
www.skyonemedical.com
China Sky One Medical Inc.
(OTCBB:CSKI)
Confidential Investment Presentation
1
Safe Harbor Statement
This presentation contains "forward-looking statements" within the meaning of the “safe-
harbor” provisions of the Private Securities Litigation Reform Act of 1995. Such
statements involve known and unknown risks, uncertainties and other factors that could
cause the actual results of the Company to differ materially from the results expressed or
implied by such statements, including changes from anticipated levels of sales, future
international, national or regional economic and competitive conditions, changes in
relationships with customers, access to capital, difficulties in developing and marketing new
products and services, marketing existing products and services, customer acceptance of
existing and new products and services and other factors. Accordingly, although the
Company believes that the expectations reflected in such forward-looking statements are
reasonable, there can be no assurance that such expectations will prove to be correct. The
Company has no obligation to update the forward-looking information contained in this
presentation.
2
Offering Summary
Use of Proceeds
Capitalization Table
Company Overview
Investment Highlights
Market Overview
Company History
Product Overview
Research & Development
Distribution Platform
Government Support
Production Capacity
Financial Highlights
Acquisition Targets
Management Profile
Overview
3
Offering Summary
Issuer: China Sky One Medical, Inc
Symbol: CSKI/OTCBB
Market Capitalization: $124.6 Million*
Shares Outstanding: 12.1 Million
Offering: Private Investment in Public Equities
Securities Offered: Common Stock
Amount : $30.0 Million
Use of Proceeds: Acquisitions & Working Capital
*Based on November 8, 2007 share price of $10.30
4
Use of Proceeds
Acquisitions
Target 1: $ 8.0 Million
Target 2: $10.0 Million
Working Capital: $ 4.0 Million
New Product Marketing: $ 8.0 Million
Total $30.0 Million
5
Capitalization Table
(Amounts in Millions)
June 30, 2007
Cash
$5.15
Debt
$0.00
Shareholder Equity
$20.98
Total Shares Outstanding
12.10
Warrants
1.64
(1)
Options
0.16
(2)
Fully Diluted
13.90
Shares held by insiders
52%
Restricted Shares
(3)
28%
Free Float
20%
(1)
Warrants with a weighted average exercise price of $2.55 @ expiration of 10/08 - 7/09
(2)
Options with a weighted average exercise price of $3.45 @ expiration of 12/08 - 1/10
(3)
Restricted stock issued May 31, 2006
6

Company Overview
China Sky One Medical
Harbin, Heilongjiang, China
45,000 Square Meters
US Auditor:
Murrell, Hall, McIntosh & Co, PLLP
US Attorney:
Hodgson Russ, LLP
Placement Agent:
Global Hunter Securities, LLC
IR Firm:
Integrated Corporate Relations, Inc
China Sky One Medical manufactures, markets and distributes
proprietary over-the-counter Traditional Chinese Medicines (“TCM”) and
biological diagnostic kits, and markets and distributes 3rd party
manufactured products.
7
Investment Highlights
Highly favorable market conditions
Pharmaceutical sales growth outpacing GDP growth
Highly robust organic top line growth
2006: 157%
2007E: 137%
Extensive distribution network
1,400 Sales Reps, 50 Distributors, >20 Countries
Diverse portfolio of SFDA approved products
70+ OTC products: TCM, Western medicines & diagnostic kits
Robust R&D pipeline
18 biological diagnostic kits to launch in near term
Well known brands
“TDR” and “Kangxi” ubiquitous
Strong government support
$3.3 Million in grants issued in 2007
Experienced management team
8
Market Overview
Rapidly Growing Industry
China’s total healthcare expenditures exceeded $100 Billion in
2005
Pharmaceutical spending outpacing GDP growth
00-04 CAGR: GDP:11.2%, Pharmaceutical spending:15.4%
World’s fifth largest pharmaceutical market by 2010
World’s second largest healthcare market by 2020
Government Imperatives
The government supports innovative projects within the
biomedical industry, including:
Biomedical engineering projects, such as oncology and
cardiology diagnostic products
Modernizing TCM production
Developing indigenous biotechnology capabilities
Changing Demographics
Rising levels of disposable income
Continued emphasis on self-care
Aging
9
Industry Overview
Traditional Chinese Medicine
The TCM Market is a large and well established component of
China’s healthcare industry and culture
TCM Market reached $16.8 Billion in 2006
Mainstream form of medicine in China; roots reaching back
thousands of years
Plant-based medicine is a component of TCM based on
materials from roots, stems and leaves
Effective with fewer side-effects
Commonly indicated for chronic and recurring diseases
Government support for modernizing TCM production
10
Favorable Industry Dynamics
Highly Fragmented Industry
Over 1,200 TCM manufacturers
Numerous privately-held companies with limited access to
growth capital
Combined sales of top three manufacturers account for only
5.5% of total market
Rigorous Regulatory Policies Force Smaller Players to
Consider M&A Alternatives
Obligation to meet Good Manufacturing Practice (“GMP”)
standards requirements drains cash
Significant Consolidation Opportunities for Companies
with:
Fully integrated infrastructure
Leveragable sales and distribution platform
Brand name recognition
Access to growth capital
11
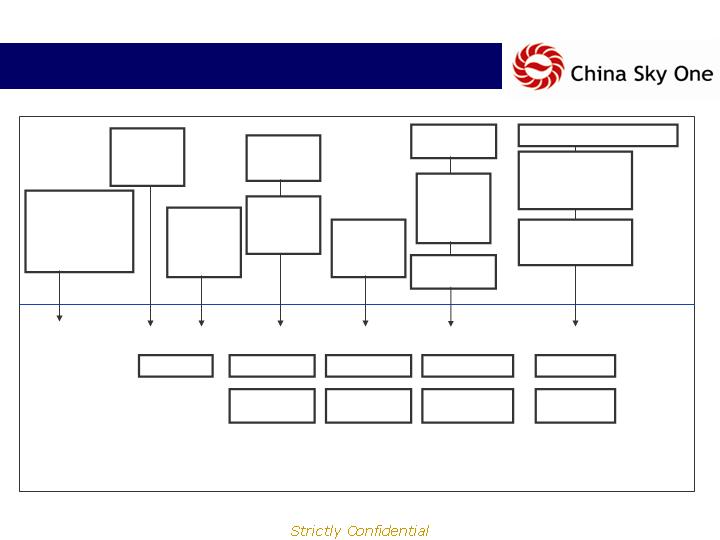
History
1996 2000 2001 2003 2005 2006 2007
Tian Di Ren
Medicine Sales
formed: TCM and
Western medicine
sales and
distribution
TDR adds
R&D and
proprietary
pipeline
Kangxi
subsidiary
formed
with 4
products
First Bio
subsidiary
formed
TDR adds
biological
R&D
initiatives
ACPG
formed for
reverse
merger
CSKI debuts
on OTCBB
Two
biological
product
lines
launched
Kangxi and
First merge
Breaks ground for
new R&D center
(30,000 sq m)
8 clinical trials in process
6 Products
18 Products
30 Products
38 Products
74 Products
300
Pharmacies
800
Pharmacies
1,600
Pharmacies
3,000
Pharmacies
Received more
than $2.5 Million
in grants from
government
12
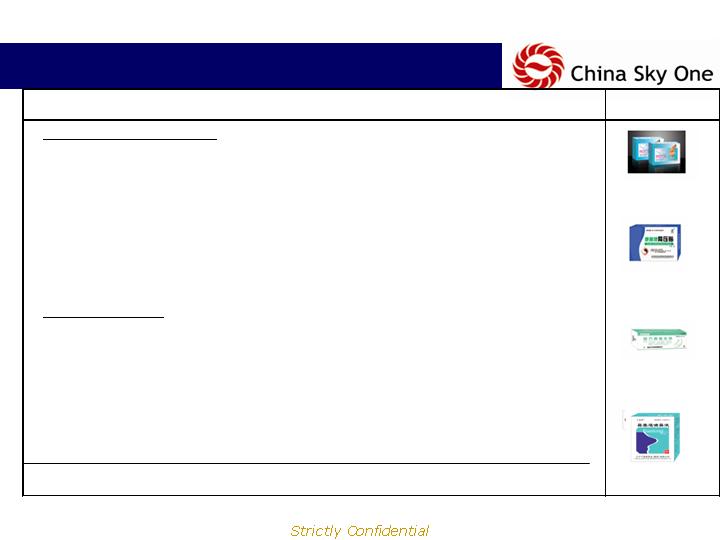
Product Portfolio:
Proprietary + 3rd Party Products
Diversified product portfolio consists of external use TCM,
traditional Western medicine and rapid diagnostic kits.
Company Manufactured Products:
External use TCM:
28 TCM products of variable topical formulations, including sprays,
ointments, creams, powders and patches
Rapid diagnostic kits:
3 products include early pregnancy tests, micro-albumin urine tests to
assess kidney functions and cardiac enzyme kits for acute myocardial
infarctions (heart attacks)
3rd Party Products:
43 products treating variety of ailments from Camphor creams for
dermatitis to sulfasalazine suppositories for different types of colitis,
including ulcerative colitis
13
Product Portfolio: Key Products
Product
Application
% Sales
2007E
Manufactured by Company
Sumei Slim Patch
TCM patch with Saponin as the
main active ingredient to promote
weight loss
19.0%
Anti-Hypertension Patch
TCM combines with modern trans-
dermal theraputic system (TTS) to
provide an effective theraputic
treatment for reducing blood
pressure
12.5%
3rd Party Products
Compound Camphor Cream
Treats dermatitis, eczema,
neurodermitis, allergic dermatitis
and popular urticaria
5.7%
Sulfasalazine
Effective for ulcerative colitis and
nonspecific chronic colitis
4.7%
Qianliming Nasal Drops
Effective for Coryza (head cold),
Ethyl ester hydroxybenzene, etc.
3.4%
45.3%
14
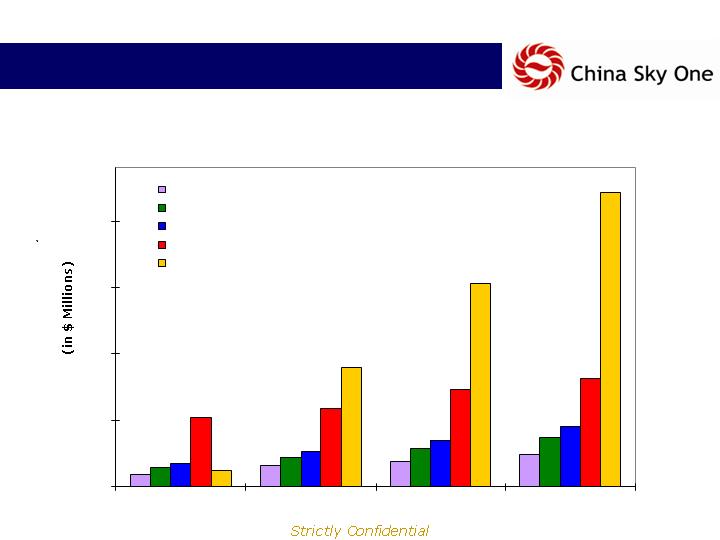
Product Portfolio: Key Products
Sales by Product
0
5
10
15
20
2006
2007
2008
2009
Qianliming Nasal Drops: 25.6% CAGR
Sulfasalazine Suppositories: 27.6% CAGR
Compound Camphor Cream: 26.2% CAGR
Anti-Hypertension Patch: 11.7% CAGR
Sumei Slim Patch: 106.6% CAGR
15
Product Portfolio: Rapid Diagnostic Kits
Diagnostic Products
Micro-Albumin Testing kit
Assess kidney functions in diabetic patients
AMI Testing Kit
Assess myocardial infarction (heart attack)
Early Pregnancy Test
Diagnostic Product Pipeline
18 Rapid Diagnostic Kits in Development:
Oncology kits (6)
Infectious disease kits (3)
Others (9)
16
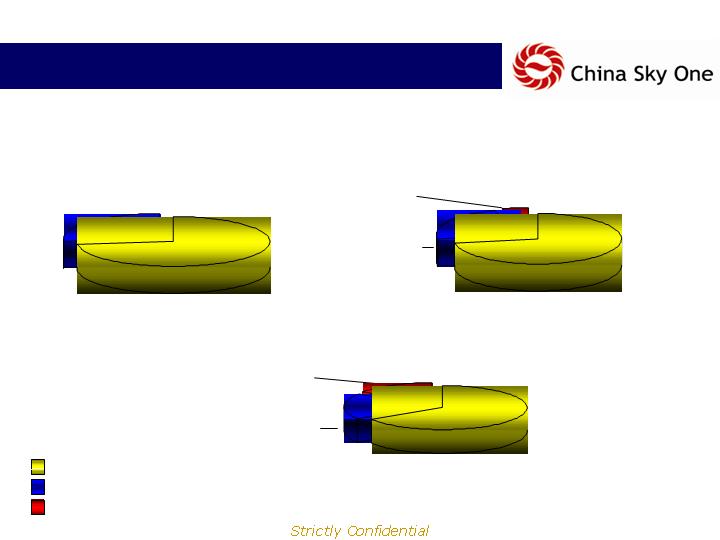
Product Sales
Sales By Product Type
2006
$19.8MM
$13.6MM
(68.9%)
2007E
$47.1MM
$32.8MM
(69.5%)
$12.2MM
(25.9%)
$2.1MM
(4.6%)
$60.2MM
(69.0%)
$16.3MM
(18.7%)
$10.8MM
(12.4%)
2008E*
$87.3MM
Manufactured by Company
3rd Party Products
Diagnostics Kits/Biologicals
$6.2MM
(31.1%)
*2008E includes Acquisitions
17
Research & Development
R&D Highlights:
Recognized leader in monoclonal antibody preparation, human
antibody and gene recombination drug studies
Well established relationships with renowned universities
including:
Harbin Medical University
Northeastern Agriculture University
Jilin University
Beijing University
New 30,000 square meter biologics R&D center under
construction
2009 completion
Awarded R&D grants by the Chinese Ministry of Science and
Technology to further develop six Early-Stage Cancer
Diagnostic Kit projects
Staff of 39
18
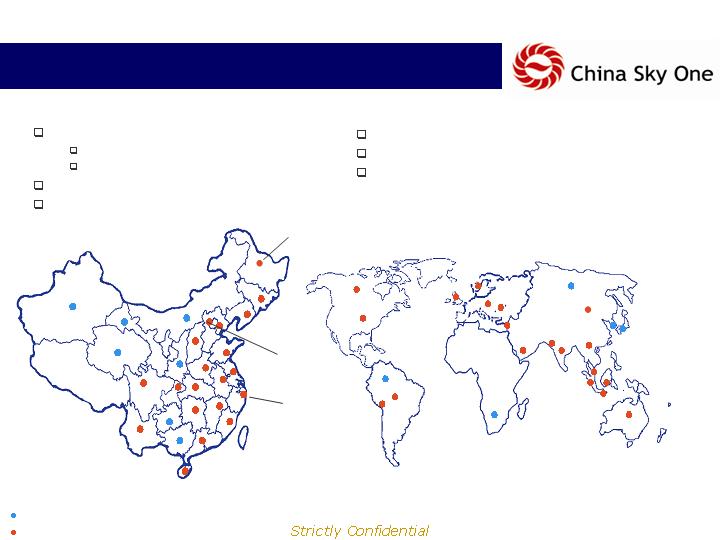
Extensive Distribution Network….
1,400 sales representatives
200 selling to pharmacies
1,200 selling from pharmacies
50 distributors
3,000 pharmacies
Current Distribution Network
Beijing
Shanghai
Harbin (HQ)
22 provincial offices
125 city offices in China
Exports to more than 20 countries
Future Distribution Network
19
…..With Room for Growth
While CSKI’s distribution platform is extensive, there’s still a
lot of runway remaining
Retail Pharmacies
China has more than 24,000 retail pharmacies
Currently, CSKI’s retail pharmacy penetration is <15%
Aggressive organic growth & acquisitions will allow CSKI to
further penetrate the retail pharmacy market
Hospitals
China has more than 2,400 hospitals
Currently, CSKI’s hospital presence is <5%
CSKI’s R&D initiatives on biological diagnostic products will
lead to an increasing penetration of hospitals
CSKI has demonstrated an ability to aggressively grow its
sales and distribution platform, and to leverage its
platform to successfully introduce new products
20
Strong Government Support
$3.3 Million in Government Grants Received in 2007
September 2007: Chinese Ministry of Science and Technology
Two year grant to develop six proprietary
Early-Stage Cancer Diagnostic Kit projects
$1.5 Million
June 2007: Harbin City Government
Annual R&D grant for the next three years through 2010
Additional R&D grant reserved for future investment
$1.3 Million
April 2007: Heilongjiang Provincial Government
First company in the province to receive government grant for
monoclonal antibody research
$0.5 Million
21
Acquisition Strategy
M&A Objectives
Accelerate CSKI’s growth
Capitalize on favorable market and industry dynamics
Leverage CSKI’s extensive sales, marketing and distribution
platform
Expand and diversify CSKI’s product portfolio
Acquisition Target Profiles
Diversified, complimentary product portfolio
High manufacturing standards
Underutilized capacity
Underdeveloped distribution platform
Flagship products
Strong R&D bench
Robust Product Pipeline
Acquisition Status
Four targets identified
Verbal agreements with highest priority targets
22
Acquisition Target 1
Target: “Heilongjiang”
Purchase Price $ 8.0MM
Revenue
2007 $ 4.0MM
2008 $10.0MM
2009 $16.0MM
Acquisition Profile
Diversified, complimentary product
portfolio
75 SFDA approved products,
including
Cardiovascular medications
Anti-gastroenteritis medications
High manufacturing standards
GMP certified
Underutilized capacity
Capacity utilization 45%
Underdeveloped distribution platform
25 sales reps
30 distributors (Northern China
focus)
500 pharmacies, <50% overlap
Flagship products
3 strong candidates
Strong R&D bench
Robust product pipeline
Near term launch of 5 products
23
Acquisition Target 2
Target: “Liaoning”
Purchase Price $10.0MM
Revenue
2007 $ 5.0MM
2008 $ 7.0MM
2009 $23.0MM
Acquisition Profile
Diversified, complimentary product
portfolio
40 SFDA approved products,
including
Anti-flu medications
Dermatologic medications
Anti-hyperlipidemic medications
High manufacturing standards
New facility, GMP certified
Underutilized capacity
Capacity utilization 40%
Underdeveloped distribution platform
15 sales reps
25 distributors
650 pharmacies, <50% overlap
Flagship products
5 strong candidates
Strong R&D bench
Robust product pipeline
24
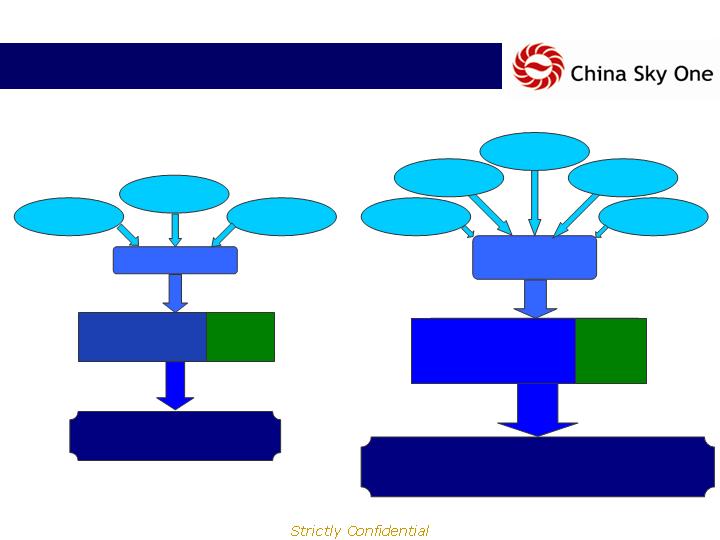
Expanded Distribution Platform
20
Hospitals
2007
43 3rd Party
Products
1,400 Sales Reps
50 Distributors
120 Million People
28 TCM
Products
3 Diagnostic
Products
Post Acquisitions (2009)
21 Diagnostic
Products
2,000 Sales Reps
100 Distributors
4,175 Pharmacies
1,200 in-store promoters
170 Million People
28 TCM
Products
40 Products
from acquisition 2
75 Products
from acquisition 1
43 3rd Party
Products
3,000
Pharmacies
4,200
Pharmacies
200
Hospitals
25
Financial Highlights
Financial Performance & Projections
(1) 2006 included one time costs for recapitalized R&D costs and RTO expenses
(2) As of 6/30/07, CSKI was designated as a high-tech development company, taxed at 15%
(3) Assumes issuance of 3 Million shares in 2008
YTD
($1,000s)
2005
2006
(1)
3Q07
2007E
w/o Acq
w/ Acq
w/o Acq
w/ Acq
Revenue
7,712
19,882
36,595
47,153
70,000
87,000
110,000
149,000
Revenue Growth
157.8%
137.2%
48.5%
84.5%
57.1%
71.3%
Gross Profit
5,498
14,819
28,491
36,657
54,600
68,904
86,900
119,945
Gross Margin
71.3%
74.5%
77.9%
77.7%
78.0%
79.2%
79.0%
80.5%
R&D
64
2,027
1,752
3,329
6,300
6,960
9,350
11,175
SG&A
2,914
10,738
12,798
16,019
21,561
26,501
33,990
46,861
PreTax Income
2,445
1,704
13,657
17,309
26,739
35,444
43,560
61,909
Income Tax
(2)
356
1,080
2,434
2,443
3,869
4,809
6,564
8,891
Net Income
2,089
624
11,223
14,866
22,870
30,635
36,996
53,018
Net Margin
27.1%
3.1%
30.7%
31.5%
32.7%
35.2%
33.6%
35.6%
Net Growth
-70.1%
2281.7%
53.8%
106.1%
61.8%
73.1%
Fully Diluted Shares
(3)
10,929
13,908
13,908
13,908
16,908
16,908
16,908
16,908
EPS
0.19
0.04
0.81
1.07
1.35
1.81
2.19
3.14
2008E
2009E
26
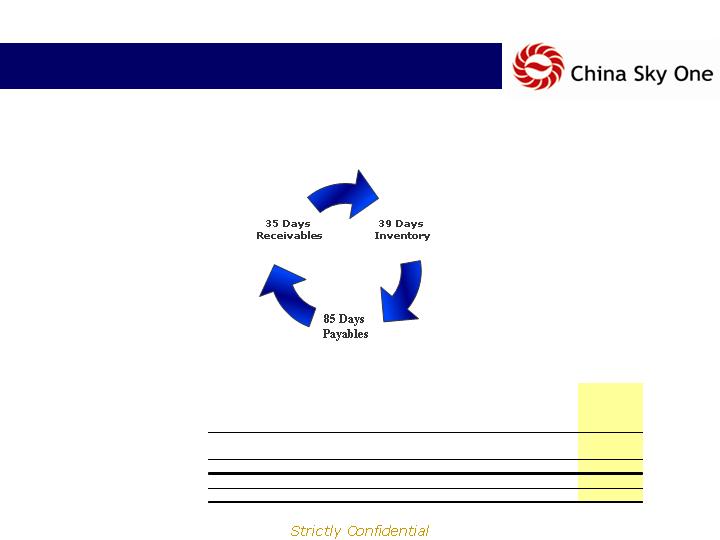
Financial Highlights
Comparable Company Operating & Cash Cycle Analysis
74 Day
Operating
Cycle
CSKI
LTM ending June 30, 2007
*CSKI 1H 07 Cash Cycle (32) days
(11) Day
Cash Cycle
Tongjitang
Chinese
Medicines
China
Medicine
Corporation
American
Oriental
Bioengineering
China
Pharma
Average
China
Sky One
Medical
Days' Receivable
124
63
29
162
95
35
Plus: Days' Inventory
79
69
99
223
117
39
Equals: Operating Cycle
203
133
128
284
212
74
Minus: Day's Payables
43
8
26
19
24
85
Equals: Cash Cycle
160
125
102
365
188
-11
27
Pharmaceutical Companies
Pharmaceutical Companies > Market Multiples
(in $ Millions, except per share data)
11/08/07
Company
Ticker
Exchange
Stock Price
China Based Healthcare Companies
Tongjitang Chinese Medicines Co.
TCM
NYSE
8.96
12.88
7.50
299.47
196.52
American Oriental Bioengineering Inc.
AOB
NYSE
12.24
14.48
6.83
911.48
806.08
Simcere Pharmaceutical Group.
SCR
NYSE
14.15
19.30
10.81
852.31
768.69
US Generic Pharmaceutical Companies
Beckman Coulter Inc.
BEC
NYSE
70.22
77.00
58.64
4,368.52
5,210.72
Bio-Rad Laboratories Inc.
BIO
AMEX
105.99
115.23
66.80
2,872.22
2,800.13
Celera Group
CRA
NYSE
15.85
16.55
11.39
1,224.48
663.06
King Pharmaceuticals Inc.
KG
NYSE
11.42
22.25
10.05
2,454.91
1,932.21
Par Pharmaceutical Companies Inc.
PRX
NYSE
17.33
30.68
16.61
614.78
596.57
ViroPharma Inc.
VPHM
NASDAQ
7.53
18.39
7.11
526.23
205.85
China Sky One Medical, Inc.
CSKI
OTC
10.30
14.35
4.75
124.72
138.10
52 Week
High
52 Week
Low
Market
Cap
TEV
28

Pharmaceutical Companies’ P/E Multiples
Company
FY 07
FY 08
FY 09
FY 07
FY 08
FY 09
China Based Healthcare Companies
Tongjitang Chinese Medicines Co.
0.84
0.92
1.08
10.7x
9.7x
8.3x
American Oriental Bioengineering Inc.
0.60
0.79
0.99
20.3x
15.6x
12.4x
Simcere Pharmaceutical Group.
0.63
0.83
0.99
22.4x
17.0x
14.3x
US Generic Pharmaceutical Companies
Beckman Coulter Inc.
3.20
3.59
3.97
21.9x
19.6x
17.7x
Bio-Rad Laboratories Inc.
3.76
4.53
5.39
28.2x
23.4x
19.7x
Celera Group
-0.32
0.16
0.44
NA
NA
NA
King Pharmaceuticals Inc.
1.81
1.38
0.97
6.3x
8.3x
11.8x
Par Pharmaceutical Companies Inc.
1.13
0.82
1.22
15.3x
21.2x
14.2x
ViroPharma Inc.
1.15
0.65
0.47
6.5x
11.7x
16.0x
Mean
1.42
1.52
1.72
16.4x
15.8x
14.3x
CSKI without Acquisition
(1)
1.07
1.35
2.19
9.6x
7.6x
4.7x
CSKI with Acquisition
(1)
1.07
1.81
3.14
9.6x
5.7x
3.3x
(1)
Assumes issuance of 3 Million shares in 2008
Earnings Per Share ($)
Price / EPS
29
Management Team
Yan-qing Liu
CEO, Chairman and Director
Over 15 years of experience in pharmaceutical sales and marketing,
new drug research & development and enterprise management
8 years of experience as a reporter for the Family Health Newspaper
Bachelor’s degree from Prophylactic Department of Harbin Medicine
University and an EMBA from Tsinghua University
Xiao-yan Han
CFO, Vice President and Director
Over 10 years of financial management experience
Appointed the general manager of TDR in 2004
MBA from Harbin Industrial University
Hai-feng Wang
Executive Vice President and Director
Joined TDR in 2003 as the manager of the international business
department
Bachelor’s degree in both international trade and English literature
from Heilongjiang University
30
Investment Summary
Highly favorable market conditions
Pharmaceutical sales growth outpacing GDP growth
Highly robust organic top line growth
2006: 157%
2007E: 137%
Extensive distribution network
1,400 Sales Reps, 50 Distributors, >20 Countries
Diverse portfolio of SFDA approved products
70+ OTC products: TCM, Western medicines & diagnostic kits
Robust R&D pipeline
18 biological diagnostic kits to launch in near term
Well known brands
“TDR” and “Kangxi” ubiquitous
Strong government support
$3.3 Million in grants issued in 2007
Experienced management team
31






























