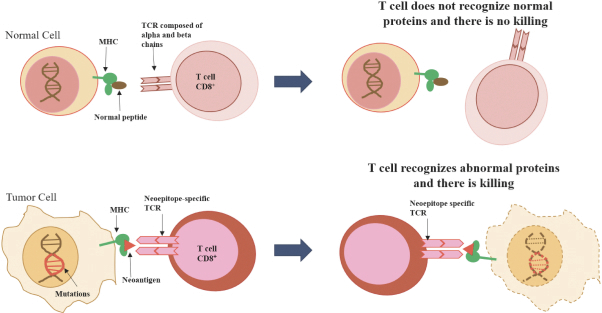
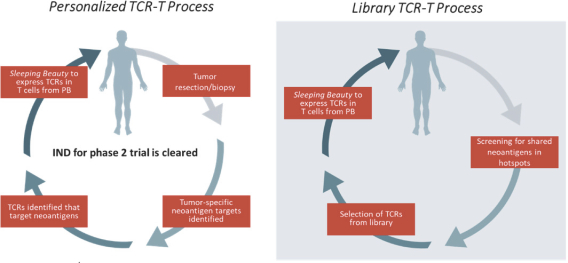
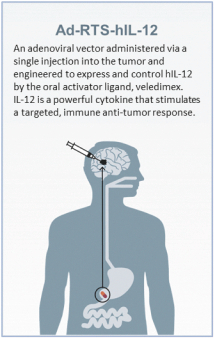
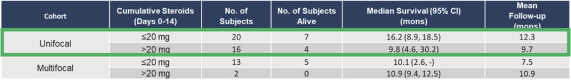
Free signup for more
- Track your favorite companies
- Receive email alerts for new filings
- Personalized dashboard of news and more
- Access all data and search results
Content analysis
?| Positive | ||
| Negative | ||
| Uncertain | ||
| Constraining | ||
| Legalese | ||
| Litigious | ||
| Readability |
H.S. sophomore Avg
|
|
New words:
accuracy, administrationof, afund, alloTCR, amountof, andBeneficial, andscalable, andsubsequent, apotent, arepursuing, ArsenalBio, asControlled, astargeting, autoTCR, baseline, believeControlled, benchmark, bleeding, BMT, borrow, borrowing, buildout, cemiplimab, choice, Circuit, cleared, ClinicalDevelopment, combinationof, combinationwith, compensatory, compliant, conditionallyproduce, customary, de, deficiency, developingControlled, EGFR, encourageuse, expresshuman, expressionof, GMP, Gorilla, Hadfield, havetested, inBlood, inClinical, incompetent, incremental, indefinitely, indemnification, India, inScience, intravenously, Iovance, Istari, Karyopharm, lab, left, libraryof, lieu, Lyell, Main, manufacturedusing, match, matching, membrane, MRI, multifocal, multitude, nonfinancial, Northwest, Novocure, Obsidian, ofControlled, ofin, ofrecombinant, ofTCR, online, Optune, ourcontrolled, PACT, partly, PB, PBMC, PGEN, potentialof, PrincipalAccounting, productionof, proper, Protheragen, pseudoprogression, reevaluate, reliant, remanded, rolling, Sath, Satyavrat, Schematic, Shukla, sixty, sublicensee, subscription, substudy, surgical, survivein, swelling, Takeda, thata, thatControlled, thelowest, Therecombinant, thisway, titledBeneficial, titledProposal, tocause, todeliver, toevaluate, Torque, transcription, transcriptional, trialof, unifocal, unilaterally, unpatentable, unqualified, upheld, useof, usinga, vendor, VIE, withRegeneron, withSleeping, worse
Removed:
aberrant, add, alive, amending, amortized, andStock, attestation, bifurcated, clinic, collateral, column, constructing, consume, dilution, diversity, dosed, double, engineer, escalation, evolving, executed, explore, favorably, feature, forin, hospital, importing, indibulin, Indonesia, inventory, issuable, Juno, landlord, LLP, Malaysia, nanosuspension, Neon, noncash, NY, organoarsenic, poster, predecessor, preliminary, proportion, pursued, redemption, representing, restatement, RSM, San, Singapore, structure, substance, subtenant, sufficiency, sustainedin, systemic, Thailand, titledStock, today, towardsin, underRule, vivopersistence, voting, York, Zealand
Filing tables
Filing exhibits
Related press release
Associated TCRT transcripts
TCRT similar filings
Filing view
External links