UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
_______________
FORM 10-Q
_______________
| x | QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the quarterly period ended June 30, 2009
| o | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from ______to______.
BENDA PHARMACEUTICAL, INC.
(Exact name of registrant as specified in Charter
| Delaware | | 000-16397 | | 41-2185030 |
(State or other jurisdiction of incorporation or organization) | | (Commission File No.) | | (IRS Employee Identification No.) |
Taibei Mingju, 4th Floor,
6 Taibei Road, Wuhan, Hubei Province, 430015, PRC
(Address of Principal Executive Offices)
_______________
+86 (27) 85494916
(Issuer Telephone number)
_______________
(Former Name or Former Address if Changed Since Last Report)
Check whether the issuer (1) has filed all reports required to be filed by Section 13 or 15(d) of the Exchange Act during the preceding 12 months (or for such shorter period that the issuer was required to file such reports), and (2)has been subject to such filing requirements for the past 90 days. Yes x No ¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every
Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files).
Yes ¨ No ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer or a smaller reporting company filer. See definition of “accelerated filer” and “large accelerated filer” in Rule 12b-2 of the Exchange Act (Check one):
Large Accelerated Filer ¨ Accelerated Filer ¨ Non-Accelerated Filer ¨ Smaller Reporting Company x
Indicate by check mark whether the registrant is a shell company as defined in Rule 12b-2 of the Exchange Act.
Yes ¨ No x
State the number of shares outstanding of each of the issuer’s classes of common equity, as of August 19, 2009: 105,155,355 shares of common stock.
BENDA PHARMACEUTICAL, INC.
FORM 10-Q
June 30, 2009
INDEX
| PART I— FINANCIAL INFORMATION | |
| | | |
| Item 1. | Financial Statements | 3 |
| Item 2. | Management’s Discussion and Analysis of Financial Condition | 4 |
| Item 3. | Quantitative and Qualitative Disclosures About Market Risk | 9 |
| Item 4T. | Control and Procedures | 9 |
| | | |
| PART II— OTHER INFORMATION | |
| | | |
| Item 1 | Legal Proceedings | 10 |
| Item 2. | Unregistered Sales of Equity Securities and Use of Proceeds | 12 |
| Item 3. | Defaults Upon Senior Securities | 13 |
| Item 4. | Submission of Matters to a Vote of Security Holders | 13 |
| Item 5. | Other Information | 13 |
| Item 6. | Exhibits and Reports on Form 8-K | 13 |
| | | |
| SIGNATURE | 14 |
Item 1. Financial Information
BENDA PHARMACEUTICAL, INC.
(an exploration stage company)
FINANCIAL STATEMENTS
AS OF JUNE 30, 2009
CONTENTS
| F1 | CONSOLIDATED BALANCE SHEETS AS OF JUNE 30, 2009 (UNAUDITED) AND AS OF DECEMEBR 31, 2008 (AUDITED). |
| | |
| F2 | CONSOLIDATED STATEMENTS OF OPERATIONS FOR THE SIX MONTHS ENDED JUNE 30, 2009 AND 2008 (UNAUDITED). |
| | |
| F5 | CONSOLIDATED STATEMENTS OF CHANGES IN EQUITY FOR THE SIX MONTHS ENDED JUNE 30, 2009 (UNAUDITED). |
| | |
| F3 | CONSOLIDATED STATEMENTS OF CASH FLOWS FOR THE SIX MONTHS ENDED JUNE 30, 2009 AND 2008 (UNAUDITED). |
| | |
| F4 | CONSOLIDATED STATEMENTS OF COMPREHENSIVE INCOME FOR THE SIX MONTHS ENDED JUNE 30, 2009 AND 2008 (UNAUDITED). |
| | |
| F6 - F37 | NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (UNAUDITED). |
Benda Pharmaceutical, Inc.
Consolidated Balance Sheets
(Amounts expressed in U.S. Dollars)
| | | June 30 | | | December 31 | |
| | | 2009 | | | 2008 | |
| | | (Unaudited) | | | | |
| Assets | | | | | | |
| Current Assets | | | | | | |
| Cash and cash equivalents | | $ | 1,453,145 | | | $ | 569,019 | |
| Trade receivables, net (Note 5) | | | 8,026,051 | | | | 9,336,915 | |
| Other receivables (Note 5) | | | 506,683 | | | | 504,349 | |
| Short-term loan receivables | | | - | | | | 146,685 | |
| VAT Prepaid | | | 1,585,289 | | | | 771,549 | |
| Due from related parties (Note 18) | | | 16,426 | | | | 11,000 | |
| Inventories (Note 7) | | | 2,720,500 | | | | 2,344,562 | |
| Prepaid expenses and deposits (Note 5) | | | 1,371,362 | | | | 918,209 | |
| Total current assets | | | 15,679,456 | | | | 14,602,288 | |
| Due from related parties (Note 18) | | | 2,984,561 | | | | 2,961,744 | |
| Property and equipments, net (Note 8) | | | 28,495,340 | | | | 29,057,225 | |
| Intangible assets, net (Note 9) | | | 5,630,480 | | | | 6,003,102 | |
| Restricted cash (Note 11) | | | 5,732,390 | | | | 6,162,849 | |
| Refundable purchase price paid (Note 6) | | | 1,200,000 | | | | 1,200,000 | |
| Other assets (Note 12) | | | 1,828,636 | | | | 1,830,634 | |
| Debt issue costs (Note 24) | | | - | | | | 55,485 | |
| Total Assets | | $ | 61,550,863 | | | $ | 61,873,327 | |
| | | | | | | | | |
| Liabilities & Shareholders' Equity | | | | | | | | |
| Current Liabilities | | | | | | | | |
| Bank indebtedness (Note 11) | | $ | 1,192,385 | | | $ | 1,288,257 | |
| Bank loans payable (current portion) (Note 13) | | | 2,981,302 | | | | 2,984,560 | |
| Long term debt payable (current portion) (Note 14) | | | 2,231,771 | | | | 2,234,210 | |
| Accounts payable and accrued liabilities (Note 15) | | | 8,581,611 | | | | 7,228,400 | |
| Commercial notes payable (Note 11) | | | 8,847,692 | | | | 9,297,169 | |
| VAT Taxes payable | | | 701,443 | | | | 516,302 | |
| Acquisition cost payable (Note 10) | | | 1,424,934 | | | | 1,426,491 | |
| Long-term convertible promissory notes (current portion) (Note 24) | | | 7,260,000 | | | | 6,395,951 | |
| Wages payable | | | 1,095,315 | | | | 630,475 | |
| Due to related parties (Note 18) | | | 1,410,075 | | | | 1,457,803 | |
| Total current liabilities | | | 35,726,528 | | | | 33,459,618 | |
| Due to related parties (Note 18) | | | 974,199 | | | | 997,593 | |
| Total Liabilities | | | 36,700,727 | | | | 34,457,211 | |
| | | | | | | | | |
| Redeemable common stock, 2,049,560 shares at $3.6 per share (Note 19) | | | 7,376,366 | | | | 7,376,366 | |
| | | | | | | | | |
| Equity | | | | | | | | |
| Preferred stock, $0.001 par value; 5,000,000 shares authorized; | | | | | | | | |
| None issued and outstanding | | | - | | | | - | |
| Common stock, $0.001 par value; 150,000,000 shares authorized; 105,155,355 shares issued and outstanding as of 6/30/2009 and 12/31/2008 respectively; | | | 105,155 | | | | 105,155 | |
| Additional paid in capital (Note 25) | | | 22,108,427 | | | | 22,108,427 | |
| Retained earnings (unrestricted) | | | (18,363,816 | ) | | | (16,047,561 | ) |
| Statutory surplus reserve fund (Note 17) | | | 2,642,775 | | | | 2,642,775 | |
| Accumulative other comprehensive income | | | 6,300,396 | | | | 6,347,547 | |
| Shares issuable for acquisition and services | | | 503,860 | | | | 503,860 | |
| Total Benda Pharmaceutical, Inc. Shareholders' Equity | | | 13,296,797 | | | | 15,660,203 | |
| Noncontrolling interest | | | 4,176,973 | | | | 4,379,547 | |
| Total Equity | | | 17,473,770 | | | | 20,039,750 | |
| | | | | | | | | |
| Total Liabilities & Equity | | $ | 61,550,863 | | | $ | 61,873,327 | |
The accompanying notes are an integral part of these consolidated financial statements.
Benda Pharmaceutical, Inc.
Consolidated Statements of Operations
(Amounts expressed in U.S. Dollars)
| | | Six Months Ended June 30 | | | Three Months Ended June 30 | |
| | | 2009 | | | 2008 | | | 2009 | | | 2008 | |
| | | (Unaudited) | | | (Unaudited) | | | (Unaudited) | | | (Unaudited) | |
| Revenue | | $ | 11,440,412 | | | $ | 13,225,623 | | | | 5,924,609 | | | | 7,278,253 | |
| Cost of goods sold | | | (6,699,079 | ) | | | (8,396,854 | ) | | | (3,556,288 | ) | | | (4,579,608 | ) |
| Gross profit | | | 4,741,333 | | | | 4,828,769 | | | | 2,368,321 | | | | 2,698,645 | |
| | | | | | | | | | | | | | | | | |
| Selling expenses | | | (1,016,366 | ) | | | (1,431,212 | ) | | | (622,173 | ) | | | (994,841 | ) |
| | | | | | | | | | | | | | | | | |
| General and administrative expenses | | | | | | | | | | | | | | | | |
| Amortization of intangible assets | | | (79,400 | ) | | | (97,948 | ) | | | (39,718 | ) | | | (46,373 | ) |
| Amortization of debt issue costs (Note 24) | | | (55,485 | ) | | | (131,757 | ) | | | - | | | | (65,878 | ) |
| Depreciation | | | (379,295 | ) | | | (243,495 | ) | | | (189,661 | ) | | | (120,275 | ) |
| Bad debts | | | (2,808,862 | ) | | | (773,893 | ) | | | (2,552,524 | ) | | | (302,596 | ) |
| Write-down of inventory to net realizable value (recovery) | | | (124,239 | ) | | | - | | | | 123,240 | | | | - | |
| Director remuneration | | | (45,000 | ) | | | (140,468 | ) | | | (22,500 | ) | | | (29,595 | ) |
| Penalty to investors | | | - | | | | (1,113,405 | ) | | | - | | | | (391,200 | ) |
| Other general and administrative expenses (Note 21) | | | (987,932 | ) | | | (2,020,274 | ) | | | (448,928 | ) | | | (922,405 | ) |
| Total general and administrative expenses | | | (4,480,213 | ) | | | (4,521,240 | ) | | | (3,130,091 | ) | | | (1,878,322 | ) |
| Research and development expenses | | | (118,083 | ) | | | (169,781 | ) | | | 183,601 | | | | (147,717 | ) |
| Total operating expenses | | | (5,614,662 | ) | | | (6,122,233 | ) | | | (3,568,663 | ) | | | (3,020,880 | ) |
| Operating income / (loss) | | | (873,329 | ) | | | (1,293,464 | ) | | | (1,200,342 | ) | | | (322,235 | ) |
| | | | | | | | | | | | | | | | | |
| Interest income / (expenses) (Note 24) | | | (1,633,247 | ) | | | (2,340,580 | ) | | | (362,866 | ) | | | (1,128,298 | ) |
| Other income / (expenses) (Note 20) | | | 45,776 | | | | 248,058 | | | | 62,976 | | | | (53,635 | ) |
| Government subsidies / grants (Note 22) | | | 26,383 | | | | - | | | | - | | | | - | |
| | | | | | | | | | | | | | | | | |
| Loss before provision for income taxes and noncontrolling interest | | | (2,434,417 | ) | | | (3,385,986 | ) | | | (1,500,232 | ) | | | (1,504,168 | ) |
| Income taxes (Note 23) | | | (84,412 | ) | | | (524,808 | ) | | | 217,966 | | | | (391,611 | ) |
| | | | | | | | | | | | | | | | | |
| Net loss | | | (2,518,829 | ) | | | (3,910,794 | ) | | | (1,282,266 | ) | | | (1,895,779 | ) |
| | | | | | | | | | | | | | | | | |
| Net loss attributable to the noncontrolling interest | | | 202,574 | | | | 88,216 | | | | 6,568 | | | | 110,614 | |
| | | | | | | | | | | | | | | | | |
| Net loss attributable to Benda Pharmaceutical, Inc. | | $ | (2,316,255 | ) | | $ | (3,822,578 | ) | | | (1,275,698 | ) | | | (1,785,165 | ) |
| | | | | | | | | | | | | | | | | |
| Basic and fully diluted loss per share | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| Net loss attributable to Benda Pharmaceutical, Inc. common shareholders | | $ | (0.02 | ) | | $ | (0.04 | ) | | | (0.01 | ) | | | (0.02 | ) |
| | | | | | | | | | | | | | | | | |
| Weighted average shares outstanding (Note 26) | | | 105,155,355 | | | | 100,558,191 | | | | 105,155,355 | | | | 100,803,509 | |
The accompanying notes are an integral part of these consolidated financial statements.
Benda Pharmaceutical, Inc.
Consolidated Statements of Cash Flows
(Amounts expressed in U.S. Dollars)
| | | Six Months Ended June 30, | |
| | | 2009 | | | 2008 | |
| | | (Unaudited) | | | (Unaudited) | |
| Cash Flows From Operating Activities | | | | | | |
| Net loss | | $ | (2,518,829 | ) | | $ | (3,910,794 | ) |
| Adjustments to reconcile net loss to net cash provided by operating activities: | | | | | | | | |
| Bad Debt provision | | | 2,808,862 | | | | 773,893 | |
| Inventory written down to net realizable value (recovery) | | | 124,239 | | | | - | |
| Depreciation | | | 980,389 | | | | 902,672 | |
| Amortization of intangible assets | | | 365,225 | | | | 373,615 | |
| Amortization of debt issue costs (Note 24) | | | 55,485 | | | | 131,757 | |
| Interest expense (amortization of debt discount) (Note 24) | | | 864,049 | | | | 1,882,244 | |
| Penalty to investors settled by issuance of common stock | | | - | | | | 230,312 | |
| Directors remuneration settled by issuance of common stock | | | - | | | | 75,900 | |
| Changes in operating assets and liabilities: | | | | | | | | |
| Trade receivables | | | (1,494,650 | ) | | | (1,855,382 | ) |
| Other receivables | | | (2,335 | ) | | | 229,758 | |
| Prepaid expenses and deposits | | | (453,153 | ) | | | (406,623 | ) |
| Inventories | | | (500,177 | ) | | | (1,298,277 | ) |
| Accounts payable and accrued liabilities | | | 1,818,056 | | | | 1,226,126 | |
| Taxes payable | | | (628,603 | ) | | | (111,647 | ) |
| Net cash provided by operating activities | | | 1,418,558 | | | | (1,756,446 | ) |
| | | | | | | | | |
| Cash Flows From Investing Activities | | | | | | | | |
| Purchases of property and equipment and construction-in-progress | | | (456,065 | ) | | | (213,176 | ) |
| Net cash used in investing activities | | | (456,065 | ) | | | (213,176 | ) |
| | | | | | | | | |
| Cash Flows From Financing Actives | | | | | | | | |
| Proceeds and repayments of borrowings under short-term loan receivables | | | 146,685 | | | | (145,947 | ) |
| Proceeds and repayments of borrowings under related parties, net | | | (99,366 | ) | | | (734,205 | ) |
| Proceeds and repayments of borrowings under government debts payable, net | | | (94,450 | ) | | | 314,677 | |
| Proceeds and repayments of borrowings under commercial bank notes, net (Note 11) | | | (20,441 | ) | | | 1,846,510 | |
| Proceeds and repayments of borrowings under bank loans, net | | | - | | | | (80,217 | ) |
| Net cash provided by (used in) financing activities | | | (67,572 | ) | | | 1,200,818 | |
| Effect of exchange rate changes on cash | | | (10,795 | ) | | | 538,495 | |
| Net increase in cash and cash equivalents | | | 884,126 | | | | (230,309 | ) |
| | | | | | | | | |
| Cash and cash equivalents, beginning of period | | | 569,019 | | | | 1,266,240 | |
| | | | | | | | | |
| Cash and cash equivalents, end of period | | $ | 1,453,145 | | | $ | 1,035,931 | |
| | | | | | | | | |
| Supplemental Disclosure of Cash Flow Information | | | | | | | | |
| Cash paid for interest | | $ | 253,139 | | | $ | 346,682 | |
| Cash paid for income taxes | | $ | 788,511 | | | $ | 636,375 | |
The accompanying notes are an integral part of these consolidated financial statements.
Benda Pharmaceutical, Inc.
Statements of Consolidated Comprehensive Income
(Amounts expressed in U.S. Dollars)
| | | Six Months Ended June 30, | |
| | | 2009 | | | 2008 | |
| | | (Unaudited) | | | (Unaudited) | |
| | | | | | | |
| Net loss | | $ | (2,518,829 | ) | | $ | (3,910,794 | ) |
| | | | | | | | | |
| Other comprehensive income (loss) | | | | | | | | |
| Foreign currency translation adjustment | | | (47,151 | ) | | | 2,866,157 | |
| | | | | | | | | |
| Comprehensive loss | | | (2,565,980 | ) | | | (1,044,637 | ) |
| Comprehensive loss attributable to the noncontrolling interest | | | 202,574 | | | | 88,216 | |
| | | | | | | | | |
| Comprehensive loss attributable to Benda Pharmaceutical, Inc. | | $ | (2,363,406 | ) | | $ | (956,421 | ) |
The accompanying notes are an integral part of these consolidated financial statements.
Benda Pharmaceutical, Inc.
Consolidated Statement of Changes in Equity
(Amounts expressed in U.S. Dollars)
| | | Common | | | | | | | | | | | | | | | | | | Statutory | | | Accumulated | | | Shares Issuable | | | | |
| | | Shares | | | | | | | | | | | | Additional | | | Retained | | | Surplus | | | Other | | | for | | | Non- | |
| | | Issued and | | | | | | Comprehensive | | | Common | | | Paid-in | | | Earnings | | | Reserve | | | Comprehensive | | | Acquisition | | | Controlling | |
| | | Outstanding | | | Total | | | Income | | | Stock | | | Capital | | | (Unrestricted) | | | Fund | | | Income | | | and Services | | | Interest | |
| Balance at December 31, 2008 | | | 105,155,355 | | | $ | 20,039,750 | | | $ | - | | | $ | 105,155 | | | $ | 22,108,427 | | | $ | (16,047,561 | ) | | $ | 2,642,775 | | | $ | 6,347,547 | | | $ | 503,860 | | | $ | 4,379,547 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Comprehensive loss: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Net loss | | | | | | | (1,236,563 | ) | | | (1,236,563 | ) | | | - | | | | - | | | | (1,040,557 | ) | | | - | | | | - | | | | - | | | | (196,006 | ) |
| Other comprehensive income (loss) | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Foreign currency translation adjustment | | | | | | | (52,320 | ) | | | (52,320 | ) | | | - | | | | - | | | | - | | | | - | | | | (52,320 | ) | | | - | | | | - | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Comprehensive loss | | | | | | | (1,288,883 | ) | | $ | (1,288,883 | ) | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Dividend paid | | | | | | | - | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Balance at March 31, 2009 (unaudited) | | | 105,155,355 | | | $ | 18,750,868 | | | | | | | $ | 105,155 | | | $ | 22,108,427 | | | $ | (17,088,117 | ) | | $ | 2,642,775 | | | $ | 6,295,227 | | | $ | 503,860 | | | $ | 4,183,541 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Comprehensive loss: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Net loss | | | | | | | (1,282,266 | ) | | | (1,282,266 | ) | | | - | | | | - | | | | (1,275,698 | ) | | | - | | | | - | | | | - | | | | (6,568 | ) |
| Other comprehensive income (loss) | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Foreign currency translation adjustment | | | | | | | 5,169 | | | | 5,169 | | | | - | | | | - | | | | - | | | | - | | | | 5,169 | | | | - | | | | - | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Comprehensive loss | | | | | | | (1,277,098 | ) | | $ | (1,277,097 | ) | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Dividend paid | | | | | | | - | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Balance at June 30, 2009 (unaudited) | | | 105,155,355 | | | $ | 17,473,770 | | | | | | | $ | 105,155 | | | $ | 22,108,427 | | | $ | (18,363,816 | ) | | $ | 2,642,775 | | | $ | 6,300,396 | | | $ | 503,860 | | | $ | 4,176,973 | |
The accompanying notes are an integral part of these consolidated financial statements.
Benda Pharmaceutical, Inc.
Notes to Consolidated Financial Statements
(Amounts expressed in U.S. Dollars)
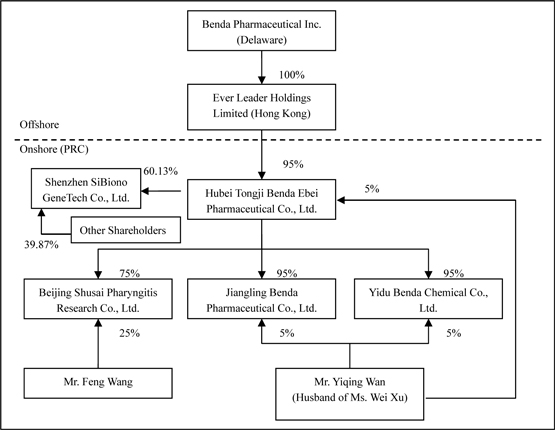
Benda Pharmaceutical, Inc. (“Benda”) is a corporation organized under the Florida Laws and headquartered in Hubei Province, the People’s Republic of China (“PRC”).
Ever Leader Holdings Limited (“Ever Leader”), a wholly owned subsidiary of Benda, is a company incorporated under the laws of Hong Kong SAR.
Ever Leader owns 95% of the issued and outstanding capital of Hubei Tongi Benda Ebei Pharmaceutical Co. Ltd. (“Benda Ebei”), a Sino-Foreign Equity Joint Venture company incorporated under the laws of PRC. Mr. Yiqing Wan owns 5% of the issued and outstanding capital stock of Benda Ebei. Benda Ebei owns: (i) 95% of the issued and outstanding capital stock of Jiangling Benda Pharmaceutical Co. Ltd., (“Jiangling Benda”) a company formed under the laws of PRC; (ii) 95% of the issued and outstanding capital stock of Yidu Benda Chemical Co. Ltd., (“Yidu Benda”) a company incorporated under the laws of PRC; and (iii) 75% of the issued and outstanding capital stock of Beijing Shusai Pharyngitis Research Co. Ltd., (“Beijing Shusai”) a company incorporated under the laws of PRC. Mr. Yiqing Wan owns: (i) 5% of the issued and outstanding capital stock of Jingling Benda; and (ii) 5% of the issued and outstanding capital stock of Yidu Benda. Mr. Feng Wang owns 25% of the issued and outstanding capital stock of Beijing Shusai.
On April 5, 2007, Benda Ebei entered into an Equity Transfer Agreements with Shenzhen Yuanzheng Investment Development Co., Ltd. and Shenzhen Yuanxing Gene City Development Co., Ltd., the then shareholders of Shenzhen SiBiono GeneTech Co., Ltd (“SiBiono”), to purchase 27.57% and 30% respectively of the shares of SiBiono’s common stock for total consideration of Rmb60 million (or $7.88 million) due and payable on or before April 30, 2007. On June 11, 2007, Benda Ebei entered into an Equity Transfer Agreements with Huimin Zhang and Yaojin Wang, the individual shareholders of SiBiono, and to purchase 1.6% and 0.96% respectively of the shares of SiBiono’s common stock for total consideration of Rmb2.56 million (or $0.34 million) due and payable on or before June 30, 2007. Altogether, the total consideration for 60.13% shares of SiBiono’s common stock was Rmb62.56 million or $9.18 million. As of June 30, 2009, an accumulated amount, approximately Rmb52.84 million or $7.76 million was paid and leaving a balance of Rmb 9.72 million or $1.42 million (please refer to Note 10 for the details).
Benda, Ever Leader, Benda Ebei, Jingling Benda, Yidu Benda, Beijing Shusai and SiBiono shall be referred to herein collectively as the “Group”. The Group is engaged principally in the business of identifying, discovering, developing, and manufacturing conventional medicines, active pharmaceuticals, bulk chemicals (or pharmaceutical immediates), and Traditional Chinese Medicines (“TCM”) for the treatment of some of the most widespread common ailments and diseases (e.g. common cold, diabetes, and cancer).
As of June 30, 2009, the organization and ownership structure of the Group is as follows:
| 2. | Basis of Preparation and Consolidation |
The unaudited consolidated financial statements of Benda and its subsidiaries have been prepared in accordance with U.S. generally accepted accounting principles for interim financial information and pursuant to the requirements for reporting on Form 10-Q. Accordingly, they do not include all the information and footnotes required by accounting principles generally accepted in the United States of America for annual financial statements. However, the information included in these interim financial statements reflects all adjustments (consisting solely of normal recurring adjustments) which are, in the opinion of management, necessary for the fair presentation of the consolidated financial position and the consolidated results of operations. Results shown for interim periods are not necessarily indicative of the results to be obtained for a full year. The consolidated balance sheet information as of December 31, 2008 was derived from the audited consolidated financial statements included in the Company's Annual Report on Form 10-K. These interim financial statements should be read in conjunction with that report.
The preparation of financial statements in conformity with US GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements, and the reported amounts of revenue and expenses during the reporting period. Actual results when ultimately realized could differ from those estimates.
| 4. | Significant Accounting Policies |
Cash and Cash Equivalents
Cash and cash equivalents include cash on hand, cash on deposit with various financial institutions, and all highly-liquid investments with original maturities of three months or less at the time of purchase.
Estimates Affecting Trade Receivables, Other Receivables, Prepaid and Deposits and Inventories
The preparation of our consolidated financial statements requires management to make estimates and assumptions that affect our reporting of assets and liabilities (and contingent assets and liabilities). However, it is explicated that the changes in estimation were not material in the preparation of our consolidation financial statements.
As of June 30, 2009 and December 31, 2008, the Group provided a $6,591,973 and $3,786,459, respectively for the allowance of doubtful accounts against trade receivables (please refer to Note 5 for details). Management's estimate of the appropriate allowance on those accounts receivable for the reporting periods was based on the aged nature of these accounts. In making its judgment, management assessed its customers' ability to continue to pay their outstanding invoices and the collectibility of those accounts on a timely basis, and whether their financial position might deteriorate significantly in the future, which would result in their inability to pay their debts to the Company.
Inventories, which are primarily comprised of raw materials, packaging materials, and finished goods, are stated at the lower of cost or net realizable value. Cost being determined on the basis of a moving average. The Group evaluates the need for reserves associated with obsolete, slow-moving and non-salable inventory by reviewing net realizable values on a periodic basis.
As of June 30, 2009 and December 31, 2008, the Group provided a reserve against its obsolete, slow-moving or non-salable inventory amounting to $4,687,738 and $4,941,372, respectively (please refer to Note 7 for details).
Property and Equipment
Property and equipment are recorded at cost and depreciated using the straight-line method, with an estimated 5% salvage value of original cost, over the estimated useful lives of the assets as follows:
| 20-30years |
| Machinery and equipment | 10-15 years |
| 5 years |
| Electronics and office equipment | 5 years |
Expenditures for repairs and maintenance, which do not improve or extend the expected useful lives of the assets, are expensed as incurred while major replacements and improvements are capitalized.
When property or equipment is retired or disposed of, the cost and accumulated depreciation are removed from the accounts, with any resulting gains or losses being included in net income or loss in the year of disposition.Impairment of Long-Lived Assets
The Group evaluates potential impairment of long-lived assets, in accordance with Statement of Financial Accounting Standards (“SFAS”) No. 144, Accounting for the Impairment or Disposal of Long-Lived Assets, which requires the Group to (a) recognize an impairment loss only if the carrying amount of a long-lived asset is not recoverable from its undiscounted cash flows and (b) measure an impairment loss as the difference between the carrying amount and fair value of the asset. As of June 30, 2009, the Group has provided a full reserve against its Goodwill in the amount of $7,904,613 (Please refer to Note 10 for details).
Intangible Assets
The Group’s intangible assets are stated at cost less accumulated amortization and are comprised of land-use rights, drug permits and licenses, patent and technology formulas know-how. Land-use rights are related to land the Group occupies in Hubei and Guangdong Province, PRC and are being amortized on a straight-line basis over a period of 40 years. Other intangible assets are being amortized on a straight-line basis over a period of 10 years.
Revenue Recognition
Among the most important accounting policies affecting the Group’s consolidated financial statements is its policy of recognizing revenue in accordance with the SEC's Staff Accounting Bulletin ("SAB") No. 104. Under this policy, all of the following criteria must be met in order for us to recognize revenue:
1. Persuasive evidence of an arrangement exists;
2. Delivery has occurred or services have been rendered;
3. The seller's price to the buyer is fixed or determinable; and
4. Collectibility is reasonably assured.
The majority of the Group's revenue results from sales contracts with distributors and revenue is recorded upon the shipment of goods. Management conducts credit background checks for new customers as a means to reduce the subjectivity of assuring collectibility. Based on these factors, the Group believes that it can apply the provisions of SAB 104 with minimal subjectivity. Sales are presented net of value added tax (VAT). No return allowance is made as products returns are insignificant based on historical experience.
Research and Development
Research and development (“R&D”) costs are expensed as incurred and consist primarily of salaries and related expenses of personnel engaged in research and development activities. The Group spent $118,083 and $169,781 on R&D efforts for the six months ended June 30, 2009 and 2008, respectively.
Income Taxes
The Group accounts for income taxes under the liability method in accordance with SFAS No. 109, Accounting for Income Taxes. Deferred tax assets and liabilities are recorded for the estimated future tax effects of temporary differences between the tax basis of assets and liabilities and amounts reported in the accompanying consolidated balance sheets. Deferred tax assets are reduced by a valuation allowance if current evidence indicates that it is considered more likely than not that these benefits will not be realized.
Comprehensive Income
The Group has adopted SFAS No. 130, Reporting Comprehensive Income, which establishes standards for reporting and displaying comprehensive income, its components, and accumulated balances in a full-set of general-purpose financial statements. Accumulated other comprehensive income represents the accumulated balance of foreign currency translation adjustments.
Going Concern
These consolidated financial statements have been prepared on a going concern basis in accordance with US GAAP. The going concern basis of presentation assumes that the Group will continue in operation for the foreseeable future and be able to realize its assets and discharge its liabilities and commitments in the normal course of business. The Group (i) has incurred consecutive losses for several years, (ii) has been in serious working capital deficit situation for an extended period of time, and (iii) has several significant loans or debts in default and without solid action plan by the Management, all that raise substantial doubt about its ability to continue as a going concern. These consolidated financial statements do not reflect adjustments that would be necessary if the going concern assumption was not appropriate. If the going concern basis was not appropriate for these consolidated financial statements, then adjustments would be necessary in the carrying value of assets and liabilities, the reported revenue and expenses and the balance sheet classifications used.
Concentration of Credit Risk
A significant portion of the Group's cash at June 30, 2009 and December 31, 2008 is maintained at various financial institutions in the PRC which do not provide insurance for amounts on deposit.
The Group has not experienced any losses in such accounts and believes it is not exposed to significant credit risk in this area.
The Group operates principally in the PRC and grants credit to its customers in this geographic region. Although the PRC is economically stable, it is always possible that unanticipated events in foreign countries could disrupt the Group’s operations.
The following table shows the individual customer’s revenue and account receivable balance which was higher than 5% of total revenue and total account receivables for the six months and three months ended June 30, 2009 and 2008:
| | | | SIX MONTHS ENDED JUNE 30, | | | THREE MONTH ENDED JUNE 30, | |
| | | | 2009 | | | 2008 | | | 2009 | | | 2008 | |
| | | | (Unaudited) | | | (Unaudited) | | | (Unaudited) | | | (Unaudited) | |
| | Revenue | | $ | 11,440,412 | | | | % | | $ | 13,225,623 | | | | % | | $ | 5,924,609 | | | | % | | $ | 7,278,253 | | | | % |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Individual customer's revenue | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| 1 | Zhuhai Gongbei Pharmaceutical Co, Ltd. | | | 2,960,739 | | | | 26 | % | | | 2,926,293 | | | | 22 | % | | | 1,176,598 | | | | 20 | % | | | 1,399,001 | | | | 19 | % |
| 2 | Shenyang Pharmaceutical Co. Ltd. | | | 1,572,583 | | | | 14 | % | | | 1,273,621 | | | | 10 | % | | | 785,787 | | | | 13 | % | | | 561,439 | | | | 8 | % |
| 3 | Shenzhen Huihua Pharmaceutical Co. Ltd. | | | 1,440,194 | | | | 13 | % | | | 1,390,396 | | | | 11 | % | | | 770,706 | | | | 13 | % | | | 515,221 | | | | 7 | % |
| 4 | Jiangxi Huiren Pharmaceutical Co. Ltd. | | | 885,238 | | | | 8 | % | | | 955,865 | | | | 7 | % | | | 406,083 | | | | 7 | % | | | 603,023 | | | | 8 | % |
| 5 | Hubei Hengchuan Health Products Co.,Ltd. | | | 701,376 | | | | 6 | % | | | 1,643,089 | | | | 12 | % | | | 420,665 | | | | 7 | % | | | 672,941 | | | | 9 | % |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Account receivable, gross | | $ | 14,618,024 | | | | % | | $ | 12,765,302 | | | | % | | | 14,618,024 | | | | % | | | 12,765,302 | | | | % |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Individual customer's account receivable gross balance | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| 1 | Zhuhai Gongbei Pharmaceutical Co, Ltd. | | | 2,785,597 | | | | 19 | % | | | 2,267,039 | | | | 18 | % | | | 2,785,597 | | | | 19 | % | | | 2,267,039 | | | | 18 | % |
| 2 | Jiangxi Huiren Pharmaceutical Co. Ltd. | | | 1,200,059 | | | | 8 | % | | | 844,413 | | | | 7 | % | | | 1,200,059 | | | | 8 | % | | | 844,413 | | | | 7 | % |
| 3 | Shenzhen Huihua Pharmaceutical Co. Ltd. | | | 1,456,728 | | | | 10 | % | | | 986,123 | | | | 8 | % | | | 1,456,728 | | | | 10 | % | | | 986,123 | | | | 8 | % |
| 4 | Shenyang Pharmaceutical Co. Ltd. | | | 1,459,949 | | | | 10 | % | | | 969,643 | | | | 8 | % | | | 1,459,949 | | | | 10 | % | | | 969,643 | | | | 8 | % |
| 5 | Hubei Hengchuan Health Products Co.,Ltd. | | | 911,834 | | | | 6 | % | | | 1,162,570 | | | | 9 | % | | | 911,834 | | | | 6 | % | | | 1,162,570 | | | | 9 | % |
Basic and Diluted Earnings Per Share
The Group adopted Statement of Financial Accounting Standards No. 128, “Earnings Per Share” (SFAS128). SFAS 128 requires the presentation of earnings per share (EPS) as Basic and Diluted EPS. Basic earnings per share are calculated by taking net income divided by the weighted average shares of common stock outstanding during the period. Diluted earnings per share is calculated by taking basic weighted average shares of common stock and increasing it for dilutive common stock equivalents such as warrants that are in the money.
Foreign Currency Translation
The functional currency of the Group is the Renminbi (“RMB”), the PRC’s currency. The Group maintains its financial statements using the functional currency. Monetary assets and liabilities denominated in currencies other than the functional currency are translated into the functional currency at rates of exchange prevailing at the balance sheet dates. Transactions denominated in currencies other than the functional currency are translated into the functional currency at the exchange rates prevailing at the dates of the transaction. Exchange gains or losses arising from foreign currency transactions are included in the determination of net income (loss) for the respective periods.
For financial reporting purposes, the financial statements of the Group, which are prepared using the RMB, are translated into the Group’s reporting currency, United States Dollars. Balance sheet accounts are translated using the closing exchange rate in effect at the balance sheet date and income and expense accounts are translated using the average exchange rate prevailing during the reporting period. Adjustments resulting from the translation, if any, are included in accumulated other comprehensive income (loss) in stockholder’s equity.
The exchange rates in effect at June 30, 2009 and 2008 were stated as follows: (for RMB 1.00):
| | | June 30, | | | June 30, | |
| | | 2009 | | | 2008 | |
| | | (Unaudited) | | | (Unaudited) | |
| Fixed rate | | $ | 0.1465 | | | $ | 0.1459 | |
| Average rate | | $ | 0.1466 | | | $ | 0.1418 | |
For the six months ended June 30, 2009 and 2008, the foreign exchange loss was $2,234 and $2,156 respectively; for the three months ended June 30, 2009 and 2008, the foreign exchange gain was $359 and $1,040, respectively.
Fair Value of Financial Instruments
Statement of Financial Accounting Standards (“SFAS”) No. 107, “Disclosures About Fair Value of Financial Instruments,” defines financial instruments and requires fair value disclosures of those financial instruments. On January 1, 2008, the Group adopted SFAS No. 157, “Fair Value Measurements,” which defines fair value, establishes a three-level valuation hierarchy for disclosures of fair value measurement and enhances disclosures requirements for fair value measures. Current assets and current liabilities qualified as financial instruments and management believes their carrying amounts are a reasonable estimate of fair value because of the short period of time between the origination of such instruments and their expected realization and if applicable, their current interest rate is equivalent to interest rates currently available. The three levels are defined as follow:
| | · | Level 1 — inputs to the valuation methodology are quoted prices (unadjusted) for identical assets or liabilities in active markets. |
| | · | Level 2 — inputs to the valuation methodology include quoted prices for similar assets and liabilities in active markets, and inputs that are observable for the assets or liability, either directly or indirectly, for substantially the full term of the financial instruments. |
| | · | Level 3 — inputs to the valuation methodology are unobservable and significant to the fair value. |
As of the balance sheet date, the estimated fair values of the financial instruments were not materially different from their carrying values as presented due to the short maturities of these instruments and that the interest rates on the borrowings approximate those that would have been available for loans of similar remaining maturity and risk profile at respective period-ends. Determining which category an asset or liability falls within the hierarchy requires significant judgment. The Group evaluates the hierarchy disclosures each quarter.
Recent Issued Accounting Pronouncements
In April 2009, the FASB issued FSP FAS 157-4, “Determining Fair Value When the Volume and Level of Activity for the Asset or Liability Have Significantly Decreased and Identifying Transactions That Are Not Orderly” (FSP FAS 157-4). FSP FAS 157-4 amends SFAS 157 and provides additional guidance for estimating fair value in accordance with SFAS 157 when the volume and level of activity for the asset or liability have significantly decreased and also includes guidance on identifying circumstances that indicate a transaction is not orderly for fair value measurements. This FSP shall be applied prospectively with retrospective application not permitted. This FSP shall be effective for interim and annual periods ending after June 15, 2009, with early adoption permitted for periods ending after March 15, 2009. An entity early adopting this FSP must also early adopt FSP FAS 115-2 and FAS 124-2, “Recognition and Presentation of Other-Than-Temporary Impairments” (FSP FAS 115-2 and FAS 124-2). Additionally, if an entity elects to early adopt either FSP FAS 107-1 and APB 28-1, “Interim Disclosures about Fair Value of Financial Instruments” (FSP FAS 107-1 and APB 28-1) or FSP FAS 115-2 and FAS 124-2, it must also elect to early adopt this FSP. The Group is currently evaluating this new FSP but do not believe that it will have a significant impact on the determination or reporting of the Group’s financial results.
In April 2009, the FASB issued FSP FAS 115-2 and FAS 124-2, “Recognition and Presentation of Other-Than-Temporary Impairment” (FSP 115-2/124-2). FSP 115-2/124-2 amends the requirements for the recognition and measurement of other-than-temporary impairment for debt securities by modifying the pre-existing “intent and ability” indicator. Under FSP 115-2/124-2, an other-than-temporary impairment is triggered when there is an intent to sell the security, it is more likely then not that the security will be required to be sold before recovery, or the security is not expected to recover the entire amortized cost basis of the security. Additionally, FSP 115-2/124-2 changes the presentation of an other-than-temporary impairment in the income statement for those impairments involving credit losses. The credit loss component will be recognized in earnings and the remainder of the impairment will be recorded in other comprehensive income. FSP 115-2/124-2 is effective for the Group beginning in the second quarter of fiscal year 2009. Upon implementation at the beginning of the second quarter of 2009, FSP 115-2/124-2 is not expected to have a significant impact on the Group’s consolidated financial statements.
In April 2009, the FASB issued FSP FAS 107-1 and APB 28-1, “Interim Disclosure about Fair Value of Financial Instruments” (“FSP 107-1/APB 28-1”). FSP 107-1/APB 28-1 requires interim disclosures regarding the fair values of financial instruments that are within the scope of FAS 107, “Disclosures about the Fair Value of Financial Instruments,” Additionally, FSP 107-1/APB 28-1 requires disclosures of the methods and significant assumptions used to estimate the fair value of financial instruments on an interim basis as well as changes in the methods and significant assumptions from prior periods. FSP 107-1/APB 28-1 does not change the accounting treatment for these financial instruments and is effective for the Group beginning in the second quarter of fiscal year 2009.
Recently Adopted Accounting Pronouncements
In the first quarter of 2009, the Group adopted Statement of Financial Accounting Standards (SFAS) No. 141 (revised 2007), “Business Combinations” (SFAS No. 141(R)) as mended by FASB staff position FSP 141(R)-1, “Accounting for Assets Acquired and Liabilities Assumed in a Business Combination.” SFAS No. 141(R) generally requires an entity to recognize the assets acquired, liabilities assumed, contingencies, and contingent consideration at their fair value on the acquisition date. In circumstances where the acquisition-date fair value for a contingency cannot be determined during the measurement period and it is concluded that it is probable that an asset or liability exists as of the acquisition date and the amount can be reasonably estimated, a contingency is recognized as of the acquisition date based on the estimated amount. It further requires that acquisition related costs be recognized separately from the acquisition and expensed as incurred, restructuring costs generally be expensed in periods subsequent to the acquisition date, and changes in accounting for deferred tax asset valuation allowances and acquired income tax uncertainties after the measurement period impact income tax expenses. In addition, acquired in-process research and development is capitalized as an intangibles asset and amortized over its estimated useful life. SFAS No. 141(R) is applicable to business combinations on a prospective basis beginning in the first quarter of 2009. The Group does not complete any business combination in the first and second quarter of 2009.
In June 2009, the FASB issued SFAS No. 167, "Amendments to FASB Interpretation No. 46(R)," which revised the consolidation guidance for variable-interest entities. The modifications include the elimination of the exemption for qualifying special purpose entities, a new approach for determining who should consolidate a variable-interest entity, and changes to when it is necessary to reassess who should consolidate a variable-interest entity. For the Company, this standard is effective January 1, 2010. The Company is currently evaluating the impact of this standard, but would not expect it to have a material impact on the Company's consolidated results of operations or financial condition.
In June 2009, the FASB issued SFAS No. 168, "The FASB Accounting Standards CodificationTM and the Hierarchy of Generally Accepted Accounting Principles a replacement of FASB Statement No. 162," and approved--the FASB Accounting Standards CodificationTM (Codification) as the single source of authoritative nongovernmental US GAAP. The Codification does not change current US GAAP, but is intended to simplify user access to all authoritative US GAAP by providing all the authoritative literature related to a particular topic in one place. All existing accounting standard documents will be superseded and all other accounting literature not included in the Codification will be considered non-authoritative. For the Company, the Codification is effective July 1, 2009 and will require future references to authoritative US GAAP to coincide with the appropriate section of the Codification. Accordingly, this standard will not have an impact on the Company's consolidated results of operations or financial condition.
In September 2006, the FASB issued SFAS No. 157, “Fair Value Measurements” (“SFAS 157”). SFAS 157 defines fair value, establishes a framework and gives guidance regarding the methods used for measuring fair value, and expands disclosures about fair value measurements. In February 2008, the FASB issued FASB Staff Position 157-1, “Application of FASB Statement No. 157 to FASB Statement No. 13 and Other Accounting Pronouncements That Address Fair Value Measurements for Purposes of Lease Classification or Measurement under Statement 13 (“FSP 157-1”) and FASB Staff Position 157-2, “Effective Date of FASB Statement No. 157” (“FSP 157-2”). FSP 157-1 amends SFAS 157 to remove certain leasing transactions from its scope. FSP 157-2 delays the effective date of SFAS 157 for all non-financial assets and non-financial liabilities, except for items that are recognized or disclosed at fair value in the financial statements on a recurring basis (at least annually), until fiscal years beginning after November 15, 2008. SFAS 157 is effective for financial statements issued for fiscal years beginning after November 15, 2007, and interim periods within those fiscal years. The Group adopted SFAS 157 effective January 1, 2008 for all financial assets and liabilities as required. The adoption of SFAS 157 was not material to the Group’s financial statements or results of operations.
Effective January 1, 2009, the Group adopted the provisions of EITF 07-5, "Determining Whether an Instrument (or Embedded Feature) is Indexed to an Entity’s Own Stock” (“EITF 07-5”). EITF 07-5 applies to any freestanding financial instruments or embedded features that have the characteristics of a derivative, as defined by SFAS No. 133, “Accounting for Derivative Instruments and Hedging Activities,” and to any freestanding financial instruments that are potentially settled in an entity’s own common stock. The adoption of EITF 07-5 was not material to the Group's financial statements or results of operations.
In July 2006, the FASB issued FASB Interpretation (“FIN”) No. 48, “Accounting for Uncertainty in Income Taxes,” which prescribes a comprehensive model for how a company should recognize, measure, present and disclose in its financial statements uncertain tax positions that the company has taken or expects to take on a tax return (including a decision whether to file or not to file a return in a particular jurisdiction). The accounting provisions of FIN No. 48 are effective for fiscal years beginning after December 15, 2006. The adoption of this Interpretation had no impact on the Group’s financial position or results of operations.
In September 2006, the FASB issued SFAS No. 157, "Fair Value Measurements" ("SFAS 157"). SFAS 157 defines fair value, establishes a framework and gives guidance regarding the methods used for measuring fair value, and expands disclosures about fair value measurements. In February 2008, the FASB issued FASB Staff Position 157-1, "Application of FASB Statement No. 157 to FASB Statement No. 13 and Other Accounting Pronouncements That Address Fair Value Measurements for Purposes of Lease Classification or Measurement under Statement 13" ("FSP 157-1") and FASB Staff Position 157-2, "Effective Date of FASB Statement No. 157" ("FSP 157-2"). FSP 157-1 amends SFAS 157 to remove certain leasing transactions from its scope. FSP 157-2 delays the effective date of SFAS 157 for all non-financial assets and non-financial liabilities, except for items that are recognized or disclosed at fair value in the financial statements on a recurring basis (at least annually), until fiscal years beginning after November 15, 2008. SFAS 157 is effective for financial statements issued for fiscal years beginning after November 15, 2007, and interim periods within those fiscal years. The Group adopted SFAS 157 effective January 1, 2008 for all financial assets and liabilities as required. The adoption of SFAS 157 was not material to the Group's financial statements or results of operations.
Reclassification
Certain amounts in the prior period financial statements have been reclassified to conform to the current period presentation. The reclassification had no effect on reported losses.
| 5. | Trade Receivables, Other Receivables, Prepaid expenses and Deposits |
As mentioned in Note 4 that the company estimates of the appropriate allowance on those accounts receivable for the reporting periods was based on the aged nature of these accounts. The table below shows the allowance for doubtful debts of the Group’s trade receivables, other receivables and prepaid expenses and deposits as of June 30, 2009 and December 31, 2008:
| | | June 30, | | | December 31, | |
| | | 2009 | | | 2008 | |
| | | (Unaudited) | | | | |
| Trade receivables, gross | | $ | 14,618,024 | | | $ | 13,123,374 | |
| Allowance for doubtful debts | | | (6,591,973 | ) | | | (3,786,459 | ) |
| Trade receivables, net | | $ | 8,026,051 | | | $ | 9,336,915 | |
| | | | | | | | | |
| | | June 30, | | | December 31, | |
| | | 2009 | | | 2008 | |
| Other receivables, gross | | $ | 506,683 | | | $ | 504,349 | |
| Allowance for doubtful debts | | | - | | | | - | |
| Other receivables, net | | $ | 506,683 | | | $ | 504,349 | |
| | | | | | | | | |
| | | June 30, | | | December 31, | |
| | | 2009 | | | 2008 | |
| Prepaid and deposits, gross | | $ | 1,371,362 | | | $ | 918,209 | |
| Allowance for doubtful debts | | | - | | | | - | |
| Prepaid and deposits, net | | $ | 1,371,362 | | | $ | 918,209 | |
The change of the allowance for doubtful debts between the reporting periods, as of June 30, 2009 and 2008, is displayed as follows:
| | | SIX MONTHS ENDED JUNE 30, | |
| | | 2009 | | | 2008 | |
| | | (Unaudited) | | | (Unaudited) | |
| Balance at beginning of period | | $ | (3,786,459 | ) | | $ | (943,647 | ) |
| Provision during the period | | | (2,808,862 | ) | | | (773,893 | ) |
| Foreign exchange difference | | | 3,348 | | | | (83,323 | ) |
| Balance at end of period | | $ | (6,591,973 | ) | | $ | (1,800,863 | ) |
The Group set full allowance for trade receivables aged over 120 days which is general credit term granted to the customers. After deducting those allowances from the gross trade receivable, based on the management’s past experience, there would be no collectability issue on the net amount.
| 6. | Refundable Purchase Price Paid |
On, December 7, 2006, Benda Ebei paid $1.2 million to SECO (Shenzhen) Biotech Co., Ltd. (“SECO”) pursuant to a purchase agreement signed between SECO and Benda Ebei on December 3, 2006 to acquire a technology know-how and drug specifications and technical parameters in producing a Gastropathy drug owned by SECO. According to the signed contract:
| · | The amount paid is refundable only if SECO fails to pass the examination conducted by Benda Ebei; |
| · | Altogether, there are three phases for the examination; however there is no specific time-table for the examination; |
| · | The contract is valid for 10 years upon the contract signed. |
As at June 30, 2009, the deal has not been closed as the product is still under the process of development. As the date when could SECO pass the examination conducted by Benda Ebei cannot be determined, thus the amount paid is reported as non-current assets as of June 30, 2009.
The Group’s inventories at June 30, 2009 and December 31, 2008 comprised as follows:
| | | June 30, | | | December 31, | |
| | | 2009 | | | 2008 | |
| | | (Unaudited) | | | | |
| Raw materials | | $ | 1,431,580 | | | $ | 874,180 | |
| Packing materials | | | 280,094 | | | | 481,724 | |
| Other materials / supplies | | | 83,562 | | | | 88,412 | |
| Finished goods | | | 373,920 | | | | 754,838 | |
| Work-in-process | | | 5,239,082 | | | | 5,086,780 | |
| Total inventories at cost | | | 7,408,238 | | | | 7,285,934 | |
| | | | | | | | | |
| Less: Reserves on inventories | | | (4,687,738 | ) | | | (4,941,372 | ) |
| | | | | | | | | |
| Total inventories, net | | $ | 2,720,500 | | | $ | 2,344,562 | |
A reserve for obsolete, slow-moving or non-salable inventory was made on raw materials, packing materials, other material and suppliers, finished goods and work-in-process at the amount of $11,078, $54,120, $57,651, $13,552 and $4,551,337 respectively as of June 30, 2009. As of December 31, 2008, there was a reserve made on raw materials, packing materials, finished goods and work-in-process at the amount of $122,433, $7,118, $269,749 and $4,542,072 respectively.
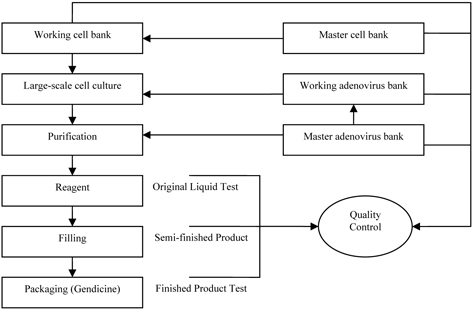
The provision of reserve on work-in-process was resulted from the manufacturing process of Gendicine, SiBiono’s sole product and SiBiono was acquired by the company in April 2007.
The following chart shows the manufacturing process of Gendicine (Ad-p53):
In the production process of finished goods, Gendicine, several working steps are needed: (i) large-scale culturing of adenovirus from master adenovirus bank; (ii) culturing of cell from master cell bank; (iii) purification. The whole process including step (i) to step (iii) takes approximately twenty-four days to make reagent (“original liquid”). This particular liquid can only be stored for approximately five years. It takes approximately another seven days for mixing and bottling original liquid to finished goods which is known as Gendicine.
Therefore, up to the stage of reagent, all the related production costs are treated as work-in-process. The major components of those production costs are: (i) direct labor; (ii) direct materials; (iii) power; (iv) supplies and other materials and (v) manufacturing overheads.
Before acquisition, as of March 31, 2007, the accumulated units of original liquid produced was 198,075 and which could be converted to approximately 226,736 vials of Gendicine. However, the accumulated vials of Gendicine sold throughout the years 2004 to three-months period ended March 31, 2007 were only approximately 18,424 vials. The accumulated production costs of $4,080,644 were remained as work-in-process as of three-month period ended March 31, 2007.
Furthermore, due to the special feature of the original liquid which can only be stored for five years, and most of the original liquid was produced in the year of 2004, and the provision of reserve on work in process was $3,696,083 as of three-month period ended March 31, 2007.
After the acquisition with the effective date April 1, 2007, the same accounting treatment was adopted for the treatment of the provision of reserve on work-in-process. As of June 30, 2009, the provision of reserve on work in process was $4,551,337.
| 8. | Property and Equipment, Net |
The Group’s property and equipment at June 30, 2009 and December 31, 2008 were comprised as fol lows:
| | | December 31, 2008 | | | Addition | | | Foreign Currency Translation Difference | | | June 30, 2009 | |
| | | | | | (Unaudited) | | | (Unaudited) | | | (Unaudited) | |
| Buildings | | $ | 9,692,441 | | | | - | | | | (9,521 | ) | | $ | 9,682,920 | |
| Machinery and equipment | | | 16,769,328 | | | | 9,835 | | | | (22,985 | ) | | | 16,756,179 | |
| Office equipment | | | 40,728 | | | | 1,099 | | | | (3,829 | ) | | | 37,998 | |
| Motor Vehicles | | | 252,163 | | | | - | | | | (338 | ) | | | 251,824 | |
| Cost | | | 26,754,660 | | | | 10,934 | | | | (36,673 | ) | | | 26,728,921 | |
| | | | | | | | | | | | | | | | | |
| Less: Accumulated Depreciation | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| Buildings | | $ | (1,559,511 | ) | | | (227,342 | ) | | | 2,585 | | | $ | (1,784,268 | ) |
| Machinery and equipment | | | (4,160,667 | ) | | | (722,043 | ) | | | 5,407 | | | | (4,877,303 | ) |
| Office equipment | | | (30,839 | ) | | | (12,128 | ) | | | 44 | | | | (42,923 | ) |
| Motor Vehicles | | | (89,863 | ) | | | (18,876 | ) | | | 102 | | | | (108,637 | ) |
| Accumulated Depreciation | | | (5,840,880 | ) | | | (980,389 | ) | | | 8,138 | | | | (6,813,131 | ) |
| | | | | | | | | | | | | | | | | |
| Construction in progress | | $ | 8,143,445 | | | | 445,131 | | | | (9,026 | ) | | $ | 8,579,550 | |
| | | | | | | | | | | | | | | | | |
| Total property and equipment, net | | $ | 29,057,225 | | | | (524,324 | ) | | | (37,561 | ) | | $ | 28,495,340 | |
As mentioned in Note 11, Benda Ebei entered into a commercial bank note issuance agreement with Shanghai Pudong Development Bank on August 14, 2007 and a supplementary agreement on January 21, 2008. Under the agreements the credit facility is secured by the buildings, machinery and equipment of Benda Ebei and Jiangling Benda. As of June 30, 2009, the net book value of pledged property and equipment was approximately Rmb126.40 million (or $18.52 million) in total.
The depreciation expense for the six months ended June 30, 2009 was calculated as follows:
| | | Original Cost | | | | | | | | | | | | | | | | |
| | | December 31, | | | June 30, | | | | | | | | | | | | Depreciation | | | Deprecation | | | | |
| | | 2008 | | | 2009 | | | Average | | | Salvage | | | Estimate | | | Calculated | | | Reported | | | Difference | |
| | | | | | (Unaduited) | | | (Unaduited) | | | Value | | | Useful Lives | | | (Unaduited) | | | (Unaduited) | | | (Unaduited) | |
| Building | | $ | 9,692,441 | | | | 9,682,920 | | | | 9,687,681 | | | | 5 | % | | | 20 | | | | 230,082 | | | | 227,342 | | | | 2,740 | |
| Property and equipment | | | 16,769,328 | | | | 16,756,179 | | | | 16,762,754 | | | | 5 | % | | | 11 | | | | 723,846 | | | | 722,043 | | | | 1,803 | |
| Office equipment | | | 40,728 | | | | 37,998 | | | | 39,363 | | | | 5 | % | | | 5 | | | | 3,739 | | | | 12,128 | | | | (8,389 | ) |
| Motor vehicle | | | 252,163 | | | | 251,824 | | | | 251,994 | | | | 5 | % | | | 5 | | | | 23,939 | | | | 18,876 | | | | 5,063 | |
| Total property and equipments | | $ | 26,754,660 | | | | 26,728,921 | | | | 26,741,791 | | | | | | | | | | | | 981,606 | | | | 980,389 | | | | 1,217 | |
The above table shows the calculation of depreciation expenses for the six months ended June 30, 2009. The difference between the depreciation calculated and depreciation reported was due to the changes of foreign exchange translation.
The estimated useful lives for buildings and property and equipments vary from 20 to 30 years and 10 to 15 years, respectively. For the six months ended June 30, 2009, the majority of the building subjected to depreciation had useful lives of 20 years and property and equipment had useful lives of 11 years.
The total depreciation expense was $980,389 and $490,324 for the six months and three months ended June 30, 2009, respectively, and is broken down as follows:
| | | SIX MONTHS ENDED JUNE 30, | | | THREE MONTHS ENDED JUNE 30, | |
| | | 2009 | | | 2008 | | | 2009 | | | 2008 | |
| | | (Unaudited) | | | (Unaudited) | | | (Unaudited) | | | (Unaudited) | |
| Cost of sales | | $ | 601,094 | | | $ | 659,177 | | | $ | 300,663 | | | $ | 329,162 | |
| Operating expenses | | | 379,295 | | | | 243,495 | | | | 189,661 | | | | 120,275 | |
| Balance at end of period | | $ | 980,389 | | | $ | 902,672 | | | $ | 490,324 | | | $ | 449,437 | |
The Group’s intangible assets at June 30, 2009 and December 31, 2008 were comprised as follows:
| | | December 31, 2008 | | | Addition | | | Foreign Currency Translation Difference | | | June 30, 2009 | |
| | | | | | (Unaudited) | | | (Unaudited) | | | (Unaudited) | |
| Land-use rights | | $ | 3,117,136 | | | | - | | | | (4,030 | ) | | $ | 3,113,106 | |
| Drugs permits and licenses | | | 2,780,254 | | | | - | | | | (3,595 | ) | | | 2,776,659 | |
| Technology formulas | | | 1,012,129 | | | | - | | | | (1,105 | ) | | | 1,011,024 | |
| Patent | | | 1,803,829 | | | | - | | | | (1,969 | ) | | | 1,801,860 | |
| Cost | | | 8,713,348 | | | | - | | | | (10,699 | ) | | | 8,702,649 | |
| | | | | | | | | | | | | | | | | |
| Land-use rights | | $ | (279,127 | ) | | | (31,032 | ) | | | 363 | | | $ | (309,796 | ) |
| Drugs permits and licenses | | | (1,664,540 | ) | | | (137,485 | ) | | | 2,043 | | | | (1,799,982 | ) |
| Technology formulas | | | (254,683 | ) | | | (50,567 | ) | | | 294 | | | | (304,956 | ) |
| Patent | | | (511,896 | ) | | | (146,141 | ) | | | 602 | | | | (657,435 | ) |
| Accumulated amortization | | | (2,710,246 | ) | | | (365,225 | ) | | | 3,302 | | | | (3,072,169 | ) |
| | | | | | | | | | | | | | | | | |
| Total intangible assets, net | | $ | 6,003,102 | | | | (365,225 | ) | | | (7,397 | ) | | $ | 5,630,480 | |
In May 2007, SiBiono enter into a co-operation agreement with DNAVEC, a Japanese gene therapy research institute. Under the terms of the agreement, DNAVEC will leverage SiBiono’s proven gene therapy manufacturing platform and will transfer the exclusive development and distribution rights of SeV-Gag in China to SiBiono. The total purchase cost was Rmb2 million or $ 0.28 million and recorded as intangible assets. In November 2008 this project was ceased and the whole cost was recoverable. Therefore the whole cost was removed from intangible assets and related amortization was reversed. As of June 30, 2009, the whole cost was received.
The amortization expense for the six months ended June 30, 2009 was calculated as follows:
| | | Original Cost | | | | | | | | | | | | | |
| | | December 31, | | | June 30, | | | | | | | | | Amortization | | | Amortization | | | | |
| | | 2008 | | | 2009 | | | Average | | | Estimated | | | Calculated | | | Reported | | | Difference | |
| | | | | | (Unaudited) | | | (Unaudited) | | | Useful lives | | | (Unaudited) | | | (Unaudited) | | | (Unaudited) | |
| Land-use rights | | $ | 3,117,136 | | | | 3,113,106 | | | | 3,115,121 | | | | 40 | | | $ | 38,939 | | | | 31,032 | | | | 7,907 | |
| Drugs permits and licenses | | | 2,780,254 | | | | 2,776,659 | | | | 2,778,457 | | | | 10 | | | | 138,923 | | | | 137,485 | | | | 1,438 | |
| Technology formulas | | | 1,012,129 | | | | 1,011,024 | | | | 1,011,577 | | | | 10 | | | | 50,579 | | | | 50,567 | | | | 12 | |
| Patent | | | 1,803,829 | | | | 1,801,860 | | | | 1,801,860 | | | | 6 | | | | 150,155 | | | | 146,141 | | | | 4,014 | |
| Total Intangible assets | | $ | 8,713,348 | | | | 8,702,649 | | | | 8,707,015 | | | | | | | $ | 378,596 | | | | 365,225 | | | | 13,371 | |
The above table shows the calculation of amortization expenses for the six months ended June 30, 2009. The difference between the amortization calculated and amortization reported was due to the changes of foreign exchange translation.
The total amortization expense for the six months and the three months ended June 30, 2009, and 2008 are broken down as follows:
| | | SIX MONTHS ENDED JUNE 30, | | | THREE MONTHS ENDED JUNE 30, | |
| | | 2009 | | | 2008 | | | 2009 | | | 2008 | |
| | | (Unaudited) | | | (Unaudited) | | | (Unaudited) | | | (Unaudited) | |
| Cost of sales | | $ | 285,825 | | | $ | 275,667 | | | $ | 142,976 | | | $ | 143,127 | |
| Operating expenses | | | 79,400 | | | | 97,948 | | | | 39,718 | | | | 46,373 | |
| Balance at end of period | | $ | 365,225 | | | $ | 373,615 | | | $ | 182,694 | | | $ | 189,500 | |
| 10. | Goodwill and Acquisition Cost Payable |
FASB 141 requires all acquisitions must be accounted for by allocating the acquisition consideration to the assets acquired based upon the fair market value of those assets. Consideration value that cannot be allocated to the acquired assets must be assigned to goodwill. In addition, it also requires eliminating the pooling treatment and eliminating the amortization of goodwill. FASB 142 requires that a company carrying goodwill on its books must revalue the assets acquired in a business combination. If there is an overall decline in the value of the acquired assets, then earlier booked goodwill is deemed “impaired” and must be written down. FASB 142 requires a two step impairment test. The fair value of a reporting unit is first compared to its carrying value, including goodwill. Then the implied fair value of the goodwill is compared to the carrying value of the goodwill. If the fair value is lower, it is considered to be impaired.
As of June 30, 2009, there was an amount approximately $7.9 million was recorded as goodwill. Meanwhile, the Group made an impairment charge at full amount on the goodwill due to the following facts (please read in conjunction with Note 13):
| a. | SiBiono has a bank loan matured and was in default, which has been sued and the court has issued a judgment on November 23, 2008 to SiBiono which required SiBiono to repay all loan balance plus penalty and interest approximately Rmb24 million immediately. As SiBiono has not paid anything yet, thus the land use right of SiBiono on its new production plant may be foreclosed for sales by the lender bank has been frozen unless the amount has been settled between the lender bank and SiBiono; |
| b. | SiBiono has serious working capital deficit situation and has reported accumulated loss of $3.6 million as of June 30, 2009; |
| c. | The management has not yet located or confirmed any feasible re-financing plan and / or equity placement as of the report date. The follow up action is still under the discussion with the lender bank. |
As of June 30, 2009, out of the total acquisition cost Rmb62.56 million (or $9.18 million), the following payments were made:
In the year of 2006, Benda, through its subsidiaries Everleader and Benda Ebei, paid Rmb19.52 million and Rmb13.03 million or totaling Rmb32.55 million to the selling shareholders of SiBiono and reported in the consolidated balance sheet and cash flow statement as “refundable purchase price paid”. It was recorded as refundable assets due to the fact that the deal was not concluded as of December 31, 2006. The acquisition was closed on April 5, 2007, and thus the total refundable amount of Rmb32.55 or $4.17 million was reclassified as investment cost.
In the year of 2007, an additional amount Rmb20.28 million was paid. The remaining balance was reported as “acquisition cost payable” on the balance sheet. As of June 30, 2009, the total amount paid was Rmb52.83 million (or $7.76 million) and the outstanding balance was Rmb9.72 million (or $1.42 million).
The Group has already obtained the oral consent from the selling shareholders of SiBiono that the remaining balance could be settled within the year of 2009.
| 11. | Restricted Cash, Bank Indebtedness and Commercial Notes Payable |
The Group’s restricted cash at June 30, 2009 and December 31, 2008 was comprised as follows:
| | | June 30, | | | December 31, | |
| | | 2009 | | | 2008 | |
| | | (Unaudited) | | | | |
| Deposits for issuance of commercial notes | | $ | 4,430,310 | | | $ | 4,859,346 | |
| Funds from government technolgy agencies | | | 1,302,080 | | | | 1,303,503 | |
| Total restricted cash | | $ | 5,732,390 | | | $ | 6,162,849 | |
| A) | On August 14, 2007, Benda Ebei entered into a commercial bank note issuable agreement with Shanghai Pudong Development Bank. Pursuant to this agreement, the following terms are included: |
| | a) | Duration of the agreement is three years; |
| | b) | It is non-interest bearing; |
| | c) | The repayment period of each commercial note payable is six months; |
| | d) | The total commercial note issuable limit is Rmb 60 million; however 50% of deposit should be made into the bank in order to secure the issuance of commercial bank note, thus the net available amount is Rmb 30 million; |
| | e) | If the net amount of each commercial bank note payable is not settled on the due date, the penalty will be the penalty rate of the PRC bank loan on daily and compound basis. |
| | f) | As mentioned in Note 8, this credit facility is guaranteed by SiBiono and secured by the buildings, machinery and equipment of Benda Ebei. On December 15, 2007, Benda Ebei received a consent letter from Pudong Bank that Pudong Bank agreed to cancel SiBiono’s guarantee toward this credit facility. |
On January 21, 2008, Benda Ebei entered into a supplementary agreement with Shanghai Pudong Development Bank, to supplement the commercial bank note issuance agreement dated on August 14, 2007. According to this supplementary agreement, the credit facility is further secured by the buildings, machinery and equipment of Jiangling Benda.
As of June 30, 2009, Benda Ebei and Jiangling Benda deposited an amount $4,430,310 in Shanghai Pudong Development Bank as deposit for the issuance of commercial bank notes. Such deposits will be released when the commercial bank notes are cleared. As of June 30, 2009, the balance of the commercial bank notes payable was $8,847,692. Thus the net commercial bank notes payable was $4,417,382 as of June 30, 2009.
| B) | The bank indebtedness was resulted from the acquisition of SiBiono with the effective date April 1, 2007. The reasons for causing bank indebtedness were stated as follows: |
| | a) | Among the cash and cash equivalents balances of SiBiono were composed of two parts; (i) unrestricted cash, which were generated from either operations, or loans from bank and financial institutions, or invested capital; (ii) restricted cash, which were obtained from the various government technology agencies as long term debt payable (see note 14 for the related details). |
| | b) | The cash obtained from the various government technology agencies as long term debt payable could only be dedicated to the related project’s research and development activities and purchase of fixed assets and construction in progress, therefore the cash balances for that part will be classified as restricted cash. |
| | c) | Due to the above reasons, SiBiono relocated the balances of restricted cash from the cash and cash equivalents balances for the reporting periods. |
| | d) | However, since the balance of the restricted cash was larger than the balance of cash and cash equivalents balances, thus bank indebtedness were resulted for the reporting periods. |
Due to the above reasons, SiBiono relocated the balances of restricted cash from the cash and cash equivalents balances with an amount $1,302,080 as of June 30, 2009. However, since the balances of the restricted cash were larger than the balance of cash and cash equivalents balances, bank indebtedness were resulted with an amount $1,192,385 as of June 30, 2009.
On November 23, 2006, Benda Ebei entered into an Equity Transfer Agreement with Xiaozhi Zhang (“Zhang”), to purchase approximately 6.24% of SiBiono’s common stock for a total consideration of Rmb12.48 million (Rmb6.24 million in cash and shares of our common stock equal to Rmb6.24 million) (or $1.71 million) which was due and payable on or before March 31, 2007.
Due to the fact that the signed agreement on November 23, 2006 was not practically executable according to the PRC regulations, Benda Ebei asked Zhang to terminate the signed agreement and sign a new agreement that was feasible under PRC regulations with essentially the same terms.
However, Zhang refused to sign the new agreement and applied to the Shenzhen Arbitration Commission (the “Commission”) in April 2007 for enforcement of the original agreement. Zhang requested the Commission to require Benda Ebei to pay for the total consideration, penalty for late payment and the related legal and arbitration expenses.
On November 27, 2007, Shenzhen Arbitration Commission determined that:
| 1. | Benda Ebei should pay for the consideration of Rmb6.24 million, equal to 50% of the total consideration set forth in the Equity Transfer Agreement. For the other 50% of the total consideration which was supposed to be settled in the form of issuing common stock, since Zhang did not make an arbitration request on how to execute the arrangement, the Arbitration Commission did not make an award on this particular part. |
| 2. | Benda Ebei should pay for the penalty of Rmb46,800; |
| 3. | Benda Eebi should pay for legal and arbitration expenses of Rmb268,971. |
Following this arbitration decision, Benda Ebei recognized the liability as total acquisition cost payable of Rmb12.48 million, plus the penalty and related legal and arbitration expenses, totaling approximately Rmb12.80 million or ($1.87 million) (Note 15). Accordingly Benda Eebi recognized the right to purchase the 6.24% equity shares in SiBiono and recorded as other assets at Rmb12.48 million or $1.83 million.
On May 22, 2008, Benda Ebei applied to Shenzhen People Court to terminate above mentioned arbitration. The termination is based on the ground that Xiaozhi Zhang does not own all 6.24% of SiBiono’s common stock. In fact, he only owns 3.28% of SiBiono’s stock. The application has been accepted by Shenzhen People Court and is waiting for its further investigation. Please also see the note descriptions in Note 29, Subsequent Events.
As of June 30, 2009 and December 31, 2008, SiBiono, had one outstanding bank loan with an amount of $2,981,302 and $2,984,560, respectively, which was used primarily to fund construction in progress projects and for general working capital purposes. This loan carries annual interest rate of 6.34% and matures on April 29, 2008. This loan is personal guaranteed by Zhaohui Peng, the former Chairman and a shareholder of SiBiono.
For the six months ended June 30, 2009, SiBiono has been sued for default on the bank loans and judgment has been made as follows:
| a. | On or before November 23, 2008 (10 days after the judgment date of November 13, 2008), SiBiono needs to repay the bank for the principal of Rmb20,346,672, accrued interest and penalty up to August 15, 2008 for Rmb1,506,994 and accrued interest from August 16, 2008 through repayment date. However, as SiBiono has not yet repaid anything yet, the accrued interest for the later period will be estimated at Rmb531,780, or total accrued interest as of June 30, 2009 was Rmb2,038,775; |
| b. | Should SiBiono did not make the full repayment; the lender bank can apply for selling the land use rights owned by SiBiono (as the collateral of the loan). Any balance after full repayment of the bank loan principal and accrued interest and penalty from the net proceeds will be returned to SiBiono; |
| c. | Zhaohui Peng should have liability to repay any insufficient amount should the net proceeds of selling the above land use rights are insufficient to repay the bank debts and then reclaimed from SiBiono; |
| d. | SiBiono and Zhaohui Peng have the obligation to repay the legal fees for this lawsuit of Rmb156,068 immediately. |
As of August 15, 2009, the Group is still under the negotiation with the bank on arranging the settlement of the bank loan. Furthermore, as of August 15, 2009, the bank has not taken any further actions to against SiBiono.
Total interest expense paid related to the Group’s outstanding bank loans was $94,476 and $92,698 for the six months ended June 30, 2009 and 2008, respectively.
| 14. | Long Term Debt Payable |
As of June 30, 2009, long term debt payable was raised due to the fact that various technology funds were obtained from various government technology agencies to support its gene therapy research and development activities during the past years and recorded as long term debt payable.
The obligations will be discharged once the examination by the various government technology agencies is conducted and most of the examination will be carried out and completed.
During the six months ended June 30, 2009, there was no long term debt payable discharged and recorded as government subsidies (see Note 22 for the related details).
| 15. | Accounts Payable and Accrued Liabilities |
The Group’s accounts payable and accrued liabilities as of June 30, 2009 and December 31, 2008 were comprised as follows:
| | | June 30, | | | December 31, | |
| | | 2009 | | | 2008 | |
| | | (Unaudited) | | | | |
| Trade payable | | $ | 1,292,367 | | | $ | 605,316 | |
| Deposits paid by customer | | | 43,852 | | | | 1,055 | |
| Acquisition cost payable following the arbitration (Note 12) | | | 1,874,904 | | | | 1,876,953 | |
| Accrued liabilities | | | 4,601,406 | | | | 3,999,155 | |
| Miscellaneous payables | | | 769,082 | | | | 745,921 | |
| Total account payables and accrued liabilities | | $ | 8,581,611 | | | $ | 7,228,400 | |
| 16. | Welfare and Employment Liabilities |
As stipulated by the relevant laws and regulations for enterprises operating in the PRC, the Group’s PRC entities are required to maintain a welfare plan for all of its employees who are residents of the PRC. Based on the wages payable and according to the labor law of the PRC, the Group accrued 14% on a monthly basis, for employees’ welfare, labor union fees, and education and training programs, respectively. As of June 30, 2009 and December 31, 2008, the Group accrued approximately $403,000 and $486,000 for the employees’ welfare respectively.
| 17. | Statutory Surplus Reserve Fund |
As stipulated by the relevant laws and regulations for enterprises operating in the PRC, the Group is required to make annual appropriations to a statutory surplus reserve fund for each of its PRC subsidiaries. Specifically, the Group is required to allocate 15% its profits after taxes at the fiscal year end, as determined in accordance with the PRC accounting standards applicable to the Group’s PRC subsidiaries, to a statutory surplus reserve until such reserve reaches 50% of the registered capital of the Group’s PRC subsidiaries. As of June 30, 2009 and December 31, 2008, the registered capital of the Group’s PRC subsidiaries was $21,719,896.
| 18. | Related Party Transactions |
Due from related parties at June 30, 2009 and December 31, 2008 were comprised as follows:
| | | | | June 30, | | | December 31, | |
| | | | | 2009 | | | 2008 | |
| | | Relationship | | (Unaudited) | | | | |
| Current | | | | | | | | |
| Qin Yu | | Vice president | | | | | | |
| Due to Shenzhen SiBiono Gene Tech Co. Ltd. | | | | $ | 10,890 | | | $ | 4,314 | |
| Xiaoji Zhang | | Minority shareholder | | | | | | | | |
| Due to Shenzhen SiBiono Gene Tech Co. Ltd. | | | | | 5,433 | | | | 5,439 | |
| Rong He | | Manager | | | | | | | | |
| Due to Shenzhen SiBiono Gene Tech Co. Ltd. | | | | | 103 | | | | 1,247 | |
| | | | | $ | 16,426 | | | $ | 11,000 | |
| | | | | | - | | | | - | |
| Non current | | | | | | | | | | |
| Yiqing Wan | | CEO & Director | | | | | | | | |
| Due to Ever Leader Holdings Co. Ltd. | | | | $ | 650,775 | | | $ | 650,791 | |
| Due to Hubei Tongji Benda Ebei Phamacetucial Co. Ltd. | | | | | 458,927 | | | | 439,719 | |
| Hubei Benda Science Development Co., Ltd. | | | | | | | | | | |
| Due to Ever Leader Holdings Co. Ltd. | | | | | - | | | | - | |
| Hubei Benda Science and Technology Co. Ltd | | Controlled by CEO | | | | | | | | |
| Due to Yidu Benda Chemicals Co. Ltd. | | | | | 1,605,419 | | | | 1,607,173 | |
| Due to Ever Leader Holdings Co. Ltd. | | | | | 231,707 | | | | 231,713 | |
| Feng Wang | | Minority shareholder | | | | | | | | |
| Due to Beijing Shusai Pharyngitis Research Co. Ltd. | | | | | 32,311 | | | | 32,348 | |
| Hua Xu | | | | | | | | | | |
| Due to Hubei Tongji Benda Ebei Phamacetucial Co. Ltd. | | | | | 5,422 | | | | - | |
| | | | | | | | | | | |
| Total due from related parties | | | | $ | 2,984,561 | | | $ | 2,961,744 | |
Due to related parties at June 30, 2009 and December 31, 2008 were comprised as follows:
| | | | | June 30, | | | December 31, | |
| | | | | 2009 | | | 2008 | |
| Current | | Relationship | | (Unaudited) | | | | |
| Wei Xu | | Vice President, CEO's Spouse & Director | | | | | | |
| Due from Shenzhen SiBiono Gene Tech Co. Ltd. | | | | $ | 195,061 | | | $ | 188,065 | |
| Due from Everleader Holding Ltd. | | | | | 967,975 | | | | 967,999 | |
| Hua Xu | | General Manager's Sister | | | | | | | | |
| Due from Shenzhen SiBiono Gene Tech Co. Ltd. | | | | | 21,196 | | | | 25,523 | |
| Yiqing, Wan | | CEO & Director | | | | | | | | |
| Due from Shenzhen SiBiono Gene Tech Co. Ltd. | | | | | 220,665 | | | | 237,781 | |
| Hua Shen | | Vice president | | | | | | | | |
| Due from Shenzhen SiBiono Gene Tech Co. Ltd. | | | | | - | | | | 33,253 | |
| Dongyi Tien | | Manager | | | | | | | | |
| Due from Shenzhen SiBiono Gene Tech Co. Ltd. | | | | | 364 | | | | 364 | |
| Pong Tsaiohuei | | Minority shareholder | | | | | | | | |
| Due from Shenzhen SiBiono Gene Tech Co. Ltd. | | | | | 4,814 | | | | 4,818 | |
| Total due to related parties | | | | $ | 1,410,075 | | | $ | 1,457,803 | |
| Non current | | | | | - | | | | | |
| Hubei Benda Science and Technology Co. Ltd | | Controlled by CEO | | | | | | | | |
| Due from Hubei Tongji Benda Ebei Phamacetucial Co. Ltd. | | | | $ | 28,572 | | | $ | 28,602 | |
| Due from Jiangliang Benda Pharamaceutical Co. Ltd. | | | | | 795,088 | | | | 811,073 | |
| Due from Beijing Shusai Pharyngitis Research Co. Ltd. | | | | | 14,133 | | | | 14,262 | |
| Wei Xu | | Vice President, CEO's Spouse & Director | | | | | | | | |
| Due from Hubei Tongji Benda Ebei Phamacetucial Co. Ltd. | | | | | 41,954 | | | | 48,922 | |
| Due from Beijing Shusai Pharyngitis Research Co. Ltd. | | | | | 65,439 | | | | 65,691 | |
| Yiqing, Wan | | CEO & Director | | | | | | | | |
| Due from Yidu Benda Chemicals Co. Ltd. | | | | | 560 | | | | 560 | |
| Hui Xu | | Manager | | | | | | | | |
| Due from Hubei Tongji Benda Ebei Phamacetucial Co. Ltd. | | | | | 28,453 | | | | 28,483 | |
| | | | | $ | 974,199 | | | $ | 997,593 | |
Except for the loans, $967,975, from the shareholder Wei Xu by Everleader which bears interest rate at 12% per annum, unsecure and matures within six months, the above advances bear no interest and the above loans due to related parties are unsecured, non-interest bearing and are not convertible into equity. Proceeds from the above loans were used primarily for general working capital purposes, among which the current portion does not have definitive terms and for those portions which are long-term debts in nature, due on December 31, 2012.
| 19. | Redeemable Common Stock |
On April 5, 2007, Benda Ebei entered into Equity Transfer Agreements with certain shareholders of SiBiono to purchase a total of approximately 57.57% of the shares of SiBiono’s common stock for total consideration of RMB 60,000,000 due and payable on or before April 30, 2007.
On June 11, 2007, Benda Ebei entered into additional Equity Transfer Agreements with Yaojin Wang (“Wang”) and Huimin Zhang “(Zhang”), also shareholders of SiBiono, for the purchase of an additional 2.56% of the shares of SiBiono’s common stock for total consideration of RMB 2,560,000 due and payable on or before June 30, 2007.
In connection with the above Equity Transfer Agreements, Benda entered into a Financial Consultancy Agreement with Super Pioneer International Limited (“Super Pioneer”) and Technical Consultancy Agreements with Wang and Zhang for financial and technical consultancy services to be rendered. Pursuant to the Financial and Technical Consultancy Agreements (the “Agreements”), Benda agreed to issue an aggregate of 2,189,560 shares of its common stock to Super Pioneer (2.1 million shares, out of which 1.96 million shares is redeemable), Wang (33,585 shares, redeemable) and Zhang (55,975 share, redeemable) within three months from the date of the Agreements. Since the issuance of common shares to Super Pioneer, Wang and Zhang was in the form of financial and technical consultancy services to be rendered, thus the corresponding amount $7,882,416 was recorded as consulting and professional fees.
Super Pioneer, Wang and Zang also agreed to refrain from selling shares of Benda’s common stock for a period of twelve months from the date of the issuance of the shares (the “Lock-up Period”). Within three months from expiration of the Lock-up Period, in the event that the public trading price of Benda’s common stock has not reach $3.60 per share and Benda’s common stock has not been listed on either the NASDAQ or AMEX stock exchanges, Super Pioneer, Wang, and Zang will have the option to require Benda to redeem an aggregate 2,049,560 shares of Benda’s common stock owned by Super Pioneer, Wang, and Zhang at a price of $3.60 per share.
In accordance EITF Topic D-98, “Classification and Measurement of Redeemable Securities” (“EITF Topic D-98”), as the Agreements governing the issuance of the 2,189,560 shares of common stock to Super Pioneer, Wang, and Zang contain provisions requiring Benda to repurchase 2,049,560 of these shares at a redemption price of $3.60 per share at the option of the holders (if certain events outside of control of the Group do not occur), these 2,049,560 shares have been classified as redeemable common stock, at their redemption price of $3.60 per share or totaling $7,376,366, outside of permanent equity at June 30, 2009.
According to the signed agreements mentioned in above, the redemption would be required to perform in September 2008. However the company has already obtained an oral consent from Super Pioneer, Wang and Zhang that the redemption would be deferred to the year of 2009.
| 20. | Other Income (expenses) |
In accordance with FASB staff position No. EITF00-19-2 the Group recorded $722,205 in Registration Delay Expense, for the six month ended June 30, 2008. Out of the total penalty, $230,312 was settled by issuance of 523,438 shares of common stock. There was no penalty to investors incurred for the six months ended June 30, 2009.
| 21. | Other General and Administrative Expenses |
For the six months and three months ended June 30, 2009 and 2008, the amount of other general and administrative expenses mainly composed of the following events:
| | | SIX MONTHS ENDED JUNE 30, | | | THREE MONTHS ENDED JUNE 30, | |
| | | 2009 | | | 2008 | | | 2009 | | | 2008 | |
| | | (Unaudited) | | | (Unaudited) | | | (Unaudited) | | | (Unaudited) | |
| Audit and accounting | | $ | 69,684 | | | $ | 201,829 | | | | 28,122 | | | | 72,495 | |
| Legal fees | | | 137,224 | | | | 114,704 | | | | 82,842 | | | | 106,651 | |
| Office expenses | | | 64,387 | | | | 292,530 | | | | 20,385 | | | | 153,559 | |
| Salaries and wages | | | 439,140 | | | | 514,993 | | | | 207,275 | | | | 165,723 | |
| Consulting fee | | | 74,802 | | | | 434,773 | | | | 49,302 | | | | 201,859 | |
| Rent & Utilities | | | 2,693 | | | | 55,830 | | | | -6,708 | | | | 20,290 | |
| Investor relation, Transfer agent and filing fees | | | 2,720 | | | | 28,123 | | | | - | | | | 9,277 | |
| Travel and transportation | | | 47,158 | | | | 160,334 | | | | 18,932 | | | | 89,771 | |
| Repair and maintanence | | | 11,357 | | | | 119,099 | | | | 4,982 | | | | 42,445 | |
| Miscellaneous | | | 138,767 | | | | 98,059 | | | | 43,796 | | | | 60,335 | |
| Total other general & administrative | | $ | 987,932 | | | $ | 2,020,274 | | | | 448,928 | | | $ | 922,405 | |
| 22. | Government Subsidies and Grants |
As mentioned in the Note 14, long term debt payable, various technology funds were obtained from various government technology agencies to support its gene therapy research and development activities during the past years and recorded as long term debt payable. According to the technology fund agreements, the various government technology agencies will examine the results of research and development according to the status of the projects. Once the examination takes place, the obligation of a particular debt payable is discharged accordingly.
According to US GAAP, once the obligation of a particular debt payable is discharged, the amount of this particular debt payable should be treated as government subsidies / grants. During the six months ended June 30, 2009, there were no long term debt payable discharged and government subsidies / grants recognized as revenue
Besides, during the six months ended June 30, 2009, there was Rmb180, 000 or $26,383 received by Jiangling Benda from the local financial bureau as subsidy for support of the enterprise with small and medium size.
Benda is subject to Delaware, United State of America tax, but no provision for income taxes were made for the six months ended June 30, 2009 and 2008 as Benda did not have reportable taxable income for the period.
Ever Leader, a wholly owned subsidiary of Benda, is subject to Hong Kong tax, but no provisions for income taxes were made for the six months ended June 30, 2009 and 2008 as Ever Leader did not have reportable taxable income for the periods.
Benda Ebei was registered as a Sino-Foreign Equity Joint Venture on May 26, 2004 and is subject to the tax laws applicable to Sino-Foreign Equity Joint Ventures in the PRC. Benda Ebei, starting from 2005, is fully exempt from PRC enterprise income tax for two years starting from the first profit-making year, followed by a 50% redsuction in income taxes, for the following three years.
Jiangling Benda and Yidu Benda are cross-municipal investment entities and enjoy the same tax treatment as Sino-Foreign Joint Ventures, starting from 2005, and were therefore exempt from PRC enterprise income tax for two years starting from the first profit-making year, followed by a 50% reduction in income taxes, for the following three years. Cross-municipal investments entities refer to entities that are incorporated in one municipal region but have investments in another municipal region.
The exemption periods for Benda Ebei, Jiangling Benda and Yidu Benda expired in the year of 2006, after which they are subject to 50% reduction in income taxes; whereas the full income tax rate is 33%. The remaining tax holidays will be expired in 2009.
However, starting and effective from January 1, 2008, the full income tax rate would be changed from 33% to 25% according to the new PRC taxation regulations. Therefore these subsidiaries will be subject to the regular full income tax rate at 25% after the tax holidays expire.
According to the new taxation regulations starting and effective from January 1, 2008, Beijing Shuhai is subject to the full income tax rate of 25%.
According to the new taxation regulations starting and effective from January 1, 2008, SiBiono, which is located in Shenzhen, a Special Economic District of PRC, is subject to the full income tax rate of 25% gradually in five years as following:
| Year | | Tax rate | |
| 2008 | | | 18 | % |
| 2009 | | | 20 | % |
| 2010 | | | 22 | % |
| 2011 | | | 24 | % |
| 2012 and thereafter | | | 25 | % |
Benda Ebei recorded $84,412 income tax for the six months ended June 30, 2009.
The provision for taxes on earnings consisted of:
| | | SIX MONTHS ENDED JUNE 30, | | | THREE MONTHS ENDED JUNE 30, | |
| | | 2009 | | | 2008 | | | 2009 | | | 2008 | |
| | | (Unaudited) | | | (Unaudited) | | | (Unaudited) | | | (Unaudited) | |
| Crurrent income taxes expenses: | | | | | | | | | | | | |
| PRC Enterprise Income | | $ | 84,412 | | | $ | 524,808 | | | $ | -217,966 | | | $ | 391,611 | |
| United States Federal Income Tax | | | - | | | | - | | | | - | | | | - | |
| | | $ | 84,412 | | | $ | 524,808 | | | $ | -217,966 | | | $ | 391,611 | |
A reconciliation between the income tax computed at the U.S statutory rate and the Groups provision for Income tax for the six month ended June 30, 2009 and 2008 are as follows:
| | | SIX MONTHS ENDED JUNE 30, | | | THREE MONTHS ENDED JUNE 30, | |
| | | 2009 | | | 2008 | | | 2009 | | | 2008 | |
| U.S Statutory rate | | | 34.0 | % | | | 34.0 | % | | | 34.0 | % | | | 34.0 | % |
| Foreign income not recognized in the U.S | | | -34.0 | % | | | -34.0 | % | | | -34.0 | % | | | -34.0 | % |
| PRC statuory rate | | | 25.0 | % | | | 25.0 | % | | | 25.0 | % | | | 25.0 | % |
| Tax relief and holidy granted to the Subsidary | | | 0.0 | % | | | -12.5 | % | | | 0.0 | % | | | -12.5 | % |
| Provision for income tax | | | 25.0 | % | | | 12.5 | % | | | 25.0 | % | | | 12.5 | % |
| 24. | Long Term Convertible Promissory Notes |
Our financial statements include enhanced note disclosure addressing the fair value of the convertible promissory notes and warrants and the appropriate accounting treatment for the overall transaction.
Additionally, the enhanced note disclosure includes a detailed discussion of the accounting for the debt discount resulting from the relative fair value of the warrants issued in conjunction with the convertible promissory notes (calculated in accordance with APB 14) and the debt discount resulting from the beneficial conversion feature / conversion discount associated with the convertible promissory notes (calculated in accordance with EITF 98-5 and EITF 00-27) as follows:
In March and April of 2007, the Group entered into Investment Agreements (“Agreements”) with certain accredited and institutional investors (“Investors”) pursuant to which the Investors purchased from the Group a total of 252 Units (“Units”), resulting in aggregate gross proceeds to the Group $7,560,000, with each Unit consisting of: (i) a Convertible Promissory Note (“Note”) in the principal amount of $30,000 and convertible into 54,087 shares of the Group's common stock; and (ii) a Common Stock Warrant (“Warrant”) to acquire 54,087 shares of the Group’s common stock at an exercise price of $0.555 per share and expiring on November 14, 2011. The Notes are immediately convertible at the option of each Investor, bear interest at a rate of 4% per annum and mature on March 28, 2009.
The Group has accounted for the Warrants issued in conjunction with the Notes in accordance with the provisions of APB No. 14, “Accounting for Convertible Debt and Debt Issued with Stock Purchase Warrants” (“APB 14”). Accordingly, the Warrants were valued using a Black-Scholes option pricing model with the following assumptions: (i) a risk free interest rate of 4.63%, (ii) a contractual life of 4.6 years, (iii) an expected volatility of 25%, and (iv) a dividend yield of zero. The relative fair value of the Warrants, based on an allocation of the value of the Notes and the value of the Warrants issued in conjunction with the Notes, was recorded as a debt discount (with a corresponding increase to additional paid-in capital) in the amount of $5,174,911, and is being amortized to interest expense over the expected term of the Notes.
Additionally, the difference between the effective conversion price of the Notes into shares of the Group’s common stock, and the fair value of the Group’s common stock on the date of issuance of the Notes, resulted in a beneficial conversion feature of $2,385,089 (capped at the $7,560,000 of gross proceeds raised less the previously calculated $5,174,911 debt discount associated with Warrants issued in conjunction with the Notes) and was calculated in accordance with EITF 98-5, “Accounting for Convertible Securities with Beneficial Conversion Features or Contingently Adjustable Conversion Ratios” and EITF 00-27, “Application of Issue No. 98-5 to Certain Convertible Instruments”. This beneficial conversion feature was recorded as an additional debt discount (with a corresponding increase to additional paid-in capital) and is being amortized to interest expense over the expected term of the Notes.
On July 10, 2008, 10 units of were converted to shares of 540,870. Therefore, as of June 30, 2009, the aggregate $7,260,000 principal balance of the notes remained outstanding and for the six months ended June 30, 2009, the Group recorded $544,500 of interest expense related to the Notes.
During the six months ended June 30, 2009, the Group recorded $864,049 in interest expense related to the amortization of the debt discount associated with the Warrants and the debt discount associated with the beneficial conversion feature.
The Group also incurred $529,200 in placement agent commissions related to the issuance of the Notes and Warrants. This amount was recorded by the Group as debt issue costs and is being amortized over expected term of the Notes. For the six months ended June 30, 2009, the Group recorded $55,485 in amortization expense related to debt issue costs.
The securities underlying the Notes and Warrants issued to the Investors are also subject to the terms of a Make Good Agreement entered into in connection with a financing the Group executed in November of 2006 (the “Make Good Agreement”). The Group further represented to the Investors that its target net income for fiscal year 2007 (“FY07 Net Income”) will be greater than or equal to $10.0 million (adjusted for a variety of non-cash charges) (the “Performance Threshold”). In the event the Performance Threshold is not attained, the Group is required to issue to the Investors a pro rata portion of 1,000,000 shares of the Group’s common stock for every one (1) cent by which the Group’s earnings per share, determined on a fully diluted basis, is less than $0.065. During the year ended December 31, 2008 the Group issued shares 3,810,976 shares of common stock in lieu of the non-achievement of the Performance Threshold at $0.07 per share, totaling to $266,768 and none for the six months ended June 30, 2009.
In the accounting for the Long Term Convertible Promissory Note, the Group’s analysis of the Performance Threshold had no effect because of the cap of the debt discounted and beneficial conversion feature as calculated in accordance with EITF 98-5.
The Group is also required to register for resale: (i) the shares of common stock underlying the Notes; and (ii) 150% of the shares of common stock underlying the Warrants, on a registration statement to be filed with the Securities and Exchange Commission (“Registration Statement”) and such Registration Statement is required to be declared effective by August 15, 2007 (the “Effectiveness Deadline”). If the Registration Statement does not become effective by the Effectiveness Deadline, or if the Group fails to maintain the effectiveness of the Registration Statement, for any reason, the Group is required to pay the Investors in cash an amount equal to 1% of the purchase price of each Unit held by the Investors on such Effectiveness Deadline or the first day of such failure to maintain the Registration Period, as applicable, and for every 30 day period (or part) thereafter, in each case until cured (“Registration Delay Payments”), provided that the Registration Delay Payments will not exceed 10% of the purchase price of the Units. In the event that the Registration Delay Payments are not made in a timely manner, such Registration Delay Payments will bear interest at a rate of 1.5% per month until paid in full.
The calculated fair value of the warrants (issued in conjunction with the convertible promissory notes) was recorded as a debt discount (reduction to the carrying value of the convertible promissory notes) with a corresponding increase (credit) to additional paid in capital.
In accordance with the registration rights granted to investors who purchased the convertible promissory notes and warrants, we are required to file a registration statement with the Securities and Exchange Commission (“Registration Statement”) attempting to register the potentially issuable shares of common stock underlying the convertible promissory notes and warrants. There is no cash settlement requirement, in the event the Company cannot deliver Registered Shares.
Specifically, in the event the Registration Statement is not declared effective by August 15, 2007 (“Effectiveness Deadline”), we are required to pay to the investors in cash an amount equal to 1% of the purchase price paid by each investor for the convertible promissory notes and warrants (“Registration Delay Payments”). Additionally, for every 30 day period (or part) thereafter that the Registration Statement is not declared effective, we are required to continue to make Registration Delay Payments, provided that such Registration Delay Payments shall not exceed 10% of the purchase price paid by each investor for the convertible promissory notes and warrants.
In the event the Registration Statement is never declared effective, the Registration Delay Payments are capped at 10% (of the purchase price paid by each investor for the convertible promissory notes and warrants). As a result, the initial requirement for the Company to deliver registered shares upon conversion of the convertible promissory notes or exercise of the warrants is negated and paragraphs 14 – 18 of EITF 00-19 are not applicable (for purposes of classifying the fair value of the warrants issued in conjunction with the convertible promissory notes as a liability with changes in fair value recorded in earnings).
| 25. | Common Stock, Preferred Stock, Additional Paid-in Capital, Warrants and Options |
As of June 30, 2009, and December 31, 2008, the Group had an aggregate of:
| (a) | None shares of preferred stock issued and outstanding; |
| (b) | The share of common stock issued and outstanding was 105,155,355 as of June 30, 2009 and December 31, 2008 respectively. |
| (c) | The following tables shows the events occurred in additional paid-in capital: |
| | | | | | Events occurred | | | | |
| | | December 31, | | | during the | | | June 30, | |
| | | 2008 | | | reporting period | | | 2009 | |
| Events | | | | | (Unaudited) | | | (Unaudited) | |
| To record the changes of par value from $0.01 to $0.001 | | | | | | | | | |
| of the outstanding common stock as of 11/17/2005 | | $ | 584,481 | | | $ | - | | | $ | 584,481 | |
| | | | | | | | | | | | | |
| To adjust the par value of outstanding common stock as of 3/31/2006 | | | (5,354 | ) | | | - | | | | (5,354 | ) |
| | | | | | | | | | | | | |
| Waiver of shareholder loan on 9/5/2006 | | | 2,298,434 | | | | - | | | | 2,298,434 | |
| | | | | | | | | | | | | |
To eliminate the common stock and additional paid-in capital of the former shell company "Applied Spectrum Technologies, Inc." on 11/15/2006 | | | 16,215,770 | | | | - | | | | 16,215,770 | |
| | | | | | | | | | | | | |
| To eliminate the accumulated deficit of the former shell company | | | | | | | | | | | | |
| "Applied Spectrum Technologies, Inc." on 11/15/2006 | | | (16,209,962 | ) | | | - | | | | (16,209,962 | ) |
| | | | | | | | | | | | | |
| Issuance of common stock, 25,961,760 shares at $0.4622 per share, on 11/15/2006 | | | 11,974,038 | | | | - | | | | 11,974,038 | |
| | | | | | | | | | | | | |
| Issuance of common stock, 64,942,369 shares at $0.001 per share on 11/15/2006 | | | (64,942 | ) | | | - | | | | (64,942 | ) |
| | | | | | | | | | | | | |
| To relocate the original common stock of Ever Leader on 11/15/2006 | | | 1,285 | | | | - | | | | 1,285 | |
| | | | | | | | | | | | | |
| To record the placement agent commission and transaction related fee | | | | | | | | | | | | |
| of reverse merger on 11/15/2006 | | | (1,694,326 | ) | | | - | | | | (1,694,326 | ) |
| | | | | | | | | | | | | |
| Issuance of common stock 706,195 at $0.4622 per share | | | 325,696 | | | | - | | | | 325,696 | |
| | | | | | | | | | | | | |
| Debt discount on beneficial conversion feature on convertible promissory notes | | | 2,385,089 | | | | - | | | | 2,385,089 | |
| | | | | | | | | | | | | |
| Debt discount on warrants issued with convertible promissory notes | | | 5,174,911 | | | | - | | | | 5,174,911 | |
| | | | | | | | | | | | | |
| Placement agent exercised 849,007 warrants at strike price through cashless arrangement | | | 470,347 | | | | - | | | | 470,347 | |
| | | | | | | | | | | | | |
| Issuance of common stock to PIPE investors as penalty for late submission of 10KSB | | | 92,461 | | | | - | | | | 92,461 | |
| | | | | | | | | | | | | |
| Issuance of common stock to directors in lieu of their remuneration | | | 75,790 | | | | - | | | | 75,790 | |
| | | | | | | | | | | | | |
| Issuance of common stock to PIPE investors as penalty for registeration delay expenses | | | 229,789 | | | | - | | | | 229,789 | |
| | | | | | | | | | | | | |
| Conversion of convertible promissory notes | | | (8,039 | ) | | | - | | | | (8,039 | ) |
| | | | | | | | | | | | | |
| Issuance of common stock to investors under "Make Good Agreement" | | | 262,957 | | | | - | | | | 262,957 | |
| Additional paid-in capital, balance for the period ended | | $ | 22,108,427 | | | $ | - | | | $ | 22,108,427 | |
| (d) | As of June 30, 2009 and December 31, 2008, 30,264,249 Warrants, each convertible into one (1) share of the Group’s Common Stock, issued and outstanding. |
| (e) | None options issued and outstanding. |
| 26. | Weighted Average Shares Outstanding |
Basic net income (loss) per share is computed by dividing net income (loss) by the weighted-average number of shares outstanding during the period.
Diluted net income (loss) per share is computed by using the weighted-average number of shares of common stock outstanding and, when dilutive, potential shares from stock options and warrants to purchase common stock, using the treasury stock method.
The following table illustrates the computation of basic and dilutive net income (loss) per share and provides a reconciliation of the number of weighted-average basic and diluted shares outstanding:
| | | SIX MONTHS ENDED JUNE 30, | | | THREE MONTHS ENDED JUNE 30, | |
| | | 2009 | | | 2008 | | | 2009 | | | 2008 | |
| | | | | | | | | | | | Restated | |
| | | (Unaudited) | | | (Unaudited) | | | (Unaudited) | | | (Unaudited) | |
| Numerator: | | | | | | | | | | | | |
| Net income (loss) | | $ | (2,316,255 | ) | | $ | (3,822,578 | ) | | $ | (1,275,698 | ) | | $ | (1,785,165 | ) |
| | | | | | | | | | | | | | | | | |
| Denominator: | | | | | | | | | | | | | | | | |
| Basic weighted-average shares outstanding | | | 105,155,355 | | | | 100,558,191 | | | | 105,155,355 | | | | 100,803,509 | |
| Effect of dilutive stock options & warrants | | | - | | | | - | | | | - | | | | - | |
| Diluted weighted-average shares outstanding | | | 105,155,355 | | | | 100,558,191 | | | | 105,155,355 | | | | 100,803,509 | |
| | | | | | | | | | | | | | | | | |
| Net income (loss) per share: | | | | | | | | | | | | | | | | |
| Basic | | $ | (0.02 | ) | | $ | (0.04 | ) | | $ | (0.01 | ) | | $ | (0.02 | ) |
| Diluted | | $ | (0.02 | ) | | $ | (0.04 | ) | | $ | (0.01 | ) | | $ | (0.02 | ) |
| 27. | Commitments and Contingencies |
As of June 30, 2009, there was an operating lease commitment and the amounts are stated as follows;
| | | June 30, | |
| | | 2009 | |
| | | (Unaudited) | |
| Rental and Property Management Fee | | | |
| Within one year | | $ | 66,256 | |
| One to two year | | | 35,166 | |
| Two to three year | | | 35,166 | |
| Over three years | | | 61,541 | |
| Total commitments payable | | $ | 198,129 | |
The Group states the segment information according to the requirement stated in paragraph 37 of SFAS 131. The Group produces five different categories of products and each category of product is produced in different subsidiaries or operation plants. The details are stated as follows:
| 1. | Benda Ebei produces conventional medicines which including banded and generic medicines; |
| 2. | Jiangling Benda produces active pharmaceutical ingredients, APIs; |
| 3. | Yidu Benda produces bulk chemicals; |
| 4. | Beijing Shusai produces pharyngitis killer therapy; and |
| 5. | SiBiono produces gene therapy medicines, Gendicine. |
Since each subsidiary produces the corresponding products by using the same production facilities of each subsidiary, therefore according to the requirement stated in paragraph of SFAS 131, the Group reports the segment information according to the un-identical products that produced in each subsidiary.
Selected financial information for each of these segments for the six months and three months ended June 30, 2009 and 2008 were as follows:
| | | SIX MONTHS ENDED JUNE 30, | | | THREE MONTHS ENDED JUNE 30, | |
| | | (Unaudited) | | | (Unaudited) | | | (Unaudited) | | | (Unaudited) | |
| Branded/Generic medicine segment | | 2009 | | | 2008 | | | 2009 | | | 2008 | |
| Revenue from external customers | | $ | 8,729,686 | | | $ | 11,466,193 | | | $ | 4,369,227 | | | $ | 5,945,084 | |
| Cost of sales | | | (5,549,051 | ) | | | (7,647,567 | ) | | | (2,889,269 | ) | | | (3,866,403 | ) |
| Gross profit | | | 3,180,636 | | | | 3,818,626 | | | | 1,479,958 | | | | 2,078,681 | |
| Gross margin | | | 36 | % | | | 33 | % | | | 34 | % | | | 35 | % |
| Research and development | | | (2,301 | ) | | | - | | | | (2,155 | ) | | | 0 | |
| Selling expense | | | (413,312 | ) | | | (773,483 | ) | | | (210,843 | ) | | | (558,832 | ) |
| General and administrative expense | | | (2,086,438 | ) | | | (486,593 | ) | | | (2,005,398 | ) | | | (44,770 | ) |
| Segment contribution | | $ | 678,584 | | | $ | 2,558,550 | | | $ | (738,438 | ) | | $ | 1,475,079 | |
| Contribution margin | | | 8 | % | | | 22 | % | | | -17 | % | | | 25 | % |
| | | | | | | | | | | | | | | | | |
| Total assets, segment | | $ | 28,928,585 | | | $ | 38,243,467 | | | $ | 28,928,585 | | | $ | 38,243,467 | |
| | | | | | | | | | | | | | | | | |
| Active pharmaceutical ingredients segment | | 2009 | | | 2008 | | | 2009 | | | 2008 | |
| Revenue from external customers | | $ | 790,062 | | | $ | 580,332 | | | $ | 367,801 | | | $ | 579,984 | |
| Cost of sales | | | (967,595 | ) | | | (638,529 | ) | | | (545,989 | ) | | | (638,529 | ) |
| Gross profit | | | (177,532 | ) | | | (58,197 | ) | | | (178,188 | ) | | | (58,545 | ) |
| Gross margin | | | -22 | % | | | -10 | % | | | -48 | % | | | -10 | % |
| Research and development | | | (73 | ) | | | - | | | | (73 | ) | | | - | |
| Selling expense | | | (14,725 | ) | | | (357 | ) | | | (10,810 | ) | | | -357 | |
| General and administrative expense | | | (321,009 | ) | | | (1,026,566 | ) | | | (59,826 | ) | | | (782,997 | ) |
| Segment contribution | | $ | (513,339 | ) | | $ | (1,085,120 | ) | | $ | (248,898 | ) | | $ | (841,899 | ) |
| Contribution margin | | | -65 | % | | | -187 | % | | | -68 | % | | | -145 | % |
| | | | | | | | | | | | | | | | | |
| Total assets, segment | | $ | 9,936,644 | | | $ | 8,436,445 | | | $ | 9,936,644 | | | $ | 8,436,445 | |
| | | | | | | | | | | | | | | | | |
| Bulk chemicals segment | | 2009 | | | 2008 | | | 2009 | | | 2008 | |
| Revenue from external customers | | $ | - | | | $ | - | | | $ | - | | | $ | - | |
| Cost of sales | | | - | | | | - | | | | - | | | | - | |
| Gross profit | | | - | | | | - | | | | - | | | | - | |
| Gross margin | | | - | | | | - | | | | - | | | | - | |
| Research and development | | | - | | | | - | | | | - | | | | - | |
| Selling expense | | | - | | | | - | | | | - | | | | - | |
| General and administrative expense | | | (220,174 | ) | | | (305,968 | ) | | | (107,999 | ) | | | (7,991 | ) |
| Segment contribution | | $ | (220,174 | ) | | $ | (305,968 | ) | | $ | (107,999 | ) | | $ | (7,991 | ) |
| Contribution margin | | | - | | | | - | | | | - | | | | - | |
| | | | | | | | | | | | | | | | | |
| Total assets, segment | | $ | 7,218,326 | | | $ | 7,694,510 | | | $ | 7,218,326 | | | $ | 7,694,510 | |
| | | | | | | | | | | | | | | | | |
| Pharynigitis killer therapy segment | | 2009 | | | 2008 | | | 2009 | | | 2008 | |
| Revenue from external customers | | $ | - | | | $ | 2,128 | | | $ | - | | | $ | 31 | |
| Cost of sales | | | - | | | | (117 | ) | | | - | | | | (2 | ) |
| Gross profit | | | - | | | | 2,011 | | | | - | | | | 29 | |
| Gross margin | | | - | | | | 95 | % | | | - | | | | 94 | % |
| Research and development | | | - | | | | - | | | | - | | | | - | |
| Selling expense | | | - | | | | (2,654 | ) | | | - | | | | (1,955 | ) |
| General and administrative expense | | | (14,475 | ) | | | (20,227 | ) | | | (7,229 | ) | | | (7,910 | ) |
| Segment contribution | | $ | (14,475 | ) | | $ | (20,870 | ) | | $ | (7,229 | ) | | $ | (9,836 | ) |
| Contribution margin | | | - | | | | -981 | % | | | - | | | | -31729 | % |
| | | | | | | | | | | | | | | | | |
| Total assets, segment | | $ | 81,597 | | | $ | 108,332 | | | $ | 81,597 | | | $ | 108,332 | |
| | | | | | | | | | | | | | | | | |
| Gendicine (Ad-p53) segment | | 2009 | | | 2008 | | | 2009 | | | 2008 | |
| Revenue from external customers | | $ | 1,920,663 | | | $ | 1,176,970 | | | $ | 1,187,580 | | | $ | 753,154 | |
| Cost of sales | | | (182,433 | ) | | | (110,651 | ) | | | (121,030 | ) | | | (74,674 | ) |
| Gross profit | | | 1,738,230 | | | | 1,066,319 | | | | 1,066,551 | | | | 678,480 | |
| Gross margin | | | 91 | % | | | 91 | % | | | 90 | % | | | 90 | % |
| Research and development | | | (115,709 | ) | | | (169,781 | ) | | | 185,828 | | | | (147,717 | ) |
| Selling expense | | | (588,329 | ) | | | (654,718 | ) | | | (400,520 | ) | | | (433,697 | ) |
| General and administrative expense | | | (1,366,012 | ) | | | (485,340 | ) | | | (737,235 | ) | | | (175,183 | ) |
| Segment contribution | | $ | (331,820 | ) | | $ | (243,520 | ) | | $ | 114,624 | | | $ | (78,117 | ) |
| Contribution margin | | | -17 | % | | | -21 | % | | | 10 | % | | | -10 | % |
| | | | | | | | | | | | | | | | | |
| Total assets, segment | | $ | 12,094,467 | | | $ | 14,048,551 | | | $ | 12,094,467 | | | $ | 14,048,551 | |
| | | SIX MONTHS ENDED JUNE 30, | | | THREE MONTHS ENDED JUNE 30, | |
| | | 2009 | | | 2008 | | | 2009 | | | 2008 | |
| | | (Unaudited) | | | (Unaudited) | | | (Unaudited) | | | (Unaudited) | |
| Total revenue from external customers | | $ | 11,440,412 | | | $ | 13,225,623 | | | $ | 5,924,608 | | | $ | 7,278,253 | |
| Cost of sales | | | (6,699,079 | ) | | | (8,396,864 | ) | | | (3,556,288 | ) | | | (4,579,608 | ) |
| Gross profit | | | 4,741,333 | | | | 4,828,759 | | | | 2,368,321 | | | | 2,698,645 | |
| Gross margin | | | 41 | % | | | 37 | % | | | 40 | % | | | 37 | % |
| Research and development | | | (118,083 | ) | | | (169,781 | ) | | | 183,600 | | | | (147,717 | ) |
| Selling expense | | | (1,016,366 | ) | | | (1,431,212 | ) | | | (622,173 | ) | | | (994,841 | ) |
| General and administrative expense | | | (4,008,108 | ) | | | (2,324,694 | ) | | | (2,917,687 | ) | | | (1,018,851 | ) |
| Segment contribution | | $ | (401,224 | ) | | $ | 903,072 | | | $ | (987,940 | ) | | $ | 537,236 | |
| Contribution margin | | | -4 | % | | | 7 | % | | | -17 | % | | | 7 | % |
| | | | | | | | | | | | | | | | | |
| Total assets, segment | | $ | 58,259,618 | | | $ | 68,531,305 | | | $ | 58,259,618 | | | $ | 68,531,305 | |
The results of the total consolidated net profit before income taxes for the reporting periods are as follows:
| | | 2009 | | | 2008 | | | 2009 | | | 2008 | |
| | | (Unaudited) | | | (Unaudited) | | | (Unaudited) | | | (Unaudited) | |
| Total segment contribution | | $ | (401,224 | ) | | $ | 903,072 | | | $ | (987,940 | ) | | $ | 537,236 | |
| Unallocated amounts: | | | | | | | | | | | | | | | | |
| Interest income/(expenses) | | | (1,633,247 | ) | | | (2,340,580 | ) | | | (362,866 | ) | | | (1,128,298 | ) |
| Government subsidy | | | 26,383 | | | | - | | | | - | | | | - | |
| Other income/(expenses) | | | 45,776 | | | | 248,058 | | | | 62,976 | | | | (53,635 | ) |
| Other corporate expenses | | | (472,105 | ) | | | (2,196,536 | ) | | | (212,403 | ) | | | (859,471 | ) |
| Total income / (loss) before noncontrolling interest and income taxes | | $ | (2,434,417 | ) | | $ | (3,385,986 | ) | | $ | (1,500,232 | ) | | $ | (1,504,168 | ) |
The other corporate expenses for the six months and three months ended June 30, 2009 and 2008 composed of the following events:
| | | SIX MONTHS ENDED JUNE 30, | | | THREE MONTHS ENDED JUNE 30, | |
| | | 2009 | | | 2008 | | | 2009 | | | 2008 | |
| | | (Unaudited) | | | (Unaudited) | | | (Unaudited) | | | (Unaudited) | |
| Penalty to investor | | $ | - | | | $ | 1,113,405 | | | $ | | | | $ | 391,200 | |
| Wages and salaries | | | 195,016 | | | | 192,675 | | | | 96,683 | | | | 40,910 | |
| Audit and accounting | | | 49,997 | | | | 106,063 | | | | 24,997 | | | | 60,757 | |
| Amortization of debt issue cost | | | 55,485 | | | | 131,757 | | | | - | | | | 65,878 | |
| Consulting fee | | | 6,216 | | | | 371,960 | | | | 3,124 | | | | 191,460 | |
| Investor relation, transfer agent and filing fees | | | 2,720 | | | | 28,123 | | | | - | | | | 9,277 | |
| Director renumeration | | | 45,000 | | | | 140,468 | | | | 22,500 | | | | 29,595 | |
| Legal fee | | | 104,567 | | | | 76,675 | | | | 61,048 | | | | 45,051 | |
| Travel and transportation | | | 2,143 | | | | 4,844 | | | | 333 | | | | - | |
| Office expense | | | - | | | | 1,120 | | | | - | | | | - | |
| Miscellaneous | | | 10,959 | | | | 29,446 | | | | 3,719 | | | | 25,343 | |
| Total | | $ | 472,105 | | | $ | 2,196,536 | | | $ | 212,403 | | | $ | 859,471 | |
For the details of information of this particular, it should be read in conjunction with the management discussion and analysis section.
The following table shows the reconciliation between the segments assets and the total assets as of June 30, 2009 and 2008:
| | | June 30, | | | June 30, | |
| | | 2009 | | | 2008 | |
| | | (Unaudited) | | | (Unaudited) | |
| Total assets, segment | | $ | 58,259,618 | | | $ | 68,531,305 | |
| | | | | | | | | |
| Total assets of corporate: | | | | | | | | |
| Cash and cash equivalent | | | 16,148 | | | | 14,823 | |
| Bank indebtedness | | | 1,192,385 | | | | 1,296,941 | |
| Prepaid expesnes and deposit | | | 230 | | | | 8,391 | |
| Refundable purchase price paid | | | 1,200,000 | | | | 1,200,000 | |
| Due from related parties | | | 882,482 | | | | 876,729 | |
| Debit issue costs: | | | | | | | | |
| placement agent commission | | | - | | | | 196,188 | |
| Total assets | | $ | 61,550,863 | | | $ | 72,124,377 | |
The following table shows how the noncontrolling interest for six months ended June 30, 2009 and 2008 was derived:
| | | Six Months Ended June 30, 2009 (Unaudited) | |
| | | Benda | | | Jiangling | | | Yidu | | | Beijing | | | | | | | |
| | | Ebei | | | Benda | | | Benda | | | Shusai | | | SiBiono | | | Total | |
| Segment operating profit / (loss) | | $ | 678,584 | | | | (513,339 | ) | | | (220,174 | ) | | | (14,475 | ) | | | (331,820 | ) | | $ | (401,224 | ) |
| Interest income/ (expenses) | | | (80,272 | ) | | | (258 | ) | | | 3 | | | | - | | | | (87,419 | ) | | | (167,946 | ) |
| Other income / (expenses) | | | (14,997 | ) | | | 99 | | | | 1,979 | | | | - | | | | 58,695 | | | | 45,776 | |
| Government subsidy | | | - | | | | 26,383 | | | | - | | | | - | | | | - | | | | 26,383 | |
| Income taxes | | | (84,412 | ) | | | - | | | | - | | | | - | | | | - | | | | (84,412 | ) |
| Income / (loss) before minority interest | | $ | 498,903 | | | | (487,115 | ) | | | (218,192 | ) | | | (14,475 | ) | | | (360,544 | ) | | $ | (581,423 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Noncontrolling interest percentage | | | 5.00 | % | | | 9.75 | % | | | 9.75 | % | | | 28.75 | % | | | 42.88 | % | | | | |
| Noncontrolling interest | | $ | 24,945 | | | | (47,494 | ) | | | (21,274 | ) | | | (4,162 | ) | | | (154,589 | ) | | $ | (202,574 | ) |
| | | Six Months Ended June 30, 2008 (Unaudited) | |
| | | Benda | | | Jiangling | | | Yidu | | | Beijing | | | | | | | |
| | | Ebei | | | Benda | | | Benda | | | Shusai | | | SiBiono | | | Total | |
| Segment operating profit / (loss) | | $ | 2,558,550 | | | | (1,085,120 | ) | | | (305,968 | ) | | | (20,870 | ) | | | (243,520 | ) | | $ | 903,072 | |
| Interest income/ (expenses) | | | (207,747 | ) | | | 1,059 | | | | 3 | | | | (8 | ) | | | (94,762 | ) | | | (301,455 | ) |
| Other income / (expenses) | | | (5,901 | ) | | | (207 | ) | | | 1,964 | | | | 6,748 | | | | 245,454 | | | | 248,058 | |
| Income taxes | | | (524,808 | ) | | | - | | | | - | | | | - | | | | - | | | | (524,808 | ) |
| Income / (loss) before minority interest | | $ | 1,820,094 | | | | (1,084,268 | ) | | | (304,001 | ) | | | (14,130 | ) | | | (92,828 | ) | | $ | 324,867 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Noncontrolling interest percentage | | | 5.00 | % | | | 9.75 | % | | | 9.75 | % | | | 28.75 | % | | | 42.88 | % | | | | |
| Noncontrolling interest | | $ | 91,005 | | | | (105,716 | ) | | | (29,640 | ) | | | (4,062 | ) | | | (39,801 | ) | | $ | (88,216 | ) |
| (a) | SiBiono patents - on January 29, 2007, SiBiono entrusted Grandall Legal Group Shenzhen Law Firm to issue a legal letter to Zhaohui Peng, one of the shareholders of Sibiono and the inventor of Gendicine, requesting him to transfer all the title of patents to SiBiono. |
On June 18, 1999, during the formation of SiBiono, Zhaohui Peng transferred the rights to the patent “A new method for manufacturing recombinant adenovirus” and related research results to SiBiono as a payment for the registered capital. In return, Zhaohui Peng was granted 32.03% of the common stock of SiBiono.
From 1999 to 2007, SiBiono successfully obtained various technology funds from various government technology agencies to support the further research and development activities of Gendicine. Due to this significant funding obtained by SiBiono, Sibiono developed five additional patents which are summarized as follows:
Item | | Patent name | | Countries / Date | | Application
Number (1) | | Publication Number (2) | | Approved Patent Number (3) | | Name of Patent Inventor (6) | | Name of Applicant (6) | | Patent Assignees |
| 1 | | A new method for manufacturing recombinant adenovirus | | | | | | | | | | | | | | |
| | | A | | China | | 98123346.5 | | CN1228474A | | ZL98123346.5 | | Peng | | Peng | | SiBiono |
| | | | | Date | | 12/14/1998 | | 9/15/1999 | | 7/3/2002 | | | | | | |
| 2 | | A recombinant constructed by a virus vector and a human tumor suppressor gene and its use | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | A | | China | | 02115228.4 | | CN1401778A | | ZL02115228.4 | | Peng / Zhang | | Peng / Zhang | | Peng / Zhang |
| | | | | Date | | 5/8/2002 | | 3/12/2003 | | 11/24/2004 | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | B | | PCT (4) | | 65 | | WO2004/078987A1 | | Not Approved | | Peng / Zhang | | Peng / Zhang | | N/A |
| | | | | Date | | 3/8/2004 | | 9/16/2004 | | N/A | | | | | | |
| 3 | | Recombinant gene medicine of adenovirus vector and and gene p54 for treating proloferative diseases | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | A | | China | | 03125129.3 | | CN1471977A | | ZL03125129.3 | | Peng / Zhang | | Peng / Zhang | | Peng / Zhang |
| | | | | Date | | 5/10/2003 | | 2/4/2004 | | 7/25/2007 | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | B | | PCT (4) | | 58 | | WO2004/104204A1 | | Not Approved | | Peng / Zhang | | Peng / Zhang | | N/A |
| | | | | Date | | 5/9/2004 | | 12/2/2004 | | N/A | | | | | | |
| 4 | | The application of recombinant adenoviral p53 as cancer vaccine (tentative title) | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | A | | China | | 200510002779.1 | | CN1679641A | | ZL200510002779.1 | | Peng / Zhang | | Peng / Zhang | | Peng / Zhang |
| | | | | Date | | 1/26/2005 | | 10/12/2005 | | 8/29/2007 | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | B | | PCT (4) | | 11 | | WO2006/079244A1 | | Not Approved | | Peng / Zhang | | Peng / Zhang | | N/A |
| | | | | Date | | 1/26/2005 | | 8/3/2006 | | N/A | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | C | | US (5) | | 11/075035 | | 2005/0281785A1 | | Not Approved | | Peng / Zhang | | Unidentified Yet | | N/A |
| | | | | Date | | 3/7/2005 | | 12/22/2005 | | N/A | | | | | | |
| 5 | | Human Embryonic Kidney (HEK) sub-clone cell line | | | | | | | | | | | | | | |
| | | A | | China | | 03126889.7 | | CN1513985A | | Not Approved | | Peng / Zhang | | Peng / Zhang | | N/A |
| | | | | Date | | 6/13/2003 | | 7/21/2004 | | N/A | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | B | | PCT (4) | | 57 | | WO2004/111239 | | Not Approved | | Peng / Zhang | | Peng / Zhang | | N/A |
| | | | | Date | | 5/9/2004 | | 12/23/2004 | | N/A | | | | | | |
| 6 | | The complex of polypeptide liposome and human VGEF gene, and its use and human VGEF gene, and its use | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | A | | China | | 02134321.7 | | CN1389269A | | Not Approved | | Peng / Zhang / Zhu | | Peng / Zhang / Zhu | | N/A |
| | | | | Date | | 7/4/2002 | | 1/8/2003 | | N/A | | | | | | |
Note:
| | (1) | Application number is obtained when application is submitted; |
| | (2) | Publication number is obtained after the first phase examination; |
| | (3) | Approved patent number is obtained after the final examination; |
| | (4) | PCT is referred to an International Patent Organization in Paris; |
| | (5) | US is referred to the application is made in United States of America alone; |
| | (6) | Peng is referred to Zhaohui Peng; Zhang is referred to Xiaozhi Zhang; Zhu is referred to Jinya Zhu. |
As indicated in the above table, Item 1, the patent “A new method for manufacturing recombinant adenovirus” had been assigned to SiBiono; however, the other approved patents (item 2 through item 4) in PRC still have not been assigned to SiBiono. The Group believes that all the above mentioned patents should be rightfully transferred to SiBiono, a subsidiary of the Group. Accordingly, the above mentioned legal letter was issued on this ground.
On August 27, 2008, the Group through its subsidiary, SiBiono filed an application to the Guongdong Province Shenzhen City (Middle) Peoples’ Court to demand Zhaozhu Peng to transfer back all the above mentioned patents to SiBiono. The case has been accepted by the Court and is waiting for its further investigation as of August 15, 2009.
| (b) | As mentioned in Note 12, following this arbitration decision, Benda Ebei has the obligation to pay the total acquisition cost payable of Rmb12.48 million, plus the penalty and related legal and arbitration expenses, totaling approximately Rmb12.80 million (or $1.88 million). |
On May 22, 2008, Benda Ebei applied to Shenzhen People Court to terminate above mentioned arbitration. The termination is based on the ground that Xiaozhi Zhang does not own all 6.24% of SiBiono’s common stock. In fact, he only owns 3.28% of SiBiono’s stock. The application has been accepted by Shenzhen People Court and is waiting for its further investigation as of August 15, 2009.
| (c) | The Company has become aware that Excalibur Limited Partnership and Excalibur Limited Partnership II (the "Plaintiffs") filed a motion for summary judgment in lieu of a complaint pursuant to CPLR § 3213 (the "Motion") with the Supreme Court of the State of New York (the "Court"), alleging that the Company has been delinquent on the payment of an aggregate sum of $600,000 and accrued interest and costs arising from the Convertible Promissory Notes that were issued to the Plaintiffs in April 2007 in connection with a $7,560,000 private placement. Pursuant to the motion, the Plaintiffs requested that the Court (1) enter summary judgment in favor of Excalibur Limited Partnership (“Excalibur Limited”) in the amount of $390,000 plus all accrued interest and costs, and, (2) enter summary judgment in favor of Excalibur Small Cap Opportunities LP (“Excalibur Small Cap”) in the amount of $210,000 and accrued interest and costs. As of June 30, 2009, the Company has not received service of such notice, and therefore, the Company does not have details regarding the content of the complaint made by the Plaintiffs. |
On July 29, 2009, the Supreme Court of the State of New York, New York County entered a judgment against the Company in favor of Excalibur Limited Partnership and Excalibur Limited Partnership II (the “Plaintiffs”) in the amount of $674,251.65 in connection with the Convertible Promissory Notes issued to the Plaintiffs in April 2007 in a $7,560,000 private placement.
| (d) | On March 4, 2009, the Company received a Notice and Default and Payment Demand letter (the "Default Letter") from Pope Investments LLC ("Pope") in connection with its convertible promissory note in the amount of $5,520,000 (the "Note") purchased in our April 2007 private placement offering. The Default Letter provided notice of default based on the Company's failure to make a required interest payment on the Note by February 20, 2009. The Default Letter further demanded full payment of all interest, liquidated damages and accrued interest thereon in the amount of $130,364.37 by March 14, 2009, or Pope will accelerate the maturity date of the full principal amount of the Note. On April 7, 2009, the Company received further Default Letters and Payment Demand from Pope, Excalibur Limited and Excalibur Small Cap demanding payment in full of the balance of the Notes, which matured on March 28, 2009. The Group has been negotiating with Pope and all of the other noteholders listed above since then and no conclusions have been reached as of June 30, 2009. |
Benda Pharmaceutical, Inc.,
Management’s Discussion and Analysis or Plan of Operations
For the Six Months and Three Months Ended June 30, 2009 and 2008
(Amount Expressed in U.S. Dollars)
Item 2. Management’s Discussion and Analysis or Plan of Operation
Critical Accounting Policies
Accounting policies discussed in this section are those that we consider to be most critical to an understanding of our financial statements because they inherently involve significant judgment and uncertainties. For all of these estimates, we caution that future events rarely develop exactly as forecast, and the best estimates routinely require adjustment. Also see Note 4, Summary of Significant Accounting Policies.
Revenue Recognition
Among the most important accounting policies affecting the Group’s consolidated financial statements is its policy of recognizing revenue in accordance with the SEC's Staff Accounting Bulletin ("SAB") No. 104. Under this policy, all of the following criteria must be met in order for us to recognize revenue:
1. Persuasive evidence of an arrangement exists;
2. Delivery has occurred or services have been rendered;
3. The seller's price to the buyer is fixed or determinable; and
4. Collectibility is reasonably assured.
The majority of the Group's revenue results from sales contracts with distributors and revenue is recorded upon the shipment of goods. Management conducts credit background checks for new customers as a means to reduce the subjectivity of assuring collectibility. Based on these factors, the Group believes that it can apply the provisions of SAB 104 with minimal subjectivity. Sales are presented net of value added tax (VAT). No return allowance is made as products returns are insignificant based on historical experience.
Estimates Affecting Accounts Receivable and Inventories
The preparation of our consolidated financial statements requires management to make estimates and assumptions that affect our reporting of assets and liabilities (and contingent assets and liabilities). However, it is explicated that the changes in estimation were not material in the preparation of our consolidation financial statements.
As of June 30, 2009 and 2008, the Group provided a $6,591,973 and $1,277,336, respectively for the allowance of doubtful accounts against trade receivables (please refer to Note 5 for details). Management's estimate of the appropriate allowance on those accounts receivable for the reporting periods was based on the aged nature of these accounts. In making its judgment, management assessed its customers' ability to continue to pay their outstanding invoices and the collectibility of those accounts on a timely basis, and whether their financial position might deteriorate significantly in the future, which would result in their inability to pay their debts to the Company.
Inventories, which are primarily comprised of raw materials, packaging materials, and finished goods, are stated at the lower of cost or net realizable value. Cost being determined on the basis of a moving average. The Group evaluates the need for reserves associated with obsolete, slow-moving and non-salable inventory by reviewing net realizable values on a periodic basis.
For the six months ended June 30, 2009 and 2008, the Group provided a reserve against its obsolete, slow-moving or non-salable inventory amounting to $4,687,738 and $3,994,628, respectively.
The provision of reserve was mainly resulted from the manufacturing process of Gendicine, SiBiono’s sole product and SiBiono was acquired by the company in April 2007.
The following chart shows the manufacturing process of Gendicine (Ad-p53):
In the production process of finished goods, Gendicine, several working steps are needed: (i) large-scale culturing of adenovirus from master adenovirus bank; (ii) culturing of cell from master cell bank; (iii) purification. The whole process including step (i) to step (iii) takes approximately twenty-four days to make reagent (“original liquid”). This particular liquid can only be stored for approximately five years. It takes approximately another seven days for mixing and bottling original liquid to finished goods which is known as Gendicine.
Therefore, up to the stage of reagent, all the related production costs are treated as work-in-progress. The major components of those production costs are: (i) direct labor; (ii) direct materials; (iii) power; (iv) supplies and other materials; and (v) manufacturing overheads.
Before acquisition, as of March 31, 2007, the accumulated units of original liquid produced was 198,075 and which could be converted to approximately 226,736 vials of Gendicine. However, the accumulated vials of Gendicine sold throughout the years 2004 to three-month period ended March 31, 2007 were only approximately 18,424 vials. Thus the accumulated production costs of $4,080,644 were remained as work-in-process as of March 31, 2007.
Furthermore, due to the special feature of the original liquid which can only be stored for five years, and most of the original liquid was produced in the year of 2004, and the provision of reserve on work- in-progress was $3,696,083 as of March 31, 2007.
After the acquisition with the effective date April 1, 2007, the same accounting treatment was adopted for the treatment of the provision of reserve on work-in-progress. As of June 30, 2009, the provision of reserve on work-in-process was $4,551,337.
A reserve for obsolete, slow-moving or non-salable inventory was made on raw materials, packing materials, other material and suppliers, finished goods and work-in-process at the amount of $11,078, $54,120, $57,651, $13,552 and $4,551,337 respectively as of June 30, 2009.
Management determination of this allowance was based on potential impairments to the current carrying value of the inventories due to potential obsolescence of aged inventories. In making its estimate, management considered the probable demand for our products in the future and historical trends in the turnover of our inventories. While the Company currently believes that there is little likelihood that actual results will differ materially from these current estimates.
Operational Results
Six months and three months ended June 30, 2009 Compared to Six months and three months ended June 30, 2008
The following table provides key components of our operational results for the six months and three months ended June 30, 2009 and 2008 for Benda Pharmaceutical, Inc.
| | | SIX MONTHS ENDED JUNE 30, | | | THREE MONTHS ENDED JUNE 30, | |
| | | 2009 | | | 2008 | | | 2009 | | | 2008 | |
| | | (Unaudited) | | | (Unaudited) | | | (Unaudited) | | | (Unaudited) | |
| Revenue | | $ | 11,440,412 | | | $ | 13,225,623 | | | $ | 5,924,609 | | | $ | 7,278,253 | |
| Cost of goods sold | | | (6,699,079 | ) | | | (8,396,854 | ) | | | (3,556,288 | ) | | | (4,579,608 | ) |
| Gross profit | | | 4,741,333 | | | | 4,828,769 | | | | 2,368,321 | | | | 2,698,645 | |
| | | | | | | | | | | | | | | | | |
| Selling expenses | | | (1,016,366 | ) | | | (1,431,212 | ) | | | (622,173 | ) | | | (994,841 | ) |
| | | | | | | | | | | | | | | | | |
| General and administravive expenses | | | (4,480,213 | ) | | | (4,521,240 | ) | | | (3,130,091 | ) | | | (1,878,322 | ) |
| Research and development expenses | | | (118,083 | ) | | | (169,781 | ) | | | 183,601 | | | | (147,717 | ) |
| Total operating expenses | | | (5,614,662 | ) | | | (6,122,233 | ) | | | (3,568,663 | ) | | | (3,020,880 | ) |
| Operating income / (loss) | | | (873,329 | ) | | | (1,293,464 | ) | | | (1,200,342 | ) | | | (322,235 | ) |
| | | | | | | | | | | | | | | | | |
| Interest expenses | | | (1,633,247 | ) | | | (2,340,580 | ) | | | (362,866 | ) | | | (1,128,298 | ) |
| Other income (expenses) | | | 45,776 | | | | 248,058 | | | | 62,976 | | | | (53,635 | ) |
| Government subsidies / grants | | | 26,383 | | | | - | | | | - | | | | - | |
| | | | | | | | | | | | | | | | | |
| Loss before provision for income taxes and noncontrolling interest | | | (2,434,417 | ) | | | (3,385,986 | ) | | | (1,500,232 | ) | | | (1,504,168 | ) |
| Income taxes | | | (84,412 | ) | | | (524,808 | | | | 217,966 | | | | (391,611 | ) |
| Net loss | | | (2,518,829 | ) | | | (3,910,794 | ) | | | (1,282,266 | ) | | | (1,895,779 | ) |
| | | | | | | | | | | | | | | | | |
| Net loss attributable to the noncontrolling interest | | | 202,574 | | | | 88,216 | | | | 6,568 | | | | 110,614 | |
| | | | | | | | | | | | | | | | | |
| Net loss attributable to Benda Pharmaceutical, Inc. | | $ | (2,316,255 | ) | | $ | (3,822,578 | ) | | $ | (1,275,698 | ) | | $ | (1,785,165 | ) |
| | | | | | | | | | | | | | | | | |
| Basic and fully diluted loss per share | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| Net loss attributable to Benda Pharmaceutical, Inc. common shareholders | | $ | (0.02 | ) | | $ | (0.04 | ) | | $ | (0.01 | ) | | $ | (0.02 | ) |
| | | | | | | | | | | | | | | | | |
| Weighted average shares outstanding, basic and fully diluted | | | 105,155,355 | | | | 100,558,191 | | | | 105,155,355 | | | | 100,803,509 | |
Net Revenue:
The Company has five core operating segments: Benda Ebei, Jiangling Benda, Yidu Benda, Beijing Shusai and SiBiono. Benda Ebei manufactures branded/generic medicines; Jiangling Benda manufactures active pharmaceutical ingredients (API); Yidu Benda manufactures bulk chemicals; Beijing Shusai operates and distributes Pharyngitis Killer Therapy; and SiBiono is a gene therapy company dedicated to the development, manufacturing and commercialization of gene therapy product, Gedicine.
Net revenue decreased by $1.79 million (or 13.5%) to $11.44 million for the six months ended June 30, 2009 from $13.23 million for the six months ended June 30, 2008, while the net revenue decreased by $1.35 million (or 18.6%) to $5.92 million for three months ended June 30, 2009 from $7.28 million for three months ended June 30, 2008 Decrease in revenue is principally attributed by the following factors:
| 1. | One of the Benda’s subsidiaries, Benda Ebei’s net revenue decreased $2.74 million (or 23.88%) and $1.57 million (or 26.43%) to $8.73 million and $4.37 million for the six months and three months ended June 30, 2009 respectively from $11.47 million and $5.94 million for the six months and three months ended June 30, 2008 respectively. It was mainly due to the keen competition in generic medicine and the seasonal factors; however the management believes that the overall year revenue of 2009 will remain the same level as year 2008. |
| 2. | One of the Benda’s subsidiaries, Jiangling Benda which resumed its production in October 2007 and achieved $0.8 million for the six months ended June 30, 2009. |
Jiangling Benda plans to produce four types of active pharmaceutical ingredients and they are Ribavirin, Asarin, Levofloxacin and Ribose whereas the production of Ribose does not require the GMP certificate, but the production of the other three products do require the GMP certificate.
On April 9, 2008, Jiangling Benda received the approved GMP Certificate from the Chinese State Food and Drug Administration ("SFDA") which authorizing the production of Ribavrin. The other two products, Asarin and Levolfozacin, are still under the stage of GMP certificate approving process. The management could not estimate the exact timing for obtaining those certificates.
| 3. | One of the Benda’s subsidiaries, Yidu Benda ceased operation due to the fact that the plant was closed since mid January of 2007 to upgrade its waster water treatment system to comply with new environmental standards enforced by PRC local government. |
Yidu Benda has completed its upgrading of the waster water system and passed the government’s verification and testing of equipments in October 2007. It is now permitted for the testing on actual production process. Once the actual products are produced, then the environmental government bodies will re-test the production results. The management could not estimate the exact timing for obtaining the final approval on the actual production process. Furthermore, the management is searching for new products to be produced in Yidu Benda which with higher profit margin.
| 4. | One of the Benda’s subsidiaries, Beijing Shusai which incorporated on July 15, 2006. China’s State Food and Drug Administration (SFDA) recently experienced an overhaul in its policies and regulatory systems in an effort to fight against corruption in Chinese pharmaceutical industry. Beijing Shusai’s operation has been adversely affected by this recent policy changes which prohibits some state-owned hospitals from forming alliances with private companies. The management could not estimate that such situation could be resolved in the coming future. |
| 5. | One of the Benda’s subsidiaries, SiBiono, acquired and effective since April 1, 2007, net revenue increased $0.74 million (or 62.71%) and $0.44 million (or 58.67%) to $1.92 million and $1.19 million for the six months ended and three months ended June 30, 2009 respectively from $1.18 million and $0.75 million for the six months and three months ended June 30, 2008 respectively. The main reason for the increase in net revenue is mainly due to the fact that SiBiono was previously underwent a process of re-engineering of the production department during the year of 2008 which improved its production process and also the company put more effort in the marketing |
SiBiono GMP - On October 16, 2003, SiBiono successfully obtained a New Drug License from the State Food & Drug Administration of China (SFDA), and then, in April 4, 2004, SiBiono obtained “Manufacture Certificate” and “Certificate of GMP for Pharmaceutical Product”, so far being fully qualified for the market launch of Recombinant Human Ad-p53 Injection, trademarked as Gendicine ® in China. Gendicine ® is the commercialized gene therapy product approved in the PRC government agency. On May 19, 2008, SiBiono received an official notice from the PRC State of SFDA in which it mentioned that during the random inspection performed by the PRC State of SFDA on April 8 to April 10, 2008, the PRC State of SFDA discovered there were several production procedures that did not meet the requirement stated in GMP, thus it required SiBiono to perform necessary improvements in order to fulfill the GMP requirements and the PRC State of SFDA collected back the distributed GMP certificate until the necessary improvements being carried out and passed the examination that conducted by SFDA. On June 10, 2008, SiBiono received another official notice from Guangdong Province SFDA and they demanded the same requirements as stated in the official notice which issued by the PRC State of SFDA dated on May 19, 2008. On November 24, 2008, SiBiono received another official notice from Guangdong Province SFDA which mentioned that after the examination conducted by Shenzhen City SFDA, the Guangdong Province SFDA consent SiBiono to carry out production on a trial basis. It further required SiBiono strictly to follow the requirements of GMP to organize trail production and follow the procedures to apply for GMP Certificate verification.
On July 14, 2009, SiBiono obtained the final approved GMP Certificate, in order words, the SFDA allows SiBiono to resume its production and sales.
Cost of Goods Sold
Cost of goods sold decreased $1.7 million (or 20.23%) and $1.02 million (or 22.27%) to $6.7 million and $3.56 million for the six months and three months ended June 30, 2009 respectively from $8.4 million and $4.58 million for the six months and three months ended June 30, 2008 respectively, primarily due to the decreased in the sales volume in Benda Ebei.
Gross Profit
Gross profit decreased $0.09 million (or 1.86%) and $0.32 million (or 11.89%) to $4.74 million and $2.37 million for the six months and three months ended June 30, 2009 respectively from $4.83 million and $2.69 million for the six months and three months ended June 30, 2008 respectively, which was mainly due to the keen competition of generic medicine products causing a slightly decrease in selling price.
Selling Expenses:
Selling expenses slightly decreased $0.41 million (or 28.67%) and $0.37 million (or 37.37%) to $1.02 million and $0.62 million for the six months and three months ended June 30, 2009 respectively from $1.43 million and $0.99 million for the six months and three months ended June 30, 2008 respectively, primarily due to the management performed cost control in the reporting period.
General and Administrative Expenses:
General and administrative for the six months ended June 30, 2009 reported at the same level as for the six months ended June 30, 2008. For the six months ended June 30, 3009, the expenses were $4.48 million while the expenses for the six months ended June 30, 2008 were $4.52 million. The General and administrative increased $1.25 million (or 66.49%) to $3.13 million for the three months ended June 30, 2009 from $1.88 million for the three months ended June 30, 2008. It was primary due to the fact that the company was facing a global financial crisis, thus an amount approximately $2.55 million was provided for bad debt provision. However, the company will put more efforts to improve and manage the situation onwards.
Operating Income / (Loss):
The Company resulted an operating loss $0.87 million and $1.2 million for the six months and three months ended June 30, 2009 respectively, while the operating loss from comparative period for 2008 was $1.29 million and $0.32 million which was mainly due to decreased in net revenue during the reporting periods.
Interest Expenses:
Interest expenses was $1.63 million and $0.36 million for the six months and three months ended June 30, 2009 respectively while the interest expenses was $2.34 million and $1.13 million for the comparative period of 2008 respectively. The main component of it was the financing cost associated with the issuance of convertible promissory note.
For the three months ended June 30, 2009, $0.54 million was incurred for the interest of the notes and $0.86 million in interest expense related to the amortization of the debt discount associated with the Warrants and the debt discount associated with the beneficial conversion feature. (Please refer to Note 24 of the Notes to Consolidation Financial Statements for details).
Other Income / (Expenses)
Due to the Registration Delay Penalty, the Group incurred an expense for the six months ended June 30, 2008. Out of the total penalty, $230,312 was settled by issuance of 523,438 shares of common stock. There was no penalty to investors incurred for the six months ended June 30, 2009.
Income Taxes:
Benda is subject to Delaware, United State of America tax, but no provision for income taxes were made for the six months and three months ended June 30, 2009 and 2008 as Benda did not have reportable taxable income for the period.
Ever Leader, a wholly owned subsidiary of Benda, is subject to Hong Kong tax, but no provisions for income taxes were made for the six months and three months ended 2009 and 2008 as Ever Leader did not have reportable taxable income for the periods.
Benda Ebei was registered as a Sino-Foreign Equity Joint Venture on May 26, 2004 and is subject to the tax laws applicable to Sino-Foreign Equity Joint Ventures in the PRC. Benda Ebei, starting from 2005, is fully exempt from PRC enterprise income tax for two years starting from the first profit-making year, followed by a 50% reduction in the state income taxes, for the following three years, commencing from the first profitable year.
Jiangling Benda and Yidu Benda are cross-municipal investment entities and enjoy the same tax treatment as Sino-Foreign Joint Ventures, starting from 2005, and were therefore exempt from PRC enterprise income tax for two years starting from the first profit-making year, followed by a 50% reduction in the state income taxes, for the following three years, commencing from the first profitable year. Cross-municipal investments entities refer to entities that are incorporated in one municipal region but have investments in another municipal region.
The exemption periods for Benda Ebei, Jiangling Benda and Yidu Benda expired in the year of 2006, after which they are subject to a 50% reduction in state income taxes, at 18%; whereas the full income tax rate is 33%. The remaining tax holidays will be expired in 2010.
However, starting and effective from January 1, 2008, the full income tax rate would be changed from 33% to 25% according to the new PRC taxation regulations. Therefore these subsidiaries will be subject to the regular full income tax rate at 25% after the tax holidays expire in 2010.
According to the new taxation regulations starting and effective from January 1, 2008, Beijing Shusai is subject to the full income tax rate of 25%.
According to the new taxation regulations starting and effective from January 1, 2008, SiBiono, which is located in Shenzhen, a Special Economic District of PRC, is subject to the full income tax rate of 25% gradually in five years as following:
| Year | | Tax rate | |
| | | | |
| 2008 | | | 18 | % |
| | | | | |
| 2009 | | | 20 | % |
| | | | | |
| 2010 | | | 22 | % |
| | | | | |
| 2011 | | | 24 | % |
| | | | | |
| 2012 and thereafter | | | 25 | % |
Benda Ebei recorded $84,412 income tax for the six months ended June 30, 2009. The prepaid income tax by the Group as of June 30, 2009 at amount of $1.59 million was recorded as taxes recoverable.
LIQUIDITY AND CAPITAL RESOURCES
Net cash provided by the operating activities was $1.42 million for the six months ended June 30, 2009, while for the six months ended June 30, 2008 was negative $1.76 million
| a) | Non-cash operating activities, reconciliation items to the net income |
For the six months ended June 30, 2008, an amount about $4.37 million non-cash operating activities was reconciled back to the net income and which mainly included amortization of debt discount and debt issue cost, penalty payment made in form of share issuance, bad debt provision, amortization of intangible assets, and depreciation.
However, for the six months ended June 30, 2009, about $5.2 million of non-cash operating activities was reconciled back to the net income and summarized as follows:
| 1. | $0.86 million that incurred as interest expenses related to the amortization of discount on long-term debt and issuance cost associated with the warrants and the beneficial conversion features (please refer to Note 24 of the Notes to Consolidated Financial Statements for details); and |
| | Other factors: $2.8 million incurred on bad debt provision; $0.98 million incurred on depreciation; and $0.36 million incurred on amortization of intangible assets. |
The net change of trade receivable was increased by $1.49 million for the six months ended June 30, 2009. The management also noticed that the net balance of the trade receivable, as of June 30, 2009, was a significant asset to the company. However, the management believes that the above situation is temporarily due to the following reasons:
Trade receivables
| a) | Customers whom have sales relationship with our company are all relatively big business wholesale enterprises and they have all passed the examination of GMP Certificate so that the collectibility from those is out of question; |
| | The management realized that it did affect the cash flow situation of the company; therefore the company will put more efforts to reduce the balance of trade receivables. |
For the six months ended June 30, 2009 and 2008, the amount spent in investing activities were $0.46 million and $0.21 million respectively. It was mainly because most of the settlements were made in the year of 2007, thus the investing activities were relatively small for the reporting periods.
Financing cash outflow was $0.06 million for the reporting period six months ended June 30, 2009 while the financing cash inflow was $1.2 million for the reporting period six months ended June 30, 2008. The major of it was a net amount $1.8 million was received from the commercial bank notes in the six months ended June 30, 2008. (Please refer to the Note 11 of the Notes to Consolidated Financial Statements for the details)
Item 3. Quantitative and Qualitative Disclosures About Market Risk
The Company is subject to certain market risks, including changes in interest rates and currency exchange rates. The Company does not undertake any specific actions to limit those exposures.
Item 4T. Evaluation of Disclosure Controls and Procedures
a) Evaluation of Disclosure Controls. Our Chief Executive Officer and Chief Accounting Officer evaluated the effectiveness of our disclosure controls and procedures as of the end of our second fiscal quarter 2009 pursuant to Rule 13a-15(b) of the Securities and Exchange Act. Disclosure controls and procedures are controls and other procedures that are designed to ensure that information required to be disclosed by us in the reports that we file or submit under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed by us in the reports that we file under the Exchange Act is accumulated and communicated to our management, as appropriate to allow timely decisions regarding required disclosure. Based on his evaluation, our Chief Executive Officer and Chief Accounting Officer concluded that our disclosure controls and procedures were effective as of June 30, 2009.
It should be noted that any system of controls, however well designed and operated, can provide only reasonable, and not absolute, assurance that the objectives of the system are met. In addition, the design of any control system is based in part upon certain assumptions about the likelihood of future events. Because of these and other inherent limitations of control systems, there can be no assurance that any design will succeed in achieving its stated goals under all potential future conditions
(b) Changes in internal control over financial reporting. There have been no changes in our internal control over financial reporting that occurred during the first fiscal quarter that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting. Our management team will continue to evaluate our internal control over financial reporting in 2009 as we implement our Sarbanes Oxley Act testing.
PART II - OTHER INFORMATION
Item 1. Legal Proceedings.
1. The Company has become aware that Excalibur Limited Partnership and Excalibur Limited Partnership II (the "Plaintiffs") filed a motion for summary judgment in lieu of a complaint pursuant to CPLR § 3213 (the "Motion") with the Supreme Court of the State of New York (the "Court"), alleging that the Company has been delinquent on the payment of an aggregate sum of $600,000 and accrued interest and costs arising from the Convertible Promissory Notes that were issued to the Plaintiffs in April 2007 in connection with a $7,560,000 private placement. Pursuant to the motion, the Plaintiffs requested that the Court (1) enter summary judgment in favor of Excalibur Limited Partnership (“Excalibur Limited”) in the amount of $390,000 plus all accrued interest and costs, and, (2) enter summary judgment in favor of Excalibur Small Cap Opportunities LP (“Excalibur Small Cap”) in the amount of $210,000 and accrued interest and costs. On July 29, 2009, the Court entered a judgment against the Company in favor of the Plaintiffs in the amount of $674,251.65 in connection with the Convertible Promissory Notes issued to the Plaintiffs in April 2007 in a private placement.
On March 4, 2009, the Company received a Notice and Default and Payment Demand letter (the "Default Letter") from Pope Investments LLC ("Pope") in connection with its convertible promissory note in the amount of $5,520,000 (the "Note") purchased in our April 2007 private placement offering. The Default Letter provided notice of default based on the Company's failure to make a required interest payment on the Note by February 20, 2009. The Default Letter further demanded full payment of all interest, liquidated damages and accrued interest thereon in the amount of $130,364.37 by March 14, 2009, or Pope will accelerate the maturity date of the full principal amount of the Note. On April 7, 2009, the Company received further Default Letters and Payment Demand from Pope, Excalibur Limited and Excalibur Small Cap demanding payment in full of the balance of the Notes, which matured on March 28, 2009. We were notified on June 15, 2009, that on May 11, 2009 Pope filed a motion for summary judgment in lieu of a complaint against us pursuant to CPLR § 3213 (the “Motion”) with the Supreme Court of the State of New York (the “Court”), alleging that we have been delinquent on the payment of an aggregate sum of $5,520,000 and accrued interest and costs arising from the Note that we issued to the Pope in April 2007. Pursuant to the motion, the Plaintiffs requested that the Court enter summary judgment in favor of Plaintiff in the amount of $5,994,617.53 constituting principal and interest, plus costs.
On June 23, 2009, we filed an Affidavit in Opposition to Motion for Summary Judgment in Lieu of Complaint with the Court requesting that Plaintiff’s Motion be denied.
On July 30, 2009, we received a Notice of Default from three additional investors in the April 2007 private placement offering holding Notes totaling $90,000.
| 2. | On November 23, 2006, Benda Ebei entered into an Equity Transfer Agreement with Xiaozhi Zhang (“Zhang”), to purchase approximately 6.24% of SiBiono’s common stock for a total consideration of Rmb12.48 million (Rmb6.24 million in cash and shares of our common stock equal to Rmb6.24 million) (or $1.71 million) which was due and payable on or before March 31, 2007. |
Due to the fact that the signed agreement on November 23, 2006 was not practically executable according to the PRC regulations, Benda Ebei asked Zhang to terminate the signed agreement and sign a new agreement that was feasible under PRC regulations with essentially the same terms.
However, Zhang refused to sign the new agreement and applied to the Shenzhen Arbitration Commission (the “Commission”) in April 2007 for enforcement of the original agreement. Zhang requested the Commission to require Benda Ebei to pay for the total consideration, penalty for late payment and the related legal and arbitration expenses.
On November 27, 2007, Shenzhen Arbitration Commission determined that:
| 1. | Benda Ebei should pay for the consideration of Rmb 6.24 million, equal to 50% of the total consideration set forth in the Equity Transfer Agreement. For the other 50% of the total consideration which was supposed to be settled in the form of issuing common stock, since Zhang did not make an arbitration request on how to execute the arrangement, the Arbitration Commission did not make an award on this particular part. |
| 2. | Benda Ebei should pay for the penalty of Rmb 46,800; |
| 3. | Benda Eebi should pay for legal and arbitration expenses of Rmb 268,971. |
On May 22, 2008, Benda Ebei applied to Shenzhen People Court to terminate above mentioned arbitration. The termination is based on the ground that Xiaozhi Zhang does not own all 6.24% of SiBiono’s common stock. In fact, he only owns 3.28% of SiBiono’s stock. The application has been accepted by Shenzhen People Court and is waiting for its further investigation..
| 3. | SiBiono patents - on January 29, 2008, SiBiono entrusted Grandall Legal Group Shenzhen Law Firm to issue a legal letter to Zhaohui Peng, one of the shareholders of Sibiono and the inventor of Gendicine, demanding that he transfer all the title of patents to SiBiono. |
On June 18, 1999, during the formation of SiBiono, Zhaohui Peng transferred the rights to the patent “A new method for manufacturing recombinant adenovirus” and related research results to SiBiono as a payment for the registered capital. In return, Zhaohui Peng was granted 32.03% of the common stock of SiBiono.
From 1999 to 2007, SiBiono successfully obtained various technology funds from various government technology agencies to support the further research and development activities of Gendicine. Due to this significant funding obtained by SiBiono, Sibiono developed five additional patents which are summarized as follows:
| Item | | Patent name | | Countries / Date | | Application Number (1) | | Publication Number (2) | | Approved Patent Number (3) | | Name of Patent Inventor (6) | | Name of Applicant (6) | | Patent Assignees |
| 1 | | A new method for manufacturing recombinant adenovirus | | | | | | | | | | | | | | |
| | | A | | China | | 98123346.5 | | CN1228474A | | ZL98123346.5 | | Peng | | Peng | | SiBiono |
| | | | | Date | | 1998/12/14 | | 1999/9/15 | | 2002/7/3 | | | | | | |
| | | | | | | | | | | | | | | | | |
| 2 | | A recombinant constructed by a virus vector and a human tumor suppressor gene and its use | | | | | | | | | | | | | | |
| | | A | | China | | 02115228.4 | | CN1401778A | | ZL02115228.4 | | Peng / Zhang | | Peng / Zhang | | Peng / Zhang |
| | | | | Date | | 2002/5/8 | | 2003/3/12 | | 2004/11/24 | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | B | | PCT (4) | | 5 | | WO2004/078987A1 | | Not Approved | | Peng / Zhang | | Peng / Zhang | | N/A |
| | | | | Date | | 2004/3/8 | | 2004/9/16 | | N/A | | | | | | |
| | | | | | | | | | | | | | | | | |
| 3 | | Recombinant gene medicine of adenovirus vector and and gene p54 for treating proloferative diseases | | | | | | | | | | | | | | |
| | | A | | China | | 03125129.3 | | CN1471977A | | ZL03125129.3 | | Peng / Zhang | | Peng / Zhang | | Peng / Zhang |
| | | | | Date | | 2003/5/10 | | 2004/2/4 | | 2007/7/25 | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | B | | PCT (4) | | 8 | | WO2004/104204A1 | | Not Approved | | Peng / Zhang | | Peng / Zhang | | N/A |
| | | | | Date | | 2004/5/9 | | 2004/12/2 | | N/A | | | | | | |
| | | | | | | | | | | | | | | | | |
| 4 | | The application of recombinant adenoviral p53 as cancer vaccine (tentative title) | | | | | | | | | | | | | | |
| | | A | | China | | 200510002779.1 | | CN1679641A | | ZL200510002779.1 | | Peng / Zhang | | Peng / Zhang | | Peng / Zhang |
| | | | | Date | | 2005/1/26 | | 2005/10/12 | | 2007/8/29 | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | B | | PCT (4) | | 1 | | WO2006/079244A1 | | Not Approved | | Peng / Zhang | | Peng / Zhang | | N/A |
| | | | | Date | | 2005/1/26 | | 2006/8/3 | | N/A | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | C | | US (5) | | 11/075035 | | 2005/0281785A1 | | Not Approved | | Peng / Zhang | | Unidentified Yet | | N/A |
| | | | | Date | | 2005/3/7 | | 2005/12/22 | | N/A | | | | | | |
| | | | | | | | | | | | | | | | | |
| 5 | | Human Embryonic Kidney (HEK) sub-clone cell line | | | | | | | | | | | | | | |
| | | A | | China | | 03126889.7 | | CN1513985A | | Not Approved | | Peng / Zhang | | Peng / Zhang | | N/A |
| | | | | Date | | 2003/6/13 | | 2004/7/21 | | N/A | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | B | | PCT (4) | | 7 | | WO2004/111239 | | Not Approved | | Peng / Zhang | | Peng / Zhang | | N/A |
| | | | | Date | | 2004/5/9 | | 2004/12/23 | | N/A | | | | | | |
| | | | | | | | | | | | | | | | | |
| 6 | | The complex of polypeptide liposome and human VGEF gene, and its use and human VGEF gene, and its use | | | | | | | | | | | | | | |
| | | A | | China | | 02134321.7 | | CN1389269A | | Not Approved | | Peng / Zhang / Zhu | | Peng / Zhang / Zhu | | N/A |
| | | | | Date | | 2002/7/4 | | 2003/1/8 | | N/A | | | | | | |
Note:
(1) Application number is obtained when application is submitted;
(2) Publication number is obtained after the first phase examination;
(3) Approved patent number is obtained after the final examination;
(4) PCT is referred to an International Patent Organization in Paris;
(5) US is referred to the application is made in United States of America alone;
(6) Peng is referred to Zhaohui Peng; Zhang is referred to Xiaozhi Zhang; Zhu is referred to Jinya Zhu.
As indicated in the above table, Item 1, the patent “A new method for manufacturing recombinant adenovirus” had been assigned to SiBiono; however, the other approved patents (item 2 through item 4) in PRC still have not been assigned to SiBiono. The Group believes that all the above mentioned patents should be rightfully transferred to SiBiono, a subsidiary of the Group. Accordingly, the above mentioned legal letter was issued on this ground.
On August 27, 2008, the Group through its subsidiary, SiBiono filed an application to the Guongdong Province Shenzhen City (Middle) Peoples’ Court and demand Zhaozhu Peng to transfer back all the mentioned patents that mentioned in above to SiBiono. The case has been accepted by the Court and is waiting for its further investigation.
Except as disclosed above, we are not aware of any pending or threatened legal proceedings in which we are involved.
Item 2. Unregistered Sales of Equity Securities and Use of Proceeds
None.
Item 3. Defaults Upon Senior Securities.
The Company has become aware that Excalibur Limited Partnership and Excalibur Limited Partnership II (the "Plaintiffs") filed a motion for summary judgment in lieu of a complaint pursuant to CPLR § 3213 (the "Motion") with the Supreme Court of the State of New York (the "Court"), alleging that the Company has been delinquent on the payment of an aggregate sum of $600,000 and accrued interest and costs arising from the Convertible Promissory Notes that were issued to the Plaintiffs in April 2007 in connection with a $7,560,000 private placement. Pursuant to the motion, the Plaintiffs requested that the Court (1) enter summary judgment in favor of Excalibur Limited Partnership (“Excalibur Limited”) in the amount of $390,000 plus all accrued interest and costs, and, (2) enter summary judgment in favor of Excalibur Small Cap Opportunities LP (“Excalibur Small Cap”) in the amount of $210,000 and accrued interest and costs. On July 29, 2009, the Court entered a judgment against the Company in favor of the Plaintiffs in the amount of $674,251.65 in connection with the Convertible Promissory Notes issued to the Plaintiffs in April 2007 in a private placement.
On March 4, 2009, the Company received a Notice and Default and Payment Demand letter (the "Default Letter") from Pope Investments LLC ("Pope") in connection with its convertible promissory note in the amount of $5,520,000 (the "Note") purchased in our April 2007 private placement offering. The Default Letter provided notice of default based on the Company's failure to make a required interest payment on the Note by February 20, 2009. The Default Letter further demanded full payment of all interest, liquidated damages and accrued interest thereon in the amount of $130,364.37 by March 14, 2009, or Pope will accelerate the maturity date of the full principal amount of the Note. On April 7, 2009, the Company received further Default Letters and Payment Demand from Pope, Excalibur Limited and Excalibur Small Cap demanding payment in full of the balance of the Notes, which matured on March 28, 2009. We were notified on June 15, 2009, that on May 11, 2009 Pope filed a motion for summary judgment in lieu of a complaint against us pursuant to CPLR § 3213 (the “Motion”) with the Supreme Court of the State of New York (the “Court”), alleging that we have been delinquent on the payment of an aggregate sum of $5,520,000 and accrued interest and costs arising from the Note that we issued to the Pope in April 2007. Pursuant to the motion, the Plaintiffs requested that the Court enter summary judgment in favor of Plaintiff in the amount of $5,994,617.53 constituting principal and interest, plus costs.
On June 23, 2009, we filed an Affidavit in Opposition to Motion for Summary Judgment in Lieu of Complaint with the Court requesting that Plaintiff’s Motion be denied.
On July 30, 2009, we received a Notice of Default from three additional investors in the April 2007 private placement offering holding Notes totaling $90,000.
Item 4. Submission of Matters to a Vote of Security Holders.
None.
Item 5. Other Information.
None
Item 6. Exhibits and Reports of Form 8-K.
(a) Exhibits
31.1 Certifications pursuant to Section 302 of Sarbanes Oxley Act of 2002
32.1 Certifications pursuant to Section 906 of Sarbanes Oxley Act of 2002
(b) Reports of Form 8-K
On May 13, 2009, the Company filed a Form 8-K with the SEC disclosing the resignation of a director.
On June 1, 2009, the Company filed a Form 8-K with the SEC disclosing that a summary judgment motion was granted against the Company.
SIGNATURES
In accordance with Section 13 or 15(d) of the Exchange Act, the registrant caused this report to be signed on its behalf by the undersigned, there unto duly authorized.
| | | BENDA PHARMACEUTICAL, INC. |
| | | Registrant |
| | | |
| Date: August 19, 2009 | | By: /s/ Yiqing Wan |
| | | Yiqing Wan |
| | | President, Chief Executive Officer |
| | | Chairman of Board of Directors |
| Date: August 19, 2009 | By: | /s/ Eric Yu |
| | | Eric Yu |
| | | Chief Financial Officer |
| | | Principal Accounting Officer |
| | | Director |