UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
| [X] | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the fiscal year ended December 31, 2010 or |
| [ ] | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the transition period from ________to ________ |
Commission File No. 000-24657
MANNATECH, INCORPORATED
(Exact Name of Registrant as Specified in its Charter)
Texas (State or other Jurisdiction of Incorporation or Organization) | 75-2508900 (I.R.S. Employer Identification No.) |
600 S. Royal Lane, Suite 200, Coppell, Texas (Address of Principal Executive Offices) | 75019 (Zip Code) |
Registrant’s Telephone Number, including Area Code: (972) 471-7400
Securities Registered Pursuant to Section 12(b) of the Act: None
Securities Registered Pursuant to Section 12(g) of the Act:
Common Stock, par value $0.0001 per share
Title of each class
Indicate by check mark if the Registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes [ ] No [X]
Indicate by check mark if the Registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Exchange Act. Yes [ ] No [X]
Indicate by check mark whether the Registrant (1) has filed all reports required to be filed by Sections 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the Registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes [X] No [ ] |
| Indicate by check mark whether the Registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (Section 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the Registrant was required to submit and post such files). Yes [X] No [ ] |
| Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (Section 229.405 of this Chapter) is not contained herein, and will not be contained, to the best of Registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. [ ] |
| Indicate by check mark whether the Registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. |
| Large accelerated filer [ ] | Accelerated filer [ ] | Non-accelerated filer [ ] | Smaller reporting company [X] |
Indicate by check mark whether the Registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes [ ] No [X]
At June 30, 2010, the aggregate market value of the common stock held by non-affiliates of the Registrant was $31,365,883 based on the closing sale price of $1.99, as reported on the NASDAQ Global Market.
The number of shares of the Registrant’s common stock outstanding as of March 4, 2011 was 26,490,466 shares.
Documents Incorporated by Reference
Mannatech, Incorporated incorporates information required by Part III (Items 10, 11, 12, 13, and 14) of this report by reference to its definitive proxy statement for its 2011 annual shareholders’ meeting to be filed pursuant to Regulation 14A no later than 120 days after the end of its fiscal year.
TABLE OF CONTENTS
| Page | |||
| Part I | |||
| Part II | |||
| Part III | |||
| Part IV | |||
Certain disclosures and analysis in this Form 10-K, including information incorporated by reference, may include forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, or the Securities Act, Section 21E of the Securities Exchange Act of 1934, as amended, (“the Exchange Act”), and the Private Securities Litigation Reform Act of 1995 that are subject to various risks and uncertainties. Opinions, forecasts, projections, guidance, or other statements other than statements of historical fact are considered forward-looking statements and reflect only current views about future events and financial performance. Some of these forward-looking statements include statements regarding:
| · | management’s plans and objectives for future operations; |
| · | existing cash flows being adequate to fund future operational needs; |
| · | future plans related to budgets, future capital requirements, market share growth, and anticipated capital projects and obligations; |
| · | the realization of net deferred tax assets; |
| · | the ability to curtail operating expenditures; |
| · | global statutory tax rates remaining unchanged; |
| · | the impact of future market changes due to exposure to foreign currency translations; |
| · | the possibility of certain policies, procedures, and internal processes minimizing exposure to market risk; |
| · | the impact of new accounting pronouncements on financial condition, results of operations, or cash flows; |
| · | the outcome of new or existing litigation matters; |
| · | the outcome of new or existing regulatory inquiries or investigations; and |
| · | other assumptions described in this report underlying such forward-looking statements. |
Although we believe that the expectations included in these forward-looking statements are reasonable, these forward-looking statements are subject to certain events, risks, assumptions, and uncertainties, including those discussed below and in the “Risk Factors” section in Item 1A of this Form 10-K, and elsewhere in this Form 10-K and the documents incorporated by reference herein. If one or more of these risks or uncertainties materialize, or if our underlying assumptions prove to be incorrect, actual results and developments could materially differ from those expressed in or implied by such forward-looking statements. For example, any of the following factors could cause actual results to vary materially from our projections:
| · | overall growth or lack of growth in the nutritional supplements industry; |
| · | plans for expected future product development; |
| · | changes in manufacturing costs; |
| · | shifts in the mix of packs and products; |
| · | the future impact of any changes to global associate career and compensation plans or incentives; |
| · | the ability to attract and retain independent associates and members; |
| · | new regulatory changes that may affect operations or products; |
| · | the competitive nature of our business with respect to products and pricing; |
| · | publicity related to our products or network marketing; and |
| · | the political, social, and economic climate. |
Forward-looking statements generally can be identified by use of phrases or terminology such as “may,” “will,” “should,” “could,” “would,” “expects,” “plans,” “intends,” “anticipates,” “believes,” “estimates,” “approximates,” “predicts,” “projects,” “potential,” and “continues” or other similar words or the negative of such terms and other comparable terminology. Similarly, descriptions of Mannatech’s objectives, strategies, plans, goals, or targets contained herein are also considered forward-looking statements. Readers are cautioned when considering these forward-looking statements to keep in mind these risks, assumptions, and uncertainties and any other cautionary statements in this report, as all of the forward-looking statements contained herein speak only as of the date of this report.
Unless stated otherwise, all financial information throughout this report and in the Consolidated Financial Statements and related Notes include Mannatech, Incorporated and all of its subsidiaries on a consolidated basis and may be referred to herein as “Mannatech,” “the Company,” “its,” “we,” “our,” or “their.”
Our products are not intended to diagnose, cure, treat, or prevent any disease and any statements about our products contained in this report have not been evaluated by the Food and Drug Administration, also referred to herein as the FDA.
1
PART I
Overview
Mannatech is a global wellness solution provider, which was incorporated and began operations in November 1993. We develop and sell innovative, high-quality, proprietary nutritional supplements, topical and skin care products, and weight-management products that target optimal health and wellness. We currently sell our products in the United States, Canada, Australia, the United Kingdom, Japan, New Zealand, the Republic of Korea, Taiwan, Denmark, Germany, South Africa, Singapore, Austria, the Netherlands, Norway, Sweden and, beginning January 24, 2011, Mexico. We conduct our business as a single operating segment and primarily sell our products and packs through a network of independent associates and members. As of December 31, 2010, we had approximately 403,000 independent associates and members who have purchased our products and packs within the last 12 months.
We sell our products through network marketing, which we believe is the most cost-effective way to quickly and effectively introduce our products and communicate information about our business to the global marketplace. Network marketing minimizes upfront costs, as compared to conventional marketing methods, and allows us to be more responsive to the ever-changing overall market conditions, as well as continue to research and develop high quality products and focus on controlled successful international expansion. We believe the network marketing channel also allows us to effectively communicate the potential benefits and unique properties of our proprietary products to our consumers. In addition, network marketing provides our business-building independent associates with an avenue to supplement their income and develop financial freedom by building their own business centered on our business philosophies and unique products.
Since our initial public offering in February 1999, our common stock has traded on the NASDAQ Global Market (formerly the NASDAQ National Market) under the symbol “MTEX”. Information for each of our five most recent fiscal years, with respect to our net sales, results of operations, and identifiable assets is set forth in “Item 6. – Selected Financial Data” of this report.
Available Information
We make available free of charge on our Internet website (https://www.mannatech.com) our annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, and certain other information filed or furnished with the Securities and Exchange Commission, (“the SEC”), as soon as reasonably practicable after electronically filing, or furnishing such material. The SEC maintains a website that contains reports, proxy and information statements, and other information regarding issuers, including Mannatech, that electronically file with the SEC at http://www.sec.gov. Additionally, such materials are available in print upon the written request of any shareholder to our principle executive office located at 600 S. Royal Lane, Suite 200, Coppell, Texas 75019, Attention: Investor Relations, or by contacting our investor relations department at (972) 471-6512 or IR@mannatech.com.
Business Segment, Products and Product Development
Business Segment. We conduct our business as a single operating segment – primarily through sales of nutritional supplements, topical and skin care products, and weight management products through network marketing distribution channels in seventeen countries. For more information with respect to the financial results and conditions of our business segment, including financial information about geographic areas, see Note 16 to our consolidated financial statements.
Products. Scientists have discovered that a healthy body consists of many sophisticated components working in harmony to achieve optimal health and wellness and requires cellular communication to function at an optimal level. In its most basic form, a body’s internal communication occurs at the cellular level, and is referred to as cell-to-cell communication. Scientists also discovered that there are more than 200 monosaccharides, also called sugar molecules, which form naturally. Specific monosaccharides are considered vital components for cellular communication in the human body. Furthermore, scientists discovered that these monosaccharides attach themselves to certain proteins, which then form a molecule called glycoprotein. Harper’s Biochemistry, a leading and nationally recognized biochemistry reference, has recognized that these molecules are found in human glycoproteins, and are believed to be essential in helping to promote and provide effective cell-to-cell communication in the human body.
2
A significant portion of our revenue is derived from our core Ambrotose® complex products which include the Ambrotose® products and Advanced Ambrotose® products. Revenue from the core Ambrotose® products were as follows for the years ended December 31, 2010 and 2009 (in thousands, except percentages):
| 2010 | 2009 | ||||||||||
Sales by product | % of total net sales | Sales by product | % of total net sales | ||||||||
Advanced Ambrotose® | $ | 61,458 | 26.9 | % | $ | 65,360 | 22.6 | % | |||
Ambrotose® | 19,078 | 8.4 | % | 25,413 | 8.8 | % | |||||
| Total | $ | 80,536 | 35.3 | % | $ | 90,773 | 31.4 | % | |||
The history of our proprietary ingredients and products is as follows:
| · | In 1994, we developed and began selling our first products containing Manapol®, an ingredient formulated to support cell-to-cell communication. |
| · | In 1996, we enhanced our products based on the study of glycoproteins and our scientists developed our own proprietary compound, Ambrotose® complex, which we patented. Our Ambrotose® complex is a blend of polysaccharides (composed of monosaccharides) that helps provide support for the immune system. |
| · | In 2001, we broadened our proprietary ingredients by developing the Ambroglycin® blend, a balanced food-mineral matrix which helps deliver nutrients to the body and which is used in our proprietary Catalyst™ and Glycentials® vitamin/mineral supplements. |
| · | In 2004, we introduced our proprietary blend of antioxidant nutrients, MTech AO Blend®, which is used in our proprietary antioxidant Ambrotose AO® product. |
| · | In 2006, we introduced a unique blend of plant-based minerals, natural vitamins, and standardized phytochemicals for use in our proprietary PhytoMatrix® product. We also introduced a compound used in reformulated Advanced Ambrotose® complex. This compound allows a more potent concentration of the full range of mannose-containing polysaccharides occurring naturally in aloe to be produced in a stable powdered form. |
| · | In 2007, we introduced into the United States market our skin care line of products that supports skin’s natural texture, beauty, and elasticity. We also launched our PhytoMatrix® caplets, Advanced Ambrotose® capsules and Manna•Bears™ supplement into international markets. |
| · | In 2008, we introduced a proprietary proteolytic enzyme and phytosterol dietary supplement that supports the body’s natural recovery processes associated with physical activity in our BounceBack™ capsules. We also introduced a proprietary version of whey protein peptide technology that assists targeted fat loss when combined with exercise and a healthy diet in our OsoLean™ powder. |
| · | In 2009, we introduced our Essential Source™ Omega-3, which features EPA/DHA essential acids, PhytoBurst™ Nutritional Chews formulated with vitamins, minerals, and phytonutrients from food-sourced ingredients, and GI-ProBalance™ Slimstick in Korea, which is a synbiotic digestive product containing probiotics, prebiotics, and digestive enzymes. In addition, we improved our Ambrotose® products to include beta-Carotene. |
| · | In 2010, we launched our Simply Delicious™ Snack Bars and our Mannatech LIFT™ Skin Care System, which is paraben-free and formulated to give skin a more natural youthful appearance. We also expanded several previously launched products from our domestic line to our international markets. |
Our product philosophy focuses on a full spectrum of quality nutritional and personal care products aimed at promoting and maintaining optimal health and wellness. We focus on producing products that are from all-natural sources, with no synthetic or chemically derived additives. There are three major categories of our products:
Health, which offers a variety of nutritional supplements that aid in optimizing overall health and wellness. This category includes a variety of daily nutritional supplements, health solutions for children, and additional nutrients designed to help keep specific body systems at optimal levels. |
Weight and Fitness, which offers products designed to curb appetite and burn fat, build lean muscle tissue, and support recovery from overexertion. |
3
Skin Care, which offers several products that are formulated with more than 30 botanical ingredients, contain our Jeunesse 7™ proprietary blend – a unique combination of montmorillonite and glyconutrients, and are designed to give the skin a more natural youthful appearance by moisturizing, hydrating and reducing the appearance of fine lines and wrinkles. |
The following table summarizes our products by category:
| Product Category | Representative Products |
| Health | Ambrotose® complex, Ambrotose AO®, Advanced Ambrotose®, PhytoMatrix®, Glyco-Bears®, MannaBears™, Catalyst™, PLUS™, Manna-C™, CardioBALANCE®, ImmunoSTART®, BounceBack™, MannaCLEANSE™, PhytAloe®, GI-Pro®, GI-Zyme®, Essential Source™ Omega-3, PhytoBurst™ Nutritional Chews, GI Pro Balance™ Slimstick, and Simply Delicious™ Snack Bars(1). |
| Weight and Fitness | OsoLean™, Accelerator3™, FiberSlim®, GlycoSlim®, AmbroStart®, SPORT™, and EM·PACT®. |
| Skin Care | Emprizone®, FIRM with Ambrotose®, LIFT™ Exfoliating Facial Cleanser, LIFT™ Multiphase Serum, LIFT™ Day Moisturizer, LIFT™ Night Repair Crème, and LIFT™ Body Lotion. |
_________________________
(1) We plan to discontinue Simply Delicious™ Snack Bars in 2011.
Product Development. Our product committee continues to focus on potential new products and compounds that help target or promote overall health and wellness. When considering new products and compounds, our product committee considers the following criteria:
| · | marketability and proprietary nature of the product; |
| · | demand for the product; |
| · | competitors’ products; |
| · | regulatory considerations; |
| · | availability of ingredients; and |
| · | data supporting claims of efficacy and safety. |
To maintain a flexible operating strategy and the ability to increase production capacity, we contract with third-parties to manufacture all of our products, which allows us to effectively respond to fluctuations in demand with minimal investment and helps control our operating costs. We believe our suppliers and manufacturers are capable of meeting our current and projected inventory requirements over the next several years. However, as a safety measure, we continue to identify and approve alternative suppliers and manufacturers to ensure that our global demands are met in a timely manner and to help minimize any risk of business interruption.
Industry Overview
Nutrition Industry
We operate in the nutritional supplement industry and distribute and sell our products through our own global network marketing channel. The nutritional supplement industry is fast-paced, highly fragmented, and intensely competitive. It includes companies that manufacture and distribute products that are intended to enhance the body’s performance and well-being. Nutritional supplements include vitamins, minerals, dietary supplements, herbs, botanicals, and compounds derived therefrom. Prior to 1990, all dietary supplements in the United States were tightly regulated by the FDA and only included essential nutrients such as vitamins, minerals, and proteins. In 1990, the Nutrition Labeling and Education Act expanded the category to include “herbs or similar nutritional substances”, but the FDA maintained control over pre-market approval. However, in 1994, the Dietary Supplement Health and Education Act of 1994 (“DSHEA”) was passed in the United States, drastically changing the dietary supplement marketplace. The DSHEA was instrumental in expanding the category of dietary supplements to further include herbal and botanical supplements and ingredients such as
4
Nutritional supplements are available through mass-market retailers, drug stores, supermarkets, discount stores, health food stores, mail order companies, and direct sales organizations. Direct selling, of which network marketing is a significant segment, has grown significantly and has been enhanced in the past decade as a distribution channel due to advancements in technology and communications resulting in improved product distribution and faster dissemination of information.
Direct Selling/Network marketing Channel
Since the 1990s, the direct selling and network marketing sales channel has grown in popularity and general acceptance, including acceptance by prominent investors and capital investment groups who have invested in direct selling companies. This has provided direct selling companies with additional recognition and credibility in the growing global marketplace. In addition, many large corporations have diversified their marketing strategy by entering the direct selling arena. Several consumer-product companies have launched their own direct selling businesses with international operations often accounting for the majority of their revenues. Consumers and investors are beginning to realize that direct selling provides unique opportunities and a competitive advantage in today’s markets. Businesses are able to quickly communicate and develop strong relationships with their customers, bypass expensive ad campaigns, and introduce products and services that would otherwise be difficult to promote through traditional distribution channels such as retail stores. Direct selling is a channel of distribution with healthy cash flow, high return on invested capital, and long-term prospects for global expansion. According to the worldwide direct sales data published by the World Federation of Direct Selling Association, in 2009 there were approximately 74 million global direct sellers who collectively generated annual retail sales of $117.6 billion.
Operating Strengths
| 1. | High-Quality, Innovative, Proprietary Products. We base our product concept on the scientific belief that certain glyconutrients, also known as monosaccharides or sugar molecules, are essential for maintaining a healthy immune system. We believe the addition of effective nutritional supplements to a well-balanced diet, coupled with an effective exercise program, will enhance and help maintain optimal health and wellness. We focus on producing products that are from all-natural sources with no synthetic or chemically derived additives. We formulate our products with predominately naturally-occurring, plant-derived, carbohydrate-based, safe ingredients that are designed to use nutrients working through normal physiology to help achieve and maintain optimal health and wellness, rather than developing common synthetic, carbohydrate-based products. |
We believe that our patented proprietary blend Ambrotose® complex included in many of our products distinguishes us as a leader in the global nutritional supplements industry and that no other combination of vitamins, minerals, amino acids, or herbals can provide the benefits found in Ambrotose® complex. We also believe the use of unique compounds found in our products allows us to effectively differentiate and distinguish our products from those of our competitors.
| 2. | Research and Development Efforts. We are steadfast in our commitment to quality-driven research and development. We use systematic processes for the research and development of our unique proprietary product formulas, as well as the identification of quality suppliers and manufacturers. Our research and quality assurance programs are outlined on our corporate websites www.mannatechscience.org, https://www.mannatech.com, and www.allaboutmannatech.com. |
Dr. Robert Sinnott, our co-CEO and chief science officer, leads our team of experienced researchers and scientists. This team continually reviews the latest published research data, attends scientific conferences, and draws upon its vast knowledge and expertise to develop new products and support existing ones. In addition, this team works in collaboration with other research firms, universities, institutes, and scientists. Our products have been the focus of numerous pre-clinical and clinical studies.
Some of our more recent collaborative research projects include:
5
| 1) | In November 2010, the Nutrition Journal published a randomized, double‐blind, placebo‐controlled crossover trial investigating the antioxidant effects of Ambrotose AO® capsules in healthy, exercise-trained and untrained men and women. In this study, scientists showed that Ambrotose AO® (4 capsules/day for 3 weeks) significantly increased two measures of antioxidant capacity—Oxygen Radical Absorbance Capacity (ORAC) and Trolox Equivalent Antioxidant Capacity (TEAC)—in the blood of healthy men and women at rest. ORAC and TEAC are standard methods for assessing the impact of dietary factors on blood antioxidant status. This study, conducted by Professor Richard Bloomer and scientists at the University of Memphis, also supports the safety of Mannatech’s Ambrotose AO® supplement at a daily dose of 4 capsules, through measurement of complete blood count, metabolic and lipid panel, and supports prior evidence for Ambrotose AO® capsules to enhance blood antioxidant capacity in healthy individuals at rest and following exercise. |
| 2) | In September 2010, Stephens & Associates, a privately owned worldwide research organization and a leading investigational site of clinical trials for cosmetic testing and dermatological and women’s health studies, published results of a 12-week clinical usage study and a number of clinical safety studies performed on the new Mannatech LIFT™ skin care system. Clinical testing revealed improvements in skin, firmness, radiance, smoothness and the appearance of fine lines and wrinkles after 8 and 12 weeks and demonstrated that all tested products were well-tolerated, non-comedogenic, and did not cause allergic contact dermatitis. |
| 3) | In April 2010, BMC Complementary and Alternative Medicine published a study demonstrating that Ambrotose AO® capsules significantly increased serum oxygen radical absorbance capacity (ORAC) in a population of healthy adults by 36.5%. The five-week combined Phase 1 and 2 open label, forced titration dose response study provides additional evidence that Ambrotose AO® capsules are safe and most effective at 4 capsules/day. The study was led by Professor Stephen Myers, PhD, BMed, ND, Director of the Australian Centre for Complementary Medicine Education and Research (ACCMER). |
| 4) | Also in April 2010, using state-of-the-art human gastrointestinal tract simulations and microbiological analyses, scientists from Ghent University and ProDigest (a company that specializes in gastrointestinal research) published their findings in the International Journal of Food Microbiology, which demonstrated that Ambrotose® complex and Advanced Ambrotose® powder exerted positive prebiotic effects. Both products were well-fermented throughout the entire simulated colon and they encouraged the growth of healthful Bifidobacteria species. The products also appeared to enhance the growth of species belonging to a group of bacteria called Bacteroidetes, which are associated with body weight management. |
| 5) | In March 2010, the Journal of the International Society of Sports Nutrition published a randomized, double-blind, placebo-controlled, crossover trial investigating the effect of creatine-free EM•PACT® sports drink on the aerobic performance of 29 healthy college students. This study, led by Professor Allyn Byars at Angelo State University, showed that a single serving of creatine-free EM•PACT® sports drink significantly improved subjects’ maximal oxygen consumption, time to exhaustion, and estimated non-protein fat substrate utilization. |
| 6) | In January 2010, Developmental Neuropsychology published a randomized, double-blind, placebo-controlled study led by Dr. Talitha Best at Flinders University. This 12-week study found that healthy middle-aged adults who took one teaspoon of Ambrotose® complex twice daily performed significantly better on memory tasks and overall had a more positive mood. |
6
To support our research and development efforts, we have strategic alliances with our suppliers, consultants, and manufacturers, which allow us to effectively identify and develop high-quality, innovative, proprietary products that increase our competitive advantage in the marketplace.
Our research and development efforts include developing and maintaining quality standards, supporting development efforts for new ingredients and compounds, and improving or enhancing existing products or ingredients. In addition, our research and development team identifies other quality-driven suppliers and manufacturers for both our global and regional needs.
| 3. | Quality Assurance Program. We use qualified manufacturing contractors to produce, test, and package our finished products. These contractors must strictly adhere to our quality standards for all markets. Our quality assurance program is designed to comply with the following regulations: |
| · | the FDA’s current Good Manufacturing Practice for manufacturing, packaging, labeling, or holding operations for dietary supplements; |
| · | the FDA’s Good Manufacturing Practices for human food; |
| · | the requirements of the Natural Health Products Directorate of Canada; |
| · | the Korean Food and Drug Administration; and |
| · | Certification by the Therapeutic Goods Administration of Australia (“TGA”) when necessary. |
We have established a quality assurance program designed to ensure compliance with regulatory requirements and to ensure that proper controls are maintained in the manufacturing, evaluation, packaging, storage, and distribution of our products. These controls include a comprehensive supplier quality program that requires frequent audits and surveillances, third-party certifications, and product monitoring.
A team of professionals, many of whom have extensive experience in the pharmaceutical industry, leads our in-house quality assurance program and continually monitors the quality assurance aspects of our products, including the production process. Our quality assurance professionals develop quality standards for raw material, components and products, and perform tests and inspections to ensure that products are safe and of high quality.
We require our dietary supplements to be packaged with seals to help minimize the risk of tampering. We also perform stability studies under controlled and accelerated temperature storage conditions to ensure the accuracy of the shelf life of our products.
We submit our products for testing by independent laboratories:
| · | Nine products are certified according to the NSF/ANSI 173 Dietary Supplement Standard—the only American National Standard for dietary supplements. This certification ensures that this product contains only the ingredients indicated on the label and is free of impurities, and that Good Manufacturing Practices were used in the manufacturing facility. |
| · | Ten products are certified Kosher by OU Kosher, the trademark with the highest certification standards worldwide. |
| · | Twenty-six products are certified gluten-free by Covance Laboratories. |
7
| 4. | High-Caliber, Industry-Leading Independent Associates. Our global team of independent associates is comprised of dedicated, hard working, high-caliber, compliance-oriented individuals, many of whom have been associated with the network marketing industry for decades and have been loyal to us since our beginning in 1993. To capitalize on their wealth of knowledge and experience, we sponsor a panel of independent associates, called the “North American Associate Advisory Council”, and a panel of international independent associates, called the “Global Advisory Council” (collectively called the “Advisory Council”), which help identify and effectively relay the needs of our independent business-building associates to us. The members of the Advisory Council are elected by their peers and serve a three-year term. The Advisory Council meets periodically with our team of senior management to recommend changes, discuss issues, and provide new ideas or concepts, including a full spectrum of innovative ideas for additional quality-driven nutritional supplements aimed at maintaining optimal health and wellness. |
| 5. | Support Philosophy for Our Independent Associates and Members. We are fully committed to providing the highest level of support services to our independent associates and members and believe that we meet expectations and build customer loyalty through the following: |
| · | providing efficient order processing centers to support operations; |
| · | offering highly-personalized and responsive customer service; |
| · | offering a 100% satisfaction guarantee product return policy for the first 180 days following the product’s purchase; |
| · | providing comprehensive corporate websites (http://www.mannatech.com, www.allaboutmannatech.com, www.mannatechscience.org, www.mannathink.com), that provide instant access to Internet ordering, marketing, technical and educational information, and unique and innovative marketing tools; |
| · | offering free personalized websites for our independent associates; |
| · | maintaining an extensive web-based downline management system called Success Tracker™ that provides access to web conferencing and downline organization reporting for our independent associates at minimal costs; |
| · | offering, in the United States and Canada, a new effective online business tool called MannaCastSM, which includes StoryCastTM, Mannapages®, and Success Tracker, and supports associates in managing and expanding business at minimal costs; |
| · | offering updated training/orientation and compliance programs for our independent associates; |
| · | providing strategically based distribution fulfillment centers to ensure products are shipped on time and at minimal cost; |
| · | inviting customer input on innovative product ideas, which is gathered and tabulated on www.mannathink.com; |
| · | sponsoring comprehensive training about our products and promotional materials, and offering a full spectrum of comprehensive educational materials; and |
| · | sponsoring several marketing events, designed to provide information, education, and motivation for our dedicated business-building associates and to help stimulate business development. These events provide an interactive venue for introducing new products and services and allow interaction between our management teams, outside researchers, and independent associates. |
| 6. | Flexible Operating Strategy. We believe efficiency, focus, and flexibility are paramount to our operations. For over a decade, we have contracted with third parties to supply and manufacture our proprietary raw materials and products, which we believe allows us to minimize capital expenditures, capitalize on such parties’ expertise, and build additional resources for strategic alliances in the areas of distribution and logistics, product registration, and export requirements. By contracting with various suppliers and manufacturers and by outsourcing distribution for all of our foreign operations, except Europe, we believe we can quickly adapt operations to current demands in a timely, efficient, and cost-effective manner. We monitor the performance of our third party contractors to ensure they maintain a high quality of service. In addition, we identify alternative sources for our raw materials suppliers and finished goods manufacturers to help prevent any risk of interruption in production should any existing contractors become unable to perform satisfactorily. |
8
| 7. | Experience and Depth of Our Management Team and Board of Directors. Our management team includes 7 individuals with over 95 years combined experience in the direct selling industry. We believe our team of executives has extensive experience in all aspects of business operations and is highly focused on our success. Our Board of Directors is composed of seven directors, including five independent directors. We believe our board members have a wealth of knowledge and experience in most aspects of our business operations and are especially well versed in network marketing, finance, nutritional products, regulatory matters, and corporate governance. Our entire management team is committed to delivering high-quality products and superior service. |
Business Strategy
Our long-term goal is to be one of the world’s leading network marketing companies founded on the best science-based proprietary products, along with a powerful global independent network distribution model. To achieve our goal, we believe we must focus on the following business priorities:
| · | Attracting New Independent Associates and Retaining Existing Independent Associates. We continually examine our global associate career and compensation plan and periodically offer incentives, such as our annual travel incentives, in order to attract, motivate, and retain independent associates. We believe our global associate career and compensation plan encourages greater associate retention, motivation, and productivity. |
| · | Carefully Planning and Executing New Market Entries. In order to expand efficiently around the globe, we must continue to present maximum opportunity to our current associates as well as those who will join us in the future. |
| · | Developing New Products and Enhancing Existing Products. We continue to focus on new areas for future product development. We continue our research efforts and strive to ensure that all of our products are made from high quality, effective ingredients that contain one or more of our proprietary compounds, which we believe supports our goal to be a cutting-edge industry leader. We expect that any future products we develop will further complement and enhance our existing products. |
| · | Strengthening our Financial Results and Adding Value to Our Shareholders and Independent Associates. We focus on improving financial results by striving to increase our revenues in both our domestic and foreign operations and to control our operating costs. |
Intellectual Property
Trademarks. We pursue registrations for all trademarks associated with our key products and protection of our legal rights concerning our trademarks. As of December 31, 2010, we had 41 registered trademarks in the United States and three trademark applications pending with the United States Patent and Trademark Office. At December 31, 2010, we also had 465 registered trademarks in 31 countries and 80 trademark applications pending in foreign jurisdictions. Globally, the protection available in foreign jurisdictions may not be as extensive as the protection available to us in the United States. Where available, we rely on common law trademark rights to protect our unregistered trademarks, even though such rights do not provide us with the same level of protection as afforded by a United States federal trademark registration. Common law trademark rights are limited to the geographic area in which the trademark is actually used. A United States federal trademark registration enables us to stop infringing use of the trademark by a third party anywhere in the United States provided the unauthorized third party user does not have superior common law rights in the trademark within a specific geographical area of a particular state or region prior to the date our mark federally registers.
Patents. We applied for patent protection in various countries for formulations and use of compositions and methods that relate to our Ambrotose® complex. As of December 31, 2010, we had obtained 48 patents for technology related to the Ambrotose® formulation, five of which are in the United States and the remainder of which are in 29 foreign jurisdictions. We have one Manapol patent in Canada. We also have 15 pending patent applications in the United States; two relate to our Ambrotose® complex technology and four of relate to our antioxidant technology. The other patent applications relate to (i) PhytoMatrix®, a vitamin and mineral supplement; (ii) our Rapid Saccharide Biomarker Assay; (iii) our Processing of Natural Saccharide Polysaccharides (Probiotic and Prebiotic); and (iv) Soluable Fiber patent. We have 103 patent applications pending in 29 foreign jurisdictions. Depending on the jurisdiction, an issued patent grants us certain rights to prevent others from making, offering to sell, using, importing or selling the patented subject matter for the term of the patent. The exclusionary rights of these patents are national in scope. Until a patent is approved and issued, we cannot exclude others from making, using, selling, offering to sell, or importing a product that falls within the scope of the claims in the application.
9
Associate Distribution System
Overview. Our sales philosophy is to distribute our products through network marketing channels where consumers purchase products for personal consumption or resale. Members purchase our products for personal use at a discounted retail value, but do not participate in our global associate career and compensation plan. Independent associates purchase our products at a discounted wholesale value and are eligible to participate in our global associate career and compensation plan. All of our associates are independent contractors. We provide each new independent associate with our policies and procedures that require the independent associates to comply with regulatory guidelines and act in a consistent and professional manner.
Our revenues are heavily dependent upon the retention and productivity of independent associates who help us achieve long-term growth. We believe the introduction of new innovative incentives, such as travel incentives, will continue to motivate our independent associates and help expand our global purchasing base. We remain actively committed to expanding the number of our independent associates through recruitment, support, motivation, and incentives. We had approximately 403,000 and 513,000 independent associates and members purchasing our products and packs within the 12 months ended December 31, 2010 and 2009, respectively.
We offer a 10% discount to independent associates and members who enroll in our automatic monthly order program to promote operating efficiencies, through which our independent associates can receive a standing order every four weeks and our members can receive a standing order once a month. Automatic monthly orders, on average, account for approximately 80% of our total orders placed during a calendar month.
Independent Associate Development. Network marketing consists of enrolling individuals who build a network of independent associates, members, and retail customers who purchase products. We support our independent associates by providing an array of support services that can be tailored to meet individual needs, including:
| · | offering educational meetings and corporate-sponsored events that emphasize business-building and compliance related information; |
| · | sponsoring various informative and science-based conference calls, web casts, and seminars; |
| · | providing automated services through the Internet and telephone that offer a full spectrum of information and business-building tools; |
| · | maintaining an efficient decentralized ordering and distribution system; |
| · | providing highly personalized and responsive order processing and customer service support accessible by multiple communication channels including telephone, Internet, or e-mail; |
| · | offering 24-hour, seven days a week access to information and ordering through the Internet; |
| · | offering Success TrackerTM, a customized business-building genealogy system, which contains graphs, maps, alerts, reports, and web video conferencing for our independent associates; |
| · | offering the MannaCastSM suite of online business tools in the United States and Canada, which includes Success TrackerTM, MannaPages®, and StoryCastTM; and |
| · | providing a wide assortment of business-building and educational materials to help stimulate product sales and simplify enrollment. |
We provide product and network marketing training and education for new independent associates. For example, we offer a unique global orientation/training program that integrates audio, video, and graphics so that associates can customize their own marketing and training program. This training program provides systematic and uniform training related to our products and related global regulatory requirements, global associate career and compensation plan, and various methods of conducting business, including ethics and compliance. We also offer a variety of brochures, monthly newsletters, and other promotional materials to associates to assist in their sales efforts, training, and continuing education. We continually update our training and promotional materials to provide our associates with the most current information and motivational tools.
Our global associate career and compensation plan consists of eight independent associate achievement levels; from lowest to highest, these include regional, national, executive, presidential, bronze, silver, gold, and platinum. These achievement levels are determined by the growth and volume of the independent associates’ direct and indirect
10
Based upon our knowledge of industry-related network marketing compensation plans, we believe our global associate career and compensation plan remains strong in the industry and is currently among the most financially rewarding plans offered. Together, our commissions and incentives range from 44% to 50% of our consolidated net sales.
Our global associate career and compensation plan pays various types of commissions and incentives based upon a point system that calculates a percentage of the independent associate’s commissionable direct and indirect net sales and the attainment of certain associate achievement levels. All payments to our independent associates are made after they have earned their commissions. We believe our global associate career and compensation plan fairly compensates our independent associates at every stage of building their business by quickly rewarding an independent associate for both the breadth and depth of their global seamless downline structure.
Our global associate career and compensation plan identifies and pays 17 types of incentive commissions to our qualified independent associates, which are based on the following:
| · | generating product sales from an independent associate’s global downline to earn certain achievement levels; |
| · | enrolling new independent associates or members who place a product order; |
| · | obtaining certain achievement levels and enrolling other independent associates in a downline who place monthly automatic orders; |
| · | obtaining and developing certain achievement levels within their downline organizations to qualify for additional bonuses; |
| · | building a team of six qualified independent associates in their global downlines who order products regularly; and |
| · | various other incentive programs, including periodic travel incentives. |
Management of Independent Associates. We actively monitor our independent associates’ sales of our products and the promotion of certain business opportunities by requiring our independent associates to abide by our policies and procedures. However, we have limited control over the actions of our independent associates. To aid in our monitoring efforts, we provide each independent associate with a copy of our policies and procedures prior to or upon signing up as an independent associate. We also use various media formats to distribute changes to our mandatory policies and procedures, including our corporate website, conference calls, educational meetings, corporate events, seminars, and webcasts.
Our legal/compliance department, in cooperation with other departments and associates, periodically evaluates the conduct of our independent associates and the need for new or revised policies and procedures. Our monitoring efforts include reviewing associates’ websites, promotional materials, and meetings. Our legal/compliance program assists in maintaining high ethical standards among our independent associates, which helps our independent associates in their sales efforts.
To help manage our associates, our legal/compliance department periodically monitors independent associates’ websites for content. In addition, associates may use our anonymous compliance reporting system to report non-compliant websites to the compliance department, which then further investigates such websites. In an effort to decrease the number of independent websites owned by our independent associates and to preserve and protect our trademarks, we offer a standardized personal Mannapages® Internet website, which helps our independent associates with their sales efforts and provides consistent, standardized information, and education.
11
Our legal/compliance program also provides our independent associates with a standardized and anonymous complaint process. When a complaint is filed against an independent associate, our legal/compliance department conducts a mandatory investigation of the allegations, if applicable. Depending on the nature of the violation, we may suspend or terminate the non-compliant associate’s agreement or we may impose various sanctions, including written warnings, probation, withholding commissions, and termination of associate status. We will terminate any associate’s agreement for making claims that our products can treat, cure, mitigate or prevent any disease, unless such claim is de minimus and isolated.
Product Return Policy. We stand behind our packs and products and believe we offer a reasonable and industry-standard product return policy to all of our customers. We do not resell returned products. Refunds are not processed until proper approval is obtained. All refunds must be processed and returned in the same form of payment that was originally used in the sale. Each country in which we operate has specific product return guidelines. However, we allow our independent associates and members to exchange products as long as the products are unopened and in good condition. Our return policies for our retail customers and our independent associates and members are as follows:
| · | Retail Customer Product Return Policy. This policy allows a retail customer to return any of our products to the original independent associate who sold the product and receive a full cash refund by the independent associate for the first 180 days following the product’s purchase if located in the United States, Canada, and South Africa, and for the first 90 days following the product’s purchase in the remaining countries. The independent associate may then return or exchange the product based on the independent associate product return policy. |
| · | Independent Associate and Member Product Return Policy. This policy allows the independent associate or member to return an order within one year of the purchase date upon terminating his/her account. If an independent associate or member returns a product unopened and in good condition, he/she may receive a full refund minus a 10% restocking fee. We may also allow the independent associate or member to receive a full satisfaction guarantee refund if they have tried the product and are not satisfied for any reason, excluding promotional materials. This satisfaction guarantee refund applies in the United States, Canada, and South Africa only for the first 180 days following the product’s purchase, and applies in the remaining countries for the first 90 days following the product’s purchase; however, any commissions earned by an independent associate will be deducted from the refund. If we discover abuse of the refund policy, we may terminate the independent associates’ or member’s account. |
Information Technology Systems
Our information technology and e-commerce systems include a transaction-processing database, financial systems, an associate management system, and comprehensive management tools that are designed to:
| · | minimize the time required to process orders and distribute products; |
| · | provide customized ordering information; |
| · | quickly respond to information requests, including providing detailed and accurate information to independent associates about qualification and downline activity; |
| · | provide detailed reports about paid commissions and incentives; |
| · | support order processing and customer service departments; and |
| · | help monitor, analyze, and report operating and financial results. |
To complement our transaction database, we developed a comprehensive management tool called Success Tracker™ that is used both internally and by our independent associates to manage and optimize their business organizations. With this tool, independent associates have constant access to graphs, maps, alerts, and reports on the status of their individual organizations, which helps to optimize their earnings.
We also maintain a written service continuity disaster recovery plan that was developed using the guidelines published by the National Institute of Standards of Technology to minimize the risk of loss due to any interruption in business. Our disaster recovery plan encompasses all critical aspects of our business and identifies contacts and resources. Additionally, we perform daily backup procedures and proactively monitor various software, hardware, and network infrastructure systems. We also perform routine maintenance procedures and periodically upgrade our software and hardware to help ensure that our systems work efficiently and effectively and to minimize the risk of business interruption.
12
We continue to enhance our information technology, websites, and e-commerce platforms to remain competitive and efficient. We launched a new website in 2009, which we believe has been more reliable and was designed to be more user-friendly than our preceding one. At December 31, 2010 and 2009, net capitalized software cost balances were $9.0 million and $15.7 million, respectively.
Government Regulations
Domestic Regulations. In the United States, governmental regulations, laws, administrative determinations, court decisions, and similar legal requirements at the federal, state, and local levels regulate companies, such as ours, and network marketing activities. Such regulations address, among other things:
| · | direct selling and network marketing systems; |
| · | transfer pricing and similar regulations affecting the amount of foreign taxes and customs duties paid; |
| · | taxation of independent associates and requirements to collect taxes and maintain appropriate records; |
| · | how a company manufactures, packages, labels, distributes, imports, sells, and stores products; |
| · | product ingredients; |
| · | product claims; |
| · | advertising; and |
| · | the extent to which companies may be responsible for claims made by independent associates. |
The following governmental agencies regulate various aspects of our business and our products in the United States:
| · | the FDA; |
| · | the Federal Trade Commission (“FTC”); |
| · | the Consumer Product Safety Commission; |
| · | the Department of Agriculture; |
| · | the Environmental Protection Agency; |
| · | the United States Postal Service; |
| · | state attorney general offices; and |
| · | various agencies of the states and localities in which our products are sold. |
The FDA regulates the formulation, manufacturing, packaging, storage, labeling, promotion, distribution, and sale of foods, dietary supplements, over-the-counter drugs, medical devices, and pharmaceuticals. In January 2000, the FDA issued a final rule called “Statements Made for Dietary Supplements Concerning the Effect of the Product on the Structure or Function of the Body”. In the rule and its preamble, the FDA distinguished between permitted claims under the Federal Food, Drug and Cosmetic Act (“the Act”) relating to the effect of dietary supplements on the structure or functions of the body, and impermissible direct or implied claims of the effect of dietary supplements on any disease. In June 2007, the FDA issued a rule, as authorized under the Act, that defined current Good Manufacturing Practices in the manufacture and holding of dietary supplements. Effective January 1, 2006, legislation required specific disclosures in labeling where a food, including a dietary supplement, contains an ingredient derived from any of eight named allergens. Legislation passed at the end of 2006 now requires us to report to the FDA any reports of “serious adverse events” associated with the use of a dietary supplement or an over-the-counter drug that is not covered by new drug approval reporting.
The Dietary Supplement Health and Education Act of 1994, referred to as DSHEA, revised the provisions of the Federal Food, Drug and Cosmetic Act concerning the composition and labeling of dietary supplements and statutorily created a new class entitled “dietary supplements.” Dietary supplements include vitamins, minerals, herbs, amino acids, and other dietary substances used to supplement diets. A majority of our products are considered dietary supplements as outlined in the Act, which requires us to maintain evidence that a dietary supplement is reasonably safe. A manufacturer of dietary supplements may make statements concerning the effect of a supplement or a dietary ingredient on the structure or any function of the body, in accordance with the regulations described above. As a result, we make such statements with respect to our products. In some cases, such statements must be accompanied by a statutory statement that the claim has not been evaluated by the FDA, and the product is not intended to treat, cure, mitigate, or prevent any disease, and the FDA must be notified of such claim within 30 days of first use.
13
The FDA oversees product safety, manufacturing, and product information, such as claims on a product’s label, package inserts, and accompanying literature. The FDA has promulgated regulations governing the labeling and marketing of dietary and nutritional supplement products. The regulations include:
| · | the identification of dietary or nutritional supplements and their nutrition and ingredient labeling; |
| · | requirements related to the wording used for claims about nutrients, health claims, and statements of nutritional support; |
| · | labeling requirements for dietary or nutritional supplements for which “high potency,” “antioxidant,” and “trans-fatty acids” claims are made; |
| · | notification procedures for statements on dietary and nutritional supplements; and |
| · | pre-market notification procedures for new dietary ingredients in nutritional supplements. |
We develop and maintain product substantiation dossiers, which contain the scientific literature pertinent to each product and its ingredients. An independent scientist reviews these dossiers, which provide the scientific basis for product claims. We periodically update our substantiation program for evidence for each of our product claims and notify the FDA of certain types of performance claims made in connection with our products.
In certain markets, including the United States, specific claims made with respect to a product may change the regulatory status of a product. For example, a product sold as a dietary supplement but marketed as a treatment, prevention, or cure for a specific disease or condition would likely be considered by the FDA or other regulatory bodies as unapproved and thus an illegal drug. To maintain the product’s status as a dietary supplement, its labeling and marketing must comply with the provisions in DSHEA and the FDA’s extensive regulations. As a result, we have procedures in place to promote and assure compliance by our employees and independent associates related to the requirements of DSHEA, the Food, Drug and Cosmetic Act, and various other regulations.
Dietary supplements are also subject to the Nutrition, Labeling and Education Act and various other acts that regulate health claims, ingredient labeling, and nutrient content claims that characterize the level of nutrients in a product. These acts prohibit the use of any specific health claim for dietary supplements unless the health claim is supported by significant scientific research and is pre-approved by the FDA.
The FTC and other regulators regulate marketing practices and advertising of a company and its products. In the past several years, regulators have instituted various enforcement actions against numerous dietary supplement companies for false and/or misleading marketing practices, as well as misleading advertising of products. These enforcement actions have resulted in consent decrees and significant monetary judgments against the companies and/or individuals involved. Regulators require a company to convey product claims clearly and accurately and further require marketers to maintain adequate substantiation for their claims. More specifically, the FTC requires such substantiation to be competent and reliable scientific evidence and requires a company to have a reasonable basis for the expressed and implied product claim before it disseminates an advertisement. A reasonable basis is determined based on the claims made, how the claims are presented in the context of the entire advertisement, and how the claims are qualified. The FTC’s standard for evaluating substantiation is designed to ensure that consumers are protected from false and/or misleading claims by requiring scientific substantiation of product claims at the time such claims are first made. The failure to have this substantiation violates the Federal Trade Commission Act.
Due to the diverse scope of regulations applicable to our products and the various regulators enforcing these requirements, determining how to conform to all requirements is often open to interpretation and debate. However, our policy is to fully cooperate with any regulatory agency in connection with any inquiries or other investigations. We can make no assurances that regulators will not question our actions in the future, even though we continue to make efforts to comply with all applicable regulations, inquiries, and investigations.
14
International Regulations. We are also subject to extensive regulations in each country in which we operate. Currently we sell our products in Canada, Australia, the United Kingdom, Japan, New Zealand, the Republic of Korea, Taiwan, Denmark, Germany, South Africa, Singapore, Austria, the Netherlands, Norway, Sweden and, beginning January 24, 2011, Mexico. Some of the country-specific regulations include the following:
| · | the National Provincial Laws, Natural Health Product Regulations of Canada, and the Federal Competition Act in Canada; |
| · | the Therapeutic Goods Administration and the Trade Practices Act in Australia; |
| · | federal and state regulations in Australia; |
| · | national regulations including the Local Trading Standards Offices in the United Kingdom; |
| · | regulations from the Ministry of International Trade and Industry in Japan; |
| · | regulations from the Commerce Commission and the Fair Trade Act of 1993 in New Zealand; |
| · | the Fair Trade Commission, which oversees the Door to Door Sales Act and the Health and Functional Food Act enforced by the Korea Food and Drug Administration in the Republic of Korea; |
| · | the Fair Trade Law, which is enforced by the Taiwan Fair Trade Commission and the Administration of Food Hygiene, Health Food Products Administration Act enforced by the Taiwan Department of Health; |
| · | the Danish Health Board, the Danish Marketing Practice Act, the Danish Consumer Ombudsman, the Danish Executive Order on Dietary Supplements, the Guidelines for food supplements, and the Danish Act on Foodstuffs in Denmark; |
| · | the German Unfair Competition Act, German Regulation on food supplements, and German Law on food and feed; |
| · | regulations governing business practices in South Africa; |
| · | the Consumer Protection Act, the Sale of Food Act, and various regulations that are governed by the Ministry of Trade and Industry in Singapore; |
| · | the Austrian Trade Law (1994), the Food Safety and Consumer Protection Law (2006), and the Food Code in Austria; |
| · | the Food and Consumer Products and the Unfair Trade Practices Act, Door to Door Selling Act and Provisions of the General Dutch Civil Code relating to terms and conditions and misleading advertising in the Netherlands; |
| · | the Consumer Sales Act, Marketing Practices Act, Distance and Doorstep Sales Act, the Product Liability Act, Product Safety Act, the Companies Act and the Food Act in Sweden; |
| · | the Law on Marketing and Contract Conditions, the Law on Repentance Right, the Statutory Order on Self Inspection of Food Provisions, the Law on Food products and Food Safety, and various guidelines from the Norwegian Consumers Agency on telephone selling and internet marketing, in Norway; |
| · | various European Union (“EU”) regulations and pronouncements address both our selling activities and the sale of food supplements in EU member nations, however, at the current time the local statutes and regulations stated above are ascendant to the EU pronouncements if in conflict; and |
| · | the Health Law and various Official Mexican Standards, the consumer protection law, the Mexican Corporate law, the Foreign Investment Law, the Federal Labor law in Mexico, as well as various municipal and state regulations and codes. |
Regulations Regarding Network Marketing System and Our Products. Our network marketing system and our global associate career and compensation plan are also subject to a number of governmental regulations including various federal and state statutes administered by the FTC, various state authorities, and foreign government agencies. The legal requirements governing network marketing organizations are directed, in part, to ensure that product sales are ultimately made to consumers. In addition, earnings within a network marketing company must be based on the sale of products rather than compensation for (i) the recruitment of distributors or associates, (ii) investments in the organization, or (iii) other non-retail sales-related criteria. For instance, some countries limit the amount associates may earn from commissions on sales by other distributors or independent associates that are not directly sponsored by that distributor or independent
15
As a network marketing company, we are also subject to regulatory oversight, including routine inquiries and enforcement actions, from various United States state attorneys general offices. Each state has specific acts referred to as Little FTC Acts. Each state act is similar to the federal laws. As a result, each state may perform its own inquiries about our organization and business practices, including allegations related to distributors or independent associates. To combat such industry-specific risk, we provide a copy of our published associate policies and procedures to each independent associate, publish these policies on our corporate website, and provide educational seminars and publications. In addition, we maintain a legal/compliance department to cooperate with all regulatory agencies and investigate allegations of improper conduct by our independent associates.
In Canada, our network marketing system is regulated by both national and provincial laws. Under Canada’s Federal Competition Act, we must make sure that any representations relating to compensation to our independent associates or made to prospective new independent associates constitute fair, reasonable, and timely disclosure and that such representations meet other legal requirements of the Federal Competition Act. All Canadian provinces and territories, other than Ontario, have legislation requiring that we register or become licensed as a direct seller within that province to maintain the standards of the direct selling industry and to protect consumers. Some other Canadian provinces require that both we and our independent associates be licensed as direct sellers.
In Australia, our network marketing system is subject to Australia’s federal and local regulations. Our global associate career and compensation plan is designed to comply with Australian law and the requirements of Australia’s Trade Practices Act. The Australian Trade Practices Administration and various other governmental entities regulate our business and trade practices, as well as those of our independent associates. Australia’s Therapeutic Goods Act, together with the Trade Practices Act, regulates any claims or representations relating to our products and our global associate career and compensation plan. An agreement to establish a joint scheme for the regulation of therapeutic products was signed by both the New Zealand and Australian governments in December 2003. The agency was initially expected to begin operating in July 2005, but on July 16, 2007, the New Zealand government announced that it will not proceed with legislation for the establishment of the joint agency because it does not have sufficient support of the New Zealand parliament. However, both the Australian and New Zealand governments remain committed to the vision of the joint agency and are expected to revisit it again in the future. The proposed harmonization of laws and regulatory bodies is anticipated to provide a more consistent approach to dietary supplement laws between the two countries.
In New Zealand, our network marketing system and our operations are subject to regulations of the Commerce Commission and the Ministry of Health, New Zealand Medical Devices Safety Authority, the Unsolicited Goods Act of 1975, the Privacy Act of 1993, and the Fair Trading Act of 1993. These regulations enforce specific kinds of business or trade practices and regulate the general conduct of network marketing companies. The Commerce Commission also enforces the Consumer Guarantees Act, which establishes specific rights and remedies with respect to transactions involving the provisions of goods and services to consumers. Finally, the New Zealand Commerce Commission and the Ministry of Health both enforce the Door-to-Door Sales Act of 1967 and the NZ Medicines Act, which govern the conduct of our independent associates.
In the United Kingdom, our network marketing system is subject to national regulations of the United Kingdom. Our global associate career and compensation plan is designed to comply with the United Kingdom’s national requirements, the requirements of the Fair Trading Act of 1973, the Data Protection Act of 1998, the Trading Schemes Regulations of 1997, and other similar regulations. The U.K. Code of Advertising and Sales Promotion regulates our business and trade practices and the activities of our independent associates, while the Trading Standards Office regulates any claims or representations relating to our operations. Our products are regulated by the Medicines and Healthcare Products Regulatory Agency.
In Japan, our network marketing system, overall business operations, trade practices, global associate career and compensation plan, and our independent associates are governed by Japan’s Door-to-Door Sales Law as enacted in 1976 by the Ministry of International Trade and Industry. Our global associate career and compensation plan is designed to meet Japan’s governmental requirements. Our product claims are subject to the Pharmaceutical Affairs Law, which prohibits the making and publication of “drug effectiveness” claims regarding products that have not received approval from Japan’s Ministry of Health, Welfare and Labor.
In the Republic of Korea, the primary body of law applicable to our operations is the Door-to-Door Sales Act, which governs the behavior of network marketing companies and affiliated distributors. The Door-to-Door Sales Act is
16
In Taiwan, our network marketing system, overall operations and trade practices are governed by the Fair Trade Law and the Consumer Protection Law. Such laws contain a wide range of provisions covering trade practices. Our products are governed by the Taiwan Department of Health and various legislation in Taiwan including the Health Food Control Act of 1999. This Act was enacted to enhance the management and supervision of matters relating to health, food, protecting the health of people and safeguarding the rights and interests of consumers.
In Denmark, the notion of door-to-door selling is prohibited. As a result, under Danish law, the trader is not allowed to contact the consumer at his home, place of work, or other non-public place in order to conclude a contract on certain subjects. However, the prohibition has an exemption when the consumer asks the trader for a contact in writing or upon written prior consent. In addition, the Danish Marketing Practices Act, the Guidelines from the Danish Consumerombudsman and the rules contained in the Danish Consumer Contracts Act govern our network marketing system. There is no requirement for pre-approval of our products in Denmark; however, our products are subject to a yearly inspection carried out by the Food authorities. Further, all our activities are subject to Self Inspection, the results of which are also controlled once a year by the Food authorities. The rules for marketing and sale of dietary supplements are covered by the Danish Executive Order on Food Supplements, as well as by the Danish Act on Foodstuffs and various EU-regulations. Denmark also subjects the marketing of a company’s food supplements to a notification procedure (with a pre-market approval process for certain substances), before a product may be lawfully marketed in Denmark. Full product compliance with all Danish provisions is reviewed by the Food authorities once a year.
In Germany, there is no specific legal regulation covering network marketing company practices. However, under certain circumstances network marketing systems may have to follow the German Unfair Competition Act. Our independent associates’ conduct is subject to the German statute that governs the conduct of a commercial agent. In addition, direct selling operations are governed by the Industrial Code, which requires direct sellers to hold itinerant trader’s cards. The German Regulation on food supplements and the German Law on food and feed govern vitamin and mineral substances and herbs and other substances, respectively.
In South Africa, there are no specific regulations for the network marketing industry. In general, the Consumer Affairs Act 1988, the Competition Act 1998, and the Advertising Standards Authority Code of Advertising Practice (a voluntary code enforced by the media) govern business practices. The products are classified as complementary medicines for which there are no specific regulations. The Foodstuffs, Cosmetics and Disinfectants Act 1972, and the Medicines and Related Substances Act 1965, currently apply.
In Singapore, the network marketing industry is governed by the Multi-Level Marketing and Pyramid Selling (Prohibition) (Amendment) Act and the accompanying Pyramid Selling (Excluded Schemes and Arrangements) Order 2000 and Order 2001. General business practices and advertising are regulated under the Consumer Protection (Fair Trading) Act 2003, as amended, and its accompanying regulations. The products are classified as food and supplements of a food nature, which are governed by the Sale of Food Act and the Singapore Food Regulations. Cosmetics and products which rise to the level of medicinal and other health-related products are regulated under various regulations such as the Medicines Act, the Poisons Act, the Sale of Drugs Act, the Medicines (Advertisement and Sale) Act and the Misuse of Drug Regulations.
In Austria, the Austrian Trade Law of 1994 (Novelle 2002) prohibits the offer of direct sale to an individual consumer of food supplement and cosmetic products. The provision, however, has generally not been enforced in recent years and sales made via the Internet or mail order or made to a non-consumer distributor do not fall under this prohibition. The Austrian Trade Law is predominantly administered through the National Ministry of Economy and Labor. Our business operations within Austria are conducted from beyond the borders of Austria which is the common practice in our industry. Our distributors qualify as “traders” for purposes of Austrian state and municipal laws. Traders are regulated by the local chambers of commerce and must obtain licenses from the respective chambers of commerce. Regulation of food supplements and cosmetics is generally harmonized throughout the EU and must conform to EU standards. Austrian-specific food regulations include the Food Safety and Consumer Protection Law (2006), supporting ordinances to this law, the Food Supplement Law, and the Austrian Food Codex, which is primarily administered by the National Ministry of Health, Office for Health and Food Security, and the Local Health Authority.
In Sweden various provisions of the Consumer Sales Act (1990), the Marketing Practices Act (2008), the Distance and Doorstep Sales Act (2005), the Product Liability Act (1992), the Product Safety Act (2004), and the Companies Act (2005) all serve to govern our multi-level marketing (“MLM”) and business activities. The Food Act (2006) provides regulations and guidelines for the sale of food and food supplements. We are subject to the authority of
17
In the Netherlands, the Food and Consumer Product and the Unfair Trade Practices Act are the most relevant legislations relating to our business practices. The first is enforced by the Food and Consumer Product Safety Authority and the latter is enforced by the Consumer Authority. Furthermore, various EU regulations apply as well as the Dutch Door to Door Selling Act, and all provisions of the Dutch Civil Code with particular emphasis to those regulations dealing with general terms and conditions, and those regarding misleading advertising.
Norway exercises a border control of products and their composition upon importation. Import products must be registered in an Import Reporting Registry, and the regulations are enforced by the customs authorities. Our products must be compliant with Norwegian regulations in order to be admitted for admission through customs into Norway. In Norway Door-to-Door Selling is allowed, provided the Guidelines from the Norwegian Consumer Agency are followed. Likewise, telephone-selling is allowed provided the agency’s guidelines are followed. Home selling in Norway is also allowed. All of our sales in Norway are subject to a 14-day right to cancel by the consumers.
In Mexico, as in many other markets, there are no specific regulations directly related to the direct selling or network marketing industry. However, all product sales and business offerings must comply with the Consumer Protection Law, which is enforced by the Consumer Protection Agency. Food supplements and medicines are subject to the Health Law and various Official Mexican Standards, which are enforced by the Health Ministry and The Federal Commission for Protection Against Sanitary Risk. Mexican Customs Law and its regulations govern the general importation of our products into Mexico. We are subject to the Mexican Corporate Law, which is enforced by the Mexican courts and to the Federal Labor Law enforced by the Labor Courts. As a company with presence, and transacting business, in Mexico, we are subject to the Foreign Investment Law and its regulations administered by the Ministry of Economy. We are required to register before the Mexican System for Business Information at the appropriate Business Chamber under the Organizations Law.
Other Regulations. Our operations are also subject to a variety of other regulations, including:
| · | social security taxes; |
| · | value added taxes; |
| · | goods and services taxes; |
| · | sales taxes; |
| · | consumption taxes; |
| · | income taxes; |
| · | customs duties; |
| · | employee/independent contractor regulations; |
| · | employment, service pay, retirement pay, and profit sharing requirements; |
| · | import/export regulations; |
| · | federal securities laws; and |
| · | antitrust laws. |
In many markets, we are limited by the types of rules we can impose on our independent associates, including rules in connection with cooling off periods and termination criteria. If we do not comply with these requirements, we may be required to pay social security, unemployment benefits, workers’ compensation, or other tax or tax-type assessments on behalf of our independent associates and may incur severance obligations if we terminate one of our independent associates.
In some countries, including the United States, we are also governed by regulations concerning the activities of our independent associates. Regulators may find that we are ultimately responsible for the conduct of our independent associates and may request or require that we take additional steps to ensure that our independent associates comply with these regulations. The types of conduct governed by these types of regulations may include:
18
| · | claims made about our products; |
| · | promises or claims of income or other promises or claims by our independent associates; and |
| · | sales of products in markets where the products have not been approved or licensed. |
In some markets, including the United States, improper product claims by independent associates could result in our products being overly scrutinized by regulatory authorities. This review could result in our products being re-classified as drugs or classified into another product category that requires stricter regulations or labeling changes.
We continuously research and monitor the laws governing the conduct of our independent associates, our operations, our global associate career and compensation plan, and our products and sales aids within each of the countries in which we sell our products. We provide education for our independent associates regarding acceptable business conduct in each market through our policies and procedures for independent associates’, seminars, and other training materials and programs. However, we cannot guarantee that our independent associates will always abide by our policies and procedures and/or act in a professional and consistent manner.
Competition
Other Nutritional Supplement Companies. The nutritional supplement industry is steadily gaining momentum and is intensely competitive. Our current direct competitors selling similar nutritional products include:
| · | Herbalife Ltd.; |
| · | Market America, Inc.; |
| · | Nature’s Sunshine Products, Inc.; |
| · | Nu Skin Enterprises, Inc.; |
| · | Reliv, International Inc; |
| · | Solgar Vitamin and Herb Company, Inc.; |
| · | Usana Health Sciences, Inc.; |
| · | Weider Nutrition; and |
| · | Schiff Nutrition International, Inc. |
Network marketing. Nutritional supplements are offered for sale in a variety of ways. Network marketing has a limited number of individuals interested in participating in the industry, and we must compete for those types of individuals. We believe network marketing is the best sales approach to sell our products for the following reasons:
| · | our products can be introduced into the global marketplace at a much lower up-front cost than through conventional methods; |
| · | our key ingredients and differential components found in our proprietary products can be better explained through network marketing; |
| · | the network marketing approach can quickly and easily adapt to changing market conditions; |
| · | consumers appreciate the convenience of ordering from home, through a sales person, by telephone, or on the Internet; and |
| · | network marketing enables independent associates to earn financial rewards. |
19
We compete with other direct selling and network marketing companies for new independent associates and for retention of continuing independent associates. Some of our competitors have longer operating histories, are better known, or have greater financial resources. These companies include:
| · | Amway Corporation; |
| · | Body Wise International, Inc.; |
| · | Envion International; |
| · | Forever Living Products, Inc.; |
| · | Herbalife International, Inc.; |
| · | Mary Kay, Inc.; |
| · | Nature’s Sunshine Products, Inc.; |
| · | New Vision International; |
| · | Nu Skin Enterprises, Inc.; |
| · | Reliv, International Inc.; |
| · | Shaklee Worldwide; and |
| · | Usana Health Sciences, Inc. |
| · | Schiff Nutrition International Inc. |
The availability of independent associates decreases when other network marketing companies successfully recruit and retain independent associates for their operations. We believe we can successfully compete for independent associates by emphasizing the following:
| · | our unique patented, proprietary blend of high-quality products; |
| · | our 17-year track record in the business of selling nutritional products; |
| · | our model which does not require our independent associates to carry inventory or accounts receivable; |
| · | our unique and financially rewarding global associate career and compensation plan; |
| · | our innovative marketing and educational tools; and |
| · | our easy and convenient delivery system. |
Employees
At December 31, 2010, we employed 490 people around the world, as set forth below:
| 2010 | 2009 | |||
| United States | 338 | 337 | ||
| Australia | 36 | 38 | ||
| United Kingdom | 30 | 34 | ||
| Japan | 28 | 33 | ||
| Republic of Korea | 27 | 34 | ||
| Taiwan | 18 | 17 | ||
| Switzerland | 6 | 7 | ||
| Mexico | 4 | — | ||
| Canada | 2 | 1 | ||
| South Africa | 1 | 1 | ||
| Total | 490 | 502 | ||
These numbers do not include our independent associates, who are independent contractors and are not considered employees.
20
Item 1A. Risk Factors
In addition to the other risks described in this report, the following risk factors should be considered in evaluating our business and future prospects:
| 1. If we are unable to attract and retain independent associates, our business may suffer. |
Our future success depends largely upon our ability to attract and retain a large active base of independent associates and members who purchase our packs and products. We cannot give any assurances that the number of our independent associates will continue at their current levels or increase in the future. Several factors affect our ability to attract and retain independent associates and members, including:
| · | on-going motivation of our independent associates; |
| · | general economic conditions; |
| · | significant changes in the amount of commissions paid; |
| · | public perception and acceptance of the wellness industry; |
| · | public perception and acceptance of network marketing; |
| · | public perception and acceptance of our business and our products, including any negative publicity; |
| · | the limited number of people interested in pursuing network marketing as a business; |
| · | our ability to provide proprietary quality-driven products that the market demands; and |
| · | competition in recruiting and retaining independent associates. |
2. The loss of key high-level independent associate leaders could negatively impact our associate growth and our revenue. |
As of December 31, 2010, we had approximately 403,000 independent associates and members who purchased our products within the last 12 months, of which 201 occupied the highest associate level under our global compensation plan. These independent associate leaders are important in maintaining and growing our revenue. As a result, the loss of a high-level independent associate or a group of leading associates in the independent associates’ networks of downlines, whether by their own choice or through disciplinary actions by us for violations of our policies and procedures, could negatively impact our associate growth and our revenue.
| 3. If our international markets are not successful, our business could suffer. |
We currently sell our products in the international markets of Canada, Australia, the United Kingdom, Japan, New Zealand, the Republic of Korea, Taiwan, Denmark, Germany, South Africa, Singapore, Austria, the Netherlands, Norway, Sweden and, beginning January 24, 2011, Mexico. Nonetheless, our international operations could experience changes in legal and regulatory requirements, as well as difficulties in adapting to new foreign cultures and business customs. If we do not adequately address such issues, our international markets may not meet growth expectations. Our international operations and future expansion plans are subject to political, economic, and social uncertainties, including:
| · | inflation; |
| · | the renegotiation or modification of various agreements; |
| · | increases in custom duties and tariffs; |
| · | changes and limits in export controls; |
| · | government regulations and laws; |
| · | trademark availability and registration issues; |
| · | changes in exchange rates; |
| · | changes in taxation; |
| · | wars and other hostilities; and |
21
| · | changes in the perception of network marketing. |
Any negative changes related to these factors could adversely affect our business, profitability, and growth prospects. Furthermore, any negative changes in our distribution channels may force us to invest significant time and money related to our distribution and sales to maintain our position in certain international markets.
4. If we are unable to protect our proprietary rights of our products, our business could suffer.
Our success and competitive position largely depends on our ability to protect the following proprietary rights:
| · | Our Ambrotose® complex, a glyconutritional dietary supplement ingredient consisting of a blend of monosaccharides, or sugar molecules, used in the majority of our products; |
| · | The MTech AO Blend®, our proprietary, patent-pending antioxidant used in the Ambrotose AO® complex; and |
| · | A compound used in our reformulated Advanced Ambrotose® complex that allows for a more potent concentration of the full range of mannose-containing polysaccharides occurring naturally in aloe. |
We have filed patent applications for the technology relating to Ambrotose®, Ambrotose AO®, Phytomatrix®, Gi-ProBalance ™ and FiberSlim® in the United States and certain foreign countries. As of December 31, 2010, we had received 48 patents for the technology relating to Ambrotose® complex, five of which were issued in the United States and the remainder in 29 foreign jurisdictions. In addition, we have entered into confidentiality agreements with our independent associates, suppliers, manufacturers, directors, officers, and consultants to help protect our proprietary rights. Nevertheless, we continue to face the risk that our patent applications for each of these products will be denied or that the patent protection we are granted is more limited than originally requested. As a precaution, we consult with outside legal counsel and consultants to help ensure that we protect our proprietary rights. However, our business, profitability, and growth prospects could be adversely affected if we fail to receive adequate protection of our proprietary rights.
| 5. Adverse or negative publicity could cause our business to suffer. |
Our business depends, in part, on the public’s perception of our integrity and the safety and quality of our products. Any adverse publicity could negatively affect the public’s perception about our industry, our products, or our reputation and could result in a significant decline in our operations and/or the number of our independent associates. Specifically, we are susceptible to adverse or negative publicity regarding:
| · | the nutritional supplements industry; |
| · | skeptical consumers; |
| · | competitors; |
| · | the safety and quality of our products and/or our ingredients; |
| · | regulatory investigations of our products or our competitors’ products; |
| · | the actions of our independent associates; |
| · | the direct selling/network marketing industry; and |
| · | scandals within the industries in which we operate. |
For instance, on July 5, 2007, the Texas Attorney General filed suit against us, MannaRelief Ministries, Samuel L.Caster, the Fisher Institute, and H. Reginald McDaniel alleging violations of the Texas Deceptive Trade Practices Act and the Texas Food, Drug and Cosmetic Act. We subsequently reached an agreement with the Texas Attorney General’s office settling an enforcement action against us, our former Chief Executive Officer and Chairman of the Board, Samuel L. Caster, and others. Without admitting any wrongdoing, we agreed to refund up to $4 million to certain members and pay $2 million to cover fees and expenses of Texas regulators. The settlement did not include any fine or penalty against the Company. We have also taken a number of actions to address concerns raised by the Texas Attorney General’s action. Although the matter has been resolved, the lawsuit created a substantial amount of adverse publicity that may have had and may continue to have a negative impact on our business.
22
| 6. If we are exposed to product liability claims, we may be liable for damages and expenses, which could affect our overall financial condition. |
We could face financial liability from product liability claims if the use of our products results in significant loss or injury. We can make no assurances that we will not be exposed to any substantial future product liability claims. Such claims may include claims that our products contain contaminants, that we provide our independent associates and consumers with inadequate instructions regarding product use, or that we provide inadequate warnings concerning side effects or interactions of our products with other substances. We believe that we, our suppliers, and our manufacturers maintain adequate product liability insurance coverage. However, a substantial future product liability claim could exceed the amount of insurance coverage or could be excluded under the terms of an existing insurance policy, which could adversely affect our overall future financial condition.
In recent years a discovery of Bovine Spongiform Encephalopathy, (“BSE”), which is commonly referred to as “Mad Cow Disease”, has caused concern among the general public. As a result, some countries have banned the importation or sale of products that contain bovine materials sourced from locations where BSE has been identified. We have changed the vast majority of our capsules to a vegetable base. However, if a vegetable base is not available or practical for use, certifications are required to ensure the capsule material is BSE-free. The higher costs could affect our financial condition, results of operations, and our cash flows.
7. If our outside suppliers and manufacturers fail to supply products in sufficient quantities and in a
timely fashion, our business could suffer.
Outside manufacturers make all of our products. Our profit margins and timely product delivery are dependent upon the ability of our outside suppliers and manufacturers to supply us with products in a timely and cost-efficient manner. Our ability to enter new markets and sustain satisfactory levels of sales in each market depends on the ability of our outside suppliers and manufacturers to provide required levels of ingredients and products and to comply with all applicable regulations. As a precaution, we have approved alternate suppliers and manufacturers for our products. However, the failure of our primary suppliers or manufacturers to supply ingredients or produce our products could adversely affect our business operations.
We believe we have dependable suppliers for all of our ingredients and we have identified alternative sources for all of our ingredients, except Arabinogalactan. Due to the unique nature of Arabinogalactan, an important component used in the formulation of our Ambrotose® complex, we are unable to identify an alternative supplier at this time. If our suppliers are unable to perform, any delay in replacing or substituting such ingredients could affect our business.
The supplier of one of our major product components announced in February 2009 that its processing facility was closed and manufacturing of the component would cease. Alternate sources of supply for this component have been identified and we are in the final stages of executing a contract, but failure to secure another source of supply will adversely affect our business operations.
| 8. Our inability to develop and introduce new products that gain associate, member, and market acceptance could harm our business. |
A critical component of our business is our ability to develop new products that create enthusiasm among our independent associates and members. If we are unable to introduce new products, our associate productivity could be harmed. In addition, if any new products fail to gain market acceptance, are restricted by regulatory requirements or have quality problems, this would harm our results of operations. Factors that could affect our ability to continue to introduce new products include, among others, government regulations, the inability to attract and retain qualified research and development staff, the termination of third-party research and collaborative arrangements, proprietary protections of competitors that may limit our ability to offer comparable products, and the difficulties in anticipating changes in consumer tastes and buying preferences.
| 9. Our failure to appropriately respond to changing consumer preferences and demand for new products or product enhancements could significantly harm our relationship with independent associates and members, product sales, as well as our financial condition and operating results. |
Our business is subject to changing consumer trends and preferences, including rapid and frequent changes in demand for products, new product introductions, and enhancements. Our failure to accurately predict these trends could
23
| · | accurately anticipate consumer needs; |
| · | innovate and develop new products or product enhancements that meet these needs; |
| · | successfully commercialize new products or product enhancements in a timely manner; |
| · | price our products competitively; |
| · | manufacture and deliver our products in sufficient volumes and in a timely manner; and |
| · | differentiate our product offerings from those of our competitors. |
If we do not introduce new products or make enhancements to meet the changing needs of our members in a timely manner, some of our products could be rendered obsolete, which could negatively impact our revenues, financial condition, and operating results.
| 10. The global nutrition industry is intensely competitive and the strengthening of any of our competitors could harm our business. |
The global nutrition industry is intensely fragmented and competitive. We compete for independent associates with other network marketing companies outside the global nutrition industry. Many of our competitors have greater name recognition and financial resources, which may give them a competitive advantage. Our competitors may also be able to devote greater resources to marketing, promotional, and pricing campaigns that may influence our continuing and potential independent associates and members to buy products from competitors rather than from us. Such competition could adversely affect our business and current market share.
| 11. A downturn in the economy has affected consumer purchases of discretionary items such as the health and wellness products that we offer, which could continue to have an adverse effect on our business, financial condition, profitability and cash flows. |
We appeal to a wide demographic consumer profile and offer a broad selection of health and wellness products. A downturn in the economy has adversely impacted consumer purchases of discretionary items such as health and wellness products. During calendar years 2008 through 2010, the United States and global economies slowed dramatically as a result of a variety of problems, including turmoil in the credit and financial markets, concerns regarding the stability and viability of major financial institutions, the state of the housing markets and volatility in worldwide stock markets. Given the significance and widespread nature of these nearly unprecedented circumstances, the U.S. and global economies could remain significantly challenged in a recessionary state for an indeterminate period of time. These economic conditions could cause many of our existing and potential associates to delay or reduce purchases of our products for some time, which in turn could continue to harm our business by adversely affecting our revenues, results of operations, cash flows and financial condition. We cannot predict the duration of these economic conditions or the impact they will have on our consumers or business. For additional information regarding current economic conditions and their impact on our results of operations, refer to Item 7, Management’s Discussion and Analysis of Financial Condition and Results of Operations.
| 12. If we incur substantial liability from litigation, complaints, or enforcement actions or incur liabilities or penalties resulting from misconduct by our independent associates, our financial condition could suffer. |
Routine enforcement actions and complaints are common in our industry. Although we believe we fully cooperate with regulatory agencies and use various means to address misconduct by our independent associates, including maintaining policies and procedures to govern the conduct of our independent associates and conducting training seminars, it is still difficult to detect and correct all instances of misconduct. Violations of our policies and procedures by our independent associates could lead to litigation, formal or informal complaints, enforcement actions, and inquiries by various federal, state, or foreign regulatory authorities against us and/or our independent associates in each country. Because we have expanded into foreign countries, our policies and procedures for our independent associates differ depending on the different legal requirements of each country in which an independent associate does business. Any future litigation, complaints, and enforcement actions involving us and/or our independent associates could consume
24
| 13. Challenges by private parties to the form of our network marketing system could harm our business. |
We may be subject to challenges by private parties, including our independent associates and members, to the form of our network marketing system or elements of our business. In the United States, the network marketing industry and regulatory authorities have relied on the implementation of distributor rules and policies designed to promote retail sales to protect consumers, prevent inappropriate activities, and distinguish between legitimate network marketing distribution plans and unlawful pyramid schemes. We have adopted rules and policies based on case law, rulings of the FTC, discussions with regulatory authorities in several states, and domestic and global industry standards. Legal and regulatory requirements concerning network marketing systems, however, involve a high level of subjectivity, are inherently fact-based, and are subject to judicial interpretation. Because of this, we can provide no assurance that we would not be harmed by the application or interpretation of statutes or regulations governing network marketing, particularly in any civil challenge by a current or former independent associate or member.
| 14. If our network marketing activities do not comply with government regulations, our business could suffer. |
Many governmental agencies regulate our network marketing activities. A government agency’s determination that our business or our independent associates have significantly violated a law or regulation could adversely affect our business. The laws and regulations for network marketing intend to prevent fraudulent or deceptive schemes. Our business faces constant regulatory scrutiny due to the interpretive and enforcement discretion given to regulators, periodic misconduct by our independent associates, adoption of new laws or regulations, and changes in the interpretation of new or existing laws or regulations. For example, in July 2007, the Texas Attorney General filed suit against us, MannaRelief Ministries, Samuel L.Caster, the Fisher Institute, and H. Reginald McDaniel alleging violations of the Texas Deceptive Trade Practices Act and the Texas Food, Drug and Cosmetic Act. We subsequently reached an agreement with the Texas Attorney General’s office settling the enforcement action. Without admitting any wrongdoing, we agreed to refund up to $4 million to certain members and paid $2 million to cover fees and expenses of Texas regulators. We also made certain corporate governance changes required by the Texas Attorney General’s office and agreed not to violate certain provisions of the Texas Deceptive Trade Practices Act and Texas Food, Drug and Cosmetic Act. If we are unable to comply fully with the provisions of the settlement, Texas regulators could pursue further remedies that may impact our business.
In addition, in the past and because of the industry in which we operate, we have experienced inquiries regarding specific independent associates.
| 15. If government regulations regarding network marketing change or are interpreted or enforced in a manner adverse to our business, we may be subject to new enforcement actions and material limitations regarding our overall business model. |
Network marketing is always subject to extensive governmental regulations, including foreign, federal, and state regulations. Any change in legislation and regulations could affect our business. Furthermore, significant penalties could be imposed on us for failure to comply with various statutes or regulations. Violations may result from:
| · | misconduct by us or our independent associates; |
| · | ambiguity in statutes; |
| · | regulations and related court decisions; |
| · | the discretion afforded to regulatory authorities and courts interpreting and enforcing laws; and |
· | new regulations or interpretations of regulations affecting our business. |
| 16. If we violate governmental regulations or fail to obtain necessary regulatory approvals, our operations could be adversely affected. |
Our operation is subject to extensive laws, governmental regulations, administrative determinations, court
decisions, and similar constraints at the federal, state, and local levels in our domestic and foreign markets. These regulations primarily involve the following:
25
| · | the formulation, manufacturing, packaging, labeling, distribution, importation, sale, and storage of our products; |
| · | the health and safety of dietary supplements, cosmetics and foods; |
| · | trade practice laws and network marketing laws; |
| · | our product claims and advertising by our independent associates; |
| · | our network marketing system; |
| · | pricing restrictions regarding transactions with our foreign subsidiaries or other related parties and similar regulations that affect our level of foreign taxable income; |
| · | the assessment of customs duties; |
| · | further taxation of our independent associates, which may obligate us to collect additional taxes and maintain additional records; and |
| · | export and import restrictions. |
Any unexpected new regulations or changes in existing regulations could significantly restrict our ability to continue operations, which could adversely affect our business. For example, changes regarding health and safety, and food and drug regulations for our nutritional products could require us to reformulate our products to comply with such regulations.
In some foreign countries, nutritional products are considered foods, while other countries consider them drugs. Future health and safety or food and drug regulations could delay or prevent our introduction of new products or suspend or prohibit the sale of existing products in a given country or marketplace. In addition, if we expand into other foreign markets, our operations or products could also be affected by the general stability of foreign governments and the regulatory environment relating to network marketing and our products. If our products are subject to high customs duties, our sales and competitive position could suffer as compared to locally produced goods. Furthermore, import restrictions in certain countries and jurisdictions could limit our ability to import products from the United States.
17. Increased regulatory scrutiny of nutritional supplements as well as new regulations that are being adopted in some of our markets with respect to nutritional supplements could result in more restrictive regulations and harm our results if our supplements or advertising activities are found to violate existing or new regulations or if we are not able to effect necessary changes to our products in a timely and efficient manner to respond to new regulations. |
There has been an increasing movement in the United States and other markets to increase the regulation of dietary supplements, which could impose additional restrictions or requirements on us and increase the cost of doing business. In several of our markets, new regulations have been adopted, or are likely to be adopted, in the near-term that will impose new requirements, make changes in some classifications of supplements under the regulations, or limit the claims we can make. In addition, there has been increased regulatory scrutiny of nutritional supplements and marketing claims under existing and new regulations. In Europe for example, we are unable to market supplements that contain ingredients that have not been previously marketed in Europe (“novel foods”) without going through an extensive registration and approval process. Europe is also expected to adopt additional regulations in the near future to set new limits on acceptable levels of nutrients. The FDA has implemented Good Manufacturing Practices for the U.S. nutritional supplement industry. Our operations could be harmed if new regulations require us to reformulate products or effect new registrations, if regulatory authorities make determinations that any of our products do not comply with applicable regulatory requirements, if the cost of complying with regulatory requirements increases materially, or if we are not able to effect necessary changes to our products in a timely and efficient manner to respond to new regulations. In addition, our operations could be harmed if governmental laws or regulations are enacted that restrict the ability of companies to market or distribute nutritional supplements, impose additional burdens, or requirements on nutritional supplement companies.
18. If our information technology system fails, our operations could suffer.
Like many companies, our business is heavily dependent upon our information technology infrastructure to effectively manage and operate many of our key business functions, including:
| · | order processing; |
| · | supply chain management; |
| · | customer service; |
26
| · | product distribution; |
| · | commission processing; |
| · | cash receipts and payments; and |
| · | financial reporting. |
These systems and operations are vulnerable to damage and interruption from fires, earthquakes, telecommunications failures, and other events. They are also subject to break-ins, sabotage, intentional acts of vandalism and similar misconduct. Although we maintain an extensive security system and disaster recovery program that was developed under the guidelines published by the National Institute of Standards of Technology, a long-term failure or impairment of any of our information technology systems could adversely affect our ability to conduct day-to-day business.
19. Currency exchange rate fluctuations could reduce our overall profits.
In 2010 and 2009, we recognized 55.9% and 51.4%, respectively, of net sales in markets outside of the United States. In preparing our consolidated financial statements, certain financial information is required to be translated from foreign currencies to the United States dollar using either the spot rate or the weighted-average exchange rate. If the United States dollar changes relative to applicable local currencies, there is a risk our reported sales, operating expenses, and net income could significantly fluctuate. We are not able to predict the degree of exchange rate fluctuations, nor can we estimate the effect any future fluctuations may have upon our future operations. To date, we have not entered into any hedging contracts or participated in any hedging or derivative activities.
| 20. We may be held responsible for certain taxes or assessments relating to the activities of our independent associates, which could harm our financial condition and operating results. |
Our independent associates are subject to taxation and, in some instances, legislation or governmental agencies impose an obligation on us to collect taxes, such as value added taxes, and to maintain appropriate tax records. In addition, we are subject to the risk in some jurisdictions of being responsible for social security and similar taxes with respect to our distributors. In the event that local laws and regulations require us to treat our independent distributors as employees, or if our distributors are deemed by local regulatory authorities to be our employees, rather than independent contractors, we may be held responsible for social security and related taxes in those jurisdictions, plus any related assessments and penalties, which could harm our financial condition and operating results.
| 21. Our Equity Line with Dutchess may not be available to us if we elect to make a draw down. |
On September 16, 2010, we entered into an Investment Agreement (the “Investment Agreement”) with Dutchess Opportunity Fund II, LP, a Delaware limited partnership (the “Investor”). Pursuant to the Investment Agreement, the Investor committed to purchase, subject to certain restrictions and conditions, up to $10,000,000 of our common stock, over a period of 36 months from the first trading day following the effectiveness of the registration statement registering the resale of shares purchased by the Investor pursuant to the Investment Agreement (the “Equity Line”). The aggregate number of shares of common stock issuable by us and purchasable by the Investor under Investment Agreement is 5,000,000. Dutchess will not be obligated to purchase shares under the Equity Line unless certain conditions are met, which include: effectiveness of the registration statement; the continued listing of our stock on the NASDAQ Global Market; our compliance with our obligations under the Investment Agreement and Registration Rights Agreement entered into with Dutchess; the absence of injunctions or other governmental actions prohibiting the issuance of common stock to Dutchess; the absence of violations of shareholder approval requirements with respect to such issuance of our common stock to Dutchess; the accuracy of representations and warranties made to Dutchess; and approval of the Equity Line transaction by our board of directors. If we are unable to access funds through the Equity Line, we may be unable to access capital on favorable terms or at all.
27
22. Any draw downs under our Equity Line with Dutchess may result in dilution to our shareholders.
If we sell shares to Dutchess under the Equity Line, it will have a dilutive effect on the holdings of our current shareholders, and may result in downward pressure on the price of our common stock. If we draw down amounts under the Equity Line, we will issue shares to Dutchess at a discount of up to 4% from the average price of our common stock. If we draw down amounts under the Equity Line when our share price is decreasing, we will need to issue more shares to raise the same amount than if our stock price was higher. Issuances in the face of a declining share price will have an even greater dilutive effect than if our share price were stable or increasing, and may further decrease our share price.
23. Our stock price is volatile and may fluctuate significantly.
The price of our common stock is subject to sudden and material increases and decreases. Decreases could adversely affect investments in our common stock. The price of our common stock and the price at which we could sell securities in the future could significantly fluctuate in response to:
| · | broad market fluctuations and general economic conditions; |
| · | fluctuations in our financial results; |
| · | future securities offerings; |
| · | changes in the market’s perception of our products or our business, including false or negative publicity; |
| · | governmental regulatory actions; |
| · | the outcome of any lawsuits; |
| · | financial and business announcements made by us or our competitors; |
| · | the general condition of the industry; and |
| · | the sale of large amounts of stock by insiders. |
In addition, the stock market has experienced extreme price and volume fluctuations in recent years that have significantly affected the quoted prices of the securities of many companies. The changes sometimes appear to occur without regard to specific operating performance. The price of our common stock in the open market could fluctuate based on factors that have little or nothing to do with us or that are outside of our control.
| 24. Certain shareholders, directors, and officers own a significant amount of our stock, which could allow them to influence corporate transactions and other matters. |
As of December 31, 2010, our directors, executive officers, and a major shareholder, collectively with their families and affiliates, beneficially owned approximately 40.5% of our total outstanding common stock. As a result, if two or more of these shareholders choose to act together based on their current share ownership, they may be able to control a significant percentage of the total outstanding shares of our common stock, which could affect the outcome of a shareholder vote on the election of directors, the adoption of stock option plans, the adoption or amendment of provisions in our articles of incorporation and bylaws, or the approval of mergers and other significant corporate transactions.
25. We have implemented anti-takeover provisions that may help discourage a change of control.
Certain provisions in our articles of incorporation, bylaws, and the Texas Business Organization Code help discourage unsolicited proposals to acquire our company, even if the proposal may benefit our shareholders. Our articles of incorporation authorize the issuance of preferred stock without shareholder approval. Our Board of Directors has the power to determine the price and terms of any preferred stock. The ability of our Board of Directors to issue one or more series of preferred stock without shareholders’ approval could deter or delay unsolicited changes of control by discouraging open market purchases of our common stock or a non-negotiated tender or exchange offer for our common stock. Discouraging open market purchases may be disadvantageous to our shareholders who may otherwise desire to participate in a transaction in which they would receive a premium for their shares.
In addition, other provisions may also discourage a change of control by means of a tender offer, open market purchase, proxy contest or otherwise. Our charter documents provide for three classes of directors on our Board of Directors with members of each class serving staggered three year terms. In addition, the Texas Business Organization Code restricts, subject to exceptions, business combinations with any “affiliated shareholder.” Any or all of these provisions could delay, deter or help prevent a takeover of our Company and could limit the price investors are willing to pay for our common stock.
28
| 26. We are not required to pay dividends, and our Board of Directors may decide not to declare dividends in the future. |
The declaration of dividends on our common stock is solely within the discretion of our Board of Directors, subject to limitations under Texas law stipulating that dividends may not be paid if payment therefore would cause the corporation to be insolvent or if the amount of the dividend would exceed the surplus of the corporation. Our Board of Directors may decide not to declare dividends or we could be prevented from declaring a dividend because of legal or contractual restrictions. The failure to pay dividends could reduce our stock price. Our Board of Directors suspended dividends in August 2009. We may not resume payment of dividends in the future.
27. Concentration Risk
A significant portion of our revenue is derived from our core Ambrotose® complex products which include the Ambrotose® products and Advanced Ambrotose® products. A decline in sales value of such legacy products could have a material adverse effect on our earnings, cash flows, and financial position. Revenue from the core Ambrotose® products were as follows for the years ended December 31, 2010 and 2009 (in thousands, except percentages):
| 2010 | 2009 | ||||||||||
Sales by product | % of total net sales | Sales by product | % of total net sales | ||||||||
Advanced Ambrotose® | $ | 61,458 | 26.9 | % | $ | 65,360 | 22.6 | % | |||
Ambrotose® | 19,078 | 8.4 | % | 25,413 | 8.8 | % | |||||
| Total | $ | 80,536 | 35.3 | % | $ | 90,773 | 31.4 | % | |||
Our business is not currently exposed to customer concentration risk given that no independent associate has ever accounted for more than 10% of our consolidated net sales.
Circumstances and conditions may change. Accordingly, additional risks and uncertainties not currently known, or that we currently deem not material, may also adversely affect our business operations.
29
None.
We lease property at several locations for our headquarters and distribution facilities, including:
| Location | Size | Original term | Expiration date | |||||
| Coppell, Texas (corporate headquarters) | 110,000 | sq. feet | 10 | years | March 2017 | |||
| Coppell, Texas (distribution center) | 75,000 | sq. feet | 10 | years | March 2017 | |||
| St. Leonards, Australia (Australian headquarters) | 850 | sq. meters(1) | 5 | years | August 2013 | |||
Didcot, Oxfordshire (combined U.K. headquarters and distribution center) | 16,631 | sq. feet | 5 | years | July 2011 | |||
| Minato-ku, Tokyo, Japan (Japanese headquarters) | 296 | Tsubos(2) | 2 | years | November 2012 | |||
| Ganganm-gu, Seoul, Korea (Republic of Korea headquarters) | 717 | Pyong (3) | 3 | years | June 2013 | |||
| Taipei, Taiwan (Taiwan headquarters) | 254 | pings (4) | 3 | years | June 2011 | |||
| Zug, Switzerland (Switzerland headquarters) | 680 | sq. meters(5) | 5 | years | October 2013 | |||
| Markham, Ontario (Canada headquarters) | 3,097 | sq. feet | 3 | years | September 2012 | |||
| Bedfordview, South Africa | 383 | sq. meters(6) | 5 | years | March 2015 | |||
| Guadalajara, Mexico (customer service center) | 1550 | sq. meters(7) | 5.25 | years | February 2016 | |||
| Mexico City, Mexico (customer service center) | 179 | sq. meters(8) | 5 | years | November 2015 | |||
______________________
(1) Approximately 9,149 square feet. |
(2) Approximately 10,538 square feet. |
(3) Approximately 25,526 square feet. |
(4) Approximately 9,021 square feet. |
(5) Approximately 7,324 square feet. |
(6) Approximately 4,119 square feet. |
(7) Approximately 16,684 square feet. |
(8) Approximately 1,926 square feet. |
Our main distribution facility is located in Coppell, Texas and consists of 75,000 square feet of leased space that houses an automated distribution system capable of processing up to 18,000 orders per day. In 2005, we opened a distribution facility in the United Kingdom, which is located in Didcot, Oxfordshire and is capable of processing up to 650 orders per day. Both distribution centers currently operate well below full capacity and are capable of supporting our planned sales volume growth in the foreseeable future.
To maximize our operating strategy and minimize costs, we continue to contract with third-party distribution and fulfillment facilities in Canada, Australia, Japan, the Republic of Korea, Taiwan, South Africa, and Mexico. By entering into these third-party distribution facility agreements, our smaller offices maintain flexible operating capacity, minimize shipping costs, and are able to process an order within 24-hours after order placement and receipt of payment.
See “Litigation” in Note 13 of the Notes to our Consolidated Financial Statement, which is incorporated herein by reference.
30
PART II
| Market for Registrant’s Common Equity, Related Shareholder Matters and Issuer Purchases of Equity Securities |
Market for Our Common Stock. On February 12, 1999, we completed our initial public offering and on February 16, 1999, our common stock began trading on the NASDAQ Global Market (formerly the NASDAQ National Market) under the symbol “MTEX.” The NASDAQ Global Select Market is a segment of the NASDAQ Global Market with the highest initial listing standards of any exchange in the world. Beginning July 3, 2006, NASDAQ moved our common stock to the NASDAQ Global Select Market. As of March 4, 2011, we had an aggregate of 26,490,466 shares of our common stock outstanding and the closing price on such date was $1.88. Below are the high and low closing prices of Mannatech’s common stock as reported on the NASDAQ for each quarter of the fiscal years ended December 31, 2010 and 2009:
| 2010: | Low | High | |||||
| First Quarter | $ | 2.78 | $ | 4.41 | |||
| Second Quarter | $ | 1.89 | $ | 4.23 | |||
| Third Quarter | $ | 2.00 | $ | 2.98 | |||
| Fourth Quarter | $ | 1.62 | $ | 2.19 | |||
| 2009: | |||||||
| First Quarter | $ | 2.50 | $ | 3.90 | |||
| Second Quarter | $ | 2.97 | $ | 4.73 | |||
| Third Quarter | $ | 3.13 | $ | 4.80 | |||
| Fourth Quarter | $ | 2.34 | $ | 3.89 | |||
Holders. As of March 4, 2011, we had 3,200 shareholders of record who held approximately 26% of our common stock directly and 130 security brokers and dealers who held approximately 74% of our common stock on behalf of approximately 9,200 shareholders.
Dividends. Our quarterly cash dividends were $0.02 per share for the first and second quarters of 2009. In the third quarter of 2009, our Board of Directors suspended the quarterly cash dividend payment to shareholders due to the recent company financial performance, protracted worldwide economic recession, and the internal funding needs of new initiatives designed to accelerate sales and associate recruitment of the Company. See “Risk Factors—We are not required to pay dividends, and our Board of Directors may decide not to declare dividends in the future” in Item 1A of this Form 10-K for further discussion related to future payment of dividends.
Stock Options. The following table provides information as of March 4, 2011 about our common stock that may be issued upon the exercise of stock options under our existing stock option plan.
| Plan Category | Number of securities to be issued upon exercise of outstanding options, warrants, and rights (a) | Weighted-average exercise price of outstanding options, warrants, and rights (b) | Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column (a)) (c) | ||||
| Equity compensation plan | 1,384,401 | $ | 2.89 | 356,998 | |||
| Equity compensation plans not approved by Shareholders | — | — | — | ||||
| Total | 1,384,401 | 356,998 | |||||
In February 2008, our Board of Directors approved our 2008 Stock Incentive Plan (the “2008 Plan”), which reserves up to 1,000,000 shares of common stock for issuance of stock options and restricted stock to our employees, board members, and consultants, plus any shares reserved under our then-existing, unexpired stock plans for which options had not been issued, and any shares underlying outstanding options under the then-existing stock option plans that terminate without having been exercised in full. The 2008 Plan was approved by our shareholders at our 2008 Annual Shareholders’ Meeting. Currently, the 2008 Plan is the one stock incentive plan under which we may grant options.
31
At the 2010 Annual Shareholders’ Meeting, shareholders approved an amendment to the 2008 Plan to permit a one-time stock option exchange program. On July 16, 2010, we commenced the option exchange program (the “Exchange Program”) whereby employees (including officers), directors, and consultants could elect to exchange their “out of the money” stock options for new options at a lower exercise price. The Exchange Program expired on August 13, 2010. On August 16, 2010, pursuant to the Exchange Program, an aggregate of 784,930 eligible options were tendered and accepted by the Company in exchange for the grant of an aggregate of 440,558 replacement options.
All of the replacement options granted pursuant to the Exchange Program have an exercise price of $2.46 and were granted under the 2008 Plan, as amended. Each replacement option vests annually in ⅓ installments starting one year from the grant date and has a term of 10 years. Incremental stock option expense resulting from the exchange was minimal because the fair value of the replacement options was approximately equal to the fair value of the surrendered options they replaced.
Sales of Unregistered Securities.
None.
Uses of Proceeds from Registered Securities.
None.
Issuer Purchases of Equity Securities.
None.
32
Performance Graph.
Our common stock began trading on the NASDAQ Global Market (formerly the NASDAQ National Market) on February 16, 1999. Set forth below is information comparing the cumulative total shareholder return and share price appreciation plus dividends on our common stock with the cumulative total return of the S&P Midcap Index and a market weighted index of publicly traded peers for the period from December 31, 2005 through December 31, 2010. The comparison assumes that $100 is invested in shares of our common stock, the S&P Midcap Index and an index of publicly traded peers on January 1, 2006, and that all dividends were reinvested. The publicly-traded companies in our peer group are Schiff Nutrition International Inc. (NYSE Symbol WNI), Herbalife Ltd. (NYSE Symbol HLF) Nature’s Sunshine Products, Inc. (NYSE Symbol NATR), USANA Health Sciences, Inc.(NADSAQ Symbol USNA), and Nu Skin Enterprises, Inc. (NYSE Symbol NUS).
The following graph also includes the S&P SmallCap Index that we will be using in the future instead of the S&P MidCap Index. We have selected this new index due to a decrease in our market capitalization in recent years. The S&P SmallCap Index includes companies whose equity securities are of comparable market capitalization. For the purposes of comparison, the stock performance graph below includes the new index and the previously reported index. The comparisons are based upon historical data and are not indicative of, nor intended to forecast, future performance of our common stock.
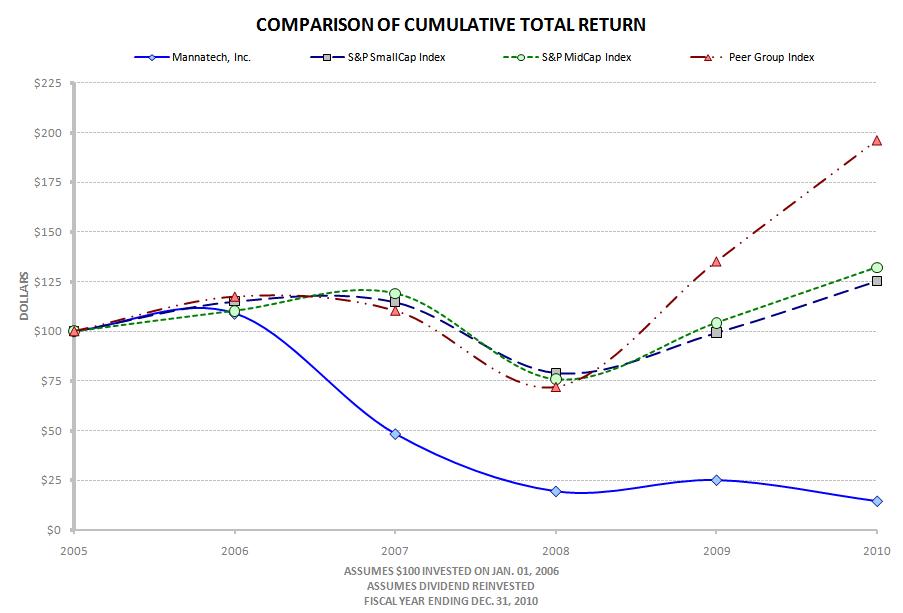
| Company/Market/Peer Group | 12/31/2005 | 12/31/2006 | 12/31/2007 | 12/31/2008 | 12/31/2009 | 12/31/2010 |
| Mannatech, Inc. | $100.00 | $108.97 | $48.44 | $19.55 | $25.18 | $14.57 |
| S&P SmallCap Index | $100.00 | $115.12 | $114.78 | $79.11 | $99.34 | $125.48 |
| S&P MidCap Index | $100.00 | $110.32 | $119.12 | $75.96 | $104.36 | $132.16 |
| Peer Group Index | $100.00 | $117.37 | $110.31 | $71.85 | $135.17 | $196.26 |
33
The Selected Financial Data set forth below for each of the five years ended December 31, have been derived from and should be read in conjunction with (A) Our Consolidated Financial Statements and related notes set forth in Item 15 of this report, and (B) Our “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” set forth in Item 7 of this report.
| 2010 | 2009 | 2008 | 2007 | 2006(1) | ||||||||||||
| Consolidated Statements of Operations Data: | (in thousands, except per share amounts) | |||||||||||||||
| Net sales | $ | 228,088 | $ | 289,705 | $ | 332,703 | $ | 412,678 | $ | 410,069 | ||||||
| Gross profit | $ | 98,015 | $ | 96,477 | $ | 134,544 | $ | 163,846 | $ | 169,393 | ||||||
| Income (loss) from operations | $ | (11,481 | ) | $ | (25,594 | ) | $ | (14,499 | ) | $ | 7,609 | $ | 44,074 | |||
| Net income (loss) | $ | (10,616 | ) | $ | (17,368 | ) | $ | (12,628 | ) | $ | 6,594 | $ | 32,390 | |||
| Earnings (loss) Per Common Share: | ||||||||||||||||
| Basic | $ | (0.40 | ) | $ | (0.66 | ) | $ | (0.48 | ) | $ | 0.25 | $ | 1.22 | |||
| Diluted | $ | (0.40 | ) | $ | (0.66 | ) | $ | (0.48 | ) | $ | 0.25 | $ | 1.19 | |||
| Weighted-Average Common Shares Outstanding: | ||||||||||||||||
| Basic | 26,488 | 26,467 | 26,461 | 26,443 | 26,598 | |||||||||||
| Diluted | 26,488 | 26,467 | 26,461 | 26,893 | 27,219 | |||||||||||
| Other Financial Data: | ||||||||||||||||
| Capital expenditures | $ | 3,764 | $ | 4,896 | $ | 5,633 | $ | 13,446 | $ | 27,216 | ||||||
| Dividends declared per common share | $ | — | $ | 0.04 | $ | 0.22 | $ | 0.36 | $ | 0.32 | ||||||
| Consolidated Balance Sheet Data: | ||||||||||||||||
| Total assets | $ | 81,423 | $ | 102,302 | $ | 124,058 | $ | 152,454 | $ | 152,235 | ||||||
| Long-term liabilities | $ | 8,103 | $ | 8,339 | $ | 9,813 | $ | 9,431 | $ | 11,402 | ||||||
______________________________________
(1) We capitalized $18.4 million of costs related to our internally-developed software projects, which were completed in April 2007. In addition, we recognized an income tax benefit of $3.3 million associated with income tax credits for our research and experimentation activities. |
34
The following discussion is intended to assist in the understanding of our consolidated financial position and our results of operations for each of the three years ended December 31, 2010, 2009, and 2008. This discussion should be read in conjunction with “Item 15. – Consolidated Financial Statements and related Notes,” beginning on page F-1 of this report and with other financial information included elsewhere in this report. Unless stated otherwise, all financial information presented below, throughout this report, and in the consolidated financial statements and related notes includes Mannatech and all of our subsidiaries on a consolidated basis.
Company Overview
Since November 1993, we have continued to develop innovative, high-quality, proprietary nutritional supplements, topical and skin care products, and weight-management products that are sold through a global network marketing system. We operate in the United States, Canada, Australia, the United Kingdom, Japan, New Zealand, the Republic of Korea, Taiwan, Denmark, Germany, South Africa, Singapore, Austria, the Netherlands, Norway, Sweden and, beginning January 24, 2011, Mexico. The Switzerland office was created to manage certain day-to-day business needs of non-North American markets.
We conduct our business as a single operating segment and primarily sell our products through a network of approximately 403,000 independent associates and members who had purchased our products and/or packs within the last 12 months, who we refer to as current independent associates and members. New recruits and pack sales are leading indicators for the long-term success of our business. New recruits include new independent associates and members purchasing our packs and products for the first time. We operate as a seller of nutritional supplements, topical and skin care products, and weight-management products through our network marketing distribution channels operating in seventeen countries. We review and analyze net sales by geographical location and by packs and products on a consolidated basis. Each of our subsidiaries sells similar products and exhibits similar economic characteristics, such as selling prices and gross margins.
Because we sell our products through network marketing distribution channels, the opportunities and challenges that affect us most are: recruitment of new and retention of independent associates and members; entry into new markets and growth of existing markets; niche market development; new product introduction; and investment in our infrastructure.
Current Economic Conditions and Recent Developments
During 2010, we continued to feel the negative impact of the weak economic environment. In addition, we experienced increased competition in certain international markets where competitors launched operations, and in some cases made attempts to attract some of our independent associate field leaders. Reduction in the number of associate recruits and loss of active associates were major factors that negatively influenced our financial results throughout 2010.
In response to adverse market conditions, we implemented various initiatives to reduce expenses over the last few years. By establishing a culture of expense control and accountability, we achieved a significant decrease in operating expenses and maintained that level of expense control through 2010. At the same time, we remained committed to our strategic plan of developing new, innovative, and scientifically validated products, international expansion, and strengthening financial results.
We continue to expand internationally and, in January 2011, began selling products in Mexico. We have office locations in both Guadalajara and Mexico City, as well as a distribution center located in Monterrey. During 2010, our operating expenses associated with the Mexico launch totaled $1.1 million. Mexico is one of the most important international market openings we have ever undertaken as we believe it has a significant sales volume potential. According to the Direct Selling Association, Mexico is the sixth largest direct selling market in the world. Our expansion into Mexico complements our existing presence in North America while highlighting our continued focus on global expansion.
We continue to focus on new product development. In late September 2010, we launched our exclusive, age-defying product line, the Mannatech LIFTTM skin care system. The Mannatech LIFTTM skin care line is an innovative system formulated to reduce the signs of aging and improve the youthful radiance of skin in three easy steps. The line is paraben-free and features our Jeunesse 7™ proprietary blend – a combination of our signature glyconutrients and a French
35
In addition to our innovative product development, we continue to invest in research for our existing products. In 2010, our collaborative research projects included various tests and studies performed on our family of Ambrotose® products, newly introduced LIFTTM skin care system, and EM·PACT® sports drink. Additional clinical and pre-clinical studies are scheduled to be conducted in 2011.
We strive to ensure that all of our products meet the strictest quality guidelines. In the third quarter of 2010, the International Sport Karate Association (ISKA) certified Mannatech as its official dietary supplement provider. Several of our products will feature the “ISKA Certified for Elite Athletes” seal on the label, which will be the only health supplements on the market to feature this seal on its packaging. The certification program was developed by ISKA to recognize high-quality dietary supplements. ISKA and Mannatech’s exclusive initial partnership will last until 2012, with an option to renew.
Even during difficult times, we maintain our focus to be a generous and socially responsible company. We continue our charitable donations to MannaRelief, a non-profit organization that provides charitable services for children in need. In 2010, MannaRelief and Mannatech partnered together to launch the Give for RealSM program. This “donation-through-consumption” initiative was designed to allow consumers and associates to help undernourished children around the world. For every purchase on an automatic order containing Ambrotose® complex powder, Advanced Ambrotose® powder, MannaBears™ gummies, PhytoMatrix® caplets, PhytoBurst® nutritional chews, or Optimal Support Packets, a donation of Mannatech’s nutritional supplements is provided through MannaRelief to children in need worldwide. In 2010, we provided an additional $166,000 in donations to MannaRelief as a result of the Give For RealSM program. MannaRelief and Mannatech’s goal is to connect one million sponsors to one million undernourished children.
We believe our aggressive cost reduction, new product introduction, international expansion, and financial discipline will help us effectively manage through the challenging economy. We believe that achieving desired levels of revenues and profitability in the future will depend largely upon our ability to attract and retain associates. In the short term, our focus is on restoring sales volume by recruiting associates and increasing consumer confidence, continued expense control, emphasizing the value and benefits of our flagship product, Ambrotose®, and maintaining charitable donations through the Give for RealSM initiative.
36
Results of Operations
Year Ended December 31, 2010 compared to Year Ended December 31, 2009
The tables below summarize our consolidated operating results in dollars and as a percentage of net sales for the years ended December 31, 2010 and 2009 (in thousands, except percentages).
| 2010 | 2009 | Change | |||||||||||||||
Total Dollars | % of net sales | Total dollars | % of net sales | Dollar | Percentage | ||||||||||||
| Net sales | $ | 228,088 | 100 | % | $ | 289,705 | 100 | % | $ | (61,617 | ) | (21.3 | )% | ||||
| Cost of sales | 32,754 | 14.4 | % | 46,813 | 16.2 | % | (14,059 | ) | (30.0 | )% | |||||||
| Commissions and incentives | 97,319 | 42.7 | % | 146,415 | 50.5 | % | (49,096 | ) | (33.5 | )% | |||||||
| 130,073 | 57.0 | % | 193,228 | 66.7 | % | (63,155 | ) | (32.7 | )% | ||||||||
| Gross profit | 98,015 | 43.0 | % | 96,477 | 33.3 | % | 1,538 | 1.6 | % | ||||||||
| Operating expenses: | |||||||||||||||||
| Selling and administrative expenses | 62,657 | 27.5 | % | 69,997 | 24.2 | % | (7,340 | ) | (10.5 | )% | |||||||
| Depreciation and amortization | 11,517 | 5.0 | % | 12,333 | 4.3 | % | (816 | ) | (6.6 | )% | |||||||
| Other operating costs | 35,322 | 15.5 | % | 39,741 | 13.7 | % | (4,419 | ) | (11.1 | )% | |||||||
| Total operating expenses | 109,496 | 48.0 | % | 122,071 | 42.1 | % | (12,575 | ) | (10.3 | )% | |||||||
| Loss from operations | (11,481 | ) | (5.0 | )% | (25,594 | ) | (8.8 | )% | 14,113 | 55.1 | % | ||||||
| Interest income | 173 | 0.1 | % | 473 | 0.2 | % | (300 | ) | (63.4 | )% | |||||||
| Other income, net | 268 | 0.1 | % | 1,046 | 0.4 | % | (778 | ) | (74.4 | )% | |||||||
| Loss before income taxes | (11,040 | ) | (4.8 | )% | (24,075 | ) | (8.3 | )% | 13,035 | 54.1 | % | ||||||
| Benefit for income taxes | 424 | 0.2 | % | 6,707 | 2.3 | % | (6,283 | ) | (93.7 | )% | |||||||
| Net loss | $ | (10,616 | ) | (4.7 | )% | $ | (17,368 | ) | (6.0 | )% | $ | 6,752 | 38.9 | % | |||
37
For geographical purposes, consolidated net sales shipped to customers by location for the years ended December 31, 2010 and 2009 were as follows (in millions, except percentages):
Net Sales in Dollars and as a Percentage of Consolidated Net Sales
| 2010 | 2009 | ||||||||||||
| United States | $ | 100.8 | 44.1 | % | $ | 140.7 | 48.6 | % | |||||
| Japan | 34.2 | 15.0 | % | 42.0 | 14.5 | % | |||||||
| Republic of Korea | 22.0 | 9.6 | % | 26.4 | 9.1 | % | |||||||
| Australia | 20.0 | 8.8 | % | 22.9 | 7.9 | % | |||||||
| Canada | 18.5 | 8.1 | % | 23.0 | 7.9 | % | |||||||
| South Africa | 12.0 | 5.3 | % | 13.2 | 4.6 | % | |||||||
| Taiwan | 6.4 | 2.8 | % | 6.6 | 2.3 | % | |||||||
| New Zealand | 3.1 | 1.4 | % | 4.3 | 1.5 | % | |||||||
| United Kingdom | 2.4 | 1.1 | % | 3.3 | 1.0 | % | |||||||
| Germany | 2.3 | 1.0 | % | 3.2 | 1.1 | % | |||||||
| Singapore | 2.1 | 0.9 | % | 1.5 | 0.5 | % | |||||||
Norway(1) | 1.6 | 0.7 | % | 0.3 | 0.1 | % | |||||||
Austria(1) | 1.1 | 0.5 | % | 0.3 | 0.1 | % | |||||||
The Netherlands(1) | 0.6 | 0.3 | % | 0.2 | 0.1 | % | |||||||
Sweden(1) | 0.5 | 0.2 | % | 0.2 | 0.1 | % | |||||||
| Denmark | 0.5 | 0.2 | % | 1.6 | 0.6 | % | |||||||
| Total | $ | 228.1 | 100 | % | $ | 289.7 | 100 | % | |||||
_________________________
(1) Austria, the Netherlands, Norway, and Sweden began operations in September 2009.
Net Sales
For the year ended December 31, 2010, our operations outside of the United States accounted for approximately 55.9% of our consolidated net sales, whereas in the same period in 2009, our operations outside of the United States accounted for approximately 51.4% of our consolidated net sales.
Consolidated net sales for the year ended December 31, 2010 decreased $61.6 million, or 21.3%, to $228.1 million, as compared to $289.7 million for the same period in 2009. Domestic sales decreased $39.9 million, while international sales decreased $21.7 million. Despite our decrease in net sales, we look for future growth as we continue to develop innovative products, actively recruit new associates, and expand internationally.
Fluctuation in foreign currency exchange rates had an overall favorable impact on our net sales of approximately $9.0 million for year ended December 31, 2010. The net sales impact is calculated as the difference between (1) the current period’s net sales in USD and (2) the current period’s net sales in local currencies converted to USD by applying average exchange rates for the year ended December 31, 2009.
38
Net sales by country in transactional currency for the year ended December 31, 2010 and 2009 were as follows (in millions, except percentages):
| Change | |||||||||||
Transactional Currency | 2010 | 2009 | Transactional currency | Percentage | |||||||
| Australia and Singapore | AUD | 23.9 | 31.1 | (7.2 | ) | (23.2 | )% | ||||
Austria(1), Germany, the Netherlands(1) | EUR | 3.0 | 2.6 | 0.4 | 15.4 | % | |||||
| Denmark | DKK | 3.0 | 8.6 | (5.6 | ) | (65.1 | )% | ||||
| Japan | JPY | 2,971.3 | 3,890.7 | (919.4 | ) | (23.6 | )% | ||||
| Korea | KRW | 25,358.9 | 33,366.8 | (8,007.9 | ) | (24.0 | )% | ||||
| New Zealand | NZD | 4.4 | 6.9 | (2.5) | (36.2 | )% | |||||
Norway(1) | NOK | 8.6 | 2.0 | 6.6 | 330.0 | % | |||||
| South Africa | ZAR | 87.6 | 109.8 | (22.2 | ) | (20.2 | )% | ||||
Sweden(1) | SEK | 3.0 | 1.2 | 1.8 | 150.0 | % | |||||
| Taiwan | TWD | 201.8 | 217.4 | (15.6 | ) | (7.2 | )% | ||||
| United Kingdom | GBP | 1.7 | 2.1 | (0.4 | ) | (19.0 | )% | ||||
_________________________
(1) Austria, the Netherlands, Norway, and Sweden began operations in September 2009.
Our total sales and sales mix can be influenced by any of the following:
| • | changes in our sales prices; |
| • | changes in consumer demand; |
| • | changes in the number of independent associates and members; |
| • | changes in competitors’ products; |
| • | changes in economic conditions; |
| • | changes in regulations; |
| • | announcements of new scientific studies and breakthroughs; |
| • | introduction of new products; |
| • | discontinuation of existing products; |
| • | adverse publicity; |
| • | changes in our commissions and incentives programs; |
| • | direct competition; and |
| • | fluctuations in foreign currency exchange rates. |
Our sales mix for the years ended December 31, was as follows (in millions, except percentages):
| Change | ||||||||||||
| 2010 | 2009 | Dollar | Percentage | |||||||||
| Consolidated product sales | $ | 187.2 | $ | 213.9 | $ | (26.7 | ) | (12.5 | )% | |||
| Consolidated pack sales | 31.3 | 62.1 | (30.8 | ) | (49.6 | )% | ||||||
| Consolidated other, including freight | 9.6 | 13.7 | (4.1 | ) | (29.9 | )% | ||||||
| Total consolidated net sales | $ | 228.1 | $ | 289.7 | $ | (61.6 | ) | (21.3 | )% | |||
Pack sales correlate to new independent associates who purchase starter packs and to continuing independent associates who purchase upgrade or renewal packs. However, there is no direct correlation between product sales and the number of new and continuing independent associates and members because independent associates and members utilize products at different volumes.
Product Sales
Substantially all of our product sales are made to independent associates at published wholesale prices. We also sell our products to independent members at discounted published retail prices.
39
For the year ended December 31, 2010, product sales decreased $26.7 million, or 12.5%, to $187.2 million, as compared to $213.9 million for the same period in 2009. The $26.7 million decrease in product sales was comprised of a decrease in existing product sales of $22.9 million and a decrease in new product sales of $3.8 million. We implemented a 2-5% price increase in most countries in late January 2010. We believe the decrease in product sales was due to the macro-economic factors negatively affecting our company and direct competition with other network marketing companies in recruiting and retaining independent associates.
The following new products were introduced during 2010:
| · | Mannatech LIFT™ Skin Care System in the United States and certain international markets; |
| · | Essential Source™ Omega 3 in several international markets; |
| · | Various promotional packages in the United States and several international markets; |
| · | Health Solutions Starter packs in Singapore and New Zealand; |
| · | GlycoSlim® drink mix (chocolate) in Japan; |
| · | Simply Delicious™ Snack Bars(1) in the United States and Canada; |
| · | PhytoBurst™ Nutritional Chews in Australia, Singapore, New Zealand, Japan, Korea, and Taiwan; |
| · | GI Pro Balance™ Slimstick in Singapore, New Zealand, Australia and Japan; and |
| · | BounceBack™ in Taiwan. |
_________________________
(1) We plan to discontinue Simply Delicious™ Snack Bars in 2011.
Pack Sales
Packs may be purchased by our independent associates who wish to build a Mannatech business. These packs are offered to our independent associates at a discount from published retail prices. There are several pack options available to our associates. In certain markets, pack sales are concluded during the associate registration process and can provide new associates with valuable training and promotional materials, as well as products for resale to retail customers, demonstration purposes, and personal consumption. Business-building associates can also purchase an upgrade pack, which provides associates with additional promotional materials and eligibility for additional commissions and incentives. Many of our business-building independent associates also choose to purchase renewal packs to satisfy annual renewal requirements to continue to earn various commissions.
The dollar amount of pack sales associated with the number of independent associates are as follows, for the years ended December 31 (in millions, except percentages):
| Change | ||||||||||||
| 2010 | 2009 | Dollar | Percentage | |||||||||
| New | $ | 21.4 | $ | 39.6 | $ | (18.2 | ) | (46.0 | )% | |||
| Continuing | 9.9 | 22.5 | (12.6 | ) | (56.0 | )% | ||||||
| Total | $ | 31.3 | $ | 62.1 | $ | (30.8 | ) | (49.6 | )% | |||
Total pack sales for the year ended December 31, 2010 decreased by $30.8 million, or 49.6%, to $31.3 million, as compared to $62.1 million for the same period in 2009.
The number of new and continuing independent associates and members who purchased our packs and/or products during the twelve months ended December 31, was as follows:
| 2010 | 2009 | |||||||||
| New | 89,000 | 22 | % | 145,000 | 28 | % | ||||
| Continuing | 314,000 | 78 | % | 368,000 | 72 | % | ||||
| Total | 403,000 | 100 | % | 513,000 | 100 | % | ||||
There was an overall decrease of 110,000, or 21.4%, for the year ended December 31, 2010 in the number of associates as compared to the same period in 2009, which was due both to a decline in the number of new independent associates and members, as well as fewer continuing independent associates and members.
40
During 2009 and 2010, we took the following actions to help increase the number of independent associates and members:
| • | registered our most popular products with the appropriate regulatory agencies in all countries of operations; |
| • | focused on new product development; |
| • | explored new international markets; |
| • | launched an aggressive marketing and educational campaign; |
| • | continued to strengthen compliance initiatives; |
| • | concentrated on publishing results of research studies and clinical trials related to our products; |
| • | initiated additional incentives; |
| • | explored new advertising and educational tools to broaden name recognition; |
| • | implemented changes to our global associate career and compensation plan; and |
| • | introduced new products in many of our global markets. |
Other Sales
Other sales consisted of (i) sales of promotional materials; (ii) training and event registration fees; (iii) monthly fees collected for Success Tracker™, a customized electronic business-building and educational materials database for our independent associates that helps stimulate product sales and provide business management; (iv) freight revenue charged to our independent associates and members; and (v) a reserve for estimated sales refunds and returns.
For the year ended December 31, 2010, other sales decreased by $4.1 million, or 29.9%, to $9.6 million as compared to $13.7 million for the same period in 2009. The decrease in other sales was primarily due to a $2.8 million decrease in freight fees for product and pack shipments, a $1.0 million decrease associated with an expiration of a transactional tax holiday for sales in certain international markets, a $0.2 million decrease in Success Tracker™ and training fees, and a $0.1 million decrease in sales of promotional materials.
Gross Profit
For the year ended December 31, 2010, gross profit increased by $1.5 million, or 1.6%, to $98.0 million, as compared to $96.5 million for the same period in 2009. For the year ended December 31, 2010, gross profit as a percentage of net sales increased to 43.0% as compared to 33.3% for the year ended December 31, 2009.
Cost of sales decreased for the year ended December 31, 2010, by 30.0%, or $14.1 million to $32.7 million as compared to $46.8 million for the same period in 2009. Cost of sales as a percentage of net sales decreased to 14.4%, as compared to 16.2% for the same period in 2009. The improvement reflected the impact of changes made to our pack sales in late 2009 and early 2010, including a reduction in the cost of packs and modification of bonus and pack components.
Commission costs decreased for the year ended December 31, 2010, by 32.2%, or $44.5 million, to $93.8 million, as compared to $138.3 million for the same period in 2009. The decrease in commissions was due to the decrease in commissionable net sales. For the year ended December 31, 2010, commissions as a percentage of net sales decreased to 41.1% as compared to 47.7% for the same period of 2009. The rate improvement was a result of changing various aspects of the Power Bonus program, as well as modification of our pack bonus structure and commission rates.
Incentive costs decreased for the year ended December 31, 2010, by 56.8%, or $4.6 million, to $3.5 million, as compared to $8.1 million for the same period in 2009. The costs of incentives, as a percentage of net sales, decreased to 1.5% for the year ended December 31, 2010, as compared to 2.8% for the same period in 2009. The decrease in total costs of annual incentives was the result of the number of independent associates who qualified for annual incentives, which decreased in 2010 by 69% to 744 as compared to 2,403 in 2009. The decrease in qualifiers for the annual incentive is primarily related to higher associate recruiting activity seen in 2009 due to the introduction of the $499 Premium/All-Star Pack.
41
Selling and Administrative Expenses
Selling and administrative expenses include a combination of both fixed and variable expenses. These expenses consist of compensation and benefits for employees, temporary and contract labor, outbound shipping and freight, and marketing-related expenses, such as monthly magazine development costs and costs related to hosting our corporate-sponsored events.
For the year ended December 31, 2010, overall selling and administrative expenses decreased $7.3 million, or 10.5%, to $62.7 million, as compared to $70.0 million for the same period in 2009. Selling and administrative expenses, as a percentage of net sales for the year ended December 31, 2010, increased to 27.5%, as compared to 24.2% for the same period in 2009. Compensation and compensation-related costs decreased $2.6 million primarily from a decrease in contract labor costs. Selling and marketing expenses decreased $2.4 million due to cost control in several areas of this category, such as advertising, public relations, promotions, and magazine publications. Freight costs decreased by $2.3 million due to a decrease in product shipments.
Other Operating Costs
Other operating costs include travel, accounting/legal/consulting fees, royalties, credit card processing fees, banking fees, off-site storage fees, utilities, and other miscellaneous operating expenses. Changes in other operating costs are associated with the changes in our net sales.
For the year ended December 31, 2010, other operating costs decreased by $4.4 million, or 11.1%, to $35.3 million as compared to $39.7 million for the same period in 2009. For the year ended December 31, 2010, other operating costs as a percentage of net sales increased to 15.5 % compared to 13.7 % for the same period in 2009. The decrease in other operating costs was primarily due to a reduction in credit card fees of $1.5 million, travel expenses of $0.9 million, and consulting fees of $0.8 million. Also contributing to the reduction in other operating costs was legal fees of $0.3 million, office expenses of $0.3 million, repairs and maintenance of $0.2 million, research and development of $0.3 million, and royalties of $0.1 million.
Depreciation and Amortization Expense
For the year ended December 31, 2010, depreciation and amortization expense was $11.5 million, as compared to $12.3 million for the same period in 2009. As a percentage of net sales, depreciation and amortization expense increased slightly to 5.0% from 4.3% for the same period in 2009.
Provision for Income Taxes
Provision for income taxes include current and deferred income taxes for both our domestic and foreign operations. Our statutory income tax rates by jurisdiction are as follows, for the years ended December 31:
| Country | 2010 | 2009 | |||
| Australia | 30.0 | % | 30.0 | % | |
| Canada | 30.0 | % | 33.0 | % | |
| Denmark | 25.0 | % | 25.0 | % | |
| Japan | 42.0 | % | 42.0 | % | |
| Mexico | 30.0 | % | — | ||
| Norway | 28.0 | % | 28.0 | % | |
| Republic of Korea | 24.2 | % | 24.2 | % | |
| Singapore | 17.0 | % | 17.0 | % | |
| South Africa | 28.0 | % | 28.0 | % | |
| Sweden | 26.3 | % | 26.3 | % | |
| Switzerland | 16.2 | % | 16.2 | % | |
| Taiwan | 17.0 | % | 25.0 | % | |
| United Kingdom | 28.0 | % | 28.0 | % | |
| United States | 37.5 | % | 37.5 | % | |
42
Income from our international operations is subject to taxation in the countries in which we operate. Although we may receive foreign income tax credits that would reduce the total amount of income taxes owed in the United States, we may not be able to utilize our foreign income tax credits in the United States.
We use the recognition and measurement provisions of FASB ASC Topic 740, Income Taxes, (“Topic 740”), to account for income taxes. The provisions of Topic 740 require a company to record a valuation allowance when the “more likely than not” criterion for realizing a deferred tax asset cannot be met. A company is to use judgment in reviewing both positive and negative evidence of realizing a deferred tax asset. Furthermore, the weight given to the potential effect of such evidence is commensurate with the extent the evidence can be objectively verified. As a result, we reviewed the operating results, as well as all of the positive and negative evidence related to realization of such deferred tax assets to evaluate the need for a valuation allowance in each tax jurisdiction.
As of December 31, 2010 and 2009, we maintained our valuation allowance for deferred tax assets in the following table (in millions), as we believe the “more likely than not” criterion for recognition and realization purposes, as defined in Topic 740, cannot be met.
| Country | 2010 | 2009 | |||
| Mexico | $ | 0.8 | $ | — | |
| Norway | 0.2 | 0.0 | |||
| Sweden | 0.1 | 0.0 | |||
| Switzerland | 0.5 | 0.3 | |||
| Taiwan | 1.0 | 0.9 | |||
| United States | 1.5 | 1.1 | |||
| Total | $ | 4.1 | $ | 2.3 | |
The dollar amount of the provisions for income taxes is directly related to our profitability and changes in taxable income among countries. For the year ended December 31, 2010, our effective income tax rate decreased to 3.8% from 27.9% for the same period in 2009. For 2010, our effective income tax rate was lower than what would be expected if the federal statutory income tax rate were applied to income before taxes primarily because of increases in the valuation allowance for deferred tax assets; increases in uncertain income tax positions and favorable differences from foreign operations. For 2009, our effective income tax rate was lower than expected if the federal statutory income tax rate were applied to income before taxes primarily because of increases in the valuation allowance for deferred tax assets and favorable differences from foreign operations.
43
Year Ended December 31, 2009 compared to Year Ended December 31, 2008
The tables below summarize our consolidated operating results in dollars and as a percentage of net sales for the years ended December 31, 2009 and 2008 (in thousands, except percentages).
| 2009 | 2008 | Change | |||||||||||||||||
Total Dollars | % of net sales | Total dollars | % of net sales | Dollar | Percentage | ||||||||||||||
| Net sales | $ | 289,705 | 100 | % | $ | 332,703 | 100 | % | $ | (42,998 | ) | (12.9 | )% | ||||||
| Cost of sales | 46,813 | 16.2 | % | 48,564 | 14.6 | % | (1,751 | ) | (3.6 | )% | |||||||||
| Commissions and incentives | 146,415 | 50.5 | % | 149,595 | 45.0 | % | (3,180 | ) | (2.1 | )% | |||||||||
| 193,228 | 66.7 | % | 198,159 | 59.6 | % | (4,931 | ) | (2.5 | )% | ||||||||||
| Gross profit | 96,477 | 33.3 | % | 134,544 | 40.4 | % | (38,067 | ) | (28.3 | )% | |||||||||
| Operating expenses: | |||||||||||||||||||
| Selling and administrative expenses | 69,997 | 24.2 | % | 81,077 | 24.4 | % | (11,080 | ) | (13.7 | )% | |||||||||
| Depreciation and amortization | 12,333 | 4.3 | % | 12,310 | 3.7 | % | 23 | 0.2 | % | ||||||||||
| Other operating costs | 39,741 | 13.7 | % | 55,656 | 16.7 | % | (15,915 | ) | (28.6 | )% | |||||||||
| Total operating expenses | 122,071 | 42.1 | % | 149,043 | 44.8 | % | (26,972 | ) | (18.1 | )% | |||||||||
| Loss from operations | (25,594 | ) | (8.8 | )% | (14,499 | ) | (4.4 | )% | (11,095 | ) | (76.5 | )% | |||||||
| Interest income | 473 | 0.2 | % | 1,604 | 0.5 | % | (1,131 | ) | (70.5 | )% | |||||||||
| Other income (expense), net | 1,046 | 0.4 | % | (5,303 | ) | (1.6 | )% | 6,349 | 119.7 | % | |||||||||
| Loss before income taxes | (24,075 | ) | (8.3 | )% | (18,198 | ) | (5.5 | )% | (5,877 | ) | (32.3 | )% | |||||||
| Benefit for income taxes | 6,707 | 2.3 | % | 5,570 | 1.7 | % | 1,137 | 20.4 | % | ||||||||||
| Net loss | $ | (17,368 | ) | (6.0 | )% | $ | (12,628 | ) | (3.8 | )% | $ | (4,740 | ) | (37.5 | )% | ||||
44
For geographical purposes, consolidated net sales primarily shipped to customers by location for the years ended December 31, 2009 and 2008 were as follows (in millions, except percentages):
Net Sales in Dollars and as a Percentage of Consolidated Net Sales
| 2009 | 2008 | ||||||||||||
| United States | $ | 140.7 | 48.6 | % | $ | 176.9 | 53.1 | % | |||||
| Japan | 42.0 | 14.5 | % | 44.8 | 13.5 | % | |||||||
| Republic of Korea | 26.4 | 9.1 | % | 35.7 | 10.7 | % | |||||||
| Canada | 23.0 | 7.9 | % | 23.6 | 7.1 | % | |||||||
| Australia | 22.9 | 7.9 | % | 26.1 | 7.8 | % | |||||||
South Africa(1) | 13.2 | 4.6 | % | 5.5 | 1.7 | % | |||||||
| Taiwan | 6.6 | 2.3 | % | 5.2 | 1.6 | % | |||||||
| New Zealand | 4.3 | 1.5 | % | 5.2 | 1.6 | % | |||||||
| United Kingdom | 3.3 | 1.0 | % | 4.7 | 1.4 | % | |||||||
| Germany | 3.2 | 1.1 | % | 3.8 | 1.1 | % | |||||||
| Denmark | 1.6 | 0.6 | % | 1.2 | 0.4 | % | |||||||
Singapore(2) | 1.5 | 0.5 | % | — | — | % | |||||||
Austria(3) | 0.3 | 0.1 | % | — | — | % | |||||||
Norway(3) | 0.3 | 0.1 | % | — | — | % | |||||||
The Netherlands(3) | 0.2 | 0.1 | % | — | — | % | |||||||
Sweden(3) | 0.2 | 0.1 | % | — | — | % | |||||||
| Total | $ | 289.7 | 100 | % | $ | 332.7 | 100 | % | |||||
_________________________
(1) South Africa began operations in May 2008.
(2) Singapore began operations in November 2008.
(3) Austria, the Netherlands, Norway, and Sweden began operations in September 2009.
Net Sales
For the year ended December 31, 2009, our operations outside of the United States accounted for approximately 51.4% of our consolidated net sales, whereas in the same period in 2008, our operations outside of the United States accounted for approximately 46.9% of our consolidated net sales.
Consolidated net sales for the year ended December 31, 2009 decreased by $43 million, or 12.9%, to $289.7 million as compared to $332.7 million for the same period in 2008. Domestic sales decreased $36.2 million, while international sales decreased $6.8 million.
Fluctuation in foreign currency exchange rates had an overall unfavorable impact on our net sales of approximately $4.6 million for year ended December 31, 2009. The net sales impact is calculated as the difference between (1) the current period’s net sales in USD and (2) the current period’s net sales in local currencies converted to USD by applying average exchange rates for the year ended December 31, 2008.
45
Net sales by country in transactional currency for the year ended December 31, 2009 and 2008 were as follows (in millions, except percentages):
| Change | |||||||||||
| Country | Transactional Currency | 2009 | 2008 | Transactional currency | Percentage | ||||||
Australia and Singapore(1) | AUD | 31.1 | 31.0 | 0.1 | 0.3 | % | |||||
Austria, Germany, the Netherlands(2) | EUR | 2.6 | 2.6 | — | — | ||||||
| Denmark | DKK | 8.6 | 6.3 | 2.3 | 36.5 | % | |||||
| Japan | JPY | 3,890.7 | 4,584.3 | (693.6 | ) | (15.1 | )% | ||||
| Korea | KRW | 33,366.8 | 38,733.4 | (5,366.6 | ) | (13.9 | )% | ||||
| New Zealand | NZD | 6.9 | 7.3 | (0.4) | (5.5 | )% | |||||
Norway(2) | NOK | 2.0 | — | 2.0 | — | ||||||
South Africa(3) | ZAR | 109.8 | 47.4 | 62.4 | 131.6 | % | |||||
Sweden(2) | SEK | 1.2 | — | 1.2 | — | ||||||
| Taiwan | TWD | 217.4 | 165.4 | 52.0 | 31.4 | % | |||||
| United Kingdom | GBP | 2.1 | 2.6 | (0.5 | ) | (19.2 | )% | ||||
_________________________
(1) Singapore began operations in November 2008.
(2) Austria, the Netherlands, Norway, and Sweden began operations in September 2009.
(3) South Africa began operations in May 2008.
Our sales mix for the years ended December 31, was as follows (in millions, except percentages):
| Change | ||||||||||||||||
| 2009 | 2008 | Dollar | Percentage | |||||||||||||
| Product sales | $ | 213.9 | $ | 260.5 | $ | (46.6 | ) | (17.9 | )% | |||||||
| Pack sales | 62.1 | 57.7 | 4.4 | 7.6 | % | |||||||||||
| Other, including freight | 13.7 | 14.5 | (0.8 | ) | (5.5 | )% | ||||||||||
| Total net sales | $ | 289.7 | $ | 332.7 | $ | (43.0 | ) | (12.9 | )% | |||||||
Although there was an overall decrease in consolidated net sales for the year ended December 31, 2009, as compared to the same period in 2008, which was primarily due to a decline in the volume of product sales, there was an increase in pack sales. Pack sales correlate to new independent associates who purchase starter packs and to continuing independent associates who purchase upgrade or renewal packs. However, there is no direct correlation between product sales and the number of new and continuing independent associates and members because independent associates and members utilize products at different volumes.
Product Sales
Substantially all of our product sales are made to independent associates at published wholesale prices. We also sell our products to independent members at discounted published retail prices.
For the year ended December 31, 2009, product sales decreased $46.6 million, or 17.9%, to $213.9 million, as compared to $260.5 million for the same period in 2008. The $46.6 million decrease in product sales was comprised of a decrease in existing product sales of $38.5 million and a decrease in new product sales of $8.1 million. We believe the decrease in product sales was primarily due to the macro-economic factors negatively impacting our company.
The following new products were introduced during 2009:
| · | Mannatech Optimal Skin Care System products in certain international markets; |
| · | OsoLean™ powder and/or OsoLean™ single use packets in all of our markets; |
| · | Various promotional packages in United States, Canada, South Africa, Taiwan, and Australia; |
| · | Health Solutions Starter packs in Australia, Singapore, and New Zealand; |
46
| · | GlycoSlim® drink mix in certain international markets; |
| · | Emprizone® in Japan; |
| · | Essential Source™ Omega 3 in United States, Canada, and South Africa; |
| · | PhytoBurst™ Nutritional Chews in United States, Canada and South Africa; |
| · | GI-ProBalance™ in South Korea; and |
| · | Various Optimal Health, Weight and Fitness products in Austria, the Netherlands, Norway and Sweden; |
Pack Sales
Packs may be purchased by our independent associates who wish to build a Mannatech business. These packs are offered to our independent associates at a discount from published retail prices. There are several pack options available to our associates. In certain markets, pack sales are concluded during the associate registration process and can provide new associates with valuable training and promotional materials, as well as products for resale to retail customers, demonstration purposes, and personal consumption. Business-building associates can also purchase an upgrade pack, which provides associates with additional promotional materials and eligibility for additional commissions and incentives. Many of our business-building independent associates also choose to purchase renewal packs to satisfy annual renewal requirements to continue to earn various commissions.
Pack sales associated with the number of independent associates can be analyzed as follows, for the years ended December 31 (in millions, except percentages):
| Change | |||||||||||||
| 2009 | 2008 | Dollar | Percentage | ||||||||||
| New | $ | 39.6 | $ | 28.0 | $ | 11.6 | 41.4 | % | |||||
| Continuing | 22.5 | 29.7 | (7.2 | ) | (24.2 | )% | |||||||
| Total | $ | 62.1 | $ | 57.7 | $ | 4.4 | 7.6 | % | |||||
Total pack sales for the year ended December 31, 2009 increased by $4.4 million, or 7.6%, to $62.1 million, as compared to $57.7 million for the same period in 2008. The overall increase in total pack sales was composed of an increase of $11.6 million related to a larger number of new independent associates purchasing starter packs, offset by a decrease of $7.2 million related to a decline in the number of renewal and upgrade packs purchased by our continuing independent associates.
The number of new and continuing independent associates and members who purchased our packs and/or products during the twelve months ended December 31, was as follows:
| 2009 | 2008 | |||||||||
| New | 145,000 | 28 | % | 133,000 | 25 | % | ||||
| Continuing | 368,000 | 72 | % | 398,000 | 75 | % | ||||
| Total | 513,000 | 100 | % | 531,000 | 100 | % | ||||
Although there was an overall decrease of 18,000, or 3.4%, for the year ended December 31, 2009 in associate activity as compared to the same period in 2008, which was due to fewer continuing independent associates and members, we had an increase in the number of new recruits. We consider new recruits to be a leading indicator of continued long-term success due to anticipated future sales. Due to the increase in new recruits, there was an increase in the number of starter pack sales as compared to the same period in 2008; but the decline in continuing independent associates resulted in a corresponding decline in renewal and upgrade pack sales as compared to the same period in 2008. The $499 Premium/All-Star Pack was successfully launched in United States, Canada, and South Africa in January 2009 and had stronger than expected sales and was responsible for an improvement in recruiting in 2009. Our new recruits increased by 9.2 % for the year ended December 31, 2009 as compared to the same period in 2008.
47
Other Sales
Other sales consisted of (i) sales of promotional materials; (ii) training and event registration fees; (iii) monthly fees collected for Success Tracker™, a customized electronic business-building and educational materials database for our independent associates that helps stimulate product sales and provide business management; (iv) freight revenue charged to our independent associates and members; and (v) a reserve for estimated sales refunds and returns.
For the year ended December 31, 2009, other sales decreased by $0.8 million, or 5.5%, to $13.7 million as compared to $14.5 million for the same period in 2008. The decrease in other sales primarily consisted of a $1.7 million decrease in freight fees due to the decrease in product shipments, as well as a $0.4 million decrease in Success Tracker™ and training fees and a $0.1 million decrease in sales of promotional materials, partially offset by a net change in sales refunds and returns of $1.3 million and a $0.1 increase in associate services.
Gross Profit
For the year ended December 31, 2009, gross profit decreased by $38 million, or 28.3%, to $96.5 million as compared to $134.5 million for the same period in 2008. For the year ended December 31, 2009, gross profit as a percentage of net sales decreased to 33.3% as compared to 40.4% for the year ended December 31, 2008. The reduction in gross profit is due to the decline in sales as well as the increase in commissions and incentives and cost of sales as a percentage of net sales.
Cost of sales decreased for the year ended December 31, 2009, by 3.6%, or $1.8 million to $46.8 million as compared to $48.6 million for the same period in 2008. Cost of sales as a percentage of net sales increased to 16.2% as compared to 14.6% for the same period in 2008. This increase was a result of an increase in freight cost, as well as the level of discount carried by the $499 Premium/All-Star Pack.
Commission costs decreased for the year ended December 31, 2009, by 2.7%, or $3.8 million, to $138.3 million as compared to $142.1 million for the same period in 2008. The decrease in commissions was primarily related to the decrease in commissionable net sales. For the year ended December 31, 2009, commissions as a percentage of net sales increased to 47.7% as compared to 42.7% for the same period of 2008, which was due to the introduction of the new $499 Premium/All-Star Pack in January of 2009 and the related pack bonuses.
Incentive costs increased for the year ended December 31, 2009, by 8%, or $0.6 million, to $8.1 million as compared to $7.5 million for the same period in 2008. The costs of incentives, as a percentage of net sales, increased to 2.8% for the year ended December 31, 2009, as compared to 2.3% for the same period in 2008.
The increase in total costs of annual incentives was the result of the number of independent associates who qualified for annual incentives, which increased in 2009 by 170.3% to 2,403 as compared to 889 in 2008. The increase in qualifiers for the annual incentive is primarily related to higher associate recruiting activity during 2009 and the introduction of the $499 Premium/All-Star Pack.
Selling and Administrative Expenses
Selling and administrative expenses include a combination of both fixed and variable expenses. These expenses consist of compensation and benefits for employees, temporary and contract labor, outbound shipping and freight, and marketing-related expenses, such as monthly magazine development costs and costs related to hosting our corporate-sponsored events.
For the year ended December 31, 2009, overall selling and administrative expenses decreased $11.1 million, or 13.7%, to $70 million as compared to $81.1 million for the same period in 2008. Selling and administrative expenses, as a percentage of net sales for the year ended December 31, 2009, decreased to 24.2%, as compared to 24.4% for the same period in 2008. Compensation and compensation-related costs decreased $9.4 million due to staff reductions in the first quarter of 2009. Freight costs decreased by $1.1 million due to a decrease in product shipments, and selling and marketing expenses decreased $0.6 million.
48
Other Operating Costs
Other operating costs include travel, accounting/legal/consulting fees, royalties, credit card processing fees, banking fees, off-site storage fees, utilities, and other miscellaneous operating expenses. Changes in other operating costs are associated with the changes in our net sales.
For the year ended December 31, 2009, other operating costs decreased by $16 million, or 28.6%, to $39.7 million as compared to $55.7 million for the same period in 2008. For the year ended December 31, 2009, other operating costs as a percentage of net sales decreased to 13.7% compared to 16.7 % for the same period in 2008. The decrease in other operating costs was primarily due to a $6.9 million reduction in legal expenses, as a result of 2008 litigation settlements, and a $5.9 million reduction in consulting fees. The decrease also consisted of a $1.2 million reduction in credit card fees, $0.6 million reduction in travel, $0.3 million reduction in repairs and maintenance, $0.1 million reduction in royalties, and $1.1 million reduction in miscellaneous other operating expenses; slightly offset by an increase of $0.1 million in research and development expense.
Depreciation and Amortization Expense
For the year ended December 31, 2009, depreciation and amortization expense was $12.3 million and remained relatively flat as compared to the same period in 2008. However, as a percentage of net sales, depreciation and amortization expense increased to 4.3% from 3.7% for the same period in 2008.
Provision for Income Taxes
Provision for income taxes include current and deferred income taxes for both our domestic and foreign operations. Our statutory income tax rates by jurisdiction are as follows, for the years ended December 31:
| Country | 2009 | 2008 | |||
| Australia | 30.0 | % | 30.0 | % | |
| Canada | 33.0 | % | 33.0 | % | |
| Denmark | 25.0 | % | 25.0 | % | |
| Japan | 42.0 | % | 42.0 | % | |
| Norway | 28.0 | % | — | ||
| Republic of Korea | 24.2 | % | 27.5 | % | |
| Singapore | 17.0 | % | 17.0 | % | |
| South Africa | 28.0 | % | 28.0 | % | |
| Sweden | 26.3 | % | — | ||
| Switzerland | 16.2 | % | 16.2 | % | |
| Taiwan | 25.0 | % | 25.0 | % | |
| United Kingdom | 28.0 | % | 28.0 | % | |
| United States | 37.5 | % | 37.5 | % | |
Income from our international operations is subject to taxation in the countries in which we operate. Although we may receive foreign income tax credits that would reduce the total amount of income taxes owed in the United States, we may not be able to utilize our foreign income tax credits in the United States.
We use the recognition and measurement provisions of FASB ASC Topic 740, Income Taxes, (“Topic 740”), to account for income taxes. The provisions of Topic 740 require a company to record a valuation allowance when the “more likely than not” criterion for realizing a deferred tax asset cannot be met. A company is to use judgment in reviewing both positive and negative evidence of realizing a deferred tax asset. Furthermore, the weight given to the potential effect of such evidence is commensurate with the extent the evidence can be objectively verified. As a result, we reviewed the operating results, as well as all of the positive and negative evidence related to realization of such deferred tax assets to evaluate the need for a valuation allowance in each tax jurisdiction.
49
In the fourth quarter of 2009, we reversed a valuation allowance against certain deferred tax assets because of new tax legislation that allowed the carry back of net operating losses for five years instead of two. This reversal resulted in an increase in tax benefit of approximately $3.0 million for the quarter and year ended December 31, 2009.
As of December 31, 2009 and 2008, we maintained our valuation allowance for deferred tax assets in the following table (in millions), as we believe the “more likely than not” criterion for recognition and realization purposes, as defined in Topic 740, cannot be met.
| Country | 2009 | 2008 | |||
| Switzerland | $ | 0.3 | $ | 0.0 | |
Taiwan(1) | 0.9 | 0.9 | |||
| United States | 1.1 | 0.0 | |||
| Total | $ | 2.3 | $ | 0.9 | |
_________________________
(1) The 2009 valuation allowance for Taiwan was adjusted to reflect the tax rate change effective for 2010. Without the rate change, the Taiwan valuation allowance would have been $1.1 million. |
The dollar amount of the provisions for income taxes is directly related to our profitability and changes in taxable income among countries. For the year ended December 31, 2009, our effective income tax rate decreased to 27.9% from 30.6% for the same period in 2008. For 2009, the Company’s effective income tax rate was lower than expected if the federal statutory income tax rate were applied to income before taxes primarily because of increases in the valuation allowance for deferred tax assets and favorable differences from foreign operations. For 2008, the Company’s effective income tax rate was lower than expected if the federal statutory income tax rate were applied to income before taxes primarily because of favorable differences from foreign operations.
Seasonality
We believe the impact of seasonality on our consolidated results of operations is minimal. We have experienced and believe we will continue to experience variations on our quarterly results of operations in response to, among other things:
| · | the timing of the introduction of new products and incentives; |
| · | our ability to attract and retain associates and members; |
| · | the timing of our incentives and contests; |
| · | the general overall economic outlook; |
| · | government regulations; |
| · | the outcome of certain lawsuits; |
| · | the perception and acceptance of network marketing; and |
| · | the consumer perception of our products and overall operations. |
As a result of these and other factors, our quarterly results may vary significantly in the future. Period-to-period comparisons should not be relied upon as an indication of future performance since we can give no assurances that revenue trends in new markets, as well as in existing markets, will follow our historical patterns. The market price of our common stock may also be adversely affected by the above factors.
Liquidity and Capital Resources
Our principal use of cash is to pay for operating expenses, including commissions and incentives, capital assets, inventory purchases, and international expansion and to pay quarterly cash dividends. In August 2009, the quarterly cash dividend was suspended and remained suspended as of December 31, 2010. We fund our business objectives, operations, and expansion of our operations through net cash flows from operations rather than incurring long-term debt. We plan to continue to fund our needs through net cash flows from operations. At December 31, 2010, we had $21.6 million in cash and cash equivalents that can be used, along with normal cash flows from operations, to fund any unanticipated shortfalls in future cash flows. Although our cash was impacted by operating losses for most of 2009, we saw our costs, particularly
50
Cash and Cash Equivalents
As of December 31, 2010, our cash and cash equivalents increased by 24.3%, or $4.2 million, to $21.6 million from $17.4 million as of December 31, 2009. The increase in cash and cash equivalents was related to the federal income tax refund of $8.1 million we received in April 2010, which is offset by the current period loss, adjusted for noncash items, purchases of property and equipment, and decreases of payables due to timing of expenses.
Working Capital
Working capital represents total current assets less total current liabilities. At December 31, 2010, our working capital increased by $1.2 million, or 5.4%, to $23.0 million from $21.8 million at December 31, 2009. The increase in working capital primarily related to an increase in cash and cash equivalents, an increase in prepaid expenses, a decrease in income tax receivable, a decrease in inventories, partially offset by a decrease in operating liabilities.
Net Cash Flows
Our net consolidated cash flows consisted of the following, for the years ended December 31 (in millions):
| 2010 | 2009 | 2008 | ||||||||||
| Provided by (used in): | ||||||||||||
| Operating activities | $ | 4.0 | $ | (10.3 | ) | $ | (19.9 | ) | ||||
| Investing activities | $ | 1.9 | $ | (1.3 | ) | $ | 7.2 | |||||
| Financing activities | $ | (1.4 | ) | $ | (1.5 | ) | $ | (5.9 | ) | |||
Our operating, investing, and financing activities are described in more detail below.
Operating Activities
For the years ended December 31, 2010, 2009, and 2008, our net operating activities provided cash of $4.0 million and used cash of $10.3 million and $19.9 million, respectively. For the years ended December 31, 2010, 2009, and 2008, net earnings adjusted for noncash activities provided cash of $1.9 million, and used cash of $1.9 million and $0.8 million, respectively, and our working capital accounts provided cash of $2.1 million and used cash of $8.4 million and $19.0 million, respectively.
We entered into an Investment Agreement with Dutchess Opportunity Fund, II, LP, a Delaware limited partnership (the “Investor”) on September 16, 2010. The Investor committed to purchase, subject to certain restrictions and conditions, up to $10 million of our common stock, over a period of 36 months from the first trading following the effectiveness of the registration statement, which was October 28, 2010 (the “Equity Line”). We may draw funds from the Equity Line by selling shares of common stock to the Investor from time to time. We will not receive any proceeds from the resale of these shares of common stock offered by the Investor. We will, however, receive proceeds from the sale of shares to the Investor pursuant to the Equity Line. The proceeds will be used for general working capital needs and for other general corporate purposes. Please see Note 14 (Shareholders’ Equity) to our consolidated financial statements for more information on this Investment Agreement.
We expect that our net operating cash flows in 2011 will be sufficient to fund our current operations. There can be no assurance, however, that we will continue to generate cash flows at or above current levels. Certain events, such as the uncertainty of the worldwide economic environment, could impact our available cash or our ability to generate cash flows from operations.
51
Investing Activities
For the years ended December 31, 2010, 2009, and 2008, our net investing activities provided cash of $1.9 million and used cash of $1.3 million and provided cash of $7.2 million, respectively.
In 2010, we used cash of $1.8 million to purchase capital assets and we had a decrease in restricted cash of $3.7 million. In 2009, we used cash of $2.8 million to purchase capital assets and we had a decrease in restricted cash of $1.5 million. In 2008, we converted our long-term investments to cash and cash equivalents, providing cash of $13.0 million, which was partially offset by the acquisition of capital assets of $5.6 million.
In 2011, we anticipate using cash of up to $3.0 million to purchase other capital assets for use in our operations, expansion of our corporate facilities, and planned international expansion.
Financing Activities
In 2010, we used cash of $1.4 million for repayment of capital lease obligations. There were no dividends declared to our shareholders in 2010.
In 2009, we used cash of $1.1 million to fund payment of cash dividends to our shareholders and $0.5 million for repayment of capital lease obligations. The amount of cash used was partially offset by the receipt of $0.1 million in stock option exercise transactions.
In 2008, we used cash of $5.9 million to fund our net financing activities. During 2008, we used cash of $5.8 million to fund payment of quarterly cash dividends to our shareholders and used cash of $0.1 million to repay capital leases.
General Liquidity and Cash Flows
We believe our existing liquidity and cash flows from operations are adequate to fund our normal expected future business operations and possible international expansion costs for the next 12 to 24 months. However, if our existing capital resources or cash flows become insufficient to meet current business plans, projections, and existing capital requirements, we may be required to raise additional funds, which may not be available on favorable terms, if at all.
Contractual Obligations. The following summarizes our future commitments and obligations associated with various agreements and contracts as of December 31, 2010, for the years ending December 31 (in thousands):
| 2011 | 2012 | 2013 | 2014 | 2015 | Thereafter | �� | Total | ||||||||||||||
| Capital lease obligations | $ | 1,445 | $ | 744 | $ | 386 | $ | 253 | $ | 3 | $ | — | $ | 2,831 | |||||||
Purchase obligations(1) | 13,772 | 2,685 | 1,200 | 1,200 | 1,200 | — | 20,057 | ||||||||||||||
| Operating leases | 3,346 | 2,972 | 1,744 | 1,094 | 1,007 | 965 | 11,128 | ||||||||||||||
| Post-employment royalty | 492 | 492 | 492 | 369 | — | — | 1,845 | ||||||||||||||
| Employment agreements | 2,290 | 133 | — | — | — | — | 2,423 | ||||||||||||||
| Total commitments and obligations | $ | 21,345 | $ | 7,026 | $ | 3,822 | $ | 2,916 | $ | 2,210 | $ | 965 | $ | 38,284 | |||||||
| _________________________ |
(1) Purchase obligations for the years 2011 and 2012 include $6.9 million, and $1.5 million, respectively, of purchase commitments under a contract terminated by the Company for an asserted breach. Pursuant to the terms of the contract, we are engaged in the arbitration process with the supplier. |
We have maintained purchase commitments with certain raw material suppliers to purchase minimum quantities and to ensure exclusivity of our raw materials and the proprietary nature of our products. Currently, we have four supply agreements that require minimum purchase commitments. We terminated one of these contracts for an asserted breach in 2009 and are now engaged in the arbitration process with the supplier. We also maintain other supply agreements and manufacturing agreements to protect our products, regulate product costs, and help ensure quality control standards. These agreements do not require us to purchase any set minimums. We have no present commitments or agreements with respect to acquisitions or purchases of any manufacturing facilities; however, management from time to time explores the possibility of the benefits of purchasing a raw material manufacturing facility to help control costs of our raw materials and help ensure quality control standards.
52
Off–Balance Sheet Arrangements
We do not have any special-purpose entity arrangements, nor do we have any off-balance sheet arrangements.
Market Risks
Please see “Quantitative and Qualitative Disclosure about Market Risk” under Item 7A of this Form 10-K for additional information about our Market Risks.
Critical Accounting Policies and Estimates
Our consolidated financial statements are prepared in accordance with accounting principles generally accepted in the United States of America (“GAAP”). The application of GAAP requires us to make estimates and assumptions that affect the reported values of assets and liabilities at the date of our financial statements, the reported amounts of revenues and expenses during the reporting period, and the related disclosures of contingent assets and liabilities. We use estimates throughout our financial statements, which are influenced by management’s judgment and uncertainties. Our estimates are based on historical trends, industry standards, and various other assumptions that we believe are applicable and reasonable under the circumstances at the time the consolidated financial statements are prepared. Our Audit Committee reviews our critical accounting policies and estimates. We continually evaluate and review our policies related to the portrayal of our consolidated financial position and consolidated results of operations that require the application of significant judgment by our management. We also analyze the need for certain estimates, including the need for such items as allowance for doubtful accounts, inventory reserves, long-lived fixed assets and capitalization of internal-use software development costs, reserve for uncertain income tax positions and tax valuation allowances, revenue recognition, sales returns, and deferred revenues, accounting for stock-based compensation, and contingencies and litigation. Historically, actual results have not materially deviated from our estimates. However, we caution readers that actual results could differ from our estimates and assumptions applied in the preparation of our consolidated financial statements. If circumstances change relating to the various assumptions or conditions used in our estimates, we could experience an adverse effect on our financial position, results of operations, and cash flows. We have identified the following applicable critical accounting policies and estimates as of December 31, 2010:
Allowance for Doubtful Accounts
Accounts receivable is carried at estimated collectible amounts and primarily consists of receivables from independent associates and members. As of December 31, 2010, net accounts receivable totaled $0.4 million. We receive payment for an order when the order ships. If the payment is rejected or if it does not match the order total, a receivable is created. We periodically review receivables for realizability and base collectability upon assumptions, historical trends, and recent account activities. If our estimates regarding estimated collectability are inaccurate or consumer trends change in an unforeseen manner, we may be exposed to additional write-offs or bad debts. As of December 31, 2010, we had an allowance for doubtful accounts of less than $0.1 million.
Inventory Reserves
Inventory consists of raw materials, work in progress, finished goods, and promotional materials that are stated at the lower of cost (using standard costs that approximate average costs) or market. We record the amounts charged by the vendors as the costs of inventory. Typically, the net realizable value of our inventory is higher than the aggregate cost. Determination of net realizable value can be complex and, therefore, requires a high degree of judgment. In order for management to make the appropriate determination of net realizable value, the following items are considered: inventory turnover statistics, current selling prices, seasonality factors, consumer demand, regulatory changes, competitive pricing, and performance of similar products. If we determine the carrying value of inventory is in excess of estimated net
realizable value, we write down the value of inventory to the estimated net realizable value.
We also review inventory for obsolescence in a similar manner and any inventory identified as obsolete is reserved or written off. Our determination of obsolescence is based on assumptions about the demand for our products, product expiration dates, estimated future sales, and general future plans. We monitor actual sales compared to original projections, and if actual sales are less favorable than those originally projected by us, we record an additional inventory reserve or write-down. Historically, our estimates have been close to our actual reported amounts. However, if our estimates regarding inventory obsolescence are inaccurate or consumer demand for our products changes in an unforeseen manner, we may be exposed to additional material losses or gains in excess of our established estimated inventory reserves. At December 31, 2010 and 2009, our inventory reserves were $1.6 million and $1.4 million, respectively.
53
Long Lived Fixed Assets and Capitalization of Software Development Costs
In addition to capitalizing long-lived fixed asset costs, we also capitalize costs associated with internally developed software projects (collectively “fixed assets”) and amortize such costs over the estimated useful lives of such fixed assets. Fixed assets are carried at cost less accumulated depreciation computed using the straight-line method over the assets’ estimated useful lives. Leasehold improvements are amortized over the shorter of the remaining lease terms or the estimated useful lives of the improvements. Expenditures for maintenance and repairs are charged to operations as incurred. If a fixed asset is sold or otherwise retired or disposed of, the cost of the fixed asset and the related accumulated depreciation or amortization is written off and any resulting gain or loss is recorded in other operating costs in our consolidated statement of operations.
We review our fixed assets for impairment whenever an event or change in circumstances indicates the carrying amount of an asset or group of assets may not be recoverable, such as plans to dispose of an asset before the end of its previously estimated useful life. Our impairment review includes a comparison of future projected cash flows generated by the asset, or group of assets, with its associated net carrying value. If the net carrying value of the asset or group of assets exceeds expected cash flows (undiscounted and without interest charges), an impairment loss is recognized to the extent the carrying amount exceeds the fair value. The fair value is determined by calculating the discounted expected future cash flows using an estimated risk-free rate of interest. Any identified impairment losses are recorded in the period in which the impairment occurs. The carrying value of the fixed asset is adjusted to the new carrying value and any subsequent increases in fair value of the fixed asset are not recorded. In addition, if we determine the estimated remaining useful life of the asset should be reduced from our original estimate, the periodic depreciation expense is adjusted prospectively, based on the new remaining useful life of the fixed asset.
The impairment calculation requires us to apply judgment and estimates concerning future cash flows, strategic plans, useful lives, and discount rates. If actual results are not consistent with our estimates and assumptions, we may be exposed to an additional impairment charge, which could be material to our results of operations. In addition, if accounting standards change, or if fixed assets become obsolete, we may be required to write off any unamortized costs of fixed assets; or if estimated useful lives change, we would be required to accelerate depreciation or amortization periods and recognize additional depreciation expense in our consolidated statement of operations.
Historically, our estimates and assumptions related to the carrying value and the estimated useful lives of our fixed assets have not materially deviated from actual results. As of December 31, 2010, the estimated useful lives and net carrying values of fixed assets are as follows:
| Estimated useful life | Net carrying value at December 31, 2010 | |||||
| Office furniture and equipment | 5 to 7 | years | $ | 1.8 million | ||
| Computer hardware and software | 3 to 5 | years | 13.5 million | |||
| Automobiles | 3 to 5 | years | 0.1 million | |||
| Leasehold improvements | 2 to 10 | years(1) | 3.0 million | |||
| Construction in progress | 2 to 10 | years(2) | 0.6 million | |||
| Total net carrying value at December 31, 2010 | $ | 19.0 million | ||||
_____________________________
(1) We amortize leasehold improvements over the shorter of the useful estimated life of the leased asset or the lease term.
(2) Construction in process includes leasehold improvements and internally developed software costs. Once placed in service, leasehold improvements will be amortized over the shorter of an asset’s useful life or the remaining lease term. Once the internally-developed software is placed in service, it will be amortized over three to five years.
The net carrying costs of fixed assets and construction in progress are exposed to impairment losses if our assumptions and estimates of their carrying values change, there is a change in estimated future cash flow, or there is a change in the estimated useful life of the fixed asset. We determined that no impairment indicators existed during the years ended December 31, 2010, 2009, and 2008.
54
Uncertain Income Tax Positions and Tax Valuation Allowances
As of December 31, 2010, we recorded $2.1 million in other long-term liabilities related to uncertain income tax positions. As required by Topic 740, we use judgments and make estimates and assumptions related to evaluating the probability of uncertain income tax positions. We base our estimates and assumptions on the potential liability related to an assessment of whether the income tax position will “more likely than not” be sustained in an income tax audit. We are also subject to periodic audits from multiple domestic and foreign tax authorities related to income tax, sales and use tax, personal property tax, and other forms of taxation. These audits examine our tax positions, timing of income and deductions, and allocation procedures across multiple jurisdictions. As part of our evaluation of these tax issues, we establish reserves in our consolidated financial statements based on our estimate of current probable tax exposures. Depending on the nature of the tax issue, we could be subject to audit over several years. Therefore, our estimated reserve balances and liability related to uncertain income tax positions may exist for multiple years before the applicable statute of limitations expires or before the taxing authority resolves an issue. We believe our tax liabilities related to uncertain tax positions are based upon reasonable judgment and estimates; however, if actual results materially differ, our effective income tax rate and cash flows could be affected in the period of discovery or resolution.
We also review the estimates and assumptions used in evaluating the probability of realizing the future benefits of our deferred tax assets and record a valuation allowance when we believe that a portion or all of the deferred tax assets may not be realized. If we are unable to realize the expected future benefits of our deferred tax assets, we are required to provide a valuation allowance. We use our history and experience, overall profitability, future management plans, and current economic information to evaluate the amount of valuation allowance to record. As of December 31, 2010, we maintained a valuation allowance for deferred tax assets arising from our operations of $4.1 million because they did not meet the “more likely than not” criteria as defined by the recognition and measurement provisions of Topic 740. In addition, as of December 31, 2010, we had deferred tax assets, after valuation allowance, totaling $5.9 million, which may not be realized if our assumptions and estimates change, which would affect our effective income tax rate and cash flows in the period of discovery or resolution.
Revenue Recognition
We derive revenue from sales of individual products, sales of starter and renewal packs, and shipping fees. Substantially all product and pack sales are made to independent associates at published wholesale prices and to members at discounted published retail prices. We record revenue net of any sales taxes and records a reserve for expected sales returns based on its historical experience.
We recognize revenue from shipped packs and products upon receipt by the customer. We recognize corporate-sponsored event revenue when the event is held. We defer certain components of our revenue. At December 31, 2010 and December 31, 2009, deferred revenue was $1.9 million and $2.8 million, respectively, and consisted primarily of revenue received from: (i) sales of packs and products shipped but not received by the customers by period end; and (ii) prepaid registration fees from customers planning to attend a future corporate-sponsored event.
We estimate a sales return reserve for expected sales refunds based on historical experience over a rolling six month period. If actual results differ from our estimated sales return reserve due to various factors, the amount of revenue recorded each period could be materially affected. Historically, sales returns have not materially changed through the years, as the majority of our customers who return their merchandise do so within the first 90 days after the original sale. Sales returns have averaged 1.5% or less of our gross sales. For the year ended December 31, 2010 our sales return reserve was composed of the following (in thousands):
| Sales reserve as of January 1, 2010 | $ | 595 | |
| Provision related to sales made in 2010 | 1,948 | ||
| Provision related to sales made prior to 2010 | 113 | ||
| Actual returns or credits in 2010 related to 2010 | (1,557 | ) | |
| Actual returns or credits in 2010 related to prior periods | (711 | ) | |
| Sales reserve as of December 31, 2010 | $ | 388 | |
55
We grant stock options to our employees, board members, and consultants. At the date of grant, we determine the fair value of a stock option award and recognize compensation expense over the requisite service period, or the vesting period of such stock option award, which is two to four years. The fair value of the stock option award is calculated using the Black-Scholes option-pricing model, (“calculated fair value”). The Black-Scholes option-pricing model requires us to apply judgment and use highly subjective assumptions, including expected stock option life, expected volatility, expected average risk-free interest rates, and expected forfeiture rates. For the year ended December 31, 2010, our assumptions and estimates used for the calculated fair value of stock options granted in 2010 were as follows:
February 2010 grant | May 2010 grant | June 2010 grant #1 | June 2010 grant #2 | September 2010 grant #1 | September 2010 grant #2 | ||||||||||||||
| Estimated fair value per share of options granted: | $ | 2.03 | $ | 1.86 | $ | 1.23 | $ | 1.18 | $ | 1.26 | $ | 1.40 | |||||||
| Assumptions: | |||||||||||||||||||
| Annualized dividend yield | 0.00 | % | 0.00 | % | 0.00 | % | 0.00 | % | 0.00 | % | 0.00 | % | |||||||
| Risk-free rate of return | 2.16 | % | 2.07 | % | 1.91 | % | 1.91 | % | 1.91 | % | 1.24 | % | |||||||
| Common stock price volatility | 71.1 | % | 70.8 | % | 71.5 | % | 71.5 | % | 72.40 | % | 72.3 | % | |||||||
| Expected average life of stock options (in years) | 4.5 | 4.5 | 4.5 | 4.5 | 4.5 | 4.5 | |||||||||||||
Historically, our estimates and underlying assumptions have not materially deviated from our actual reported results and rates. However, we base assumptions we use on our best estimates, which involves inherent uncertainties based on market conditions that are outside of our control. If actual results are not consistent with the assumptions we use, the stock-based compensation expense reported in our consolidated financial statements may not be representative of the actual economic cost of stock-based compensation. For example, if actual employee forfeitures significantly differ from our estimated forfeitures, we may be required to make an adjustment to our consolidated financial statements in future periods. As of December 31, 2010, using our current assumptions and estimates, we anticipate recognizing $0.6 million in gross compensation expense through 2013 related to unvested stock options outstanding.
If we grant additional stock options in the future, we would be required to recognize additional compensation expense over the vesting period of such stock options in our consolidated statement of operations. Gross compensation expense would equal the calculated fair value of such stock options, which is dependent on the assumptions used to calculate such fair value, but ranges between 34% to 69% of the exercise price multiplied by the number of stock options awarded. As of December 31, 2010, we had 334,812 shares available for grant in the future.
Contingencies and Litigation
Each quarter, we evaluate the need to establish a reserve for any legal claims or assessments. We base our evaluation on our best estimates of the potential liability in such matters. The legal reserve includes an estimated amount for any damages and the probability of losing any threatened legal claims or assessments. The legal reserve is developed in consultation with our general and outside counsel and is based upon a combination of litigation and settlement strategies. Although we believe that our legal reserves and accruals are based on reasonable judgments and estimates, actual results could differ, which may expose us to material gains or losses in future periods. If actual results differ, if circumstances change, or if we experience an unanticipated adverse outcome of any legal action, including any claim or assessment, we would be required to recognize the estimated amount that could reduce net income, earnings per share, and cash flows.
Recent Accounting Pronouncements
See “Recent Accounting Pronouncements” in Note 2 of the Notes to our Consolidated Financial Statements, which is incorporated herein by reference.
Item 7A. Quantitative and Qualitative Disclosures About Market Risk
56
We do not engage in trading market risk sensitive instruments and do not purchase investments as hedges or for purposes “other than trading” that are likely to expose us to certain types of market risk, including interest rate, commodity price, or equity price risk. Although we have investments, we believe there has been no material change in our exposure to interest rate risk. We have not issued any debt instruments, entered into any forward or futures contracts, purchased any options, or entered into any swap agreements.
We are exposed, however, to other market risks, including changes in currency exchange rates as measured against the United States dollar. Because the change in value of the United States dollar measured against foreign currency may affect our consolidated financial results, changes in foreign currency exchange rates could positively or negatively affect our results as expressed in United States dollars. For example, when the United States dollar strengthens against foreign currencies in which our products are sold or weakens against foreign currencies in which we may incur costs, our consolidated net sales or related costs and expenses could be adversely affected.
We believe inflation has not had a material impact on our consolidated operations or profitability. We expanded into Canada in 1996, into Australia in 1998, into the United Kingdom in 1999, into Japan in 2000, into New Zealand in 2002, into the Republic of Korea in 2004, into Taiwan and Denmark in 2005, into Germany in 2006, into South Africa and Singapore in 2008, into Austria, the Netherlands, Norway, and Sweden in September 2009, and into Mexico in January 2011. We translate our revenues and expenses in foreign markets using an average rate.
We maintain policies, procedures, and internal processes in an effort to help monitor any significant market risks and we do not use any financial instruments to manage our exposure to such risks. We assess the anticipated foreign currency working capital requirements of our foreign operations and maintain a portion of our cash and cash equivalents denominated in foreign currencies sufficient to satisfy most of these anticipated requirements.
We caution that we cannot predict with any certainty our future exposure to such currency exchange rate fluctuations or the impact, if any, such fluctuations may have on our future business, product pricing, operating expenses, and on our consolidated financial position, results of operations, or cash flows. However, to combat such market risk, we closely monitor our exposure to currency fluctuations. The foreign currencies in which we currently have exposure to foreign currency exchange rate risk include the currencies of Canada, Australia, the United Kingdom, Japan, New Zealand, the Republic of Korea, Taiwan, Denmark, Germany, South Africa, Singapore, Austria, the Netherlands, Norway, Sweden, and Mexico. The current (spot) rate, average currency exchange rates, and the low and high of such currency exchange rates as compared to the United States dollar, for each of these countries as of and for the year ended December 31, 2010 were as follows:
| Country (foreign currency name) | Low | High | Average | Spot | |||||
| Australia (Dollar) | 0.81520 | 1.01640 | 0.91994 | 1.01630 | |||||
| Austria, Germany, the Netherlands (Euro) | 1.19440 | 1.45130 | 1.32788 | 1.32530 | |||||
| Canada (Dollar) | 0.92880 | 1.00340 | 0.97101 | 1.00010 | |||||
| Denmark (Krone) | 0.16060 | 0.19520 | 0.17828 | 0.17800 | |||||
| Japan (Yen) | 0.01057 | 0.01245 | 0.01142 | 0.01226 | |||||
| Mexico (Peso) | 0.07580 | 0.08220 | 0.07913 | 0.08070 | |||||
| New Zealand (Dollar) | 0.66310 | 0.79610 | 0.72191 | 0.76900 | |||||
| Norway (Krone) | 0.14970 | 0.17800 | 0.16580 | 0.16960 | |||||
| Republic of Korea (Won) | 0.00080 | 0.00092 | 0.00087 | 0.00088 | |||||
| Singapore (Dollar) | 0.70340 | 0.77900 | 0.73436 | 0.77480 | |||||
| South Africa (Rand) | 0.12600 | 0.15090 | 0.13721 | 0.15090 | |||||
| Sweden (Krona) | 0.12370 | 0.15330 | 0.13921 | 0.14760 | |||||
| Switzerland (Franc) | 0.86000 | 1.06320 | 0.96144 | 1.06320 | |||||
| Taiwan (Dollar) | 0.03074 | 0.03444 | 0.03175 | 0.03444 | |||||
| United Kingdom (British Pound) | 1.43380 | 1.63820 | 1.54631 | 1.54710 |
57
Item 8. Financial Statements and Supplementary Data
Our Consolidated Financial Statements and Supplementary Data required by this Item 8 are set forth in Item 15 of this report.
The following table sets forth our unaudited quarterly Consolidated Statements of Operations data for the periods indicated. In our opinion, this information has been prepared on the same basis as our audited consolidated financial statements set forth in this report and includes all adjustments that are considered necessary to present fairly this information in accordance with generally accepted accounting principles. The reader should read this information in conjunction with Item 15 in this report.
Mar. 31, 2010 | June 30, 2010 | Sept. 30, 2010 | Dec. 31, 2010 | Mar. 31, 2009 | June 30, 2009 | Sept. 30, 2009 | Dec. 31, 2009) | ||||||||||||||||||
| (in millions, except per share information) | |||||||||||||||||||||||||
| Net sales | $ | 60.7 | $ | 57.6 | $ | 54.9 | $ | 54.9 | $ | 70.7 | $ | 77.6 | $ | 71.3 | $ | 70.1 | |||||||||
| Gross profit | $ | 25.0 | $ | 25.0 | $ | 23.8 | $ | 24.2 | $ | 25.2 | $ | 18.9 | $ | 24.1 | $ | 28.2 | |||||||||
| Income (loss) before income taxes | $ | (2.8 | ) | $ | (2.8 | ) | $ | (3.3 | ) | $ | (2.1 | ) | $ | (7.0 | ) | $ | (9.6 | ) | $ | (7.7 | ) | $ | 0.2 | ||
| (Provision) benefit for income taxes | $ | — | $ | (1.0 | ) | $ | 2.0 | $ | (0.6 | ) | $ | (2.2 | ) | $ | (4.1 | ) | $ | 1.5 | $ | (1.9 | ) | ||||
| Net income (loss) | $ | (2.8 | ) | $ | (3.8 | ) | $ | (1.3 | ) | $ | (2.7 | ) | $ | (4.8 | ) | $ | (5.5 | ) | $ | (9.2 | ) | $ | 2.1 | ||
| Earnings (loss) per share: | |||||||||||||||||||||||||
| Basic | $ | (0.11 | ) | $ | (0.14 | ) | $ | (0.05 | ) | $ | (0.10 | ) | $ | (0.18 | ) | $ | (0.21 | ) | $ | (0.35 | ) | $ | 0.08 | ||
| Diluted | $ | (0.11 | ) | $ | (0.14 | ) | $ | (0.05 | ) | $ | (0.10 | ) | $ | (0.18 | ) | $ | (0.21 | ) | $ | (0.35 | ) | $ | 0.08 | ||
None.
Evaluation of Disclosure Controls and Procedures
Our management, with the participation of our Co-Chief Executive Officer (principal executive officer) and our Chief Financial Officer (principal financial officer) have concluded, based on their evaluation as of the end of the period covered by this report, that our disclosure controls and procedures (as defined in Rule 13a-15(e) or Rule 15d – 15(e) under the Exchange Act) are effective to ensure that information required to be disclosed by us in reports filed or submitted under the Exchange Act is recorded, processed, summarized, and reported within the time periods specified in the SEC’s rules and forms and include controls and procedures designed to ensure that information required to be disclosed by us in such reports is accumulated and communicated to our management, including our principal executive and financial officers, as appropriate, to allow timely decisions regarding required disclosure.
Changes in Internal Control over Financial Reporting
During the quarter ended December 31, 2010, there were no changes in our internal control over our financial reporting that we believe materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
58
REPORT OF MANAGEMENT ON INTERNAL CONTROL OVER FINANCIAL REPORTING
Our management is responsible for establishing and maintaining adequate internal control over financial reporting (as defined in Rule 13a – 13(f) or Rule 15d-15(f) under the Exchange Act) for the Company. Internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of our financial reporting for external purposes in accordance with accounting principles generally accepted in the United States of America. Internal control over financial reporting includes maintaining records that in reasonable detail accurately and fairly reflect our transactions; providing reasonable assurance that transactions are recorded as necessary for preparation of our consolidated financial statements; providing reasonable assurance that receipts and expenditures of company assets are made in accordance with management authorization; and providing reasonable assurance that unauthorized acquisition, use or disposition of company assets that could have a material effect on our consolidated financial statements would be prevented or detected on a timely basis. Because of its inherent limitations, internal control over financial reporting is not intended to provide absolute assurance that a misstatement of our consolidated financial statements would be prevented or detected.
Management conducted an evaluation of the effectiveness of our internal control over financial reporting based on the framework in Internal Control — Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on this evaluation, management concluded that the Company’s internal control over financial reporting was effective as of December 31, 2010.
59
None.
The information required by Items 10, 11, 12, 13, and 14 of Part III is incorporated by reference to our definitive proxy statement to be filed with the SEC no later than April 30, 2011.
PART IV
(a) Documents filed as a part of the report:
1. Consolidated Financial Statements
The following financial statements and the Reports of Independent Registered Public Accounting Firms are filed as a part of this report on the pages indicated:
2. Financial Statement Schedule
The financial statement schedule required by this item is included as an Exhibit to this Annual Report on Form 10-K.
Report of Independent Registered Public Accounting Firm on Financial Statement Schedule.
3. Exhibit List
See Index to Exhibits following our Consolidated Financial Statements contained in this Annual Report on Form 10-K.
60
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| Mannatech, Incorporated | |||
Dated: March 10, 2011 | By: | /s/ Stephen D. Fenstermacher | |
Stephen D. Fenstermacher Co-Chief Executive Officer and Chief Financial Officer | |||
Dated: March 10, 2011 | By: | /s/ Robert A. Sinnott | |
Robert A. Sinnott Co-Chief Executive Officer and Chief Science Officer | |||
61
POWER OF ATTORNEY
The undersigned directors and officer of Mannatech, Incorporated hereby constitute and appoint Larry A. Jobe and Stephen D. Fenstermacher, and each of them, with the power to act without the other and with full power of substitution and resubstitution, our true and lawful attorneys-in fact and agents with full power to execute in our name and behalf in the capacities indicated below any and all amendments to this report and to file the same, with all exhibits and other documents relating thereto and hereby ratify and confirm all that such attorneys-in-fact, or either of them, or their substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed by the following persons on behalf of the registrant and in the capacities indicated:
| Signature | Title | Date | ||
| /s/ Stephen D. Fenstermacher | Co-Chief Executive Officer and Chief Financial Officer (principal financial and accounting officer) | March 10, 2011 | ||
| Stephen D. Fenstermacher | ||||
| /s/ Robert A. Sinnott | Co-Chief Executive Officer and Chief Science Officer (principal executive officer) | March 10, 2011 | ||
| Robert A. Sinnott | ||||
| /s/ J. Stanley Fredrick | Chairman of the Board | March 10, 2011 | ||
| J. Stanley Fredrick | ||||
| /s/ Patricia A. Wier | Director | March 10, 2011 | ||
| Patricia A. Wier | ||||
| /s/ Alan D. Kennedy | Director | March 10, 2011 | ||
| Alan D. Kennedy | ||||
| /s/ Gerald E. Gilbert | Director | March 10, 2011 | ||
| Gerald E. Gilbert | ||||
| /s/ Marlin Ray Robbins | Director | March 10, 2011 | ||
| Marlin Ray Robbins | ||||
| /s/ Larry A. Jobe | Director | March 10, 2011 | ||
| Larry A. Jobe | ||||
| /s/ Robert A. Toth | Director | March 10, 2011 | ||
| Robert A. Toth | ||||
62
| Page | |
F-1
Board of Directors and Shareholders
Mannatech Incorporated
Coppell, Texas
We have audited the accompanying consolidated balance sheets of Mannatech, Incorporated as of December 31, 2010 and 2009 and the related consolidated statements of operations, shareholders’ equity and comprehensive income, and cash flows for each of the three years in the period ended December 31, 2010. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of their internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements and schedules. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of Mannatech, Incorporated and subsidiaries at December 31, 2010 and 2009 and the results of their operations and their cash flows for each of the three years in the period ended December 31, 2010 in conformity with accounting principles generally accepted in the United States of America.
As discussed in Note 2 to the consolidated financial statements, the Company has adopted FASB Accounting Standards Codification Topic 820, Fair Value Measurements and Disclosures (formerly FASB Statement No. 157, Fair Value Measurements) as of January 1, 2008.
/s/ BDO USA, LLP
Dallas, Texas
March 10, 2011
F-2
CONSOLIDATED BALANCE SHEETS
(in thousands, except share and per share information)
| December 31, | |||||||
| 2010 | 2009 | ||||||
| ASSETS | |||||||
| Cash and cash equivalents | $ | 21,584 | $ | 17,367 | |||
| Restricted cash | 1,265 | 1,288 | |||||
| Accounts receivable, net of allowance of $21 and $16 in 2010 and 2009, respectively | 416 | 664 | |||||
| Income tax receivable | 917 | 8,075 | |||||
| Inventories, net | 24,070 | 31,290 | |||||
| Prepaid expenses and other current assets | 4,356 | 3,139 | |||||
| Deferred tax assets | 2,607 | 2,662 | |||||
| Total current assets | 55,215 | 64,485 | |||||
| Property and equipment, net | 18,449 | 27,144 | |||||
| Construction in progress | 524 | 317 | |||||
| Long-term restricted cash | 3,532 | 7,201 | |||||
| Other assets | 3,054 | 2,503 | |||||
| Long-term deferred tax assets | 649 | 652 | |||||
| Total assets | $ | 81,423 | $ | 102,302 | |||
| LIABILITIES AND SHAREHOLDERS’ EQUITY | |||||||
| Current portion of capital leases | $ | 1,328 | $ | 847 | |||
| Accounts payable | 5,534 | 11,319 | |||||
| Accrued expenses | 10,318 | 14,231 | |||||
| Commissions and incentives payable | 9,166 | 10,624 | |||||
| Taxes payable | 3,721 | 2,577 | |||||
| Current deferred tax liability | 243 | 274 | |||||
| Deferred revenue | 1,930 | 2,807 | |||||
| Total current liabilities | 32,240 | 42,679 | |||||
| Capital leases, excluding current portion | 1,204 | 1,068 | |||||
| Long-term deferred tax liabilities | 1,903 | 3,923 | |||||
| Other long-term liabilities | 4,996 | 3,348 | |||||
| Total liabilities | 40,343 | 51,018 | |||||
| Commitments and contingencies | |||||||
| Shareholders’ equity: | |||||||
| Preferred stock, $0.01 par value, 1,000,000 shares authorized, no shares issued or outstanding | — | — | |||||
Common stock, $0.0001 par value, 99,000,000 shares authorized, 27,697,560 shares issued and 26,490,466 shares outstanding as of December 31, 2010 and 27,687,882 shares issued and 26,480,788 shares outstanding as of December 31, 2009 | 3 | 3 | |||||
| Additional paid-in capital | 42,049 | 41,442 | |||||
| Retained earnings | 15,127 | 25,743 | |||||
| Accumulated other comprehensive loss | (1,308 | ) | (1,113 | ) | |||
| Less treasury stock, at cost, 1,207,094 shares in 2010 and 2009 | (14,791 | ) | (14,791 | ) | |||
| Total shareholders’ equity | 41,080 | 51,284 | |||||
| Total liabilities and shareholders’ equity | $ | 81,423 | $ | 102,302 | |||
See accompanying notes to consolidated financial statements.
F-3
CONSOLIDATED STATEMENTS OF OPERATIONS
(in thousands, except per share information)
| For the years ended December 31, | |||||||||||
| 2010 | 2009 | 2008 | |||||||||
| Net sales | $ | 228,088 | $ | 289,705 | $ | 332,703 | |||||
| Cost of sales | 32,754 | 46,813 | 48,564 | ||||||||
| Commissions and incentives | 97,319 | 146,415 | 149,595 | ||||||||
| 130,073 | 193,228 | 198,159 | |||||||||
| Gross profit | 98,015 | 96,477 | 134,544 | ||||||||
| Operating expenses: | |||||||||||
| Selling and administrative expenses | 62,657 | 69,997 | 81,077 | ||||||||
| Depreciation and amortization | 11,517 | 12,333 | 12,310 | ||||||||
| Other operating costs | 35,322 | 39,741 | 55,656 | ||||||||
| Total operating expenses | 109,496 | 122,071 | 149,043 | ||||||||
| Loss from operations | (11,481 | ) | (25,594 | ) | (14,499 | ) | |||||
| Interest income | 173 | 473 | 1,604 | ||||||||
| Other income (expense), net | 268 | 1,046 | (5,303 | ) | |||||||
| Loss before income taxes | (11,040 | ) | (24,075 | ) | (18,198 | ) | |||||
| Benefit from income taxes | 424 | 6,707 | 5,570 | ||||||||
| Net loss | $ | (10,616 | ) | $ | (17,368 | ) | $ | (12,628 | ) | ||
| Loss per common share: | |||||||||||
| Basic | $ | (0.40 | ) | $ | (0.66 | ) | $ | (0.48 | ) | ||
| Diluted | $ | (0.40 | ) | $ | (0.66 | ) | $ | (0.48 | ) | ||
| Weighted-average common shares outstanding: | |||||||||||
| Basic | 26,488 | 26,467 | 26,461 | ||||||||
| Diluted | 26,488 | 26,467 | 26,461 | ||||||||
See accompanying notes to consolidated financial statements.
F-4
CONSOLIDATED STATEMENTS OF SHAREHOLDERS’ EQUITY AND
COMPREHENSIVE INCOME (LOSS)
(in thousands, except per share information)
Common Stock Outstanding | Additional paid in capital | Retained earnings | Accumulated other comprehensive income (loss) | Treasury stock | Total shareholders’ equity | ||||||||||||||||||
| Shares | Par value | Shares | Amounts | ||||||||||||||||||||
| Balance at January 1, 2008 | 26,461 | 3 | 40,146 | 62,620 | (1,123 | ) | 1,207 | (14,791 | ) | 86,855 | |||||||||||||
Tax shortfall from expiration of stock options | — | — | (120 | ) | — | — | — | — | (120 | ) | |||||||||||||
Charge related to stock-based compensation | — | — | 727 | — | — | — | — | 727 | |||||||||||||||
| Declared dividends of $0.22 per share | — | — | — | (5,822 | ) | — | — | — | (5,822 | ) | |||||||||||||
| Components of comprehensive loss: | |||||||||||||||||||||||
| Foreign currency translation | — | — | — | — | (318 | ) | — | — | (318 | ) | |||||||||||||
| Pension obligations, net of tax of $26 | — | — | — | — | 35 | — | — | 35 | |||||||||||||||
| Net loss | — | — | — | (12,628 | ) | — | — | — | (12,628 | ) | |||||||||||||
| Total comprehensive loss | — | — | — | — | — | — | — | (12,911 | ) | ||||||||||||||
| Balance at December 31, 2008 | 26,461 | 3 | 40,753 | 44,170 | (1,406 | ) | 1,207 | (14,791 | ) | 68,729 | |||||||||||||
Tax shortfall from expiration of stock options | — | — | (13 | ) | — | — | — | — | (13 | ) | |||||||||||||
| Proceeds from stock options exercised | 20 | — | 66 | — | — | — | — | 66 | |||||||||||||||
Charge related to stock-based compensation | — | — | 636 | — | — | — | — | 636 | |||||||||||||||
| Declared dividends of $0.04 per share | — | — | — | (1,059 | ) | — | — | — | (1,059 | ) | |||||||||||||
| Components of comprehensive loss: | |||||||||||||||||||||||
| Foreign currency translation | — | — | — | — | 276 | — | — | 276 | |||||||||||||||
| Pension obligations, net of tax $12 | 17 | 17 | |||||||||||||||||||||
| Net loss | — | — | — | (17,368 | ) | — | — | — | (17,368 | ) | |||||||||||||
| Total comprehensive loss | (17,075 | ) | |||||||||||||||||||||
| Balance at December 31, 2009 | 26,481 | $ | 3 | $ | 41,442 | $ | 25,743 | $ | (1,113 | ) | 1,207 | $ | (14,791 | ) | $ | 51,284 | |||||||
Tax shortfall from expiration of stock options | — | — | (51 | ) | — | — | — | — | (51 | ) | |||||||||||||
| Proceeds from stock options exercised | 9 | — | 28 | — | — | — | — | 28 | |||||||||||||||
Charge related to stock-based compensation | — | — | 630 | — | — | — | — | 630 | |||||||||||||||
| Components of comprehensive loss: | |||||||||||||||||||||||
| Foreign currency translation | — | — | — | — | (299 | ) | — | — | (299 | ) | |||||||||||||
| Pension obligations, net of tax $75 | 104 | 104 | |||||||||||||||||||||
| Net loss | — | — | — | (10,616 | ) | — | — | — | (10,616 | ) | |||||||||||||
| Total comprehensive loss | (10,811 | ) | |||||||||||||||||||||
| Balance at December 31, 2010 | 26,490 | $ | 3 | $ | 42,049 | $ | 15,127 | $ | (1,308 | ) | 1,207 | $ | (14,791 | ) | $ | 41,080 | |||||||
See accompanying notes to consolidated financial statements.
F-5
CONSOLIDATED STATEMENTS OF CASH FLOWS
(in thousands)
| For the years ended December 31, | ||||||||||||
| 2010 | 2009 | 2008 | ||||||||||
| CASH FLOWS FROM OPERATING ACTIVITIES: | ||||||||||||
| Net loss | $ | (10,616 | ) | $ | (17,368 | ) | $ | (12,628 | ) | |||
Adjustments to reconcile net loss to net cash provided by (used in) operating activities: | ||||||||||||
| Depreciation and amortization | 11,517 | 12,333 | 12,310 | |||||||||
| Provision for inventory losses | 2,266 | 1,544 | 1,321 | |||||||||
| Provision for doubtful accounts | 51 | 33 | 23 | |||||||||
| Loss on disposal of assets | 71 | 102 | 468 | |||||||||
| Accounting charge related to stock-based compensation expense | 630 | 636 | 727 | |||||||||
| Deferred income taxes | (2,044 | ) | 761 | (3,062 | ) | |||||||
| Changes in operating assets and liabilities: | ||||||||||||
| Accounts receivable | 197 | (405 | ) | 316 | ||||||||
| Income tax receivable | 7,158 | (4,525 | ) | (1,395 | ) | |||||||
| Inventories | 4,954 | (1,198 | ) | (9,512 | ) | |||||||
| Prepaid expenses and other current assets | (501 | ) | 2,821 | 1,927 | ||||||||
| Other assets | (551 | ) | (1,019 | ) | (9 | ) | ||||||
| Accounts payable | (5,785 | ) | 6,245 | 1,407 | ||||||||
| Accrued expenses | (2,161 | ) | (10,345 | ) | (5,947 | ) | ||||||
| Taxes payable | 1,144 | 1,643 | (4,901 | ) | ||||||||
| Commissions and incentives payable | (1,458 | ) | (898 | ) | 362 | |||||||
| Deferred revenue | (877 | ) | (670 | ) | (1,295 | ) | ||||||
| Net cash provided by (used in) operating activities | 3,995 | (10,310 | ) | (19,888 | ) | |||||||
| CASH FLOWS FROM INVESTING ACTIVITIES: | ||||||||||||
| Acquisition of property and equipment | (1,766 | ) | (2,797 | ) | (5,614 | ) | ||||||
| Proceeds from sale of assets | — | 37 | 3 | |||||||||
| Change in restricted cash | 3,692 | 1,473 | (139 | ) | ||||||||
| Sale of investments | — | — | 20,350 | |||||||||
| Purchase of investments | — | — | (7,400 | ) | ||||||||
| Net cash provided by (used in) investing activities | 1,926 | (1,287 | ) | 7,200 | ||||||||
| CASH FLOWS FROM FINANCING ACTIVITIES: | ||||||||||||
| Proceeds from stock options exercised | 28 | 66 | — | |||||||||
| Payment of cash dividends | — | (1,059 | ) | (5,822 | ) | |||||||
| Repayment of capital lease obligation | (1,387 | ) | (473 | ) | (115 | ) | ||||||
| Net cash used in financing activities | (1,359 | ) | (1,466 | ) | (5,937 | ) | ||||||
| Effect of currency exchange rate changes on cash and cash equivalents | (345 | ) | (515 | ) | 2,467 | |||||||
| Net increase (decrease) in cash and cash equivalents | 4,217 | (13,578 | ) | (16,158 | ) | |||||||
| Cash and cash equivalents at the beginning of year | 17,367 | 30,945 | 47,103 | |||||||||
| Cash and cash equivalents at the end of year | $ | 21,584 | $ | 17,367 | $ | 30,945 | ||||||
| SUPPLEMENTAL DISCLOSURE OF CASH FLOW INFORMATION: | ||||||||||||
| Income taxes received (paid), net | $ | 7,126 | $ | 2,441 | $ | (1,266 | ) | |||||
| Interest paid on capital leases | $ | 117 | $ | 50 | $ | 17 | ||||||
| Summary of non-cash investing and financing activities: | ||||||||||||
| Assets acquired through capital leases | $ | 1,998 | $ | 2,099 | $ | 30 | ||||||
| Unrealized gains from investments | $ | — | $ | — | $ | — | ||||||
See accompanying notes to consolidated financial statements.
F-6
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
| NOTE 1: ORGANIZATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES |
Mannatech, Incorporated (together with its subsidiaries, the “Company”), located in Coppell, Texas, was incorporated in the state of Texas on November 4, 1993 and is listed on the NASDAQ Global Select Market under the symbol “MTEX”. The Company develops, markets, and sells high-quality, proprietary nutritional supplements, topical and skin care products, and weight-management products that are primarily sold to independent associates and members located in the United States, Canada, Australia, the United Kingdom, Japan, New Zealand, the Republic of Korea, Taiwan, Denmark, Germany, South Africa, Singapore, Austria, the Netherlands, Norway, Sweden and, beginning January 24, 2011, Mexico.
Independent associates (“associates”) purchase the Company’s products at published wholesale prices to either sell to retail customers or for personal use. Members purchase the Company’s products at a discount from published retail prices primarily for personal consumption. The Company cannot distinguish its personal consumption sales from its other sales because it is not involved with the products after delivery, other than usual and customary product warranties and returns. Only independent associates are eligible to earn commissions and incentives.
Principles of Consolidation
The consolidated financial statements and footnotes include the accounts of the Company and its wholly-owned subsidiaries. All intercompany balances and transactions have been eliminated in consolidation.
Use of Estimates
The preparation of the Company’s consolidated financial statements in accordance with generally accepted accounting principles requires the use of estimates that affect the reported value of assets, liabilities, revenues and expenses. These estimates are based on historical experience and various other factors. The Company continually evaluates the information used to make these estimates as the business and economic environment changes. Historically, actual results have not varied materially from the Company’s estimates and the Company does not currently anticipate a significant change in its assumptions related to these estimates. Actual results may differ from these estimates under different assumptions or conditions.
The use of estimates is pervasive throughout the consolidated financial statements, but the accounting policies and estimates considered the most significant are described in this note to the consolidated financial statements, Organization and Summary of Significant Accounting Policies.
Cash and Cash Equivalents
The Company considers all highly liquid investments with original maturities of three months or less to be cash equivalents. The Company includes in its cash and cash equivalents credit card receivables due from its credit card processor, as the cash proceeds from credit card receivables are received within 24 to 72 hours. As of December 31, 2010 and 2009, credit card receivables were $0.9 million and $2.8 million, respectively. Additionally, as of December 31, 2010 and 2009, cash and cash equivalents held in bank accounts in foreign countries totaled $12.7 million and $10.2 million, respectively.
Restricted Cash
The Company is required to restrict cash for (i) direct selling insurance premiums and credit card sales in the Republic of Korea; (ii) reserve on credit card sales in United States and Canada; and (iii) Australia building lease collateral. As of December 31, 2010 and 2009, our total restricted cash was $4.8 million and $8.5 million, respectively. The decrease in restricted cash was primarily related to the partial refund of direct selling insurance premiums in Korea.
F-7
Accounts Receivable
Accounts receivable are carried at their estimated collectible amounts. Receivables are created upon shipment of an order if the credit card payment is rejected or does not match the order total. As of December 31, 2010 and 2009, receivables consisted primarily of amounts due from members and associates. The Company periodically evaluates its receivables for collectability based on historical experience, recent account activities, and the length of time receivables are past due and writes-off receivables when they become uncollectible. At December 31, 2010 and 2009, the Company held an allowance for doubtful accounts of less than $0.1 million.
Property and Equipment
Property and equipment are stated at cost, less accumulated depreciation and amortization computed using the straight-line method over the estimated useful life of each asset. Leasehold improvements are amortized over the shorter of the lease term or the estimated useful life of the improvements. Expenditures for maintenance and repairs are charged to expense as incurred. The cost of property and equipment sold or otherwise retired and the related accumulated depreciation are removed from the accounts and any resulting gain or loss is included in other operating costs in the accompanying Consolidated Statements of Operations. The estimated useful lives of fixed assets are as follows:
| Estimated useful life | ||
| Office furniture and equipment | 5 to 7 | years |
| Computer hardware and software | 3 to 5 | years |
| Automobiles | 3 to 5 | years |
Leasehold improvements(1) | 2 to 10 | years |
Construction in progress(2) | ||
___________________
(1) We amortize leasehold improvements over the shorter of the useful estimated life of the leased asset or the lease term.
(2) Construction in process includes leasehold improvements and internally developed software costs. Once placed in service, leasehold improvements will be amortized over the shorter of an asset’s useful life or the remaining lease term. Once the internally-developed software is placed in service, it will be amortized over three to five years.
Property and equipment are reviewed for impairment whenever an event or change in circumstances indicates that the carrying amount of an asset or group of assets may not be recoverable. The impairment review includes a comparison of future projected cash flows generated by the asset or group of assets with its associated net carrying value. If the net carrying value of the asset or group of assets exceeds expected cash flows (undiscounted and without interest charges), an impairment loss is recognized to the extent the carrying amount of the asset exceeds its fair value. We determined that no impairment indicators existed during the years ended December 31, 2010 and 2009.
Inventories
Inventories consist of raw materials, work in progress, finished goods, and promotional materials that are stated at the lower of cost or market (using standard costs that approximate average costs). The Company periodically reviews inventories for obsolescence and any inventories identified as obsolete are reserved or written off.
Other Assets
As of December 31, 2010 and 2009, other assets of $3.1 million and $2.5 million primarily consisted of deposits for building leases in various locations and certain intangible assets. Also included in December 31, 2010 balance was a $0.9 million deposit with Mutual Aid Cooperative and Consumer (“MAC&CO”) in Republic of Korea, an organization established by the Republic of Korea’s Fair Trade Commission to protect consumers who participate in network marketing activities.
Commissions and Incentives
Independent associates earn commissions and incentives based on their direct and indirect commissionable net sales over 13 business periods. Each business period equals 28 days. The Company accrues commissions and incentives when earned by independent associates and pays commissions on product sales three weeks following the business period end and pays commissions on its pack sales five weeks following the business period end.
F-8
Other Long-Term Liabilities
In August 2003, the Company entered into a Long-Term Post-Employment Royalty Agreement with Dr. Bill McAnalley, the Company’s former Chief Science Officer, pursuant to which the Company is required to pay Dr. McAnalley, or his heirs, royalties for ten years beginning September 2005 through August 2015. Quarterly payments related to this Long-Term Post-Employment Royalty Agreement are based on certain applicable annual global product sales by the Company in excess of $105.4 million. At the time the Company entered into this Long-Term Post-Employment Royalty Agreement, it was considered a post-employment benefit and the Company was required to measure and accrue the present value of the estimated future royalty payments related to the post-employment royalty benefit and recognize it over the life of Dr. McAnalley’s employment agreement, which was two years. As of December 31, 2010 and 2009, the Company’s liability related to this royalty agreement was $1.6 million and $2.0 million, respectively, of which $0.3 million was currently due and included in accrued expenses.
Certain operating leases for the Company’s regional office facilities contain a restoration clause that requires the Company to restore the premises to its original condition. As of December 31, 2010 and 2009, accrued restoration costs related to these leases amounted to $0.4 million. At December 31, 2010 and 2009, the Company also recorded a long-term liability for an estimated deferred benefit obligation related to a deferred benefit plan for its Japan operations of $1.0 million and $0.8 million, respectively.
Income Taxes
The Company accounts for income taxes using the asset and liability method. Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases and operating loss and tax credit carryforwards. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized as income in the period that includes the enactment date. The Company evaluates the probability of realizing the future benefits of its deferred tax assets and provides a valuation allowance for the portion of any deferred tax assets where the likelihood of realizing an income tax benefit in the future does not meet the more likely than not criterion for recognition.
Revenue Recognition
The Company’s revenue is derived from sales of individual products, sales of its starter and renewal packs, and shipping fees. Substantially all of the Company’s product and pack sales are made to independent associates at published wholesale prices and to members at discounted published retail prices. The Company records revenue net of any sales taxes and records a reserve for expected sales returns based on its historical experience.
The Company recognizes revenue from shipped packs and products upon receipt by the customer. Corporate-sponsored event revenue is recognized when the event is held. The Company defers certain components of its revenue. At December 31, 2010 and December 31, 2009, the Company’s deferred revenue was $1.9 million and $2.8 million, respectively, and consisted primarily of revenue received from: (i) sales of packs and products shipped but not received by the customers by period end; and (ii) prepaid registration fees from customers planning to attend a future corporate-sponsored event.
We estimate a sales return reserve for expected sales refunds based on our historical experience over a rolling six- month period. If actual results differ from our estimated sales return reserve due to various factors, the amount of revenue recorded each period could be materially affected. Historically, our sales returns have not materially changed through the years, as the majority of our customers who return their merchandise do so within the first 90 days after the original sale. Sales returns have averaged 1.5% or less of our gross sales. For the year ended December 31, 2010 our sales return reserve was composed of the following (in thousands):
| Sales reserve as of January 1, 2010 | $ | 595 | |
| Provision related to sales made in 2010 | 1,948 | ||
| Provision related to sales made prior to 2010 | 113 | ||
| Actual returns or credits in 2010 related to 2010 | (1,557 | ) | |
| Actual returns or credits in 2010 related to prior periods | (711 | ) | |
| Sales reserve as of December 31, 2010 | $ | 388 |
F-9
Shipping and Handling Costs
The Company records freight and shipping fees collected from its customers as revenue. The Company records inbound freight as a component of inventory and cost of sales and records shipping and handling costs associated with shipping products to its customers as selling and administrative expenses. Total shipping and handling costs included in selling and administrative expenses were approximately $11.7 million, $14.0 million, and $15.1 million for the years ended December 31, 2010, 2009, and 2008, respectively.
Advertising Costs
The Company expenses advertising and promotions in selling and administrative expenses when incurred. Advertising and promotional expenses were approximately $5.6 million, $8.0 million, and $8.6 million for the years ended December 31, 2010, 2009, and 2008, respectively. Educational and promotional items, called sales aids, are sold to independent associates to assist in their sales efforts and are included in inventories and charged to cost of sales when sold.
Accounting for Stock-Based Compensation
The Company currently has one active stock-based compensation plan, which was approved by its shareholders at its 2008 Annual Shareholder’s meeting held on June 18, 2008. The Company grants stock options to its employees, consultants, and board members with an exercise price equal to the closing price of its common stock on the date of grant with a term no greater than 10 years. The majority of stock options vest over two or three years. Incentive stock options granted to shareholders who own 10% or more of the Company’s outstanding stock are granted at an exercise price that may not be less than 110% of the closing price of the Company’s common stock on the date of grant and have a term no greater than five years. At the date of grant, the Company determines the fair value of the stock option award and recognizes compensation expense over the requisite service period, or the vesting period of the award. The fair value of the stock option award is calculated using the Black-Scholes option-pricing model.
Research and Development Costs
The Company expenses research and development costs when incurred. Research and development costs related to new product development, enhancement of existing products, clinical studies and trials, Food and Drug Administration compliance studies, general supplies, internal salaries, third-party contractors, and consulting fees were approximately $3.6 million, $4.1 million, and $5.0 million for the years ended December 31, 2010, 2009, and 2008, respectively. Salaries and contract labor are included in selling and administrative expenses and all other research and development costs are included in other operating costs.
Software Development Costs
The Company capitalizes qualifying internal payroll and external contracting and consulting costs related to the development of internal use software that are incurred during the application development stage, which includes design of the software configuration and interfaces, coding, installation, and testing. Costs incurred during the preliminary project along with post-implementation stages of internal use software are expensed as incurred. The Company amortizes such costs over the estimated useful life of the software, which is three to five years once the software is placed in service.
Concentration Risk
A significant portion of the Company’s revenue is derived from core Ambrotose® complex products, which include the Ambrotose® products and Advanced Ambrotose® products. For the years ended December 31, 2010, 2009, and 2008, revenue from the core Ambrotose® products accounted for 35.3%, 31.4%, and 35.9% of the Company’s consolidated net sales, respectively.
Financial instruments, which potentially subject the Company to concentrations of credit risk, consist principally of cash and cash equivalents, investments, receivables, and restricted cash. The Company utilizes financial institutions that the Company considers to be of high credit quality.
F-10
Fair Value of Financial Instruments
The fair value of the Company’s financial instruments, including cash and cash equivalents, restricted cash, time deposits, investments, receivables, payables, and accrued expenses, approximate their carrying values due to their relatively short maturities. See Note 3 (“Fair Value”) for more information.
Comprehensive Income (Loss) and Accumulated Other Comprehensive Income (Loss)
Comprehensive income (loss) is defined as the change in equity of a business enterprise during a period from transactions and other events and circumstances from non-owner sources and includes all changes in equity during a period except those resulting from investments by owners and distributions to owners. The Company’s comprehensive income (loss) consists of the Company’s net income (loss), foreign currency translation adjustments from its Japan, Republic of Korea, Taiwan, Norway, and Sweden operations, and changes in the pension obligation for its Japanese employees.
Foreign Currency Translation
The United States dollar is the functional currency for the majority of the Company’s foreign subsidiaries. As a result, nonmonetary assets and liabilities are translated at their approximate historical rates, monetary assets and liabilities are translated at exchange rates in effect at the end of the year, and revenues and expenses are translated at weighted-average exchange rates for the year. Transaction gains (losses) totaled approximately $0.3 million, $1.1 million, and $(5.2) million, for the years ended December 31, 2010, 2009, and 2008, respectively, and are included in other income (expense), net in the Company’s Consolidated Statements of Operations.
The local currency is the functional currency of our subsidiaries in Japan, Republic of Korea, Taiwan, Norway, Sweden, and Mexico. These subsidiaries’ assets and liabilities are translated into United States dollars at exchange rates existing at the balance sheet dates, revenues and expenses are translated at weighted-average exchange rates, and shareholders’ equity and intercompany balances are translated at historical exchange rates. The foreign currency translation adjustment is recorded as a separate component of shareholders’ equity and is included in accumulated other comprehensive income (loss).
NOTE 2: RECENT ACCOUNTING PRONOUNCEMENTS
In February 2010, the FASB issued ASU 2010-09, Amendments to Certain Recognition and Disclosure Requirements, (“ASU 2010-09”), which eliminated the requirement under FASB ASC Topic 855, “Subsequent Events” for SEC registrants to disclose the date through which they have evaluated subsequent events in the financial statements. ASU 2010-09 was effective upon issuance, and the Company adopted its provisions as of the issuance of the Quarterly Report on the Form 10-Q for the period ended March 31, 2010. The adoption of ASU 2010-09 was for disclosure purposes only and did not have any effect on the Company’s financial position or results of operations.
In January 2010, the FASB issued Accounting Standards Update No 2010-06, “Improving Disclosures about Fair Value Measurements” (“ASU 2010-06”), which amended FASB ASC Topic 820, Fair Value Measurements and Disclosures (“ASC Topic 820”), to require a number of additional disclosures regarding fair value measurements, including the amount of transfers between Levels 1 and 2 of the fair value hierarchy, the reasons for transfers in or out of Level 3 of the fair value hierarchy and activity for recurring Level 3 measures. ASU 2010-06 also clarifies certain existing disclosure requirements related to the level at which fair value disclosures should be disaggregated and the requirement to provide disclosures about the valuation techniques and inputs used in determining the fair value of assets or liabilities classified as Levels 2 or 3. The adoption of the new guidance had no impact on the Company’s consolidated financial statements and disclosures.
In October 2009, the FASB issued Accounting Standards Update No. 2009-13, “Revenue Recognition—Multiple Deliverable Revenue Arrangements” (“ASU 2009-13”). ASU 2009-13 updated the multiple-element revenue arrangements guidance, which was previously included in FASB ASC 605-25. The revised guidance provides for two significant changes to the existing multiple element revenue arrangements guidance. The first change relates to the determination of when the individual deliverables included in a multiple-element arrangement may be treated as separate units of accounting. The second change modifies the manner in which the transaction consideration is allocated across the separately identified deliverables. The revised guidance also expands the disclosures required for multiple-element revenue arrangements. The revised multiple-element revenue arrangements guidance was effective beginning January 1, 2011. The adoption of ASU 2009-13 had no impact on the Company’s financial position or results of operations.
F-11
In January 2009, the Securities and Exchange Commission issued Release No. 33-9002, “Interactive Data to Improve Financial Reporting.” The final rule requires companies to provide their financial statements and financial statement schedules to the Securities and Exchange Commission in interactive data format using the eXtensible Business Reporting Language (“XBRL”). The rule was adopted by the Securities and Exchange Commission to improve the ability of financial statement users to access and analyze financial data. The Securities and Exchange Commission adopted a phase-in schedule indicating when registrants must furnish interactive data. Under this schedule, the Company will be required to submit filings with financial statement information using XBRL commencing with our June 30, 2011 quarterly report on Form 10-Q. We began furnishing financial information in XBRL format starting with our quarterly report on Form 10-Q for the period ended September 30, 2009. As an earlier XBRL adopter, the Company may choose to discontinue furnishing XBRL data in the future until the required compliance date of June 30, 2011.
Other accounting pronouncements recently issued by the FASB, the AICPA, and the SEC did not or are not believed by management to have a material impact on the Company's present or future financial statements.
NOTE 3: FAIR VALUE
The Company utilizes fair value measurements to record fair value adjustments to certain financial assets and to determine fair value disclosures.
Fair Value Measurements and Disclosure Topic of the FASB ASC establishes a fair value hierarchy that requires the use of observable market data, when available, and prioritizes the inputs to valuation techniques used to measure fair value in the following categories:
· Level 1—Quoted unadjusted prices for identical instruments in active markets.
· Level 2—Quoted prices for similar instruments in active markets, quoted prices for identical or similar instruments in markets that are not active and model-derived valuations in which all observable inputs and significant value drivers are observable in active markets.
· Level 3—Model derived valuations in which one or more significant inputs or significant value drivers are unobservable, including assumptions developed by the Company.
The primary objective of the Company’s investment activities is to preserve principal while maximizing yields without significantly increasing risk. The investment instruments held by the Company are money market funds and interest bearing deposits for which quoted market prices are readily available. The Company considers these highly liquid investments to be cash equivalents. These investments are classified within Level 1 of the fair value hierarchy because they are valued based on quoted market prices in active markets. The table below presents the recorded amount of financial assets measured at fair value (in thousands) on a recurring basis as of December 31, 2010. The Company does not have any material financial liabilities that were required to be measured at fair value on a recurring basis at December 31, 2010.
| Level 1 | Level 2 | Level 3 | Total | |||||||||
| Assets | ||||||||||||
| Money Market Funds – Fidelity, US | $ $ | 6,032 | $ $ | — | $ $ | — | $ $ | 6,032 | ||||
| Interest bearing deposits – various banks, Korea | 2,721 | — | — | 2,721 | ||||||||
| Total assets | $ | 8,753 | $ | — | $ | — | $ | 8,753 | ||||
| Amounts included in: | ||||||||||||
| Cash and cash equivalents | $ $ | 6,220 | $ $ | — | $ $ | — | $ $ | 6,220 | ||||
| Long-term restricted cash | 2,533 | — | — | 2,533 | ||||||||
| Total | $ | 8,753 | $ | — | $ | — | $ | 8,753 | ||||
F-12
NOTE 4: INVENTORIES
Inventories consist of raw materials, work in progress, and finished goods, including promotional materials. The Company provides an allowance for any slow-moving or obsolete inventories. Inventories as of December 31, 2010 and 2009, consisted of the following (in thousands):
| 2010 | 2009 | |||||||
| Raw materials | $ | 8,846 | $ | 10,819 | ||||
| Finished goods | 16,785 | 21,844 | ||||||
| Inventory reserves for obsolescence | (1,561 | ) | (1,373 | ) | ||||
| Total | $ | 24,070 | $ | 31,290 | ||||
NOTE 5: PROPERTY AND EQUIPMENT
As of December 31, 2010 and 2009, property and equipment consisted of the following (in thousands):
| 2010 | 2009 | |||||
| Office furniture and equipment | $ | 10,955 | $ | 10,944 | ||
| Computer hardware | 15,070 | 14,324 | ||||
| Computer software | 46,860 | 46,901 | ||||
| Automobiles | 141 | 115 | ||||
| Leasehold improvements | 11,958 | 11,726 | ||||
| 84,984 | 84,010 | |||||
| Less accumulated depreciation and amortization | (66,535 | ) | (56,866 | ) | ||
| Property and equipment, net | 18,449 | 27,144 | ||||
| Construction in process | 524 | 317 | ||||
| Total | $ | 18,973 | $ | 27,461 | ||
At December 31, 2010, construction in progress consisted of capitalized software costs of $0.2 million and leasehold improvements related to our new offices in Mexico of $0.3 million and our office in South Africa of less than $0.1 million. At December 31, 2009, construction in progress consisted of $0.3 million for in-process leasehold improvements for its corporate facility and capitalized software costs of less than $0.1 million.
NOTE 6: CAPITAL LEASE OBLIGATIONS
As of December 31, 2010 and 2009, the net book value of leased assets was $2.3 million and $1.9 million, respectively for leased equipment, purchased licenses, and corporate insurance. The future minimum lease payments (in thousands) are as follows:
| 2011 | $ | 1,445 | |
| 2012 | 744 | ||
| 2013 | 386 | ||
| 2014 | 253 | ||
| 2015 | 3 | ||
| Total future minimum lease payments | 2,831 | ||
| Less: Amounts representing interest (effective interest rate 7.8%) | (299 | ) | |
| Present value of minimum lease payments | 2,532 | ||
| Current portion of capital lease obligations | (1,328 | ) | |
| Long-term portion of capital lease obligations | $ | 1,204 |
F-13
NOTE 7: ACCRUED EXPENSES
As of December 31, 2010 and 2009, accrued expenses consisted of the following (in thousands):
| 2010 | 2009 | |||||
| Accrued inventory purchases | $ | 1,786 | $ | 2,562 | ||
| Accrued compensation | 2,887 | 2,579 | ||||
| Accrued royalties | 415 | 362 | ||||
| Accrued sales and other taxes | 314 | 425 | ||||
| Other accrued operating expenses | 1,650 | 4,255 | ||||
| Customer deposits and sales returns | 398 | 607 | ||||
| Accrued travel expenses related to corporate events | 1,034 | 892 | ||||
| Accrued shipping and handling costs | 545 | — | ||||
| Fixed asset purchases | 29 | 185 | ||||
| Rent expense | 70 | — | ||||
| Accrued legal and accounting fees | 1,195 | 2,364 | ||||
| $ | 10,323 | $ | 14,231 | |||
NOTE 8: INCOME TAXES
The components of the Company’s income (loss) before income taxes are attributable to the following jurisdictions for the years ended December 31 (in thousands):
| 2010 | 2009 | 2008 | |||||||||
| United States | $ | (7,920 | ) | $ | (23,945 | ) | $ | (20,297 | ) | ||
| Foreign | (3,120 | ) | (130 | ) | 2,099 | ||||||
| $ | (11,040 | ) | $ | (24,075 | ) | $ | (18,198 | ) | |||
The components of the Company’s income tax provision (benefit) for the years ended December 31 are as follows (in thousands):
| Current provision (benefit): | 2010 | 2009 | 2008 | |||||||||
| Federal | $ | 799 | $ | (8,521 | ) | $ | (3,876 | ) | ||||
| State | 235 | (1 | ) | (95 | ) | |||||||
| Foreign | 490 | 886 | 1,583 | |||||||||
| 1,524 | (7,636 | ) | (2,388 | ) | ||||||||
| Deferred provision (benefit): | ||||||||||||
| Federal | (1,607 | ) | 269 | (2,411 | ) | |||||||
| State | (191 | ) | 140 | (299 | ) | |||||||
| Foreign | (150 | ) | 520 | (472 | ) | |||||||
| (1,948 | ) | 929 | (3,182 | ) | ||||||||
| $ | (424 | ) | $ | (6,707 | ) | $ | (5,570 | ) | ||||
F-14
A reconciliation of the Company’s effective income tax rate and the United States federal statutory income tax rate is summarized as follows, for the years ended December 31:
| 2010 | 2009 | 2008 | |||||||||
| Federal statutory income taxes | 35.0 | % | 35.0 | % | 35.0 | % | |||||
| State income taxes, net of federal benefit | 1.3 | 1.6 | 0.4 | ||||||||
| Difference in foreign and United States tax on foreign operations | (5.7 | ) | (3.0 | ) | (0.6 | ) | |||||
| Effect of changes in valuation allowance for net operating loss carryforwards | (10.3 | ) | (7.2 | ) | (1.1 | ) | |||||
| Effect of change in uncertain tax positions (net) | (17.5 | ) | 0.9 | 5.5 | |||||||
| Federal Sub-Part F Income from foreign operations | (6.3 | ) | 0.0 | (5.9 | ) | ||||||
| Research and experimentation income tax credits | 8.0 | 0.0 | 0.0 | ||||||||
| Other | (0.7 | ) | 0.6 | (2.7 | ) | ||||||
| 3.8 | % | 27.9 | % | 30.6 | % | ||||||
For 2010, the Company’s effective income tax rate was lower than what would be expected if the federal statutory income tax rate were applied to income before taxes primarily because of increases in the valuation allowance for deferred tax assets; increases in uncertain income tax positions and favorable differences from foreign operations. For 2009, the Company’s effective income tax rate was lower than expected if the federal statutory income tax rate were applied to income before taxes primarily because of increases in the valuation allowance for deferred tax assets and favorable differences from foreign operations. For 2008, the Company’s effective income tax rate was lower than expected if the federal statutory income tax rate were applied to income before income taxes primarily because of favorable differences from foreign operations.
F-15
Deferred income taxes reflect the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. Significant components of the Company’s deferred tax assets and liabilities consisted of the following at December 31 (in thousands):
| 2010 | 2009 | |||||||
| Deferred tax assets: | ||||||||
| Current: | ||||||||
| Deferred revenue | $ | 25 | $ | 1 | ||||
| Inventory capitalization | 917 | 428 | ||||||
| Inventory reserves | 430 | 314 | ||||||
| Accrued expenses | 1,158 | 1,953 | ||||||
| Net operating loss | — | — | ||||||
| Other | 1,400 | 1,123 | ||||||
| Total current deferred tax assets | 3,930 | 3,819 | ||||||
| Noncurrent: | ||||||||
| Depreciation and amortization | — | — | ||||||
Net operating loss(1) | 3,251 | 2,049 | ||||||
| Deferred royalty | 625 | 746 | ||||||
| Non-cash accounting charges related to stock options and warrants | 604 | 519 | ||||||
| Accrued expenses | 178 | 191 | ||||||
| Other | 1,333 | 744 | ||||||
| Total noncurrent deferred tax assets | 5,991 | 4,249 | ||||||
| Total deferred tax assets | 9,921 | 8,068 | ||||||
| Valuation allowance | (4,059 | ) | (2,290 | ) | ||||
| Total deferred tax assets, net of valuation allowance | $ | 5,862 | $ | 5,778 | ||||
| Deferred tax liabilities: | ||||||||
| Current: | ||||||||
| Prepaid expenses | $ | 608 | $ | 670 | ||||
| Other | 1,066 | 1,124 | ||||||
| Total current deferred tax liabilities | 1,674 | 1,794 | ||||||
| Noncurrent: | ||||||||
| Internally-developed software | 1,160 | 2,873 | ||||||
| Depreciation and amortization | 963 | 1,994 | ||||||
| Sub-Part F Income Deferred | 801 | — | ||||||
| Other | 154 | — | ||||||
| Total noncurrent deferred tax liabilities | 3,078 | 4,867 | ||||||
| Total deferred tax liabilities | $ | 4,752 | $ | 6,661 | ||||
__________________________________________
(1) The net operating loss for the Company will expire as follows (amounts in thousands): |
| Jurisdiction | NOL | Expiration Years |
| Denmark | 4 | Indefinite |
| Mexico | 833 | 2020 |
| Norway | 170 | Indefinite |
| Singapore | 3 | Indefinite |
| Sweden | 97 | Indefinite |
| Switzerland | 505 | 2016-2017 |
| Taiwan | 997 | 2016-2020 |
| United States (federal) | 88 | 2030 |
| United States (states) | 554 | 2015-2030 |
F-16
At December 31, 2010 and 2009, the Company’s valuation allowance was $4.1 million and $2.3 million, respectively. The provisions of Topic 740 require a company to record a valuation allowance when the “more likely than not” criterion for realizing a deferred tax asset cannot be met. A company is to use judgment in reviewing both positive and negative evidence of realizing a deferred tax asset. Furthermore, the weight given to the potential effect of such evidence is commensurate with the extent the evidence can be objectively verified.
The valuation allowances presented below (in thousands) at December 31, 2010 and 2009, represented a reserve against the Company’s net deferred tax asset the Company believed the “more likely than not” criterion for recognition purposes could not be met.
| Country | 2010 | 2009 | |||
| Mexico | $ | 834 | $ | — | |
| Norway | 170 | 16 | |||
| Sweden | 98 | 10 | |||
| Switzerland | 505 | 346 | |||
| Taiwan | 995 | 802 | |||
| United States | 1,457 | 1,116 | |||
| Total | $ | 4,059 | $ | 2,290 | |
At December 31, 2010 and 2009, the Company did not record a provision for any United States or foreign withholding taxes on its undistributed earnings related to its foreign subsidiaries because it is the intention of the Company to reinvest its undistributed earnings indefinitely in its foreign operations. Generally, such earnings become subject to United States income tax upon the remittance of dividends and under certain other circumstances. At December 31, 2010, it is not practicable to estimate the amount of deferred tax liability on such undistributed earnings.
Deferred tax assets (liabilities) are classified in the accompanying Consolidated Balance Sheets of December 31 as follows (in thousands):
| 2010 | 2009 | |||||||
| Current deferred tax assets | $ | 2,607 | $ | 2,662 | ||||
| Noncurrent deferred tax assets | 649 | 652 | ||||||
| Current deferred tax liabilities | (243 | ) | (274 | ) | ||||
| Noncurrent deferred tax liabilities | (1,903 | ) | (3,923 | ) | ||||
| Net deferred tax assets (liabilities) | $ | 1,110 | $ | (883 | ) | |||
On January 1, 2007, the Company adopted FIN 48, which was codified into Topic 740, which prescribes a comprehensive model for how a company should recognize, measure, present, and disclose in its financial statements, uncertain tax positions that it has taken or expects to take on a tax return. Topic 740 requires that a company recognize in its financial statements the impact of tax positions that meet a “more likely than not” threshold, based on the technical merits of the position. The tax benefits recognized in the financial statements from such a position should be measured based on the largest benefit that has a greater than fifty percent likelihood of being realized upon ultimate settlement. As of December 31, 2010, the Company recorded $2.1 million in other long-term liabilities related to uncertain income tax positions and income tax reserves associated with various audits. At December 31, 2010, the Company had gross tax-affected unrecognized tax benefits of $2.1 million that, if recognized, would impact the effective tax rate. The Company recognizes penalties and interest charges related to unrecognized tax benefits in current tax expense. A reconciliation of the beginning and ending amount of unrecognized tax benefits is as follows, for the years ended December 31, 2010 and 2009 (in thousands):
| 2010 | 2009 | ||||||
| Balance as of January 1 | $ | 178 | $ | 596 | |||
| Additions for tax positions related to the current year | — | 31 | |||||
| Additions for tax positions of prior years | 1,936 | 72 | |||||
| Reductions of tax positions of prior years | — | (521 | ) | ||||
| Balance as of December 31 | $ | 2,114 | $ | 178 | |||
F-17
Our 2005-2009 tax years remain subject to examination by the IRS for U.S. federal tax purposes. The majority of items identified by the IRS as a result of the ongoing review were related to uncertain tax positions and included in other long-term liabilities balance as of December 31, 2010. There are other ongoing audits in various international jurisdictions that are not material to our financial statements.
The Company files income tax returns in the United States federal jurisdiction and various state and foreign jurisdictions. As of December 31, 2010, the tax years that remained subject to examination by a major tax jurisdiction for the Company’s most significant subsidiaries were as follows:
| Jurisdiction | Open Years | |
| Australia | 2006-2010 | |
| Canada | 2004-2010 | |
| Denmark | 2008-2010 | |
| Japan | 2005-2010 | |
| Mexico | 2009-2010 | |
| Norway | 2009-2010 | |
| Republic of Korea | 2005-2010 | |
| Singapore | 2008-2010 | |
| South Africa | 2008-2010 | |
| Sweden | 2009-2010 | |
| Switzerland | 2008-2010 | |
| Taiwan | 2005-2010 | |
| United Kingdom | 2004-2010 | |
| United States | 2005-2010 |
The Company does not anticipate a decrease in the unrecognized income tax benefits in 2011 due to the closure of tax years by expiration of the statute of limitations. Any decrease may have a favorable impact on the Company’s consolidated financial statements.
NOTE 9: TRANSACTIONS WITH RELATED PARTIES AND AFFILIATES
Transactions involving J. Stanley Fredrick
During 2010, we paid employment compensation of approximately $143,000 in salary, bonus, auto allowance, and other compensation to Landen Fredrick, son of J. Stanley Fredrick, the Company’s Chairman of the Board and a major shareholder. In addition, Landen Fredrick participated in the employee health care benefit plans available to all employees of the Company. Landen Fredrick has served as vice president, North American sales since February of 2010. Prior to his promotion, Mr. Fredrick served as senior director of tools and training. In connection with his promotion, Mr. Fredrick was granted 25,000 stock options on February 24, 2010.
Transactions involving Samuel Caster
Mr. Caster, the Company’s founder, major stockholder, and former Chairman of the Board, founded MannaRelief in 1999 and served as its Chairman from 1999 through August 2007. William A. Mullens, Mr. Caster’s brother-in-law, is employed by MannaRelief as the Executive Director. Linda Caster, Mr. Caster’s wife, serves as MannaRelief’s Chairman of the Board. MannaRelief is a 501(c)(3) charitable organization that provides charitable services for children.
Historically, the Company has made cash donations to MannaRelief, sold products to MannaRelief at cost plus shipping and handling charges, and shipped products purchased by MannaRelief to its chosen recipients. In addition, certain Company employees and consultants periodically volunteer to work or host various fund raising projects and events for MannaRelief at no cost to MannaRelief. The Company has made cash donations and sold products to MannaRelief as follows:
| 2010 | 2009 | 2008 | |||||||||
| Sold Products | $ | 0.5 | million | $ | 0.7 | million | $ | 0.8 | million | ||
| Contributed Cash Donations | $ | 0.5 | million | $ | 0.3 | million | $ | 0.8 | million | ||
F-18
On March 17, 2010, the Company renewed its Consulting Agreement with Wonder Enterprises, LLC (f/k/a Salinda Enterprises, LLC; hereinafter “Wonder”) for the consulting services of Mr. Caster who is an employee of Wonder. Pursuant to the terms of the Consulting Agreement, the Company will pay Wonder $650,000 a year for consulting services performed by Mr. Caster. The Consulting Agreement has a term of one year and may be renewed by the Company upon 60 days’ written notice to Wonder before the expiration of the current term.
Transactions involving Ray Robbins
Mr. Ray Robbins is a member of the Company’s Board of Directors and a major shareholder. Mr. Robbins holds positions in the Company’s associate global downline network marketing system. The Company pays commissions and incentives to its independent associates and during 2010, 2009, and 2008, the Company paid commissions and incentives to Mr. Robbins totaling $3.0 million, $3.4 million, and $3.4 million, respectively. In addition, several of Mr. Robbins’ family members are independent associates and were paid associate commissions and earned aggregate incentives of approximately $0.4 million, $0.5 million, and $0.5 million for 2010, 2009, and 2008, respectively. The $0.4 million for the year ended December 31, 2010 includes his son, Kevin Robbins, who earned approximately $227,000, as well as his daughter, Marla Finley, and daughter-in-law, Demra Robbins, who both share an account that totaled approximately $207,000 in earnings. All commissions and incentives were paid to Mr. Robbins and his family members in accordance with the Company’s global associate career and compensation plan.
NOTE 10: EMPLOYEE BENEFIT PLANS
Employee Retirement Plan
Effective May 9, 1997, the Company adopted a Defined Contribution 401(k) and Profit Sharing Plan (the “401(k) Plan”) for its United States employees. The 401(k) Plan covers all full-time employees who have completed three months of service and attained the age of twenty-one. United States employees can contribute up to 100 percent of their annual compensation but are limited to the maximum annual dollar amount allowable under the Internal Revenue Code. The 401(k) plan permits matching and discretionary employer contributions, although in response to adverse market conditions the Company suspended the matching contributions under the 401(k) Plan in the first quarter of 2009 through June 30, 2010. The Company’s matching contributions for its United States employees vest ratably over a five-year period. During the years ended December 31, 2010 and 2008, the Company contributed approximately $0.1 million and $0.4 million, respectively, to the 401(k) Plan for matching contributions.
The Company also sponsors a non-U.S. defined benefit plan covering its employees in its Japan subsidiary (“the Benefit Plan”). Pension benefits under the Benefit Plan are based on years of service and annual salary. The Company utilizes actuarial methods. Inherent in the application of these actuarial methods are key assumptions, including, but not limited to, discount rates and expected long-term rates of return on plan assets. Changes in the related Benefit Plan costs may occur in the future due to changes in the underlying assumptions, changes in the number and composition of plan participants, and changes in the level of benefits provided. The Company uses a measurement date of December 31 to evaluate and record any post-retirement benefits related to the Benefit Plan.
F-19
Projected Benefit Obligation and Fair Value of Plan Assets
The Benefit Plan’s projected benefit obligation and valuation of plan assets are as follows for the years ended December 31 (in thousands):
| Projected benefit obligation: | 2010 | 2009 | ||||||
| Balance, beginning of year | $ | 846 | $ | 792 | ||||
| Service cost | 217 | 196 | ||||||
| Interest cost | 20 | 18 | ||||||
| Liability (gains) and losses | (162 | ) | (23 | ) | ||||
| Benefits paid to participants | (28 | ) | (123 | ) | ||||
| Foreign currency | 115 | (14 | ) | |||||
| Balance, end of year | $ | 1,008 | $ | 846 | ||||
| Plan assets: | ||||||||
| Fair value, beginning of year | $ | — | $ | — | ||||
| Company contributions | 28 | 123 | ||||||
| Benefits paid to participants | (28 | ) | (123 | ) | ||||
| Fair value, end of year | $ | — | $ | — | ||||
Funded status of the Benefit Plan as of December 31 (in thousands): | 2010 | 2009 | |||||
| Benefit obligation | $ | (1,008 | ) | $ | (846 | ) | |
| Fair value of plan assets | — | — | |||||
| Excess of benefit obligation over fair value of plan assets | $ | (1,008 | ) | $ | (846 | ) | |
Amounts recognized in the accompanying Consolidated Balance Sheets consist of, as of December 31 (in thousands): | 2010 | 2009 | |||||
| Accrued benefit liability | $ | (1,209 | ) | $ | (865 | ) | |
| Transition obligation | 201 | 19 | |||||
| Net amount recognized in the consolidated balance sheets | $ | (1,008 | ) | $ | (846 | ) | |
| Non-current liabilities | $ | (1,008 | ) | $ | (846 | ) | |
| Years Ended December 31, | ||||||||||||
Other changes recognized in other comprehensive income/loss (in thousands) | 2010 | 2009 | 2008 | |||||||||
| Net periodic cost | $ | 242 | $ | 219 | $ | 215 | ||||||
| Other changes in plan assets and benefit obligations | — | — | — | |||||||||
| Current year actuarial gain | (162 | ) | (23 | ) | (56 | ) | ||||||
| Current year prior service benefit | — | — | — | |||||||||
| Amortization of actuarial gain | — | — | — | |||||||||
| Amortization of transition obligation | (5 | ) | (5 | ) | (5 | ) | ||||||
| Total recognized in other comprehensive income | (167 | ) | (28 | ) | (61 | ) | ||||||
| Total | $ | 75 | $ | 191 | $ | 154 | ||||||
| As of December 31, | ||||||||
Amounts not yet reflected in net periodic benefit cost and included in accumulated other comprehensive gain/loss (in thousands): | 2010 | 2009 | ||||||
| Net actuarial gain/loss | $ | 175 | $ | 79 | ||||
| Transition obligation | 27 | (60 | ) | |||||
| Total recognized in accumulated other comprehensive loss | $ | 201 | $ | 19 | ||||
F-20
2011 estimated amounts amortized from accumulated other comprehensive income/loss, net into net periodic cost (in thousands) | ||||
| Transition obligation | $ | (5 | ) | |
| As of December 31, | |||||||
| 2010 | 2009 | ||||||
Aggregate Benefit Plan information and accumulated benefit obligation in excess of plan assets (in thousands): | |||||||
| Projected benefit obligation | $ | 1,008 | $ | 846 | |||
| Accumulated benefit obligation | 705 | 491 | |||||
| Fair value of plan assets | — | — | |||||
The weighted-average assumptions to determine the benefit obligation and net cost are as follows:
| 2010 | 2009 | |||||
| Discount rate | 1.75 | % | 2.25 | % | ||
| Rate of increase in compensation levels | 3.0 | % | 3.0 | % | ||
Components of Expense
Pension expense for the Benefit Plan is included in selling, general and administrative expenses in the Consolidated Statements of Operations and is comprised of the following for the years ended December 31 (in thousands):
| 2010 | 2009 | 2008 | |||||||
| Service cost | $ | 217 | $ | 196 | $ | 194 | |||
| Interest cost | 20 | 18 | 16 | ||||||
| Amortization of transition obligation | 5 | 5 | 5 | ||||||
| Amortization of unrecognized loss | — | — | — | ||||||
| Total pension expense | $ | 242 | $ | 219 | $ | 215 | |||
Estimated Benefits and Contributions
The Company expects to contribute approximately $50,000 to the plan in 2011. As of December 31, 2010, benefits expected to be paid by the Benefit Plan for the next ten years is approximately as follows (in thousands):
| 2011 | $ | 50 |
| 2012 | 54 | |
| 2013 | 53 | |
| 2014 | 95 | |
| 2015 | 196 | |
| Next five years | 185 | |
| Total expected benefits to be paid | $ | 633 |
F-21
NOTE 11: STOCK OPTION PLAN
Summary of Stock Plan
The Company currently has one active stock-based compensation plan, which was approved by shareholders. The Company grants stock options to employees, consultants, and board members at the fair market value of its common stock, on the date of grant, with a term no greater than ten years. The majority of stock options vest over two or three years. Shareholders who own 10% or more of the Company’s outstanding stock are granted incentive stock options at an exercise price that may not be less than 110% of the fair market value of the Company’s common stock on the date of grant and have a term no greater than five years.
In February 2008, the Company’s Board of Directors approved the Mannatech, Incorporated 2008 Stock Incentive Plan (the “2008 Plan”), which reserves up to 1,000,000 shares of common stock for issuance of stock options and restricted stock to our employees, board members, and consultants, plus any shares reserved under the Company’s then-existing, unexpired stock plans for which options had not yet been issued, and any shares underlying outstanding options under the then-existing stock option plans that terminate without having been exercised in full. The 2008 Plan was approved by the Company’s shareholders at the 2008 Annual Shareholders’ Meeting. As of December 31, 2010, the 2008 Plan had 334,812 stock options available for grant before the plan expires on February 20, 2018.
At the 2010 Annual Shareholders’ Meeting, shareholders approved an amendment to the 2008 Plan to permit a one-time stock option exchange program. On July 16, 2010, the Company commenced the option exchange program (the “Exchange Program”) whereby employees (including officers), directors, and consultants could elect to exchange their “out of the money” stock options for fewer options at a lower exercise price. The Exchange Program expired on August 13, 2010. On August 16, 2010, pursuant to the Exchange Program, an aggregate of 784,930 eligible options were tendered and accepted by the Company in exchange for the grant of an aggregate of 440,558 replacement options.
All of the replacement options granted pursuant to the Exchange Program have an exercise price of $2.46 and were granted under the 2008 Plan, as amended. Each replacement option vests annually in ⅓ installments starting one year from the grant date and has a term of 10 years. Incremental stock option expense resulting from the exchange was minimal because the fair value of the replacement options was approximately equal to the fair value of the surrendered options they replaced.
A summary of changes in stock options outstanding during the year ended December 31, 2010, is as follows:
| 2010 | |||||||||
Number of Options (in thousands) | Weighted average exercise price | Weighted average remaining contractual life (in years) | Aggregate intrinsic value (in thousands) | ||||||
| Outstanding at beginning of year | 1,539 | $ | 5.07 | ||||||
| Granted | 772 | $ | 2.65 | ||||||
| Exercised | (10 | ) | $ | 2.63 | |||||
Forfeited or expired(1) | (895 | ) | $ | 6.44 | |||||
| Outstanding at end of year | 1,406 | $ | 2.89 | 7.7 | — | ||||
| Options exercisable at year end | 583 | $ | 3.20 | 5.9 | — | ||||
(1) Includes 784,930 options tendered in the exchange program.
The Company issues new shares upon the exercise of options. Options exercised during the year ended December 31, 2010 had a total intrinsic value, calculated as the difference between the exercise date stock price and the exercise price of the option, of less than $0.1 million.
Valuation and Expense Information Under FASB ASC Topic 718 Compensation – Stock Compensation
Under the provisions of FASB ASC Topic 718, the Company is required to measure and recognize compensation expense related to any outstanding and unvested stock options previously granted, and thereafter recognize, in its consolidated financial statements, compensation expense related to any new stock options granted after implementation using a calculated fair-value based option-pricing model.
F-22
The Company uses the Black-Scholes option-pricing model to calculate the fair value of all of its stock options and its assumptions are based on historical information. The following assumptions were used to calculate the compensation expense and the calculated fair value of stock options granted each year:
| 2010 | 2009 | 2008 | |||||||||||||
| Dividend yield: | —(1) | 1.05 — 2.87 | % | 3.2 — 6.1 | % | ||||||||||
| Risk-free interest rate: | 1.9 — 2.16 | % | 1.53 — 2.7 | % | 1.8 — 3.6 | % | |||||||||
| Expected market price volatility: | 70.8 — 72.4 | % | 65.9 — 70.8 | % | 59.8 — 63.8 | % | |||||||||
| Average expected life of stock options: | 4.5 years | 4.5 years | 4.5 years | ||||||||||||
_________________________
(1) There were no dividends declared in 2010.
The computation of the expected volatility assumption used in the Black-Scholes calculations for new grants is based on historical volatilities of the Company’s stock. The expected life assumptions are based on the Company’s historical employee exercise and forfeiture behavior.
The weighted-average grant-date fair value of stock options granted during the years ended December 31, 2010, 2009, and 2008 was $1.05, $1.56, and $1.74 per share, respectively. The total fair value of shares vested during the years ended December 31, 2010, 2009, and 2008 was $0.5 million, $0.6 million, and $0.7 million, respectively.
The Company recorded the following amounts related to the expense of the fair values of options during the years ended December 31, 2010, 2009, and 2008 (in thousands):
| 2010 | 2009 | 2008 | |||||||
| Selling, general and administrative expenses and income (loss) from operations before income taxes | $ | 630 | $ | 636 | $ | 706 | |||
| (Provision) Benefit for income taxes | 85 | 134 | (79 | ) | |||||
| Effect on net loss | $ | 545 | $ | 502 | $ | 785 | |||
As of December 31, 2010, the Company had approximately $0.6 million of total unrecognized compensation expense related to stock options currently outstanding, to be recognized in future years, ending December 31,
as follows (in thousands):
Total gross unrecognized compensation expense | Total tax benefit associated with unrecognized compensation expense | Total net unrecognized compensation expense | |||||||||
| 2011 | $ | 370 | $ | 50 | $ | 320 | |||||
| 2012 | 184 | 21 | 163 | ||||||||
| 2013 | 71 | 7 | 64 | ||||||||
| $ | 625 | $ | 78 | $ | 547 | ||||||
NOTE 12: COMMITMENTS AND CONTINGENCIES
Operating Leases
The Company leases certain office space, automobiles, computer hardware, and warehouse equipment under various noncancelable operating leases. Some of these leases have renewal options. All of the Company’s leases expire at various times through March 2017. The Company also leases equipment under various month-to-month cancelable operating leases. For the years ended December 31, 2010, 2009, and 2008, total rent expense was approximately $4.2 million, $4.0 million, and $4.1 million, respectively.
F-23
Approximate future minimum rental commitments for non-cancelable operating leases (in millions) are as follows:
| Years ending December 31, | ||
| 2011 | $ | 3.3 |
| 2012 | 3.0 | |
| 2013 | 1.7 | |
| 2014 | 1.1 | |
| 2015 | 1.0 | |
| Thereafter | 1.0 | |
| $ | 11.1 | |
Purchase Commitments
The Company maintains supply agreements with its suppliers and manufacturers. Some of the supply agreements contain exclusivity clauses and/or minimum annual purchase requirements. Purchase agreements with suppliers that contain minimum purchase clauses are as follows:
| · | In January 2006, the Company entered into a five-year supply agreement with Larex, Inc. to exclusively purchase Arabinogalactan, an important component used in the formulation of its Ambrotose® complex. In order to retain exclusive rights to purchase Arabinogalactan, the Company is required to purchase a minimum monthly quantity over the five year agreement. Effective December 31, 2010 the supply agreement with Larex, Inc. was terminated. The Company is negotiating a new agreement with Larex, Inc., and continues to purchase Arabinogalactan from Larex, Inc. |
| · | In March 2006, the Company entered into a ten-year supply agreement to purchase plant-derived mineral nutrition products from InB:Biotechnologies, Inc. As of December 31, 2010, the Company is required to purchase an aggregate of $7.2 million through 2016. |
| · | In April 2007, the Company entered into a supply agreement with Marinova PTY Limited to purchase raw materials used in its products through 2012. On August 13, 2009, the Company terminated the contract for an asserted breach by Marinova PTY Limited. Pursuant to the terms of the contract, the parties are currently engaged in the arbitration process. |
| · | In June of 2008, the Company entered into a three-year supply agreement with Improve U.S.A. to purchase an aloe vera powder. As of December 31, 2010, the Company is required to purchase an aggregate of $4.5 million through 2011 under the terms of the agreement. |
Royalty and Consulting Agreements
In 2001, the Company entered into a royalty agreement with a high level associate and shareholder, whereby the Company agreed to pay royalties totaling $1.6 million related to the sale of certain sales aids developed by the associate and sold by the Company. Pursuant to this royalty agreement, the Company has paid an aggregate of $1.4 million through December 31, 2010, of which less than $0.1 million was paid in 2010 and approximately $0.1 million was paid in each of the years 2009 and 2008.
The Company also utilizes royalty agreements with individuals and entities to provide compensation for items such as reprints of articles or speeches relating to the Company, sales of promotional videos featuring sports personalities, and promotional efforts used by the Company for product sales or attracting new associates. The Company paid royalties for such royalty agreements of approximately $0.3 million in 2010, 2009 and 2008.
Employment Agreements
The Company has non-cancellable employment agreements with certain executives. If the employment relationships with these executives were terminated, as of December 31, 2010, the Company would continue to be indebted to the executives for $2.4 million, payable through 2012.
F-24
NOTE 13: LITIGATION
Patent Infringement Litigation
Mannatech, Inc. v. Country Life, LLC, et al., Case No. 3:10-cv-00533-O, United States District Court, Northern District of Texas, Dallas Division
On March 16, 2010, Mannatech, Inc. filed a patent infringement lawsuit against Country Life, LLC; Country Life Manufacturing, LLC; EvenBetterNow, LLC; Micro Health Solutions, LLC; New Sun, Inc.; Oasis Advanced Wellness, LLC; Roex, Inc.; VDF FutureCeuticals, Inc.; and John Does 1-20, alleging the defendants infringed on one or more of the following patents: United States Patent Nos. 6,929,807, 7,157,431, and 7,202,220, all entitled “Compositions of Plant Carbohydrates as Dietary Supplements” (“Patents-in-Suit”), and seeking to stop their manufacture, offer, and sale of infringing glyconutritional dietary supplement products. On May 14, 2010, Mannatech amended its complaint to add patent infringement claims against Angel Care USA, Inc.; Florida Nutri Labs, LLC; and Wy’s Enterprises of Springfield, Inc.
Mannatech has settled with nine of the eleven defendants: EvenBetterNow, Oasis Advanced Wellness, Micro Health Solutions, Wy's Enterprises of Springfield, Florida Nutri Labs, Angel Care, USA, New Sun, Country Life, and VDF. In accordance with these settlements, Agreed Judgments were entered prohibiting the defendants from making, using, offering to sell, or otherwise distributing the accused infringing products or colorable imitations thereof (the “Enjoined Products”); inducing infringement of the patents by encouraging or assisting others in selling the Enjoined Products; or supplying or causing to be supplied all or a substantial portion of the components of the Enjoined Products, for the term of the Patents-in-Suit.
On June 25, 2010, Mannatech filed an unopposed motion to drop defendant Country Life Manufacturing, LLC because Mannatech discovered that Country Life Manufacturing was no longer active or associated with Country Life, LLC. On June 29, 2010, Mannatech’s motion was granted and Country Life Manufacturing, LLC was dismissed from the lawsuit without prejudice.
The lawsuit is now proceeding against the sole remaining defendant, Roex. On January 31, 2011, counsel for Roex filed an Unopposed Motion to Withdraw as Counsel, representing to the court that Roex is insolvent and no longer exists as an entity. While Roex’s website is still functional, and it continues to sell other products, Roex stopped selling its infringing products upon the commencement of this lawsuit. Mannatech is now conducting discovery in order to determine Roex’s current status. On February 7, 2011, Mannatech subpoenaed documents from the purported assignee of Roex’s assets, PCG Consultants, Inc., and noticed the deposition of a corporate representative for Roex to take place on February 25, 2011. When this discovery is complete, Mannatech will advise the court whether it intends to continue to prosecute its claims against Roex or to seek dismissal of those claims without prejudice.
Business Arbitration and Litigation
Marinova Pty. Limited v. Mannatech, Incorporated & Mannatech (International) Limited, Case No. 50-122-T-00635-09, International Centre for Dispute Resolution, a division of the American Arbitration Association
On December 10, 2009, Marinova Pty. Limited (“Marinova”), a company duly organized and operating under the laws of Australia, filed a Notice of Arbitration and Statement of Claim with the International Centre for Dispute Resolution, which is a division of the American Arbitration Association, against Mannatech Incorporated and Mannatech (International) Limited (collectively, “Mannatech”). Marinova’s claims stem from the parties’ April 27, 2007 purchase agreement, which was entered into between Marinova and Mannatech (International) Limited and executed by Marinova and Mannatech, Incorporated. Through the purchase agreement, Marinova agreed to sell and Mannatech agreed to buy set quantities of glyconutrient powder that Mannatech uses to manufacture some of its products. Marinova claims that Mannatech breached the purchase agreement by not buying certain quantities of Marinova’s product. Marinova alternatively claims that Mannatech, Incorporated tortiously interfered with the purchase agreement. Finally, Marinova claims that Mannatech, Incorporated made fraudulent representations to Marinova upon which Marinova claims it relied in executing the purchase agreement. Marinova claims that Mannatech’s actions have caused them over $5,000,000 in damages, as well as attorneys’ fees and costs.
On January 15, 2010, Mannatech filed its Answering Statement and Counterclaims, through which Mannatech asserted affirmative defenses in response to Marinova’s claims, including that Marinova’s own actions or omissions contributed to or caused Marinova’s alleged injury. Mannatech also filed a counterclaim for breach of contract, through which Mannatech alleges that Marinova sold Mannatech non-conforming powder and then refused to reimburse
F-25
On August 25, 2010, the panel of three arbitrators held their preliminary hearing, during which Mannatech requested the opportunity to brief a motion to strike certain portions of Marinova’s Statement of Claims that Mannatech thought were brought solely for the purposes of prejudicing the panel against Mannatech. Those portions of Marinova’s Statement of Claims related to lawsuits filed against Mannatech several years ago, all of which have now settled and bear no relation to Marinova’s claims. The panel granted Mannatech leave to file a motion to strike.
In response, Marinova sought leave and was granted the opportunity to re-plead its Statement of Claims. On August 31, 2010, Marinova filed its Amended Statement of Claims, in which it claims that, in addition to its other claims against Mannatech, Mannatech breached the purchase agreement because Mannatech allegedly falsely warranted that it was not the subject of any lawsuits or investigations. Accordingly, on September 15, 2010, Mannatech filed its Motion to Dismiss and Strike, through which it seeks to strike Marinova’s newly-added claim because (a) Marinova never raised the claim prior to its re-pleading; (b) Marinova accepted millions of dollars from Mannatech after it became aware of Mannatech’s alleged breach; and (c) Marinova is asserting its claim solely in an attempt to prejudice the panel. Marinova subsequently filed a response and Mannatech filed a reply in support of its Motion to Dismiss and Strike on October 12, 2010. The panel has not yet ruled on the Motion to Dismiss and Strike.
Mannatech intends to vigorously defend against Marinova’s claims and prosecute its counterclaim.
Litigation in General
The Company has several pending claims incurred in the normal course of business. In the Company’s opinion, such claims can be resolved without any material adverse effect on its consolidated financial position, results of operations, or cash flows.
The Company maintains certain liability insurance; however, certain costs of defending lawsuits, such as those below the insurance deductible amount, are not covered by or only partially covered by its insurance policies, or its insurance carriers could refuse to cover certain of these claims in whole or in part. The Company accrues costs to defend itself from litigation as it is incurred or as it becomes determinable.
The outcome of litigation may not be assured, and despite management’s views of the merits of any litigation, or the reasonableness of the Company’s estimates and reserves, the Company’s financial statements could nonetheless be materially affected by an adverse judgment. The Company believes it has adequately reserved for the contingencies arising from the above legal matters where an outcome was deemed to be probable, and the loss amount could be reasonably estimated. While it is not possible to predict with certainty what liability or damages the Company might incur in connection with any of the above-described lawsuits, based on the advice of counsel and a management review of the existing facts and circumstances related to these lawsuits, the Company has accrued $0.2 million as of December 31, 2010 for these matters, which is included in accrued expenses in its Consolidated Balance Sheet.
NOTE 14: SHAREHOLDERS’ EQUITY
Equity Line
On September 16, 2010, the Company entered into an Investment Agreement (the “Investment Agreement”) with Dutchess Opportunity Fund, II, LP, a Delaware limited partnership (the “Investor”). Pursuant to the Investment Agreement, the Investor committed to purchase, subject to certain restrictions and conditions, up to $10,000,000 of the Company’s common stock, over a period of 36 months from the first trading day following the effectiveness of the registration statement registering the resale of shares purchased by the Investor pursuant to the Investment Agreement (the “Equity Line”). The aggregate number of shares of common stock issuable by the Company and purchasable by the Investor under the Investment Agreement is 5,000,000.
F-26
The Company may draw on the Equity Line from time to time, as and when it determines appropriate in accordance with the terms and conditions of the Investment Agreement. The maximum amount that the Company is entitled to put to the Investor in any one draw down notice is the greater of (i) 200% of the average lowest daily volume of the Company’s common stock traded on the NASDAQ Global Market for the 3 trading days prior to the date of delivery of the applicable draw down notice, multiplied by the average of the closing prices for such trading days or (ii) $500,000. The purchase price will be set at 96% of the lowest daily volume weighted average price (VWAP) of the Company’s common stock during the 5 consecutive trading day period beginning on the date of delivery of the applicable draw down notice. Before the closing of an exercised put, the Company (not the Investor) has the right to withdraw all or any portion of the put; provided that the Company may not withdraw any portion of a put that the Investor has already committed to resell to a third party. There are put restrictions applied on days between the draw down notice date and the closing date with respect to that particular put. During such time, the Company will not be entitled to deliver another draw down notice. In addition, the Investor will not be entitled to purchase shares if the Investor’s total number of shares beneficially held at that time would exceed 4.99% of the number of shares of the Company’s common stock as determined in accordance with Rule 13d-1(j) of the Securities Exchange Act of 1934, as amended. In addition, the Company is not permitted to draw on the Equity Line unless there is an effective registration statement (as further explained below) to cover the resale of the shares.
The Investment Agreement further provides that the Company and the Investor are each entitled to customary indemnification from the other for, among other things, any losses or liabilities they may suffer as a result of any breach by the other party of any provisions of the Investment Agreement or Registration Rights Agreement (as defined below), or as a result of any lawsuit brought by a third-party arising out of or resulting from the other party’s execution, delivery, performance or enforcement of the Investment Agreement or the Registration Rights Agreement.
The Investment Agreement also contains customary representations and warranties of each of the parties. Investors should read the Investment Agreement together with the other information concerning the Company that the Company publicly files in reports and statements with the SEC.
Pursuant to the terms of a Registration Rights Agreement dated September 16, 2010 between the Company and the Investor (the “Registration Rights Agreement”), the Company is obligated to file one or more registration statements with the SEC to register the resale by Investor of the shares of common stock issued or issuable under the Investment Agreement. On October 28, 2010, the SEC declared effective the Company’s Registration Statement on Form S-3 (File No. 333-169774), which registers up to 5,000,000 shares of common stock that may be resold by the Investor pursuant to the Investment Agreement. As of March 10, 2011, no shares of common stock have been issued pursuant to the Investment Agreement.
In connection with the preparation of the Investment Agreement and the Registration Rights Agreement, the Company paid the Investor a document preparation fee in the amount of $15,000.
Preferred Stock
On April 8, 1998, the Company amended its Articles of Incorporation to reduce the number of authorized shares of common stock from 100.0 million to 99.0 million and the Company authorized 1.0 million shares of preferred stock with a par value of $0.01 per share. No shares of preferred stock have ever been issued or outstanding.
Treasury Stock
On June 30, 2004, the Company’s Board of Directors authorized the Company to repurchase, in the open market, up to 5% of its outstanding shares, or approximately 1.3 million shares, of its common stock to help manage any dilutive effects of its common stock in the open market. On August 28, 2006, a second program permitting the Company to purchase, in the open market, up to $20 million of its outstanding shares was approved by our Board of Directors. During 2005 and 2006, the Company had repurchased 1,121,444 common shares in the open market with an approximate cost of $14.0 million and an average price paid per share of $12.48.
As of December 31, 2010, the maximum number of shares available for repurchase under the June 2004 plan, previously approved by the Company’s Board of Directors, was 196,124. The Company is also authorized to purchase up to $20 million of its outstanding common stock, in the open market, under its August 2006 program.
F-27
Accumulated Other Comprehensive Income (Loss)
Accumulated other comprehensive income (loss), net, which is displayed in the Consolidated Statement of Shareholders’ Equity and Comprehensive Income (loss), represents net income (loss) plus the results of certain shareholders’ equity changes not reflected in the consolidated statements of operations. Such items include foreign currency translation and certain pension and postretirement benefit obligations.
The after-tax components of accumulated other comprehensive income (loss), are as follows (in thousands):
Foreign Currency Translation | Pension Postretirement Benefit Obligation | Accumulated Other Comprehensive Income (Loss), Net | ||||||||||||||
| Balance as of December 31, 2007 | $ | (1,091 | ) | $ | (32 | ) | $ | (1,123 | ) | |||||||
| Current-period change | (318 | ) | 35 | (283 | ) | |||||||||||
| Balance as of December 31, 2008 | (1,409 | ) | 3 | (1,406 | ) | |||||||||||
| Current-period change | 276 | 17 | 293 | |||||||||||||
| Balance as of December 31, 2009 | (1,133 | ) | 20 | (1,113 | ) | |||||||||||
| Current-period change | (299 | ) | 104 | (195 | ) | |||||||||||
| Balance as of December 31, 2010 | $ | (1,432 | ) | $ | 124 | $ | (1,308 | ) | ||||||||
NOTE 15: EARNINGS (LOSS) PER SHARE
Basic Earnings (Loss) Per Share (“EPS”) calculations are based on the calculated weighted-average number of the Company’s common shares outstanding during the period. Diluted EPS calculations are based on the calculated weighted-average number of common shares and dilutive common share equivalents outstanding during each period.
The following data shows the amounts used in computing the Company’s EPS and their effect on the Company’s weighted-average number of common shares and dilutive common share equivalents for the years ended December 31, 2010, 2009 and 2008. For 2010, approximately 1.6 million of the Company’s common stock options were excluded from its diluted EPS calculation using average close price of $2.67 per share, as their effect was anti-dilutive. For 2009, approximately 1.4 million of the Company’s common stock options were excluded from its diluted EPS calculation using average close price of $3.39 per share, as their effect was anti-dilutive. For 2008, approximately 1.3 million of the Company’s common stock options were excluded from its diluted EPS calculation using average close price of $5.37 per share, as their effect was anti-dilutive. The amounts are rounded to the nearest thousands, except per share amounts.
| 2010 | 2009 | 2008 | ||||||||||||||||||||||
Loss (Numerator) | Shares (Denominator) | Per Share Amount) | Loss (Numerator) | Shares (Denominator) | Per Share Amount | Loss (Numerator) | Shares (Denominator) | Per Share Amount | ||||||||||||||||
| Basic EPS: | ||||||||||||||||||||||||
Net loss attributable to common shareholders | $ | (10,616 | ) | 26,488 | $ | (0.40 | ) | $ | (17,368 | ) | 26,467 | $ | (0.66 | ) | $ | (12,628 | ) | 26,461 | $ | (0.48 | ) | |||
Effect of dilutive securities – | ||||||||||||||||||||||||
| Stock options | — | — | — | — | — | — | — | — | — | |||||||||||||||
Stock warrants(1) | — | — | — | — | — | — | — | — | — | |||||||||||||||
| Diluted EPS: | ||||||||||||||||||||||||
Net loss attributable to common shareholders plus assumed conversions | $ | (10,616 | ) | 26,488 | $ | (0.40 | ) | $ | (17,368 | ) | 26,467 | $ | (0.66 | ) | $ | (12,628 | ) | 26,461 | $ | (0.48 | ) | |||
_________________________
(1) In 2001, as part of a separation agreement, the Company granted an officer 213,333 stock warrants for common stock at exercise prices ranging from $1.75 to $4.00 per share. The stock warrants vested immediately and expired on February 28, 2008. |
F-28
The Company’s quarterly cash dividends were $0.02 per share for the first and second quarters of 2009. In the third quarter of 2009, the Board of Directors suspended the quarterly cash dividend payment to shareholders due to company financial performance, protracted worldwide economic recession, and the internal funding needs of new initiatives designed to accelerate sales and associate recruitment of the Company. The quarterly cash dividend remained suspended through year end December 31, 2010. The Company’s quarterly cash dividends were $0.09 per share for the first and second quarters of 2008 and $0.02 per share for the third and fourth quarters of 2008. The dividend policy is periodically re-evaluated based on consolidated results of operations, financial position, cash requirements, and other relevant factors.
NOTE 16: SEGMENT INFORMATION
The Company conducts its business as a single operating segment, consolidating all of its business units into a single reportable entity, as a seller of proprietary nutritional supplements, topical and skin care products, and weight-management products through its network marketing distribution channels operating in seventeen countries. Each of the Company’s business units sells similar packs and products and possesses similar economic characteristics, such as selling prices and gross margins. In each country, the Company markets its products and pays commissions and incentives in similar market environments. The Company’s management reviews its financial information by country and focuses its internal reporting and analysis of revenues by packs and product sales. The Company sells its products through its independent associates and distributes its products through similar distribution channels in each country. No single independent associate has ever accounted for more than 10% of the Company’s consolidated net sales.
The Company operates facilities in ten countries and sells product in seventeen countries around the world. These facilities are located in the United States, Canada, Switzerland, Australia, the United Kingdom, Japan, the Republic of Korea (South Korea), Taiwan, South Africa and beginning January 24, 2011, Mexico. Each facility services different geographic areas. The Switzerland office was created to manage certain day-to-day business needs of non-North American markets.
By country of operation, consolidated net sales shipped to customers in these locations, along with pack and product information for the years ended December 31, are as follows (in millions, except percentages):
| 2010 | 2009 | 2008 | ||||||||||||||||
| United States | $ | 100.8 | 44.1 | % | $ | 140.7 | 48.6 | % | $ | 176.9 | 53.1 | % | ||||||
| Japan | 34.2 | 15.0 | % | 42.0 | 14.5 | % | 44.8 | 13.5 | % | |||||||||
| Republic of Korea | 22.0 | 9.6 | % | 26.4 | 9.1 | % | 35.7 | 10.7 | % | |||||||||
| Australia | 20.0 | 8.8 | % | 22.9 | 7.9 | % | 26.1 | 7.8 | % | |||||||||
| Canada | 18.5 | 8.1 | % | 23.0 | 7.9 | % | 23.6 | 7.1 | % | |||||||||
| South Africa | 12.0 | 5.3 | % | 13.2 | 4.6 | % | 5.5 | 1.7 | % | |||||||||
| Taiwan | 6.4 | 2.8 | % | 6.6 | 2.3 | % | 5.2 | 1.6 | % | |||||||||
| New Zealand | 3.1 | 1.4 | % | 4.3 | 1.5 | % | 5.2 | 1.6 | % | |||||||||
| United Kingdom | 2.4 | 1.1 | % | 3.3 | 1.0 | % | 4.7 | 1.4 | % | |||||||||
| Germany | 2.3 | 1.0 | % | 3.2 | 1.1 | % | 3.8 | 1.1 | % | |||||||||
| Singapore | 2.1 | 0.9 | % | 1.5 | 0.5 | % | — | — | % | |||||||||
Norway(1) | 1.6 | 0.7 | % | 0.3 | 0.1 | % | — | — | % | |||||||||
Austria(1) | 1.1 | 0.5 | % | 0.3 | 0.1 | % | — | — | % | |||||||||
The Netherlands(1) | 0.6 | 0.3 | % | 0.2 | 0.1 | % | — | — | % | |||||||||
Sweden(1) | 0.5 | 0.2 | % | 0.2 | 0.1 | % | — | — | % | |||||||||
| Denmark | 0.5 | 0.2 | % | 1.6 | 0.6 | % | 1.2 | 0.4 | % | |||||||||
| Total | $ | 228.1 | 100 | % | $ | 289.7 | 100 | % | $ | 332.7 | 100 | % | ||||||
__________________________________
(1) Austria, the Netherlands, Norway, and Sweden began operations in September 2009.
F-29
| 2010 | 2009 | 2008 | ||||||
| Consolidated product sales | $ | 187.2 | $ | 213.9 | $ | 260.5 | ||
| Consolidated pack sales | 31.3 | 62.1 | 57.7 | |||||
| Consolidated other, including freight | 9.6 | 13.7 | 14.5 | |||||
| Total | $ | 228.1 | $ | 289.7 | $ | 332.7 | ||
Long-lived assets, which include property and equipment and construction in progress for the Company and its subsidiaries, as of December 31, reside in the following countries, as follows (in millions):
| Country | 2010 | 2009 | |||
| Australia | $ | 0.3 | $ | 0.3 | |
| Canada | 0.1 | 0.1 | |||
| Japan | 0.2 | 0.3 | |||
Mexico(1) | 0.3 | — | |||
| Republic of Korea | 0.8 | 0.6 | |||
| South Africa | 0.1 | — | |||
| Switzerland | 0.4 | 0.5 | |||
| Taiwan | 0.1 | 0.1 | |||
| United Kingdom | 0.1 | 0.1 | |||
| United States | 16.6 | 25.5 | |||
| Total | $ | 19.0 | $ | 27.5 | |
__________________________________
(1) Mexico began operations in January 2011.
Inventory balances, which consist of raw materials, work in progress, and finished goods, including promotional materials, and offset by obsolete inventories, for the Company and its subsidiaries, reside in the following countries as of December 31, as follows (in millions):
| Country | 2010 | 2009 | |||
| Australia | $ | 1.5 | $ | 2.5 | |
| Canada | 1.5 | 1.5 | |||
| Japan | 1.6 | 1.5 | |||
| Mexico | 0.8 | — | |||
| Republic of Korea | 0.8 | 1.9 | |||
| South Africa | 1.3 | 1.2 | |||
| Switzerland | 0.3 | 1.0 | |||
| Taiwan | 0.4 | 0.5 | |||
| United Kingdom | 1.1 | 0.7 | |||
| United States | 14.8 | 20.5 | |||
| Total | $ | 24.1 | $ | 31.3 | |
F-30
INDEX TO EXHIBITS
| Incorporated by Reference | |||||
Exhibit Number | Exhibit Description | Form | File No. | Exhibit (s) | Filing Date |
| 3.1 | Amended and Restated Articles of Incorporation of Mannatech, dated May 19, 1998. | S-1 | 333-63133 | 3.1 | October 28, 1998 |
| 3.2 | Fourth Amended and Restated Bylaws of Mannatech, dated August 8, 2001 (Corrected). | 10-K | 000-24657 | 3.2 | March 16, 2007 |
| 3.3 | First Amendment to the Fourth Amended and Restated Bylaws of Mannatech, effective November 30, 2007. | 8-K | 000-24657 | 3.1 | December 6, 2007 |
| 4.1 | Specimen Certificate representing Mannatech’s common stock, par value $0.0001 per share. | S-1 | 333-63133 | 4.1 | October 28, 1998 |
| 10.1 | Amended and Restated 1997 Stock Option Plan, dated August 7, 2004. | 10-K | 000-24657 | 10.1 | March 15, 2004 |
| 10.2 | First Amendment to the Mannatech, Incorporated 2008 Stock Incentive Plan | 8-K | 000-24657 | 99.1 | June 11, 2010 |
| 10.3 | Investment Agreement by and between Mannatech, Incorporated and Dutchess Opportunity Fund, II, LP dated September 16, 2010. | 8-K | 000-24657 | 10.1 | September 21, 2010 |
| 10.4 | Amendment to Investment Agreement, dated as of October 4, 2010, by and between Mannatech, Incorporated and Dutchess Opportunity Fund, II, LP. | 8-K | 000-24657 | 10.1 | October 5, 2010 |
| 10.5 | Amended and Restated 1998 Incentive Stock Option Plan, dated August 7, 2004. | 10-K | 000-24657 | 10.1 | March 15, 2004 |
| 10.6 | Registration Rights Agreement by and between Mannatech, Incorporated and Dutchess Opportunity Fund, II, LP dated September 16, 2010. | 8-K | 000-24657 | 10.2 | September 21, 2010 |
| 10.7 | Amended and Restated 2000 Option Plan, dated August 7, 2004. | 10-K | 000-24657 | 10.1 | March 15, 2004 |
| 10.8 | 2008 Stock Incentive Plan. | DEF 14A | 000-24657 | Appendix B | April 29, 2008 |
| 10.9 | Form of Indemnification Agreement between Mannatech and each member of the Board of Directors of Mannatech Korea Ltd., dated March 3, 2004. | 10-Q | 000-24657 | 10.2 | August 9, 2004 |
| 10.10 | Form of Indemnification Agreement between Mannatech, and its Board of Directors, dated September 10, 1998. | S-1 | 333-63133 | 10.8 | September 10, 1998 |
| 10.11 | Commercial Lease Agreement between Mannatech and MEPC Quorum Properties II Inc., dated November 7, 1996, as amended by the First Amendment thereto dated May 29, 1997 and the Second Amendment thereto dated November 13, 1997. | S-1 | 333-63133 | 10.13 | September 10, 1998 |
| 10.12 | Second Amendment to the Commercial Lease Agreement between Mannatech and Texas Dugan Limited Partnership, dated September 22, 2005. | 10-Q | 000-24657 | 10.1 | November 9, 2005 |
| 10.13 | Commercial Lease Agreement between Mannatech and MEPC Quorum Properties II Inc., dated May 29, 1997 as amended by the First Amendment thereto dated November 6, 1997. | S-1 | 333-63133 | 10.14 | September 10, 1998 |
| 10.14 | Third Amendment to the Commercial Lease Agreement between Mannatech and Texas Dugan Limited Partnership, dated September 22, 2005. | 10-Q | 000-24657 | 10.2 | November 9, 2005 |
| 10.15 | Trademark License and Supply Agreement between Mannatech and Carrington Laboratories, Inc., dated January 25, 2007. (Portions of this exhibit were omitted pursuant to a confidential treatment request submitted pursuant to Rule 24b-2 of the Exchange Act.) | 8-K | 000-24657 | 10.1 | January 31, 2007 |
| 10.16 | Supply Agreement between Mannatech (International) Limited and Marinova Pty. Limited, effective August 9, 2007 and dated May 7, 2007, (Portions of this exhibit were omitted pursuant to a confidential treatment request submitted pursuant to Rule 24b-2 of the Exchange Act). | 10-Q | 000-24657 | 10.3 | May 10, 2007 |
| 10.17 | Amendment to Purchase Agreement between Mannatech and Marinova PTY, Limited, dated May 6, 2008 (Portions of this exhibit were omitted pursuant to a confidential treatment request submitted pursuant to Rule 24b-2 of the Exchange Act). | 10-Q | 000-24657 | 10.4 | August 11, 2008 |
| Incorporated by Reference | |||||
Exhibit Number | Exhibit Description | Form | File No. | Exhibit (s) | Filing Date |
| 10.18 | Purchase Agreement between Mannatech and Larex, Inc., dated January 1, 2006. (Portions of this exhibit were omitted pursuant to a confidential treatment request submitted pursuant to Rule 24b-2 of the Exchange Act.) | 10-K | 000-24657 | 10.18 | March 16, 2006 |
| 10.19 | Purchase Agreement between Mannatech and Wellness Enterprises, LLC, dated February 1, 2006. (Portions of this exhibit were omitted pursuant to a confidential treatment request submitted pursuant to Rule 24b-2 of the Exchange Act.) | 10-K | 000-24657 | 10.19 | March 16, 2006 |
| 10.20 | Supply Agreement between Mannatech and Coradji PTY. Limited, dated March 29, 2004. (Portions of this exhibit were omitted pursuant to a confidential treatment request submitted pursuant to Rule 24b-2 of the Exchange Act.) | 10-Q/A | 000-24657 | 10.1 | March 29, 2005 |
| 10.21 | Supply License Agreement between Mannatech and InB:Biotechnologies, Inc., dated March 22, 2006. (Portions of this exhibit were omitted pursuant to a confidential treatment request submitted pursuant to Rule 24b-2 of the Exchange Act.) | 10-Q | 000-24657 | 10.2 | May 10, 2006 |
| 10.22 | Initial Commercial Supply and Manufacturing Agreement between Mannatech and Fine Chemetics, Inc., dated March 29, 2006. (Portions of this exhibit were omitted pursuant to a confidential treatment request submitted pursuant to Rule 24b-2 of the Exchange Act.) | 10-Q | 000-24657 | 10.3 | May 10, 2006 |
| 10.23 | Supply Agreement between Mannatech, Incorporated, and Improve U.S.A., Inc., effective June 1, 2008, and executed May 2, 2008. (Portions of this exhibit were omitted pursuant to a confidential treatment request submitted pursuant to Rule 24b-2 of the Exchange Act.) | 8-K | 000-24657 | 10.1 | May 8, 2008 |
| 10.24 | Amended and Restated Employment Agreement between Terry L. Persinger and Mannatech, dated June 16, 2008. | 8-K | 000-24657 | 10.1 | June 20, 2008 |
| 10.25 | Employment Agreement between Robert A. Sinnott, Ph.D. and Mannatech, dated October 5, 2007. | 8-K | 000-24657 | 10.3 | October 11, 2007 |
| 10.26 | Employment Agreement between Mannatech and Mr. Samuel L. Caster, dated January 23, 2006. | 10-K | 000-24657 | 10.32 | March 16, 2006 |
| 10.27 | Employment Agreement between Stephen D. Fenstermacher and Mannatech, dated October 5, 2007. | 8-K | 000-24657 | 10.2 | October 11, 2007 |
| 10.28 | First Amendment to Employment Agreement between Stephen D. Fenstermacher and Mannatech, dated December 18, 2008. | 10-K | 000-24657 | 10.24 | March 12, 2009 |
| 10.29 | Employment Agreement between Terence L. O’Day and Mannatech, dated October 5, 2007. | 8-K | 000-24657 | 10.1 | October 11, 2007 |
| 10.30 | Employment Agreement between B. Keith Clark and Mannatech, dated October 5, 2007. | 8-K | 000-24657 | 10.4 | October 11, 2007 |
| 10.31 | Employment Agreement between Wayne L. Badovinus and Mannatech, dated June 4, 2008. | 8-K | 000-24657 | 10.1 | June 9, 2008 |
| 10.32 | Employment Agreement between Terri F. Maxwell and Mannatech, dated August 28, 2008. | 8-K | 000-24657 | 10.1 | September 2, 2008 |
| 10.33 | Lock-up Agreement between Mannatech and J. Stanley Fredrick, dated November 6, 2003. | 10-K | 000-24657 | 10.36 | March 15, 2004 |
| 10.34 | Termination of Lock-up Agreement between Mannatech and J. Stanley Fredrick, dated March 6, 2009. | 8-K | 000-24657 | 10.1 | March 10, 2009 |
| 10.35 | Follow-Up Agreement to Letter of Intent Agreement between Mannatech and Jett, dated September 10, 2001. | 10-Q | 000-24657 | 10.4 | November 14, 2001 |
| 10.36 | Letter of Understanding between Mannatech and Dr. John Axford, dated April 19, 2006. | 8-K | 000-24657 | 99.1 | April 21, 2006 |
| 10.37 | Extension of the Letter of Spokesperson Arrangement between Mannatech and Dr. John Axford, dated February 18, 2007. | 8-K | 000-24657 | 99.1 | February 21, 2007 |
| 10.38 | Employment Agreement between Alfredo Bala and Mannatech, effective October 1, 2007, dated September 18, 2007. | 8-K | 000-24657 | 10.1 | September 24, 2007 |
| 10.39 | Amendment to Employment Agreement between Alfredo Bala and Mannatech, dated October 11, 2007. | 8-K | 000-24657 | 10.1 | October 17, 2007 |
| Incorporated by Reference | |||||
Exhibit Number | Exhibit Description | Form | File No. | Exhibit (s) | Filing Date |
| 10.40 | Clinical Research Agreement dated January 3, 2007 by and between St. George’s Hospital Medical School (trading as St George’s, University of London), and Mannatech, Inc. | 10-K | 000-24657 | 10.39 | March 17,2008 |
| 10.41 | Employment Agreement, effective March 2, 2009, by and between Mannatech and Randy S. Bancino. | 8-K | 000-24657 | 10.1 | March 6, 2009 |
| 10.42 | First Amendment to Employment Agreement, dated as of December 16, 2009, by and between Mannatech and Randy S. Bancino. | 8-K | 000-24657 | 10.4 | December 18, 2009 |
| 10.43 | Consulting Agreement, dated March 17, 2009, between Mannatech, Salinda Enterprises, LLC and Samuel L. Caster. | 8-K | 000-24657 | 10.1 | March 19, 2009 |
| 10.44 | Separation and Release Agreement, dated July 17, 2009 between Mannatech and Terri F. Maxwell. | 8-K | 000-24657 | 10.1 | July 21, 2009 |
| 10.45 | Second Amendment to Employment Agreement, dated as of December 16, 2009, by and between Mannatech and Stephen D. Fenstermacher. | 8-K | 000-24657 | 10.1 | December 18, 2009 |
| 10.46 | Second Amendment to Employment Agreement, dated as of December 16, 2009, by and between Mannatech and Robert A. Sinnott, Ph.D. | 8-K | 000-24657 | 10.2 | December 18, 2009 |
| 10.47 | Second Amendment to Employment Agreement, dated as of December 16, 2009, by and between Mannatech and B. Keith Clark. | 8-K | 000-24657 | 10.3 | December 18, 2009 |
| 14.1 | Code of Ethics. | 10-K | 000-24657 | 14.1 | March 16, 2007 |
| 21* | List of Subsidiaries. | * | * | * | * |
| 23.1* | Consent of BDO USA, LLP. | * | * | * | * |
| 23.2* | Report of Independent Registered Public Accounting Firm on Financial Statement Schedule. | * | * | * | * |
| 24* | Power of Attorney, which is included on the signature page of this annual report on Form 10-K. | * | * | * | * |
| 31.1* | Certification pursuant to Section 302 of the Sarbanes-Oxley Act of 2002, of the Co-Chief Executive Officer of Mannatech. | * | * | * | * |
| 31.2* | Certification pursuant to Section 302 of the Sarbanes-Oxley Act of 2002, of the Co-Chief Financial Officer of Mannatech. | * | * | * | * |
| 32.1* | Certification pursuant to Section 906 of the Sarbanes-Oxley Act of 2002, of the Co-Chief Executive Officer of Mannatech. | * | * | * | * |
| 32.2* | Certification pursuant to Section 906 of the Sarbanes-Oxley Act of 2002, of the Co-Chief Financial Officer of Mannatech. | * | * | * | * |
| 99.1* | Financial Statement schedule regarding Valuation and Qualifying Accounts. | * | * | * | * |
| 101.INS** | XBRL Instance Document | ** | ** | ** | ** |
| 101.SCH** | XBRL Taxonomy Extension Schema Document | ** | ** | ** | ** |
| 101.CAL** | XBRL Taxonomy Extension Calculation Linkbase Document | ** | ** | ** | ** |
| 101.LAB** | XBRL Taxonomy Extension Label Linkbase Document | ** | ** | ** | ** |
| 101.PRE** | XBRL Taxonomy Extension Presentation Linkbase Document | ** | ** | ** | ** |
| 101.DEF** | XBRL Taxonomy Extension Definition Linkbase Document | ** | ** | ** | ** |
_____________
* Filed herewith.
** Furnished herewith. In accordance with Rule 406T of Regulation S-T, the information in these exhibits shall not be deemed to be “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, or otherwise subject to liability under that section, and shall not be incorporated by reference into any registration statement or other document filed under the Securities Act of 1933, as amended, except as expressly set forth by specific reference in such filing.