December 31, 2009
Via Edgar
United States Securities and Exchange Commission
Division of Corporation Finance
100 F Street, N.E.
Washington, D.C. 20549
Mail Stop 4720
Senior Assistant Chief Accountant
Re: China Sky One Medical, Inc.
Form 10-K for the Period Ended December 31, 2008
Filed April 15, 2009
File No. 001-34080
Ladies and Gentlemen:
We are in receipt of the additional comments of the Securities and Exchange Commission (the “Commission”) to the Form 10-K for the year ended December 31, 2008 of China Sky One Medical, Inc. (the “Company”), filed April 15, 2009 (the “Form 10-K”), by letter dated December 4, 2009, to Mr. Yan-Qing Liu, the Company’s Chairman, Chief Executive Officer and President, and have set forth below the Company’s responses. The responses correspond to the numbered items in the Commission’s letter. For your convenience, we have also inserted each of your comments above the corresponding response.
General
| 1. | SEC Comment: We acknowledge your intent to amend your previous filings in response to our comments one, four, seven, nine, ten, 12, 13, 14, 15 and 18. Please provide us with your proposed revised disclosures before amending your filings. |
Response: Immediately following this letter are the Company’s proposed drafts of Amendment No. 1 to the Form 10-K for the year ended December 31, 2008 (the “Form 10-K/A”) and Forms 10-Q for the quarters ended March 31, 2009 and June 30, 2009 (the Form 10-Q/As”), which reflect, where appropriate, marked revisions made in response to the Staff’s September 18, 2009 and December 4, 2009 comment letters.
Securities and Exchange Commission
December 31, 2009
Page 2 of 8
| 2. | SEC Comment: In your response to prior comment one, you state that “the March 31, 2009 information..., which appears in the beneficial ownership table of the 2008 Form 10-K, does not necessarily include persons the company deemed to be “affiliates” as of June 30, 2008 due to their “control” positions with respect to the company and its management.” Please revise your beneficial ownership table on page 59 to include the shares held by your interim CFO and all shares deemed beneficially owned by your Chairman and Vice Chairman. If you believe that the shares held by your Chairman’s wife and daughter and your Vice Chairman’s daughter are not deemed beneficially owned by your Chairman and Vice Chairman, respectively, then please provide us with a detailed analysis which explains such wife’s and daughters’ “control” positions. See also Question 105.05 of the Exchange Act Sections 13(d) and 13(g) and Regulation 13D-G Beneficial Ownership Reporting Compliance and Disclosure Interpretations. |
Response: Given the circumstances, the Company does not believe any revisions are required to the beneficial ownership table of the Form 10-K.
First, Zhang Yu-kun only served as the Company’s interim Chief Financial Officer between June 10, 2008 and November 25, 2008, when he resigned. Therefore the 20,617 shares of common stock Mr. Zhang beneficially owns should be included for the purpose of calculating the Company’s public float as of June 30, 2008, but shares he owns should not be included in the beneficial ownership table as of March 31, 2009.
In addition, the Company believes that, in accordance with Rule 13d-3 promulgated under the Securities Exchange Act of 1934 (the “Exchange Act”), the shares that were held by Liu Yan-qing’s daughter were not beneficially owned by Mr. Liu and the shares held by Han Xiao-yan’s daughter were not beneficially owned by Ms. Han.
Rule 13d-3 provides in part:
| | (a) | For the purposes of Sections 13(d) and 13(g) of the Act, a beneficial owner of a security includes any person who, directly or indirectly, through any contract, arrangement, understanding, relationship, or otherwise has or shares: |
| | (1) | Voting power which includes the power to vote, or to direct the voting of, such security; and/or |
Securities and Exchange Commission
December 31, 2009
Page 3 of 8
| | (2) | Investment power which includes the power to dispose, or to direct the disposition of, such security. |
The Staff has indicated in Question 105.5 of the Exchange Act Sections 13(d) and 13(g) and Regulation 13D-G Beneficial Ownership Reports Compliance and Disclosure Interpretations:
“For purposes of Regulation 13D-G, an analysis of the facts and circumstances is necessary in determining whether a husband, wife or child beneficially owns shares held by another family member sharing the same household. The relationship between family members should be analyzed to determine whether a family member directly or indirectly either has or shares voting and/or dispositive power over the shares held by any other family member living in the same household.”
Mr. Liu did not have or share voting or investment power over the shares held by his daughter, as she retains sole voting and investment power. Similarly, Ms. Han does not have or share voting or investment power over the shares held by her daughter, as Ms. Han’s daughter has sole voting and investment power over the shares held by her. Mr. Liu’s and Ms. Han’s daughters were each 19 years old at June 30, 2008, above the age of independence in the People’s Republic of China (the “PRC”).
Notwithstanding the fact that Mr. Liu’s and Ms. Han’s daughters are the sole beneficial owners of the stock held by them, the Company considers their shares to be part of a “control” group. In this regard, members of a family with an aggregate amount of stock that is significant in relation to the number of shares outstanding are often viewed as affiliates, even though the parties may not actually combine their holdings. Although this question is one of fact, the Staff has, in the past, considered and concurred with this general perception. The aggregate ownership of Mr. Liu, his wife and daughter as of June 30, 2008 was approximately 38.0% and the aggregate ownership of Ms. Han and her daughter as of June 30, 2008 was approximately 12.9%. Moreover, while each daughter attends college abroad, their legal addresses are with Mr. Liu and Ms. Han, as the case may be, including for purposes of the PRC census. Further, Mr. Liu and Ms. Han pay for their respective daughter’s educations and travel to and from school, and otherwise provide financial support to them.
Securities and Exchange Commission
December 31, 2009
Page 4 of 8
Accordingly, as noted above, while Mr. Liu did not and Ms. Han does not have voting or investment power over their respective daughter’s stock and therefore are not deemed beneficial owners of such shares, based upon the foregoing facts, the Company considers each daughter to be part of a “control group” and therefore an affiliate within the meaning of Rule 144(a)(1). Therefore the 507,531 and 526,170 shares of common stock beneficially owned by Mr. Liu’s and Ms. Han’s daughters, respectively, should be included for the purpose of calculating the Company’s public float as of June 30, 2008, but shares they own should not be included in the beneficial ownership table as of March 31, 2009.
Lastly, the 526,170 shares held by Mr. Liu’s wife as of June 30, 2008, are not included in Mr. Liu’s beneficial ownership as of March 31, 2009, as reflected on the beneficial ownership table, because she transferred her shares to a third party as a bona fide gift as of February 9, 2009.
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operation
Liquidity and Capital Resources, page 45
| 3. | SEC Comment: Refer to your response to comment ten. Your sales have fluctuated significantly during each of the years presented. In calculating your days sales, it appears that quarterly days sales may be more meaningful than an annual calculation. If so, please revise to use quarterly days sales in your disclosure. |
| | Response: The Company will add disclosure regarding turnover and quarterly days sales to the “Liquidity and Capital Resources” section in the Form 10-K/A and Form 10-Q/As. |
Notes to Consolidated Financial Statements
2. Acquisition of Business, page F-8
| 4. | SEC Comment: Refer to your response to comment 11. Please address the following comments: |
| a. | Please tell us why the grand total of the intangible assets listed in the table agree to your balance sheet as of December 31, 2008, while the amounts for each individual acquisition do not agree to your separate footnote disclosure. |
| | b. | You indicate in your response that you never performed an appraisal of the “Kang Xi” trademark nor did you assign any value to that trademark. Please explain to us how you acquired the “Kang Xi” trademark. If you acquired this trademark in a business combination, please clarify which one. In addition, please reference for us the authoritative literature you relied upon to not record an intangible asset for this trademark. |
Securities and Exchange Commission
December 31, 2009
Page 5 of 8
| | c. | As previously requested, please explain to us why you have not apparently recorded an intangible asset for the Good Supply Practice license issued by the SFDA acquired in your business combination with Haina. Please reference the authoritative literature you rely upon to support your accounting. |
Response:
| | a. | The information provided in footnote 2 to the Company’s 2008 audited consolidated financial statements represents the value of the intangible assets acquired at the time of each acquisition. There was, however, one typographical error in footnote 2, with respect to the information provided for Heilongjiang Haina Pharmaceutical Inc. (“Haina”). The Company acquired Haina, which had registered capital of $84,307, for a cash payment of $437,375. The Company recorded the difference of $353,068 under the category of Goodwill. The Company will change “$437,375” to “$353,068” in the Form 10-K/A and Form 10-Q/As. |
Following the three business acquisitions the Company made in fiscal 2008, it further developed each of the acquired subsidiaries’ technologies. This resulted in an increased value of the intangible asset for each. These increases are documented by the following table:
| Intangible Assets, Net |
| As of 2008-12-31 | | Tian Long | | | Haina | | | Peng Lai | |
| Patent | | $ | 1,948,362 | | | $ | 0 | | | $ | 4,935,863 | |
| Goodwill | | $ | 0 | | | $ | 353,068 | | | $ | 0 | |
| Total | | $ | 1,948,362 | | | $ | 353,068 | | | $ | 4,935,863 | |
| | | | | | | | | | | | | |
As of Each Acquisition Date | | | | | | | | | | | | |
| 2008-4-3 | | $ | 1,786,990 | | | | | | | | | |
| 2008-4-18 | | | | | | $ | 353,068 | | | | | |
| 2008-9-5 | | | | | | | | | | $ | 2,917,386 | |
A breakdown of the Company’s intangible assets as of December 31, 2008, as reflected on the balance sheet in the Form 10-K is as follows:
Securities and Exchange Commission
December 31, 2009
Page 6 of 8
| Intangible Assets, net as of December 31, 2008 |
| | | TDR | | | First | | | Tianlong | | | Haina | | | Peng Lai | | | Total | |
| Patent | | $ | 1,470,631 | | | $ | 6,738,763 | | | $ | 1,948,362 | | | $ | 0 | | | $ | 4,935,863 | | | $ | 15,093,619 | |
| Goodwill | | $ | 405,078 | | | $ | 0 | | | $ | 0 | | | $ | 353,068 | | | $ | 0 | | | $ | 758,146 | |
| Total | | $ | 1,875,709 | | | | 6,738,763 | | | $ | 1,948,362 | | | $ | 353,068 | | | | 4,935,863 | | | $ | 15,851,765 | |
Disclosure has been added to Note 11 to Notes to Financial Statements in the Form 10-K/A to reconcile the balances.
| | b. | The “Kang Xi” trademark was developed internally and registered by the Company’s subsidiary, Harbin Tian Di Ren Medical Science and Technology Company (“TDR”) before the Company became a public company. The Company’s cost basis in the trademark is nominal. |
| | c. | As mentioned above, the Company acquired Haina for a total purchase price of $437,375. The Company recorded the difference between the purchase price and fair value under the category of Goodwill. The Good Supply Practice license issued by the SFDA and goodwill were the only intangible assets the Company acquired in the purchase of Haina. Since this license must be certified and confirmed by the SFDA on an annual basis to be renewed, management assigned a nominal value to it. |
3. Summary of Significant Accounting Policies
Advertising, page F-12
| 5. | SEC Comment: Please revise your response to comment 14 to explain what you mean by expensing advertising costs “in the period in which the service is used.” In your response, separately explain to us how this accounting complies with the requirements of FASB ASC 720-35-05-1 to expense the costs of advertising either as incurred or the first time the advertising takes place. |
Response: Generally, the Company signs contracts with agents who then place its advertising in the mediums of television, radio and internet. Advertising expense is incurred in the period the advertisements take place. Thus, costs of advertising are expensed as incurred, in compliance with FASB Concept Statement #6 paragraph 81 and ASC 720-35-05-1. Historically, the Company’s direct-response advertising costs has been immaterial. Total advertising costs for the years ended December 31, 2008 and 2007 were $7,299,367 and $4,385,045, respectively. Advertising costs are reported as part of selling, general and administrative expenses in the statement of operations.
Securities and Exchange Commission
December 31, 2009
Page 7 of 8
The Company will revise the disclosure in Note 3 to Notes to Financial Statements in the Form 10-K/A and in the corresponding Notes to Financial Statements in the Forms 10-Q/As as requested.
8. Outstanding Warrants and Options, page F-19
| 6. | SEC Comment: In your response to comment 16 you indicate that “according to published accounting guidance, equity-linked financial instruments (or embedded features) that have antidilution provisions that adjust the exercise or conversion price only to the extent of any economic dilution directly attributable to a company-initiated transaction do qualify as being indexed to a company’s own stock.” However, you do not appear to indicate the source for this statement. Please reference for us the specific authoritative literature you rely upon to support your contention. In your response, please explain why the guidance provided in Example 8 at FASB ASC 815-40-55-33 and -34 is not applicable. This example appears to contradict your contention. |
Response: The Company has reviewed Example 8 at FASB ASC 815-40-55-33 and -34 and withdraws the section of its response related to EITF 07-05. However, management has no current or future plans to sell additional equity at a value below the $12.50 exercise price of its outstanding warrants. Management believes it has adequate working capital to fund its operations for the foreseeable future. Further, while the Company has no current plans to make any major capital expenditures, as of September 30, 2009, the Company had approximately $56.3 million of cash and cash equivalents available for such purposes and such amount has continued to increase, as the Company has continuously presented positive cash flow. Finally, the Company has unofficial commitments from local banks in the PRC to lend the Company in excess of $100 million if requested, subject only to satisfactory due diligence review. Therefore, the Company does not foresee any situation in which the weighted-average adjustment will be triggered.
13. Income Taxes, page F-22
| 7. | SEC Comment: We acknowledge your response to comment 18b. As previously requested, for each period presented please disclose the impact on net income and earnings per share of your special tax rate compared to the 25% enterprise tax rate in the People’s Republic of China. Please see SAB 11:C. |
Securities and Exchange Commission
December 31, 2009
Page 8 of 8
| | Response: The Company will revise the disclosure in Note 13 to Notes to Financial Statements as requested. |
In connection with the Company’s responses to the Commission’s comments, the Company acknowledges that:
| | · | the Company is responsible for the adequacy and accuracy of the disclosure in the filings; |
| | · | staff comments or changes to disclosure in response to staff comments do not foreclose the Commission from taking any action with respect to the filings; and |
| | · | the Company may not assert staff comments as a defense in any proceeding initiated by the Commission or any person under the federal securities laws of the United States. |
Please do not hesitate to contact the undersigned with any questions or further comments.
| Very truly yours, |
| |
| /s/ Liu Yan-qing |
| |
| Liu Yan-qing |
| Chairman, Chief Executive Officer and President |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
AMENDMENT NO. 1
TO
FORM 10-K
(Mark One)
| x | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended: December 31, 2008
x | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from ____________ to ____________
Commission file number: 000-26059
CHINA SKY ONE MEDICAL, INC.
(Exact name of registrant as specified in its charter)
| | Nevada | | 87-0430322 | |
| | (State or other jurisdiction of incorporation or organization) | | (I.R.S. Employer Identification No.) | |
| | Room 1706, Di Wang Building, No. 30 Gan Shui Road, Nangang District, Harbin, People’s Republic of China | | 150001 | |
| | (Address of principal executive offices) | | (Zip Code) | |
| Registrant’s telephone number, including area code: | 86-451-53994069 (China) | |
Securities registered pursuant to Section 12(b) of the Act:
Title of each class None | | Name of each exchange on which registered Not Applicable | |
Securities registered pursuant to Section 12(g) of the Act:
| | Common Stock | |
| | (Title of Class) | |
Indicate by check mark if the registrant is a well-known seasonal issuer, as defined in Rule 405 of the Securities Act.
Yes o No x
Indicate by check mark if the registrant is not required to file reports pursuant to section 13 or Section 15(d) of the Act.
Yes o No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
Yes x No o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§229.405 of this chapter) is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K.
x
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a small reporting company:
Large accelerated filer o | Accelerated filer o |
| | |
Non-accelerated filer o (do not check if a smaller reporting company) | Smaller reporting company x |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act).
Yes x No o
As of June 30, 2008, the aggregate market value of the voting and non-voting common equity held by non-affiliates was approximately $ 62,853,570,69,711,542, based on the last closing price of $11.13 per share, as quoted on the American Stock Exchange.
As of March 31, 2009, the registrant had 16,446,467 shares of common stock issued and outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
A description of “Documents Incorporated by Reference” is contained in Item 15 of this report.
CHINA SKY ONE MEDICAL, INC.
ANNUAL REPORT ON FORM 10-K
TABLE OF CONTENTS
| | | PAGE |
| | | |
| Special Note Regarding Forward-Looking Statements | 1 |
| | |
| PART I | | 2 |
| Item 1. | Business | 2 |
| Item 1A. | Risk Factors | 20 |
| Item. 1B. | Unresolved Staff Comments | 2932 |
| Item 2. | Properties | 2932 |
| Item 3. | Legal Proceedings | 3033 |
| Item 4. | Submission of Matters to a Vote of Security Holders | 3033 |
| | | |
| PART II | | 3134 |
| Item 5. | Market for Common Equity, Related Stockholder Matters and Small Business Issuer Purchases of Equity Securities | 3134 |
| Item 6. | Selected Financial Data | 3639 |
| Item 7. | Management’s Discussion and Analysis of Financial Condition and Results of Operation | 3740 |
| Item 7A. | Quantitative and Qualitative Disclosures About Market Risk | 4449 |
| Item 8. | Financial Statements and Supplementary Data | F-1 |
| Item 9. | Changes In and Disagreements With Accountants on Accounting and Financial Disclosure | 4150 |
| Item 9A. | Controls and Procedures | 4150 |
| Item 9B. | Other Information | 4251 |
| | | |
| PART III | | 4352 |
| Item10. | Directors, Executive Officers and Corporate Governance | 4352 |
| Item 11. | Executive Compensation | 4857 |
| Item 12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters | 5161 |
| Item 13. | Certain Relationships and Related Transactions, and Director Independence | 5262 |
| Item 14. | Principal Accounting Fees and Services | 5363 |
| Item 15. | Exhibits, Financial Statement Schedules | 5464 |
| | | |
| Signatures | | 66 |
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This Annual Report on Form 10-K, together with other statements and information we publicly disseminate, contains certain forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. We intend such forward-looking statements to be covered by the safe harbor provisions for forward-looking statements contained in the Private Securities Litigation Reform Act of 1995, and include this statement for purposes of complying with these safe harbor provisions.
Forward-looking statements, which are based on certain assumptions and describe our future plans, strategies and expectations, are generally identifiable by use of the words “believe,” “expect,” “intend,” “anticipate,” “estimate,” “project” or similar expressions. You should not rely on forward-looking statements since they involve known and unknown risks, uncertainties and other factors that are, in some cases, beyond our control and which could materially affect actual results, performances or achievements.
Factors that may cause actual results to differ materially from current expectations include, but are not limited to the “Risk Factors” discussed in Part 1, Item 1A of this Annual Report on Form 10-K. Accordingly, there is no assurance that our expectations will be realized. Except as otherwise required by the federal securities laws, we disclaim any obligations or undertaking to publicly release any updates or revisions to any forward-looking statement contained herein (or elsewhere) to reflect any change our expectations with regard thereto, or any change in events, conditions or circumstances on which any such statement is based.
The terms “the Company,” “we,” “us” and “our” refer to China Sky One Medical, Inc., together with our consolidated subsidiaries.
PART I
Item 1. Business.
General
We are engaged, through our China based indirect subsidiaries described below, in the development, manufacture, marketing and sale of over-the-counter, branded nutritional supplements and over-the-counter plant and herb based pharmaceutical and medicinal products. Our principal products are external use Traditional Chinese Herbal Remedies/Medicines commonly referred to in the industry as “TCM.” We have evolved into an integrated manufacturer, marketer and distributor of external use Chinese medicine products sold primarily in China and through Chinese domestic pharmaceutical chains and have been expanding our worldwide sales effort as well. We sell both our own manufactured products, as well as medicinal and pharmaceutical products manufactured by others in China.
Corporate History
China Sky One Medical, Inc. (“China Sky One”), is a Nevada corporation formed on February 7, 1986, formerly known as Comet Technologies, Inc. On July 26, 2006, after our acquisition of a China based nutritional supplements business, we changed our name to “China Sky One Medical, Inc.”
ACPG, our non-operating United States holding company subsidiary, was incorporated on December 16, 2003, in the State of California, under the name “QQ Group, Inc.” It changed its name to “American California Pharmaceutical Group, Inc.” in anticipation of the Stock Exchange Agreement with China Sky One (then known as “Comet Technologies, Inc.”) and TDR, described herein. On December 8, 2005, ACPG completed a stock exchange transaction with TDR an operating company based in the People’s Republic of China (“PRC”) and TDR’s subsidiaries, each of which were fully operating companies in the PRC. Under the terms of the agreement, ACPG exchanged 100% of its issued and outstanding common stock for 100% of the capital stock of TDR and its subsidiaries, described below.
Thereafter, on May 11, 2006, ACPG entered into a Stock Exchange Agreement (the “Exchange Agreement”) with the shareholders of China Sky One. The terms of the Exchange Agreement were consummated and the acquisition was completed on May 30, 2006. As a result of the transaction, we issued a total of 10,193,377 shares of our common voting stock to the stockholders of ACPG, in exchange for 100% of the capital stock of ACPG, resulting in ACPG becoming our wholly-owned subsidiary.
TDR, formerly known as “Harbin City Tian Di Ren Medical Co.,” was originally formed in 1994 and maintained its principal executive office in Harbin City of Heilongjiang Province, in the PRC. TDR was reorganized and incorporated as a limited liability company on December 29, 2000, under the “Corporation Laws and Regulations” of the PRC. At the time of the TDR Acquisition by ACPG in December of 2005, TDR had two wholly-owned subsidiaries, Harbin First Bio-Engineering Company Limited and Kangxi Medical Care Product Factory, until July, 2006, when the two were merged, with Harbin First Bio-Engineering Company Limited as the surviving subsidiary of TDR.
As of October 16, 2006, we organized Harbin Tian Qing Biotech Application Company as a wholly-owned PRC subsidiary of TDR, to conduct research and development in the areas of tissue and stem cell banks, which is described in more detail below.
On April 3, 2008, TDR completed its acquisition of Heilongjiang Tianlong Pharmaceutical, Inc., a corporation with a variety of SFDA approved medicines and new medicine applications, organized under the laws of the PRC (“Tianlong”), which is in the business of manufacturing external-use pharmaceuticals. TDR previously acquired the Beijing sales office of Tianlong in mid-2006. TDR acquired 100% of the issued and outstanding capital stock of Tianlong from its sole stockholder, in consideration for an aggregate purchase price of approximately $8,300,000, consisting of (i) $8,000,000 in cash, and (ii) 23,850 shares of our common stock.
On April 18, 2008, TDR consummated its acquisition of Heilongjiang Haina Pharmaceutical Inc., a recently formed corporation organized under the laws of the PRC (“Haina”) licensed as a wholesaler of TCD, bio-medicines, bio-products, medicinal devices, antibiotics and chemical medicines. Haina did not have an established sales network and was acquired for its primary asset, a Good Supply Practice (GSP) license (License No. A-HLJ03-010), issued by the Heilongjiang province office of the SFDA. The SFDA recently started issuing such licenses to resellers of medicines that maintain certain quality controls. The GSP license was issued as of December 21, 2006 and will expire on January 29, 2012 and will enable us to expand its sales of medicinal products without having to go through a lengthy license application process. TDR acquired 100% of the issued and outstanding capital stock of Haina from its three stockholders in consideration for payment of approximately $437,375. TDR had been overseeing the operations of Haina Pharmaceutical since January of 2008, as part of our due diligence prior to closing of this acquisition.
On September 5, 2008, TDR acquired Peng Lai Jin Chuang Pharmaceutical Company (“Jin Chuang”), a corporation organized under the laws of the PRC, from Peng Lai Jin Chuang Group Corporation (the “Seller”). Jin Chuang, which has received Good Manufacturing Practice certification from the SFDA, was organized to develop, manufacture and distribute pharmaceutical, medicinal and diagnostic products in the PRC. In connection with the acquisition of Jin Chuang, TDR acquired all of Jin Chuang’s assets, including, without limitation, franchise, production and operating rights to a portfolio of twenty (20) medicines approved by the SFDA, for an aggregate purchase price of approximately $7.1 million, consisting of (i) approximately $2.5 million in cash, and (ii) 381,606 shares of our common stock.
Principal Products and Markets
We are engaged, through TDR and its respective subsidiaries in the PRC, in the development, manufacture, marketing and sale of over-the-counter, branded nutritional supplements and over-the-counter plant and herb based pharmaceutical and medicinal products. We have evolved into an integrated manufacturer, marketer and distributor of external use Chinese medicine products sold primarily to and through China domestic pharmaceutical chains. The Company sells both its own manufactured products, and medicinal and pharmaceutical products manufactured by others in the PRC.
With the exception of Jin Chuang, which is located in Shan Dong Province, PRC, all of our manufacturing facilities are located in Heilongjiang Province, PRC. In addition, we have sales offices located in 22 provinces across China.
Our principal products are external use Traditional Chinese Herbal Remedies/Medicines (“TCM”). Using various formulas, we produce a number of TCM products with several forms of delivery including creams and ointments, powders, sprays, various medicated skin patch products, and herbs believed to have complimentary effects. We intend to concentrate many of our efforts during the next several years on development, production and sales of TCM products and biological test kits and in particular, tissue and our stem cell research as described more fully below.
Our principal operations are in China, where TDR and its subsidiaries have manufacturing facilities and sales distribution covering most of China and the Hong Kong Special Administration Region. Our overall revenues were $91,816,183 in 2008, of which, export sales for our main countries of export (in order of revenues during the year ended 2008) were as follows:
| | | |
| Malaysia | | $ | 8,821,616 | |
Germany | | $ | 23,445 | |
Russia | | $ | 2,897 | |
TaiwanOther | | $ | 6,08737,575 | |
| TOTAL: | | $ | 8,859,191 | |
TDR has also established several long-term relationships with well-known universities and enterprises in the PRC, as described below under “- Current Research and Development.” Through these relationships, we hope to develop a number of additional products that we will be able to manufacture and market both in the PRC and in other countries.
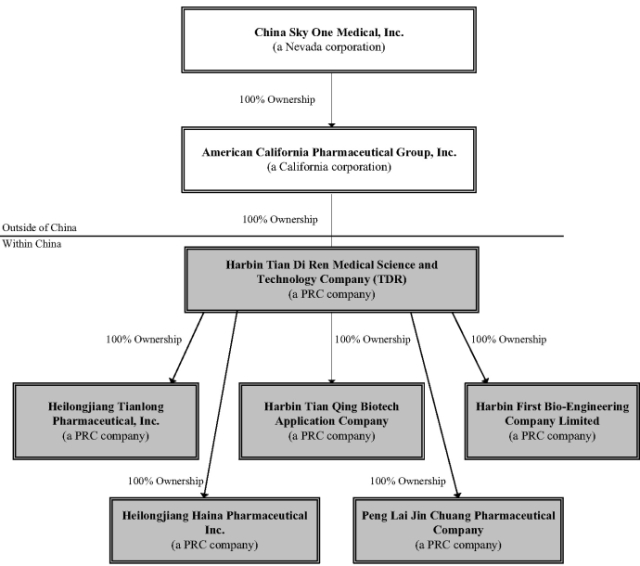
Below is a chart depicting our corporate organization:
SFDA Licenses
The State Food and Drug Administration of the government of Heilongjiang Province, PRC (“SFDA”) issues the licenses and petitions for permission to manufacture and market pharmaceutical products in the PRC. Our licenses relate primarily to medical machine producing licenses which are needed mainly for topical products, ointments and external test kits. TCM products also require a permit for sales, which permits are generally granted on a non-exclusive basis for four to five years depending on the TCM. TDR has been granted 29 product licenses and permits, inclusive of our recently approved Cardiac Arrest Early Examination, Micro-Albuminuria Diagnostic Kit, and Early Pregnancy Test Kit, which have allowed TDR to commercialize a total of 29 products. TDR is undertaking efforts to develop a series of 25 new products, and is planning to register these products with the SFDA over the next 5 years. TDR has also registered 7 patents with the State Intellectual Property Rights Bureau of the PRC, which includes packing design patents as well as product ingredients patents. TDR plans to continue registering patents resulting from its ongoing product research and development.
In addition to the above:
| · | as a result of TDR’s acquisition of Tianlong in April 2008, TDR acquired all of Tianlongs’s assets, which included, among other things, 69 SFDA-approved medicines, and an additional 20 new medicines, which have been submitted for approval to the SFDA; and |
| · | as a result of TDR’s acquisition of Jin Chuang in September 2008, TDR acquired franchise, production and operating rights to a portfolio of 20 medicines approved by the SFDA, and an additional 2 new medicines, which have been submitted for approval to the SFDA. |
Our TDR Subsidiary Owns the Following Subsidiaries in China
Harbin First Bio-Engineering
On September 26, 2003, TDR formed Harbin First Bio-Engineering Company Limited (referred to herein as “Harbin Bio-Engineering”) as its wholly owned subsidiary, with an authorized capital of $241,546 (2 million RMB). Harbin Bio-Engineering focuses on research and development of the use of natural medicinal plants and biological technology products, such as Endothelin-1. Harbin Bio-Engineering, which officially commenced production on July 21, 2006, is one of the first companies in Heilongjiang Province conducting research and development of high technology biological products. Harbin Bio-Engineering has two production lines:
| | · | an enzyme immunity reagent kit production line; and |
| | · | a colloid gold production line. |
Harbin Tian Qing Biotech Application
On October 16, 2006, TDR organized Harbin Tian Qing Biotech Application Company (referred to herein as “Tian Qing Biotech”) as its wholly-owned subsidiary, to conduct research and development in the areas of tissue and stem cell banks, which is described in more detail below. (See “Research and Development” below.)
Heilongjiang Tianlong Pharmaceutical
On April 3, 2008, TDR completed the acquisition of Heilongjiang Tianlong Pharmaceutical, Inc., a corporation organized under the laws of the PRC (referred to herein as “Tianlong”), which is in the business of manufacturing external-use pharmaceuticals. Previously, in 2006, TDR had acquired the Beijing office of Tianlong. Tianlong’s assets included, among other things, GMP-certified manufacturing facilities, state-of-the-art manufacturing equipment, an research and development center, sixty-nine (69) SFDA-approved medicines, and an additional thirty-eight (38) new medicines, which have been submitted for approval to the SFDA.
Heilongjiang Haina Pharmaceutical
On April 18, 2008, TDR consummated its acquisition of Heilongjiang Haina Pharmaceutical Inc., a corporation organized under the laws of the PRC (referred to herein as “Haina”), which is licensed as a wholesaler of TCD, bio-medicines, bio-products, medicinal devices, antibiotics and chemical medicines. At the time of the acquisition, Haina did not have an established sales network and was acquired for its primary asset, a Good Supply Practice (GSP) license (License No. A-HLJ03-010), issued by the Heilongjiang province office of the SFDA. The SFDA recently started issuing such licenses to resellers of medicines that maintain certain quality controls. The GSP license was issued as of December 21, 2006 and will expire on January 29, 2012. This license will enable us to expand Haina’s sales of medicinal products without having to go through a lengthy license application process. TDR had been overseeing the operations of Haina Pharmaceutical since January of 2008, as part of our due diligence prior to closing of this acquisition.
Peng Lai Jin Chuang Pharmaceutical
On September 5, 2008, TDR acquired Peng Lai Jin Chuang Pharmaceutical Company, a corporation organized under the laws of the PRC (referred to herein as “Jin Chuang”), from Peng Lai Jin Chuang Group Corporation. Jin Chuang, which has received Good Manufacturing Practice certification from the SFDA, was organized to develop, manufacture and distribute pharmaceutical, medicinal and diagnostic products in the PRC. In connection with the acquisition of Jin Chuang, TDR acquired all of Jin Chuang’s assets, including, without limitation, franchise, production and operating rights to a portfolio of twenty (20) medicines approved by the SFDA.
Product Line
We manufacture over 90 branded products, which management believes enables us to maintain better control over product quality and availability while also reducing production costs. We also sell a total of 7 products manufactured by other firms (See “Other Products,” below). Our manufacturing operations are conducted in our indirect subsidiaries’ facilities located in Heilongjiang Province and Shan Dong Province in the PRC. Additionally, we maintain a working relationship with a number of outside manufacturers, including softgel manufacturers and packagers, and utilize these outside sources from time to time.
We sell our products under six main categories:
| | · | Diagnostic Kits (3 items); |
| | · | Contract Sales (7 items); and |
A description of our main product lines follows.
Sumei Slim Patch
The Sumei Slim Patch is marketed and sold within and outside the PRC as a more natural way to lose weight. The Sumei Slim Patch uses Saponin, believed to regulate and restrain the excessive secretion of certain hormones, while promoting others. The Sumei Slim Patch is also believed to foster weight loss and prevent weight gain.
Pain Killer Patch
A pain killer patch applied to the neck, shoulder and waist, this product is a treatment to fend off fever, promote well-being and to relieve diarrhea. The patch is used for a number of ailments, including fever, headache, dysentery of a heat type, diarrhea and stiffness and pain in the neck caused by hypertension.
Anti-Hypertension Patch
The anti-hypertension patch is based on five thousand years of Chinese herbal vein therapy that has been adapted to a modern trans-dermal therapeutic system (TTS). The product utilizes a Body-Yong-Guan point technique, which is believed to maximize the effectiveness of the medicinal ingredients. The product is believed to stimulate blood capillaries and is believed to be effective in improving circulation and in reducing blood pressure.
Dysmenorrheal Patch
This is a soft patch, applied externally, for pain relief from dysmenorrheal (menstrual cramps) that combines traditional Chinese point therapy and modern trans-dermal technology. This product contains a pure herb formula selected from rare Chinese herbs or plants which is refined to extract the effective ingredients. This product is believed to be effective in regulating microcirculation, in balancing the functions of the human body and in enhancing the immunity response of women. It is believed to be effective in treating the dysmenorrheal (cramping) in a woman’s critical days, and in regulating pain and catamenia (menstruation period).
Yin Ke Psoriasis Spray
Psoriasis is a skin disease that is difficult to treat. Our research scientists have focused their efforts in finding treatments for this disease. Yin Ke Psoriasis Spray is a spray that contains Chinese herbal ingredients that are believed to be effective in killing pathogenic ringworms inside or under the skin, causing scale-like skin to fall off, and allowing healthy skin to grow.
Wart Removing Spray
This product has been developed to eliminate the viruses in a tumors or warts. The product is effective in removing warts, through a strong permeation and sterilization process. The product is a highly concentrated washing liquid that is applied topically to the affected area.
Chilblain Ointment
This product contains Rhizoma Paridis, Rhizoina Bletilae and Camphor, and is refined from Chinese herbal materials. It is believed to be effective in improving blood circulation, and in eliminating various symptoms of Chilblain (a cold injury that appears as an inflamed swelling on the extremities), including itching and swelling.
Hemorrhoids Ointment
This product contains Acetate, Radix Notoginseng, and Rhizoma Coptidis. The product is made in a soft ointment that is effective in sterilizing and relieving hemorrhoid symptoms, including itching, distending pain, burning, and bleeding.
Tinea Pedis Spray, Ointment and Powder
This product contains Cortex Pseudolaricis and Cortex Phellodendri, and is a treatment for killing various pathogens on the skin surface and subcutaneously, such as mycete (a fungus), trichopytic, staphylococcal bacteria aureus, bacillus coli, and candida albicans (thrush).
Dermatitis Spray
This product is effective in sterilization and in relieving itching in various kinds of skin pruritis (intense itching condition) caused by eczema, urticaria (hives), seborrheic dermatitis (flaking of skin, dandruff), herpes zoster (shingles), neurodermitis and allergic dermatitis.
Dandruff Treatment Herbal Shampoo
This product has been specifically designed to treat dandruff, and is not intended for use as an ordinary shampoo. The product is believed to be effective in killing fungi and providing nutrition to pallium cells.
Runze Eye Drop
This product is refined from active ingredients extracted from natural herbs or plants, and functions as a protection from infection, tiredness of optic nerves and myopia.
Testing Kits
Cardiac Arrest Early Examination Kit
This product is used for early stage diagnosis of myocardial infarction (heart attacks). We completed SFDA clinical testing of the Cardiac Arrest Early Examination Kit and began sales of this product in 2007. This kit is patented in PRC.
Kidney Disease Testing Kit
The Urinate Micro Albumin Examination Testing Kit is used in connection with early stage diagnosis for primary kidney disease, hypertension and diabetes. We completed SFDA clinical testing for the Urinate Micro Albumin Examination Testing Kit and commenced sales of this product in 2007. This kit is patented in PRC.
Early Pregnancy Test Kit
The early pregnancy test kit is used to determine pregnancy through a urine sample. We completed SFDA clinical testing for this kit and commenced sales of this product in 2007. This kit is patented in PRC.
Other Products
TDR offers a number of additional products made from Chinese herbs and plants, including a leukoderma ointment, rheumatism spray, Coryza powder, Hircus removing spray, gonorrheal cleaning spray, a snoring retardant, deodorants, diet tea, cough arresting patch, pharyngitis spray, Clindamycin Metronidazole Liniment, Ganciclovir Injection, Loquat Syrup, Indigowoad Root Granule, and others.
Historically we have sold only products that we manufactured. However, during the 2007 fiscal year, we began an initiative to sell medicinal products manufactured by other companies under exclusive sales and marketing arrangements. Set forth in the table below is information concerning these products and the intended treatment applications.
| | | | |
| Ofloxacin Eye Drops | | Conjunctivitis, Keratitis | | Ofloxacin |
| Ribavirin Nasal Drops | | Influenza | | Ribavirin |
| Econazole Nitrate Suppositories | | Colpitis (inflammation of the vagina) | | Econazole Nitrate |
| Qianliming Nasal Drops | | Coryza (head cold) | | Ethyl Ester Hydroxybenzene, etc. |
| Terbinafine Hydrochloride Liquor | | Tinea (scalp ringworm) | | Terbinafine Hydrochloride |
| Compound Camphor Cream | | Eczema, dermatitis, etc. | | Camphor, Menthol, Methyl Salicylate |
| Terbinafine Hydrochloride Cream | | Tinea (scalp ringworm) | | Terbinafine Hydrochloride |
| Sulfasalazine Suppositories | | Colonitis | | Sulfasalazine |
Total sales in 2008 from products manufactured by other companies under exclusive sales arrangements totaled approximately $5,642,182 or approximately 6% of total sales in the year ended December 31, 2008, as compared to $12,998,000 for the year ended December 31, 2007. We market and sell these products through our existing distribution channels to our customers throughout the world and primarily in China. We intend to expand our product line under sales and manufacturing contracts with third-party manufacturers with a goal of increasing sales revenue from current and new pharmaceutical and medicinal products manufactured by other companies.
Revenues by General Product Lines
Management believes that the most accurate benchmark of revenue breakdown is based on the method of application as different applications have different sales channels. Below is a breakdown of our revenues for 2008 based on application and application usage.
Revenues based on Application Category
Our total revenues during fiscal 2008 and 2007 were approximately $91,801,000 and $49,318,308, respectively. The following table sets forth our principal product categories based on application type and the approximate amount and percentage of revenue from each of such product categories, during the fiscal year ended December 31, 2008 and 2007:
| | | | | | | | For the Year Ended December 31 | | | | |
| | | | | | | | 2008 | | | | | | | | | 2007 | | | | |
Product Category (97 products) | | Subsidiary | | Quantity (Unit) | | | Sales USD | | | % of Sales | | | Quantity (Unit) | | | Sales USD | | | % of Sales | |
| Patch (5 products) | | TDR | | | 9,494,535 | | | $ | 35,484,230 | | | | 39 | % | | | 2,294,901 | | | $ | 19,690,051 | | | | 39 | % |
| Ointment (20 products) | | TDR & TL | | | 11,478,130 | | | | 23,068,210 | | | | 25 | % | | | 3,037,022 | | | | 6,190,003 | | | | 13 | % |
| Spray (19 products) | | TDR & TL | | | 3,941,295 | | | | 10,612,679 | | | | 12 | % | | | 3,580,266 | | | | 9,210,233 | | | | 19 | % |
| Diagnostic Kits (3 products) | | First | | | 2,184,013 | | | | 8,780,990 | | | | 10 | % | | | 739,151 | | | | 2,990,664 | | | | 6 | % |
| Contract Sales (7 products) | | Haina | | | 3,837,578 | | | | 5,655,085 | | | | 6 | % | | | 5,718,652 | | | | 8,197,758 | | | | 17 | % |
| others (43 products) | | | | | 4,306,972 | | | | 8,214,989 | | | | 8 | % | | | 1,896,193 | | | | 3,039,599 | | | | 6 | % |
| Total | | | | | 35,242,523 | | | $ | 91,816,183 | | | | 100 | % | | | 17,266,185 | | | $ | 49,318,308 | | | | 100 | % |
Research and Development
We currently conduct all of our research and development (“R&D”) activities, either internally or through collaborative arrangements with universities and research institutions in the PRC. We have our own research, development and laboratory facilities located at TRD’s principal headquarters in the city of Harbin, Heilongjiang Province, PRC. We have also recently organized Harbin Tian Qing Biotech Application Company (“Tian Qing Biotech”) as a wholly-owned PRC subsidiary of TDR, to conduct research and development in the areas of tissue and stem cell banks, which is described in more detail below. In all, our internal R&D team currently consists of approximately 35 people, of which 25 are full time researchers and 10 are part time technical experts. Many of our team members are professors affiliated with universities in the PRC.
Additionally, we have established several long-term partnerships with well-known universities and enterprises in the PRC. We have:
| | · | built a gene medicine laboratory through a collaborative effort with Harbin Medical University; |
| | · | established a cell laboratory with North East Agricultural University; and |
| | · | founded a monoclonal antibody laboratory with Jilin University. |
Under our partnership arrangements with other universities and research institutions, we will generally hold the intellectual property rights to any developed technology. As a result of one of these collaborations with Harbin Medical University, a product known as “Endothelin-1” is currently under development as a cancer suppressing product. Additional information relating to this product and other products being developed is set forth under “Products Under Development” below and under the general product descriptions throughout this prospectus.
During the year ended December 31, 2008, we invested approximately $7,415,000 in R&D. Our R&D investments in 2007 were approximately $3,158,000. Additional information about our R&D investments is included in the financial statements to this prospectus (and notes thereto) and our “Management Discussion and Analysis on Financial Condition and Results of Operations” section below.
Products Under Development
At present, our ongoing research is divided into five general areas:
| | · | the development of an enzyme linked immune technique to prepare extraneous diagnostic kits (see table below); |
| | · | the development of an enzyme linked gold colloid technique to prepare extraneous rapid diagnostic test strip; |
| | · | the development of a gene recombination technique to prepare gene drug; |
| | · | the development of a biology protein chip for various tumor diagnostic applications; and |
| | · | the development of a cord blood stem cell bank described below. |
Biological Products - Examination and Diagnosis Kits
We currently have various biological products under development at various stages of clinical testing and development. The development of some of these products are expected to be completed as early as the end of fiscal 2009. A summary of each of these products is set forth in the table below.
| | Clinical Experiment and Status | | | | Patent or Intellectual Property (IP) |
| AIDS Early Examination Kit | | Completed clinical testing; application for manufacturing certificate submitted. | | Early stage diagnosis for AIDS | | Method of Anti-body preparation is our IP. |
| | | | | | | |
| Carcinoma Cervix Early Examination Kit | | Research completed and application for manufacturing certificate submitted. | | Early stage diagnosis for Carcinoma Cervix | | Anti-body preparation is our IP. |
| | | | | | | |
| Breast Cancer Early Examination Kit | | Research on product formula completed; application for production permit submitted. | | Early stage diagnosis for Breast Cancer. | | Anti-body preparation is our IP. |
| | | | | | | |
| Liver Cancer Early Examination Kit | | Research on product formula completed; clinical experiment in process. | | Early stage diagnosis for Liver Cancer. | | Anti-body preparation is our IP. |
| | | | | | | |
| Rectal Cancer Early Examination Kit | | Research on product formula completed; clinical experiment in process. | | Early stage diagnosis for Rectal Cancer. | | Anti-body preparation is our IP. |
| | | | | | | |
| Stomach Cancer Early Examination Kit | | Product research completed; clinical experiment in process. | | Early stage diagnosis for Stomach Cancer. | | Anti-body preparation is our IP. |
| | | | | | | |
| Multi-tumor Marker Protein Chip Assay Kit | | Product research in process. | | Early stage diagnosis for multiple cancers. | | Anti-body preparation is our IP. |
| | | | | | | |
| New Endostatin | | Toxicology test, teratogenicity test and quality standard completed; product research in process. | | Early stage diagnosis for cancer. | | Anti-body preparation is our IP. |
New Products
We are currently conducting toxicology experiments, quality standard measurement and other experimentation for our products under development. It is estimated that the experimental time takes about another seven to eight months for each product. We also hope to commence with clinical testing of 8 testing kit products in 2009 for uterine cancer, cervical cancer, ovulatory cancer, liver cancer, breast cancer and neisseria gonorrhea. We cannot predict whether, and when, these efforts will be successful, or the likelihood and/or timing of receiving SFDA approval of each product.
Research and Development
Research and Development for Endothelin-1
One of our various products under development is Endothelin-1. We have already completed oxicology and teratogenicity testing, and have established quality standards, and further developments are underway to improve the product quality of Endothelin-1. In collaboration with Harbin Medical University, we have completed a laboratory experimental study pertaining to Endothelin-1, which is required prior to clinical trials, and are currently applying for approval to enter clinical experiments. At such time as development and clinical testing is successfully completed, we will commence efforts to market Endothelin-1 in the PRC and, where legal, as a new anti-cancer medicine. There can be no assurance, of course, that these development efforts, or that any subsequent efforts to obtain SFDA approval (or other foreign drug regulatory authority approval where we may wish to market this drug) of the product, will be successful. We hope to develop Endothelin-1 as a cancer treatment drug that works by “starving” cancer cells by restricting the generation of blood vessels around cancer lesions, thereby inhibiting, to a degree, the source of nutrients upon which the cancer cells survive. Endothelin-1 has been recognized by the PRC medical industry as a “Top Category in New Medicine.” In order to qualify as the “Top Category in New Medicine,” a company must have intellectual property rights, high technology involvement, strong innovation, and the medicine must be the first of its kind to be introduced to the PRC. TDR has ownership of the intellectual property rights pertaining to this technology, and has obtained an invention patent in China for Endothelin-1. We expect that research and development and testing will be completed for manufacturing in 2009. To date we have expensed over approximately $3,163,218 (unaudited) on research and development for Endothelin-1.
Research and Development for Cord Blood Stem Cell Bank
In 2006, we began implementing a plan to establish a cord blood stem cell bank in the PRC, for the treatment of various diseases such as leukemia, lymphoma and rebirth anemia. We are now in the process of perfecting our cultivation methods and freezing/storage of stem cells. It is expected that these efforts will continue over the next two years or more in particular in the research and development of technology, applications and methodology for the establishment of a cord blood stem cell bank. We have recently organized Harbin Tian Qing Biotech Application Company (referred to herein as “Harbin Biotech”) as a wholly-owned subsidiary, to conduct research and development in the areas of tissue and stem cell banks. This project will involve substantial expense and involve numerous risks. We entered into a development agreement with the Heilongjiang Provincial Red Cross out-patient department for purposes of defraying the costs of developing and marketing this product and are seeking additional R&D partners with laboratories having substantial experience in this area for this purpose as well.
Exclusive Regional License for Stem Cell Research
Research in biotechnology areas such as tissue and stem cell banks has historically been controlled tightly by the government of the PRC. Recently, however, the PRC government has altered its policies to allow one company per each geographic area in China to become actively engaged in research in these areas, with the result that many companies have applied to become engaged in this area of research and development.
In August 2006, we applied with the Ministry of Health of the PRC to become engaged in the research and development of stem cell and tissue banks and related biotechnology areas. Following an extensive review by the applicable local office of the Health Department of Heilongjiang Province, our application was approved on October 16, 2006, granting us, through our subsidiary, the exclusive right and license to become engaged in tissue and stem cell bank activities in Heilongjiang Province, PRC, through December 2010. We intend to renew this license from time to time as necessary. We organized Harbin Biotech to conduct these business operations, as required by Heilongjiang Province. Cord blood stem cells have been shown to be effective in treating a number of diseases, including but not limited to: (a) various forms of blood diseases, including Mediterranean anemia, Dresbach’s anemia, hypoplastic anemia, inborn cell deficiency, Evan’s syndrome, Fanconi’s anemia, Kostmann’s syndrome, and Blackfan-Diamond’s anemia; (b) various malignant diseases, including encephaloma, lymphoma, acute and chronic leukemia, Ewing myoma, Neuoblastoma, germ cell tumor, and multiple myeloma; (c) metabolism defects, including congenital dyskeratosis, Gunter’s disease, and Lesch-Nyhan’s disease; (d) immunodeficiency disease, including chronic granuloma disease and Wiskott-Aldrich syndrome; and (e) various auto-immune diseases.
Our Stem Cell Research
There are numerous advantages of cord blood stem cell banks over traditional marrow transplants, including: a high success rate; low rejection rate; rich source of cord blood; absence of suffering of recipient; simple inspection and quick application; and low matching requirements. While we are not aware of a method to calculate the size of the stem cell market, management believes that the market for this business in PRC and elsewhere is potentially very large. The entry into this business will require strict examination and approval by PRC and local governmental agencies and will require close collaboration with medical institutions and academies.
Blood from umbilical cords—a byproduct of normal childbirth—is a good source of potentially life-saving stem cells, called Hematopoietic progenitor cells (HPCs), the type of stem cells also found in bone marrow and mobilized peripheral blood that give rise to various kinds of blood cells. Transplants of these stem cells have been effective in treating diseases of the blood and immune system, such as anemia and leukemia. Consequently, in many parts of the world, cord blood, once seen as a waste to be discarded after a birth, is now viewed as a valuable resource.
Over the past decade, several public and private cord blood banks have been established in other parts of the world to provide for the collection and preservation of these cells. The PRC is now making these activities available to a limited number of private enterprises in different parts of the PRC, including the Heilongjiang Province where the Company conducts its principal operations. As indicated, our Harbin Biotech subsidiary will have the exclusive right and license to establish a research and development business in this area in northeast China through 2010.
Typically, public cord blood banks collect and store umbilical cord blood donated by women at the birth of a child. This blood is preserved and stored and made available for a significant fee to anyone who needs it in the future. The children of the donor may, in turn, be able to use the stored stem cells to fight various diseases, immune deficiencies and genetic disorders. Storing the stem cells will come at a cost to the donor, consisting of a sizable initial fee and an annual maintenance fee for each year of storage.
Through Harbin Biotech, we are in the process of implementing a plan to establish a cord stem cell and tissue bank at a new facility we are constructing outside Harbin, Heilongjiang Province, PRC. The first stage of construction is expected to be complete in late 2009.
This project represents a substantial commitment and consequently involves a number of significant risks, including, without limitation:
| | · | our need to raise substantial additional capital to fund our stem cell R&D project over the next two or more years, through borrowings, the sale of equity or from income from operations, which, if not obtained on a timely basis, the could severely compromise this project and our rights, |
| | · | our continued compliance with laws and requirements of the PRC and reliance on a license from the PRC government to engage in these research and business operations in northeast China on an exclusive basis, |
| | · | the developing nature of stem cell banking and research, and numerous technical and development challenges, including issues pertaining to the long-term viability of cryogenically frozen cord blood, and |
| | · | our reliance on the efforts of management, in particular Liu Yan-Qing, our President to continue to manage our stem sell research. |
There can be no assurance we will be successful in obtaining capital when needed, or on favorable terms or that the PRC government will not restrict or cancel our rights, or allow other competitors to become engaged in this business in northeast China, which would make it more difficult for us to compete.
While we do not expect that our research and development in this area will have a negative impact on our current core business – the manufacture, marketing and sale of nutritional and medicinal products – the development of this business will require substantial managerial, technical and financial resources.
During the 2008 fiscal year, we had capital expenditures of over $4,358,265 on equipment and machinery; $8,590,651 on construction; as well as additional costs in previous periods on equipment, construction and R&D described in this report.
Sales Approach
We have established a domestic marketing network for our products covering most of the PRC mainland, and have employed sales agents in these areas. Our target customers are chain drug stores and hospitals in all cities. We use distributors to sell products in those countries and remote regions where we do not have sales agents. We have established a marketing network through independent agents to develop an international market. At present, while our primary initial growth focus remains mainland PRC, we have also established over 20 international agents to sell our products, and are expanding our overseas sales efforts. Outside of China, our products are currently sold in Malaysia, the United Kingdom, Hong Kong, the United Arab Emirates, the United States, Russia, Sweden and Ireland.
Materials and Suppliers
We employ a purchasing staff with extensive knowledge of our products who work with marketing, product development, and formulations and quality control personnel to source raw materials for products and other items. Raw materials are sourced principally in the PRC, and are generally available from a variety of suppliers. NoFor the year ended December 31, 2008, one supplier accounts for approximately 33% of our total raw material purchases and no other supplier accounted for 10% or more than 80% of our total raw material purchases. We seek to mitigate the risk of a shortage of raw materials, through identification of alternative suppliers for the same or similar raw materials, where available. We manufacture bulk branded products to allow more extensive vertical integration and to improve the quality and consistency of raw materials.
Customers and Distribution
Currently, our products are sold primarily in the PRC and, to a lesser extent, in [seven]several other locationscountries listed above. Approximately 90% of our revenues in 2008 were from the sale of products in China and Hong Kong, with Malaysia marking our largest country of export.
Over the past several years, we have continuously expanded our distribution channels for our products. As a result, we have established representative sales offices in 22 provinces and 125 municipalities, and deployed sales managers and representatives in each of these markets.
OurIn fiscal 2007, our sales model was focused on the creation of our own distribution channels. Therefore, we sold products are sold directly to many small distributors and retail stores, including pharmacies and drug store chains, and through independent distributors. store locations. In fiscal 2008, we changed our business model and entered into distribution agreements with larger regional sales agents, who resell to smaller distributors and retail store locations. In addition, we entered into contracts with nationwide chain pharmacies. Through the extensive sales networks, of these nationwide chains, we were able to reach all major metropolitan areas throughout the PRC. These changes to our product distribution channels resulted in our direct customer base decreasing from 943 customers at December 31, 2007 to 233 customers (not including branches of retail and drug supply chains) at December 31, 2008. We currently have 233 customers, not including branches of retail and drug supply chains. Our change of sale strategy in fiscal 2008 was initiated to improve product channel efficiencies, and to give us access to an increased number of ultimate purchasers. We believe that these changes will lead to further increased revenue by extending the reach of our distribution network. We also believe that by reducing the number of customers we sell to directly, we will be able to streamline our accounts receivable management and collection, and reduce channel distribution costs. These favorable cost variances are expected to be partially offset by product price incentives we grant to the larger agents with which we have contracted with.
Four customers accounted for approximately 4042 % of our total revenues in 2008.2008, including Shanxi Xintai and Harbin Shiji Baolong, which accounted for approximately 15% and 12% of our total revenues, respectively. In 2007, Ningbo Yuehua Trading Co. and Guangzhou Xhinghe Trading Co. accounted for approximately 14% and 11% of our total revenues, respectively. No other customers accounted for 10% or more of our total revenues in 2008 or 2007.
As a means of accelerating our distribution into other countries, we expect that we will enter into strategic marketing arrangements with firms that have distribution channels, brand name recognition, or other unique marketing strengths. Under a typical arrangement, we expect to will grant limited exclusivity to a sales agent or distributor to certain products in a specified territory, subject to the agent meeting specified minimum monthly or annual sales numbers. Consistent with this approach, in March 2007, we entered into an exclusive strategic agreement with Takasima Industries (“Takasima”), under the terms of which Takasima has been engaged as the exclusive sales agent of our patch products in Malaysia. Takasima will offer our Slim Patch products in Malaysia, under Takasima’s name brand.
We also export a number of our products to various countries, including Malaysia, United Arab Emirates, United Kingdom, Hong Kong, the United States, and others, and utilize agents and independent distributors for these marketing and sales efforts.
We will continue efforts to expand our markets into other provinces and larger cities in the PRC, and to other markets worldwide.
Competition
Competition in the TCM, pharmaceutical, and over-the-counter nutraceutical business is intense in China and throughout the world. We compete with various firms, many of which produce and market products similar to our products, and many of which have greater resources than us in terms of manufacturing and marketing capabilities, management expertise and breadth, and financial wherewithal. Some of these competitors are far larger, have more resources then us and have stronger sales and distribution networks.
Our direct competitors are other domestic firms engaged in developing, manufacturing and marketing TCM and nutraceutical products. There are many of these companies in the PRC, in Heilongjiang Province, and even in the city of Harbin.
We expect that the competition for medicinal products in the PRC and other world markets will become more intense over the next few years both from existing competitors and new market entrants. We will also face competition from foreign companies who may have established products, a strong proprietary pipeline and strong financial resources. Our management believes that we have certain competitive advantages in introducing new products to market due to key focus areas for development, our existing distribution channels, research and development capabilities and our relationship with certain universities and other research institutions. However, there can be no assurance that we will be able to compete and continue to grow in this highly competitive environment. Additional information relating to competition in the PRC can be found in the “Risk Factors” section below.
Government Regulation
Regulatory Environment
Our principal sales market is in the PRC. We are subject to the Pharmaceutical Administrative Law of the PRC, which governs the licensing, manufacturing, marketing and distribution of pharmaceutical products in the PRC, and sets penalties for violations. Our business is subject to various regulations and permit systems of the government of the PRC. Additionally, we are subject to government licensing rights and regulations, which relating to our stem cell R&D license. Permits we attain for TCM products are granted on a non-exclusive basis and one limited for four to five years.
The governmental approval process in the PRC for a newly developed health product can be lengthy and difficult. A product sample is first sent to a clinical testing agent designated by the Ministry of Health, which conducts extensive clinical testing and examinations of the product to verify if it has the specified functions as stated by the company producing the product. A report will then be prepared and issued by the clinical testing agent confirming or negating such functions. It generally takes six months to one year for a report to be issued by the testing agent, after submittal to the agent. The report must then be submitted to a provincial Health Management Commission for approval. Following this submittal, a letter of approval issued by such commission will be submitted to the Ministry of Health for the issuance of a certificate that authorizes sale and marketing of the product in the PRC.
This entire process will generally take between eighteen months and two years. The approval process will depend to a certain extent on whether a specified product is a plant based pharmaceutical (“PBP”), or a plant based nutraceutical (“PBN”). PBPs are products composed of herbs, roots and plants that do not use synthetic chemicals, with certain medicinal functions for treatment of one or more illnesses. PBPs are generally prescription-based but in some cases may be sold over-the-counter. PBNs, also frequently known as “dietary supplements” or “nutritional supplements,” are also composed of herbs, roots and plants, but are essentially prophylactic or preventive in nature. All PBNs are available over-the-counter without a prescription. In the PRC, PBPs require the approval of the SFDA, and PBNs only require the approval of state and local governments prior to manufacturing and sale. Obtaining the approval from the SFDA is generally more complex and lengthy.
Because we and our subsidiaries are wholly-owned enterprises, we are subject to the law of foreign investment enterprises in the PRC, and the foreign company provisions of the Company Law of China, which governs the conduct of our wholly-owned subsidiaries and their officers and directors, and also limits our ability to pay dividends.
Compliance with Environmental Law
We comply with the Environmental Protection Law of the PRC, as well as applicable local regulations. In addition to compliance with the PRC law and local regulations, we consistently undertake active efforts to ensure the environmental sustainability of our operations. Because the manufacturing of herb and plant-based products does not generally cause significant damage or pollution to the environment, the cost of complying with applicable environmental laws is not material. In the event we fail to comply with applicable laws, we may be subject to penalties.
Intellectual Property
We regard our service marks, trademarks, trade secrets, patents and similar intellectual property (“IP”) as critical to our business. We have relied, and will continue to rely, on patent, trademark and trade secret law, as well as confidentiality and license agreements with certain of our employees, consultants, customers and others, to protect our proprietary rights.
Under the PRC State Protection law, certain herbal medicine products which have received approval from the SFDA, have automatic protected IP rights for a seven-year period from the date of grant of such approval. An application can be submitted to extend such protection for up to three consecutive seven-year periods. Once this protection period has expired, an applicant may apply for patent protection in the PRC which lasts for up to 20 years for traditional medicines depending on the type of patent, and is renewable for indefinite number of times. Patents for arts and crafts and packaging have 10 year patent protection periods which are also renewable. To a large extent, we rely on such State Protection law to protect our IP rights with respect to our products. In addition, as of the date of this filing, we own a total of 7 patents in the PRC pertaining to our TCMs and biotech diagnostic kits and drugs, as follows:
| | · | Package foil bag design patent of Sumei slim patch, registered December 4, 2001; |
| | · | Package box design patent for all TCM products, registered December 4, 2001; |
| | · | Arts and crafts patent of Human Urinary Albumin Elisa Kit, registered August 24, 2004; |
| | · | Arts and crafts patent of Sumei slim patch, registered in 2001; |
| | · | Arts and crafts design patent of myocardial infarction testing kit, registered March 16, 2004; |
| | · | Arts and crafts patent of Suning cough removing patch, initially registered December 4, 2001; and |
| | · | Endothelin-1 patent relating to anti-tumor technology (application for public instruction made), registered October 4, 2006; |
We have received awards and grants from the government of the PRC for R&D in 2007 for the below listed products, resulting in a total amount of $2,141,022 (15,000,000 RMB) of which $42,492 (300,000 RMB) has been paid with the remaining amount anticipated to be available to us in 2008:
| | · | High Technology products certificates by Heilongjiang High Technology Products Committee covering the following products: |
| ¡ | Gonorrhea Cleaning Spray; |
| | ¡ | Suning Cough removing patch; and |
| | · | National Class Torch Project (pertaining to the Sumei slim patch); |
| | · | Excellence Products Award for Human Urinary Albumin Elisa Kit by The 6th New & High Technology Fruits Fair Shen Zhen and National Commercial Department; |
| | · | 100 important pre-phase projects in Heilongjiang Province covering various medical diagnostics kits; |
| | · | Material Medical Technology Research and Development Company (by Heilongjiang provincial Science and Technology Bureau); and |
| | · | High Technology Industrialized Base of Medical Area, by Heilongjiang Provincial Development and Reform Committee (March of 2006). |
Trademarks
We have registered “Kang Xi” as our trademark, which is used for all of our TCM products.
Employees
The number of our employees has increased over the past two years, due to growth, increased research and development and expanded marketing and distribution of products Currently we have a total of 1,804 full time employees and manufacturers’ representatives, generally falling into the following categories:
By subsidiary company:
| Company | | Number of Employees | |
| TDR | | | 1515 | |
| Harbin Biotech | | | 0 | |
| Harbin Bio-Engineering | | | 97 | |
| Tianlong | | | 97 | |
| Haina | | | 24 | |
| Jin Chuang | | | 71 | |
| TOTAL: | | | 1,804 | |
By nature of job:
| Type of Job | | Number of Employees | |
| Executives and Managers | | | 146 | |
| Production and Clerical | | | 359 | |
| Sales and Marketing | | | 1,261 | |
| Research and Development, Technology | | | 38 | |
| TOTAL: | | | 1,804 | |
* Includes manufacturers’ representatives.
** Does not include 10 part time technical researchers.
We do not have any employment agreements in place with our executive officers. None of the employees are covered by a collective bargaining agreement, however, we believe our relationship with employees is good.
Available Information
We file various reports with the SEC, including Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q and Current Reports on From 8-K, which are available though the SEC’s electronic data gathering, analysis and retrieval system by accessing the SEC’s home page (http://www.sec.gov). The documents are also available to be read or copied at the SEC’s Public Reference Room located at 100 F Street, NE, Washington, D.C., 20549. Information on the Public Reference Room may be obtained by calling the SEC at 1-800-SEC-0330.
Item 1A. Risk Factors.
We are subject to certain risks and uncertainties as described below. These risks and uncertainties may not be the only ones we face. There may be additional risks that we do not presently know of, or that we currently consider immaterial. All of these risks could adversely affect our business, financial condition, results of operations and cash flows. Our business and operations may be adversely affected if any of such risks are realized. All investors should consider the following risk factors before deciding to purchase or sell our securities.
Risks Related to Our Business
Adverse economic conditions may harm our business.
In 2008, general worldwide economic conditions declined due to sequential effects of the sub prime lending crisis, general credit market crisis, collateral effects on the finance and banking industries, concerns about inflation, slower economic activity, decreased consumer confidence, reduced corporate profits and capital spending, adverse business conditions and liquidity concerns. This global economic downturn poses a risk as consumers and businesses may postpone spending, or seek new ways to eliminate spending, in response to these uncertain and challenging economic conditions. In addition, there could be a number of follow-on effects including foreign currency exchange rate fluctuations, insolvency of key suppliers and customer insolvencies. We cannot predict the timing or duration of any economic slowdown or recession or the timing or strength of a subsequent recovery, worldwide, or in the specific markets we serve. If the markets for our products significantly deteriorate due to these economic effects, our business, financial condition and results of operations may be materially and adversely affected.
Certain officers and directors have significant control over our company.
Dr. Liu Yan-qing and Ms. Han Xiao-yan, who are officers and directors of ours, also serve as officers and directors of ACPG and TDR. As of the date hereof, Dr. Liu and Ms. Han own, in the aggregate, approximately 36.7% of the issued and outstanding shares of our common stock. As a result, these shareholders are effectively able to control certain corporate governance matters requiring shareholders’ approval. Such matters may include transactions in which they have an interest other than as a shareholder of ours, the approval of significant corporate transactions such as increasing the authorized number of our shares to complete acquisitions or raise capital, if necessary, and any other transactions requiring a majority vote without seeking other shareholders’ approval. These persons also have the ability to control other matters requiring shareholder approval including our election of directors which could result in the entrenchment of management.
We depend on our key management personnel and the loss of their services could adversely affect our business.
We place substantial reliance upon the efforts and abilities of our executive officers, Liu Yan-qing, President, Chief Executive Officer and Chairman of the Board, Han Xiao-yan, Vice Chairman, and Hao Yu-bo, Chief Financial Officer and Secretary. We do not have employment agreements with these members of management. Accordingly, if any of these persons should leave the company we would have no remedy or protections in place and would not be able to prevent them from competing with us or working for competitors. The loss of the services of any of these executive officers could have a material adverse effect on our business, operations, revenues or prospects. In addition, we do not maintain key man life insurance on the lives of these individuals.
Our expansion plan may not be successful.
Part of our strategy is to grow through increasing the distribution and sales of our products by penetrating existing markets in the PRC and Hong Kong, and entering new geographic markets in the PRC as well as Asia, the United States and other countries. However, many obstacles to entering such new markets exist, including, but not limited to, international trade and tariff barriers, regulatory constraints, product liability concerns, shipping and delivery costs, costs associated with marketing efforts abroad and maintaining attractive foreign exchange ratios. Moreover, our expansion strategy may be based on incorrect assumptions and may be flawed, and may even damage our performance, competitive position in the market and ultimately even our ability to survive in the marketplace. Even if the strategy is correct, we may never be able to successfully implement our strategy. We cannot, therefore, assure shareholders that we will be able to successfully overcome such obstacles and establish our products in any additional markets. Our inability to implement this growth strategy successfully may have a negative impact on growth, future financial condition, results of operations or cash flows.
There are many safety risks involved in our products and services that could expose us to liability or inhibit our ability to secure insurance.
Our products and services involve direct or indirect impact on human health and life. The drugs, products and services we manufacture and sell may be flawed and cause dangerous side effects and even fatality in certain cases, and lead to major business losses and legal and other liabilities and damages to our company. In the event that any of our products are alleged to have adverse side effects, we could be subject to product liability claims. In addition to the threat of liability, there may be insurance costs if we enter into certain markets or may not be able to obtain insurance for certain products in some countries. Some distributors may refuse to sell our products in certain countries if they perceive such products to have a high risk or to be uninsurable.
We do not maintain any insurance and are exposed to all risks of loss, including resulting form product liability, property loss or damages or other harm that we may cause to customers, vendors, suppliers and other third parties and securities law claims.
We do not maintain liability or property insurance coverage or director and officer insurance coverage and, therefore, we are self-insured for all risks of loss. Although we seek to reduce potential liability through measures such as contractual indemnification provisions with distributors and suppliers, we cannot assure you that such measures will be enforced or effective. Our policy is to record losses associated with our lack of insurance coverage at such time as realized loss is incurred. Historically, we have not had any material losses in connection with our lack of insurance coverage and are not party to any material pending legal proceedings as of the date of this report. Management’s intention is to use out working capital to fund any such losses incurred due to our exposure to inadequate insurance coverage. Our operating results could be materially and adversely affected if we were to pay significant damages or incur significant defense costs in connection with a claim.
We are highly dependent upon the public perception and quality of our products. Additionally, anti-corruption measures taken by the government to correct corruptive practices in the pharmaceutical industry could adversely affect our sales and reputation.
We are highly dependent upon consumers’ perception of the safety and quality of our products as well as similar products distributed by other companies. Thus, the mere publication of reports asserting that such products may be harmful could have a material adverse effect on our business, regardless of whether these reports are scientifically supported.
The government has recently taken anti-corruption measures to correct corrupt practices. In the pharmaceutical industry, such practices include, among others, acceptance of kickbacks, bribery or other illegal gains or benefits by the hospitals and medical practitioners from pharmaceutical distributors in connection with the prescription of a certain drug. Substantially all of our sales to our ultimate customers are conducted through third-party distributors. We have no control over our third-party distributors, who may engage in corrupt practices to promote our products. While we maintain strict anti-corruption policies applicable to our internal sales force and third-party distributors, these policies may not be effective. If any of our third-party distributors engage in such practices and the government takes enforcement action, our products may be seized and our own practices, and involvement in the distributors’ practices may be investigated. If this occurs, our sales and reputation may be materially and adversely affected.
Our success will depend on our research and the ability to develop new products.
Our growth depends on our ability to consistently discover, develop and commercialize new products and find new and improve on existing technologies, platforms and products. As such, if we fail to make sufficient investments in research, to be attentive to consumer needs, or fail to focus on the most advanced technologies, our current and future products could be surpassed by more effective or advanced products of other companies.
We currently rely on third parties to supply the key raw materials we use to produce our products.
Our business depends upon the availability of key raw materials. We rely on only external suppliers for these raw materials. In fiscal year 2008, we purchased approximately 33% of our total raw materials from Heilongjiang Kangda Medicine Co. (“HKM”). Our purchases through September 30, 2009 were or from similar sources and in increased amounts commensurate with the increase in demand for our products. For the 2010 fiscal year we expect that our raw material suppliers will be substantially similar to past years and the amount of raw materials will increase commensurate with the increase in the demand of our products. Management believes there are alternative suppliers available to fulfill our raw material needs. However, if HKM or any of our other suppliers were to default or become unable to deliver the raw materials in sufficient quantities, we may be unable to purchase these raw materials from alternative sources on the same or similar terms, which could result in a significant decrease in our operating costs. In addition, any disruption in the supply of our raw materials could cause delay in the delivery of our products which would be harmful to our sales reputation and business. If supply is disrupted the increased amount we have to pay for raw materials could negatively impact our margins, cause us to cease production if an alternate supplier cannot be found. If we are unable to procure replacement supplies, our ability to meet the production demands of our customers could cause the loss of costumers and/or market share. Our financial results could be negatively impacted by the lost sales or decrease margins.
Significant competition from existing and new entities could adversely affect revenues and profitability.
We compete with other companies, many of which are offering and/or developing, or can be expected to develop and offer, products similar to ours. Our market is a large market with many competitors. Many of our competitors are more established than we are, and have significantly greater financial, technical, marketing and other resources than our company. Some of our competitors have greater name recognition and a larger customer base. These competitors may be able to respond more quickly to new or changing opportunities and customer requirements and may be able to undertake more extensive promotional activities, offer more attractive terms to customers, and adopt more aggressive pricing policies. We cannot assure investors that we will be able to compete effectively with current or future competitors or that the competitive pressures we face will not harm our business.
We may not be able to obtain sufficient financing, and may not be able to develop our product candidates.
We may need to incur debt or issue equity in order to fund research and other expenditures as well as to make acquisitions and other investments. We cannot assure you that debt or equity financing will be available to us on acceptable terms or at all. If we cannot or are limited in the ability to incur debt, issue equity or enter in strategic collaborations, we may be unable to fund discovery and development of our product candidates, address gaps in our product offerings or improve our technologies.
We anticipate that we will need to raise substantial amounts of money to fund a variety of future activities integral to the development of our business, which may include but are not limited to the following:
| | · | obtaining regulatory approval for our products and conducting research and development to successfully develop our stem cell and other technologies; |
| | · | filing and prosecuting patent applications and defending and assessing patents to protect our technologies; |
| | · | retaining qualified employees, particularly in light of intense competition for qualified scientists; |
| | · | manufacturing products ourselves or through third parties; |
| | · | marketing our products, either through building our own sales and distribution capabilities or relying on third parties; and |
| | · | acquiring new technologies, licenses or products. |
We cannot assure you that any needed financing will be available to us on acceptable terms or at all. If we cannot obtain additional financing in the future, our operations may be restricted and we may ultimately be unable to continue to develop and potentially commercialize our product candidates.
We are subject to market and channel risks.
Over 85% of our sales are made in the PRC, where we primarily sell our products through drug chain stores. Because of this, we are dependent to a large degree upon the success of our PRC based distribution channel as well as the success of specific retailers in the distribution channel. Many of the drug stores are individual stores or very small chains, and only a few are large chain drug stores. We rely on these distribution channels to purchase, market, and sell our products. Our success is dependent, to a large degree, on the growth and success of the drug stores, which may be outside our control. There can be no assurance that the drug store distribution channels will be able to grow or prosper as it faces price and service pressure from other channels, including the mass market. There can be no assurance that retailers in the drug store distribution channel, in the aggregate, will respond or continue to respond to our marketing commitment in these channels.
We may have difficulty in defending intellectual property rights from infringement.
Our TCM products are generally not protected by patents but by trade secrets. Certain TCM license agreements are made on a non-exclusive basis. Our success depends, in large part, on our ability to protect current and future technologies and products and to defend our intellectual property rights. If we fail to protect our intellectual property adequately, competitors may manufacture and market similar products. We continually file, patent applications seeking to protect newly developed technologies and products in various countries, particularly in the PRC. Some patent applications in the PRC are maintained in secrecy until the patent is issued. Because the publication of discoveries tends to follow their actual discovery by many months, we may not be the first to invent, or file patent applications on any of its discoveries. Patents may not be issued with respect to any of our patent applications and existing or future patents issued to or licensed by us may not provide competitive advantages for its products. Patents that are issued may be challenged, invalidated or circumvented by competitors. Furthermore, our patent rights may not prevent our competitors from developing, using or commercializing products that are similar or functionally equivalent to our products.
To the extent that we market products in other countries, we may have to take additional action to protect our intellectual property. The measures we take to protect our proprietary rights may be inadequate, and we cannot provide any assurance that our competitors will not independently develop formulations and processes that are substantially equivalent or superior to our products or copy our products.
We also rely on trade secrets, non-patented proprietary expertise and continuing technological innovation that we seek to protect, in part, by entering into confidentiality agreements with licensees, suppliers, employees and consultants. These agreements may be breached and there may not be adequate remedies in the event of a breach. Disputes may arise concerning the ownership of intellectual property or the applicability of confidentiality agreements. Moreover, trade secrets and proprietary technology may otherwise become known or be independently developed by competitors. If patents are not issued with respect to products arising from research, we may not be able to maintain the confidentiality of information relating to these products.
We will be subject to risks relating to third parties that may claim that we infringe on their proprietary rights and may prevent us from manufacturing and selling certain of our products.
There has been substantial litigation in the pharmaceutical and nutraceutical industries with respect to the manufacturing, use and sale of new products. These lawsuits relate to the validity and infringement of patents or proprietary rights of third parties. We may be required to commence or defend against charges relating to the infringement of patent or proprietary rights. Any such litigation could involve or result in:
| | · | the incurrence of substantial expense, even if we are successful in the litigation; |
| | · | a diversion of significant time and effort of technical and management personnel; |
| | · | the loss of our rights to develop or make certain products; and |
| | · | the payment of substantial monetary damages or royalties in order to license proprietary rights from third parties. |
Although patent and intellectual property disputes within these industries have often been settled through licensing or similar arrangements, costs associated with these arrangements may be substantial and could include the long-term payment of royalties. These arrangements may be investigated by regulatory agencies and, if improper, may be invalidated. Also, the required licenses may not be made available to our company on acceptable terms. Accordingly, an adverse determination in a judicial or administrative proceeding or a failure to obtain necessary licenses could prevent our company from manufacturing and selling some of our products or increase costs to market these products.
In addition, when seeking regulatory approval for some of our products, we are required to certify to regulatory authorities, including the SFDA that such products do not infringe upon third party patent rights. Filing a certification against a patent gives the patent holder the right to bring a patent infringement lawsuit against our company. Any lawsuit would delay regulatory approval by the SFDA. A claim of infringement and the resulting delay could result in substantial expenses and even prevent us from manufacturing and selling certain of our products.
The launch of a product prior to a final court decision or the expiration of a patent held by a third party may result in substantial damages to our company. Depending upon the circumstances, a court may award the patent holder damages equal to three times their loss of income. If our company is found to infringe a patent held by a third party and become subject to such treble damages, these damages could have a material adverse effect on our results of operations and financial condition.
Our failure to comply with accounting policies and regulations in making reasonable estimates and judgments could negatively impact our financial position and results of operation.
We will be subject to critical accounting policies and actual results may vary from estimates. We have followed, and will continue to follow, generally accepted accounting principles for the United States in preparing financial statements. As part of this work, we must make many estimates and judgments concerning future events. These affect the value of the assets and liabilities, contingent assets and liabilities, and revenue and expenses reported in such financial statements. We believe that these estimates and judgments are reasonable, and we have made them in accordance with accounting policies based on information available at the time. However, actual results could differ from estimates, and this could require us to record adjustments to expenses or revenues that could be material to our financial position and results of operations in the future.
Our business is subject to many governmental regulatory and policy risks.
Our business must be conducted in compliance with various government regulations and in particular, the PRC SFDA regulations. Government regulations may have material impact on our operations, increase costs and could prevent or delay the manufacturing and selling of our products. Research, development, testing, manufacturing and marketing activities are subject to various governmental regulations in China, including health and drug regulations. Government regulations, among other things, cover the inspection of and controls over testing, manufacturing, safety and environmental considerations, efficacy, labeling, advertising, promotion, record keeping and sale and distribution of pharmaceutical products. We will not be able to license, manufacture, sell and distribute the vast majority of its products without a proper approval from government agencies and in particular the SFDA. There is no assurance that we will obtain such approvals.
In addition, delays or rejections may be encountered based upon additional government regulation from future legislation, administrative action or changes in governmental policy and interpretation during the period of product development and product assessment. Although we have, so far, obtained the rights to sell our products in the PRC, we may not continue to receive and maintain regulatory approvals for the sales of these products. Our marketing activities are also subject to government regulations with respect to the prices that it intends to charge or any other marketing and promotional related activities. Government regulations may substantially increase the costs for developing, licensing, manufacturing and selling products, impacting negatively our operations, revenue, income and cash flow. For more specific risks relating to doing business in the PRC see “Risks Related to Doing Business in China” below.
There could be changes in government regulations towards the pharmaceutical and nutraceutical industries that may adversely affect our business.
The manufacture and sale of pharmaceutical and nutraceutical products in the PRC is heavily regulated by many state, provincial and local authorities. These regulations significantly increased the difficulty and costs involved in obtaining and maintaining regulatory approvals for marketing new and existing products. Our future growth and profitability depends to a large extent on our ability to obtain regulatory approvals.
The SFDA of China implemented new guidelines for licensing of pharmaceutical products. All existing manufacturers with licenses, which are currently valid under the previous guidelines, were required to apply for the Good Manufacturing Practices “GMP” certifications by June 30, 2004, and to receive approvals by December 31, 2004. We received certifications for our current products. However, should we fail to maintain the GMP certifications under the new guidelines in the future, or for new products, our businesses would be materially and adversely affected.
Moreover, the laws and regulations regarding acquisitions of the pharmaceutical and nutraceutical industries in the PRC may also change and may significantly impact our ability to grow through acquisitions. For more specific risks relating to doing business in the PRC see “Risks related to Doing Business in China” below.
We need to manage growth in operations to maximize our potential growth and achieve our expected revenues.
Our success depends on our ability to achieve continued growth. In order to maximize potential growth in current and potential markets, we believe that we must expand our manufacturing and marketing operations. This expansion will place a significant strain on management and operational, accounting and information systems and will require substantial additional capital. We will need to continue to improve financial controls, operating procedures, and management information systems if and as we grow. We will also need to effectively train, motivate, and manage our employees. A failure to manage our growth could disrupt operations and ultimately prevent us from generating the revenues we expect.
International operations require our company to comply with a number of U.S. and international regulations.
We are required to comply with a number of international regulations in countries outside of the United States. In addition, we must comply with the Foreign Corrupt Practices Act, or FCPA, which prohibits U.S. companies or their agents and employees from providing anything of value to a foreign official for the purposes of influencing any act or decision of these individuals in their official capacity to help obtain or retain business, direct business to any person or corporate entity or obtain any unfair advantage. Any failure to adopt appropriate compliance procedures and ensure that our employees and agents comply with the FCPA and applicable laws and regulations in foreign jurisdictions could result in substantial penalties and/or restrictions in our ability to conduct business in certain foreign jurisdictions. The U.S. Department of The Treasury’s Office of Foreign Asset Control, or OFAC, administers and enforces economic and trade sanctions against targeted foreign countries, entities and individuals based on U.S. foreign policy and national security goals. As a result, we are restricted from entering into transactions with certain targeted foreign countries, entities and individuals except as permitted by OFAC which may reduce our future growth.
We may incur significant costs to ensure compliance with U.S. corporate governance and accounting requirements.
We are a public reporting company, and, as such, we will incur significant costs associated with public company reporting requirements, costs associated with newly applicable corporate governance requirements, including requirements under the Sarbanes-Oxley Act of 2002 and other rules implemented by the U.S. Securities and Exchange Commission. All of these applicable rules and regulations can be expected to increase legal and financial compliance costs and to make some activities more time consuming and costly. Management also expects that these applicable rules and regulations may make it more difficult and more expensive to obtain director and officer liability insurance and we may be required to accept reduced policy limits and coverage or incur substantially higher costs to obtain the same or similar coverage. As a result, it may be more difficult for our company to attract and retain qualified individuals to serve on our board of directors or as executive officers.
We may have difficulty raising necessary capital to fund operations as a result of market price volatility for our shares of common stock.
In recent years, the securities markets in the United States have experienced a high level of price and volume volatility, and the market price of securities of many companies have experienced wide fluctuations that have not necessarily been related to the operations, performances, underlying asset values or prospects of such companies. For these reasons, our shares of common stock can also be expected to be subject to volatility resulting from purely market forces over which we will have no control. If our business development plans are successful, we may require additional financing to continue to develop and exploit existing and new technologies and to expand into new markets. The exploitation of existing and new technologies may, therefore, be dependent upon our ability to obtain financing through debt and equity or other means.
We are obligated to indemnify our officers and directors for certain losses they suffer.
To the fullest extent permitted by Chapter 78 of the Nevada Revised Statues, we may, if and to the extent authorized by our board of directors, indemnify our officers and any other persons who we have power to indemnify against liability, reasonable expense or other matter whatsoever. If we are required to indemnify any persons under this policy, we may have to pay indemnity in a substantial amount which we may be unable to recover at all.
Risks Related to Doing Business in China
Our business will be affected by the government regulation and Chinese economic environment because most of our sales will be in the China market.
The manufacture and sale of pharmaceutical products in China is heavily regulated by many state, provincial and local authorities. The SFDA of China requires pharmaceutical manufacturers to obtain Good Manufacturing Practices, or GMP, certifications. We currently have the certifications needed for our current operations. However, should we fail to receive or maintain the GMP certifications in the future, we would no longer be able to manufacture pharmaceuticals in China, and our businesses would be materially and adversely affected. These regulations significantly increase the difficulty and costs involved in obtaining and maintaining regulatory approvals for marketing new and existing products. Our future growth and profitability depend to a large extent on our ability to obtain regulatory approvals. Additionally, the law could change so as to prohibit the use of certain pharmaceuticals. If one of our products becomes prohibited, this change would cease the productivity of that product. The China National Development and Reform Commission, or CNDRC, has recently implemented price adjustments on many marketed pharmaceutical products. We have no control over such governmental policies, which may impact the pricing and profitability of our products.
Although we have started exporting products to other countries, most of our sales are in the PRC and Hong Kong. It is anticipated that our products in the PRC will continue to represent a significant portion of sales in the near future. As a result of our reliance on the PRC markets, our operating results and financial performance could be affected by any adverse changes in economic, political and social conditions in the PRC.
The modernization of regulations for the pharmaceutical industry is relatively new in the PRC, and the manner and extent to which it is regulated will continue to evolve. As a pharmaceutical company, we are subject to the Pharmaceutical Administrative Law, which governs the licensing, manufacture, marketing and distribution of pharmaceutical products in the PRC, and sets penalty provisions for violations of provisions of the Pharmaceutical Administrative Law. In addition as a “Foreign Owned Enterprise,” we will be subject to the Foreign Company provisions of the Company Law of the PRC. Changes in these laws or new interpretations of existing laws may have a significant impact our methods and our cost of doing business. For example, if legislative proposals for pharmaceutical product pricing, reimbursement levels, approval criteria or manufacturing requirements should be proposed and adopted, such new legislation or regulatory requirements may have a material adverse effect on our financial condition, results of operations or cash flows. In addition, we are subject to varying degrees of regulation and licensing by governmental agencies in China. At this time, we are unaware of any China legislative proposals that could adversely affect our business. There can be no assurance that future regulatory, judicial and legislative changes will not have a material adverse effect on our operations, that regulators or third parties will not raise material issues with regard to compliance or non-compliance with applicable laws or regulations, or that any changes in applicable laws or regulations will not have a material adverse effect on our business.
Certain political and economic considerations relating to China could adversely affect our company.
China is transitioning from a planned economy to a market economy. While the PRC government has pursued economic reforms since its adoption of the open-door policy in 1978, a large portion of the Chinese economy is still operating under five-year plans and annual state plans. Through these plans and other economic measures, such as control on foreign exchange, taxation and restrictions on foreign participation in the domestic market of various industries, the PRC government exerts considerable direct and indirect influence on the economy. Many of the economic reforms carried out by the PRC government are unprecedented or experimental, and are expected to be refined and improved. Other political, economic and social factors can also lead to further readjustment of such reforms. This refining and readjustment process may not necessarily have a positive effect on our operations or future business development. Our operating results may be adversely affected by changes in China’s economic and social conditions as well as by changes in the policies of the PRC government, such as changes in laws and regulations, or the official interpretation thereof, which may be introduced to control inflation, changes in the interest rate or method of taxation, and the imposition of additional restrictions on currency conversion.
Accordingly, government actions in the future, including any decision not to continue to support recent economic reforms and to return to a more centrally planned economy or regional or local variations in the implementation of economic policies, could have a significant effect on economic conditions in China or particular regions thereof, and could require us to divest ourselves of any interest we then hold in Chinese properties or joint ventures.
There are risks inherent in doing business in China.
The PRC is a developing country with a young market economic system overshadowed by the state under heavy regulation and scrutiny. Its political and economic systems are very different from the more developed countries. China also faces many social, economic and political challenges that may produce major shocks and instabilities and even crises, in both its domestic arena and in its relationship with other countries, including but not limited to the United States. Such shocks, instabilities and crises may in turn significantly and adversely affect our performance.
The recent nature and uncertain application of many PRC laws applicable to our company create an uncertain environment for business operations and they could have a negative effect on our business and operations.
The PRC legal system is a civil law system. Unlike the common law system, the civil law system is based on written statutes in which decided legal cases have little value as precedents. In 1979, the PRC began to promulgate a comprehensive system of laws and has since introduced many laws and regulations to provide general guidance on economic and business practices in the PRC and to regulate foreign investment. Progress has been made in the promulgation of laws and regulations dealing with economic matters such as corporate organization and governance, foreign investment, commerce, taxation and trade. The promulgation of new laws, changes of existing laws and the abrogation of local regulations by national laws could have a negative impact on our business, business prospects and operations. In addition, as these laws, regulations and legal requirements are relatively recent, their interpretation and enforcement involve significant uncertainty.
It may be difficult to effect service of process and enforcement of legal judgments upon our company and its officers and directors because they reside outside the United States.
As our operations are presently based in the PRC and our directors and officers reside in the PRC, service of process on our company and such directors and officers may be difficult to effect within the United States. Also, substantially all of our assets are located in the PRC and any judgment obtained in the United States against our company may not be enforceable outside the United States.
Our business may be affected by unexpected changes in regulatory requirements in the jurisdictions in which we operate.
Our company, and its subsidiaries, are subject to many general regulations governing business entities and their behavior in China and in other jurisdictions in which we and our subsidiaries have, or plan to have, operations and market products. In particular, we are subject to laws and regulations covering food, dietary supplements and pharmaceutical products. Such regulations typically deal with licensing, approvals and permits. Any change in product licensing may make our products more or less available on the market. Such changes may have a positive or negative impact on the sale of our products and may directly impact the associated costs in compliance and our operational and financial viability. Such regulatory environment also covers any existing or potential trade barriers in the form of import tariff and taxes that may make it difficult for us to import our products to certain countries and regions, such as Hong Kong, which would limit its international expansion.
We may have difficulty attracting talent in foreign countries.
Currently, over 85% of our sales are in the PRC and in Hong Kong. We are in the process of attempting to establish marketing and sales presence in the United States and other countries. We expect to establish an office in the United States for investor relations. In the future, we may explore expanding its operations in the United States, as well as other countries throughout the world. Upon effecting any such expansion, we may not be able to identify and retain qualified personnel due to its lack of understanding of different cultures and lack of local contacts. This may impede international expansion.
Currency conversion and exchange rate volatility could adversely affect our financial condition, by making acquisitions in China or of Chinese products more expensive.
The PRC government imposes control over the conversion of RMB into foreign currencies. Under the current unified floating exchange rate system, the People’s Bank of China publishes an exchange rate, referred to as the PBOC exchange rate, based on the previous day’s dealings in the inter-bank foreign exchange market. Financial institutions authorized to deal in foreign currency may enter into foreign exchange transactions at exchange rates within an authorized range above or below the PBOC exchange rate according to market conditions.
Pursuant to the Foreign Exchange Control Regulations of the PRC issued by the State Council which came into effect on April 1, 1996, and the Regulations on the Administration of Foreign Exchange Settlement, Sale and Payment of the PRC which came into effect on July 1, 1996, regarding foreign exchange control, conversion of RMB into foreign exchange by Foreign Investment Enterprises, or FIE’s, for use on current account items, including the distribution of dividends and profits to foreign investors, is permissible. FIEs are permitted to convert their after-tax dividends and profits to foreign exchange and remit such foreign exchange to their foreign exchange bank accounts in the PRC.
Conversion of RMB into foreign currencies for capital account items, including direct investment, loans, and security investment, is still subject to certain restrictions. On January 14, 1997, the State Council amended the Foreign Exchange Control Regulations and added, among other things, an important provision, which provides that the PRC government shall not impose restrictions on recurring international payments and transfers under current account items. These rules are subject to change.
Enterprises in the PRC (including FIEs) which require foreign exchange for transactions relating to current account items, may, without approval of the State Administration of Foreign Exchange, or SAFE, effect payment from their foreign exchange account or convert and pay at the designated foreign exchange banks by providing valid receipts and proofs.
Convertibility of foreign exchange in respect of capital account items, such as direct investment and capital contribution, is still subject to certain restrictions, and prior approval from the SAFE or its relevant branches must be sought.
Our company is a FIE to which the Foreign Exchange Control Regulations are applicable. There can be no assurance that we will be able to obtain sufficient foreign exchange to pay dividends or satisfy other foreign exchange requirements in the future.
Since 1994, the exchange rate for RMB against the United States dollars has remained relatively stable, most of the time in the region of approximately RMB8.00 to US$1.00. However, in 2005, the Chinese government announced that would begin pegging the exchange rate of the Chinese RMB against a number of currencies, rather than just the U.S. dollar. Currently, exchange rates are approximately RMB 1.44 to US$1.00 resulting in the increase in price of Chinese products to U.S purchasers. As our operations are primarily in China, any significant revaluation of the Chinese RMB may materially and adversely affect cash flows, revenues and financial condition. For example, to the extent that we need to convert United States dollars into Chinese RMB for operations, appreciation of this currency against the United States dollar could have a material adverse effect on our business, financial condition and results of operations. Conversely, if we decide to convert Chinese RMB into United States dollars for other business purposes and the United States dollar appreciates against this currency, the United States dollar equivalent of the Chinese RMB that we convert would be reduced.
We are required to be in compliance with the registered capital requirements of the PRC.
Under the Company Law of the PRC, our company will be required to contribute a certain amount of “registered capital” to our wholly owned subsidiary. By law, our subsidiaries are required to contribute at least 10% of after tax net income (as determined in accordance with Chinese GAAP) into a statutory surplus reserve until the reserve is equal to 50% of the Company and its subsidiaries’ registered capital, and between 5% and 10% of its after tax net income, as determined by our board of directors, into a public welfare fund. These reserve funds are recorded as part of shareholders’ equity but are not available for distribution to shareholders other than in the case of liquidation. As a result of this requirement, the amount of net income available for distribution to shareholders will be limited.
Since most of our assets are located in the PRC, any dividends or proceeds from liquidation are subject to the approval of the relevant PRC government agencies. We are not likely to declare dividends in the near future and would need regulatory approval to do so.
Because our assets are predominantly located inside the PRC, we will be subject to the law of the PRC in determining dividends. Under the laws governing foreign invested enterprises in the PRC, dividend distribution and liquidation are allowed but subject to special procedures under the relevant laws and rules. Any dividend payment will be subject to the decision of the board of directors and subject to foreign exchange rules governing such repatriation. Any liquidation is subject to both the relevant government agency’s approval and supervision as well the foreign exchange control. This may generate additional risk for investors in case of dividend payment and liquidation.
Risks Relating to the Market for Our Common Stock
Our stock price is likely to be highly volatile.
The trading price of our common stock has been highly volatile. Failure to meet market expectations in our financial results could cause our stock price to decline. Moreover, factors that are not related to our operating performance could cause our stock price to decline. The stock market has recently experienced significant price and volume fluctuations that have affected the market prices for securities of technology and communications companies. Consequently, you may experience a decrease in the market value of your common stock, regardless of our operating performance or prospects.
We do not plan to declare or pay any dividends to our shareholders in the near future and would need regulatory approval to do so.
We have not declared any dividends in the past, and we do not intend to distribute dividends in the near future. The declaration, payment and amount of any future dividends will be made at the discretion of the board of directors and subject to PRC law, and will depend upon, among other things, the results of operations, cash flows and financial condition, operating and capital requirements, and other factors as the board of directors considers relevant. There is no assurance that future dividends will be paid, and if dividends are paid, there is no assurance with respect to the amount of any such dividend.
We have the right to issue up to 5,000,000 shares of "blank check" preferred stock, which may adversely affect the voting power of the holders of other of our securities and may deter hostile takeovers or delay changes in management control.
Our articles of incorporation provides that we may issue up to 5,000,000 shares of preferred stock from time to time in one or more series, and with such rights, preferences and designations as our board of directors may determinate from time to time. Our board of directors, without further approval of our common stockholders, is authorized to fix the dividend rights and terms, conversion rights, voting rights, redemption rights, liquidation preferences and other rights and restrictions relating to any series of our preferred stock. Issuances of shares of preferred stock could, among other things, adversely affect the voting power of the holders of other of our securities and may, under certain circumstances, have the effect of deterring hostile takeovers or delaying changes in management control. Such an issuance would dilute existing stockholders, and the securities issued could have rights, preferences and designations superior to our Common Stock.
Sales of our common stock may have an adverse effect on the market price of our common stock. Additionally, we may issue shares upon exercise of outstanding warrants and stock options that are exercisable at prices that are below current market prices which will be dilutive to the common stock.
As of March 31, 2009, we had 16,446,467 shares of common stock outstanding, many of which are freely transferable under Rule 144. The sale of these shares may have an adverse effect on the market price for our common stock.
In addition, we currently have issued and outstanding warrants and stock options to purchase an aggregate of 1,013,500 shares of our common stock, of which (i) 750,000 are exercisable at a price of $12.50 per share; (ii) 150,000 are exercisable at a price of $2.00 per share; and (iii) 113,500 are exercisable at a price of $3.65 per share. All of these warrants are also exercisable on a “cashless” basis under certain circumstances. Our issuance of additional shares of common stock upon exercise of our outstanding warrants will substantially reduce the percentage equity ownership of holders of shares of our common stock. Further, the exercise of a significant number of warrants, and subsequent sale of shares of common stock received upon such exercise, could cause a sharp decline in the market price of our common stock.
FORWARD-LOOKING STATEMENTS
Some of the statements contained in this prospectus are not statements of historical or current fact. As such, they are "forward-looking statements" based on our current expectations, which are subject to known and unknown risks, uncertainties and assumptions. They include statements relating to:
| | · | future sales and financings; |
| | · | the future development of our business; |
| | · | our ability to execute our business strategy; |
| | · | projected expenditures; and |
| | · | the market for our products. |
You can identify forward-looking statements by terminology such as "may," "will," "should," "could," "expects," "intends," "plans," "anticipates," "believes," "estimates," "predicts," "potential" or "continue" or the negative of these terms or other comparable terminology. These statements are not predictions. Actual events or results may differ materially from those suggested by these forward-looking statements. In evaluating these statements and our prospects generally, you should carefully consider the factors set forth below. All forward-looking statements attributable to us or persons acting on our behalf are expressly qualified in their entirety by these cautionary factors and to others contained throughout this prospectus. We are under no duty to update any of the forward-looking statements after the date of this prospectus or to conform these statements to actual results.
Although it is not possible to create a comprehensive list of all factors that may cause actual results to differ from the results expressed or implied by our forward-looking statements or that may affect our future results, some of these factors are set forth under "Risk Factors" in this report and in our periodic filings made with the SEC.
Item. 1B. Unresolved Staff Comments.
None.
Item 2. Properties.
Our facilities are located on approximately 92,000 square meters of land, including two buildings in the city of Harbin, Heilongjiang Province, PRC. We also have a sales and marketing facility in Beijing, PRC.
Under Chinese law, the government owns all of the land in the PRC and companies and individuals are authorized to use the land only through land use rights granted by the PRC government. The PRC has granted TDR a land use grant covering the land and facilities in which its headquarters are located in downtown Harbin City, which expires in 2046. The PRC has granted land use rights on TDR’s two production and warehouse facilities, expiring in 2048 and 2053, respectively. TDR’s two buildings contain GMP production certified facilities, and are used for manufacturing office, warehousing and staff operations.
Description of Production and Other Facilities
We have two separate facilities, headquartered in the city of Harbin in the Heilongjiang Province of China. The older facility includes 3,000 square meters of production space, and 1,000 square meters of warehouse. The facility also includes an extraction workshop (approximately 1,200 square meters) and filling workshop (approximately 500 square meters) for traditional Chinese medicines; a patches production line (approximately 500 square meters), packing workshop (approximately 500 square meters), testing workshop (approximately 50 square meters), examination laboratory (approximately 100 square meters), sample laboratory (approximately 50 square meters), refining room (approximately 100 square meters), and a work-in-process warehouse (approximately 300 square meters); finished product warehouse (approximately 200 square meters); materials warehouse (approximately 100 square meters); and a packing warehouse (approximately 400 square meters).
The newer facility consists of a four floor office building (1,500 square meters for office purpose, 1,200 square meters for R&D center, 800 square meters for central examination lab, dormitory and eatery 1,000 square meters), total 4,500 square meters construction area, and a factory of 3,500 square meters. The facility also include: an enzyme immunity reagent kit production workshop (1,500 square meters) and a colloid gold production workshop (600 square meters); a packing workshop (800 square meters); and an examination lab (500 square meters). The newer facility also includes a research center covering approximately 1,200 square meters, for research pertaining to the development of various products, including traditional Chinese medicinals (TCM), biological medicine, gene medicine, immune body research, and vitro diagnosis reagent. These facilities also include an electricity room, heating and boiler room and garage. Our enzyme immunity examination reagent kit production workshop includes antigen and immune body areas, disinfection room, aseptic clothes room, cushion room, weighing room, separation room, cleaning equipment room, a Wan Ji flow cushion room, and antigen and immune body sign room. The enzyme sign processing area has a cushion room, cloth cleaning room, cleaning equipment room, packing material temporary storage room, raw material temporary storage room, equipment storage room, weighing room, seal protection room, seal foster room, drying room, packing room, and middle cooler room. The work fluid separation loading room includes a disinfection clean room, storage room, weighting room, loading room, and immune body purification room. The colloid gold production workshop has a darkroom, sample room, seal room, cementation room, cutting room, and a packing room. The packing workshop includes a central equipment room, a cooler room, material relay room, label and temporary storage room, a packing material temporary storage room, two examination cooler rooms, and two finished product cooler rooms.
We also have a sales office in Beijing, which TDR acquired in December of 2006, when it completed the acquisition of the products, dealership and marketing network of Heilongjiang. In addition to the above, our properties include GMP-certified manufacturing facilities of Heilongjiang and manufacturing equipment which was acquired on April 3, 2008. (See “Corporate History” above and “Recent Developments” and “Management’s Discussion and Analysis or Plan of Operation” below).
Our production facilities are operated in accordance with “good manufacturing practices” (“GMP”).
Item 3. Legal Proceedings.
We are not a party to any material pending legal proceedings, and to the best of our knowledge, no such proceedings by or against the Company have been threatened.
Item 4. Submission of Matters to a Vote of Security Holders.
During the fourth quarter of the fiscal year ended December 31, 2008, and subsequent periods through the date hereof, no matters were submitted to a vote of our security holders, except as follows:
On September 30, 2008 (the “Record Date”), we obtained the written consent of the holders of 8,158,251 shares of our common stock, which as of the Record Date represented 51.3% of our outstanding voting securities, to increase our number of authorized shares of common stock from 20,000,000 shares to 50,000,000 shares. The increase was effective upon filing of the Certificate of Amendment with the Secretary of State of the State of Nevada on November 17, 2008.
PART II
Item 5. Market for Common Equity, Related Stockholder Matters and Small Business Issuer Purchases of Equity Securities.
Market Information
Until May 28, 2008, our common stock was traded on FINRA’s Over-the-Counter Bulletin Board under the trading symbol “CSKI.” The range of high and low sales prices for each quarter during the last two fiscal years, as quoted on the OTC Bulletin Board for the periods discussed below, reflect inter-dealer prices, without retail mark-up, mark-down, or commission and may not necessarily represent actual transactions. On May 28, 2008, our common stock commenced trading on the American Stock Exchange under the trading symbol “CSY”. As of September 14, 2008, we terminated our listing on the American Stock Exchange and became listed on the Nasdaq Global Market under the trading symbol “CSKI.” The high and low prices for our common stock in the two prior fiscal years were as follows:
| | | Year Ended December 31, 2008 | | | Year Ended December 31, 2007 | |
| | | | | | | | | | | | | |
| 1st Quarter | | $ | 13.75 | | | $ | 9.40 | | | $ | 10.00 | | | $ | 7.00 | |
| 2nd Quarter | | $ | 17.10 | | | $ | 9.50 | | | $ | 14.20 | | | $ | 6.00 | |
| 3rd Quarter | | $ | 14.95 | | | $ | 9.48 | | | $ | 14.35 | | | $ | 10.00 | |
| 4th Quarter | | $ | 16.09 | | | $ | 6.77 | | | $ | 15.50 | | | $ | 9.00 | |
As of March 31, 2009, the closing price for our common stock was $11.50.
Dividends
Since inception, no dividends have been paid on our common stock. We intend to retain any earnings for use in our business, so it is not expected that any dividends on the common stock will be declared and paid in the foreseeable future. We do not currently have any restrictions that would limit our ability to pay dividends, and we are not currently aware of any restrictions that are likely to limit our ability to pay dividends in the future.
Holders
At March 31, 2009, there were approximately 379 holders of record of our common stock, with 16,446,467 shares issued and outstanding.
Securities Authorized For Issuance Under Equity Compensation Plan
As of December 31, 2008, we had only one stock option, bonus, profit sharing, pension or similar plan in place, which is our 2006 Stock Incentive Plan.
EQUITY COMPENSATION PLAN INFORMATION
| | | (a) | | | (b) | | | (c) | |
| Plan Category | | Number of securities to be issued upon exercise of outstanding options, warrants and rights | | | Weighted- average exercise price of outstanding options, warrants and rights | | | Number of securities remaining available for futrefuture issuance under equity compensation plans (excluding securities reflected in column (a)) | |
Equity compensation plans approved by security holders (1) | | | 113,500 | | | $ | 3.65 | | | | 1,326,437 | (3) |
Equity compensation plans not approved by security holders (2) | | | 0 | | | | N/A | | | | 0 | |
| Total | | | 113,500 | | | $ | 3.65 | | | | 1,326,437 | |
| | (1) | The Company’s board of directors adopted the 2006 Stock Incentive Plan (the “Plan”), to be effective on July 31, 2006. The Plan was approved by the shareholders on July 31, 2006. |
| | (2) | We do not have any equity compensation plans not approved by the security holders. |
| | (3) | The Plan reserves an aggregate of 1,500,000 shares of our common stock for awards of stock options, stock appreciation rights, restricted stock, performance stock and bonus stock granted thereunder. As of the date hereof, 60,063 shares of restricted stock have been granted under the Plan. |
Sales of Unregistered Securities
The following is a list of securities we have sold or issued during the past year. We believe that each of these transactions was exempt from registration under the Securities Act of 1933, as amended, pursuant to Section 4(2), or Regulation D promulgated thereunder, as a transaction by an issuer not involving a public offering. There were no underwriting discounts or commissions paid in connection with the sale of these securities, except as otherwise noted.
Private Offering
On January 31, 2008 (the “Closing Date”), we entered into a Securities Purchase Agreement (the “Purchase Agreement”) with certain accredited investors (the “Investors”), for the purchase and sale of 2,500,000 units of our securities (“Units”) consisting of an aggregate of: (i) 2,500,000 shares of our common stock (the “Purchased Shares”), and (ii) Class A Warrants to purchase 750,000 additional shares of our common stock, at an exercise price of $12.50 per share (the “Purchased Warrants”), for a purchase price of $10.00 per unit (the “Unit Purchase Price”), or aggregate of $25,000,000 (the “Offering”).
Pursuant to the Purchase Agreement, among other things:
| | · | The lead investor in the Offering (the “Investor Agent”), was granted a right of first refusal, for a period of eighteen (18) months after the later of the Closing Date, or the effective date of the Registration Statement (defined below), to purchase up to a maximum of $15,000,000 of any securities we offer in any proposed offering of our common stock, or other securities or debt obligations, except certain Excepted Issuances (as defined in the Purchase Agreement); and |
| | · | We agreed that, if and whenever, within twelve (12) months of the Closing Date, it issues or sells, or is deemed to have issued or sold, any shares of common stock, or securities convertible into or exercisable for shares of common stock, or modifies any of the foregoing which may be outstanding (with the exception of certain excluded securities), to any person or entity at a price per share, or conversion or exercise price per share less than the Unit Purchase Price, then we shall issue, for each such occasion, additional shares of our common stock to the Investors in such number so that the average per share purchase price of the shares of common stock purchased by the Investors in the Offering shall automatically be reduced to such other lower price per share (in each case, the “Additional Shares”). In addition, the Investors will have the registration rights described in the Registration Rights Agreement with respect to such Additional Shares. |
In connection with the Initial Placement, we paid a placement agent (the “Placement Agent”) a fee of five percent (5%) of the Offering Proceeds. In addition, we paid the Placement Agent’s legal fees and additional out-of-pocket expenses related to the Offering.
We used the net proceeds from the Offering primarily for: (a) acquisitions, (b) new product marketing, (c) expenses related to the Offering and the Registration Statement (defined below), and (d) general working capital purposes.
As of the Closing Date, we entered into a Registration Rights Agreement (the “Registration Rights Agreement”) with the Investors, pursuant to which it agreed that within sixty (60) calendar days of the Closing Date (the “Filing Date”), we would file a registration statement (the “Registration Statement”) with the SEC, on the appropriate form, covering the resale of (i) the Purchased Shares, and (ii) the common stock issuable upon exercise of the Purchased Warrants (the “Warrant Shares”) (collectively (i) and (ii), the “Registrable Securities”). Further, we agreed to use our best efforts to (a) cause the Registration Statement to be declared effective within one hundred twenty (120) calendar days from the Filing Date, or, if reviewed by the Commission, within one hundred fifty (150) calendar days after the Filing Date, and (b) keep the Registration Statement continuously effective until all of the Registrable Securities have been sold, or may be sold without volume restrictions pursuant to Rule 144. We have not yet satisfied these registration requirements. We engaged an independent third-party consultant to calculate the derivative liability resulting from our failure to register the Registrable Securities for resale. The calculation was made using the Black-Scholes model. The liability was deemed to be immaterial as of December 31, 2008.
Notwithstanding anything to the contrary stated in the Registration Rights Agreement, the Company shall be entitled to limit the Registrable Securities to the extent necessary to avoid any issues arising from the recent interpretations by the Commission of Rule 415 of the Securities Act of 1933, as amended.
In addition, as of the Closing Date, we entered into a Make Good Agreement (the “Make Good Agreement”) with Liu Yan-Qing, our Chairman, Chief Executive Officer and President, and a principal shareholder of ours (the “CSKI Shareholder”) and the Investor Agent (collectively, the “Make Good Parties”), pursuant to which the CSKI Shareholder placed 3,000,000 shares of our common stock (the “Escrow Shares”) into escrow for the benefit of the Investors, in the event that we failed to attain Earnings Per Share, as adjusted (“Adjusted EPS”) of at least (i) $1.05 per share for the fiscal year ending December 31, 2007, based on fully diluted shares outstanding (an aggregate of 13,907,696 shares, including all outstanding common shares, preferred shares, any convertible security, options, and warrants) and/or (ii) $1.63 per share for the fiscal year ending December 31, 2008, based on fully diluted shares outstanding (an aggregate of 16,907,696 shares, including all outstanding common shares, preferred shares, any convertible security, options, and warrants, excluding the 750,000 warrants issued in the Offering), based upon annual audits conducted in conformity with United States generally accepted accounting principles.
In each case, the Investors shall have the right to receive a pro rata share of any Escrow Shares released pursuant to the terms and conditions of the Make Good Agreement and a Make Good Escrow Agreement (the “Make Good Escrow Agreement”), which the Make Good Parties entered into with our transfer agent, as escrow agent. Notwithstanding anything to the contrary set forth in the Make Good Agreement, upon any Investor’s exercise of its Put Right (defined below), such Investor’s right to receive a pro rata share of the Escrow Shares automatically and permanently terminates.
We deem the Escrow Shares arrangement as analogous to the issuance of a fixed number of warrants in an equity transaction. Under the Make Good Agreement these Escrow Shares would have been reallocated on a pro rata basis to the Investors only if the Adjusted EPS targets were not achieved in years 2007 and 2008. If the earnings targets were met, the Escrow Shares would automatically be released to the CSKI Shareholder. As of January 31, 2008, the date the common shares were placed into escrow, we achieved the 2007 earnings target and, based upon internal forecasts, we were confident the 2008 target would also be met. Based upon certain assumptions, including the low probability that the Escrow Shares would be released to the Investors and not be returned to the CSKI Shareholder, we considered the fair value of the right held by the Investors through the Escrow Shares provision under the Make Good Agreement to be immaterial. As of December 31, 2008, we satisfied the earnings per common share targets for each of fiscal 2007 and 2008 as defined under the Make Good Agreement and, as such, the Escrow Shares shall be released to the CSKI Shareholder in 2009.
Further, as of the Closing Date, we entered into a Put Agreement (the “Put Agreement”) with the Investors, pursuant to which each Investor has the right to cause us to repurchase all, but not less than all of its Purchased Shares (the “Put Right”), for a price of $10.00 per share (the “Repurchase Price”), in the event that:
| | · | our Adjusted EPS for the fiscal year ending December 31, 2007 is less than $0.80 per share, as set forth in our audited financial statements; or |
| | · | our accounts receivable exceeds $12,000,000 at the end of fiscal 2007, as set forth in our audited financial statements. |
Upon our receipt of a notice of exercise from any Investor, (i) such Investor’s right to receive a pro rata shares of the Escrow Shares shall automatically and permanently terminate, subject only to the satisfaction of our obligations under the Put Agreement; and (ii) such Investor’s right to exercise the Purchased Warrants shall be suspended pending the satisfaction of our obligation to pay the Repurchase Price in full, and any interest accrued thereon, to the applicable Investor.
After exercise of the Put Right, and upon delivery to the Investor of the applicable Repurchase Price, such Investor shall no longer be deemed to be the owner of the Purchased Shares or Purchased Warrants. The Purchased Shares shall be placed in our treasury and the Purchased Warrants shall be cancelled on our books.
We satisfied the Adjusted EPS and accounts receivable requirements as of the end of fiscal 2007. Therefore, the Investors’ Put Right has terminated.
Lastly, as of the Closing Date, we entered into a Lock-up Agreement (the “Lock-up Agreement”) with two of our stockholders, whom are also members of our management (the “Principal Stockholders”), pursuant to which the Principal Stockholders agreed not to sell, assign, transfer, pledge, hypothecate, or otherwise dispose of any of their aggregate of 6,063,502 shares of our common stock (the “Lock-up Shares”) until twelve (12) months from the effective date of the Registration Statement (the “Lock-Up Period”). Anything to the contrary notwithstanding, the Principal Stockholders are entitled to sell, in the aggregate, 136,000 of the Lock-up Shares pursuant to Rule 144 under the Securities Act of 1933, as amended (“Rule 144”).
If any of the Escrow Shares are released to the Investors pursuant to the terms and conditions of the Make Good Agreement and Make Good Escrow Agreement (“Released Shares”), the Lock-Up Period shall be deemed to have automatically and permanently terminated with respect to such Released Shares.
The Class A Warrants represent the right to purchase an aggregate of 750,000 shares of our Common Stock, at an exercise price of $12.50 per share (the “Exercise Price”), and have the following additional characteristics:
| | · | The Class A Warrants shall be exercisable beginning on the six-month anniversary of the Closing Date and will expire three years thereafter (the “Expiration Date”). |
| | · | Commencing on one-year anniversary of the Closing Date, in the event the Warrant Shares may not be freely sold by the holders (the “Warrantholders”) due to our failure to satisfy our registration requirements, and an exemption for such sale is not otherwise available to the Warrantholders under Rule 144, the Class A Warrants will be exercisable on a cashless basis. |
| | · | The Exercise Price and number of Warrant Shares will be subject to adjustment for standard dilutive events, including the issuance of common stock, or securities convertible into or exercisable for shares of common stock, at a price per share, or conversion or exercise price per share less than the Exercise Price. |
| | · | At anytime following the date a Registration Statement covering the Warrant Shares is declared effective, we will have the ability to call the Class A Warrants at a price of $0.01 per Class A Warrant, upon thirty (30) days prior written notice to the holders of the Class A Warrants, provided (i) the closing price of the common stock exceeded $18.75 for each of the ten (10) consecutive trading days immediately preceding the date that the call notice is given by us, and (ii) we have attained an Adjusted EPS of at least $1.75 per share for the fiscal year ending December 31, 2008, as set forth in our audited financial statements. |
| | · | If, among other things, we fail to cause a Registration Statement covering the Warrant Shares to be declared effective prior to the applicable dates set forth in the Registration Rights Agreement (the “Effectiveness Deadlines”), the Expiration Date of the Class A Warrants shall be extended one day for each day beyond the Effectiveness Deadlines. |
| | · | If a Warrantholder exercises its Put Right, such Warrantholder’s right to exercise the Class A Warrants shall be suspended, pending the satisfaction of our obligations to pay the Warrantholder the applicable Repurchase Price. Upon receipt of the Repurchase Price in full by the Warrantholder, the Warrantholder’s right to exercise the Class A Warrants shall automatically and permanently terminate and expire, and the Class A Warrants shall be immediately cancelled on our books. |
| | · | The Warrantholder shall not be entitled to exercise a number of Class A Warrants in excess of the number of Class A Warrants upon exercise of which would result in beneficial ownership by the Warrantholder and its affiliates of more than 9.9% of the outstanding shares of our common stock. This limitation on exercise may be waived by written agreement between the Warrantholder and us; provided, however, such waiver may not be effective less than sixty-one (61) days from the date thereof. |
Exercises of Warrants and Stock Options
In fiscal 2008, we issued an aggregate of 1,142,302 shares of our common stock in connection with their exercise of outstanding warrants and stock options of ours, as follows:
| | · | Between February 2008 and October 2008, warrants to purchase an aggregate of 355,002 shares of our common stock, which we issued to “accredited” investors in connection with the private offering we completed in October 2006 (the “2006 Offering”), were exercised at a price of $3.50 per share, for an aggregate of $1,642,091. |
| | · | In April 2008, warrants to purchase an aggregate of 100,000 shares of our common stock, which we issued to a consultant in consideration for services rendered in connection with the 2006 Offering, were exercised at a price of $3.00 per share, for an aggregate of $300,000. In October 2008, warrants to purchase an additional 50,000 shares of our common stock were exercised by the consultant, at a price of $3.50 per share, for an aggregate of $475,000. |
| | · | In September 2008, warrants to purchase an aggregate of 500,000 shares of our common stock, which we issued to a consultant in consideration for services rendered in connection with the 2006 Offering, were exercised on a cashless basis. In connection with the cashless exercise, the warrant holder was deemed to have paid an amount equal to the difference between the exercise price ($2.00 per share) and the average closing price of a share of our common stock during the ten (10) trading days ending on the date of exercise ($12.67 per share). As a result of the cashless exercise, we issued an aggregate of 421,055 shares of our common stock to the warrant holder and its designees. |
| | · | In October 2008, stock options to purchase 50,000 shares of our common stock, which we issued to a former executive officer in fiscal 2006 in consideration for consulting services he performed following the termination of his employment, were exercised at a price of $3.00 per share, for an aggregate of $150,000. |
| | · | In October 2008, warrants to purchase an aggregate of 200,000 shares of our common stock, which we issued to a consultant in consideration for services rendered in connection with the share exchange transaction we consummated in May 2006, were exercised on a cashless basis. In connection with the cashless exercise, the warrant holder was deemed to have paid an amount equal to the difference between the exercise price ($2.00 per share) and the average closing price of a share of our common stock during the ten (10) trading days ending on the date of exercise ($11.85 per share). As a result of such cashless exercise, we issued an aggregate of 166,245 shares of our common stock to the warrant holder. Following this exercise, the warrant holder still held warrants to purchase an additional 300,000 shares of our common stock, which could be exercised in whole, or in part, for cash, or on a cashless basis. |
Shares Issued in Connection with Acquisitions
In fiscal 2008, we issued an aggregate of 408,456 shares of our common stock in connection with acquisitions we completed, as follows:
| | · | On April 3, 2008, Harbin Tian Di Ren Medical Science and Technology Company, our indirect subsidiary (“TDR”), acquired 100% of the equity of Heilongjiang Tianlong Pharmaceutical, Inc., a corporation organized under the laws of the PRC, from its sole shareholder, in consideration for a purchase price of $8.3 million, consisting of: (i) approximately $8.0 million in cash, and (ii) 23,850 shares of our common stock (valued at $12.00 per share). |
| | · | On September 5, 2008, TDR acquired 100% of the equity of Peng Lai Jin Chuang Pharmaceutical Company, a corporation organized under the laws of the PRC, from its sole shareholder, in consideration for a purchase price of $7.1 million, consisting of (i) $2.5 million in cash, and (ii) 381,606 shares of our common stock (valued at $12.00 per share). |
Restricted Shares Issued Under 2006 Equity Incentive Plan
As of July 15, 2008, we issued 30,063 “restricted” shares of our common stock to certain employees, directors and advisors of ours, pursuant to our 2006 Stock Incentive Plan.
Item 6. Selected Financial Data.
We are a smaller reporting company as defined by Rule 12b-2 of the Securities Exchange Act of 1934 and are not required to provide the information under this item.
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operation.
The financial and business analysis in this Annual Report on Form 10-K (the “Report”) provides information we believe is relevant to an assessment and understanding of our financial condition and results of operations. The following discussion should be read in conjunction with our consolidated financial statements and related notes included in Part II, Item 8 of this Report.
FORWARD LOOKING STATEMENTS
The following discussion should be read in conjunction with the information contained in our consolidated financial statements and the notes thereto appearing elsewhere herein and in the risk factors and “Forward Looking Statements” summary set forth in the forepart of this Annual Report as well as the “Risk Factors” section above and are afforded the safe harbor provisions of Section 27A of the Securities Act and Section 21E of the Securities Exchange Act of 1934, as amended. Readers should carefully review the risk factors disclosed in this Annual Report and other documents filed by us with the SEC.
DISCUSSION
We primarily generate revenues, through our China based indirect subsidiaries described below, in the development, manufacture, marketing and sale of over-the-counter, branded nutritional supplements and over-the-counter plant and herb based pharmaceutical and medicinal products. Our principal products are external use Traditional Chinese Herbal Remedies/Medicines commonly referred to in the industry as “TCM.” We have evolved into an integrated manufacturer, marketer and distributor of external use Chinese medicine products sold primarily in China and through Chinese domestic pharmaceutical chains and have been expanding our worldwide sales effort as well. We sell both our own manufactured products, as well as medicinal and pharmaceutical products manufactured by others in China.
We achieved continuing growth on the sale of both our own product line and a contract service line of manufacturer’s products which we sell through our distribution channel. For the year ended December 31, 2008, total revenue was $91,816,183, an 86% increase over the same period in 2007, and net income was $28,835,207, or $1.87 per share compared to net income of $15,332,945, or $1.15 per share on a diluted basis in the same period in 2007.
All of our business is conducted through our wholly-owned subsidiary, ACPG which, in turn, wholly owns Harbin Tian Di Ren Medical Science and Technology Company (referred to herein as “TDR”), a company organized in the PRC, and TDR’s subsidiaries.
TDR, formerly known as “Harbin City Tian Di Ren Medical Co.,” was originally formed in 1994 with its principal executive office in Harbin City of Heilongjiang Province, in the PRC. TDR was reorganized and incorporated as a limited liability company on December 29, 2000, under the “Corporation Laws and Regulations” of the PRC. At the time of the TDR Acquisition by ACPG in December of 2005, TDR had two wholly-owned subsidiaries, Harbin First Bio-Engineering Company Limited and Kangxi Medical Care Product Factory, until July, 2006, when the two were merged, with Harbin First Bio-Engineering Company Limited (“First” or “Harbin Bio Engineering”) as the surviving subsidiary of TDR.
As of October 16, 2006, we organized Harbin Tian Qing Biotech Application Company as a wholly-owned PRC subsidiary of TDR, to conduct research and development in the areas of tissue and stem cell banks, which is described in more detail below.
Recent Developments
On April 3, 2008, TDR completed its acquisition of Heilongjiang Tianlong Pharmaceutical, Inc., a corporation with a variety of SFDA approved medicines and new medicine applications, organized under the laws of the PRC (“Tianlong”), which is in the business of manufacturing external-use pharmaceuticals. TDR previously acquired the Beijing sales office of Tianlong in mid-2006. TDR acquired 100% of the issued and outstanding capital stock of Tianlong from its sole stockholder, in consideration for an aggregate purchase price of approximately $8,300,000, consisting of (i) $8,000,000 in cash, and (ii) 23,850 shares of our common stock.
On April 18, 2008, TDR consummated its acquisition of Heilongjiang Haina Pharmaceutical Inc., a recently formed corporation organized under the laws of the PRC (“Haina”) licensed as a wholesaler of TCD, bio-medicines, bio-products, medicinal devices, antibiotics and chemical medicines. Haina did not have an established sales network and was acquired for its primary asset, a Good Supply Practice (GSP) license (License No. A-HLJ03-010), issued by the Heilongjiang province office of the SFDA. The SFDA recently started issuing such licenses to resellers of medicines that maintain certain quality controls. The GSP license was issued as of December 21, 2006 and will expire on January 29, 2012 and will enable us to expand its sales of medicinal products without having to go through a lengthy license application process. TDR acquired 100% of the issued and outstanding capital stock of Haina from its three stockholders in consideration for payment of approximately $437,375. TDR had been overseeing the operations of Haina Pharmaceutical since January of 2008, as part of our due diligence prior to closing of this acquisition.
On September 5, 2008, TDR acquired Peng Lai Jin Chuang Pharmaceutical Company (“Jin Chuang”), a corporation organized under the laws of the PRC, from Peng Lai Jin Chuang Group Corporation (the “Seller”). Jin Chuang, which has received Good Manufacturing Practice certification from the SFDA, was organized to develop, manufacture and distribute pharmaceutical, medicinal and diagnostic products in the PRC. In connection with the acquisition of Jin Chuang, TDR acquired all of Jin Chuang’s assets, including, without limitation, franchise, production and operating rights to a portfolio of twenty (20) medicines approved by the SFDA, for an aggregate purchase price of approximately $7.1 million, consisting of (i) approximately $2.5 million in cash, and (ii) 381,606 shares of our common stock.
Summary of Our Research and Development Activities
We currently conduct all of our research and development (“R&D”) activities, either internally or through collaborative arrangements with universities and research institutions in the PRC. We have our own research, development and laboratory facilities located at TRD’s principal executive.
Additionally, we have established several long-term partnerships with well-known universities and enterprises in the PRC. We have built a gene medicine laboratory through a collaborative effort with Harbin Medical University; established a cell laboratory with North East Agricultural University; and founded a monoclonal antibody laboratory with Jilin University. Under our partnership arrangements with other universities and research institutions, we will generally hold the intellectual property rights to any developed technology. As a result of one of these collaborations with Harbin Medical University, a product known as “Endothelin-1” is currently under development as a cancer suppressing product. Additional information relating to this product and other products being developed is set forth under “Products Under Development” below and under the general product descriptions in the “Business” section above, which is incorporated by reference herein.
In collaboration with Harbin Medical University, we have completed a laboratory experimental study pertaining to Endothelin-1, which is required prior to clinical trials, and we are currently applying for approval to enter clinical experiments. This medicine has been recognized by the PRC as the “Top Category in New Medicine.” In order to qualify as the “Top Category in New Medicine,” a company must have intellectual property rights, high technology involvement, strong innovation, and the medicine must be the first of its kind to be introduced to the PRC. We hold the intellectual property rights pertaining to this technology, and we have obtained an invention patent to this intellectual property in the PRC. Under our partnership arrangements with other universities and research institutions, we will generally hold the intellectual property rights to any developed technology.
At present, our ongoing research is divided into five general areas:
| | · | the development of an enzyme linked immune technique to prepare extraneous diagnostic kits (see table below); |
| | · | the development of an enzyme linked gold colloid technique to prepare extraneous rapid diagnostic test strip; |
| | · | the development of a gene recombination technique to prepare gene drug; |
| | · | the development of a biology protein chip for various tumor diagnostic applications; and |
| | · | the development of a cord blood stem cell bank, as more fully described in other reports we have filed. |
We currently have eight biological products under development: HIV detection kit; a uterus cancer diagnostic kit; a breast cancer diagnostic kit; a liver cancer diagnostic kit; a rectum cancer diagnostic kit; a gastric cancer diagnostic kit; a gene recombination drug; and a multi-tumor marker protein chip detection kit. We are also working to establish additional sales networks and cell banks covering domestic and international markets.
Testing Kits and Other Products in Production
We also have three products: AMI Diagnostic Kit, Human Urinary Albumin Elisa Kit and Early Pregnancy Diagnostic Kit that passed the final stages of national inspection in 2006 or 2007. These diagnostic kits are being sold through drug stores, hospitals, examination stations and independent sales agents throughout the PRC. We also plan to market these products in Vietnam, Indonesia, Philippines and eventually in Africa. Our sales in this product category increased in mid 2008.
Our AMI Diagnostic Kit, which entered markets in 2007, is used for early diagnosis of Myocardial Infarction (MI), also known as heart disease. All the test kits require users to place a blood or urine sample on the marker and a positive (+) or negative (-) reaction signal will result, showing if a user should consult his or her doctor for further testing. According to the China Medical Newspaper, Several million people die from MI every year. MI often occurs to people who are, but not limited to, smokers, over-weight and diabetic. There are approximately 8 million new MI patients in China every year. Recent medical studies have shown that heart failure or heart attacks are increasing among younger people in China. This is a result from a more modern life style, the fast pace of city life and increased pressure from work or school. The use of AMI Diagnostic Kits will help in early detection that can help in reducing these statistics.
We are continuing our marketing efforts with respect to these testing kits which resulted in continued increased sales of these products in 2008.
Significant Accounting Estimates and Policies
The discussion and analysis of our financial condition and results of operations is based upon our financial statements which have been prepared in accordance with accounting principles generally accepted in the United States. The preparation of these financial statements requires us to make estimates and judgments that affect the reported amounts of assets and liabilities. On an on-going basis, we evaluate our estimates including the allowance for doubtful accounts and inventories, the salability and recoverability of our products, income taxes and contingencies and remaining useful lives of our tangible and certain intangible assets. We base our estimates on historical experience and on other assumptions that we believes to be reasonable under the circumstances, the results of which form our basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions.
Property and equipment are evaluated for impairment whenever indicators of impairment exist. Accounting standards require that if an impairment indicator is present, we must assess whether the carrying amount of the asset is unrecoverable by estimating the sum of the future cash flows expected to result from the asset, undiscounted and without interest charges. If the recoverable amount is less than the carrying amount, an impairment charge must be recognized based on the fair value of the asset.
As part of the process of preparing our financial statements, we are required to estimate our income taxes. This process involves estimating our current tax exposure together with assessing temporary differences resulting from differing treatment of items for tax and accounting purposes. These differences result in deferred tax assets and liabilities. We must then assess the likelihood that our deferred tax assets will be recovered from future taxable income, and, to the extent we believe that recovery is not likely, we must establish a valuation allowance. To the extent that we establish a valuation allowance or increase this allowance in a period, we must include a tax provision or reduce our tax benefit in the statements of operations. We use our judgment to determine our provision or benefit for income taxes, deferred tax assets and liabilities and any valuation allowance recorded against our net deferred tax assets. We believe, based on a number of factors including historical operating losses, which we will not realize the future benefits of a significant portion of our net deferred tax assets and we have accordingly provided a full valuation allowance against our deferred tax assets. However, various factors may cause those assumptions to change in the near term.
We cannot predict what future laws and regulations might be passed that could have a material effect on our results of operations. We assess the impact of significant changes in laws and regulations on a regular basis and update the assumptions and estimates used to prepare our financial statements when we deem it necessary.
We have determined the significant principles by considering accounting policies that involve the most complex or subjective decisions or assessments. Our most significant accounting policies are those related to intangible assets and research and development.
Intangible assets
Intangible assets consist of patents and goodwill. Patent costs are being amortized over the remaining term of the patent. As of December 31, 2008, the remaining weighted average life of our patents is approximately 9 years.
Intangible assets are accounted for in accordance with Statement of Financial Accounting Standards No. 142, Goodwill and Other Intangible Assets (“SFAS 142”). Intangible assets with finite useful lives are amortized while intangible assets with indefinite useful lives are not amortized. As prescribed by SFAS 142, goodwill and intangible assets are tested periodically for impairment. The Company adopted SFAS No. 144, "Accounting for the Impairment or Disposal of Long- Lived Assets," effective January 1, 2002. Accordingly, the Company reviews its long-lived assets, including property and equipment and finite-lived intangible assets for impairment whenever events or changes in circumstances indicate that the carrying amount of the assets may not be fully recoverable. To determine recoverability of its long-lived assets, the Company evaluates the probability that future undiscounted net cash flows will be less than the carrying amount of the assets. Impairment costs, if any, are measured by comparing the carrying amount of the related assets to their fair value.
Research and development
Research and development expenses include the costs associated with the Company’s internal research and development as well as research and development conducted by third parties. These costs primarily consist of salaries, clinical trials, outside consultants, and materials. All research and development costs are expensed as incurred.
Third-party expenses were reimbursed under non-refundable research and development contracts, and are recorded as a reduction to research and development costs in the statement of operations.
We recognize in-process research and development in accordance with FASB Interpretation No. 4, Applicability of FASB Statement No. 2 to Business Combinations Accounted for by the Purchase Method and the AICPA Technical Practice Aid, Assets Acquired in a Business Combination to be used in Research and Development Activities: A Focus on Software, Electronic Devices, and Pharmaceutical Industries. Assets to be used in research and development activities, specifically, compounds that have yet to receive new drug approval and would have no alternative use, should approval not be given, are immediately charged to expense when acquired. Certain assets and high technologies acquired that has a foreseeable future cash flows are capitalized as intangible assets. Such intangible assets are amortized starting from the year revenue is generated and amortize over a period of 10 years.
For the year ended December 31, 2008 and 2007, the Company incurred $7,412,895 and $3,158,351, respectively, in research and development expenditures.
Results Of Operations
For the year ended December 31, 2008 as compared to December 31, 2007
Our principal business operations are conducted through our wholly owned subsidiary, TDR, and TDR’s wholly-owned subsidiaries.
| | | December 31, | | | December 31 | | | December 31. | |
| | | | | | | | | | |
| REVENUES | | | | | | | | | |
| Product Sales (net of sales allowance) | | $ | 86,161,098 | | | | 137 | % | | $ | 36,320,156 | |
| Contract Sales | | | 5,655,085 | | | | (57 | )% | | | 12,998,152 | |
| Total revenues | | $ | 91,816,183 | | | | 86 | % | | | 49,318,308 | |
| | | | | | | | | | | | | |
| COST OF GOODS SOLD | | | | | | | | | | | | |
| Cost of goods sold | | | 22,403,303 | | | | 105 | % | | | 10,939,531 | |
| Gross Profit | | $ | 69,412,880 | | | | 81 | % | | $ | 38,378,777 | |
Total revenues increased by 86% in the year ended December 31, 2008 compared to 2007. The $42.5 million increase in revenue is attributable to strong performances from our sales distribution channels.
Product sales increased by 137% in the year ended December 31, 2008, to $86,161,098 from $36,320,156 in 2007. This growth in sales is attributable to volume and our efforts to continue to develop our distribution channels by hiring additional direct territory managers and sales agents to assure that our products and their associated benefits are seen by those making or influencing the purchasing decisions.
Contract sales of non-manufactured products amounted to $5,655,085 in the year ended December 31, 2008, or a significant decrease of $7,343,067 from sales of $12,998,152 in 2007.
In 2007, our sales model was focused on the creation of our own distribution channels. Therefore, we sold products directly to many smaller distributors and retail store locations. In 2008, we changed our business model and entered into distribution agreements with larger regional sales agents, that resell to smaller distributors and retail store locations. In addition, we began entering into contracts with nationwide chain pharmacies. In 2008, TDR began to discontinue contract sales as part of its strategic goals.
Our change of sales strategy in fiscal 2008 was initiated to improve product channel efficiencies, and to give us access to an increased number of ultimate purchasers. We believe that these changes will lead to further increased revenue by extending the reach of our distribution network. We also believe that, by reducing the number of customers we sell to directly, we will be able to streamline our accounts receivable management and collection, and reduce channel distribution costs. These favorable cost variances are expected be partially offset by product price incentives we grant to the larger agents with which we have contracted.
Sales by Product Line
A break-down of our sales by major product line for each of the years ended December 31, 2008 and 2007 is as follows:
| | | | | For the Year Ended December 31 31 , | |
| | | | | 2008 | | | 2007 | |
Product Category (97 products) | | | | | | | | | | | | | | | | | | | | |
| Patch (5 products) | | TDR | | | 9,494,535 | | | $ | 35,484,230 | | | | 39 | % | | | 2,294,901 | | | $ | 19,690,051 | | | | 39 | % |
| Ointment (20 products) | | TDR & TL | | | 11,478,130 | | | | 23,068,210 | | | | 25 | % | | | 3,037,022 | | | | 6,190,003 | | | | 13 | % |
| Spray (19 products) | | TDR & TL | | | 3,941,295 | | | | 10,612,679 | | | | 12 | % | | | 3,580,266 | | | | 9,210,233 | | | | 19 | % |
| Diagnostic Kits (3 products) | | First | | | 2,184,013 | | | | 8,780,990 | | | | 10 | % | | | 739,151 | | | | 2,990,664 | | | | 6 | % |
| Contract Sales (7 products) | | Haina | | | 3,837,578 | | | | 5,655,085 | | | | 6 | % | | | 5,718,652 | | | | 8,197,758 | | | | 17 | % |
| others (43 products) | | | | | 4,306,972 | | | | 8,214,989 | | | | 8 | % | | | 1,896,193 | | | | 3,039,599 | | | | 6 | % |
| Total | | | | | 35,242,523 | | | $ | 91,816,183 | | | | 100 | % | | | 17,266,185 | | | $ | 49,318,308 | | | | 100 | % |
During the year 2008, we acquired Tianlong Pharmaceutical. As a result, Tianlong’s contract sales prior to the business acquisition have been realocated to the applicable product category of sale to enhance financial data analysis.
Our internal growth was driven by increases in the revenues of TDR, which increased from $33,269,322 in 2007 to $60,078,236 in 2008; and First, which increased from $2,989,228 in 2007 to $8,780,990 in 2008. These increases were partially offset by a decrease in our contract sales of $7,357,793, or 57% from $12,998,152 in fiscal 2007 to $5,640,359 in fiscal 2008, due primarily to our discontinuance of contract sales of Tialong products following the acquisition of Tianlong as of April 3, 2008.
In 2008, before TDR acquired Tianlong, the majority of our contracts sales consisted of products purchased from Tianlong. In 2008, TDR began to discontinue contract sales, and in 2009, TDR discontinued contract sales as part of its strategic goals and, in 2009 TDR discontinued contact sales. Revenues derived from the sale of a Tianlong product of $4,804,814 and $1,477,376 for 2007 and 2008 respectively, have been reallocated to each of the appropriate product categories to present a more appropriate measure of our revenues by product line.
Following the Tianlong acquisition, we were able to fully integrate Tianlong’s products into our marketing and distribution channels and increase overall sales. As a result, we derived an aggregate of $15,137,995 from the sale of Tianlong’s products for the remainder of 2008, in addition to the $1,447,376 of contract sales of Tianlong’s products from January 1, 2008 through the Tianlong acquisition.
Prior to our acquisition of Peng Lai, as of September 5, 2008, Peng lai had nominal production and operations. Following the acquisition, Peng Lai contributed revenue of $2,163,877 to our total revenue in 2008.
Cost of Goods Sold and Product Gross Margin
| | | For the Year ended December 31 | |
| | | | | | | | | | |
| Revenues | | $ | 91,816,183 | | | | 86 | % | | $ | 49,318,308 | |
| Cost of goods sold | | | 22,403,303 | | | | 105 | % | | | 10,939,531 | |
| Gross profit | | $ | 69,412,880 | | | | 171 | % | | $ | 38,378,777 | |
| Products gross margin | | | 76 | % | | | | | | | 78 | % |
Cost of goods sold increased by $11,463,772, or 105%, from $10,939,531 in year ended December 31, 2007, to $22,403,303 for the year ended December 31, 2008 as a direct result of increased sales activities, partially offset by a higher gross margin on our sales of Tianlong products following the acquisition in April 2008. Overall, our product gross margins decreased slightly to 76% for the year ended December 31, 2008 from 78% for the year ended December 31, 2007. From January 1, 2008 thought April 2, 2008, revenues from Tianlong contract sales were $1,477,376, and gross profit from these sales were $1,173,185. The gross margin from these sales were approximately 79.4%, After our acquisition of Tianlong, revenues from sales of Tianlong products were $13,802,565, and gross profit from these sales were $12,298,495. The gross margin from these sales was approximately 89.1%. This increase in gross margin from sales of Tianlong’s products following the acquisition was offset by the decrease in gross margins related to sales of certain TDR’s products due to our reduction in the sales prices of certain of our products to be competitive in the PRC market.
Operating Expenses
The following table summarizes the changes in our operating expenses from $19,764,991 to $33,753,502 for each of the year ended December 31, 2007 and 2008, respectively:
| | | For the Year ended December 31 | |
| | | | | | | | | | |
| Operating Expenses | | | | | | | | | |
| Selling , General and Administrative expenses | | $ | 25,482,201 | | | | 58 | % | | $ | 16,163,577 | |
| Depreciation and amortization | | | 858,406 | | | | 94 | % | | | 443,063 | |
| R&D Expenses | | $ | 7,412,895 | | | | 135 | % | | $ | 3,158,351 | |
| Total operating expenses | | | 33,753,502 | | | | 71 | % | | | 19,764,991 | |
Total operating expenses for the year ended December 31, 2008 increased approximately $14 million or 71% over the same period in 2007. The higher operating expenses were primarily attributable to the increased costs of marketing and distribution of our products for sale to generate our increased product sales from $49,318,308 in 2007 to $91,816,183 in 2008.
Research and development expenses were $7,412,895 in the year ended December 31, 2008 compared to $3,158,351 for 2007. The increased R&D expenses in 2008 were primarily due to additional clinical trials and development of patents to generate continued sales growth. During 2008, we initiated clinical trials and clinical irritation tests for 38 products whereas in 2007, substantially all of our research and development expenses spent on earlier stage clinical observations for eight diagnostic kits.
2009 Outlook
We estimate our total revenues in 2009 versus 2008 to increase by 40% or approximately $37 million with growth in all categories of our product sales. Our gross profit margin in 2009 is expected to be approximately 74% due to raw material inflation. Operating expenses will increase due to higher percentage of R&D investment as well as expanding our own distribution channels. We estimate our overall 2009 net profit margins to be approximately 30%.
Liquidity and Capital Resources
The following table summarizes our cash and cash equivalents position, our working capital, and our cash flow activity as of December 31, 2008 and 2007 and for each of the years then ended:
| | | | | | | |
| As of December 31: | | | | | | |
| Cash and cash equivalents | | $ | 40,288,116 | | | $ | 9,190,870 | |
| Working capital | | $ | 58,022,750 | | | $ | 15,447,162 | |
| Inventories | | $ | 462,351 | | | $ | 371,672 | |
| Year Ended December 31: | | | | | | | | |
| Cash provided by (used in): | | | | | | | | |
| Operating activities | | $ | 27,538,021 | | | $ | 11,601,480 | |
| Investing activities | | $ | (23,114,522 | ) | | $ | (10,260,933 | ) |
| Financing activities | | $ | 25,355,470 | | | $ | (32,516 | ) |
As of December 31, 2008, cash and cash equivalents were approximately $40.3 million as compared to $9.2 million at December 31, 2007. The increased cash and cash equivalents position of approximately $31.1 million at December 31, 2008 was primarily due to our cash flows provided by operating activities in 2008 of approximately $27.5 million and the receipt of net proceeds of approximately $23.5 million from the 2008 issuance of 2,500,000 shares of our common stock. These favorable variances were partially offset by the outlay of funds of approximately $10.9 million related to our 2008 business acquisitions and our construction in progress expenditures of approximately $2.0 million for our new corporate headquarters being built in Harbin. Our Corporate headquarters is expected to be completed in 2009 at an additional estimated cost of approximately $5 million.
We plan to fund our corporate headquarters construction project using internal funds.
Our current ratio was 10.17 versus 4.07 and the quick ratio was 8.80 versus 4.05 at December 31, 2008 and 2007, respectively. Management endeavors to ensure that funds are available to take advantage of new investment opportunities and that funds are sufficient to meet future liquidity and capital needs.
We calculate accounts receivable turnover by averaging the opening and closing balances of out accounts receivable during that period and dividing that amount by our average daily sales during that period. Since accounts receivables fluctuate over the course of each quarter, in order to determine a more representative accountant receivables collection days, management calculates the turnover rate on a quarter-by-quarter basis. Our average daily sales and turnover for each quarter during 2007 and 2008 were as follows:
Quarter Ended | | Average Daily Sales | | | Turnover (days) | |
| March 31, 2007 | | $ | 57,546 | | | | 66.3 | |
| June 30, 2007 | | $ | 160,937 | | | | 25.4 | |
| September 30, 2007 | | $ | 180,289 | | | | 31.6 | |
| December 31, 2007 | | $ | 138,298 | | | | 67.4 | |
| March 31, 2008 | | $ | 136,441 | | | | 74.5 | |
| June 30, 2008 | | $ | 260,974 | | | | 35.9 | |
| September 30, 2008 | | $ | 322,818 | | | | 28.8 | |
| December 31, 2008 | | $ | 282,118 | | | | 43.0 | |
In 2008, due to our change in business model, we reduced the number of our customers from 943 to 233. As a result, our accounts receivable turnover has improved significantly.
During 2007 and 2008, our average inventory turnover was approximately 30 and 18 days, respectively. Since sales and costs of goods sold fluctuate over the course of each quarter, in order to determine a more representative inventory rate, management calculates inventory rate on a quarter-by-quarter basis, and then take the average of the resulting numbers. Management calculates our inventory turnover rate using total inventory rather than just finished goods, because our production cycle is of an extremely short duration. Our average inventory and turnover for each quarter during 2007 and 2008 were as follows:
Quarter Ended | | Average Inventory | | | Turnover (days) | |
| March 31, 2007 | | $ | 580,062 | | | | 46.3 | |
| June 30, 2007 | | $ | 944,100 | | | | 26.0 | |
| September 30, 2007 | | $ | 1,039,032 | | | | 25.8 | |
| December 31, 2007 | | $ | 674,993 | | | | 21.7 | |
| March 31, 2008 | | $ | 583,201 | | | | 18.6 | |
| June 30, 2008 | | $ | 1,109,443 | | | | 18.3 | |
| September 30, 2008 | | $ | 1,613,792 | | | | 20.2 | |
| December 31, 2008 | | $ | 1,132,890 | | | | 15.7 | |
At December 31, 2008, there are no restrictive bank deposits pledged as security.
Cash flows provided by operating activities was approximately $27.5 million for the year ended December 31, 2008 compared to $11.6 million for the same period in 2007. The increase in cash provided by operating activities of approximately $15.9 million is primarily attributable to the increased net income of approximately $28.8 million in 2008 versus $15.3 million in 2007.
Our working capital position at December 31, 2008 was approximately $58.0 million, compared to $15.4 million at December 31, 2007. Our increased working capital position in 2008 was principally funded by the increased cash flows generated from our operating activities ($15.9 million) and the rate of our common stock under the January 2008 private placement (23.5 million) in 2008. Management considers current working capital and borrowing capabilities adequate to cover our current operating and capital requirements for the full year 2009.
Currency Exchange Fluctuations
All of our revenues and majority of the expenses during the year ended December 31, 2008 were denominated primarily in Renminbi (“RMB”), the currency of China, and was converted into US dollars at the exchange rate of 6.96225 RMB to 1 U.S. Dollar. In the third quarter of 2005, the Renminbi began to rise against the US dollar. There can be no assurance that RMB-to-U.S. dollar exchange rates will remain stable. A devaluation of RMB relative to the U.S. dollar would adversely affect our business, financial condition and results of operations. We do not engage in currency hedging.
Off-Balance Sheet Arrangements
We do not have any off-balance sheet arrangements that are currently material or reasonably likely to be material to our financial position or results of operations.
Item 7A. Quantitative and Qualitative Disclosures About Market Risk
We are a smaller reporting company as defined by Rule 12b-2 of the Securities Exchange Act of 1934 and are not required to provide the information under this item.
Item 8. Financial Statements and Supplementary Data.
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
| Report of Independent Registered Public Accounting Firm | | F-2 |
| | | |
| Report of Independent Registered Public Accounting Firm | | F-3 |
| | | |
| Consolidated Balance Sheets | | F-4 |
| | | |
| Consolidated Statements of Operations and Comprehensive Income | | F-5 |
| | | |
| Consolidated Statements of Stockholders’ Equity | | F-6 |
| | | |
| Consolidated Statements of Cash Flows | | F-7 |
| | | |
| Notes to Consolidated Financial Statements | | F-8 - F-28 |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of
China Sky One Medical, Inc. and Subsidiaries
We have audited the accompanying consolidated balance sheet of China Sky One Medical, Inc. and Subsidiaries (the “Company”) as of December 31, 2008 and the related consolidated statements of operations and comprehensive income, stockholders' equity, and cash flows for the year then ended. These consolidated financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these consolidated financial statements based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audit provides a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of China Sky One Medical, Inc. and Subsidiaries as of December 31, 2008 and the results of their operations and their cash flows for the year then ended in conformity with United States generally accepted accounting principles.
We were not engaged to examine management’s assessment of the effectiveness of the Company’s internal control over financial reporting as of December 31, 2008, included in the accompanying Management’s Report on Internal Control Over Financial Reporting and accordingly, we do not express an opinion thereon.
| /s/ MSPC |
| MSPC |
| Certified Public Accountants and Advisors |
| A Professional Corporation |
New York, New York
March 25, 2009
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
[Letterhead of Sherb & Co., LLP]
To the Board of Directors and Stockholders of
China Sky One Medical, Inc.
We have audited the accompanying consolidated balance sheets of China Sky One Medical, Inc. and its Subsidiaries as of December 31, 2007 and the related consolidated statements of operations, stockholders’ equity and cash flows for the year ended December 31, 2007. China Sky One Medical, Inc. management is responsible for these financial statements. Our responsibility is to express an opinion on these consolidated financial statements based on our audit.
We conducted our audit in accordance with standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, and audit of its internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purposes of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audit provides a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of China Sky One Medical, Inc. as of December 31, 2007 and the results of its operations and its cash flows for the year ended December 31, 2007 in conformity with accounting principles generally accepted in the United States.
| /s/ Sherb & Co., LLP |
| Certified Public Accountants |
Boca Raton, Florida
March 25, 2008
China Sky One Medical, Inc. and Subsidiaries
Consolidated Balance Sheets
| | | | | | | |
| ASSETS | | | | | | |
| Current Assets | | | | | | |
| Cash and cash equivalents | | $ | 40,288,116 | | | $ | 9,190,870 | |
| Accounts receivable, net | | | 14,978,648 | | | | 10,867,106 | |
| Inventories | | | 462,351 | | | | 371,672 | |
| Prepaid and other current assets | | | 106,386 | | | | 57,907 | |
| Land and construction deposit | | | 8,513,284 | | | | - | |
| Total current assets | | | 64,348,785 | | | | 20,487,555 | |
| | | | | | | | | |
| Property and equipment, net | | | 21,058,779 | | | | 6,861,432 | |
| Land and construction deposit | | | - | | | | 8,003,205 | |
| Intangible assets, net | | | 15,851,765 | | | | 1,933,014 | |
| | | $ | 101,259,329 | | | $ | 37,285,206 | |
| LIABILITIES AND STOCKHOLDERS' EQUITY | | | | | | | | |
| Current Liabilities | | | | | | | | |
| Accounts payable and accrued expenses | | $ | 2,937,068 | | | $ | 3,448,701 | |
| Taxes payable | | | 3,362,888 | | | | 1,567,188 | |
| Deferred revenues | | | 26,079 | | | | 24,504 | |
| Total current liabilities | | | 6,326,035 | | | | 5,040,393 | |
| | | | | | | | | |
| Property and equipment, net | | | 21,058,779 | | | | 6,861,432 | |
| Land and construction deposit | | | - | | | | 8,003,205 | |
| Intangible assets, net | | | 15,851,765 | | | | 1,933,014 | |
| | | $ | 101,259,329 | | | $ | 37,285,206 | |
| | | | | | | | | |
| Commitments and Contingencies | | | | | | | | |
| | | | | | | | | |
| Stockholders' Equity | | | | | | | | |
| Preferred stock ($0.001 par value, 5,000,000 shares authorized, none issued and outstanding) | | | - | | | | - | |
| Common stock ($0.001 par value, 50,000,000 shares authorized, 16,306,184 and 12,228,363 issued and outstanding, respectively) | | | 16,306 | | | | 12,228 | |
| Additional paid-in capital | | | 40,105,134 | | | | 9,572,608 | |
| Accumulated other comprehensive income | | | 5,566,806 | | | | 2,271,843 | |
| Retained earnings | | | 49,245,048 | | | | 20,388,134 | |
| Total stockholders' equity | | | 94,933,294 | | | | 32,244,813 | |
| | | $ | 101,259,329 | | | $ | 37,285,206 | |
The accompanying notes are an integral part of these consolidated financial statements.
China Sky One Medical, Inc. and Subsidiaries
Consolidated Statements of Operations and Comprehensive Income
| | | | |
| | | | | | | |
| Revenues | | $ | 91,816,183 | | | $ | 49,318,308 | |
| Cost of Goods Sold | | | 22,403,303 | | | | 10,939,531 | |
| Gross Profit | | | 69,412,880 | | | | 38,378,777 | |
| | | | | | | | | |
| Operating Expenses | | | | | | | | |
| Selling, general and administrative | | | 25,482,201 | | | | 16,163,577 | |
| Depreciation and amortization | | | 858,406 | | | | 443,063 | |
| Research and development | | | 7,412,895 | | | | 3,158,351 | |
| Total operating expenses | | | 33,753,502 | | | | 19,764,991 | |
| | | | | | | | | |
| Income From Operations | | | 35,659,378 | | | | 18,613,786 | |
| | | | | | | | | |
| Other Income | | | | | | | | |
MisellaneousMiscellaneous income | | | 813,705 | | | | 38,332 | |
| | | | | | | | | |
| Net Income Before Provision for Income Tax | | | 36,473,082 | | | | 18,652,118 | |
| | | | | | | | | |
| Provision for Income Taxes | | | | | | | | |
| Current | | | 7,616,169 | | | | 3,319,173 | |
| | | | | | | | | |
| Income | | | 28,856,914 | | | | 15,332,945 | |
| | | | | | | | | |
| The Components of Other Comprehensive Income | | | | | | | | |
| Foreign currency translation adjustment | | | 3,294,963 | | | | 1,849,724 | |
| | | | | | | | | |
| Comprehensive Income | | $ | 32,151,877 | | | $ | 17,182,669 | |
| | | | | | | | | |
| Basic Earnings Per Share | | $ | 1.91 | | | $ | 1.27 | |
| Basic Weighted Average Shares Outstanding | | | 15,101,833 | | | | 12,094,949 | |
| | | | | | | | | |
| Diluted Earnings Per Share | | $ | 1.87 | | | $ | 1.15 | |
| Basic Weighted Average Shares Outstanding | | | 15,429,136 | | | | 13,370,528 | |
The accompanying notes are an integral part of these consolidated financial statements.
China Sky One Medical, Inc. and Subsidiaries
Consolidated Statements of Stockholders' Equity
For the Years Ended December 31, 2008 and 2007
| | | | | | | | | | | | | | | | | | Total | |
| | | | | | Additional | | | Retained | | | Comprehensive | | | Stockholders’ | |
| | | | | | | | | | | | | | | | | | | |
| Balance at January 1, 2007 | | | 12,031,536 | | | $ | 12,032 | | | $ | 8,821,502 | | | $ | 5,055,189 | | | $ | 422,119 | | | $ | 14,310,842 | |
| Issuance of common stock for service | | | 30,000 | | | | 30 | | | | 194,970 | | | | | | | | | | | | 195,000 | |
| Warrants exercised | | | 166,827 | | | | 167 | | | | 515,667 | | | | | | | | | | | | 515,834 | |
| Employee stock options | | | | | | | | | | | 40,468 | | | | | | | | | | | | 40,468 | |
| Foreign currency translation adjustment | | | | | | | | | | | | | | | | | | | 1,849,724 | | | | 1,849,724 | |
| Net income | | | | | | | | | | | | | | | 15,332,945 | | | | | | | | 15,332,945 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Balance at December 31, 2007 | | | 12,228,363 | | | | 12,229 | | | | 9,572,607 | | | | 20,388,134 | | | | 2,271,843 | | | | 32,244,813 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Issuance of common stock through private placement | | | 2,500,000 | | | | 2,500 | | | | 23,485,463 | | | | | | | | | | | | 23,487,963 | |
| Warrants and options exercised under cash and cashless options | | | 1,142,302 | | | | 1,142 | | | | 1,866,365 | | | | | | | | | | | | 1,867,507 | |
Issuance of common stock for acquistionsacquisitions | | | 405,456 | | | | 405 | | | | 4,865,067 | | | | | | | | | | | | 4,865,472 | |
| Stock-based compensation | | | 30,063 | | | | 30 | | | | 315,632 | | | | | | | | | | | | 315,662 | |
| Foreign currency translation adjustment | | | | | | | | | | | | | | | | | | | 3,294,963 | | | | 3,294,963 | |
| Net income | | | | | | | | | | | | | | | 28,856,914 | | | | | | | | 28,856,914 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Balance at December 31, 2008 | | | 16,306,184 | | | $ | 16,306 | | | $ | 40,105,134 | | | $ | 49,245,048 | | | $ | 5,566,806 | | | $ | 94,933,294 | |
The accompanying notes are an integral part of these consolidated financial statements.
China Sky One Medical, Inc. and Subsidiaries
Consolidated Statements of Cash Flows
For the Years Ended December 31, 2008 and 2007
| | | | | | | |
| CASH FLOWS FROM OPERATING ACTIVITIES | | | | | | |
| Net income | | $ | 28,856,914 | | | $ | 15,332,945 | |
| Adjustments to reconcile net income to net cash provided (used) by operating activities: | | | | | | | | |
| Allowance for bad debt | | | 37,883 | | | | - | |
| Depreciation and amortization | | | 858,406 | | | | 443,063 | |
| Stock-based compensation | | | 315,662 | | | | 235,468 | |
| | | | | | | | | |
| Decrease (increase) in operating assets: | | | | | | | | |
| Accounts receivable | | | (3,398,228 | ) | | | (7,478,964 | ) |
| Inventories | | | (65,762 | ) | | | (73,142 | ) |
| Prepaid expenses and others | | | (23,840 | ) | | | 93,463 | |
| Decrease (increase) in operating assets: | | | | | | | | |
| Accounts receivable | | | (3,398,228 | ) | | | (7,478,964 | ) |
| Inventories | | | (65,762 | ) | | | (73,142 | ) |
| Prepaid expenses and others | | | (23,840 | ) | | | 93,463 | |
| Increase (decrease) in operating liabilities: | | | | | | | | |
| Accounts payable and accrued liabilities | | | (677,722 | ) | | | 2,136,356 | |
| Taxes payable | | | 1,660,382 | | | | 960,170 | |
| Deferred revenues | | | (25,674 | ) | | | (47,879 | ) |
| Net cash provided by operating activities | | | 27,538,021 | | | | 11,601,480 | |
| | | | | | | | | |
| CASH FLOWS FROM INVESTING ACTIVITIES | | | | | | | | |
| Purchase of property, plant and equipment | | | (11,167,396 | ) | | | (2,222,448 | ) |
| Land and construction deposits | | | 4,084 | | | | (8,003,205 | ) |
| Purchase of intangible assets | | | (11,951,210 | ) | | | (35,280 | ) |
| Net cash used in investing activities | | | (23,114,522 | ) | | | (10,260,933 | ) |
| | | | | | | | | |
| CASH FLOWS FROM FINANCING ACTIVITIES | | | | | | | | |
| Sales of common stock for cash, net of offering costs | | | 23,487,963 | | | | - | |
| Proceeds from warrants and options exercised | | | 1,867,507 | | | | 515,834 | |
| Payment of short-term loan | | | - | | | | (548,350 | ) |
| Net cash provided (used) by financing activities | | | 25,355,470 | | | | (32,516 | ) |
| | | | | | | | | |
| Effect of exchange rate changes on cash | | | 1,318,277 | | | | 1,296,039 | |
| | | | | | | | | |
| NET INCREASE IN CASH AND CASH EQUIVALENTS | | $ | 31,097,246 | | | $ | 2,604,070 | |
| | | | | | | | | |
| CASH AND CASH EQUIVALENTS AT BEGINNING OF YEAR | | | 9,190,870 | | | | 6,586,800 | |
| | | | | | | | | |
| CASH AND CASH EQUIVALENTS AT END OF YEAR | | $ | 40,288,116 | | | $ | 9,190,870 | |
| | | | | | | | | |
| Supplemental disclosure of cash flow information | | | | | | | | |
| Interest paid | | $ | 135,136 | | | $ | 10,457 | |
| Taxes paid | | $ | 6,630,480 | | | $ | 2,359,003 | |
| | | | | | | | | |
| On April 3, 2008, the Company acquired a 100% ownership interest in Heilongjiang Tianlong Pharmaceutical. Approximate net assets acquired (see note 2) consisted of the following: | | | | | | | | |
| | | | | | | | | |
| Fixed assets | | $ | 6,314,871 | | | | | |
| Intangible assets | | | 1,786,990 | | | | | |
| Other | | | 170,000 | | | | | |
| Net assets acquired | | $ | 8,271,861 | | | | | |
| | | | | | | | | |
| On April 18, 2008, the Company acquired Heilongjiang Haina ("Haina") Pharmaceutical Inc. Approximate net assets acquired (see note 2) consisted of the following: | | | | | | | | |
| | | | | | | | | |
| Intangible assets | | $ | 437,375 | | | | | |
| | | | | | | | | |
| On September 5, 2008, the Company acquired a 100% ownership interest in Peng Lai Jin Chuang Company. Approximate net assets acquired (see note 2) consisted of the following: | | | | | | | | |
| | | | | | | | | |
| Fixed assets | | $ | 4,176,922 | | | | | |
| Intangible assets | | | 2,917,386 | | | | | |
| Net assets acquired | | $ | 7,094,308 | | | | | |
| Other | | | 170,000 | | | | | |
The accompanying notes are an integral part of these consolidated financial statements.
China Sky One Medical, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
| 1. | Description of Business |
China Sky One Medical Inc., ("China Sky One" or the “Company”), a Nevada corporation, was formed on February 7, 1986, and formerly known as Comet Technologies, Inc. (“Comet”). On July 26, 2006, the Company changed the name of the reporting company from "Comet Technologies, Inc." to "China Sky One Medical, Inc."
American California Pharmaceutical Group, Inc. (“ACPG”), our non operating United States holding company subsidiary, was incorporated on December 16, 2003, in the State of California, under the name “QQ Group, Inc.” QQ Group, Inc. changed its name to “American California Pharmaceutical Group, Inc.” in anticipation of the Stock Exchange Agreement with China Sky One (then known as “Comet Technologies, Inc.”) and TDR, described herein. On December 8, 2005, ACPG completed a stock exchange transaction with TDR a People’s Republic of China (“PRC”) based operating company and TDR’s subsidiaries (the “TDR Acquisition”), each of which were fully operating companies in the PRC. Under the terms of the agreement, ACPG exchanged 100% of its issued and outstanding common stock for 100% of the capital stock of TDR and its subsidiaries, described below.
Thereafter, on May 11, 2006, ACPG entered into a Stock Exchange Agreement (the “Exchange Agreement”) with the shareholders of China Sky One. The terms of the Exchange Agreement were consummated and the acquisition was completed on May 30, 2006. As a result of the transaction, the Company issued a total of 10,193,377 shares of its common voting stock to the stockholders of ACPG, in exchange for 100% of the capital stock of ACPG resulting in ACPG becoming our wholly-owned subsidiary. The transaction is treated as a reverse merger for accounting purposes.
TDR, formerly known as “Harbin City Tian Di Ren Medical Co.,” was originally formed in 1994 and maintained its principal executive office in Harbin City of Heilongjiang Province, in the People’s Republic of China (“PRC”). TDR was reorganized and incorporated as a limited liability company on December 29, 2000, under the “Corporation Laws and Regulations” of the PRC. At the time of the TDR Acquisition by ACPG in December of 2005, TDR had two wholly-owned subsidiaries, Harbin First Bio-Engineering Company Limited and Kangxi Medical Care Product Factory, until July, 2006, when the two were merged, with Harbin First Bio-Engineering Company Limited (“First”) as the surviving subsidiary of TDR. The principal activities of TDR and First are the research, manufacture and sale of over-the-counter non-prescription health care products. TDR commenced its business in the sale of branded nutritional supplements and over-the-counter pharmaceutical products in the Heilongjiang Province. TDR has subsequently evolved into an integrated manufacturer, marketer, and distributor of external use natural Chinese medicine products sold primarily to and through China’s various domestic pharmaceutical chain stores.
China Sky One is a holding company whose principal operations are through its wholly-owned subsidiaries; it has no revenues separate from its subsidiaries, and has nominal expenses related to its status as a public reporting company and to its ownership interest in ACPG and TDR.
On September 30, 2008 (the “Record Date”), we obtained the written consent of the holders of 8,158,251 shares of our common stock, which as of the Record Date, represented 51.3% of our outstanding voting securities, to increase our number of authorized shares of common stock from twenty million (20,000,000) to fifty million (50,000,000) shares.
| 2. | Acquisition of Businesses |
On April 3, 2008, TDR completed an acquisition pursuant to an Equity Transfer Agreement dated February 22, 2008, between TDR and Heilongjiang Tianlong Pharmaceutical, Inc., a corporation with a multitude of SFDA approved medicines and new medicine applications, organized under the laws of the PRC (“Tianlong”), which is in the business of manufacturing external-use pharmaceuticals. Our TDR subsidiary previously acquired the Beijing sales office of Tianlong in mid 2006. Pursuant to the Equity Transfer Agreement, TDR acquired 100% of the issued and outstanding capital stock of Tianlong from Heilongjiang’s sole stockholder Wu Jiechen, a resident of China, in consideration for an aggregate purchase price of approximately $8,300,000, consisting of (i) $8,000,000 in cash, and (ii) 23,850 shares of China Sky One (fair value at April 3, 2008 of approximately $270,000). The acquisition received regulatory approval and closed on April 3, 2008.
China Sky One Medical, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
| 2. | Acquisition of Businesses (Continued) |
The following table summarizes the approximate estimated fair values of the assets acquired in the Tianlong acquisition.
| Fixed assets | | $ | 6,314,871 | |
| Intangible assets | | | 1,786,990 | |
| Other | | | 170,000 | |
| Net assets acquired | | $ | 8,271,861 | |
On April 18, 2008, China Sky One through its subsidiary TDR consummated a share acquisition pursuant to an Equity Transfer Agreement with the shareholders of Heilongjiang Haina Pharmaceutical Inc., a recently formed corporation organized under the laws of the PRC (“Haina”) licensed as a wholesaler of TCD, bio-medicines, bio-products, medicinal devices, antibiotics and chemical medicines. Haina does not have an established sales network and was acquired for its primary asset, a Good Supply Practice (GSP) license (License No. A-HLJ03-010) issued by the Heilongjian office of the SFDA. The SFDA recently started issuing such licenses to resellers of medicines that maintain certain quality controls. The GSP license was issued as of December 21, 2006 and will expire on January 29, 2012 and will enable the Company to expand its sales of medicinal products without having to go through a lengthy license application process.
The following table summarizes the approximate estimated fair values of the assets acquired in the Haina acquisition.
| Cash | | $ | 84,307 | |
| Intangible assets | | $ | 1,786,990353,068 | |
| Net assets acquired | | $ | 437,375 | |
Pursuant to the Equity Transfer Agreement, TDR acquired 100% of the issued and outstanding capital stock of Haina from its three stockholders in consideration for payment of 3,000,000 RMB (approximately $437,375). TDR has been overseeing the operations of Haina since January of 2008 as part of its due diligence prior to closing of this acquisition.
On June 9, 2008, TDR entered into a Merger and Acquisition Agreement (the “Acquisition Agreement”) with Peng Lai Jin Chuang Company, a corporation organized under the laws of the People’s Republic of China (“Peng Lai”), which was recently organized to develop, manufacture and distribute pharmaceutical, medicinal and diagnostic products in the PRC. Pursuant to the Acquisition Agreement, TDR acquired all of the assets of Peng Lai in consideration for an aggregate of approximately (i) US$2.5 million in cash, and (ii) 381,606 shares of the Company’s common stock with a fair value of approximately $4.6 million (at $12 per share). The acquisition of Peng Lai closed on September 5, 2008.
The following table summarizes the approximate estimated fair values of the assets acquired in the Peng Lai acquisition.
| Fixed assets | | $ | 4,176,922 | |
| Intangible assets | | | 2,917,386 | |
| Net assets acquired | | $ | 7,094,308 | |
The following table contains pro forma condensed consolidated statement of operations information assuming the Tianlong, Haina and Jin Chuang transactions closed on January 1, 2007, for the years ended December 31, 2008 and 2007. Jin Chuang had dormant operations until October 2008.
| | | | | | | |
| Revenue | | $ | 92,377,568 | | | $ | 51,334,371 | |
| Operating income | | $ | 35,746,742 | | | $ | 17,143,333 | |
| Net income | | $ | 28,933,581 | | | $ | 13,822,150 | |
| Basic earnings per common share | | $ | 1.92 | | | $ | 1.14 | |
| Basic weighted average shares outstanding | | | 15,358,843 | | | | 12,500,405 | |
| Diluted earnings per common share | | $ | 1.88 | | | $ | 1.03 | |
| Diluted weighted average shares outstanding | | | 15,686,146 | | | | 13,775,984 | |
China Sky One Medical, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
| 3. | Summary of Significant Accounting Policies |
Principles of Consolidation – The accompanying consolidated financial statements include the accounts of the Company and its wholly-owned subsidiaries, ACPG, TDR, First, Haina, Tianlong, and Peng Lai. All significant inter-company transactions and balances were eliminated.
These financial statements are stated in U.S. Dollars and have been prepared in accordance with accounting principles generally accepted in the United States of America. This basis of accounting differs from that used under applicable accounting requirements in the PRC. No material adjustment was required.
Certain items in the 2007 financial statements have been reclassified to conform with the 2008 financial statements presentation.
Use of estimates – The preparation of these financial statements in conformity with accounting principles generally accepted in the United States of America, requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, disclosure of contingent assets and liabilities at the dates of the financial statements, and the reported amounts of revenues and expenses during the reported periods.
Significant estimates include values and lives assigned to acquiredacquire tangible and intangible assets under our business acquisitions, reserves for customer returns and allowances, uncollectible accounts receivable, share-based compensation, impairment testing of goodwill and other intangible assets and slow moving and/or obsolete/damaged inventory. Actual results may differ from these estimates.
Earnings per share - Basic earnings per common share is computed by dividing net earnings applicable to common shareholders by the weighted-average number of common shares outstanding during the period. When applicable, diluted earnings per common share is determined using the weighted-average number of common shares outstanding during the period, adjusted for the dilutive effect of common stock equivalents, consisting of shares that might be issued upon exercise of common stock options and warrants.
Potential common shares issued are calculated using the treasury stock method, which recognizes the use of proceeds that could be obtained upon the exercise of options and warrants in computing diluted earnings per share. It assumes that any proceeds would be used to purchase common stock at the average market price of the common stock during the period.
Cash and cash equivalents – The Company considers all highly liquid debt instruments purchased with maturity period of sixthree months or less to be cash equivalents. The carrying amounts reported in the accompanying consolidated balance sheets for cash and cash equivalents approximate their fair value.
Accounts receivable – Accounts receivable are stated at net realizable value, net of an allowance for doubtful accounts. Provision of allowance is made for estimated bad debts based on a periodic analysis of individual customer balances including an evaluation of days of sales outstanding, payment history, recent payment trends, and perceived credit worthiness. At December 31, 2008, the Company’s allowance for doubtful accounts was approximately $50,000. At December 31, 2007, the Company had no allowance for doubtful accounts.
Inventories – Inventories include finished goods, raw materials, freight-in, packing materials, labor, and overhead costs and are valued at the lower of cost or market using the first-in, first-out method. Inventory units are valued using the weighted average method. Provisions are made for slow moving, obsolete and/or damaged inventory based on a periodic analysis of individual inventory items including an evaluation of historical usage and/or movement, age, expiration date, and general conditions. There was no inventory reserve provision recorded at December 31, 2008 and December 31, 2007.
Property and equipment – Property and equipment are stated at historical cost less accumulated depreciation. Depreciation on property and equipment is provided using the straight-line method over the estimated useful lives of the assets. The Company uses an estimated residual value of 5% of cost, or valuation for both financial and income tax reporting purposes. The estimated lengths of useful lives are as follows:
China Sky One Medical, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
| 3. | Summary of Significant Accounting Policies (Continued) |
| Building and Improvements | 30 years |
| Land use rights | 50 years |
| Furniture & Equipment | 5 to 7 years |
| Transportation Equipment | 5 to 15 years |
| Machinery and Equipment | 7 to 14 years |
Expenditures for renewals and betterments are capitalized while repairs and maintenance costs are normally charged to the statement of operations in the year in which they were incurred. In situations where it can be clearly demonstrated that the expenditure has resulted in an increase in the future economic benefits expected to obtain from the use of the asset, the expenditure is capitalized as an additional cost of the asset. Upon sale or disposal of an asset, the historical cost and related accumulated depreciation or amortization of such asset is removed from their respective accounts, and any gain or loss is recorded in the Consolidated Statement of Operations.
Property and equipment are evaluated for impairment in value whenever an event or change in circumstances indicates that the carrying values may not be recoverable. If such an event or change in circumstances occurs and potential impairment is indicated because the carrying values exceed the estimated future undiscounted cash flows of the asset, the Company will measure the impairment loss as the amount by which the carrying value of the asset exceeds its fair value. The Company did not record any impairment charges during each of the years ended December 31, 2008 and 2007.
Construction-in-progress – Properties currently under development are accounted for as construction-in-progress. Construction-in-progress is recorded at acquisition cost, including land rights cost, development expenditures, professional fees, and capitalized interest costs during the course of construction.
Upon completion and readiness for use of the project, the cost of construction-in-progress is transferred to the facility. In the case of construction-in-progress, management takes into consideration the estimated cost to complete the project when making the lower of cost or market calculation.
Intangible assets – Intangible assets consists of patents and goodwill. Patent costs are amortized over an estimated life of ten years. As of December 31, 2008, the remaining weighted average life of our patents is approximately 9 years.
Intangible assets are accounted for in accordance with Statement of Financial Accounting Standards No. 142, Goodwill and Other Intangible Assets (“SFAS 142”). Intangible assets with finite useful lives are amortized while intangible assets with indefinite useful lives are not amortized. As prescribed by SFAS 142, goodwill and intangible assets are tested periodically for impairment. The Company adopted SFAS No. 144, "Accounting for the Impairment or Disposal of Long- Lived Assets," effective January 1, 2002. Accordingly, the Company reviews its long-lived assets, including property and equipment and finite-lived intangible assets for impairment whenever events or changes in circumstances indicate that the carrying amount of the assets may not be fully recoverable. To determine recoverability of its long-lived assets, the Company evaluates the probability that future undiscounted net cash flows will be less than the carrying amount of the assets. Impairment costs, if any, are measured by comparing the carrying amount of the related assets to their fair value. The Company recognizes an impairment loss based on the excess of the carrying amount of the assets over their respective fair values. Fair value is determined by discounted future cash flows, appraisals or other methods. If the assets determined to be impaired are to be held and used, the Company recognizes an impairment loss thru a charge to operating results to the extent the present value of anticipated cash flows attributable to the assets are less than the asset’s carrying value. The Company would depreciate the remaining the remaining value over the remaining estimated useful life of the asset to operating results. There were no impairments during each of the years ended December 31, 2008 and 2007.
Foreign Currency - The Company’s principal country of operations is in The People’s Republic of China. The financial position and results of operations of the Company are recorded in RMB as the functional currency. The results of operations denominated in foreign currency are translated at the average rate of exchange during the reporting period.
China Sky One Medical, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
| 3. | Summary of Significant Accounting Policies (Continued) |
Assets and liabilities denominated in foreign currencies at the balance sheet date are translated at the market rate of exchange at that date. The registered equity capital denominated in the functional currency is translated at the historical rate of exchange at the time of the capital contribution. All translation adjustments resulting from the translation of the financial statements into the reporting currency (“US Dollars”) are recorded as accumulated other comprehensive income, a component of stockholders’ equity.
Revenue recognition — Revenue is recognized when the following criteria are met: (1) persuasive evidence of an arrangement exists; (2) the product has been shipped and the customer takes ownership and assumes the risk of loss; (3) the selling price is fixed or determinable; and (4) collection of the resulting receivable is reasonably assured. The Company believes that these criteria are satisfied upon shipment from its facilities. Revenue is reduced by provisions for estimated returns and allowances as well as specific known claims, if any , which are based on historical averages that have not varied significantly for the periods presented. . Historically, the Company’s estimated returns, allowances and claims have been deemed immaterial. The Company’s sale agreements only allow a return if the product has quality related issues. In such event, the Company accepts the return for equivalent product exchange from inventory only.
The Company occasionally applies to various government agencies for research grants. Revenue from such research grants is recognized when earned. In situations where TDR receives payment in advance for the performance of research and development services, such amounts are deferred and recognized as revenue as the related services are performed.
Deferred revenues — The Company recognizes revenues as earned. Amounts billed in advance of the period in which goods are delivered are recorded as a liability under “Deferred revenues.”
Research and development — Research and development expenses include the costs associated with the Company’s internal research and development as well as research and development conducted by third parties. These costs primarily consist of salaries, clinical trials, outside consultants, and materials. All research and development costs are expensed as incurred.
Third-party expenses reimbursed under non-refundable research and development contracts are recorded as a reduction to research and development expense in the statement of operations.
The Company recognizes in-process research and development in accordance with FASB Interpretation No. 4, Applicability of FASB Statement No. 2 to Business Combinations Accounted for by the Purchase Method and the AICPA Technical Practice Aid, Assets Acquired in a Business Combination to be used in Research and Development Activities: A Focus on Software, Electronic Devices, and Pharmaceutical Industries. Assets to be used in research and development activities, specifically, compounds that have yet to receive new drug approval and would have no alternative use, should approval not be given, are immediately charged to expense when acquired. Certain assets and high technologies acquired that has a foreseeable future cash flows are capitalized as intangible assets. Such intangible assets are amortized starting from the year revenue is generated and amortize over a period of 10 years.
For the year ended December 31, 2008, the Company incurred $7,412,895 in research and development costs and $3,158,351 for the year ended December 31, 2007.
Advertising - Advertising and promotion costs – Generally, the Company signs contracts with agents who then place its advertising in the mediums of television, radio and internet. Advertising expense is incurred in the period the advertisements take place. Thus, costs of advertising are expensed as incurred , in compliance with FASB Concept Statement #6 paragraph 81 and ASC 720-35-05-1. Historically, the Company’s direct-response advertising costs has been immaterial . Total advertising costs for the years ended December 31, 2008 and 2007 waswere $7,299,367 and $4,385,045, respectively. Advertising costs are reported as part of selling, general and administrative expenses in the statementsstatement of operations.
Slotting fees — From time to time, the Company enters into arrangements with customers in exchange for obtaining rights to place our products on customers' shelves for resale. The Company also engages in promotional discount programs in order to enhance sales in specific channels. These payments, discounts and allowances reduce our reported revenue in accordance with the guidelines set forth in EITF 01-9 and SEC Staff Accounting Bulletin No. 104.
China Sky One Medical, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
| 3. | Summary of Significant Accounting Policies (Continued) |
The Company records its obligations under each arrangement at inception and amortizes the total required payments using the straight-line method over a term of time, which is normally the minimum time that we are allowed to sell our products in given retail outlets. The Company records the balance of the amortized slotting fee not used in the current period as prepaid expenses. As the Company applies the amortized slotting fee as contra revenue, it reduces the reported gross revenue by an amount equal to the reduction in prepaid expenses. For the years ended December 31, 2008 and 2007, the Company did not incur any slotting fees.
Taxation – The Company uses the asset and liability method of accounting for deferred income taxes. The Company’s provision for income taxes includes income taxes currently payable and those deferred because of temporary differences between the financial statement and tax bases of assets and liabilities. The Company records liabilities for income tax contingencies based on our best estimate of the underlying exposures.
The Company periodically estimates its tax obligations using historical experience in tax jurisdictions and informed judgments. There are inherent uncertainties related to the interpretation of tax regulations in the jurisdictions in which the Company transacts business. The judgments and estimates made at a point in time may change based on the outcome of tax audits, as well as changes to, or further interpretations of, regulations. The Company adjusts income tax expense in the period in which these events occur.
Provision for the PRC’s enterprise income tax is calculated at the prevailing rate based on the estimated assessable profits less available tax relief for losses brought forward.
Provision for the PRC enterprise income tax is calculated at the prevailing rate based on the estimated assessable profits less available tax relief for losses brought forward. The Company does not accrue taxes on unremitted earnings from foreign operations as it is the Company’s intention to invest these earnings in the foreign operations indefinitely.
Enterprise income tax
Under the Provisional Regulations of PRC Concerning Income Tax on Enterprises promulgated by the PRC, income tax is payable by enterprises at a rate of 25% of their taxable income. Preferential tax treatment may, however, be granted pursuant to any law or regulations from time to time promulgated by the State Council.
According to “Enterprise Income Tax and Certain Preferential Policies Notice” published by the Ministry of Finance and the National Tax Affairs Bureau, if the enterprise is authorized by the State Council as a special entity, the enterprise income tax rate is reduced to 15%. The income tax rate for TDR and Tianlong is 15% and 12% respectively, based on State Council approval.
Value added tax
The Provisional Regulations of PRC Concerning Value Added Tax promulgated by the State Council came into effect on January 1, 1994. Under these regulations and the Implementing Rules of the Provisional Regulations of the PRC Concerning Value Added Tax, value added tax is imposed on goods sold in, or imported into, the PRC and on processing, repair and replacement services provided within the PRC.
Value added tax payable in the PRC is charged on an aggregated basis at a rate of 13% or 17% (depending on the type of goods involved) on the full price collected for the goods sold or, in the case of taxable services provided, at a rate of 17% on the charges for the taxable services provided, but excluding, in respect of both goods and services, any amount paid in respect of value added tax included in the price or charges, and less any deductible value added tax already paid by the taxpayer on purchases of goods and services in the same financial year.
According to “Agriculture Product Value Added Tax Rate Adjustment and Certain Items’ Value Added Tax Waiver” published by the Ministry of Finance and the National Tax Affairs Bureau, the value added tax for agriculture related products is to be taxed at 13%. Furthermore, traditional Chinese medicine and medicinal plant are by definition agriculture related products.
China Sky One Medical, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
| 3. | Summary of Significant Accounting Policies (Continued) |
Accounting for uncertainty in income taxes – In June 2006, the Financial Accounting Standards Board (FASB) issued Interpretation No. 48, Accounting for Uncertainty in Income Taxes - an Interpretation of FASB Statement No. 109 (FIN 48). FIN 48 is intended to clarify the accounting for uncertainty in income taxes recognized in a company’s financial statements and prescribes the recognition and measurement of a tax position taken or expected to be taken in a tax return. FIN 48 also provides guidance on de-recognition, classification, interest and penalties, accounting in interim periods, disclosure and transition.
Under FIN 48, evaluation of a tax position is a two-step process. The first step is to determine whether it is more-likely-than-not that a tax position will be sustained upon examination, including the resolution of any related appeals or litigation based on the technical merits of that position. The second step is to measure a tax position that meets the more-likely-than-not threshold to determine the amount of benefit to be recognized in the financial statements. A tax position is measured at the largest amount of benefit that is greater than 50% likely of being realized upon ultimate settlement.
Tax positions that previously failed to meet the more-likely-than-not recognition threshold should be recognized in the first subsequent period in which the threshold is met. Previously recognized tax positions that no longer meet the more-likely-than-not criteria should be de-recognized in the first subsequent financial reporting period in which the threshold is no longer met.
The adoption of FIN 48 at January 1, 2007 did not have a material effect on the Company’s results of operations and financial position.
Comprehensive income – Comprehensive income consists of net income and other gains and losses affecting stockholders’ equity that, under generally accepted accounting principles are excluded from net income. For the Company, such items consist entirely of foreign currency translation gains and losses.
Related companies – A related company is a company in which the director has beneficial interests in and in which the Company has significant influence.
Retirement benefit costs – According to the PRC regulations on pension plans, the Company contributes to a defined contribution retirement plan organized by municipal government in the province in which the Company was registered and all qualified employees as defined by statutory regulations are eligible to participate in the plan. Retirement benefit costs incurred by the Company for each of years ended December 31, 2008 and 2007, respectively, amounted to $53,476 and $30,717.
Contributions to the pension or retirement plan are calculated at 22.5% of the employees’ salaries above a fixed threshold amount. The employees contribute between 2% to 8% to the pension plan, and the Company contributes the balance. The Company has no other material obligations for the payment of retirement benefits beyond the annual contributions under this plan.
Fair value of financial instruments – The carrying amounts of certain financial instruments, including cash and cash equivalents, accounts receivable, other receivables, accounts payable, accrued expenses, and other payables approximate their fair values at December 31, 2008 and 2007 because of the relatively short-term maturity of these instruments.
Recent accounting pronouncements:
| - | In June 2008, the FASB issued FSP Emerging Issues Task Force (“EITF”) Issue No. 03-6-1, “Determining Whether Instruments Granted in Share-Based Payment Transactions Are Participating Securities.” The EITF addresses whether instruments granted in share-based payment transactions are participating securities prior to vesting and, therefore, need to be included in the earnings allocation in computing earnings per share under the two-class method. The EITF affects entities that accrue dividends on share-based payment awards during the awards’ service period when the dividends do not need to be returned if the employees forfeit the award. This EITF is effective for fiscal years beginning after December 15, 2008. The Company is currently assessing the impact of FSP EITF 03-6-1 on its financial position and results of operations. |
China Sky One Medical, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
| 3. | Summary of Significant Accounting Policies (Continued) |
| - | In June 2008, the FASB ratified EITF Issue No. 07-5, "Determining Whether an Instrument (or an Embedded Feature) Is Indexed to an Entity's Own Stock" (EITF 07-5). EITF 07-5 provides that an entity should use a two step approach to evaluate whether an equity-linked financial instrument (or embedded feature) is indexed to its own stock, including evaluating the instrument's contingent exercise and settlement provisions. It also clarifies on the impact of foreign currency denominated strike prices and market-based employee stock option valuation instruments on the evaluation. EITF 07-5 is effective for fiscal years beginning after December 15, 2008. The Company is currently assessing the impact of EITF 07-5 on its financial position and results of operations. |
| - | In May 2008, the FASB issued SFAS No. 162, "The Hierarchy of Generally Accepted Accounting Principles" (FAS No.162). SFAS No. 162 identifies the sources of accounting principles and the framework for selecting the principles used in the preparation of financial statements. SFAS No. 162 is effective 60 days following the SEC's approval of the Public Company Accounting Oversight Board amendments to AU Section 411, "The Meaning of Present Fairly in Conformity with Generally Accepted Accounting Principles". The implementation of this standard will not have a material impact on the Company's financial position and results of operations. |
| - | In April 2008, the FASB issued FASB Staff Position on Financial Accounting Standard (“FSP FAS”) No. 142-3, “Determination of the Useful Life of Intangible Assets”, which amends the factors that should be considered in developing renewal or extension assumptions used to determine the useful life of intangible assets under SFAS No. 142 “Goodwill and Other Intangible Assets”. The intent of this FSP is to improve the consistency between the useful life of a recognized intangible asset under SFAS No. 142 and the period of the expected cash flows used to measure the fair value of the asset under SFAS No. 141 (revised 2007) “Business Combinations” and other U.S. generally accepted accounting principles. The Company is currently evaluating the potential impact of FSP FAS No. 142-3 on its financial statements. |
| - | In March 2008, the FASB issued Statement No. 161, Disclosures about Derivative Instruments and Hedging Activities an amendment of SFAS 133 ("SFAS 161"). This Statement will require enhanced disclosures about derivative instruments and hedging activities to enable investors to better understand their effects on an entity's financial position, financial performance, and cash flows. It is effective for financial statements issued for fiscal years and interim periods beginning after November 15, 2008, with early application encouraged. The Company does not expect the adoption of SFAS 161 to have a material impact on its financial position, results of operations or cash flows. |
| - | In December 2007, the FASB issued SFAS No. 141 (revised 2007), “Business Combinations” (“SFAS 141(R)”). SFAS 141(R) will change the accounting for business combinations. Under SFAS No. 141(R), an acquiring entity will be required to recognize all the assets acquired and liabilities assumed in a transaction at the acquisition-date fair value with limited exceptions. SFAS No. 141(R) will change the accounting treatment and disclosure for certain specific items in a business combination. SFAS 141R requires earn-outs and other contingent consideration to be recorded at fair value on acquisition date and contingencies to be recorded at fair value on acquisition date with subsequent remeasurement. SFAS 141R requires acquisition costs to be expensed as incurred and generally requires restructuring costs to be expensed in periods after the acquisition date. SFAS 141R requires amounts previously called “negative goodwill” which result from a bargain purchase in which acquisition date fair value of identifiable net assets acquired exceeds the fair value of consideration transferred plus any non controlling interest in the acquirer to be recognized in earnings as a gain attributable to the acquirer. SFAS No. 141(R) applies prospectively to business combinations for which the acquisition date is on or after the beginning of the first annual reporting period beginning on or after December 15, 2008. SFAS 141(R) will impact the Company in the event of any acquisition after December 31, 2008. |
China Sky One Medical, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
| 3. | Summary of Significant Accounting Policies (Continued) |
| - | In December 2007, the FASB issued SFAS No. 160, “Non-controlling Interests in Consolidated Financial Statements—an amendment of Accounting Research Bulletin No. 51” (“SFAS 160”). SFAS 160 requires noncontrolling interests to be reported in the equity section of financial statements and requires that net earnings include the amounts attributable to both the parent and the noncontrolling interests with disclosure on the face of the statement of operations of the net earnings attributable to the parent and to the noncontrolling interests, with any losses attributable to the noncontrolling interests in excess of the noncontrolling interests’ equity to be allocated to the noncontrolling interest. Calculation of earnings per share amounts in the financial statements will continue to be based on amounts attributable to the parent. SFAS No. 160 is effective for fiscal years beginning on or after December 15, 2008. The Company does not believe that SFAS 160 will have a material impact on the Company’s financial statements. |
| - | In February 2007, the FASB issued Statement No. 159 “The Fair Value Option for Financial Assets and Financial Liabilities” (SFAS 159). This statement permits companies to choose to measure many financial assets and liabilities at fair value. Unrealized gains and losses on items for which the fair value option has been elected are reported in earnings. SFAS 159 is effective for fiscal years beginning after November 15, 2007. The adoption of SFAS 159, effective January 1, 2008, did not have a material impact on the Company’s financial statements. |
| - | In September 2006, the FASB issued Statement of Financial Accounting Standards No. 157, Fair Value Measurements (“SFAS 157”). The statement provides enhanced guidance for using fair value to measure assets and liabilities and also responds to investors’ requests for expanded information about the extent to which company’s measure assets and liabilities at fair value, the information used to measure fair value, and the effect of fair value measurements on earnings. While the standard applies whenever other standards require (or permit) assets or liabilities to be measured at fair value, it does not expand the use of fair value in any new circumstances. Statement No. 157 is effective for financial statements issued for fiscal periods beginning after November 15, 2007. The Company adopted SFAS 157 effective January 1, 2008, except for the nonfinancial assets and liabilities that are subject to a one-year deferral allowed by FASB Staff Position (FSP) SFAS 157-2 (“ FSP SFAS 157-2”). FSP SFAS 157-2 delays the effective date of SFAS 157 until fiscal years beginning after November 15, 2008 for nonfinancial assets and liabilities that are not recognized or disclosed at fair value in the financial statements on a recurring basis (at least annually). The adoption of SFAS 157 did not have a material effect on our financial statements. The Company does not expect the adoption of SFAS 157 will have a material effect on our financial statements beginning in year 2009 as it relates to the items subject to the one-year deferral allowed by FSP SFAS 157-2. |
| 4. | Revenue By Product Category and Geographic Region |
The Company’s total revenues during fiscal 2008 and 2007 were approximately $91,801,000 and $49,318,308, respectively. The following table sets forth the Company’s principal product categories based on application type and the approximate amount and percentage of revenue from each of such product categories, during the fiscal year ended December 31, 2008 and 2007:
| | | | | | | | For the Year Ended December 31 | | | | |
| | | | | | | | 2008 | | | | | | | | | 2007 | | | | |
Product Category (97 products) | | Subsidiary | | Quantity (Unit) | | | Sales | | | % of Sales | | | Quantity (Unit) | | | Sales | | | % of Sales | |
| Patch (5 products) | | TDR | | | 9,494,535 | | | $ | 35,484,230 | | | | 39 | % | | | 2,294,901 | | | $ | 19,690,051 | | | | 39 | % |
| Ointment (20 products) | | TDR & TL | | | 11,478,130 | | | | 23,068,210 | | | | 25 | % | | | 3,037,022 | | | | 6,190,003 | | | | 13 | % |
| Spray (19 products) | | TDR & TL | | | 3,941,295 | | | | 10,612,679 | | | | 12 | % | | | 3,580,266 | | | | 9,210,233 | | | | 19 | % |
| Diagnostic Kits (3 products) | | First | | | 2,184,013 | | | | 8,780,990 | | | | 10 | % | | | 739,151 | | | | 2,990,664 | | | | 6 | % |
| Contract Sales (7 products) | | Haina | | | 3,837,578 | | | | 5,655,085 | | | | 6 | % | | | 5,718,652 | | | | 8,197,758 | | | | 17 | % |
| Others (43 products) | | | | | 4,306,972 | | | | 8,214,989 | | | | 8 | % | | | 1,896,193 | | | | 3,039,599 | | | | 6 | % |
| Total | | | | | 35,242,523 | | | $ | 91,816,183 | | | | 100 | % | | | 17,266,185 | | | $ | 49,318,308 | | | | 100 | % |
China Sky One Medical, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
Export sales for our main countries of export (in order of revenues during the year ended 2008) were as follows:
| | | |
| Malaysia | | $ | 8,821,616 | |
| Other | | $ | 37,575 | |
| TOTAL: | | $ | 8,859,191 | |
| 5. | 4. Concentrations of Business and Credit riskRisk |
The Company maintains certain bank accounts in the PRC which are not protected by FDIC insurance or other insurance. As of December 31, 2008 the Company held approximately $2,729,000 of cash balances within the United States of which approximately $577,000 was in excess of FDIC insurance limits. At December 31, 2008, the Company had approximately $37,579,000, in China bank deposits, which is not insured. As of December 31, 2007, the Company held $583,495 of cash balances within the United States of which $432,798 was in excess of FDIC insurance limits. At December 31, 2007, the Company had approximately $8,605,000 in China bank deposits, which may not be insured. Historically, the Company has not experienced any losses in such accounts.
A significant amount of the Company’s sales are concentrated in China. Accordingly, the Company is susceptible to fluctuations in its business caused by adverse economic conditions in this country. Difficult economic conditions in other geographic areas into which the Company may expand may also adversely affect its business, operations and finances.
The Company provides credit in the normal course of business. The Company performs ongoing credit evaluations of its customers and maintains allowances for doubtful accounts based on factors surrounding the credit risk of specific customers, historical trends, and other information.
Substantially all of the Company's fixedlong-lived assets and business operations are located in the PRC.
The Company is self-insured for all risks and carries no liability or property insurance coverage of any kind. The Company does not set aside any reserves for product liability risks or other potential claims. The Company’s policy is to record losses associated with its lack of insurance coverage at such time as a realized loss is incurred. Historically, the Company has not had any material losses in connection with its lack of insurance coverage and was not party to any material pending legal proceedings as of December 31, 2008. Management’s intention is to use the Company’s working capital to fund any such losses incurred due to the Company’s exposure to inadequate insurance coverage.
| 4. | Concentrations of Business and Credit risk (Continued) |
Payments of dividends may be subject to some restrictions due to the Company’s operating subsidiaries all being located in the PRC.
Major Customers
For the year ended December 31, 2008, Shanxi Xintai and Harbin Shiji Baolong accounted for 15% and 12% respectively of salestotal revenues. Harbin Shiji Baolong and Shanxi Xintai accounted for approximately 29% and 11% respectively of all accounts receivable. For the year ended December 31, 2007, Ningbo Yuehua Trading Co. and Guangzhou Xinghe Trading Co. accounted for approximately 14% and 11% of sales revenuetotal revenues respectively. OneHua Li Jiu Zhou accounted for approximately 11% of all accounts receivable. No other customer accounted for 10% of allor more of our total revenues in 2008 or 2007 or accounts receivable . as of December 31, 2008 or 2007.
China Sky One Medical, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
Major Suppliers
Heilongjiang Kangda Medicine Co. accounted for approximately 33% of the Company’s inventory purchases for the year ended December 31, 2008. Purchases from Harbin Yongheng Printing Ltd. for the year ended December 31, 2007 represented approximately 23%. Payments of dividends may be subject to some restrictions due to the Company’s operating subsidiaries all being located in the PRC. Management believes that, if necessary, there are alternative suppliers available to fulfill the Company’s raw material needs.
We have applied SFAS No. 128, “Earnings Per Share” in our calculation and presentation of earnings per share - “basic” and “diluted”. Basic earnings per share are computed by dividing net earnings available to common shareholders (the numerator) by the weighted average number of common shares (the denominator) for the period presented. The computation of diluted earnings per share is similar to basic earnings per share, except that the denominator is increased to include the number of additional common shares that would have been outstanding if the potentially dilutive common shares had been issued.
Stock warrants and options to purchase 1,151,000 shares of common stock were outstanding and exercisable as of December 31, 2008. Stock warrants and options to purchase 1,617,483 shares of common stock, all were exercisable and outstanding during the year ended December 31, 2007. These common stock equivalents were included in the computation of diluted earnings per share because the option exercise prices were less than the average market price of our common stock during these periods.
The dilutive potential common shares on warrants and options is calculated in accordance with the treasury stock method, which assumes that proceeds from the exercise of all warrants and options are used to repurchase common stock at the average market valueprice of the common stock during the relevant period . The amount of shares remaining after the proceeds are exhausted represent s the potential dilutive effect of the securities.
China Sky One Medical, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
| 5. | Earnings per Share (Continued) |
The following table sets forth our computation of basic and diluted net income per share:
| | | For the year ended December 31, | |
Numerator: | | | | | | |
| Net income used in calculation of basic and diluted earnings per share | | $ | 28,856,914 | | | $ | 15,332,945 | |
| | | | | | | | | |
Denominator: | | | | | | | | |
| Weighted-average common shares outstanding used in calculation of basic earnings per share | | | 15,101,833 | | | | 12,094,949 | |
| Effect of dilutive securities: | | | | | | | | |
| Stock options and equivalents | | | 327,303 | | | | 1,275,579 | |
| Weighted-average common shares used in calculation of diluted earnings per share | | | 15,429,136 | | | | 13,370,528 | |
| | | | | | | | | |
| Net income per share: | | | | | | | | |
| Basic | | $ | 1.91 | | | $ | 1.27 | |
| Diluted | | $ | 1.87 | | | $ | 1.15 | |
| 7. | 6. Equity and Share-based Compensation |
Effective January 1, 2006, we adopted the fair value recognition provisions of SFAS No. 123R, Share-Based Payment (“SFAS No. 123R”), for options granted to employees and directors, using the modified prospective transition method, and therefore have not restated results from prior periods. Compensation cost for all stock-based compensation awards granted is based on the grant date fair value estimated in accordance with the provisions of SFAS No. 123R. Under the fair value recognition provisions of SFAS No. 123R, we recognize stock-based compensation net of an estimated forfeiture rate and only recognize compensation cost for those shares expected to vest on a straight-line prorated basis over the requisite service period of the award. In March 2005, the SEC issued Staff Accounting Bulletin (“SAB”) No. 107, Share-Based Payment (“SAB No. 107”), regarding the SEC’s guidance on SFAS No. 123R and the valuation of share-based payments for public companies. We have applied the provisions of SAB No. 107 in the adoption of SFAS No. 123R.
In July 2006, the Company’s stockholders approved the 2006 Stock Incentive Plan (the “2006 Plan”). The 2006 Plan, provides for the grant of stock options, restricted stock awards, and performance shares to qualified employees, officers, directors, consultants and other service providers. The 2006 Plan originally authorized the Company to grant options and/or rights to purchase up to an aggregate of 1,500,000 shares of common stock. As of December 31, 2008, non-qualified options to purchase a total of 113,500 shares have been granted under the 2006 Stock Incentive Plan. All options were granted in October 2006. All options have an exercise price of $3.65 per share, the weighted fair market value on the date of grant was $4.25 per share. Of these 113,500 options a total of 60,500 were granted to employees and a total of 53,000 were granted to consultants. These options were valued using the Black-Scholes option-pricing model with the following assumptions: no dividends; risk-free interest rate of 4%; a contractual life of 5 years and volatility of 39%. All 113,500 options vest over various periods for the options granted to employees and consultants. There were no options granted during the years ended December 31, 2007 and 2008. In accordance with FAS 123(R), "Share-Based Payment," compensation expenses to employees for the years ended December 31, 2007 and 2008 were $235,468 and $315,662, respectively.
China Sky One Medical, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
6. 7. Equity and Share-based Compensation (Continued)
Options or stock awards issued to non-employees and consultants are recorded at their fair value as determined in accordance with SFAS No. 123R and EITF No. 96-18, “Accounting for Equity Instruments That Are Issued to Other Than Employees for Acquiring, or in Conjunction with Selling, Goods or Services”, and recognized over the related vesting or service period. In connection with closing of the Stock Exchange Agreement, the Company agreed to grant warrants to advisors for the services they already performed for the reverse merger in July 2006, entitling them to purchase up to 500,000 shares on or before July 31, 2009, at a price of $2.00 per share and options to purchase up to 50,000 shares on or before December 20, 2008 at a price of $3.00 per share. The fair value of these warrants and options were determined to be $772,275 and deducted as expenses using the Black-Scholes option-pricing model with the following weighted assumptions: no dividends; risk-free interest rate of 4%; a contractual life of 2.5-3.5 years and volatility of 39%. The Company based its estimate of expected volatility on the historical, expected or implied volatility of similar entities whose share or option prices are publicly available.
On January 3, 2007, the holder of 50,000 options dated March 11, 1999, granted prior to the May 30, 2006 company reorganization, exercised the warrants by electing to use cashless conversion provision of the warrants and acquired 5,160 shares of the Company common stock (after giving effect to the 8 to 1 reverse stock split effected after the warrants were issued). .
At various times during the year ended December 31, 2008, warrant holders exercised warrants for 505,002 shares of the Company’s common stock, at various exercise prices, for total proceeds of approximately $1,717,507. Warrant holders also exercised 700,000 shares of warrants on a cashless basis for a total of 587,300 shares of the Company’s common stock. During the year ended December 31, 2008, option holders exercised 50,000 stock options for 50,000 shares of the Company’s common stock at an exercise price of $3.00 per share for total proceeds of $150,000.
| 8. | 7. Securities Purchase Agreement and Related Transaction |
On January 31, 2008 (the “Closing Date”), the Company entered into a Securities Purchase Agreement (the “Purchase Agreement”) with certain accredited investors (the “Investors”), for the purchase and sale of units consisting of an aggregate of: (i) 2,500,000 shares of the Company’s common stock, and (ii) Class A Warrants to purchase 750,000 additional shares of the Company’s common stock exercisable at $12.50 per share, and expiring on July 31, 2011 (the “Class A Warrants”), for a purchase price of $10.00 per unit (the “Unit Purchase Price”), or gross offering proceeds of $25.0 million (the “2008 Offering”). The Company received net proceeds of approximately $23.5 million in connection with the 2008 Offering.
Pursuant to the Purchase Agreement, among other things, if, and whenever, within twelve (12) months of the Closing Date, the Company issues or sells, or is deemed to have issued or sold, any shares of common stock, or securities convertible into or exercisable for shares of common stock, or modifies any of the foregoing which may be outstanding (with the exception of certain excluded securities), to any person or entity at a price per share, or conversion or exercise price per share less than the Unit Purchase Price, then the Company shall issue, for each such occasion, additional shares of its common stock to the Investors in such number so that the average per share purchase price of the shares of common stock purchased by the Investors in the 2008 Offering shall automatically be reduced to such other lower price per share.
In addition, as of the Closing Date, the Company entered into a Make Good Agreement (the “Make Good Agreement”) with Liu Yan-Qing, its Chairman, Chief Executive Officer and President, and a principal shareholder of the Company, (the “Principal Shareholder”) and the Investors (collectively, the “Make Good Parties”), pursuant to which the Principal Shareholder deposited 3,000,000 shares of his common stock of the Company (the “Escrow Shares”) into escrow, to be released to the Investors in an amount pro rata pro to their initial investments in the 2008 Offering, in the event the Company failed to attain earnings per share, as adjusted, of at least (i) $1.05 per share for the fiscal year ending December 31, 2007 (based on an aggregate of 13,907,696 shares outstanding), and/or (ii) $1.63 per share for the fiscal year ending December 31, 2008 (based on 16,907,696 shares outstanding).
China Sky One Medical, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
7.8. Securities Purchase Agreement and Related Transaction (Continued)
The Company deems the Escrow Shares arrangement as analogous to the issuance of a fixed number of warrants in an equity transaction. Under the Make Good Agreement these Escrow Shares would have been reallocated on a pro rata basis to the Investors only if certain earnings targets were not achieved in years 2007 and 2008. If the earnings targets were met, the Escrow Shares would automatically be released to the Principal Shareholder. As of January 31, 2008, the date the common shares were placed into escrow, the Company achieved the 2007 earnings target and, based upon internal forecasts, was confident the 2008 target would also be met. Based upon certain assumptions, including the low probability that the Escrow Shares would be released to the Investors and not be returned to the Principal Shareholder, the Company considered the fair value of the right held by the Investors through the Escrow Shares provision under the Make Good Agreement to be immaterial. As of December 31, 2008, the Company satisfied the earnings per common share targets for each of fiscal 2007 and 2008 as defined under the Make Good Agreement and, as such, the Escrow Shares shall be released to the Principal Shareholder in 2009.
The Class A Warrants represent the right to purchase an aggregate of 750,000 shares of common stock, at an exercise price of $12.50 per share. Additional information relating to these Class A Warrants is provided in Note 8.
| 9. | 8. Outstanding Warrants and Options |
| | | | | | Weighted average Exercise Price Warrants | | | | | | Weighted average Exercise Price Options | |
| Outstanding as of January 1, 2006 | | | 25,000 | | | $ | 1.50 | | | | - | | | | |
| Granted | | | 1,650,000 | | | | 2.58 | | | | 163,500 | | | $ | 3.45 | |
| Exercised | | | - | | | | - | | | | - | | | | - | |
| Expired or cancelled | | | - | | | | - | | | | - | | | | - | |
| Outstanding as of December 31, 2006 | | | 1,675,000 | | | | 2.57 | | | | 163,500 | | | | 3.45 | |
| Granted | | | - | | | | - | | | | - | | | | - | |
| Exercised | | | - | | | | - | | | | - | | | | - | |
| Expired or cancelled | | | (161,667 | ) | | | 3.19 | | | | - | | | | - | |
| Outstanding as of December 31, 2007 | | | 1,513,333 | | | $ | 2.48 | | | | 163,500 | | | $ | 3.45 | |
| Granted | | | 750,000 | | | | 12.50 | | | | - | | | | - | |
| Exercised (See Note 6) | | | (1,205,002 | ) | | | (50,000 | ) | | | - | | | | | |
| Expired or cancelled | | | (8,331 | ) | | | - | | | | - | | | | - | |
| Outstanding as of December 31, 2008 | | | 1,050,000 | | | $ | 9.50 | | | | 113,500 | | | $ | 3.45 | |
The following table summarizes information about stock warrants outstanding and exercisable as of December 31, 2008.
| | | | Outstanding December 31, 2008 | | | Weighted Average Remaining Life in Years | | | | |
| $ | 2.00 | | | 300,000 | | | | 0.50 | | | | 300,000 | |
| $ | 12.50 | | | 750,000 | | | | 2.50 | | | | 750,000 | |
| | | | | 1,050,000 | | | | | | | | 1,050,000 | |
China Sky One Medical, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
| 8. | 9. Outstanding Warrants and Options (Continued) |
Out of the 1,050,000 outstanding warrants, all were exercisable as of December 31, 2008. The remaining Class A Warrants represent the right to purchase an aggregate of 750,000 shares of Common Stock of the Company granted with the Securities Purchase Agreement, at an exercise price of $12.50 per share, and have the following additional characteristics:
The Class A Warrants issued in our January 2008 Offering described in Note 7 above, represent the right to purchase an aggregate of 750,000 shares of common stock, at an exercise price of $12.50 per share, and have the following additional characteristics:
| | · | The Class A Warrants are exercisable beginning on the six-month anniversary of the closing of the January 2008 Offering and will expire July 31, 2011. |
| | · | Commencing on one-year anniversary of the Closing Date, in the event the Warrant Shares may not be freely sold by the holders of the Class A Warrants due to the Company’s failure to satisfy its registration requirements, and an exemption for such sale is not otherwise available to the Warrant-holders under Rule 144, the Class A Warrants will be exercisable on a cashless basis. |
| | · | The Exercise Price and number of Warrant Shares will be subject to adjustment for standard dilutive events, including the issuance of common stock, or securities convertible into or exercisable for shares of common stock, at a price per share, or conversion or exercise price per share less than the Class A Warrant exercise price of $12.50 per share. There was no issuance of securities during 2008, which would have resulted in an adjustment to the Exercise Price or number of Warrant Shares. |
| | · | At anytime following the date a Registration Statement covering the Warrant Shares is declared effective, we will have the ability to call the Class A Warrants at a price of $0.01 per Class A Warrant, upon thirty (30) days prior written notice to the holders of the Class A Warrants, provided (i) the closing price of the Common stock exceeded $18.75 for each of the ten (10) consecutive trading days immediately preceding the date that the call notice is given by the Company, and (ii) the Company has attained an Adjusted EPS of at least $1.75 per share for the fiscal year ending December 31, 2008, as set forth in our audited financial statements of the Company. |
| | · | If, among other things, we fail to cause a Registration Statement covering the Warrant Shares to be declared effective prior to the applicable dates set forth in the Registration Rights Agreement, the expiration date of the Class A Warrants shall be extended one day for each day beyond the Effectiveness Deadlines. The Company engaged an independent third-party consultant to calculate the derivative liability resulting from its failure to register the Warrant Shares. The calculation was made using the Black-Scholes model. The liability was deemed to be immaterial as of December 31, 2008. |
| | · | If a Warrant-holder exercises its Put Right under the Put Agreement (as previously defined above), such Warrant-holder’s right to exercise the Class A Warrants shall be suspended, pending the satisfaction of our obligations to pay the Warrant-holder the applicable Repurchase Price. Upon receipt of the Repurchase Price in full by the Warrant-holder, the Warrant-holder’s right to exercise the Class A Warrants shall automatically and permanently terminate and expire, and the Class A Warrants shall be immediately cancelled on the books of the Company. |
China Sky One Medical, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
8. Outstanding Warrants and Options (Continued) The following table summarizes information about stock options outstanding and exercisable as of December 31, 2008.
| | | | Outstanding December 31, 2008 | | | Weighted Average Remaining Life in Years | | | | |
| $ | 3.65 | | | 113,500 | | | | 3.00 | | | | 12,500 | |
| | | | | 1,050,000 | | | | | | | | 1,050,000 | |
The Company values its inventories at the lower of cost and market method. Inventories are accounted for using the first-in, first-out method. Inventories include packing materials, raw materials, supplemental materials, work-in-process, and finished products.
As of December 31, 2008 and 2007, inventories consist of the following:
| | | | | | | |
| Raw Material | | $ | 330,275 | | | $ | 284,614 | |
| Work-in-Process | | | 76,462 | | | | 57,337 | |
| Finished Products | | | 55,614 | | | | 29,721 | |
| Total Inventories | | $ | 462,351 | | | $ | 371,672 | |
| 11. | 10. Property and Equipment |
As of December 31, 2008 and 2007, Property and Equipment, net consist of the following:
| | | December 31, 2008 | | | December 31, 2007 | |
| Buildings and improvements | | $ | 9,961,820 | | | $ | 2,861,011 | |
| Machinery and equipment | | | 4,946,247 | | | | 1,568,958 | |
| Land use rights | | | 1,945,209 | | | | 563,469 | |
| Transportation equipment | | | 885,880 | | | | 318,779 | |
| Furniture and equipment | | | 299,467 | | | | 96,501 | |
| Construction in progress (See Note 14) | | | 4,317,265 | | | | 2,113,957 | |
| Total Property and Equipment | | | 22,355,888 | | | | 7,522,675 | |
| Less: Accumulated Depreciation | | | (1,297,109 | ) | | | (661,243 | ) |
| Property and Equipment, Net | | | 21,058,779 | | | $ | 6,861,432 | |
For the year ended December 31, 2008 and 2007, annual depreciation expense totaled $584,171 and $187,231 respectively. Depreciation expense recorded under Cost of Goods Sold for 2008 amounted to $225,884.
China Sky One Medical, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
As of December 31, 2008 and 2007, the Company’s net unamortized intangible assets consist of:
| | | | | | | |
| Patents | | $ | 15,093,718 | | | $ | 1,599,814 | |
| Goodwill | | | 758,047 | | | | 333,200 | |
| Total Intangible Assets, net | | $ | 15,851,765 | | | $ | 1,933,014 | |
The Company further developed the technologies acquired in the Tianlong and Peng Lei acquisitions, increasing the carrying value of their patents by $197,372 and $2,016,477 respectively, during 2008. The following table sets forth the intangible assets, net by subsidiary as of December 31, 2008:
| | | TDR | | | First | | | Tianlong | | | Haina | | | Peng Lai | | | Total | |
| Patent | | $ | 1,470,631 | | | $ | 6,738,763 | | | $ | 1,948,362 | | | $ | 0 | | | $ | 4,935,863 | | | $ | 15,093,619 | |
| Goodwill | | $ | 405,078 | | | $ | 0 | | | $ | 0 | | | $ | 353,068 | | | $ | 0 | | | $ | 758,146 | |
| Total | | $ | 1,875,709 | | | $ | 6,738,763 | | | $ | 1,948,362 | | | $ | 353,068 | | | $ | 4,935,863 | | | $ | 15,851,765 | |
Amortization expense for the year ended December 31, 2008 and 2007 was $274,235 and $255,832 respectively. Future amortization of the patents with finite useful lives are as follows:
China Sky One Medical, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
| | | | | | | |
| Patents | | $ | 15,093,718 | | | $ | 1,599,814 | |
| Goodwill | | | 758,047 | | | | 333,200 | |
| Total Intangible Assets, net | | $ | 15,851,765 | | | $ | 1,933,014 | |
Amortization expense for the year ended December 31, 2008 and 2007 was $274,235 and $255,832 respectively. Future amortization of the patents with finite useful lives are as follows:
| | | |
| 2009 | | $ | 695,300 | |
| 2010 | | | 695,300 | |
| 2011 | | | 695,300 | |
| 2012 | | | 695,300 | |
| 2013 | | | 695,300 | |
| Thereafter | | $ | 11,617,200 | |
| | | | 15,093,700 | |
Taxes payable consists of the following:
| | | | | | | |
| Value Added Tax, net | | $ | 1,179,383 | | | $ | 612,602 | |
| Enterprise Income Tax | | | 2,106,956 | | | | 940,819 | |
| City Tax | | | 32,013 | | | | 4,789 | |
| Other Taxes and additions | | | 44,536 | | | | 8,978 | |
| Total Taxes Payable | | $ | 3,362,888 | | | $ | 1,567,188 | |
Under the Provisional Regulations of PRC Concerning Income Tax on Enterprises promulgated by the PRC, income tax is payable by enterprises at a rate of 25% of their taxable income. Preferential tax treatment may, however, be granted pursuant to any law or regulations from time to time promulgated by the State Council.
| 14. | Income Taxes (Continued) |
According to “Enterprise Income Tax and Certain Preferential Policies Notice” published by the Ministry of Finance and the National Tax Affairs Bureau, if the enterprise is authorized by the State Council as a special entity, the enterprise income tax rate is reduced to 15%. The income tax rate for TDR and Tianlong in fiscal 2008 was 15% and 12%, respectively, based on State Council approval. As a result of the special income tax rates for TDR and Tianlong, the Company’s effective tax rate was approximately 20.9% in 2008. If the Company’s effective tax rate was 25% in 2008, its net income, basic earnings per share and diluted earnings per share would have been $27,354,182, $1.81 and $1.77, respectively in 2008. During 2007, TDR and First were authorized as a special entity and had special income tax rates of 15%. As a result, the Company’s effective tax rate was approximately 17.8% for 2007. If the Company’s effective tax rate was 25% in 2007, its net income increase basic earnings per share and diluted earnings per share would have been $13,989,089, $1.15 and $1.05, respectively .
We record a full valuation allowance to reduce our deferred tax assets to the amount that is more likely than not to be realized. While we have considered future taxable income and ongoing prudent and feasible tax planning strategies in assessing the need for the valuation allowance, in the event we were to determine that we would be able to realize our deferred tax assets in the future in excess of its net recorded amount, an adjustment to the deferred tax asset would increase income in the period such determination was made.
China Sky One Medical, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
13. | Income Taxes (Continued) |
Pursuant to Sections 382 and 383 of the Internal Revenue Code (“IRC”), annual use of the Company’s net operating losses and tax credit carryforwards may be limited because of cumulative changes in ownership of more than 50% that have occurred. As of December 31, 2008, the Company has US net operating loss carryforward’s of approximately $4.84 million which will begin to expire in 2028. Accordingly, as mentioned above, any deferred tax asset that would result from these carryforwards have been fully reserved as of December 31, 2008.
Significant components of the Company’s deferred tax assets are shown below. Nil valuation allowance has been established to offset the deferred tax assets, as realization of such assets is certain per the management valuation.
Provision for the PRC enterprise income tax is calculated at the prevailing rate based on the estimated assessable profits less available tax relief for losses brought forward. The Company does not accrue taxes on unremitted earnings from foreign operations as it is the Company’s intention to invest these earnings in the foreign operations indefinitely.
Net deferred tax assets consist of the following components as of December 31, 2008 and 2007:
| | | December 31, | |
| | | | | | | |
Deferred tax assets: | | | | | | |
| NOL Carryover from China Sky One (formerly known as Comet) | | $ | 4,840,994 | | | $ | 246,000 | |
| Share-based compensation expenses based on SFAS123R | | | 356,130 | | | | 639,000 | |
| | | | 5,197,124 | | | | 885,000 | |
| Less valuation allowance | | | (5,197,124 | ) | | | (885,000 | ) |
| Net deferred tax asset | | $ | - | | | $ | - | |
As of December 31, 2008, the Company has US net operating loss carryforward’s of approximately $4.84 million which will begin to expire in 2028.
The Company recognizes that virtually all tax positions in the PRC are not free of some degree of uncertainty due to tax law and policy changes by the state. However, the Company cannot reasonably quantify political risk factors and thus must depend on guidance issued by current state officials.
Based on all known facts and circumstances and current tax law, the Company believes that the total amount of unrecognized tax benefits as of December 31, 2008, is not material to its results of operations, financial condition or cash flows. The Company also believes that the total amount of unrecognized tax benefits as of December 31, 2008, if recognized, would not have a material effect on its effective tax rate. The Company further believes that there are no tax positions for which it is reasonably possible, based on current Chinese tax law and policy, that the unrecognized tax benefits will significantly increase or decrease over the next 12 months producing, individually or in the aggregate, a material effect on the company’s results of operations, financial position or cash flows.
| 15. | 14. Land Use Rights Purchase Agreement and Construction in progress |
During the second quarter in 2007 TDR entered into an agreement with the Development and Construction Administration Committee of Harbin Song Bei New Development district to purchase the land use rights for 50 years for development of a new biotech engineering project. Terms of the agreement called for a deposit of 30% of the total land price within 15 days after signing the agreement, 40% payment 7 days prior to the start of construction and the balance of 30% 7 days after getting the formal land use right.
China Sky One Medical, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
14. | Land Use Rights Purchase Agreement and Construction in progress (Continued) |
The project consists of two phases:
| | (1) | Construction of main workshop, R&D center and office using land area of 30,000 square meters. Construction started in May 2007 and is estimated to be completed by the end of 2009. |
| | (2) | Construction of Second workshop and show room using land area of 20,000 square meters. Construction is expected to start in September 2008 to be completed by December 2009. |
TDR has committed to the Development and Construction Administration Committee of the Harbin Song Bei New Development District that the minimum investment per square meter will be $394.
Management plans to use the internal generated funds to fund the project. The estimated additional cost is approximately $5 million.
As of December 31, 2008 and 2007, the Company has deposits totaling $8,513,284 and 8,003,205 respectively, related to the land and construction deposits.
| 16. | 15. Commitments and Contingencies |
The formulation, manufacturing, processing, packaging, labeling, advertising, distribution and sale of external use Chinese medicine such as those sold by the Company are subject to regulations by one or more federal agencies. The principal federal agencies include the State Food and Drug Administration of the Government of the Peoples Republic of China, the Food and Drug Administration (the “FDA”), Heilongjiang Provincial Food and Drug Administration of the People's Republic of China (PFDA), National Biology Products Inspection Institute (NBPI) and the National Food and Drug Administration (NFDA) of the People's Republic of China and, to a lesser extent, the Consumer Product Safety Commission. These activities are also regulated by various governmental agencies for the countries, states and localities in which the Company’s products are sold.
Although management believes that the Company is in material compliance with the statutes, laws, rules and regulations of every jurisdiction in which it operates, no assurance can be given that the Company’s compliance with the applicable statutes, laws, rules and regulations will not be challenged by governing authorities or private parties, or that such challenges will not lead to material adverse effects on the Company’s financial position, results of operations, or cash flows.
The Company, like any other distributor or manufacturer of products is exposed to the inherent risk of product liability claims in the events of possible injuries caused by the use of its products. The Company does not have liability insurance with respect to product liability claims; the insurance environment of China is neither sufficient nor mature. Inadequate insurance or lack of contractual indemnification from parties supplying raw materials or marketing its products, and product liabilities related to defective products could have a material adverse effects on the Company.
The Company is not involved in any legal matters arising in the normal course of business. While incapable of estimation, in the opinion of the management, the individual regulatory and legal matters in which the Company might be involved in the future are not expected to have a material adverse effect on the Company’s financial position, results of operations, or cash flows.
Rental expense is approximately $25,000 per year for each of the years ended December 31, 2008 and 2007 respectively. The rental commitment for the year 2009 is approximately $25,000. The Company is expecting the corporate headquarters currently under construction to be completed by 2009. As a result, there is no rental commitment made by the Company for the year 2010 and thereafter.
China Sky One Medical, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
| 17. | 16. Quarterly Results (Unaudited) |
The following table presents the Company’s selected unaudited quarterly operating results for the eight quarters ended December 31, 2008. The Company believes that all adjustments of a normal recurring natural have been made to present fairly the related quarterly results:
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
| Revenues | | $ | 12,413,430 | | | $ | 23,748,592 | | | $ | 29,699,282 | | | $ | 25,954,879 | | | $ | 91,816,183 | |
| Gross profit | | $ | 9,553,002 | | | $ | 18,226,278 | | | $ | 22,333,223 | | | $ | 19,300,377 | | | $ | 69,412,880 | |
| Income from operations | | $ | 4,850,026 | | | $ | 10,127,636 | | | $ | 11,751,105 | | | $ | 8,930,611 | | | $ | 35,659,378 | |
| Net income | | $ | 3,864,911 | | | $ | 8,110,667 | | | $ | 9,943,435 | | | $ | 6,937,901 | | | $ | 28,856,914 | |
| Basic EPS | | $ | 0.26 | | | $ | 0.54 | | | $ | 0.66 | | | $ | 0.45 | | | $ | 1.91 | |
| Diluted EPS | | $ | 0.25 | | | $ | 0.53 | | | $ | 0.64 | | | $ | 0.45 | | | $ | 1.87 | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
| Revenues | | $ | 5,179,116 | | | $ | 14,645,247 | | | $ | 16,770,570 | | | $ | 12,723,375 | | | $ | 49,318,308 | |
| Gross profit | | $ | 4,052,421 | | | $ | 11,336,599 | | | $ | 13,101,558 | | | $ | 9,888,199 | | | $ | 38,378,777 | |
| Income from operations | | $ | 1,910,080 | | | $ | 5,164,183 | | | $ | 6,589,801 | | | $ | 4,949,722 | | | $ | 18,613,786 | |
| Net income | | $ | 1,549,321 | | | $ | 4,227,524 | | | $ | 5,446,271 | | | $ | 4,109,829 | | | $ | 15,332,945 | |
| Basic EPS | | $ | 0.13 | | | $ | 0.35 | | | $ | 0.45 | | | $ | 0.34 | | | $ | 1.27 | |
| Diluted EPS | | $ | 0.12 | | | $ | 0.34 | | | $ | 0.44 | | | $ | 0.35 | | | $ | 1.15 | |
Item 9. Changes In and Disagreements With Accountants on Accounting and Financial Disclosure.
None.
Item 9A. Controls and Procedures.
Management’s Evaluation of Disclosure Controls and Procedures
Our management, with the participation of our chief executive officer and chief financial officer, evaluated the effectiveness of our disclosure controls and procedures as of December 31, 2008. The term “disclosure controls and procedures,” as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act, means controls and other procedures of a company that are designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Securities Exchange Act are recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act are accumulated and communicated to the company’s management, including its principal executive and principal financial officers, as appropriate to allow timely decisions regarding required disclosure. Our management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving their objectives and management necessarily applies its judgment in evaluating the cost-benefit relationship of possible controls and procedures. Our disclosure controls and procedures are designed to provide reasonable assurance of achieving their objectives as described above. Based on this evaluation, our management, including our chief executive officer and chief financial officer, concluded that as of December 31, 2008, our disclosure controls and procedures were effective at the reasonable assurance level.
Management’s Annual Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over our financial reporting. Internal control over financial reporting is defined in Rule 13a-15(f) or 15d-15(f) under the Securities Exchange Act as a process designed by, or under the supervision of, a company’s principal executive and principal financial officers and effected by a company’s board of directors, management and other personnel to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements in accordance with U.S. generally accepted accounting principles and includes those policies and procedures that:
| | · | pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of a company; |
| | · | provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with U.S. generally accepted accounting principles and that receipts and expenditures of a company are being made only in accordance with authorizations of management and directors of a company; and |
| | · | provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of a company’s assets that could have a material effect on the financial statements. |
Our internal control system was designed to provide reasonable assurance to our management and board of directors regarding the preparation and fair presentation of published financial statements. All internal control systems, no matter how well designed, have inherent limitations which may not prevent or detect misstatements. Therefore, even those systems determined to be effective can provide only reasonable assurance with respect to financial statement preparation and presentation. Projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Our management assessed the effectiveness of our internal control over financial reporting as of December 31, 2008. In making this assessment, management used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission in Internal Control-Integrated Framework.
Based on this assessment, management concluded that, as of December 31, 2008, our internal control over financial reporting was effective based on those criteria.
Attestation Report of the Registered Public Accounting Firm
This annual report does not include an audit report of our registered public accounting firm regarding internal control over financial reporting. In addition, management’s report on internal control over financial reporting is not subject to attestation by our registered public accounting firm pursuant to temporary rules of the SEC that require us to provide only management’s report in this annual report.
Changes in Internal Control Over Financial Report
During our fourth fiscal quarter, there was no change in our internal control over financial reporting that has materially affected, or is reasonably likely to materially affect, our internal controls over financial reporting.
Item 9B. Other Information.
Except as set forth below, there was no information we were required to disclose in a report on Form 8-K during the fourth quarter of our fiscal year ended December 31, 2008, or subsequent period through the date hereof, which was not so reported.
PART III
Item 10. Directors, Executive Officers and Corporate Governance.
Directors and Officers
The following table sets forth certain information regarding our directors and executive officers during the fiscal year ended December 31, 2008, and the subsequent period through the date hereof:
| | | | | |
| | | | | | |
| Liu Yan-qing | | 44 | | Chief Executive Officer, President and Director | |
| | | | | | |
| Han Xiao-yan | | 41 | | Vice Chairman and Director | |
| | | | | | |
| Hao Yu-Bo | | 38 | | Chief Financial Officer and, Secretary and Director | |
| | | | | | |
| Song Chun Fan | | 69 | | Director | |
| | | | | | |
| Jiang Qi Feng | | 26 | | Director | |
| | | | | | |
| Zhao Jie | | 46 | | Director | |
| | | | | | |
| Qian Xu Feng | | 41 | | Director | |
The following information reflects the business background and experience of each director and officer.
Liu Yan-qing is our Chief Executive Officer and President, Director of TDR, and General Manager of our Harbin Bio-Engineering subsidiary. Mr. Liu graduated from Prophylactic Department of Harbin Medicine University, where he obtained his bachelor’s degree. In 2005, he studied at Tsing Hua University and earned an Executive Masters of Business. Before establishing his own company, he had 8 years of experience as a reporter of Family Health Newspaper, and has over 10 years of experience in drug marketing, research and development of new drugs and enterprise management. Mr. Liu has been instrumental in establishing TDR’s sales program and sales network covering the PRC.
Han Xiao-yan is our Vice Chairman and Director, General Manager of TDR, and Vice Director of Harbin Bio-Engineering. Ms. Han received a master of business administration at Harbin Industrial University. She had five years of hygiene and medical media experience before becoming employed by TDR, and has been instrumental in developing and marketing TDR’s products and expanding its sales. She also serves as our senior marketing manager and administrative manager. She has over 10 years of financial management experience. In 2004, she was appointed the general manager of TDR, with responsibility for financing, production, quality control and purchasing. In 2003, she was appointed vice director of Harbin Bio-Engineering.
Hao Yu-bo has been employed by us in various capacities since June 2008 and as a director since November 2008. From January 2006 through June 2008, Mr. Hao served as President’s Assistant and Financial Officer for Sumitomo Group Canadian Branch, an integrated trading company. Prior to this, commencing in September 2004, Mr. Hao served as Marketing Executive and Canadian Market Analyst for MGM Mirage, an entertainment company which owns and operates casino properties. From September 1997 through the time he joined MGM Mirage, he was Chief Executive Officer of SunnyZone Consulting Co. Ltd., a financial consulting company he co-founded. Mr. Hao holds Bachelor’s degrees in Economics and Arts from Beijing Union University and an MBA from the University of Phoenix.
Song Chun Fan joined our board of directors on February 22, 2008. From 1964 to the present, Mr. Song has been employed by the First Clinical College of Harbin Medical University in Heilongjiang, China, where he has served as the Director of the Surgery Research Room and the Director of graduate students of the Surgery Department since 1996. From 1998 to the present, he has been the acting Director of the Heilongjiang Professional Surgery Committee, the Commissary of the Degree Commission of China, the Director of the Key Laboratory of Cell Transplantation of the Ministry of Public Health of China, the Vice-Chairman of the Heilongjiang Medicine Association, the Vice-Chairman of the Heilongjiang Physician Association, and the Director of Heilongjiang (Special) Medical Treatment Application Administration Committee. Mr. Song received a Bachelor’s Degree in Medical Treatment from Harbin Medical University in 1964.
Jiang Qi Feng joined our board of directors on February 22, 2008. From September 2006 to the present, Mr. Jiang has served as a Teaching Assistant and a Research Assistant at Simon Fraser University in Canada, where he specializes in biology statistics, biology research and probability. He received a Masters Degree in Computer Science from Simon Fraser University in 2006, and Bachelor’s Degrees in Bio-Statistics and Mathematics from the University of British Columbia in 2005.
Zhao Jie joined our board of directors on February 22, 2008. From 1999 to the present, Mr. Zhao has served as the Tissue Specialist of the Replant Department of Capital Health Transplant Services in Alberta, Canada, responsible for various aspects of tissue transplantation, including determining donee acceptability, processing and preserving tissue, performing surgical procedures, and quality control. In addition, he has written and published several books and articles regarding tissue transplantation. Mr. Zhao has received awards from Capital Health for Quality and Safety (2006), Recognition of Excellence and Achievement (2002), and Teamwork (2002). He received a Bachelor’s Degree in Medicine from Harbin Medical University in 1988.
Qian Xu Feng joined our board of directors on February 22, 2008. From March 2005 to the present, Mr. Qian has been employed by Moody’s Investors Service; from May 2007 to the present, as the Vice President and Senior Analyst, from May 2006 to May 2007, as the Assistant Vice President and Quantitative Analyst, and from March 2005 to April 2006, as the Quantitative Analyst. Prior to that, from June 2004 until February 2005, he was the Research Fellow of the Furman Center for Real Estate and Urban Policy of New York University, where she conducted empirical quantitative research in various aspects of commercial and residential properties. From September 1990 to July 1996, Mr. Qian was an Assistant Professor of Economics at the Beijing Normal University. He received a Ph.D. in Economics from Rutgers University in 2004, a Masters Degree in Economics from Rutgers University in 2001, a Masters Degree in Accounting from City University of New York in 1999, and a Bachelor’s Degree in Economics from Beijing Normal University in 1990.
Significant Employees
As of December 31, 2008, we had 1,804 employees. Other than our members of management, only the individuals listed below are expected to make significant contributions to our business:
Zhang Wen Chao has been our Director of Scientific and Technological Development since 2005. Mr. Zhang graduated with a PhD in biology pharmaceuticals from South China University of Technology in 1997. He has been employed in various R&D roles since his graduation. Mr. Zhang completed our gene recombination medicine independently and has been responsible for researching and developing various products that have been launched by the Company since 2005.
Family Relationships
There are no family relationships among our directors, executive officers, or persons nominated to become directors of executive officers.
Involvement in Certain Legal Proceedings
There have been no events under any bankruptcy act, no criminal proceedings and no judgments, injunctions, orders or decrees material to the evaluation of the ability and integrity of any director, executive officer, promoter or control person of Registrant during the past five years.
Compliance with Section 16(a) of the Exchange Act
To our knowledge, based solely on a review of such materials as are required by the Securities and Exchange Commission, none of our officers, directors or beneficial holders of more than 10% of our issued and outstanding shares of common stock failed to timely file with the Securities and Exchange Commission any form or report required to be so filed pursuant to Section 16(a) of the Securities Exchange Act of 1934, during the fiscal year ended December 31, 2008.
Code of Ethics
We have adopted a Code of Ethics that applies to our principal chief executive officer, principal financial officer, principal accounting officer or controller, or persons performing similar functions, as well as other employees (the “Code of Ethics”). A copy of the Code of Ethics is appended as an exhibit to our Amended Report on Form 10-KSB for the year ended December 31, 2006. The Code of Ethics is being designed with the intent to deter wrongdoing, and to promote the following:
| | · | Honest and ethical conduct, including the ethical handling of actual or apparent conflicts of interest between personal and professional relationships, |
| | · | Full, fair, accurate, timely and understandable disclosure in reports and documents that a small business issuer files with, or submits to, the Commission and in other public communications made by the small business issuer, |
| | · | Compliance with applicable governmental laws, rules and regulations, |
| | · | The prompt internal reporting of violations of the code to an appropriate person or persons identified in the code, |
| | | Accountability for adherence to the code. |
Board of Directors
We have 6 members serving on our Board of Directors. Each board member is nominated for election at our annual meeting to serve until the next annual meeting of stockholders and until their successors are duly elected and qualified.
Board Committees
The Board of Directors has an Audit Committee, Nominating and Governance Committee, Executive Committee, a Finance Committee and a Compensation Committee, all of which were created on February 22, 2008. A description of each committee follows.
Audit Committee
We have a separately designated standing Audit Committee and adopted an Audit Committee Charter on February 22, 2008. The purpose of the Audit Committee is to recommend to the board of directors the annual engagement of a firm of independent accountants and reviews with the independent accountants the scope and results of audits, our internal accounting controls and audit practices and professional services rendered to us by our independent accountants. The Audit Committee has been created after the appointment of the auditors however, have approved the inclusion of their report herewith. The Audit Committee also reviews and discusses with management and the board of directors, such matters as accounting policies, internal accounting controls and procedures for preparation of financial statements. The Audit Committee is required, at all times to be composed exclusively of directors who, in the opinion of our board of directors, are free from any relationship that would interfere with the exercise of independent judgment as a committee member and who posses an understanding of financial statements and generally accepted accounting principles. The Audit Committee is comprised of solely independent directors, Messrs. Jiang Qi Feng, Zhao Jie and Qian Xu Feng. Management believes, in good faith, that each of these members are considered “independent” under applicable Nasdaq rules, and that Jiang Qi Feng qualifies as an “audit committee financial expert” as defined under Item 401(c) of Regulation S-K. A copy of the Audit Committee Charter is filed as an exhibit to this report and can be made available in print free of charge to any shareholder who requests it.
Compensation Committee
We have designated a Compensation Committee of Board of Directors and adopted a Compensation Committee Charter on February 22, 2008. The Compensation Committee is responsible for (a) reviewing and recommending to the board of directors on matters relating to employee compensation and benefit plans, and (b) assisting the board in determining the compensation of the CEO and other executives and make recommendations to the board with respect to the compensation of the CFO, other executive officers of the Company and independent directors. The Compensation Committee is comprised independent directors, Messrs. Jiang Qi Feng, Qian Xu Feng and Song Chun Fan.
Nominating and Governance Committee
We created a Nominating and Governance Committee and adopted a Nominating and Governance Committee Charter on February 22, 2008. The purpose of this committee is to assist the board of directors in identifying qualified individuals to become board members, in determining the composition of the board of directors and in monitoring the process to assess Board effectiveness. The Nominating and Governance Committee of the board of directors comprised of independent directors Zhao Jie, Qian Xu Feng and Song Chun Fan. A copy of the Nominating and Governance Committee Charter is filed as an exhibit to this prospectus and can be made available in print free of charge to any shareholder who requests it.
Executive Committee
We have created an Executive Committee of the Board of Directors, comprised solely of independent directors. Song Chun Fan, Zhao Jie and Jiang Qi Feng serve as members of the Executive Committee.
Finance Committee
We have created a Finance Committee of the Board of directors, comprised solely of independent directors. Qian Xu Feng, Jiang Qi Feng and Song Chun Fan serve as members of the Finance Committee.
Director Independence
Our common stock trades on the Nasdaq Global Market. Under applicable Nasdaq rules, a director will only qualify as an “independent director” if, in the opinion of our board, that person does not have a relationship which would interfere with the exercise of independent judgment in carrying out the responsibilities of a director.
Our board of directors has determined that none of Song Chun Fan, Jiang Qi Feng, Zhao Jie and Qian Xu Feng has a relationship which would interfere with the exercise of independent judgment in carrying out the responsibilities of a director, and that each of these directors is an “independent director,” as defined under Rule 4200(a)(15) of the Nasdaq Stock Market, Inc. Marketplace Rules.
Indemnification
Under Chapter 78 of the Nevada Revised Statutes, we have broad powers to indemnify and insure our directors and officers against liabilities they may incur in their capacities as such. Article VII of our articles of incorporation provides, in part, that we must indemnify our directors and officers, and their respective heirs, administrators, successors and assigns against any and all expenses, including amounts paid upon judgments, counsel fees and amounts paid in settlement by reason of their being or having been directors of officers. This indemnification is in addition to any rights to which those indemnified may be entitled under any law, by law, agreement, vote of shareholder or otherwise.
This indemnification provisions may be sufficiently broad to permit indemnification of our directors and officers for liabilities (including reimbursement of expenses incurred) arising under the Securities Act. Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers, or persons controlling the registrant pursuant to the foregoing provisions, we have been informed that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable.
The indemnity provisions may discourage stockholders from bringing a lawsuit against our directors for breach of their fiduciary duty. These provisions may also have the effect of reducing the likelihood of derivative litigation against directors and officers, even though such an action, if successful, might otherwise benefit us and our stockholders. Furthermore, a stockholder’s investment may be adversely affected to the extent we pay the costs of settlement and damage awards against directors and officers pursuant to these indemnification provisions. We believe that these provisions, the indemnification agreements and the insurance are necessary to attract and retain talented and experienced directors and officers.
At present, there is no pending litigation or proceeding involving any of our directors or officers where indemnification will be required or permitted. We are not aware of any threatened litigation or proceeding that might result in a claim for such indemnification.
Anti-Takeover Provisions
Provisions of Nevada law, our articles of incorporation, or our bylaws could have the effect of delaying or preventing a third party from acquiring us, even if the acquisition would benefit our stockholders. The provisions of Nevada law and in our articles of incorporation and bylaws are intended to enhance the likelihood of continuity and stability in the composition of our Board of Directors and in the policies formulated by the Board of Directors and to discourage certain types of transactions that may involve an actual or threatened change of control of China Sky One. These provisions are designed to reduce our vulnerability to an unsolicited proposal for a takeover that does not contemplate the acquisition of all of our outstanding shares, or an unsolicited proposal for the restructuring or sale of all or part of our company. See the Subsection titled “Anti-Takeover Provisions” in the “Description of Capital Stock” Section below.
Director Fiduciary Duty and Business Judgment Provisions
Nevada has enacted several statutes governing the fiduciary duty and business judgment of our directors and officers including a provision that our directors and officers must exercise their powers in good faith and with a view to our interests. In the same section, the Nevada Revised Statutes state that our directors and officers, in deciding upon matters of business, are presumed to act in good faith, on an informed basis and with a view to our interests. They may rely on information, opinions, reports, financial statements and other financial data, that are prepared or presented by our directors, officers or employees who are reasonably believed to be reliable and competent.
Limitation on Liability
Section 78.138(7) of the Nevada Revised Statutes provides that our directors and officers will not be individually liable to us or our stockholders or our creditors for any damages as a result of any act or failure to act in their capacity as a director or officer unless it is proven that the act or failure to act breached fiduciary duties as a director or officer and such breach involved intentional misconduct, fraud or a knowing violation of law. As a result, neither we nor our stockholders nor our creditors have the right to recover damages against a director or officer for any act or failure to act in his capacity as a director or officer, except in the situations described above and except under very limited circumstances.
Item 11. Executive Compensation.
Summary Compensation Table
The following table sets forth the cash compensation paid by the Company to its President and all other executive officers who earned annual compensation exceeding $100,000 for services rendered during the 2008, 2007 and 2006 fiscal years.
SUMMARY COMPENSATION TABLE
Name and Principal Position | | | | | | | | | | | | | | | | Non-Equity Incentive Plan Compensation ($) | | | Nonqualified Deferred Compensation Earnings ($) | | | | | | | |
| Liu Yan-Qing | | 2008 | | | 34,320 | | | | - | | | | 51,380 | | | | - | | | | - | | | | - | | | | - | | | | 85,700 | |
| Chairman, Chief | | 2007 | | | 68,512 | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | 68,512 | |
Executive Officer and President | | 2006 | | | 19,500 | | | | - | | | | - | | | | 62,050 | | | | - | | | | - | | | | - | | | | 81,550 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Han Xiao-Yan Vice | | 2008 | | | 25,680 | | | | - | | | | 40,120 | | | | - | | | | - | | | | - | | | | - | | | | 65,800 | |
| Chairman and Director | | 2007 | | | 54,810 | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | 54,810 | |
| | | 2006 | | | 16,500 | | | | - | | | | - | | | | 43,800 | | | | - | | | | - | | | | - | | | | 60,300 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Hao Yu-Bo Chief | | 2008 | | | 17,241 | | | | - | | | | 11,424 | | | | - | | | | - | | | | - | | | | - | | | | 28,665 | |
| Financial Officer and | | 2007 | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | |
| Secretary | | 2006 | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Richard B. Stuart | | 2008 | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | |
| former Chief Executive | | 2007 | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | |
| Officer and Director | | 2006 | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | 28,200 | (3) | | | 28,200 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Jack M. Gertino former | | 2008 | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | |
| Chief Financial Officer | | 2007 | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | |
| and Director | | 2006 | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | 56,325 | (3) | | | 56,325 | |
| | (1) | As of July 15, 2008, we issued an aggregate of 30,063 shares of restricted stock to certain employees, directors an advisors pursuant to our 2006 Stock Incentive Plan. |
| | (2) | Option Awards represent the aggregate grant date fair value of options to purchase 17,000 common shares for Mr. Liu and 12,000 common shares for Ms. Han, computed in accordance with FAS 123R. The grant, vesting and forfeiture information and assumption made in valuation may be found in Note 6 to our financial statements for the year ended December 31, 2008, which are attached hereto, beginning on Page F-1, and in the notes following the table below. |
| | (3) | Mr. Stuart and Mr. Gertino were former officers and directors of ours through May 2006. We recorded compensation expense for Richard B. Stuart and Jack M. Gertino, computed on an hourly basis, in the amounts indicated, for their efforts in reviewing specific business opportunities for a possible business combination during the fiscal year, participating in meetings and conference calls in connection with such opportunities, and undertaking related activities. |
Summary of Employment Agreements and Arrangements
We do not have formal employment agreements with any members of management.
OUTSTANDING EQUITY AWARDS AT FISCAL YEAR-END
| | Number of Securities Underlying Unexercised Options (#) Exercisable | | | Number of Securities Underlying Unexercised Options (#) Unexercisable | | | Equity Incentive Plan Awards: Number of Securities Unexercised Unearned Options (#) | | | | | | | Number of Shares or Units of Stock That Have Not Vested (#) | | | Market Value of Shares or Units of Stock That Have Not Vested ($) | | | Equity Incentive Plan Awards: Number of Unearned Shares, Units or Other Rights That Have Not Vested (#) | | | Equity Incentive Plan Awards: Market or Payout Value of Unearned Shares, Units or Other Rights That Have Not Vested ($) | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Liu Yan-Qing Principal Executive Officer and Director | | | 17,000 | | | | 0 | | | | 0 | | | $ | 3.65 | | October 26, 2011 | | | 0 | | | | 0 | | | | 0 | | | | 0 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Han Xiao-Yan former Principal Financial Officer and Director | | | 12,000 | | | | 0 | | | | 0 | | | $ | 3.65 | | October 26, 2011 | | | 0 | | | | 0 | | | | 0 | | | | 0 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Hao Yu-Bo Principal Financial Officer | | | 0 | | | | 0 | | | | 0 | | | | n/a | | n/a | | | n/a | | | | n/a | | | | n/a | | | | n/a | |
(1) On October 26, 2006, we issued to Liu Yan-Qing under our 2006 Incentive Stock Plan (the “Plan”), options to purchase 17,000 shares of common stock, 6,000 options vesting June 30, 2007, and the remaining 11,000 options vesting on June 30, 2008. These options are exercisable for a five-year period from the date of grant at an exercise price of $3.65 per share.
(2) On October 26, 2006, we issued to Han Xiao-Yan under our Plan, options to purchase 12,000 shares of common stock, 5,000 options vesting June 30, 2007, and the remaining 7,000 options vesting on June 30, 2008. These options are exercisable for a five-year period from the date of grant at an exercise price of $3.65 per share.
Equity Compensation Plan Information
Our Board of Directors adopted a 2006 Stock Incentive Plan (the “Plan”), to be effective on July 31, 2006. The Plan was approved by our shareholders on July 31, 2006. The Plan authorizes the granting of incentive stock options and nonqualified stock options to purchase common stock, stock appreciation rights (“SARs”), restricted stock, performance stock and bonus stock, to key executives and other key employees and consultants of ours, including officers of our subsidiaries. The purpose of the Plan is to attract and retain key employees, to motivate key employees to achieve long-range goals and to further identify the interests of key employees with those of the other shareholders of ours. The Plan authorizes the award of 1,500,000 shares of common stock to be used for stock, SARs, restricted stock and performance and bonus stock. If an award made under the Plan expires, terminates or is forfeited, canceled or settled in cash, without issuance of shares covered by the award, those shares will be available for future awards under the Plan. The Plan will terminate on July 31, 2017. The Plan is intended to qualify for favorable treatment under Section 16 of the Exchange Act, as amended, pursuant to Rule 16b-3 promulgated thereunder (“Rule 16b-3”). The Plan provides for the grant of “incentive stock options,” as defined in Section 422 of the Internal Revenue Code (“Code”) and nonqualified stock options.
The Plan designates a Stock Option Committee appointed by the Board of Directors (which may be the Compensation Committee) and authorizes the Stock Option committee to grant or award to eligible participants stock options, SARs, restricted stock performance stock awards and bonus stock awards for up to 1,500,000 shares of our common stock. The initial members of the Stock Option Committee are the Board of Directors.
On October 26, 2006, we granted a total of 113,500 non-qualified options under the Plan to key employees, including certain of our officers. These options are exercisable at a price of $3.65 per share, or 85% of the fair market value of the shares covered by the options as of the date of grant. The options begin to vest in June 2007, and typically vest in increments over either a two-year or three year period, and expire in October, 2011, unless earlier terminated by their terms. The outstanding options include a total of 29,000 options held by current officers and directors.
The following table provides certain information with respect to our equity compensation plans in effect as of December 31, 2008.
| | (a) Number of securities to be issued upon exercise of outstanding options, warrants and rights | | | (b) Weighted-average exercise price of outstanding options, warrants and rights | | | (c) Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column (a) | |
| | | | | | | | | | | | | |
| Equity compensation plans approved by security holders | | | 113,500 | | | $ | 3.65 | | | | 1,386,500 | |
| Equity compensation plans not approved by security holders | | None | | | | | | | | | |
| Total | | | 113,500 | | | | | | | | 1,386,500 | |
Director Compensation
We do not currently pay any cash fees to our directors . During 2008, there was no director compensation paid other than the stock grants. The following table sets forth certain information regarding our independent directors compensation for the year ended December 31, 2008:
| Name | | Stock Awards | | | Total | |
| | | | | | | |
| Song Chung Fang, Director | | $ | 11,424 | | | $ | 11,424 | |
| | | | | | | | | |
| Jiang Qu Feng, Director | | $ | 11,424 | | | $ | 11,424 | |
| | | | | | | | | |
| Zhao Jie, Director | | $ | 11,424 | | | $ | 11,424 | |
| | | | | | | | | |
| Qian Zu Feng, Director | | $ | 11,424 | | | $ | 11,424 | |
All Stock grants vested immediately upon grant. The independent directors joined our Board of Directors on February 22, 2008, thus there was no independent director compensation for the year of 2007.
Limitation on Liability and Indemnification of Directors and Officers
Chapter 78 of the Nevada General Corporation Law ("NGCL") provides that a corporation may indemnify any person who was or is a party or is threatened to be made a party to any threatened, pending or completed action, suit or proceeding whether civil, criminal, administrative or investigative (other than an action by or in the right of the corporation) by reason of the fact that he is or was a director, officer, employee or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise, against expenses (including attorneys' fees), judgments, fines and amounts paid in settlement actually and reasonably incurred by him in connection with such action, suit or proceeding if he is not liable pursuant to NGCL Section 78.138 or acted in good faith and in a manner he reasonably believed to be in or not opposed to the best interests of the corporation, and, with respect to any criminal action or proceeding, had no reasonable cause to believe his conduct was unlawful. NGCL Chapter 78 further provides that a corporation similarly may indemnify any such person serving in any such capacity who was or is a party or is threatened to be made a party to any threatened, pending or completed action or suit by or in the right of the corporation to procure a judgment in its favor by reason of the fact that he is or was a director, officer, employee or agent of the corporation or is or was serving at the request of the corporation as a director, officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise, against expenses (including attorneys' fees) actually and reasonably incurred in connection with the defense or settlement of such action or suit if he is not liable pursuant to NGCL Section 78.138 or acted in good faith and in a manner he reasonably believed to be in or not opposed to the best interests of the corporation and except that no indemnification shall be made in respect of any claim, issue or matter as to which such person shall have been adjudged to be liable to the corporation unless and only to the extent that the court or other court of competent jurisdiction in which such action or suit was brought shall determine upon application that, despite the adjudication of liability but in view of all of the circumstances of the case, such person is fairly and reasonably entitled to indemnity for such expenses which the court or other court of competent jurisdiction shall deem proper.
Our Articles of Incorporation and By-laws provide that we may indemnify our officers, directors, agents and any other persons to the fullest extent permitted by the NGCL.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters.
Security Ownership of Certain Beneficial Owners
The following table sets forth certain information regarding the beneficial ownership of our common stock, by (i) each person who, to our knowledge, owns more than 5% of our common stock, (ii) each of our named executive officers and directors, and (iii) all of our named executive officers and directors as a group. Shares of our common stock subject to options, warrants, or other rights currently exercisable, or exercisable within 60 days of the date hereof, are deemed to be beneficially owned and outstanding for computing the share ownership and percentage of the person holding such options, warrants or other rights, but are not deemed outstanding for computing the percentage of any other person. As of March 31, 2009, the Company had 16,446,467 shares of Common Stock issued and outstanding.
| | | Common Stock Beneficially Owned | |
Name, Title and Address(1) | | | | | | |
| 5% Stockholders | | | | | | |
| | | | | | | |
Pope Investments II LLC(3) 5100 Poplar Avenue, Suite 805 Memphis, TN 38137 | | | 1,391,000 | | | | 8.38.5 | % |
| | | | | | | | | |
| 5% Stockholders | | | | | | | | |
| | | | | | | | | |
Liu Yan Qing(4) Chief Executive Officer, President and Chairman of the Board of Directors | | | 4,677,5954,682,493 | | | | 28.428.5 | % |
| | | | | | | | | |
Han Xiao Yan(5) Vice Chairman of the Board of Directors | | | 1,415,0571,418,719 | | | | 8.6 | % |
| | | | | | | | | |
Hao Yu-Bo Chief Financial Officer and Secretary | | | 1,088 | | | | * | |
| | | | | | | | | |
Song Chun Fang Director | | | 1,088 | | | | * | |
| | | | | | | | | |
Jiang Qi Feng Director | | | 1,088 | | | | * | |
| | | | | | | | | |
Zhao Jie Director | | | 1,088 | | | | * | |
| | | | | | | | | |
Qian Xu Feng Director | | | 1,088 | | | | * | |
| | | | | | | | | |
All Named Executive Officers and Directors as a Group (7 persons)( 76 ) | | | 6,098,0926,106,650 | | | | 37.037.1 | % |
*Less than 1%
(1) Unless otherwise indicated, each person named in the table has sole voting and investment power and that person’s address is c/o the Company, at Room 1706, No. 30 Di Wang Building, Gan Shui Road, Nangang District, Harbin, People’s Republic of China 150001.
(2) All shares are held of record and beneficially.
(3) Includes 321,000 shares underlying currently exercisable warrants held by Pope Investments II LLC. William D. Wells is the Managing Member of Pope Investments II LLC and has sole voting and investment power over the shares owned by such entity. Mr. Wells disclaims beneficial ownership of these shares, except to the extent of his pecuniary interest therein.
(4) Includes 17,000 shares underlying currently exercisable stock options held by Mr. Liu. There are 3,000,000 of Mr. Liu’s shares are held in escrow in connection with a Make Good Agreement entered into with investors in a private offering the Company closed in January 2008, pursuant to which such shares may be distributed to such investors if the Company does not attain certain earnings per share targets for fiscal 2007 and 2008, based on fully diluted shares outstanding at the time of the offering excluding warrants issued in such offering. The Company has satisfied the earnings per share targets for each of fiscal 2007 and 2008. Therefore, the The 3,000,000 shares shall bewere released to Mr. Liu in 2009.
(5) Includes 12,000 shares underlying currently exercisable stock options held by Ms. Han. Does not include 526,170 shares held by Ms. Han’s mother or 507,531 shares held by Ms. Han’s daughter, since each of Ms. Han’s mother and daughter has sole voting and investment power over the shares held by her. Ms. Han disclaims beneficial ownership of the shares held by her mother and daughter.
( 76 ) Includes (i) 17,000 shares underlying currently exercisable stock options held by Mr. Liu, and (ii) 12,000 shares underlying currently exercisable stock options held by Ms. Han.
Item 13. Certain Relationships and Related Transactions, and Director Independence.
Since the beginning of our last fiscal year, there have been no transactions between members of management, five percent stockholders, “affiliates,” promoters and finders, except as set forth below. Each of the transactions listed below was negotiated on an “arm’s length” basis.
Transactions with Management and Others
Effective May 30, 2006, we acquired 100% of ACPG in a stock-for-stock exchange. The transaction was treated as a reverse merger and a recapitalization of ACPG for financial reporting purposes. As part of the exchange, Liu Yan-qing and Han Xiao-yan, officers and directors of ACPG and TDR, received 4,660,595 and 1,402,907 shares, respectively, of our common stock in exchange for their ownership interest in ACPG. The other shareholders of ACPG received a total of 4,129,875 shares of our common stock for their ownership interest in ACPG, including American Eastern Group, Inc., American Eastern Securities, Inc., and its principal, Trang Chong (Charles) Hung, who received 87,685 shares, 54,803 shares, and 87,685 shares of our common stock, respectively, in connection with the transaction.
In connection with the closing of the stock exchange described above, we entered into a consulting agreement, as of May 11, 2006, with Jack M. Gertino and Richard B. Stuart, officers and directors who resigned at closing, providing for their services as consultants. For such services, we agreed to compensate Messrs. Gertino and Stuart as follows: (a) the sum of $3,000 per month for a period of two years; (b) the issuance of a total of 219,212 shares of our restricted common stock (or 109,606 shares each). In addition, under this arrangement, we granted to Mr. Gertino an option to purchase a total of 50,000 shares at a price of $3.00 per share at any time before December 20, 2008.
We incurred fees to American Eastern Group, Inc. (“AEG”), and Shenzhen DRB Investment Consultant Limited (“DRB”), in the amount of $200,000 in cash for services rendered in connection with the Exchange Agreement. In addition, in October, 2006, we granted to AES and DRB warrants to purchase an aggregate of 1,000,000 shares of common stock (500,000 warrants each) exercisable at any time before July 31, 2009, at a price of $2.00 per share. One-half of such warrants were deemed to be earned as of the completion of the Exchange Agreement, and the other one-half was deemed earned after completion of our private offering in October, 2006. The fair value of the above warrants was calculated as $1,469,190 as of December 31, 2006, based on the Black-Scholes model of accounting, and the total value of compensation to AES and DRB has been determined to be $2,317,128.
American Eastern Securities, Inc., acted as placement agent for us in connection with our private offering completed in October, 2006. We sold a total of 200 Units in the private offering, at a price of $15,000 per Unit, for total gross proceeds of $3,000,000 and net cash proceeds of approximately $2,745,000. Each Unit in the private offering consisted of a total of 5,000 shares of common stock and a warrant to purchase an additional 2,500 shares of common stock at any time prior to October 10, 2008, at a price of $3.50 per share. As a result, we sold a total of 1,000,000 shares of common stock, and issued warrants to purchase an additional 500,000 shares of common stock. As placement agent, American Eastern Securities earned a placement fee of $270,000 or 9% of the total proceeds from the private offering, and was granted a warrant to purchase Units equivalent to 10% of the Units sold in the offering in October 2006, or a total of 100,000 shares at a price of $3.00 per share, and, subject to exercising the foregoing $3.00 Warrants in full, a warrant for an additional 50,000 shares exercisable at $3.50 per share. The $3.00 warrants were exercised in full. All of the warrants described above have an expiration date of October 10, 2008.
Review, Approval and Ratification of Related Party Transactions
Given our small size and limited financial resources, we had not adopted prior to our Reverse Merger formal policies and procedures for the review, approval or ratification of transactions, such as those described above, with our executive officers, directors and significant shareholders. However, we intend that such transactions will, on a going-forward basis, be subject to the review, approval or ratification of our board of directors, or an appropriate committee thereof.
As of the date hereof, we have not adopted a written conflict of interest policy that applies to our executive officers and directors. We intend to adopt a written conflict of interest policy in the near future.
Item 14. Principal Accounting Fees and Services
Murrell, Hall, McIntosh & Co., PLLP served as the principal accountant to audit our financial statements through December 18, 2007, when they were replaced by the firm of Sherb & Co., LLP. Effective as of May 21, 2008, we dismissed Sherb & Co., LLP as our independent registered public accounting firm, and engaged the firm of MSPC, Certified Public Accountants and Advisors LLP.
The following is a summary of the combined fees billed to us by Murrell, Hall, McIntosh & Co., PLLP, Sherb & Co., LLP and MSPC, Certified Public Accountants and Advisors LLP for professional services rendered for the fiscal years ended December 31, 2008 and 2007:
Aggregate fees rendered for the fiscal years ended December 2008 and 2007 were as follows:
| | | | | | | |
| | | | | | | |
| Audit Fees | | $ | 161,1066 | | | $ | 135,442 | |
| Audit Related Fees | | $ | 29,600 | | | $ | 21,500 | |
| Tax Fees | | | - | | | | - | |
| Other Fees | | | - | | | | - | |
| Total Fees: | | $ | 190,706 | | | $ | 156,942 | |
Audit Fees. Consists of fees billed for professional services rendered for the audit of our consolidated financial statements and review of the interim consolidated financial statements included in quarterly reports, services that are normally provided by our independent registered public accounting firm in connection with statutory and regulatory filings or engagements.
Audit-Related Fees. Consists of fees billed for assurance and related services that are reasonably related to the performance of the audit or review of our consolidated financial statements and are not reported under "Audit Fees."
Tax Fees. Consists of fees billed for professional services for our corporate tax returns and extensions, tax compliance, tax advice and tax planning. No such fees were billed by our independent registered public accounting firm in fiscal 2008 or 2007.
All Other Fees. No fees were billed to us by our independent registered public accounting firm for products and services other than the services reported above. No such fees were billed by our independent registered public accounting firm in fiscal 2008 or 2007.
The board of directors has the sole authority to review in advance and grant any pre-approvals of (i) all auditing services to be provided by the independent auditor, (ii) all significant non-audit services to be provided by the independent auditors as permitted by Section 10A of the Securities Exchange Act of 1934, and (iii) all fees and the terms of engagement with respect to such services. All audit and non-audit services performed by Murrell, Hall, McIntosh & Co., PLLP, Sherb & Co., LLP and MSPC, Certified Public Accountants and Advisors LLP during fiscal 2008 and 2007 were pre-approved pursuant to the procedures outlined above.
Item 15. Exhibits, Financial Statement Schedules
| 3.1 | Articles of Incorporation, as amended (1) |
| 3.2 | Restated Articles of Incorporation, as filed with the Secretary of State of Nevada on July 11, 2008 (8) |
| 3.3 | Certificate of Amendment to Articles of Incorporation (9) |
| 3.4 | By-Laws of the Company (1) |
| 3.3 | Finance Committee Charter (2) |
| 3.4 | Audit Committee Charter (2) |
| 3.5 | Compensation Committee Charter (2) |
| 3.6 | Nominating and Governance Committee Charter (2) |
| 3.7 | Executive Committee Charter (2) |
| 4.1 | Form of Class A Warrant issued to investors in connection with January 2008 private offering (3) |
| 10.1 | Form of Securities Purchase Agreement between Company and investors, dated as of January 31, 2008 (3) |
| 10.2 | Form of Registration Rights Agreement between Company and investors, dated as of January 31, 2008 (3) |
| 10.3 | Form of Make Good Agreement between Pope Asset Management LLC, as the authorized agent of the investors, the Company and Liu Yan-Qin, dated as of January 31, 2008 (3) |
| 10.4 | Form of Make Good Escrow Agreement between Pope Asset Management LLC, as the authorized agent of the investors, the Company, Liu Yan-Qing and Interwest Transfer, dated as of January 31, 2008 (3) |
| 10.5 | Form of Put Agreement between Company and investors, dated as of January 31, 2008 (3) |
| 10.6 | Equity Transfer Agreement, dated as of February 22, 2008, relating to acquisition of Heilongjiang Tianlong Pharmaceutical, Inc. (4) |
| 10.7 | Equity Transfer Agreement, dated as of April 18, 2008, relating to acquisition of Heilongjiang Haina Pharmaceutical Inc. (5) |
| 10.8 | Acquisition Agreement, dated as of June 9, 2008, relating to acquisition of Peng Lai Jin Chuang Pharmaceutical Company (7) |
| 16.1 | Letter from Sherb & Co., LLP dated as of June 6, 2008 (6) |
| 31.1 | Certification of Principal Executive Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 (10) |
| 31.2 | Certification of Principal Financial Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 (10) |
| 32.1 | Certification of Chief Executive Officer pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 (10) |
| 32.2 | Certification of Chief Financial Officer pursuant to Section 906 of Sarbanes-Oxley Act of 2002 (10) |
| | (1) | Incorporated by reference to the Registrant’s Registration Statement on Form 10-SB, as filed on May 13, 1999. |
| | (2) | Incorporated by reference to the Registrant’s Annual Report on Form 10-KSB, for the fiscal year ended December 31, 2007. |
| | (3) | Incorporated by reference from exhibits filed with Current Report on Form 8-K, Date of Event of January 31, 2008. |
| | (4) | Incorporated by reference to the Registrant’s Form 8-K/A, filed on April 9, 2008 |
| | (5) | Incorporated by reference to the Registrant’s Form 8-K, filed on April 24, 2008 |
| | (6) | Incorporated by reference to the Registrant’s Form 8-K/A, filed on June 10, 2008 |
| | (7) | Incorporated by reference to the Registrant’s Form 8-K, filed on June 11, 2008 |
| | (8) | Incorporated by reference to the Registrant’s Quarterly Report on Form 10-Q, for the fiscal quarter ended June 30, 2008 |
| | (9) | Incorporated by reference to the Registrant’s Form 8-K, filed on November 21, 2008 |
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| | CHINA SKY ONE MEDICAL, INC. |
| | |
Dated: April 15, 2009______________, 2010 | By: | /s/ Liu Yan-Qing |
| | | Liu Yan-Qing |
| | | Chairman, Chief Executive Officer and |
| | | President (Authorized Representative) |
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| Name | | Title | | Date |
| | | | | |
| | President, Chief Executive Officer | | April 15, 2009 _____________________, 2010 |
| Liu Yan-Qing | | and Director (Principal Executive Officer) | | |
| | | | | |
| | Chief Financial Officer and | | April 15, 2009 _____________________, 2010 |
| Hao Yu-Bo | | Secretary (Principal Financial and Accounting Officer) | | |
| | | | | |
| | Vice Chairman and Director | | April 15, 2009 _____________________, 2010 |
| Han Xiao-Yan | | Secretary (Principal Financial and Accounting Officer) | | |
| | | | | |
| | Director | | April 15, 2009 _____________________, 2010 |
| Song Chun Fan | | | | |
| | | | | |
| | Director | | April 15, 2009 _____________________, 2010 |
| Jiang Qi Feng | | | | |
| | | | | |
| | Director | | April 15, 2009 _____________________, 2010 |
| Zhao Jie | | | | |
| | | | | |
| | Director | | April 15, 2009 _____________________, 2010 |
| Qian Xu Feng | | | | |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
AMENDMENT NO. 1
TO
FORM 10-Q
(Mark One)
| x | QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
| | |
For the quarterly period ended | March 31, 2009 |
or
For the transition period from | | to | |
Commission File Number: | 000-26059 |
| CHINA SKY ONE MEDICAL, INC. |
(Exact name of registrant as specified in its charter) |
| o | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
| | |
| For the transition period from | | to | |
| Commission File Number: | 000-26059 |
| CHINA SKY ONE MEDICAL, INC. |
| (Exact name of registrant as specified in its charter) |
| Nevada | | 87-0430322 |
| (State or other jurisdiction of incorporation or organization) | | (I.R.S. Employer Identification No.) |
| | | | |
Room 1706, Di Wang Building, No. 30 Gan Shui Road, Nangang District, Harbin, People’s Republic of China | | 150001 |
| (Address of principal executive offices) | | (Zip Code) |
| |
86-451-53994069 (China) |
| (Registrant’s telephone number, including area code) |
| |
| |
| (Former name, former address and former fiscal year, if changed since last report) |
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. x Yes o No
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every InteracticeInteractive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files).
o Yes o No
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company.
Large accelerated filer o | Accelerated filer o |
| | |
Non-accelerated filer o | Smaller reporting company x |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act).
The registrant had 16,576,128 shares of Common Stock issued and outstanding as of May 13, 2009.
QUARTERLY REPORT ON FORM 10-Q
OF CHINA SKY ONE MEDICAL, INC. AND SUBSIDIARIES
FOR THE PERIOD ENDED MARCH 31, 2009
TABLE OF CONTENTS
| | | | | PAGE |
| | | | | |
| PART I | - | | FINANCIAL INFORMATION | |
| Item 1. | | | Financial Statements | |
| | | | Condensed Consolidated Balance Sheets as of March 31, 2009 (unaudited) and December 31, 2008 | 1 |
| | | | Condensed Consolidated Statements of Operations and Comprehensive Income for the Three Months Ended March 31, 2009 and 2008 (unaudited) | 2 |
| | | | Condensed Consolidated Statements of Cash Flows for the Three Months Ended March 31, 2009 and 2008 (unaudited) | 3 |
| | | | Notes to Condensed Consolidated Financial Statements (unaudited) | 4 |
| Item 2. | | | Management’s Discussion and Analysis of Financial Condition and Results of Operations | 22 |
| Item 3. | | | Quantitative and Qualitative Disclosures About Market Risk | 32 |
| Item 4. | | | Controls and Procedures | 32 |
| | | | | |
| PART II | - | | OTHER INFORMATION | |
| Item 1. | | | Legal Proceedings | 33 |
| Item 1A. | | | Risk Factors | 33 |
| Item 2. | | | Unregistered Sales of Equity Securities and Use of Proceeds | 33 |
| Item 3. | | | Defaults Upon Senior Securities | 33 |
| Item 4. | | | Submission of Matters to a Vote of Security Holders | 33 |
| Item 5. | | | Other Information | 33 |
| Item 6. | | | Exhibits | 34 |
| | |
| Signatures | 35 |
PART I. FINANCIAL INFORMATION
Item 1. Financial Statements
China Sky One Medical, Inc. and Subsidiaries
Condensed Consolidated Balance Sheets
| | | March 31, 2009 | | | December 31, 2008 (A) | |
| | | (Unaudited) | | | | |
| ASSETS | | | | | | |
| Current Assets | | | | | | |
| Cash and cash equivalents | | $ | 48,788,597 | | | $ | 40,288,116 | |
| Accounts receivable, net | | | 14,077,827 | | | | 14,978,648 | |
| Inventories | | | 1,320,295 | | | | 462,351 | |
| Prepaid and other current assets | | | 77,726 | | | | 106,386 | |
| Land and construction deposit | | | 8,523,979 | | | | 8,513,284 | |
| Total current assets | | | 72,788,424 | | | | 64,348,785 | |
| | | | | | | | | |
| Property and equipment, net | | | 20,907,074 | | | | 21,058,779 | |
| Intangible assets, net | | | 15,531,498 | | | | 15,851,765 | |
| | | $ | 109,226,996 | | | $ | 101,259,329 | |
| | | | | | | | | |
| LIABILITIES AND STOCKHOLDERS' EQUITY | | | | | | | | |
| | | | | | | | | |
| Current Liabilities | | | | | | | | |
| Accounts payable and accrued expenses | | $ | 3,702,703 | | | $ | 2,937,068 | |
| Taxes payable | | | 3,202,051 | | | | 3,362,888 | |
| Deferred revenues | | | - | | | | 26,079 | |
| Total current liabilities | | | 6,904,754 | | | | 6,326,035 | |
| | | | | | | | | |
| Commitments and Contingencies | | | | | | | | |
| | | | | | | | | |
| Stockholders' Equity | | | | | | | | |
| Preferred stock ($0.001 par value, 5,000,000 shares authorized, none issued and outstanding) | | | - | | | | - | |
| Common stock ($0.001 par value, 50,000,000 shares authorized, 16,446,467 and 16,306,184 issued and outstanding, respectively) | | | 16,446 | | | | 16,306 | |
| Additional paid-in capital | | | 40,134,162 | | | | 40,105,134 | |
| Accumulated other comprehensive income | | | 5,683,748 | | | | 5,566,806 | |
| Retained earnings | | | 56,487,886 | | | | 49,245,048 | |
| Total stockholders' equity | | | 102,322,242 | | | | 94,933,294 | |
| | | $ | 109,226,996 | | | $ | 101,259,329 | |
See accompanying summary of accounting policies and notes to the condensed consolidated financial statements.
(A) Reference is made to the Company’s Annual Report on Form 10-K for the year ended December 31, 2008 filed with the Securities and Exchange Commission on April 15, 2009
China Sky One Medical, Inc. and Subsidiaries
Condensed Consolidated Statements of Operations and Comprehensive Income
(Unaudited)
| | | Three Months Ended March 31, | |
| | | 2009 | | | 2008 | |
| Revenues | | $ | 24,833,692 | | | $ | 12,413,430 | |
| Cost of Goods Sold | | | 6,040,918 | | | | 2,860,428 | |
| Gross Profit | | | 18,792,774 | | | | 9,553,002 | |
| | | | | | | | | |
| Operating Expenses | | | | | | | | |
| Selling, general and administrative | | | 6,877,468 | | | | 3,956,795 | |
| Depreciation and amortization | | | 451,372 | | | | 76,348 | |
| Research and development | | | 2,412,780 | | | | 669,833 | |
| Total operating expenses | | | 9,741,620 | | | | 4,702,976 | |
| | | | | | | | | |
| Other Income (Expense) | | | | | | | | |
| Other income | | | - | | | | 63,048 | |
| Interest expense | | | 11,823 | | | | (1,147 | ) |
| Total other income (expense) | | | 11,823 | | | | 61,901 | |
| | | | | | | | | |
| Net Income Before Provision for Income Tax | | | 9,062,977 | | | | 4,911,927 | |
| | | | | | | | | |
| Provision for Income Taxes | | | | | | | | |
| Current | | | 1,820,139 | | | | 1,047,016 | |
| | | | | | | | | |
| Net Income | | $ | 7,242,838 | | | $ | 3,864,911 | |
| | | | | | | | | |
| Basic Earnings Per Share | | $ | 0.44 | | | $ | 0.28 | |
| | | | | | | | | |
| Basic Weighted Average Shares Outstanding | | | 16,413,920 | | | | 13,732,269 | |
| | | | | | | | | |
| Diluted Earnings Per Share | | $ | 0.43 | | | $ | 0.26 | |
| | | | | | | | | |
| Diluted Weighted Average Shares Outstanding | | | 16,665,221 | | | | 14,888,310 | |
| | | | | | | | | |
| Comprehensive Income | | | | | | | | |
| Net Income | | $ | 7,242,838 | | | $ | 3,864,911 | |
| Foreign currency translation adjustment | | | 116,942 | | | | 1,620,516 | |
| | | | | | | | | |
| Comprehensive Income | | $ | 7,359,780 | | | $ | 5,485,427 | |
See accompanying summary of accounting policies and notes to the condensed consolidated financial statements.
China Sky One Medical, Inc. and Subsidiaries
Condensed Consolidated Statements of Cash Flows
(Unaudited)
| | | Three Months Ended March 31, | |
| | | 2009 | | | 2008 | |
| Cash flows from operating activities | | | | | | |
| Net Income | | $ | 7,242,838 | | | $ | 3,864,911 | |
| Adjustments to reconcile net cash provided by operating activities | | | | | | | | |
| Depreciation and amortization | | | 588,066 | | | | 140,009 | |
| Share-based compensation expense | | | - | | | | 10,117 | |
| Net change in assets and liabilities | | | | | | | | |
| Accounts receivables | | | 912,487 | | | | 1,859,639 | |
| Inventories | | | (857,238 | ) | | | (408,079 | ) |
| Prepaid expenses and other current assets | | | 35,805 | | | | 5,526 | |
| Accounts payable and accrued expenses | | | 735,730 | | | | (370,378 | ) |
| Taxes payable | | | (165,038 | ) | | | 102,786 | |
| Deferred revenues | | | - | | | | (6,952 | ) |
| Net cash provided by operating activities | | | 8,492,650 | | | | 5,197,579 | |
| | | | | | | | | |
| Cash flows from investing activities | | | | | | | | |
| Purchases of fixed assets | | | (66,148 | ) | | | (42,782 | ) |
| Land and construction deposit | | | - | | | | (710,656 | ) |
| Purchase of subsidiary-Heilongjiang Haina Pharmaceutical, Inc. | | | - | | | | (427,838 | ) |
| Cash of subsidiary upon acquisition | | | - | | | | 82,715 | |
| Purchase of intangible assets | | | (3,651 | ) | | | (7,139 | ) |
| Net cash used in investing activities | | | (69,799 | ) | | | (1,105,700 | ) |
| | | | | | | | | |
| Cash flows from financing activities | | | | | | | | |
| Sale of common stock for cash, net of offering costs | | | - | | | | 23,487,963 | |
| Proceeds from warrants conversion | | | 29,169 | | | | 739,588 | |
| Net cash provided by financing activities | | | 29,169 | | | | 24,227,551 | |
| | | | | | | | | |
| Effect of exchange rate changes on cash | | | 48,462 | | | | 727,694 | |
| | | | | | | | | |
| Net increase in cash and cash equivalents | | | 8,500,481 | | | | 29,047,124 | |
| | | | | | | | | |
| Cash and cash equivalents at beginning of period | | | 40,288,116 | | | | 9,190,870 | |
| | | | | | | | | |
| Cash and cash equivalents at end of period | | $ | 48,788,597 | | | $ | 38,237,994 | |
| | | | | | | | | |
| Supplemental disclosure of cash flow information | | | | | | | | |
| Interest paid | | $ | - | | | $ | 1,157 | |
| Taxes paid | | $ | 2,106,956 | | | $ | 944,230 | |
See accompanying summary of accounting policies and notes to the condensed consolidated financial statements.
China Sky One Medical, Inc. and Subsidiaries
Notes to Unaudited Condensed Consolidated Financial Statements
1. Description of Business
China Sky One Medical, ("China Sky One" or the “Company”), a Nevada corporation, was formed on February 7, 1986, and formerly known as Comet Technologies, Inc. (“Comet”). On July 26, 2006, the Company changed the name of the reporting company from "Comet Technologies, Inc." to "China Sky One Medical, Inc."
American California Pharmaceutical Group, Inc. (“ACPG”), our non operating United States holding company subsidiary, was incorporated on December 16, 2003, in the State of California, under the name “QQ Group, Inc.” QQ Group, Inc. changed its name to “American California Pharmaceutical Group, Inc.” in anticipation of the Stock Exchange Agreement with China Sky One (then known as “Comet Technologies, Inc.”) and TDR, described herein. On December 8, 2005, ACPG completed a stock exchange transaction with TDR a People’s Republic of China (“PRC”) based operating company and TDR’s subsidiaries (the “TDR Acquisition”), each of which were fully operating companies in the PRC. Under the terms of the agreement, ACPG exchanged 100% of its issued and outstanding common stock for 100% of the capital stock of TDR and its subsidiaries, described below.
Thereafter, on May 11, 2006, ACPG entered into a Stock Exchange Agreement (the “Exchange Agreement”) with the shareholders of China Sky One. The terms of the Exchange Agreement were consummated and the acquisition was completed on May 30, 2006. As a result of the transaction, the Company issued a total of 10,193,377 shares of its common voting stock to the stockholders of ACPG, in exchange for 100% of the capital stock of ACPG resulting in ACPG becoming our wholly-owned subsidiary. The transaction is treated as a reverse merger for accounting purposes.
TDR, formerly known as “Harbin City Tian Di Ren Medical Co.,” was originally formed in 1994 and maintained its principal executive office in Harbin City of Heilongjiang Province, in the People’s Republic of China. TDR was reorganized and incorporated as a limited liability company on December 29, 2000, under the “Corporation Laws and Regulations” of the PRC. At the time of the TDR Acquisition by ACPG in December of 2005, TDR had two wholly-owned subsidiaries, Harbin First Bio-Engineering Company Limited and Kangxi Medical Care Product Factory, until July, 2006, when the two were merged, with Harbin First Bio-Engineering Company Limited (“First”) as the surviving subsidiary of TDR. The principal activities of TDR and First are the research, manufacture and sale of over-the-counter non-prescription health care products. TDR commenced its business in the sale of branded nutritional supplements and over-the-counter pharmaceutical products in the Heilongjiang Province. TDR has subsequently evolved into an integrated manufacturer, marketer, and distributor of external use natural Chinese medicine products sold primarily to and through China’s various domestic pharmaceutical chain stores.
China Sky One is a holding company whose principal operations are through its wholly-owned subsidiaries; it has no revenues separate from its subsidiaries, and has nominal expenses related to its status as a public reporting company and to its ownership interest in ACPG and TDR.
On September 30, 2008 (the “Record Date”), we obtained the written consent of the holders of 8,158,251 shares of our common stock, which as of the Record Date, represented 51.3% of our outstanding voting securities, to increase our number of authorized shares of common stock from twenty million (20,000,000) to fifty million (50,000,000) shares.
2. Acquisition of Businesses
On April 3, 2008, TDR completed an acquisition pursuant to an Equity Transfer Agreement dated February 22, 2008, between TDR and Heilongjiang Tianlong Pharmaceutical, Inc., a corporation with a multitude of SFDA approved medicines and new medicine applications, organized under the laws of the PRC (“Tianlong”), which is in the business of manufacturing external-use pharmaceuticals. Our TDR subsidiary previously acquired the Beijing sales office of Tianlong in mid 2006. Pursuant to the Equity Transfer Agreement, TDR acquired 100% of the issued and outstanding capital stock of Tianlong from Tianlong ’s sole stockholder Wu Jiechen, a resident of China, in consideration for an aggregate purchase price of approximately $8,300,000, consisting of (i) $8,000,000 in cash, and (ii) 23,850 shares of the Company’s common stock (at $12 per share). The acquisition received regulatory approval and closed on April 3, 2008.
China Sky One Medical, Inc. and Subsidiaries
Notes to Unaudited Condensed Consolidated Financial Statements
2. Acquisition of Businesses (Continued)
The following table summarizes the approximate estimated fair values of the assets acquired in the Tianlong acquisition.
| Fixed assets | | $ | 6,314,871 | |
| Intangible assets | | | 1,786,990 | |
| Other | | | 170,000 | |
| Net assets acquired | | $ | 8,271,861 | |
On April 18, 2008, China Sky One through its subsidiary TDR consummated a share acquisition pursuant to an Equity Transfer Agreement with the shareholders of Heilongjiang Haina Pharmaceutical Inc., a recently formed corporation organized under the laws of the PRC (“Haina”) licensed as a wholesaler of TCD, bio-medicines, bio-products, medicinal devices, antibiotics and chemical medicines. Haina does not have an established sales network and was acquired for its primary asset, a Good Supply Practice (GSP) license (License No. A-HLJ03-010) issued by the Heilongjiang office of the SFDA. The SFDA recently started issuing such licenses to resellers of medicines that maintain certain quality controls. The GSP license was issued as of December 21, 2006 and will expire on January 29, 2012 and will enable the Company to expand its sales of medicinal products without having to go through a lengthy license application process. Although the transaction did not close until April 18, 2008, TDR began overseeing the operations of Haina in January of 2008 as part of its due diligence prior to closing of this acquisition.
The following table summarizes the approximate estimated fair values of the assets acquired in the Haina acquisition.
| Cash | | $ | 84,307 | |
| Intangible assets | | $ | 437,375353,068 | |
| Net assets acquired | | $ | 437,375 | |
Pursuant to the Equity Transfer Agreement, TDR acquired 100% of the issued and outstanding capital stock of Haina from its three stockholders in consideration for payment of 3,000,000 RMB (approximately $437,375).
On June 9, 2008, TDR entered into a Merger and Acquisition Agreement (the “Acquisition Agreement”) with Peng Lai Jin Chuang Company, a corporation organized under the laws of the People’s Republic of China (“Peng Lai”), which was recently organized to develop, manufacture and distribute pharmaceutical, medicinal and diagnostic products in the PRC. Pursuant to the Acquisition Agreement, TDR acquired all of the assets of Peng Lai in consideration for an aggregate of approximately (i) US$2.5 million in cash, and (ii) 381,606 shares of the Company’s common stock with a fair value of approximately $4.6 million (at $12 per share). The acquisition of Peng Lai closed on September 5, 2008.
The following table summarizes the approximate estimated fair values of the assets acquired in the Peng Lai acquisition.
| Fixed assets | | $ | 4,176,922 | |
| Intangible assets | | | 2,917,386 | |
| Net assets acquired | | $ | 7,094,308 | |
China Sky One Medical, Inc. and Subsidiaries
Notes to Unaudited Condensed Consolidated Financial Statements
2. Acquisition of Businesses (Continued)
The following table contains pro forma condensed consolidated statements of operations information assuming the Tianlong, Haina and Peng Lai transactions closed on January 1, 2008 for the three months ended March 31, 2008. Peng Lai had dormant operations until October 2008.
| Three Months Ended March 31, 2008 | |
| Revenues | | $ | 12,982,982 | |
| Operating income | | $ | 4,940,468 | |
| Net income | | $ | 3,944,500 | |
| Basic earnings per common share | | $ | 0.28 | |
| Basic weighted average shares outstanding | | | 14,137,725 | |
| Diluted earnings per share | | $ | 0.26 | |
| Diluted weighted average shares outstanding | | | 15,293,766 | |
3. Summary of Significant Accounting Policies
Principles of Consolidation – The accompanying consolidated financial statements include the accounts of the Company and its wholly-owned subsidiaries, ACPG, TDR, First, Haina, Tianlong, and Peng Lai. All significant inter-company transactions and balances were eliminated.
These financial statements are stated in U.S. Dollars and have been prepared in accordance with accounting principles generally accepted in the United States of America. This basis of accounting differs from that used under applicable accounting requirements in the PRC. No material adjustment was required.
Certain items in the 2008 financial statements have been reclassified to conform with the 2009 financial statements presentation.
The financial summaries for the three months ended March 31, 2009 and March 31, 2008 are unaudited and include, in the opinion of management of the Company , all adjustments (consisting of normal recurring accruals) necessary to present fairly the earnings for such periods. Operating results for the three months ended March 31, 2009 are not necessarily indicative of the results that may be expected for the entire year ending December 31, 2009.
While the Company believes that the disclosures presented are adequate to make the information not misleading, it is suggested that these consolidated financial statements be read in conjunction with the financial statements and notes included in the Company’s audited financial statements for the year ended December 31, 2008.
Use of estimates – The preparation of these financial statements in conformity with accounting principles generally accepted in the United States of America, requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, disclosure of contingent assets and liabilities at the dates of the financial statements, and the reported amounts of revenues and expenses during the reported periods.
Significant estimates include values and lives assigned to acquired tangible and intangible assets under our business acquisitions, reserves for customer returns and allowances, uncollectible accounts receivable, share-based compensation, impairment testing of goodwill and other intangible assets and slow moving and/or obsolete/damaged inventory. Actual results may differ from these estimates.
Earnings per share - Basic earnings per common share is computed by dividing net earnings applicable to common shareholders by the weighted-average number of common shares outstanding during the period. When applicable, diluted earnings per common share is determined using the weighted-average number of common shares outstanding during the period, adjusted for the dilutive effect of common stock equivalents, consisting of shares that might be issued upon exercise of common stock options and warrants.
China Sky One Medical, Inc. and Subsidiaries
Notes to Unaudited Condensed Consolidated Financial Statements
3. Summary of Significant Accounting Policies (continued)
Potential common shares issued are calculated using the treasury stock method, which recognizes the use of proceeds that could be obtained upon the exercise of options and warrants in computing diluted earnings per share. It assumes that any proceeds would be used to purchase common stock at the average market price of the common stock during the period.
Cash and cash equivalents – The Company considers all highly liquid debt instruments purchased with maturity period of sixthree months or less to be cash equivalents. The carrying amounts reported in the accompanying consolidated balance sheets for cash and cash equivalents approximate their fair value.
Accounts receivable – Accounts receivable are stated at net realizable value, net of an allowance for doubtful accounts. Provision of allowance is made for estimated bad debts based on a periodic analysis of individual customer balances including an evaluation of days of sales outstanding, payment history, recent payment trends, and perceived credit worthiness. At each of March 31, 2009 and December 31, 2008, the Company’s allowance for doubtful accounts was approximately $50,000.
Inventories – Inventories include finished goods, raw materials, freight-in, packing materials, labor, and overhead costs and are valued at the lower of cost or market using the first-in, first-out method. Inventory units are valued using the weighted average method. Provisions are made for slow moving, obsolete and/or damaged inventory based on a periodic analysis of individual inventory items including an evaluation of historical usage and/or movement, age, expiration date, and general conditions. There was no inventory reserve provision recorded at March 31, 2009 and December 31, 2008.
Property and equipment – Property and equipment are stated at historical cost less accumulated depreciation. Depreciation on property and equipment is provided using the straight-line method over the estimated useful lives of the assets. The Company uses an estimated residual value of 5% of cost, or valuation for both financial and income tax reporting purposes. The estimated lengths of useful lives are as follows:
| Building and Improvements | 30 years |
| Land use rights | 50 years |
| Furniture & Equipment | 5 to 7 years |
| Transportation Equipment | 5 to 15 years |
| Machinery and Equipment | 7 to 14 years |
Expenditures for renewals and betterments are capitalized while repairs and maintenance costs are normally charged to the statement of operations in the year in which they were incurred. In situations where it can be clearly demonstrated that the expenditure has resulted in an increase in the future economic benefits expected to obtain from the use of the asset, the expenditure is capitalized as an additional cost of the asset. Upon sale or disposal of an asset, the historical cost and related accumulated depreciation or amortization of such asset is removed from their respective accounts, and any gain or loss is recorded in the Consolidated Statements of Operations.
Property and equipment are evaluated for impairment in value whenever an event or change in circumstances indicates that the carrying values may not be recoverable. If such an event or change in circumstances occurs and potential impairment is indicated because the carrying values exceed the estimated future undiscounted cash flows of the asset, the Company will measure the impairment loss as the amount by which the carrying value of the asset exceeds its fair value. The Company did not record any impairment charges during each of the three months ended March 31, 2009 and 2008.
Construction-in-progress – Properties currently under development are accounted for as construction-in-progress. Construction-in-progress is recorded at acquisition cost, including land rights cost, development expenditures, professional fees, and capitalized interest costs during the course of construction.
China Sky One Medical, Inc. and Subsidiaries
Notes to Unaudited Condensed Consolidated Financial Statements
3. Summary of Significant Accounting Policies (Continued)
Upon completion and readiness for use of the project, the cost of construction-in-progress is transferred to the facility. In the case of construction-in-progress, management takes into consideration the estimated cost to complete the project when making the lower of cost or market calculation.
Intangible assets – Intangible assets consists of patents and goodwill. Patent costs are amortized over an estimated life of ten years.
Intangible assets are accounted for in accordance with Statement of Financial Accounting Standards No. 142, Goodwill and Other Intangible Assets (“SFAS 142”). Intangible assets with finite useful lives are amortized while intangible assets with indefinite useful lives are not amortized. As prescribed by SFAS 142, goodwill and intangible assets are tested periodically for impairment. The Company adopted SFAS No. 144, "Accounting for the Impairment or Disposal of Long- Lived Assets," effective January 1, 2002. Accordingly, the Company reviews its long-lived assets, including property and equipment and finite-lived intangible assets for impairment whenever events or changes in circumstances indicate that the carrying amount of the assets may not be fully recoverable. To determine recoverability of its long-lived assets, the Company evaluates the probability that future undiscounted net cash flows will be less than the carrying amount of the assets. Impairment costs, if any, are measured by comparing the carrying amount of the related assets to their fair value. The Company recognizes an impairment loss based on the excess of the carrying amount of the assets over their respective fair values. Fair value is determined by discounted future cash flows, appraisals or other methods. If the assets determined to be impaired are to be held and used, the Company recognizes an impairment loss thru a charge to operating results to the extent the present value of anticipated cash flows attributable to the assets are less than the asset’s carrying value. The Company would depreciate the remaining the remaining value over the remaining estimated useful life of the asset to operating results. The company did not record any impairment charges during each of the three months ended March 31, 2009 and 2008.
Foreign Currency - The Company’s principal country of operations is in The People’s Republic of China. The financial position and results of operations of the Company are recorded in RMB as the functional currency. The results of operations denominated in foreign currency are translated at the average rate of exchange during the reporting period.
Assets and liabilities denominated in foreign currencies at the balance sheet date are translated at the market rate of exchange at that date. The registered equity capital denominated in the functional currency is translated at the historical rate of exchange at the time of the capital contribution. All translation adjustments resulting from the translation of the financial statements into the reporting currency (“US Dollars”) are recorded as accumulated other comprehensive income, a component of stockholders’ equity.
Revenue recognition– Revenue is recognized when the following criteria are met: (1) persuasive evidence of an arrangement exists; (2) the product has been shipped and the customer takes ownership and assumes the risk of loss; (3) the selling price is fixed or determinable; and (4) collection of the resulting receivable is reasonably assured. The Company believes that these criteria are satisfied upon shipment from its facilities. Revenue is reduced by provisions for estimated returns and allowances as well as specific known claims, if any , which are based on historical averages that have not varied significantly for the periods presented. Historically, the Company’s estimated returns, allowances and claims have been deemed immaterial. The Company’s safe agreements only allow a return if the product has quality related issues. In such event, the Company accepts the return for equivalent product exchange from inventory only.
The Company occasionally applies to various government agencies for research grants. Revenue from such research grants is recognized when earned. In situations where TDR receives payment in advance for the performance of research and development services, such amounts are deferred and recognized as revenue as the related services are performed.
Deferred revenues - The Company recognizes revenues as earned. Amounts billed in advance of the period in which goods are delivered are recorded as a liability under “Deferred revenues.”
China Sky One Medical, Inc. and Subsidiaries
Notes to Unaudited Condensed Consolidated Financial Statements
3. Summary of Significant Accounting Policies (Continued)
Research and development—Research and development expenses include the costs associated with the Company’s internal research and development as well as research and development conducted by third parties. These costs primarily consist of salaries, clinical trials, outside consultants, and materials. All research and development costs are expensed as incurred.
Third-party expenses reimbursed under non-refundable research and development contracts are recorded as a reduction to research and development expense in the statement of operations.
The Company recognizes in-process research and development in accordance with FASB Interpretation No. 4, Applicability of FASB Statement No. 2 to Business Combinations Accounted for by the Purchase Method and the AICPA Technical Practice Aid, Assets Acquired in a Business Combination to be used in Research and Development Activities: A Focus on Software, Electronic Devices, and Pharmaceutical Industries. Assets to be used in research and development activities, specifically, compounds that have yet to receive new drug approval and would have no alternative use, should approval not be given, are immediately charged to expense when acquired. Certain assets and high technologies acquired that has a foreseeable future cash flows are capitalized as intangible assets. Such intangible assets are amortized starting from the year revenue is generated and amortize over a period of 10 years. Should under any circumstances these capitalized intangible assets are valued with no future benefit, the Company will charge to expense immediately.
The Company incurred $2,412,780 and $669,833 in research and development costs for each of the three months ended March 31, 2009 and 2008, respectively.
Advertising— Advertising and promotion costs are expensed as incurred Generally, the Company signs contracts with agents who then place its advertising in the mediums of television, radio and internet. Advertising expense is incurred in the period the advertisements take place. Thus, costs of advertising are expenses as incurred, in compliance with FASB Concept Statement #6 paragraph 81 and ASC 720-35-05-01. Historically, the Company’s direct-response advertising costs have been immaterial. Total advertising costs for the three months ended March 31, 2009 and 2008 was $2,776,126 and $369,995, respectively. Advertising costs are reported as part of selling, general and administrative expenses in the consolidated statement of operations.
Taxation – The Company uses the asset and liability method of accounting for deferred income taxes. The Company’s provision for income taxes includes income taxes currently payable and those deferred because of temporary differences between the financial statement and tax bases of assets and liabilities. The Company records liabilities for income tax contingencies based on our best estimate of the underlying exposures.
The Company periodically estimates its tax obligations using historical experience in tax jurisdictions and informed judgments. There are inherent uncertainties related to the interpretation of tax regulations in the jurisdictions in which the Company transacts business. The judgments and estimates made at a point in time may change based on the outcome of tax audits, as well as changes to, or further interpretations of, regulations. The Company adjusts income tax expense in the period in which these events occur.
Provision for the PRC’s enterprise income tax is calculated at the prevailing rate based on the estimated assessable profits less available tax relief for losses brought forward.
Provision for the PRC enterprise income tax is calculated at the prevailing rate based on the estimated assessable profits less available tax relief for losses brought forward. The Company does not accrue taxes on unremitted earnings from foreign operations as it is the Company’s intention to invest these earnings in the foreign operations indefinitely ..
Enterprise income tax
Under the Provisional Regulations of PRC Concerning Income Tax on Enterprises promulgated by the PRC, income tax is payable by enterprises at a rate of 25% of their taxable income. Preferential tax treatment may, however, be granted pursuant to any law or regulations from time to time promulgated by the State Council. Income tax for Peng Lai is regulated by the local government and has been charged at the rate of 2% of total revenues commencing January 1, 2009. Peng Lai had no business operations during the first quarter of 2008.
China Sky One Medical, Inc. and Subsidiaries
Notes to Unaudited Condensed Consolidated Financial Statements
3. Summary of Significant Accounting Policies (Continued)
According to “Enterprise Income Tax and Certain Preferential Policies Notice” published by the Ministry of Finance and the National Tax Affairs Bureau, if the enterprise is authorized by the State Council as a special entity, the enterprise income tax rate is reduced to 15%. The income tax rate for TDR, First, and Tianlong is 15%, based on State Council approval.
Value added tax
The Provisional Regulations of PRC Concerning Value Added Tax promulgated by the State Council came into effect on January 1, 1994. Under these regulations and the Implementing Rules of the Provisional Regulations of the PRC Concerning Value Added Tax, value added tax is imposed on goods sold in, or imported into, the PRC and on processing, repair and replacement services provided within the PRC.
Value added tax payable in the PRC is charged on an aggregated basis at a rate of 13% or 17% (depending on the type of goods involved) on the full price collected for the goods sold or, in the case of taxable services provided, at a rate of 17% on the charges for the taxable services provided, but excluding, in respect of both goods and services, any amount paid in respect of value added tax included in the price or charges, and less any deductible value added tax already paid by the taxpayer on purchases of goods and services in the same financial year.
According to “Agriculture Product Value Added Tax Rate Adjustment and Certain Items’ Value Added Tax Waiver” published by the Ministry of Finance and the National Tax Affairs Bureau, the value added tax for agriculture related products is to be taxed at 13%. Furthermore, traditional Chinese medicine and medicinal plant are by definition agriculture related products.
Accounting for uncertainty in income taxes – In June 2006, the Financial Accounting Standards Board (FASB) issued Interpretation No. 48, Accounting for Uncertainty in Income Taxes - an Interpretation of FASB Statement No. 109 (FIN 48). FIN 48 is intended to clarify the accounting for uncertainty in income taxes recognized in a company’s financial statements and prescribes the recognition and measurement of a tax position taken or expected to be taken in a tax return. FIN 48 also provides guidance on de-recognition, classification, interest and penalties, accounting in interim periods, disclosure and transition.
Under FIN 48, evaluation of a tax position is a two-step process. The first step is to determine whether it is more-likely-than-not that a tax position will be sustained upon examination, including the resolution of any related appeals or litigation based on the technical merits of that position. The second step is to measure a tax position that meets the more-likely-than-not threshold to determine the amount of benefit to be recognized in the financial statements. A tax position is measured at the largest amount of benefit that is greater than 50% likely of being realized upon ultimate settlement.
Tax positions that previously failed to meet the more-likely-than-not recognition threshold should be recognized in the first subsequent period in which the threshold is met. Previously recognized tax positions that no longer meet the more-likely-than-not criteria should be de-recognized in the first subsequent financial reporting period in which the threshold is no longer met.
The adoption of FIN 48 at January 1, 2007 did not have a material effect on the Company’s results of operations and financial position.
Comprehensive income – Comprehensive income consists of net income and other gains and losses affecting stockholders’ equity that, under generally accepted accounting principles are excluded from net income. For the Company, such items consist entirely of foreign currency translation gains and losses.
Related companies – A related company is a company in which the director has beneficial interests in and in which the Company has significant influence.
China Sky One Medical, Inc. and Subsidiaries
Notes to Unaudited Condensed Consolidated Financial Statements
3. Summary of Significant Accounting Policies (Continued)
Retirement benefit costs – According to the PRC regulations on pension plans, the Company contributes to a defined contribution retirement plan organized by municipal government in the province in which the Company was registered and all qualified employees as defined by statutory regulations are eligible to participate in the plan.
Contributions to the pension or retirement plan are calculated at 22.5% of the employees’ salaries above a fixed threshold amount. The employees contribute between 2% to 8% to the pension plan, and the Company contributes the balance. The Company has no other material obligations for the payment of retirement benefits beyond the annual contributions under this plan. The Company incurred costs of $44,211 and $7,472 for the three months ended March 31, 2009 and 2008, respectively.
Fair value of financial instruments – The carrying amounts of certain financial instruments, including cash and cash equivalents, accounts receivable, other receivables, accounts payable, accrued expenses, and other payables approximate their fair values at March 31, 2009 and 2008 because of the relatively short-term maturity of these instruments.
Recent accounting pronouncements
In March 2008, the FASB issued Statement No. 161, Disclosures about Derivative Instruments and Hedging Activities an amendment of SFAS 133 ("SFAS 161"). This Statement will require enhanced disclosures about derivative instruments and hedging activities to enable investors to better understand their effects on an entity's financial position, financial performance, and cash flows. It is effective for financial statements issued for fiscal years and interim periods beginning after November 15, 2008, with early application encouraged. SFAS 161 became effective for the Company in 2009 and did not have a material impact on its financial position, results of operations or cash flows.
In December 2007, the FASB issued SFAS No. 141 (revised 2008), “Business Combinations” (“SFAS 141(R)”). SFAS 141(R) will change the accounting for business combinations. Under SFAS No. 141(R), an acquiring entity will be required to recognize all the assets acquired and liabilities assumed in a transaction at the acquisition-date fair value with limited exceptions. SFAS No. 141(R) will change the accounting treatment and disclosure for certain specific items in a business combination. SFAS 141R requires earn-outs and other contingent consideration to be recorded at fair value on acquisition date and contingencies to be recorded at fair value on acquisition date with subsequent remeasurement. SFAS 141R requires acquisition costs to be expensed as incurred and generally requires restructuring costs to be expensed in periods after the acquisition date. SFAS 141R requires amounts previously called “negative goodwill” which result from a bargain purchase in which acquisition date fair value of identifiable net assets acquired exceeds the fair value of consideration transferred plus any non controlling interest in the acquirer to be recognized in earnings as a gain attributable to the acquirer. SFAS No. 141(R) became effective for the Company in 2009. SFAS 141(R) will impact the Company in the event of any acquisition after December 31, 2008.
In December 2007, the FASB issued SFAS No. 160, “Non-controlling Interests in Consolidated Financial Statements—an amendment of Accounting Research Bulletin No. 51” (“SFAS 160”). SFAS 160 requires noncontrolling interests to be reported in the equity section of financial statements and requires that net earnings include the amounts attributable to both the parent and the noncontrolling interests with disclosure on the face of the statement of operations of the net earnings attributable to the parent and to the noncontrolling interests, with any losses attributable to the noncontrolling interests in excess of the noncontrolling interests’ equity to be allocated to the noncontrolling interest. Calculation of earnings per share amounts in the financial statements will continue to be based on amounts attributable to the parent. SFAS No. 160 became effective for the Company in 2009 and did not have a material impact on the Company’s financial statements.
In February 2007, the FASB issued Statement No. 159 “The Fair Value Option for Financial Assets and Financial Liabilities” (SFAS 159). This statement permits companies to choose to measure many financial assets and liabilities at fair value. Unrealized gains and losses on items for which the fair value option has been elected are reported in earnings. SFAS 159 is effective for fiscal years beginning after November 15, 2007. SFAS 159 became effective on January 1, 2008 and did not have a material impact on the Company’s financial statements.
China Sky One Medical, Inc. and Subsidiaries
Notes to Unaudited Condensed Consolidated Financial Statements
3. Summary of Significant Accounting Policies (Continued)
In September 2006, the FASB issued Statement of Financial Accounting Standards No. 157, Fair Value Measurements (“SFAS 157”). The statement provides enhanced guidance for using fair value to measure assets and liabilities and also responds to investors’ requests for expanded information about the extent to which company’s measure assets and liabilities at fair value, the information used to measure fair value, and the effect of fair value measurements on earnings. While the standard applies whenever other standards require (or permit) assets or liabilities to be measured at fair value, it does not expand the use of fair value in any new circumstances. Statement No. 157 is effective for the Company beginning in 2008 except for the nonfinancial assets and liabilities that are subject to a one-year deferral allowed by FASB Staff Position (FSP) SFAS 157-2 (“ FSP SFAS 157-2”). FSP SFAS 157-2 delays the effective date of SFAS 157 until fiscal years beginning after November 15, 2008 for nonfinancial assets and liabilities that are not recognized or disclosed at fair value in the financial statements on a recurring basis (at least annually). The adoption of SFAS 157 nor the adoption relating to the deferral provision of FSP SFAS 157-2 had a material effect on our consolidated financial statements.
4. Revenue by Product Category
The following table sets forth the Company’s principal product categories based an application type and the approximate amount and percentage of revenue from each of such product categories during the three months ended march 31, 2009 and 2008:
| | | | | For the Three Months Ended March 31, | |
| | | | | 2009 | | | 2008 | |
| Product | | Subsidiary | | Sales | | | % of Sales | | | Sales | | | % of Sales | |
| Patch | | TDR | | $ | 9,121,961 | | | | 37 | % | | $ | 3,797,989 | | | | 30 | % |
| Ointment | | TDR & TL | | | 5,081,978 | | | | 20 | % | | | 1,487,146 | | | | 12 | % |
| Spray | | TDR & TL | | | 2,902,074 | | | | 12 | % | | | 1,863,371 | | | | 15 | % |
| Bio-Engineering | | First | | | 3,100,775 | | | | 12 | % | | | 1,825,615 | | | | 15 | % |
| Contract Sales | | | | | - | | | | - | | | | 2,946,016 | | | | 24 | % |
| Others | | TDR & TL & PL | | | 4,626,904 | | | | 19 | % | | | 493,293 | | | | 4 | % |
| Total | | | | $ | 24,833,692 | | | | 100 | % | | $ | 12,413,430 | | | | 100 | % |
5. Concentrations of Business and Credit Risk
The Company maintains certain bank accounts in the PRC which are not protected by FDIC insurance or other insurance. As of March 31, 2009 the Company held approximately $2,562,000 of cash balances within the United States of which approximately $404,000 was in excess of FDIC insurance limits. At March 31, 2009, the Company had approximately $46,212,000, in China bank deposits, which is not insured. Historically, the Company has not experienced any losses in such accounts.
Nearly all of the Company’s sales are concentrated in China. Accordingly, the Company is susceptible to fluctuations in its business caused by adverse economic conditions in China. Difficult economic conditions in other geographic areas into which the Company may expand may also adversely affect its business, operations and finances.
The Company provides credit in the normal course of business. Substantially all customers are located in PRC. The Company performs ongoing credit evaluations of its customers and maintains allowances for doubtful accounts based on factors surrounding the credit risk of specific customers, historical trends, and other information.
Substantially all of the Company's fixedlong-lived assets and operations are located in the PRC.
The Company is self-insured for all risks and carries no liability or property insurance coverage of any kind. The Company does not set aside any reserves for product liability risks or other potential claims. The Company’s policy is to record losses associated with its lack of insurance coverage at such time as a realized loss is incurred. Historically, the Company has not had any material losses in connection with its lack of insurance coverage and was not party to any material pending legal proceedings as of March 31, 2009. Management’s intention is to use the Company’s working capital to fund any such losses incurred due to the Company’s exposure to inadequate insurance coverage.
Substantially all of the Company's businesses are generated from operations in mainland China.
Major Customers
For the three months ended March 31, 2009, Shanxi Xintai and Harbin Shiji Baolong accounted for approximately 22% and 20%, respectively, of salestotal revenues. For the three months ended March 31, 2008, Xinteyao Ltd. accounted for approximately 11% of salestotal revenues. Shanxi Xintai and Harbin Shiji Baolong accounted for approximately 25% and 29%, respectively, of all account receivable, for the three months ended March 31, 2009. No other customer accounted for 10% or more of the Company’s total revenues for the three months ended March 31, 2009 or 2008 or accounts receivable as of March 31, 2009 or 2008.
Major Suppliers
Heilongjiang Kangda Medicine Co. accounted for approximately 43% of the Company’s inventory purchases for the three months ended March 31, 2009 and 45% for the three months ended March 31, 2008.
China Sky One Medical, Inc. and Subsidiaries
Notes to Unaudited Condensed Consolidated Financial Statements
5.6. Earnings Per Share
We have applied SFAS No. 128, “Earnings Per Share” in our calculation and presentation of earnings per share - “basic” and “diluted”. Basic earnings per share are computed by dividing net earnings available to common shareholders (the numerator) by the weighted average number of common shares (the denominator) for the period presented. The computation of diluted earnings per share is similar to basic earnings per share, except that the denominator is increased to include the number of additional common shares that would have been outstanding if the potentially dilutive common shares had been issued.
Stock warrants and options to purchase 1,001,000 shares and 1,452,665 of common stock were outstanding and exercisable as of March 31, 2009 and 2008, respectively. These common stock equivalents were included in the computation of diluted earnings per share because the option exercise prices were less than the average market price of our common stock during these periods.
The dilutive potential common shares on warrants and options is calculated in accordance with the treasury stock method, which assumes that proceeds from the exercise of all warrants and options are used to repurchase common stock at the average market valueprice of the common stock during the relevant period. The amount of shares remaining after the proceeds are exhausted represents the potential dilutive effect of the securities.
The following table sets forth our computation of basic and diluted net income per share:
| | | For the three months ended March 31, | |
| | | 2009 | | | 2008 | |
Numerator: | | | | | | |
| Net income used in calculation of basic and diluted earnings per share | | $ | 7,242,838 | | | $ | 3,864,911 | |
| | | | | | | | | |
Denominator: | | | | | | | | |
| Weighted-average common shares outstanding used in calculation of basic earnings per share | | | 16,413,920 | | | | 13,732,269 | |
| | | | | | | | | |
| Effect of dilutive securities: | | | | | | | | |
| Stock options and equivalents | | | 251,301 | | | | 1,156,041 | |
| Weighted-average common shares used in calculation of diluted earnings (loss) per share | | | 16,665,221 | | | | 14,888,310 | |
| | | | | | | | | |
| Net income per share: | | | | | | | | |
| Basic | | $ | 0.44 | | | $ | 0.28 | |
| Diluted | | $ | 0.43 | | | $ | 0.26 | |
6.7. Equity and Share-Based Compensation
Effective January 1, 2006, we adopted the fair value recognition provisions of SFAS No. 123R, Share-Based Payment (“SFAS No. 123R”), for options granted to employees and directors, using the modified prospective transition method, and therefore have not restated results from prior periods. Compensation cost for all stock-based compensation awards granted is based on the grant date fair value estimated in accordance with the provisions of SFAS No. 123R. Under the fair value recognition provisions of SFAS No. 123R, we recognize stock-based compensation net of an estimated forfeiture rate and only recognize compensation cost for those shares expected to vest on a straight-line prorated basis over the requisite service period of the award. In March 2005, the SEC issued Staff Accounting Bulletin (“SAB”) No. 107, Share-Based Payment (“SAB No. 107”), regarding the SEC’s guidance on SFAS No. 123R and the valuation of share-based payments for public companies. We have applied the provisions of SAB No. 107 in the adoption of SFAS No. 123R.
China Sky One Medical, Inc. and Subsidiaries
Notes to Unaudited Condensed Consolidated Financial Statements
6.7. Equity and Share-Based Compensation (Continued)
In July 2006, the Company’s stockholders approved the 2006 Stock Incentive Plan (the “2006 Plan”). The 2006 Plan, provides for the grant of stock options, restricted stock awards, and performance shares to qualified employees, officers, directors, consultants and other service providers. The 2006 Plan originally authorized the Company to grant options and/or rights to purchase up to an aggregate of 1,500,000 shares of common stock. As of March 31, 2009, non-qualified options to purchase a total of 113,500 shares have been granted under the 2006 Stock Incentive Plan. All options were granted in October 2006. All options have an exercise price of $3.65 per share, the weighted fair market value on the date of grant was $4.25 per share. Of these 113,500 options a total of 60,500 were granted to employees and a total of 53,000 were granted to consultants. These options were valued using the Black-Scholes option-pricing model with the following assumptions: no dividends; risk-free interest rate of 4%; a contractual life of 5 years and volatility of 39%. All 113,500 options vest over various periods for the options granted to employees and consultants.
Options or stock awards issued to non-employees and consultants are recorded at their fair value as determined in accordance with SFAS No. 123R and EITF No. 96-18, “Accounting for Equity Instruments That Are Issued to Other Than Employees for Acquiring, or in Conjunction with Selling, Goods or Services”, and recognized over the related vesting or service period. In connection with closing of the Stock Exchange Agreement, the Company agreed to grant warrants to advisors for the services they already performed for the reverse merger in July 2006, entitling them to purchase up to 500,000 shares on or before July 31, 2009, at a price of $2.00 per share and options to purchase up to 50,000 shares on or before December 20, 2008 at a price of $3.00 per share. The fair value of these warrants and options were determined to be $772,275 and deducted as expenses using the Black-Scholes option-pricing model with the following weighted assumptions: no dividends; risk-free interest rate of 4%; a contractual life of 2.5-3.5 years and volatility of 39%. The Company based its estimate of expected volatility on the historical, expected or implied volatility of similar entities whose share or option prices are publicly available .
During the three months ended March 31, 2009, warrant holders exercised 158,334 warrants in total. 150,000 of the warrants were exercised on a cashless basis for a total of 131,949 shares of the Company’s common stock. 8,334 of the warrants were exercised for cash, at an exercise price of $3.50 per share, for total proceeds of $29,169.
7.8. Securities Purchase Agreement and Related Transaction
On January 31, 2008 (the “Closing Date”), the Company entered into a Securities Purchase Agreement (the “Purchase Agreement”) with certain accredited investors (the “Investors”), for the purchase and sale of units consisting of an aggregate of: (i) 2,500,000 shares of the Company’s common stock, and (ii) Class A Warrants to purchase 750,000 additional shares of the Company’s common stock exercisable at $12.50 per share, and expiring on July 31, 2011 (the “Class A Warrants”), for a purchase price of $10.00 per unit (the “Unit Purchase Price”), or gross offering proceeds of $25.0 million (the “2008 Offering”). The Company received net proceeds of approximately $23.5 million in connection with the 2008 Offering.
Pursuant to the Purchase Agreement, among other things, if, and whenever, within twelve (12) months of the Closing Date, the Company issues or sells, or is deemed to have issued or sold, any shares of common stock, or securities convertible into or exercisable for shares of common stock, or modifies any of the foregoing which may be outstanding (with the exception of certain excluded securities), to any person or entity at a price per share, or conversion or exercise price per share less than the Unit Purchase Price, then the Company shall issue, for each such occasion, additional shares of its common stock to the Investors in such number so that the average per share purchase price of the shares of common stock purchased by the Investors in the 2008 Offering shall automatically be reduced to such other lower price per share.
China Sky One Medical, Inc. and Subsidiaries
Notes to Unaudited Condensed Consolidated Financial Statements
7.8. Securities Purchase Agreement and Related Transaction (Continued)
In addition, as of the Closing Date, the Company entered into a Make Good Agreement (the “Make Good Agreement”) with Liu Yan-Qing, its Chairman, Chief Executive Officer and President, and a principal shareholder of the Company, (the “Principal Shareholder”) and the Investors (collectively, the “Make Good Parties”), pursuant to which the Principal Shareholder deposited 3,000,000 shares of his common stock of the Company (the “Escrow Shares”) into escrow, to be released to the Investors in an amount pro rata pro to their initial investments in the 2008 Offering, in the event the Company failed to attain earnings per share, as adjusted, of at least (i) $1.05 per share for the fiscal year ending December 31, 2007 (based on an aggregate of 13,907,696 shares outstanding), and/or (ii) $1.63 per share for the fiscal year ending December 31, 2008 (based on 16,907,696 shares outstanding).
The Company deems the Escrow Shares arrangement as analogous to the issuance of a fixed number of warrants in an equity transaction. Under the Make Good Agreement these Escrow Shares would have been reallocated on a pro rata basis to the Investors only if certain earnings targets were not achieved in years 2007 and 2008. If the earnings targets were met, the Escrow Shares would automatically be released to the Principal Shareholder. As of January 31, 2008, the date the common shares were placed into escrow, the Company achieved the 2007 earnings target and, based upon internal forecasts, was confident the 2008 target would also be met. Based upon certain assumptions, including the low probability that the Escrow Shares would be released to the Investors and not be returned to the Principal Shareholder, the Company considered the fair value of the right held by the Investors through the Escrow Shares provision under the Make Good Agreement to be immaterial. As of December 31, 2008, the Company satisfied the earnings per common share targets for each of fiscal 2007 and 2008 as defined under the Make Good Agreement and, as such, the Escrow Shares shall be released to the Principal Shareholder in 2009.
The Class A Warrants represent the right to purchase an aggregate of 750,000 shares of common stock, at an exercise price of $12.50 per share. Additional information relating to these Class A Warrants is provided in Note 8.
8.9. Outstanding Warrants and Options
| | | Shares Underlying Warrants | | | Weighted average Exercise Price Warrants | | | Shares underlying Options | | | Weighted average Exercise Price Options | |
| Outstanding as of January 1, 2006 | | | 25,000 | | | $ | 1.50 | | | | - | | | | |
| Granted | | | 1,650,000 | | | | 2.58 | | | | 163,500 | | | $ | 3.45 | |
| Exercised | | | - | | | | - | | | | - | | | | - | |
| Expired or cancelled | | | - | | | | - | | | | - | | | | - | |
| Outstanding as of December 31, 2006 | | | 1,675,000 | | | | 2.57 | | | | 163,500 | | | $ | 3.45 | |
| Granted | | | - | | | | - | | | | - | | | | - | |
| Exercised | | | - | | | | - | | | | - | | | | - | |
| Expired or cancelled | | | (161,667 | ) | | | 3.19 | | | | - | | | | - | |
| Outstanding as of December 31, 2007 | | | 1,513,333 | | | $ | 2.48 | | | | 163,500 | | | $ | 3.45 | |
| Granted | | | 750,000 | | | | 12.50 | | | | - | | | | - | |
| Exercised | | | (1,205,002 | ) | | | | | | | (50,000 | ) | | | - | |
| Expired or cancelled | | | - | | | | - | | | | - | | | | - | |
| Outstanding as of December 31, 2008 | | | 1,058,334 | | | $ | 9.50 | | | | 113,500 | | | $ | 3.45 | |
| Granted | | | - | | | | - | | | | - | | | | - | |
| Exercised | | | (158,334 | ) | | | | | | | - | | | | - | |
| Expired or cancelled | | | - | | | | - | | | | - | | | | - | |
| Outstanding as of March 31, 2009 | | | 900,000 | | | $ | 9.50 | | | | 113,500 | | | $ | 3.45 | |
China Sky One Medical, Inc. and Subsidiaries
Notes to Unaudited Condensed Consolidated Financial Statements
8.9. Outstanding Warrants and Options (Continued)
As of January 20, 2009, warrants to purchase 8,334 shares of our common stock, which we issued to “accredited” investors in connection with the private offering we completed in October 2006, were exercised at a price of $3.50 per share, for an aggregate of $29,169.
As of January 22, 2009, warrants to purchase an aggregate of 150,000 shares of our common stock, which we issued to a consultant in consideration for services rendered in connection with the share exchange transaction we consummated in May 2006, were exercised on a cashless basis. In connection with the cashless exercise, the warrant holder was deemed to have paid an amount equal to the difference between the exercise price ($2.00 per share) and the average closing price of a share of our common stock during the ten (10) trading days ending on the date of exercise ($16.62 per share). As a result of such cashless exercise, we issued an aggregate of 131,949 shares of our common stock to the warrant holder. Following this exercise, the warrant holder still held warrants to purchase an additional 150,000 shares of our common stock, at an exercise price of $2.00 per share, which may be exercised in whole, or in part, for cash, or on a cashless basis.
The following table summarizes information about stock warrants outstanding and exercisable as of March 31, 2009.
| | Exercise Price | | Outstanding March 31, 2009 | | | Weighted Average Remaining Life in Years | | | Number exercisable | |
| $ | 2.00 | | | 150,000 | | | | 0.25 | | | | 150,000 | |
| $ | 12.50 | | | 750,000 | | | | 2.25 | | | | 750,000 | |
| | | | | 900,000 | | | | | | | | 900,000 | |
Out of the 900,000 outstanding warrants, all were exercisable as of March 31, 2009. The Class A Warrants represent the right to purchase an aggregate of 750,000 shares of Common Stock of the Company granted with the Securities Purchase Agreement, at an exercise price of $12.50 per share, and have the following additional characteristics:
The Class A Warrants issued in our January 2008 Offering described in Note 8 above, represent the right to purchase an aggregate of 750,000 shares of common stock, at an exercise price of $12.50 per share (the “Exercise Price”), and have the following additional characteristics:
| · | The Class A Warrants became exercisable beginning on the six-month anniversary of the closing of the January 2008 Offering and will expire July 31, 2011. |
| · | Commencing on the one-year anniversary of the date of issuance, in the event the shares underlying the Class A Warrants (the “Warrant Shares”) are not freely sold by the holders due to the Company’s failure to satisfy its registration requirements, and an exemption for such sale is not otherwise available to the Warrant-holders under Rule 144, the Class A Warrants will be exercisable on a cashless basis. There was no issuance of securities during 2008 or the three months ended March 31, 2009 which would have resulted in an adjustment to the Exercise Price and number of Warrant Shares. |
| · | The Exercise Price and number of Warrant Shares are subject to adjustment for standard dilutive events, including the issuance of Common stock, or securities convertible into or exercisable for shares of Common stock, at a price per share, or conversion or exercise price per share less than the Class A Warrant exercise price of $12.50 per share. |
| · | At any time following the date a Registration Statement covering the Warrant Shares is declared effective, the Company will have the ability to call the Class A Warrants at a price of $0.01 per Class A Warrant, upon thirty (30) days prior written notice to the holders of the Class A Warrants, provided (i) the closing price of the Common stock exceeded $18.75 for each of the ten (10) consecutive trading days immediately preceding the date that the call notice is given by the Company, and (ii) the Company has attained earnings per share of at least $1.63 per share (as adjusted pursuant to the terms of the Make Good Agreement describer above) for the fiscal year ending December 31, 2008. |
China Sky One Medical, Inc. and Subsidiaries
Notes to Unaudited Condensed Consolidated Financial Statements
8.9. Outstanding Warrants and Options (Continued)
| · | If, among other things, the Company fails to cause a Registration Statement covering the Warrant Shares to be declared effective prior to the applicable dates set forth in the Registration Rights Agreement, the expiration date of the Class A Warrants shall be extended one day for each day beyond the Effectiveness Deadlines. The Company engaged an independent third-party consultant to calculate the derivative liability resulting from its failure to register the Warrant Shares. The calculation was made using the Black-Scholes model. The liability was deemed to be immaterial as of March 31, 2009. |
The following table summarizes information about stock options outstanding and exercisable as of March 31, 2009.
| | Exercise Price | | Outstanding March 31, 2009 | | | Weighted Average Remaining Life in Years | | | Exercisable Options | | | Unvested Options | |
| $ | 3.65 | | | 113,500 | | | | 2.75 | | | | 101,000 | | | | 12,500 | |
9.10. Inventories
The Company values its inventories at the lower of cost and market method. Inventories are accounted for using the first-in, first-out method. Inventories include packing materials, raw materials, supplemental materials, work-in-process, and finished products.
As of March 31, 2009 and December 31, 2008, inventories consist of the following:
| | | March 31,
2009 | | | December 31, 2008 (Audited) | |
| Raw Material | | $ | 357,872 | | | $ | 330,275 | |
| Work-in-Process | | | 464,328 | | | | 76,462 | |
| Finished Products | | | 498,095 | | | | 55,614 | |
| Total Inventories | | $ | 1,320,295 | | | $ | 462,351 | |
10.11. Property and Equipment
As of March 31, 2009 and December 31, 2008, Property and Equipment, net consist of the following:
| | | March 31,
2009 | | | December 31, 2008 (Audited) | |
| Buildings and improvements | | $ | 9,548,869 | | | | 9,961,820 | |
| Machinery and equipment | | | 5,446,472 | | | | 4,946,247 | |
| Land use rights | | | 1,929,579 | | | | 1,945,209 | |
| Transportation equipment | | | 886,993 | | | | 885,880 | |
| Furniture and equipment | | | 297,456 | | | | 299,467 | |
| Construction in progress (See Note 14) | | | 4,326,340 | | | | 4,317,265 | |
| Total Property and Equipment | | | 22,435,709 | | | | 22,355,888 | |
| Less: Accumulated Depreciation | | | (1,528,635 | ) | | | (1,297,109 | ) |
| Property and Equipment, Net | | $ | 20,907,074 | | | $ | 21,058,779 | |
China Sky One Medical, Inc. and Subsidiaries
Notes to Unaudited Condensed Consolidated Financial Statements
10.11. Property and Equipment (Continued)
For the three months ended March 31, 2009 and 2008, depreciation expense totaled $247,934 and $70,560 respectively. Depreciation expense for cost of goods sold amounted to $136,694 and $39,115 for the three months ended March 31, 2009 and 2008, respectively.
11.12. Intangible Assets
As of March 31, 2009 and December 31, 2008, the Company’s net unamortized intangible assets consist of:
| | | March 31, 2009 | | | December 31, 2008 (Audited) | |
| Patents | | $ | 14,772,399 | | | $ | 15,093,718 | |
| Goodwill | | | 759,099 | | | | 758,047 | |
| Total Intangible Assets, net | | $ | 15,531,498 | | | $ | 15,851,765 | |
Amortization expense for the three months ended March 31, 2009 and 2008 was $340,132 and $69,449, respectively.
12.13. Taxes Payable
Taxes payable consists of the following:
| | | March 31, 2009 | | | December 31, 2008 (Audited) | |
| Value Added Tax, net | | $ | 1,294,150 | | | $ | 1,179,383 | |
| Enterprise Income Tax | | | 1,820,139 | | | | 2,106,956 | |
| City Tax | | | 34,672 | | | | 32,013 | |
| Other Taxes and additions | | | 53,090 | | | | 44,536 | |
| Total Taxes Payable | | $ | 3,202,051 | | | $ | 3,362,888 | |
13.14. Income Taxes
Under the Provisional Regulations of PRC Concerning Income Tax on Enterprises promulgated by the PRC, income tax is payable by enterprises at a rate of 25% of their taxable income. Preferential tax treatment may, however, be granted pursuant to any law or regulations from time to time promulgated by the State Council.
According to “Enterprise Income Tax and Certain Preferential Policies Notice” published by the Ministry of Finance and the National Tax Affairs Bureau, if the enterprise is authorized by the State Council as a special entity, the enterprise income tax rate is reduced to 15%. The income tax rate for TDR, First, and Tianlong is 15%, based on State Council approval. Income tax for Peng Lai is regulated by the local government and has been charged at the rate of 2% of total revenues commencing January 1, 2009. Peng Lai had no business operations during the first quarter of 2008. As a result of the special income tax rates for TDR and Tianlong, the Company’s effective tax rate was approximately 20.1% for the three months ended March 31, 2009. If the Company’s effective tax rate was 25% during the three months ended March 31, 2009, its net income, basic earnings per share and diluted earnings per share would have been $6,797,233, $0.41 and $0.41, respectively. If the Company’s effective tax rate was 25% during the three months ended March 31, 2008, its net income, basic earnings per share and diluted earnings per share would have been $2,898,683, $0.21 and $0.19, respectively.
We record a full valuation allowance to reduce our deferred tax assets to the amount that is more likely than not to be realized. While we have considered future taxable income and ongoing prudent and feasible tax planning strategies in assessing the need for the valuation allowance, in the event we were to determine that we would be able to realize our deferred tax assets in the future in excess of its net recorded amount, an adjustment to the deferred tax asset would increase income in the period such determination was made.
China Sky One Medical, Inc. and Subsidiaries
Notes to Unaudited Condensed Consolidated Financial Statements
13.14. Income Taxes (Continued)
Pursuant to Sections 382 and 383 of the Internal Revenue Code (“IRC”), annual use of the Company’s net operating losses and tax credit carryforwards may be limited because of cumulative changes in ownership of more than 50% that have occurred. As of March 31, 2009, the Company has US net operating loss carryforward’s of approximately $5.0 million which will begin to expire in 2028. Accordingly, as mentioned above, any deferred tax asset that would result from these carryforwards has been fully reserved as of March 31, 2009.
Significant components of the Company’s deferred tax assets are shown below. Nil valuation allowance has been established to offset the deferred tax assets, as realization of such assets is certain per the management valuation.
Provision for the PRC enterprise income tax is calculated at the prevailing rate based on the estimated assessable profits less available tax relief for losses brought forward . The Company does not accrue taxes on unremitted earnings from foreign operations as it is the Company’s intention to invest these earnings in the foreign operations indefinitely .
As of March 31, 2009, the Company has US net operating loss carryforward’s of approximately $5.0 million which will begin to expire in 2028.
As of March 31, 2009, the company has U.S. net operating loss carryforwards of approximately $5.0 million which will begin to expire in 2028. At March 31, 2009, the Company has recorded a full valuation allowance against these net operating loss carryforwards.
In 2006, the Financial Accounting Standards Board (FASB) issued FIN 48, which clarifies the application of SFAS 109 by defining a criterion that an individual income tax position must meet for any part of the benefit of that position to be recognized in an enterprise’s financial statements and provides guidance on measurement, derecognition, classification, accounting for interest and penalties, accounting in interim periods, disclosure and transition. In accordance with the transition provisions, the company adopted FIN 48 effective January 1, 2007.
The Company recognizes that virtually all tax positions in the PRC are not free of some degree of uncertainty due to tax law and policy changes by the state. However, the Company cannot reasonably quantify political risk factors and thus must depend on guidance issued by current state officials.
Based on all known facts and circumstances and current tax law, the company believes that the total amount of unrecognized tax benefits as of March 31, 2009, is not material to its results of operations, financial condition or cash flows. The company also believes that the total amount of unrecognized tax benefits as of March 31, 2009, if recognized, would not have a material effect on its effective tax rate. The Company further believes that there are no tax positions for which it is reasonably possible, based on current Chinese tax law and policy, that the unrecognized tax benefits will significantly increase or decrease over the next 12 months producing, individually or in the aggregate, a material effect on the company’s results of operations, financial position or cash flows.
14.15. Land Use Rights Purchase Agreement and Construction in Progress
During the second quarter in 2007 TDR entered into an agreement with the Development and Construction Administration Committee of Harbin Song Bei New Development district to purchase the land use rights for 50 years for development of a new biotech engineering project. Terms of the agreement called for a deposit of 30% of the total land price within 15 days after signing the agreement, 40% payment 7 days prior to the start of construction and the balance of 30% 7 days after getting the formal land use right.
China Sky One Medical, Inc. and Subsidiaries
Notes to Unaudited Condensed Consolidated Financial Statements
14.15. Land Use Rights Purchase Agreement and Construction in Progress (Continued)
The project consists of two phases:
| (1) | Construction of main workshop, R&D center and office using land area of 30,000 square meters. Construction started in May 2007 and is estimated to be completed by the end of 2009. |
| (2) | Construction of Second workshop and show room using land area of 20,000 square meters. Construction is expected to start in September 2008 to be completed by December 2009. |
TDR has committed to the Development and Construction Administration Committee of the Harbin Song Bei New Development District that the minimum investment per square meter will be $394.
Management plans to use the internal generated funds to fund the project. The estimated additional cost is approximately $5.0 million.
As of March 31, 2009 and December 31, 2008, the Company has deposits totaling $8,523,979 and 8,513,284 respectively, related to the land and construction deposits.
15.16. Commitments and Contingencies
The formulation, manufacturing, processing, packaging, labeling, advertising, distribution and sale of external use Chinese medicine such as those sold by the Company are subject to regulations by one or more federal agencies. The principal federal agencies include the State Food and Drug Administration of the Government of the Peoples Republic of China, the Food and Drug Administration (the “FDA”), Heilongjiang Provincial Food and Drug Administration of the People's Republic of China (PFDA), National Biology Products Inspection Institute (NBPI) and the National Food and Drug Administration (NFDA) of the People's Republic of China and, to a lesser extent, the Consumer Product Safety Commission. These activities are also regulated by various governmental agencies for the countries, states and localities in which the Company’s products are sold.
Although management believes that the Company is in material compliance with the statutes, laws, rules and regulations of every jurisdiction in which it operates, no assurance can be given that the Company’s compliance with the applicable statutes, laws, rules and regulations will not be challenged by governing authorities or private parties, or that such challenges will not lead to material adverse effects on the Company’s financial position, results of operations, or cash flows.
The Company, like any other distributor or manufacturer of products is exposed to the inherent risk of product liability claims in the events of possible injuries caused by the use of its products. The Company does not have liability insurance with respect to product liability claims; the insurance environment of China is neither sufficient nor mature. Inadequate insurance or lack of contractual indemnification from parties supplying raw materials or marketing its products, and product liabilities related to defective products could have a material adverse effects on the Company.
The Company is not involved in any legal matters arising in the normal course of business. While incapable of estimation, in the opinion of the management, the individual regulatory and legal matters in which the Company might be involved in the future are not expected to have a material adverse effect on the Company’s financial position, results of operations, or cash flows.
The rental commitment for the year 2009 is approximately $25,000. The Company is expecting the corporate headquarters currently under construction to be completed by 2009. As a result, there is no rental commitment made by the Company for the year 2010 and thereafter.
Item 2. Management’s Discussion and Analysis of Financial Condition and Results of Operations
FORWARD LOOKING STATEMENTS
The following discussion should be read in conjunction with the information contained in the consolidated financial statements of the Company and the notes thereto appearing elsewhere herein and in the risk factors and “Forward Looking Statements” summary set forth in the forepart of our Annual Report for the year ended December 31, 2008. This quarterly report on Form 10-Q contains forward-looking statements and is afforded the safe harbor provisions of Section 27A of the Securities Act and Section 21E of the Securities Exchange Act of 1934, as amended. Readers should carefully review the risk factors disclosed in our Annual Report for the year ended December 31, 2008 and other documents filed by us with the SEC.
DISCUSSION
We primarily generate revenues, through our China based indirect subsidiaries described below, in the development, manufacture, marketing and sale of over-the-counter, branded nutritional supplements and over-the-counter plant and herb based pharmaceutical and medicinal products. Our principal products are external use Traditional Chinese Herbal Remedies/ Medicines commonly referred to in the industry as “TCM.” We have evolved into an integrated manufacturer, marketer and distributor of external use Chinese medicine products sold primarily in China and through Chinese domestic pharmaceutical chains and have been expanding our worldwide sales effort as well. We sell both our own manufactured products, as well as medicinal and pharmaceutical products manufactured by others in China.
We achieved continuing growth on the sale of both our own product line and a contract service line of manufacturer’s products which we sell through our distribution channel. For the three months ended March 31, 2009, total revenue was $24,833,692, as compared to $12,413,430 for the three months ended March 31, 2008, or a 100% increase over the same period in 2008, and net income was $7,242,838, or $0.43 per share compared to net income of $3,864,911, or $0.26 per share on a diluted basis in the same period in 2008.
All of our business is conducted through our wholly-owned subsidiary, ACPG which, in turn, wholly owns Harbin Tian Di Ren Medical Science and Technology Company (referred to herein as “TDR”) a company organized in the PRC and TDR’s subsidiaries.
TDR, formerly known as “Harbin City Tian Di Ren Medical Co.,” was originally formed in 1994 with its principal executive office in Harbin City of Heilongjiang Province, in the PRC. TDR was reorganized and incorporated as a limited liability company on December 29, 2000, under the “Corporation Laws and Regulations” of the PRC. At the time of the TDR Acquisition by ACPG in December of 2005, TDR had two wholly-owned subsidiaries, Harbin First Bio-Engineering Company Limited and Kangxi Medical Care Product Factory, until July, 2006, when the two were merged, with Harbin First Bio-Engineering Company Limited (“First” or “Harbin Bio Engineering”) as the surviving subsidiary of TDR.
Recent Developments
On April 3, 2008, TDR completed its acquisition of Heilongjiang Tianlong Pharmaceutical, Inc., a corporation with a variety of SFDA approved medicines and new medicine applications, organized under the laws of the PRC (“Tianlong”), which is in the business of manufacturing external-use pharmaceuticals. TDR previously acquired the Beijing sales office of Tianlong in mid-2006. TDR acquired 100% of the issued and outstanding capital stock of Tianlong from its sole stockholder, in consideration for an aggregate purchase price of approximately $8,300,000, consisting of (i) $8,000,000 in cash, and (ii) 23,850 shares of our common stock.
On April 18, 2008, TDR consummated its acquisition of Heilongjiang Haina Pharmaceutical Inc., a recently formed corporation organized under the laws of the PRC (“Haina”) licensed as a wholesaler of TCD, bio-medicines, bio-products, medicinal devices, antibiotics and chemical medicines. Haina did not have an established sales network and was acquired for its primary asset, a Good Supply Practice (GSP) license (License No. A-HLJ03-010), issued by the Heilongjiang province office of the SFDA. The SFDA recently started issuing such licenses to resellers of medicines that maintain certain quality controls. The GSP license was issued as of December 21, 2006 and will expire on January 29, 2012 and will enable us to expand its sales of medicinal products without having to go through a lengthy license application process. TDR acquired 100% of the issued and outstanding capital stock of Haina from its three stockholders in consideration for payment of approximately $437,375. TDR had been overseeing the operations of Haina Pharmaceutical since January of 2008, as part of our due diligence prior to closing of this acquisition.
On September 5, 2008, TDR acquired Peng Lai Jin Chuang Pharmaceutical Company (“Peng Lai”), a corporation organized under the laws of the PRC, from Peng Lai Jin Chuang Group Corporation (the “Seller”). Peng Lai, which has received Good Manufacturing Practice certification from the SFDA, was organized to develop, manufacture and distribute pharmaceutical, medicinal and diagnostic products in the PRC. In connection with the acquisition of Peng Lai, TDR acquired all of Peng Lai’s assets, including, without limitation, franchise, production and operating rights to a portfolio of twenty (20) medicines approved by the SFDA, for an aggregate purchase price of approximately $7.1 million, consisting of (i) approximately $2.5 million in cash, and (ii) 381,606 shares of our common stock.
Testing Kits and Other Products in Production
We have three products: AMI Diagnostic Kit, Human Urinary Albumin Elisa Kit and Early Pregnancy Diagnostic Kit that passed the final stages of national inspection in 2006 or 2007. These diagnostic kits are being sold through drug stores, hospitals, examination stations and independent sales agents throughout the PRC. We also plan to market these products in Vietnam, Indonesia, Philippines and eventually in Africa. Our sales in this product category increased in mid 2008.
Our AMI Diagnostic Kit, which entered markets in 2007, is used for early diagnosis of Myocardial Infarction (MI), also known as heart disease. All the test kits require users to place a blood or urine sample on the marker and a positive (+) or negative (-) reaction signal will result, showing if a user should consult his or her doctor for further testing. According to the China Medical Newspaper, Several million people die from MI every year. MI often occurs to people who are, but not limited to, smokers, over-weight and diabetic. There are approximately 8 million new MI patients in China every year. Recent medical studies have shown that heart failure or heart attacks are increasing among younger people in China. This is a result from a more modern life style, the fast pace of city life and increased pressure from work or school. The use of AMI Diagnostic Kits will help in early detection that can help in reducing these statistics.
We are continuing our marketing efforts with respect to these testing kits which resulted in continued increased sales of these products in 2008.
Summary of Our Research and Development Activities
We currently conduct all of our research and development (“R&D”) activities, either internally or through collaborative arrangements with universities and research institutions in the PRC. We have our own research, development and laboratory facilities located at TDR’s principal executive.
At present, our ongoing research is divided into five general areas:
| · | the development of an enzyme linked immune technique to prepare extraneous diagnostic kits; |
| · | the development of an enzyme linked gold colloid technique to prepare extraneous rapid diagnostic test strip; |
| · | the development of a gene recombination technique to prepare gene drug; |
| · | the development of a biology protein chip for various tumor diagnostic applications; and |
| · | the development of a cord blood stem cell bank, as more fully described in other reports we filed. |
We currently have eight biological products under development: HIV detection kit; a uterus cancer diagnostic kit; a breast cancer diagnostic kit; a liver cancer diagnostic kit; a rectum cancer diagnostic kit; a gastric cancer diagnostic kit; a gene recombination drug; and a multi-tumor marker protein chip detection kit. We are also working to establish additional sales networks and cell banks covering domestic and international markets.
Significant Accounting Estimates and Policies
The discussion and analysis of our financial condition and results of operations is based upon our financial statements which have been prepared in accordance with accounting principles generally accepted in the United States. The preparation of these financial statements requires us to make estimates and judgments that affect the reported amounts of assets and liabilities. On an on-going basis, we evaluate our estimates including the allowance for doubtful accounts and inventories, the salability and recoverability of our products, income taxes and contingencies and remaining useful lives of our tangible and certain intangible assets. We base our estimates on historical experience and on other assumptions that we believes to be reasonable under the circumstances, the results of which form our basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions.
Property and equipment are evaluated for impairment whenever indicators of impairment exist. Accounting standards require that if an impairment indicator is present, we must assess whether the carrying amount of the asset is unrecoverable by estimating the sum of the future cash flows expected to result from the asset, undiscounted and without interest charges. If the recoverable amount is less than the carrying amount, an impairment charge must be recognized based on the fair value of the asset.
As part of the process of preparing our financial statements, we are required to estimate our income taxes. This process involves estimating our current tax exposure together with assessing temporary differences resulting from differing treatment of items for tax and accounting purposes. These differences result in deferred tax assets and liabilities. We must then assess the likelihood that our deferred tax assets will be recovered from future taxable income, and, to the extent we believe that recovery is not likely, we must establish a valuation allowance. To the extent that we establish a valuation allowance or increase this allowance in a period, we must include a tax provision or reduce our tax benefit in the statements of operations. We use our judgment to determine our provision or benefit for income taxes, deferred tax assets and liabilities and any valuation allowance recorded against our net deferred tax assets. We believe, based on a number of factors including historical operating losses, which we will not realize the future benefits of a significant portion of our net deferred tax assets and we have accordingly provided a full valuation allowance against our deferred tax assets. However, various factors may cause those assumptions to change in the near term.
We cannot predict what future laws and regulations might be passed that could have a material effect on our results of operations. We assess the impact of significant changes in laws and regulations on a regular basis and update the assumptions and estimates used to prepare our financial statements when we deem it necessary.
We have determined the significant principles by considering accounting policies that involve the most complex or subjective decisions or assessments. Our most significant accounting policies are those related to intangible assets and research and development.
Intangible assets - Intangible assets consists of patents and goodwill. Patent costs are amortized over an estimated life of ten years.
Intangible assets are accounted for in accordance with Statement of Financial Accounting Standards No. 142, Goodwill and Other Intangible Assets (“SFAS 142”). Intangible assets with finite useful lives are amortized while intangible assets with indefinite useful lives are not amortized. As prescribed by SFAS 142, goodwill and intangible assets are tested periodically for impairment. The Company adopted SFAS No. 144, "Accounting for the Impairment or Disposal of Long- Lived Assets," effective January 1, 2002. Accordingly, the Company reviews its long-lived assets, including property and equipment and finite-lived intangible assets for impairment whenever events or changes in circumstances indicate that the carrying amount of the assets may not be fully recoverable. To determine recoverability of its long-lived assets, the Company evaluates the probability that future undiscounted net cash flows will be less than the carrying amount of the assets. Impairment costs, if any, are measured by comparing the carrying amount of the related assets to their fair value.
Research and development—Research and development expenses include the costs associated with the Company’s internal research and development as well as research and development conducted by third parties. These costs primarily consist of salaries, clinical trials, outside consultants, and materials. All research and development costs are expensed as incurred.
Third-party expenses were reimbursed under non-refundable research and development contracts, and are recorded as a reduction to research and development costs in the statement of operations.
The Company recognizes in-process research and development in accordance with FASB Interpretation No. 4, Applicability of FASB Statement No. 2 to Business Combinations Accounted for by the Purchase Method and the AICPA Technical Practice Aid, Assets Acquired in a Business Combination to be used in Research and Development Activities: A Focus on Software, Electronic Devices, and Pharmaceutical Industries. Assets to be used in research and development activities, specifically, compounds that have yet to receive new drug approval and would have no alternative use, should approval not be given, are immediately charged to expense when acquired. Certain assets and high technologies acquired that has a foreseeable future cash flows are capitalized as intangible assets.
Such intangible assets are amortized starting from the year revenue is generated and amortize over a period of 10 years. Should under any circumstances these capitalized intangible assets are valued with no future benefit, the Company will charge to expense immediately.
For the three months ended March 31, 2009 and 2008, the Company incurred $2,412,780 and $669,833, respectively, in research and development expenditures.
RESULTS OF OPERATIONS
For the three months ended March 31, 2009 as compared to March 31, 2008
Our principal business operations are conducted through our wholly-owned subsidiary, TDR, and TDR’s wholly-owned subsidiaries.
| | | For the Three Months Ended March 3131, | |
| | | 2009 | | | Variance | | | 2008 | |
| REVENUES | | | | | | | | | |
| Product Sales (net of sales allowance) | | $ | 24,833,692 | | | | 162 | % | | $ | 9,467,414 | |
| Contract Sales | | | - | | | | - | % | | | 2,946,016 | |
| Total revenues | | | 24,833,692 | | | | 100 | % | | | 12,413,430 | |
| | | | | | | | | | | | | |
| COST OF GOODS SOLD | | | | | | | | | | | | |
| Cost of goods sold | | | 6,040,918 | | | | 111 | % | | | 2,860,428 | |
| Gross Profit | | $ | 18,792,774 | | | | 97 | % | | $ | 9,553,002 | |
Total revenues increased by 100% during the three months ended March 31, 2009 as compared to 2008. The $12.4 million increase in revenues in 2009 versus 2008 is primarily attributable to strong performances from our sales distribution channels and the results of our several successful business acquisitions in 2008.
Product sales increased by 162% during the three months ended March 31, 2009, to $24,833,692 from $9,467,414 in 2008. This growth in sales is attributable to volume and our efforts to continue to develop our distribution channels by hiring additional direct territory managers and sales agents to assure that our products and their associated benefits are seen by those making or influencing the purchasing decisions.
In the fourth quarter of fiscal 2008, management determined that contract sales were no longer within our strategic plans. Therefore, effective as of January 1, 2009, we discontinued sales of products manufactured by other companies.
Sales by Product Line
A break-down of our sales by product line for each of the three months ended March 31, 2009 and 2008 is as follows:
| | | | | For the Three Months Ended March 31, | |
| | | | | 2009 | | | 2008 | |
| Product | | Subsidiary | | Sales | | | % of Sales | | | Sales | | | % of Sales | |
| Patch | | TDR | | $ | 9,121,961 | | | | 37 | % | | $ | 3,797,989 | | | | 30 | % |
| Ointment | | TDR & TL | | | 5,081,978 | | | | 20 | % | | | 1,487,146 | | | | 12 | % |
| Spray | | TDR & TL | | | 2,902,074 | | | | 12 | % | | | 1,863,371 | | | | 15 | % |
| Bio-Engineering | | First | | | 3,100,775 | | | | 12 | % | | | 1,825,615 | | | | 15 | % |
| Contract Sales | | | | | - | | | | - | | | | 2,946,016 | | | | 24 | % |
| Others | | TDR & TL & PL | | | 4,626,904 | | | | 19 | % | | | 493,293 | | | | 4 | % |
| Total | | | | $ | 24,833,692 | | | | 100 | % | | $ | 12,413,430 | | | | 100 | % |
There were various changes in the break-down of sales among our product lines over the three months ended March 31, 2009 as we continue to develop new products and expand into new markets. As shown in the table above, sales volume for all products increased as compared to the three months ended March 31, 2008. To maintain our competitiveness in the PRC markets, unit selling prices were reduced in 2008. However, the Company waswe were able to negotiate a lower purchase price from our suppliers which negated the decrease in the selling prices of our products.
Our internal growth was driven by increases in the revenues of TDR, which increased from $7,642,000 for the three months ended March 31, 2008 to $14,981,000 for the three months ended March 31, 2009; and First, which increased from $1,826,000 for the three months ended March 31, 2008 to $3,101,000 for the three months ended June 30, 2009.
For the three months ended March 31, 2008, $2,946,016, representing 24% of our total sales, was attributable to Contract Sales. Since early 2009, the Companycontract sales. In 2008, before TDR acquired Tianlong, the majority of our contracts sales consisted of products purchased from Tianlong. In 2008, TDR began to discontinue its contract sales as part of its strategic goals. For the three months ended March 31, 2009, the Companywe did not have any contract sales. Overall, the Company’s product gross margins were at 76% and 77% during the Revenues derived from the sale of a Tianlong product of $4,599,000 for three months ended March 31, 2009 and 2008, respectively.have been reallocated to each of the appropriate product categories to present a more appropriate measure of our revenues by product line.
Prior to our acquisition of Peng Lai, as of September 5, 2008, Peng Lai had nominal production and operations. Peng Lai contributed revenue of $2,153,000 to our total revenue for the three months ended March 31, 2009.
Cost of Goods Sold and Product Gross Margin
| | | For the Three Months ended March 3131, | |
| | | 2009 | | | Variance | | | 2008 | |
| Total revenue | | $ | 24,833,692 | | | | 100 | % | | $ | 12,413,430 | |
| Cost of goods sold | | $ | 6,040,918 | | | | 111 | % | | $ | 2,860,428 | |
| Products gross margin | | | 76 | % | | | | | | | 77 | % |
The decrease in gross margins related to sales of certain TDR’s products due to the competitive of our sales prices in the PRC market.
Operating Expenses
The following table summarizes the changes in our operating expenses from $4,702,976 to $9,685,066 for each of the three months ended March 31, 2008 and 2009, respectively:
| | | For the Three Month ended March 3131, | |
| | | 2009 | | | Variance | | | 2008 | |
| Operating expenses | | | | | | | | | |
| Selling, general and administrative | | $ | 6,877,468 | | | | 74 | % | | $ | 3,956,795 | |
| Depreciation and amortization | | | 451,372 | | | | 491 | % | | | 76,348 | |
| Research and development | | | 2,412,780 | | | | 260 | % | | | 669,833 | |
| Total operating expenses | | $ | 9,741,620 | | | | 107 | % | | $ | 4,702,976 | |
Selling, general and administrative expenses for the three months ended March 31, 2009 increased approximately $2.9 million or 74% over the same period in 2008. The higher selling, general and administrative expenses were primarily attributable to the increased costs of marketing our products from approximately $3.4 million in the three month period ended March 31, 2008, to $6.2 million in the same period in 2009.
Depreciation and amortization expenses in the first quarter of 2009 were $451,372, an increase of $ 375,024 ,375,024, or 491%, from $76,348 in the same period in the prior year. The increase was the result of the three business acquisitions we consummated in fiscal 2008.
Research and development expenses were $2,412,780 in the three months ended March 31, 2009 compared to $669,833 for 2008. The increased R&D expenses of approximately $1.7 million in 2009 versus 2008 were primarily due to additional clinical trials and development of patents to generate continued sales growth.
Liquidity and Capital Resources
The following table summarizes our cash and cash equivalents position, our working capital, and our cash flow activity as of March 31, 2009 and 2008 and for each of the three months then ended:
| | | 2009 | | | 2008 | |
| As of March 31: | | | | | | |
| Cash, cash equivalents and marketable securities | | $ | 48,788,597 | | | $ | 38,237,994 | |
| Working capital | | $ | 65,883,670 | | | $ | 43,563,886 | |
| Inventories | | $ | 1,320,295 | | | $ | 794,730 | |
| Three Months Ended March 31: | | | | | | | | |
| Cash provided by (used in): | | | | | | | | |
| Operating activities | | $ | 8,492,650 | | | $ | 5,197,579 | |
| Investing activities | | $ | (69,799 | ) | | $ | (1,105,700 | ) |
| Financing activities | | $ | 29,169 | | | $ | 24,227,551 | |
As of March 31, 2009, cash and cash equivalents were approximately $48.8 million as compared to $38.2 million at March 31, 2008. The increased cash and cash equivalents position of approximately $10.6 million at March 31, 2009 was primarily due to our cash flows provided by operating activities in 2009 of approximately $8.5 million and reduced investing activities for the purchase of fixed assets and intangible assets.
Our corporate headquarters is expected to be completed in 2009 at an additional estimated cost of approximately $5.0 million. The Company plans to fund our corporate headquarters construction project using internal funds.
The Company’s current ratio was 10.54 versus 9.77 and the quick ratio was 9.11 versus 9.60 at March 31, 2009 and 2008, respectively. Management endeavors to ensure that funds are available to take advantage of new investment opportunities and that funds are sufficient to meet future liquidity and capital needs.
We calculate accounts receivable turnover by averaging the opening and closing balances of out accounts receivable during that period and dividing that amount by our average daily sales during that period. Since accounts receivables fluctuate over the course of each quarter, in order to determine a more representative accountant receivables collection days, management calculates the turnover rate on a quarter-by-quarter basis. Our average daily sales and turnover for each of the three months ended March 31, 2008 and 2009 were as follows:
Quarter Ended | | Average Daily
Sales | | | Turnover
(days) | |
| March 31, 2008 | | $ | 136,441 | | | | 74.5 | |
| March 31, 2009 | | $ | 275,930 | | | | 52.7 | |
Since sales and costs of goods sold fluctuate over the course of each quarter, in order to determine a more representative inventory rate, management calculates inventory rate on a quarter-by-quarter basis. Management calculates our inventory turnover rate using total inventory rather than just finished goods, because our production cycle is of an extremely short duration. Our average inventory and turnover of the three months ended March 31, 2008 and 2009 were as follows:
| | Average
Inventory | | | Turnover
(days) | |
| March 31, 2008 | | $ | 583,201 | | | | 18.6 | |
| March 31, 2009 | | $ | 891,323 | | | | 13.4 | |
At March 31, 2009, there are no restrictive bank deposits pledged as security.
Cash flows provided by operating activities was approximately $8.5 million for the three months ended March 31, 2009, compared to $5.2 million for the same period in 2008. The increase in cash provided by operating activities of approximately $3.3 million is primarily attributable to the increased net income of approximately $7.2 million in the three months ended March 31, 2009 versus $3.9 million in the same period of 2008.
Our working capital position at March 31, 2009 was approximately $65.9 million, compared to $43.6 million at March 31, 2008. Our increased working capital position in 2009 resulted principally from the cash flows generated from our operating activities of approximately $8.5 million in the three months ended March 31, 2009. Management considers current working capital and borrowing capabilities adequate to cover our current operating and capital requirements for the full year 2009.
In the first quarter of fiscal 2008 we received aggregate net proceeds of $23.5 million from the consummation of a private placement of our securities. The net proceeds from the private offering were used to fund three business acquisitions we completed in fiscal 2008 and other working capital needs. There was no similar financing in the three month period ended March 31, 2009.
Currency Exchange Fluctuations
All of Company’s revenues and majority of the expenses during the three months ended March 31, 2009 were denominated primarily in Renminbi (“RMB”), the currency of China, and was converted into US dollars at the exchange rate of 6.84659 RMB to 1 U.S. Dollar. In the third quarter of 2005, the Renminbi began to rise against the US dollar. The exchange rate as of December 31, 2007 and 2008 was 7.3141 and 6.8542 RMB to 1 U.S. Dollar respectively. The exchange rate as of March 31, 2008 was 7.0222 RMB to 1 U.S. Dollar. There could be no assurance that RMB-to-U.S. dollar exchange rates will remain stable. A devaluation of RMB relative to the U.S. dollar would adversely affect our business, financial condition and results of operations. We do not engage in currency hedging.
Off-Balance Sheet Arrangements
We do not have any off-balance sheet arrangements that are currently material or reasonably likely to be material to our financial position or results of operations.
Item 3. Quantitative and Qualitative Disclosures About Market Risk.
We are a smaller reporting company as defined by Rule 12b-2 of the Securities Exchange Act of 1934 and are not required to provide the information under this item.
Item 4. Controls and Procedures.
Disclosure Controls and Procedures
Our management, with the participation of our chief executive officer and interim chief financial officer, evaluated the effectiveness of our disclosure controls and procedures pursuant to Rule 13a-15 under the Securities Exchange Act of 1934 as of March 31, 2009. In designing and evaluating the disclosure controls and procedures, management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving the desired control objectives. In addition, the design of disclosure controls and procedures must reflect the fact that there are resource constraints and that management is required to apply its judgment in evaluating the benefits of possible controls and procedures relative to their costs.
Based on our evaluation, our chief executive officer and interim chief financial officer concluded that our disclosure controls and procedures are designed at a reasonable assurance level and are effective to provide reasonable assurance that information we are required to disclose in reports that we file or submit under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in Securities and Exchange Commission rules and forms, and that such information is accumulated and communicated to our management, including our chief executive officer and interim chief financial officer, as appropriate, to allow timely decisions regarding required disclosure.
Changes in Internal Control Over Financial Reporting
There was no change in our internal control over financial reporting that occurred during our first quarter of fiscal 2009, that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
PART II - OTHER INFORMATION
Item 1. Legal Proceedings.
We are not a party to any material pending legal proceedings.
Item 1A. Risk Factors.
We are a smaller reporting company as defined by Rule 12b-2 of the Securities Exchange Act of 1934 and are not required to provide the information under this item.
Item 2. Unregistered Sales of Equity Securities and Use of Proceeds.
In the three-month period ended March 31, 2009, and subsequent period through the date hereof, we did not engage in any unregistered sales of equity securities other than as set forth below:
As of January 20, 2009, warrants to purchase 8,334 shares of our common stock, which we issued to “accredited” investors in connection with the private offering we completed in October 2006, were exercised at a price of $3.50 per share, for an aggregate of $29,169.
As of January 22, 2009, warrants to purchase an aggregate of 150,000 shares of our common stock, which we issued to a consultant in consideration for services rendered in connection with the share exchange transaction we consummated in May 2006, were exercised on a cashless basis. In connection with the cashless exercise, the warrant holder was deemed to have paid an amount equal to the difference between the exercise price ($2.00 per share) and the average closing price of a share of our common stock during the ten (10) trading days ending on the date of exercise ($16.62 per share). As a result of such cashless exercise, we issued an aggregate of 131,949 shares of our common stock to the warrant holder. Following this exercise, the warrant holder still held warrants to purchase an additional 150,000 shares of our common stock, could be exercised in whole, or in part, for cash, or on a cashless basis.
As of April 29, 2009, the warrant holder referred to in the preceding paragraph exercised his remaining 150,000 warrants to purchase shares of our common stock, on a cashless basis. In connection with the cashless exercise, the warrant holder was deemed to have paid an amount equal to the difference between the exercise price ($2.00 per share) and the average closing price of a share of our common stock during the ten (10) trading days ending on the date of exercise ($14.75 per share). As a result of such cashless exercise, we issued an aggregate of 129,661 shares of our common stock to the warrant holder.
We believe that these transactions are exempt from registration under the Securities Act of 1933, as amended, pursuant to Section 4(2), or Regulation D promulgated thereunder, as transactions by an issuer not involving a public offering.
Item 3. Defaults Upon Senior Securities.
In the three-month period ended March 31, 2009, and subsequent period through the date hereof, we did not default upon any senior securities.
Item 4. Submission of Matters to a Vote of Security Holders.
In the three-month period ended March 31, 2009, and subsequent period through the date hereof, we did not submit any matters to a vote of our stockholders.
Item 5. Other Information.
There was no information we were required to disclose in a report on Form 8-K during the three-month period ended March 31, 2009, or subsequent period through the date hereof, which was not so reported.
| Item 6. | | Exhibits |
| Exhibit No. | | Description of Exhibit |
| 31.1 | | Certification of Principal Executive Officer pursuant to Rule 13a-14 and Rule 15d-14(a), promulgated under the Securities and Exchange Act of 1934, as amended* |
| 31.2 | | Certification of Interim Principal Financial and Accounting Officer pursuant to Rule 13a-14 and Rule 15d-14(a), promulgated under the Securities and Exchange Act of 1934, as amended* |
| 32.1 | | Certification pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 (Principal Executive Officer)* |
| 32.2 | | Certification pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 (Interim Principal Financial and Accounting Officer)* |
| * Filed herewith |
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| | | CHINA SKY ONE MEDICAL, INC. |
| | | |
Dated: May 13, 2009_______________, 2010 | | By: | /s/ Liu Yan Qing |
| | | | Liu Yan Qing President and Chief Executive Officer |
| | | | |
Dated: May 13, 2009_______________, 2010 | | By: | /s/ Liu Yan Qing |
| | | | Stanley Hao Interim Chief Financial Officer |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
AMENDMENT NO. 1
TO
FORM 10-Q
(Mark One)
x | QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
| For the quarterly period ended | June 30, 2009 |
or
o | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
| For the transition period from _______________________________ to ___________________________ |
| |
| Commission File Number: | 000-26059 |
| CHINA SKY ONE MEDICAL, INC. |
| (Exact name of registrant as specified in its charter) |
| Nevada | | 87-0430322 |
| (State or other jurisdiction of incorporation or organization) | | (I.R.S. Employer Identification No.) |
| | | |
Room 1706, Di Wang Building, No. 30 Gan Shui Road, Nangang District, Harbin, People’s Republic of China | | 150001 |
| (Address of principal executive offices) | | (Zip Code) |
| |
86-451-53994069 (China) |
| (Registrant’s telephone number, including area code) |
| |
| (Former name, former address and former fiscal year, if changed since last report) |
Nevada | | 87-0430322 |
(State or other jurisdiction of incorporation or organization) | | (I.R.S. Employer Identification No.) |
| | | |
Room 1706, Di Wang Building, No. 30 Gan Shui Road, Nangang District, Harbin, People’s Republic of China | | 150001 |
(Address of principal executive offices) | | (Zip Code) |
| |
86-451-53994069(China) |
(Registrant’s telephone number, including area code) |
| |
| |
(Former name, former address and former fiscal year, if changed since last report) |
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
x Yes o No
o Yes o No
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filed,” “accelerated filer” and “smaller reporting company in Rule 12b-2 of the Exchange Act.
| | Large accelerated filer o | Accelerated filer o | |
| | | | |
| | Non-accelerated filer o (Do not check if a smaller reporting company) | Smaller reporting company x | |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act).
o Yes x No
The registrant had 16,652,016 shares of Common Stock issued and outstanding as of August 13, 2009.
QUARTERLY REPORT ON FORM 10-Q
OF CHINA SKY ONE MEDICAL, INC. AND SUBSIDIARIES
FOR THE PERIOD ENDED JUNE 30, 2009
TABLE OF CONTENTS
| | | | | | PAGE | |
| | | | | |
| PART I | - | FINANCIAL INFORMATION | | |
| Item 1. | | Financial Statements | | |
| | | Condensed Consolidated Balance Sheets as of June 30, 2009 (unaudited) and December 31, 2008 | | 1 |
| | | Condensed Consolidated Statements of Operations and Comprehensive Income for the Three and Six Months Ended June 30, 2009 and 2008 (unaudited) | | 2 |
| | | Condensed Consolidated Statements of Cash Flows for the Six Months Ended June 30, 2009 and 2008 (unaudited) | | 3 |
| | | Notes to Condensed Consolidated Financial Statements (unaudited) | | 4 |
| Item 2. | | Management’s Discussion and Analysis of Financial Condition and Results of Operations | | 30 |
| Item 3. | | Quantitative and Qualitative Disclosures About Market Risk | | 41 |
| Item 4. | | Controls and Procedures | | 42 |
| PART II | - | OTHER INFORMATION | | |
| Item 1. | | Legal Proceedings | | 43 |
| Item 1A. | | Risk Factors | | 43 |
| Item 2. | | Unregistered Sales of Equity Securities and Use of Proceeds | | 43 |
| Item 3. | | Defaults Upon Senior Securities | | 43 |
| Item 4. | | Submission of Matters to a Vote of Security Holders | | 43 |
| Item 5. | | Other Information | | 44 |
| Item 6. | | Exhibits | | 44 |
| Signatures | | 45 |
PART I. FINANCIAL INFORMATION
Item 1. Financial Statements
China Sky One Medical, Inc. and Subsidiaries
Condensed Consolidated Balance Sheets
| | | June 30, 2009 | | | December 31, 2008 | |
| | | (Unaudited) | | | (Audited) | |
| ASSETS | | | | | | |
| Current Assets | | | | | | |
| Cash and cash equivalents | | $ | 48,233,324 | | | $ | 40,288,116 | |
| Accounts receivable, net | | | 16,172,625 | | | | 14,978,648 | |
| Inventories | | | 1,571,465 | | | | 462,351 | |
| Prepaid and other current assets | | | 76,763 | | | | 106,386 | |
| Land and construction deposit | | | 8,525,025 | | | | 8,513,284 | |
| Total current assets | | | 74,579,202 | | | | 64,348,785 | |
| | | | | | | | | |
| Property and equipment, net | | | 30,553,224 | | | | 21,058,779 | |
| Intangible assets, net | | | 15,193,190 | | | | 15,851,765 | |
| | | $ | 120,325,616 | | | $ | 101,259,329 | |
| | | | | | | | | |
| LIABILITIES AND STOCKHOLDERS' EQUITY | | | | | | | | |
| | | | | | | | | |
| Current Liabilities | | | | | | | | |
| Accounts payable and accrued expenses | | $ | 4,274,858 | | | $ | 2,937,068 | |
| Taxes payable | | | 4,265,075 | | | | 3,362,888 | |
| Deferred revenues | | | - | | | | 26,079 | |
| Total current liabilities | | | 8,539,933 | | | | 6,326,035 | |
| | | | | | | | | |
| Commitments and Contingencies | | | - | | | | - | |
| | | | | | | | | |
| Stockholders' Equity | | | | | | | | |
| Preferred stock ($0.001 par value, 5,000,000 shares authorized, none issued and outstanding) | | | - | | | | - | |
| Common stock ($0.001 par value, 50,000,000 shares authorized, 16,652,016 and 16,306,184 issued and outstanding, respectively) | | | 16,652 | | | | 16,306 | |
| Additional paid-in capital | | | 40,133,957 | | | | 40,105,134 | |
| Accumulated other comprehensive income | | �� | 5,690,195 | | | | 5,566,806 | |
| Retained earnings | | | 65,944,879 | | | | 49,245,048 | |
| Total stockholders' equity | | | 111,785,683 | | | | 94,933,294 | |
| | | $ | 120,325,616 | | | $ | 101,259,329 | |
See accompanying summary of accounting policies and notes to the condensed consolidated financial statements.
China Sky One Medical, Inc. and Subsidiaries
Condensed Consolidated Statements of Operations and Comprehensive Income
(Unaudited)
| | | Three Months Ended June 30, | | | Six Months Ended June 30, | |
| | | 2009 | | | 2008 | | | 2009 | | | 2008 | |
| Revenues | | $ | 32,181,590 | | | $ | 23,748,592 | | | $ | 57,015,282 | | | $ | 36,162,022 | |
| Cost of Goods Sold | | | 7,752,371 | | | | 5,522,314 | | | | 13,793,289 | | | | 8,382,742 | |
| Gross Profit | | | 24,429,219 | | | | 18,226,278 | | | | 43,221,993 | | | | 27,779,280 | |
| | | | | | | | | | | | | | | | | |
| Operating Expenses | | | | | | | | | | | | | | | | |
| Depreciation and amortization | | | 449,294 | | | | 139,004 | | | | 900,666 | | | | 215,352 | |
| Research and development | | | 3,681,914 | | | | 1,372,579 | | | | 6,094,694 | | | | 2,042,412 | |
| Selling, general and administrative | | | 8,216,348 | | | | 6,587,059 | | | | 15,093,816 | | | | 10,543,854 | |
| Total operating expenses | | | 12,347,556 | | | | 8,098,642 | | | | 22,089,176 | | | | 12,801,618 | |
| | | | | | | | | | | | | | | | | |
| Other Income (Expense) | | | | | | | | | | | | | | | | |
| Other income (expense) | | | (1,918 | ) | | | (35,539 | ) | | | (1,128 | ) | | | 27,509 | |
| Interest income (expense), net | | | 15,921 | | | | ( 132,495 | ) | | | 26,954 | | | | ( 133,642 | ) |
| Total other income (expense) | | | 14,003 | | | | ( 168,034 | ) | | | 25,826 | | | | ( 106,133 | ) |
| | | | | | | | | | | | | | | | | |
| Net Income Before Provision for Income Tax | | | 12,095,666 | | | | 9,959,602 | | | | 21,158,643 | | | | 14,871,529 | |
| | | | | | | | | | | | | | | | | |
| Provision for Income Taxes | | | | | | | | | | | | | | | | |
| Current | | | 2,638,673 | | | | 1,848,935 | | | | 4,458,812 | | | | 2,895,951 | |
| | | | | | | | | | | | | | | | | |
| Net Income | | $ | 9,456,993 | | | $ | 8,110,667 | | | $ | 16,699,831 | | | $ | 11,975,578 | |
| | | | | | | | | | | | | | | | | |
| Basic Earnings Per Share | | $ | 0.57 | | | $ | 0.54 | | | $ | 1.01 | | | $ | 0.84 | |
| | | | | | | | | | | | | | | | | |
| Basic Weighted Average Shares Outstanding | | | 16,537,066 | | | | 14,971,652 | | | | 16,475,833 | | | | 14,253,547 | |
| | | | | | | | | | | | | | | | | |
| Diluted Earnings Per Share | | $ | 0.57 | | | $ | 0.50 | | | $ | 1.01 | | | $ | 0.78 | |
| | | | | | | | | | | | | | | | | |
| Diluted Weighted Average Shares Outstanding | | | 16,628,892 | | | | 16,090,211 | | | | 16,547,037 | | | | 15,372,106 | |
| | | | | | | | | | | | | | | | | |
| Comprehensive Income | | | | | | | | | | | | | | | | |
| Net Income | | $ | 9,456,993 | | | $ | 8,110,667 | | | $ | 16,699,831 | | | $ | 11,975,578 | |
| Foreign currency translation adjustment | | | 6,447 | | | | 1,507,587 | | | | 123,389 | | | | 3,155,780 | |
| | | | | | | | | | | | | | | | | |
| Comprehensive Income | | $ | 9,463,440 | | | $ | 9,618,254 | | | $ | 16,823,220 | | | $ | 15,131,358 | |
See accompanying summary of accounting policies and notes to the condensed consolidated financial statements.
China Sky One Medical, Inc. and Subsidiaries
Condensed Consolidated Statements of Cash Flows
(Unaudited)
| | | Six Months Ended June 30, | |
| | | 2009 | | | 2008 | |
| Cash flows from operating activities | | | | | | |
| Net Income | | $ | 16,699,831 | | | $ | 11,975,578 | |
| Adjustments to reconcile net cash provided by operating activities | | | | | | | | |
| Depreciation and amortization | | | 1,168,601 | | | | 352,833 | |
| Share-based compensation expense | | | - | | | | 20,234 | |
| Net change in assets and liabilities | | | | | | | | |
| Accounts receivables | | | (1,184,343 | ) | | | 1,972,239 | |
| Inventories | | | (1,108,724 | ) | | | (807,993 | ) |
| Prepaid expenses and other current assets | | | 40,536 | | | | (14,607 | ) |
| Accounts payable and accrued expenses | | | 1,307,906 | | | | 2,474,459 | |
| Taxes payable | | | 897,750 | | | | 1,872,324 | |
| Deferred revenues | | | - | | | | (24,504 | ) |
| Net cash provided by operating activities | | | 17,821,557 | | | | 17,820,563 | |
| | | | | | | | | |
| Cash flows from investing activities | | | | | | | | |
| Purchase of fixed assets | | | (83,687 | ) | | | (667,432 | ) |
| Purchase of subsidiary-TianLong and Haina | | | - | | | | (8,437,375 | ) |
| Purchase of construction in progress | | | (9,877,830 | ) | | | - | |
| Purchase of intangible assets | | | - | | | | (7,139 | ) |
| Net cash used in investing activities | | | (9,961,517 | ) | | | (9,111,946 | ) |
| | | | | | | | | |
| Cash flows from financing activities | | | | | | | | |
| Sale of common stock for cash, net of offering costs | | | - | | | | 23,487,963 | |
| Proceeds from warrants conversion | | | 29,169 | | | | 840,000 | |
| Net cash provided by financing activities | | | 29,169 | | | | 24,327,963 | |
| | | | | | | | | |
| Effect of exchange rate changes on cash | | | 55,997 | | | | 303,955 | |
| | | | | | | | | |
| Net increase in cash and cash equivalents | | | 7,945,206 | | | | 33,340,535 | |
| | | | | | | | | |
| Cash and cash equivalents at beginning of period | | | 40,288,117 | | | | 9,190,870 | |
| | | | | | | | | |
| Cash and cash equivalents at end of period | | $ | 48,233,324 | | | $ | 42,531,405 | |
| | | | | | | | | |
| Supplemental disclosure of cash flow information | | | | | | | | |
| Interest paid | | $ | - | | | $ | 1,157 | |
| Taxes paid | | $ | 3,928,870 | | | $ | 6,366,350 | |
See accompanying summary of accounting policies and notes to the condensed consolidated financial statements.
China Sky One Medical, Inc. and Subsidiaries
Notes to Unaudited Condensed Consolidated Financial Statements
| 1. | Description of Business |
The accompanying unaudited consolidated financial statements of China Sky One Medical, Inc., a Nevada corporation, and subsidiaries have been prepared in accordance with generally accepted accounting principles ("GAAP") for interim financial information and with the instructions to Form 10-Q and Article 10 of Regulation S-X. Accordingly, they do not include all of the information and notes required by U.S. GAAP for complete financial statements. The financial statements for the periods ended June 30, 2009 and 2008 are unaudited and include all adjustments necessary for a fair statement of the results of operations for the periods then ended. All such adjustments are of a normal recurring nature. The results of the company's operations for any interim period are not necessarily indicative of the results of the company's operations for a full fiscal year. For further information, refer to the financial statements and notes thereto included in the company's annual report on Form 10-K for the year ended December 31, 2008 as filed with the Securities and Exchange Commission ("SEC") on April 15, 2009.
China Sky One Medical, ("China Sky One" or the “Company”), a Nevada corporation, was formed on February 7, 1986, and formerly known as Comet Technologies, Inc. (“Comet”). On July 26, 2006, the Company changed the name of the reporting company from "Comet Technologies, Inc." to "China Sky One Medical, Inc."
American California Pharmaceutical Group, Inc. (“ACPG”), our non operating United States holding company subsidiary, was incorporated on December 16, 2003, in the State of California, under the name “QQ Group, Inc.” QQ Group, Inc. changed its name to “American California Pharmaceutical Group, Inc.” in anticipation of the Stock Exchange Agreement with China Sky One (then known as “Comet Technologies, Inc.”) and TDR, described herein. On December 8, 2005, ACPG completed a stock exchange transaction with Harbin Tian Di Ren Medical Science and Technology Company (“TDR”), a company organized under the laws of the People’s Republic of China (“PRC”) and TDR’s subsidiaries (the “TDR Acquisition”), each of which were fully operating companies in the PRC. Under the terms of the agreement, ACPG exchanged 100% of its issued and outstanding common stock for 100% of the capital stock of TDR and its subsidiaries, described below.
Thereafter, on May 11, 2006, ACPG entered into a Stock Exchange Agreement (the “Exchange Agreement”) with the shareholders of China Sky One. The terms of the Exchange Agreement were consummated and the acquisition was completed on May 30, 2006. As a result of the transaction, the Company issued a total of 10,193,377 shares of its common stock to the stockholders of ACPG, in exchange for 100% of the capital stock of ACPG resulting in ACPG becoming our wholly-owned subsidiary. The transaction was treated as a reverse merger for accounting purposes.
TDR, formerly known as “Harbin City Tian Di Ren Medical Co.,” was originally formed in 1994 and maintained its principal executive office in Harbin City of Heilongjiang Province, in the PRC. TDR was reorganized and incorporated as a limited liability company on December 29, 2000, under the “Corporation Laws and Regulations” of the PRC. At the time of the TDR Acquisition by ACPG in December of 2005, TDR had two wholly-owned subsidiaries, Harbin First Bio-Engineering Company Limited and Kangxi Medical Care Product Factory, until July, 2006, when the two were merged, with Harbin First Bio-Engineering Company Limited (“First”) as the surviving subsidiary of TDR. The principal activities of TDR and First are the research, manufacture and sale of over-the-counter non-prescription health care products. TDR commenced its business in the sale of branded nutritional supplements and over-the-counter pharmaceutical products in the Heilongjiang Province. TDR has subsequently evolved into an integrated manufacturer, marketer, and distributor of external use natural Chinese medicine products sold primarily to and through China’s various domestic pharmaceutical chain stores.
China Sky One is a holding company whose principal operations are through its wholly-owned subsidiaries; it has no revenues separate from its subsidiaries, and has nominal expenses related to its status as a public reporting company and to its ownership interest in ACPG and TDR.
China Sky One Medical, Inc. and Subsidiaries
Notes to Unaudited Condensed Consolidated Financial Statements
| 1. | Description of Business (Continued) |
On September 30, 2008 (the “Record Date”), we obtained the written consent of the holders of 8,158,251 shares of our common stock, which as of the Record Date, represented 51.3% of our outstanding voting securities, to increase our number of authorized shares of common stock from twenty million (20,000,000) to fifty million (50,000,000) shares.
| 2. | Acquisition of Businesses |
On April 3, 2008, TDR completed an acquisition pursuant to an Equity Transfer Agreement dated February 22, 2008, between TDR and Heilongjiang Tianlong Pharmaceutical, Inc., a corporation with a multitude of medicines approved by China’s State Food and Drug Administration (“SFDA”) and new medicine applications, organized under the laws of the PRC (“TianLong”), which is in the business of manufacturing external-use pharmaceuticals. Our TDR subsidiary previously acquired the Beijing sales office of TianLong in mid 2006. Pursuant to the Equity Transfer Agreement, TDR acquired 100% of the issued and outstanding capital stock of TianLong from its sole stockholder Wu Jiechen, a resident of China, in consideration for an aggregate purchase price of approximately $8,300,000, consisting of (i) $8,000,000 in cash, and (ii) 23,850 shares of China Sky One’s common stock (valued at $12 per share or approximately $286,000). The acquisition received regulatory approval and closed on April 3, 2008.
The following table summarizes the approximate estimated fair values of the assets acquired in the TianLong acquisition.
| Fixed assets | | $ | 6,314,871 | |
| Intangible assets | | | 1,786,990 | |
| Other | | | 170,000 | |
| Net assets acquired | | $ | 8,271,861 | |
On April 18, 2008, China Sky One, through its subsidiary TDR, consummated a share acquisition pursuant to an Equity Transfer Agreement with the shareholders of Heilongjiang Haina Pharmaceutical Inc., a recently formed corporation organized under the laws of the PRC (“Haina”) licensed as a wholesaler of Traditional Chinese Herbal Remedies/Medicines “TCM”, bio-medicines, bio-products, medicinal devices, antibiotics and chemical medicines. Haina does not have an established sales network and was acquired for its primary asset, a Good Supply Practice (GSP) license (License No. A-HLJ03-010) issued by the Heilongjiang office of the State Food and Drug Administration (SFDA). The SFDA recently started issuing such licenses to resellers of medicines that maintain certain quality controls. The GSP license was issued as of December 21, 2006 and will expire on January 29, 2012 and will enable the Company to expand its sales of medicinal products without having to go through a lengthy license application process.
The following table summarizes the approximate estimated fair values of the assets acquired in the Haina acquisition.
| Cash | | $ | 84,307 | |
| Intangible assets | | $ | 437,375 353,068 | |
| Net assets acquired | | $ | 437,375 | |
Pursuant to the Equity Transfer Agreement, TDR acquired 100% of the issued and outstanding capital stock of Haina from its three stockholders in consideration for payment of 3,000,000 RMB (approximately $437,000). TDR has been overseeing the operations of Haina since January of 2008 as part of its due diligence prior to closing of this acquisition.
China Sky One Medical, Inc. and Subsidiaries
Notes to Unaudited Condensed Consolidated Financial Statements
2. | Acquisition of Businesses (Continued) |
On June 9, 2008, TDR entered into a Merger and Acquisition Agreement (the “Acquisition Agreement”) with Peng Lai Jin Chuang Company, a corporation organized under the laws of the PRC (“Peng Lai”), which was organized to develop, manufacture and distribute pharmaceutical, medicinal and diagnostic products in the PRC. Pursuant to the Acquisition Agreement, TDR acquired all of the assets of Peng Lai in consideration for an aggregate of approximately (i) US$2.5 million in cash, and (ii) 381,606 shares of the Company’s common stock with a fair value of approximately $4.6 million (value at $12 per share). The acquisition of Peng Lai closed on September 5, 2008.
The following table summarizes the approximate estimated fair values of the assets acquired in the Peng Lai acquisition.
| Fixed assets | | $ | 4,176,922 | |
| Intangible assets | | | 2,917,386 | |
| Net assets acquired | | $ | 7,094,308 | |
China Sky One Medical, Inc. and Subsidiaries
Notes to Unaudited Condensed Consolidated Financial Statements
| 2. | Acquisition of Businesses (Continued) |
The following table contains pro forma condensed consolidated statements of operations information assuming the TianLong, Haina and Peng Lai transactions closed on January 1, 2008 for the six months ended June 30, 2008. Peng Lai had dormant operations until October 2008.
| | | Six Months Ended June 30, 2008 | |
| Revenue | | $ | 36,723,407 | |
| Operating income | | $ | 15,065,026 | |
| Net income | | $ | 12,052,245 | |
| Basic earnings per common share | | $ | 0.85 | |
| Basic weighted average shares outstanding | | | 14,253,547 | |
| Diluted earnings per common share | | $ | 0.78 | |
| Diluted weighted average shares outstanding | | | 15,372,106 | |
| 3. | Summary of Significant Accounting Policies |
Principles of Consolidation – The accompanying consolidated financial statements include the accounts of the Company and its wholly-owned subsidiaries, ACPG, TDR, First, Haina, TianLong, and Peng Lai. All significant inter-company transactions and balances were eliminated.
These financial statements are stated in U.S. Dollars and have been prepared in accordance with accounting principles generally accepted in the United States of America. This basis of accounting differs from that used under applicable accounting requirements in the PRC. No material adjustment was required.
Certain items in the 2008 financial statements have been reclassified to conform with the 2009 financial statements presentation.
Use of estimates – The preparation of these financial statements in conformity with accounting principles generally accepted in the United States of America, requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, disclosure of contingent assets and liabilities at the dates of the financial statements, and the reported amounts of revenues and expenses during the reported periods.
Significant estimates include assigned lives to tangible and intangible assets, reserves for customer returns and allowances, uncollectible accounts receivable, and impairment testing of tangible and intangible assets. Actual results may differ from these estimates.
China Sky One Medical, Inc. and Subsidiaries
Notes to Unaudited Condensed Consolidated Financial Statements
| 3. | Summary of Significant Accounting Policies (Continued) |
Earnings per share - Basic earnings per common share is computed by dividing net earnings applicable to common shareholders by the weighted-average number of common shares outstanding during the period. When applicable, diluted earnings per common share is determined using the weighted-average number of common shares outstanding during the period, adjusted for the dilutive effect of common stock equivalents, consisting of shares that might be issued upon exercise of common stock options and warrants.
Potential common shares issued are calculated using the treasury stock method, which recognizes the use of proceeds that could be obtained upon the exercise of options and warrants in computing diluted earnings per share. It assumes that such proceeds would be used to purchase common stock at the average market price of the common stock during the period.
Cash and cash equivalents – The Company considers all highly liquid debt instruments purchased with maturity period of sixthree months or less to be cash equivalents. The carrying amounts reported in the accompanying consolidated balance sheets for cash and cash equivalents approximate their fair value.
A significant amount of our cash and cash equivalents are held in China’s commercial bank checking accounts and earned an annual interest income yield of approximately 0.36% for the six months ended June 30, 2009. For all the bank accounts in China and overseas, the Company earned interests of approximately $26,000 and $79,000 for the six months ended June 30, 2009 and 2008, respectively.
Accounts receivable – Accounts receivable are stated at net realizable value, net of an allowance for doubtful accounts. The allowance for estimated bad debts is based upon the periodic analysis of individual customer balances including an evaluation of days of sales outstanding, payment history, recent payment trends, and perceived credit worthiness. At June 30, 2009 and December 31, 2008, the Company’s allowance for doubtful accounts was $50,000.
Inventories – Inventories include finished goods, raw materials, freight-in, packing materials, labor, and overhead costs and are valued at the lower of cost or market using the first-in, first-out method. Inventory units are valued using the weighted average method. Provisions are made for slow moving, obsolete and/or damaged inventory based upon the periodic analysis of individual inventory items including an evaluation of historical usage and/or movement, age, expiration date, and general conditions. There was no inventory reserve provision recorded at June 30, 2009 and December 31, 2008.
Property and equipment – Property and equipment are stated at historical cost less accumulated depreciation. Depreciation on property and equipment is provided using the straight-line method over the estimated useful lives of the assets. The Company uses an estimated residual value of 5% of cost, or valuation for both financial and income tax reporting purposes. The estimated lengths of useful lives are as follows:
| Building and Improvements | 30 years |
| Land use rights | 50 years |
| Furniture & Equipment | 5 to 7 years |
| Transportation Equipment | 5 to 15 years |
| Machinery and Equipment | 7 to 14 years |
Expenditures for renewals and betterments are capitalized while repairs and maintenance costs are normally charged to the statement of operations in the year in which they were incurred. In situations where it can be clearly demonstrated that the expenditure has resulted in an increase in the future economic benefits expected to be obtained from the use of the asset, the expenditure is capitalized as an additional cost of the asset. Upon sale or disposal of an asset, the historical cost and related accumulated depreciation or amortization of such asset is removed from their respective accounts, and any gain or loss is recorded in the consolidated statements of operations.
China Sky One Medical, Inc. and Subsidiaries
Notes to Unaudited Condensed Consolidated Financial Statements
| 3. | Summary of Significant Accounting Policies (Continued) |
Property and equipment are evaluated for impairment in value whenever an event or change in circumstances indicates that the carrying values may not be recoverable. If such an event or change in circumstances occurs and potential impairment is indicated because the carrying values exceed the estimated future undiscounted cash flows of the asset, the Company will measure the impairment loss as the amount by which the carrying value of the asset exceeds its fair value. The Company did not record any impairment charges during the three and six months ended June 30, 2009 and 2008.
Construction-in-progress – Properties currently under development are accounted for as construction-in-progress. Construction-in-progress is recorded at acquisition cost, including land rights cost, development expenditures, professional fees, and capitalized interest costs during the course of construction.
Upon completion and readiness for use of the project, the cost of construction-in-progress is transferred to the facility. In the case of construction-in-progress, management takes into consideration the estimated cost to complete the project when making the lower of cost or market calculation (see Note 14).
Intangible assets – Intangible assets consists of patents and goodwill. Patent costs are amortized over an estimated life of ten years.
Intangible assets are accounted for in accordance with Statement of Financial Accounting Standards No. 142, Goodwill and Other Intangible Assets (“SFAS 142”). Intangible assets with finite useful lives are amortized while intangible assets with indefinite useful lives are not amortized. As prescribed by SFAS 142, goodwill and intangible assets are tested periodically for impairment. The Company adopted SFAS No. 144, "Accounting for the Impairment or Disposal of Long- Lived Assets," effective January 1, 2002. Accordingly, the Company reviews its long-lived assets, including property and equipment and finite-lived intangible assets for impairment whenever events or changes in circumstances indicate that the carrying amount of the assets may not be fully recoverable. To determine recoverability of its long-lived assets, the Company evaluates the probability that future undiscounted net cash flows will be less than the carrying amount of the assets. Impairment costs, if any, are measured by comparing the carrying amount of the related assets to their fair value. The Company recognizes an impairment loss based on the excess of the carrying amount of the assets over their respective fair values. Fair value is determined by discounted future cash flows, appraisals or other methods. If the assets determined to be impaired are to be held and used, the Company recognizes an impairment loss thru a charge to operating results to the extent the present value of anticipated cash flows attributable to the assets are less than the asset’s carrying value. The Company would depreciate the remaining value over the remaining estimated useful life of the asset to operating results. The Company did not record any impairment charges during the three and six months ended June 30, 2009 and 2008.
Foreign Currency - The Company’s principal country of operations is in the PRC. The financial position and results of operations of the Company are recorded in RMB as the functional currency. The results of operations denominated in foreign currency are translated at the average rate of exchange during the reporting period.
Assets and liabilities denominated in foreign currencies at the balance sheet date are translated at the market rate of exchange at that date. The registered equity capital denominated in the functional currency is translated at the historical rate of exchange at the time of the capital contribution. All translation adjustments resulting from the translation of the financial statements into the reporting currency (“US Dollars”) are recorded as accumulated other comprehensive income, a component of stockholders’ equity.
China Sky One Medical, Inc. and Subsidiaries
Notes to Unaudited Condensed Consolidated Financial Statements
3. | Summary of Significant Accounting Policies (Continued) |
Revenue recognition - Revenue is recognized when the following criteria are met: (1) persuasive evidence of an arrangement exists; (2) the product has been shipped and the customer takes ownership and assumes the risk of loss; (3) the selling price is fixed or determinable; and (4) collection of the resulting receivable is reasonably assured. The Company believes that all of these criteria are satisfied upon shipment from its facilities. Revenue is reduced by provisions for estimated returns and allowances as well as specific known claims, if any , which are based on historical trends that have not varied significantly for the periods presented. Historically, the Company’s estimated returns, allowances and claims have been deemed immaterial. The Company’s sale agreements only allow a return if the product has quality related issues. In such event, the Company accepts the return for equivalent product exchange from inventory only.
The Company occasionally applies to various government agencies for research grants. Revenue from such research grants is recognized when earned. In situations where the Company receives payment in advance for the performance of research and development services, such amounts are deferred and recognized as revenue as the related services are performed.
Deferred revenues - The Company recognizes revenues as earned. Amounts billed in advance of the period in which goods are delivered are recorded as a liability under “Deferred revenues.”
China Sky One Medical, Inc. and Subsidiaries
Notes to Unaudited Condensed Consolidated Financial Statements
| 3. | Summary of Significant Accounting Policies (Continued) |
Research and development - Research and development expenses include the costs associated with the Company’s internal research and development as well as research and development conducted by third parties. These costs primarily consist of salaries, clinical trials, outside consultants, and materials. All research and development costs are expensed as incurred.
Third-party expenses reimbursed under non-refundable research and development contracts are recorded as a reduction to research and development expense in the consolidated statement of operations.
The Company recognizes in-process research and development in accordance with Financial Accounting Standard Board (“FASB”) Interpretation No. 4, Applicability of FASB Statement No. 2 to Business Combinations Accounted for by the Purchase Method and the AICPA Technical Practice Aid, Assets Acquired in a Business Combination to be used in Research and Development Activities: A Focus on Software, Electronic Devices, and Pharmaceutical Industries. Assets to be used in research and development activities, specifically, compounds that have yet to receive new drug approval and would have no alternative use, should approval not be given, are immediately charged to expense when acquired. Certain assets and other technologies acquired that has foreseeable future cash flows are capitalized as intangible assets. Such intangible assets are amortized starting from the year revenue is generated and amortized over a period of 10 years. Should under any circumstances these capitalized intangible assets have no future benefit, the Company will record an immediate write-off for the remaining net carrying value within the consolidated statement of operations.
The Company incurred $3,681,914, $6,094,694, $1,372,579 and $2,042,412 in research and development costs for the three and six months ended June 30, 2009 and 2008, respectively.
Advertising – Advertising and promotion costs are expensed as incurred.Generally, the Company signs contracts with agents who then place its advertising in the mediums of television, radio and internet. Advertising expense is incurred in the period the advertisements take place. Thus, costs of advertising are expenses as incurred, in compliance with FASB Concept Statement #6 paragraph 81 and ASC 720-35-05-01. Historically, the Company’s direct-response advertising costs have been immaterial. Total advertising costs for the three and six months ended June 30, 2009 and 2008 was $3,437,828, $6,213,958, $2,796,123 and $3,165,426, respectively. Advertising costs are reported as part of selling, general and administrative expenses in the statements of operations.
Taxation – The Company uses the asset and liability method of accounting for deferred income taxes. The Company’s provision for income taxes includes income taxes currently payable and those deferred because of temporary differences between the financial statement and tax bases of assets and liabilities. The Company records liabilities for income tax contingencies based on our best estimate of the underlying exposures.
China Sky One Medical, Inc. and Subsidiaries
Notes to Unaudited Condensed Consolidated Financial Statements
3. | Summary of Significant Accounting Policies (Continued) |
The Company periodically estimates its tax obligations using historical experience in tax jurisdictions and informed judgments. There are inherent uncertainties related to the interpretation of tax regulations in the jurisdictions in which the Company transacts business. The judgments and estimates made at a point in time may change based on the outcome of tax audits, as well as changes to, or further interpretations of, regulations. The Company adjusts income tax expense in the period in which these events occur.
Provision for the PRC enterprise income tax is calculated at the prevailing rate based on the estimated assessable profits less available tax relief for losses brought forward. The Company does not accrue taxes on unremitted earnings from foreign operations as it is the Company’s intention to invest these earnings in the foreign operations indefinitely ..
Enterprise income tax
Under the Provisional Regulations of PRC Concerning Income Tax on Enterprises promulgated by the PRC, income tax is payable by enterprises at a rate of 25% of their taxable income. Preferential tax treatment may, however, be granted pursuant to any law or regulations from time to time promulgated by the State Council.
According to “Enterprise Income Tax and Certain Preferential Policies Notice” published by the Ministry of Finance and the National Tax Affairs Bureau, if the enterprise is authorized by the State Council as a special entity, the enterprise income tax rate is reduced to 15%. In 2009, the income tax rate for TDR, TianLong, and First is 15% based on State Council approval. The income tax rate for Haina is 25%. The income tax rate for Peng Lai is regulated by local government at 2% of total revenue commencing January 1, 2009.
China Sky One Medical, Inc. and Subsidiaries
Notes to Unaudited Condensed Consolidated Financial Statements
| 3. | Summary of Significant Accounting Policies (Continued) |
In 2008, the income tax rate for TDR and TianLong was 15% and 12%, respectively. The income tax rate for First, Haina, and Peng Lai was 25%.
Value added tax
The Provisional Regulations of PRC Concerning Value Added Tax promulgated by the State Council came into effect on January 1, 1994. Under these regulations and the Implementing Rules of the Provisional Regulations of the PRC Concerning Value Added Tax, value added tax is imposed on goods sold in, or imported into, the PRC and on processing, repair and replacement services provided within the PRC.
Value added tax payable in the PRC is charged on an aggregated basis at a rate of 13% or 17% (depending on the type of goods involved) on the full price collected for the goods sold or, in the case of taxable services provided, at a rate of 17% on the charges for the taxable services provided, but excluding, in respect of both goods and services, any amount paid in respect of value added tax included in the price or charges, and less any deductible value added tax already paid by the taxpayer on purchases of goods and services in the same financial year.
According to “Agriculture Product Value Added Tax Rate Adjustment and Certain Items’ Value Added Tax Waiver” published by the Ministry of Finance and the National Tax Affairs Bureau, the value added tax for agriculture related products is to be taxed at 13%. Furthermore, traditional Chinese medicine and medicinal plant are by definition agriculture related products.
Accounting for uncertainty in income taxes – In June 2006, the FASB issued Interpretation No. 48, Accounting for Uncertainty in Income Taxes - an Interpretation of FASB Statement No. 109 (FIN 48). FIN 48 is intended to clarify the accounting for uncertainty in income taxes recognized in a company’s financial statements and prescribes the recognition and measurement of a tax position taken or expected to be taken in a tax return. FIN 48 also provides guidance on de-recognition, classification, interest and penalties, accounting in interim periods, disclosure and transition.
China Sky One Medical, Inc. and Subsidiaries
Notes to Unaudited Condensed Consolidated Financial Statements
3. | Summary of Significant Accounting Policies (Continued) |
Under FIN 48, evaluation of a tax position is a two-step process. The first step is to determine whether it is more-likely-than-not that a tax position will be sustained upon examination, including the resolution of any related appeals or litigation based on the technical merits of that position. The second step is to measure a tax position that meets the more-likely-than-not threshold to determine the amount of benefit to be recognized in the financial statements. A tax position is measured at the largest amount of benefit that is greater than 50% likely of being realized upon ultimate settlement.
Tax positions that previously failed to meet the more-likely-than-not recognition threshold should be recognized in the first subsequent period in which the threshold is met. Previously recognized tax positions that no longer meet the more-likely-than-not criteria should be de-recognized in the first subsequent financial reporting period in which the threshold is no longer met.
The Company did not have any FIN 48 income tax issues during the six months ended June 30, 2009 and 2008.
Comprehensive income – Comprehensive income consists of net income and other gains and losses affecting stockholders’ equity that, under generally accepted accounting principles are excluded from net income. For the Company, such items consist entirely of foreign currency translation gains and losses.
Related companies – A related company is a company in which the director has beneficial interests in and in which the Company has significant influence.
Retirement benefit costs – According to the PRC regulations on pension plans, the Company contributes to a defined contribution retirement plan organized by municipal government in the province in which the Company was registered and all qualified employees as defined by statutory regulations are eligible to participate in the plan.
Contributions to the pension or retirement plan are calculated at 22.5% of the employees’ salaries above a fixed threshold amount. The employees contribute between 2% to 8% to the pension plan, and the Company contributes the balance. The Company has no other material obligations for the payment of retirement benefits beyond the annual contributions under this plan. The Company incurred costs of $50,868, $89,972, $17,737 and $25,186 for the three and six months ended June 30, 2009 and 2008, respectively.
China Sky One Medical, Inc. and Subsidiaries
Notes to Unaudited Condensed Consolidated Financial Statements
| 3. | Summary of Significant Accounting Policies (Continued) |
Fair value of financial instruments – The carrying amounts of certain financial instruments, including cash and cash equivalents, accounts receivable, other receivables, accounts payable, accrued expenses, and other payables approximate their fair values at June 30, 2009 and 2008 because of the relatively short-term maturity of these instruments.
Recent accounting pronouncements:
In June 2009, the FASB issued SFAS No. 168, The FASB Accounting Standards Codification and the Hierarchy of Generally Accepted Accounting Principles—a replacement of FASB Statement No. 162 . This standard establishes only two levels of U.S. generally accepted accounting principles (“GAAP”), authoritative and non-authoritative. The FASB Accounting Standards Codification (the “Codification”) will become the source of authoritative, nongovernmental GAAP, except for rules and interpretive releases of the SEC, which are sources of authoritative GAAP for SEC registrants. All other nongrandfathered, non-SEC accounting literature not included in the Codification will become nonauthoritative. This standard is effective for financial statements for interim or annual reporting periods ending after September 15, 2009. The Company will begin to use the new guidelines and numbering system prescribed by the Codification when referring to GAAP beginning in the period ended September 30, 2009. As the Codification was not intended to change or alter existing GAAP, it is not otherwise expected to have any impact on the Company’s consolidated financial statements ..
China Sky One Medical, Inc. and Subsidiaries
Notes to Unaudited Condensed Consolidated Financial Statements
3. | Summary of Significant Accounting Policies (Continued) |
In May 2009, the FASB issued SFAS No. 165, “Subsequent Events” (“SFAS 165”). SFAS 165 establishes general standards of accounting for and disclosures of subsequent events that occurred after the balance sheet date but prior to the issuance of financial statements. SFAS 165 is effective for financial statements issued for interim or fiscal years ending after June 15, 2009. The adoption of SFAS 165, effective June 2009, did not affect the consolidated financial position, results of operations or cash flows of the Company.
In April 2009, the FASB issued FSP No. FAS 141(R)-1, Accounting for Assets Acquired and Liabilities Assumed in a Business Combination That Arise from Contingencies , to address some of the application issues under SFAS 141(R). The FSP deals with the initial recognition and measurement of an asset acquired or a liability assumed in a business combination that arises from a contingency provided the asset or liability’s fair value on the date of acquisition can be determined. When the fair value can’t be determined, the FSP requires using the guidance under SFAS No. 5, Accounting for Contingencies, and FASB Interpretation (FIN) No. 14, Reasonable Estimation of the Amount of a Loss . This FSP was effective for assets or liabilities arising from contingencies in business combinations for which the acquisition date is on or after January 1, 2009. The adoption of this FSP has not had a material impact on the Company’s financial position, results of operations, or cash flows during the first six months ended June 30, 2009.
In April 2008, the FASB issued FASB FSP No. 142-3, Determination of the Useful Life of Intangible Assets (“FSP FAS 142-3”). FSP FAS 142-3 removed the requirement of SFAS No. 142, Goodwill and Other Intangible Assets (“SFAS 142”), for an entity to consider, when determining the useful life of an intangible asset, whether the intangible asset can be renewed without substantial cost or material modification to the existing terms and conditions associated with the intangible asset. FSP FAS 142-3 replaces the previous useful life assessment criteria with a requirement that an entity considers its own experience in renewing similar arrangements. If the entity has no relevant experience, it would consider market participant assumptions regarding renewal. This should lead to greater consistency between the useful life of recognized intangibles under SFAS 142 and the period of expected cash flows used to measure fair value of such assets under SFAS No. 141(R), Business Combinations. FSP FAS 142-3 is being applied prospectively beginning January 1, 2009. The adoption of this Statement has not had a material impact on the Company’s financial position, results of operations, or cash flows during the first six months ended June 30, 2009.
In December 2007, the FASB issued SFAS No. 141 (revised 2007), “Business Combinations” (“SFAS 141(R)”). SFAS 141(R) will change the accounting for business combinations. Under SFAS No. 141(R), an acquiring entity will be required to recognize all the assets acquired and liabilities assumed in a transaction at the acquisition-date fair value with limited exceptions. SFAS No. 141(R) will change the accounting treatment and disclosure for certain specific items in a business combination. SFAS 141R requires earn-outs and other contingent consideration to be recorded at fair value on acquisition date and contingencies to be recorded at fair value on acquisition date with subsequent re-measurement. SFAS 141R requires acquisition costs to be expensed as incurred and generally requires restructuring costs to be expensed in periods after the acquisition date. SFAS 141R requires amounts previously called “negative goodwill” which result from a bargain purchase in which acquisition date fair value of identifiable net assets acquired exceeds the fair value of consideration transferred plus any non controlling interest in the acquirer to be recognized in earnings as a gain attributable to the acquirer. SFAS No. 141(R) applies prospectively to business combinations for which the acquisition date is on or after the beginning of the first annual reporting period beginning on or after December 15, 2008. SFAS 141(R) will impact the Company in the event of any new business acquisition after December 31, 2008.
China Sky One Medical, Inc. and Subsidiaries
Notes to Unaudited Condensed Consolidated Financial Statements
| 3. | Summary of Significant Accounting Policies (Continued) |
In December 2007, the FASB issued SFAS No. 160, “Non-controlling Interests in Consolidated Financial Statements—an amendment of Accounting Research Bulletin No. 51” (“SFAS 160”). SFAS 160 establishes accounting and reporting standards for the non-controlling interest in a subsidiary. SFAS 160 also requires that upon the deconsolidation of a subsidiary, a retained non-controlling interest be initially measured at its fair value. Upon adoption of SFAS 160, non-controlling interests shall be reported in the equity section of financial statement and requires that net earnings include the amounts attributive to both the parent and to the non-controlling interests with disclosure on the face of the statement of operations of the net earnings attributable to the parent and to the non-controlling interests, with any losses attributable to the non-controlling interests in excess of the non-controlling interests’ equity to be allocated to the non-controlling interest. Calculation of earnings per share amounts in the financial statements will continue to be based on amounts attributable to the parent. SFAS No. 160 is effective for fiscal years beginning on or after December 15, 2008. SFAS No. 160 requires retroactive adoption of the presentation and disclosure requirements for existing minority interests. All other requirement are to be applied prospectively. The adoption of SFAS No. 160, effective January 1, 2009, did not have a material impact on the Company’s consolidated financial statements.
| 4. | Revenue by Product Category |
The following tables set forth the Company’s principal product categories based on application type and the approximate amount and percentage of revenue from each of such product categories during the three and six months ended June 30, 2009 and 2008:
| | | For the Three Months Ended June 30 | |
| | | | | 2009 | | | | 2008 | |
Product (Number of Products) | | Subsidiary | | Sales (USD) | | | % of Sales | | Product (Number of Products) | | Sales (USD) | | | % of Sales | |
| Patch (5) | | TDR | | $ | 9,937,203 | | | | 30.9 | | Patch (4) | | $ | 8,781,903 | | | | 37.0 | |
| Ointment (18) | | TDR&TL | | | 7,658,146 | | | | 23.8 | | Ointment (11) | | | 6,486,619 | | | | 27.3 | |
| Spray (15) | | TDR&TL | | | 4,808,652 | | | | 14.9 | | Spray (19) | | | 2,840,196 | | | | 12.0 | |
| Bio-Engineering (3) | | FIRST | | | 3,687,991 | | | | 11.5 | | Bio-Engineering (3) | | | 2,145,355 | | | | 9.0 | |
| Others (48) | | TDR&TL&PL | | | 6,089,598 | | | | 18.9 | | Others (31) | | | 3,494,518 | | | | 14.7 | |
| Total (89 products) | | | | $ | 32,181,590 | | | | 100 | | Total (68 products) | | $ | 23,748,592 | | | | 100 | |
| | | For the Six Months Ended June 30 | |
| | | | | 2009 | | | | 2008 | |
Product (Number of Products) | | Subsidiary | | Sales (USD) | | | % of Sales | | Product (Number of Products) | | Sales (USD) | | | % of Sales | |
| Patch (5) | | TDR | | $ | 19,059,164 | | | | 33.4 | | Patch (4) | | $ | 12,573,362 | | | | 34.7 | |
| Ointment (18) | | TDR&TL | | | 12,740,124 | | | | 22.3 | | Ointment (11) | | | 8,796,305 | | | | 24.3 | |
| Spray (15) | | TDR&TL | | | 7,710,726 | | | | 13.5 | | Spray (19) | | | 4,878,058 | | | | 13.5 | |
| Bio-Engineering (3) | | FIRST | | | 6,788,766 | | | | 11.9 | | Bio-Engineering (3) | | | 3,982,860 | | | | 11.0 | |
| Others (48) | | TDR&TL&PL | | | 10,716,502 | | | | 18.9 | | Others (31) | | | 5,931,437 | | | | 16.5 | |
| Total (89 products) | | | | $ | 57,015,282 | | | | 100 | | Total (68 products) | | $ | 36,162,022 | | | | 100 | |
4.5. | Concentrations of Business and Credit Risk |
The Company maintains certain bank accounts in the PRC which are not protected by FDIC insurance or other insurance. As of June 30, 2009, the Company held approximately $2,418,000 of cash balances within the United States of which was all insured. At June 30, 2009, the Company had approximately $45,815,000, in China bank deposits, which is not insured. Historically, the Company has not
experienced any losses in such accounts.
China Sky One Medical, Inc. and Subsidiaries
Notes to Unaudited Condensed Consolidated Financial Statements
| 5. | Concentrations of Business and Credit Risk (Continued) |
Nearly all of the Company’s sales are concentrated in China. Accordingly, the Company is susceptible to fluctuations in its business caused by adverse economic conditions in this country. Difficult economic conditions in other geographic areas into which the Company may expand may also adversely affect its business, operations and finances.
The Company provides credit in the normal course of business. Substantially all customers are located in PRC. The Company performs ongoing credit evaluations of its customers and maintains allowances for doubtful accounts based on factors surrounding the credit risk of specific customers, historical trends, and other information.
Substantially all of the Company's fixedlong-lived assets and operations are located in the PRC.
The Company is self-insured for all risks and carries no liability or property insurance coverage of any kind. The Company does not set aside any reserves for product liability risks or other potential claims. The Company’s policy is to record losses associated with its lack of insurance coverage at such time as a realized loss is incurred. Historically, the Company has not had any material losses in connection with its lack of insurance coverage and was not party to any material pending legal proceedings as of June 30, 2009. Management’s intention is to use the Company’s working capital to fund any such losses incurred due to the Company’s exposure to inadequate insurance coverage.
Substantially all of the Company's businesses are generated from operations in mainland China.
Payments of dividends may be subject to some restrictions due to the Company’s operating subsidiaries all being located in the PRC.
Major Customers
For the six months ended June 30, 2009, Harbin Shiji Baolong and Shanxi Xintai accounted for approximately 21% and 18% , respectively of sales, of total revenues and no other customer accounted for 10% or more of the Company’s total revenues. For the six months ended June 30, 2008, no individual customer accounted for more than 10% of salestotal revenues. As of June 30, 2009, Harbin Shiji Baolong and Shanxi Xintai accounted for approximately 34% and 15% of the Company’s outstanding accounts receivables, respectively. As of June 30, 2008, Harbin Shiji Baolong accounted for approximately 15% of the Company’s outstanding accounts receivables. No other customer accounted for 10% or more of the Company’s outstanding accounts receivables as of June 30, 2009 or 2008.
China Sky One Medical, Inc. and Subsidiaries
Notes to Unaudited Condensed Consolidated Financial Statements
4. | Concentrations of Business and Credit Risk (Continued) |
Major Suppliers
Heilongjiang Kangda Medicine Co. accounted for approximately 41% of the Company’s inventory purchases for the six months ended June 30, 2009 and 45% for the six months ended June 30, 2008.
We have applied SFAS No. 128, “Earnings Per Share” in our calculation and presentation of earnings per share - “basic” and “diluted”. Basic earnings per share are computed by dividing net earnings available to common shareholders (the numerator) by the weighted average number of common shares (the denominator) for the period presented. The computation of diluted earnings per share is similar to basic earnings per share, except that the denominator is increased to include the number of additional common shares that would have been outstanding if the potentially dilutive common shares had been issued.
Stock warrants to purchase 750,000 shares of common stock were outstanding and exercisable as of June 30, 2009. In addition, options to purchase 12,500 shares of common stock were outstanding and exercisable as of June 30, 2009. Stock warrants and options to purchase 1,151,000 shares of common stock outstanding during the year ended December 31, 2008, all of which were exercisable. These common stock equivalents were included in the computation of diluted earnings per share because the option exercise prices were less than the average market price of our common stock during these periods.
The dilutive potential common shares on warrants and options is calculated in accordance with the treasury stock method, which assumes that proceeds from the exercise of all warrants and options are used to repurchase common stock at the average market valueprice of the common stock during the relevant period . The amount of shares remaining after the proceeds are exhausted represent s the potential dilutive effect of the securities.
The following table sets forth our computation of basic and diluted net income per share:
| | | For the three months ended June 30, | |
| | | 2009 | | | 2008 | |
Numerator: | | | | | | |
| Net income used in calculation of basic and diluted earnings per share | | $ | 9,456,993 | | | $ | 8,110,667 | |
| | | | | | | | | |
Denominator: | | | | | | | | |
Weighted-average common shares outstanding used in calculation of basic earnings per share | | | 16,537,066 | | | | 14,971,652 | |
Effect of dilutive securities: | | | | | | | | |
Stock options and equivalents | | | 91,826 | | | | 1,118,559 | |
Weighted-average common shares used in calculation of diluted earnings per share | | | 16,628,892 | | | | 16,090,211 | |
| | | | | | | | | |
Net income per share: | | | | | | | | |
Basic | | $ | 0.57 | | | $ | 0.54 | |
Diluted | | $ | 0.57 | | | $ | 0.50 | |
China Sky One Medical, Inc. and Subsidiaries
Notes to Unaudited Condensed Consolidated Financial Statements
5.6. | Earnings per Share (Continued) |
The following table sets forth our computation of basic and diluted net income per share:
| | | For the three months ended June 30, | |
| | | 2009 | | | 2008 | |
Numerator: | | | | | | |
| Net income used in calculation of basic and diluted earnings per share | | $ | 9,456,993 | | | $ | 8,110,667 | |
| | | | | | | | | |
Denominator: | | | | | | | | |
| Weighted-average common shares outstanding used in calculation of basic earnings per share | | | 16,537,066 | | | | 14,971,652 | |
| Effect of dilutive securities: | | | | | | | | |
| Stock options and equivalents | | | 91,826 | | | | 1,118,559 | |
| Weighted-average common shares used in calculation of diluted earnings per share | | | 16,628,892 | | | | 16,090,211 | |
| | | | | | | | | |
| Net income per share: | | | | | | | | |
| Basic | | $ | 0.57 | | | $ | 0.54 | |
| Diluted | | $ | 0.57 | | | $ | 0.50 | |
The following table sets forth our computation of basic and diluted net income per share:
| | | For the six months ended June 30, | |
| | | 2009 | | | 2008 | |
Numerator: | | | | | | |
| Net income used in calculation of basic and diluted earnings per share | | $ | 16,699,831 | | | $ | 11,975,578 | |
| | | | | | | | | |
Denominator: | | | | | | | | |
| Weighted-average common shares outstanding used in calculation of basic earnings per share | | | 16,475,833 | | | | 14,253,547 | |
| Effect of dilutive securities: | | | | | | | | |
| Stock options and equivalents | | | 71,154 | | | | 1,118,559 | |
| Weighted-average common shares used in calculation of diluted earnings per share | | | 16,547,037 | | | | 15,372,106 | |
| | | | | | | | | |
| Net income per share: | | | | | | | | |
| Basic | | $ | 1.01 | | | $ | 0.84 | |
| Diluted | | $ | 1.01 | | | $ | 0.78 | |
| 7. | Equity and Share-based Compensation |
6. | Equity and Share-based Compensation |
Effective January 1, 2006, the Company adopted the fair value recognition provisions of SFAS No. 123R, Share-Based Payment (“SFAS No. 123R”), for options granted to employees and directors, using the modified prospective transition method, and therefore have not restated results from prior periods. Compensation cost for all stock-based compensation awards granted is based on the grant date fair value estimated in accordance with the provisions of SFAS No. 123R. Under the fair value recognition provisions of SFAS No. 123R, the Company recognizes stock-based compensation net of an estimated forfeiture rate and only recognize compensation cost for those shares expected to vest on a straight-line prorated basis over the requisite service period of the award. In March 2005, the SEC issued Staff Accounting Bulletin (“SAB”) No. 107, Share-Based Payment (“SAB No. 107”), regarding the SEC’s guidance on SFAS No. 123R and the valuation of share-based payments for public companies. The Company has applied the provisions of SAB No. 107 in the adoption of SFAS No. 123R.
China Sky One Medical, Inc. and Subsidiaries
Notes to Unaudited Condensed Consolidated Financial Statements
| 7. | Equity and Share based Compensation (Continued) |
In July 2006, the Company’s stockholders approved the 2006 Stock Incentive Plan (the “2006 Plan”). The 2006 Plan, provides for the grant of stock options, restricted stock awards, and performance shares to qualified employees, officers, directors, consultants and other service providers. The 2006 Plan the Company to grant options and/or rights to purchase up to an aggregate of 1,500,000 shares of common stock. As of June 30, 2009, non-qualified options to purchase a total of 113,500 shares have been granted under the 2006 Stock Incentive Plan. All of these options were granted in October 2006. All options have an exercise price of $3.65 per share, the weighted fair market value on the date of grant was $4.25 per share. Of these 113,500, options a total of 60,500 were granted to employees and a total of 53,000 were granted to consultants. These options were valued using the Black-Scholes option-pricing model with the following assumptions: no dividends; risk-free interest rate of 4%; a contractual life of 5 years and volatility of 39%. All 113,500 options vested over various periods. As of June 30, 2009, 101,000 options were exercised on a cashless basis resulting in the issuance of a total of 75,888 shares of the Company’s common stock. As of June 30, 2009, 12,500 options remained outstanding.
China Sky One Medical, Inc. and Subsidiaries
Notes to Unaudited Condensed Consolidated Financial Statements
6. | Equity and Share-based Compensation (Continued) |
Options or stock awards issued to non-employees and consultants are recorded at their fair value as determined in accordance with SFAS No. 123R and EITF No. 96-18, “Accounting for Equity Instruments That Are Issued to Other Than Employees for Acquiring, or in Conjunction with Selling, Goods or Services”, and recognized over the related vesting or service period. In connection with closing of the Stock Exchange Agreement, the Company agreed to grant warrants to advisors for the services they already performed for the reverse merger in July 2006, entitling them to purchase up to 500,000 shares on or before July 31, 2009, at a price of $2.00 per share (the “Advisor Warrants”) and options to purchase up to 50,000 shares on or before December 20, 2008 at a price of $3.00 per share. The fair value of these warrants and options were determined to be $772,275 and deducted as expenses using the Black-Scholes option-pricing model with the following weighted assumptions: no dividends; risk-free interest rate of 4%; a contractual life of 2.5-3.5 years and volatility of 39%. The Company based its estimate of expected volatility on the historical, expected or implied volatility of similar entities whose share or option prices are publicly available ..
In fiscal 2008, the holder of the Advisor Warrants exercised 200,000 of the Advisor Warrants on a cashless basis, resulting in the issuance of 166,245 shares of the Company’s common stock. In the six months ended June 30, 2009, the holder of the Advisor Warrants exercised the remaining 300,000 Advisor Warrants on a cashless basis, resulting in the issuance of 261,610 shares of the Company’s common stock.
In addition, in the six months ended June 30, 2009, a warrant holder exercised warrants to purchase 8,334 shares of the Company’s common stock, at an exercise price of $3.50 per share. These warrants were originally issued in a private placement the Company consummated in October 2006.
7.8. | Securities Purchase Agreement and Related Transaction |
On January 31, 2008 China Sky One entered into a Securities Purchase Agreement (the “Purchase Agreement”) with certain accredited investors, for the purchase and sale of units consisting of: (i) one (1) share of the Company’s common stock; and (ii) 750,000 Class A Warrants exercisable at $12.50 per share, and expiring on July 31, 2011 (the “Class A Warrants”), for a purchase price of $10.00 per Unit (the “Unit Purchase Price”), or gross offering proceeds of $25.0 million (the “2008 Offering”). The Company received net proceeds of approximately $23.5 million in connection with the 2008 offering.
Pursuant to the Purchase Agreement, among other things, if, and whenever, within twelve (12) months of the Closing Date, the Company issues or sells, or is deemed to have issued or sold, any shares of common stock, or securities convertible into or exercisable for shares of common stock, or modifies any of the foregoing which may be outstanding (with the exception of certain excluded securities), to any person or entity at a price per share, or conversion or exercise price per share less than the Unit Purchase Price, then the Company shall issue, for each such occasion, additional shares of its common stock to the Investors in such number so that the average per share purchase price of the shares of common stock purchased by the Investors in the 2008 Offering shall automatically be reduced to such other lower price per share.
In addition, as of the Closing Date, the Company entered into a Make Good Agreement (the “Make Good Agreement”) with Liu Yan-Qing, its Chairman, Chief Executive Officer and President, and a principal shareholder of the Company, (the “Principal Shareholder”) and the Investors (collectively, the “Make Good Parties”), pursuant to which the Principal Shareholder deposited 3,000,000 shares of his common stock of the Company (the “Escrow Shares”) into escrow, to be released to the Investors in an amount pro rata pro to their initial investments in the 2008 Offering, in the event the Company failed to attain earnings per share, as adjusted, of at least (i) $1.05 per share for the fiscal year ending December 31, 2007 (based on an aggregate of 13,907,696 shares outstanding), and/or (ii) $1.63 per share for the fiscal year ending December 31, 2008 (based on 16,907,696 shares outstanding).
China Sky One Medical, Inc. and Subsidiaries
Notes to Unaudited Condensed Consolidated Financial Statements
7.8. | Securities Purchase Agreement and Related Transaction (Continued) |
The Company deems the Escrow Shares arrangement as analogous to the issuance of a fixed number of warrants in an equity transaction. Under the Make Good Agreement these Escrow Shares would have been reallocated on a pro rata basis to the Investors only if certain earnings targets were not achieved in years 2007 and 2008. If the earnings targets were met, the Escrow Shares would automatically be released to the Principal Shareholder. As of January 31, 2008, the date the common shares were placed into escrow, the Company achieved the 2007 earnings target and, based upon internal forecasts, was confident the 2008 target would also be met. Based upon certain assumptions, including the low probability that the Escrow Shares would be released to the Investors and not be returned to the Principal Shareholder, the Company considered the fair value of the right held by the Investors through the Escrow Shares provision under the Make Good Agreement to be immaterial. As of December 31, 2008, the Company satisfied the earnings per common share targets for each of fiscal 2007 and 2008 as defined under the Make Good Agreement and, as such, the Escrow Shares had been released in May 2009.
The Class A Warrants represent the right to purchase an aggregate of 750,000 shares of common stock, at an exercise price of $12.50 per share. Additional information relating to these Class A Warrants is provided in Note 8.
8.9. | Outstanding Warrants and Options |
| | Shares Underlying Warrants | | Weighted average Exercise Price Warrants | | Shares underlying Options | | Weighted average Exercise Price Options | |
| Outstanding as of January 1, 2006 | 25,000 | | $ | 1.50 | | - | | | | |
| Granted | 1,650,000 | | | 2.58 | | 163,500 | | $ | 3.45 | |
| Exercised | - | | | - | | - | | | - | |
| Expired or cancelled | - | | | - | | - | | | - | |
| Outstanding as of December 31, 2006 | 1,675,000 | | | 2.57 | | 163,500 | | $ | 3.45 | |
| Granted | - | | | - | | - | | | - | |
| Exercised | - | | | - | | - | | | - | |
| Expired or cancelled | (161,667) | | | 3.19 | | - | | | - | |
| Outstanding as of December 31, 2007 | 1,513,333 | | $ | 2.48 | | 163,500 | | $ | 3.45 | |
| Granted | 750,000 | | | 12.50 | | - | | | - | |
| Exercised | (1,204,999) | | | | | (50,000) | | | - | |
| Expired or cancelled | - | | | - | | - | | | - | |
| Outstanding as of December 31, 2008 | 1,058,334 | | $ | 9.50 | | 113,500 | | $ | 3.65 | |
| Granted | - | | | - | | - | | | - | |
| Exercised | (308,334) | | | | | (101,000) | | | | |
| Expired or cancelled | - | | | - | | - | | | - | |
| Outstanding as of June 30, 2009 | 750,000 | | $ | 12.50 | | 12,500 | | $ | 3.65 | |
China Sky One Medical, Inc. and Subsidiaries
Notes to Unaudited Condensed Consolidated Financial Statements
8.9. | Outstanding Warrants and Options (Continued) |
The following table summarizes information about stock warrants outstanding and exercisable as of June 30, 2009.
Exercise Price | | Outstanding June 30, 2009 | | Weighted Average Remaining Life in Years | | | Number exercisable | |
| $ | 12.50 | | 750,000 | | | 2.00 | | | | 750,000 | |
| | | 750,000 | | | | | | | 750,000 | |
Out of the 750,000 outstanding warrants, all were exercisable as of June 30, 2009. These Class A Warrants represent the right to purchase an aggregate of 750,000 shares of common stock of the Company granted with the Purchase Agreement, at an exercise price of $12.50 per share (the “Exercise Price”), and have the following additional characteristics:
The Class A Warrants issued in our January 2008 Offering described in Note 8 above, represent the right to purchase an aggregate of 750,000 shares of common stock and have the following additional characteristics:
| | · | The Class A Warrants are exercisable beginning on the six-month anniversary of the closing of the January 2008 Offering and will expire July 31, 2011. |
| | · | Commencing on one-year anniversary of the Closing Date, in the event the shares underlying the Class A Warrants (the “Warrant Shares”) may not be freely sold by the holders of the Class A Warrants due to the Company’s failure to satisfy its registration requirements, and an exemption for such sale is not otherwise available to the Warrant-holders under Rule 144, the Class A Warrants will be exercisable on a cashless basis. There was no issuance of securities during 2008 or the six months ended June 30, 2009 which would have resulted in an adjustment to the Exercise Price and number of Warrant Shares. |
| | · | The Exercise Price and number of Warrant Shares will be subject to adjustment for standard dilutive events, including the issuance of Common stock, or securities convertible into or exercisable for shares of Common stock, at a price per share, or conversion or exercise price per share less than the Class A Warrant exercise price of $12.50 per share. |
| | · | At anytime following the date a Registration Statement covering the Warrant Shares is declared effective, we will have the ability to call the Class A Warrants at a price of $0.01 per Class A Warrant, upon thirty (30) days prior written notice to the holders of the Class A Warrants, provided (i) the closing price of the Common stock exceeded $18.75 for each of the ten (10) consecutive trading days immediately preceding the date that the call notice is given by the Company, and (ii) the Company has attained an Adjusted EPS of at least $1.75 per share for the fiscal year ending December 31, 2008, as set forth in our audited financial statements of the Company. |
| | · | If, among other things, we fail to cause a Registration Statement covering the Warrant Shares to be declared effective prior to the applicable dates set forth in the Registration Rights Agreement, the expiration date of the Class A Warrants shall be extended one day for each day beyond the Effectiveness Deadlines. The Company engaged an independent third-party consultant to calculate the derivative liability resulting from its failure to register the Warrant Shares. The calculation was made using the Black-Scholes model. The liability was deemed to be immaterial as of June 30, 2009. |
China Sky One Medical, Inc. and Subsidiaries
Notes to Unaudited Condensed Consolidated Financial Statements
| 9. | Outstanding Warrants and Options (Continued) |
| | |
| | · | If a Warrant-holder exercises its Put Right under the Put Agreement (as previously defined above), such Warrant-holder’s right to exercise the Class A Warrants shall be suspended, pending the satisfaction of our obligations to pay the Warrant-holder the applicable Repurchase Price. Upon receipt of the Repurchase Price in full by the Warrant-holder, the Warrant-holder’s right to exercise the Class A Warrants shall automatically and permanently terminate and expire, and the Class A Warrants shall be immediately cancelled on the books of the Company. |
China Sky One Medical, Inc. and Subsidiaries
Notes to Unaudited Condensed Consolidated Financial Statements
8. | Outstanding Warrants and Options (Continued) |
| | · | If a Warrant-holder exercises its Put Right under the Put Agreement (as previously defined above), such Warrant-holder’s right to exercise the Class A Warrants shall be suspended, pending the satisfaction of our obligations to pay the Warrant-holder the applicable Repurchase Price. Upon receipt of the Repurchase Price in full by the Warrant-holder, the Warrant-holder’s right to exercise the Class A Warrants shall automatically and permanently terminate and expire, and the Class A Warrants shall be immediately cancelled on the books of the Company. |
The following table summarizes information about stock options outstanding and exercisable as of June 30, 2009.
Exercise Price | | Outstanding June 30, 2009 | | Weighted Average Remaining Life in Years | | | Exercisable Options | | | Vested Options | |
| $ | 3.65 | | 12,500 | | | 2.50 | | | | - | | | | 12,500 | |
| | | 12,500 | | | | | | | - | | | | 12,500 | |
The Company values its inventories at the lower of cost and market method. Inventories are accounted for using the first-in, first-out method. Inventories include packing materials, raw materials, supplemental materials, work-in-process, and finished products.
As of June 30, 2009 and December 31, 2008, inventories consist of the following:
| | | June 30, 2009 | | | December 31, 2008 (Audited) | |
| Raw Material | | $ | 809,803 | | | $ | 330,275 | |
| Work-in-Process | | | 138,862 | | | | 76,462 | |
| Finished Products | | | 622,800 | | | | 55,614 | |
| Total Inventories | | $ | 1,571,465 | | | $ | 462,351 | |
As of June 30, 2009 and December 31, 2008, the Company had no inventory reserve.
10.11. | Property and Equipment |
As of June 30, 2009 and December 31, 2008, Property and Equipment, net consist of the following:
| | June 30, 2009 | | | December 31, 2008 (Audited) | |
| Buildings and improvements | | $ | 9,550,042 | | | $ | 9,961,820 | |
| Machinery and equipment | | | 5,447,140 | | | | 4,946,247 | |
| Land use rights | | | 1,919,911 | | | | 1,945,209 | |
| Transportation equipment | | | 887,102 | | | | 885,880 | |
| Furniture and equipment | | | 314,994 | | | | 299,467 | |
| Construction in progress (See Note 14) | | | 14,192,855 | | | | 4,317,265 | |
| Total Property and Equipment | | | 32,312,044 | | | | 22,355,888 | |
| Less: Accumulated Depreciation | | | (1,758,820 | ) | | | (1,297,109 | ) |
| Property and Equipment, Net | | $ | 30,553,224 | | | $ | 21,058,779 | |
China Sky One Medical, Inc. and Subsidiaries
Notes to Unaudited Condensed Consolidated Financial Statements
10.11. | Property and Equipment (Continued) |
For the six months ended June 30, 2009 and 2008, depreciation expense totaled $461,711 and $213,935 respectively. Depreciation expense of approximately $268,000 and $137,000 is included as part of cost of goods sold for the six months ended June 30, 2009 and 2008, respectively.
As of June 30, 2009 and December 31, 2008, the Company’s net unamortized intangible assets consist of:
| | | June 30, 2009 | | | December 31, 2008 (Audited) | |
| Patents | | $ | 14,433,828 | | | $ | 15,093,718 | |
| Goodwill | | | 759,362 | | | | 758,047 | |
| Total Intangible Assets, net | | $ | 15,193,190 | | | $ | 15,851,765 | |
Amortization expense for the six months ended June 30, 2009 and 2008 was $706,890 and $138,898 respectively.
Taxes payable consists of the following:
| Taxes payable consists of the following: | | June 30, 2009 | | | December 31, 2008 (Audited) | |
| Value Added Tax, net | | $ | 1,505,459 | | | $ | 1,179,383 | |
| Enterprise Income Tax | | | 2,637,189 | | | | 2,106,956 | |
| City Tax | | | 49,678 | | | | 32,013 | |
| Other Taxes and additions | | | 72,748 | | | | 44,536 | |
| Total Taxes Payable | | $ | 4,265,075 | | | $ | 3,362,888 | |
Under the Provisional Regulations of PRC Concerning Income Tax on Enterprises promulgated by the PRC, income tax is payable by enterprises at a rate of 25% of their taxable income. Preferential tax treatment may, however, be granted pursuant to any law or regulations from time to time promulgated by the State Council.
According to “Enterprise Income Tax and Certain Preferential Policies Notice” published by the Ministry of Finance and the National Tax Affairs Bureau, if the enterprise is authorized by the State Council as a special entity, the enterprise income tax rate is reduced to 15%. In 2009, the income tax rate for TDR, TianLong, and First is 15% based on State Council approval. The income tax rate for Haina is 25%. The income tax rate for Peng Lai is regulated by local government at 2% of total revenue commencing January 1, 2009. In 2008, the income tax rate for TDR and TianLong was 15% and 12%, respectively. The income tax rate for First, Haina, and Peng Lai was 25%. As a result of the special income tax rates for TDR, Tianlong and First, the Company’s effective tax rate was approximately 21.8% and 21.1% for the three and six months ended June 30, 2009, respectively, and 18.6% and 19.5% for the three and six months ended June 30, 2008, respectively. If the Company’s effective tax rate was 25%, its net income, basic earnings per share and diluted earnings per share for the three months ended June 30, 2009 would have been $9,071,750, $0.55 and $0.55, respectively, and its net income, basic earnings per share and diluted earnings per share for the six months ended June 30, 2009 would have been $15,868,982, $0.96 and $0.96, respectively. If the Company’s effective tax rate was 25%, its net income, basic earnings per share and diluted earnings per share for the six months ended June 30, 2008 would have been $11,153,647, $0.78 and $0.73, respectively, and for the three months ended June 30, 2008 its net income, basic earnings per share and diluted earnings per share would have been $7,469,702, $0.50 and $0.46, respectively.
We record a full valuation allowance to reduce our deferred tax assets to the amount that is more likely than not to be realized. While we have considered future taxable income and ongoing prudent and feasible tax planning strategies in assessing the need for the valuation allowance, in the event we were to determine that we would be able to realize our deferred tax assets in the future in excess of its net recorded amount, an adjustment to the deferred tax asset would increase income in the period such determination was made.
China Sky One Medical, Inc. and Subsidiaries
Notes to Unaudited Condensed Consolidated Financial Statements
13. | Income Taxes (Continued) |
Pursuant to Sections 382 and 383 of the Internal Revenue Code of 1986 (“IRC”), annual use of the Company’s net operating losses and tax credit carryforwards may be limited because of cumulative changes in ownership of more than 50% that have occurred.
Provision for the PRC enterprise income tax is calculated at the prevailing rate based on the estimated assessable profits less available tax relief for losses brought forward . The Company does not accrue taxes on unremitted earnings from foreign operations as it is the Company’s intention to invest these earnings in the foreign operations indefinitely .
In 2006, the FASB issued FIN 48, which clarifies the application of SFAS 109 by defining a criterion that an individual income tax position must meet for any part of the benefit of that position to be recognized in an enterprise’s financial statements and provides guidance on measurement, derecognition, classification, accounting for interest and penalties, accounting in interim periods, disclosure and transition. In accordance with the transition provisions, the Company adopted FIN 48 effective January 1, 2007.
The Company recognizes that virtually all tax positions in the PRC are not free of some degree of uncertainty due to tax law and policy changes by the state. However, the Company cannot reasonably quantify political risk factors and thus must depend on guidance issued by current state officials.
Based on all known facts and circumstances and current tax law, the Company believes that the total amount of unrecognized tax benefits as of June 30, 2009, is not material to its results of operations, financial condition or cash flows. The Company also believes that the total amount of unrecognized tax benefits as of June 30, 2009, if recognized, would not have a material effect on its effective tax rate. The Company further believes that there are no tax positions for which it is reasonably possible, based on current Chinese tax law and policy, that the unrecognized tax benefits will significantly increase or decrease over the next 12 months producing, individually or in the aggregate, a material effect on the Company’s results of operations, financial position or cash flows
14.15. | Land Use Rights Purchase Agreement and Construction in Progress |
During the second quarter in 2007 TDR entered into an agreement with the Development and Construction Administration Committee of Harbin Song Bei New Development district to purchase certain land use rights for 50 years in conjunction with the Company’s development of a new headquarter and a new biotech engineering lab at a construction cost of approimatelyapproximately $10.5 million. The cost of the land use rights amounted to approximately $2.5 million. Terms of the agreement called for a deposit of 30% within 15 days after signing the agreement, 40% payment 7 days prior to the start of construction and the balance of 30% 7 days after getting the formal land use right.
The project consists of two phases:
| | (1) | Construction of a main workshop, R&D center and our principle corporate office using land area of 30,000 square meters. Construction started in May 2007 and is estimated to be completed by the end of 2009. |
| | (2) | Construction of Second workshop and show room using land area of 20,000 square meters. Construction is expected to start in September 2008 and is estimated to be completed by December 2009. | |
As of June 30, 2009, the Company remitted deposits totaling $8,525,025 under the terms of the above agreement. Within this deposit, there are approximately $5.8 million for construction. Upon the completion of the project, this construction deposits shall be released and returned to the Company.
China Sky One Medical, Inc. and Subsidiaries
Notes to Unaudited Condensed Consolidated Financial Statements
15.16. | Commitments and Contingencies |
The formulation, manufacturing, processing, packaging, labeling, advertising, distribution and sale of external use Chinese medicine such as those sold by the Company are subject to regulations by one or more federal agencies. The principal federal agencies include the SFDA, the Food and Drug Administration (the “FDA”), Heilongjiang Provincial Food and Drug Administration of the People's Republic of China (PFDA), National Biology Products Inspection Institute (NBPI) and the National Food and Drug Administration (NFDA) of the People's Republic of China and, to a lesser extent, the Consumer Product Safety Commission. These activities are also regulated by various governmental agencies for the countries, states and localities in which the Company’s products are sold.
Although management believes that the Company is in material compliance with the statutes, laws, rules and regulations of every jurisdiction in which it operates, no assurance can be given that the Company’s compliance with the applicable statutes, laws, rules and regulations will not be challenged by governing authorities or private parties, or that such challenges will not lead to material adverse effects on the Company’s financial position, results of operations, or cash flows.
The Company, like any other distributor or manufacturer of products is exposed to the inherent risk of product liability claims in the events of possible injuries caused by the use of its products. The Company does not have liability insurance with respect to product liability claims; the insurance environment of China is neither sufficient nor mature. Inadequate insurance or lack of contractual indemnification from parties supplying raw materials or marketing its products, and product liabilities related to defective products could have a material adverse effects on the Company.
The Company is not involved in any legal matters arising in the normal course of business. While incapable of estimation, in the opinion of the management, the individual regulatory and legal matters in which the Company might be involved in the future are not expected to have a material adverse effect on the Company’s financial position, results of operations, or cash flows.
The Company’s sole rental commitment is office space for the year 2009 is approximately $25,000. The Company is expecting the corporate headquarters currently under construction to be completed by 2009. As a result, there is no rental commitment made by the Company for the year 2010 and thereafter.
Item 2. Management’s Discussion and Analysis of Financial Condition and Results of Operations
FORWARD LOOKING STATEMENTS
The following discussion should be read in conjunction with the information contained in the consolidated financial statements of the Company and the notes thereto appearing elsewhere herein and in the risk factors and “Forward Looking Statements” summary set forth in the forepart of our Annual Report for the year ended December 31, 2008 (“Annual Report”). This quarterly report on Form 10-Q contains forward-looking statements and is afforded the safe harbor provisions of Section 27A of the Securities Act and Section 21E of the Securities Exchange Act of 1934, as amended. Readers should carefully review the risk factors disclosed in our Annual Report and other documents filed by us with the Securities and Exchange Commission (“SEC”).
DISCUSSION
We primarily generate revenues, through our China based indirect subsidiaries described below, in the development, manufacture, marketing and sale of over-the-counter, branded nutritional supplements and over-the-counter plant and herb based pharmaceutical and medicinal products. Our principal products are external use Traditional Chinese Herbal Remedies/Medicines commonly referred to in the industry as “TCM.” We have evolved into an integrated manufacturer, marketer and distributor of external use Chinese medicine products sold primarily in China and through Chinese domestic pharmaceutical chains and have been expanding our worldwide sales effort as well. We sell both our own manufactured products, as well as medicinal and pharmaceutical products manufactured by others in China.
We have achieved continued growth of our line of products. For the three months ended June 30, 2009, total revenue was $32,181,590 , a 36% increase over the same period in 2008, and net income was $9,456,993 , or $0.57 per share compared to net income of $8,110,667, or $0.50 per share on a diluted basis in the same period in 2008. For the six months ended June 30, 2009, total revenue was $57,015,282, a 58% increase over the same period in 2008, and net income was $16,699,831, or $1.01 per common share compared to net income of $11,975,578, or $0.78 per common share on a diluted basis in the same period in 2008.
All of our business is conducted through our wholly-owned subsidiary, ACPG which, in turn, wholly owns Harbin Tian Di Ren Medical Science and Technology Company (referred to herein as “TDR”), a company organized in the PRC, and TDR’s subsidiaries.
TDR, formerly known as “Harbin City Tian Di Ren Medical Co.,” was originally formed in 1994 with its principal executive office in Harbin City of Heilongjiang Province, in the People’s Republic of China (“PRC”). TDR was reorganized and incorporated as a limited liability company on December 29, 2000, under the “Corporation Laws and Regulations” of the PRC. At the time of the TDR Acquisition by ACPG in December of 2005, TDR had two wholly-owned subsidiaries, Harbin First Bio-Engineering Company Limited and Kangxi Medical Care Product Factory, until July, 2006, when the two were merged, with Harbin First Bio-Engineering Company Limited (“First” or “Harbin Bio Engineering”) as the surviving subsidiary of TDR.
Year 2008 Business Acquisitions
On April 3, 2008, TDR completed its acquisition of Heilongjiang TianL ongTianLong Pharmaceutical, Inc., a c orporationcorporation with a variety of medicines approved by China’ s State Food and Drug Administration (“SFDA”) and new medicine applications, organized under the laws of the PRC (“TianLong” ), which is in the business of manufacturing external-use pharmaceuticals. TDR previously acquired the Beijing sales office of TianL ongTianLong in mid-2006. TDR acquired 100% of the issued and outstanding capital stock of TianL ongTianLong from its sole stockholder, in consideration for an aggregate purchase price of approximately $8,300,000, consisting of (i) $8,000,000 in cash, and (ii) 23,850 shares of our common stock.
On April 18, 2008, TDR consummated its acquisition of Heilongjiang Haina Pharmaceutical Inc., a corporation which had been recently organized under the laws of the PRC (“Haina”), licensed as a wholesaler of TCM, bio-medicines, bio-p roductsproducts , medicinal devices, antibiotics and chemical medicines. Haina did not have an established sales network and was acquired for its primary asset, a Good Supply Practice (GSP) license (License No. A-HLJ03-010), issued by the Heilongjiang province offi ceoffice of the SFDA. The SFDA recently started issuing such licenses to resellers of medicines that maintain certain quality controls. The GSP license was issued as of December 21, 2006 and will expire on January 29, 2012, and will enable us to expand our sales of medicinal products without having to go through a lengthy license application process. TDR acquired 100% of the issued and outstanding capital stock of Haina from its three stockholders in consideration for payment of approximately $437,000. TDR had been overseeing the operations of Haina since January of 2008, as part of our due diligence prior to closing of this acquisition.
On September 5, 2008, TDR acquired Peng Lai Jin Chuang Pharmaceutical Company, a corporation organized under the laws of the PRC (“Peng Lai”), from Peng Lai Jin Chuang Group Corporation. Peng Lai, which received Good Manufacturing Practice certification from the SFDA, was organized to develop, manufacture and distribute pharmaceutical, medicinal and diagnostic products in the PRC . In connection with the acquisition of Peng Lai, TDR acquired all of Peng Lai’ s assets, including, without limitation, franchise, production and operating rights to a portfolio of twenty (20) medicines approved by the SFDA, for an aggregate purchase price of approximately $7.1 million, consisting of (i) approximately $2.5 million in cash, and (ii) 381,606 shares of our common stock.
Testing Kits and Other Products in Production
Our AMI Diagnostic Kit, Human Urinary Albumin Elisa Kit and Early Pregnancy Diagnostic Kit each passed the final stages of a national inspection in 2006 or 2007. These diagnostic kits are being sold through drug stores, hospitals, examination stations and independent sales agents throughout the PRC.
Our AMI Diagnostic Kit, which entered markets in 2007, is used for early diagnosis of Myocardial Infarction (MI), also known as heart disease. All the test kits require users to place a blood or urine sample on the marker and a positive (+) or negative (-) reaction signal will result, showing if a user should consult his or her doctor for further testing. According to the China Medical Newspaper, several million people die from MI every year. MI often occurs to people who are, but not limited to, smokers, over-weight and diabetic. There are approximately 8 million new MI patients in China every year. Recent medical studies have shown that heart failure or heart attacks are increasing among younger people in China. This is believed to be the result of a more modern life style, the fast pace of city life and increased pressure from work or school. The use of AMI Diagnostic Kits will help in early detection of MI that can help in reducing these incidences.
We are continuing our marketing efforts with respect to these testing kits which have contributed to increase sales of these products in 2009 versus 2008. Sales generated under these products during the six month periods ended June 30, 2009 and 2008 amounted to approximately $6,789,000 and $3,983,000, respectively.
Summary of Our Resear chResearch and Development Activities
We currently conduct all of our research and development (“R&D” ) activities, either internally or through collaborative arrangements with universities and research institutions in the PRC. We have our own research, developme ntdevelopment and laboratory facilities located at TDR’s principal executive offices.
At present, our ongoing research is divided into five general areas:
| | · | the development of an enzyme linked immune technique to prepare extraneous diagnostic kits; |
| | · | the development of an enzyme linked gold colloid technique to prepare an extraneous rapid diagnostic test strip; |
| | · | the development of a g enegene recombination technique to prepare a gene drug; |
| | · | the development of a biology protein chip for various tumor diagnostic applications; and |
| | · | the development of a cord blood stem cell bank, as more fully described in our Annual R eportReport and other reports filed by us with the SEC. |
We currently have the following eight biological products under development: an HIV detection kit; a uterus cancer diagnostic kit; a breast cancer diagnostic kit; a liver cancer diagnostic kit; a rectum cancer diagnostic kit; a gastric cancer diagnostic kit; a gene recombination drug; and a multi-tumor marker protein chip detection kit. I n addition, w e are also working to establish additional sales networks and cell banks covering domestic and internation alinternational markets.
Due to cont inuedcontinued changes in the industry , Management cannot guarantee any of the above research and development activities will be successful or generate future revenues. We incurred research and development costs of $6,094,694 and $2,042,412 during each of the six month periods ended June 30, 2009 and 2008, respectively.
Significant Accounting Estimates and Policies
The discussion and analysis of our financial condition and results of operations is based upon our financial statements which have been prepared in accordance with accounting principles generally accepted in the United States of America. The preparation of these financial statements requires us to make estimates and judgments that affect the reported amounts of assets and liabilities. On an on-going basis, we evaluate our methodologies and assumptions used to derive these estimates. Estimates include the reserve allowance for doubtful accounts and inventories, the salability and recoverability of our products, our impairment test for tangible and intangible assets, income taxes and contingencies and the remaining useful lives of our tangible and certain intangible assets. We base our estimates on historical experience and on other assumptions that we believes to be reasonable under the circumstances, the results of which form our basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates. Our significant estimates include:
Property and equipment are evaluated for impairment whenever indicators of impairment exist. Accounting standards require that if an impairment indicator is present, we must assess whether the carrying amount of the asset is unrecoverable by estimating the sum of the future cash flows expected to result from the asset, undiscounted and without interest charges. If the recoverable amount is less than the carrying amount, an impairment charge must be recognized based on the fair value of the asset.
As part of the process of preparing our financial statements, we are required to estimate our income taxes. This process involves estimating our current tax exposure together with assessing temporary differences resulting from differing treatment of items for tax and accounting purposes. These differences result in deferred tax assets and liabilities. We must then assess the likelihood that our deferred tax assets will be recovered from future taxable income, and, to the extent we believe that recovery is not likely, we must establish a valuation allowance. To the extent that we establish a valuation allowance or increase this allowance in a period, we must include a tax provision or reduce our tax benefit in the statements of operations. We use our judgment to determine our provision or benefit for income taxes, deferred tax assets and liabilities and any valuation allowance recorded against our net deferred tax assets. We believe, based on a number of factors including historical operating losses, which we will not realize the future benefits of a significant portion of our net deferred tax assets and we have accordingly provided a full valuation allowance against our deferred tax assets. However, various factors may cause those assumptions to change in the near term.
We cannot predict what future laws and regulations might be passed that could have a material effect on our results of operations. We assess the impact of significant changes in laws and regulations on a regular basis and update the assumptions and estimates used to prepare our financial statements when we deem it necessary.
We have determined the significant principles by considering accounting policies that involve the most complex or subjective decisions or assessments. Our most significant accounting policies are those related to intangible assets and research and development.
Intangible assets – Our intangible assets primarily consists of patents. Patent costs are amortized over an estimated life of approximately ten years.
Intangible assets are accounted for in accordance with Statement of Financial Accounting Standards No. 142, Goodwill and Other Intangible Assets (“SFAS 142”). Intangible assets with finite useful lives are amortized while intangible assets with indefinite useful lives are not amortized. As prescribed by SFAS 142, goodwill and intangible assets are tested periodically for impairment. We adopted SFAS No. 144, Accounting for the Impairment or Disposal of Long- Lived Assets , effective January 1, 2002. Accordingly, we review our long-lived assets, including property and equipment and finite-lived intangible assets for impairment whenever events or changes in circumstances indicate that the carrying amount of the assets may not be fully recoverable. To determine recoverability of its long-lived assets, we evaluate the probability that future undiscounted net cash flows will be less than the carrying amount of the assets. Impairment costs, if any, are measured by comparing the carrying amount of the related assets to their fair value.
Research and development—Research and development expenses include the costs associated with our internal research and development as well as research and development conducted by third parties. These costs primarily consist of salaries, clinical trials, outside consultants, and materials. All research and development costs are expensed as incurred.
Third-party expenses reimbursed under non-refundable research and development contracts are recorded as a reduction to research and development costs in the statement of operations.
We recognize in-process research and development in accordance with FASB Interpretation No. 4, Applicability of FASB Statement No. 2 to Business Combinations Accounted for by the Purchase Method and the AICPA Technical Practice Aid, Assets Acquired in a Business Combination to be used in Research and Development Activities: A Focus on Software, Electronic Devices , and Pharmaceutical Industries. Assets to be used in research and development activities, specifically, compounds that have yet to receive new drug approval and would have no alternative use, should approval not be given, are immediately charged to expense when acquired. Certain assets and high technologies acquired that has a foreseeable future cash flows are capitalized as intangible assets. Such intangible assets are amortized starting from the year revenue is generated and amortized over a period of 10 years. If a capitalized intangible asset is deemed to have no future benefit, the unamortized carrying value will be expensed.
For the three months ended June 30, 2009 and 2008, we incurred $3,681,914 and $1,372,579, respectively, in research and development expenditures. For the six months ended June 30, 2009 and 2008, we incurred $6,094,694 and $2,042,412, respectively, in research and development expenditures.
RESULTS OF OPERATIONS
For the three months ended June 30, 2009 as compared to June 30, 2008
Our principal business operations are conducted through our wholly owned subsidiary, TDR, and TDR’s wholly-owned subsidiaries.
| | For Three Months Ended June 30 |
| | 2009 | | % of Sales | | 2008 | | % of Sales |
| Revenues | | $ | 32,181,590 | | | | 100.0 | | | $ | 23,748,592 | | 100.0 |
| Cost of goods sold | | | 7,752,371 | | | | 24.1 | | | | 5,522,314 | | 23.3 |
| Gross Profit | | $ | 24,429,219 | | | | 75.9 | | | $ | 18,226,278 | | 76.7 |
Total revenues increased approximately $8.4 million or 36% during the three months ended June 30, 2009 as compared to 2008. Our revenue increase is primarily attributable to strong performances from our sales distribution channels due to our hiring of additional direct territory managers and sales agents to help market our products and their associated benefits to those individuals making or influencing purchasing decisions, as well as the results of our several successful business acquisitions in 2008 as previous discussed.
Revenues by Product Line
A break-down of our revenues by product line for each of the three months ended June 30, 2009 and 2008 is as follows:
| | | For the Three Months Ended June 3030, | |
| | | | | 2009 | | | | 2008 | |
Product (Number of Products) | | Subsidiary | | Sales (USD) | | | % of Sales | | Product (Number of Products) | | Sales (USD) | | | % of Sales | |
| Patch (5) | | TDR | | $ | 9,937,203 | | | | 30.9 | | Patch (4) | | $ | 8,781,903 | | | | 37.0 | |
| Ointment (18) | | TDR&TL | | | 7,658,146 | | | | 23.8 | | Ointment (11) | | | 6,486,619 | | | | 27.3 | |
| Spray (15) | | TDR&TL | | | 4,808,652 | | | | 14.9 | | Spray (19) | | | 2,840,196 | | | | 12.0 | |
| Bio-Engineering (3) | | FIRST | | | 3,687,991 | | | | 11.5 | | Bio-Engineering (3) | | | 2,145,355 | | | | 9.0 | |
| Others (48) | | TDR&TL&PL | | | 6,089,598 | | | | 18.9 | | Others (31) | | | 3,494,518 | | | | 14.7 | |
| Total (89 products) | | | | $ | 32,181,590 | | | | 100 | | Total (68 products) | | $ | 23,748,592 | | | | 100 | |
In 2009 TDR discontinued its contract revenues as part of its strategic goals. Contract revenues of $1,861,225 for the three months ended June 30, 2008 have been reallocated to each of the applicable product to present a m ore appropriate measure of our revenues by product line.
Our internal growth was driven by increases in the revenues of TDR, which increased from $16,319,000 for the three months ended June 30, 2008 to $17,525,000 for the three months ended June 30, 2009; and First, which increased from $2,145,000 for the three months ended June 30, 2008 to $3,688,000 for the three months ended June 30, 2009.
For the three months ended June 30, 2008 contract sales were $1,861,000. In 2008, before TDR acquired Tianlong, the majority of contracts sales consisted of products purchased from Tianlong. In 2008, TDR began to discontinue contract sales as part of its strategic goals.
Overall, our product gross margins were at approximately 75.9% and 76.7% during eachFollowing the Tianlong acquisition, we were able to fully integrate Tianlong’s products into our marketing and distribution channels and increase overall sales. As a result, we derived an aggregate of $3,423,000 from the sale of Tianlong’s products for the remainder of the three months ended June 30, 2009 and 2008, respectively. Our lower product gross margins in 2009 versus 2008 were principally attributable to the competivenessin addition to the $1,447,000 of contract sales of Tianlong’s products from January 1, 2008 through the Tianlong acquisition. Revenues from the sale of Tianlong products were $9,541,000 for the three months ended June 30, 2009.
Prior to our acquisition of Peng Lai, as of September 5, 2008, Peng Lai had nominal production and operations. Peng Lai contributed revenue of $1,431,000 to our total revenue for the three months ended June 30, 2009.
Cost of Goods Sold and Product Gross Margin
| | | For the Three Months Ended June 30, | |
| | | | | | | | | | |
| Revenues | | $ | 32,181,590 | | | | 36 | % | | $ | 23,748,592 | |
| Cost of goods sold | | | 7,752,371 | | | | 40 | % | | | 5,522,314 | |
| Gross profit | | $ | 24,429,219 | | | | 34 | % | | $ | 18,226,278 | |
| Products gross margin | | | 76 | % | | | | | | | 77 | % |
The decrease in gross margins related to sales of certain TDR’s products due to the competitive of our sales prices in the PRC market.
Operating Expenses
The following table summarizes the changes in our operating expenses from $8,098,642 to $12,347,556 for each of the three months ended June 30, 2008 and 2009, respectively:
| | | For the Three Months Ended June 3030, | |
| | | 2009 | | | % of Sales | | | 2008 | | % of Sales | |
| Operating Expenses | | | | | | | | | | | | |
| Depreciation and amortization | | $ | 449,294 | | | | 1.4 | | | $ | 139,004 | | | | 0.6 | |
| Research and development | | | 3,681,914 | | | | 11.4 | | | | 1,372,579 | | | | 5.8 | |
| Selling, general and administrative | | | 8,216,348 | | | | 25.5 | | | | 6,587,059 | | | | 27.7 | |
| Total operating expenses | | $ | 12,347,556 | | | | 38.3 | | | $ | 8,098,642 | | | | 34.1 | |
Depreciation and amortization for the three months ended June 30, 2009 amounted to approximately $449,000 as compared to $ 139,000 during the same period in 2008. The higher costs in 2009 are primarily attributable to the additional depreciation and amortization costs associated with the tangible and intangible assets acquired under our 2008 strategic business acquisitions.
Research and development expenses were approximately $3.7 million for the three months ended June 30, 2009 as compared to $1.4 million for the same period in 2008. The increased costs in 2009 is primarily associated with the ongoing clinical trials for proposed products and studies under the patents, licenses and other technologies acquired from our 2008 strategic business acquisitions.
Selling, general and administrative expenses were $8.2 million for the three months ended June 30, 2009 versus $6.6 mil lion for the same period in 2008. The higher selling, general and administrative expenses were primarily attributable to increased marketing and selling costs to support our revenue growth from $23.7 million in 2008 to $32.2 million in 2009.
For the six months ended June 30, 2009 as compared to June 30, 2008
Our principal business operations are conducted through our wholly owned subsidiary, TDR, and TDR’s wholly-owned subsidiaries.
| | For the Six Months Ended June 3030, | |
| | 2009 | | % of Sales | | 2008 | | % of Sales | |
| Revenues | | $ | 57,015,282 | | | | 100.0 | | | $ | 36,162,022 | | | | 100.0 | |
| Cost of goods sold | | | 13,793,289 | | | | 24.2 | | | | 8,382,742 | | | | 23.2 | |
| Gross Profit | | $ | 43,221,993 | | | | 75.8 | | | $ | 27,779,280 | | | | 76.8 | |
Total revenues increased by 58% during the six months ended June 30, 2009 as compared to 2008. The $20.9 million increase in revenue is attributable to strong performances from our sales distribution channels and the results of our several successful acquisitions.
As part of our strategic goals, contract sales of non-manufactured products were discontinued from early 2009.
Revenues by Product Line
A break-down of our revenues by product line for each of the six months ended June 30, 2009 and 2008 is as follows:
| | | For the Six Months Ended June 3030, | |
| | | | | 2009 | | | | 2008 | |
Product (Number of Products) | | Subsidiary | | Sales (USD) | | | % of Sales | | Product (Number of Products) | | Sales (USD) | | | % of Sales | |
| Patch (5) | | TDR | | $ | 19,059,164 | | | | 33.4 | | Patch (4) | | $ | 12,573,362 | | | | 34.7 | |
| Ointment (18) | | TDR&TL | | | 12,740,124 | | | | 22.3 | | Ointment (11) | | | 8,796,305 | | | | 24.3 | |
| Spray (15) | | TDR&TL | | | 7,710,726 | | | | 13.5 | | Spray (19) | | | 4,878,058 | | | | 13.5 | |
| Bio-Engineering (3) | | FIRST | | | 6,788,766 | | | | 11.9 | | Bio-Engineering (3) | | | 3,982,860 | | | | 11.0 | |
| Others (48) | | TDR&TL&PL | | | 10,716,502 | | | | 18.9 | | Others (31) | | | 5,931,437 | | | | 16.5 | |
| Total (89 products) | | | | $ | 57,015,282 | | | | 100 | | Total (68 products) | | $ | 36,162,022 | | | | 100 | |
As shown in the table above, revenues for all products increased as compared to the six months ended June 30, 2008. Con tract sales of $4,836,072 for the six months ended June 30, 2008 have been reallocated to each of the applicable sale products to present a more appropriate measure of our sales by product line.
In the six months ended June 30, 2009, we remained focused on expanding our market coverage. Our sales representatives increased from approximately 1,300 to 1,500, along with the increase of approximately 1,000 pharmacies newly opened in 2009. Our total pharmacy coverage number in 2009 reached approximately 5,500 over 24 provinces in China versus 4,500 over 22 provinces in China in 2008.
Overall, our product gross margins were at 75.8% and 76.8% during the six months ended June 30, 2009 and 2008, respectively. Our lower product gross margins in 2009 were principally attributable to the competiveness of our sales prices in the PRC market.
Our internal growth was driven by increases in the revenues of TDR, which increased from $22,517,000 for the six months ended June 30, 2008 to $32,505,000 for the six months ended June 30, 2009; and First, which increased from $3,981,000 for the six months ended June 30, 2008 to $6,789,000 for the six months ended June 30, 2009. For the six months ended June 30, 2008 contract sales were $4,836,000.
In 2008, before TDR acquired Tianlong, the majority of our contracts sales consisted of products purchased from Tianlong. In 2008, TDR began to discontinue contract sales as part of its strategic goals.
Following the Tianlong acquisition, we were able to fully integrate Tianlong’s products into our marketing and distribution channels and increase overall sales. As a result, we derived an aggregate of $3,423,000 from the sale of Tianlong’s products for the remainder of the three months ended June 30, 2008, in addition to the $1,447,376 of contract sales of Tianlong’s products from January 1, 2008 through the Tianlong acquisition. Revenues from the sale of Tialong products were $14,137,000 for the six months ended June 30, 2009.
Prior to our acquisition of Peng Lai, as of September 5, 2008, Peng Lai had nominal production and operations. Peng Lai contributed revenue of $3,584,000 to our total revenue for the six months ended June 30, 2009.
Cost of Goods Sold and Product Gross Margin
| | | For the Six Months Ended June 30, | |
| | | | | | | | | | |
| Revenues | | $ | 57,015,282 | | | | 58 | % | | $ | 36,162,022 | |
| Cost of goods sold | | | 13,793,289 | | | | 65 | % | | $ | 8,382,742 | |
| Gross profit | | $ | 43,221,993 | | | | 56 | % | | $ | 27,779,280 | |
| Products gross margin | | | 76 | % | | | | | | | 77 | % |
From January 1, 2008 thought April 2, 2008, revenues from Tianlong contract sales were $1,477,000, and gross profit from these sales were $1,173,000. The gross margin from these sales were approximately 79.4%, After our acquisition of Tianlong, revenues from sales of Tianlong products were $3,584,000, and gross profit from these sales were $3,026,000. The gross margin from these sales was approximately 84.4%. This increase in gross margin from sales of Tianlong’s products following the acquisition was offset by the decrease in gross margins related to sales of certain TDR’s products due to the competitive of our sales prices in the PRC market.
Operating Expenses
The following table summarizes the changes in our operating expenses from $12,801,618 to $22,089,177 for each of the six months ended June 30, 2008 and 2009, respectively:
| | | For the Six Months Ended June 30 | |
| | | 2009 | | | % of Sales | | | 2008 | | | % of Sales | |
| Operating Expenses | | | | | | | | | | | | |
| Depreciation and amortization | | $ | 900,666 | | | | 1.6 | | | $ | 215,352 | | | | 0.6 | |
| Research and development | | | 6,094,694 | | | | 10.7 | | | | 2,042,412 | | | | 5.6 | |
| Selling, general and administrative | | | 15,093,816 | | | | 26.4 | | | | 10,543,854 | | | | 29.2 | |
| Total operating expenses | | $ | 22,089,176 | | | | 38.7 | | | $ | 12,801,618 | | | | 35.4 | |
Depreciation and amortization for the six months ended June 30, 2009 amounted to approximately $900,000 as compared to $215,000 during the same period in 2008. The higher costs in 2009 are primarily attributable to the additional depreciation and amortization costs associated with the tangible and intangible assets acquired under our 2008 strategic business acquisitions.
Research and development expenses were approximately $6.1 million for the six months ended June 30, 2009 compared to $2.0 million for 2008. The increased costs in 2009 is primarily associated with the ongoing clinical trials and studies under the patents, licenses and other technologies acquired from our 2008 strategic business acquisitions.
Selling, general and administrative expenses for the six months ended June 30, 2009 amounted to $15.1 million in 2009 versus $10.5 million over the same period in 2008. The higher selling, general and administrative expenses were primarily attributable to the increased costs of marketing and sales to support our product revenues growth from $36.2 million in 2008 to $57.0 million in 2009.
FULL YEAR 2009 OUTLOOK
We are affirming our 2009 annual guidance which was disclosed in our Annual Report.
We estimate our tota ltotal revenue in 2009 versus 2008 will increase by 40%, or approximately $37 million, with growth in all categories of our product sales. Our gross profit margin in 2009 is expected to be approximately 75% due to possible increase in prices of raw materials. Operating expenses are expected to increase due to a higher percentage of R&D investment, as well as the additional costs to support our expanding distribution channels and sales growth. We estimate our overall 2009 net profit margin to be approximately 30%.
Our new corporate headquarter is currently under construction and we plan to occupy the space by the end of fiscal 2009. As a result , we will no longer rent office space. Another benefit of the corporate headquarters is that all organizational departments will be consolidated into one main central place. This should create a more productive and efficient working environment for management operations, as well as all other business activities. Our new facilities will feature a dining area , gymnasium, basketball court, dormitor iesdormitories , and guest rooms to provide accommodation s and services to our staff and to all our guests visiting us. The cost outlays for our new corporate headquarter are planned to be entirely funded without borrowed funds.
LIQUIDITY AND CAPITAL RESOURCES
Certain of our liquidity and capital ratios are outlined below for the six month periods ended June 30, 2009 and 2008:
| | | 2009 | | | 2008 |
| | | | | | |
| Working capital ratio | | | 8.7 | | | | 6.3 | |
| Quick ratio | | | 7.6 | | | | 6.1 | |
| Average accounts receivable collection (days) | | | 47.6 | | | | 49.2 | |
| Average inventory turnover (days) | | | 14.6 | | | | 18.5 | |
| Outstanding debt | | $ | - | | | $ | - | |
| Stockholders’ equity per common share | | $ | 6.71 | | | $ | 4.79 | |
| | | | | | | | | |
| The following table summarizes our cash and cash equivalents position, our working capital, and our cash flow activity as of June 30, 2009 and 2008 and for each of the six months then ended: | | | | | | | | |
As of June 30: | | | | | | |
| Working capital | | $ | 66,039,269 | | | $ | 44,861,048 | |
| Inventories | | $ | 1,571,465 | | | $ | 1,424,155 | |
| | | | | | | | | |
| Six Months Ended June 30: | | | | | | | | |
| Cash provided by (used in): | | | | | | | | |
| Operating activities | | $ | 17,821,557 | | | $ | 17,820,563 | |
| Investing activities | | $ | (9,961,517 | ) | | $ | (9,111,946 | ) |
| Financing activities | | $ | 29,169 | | | $ | 24,327,963 | |
As of June 30: | | | | | | |
Working capital | | $ | 66,039,269 | | | $ | 44,861,048 | |
Inventories | | $ | 1,571,465 | | | $ | 1,424,155 | |
| | | | | | | | | |
Six Months Ended June 30: | | | | | | | | |
Cash provided by (used in): | | | | | | | | |
Operating activities | | $ | 17,821,557 | | | $ | 17,820,563 | |
Investing activities | | $ | (9,961,517 | ) | | $ | (9,111,946 | ) |
Financing activities | | $ | 29,169 | | | $ | 24,327,963 | |
As of Ju neJune 30, 2009, cash and cash equivalents were approximately $48.23 million.
As of June 30, 2009, we have spent approximately $9.9 million in construction costs related to our corporate headquarters which is planned to be completed by the end of fiscal 2009. We plan to fund our corporate headquarters construction project using internal funds. The estimated cost under this project is approximately $13 million.
Our working capital ratio is 8.7 versus 6.3 and quick ratio is 7.6 versus 6.1 at June 30, 2009 and 2008, respectively. Management endeavors to ensure that funds are available to take advantage of new strategic business alliances and that funds are sufficient to meet future liquidity and capital needs.
At June 30, 2009, there are no restrictiv e bank deposits pledged as security.
We calculate accounts receivable turnover by averaging the opening and closing balances of out accounts receivable during that period and dividing that amount by our average daily sales during that period. Since accounts receivables fluctuate over the course of each quarter, in order to determine a more representative accountant receivables collection days, management calculates the turnover rate on a quarter-by-quarter basis. Our average daily sales and turnover for each of the first two quarters of 2008 and 2009 were as follows:
Quarter Ended | | Average Daily Sales | | | Turnover (days) | |
| March 31, 2008 | | $ | 136,441 | | | | 74.5 | |
| June 30, 2008 | | $ | 260,974 | | | | 35.9 | |
| March 31, 2009 | | $ | 275,930 | | | | 52.7 | |
| June 30, 2009 | | $ | 353,644 | | | | 42.8 | |
Since sales and costs of goods sold fluctuate over the course of each quarter, in order to determine a more representative inventory rate, management calculates inventory rate on a quarter-by-quarter basis. Management calculates our inventory turnover rate using total inventory rather than just finished goods, because our production cycle is of an extremely short duration. Our average inventory and turnover for the first and second quarters of fiscal 2008 and 2009 were as follows:
Quarter Ended | | Average Inventory | | | Turnover (days) | |
| March 31, 2008 | | $ | 583,201 | | | | 18.6 | |
| June 30, 2008 | | $ | 1,109,443 | | | | 18.3 | |
| March 31, 2009 | | $ | 891,323 | | | | 13.4 | |
| June 30, 2009 | | $ | 1,445,880 | | | | 17.0 | |
At June 30, 2009, there are no restrictive bank deposits pledged as security.
Cash flows provided by operating activities was approximately $17.8 million for the six months ended June 30, 2009 and for the same period in 2008.
Our working capital at June 30, 2009 was appr oximatelyapproximately $66.0 million, compared to $44.9 million at June 30, 2008. Our increased working capital position in 2009 was principally funded by the cash flows generated from our operating activities of approximately $17.8 million in the six months ended June 30, 2009. Management considers current working capital and borrowing capabilities adequate to cover our current operating and capital requirements for the full year 2009.
In the first quarter of fiscal 2008 we received aggregate net proceeds of approxim atelyapproximately $23.5 million from the consummation of a private placement of our securities. The net pr oceedsproceeds from the private placement were used to fund three business acquisitions we completed in fiscal 2008 and other working capital needs. There was no similar financing in the six month period ended June 30, 2009.
Currency Exchange Fluctuations
All of our revenues and majority of the expenses during the six months ended June 30, 2009 were denominated primarily in Renminbi (“RMB”), the currency of China, and were converted into U.S. dollars at the exchange rate of 6.84323 RMB to 1 U.S. Dollar. For the three months ended June 30, 2009, the exchange rate is 6.83992 RMB to 1 U.S. Dollar. In the third quarter of 2005, the RMB began to rise against the US dollar. There could be no assurance that RMB-to-U.S. dollar exchange rates will remain stable. A devaluation of RMB relative to the U.S. dollar would adversely affect our business, financial condition and results of operations. We do not engage in currency hedging.
Off-Balance Sheet Arrangements
We do not have any off-balance sheet arrangements that are currently material or reasonably likely to be material to our financial position or results of operations.
Item 3. Quantitative and Qualitative Disclosures About Market Risk.
We are a smaller reporting company as defined by Rule 12b-2 of the Securities Exchange Act of 1934 (the “Exchange Act”) and are not required to provide the information under this item.
Disclosure Controls and Procedures
Our management, with the participation of our chief executive officer and interim chief financial officer, evaluated the effectiveness of our disclosure controls and procedures pursuant to Rule 13a-15 under the Exchange Act as of June 30, 2009. In designing and evaluating the disclosure controls and procedures, management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving the desired control objectives. In addition, the design of disclosure controls and procedures must reflect the fact that there are resource constraints and that management is required to apply its judgment in evaluating the benefits of possible controls and procedures relative to their costs.
Based on our evaluation, our chief executive officer and interim chief financial officer concluded that our disclosure controls and procedures are designed at a reasonable assurance level and are effective to provide reasonable assurance that information we are required to disclose in reports that we file or submit under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in Securities and Exchange Commission rules and forms, and that such information is accumulated and communicated to our management, including our chief executive officer and interim chief financial officer, as appropriate, to allow timely decisions regarding required disclosure.
Changes in Internal Control Over Financial Reporting
There was no change in our internal control over financial reporting that occurred during our second quarter of fiscal 2009, that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
PART II - OTHER INFORMATION
Item 1. Legal Proceedings.
We are not a party to any material pending legal proceedings.
Item 1A. Risk Factors.
We are a smaller reporting company as defined by Rule 12b-2 of the Securities Exchange Act of 1934 and are not required to provide the information under this item.
Item 2. Unregistered Sales of Equity Securities and Use of Proceeds.
In the three-month period ended June 30, 2009, and subsequent period through the date hereof, we did not engage in any unregistered sales of equity securities other than as set forth below:
Cashless Exercise of Warrants
As of April 29, 2009, warrants to purchase an aggregate of 150,000 shares of our common stock, which we issued to a consultant in consideration for services rendered in connection with the share exchange transaction we consummated in May 2006, were exercised on a cashless basis. In connection with the cashless exercise, the warrant holder was deemed to have paid an amount equal to the difference between the exercise price ($2.00 per share) and the average closing price of a share of our common stock during the ten (10) trading days ending on the date of exercise ($14.75 per share). As a result of such cashless exercise, we issued an aggregate of 129,661 shares of our common stock to the warrant holder.
We believe that this transaction is exempt from registration under the Securities Act of 1933, as amended, pursuant to Section 4(2), or Regulation D promulgated thereunder, as transactions by an issuer not involving a public offering.
Cashless Exercise of Stock Options
As of June 30, 2009, stock options to purchase an aggregate of 101,000 shares of our common stock, which we issued to pursuant to our 2006 Stock Incentive Plan on October 25, 2006, were exercised on a cashless basis by 36 optionees. In connection with the cashless exercises, the optionees were deemed to have paid an amount equal to the difference between the exercise price ($3.65 per share) and the fair market value of a share of our common stock on the date of exercise ($14.68 per share). As a result of such cashless exercises, we issued an aggregate of 75,888 shares of our common stock to the optionees.
We believe that these transactions are exempt from registration under the Securities Act of 1933, as amended, pursuant to Section 4(2), or Regulation D promulgated thereunder, as transactions by an issuer not involving a public offering.
Item 3. Defaults Upon Senior Securities.
In the three-month period ended June 30, 2009, and subsequent period through the date hereof, we did not default upon any senior securities.
Item 4. Submission of Matters to a Vote of Security Holders.
In the three-month period ended June 30, 2009, and subsequent period through the date hereof, we did not submit any matters to a vote of our stockholders.
Item 5. Other Information.
There was no information we were required to disclose in a report on Form 8-K during the three-month period ended June 30, 2009, or subsequent period through the date hereof, which was not so reported.
Item 6. Exhibits
| Exhibit No. | | | Description of Exhibit | |
| 31.1 | | Certification of Principal Executive Officer pursuant to Rule 13a-14 and Rule 15d-14(a), promulgated under the Securities and Exchange Act of 1934, as amended* |
| 31.2 | | Certification of Interim Principal Financial and Accounting Officer pursuant to Rule 13a-14 and Rule 15d-14(a), promulgated under the Securities and Exchange Act of 1934, as amended* |
| 32.1 | | Certification pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 (Principal Executive Officer)* |
| 32.2 | | Certification pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 (Interim Principal Financial and Accounting Officer)* |
| * Filed herewith |
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| | CHINA SKY ONE MEDICAL, INC. |
| | |
Dated: August 14, 2009____________________, 2010 | By: | /s/ Liu Yan Qing | |
| | | Liu Yan Qing Chairman, Chief Executive Officer and President | |
| | | | |
Dated: August 14, 2009____________________, 2010 | By: | /s/ Stanley Hao | |
| | | Stanley Hao Chief Financial Officer and Secretary | |