Table of Contents
UNITED STATES SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 10-K
| þ | Annual Report Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 |
For the fiscal year ended December 31, 2006
or
| o | Transition Report Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 |
For the transition period from to .
Commission File Number 0-27316
MOLECULAR DEVICES CORPORATION
(Exact name of Registrant as Specified in Its Charter)
| DELAWARE | 94-2914362 | |
| (State or Other Jurisdiction of | (I.R.S. Employer Identification No.) | |
| Incorporation or Organization) | ||
| 1311 ORLEANS DRIVE, SUNNYVALE, CALIFORNIA | 94089 | |
| (Address of Principal Executive Offices) | (Zip code) |
(408) 747-1700
(Registrant’s Telephone Number, Including Area Code)
(Registrant’s Telephone Number, Including Area Code)
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Name of each exchange on which registered | |
| Common Stock, $.001 par value | The NASDAQ Stock Market LLC |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.
YESo NOþ
YESo NOþ
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act.
YESo NOþ
YESo NOþ
Indicate by check mark whether the registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
YESþ NOo
YESþ NOo
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K.o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer or a non-accelerated filer. (check one):
Large accelerated filero Accelerated filerþ Non-accelerated filero
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act).
YESo NOþ
YESo NOþ
The aggregate market value of the voting and non-voting stock held by non-affiliates of the registrant as of June 30, 2006, based upon the last sale price reported for such date on The NASDAQ Global Select Market was $352,890,225.*
The number of outstanding shares of the registrant’s Common Stock as of March 9, 2007 was 16,580,097.
DOCUMENTS INCORPORATED BY REFERENCE
None
None
| * | Excludes approximately 5,183,945 shares of common stock held by directors, officers and holders of 5% or more of the registrant’s outstanding common stock at June 30, 2006. Exclusion of shares held by any person should not be construed to indicate that such person possesses the power, direct or indirect, to direct or cause the direction of the management or policies of the registrant, or that such person is controlled by or under common control with the registrant. |
TABLE OF CONTENTS
| 1 | ||||||||
| 11 | ||||||||
| 21 | ||||||||
| 21 | ||||||||
| 21 | ||||||||
| 21 | ||||||||
| 22 | ||||||||
| 23 | ||||||||
| 24 | ||||||||
| 33 | ||||||||
| 34 | ||||||||
| 35 | ||||||||
| 35 | ||||||||
| 35 | ||||||||
| 36 | ||||||||
| 38 | ||||||||
| 55 | ||||||||
| 58 | ||||||||
| 59 | ||||||||
| 59 | ||||||||
| 62 | ||||||||
| EXHIBIT 21.1 | ||||||||
| EXHIBIT 23.1 | ||||||||
| EXHIBIT 31.1 | ||||||||
| EXHIBIT 31.2 | ||||||||
| EXHIBIT 32.1 | ||||||||
Table of Contents
Except for the historical information contained herein, this Annual Report on Form 10-K contains “forward-looking” statements within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended, and Section 27A of the Securities Act of 1933, as amended. For this purpose, any statements that are not statements of historical fact may be deemed to be forward-looking statements. Words such as “believes,” “anticipates,” “plans,” “predicts,” “expects,” “estimates,” “intends,” “will,” “continue,” “may,” “potential,” “should” and similar expressions are intended to identify forward-looking statements. There are a number of important factors that could cause our results to differ materially from those indicated by these forward-looking statements, including, among others, those discussed in Item 1A — “Risk Factors,” Item 7 — “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” Item 7A — “Quantitative and Qualitative Disclosures About Market Risk,” and the risks detailed from time to time in the Company’s future U.S. Securities and Exchange Commission (“SEC”) reports. Neither we nor any other person assumes responsibility for the accuracy and completeness of these statements. We assume no duty to update any of the forward-looking statements after the date of this report or to conform these statements to actual results. Accordingly, we caution readers not to place undue reliance on these statements.
PART I
Item 1.Business.
The Company
We are a leading supplier of high-performance bioanalytical measurement systems that accelerate and improve drug discovery and other life sciences research. Our systems and consumables enable pharmaceutical and biotechnology companies to leverage advances in genomics, proteomics and parallel chemistry by facilitating the high-throughput and cost-effective identification and evaluation of drug candidates. Our solutions are based on our advanced core technologies that integrate our expertise in engineering, molecular and cell biology and chemistry. We enable our customers to improve research productivity and effectiveness, which ultimately accelerates the complex process of discovering and developing new drugs. We were incorporated in California in 1983 and reincorporated in Delaware in 1995.
On January 28, 2007, we entered into a definitive agreement and plan of merger with MDS Inc. (“MDS”) and Monument Acquisition Corp. (“Monument”), a subsidiary of MDS, which contemplates the acquisition by MDS, through Monument, of all of our outstanding common stock in a two-step transaction comprised of a cash tender offer for all of our issued and outstanding shares of common stock, followed by a merger of Monument with and into us. Pursuant to the definitive agreement, and upon the terms and subject to the conditions thereof, Monument has commenced a tender offer to acquire all of the outstanding shares of our common stock at a price of $35.50 per share, net to the holder thereof in cash. Pursuant to the definitive agreement, as soon as practicable after the consummation of the tender offer and subject to the satisfaction or waiver of certain conditions set forth in the definitive agreement, Monument will merge with and into us and we will become an indirect wholly-owned subsidiary of MDS. In this merger, each share of our common stock remaining outstanding following the consummation of the tender offer, other than each share held by MDS or Monument or by any stockholder who has validly exercised appraisal rights under Delaware law, will be converted into the right to receive $35.50 in cash. The obligation of Monument to accept for payment and pay for the shares tendered in the tender offer is subject to the satisfaction or waiver of a number of closing conditions set forth in the definitive agreement, including among others, the expiration of the waiting period under the Hart-Scott-Rodino Antitrust Improvements Act. In addition, it is also a condition to Monument’s obligation to accept for payment and pay for the shares tendered in the tender offer that at least a majority of the then-outstanding shares of our common stock (determined on a fully diluted basis including any unvested stock options that would vest by their terms on or before June 30, 2007, but disregarding any other unvested stock options) will have been validly tendered in accordance with the terms of the tender and not validly withdrawn. The closing of the merger contemplated by the definitive agreement is subject to customary closing conditions, and, depending on the number of shares held by MDS and Monument after Monument’s acceptance of the shares properly tendered in connection with the tender offer, approval of the merger contemplated by the definitive agreement by the holders of the outstanding shares of our common stock remaining after the completion of the tender offer may be required.
Industry Background
In recent years, research in the life sciences industry has accelerated as funding from major private and public sources has significantly increased. This expansion of research activity has yielded discoveries that are currently fueling a revolution in our understanding of human health and disease. One major milestone, the sequencing of the human genome, was widely celebrated not only as an important scientific advance in itself, but also as a starting point for a much broader exploration of fundamental biological processes. The genome map is significant because genes and the proteins they encode play a central role in every aspect of the body’s functioning. Learning which genes code for various proteins, which proteins are involved in different biological events and how these proteins function or malfunction are all challenges that are now being tackled by scientists on an unprecedented scale. By better understanding biology at the level of genes, proteins and cells, researchers hope to discover the underlying causes of human diseases and determine new ways to treat them.
1
Table of Contents
Because of their critical role in the body, proteins that malfunction or are present in abnormal quantities can cause problems that are manifested as diseases. Drugs typically fight illness by binding to such proteins, known as “targets,” and modifying their behavior to reduce their disease-causing effects. Collectively, all of the pharmacologically active drugs currently on the market are aimed at approximately 400 distinct protein targets in the human body. The human genome map has revealed the existence of an additional 3,000 to 5,000 targets that may be associated with a variety of diseases. This expansion in the number of potential drug targets is driving increased activity in two areas of scientific inquiry, basic life sciences research and drug discovery.
Understanding the role of genes and proteins in human health is a goal of basic life sciences research, which is conducted by a variety of pharmaceutical, biotechnology, academic and other organizations. The proliferation of genomic information and uncharacterized protein targets has enhanced the importance of such basic research in all of these settings. The process of gathering this wide range of new biological information involves a variety of techniques and tools. To identify genes and understand their functioning, researchers use microarray scanners, instruments that are designed to read gene chips. Studying proteins can involve a large number of “test tube” experiments; today, many such experiments are no longer performed in test tubes, but in plates with an array of wells known as “microplates.” With microplates, data can be obtained much more quickly than in the past using instruments called microplate readers. In addition to gene and isolated protein studies, biologists often conduct research on cells using the techniques of microscopy and electrophysiology. Electrophysiology is concerned with the flow of ions into and out of cells and utilizes electrical recording to measure this flow. The large toolkit available to life sciences researchers, including the methods mentioned above, is currently enabling a significant expansion in our understanding of disease processes.
Once a protein’s link to a disease is understood, the task of finding a drug that acts on the protein and treats the disease is undertaken primarily by pharmaceutical and biotechnology companies. These companies typically own “libraries” of drug candidates comprising hundreds of thousands, or even millions, of chemical compounds. In order to determine which compounds are effective against a particular target, a test, or assay, must be developed to detect whether a compound has modified the behavior of the target. Then, in a process known as “screening,” this test must be repeated for each compound in the library. As life sciences research continues to unveil new targets, the task of screening large libraries of compounds against these targets represents both a great opportunity and a technological challenge for pharmaceutical companies. Accordingly, these companies have invested heavily in systems that automate and accelerate this process, often implementing drug screening “factories” where compounds are run through a battery of cellular and biochemical tests on high-speed equipment around the clock. This trend toward industrializing drug candidate screening has gained momentum among pharmaceutical companies in recent years and continues to be a major driver of spending in the drug discovery market.
Our Products
We offer a full range of high-performance bioanalytical tools including automated systems for pharmaceutical screening and a variety of general-purpose research instruments. We group our product offerings into two families, life sciences research and drug discovery, to reflect the markets they primarily address. We operate in a single industry segment. We had revenues of $186.4 million in 2006, $182.0 million in 2005 and $149.1 million in 2004. Most of our products use optical technologies to detect the results of biological tests that occur in microplates. A microplate is a disposable vessel comprised of a standardized array of 96, 384 or 1,536 wells that are similar to small test tubes. This format has been widely adopted by scientists because it allows many experiments to be performed in parallel, enhancing the efficiency of research efforts. In recent years, customers have increasingly sought the cost and throughput benefits of the higher-density 384 and 1,536 well configurations, a trend that we expect to continue.
Life Sciences Research Products
Our life sciences research products, which represented 63% of total revenues in 2006, 60% of total revenues in 2005 and 59% of total revenues in 2004, encompass our SpectraMax®, GenePix®, MetaMorph®, Laser Capture Microdissection, Cellular Neurosciences, Liquid Handling and Threshold® product lines.
Microplate Detection Products
Our microplate detection products consist of our SpectraMax® and FlexStation® families of advanced microplate readers. Microplate readers have become one of the most fundamental tools used in life sciences research by addressing the increasing need for the acquisition and processing of large quantities of biochemical and biological data. Because of the productivity gains offered by their multi-sample format, microplates have largely replaced test tubes and cuvettes for many life sciences applications.
2
Table of Contents
The basic principles of microplate readers are that light from an appropriate source is directed to a wavelength selection device, such as a monochromator, and its intensity is measured either before and after, or just after, passing through each of the sample wells of a microplate. Application of a mathematical formula to the light intensity measurements of each microplate well provides a measure of the sample present in the well. One type of measurement, known as optical density, is proportional to the concentration of the substance that is being measured. Other important types of light intensity measurement are fluorometry and luminometry, both of which provide quantitative information comparing the different samples in a microplate with each other.
SpectraMax
Our SpectraMax strategy has been to continue to introduce new products that include first-of-a-kind features, as well as to offer varying feature sets and price points to address different market segments. We have historically focused on the premium end of the microplate reader market through offering products with advanced capabilities. Some of the first-of-a-kind features that we have pioneered include the first reader and software capable of kinetic analysis, the first monochromator-based reader that enabled continuous wavelength selection and the first reader capable of performance comparable to a spectrophotometer. In each case, we believe that the innovation helped expand the utility of microplate readers and, more broadly, the available market for microplate readers. Our SpectraMax family currently includes the following products:
| • | EMax®.This product is aimed at the market for traditional microplate readers that do not require kinetic capability. We introduced it to provide a reader for customers in academia and other customers with restricted capital budgets. | ||
| • | VMax®.This product was the first microplate reader to offer kinetic read capability and is designed to address the needs of biochemists. | ||
| • | VersaMaxTM. The VersaMax is our low cost variable wavelength offering that provides kinetic capability and temperature control. | ||
| • | SpectraMax 340PC384.This product is a visible range microplate spectrophotometer, offering tunability and the additional capability of our patented PathCheck® Sensor technology, which corrects common variability problems across wells of microplates. | ||
| • | SpectraMax 190.The predecessor to this product was the first microplate reader that incorporated a monochromator for continuous wavelength selection. Wavelength selection provides for enhanced convenience and flexibility in assay design. In addition, the SpectraMax 190 also includes our patented PathCheck Sensor technology. | ||
| • | SpectraMax Plus384.The SpectraMax Plus384 combines the high-throughput of a microplate reader with the performance of a cuvette-based spectrophotometer as a result of our patented PathCheck Sensor technology. It is capable of reading wavelengths as short as 190 nanometers and as long as 1,000 nanometers, the equivalent range to a spectrophotometer, and is compatible with both 96-well and 384-well microplates. | ||
| • | Gemini XPS.Gemini was the world’s first dual-scanning microplate spectrofluorometer, a configuration that allows the user to automatically optimize the instrument for particular assays. The Gemini XPS, introduced in 2004, is the most sensitive version of Gemini yet and is capable of fluorescence, luminescence and time-resolved fluorescence measurements. | ||
| • | Gemini EM.The Gemini EM features the ability to read microplates from either the top or the bottom, a capability that enables it to perform complex cell-based assays. | ||
| • | LMax II384.LMax was our first reader to offer customers sensitive luminescence detection in a bench-top instrument. The second generation of LMax adds improved sensitivity, 384-well detection and the capability to integrate with laboratory robots. | ||
| • | SpectraMax M2/M2e. These instruments incorporate the dual-scanning monochromator technology of the Gemini family into a multimode reader with both absorbance and fluorescence detection. SpectraMax M2/M2e can read 96 and 384 well microplates using any of four different scanning techniques. This combination of features makes these highly versatile instruments capable of a wide range of applications. The SpectraMax M2eoffers the additional option ofbottom-reading capability. | ||
| • | SpectraMax M5/M5e.These instruments expand our multimode reader family by offering the performance features of SpectraMax M2/M2e with the flexibility of five different detection modes. Our most versatile benchtop readers, SpectraMax M5/M5e can measure absorbance, fluorescence intensity, fluorescence polarization, time-resolved fluorescence and luminescence. The SpectraMax M5e offers the additional benefit of being certified for a popular assay known as HTRF. | ||
| • | StakMax.Introduced in 2006, the StakMax is a microplate handling system that is compatible with all of our SpectraMax readers. This product allows users to automatically process magazines of 20, 40 or 50 microplates, greatly increasing the efficiency of laboratory operations. |
3
Table of Contents
FlexStation
Our FlexStation system is a bench top workstation that integrates liquid handling and detection and has applications in drug discovery as well as life sciences research. This product offers flexibility to address a wide range of research applications by combining both multi-channel, plate-to-plate fluid transfer and fluorescence measurement in one system. For drug discovery applications, FlexStation provides a convenient means of developing assays for later transfer to higher-throughput screening. For basic and applied research in life sciences, the flexibility of this system enables scientists to develop, optimize and run their assays on one system with the same small footprint as a standard benchtop microplate reader. The FlexStation family comprises the following instruments and reagents:
| • | FlexStation II/II 384. Introduced in 2003, these instruments represent the second generation of FlexStation technology with dual-monochrometer scanning, 8- or 16-channel fluid transfer and top or bottom reading. FlexStation II is compatible with 96-well microplates, while FlexStation II384 addresses both 96-well and 384-well formats. In addition to instrumentation, we offer proprietary reagent kits based on our successful FLIPR assay technology and optimized to perform on the FlexStation platform. These products are our FlexStation Calcium Assay Kit, FlexStation Calcium 3 Assay Kit, two formulations of the FlexStation Membrane Potential Assay Kit and our QBTTM Fatty Acid Uptake Assay Kit. | ||
| • | FlexStation 3. This instrument, introduced in 2006, adds liquid handling capabilities to our popular SpectraMax M5/M5eplatform, enabling an even wider variety of applications than previous generations of FlexStation. In addition to the assay kits that are compatible with FlexStation II/ II384, FlexStation 3 is capable of running our Calcium 4 Assay Kit and IMAP assays. |
GenePix Microarray Systems
Because of the central role that genes play in human health and disease, the study of genes, or genomics, is a fundamental area of life sciences research. By studying the varying levels of gene expression among members of a population or between healthy and diseased tissues, researchers can learn the functions of tens of thousands of genes that constitute the genomes of humans and other complex organisms. Microarrays, which allow the high-throughput identification of large numbers of genes, have been an enabling technology in this field. Our GenePix® family, a complete line of instruments and software for analyzing microarrays, includes the following products:
| • | GenePix 4000B.The GenePix 4000B is the fastest and most compact microarray scanner on the market today. Unlike most commercially available scanners, this instrument acquires data simultaneously at two wavelengths, resulting in superior speed and a low error rate. Like all of our microarray scanners, the GenePix 4000B comes with our popular and user-friendly GenePix Pro software for data acquisition and analysis. | ||
| • | GenePix Professional 4200A.This instrument offers the performance of the GenePix 4000B with additional features such as four-color scanning and enhanced flexibility. An optional accessory, the Autoloader 4200AL, automates the process of introducing microarrays to the instrument, an important feature for high-volume laboratory environments. | ||
| • | GenePix Personal 4100A.This instrument offers the superior performance of the GenePix family in a lower-cost, more compact version that addresses the needs of the individual researcher. | ||
| • | Acuity® 4.0. This software package provides applications for advanced users, including data warehousing, sophisticated data analysis and visualization applications. It is compatible with the GenePix family of scanners as well as other commercially available microarray platforms. |
MetaMorph Imaging Software
We offer software that works in tandem with microscope and camera systems to enable researchers to acquire images of cells and quantify and analyze the images in a variety of ways. This product family consists of the following software packages:
| • | MetaMorph®.MetaMorph is a state-of-the-art software package for capturing and analyzing cellular images. MetaMorph’s functions include control of a wide variety of imaging devices as well as a large menu of tools for image processing and analysis. | ||
| • | MetaFluor®.MetaFluor software allows researchers to image and analyze ratiometric indicators of intracellular events. | ||
| • | MetaVueTM. A lower-cost version of MetaMorph, MetaVue is an entry-level product tailored to common imaging applications. |
4
Table of Contents
We sell MetaMorph either as a stand-alone product or as part of an integrated system including a camera, software and peripherals. We are an authorized reseller of cameras and peripheral equipment for several major manufacturers, including Nikon Corporation and Roper Industries, Inc. Additionally, we have authorized several value-added resellers, who integrate multiple components to create complete imaging systems, to distribute MetaMorph.
Laser Capture Microdissection
Laser Capture Microdissection (“LCM”) is a sophisticated sample preparation technology for genomic and imaging analysis that is an upstream step for many of our GenePix and MetaMorph customers. LCM allows researchers to visualize and extract individual cells or groups of cells from tissue samples with minimal damage. The selective capture of specific cells through LCM allows researchers to quickly and accurately utilize the smallest and purest cellular samples for further RNA, DNA or protein analysis. In 2006, we acquired the LCM business from Arcturus Bioscience, Inc. (“Arcturus”), and we now offer the following systems and reagents:
| • | Arcturus XTTM. This open-architecture system is built upon the Nikon TE2000U(R) inverted research grade microscope and combines infrared laser-enabled LCM and ultraviolet laser cutting in a single modular instrument, offering researchers superior speed, precision and flexibility. It is completely modular and fully upgradeable, allowing the system utility to expand as research requirements grow and microdissection technologies emerge. | ||
| • | PixCell® IIe. This is a dedicated LCM instrument that offers researchers a simple, rapid and effective tool for selecting cells of interest from a tissue sample. The system, like all of our LCM instruments, utilizes our CapSure® disposable caps for clean and accurate retrieval of user-defined cell populations. | ||
| • | VeritasTM. This system offers both LCM and laser cutting, enabling researchers to prepare samples from a wide variety of tissue types. Veritas is highly automated, simplifying the LCM process and enabling researchers to operate the system with minimal training. | ||
| • | Reagents. We offer several reagent kits that are optimized for the Veritas and PixCell IIe and allow researchers to isolate RNA and DNA and amplify RNA from a variety of tissue types. |
Cellular Neurosciences Research
We are a leading supplier of signal amplification instruments and related software for cellular neurosciences research, offering a range of products for voltage recording, current and voltage clamping and patch clamping. Our microelectrode amplifiers are more sensitive than any competing product, enabling scientists to perform experiments that would otherwise be impossible. In addition, we offer software packages for the acquisition and analysis of electrophysiological data and various accessories for the electrophysiology workstation. Our products for cellular neurosciences research consist of the following:
| • | Digidata 1440A.The Digidata 1440A is a high-speed, low-noise data acquisition system that is compatible with our pCLAMP 10.0 software. | ||
| • | pCLAMP 10.0.Introduced in 2006, pCLAMP 10.0 is our latest-generation software package for acquisition and analysis of electrophysiological data. | ||
| • | MultiClamp 700B.MultiClamp 700B is a computer-controlled microelectrode amplifier that is used for high-speed current clamp, patch clamp, voltammetry/amperometry, ion-selective measurement, and bilayer recordings. | ||
| • | Axopatch 200B.Axopatch 200B patch clamp is the latest version of our premier patch-clamp amplifier, incorporating capacitor-feedback technology for single-channel recording as well as resistive-feedback for whole-cell recording. | ||
| • | Axoclamp 900A. Axoclamp 900A is a computer-controlled microelectrode current-clamp and voltage-clamp amplifier, useful for a wide range of intracellular microelectrode recording techniques with several modes of operation. |
Liquid Handling Systems
We offer a line of liquid handling systems that includes a variety of cell and plate washers with 96, 384 and 1,536 well dispensing and washing capabilities. Washers dispense and remove fluid from microplates and are used as an integral step during the course of many assays. Our liquid handling systems bring a complete line of state-of-the-art microplate washers and other related tools, including cell harvesters, to the life sciences research product family.
5
Table of Contents
Threshold System
As a result of the growing number of biopharmaceutical therapeutics both entering clinical trials and receiving regulatory approval for commercial sale, there is demand for systems that can quantitate contaminants in the manufacturing and quality control of bioengineered products. Our Threshold system, which comprises a detection instrument and reagents, incorporates a proprietary technology to quantitate a variety of biomolecules such as DNA, proteins and mRNA rapidly and accurately. The Threshold system emerged from a need by biopharmaceutical companies for more sensitive and reproducible methods to detect contaminants in biopharmaceuticals during the manufacturing and quality control process. The Threshold family of products includes a workstation, software and consumable reagent kits.
Drug Discovery Products
Our drug discovery systems, which represented 37% of our total revenues in 2006, 40% of our total revenues in 2005 and 41% of our total revenues in 2004, are used to screen large numbers of chemical compounds to assess their effects on disease targets. This category includes our FLIPR, Automated Electrophysiology, High-Throughput Imaging and Analyst product families.
FLIPR System and Reagent Kits
Since its introduction in 1995, our FLIPR system has become the industry standard for the automated testing of compounds in live cells. Because they provide highly biologically relevant results, live cell assays are valuable tools for drug discovery. Many important target classes are best studied in live cells, including the most popular target class, G-protein-coupled receptors (“GPCRs”). FLIPR addresses a key market need for automating a common GPCR test based on the phenomenon of calcium flux. The activation of many types of GPCRs triggers a release of calcium within the cell, an event that can be detected using a calcium-sensitive fluorescent dye. Because this assay requires live cells and produces only a brief signal, it cannot be performed on standard bioanalytical instruments. FLIPR’s optical and fluidic systems are specialized for this type of assay, automating the process and enabling multiple experiments to be performed simultaneously in microplates. Because of its unique configuration, FLIPR is also able to perform other complex live cell assays, such as detecting changes in cellular membrane potential. The latest generation of FLIPR is engineered to meet the industrialization requirements of our pharmaceutical company customers. Our FLIPR instrumentation is complemented by our FLIPR reagent kits, which use a proprietary technology to reduce the number of steps involved in live cell testing.
| • | FLIPRTETRA.This product is the fourth generation FLIPR instrument. It combines all of the benefits of preceding versions of FLIPR with new capabilities in a completely redesigned, compact platform. FLIPRTETRA features simultaneous liquid transfer of samples in 96, 384 and 1,536 well microplates, allowing customers to screen as many as 250,000 samples daily with little human intervention. In addition, the instrument enables excitation and detection at multiple wavelengths, enabling a broader set of applications, and incorporates interfaces that allow it to integrate into automated screening lines. | ||
| • | FLIPR Assay Kits.This product family includes the FLIPR Calcium Assay Kit, the FLIPR Calcium 3 Kit, the FLIPR Calcium 4 Assay Kit, the QBT Fatty Acid Uptake Assay Kit and two formulations of the FLIPR Membrane Potential Assay Kit. By eliminating a step in the assay protocol, these kits can significantly increase throughput, reduce costs and increase screening efficiency. The FLIPR Calcium Assay Kit addresses the most popular assay performed on the FLIPR system for detecting the activation of GPCRs. The FLIPR Calcium 3 Assay Kit extends the applicability of this assay by allowing researchers to test problematic but important targets such as chemokines and other small peptides. The FLIPR Calcium 4 Assay Kit, introduced in 2006, features a new dye which allows researchers to detect very small calcium responses. The QBT Fatty Acid Uptake Assay Kit offers the only kinetic uptake assay in a one-step, fluorescent format for detecting processes associated with diabetes and cardiovascular disease. The FLIPR Membrane Potential Assay Kits allow researchers to measure changes in the electrical potential across live cell membranes, a key indicator of ion channel activity. |
Automated Electrophysiology Systems
In recent years, several drugs have been withdrawn from the market due to safety concerns, resulting in financial losses, negative publicity and ongoing litigation for some major pharmaceutical companies. As a result, drug companies are strengthening their efforts to understand more thoroughly the “safety profile” of prospective drugs before they are launched. Developing such a profile requires increased testing of a candidate compound to determine whether it interacts with certain biological pathways that are associated with adverse side-effects. Among the most important of these pathways are those involving ion channels, cellular structures that mediate many key biological processes such as heart muscle contractions. Accordingly, ion channel testing is receiving increased attention as a way of identifying compounds that may put patients at risk for cardiac arrest.
6
Table of Contents
We are an industry leader in automated tools for testing ion channels, which are important both as a target class and for their role in producing drug side-effects. Traditionally, the most valuable information on ion channel activity has been obtained through patch clamping, a time-consuming, low-throughput method that is best performed by highly-skilled scientists. Few high-throughput alternatives exist for direct patch clamping. Those that do use indirect methods to assay ion channels, which yield lower quality data than patch clamping. Our Automated Electrophysiology products are automated systems that obtain the same high-quality information from cells as conventional patch clamping, but at a much faster rate and requiring far less operator skill. While traditional patch clamping may allow researchers to test only 5-15 compounds per day, our Automated Electrophysiology systems operate at speeds ranging from hundreds to thousands of compounds per day.
| • | IonWorks Quattro.This is a turnkey system for drug discovery screening and safety profiling that enables customers to obtain up to 400,000 data points per year, the highest throughput currently available. The system consists of an instrument and a family of proprietary consumables. | ||
| • | PatchXpress 7000A.This system offers automated patch clamping with a high degree of flexibility. It is capable of performing tests on the two major types of ion channels, voltage-gated and ligand-gated, and generating an entire response curve for each cell tested. Like the IonWorks Quattro, this system operates using its own proprietary consumable plate. | ||
| • | OpusXpress 6000A.OpusXpress 6000A is an automated solution for the early-stage process of ion channel target identification, and enables efficient pharmacological testing in the later stages of drug discovery research. |
High-Throughput Imaging Systems
One of the most powerful ways to study the activity of unknown or poorly-characterized proteins is to visualize their movements within cells. By capturing images of proteins at work, researchers obtain more detailed information about potential drug targets than is possible with non-image-based methods. Life sciences researchers have used microscopes to visualize cellular events for centuries; more recently, digital cameras and sophisticated image analysis software have greatly enhanced this process. The latest advance in this field has been the automation of image capture and analysis to allow tens of thousands of microscopic cellular assays to be performed in a single day. This technology is particularly attractive to pharmaceutical companies that require high-throughput imaging to capitalize on new drug targets and to run these tests on thousands or even millions of chemical compounds. To address this need, we offer a family of imaging systems that enable customers to conduct microscope studies in an automated, high-throughput fashion. Our products include the following advanced instruments, software and proprietary technology for performing cell-based assays:
| • | ImageXpress® Micro.This fully-integrated hardware and software system for high-throughput imaging features custom optics for the most efficient light path and highest resolution possible. It is our most compact, cost-effective and modular imaging system to date. The system design is optimized for speed, utilizing an all-new high-speed laser auto-focus option that allows for the acquisition of more than 50,000 wells per day. Its small footprint requires minimal lab space, and it features an automated door for robotic plate-loading access. | ||
| • | ImageXpress UltraTM. The ImageXpress Ultra imaging system is a fully-integrated true point-scanning confocal system for automated acquisition and analysis of images for high-throughput cell-based screening. The bench-top system utilizes up to four configurable solid-state lasers. The throughput and resolution of the ImageXpress Ultra is comparable to larger, more expensive confocal commercial systems. | ||
| • | ImageXpress® 5000A.ImageXpress 5000A is an integrated hardware and software system capable of very rapid image acquisition and sophisticated image analysis. The system can be easily upgraded to allow environmental control and liquid handling, both of which enable live cell imaging applications. | ||
| • | MetaXpress and AcuityXpress.Our imaging systems incorporate MetaXpress software, which is built on our industry-leading MetaMorph® software for cellular imaging. In addition, an option available with all of our imaging systems is AcuityXpress cellular informatics software, designed for enterprise-level data mining of high-throughput screening experiments. | ||
| • | Transfluor®.Transfluor is a patented, cell-based fluorescence technology that is used in conjunction with high-throughput imaging systems. It is the most versatile technology available for screening GPCRs. It has been successfully validated on over 90 GPCRs and is unique in its ability to test all of the different categories of GPCRs. Transfluor has been established as a “gold-standard” high-throughput imaging assay by many pharmaceutical and biotechnology companies and is considered an integral part of their drug discovery processes. |
7
Table of Contents
Analyst System and Reagent Kits
Our Analyst family of products provides industry-leading flexibility and throughput for a wide range of biochemical assays. Instruments in this family feature several different detection technologies, allowing customers to choose the one that is optimal for their particular screen. One of these detection modes, fluorescence polarization (“FP”), has become popular in recent years because it enables assays to be performed with greatly simplified protocols. We were pioneers in developing the market for FP and we have successfully applied this technology to screening kinases, an important target class. Traditionally, tests of kinase activity have been performed using multi-step protocols that involve radioactive labels or highly specific antibodies. Because radioactivity is hazardous and antibody production is practical for only a small number of kinases, customers have sought better assays as the popularity of kinase targets has increased. To address this, we developed IMAP, a simple, non-radioactive, antibody-free technology that allows accurate determination of enzyme activity for a wide variety of kinases and phosphatases.
| • | Analyst GT.This multi-mode system features seven detection options and the ability to read 96, 384 and 1,536 well microplates. With the capacity to test over 400,000 wells per day, Analyst GT is designed to address the industrialized screening needs of the drug discovery market. | ||
| • | IMAP Technology.A proprietary bead-based platform, IMAP allows researchers to determine the activity of kinases, phosphatases and phosphodiesterases in a simple, non-radioactive format. We offer optimized kits for screening several of the most popular kinases as well as kits that enable our customers to apply this extremely flexible technology to their own protein kinase targets. IMAP assays were developed using FP technology, and in 2006 we extended the IMAP platform to incorporate TR-FRET, another popular detection technology. |
Customer Service
Our service and support offerings include field service, customer support, applications assistance and training through an organization of factory-trained and educated service and application support personnel around the world. We offer services to our installed base of customers on both a contract and time and materials basis and we offer a variety of post-warranty contract options for all of our instrument offerings. Our installed base provides us with stable, recurring after-market service and support revenue, as well as product upgrade and replacement opportunities.
Research and Development
Our research and development team included 117 full time employees as of December 31, 2006. We have typically invested 13% to 15% of our revenues in research and development, which has resulted in a strong track record of technological innovation. In 2006, 65% of our revenues were derived from products that we introduced in the last three years. Our research and development expenditures were approximately $23.4 million in 2006, $25.3 million in 2005 and $22.0 million in 2004. We also incurred $4.3 million and $5.0 million of acquired in-process research and development charges in 2006 and 2004, respectively.
Our research and development activities are focused on:
| • | broadening our technology solution, including development of new proprietary reagent kits and additional solutions for automated ion channel testing and high-throughput imaging; | ||
| • | providing more sensitive quantitative evaluation of biological events; | ||
| • | providing greater throughput capability, especially with smaller sample volumes; and | ||
| • | developing increasingly sophisticated data management and analysis capability. |
Marketing and Customers
As of December 31, 2006, our sales and marketing organization included 274 full time employees in North America, Europe, Asia, Australia and South America. We distribute our products primarily through direct sales representatives in North America. We have subsidiaries in the United Kingdom, Germany, Japan, China, South Korea, Australia and Brazil responsible for direct selling and servicing of our products. Our direct sales effort is supported by a team of service, technical and applications specialists employed by us. We also sell our products through international distributors, most of which enter into distribution agreements with us that provide for exclusive distribution arrangements and minimum purchase targets. Such agreements also generally prohibit the distributors from designing, manufacturing, promoting or selling any products that are competitive with our products.
8
Table of Contents
Our customers include leading pharmaceutical and biotechnology companies as well as medical centers, universities, government research laboratories and other institutions throughout the world. Information on net sales to unaffiliated customers and identifiable assets attributable to our geographic regions is included in Note 10 of the Notes to Consolidated Financial Statements. In 2006 and 2005, no single customer accounted for more than 5% of our total revenues.
Sales to customers outside the United States accounted for 47% of total revenues in 2006, 44% of total revenues in 2005 and 42% of total revenues in 2004, and total sales denominated in foreign currencies accounted for 32%, 29% and 31% of total revenues in 2006, 2005 and 2004, respectively. We anticipate that international sales will account for an increasing percentage of revenues in the future. We expect to continue expanding our international operations in order to take advantage of increasing international market opportunities resulting from worldwide growth in the life sciences industry. Our international operations expose us to a number of risks inherent in international business activities that are described under Item 1A — “Risk Factors” below, including:
| • | political, social and economic instability; | ||
| • | trade restrictions and changes in tariffs; | ||
| • | the impact of business cycles and downturns in economies outside of the United States; | ||
| • | unexpected changes in regulatory requirements that may limit our ability to export our products or sell into particular jurisdictions; | ||
| • | import and export license requirements and restrictions; | ||
| • | difficulties and costs of staffing, managing and monitoring geographically disparate operations; | ||
| • | difficulties in maintaining effective communications with employees and customers due to distance, language and cultural barriers; | ||
| • | disruptions in international transport or delivery; | ||
| • | difficulties in protecting our intellectual property rights, particularly in countries where the laws and practices do not protect proprietary rights to as great an extent as do the laws and practices of the United States; | ||
| • | difficulties in enforcing agreements through non-U.S. legal systems; | ||
| • | longer payment cycles and difficulties in collecting receivables; | ||
| • | potentially adverse tax consequences; and | ||
| • | foreign currency exchange fluctuations. |
Manufacturing
We manufacture our products at our facilities in Sunnyvale and Union City, both in California, and in Shanghai, China. Our California facilities are ISO 9001:2000 certified. We assemble Meta systems at our facility in Downingtown, Pennsylvania, which is also ISO 9001:2000 certified. We manufacture our own components where we believe it adds significant value, but we rely on suppliers for the manufacture of some of the consumables, components and subassemblies used with or included in our systems, which are manufactured to our specifications. We conduct all final testing and inspection of our products. We have established a quality control program, including a set of standard manufacturing and documentation procedures intended to ensure that, where required, our products are manufactured to comply with good manufacturing practices.
Patents and Proprietary Technologies
We protect our proprietary rights from unauthorized use by third parties to the extent that our proprietary rights are covered by valid and enforceable patents or are effectively maintained as trade secrets. Patents and other proprietary rights are an essential element of our business. Our policy is to file patent applications and to protect technology, inventions and improvements to inventions that are commercially important to the development of our business. As of December 31, 2006, we held 132 U.S. patents and other corresponding foreign patents based on our discoveries. These patents expire at various dates between 2007 and 2025. In addition, as of December 31, 2006, we had 44 and 46 patent applications pending in the United States and foreign countries, respectively.
9
Table of Contents
We are a party to various license agreements that give us rights to use certain technologies. We pay royalties to the parties from which we licensed or acquired the core technologies.
We also rely on trade secret, employee and third-party nondisclosure agreements and other protective measures to protect our intellectual property rights pertaining to our products and technology.
Competition
The market for bioanalytical instrumentation is highly competitive, and we expect competition to increase. We compete for the allocation of customer capital funds with many other companies marketing capital equipment, including those not directly competitive with any of our products. Some of our products also compete directly with similar products from other companies.
The life sciences research market is very competitive and includes a number of companies, such as Agilent Technologies Inc., Bio-Tek Instruments, Inc., PerkinElmer, Inc., Tecan Group Ltd., and Thermo Fisher Scientific, Inc., that offer, or may in the future offer, products with performance capabilities generally similar to those offered by our products. We expect that competition is likely to increase in the future, as several current and potential competitors have the technological and financial ability to enter the markets that we serve. Many of our life sciences research products are priced at a premium; in these markets, we compete primarily on the basis of performance and productivity. Many companies, research institutions and government organizations that might otherwise be customers for our products employ methods for bioanalytical analysis that are internally developed.
The drug discovery market is characterized by intense competition among a number of companies, including GE Healthcare, a division of General Electric Company, Applied Biosystems, PerkinElmer, Inc., and Tecan Group Ltd., that offer, or may in the future offer, products with performance capabilities generally similar to those offered by our products. We believe that the primary competitive factors in the market for our products are throughput, quantitative accuracy, breadth of applications, ease-of-use, productivity enhancement, quality, support and price/performance. We believe that we compete favorably with respect to these factors.
Many of our competitors have significantly greater financial, technical, marketing, sales and other resources than we do. In addition to competing with us with respect to product sales, these companies and institutions compete with us in recruiting and retaining highly qualified scientific and management personnel.
Government Regulation
In the United States, the development, manufacturing, distribution, labeling and advertising of products intended for use in the diagnosis of disease or other conditions is extensively regulated by the U.S. Food and Drug Administration (“FDA”). These products generally require FDA clearance before they may be marketed, and also are subject to post-market manufacturing, reporting and labeling requirements. With the exception of certain of our SpectraMax microplate readers, none of our products is intended for use in the diagnosis of disease or other conditions, and, therefore, they are not currently subject to FDA regulation. The SpectraMax readers intended for diagnostic uses are the subject of an FDA marketing clearance. If we were to offer any of our other products for diagnostic uses, those products would become subject to FDA regulations.
Employees
As of December 31, 2006, we employed 631 persons full time, including 117 in research and development, 174 in manufacturing, 274 in marketing and sales and 66 in general administration and finance. Of these employees, 139 hold Ph.D. or other advanced degrees. None of our employees is covered by collective bargaining agreements, and we consider relations with our employees to be good.
Available Information
We make available, free of charge, on or through our Internet address located at www.moleculardevices.com our annual report on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), as soon as reasonably practicable after we electronically file that material with, or furnish it to, the SEC. Materials we file with the SEC may be read and copied at the SEC’s Public Reference Room at 100 F Street, NE, Washington, D.C. 20549. This information may also be obtained by calling the SEC at 1-800-SEC-0330. The SEC also maintains an internet website that contains reports, proxy and information statements, and other information regarding issuers that file electronically with the SEC at http://www.sec.gov. We will provide a copy of any of the foregoing documents to stockholders upon request.
10
Table of Contents
Item 1A.Risk Factors.
Our business faces significant risks and the risks described below may not be the only risks we face. Additional risks that we do not know of or that we currently think are immaterial may also impair our business operations. If any of the events or circumstances described in the following risks actually occurs, our business, financial condition or results of operations could be harmed and the trading price of our common stock could decline.
Risks Related to the Proposed Acquisition by MDS Inc.
On January 28, 2007, we entered into a definitive agreement and plan of merger with MDS Inc. (“MDS”) and Monument Acquisition Corp. (“Monument”), a subsidiary of MDS, which contemplates the acquisition by MDS, through Monument, of all of our outstanding common stock in a two-step transaction comprised of a cash tender offer for all of our issued and outstanding shares of common stock, followed by a merger of Monument with and into us. The obligation of Monument to accept for payment and pay for the shares tendered in the tender offer, as well as the completion of the merger, are subject to the satisfaction or waiver of a number of closing conditions set forth in the definitive agreement, including among others, the expiration of the waiting period under the Hart-Scott-Rodino Antitrust Improvements Act and the receipt of all material governmental authorizations, consents and approvals, as well as the absence of certain injunctions by court order that would restrain or prohibit the consummation of the merger. In addition, it is also a condition to Monument’s obligation to accept for payment and pay for the shares tendered in the tender offer that at least a majority of the then-outstanding shares of our common stock (determined on a fully diluted basis including any unvested stock options that would vest by their terms on or before June 30, 2007, but disregarding any other unvested stock options) will have been validly tendered in accordance with the terms of the tender and not validly withdrawn. We cannot assure you that each of the conditions to the merger or Monument’s obligation to accept for payment and pay for the shares tendered in the tender offer will be satisfied. Further, depending on the number of shares held by MDS and Monument after Monument’s acceptance of the shares properly tendered in connection with the tender offer, approval of the merger contemplated by the definitive agreement by the holders of the outstanding shares of our common stock remaining after the completion of the tender offer may be required. In the event stockholder approval of the merger is required, the consummation of the merger would be significantly delayed and the uncertainty resulting from any delay in consummating the merger could erode customer and employee confidence in us and divert our management’s focus and resources from other operational matters.
Our business and results of operations are likely to be affected by the announcement of the proposed merger with MDS. The announcement of the proposed merger could have an adverse effect on our business in the near term if our customers delay, defer or cancel purchases pending consummation of the proposed merger. Although we are attempting to mitigate this risk through communications with our customers, our customers could be reluctant to purchase our products or services due to uncertainty about the direction of the combined company’s product offerings and its support and service of our existing products after the proposed merger. To the extent that our announcement of the proposed merger creates uncertainty among our customers such that any of our larger customers delay purchase decisions pending consummation of the proposed merger, our results of operations could be negatively affected. The proposed merger could also adversely affect our ability to attract and retain key management, research and development, manufacturing, sales and marketing and other personnel. In addition, activities relating to the proposed merger and related uncertainties could divert the attention of our management and employees from our day-to-day business, which could cause disruptions among our relationships with customers, and cause our employees to seek alternative employment, all of which could detract from our ability to generate revenue and control costs.
The definitive agreement with MDS imposes certain restrictions on the operations of our business. Without MDS’ consent, we may be restricted from making certain acquisitions and taking other specified actions until the merger occurs or the definitive agreement has terminated. These restrictions may prevent us from pursuing otherwise attractive business opportunities and making other changes to our business prior to completion of the merger or the termination of the definitive agreement. In particular, we agreed not to solicit or engage in discussions with third parties regarding other proposals to acquire us, subject to specified exceptions. In connection with the termination of the definitive agreement under specified circumstances involving competing transactions or a change in our Board of Directors’ recommendation of the transaction to our stockholders, we may be required to pay MDS a termination fee of $23.0 million, and we may also be required to reimburse MDS transaction expenses up to $3.5 million, each subject to specified limitations. These provisions may deter third parties from proposing or pursuing alternative business combinations that might result in greater value to our stockholders than the transactions contemplated by the definitive agreement.
11
Table of Contents
Variations in the amount of time it takes for us to sell our products and collect accounts receivable and the timing of customer orders may cause fluctuations in our operating results, which could cause our stock price to decline.
The timing of capital equipment purchases by our customers has been and is expected to continue to be uneven and difficult to predict. Our products represent major capital purchases for our customers. Accordingly, our customers generally take a relatively long time to evaluate our products, and a significant portion of our revenue is typically derived from sales of a small number of relatively high-priced products. Purchases are generally made by purchase orders and not long-term contracts. Delays in receipt of anticipated orders for our relatively high-priced products could lead to substantial variability from quarter to quarter. Furthermore, we have historically received purchase orders and made a significant portion of each quarter’s product shipments near the end of the quarter. If that pattern continues, even short delays in the receipt of orders or shipment of products at the end of a quarter could have a material adverse affect on results of operations for that quarter.
We expend significant resources educating and providing information to our prospective customers regarding the uses and benefits of our products. Because of the number of factors influencing the sales process, the period between our initial contact with a customer and the time when we recognize revenues from that customer, if ever, varies widely. Our sales cycles typically range from three to six months, but can be much longer. During these cycles, we commit substantial resources to our sales efforts in advance of receiving any revenues, and we may never receive any revenues from a customer despite our sales efforts.
The relatively high purchase price for a customer order contributes to collection delays that result in working capital volatility. While the terms of our sales orders generally require payment within 30 days of product shipment and do not provide return rights, in the past we have experienced significant collection delays. We cannot predict whether we will continue to experience similar or more severe delays.
The capital spending policies of our customers have a significant effect on the demand for our products. Those policies are based on a wide variety of factors, including resources available to make purchases, spending priorities, and policies regarding capital expenditures during industry downturns or recessionary periods. Any decrease in capital spending by our customers resulting from any of these factors could harm our business.
We depend on orders that are received and shipped in the same quarter and therefore have limited visibility of future product shipments.
Our net sales in any given quarter depend upon a combination of orders received in that quarter for shipment in that quarter and shipments from backlog. Our products are typically shipped within ninety days of purchase order receipt. As a result, we do not believe that the amount of backlog at any particular date is indicative of our future level of sales. Our backlog at the beginning of each quarter does not include all product sales needed to achieve expected revenues for that quarter. Consequently, we are dependent on obtaining orders for products to be shipped in the same quarter that the order is received. Moreover, customers may reschedule shipments, and production difficulties could delay shipments. Accordingly, we have limited visibility of future product shipments, and our results of operations are subject to significant variability from quarter to quarter.
Many of our current and potential competitors have significantly greater resources than we do, and increased competition could impair sales of our products.
We operate in a highly competitive industry and face competition from companies that design, manufacture and market instruments for use in the life sciences research industry, from genomic, pharmaceutical, biotechnology and diagnostic companies and from academic and research institutions and government or other publicly-funded agencies, both in the United States and elsewhere. We may not be able to compete effectively with all of these competitors. Many of these companies and institutions have greater financial, engineering, manufacturing, marketing and customer support resources than we do. As a result, our competitors may be able to respond more quickly to new or emerging technologies or market developments by devoting greater resources to the development, promotion and sale of products, which could impair sales of our products. Moreover, there has been significant merger and acquisition activity among our competitors and potential competitors. These transactions by our competitors and potential competitors may provide them with a competitive advantage over us by enabling them to rapidly expand their product offerings and service capabilities to meet a broader range of customer needs. Many of our customers and potential customers are large companies that require global support and service, which may be easier for our larger competitors to provide.
We believe that competition within the markets we serve is primarily driven by the need for innovative products that address the needs of customers. We attempt to counter competition by seeking to develop new products and provide quality products and services that meet customers’ needs. We cannot assure you, however, that we will be able to successfully develop new products or that our existing or new products and services will adequately meet our customers’ needs.
12
Table of Contents
Rapidly changing technology, evolving industry standards, changes in customer needs, emerging competition and frequent new product and service introductions characterize the markets for our products. To remain competitive, we will be required to develop new products and periodically enhance our existing products in a timely manner. We are facing increased competition as new companies entering the market with new technologies compete, or will compete, with our products and future products. We cannot assure you that one or more of our competitors will not succeed in developing or marketing technologies or products that are more effective or commercially attractive than our products or future products, or that would render our technologies and products obsolete or uneconomical. Our future success will depend in large part on our ability to maintain a competitive position with respect to our current and future technologies, which we may not be able to do. In addition, delays in the launch of our new products may result in loss of market share due to our customers’ purchases of competitors’ products during any delay.
If we are not successful in developing new and enhanced products, we may lose market share to our competitors.
The life sciences research instrumentation market is characterized by rapid technological change and frequent new product introductions. In the twelve months ended December 31, 2006, 65% of our revenues were derived from the sale of products that were introduced in the last three years, and our future success will depend on our ability to enhance our current products and to develop and introduce, on a timely basis, new products that address the evolving needs of our customers. We may experience difficulties or delays in our development efforts with respect to new products, and we may not ultimately be successful in developing or commercializing them, which would harm our business. Any significant delay in releasing new systems could cause our revenues to suffer, adversely affect our reputation, give a competitor a first-to-market advantage or cause a competitor to achieve greater market share. In addition, our future success depends on our continued ability to develop new applications for our existing products. If we are not able to complete the development of these applications, or if we experience difficulties or delays, we may lose our current customers and may not be able to attract new customers, which could seriously harm our business and our future growth prospects.
We must expend a significant amount of time and resources to develop new products, and if these products do not achieve commercial acceptance, our operating results may suffer.
We expect to spend a significant amount of time and resources to develop new products and refine existing products. In light of the long product development cycles inherent in our industry, these expenditures will be made well in advance of the prospect of deriving revenues from the sale of new products. Our ability to commercially introduce and successfully market new products is subject to a wide variety of challenges during this development cycle that could delay introduction of these products. In addition, since our customers are not obligated by long-term contracts to purchase our products, our anticipated product orders may not materialize, or orders that do materialize may be canceled. As a result, if we do not achieve market acceptance of new products, our operating results will suffer. Our products are also generally priced higher than competitive products, which may impair commercial acceptance. We cannot predict whether new products that we expect to introduce will achieve commercial acceptance.
We obtain some of the consumables, components and subassemblies used with or included in our systems from a single source or limited group of suppliers, and the partial or complete loss of one of these suppliers could cause customer supply or production delays and a substantial loss of revenues.
We rely on outside vendors to manufacture some of the consumables, components and subassemblies used with or included in our systems. Certain consumables, components, subassemblies and services necessary for the manufacture of our systems are obtained from a sole supplier or limited group of suppliers, some of which are our competitors. Additional components, such as optical, electronic and pneumatic devices, are currently purchased in configurations specific to our requirements and, together with certain other components, such as computers, are integrated into our products. We maintain only a limited number of long-term supply agreements with our suppliers, and some of these agreements provide that the supplier is the exclusive supplier of a particular consumable, component or subassembly.
Our reliance on a sole or a limited group of suppliers involves several risks, including the following:
| • | our suppliers may cease or interrupt production of required consumables, components or subassemblies or otherwise fail to supply us with an adequate supply of required consumables, components or subassemblies for a number of reasons, including contractual disputes with our suppliers or adverse financial developments at or affecting the supplier; | ||
| • | we have reduced control over the pricing of third party-supplied consumables, components and subassemblies, and our suppliers may be unable or unwilling to supply us with required consumables, components and subassemblies on commercially acceptable terms, or at all; | ||
| • | we have reduced control over the timely delivery of third party-supplied consumables, components and subassemblies; and | ||
| • | our suppliers may be unable to develop technologically advanced products to support our growth and development of new systems. |
13
Table of Contents
Because the manufacturing of certain of these consumables, components and subassemblies involves extremely complex processes and requires long lead times, we may experience delays or shortages caused by suppliers. We believe that alternative sources could be obtained and qualified, if necessary, for most sole and limited source parts, subject in certain cases to the terms of exclusive supply agreements with suppliers. However, if we were forced to seek alternative sources of supply or to manufacture such consumables, components or subassemblies internally, we may be forced to redesign our systems, which could prevent us from shipping our systems to customers on a timely basis, and we may be liable to suppliers under the terms of existing supply agreements. Some of our suppliers have relatively limited financial and other resources. Any inability to obtain adequate deliveries, or any other circumstance that would restrict our ability to ship our products, could damage relationships with current and prospective customers and could harm our business, and any disputes with suppliers could have an adverse impact on our business, financial condition or results of operations.
We may encounter manufacturing and assembly problems or delays, which could result in lost revenues.
We manufacture our products at our facilities in Sunnyvale and Union City, both in California, and in Shanghai, China. Our manufacturing and assembly processes are highly complex and require sophisticated, costly equipment and specially designed facilities. As a result, any prolonged disruption in the operations of our manufacturing facilities could seriously harm our ability to satisfy our customer order deadlines. If we cannot deliver our systems in a timely manner, our revenues will likely suffer.
Our product sales depend in part upon manufacturing yields. We currently have limited manufacturing capacity and experience variability in manufacturing yields. We are currently manufacturing high-throughput instruments in-house, in limited volumes and with largely manual assembly. If demand for our high-throughput instruments increases, we will either need to expand our in-house manufacturing capabilities or outsource to other manufacturers. If we fail to deliver our products in a timely manner, our relationships with our customers could be seriously harmed, and our revenues could decline.
As we develop new products, we must transition the manufacture of a new product from the development stage to commercial manufacturing. We cannot predict whether we will be able to complete these transitions on a timely basis and with commercially reasonable costs. We cannot assure you that manufacturing or quality control problems will not arise as we attempt to scale-up our production for any future new products or that we can scale-up manufacturing and quality control in a timely manner or at commercially reasonable costs. If we are unable to consistently manufacture our products on a timely basis because of these or other factors, our product sales will decline.
If we deliver products with defects, our credibility will be harmed and the sales and market acceptance of our products will decrease.
Our products are complex and have at times contained errors, defects and bugs when introduced. If we deliver products with errors, defects or bugs, our credibility and the market acceptance and sales of our products would be harmed. Further, if our products contain errors, defects or bugs, we may be required to expend significant capital and resources to alleviate such problems. Defects could also lead to product liability as a result of product liability lawsuits against us or against our customers. We have agreed to indemnify our customers in some circumstances against liability arising from defects in our products. In the event of a successful product liability claim, we could be obligated to pay damages significantly in excess of our product liability insurance limits.
We have significantly expanded our international operations, which exposes us to risks inherent in international business activities.
We maintain facilities in the United Kingdom, Germany, Japan, South Korea, Australia, China and Brazil. In addition to the increase in our international operations, we are also deriving an increasing portion of our revenues from customers located outside of the United States. Sales to customers outside of the United States accounted for approximately 47% of our revenues during the year ended December 31, 2006 and we anticipate that international sales will continue to account for a significant portion of our revenues. A key aspect of our business strategy has been and is to expand our sales and support organizations internationally in order to take advantage of increasing international market opportunities resulting from worldwide growth in the life sciences research industry.
14
Table of Contents
Our reliance on international sales and operations exposes us to a number of risks associated with conducting operations internationally, including:
| • | political, social and economic instability; | ||
| • | trade restrictions and changes in tariffs; | ||
| • | the impact of business cycles and downturns in economies outside of the United States; | ||
| • | unexpected changes in regulatory requirements that may limit our ability to export our products or sell into particular jurisdictions; | ||
| • | import and export license requirements and restrictions; | ||
| • | difficulties and costs of staffing, managing and monitoring geographically disparate operations; | ||
| • | difficulties in maintaining effective communications with employees and customers due to distance, language and cultural barriers; | ||
| • | disruptions in international transport or delivery; | ||
| • | difficulties in protecting our intellectual property rights, particularly in countries where the laws and practices do not protect proprietary rights to as great an extent as do the laws and practices of the United States; | ||
| • | difficulties in enforcing agreements through non-U.S. legal systems; | ||
| • | longer payment cycles and difficulties in collecting receivables; and | ||
| • | potentially adverse tax consequences. |
If any of these risks materialize, our international sales could decrease and our foreign operations could suffer.
In addition, all of our sales to international distributors are denominated in U.S. dollars. Most of our direct sales in the United Kingdom, Germany, France, the Benelux, Scandinavia, Canada, Japan, Australia and South Korea are denominated in local currencies and totaled $60.3 million (32% of total revenues) for the year ended December 31, 2006. To the extent that our sales and operating expenses are denominated in foreign currencies, our operating results may be adversely affected by changes in exchange rates. Owing to the number of currencies involved, the substantial volatility of currency exchange rates, and our constantly changing currency exposures, we cannot predict the effect of exchange rate fluctuations on our future operating results. We do not currently engage in foreign currency hedging transactions, but may do so in the future.
Most of our current and potential customers are from the pharmaceutical and biotechnology industries and are subject to risks faced by those industries.
We derive a significant portion of our revenues from sales to pharmaceutical and biotechnology companies. We expect that sales to pharmaceutical and biotechnology companies will continue to be a primary source of revenues for the foreseeable future. As a result, we are subject to risks and uncertainties that affect the pharmaceutical and biotechnology industries, such as availability of capital and reduction and delays in research and development expenditures by companies in these industries, pricing pressures as third-party payers continue challenging the pricing of medical products and services, government regulation, and the uncertainty resulting from technological change.
In addition, our future revenues may be adversely affected by the ongoing consolidation in the pharmaceutical and biotechnology industries, which would reduce the number of our potential customers. Furthermore, we cannot assure you that the pharmaceutical and biotechnology companies that are our customers will not develop their own competing products or in-house capabilities.
Our products could infringe on the intellectual property rights of others, which may cause us to engage in costly litigation and, if we are not successful, could also cause us to pay substantial damages and prohibit us from selling our products.
Third parties may assert infringement or other intellectual property claims against us. We may have to pay substantial damages for infringement if it is ultimately determined that our products infringe on a third party’s proprietary rights. Further, any legal action against us could, in addition to subjecting us to potential liability for damages, prohibit us from selling our products before we obtain a license to do so from the party owning the intellectual property, which, if available at all, may require us to pay substantial royalties. Even if these claims are without merit, defending a lawsuit takes significant time, may be expensive and may divert management attention from other business concerns. There may be third-party patents that may relate to our technology or potential products. Any public announcements related to litigation or interference proceedings initiated or threatened against us could cause our stock price to decline. We believe that there may be significant litigation in the industry regarding patent and other intellectual property rights. If we become involved in litigation, it could consume a substantial portion of our managerial and financial resources.
15
Table of Contents
We may need to initiate lawsuits to protect or enforce our patents, which would be expensive and, if we lose, may cause us to lose some of our intellectual property rights, which would reduce our ability to compete in the market.
We rely on patents to protect a large part of our intellectual property and our competitive position. In order to protect or enforce our patent rights, we may initiate patent litigation against third parties, such as infringement suits or interference proceedings. Litigation may be necessary to:
| • | assert claims of infringement; | ||
| • | enforce our patents; | ||
| • | protect our trade secrets or know-how; or | ||
| • | determine the enforceability, scope and validity of the proprietary rights of others. |
Lawsuits could be expensive, take significant time and divert management’s attention from other business concerns. They would put our patents at risk of being invalidated or interpreted narrowly and our patent applications at risk of not issuing. We may also provoke third parties to assert claims against us. Patent law relating to the scope of claims in the technology fields in which we operate is still evolving and, consequently, patent positions in our industry are generally uncertain. If initiated, we cannot assure you that we would prevail in any of these suits or that the damages or other remedies awarded, if any, would be commercially valuable. During the course of these suits, there could be public announcements of the results of hearings, motions and other interim proceedings or developments in the litigation. If securities analysts or investors were to perceive any of these results to be negative, our stock price could decline.
The rights we rely upon to protect our intellectual property underlying our products may not be adequate, which could enable third parties to use our technology and would reduce our ability to compete in the market.
Our success will depend in part on our ability to obtain commercially valuable patent claims and to protect our intellectual property. Our patent position is generally uncertain and involves complex legal and factual questions. Legal standards relating to the validity and scope of claims in our technology field are still evolving. Therefore, the degree of future protection for our proprietary rights is uncertain.
The risks and uncertainties that we face with respect to our patents and other proprietary rights include the following:
| • | the pending patent applications we have filed or to which we have exclusive rights may not result in issued patents or may take longer than we expect to result in issued patents; | ||
| • | the claims of any patents which are issued may not provide meaningful protection; | ||
| • | we may not be able to develop additional proprietary technologies that are patentable; | ||
| • | the patents licensed or issued to us or our customers may not provide a competitive advantage; | ||
| • | other companies may challenge patents licensed or issued to us or our customers; | ||
| • | patents issued to other companies may harm our ability to do business; | ||
| • | other companies may independently develop similar or alternative technologies or duplicate our technologies; and | ||
| • | other companies may design around the technologies we have licensed or developed. |
In addition to patents, we rely on a combination of trade secrets, nondisclosure agreements and other contractual provisions and technical measures to protect our intellectual property rights. Nevertheless, these measures may not be adequate to safeguard the proprietary technology underlying our products. If these measures do not protect our rights, third parties could use our technology, and our ability to compete in the market would be reduced. In addition, employees, consultants and others who participate in the development of our products may breach their agreements with us regarding our intellectual property, and we may not have adequate
16
Table of Contents
remedies for the breach. We also may not be able to effectively protect our intellectual property rights in some foreign countries. For a variety of reasons, we may decide not to file for patent, copyright or trademark protection or prosecute potential infringements of our patents. We also realize that our trade secrets may become known through other means not currently foreseen by us. Notwithstanding our efforts to protect our intellectual property, our competitors may design around our proprietary technologies or may independently develop similar or alternative technologies or products that are equal or superior to our technology and products without infringing on any of our intellectual property rights.
We may have difficulty managing our growth.
We expect to experience significant growth in the number of our employees and customers and the scope of our operations, including as a result of potential acquisitions. This growth may continue to place a significant strain on our management and operations. Our ability to manage this growth will depend upon our ability to broaden our management team and our ability to attract, hire and retain skilled employees. Our success will also depend on the ability of our officers and key employees to continue to implement and improve our operational and other systems, to manage multiple, concurrent customer relationships and to hire, train and manage our employees. Our future success is heavily dependent upon growth and acceptance of new products. If we cannot scale our business appropriately or otherwise adapt to anticipated growth and new product introductions, a key part of our strategy may not be successful.
We rely upon distributors for product sales and support outside of North America.
In the year ended December 31, 2006, approximately 10% of our sales were made through distributors. We often rely upon distributors to provide customer support to the ultimate end users of our products. As a result, our success depends on the continued sales and customer support efforts of our network of distributors. The use of distributors involves certain risks, including risks that distributors will not effectively sell or support our products, that they will be unable to satisfy financial obligations to us and that they will cease operations. Any reduction, delay or loss of orders from our significant distributors could harm our revenues. We also do not currently have distributors under contract in a number of international markets and may need to establish additional international distribution relationships. There can be no assurance that we will engage qualified distributors in a timely manner, and the failure to do so could have a material adverse affect on our business, financial condition and results of operations.
If we choose to acquire new and complementary businesses, products or technologies instead of developing them ourselves, we may be unable to complete these acquisitions or may not be able to successfully integrate an acquired business or technology in a cost-effective and non-disruptive manner.
Our success depends on our ability to continually enhance and broaden our product offerings in response to changing technologies, customer demands and competitive pressures. To this end, from time to time we have acquired complementary businesses, products or technologies instead of developing them ourselves, and we may choose to do so in the future. For example, we acquired the LCM business from Arcturus Bioscience, Inc. (“Arcturus”) in April 2006, intellectual property relating to the Transfluor® technology from Xsira Pharmaceuticals, Inc. (“Xsira”) in March 2005 and acquired Axon Instruments, Inc. (“Axon”) in July 2004. We do not know if we will be able to complete any additional acquisitions, or whether we will be able to successfully integrate any acquired business, operate it profitably or retain its key employees. Integrating any business, product or technology we acquire involves considerable operational and financial risks and strains, including:
| • | the potential disruption of our ongoing business and distraction of our management; | ||
| • | the potential strain on our financial and managerial controls and reporting systems and procedures; | ||
| • | unanticipated expenses and potential delays related to integration of the operations, technology and other resources of acquired businesses; | ||
| • | the impairment of relationships with employees, suppliers and customers as a result of any integration of new management personnel; | ||
| • | greater than anticipated costs and expenses related to restructuring, including employee severance or relocation costs and costs related to vacating leased facilities; and | ||
| • | potential unknown liabilities associated with any acquisition, including higher than expected integration costs, which may cause our quarterly and annual operating results to fluctuate. |
17
Table of Contents
We may not succeed in addressing these risks or any other problems encountered in connection with the acquisition of complementary businesses, products or technologies. If we are unable to successfully integrate the operations, products, technology and personnel of acquired businesses in a timely manner or at all, or if we do not achieve the perceived benefits of any acquisition as rapidly as, or to the extent anticipated by, financial analysts or investors, the market price of our common stock could decline.
In addition, in order to finance any acquisitions, we might need to raise additional funds through public or private equity or debt financings. In that event, we could be forced to obtain financing on terms that are not favorable to us and, in the case of equity financing, that may result in dilution to our stockholders. In addition, any impairment of goodwill and amortization of other intangible assets or charges resulting from the costs of acquisitions could harm our business and operating results.
We depend on our key personnel, the loss of whom would impair our ability to compete.
We are highly dependent on the principal members of our management, engineering and scientific staff. The loss of the service of any of these persons could seriously harm our product development and commercialization efforts. In addition, research, product development and commercialization will require additional skilled personnel in areas such as chemistry and biology, and software and electronic engineering. Our corporate headquarters are located in Sunnyvale, California, where demand for personnel with these skills is extremely high and is likely to remain high. As a result, competition for and retention of personnel, particularly for employees with technical expertise, is intense and the turnover rate for qualified personnel is high. If we are unable to hire, train and retain a sufficient number of qualified employees, our ability to conduct and expand our business could be seriously reduced. The inability to retain and hire qualified personnel could also hinder the planned expansion of our business.
Changes to financial accounting standards or interpretations of those standards, may affect our results of operations and cause us to change our business practices.
We prepare our financial statements to conform with U.S. generally accepted accounting principles. These accounting principles are subject to interpretation by the American Institute of Certified Public Accountants, the Public Company Accounting Oversight Board, the SEC and various other bodies formed to interpret and create appropriate accounting principles. A change in those principles can have a significant effect on our reported results and may affect our reporting of transactions completed before a change is announced. Changes to those principles, the interpretation of those principles, or the questioning of current practices, may adversely affect our reported financial results or may require us to reclassify, restate or otherwise change or revise our financial statements, and could cause us to change the way we conduct our business. For example, accounting principles affecting many aspects of our business, including rules relating to equity-related compensation, have recently been revised or are under review. The Financial Accounting Standards Board and other agencies have finalized changes to U.S. generally accepted accounting principles that required us, effective January 1, 2006, to record a charge to earnings for employee stock option grants and other equity incentives. We will have significant and ongoing accounting charges resulting from option grant and other equity incentive expensing that could reduce our overall net income. In addition, since we historically have used equity-related compensation as a component of our total employee compensation program, the accounting change could make the use of equity-related compensation less attractive to us and therefore make it more difficult to attract and retain employees.
Changes in our effective income tax rate could reduce our earnings.
Various factors may have favorable or unfavorable effects on our effective income tax rate. These factors include, but are not limited to, interpretations of existing tax laws, our adoption of the Financial Accounting Standards Board’s Statement No. 123 (revised 2004),“Share-Based Payment”(“FAS 123R”) relating to the accounting for stock options and other share-based payments, changes in tax laws and rates, future levels of research and development spending, changes in accounting standards, future levels of capital expenditures, changes in the mix of earnings in the various tax jurisdictions in which we operate and changes in overall levels of pre-tax earnings. The impact on our income tax provision resulting from the above-mentioned factors may be significant and could have a negative impact on our results of operations.
Our operating results fluctuate and any failure to meet financial expectations may disappoint securities analysts or investors and result in a decline in our stock price.
We have experienced and in the future may experience a shortfall in revenues or earnings or otherwise fail to meet public market expectations, which could materially and adversely affect our business and the market price of our common stock. Our total revenues and operating results may fluctuate significantly because of a number of factors, many of which are outside of our control. These factors include:
| • | customer confidence in the economy, evidenced, in part, by stock market levels; | ||
| • | changes in the domestic and international economic, business and political conditions; |
18
Table of Contents
| • | economic conditions within the pharmaceutical and biotechnology industries; | ||
| • | levels of product and price competition; | ||
| • | market acceptance of and demand for our products; | ||
| • | the length of our sales cycle and customer buying patterns; | ||
| • | the size and timing of individual transactions; | ||
| • | the timing of new product introductions and product enhancements; | ||
| • | the mix of products sold; | ||
| • | levels of international transactions; | ||
| • | activities of and acquisitions by competitors; | ||
| • | the timing of new hires and the allocation of our resources; | ||
| • | changes in foreign currency exchange rates; | ||
| • | our ability to develop and market new products and control costs; and | ||
| • | changes in U.S. generally accepted accounting principles. |
One or more of the foregoing factors may cause our operating expenses to be disproportionately high during any given period or may cause our revenues and operating results to fluctuate significantly. In particular, we typically experience a decrease in the level of sales in the first calendar quarter as compared to the fourth quarter of the preceding year because of budgetary and capital equipment purchasing patterns in the life sciences research industry. Our quarterly operating results have fluctuated in the past, and we expect they will fluctuate in the future as a result of many factors, some of which are outside of our control.
In addition, we manufacture our products based on forecasted orders rather than on outstanding orders. Accordingly, our expense levels are based, in part, on expected future sales, and we generally cannot quickly adjust operating expenses. For example, research and development and general and administrative expenses are not directly affected by variations in revenues. As a result, if sales levels in a particular quarter do not meet expectations, we may not be able to adjust operating expenses in a sufficient timeframe to compensate for the shortfall, and our results of operations for that quarter may be seriously harmed. Likewise, our manufacturing processes may in certain instances create a risk of excess or inadequate inventory levels if orders do not match forecasts.
Due to the preceding factors, we may experience a shortfall in revenues or earnings or otherwise fail to meet public market expectations, which could materially and adversely affect our business, financial condition, results of operations and the market price of our common stock. Because our revenues and operating results are difficult to predict, we believe that period-to-period comparisons of our results of operations are not a good indication of our future performance.
Failure to maintain effective internal controls in accordance with Section 404 of the Sarbanes-Oxley Act of 2002 could have a material adverse effect on our stock price.
Section 404 of the Sarbanes-Oxley Act of 2002 and the related rules and regulations of the SEC require annual management assessments of the effectiveness of our internal control over financial reporting and a report by our independent registered public accounting firm attesting to and reporting on these assessments. If we fail to maintain the adequacy of our internal control over financial reporting, as such standards are modified, supplemented or amended from time to time, we may not be able to ensure that we can conclude on an ongoing basis that we have effective internal control over financial reporting in accordance with Section 404 of the Sarbanes-Oxley Act of 2002 and the related rules and regulations of the SEC. If we cannot favorably assess, or our independent registered public accounting firm is unable to provide an unqualified attestation report on our assessment of, the effectiveness of our internal control over financial reporting, investor confidence in the reliability of our financial reports may be adversely affected, which could have a material adverse effect on our stock price.
19
Table of Contents
Our stock price is volatile, which could cause stockholders to lose a substantial part of their investment in our stock.
The stock market in general, and the stock prices of technology companies in particular, have recently experienced volatility which has often been unrelated to the operating performance of any particular company or companies. In the twelve months ended December 31, 2006, the closing sales price of our common stock ranged from $18.03 to $35.92. Our stock price could decline regardless of our actual operating performance, and stockholders could lose a substantial part of their investment as a result of industry or market-based fluctuations. In the past, our stock has traded relatively thinly. If a more active public market for our stock is not sustained, it may be difficult for stockholders to resell shares of our common stock. Because we do not anticipate paying cash dividends on our common stock for the foreseeable future, stockholders will not be able to receive a return on their shares unless they sell them.
The market price of our common stock will likely fluctuate in response to a number of factors, including the following:
| • | developments with or related to the transactions contemplated by the definitive agreement and plan of merger with MDS; | ||
| • | domestic and international economic, business and political conditions; | ||
| • | economic conditions within the pharmaceutical and biotechnology industries; | ||
| • | our failure to meet our performance estimates or the performance estimates of securities analysts; | ||
| • | changes in financial estimates of our revenues and operating results by us or securities analysts; | ||
| • | changes in buy/sell recommendations by securities analysts; and | ||
| • | the timing of announcements by us or our competitors of significant products, contracts or acquisitions or publicity regarding actual or potential results or performance thereof. |
Provisions of our charter documents and Delaware law may inhibit a takeover, which could limit the price investors might be willing to pay in the future for our common stock.
Provisions in our certificate of incorporation and bylaws may have the effect of delaying or preventing an acquisition or merger in which we are not the surviving company or which results in changes in our management. For example, our certificate of incorporation gives our Board of Directors the authority to issue shares of preferred stock and to determine the price, rights, preferences and privileges and restrictions, including voting rights, of those shares without any further vote or action by our stockholders. The rights of the holders of common stock will be subject to, and may be harmed by, the rights of the holders of any shares of preferred stock that may be issued in the future. The issuance of preferred stock may delay, defer or prevent a change in control, as the terms of the preferred stock that might be issued could potentially prohibit our consummation of any merger, reorganization, sale of substantially all of our assets, liquidation or other extraordinary corporate transaction without the approval of the holders of the outstanding shares of preferred stock. The issuance of preferred stock could also have a dilutive effect on our stockholders. In addition, because we are incorporated in Delaware, we are governed by the provisions of Section 203 of the Delaware General Corporation Law. These provisions may prohibit large stockholders, in particular those owning 15% or more of the outstanding voting stock, from consummating a merger or combination involving us. Further, in October 2001, our Board of Directors adopted a stockholder rights plan, commonly known as a “poison pill.” We amended the terms of our stockholder rights plan in January 2007 in order to prevent the definitive agreement and plan of merger with MDS, the tender offer and the merger contemplated by the definitive agreement, from triggering the distribution and/or exercise of the rights provided for under the stockholder rights plan. These provisions described above and our stockholder rights plan could limit the price that investors might be willing to pay in the future for our common stock.
Our actual results could differ materially from those anticipated in our forward-looking statements.
This report contains forward-looking statements within the meaning of the federal securities laws that relate to future events or our future financial performance. When used in this report, you can identify forward-looking statements by terminology such as “believes,” “anticipates,” “plans,” “predicts,” “expects,” “estimates,” “intends,” “will,” “continue,” “may,” “potential,” “should” and similar expressions. These statements are only predictions. Our actual results could differ materially from those anticipated in our forward-looking statements as a result of many factors, including those set forth above and elsewhere in this report. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we cannot guarantee future results, levels of activity, performance or achievements. Neither we nor any other person assumes responsibility for the accuracy and completeness of these statements. We assume no duty to update any of the forward-looking statements after the date of this report or to conform these statements to actual results. Accordingly, we caution readers not to place undue reliance on these statements.
20
Table of Contents
Item 1B.Unresolved Staff Comments.
None.
Item 2.Properties.
We lease two facilities in Sunnyvale, California, two in Union City, California, and one facility in Downingtown, Pennsylvania which include laboratory, manufacturing and administrative space. We also lease sales and service offices in the United Kingdom, Germany, Japan, South Korea, Australia, China and Brazil, and a manufacturing facility in China. We believe that our current facilities will be sufficient for our operations through at least 2007. These properties are described below:
| Location (1) | Ownership | Facilities | Lease Expiration | |||
| 1311 Orleans Drive Sunnyvale, CA 94089 | Leased | Approximately 60,000 square feet of office and laboratory space | February 28, 2013 | |||
| 3280 Whipple Road Union City, CA 94587 | Leased | Approximately 76,214 square feet of office, laboratory and manufacturing space | February 16, 2011 | |||
| 1312 Crossman Avenue Sunnyvale, CA 94089 | Leased | Approximately 54,500 square feet of office, laboratory and manufacturing space | February 28, 2013 | |||
| 402 Boot Road Downingtown, PA 19335 | Leased | Approximately 27,900 square feet of office, laboratory and manufacturing space | November 15, 2010 | |||
| Nanhui, Shanghai, China | Leased | Approximately 19,000 square feet of manufacturing space | December 31, 2010 | |||
| Wokingham, England | Leased | Approximately 14,048 square feet of office space | September 2, 2015 | |||
| Tokyo, Japan | Leased | Approximately 4,300 square feet of office space | June 30, 2007 | |||
| Osaka, Japan | Leased | Approximately 3,700 square feet of office space | March 31, 2007 | |||
| Shanghai, China | Leased | Approximately 3,500 square feet of office space | April 9, 2007 | |||
| Munich, Germany | Leaased | Approximately 3,500 square feet of office space | Month to month | |||
| Melbourne, Australia | Leased | Approximately 2,200 square feet of office space | May 31, 2007 | |||
| Seoul, South Korea | Leased | Approximately 2,100 square feet of office space | November 14, 2008 | |||
| Beijing, China | Leased | Approximately 2,000 square feet of office space | May 31, 2008 | |||
| Sao Paulo, Brazil | Leased | Approximately 2,000 square feet of office space | February, 2008 | |||
| (1) | In addition to the properties described above, we lease approximately 20,000 square feet of property in Union City, California, which we sublease to a third party. We do not use this property in our current operations and have not included it in the table above. |
Item 3.Legal Proceedings.
None.
Item 4.Submission of Matters to a Vote of Security Holders.
None.
21
Table of Contents
PART II
Item 5.Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities.
Market Information
Our common stock is traded on The NASDAQ Global Select Market (“NASDAQ”) under the symbol “MDCC.”
The prices per share reflected on the table below represent the range of high and low closing sales prices of our common stock on NASDAQ, for the period indicated.
| 2006 | 2005 | |||||||||||||||
| High | Low | High | Low | |||||||||||||
| First Quarter | $ | 33.30 | $ | 28.00 | $ | 21.32 | $ | 18.27 | ||||||||
| Second Quarter | 35.92 | 27.79 | 22.01 | 18.16 | ||||||||||||
| Third Quarter | 30.21 | 18.03 | 21.80 | 19.95 | ||||||||||||
| Fourth Quarter | 22.82 | 18.15 | 28.93 | 19.45 | ||||||||||||
Holders
As of March 9, 2007, we had approximately 6,300 stockholders of record. In addition, we believe that a significant number of beneficial owners of our common stock hold their shares in street name. On March 9, 2007, the last sale price reported on NASDAQ for our common stock was $35.37 per share.
Dividends
Historically, we have not paid cash dividends on our common stock and do not intend to pay any cash dividends in the foreseeable future. Our Board of Directors will determine any future cash dividends.
Performance Graph
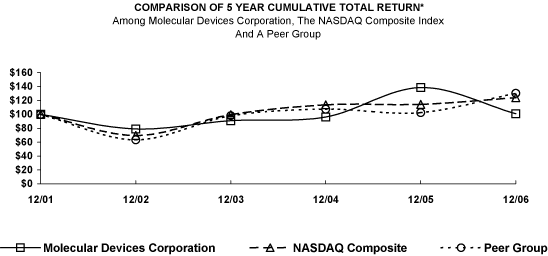
22
Table of Contents
Item 6.Selected Consolidated Financial Data.
The following table sets forth our selected historical financial information, certain portions of which are based on, and should be read in conjunction with, our audited consolidated financial statements that are filed as a part of this report.
Consolidated Statements of Operations Data (in thousands):
| Years ended December 31, | ||||||||||||||||||||
| 2006 | 2005 | 2004 | 2003 | 2002 | ||||||||||||||||
| Revenues (1) | $ | 186,412 | $ | 181,951 | $ | 149,095 | $ | 116,008 | $ | 102,570 | ||||||||||
| Cost of revenues (1) | 70,065 | 70,267 | 56,840 | 43,683 | 40,974 | |||||||||||||||
| Gross profit | 116,347 | 111,684 | 92,255 | 72,325 | 61,596 | |||||||||||||||
| Operating expenses: | ||||||||||||||||||||
| Research and development | 23,362 | 25,281 | 22,038 | 18,679 | 18,002 | |||||||||||||||
| Selling, general and administrative | 71,826 | 59,885 | 52,469 | 43,457 | 35,435 | |||||||||||||||
| Acquired in-process research and development (2) | 4,272 | — | 5,000 | — | — | |||||||||||||||
| Restructuring and other charges (2) | 331 | 1,427 | 1,157 | — | — | |||||||||||||||
| Total operating expenses | 99,791 | 86,593 | 80,664 | 62,136 | 53,437 | |||||||||||||||
| Income from operations (2) | 16,556 | 25,091 | 11,591 | 10,189 | 8,159 | |||||||||||||||
| Gain on sale of equity securities | 2,235 | — | 18,288 | — | — | |||||||||||||||
| Translation gain on liquidation of subsidiaries (2) | 1,413 | — | — | — | — | |||||||||||||||
| Interest expense | (85 | ) | (85 | ) | (187 | ) | — | — | ||||||||||||
| Interest and other income (expense), net | 524 | (530 | ) | 319 | 872 | 1,562 | ||||||||||||||
| Income before income taxes | 20,643 | 24,476 | 30,011 | 11,061 | 9,721 | |||||||||||||||
| Income tax provision | 9,816 | 8,580 | 12,778 | 3,319 | 2,916 | |||||||||||||||
| Net income | $ | 10,827 | $ | 15,896 | $ | 17,233 | $ | 7,742 | $ | 6,805 | ||||||||||
| Basic net income per share | $ | 0.65 | $ | 0.95 | $ | 1.08 | $ | 0.51 | $ | 0.44 | ||||||||||
| Diluted net income per share | $ | 0.64 | $ | 0.93 | $ | 1.04 | $ | 0.51 | $ | 0.44 | ||||||||||
| Shares used in computing basic net income per share | 16,676 | 16,783 | 16,028 | 15,067 | 15,348 | |||||||||||||||
| Shares used in computing diluted net income per share | 17,047 | 17,147 | 16,532 | 15,179 | 15,457 | |||||||||||||||
Consolidated Balance Sheets Data (in thousands):
| As of December 31, | ||||||||||||||||||||
| 2006 | 2005 | 2004 | 2003 | 2002 | ||||||||||||||||
| Cash and cash equivalents | $ | 22,824 | $ | 28,908 | $ | 30,175 | $ | 60,110 | $ | 53,783 | ||||||||||
| Working capital | 60,416 | 62,664 | 67,556 | 87,305 | 84,851 | |||||||||||||||
| Total assets | 266,778 | 257,416 | 255,229 | 166,913 | 162,901 | |||||||||||||||
| Long-term liabilities | 9,630 | 5,479 | 6,776 | — | — | |||||||||||||||
| Retained earnings (accumulated deficit) | 17,850 | 7,023 | (8,873 | ) | (26,106 | ) | (33,848 | ) | ||||||||||||
| Total stockholders’ equity | 217,986 | 213,073 | 210,620 | 145,538 | 142,804 | |||||||||||||||
| (1) | Revenues include increases of $0.7 million, $0.6 million, $0.4 million and $0.4 million for the years ended 2005, 2004, 2003 and 2002, respectively, related to the reclassification of freight charges recovered from customers from Cost of Revenues to Revenues. Cost of Revenues has been increased accordingly, and there was no effect on gross profit, operating income or net income for any of the periods presented. | |
| (2) | Our 2006 income from operations included a $4.3 million write-off for the acquisition of in-process research and development costs related to the acquisition of the LCM business from Arcturus, a $0.3 million restructuring charge related to the closure of our |
23
Table of Contents
| Norway facility and a $1.4 million translation gain from the reversal of cumulative translation adjustments related to the liquidation of our Norway and Swiss subsidiaries. Our 2005 income from operations included a $1.4 million charge related to restructuring, associated with the termination of employees and the restructuring of an agreement. Our 2004 income from operations included a $5.0 million write-off for the acquisition of in-process research and development costs and a $1.2 million charge related to restructuring, associated with the acquisition of Axon. | ||
Item 7.Management’s Discussion and Analysis of Financial Condition and Results of Operations.
Overview
Except for the historical information contained herein, the following discussion contains “forward-looking” statements. For this purpose, any statements that are not statements of historical fact may be deemed to be forward-looking statements. Words such as “believes,” “anticipates,” “plans,” “predicts,” “expects,” “estimates,” “intends,” “will,” “continue,” “may,” “potential,” “should” and similar expressions are intended to identify forward-looking statements. There are a number of important factors that could cause our results to differ materially from those indicated by these forward-looking statements, including, among others, those discussed in this section as well as under Item 1A — “Risk Factors” and Item 7A — “Quantitative and Qualitative Disclosures About Market Risk” and the risks detailed from time to time in the Company’s future SEC reports.
On January 28, 2007, we entered into a definitive agreement and plan of merger with MDS Inc. (“MDS”) and Monument Acquisition Corp. (“Monument”), a subsidiary of MDS, which contemplates the acquisition by MDS, through Monument, of all of our outstanding common stock in a two-step transaction comprised of a cash tender offer for all of our issued and outstanding shares of common stock, followed by a merger of Monument with and into us. Pursuant to the definitive agreement, and upon the terms and subject to the conditions thereof, Monument has commenced a tender offer to acquire all of the outstanding shares of our common stock at a price of $35.50 per share, net to the holder thereof in cash. Pursuant to the definitive agreement, as soon as practicable after the consummation of the tender offer and subject to the satisfaction or waiver of certain conditions set forth in the definitive agreement, Monument will merge with and into us and we will become an indirect wholly-owned subsidiary of MDS. Under the terms of the agreement, our stockholders will receive $35.50 per share in cash for each outstanding share of our common stock through a tender offer commenced by MDS and its indirect wholly-owned subsidiary. The obligation of Monument to accept for payment and pay for the shares tendered in the tender offer is subject to the satisfaction or waiver of a number of closing conditions set forth in the definitive agreement, including among others, the expiration of the waiting period under the Hart-Scott-Rodino Antitrust Improvements Act. The closing of the merger contemplated by the definitive agreement is subject to customary closing conditions, and, depending on the number of shares held by MDS and Monument after Monument’s acceptance of the shares properly tendered in connection with the tender offer, approval of the merger contemplated by the definitive agreement by the holders of the outstanding shares of our common stock remaining after the completion of the tender offer may be required. See Item 1A — “Risk Factors – Risks Related to the Proposed Acquisition by MDS Inc.”
We are a leading supplier of high-performance bioanalytical measurement systems that accelerate and improve drug discovery and other life sciences research. Our systems and consumables enable pharmaceutical and biotechnology companies to leverage advances in genomics, proteomics and parallel chemistry by facilitating the high-throughput and cost-effective identification and evaluation of drug candidates. Our solutions are based on our advanced core technologies that integrate our expertise in engineering, molecular and cell biology, and chemistry. We enable our customers to improve research productivity and effectiveness, which ultimately accelerates the complex process of discovering and developing new drugs.
Our customers include leading pharmaceutical and biotechnology companies as well as medical centers, universities, government research laboratories and other institutions throughout the world. The success of our business is impacted by research and development spending trends of these customers, which has been unpredictable over the last three years and remains unpredictable in the near term. We focus on generating revenue growth through the development of innovative products for these customers. In each of the last five years, our internal research and development efforts have enabled us to exceed our goal of generating over 50% of annual revenues from products introduced in the last three years.
We divide our revenues into two product families based primarily on the customers to which they are sold. The drug discovery product family includes systems that integrate detection, liquid handling and automation, have price points in excess of $200,000, and are primarily sold to large pharmaceutical and biotechnology companies. Product lines included in the drug discovery family are the FLIPR, Automated Electrophysiology, High-Throughput Imaging and Analyst product families. The life sciences research product family consists of SpectraMax, GenePix, MetaMorph, Laser Capture Microdissection, Cellular Neurosciences, Liquid Handling and Threshold product lines. These single-purpose instruments generally cost less than $60,000 and are sold throughout our entire customer base. We recognize revenue on the sale of these products, when collectibility is reasonably assured, at the time of shipment and transfer of title to customers and distributors. There are no significant customer acceptance requirements or post shipment obligations on our part.
24
Table of Contents
We are deriving an increasing portion of our revenues from overseas operations. Sales to customers outside of the United States accounted for 47% of total revenue in 2006, 44% in 2005, and 42% in 2004. We currently have sales and services offices in the United Kingdom, Germany, Japan, China, South Korea, Australia and Brazil. In addition, we employ sales and service personnel in Canada, France, the Benelux, Scandinavia and Spain. Additional international sales are conducted through distributors around the world. We anticipate that international sales will account for an increasing percentage of revenues in the future, and we expect to continue expanding our international operations in order to take advantage of increasing international market opportunities. Our international business exposes us to a number of risks that are described under Item 1A — “Risk Factors” above, including:
| • | political, social and economic instability; | ||
| • | trade restrictions and changes in tariffs; | ||
| • | the impact of business cycles and downturns in economies outside of the United States; | ||
| • | unexpected changes in regulatory requirements that may limit our ability to export our products or sell into particular jurisdictions; | ||
| • | import and export license requirements and restrictions; | ||
| • | difficulties and costs of staffing, managing and monitoring geographically disparate operations; | ||
| • | difficulties in maintaining effective communications with employees and customers due to distance, language and cultural barriers; | ||
| • | disruptions in international transport or delivery; | ||
| • | difficulties in protecting our intellectual property rights, particularly in countries where the laws and practices do not protect proprietary rights to as great an extent as do the laws and practices of the United States; | ||
| • | difficulties in enforcing agreements through non-U.S. legal systems; | ||
| • | longer payment cycles and difficulties in collecting receivables; | ||
| • | potentially adverse tax consequences; and | ||
| • | foreign currency exchange fluctuations. |
On April 3, 2006, we acquired the LCM business from Arcturus. The total cost of the acquisition was $11.3 million, including cash paid of $10.3 million and transaction costs of $1.0 million. The acquisition was accounted for under the purchase method of accounting. The results of operations of the LCM business have been included in the accompanying consolidated financial statements from the date of acquisition. This strategic acquisition expanded our life sciences product portfolio to include complete systems and reagents for LCM. This acquisition did not cause us to create a new business segment.
We allocated the preliminary purchase price based on the fair value of the assets acquired and liabilities assumed. A valuation of the purchased intangible assets was undertaken by a third party valuation specialist to assist us in determining the estimated fair value of each identifiable asset and in allocating the purchase price among acquired assets, including the portion of the purchase price attributed to acquired in-process research and development projects. The analysis resulted in $4.3 million of the purchase price being allocated to acquired in-process research and development, using a discount rate of 25%, and charged to earnings. The in-process research and development acquired from Arcturus consisted of product development initiatives. We estimated that the in-process projects related to these products were approximately 81% complete.
The value assigned to acquired in-process research and development was determined by considering the importance of the project to the overall development plan, estimating the costs to develop the purchased in-process research and development into commercially viable products, estimating the resulting net cash flows from the projects when completed, and discounting the net cash flows to their present value. The revenue estimates used to value the acquired in-process research and development were based on estimates of relevant market sizes and growth factors, expected trends in technology and the nature and expected timing of new product introductions by Arcturus and its competitors. The rates used to discount the net cash flows to their present value were based on Arcturus’ internal rate of return. The internal rate of return was adjusted to reflect the difficulties and uncertainties in completing each project and thereby achieving technological feasibility, the percentage of completion of each project, anticipated market acceptance and penetration, and market growth rates and risks related to the impact of potential changes in future target markets.
25
Table of Contents
In March 2005, we completed the purchase of certain assets from Xsira relating to the Transfluor® technology, a cell-based fluorescent assay system for monitoring the function of G-protein coupled receptors. We acquired $11.2 million of intangible assets, including $11.0 million of developed technology and tradenames valued at $0.2 million. We also acquired $0.1 million of other current assets consisting of receivables assigned to us in the purchase.
On July 1, 2004, we acquired all of the outstanding capital stock of Axon. This acquisition expanded our product portfolio with systems for cellular neurosciences and genomics and combined complementary product lines in High-Throughput Imaging and Automated Electrophysiology. This acquisition did not cause us to create a new business segment. The total cost of the acquisition was $139.3 million including cash and stock paid, options assumed, and direct transaction costs. As a result of the acquisition, we received $22.1 million in cash that had been on the balance sheet of Axon. The acquisition was accounted for under the purchase method of accounting. The results of operations of Axon have been included in the accompanying consolidated financial statements from the date of acquisition. We allocated the purchase price based on the estimated fair value of the assets acquired and liabilities assumed. A valuation of the purchased intangible assets was undertaken by a third party valuation specialist to assist us in determining the estimated fair value of each identifiable asset and in allocating the purchase price among acquired assets, including the portion of the purchase price attributed to acquired in-process research and development projects. The analysis resulted in $5.0 million of the purchase price being allocated to acquired in-process research and development and charged to earnings.
Critical Accounting Policies
Management’s discussion and analysis of our financial condition and results of operations are based upon our consolidated financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States. The preparation of these financial statements requires management to make estimates and judgments that affect the reported amounts of assets, liabilities, revenues and expenses and related disclosure of contingent assets and liabilities. On an ongoing basis, management evaluates its estimates, including those related to revenue recognition, warranty obligations, bad debts, inventories, intangible assets, equity investments and income taxes. Management bases its estimates on historical experience and on various assumptions that are believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions.
We believe the following critical accounting policies affect the more significant judgments and estimates used in the preparation of our consolidated financial statements:
Revenue Recognition
We apply the provisions of the following authoritative literature in the development of our revenue recognition policies:
| • | Emerging Issues Task Force, Issue No. 00-21, “Revenue Arrangements with Multiple Deliverables.” Revenue arrangements with multiple elements are divided into separate units of accounting if the deliverables in the arrangement have value to the customer on a stand alone basis, there is objective and reliable evidence of the fair value of the undelivered elements and there are no rights of return or additional performance guarantees by us. | ||
| • | Statement of Position 97-2, “Software Revenue Recognition.” Revenue earned on software arrangements involving multiple elements is allocated to each element based on the relative fair values of the elements as determined by means of our quoting process and published price lists. | ||
| • | Staff Accounting Bulletin (“SAB”) No. 104, “Revenue Recognition.” Revenue is recognized when the following four criteria are met: (1) persuasive evidence of an arrangement exists; (2) delivery has occurred or services have been rendered; (3) the seller’s price is fixed or determinable; and (4) collectibility is reasonably assured. |
A majority of our revenue is derived from the sale of instruments to end-users with a one-year warranty. These arrangements include a single element (the instrument). Other single-element arrangements include the sale of consumables, software, service, technology licenses, installation or training. Arrangements incorporating multiple elements may exist, either through one invoice or through separate invoices entered into with a single customer at or near the same time. These multiple-element arrangements can include any combination of the previously described products or services. If there are multiple elements not delivered together, we recognize revenue on delivered elements when the fair value of any undelivered elements is known.
26
Table of Contents
All single-element and multiple-element arrangements are evidenced by an invoice in response to a written purchase order or license agreement. Each element of an arrangement is invoiced at fair value as determined by means of our quoting process and published price lists. Standard end-user terms include: risk of loss transferring to the purchaser at the time of shipment, net 30 day payment terms, no right of return or exchange, no right to upgrades and no acceptance provisions.
We do not enter into arrangements that require performance in excess of our published specifications.
Our revenue recognition criteria are as follows. In multiple element arrangements, each element is invoiced at fair value, and our revenue recognition criteria are applied to each element of the arrangement.
| • | Instruments, software and consumables — We recognize revenue with respect to sales of instruments, software and consumables at the time that an instrument, software or consumable is shipped, in accordance with the shipping terms of the invoice. Under FOB Destination terms, we do not recognize revenue until the product arrives at the customer site. We determine that the SAB 104 criteria have been met through receipt of a valid purchase order and issuance by us of either a sales order confirmation or an invoice, confirmation of product shipment (or receipt, when FOB Destination terms apply), issuance of an invoice indicating the price and determination of credit-worthiness or, in certain circumstances, receipt of prior payment. | ||
| • | Service, installation and training — Revenue from service events not covered by warranty or a service contract is recognized upon completion of the service. Service can be provided in the field or at our service depot. For a small number of products, we offer the option to purchase installation services. Installation is billed separately at the time of performance and is not part of a package price for instruments. We have established a fair value for installation services, as installation can be purchased with or without an instrument. Further, a third party or the customer can perform the installation. Training is billed separately at the time of performance and is not part of a package price for instruments. Training revenue is recognized upon completion of the training. We determine that the SAB 104 criteria have been met through receipt of a valid purchase order and issuance by us of either a sales order confirmation or an invoice, receipt of a customer acknowledgment that the service, installation or training has been completed, issuance of an invoice indicating the price and determination of credit-worthiness or, in certain circumstances, receipt of prior payment. | ||
| • | Service contracts — Revenue from service contracts for our instruments, generally with a one-year term, is recognized ratably over the period of coverage. We determine that the SAB 104 criteria have been met through receipt of a valid purchase order and issuance by us of either a sales order confirmation or an invoice, issuance of an invoice indicating the price and determination of credit-worthiness or, in certain circumstances, receipt of prior payment. | ||
| • | Technology license agreements — Revenue from technology license agreements is recognized upon completion of our obligations to the licensee. We determine that the SAB 104 criteria have been met through receipt of an executed license agreement and determination of credit-worthiness or, in certain circumstances, receipt of prior payment. We have no ongoing obligations under our current technology license agreements. |
Warranty
Future warranty costs are estimated based on historical experience and provided for at the time of sale.
Accounts Receivable
We sell our products primarily to corporations, academic institutions, government entities and distributors within the drug discovery and life sciences research markets. We perform ongoing credit evaluations of our customers and generally do not require collateral. We provide reserves against trade receivables for estimated losses that may result from customers’ inability to pay. The amount of the reserve is determined by analyzing known uncollectible accounts, aged receivables, economic conditions in the customers’ country or industry, historical losses and customer credit-worthiness. Amounts later determined and specifically identified to be uncollectible are charged or written off against the reserve. Estimated losses have historically been within our expectations.
Inventories
Inventories are stated on a first-in, first-out basis at the lower of cost or market. We write down our inventory for estimated obsolescence or unmarketable inventory equal to the difference between the cost of inventory and the estimated market value based upon assumptions about future demand and market conditions. If actual market conditions are less favorable than those we project, additional inventory write-downs may be required. Such write-downs have historically been within our expectations.
27
Table of Contents
Goodwill and Other Intangible Assets
Our business acquisitions have resulted in goodwill and other intangible assets, and the recorded value of those assets may become impaired in the future. As of December 31, 2006, our goodwill and other intangible assets, net of accumulated amortization, were $107.3 million and $41.8 million, respectively. The determination of the value of such assets requires management to make estimates and assumptions that affect our consolidated financial statements. Annually, and more frequently if an event or circumstance indicates that impairment has occurred, we assess impairment of goodwill and other intangible assets. We assess potential impairments to other intangible assets when there is evidence that events or changes in circumstances indicate that the carrying amount of an asset may not be recovered. Our judgments regarding the existence of impairment indicators and future cash flows related to intangible assets are based on operational performance of our business, market condition and other factors. Although there are inherent uncertainties in this assessment process, the estimates and assumptions we use are consistent with our internal planning. If these estimates or their related assumptions change in the future, we may be required to record an impairment charge on all or a portion of our goodwill and intangible assets. Furthermore, we cannot predict the occurrence of future impairment-triggering events nor the impact such events might have on our reported asset values. Future events could cause us to conclude that impairment indicators exist and that goodwill or other intangible assets associated with our acquired businesses is impaired. Any resulting impairment loss could have an adverse impact on our results of operations.
Equity Investments
We have invested in equity instruments of privately held companies for business and strategic purposes. As of December 31, 2006, we had an investment in one company with a carrying value of $1.2 million, which is included in intangible and other assets. This investment is accounted for under the cost method because our ownership is less than 20 percent of voting securities and we do not have the ability to exercise significant influence over operations. We regularly review the assumptions underlying the operating performance and cash flow forecasts in assessing the estimated fair values of our non-marketable investments. We monitor the preceding factors to identify events or circumstances that would cause us to test for other than temporary impairment and revise our assumptions for the estimated recovery of equity investments.
Income Taxes
Income taxes are accounted for under the liability method whereby deferred tax asset or liability account balances are calculated at the balance sheet date using current tax laws and rates in effect for the year in which the differences are expected to affect taxable income. Valuation allowances are established when necessary to reduce deferred tax assets to the amounts expected to be realized in the future. Significant estimates are required in determining our provision for income taxes. Some of these estimates are based on interpretations of existing tax laws or regulations. We believe that our estimates are reasonable and that our reserves for income tax related uncertainties are adequate.
At December 31, 2006, we had net deferred tax assets of $0.8 million. Realization of these assets is dependent on our ability to generate significant future taxable income. We believe that sufficient income will be earned in the future to realize these assets. We will evaluate the realizability of the deferred tax assets and assess the need for valuation allowances periodically.
Various factors may have favorable or unfavorable effects upon our effective tax rate in the future. These factors include, but are not limited to, interpretations of existing tax laws, changes in tax laws and rates, future levels of research and development spending, future levels of capital expenditures, international operations and our success in research and development and commercializing products.
Stock-Based Compensation
Effective January 1, 2006, we adopted the fair value recognition provisions of FAS 123R using the modified prospective transition method and therefore have not restated prior periods. Prior to January 1, 2006, we accounted for our employee stock option plans under the recognition and measurement provisions of Accounting Principles Board Opinion No. 25 and related interpretations, as permitted by FASB Statement No. 123, “Accounting for Stock-Based Compensation,” as amended. Accordingly, no compensation expense was recognized for stock option grants with an exercise price equal to the fair market value of the shares at the date of grant.
As part of our adoption of FAS 123R, we reevaluated our assumptions used in estimating the fair value of stock options granted. As part of this assessment, we determined that a combination of implied and historical volatility is a better indicator of our expected volatility than historical volatility alone, which we previously used to value our options. Further, we examined the historical pattern of option exercises in order to determine if there were any discernable activity patterns based on certain employee populations. From this analysis, we identified two employee populations. We used the Black Scholes option-pricing model to value the options for both of the employee populations.
28
Table of Contents
In 2006, we recognized pre-tax stock-based compensation expense of $5.0 million. As of December 31, 2006, $7.5 million of total unrecognized stock-based compensation expense related to stock options was expected to be recognized over a weighted-average period of 1.70 years.
Results of Operations
The following table summarizes our consolidated statements of operations as a percentage of revenues:
| Years Ended December 31, | ||||||||||||
| 2006 | 2005 | 2004 | ||||||||||
| Revenues | 100.0 | % | 100.0 | % | 100.0 | % | ||||||
| Cost of revenues | 37.6 | 38.6 | 38.1 | |||||||||
| Gross profit | 62.4 | 61.4 | 61.9 | |||||||||
| Operating expenses: | — | — | — | |||||||||
| Research and development | 12.5 | 13.9 | 14.8 | |||||||||
| Selling, general and administrative | 38.5 | 32.9 | 35.2 | |||||||||
| Acquired in-process research and development | 2.3 | — | 3.4 | |||||||||
| Restructuring and other charges | 0.2 | 0.8 | 0.8 | |||||||||
| Total operating expenses | 53.5 | 47.6 | 54.1 | |||||||||
| Income from operations | 8.9 | 13.8 | 7.8 | |||||||||
| Gain on sale of equity securities | 1.2 | — | 12.3 | |||||||||
| Translation gain on liquidation of subsidiaries | 0.8 | — | — | |||||||||
| Interest expense | — | — | (0.1 | ) | ||||||||
| Interest and other income (expense), net | 0.3 | (0.3 | ) | 0.2 | ||||||||
| Income before income taxes | 11.1 | 13.5 | 20.1 | |||||||||
| Income tax provision | 5.3 | 4.7 | 8.6 | |||||||||
| Net income | 5.8 | % | 8.7 | % | 11.6 | % | ||||||
Years Ended December 31, 2006 and 2005
Revenues
Revenues in 2006 increased 2% to $186.4 million from $182.0 million in 2005. Drug discovery product family revenues in 2006 decreased by 7% compared to 2005, and represented 37% of total revenue. Life sciences research product family revenues in 2006 increased by 9% compared to 2005, and represented 63% of total revenue. The $4.4 million increase in revenues was primarily due to sales of products from our acquired LCM business, as well as growth in our SpectraMax and High-Throughput Imaging product lines, offset by decreases across several product lines in drug discovery.
Gross margin
Gross margin increased to 62.4% in 2006 from 61.4% in 2005 primarily due to the addition of revenues from the LCM acquired products without significant additions to overhead.
Research and development expenses
Research and development expenses in 2006 decreased by 8% to $23.4 million from $25.3 million in 2005. This decrease was primarily due to decreases of $2.3 million in salaries, benefits, and associated costs due to reduced headcount and a reduction of $0.5 million in consulting fees related to our Automated Electrophysiology product line, partially offset by FAS 123R stock-based compensation expense of $1.1 million.
Selling, general and administrative expenses
Selling, general and administrative expenses in 2006 increased by 20% to $71.8 million from $59.9 million in 2005. The increase was primarily due to $6.3 million of additional salaries and associated costs of increased headcount associated with international expansion and the LCM business acquisition, $3.5 million of FAS 123R stock-based compensation expense, amortization of $0.7 million primarily for the intangible assets acquired from Arcturus, and $1.1 million for consulting costs related to FAS 123R implementation and other projects.
29
Table of Contents
Acquired in-process research and development
In 2006, a $4.3 million write-off of in-process research and development occurred in conjunction with the acquisition of the LCM business from Arcturus. There was no similar write-off in 2005.
Restructuring and other charges
In 2006, we recorded a restructuring charge of $0.3 million related to the liquidation and closing of a foreign subsidiary, primarily due to employee severance. This restructuring will not have a material impact on future results of operations nor cash flows.
In 2005, we implemented a restructuring plan which included the termination of employees and the restructuring of a development and license agreement with a supplier. Costs for this plan associated with employee severance totaled $0.6 million. Costs for the restructuring of the agreement totaled $0.8 million, including the return of our equity investment in the supplier valued at $0.5 million and the transfer of $0.3 million of inventory. The total of $1.4 million was recognized as expense in restructuring and other charges in the Consolidated Statements of Income.
Gain on sale of equity securities
In the fourth quarter of 2004, we disposed of our investment in Serologicals Corporation (“Serologicals”) subsequent to its acquisition of Upstate Group, Inc. (“Upstate”). During 2006, we received additional cash consideration for our Upstate investment in the amount of $2.2 million from the escrow account established by Serologicals in connection with its acquisition of Upstate in 2004. There was no similar gain in 2005.
Translation gain on liquidation of subsidiaries
We recorded a gain in 2006 of $1.4 million from the reversal of cumulative translation adjustments upon the liquidation of two foreign subsidiaries.
Interest expense
Interest expense remained consistent in 2006 and 2005. Interest expense represents interest and fees on our revolving credit facility initially entered into to finance our acquisition of Axon.
Interest and other income (expense), net
Interest and other income (expense), net increased in 2006 to $0.5 million from $(0.5) million in 2005. This increase was due to an increase in interest income due to higher interest rates earned on our cash and cash equivalent portfolio and lower foreign currency transaction losses on short-term intercompany receivables and payables.
Income tax provision
We recorded income tax provisions of $9.8 million (an effective tax rate of 48%) and $8.6 million (an effective tax rate of 35%) for 2006 and 2005, respectively. The increase in our 2006 effective tax rate when compared to 2005 was primarily due to non-deductible stock based compensation expense, the recording of a deferred tax liability in connection with the liquidation and closing of a foreign subsidiary and an adjustment to deferred tax assets associated with non-deductible intangible assets. The effective tax rates for 2006 and 2005 were calculated on profit before tax.
Years Ended December 31, 2005 and 2004
Revenues
Revenues in 2005 increased 22% to $182.0 million from $149.1 million in 2004. Drug discovery product family revenues in 2005 increased by 18% compared to 2004, and represented 40% of total revenue. Life sciences research product family revenues in 2005 increased by 25% compared to 2004, and represented 60% of total revenue. The $32.9 million increase in revenue was due to $22.2 million of sales of our acquired Axon product lines, including PatchXpress, ImageXpress, Cellular Neurosciences and GenePix, $7.7 million in growth in life sciences research products driven by our SpectraMax products including our SpectraMax M5, and increases in drug discovery of $3.0 million attributed to increases in the Automated Electrophysiology and FLIPR product families.
30
Table of Contents
Gross margin
Gross margin decreased to 61.4% in 2005 from 61.9% in 2004 due to a full year of revenue on lower margin products acquired from Axon in July 2004.
Research and development expenses
Research and development expenses in 2005 increased by 15% to $25.3 million from $22.0 million in 2004. This $3.3 million increase was the result of a full year of salary, benefits and other expenses of the acquired Axon research and development activities.
Selling, general and administrative expenses
Selling, general and administrative expenses in 2005 increased by 14% to $59.9 million from $52.5 million in 2004. This increase was due to $3.1 million of domestic salary, benefits, facility and other expenses of the acquired Axon selling, general and administrative activities; $2.0 million of increased costs associated with our international operations, including European expansion and new offices in China and South Korea; and increased amortization of $1.8 million for the intangible assets acquired from Axon and Xsira.
Acquired in-process research and development
In 2004, a $5.0 million write-off of in-process research and development occurred in conjunction with the acquisition of Axon. There was no similar write-off in 2005.
Restructuring and other charges
In 2005, we implemented a restructuring plan which included the termination of employees and the restructuring of a development and license agreement with a supplier. Costs for this plan associated with employee severance totaled $0.6 million. Costs for the restructuring of the agreement totaled $0.8 million, including the return of our equity investment in the supplier valued at $0.5 million and the transfer of $0.3 million of inventory. The total of $1.4 million was recognized as expense in restructuring and other charges in the Consolidated Statements of Income.
In connection with the acquisition of Axon in 2004, we implemented an integration plan which included the termination of Axon and Molecular Devices employees, the relocation or transfer of employees to other sites and the closure of duplicate facilities. Termination costs related to Molecular Devices employees totaled $1.2 million and was expensed as restructuring and other charges.
Gain on sale of equity securities
As a result of the acquisition of Upstate by Serologicals in October 2004, we received cash and shares of common stock in Serologicals. Subsequently, we disposed of our entire equity interest in Serologicals. The net gain as a result of these transactions was $18.3 million, which was reported as gain on sale of equity securities in 2004. There was no similar gain in 2005.
Interest expense
Interest expense was $85,000 and $0.2 million in 2005 and 2004, respectively. Interest expense represents interest and fees on our revolving credit facility entered into to finance our acquisition of Axon.
Interest and other income (expense), net
Interest and other income (expense), net decreased in 2005 to $(0.5) million from $0.3 million in 2004. This decrease was due to foreign currency transaction losses on short-term intercompany receivables and payables and a decrease in interest income due to lower cash and investment balances.
Income tax provision
We recorded income tax provisions of $8.6 million (an effective tax rate of 35%) and $12.8 million (an effective tax rate of 43%) for 2005 and 2004, respectively. The decrease in our 2005 effective tax rate when compared to 2004 was primarily due to changes in estimates regarding certain current and prior year’s state income tax items and the absence of a non-deductible acquired in-process research and development expense which was recorded in 2004 in connection with our acquisition of Axon. The effective tax rates for 2005 and 2004 were calculated on profit before tax.
31
Table of Contents
Liquidity and Capital Resources
As of December 31, 2006 we had $22.8 million in cash and cash equivalents compared to $28.9 million and $30.2 million as of December 31, 2005 and December 31, 2004, respectively.
Net cash provided by operating activities was $21.7 million for the year ended December 31, 2006, compared to $24.6 million for the year ended December 31, 2005, and $23.1 million for the year ended December 31, 2004. The cash provided during 2006 was primarily the result of net income of $10.8 million plus net non-cash charges of $14.4 million, less net changes in operating assets and liabilities of $3.5 million. The non-cash charges included depreciation and amortization of $10.2 million, $3.5 million of FAS123R expense, net of tax benefits from stock-based compensation, a $4.3 million charge for the write-off of acquired in-process research and development, the gain on the sale of equity securities of $2.2 million, and a $1.4 million translation gain on the liquidation of subsidiaries.
Net cash used in investing activities was $15.4 million for the year ended December 31, 2006, compared to $13.1 million for the year ended December 31, 2005 and $16.8 million for the year ended December 31, 2004. We received $2.2 million of additional cash consideration for our Upstate investment from the escrow account established by Serologicals in connection with its acquisition of Upstate in 2004. We used $11.3 million of cash in 2006 to purchase the LCM business from Arcturus. In 2006 we also used $5.1 million to purchase capital equipment and $1.3 million to purchase intangible assets. In March 2005, we completed the purchase of certain assets from Xsira relating to the Transfluor technology and used $10.1 million of cash to acquire these assets. In 2005, we also used $2.9 million to purchase capital equipment. In 2004, we used $48.5 million to acquire Axon, net of cash received and $4.8 million to purchase capital equipment. We also received $28.3 million from the sale of equity securities and $9.9 million from the sales and maturity of investments.
Net cash used in financing activities was $12.3 million in 2006, compared to $12.6 million and $27.5 million in 2005 and 2004, respectively. In 2006, we used $27.0 million to purchase 0.9 million shares of our common stock, partially offset by $13.1 million of proceeds from the issuance of common stock for options exercised and $1.5 million of excess tax benefits related to stock-based compensation. In 2005, we used $19.9 million to purchase 1.0 million shares of our common stock, offset by $7.3 million of proceeds from the issuance of common stock for options exercised and employee stock purchases. The share repurchases occurred throughout 2006 and 2005, and accounted for approximately 5.6% and 5.8% of the shares of our common stock outstanding as of December 31, 2006 and 2005, respectively. Approximately 0.2 million shares remained available for repurchase at December 31, 2006 under the stock repurchase program initially approved by our Board of Directors in August 2001.
On July 1, 2004, we acquired all of the outstanding capital stock of Axon. In connection with the acquisition of Axon, we entered into a senior unsecured credit facility with Union Bank of California, N.A., which provides us with a revolving credit facility in the amount of up to $30.0 million. Borrowings under our revolving credit facility are guaranteed by our domestic subsidiaries. All loans outstanding under the senior unsecured credit facility will bear interest at a rate per annum equal to, at our option, either the base rate plus 0.50% or the London InterBank Offered Rate (LIBOR) plus 1.25%. The revolving credit facility may be drawn, paid and reborrowed at our option, and matures on July 1, 2007. We initially used $15.0 million of this credit facility to partially finance the cash portion of the merger consideration paid to Axon shareholders and certain optionholders. The $15.0 million drawdown was repaid and the revolving credit facility had no outstanding balance as of December 31, 2006 or 2005.
We believe that our existing cash and anticipated cash flow from our operations will be sufficient to support our current operating plan for the foreseeable future. Our ability to generate our anticipated cash flow from operations is subject to the risks and uncertainties discussed above under Item 1A — “Risk Factors” including, in particular, variations in the amount of time it takes for us to sell our products and collect accounts receivable, the timing of customer orders and our dependence on orders that are shipped in the same quarter which gives us limited visibility of future product shipments, competition, risks associated with the pharmaceutical and biotechnology industries, supplier or manufacturing problems or delays, risks associated with past and potential future acquisitions, and risks associated with our need to develop new and enhanced products and market acceptance of such products.
32
Table of Contents
Likewise, our forecast of the period of time through which our financial resources will be adequate to support our operations is a forward-looking statement that involves risks and uncertainties, and actual results could vary as a result of a number of factors. We have based this estimate on our current plans which may change and assumptions that may prove to be wrong. Our future capital requirements will depend on many factors, including:
| • | the progress of our research and development; | ||
| • | the number and scope of our research and development programs; | ||
| • | our need to develop new and enhanced products; | ||
| • | market acceptance and demand for our products; | ||
| • | the costs that may be involved in enforcing our patent claims and other intellectual property rights; | ||
| • | potential acquisition and technology licensing opportunities; | ||
| • | the costs associated with repurchasing shares of our common stock; | ||
| • | manufacturing capacity requirements; and | ||
| • | the costs of expanding our sales, marketing and distribution capabilities both in the United States and abroad. |
We have generated sufficient cash flow to fund our capital requirements primarily through operating and financing activities over the last three years. However, we cannot assure you that we will not require additional financing in the future to support our existing operations or potential acquisition and technology licensing opportunities that may arise. Therefore, we may in the future seek to raise additional funds through bank facilities, debt or equity offerings or other sources of capital. Additional financing may not be available on favorable terms or at all, and may be dilutive to our then-current stockholders.
Our cash and investments policy emphasizes liquidity and preservation of principal over other portfolio considerations. We select investments that maximize interest income to the extent possible given these two constraints. We satisfy liquidity requirements by investing excess cash in securities with different maturities to match projected cash needs and limit concentration of credit risk by diversifying our investments among a variety of high credit-quality issuers. We believe that our existing cash and cash equivalents and anticipated cash flow from our operations will be sufficient to support our current operating plan for the foreseeable future.
Contractual Obligations
Our facilities are leased under noncancelable operating leases. In addition, we have contractual commitments for the purchase of products, components and services ending in 2007. As of December 31, 2006, the following is a summary of our contractual obligations (in millions):
| Payments Due by Period | ||||||||||||||||||||
| 2012 and | ||||||||||||||||||||
| Total | 2007 | 2008 to 2009 | 2010 to 2011 | thereafter | ||||||||||||||||
| Operating leases | $ | 31.2 | $ | 6.3 | $ | 11.2 | $ | 9.2 | $ | 4.5 | ||||||||||
| Unconditional purchase obligations | 14.1 | 14.1 | — | — | — | |||||||||||||||
| Total contractual cash obligations | $ | 45.3 | $ | 20.4 | $ | 11.2 | $ | 9.2 | $ | 4.5 | ||||||||||
Recent Accounting Pronouncements
For a description of recent accounting pronouncements, see Note 1 (under the heading “Recent Accounting Pronouncements”) of the Notes to Consolidated Financial Statements included in this report.
Item 7A.Quantitative and Qualitative Disclosures About Market Risk.
We are exposed to market risk, including changes in interest rates and foreign currency exchange rates. The primary objective of our investment activities is to preserve principal while at the same time maximizing the income we receive from our investments without significantly increasing risk. A discussion of our accounting policies for financial instruments and further disclosures relating to financial investments is included in Note 1 of the Notes to Consolidated Financial Statements included in this report.
33
Table of Contents
Foreign Currency Exchange
We are exposed to changes in foreign currency exchange rates primarily in the United Kingdom, Germany, France, the Benelux, Scandinavia, Canada, Switzerland, Japan, Australia and South Korea, where we sell many of our products directly in local currencies. All other foreign sales are denominated in U.S. dollars and bear no exchange rate risk. However, a strengthening of the U.S. dollar could make our products less competitive in overseas markets. A sensitivity analysis assuming a hypothetical 10% movement in exchange rates applied to our projected foreign sales for the fiscal year 2007, indicated that such movement would not have a material effect on our business, operating results or financial condition. However, owing to the number of currencies involved, the substantial volatility of currency exchange rates, and our constantly changing currency exposures, we cannot predict with certainty the effect of exchange rate fluctuations on our future operating results. We do not currently engage in foreign currency hedging transactions, but may do so in the future.
Interest and Investment Income
Our interest and investment income is subject to changes in the general level of interest rates, primarily U.S. interest rates. In this regard, changes in U.S. interest rates affect the interest earned on our cash equivalents and short-term investments. We invest our excess cash primarily in demand deposits with U.S. banks and money market accounts. We have not experienced any significant losses on the investments. A sensitivity analysis assuming a hypothetical 10% movement in interest rates applied to our investment balances at December 31, 2006 indicated that such market movement would not have a material effect on our business, operating results or financial condition. Actual gains or losses in the future may differ materially from this analysis, depending upon actual balances and changes in the timing and amount of interest rate movements.
Debt and Interest Expense
In connection with the acquisition of Axon in 2004, we entered into a senior unsecured credit facility with Union Bank of California, N.A., which provides us with a revolving credit facility in the amount of up to $30.0 million. Borrowings under our revolving credit facility are guaranteed by our domestic subsidiaries. All loans outstanding under the senior unsecured credit facility will bear interest at a rate per annum equal to, at our option, either the base rate plus 0.50% or the London InterBank Offered Rate plus 1.25%. A sensitivity analysis assuming a hypothetical 10% movement in interest rates applied to our debt balance at December 31, 2006 indicated that such market movement would not have a material effect on our business, operating results or financial condition, as there was no balance outstanding at year end. Actual gains or losses in the future may differ materially from this analysis, depending on the level of our outstanding debt and changes in the timing and amount of interest rate movements.
Item 8.Financial Statements and Supplementary Data.
Our consolidated financial statements and financial statement schedule as listed below are attached to this Annual report on Form 10-K as pages 63 through 88.
Financial Statements:
| • | Reports of Ernst & Young LLP, Independent Registered Public Accounting Firm; | ||
| • | Consolidated Balance Sheets as of December 31, 2006 and 2005; | ||
| • | Consolidated Statements of Income for each of the three years in the period ended December 31, 2006; | ||
| • | Consolidated Statements of Stockholders’ Equity for each of the three years in the period ended December 31, 2006; | ||
| • | Consolidated Statements of Cash Flows for each of the three years in the period ended December 31, 2006; and | ||
| • | Notes to Consolidated Financial Statements. |
Financial Statement Schedules:
Schedule II — Valuation and Qualifying Accounts
All other schedules are omitted because they are not applicable or the required information is shown in the consolidated financial statements or notes thereto.
34
Table of Contents
Item 9.Changes in and Disagreements with Accountants on Accounting and Financial Disclosure.
None.
Item 9A.Controls and Procedures.
Evaluation of Disclosure Controls and Procedures
Based on our management’s evaluation (with the participation of our chief executive officer and chief financial officer), as of the end of the period covered by this report, our chief executive officer and chief financial officer have concluded that our disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) are effective to ensure that the information required to be disclosed by us in reports that we file or submit under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the SEC rules and forms and that such information is accumulated and communicated to our management, including our chief executive officer and chief financial officer, as appropriate, to allow timely decisions regarding required disclosure.
Management’s Annual Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Exchange Act Rules 13a -15(f) and 15d-15(f). Under the supervision and with the participation of our management, including our chief executive officer and chief financial officer, we conducted an evaluation of the effectiveness of our internal control over financial reporting as of December 31, 2006 based on the framework inInternal Control — Integrated Frameworkissued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). Based on our evaluation, our management concluded that our internal control over financial reporting was effective as of December 31, 2006.
Management’s assessment of the effectiveness of internal control over financial reporting as of December 31, 2006 has been audited by Ernst & Young LLP, an independent registered public accounting firm, as stated in their report which appears below.
Attestation Report of the Registered Public Accounting Firm
Report of Ernst & Young LLP, Independent Registered Public Accounting Firm
The Board of Directors and Stockholders of Molecular Devices Corporation
We have audited management’s assessment, included in the accompanying “Management’s Annual Report on Internal Control over Financial Reporting”, that Molecular Devices Corporation maintained effective internal control over financial reporting as of December 31, 2006, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (the COSO criteria). Molecular Devices Corporation’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting. Our responsibility is to express an opinion on management’s assessment and an opinion on the effectiveness of the company’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, evaluating management’s assessment, testing and evaluating the design and operating effectiveness of internal control, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, management’s assessment that Molecular Devices Corporation maintained effective internal control over financial reporting as of December 31, 2006, is fairly stated, in all material respects, based on the COSO criteria. Also, in our opinion, Molecular Devices Corporation maintained, in all material respects, effective internal control over financial reporting as of December 31, 2006, based on the COSO criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), consolidated balance sheets of Molecular Devices Corporation and subsidiaries as of December 31, 2006 and 2005, and the related consolidated statements of income, stockholders’ equity, and cash flows for each of the three years in the period ended December 31, 2006 and our report dated March 15, 2007 expressed an unqualified opinion thereon.
/s/ Ernst & Young LLP
Palo Alto, California
March 15, 2007
March 15, 2007
Changes in Internal Control over Financial Reporting
There was no change in our internal control over financial reporting during our fourth fiscal quarter that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
Item 9B.Other Information.
None.
35
Table of Contents
PART III
Item 10.Directors, Executive Officers and Corporate Governance.
Identification of Directors and Executive Officers
Our executive officers and directors and their respective ages and positions as of January 31, 2007 are as follows:
| Name | Age | Position Held with Molecular Devices | ||||
| Joseph D. Keegan, Ph.D. | 53 | President and Chief Executive Officer; Director | ||||
| Moshe H. Alafi | 77 | Director | ||||
| David L. Anderson | 63 | Director | ||||
| A. Blaine Bowman | 60 | Director | ||||
| Alan Finkel, Ph.D. | 54 | Director | ||||
| André F. Marion | 71 | Director | ||||
| Harden M. McConnell, Ph.D. | 79 | Director | ||||
| J. Allan Waitz, Ph.D. | 71 | Director | ||||
| Timothy A. Harkness | 40 | Chief Financial Officer and Senior Vice President Finance and Operations | ||||
| Steven Davenport | 41 | Vice President European Operations | ||||
| Jan Hughes | 46 | Vice President Worldwide Marketing | ||||
| Gillian M.K. Humphries, Ph.D. | 68 | Vice President Strategic Affairs | ||||
| Robert J. Murray | 58 | Vice President Worldwide Operations | ||||
| Thomas J. O’Lenic | 43 | Vice President North American Sales and Service | ||||
| Patricia C. Sharp | 63 | Vice President Human Resources | ||||
| J. Richard Sportsman, Ph.D. | 54 | Vice President Assay and Reagent Research and Development | ||||
| Robert H. Wicke | 37 | Vice President Product Development | ||||
Joseph D. Keegan, Ph.D., has been a member of our Board of Directors since 1998. Dr. Keegan was appointed as our President and Chief Executive Officer effective March 30, 1998. From 1992 to 1998, Dr. Keegan served in various positions at Becton Dickinson and Company, a research and diagnostic company, including the positions of Vice President, Sales and Service, Vice President, General Manager of the Immunocytometry Systems Division and, most recently, President of the Worldwide Tissue Culture Business. From 1987 to 1992, he was employed by LEICA, Inc., a microscope manufacturer, where he held various senior management positions. Dr. Keegan holds a Ph.D. in Chemistry from Stanford University.
Moshe H. Alafihas been a member of our Board of Directors since 1985. Mr. Alafi has been the General Partner of Alafi Capital Company, which specializes in forming new companies in the medical, pharmaceutical and biological fields, since January 1984.
David L. Andersonhas been a member of our Board of Directors since 1983. Mr. Anderson has been a Managing Director of the General Partner of Sutter Hill Ventures, a California Limited Partnership, a venture capital company, since 1974. Mr. Anderson is also a director of Dionex Corporation, a leading supplier of analytical instrumentation.
A. Blaine Bowmanhas been a member of our Board of Directors since 1985. Mr. Bowman is also a director of Dionex Corporation and Illumina, Inc. Previously, Mr. Bowman was chairman of the board of directors of Dionex Corporation from 2002 to 2005, and President, Chief Executive Officer and a director of Dionex Corporation from 1980 to 2002.
Alan Finkel, Ph.D., has been a member of our Board of Directors since November 2005. From July 2004 to December 2005, Dr. Finkel served initially as our Vice President and Chief Technical Officer and most recently as our Senior Vice President and Chief Technology Officer. Dr. Finkel was the founder of Axon Instruments, Inc. and served as its Chief Executive Officer from 1983 until the acquisition of Axon Instruments by us in July 2004. From 2000 to 2003, Dr. Finkel was Adjunct Professor in the School of Biomedical Sciences at the University of Queensland and a Member of the John Curtin School of Medical Research Strategic Advisory Committee. From 1984 to 1987, Dr. Finkel was a Consulting Assistant Professor of Surgery, Stanford University. In 1981 and 1982, Dr. Finkel was a Research Fellow at the John Curtin School of Medical Research at the Australian National University in Canberra. Dr. Finkel received his doctorate in Electrical Engineering at Monash University, Australia, in 1981.
André F. Marionhas been a member of our Board of Directors since September 1995. Mr. Marion was a founder of Applied Biosystems, Inc., a supplier of instruments for biotechnology research, and served as its Chief Operating Officer from 1983 to 1986, its President from 1985 to 1993, its Chief Executive Officer from 1986 to 1993 and its Chairman of the board from 1987 to February 1993, when it merged with the Perkin-Elmer Corporation, a manufacturer of analytical instruments. Mr. Marion served as Vice President of Perkin-Elmer Corporation and President of its Applied Biosystems Division until his retirement in February 1995. Mr. Marion is presently a management consultant, and also serves as a director of several privately held corporations.
36
Table of Contents
Harden M. McConnell, Ph.D., is our founder and has been a member of our Board of Directors since our inception in July 1983. He is the Robert Eckles Swain Professor of Physical Chemistry, Emeritus at Stanford University and is a member of the National Academy of Sciences. Dr. McConnell has received many awards in recognition of his scientific work. Most recently these include the 1987 Pauling Medal for Chemistry and, in 1988, the National Academy of Sciences Award in Chemical Sciences. Dr. McConnell has also received the Wolf Prize (1984), the Wheland Medal (1988), the National Medal of Science (1989), the Peter Debeye Award in Physical Chemistry (1990), the Bruker Prize of the Royal Society of Chemistry (1995) and the Welch Award (2002). Dr. McConnell holds a Ph.D. degree from the California Institute of Technology.
J. Allan Waitz, Ph.D., has been a member of our Board of Directors since 1990. Dr. Waitz was President and Chief Executive Officer of DNAX Research Institute of Molecular and Cellular Biology, Inc., a subsidiary of Schering-Plough Corporation, a pharmaceutical company, until his retirement in 1992. From 1991 through December 1996, Dr. Waitz served as chairperson of the Area Committee on Microbiology of the National Committee for Clinical Laboratory Standards.
Timothy A. Harknesshas served as our Chief Financial Officer since July 1998, served as our Senior Vice President and Chief Financial Officer from August 2004 to July 2005 and was appointed as our Chief Financial Officer and Senior Vice President Finance and Operations effective August 1, 2005. From 1997 to 1998, Mr. Harkness was Vice President of Business Development at Vivra Specialty Partners, a physician practice management company. Previously, Mr. Harkness was with Montgomery Securities in the Health Care Investment Banking Group from 1994 to 1997 and with Arthur Andersen & Co. from 1989 to 1992. Mr. Harkness holds an M.B.A. from Stanford University Graduate School of Business, a B.B.A. from the University of Wisconsin-Madison, and is a C.P.A.
Steven Davenporthas served as our Vice President European Operations since February 2005. Mr. Davenport joined us in March 2002 and served as our General Manager European Operations. From 1989 to 2002, Mr. Davenport was employed by Amersham plc and Amersham Biosciences (now a part of GE Healthcare) in a variety of roles involving sales of technologies for genomics, proteomics and high throughput screening to pharmaceutical companies throughout Europe. From August 2001 to March 2002, Mr. Davenport held the post of European Business Director Technology Programs with Amersham Biosciences. Mr. Davenport holds a first class honors degree in Applied Chemistry from the University of Wales Institute of Science and Technology, Cardiff, U.K.
Jan Hugheshas served as our Vice President Worldwide Marketing since February 2005. Mr. Hughes joined us in September 2003 as Director of Product Development, IonWorks and was subsequently promoted to Vice President of Product Development, IonWorks in March 2004. In 1994, Mr. Hughes co-founded Argonaut Technologies, Inc., a bioanalytical instrument company, where he served in various capacities until September 2003, including most recently as Senior Vice President and Chief Technology Officer. Prior to co-founding Argonaut, Mr. Hughes was employed by Applied Biosystems, a bioanalytical instrument company, for approximately ten years where he led instrument product development efforts in protein sequencing, amino acid analysis and both DNA and protein synthesis. Mr. Hughes holds a B.S. in Mechanical Engineering from Cal Poly, San Luis Obispo.
Gillian M.K. Humphries, Ph.D., has served as our Vice President Strategic Affairs since March 1990. Dr. Humphries served as our consultant since our inception in 1983. In 1984, Dr. Humphries joined us on a full time basis as a research scientist and, from 1985 to 1990, she served as Director of MAXline and Cytosensor Development. Dr. Humphries holds a Ph.D. in Biochemistry from Stanford University and an M.S. in Biochemistry from San Jose State University.
Robert J. Murrayhas served as our Vice President Worldwide Operations since July 1995 and our Director of Operations from October 1993 to July 1995. Prior to joining us, Mr. Murray held general management and executive positions at a variety of companies, including Electromer Corporation, Comptronix Corp. and Gould/Biomation, Inc. Mr. Murray holds an M.B.A. from the University of California at Berkeley, an M.S. in Electrical Engineering from San Jose State University, and a B.S. in Science from the University of California at Davis.
Thomas J. O’Lenichas served as our Vice President North American Sales and Service since January 2002. From 1995 to 2002, Mr. O’Lenic served in various Sales Management positions with us, most recently as our Director of North American Sales, Life Sciences Division. From 1994 to 1995, Mr. O’Lenic was with PerSeptive Biosystems, a bioanalytical instrument company. From 1990 to 1994, Mr. O’Lenic worked for Millipore Corporation, a multinational bioscience company, and from 1989 to 1990, he worked for Bios Corporation, a life science products company. Mr. O’Lenic holds a B.S. in Biology from the University of South Florida.
Patricia C. Sharphas served as our Vice President Human Resources since September 2000. From 1997 to 2000, Ms. Sharp served as Human Resources consultant at Sharp Associates Consulting specializing in Human Resources management, leadership and organizational development. Previously, Ms. Sharp worked at Apple Computer, Inc. as Senior Vice President, Human Resources. Ms. Sharp has a B.A. in Behavioral Sciences from San Jose State University.
37
Table of Contents
J. Richard Sportsman, Ph.D., has served as our Vice President Assay and Reagent Research and Development since August 2002. From 2000 to 2002, Dr. Sportsman served as our Director of Biochemistry. From 1998 to 2000, Dr. Sportsman served as Senior Director, Assay Systems, at LJL BioSystems. From 1993 to 1998, Dr. Sportsman served as a Staff Scientist and Director of Molecular Recognition at Telik, Inc. (formerly Terrapin Technologies, Inc.).
Robert H. Wickehas served as our Vice President Product Development since June 2006. Mr. Wicke joined us in July 2004 as our Vice President Software Engineering. From 1994 to July 2004, Mr. Wicke was Vice President of Software Engineering at Axon Instruments, where he was responsible for the design, implementation and vision of software development, creating pClamp for electrophysiology, Genepix Pro for microarray detection and AcuityXpress for high-end bioinformatics analysis. In 1995, Mr. Wicke co-founded Anywhere Fast, a start-up company that specialized in imaging and language software. Mr. Wicke holds an M.S. in Computer Science from the University of Oregon and a B.S. in Mathematics and Computer Science from Drew University.
Audit Committee
Our Audit Committee is currently composed of Mr. Bowman, Dr. Waitz and Mr. Marion. Mr. Bowman serves as the chair of our Audit Committee. Our Board of Directors annually reviews the NASDAQ listing standards definition of independence for our Audit Committee members and has determined that all members of our Audit Committee are independent (as independence is currently defined in Rule 4350(d)(2)(A)(i) and (ii) of the NASDAQ listing standards). Our Board of Directors has also determined that each of Messrs. Bowman and Marion qualify as an “audit committee financial expert,” as defined in the SEC rules.
Schedule 16(a) Beneficial Ownership Reporting Compliance
Section 16(a) of the Exchange Act requires our executive officers and directors, and persons who own more than ten percent of a registered class of our equity securities, to file with the SEC initial reports of ownership and reports of changes in ownership of our common stock and other equity securities. Officers, directors and greater than ten percent stockholders are required by SEC regulations to furnish us with copies of all Section 16(a) forms they file.
To our knowledge, based solely on a review of the copies of such reports and written representations that no other reports were required, during the fiscal year ended December 31, 2006, we believe that all Section 16(a) filing requirements applicable to our executive officers, directors and greater than ten percent beneficial owners were complied with, except that Jan Hughes, Vice President Worldwide Marketing, filed a late report on Form 4, covering two transactions. In addition, Robert Wicke, Vice President Product Development, timely filed a Form 3, but the Form 3 was not filed using the correct Central Index Key code. This error was corrected by amending the previous Form 3 report.
Code of Conduct
We have adopted the Molecular Devices Corporation Code of Conduct that applies to all officers, directors and employees. The Code of Conduct is available in the Corporate Governance section of the Investor Relations section of our website at www.moleculardevices.com. We intend to satisfy the disclosure requirements under Item 5.05 of Form 8-K regarding an amendment to, or waiver from, a provision of the Code of Conduct by posting such information on our website at the address specified above.
Item 11.Executive Compensation.
Compensation Discussion and Analysis
Overview
The goals of our executive compensation program are to align compensation with business objectives and performance, to enable us to attract, retain and reward executive officers and other key employees who contribute to our long-term success, and to establish an appropriate relationship between executive compensation and the creation of stockholder value. Specifically, we have created a compensation program that combines short and long-term components, cash and equity, and fixed and contingent payments, in the proportions that we believe are most appropriate to incentivize and reward our executive officers and other key employees for achieving these goals. Our executive compensation program not only aims to be competitive in its industry, but also aims to be fair relative to compensation paid to other professionals within our organization, relative to short and long-term performance and relative to the value we deliver to our stockholders.
38
Table of Contents
As discussed in further detail below, our executive compensation program for 2006 consisted of, and was intended to strike a balance among, the following three principal components:
| • | Base Salary.Salary for each of our executive officers was based principally on an assessment of the executive officer’s current salary against individual performance and peer company pay levels. | ||
| • | Bonus.Annual cash bonuses are awarded to our executive officers based on the achievement of individual and company-wide performance objectives. | ||
| • | Long-Term Incentive Compensation.Long-term incentive awards, comprised of stock option grants, are designed to ensure that incentive compensation is linked to our long-term performance and to align our executive officers’ performance objectives with the interests of our stockholders. |
Our Compensation Committee has not established any formal policies or guidelines for allocating compensation between current and long-term incentive compensation, or between cash and non-cash compensation. However, commensurate with our philosophy of establishing a link between compensation and corporate performance, our Compensation Committee believes that a greater component of overall cash compensation for executive officers relative to other employees should be performance-based.
Role of the Compensation Committee in Setting Executive Compensation
Our Compensation Committee is responsible for recommending to our Board of Directors for approval the compensation packages offered to our executive officers. For executive compensation decisions, including decisions relating to the grant of stock options to executive officers, our Compensation Committee typically considers the recommendations of Dr. Keegan, our President and Chief Executive Officer, and he often participates in the Compensation Committee’s deliberations about executive compensation matters. However, Dr. Keegan does not participate in the determination of his own compensation or the compensation of directors, nor does he participate in deliberations with respect thereto. In determining his recommendations, Dr. Keegan solicits the input of, and receives documentary support from, Patricia Sharp, our Vice President Human Resources. The Compensation Committee also receives documentary support from Ms. Sharp. In addition, Timothy Harkness, our Chief Financial Officer, recommends to Dr. Keegan individual performance objectives for and compensation of Robert Murray, our Vice President Worldwide Operations. Other than Dr. Keegan, Ms. Sharp and Mr. Harkness, no other executive officers participate in the determination or recommendation of the amount or form of executive officer compensation. The Compensation Committee does not delegate any of its functions to others in determining executive and/or director compensation and we do not currently engage any consultants with respect to executive and/or director compensation matters.
Benchmarking of Cash and Long-Term Incentive Compensation
Our Compensation Committee believes that it is important when making its compensation decisions to be informed as to the current practices of comparable, publicly-held companies. To this end, our Compensation Committee reviewed a peer group compensation data report compiled by Equilar, Inc., a provider of compensation benchmarking solutions, in determining its 2006 compensation recommendations for our executive officers. The benchmarking report provided by Equilar reviewed base salary, bonus and equity compensation data, as well as certain financial data, from 16 comparable, publicly-held companies which included Actel Corporation, Atheros Communications, Inc., Bruker BioSciences Corporation, Caliper Life Sciences, Inc., Cepheid, Dionex Corporation, Harvard Bioscience, Inc., Illumina, Inc., IXYS Corporation, Micrel, Incorporated, Orchid Cellmark Inc., PMC-Sierra, Inc., Serologicals Corporation, Silicon Image, Inc., Stratagene Corporation and Techne Corporation. These companies were chosen for inclusion in the report by our Compensation Committee based on locale and certain business characteristics similar to our company, including revenues, net income, total assets and market capitalization. In addition to benchmarking data, our Compensation Committee has historically taken into account input from other sources, including input from other independent members of our Board of Directors and publicly-available data relating to the compensation practices and policies of other companies within and outside of the life sciences industry. The Compensation Committee realizes that benchmarking our executive compensation program against compensation earned at comparable companies may not always be appropriate as a stand-alone tool for setting compensation due to the aspects of our business and objectives that may be unique to our company; however, our Compensation Committee generally believes that gathering this information is an important part of its decision-making process with respect to our executive compensation program.
Executive Compensation Program
Our executive compensation program consists of three principal components: base salary, annual cash bonuses and long-term incentive compensation. We also provide our executive officers with certain severance and change in control benefits. Finally, we offer to our executive officers participation (with all other eligible employees) in our 401(k) Plan and certain other benefits available generally to our employees, as well as certain perquisites.
39
Table of Contents
Base Salary.In setting or adjusting base salaries for 2006, our Compensation Committee assessed each executive officer’s current salary against a number of factors, including corporate and individual performance during 2005, his or her tenure, his or her pay level compared to our other executive officers, base salary benchmarking against comparable companies, as well as general economic factors such as increases in the cost of living. As noted above, our Compensation Committee also considers the recommendations of Dr. Keegan in setting or adjusting base salaries for our other executive officers. Our Compensation Committee neither based its considerations on any single factor nor did it specifically assign relative weights to factors but rather considered a mix of factors and evaluated individual salaries against that mix. In January 2007, our Board of Directors, upon recommendation of our Compensation Committee, set base salaries for our executive officers for 2007 based on substantially similar criteria. Merit increases, if any, normally take effect on March 1 of each year.
Bonus.We maintain an annual cash bonus award program to reward executive officers and other employees for attaining company-wide and individual performance objectives. Commensurate with our philosophy of establishing a link between compensation and corporate performance, bonuses represent a greater component of overall cash compensation for executive officers than for other employees. For 2006, bonus awards for executive officers depended on the achievement of various weighted performance objectives as follows:
| • | a revenue growth target, which was weighted at 25%; | ||
| • | an operating margin target, which was weighted at 25%; | ||
| • | the performance objectives specified in our 2006 Balanced Scorecard (described below) which collectively were weighted at 30%; and | ||
| • | personal performance objectives for each executive officer individually, which were weighted at 20%. |
Our 2006 Balanced Scorecard sets forth a number of objectives established by our Board of Directors based on recommendations of management that applied generally to our employees in determining their bonuses. These company performance objectives were divided into four primary categories—financial objectives, customer objectives, internal business objectives and learning and growth objectives. The financial objectives related to revenue and EPS growth targets, including consumable revenue growth and revenue per employee growth, gross and operating margin targets, and certain working capital targets. Customer objectives related to maintaining new product leadership by attaining product shipment goals, maintaining competitive customer satisfaction levels, fostering the growth of customer user meetings, establishing a global sales presence and enhancing our overall customer experience. Internal business objectives included manufacturing cost improvement, new product solution development and product quality and process improvement. Finally, learning and growth objectives included employee development and retention and employee safety objectives. Individual performance objectives were set forth in each executive officer’s individual Balanced Scorecard (other than Dr. Keegan), which was determined in consultation with Dr. Keegan. Each executive officer’s individual Balanced Scorecard contained individual performance objectives relevant to his or her principal business unit. Dr. Keegan’s individual performance objectives were based on the objectives set forth in our 2006 Balanced Scorecard, and the individual performance component of the bonus awarded to Dr. Keegan for 2006 was based on our Compensation Committee’s subjective assessment of Dr. Keegan’s role in helping to achieve the company-wide performance objectives set forth in the 2006 Balanced Scorecard as well as overall corporate goals.
For fiscal 2006, each executive officer was assigned a target bonus ranging from 40% to 100% of his or her base salary which would be earned by the officer upon achievement of his or her respective performance target at the 100% level. Performance below 100% of target could result in a reduced bonus or no bonus. Performance above target, on the other hand, could result in bonuses above the target bonus level. The target bonuses for each of the named executive officers in 2006 are set forth below in the table captioned “2006 Grants of Plan-Based Awards Table.” Target bonuses for Dr. Keegan and Mr. Harkness were established pursuant to employment agreements entered into with us in July 2004 and December 2004, respectively. Our Board of Directors also retains the discretion to increase or decrease bonuses based on individual or company-wide circumstances not addressed or contemplated at the time when the individual and company-wide performance objectives were established. For instance, in 2007, our Compensation Committee recommended, and our Board of Directors approved, increases above the amounts our executive officers would have otherwise been awarded under the annual cash bonus program for 2006 performance to acknowledge the contribution level and effort of each of the executive officers during 2006, particularly in connection with the strategic review process that ultimately culminated in the Merger Agreement with MDS.
Our Compensation Committee has not considered whether it would adjust or attempt to recover bonus awards paid to our executive officers if the relevant performance objectives upon which such bonus awards were based were to be restated or otherwise adjusted in a manner that would have the effect of reducing the amounts payable or paid. However, in accordance with Section 304 of the Sarbanes-Oxley Act of 2002, if we are required to restate our financial statements due to our material noncompliance with any
40
Table of Contents
financial reporting requirement under the federal securities laws, as a result of misconduct, Mr. Harkness, our Chief Financial Officer, and Dr. Keegan may be legally required to reimburse us for any bonus or other incentive-based or equity-based compensation he or they receive from us during the 12-month period following the first public issuance or filing with the SEC of the financial document embodying such financial reporting requirement, as well as any profits they realize from the sale of our securities during this 12-month period.
We have not historically paid any automatic or guaranteed bonuses to our executive officers. However, we have from time to time paid signing or promotion bonuses in connection with the initial hiring or appointment of an executive officer, or a change in a person’s position or responsibilities with us. We did not pay any such signing or promotion bonuses to any of our executive officers in 2006.
Long-Term Incentive Compensation.We believe that providing a significant portion of our executives’ total compensation package in stock options aligns the incentives of our executive officers with the interests of our stockholders and with our long-term success. At present, our long-term compensation program consists solely of the grant of stock options carrying service-based vesting conditions. We grant stock options to our executive officers through the Molecular Devices Corporation 2005 Equity Incentive Plan (the “2005 Equity Plan”). The 2005 Equity Plan was established to provide our employees with an opportunity to participate, along with our other stockholders, in our long-term performance. Under the 2005 Equity Plan, initial grants of stock options are generally made to eligible employees in connection with their commencement of employment, with additional grants being made to certain employees periodically or following a significant change in the job responsibilities, scope or title of such employment. Stock options granted under the 2005 Equity Plan are subject to service-based vesting conditions, generally over a four or five-year period from the date of grant, and expire ten years from the date of grant. The exercise price of stock options granted under the 2005 Equity Plan is equal to the market price on the date of grant.
The guidelines for the number of stock options for each participant under the 2005 Equity Plan are generally determined by applying several factors to the salary and performance level of each participant and then related to the approximate market price of the stock at the time of grant. In recommending stock option grants for the executive officers to our Board of Directors, our Compensation Committee considers individual performance, overall contribution to our company, internal pay equity, officer retention, the number of unvested stock options held by the officer and the total number of stock options to be awarded. As noted above, our Compensation Committee also considers the recommendations of Dr. Keegan in determining stock option grant recommendations for our other executive officers.
We do not have any programs, plans or practices with respect to the timing of stock option grants in coordination with the release of material nonpublic information and our Board of Directors generally grants all stock options at regularly-scheduled meetings of the Board. Likewise, we do not time the release of material nonpublic information for the purpose of affecting the value of equity or other compensation granted to our executive officers. With respect to annual incentive stock option grants for our executive officers, our Board of Directors generally grants stock options to our executive officers at the first regularly-scheduled Board meeting of each fiscal year.
Severance and Change in Control Benefits.We have adopted a Change in Control Severance Benefit Plan and have entered into employment agreements with our executive officers, including Dr. Keegan and Mr. Harkness, providing for severance and change in control benefits, the terms of which are described in more detail below in the sections below entitled “Employment Agreements and Arrangements” and “Potential Payments Upon Termination or Change in Control.” We believe that these severance and change in control benefits are an important element of our executive compensation and retention program, with particular importance in the context of a change in control. Change in control benefits, including stock option vesting acceleration, are structured on a “double-trigger” basis, meaning that the executive officer must experience a constructive termination or a termination without cause in connection with the change in control in order for the change in control benefits to become due. It is our Board of Directors’ belief that providing change in control benefits should eliminate, or at least reduce, the reluctance of our executive officers to diligently consider and pursue potential change in control transactions that may be in the best interests of our stockholders.
Other Benefits.We believe that establishing competitive benefit packages for our employees is an important factor in attracting and retaining highly-qualified personnel. Our executive officers are eligible to participate in all of our employee benefit plans, such as medical, dental, vision, group life, short-term disability insurance and our 401(k) plan, in each case generally on the same basis as other employees. We do not generally offer pension or other retirement benefits unless required by the laws of the foreign jurisdictions in which we operate and have employees.
Perquisites.Our Compensation Committee annually reviews the perquisites that our executive officers receive. The primary perquisite for the executive officers during 2006 was the reimbursement of supplemental health plan expenses. In addition, Dr. Keegan and Mr. Harkness were provided with a car allowance during 2006, and we also reimbursed certain health club membership dues paid by Dr. Keegan, all pursuant to negotiated employment agreements with Dr. Keegan and Mr. Harkness as described under “Employment Agreements and Arrangements.”
41
Table of Contents
Compensation Actions for Our Executive Officers for 2006
Our compensation actions for Dr. Keegan and the other named executive officers are summarized as follows:
Joseph D. Keegan, Ph.D. — President and Chief Executive Officer
Our Compensation Committee used the approach described above in recommending to our Board of Directors for determination Dr. Keegan’s base salary, annual bonus and long-term incentive compensation for fiscal 2006. In addition, our Compensation Committee considered the status of Dr. Keegan as our most senior officer, reviewed reported cash and incentive compensation for chief executive officers of certain peer companies within and outside of the life sciences industry as described above, and evaluated the role he plays in helping to achieve the company-wide performance objectives set forth in our 2006 Balanced Scorecard as well as overall corporate goals.
For fiscal 2006, Dr. Keegan’s base salary was set at $445,000, or a 6% increase from his prior year’s base salary of $420,000. Dr. Keegan’s base salary for fiscal 2007 was set at $467,250, or a 5% increase from his 2006 base salary. Dr. Keegan was also awarded a cash bonus under our annual cash bonus award program. With respect to this cash bonus award, our Compensation Committee determined that the company-wide performance objectives under the annual cash bonus award program had not been met at the 100% level. In particular, the revenue growth target had not been met, the operating margin target had been met at the 50% level, and the 2006 Balanced Scorecard objectives had been met at the 80% level. Our Compensation Committee determined that Dr. Keegan had performed at a level above target with respect to his individual performance objectives, which, together with the results of thecompany-wide performance objectives, would result in a bonus award of $293,154, or approximately 67% of his target bonus. Nevertheless, in recognition of his efforts and contributions not addressed by these performance objectives, particularly in connection with the strategic review process that ultimately culminated in the Merger Agreement with MDS, our Board of Directors, upon recommendation of our Compensation Committee, determined to increase Dr. Keegan’s bonus award for 2006 by $175,000, for a total bonus award of $468,154. Our Compensation Committee also reviewed perquisites and other compensation paid to Dr. Keegan for 2006, which included $9,467 in supplemental health plan expenses reimbursed by us, $2,249 in health club membership dues reimbursed by us and $12,000 for the use of an automobile, and found these amounts to be reasonable. Although Dr. Keegan, along with the other named executive officers, would have ordinarily been eligible to receive an incentive stock option award for 2006 performance, our Board of Directors did not award any stock options to executive officers for 2006 performance given the restrictions on the grant of such stock option awards under the Merger Agreement with MDS. However, each of the named executive officers, including Dr. Keegan, was granted incentive stock option awards in 2006 for 2005 performance. These stock option awards are set forth in the table captioned “2006 Grants of Plan-Based Awards Table” below.
Timothy A. Harkness — Chief Financial Officer and Senior Vice President Finance and Operations
For fiscal 2006, Mr. Harkness’ base salary was set at $333,506, or a 5% increase from his prior year’s base salary of $317,625. Mr. Harkness’ base salary for fiscal 2007 was set at $350,181, or a 5% increase from his 2006 base salary. Mr. Harkness was also awarded a cash bonus pursuant to our annual cash bonus program. Our Compensation Committee determined that Mr. Harkness had performed at a level above target with respect to his individual performance objectives, which, together with the results of the company-wide performance objectives, would result in a bonus award of $132,012, or approximately 67% of his target bonus. Nevertheless, in recognition of his efforts and contributions not addressed by these performance objectives, particularly in connection with the strategic review process that ultimately culminated in the Merger Agreement with MDS, our Board of Directors, upon recommendation of our Compensation Committee, determined to increase Mr. Harkness’ bonus award for 2006 by $150,000, for a total bonus award of $282,012. Our Compensation Committee also reviewed perquisites and other compensation paid to Mr. Harkness for 2006, which included $10,466 in supplemental health plan expenses reimbursed by us and $9,000 for the use of an automobile, and found these amounts to be reasonable.
Thomas J. O’Lenic — Vice President North American Sales and Service
For fiscal 2006, Mr. O’Lenic’s base salary was set at $222,685, or a 6% increase from his prior year’s base salary of $210,080. Mr. O’Lenic’s base salary for fiscal 2007 was set at $233,819, or a 5% increase from his 2006 base salary. Mr. O’Lenic was also awarded a cash bonus pursuant to our annual cash bonus program. Our Compensation Committee determined that Mr. O’Lenic had performed at a level above target with respect to his individual performance objectives, which, together with the results of the company-wide performance objectives, would result in a bonus award of $54,264, or approximately 62% of his target bonus. Nevertheless, in recognition of his efforts and contributions not addressed by these performance objectives, particularly in connection with the strategic review process that ultimately culminated in the Merger Agreement with MDS, our Board of Directors, upon recommendation of our Compensation Committee, determined to increase Mr. O’Lenic’s bonus award for 2006 by $75,000, for a total bonus award of $129,264. Our Compensation Committee also reviewed perquisites and other compensation paid to Mr. O’Lenic for 2006, which included $4,120 in supplemental health plan expenses reimbursed by us, and found these amounts to be reasonable.
42
Table of Contents
Patricia C. Sharp — Vice President Human Resources
For fiscal 2006, Ms. Sharp’s base salary was set at $224,926, or a 4% increase from her prior year’s base salary of $210,080. Ms. Sharp’s base salary for fiscal 2007 was set at $236,172, or a 5% increase from her 2006 base salary. Ms. Sharp was also awarded a cash bonus pursuant to our annual cash bonus program. Our Compensation Committee determined that Ms. Sharp had performed at a level above target with respect to her individual performance objectives, which, together with the results of the company-wide performance objectives, would result in a bonus award of $59,447, or approximately 67% of her target bonus. Nevertheless, in recognition of her efforts and contributions not addressed by these performance objectives, particularly in connection with the strategic review process that ultimately culminated in the Merger Agreement with MDS, our Board of Directors, upon recommendation of our Compensation Committee, determined to increase Ms. Sharp’s bonus award for 2006 by $80,000, for a total bonus award of $139,447. Our Compensation Committee also reviewed perquisites and other compensation paid to Ms. Sharp for 2006, which included $815 in supplemental health plan expenses reimbursed by us, and found these amounts to be reasonable.
Robert J. Murray — Vice President Worldwide Operations
For fiscal 2006, Mr. Murray’s base salary was set at $227,089, or a 5% increase from his prior year’s base salary of $216,275 Mr. Murray’s base salary for fiscal 2007 was set at $238,443, or a 5% increase from his 2006 base salary. Mr. Murray was also awarded a cash bonus pursuant to our annual cash bonus program. Our Compensation Committee determined that Mr. Murray had performed at a level above target with respect to his individual performance objectives, which, together with the results of the company-wide performance objectives, would result in a bonus award of $59,926, or approximately 67% of his target bonus. Nevertheless, in recognition of his efforts and contributions not addressed by these performance objectives, particularly in connection with the strategic review process that ultimately culminated in the Merger Agreement with MDS, our Board of Directors, upon recommendation of our Compensation Committee, determined to increase Mr. Murray’s bonus award for 2006 by $50,000, for a total bonus award of $109,926. our Compensation Committee also reviewed perquisites and other compensation paid to Mr. Murray for 2006, which included $2,639 in supplemental health plan expenses reimbursed by us, and found these amounts to be reasonable.
Accounting and Tax Considerations
Effective January 1, 2006, we adopted the fair value recognition provisions of FAS 123R. Under FAS 123R, we are required to estimate and record an expense for each award of equity compensation over the vesting period of the award. Although we assessed the desirability of granting shares of restricted stock to our executive officers and employees in lieu of stock option grants in light of the accounting impact of FAS 123R, we ultimately determined to retain our stock option granting program as the sole component of our long-term compensation program. Accounting rules also require us to record cash compensation as an expense at the time the obligation is incurred.
Section 162(m) of the Internal Revenue Code of 1986 limits us to a deduction for federal income tax purposes of up to $1 million of compensation paid to certain named executive officers in a taxable year. Compensation above $1 million may be deducted if it is “performance-based compensation.” Stock option awards under the 2005 Equity Plan, to the extent our Board of Directors or the committee of the Board granting such stock awards is composed solely of “outside directors,” are performance-based compensation within the meaning of Section 162(m) and, as such, are fully deductible. To maintain flexibility in compensating executive officers in a manner designed to promote varying corporate goals, our Compensation Committee has not adopted a policy requiring all compensation to be deductible. Our Compensation Committee intends to continue to evaluate the effects of the compensation limits of Section 162(m) and to grant compensation awards in the future in a manner consistent with the best interests of our company and our stockholders.
Summary
Through the compensation arrangements described above, a significant portion of our executive compensation program is contingent upon the individual and company-wide performance, and realization of benefits by our executive officers is closely linked to increases in long-term stockholder value. We remain committed to this philosophy of pay-for-performance, recognizing that the competitive market for talented executives and the volatility of our business may result in highly variable compensation during any given annual period.
43
Table of Contents
Summary Compensation Table
The following table sets forth all of the compensation awarded to, earned by, or paid to our principal executive officer, principal financial officer and our three other highest paid executive officers for the year ended December 31, 2006. The officers listed in the table below are referred to in this Annual Report on Form 10-K as our “named executive officers.”
2006 SUMMARY COMPENSATION TABLE
| Non-Equity | ||||||||||||||||||||||||||||
| Option | Incentive Plan | All Other | ||||||||||||||||||||||||||
| Salary | Bonus | Awards | Compensation | Compensation | Total | |||||||||||||||||||||||
| Name and Principal Position | Year | ($) | ($)(1) | ($)(2) | ($)(3) | ($) | ($) | |||||||||||||||||||||
| Joseph D. Keegan, Ph.D. | 2006 | 440,833 | 175,000 | 727,188 | 293,154 | 26,972 | (4) | 1,663,147 | ||||||||||||||||||||
| President and Chief Executive Officer | ||||||||||||||||||||||||||||
| Timothy A. Harkness | 2006 | 330,859 | 150,000 | 464,366 | 132,012 | 22,218 | (5) | 1,099,455 | ||||||||||||||||||||
| Chief Financial Officer and Senior Vice President Finance and Operations | ||||||||||||||||||||||||||||
| Thomas J. O’Lenic | 2006 | 220,584 | 75,000 | 340,927 | 54,264 | 7,182 | (6) | 697,957 | ||||||||||||||||||||
| Vice President North American Sales and Service | ||||||||||||||||||||||||||||
| Patricia C. Sharp | 2006 | 223,484 | 80,000 | 327,344 | 59,447 | 3,882 | (7) | 694,157 | ||||||||||||||||||||
| Vice President Human Resources | ||||||||||||||||||||||||||||
| Robert J. Murray | 2006 | 225,287 | 50,000 | 300,966 | 59,926 | 5,712 | (8) | 641,891 | ||||||||||||||||||||
| Vice President Worldwide Operations | ||||||||||||||||||||||||||||
| (1) | Amounts in this column represent discretionary bonus award increases above the amounts earned by the named executive officers under our annual cash bonus award program. These increases were awarded in recognition of the efforts and contributions of the named executive officers that were not addressed by the performance objectives under our annual cash bonus award program, particularly in connection with the strategic review process that ultimately culminated in the Merger Agreement with MDS. | |
| (2) | The dollar amounts in this column represent the compensation cost for the year ended December 31, 2006 of stock option awards granted in and prior to 2006. These amounts have been calculated in accordance with FAS 123R using the Black Scholes option-pricing model and the assumptions outlined in Note 7 to the Notes to Consolidated Financial Statements included in this Annual Report on Form 10-K. | |
| (3) | See footnote (1) to the 2006 Grants of Plan-Based Awards Table below. | |
| (4) | Consists of $9,467 in supplemental health plan expenses reimbursed by us, $2,249 in health club membership dues reimbursed by us, $12,000 for the use of an automobile, $756 in group life insurance paid by us, and $2,500 in matching contributions made by us to Dr. Keegan’s 401(k) account. | |
| (5) | Consists of $10,466 in supplemental health plan expenses reimbursed by us, $9,000 for the use of an automobile, $252 in group life insurance paid by us, and $2,500 in matching contributions made by us to Mr. Harkness’ 401(k) account. | |
| (6) | Consists of $4,120 in supplemental health plan expenses reimbursed by us, $562 in group life insurance paid by us, and $2,500 in matching contributions made by us to Mr. O’Lenic’s 401(k) account. | |
| (7) | Consists of $815 in supplemental health plan expenses reimbursed by us, $567 in group life insurance paid by us, and $2,500 in matching contributions made by us to Ms. Sharp’s 401(k) account. | |
| (8) | Consists of $2,639 in supplemental health plan expenses reimbursed by us, $573 in group life insurance paid by us, and $2,500 in matching contributions made by us to Mr. Murray’s 401(k) account. |
Grants of Plan-Based Awards
The following table sets forth certain information regarding grants of plan-based awards to our named executive officers during the year ended December 31, 2006.
44
Table of Contents
2006 GRANTS OF PLAN-BASED AWARDS TABLE
| All Other | ||||||||||||||||||||
| Option Awards: | ||||||||||||||||||||
| Estimated PossiblePayouts | Number of | Exercise or | Grant | |||||||||||||||||
| Under Non-Equity | Securities | Base Price | Date Fair | |||||||||||||||||
| Incentive Plan Awards | Underlying | of Option | Value of | |||||||||||||||||
| Grant | Target | Options | Awards | Option | ||||||||||||||||
| Name | Date | ($)(1) | (#)(2) | ($/Sh) | Awards($)(3) | |||||||||||||||
| Joseph D. Keegan, Ph.D. | — | 440,833 | — | — | — | |||||||||||||||
| 02/09/06 | — | 50,000 | 29.45 | 755,000 | ||||||||||||||||
| Timothy A. Harkness | — | 198,515 | — | — | — | |||||||||||||||
| 02/09/06 | — | 35,000 | 29.45 | 528,500 | ||||||||||||||||
| Thomas J. O’Lenic | — | 88,234 | — | — | — | |||||||||||||||
| 02/09/06 | — | 25,000 | 29.45 | 377,500 | ||||||||||||||||
| Patricia C. Sharp | — | 89,394 | — | — | — | |||||||||||||||
| 02/09/06 | — | 20,000 | 29.45 | 302,000 | ||||||||||||||||
| Robert J. Murray | — | 90,115 | — | — | — | |||||||||||||||
| 02/09/06 | — | 20,000 | 29.45 | 302,000 | ||||||||||||||||
| (1) | This column sets forth the target amount of each named executive officer’s annual cash bonus award for the year ended December 31, 2006 under our annual cash bonus award program. The actual cash bonus award earned for the year ended December 31, 2006 for each named executive officer is set forth in the 2006 Summary Compensation Table above. As such, the amounts set forth in this column do not represent additional compensation earned by our named executive officers for the year ended December 31, 2006. For a description of our annual cash bonus award program, see “Compensation Discussion and Analysis—Executive Compensation Program—Bonus.” | |
| (2) | Stock options were granted pursuant to the 2005 Equity Plan. 25% of the shares subject to the stock option vest on the first anniversary of the grant date, and 6.25% of the shares subject to the stock option vest quarterly thereafter. Vesting is contingent upon continued service with us (or our affiliate). For a description of the terms of stock options granted under the 2005 Equity Plan, see “Employment Agreements and Arrangements—Stock Option Awards.” | |
| (3) | Represents the grant date fair value of such option award as determined in accordance with FAS 123R. These amounts have been calculated in accordance with FAS 123R using the Black Scholes option-pricing model and the assumptions outlined in Note 7 to the Notes to Consolidated Financial Statements included in this Annual Report on Form 10-K. |
Employment Agreements and Arrangements
Chief Executive Officer Key Employee Agreement
On July 29, 2004, we entered into an Amended Key Employee Agreement with Joseph D. Keegan, Ph.D., our President and Chief Executive Officer. The agreement supersedes and replaces Dr. Keegan’s original Key Employment Agreement with us and Dr. Keegan’s participation in our Change in Control Severance Benefit Plan. Pursuant to the terms of the agreement, Dr. Keegan’s base salary was initially set at $400,000, which is reviewed annually. Dr. Keegan is also eligible for a variable discretionary performance bonus based on the attainment of certain corporate goals established in agreement with our Board of Directors, which is intended to result in a bonus equal to 100% of his base salary if performance is at 100% of target. The agreement also provides for the provision of standard employee benefits as well as a $1,000 monthly car allowance, the payment of certain administrative expenses incurred by Dr. Keegan (not to exceed $15,000 per year), and the reimbursement of cell phone service and health club membership dues (not to exceed $500 per month).
45
Table of Contents
In the event Dr. Keegan is terminated without cause or Dr. Keegan resigns from Molecular Devices for good reason, other than in connection with a change in control, Dr. Keegan will, subject to certain conditions (including the signing of a general release of all claims against us), be entitled to receive certain severance benefits, including the following:
| • | we will retain Dr. Keegan as a consultant for a period of one year following his termination, during which time he will be entitled to a monthly cash payment equal to 1/12th of the sum of (i) his annual base salary in effect at the time of his termination plus (ii) the bonus amount Dr. Keegan would have earned at 100% of target for the year in which the termination occurs. Dr. Keegan agreed to abide by certain noncompetition covenants during the one-year consulting period; | ||
| • | the outstanding stock options held by Dr. Keegan as of the date of his termination will become fully vested and exercisable as of that date; | ||
| • | if Dr. Keegan timely elects continuation of his then-current medical, dental and vision insurance under the Consolidated Omnibus Budget Reconciliation Act of 1985, (“COBRA”), then we will pay the portion of the premiums it paid prior to the date of his termination for up to one year; | ||
| • | we will pay for an executive assistance program for a period not to exceed three months and at a cost not to exceed $7,500; and | ||
| • | we will provide reasonable secretarial support to Dr. Keegan during his one-year consulting period from its own support staff, or will reimburse Dr. Keegan for reasonable secretarial services provided to him during this time, up to a maximum of $1,250 per month. |
In the event Dr. Keegan is terminated without cause or Dr. Keegan resigns from Molecular Devices for good reason within two months prior to, or 24 months after, a change in control, Dr. Keegan will, subject to certain conditions (including the signing of a general release of all claims against us), be entitled to receive certain severance benefits (in lieu of the severance benefits described above), including the following:
| • | Dr. Keegan will be entitled to a single lump-sum payment equal to the sum of (i) 24 months of his annual base salary in effect at the time of his termination, plus (ii) a bonus amount equal to twice what Dr. Keegan would have earned at 100% of target for the year in which the termination occurs; | ||
| • | the outstanding stock options held by Dr. Keegan as of the date of his termination will become fully vested and exercisable as of that date; | ||
| • | if Dr. Keegan timely elects continuation of his then-current medical, dental and vision insurance under COBRA, then we will pay the portion of the premiums it paid prior to the date of his termination for up to 24 months; | ||
| • | we will pay for an executive assistance program for a period not to exceed three months and at a cost not to exceed $7,500; and | ||
| • | we will provide reasonable secretarial support to Dr. Keegan during the twenty-four month period following his termination from its own support staff, or will reimburse Dr. Keegan for reasonable secretarial services provided to him during this time, up to a maximum of $1,250 per month. |
If the total amount of payments and benefits to be provided to Dr. Keegan under the agreement in connection with a change in control would cause Dr. Keegan to incur “golden parachute” excise tax liability, then the payments and benefits will be reduced to the extent necessary to leave him in a better after-tax position than if no such reduction had occurred. The agreement does not provide for any tax “gross-up” payments to Dr. Keegan.
In the event Dr. Keegan’s employment relationship terminates due to his death or disability, we will continue to pay Dr. Keegan (or his heirs) Dr. Keegan’s then-current base salary for a period of three months after his termination date, and will pay the COBRA premiums necessary to continue Dr. Keegan’s then-current health insurance coverage (if applicable) for a period of three months after his termination date.
During the two year period following the termination of Dr. Keegan’s employment relationship with us, Dr. Keegan agreed that he would not interfere with our business by, among other things, soliciting our employees and customers.
Chief Financial Officer Employment Agreement
On December 17, 2004, we entered into an Amended Key Employee Agreement with Timothy A. Harkness, our Chief Financial Officer and Senior Vice President Finance and Operations. The agreement supersedes and replaces Mr. Harkness’ original offer letter agreement with us and Mr. Harkness’ participation in our Change in Control Severance Benefit Plan. Pursuant to the terms of the agreement, Mr. Harkness’ base salary was initially set at $275,000, which is reviewed annually. Mr. Harkness is also eligible for a
46
Table of Contents
variable discretionary performance bonus based on the attainment of certain corporate goals established in agreement with our Chief Executive Officer, which is intended to result in a bonus equal to 60% of his base salary if performance is at 100% of target. The agreement also provides for the provision of standard employee benefits as well as a $750 monthly car allowance, the payment of certain administrative expenses incurred by Mr. Harkness (not to exceed $5,000 per year) and the reasonable cost of cell phone service.
In the event Mr. Harkness is terminated without cause or Mr. Harkness resigns from Molecular Devices for good reason, other than in connection with a change in control, Mr. Harkness will, subject to certain conditions (including the signing of a general release of all claims against us), be entitled to receive certain severance benefits, including the following:
| • | we will retain Mr. Harkness as a consultant for a period of one year following his termination, during which time he will be entitled to a monthly cash payment equal to 1/12th of the sum of (i) his annual base salary in effect at the time of his termination plus (ii) the bonus amount Mr. Harkness would have earned at 100% of target for the year in which the termination occurs. Mr. Harkness agreed to abide by certain noncompetition covenants during the one-year consulting period; | ||
| • | the outstanding stock options held by Mr. Harkness as of the date of his termination will become fully vested and exercisable as of that date; and | ||
| • | if Mr. Harkness timely elects continuation of his then-current medical, dental and vision insurance under COBRA, then we will pay the portion of the premiums it paid prior to the date of his termination for up to one year. |
In the event Mr. Harkness is terminated without cause or Mr. Harkness resigns from Molecular Devices for good reason within two months prior to, or 24 months after, a change in control, Mr. Harkness will, subject to certain conditions (including the signing of a general release of all claims against us), be entitled to receive certain severance benefits (in lieu of the severance benefits described above), including the following:
| • | Mr. Harkness will be entitled to a single lump-sum payment equal to the sum of (i) 18 months of his annual base salary in effect at the time of his termination, plus (ii) 1.5 times the bonus amount Mr. Harkness would have earned at 100% of target for the year in which the termination occurs; | ||
| • | the outstanding stock options held by Mr. Harkness as of the date of his termination will become fully vested and exercisable as of that date; and | ||
| • | if Mr. Harkness timely elects continuation of his then-current medical, dental and vision insurance under COBRA, then we will pay the portion of the premiums it paid prior to the date of his termination for up to 18 months. |
If the total amount of payments and benefits to be provided to Mr. Harkness under the agreement in connection with a change in control would cause Mr. Harkness to incur “golden parachute” excise tax liability, then the payments and benefits will be reduced to the extent necessary to leave him in a better after-tax position than if no such reduction had occurred. The agreement does not provide for any tax “gross-up” payments to Mr. Harkness.
In the event Mr. Harkness’ employment relationship terminates due to his death or disability, we will continue to pay Mr. Harkness (or his heirs) Mr. Harkness’ then-current base salary for a period of three months after his termination date, and will pay the COBRA premiums necessary to continue Mr. Harkness’ then-current health insurance coverage (if applicable) for a period of three months after his termination date.
During the two year period following the termination of Mr. Harkness’ employment relationship with us, Mr. Harkness agreed that he would not interfere with our business by, among other things, soliciting our employees and customers.
47
Table of Contents
Vice President Employment Agreements
On July 25, 2000, we have entered into an employment letter agreement with Patricia C. Sharp, our Vice President Human Resources, that provided for the following:
| • | an initial annual base salary of $185,000 per year, subject to annual review; | ||
| • | signing bonuses totaling $50,000; | ||
| • | participation in our annual cash bonus award program; | ||
| • | the grant of a stock option to purchase 47,500 shares of our common stock, which vested over a period of four years; | ||
| • | the grant of an aggregate of 2,500 shares of our restricted common stock, which vested over a period of two years; and | ||
| • | the provision of standard employee benefits. |
On January 10, 2002, we entered into an employment letter agreement with Thomas J. O’Lenic, our Vice President North America Sales and Service. The agreement provides for an annual base salary of $185,000 per year and Mr. O’Lenic’s eligibility to participate in our annual cash bonus award program and Change in Control Severance Benefit Plan. The agreement also provides for certain relocation benefits in connection with Mr. O’Lenic’s relocation to California from Georgia. Pursuant to the agreement, Mr. O’Lenic was entitled to receive options to purchase 40,000 shares of our common stock. The options vested over a period of four years.
On October 4, 1993, we entered into an employment letter agreement with Robert J. Murray, our Vice President Worldwide Operations. The agreement provides for an annual base salary of $100,000 per year and Mr. Murray’s eligibility to participate in our annual cash bonus award. Pursuant to the agreement, Mr. Murray was entitled to receive options to purchase 20,000 shares of our common stock. The options vested over a period of four years.
Change in Control Severance Benefit Plan
In February 2001, our Board of Directors adopted a Change in Control Severance Benefit Plan to provide certain benefits and protections to designated executive officers, currently including Ms. Sharp and Messrs. O’Lenic and Murray, who have not entered into individual severance benefit or change in control agreements with us. The plan provides that in the event of a constructive or involuntarily termination without cause within 13 months after a change in control, such terminated executive officer will, subject to certain conditions (including the signing of a general release of all claims against us), receive the following payments and benefits:
| • | a lump sum payment equal to 12 months’ salary; | ||
| • | a lump sum bonus payment equal to what would have been earned at 100% of target for the year of termination; | ||
| • | if the executive timely elects continuation of his or her then-current medical, dental and vision insurance under COBRA, we will pay the portion of premiums it paid prior to the date of his or her termination for up to 18 months; | ||
| • | the outstanding stock options held by the executive officer as of the date of his or her termination will become fully vested and exercisable as of that date; and | ||
| • | we will pay for an executive assistance program for a period not to exceed three months and at a cost not to exceed $7,500. |
The payments and benefits described above are subject to certain reductions and offsets if, for example, the executive officer received severance benefits from us pursuant to any other policy, program, plan or arrangement. In addition, if the total amount of payments and benefits under the plan would cause the executive officer to incur “golden parachute” excise tax liability in connection with the change in control, then the payments and benefits will be reduced to the extent necessary to leave him or her in a better after-tax position than if no such reduction had occurred. The plan does not provide for any tax “gross-up” payments to the executive officers. All benefits under the plan would terminate immediately with respect to an executive officer if that executive officer violates any proprietary or confidentiality obligation to us.
Stock Option Awards
We currently grant stock options to our executive officers through the 2005 Equity Plan. The 2005 Equity Plan was established to provide our employees with an opportunity to participate, along with our other stockholders, in our long-term performance. The following is a description of the permissible terms of options under the 2005 Equity Plan:
48
Table of Contents
Exercise Price.The exercise price of incentive stock options may not be less than 100% of the fair market value of the stock subject to the option on the date of grant and, in some cases, may not be less than 110% of such fair market value. The exercise price of nonstatutory stock options may not be less than 100% of the fair market value of the stock on the date of grant.
Consideration.The exercise price of options granted under the 2005 Equity Plan must be paid, to the extent permitted by applicable law and at the discretion of our Board of Directors, (i) by cash or check, (ii) pursuant to a broker-assisted cashless exercise, (iii) by delivery of other common stock of Molecular Devices, (iv) pursuant to a net exercise arrangement, or (iv) in any other form of legal consideration acceptable to our Board of Directors.
Vesting.Options granted under the 2005 Equity Plan may become exercisable in cumulative increments, or “vest,” as determined by our Board of Directors. Vesting typically will occur during the optionholder’s continued service with us or an affiliate of ours, whether such service is performed in the capacity of an employee or director and regardless of any change in the capacity of the service performed. Shares covered by different options granted under the 2005 Equity Plan may be subject to different vesting terms. Shares subject to stock options granted to executive officers during the year ended December 31, 2006 vest 25% on the one-year anniversary of the date of grant and 6.25% on a quarterly basis thereafter, subject to continued service. Our Board of Directors has the authority to accelerate the time during which an option may vest or be exercised. In addition, the 2005 Equity Plan provides for vesting acceleration upon certain corporate transaction and change in control events. Further, as described above, our executive officers are parties to agreements with us that provide for vesting acceleration in connection with certain termination and change in control events. Options granted under the 2005 Equity Plan may permit exercise prior to vesting. However, any unvested shares acquired under such an early exercise arrangement will be subject to repurchase by us, should the participant’s service terminate before vesting.
Tax Withholding.To the extent provided by the terms of a stock option agreement, a participant may satisfy any federal, state or local tax withholding obligation relating to the exercise of the option by a cash payment upon exercise, by authorizing us to withhold a portion of the stock otherwise issuable to the participant, by delivering already-owned common stock of Molecular Devices, or by a combination of these means.
Term.The maximum term of options granted under the 2005 Equity Plan is ten years, except that in certain cases, the maximum term is five years.
Termination of Service.Options granted under the 2005 Equity Plan generally terminate three months after termination of the participant’s service unless (i) termination is due to the participant’s disability, in which case the option may be exercised (to the extent the option was exercisable at the time of the termination of service) at any time within 12 months following termination; (ii) the participant dies before the participant’s service has terminated, or within generally three months after termination of service, in which case the option may be exercised (to the extent the option was exercisable at the time of the participant’s death) within 18 months following the participant’s death by the person or persons to whom the rights to such option have passed; or (iii) the option by its terms specifically provides otherwise. Under the 2005 Equity Plan, the option term may be extended in the event that exercise of the option following termination of service is prohibited by applicable securities laws. In no event, however, may an option be exercised beyond the expiration of its term.
Restrictions on Transfer.Unless provided otherwise by our Board of Directors, a participant in the 2005 Equity Plan may not transfer an option other than by will or by the laws of descent and distribution or pursuant to a domestic relations order. During the lifetime of the participant, only the participant (or the transferee pursuant to a domestic relations order) may exercise an option. A participant may also designate a beneficiary who may exercise an option following the participant’s death. Shares subject to repurchase by us pursuant to an early exercise arrangement may be subject to restrictions on transfer that our Board of Directors deems appropriate.
Annual Cash Bonus Awards
We maintain an annual cash bonus award program to reward our executive officers and other employees for attaining company-wide and individual performance objectives. For more information regarding our annual cash bonus award program, see “Compensation Discussion and Analysis—Executive Compensation Program—Bonus.”
Other Arrangements
Our executive officers are eligible to participate in all of our employee benefit plans, such as medical, dental, vision, group life, short-term disability insurance and our 401(k) plan, in each case generally on the same basis as other employees. Our executive officers are also provided with certain perquisites as described under “Compensation Discussion and Analysis—Executive Compensation Program—Perquisites.”
49
Table of Contents
Outstanding Equity Awards at December 31, 2006
The following table sets forth certain information regarding stock options granted to our named executive officers that were outstanding as of December 31, 2006.
2006 OUTSTANDING EQUITY AWARDS AT FISCAL YEAR-END TABLE
| Option Awards | ||||||||||||||||||||
| Number of | Number of | |||||||||||||||||||
| Securities | Securities | |||||||||||||||||||
| Underlying | Underlying | |||||||||||||||||||
| Unexercised | Unexercised | Option | ||||||||||||||||||
| Options | Options | Exercise | Option | |||||||||||||||||
| (#) | (#) | Price | Expiration | |||||||||||||||||
| Name | Exercisable | Unexercisable | ($) | Date | ||||||||||||||||
| Joseph D. Keegan, Ph.D. | (1 | ) | 25,000 | — | 19.12 | 03/30/08 | ||||||||||||||
| (2 | ) | 50,000 | — | 26.63 | 05/20/09 | |||||||||||||||
| (2 | ) | 75,000 | — | 48.00 | 02/08/10 | |||||||||||||||
| (3 | ) | 60,000 | — | 22.95 | 05/29/11 | |||||||||||||||
| (3 | ) | 60,000 | — | 20.20 | 01/31/12 | |||||||||||||||
| (3 | ) | 30,531 | 2,969 | 15.85 | 02/10/13 | |||||||||||||||
| (3 | ) | 41,250 | 18,750 | 19.66 | 02/11/14 | |||||||||||||||
| (3 | ) | 22,968 | 29,532 | 21.32 | 02/16/15 | |||||||||||||||
| (3 | ) | — | 50,000 | 29.45 | 02/09/16 | |||||||||||||||
| Timothy A. Harkness | (2 | ) | 30,000 | — | 48.00 | 02/08/10 | ||||||||||||||
| (3 | ) | 10,000 | — | 20.20 | 01/31/12 | |||||||||||||||
| (3 | ) | 10,468 | 2,032 | 15.85 | 02/10/13 | |||||||||||||||
| (3 | ) | 24,062 | 10,938 | 19.66 | 02/11/14 | |||||||||||||||
| (3 | ) | 14,218 | 18,282 | 21.32 | 02/16/15 | |||||||||||||||
| (3 | ) | — | 35,000 | 29.45 | 02/09/16 | |||||||||||||||
| Thomas J. O’Lenic | (2 | ) | 5,000 | — | 48.00 | 02/08/10 | ||||||||||||||
| (3 | ) | 4,218 | 1,407 | 15.85 | 02/10/13 | |||||||||||||||
| (3 | ) | 4,687 | 7,813 | 19.66 | 02/11/14 | |||||||||||||||
| (3 | ) | 10,937 | 14,063 | 21.32 | 02/16/15 | |||||||||||||||
| (3 | ) | — | 25,000 | 29.45 | 02/09/16 | |||||||||||||||
| Patricia C. Sharp | (2 | ) | 47,500 | — | 77.69 | 10/26/10 | ||||||||||||||
| (3 | ) | 15,000 | — | 22.95 | 05/29/11 | |||||||||||||||
| (3 | ) | 17,500 | — | 20.20 | 01/31/12 | |||||||||||||||
| (3 | ) | 7,937 | 1,563 | 15.85 | 02/10/13 | |||||||||||||||
| (3 | ) | 7,187 | 7,813 | 19.66 | 02/11/14 | |||||||||||||||
| (3 | ) | 10,937 | 14,063 | 21.32 | 02/16/15 | |||||||||||||||
| (3 | ) | — | 20,000 | 29.45 | 02/09/16 | |||||||||||||||
| Robert J. Murray | (2 | ) | 15,000 | — | 26.63 | 05/20/09 | ||||||||||||||
| (2 | ) | 22,500 | — | 48.00 | 02/08/10 | |||||||||||||||
| (3 | ) | 25,000 | — | 22.95 | 05/29/11 | |||||||||||||||
| (3 | ) | 25,000 | — | 20.20 | 01/31/12 | |||||||||||||||
| (3 | ) | 21,093 | 1,407 | 15.85 | 02/10/13 | |||||||||||||||
| (3 | ) | 17,187 | 7,813 | 19.66 | 02/11/14 | |||||||||||||||
| (3 | ) | 8,750 | 11,250 | 21.32 | 02/16/15 | |||||||||||||||
| (3 | ) | — | 20,000 | 29.45 | 02/09/16 | |||||||||||||||
(1) The stock option vested as to 20% of the shares of common stock subject to the stock option on the first anniversary of the vesting commencement date, and vested as to 5% of the shares subject to the stock option each quarterly period thereafter.
(2) The stock option vested as to 25% of the shares of common stock subject to the stock option on an annual basis commencing on the first anniversary of the vesting commencement date.
(3) The stock option vested or vests, as the case may be, as to 25% of the shares of common stock subject to the stock option on the first anniversary of the vesting commencement date, and vested or vests, as the case may be, as to 6.25% of the shares of common stock subject to the stock option each quarterly period thereafter.
50
Table of Contents
Option Exercises During 2006
The following table provides certain information with respect to the exercise of stock options by our named executive officers during the year ended December 31, 2006.
2006 OPTION EXERCISES TABLE
| Option Awards | ||||||||
| Number of Shares | Value Realized | |||||||
| Acquired on Exercise | on Exercise | |||||||
| Name | (#) | ($) | ||||||
| Joseph D. Keegan, Ph.D. | 65,500 | 873,735 | ||||||
| Timothy A. Harkness | 140,000 | 1,532,711 | ||||||
| Thomas J. O’Lenic | 36,235 | 398,271 | ||||||
| Patricia C. Sharp | 52,000 | 605,619 | ||||||
| Robert J. Murray | 3,000 | 57,797 | ||||||
Potential Payments Upon Termination or Change in Control
See “Employment Agreements and Arrangements” above for a description of the severance and change in control arrangements for each of our named executive officers. The amount of compensation and benefits payable to each named executive officer in various termination and change in control situations has been estimated in the tables below.
Joseph D. Keegan, Ph.D.
The following table describes the potential payments and benefits upon employment termination for Dr. Keegan, our President and Chief Executive Officer, as if his employment had terminated as of December 29, 2006, the last business day of our last fiscal year.
| No Change in Control | Change in Control | |||||||||||
| Termination | Termination | |||||||||||
| without Cause or for | without Cause or for | |||||||||||
| Good Reason | Good Reason | Death or Disability | ||||||||||
| Compensation and Benefits | ($) | ($) | ($) | |||||||||
| Base Salary and Bonus | — | 1,771,666 | 111,250 | |||||||||
| Consulting Agreement | 885,833 | — | — | |||||||||
| COBRA Premiums | 12,924 | 25,853 | 3,231 | |||||||||
| Vesting Acceleration (1) | 41,936 | 41,936 | — | |||||||||
| Executive Assistance Program | 7,500 | 7,500 | — | |||||||||
| Secretarial Support | 14,400 | 28,800 | — | |||||||||
(1) The value of vesting acceleration is based on the closing price of our common stock on December 29, 2006 ($21.07) with respect to in-the-money unvested option shares minus the exercise price of the unvested option shares.
51
Table of Contents
Timothy A. Harkness
The following table describes the potential payments and benefits upon employment termination for Mr. Harkness, our Chief Financial Officer, as if his employment had terminated as of December 29, 2006, the last business day of our last fiscal year.
| No Change in Control | Change in Control | |||||||||||
| Termination | Termination | |||||||||||
| without Cause or for | without Cause or for | |||||||||||
| Good Reason | Good Reason | Death or Disability | ||||||||||
| Compensation and Benefits | ($) | ($) | ($) | |||||||||
| Base Salary and Bonus | — | 798,032 | 83,377 | |||||||||
| Consulting Agreement | 532,021 | — | — | |||||||||
| COBRA Premiums | 12,876 | 19,360 | 3,219 | |||||||||
| Vesting Acceleration (1) | 26,030 | 26,030 | — | |||||||||
(1) The value of vesting acceleration is based on the closing price of our common stock on December 29, 2006 ($21.07) with respect to in-the-money unvested option shares minus the exercise price of the unvested option shares.
Other Named Executive Officers
The following table describes the potential payments and benefits upon employment termination for each of our named executive officers, other than Dr. Keegan and Mr. Harkness, as if such named executive officer’s employment terminated as of December 29, 2006, the last business day of our last fiscal year.
| Bonus | Vesting | Executive Assistance | ||||||||||||||||||
| Base Salary | Payment | COBRA Premiums | Acceleration | Program | ||||||||||||||||
| Name | ($) | ($) | ($) | ($)(1) | ($) | |||||||||||||||
| Thomas J. O’Lenic | 222,685 | 88,234 | 19,266 | 18,361 | 7,500 | |||||||||||||||
| Patricia C. Sharp | 224,926 | 89,394 | 9,720 | 19,175 | 7,500 | |||||||||||||||
| Robert J. Murray | 227,089 | 90,115 | 11,488 | 18,361 | 7,500 | |||||||||||||||
(1) The value of vesting acceleration is based on the closing price of our common stock on December 29, 2006 ($21.07) with respect to in-the-money unvested option shares minus the exercise price of the unvested option shares.
Compensation of Directors
Cash Compensation Arrangements
Each of our non-employee directors receives an annual retainer of $15,000. In addition, the chair of the Audit Committee of our Board of Directors (currently Mr. Bowman) receives a supplemental annual retainer of $5,000, the chair of our Compensation Committee (currently Mr. Anderson) receives a supplemental annual retainer of $3,000, and any non-employee director designated as Lead Independent Director (currently Mr. Bowman) receives a supplemental annual retainer of $5,000; provided, however, that the maximum annual retainer for any non-employee director will not exceed $20,000 in the aggregate. Each of our non-employee directors also receives $1,500 for each Board meeting attended in person by such director, and $750 for each meeting attended via teleconference. Each non-employee director committee member receives $1,000 for each committee meeting attended in person and $500 for each meeting attended via teleconference. In addition, each of our non-employee directors may be reimbursed for out-of-pocket and travel expenses incurred in connection with attendance at Board and committee meetings.
Equity Compensation Arrangements
Each of our non-employee directors is eligible to receive automatic stock option grants under the 2005 Equity Plan. However, Dr. Finkel has declined for the time being any equity compensation for his service as a non-employee director. In connection with the adoption of the 2005 Equity Plan in April 2005, our Board of Directors adopted resolutions setting forth the terms of options to be granted to our non-employee directors, which are described below. Our Board of Directors may at any time, however, adopt resolutions modifying, amending or otherwise changing the terms of the options to be granted to our non-employee directors under the 2005 Equity Plan, subject to any applicable stockholder approval requirements. Under these resolutions, each person who is elected for the first time to be a non-employee director automatically receives, at the time of his or her initial election to the Board of Directors, an option to purchase 10,000 shares of our common stock. In addition, each non-employee director automatically receives
52
Table of Contents
an additional option to purchase 4,000 shares of our common stock immediately following each annual meeting of stockholders. In this regard, immediately following the 2006 Annual Meeting of Stockholders, we granted each non-employee director continuing in office (other than Dr. Finkel) an option under the 2005 Equity Plan covering 4,000 shares of common stock at an exercise price per share of $29.96, the fair market value of such shares based on the closing sales price reported on the NASDAQ on the date of grant. The exercise price of each option granted to the non-employee directors is 100% of the fair market value of the common stock subject to the option on the date of grant. Prior to July 2006, all options granted to non-employee directors under the 2005 Equity Plan became exercisable in equal installments over a period of four years from the date of grant, commencing on the date one year after the date of the grant. However, our Board of Directors amended the terms of the annual option grants to non-employee directors in July 2006 to provide that 25% of the shares subject to all future annual option grants will become exercisable on the day prior to each annual meeting of stockholders following the date of grant so that non-employee directors who will not be continuing office following an annual meeting of stockholders do not lose vesting credit as a result of annual meeting scheduling. In all cases, attendance at no less than two-thirds of the regularly scheduled Board of Directors meetings occurring during a vesting installment period is required for the options to become vested with respect to such installment. Failure to satisfy this requirement during any particular installment period will result in an abatement of the vesting of the options during the applicable installment period and the aggregate vesting period of the options will be increased by one additional year in the case of initial option grants, and one additional period between annual meetings in the case of annual option grants. In the event of certain change in control events, as specified in the 2005 Equity Plan, any outstanding options to a non-employee director will be fully accelerated and the option will terminate if not exercised prior to such event. Vesting will cease upon the termination of service of a non-employee director. The maximum term of options granted to the non-employee directors under the 2005 Equity Plan is ten years.
The table below summarizes the compensation paid by us to our non-employee directors for the fiscal year ended December 31, 2006.
2006 DIRECTOR COMPENSATION TABLE
| Fees Earned or | All Other | |||||||||||||||
| Paid in Cash | Option Awards | Compensation | Total | |||||||||||||
| Name | ($) | ($)(1)(2)(3) | ($) | ($) | ||||||||||||
| Moshe H. Alafi | 25,250 | 67,075 | — | 92,325 | ||||||||||||
| David L. Anderson | 28,500 | 67,075 | — | 95,575 | ||||||||||||
| A. Blaine Bowman | 34,250 | 67,075 | — | 101,325 | ||||||||||||
| Alan Finkel, Ph.D. | 24,500 | — | 7,422 | (5) | 31,922 | |||||||||||
| Paul Goddard, Ph.D.(4) | 9,250 | 57,227 | 41,560 | (6) | 108,037 | |||||||||||
| André F. Marion | 31,000 | 67,075 | — | 98,075 | ||||||||||||
| Harden M. McConnell, Ph.D. | 26,750 | 67,075 | — | 93,825 | ||||||||||||
| J. Allan Waitz, Ph.D. | 26,250 | 67,075 | 1,408 | (5) | 94,733 | |||||||||||
(1) The dollar amounts in this column represent the compensation cost for the year ended December 31, 2006 of stock option awards granted in and prior to 2006. These amounts have been calculated in accordance with FAS 123R using the Black Scholes option-pricing model and the assumptions outlined in Note 7 to Notes to Consolidated Financial Statements included in this Annual Report on Form 10-K.
(2) The aggregate number of shares subject to outstanding stock options held by each of the directors listed in the table above as of December 31, 2006 was as follows: Mr. Alafi, 46,500 shares; Mr. Anderson, 46,500 shares; Mr. Bowman, 46,500 shares; Dr. Finkel, no shares; Dr. Goddard, no shares; Mr. Marion, 30,000 shares; Dr. McConnell, 46,500 shares; and Mr. Waitz, 46,500 shares.
(3) The grant date fair value, as determined in accordance with FAS 123R, of the stock option awards granted during the year ended December 31, 2006 for each of the directors listed in the table above, with the exception of Drs. Finkel and Goddard, was $61,440. We did not grant any stock options to Drs. Finkel and Goddard during the year ended December 31, 2006.
(4) Dr. Goddard’s term of service as director expired on May 11, 2006, the date of our 2006 Annual Meeting of Stockholders.
(5) Represents the reimbursement of travel expenses.
(6) Represents remuneration paid to Dr. Goddard in recognition of his past service as our director and in connection with Dr. Goddard’s loss of stock option vesting credit due to Annual Meeting scheduling. As described above, our Board of Directors amended the terms of annual option grants to non-employee directors under the 2005 Equity Plan in July 2006 in order to ensure that non-employee directors who will not be continuing in office following an annual meeting of stockholders will not lose vesting credit as a result of annual meeting scheduling.
53
Table of Contents
Compensation Committee Interlocks and Insider Participation
Our Compensation Committee is composed of three non-employee directors: Dr. Finkel and Messrs. Anderson and Marion. Dr. Waitz served on the Compensation Committee until May 2006, at which time he was replaced on the Compensation Committee by Dr. Finkel. From July 2004 to December 2005, Dr. Finkel served initially as our Vice President and Chief Technical Officer and most recently as our Senior Vice President and Chief Technology Officer. Mr. Marion served as our interim chief executive officer from October 1997 to March 1998. No interlocking relationship exists between our Board of Directors or Compensation Committee and the board of directors or compensation committee of any other company, nor has such interlocking relationship existed in the past.
Compensation Committee Report
Our Compensation Committee has reviewed and discussed with management the Compensation Discussion and Analysis included in this Annual Report on Form 10-K. Based on this review and discussion, our Compensation Committee has recommended to our Board of Directors that the Compensation Discussion and Analysis be included in this Annual Report on Form 10-K.
THE COMPENSATION COMMITTEE
David L. Anderson
Alan Finkel, Ph.D.
André F. Marion
Alan Finkel, Ph.D.
André F. Marion
54
Table of Contents
Item 12.Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters.
Security Ownership of Management and Certain Beneficial Owners
The following table sets forth certain information regarding the ownership of our common stock as of January 31, 2007 (except as noted) by: (i) each of our directors; (ii) each of our named executive officers; (iii) all of our executive officers and directors as a group; and (iv) all those known by us to be beneficial owners of more than five percent of our outstanding common stock at January 31, 2007.
| Beneficial Ownership (1) | ||||||||
| Percent of | ||||||||
| Beneficial Owner | Number of Shares | Total (%) | ||||||
| Franklin Resources, Inc.(2) | ||||||||
| One Franklin Parkway | ||||||||
| San Mateo, California 94403-1906 | 1,563,193 | 9.5 | ||||||
| OppenheimerFunds, Inc.(3) | ||||||||
| Two World Financial Center | ||||||||
| 225 Liberty Street, 11th Floor | ||||||||
| New York, New York 10281-1008 | 1,174,903 | 7.1 | ||||||
| Barclays Global Investors, NA(4) | ||||||||
| 45 Fremont Street | ||||||||
| San Francisco, California 94105 | 1,050,229 | 6.4 | ||||||
| Brown Capital Management, Inc.(5) | ||||||||
| 1201 N. Calvert Street | ||||||||
| Baltimore, Maryland 21202 | 999,000 | 6.0 | ||||||
| WEDGE Capital Management L.L.P.(6) | ||||||||
| 301 South College Street, Suite 2920 | ||||||||
| Charlotte, North Caroline 28202-6002 | 858,901 | 5.2 | ||||||
| Joseph D. Keegan, Ph.D.(7) | 388,099 | 2.3 | ||||||
| Moshe H. Alafi(8) | 359,040 | 2.2 | ||||||
| Harden M. McConnell, Ph.D.(9) | 246,300 | 1.5 | ||||||
| Robert J. Murray(10) | 171,970 | 1.0 | ||||||
| Patricia C. Sharp(11) | 119,258 | * | ||||||
| Timothy A. Harkness(12) | 110,269 | * | ||||||
| A. Blaine Bowman(13) | 76,500 | * | ||||||
| J. Allan Waitz, Ph.D.(14) | 43,000 | * | ||||||
| David L. Anderson(15) | 41,500 | * | ||||||
| Thomas J. O’Lenic(16) | 36,397 | * | ||||||
| André F. Marion(17) | 21,000 | * | ||||||
| Alan Finkel, Ph.D. | — | — | ||||||
| All directors and executive officers as a group (17 persons)(18) | 1,782,177 | 10.1 | ||||||
* Less than one percent.
| (1) | This table is based upon information supplied by officers and directors and Schedules 13G and 13G/A filed with the SEC. Unless otherwise indicated in the footnotes to this table and subject to community property laws where applicable, we believe that each of the stockholders named in this table has sole voting and investment power with respect to the shares indicated as beneficially owned. Applicable percentages are based on 16,527,156 shares outstanding on January 31, 2007, adjusted as required by rules promulgated by the SEC. Unless otherwise indicated, the address of each of the individuals and entities listed in this table is c/o Molecular Devices, 1311 Orleans Drive, Sunnyvale, CA 94089-1136. |
| (2) | Based upon a Schedule 13G/A filed with the SEC on February 6, 2007 by Franklin Resources, Inc., or FRI. According to the Schedule 13G/A filed by FRI, such shares are beneficially owned by one or more open or closed-end investment companies or other managed accounts that are investment advisory clients of investment advisers that are direct or indirect subsidiaries of FRI. FRI, Charles B. Johnson and Rupert H. Johnson, Jr., each an owner of more than 10% of the outstanding common stock of FRI, may be deemed to be beneficial owners of securities held by persons and entities advised by the direct or indirect subsidiaries of FRI. Pursuant to the Schedule 13G/A filed by FRI, each of FRI, the direct or indirect subsidiaries of FRI, Charles B. Johnson and Rupert H. Johnson, Jr. disclaim beneficial ownership of such shares. According to the Schedule 13G/A filed by FRI, FRI has shared voting power over 1,540,493 of such shares and has shared dispositive power over all of such shares. The Schedule 13G/A filed by FRI provides information only as of December 31, 2006 and, consequently, FRI’s beneficial ownership of our common stock may have changed between December 31, 2006 and January 31, 2007. |
55
Table of Contents
| (3) | Based upon a Schedule 13G filed with the SEC on February 6, 2007 by OppenheimerFunds, Inc., or OFI. The Schedule 13G filed by OFI was filed on behalf of itself and Oppenheimer Global Opportunities Fund. Pursuant to the Schedule 13G filed by OFI, OFI has shared voting and dispositive power over such shares, and Oppenheimer Global Opportunities Fund has shared voting and dispositive power over 1,000,000 of such shares. The Schedule 13G filed by OFI provides information only as of December 31, 2006 and, consequently, OFI’s beneficial ownership of our common stock may have changed between December 31, 2006 and January 31, 2007. | |
| (4) | Based upon a Schedule 13G filed with the SEC on January 23, 2007 by Barclays Global Investors, NA, or Barclays. The Schedule 13G filed by Barclays was filed on behalf of itself and Barclays Global Fund Advisors, Barclays Global Investors, Ltd., Barclays Global Investors Japan Trust and Banking Company Limited, and Barclays Global Investors Japan Limited. Pursuant to the Schedule 13G filed by Barclays, Barclays and the foregoing affiliates of Barclays have sole voting power over 957,888 of such shares, and have sole dispositive power over all of such shares. The Schedule 13G filed by Barclays provides information only as of December 31, 2006 and, consequently, Barclays beneficial ownership of our common stock may have changed between December 31, 2006 and January 31, 2007. | |
| (5) | Based upon a Schedule 13G/A filed with the SEC on February 5, 2007 by Brown Capital Management, Inc., or BCM. According to the Schedule 13G/A filed by BCM, such shares are owned by various investment advisory clients of BCM, which is deemed to be a beneficial owner of such shares due to its discretionary power to make investment decisions over such shares for its clients and its ability to vote such shares. Pursuant to the Schedule 13G/A filed by BCM, BCM has sole dispositive power over all of such shares and has sole voting power over 503,500 of such shares. The Schedule 13G/A filed by BCM provides information only as of December 31, 2006 and, consequently, BCM’s beneficial ownership of our common stock may have changed between December 31, 2006 and January 31, 2007. | |
| (6) | Based upon a Schedule 13G filed with the SEC on February 13, 2006 by WEDGE Capital Management L.L.P., or WCM. Pursuant to the Schedule 13G filed by WCM, WCM has sole dispositive power over all of such shares, and has sole voting power over 853,321 of such shares. The Schedule 13G filed by WCM provides information only as of December 31, 2006 and, consequently, WCM’s beneficial ownership of our common stock may have changed between December 31, 2006 and January 31, 2007. | |
| (7) | Consists of 849 shares held by the Keegan 1990 Revocable Trust UAD 4/27/90, of which Dr. Keegan is a trustee, and 387,250 shares that may be acquired within 60 days after January 31, 2007 pursuant to outstanding stock options. | |
| (8) | Includes 306,040 shares held by Alafi Capital Company, of which Mr. Alafi is a general partner, and 36,500 shares that may be acquired within 60 days after January 31, 2007 pursuant to outstanding stock options. | |
| (9) | Consists of 177,400 shares held by the Harden M. McConnell and Sophia G. McConnell Trust DTD 3/29/82, of which Dr. McConnell is a co-trustee, 11,200 shares held by the Jane R. McConnell 1999 Irrevocable Trust, of which Dr. McConnell is a trustee, 11,200 shares held by the Trevor M. McConnell 1999 Irrevocable Trust, of which Dr. McConnell is a trustee, 10,000 shares held by the Hunter McConnell 1999 Irrevocable Trust, of which Dr. McConnell is a trustee, and 36,500 shares that may be acquired within 60 days after January 31, 2007 pursuant to outstanding stock options. | |
| (10) | Includes 48 shares held in joint tenancy with Mr. Murray’s spouse, and 143,750 shares that may be acquired within 60 days after January 31, 2007 pursuant to outstanding stock options. | |
| (11) | Includes 115,750 shares that may be acquired within 60 days after January 31, 2007 pursuant to outstanding stock options. | |
| (12) | Includes 103,750 shares that may be acquired within 60 days after January 31, 2007 pursuant to outstanding stock options. | |
| (13) | Includes 36,500 shares that may be acquired within 60 days after January 31, 2007 pursuant to outstanding stock options. | |
| (14) | Includes 36,500 shares that may be acquired within 60 days after January 31, 2007 pursuant to outstanding stock options. | |
| (15) | Consists of 5,000 shares held by the Anderson Living Trust UAD 1/22/98, of which Mr. Anderson is a trustee, and 36,500 shares that may be acquired within 60 days after January 31, 2007 pursuant to outstanding stock options. Mr. Anderson disclaims beneficial ownership of the shares held by the Anderson Living Trust UAD 1/22/98 except to the extent of his pecuniary interest therein. | |
| (16) | Includes 35,625 shares that may be acquired within 60 days after January 31, 2007 pursuant to outstanding stock options. | |
| (17) | Includes 20,000 shares that may be acquired within 60 days after January 31, 2007 pursuant to outstanding stock options. | |
| (18) | Includes 522,311 shares held by persons and/or entities affiliated with certain directors and executive officers and 1,156,839 shares that certain directors and executive officers have the right to acquire within 60 days after January 31, 2007 pursuant to outstanding stock options. |
56
Table of Contents
Agreement and Plan of Merger with MDS
On January 28, 2007, we entered into a definitive agreement and plan of merger with MDS Inc. (“MDS”) and Monument Acquisition Corp. (“Monument”), a subsidiary of MDS, which contemplates the acquisition by MDS, through Monument of all of our outstanding common stock in a two-step transaction comprised of a cash tender offer for all of our issued and outstanding shares of common stock, followed by a merger of Monument with and into us. Pursuant to the definitive agreement, and upon the terms and subject to the conditions thereof, Monument has commenced a tender offer to acquire all of the outstanding shares of our common stock at a price of $35.50 per share, net to the holder thereof in cash. Pursuant to the definitive agreement, as soon as practicable after the consummation of the tender offer and subject to the satisfaction or waiver of certain conditions set forth in the definitive agreement, Monument will merge with and into us and we will become an indirect wholly-owned subsidiary of MDS. Under the terms of the agreement, our stockholders will receive $35.50 per share in cash for each outstanding share of our common stock through a tender offer commenced by MDS and its indirect wholly-owned subsidiary. The obligation of Monument to accept for payment and pay for the shares tendered in the tender offer is subject to the satisfaction or waiver of a number of closing conditions set forth in the definitive agreement, including among others, the expiration of the waiting period under the Hart-Scott-Rodino Antitrust Improvements Act. The closing of the merger contemplated by the definitive agreement is subject to customary closing conditions, and, depending on the number of shares held by MDS and Monument after Monument’s acceptance of the shares properly tendered in connection with the tender offer, approval of the merger contemplated by the definitive agreement by the holders of the outstanding shares of our common stock remaining after the completion of the tender offer may be required.
Securities Authorized for Issuance Under Equity Compensation Plans
The following table provides certain information with respect to all of our equity compensation plans in effect as of December 31, 2006:
| Number of | ||||||||||||
| securities remaining | ||||||||||||
| Number of | available for future | |||||||||||
| securities to be | issuance under | |||||||||||
| issued upon | Weighted-average | equity | ||||||||||
| exercise of | exercise price of | compensation plans | ||||||||||
| outstanding options, | outstanding | (excluding | ||||||||||
| warrants and rights | options, warrants | securities reflected | ||||||||||
| Plan category | (a) | and rights (b) | in column (a))(c) | |||||||||
| Equity compensation plans approved by security holders | 2,248,598 | $ | 27.34 | 930,865 | ||||||||
| Equity compensation plans not approved by security holders (1) | 64,554 | $ | 18.24 | 11,706 | ||||||||
| Total | 2,313,152 | 942,571 | ||||||||||
(1) Represents shares issuable upon the exercise of outstanding options under the Molecular Devices Corporation 2001 Stock Option Plan and shares remaining available for issuance thereunder. The table does not include information with respect to shares subject to outstanding options granted under equity compensation arrangements assumed by us in connection with the acquisition of LJL BioSystems, Inc. in August 2000 and Axon Instruments, Inc. in July 2004. As of December 31, 2006, a total of 177,340 shares of our common stock were issuable upon the exercise of outstanding options under those assumed arrangements at a weighted average exercise price of $28.51. No additional options may be granted under those assumed arrangements.
Molecular Devices Corporation 2001 Stock Option Plan
The Molecular Devices Corporation 2001 Stock Option Plan, or 2001 Plan, was adopted by our Board of Directors in July 2001 without the approval of our security holders. An aggregate of 100,000 shares of our common stock have been reserved for issuance under the 2001 Plan. As of December 31, 2006, options to purchase 64,554 shares of common stock were outstanding under the 2001 Plan, and 11,706 shares (plus any shares that might in the future be returned to the 2001 Plan as a result of cancellations or expiration of options) remained available for grant. The 2001 Plan provides for the grant of nonstatutory stock options only to employees who, at
57
Table of Contents
the time of grant, are working or residing outside of the U.S. and are not our officers or directors. The exercise price of nonstatutory stock options granted under the 2001 Plan may not be less than 85% of the fair market value of a share of our common stock on the date of grant. All stock options have a maximum term of twelve years and typically vest over a four-year period. Options may be exercised prior to vesting, subject to repurchase rights in our favor that expire over the vesting period. Our Board of Directors at any time, and from time to time, may amend the 2001 Plan, provided that the rights of an option holder under the 2001 Plan cannot be impaired without the option holder’s consent. In the event of: (1) a dissolution, liquidation or sale of substantially all of our assets; (2) a merger or consolidation in which we are not the surviving corporation; or (3) a reverse merger in which we are the surviving corporation but our shares of common stock outstanding immediately preceding the merger are converted by virtue of the merger into other property, whether in the form of securities, cash or otherwise, then to the extent permitted by applicable law, any surviving corporation shall either assume the options or shall substitute similar options for those outstanding under the 2001 Plan, or such options shall terminate to the extent not exercised prior to such event; provided, however, that at the discretion of our Board of Directors the options may be accelerated prior to such event.
Item 13.Certain Relationships and Related Transactions, and Director Independence.
Policies and Procedures for Review of Related Party Transactions
Pursuant to the requirements set forth in applicable NASDAQ listing standards and as set forth in the charter of our Audit Committee, our Audit Committee is charged with reviewing all related party transactions for potential conflict of interest situations and is also responsible for approving such related party transactions. Pursuant to our Code of Conduct, all of our executive officers, directors and employees are required to report to the Compliance Officer under our Code of Conduct any such related party transaction. In approving or rejecting a proposed related party transaction, our Audit Committee will consider the relevant facts and circumstances available and deemed relevant to the Audit Committee, including, but not limited to the risks, costs and benefits to us, the terms of the transaction, the availability of other sources for comparable services or products, and, if applicable, the impact on a director’s independence. Our Audit Committee will approve only those related party transactions that, in light of known circumstances, are in, or are not inconsistent with, our best interests, as the Audit Committee determines in the good faith exercise of its discretion.
Indemnity Agreements; Insurance
We have entered into indemnity agreements with certain of our officers and directors which provide, among other things, that we will indemnify such officer or director, under the circumstances and to the extent provided for therein, for expenses, damages, judgments, fines and settlements he or she may be required to pay in actions or proceedings to which he or she is or may be made a party by reason of his or her position as our director, officer or other agent, and otherwise to the fullest extent permitted under Delaware law and our bylaws. We also maintain a directors’ and officers’ liability insurance policy.
Independence of Our Board of Directors
As required under the NASDAQ listing standards, a majority of the members of a listed company’s board of directors must qualify as “independent,” as affirmatively determined by the board of directors. Our Board of Directors consults with our counsel to ensure that the board’s determinations are consistent with all relevant securities and other laws and regulations regarding the definition of “independent,” including those set forth in pertinent listing standards of the NASDAQ, as in effect time to time. Consistent with these considerations, our Board of Directors has affirmatively determined that all of our directors are independent directors within the meaning of the applicable NASDAQ listing standards, except for Dr. Keegan, our President and Chief Executive Officer, and Dr. Finkel, our former Senior Vice President and Chief Technology Officer. Our Board of Directors had also determined that Dr. Goddard, whose term of service expired on May 11, 2006, was an independent director within the meaning of the applicable NASDAQ listing standards.
58
Table of Contents
Item 14.Principal Accountant Fees and Services.
Principal Accountant Fees
The following is a summary of the aggregate fees billed as of January 31, 2007 to us by Ernst & Young LLP for professional services rendered during the fiscal years ended December 31, 2006 and 2005.
| Fiscal Year Ended December 31, | ||||||||
| 2006 | 2005 | |||||||
| Audit Fees | $ | 1,305,000 | $ | 1,257,000 | ||||
| Audit-Related Fees | 140,000 | — | ||||||
| Tax Fees | 12,000 | 9,000 | ||||||
| All Other Fees | — | — | ||||||
| Total Fees | $ | 1,457,000 | $ | 1,266,000 | ||||
Audit Fees.Consists of fees billed for professional services rendered for the audit of our financial statements and review of the interim financial statements included in quarterly reports and services that are normally provided by Ernst & Young LLP in connection with our statutory and regulatory filings or engagements.
Audit-Related Fees.Consists of fees billed for assurance and related services that are reasonably related to the performance of the audit or review of our financial statements and are not reported under “Audit Fees.” During the year ended December 31, 2006, these services included assurance and related services associated with the acquisition of the LCM business from Arcturus. No audit-related fees were billed by Ernst & Young LLP during the fiscal year ended December 31, 2005.
Tax Fees.Consists of fees billed for professional services for tax compliance, tax advice and tax planning. During the fiscal years ended December 31, 2006 and 2005, these services included tax return preparation for certain international entities and acquired entities.
All Other Fees.During each of the fiscal years ended December 31, 2006 and 2005, no fees were billed by Ernst & Young LLP other than as set forth under “Audit Fees,” “Audit-Related Fees” and “Tax Fees” above.
All fees described above were approved by the Audit Committee.
Pre-Approval of Audit and Non-Audit Services
The Audit Committee pre-approves all audit and permissible non-audit services provided by our independent registered public accounting firm. These services may include audit services, audit-related services, tax services and other services. Pre-approval may be given as part of the Audit Committee’s approval of the scope of the engagement of the independent registered public accounting firm or on an individual explicit case-by-case basis. The Chairman of the Audit Committee is also authorized to pre-approve any services, provided the Audit Committee is advised at its next meeting of any services pre-approved by the Chairman.
The Audit Committee has determined that the rendering of the services other than audit services by Ernst & Young LLP is compatible with maintaining the principal accountant’s independence.
PART IV
Item 15.Exhibits and Financial Statement Schedules.
(a) The following documents are filed as a part of this report:
1. Financial Statements — See Index to Consolidated Financial Statements as Item 8 on page 34 of this report.
2. Financial Statement Schedule — See Index to Consolidated Financial Statements as Item 8 on page 34 of this report.
(b) Exhibits:
59
Table of Contents
| EXHIBIT | ||
| NUMBER | DESCRIPTION OF DOCUMENT | |
| 2.1(19) | Agreement and Plan of Merger, dated as of January 28, 2007, by and among MDS Inc., a company organized under the laws of Canada, Monument Acquisition Corp., a Delaware corporation, and an indirect wholly-owned subsidiary of MDS and direct wholly owned subsidiary of MDS (US) Inc., a Delaware corporation, and Registrant | |
| 3.1(1) | Amended and Restated Certificate of Incorporation of Registrant | |
| 3.2(1) | Bylaws of the Registrant | |
| 3.3(5) | Certificate of Amendment to Certificate of Incorporation | |
| 3.4(8) | Certificate of Designation of Series A Junior Participating Preferred Stock of Registrant | |
| 4.1(1) | Specimen Certificate of Common Stock of Registrant | |
| 4.2(8) | Rights Agreement, dated October 25, 2001, among the Registrant and EquiServe Trust Company, N.A. | |
| 4.3(19) | Amendment No. 1 to Rights Agreement, dated as of January 28, 2007, by and between Computershare Trust Company, N.A. (successor-in-interest to EquiServe Trust Company, N.A.) and Registrant. | |
| 10.1(1)* | 1988 Stock Option Plan | |
| 10.2(1)* | Form of Incentive Stock Option under the 1988 Stock Option Plan | |
| 10.3(1)* | Form of Supplemental Stock Option under the 1988 Stock Option Plan | |
| 10.4(5)* | 1995 Employee Stock Purchase Plan | |
| 10.6(1)* | Form of Nonstatutory Stock Option under the 1995 Non-Employee Directors’ Stock Option Plan | |
| 10.8(1)* | Form of Incentive Stock Option under the 1995 Stock Option Plan | |
| 10.9(1)* | Form of Nonstatutory Stock Option under the 1995 Stock Option Plan | |
| 10.10(1)* | Form of Early Exercise Stock Purchase Agreement under the 1995 Stock Option Plan | |
| 10.11(1)* | Form of Indemnity Agreement between the Registrant and its Directors and Executive Officers | |
| 10.19(10)* | Amended Key Employee Agreement for Joseph D. Keegan, Ph.D., dated July 29, 2004. | |
| 10.20(2) | Exclusive License and Technical Support Agreement, dated August 28, 1998, by and between the Registrant and Affymax | |
| 10.21(2)* | Employee Offer Letter for Timothy A. Harkness | |
| 10.24(10)* | 1995 Non-Employee Director’s Stock Option Plan, as amended | |
| 10.25(10)* | 1995 Stock Option Plan, as amended | |
| 10.26(3)* | Employee Offer Letter for Patricia Sharp | |
| 10.27(4)* | LJL BioSystems 1994 Equity Incentive Plan and Forms of Agreements | |
| 10.28(4)* | LJL BioSystems 1997 Stock Plan and Forms of Agreements | |
| 10.29(4)* | LJL BioSystems 1998 Directors’ Stock Option Plan and Forms of Agreements | |
| 10.33(6) | Lease Agreement, dated May 26, 2000, by and between Aetna Life Insurance Company and the Registrant | |
| 10.34(7)* | Change in Control Severance Benefit Plan | |
| 10.37(5)* | Key Employee Agreement for Tom O’Lenic | |
| 10.38(10)* | 2001 Stock Option Plan, as amended | |
| 10.39(9) | Lease dated May 28, 2002 by and between The Irvine Company and the Registrant | |
| 10.40(9)* | Letter Agreement, dated April 11, 2002, by and between the Registrant and Joseph D. Keegan, Ph.D. | |
| 10.43(11)* | Amended Key Employment Agreement for Timothy A. Harkness | |
| 10.44(11)* | Employee Offer Letter for Alan Finkel | |
| 10.45(11)* | Employee Offer Letter for Steven Davenport | |
| 10.46(11)* | Employee Offer Letter for Jan Hughes | |
| 10.47(11)* | Amended Employee Offer Letter for Jan Hughes | |
| 10.50(12)* | Form of Stock Option Agreement for Non-Employee Director under the 1995 Equity Incentive Plan | |
| 10.51(13)† | Asset Purchase Agreement, dated as of March 9, 2005, by and between Molecular Devices Corporation and Xsira Pharmaceuticals, Inc. | |
| 10.52(14)* | 2005 Equity Incentive Plan | |
| 10.53(14)* | Form of Stock Option Agreement under the 2005 Equity Incentive Plan | |
| 10.54(15)* | Form of Stock Option Agreement for Non-Employee Director under the 2005 Equity Incentive Plan | |
| 10.55(16) | First Amendment to Lease, dated January 27, 2006, by and between the Registrant and Moffett Office Park Investors LLC (successor-in-interest to Aetna Life Insurance Company) | |
| 10.56(16) | First Amendment to Lease, dated January 30, 2006, by and between the Registrant and The Irvine Company LLC | |
| 10.57(17)* | Executive Officer Compensation Arrangements | |
| 10.58(18)* | Non-Employee Director Cash Compensation Arrangements | |
| 21.1 | Subsidiaries of the Registrant | |
| 23.1 | Consent of Ernst & Young LLP, Independent Registered Public Accounting Firm | |
| 24.1 | Power of Attorney (contained on the signature pages hereto) | |
| 31.1 | Certification required by Rule 13a-14(a) or Rule 15d-14(a) | |
| 31.2 | Certification required by Rule 13a-14(a) or Rule 15d-14(a) | |
| 32.1** | Certifications required by Rule 13a-14(b) or Rule 15d-14(b) and Section 1350 of Chapter 63 of Title 18 of the United States Code (18 U.S.C 1350) |
(1) Incorporated by reference to the similarly described exhibit in our Registration Statement on Form S-1 (File No. 33-98926), as amended.
(2) Incorporated by reference to the similarly described exhibit in our Form 10-Q Quarterly Report dated September 30, 1998, and filed November 13, 1998.
60
Table of Contents
(3) Incorporated by reference to the similarly described exhibit in our Form 10-Q Quarterly Report dated September 30, 2000 and filed on November 13, 2000.
(4) Incorporated by reference to the similarly described exhibit filed with LJL BioSystems’ Registration Statement on Form S-1 (File No. 333-43529) declared effective on March 12, 1998.
(5) Incorporated by reference to the similarly described exhibit in our Form 10-K Annual Report dated December 31, 2001 and filed on April 1, 2002.
(6) Incorporated by reference to the similarly described exhibit in our Form 10-K Annual Report dated December 31, 2000 and filed on March 30, 2001.
(7) Incorporated by reference to the similarly described exhibit in our Form 10-Q Quarterly Report dated March 31, 2001 and filed on May 11, 2001.
(8) Incorporated by reference to the similarly described exhibit in our Current Report on Form 8-K filed October 30, 2001.
(9) Incorporated by reference to the similarly described exhibit in our Form 10-K Annual Report dated December 31, 2003 and filed on March 27, 2003.
(10) Incorporated by reference to the similarly described exhibit in our Form 10-Q Quarterly Report dated September 30, 2004, and filed on November 9, 2004.
(11) Incorporated by reference to the similarly described exhibit in our Form 10-K Annual Report dated December 31, 2004, and filed on March 16, 2005
(12) Incorporated by reference to the similarly described exhibit in our Current Report on Form 8-K filed on April 18, 2005.
(13) Incorporated by reference to the similarly described exhibit in our Quarterly Report on Form 10-Q dated March 31, 2005 and filed on May 4, 2005.
(14) Incorporated by reference to the similarly described exhibit in our Current Report on Form 8-K filed on June 1, 2005.
(15) Incorporated by reference to the similarly described exhibit in our Quarterly Report on Form 10-Q dated November 7, 2006.
(16) Incorporated by reference to the similarly described exhibit in our Form 10-K Annual Report dated December 31, 2005, and filed on March 16, 2006.
(17) Incorporated by reference to the information in our Current Reports on Form 8-K filed on February 15, 2006 and February 6, 2007, as amended.
(18) Incorporated by reference to the information in our Current Report on Form 8-K filed on May 17, 2006.
(19) Incorporated by reference to the similarly described exhibit in our Current Report on Form 8-K filed on January 29, 2007.
* Management contract or compensatory plan or arrangement.
† Confidential treatment has been requested with respect to certain portions of this exhibit. Omitted portions have been filed separately with the Securities and Exchange Commission.
** The certification attached as Exhibit 32.1 accompanies the Annual Report on Form 10-K pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 and shall not be deemed “filed” by the Company for purposes of Section 18 of the Securities Exchange Act of 1934, as amended.
61
Table of Contents
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized on March 16, 2007.
| Molecular Devices Corporation | ||||
| By: | /s/ Joseph D. Keegan, Ph.D. | |||
| Joseph D. Keegan, Ph.D. | ||||
| President and Chief Executive Officer | ||||
KNOW ALL PERSONS BY THESE PRESENT, that each person whose signature appears below constitutes and appoints Joseph D. Keegan, Ph.D. and Timothy A. Harkness, and each of them, as his true and lawful attorneys-in-fact and agents, with full power of substitution for him, and in his name in any and all capacities, to sign any and all amendments to this Annual Report on Form 10-K, and to file the same, with exhibits thereto and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and thing requisite and necessary to be done therewith, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, and any of them or his or her substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the Registrant and in the capacities and on the dates indicated.
| Signature | Title | Date | ||
/s/ Joseph D. Keegan, Ph.D | President, Chief Executive Officer and Director (Principal Executive Officer) | March 16, 2007 | ||
| /s/ Timothy A. Harkness | Chief Financial Officer and Senior Vice President Finance and Operations (Principal Financial and Accounting Officer) | March 16, 2007 | ||
| /s/ Moshe H. Alafi | Director | March 16, 2007 | ||
| /s/ David L. Anderson | Director | March 16, 2007 | ||
| /s/ A. Blaine Bowman | Director | March 16, 2007 | ||
| /s/ Andre F. Marion | Director | March 16, 2007 | ||
| /s/ Harden M. McConnell, Ph.D. | Director | March 16, 2007 | ||
| /s/ J. Allan Waitz, Ph.D. | Director | March 16, 2007 | ||
| /s/ Alan Finkel, Ph.D. | Director | March 16, 2007 |
62
Table of Contents
REPORT OF ERNST & YOUNG LLP, INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
The Board of Directors and Stockholders of Molecular Devices Corporation
We have audited the accompanying consolidated balance sheets of Molecular Devices Corporation and subsidiaries as of December 31, 2006 and 2005, and the related consolidated statements of income, stockholders’ equity, and cash flows for each of the three years in the period ended December 31, 2006. Our audits also included the financial statement schedule listed in the Index at Item 15(a)2. These financial statements and schedule are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements and schedule based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the consolidated financial position of Molecular Devices Corporation and subsidiaries at December 31, 2006 and 2005, and the consolidated results of their operations and their cash flows for each of the three years in the period ended December 31, 2006, in conformity with U.S. generally accepted accounting principles. Also, in our opinion, the related financial statement schedule, when considered in relation to the basic financial statements taken as a whole, presents fairly in all material respects the information set forth therein.
As discussed in Note 1 to the consolidated financial statements, Molecular Devices Corporation adopted Statement of Financial Accounting Standards No. 123 (revised 2004), “Share-Based Payment”, effective January 1, 2006.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the effectiveness of Molecular Devices Corporation’s internal control over financial reporting as of December 31, 2006, based on criteria established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission, and our report dated March 15, 2007 expressed an unqualified opinion thereon.
/s/ Ernst & Young LLP
Palo Alto, California
March 15, 2007
March 15, 2007
63
Table of Contents
MOLECULAR DEVICES CORPORATION AND SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS
| December 31, | ||||||||
| 2006 | 2005 | |||||||
| (In thousands, except share and per share | ||||||||
| amounts) | ||||||||
| ASSETS | ||||||||
| Current assets: | ||||||||
| Cash and cash equivalents | $ | 22,824 | $ | 28,908 | ||||
| Accounts receivable, net of allowance for doubtful accounts of $182 and $442 | 39,098 | 41,197 | ||||||
| Inventories, net | 29,435 | 23,197 | ||||||
| Deferred tax assets | 4,326 | 5,873 | ||||||
| Prepaid and other current assets | 3,895 | 2,353 | ||||||
| Total current assets | 99,578 | 101,528 | ||||||
| Equipment and leasehold improvements, net | 10,774 | 9,902 | ||||||
| Goodwill | 107,302 | 102,835 | ||||||
| Developed technology | 24,442 | 25,152 | ||||||
| Intangible and other assets | 24,682 | 17,999 | ||||||
| $ | 266,778 | $ | 257,416 | |||||
| LIABILITIES AND STOCKHOLDERS’ EQUITY | ||||||||
| Current liabilities: | ||||||||
| Accounts payable | $ | 7,091 | $ | 7,676 | ||||
| Accrued compensation | 9,415 | 9,759 | ||||||
| Other accrued liabilities | 13,680 | 13,623 | ||||||
| Deferred revenue | 8,976 | 7,806 | ||||||
| Total current liabilities | 39,162 | 38,864 | ||||||
| Deferred tax liabilities | 8,341 | 4,486 | ||||||
| Other long-term liabilities | 1,289 | 993 | ||||||
| Total liabilities | 48,792 | 44,343 | ||||||
| Commitments and contingencies (Note 3) | ||||||||
| Stockholders’ equity | ||||||||
| Preferred stock, $.001 par value; 3,000,000 shares authorized, no shares issued or outstanding | — | — | ||||||
| Common stock, $.001 par value; 60,000,000 shares authorized; 20,803,632 and 20,076,495 shares issued and 16,486,302 and 16,681,727 outstanding at December 31, 2006 and 2005, respectively | 21 | 20 | ||||||
| Additional paid-in capital | 292,375 | 271,300 | ||||||
| Retained earnings | 17,850 | 7,023 | ||||||
| Treasury stock, at cost; 4,317,330 and 3,394,768 shares at December 31, 2006 and 2005, respectively | (93,523 | ) | (66,519 | ) | ||||
| Accumulated other comprehensive income | 1,263 | 1,249 | ||||||
| Total stockholders’ equity | 217,986 | 213,073 | ||||||
| $ | 266,778 | $ | 257,416 | |||||
See accompanying Notes to Consolidated Financial Statements.
64
Table of Contents
MOLECULAR DEVICES CORPORATION AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF INCOME
| Years Ended December 31, | ||||||||||||
| 2006 | 2005 | 2004 | ||||||||||
| (In thousands, except per share amounts) | ||||||||||||
| Revenues | $ | 186,412 | $ | 181,951 | $ | 149,095 | ||||||
| Cost of revenues | 70,065 | 70,267 | 56,840 | |||||||||
| Gross profit | 116,347 | 111,684 | 92,255 | |||||||||
| Operating expenses: | ||||||||||||
| Research and development | 23,362 | 25,281 | 22,038 | |||||||||
| Selling, general and administrative | 71,826 | 59,885 | 52,469 | |||||||||
| Acquired in-process research and development | 4,272 | — | 5,000 | |||||||||
| Restructuring and other charges | 331 | 1,427 | 1,157 | |||||||||
| Total operating expenses | 99,791 | 86,593 | 80,664 | |||||||||
| Income from operations | 16,556 | 25,091 | 11,591 | |||||||||
| Gain on sale of equity securities | 2,235 | — | 18,288 | |||||||||
| Translation gain on liquidation of subsidiaries | 1,413 | — | — | |||||||||
| Interest expense | (85 | ) | (85 | ) | (187 | ) | ||||||
| Interest and other income (expense), net | 524 | (530 | ) | 319 | ||||||||
| Income before income taxes | 20,643 | 24,476 | 30,011 | |||||||||
| Income tax provision | 9,816 | 8,580 | 12,778 | |||||||||
| Net income | $ | 10,827 | $ | 15,896 | $ | 17,233 | ||||||
| Basic net income per share | $ | 0.65 | $ | 0.95 | $ | 1.08 | ||||||
| Diluted net income per share | $ | 0.64 | $ | 0.93 | $ | 1.04 | ||||||
See accompanying Notes to Consolidated Financial Statements.
65
Table of Contents
MOLECULAR DEVICES CORPORATION AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY
| Retained | Accumulated | ||||||||||||||||||||||||||||||||
| Earnings | Other | Total | |||||||||||||||||||||||||||||||
| Common Stock | Additional Paid- | (Accumulated | Treasury Stock | Deferred Stock | Comprehensive | Stockholders' | |||||||||||||||||||||||||||
| Shares | Amount | In Capital | Deficit) | (at cost) | Compensation | Income | Equity | ||||||||||||||||||||||||||
| (In thousands, except share amounts) | |||||||||||||||||||||||||||||||||
| Balance at December 31, 2003 | 14,778,837 | $ | 16 | $ | 184,956 | $ | (26,106 | ) | $ | (14,968 | ) | $ | — | $ | 1,640 | $ | 145,538 | ||||||||||||||||
| Comprehensive income | |||||||||||||||||||||||||||||||||
| Net income | — | — | — | 17,233 | — | — | — | 17,233 | |||||||||||||||||||||||||
| Currency translation, net of tax | — | — | — | — | — | — | 1,866 | 1,866 | |||||||||||||||||||||||||
| Total comprehensive income | 19,099 | ||||||||||||||||||||||||||||||||
| Issuance of common stock to acquire Axon Instruments, Inc., net of issuance costs of $784 | 3,582,655 | 3 | 69,648 | — | — | — | — | 69,651 | |||||||||||||||||||||||||
| Issuance of shares of common stock for options exercised | 290,619 | — | 3,266 | — | — | — | — | 3,266 | |||||||||||||||||||||||||
| Issuance of shares of common stock under Employee Stock Purchase Plan | 55,499 | — | 872 | — | — | — | — | 872 | |||||||||||||||||||||||||
| Income tax benefit associated with the exercise of stock options | — | — | 3,934 | — | — | — | — | 3,934 | |||||||||||||||||||||||||
| Repurchase of shares of common stock | (1,555,000 | ) | — | — | — | (31,627 | ) | — | — | (31,627 | ) | ||||||||||||||||||||||
| Deferred stock compensation | — | — | — | — | — | (226 | ) | — | (226 | ) | |||||||||||||||||||||||
| Amortization of deferred stock compensation | — | — | — | — | — | 113 | — | 113 | |||||||||||||||||||||||||
| Balance at December 31, 2004 | 17,152,610 | 19 | 262,676 | (8,873 | ) | (46,595 | ) | (113 | ) | 3,506 | 210,620 | ||||||||||||||||||||||
| Comprehensive income | |||||||||||||||||||||||||||||||||
| Net income | — | — | — | 15,896 | — | — | — | 15,896 | |||||||||||||||||||||||||
| Currency translation, net of tax | — | — | — | — | — | — | (2,257 | ) | (2,257 | ) | |||||||||||||||||||||||
| Total comprehensive income | 13,639 | ||||||||||||||||||||||||||||||||
| Issuance of shares of common stock for options exercised | 426,567 | 1 | 6,216 | — | — | — | — | 6,217 | |||||||||||||||||||||||||
| Issuance of shares of common stock under Employee Stock Purchase Plan | 67,872 | — | 1,098 | — | — | — | — | 1,098 | |||||||||||||||||||||||||
| Income tax benefit associated with the exercise of stock options | — | — | 1,310 | — | — | — | — | 1,310 | |||||||||||||||||||||||||
| Repurchase of shares of common stock | (965,322 | ) | — | — | — | (19,924 | ) | — | — | (19,924 | ) | ||||||||||||||||||||||
| Amortization of deferred stock compensation | — | — | — | — | — | 113 | — | 113 | |||||||||||||||||||||||||
| Balance at December 31, 2005 | 16,681,727 | 20 | 271,300 | 7,023 | (66,519 | ) | — | 1,249 | 213,073 | ||||||||||||||||||||||||
| Comprehensive income | |||||||||||||||||||||||||||||||||
| Net income | — | — | — | 10,827 | — | — | — | 10,827 | |||||||||||||||||||||||||
| Gain on liquidation of subsidiaries | (1,413 | ) | (1,413 | ) | |||||||||||||||||||||||||||||
| Currency translation, net of tax | — | — | — | — | — | — | 1,427 | 1,427 | |||||||||||||||||||||||||
| Total comprehensive income | 10,841 | ||||||||||||||||||||||||||||||||
| Issuance of shares of common stock for options exercised | 727,137 | 1 | 13,144 | — | — | — | — | 13,145 | |||||||||||||||||||||||||
| Employee stock based compensation expense | 4,676 | 4,676 | |||||||||||||||||||||||||||||||
| Income tax benefit associated with the exercise of stock options | — | — | 3,255 | — | — | — | — | 3,255 | |||||||||||||||||||||||||
| Repurchase of shares of common stock | (922,562 | ) | — | — | — | (27,004 | ) | — | — | (27,004 | ) | ||||||||||||||||||||||
| Balance at December 31, 2006 | 16,486,302 | $ | 21 | $ | 292,375 | $ | 17,850 | $ | (93,523 | ) | $ | — | $ | 1,263 | $ | 217,986 | |||||||||||||||||
See accompanying Notes to Consolidated Financial Statements.
66
Table of Contents
MOLECULAR DEVICES CORPORATION AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
| Years Ended December 31, | ||||||||||||
| 2006 | 2005 | 2004 | ||||||||||
| (In thousands, except share amounts) | ||||||||||||
| Cash flows from operating activities: | ||||||||||||
| Net income: | $ | 10,827 | $ | 15,896 | $ | 17,233 | ||||||
| Adjustments to reconcile net income to net cash provided by operating activities: | ||||||||||||
| Depreciation and amortization | 6,446 | 6,494 | 5,818 | |||||||||
| Amortization of intangible assets | 3,767 | 3,032 | 1,247 | |||||||||
| Amortization of deferred stock compensation | — | 113 | 113 | |||||||||
| Charge for acquired in-process research and development | 4,272 | — | 5,000 | |||||||||
| Non-cash restructuring and other expenses | — | 823 | — | |||||||||
| Gain on sale of equity securities | (2,235 | ) | — | (18,288 | ) | |||||||
| Loss on disposal of fixed assets | 31 | 5 | 23 | |||||||||
| Equity investment exchanged for services | 524 | 653 | ||||||||||
| Employee stock-based compensation | 5,016 | — | — | |||||||||
| Excess tax benefits from stock-based compensation | (1,513 | ) | 1,310 | 3,934 | ||||||||
| Translation gain on liquidation of subsidiaries | (1,413 | ) | — | — | ||||||||
| (Increase) decrease in assets: | ||||||||||||
| Accounts receivable | 3,431 | (5,953 | ) | (2,715 | ) | |||||||
| Inventories | (6,317 | ) | (67 | ) | 1,188 | |||||||
| Prepaid and other current assets | (1,466 | ) | 375 | (233 | ) | |||||||
| Deferred tax assets | (1,142 | ) | 1,783 | 4,297 | ||||||||
| Increase (decrease) in liabilities: | ||||||||||||
| Accounts payable | (602 | ) | 626 | (576 | ) | |||||||
| Accrued liabilities | 2,645 | (1,120 | ) | 5,068 | ||||||||
| Deferred revenue | (44 | ) | 776 | 377 | ||||||||
| Net cash provided by operating activities | 21,703 | 24,617 | 23,139 | |||||||||
| Cash flows from investing activities: | ||||||||||||
| Proceeds from sales and maturities of available-for-sale investments | — | — | 9,850 | |||||||||
| Proceeds from sale of equity securities | 2,235 | — | 28,288 | |||||||||
| Capital expenditures | (5,054 | ) | (2,850 | ) | (4,773 | ) | ||||||
| Acquisitions | (11,264 | ) | — | (48,533 | ) | |||||||
| Purchase of intangible and other assets | (1,292 | ) | (10,221 | ) | (1,600 | ) | ||||||
| Net cash used in investing activities | (15,375 | ) | (13,071 | ) | (16,768 | ) | ||||||
| Cash flows from financing activities: | ||||||||||||
| Proceeds from borrowings on credit facility | — | — | 15,000 | |||||||||
| Repayment of borrowings | — | — | (15,000 | ) | ||||||||
| Issuance of common stock from stock option exercises | 13,146 | 7,314 | 4,138 | |||||||||
| Purchase of treasury stock | (27,004 | ) | (19,924 | ) | (31,627 | ) | ||||||
| Excess tax benefits from stock-based compensation | 1,513 | — | — | |||||||||
| Net cash used in financing activities | (12,345 | ) | (12,610 | ) | (27,489 | ) | ||||||
| Effect of exchange rate changes on cash and cash equivalents | (67 | ) | (203 | ) | 1,033 | |||||||
| Net decrease in cash and cash equivalents | (6,084 | ) | (1,267 | ) | (20,085 | ) | ||||||
| Cash and cash equivalents at beginning of year | 28,908 | 30,175 | 50,260 | |||||||||
| Cash and cash equivalents at end of year | $ | 22,824 | $ | 28,908 | $ | 30,175 | ||||||
| Supplemental cash flow information: | ||||||||||||
| Cash paid during the year for: | ||||||||||||
| Interest | $ | 85 | $ | 85 | $ | 186 | ||||||
| Income taxes | $ | 7,118 | $ | 5,257 | $ | 2,972 | ||||||
| Issuance of 3,582,655 shares of common stock in conjunction with the acquisition of Axon Instruments, Inc. in July 2004. | $ | — | $ | — | $ | 69,648 | ||||||
See accompanying Notes to Consolidated Financial Statements.
67
Table of Contents
MOLECULAR DEVICES CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Note 1. Summary of Significant Accounting Policies
Basis of Presentation
Molecular Devices Corporation (“Molecular Devices,” “the Company,” “our,” “us” or “we”), a Delaware corporation, is principally involved in the design, development, manufacture, sale and service of bioanalytical measurement systems that accelerate and improve drug discovery and other life sciences research. The customers for our products include leading pharmaceutical and biotechnology companies as well as medical centers, universities, government research laboratories and other institutions throughout the world.
Principles of Consolidation
The consolidated financial statements include the accounts of Molecular Devices and its wholly owned subsidiaries. All significant intercompany balances and transactions have been eliminated.
Use of Estimates
The preparation of financial statements in conformity with U.S. generally accepted accounting principles requires management to make estimates and assumptions that affect the amounts reported in the financial statements and accompanying notes. Actual results could differ from those estimates.
Cash Equivalents
Cash equivalents consist of highly liquid investments in money market accounts.
Concentration of Credit Risk
Financial instruments that potentially subject us to concentrations of credit risk are primarily cash, cash equivalents, and accounts receivable. We deposit cash with high credit quality financial institutions.
Accounts Receivable
We sell our products primarily to corporations, academic institutions, government entities and distributors within the drug discovery and life sciences research markets. We perform ongoing credit evaluations of our customers and generally do not require collateral. We provide reserves against trade receivables for estimated losses that may result from customers’ inability to pay. The amount of the reserve is determined by analyzing known uncollectible accounts, aged receivables, economic conditions in the customers’ country or industry, historical losses and customer credit-worthiness. Amounts later determined and specifically identified to be uncollectible are charged or written off against the reserve. Estimated losses have historically been within our expectations. In 2006 and 2005, no single customer accounted for more than 5% of sales.
Inventories
Inventories are stated on a first-in, first-out basis at the lower of cost or market. We write down our inventory for estimated obsolescence or unmarketable inventory equal to the difference between the cost of inventory and the estimated market value based upon assumptions about future demand and market conditions. If actual market conditions are less favorable than those projected by management, additional inventory write-downs may be required. Such write-downs have historically been within our expectations.
Equipment and Leasehold Improvements
Equipment is recorded at cost and depreciated using the straight-line method over the estimated useful lives of the assets (ranging from three to five years). Leasehold improvements are amortized over the remaining term of the lease, or the life of the asset, whichever is shorter. Maintenance and repairs are expensed as incurred. Depreciation expense for 2006, 2005 and 2004 was $4.4 million, $4.6 million and $4.3 million, respectively.
68
Table of Contents
Goodwill
Goodwill represents the difference between the purchase price and the fair value of net assets when accounted for by the purchase method of accounting. Financial Accounting Standards Board (“FASB”) Statement No. 142,“Goodwill and Other Intangible Assets”(“FAS 142”) requires periodic evaluations for impairment of goodwill balances. We perform our goodwill impairment tests annually at the Company level, which is the reporting unit, based on the market capitalization approach, during the third quarter of our fiscal year, and more frequently if an event or circumstance indicates that impairment has occurred. Based on our annual evaluations for impairment of goodwill, we determined that no impairment of goodwill existed at any period presented.
Developed Technology, Intangible and Other Assets
Intangible and other assets include patents, license fees, order backlog, distribution rights, tradenames and strategic investments in privately held companies that have been accounted for under the cost method. Patents, certain developed technology, license fees, and certain tradenames are amortized over their expected useful life of ten years. Order backlog is amortized over a life of one year. Certain tradenames and distribution rights are assessed to have an indefinite life and therefore are not subject to amortization.
Impairment of Long-Lived Assets
We evaluate indefinite-life intangibles for impairment annually based on expected discounted cash flows attributable to that asset. We evaluate other long-lived assets, including investments accounted for under the cost method, for impairment whenever events or changes in circumstances indicate that the carrying value of an asset may not be recoverable based on expected discounted cash flows attributable to that asset. The amount of any impairment is measured as the difference between the carrying value and the fair value of the impaired asset. Fair value is be measured based on quantitative and qualitative analyses. There were no assets that were considered to be impaired during any period presented.
Equity Investments
We have invested in equity instruments of privately held companies for business and strategic purposes. As of December 31, 2006, we had an investment in one company with a carrying value of $1.2 million, which is included in intangible and other assets in the Consolidated Balance Sheets. This investment is accounted for under the cost method because our ownership is less than 20 percent of voting securities and we do not have the ability to exercise significant influence over operations. We regularly review the assumptions underlying the operating performance and cash flow forecasts in assessing the estimated fair values of our non-marketable investments. We monitor the preceding factors to identify events or circumstances which would cause us to test for other than temporary impairment and revise our assumptions for the estimated recovery of equity investments. There were no investments considered impaired during any of the periods presented.
In October 2004, Serologicals Corporation (“Serologicals”) purchased Upstate Group, Inc. (“Upstate”), a privately held company in which we held an equity interest. As a result of the acquisition, we received cash of $9.6 million and shares of common stock in Serologicals. Subsequently, we disposed of our entire equity investment in Serologicals. The net gain as a result of these transactions was $18.3 million, which was reported as gain on sale of equity securities in 2004 in the Consolidated Statements of Income. In 2006, we received additional cash consideration of $2.2 million from the escrow account established by Serologicals in connection with its acquisition of Upstate in 2004.
Income Taxes
Income taxes are accounted for under the liability method whereby deferred tax asset or liability account balances are calculated at the balance sheet date using current tax laws and rates in effect for the year in which the differences are expected to affect taxable income. Valuation allowances are established when necessary to reduce deferred tax assets to the amounts expected to be realized in the future. Significant estimates are required in determining our provision for income taxes. Some of these estimates are based on interpretations of existing tax laws or regulations. We believe that our estimates are reasonable and that our reserves for income tax related uncertainties are adequate.
Foreign Currency Translation
We translate the assets and liabilities of our foreign subsidiaries into U.S. dollars at the rates of exchange in effect at the end of the period and translate revenues and expenses using average rates in effect during the period. Gains and losses from these translations, including translation of our long-term investments in our foreign subsidiaries, are accumulated as a separate component of stockholders’ equity. Many of our subsidiaries conduct a portion of their business in currencies other than their functional currency. These transactions give rise to receivables and payables that are denominated in currencies other than their functional currency. The
69
Table of Contents
value of these receivables and payables is subject to changes in currency exchange rates. Both realized and unrealized gains or losses in the value of these receivables and payables are included in the determination of net income. Foreign currency transaction gains (losses) recognized were $0.2 million, $(0.9) million and $(0.2) million in 2006, 2005 and 2004, respectively, and are included in interest and other income (expense), net, in the Consolidated Statements of Income.
In 2006, we substantially liquidated investments in two of our foreign subsidiaries, and recognized a gain from the reversal of cumulative translation adjustments for these subsidiaries of $1.4 million, included as translation gain on liquidation of subsidiaries in the Consolidated Statements of Income.
Revenue Recognition and Warranty
We apply the provisions of the following authoritative literature in the development of our revenue recognition policies:
| • | Emerging Issues Task Force, Issue No. 00-21,“Revenue Arrangements with Multiple Deliverables.”Revenue arrangements with multiple elements are divided into separate units of accounting if the deliverables in the arrangement have value to the customer on a stand alone basis, there is objective and reliable evidence of the fair value of the undelivered elements and there are no rights of return or additional performance guarantees by the Company. | ||
| • | Statement of Position 97-2,“Software Revenue Recognition.”Revenue earned on software arrangements involving multiple elements is allocated to each element based on the relative fair values of the elements as determined by means of our quoting process and published price lists. | ||
| • | Staff Accounting Bulletin (“SAB”) No. 104,“Revenue Recognition.”Revenue is recognized when the following four criteria are met: (1) persuasive evidence of an arrangement exists; (2) delivery has occurred or services have been rendered; (3) the seller’s price is fixed or determinable; and (4) collectibility is reasonably assured. |
A majority of our revenue is derived from the sale of instruments to end-users with a one-year warranty. These arrangements include a single element (the instrument). Other single-element arrangements include the sale of consumables, software, service, technology licenses, installation or training. Arrangements incorporating multiple elements may exist, either through one invoice or through separate invoices entered into with a single customer at or near the same time. These multiple-element arrangements can include any combination of the previously described products or services. If there are multiple elements not delivered together, we recognize revenue on delivered elements when the fair value of any undelivered elements is known.
All single-element and multiple-element arrangements are evidenced by an invoice in response to a written purchase order or license agreement. Each element of an arrangement is invoiced at fair value as determined by means of our quoting process and published price lists. Standard end-user terms include: risk of loss transferring to the purchaser at the time of shipment, net 30 day payment terms, no right of return or exchange, no right to upgrades and no acceptance provisions.
We do not enter into arrangements that require performance in excess of our published specifications.
Our revenue recognition criteria are as follows. In multiple element arrangements, each element is invoiced at fair value, and our revenue recognition criteria are applied to each element of the arrangement.
| • | Instruments, software and consumables — We recognize revenue with respect to sales of instruments, software and consumables at the time that an instrument, software or consumable is shipped, in accordance with the shipping terms of the invoice. Under FOB Destination terms, we do not recognize revenue until the product arrives at the customer site. We determine that the SAB 104 criteria have been met through receipt of a valid purchase order and issuance by us of either a sales order confirmation or an invoice, confirmation of product shipment (or receipt, when FOB Destination terms apply), issuance of an invoice indicating the price and determination of credit-worthiness or, in certain circumstances, receipt of prior payment. | ||
| • | Service, installation and training — Revenue from service events not covered by warranty or a service contract is recognized upon completion of the service. Service can be provided in the field or at our service depot. For a small number of products, we offer the option to purchase installation services. Installation is billed separately at the time of performance and is not part of a package price for instruments. We have established a fair value for installation services, as installation can be purchased with or without an instrument. Further, a third party or the customer can perform the installation. Training is billed separately at the time of performance and is not part of a package price for instruments. Training revenue is recognized upon completion of the training. We determine that the SAB 104 criteria have been met through receipt of a valid purchase order and issuance by us of either a sales order confirmation or an invoice, receipt of a customer acknowledgment that the service, installation or training has been completed, issuance of an invoice indicating the price and determination of credit-worthiness or, in certain circumstances, receipt of prior payment. |
70
Table of Contents
| • | Service contracts — Revenue from service contracts for our instruments, generally with a one-year term, is recognized ratably over the period of coverage. We determine that the SAB 104 criteria have been met through receipt of a valid purchase order and issuance by us of either a sales order confirmation or an invoice, issuance of an invoice indicating the price and determination of credit-worthiness or, in certain circumstances, receipt of prior payment. | ||
| • | Technology license agreements — Revenue from technology license agreements is recognized upon completion of our obligations to the licensee. We determine that the SAB 104 criteria have been met through receipt of an executed license agreement and determination of credit-worthiness or, in certain circumstances, receipt of prior payment. We have no ongoing obligations under our current technology license agreements. |
Future warranty costs are estimated based on historical experience and provided for at the time of sale. Shipping and handling costs for revenue-generating shipments are charged to costs of goods sold.
Advertising Costs
We expense the cost of advertising as incurred. Such costs approximated $1.4 million, $1.2 million and $1.1 million for 2006, 2005 and 2004, respectively.
Recent Accounting Pronouncements
In December 2004, the FASB issued Statement No. 123 (revised 2004),“Share-Based Payment” (“FAS 123R”) which is a revision of FASB Statement No. 123,“Accounting for Stock-Based Compensation”(“FAS 123”), and supersedes APB Opinion No. 25, “Accounting for Stock Issued to Employees” (“APB Opinion 25”). FAS 123R requires all share-based payments to employees, including grants of employee stock options, to be recognized in the financial statements based on their fair values, beginning with the first interim or annual period after June 15, 2005, with early adoption encouraged. On April 14, 2005, the SEC adopted a new rule that amended the compliance dates for FAS 123R such that we adopted the new standard effective January 1, 2006. The pro forma disclosures previously permitted under FAS 123 are no longer an alternative to financial statement recognition.
In September 2006, the FASB issued Statement No. 157,“Fair Value Measurements”(“FAS 157”). FAS 157 provides guidance for using fair value to measure assets and liabilities. The standard also responds to investors’ request for expanded information about the extent to which a company measures assets and liabilities at fair value, the information used to measure fair value, and the effect of fair value measurements on earnings. FAS 157 will be effective for the Company’s fiscal years beginning January 1, 2008. We are still evaluating what impact, if any, the adoption of this standard will have on our financial position and results of operations.
In June 2006, the FASB issued FASB Interpretation No. 48 (“FIN48”),“Accounting for Uncertainty in Income Taxes — an interpretation of FASB Statement No. 109”(“FAS 109”). FIN 48 clarifies the accounting for uncertainty in income taxes recognized in an enterprise’s financial statements in accordance with FAS 109 and prescribes a recognition threshold and measurement attribute for the financial statement recognition and measurement of tax positions taken or expected to be taken in a tax return. FIN 48 also provides guidance on derecognition, classification, interest and penalties, accounting in interim periods, disclosures and transition. FIN 48 is effective for fiscal years beginning after December 15, 2006. We will be required to adopt FIN 48 as of January 1, 2007, with any cumulative effect of the change in accounting principles recorded as an adjustment to opening retained earnings. We are still evaluating what impact, if any, the adoption of this standard will have on our financial position and results of operations.
Earnings Per Share
Basic net income per share is computed based on the weighted average number of shares of our common stock outstanding. Diluted net income per share is computed based on the weighted average number of shares of our common stock outstanding and other dilutive securities. Dilutive securities consist of the incremental common shares issuable upon the exercise of stock options (using the treasury stock method).
71
Table of Contents
Computation of diluted earnings per share was as follows (in thousands, except per share amounts):
| Years Ended December 31, | ||||||||||||
| 2006 | 2005 | 2004 | ||||||||||
| Weighted average common shares outstanding for the period | 16,676 | 16,783 | 16,028 | |||||||||
| Common equivalent shares assuming the exercise of stock options under the treasury stock method | 371 | 364 | 504 | |||||||||
| Shared used in computing diluted net income per share | 17,047 | 17,147 | 16,532 | |||||||||
| Net income | $ | 10,827 | $ | 15,896 | $ | 17,233 | ||||||
| Basic net income per share | $ | 0.65 | $ | 0.95 | $ | 1.08 | ||||||
| Diluted net income per share | $ | 0.64 | $ | 0.93 | $ | 1.04 | ||||||
Options to purchase 893,384 shares of common stock at a weighted average per share exercise price of $34.05 were outstanding during 2006, but were not included in the computation of diluted earnings per share for that year as the options exercise prices were greater than the average market price of the common shares and, therefore, the effect would have been anti-dilutive. In 2005 and 2004, the total number of shares excluded from the calculations of diluted net income per share were 1,468,790 and 1,428,219 respectively. Such securities, had they been dilutive, would have been included in the computations of diluted net income per share using the treasury stock method.
Comprehensive Income
Comprehensive income is comprised of net income and other items of comprehensive income. Other comprehensive income includes cumulative translation adjustments from the translation of foreign subsidiaries’ financial statements, and unrealized gains and losses on available-for-sale securities, if material.
Reclassifications
Revenues includes an increase of $0.7 million and $0.6 million for the years ended 2005 and 2004, respectively, related to the reclassification of freight charges recovered from customers from Cost of Revenues to Revenues. Cost of Revenues has been increased accordingly, and there was no effect on gross profit, operating income or net income for any of the periods presented.
72
Table of Contents
Note 2. Balance Sheet Amounts
| December 31, | ||||||||
| 2006 | 2005 | |||||||
| (in thousands) | ||||||||
| Inventories, net: | ||||||||
| Raw materials | $ | 15,519 | $ | 11,350 | ||||
| Work-in-process | 1,667 | 2,127 | ||||||
| Finished goods and demonstration equipment, net | 12,249 | 9,720 | ||||||
| $ | 29,435 | $ | 23,197 | |||||
| Equipment and leasehold improvements, net: | ||||||||
| Machinery and equipment | $ | 22,455 | $ | 21,862 | ||||
| Software | 5,327 | 4,617 | ||||||
| Furniture and fixtures | 4,749 | 4,604 | ||||||
| Leasehold improvements | 9,371 | 8,898 | ||||||
| 41,902 | 39,981 | |||||||
| Less accumulated depreciation and amortization | (31,128 | ) | (30,079 | ) | ||||
| $ | 10,774 | $ | 9,902 | |||||
| Intangible and other assets: | ||||||||
| Equity investments | $ | 1,177 | $ | 1,177 | ||||
| Intangible assets | 17,389 | 13,481 | ||||||
| Other assets | 6,116 | 3,341 | ||||||
| $ | 24,682 | $ | 17,999 | |||||
| Other accrued liabilities: | ||||||||
| Accrued income tax | $ | 4,291 | $ | 3,051 | ||||
| Warranty accrual | 2,448 | 2,663 | ||||||
| Other | 6,941 | 7,909 | ||||||
| $ | 13,680 | $ | 13,623 | |||||
Note 3. Commitments and Contingencies
Operating Leases
Our facilities are leased under noncancelable operating leases. The leases generally require payment of taxes, insurance and maintenance costs on leased facilities. The total amount of rental payments due over the initial lease term is being charged to rent expense on the straight-line method over the term of the lease. The difference between rent expense recorded and the net amount paid is recorded in deferred rent, which is included in other accrued liabilities in the accompanying Consolidated Balance Sheets. In May 2005, we entered into a sublease agreement whereby we lease a portion of our office space to a third party through February 2011. Operating lease terms range from one to ten years, and as of December 31, 2006, minimum annual rental commitments under these noncancelable operating leases, net of sublease income, were as follows (in thousands):
| For the year ended | Minimum Lease | |||||||||||
| December 31, | Payments | Sublease Income | Net | |||||||||
| 2007 | $ | 6,291 | $ | 289 | $ | 6,002 | ||||||
| 2008 | 5,604 | 312 | 5,292 | |||||||||
| 2009 | 5,630 | 323 | 5,307 | |||||||||
| 2010 | 5,774 | 334 | 5,440 | |||||||||
| 2011 | 3,402 | 43 | 3,359 | |||||||||
| Thereafter | 4,509 | — | 4,509 | |||||||||
| $ | 31,210 | $ | 1,301 | $ | 29,909 | |||||||
Net rental expense under operating leases related to our facilities was approximately $6.1 million, $7.3 million and $6.7 million, respectively, for each of the three years ended December 31, 2006, 2005 and 2004.
73
Table of Contents
Purchase Obligations
We have contractual commitments for the purchase of products, components and services, ending in 2007. The minimum purchase commitments are based on a set percentage of our forecasted production, and for 2007, at current prices, were approximately $14.1 million. These purchase commitments are not expected to result in a material loss.
Warranty
At the time of sale, we record an estimate for warranty costs that may be incurred under product warranties. Warranty expense and activity are estimated based on historical experience. The warranty accrual is evaluated periodically and adjusted for changes in experience. Changes in the warranty liability during the years ended December 31, 2006 and 2005 were as follows (in thousands):
| Balance December 31, 2004 | $ | 2,276 | ||
| New warranties issued during the period | 2,738 | |||
| Cost of warranties incurred during the period | (2,351 | ) | ||
| Balance December 31, 2005 | 2,663 | |||
| New warranties issued during the period | 2,195 | |||
| Warranties assumed through the acquisition of LCM business | 186 | |||
| Cost of warranties incurred during the period | (2,596 | ) | ||
| Balance December 31, 2006 | $ | 2,448 | ||
Guarantees
Our charter and bylaws provide for the mandatory indemnification of our directors, officers, and to the extent authorized by our board of directors, employees and other agents, to the maximum extent permitted by the Delaware General Corporation Law. We have entered into indemnity agreements with certain officers and directors which provide, among other things, that we will indemnify such officer or director, under the circumstances and to the extent provided for therein, for expenses, damages, judgments, fines and settlements he or she may be required to pay in actions or proceedings to which he or she is or may be made a party by reason of his or her position as one of our directors, officers or other agents, and otherwise to the fullest extent permitted under Delaware law. The maximum potential amount of future payments that we could be required to make under these indemnification agreements and the relevant provisions of our charter and bylaws is unlimited; however, we have director’s and officer’s liability insurance policies that, in most cases, would limit our exposure and enable us to recover a portion of any future amounts paid. The estimated fair value of these indemnification provisions was minimal. Most of these indemnification provisions were grandfathered under the provisions of FASB Interpretation No. 45,“Guarantor’s Accounting and Disclosure Requirements for Guarantees, Including Indirect Guarantees of Indebtedness of Others,”as they were in effect prior to the year ended December 31, 2003. Accordingly, we had no liabilities recorded for these provisions as of December 31, 2006.
We are subject to indemnification provisions under our agreements with third parties in the ordinary course of business, typically with business partners, contractors, clinical sites and customers. Under these provisions we generally indemnify and hold harmless such third party for losses suffered or incurred by it as a result of our conduct, including breach of representations and warranties in the agreements or infringement on intellectual property rights. These indemnification provisions generally survive termination of the underlying agreement. The maximum potential amount of future payments we could be required to make under these indemnification provisions is unlimited. We have not incurred material costs to defend lawsuits or settle claims related to these indemnification obligations. As a result, the estimated fair value of these indemnification obligations was minimal. Accordingly, we had no liabilities recorded for these indemnification obligations as of December 31, 2006.
Borrowings under our revolving credit facility, discussed in Note 9, are guaranteed by our domestic subsidiaries.
Note 4. Acquisitions and Restructuring
Acquisition of LCM Business from Arcturus Bioscience, Inc.
On April 3, 2006, we acquired the Laser Capture Microdissection (“LCM”) business from Arcturus Bioscience, Inc. (“Arcturus”) for $11.3 million. This strategic acquisition expanded our product portfolio to include complete systems and reagents for LCM.
The acquisition was accounted for under the purchase method of accounting. The results of operations of the LCM business have been included in the accompanying consolidated financial statements from the date of the acquisition. The total cost of the acquisition was $11.3 million, including cash paid of $10.3 million and transaction costs of $1.0 million.
74
Table of Contents
As of December 31, 2006, the purchase price allocation was as follows (in thousands):
| In-process research and development | $ | 4,272 | ||
| Acquired goodwill | 1,354 | |||
| Acquired developed technology (amortized over five to ten years) | 2,762 | |||
| Acquired trade name | 1,002 | |||
| Acquired patents (amortized over ten years) | 1,797 | |||
| Net book value of acquired assets and liabilities which approximate fair value | 72 | |||
| Total purchase price | $ | 11,259 | ||
We allocated the purchase price based on the fair value of the assets acquired and liabilities assumed. A valuation of the purchased intangible assets was undertaken by a third party valuation specialist to assist us in determining the estimated fair value of each identifiable asset and in allocating the preliminary purchase price among acquired assets, including the portion of the purchase price attributed to acquired in-process research and development projects. Projects that qualify as in-process research and development represent those that have not yet reached technological feasibility and which have no alternative use. The valuation analysis resulted in $4.3 million of the purchase price being allocated to acquired in-process research and development and charged to earnings using a discount rate of 25%. The in-process research and development acquired from Arcturus consisted of product development initiatives. We estimated that the in-process projects related to these products were approximately 81% complete.
The value assigned to acquired in-process research and development was determined by considering the importance of the project to the overall development plan, estimating costs to develop the purchased in-process research and development into commercially viable products, estimating the resulting net cash flows from the projects when completed and discounting the net cash flows to their present value. The revenue estimates used to value the acquired in-process research and development were based on estimates of relevant market sizes and growth factors, expected trends in technology and the nature and expected timing of new product introductions by Arcturus and its competitors. The rates used to discount the net cash flows to their present value were based on Arcturus’ internal rate of return. The internal rate of return was adjusted to reflect the difficulties and uncertainties in completing each project and thereby achieving technological feasibility, the percentage of completion of each project, anticipated market acceptance and penetration, and market growth rates and risks related to the impact of potential changes in future target markets.
Restructuring and Other Charges
In the fourth quarter of 2006, we implemented a restructuring plan which included the closing of facilities in Norway and the termination of employees at that site. Costs for this plan for employee severance totaled $0.2 million. Other costs totaled $0.1 million. The total of $0.3 million was recognized as expense in restructuring and other charges in the Consolidated Statements of Income. Activity for these charges for the year ended December 31, 2006 was as follows (in thousands):
| Non-cash | ||||||||||||
| Charges and | Balance at | |||||||||||
| Cash | December 31, | |||||||||||
| Initial Cost | Payments | 2006 | ||||||||||
| Molecular Devices employee severance | $ | 240 | $ | (181 | ) | $ | 59 | |||||
| Other costs | 91 | (91 | ) | — | ||||||||
| Total | $ | 331 | $ | (272 | ) | $ | 59 | |||||
In the fourth quarter of 2005, we implemented a restructuring plan which included the termination of employees and the restructuring of a development and license agreement with a supplier. Costs for this plan associated with employee severance totaled $0.6 million. Costs for the restructuring of the agreement totaled $0.8 million, including the return of our equity investment in the supplier valued at $0.5 million and the transfer of $0.3 million of inventory. The total of $1.4 million was recognized as expense in restructuring and other charges in the Consolidated Statements of Income. Activity for these charges for the years ended December 31, 2005 and 2006 was as follows (in thousands):
75
Table of Contents
| Non-cash | Non-cash | |||||||||||||||||||
| Charges and | Balance at | Charges and | Balance at | |||||||||||||||||
| Cash | December 31, | Cash | December 31, | |||||||||||||||||
| Initial Cost | Payments | 2005 | Payments | 2006 | ||||||||||||||||
| Molecular Devices employee severance | $ | 604 | $ | (201 | ) | $ | 403 | $ | (403 | ) | $ | — | ||||||||
| Termination of agreement | 823 | (823 | ) | — | 0 | — | ||||||||||||||
| Total | $ | 1,427 | $ | (1,024 | ) | $ | 403 | $ | (403 | ) | $ | — | ||||||||
Concurrent with the Axon acquisition in 2004, we implemented an integration plan which included the termination of Axon employees, the relocation or transfer to other sites of employees and the closure of duplicate facilities. Costs for this plan associated with employee severance and relocation totaled $0.5 million. Costs for the closure of duplicate facilities, representing an unfavorable lease liability associated with one of Axon’s remaining leases, totaled $2.2 million.
In the fourth quarter of 2004, we implemented an integration plan which included the termination of additional employees. Costs necessary to integrate the businesses of Molecular Devices and Axon that were expected to benefit future operations were expensed as restructuring and other charges in the Consolidated Statements of Income. Termination costs related to Molecular Devices employees totaled $1.2 million for the year ended December 31, 2004.
Activity for these restructuring charges for the years ended December 31, 2006, 2005 and 2004, was as follows (in thousands):
| Non-cash | Non-cash | Non-cash | ||||||||||||||||||||||||||||||
| Charges and | Balance at | Charges and | Adjustments | Balance at | Charges and | Balance at | ||||||||||||||||||||||||||
| Cash | December 31, | Cash | Included in | December 31, | Cash | December 31, | ||||||||||||||||||||||||||
| Initial Cost | Payments | 2004 | Payments | Net Income | 2005 | Payments | 2006 | |||||||||||||||||||||||||
| Axon employee severance and relocation | $ | 500 | $ | (365 | ) | $ | 135 | $ | (35 | ) | $ | (100 | ) | $ | — | $ | — | $ | — | |||||||||||||
| Closure of duplicate facilities | 2,200 | — | 2,200 | (857 | ) | (54 | ) | 1,289 | (304 | ) | 985 | |||||||||||||||||||||
| Molecular Devices employee severance | 1,157 | (124 | ) | 1,033 | (972 | ) | (61 | ) | — | — | — | |||||||||||||||||||||
| Total | $ | 3,857 | $ | (489 | ) | $ | 3,368 | $ | (1,864 | ) | $ | (215 | ) | $ | 1,289 | $ | (304 | ) | $ | 985 | ||||||||||||
Note 5. Goodwill, Developed Technology and Intangible and Other Assets
In March 2005, we completed the purchase of certain assets from Xsira Pharmaceuticals, Inc. (“Xsira”) relating to the Transfluor® technology, a cell-based fluorescent assay system for monitoring the function of G-protein coupled receptors. We acquired $11.2 million of intangible assets, including $11.0 million of developed technology and tradenames valued at $0.2 million. These intangible assets will be amortized over their estimated useful lives of ten years. We also acquired $0.1 million of other current assets consisting of receivables assigned to us in the purchase.
Goodwill was $107.3 million and $102.8 million at December 31, 2006 and 2005, respectively. In 2006, we increased goodwill by $3.5 million to correct the purchase price allocation for our 2004 acquisition of Axon and to recognize a deferred tax liability on an acquired tradename. The correction had no impact on our results of operations or cash flows. At December 31, 2006 and 2005, purchased intangible assets not subject to amortization totaled $11.8 million and consisted of tradenames valued at $10.3 million and distribution rights valued at approximately $1.5 million.
Purchased intangible assets subject to amortization over ten years consisted of patents, certain developed technology, license fees, and certain tradenames. Certain developed technology is amortized over a five year life. Purchased intangible assets subject to amortization over one year consisted of order backlog. The gross and net carrying value of these assets were as follows (in thousands):
79
Table of Contents
| December 31, 2006 | December 31, 2005 | |||||||||||||||||||||||
| Gross Carrying | Accumulated | Net Carrying | Gross Carrying | Accumulated | Net Carrying | |||||||||||||||||||
| Value | Amortization | Value | Value | Amortization | Value | |||||||||||||||||||
| Developed technology | $ | 31,505 | $ | 7,063 | $ | 24,442 | $ | 28,743 | $ | 3,591 | $ | 25,152 | ||||||||||||
| License fees | 4,285 | 1,165 | 3,120 | 2,688 | 772 | 1,916 | ||||||||||||||||||
| Patents | 3,169 | 867 | 2,302 | 1,372 | 610 | 762 | ||||||||||||||||||
| Tradename | 200 | 35 | 165 | 200 | 15 | 185 | ||||||||||||||||||
| Order Backlog | 100 | 100 | — | 100 | 100 | — | ||||||||||||||||||
| Total | $ | 39,259 | $ | 9,230 | $ | 30,029 | $ | 33,103 | $ | 5,088 | $ | 28,015 | ||||||||||||
Amortization expense was $3.8 million, $3.0 million and $1.2 million for the years ended December 31, 2006, 2005 and 2004, respectively. The estimated future amortization expense of purchased intangible assets and license fees is as follows (in thousands):
| For the Year Ended December 31, | Amortization Expense | |||
| 2007 | $ | 3,987 | ||
| 2008 | 3,987 | |||
| 2009 | 3,987 | |||
| 2010 | 3,987 | |||
| 2011 | 3,740 | |||
| Thereafter | 10,341 | |||
| $ | 30,029 | |||
80
Table of Contents
Note 6. Stockholders’ Equity
In 2006, we repurchased 0.9 million shares of our common stock. The repurchases occurred at various times throughout the year. As of December 31, 2006, approximately 0.2 million shares remained available for repurchase under the stock repurchase program initially approved by the Board of Directors in August 2001.
Note 7. Stock Option and Employee Incentive Plans
In April 2005, we established the 2005 Equity Incentive Plan (“2005 Plan”), which is an amendment and restatement of our 1995 Stock Option Plan. The 2005 Plan provides for the grant of incentive stock options, nonstatutory stock options, stock appreciation rights, stock purchase awards, stock bonus awards, stock unit awards, and other forms of equity awards. There were an aggregate of 4,333,011 shares of common stock reserved for issuance under the 2005 Plan. Stock options generally expire in ten years and become exercisable in increments as specified in the option agreements. Terms and vesting schedules of other types of equity awards are pursuant to the various agreements under which such awards are made. We intend to settle employee stock option exercises with newly issued common shares rather than shares held in treasury.
In July 2001, we established the 2001 Stock Option Plan (the “2001 Plan”). Under the 2001 Plan, we are authorized to grant options to purchase up to 100,000 shares of common stock to employees who are working or residing outside of the U.S. and are not officers or directors. Stock options generally expire in twelve years and become exercisable in increments over a period of four to five years from the date of grant. Options may be granted with different vesting terms from time to time.
As a result of our acquisition of Axon in 2004, we assumed options issued by Axon under Axon’s 1993 Stock Option Plan, as amended, and Axon’s 2001 Equity Incentive Plan (“Axon employee stock options”). The Axon employee stock options vest over a maximum period of five years and expire ten years from the date of grant.
Prior to January 1, 2006, these stock option plans were accounted for under the recognition and measurement provisions of APB Opinion 25 and related interpretations, as permitted by FAS 123. Accordingly, no compensation expense was recognized for stock option grants with an exercise price equal to the fair market value of the shares at the date of grant. Effective January 1, 2006, we adopted the fair value recognition provisions of FAS 123R using the modified prospective transition method and therefore have not restated prior periods. Under this transition method, stock-based compensation expense for the year ended December 31, 2006 included compensation expense for all stock-based compensation awards granted prior to, but not yet vested as of, January 1, 2006, based on the grant date fair value estimated in accordance with the provisions of FAS 123 and compensation expense for all stock-based compensation awards granted after January 1, 2006 based on the grant-date fair value estimated in accordance with the provisions of FAS 123R. We recognize stock-based compensation expense net of estimated forfeitures on a straight-line basis over the requisite service period of the award, which is generally the option vesting term of four years.
The following table shows total stock-based compensation expense recorded under FAS 123R included in the Consolidated Statements of Operations for the year ended December 31, 2006 (in thousands, except per share amounts):
| Year Ended | ||||
| December 31, 2006 | ||||
| Cost of revenues | $ | 408 | ||
| Research and development | 1,097 | |||
| Selling, general and administrative | 3,511 | |||
| Income before taxes | 5,016 | |||
| Income tax benefit | (930 | ) | ||
| Effect on net income | $ | 4,086 | ||
| Basic net income per share | $ | 0.25 | ||
| Diluted net income per share | $ | 0.24 | ||
Prior to the adoption of FAS 123R, we presented all tax benefits of deductions resulting from the exercise of stock options as operating cash flows in the Condensed Consolidated Statements of Cash Flows. FAS 123R requires the excess tax benefit associated with an individual share-based payment award be included in the statements of cash flows as a cash inflow from financing activities and a cash outflow from operating activities. For the year ended December 31, 2006, the benefit realized as a result of employee exercises of stock options of $1.5 million was classified as a financing cash flow.
81
Table of Contents
The table below presents net income and basic and diluted net income per share as if we had elected to recognize compensation expense based on the fair value of the options granted on their grant date and shares issued under our employee stock purchase plans as prescribed by FAS 123 for the years ended December 31, 2005 and 2004 (in thousands, except per share amounts):
| Years ended December 31, | ||||||||
| 2005 | 2004 | |||||||
| Net income — as reported | $ | 15,896 | $ | 17,233 | ||||
| Plus: Stock based compensation expense included in reported net income, net of tax | 65 | 65 | ||||||
| Less: Stock based compensation expense determined using the fair value method, net of tax | (5,759 | ) | (6,301 | ) | ||||
| Net income — pro forma | $ | 10,202 | $ | 10,997 | ||||
| Net income per share: | ||||||||
| Basic — as reported | $ | 0.95 | $ | 1.08 | ||||
| Basic — pro forma | $ | 0.61 | $ | 0.69 | ||||
| Diluted — as reported | $ | 0.93 | $ | 1.04 | ||||
| Diluted — pro forma | $ | 0.59 | $ | 0.67 | ||||
As part of our adoption of FAS 123R, we reevaluated our assumptions used in estimating the fair value of stock options granted. As part of this assessment, we determined that a combination of implied and historical volatility is a better indicator of our expected volatility than historical volatility alone, which we previously used to value our options. Further, we examined the historical pattern of option exercises in order to determine if there were any discernable activity patterns based on certain employee populations. From this analysis, we identified two employee populations. We used the Black Scholes option-pricing model to value the options for both of the employee populations. The table below presents the weighted average expected term in years of the two identified employee populations. The expected term is based on historical exercise patterns and post-vesting termination behavior within both of the populations identified. The interest rates for periods within the contractual life of the awards are based on the U.S. Treasury yield curve in effect at the time of grant.
The fair value of each option was estimated on the date of grant using the following assumptions:
| Years Ended December 31, | ||||||||||||
| 2006 | 2005 | 2004 | ||||||||||
| Expected dividend yield | 0 | % | 0 | % | 0 | % | ||||||
| Expected stock price volatility | 49 | % | 77 | % | 86 | % | ||||||
| Risk-free interest rate | 4.3 | % | 4.0 | % | 4.1 | % | ||||||
| Expected life of options | 5.2 years | 6.0 years | 5.2 years | |||||||||
82
Table of Contents
The following table summarizes the activity under all of our stock option plans:
| Shares Available for | Weighted Average | |||||||||||
| Future Grant | Options Outstanding | Exercise Price | ||||||||||
| Balance at December 31, 2003 | 1,064,355 | 2,952,571 | $ | 25.55 | ||||||||
| Authorized | 300,000 | — | — | |||||||||
| Granted | (522,500 | ) | 522,500 | 19.54 | ||||||||
| Assumed through acquisition of Axon | — | 572,570 | 18.55 | |||||||||
| Exercised | — | (270,258 | ) | 12.18 | ||||||||
| Cancelled | 289,768 | (289,768 | ) | 24.74 | ||||||||
| Balance at December 31, 2004 | 1,131,623 | 3,487,615 | 24.59 | |||||||||
| Granted | (411,500 | ) | 411,500 | 20.82 | ||||||||
| Exercised | — | (458,190 | ) | 14.29 | ||||||||
| Cancelled | 356,595 | (356,595 | ) | 27.69 | ||||||||
| Balance at December 31, 2005 | 1,076,718 | 3,084,330 | 25.15 | |||||||||
| Granted | (336,250 | ) | 336,250 | 29.49 | ||||||||
| Exercised | — | (727,985 | ) | 18.00 | ||||||||
| Cancelled | 202,103 | (202,103 | ) | 33.98 | ||||||||
| Balance at December 31, 2006 | 942,571 | 2,490,492 | $ | 27.18 | ||||||||
The following table is a summary of our outstanding and exercisable options at December 31, 2006:
| Options Outstanding | Options Exercisable | |||||||||||||||||||||||
| Weighted Average | ||||||||||||||||||||||||
| Weighted Average | Remaining | |||||||||||||||||||||||
| Range of Exercise | Remaining Contractual | Weighted Average | Number | Contractual Life | Weighted Average | |||||||||||||||||||
| Prices | Number Outstanding | Life (Yr.) | Exercise Price | Exercisable | (Yr.) | Exercise Price | ||||||||||||||||||
| $0.00 to $12.58 | 87,959 | 3.9 | $ | 7.73 | 86,887 | 3.9 | $ | 7.69 | ||||||||||||||||
| $12.59 to $25.16 | 1,509,149 | 6.0 | 19.63 | 1,161,366 | 5.5 | 19.49 | ||||||||||||||||||
| $25.17 to $37.74 | 459,235 | 7.1 | 28.86 | 144,485 | 2.5 | 27.50 | ||||||||||||||||||
| $37.75 to $50.33 | 274,956 | 3.2 | 47.09 | 274,956 | 3.2 | 47.09 | ||||||||||||||||||
| $50.34 to $62.91 | 43,594 | 3.6 | 53.15 | 43,594 | 3.6 | 53.15 | ||||||||||||||||||
| $62.92 to $75.49 | 41,250 | 4.1 | 75.27 | 41,250 | 4.1 | 75.27 | ||||||||||||||||||
| $75.50 to $88.08 | 74,349 | 3.8 | 77.74 | 74,349 | 3.8 | 77.74 | ||||||||||||||||||
| 2,490,492 | $ | 27.18 | 1,826,887 | $ | 28.15 | |||||||||||||||||||
There were 2,297,101 and 2,497,777 options exercisable under the various plans at December 31, 2005 and 2004, respectively.
The weighted average grant date fair value of options granted during the years ended December 31, 2006, 2005 and 2004 was $14.48, $14.44 and $9.89 per share, respectively. The intrinsic value of options exercised during the year ended December 31, 2006, 2005 and 2004 was $9.3 million, $3.9 million and $3.3 million, respectively. The intrinsic value of options outstanding and exercisable at December 31, 2006 was $3.9 million and $3.5 million, respectively. As of December 31, 2006, $7.5 million of total unrecognized compensation expense related to stock options was expected to be recognized over a weighted-average period of 1.70 years.
401(k) Plan
Our 401(k) Plan (the “Plan”) covers substantially all of our U.S. based employees. Under the Plan, as amended in November 2004, eligible employees may make contributions subject to certain Internal Revenue Service restrictions. We began matching a portion of employee contributions in 1997, up to a maximum of $2,500 of each employee’s eligible compensation. The match, which is subject to board approval based on a number of factors, is effective December 31 of each year and vests over a period of four years of service. For the years ended December 31, 2006, 2005 and 2004, we recognized as expense approximately $0.8 million, $0.7 million and $0.5 million, respectively, under the Plan.
83
Table of Contents
Stock Reserved for Issuance
As of December 31, 2006, we had 3,433,063 shares of common stock reserved for issuance under our stock option plans.
Note 8. Income Taxes
The components of the provision for income taxes are as follows (in thousands):
| Years ended December 31, | ||||||||||||
| 2006 | 2005 | 2004 | ||||||||||
| Current: | ||||||||||||
| US Federal | $ | 7,732 | $ | 3,015 | $ | 3,374 | ||||||
| State | 1,046 | 469 | 1,755 | |||||||||
| Foreign | 1,767 | 914 | 650 | |||||||||
| 10,545 | 4,398 | 5,779 | ||||||||||
| Deferred: | ||||||||||||
| US Federal | (1,079 | ) | 3,389 | 6,089 | ||||||||
| State | 381 | 368 | 730 | |||||||||
| Foreign | (31 | ) | 425 | 180 | ||||||||
| (729 | ) | 4,182 | 6,999 | |||||||||
| Total | $ | 9,816 | $ | 8,580 | $ | 12,778 | ||||||
The provision for income taxes differs from the amounts computed by applying the statutory federal income tax rate to income before taxes. The source and tax effects of the differences are as follows (in thousands):
| Years ended December 31, | ||||||||||||
| 2006 | 2005 | 2004 | ||||||||||
| Income before provisions for income taxes | $ | 20,643 | $ | 24,476 | $ | 30,011 | ||||||
| Income tax at statutory rate (35%) | 7,225 | 8,567 | 10,504 | |||||||||
| Non-deductible acquired in-process research and development | — | — | 1,750 | |||||||||
| State income tax, net of federal benefit | 1,180 | 555 | 1,615 | |||||||||
| Extraterritorial income exclusion benefit | (238 | ) | (414 | ) | (410 | ) | ||||||
| Qualified production activities income deduction | (123 | ) | (201 | ) | — | |||||||
| Research and development credits | (301 | ) | (301 | ) | (688 | ) | ||||||
| Non-deductible stock based compensation expense | 691 | — | — | |||||||||
| Foreign subsidiary liquidation | 585 | — | — | |||||||||
| Translation gain on subsidiary liquidation | (495 | ) | — | — | ||||||||
| Adjustment to deferred tax assets relating to non- deductible intangible assets | 1,184 | — | — | |||||||||
| Other | 108 | 374 | 7 | |||||||||
| $ | 9,816 | $ | 8,580 | $ | 12,778 | |||||||
Foreign pretax income was $6.3 million, $2.8 million and $1.4 million in 2006, 2005 and 2004, respectively.
84
Table of Contents
Deferred income taxes reflect the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting and the amount used for income tax purposes. The tax effects of temporary differences and carryforwards which give rise to significant portions of the deferred tax assets and liabilities are as follows (in thousands):
| 2006 | 2005 | |||||||
| Deferred Tax Assets: | ||||||||
| Deferred revenue and reserves not currently deductible | $ | 2,210 | $ | 1,127 | ||||
| Warranty and accrued expenses | 2,885 | 3,730 | ||||||
| Stock based compensation | 1,072 | — | ||||||
| Depreciation and amortization | 2,519 | 1,562 | ||||||
| Net operating loss carryforwards | — | 82 | ||||||
| Foreign loss carryforwards | 204 | 288 | ||||||
| Tax credit carryforwards | 354 | 624 | ||||||
| Intercompany transactions | 258 | 512 | ||||||
| Other | 182 | 22 | ||||||
| Total deferred taxes | 9,684 | 7,947 | ||||||
| Deferred Tax Liabilities: | ||||||||
| Acquired intangible assets | (8,341 | ) | (4,486 | ) | ||||
| Depreciation and amortization | — | — | ||||||
| Other | (587 | ) | — | |||||
| Total deferred tax liabilities | (8,928 | ) | (4,486 | ) | ||||
| Net deferred tax assets | $ | 756 | $ | 3,461 | ||||
As of December 31, 2006, we had California research and development credits of approximately $0.5 million which carry forward indefinitely.
As of December 31, 2006, we had unrecognized deferred tax liabilities of approximately $0.2 million related to approximately $8.8 million of cumulative net undistributed earnings of foreign subsidiaries. These earnings are considered to be permanently reinvested in operations outside the United States.
Note 9. Revolving Credit Facility
We have a senior unsecured credit facility with Union Bank of California, N.A., which provides us with a revolving credit facility in the amount of up to $30.0 million. Borrowings under the revolving credit facility are guaranteed by our domestic subsidiaries. All loans outstanding under the senior unsecured credit facility will bear interest at a rate per annum equal to, at our option, either the base rate plus 0.50% or the London InterBank Offered Rate (LIBOR) plus 1.25%. The revolving credit facility may be drawn, paid and reborrowed at our option, and matures on July 1, 2007. We initially used $15.0 million of this credit facility to partially finance the cash portion of the merger consideration paid to Axon shareholders and certain optionholders. The $15.0 million drawdown was repaid and the revolving credit facility had no outstanding balance as of December 31, 2006 or 2005. At December 31, 2006, we were in compliance with all of the credit facility covenants.
Note 10. Industry Segment, Geographic and Customer Information
We operate in a single industry segment, and our chief operating decision maker views our operations as follows: the design, development, manufacture, sale and service of bioanalytical measurement systems for drug discovery and life sciences research applications.
85
Table of Contents
Foreign subsidiaries’ operations consist of research and development, sales, service, manufacturing and distribution. Summarized data for our domestic and international operations was as follows (in thousands):
| Adjustments and | ||||||||||||||||
| United States | International | Eliminations | Total | |||||||||||||
| Year ended December 31, 2004 | ||||||||||||||||
| Revenues | $ | 134,228 | $ | 44,577 | $ | (29,710 | ) | $ | 149,095 | |||||||
| Income from operations | 10,118 | 1,624 | (151 | ) | 11,591 | |||||||||||
| Identifiable assets | 248,996 | 31,577 | (25,344 | ) | 255,229 | |||||||||||
| Year ended December 31, 2005 | ||||||||||||||||
| Revenues | 162,345 | 59,970 | (40,364 | ) | 181,951 | |||||||||||
| Income from operations | 21,846 | 3,150 | 95 | 25,091 | ||||||||||||
| Identifiable assets | 255,146 | 33,652 | (31,382 | ) | 257,416 | |||||||||||
| Year ended December 31, 2006 | ||||||||||||||||
| Revenues | 164,822 | 70,988 | (49,398 | ) | 186,412 | |||||||||||
| Income from operations | 11,288 | 4,469 | 799 | 16,556 | ||||||||||||
| Identifiable assets | 261,203 | 43,629 | (38,054 | ) | 266,778 | |||||||||||
Our products are broken into two product families. The drug discovery family includes our FLIPR, Automated Electrophysiology, High-Throughput Imaging and Analyst product families. The life sciences research family includes the SpectraMax, GenePix, MetaMorph, Laser Capture Microdissection, Cellular Neurosciences, Liquid Handling and Threshold product lines. Consolidated revenue from our product families was as follows (in thousands):
| Years Ended December 31, | ||||||||||||
| 2006 | 2005 | 2004 | ||||||||||
| Drug discovery | $ | 68,198 | $ | 73,074 | $ | 61,691 | ||||||
| Life sciences research | 118,214 | 108,877 | 87,404 | |||||||||
| Total revenues | $ | 186,412 | $ | 181,951 | $ | 149,095 | ||||||
Sources of consolidated revenue from significant geographic regions were as follows (in thousands):
| Years Ended December 31, | ||||||||||||
| 2006 | 2005 | 2004 | ||||||||||
| North America | $ | 103,288 | $ | 106,298 | $ | 91,020 | ||||||
| Europe | 49,206 | 46,231 | 38,515 | |||||||||
| Rest of World | 33,918 | 29,422 | 19,560 | |||||||||
| Total revenues | $ | 186,412 | $ | 181,951 | $ | 149,095 | ||||||
Note 11. Related Party Transactions
Our Chief Executive Officer is a member of the Board of Directors of Essen Instruments (“Essen”) and we are a minority investor in Essen. We paid Essen $0.7 million, $0.5 million and $0.3 million in royalties in the years ended December 31, 2006, 2005 and 2004, respectively. At December 31, 2006, we owed Essen $0.3 million for royalties payable.
In December 2003, we entered into a services agreement with Essen whereby Essen provided certain consulting services to us for two years in exchange for the return of a portion of the Essen shares owned by us. Through December 31, 2005, $1.2 million in equity was returned to Essen in exchange for consulting services provided, and the services agreement was completed.
We had an equity investment in Upstate until October 2004. We paid Upstate $0.1 million for royalties and $90,000 for inventory during the year ended December 31, 2004.
We had an equity investment in Aviva Biosciences Corporation (“Aviva”) until December 2005, when we returned it as part of our restructuring as described in Note 4. For the years ended December 31, 2005 and 2004, we purchased $3.3 million and $0.8 million of inventory from Aviva.
86
Table of Contents
Note 12. Subsequent Event
On January 28, 2007, we entered into a definitive agreement and plan of merger with MDS Inc.(“MDS”) and Monument Acquisition Corp. (“Monument”), a subsidiary of MDS, which contemplates the acquisition by MDS, through Monument, of all of our outstanding common stock in a two-step transaction comprised of a cash tender offer for all of our issued and outstanding shares of common stock, followed by a merger of Monument with and into us. Pursuant to the definitive agreement, and upon the terms and subject to the conditions thereof, Monument has commenced a tender offer to acquire all of the outstanding shares of our common stock at a price of $35.50 per share, net to the holder thereof in cash. Pursuant to the definitive agreement, as soon as practicable after the consummation of the tender offer and subject to the satisfaction or waiver of certain conditions set forth in the definitive agreement, Monument will merge with and into us and we will become an indirect wholly-owned subsidiary of MDS. In this merger, each share of our common stock remaining outstanding following the consummation of the tender offer, other than each share held by MDS or Monument or by any stockholder who has validly exercised appraisal rights under Delaware law, will be converted into the right to receive $35.50 in cash. The obligation of Monument to accept for payment and pay for the shares tendered in the tender offer is subject to the satisfaction or waiver of a number of closing conditions set forth in the definitive agreement, including among others, the expiration of the waiting period under the Hart-Scott-Rodino Antitrust Improvements Act. In addition, it is also a condition to Monument’s obligation to accept for payment and pay for the shares tendered in the tender offer that at least a majority of the then-outstanding shares of our common stock (determined on a fully diluted basis including any unvested stock options that would vest by their terms on or before June 30, 2007, but disregarding any other unvested stock options) will have been validly tendered in accordance with the terms of the tender and not validly withdrawn. The closing of the merger contemplated by the definitive agreement is subject to customary closing conditions, and, depending on the number of shares held by MDS and Monument after Monument’s acceptance of the shares properly tendered in connection with the tender offer, approval of the merger contemplated by the definitive agreement by the holders of the outstanding shares of our common stock remaining after the completion of the tender offer may be required.
Note 13. Quarterly Financial Data (Unaudited)
Summarized quarterly financial data is as follows:
| First | Second | Third | Fourth | |||||||||||||
| (in thousands, except per share amounts) | ||||||||||||||||
| Year ended December 31, 2005 | ||||||||||||||||
| Revenues | $ | 39,230 | $ | 44,727 | $ | 45,305 | $ | 52,689 | ||||||||
| Gross profit | 24,018 | 26,937 | 27,763 | 32,966 | ||||||||||||
| Net income | 1,844 | 3,665 | 4,631 | 5,756 | ||||||||||||
| Basic net income per share | 0.11 | 0.22 | 0.28 | 0.35 | ||||||||||||
| Diluted net income per share | 0.11 | 0.21 | 0.27 | 0.34 | ||||||||||||
| Year ended December 31, 2006 | ||||||||||||||||
| Revenues | $ | 40,159 | $ | 47,643 | $ | 45,765 | $ | 52,845 | ||||||||
| Gross profit | 24,535 | 29,760 | 28,678 | 33,374 | ||||||||||||
| Net income | 2,846 | 1,228 | 2,782 | 3,971 | ||||||||||||
| Basic net income per share | 0.17 | 0.07 | 0.17 | 0.24 | ||||||||||||
| Diluted net income per share | 0.16 | 0.07 | 0.17 | 0.24 | ||||||||||||
For the first, second, third and fourth quarters of 2005, Revenues include increases of $0.2 million, $0.2 million, $0.1 million and $0.2 million, respectively, related to the reclassification of freight charges recovered from customers from Cost of Revenues to Revenues. For the first, second and third quarters of 2006, Revenues included an increase of $0.2 million, $0.3 million and $0.3 million, respectively, related to the reclassification of freight charges recovered from customers from Cost of Revenues to Revenues. Cost of Revenues has been increased accordingly, and there was no effect on gross profit, operating income or net income for any of the periods presented.
Adjustments have been made to amounts previously reported for net income, basic net income per share, and diluted net income per share in the second quarter of 2006. During the preparation of our year-end financial statements, we reduced income tax expense by $1.7 million in the second quarter of 2006 associated with deferred tax assets on the acquired in-process research and development from the acquisition of the LCM business from Arcturus. This adjustment increased basic and diluted net income per share by $0.10 in the second quarter of 2006.
87
Table of Contents
SCHEDULE II — VALUATION AND QUALIFYING ACCOUNTS
| Balance at | Charged to Costs and | Balance at End of | ||||||||||||||
| Description | Beginning of Year | Expenses | Deductions | Year | ||||||||||||
| (In thousands) | ||||||||||||||||
| Allowance for doubtful accounts receivable | ||||||||||||||||
| Year ended December 31, 2004 | $ | 408 | $ | 436 | (A) | $ | (505 | ) | $ | 339 | ||||||
| Year ended December 31, 2005 | $ | 339 | $ | 255 | $ | (152 | ) | $ | 442 | |||||||
| Year ended December 31, 2006 | $ | 442 | $ | 82 | $ | (342 | ) | $ | 182 | |||||||
| (A) | Of this amount, $0.2 million in reserves was associated with receivables acquired from Axon (see Note 4). |
88
Table of Contents
EXHIBIT INDEX
| EXHIBIT | ||
| NUMBER | DESCRIPTION OF DOCUMENT | |
| 2.1(19) | Agreement and Plan of Merger, dated as of January 28, 2007, by and among MDS Inc., a company organized under the laws of Canada, Monument Acquisition Corp., a Delaware corporation, and an indirect wholly-owned subsidiary of MDS and direct wholly owned subsidiary of MDS (US) Inc., a Delaware corporation, and Registrant | |
| 3.1(1) | Amended and Restated Certificate of Incorporation of Registrant | |
| 3.2(1) | Bylaws of the Registrant | |
| 3.3(5) | Certificate of Amendment to Certificate of Incorporation | |
| 3.4(8) | Certificate of Designation of Series A Junior Participating Preferred Stock of Registrant | |
| 4.1(1) | Specimen Certificate of Common Stock of Registrant | |
| 4.2(8) | Rights Agreement, dated October 25, 2001, among the Registrant and EquiServe Trust Company, N.A. | |
| 4.3(19) | Amendment No. 1 to Rights Agreement, dated as of January 28, 2007, by and between Computershare Trust Company, N.A. (successor-in-interest to EquiServe Trust Company, N.A.) and Registrant. | |
| 10.1(1)* | 1988 Stock Option Plan | |
| 10.2(1)* | Form of Incentive Stock Option under the 1988 Stock Option Plan | |
| 10.3(1)* | Form of Supplemental Stock Option under the 1988 Stock Option Plan | |
| 10.4(5)* | 1995 Employee Stock Purchase Plan | |
| 10.6(1)* | Form of Nonstatutory Stock Option under the 1995 Non-Employee Directors’ Stock Option Plan | |
| 10.8(1)* | Form of Incentive Stock Option under the 1995 Stock Option Plan | |
| 10.9(1)* | Form of Nonstatutory Stock Option under the 1995 Stock Option Plan | |
| 10.10(1)* | Form of Early Exercise Stock Purchase Agreement under the 1995 Stock Option Plan | |
| 10.11(1)* | Form of Indemnity Agreement between the Registrant and its Directors and Executive Officers | |
| 10.19(10)* | Amended Key Employee Agreement for Joseph D. Keegan, Ph.D., dated July 29, 2004. | |
| 10.20(2) | Exclusive License and Technical Support Agreement, dated August 28, 1998, by and between the Registrant and Affymax | |
| 10.21(2)* | Employee Offer Letter for Timothy A. Harkness | |
| 10.24(10)* | 1995 Non-Employee Director’s Stock Option Plan, as amended | |
| 10.25(10)* | 1995 Stock Option Plan, as amended | |
| 10.26(3)* | Employee Offer Letter for Patricia Sharp | |
| 10.27(4)* | LJL BioSystems 1994 Equity Incentive Plan and Forms of Agreements | |
| 10.28(4)* | LJL BioSystems 1997 Stock Plan and Forms of Agreements | |
| 10.29(4)* | LJL BioSystems 1998 Directors’ Stock Option Plan and Forms of Agreements | |
| 10.33(6) | Lease Agreement, dated May 26, 2000, by and between Aetna Life Insurance Company and the Registrant | |
| 10.34(7)* | Change in Control Severance Benefit Plan | |
| 10.37(5)* | Key Employee Agreement for Tom O’Lenic | |
| 10.38(10)* | 2001 Stock Option Plan, as amended | |
| 10.39(9) | Lease dated May 28, 2002 by and between The Irvine Company and the Registrant | |
| 10.40(9)* | Letter Agreement, dated April 11, 2002, by and between the Registrant and Joseph D. Keegan, Ph.D. | |
| 10.43(11)* | Amended Key Employment Agreement for Timothy A. Harkness | |
| 10.44(11)* | Employee Offer Letter for Alan Finkel | |
| 10.45(11)* | Employee Offer Letter for Steven Davenport | |
| 10.46(11)* | Employee Offer Letter for Jan Hughes | |
| 10.47(11)* | Amended Employee Offer Letter for Jan Hughes | |
| 10.50(12)* | Form of Stock Option Agreement for Non-Employee Director under the 1995 Equity Incentive Plan | |
| 10.51(13)† | Asset Purchase Agreement, dated as of March 9, 2005, by and between Molecular Devices Corporation and Xsira Pharmaceuticals, Inc. | |
| 10.52(14)* | 2005 Equity Incentive Plan | |
| 10.53(14)* | Form of Stock Option Agreement under the 2005 Equity Incentive Plan | |
| 10.54(15)* | Form of Stock Option Agreement for Non-Employee Director under the 2005 Equity Incentive Plan | |
| 10.55(16) | First Amendment to Lease, dated January 27, 2006, by and between the Registrant and Moffett Office Park Investors LLC (successor-in-interest to Aetna Life Insurance Company) | |
| 10.56(16) | First Amendment to Lease, dated January 30, 2006, by and between the Registrant and The Irvine Company LLC | |
| 10.57(17)* | Executive Officer Compensation Arrangements | |
| 10.58(18)* | Non-Employee Director Cash Compensation Arrangements | |
| 21.1 | Subsidiaries of the Registrant | |
| 23.1 | Consent of Ernst & Young LLP, Independent Registered Public Accounting Firm | |
| 24.1 | Power of Attorney (contained on the signature pages hereto) | |
| 31.1 | Certification required by Rule 13a-14(a) or Rule 15d-14(a) | |
| 31.2 | Certification required by Rule 13a-14(a) or Rule 15d-14(a) | |
| 32.1** | Certifications required by Rule 13a-14(b) or Rule 15d-14(b) and Section 1350 of Chapter 63 of Title 18 of the United States Code (18 U.S.C 1350) |
| (1) | Incorporated by reference to the similarly described exhibit in our Registration Statement on Form S-1 (File No. 33-98926), as amended. | |
| (2) | Incorporated by reference to the similarly described exhibit in our Form 10-Q Quarterly Report dated September 30, 1998, and filed November 13, 1998. |
89
Table of Contents
| (3) | Incorporated by reference to the similarly described exhibit in our Form 10-Q Quarterly Report dated September 30, 2000 and filed on November 13, 2000. | |
| (4) | Incorporated by reference to the similarly described exhibit filed with LJL BioSystems’ Registration Statement on Form S-1 (File No. 333-43529) declared effective on March 12, 1998. | |
| (5) | Incorporated by reference to the similarly described exhibit in our Form 10-K Annual Report dated December 31, 2001 and filed on April 1, 2002. | |
| (6) | Incorporated by reference to the similarly described exhibit in our Form 10-K Annual Report dated December 31, 2000 and filed on March 30, 2001. | |
| (7) | Incorporated by reference to the similarly described exhibit in our Form 10-Q Quarterly Report dated March 31, 2001 and filed on May 11, 2001. | |
| (8) | Incorporated by reference to the similarly described exhibit in our Current Report on Form 8-K filed October 30, 2001. | |
| (9) | Incorporated by reference to the similarly described exhibit in our Form 10-K Annual Report dated December 31, 2003 and filed on March 27, 2003. | |
| (10) | Incorporated by reference to the similarly described exhibit in our Form 10-Q Quarterly Report dated September 30, 2004, and filed on November 9, 2004. | |
| (11) | Incorporated by reference to the similarly described exhibit in our Form 10-K Annual Report dated December 31, 2004, and filed on March 16, 2005 | |
| (12) | Incorporated by reference to the similarly described exhibit in our Current Report on Form 8-K filed on April 18, 2005. | |
| (13) | Incorporated by reference to the similarly described exhibit in our Quarterly Report on Form 10-Q dated March 31, 2005 and filed on May 4, 2005. | |
| (14) | Incorporated by reference to the similarly described exhibit in our Current Report on Form 8-K filed on June 1, 2005. | |
| (15) | Incorporated by reference to the similarly described exhibit in our Quarterly Report on Form 10-Q dated November 7, 2006. | |
| (16) | Incorporated by reference to the similarly described exhibit in our Form 10-K Annual Report dated December 31, 2005, and filed on March 16, 2006. | |
| (17) | Incorporated by reference to the information in our Current Reports on Form 8-K filed on February 15, 2006 and February 6, 2007, as amended. | |
| (18) | Incorporated by reference to the information in our Current Report on Form 8-K filed on May 17, 2006. | |
| (19) | Incorporated by reference to the similarly described exhibit in our Current Report on Form 8-K filed on January 29, 2007. | |
| * | Management contract or compensatory plan or arrangement. | |
| † | Confidential treatment has been requested with respect to certain portions of this exhibit. Omitted portions have been filed separately with the Securities and Exchange Commission. | |
| ** | The certification attached as Exhibit 32.1 accompanies the Annual Report on Form 10-K pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 and shall not be deemed “filed” by the Company for purposes of Section 18 of the Securities Exchange Act of 1934, as amended. |
90