prepaid expesnes and deposit | | | 851 | | | - | |
| Refundable purchase price paid | | | 1,200,000 | | | 5,367,801 | |
| Due from related parties | | | 876,589 | | | 665,824 | |
| Debit issue costs: | | | | | | | |
| placement agent commission | | | 327,945 | | | - | |
| | | | | | | | |
| Total assets | | $ | 65,917,572 | | $ | 32,053,827 | |
The following table shows how the minority interest for year ended December 31, 2007 and 2006 was derived:
| | | Year Ended December 31, 2007 | |
| | | Benda | | Jiangling | | Yidu | | Beijing | | | | | |
| | | Ebei | | Benda | | Benda | | Shusai | | SiBiono | | Total | |
Segment profit | | $ | 5,959,990 | | | (222,560 | ) | | (345,019 | ) | | (118,956 | ) | | 2,697,169 | | $ | 7,970,624 | |
| Loss on disposal of assets | | | - | | | (24,852 | ) | | (5,175 | ) | | - | | | - | | | (30,027 | ) |
| Interest income/ (expenses) | | | (88,077 | ) | | 2,547 | | | (23,748 | ) | | (23 | ) | | (189,423 | ) | | (298,724 | ) |
| Other income / (expenses) | | | (18,449 | ) | | (12,273 | ) | | 3,555 | | | (1,251 | ) | | (5,127 | ) | | (33,545 | ) |
| Prior year adjustment | | | - | | | - | | | - | | | - | | | 89,509 | | | 89,509 | |
| Government subsidies | | | - | | | - | | | - | | | - | | | 2,276,574 | | | 2,276,574 | |
| Income taxes | | | (965,183 | ) | | - | | | - | | | - | | | - | | | (965,183 | ) |
Income before minority interest | | $ | 4,888,281 | | | (257,138 | ) | | (370,387 | ) | | (120,230 | ) | | 4,868,702 | | $ | 9,009,228 | |
| | | | | | | | | | | | | | | | | | | | |
| MI percentage | | | 5.00 | % | | 9.75 | % | | 9.75 | % | | 28.75 | % | | 42.88 | % | | | |
| MI interest | | $ | 244,414 | | | (25,071 | ) | | (36,113 | ) | | (34,566 | ) | | 2,087,529 | | $ | 2,236,194 | |
| | | Year Ended December 31, 2006 | |
| | | Benda | | Jiangling | | Yidu | | Beijing | | | | | |
| | | Ebei | | Benda | | Benda | | Shusai | | SiBiono | | Total | |
Segment profit | | $ | 2,976,038 | | | (169,979 | ) | | 2,343,081 | | | (39,337 | ) | | - | | $ | 5,109,803 | |
| Loss on disposal of assets | | | - | | | (249,381 | ) | | - | | | - | | | - | | | (249,381 | ) |
| Interest income/ (expenses) | | | (87,706 | ) | | (78 | ) | | (26,527 | ) | | 10 | | | - | | | (114,301 | ) |
| Other income / (expenses) | | | (3,671 | ) | | 369 | | | 1,695 | | | 793 | | | - | | | (814 | ) |
| Prior year adjustment | | | - | | | - | | | - | | | - | | | - | | | - | |
| Government subsidies | | | - | | | - | | | - | | | - | | | - | | | - | |
| Income taxes | | | - | | | - | | | - | | | - | | | - | | | - | |
Income before minority interest | | $ | 2,884,661 | | | (419,069 | ) | | 2,318,249 | | | (38,534 | ) | | - | | $ | 4,745,307 | |
| | | | | | | | | | | | | | | | | | | | |
| MI percentage | | | 5.00 | % | | 9.75 | % | | 9.75 | % | | 28.75 | % | | | | | | |
| MI interest | | $ | 144,233 | | | (40,859 | ) | | 226,029 | | | (11,079 | ) | | - | | $ | 322,590 | |
Further, set forth below is a breakdown of the various product segments:
Manufacturer | | Product | | Type | | Function |
| Benda Ebei | | Jixuening injection vial | | Generic | | Haemostatic (stops bleeding) |
| | | | | | | |
| Benda Ebei | | Xujing injection vial | | Generic | | Haemostatic |
| | | | | | | |
| Benda Ebei | | Nokeqing injection vial | | Generic | | Used to treat hepatitis |
| | | | | | | |
| Benda Ebei | | Yidingshu injection vial | | Generic | | Vitamin to treat lack of Riboflavin |
| | | | | | | |
| Benda Ebei | | Shusai-A injection vial | | Generic | | Anti-inflammatory analgesic |
| | | | | | | |
| Benda Ebei | | Suzheng-B injection vial | | Generic | | Vitamin; complementary medicine used to treat hepatitis |
| | | | | | | |
| Benda Ebei | | Ribavirin injection vial | | Generic | | Anti-virus, to treat acute upper respiratory tract infection |
| | | | | | | |
| Benda Ebei | | Gentamycin Sulfate Injection vial | | Generic | | Broad spectrum antibiotic |
| | | | | | | |
| Benda Ebei | | Vitamin B6 injection vial | | Generic | | Vitamin; complementary medicine used to treat hepatitis |
| | | | | | | |
| Benda Ebei | | Inosine injection vial | | Generic | | Nutrition, complementary medicine used to treat hepatitis |
| | | | | | | |
| Benda Ebei | | Vitamin C injection vial | | Generic | | To treat deficiency of vitamin C |
| | | | | | | |
| Jiangling Benda | | Ribavirin API (1) | | API | | Ribavirin drug manufacture. |
| | | | | | | |
| Jiangling Benda | | Asarin API (1) | | API | | Asarin manufacture to treat acute upper respiratory system infection |
| | | | | | | |
| Jiangling Benda | | Levofloxacin Mesylate API (1) | | API | | Broad spectrum antibiotic drug manufacture |
| | | | | | | |
| Yidu Benda | | Triazol carboxylic acid methyl ester (“TCA”) | | Bulk chemical | | Ribavirin manufacture, anti-virus |
| | | | | | | |
| Yidu Benda | | L-methionine | | Bulk chemical Nutrition | | An essential amino acid for humans |
| | | | | | | |
| Yidu Benda | | Tricabroxylic acid amide (“TAA”) | | Bulk chemical | | Ribavirin manufacture, anti-virus drug manufacture |
| | | | | | | |
| Yidu Benda | | 1,2,3,5-Tetraacetyl-ß-D-Ribose | | Bulk chemical | | Ribavirin manufacture, anti-virus drug manufacture |
| (c) | SiBiono patents - on January 29, 2007, SiBiono entrusted Grandall Legal Group Shenzhen Law Firm to issue a legal letter to Zhaohui Peng, one of the shareholders of Sibiono and the inventor of Gendicine, requesting him to transfer all the title of patents to SiBiono. |
On June 18, 1999, during the formation of SiBiono, Zhaohui Peng transferred the rights to the patent “A new method for manufacturing recombinant adenovirus” and related research results to SiBiono as a payment for the registered capital. In return, Zhaohui Peng was granted 32.03% of the common stock of SiBiono.
From 1999 to 2007, SiBiono successfully obtained various technology funds from various government technology agencies to support the further research and development activities of Gendicine. Due to this significant funding obtained by SiBiono, Sibiono developed five additional patents which are summarized as follows:
Item | | Patent name | | Countries / Date | | Application Number (1) | | Publication Number (2) | | Approved Patent Number (3) | | Name of Patent Inventor (6) | | Name of Applicant (6) | | Patent Assignees |
| 1 | | A new method for manufacturing recombinant adenovirus | | | | | | | | | | | | | | |
| | | A | | China | | 98123346.5 | | CN1228474A | | ZL98123346.5 | | Peng | | Peng | | SiBiono |
| | | | | Date | | 12/14/1998 | | 9/15/1999 | | 7/3/2002 | | | | | | |
| 2 | | A recombinant constructed by a virus vector and a | | | | | | | | | | | | | | |
| | | human tumor suppressor gene and its use | | | | | | | | | | | | | | |
| | | A | | China | | 02115228.4 | | CN1401778A | | ZL02115228.4 | | Peng / Zhang | | Peng / Zhang | | Peng / Zhang |
| | | | | Date | | 5/8/2002 | | 3/12/2003 | | 11/24/2004 | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | B | | PCT (4) | | PCT/CN/2004/000465 | | WO2004/078987A1 | | Not Approved | | Peng / Zhang | | Peng / Zhang | | N/A |
| | | | | Date | | 3/8/2004 | | 9/16/2004 | | N/A | | | | | | |
| 3 | | Recombinant gene medicine of adenovirus vector and | | | | | | | | | | | | | | |
| | | and gene p54 for treating proloferative diseases | | | | | | | | | | | | | | |
| | | A | | China | | 03125129.3 | | CN1471977A | | ZL03125129.3 | | Peng / Zhang | | Peng / Zhang | | Peng / Zhang |
| | | | | Date | | 5/10/2003 | | 2/4/2004 | | 7/25/2007 | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | B | | PCT (4) | | PCT/CN/2004/000458 | | WO2004/104204A1 | | Not Approved | | Peng / Zhang | | Peng / Zhang | | N/A |
| | | | | Date | | 5/9/2004 | | 12/2/2004 | | N/A | | | | | | |
| 4 | | The application of recombinant adenoviral p53 | | | | | | | | | | | | | | |
| | | as cancer vaccine (tentative title) | | | | | | | | | | | | | | |
| | | A | | China | | 200510002779.1 | | CN1679641A | | ZL200510002779.1 | | Peng / Zhang | | Peng / Zhang | | Peng / Zhang |
| | | | | Date | | 1/26/2005 | | 10/12/2005 | | 8/29/2007 | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | B | | PCT (4) | | PCT/CN/2005/000111 | | WO2006/079244A1 | | Not Approved | | Peng / Zhang | | Peng / Zhang | | N/A |
| | | | | Date | | 1/26/2005 | | 8/3/2006 | | N/A | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | C | | US (5) | | 11/075035 | | 2005/0281785A1 | | Not Approved | | Peng / Zhang | | Unidentified Yet | | N/A |
| | | | | Date | | 3/7/2005 | | 12/22/2005 | | N/A | | | | | | |
| 5 | | Human Embryonic Kidney (HEK) sub-clone cell line | | | | | | | | | | | | | | |
| | | A | | China | | 03126889.7 | | CN1513985A | | Not Approved | | Peng / Zhang | | Peng / Zhang | | N/A |
| | | | | Date | | 6/13/2003 | | 7/21/2004 | | N/A | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | B | | PCT (4) | | PCT/CN/2004/000457 | | WO2004/111239 | | Not Approved | | Peng / Zhang | | Peng / Zhang | | N/A |
| | | | | Date | | 5/9/2004 | | 12/23/2004 | | N/A | | | | | | |
| 6 | | The complex of polypeptide liposome and human VGEF | | | | | | | | | | | | | | |
| | | gene, and its use and human VGEF gene, and its use | | | | | | | | | | | | | | |
| | | A | | China | | 02134321.7 | | CN1389269A | | Not Approved | | Peng / Zhang / Zhu | | Peng / Zhang / Zhu | | N/A |
| | | | | Date | | 7/4/2002 | | 1/8/2003 | | N/A | | | | | | |
Note:
| | (1) | Application number is obtained when application is submitted; |
| | (2) | Publication number is obtained after the first phase examination; |
| | (3) | Approved patent number is obtained after the final examination; |
| | (4) | PCT is referred to an International Patent Organization in Paris; |
| | (5) | US is referred to the application is made in United States of America alone; |
| | (6) | Peng is referred to Zhaohui Peng; Zhang is referred to Xiaozhi Zhang; Zhu is referred to Jinya Zhu. |
As indicated in the above table, Item 1, the patent “A new method for manufacturing recombinant adenovirus” had been assigned to SiBiono; however, the other approved patents (item 2 through item 4) in PRC still have not been assigned to SiBiono. The Group believes that all the above mentioned patents should be rightfully transferred to SiBiono, a subsidiary of the Group. Accordingly, the above mentioned legal letter was issued on this ground.
The Group and Zhaohui Peng are currently in negotiations regarding the patent transfers. However, if Zhaozhui Peng refuses to transfer all the patents’s title to SiBiono, the Group will take a legal action against him.
| (d) | As mentioned in Note 12, following this arbitration decision, Benda Ebei has the obligation to pay the total acquisition cost payable of Rmb 12.48 million, plus the penalty and related legal and arbitration expenses, totaling approximately Rmb 12.80 million (or $1.75 million). Up to now, since both parties are still in the process of negotiation about the date of the payment, no actual equity transfer is made yet. |
| (e) | On January 21, 2008, Benda Ebei entered into a supplementary agreement with Shanghai Pudong Development Bank, to supplement the commercial bank note issuance agreement dated on August 14, 2007 (see Note 8). According to this supplementary agreement, the credit facility is further secured by the buildings, machinery and equipment of Jiangling Benda. As of December 31, 2007, the net book value of secured property and equipment was approximately Rmb67.33 million (or $9.2 million). |
2,106,561 SHARES COMMON STOCK
WARRANTS TO PURCHASE 15,774,375 SHARES OF COMMON STOCK
PROSPECTUS
__________, 2008
YOU SHOULD RELY ONLY ON THE INFORMATION CONTAINED IN THIS PROSPECTUS TO MAKE YOUR INVESTMENT DECISION. WE HAVE NOT AUTHORIZED ANYONE TO PROVIDE YOU WITH DIFFERENT INFORMATION. THIS PROSPECTUS MAY BE USED ONLY WHERE IT IS LEGAL TO SELL THESE SECURITIES. YOU SHOULD NOT ASSUME THAT THE INFORMATION IN THIS PROSPECTUS IS ACCURATE AS OF ANY DATE OTHER THAN THE DATE OF THIS PROSPECTUS.
PART II
ITEM 24. INDEMNIFICATION OF DIRECTORS AND OFFICERS.
Section of the Delaware Statutes provides for the indemnification of officers, directors, employees, and agents. A corporation shall have power to indemnify any person who was or is a party to any proceeding (other than an action by, or in the right of, the corporation), by reason of the fact that he or she is or was a director, officer, employee, or agent of the corporation or is or was serving at the request of the corporation as a director, officer, employee, or agent of another corporation, partnership, joint venture, trust, or other enterprise against liability incurred in connection with such proceeding, including any appeal thereof, if he or she acted in good faith and in a manner he or she reasonably believed to be in, or not opposed to, the best interests of the corporation and, with respect to any criminal action or proceeding, had no reasonable cause to believe his or her conduct was unlawful. The termination of any proceeding by judgment, order, settlement, or conviction or upon a plea of nolo contendere or its equivalent shall not, of itself, create a presumption that the person did not act in good faith and in a manner which he or she reasonably believed to be in, or not opposed to, the best interests of the corporation or, with respect to any criminal action or proceeding, had reasonable cause to believe that his or her conduct was unlawful.
We have agreed to indemnify each of our directors and certain officers against certain liabilities, including liabilities under the Securities Act of 1933.Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to our directors, officers and controlling persons pursuant to the provisions described above, or otherwise, we have been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act of1933 and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than our payment of expenses incurred or paid by our director, officer or controlling person in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, we will, unless in the opinion of our counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
ITEM 25. OTHER EXPENSES OF ISSUANCE AND DISTRIBUTION.
The following table sets forth an estimate of the costs and expenses payable by Applied Spectrum in connection with the offering described in this registration statement. All of the amounts shown are estimates except the Securities and Exchange Commission Registration Fee:
| Securities and Exchange Commission Registration Fee | | $ | 3,969 | |
| Printing Fees | | $ | 5,000 | |
| Accounting Fees and Expenses | | $ | 100,000 | |
| Legal Fees and Expenses | | $ | 100,000 | |
| Miscellaneous | | $ | 5,000 | |
| Total | | $ | 213,969 | |
ITEM 26. RECENT SALES OF UNREGISTERED SECURITIES.
On October 12, 2007, we issued 166,898 shares of our common stock pro rata to the investors in our November 2006 offering as a penalty payment for failing to meet the filing date of the registration statement. The above shares of common stock were issued under an exemption from registration under Section 4(2) of the Securities Act of 1933, as amended ("Securities Act"). As such, the above shares of common stock will be restricted shares, and the holder thereof may not sell, transfer or otherwise dispose of such shares without registration under the Securities Act or an exemption therefrom.
On October 4, 2007, we issued 236,813 shares of our common stock in connection with the exercise of warrants issued to our placement agent. The above shares of common stock were issued under an exemption from registration under Section 4(2) of the Securities Act of 1933, as amended ("Securities Act"). As such, the above shares of common stock will be restricted shares, and the holder thereof may not sell, transfer or otherwise dispose of such shares without registration under the Securities Act or an exemption therefrom.
On August 16, 2007, we issued 65,200 shares of our common stock in connection with the exercise of warrants issued to our placement agent. The above shares of common stock were issued under an exemption from registration under Section 4(2) of the Securities Act of 1933, as amended ("Securities Act"). As such, the above shares of common stock will be restricted shares, and the holder thereof may not sell, transfer or otherwise dispose of such shares without registration under the Securities Act or an exemption therefrom.
On July 31, 2007, we issued 546,994 shares of our common stock in connection with the exercise of warrants issued to our placement agent. The above shares of common stock were issued under an exemption from registration under Section 4(2) of the Securities Act of 1933, as amended ("Securities Act"). As such, the above shares of common stock will be restricted shares, and the holder thereof may not sell, transfer or otherwise dispose of such shares without registration under the Securities Act or an exemption therefrom.
On June 11, 2007, we issued 33,585 shares of our common stock to Yaojin Wang and 55,975 shares of our common stock to Huimin Zhang in exchange for 2.56% of the outstanding shares of Shenzhen SiBiono Gene Technology Co., Ltd. The above shares of common stock were issued under an exemption from registration under Section 4(2) of the Securities Act of 1933, as amended ("Securities Act"). As such, the above shares of common stock will be restricted shares, and the holder thereof may not sell, transfer or otherwise dispose of such shares without registration under the Securities Act or an exemption therefrom.
On April 5, 2007, we issued 2,100,000 shares of our common stock to Super Pioneer International Limited in exchange for 57.57% of the outstanding shares of Shenzhen SiBiono Gene Technology Co., Ltd. The above shares of common stock were issued under an exemption from registration under Section 4(2) of the Securities Act of 1933, as amended ("Securities Act"). As such, the above shares of common stock will be restricted shares, and the holder thereof may not sell, transfer or otherwise dispose of such shares without registration under the Securities Act or an exemption therefrom.
Pursuant to the Investment Agreement, on April 5, 2007, we issued to the Investors a total of 252 Units for $7,560,000 with each Unit consisting of (1) a convertible promissory note (the “Note”) in the principal amount of Thirty Thousand Dollars ($30,000) which shall be convertible into 54,087 shares of the Company's common stock, par value $0.001 per share (the "Common Stock"), and (ii) a warrant (a “Warrant”) to acquire 54,087 shares of Common Stock at an exercise price of $0.555 per share. Such securities were not registered under the Securities Act of 1933. The issuance of these shares was exempt from registration, in part pursuant to Regulation S and Regulation D under the Securities Act of 1933 and in part pursuant to Section 4(2) of the Securities Act of 1933. We made this determination based on the representations of Benda which included, in pertinent part, that such shareholders were either (a) "accredited investors" within the meaning of Rule 501 of Regulation D promulgated under the Securities Act, or (b) not a "U.S. person" as that term is defined in Rule 902(k) of Regulation S under the Act, and that such shareholders were acquiring our common stock, for investment purposes for their own respective accounts and not as nominees or agents, and not with a view to the resale or distribution thereof, and that the Ever Leader Shareholders understood that the shares of our common stock may not be sold or otherwise disposed of without registration under the Securities Act or an applicable exemption therefrom.
In February 2007, we issued 706,195 shares of our common stock to the various consultants who rendered services related to the reverse merger. The above shares of common stock were issued under an exemption from registration under Section 4(2) of the Securities Act of 1933, as amended ("Securities Act"). As such, the above shares of common stock will be restricted shares, and the holder thereof may not sell, transfer or otherwise dispose of such shares without registration under the Securities Act or an exemption therefrom.
Pursuant to the Exchange Agreement, on November 15, 2006, we issued 64,942,360 shares of our Common Stock to the Ever Leader shareholders in exchange for 100% of the outstanding shares of Ever Leader Holdings Limited. Such securities were not registered under the Securities Act of 1933. The issuance of these shares was exempt from registration, in part pursuant to Regulation S and Regulation D under the Securities Act of 1933 and in part pursuant to Section 4(2) of the Securities Act of 1933. We made this determination based on the representations of Benda which included, in pertinent part, that such shareholders were either (a) "accredited investors" within the meaning of Rule 501 of Regulation D promulgated under the Securities Act, or (b) not a "U.S. person" as that term is defined in Rule 902(k) of Regulation S under the Act, and that such shareholders were acquiring our common stock, for investment purposes for their own respective accounts and not as nominees or agents, and not with a view to the resale or distribution thereof, and that the Ever Leader Shareholders understood that the shares of our common stock may not be sold or otherwise disposed of without registration under the Securities Act or an applicable exemption therefrom.
Pursuant to the Financing, on November 15, 2006, we issued 480 Units to the Investors, with each Unit consisting of 54,087 shares of our Common Stock and a Warrant to purchase 54,087 shares of our Common Stock at an exercise price of $0.555 per share, in exchange for gross proceeds of $12,000,000 (less expenses including placement agent fees and non-accountable expenses) that the Company received pursuant to Subscription Agreements entered into with the Investors. Such securities were not registered under the Securities Act of 1933. The issuance of these securities was exempt from registration under Regulation D and Section 4(2) of the Securities Act. We made this determination based on the representations of Benda, which included, in pertinent part, that such shareholders were either (a) "accredited investors" within the meaning of Rule 501 of Regulation D promulgated under the Securities Act, or (b) not a "U.S. person" as that term is defined in Rule 902(k) of Regulation S under the Act, and that such shareholders were acquiring our common stock, for investment purposes for their own respective accounts and not as nominees or agents, and not with a view to the resale or distribution thereof, and that Benda understood that the shares of our common stock may not be sold or otherwise disposed of without registration under the Securities Act or an applicable exemption therefrom.
Pursuant to the Financing, on November 15, 2006, we issued to the Placement Agent five year warrants to purchase 2,597,401 shares of our Common Stock at an exercise price of $0.555 per share. Such securities were not registered under the Securities Act of 1933. The issuance of these securities was exempt from registration under Section 4(2) of the Securities Act. We made this determination based on the representations of the Placement Agent, which included, in pertinent part, that such Placement Agent was an "accredited investors" within the meaning of Rule 501 of Regulation D promulgated under the Securities Act and that the Placement Agent was acquiring our common stock for investment purposes for its own respective accounts and not as nominees or agents, and not with a view to the resale or distribution thereof, and that the Placement Agent understood that the shares of our common stock may not be sold or otherwise disposed of without registration under the Securities Act or an applicable exemption therefrom.
On November 15, 2006, the Company issued a total of 423,292 shares of our common stock to Anslow & Jaclin, LLP for legal services rendered. The above shares of common stock were issued under an exemption from registration under Section 4(2) of the Securities Act of 1933, as amended ("Securities Act"). As such, the above shares of common stock will be restricted shares, and the holder thereof may not sell, transfer or otherwise dispose of such shares without registration under the Securities Act or an exemption therefrom.
On March 10, 2006, the Company issued additional 700,000 shares of its common stock to KI Equity for a purchase price of $35,000, or $0.05 per share. The above shares of common stock were issued under an exemption from registration under Section 4(2) of the Securities Act of 1933, as amended ("Securities Act"). As such, the above shares of common stock will be restricted shares, and the holder thereof may not sell, transfer or otherwise dispose of such shares without registration under the Securities Act or an exemption therefrom. The Company has agreed to grant "piggyback" registration rights to the holders with respect to the above shares.
On January 4, 2006, the Company issued 1,500,000 shares of its common stock to KI Equity for a purchase price of $75,000, or $0.05 per share. The proceeds from the purchase price were intended as working capital to pay expenses to maintain the reporting status of the Company. Concurrently, the Company issued 100,000 shares of its common stock to Kevin R. Keating, the sole officer and director of the Company, for services rendered to the Company valued at $5,000, or $0.05 per share. The Company also issued 100,000 shares of its common stock to a consulting firm for financial consulting services rendered to the Company valued at $5,000, or $0.05 per share. The above shares of common stock were issued under an exemption from registration under Section 4(2) of the Securities Act of 1933, as amended ("Securities Act"). As such, the above shares of common stock will be restricted shares, and the holder thereof may not sell, transfer or otherwise dispose of such shares without registration under the Securities Act or an exemption therefrom. The Company has agreed to grant "piggyback" registration rights to the holders with respect to the above shares.
ITEM 27. EXHIBITS.
EXHIBIT INDEX
The following exhibits are incorporated by reference or included as part of this report:
Exhibit Number | | Description |
| 2.1 | | Articles of Merger between Applied Spectrum Technologies, Inc., a Minnesota corporation, and Applied Spectrum Technologies, Inc., a Delaware corporation, together with the Agreement and Plan of Merger (1) |
| | | |
| 2.2 | | Exchange Agreement by and among Applied Spectrum Technologies, Inc. (“Applied Spectrum”); KI Equity Partners, III, LLC (“KI Equity”); Ever Leader Holdings Limited (“Ever Leader”); and each of the equity owners of Ever Leader Shareholders, dated September 7, 2006 (2) |
| | | |
| 2.3 | | Voting Agreement by and among the Ever Leader Shareholders and KI Equity, dated November 15, 2006 (4) |
| | | |
| 2.4 | | Escrow Agreement by and among Applied Spectrum, Ever Leader, Keating Securities, LLC and Steele Street State Bank, the escrow agent, dated November 15, 2006 (4) |
| | | |
| 2.5 | | Make Good Agreement by and among Keating Securities, LLC, Applied Spectrum, Ever Leader, Mr. Yiqing Wan and Ms. Wei Xu, and Moveup Investments Limited, dated November 15, 2006 (4) |
| 2.6 | | Make Good Escrow Agreement by and among Keating Securities, LLC, Applied Spectrum, Ever Leader, Mr. Yiqing Wan and Ms. Wei Xu, and Moveup Investments Limited, dated November 15, 2006 (4) |
| | | |
| 3.1 | | Certificate of Incorporation of Applied Spectrum Technologies, Inc., a Delaware corporation. (1) |
| | | |
| 3.2 | | Bylaws of Applied Spectrum Technologies, Inc., a Delaware corporation. (1) |
| | | |
| 4.1 | | Lock-Up Agreement amongst Applied Spectrum, Keating Securities, LLC, Yiqing Wan, Wei Xu and Moveup Investments Limited (4) |
| | | |
| 4.2 | | Specimen Stock Certificate for Shares of Common Stock of the Company (3) |
| | | |
| 5.1 | | Opinion of Anslow & Jaclin, LLP |
| | | |
| 10.1 | | Placement Agent Agreement dated October 17, 2006 between Applied Spectrum and Keating Securities, LLC (4) |
| | | |
| 10.2 | | Securities Purchase Agreement by and among Applied Spectrum, Ever Leader and the Investor listed on the attached Schedule of Buyers (4) |
| | | |
| 10.3 | | Registration Rights Agreement (4) |
| | | |
| 10.4 | | Form of Common Stock Purchase Warrant (4) |
| | | |
| 10.5 | | Form of Placement Agent Stock Purchase Warrant (4) |
| | | |
| 10.6 | | Employment Agreement with Yiqing Wan (4) |
| | | |
| 10.7 | | Employment Agreement with Wei Xu (4) |
| | | |
| 10.8 | | Employment Agreement with Hui Long (4) |
| | | |
| 10.9 | | Employment Agreement with Daping Gu (4) |
| | | |
| 10.10 | | Employment Agreement with Ruilu Song (4) |
| | | |
| 10.11 | | Employment Agreement with Jingbo Wu (4) |
| | | |
| 10.12 | | Form Investment Agreement between the Company and Buyers (5) |
| | | |
| 10.13 | | Equity Transfer Agreement with Shenzhen Yuanzheng Investment Development Co., Ltd (5) |
| | | |
| 10.14 | | Financial Consultancy Agreement (5) |
| 10.15 | | Equity Transfer Agreement with Shenzhen Yuanxing Gene City Development Co., Ltd. (5) |
| | | |
| 10.16 | | Modification and Amendment Agreement dated April 5, 2007 (5) |
| | | |
| 10.17 | | Technical Consultancy Agreement with Huimin Zhang (6) |
| | | |
| 10.18 | | Equity Transfer Agreement with Huimin Zhang (6) |
| | | |
| 10.19 | | Technical Consultancy Agreement with Yaojin Wang (6) |
| | | |
| 10.20 | | Equity Transfer Agreement with Yaojin Wang (6) |
| | | |
| 10.21 | | Interview by Eric Yu for WallSt.net dated July 26, 2007 (7) |
| | | |
| 10.22 | | Rights Purchase Agreement with Dr. Yan Li (7) |
| | | |
| 10.23 | | The Science and Technology Cooperation Agreement between College of Chemistry and Life Science of China Three Gorges University and Yidu Benda (7) |
| | | |
| 23.1 | | Consent of Kempisty & Company Certified Public Accountants, P.C. |
| | | |
| 24.1 | | Consent of Counsel, as in Exhibit 5.1 |
| (1) | Incorporated by reference to the Company's Current Report on Form 8-K dated November 17, 2005 and filed on November 22, 2005 (SEC File No. 000-16397). |
| | |
| (2) | Incorporated by reference to the Company's Current Report on Form 8-K dated September 7, 2006 and filed on September 7, 2006 (SEC File No. 000-16397). |
| | |
| (3) | Incorporated by reference to the Company’s Registration Statement on Form SB-2 (SEC File No. 33-17959). |
| | |
| (4) | Incorporated by reference to the Company's Current Report on Form 8-K dated November 15, 2006 and filed on November 17, 2006 (SEC File No. 000-16397). |
| | |
| (5) | Incorporated by reference to the Company’s Current Report on Form 8-K dated April 5, 2007 and filed on April 6, 2007 (SEC File No. 000-16397). |
| | |
| (6) | Incorporated by reference to the Company’s Current Report on Amendment No. 1 to Form 8-K dated April 5, 2007 and filed on June 15, 2007 (SEC File No. 000-16397). |
| | |
| (7) | Incorporated by reference to the Company’s Amendment No. 2 to Registration Statement on Form SB-2 (SEC File No. 33-17959). |
ITEM 28. UNDERTAKINGS.
| (a) | The undersigned registrant hereby undertakes: |
| | (1) | To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement: |
| | (i) | To include any prospectus required by Section 10(a)(3) of the Securities Act of 1933 (the "Securities Act"); |
| | | |
| | (ii) | To reflect in the prospectus any facts or events which, individually or together, represent a fundamental change in the information in this registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus file with the Securities and Exchange Commission ("SEC") pursuant to Rule 424(b), if in the aggregate, the changes in volume and price represent no more than a 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement; and |
| | (iii) | Include any additional or changed material information on the plan of distribution. |
| | (2) | For purposes of determining liability under the Securities Act, to treat each post-effective amendment as a new registration statement of the securities offered, and the offering of the securities at that time to be the initial bona fide offering. |
| | (3) | To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering. |
| | (4) | For determining liability of the undersigned small business issuer under the Securities Act to any purchaser in the initial distribution of the securities, the undersigned small business issuer undertakes that in a primary offering of securities of the undersigned small business issuer pursuant to this registration statement, regardless of the underwriting method used to sell the securities to purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned small business issuer will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser: |
| | (i) | any preliminary prospectus or prospectus of the undersigned small business issuer relating to the offering required to be filed pursuant to Rule 424; |
| | (ii) | any free writing prospectus relating to the offering prepared by or on behalf of the undersigned small business issuer or used or referred to by the undersigned small business issuer; |
| | | |
| | (iii) | the portion of any other free writing prospectus relating to the offering containing material information about the undersigned small business issuer or its securities provided by or on behalf of the undersigned small business issuer; and |
| | | |
| | (iv) | any other communication that is an offer in the offering made by the undersigned small business issuer to the purchaser. |
| | (5) | For determining any liability under the Securities Act, treat the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the small business issuer under Rule 424(b)(1), or (4) or 497(h) under the Securities Act as part of this registration statement as of the time the SEC declared it effective. |
| | (6) | For determining any liability under the Securities Act, treat each post-effective amendment that contains a form of prospectus as a new registration statement for the securities offered in the registration statement, and that offering of the securities at that time as the initial bona fide offering of those securities. |
| (b) | Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question of whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue. |
[The remainder of this page is left blank intentionally.]
SIGNATURES
In accordance with the requirements of the Securities Act of 1933, as amended, the registrant certifies that it has reasonable grounds to believe that it meets all of the requirements for filing on Form S-1 to be signed on its behalf by the undersigned, in the Hubei Province, China S.A.R. on August 1, 2008.
| | BENDA PHARMACEUTICAL, INC. |
| | | |
| | By: | /s/ Yiqing Wan |
| Date: August 1, 2008 | | Yiqing Wan |
| | | Chief Executive Officer and President |
POWER OF ATTORNEY
Each person whose signature appears below constitutes and appoints Mr. Yiqing Wan as his true and lawful attorneys-in-fact and agents, each acting alone, with full power of substitution and resubstitution, for him and in his name, place and stead, in any and all capacities, to sign any or all amendments (including post-effective amendments) to the Registration Statement, and to sign any registration statement for the same offering covered by this Registration Statement that is to be effective upon filing pursuant to Rule 462(b) under the Securities Act of 1933, as amended, and all post-effective amendments thereto, and to file the same, with all exhibits thereto, and all documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, each acting alone, or his substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
PURSUANT TO THE REQUIREMENTS OF THE SECURITIES ACT OF 1933, THIS REGISTRATION STATEMENT HAS BEEN SIGNED BY THE FOLLOWING PERSONS IN THE CAPACITIES AND ON THE DATES INDICATED:
| Name | | Title | | Date |
| | | | | |
| /s/ Yiqing Wan | | Chief Executive Officer and | | August 1, 2008 |
| Yiqing Wan | | President / Director | | |
| | | | | |
| /s/ Dr. Q.Y. Ma | | Director | | August 1, 2008 |
| Dr. Q.Y. Ma | | | | |
| | | | | |
| /s/ Eric Yu | | Chief Financial Officer, Principal | | August 1, 2008 |
| Eric Yu | | Accounting Officer, and Director | | |
| | | | | |
| /s/ Jun Tang | | Director | | August 1, 2008 |
| June Tang | | | | |