Table of Contents
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
| | |
| (Mark One) | | |
ý |
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2008 |
Or |
o |
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
|
Commission file number: 0-32259
ALIGN TECHNOLOGY, INC.
(Exact name of registrant as specified in its charter)
| | |
| Delaware | | 94-3267295 |
(State or other jurisdiction of
incorporation or organization) | | (I.R.S. Employer Identification Number) |
881 Martin Avenue
Santa Clara, California 95050
(Address of principal executive offices) |
(408) 470-1000
(Registrant's telephone number, including area code)
|
Securities registered pursuant to Section 12(b) of the Act:
| | |
| Title of each class | | Name of each exchange on which registered |
|---|
Common Stock, $0.0001 par value
(Including associated Preferred Stock Purchase Rights) | | The NASDAQ Stock Market LLC
(NASDAQ Global Market) |
Securities registered pursuant to Section 12(g) of the Act:
None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ý No o
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Exchange Act. Yes o No ý
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ý No o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant's knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See definitions of "large accelerated filer," "accelerated filer," and "smaller reporting company" in Rule 12b-2 of the Exchange Act. (Check one):
| | | | | | |
| Large accelerated filer ý | | Accelerated filer o | | Non-accelerated filer o
(Do not check if a
smaller reporting company) | | Smaller reporting company o |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes o No ý
The aggregate market value of the registrant's common stock held by non-affiliates of the registrant was $601,665,751 as of June 30, 2008 based on the closing sale price of the registrant's common stock on the NASDAQ Global Market on such date. Shares held by persons who may be deemed affiliates have been excluded. This determination of affiliate status is not necessarily a conclusive determination for other purposes.
On February 18, 2009, 66,055,143 shares of the registrant's common stock were outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the registrant's definitive Proxy Statement relating to its 2009 Annual Stockholders' Meeting to be filed pursuant to Regulation 14A within 120 days after the registrant's fiscal year end of December 31, 2008 are incorporated by reference into Part III of this Annual Report on Form 10-K.
Table of Contents
ALIGN TECHNOLOGY, INC.
FORM 10-K
For the Year Ended December 31, 2008
TABLE OF CONTENTS
| | | | |
| |
| | Page |
|---|
PART I | | 3 |
Item 1. | | Business | | 3 |
| | Executive Officers of the Registrant | | 19 |
Item 1A. | | Risk Factors | | 20 |
Item 1B. | | Unresolved Staff Comments | | 33 |
Item 2. | | Properties | | 33 |
Item 3. | | Legal Proceedings | | 34 |
Item 4. | | Submission of Matters to a Vote of Security Holders | | 36 |
PART II | |
37 |
Item 5. | | Market for Registrant's Common Equity and Related Stockholder Matters and Issuer Purchases of Equity Securities | | 37 |
Item 6. | | Selected Consolidated Financial Data | | 39 |
Item 7. | | Management's Discussion and Analysis of Financial Condition and Results of Operations | | 41 |
Item 7A. | | Quantitative and Qualitative Disclosures About Market Risk | | 55 |
Item 8. | | Consolidated Financial Statements and Supplementary Data | | 57 |
Item 9. | | Changes In and Disagreements With Accountants on Accounting and Financial Disclosure | | 93 |
Item 9A. | | Controls and Procedures | | 93 |
Item 9B. | | Other Information | | 93 |
PART III | |
93 |
Item 10. | | Directors, Executive Officers and Corporate Governance | | 93 |
Item 11. | | Executive Compensation | | 94 |
Item 12. | | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters | | 94 |
Item 13. | | Certain Relationships and Related Transactions and Director Independence | | 95 |
Item 14. | | Principal Accounting Fees and Services | | 95 |
PART IV | |
96 |
Item 15. | | Exhibits and Financial Statement Schedules | | 96 |
Signatures | | 103 |
Invisalign, Align, ClinCheck, Invisalign Assist, Invisalign Teen and Vivera, amongst others, are trademarks belonging to Align Technology, Inc. and are pending or registered in the United States and other countries.
Table of Contents
In addition to historical information, this annual report on Form 10-K contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. These statements include, among other things, statements concerning our expectations regarding the expected impact our new products and product enhancements will have on doctor utilization and our market share, our expectations regarding product mix and product adoption, our expectations regarding the existence and impact of seasonality, our expectations regarding the relocation of several customer facing organizations from our Santa Clara, California facility to our facility in Costa Rica, including the timing of such relocation, our expectation that our utilization rate will improve over time, our expectations regarding our average selling prices and gross profits in 2009, the expected timing of the completion of our transition from our reliance on a shelter service provider in Juarez, Mexico to a direct manufacturer of our product, our expectations regarding the continued growth of our international markets, our expectations regarding the impact of increased consumer marketing programs in Europe, our expectations that the decline in general economic conditions in 2009 may result in a decline in our product volumes and revenues compared to 2008, the anticipated level of our operating expenses, and other factors beyond our control, as well as other statements regarding our future operations, financial condition and prospects and business strategies. These statements may contain words such as "expects," "anticipates," "intends," "plans," "believes," "estimates," or other words indicating future results. These forward-looking statements are subject to certain risks and uncertainties that could cause actual results to differ materially from those reflected in the forward-looking statements. Factors that could cause or contribute to such differences include, but are not limited to, those discussed in Item 7 "Management's Discussion and Analysis of Financial Condition and Results of Operations", and in particular, the risks discussed below in Part I, Item 1A "Risk Factors". We undertake no obligation to revise or update these forward-looking statements. Given these risks and uncertainties, readers are cautioned not to place undue reliance on such forward-looking statements.
PART I
ITEM 1. BUSINESS
Our Company
Align Technology, Inc. designs, manufactures and markets the Invisalign system, a proprietary method for treating malocclusion, or the misalignment of teeth. Invisalign corrects malocclusion using a series of clear, nearly invisible, removable appliances that gently move teeth to a desired final position. Because it does not rely on the use of metal or ceramic brackets and wires, Invisalign significantly reduces the aesthetic and other limitations associated with metal arch wires and brackets, commonly referred to as braces. We received the United States Food and Drug Administration ("FDA") clearance to market Invisalign in 1998. The Invisalign system is regulated by the FDA as a Class II medical device.
We distribute the vast majority of our products directly to our customers: the orthodontist and the general practitioner dentist, or GP. Orthodontists and GPs must complete an Invisalign training course in order to provide the Invisalign treatment solution to their patients. The Invisalign system is sold in North America, Europe, Asia Pacific, Latin America and Japan. We use a distributor model for the sale of our products in parts of the Asia Pacific and Latin American region.
We were incorporated in Delaware in April 1997. Our headquarters are located at 881 Martin Avenue, Santa Clara, California 95050, and our telephone number is 408-470-1000.
3
Table of Contents
Industry Background
Malocclusion, or the misalignment of teeth, is one of the most prevalent clinical dental conditions, affecting over 195 million individuals, or approximately 65% of the U.S. population. Approximately 2.1 million people annually elect treatment by orthodontists in the U.S. While most individuals seek orthodontic treatment to improve their appearance, malocclusion may also be responsible for dental problems such as tooth decay, tooth loss, gum disease, jaw joint pain and headaches. Because of the compromised aesthetics, discomfort and other drawbacks associated with traditional orthodontic treatments, only a relatively small proportion of people with malocclusion seek treatment.
In the U.S., dental professionals treat malocclusion primarily with metal arch wires and brackets, commonly referred to as braces. Occasionally, dental professionals attempt to improve treatment aesthetics by using ceramic, tooth-colored brackets or bond brackets on the inside, or lingual surfaces, of the patient's teeth. Dental professionals also augment braces with elastics, metal bands, headgear and other ancillary devices.
The average treatment takes approximately 12 to 24 months to complete and requires several hours of direct dental professional involvement, or chair time. To initiate treatment, a dental professional will diagnose a patient's condition and create an appropriate treatment plan. In a subsequent visit, the dental professional will bond brackets to the patient's teeth with cement and attach an arch wire to the brackets. Thereafter, by tightening or otherwise adjusting the braces approximately every six weeks, the dental professional is able to exert sufficient force on the patient's teeth to achieve desired tooth movement. Because of the length of time between visits, the dental professional must tighten the braces to a degree sufficient to achieve sustained tooth movement during the interval. In a final visit, the dental professional removes each bracket and residual cement from the patient's teeth. Upon completion of the treatment, the dental professional may, at his or her discretion, have the patient use a retainer.
Fees for traditional orthodontic treatment typically range between U.S. $3,500 to $7,000 with a median fee of approximately $5,000; generally only a portion of the fee is reimbursed by insurance. In addition, dental professionals commonly charge a premium for lingual or ceramic alternatives. Fees are based on the difficulty of the particular case and on the dental professional's estimate of chair time, and are generally negotiated in advance. A treatment that exceeds the dental professional's estimate of chair time generally results in decreased fees per hour of chair time, and reduced profitability for the dental professional.
Although braces are generally effective in correcting a wide range of malocclusions, they are subject to many limitations and disadvantages. Traditional orthodontic treatment is associated with:
- •
- Unattractive appearance. Braces call attention to the patient's condition and treatment. In addition, braces trap food, which can further compromise appearance. Braces can also result in permanent discoloration of teeth. Many adults associate braces with adolescence. As a result of these and other limitations, relatively few adults with malocclusion elect traditional orthodontic treatment annually.
- •
- Oral discomfort. Braces are sharp and bulky and can abrade and irritate the interior surfaces of the mouth. The tightening or adjustment of braces results in root and gum soreness and discomfort, especially in the few days immediately following an orthodontic visit.
4
Table of Contents
- •
- Poor oral hygiene. Braces compromise oral hygiene by making it more difficult to brush and floss. These problems can result in tooth decay and periodontal damage. Additionally, the bonding of brackets to teeth can cause permanent markings on the teeth.
- •
- Inability to project treatment. Historically, dental professionals have not had a means to model the movement of teeth over a course of treatment. Accordingly, dental professionals must rely on intuition and judgment to plan and project treatment. As a result, they cannot be precise about the direction or distance of expected tooth movement between patient visits. This lack of predictability may result in unwanted tooth movements and can limit the dental professional's ability to estimate the duration of treatment. Because most orthodontic treatment is performed on a fixed price basis, extended treatment duration reduces profitability for the dental professional.
- •
- Physical demands on dental professional. The manipulation of wires and brackets requires sustained manual dexterity and visual acuity, and may place other physical burdens on the dental professional.
- •
- Root resorption. The sustained high levels of force associated with traditional treatment can result in root resorption, which is a shortening of tooth roots. This shortening can have substantial adverse periodontal consequences for the patient.
- •
- Emergencies. At times, braces need to be repaired or replaced on an emergency basis. Such emergencies cause significant inconvenience to both the patient and the dental professional.
Due to the poor aesthetics, discomfort and other limitations of braces, relatively few people with malocclusion elect traditional orthodontic treatment. Accordingly, we believe there is a large unmet need for an orthodontic system that addresses these patient concerns. We also believe there is an unmet need among dental professionals for a treatment system that increases the predictability and efficiency of treatment and enhances practice profitability.
The Align Solution
Invisalign is a proprietary system for treating malocclusion. The Invisalign system is comprised of several phases, the principal steps of which are the creation of digital treatment plans using proprietary software known as ClinCheck, and the manufacturing of customized Invisalign aligners.
Orthodontic diagnosis and transmission of treatment data to us. In an initial patient visit, the dental professional determines whether Invisalign is an appropriate treatment. The dental professional then prepares a treatment data package which consists of a polyvinyl-siloxane, or PVS impression of the relevant dental arches, x-rays of the patient's dentition, photographs of the patient, a bite impression depicting the relationship between the patient's upper and lower dental arches and an Invisalign treatment planning form, or prescription. The impression is a critical component of the Invisalign system as it depicts the three-dimensional geometry of the patient's teeth and hence forms the basis for our computer models and subsequent molds and aligners. An impression requires the patient to bite into a viscous material. This material hardens, capturing the shape of the patient's teeth. The prescription is also a critical component of the Invisalign system, describing the desired positions and movement of the patient's teeth. The dental professional sends the treatment data to our facility in Juarez, Mexico. Manufacturing services are currently provided by International Manufacturing Solutions Operaciones, S.R.L., or IMS, a third party shelter services provider in Juarez, Mexico. On December 22, 2008, we notified IMS of our intention to terminate this shelter services arrangement effective April 2009. At that time, we will become a direct manufacturer of our clear aligners at the facility in Juarez, Mexico.
Preparation of three-dimensional computer models of the patient's initial malocclusion. Upon receipt, we use the treatment data to construct digital models of the patient's dentition. Using computed
5
Table of Contents
tomography, known as CT scanning, we scan the PVS impression to develop a digital, three-dimensional computer model of the patient's current dentition. We then transmit this initial computer model together with the dental professional's prescription and supplemental materials electronically to our facilities in San Jose, Costa Rica.
Preparation of computer-simulated treatment and viewing of treatment using ClinCheck. In Costa Rica, we transform this initial digital model into a proposed customized, three-dimensional treatment plan that simulates appropriate tooth movement in a series of two-week increments. This simulated treatment plan, called ClinCheck, is an internally developed and proprietary computer modeling program that allows dental professionals to diagnose and plan treatments for their patients. This ClinCheck simulation is then reviewed for adherence to prescribed clinical treatment and quality standards. Upon completion of the review, the patient's ClinCheck is then made available to the prescribing dental professional via Virtual Invisalign Practice (VIP), our proprietary customer interfacing software portal, which is available on our websites located atwww.invisalign.com andwww.aligntech.com. The dental professional then reviews the ClinCheck and can either accept the proposed treatment or request modifications and adjustments until satisfied with the treatment plan. ClinCheck allows the dental professional to view this three-dimensional simulation with a high degree of magnification and from any angle. Accordingly, ClinCheck enables the dental professional to project tooth movement with a level of accuracy not previously possible with metal arch wires and brackets. By reviewing and amending the treatment simulation, the dental professional retains control over the treatment plan and, thus participates in the customized design of the aligners. At this point, the dental professional may also invite the patient to view the ClinCheck treatment plan, allowing the patient to see the projected course of treatment. The dental professional's final approval of the proposed ClinCheck treatment engages us to manufacture the corresponding molds and aligners.
Construction of molds corresponding to each step of treatment. Upon the dental professional's approval of the ClinCheck simulation, we use the data underlying the simulation, in conjunction with stereolithography technology, to construct a series of molds depicting the future position of the patient's teeth. Each mold is a replica of the patient's teeth at each two-week stage of the simulated course of treatment. IMS currently manufactures the molds and then uses these molds to fabricate the patient's aligners. As stated above, in April 2009, we will become a direct manufacturer of our clear aligners at the facility in Juarez, Mexico.
Manufacture of aligners and shipment to the dental professional. From these molds, aligners are fabricated by pressure-forming polymeric sheets over each mold. Aligners are custom-manufactured, thin, clear plastic, removable dental appliances that are manufactured in a series to correspond to each two-week stage of the ClinCheck simulation. Aligners are customized to perform the treatment prescribed for an individual patient by dental professionals using ClinCheck. Each aligner covers a patient's teeth and is nearly invisible when worn. Aligners are commonly worn in pairs, over the upper and lower dental arches. Aligners are generally worn for consecutive two-week periods which correspond to the approved ClinCheck treatment simulation. After two weeks of use, the patient replaces them with the next pair in the series, advancing the teeth movement with each aligner stage. This process is repeated until the final aligners are used and treatment is complete. When treating with Invisalign Full, Invisalign Express and Invisalign Teen, aligners are manufactured and then delivered to the dental professionals in a single shipment. For Invisalign Assist, aligners are manufactured in batches based on a progress tracking feature integrated into Invisalign Assist. When the progress tracking feature is selected, aligners are shipped to the dental professional after every nine stages. In certain cases, dental professionals may use Invisalign in conjunction with tooth-colored attachments bonded to the patient's teeth. These attachments are used to increase the force applied to a tooth or teeth in circumstances where the aligners alone may have difficulty in effecting the desired movement. In certain cases, we provide an aligner-like template to the dental professionals to aid the placement of bonding attachments to the patient's teeth. Also, in cases where interproximal reduction, or IPR, is
6
Table of Contents
requested by the dental professional, we provide an IPR treatment form, quantifying the amount of space to be created through enamel reduction, location, and timing of IPR.
Retention. Upon completion of the treatment, the patient may be prescribed our single clear retainer product or our Vivera retainer product. New Vivera retainers are shipped every three months over the one year period.
Our Products
Our revenues are generated from the sale of the following product offerings.
Invisalign Full. Commercial sales of Invisalign Full commenced in the U.S. in July 1999. Our traditional, Invisalign Full is intended to be used as a complete treatment for a broad range of malocclusions. Each treatment plan is unique to the individual patient and will consist of as many aligners as indicated by ClinCheck in order to achieve the doctor's treatment goals. For Invisalign Full, aligners are manufactured and then delivered to the dental professionals in a single shipment. In fiscal 2008, approximately 85% of our net revenues were generated by the sale of Invisalign Full.
Invisalign Express. In the third quarter of 2005, we launched Invisalign Express, a lower-cost solution for less complex orthodontic cases. Invisalign Express is a dual arch orthodontic treatment for cases that meet certain predetermined clinical criteria and consist of up to ten sets of aligners. Invisalign Express is intended to help a broader range of patients elect orthodontic treatment by providing a lower-cost option for adult relapse cases, for minor crowding and spacing, or as a pre-cursor to restorative or cosmetic treatments such as veneers. For Invisalign Express, aligners are manufactured and then delivered to the dental professionals in a single shipment.
Retention. In addition to our traditional single retainer product, in January 2008, we launched Vivera retainers, where we deliver a new replacement retainer to orthodontic patients every three months for one year. Vivera retainers are produced using the same proprietary technology and material as the Invisalign aligners, and offer an effective, aesthetic retention solution for both Invisalign and non-Invisalign patients.
Invisalign Teen. In July 2008, we launched Invisalign Teen to meet the specific needs of the non-adult comprehensive or teen treatment market. Invisalign Teen includes features such as an aligner wear indicator to help gauge patient compliance and specially engineered aligner features to address lingual root control issues and the natural eruption of key teeth common in teen patients. Predominantly marketed to orthodontists who treat the vast majority of malocclusion in teen patients, these features are intended to meet the treatment needs of those younger patients. As part of Invisalign Teen, we include up to six free individual replacement aligners during active treatment to cover potential aligner loss. For Invisalign Teen, aligners (other than the replacement aligners) are manufactured and then delivered to the dental professionals in a single shipment.
Invisalign Assist. In October 2008, we launched Invisalign Assist. Invisalign Assist is designed specifically for GPs who want an integrated approach to selecting, monitoring and finishing Invisalign cases. Intended to help newly-trained and low volume GPs accelerate the adoption and frequency of use of Invisalign into their practice, Invisalign Assist is intended to make it easier for GPs to select appropriate cases for their experience level or treatment approach, submit cases more efficiently and manage appointments with suggested tasks. In addition, new progress tracking features allow GPs to submit new impressions every nine stages. When the progress tracking feature is selected, aligners are shipped to the dental professional after every nine stages. We believe Invisalign Assist will help GPs increase their confidence in prescribing Invisalign treatment.
7
Table of Contents
Proprietary software mentioned in this Annual Report on Form 10-K such as ClinCheck and VIP (Virtual Invisalign Practice) are included as part of the Invisalign system and are not sold separately nor do they contribute as individual items of revenue.
Ancillary and Other. The remaining net revenues are generated by training fees and sales of ancillary products, such as cleaning material and adjusting tools used by dental professionals during the course of treatment.
Benefits of Invisalign
We believe that Invisalign provides benefits to dental professionals and patients that have the potential to establish Invisalign as the preferred alternative to traditional braces.
8
Table of Contents
- •
- Improved oral hygiene. Patients can remove aligners for tasks that are difficult with traditional braces, such as eating, brushing and flossing. We believe this feature has the potential to reduce tooth decay and periodontal damage during treatment, which may result from traditional braces.
- •
- Potentially reduced overall treatment time. Aligners control force by distributing it broadly over the exposed surfaces of the teeth. In addition, the ClinCheck simulation from which aligners are produced is designed to reduce unintended and unnecessary tooth movements. Together, these factors may reduce overall treatment time relative to traditional braces.
- •
- Potentially reduced root resorption. We believe that controlling force and shortening treatment time has the potential to reduce the incidence of root resorption, which is the breakdown or destruction of root structure.
- •
- Reduced incidence of emergencies. Typically, a lost or broken aligner is simply replaced with the next aligner in the treatment series, minimizing inconvenience to both the patient and the dental professional.
We believe that these benefits will prove attractive to people who currently do not seek treatment because of the limitations of traditional braces.
Limitations of Invisalign
In some instances, the Invisalign system may have certain limitations relative to traditional treatment. Aligners cost more to produce than traditional braces, and we charge dental professionals more than they generally pay for the supplies used in traditional treatment. Depending on the individual pricing policies of each dental professional and the treatment selected, the cost of Invisalign treatment to the patient may be greater than for traditional braces. Dental professionals must also incorporate our manufacturing cycle times into their overall treatment plan. Once a dental professional submits a case to us, there is generally a turn-around time of a month or more before the corresponding aligners are delivered. Aligners may not be appropriate for all cases, such as severe malocclusion, which may require aligners to be used in combination with traditional braces for optimal results. In addition, because aligners are removable, treatment using Invisalign depends on patients wearing their aligners as recommended. Some patients may experience a temporary period of adjustment to wearing aligners that may mildly affect speech. In some instances, patients have experienced scratched or irritated gums, cheeks and lips and in some rare instances allergic reactions have occurred. We believe that these limitations are generally outweighed by the many benefits of Invisalign to both patients and dental professionals.
Our Target Market and Patient Base
Approximately 2.1 million people annually elect treatment by orthodontists in the U.S., of which approximately 1.2 million have mild to moderate malocclusion and are applicable to Invisalign—our served market. Twenty-six percent of these patients, or approximately 300 thousand, have mature dentition (adults and older teens), with fully-erupted second molars and substantially completed jaw growth. Seventy-four percent, or approximately 850 thousand, have erupting dentition (non-adult comprehensive, or younger teens), with partially-erupted second molars, cuspid and second bicuspid teeth. As of December 31, 2008, our share of the 2.1 million case starts is approximately 3% and approximately 6% of the 1.2 million patients of our served market.
Our market research indicates that the majority of people with malocclusion who desire treatment forgo treatment rather than elect traditional treatment due to its many limitations. We believe that since Invisalign addresses the primary limitations of braces, adults, who are particularly sensitive to aesthetic limitations of traditional treatment, will be more likely to seek treatment and therefore represent our most immediate market expansion opportunity. With the launch of Invisalign Teen in July
9
Table of Contents
2008, we now offer a product designed to meet the needs of the non-adult comprehensive, or younger teen, treatment market. Even though we have not previously marketed our product to treat younger patients, approximately 18% of our total case starts since 2007 were with individuals younger than 19. Launching Invisalign Teen makes our treatment more applicable to an orthodontist's patient base, which we believe will provide us the opportunity to increase our penetration into and our share of the teen treatment market.
Published market data for GPs providing treatment for malocclusion is limited, however, as the primary care provider, GPs have access to a greater number of patients than orthodontists and possess a unique opportunity to introduce Invisalign and expand their practice and patient base. We believe GPs represent a significant market expansion opportunity.
As of December 31, 2008, approximately 944,000 patients worldwide have started treatment using Invisalign. The Invisalign system is sold in North America, Europe, Asia Pacific, Latin America and Japan. International sales accounted for 21%, 17% and 16% of our net revenues in 2008, 2007 and 2006, respectively. A geographic breakdown of our net revenues is summarized in Note 17 "Segments and Geographic Information" in the Notes to our Consolidated Financial Statements. We operate as one reportable segment—the design, development, manufacturing and marketing of Invisalign, a proprietary method for treating malocclusion, or the misalignment of teeth.
No single customer accounted for 10% or more of our total net revenues in 2008, 2007 and 2006.
Business Strategy
Our goal is to establish Invisalign as the standard method for treating malocclusion ultimately driving increased product adoption by dental professionals by focusing on the following four key objectives: driving product innovation, enhancing the customer experience, generating consumer demand and expanding our international markets.
Product innovation and enhancements to existing products. We believe that product performance and innovation is a cornerstone to our future long-term goal to drive and sustain product adoption. Until 2008, the Invisalign system was a single offering used by our primary channels—GPs and orthodontists—each with distinct and separate needs. In 2008, we launched additional products to better meet those distinct needs. Specifically, orthodontists want a more robust set of tools for greater predictability, wider applicability and more flexibility in the use of the Invisalign system. On the other hand, typical GPs want greater ease of use, more efficient and simplified diagnostic tools, guidance through the case set-up process, minimal treatment intervention and self-help tools designed to simplify treatment of cases of mild to moderate malocclusion. Based on this knowledge, in July 2008, we announced the release of Invisalign Teen, predominantly marketed to the orthodontist. In October 2008, we announced the release of Invisalign Assist, predominantly marketed to the GP.
With the introduction of Invisalign Teen, our Invisalign product family now includes a product designed to meet the specific needs of the non-adult comprehensive or younger teen market. Invisalign Teen features include an aligner wear indicator to help gauge patient compliance and specially engineered aligner features to address the natural eruption of key teeth and lingual root control. Predominantly marketed to orthodontists who treat the vast majority of malocclusion in teen patients, these features make it easier and more efficient for orthodontists to treat those younger patients. The launch of a teen-specific product makes the Invisalign system more applicable to an orthodontist's patient base, which we believe will increase our penetration into and our share of the teen treatment market over time. We expect that orthodontists will adopt Invisalign Teen slowly, after they experience multiple successful treatment outcomes. As a result, we anticipate that Invisalign Teen volume may increase gradually and will not constitute a significant portion of our total product mix in the near-term.
10
Table of Contents
Our most recently launched product, Invisalign Assist, is intended to help newly-trained and low volume Invisalign GPs accelerate the adoption and frequency of use of Invisalign into their practice. Invisalign Assist features are intended to make it easier for doctors to select appropriate cases for their experience level or treatment approach. In addition, GPs can plan and submit cases efficiently, manage appointments with suggested tasks, and receive batch shipments of aligners based on treatment progress. We believe Invisalign Assist will help GPs increase their confidence in prescribing Invisalign treatment.
We believe continuing to introduce new products and product features as well as enhancing the user experience will keep us at the forefront of the market and increase adoption of Invisalign. The recent launch of Invisalign Teen and Invisalign Assist and other future products will rely on new features, tools and delivery options to meet specific clinical demands while providing a family of end-to-end solutions for our customers. Enhanced product performance and innovation should continue to drive the adoption and frequency of use (what we call utilization). Although we believe new product introduction to be a cornerstone to our future long-term growth, we expect that adoption of these new products will increase gradually over a number of years.
Enhancing the customer experience. We are committed to enhancing the customer experience by focusing on specific customer "touch points", or areas where we interact directly with our customers. Specifically, we are focused on improving our pre-selection process in order to attract new doctors that are motivated to become Invisalign providers and committed to making Invisalign a key part of their practices and strengthening our training programs in order to increase the rate that these newly trained customers submit Invisalign cases, as well as increase the rate that they move up the adoption curve to ultimately become leading Invisalign providers, or what we call promoters.
- •
- Improving Training Programs. Increasing the number of Invisalign trained doctors and ensuring that these doctors are confident in using the Invisalign system is a key driver toward our ultimate goal of increasing product adoption. We continuously update our training programs to address the needs of our customers. For instance, we developed a pre-training course intended to familiarize doctors with the Invisalign system prior to attending the full training course. In addition, we recently updated our initial training program by focusing on Invisalign Assist, instead of Invisalign Full, since we believe Invisalign Assist is the right product for newly trained GPs. We anticipate that by using Invisalign Assist, newly trained GPs will exit this initial training program with increased confidence in prescribing Invisalign treatment. We have also incorporated the Invisalign technique into the curriculum of 38 university programs. By educating dental students and orthodontic residents on the benefits of the Invisalign technique, we believe they will be more likely to use this technology in their future practices and offer Invisalign as a treatment option.
- •
- Moving from Invisalign provider to a leading Invisalign provider. Once a doctor is trained, our goal is to assist the doctor to move up the adoption curve to ultimately become a leading Invisalign provider, or a promoter. In order to increase the number of Invisalign promoters, we provide additional services to help our customers increase their confidence in using the Invisalign system through continuing education and clinical support as well as improving their practice management skills. In early 2008, we announced the introduction of the Aligntech Institute program (www.aligntechinstitute.com), which is an interactive website that provides clinical education and practice development training. These clinical education and practice development training opportunities include instructor-led training classes, seminars and workshops, conference calls, web-based videos, case studies, and other clinical resources. Many of these courses and resources are eligible for continuing education (CE) credits. Additionally, our VIP portal (Virtual Invisalign Practice) provides our trained doctors and their staff access to thousands of Invisalign cases and best practices as well as up-to-date support information, programs and marketing materials for continuous support and information access. Lastly, as trained Invisalign
11
Table of Contents
providers grow their case starts with Invisalign, programs such as the Advantage Program provide tiered benefits including volume rebates, dedicated clinical support and a premium website position on the Invisalign Doctor Locator website to those leading providers. By participating in these programs and the various events and educational offerings, we believe that our customers will emerge with a better understanding of the product and its applicability, and with a greater aptitude for starting and finishing Invisalign cases successfully.
Consumer demand generation for Invisalign. Marketing to the consumer and creating demand is one of our key strategic objectives to driving long-term growth. Our market research indicates that the majority of people with malocclusion who desire treatment forgo treatment rather than elect traditional treatment due to its many limitations, such as compromised aesthetics and oral discomfort. By communicating the benefits of Invisalign to both dental professionals and consumers, we intend to increase the number of patients who seek treatment using Invisalign. Historically, our marketing programs have been directed to an adult audience, however, with the introduction of Invisalign Teen, we will for the first time direct our communication efforts directly to teens and their parents. Despite the continuing challenges in the U.S. economy and weak consumer spending, we believe that consumer demand creation is a key component to our long-term growth. As a result, we will continue to invest in efforts to increase consumer awareness of Invisalign through a variety of media outlets. We will continue to drive consumer demand among the adult population through our traditional TV advertising, as well as digital online media. In 2009, we will focus our efforts on the introduction of a new public relations program for Invisalign Teen intended to access print, TV and online media. We also have a teen specific website and will increasingly leverage widgets, social media and blogs to directly target teens.
Growth of international markets. We will continue to focus our efforts towards increasing adoption of Invisalign by dental professionals in our key international markets, Europe and Japan. Similar to the domestic market, our objective internationally is to increase the number of doctors that are motivated to becoming an Invisalign provider and committed to making Invisalign a key part of their practices. Through December 31, 2008, we have trained over 14,000 doctors, predominantly orthodontists in core Europe, our primary international market. Product line expansion is key to providing doctors a solution that addresses a wider range of potential patient needs with greater treatment flexibility. In October 2008, we launched Invisalign Express in Europe expanding our international product offerings. In Europe, the vast majority of orthodontic case starts are children and teens. With the introduction of Invisalign Teen in Europe, planned for mid-2009, we expect the addressable market for our product to expand and ultimately increase adoption. In addition, we will carry on our efforts to increase brand awareness and consumer demand in Europe by continuing our consumer advertising campaign that was first launched in March 2007. Additionally, although the vast majority of our international revenues are from direct sales, approximately 9% of our international sales are through distributors covering smaller international markets, specifically Asia Pacific and Latin America. We will consider selling through distributors in other smaller or less strategic markets as well as consider expanding directly into additional countries on a case-by-case basis. With these efforts, we expect our international revenues to continue to increase in absolute dollars and as a percentage of total net revenues in the foreseeable future. In 2008, our international sales increased from 17% of net revenues to 21% of net revenues, an increase of approximately 34%.
Manufacturing
To produce our highly customized, highly precise, medical quality products in volume, we have developed a number of proprietary processes and technologies. These technologies include complex software solutions, CT scanning, stereolithography and automated aligner fabrication.
Manufacturing administration is located in Santa Clara, California; however, our manufacturing facilities are located outside of the U.S, in San Jose, Costa Rica and Juarez, Mexico. As of
12
Table of Contents
December 31, 2008, our manufacturing and operations staff in the U.S. and Costa Rica consisted of 747 people. At our facility in Costa Rica, technicians use a sophisticated, internally developed computer-modeling program to prepare digital treatment plans. Upon acceptance of the treatment plan by the dental professional, these plans are then transmitted electronically to Juarez, Mexico. These digital files form the basis of ClinCheck and are used to manufacture SLA (stereolithography) aligner molds. Our order acquisition operations, the manufacturing of aligner molds and aligners, as well as the packaging and shipment of aligners, are currently conducted by International Manufacturing Solutions Operaciones, S.R.L., or IMS, a third party shelter services provider in Juarez, Mexico. On December 22, 2008, we notified IMS of our intention to terminate IMS' shelter services arrangement effective April 2009. At that time, we will become a direct manufacturer of our clear aligners at the facility in Juarez, Mexico. Upon the completion of this transition, we will directly employ approximately 500 people in Mexico, each of whom is currently employed by IMS. Information regarding risks associated with our manufacturing process and foreign operations may be found in Item 1A of this Annual Report on Form 10-K under the heading "Risk Factors."
We rely on two vendors who are each the sole source of the polymer and resin used in our manufacturing process. In the event that either of these vendors becomes unable for any reason to supply us with their respective products, we would experience a manufacturing disruption while we qualify and obtain an alternate source.
Throughput Management
Because we manufacture each case on a build-to-order basis, we must conservatively build manufacturing capacity for anticipated demand. To increase throughput, we must improve the efficiency and increase the scale of our manufacturing processes.
In order to increase the efficiency of our manufacturing processes, we focus our efforts on software development and the improvement of rate-limiting processes, or bottlenecks. We continue to upgrade our proprietary, three-dimensional treatment-planning software to enhance computer analysis of treatment data and to reduce time spent on manual and judgmental tasks for each case, thereby increasing the efficiency of our technicians in Costa Rica. We are also continuing the development of automated systems for the fabrication and packaging of aligners manufactured in Juarez, Mexico. In order to scale our manufacturing capacity, we expect that we will continue to invest in capital equipment.
Quality Assurance
Align's quality system is in compliance with Food & Drug Administration's Medical Device regulations, 21CFR Part 820, and Health Canada's Medical Device Regulations. We are certified to EN ISO 13485:2003, internationally recognized standards for Medical Device manufacturing and of the Council of Canada. We have a formal, documented quality system by which quality objectives are defined, understood and achieved. Systems, processes and procedures are implemented to ensure high levels of product and service quality. We monitor the effectiveness of the quality system based on internal data and direct customer feedback and strive to continually improve our systems and processes, taking corrective action, as needed.
Since we custom manufacture aligners on a build-to-order basis, we do not offer refunds on our products. Because each ClinCheck and each aligner is unique, we inspect the product at various points during the manufacturing process, to ensure that the product meets our customers' expectations. Aligners are subject to the Invisalign product warranty, which covers defects in materials and workmanship. Our materials and workmanship warranty is in force until the Invisalign case is completed. In the event aligners fall within the scope of the Invisalign product warranty, we will replace the aligners at our expense. Our warranty is contingent upon proper use of the aligners for the
13
Table of Contents
purposes for which they are intended. If a patient chooses not to wear the aligners, and as a result, requests additional Invisalign treatment, the dental professional pays the additional expense of the replacement aligners. Warranty treatment requires that the dental professional submit new impressions of the patient's dentition to us. We use the impressions to create a new ClinCheck treatment plan for the dental professional to approve, from which a successive series of aligners will be produced that will allow the patient to finish treatment.
The Invisalign product warranty does not provide any assurances regarding the outcome of treatment using Invisalign. Actual treatment results may deviate significantly from the approved ClinCheck treatment plan. Deviations not covered under warranty have typically been the result of unpredictable biological factors, such as variations in bone density or tooth topography and abnormal jaw growth.
Sales and Marketing
We market Invisalign by communicating Invisalign's benefits to dental professionals through our training programs, mail campaigns, trade shows, trade journals and print media, and to consumers through a nationwide advertising campaign. Based on our experience with advertising and commercial sales, we believe that making consumers aware of Invisalign as a new treatment alternative generates significant demand for Invisalign. In order to serve anticipated demand, we continue to train a broad base of dental professionals.
Our sales and support staff has been engaged in marketing Invisalign to orthodontists since July 1999. In 2001, we began marketing Invisalign to GPs in our North American market. As of December 31, 2008 our North American sales organization consisted of 161 people, of which 134 were direct sales representatives and 27 were sales administration and management. Internationally, we had 41 people engaged in sales and sales support as of December 31, 2008. We continually evaluate cost effective ways to support our customers in smaller markets. For instance, during 2007, we transitioned the sales of our products in part of the Asia Pacific and Latin American regions to a distributor model. We will consider selling through a distributor in other smaller markets as well as consider expanding directly into additional countries on a case-by-case basis.
We provide training, marketing and clinical support to orthodontists and GPs in the U.S. and Canada, which we consider our North American market, and internationally. As of December 31, 2008, we had trained 55,510 dental professionals worldwide to use Invisalign. Of those trained dental professionals, approximately 74% are dental professionals in our North American market. Within our North American market, we have trained 8,670 orthodontists and 32,660 GPs cumulatively through the end of 2008.
Invisalign relies on the same orthodontic principles that apply to traditional treatment. Our sales and orthodontic teams conduct training primarily in a workshop format. The key topics covered in training include Invisalign applicability, instructions on filling out the Invisalign treatment form, clinical tips and techniques, guidance on pricing and instructions on interacting with our ClinCheck software and the many other features of our website.
After doctors complete their training, sales representatives may follow up with the dental professional to ensure that their staff is prepared to handle Invisalign cases. These practice development activities may include assisting the dental professional in taking dental impressions, treatment planning processes and familiarizing them with our dental online portals and tools. Sales representatives may also provide practice-building assistance, including helping the dental professional to market Invisalign to prospective patients through direct mail or other forms of media. Many dental professionals have commenced promotional activity in their local region with our assistance.
14
Table of Contents
Our experience indicates that prospective patients seek information from six primary sources:
- •
- an orthodontist;
- •
- a GP;
- •
- consumer marketing and advertising;
- •
- our website, which can be accessed at eitherwww.invisalign.com orwww.aligntech.com;
- •
- direct-to-consumer mail advertising and public relations efforts; and
- •
- other Invisalign patients.
In 2009, we expect higher marketing spending in Europe with a focus on consumer advertising, including television and print media to raise the profile of Invisalign and drive more consumers to our most experienced doctors. We will continue to incur additional costs related to commercialization and launch of new products to market in the United States and Europe.
Research and Development
Our research and development effort is focused on extending the range of dental applicability of Invisalign, enhancing the software used in the manufacturing process and enhancing our Invisalign system product lines, including the development of distinct product platforms for the GPs and orthodontists such as Invisalign Assist and Invisalign Teen. Our research and development expenses were $26.2 million for 2008, $25.7 million for 2007, and $18.5 million for 2006.
In an effort to demonstrate Invisalign's broad treatment capabilities, various clinical case studies and articles have been published that highlight the applicability of Invisalign to malocclusion cases, including those of severe complexity. We are also undertaking post-marketing studies and making additional technological improvements to the product and manufacturing process. As mentioned in our Business Strategy, we are making investments in the development of new products and enhancements of existing products to meet the needs of our customers and increase adoption and utilization of Invisalign.
Intellectual Property
We believe our intellectual property position represents a substantial business advantage. As of December 31, 2008, we had 118 issued U.S. patents, 181 pending U.S. patent applications, and numerous foreign issued patents, as well as pending foreign patent applications.See Item 3 "Legal Proceedings" for a discussion on Reexamination Proceedings pending with the United States Patent and Trademark Office.
We continue to pursue further intellectual property protection through U.S. and foreign patent applications and non-disclosure agreements. We also seek to protect our software, documentation and other written materials under trade secret and copyright laws. We cannot be certain that patents will be issued as a result of any patent application or that patents that have been issued to us or that may be issued in the future will be found to be valid and enforceable and sufficient to protect our technology or products. Our intellectual property rights may not be successfully asserted in the future or may be invalidated, circumvented or challenged. In addition, the laws of various foreign countries where Invisalign is distributed do not protect our intellectual property rights to the same extent as U.S. laws. Our inability to protect our proprietary information could harm our business. Information regarding risks associated with failing to protect our proprietary technology and our intellectual property rights may be found in Item 1A of this Annual Report on Form 10-K under the heading "Risk Factors."
15
Table of Contents
Seasonal Fluctuations
Seasonal fluctuations in the number of doctors in their offices and available to take appointments have affected, and are likely to continue to affect, our business. Specifically, our customers often take vacation or are on holiday during the summer months and therefore tend to start fewer cases. These seasonal trends have caused and will likely continue to cause, fluctuations in our quarterly results, including fluctuations in sequential revenue growth rates.
Backlog
Due to the nature of our business, we maintain relatively low levels of backlog. The period from which treatment data (or "a case") is received until the acceptance of the digital treatment plan, or ClinCheck, is dependent on the dental professional's discretion to modify, accept or cancel the treatment plan. Therefore, we consider the case a firm order to manufacture aligners once the dental professional has approved ClinCheck. Our backlog consists of ClinCheck-approved cases, which are generally shipped within a short period of time. As a result, we believe that backlog is not a good indicator of future sales, and our quarterly revenues depend largely on the timing of ClinCheck approvals and the impact on cases shipped in that quarter.
Competition
We compete for the attention of dental professionals with manufacturers of traditional orthodontic appliances (or wires and brackets), which include 3M Company, Sybron Dental Specialties and Dentsply International, Inc. We also compete directly with established companies that manufacture and distribute products that are similar in use to Invisalign, including the products manufactured and distributed by Ormco Orthodontics, a division of Sybron Dental Specialties (a division of Danaher Corporation). SeeItem 3 "Legal Proceedings" for a summary of our litigation with Ormco. In the future, we may face further competition from early stage and more mature companies who enter our target markets to manufacture and distribute products that are similar in use to Invisalign. Information regarding risks associated with increased competition may be found in Item 1A of this Annual Report on Form 10-K under the heading "Risk Factors."
We believe that in addition to price, the principal competitive factors in the market for orthodontic appliances include the following:
- •
- aesthetic appeal of the treatment method;
- •
- effectiveness of treatment;
- •
- customer support;
- •
- comfort associated with the treatment method;
- •
- oral hygiene;
- •
- ease of use; and
- •
- dental professionals' chair time.
We believe that Invisalign compares favorably with our competitors' products with respect to each of these factors.
16
Table of Contents
Government Regulation
FDA's Quality System Regulation for Medical Devices. The Invisalign system is classified as a Class II medical device. In 1998, we received pre-market clearance from the FDA pursuant to the 510(k) pre-market notification procedure, allowing us to market the product in the U.S. The Invisalign system was originally cleared for use by the FDA in patients with permanent teeth and contraindicated the device for patients presenting with mixed dentition, severe overbite, severe overjet, tooth malocclusion requiring surgical correction, adolescent patients with a skeletally narrow jaw, and adult patients with dental prosthetics/implants. In 2008, the FDA cleared new labeling for the Invisalign system, by removing the permanent dentition limitation from the indications for use. In addition, certain conditions previously listed as contraindications will now be listed as precautions. We believe our Invisalign system is in compliance in all material respects with applicable quality system regulations, record keeping and reporting requirements in the production and distribution of the Invisalign system. Our aligners are currently manufactured by IMS, a third party shelter services provider based in Juarez, Mexico. IMS is registered with the FDA as a medical device manufacturer and is certified to ISO 13485:2003 under Align's quality management system. We have also ensured that our quality system procedures and processes have been implemented at IMS to comply with the FDA's Quality Systems standards. IMS has dedicated an area in its facilities and trained personnel in the manufacture and distribution of Invisalign. We and IMS are subject to routine inspections by the FDA and state agencies to determine compliance with Quality System requirements. We are registered with the State of California as a medical device manufacturer. On December 22, 2008, we notified IMS of our intention to terminate IMS' shelter services arrangement effective April 2009. At that time, we will become a direct manufacturer of our clear aligners at the facility in Juarez, Mexico.
If the FDA determines that we or IMS failed to comply with the applicable FDA regulations, it can institute a wide variety of enforcement actions against us, ranging from a public Warning Letter to more severe sanctions, including but not limited to financial penalties, withdrawal of our right to market our products and criminal prosecution.
Health Canada's Medical Device Regulations. In Canada, we are required to comply with Health Canada's Medical Device Regulations. Our products are registered with Health Canada. We believe we are in compliance with their regulations and have been granted clearance to market our products in Canada.
European Union's MDD Requirements & ISO 13485:2003. In Europe, Invisalign is regulated as a custom device and as such, we follow the requirements of the Medical Device Directives. We are ISO 13485:2003 certified, which facilitates commercialization of Invisalign outside the United States and especially in Europe.
Health Insurance Portability and Accountability Act of 1996. Under the Health Insurance Portability and Accountability Act of 1996, or HIPAA, Congress mandated a package of interlocking administrative simplification rules to establish standards and requirements for electronic transmission of certain health information. Confidentiality of patient records and the circumstances under which these records may be released are subject to substantial regulations under the HIPAA Standards for Privacy of Individually Identifiable Health Information, referred to as the Privacy Standard, and other state laws and regulations. The Privacy Standard governs both the disclosure and the use of confidential patient medical information. Although compliance is the responsibility of the hospital, physician or other healthcare provider, we understand the importance to our customers and their patients of maintaining the confidentiality of patient information. Accordingly, we have designed our product and service offerings to be consistent with the requirements of the Privacy and Security standards under HIPAA and applicable corresponding state laws and regulations. Maintaining systems that are consistent with these laws and regulations is costly and could require complex changes in the way we do
17
Table of Contents
business or provide services to our patients. Additionally, our success may be dependent on the success of healthcare participants in dealing with HIPAA requirements.
Other Federal and State Laws. As a participant in the health care industry we are subject to extensive and frequently changing regulation under many other laws administered by governmental entities at the federal, state and local levels, some of which are, and others of which may be, applicable to our business. We are a medical device manufacturer subject to U.S. Food and Drug Administration regulations. These regulations, among other things, require that we maintain device and facilities registrations and listings as well as promote our products as permitted by our FDA clearances. Furthermore, our health care service provider customers are also subject to a wide variety of laws and regulations that could affect the nature and scope of their relationships with us. Laws regulating medical device manufacturers and health care providers cover a broad array of subjects. For example, the confidentiality of patient medical information and the circumstances under which such information may be used by us, released for inclusion in our databases, or released by us to third parties, are subject to substantial regulation by state governments. These state laws and regulations govern both the disclosure and the use of confidential patient medical information and are evolving rapidly. In addition, provisions under the federal anti-kickback statute prohibit, among other things, paying or offering to pay any remuneration in exchange for the referral of patients to a person participating in, or for the order, purchase or recommendation of items or services that are subject to reimbursement by, Medicare, Medicaid and other similar federal or state health care programs. Most states have also enacted illegal remuneration laws that are similar to the federal laws. These laws, which are evolving at the federal and state levels, are applicable to our financial relationships with, and any marketing or other promotional activities involving, our dental professional customers. Violations of any of these laws or regulations could subject us to a variety of civil and criminal sanctions.
Employees
As of December 31, 2008, we had 1,394 employees, including 747 in manufacturing and operations, 339 in sales and marketing, 137 in research and development and 171 in general and administrative functions. We had 503 employees in North America, 721 employees in Costa Rica, 160 employees in Europe and 10 employees in Japan.
Available Information
Our website is located atwww.aligntech.com, and our investor relations website is located athttp://investor.aligntech.com. The information on or accessible through our websites is not part of this Annual Report on Form 10-K. Our Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K, our proxy statement on Schedule 14A for our annual stockholders' meeting and amendments to such reports are available, free of charge, on our investor relations website as soon as reasonably practicable after we electronically file or furnish such material with the SEC. Further, a copy of this Annual Report on Form 10-K is located at the SEC's Public Reference Room at 100 F Street, NE, Washington, D.C. 20549. Information on the operation of the Public Reference Room can be obtained by calling the SEC at 1-800-SEC-0330. The SEC maintains an internet site that contains reports, proxy and information statements and other information regarding our filings atwww.sec.gov.
18
Table of Contents
Executive Officers of the Registrant
The following table sets forth certain information regarding our executive officers as of February 26, 2009:
| | | | | |
Name | | Age | | Position |
|---|
Thomas M. Prescott | | | 53 | | President and Chief Executive Officer |
Kenneth B. Arola | | | 53 | | Vice President, Finance and Chief Financial Officer |
Dana Cambra | | | 51 | | Vice President, Research & Development and Information Technology |
Dan S. Ellis | | | 57 | | Vice President, North American Sales |
Roger E. George | | | 43 | | Vice President, Legal and Corporate Affairs General Counsel and Corporate Secretary |
Len M. Hedge | | | 51 | | Senior Vice President, Business Operations |
Gil Laks | | | 43 | | Vice President, International |
Emory Wright | | | 39 | | Vice President, Operations |
Thomas M. Prescott has served as our President and Chief Executive Officer and as a member of our Board of Directors since March 2002. Prior to joining us, Mr. Prescott was President and Chief Executive Officer of Cardiac Pathways, Inc., a publicly-traded medical device company, from May 1999 until its acquisition by Boston Scientific in August 2001. Mr. Prescott then worked as a consultant for Boston Scientific Corporation until January 2002. Prior to working at Cardiac Pathways, Mr. Prescott held various sales, general management and executive roles at Nellcor Puritan Bennett, Inc. from April 1994 to May 1999. Mr. Prescott serves as a director of Interventional Rhythm Management, Inc., a privately held company.
Kenneth B. Arola has served as our Vice President of Finance and Chief Financial Officer since December 2007. He joined us as Vice President of Finance and Corporate Controller in August 2005. Prior to joining us, Mr. Arola served for fourteen years at Adaptec, Inc, an electronic data storage equipment company, where he held various senior finance management positions, most recently as Vice President of Finance and Corporate Controller. His experience also includes positions of increasing responsibility in various financial roles at Varian Associates and Cooper Labs.
Dana C. Cambra our Vice President, Research & Development and Information Technology has been with Align since June 2008. Prior to joining us, Mr. Cambra served as Senior Vice President, Research and Development for Pharsight Corporation, a provider of simulation and modeling software for pharmaceutical and biotechnology companies from March 2007 to June 2008. Prior to his role at Pharsight, Mr. Cambra was Vice President, Engineering at Stentor Inc., a medical image and information management software provider from October 2002 to February 2006. Earlier roles included executive engineering and operations positions at Visto Corporation and iScribe, Inc. Mr. Cambra also spent several years in positions of increasing responsibility at Acuson Corporation, now a Siemens Company.
Dan S. Ellis has served as our Vice President, North American Sales since June 2005. Prior to joining us, Mr. Ellis was Vice President, Sales for privately-held BARRx Medical, a medical device company, from September 2004 to June 2005. From June 1999 to May 2004, Mr. Ellis was at Fusion Medical Technologies, a division of Baxter Healthcare, most recently as Vice President, BioSurgery US. From January 1998 to June 1999, Mr. Ellis served as Vice President, Sales & Marketing for Cardiac Pathways, Inc. Earlier in his career, Mr. Ellis held national sales positions of increasing scope and responsibility at Fusion Medical Technologies and Eli Lilly MDD/Guidant Corporation.
Roger E. George has served as our Vice President, Legal and Corporate Affairs, General Counsel and Corporate Secretary since July 2002. Prior to joining us, Mr. George was the Chief Financial Officer, Vice President of Finance and Legal Affairs and General Counsel of SkyStream Networks, a
19
Table of Contents
privately held broadband and broadcast network equipment company. Prior to SkyStream, Mr. George was a partner at Wilson Sonsini Goodrich & Rosati, P.C. in Palo Alto, California.
Len M. Hedge has served as our Senior Vice President, Business Operations since December 2007. He joined us as our Vice President, of Manufacturing in January 1999 and was our Vice President, of Operations from March 2002 to December 2007. Prior to joining us, Mr. Hedge served as Vice President of Operations for Plynetics Express Corporation, a rapid-prototyping and stereolithography services supplier, from December 1996 to December 1998. From October 1991 to December 1996, Mr. Hedge worked at Beckman Instruments Corporation as Manager for Prototype Manufacturing and Process Development.
Gil Laks has served as our Vice President, International since September 2005, and served as our Vice President, Europe since June 2001. Prior to joining us, Mr. Laks was Vice President, Business Development for the diagnostic imaging division of Singapore Technologies, from November 1999 to May 2001. He also served as Director of International for ISIX, Ltd., an educational computing services firm, from October 1996 to October 1999.
Emory M. Wright has served as our Vice President, Operations since December 2007. He has been with us since March 2000, predominantly in manufacturing and operations roles. Previously, Mr. Wright served as Vice President, Manufacturing and most recently was General Manager of New Product Development. Prior to joining us, Mr. Wright was Senior Manufacturing Manager at Metrika, Inc. a medical device manufacturer, from May 1999 to March 2000. From July 1994 to May 1999, Mr. Wright served as Manager of Manufacturing and Process Development for Metra Biosystems Inc.
ITEM 1A. RISK FACTORS
We have only recently returned to profitability. If we fail to sustain or increase profitability or revenue growth in future periods, the market price for our common stock may decline.
Although we maintained profitability in 2008, if we are to sustain or increase profitability in future periods, we will need to continue to increase our revenues, while controlling our expenses. While we generated positive operating cash flow in 2008, we cannot be certain that we will be able to achieve positive cash flow from operations, from period to period, in the future. Because our business is evolving, it is difficult to predict our future operating results or levels of growth, and we have in the past not been and may in the future not be able to sustain our historical growth rates. If we do not increase profitability or revenue growth or otherwise meet the expectations of securities analysts or investors, the market price of our common stock will likely decline.
Our financial results have fluctuated in the past and may fluctuate in the future which may cause volatility in our stock price.
Our operating results have fluctuated in the past and we expect our future quarterly and annual operating results to fluctuate as we focus on increasing doctor and consumer demand for our products. These fluctuations could cause our stock price to decline. Some of the factors that could cause our operating results to fluctuate include:
- •
- limited visibility into and difficulty predicting the level of activity in our customers' practices from quarter to quarter;
- •
- weakness in consumer spending as a result of the slowdown in the United States economy and global economies;
- •
- changes in the timing of receipt of case product orders during a given quarter which, given our cycle time and the delay between case receipts and case shipments, could have an impact on which quarter revenue can be recognized;
20
Table of Contents
- •
- changes in product mix;
- •
- seasonal fluctuations in the number of doctors in their offices and their availability to take appointments;
- •
- success of marketing programs from quarter to quarter;
- •
- changes in the timing of when revenue is recognized as a result of the introduction of new products such as Invisalign Assist and Invisalign Teen;
- •
- changes to our effective tax rate;
- •
- unanticipated delays in production caused by insufficient capacity;
- •
- any disruptions in the manufacturing process, including unexpected turnover in the labor force or the introduction of new production processes or natural or other disasters beyond our control;
- •
- the development and marketing of directly competitive products by existing and new competitors;
- •
- aggressive price competition from competitors;
- •
- costs and expenditures in connection with litigation;
- •
- inaccurate forecasting of revenues, production and other operating costs; and
- •
- investments in research and development to develop new products and enhancements to Invisalign.
To respond to these and other factors, we may need to make business decisions that could adversely affect our operating results such as modifications to our pricing policy, business structure or operations. Most of our expenses, such as employee compensation and lease payment obligations, are relatively fixed in the short term. Moreover, our expense levels are based, in part, on our expectations regarding future revenue levels. As a result, if our revenues for a particular period fall below our expectations, whether caused by changes in consumer spending, consumer preferences, weakness in the U.S. or global economies, changes in customer behavior related to advertising and prescribing our product, or other factors, we may be unable to adjust spending quickly enough to offset any shortfall in revenues. Due to these and other factors, we believe that quarter-to-quarter comparisons of our operating results may not be meaningful. You should not rely on our results for any one quarter as an indication of our future performance.
We depend on the sale of the Invisalign system for the vast majority of our revenues, and any decline in sales of Invisalign for any reason, including as a result of a decline in general economic conditions, or a decline in average selling prices would adversely affect revenues, gross margin and net profits.
We expect that revenues from the sale of the Invisalign system will continue to account for the vast majority of our total revenues for the foreseeable future. Continued and widespread market acceptance of Invisalign by orthodontists, GPs and consumers is critical to our future success. If orthodontists and GPs experience a reduction in consumer demand for orthodontic services, if consumers prove unwilling to adopt Invisalign as rapidly as we anticipate or in the volume that we anticipate, if orthodontists or GPs choose to use a competitive product rather than Invisalign or if the average selling price of our product declines, our operating results would be harmed. Factors that could cause adoption of Invisalign at a lower rate than we expect, as well as the risk related to declining average selling prices are described more fully below.
21
Table of Contents
Consumers may not adopt Invisalign as rapidly as we anticipate due to a variety of factors including a continued decline in general economic conditions.
Consumer spending habits are affected by, among other things, prevailing economic conditions, levels of employment, salaries and wage rates, gas prices, consumer confidence and consumer perception of economic conditions. A general slowdown in the United States economy and certain international economies or an uncertain economic outlook would adversely affect consumer spending habits which may among other things result in a decrease in the number of overall orthodontic case starts or a reduction in the demand for Invisalign generally either of which would have a material adverse effect on our sales and operating results. In addition, Invisalign represents a significant change from traditional orthodontic treatment, and consumers may be reluctant to accept it or may not find it preferable to traditional treatment We have generally received positive feedback from both orthodontists, GPs and consumers regarding Invisalign as both an alternative to braces and as a clinical method for treatment of malocclusion, but a number of dental professionals believe that Invisalign is appropriate for only a limited percentage of their patients. Market acceptance will depend in part upon the recommendations of dental professionals, as well as other factors including effectiveness, safety, ease of use, reliability, aesthetics, greater comfort and hygiene compared to traditional orthodontic products and price for Invisalign compared to competing products.
Orthodontists and GPs may not adopt Invisalign in sufficient numbers or as rapidly as we anticipate.
Our success depends upon increasing acceptance and frequency of use of the Invisalign system by dental professionals (what we refer to as utilization). Invisalign requires orthodontists, GPs and their staff to undergo special training and learn to interact with patients in new ways. Increasing adoption and cumulative use by orthodontists and GPs will depend on factors such as the capability, safety, efficacy, ease of use, price, quality and reliability of our products, our ability to provide effective sales support, training and service and the availability of competing products, technologies and alternative treatments. In addition, unanticipated poor clinical performance of Invisalign could result in significant adverse publicity and, consequently, reduced acceptance by dental professionals. Also increased competition from direct competitors could cause us to lose market share and reduce dental professionals' efforts and commitment to expand their Invisalign practice. If adoption and utilization does not increase as we anticipate, our revenues may fail to grow as expected and our operating results may be harmed.
The frequency of use by orthodontists or GPs may not increase at the rate that we anticipate or at all.
One of our key objectives is to continue to increase utilization, or the adoption and frequency of use, of the Invisalign system by new and existing customers. If utilization of Invisalign by our existing and newly trained orthodontists or GPs does not occur or does not occur as quickly as we anticipate, our operating results could be harmed.
We may experience declines in average selling prices of our products.
In response to challenges in our business, including increased competition, we have in the past reduced the list price of our products. In addition, we provide volume based discount programs to our doctors. If we are to introduce any price reductions or expand our discount programs in the future or if participation in these programs increases, our revenues, gross margin and net profits (losses) may be adversely affected. Furthermore, although the U.S. dollar is our reporting currency, a portion of our revenues and profits are generated in foreign currencies. Revenues and profits generated by subsidiaries operating outside of the United States are translated into U.S. dollars using exchange rates effective during the respective period and as a result are affected by changes in exchange rates. As a result, negative movements in currency exchange rates against the U.S. dollar will adversely affect our average selling price and consequently the amount of revenues and profits in our consolidated financial statements.
22
Table of Contents
We may experience unexpected problems and expenses associated with the phased-relocation of our customer facing organizations to Costa Rica.
In October 2008, we announced a restructuring plan to increase efficiencies across the organization and lower our overall cost structure. In addition to headcount reduction, the restructuring plan included the phased-relocation of our customer care, accounts receivable, credit and collections and customer event registration organizations currently located in Santa Clara, California, to our facility in Costa Rica. We expect this relocation to be completed by the end of July 2009. This relocation is accompanied by a number of risks and uncertainties that may affect our results of operations and statement of cash flows, including:
- •
- failure to successfully coordinate and phase the relocation of these customer facing organizations may cause our customers to experience decrease in service levels;
- •
- the relocation may absorb significant management and key employee attention and resources that would otherwise be available for the ongoing business operations;
- •
- failure to retain key employees who possess specific knowledge or expertise and upon whom we are depending upon for the timely and successful transition to Costa Rica; and
- •
- difficulties in hiring employees in Costa Rica with the necessary skills to perform these customer facing functions.
If any of these risks materialize in the future, our operating results, statement of operations and cash flows may be adversely affected.
Our future success may depend on our ability to develop, successfully introduce and achieve market acceptance of new products.
Our future success may depend on our ability to develop, manufacture, market, and obtain regulatory approval or clearance of new products. We launched Invisalign Teen in July 2008 and Invisalign Assist in October 2008. There can be no assurance that we will be able to successfully develop, sell and achieve market acceptance of these and other new products and applications and enhanced versions of our existing product. The extent of, and rate at which, market acceptance and penetration are achieved by future products is a function of many variables, which include, among other things, our ability to include functionality and features that address customer requirements, the availability of third-party reimbursement of procedures using our new products, the existence of competing products and general economic conditions affecting purchasing patterns. In addition, even if our new products are successfully introduced, it is unlikely that they will rapidly gain market share and acceptance primarily due to the relatively long period of time it takes to successfully treat a patient. Since it takes approximately 12 to 24 months to treat a patient, our customers may be unwilling to rapidly adopt our new products until they successfully complete at least one case or until more historical clinical results are available.
Our ability to market and sell new products may also be subject to government regulation, including approval or clearance by the United States Food and Drug Administration ("FDA"), and foreign government agencies. Any failure in our ability to successfully develop and introduce or achieve market acceptance of our new products or enhanced versions of existing products could have a material adverse effect on our operating results and could cause our revenues to decline.
A disruption in the operations of our primary freight carrier or higher shipping costs could cause a decline in our revenues or a reduction in our earnings.
We are dependent on commercial freight carriers, primarily UPS, to deliver our products. If the operations of these carriers are disrupted for any reason, we may be unable to deliver our products to
23
Table of Contents
our customers on a timely basis. If we cannot deliver our products in an efficient and timely manner, our customers may reduce their orders from us and our revenues and operating profits could materially decline. In a rising fuel cost environment, our freight costs will increase. If freight costs materially increase and we are unable to pass that increase along to our customers for any reason or otherwise offset such increases in our cost of revenues, our gross margin and financial results could be adversely affected.
We are dependent on our international operations, which exposes us to foreign operational, political and other risks that may harm our business.
Our key production steps are performed in operations located outside of the U.S. At our facility in Costa Rica, technicians use a sophisticated, internally developed computer-modeling program to prepare digital treatment plans, which are then transmitted electronically to the Juarez, Mexico. These digital files form the basis of ClinCheck and are used to manufacture aligner molds. Our order acquisition, aligner fabrication and shipping operations are conducted in Juarez, Mexico. In addition to the research and development efforts conducted in our Santa Clara, California facility, we also carry out research and development at locations in San Jose, Costa Rica and Moscow, Russia. In October 2008, we announced the phased-consolidation of our customer-care, accounts receivable, credit and collections and customer event registration organizations, which are currently located in Santa Clara, California, to our facility in Costa Rica. We expect this relocation to be completed by the end of July 2009. Our increasing reliance on international operations exposes us to risks and uncertainties that may affect our business or results of operation, including:
- •
- difficulties in hiring and retaining employees generally, as well as difficulties in hiring and retaining employees with the necessary skills to perform the more technical aspects of our operations;
- •
- difficulties in managing international operations;
- •
- fluctuations in currency exchange rates;
- •
- import and export license requirements and restrictions;
- •
- controlling production volume and quality of the manufacturing process;
- •
- political, social and economic instability, including as a result of increased levels of violence in Juarez, Mexico;
- •
- acts of terrorism and acts of war;
- •
- interruptions and limitations in telecommunication services;
- •
- product or material transportation delays or disruption;
- •
- burdens of complying with a wide variety of local country and regional laws;
- •
- trade restrictions and changes in tariffs; and
- •
- potential adverse tax consequences.
If any of these risks materialize in the future, we could experience production delays and lost or delayed revenue.
24
Table of Contents
We currently are transitioning from reliance on a shelter service arrangement to become a direct manufacturer of our products. If we fail to successfully manage this transition, our business may be harmed.
We currently rely on IMS, a third party shelter services provider located in Juarez, Mexico, for order acquisition, to fabricate aligner molds as well as finished aligners and to ship the completed product to customers. On December 22, 2008, we announced the termination of our shelter services arrangement with IMS effective April 1, 2009. In addition to the risks related to international operations described in the risk factor above, any difficulties encountered by us with respect to a transition from a third party shelter services arrangement to manufacture, including difficulties hiring and retaining qualified personnel could disrupt our ability to deliver our products in a timely manner which could harm our business. In addition, any delay in completing the transition could result in increased transition costs, which could decrease the amount of expected savings.
A key step in our manufacturing process relies on sophisticated computer technology that requires new technicians to undergo a relatively long training process. If we are unable to accurately predict our volume growth, and fail to hire a sufficient number of technicians in advance of such demand, the delivery time of our products could be delayed which could adversely affect our results of operations.
Training technicians to use our sophisticated computer modeling program that produces the digital treatment plan that forms the basis of ClinCheck takes approximately 90 to 120 days. As a result, if we are unable to accurately predict our volume growth, we may not have a sufficient number of trained technicians to timely create ClinCheck treatment plans within the timeframe our customers expect. Any delay in ClinCheck processing time could delay the ultimate delivery of finished aligners to our customers. Such a delay could cause us to lose existing customers or fail to attract new customers. This could cause a decline in our revenues and net profits and could adversely affect our results of operations.
Our headquarters, ClinCheck setup and other manufacturing processes are all principally located in regions that are subject to earthquakes and other natural disasters.
Our digital dental modeling is processed in our facility located in San Jose, Costa Rica. The operations team in Costa Rica creates ClinCheck treatment plans using sophisticated computer software. We are also transitioning our customer facing operations from Santa Clara, California to Costa Rica. In addition, our aligner molds and finished aligners are fabricated in Juarez, Mexico. Both Costa Rica and Mexico are in earthquake zones and may be subject to other natural disasters. If there is a major earthquake or any other natural disaster in a region where one of these facilities is located, our ability to create ClinCheck treatment plans or manufacture and ship our aligners could be compromised which could result in our customers experiencing a significant delay in receiving their completed aligners. In addition, our headquarters facility is located in the San Francisco Bay Area. An earthquake or other natural disaster in this region could result in a disruption in our operations. Any such business interruption could materially and adversely affect our business, financial condition and results of operations.
We experience competition from manufacturers of traditional braces and expect aggressive competition from these and other companies that may introduce new technologies in the future.
Currently, our Invisalign product competes directly against products manufactured and distributed by Ormco Orthodontics, a division of Sybron Dental Specialties (a Danaher Corporation subsidiary), and traditional braces manufactured by 3M Company and Dentsply International. These manufacturers have substantially greater financial resources and manufacturing and marketing experience than we do and may, in the future, attempt to develop an orthodontic system similar to ours or combine technologies that make our product economically unattractive. Large consumer product companies may
25
Table of Contents
also enter the orthodontic supply market. Furthermore, we may face competition in the future from new companies that may introduce new technologies. We may be unable to compete with these competitors and one or more of these competitors may render our technology obsolete or economically unattractive. If we are unable to compete effectively with existing products or respond effectively to any products developed by new or existing competitors, our business could be harmed. Increased competition has resulted in the past and may in the future result in volume discounting and price reductions, reduced gross margins, reduced profitability and loss of market share, any of which could have a material adverse effect on our revenues, volume growth, net profit (losses) and stock price. We cannot assure you that we will be able to compete successfully against our current or future competitors or that competitive pressures will not have a material adverse effect on our business, results of operations and financial condition.
Our information technology systems are critical to our business. System integration and implementation issues and system security risks could disrupt our operations, which could have a material adverse impact on our business and operating results.
We rely on the efficient and uninterrupted operation of complex information technology systems. All information technology systems are vulnerable to damage or interruption from a variety of sources. As our business has grown in size and complexity, the growth has placed, and will continue to place, significant demands on our information technology systems. To effectively manage this growth, we will need to continually upgrade and enhance our information systems. In addition, experienced computer programmers and hackers may be able to penetrate our network security and misappropriate our confidential information or that of third parties, create system disruptions or cause shutdowns. Furthermore, sophisticated hardware and operating system software and applications that we either internally develop or procure from third parties may contain defects in design and manufacture, including "bugs" and other problems that can unexpectedly interfere with the operation of the system. The costs to eliminate or alleviate security problems, viruses and bugs could be significant, and the efforts to address these problems could result in interruptions that may have a material adverse impact on our operations, revenues and operating results.
We are currently focused on adding more functionality into our business enterprise systems to more efficiently integrate these systems with our other system applications, such as customer facing and manufacturing tools, and intend to continue this effort for the foreseeable future. System upgrades and enhancements require significant expenditures and allocation of valuable employee resources. Delays in integration or disruptions to our business from implementation of these new or upgraded systems could have a material adverse impact on our financial condition and operating results. Furthermore, we continuously upgrade our customer facing software applications, specifically ClinCheck and VIP. Software applications frequently contain errors or defects, especially when they are first introduced or when new versions are released. The discovery of a defect or error in a new upgraded version or the failure of our primary information systems may result in the following consequences, among others: loss of revenue or delay in market acceptance, damage to our reputation or increased service costs, any of which could have a material adverse effect on our business, financial condition or results of operations.
Our success depends in part on our proprietary technology, and if we are unable to successfully enforce our intellectual property rights, our competitive position may be harmed. Litigating claims of this type is costly and could distract our management and cause a decline in our results of operations and stock price.
Our success will depend in part on our ability to maintain existing intellectual property and to obtain and maintain further intellectual property protection for our products, both in the U.S. and in other countries. Our inability to do so could harm our competitive position. As of December 31, 2008, we had 118 issued U.S. patents, 181 pending U.S. patent applications, and 47 issued foreign patents, and 142 pending foreign patent applications.
26
Table of Contents
We intend to rely on our portfolio of issued and pending patent applications in the U.S. and in other countries to protect a large part of our intellectual property and our competitive position. However, our currently pending or future patent filings may not result in the issuance of patents. Additionally, any patents issued to us may be challenged, invalidated, held unenforceable, circumvented, or may not be sufficiently broad to prevent third parties from producing competing products similar in design to our products. During fiscal 2005 and 2006, requests were filed with the United States Patent and Trademark Office ("USPTO") by a San Francisco, California law firm, acting on behalf of an unnamed party and in some instances acting on behalf of OrthoClear, requesting re-examination of a number of our patents. In addition, any protection afforded by foreign patents may be more limited than that provided under U.S. patents and intellectual property laws. We also rely on protection of our copyrights, trade secrets, know-how and proprietary information. We generally enter into confidentiality agreements with our employees, consultants and our collaborative partners upon commencement of a relationship with us. However, these agreements may not provide meaningful protection against the unauthorized use or disclosure of our trade secrets or other confidential information, and adequate remedies may not exist if unauthorized use or disclosure were to occur. Our inability to maintain the proprietary nature of our technology through patents, copyrights or trade secrets would impair our competitive advantages and could have a material adverse effect on our operating results, financial condition and future growth prospects. In particular, a failure to protect our proprietary rights might allow competitors to copy our technology, which could adversely affect our pricing and market share. In addition, in an effort to protect our intellectual property we have in the past been and may in the future be involved in litigation. The potential effects on our business operations resulting from litigation that we may participate in the future, whether or not ultimately determined in our favor or settled by us, are costly and divert the efforts and attention of our management and technical personnel from normal business operations. Any of these results from our litigation could adversely affect our results of operations and stock price.
We are currently a party to various other legal proceedings and claims. Management does not believe that the ultimate outcome of these other legal proceedings and claims will have a material adverse effect on our financial position or results of operations. In addition, litigation is subject to inherent uncertainties and unfavorable rulings could occur. An unfavorable ruling could include monetary damages or, in cases where injunctive relief is sought, an injunction prohibiting us from selling our products. Any of these results from our litigation could adversely affect our results of operations and stock price.See Part I, Item 3 of this Annual Report on Form 10-K for a summary of our material pending legal proceedings.
While we believe we currently have adequate internal control over financial reporting, we are required to assess our internal control over financial reporting on an annual basis and any future adverse results from such assessment could result in a loss of investor confidence in our financial reports and have an adverse effect on our stock price.
Pursuant to the Sarbanes-Oxley Act of 2002 and the rules and regulations promulgated by the SEC, we are required to furnish in our Form 10-K an Annual Report by our management regarding the effectiveness of our internal control over financial reporting. The report includes, among other things, an assessment of the effectiveness of our internal control over financial reporting as of the end of our fiscal year, including a statement as to whether or not our internal control over financial reporting is effective. This assessment must include disclosure of any material weaknesses in our internal control over financial reporting identified by management. While we currently believe our internal control over financial reporting is effective, the effectiveness of our internal controls to future periods is subject to the risk that our controls may become inadequate because of changes in conditions, and, as a result, the degree of compliance of our internal control over financial reporting with the policies or procedures may deteriorate. If we are unable to assert that our internal control over financial reporting is effective in any future period (or if our auditors are unable to express an
27
Table of Contents
opinion on the effectiveness of our internal controls or conclude that our internal controls are ineffective), we could lose investor confidence in the accuracy and completeness of our financial reports, which could have an adverse effect on our stock price.
If we lose our key personnel or are unable to attract and retain key personnel, we may be unable to pursue business opportunities or develop our products.
We are highly dependent on the key employees in our clinical engineering, technology development, sales and marketing personnel and management teams. The loss of the services provided by those individuals may significantly delay or prevent the achievement of our product development and other business objectives and could harm our business. Our future success will also depend on our ability to identify, recruit, train and retain additional qualified personnel, including orthodontists. Few orthodontists are accustomed to working in a manufacturing environment since they are generally trained to work in private practices, universities and other research institutions. Thus, we may be unable to attract and retain personnel with the advanced qualifications necessary for the further development of our business. Furthermore, we may not be successful in retaining our key personnel or their services. If we are unable to attract and retain key personnel, our business could be materially harmed.
If we infringe the patents or proprietary rights of other parties or are subject to a patent infringement claim, our ability to grow our business may be severely limited.
Extensive litigation over patents and other intellectual property rights is common in the medical device industry. We have been sued for infringement of third party's patents in the past and we may be the subject of patent or other litigation in the future. From time to time, we have received and may in the future receive letters from third parties drawing our attention to their patent rights. While we do not believe that we infringe upon any valid and enforceable rights that have been brought to our attention, there may be other more pertinent rights of which we are presently unaware. The defense and prosecution of intellectual property suits, interference proceedings and related legal and administrative proceedings could result in substantial expense to us and significant diversion of effort by our technical and management personnel. An adverse determination of any litigation or interference proceeding to which we may become a party could subject us to significant liabilities. An adverse determination of this nature could also put our patents at risk of being invalidated or interpreted narrowly or require us to seek licenses from third parties. Licenses may not be available on commercially reasonable terms or at all, in which event, our business would be materially adversely affected.
We maintain single supply relationships for certain of our key machines and materials technologies, and our business and operating results could be harmed if supply is restricted or ends or the price of raw materials used in our manufacturing process increases.
We are highly dependent on manufacturers of specialized scanning equipment, rapid prototyping machines, resin and other advanced materials. We maintain single supply relationships for many of these machines and materials technologies. In particular, our scanning and stereolithography equipment are provided by a single supplier. We are also committed to purchasing all of our resin and polymer, the primary raw materials used in our manufacturing process, from a single-source. In addition, technology changes by our vendors could disrupt access to required manufacturing capacity or require expensive, time consuming development efforts to adapt and integrate new equipment or processes. Our growth may exceed the capacity of one or more of these manufacturers to produce the needed equipment and materials in sufficient quantities to support our growth. In the event of technology changes, delivery delays, or shortages of or increases in price for these items, our business and growth prospects may be harmed.
28
Table of Contents
We rely on our direct sales force to sell our products, and any failure to maintain our direct sales force could harm our business.
Our ability to sell our products and generate revenues depends upon our direct sales force within our North American and international markets. As of December 31, 2008, our North American sales organization consisted of 161 people, of which 134 were direct sales representatives and 27 were sales administration and regional sales management. Internationally, we had 41 people engaged in sales and sales support as of December 31, 2008. We do not have any long-term employment contracts with the members of our direct sales force. The loss of the services provided by these key personnel may harm our business. If we are unable to retain our direct sales force personnel or replace them with individuals of equivalent technical expertise and qualifications, or if we are unable to successfully instill such technical expertise or if we fail to establish strong relationships with our customers within a relatively short period of time, our revenues and our ability to maintain market share could be materially harmed. In addition, due to our large and fragmented customer base, we may not be able to provide all of our customers with product support immediately upon the launch of a new product. As a result, adoption of new products by our customers may be slower than anticipated and our ability to grow market share and increase our revenues may be harmed.
Complying with regulations enforced by the FDA and other regulatory authorities is an expensive and time-consuming process, and any failure to comply could result in substantial penalties.
Our products are medical devices and are subject to extensive regulation in the U.S. and internationally. FDA regulations are wide ranging and govern, among other things:
- •
- product design, development, manufacturing and testing;
- •
- product labeling;
- •
- product storage;
- •
- pre-market clearance or approval;
- •
- advertising and promotion; and
- •
- product sales and distribution.
Our failure to comply with applicable regulatory requirements could result in enforcement action by the FDA or state agencies, which may include any of the following sanctions:
- •
- warning letters, fines, injunctions, consent decrees and civil penalties;
- •
- repair, replacement, refunds, recall or seizure of our products;
- •
- operating restrictions or partial suspension or total shutdown of production;
- •
- refusing our requests for 510(k) clearance or pre-market approval of new products, new intended uses, or modifications to existing products;
- •
- withdrawing clearance or pre-market approvals that have already been granted; and
- •
- criminal prosecution.
If any of these events were to occur, they could harm our business. We must comply with facility registration and product listing requirements of the FDA and adhere to applicable Quality System regulations. The FDA enforces its Quality System regulations through periodic unannounced inspections. Our failure or the failure of IMS to take satisfactory corrective action in response to an adverse inspection or the failure to comply with applicable manufacturing regulations could result in enforcement action, and we may be required to find alternative manufacturers, which could be a long and costly process.
29
Table of Contents
Before we can sell a new medical device in the U.S., or market a new use of or claim for an existing product we must obtain FDA clearance or approval, unless an exemption applies. Obtaining regulatory clearances or approvals can be a lengthy and time-consuming process. Even though the devices we market have obtained the necessary clearances from the FDA, we may be unable to maintain such clearances in the future. Furthermore, we may be unable to obtain the necessary clearances for new devices that we intend to market in the future. Our inability to maintain or obtain regulatory clearances or approvals could materially harm our business.
If the security of our customer and patient information is compromised, patient care could suffer, and we could be liable for related damages, and our reputation could be impaired.
We retain confidential customer and patient information in our processing centers. Therefore, it is critical that our facilities and infrastructure remain secure and that our facilities and infrastructure are perceived by the marketplace and our customers to be secure. Despite the implementation of security measures, our infrastructure may be vulnerable to physical break-ins, computer viruses, programming errors, attacks by third parties or similar disruptive problems. If we fail to meet our clients' expectations regarding the security of healthcare information, we could be liable for damages and our reputation could be impaired. In addition, patient care could suffer, and we could be liable if our systems fail to deliver correct information in a timely manner. Our insurance may not protect us from this risk.
If compliance with healthcare regulations becomes costly and difficult for our customers or for us, we may not be able to grow our business.
Participants in the healthcare industry are subject to extensive and frequently changing regulations under numerous laws administered by governmental entities at the federal, state and local levels, some of which are, and others of which may be, applicable to our business. Furthermore, our healthcare provider customers are also subject to a wide variety of laws and regulations that could affect the nature and scope of their relationships with us.
The healthcare market itself is highly regulated and subject to changing political, economic and regulatory influences. Regulations implemented pursuant to the Health Insurance Portability and Accountability Act (HIPAA), including regulations affecting the security and privacy of patient healthcare information held by healthcare providers and their business associates may require us to make significant and unplanned enhancements of software applications or services, result in delays or cancellations of orders, or result in the revocation of endorsement of our products and services by healthcare participants. The effect of HIPAA and newly enforced regulations on our business is difficult to predict, and there can be no assurance that we will adequately address the business risks created by HIPAA and its implementation or that we will be able to take advantage of any resulting business opportunities.
Extensive and changing government regulation of the healthcare industry may be expensive to comply with and exposes us to the risk of substantial government penalties.
In addition to medical device laws and regulations, numerous state and federal healthcare-related laws regulate our business, covering areas such as:
- •
- storage, transmission and disclosure of medical information and healthcare records;
- •
- prohibitions against the offer, payment or receipt of remuneration to induce referrals to entities providing healthcare services or goods or to induce the order, purchase or recommendation of our products; and
- •
- the marketing and advertising of our products.
30
Table of Contents
Complying with these laws and regulations could be expensive and time-consuming, and could increase our operating costs or reduce or eliminate certain of our sales and marketing activities or our revenues.
We face risks related to our international sales, including the need to obtain necessary foreign regulatory clearance or approvals.
We currently sell our products in Europe, Asia Pacific, Latin America and Japan and may expand into other countries from time to time. We do not know whether orthodontists, GPs and consumers outside our North American market will adopt Invisalign in sufficient numbers or as rapidly as we anticipate. In addition, sales of our products outside the U.S. are subject to foreign regulatory requirements that vary widely from country to country. The time required to obtain clearances or approvals required by other countries may be longer than that required for FDA clearance or approval, and requirements for such approvals may differ from FDA requirements. We may be unable to obtain regulatory approvals in one or more of the other countries in which we do business or in which we may do business in the future. We may also incur significant costs in attempting to obtain and maintain foreign regulatory approvals. If we experience delays in receipt of approvals to market our products outside of the U.S., or if we fail to receive these approvals, we may be unable to market our products or enhancements in international markets in a timely manner, if at all.
Our business exposes us to potential product liability claims, and we may incur substantial expenses if we are subject to product liability claims or litigation.
Medical devices involve an inherent risk of product liability claims and associated adverse publicity. We may be held liable if any product we develop or any product that uses or incorporates any of our technologies causes injury or is otherwise found unsuitable. Although we intend to continue to maintain product liability insurance, adequate insurance may not be available on acceptable terms, if at all, and may not provide adequate coverage against potential liabilities. A product liability claim, regardless of its merit or eventual outcome, could result in significant legal defense costs. These costs would have the effect of increasing our expenses and diverting management's attention away from the operation of our business, and could harm our business.
Historically, the market price for our common stock has been volatile.
The market price of our common stock could be subject to wide price fluctuations in response to various factors, many of which are beyond our control. The factors include:
- •
- quarterly variations in our results of operations and liquidity;
- •
- changes in recommendations by the investment community or in their estimates of our revenues or operating results;
- •
- speculation in the press or investment community concerning our business and results of operations;
- •
- strategic actions by our competitors, such as product announcements or acquisitions;
- •
- announcements of technological innovations or new products by us, our customers or competitors; and
- •
- general economic market conditions.
31
Table of Contents
In addition, the stock market in general, and the market for technology and medical device companies in particular, have experienced extreme price and volume fluctuations that have often been unrelated to or disproportionate to the operating performance of those companies. These broad market and industry factors may seriously harm the market price of our common stock, regardless of our operating performance. In the past, class action litigation has often been brought against the issuing company following periods of volatility in the market price of a company's securities. If a securities class action suit is filed against us in the future, we would incur substantial legal fees, and our management's attention and resources would be diverted from operating our business in order to respond to the litigation.
Future sales of significant amounts of our common stock may depress our stock price.
A large percentage of our outstanding common stock is currently owned by a small number of significant stockholders. These stockholders have sold in the past, and may sell in the future, large amounts of common stock over relatively short periods of time. Sales of substantial amounts of our common stock in the public market by our existing stockholders may adversely affect the market price of our common stock. Such sales could create public perception of difficulties or problems with our business and may depress our stock price.
Changes in, or interpretations of, accounting rules and regulations, could result in unfavorable accounting charges.
We prepare our consolidated financial statements in conformity with accounting principles generally accepted in the United States of America. These principles are subject to interpretation by the SEC and various bodies formed to interpret and create appropriate accounting policies. A change in these policies can have a significant effect on our reported results and may even retroactively affect previously reported transactions. Our accounting policies that recently have been or may be affected by changes in the accounting rules are as follows:
- •
- revenue recognition;
- •
- accounting for share-based payments; and
- •
- accounting for income taxes.
If we fail to manage our exposure to global financial and securities market risk successfully, our operating results and financial statements could be materially impacted.
The primary objective of most of our investment activities is to preserve principal. To achieve this objective, a majority of our marketable investments are investment grade, liquid, short-term fixed-income securities and money market instruments denominated in U.S. dollars. If the carrying value of our investments exceeds the fair value, and the decline in fair value is deemed to be other-than-temporary, we will be required to write down the value of our investments, which could materially harm our results of operations and financial condition. Moreover, the performance of certain securities in our investment portfolio correlates with the credit condition of the U.S. financial sector. With the current unstable credit environment, we might incur significant realized, unrealized or impairment losses associated with these investments.
We have adopted a shareholders rights' plan to limit the possibility that we are acquired, which may mean that a transaction that shareholders are in favor of or are benefited by may be prevented.
Our board of directors has the authority to issue up to 5,000,000 shares of preferred stock and to determine the rights, preferences, privileges and restrictions of such shares without any further vote or action by our shareholders. To date, our board of directors has designated 200,000 shares as Series A
32
Table of Contents
participating preferred stock in connection with our shareholder rights' plan. The issuance of preferred stock under certain circumstances could have the effect of delaying or preventing an acquisition of the company or otherwise adversely affecting the rights of the holders of our stock. The shareholder rights' plan may have the effect of rendering more difficult or discouraging an acquisition of our company which is deemed undesirable by our board of directors. The shareholder rights' plan may cause substantial dilution to a person or group attempting to acquire us on terms or in a manner not approved by our board of directors, except pursuant to an offer conditioned on the negation, purchase or redemption of the rights issued under the shareholder rights' plan.
Our effective tax rate may vary significantly from period to period.
Various internal and external factors may have favorable or unfavorable effects on our future effective tax rate. These factors include, but are not limited to, changes in tax laws, regulations and/or rates, changing interpretations of existing tax laws or regulations, the future levels of tax benefits of stock option deductions relating to incentive stock options and employee stock purchase plans and changes in overall levels of pretax earnings. In addition, we have negotiated tax incentives with the Costa Rica Ministry of Foreign Trade, an agency of the Government of Costa Rica. Under these incentives, all of the income we earn in Costa Rica during these eight to twelve year incentive periods is subject to reduced rates of Costa Rica income tax. The incentive tax rates will expire in various years beginning in 2010. The Costa Rica corporate income tax rate that would apply, absent the incentives, is 30% for 2009. As a result of these incentives, income taxes decreased by $1.3 million in 2008. In order to receive the benefit of the incentives, we must hire specified numbers of employees and maintain minimum levels of fixed asset investment in Costa Rica. If we do not fulfill these conditions for any reason, our incentive could lapse and our income in Costa Rica would be subject to taxation at higher rates, which could have a negative impact on our operating results.
ITEM 1B. UNRESOLVED STAFF COMMENTS
None.
ITEM 2. PROPERTIES
Our headquarters are located in Santa Clara, California. We lease approximately 127,000 square feet of space where we house our research and development and administrative personnel. We lease our Santa Clara facilities under four leases, which expire in June 2010. The combined monthly rent for the Santa Clara facilities is approximately $64,000. Commencing July 1, 2005 and continuing on the first day of each calendar month thereafter, $11,000 will be deducted from the $1.3 million security deposit previously paid by us to the lessor and such amount will be applied against the monthly base rent for the Santa Clara facilities.
We operate a facility in San Jose, Costa Rica. The facility comprises approximately 63,000 square feet of manufacturing and office space. The monthly rent for the Costa Rica facility is approximately $78,000. We renewed the lease in March 2008 for an additional five year term, which commenced in October 2008 and expires in September 2013. Our European headquarters are located in Amsterdam, The Netherlands. On August 3, 2007, we entered into an amendment to the original lease agreement to expand the Amsterdam facility to approximately 16,000 square feet of office space. This lease will expire in June 2012, with an option to renew for an additional five year term. We may also terminate this lease in June 2012 for a fee of $125,000. The monthly rent for the Amsterdam facility is approximately $48,000.
On December 22, 2008, we notified IMS of our intention to terminate IMS' shelter services arrangement effective April 2009. At that time, we will become a direct manufacturer of our clear
33
Table of Contents
aligners at the facility in Juarez, Mexico and we will also assume IMS' lease with its landlord. The monthly rent for this 68,000 square foot facility is $30,000 and the lease expires in July 2013.
We believe that our existing facilities are adequate to meet current requirements and that additional or substitute space will be available as needed to accommodate any expansion of operations.
ITEM 3. LEGAL PROCEEDINGS
Ormco
On January 6, 2003, Ormco Corporation ("Ormco"), a division of Sybron Dental Specialties (a Danaher Corporation subsidiary), filed suit against us in the United States District Court for the Central District, Orange County Division, asserting infringement of U.S. Patent Nos. 5,447,432, 5,683,243 and 6,244,861. The complaint sought unspecified monetary damages and injunctive relief. Also in 2003, we counterclaimed for infringement of our U.S. Patent No. 6,398,548, seeking unspecified monetary damages and injunctive relief. Ormco filed a first amended complaint for infringement of U.S. Patent No. 6,616,444 and we filed an answer to Ormco's first amended complaint and a counterclaim for invalidity and non-infringement of U.S. Patent No. 6,616,444 and for infringement of U.S. Patent No. 6,554,611.
In connection with these claims, in 2004, the Court granted five motions for summary judgment that we filed. First, the Court granted our motion for summary judgment of non-infringement, finding that our Invisalign system does not infringe any of the asserted Ormco patents (5,447,432, 5,683,243, 6,244,861 and 6,616,444). Second, the Court granted in part our motion for summary judgment of infringement, finding that Ormco and its subsidiary, Allesee Orthodontic Appliances, Inc. ("AOA") infringe certain, but not all, claims of our patents Nos. 6,398,548 and 6,554,611 through the manufacture and sale of Red, White & Blue appliances. Third, the Court granted our motion for summary judgment of invalidity of Ormco's asserted patents claims (5,447,432, 5,683,243, 6,244,861 and 6,616,444). As noted above, the Court earlier found that we do not infringe these patents. In addition, the Court also denied Ormco's and AOA's motion for summary judgment seeking a finding of invalidity of our asserted patent claims (6,398,548 and 6,554,611). Fourth, the Court granted our summary judgment motion that our asserted patent claims are not invalid based on the evidence currently before the Court. Although the Court granted that motion, it reopened discovery on two additional invalidity arguments Ormco and AOA asserted. Fifth, the Court also granted our summary judgment motion that our patents are not unenforceable and granted Ormco's and AOA's summary judgment motion that Ormco and AOA did not willfully infringe our patents.
On February 24, 2005, the Court, on further summary judgment, confirmed the validity of all of the asserted claims of our 6,554,611 patent and two of the asserted claims of our 6,398,548 patent. The Court also found certain claims of our 6,398,548 patent to be invalid in view of prior use evidence. On May 26, 2005, the Court issued a permanent injunction (the "Permanent Injunction") to enjoin Ormco and AOA from further infringement of Claims 10 and 17 of our 6,398,548 patent and Claims 1-3 and 7 of our 6,554,611 patent. On May 31, 2005, Ormco and AOA filed a notice of appeal with the Federal Circuit from the Permanent Injunction.
There have been two appeals. After the Permanent Injunction was entered, Ormco and AOA appealed that injunction and the orders of the District Court on summary judgment on which the injunction was based. In April 2006, the U.S. Court of Appeals for the Federal Circuit ("CAFC") issued a ruling declaring two out of a total of seventy-one claims in our US Patent No. 6,398,548 and four out of a total of ten claims in US Patent No. 6,554,611 to be invalid as "obvious." The CAFC's decision reverses the California District Court summary judgment order of validity.
The 6,398,548 patent consists of 71 claims; only claims 10 and 17 were at issue in the first appeal and CAFC ruling. These two claims are directed to a system of appliances and method of repositioning
34
Table of Contents
teeth from an initial to a final tooth arrangement where at least some of the appliances are marked to show order of use. These claims contain further limitations requiring instructions as to the order in which the appliances are to be worn and use of the appliances in intervals of 2-20 days.
The 6,554,611 patent consists of ten claims directed to a system for repositioning teeth that includes one or more intermediate appliances and a final appliance, provided in a single package, as well as instructions which set forth the order in which the appliances are to be worn. The CAFC's ruling pertains only to claims 1, 2, 3 and 7 in the patent.
The second appeal was from the final judgment. Ormco appealed the ruling of the District Court that 92 claims in four of its patents are not infringed by us and that the asserted claims are invalid. We appealed the ruling of the District Court that certain claims of our 6,398,548 patent which were found to be infringed by Ormco's and AOA's Red, White & Blue appliances were invalid. The CAFC issued a ruling on August 24, 2007, affirming the District Court's ruling that 86 out of 92 claims in Ormco's 5,447,432, 5,683,243, 6,244,861 and 6,616,444 patents are invalid and not infringed by us. The CAFC reversed the District Court's non-infringement and invalidity rulings on six claims in Ormco's 6,616,444 patent. Ormco filed a petition for review with the U.S. Supreme Court with respect to the portion of the CAFC's opinion that affirmed the District Court's ruling of non-infringement and non-enablement of Ormco's 86 claims. The Supreme Court denied Ormco's petition, and the case on the six claims in Ormco's 6,616,444 patent were returned to the District Court for a determination of validity and infringement of those claims. The District Court issued orders construing the claim terms at issue and granting our motion to amend our answer and counterclaim to assert Ormco's 6,616,444 patent is unenforceable due to inequitable conduct. The parties are currently completing fact discovery.
On February 25, 2009, the District Court issued rulings on various Summary Judgment and expert related motions. In summary, the District Court granted one of Ormco's motions on one theory of infringement and granted our motion on two theories of non-infringement. Our invalidity argument supported by over fifty prior art references was unaffected. The District Court also ruled that one of our inequitable conduct theories should be resolved at trial. A finding of inequitable conduct at trial could render the six claims at issue and possibly the family of Ormco patents related to the 6,616,444 patent unenforceable.
Trial on liability issues is scheduled for June 2, 2009. Despite the District Court's ruling of infringement on one of Ormco's theories, if the jury finds Ormco's six claims to be invalid or unenforceable, there can be no liability for infringement. We intend to vigorously pursue our invalidity and inequitable conduct counterclaims at trial.
Other matters
During fiscal 2005 and 2006, requests were filed with the United States Patent and Trademark Office ("USPTO") by a San Francisco, California, law firm, acting on behalf of an unnamed party, requesting Ex Parte re-examination of our patents. A Reexamination Certificate has been issued regarding the 6,309,215, 6,398,548, 6,705,863, 6,217,325, 6,722,880, 6,318,994 and 5,975,893 patents and
35
Table of Contents
therefore these patents are no longer in reexamination. We received an Action Closing Prosecution on the 6,685,469 patent. The status of the 6,629,840 patent is as follows:
| | | | | | |
Patent No. | | Request for
Reexamination
Granted? | | Initial Office
Actions
Received? | | Status |
|---|
| 6,629,840 | | Yes | | Yes | | In this initial Office Action dated June 13, 2006, the examiners confirmed the validity of eight of the eleven claims of U.S. Patent No. 6,629,840 (the '840 patent) without amendment and preliminarily rejected the remaining claims of the patents. The non-final initial Office Action presented us with the first opportunity to respond to the USPTO's review and interpretation of the prior art. On September 13, 2006, we submitted a response to the initial Office Action. A petition seeking a waiver was filed on February 15, 2007 and was granted on April 17, 2007, granting a single interview. The interview was held on May 22, 2007, and an Interview Summary was filed with the USPTO on June 21, 2007. We are awaiting further action by the USPTO. |
As part of the OrthoClear Agreement, OrthoClear agreed to take no further action with respect to the Inter Parte Requests, including the 6,629,840 patent.
On May 18, 2007, Debra A. Weber filed a consumer class action lawsuit against us, OrthoClear, Inc. and OrthoClear Holdings, Inc. (d/b/a OrthoClear, Inc.) in Syracuse, New York, U.S. District Court. The complaint alleges two causes of action against the OrthoClear defendants and one cause of action against us for breach of contract. The cause of action against us titled "Breach of Third Party Benefit Contract" references our agreement to make Invisalign treatment available to OrthoClear patients, alleging that we failed "to provide the promised treatment to Plaintiff or any of the class members".
On July 3, 2007, we filed our answer to the complaint and asserted 17 affirmative defenses. On July 20, 2007, we filed a motion for summary judgment on the Third Cause of Action (the only cause of action alleged against us). On August 24, 2007, Weber filed a motion for class certification. On October 1, 2007, we filed an opposition to the motion of class certification and we are currently awaiting rulings from the Court. OrthoClear has filed a motion to dismiss. The initial case management conference and all discovery has been stayed pending the Court's decision on the motion for class certification, OrthoClear's motion to dismiss and our motion for summary judgment.
Litigating claims of the types discussed in this Annual Report on Form 10-K, whether or not ultimately determined in our favor or settled by us, is costly and diverts the efforts and attention of our management and technical personnel from normal business operations. Any of these results from litigation could adversely affect our results of operations and stock price. From time to time, we have received, and may again receive, letters from third parties drawing our attention to their patent rights. While we do not believe that we infringe any such rights that have been brought to our attention, there may be other more pertinent proprietary rights of which we are presently unaware.
ITEM 4. SUBMISSION OF MATTERS TO A VOTE OF SECURITY HOLDERS
There were no matters submitted to a vote of security holders during the fourth quarter of 2008.
36
Table of Contents
PART II
ITEM 5. MARKET FOR REGISTRANT'S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Our common stock is listed on the NASDAQ Global Market under the symbol "ALGN." Public trading of our common stock commenced on January 26, 2001. Prior to that date, there was no public market for our common stock. The following table shows, for the periods indicated, the high and low per share closing prices of our common stock, as reported by the NASDAQ Global Market:
| | | | | | | |
| | High | | Low | |
|---|
Year Ended December 31, 2008: | | | | | | | |
Fourth quarter | | $ | 10.48 | | $ | 5.00 | |
Third quarter | | $ | 13.48 | | $ | 10.01 | |
Second quarter | | $ | 13.19 | | $ | 9.84 | |
First quarter | | $ | 16.55 | | $ | 10.34 | |
Year Ended December 31, 2007: | | | | | | | |
Fourth quarter | | $ | 28.70 | | $ | 14.69 | |
Third quarter | | $ | 27.69 | | $ | 22.55 | |
Second quarter | | $ | 24.89 | | $ | 15.74 | |
First quarter | | $ | 17.88 | | $ | 13.35 | |
On February 18, 2009, the closing price of our common stock on the NASDAQ Global Market was $8.03 per share. As of February 18, 2009 there were approximately 184 holders of record of our common stock. Because the majority of our shares of outstanding common stock is held by brokers and other institutions on behalf of stockholders, we are unable to estimate the total number of stockholders represented by these record holders.
We have never declared or paid any cash dividends on our common stock. We currently intend to retain any future earnings to fund the development and growth of our business and do not anticipate paying any cash dividends in the foreseeable future. Our credit facility contains certain restrictive loan covenants, including restrictions on our ability to pay dividends.See Item 7 "Management's Discussion and Analysis of Financial Condition and Results of Operations—Liquidity and Capital Resources".
Following is a summary of stock repurchases for the three months ended December 31, 2008 (1):
| | | | | | | | | | | | | |
Period | | Total Number of
Shares
Repurchased | | Average Price Paid
per Share | | Total Number of
Shares Repurchased as
Part of Publicly
Announced Program | | Approximate Dollar Value
of Shares that May
Yet Be Repurchased Under
the Program | |
|---|
November 1, 2008 to November 30, 2008 | | | 1,064,021 | | $ | 6.83 | | | 1,064,021 | | $ | 3,431,000 | |
December 1, 2008 to December 31, 2008 | | | 477,500 | | $ | 7.22 | | | 477,500 | | $ | — | |
| | | | | | | | | | | | |
Total | | | 1,541,521 | | $ | 6.95 | | | 1,541,521 | | | | |
| | | | | | | | | | | | |
In April 2008, our Board of Directors approved a common stock repurchase program authorizing management to purchase up to $50.0 million of our outstanding shares of common stock. Purchases under the program were made, from time to time, in the open market. As of December 31, 2008, we had repurchased approximately 4.7 million shares of common stock at an average price of $10.76 per
37
Table of Contents
share. In December 2008, we completed the stock repurchase program, and all repurchased shares have been retired.
- (1)
- All shares were repurchased pursuant to the publicly announced repurchase program described above.
Notwithstanding any statement to the contrary in any of our previous or future filings with the SEC, the following information relating to the price performance of our common stock shall not be deemed "filed" with the SEC or "Soliciting Material" under the Securities Exchange Act of 1934, as amended, or subject to Regulation 14A or 14C, or to liabilities of Section 18 of the Exchange Act except to the extent we specifically request that such information be treated as soliciting material or to the extent we specifically incorporate this information by reference.
The following graph compares the cumulative total stockholder return on our common stock with that of the NASDAQ Stock Market US Index, a broad market index published by the National Association of Securities Dealers, Inc. and a peer group that reflects the labor market in which we compete. The comparison for each of the periods assumes that $100 was invested on January 1, 2003 in our common stock, the stocks in the NASDAQ Stock Market US Index, and the stocks in the peer group index, and that all dividends were reinvested.
COMPARISON OF 5 YEAR CUMULATIVE TOTAL RETURN*
Among Align Technology, Inc., The NASDAQ Composite Index
And A Peer Group
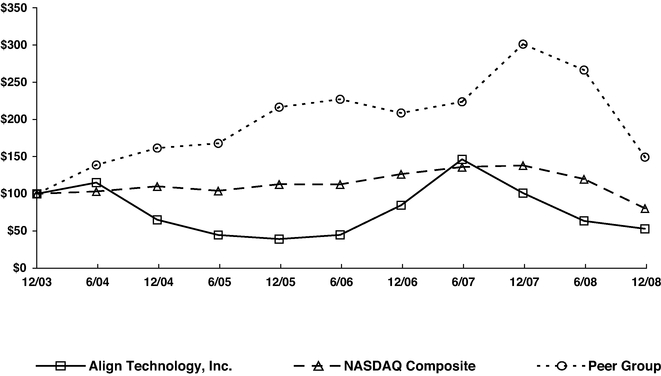
38
Table of Contents
Our peer group consists of 13 companies that are predominantly (although not exclusively) located in the San Francisco Bay Area, the geographic location in which we operate and compete for executive talent. In addition to geographic location, these companies were chosen using the following principles:
- •
- companies that are close industry competitors;
- •
- medical device companies that are generally between 0.5 to 2.5 times Align's revenue (with Align at approximately the median); and
- •
- technology companies with similar growth potential and technology development needs for software and enterprise system designers.
The following companies made up the peer group for 2008:
| | |
Advanced Medical Optics Inc.* | | Natus Medical Inc. |
American Medical Systems Holdings Inc. | | Nektar Therapeutics |
Ansys Inc. | | Nuvasive Inc. |
ArthroCare Corp. | | Sirona Dental Systems Inc. |
Integra Lifesciences Holdings Corp. | | Sonosite |
Intuitive Surgical Inc. | | Vital Images Inc. |
Mentor Corp.* | | |
- *
- Each of these companies was acquired in 2008 and will no longer form part of our peer group as public information for these companies are no longer publicly available.
ITEM 6. SELECTED CONSOLIDATED FINANCIAL DATA
The following tables set forth the selected consolidated financial data for each of the years in the five-year period ended December 31, 2008. The selected consolidated financial data is qualified in its entirety and should be read in conjunction with the Consolidated Financial Statements and related Notes thereto set forth on pages 50 to 78 and "Management's Discussion and Analysis of Financial Condition and Results of Operations" beginning on page 35. We have derived the statement of operations data for the years ended December 31, 2008, 2007 and 2006 and the balance sheet data as of December 31, 2008 and 2007 from the consolidated audited financial statements included elsewhere in this Annual Report on Form 10-K. The statement of operations data for the years ended December 31, 2005 and 2004 and the balance sheet data as of December 31, 2006, 2005 and 2004 were derived from the consolidated audited financial statements that are not included in this Annual Report on Form 10-K.
39
Table of Contents
SELECTED CONSOLIDATED FINANCIAL DATA
(in thousands, except per share data)
| | | | | | | | | | | | | | | | |
| | Years Ended December 31, | |
|---|
| | 2008 | | 2007 | | 2006 | | 2005 | | 2004 | |
|---|
Consolidated Statement of Operations Data: | | | | | | | | | | | | | | | | |
Net revenues | | $ | 303,976 | | $ | 284,332 | | $ | 206,354 | | $ | 207,125 | | $ | 172,830 | |
| | | | | | | | | | | | |
Gross profit | | $ | 225,126 | | $ | 209,297 | | $ | 141,579 | | $ | 143,341 | | $ | 115,304 | |
| | | | | | | | | | | | |
Profit (loss) from operations(1)(2) | | | 15,514 | | | 33,855 | | | (37,536 | ) | | 2,446 | | | 9,765 | |
Other income (expense), net | | | 1,562 | | | 3,095 | | | 3,401 | | | 283 | | | (3 | ) |
| | | | | | | | | | | | |
Net profit (loss) before provision for (benefit from) income taxes(1)(2) | | | 17,076 | | | 36,950 | | | (34,135 | ) | | 2,729 | | | 9,762 | |
Provision for (benefit from) income taxes | | | (62,911 | ) | | 1,226 | | | 828 | | | 1,316 | | | 994 | |
| | | | | | | | | | | | |
Net profit (loss)(1)(2)(3) | | $ | 79,987 | | $ | 35,724 | | $ | (34,963 | ) | $ | 1,413 | | $ | 8,768 | |
| | | | | | | | | | | | |
Net profit (loss) per share | | | | | | | | | | | | | | | | |
Basic | | $ | 1.20 | | $ | 0.53 | | $ | (0.55 | ) | $ | 0.02 | | $ | 0.15 | |
| | | | | | | | | | | | |
Diluted | | $ | 1.18 | | $ | 0.50 | | $ | (0.55 | ) | $ | 0.02 | | $ | 0.14 | |
| | | | | | | | | | | | |
Shares used in computing net profit (loss) per share: | | | | | | | | | | | | | | | | |
Basic | | | 66,812 | | | 67,176 | | | 63,246 | | | 61,644 | | | 59,963 | |
| | | | | | | | | | | | |
Diluted | | | 68,064 | | | 71,444 | | | 63,246 | | | 63,152 | | | 64,089 | |
| | | | | | | | | | | | |
| | | | | | | | | | | | | | | | |
| | December 31, | |
|---|
| | 2008 | | 2007 | | 2006 | | 2005 | | 2004 | |
|---|
Consolidated Balance Sheet Data: | | | | | | | | | | | | | | | | |
Working capital | | $ | 117,335 | | $ | 123,058 | | $ | 40,306 | | $ | 62,978 | | $ | 61,886 | |
Total assets | | | 279,341 | | | 222,761 | | | 151,558 | | | 142,110 | | | 130,712 | |
Total long-term liabilities | | | 229 | | | 148 | | | 219 | | | 64 | | | 25 | |
Stockholders' equity | | $ | 218,540 | | $ | 161,154 | | $ | 83,556 | | $ | 93,438 | | $ | 85,739 | |
- (1)
- Profit (loss) from operations, net profit (loss) before provision for income taxes and net profit (loss) for the years ended December 31, 2007 and 2006 included a $1.8 million credit and a $14.3 million charge, respectively, for the Patients First Program and settlement costs. SeeNote 5 "Patients First Program and settlement costs" in the Notes to Consolidated Financial Statements for additional information.
- (2)
- Profit from operations and net profit before benefit from income taxes included a $6.2 million restructuring charge for the year ended December 31, 2008. In addition, 2008 net profit included a $6.1 million restructuring charge net of taxes of $129,000. SeeNote 18 "Restructurings" in the Notes to Consolidated Financial Statements for additional information.
- (3)
- Net profit for the year ended December 31, 2008 included a $64.6 million benefit to income taxes as a result of the release of a tax valuation allowance on most of the deferred tax assets. SeeNote 13 "Income Taxes" in the Notes to Consolidated Financial Statements for additional information.
40
Table of Contents
ITEM 7. MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion and analysis of our financial condition and results of operations should be read together with "Selected Consolidated Financial Data" and our consolidated financial statements and related notes included elsewhere in this Annual Report on Form 10-K.
Overview
Align Technology, Inc. designs, manufactures and markets the Invisalign system, a proprietary method for treating malocclusion, or the misalignment of teeth. Invisalign corrects malocclusion using a series of clear, nearly invisible, removable appliances that gently move teeth to a desired final position. Because it does not rely on the use of metal or ceramic brackets and wires, Invisalign significantly reduces the aesthetic and other limitations associated with metal arch wires and brackets, commonly referred to as braces. We received the United States Food and Drug Administration ("FDA") clearance to market Invisalign in 1998. The Invisalign system is regulated by the FDA as a Class II medical device.
Each Invisalign treatment plan is unique to the individual patient. Our Invisalign Full treatment consists of as many aligners as indicated by ClinCheck in order to achieve the doctors' treatment goals. Our Invisalign Express is a dual arch orthodontic treatment for cases that meet certain predetermined clinical criteria and consist of up to ten sets of aligners. Invisalign Express treatment is intended to assist dental professionals to treat a broader range of patients by providing a lower-cost option for adult relapse cases, for minor crowding and spacing, or as a pre-cursor to restorative or cosmetic treatments such as veneers. Invisalign Teen, which was launched in July 2008, is designed to meet the specific needs of the non-adult comprehensive or teen treatment market. Invisalign Assist, launched in October 2008, is intended to help newly-trained and low volume Invisalign GPs accelerate the adoption and frequency of use of Invisalign into their practice. Upon completion of an Invisalign or non-Invisalign treatment, the patient may be prescribed our traditional retainer product, or our Vivera retainers, a clear aligner set designed for ongoing retention.
Our goal is to establish Invisalign as the standard method for treating malocclusion ultimately driving increased product adoption by dental professionals by focusing on the four key objectives: driving product innovation, enhancing the customer experience, generating consumer demand and expanding into international markets. Each of these four key objectives is described more fully in "Item I—Business—Business Strategy" of this Annual Report on Form 10-K and is incorporated herein by this reference. In addition to whether we successfully execute our business strategy, a number of other factors, the most important of which are set forth below, may affect our results during the remainder of 2009 and beyond.
- •
- Impact on consumer spending due to a decline in general economic conditions. Consumer spending habits are affected by, among other things, prevailing economic conditions, levels of employment, salaries and wage rates, gas prices, consumer confidence and consumer perception of economic conditions. A general slowdown in the United States economy and certain international economies as well as an uncertain economic outlook have adversely affected consumer spending habits. As a result of the decline in general economic conditions, we expect that our product volumes and revenues may decline in 2009 compared to 2008. In addition, the decline in general economic conditions may further have the impact of decreasing the number of orthodontic case starts overall.
- •
- Utilization Rates. Our goal is to establish Invisalign as the standard method for treating malocclusion ultimately driving increased product adoption and frequency of use by dental
41
Table of Contents
professionals, or utilization. Our quarterly utilization rates from the years ended December 31, 2008, 2007 and 2006 are as follows:
Utilization Rates*
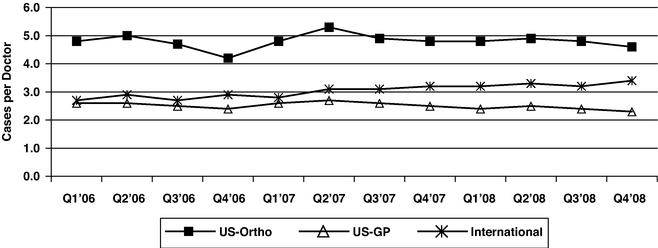
Utilization rates = # of cases shipped divided by # of doctors cases were shipped to
As set forth in the chart above, year over year utilization rates declined slightly for our domestic channel in each quarter of 2008 compared to the same quarter in 2007. We believe that the continued economic slowdown has negatively impacted many of our North America customers. International utilization rates for the same periods, however, increased slightly.
- •
- Impact of new products on deferred revenue. We launched three new products in 2008: Vivera retainers in January 2008, Invisalign Teen in July 2008, and Invisalign Assist in October 2008. As a result of and depending upon customer adoption of these new products, we expect our mix of products to begin shifting gradually. Key features of these new products include staged delivery of retainers with Vivera, up to six free individual replacement aligners with Invisalign Teen and staged delivery of aligners with Invisalign Assist. As a result of these features, these new products will have a significantly higher amount of deferred revenue as a percentage of their average selling prices compared to Invisalign Full.
The Vivera retainer includes four shipments per year, and revenue is deferred upon the first shipment and then recognized as each shipment occurs. Revenue for the six replacement aligners included in Invisalign Teen will be deferred based on their fair market value until the earlier of the replacement aligners being used or until the case is completed. For Invisalign Assist, when the progress tracking feature is selected, aligners are shipped to the dental professional after every nine stages. As a result, for these cases, revenue will be deferred upon the first staged shipment and will be recognized upon shipment of the final staged shipment. In addition, included in the price of Invisalign Full treatment, we offer case refinement, which is a finishing tool used to adjust a patient's teeth to the desired final position. Invisalign Teen, Invisalign Assist, and Invisalign Full include a deferral for case refinement. As these new products increase as a percentage of our total case volume, deferred revenue on our balance sheet will increase.
- •
- Reliance on international manufacturing operations. Our manufacturing efficiency has been and will continue to be an important factor in our future profitability. Currently, two of our key production steps are performed in operations located outside of the U.S. At our facility in Costa Rica, dental technicians use a sophisticated, internally developed computer-modeling program to prepare digital treatment plans. We currently rely on IMS, a third party shelter services provider located in Juarez, Mexico, for order acquisition, to fabricate aligner molds as well as finished
42
Table of Contents
aligners and to ship the completed product to customers. On December 22, 2008, we notified IMS of our intention to terminate this shelter services arrangement effective in April 2009. At that time, we will become a direct manufacturer of our clear aligners at the facility in Juarez, Mexico. Our success will depend in part on the efforts and abilities of management to effectively manage these international operations, including any difficulties encountered by us with respect to a transition from a third party shelter services arrangement to a direct manufacturer, including difficulties hiring and retaining qualified personnel. If our management fails in any of these respects, we could experience production delays and lost or delayed revenue. In addition, even if we have case submissions, we may not have a sufficient number of trained dental technicians in Costa Rica to create the ClinCheck treatments, or if we are unable to ship our product to our customers on a timely basis, our revenue will be delayed or lost, which will cause our operating results to fluctuate. See Part I, Item 1A—"Risk Factors" for risks related to our international operations as well as risks related to transitioning from reliance on shelter service arrangement to independent manufacturer.
- •
- Seasonal Fluctuations. Seasonal fluctuations in the number of doctors in their offices and available to take appointments have affected, and are likely to continue to affect, our business. Specifically, our customers often take vacation or are on holiday during the summer months and therefore tend to start fewer cases. These seasonal trends have caused and will likely continue to cause, fluctuations in our quarterly results, including fluctuations in sequential revenue growth rates.
- •
- Foreign Exchange Rates. Although the U.S. dollar is our reporting currency, a portion of our revenues and profits are generated in foreign currencies. Revenues and profits generated by subsidiaries operating outside of the United States are translated into U.S. dollars using exchange rates effective during the respective period and as a result are affected by changes in exchange rates. We have generally accepted the exposure to exchange rate movements without using derivative financial instruments to manage this risk. Therefore, both positive and negative movements in currency exchanges rates against the U.S. dollar will continue to affect the reported amount of revenues and profits in our consolidated financial statements.
- •
- Restructurings. During 2008, we announced restructuring plans in July and October to increase efficiencies across the organization and lower the overall cost structure. In July 2008, we implemented a restructuring plan to reduce our full time headcount by 67 employees including initiating a phased-consolidation of order acquisition operations from our corporate headquarters in Santa Clara, California to Juarez, Mexico, which was completed by the end of 2008. The October restructuring plan included a total reduction of 111 full time headcount in Santa Clara, California by July 2009 as we create a new shared services organization in our existing Costa Rica facility that will consolidate customer care, accounts receivable, credit and collections, and customer event registration organizations, which are currently located in Santa Clara, California. We anticipate phasing the relocation to Costa Rica in an attempt to minimize disruptions to customer service levels and expect the relocation to be completed by the end of July 2009. These actions resulted in a restructuring charge of approximately $6.2 million in 2008, and we expect to incur additional restructuring charges of approximately $1.8 million over the first half of 2009. We expect to reinvest the annualized savings of these two restructurings in the execution of our key strategic initiatives. See Part I, Item 1A—"Risk Factors" for risks related to the October restructuring, including the phased-relocation of our customer facing operations to Costa Rica.
- •
- Review of our investment portfolio and policies. Our cash equivalent and short-term investment portfolio as of the date of this Form 10-K consisted of U.S. government notes and bonds, corporate bonds and certificates of deposits, agency bonds and discount notes and commercial paper. We follow an established investment policy and set of guidelines to monitor, manage and
43
Table of Contents
limit our exposure to interest rate, liquidity and credit risk. The policy sets forth credit quality standards and limits our exposure to any one issuer, as well as our maximum exposure to various asset classes. As a result of current adverse financial market conditions, investments in some financial instruments, such as structured investment vehicles, sub-prime mortgage-backed securities and collateralized debt obligations, may pose risks arising from liquidity and credit concerns. As of the date of this Form 10-K, we had no direct holdings in these categories of investments and our indirect exposure to these financial instruments through our holdings in money market mutual funds was immaterial. As of the date of this Form 10-K, we had no impairment charge associated with our short-term investment portfolio relating to such adverse financial market conditions. Although we believe our current investment portfolio has very little risk of impairment, we cannot predict future market conditions or market liquidity and can provide no assurance that our investment portfolio will remain unimpaired. See Part I, Item 1A—"Risk Factors" for risks related to global financial and securities markets.
Our short-term marketable securities as of December 31, 2008 are as follows (in thousands):
| | | | | | | | | | | | | |
December 31, 2008 | | Amortized
Costs | | Gross
Unrealized
Gains | | Gross
Unrealized
Losses | | Fair Value | |
|---|
U.S. government notes and bonds | | $ | 9,971 | | $ | 25 | | $ | — | | $ | 9,996 | |
Corporate bonds and certificates of deposit | | | 3,774 | | | 1 | | | (24 | ) | | 3,751 | |
Agency bonds and discount notes | | | 8,499 | | | 20 | | | — | | | 8,519 | |
Commercial paper | | | 800 | | | — | | | — | | | 800 | |
| | | | | | | | | | |
Total | | $ | 23,044 | | $ | 46 | | $ | (24 | ) | $ | 23,066 | |
| | | | | | | | | | |
Our long-term marketable securities as of December 31, 2008 are as follows (in thousands):
| | | | | | | | | | | | | |
September 30, 2008 | | Amortized
Costs | | Gross
Unrealized
Gains | | Gross
Unrealized
Losses | | Fair Value | |
|---|
Agency bonds | | $ | 1,000 | | $ | 1 | | | | | $ | 1,001 | |
Corporate bonds | | | 1,897 | | | 0 | | | (35 | ) | | 1,862 | |
| | | | | | | | | | |
Total | | | 2,897 | | | 1 | | | (35 | ) | | 2,863 | |
| | | | | | | | | | |
- •
- Tax Valuation Allowance. We continually evaluate both the positive and negative evidence in assessing our need for a tax valuation allowance. As a result of our analysis, we released the tax valuation allowance on most of the deferred tax assets with the exception of certain capital loss and foreign net operating loss carryforwards as of December 31, 2008.
- •
- Effective Tax Rate. Our effective tax rate may vary significantly from period to period. Various internal and external factors may have favorable or unfavorable effects on our future effective tax rate. These factors include, but are not limited to, changes in tax laws, regulations and/or rates, changing interpretations of existing tax laws or regulations, the future levels of tax benefits of stock option deductions relating to incentive stock options and employee stock purchase plans and changes in overall levels of pretax earnings.
- •
- Stock-based compensation. We implemented Statement of Financial Accounting Standards ("FAS") No. 123 (revised 2004), "Share-based Payment" ("FAS 123R") in 2006, and we expect stock-based compensation to increase until at least 2010, which corresponds to our standard 4-year vesting term. Thereafter, new grants will be expensed over the vesting period, however, this expense may be offset by fully vested grants that are no longer expensed. For the years
44
Table of Contents
ended December 31, 2008, 2007 and 2006, stock-based compensation expense recognized in accordance with FAS 123R is as follows (in thousands):
| | | | | | | | | | | | | | | | | | | |
| | Year Ended
December 31, 2008 | | Year Ended
December 31, 2007 | | Year Ended
December 31, 2006 | |
|---|
| | Stock-based
Compensation | | % of net
revenues | | Stock-based
Compensation | | % of net
revenues | | Stock-based
Compensation | | % of net
revenues | |
|---|
Cost of revenues | | $ | 1,753 | | | 0.6 | % | $ | 994 | | | 0.4 | % | $ | 700 | | | 0.3 | % |
Sales and marketing | | | 5,289 | | | 1.7 | % | | 4,225 | | | 1.5 | % | | 2,862 | | | 1.4 | % |
General and administrative | | | 8,011 | | | 2.6 | % | | 5,443 | | | 1.9 | % | | 4,054 | | | 2.0 | % |
Research and development | | | 2,004 | | | 0.7 | % | | 1,549 | | | 0.5 | % | | 1,294 | | | 0.6 | % |
| | | | | | | | | | | | | | |
Total stock-based compensation expense | | $ | 17,057 | | | 5.6 | % | $ | 12,211 | | | 4.3 | % | $ | 8,910 | | | 4.3 | % |
| | | | | | | | | | | | | | |
Results of Operations
Comparison of Years Ended December 31, 2008, 2007 and 2006:
Invisalign product revenues by channel and other revenues, which represent training and sales of ancillary products for the years ended December 31, 2008, 2007 and 2006, are as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Years Ended December 31, | |
|---|
Net revenues | | 2008 | | Net
Change | | %
Change | | 2007 | | Net
Change | | %
Change | | 2006 | |
|---|
| | (in millions)
| |
|---|
North America: | | | | | | | | | | | | | | | | | | | | | | |
| | | Ortho | | $ | 89.5 | | $ | (0.8 | ) | | (0.9 | )% | $ | 90.3 | | $ | 21.7 | | | 31.7 | % | $ | 68.6 | |
| | | GP | | | 140.2 | | | 5.7 | | | 4.2 | % | | 134.5 | | | 40.6 | | | 43.2 | % | | 93.9 | |
| | | | | | | | | | | | | | | | |
Total North American Invisalign | | | 229.7 | | | 4.9 | | | 2.2 | % | | 224.8 | | | 62.3 | | | 38.3 | % | | 162.5 | |
International Invisalign | | | 61.9 | | | 15.3 | | | 32.8 | % | | 46.6 | | | 14.5 | | | 45.1 | % | | 32.1 | |
| | | | | | | | | | | | | | | | |
Total Invisalign revenues | | | 291.6 | | | 20.2 | | | 7.4 | % | | 271.4 | | | 76.8 | | | 39.5 | % | | 194.6 | |
| | | | | Other revenues | | | 12.4 | | | (0.5 | ) | | (3.9 | )% | | 12.9 | | | 1.1 | | | 10.3 | % | | 11.8 | |
| | | | | | | | | | | | | | | | |
Total net revenues | | $ | 304.0 | | $ | 19.7 | | | 6.9 | % | $ | 284.3 | | $ | 77.9 | | | 37.8 | % | $ | 206.4 | |
| | | | | | | | | | | | | | | | |
Case volume data which represents Invisalign case shipments by channel, for the years ended December 31, 2008, 2007 and 2006 are as follows:
| | | | | | | | | | | | | | | | | | | | | | | | |
| | Years Ended December 31, | |
|---|
Invisalign case volume | | 2008 | | Net
Change | | %
Change | | 2007 | | Net
Change | | %
Change | | 2006 | |
|---|
| | (in thousands)
| |
|---|
North America: | | | | | | | | | | | | | | | | | | | | | | |
| | | Ortho | | | 70.6 | | | (2.3 | ) | | (3.2 | )% | | 72.9 | | | 17.4 | | | 31.3 | % | | 55.5 | |
| | | GP | | | 103.5 | | | 1.5 | | | 1.5 | % | | 102.0 | | | 26.6 | | | 35.3 | % | | 75.4 | |
| | | | | | | | | | | | | | | | |
Total North American Invisalign | | | 174.1 | | | (0.8 | ) | | (0.5 | )% | | 174.9 | | | 44.0 | | | 33.6 | % | | 130.9 | |
| | | | | | | | | | | | | | | | |
International Invisalign | | | 37.9 | | | 9.9 | | | 35.4 | % | | 28.0 | | | 8.8 | | | 46.1 | % | | 19.2 | |
| | | | | | | | | | | | | | | | |
Total Invisalign case volume | | | 212.0 | | | 9.1 | | | 4.5 | % | | 202.9 | | | 52.8 | | | 35.2 | % | | 150.1 | |
| | | | | | | | | | | | | | | | |
45
Table of Contents
Total net revenues increased in 2008 compared to 2007 primarily as a result of increased growth in our international Invisalign revenue and our North American GP revenue.
Net revenues from our North American Invisalign orthodontic channel decreased in 2008 compared to 2007 due to decreased case volume and higher revenue deferrals related to new products introduced in 2008 partially offset by lower participation in volume-based discount program rebates. Net revenues from our North American Invisalign GP channel increased due to higher case volume and lower participation in volume-based discount rebate programs.
Revenues from both our North American orthodontic and GP channels increased in 2007 compared to 2006 primarily as a result of an overall increase in case volume and a favorable product mix shift towards Invisalign Full. This product mix shift towards Invisalign Full began in the fourth quarter of 2006 after we removed the cancellation fees on Invisalign Full cases prior to ClinCheck approval and clarified clinical protocols surrounding what is an appropriate Invisalign Express case. The increase in Invisalign Full revenues is partially offset by increased participation in our volume-based discount programs.
The increase in our international Invisalign revenues in 2008 compared to 2007 was predominantly due to higher case volumes partially offset by unfavorable exchange rates and increased participation in volume-based discount programs.
The increase in our international Invisalign revenues in 2007 compared to 2006 was attributable to an increase in case volume. Additionally, our international revenues benefited from favorable exchange rates in 2007. These increases were partially offset by increased participation in volume-based discount programs.
Other revenues, consisting of training fees and sales of ancillary products, were lower in 2008 compared to 2007 primarily due to North American training discount programs. Other revenues were higher in 2007 compared to 2006 as a result of an increased number of doctors trained year over year.
For 2009, we expect our total net revenues to decrease compared to 2008 primarily due to case volume decreases in North America partially offset by expected growth in international revenue. We expect our mix of products to continue to shift in 2009 due to the introduction of new products during the second half of 2008. These new products have a significantly higher amount of deferred revenue as a percentage of their average selling price compared to Invisalign Full.
| | | | | | | | | | | | | | | | |
| | Years Ended December 31, | |
|---|
| | 2008 | | Change | | 2007 | | Change | | 2006 | |
|---|
| | (in millions)
| |
|---|
Cost of revenues | | $ | 78.9 | | $ | 3.9 | | $ | 75.0 | | $ | 10.2 | | $ | 64.8 | |
% of net revenues | | | 25.9 | % | | | | | 26.4 | % | | | | | 31.4 | % |
Gross profit | | $ | 225.1 | | $ | 15.8 | | $ | 209.3 | | $ | 67.7 | | $ | 141.6 | |
Gross margin % | | | 74.1 | % | | | | | 73.6 | % | | | | | 68.6 | % |
Cost of revenues includes salaries for staff involved in the production process, costs incurred by IMS, a third party shelter service provider in Juarez, Mexico, the cost of materials, packaging, shipping costs, depreciation on capital equipment used in the production process, training costs and stock-based compensation expense.
Gross margin improved in 2008 compared to 2007 primarily due to an increase in case volume over our relatively fixed cost structure and improved operating efficiencies.
Gross margin improved in 2007 compared to 2006 primarily as a result of increased case volume over our relatively fixed cost structure resulting in decreases in our per unit standard cost. Additionally,
46
Table of Contents
cost reductions from improved operating efficiencies also contributed to the increase in 2007 gross margin.
We believe that gross margin in 2009 will be slightly lower than in 2008 as we expect overall case volume to decrease year over year partially offset by a benefit from our transition from a third party shelter arrangement to direct manufacturing in Juarez, Mexico effective April 2009. However, with our relatively fixed manufacturing cost structure, quarterly gross margin will fluctuate based on case volume and product mix. In addition, should we experience continuing unfavorable exchange rates, our gross margin may be adversely affected.
| | | | | | | | | | | | | | | | |
| | Years Ended December 31, | |
|---|
| | 2008 | | Change | | 2007 | | Change | | 2006 | |
|---|
| | (in millions)
| |
|---|
Sales and marketing | | $ | 115.1 | | $ | 16.9 | | $ | 98.2 | | $ | 16.2 | | $ | 82.0 | |
% of net revenues | | | 37.9 | % | | | | | 34.5 | % | | | | | 39.7 | % |
Sales and marketing expense includes sales force compensation (including travel-related costs), marketing personnel-related costs, media and advertising, clinical education, product marketing and stock-based compensation expense.
Sales and marketing expense increased during 2008 compared to 2007 due to higher payroll-related expenses of $7.0 million, including stock-based compensation of $1.1 million, as a result of the full year effect of additional headcount hired in the fourth quarter of 2007. We also incurred higher product marketing expenses of $9.0 million, associated with our new product launches and commercialization, professional marketing programs, clinical education and media costs.
Our sales and marketing expense increased during 2007 compared to 2006 predominantly as a result of a $10.3 million increase in media, advertising and product marketing expenses and an increase in payroll-related expenses of $5.1 million, of which $3.7 million was attributable to additional headcount and $1.4 million to stock-based compensation.
We expect sales and marketing expense levels in 2009 to be comparable to 2008. In 2009, we expect to invest in our international channel, including consumer advertising and sales force expansion, and continue commercialization of new products in North America, offset by benefits from the transition of our shared services organizations to Costa Rica, beginning in the second half of 2009.
| | | | | | | | | | | | | | | | |
| | Years Ended December 31, | |
|---|
| | 2008 | | Change | | 2007 | | Change | | 2006 | |
|---|
| | (in millions)
| |
|---|
General and administrative | | $ | 62.2 | | $ | 8.9 | | $ | 53.3 | | $ | (11.0 | ) | $ | 64.3 | |
% of net revenues | | | 20.5 | % | | | | | 18.7 | % | | | | | 31.2 | % |
General and administrative expense includes salaries for administrative personnel, outside consulting services, legal expenses and stock-based compensation expense.
General and administrative expenses increased during 2008 compared to 2007 primarily due to higher payroll-related expenses of $4.5 million, including increased stock-based compensation expense of $2.6 million, resulting from additional headcount. Management decided to no longer invest in an internally developed software tool for business process management resulting in an asset impairment charge of $1.7 million in the fourth quarter of 2008. In addition, legal and other professional fees were
47
Table of Contents
higher by $3.4 million compared to 2007 primarily due to a $1.6 million credit in 2007 from an insurance reimbursement we received associated with the OrthoClear litigation.
General and administrative expense decreased in 2007 compared to 2006 largely due to a $20.6 million decline in external legal fees following the settlement of the OrthoClear litigation in the fourth quarter of 2006. As mentioned above, our 2007 legal expense included a $1.6 million credit for an insurance reimbursement of legal costs also associated with the OrthoClear litigation. This decrease was partially offset by a $3.5 million increase in additional headcount and incentive compensation, a $1.7 million increase in consulting fees, and a $1.4 million increase in stock-based compensation expense. Additionally, amortization expense increased $2.2 million in 2007 compared to 2006 related to the amortization of the non-compete agreements we received in connection with the OrthoClear settlement.
We expect general and administrative expense in 2009 to be lower than 2008 levels as we begin to benefit from the transition of our shared services organizations to Costa Rica, beginning in the second half of 2009.
| | | | | | | | | | | | | | | | |
| | Years Ended December 31, | |
|---|
| | 2008 | | Change | | 2007 | | Change | | 2006 | |
|---|
| | (in millions)
| |
|---|
Research and development | | $ | 26.2 | | $ | 0.5 | | $ | 25.7 | | $ | 7.2 | | $ | 18.5 | |
% of net revenues | | | 8.6 | % | | | | | 9.0 | % | | | | | 9.0 | % |
Research and development expense includes the personnel-related costs and outside consulting expenses associated with the research and development of new products and enhancements to existing products, conducting clinical and post-marketing trials and stock-based compensation expense.
Research and development expenses increased slightly in 2008 compared to the same period in 2007 primarily due to higher payroll-related expenses, including stock-based compensation, as a result of increased headcount in the first half of 2008, partially offset by lower consulting fees.
Research and development expense increased in 2007 compared to 2006 predominantly from a $5.0 million increase resulting from higher headcount and incentive compensation. Additionally, a $1.5 million increase in outside services also contributed to the increase in 2007 research and development costs.
We expect research and development expense in 2009 to be lower than 2008 levels as a result of lower headcount and reduced consulting expenses.
| | | | | | | | | | | | | | | | |
| | Years Ended December 31, | |
|---|
| | 2008 | | Change | | 2007 | | Change | | 2006 | |
|---|
| | (in millions)
| |
|---|
Restructurings | | $ | 6.2 | | $ | 6.2 | | $ | — | | $ | — | | $ | — | |
% of net revenues | | | 2.1 | % | | n/a | | | | | | | | | | |
During 2008, we announced restructuring plans in July and October to increase efficiencies across the organization and lower the overall cost structure. In July 2008, we implemented a restructuring plan to reduce our full time headcount by 67 employees including a phased-consolidation of order acquisition operations from our corporate headquarters in Santa Clara, California to Juarez, Mexico, which was completed by the end of 2008. The October restructuring plan included a total reduction of 111 full time headcount in Santa Clara, California by July 2009 as we move customer care, accounts
48
Table of Contents
receivable, credit and collections, and customer event registration organizations in Santa Clara, California to our existing facilities in Costa Rica.
In 2008, we incurred approximately $6.2 million in restructuring expenses relating to these actions which includes $0.7 million related to the acceleration of stock option vesting and $5.5 million related to severance and termination benefits, of which $3.0 million was paid during the year.
We are phasing in the relocation to Costa Rica of our shared services group in an attempt to minimize disruptions to customer service levels and expect the relocation to be completed by July 2009. We expect to incur additional restructuring charges of approximately $1.8 million during the first half of 2009.
| | | | | | | | | | | | | | | | |
| | Years Ended December 31, | |
|---|
| | 2008 | | Change | | 2007 | | Change | | 2006 | |
|---|
| | (in millions)
| |
|---|
Patients First Program | | $ | — | | $ | 1.8 | | $ | (1.8 | ) | $ | (10.1 | ) | $ | 8.3 | |
Settlement costs | | | — | | | | | | — | | | (6.0 | ) | | 6.0 | |
| | | | | | | | | | | | |
Total Patients First Program and settlement costs | | $ | — | | $ | 1.8 | | $ | (1.8 | ) | $ | (16.1 | ) | $ | 14.3 | |
| | | | | | | | | | | | |
% of net revenues | | | — | | | | | | (0.6 | )% | | | | | 7.0 | % |
As part of the OrthoClear Agreement in October 2006, OrthoClear agreed to stop the importation of aligners into the United States and discontinue all aligner business operations worldwide. As a result, most OrthoClear patients were unable to complete their orthodontic treatment with OrthoClear. In an attempt to help minimize treatment disruptions for the OrthoClear patients and their doctors, we committed to make treatment available to these patients at no additional cost under the "Patients First Program". In the fourth quarter of 2006, we recorded an $8.3 million charge for the anticipated costs of completing this program. Subsequently, in the first quarter of 2007, we reduced our Patients First Program accrual by $1.8 million to reflect a reduction of our initial estimate to the number of cases actually received by the case submission deadline. We shipped all Patients First Program cases by June 30, 2007.
We paid $20.0 million to OrthoClear during the fourth quarter of 2006 in accordance with the terms of the OrthoClear Agreement, of which $14.0 million was capitalized on our balance sheet representing the fair value of the non-compete agreements and is being amortized over 5 years. In accordance with Emerging Issues Task Force 04-01 "Accounting for Pre-existing Contractual Relationships between the Parties to a Purchase Business Combination" ("EITF 04-01"), we recorded the remaining $6.0 million as settlement costs in the fourth quarter of fiscal 2006.
Interest and other income (expense), net:
| | | | | | | | | | | | | | | | |
| | Years Ended December 31, | |
|---|
| | 2008 | | Change | | 2007 | | Change | | 2006 | |
|---|
| | (in millions)
| |
|---|
Interest income | | $ | 3.1 | | $ | (1.1 | ) | $ | 4.2 | | $ | 1.0 | | $ | 3.2 | |
Interest (expense) | | | — | | | 0.3 | | | (0.3 | ) | | — | | | (0.3 | ) |
Other income (expense), net | | | (1.5 | ) | | (0.7 | ) | | (0.8 | ) | | (1.3 | ) | | 0.5 | |
| | | | | | | | | | | | |
Total interest and other income(expense), net | | $ | 1.6 | | $ | (1.5 | ) | $ | 3.1 | | $ | (0.3 | ) | $ | 3.4 | |
| | | | | | | | | | | | |
49
Table of Contents
Interest and other income (expense), net, includes interest income earned on cash balances, interest expense on debt, foreign currency translation gains and losses and other miscellaneous charges.
Interest income, net in 2008 decreased by $1.1 million compared to 2007 primarily due to lower average cash, cash equivalents and marketable securities balances resulting from the $50.0 million stock repurchase program and lower interest rates. In 2008, we shifted our investments into more conservative instruments principally, U.S. government securities, which bear lower interest rates. We incurred no interest expense in 2008 compared to 2007. In 2007 we incurred interest expense on the outstanding balance of our line of credit during 2007 which was repaid during 2007. We had no outstanding borrowings as of December 31, 2008.
Other income (expense), net, increased in 2008 compared to 2007 reflecting increases in foreign currency translation losses.
Interest income, net increased in 2007 compared to 2006 primarily due to higher average cash, cash equivalents and marketable securities balances in 2007.
Other income (expense), net, decreased in 2007 compared to 2006 reflecting the decrease in foreign currency translation gains. In January 2007, we began to record the adjustments from translating certain European subsidiaries' financial statements from the local currency into the U.S. dollar as a separate component of shareholders' equity on our Consolidated Balance Sheets. SeeItem 7A "Quantitative And Qualitative Disclosures About Market Risk" under the heading "Currency Rate Risk" for additional information on the change in functional currency.
| | | | | | | | | | | | | | | | |
| | Years Ended December 31, | |
|---|
| | 2008 | | Change | | 2007 | | Change | | 2006 | |
|---|
| | (in millions)
| |
|---|
Provision for (benefit from) income taxes | | $ | (63.0 | ) | $ | (64.2 | ) | $ | 1.2 | | $ | 0.4 | | $ | 0.8 | |
We recorded an income tax benefit of $63.0 million for 2008 and income tax provisions of $1.2 million and $0.8 million for 2007 and 2006, respectively. These represented effective tax rates of (368.4)%, 3.3%, and (2.4)%, in 2008, 2007 and 2006, respectively. Our income tax provision is based upon our operating results for each taxable jurisdiction in which we operate and the amount of statutory tax that we incur in each jurisdiction. We exercised significant judgment in regards to estimates of future market growth, forecasted earnings and projected taxable income, in determining the provision for income taxes, and for purposes of assessing our ability to utilize any future benefit from deferred tax assets. At December 31, 2008, based on available positive evidence, we determined that most of our deferred tax assets would be realized with the exception of certain capital loss and foreign net operating loss carryforwards as we cannot forecast sufficient future capital gains or foreign source income to realize these deferred tax assets. Therefore, we recorded a tax valuation allowance release of $64.6 million in 2008. The remaining valuation allowance of approximately $6.2 million relating to capital loss and foreign net operating loss carryforwards as of December 31, 2008, will result in an income tax benefit if and when we conclude it is more likely than not that the related deferred tax assets will be realized.
At December 31, 2008, we had net operating loss carryforwards of approximately $191.1 million for federal tax purposes and $68.1 million for California state tax purposes. If not utilized, these carryforwards will begin to expire in 2020 for federal purposes and 2010 for California purposes. FAS 123R prohibits recognition of a deferred income tax asset for excess tax benefits due to stock option exercises that have not yet been realized through a reduction in income taxes payable. Such unrecognized deferred tax benefits totaled $7.5 million as of December 31, 2008 and will be accounted for as a credit to additional paid-in capital, if and when realized through a reduction in income taxes
50
Table of Contents
payable. The Internal Revenue Code imposes an annual limitation on the use of a corporation's tax attributes if a corporation undergoes an ownership change for tax purposes. If an ownership change is determined to have occurred, our ability to use the net operating loss carryforwards would be subject to an annual limitation. However, based on our current estimate of the total net operating losses at December 31, 2008 and our current estimate of the annual limitation, we do not expect our net operating loss carryforwards to be limited. At December 31, 2008, we had research credit carryforwards of approximately $3.5 million for federal purposes and $4.3 million for California state tax purposes. If not utilized, the federal credit carryforwards will begin to expire in 2017. The California state credit can be carried forward indefinitely.
We have not provided additional U.S. income taxes on undistributed earnings from non- U.S. operations as of December 31, 2008 because such earnings are intended to be reinvested indefinitely outside of the United States.
Liquidity and Capital Resources
We fund our operations from product sales, the proceeds of the sale of our common stock, and from occasional borrowings under our available credit facility. As of December 31, 2008, 2007 and 2006, we had the following cash and cash equivalents, restricted cash and short-term investments:
| | | | | | | | | | |
| | Years Ended December 31, | |
|---|
| | 2008 | | 2007 | | 2006 | |
|---|
| | (in thousands)
| |
|---|
Cash and cash equivalents | | $ | 87,100 | | $ | 89,119 | | $ | 55,113 | |
Restricted cash | | | — | | | 21 | | | 93 | |
Short-term investments | | | 23,066 | | | 38,771 | | | 8,931 | |
| | | | | | | | |
Total | | $ | 110,166 | | $ | 127,911 | | $ | 64,137 | |
| | | | | | | | |
Net cash provided by operating activities for the year ended December 31, 2008 was $39.7 million, resulting primarily from our net profit of $80.0 million and non-cash items totaling $32.7 million, which included depreciation, amortization of intangibles, option acceleration charges for terminated executives and stock-based compensation expense. Also included in non-cash items was an asset impairment charge of $1.7 million relating to management's decision to no longer invest in an internally developed software tool for business process management. These increases were offset by the release of a non-cash tax valuation allowance of $64.6 million on most of the deferred tax assets. Cash flows from operating activities also resulted from a $4.6 million increase in deferred revenue and a $0.9 million decrease in inventories. These increases in cash flows were offset by an $8.0 increase in accounts receivable and a decrease of $6.1 million in accounts payable and accrued liabilities.
Net cash provided by operating activities for the year ended December 31, 2007 was $52.8 million, resulting primarily from our net income of $35.7 million and non-cash items such as depreciation and amortization, stock-based compensation, and amortization of intangibles totaling $25.6 million. Additionally, a $2.7 million increase in accounts payable also contributed to the increase in net cash provided by operating activities. These increases in cash flows from operating activities were partially offset by a $10.7 million increase in accounts receivable.
Net cash used in operating activities for the year ended December 31, 2006 was $14.0 million, resulting primarily from our net loss of $35.0 million and non-cash items such as depreciation and amortization, stock-based compensation, and amortization of intangibles totaling $19.2 million. Additionally, a $6.4 million increase in current assets and a $5.8 million reduction in deferred revenue partially offset by a $14.3 million increase in accounts payable and accrued liabilities also contributed to the cash used in operating activities. The increase in accrued liabilities was primarily due to the
51
Table of Contents
$6.8 million accrual as of December 31, 2006 for the anticipated costs of completing the Patients First Program.
Net cash used in investing activities was $1.1 million for the year ended December 31, 2008 and consisted of $14.3 million for the purchase of property and equipment offset by $12.9 million of net maturities of marketable securities. As a result of current adverse financial market conditions, investments in some financial instruments may pose risks arising from liquidity and credit concerns. Although we believe our current investment portfolio has very little risk of impairment, we cannot predict future market conditions or market liquidity and can provide no assurance that our investment portfolio will remain unimpaired.
Net cash used in investing activities was $36.8 million for the year ended December 31, 2007, which largely consisted of $29.9 million of net purchases of short-term marketable securities and $7.4 million used for the purchase of capital assets.
Net cash used in investing activities was $32.8 million for the year ended December 31, 2006, primarily due to a $14.0 million purchase of intangible assets resulting from the OrthoClear Agreement, $10.0 million for the purchase of capital assets and $8.9 million for net purchases of short-term marketable securities.
Net cash used in financing activities was $40.4 million for the year ended December 31, 2008 and resulted primarily from our $50.1 million stock repurchase including commissions offset by $10.5 million in proceeds from the issuance of our common stock, principally from exercises of employee stock options and purchases under the employee stock purchase plan.
Net cash provided by financing activities was $17.5 million for the year ended December 31, 2007 and consisted of $29.0 million in proceeds from the issuance of our common stock, primarily from exercises of employee stock options. This increase was partially offset by the repayment of $11.5 million against the outstanding balance on our line of credit. Net cash provided by financing activities was $27.7 million for the year ended December 31, 2006 and consisted of $16.2 million in proceeds from the issuance of our common stock, primarily from exercises of employee stock options and $11.5 million in net borrowings from our line of credit.
Net proceeds from the issuance of our common stock related to the exercise of employee stock options have historically been a significant component of our liquidity. However, in 2006, we began granting restricted stock units ("RSUs") which, unlike stock options, do not generate cash from exercise. As a result, we will likely generate less cash from the proceeds of the sale of our common stock in future periods. In addition, because RSUs are taxable to the individuals when they vest, the number of shares we issue to each of our executive officers will be net of applicable withholding taxes which will be paid by us on their behalf. During 2008 and 2007, we paid $0.5 million and $0.4 million of taxes related to RSUs that vested during the period for executive officers.
On December 5, 2008, we renegotiated and amended our existing credit facility with Comerica Bank. Under this revolving line of credit, we have $25.0 million of available borrowings with a maturity date of December 31, 2010. This credit facility requires a quick ratio covenant and also requires us to maintain a minimum unrestricted cash balance of $10.0 million. As of December 31, 2008, we had no outstanding borrowings and we were in compliance with the financial covenant of this credit facility.
On April 29, 2008, we announced that our Board of Directors had approved a stock repurchase program of up to $50 million. During the year ended December 31, 2008, we repurchased 4.7 million shares of common stock at an average price of $10.76 per share for an aggregate purchase price of $50.1 million including commissions. As of December 31, 2008, we had completed repurchases under the stock repurchase authorization.
52
Table of Contents
Contractual Obligations/Off Balance Sheet Arrangements
The impact that our contractual obligations as of December 31, 2008 are expected to have on our liquidity and cash flows in future periods is as follows (in thousands):
| | | | | | | | | | | | | | | | |
| |
| | Payments Due by Period | |
|---|
| | Total | | Less than
1 Year | | 1-2
Years | | 3-5
Years | | More than
5 Years | |
|---|
Operating lease obligations | | $ | 9,687 | | $ | 3,234 | | $ | 4,205 | | $ | 2,248 | | $ | — | |
Computer support services | | | 388 | | | 388 | | | — | | | — | | | — | |
| | | | | | | | | | | | |
Total | | $ | 10,075 | | $ | 3,622 | | $ | 4,205 | | $ | 2,248 | | $ | — | |
| | | | | | | | | | | | |
In July 2008, we entered into an agreement in favor of and for the benefit of Elamex de Juarez, S.A. DE C.V., or Elamex, landlord to IMS, our third party shelter services provider. Under this agreement, we guarantee IMS' lease payments for its facility located in Juarez, Mexico. The monthly rent for this 68,000 square foot facility is $30,000 and the lease expires in July 2013. Pursuant to the guarantee, we are obligated to pay Elamex for any rental payments in default by IMS. During the year ended December 31, 2008, there were no rental payment defaults by IMS.
On December 22, 2008, we notified IMS of our intention to terminate IMS' shelter services arrangement effective April 2009. At that time, we will become a direct manufacturer of our clear aligners at the facility in Juarez, Mexico and we will also assume IMS' lease with Elamex.
We had no off-balance sheet arrangements as defined in Regulation S-K Item 303(a) (4) as of December 31, 2008.
We believe that our current cash and cash equivalents combined with our existing borrowing capacity will be sufficient to fund our operations for at least the next 12 months. If we are unable to generate adequate operating cash flows, we may need to seek additional sources of capital through equity or debt financing, collaborative or other arrangements with other companies, bank financing and other sources in order to realize our objectives and to continue our operations. There can be no assurance that we will be able to obtain additional debt or equity financing on terms acceptable to us, or at all. If adequate funds are not available, we may need to make business decisions that could adversely affect our operating results such as modifications to our pricing policy, business structure or operations. Accordingly, the failure to obtain sufficient funds on acceptable terms when needed could have a material adverse effect on our business, results of operations and financial condition.
Critical Accounting Policies and Estimates
Management's discussion and analysis of our financial condition and results of operations is based upon our consolidated financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States of America. The preparation of financial statements requires our management to make estimates and judgments that affect the reported amounts of assets and liabilities, revenues and expenses and disclosures at the date of the financial statements. We evaluate our estimates on an on-going basis, including those related to revenue recognition, stock-based compensation and income taxes. We use authoritative pronouncements, historical experience and other assumptions as the basis for making estimates. Actual results could differ from those estimates.
We believe the following critical accounting policies and estimates affect our more significant judgments used in the preparation of our consolidated financial statements.
53
Table of Contents
We recognize revenue in accordance with SEC Staff Accounting Bulletin No. 104 "Revenue Recognition" ("SAB 104"), and Emerging Issues Task Force ("EITF") No. 00-21 "Revenue Arrangements with Multiple Deliverables" ("EITF 00-21"). SAB 104 requires that four basic criteria must be met before revenue can be recognized: persuasive evidence of an arrangement exists, delivery has occurred, the price is fixed or determinable, and collectibility is reasonably assured.
Revenues are recognized from product sales, net of discounts and rebates. Service revenues related to the training of dental professionals and staff on the Invisalign treatment process are recorded when the services are completed.
We enter into arrangements that involve multiple product deliveries in the future. Included in the price of Invisalign Full, Invisalign Teen and Invisalign Assist, we offer case refinement, which is a finishing tool used to adjust a patient's teeth to the desired final position. Case refinement may be elected by the dental professional in the last stages of orthodontic treatment. We use vendor specific objective evidence of fair value to allocate revenue to the case refinement deliverable and recognize the residual revenue upon shipment. We defer the fair value of case refinement upon shipment based on a breakage factor, which is determined by sufficient historical experience of case refinement utilization. We believe that the use of a breakage factor is reasonable and appropriate because of the relative stability of case refinement utilization since case refinement was first offered. Actual utilization rates could differ from the historical breakage factor requiring future adjustments to revenue.
Revenues are deferred for certain products that include staged delivery. Depending on the product, revenues are recognized based on usage, case completion, ratably over a delivery period or upon shipment of the final staged shipment. Revenue for the six replacement aligners included in Invisalign Teen is deferred based on the fair market value until the earlier of replacement aligners being used or until the case is completed. The Vivera retainer includes four shipments per year, and revenue is deferred upon the first shipment and recognized ratably over the one year delivery period. For Invisalign Assist, when the progress tracking feature is selected, aligners are shipped to the dental professional after every nine stages. For these cases, revenue is deferred upon the first staged shipment and will be recognized upon shipment of the final staged shipment.
We estimate and record a provision for amounts of estimated losses on sales, if any, in the period such sales occur. Provisions for discounts and rebates to customers are provided for in the same period that the related product sales are recorded based upon historical discounts and rebates.
We adopted the fair value recognition provisions of Statement of Financial Accounting Standards ("FAS") No. 123 (revised 2004), "Share-Based Payment" ("FAS 123R") in 2006. Accordingly, we recognize compensation cost for only those shares ultimately expected to vest on a straight-line basis over the requisite service period of the award, net of an estimated forfeiture rate. We estimate the fair value of stock options using a Black-Scholes valuation model, which requires the input of highly subjective assumptions, including the option's expected term and stock price volatility. In addition, judgment is also required in estimating the number of stock-based awards that are expected to be forfeited. Forfeitures are estimated based on historical experience at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates. The assumptions used in calculating the fair value of share-based payment awards represent management's best estimates, but these estimates involve inherent uncertainties and the application of management's judgment. As a result, if factors change and we use different assumptions, our stock-based compensation expense could be materially different in the future. Prior to FAS 123R adoption, we accounted for share-based payment awards under Accounting Principles Board Opinion No. 25, "Accounting for Stock Issued to
54
Table of Contents
Employees" ("APB 25") and its related interpretations.See Note 10 "Stockholders' Equity" in the Notes to Consolidated Financial Statements for additional information.
Long-lived assets, including finite lived purchased intangible assets
Long-lived assets, including intangible assets other than goodwill are amortized over their useful lives, unless these lives are determined to be indefinite. Intangible assets are carried at cost less accumulated amortization. Long-lived assets are reviewed for impairment in accordance with Statement of Financial Accounting Standards No. 144, "Accounting for the Impairment or Disposal of Long-Lived Assets" ("FAS 144"). We perform an impairment test whenever events or changes in circumstances indicate that the carrying value of such assets may not be recoverable. Examples of such events or circumstances include significant underperformance relative to historical or projected future operating results, significant changes in the manner of use of the acquired assets or the strategy for its business, significant negative industry or economic trends, or a significant decline in our stock price for a sustained period. Impairments are recognized based on the difference between the fair value of the asset and its carrying value, and fair value is generally measured based on discounted cash flow analyses. Management decided to no longer invest in an internally developed software tool for business process management resulting in an asset impairment charge of $1.7 million which was recorded in general and administrative expense in the fourth quarter of 2008. No intangible asset impairment was recorded for the periods presented.
We consider all available evidence, both positive and negative including historical levels of income, expectations and risks associated with estimates of future taxable income and ongoing prudent and feasible tax planning strategies in assessing the need for a valuation allowance. In the fourth quarter of 2008, with the exception of certain capital loss and foreign net operating loss carryforwards, we determined that it was more likely than not the deferred tax assets would be realized. Accordingly, we released the tax valuation allowance on most of the deferred tax assets and recorded an income tax benefit of $64.6 million for the year ended December 31, 2008.
As of December 31, 2008, we believed that the amount of deferred tax assets recorded on our balance sheet would ultimately be realized. However, should there be a change in our ability to recover our deferred tax assets, our tax provision would increase in the period in which we determine that it is more likely than not that we cannot recover our deferred tax assets.
See Note 1 "Summary of Significant Accounting Policies" in the Notes to Consolidated Financial Statements in Item 8 for a full description of recent accounting pronouncements, including the expected dates of adoption and estimated effects on results of operations and financial condition, which is incorporated herein.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
In the normal course of business, we are exposed to foreign currency exchange rate and interest rate risks that could impact our financial position and results of operations.
Changes in interest rates could impact our anticipated interest income on our cash equivalents and investments in marketable securities. Our cash equivalents and investments are in fixed-rate, short-term securities. Fixed-rate securities may have their fair market value adversely impacted due to a rise in interest rates, and as a result, our future investment income may fall short of expectations due to
55
Table of Contents
changes in interest rates or we may suffer losses in principal if forced to sell securities which have declined in market value due to changes in interest rates. As of December 31, 2008, we had $23.1 million invested in available-for-sale marketable securities. An immediate 10% increase in interest rates would not have a material adverse impact on our future operating results and cash flows.
We do not have interest bearing liabilities as of December 31, 2008 and therefore, we are not subject to risks from immediate interest rate decreases.
We operate in North America, Europe, Asia-Pacific, Costa Rica and Japan. As a result of our international business activities, our financial results could be affected by factors such as changes in foreign currency exchange rates or economic conditions in foreign markets, and there is no assurance that exchange rate fluctuations will not harm our business in the future. We sell our products in the local currency for the respective country. This provides some natural hedging because most of the subsidiaries' operating expenses are denominated in their local currencies as discussed further below. Regardless of this natural hedging, our results of operations may be adversely impacted by the exchange rate fluctuation. Although we will continue to monitor our exposure to currency fluctuations, and, where appropriate, may use financial hedging techniques in the future to minimize the effect of these fluctuations, we are not currently engaged in any financial hedging transactions. The impact of an aggregate decline of 10% in foreign currency exchange rates relative to the U.S. dollar on our results of operations and financial position could be material.
Prior to January 1, 2007, the functional currency of Align and our subsidiaries was the U.S. dollar, and accordingly, gains and losses resulting from the remeasurement of monetary assets and liabilities denominated in Euros, Costa Rican Colones, and other currencies were reflected in other income (expense). During the first quarter of 2007, we analyzed the various economic factors of our international subsidiaries in accordance with FAS 52 and determined that there had been a significant change in facts and circumstances to warrant a change in the functional currency for some of our European subsidiaries from the U.S. dollar to the local currency. Effective January 1, 2007, the adjustment from translating certain European subsidiaries' financial statements from the local currency into the U.S. dollar was recorded as a separate component of accumulated other comprehensive income, net in the stockholder's equity section of our Consolidated Balance Sheets.
56
Table of Contents
ITEM 8. CONSOLIDATED FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
Quarterly Results of Operations
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | Three Months Ended | |
|---|
| | 2008 | | 2007 | |
|---|
| | 31-Dec | | 30-Sep | | 30-Jun | | 31-Mar | | 31-Dec | | 30-Sep | | 30-Jun | | 31-Mar | |
|---|
| | (in thousands, except per share data)
| |
|---|
| | (unaudited)
| |
|---|
Net revenues | | $ | 74,125 | | $ | 75,173 | | $ | 79,902 | | $ | 74,776 | | $ | 72,517 | | $ | 71,451 | | $ | 76,603 | | $ | 63,761 | |
Gross profit | | | 53,892 | | | 56,407 | | | 59,659 | | | 55,168 | | | 53,390 | | | 53,319 | | | 56,356 | | | 46,232 | |
Profit from operations(1)(2) | | | 1,325 | | | 5,691 | | | 3,872 | | | 4,626 | | | 5,012 | | | 8,395 | | | 13,448 | | | 7,000 | |
Net profit(1)(2)(3) | | $ | 65,496 | | $ | 5,157 | | $ | 4,030 | | $ | 5,304 | | $ | 5,668 | | $ | 9,460 | | $ | 13,618 | | $ | 6,978 | |
Net profit per share: | | | | | | | | | | | | | | | | | | | | | | | | | |
Basic | | $ | 0.99 | | $ | 0.08 | | $ | 0.06 | | $ | 0.08 | | $ | 0.08 | | $ | 0.14 | | $ | 0.20 | | $ | 0.11 | |
Diluted | | $ | 0.98 | | $ | 0.08 | | $ | 0.06 | | $ | 0.07 | | $ | 0.08 | | $ | 0.13 | | $ | 0.19 | | $ | 0.10 | |
Shares used in computing net profit per share: | | | | | | | | | | | | | | | | | | | | | | | | | |
Basic | | | 66,440 | | | 67,367 | | | 68,581 | | | 69,053 | | | 68,562 | | | 67,970 | | | 66,696 | | | 65,433 | |
Diluted | | | 66,816 | | | 68,704 | | | 69,916 | | | 70,860 | | | 71,864 | | | 72,230 | | | 71,207 | | | 69,331 | |
- (1)
- March 2007 profit from operations and net profit included a $1.8 million credit for the Patients First Program and settlement costs.See Note 5 "Patients First Program and settlement costs" in the Notes to Consolidated Financial Statements for additional information.
- (2)
- September and December 2008 profit from operations and net profit included $2.2 million and 4.0 million, respectively, for restructuring charges. SeeNote 18 "Restructurings" in the Notes to Consolidated Financial Statements for additional information.
- (3)
- December 2008 net profit included a $64.6 million benefit to income taxes as a result of the release of a tax valuation allowance on most of the deferred tax assets. SeeNote 13 "Income Taxes" in the Notes to Consolidated Financial Statements for additional information.
57
Table of Contents
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
| | |
| | Page |
|---|
Report of Management on Internal Control over Financial Reporting | | 59 |
Report of Independent Registered Public Accounting Firm | |
60 |
Consolidated Statements of Operations | |
61 |
Consolidated Balance Sheets | |
62 |
Consolidated Statements of Stockholders' Equity | |
63 |
Consolidated Statements of Cash Flows | |
64 |
Notes to Consolidated Financial Statements | |
65 |
Invisalign, Align, ClinCheck, Invisalign Assist, Invisalign Teen and Vivera, amongst others, are trademarks belonging to Align Technology, Inc. and are pending or registered in the United States and other countries.
58
Table of Contents
REPORT OF MANAGEMENT ON INTERNAL CONTROL OVER FINANCIAL REPORTING
Management of Align is responsible for establishing and maintaining adequate internal control over financial reporting as defined in Rules 13a-15(f) and 15d-15(f) under the Securities Exchange Act of 1934. Align's internal control over financial reporting is designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. Internal control over financial reporting includes those policies and procedures that:
- •
- pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company;
- •
- provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and
- •
- provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the company's assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. In addition, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions and that the degree of compliance with the policies or procedures may deteriorate.
Management assessed the effectiveness of Align's internal control over financial reporting as of December 31, 2008. In making this assessment, management used the criteria set forth in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO).
Based on its assessment and those criteria, management has concluded that, as of December 31, 2008, Align's internal control over financial reporting was effective to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles.
The Company's internal control over financial reporting as of December 31, 2008 has been audited by PricewaterhouseCoopers LLP, an independent registered public accounting firm, as stated in their report which is included herein, which expresses an unqualified opinion on the effectiveness of the Company's internal control over financial reporting as of December 31, 2008.
| | |
/s/THOMAS M. PRESCOTT
Thomas M. Prescott
President and Chief Executive Officer | | |
February 27, 2009 |
|
|
/s/KENNETH B. AROLA
Kenneth B. Arola
Vice President, Finance and Chief Financial Officer |
|
|
February 27, 2009
|
|
|
59
Table of Contents
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Stockholders and Board of Directors of Align Technology, Inc. and subsidiaries:
In our opinion, the consolidated financial statements listed in the index under item 15(a)(1) present fairly, in all material respects, the financial position of Align Technology, Inc. and its subsidiaries at December 31, 2008 and 2007, and the results of their operations and their cash flows for each of the three years in the period ended December 31, 2008 in conformity with accounting principles generally accepted in the United States of America. In addition, in our opinion, the financial statement schedule listed in the accompanying index appearing under item 15(a)(2) presents fairly, in all material respects, the information set forth therein when read in conjunction with the related consolidated financial statements. Also in our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2008, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). The Company's management is responsible for these financial statements and financial statement schedule, for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Management's Report on Internal Control Over Financial Reporting. Our responsibility is to express opinions on these financial statements, on the financial statement schedule, and on the Company's internal control over financial reporting based on our integrated audits. We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement and whether effective internal control over financial reporting was maintained in all material respects. Our audits of the financial statements included examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audits also included performing such other procedures as we considered necessary in the circumstances. We believe that our audits provide a reasonable basis for our opinions.
A company's internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company's internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company's assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
/s/ PricewaterhouseCoopers LLP
San Jose, CA
February 27, 2009
60
Table of Contents
ALIGN TECHNOLOGY, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF OPERATIONS
(in thousands, except per share data)
| | | | | | | | | | | | |
| | Years Ended December 31, | |
|---|
| | 2008 | | 2007 | | 2006 | |
|---|
Net revenues: | | | | | | | | | | |
| | Invisalign | | $ | 291,630 | | $ | 271,350 | | $ | 194,582 | |
| | Ancillary products and other services | | | 12,346 | | | 12,982 | | | 11,772 | |
| | | | | | | | |
| | | Total net revenues | | | 303,976 | | | 284,332 | | | 206,354 | |
| | | | | | | | |
Cost of revenues: | | | | | | | | | | |
| | Invisalign | | | 70,186 | | | 65,490 | | | 55,759 | |
| | Ancillary products and other services | | | 8,664 | | | 9,545 | | | 9,016 | |
| | | | | | | | |
| | | Total cost of revenues | | | 78,850 | | | 75,035 | | | 64,775 | |
Gross profit | | |
225,126 | | |
209,297 | | |
141,579 | |
| | | | | | | | |
Operating expenses: | | | | | | | | | | |
| | Sales and marketing | | | 115,062 | | | 98,231 | | | 81,993 | |
| | General and administrative | | | 62,154 | | | 53,280 | | | 64,305 | |
| | Research and development | | | 26,165 | | | 25,727 | | | 18,474 | |
| | Patients First Program and settlement costs | | | — | | | (1,796 | ) | | 14,343 | |
| | Restructurings | | | 6,231 | | | — | | | — | |
| | | | | | | | |
| | | Total operating expenses | | | 209,612 | | | 175,442 | | | 179,115 | |
| | | | | | | | |
Profit (loss) from operations | | | 15,514 | | | 33,855 | | | (37,536 | ) |
Interest income | | |
3,052 | | |
4,195 | | |
3,179 | |
Interest expense | | | (24 | ) | | (342 | ) | | (296 | ) |
Other income (expense) | | | (1,466 | ) | | (758 | ) | | 518 | |
| | | | | | | | |
Net profit (loss) before provision for income taxes | | | 17,076 | | | 36,950 | | | (34,135 | ) |
Provision for (benefit from) income taxes | | | (62,911 | ) | | 1,226 | | | 828 | |
| | | | | | | | |
Net profit (loss) | | $ | 79,987 | | $ | 35,724 | | $ | (34,963 | ) |
| | | | | | | | |
Net profit (loss) per share: | | | | | | | | | | |
| | Basic | | $ | 1.20 | | $ | 0.53 | | $ | (0.55 | ) |
| | | | | | | | |
| | Diluted | | $ | 1.18 | | $ | 0.50 | | $ | (0.55 | ) |
| | | | | | | | |
Shares used in computing net profit (loss) per share: | | | | | | | | | | |
| | Basic | | | 66,812 | | | 67,176 | | | 63,246 | |
| | | | | | | | |
| | Diluted | | | 68,064 | | | 71,444 | | | 63,246 | |
| | | | | | | | |
The accompanying notes are an integral part of these consolidated financial statements.
61
Table of Contents
ALIGN TECHNOLOGY, INC. AND SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS
(in thousands, except per share data)
| | | | | | | | | |
| | December 31, | |
|---|
| | 2008 | | 2007 | |
|---|
ASSETS | | | | | | | |
Current assets: | | | | | | | |
| | Cash and cash equivalents | | $ | 87,100 | | $ | 89,119 | |
| | Restricted cash | | | — | | | 21 | |
| | Marketable securities, short-term | | | 23,066 | | | 38,771 | |
| | Accounts receivable, net of allowance for doubtful accounts of $612 and $760, respectively | | | 52,362 | | | 44,850 | |
| | Inventories, net | | | 1,965 | | | 2,910 | |
| | Prepaid expenses and other current assets | | | 13,414 | | | 8,846 | |
| | | | | | |
| | | Total current assets | | | 177,907 | | | 184,517 | |
Property and equipment, net | | |
26,979 | | |
25,320 | |
Goodwill | | | 478 | | | 478 | |
Intangible assets, net | | | 7,788 | | | 10,615 | |
Deferred tax asset | | | 61,696 | | | — | |
Other assets | | | 4,493 | | | 1,831 | |
| | | | | | |
| | | Total assets | | $ | 279,341 | | $ | 222,761 | |
| | | | | | |
LIABILITIES AND STOCKHOLDERS' EQUITY | | | | | | | |
Current liabilities: | | | | | | | |
| | Accounts payable | | $ | 5,580 | | $ | 9,222 | |
| | Accrued liabilities | | | 38,282 | | | 39,875 | |
| | Deferred revenues | | | 16,710 | | | 12,362 | |
| | | | | | |
| | | Total current liabilities | | | 60,572 | | | 61,459 | |
Other long-term liabilities | | |
229 | | |
148 | |
| | | | | | |
| | | Total liabilities | | | 60,801 | | | 61,607 | |
Commitments and contingencies (Notes 7 and 9) | | | | | | | |
Stockholders' equity: | | | | | | | |
| | Preferred stock, $0.0001 par value (5,000 shares authorized; none issued) | | |
— | | |
— | |
| | Common stock, $0.0001 par value (200,000 shares authorized; 65,633 and 68,682 shares issued, respectively; 65,633 and 68,642 shares outstanding, respectively) | | | 7 | | | 7 | |
| | Additional paid-in capital | | | 439,494 | | | 450,140 | |
| | Accumulated other comprehensive income, net | | | 269 | | | 657 | |
| | Accumulated deficit | | | (221,230 | ) | | (289,650 | ) |
| | | | | | |
| | | Total stockholders' equity | | | 218,540 | | | 161,154 | |
| | | | | | |
| | | Total liabilities and stockholders' equity | | $ | 279,341 | | $ | 222,761 | |
| | | | | | |
The accompanying notes are an integral part of these consolidated financial statements.
62
Table of Contents
ALIGN TECHNOLOGY, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF STOCKHOLDERS' EQUITY
For the years ended December 31, 2008, 2007 and 2006
(in thousands)
| | | | | | | | | | | | | | | | | | | |
| | Common Stock | |
| | Accumulated
Other
Comprehensive
Income (Loss) | |
| |
| |
|---|
| | Additional
Paid in
Capital | | Accumulated
Deficit | |
| |
|---|
| | Shares | | Amount | | Total | |
|---|
Balances at December 31, 2005 | | | 62,080 | | $ | 6 | | $ | 383,836 | | $ | 7 | | $ | (290,411 | ) | $ | 93,438 | |
Net loss | | |
— | | |
— | | |
— | | |
— | | |
(34,963 |
) | |
(34,963 |
) |
Net change in unrealized loss from available-for sale securities | | | — | | | — | | | — | | | (4 | ) | | — | | | (4 | ) |
| | | | | | | | | | | | | | | | | | | |
Comprehensive net loss | | | | | | | | | | | | | | | | | | (34,967 | ) |
| | | | | | | | | | | | | | | | | | | |
Issuance of common stock relating to employee stock purchase plan | | | 462 | | | — | | | 2,583 | | | — | | | — | | | 2,583 | |
Issuance of common stock upon exercise of stock options | | | 2,317 | | | — | | | 13,592 | | | — | | | — | | | 13,592 | |
Stock-based compensation | | | — | | | — | | | 8,910 | | | — | | | — | | | 8,910 | |
| | | | | | | | | | | | | | |
Balances at December 31, 2006 | | | 64,859 | | $ | 6 | | $ | 408,921 | | $ | 3 | | $ | (325,374 | ) | $ | 83,556 | |
Net profit | | | | | | | | | | | | | | |
35,724 | | |
35,724 | |
Net change in cumulative translation adjustment | | | — | | | — | | | — | | | 703 | | | — | | | 703 | |
Net change in unrealized loss from available-for sale securities | | | — | | | — | | | — | | | (49 | ) | | — | | | (49 | ) |
| | | | | | | | | | | | | | | | | | | |
Comprehensive net income | | | | | | | | | | | | | | | | | | 36,378 | |
| | | | | | | | | | | | | | | | | | | |
Issuance of common stock relating to employee stock purchase plan | | | 580 | | | — | | | 3,434 | | | — | | | — | | | 3,434 | |
Issuance of common stock upon exercise of stock options | | | 3,048 | | | 1 | | | 25,558 | | | — | | | — | | | 25,559 | |
Issuance of common stock in settlement of restricted stock units, net of shares withheld for employees' taxes | | | 155 | | | — | | | (433 | ) | | — | | | — | | | (433 | ) |
Excess tax benefit from share-based payment arrangements | | | — | | | — | | | 449 | | | — | | | — | | | 449 | |
Stock-based compensation | | | — | | | — | | | 12,211 | | | — | | | — | | | 12,211 | |
| | | | | | | | | | | | | | |
Balances at December 31, 2007 | | | 68,642 | | $ | 7 | | $ | 450,140 | | $ | 657 | | $ | (289,650 | ) | $ | 161,154 | |
Net profit | | | | | | | | | | | | | | |
79,987 | | |
79,987 | |
Net change in unrealized gain from available-for sale securities | | | | | | | | | | | | 33 | | | | | | 33 | |
Net change in cumulative translation adjustment | | | | | | | | | | | | (421 | ) | | | | | (421 | ) |
| | | | | | | | | | | | | | | | | | | |
Comprehensive net income | | | | | | | | | | | | | | | | | | 79,599 | |
| | | | | | | | | | | | | | | | | | | |
Issuance of common stock relating to employee stock purchase plan | | | 523 | | | | | | 4,457 | | | | | | | | | 4,457 | |
Issuance of common stock upon exercise of stock options | | | 912 | | | | | | 6,049 | | | | | | | | | 6,049 | |
Issuance of common stock in settlement of restricted stock units, net of shares withheld for employees' taxes | | | 216 | | | | | | (460 | ) | | | | | | | | (460 | ) |
Common stock repurchased | | | (4,660 | ) | | | | | (38,571 | ) | | | | | (11,567 | ) | | (50,138 | ) |
Excess tax benefit from share-based payment arrangements | | | | | | | | | 144 | | | | | | | | | 144 | |
Stock-based compensation | | | | | | | | | 17,057 | | | | | | | | | 17,057 | |
Acceleration of stock-based compensation | | | | | | | | | 678 | | | | | | | | | 678 | |
| | | | | | | | | | | | | | |
Balances at December 31, 2008 | | | 65,633 | | $ | 7 | | $ | 439,494 | | $ | 269 | | $ | (221,230 | ) | $ | 218,540 | |
| | | | | | | | | | | | | | |
The accompanying notes are an integral part of these consolidated financial statements.
63
Table of Contents
ALIGN TECHNOLOGY, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
(in thousands)
| | | | | | | | | | | | |
| | Years Ended December 31, | |
|---|
| | 2008 | | 2007 | | 2006 | |
|---|
CASH FLOWS FROM OPERATING ACTIVITIES: | | | | | | | | | | |
| | Net profit (loss) | | $ | 79,987 | | $ | 35,724 | | $ | (34,963 | ) |
| | Adjustments to reconcile net profit (loss) to net cash provided by (used in) operating activities: | | | | | | | | | | |
| | | Deferred taxes | | | (64,608 | ) | | — | | | — | |
| | | Depreciation and amortization | | | 9,964 | | | 10,176 | | | 9,279 | |
| | | Stock-based compensation | | | 17,057 | | | 12,211 | | | 8,910 | |
| | | Acceleration of stock-based compensation | | | 678 | | | — | | | — | |
| | | Amortization of intangibles | | | 2,827 | | | 3,209 | | | 992 | |
| | | Provision for doubtful accounts | | | 71 | | | 46 | | | (288 | ) |
| | | Loss on retirement and disposal of fixed assets | | | 513 | | | 24 | | | 40 | |
| | | Loss on impairment of fixed assets | | | 1,712 | | | — | | | — | |
| | | Excess tax benefit from share-based payment arrangements | | | (144 | ) | | (449 | ) | | — | |
| | Changes in assets and liabilities, net of acquisition effect: | | | | | | | | | | |
| | | Accounts receivable | | | (7,951 | ) | | (10,703 | ) | | (4,042 | ) |
| | | Inventories | | | 943 | | | 186 | | | (160 | ) |
| | | Prepaid expenses and other current assets | | | 276 | | | (1,480 | ) | | (2,245 | ) |
| | | Accounts payable | | | (2,651 | ) | | 2,738 | | | 2,997 | |
| | | Accrued and other long-term liabilities | | | (3,487 | ) | | (295 | ) | | 11,255 | |
| | | Deferred revenues | | | 4,559 | | | 1,390 | | | (5,805 | ) |
| | | | | | | | |
Net cash provided by (used in) operating activities | | | 39,746 | | | 52,777 | | | (14,030 | ) |
| | | | | | | | |
CASH FLOWS FROM INVESTING ACTIVITIES: | | | | | | | | | | |
| | Purchase of property and equipment | | | (14,334 | ) | | (7,429 | ) | | (10,028 | ) |
| | Proceeds from sale of property and equipment | | | 189 | | | — | | | 366 | |
| | Restricted cash | | | 21 | | | 77 | | | 57 | |
| | Purchase of marketable securities | | | (75,050 | ) | | (64,686 | ) | | (18,416 | ) |
| | Maturities of marketable securities | | | 87,926 | | | 34,797 | | | 9,481 | |
| | Payments for acquisition, net of cash acquired | | | — | | | — | | | — | |
| | Purchase of intangible assets | | | — | | | — | | | (14,000 | ) |
| | Other assets | | | 193 | | | 462 | | | (211 | ) |
| | | | | | | | |
Net cash used in investing activities | | | (1,055 | ) | | (36,779 | ) | | (32,751 | ) |
| | | | | | | | |
CASH FLOWS FROM FINANCING ACTIVITIES: | | | | | | | | | | |
| | Proceeds from issuance of common stock | | | 10,506 | | | 28,993 | | | 16,175 | |
| | Proceeds from line of credit | | | — | | | — | | | 15,000 | |
| | Payments on line of credit | | | — | | | (11,500 | ) | | (3,500 | ) |
| | Payments on short-term obligations | | | (407 | ) | | | | | | |
| | Repurchases of common stock | | | (50,138 | ) | | — | | | — | |
| | Excess tax benefit from share-based payment arrangements | | | 144 | | | 449 | | | — | |
| | Employees' taxes paid upon the vesting of restricted stock units | | | (460 | ) | | (433 | ) | | — | |
| | | | | | | | |
| | Net cash provided by (used in) financing activities | | | (40,355 | ) | | 17,509 | | | 27,675 | |
| | | | | | | | |
| | Effect of foreign exchange rate changes on cash and cash equivalents | | | (355 | ) | | 499 | | | — | |
Net increase (decrease) in cash and cash equivalents | | | (2,019 | ) | | 34,006 | | | (19,106 | ) |
Cash and cash equivalents, beginning of year | | | 89,119 | | | 55,113 | | | 74,219 | |
| | | | | | | | |
Cash and cash equivalents, end of year | | $ | 87,100 | | $ | 89,119 | | $ | 55,113 | |
| | | | | | | | |
The accompanying notes are an integral part of these consolidated financial statements.
64
Table of Contents
ALIGN TECHNOLOGY, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Note 1. Summary of Significant Accounting Policies
Align Technology, Inc. ("Align" or the "Company") was incorporated in April 1997 and designs, manufactures and markets the Invisalign system, a proprietary method for treating malocclusion, or the misalignment of teeth. Invisalign corrects malocclusion using a series of clear, nearly invisible, removable appliances that gently move teeth to a desired final position.
The consolidated financial statements include the accounts of the Company and its wholly-owned subsidiaries after elimination of intercompany transactions and balances.
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires the Company's management to make estimates and assumptions that affect the amounts reported in the consolidated financial statements and accompanying notes. Actual results could differ materially from those estimates.
The carrying amounts of the Company's cash and cash equivalents, accounts receivable, accounts payable and other current liabilities approximate the fair value.
Cash equivalents consist of highly liquid instruments purchased with an original maturity of three months or less. The Company invests primarily in money market funds, commercial paper, and United States government securities, accordingly, these investments are subject to minimal credit and market risks.
Marketable securities are classified as available-for-sale and are carried at fair value. Marketable securities classified as current assets have maturities of less than one year. Unrealized gains or losses on such securities are included in accumulated other comprehensive income (loss) in stockholders' equity. Realized gains and losses from maturities of all such securities are reported in earnings and computed using the specific identification cost method. Realized gains or losses and charges for other-than-temporary declines in value, if any, on available-for-sale securities are reported in other income (expense) as incurred. The Company periodically evaluates these investments for other-than-temporary impairment.
The Company adopted the provisions of Statement of Financial Accounting Standards No. 157 ("FAS"), "Fair Value Measurements" ("FAS 157"), for measuring its cash equivalents and marketable securities effective January 1, 2008. FAS 157 defines fair value as the price that would be received from selling an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. FAS 157 establishes a fair value hierarchy that prioritizes the inputs to valuation techniques used to measure fair value. This hierarchy requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value.
65
Table of Contents
ALIGN TECHNOLOGY, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Note 1. Summary of Significant Accounting Policies (Continued)
The Company also adopted FAS No. 159, "The Fair Value Option for Financial Assets and Financial Liabilities—Including an amendment of FAS No. 115" ("FAS 159"). FAS 159 expands the use of fair value accounting but does not affect existing standards which require assets or liabilities to be carried at fair value. Under FAS 159, a company may elect to use fair value to measure accounts and loans receivable, available-for-sale and held-to-maturity securities, equity method investments, accounts payable, guarantees and issued debt. Other eligible items include firm commitments for financial instruments that otherwise would not be recognized at inception and non-cash warranty obligations where a warrantor is permitted to pay a third party to provide the warranty goods or services. If the use of fair value is elected, any upfront costs and fees related to the item must be recognized in earnings and cannot be deferred, e.g., debt issue costs. The fair value election is irrevocable and generally made on an instrument-by-instrument basis, even if a company has similar instruments that it elects not to measure based on fair value. At the adoption date, unrealized gains and losses on existing items for which fair value has been elected are reported as a cumulative adjustment to beginning retained earnings. Subsequent to the adoption of FAS 159, changes in fair value are recognized in earnings. The adoption of FAS 159 did not have a material impact on the Company's consolidated financial position, results of operations or cash flows as the fair value option was not elected for any of the Company's financial assets or financial liabilities at the date of adoption.
The Company's restricted cash as of December 31, 2007 was $21,000 and was primarily comprised of a security deposit for a leasing arrangement in Europe. There was no restricted cash balance as of December 31, 2008.
The Company accounts for both the translation and remeasurement of balance sheet and income statement items into the U.S. dollar in accordance with FAS No. 52, "Foreign Currency Translation" ("FAS 52"). The Company analyzes the functional currency for each of its international subsidiaries on an annual basis, or more often if necessary, to determine if a significant change in facts and circumstances indicate that the primary economic currency has changed.
During the first quarter of 2007, the Company analyzed the various economic factors of its international subsidiaries in accordance with FAS 52 and determined that there had been a significant change in facts and circumstances to warrant a change in the functional currency for some of its European subsidiaries from the U.S. dollar to the local currency. Effective January 1, 2007, the adjustment from translating certain European subsidiaries' financial statements from the local currency to the U.S. dollar was recorded as a separate component of accumulated other comprehensive income (loss), net in the stockholders' equity section of the Consolidated Balance Sheets. This foreign currency translation adjustment reflects the translation of the balance sheet at period end exchange rates, and the income statement at an average exchange rate in effect during the period. As of December 31, 2008 and 2007, the Company had $0.3 million and $0.7 million, respectively, in accumulated other comprehensive income (loss), net related to the translation of its foreign subsidiaries' financial statements. SeeNote 15 "Comprehensive Income (Loss)"for additional disclosures.
Align's other international entities operate in a U.S. dollar functional currency environment, and therefore, the foreign currency assets and liabilities are remeasured into the U.S. dollar at current
66
Table of Contents
ALIGN TECHNOLOGY, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Note 1. Summary of Significant Accounting Policies (Continued)
exchange rates except for non-monetary assets and liabilities which are remeasured at historical exchange rates. Revenues and expenses are generally remeasured at an average exchange rate in effect during each period. Gains or losses from foreign currency remeasurement are included in other income (expense). Prior to January 1, 2007, all of Align's subsidiaries used the U.S. dollar as its functional currency, and accordingly, gains and losses resulting from remeasurement were included in other income (expense).
For the years ended December 31, 2008, 2007 and 2006, the Company included in other income (expense) a loss of $0.4 million, a loss of $0.1 million and a gain of $1.1 million, respectively.
The Company's operating results depend to a significant extent on the Company's ability to market and develop its products. The life cycles of the Company's products are difficult to estimate due in part to the effect of future product enhancements and competition. The inability of the Company to successfully develop and market its products as a result of competition or other factors would have a material adverse effect on the Company's business, financial condition and results of operations.
Financial instruments which potentially expose the Company to concentrations of credit risk consist primarily of cash equivalents, marketable securities and accounts receivable. The Company invests excess cash primarily in money market funds of major financial institutions, commercial paper and notes and government securities. If the carrying value of the Company's investments exceeds the fair value, and the decline in fair value is deemed to be other-than-temporary, the Company will be required to write down the value of its investments, which could materially harm the Company's results of operations and financial condition. Moreover, the performance of certain securities in the Company's investment portfolio correlates with the credit condition of the U.S. financial sector. With the current unstable credit environment, the Company might incur significant realized, unrealized or impairment losses associated with these investments. The Company provides credit to customers in the normal course of business. Collateral is not required for accounts receivable, but ongoing evaluations of customers' credit worthiness are performed. The Company maintains reserves for potential credit losses and such losses have been within management's expectations. No individual customer accounted for 10% or more of the Company's accounts receivable at December 31, 2008 and 2007, or net revenues in 2008, 2007 and 2006.
In the United States of America, the Food and Drug Administration ("FDA") regulates the design, manufacture, distribution, preclinical and clinical study, clearance and approval of medical devices. Products developed by the Company may require approvals or clearances from the FDA or other international regulatory agencies prior to commercialized sales. There can be no assurance that the Company's products will receive any of the required approvals or clearances. If the Company was denied approval or clearance or such approval was delayed, it may have a material adverse impact on the Company.
The Company has manufacturing operations located outside the United States of America. The Company currently relies on its manufacturing facilities in Costa Rica to prepare digital treatment plans using a sophisticated, internally developed computer-modeling program. In addition, the Company relies on a third party shelter services provider in Juarez, Mexico to fabricate aligners and to ship the completed product to the Company's customers. On December 22, 2008, the Company announced the termination of its shelter services arrangement with the third party shelter services
67
Table of Contents
ALIGN TECHNOLOGY, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Note 1. Summary of Significant Accounting Policies (Continued)
provider effective April 1, 2009. At that time, the Company will become a direct, independent manufacturer of its clear aligners at the facility in Juarez, Mexico. The Company's reliance on international operations exposes it to related risks and uncertainties, including difficulties in staffing and managing international operations; difficulties in managing the transition from a third party shelter services arrangement to becoming an independent manufacturer, including difficulties in hiring and retaining qualified personnel; controlling production volume and quality of manufacture; political, social and economic instability, including as a result of increased levels of violence in Juarez, Mexico; interruptions and limitations in telecommunication services; product and material transportation delays or disruption; trade restrictions and changes in tariffs; import and export license requirements and restrictions; fluctuations in foreign currency exchange rates; and potential adverse tax consequences. If any of these risks materialize, the Company's international manufacturing operations, as well as its operating results, may be harmed.
The Company purchases certain inventory from sole suppliers. Additionally, the Company relies on a limited number of hardware manufacturers. The inability of any supplier or manufacturer to fulfill supply requirements of the Company could materially and adversely impact future operating results.
Inventories are valued at the lower of cost or market, with cost computed on a first-in, first-out basis.
Property and equipment are stated at historical cost less accumulated depreciation and amortization. Depreciation and amortization are computed using the straight-line method over the estimated useful lives of the assets. Upon sale or retirement, the asset's cost and related accumulated depreciation are removed from the general ledger and any related gains or losses are reflected in the Consolidated Statements of Operations. Maintenance and repairs are expensed as incurred.
The Company evaluates the recoverability of property and equipment in accordance with FAS No. 144, "Accounting for the Impairment or Disposal of Long-Lived Assets" ("FAS 144"), whenever events or changes in circumstances indicate that the carrying value of a long-lived asset may not be recoverable. Examples of such events or circumstances include significant underperformance relative to historical or projected future operating results, significant changes in the manner of use of the acquired assets or the strategy for its business, significant negative industry or economic trends, or a significant decline in our stock price for sustained period. The carrying amount of a long-lived asset is not recoverable if it exceeds the sum of the projected undiscounted cash flows expected to result from the use and eventual disposal of the asset. The Company also compares the carrying amount of the asset to its fair market value in accordance with FAS 157. FAS 157 defines fair value as the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. In the event that the projected undiscounted cash flows are not sufficient to recover the carrying value of the assets, the assets are written down to their estimated fair values. The Company recorded an asset impairment charge of $1.7 million in connection with an internally developed software in fourth quarter of 2008. See Note 4 "Impairment of Long-Lived Assets" for additional information.
68
Table of Contents
ALIGN TECHNOLOGY, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Note 1. Summary of Significant Accounting Policies (Continued)
Costs relating to internal use software are accounted for in accordance with the provisions of Statement of Position 98-1, "Accounting for the Costs of Computer Software Developed or Obtained for Internal Use" ("SOP 98-1"). As of December 31, 2008 and 2007, capitalized internal use software at cost was $5.3 million and $5.0 million, respectively. The associated accumulated amortization was $4.7 million and $4.4 million as of December 31, 2008 and 2007, respectively. Capitalized software costs are amortized over the estimated useful lives of three years.
Goodwill represents the excess of the purchase price paid over the fair value of tangible and identifiable intangible net assets acquired in business combinations. Goodwill is reviewed annually in the fourth quarter and whenever events or circumstances occur which indicate that goodwill might be impaired. The Company completed its annual evaluation of goodwill during the fourth quarter of 2008 and determined that there was no impairment.
Long-lived assets, including finite lived purchased intangible assets
Other intangible assets primarily consist of intangible assets purchased as part of the OrthoClear Agreement. These assets are amortized using the straight-line method over their estimated useful lives of three to five years, reflecting the period in which the economic benefits of the assets are expected to be realized.
The Company performs an impairment test whenever events or changes in circumstances indicate that the carrying value of such assets may not be recoverable. Examples of such events or circumstances include significant underperformance relative to historical or projected future operating results, significant changes in the manner of use of acquired assets or the strategy for its business, significant negative industry or economic trends, or a significant decline in the Company's stock price for a sustained period. Impairments are recognized based on the difference between the fair value of the asset and its carrying value, and fair value is generally measured based on discounted cash flow analyses. No intangible asset impairment was recorded for the periods presented.
The Company warrants its products against material defects until the Invisalign cases are completed. The Company accrues for product warranty in cost of revenues upon shipment of products. Product warranty costs are primarily based on historical experience as to product failures as well as current information on repair costs. Actual warranty costs could differ materially from the estimated amounts. The Company regularly reviews the accrued balances and updates these balances based on historical warranty cost trends. Actual warranty costs incurred have not materially differed from those accrued.
The Company maintains an allowance for doubtful accounts for estimated losses resulting from the inability of the Company's customers to make payments. The Company periodically reviews these allowances, including an analysis of the customers' payment history and information regarding the
69
Table of Contents
ALIGN TECHNOLOGY, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Note 1. Summary of Significant Accounting Policies (Continued)
customers' creditworthiness. Actual write-offs have not materially differed from the estimated allowance.
Align recognizes revenue in accordance with SEC Staff Accounting Bulletin No. 104 "Revenue Recognition" ("SAB 104"), and Emerging Issues Task Force ("EITF") No. 00-21 "Revenue Arrangements with Multiple Deliverables" ("EITF 00-21"). SAB 104 requires that four basic criteria must be met before revenue can be recognized: persuasive evidence of an arrangement exists, delivery has occurred, the price is fixed or determinable, and collectibility is reasonably assured.
Revenues are recognized from product sales, net of discounts and rebates. Service revenues related to the training of dental professionals and staff on the Invisalign treatment process are recorded when the services are completed.
Align enters into arrangements that involve multiple product deliveries in the future. Included in the price of Invisalign Full, Invisalign Teen and Invisalign Assist, the Company offers case refinement, which is a finishing tool used to adjust a patient's teeth to the desired final position. Case refinement may be elected by the dental professional in the last stages of orthodontic treatment. The Company uses vendor specific objective evidence of fair value to allocate revenue to the case refinement deliverable and recognizes the residual revenue upon shipment. Align defers the fair value of case refinement upon shipment based on a breakage factor, which is determined by sufficient historical experience of case refinement utilization. The Company believes that the use of a breakage factor is reasonable and appropriate because of the relative stability of case refinement utilization since case refinement was first offered. Actual utilization rates could differ from the historical breakage factor requiring future adjustments to revenue.
Revenues are deferred for certain products that include staged delivery. Depending on the product, revenues are recognized based on usage, case completion, ratably over a subscription period or upon shipment of the final staged shipment. Revenue for the six replacement aligners included in Invisalign Teen is deferred based on the fair market value until the earlier of replacement aligners being used or until the case is completed. The Vivera retainer includes four shipments per year, and revenue is deferred upon the first shipment and recognized as each shipment occurs. For Invisalign Assist, when the progress tracking feature is selected, aligners are shipped to the dental professional after every nine stages. For these cases, revenue is deferred upon the first staged shipment and will be recognized upon shipment of the final staged shipment.
The Company estimates and records a provision for amounts of estimated losses on sales, if any, in the period such sales occur. Provisions for discounts and rebates to customers are provided for in the same period that the related product sales are recorded based upon historical discounts and rebates.
Shipping and handling charges to customers are included in net revenues, and the associated costs incurred are recorded in cost of revenues for all periods presented.
Research and development costs are expensed as incurred.
70
Table of Contents
ALIGN TECHNOLOGY, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Note 1. Summary of Significant Accounting Policies (Continued)
The cost of advertising and media is expensed as incurred. For the years ended December 31, 2008, 2007 and 2006 advertising costs totaled $18.3 million, $15.9 million, and $9.2 million, respectively.
The Company estimates income taxes based on the various jurisdictions where business is conducted. Significant judgment is required in determining the income tax provision. Deferred tax assets and liabilities are recognized for differing treatments of certain items for tax and accounting purposes. These differences result in deferred tax assets and liabilities. The Company must then assess the likelihood that its deferred tax assets will be realized. To the extent the Company believes that realization is not likely, it will establish a valuation allowance.
On January 1, 2007, the Company adopted the provision of Financial Accounting Standards Board ("FASB") Interpretation No. 48, "Accounting for Uncertain Income Taxes—An Interpretation of FASB Statement No. 109" ("FIN 48"). FIN 48 clarifies the accounting for uncertainty in income taxes recognized in an entity's financial statements in accordance with FAS No. 109, "Accounting for Income Taxes" ("FAS 109") and prescribes a recognition threshold and measurement attributes for financial statement disclosure of tax positions taken or expected to be taken on a tax return. Under FIN 48, the impact of an uncertain income tax position on the income tax return must be recognized at the largest amount that is more-likely-than-not to be sustained upon audit by the relevant taxing authority. An uncertain income tax position will not be recognized if it has less than a 50% likelihood of being sustained. Additionally, FIN 48 provides guidance on de-recognition, classification, interest and penalties, accounting in interim periods, disclosure and transition.
The Company adopted the fair value recognition provisions of FAS No. 123 (revised 2004), "Share-Based Payment" ("FAS 123R") in 2006. Accordingly, the Company recognizes compensation cost for only those shares ultimately expected to vest on a straight-line basis over the requisite service period of the award, net of an estimated forfeiture rate. The Company estimates the fair value of stock options using a Black-Scholes valuation model, which requires the input of highly subjective assumptions, including the option's expected term and stock price volatility. In addition, judgment is also required in estimating the number of stock-based awards that are expected to be forfeited. Forfeitures are estimated based on historical experience at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates. The assumptions used in calculating the fair value of share-based payment awards represent management's best estimates, but these estimates involve inherent uncertainties and the application of management's judgment. As a result, if factors change and the Company uses different assumptions, its stock-based compensation expense could be materially different in the future. Prior to FAS 123R adoption, the Company accounted for share-based payment awards under Accounting Principles Board Opinion ("APB") No. 25, "Accounting for Stock Issued to Employees" ("APB 25") and its related interpretations.See Note 10 "Stockholders' Equity" for additional information.
71
Table of Contents
ALIGN TECHNOLOGY, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Note 1. Summary of Significant Accounting Policies (Continued)
Comprehensive income (loss) includes all changes in equity during a period from non-owner sources. Comprehensive income (loss), including unrealized gains and losses on available-for-sale securities and foreign currency translation adjustments, are reported net of their related tax effect.
In December 2007, the FASB issued FAS No. 141 (revised 2007), "Business Combinations" ("FAS 141R"). FAS 141R establishes principles and requirements for how the acquirer in a business combination recognizes and measures in its financial statements the fair value of identifiable assets acquired, the liabilities assumed and any noncontrolling interest in the acquiree at the acquisition date. FAS 141R determines what information to disclose to enable users of the financial statements to evaluate the nature and financial effects of the business combination. FAS 141R applies prospectively and is effective for fiscal years beginning on or after December 15, 2008. The Company is currently evaluating the potential impact, if any, of the adoption of FAS 141R on its consolidated financial position, results of operations and cash flows.
In December 2007, the FASB issued FAS No.160, "Noncontrolling Interests in Consolidated Financial Statements" ("FAS 160"), an amendment of Accounting Research Bulletin No. 51, "Consolidated Financial Statements" ("ARB 51"). FAS 160 changes the accounting and reporting for minority interests, which will be recharacterized as noncontrolling interests and classified as a component of equity. This new consolidation method significantly changes the accounting for transactions with minority interest holders. FAS 160 is effective for fiscal years beginning after December 15, 2008. The Company plans to adopt FAS 160 beginning in the first quarter of 2009. The Company is evaluating the impact the adoption of FAS 160 will have on its consolidated financial position and results of operations.
In April 2008, the FASB issued FSP FAS No. 142-3, "Determination of the Useful Life of Intangible Assets" ("FSP FAS 142-3"). FSP FAS 142-3 amends the factors that should be considered in developing renewal or extension assumptions used to determine the useful life of a recognized intangible asset under FAS No. 142, "Goodwill and Other Intangible Assets." FSP FAS No. 142-3 is effective for fiscal years beginning after December 15, 2008. The guidance for determining the useful life of an intangible asset must be applied prospectively to intangible assets acquired after the effective date. The Company is currently evaluating the impact of the pending adoption of FSP FAS No. 142-3 on its consolidated financial statements.
In August 2008, the U.S. Securities and Exchange Commission ("SEC") announced that they will issue for comment a proposed roadmap regarding the potential use by U.S. issuers of financial statements prepared in accordance with International Financial Reporting Standards ("IFRS"). IFRS is a comprehensive series of accounting standards published by the International Accounting Standards Board ("IASB"). Under the proposed roadmap, the Company could be required in fiscal 2014 to prepare financial statements in accordance with IFRS, and the SEC will make a determination in 2011 regarding the mandatory adoption of IFRS. The Company will assess the impact that this potential change would have on its consolidated financial statements and will monitor the development of the potential implementation of IFRS.
72
Table of Contents
ALIGN TECHNOLOGY, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Note 1. Summary of Significant Accounting Policies (Continued)
Other recent accounting pronouncements issued by the FASB (including its Emerging Issues Task Force), the American Institute of Certified Public Accountants and the SEC did not or are not believed by management to have a material impact on the Company's present or future consolidated financial statements.
Note 2. Marketable Securities and Fair Value Measurements
The Company has the following short-term investments as of December 31, 2008 and 2007 (in thousands):
| | | | | | | | | | | | | |
December 31, 2008 | | Amortized
Costs | | Gross
Unrealized
Gains | | Gross
Unrealized
Losses | | Fair Value | |
|---|
U.S. government notes and bonds | | $ | 9,971 | | $ | 25 | | $ | — | | $ | 9,996 | |
Corporate bonds and certificates of deposit | | | 3,774 | | | 1 | | | (24 | ) | | 3,751 | |
Agency bonds and discount notes | | | 8,499 | | | 20 | | | — | | | 8,519 | |
Commercial paper | | | 800 | | | — | | | — | | | 800 | |
| | | | | | | | | | |
Total | | $ | 23,044 | | $ | 46 | | $ | (24 | ) | $ | 23,066 | |
| | | | | | | | | | |
| | | | | | | | | | | | | |
December 31, 2007 | | Amortized
Costs | | Gross
Unrealized
Gains | | Gross
Unrealized
Losses | | Fair Value | |
|---|
U.S. government notes and bonds | | $ | 4,081 | | $ | 6 | | $ | — | | $ | 4,087 | |
Corporate bonds | | | 6,983 | | | — | | | — | | | 6,983 | |
Commercial paper and asset-backed securities | | | 27,754 | | | — | | | (53 | ) | | 27,701 | |
| | | | | | | | | | |
Total | | $ | 38,818 | | $ | 6 | | $ | (53 | ) | $ | 38,771 | |
| | | | | | | | | | |
As of December 31, 2008, all short-term investments have maturity dates of less than one year. For the years ended December 31, 2008 and 2007, realized losses were immaterial.
The Company has the following long-term investments as of December 31, 2008 (in thousands):
| | | | | | | | | | | | | |
December 31, 2008 | | Amortized
Costs | | Gross
Unrealized
Gains | | Gross
Unrealized
Losses | | Fair Value | |
|---|
Agency bonds | | $ | 1,000 | | $ | 1 | | $ | — | | $ | 1,001 | |
Corporate bonds | | | 1,897 | | | 0 | | | (35 | ) | | 1,862 | |
| | | | | | | | | | |
Total | | $ | 2,897 | | $ | 1 | | $ | (35 | ) | $ | 2,863 | |
| | | | | | | | | | |
The long-term marketable securities are included in Other assets in the consolidated balance sheets. As of December 31, 2007, the Company did not hold any long-term marketable securities.
73
Table of Contents
ALIGN TECHNOLOGY, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Note 2. Marketable Securities and Fair Value Measurements (Continued)
The Company adopted the provisions of FAS 157 for measuring its cash equivalents and marketable securities effective January 1, 2008. FAS 157 defines fair value as the price that would be received from selling an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. FAS 157 establishes a fair value hierarchy that prioritizes the inputs to valuation techniques used to measure fair value. This hierarchy requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value. FAS 157 establishes three levels of inputs that may be used to measure fair value:
Level 1—Quoted (unadjusted) prices in active markets for identical assets or liabilities.
The Company's Level 1 assets and liabilities consist of U.S. government debt securities and money market funds.
Level 2—Observable inputs other than quoted prices included in Level 1, such as quoted prices for similar assets or liabilities in active markets; quoted prices for identical or similar assets or liabilities in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the asset or liability.
The Company's Level 2 assets and liabilities consist of agency bonds and discount notes, corporate bonds and certificates of deposit and commercial paper.
Level 3—Unobservable inputs to the valuation methodology that are supported by little or no market activity and that are significant to the measurement of the fair value of the assets or liabilities. Level 3 assets and liabilities include those whose fair value measurements are determined using pricing models, discounted cash flow methodologies or similar valuation techniques, as well as significant management judgment or estimation.
The Company did not hold any Level 3 assets and liabilities during the year ended December 31, 2008.
74
Table of Contents
ALIGN TECHNOLOGY, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Note 2. Marketable Securities and Fair Value Measurements (Continued)
The following table summarizes the Company's financial assets measured at fair value on a recurring basis in accordance with FAS 157 as of December 31, 2008 (in thousands):
| | | | | | | | | | |
Description | | Balance as of
December 31, 2008 | | Quoted Prices in
Active Markets for
Identical Assets
(Level 1) | | Significant Other
Observable Inputs
(Level 2) | |
|---|
Cash equivalents: | | | | | | | | | | |
Money market funds | | $ | 39,457 | | $ | 39,457 | | $ | — | |
U.S. government debt securities | | | 20,000 | | | 20,000 | | | — | |
Short-term investments: | | | | | | | | | | |
Corporate bonds and certificates of deposit | | | 3,751 | | | — | | | 3,751 | |
U.S. government debt securities | | | 9,996 | | | 9,996 | | | — | |
Agency bonds and discount notes | | | 8,519 | | | | | | 8,519 | |
Commercial paper | | | 800 | | | — | | | 800 | |
Long-term investments: | | | | | | | | | | |
Corporate bonds and certificates of deposit | | | 1,862 | | | — | | | 1,862 | |
Agency bonds and discount notes | | | 1,001 | | | — | | | 1,001 | |
| | | | | | | | |
| | $ | 85,386 | | $ | 69,453 | | $ | 15,933 | |
| | | | | | | | |
Note 3. Balance Sheet Components
Inventories consist of the following (in thousands):
| | | | | | | |
| | December 31, | |
|---|
| | 2008 | | 2007 | |
|---|
Raw materials | | $ | 1,066 | | $ | 1,983 | |
Work in process | | | 416 | | | 631 | |
Finished goods | | | 483 | | | 296 | |
| | | | | | |
| | $ | 1,965 | | $ | 2,910 | |
| | | | | | |
Work in process includes costs to produce the Invisalign product. Finished goods primarily represent ancillary products that support the Invisalign system.
75
Table of Contents
ALIGN TECHNOLOGY, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Note 3. Balance Sheet Components (Continued)
Property and equipment consist of the following (in thousands):
| | | | | | | | | | |
| |
| | December 31, | |
|---|
| | Useful Life
(in years) | |
|---|
| | 2008 | | 2007 | |
|---|
Clinical and manufacturing equipment | | | 5 | | $ | 45,125 | | $ | 44,230 | |
Computer hardware | | | 3 | | | 13,548 | | | 10,444 | |
Computer software | | | 3 | | | 10,092 | | | 8,493 | |
Furniture and fixtures | | | 5 | | | 5,584 | | | 5,013 | |
Leasehold improvements | | | Term of the lease | | | 9,918 | | | 9,701 | |
Construction in progress | | | — | | | 4,075 | | | 1,198 | |
| | | | | | | | | |
| | | | | $ | 88,342 | | $ | 79,079 | |
Less: Accumulated depreciation and amortization | | | | | | (61,363 | ) | | (53,759 | ) |
| | | | | | | | | |
| | | | | $ | 26,979 | | $ | 25,320 | |
| | | | | | | | | |
As of December 31, 2008, construction in progress consisted primarily of costs for capital equipment expected to be placed in service in the next year. Depreciation and amortization was $10.0 million, $10.2 million, and $9.3 million, for the years ended December 31, 2008, 2007 and 2006, respectively.
Accrued liabilities consist of the following (in thousands):
| | | | | | | |
| | December 31, | |
|---|
| | 2008 | | 2007 | |
|---|
Accrued payroll and benefits | | $ | 17,795 | | $ | 22,165 | |
Accrued restructuring | | | 2,501 | | | — | |
Accrued sales and marketing expenses | | | 2,449 | | | 2,910 | |
Accrued sales rebate | | | 2,205 | | | 3,724 | |
Accrued warranty | | | 2,031 | | | 2,035 | |
Accrued Patients First Program costs | | | 100 | | | 996 | |
Other | | | 11,201 | | | 8,045 | |
| | | | | | |
| | $ | 38,282 | | $ | 39,875 | |
| | | | | | |
Warranty accrual as of December 31, 2008 and 2007 consists of the following activity (in thousands):
| | | | | |
Warranty accrual, December 31, 2006 | | $ | 2,094 | |
| | Charged to cost of revenues | | | 2,086 | |
| | Actual warranty expenditures | | | (2,145 | ) |
| | | | |
Warranty accrual, December 31, 2007 | | $ | 2,035 | |
| | Charged to cost of revenues | | | 2,484 | |
| | Actual warranty expenditures | | | (2,488 | ) |
| | | | |
Warranty accrual, December 31, 2008 | | $ | 2,031 | |
| | | | |
76
Table of Contents
ALIGN TECHNOLOGY, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Note 4. Impairment of Long-Lived Assets
The Company evaluates the recoverability of property and equipment in accordance with FAS 144 whenever events or changes in circumstances indicate that the carrying value of a long-lived asset may not be recoverable. The Company's management decided to no longer invest in an internally developed software tool for business process management resulting in an asset impairment charge of $1.7 million which was recorded in general and administrative expense in the fourth quarter of 2008. The impairment indicators which management considered included the fact that this internally developed software tool was not completed and as no market participant would be willing to purchase an unfinished customized application, the fair value was determined to be zero, and the fully capitalized amount of the software tool was written off consistent with the related provisions of FAS 144 and FAS 157.
Note 5. Patients First Program and settlement costs
In October 2006, the Company entered into a formal agreement with OrthoClear, Inc., OrthoClear Holdings, Inc., and OrthoClear Pakistan Pvt. Ltd. ("OrthoClear"), together with certain individuals associated with OrthoClear (the "OrthoClear Agreement") to end all pending litigation between the parties. In addition, OrthoClear agreed to stop the importation of aligners into the United States and discontinue all aligner business operations worldwide. As a result, most OrthoClear patients were unable to complete their orthodontic treatment with OrthoClear. OrthoClear also agreed to assign and transfer to Align all intellectual property rights with application to the correction of malocclusion and certain OrthoClear principals also signed five year non-compete agreements. The Company evaluated this transaction under the provisions of EITF No. 98-3 "Determining Whether a Non-Monetary Transaction Involves a Receipt of Productive Assets or of a Business" ("EITF 98-3") and concluded that this transaction was not a business acquisition and was accounted for as an asset purchase.
In an attempt to help minimize treatment disruptions for the OrthoClear patients and their doctors, the Company committed to make treatment available to these patients at no additional cost under the "Patients First Program". In 2006, the Company recorded an $8.3 million charge for the anticipated costs of completing this program. Subsequently, in the first quarter of 2007, the Company reduced its Patients First Program accrual by $1.8 million to reflect a reduction of its initial estimate of the number of cases actually received by the case submission deadline. During 2007, the Company shipped virtually all Patients First Program cases. The accrued Patients First Program balance as of December 31, 2008 and 2007 was $0.1 million and $1.0 million, respectively, and principally consisted of estimated future warranty and case refinement costs.
The Company paid $20.0 million to OrthoClear during 2006 in accordance with the terms of the OrthoClear Agreement, of which $14.0 million was capitalized on the Company's balance sheet representing the fair value of the non-compete agreements and is being amortized over 5 years. The Company recorded the remaining $6.0 million as settlement costs in 2006 in accordance with EITF No. 04-01 "Accounting for Pre-existing Contractual Relationships between the Parties to a Purchase Business Combination" ("EITF 04-01").
77
Table of Contents
ALIGN TECHNOLOGY, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Note 6. Intangible Assets
The following is a summary of the Company's purchased intangible assets as of December 31, 2008 and 2007 (in thousands):
| | | | | | | | | | | | | | | | | | | | | | |
| |
| | December 31, 2008 | | December 31, 2007 | |
|---|
| | Estimated
Useful Life
(in years) | | Gross
Carrying
Value | | Accumulated
Amortization | | Net
Carrying
Value | | Gross
Carrying
Value | | Accumulated
Amortization | | Net
Carrying
Value | |
|---|
Non-compete agreements | | | 5 | | $ | 14,000 | | $ | 6,212 | | $ | 7,788 | | $ | 14,000 | | $ | 3,412 | | $ | 10,588 | |
Consultant relationships | | | 3 | | | 980 | | | 980 | | | — | | | 980 | | | 980 | | | — | |
Patent | | | 5 | | | 180 | | | 180 | | | — | | | 180 | | | 153 | | | 27 | |
Other | | | 3 | | | 55 | | | 55 | | | — | | | 55 | | | 55 | | | — | |
| | | | | | | | | | | | | | | | | |
Total | | | | | $ | 15,215 | | $ | 7,427 | | $ | 7,788 | | $ | 15,215 | | $ | 4,600 | | $ | 10,615 | |
| | | | | | | | | | | | | | | | | |
Non-compete agreements represent the fair value of assets received in conjunction with the OrthoClear Agreement. These intangible assets are being amortized on a straight-line basis over the expected useful life of five years beginning in 2006. SeeNote 5 "Patients First Program and settlement costs" for additional information.
Consultant relationships and patents and other intangible assets represent the fair value of intangible assets acquired as the result of the acquisition of General Orthodontics, LLC ("GO") in 2005. Upon the integration of GO, Align included GO's consulting services in its clinical education and training programs under the name of Invisalign Consulting Services ("ICS"). During 2007, the Company announced the discontinuation of ICS, and the net carrying values of the consultant relationships and other intangible assets related to ICS were fully amortized as of December 31, 2007.
For the years ended December 31, 2008, 2007 and 2006, total amortization expense for intangible assets was $2.8 million, $3.2 million and $1.0 million respectively. The total estimated annual future amortization expense for these intangible assets is as follows (in thousands):
| | | | |
Years Ending December 31, | |
| |
|---|
2009 | | $ | 2,800 | |
2010 | | | 2,800 | |
2011 | | | 2,188 | |
| | | | |
Total | | $ | 7,788 | |
| | | | |
Note 7. Legal Proceedings
Ormco
On January 6, 2003, Ormco Corporation ("Ormco"), a division of Sybron Dental Specialties (a Danaher Corporation subsidiary), filed suit against the Company in the United States District Court for the Central District, Orange County Division, asserting infringement of certain patents. The complaint sought unspecified monetary damages and injunctive relief. On February 18, 2003, the Company answered the complaint and asserted counterclaims seeking a declaration by the Court of invalidity and non-infringement of the asserted patents. In addition, the Company counterclaimed for infringement of one of its patents, seeking unspecified monetary damages and injunctive relief. Ormco filed a reply to its counterclaims on March 10, 2003 and asserted counterclaims against the Company seeking a declaration by the Court of invalidity and non-infringement of the patent.
78
Table of Contents
ALIGN TECHNOLOGY, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Note 7. Legal Proceedings (Continued)
There have been two appeals. After the permanent injunction was entered, Ormco and Allesee Orthodontic Appliances, Inc. ("AOA") appealed that injunction and the orders of the District Court on summary judgment on which the injunction was based. Oral arguments took place on April 3, 2006. Following oral arguments, the U.S. Court of Appeals for the Federal Circuit ("CAFC") issued a ruling declaring two out of a total of seventy-one claims in the Company's US Patent No. 6,398,548 and four out of a total of ten claims in US Patent No. 6,554,611 to be invalid as "obvious." The CAFC's decision reverses the California District Court summary judgment order of validity.
The second appeal was from the final judgment. Ormco appealed the ruling of the District Court that 92 claims in four of its patents are not infringed by the Company and that the asserted claims are invalid. Align appealed the ruling of the District Court that certain claims of its 6,398,548 patent which were found to be infringed by Ormco's and AOA's Red, White & Blue appliances were invalid. The CAFC issued a ruling on August 24, 2007, affirming the District Court's ruling that 86 out of 92 claims in the four asserted Ormco patents are invalid and not infringed by Align. The CAFC reversed the District Court's non-infringement rulings on six claims in Ormco's 6,616,444 patent.
Ormco has filed a petition with the U.S. Supreme Court asking for an extension of time in which to file a petition for review by the U.S. Supreme Court with respect to the portion of the CAFC's opinion that affirmed the District Court's ruling of non-infringement and non-enablement of the 86 claims. The Supreme Court denied Ormco's petition, and the case on the six claims in Ormco's patent were returned to the District Court for a determination of validity and infringement of those claims. The District Court issued orders construing the claim terms at issue and granting the Company's motion to amend its answer and counterclaim to assert Ormco's patent is unenforceable due to inequitable conduct. The parties are currently conducting discovery. Trial on liability issues is scheduled for June 2, 2009.
On February 25, 2009, the District Court issued rulings on various Summary Judgment and expert related motions. In summary, the District Court granted one of Ormco's motions on one theory of infringement and granted the Company's motion on two theories of non-infringement. The Company's invalidity argument supported by over fifty prior art references was unaffected. The District Court also ruled that one of the Company's inequitable conduct theories should be resolved at trial. A finding of inequitable conduct at trial could render the six claims at issue and possibly the family of Ormco patents related to the 6,616,444 patent unenforceable.
Trial on liability issues is scheduled for June 2, 2009. Despite the District Court's ruling of infringement on one of Ormco's theories, if the jury finds Ormco's six claims to be invalid or unenforceable, there can be no liability for infringement. The Company intends to vigorously pursue its invalidity and inequitable conduct counterclaims at trial.
On May 18, 2007, Debra A. Weber filed a consumer class action lawsuit against Align, OrthoClear, Inc. and OrthoClear Holdings, Inc. (d/b/a OrthoClear, Inc.) in Syracuse, New York, U.S. District Court. The complaint alleges two causes of action against the OrthoClear defendants and one cause of action against Align for breach of contract. The cause of action against the Company, titled "Breach of Third Party Benefit Contract" references Align's agreement to make Invisalign treatment available to OrthoClear patients, alleging that the Company failed "to provide the promised treatment to Plaintiff or any of the class members".
79
Table of Contents
ALIGN TECHNOLOGY, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Note 7. Legal Proceedings (Continued)
On July 3, 2007, the Company filed an answer to the complaint and asserted 17 affirmative defenses. On July 20, 2007, the Company filed a motion for summary judgment on the Third Cause of Action (the only cause of action alleged against Align). On August 24, 2007, Weber filed a motion for class certification. On October 1, 2007, the Company filed an opposition to the motion for class certification and it is currently awaiting rulings from the Court. OrthoClear has filed a motion to dismiss. The initial case management conference and all discovery has been stayed pending the Court's decision on the motion for class certification, OrthoClear's motion to dismiss and the Company's motion for summary judgment.
Litigating claims of these types, whether or not ultimately determined in the Company's favor or settled by the Company, are costly and divert the efforts and attention of the Company's management and technical personnel from normal business operations. Any of these results from litigation could adversely affect the Company's results of operations. From time to time, the Company has received, and may again receive, letters from third parties drawing the Company's attention to their patent rights. While the Company does not believe that it infringes any such rights that have been brought to the Company's attention, there may be other more pertinent proprietary rights of which the Company is presently unaware.
Note 8. Credit Facilities
On December 5, 2008, the Company renegotiated and amended its existing credit facility with Comerica Bank. Under this revolving line of credit, the Company has $25.0 million of available borrowings with a maturity date of December 31, 2010. This credit facility requires a quick ratio covenant and also requires the Company to maintain a minimum unrestricted cash balance of $10.0 million. The interest rate on borrowings will range from Libor plus 1.5% to 2.0% depending upon the amount of unrestricted cash the Company maintains at Comerica Bank above the $10.0 million minimum.
As of December 31, 2008, the Company had no outstanding borrowings under this credit facility and is in compliance with the financial covenant.
Note 9. Commitments and Contingencies
Align rents its facilities and certain equipment and automobiles under non-cancelable operating lease arrangements. Facility leases expire at various dates through 2013 and provide for pre-negotiated fixed rental rates during the terms of the lease.
In February 2005, the Company renewed its Santa Clara headquarters lease allowing it to utilize the security deposit of $1.3 million paid at the inception of the lease on July 1, 2000, to reduce the monthly rent payment by $11,000. By the end of the lease term on June 30, 2010, the security deposit balance will be reduced to $0.6 million.
The Company has a facility in San Jose, Costa Rica. The facility comprises approximately 63,000 square feet of manufacturing and office space. The monthly rent for the Costa Rica facility is approximately $78,000. The Company renewed the lease in March 2008 for an additional five year term, which commenced in October 2008 and expires in September 2013.
The Company's European headquarters are located in Amsterdam, The Netherlands. On August 3, 2007, the original lease agreement was amended to expand its Amsterdam facility to approximately
80
Table of Contents
ALIGN TECHNOLOGY, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Note 9. Commitments and Contingencies (Continued)
16,000 square feet of office space. This lease will expire in June 2012, with an option to renew for an additional five year term. The Company may also terminate this lease in June 2012 for a fee of $125,000. The monthly rent for the Amsterdam facility is approximately $48,000.
The Company recognizes rent expense on a straight-line basis over the lease period, and has accrued for rent expense incurred but not paid. Total rent expense was $3.8 million, $3.4 million, and $3.8 million, for the years ended December 31, 2008, 2007 and 2006, respectively.
Minimum future lease payments for non-cancelable leases as of December 31, 2008, are as follows (in thousands):
| | | | |
Years Ending December 31, | |
| |
|---|
2009 | | $ | 3,234 | |
2010 | | | 2,411 | |
2011 | | | 1,794 | |
2012 | | | 1,373 | |
2013 | | | 875 | |
| | | | |
Total | | $ | 9,687 | |
| | | | |
In July 2008, the Company entered into an agreement in favor of and for the benefit of Elamex de Juarez, S.A. DE C.V., or Elamex, landlord to International Manufacturing Solutions Operaciones, S.R.L. ("IMS"), the Company's third party shelter services provider. Under this agreement, the Company guaranteed IMS' lease payments for its facility located in Juarez, Mexico. The monthly rent for this 68,000 square foot facility is $30,000 and the lease expires in July 2013. Pursuant to the guarantee, the Company is obligated to pay Elamex for any rental payments in default by IMS. During the year ended December 31, 2008, there were no rental payment defaults by IMS. On December 22, 2008, the Company notified IMS of its intention to terminate IMS' shelter services arrangement effective April 2009. At that time, the Company will become a direct manufacturer of its clear aligners at the facility in Juarez, Mexico, and the Company will also assume IMS' lease with Elamex.
Note 10. Stockholders' Equity
The Preferred Stock Rights Agreement (the "Rights Agreement") is intended to protect stockholders from unfair or coercive takeover practices. In accordance with the Rights Agreement, the Board of Directors declared a dividend distribution of one preferred stock purchase right (a "Right") for each outstanding share of Align's common stock to stockholders of record on November 22, 2005. Each Right entitles stockholders to buy one one-thousandth of a share of Align's Series A Participating Preferred Stock, par value $0.0001 per share, at an exercise price of $37.00, subject to adjustment. Rights will become exercisable in certain circumstances, including upon a person or group acquiring or announcing the intention to acquire beneficial ownership of 15% or more of the then outstanding common stock without the approval of the Board of Directors. Each holder of a Right will have the right to receive, upon exercise, shares of common stock having a value equal to two times the purchase price. The Rights will expire on November 22, 2015 or upon the exercise of the Rights, whichever occurs earlier.
81
Table of Contents
ALIGN TECHNOLOGY, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Note 10. Stockholders' Equity (Continued)
The holders of common stock are entitled to receive dividends whenever funds are legally available and when and if declared by the Board of Directors. The Company has never declared or paid dividends on its common stock.
Align's Employee Stock Purchase Plan (the "Purchase Plan") consists of overlapping twenty-four month offering periods with four six-month purchase periods in each offering period. Employees purchase shares at 85% of the fair market value of the common stock at either the beginning of the purchase period or the end of the purchase period, whichever price is lower. The Purchase Plan provides that the number of shares of the Company's common stock reserved for issuance thereunder will automatically increase on the first trading day of January in each calendar year by an amount equal to three percent (3%) of the total number of shares of common stock outstanding on the last trading day in December of the immediately preceding calendar year, with this annual increase not to exceed 1,500,000 shares. The maximum number of shares that can be granted under the Purchase Plan in any one year is 800,000 shares of common stock.
During the year ended December 31, 2008, 522,829 shares were issued under the Purchase Plan. As of December 31, 2008, the Company had reserved 11,933,456 shares of common stock for future issuance and 9,169,883 shares remain available for future issuance.
As of December 31, 2008, there was $2.6 million of total unamortized compensation costs related to employee stock purchases. These costs are expected to be recognized over a weighted average period of 0.6 years.
In May 2005, the 2005 Incentive plan ("2005 Plan") replaced the 2001 stock incentive Plan ("2001 Plan"). The 2005 Plan, which expires December 31, 2010, provides for the granting of incentive stock options, non-statutory stock options, restricted stock units, stock appreciation rights, performance units and performance shares. Employees, non-employee directors and consultants are eligible to receive grants under the 2005 Plan. The options are granted for periods not exceeding ten years and generally vest over 4 years with 25% vesting one year from the date of grant and 1/48th each month thereafter. The Plan Administrator may, however, grant options with different vesting schedules.
In 2006, the Compensation Committee of the Board of Directors approved the grant of restricted stock units (contracts that give the recipients the right to receive shares as the units vest) to its employees and director(s) in addition to stock options. Each restricted stock unit award generally vests over 4 years with 25% on the one year anniversary of the date of grant and 6.25% vesting quarterly thereafter. Each grant of a restricted stock unit will reduce shares available for grant by 2 shares. In October 2007, the Compensation Committee of the Board of Directors approved a change in vesting for prospective grants of restricted stock units to 25% annually.
The 2005 Plan has 9,983,379 shares of the Company's common stock reserved for issuance, plus up to an aggregate of 5,000,000 shares that have been or will be returned to the 2001 Plan as a result of termination of outstanding options or repurchase of shares granted under the 2001 Plan on or after March 28, 2005. As of December 31, 2008, 2,311,367 shares have been transferred to and 4,035,602 shares remain available for issuance under the 2005 Plan.
82
Table of Contents
ALIGN TECHNOLOGY, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Note 10. Stockholders' Equity (Continued)
In January 2001, the stockholders approved two option grants to purchase 1,000,000 shares of the Company's common stock at an exercise price of $15.00 per share to each of the Company's then Chief Executive Officer and President. The options were granted outside of the 1997 Equity Incentive Plan and prior to the adoption of the 2001 Plan or the 2005 Plan. As of January 1, 2006, 1,500,000 shares had been cancelled. The remaining 500,000 shares were cancelled in May 2006. As of December 31, 2008, no options to purchase shares of common stock were outstanding under these grants.
Activity for the years ended December 31, 2008, 2007 and 2006 under the stock option plans and the executive grants are set forth below (in thousands, except per share data):
| | | | | | | | | | | | | | |
| | Stock Options | |
|---|
| | Number of Shares
Underlying
Stock Options
(in thousands) | | Weighted
Average
Exercise
Price | | Weighted
Average
Remaining
Contractual
Term (in years) | | Aggregate
Intrinsic
Value
(in thousands) | |
|---|
Outstanding at December 31, 2005 | | | 11,304 | | $ | 8.76 | | | | | | | |
| | Granted | | | 1,794 | | | 8.74 | | | | | | | |
| | Exercised | | | (2,317 | ) | | 5.87 | | | | | | | |
| | Cancelled or expired | | | (1,603 | ) | | 12.37 | | | | | | | |
| | | | | | | | | | | | |
Outstanding at of December 31, 2006 | | | 9,178 | | $ | 8.86 | | | | | | | |
| | Granted | | | 1,517 | | | 18.97 | | | | | | | |
| | Exercised | | | (3,048 | ) | | 8.52 | | | | | | | |
| | Cancelled or expired | | | (514 | ) | | 11.13 | | | | | | | |
| | | | | | | | | | | | |
Outstanding at December 31, 2007 | | | 7,133 | | $ | 10.99 | | | | | | | |
| | Granted | | | 2,182 | | | 12.78 | | | | | | | |
| | Exercised | | | (912 | ) | | 6.50 | | | | | | | |
| | Cancelled or expired | | | (1,094 | ) | | 14.02 | | | | | | | |
| | | | | | | | | | | | |
Outstanding at of December 31, 2008 | | | 7,309 | | $ | 11.63 | | | 6.6 | | $ | 6,347 | |
| | | | | | | | | | |
Vested and expected to vest at December 31, 2008 | | | 7,131 | | $ | 11.58 | | | 6.6 | | $ | 6,335 | |
| | | | | | | | | | |
Exercisable at December 31, 2008 | | | 4,470 | | $ | 10.54 | | | 5.4 | | $ | 6,004 | |
| | | | | | | | | | |
The aggregate intrinsic value in the table above represents the total pre-tax intrinsic value (the difference between Align's closing stock price on the last trading day in 2008 and the exercise price, multiplied by the number of in-the-money options) that would have been received by the option holders had all option holders exercised their options on the last day of each fiscal year. This amount will fluctuate based on the fair market value of Align's stock. The total intrinsic value of stock options exercised for the years ended December 31, 2008, 2007 and 2006 was $5.2 million, $43.1 million, and $13.2 million, respectively. The Company issues new shares upon the exercise of options.
As of December 31, 2008, there was $19.7 million of total unamortized compensation costs related to stock options and these costs are expected to be recognized over a weighted average period of
83
Table of Contents
ALIGN TECHNOLOGY, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Note 10. Stockholders' Equity (Continued)
2.4 years. For the year ended December 31, 2008, the total recognized tax benefit from exercised options was $52,000.
The options outstanding and exercisable by exercise price at December 31, 2008 are as follows:
| | | | | | | | | | | | | | | | |
| | Options Outstanding | | Options Exercisable | |
|---|
Range of Exercise Prices | | Shares | | Weighted
Average
Remaining
Contractual
Term (in years) | | Weighted
Average
Exercise
Price | | Shares | | Weighted
Average
Exercise
Price | |
|---|
$ 0.15 – $ 6.57 | | | 1,235,676 | | | 3.74 | | $ | 5.19 | | | 1,178,148 | | $ | 5.13 | |
6.70 – 7.97 | | | 1,219,487 | | | 5.89 | | | 7.41 | | | 1,157,664 | | | 7.43 | |
8.13 – 12.30 | | | 1,241,471 | | | 7.08 | | | 9.43 | | | 700,616 | | | 9.02 | |
12.40 – 13.00 | | | 1,550,727 | | | 8.79 | | | 12.97 | | | 57,961 | | | 12.61 | |
13.04 – 17.88 | | | 1,173,427 | | | 7.88 | | | 17.39 | | | 531,161 | | | 17.32 | |
18.35 – 27.06 | | | 888,339 | | | 5.58 | | | 19.50 | | | 844,258 | | | 19.24 | |
| | | | | | | | | | | | |
| | | 7,309,127 | | | 6.63 | | $ | 11.63 | | | 4,469,808 | | $ | 10.54 | |
| | | | | | | | | | | | |
In 2006, the Company began granting restricted stock units ("RSUs"). The fair value of each unit is based on the Company's closing stock price on the date of grant. A summary of the nonvested shares for the years ended December 31, 2008, 2007 and 2006 is as follows (in thousands, except per share amounts):
| | | | | | | | | | | | | | |
| | Number of
Shares
Underlying RSUs
(in thousands) | | Weighted
Average Grant
Date Fair
Value | | Weighted Average
Remaining
Contractual Term
(in years) | | Aggregate
Intrinsic
Value | |
|---|
Nonvested as of December 31, 2005 | | | — | | $ | — | | | | | | | |
| | Granted | | | 442 | | | 8.69 | | | | | | | |
| | Vested and released | | | — | | | — | | | | | | | |
| | Forfeited | | | (23 | ) | | 8.22 | | | | | | | |
| | | | | | | | | | | | |
Nonvested as of December 31, 2006 | | | 419 | | $ | 8.71 | | | | | | | |
| | | | | | | | | | | | |
| | Granted | | | 480 | | | 19.17 | | | | | | | |
| | Vested and released | | | (178 | ) | | 9.27 | | | | | | | |
| | Forfeited | | | (70 | ) | | 13.31 | | | | | | | |
| | | | | | | | | | | | |
Nonvested as of December 31, 2007 | | | 651 | | $ | 15.78 | | | | | | | |
| | | | | | | | | | | | |
| | Granted | | | 685 | | | 12.78 | | | | | | | |
| | Vested and released | | | (258 | ) | | 15.55 | | | | | | | |
| | Forfeited | | | (206 | ) | | 15.00 | | | | | | | |
| | | | | | | | | | | | |
Nonvested as of December 31, 2008 | | | 872 | | $ | 13.68 | | | 1.5 | | $ | 7,628 | |
| | | | | | | | | | |
84
Table of Contents
ALIGN TECHNOLOGY, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Note 10. Stockholders' Equity (Continued)
The aggregate intrinsic value in the table above represents the total pre-tax intrinsic value (calculated by using Align's closing stock price on the last trading day of 2008 multiplied by the number of non-vested RSUs) that would have been received by the unit holders had all RSUs been vested and released on the last day of each fiscal year. This amount will fluctuate based on the fair market value of Align's stock. During 2008, of the 258,318 shares vested and released, approximately 42,816 vested shares were withheld for executive RSU tax payments, resulting in a net issuance of 215,502 shares.
The total intrinsic value of RSUs vested and released during 2008 and 2007 was $2.9 million and $3.4 million. There were no RSUs vested and released during 2006. As of December 31, 2008, there was $11.1 million of total unamortized compensation costs related to RSUs, and these costs are expected to be recognized over a weighted average period of 2.5 years.
Effective January 1, 2006, the Company adopted Statement of Financial Accounting Standards No. 123 (Revised 2004), "Share-based Payment" ("FAS 123R") using the modified prospective transition method, which requires the measurement and recognition of compensation expense for all share-based payment awards based on estimated fair values over the requisite service period. In accordance with the modified prospective transition method, our financial statements for the prior periods have not been restated to reflect and do not include the impact of FAS 123R stock options.
The fair value of stock options granted and the option component of the Purchase Plan shares were estimated at the grant date using the Black-Scholes option pricing model with the following weighted average assumptions:
| | | | | | | | | | |
| | 2008 | | 2007 | | 2006 | |
|---|
Stock options: | | | | | | | | | | |
Expected term (in years) | | | 4.4 | | | 4.5 | | | 5.0 | |
Expected volatility | | | 60.0 | % | | 68.0 | % | | 76.2 | % |
Risk-free interest rate | | | 2.8 | % | | 4.4 | % | | 4.6 | % |
Expected dividend | | | — | | | — | | | — | |
Weighted average fair value at grant date | | $ | 6.40 | | $ | 10.82 | | $ | 5.67 | |
Employee stock purchase plan: | | | | | | | | | | |
Expected term (in years) | | | 1.2 | | | 1.2 | | | 1.3 | |
Expected volatility | | | 67.2 | % | | 55.8 | % | | 48.2 | % |
Risk-free interest rate | | | 2.2 | % | | 4.8 | % | | 5.0 | % |
Expected dividend | | | — | | | — | | | — | |
Weighted average fair value at grant date | | $ | 4.89 | | $ | 9.42 | | $ | 2.69 | |
The expected term of stock options represents the weighted-average period the stock options are expected to remain outstanding. Upon the adoption of FAS 123R, the Company used a mid-point model to determine the expected term of stock options based on the Company's historical exercise, post-vesting cancellation experience, and the remaining contractual term of its outstanding options.
85
Table of Contents
ALIGN TECHNOLOGY, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Note 10. Stockholders' Equity (Continued)
For the years ended December 31, 2008 and 2007, the Company used its own historical volatility when determining the expected volatility. In 2006, the Company used a combination of historical volatility and peer group volatility in deriving its expected volatility assumption as allowed under FAS 123R and SAB 107.
The risk-free interest rate is based on the implied yield on a U.S. Treasury zero-coupon issue with a remaining term equal to the expected term of the option.
The dividend yield reflects that the Company has not paid any cash dividends since inception and does not anticipate paying cash dividends in the foreseeable future.
Stock-based compensation expense recognized in the Consolidated Statements of Operations for the years ended December 31, 2008, 2007 and 2006 is based on awards ultimately expected to vest, net of estimated forfeitures. Forfeitures are estimated based on historical experience at the time of grant and may be revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates. The stock-based compensation expense related to all of the Company's stock-based awards and employee stock purchases under FAS 123R for the years ended December 31, 2008, 2007, 2006 is as follows:
| | | | | | | | | | |
| | For the Years Ended December 31, | |
|---|
| | 2008 | | 2007 | | 2006 | |
|---|
| | (in thousands)
| |
|---|
Cost of revenues | | $ | 1,753 | | $ | 994 | | $ | 700 | |
Sales and marketing | | | 5,289 | | | 4,225 | | | 2,862 | |
General and administrative | | | 8,011 | | | 5,443 | | | 4,054 | |
Research and development | | | 2,004 | | | 1,549 | | | 1,294 | |
| | | | | | | | |
Total stock-based compensation | | $ | 17,057 | | $ | 12,211 | | $ | 8,910 | |
| | | | | | | | |
Note 11. Common Stock Repurchase Program
In April 2008, the Company's Board of Directors approved a common stock repurchase program authorizing management to repurchase up to $50 million of the Company's outstanding common stock. Purchases under the program were made, from time to time, in the open market. During 2008, the Company purchased approximately 4.7 million shares of common stock at an average price of $10.76 per share for an aggregate purchase price of $50.1 million including commissions and completed the stock repurchase program. The common stock repurchases reduced additional paid-in capital by $38.6 million and increased accumulated deficit by $11.6 million. All repurchased shares were retired.
Note 12. Employee Benefit Plan
In January 1999, the Company adopted a defined contribution retirement plan under Section 401(k) of the Internal Revenue Code. This plan covers substantially all employees who meet minimum age and service requirements and allows participants to defer a portion of their annual compensation on a pre-tax basis. Company contributions to the plan may be made at the discretion of
86
Table of Contents
ALIGN TECHNOLOGY, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Note 12. Employee Benefit Plan (Continued)
the Board of Directors. There have been no contributions by the Company since the inception of the plan.
Note 13. Income Taxes
Deferred tax assets and liabilities were as follows (in thousands):
| | | | | | | | |
| | Years Ended December 31, | |
|---|
| | 2008 | | 2007 | |
|---|
Deferred tax assets, net: | | | | | | | |
| | Net operating loss carryforwards | | $ | 48,583 | | $ | 73,440 | |
| | Credit carryforwards | | | 6,836 | | | 6,053 | |
| | Reserves & accruals | | | 4,807 | | | 5,006 | |
| | Depreciation and amortization | | | 6,100 | | | 6,843 | |
| | Other | | | 6,377 | | | 3,698 | |
| | | | | | |
| | $ | 72,703 | | $ | 95,040 | |
Deferred tax liabilities: | | | | | | | |
| | Prepaid expenses | | $ | 1,752 | | $ | 1,883 | |
| | Translation gains | | | 143 | | | — | |
| | | | | | |
| | | 1,895 | | | 1,883 | |
| | | | | | |
Net deferred tax assets before valuation allowance | | | 70,808 | | | 93,157 | |
| | Valuation allowance | | | (6,200 | ) | | (93,157 | ) |
| | | | | | |
Net deferred tax assets | | $ | 64,608 | | $ | — | |
| | | | | | |
With the exception of certain capital loss and foreign net operating loss carryforwards, the Company released the tax valuation allowance on most of the deferred tax assets and recorded an income tax benefit of $64.6 million for the year ended December 31, 2008. As of December 31, 2008, the Company believed, except for the items noted above, that the amount of deferred tax assets recorded on the balance sheet will be realized. However, should there be a change in the Company's ability to recover its deferred tax assets, the tax provision would increase in the period in which it is more likely than not that the Company cannot recover its deferred tax assets.
At December 31, 2008, the Company had net operating loss carryforwards of approximately $191.1 million for federal purposes and $68.1 million for California state tax purposes. If not utilized, these carryforwards will begin to expire beginning in 2020 for federal purposes and 2010 for California purposes.
The Company has research credit carryforwards of approximately $3.5 million for federal purposes and $4.3 million for California state tax purposes. If not utilized, the federal credit carryforwards will begin to expire in 2017. The California state credit can be carried forward indefinitely.
Effective January 1, 2007, the Company adopted FASB Interpretation No. 48, "Accounting for Uncertainty in Income Taxes" ("FIN 48"). This interpretation clarifies the criteria for recognizing income tax benefits under FASB Statement No. 109, "Accounting for Income Taxes", and requires additional disclosures about uncertain tax positions. Under FIN 48, the financial statement recognition
87
Table of Contents
ALIGN TECHNOLOGY, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Note 13. Income Taxes (Continued)
of the benefit for a tax position is dependent upon the benefit being more-likely-than-not to be sustainable upon audit by the applicable taxing authority. If this threshold is met, the tax benefit is then measured and recognized at the largest amount that is greater than 50 percent likely of being realized upon ultimate settlement.
The following is a rollforward of the Company's total gross unrecognized tax benefit for 2008 (in thousands):
| | | | | |
Balance as of January 1, 2008 | | $ | 2,816 | |
Tax positions related to current year: | | | | |
| | Additions for tax positions related to R&D credits | | | 314 | |
Tax positions related to prior year: | | | | |
| | Reductions for tax positions related to R&D credits | | | (168 | ) |
| | Other positions | | | (84 | ) |
| | | | |
Balance as of December 31, 2008 | | $ | 2,878 | |
| | | | |
The unrecognized tax benefits of $2.9 million include $0.3 million of uncertain tax positions that would impact the Company's effective tax rate if recognized. In accordance with FIN 48, the Company recognizes interest and penalties related to unrecognized tax benefits as a component of income taxes. Interest and penalties are immaterial at the date of adoption and are included in the unrecognized tax benefits.
The Company is subject to taxation in the U.S. and various states and foreign jurisdictions. All of the Company's tax years will be open to examination by the U.S. federal and most state tax authorities due to the Company's net operating loss and overall credit carryforward position. With few exceptions, the Company is no longer subject to examination by foreign tax authorities for years before 2004.
The differences between income taxes using the federal statutory income tax rate of 35% and the Company's effective tax rate were as follows:
| | | | | | | | | | |
| | Years Ended December 31, | |
|---|
| | 2008 | | 2007 | | 2006 | |
|---|
U.S. federal statutory income tax rate | | | 35.00 | % | | 35.00 | % | | 35.00 | % |
State income taxes, net of federal tax benefit | | | 7.74 | % | | 5.04 | % | | 5.15 | % |
Deferred tax benefits utilized | | | (67.04 | )% | | (44.53 | )% | | (21.59 | )% |
Foreign losses not benefited | | | 4.16 | % | | 2.40 | % | | (12.25 | )% |
Impact of differences in foreign tax rates | | | (8.13 | )% | | (7.23 | )% | | 1.27 | % |
Amortization of stock-based compensation | | | 32.34 | % | | 7.89 | % | | (7.67 | )% |
Non-deductible foreign exchange losses | | | (0.91 | )% | | — | | | (0.74 | )% |
Non-deductible meals & entertainment charges | | | 3.21 | % | | 1.21 | % | | (1.29 | )% |
Valuation allowance release | | | (378.34 | )% | | — | | | — | |
Other items not individually material | | | 3.57 | % | | 3.54 | % | | (0.31 | )% |
| | | | | | | | |
| | | (368.40 | )% | | 3.32 | % | | (2.43 | )% |
| | | | | | | | |
88
Table of Contents
ALIGN TECHNOLOGY, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Note 13. Income Taxes (Continued)
The provision for (benefit from) income taxes consisted of the following (in thousands):
| | | | | | | | | | | |
| | Years Ended December 31 | |
|---|
| | 2008 | | 2007 | | 2006 | |
|---|
Federal | | | | | | | | | | |
| | Current | | $ | 268 | | $ | 276 | | $ | (27 | ) |
| | Deferred | | | (56,934 | ) | | | | | | |
| | | | | | | | |
| | | (56,666 | ) | | 276 | | | (27 | ) |
| | | | | | | | |
State | | | | | | | | | | |
| | Current | | | 784 | | | 309 | | | 55 | |
| | Deferred | | | (7,674 | ) | | | | | | |
| | | | | | | | |
| | | (6,890 | ) | | 309 | | | 55 | |
| | | | | | | | |
Foreign | | | | | | | | | | |
| | Current | | | 645 | | | 641 | | | 800 | |
| | Deferred | | | — | | | — | | | — | |
| | | | | | | | |
| | | 645 | | | 641 | | | 800 | |
| | | | | | | | |
Provision for (benefit from) income taxes | | $ | (62,911 | ) | $ | 1,226 | | $ | 828 | |
| | | | | | | | |
Note 14. Net Profit (Loss) per Share
Basic net profit (loss) per share is computed using the weighted average number of shares of common stock during the year less unvested common shares subject to repurchase. Diluted net profit (loss) per share is computed using the weighted average number of shares of common stock, adjusted for the dilutive effect of potential common stock. Potential common stock, computed using the treasury stock method, includes options, restricted stock units, and the dilutive component of Purchase Plan shares.
The following table sets forth the computation of basic and diluted net profit (loss) per share attributable to common stock (in thousands, except per share amounts):
| | | | | | | | | | | | |
| | Years Ended December 31, | |
|---|
| | 2008 | | 2007 | | 2006 | |
|---|
Numerator: | | | | | | | | | | |
| | Net profit (loss) | | $ | 79,987 | | $ | 35,724 | | $ | (34,963 | ) |
| | | | | | | | |
Denominator: | | | | | | | | | | |
| | Weighted-average common shares outstanding, basic | | | 66,812 | | | 67,176 | | | 63,246 | |
| | Dilutive effect of potential common stock | | | 1,252 | | | 4,268 | | | — | |
| | | | | | | | |
| | | Total shares, diluted | | | 68,064 | | | 71,444 | | | 63,246 | |
| | | | | | | | |
| | | Net profit (loss) per share, basic | | $ | 1.20 | | $ | 0.53 | | $ | (0.55 | ) |
| | | | | | | | |
| | | Net profit (loss) per share, diluted | | $ | 1.18 | | $ | 0.50 | | $ | (0.55 | ) |
| | | | | | | | |
89
Table of Contents
ALIGN TECHNOLOGY, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Note 14. Net Profit (Loss) per Share (Continued)
For the years ended December 31, 2008, 2007, and 2006, stock options and restricted stock units totaling 5.1 million, 1.0 million, and 6.5 million respectively, were excluded from diluted net profit (loss) per share because of their anti-dilutive effect.
Note 15. Comprehensive Income (Loss)
Comprehensive income (loss) includes net profit (loss), foreign currency translation adjustments and unrealized gains and losses on available-for-sale securities. The components of comprehensive income (loss) are as follows (in thousands):
| | | | | | | | | | | |
| | Years Ended December 31, | |
|---|
| | 2008 | | 2007 | | 2006 | |
|---|
Net profit (loss) | | $ | 79,987 | | $ | 35,724 | | $ | (34,963 | ) |
| | Foreign currency translation adjustments | | | (421 | ) | | 703 | | | — | |
| | Unrealized gain/(loss) on available-for-sale securities | | | 33 | | | (49 | ) | | (4 | ) |
| | | | | | | | |
Comprehensive income (loss) | | $ | 79,599 | | $ | 36,378 | | $ | (34,967 | ) |
| | | | | | | | |
Note 16. Supplemental Cash Flow Information
The supplemental cash flow information consists of the following (in thousands):
| | | | | | | | | | | |
| | Years Ended December 31, | |
|---|
| | 2008 | | 2007 | | 2006 | |
|---|
Taxes paid | | $ | 1,510 | | $ | 1,632 | | $ | 992 | |
| | | | | | | | |
Interest paid | | | — | | $ | 415 | | $ | 223 | |
| | | | | | | | |
Non-cash investing and financing activities: | | | | | | | | | | |
| | Fixed assets acquired with accounts payable, accrued liabilities, or through financing | | $ | 1,322 | | $ | 1,135 | | $ | 541 | |
| | | | | | | | |
Note 17. Segments and Geographical Information
The Company reports segment data based on the management approach which designates the internal reporting that is used by management for making operating decisions and assessing performance as the source of the Company's reportable operating segments. During all periods presented, the Company operated as a single business segment.
90
Table of Contents
ALIGN TECHNOLOGY, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Note 17. Segments and Geographical Information (Continued)
Net revenues and long-lived assets are presented below by geographic area (in thousands):
| | | | | | | | | | | |
| | For the Years Ended December 31, | |
|---|
| | 2008 | | 2007 | | 2006 | |
|---|
Net revenues: | | | | | | | | | | |
| | North America | | $ | 240,210 | | $ | 236,758 | | $ | 172,635 | |
| | Europe | | | 61,652 | | | 45,047 | | | 28,076 | |
| | Other international | | | 2,114 | | | 2,527 | | | 5,643 | |
| | | | | | | | |
Total net revenues | | $ | 303,976 | | $ | 284,332 | | $ | 206,354 | |
| | | | | | | | |
| | | | | | | | |
| | As of December 31, | |
|---|
| | 2008 | | 2007 | |
|---|
Long-lived assets: | | | | | | | |
| | North America | | $ | 99,086 | | $ | 35,632 | |
| | Europe | | | 960 | | | 1,081 | |
| | Other international | | | 1,388 | | | 1,531 | |
| | | | | | |
Total long-lived assets | | $ | 101,434 | | $ | 38,244 | |
| | | | | | |
Note 18. Restructurings
During 2008, the Company announced restructuring plans in July and October to increase efficiencies across the organization and lower the overall cost structure. In July 2008, the Company implemented a restructuring plan to reduce its full time headcount by 67 employees including a phased-consolidation of order acquisition operations from its corporate headquarters in Santa Clara, California to Juarez, Mexico, which was completed by the end of 2008. The October restructuring plan included a total reduction of 111 full time headcount in Santa Clara, California by July 2009 as the Company moves its customer care, accounts receivable, credit and collections, and customer event registration organizations, in Santa Clara, California to existing facilities in Costa Rica.
In 2008, the Company incurred approximately $6.2 million in restructuring expenses relating to these actions which included $0.7 million related to the acceleration of stock option vesting and $5.5 million related to severance and termination benefits, of which $3.0 million was paid during the year.
The Company anticipates phasing in the relocation to Costa Rica of its shared services group in an attempt to minimize disruptions to customer service levels and expect the relocation to be completed by July 2009. The Company expects to incur additional restructuring charges of approximately $1.8 million during the first half of 2009.
91
Table of Contents
ALIGN TECHNOLOGY, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Note 18. Restructurings (Continued)
Activity and liability balances related to restructuring activity for the year ended December 31, 2008 are as follows (in thousands):
| | | | |
| | Severance and
Benefits | |
|---|
Balance at January 1, 2008 | | $ | — | |
Restructuring accrual | | | 5,568 | |
Cash payments | | | (3,067 | ) |
| | | | |
Balance at December 31, 2008 | | $ | 2,501 | |
| | | | |
The Company has included this amount in accrued liabilities in the Consolidated Balance Sheets.
92
Table of Contents
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
None
ITEM 9A. CONTROLS AND PROCEDURES
Under the supervision and with the participation of our management, including our Chief Executive Officer and our Chief Financial Officer, we have evaluated the effectiveness of the design and operation of our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act). Based upon that evaluation, our Chief Executive Officer and our Chief Financial Officer have concluded that our disclosure controls and procedures are effective as of December 31, 2008 to provide reasonable assurance that information required to be disclosed by us in the reports that we file or submit under the Exchange Act is accumulated and communicated to our management, including our Chief Executive Officer and our Chief Financial Officer, as appropriate, to allow timely decisions regarding required disclosure, and that such information is recorded, processed, summarized and reported within the time periods specified in the Securities and Exchange Commission rules and forms.
See "Report of Management on Internal Control over Financial Reporting" on page 59 of this Annual Report on Form 10-K, which is incorporated herein by reference.
There have been no changes in our internal control over financial reporting during the year ended December 31, 2008 that have materially affected or are reasonably likely to materially affect, our internal control over financial reporting.
ITEM 9B. OTHER INFORMATION
None.
PART III
Certain information required by Part III is omitted from this Form 10-K because we intend to file a definitive Proxy Statement for our 2009 Annual Meeting of Stockholders (the "Proxy Statement") not later than 120 days after the end of the fiscal year covered by this Annual Report on Form 10-K, and certain information to be included therein is incorporated herein by reference.
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
The information required by Item 401 of Regulation S-K concerning our directors is incorporated by reference to the Proxy Statement under the section captioned "Election of Directors." The information required by Item 401 of Regulation S-K concerning our executive officers is set forth in Item 1—"Business" of this Annual Report on Form 10-K. The information required by Item 405 of Regulation S-K is incorporated by reference to the section entitled "Section 16(a) Beneficial Ownership Reporting Compliance" contained in the Proxy Statement. The information required by Item 407(d)(5) of Regulation S-K is incorporated by reference to the Proxy Statement under the section entitled "Corporate Governance—Board of Directors & Committee Meetings—Audit Committee".
93
Table of Contents
Code of Ethics
We have a code of ethics that applies to all of our employees, including our principal executive officer, principal financial officer and principal accounting officer. This code of ethics is posted on our Internet website. The Internet address for our website iswww.aligntech.com, and the code of ethics may be found on the "Corporate Governance" section of our "Investor Relations" webpage.
We intend to satisfy the disclosure requirement under Item 5.05 of Form 8-K regarding an amendment to, or waiver from, a provision of this code of ethics by posting such information on our website, at the address and location specified above, or as otherwise required by the NASDAQ Global Market.
ITEM 11. EXECUTIVE COMPENSATION
The information required by Item 402 of Regulation S-K is incorporated by reference to the Proxy Statement under the section captioned "Executive Compensation". The information required by Items 407(e)(4) and (e)(5) is incorporated by reference to the Proxy Statement under the section captioned "Corporate Governance—Compensation Committee Interlocks" and "Compensation Committee Report", respectively.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
The information required by Item 403 of Regulation S-K is incorporated by reference to the Proxy Statement under the section captioned "Security Ownership of Certain Beneficial Owners and Management."
The following table provides information as of December 31, 2008 about our common stock that may be issued upon the exercise of options and rights granted to employees, consultants or members of our Board of Directors under all existing equity compensation plans, including the 1997 Equity Incentive Plan, the Employee Stock Purchase Plan, the 2001 Stock Incentive Plan and the 2005 Incentive Plan, each as amended, and certain individual arrangements.
| | | | | | | | | | |
Plan Category | | Number of
securities to be
issued upon
exercise of
outstanding
options and
restricted
stock units(a) | | Weighted average
exercise price of
outstanding
options(b) | | Number of securities
remaining available for
future issuance under
equity compensation plans
(excluding securities
reflected in column(a)) | |
|---|
Equity compensation plans approved by security holders | | | 8,180,904 | (1)(2) | $ | 11.63 | | | 13,205,485 | (3) |
Equity compensation plans not approved by security holders | | |
— | | |
— | | |
— | |
Total | | |
8,180,904 | |
$ |
11.63 | | |
13,205,485 | |
- (1)
- This number reflects the number of securities to be issued upon exercise of outstanding options and restricted stock units under the 1997 Equity Incentive Plan, the 2001 Stock Incentive Plan, and the 2005 Incentive Plan. The 871,777 restricted stock units included in this number have an exercise price of zero.
94
Table of Contents
- (2)
- We are unable to ascertain with specificity the number of securities to be issued upon exercise of outstanding rights under the Employee Stock Purchase Plan or the weighted average exercise price of outstanding rights under the Employee Stock Purchase Plan.
- (3)
- This number reflects securities available for future issuance under the 2005 Stock Incentive Plan and the Employee Stock Purchase Plan. In January 2001, all outstanding options under the 1997 Equity Incentive Plan were subsumed under the 2001 Stock Incentive Plan. Since that date no options have been granted under the 1997 Equity Incentive Plan. In May 2005, stockholder approval was obtained for the 2005 Incentive Plan and the 2001 Stock Incentive Plan was terminated. Since that date, no further options have been granted under the 2001 Stock Incentive Plan. The 2005 Incentive Plan has 9,983,379 shares of common stock reserved for issuance, plus up to an aggregate of 5,000,000 shares that are or would have been returned to the 2001 Stock Incentive Plan as a result of termination of outstanding options or repurchase of shares granted under the 2001 Stock Incentive Plan after March 28, 2005. As of December 31, 2008, 2,311,367 shares have been transferred to the 2005 Incentive Plan. As of December 31, 2008, the number of shares available for future issuance under the 2005 Incentive Plan was 4,035,602. Any grants of restricted stock units will reduce shares available for grant at a 2:1 ratio. The Employee Stock Purchase Plan provides that the number of shares of our common stock reserved for issuance thereunder will automatically increase on the first trading day of January in each calendar year by an amount equal to three percent (3%) of the total number of shares of common stock outstanding on the last trading day in December of the immediately preceding calendar year, with this annual increase not to exceed 1,500,000 shares. The maximum number of shares that can be granted under the Employee Stock Purchase Plan in any one year is 800,000 shares of common stock. As of December 31, 2008, the total number of shares of our common stock available for future issuance under the Employee Stock Purchase Plan was 9,169,883.
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
The information required by Item 404 and Item 407 of Regulation S-K is incorporated by reference to the Proxy Statement under the sections captioned "Certain Relationships and Related Party Transactions" and "Corporate Governance—Director Independence", respectively.
ITEM 14. PRINCIPAL ACCOUNTING FEES AND SERVICES
The information required by Item 9(e) of Schedule 14A of the Securities Act of 1934, as amended, is incorporated by reference to the Proxy Statement under the section captioned "Ratification of Appointment of Independent Registered Public Accountants."
95
Table of Contents
PART IV
ITEM 15. EXHIBITS, FINANCIAL STATEMENT SCHEDULES
- (a)
- Financial Statements
- 1.
- Consolidated Financial Statements
The following documents are filed as part of this Annual Report on Form 10-K:
| | | | | | |
| | | Report of Independent Registered Public Accounting Firm | | | 60 | |
| | | Consolidated Statements of Operations for the years ended December 31, 2008, 2007 and 2006 | | |
61 | |
| | | Consolidated Balance Sheets as of December 31, 2008 and 2007 | | |
62 | |
| | | Consolidated Statements of Stockholders' Equity for the years ended December 31, 2008, 2007 and 2006 | | |
63 | |
| | | Consolidated Statements of Cash Flows for the years ended December 31, 2008, 2007 and 2006 | | |
64 | |
| | | Notes to Consolidated Financial Statements | | |
65 | |
- 2.
- The following financial statement schedule is filed as part of this Annual Report on Form 10-K:
All other schedules have been omitted as they are not required, not applicable, or the required information is otherwise included.
96
Table of Contents
SCHEDULE II: VALUATION AND QUALIFYING ACCOUNTS AND RESERVES
| | | | | | | | | | | | | | | | | |
| | Balance
at
Beginning
of Period | | Additions
(reductions)
to Costs
and
Expenses | | Write
offs | | Reclass
from
Other
Accounts | | Balance
at End
of
Period | |
|---|
| | (in thousands)
| |
|---|
Allowance for doubtful accounts: | | | | | | | | | | | | | | | | |
| | Year ended December 31, 2006 | | $ | 1,626 | | $ | (332 | ) | $ | (450 | ) | $ | — | | $ | 844 | |
| | Year ended December 31, 2007 | | $ | 844 | | $ | 46 | | $ | (184 | ) | $ | 54 | | $ | 760 | |
| | Year ended December 31, 2008 | | $ | 760 | | $ | 71 | | $ | (184 | ) | $ | (35 | ) | $ | 612 | |
Allowance for deferred tax assets: | | | | | | | | | | | | | | | | |
| | Year ended December 31, 2006 | | $ | 89,988 | | $ | 12,165 | | $ | — | | $ | — | | $ | 102,153 | |
| | Year ended December 31, 2007 | | $ | 102,153 | | $ | (8,996 | ) | $ | — | | $ | — | | $ | 93,157 | |
| | Year ended December 31, 2008 | | $ | 93,157 | | $ | (86,957 | ) | $ | — | | $ | — | | $ | 6,200 | |
Allowance for excess and obsolete inventory and abandoned product: | | | | | | | | | | | | | | | | |
| | Year ended December 31, 2006 | | $ | 178 | | $ | 10 | | $ | — | | $ | — | | $ | 188 | |
| | Year ended December 31, 2007 | | $ | 188 | | $ | 47 | | $ | (19 | ) | $ | — | | $ | 216 | |
| | Year ended December 31, 2008 | | $ | 216 | | $ | 110 | | $ | (188 | ) | $ | — | | $ | 138 | |
97
Table of Contents
- (b)
- The following Exhibits are included in this Annual Report on Form 10-K:
| | | | | | | | | | | | |
Exhibit
Number | | Description | | Form | | Date | | Exhibit
Number
Incorporated
by reference
herein | | Filed herewith |
|---|
| | 3.1 | | Amended and Restated Certificate of Incorporation of registrant. | | Form S-1, as amended (File No. 333-49932) | | | 12/28/2000 | | 3.1 | | |
| | 3.2 | | Amended and Restated Bylaws of registrant. | | Form S-1, as amended (File No. 333-49932) | | |
12/28/2000 | | 3.2 | | |
| | 3.2A | | Amendment to Amended and Restated Bylaws of registrant | | Form 8-K (item 5.03 only) | | |
12/18/2007 | | 3.1 | | |
| | 3.3 | | Certificate of Designations of Rights, Preferences and Privileges of Series A Participating Preferred Stock registrant. | | Form 8-K | | |
10/27/2005 | | 3.1 | | |
| | 4.1 | | Form of Specimen Common Stock Certificate. | | Form S-1, as amended (File No. 333-49932) | | |
01/17/2001 | | 4.1 | | |
| | 4.2 | | Preferred Stock Rights Agreement dated October 25 between the registrant and EquiServe Trust Company, N.A. | | Form 8-K | | |
10/27/2005 | | 4.1 | | |
| | 10.1 | | Lease Agreement by and between James Lindsey and registrant, dated June 20, 2000, for office space located at 881 Martin Avenue, Santa Clara, CA. | | Form S-1, as amended (File No. 333-49932) | | |
11/14/2000 | | 10.4 | | |
| | 10.2 | | First Amendment to Lease Agreement dated February 2, 2005 for office space located at 881 Martin Avenue, Santa Clara, CA. | | Form 8-K | | |
02/09/2005 | | 10.1 | | |
| | 10.3 | | Lease Agreement dated August 30, 2001 by and between James S. Lindsey and registrant for office space located at 821 Martin Avenue, Santa Clara, CA. | | Form 10-K | | |
03/27/2003 | | 10.28 | | |
| | 10.4 | | First Amendment to Lease Agreement dated February 2, 2005 for office space located at 821 Martin Avenue, Santa Clara, CA. | | Form 8-K | | |
02/09/2005 | | 10.3 | | |
98
Table of Contents
| | | | | | | | | | | | |
Exhibit
Number | | Description | | Form | | Date | | Exhibit
Number
Incorporated
by reference
herein | | Filed herewith |
|---|
| | 10.5 | | Lease Agreement dated March 4, 2004 by and between James S. Lindsey and registrant for office space located at 831 Martin Avenue, Santa Clara, CA. | | Form 10-Q | | | 05/06/2004 | | 10.40 | | |
| | 10.6 | | First Amendment to Lease Agreement dated February 2, 2005 for office space located at 831 Martin Avenue, Santa Clara, CA. | | Form 8-K | | |
02/09/2005 | | 10.2 | | |
| | 10.7 | | Shelter Agreement dated December 22, 2005 between registrant and International Manufacturing Solutions Operaciones, S.R.L. | | Form 8-K | | |
12/28/2005 | | 10.1 | | |
| | 10.8 | | Amended and Restated Loan and Security Agreement dated December 16, 2005 between registrant and Comerica Bank. | | Form 8-K. | | |
12/19/2005 | | 10.1 | | |
| | 10.8A | | Amendment to Amended and Restated Loan and Security Agreement dated March 7, 2007 between registrant and Comerica Bank. | | Form 10-K | | |
03/12/2007 | | 10.8A | | |
| | 10.8B | | Amendment to Amended and Restated Loan and Security Agreement dated March 7, 2007 between registrant and Comerica Bank. | | Form 8-K | | |
04/29/2008 | | 10.1 | | |
| | 10.8C | | Amendment to Amended and Restated Loan and Security Agreement dated March 7, 2007 between registrant and Comerica Bank. | | Form 8-K | | |
01/13/2009 | | 10.1 | | |
| | 10.10† | | Registrant's 2001 Stock Incentive Plan. | | Form S-1 as amended (File No. 333-49932) | | |
12/28/2000 | | 10.13 | | |
| | 10.11† | | Form of option agreement under Align's 2001 Stock Incentive Plan. | | Form 10-Q | | |
11/05/2004 | | 10.13.1 | | |
| | 10.12† | | Registrant's Employee Stock Purchase Plan. | | Form S-8 | | |
02/05/2001 | | 99.2 | | |
99
Table of Contents
| | | | | | | | | | | | |
Exhibit
Number | | Description | | Form | | Date | | Exhibit
Number
Incorporated
by reference
herein | | Filed herewith |
|---|
| | 10.13† | | Form of Indemnification Agreement by and between registrant and its Board of Directors and its executive officers. | | Form S-1 as amended (File No. 333-49932) | | | 01/17/2001 | | 10.15 | | |
| | 10.14† | | Amended and restated 2005 Incentive Plan. | | Form 10-K | | |
03/12/2007 | | 10.14 | | |
| | 10.14A† | | Form of restricted stock unit award agreement under registrant's 2005 Incentive Plan | | Form 10-Q | | |
11/05/2007 | | 10.1A, 10.1B, 10.1C | | |
| | | | (General Form; Officer Form: Director Form). | | | | | | | | | |
| | 10.14B† | | Form of option award agreement under registrant's 2005 Incentive Plan. | | Form 10-Q | | |
08/04/2005 | | 10.4 | | |
| | 10.14C† | | Form of restricted stock unit award agreement under registrant's 2005 Incentive Plan with Thomas M. Prescott. | | Form 10-K | | |
03/12/2007 | | 10.14C | | |
| | 10.14D† | | Form of restricted stock unit award agreement amendment under registrant's 2005 Incentive Plan with Thomas M. Prescott. | | Form 10-K | | |
03/12/2007 | | 10.14D | | |
| | 10.15† | | Amended and Restated Employment Agreement dated May 5, 2008 between Thomas M. Prescott and registrant. | | Form 10-Q | | |
04/08/2008 | | 10.4 | | |
| | 10.16† | | Form of Employment Agreement entered into by and between registrant and each of executive officer (other than CEO). | | Form 10-Q | | |
05/08/2008 | | 10.3 | | |
| | 10.17† | | Amended and Restated Employment Agreement between registrant and Thomas M. Prescott dated May 5, 2008. | | Form 10-Q | | |
05/08/2008 | | 10.4 | | |
| | 10.18† | | Separation and Release Agreement between Afsaneh Azadeh and Align dated August 1, 2008. | | Form 10-Q. | | |
11/05/2008 | | 10.1 | | |
100
Table of Contents
| | | | | | | | | | | | |
Exhibit
Number | | Description | | Form | | Date | | Exhibit
Number
Incorporated
by reference
herein | | Filed herewith |
|---|
| | 10.19† | | Separation and Release Agreement between Sonia Clark and Align dated December 31, 2008. | | | | | | | | | * |
| | 10.20 | | Lease Agreement dated February 26, 2003 between KPMG FIDES (Costa Rica) S.A., Parque Global S.A.A. and registrant. | | Form 10-Q | | |
05/13/2003 | | 10.36 | | |
| | 10.20A | | Omnibus Amendment to Lease and Service Agreement between KPMG FIDES (Costa Rica) S.A., Parque Global S.A. and Align dated June 24, 2008. | | Form 8-K | | |
06/26/2008 | | 10.1 | | |
| | 10.21 | | Lease Agreement between Schootsepoort Onroerendgoed Beheer, for Stichting Philips Pensioenfonds and Align. | | Form 10-Q | | |
08/05/2004 | | 10.41 | | |
| | 10.21A | | Amendment to Lease Agreement between Align Technology, B.V. and TT Amsterdam Project Company (formerly Stichting Philips Pensioenfonds). | | Form 10-Q | | |
08/03/2007 | | 10.4 | | |
| | 10.22 | | Lease Agreement between International Manufacturing Solutions Operaciones, S.R.L. and Elamex de Juarez, S.A. de C.V. dated July 31, 2008 (assigned to Align as Lessee effective April 1, 2009). | | Form 8-K | | |
12/22/2008 | | 10.1 | | |
| | 10.23† | | Summary of 2008 Incentive Awards for Named Executive Officers. | | Form 8-K and Form 8-K/A | | |
01/13/2009 and 01/22/2009 | | Item 5.02 only | | |
| | 21.1 | | Subsidiaries of the registrant. | | | | | | | | | * |
| | 23.1 | | Consent of PricewaterhouseCoopers LLP, Independent Registered Public Accounting Firm. | | | | | | | | | * |
101
Table of Contents
| | | | | | | | | | | | |
Exhibit
Number | | Description | | Form | | Date | | Exhibit
Number
Incorporated
by reference
herein | | Filed herewith |
|---|
| | 31.1 | | Certifications of Chief Executive Officer pursuant to Exchange Act Rules 13a-14(a) and 15d-14(a), as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2003. | | | | | | | | | * |
| | 31.2 | | Certifications of Chief Financial Officer pursuant to Exchange Act Rules 13a-14(a) and 15d-14(a), as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2003. | | | | | | | | | * |
| | 32 | | Certification of Chief Executive Officer and Chief Financial Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2003. | | | | | | | | | * |
- †
- Management contract or compensatory plan or arrangement filed as an Exhibit to this form pursuant to Items 14(a) and 14(c) of Form 10-K.
102
Table of Contents
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized, on February 27, 2009.
| | | | |
| | | ALIGN TECHNOLOGY, INC. |
|
|
By: |
|
/s/ THOMAS M. PRESCOTT
Thomas M. Prescott
President and Chief Executive Officer |
Know All Men By These Presents, that each person whose signature appears below constitutes and appoints Thomas M. Prescott, his or her attorney-in-fact, with the power of substitution, for him or her in any and all capacities, to sign any amendments to this Report on Form 10-K and to file the same, with exhibits thereto and other documents in connection therewith, with the Securities and Exchange Commission, hereby ratifying and confirming all that each of said attorneys-in-fact, or his or her substitute or substitutes, may do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the Registrant and in the capacities and on the dates indicated.
| | | | |
Signature | | Title | | Date |
|---|
| | | | | |
/s/ THOMAS M. PRESCOTT
Thomas M. Prescott |
|
President and Chief Executive Officer (Principal Executive Officer) |
|
February 27, 2009 |
/s/ KENNETH B. AROLA
Kenneth B. Arola |
|
Chief Financial Officer and Vice President, Finance (Principal Financial Officer and Principal Accounting Officer) |
|
February 27, 2009 |
/s/ DAVID E. COLLINS
David E. Collins |
|
Director |
|
February 27, 2009 |
/s/ JOSEPH LACOB
Joseph Lacob |
|
Director |
|
February 27, 2009 |
/s/ C. RAYMOND LARKIN
C. Raymond Larkin |
|
Director |
|
February 27, 2009 |
/s/ GEORGE J. MORROW
George J. Morrow |
|
Director |
|
February 27, 2009 |
/s/ GREG J. SANTORA
Greg J. Santora |
|
Director |
|
February 27, 2009 |
/s/ WARREN S. THALER
Warren S. Thaler |
|
Director |
|
February 27, 2009 |
103
Table of Contents
Exhibit Index
| | | | | | | | | | | | |
Exhibit
Number | | Description | | Form | | Date | | Exhibit
Number
Incorporated
by reference
herein | | Filed
herewith |
|---|
| | 3.1 | | Amended and Restated Certificate of Incorporation of registrant. | | Form S-1, as amended (File No. 333-49932) | | | 12/28/2000 | | 3.1 | | |
| | 3.2 | | Amended and Restated Bylaws of registrant. | | Form S-1, as amended (File No. 333-49932) | | |
12/28/2000 | | 3.2 | | |
| | 3.2A | | Amendment to Amended and Restated Bylaws of registrant | | Form 8-K (item 5.03 only) | | |
12/18/2007 | | 3.1 | | |
| | 3.3 | | Certificate of Designations of Rights, Preferences and Privileges of Series A Participating Preferred Stock registrant. | | Form 8-K | | |
10/27/2005 | | 3.1 | | |
| | 4.1 | | Form of Specimen Common Stock Certificate. | | Form S-1, as amended (File No. 333-49932) | | |
01/17/2001 | | 4.1 | | |
| | 4.2 | | Preferred Stock Rights Agreement dated October 25 between the registrant and EquiServe Trust Company, N.A. | | Form 8-K | | |
10/27/2005 | | 4.1 | | |
| | 10.1 | | Lease Agreement by and between James Lindsey and registrant, dated June 20, 2000, for office space located at 881 Martin Avenue, Santa Clara, CA. | | Form S-1, as amended (File No. 333-49932) | | |
11/14/2000 | | 10.4 | | |
| | 10.2 | | First Amendment to Lease Agreement dated February 2, 2005 for office space located at 881 Martin Avenue, Santa Clara, CA. | | Form 8-K | | |
02/09/2005 | | 10.1 | | |
| | 10.3 | | Lease Agreement dated August 30, 2001 by and between James S. Lindsey and registrant for office space located at 821 Martin Avenue, Santa Clara, CA. | | Form 10-K | | |
03/27/2003 | | 10.28 | | |
| | 10.4 | | First Amendment to Lease Agreement dated February 2, 2005 for office space located at 821 Martin Avenue, Santa Clara, CA. | | Form 8-K | | |
02/09/2005 | | 10.3 | | |
Table of Contents
| | | | | | | | | | | | |
Exhibit
Number | | Description | | Form | | Date | | Exhibit
Number
Incorporated
by reference
herein | | Filed
herewith |
|---|
| | 10.5 | | Lease Agreement dated March 4, 2004 by and between James S. Lindsey and registrant for office space located at 831 Martin Avenue, Santa Clara, CA. | | Form 10-Q | | | 05/06/2004 | | 10.40 | | |
| | 10.6 | | First Amendment to Lease Agreement dated February 2, 2005 for office space located at 831 Martin Avenue, Santa Clara, CA. | | Form 8-K | | |
02/09/2005 | | 10.2 | | |
| | 10.7 | | Shelter Agreement dated December 22, 2005 between registrant and International Manufacturing Solutions Operaciones, S.R.L. | | Form 8-K | | |
12/28/2005 | | 10.1 | | |
| | 10.8 | | Amended and Restated Loan and Security Agreement dated December 16, 2005 between registrant and Comerica Bank. | | Form 8-K | | |
12/19/2005 | | 10.1 | | |
| | 10.8A | | Amendment to Amended and Restated Loan and Security Agreement dated March 7, 2007 between registrant and Comerica Bank. | | Form 10-K | | |
03/12/2007 | | 10.8A | | |
| | 10.8B | | Amendment to Amended and Restated Loan and Security Agreement dated March 7, 2007 between registrant and Comerica Bank. | | Form 8-K | | |
04/29/2008 | | 10.1 | | |
| | 10.8C | | Amendment to Amended and Restated Loan and Security Agreement dated March 7, 2007 between registrant and Comerica Bank. | | Form 8-K | | |
01/13/2009 | | 10.1 | | |
| | 10.10† | | Registrant's 2001 Stock Incentive Plan. | | Form S-1 as amended (File No. 333-49932) | | |
12/28/2000 | | 10.13 | | |
| | 10.11† | | Form of option agreement under Align's 2001 Stock Incentive Plan. | | Form 10-Q | | |
11/05/2004 | | 10.13.1 | | |
| | 10.12† | | Registrant's Employee Stock Purchase Plan. | | Form S-8 | | |
02/05/2001 | | 99.2 | | |
Table of Contents
| | | | | | | | | | | | |
Exhibit
Number | | Description | | Form | | Date | | Exhibit
Number
Incorporated
by reference
herein | | Filed
herewith |
|---|
| | 10.13† | | Form of Indemnification Agreement by and between registrant and its Board of Directors and its executive officers. | | Form S-1 as amended (File No. 333-49932) | | | 01/17/2001 | | 10.15 | | |
| | 10.14† | | Amended and restated 2005 Incentive Plan. | | Form 10-K | | |
03/12/2007 | | 10.14 | | |
| | 10.14A† | | Form of restricted stock unit award agreement under registrant's 2005 Incentive Plan. | | Form 10-Q | | |
11/05/2007 | | 10.1A, 10.1B, 10.1C | | |
| | | | (General Form; Officer Form: Director Form). | | | | | | | | | |
| | 10.14B† | | Form of option award agreement under registrant's 2005 Incentive Plan. | | Form 10-Q | | |
08/04/2005 | | 10.4 | | |
| | 10.14C† | | Form of restricted stock unit award agreement under registrant's 2005 Incentive Plan with Thomas M. Prescott. | | Form 10-K | | |
03/12/2007 | | 10.14C | | |
| | 10.14D† | | Form of restricted stock unit award agreement amendment under registrant's 2005 Incentive Plan with Thomas M. Prescott. | | Form 10-K | | |
03/12/2007 | | 10.14D | | |
| | 10.15† | | Amended and Restated Employment Agreement dated May 5, 2008 between Thomas M. Prescott and registrant. | | Form 10-Q | | |
04/08/2008 | | 10.4 | | |
| | 10.16† | | Form of Employment Agreement entered into by and between registrant and each of executive officer (other than CEO). | | Form 10-Q | | |
05/08/2008 | | 10.3 | | |
| | 10.17† | | Amended and Restated Employment Agreement between registrant and Thomas M. Prescott dated May 5, 2008. | | Form 10-Q | | |
05/08/2008 | | 10.4 | | |
| | 10.18† | | Separation and Release Agreement between Afsaneh Azadeh and Align dated August 1, 2008. | | Form 10-Q | | |
11/05/2008 | | 10.1 | | |
| | 10.19† | | Separation and Release Agreement between Sonia Clark and Align dated December 31, 2008. | | | | | | | | | * |
Table of Contents
| | | | | | | | | | | | |
Exhibit
Number | | Description | | Form | | Date | | Exhibit
Number
Incorporated
by reference
herein | | Filed
herewith |
|---|
| | 10.20 | | Lease Agreement dated February 26, 2003 between KPMG FIDES (Costa Rica) S.A., Parque Global S.A.A. and registrant. | | Form 10-Q | | | 05/13/2003 | | 10.36 | | |
| | 10.20A | | Omnibus Amendment to Lease and Service Agreement between KPMG FIDES (Costa Rica) S.A., Parque Global S.A. and Align dated June 24, 2008. | | Form 8-K | | |
06/26/2008 | | 10.1 | | |
| | 10.21 | | Lease Agreement between Schootsepoort Onroerendgoed Beheer, for Stichting Philips Pensioenfonds and Align. | | Form 10-Q | | |
08/05/2004 | | 10.41 | | |
| | 10.21A | | Amendment to Lease Agreement between Align Technology, B.V. and TT Amsterdam Project Company (formerly Stichting Philips Pensioenfonds). | | Form 10-Q | | |
08/03/2007 | | 10.4 | | |
| | 10.22 | | Lease Agreement between International Manufacturing Solutions Operaciones, S.R.L. and Elamex de Juarez, S.A. de C.V. dated July 31, 2008 (assigned to Align as Lessee effective April 1, 2009). | | Form 8-K | | |
12/22/2008 | | 10.1 | | |
| | 10.23† | | Summary of 2008 Incentive Awards for Named Executive Officers. | | Form 8-K and Form 8-K/A | | |
01/13/2009 and 01/22/2009 | | Item 5.02 only | | |
| | 21.1 | | Subsidiaries of the registrant. | | | | | | | | | * |
| | 23.1 | | Consent of PricewaterhouseCoopers LLP, Independent Registered Public Accounting Firm. | | | | | | | | | * |
| | 31.1 | | Certifications of Chief Executive Officer pursuant to Exchange Act Rules 13a-14(a) and 15d-14(a), as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2003. | | | | | | | | | * |
| | 31.2 | | Certifications of Chief Financial Officer pursuant to Exchange Act Rules 13a-14(a) and 15d-14(a), as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2003. | | | | | | | | | * |
Table of Contents
| | | | | | | | | | | | |
Exhibit
Number | | Description | | Form | | Date | | Exhibit
Number
Incorporated
by reference
herein | | Filed
herewith |
|---|
| | 32 | | Certification of Chief Executive Officer and Chief Financial Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2003. | | | | | | | | | * |
- †
- Management contract or compensatory plan or arrangement filed as an Exhibit to this form pursuant to Items 14(a) and 14(c) of Form 10-K.

