UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 10-K | | | | | | | | |
| ☑ | | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 29, 2024
or
| | | | | | | | |
| ☐ | | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
| | For the transition period from to |
Commission file number: 001-35406
Illumina, Inc.
(Exact name of registrant as specified in its charter)
| | | | | | | | |
| Delaware | | 33-0804655 |
| (State or other jurisdiction of incorporation or organization) | (I.R.S. Employer Identification No.) |
| |
| |
5200 Illumina Way, San Diego, CA 92122
(Address of principal executive offices) (Zip code)
Registrant’s telephone number, including area code: (858) 202-4500
Securities registered pursuant to Section 12(b) of the Act:
| | | | | | | | |
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered |
| Common Stock, $0.01 par value | ILMN | The Nasdaq Stock Market LLC |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes þ No o
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes o No þ
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes þ No o
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes þ No o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Large accelerated filer | þ | Accelerated filer | ☐ | Non-accelerated filer | ☐ | Smaller reporting company | ☐ | Emerging growth company | ☐ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13a of the Exchange Act. o
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ☑
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements.o
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b).o
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No þ
As of February 7, 2025, there were 158.4 million shares (excluding 41.8 million shares held in treasury) of the registrant’s common stock outstanding. The aggregate market value of the common stock held by non-affiliates of the registrant as of June 30, 2024 (the last business day of the registrant’s most recently completed second quarter), based on the closing price for the common stock on The Nasdaq Global Select Market on June 28, 2024 (the last trading day before June 30, 2024), was $14.7 billion. This excludes an aggregate of 18.3 million shares of common stock held by officers and directors and each person known by the registrant to own 10% or more of the outstanding common stock. Exclusion of shares held by any person should not be construed to indicate that such person possesses the power, directly or indirectly, to direct or cause the direction of the management or policies of the registrant, or that the registrant is controlled by or under common control with such person.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the registrant’s proxy statement for the 2025 annual meeting of stockholders are incorporated by reference into Items 10 through 14 of Part III of this Report.
ILLUMINA, INC.
FORM 10-K
FOR THE FISCAL YEAR ENDED DECEMBER 29, 2024
TABLE OF CONTENTS
| | | | | |
| BUSINESS & MARKET INFORMATION | PAGE |
| |
| |
| |
| |
| |
| |
| |
| MANAGEMENT’S DISCUSSION & ANALYSIS | |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| CONSOLIDATED FINANCIAL STATEMENTS | |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| OTHER KEY INFORMATION | |
| |
| |
| |
| |
| |
| |
| |
| |
| |
See “Form 10-K Cross-Reference Index” within Other Key Information for a cross-reference to the parts and items requirements of the Securities and Exchange Commission Annual Report on Form 10-K.
Consideration Regarding Forward-Looking Statements
This annual report on Form 10-K contains, and our officers and representatives may from time to time make, “forward-looking statements” within the meaning of the safe harbor provisions of the U.S. Private Securities Litigation Reform Act of 1995. Words such as: “anticipate,” “intend,” “plan,” “goal,” “seek,” “believe,” “continue,” “project,” “estimate,” “expect,” “strategy,” “future,” “likely,” “may,” “potential,” “predict,” “should,” “will,” or similar words or phrases, or the negatives of these words, may identify forward-looking statements, but the absence of these words does not necessarily mean that a statement is not forward looking. Examples of forward-looking statements include, among others, statements we make regarding:
•our expectations as to our future financial performance, results of operations, or other operational results or metrics;
•the benefits that we expect will result from our business activities and certain transactions we have completed, or may complete, such as product introductions, increased revenue, decreased expenses, and avoided expenses and expenditures;
•our expectations of the effect on our financial condition of claims, litigation, contingent liabilities, and governmental investigations, proceedings, and regulations;
•our strategies or expectations for product development, market position, financial results, and reserves;
•our ability to successfully implement cost reduction plans in a timely manner and the possibility that costs associated with our cost reduction plans are greater than we anticipate;
•risks relating to the recent divestiture of GRAIL, Inc. (f/k/a GRAIL, LLC) (GRAIL); and
•other expectations, beliefs, plans, strategies, anticipated developments, and other matters that are not historical facts.
Forward-looking statements are neither historical facts nor assurances of future performance. Instead, they are based only on our current beliefs, expectations, and assumptions regarding the future of our business, future plans and strategies, projections, anticipated events and trends, the economy, and other future conditions. Because forward-looking statements relate to the future, they are subject to inherent uncertainties, risks, and changes in circumstances that are difficult to predict and many of which are outside of our control. Our actual results and financial condition may differ materially from those indicated in the forward-looking statements. Therefore, you should not rely on any of these forward-looking statements. Important factors that could cause our actual results and financial condition to differ materially from those indicated in the forward-looking statements include, among others, the following:
•our expectations and beliefs regarding prospects and growth for our business and the markets in which we operate;
•the timing and mix of customer orders among our products and services;
•challenges inherent in developing, manufacturing, and launching new products and services, including expanding manufacturing operations and reliance on third-party suppliers for critical components;
•uncertainty regarding the impact of our recent inclusion by the China Ministry of Commerce announcement that Illumina is included on its “unreliable entities list”;
•any reductions or potential reductions in funding for the National Institutes of Health, or targeted cancellations by the U.S. federal government of certain grants or contracts, could negatively impact our customers and reduce demand for our products and services;
•the impact of recently launched or pre-announced products and services on existing products and services;
•uncertainty regarding, or potential changes in, diplomatic and trade relationships, for example, as a result of the recent change in the U.S. government administration;
•risks and uncertainties regarding legal and regulatory proceedings;
•the impact of tariffs recently imposed by the U.S. government and its trading partners in response, other possible tariffs or trade protection measures, import or export licensing requirements, new or different customs duties, trade embargoes and sanctions and other trade barriers;
•risks associated with contracts or other agreements containing provisions that might be implicated by the divestiture of GRAIL, including our ability to fully realize the anticipated economic benefits of our commercial arrangements with GRAIL and our obligations with respect to contingent value rights (the CVRs) issued by us in connection with the GRAIL acquisition, which may adversely affect us and our business and/or the market value of the CVRs;
•the risk of additional litigation arising against us in connection with the GRAIL acquisition;
•the assumptions underlying our critical accounting policies and estimates;
•our assessments and estimates that determine our effective tax rate;
•our assessments and beliefs regarding the outcome of pending legal proceedings and any liability that we may incur as a result of those proceedings, as well as the cost and potential diversion of management resources associated with these proceedings;
•uncertainty, or adverse economic and business conditions, including as a result of slowing or uncertain economic growth, public health crisis, or armed conflict; and
•other factors detailed in our filings with the Securities and Exchange Commission (SEC), including the risks, uncertainties, and assumptions described in Risk Factors within the Business & Market Information section of this report, or in information disclosed in public conference calls, the date and time of which are released beforehand. Any forward-looking statement made by us in this annual report on Form 10-K is based only on information currently available to us and speaks only as of the date on which it is made. We undertake no obligation, and do not intend, to publicly update any forward-looking statement, whether written or oral, that may be made from time to time, or to review or confirm analysts’ expectations, or to provide interim reports or updates on the progress of any current financial quarter, in each case whether as a result of new information, future developments, or otherwise.
Available Information
Our annual report on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, and all amendments to those reports are available free of charge on our website, www.illumina.com. The information on our website is not incorporated by reference into this report. Such reports are made available as soon as reasonably practicable after filing with, or furnishing to, the SEC. The SEC also maintains an Internet site at www.sec.gov that contains reports, proxy and information statements, and other information regarding issuers that electronically file with the SEC. Copies of our annual report on Form 10-K will be made available, free of charge, upon written request.
_______________________________________
Assign, BaseSpace, BeadArray, Bluebee, BlueFuse, BlueGnome, cBot, Clarity LIMS, CircLigase, COVIDSeq, DesignStudio, DRAGEN, DRAGEN ORA, Emedgene, Enancio, FastTrack, Flow, Fluent Biosciences, Genetic Energy, GenomeStudio, Genomics Suite, Golden Gate, HiSeq, iHope, Illumina, Illumina Connected Analytics, Illumina Propel Certified, Infinium, iScan, iSelect, iSeq, MiniSeq, MiSeq, MiSeq FGx, Nextera, NextSeq, NovaSeq, Partek, Pattern Visualization System, PIPseq, Powered by Illumina, Praxis, Ribo-Zero, SureCell, The Analytical Spreadsheet, TruGenome, TruSeq, TruSight, Turning Data Into Discovery, Verifi, Verinata, Verinata Health, VeriSeq, XLEAP-SBS, the pumpkin orange color, and the Genetic Energy / streaming bases design are trademarks or registered trademarks of Illumina, Inc.
“GRAIL,” the GRAIL logos, and other trade names, trademarks, or service marks of GRAIL are the property of GRAIL. The “Galleri” mark and logo are registered in numerous countries including the United States and the United Kingdom. Applications to register the “Galleri” mark and logo, the “GRAIL” mark and the logo, and marks associated with GRAIL are also pending in a variety of countries.
_______________________________________
Unless the context requires otherwise, references in this annual report on Form 10-K to “Illumina,” the “Company,” “we,” “us,” and “our” refer to Illumina, Inc. and its consolidated subsidiaries.
_______________________________________
Our fiscal year is the 52 or 53 weeks ending the Sunday closest to December 31, with quarters of 13 or 14 weeks ending the Sunday closest to March 31, June 30, September 30, and December 31. References to 2024, 2023, and 2022 refer to fiscal years ended December 29, 2024, December 31, 2023, and January 1, 2023, respectively, which were all 52 weeks.
| | |
| BUSINESS & MARKET INFORMATION |
BUSINESS OVERVIEW
We are a global leader in sequencing- and array-based solutions for genetic and genomic analysis. Our products and services serve customers in a wide range of markets, enabling the adoption of genomic solutions in research and clinical settings. We were incorporated in California in April 1998 and reincorporated in Delaware in July 2000. Our principal executive offices are located at 5200 Illumina Way, San Diego, California 92122.
Our customers include leading genomic research centers, academic institutions, government laboratories, and hospitals, as well as pharmaceutical, biotechnology, commercial molecular diagnostic laboratories, and consumer genomics companies. Our portfolio of integrated sequencing and microarray systems, consumables, and analysis tools is designed to accelerate and simplify genetic analysis and addresses the range of genomic complexity, price points, and throughput, enabling customers to select the best solution for their research or clinical application.
On June 24, 2024, we completed the separation (the Spin-Off) of GRAIL into a new public company through the distribution of 26,547,021 shares of GRAIL common stock to Illumina stockholders on a pro rata basis. The distribution reflected approximately 85.5% of the outstanding common stock of GRAIL as of 5:00 p.m. New York time on June 13, 2024, the record date for the distribution (the Record Date). We retained approximately 14.5% of the shares of GRAIL common stock immediately following the Spin-Off. The disposition of GRAIL did not meet the criteria to be reported as a discontinued operation and accordingly, GRAIL’s assets, liabilities, results of operations and cash flows have not been reclassified. Refer to note 2. GRAIL Spin-Off for additional details. Genetics Primer
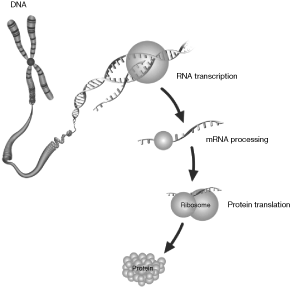
The instruction set for all living cells is encoded in deoxyribonucleic acid, or DNA. The complete set of DNA for any organism is referred to as its genome. DNA contains small regions called genes, which comprise a string of nucleotide bases labeled A, C, G, and T, representing adenine, cytosine, guanine, and thymine, respectively. These nucleotide bases occur in a precise order known as the DNA sequence. When a gene is “expressed,” a copy of a portion of its DNA sequence called messenger RNA (mRNA) is used as a template to direct the synthesis of a particular protein. Proteins, in turn, direct all cellular function. The illustration below is a simplified gene expression schematic.
Variations among organisms are due, in large part, to differences in their DNA sequences. Changes can result from insertions, deletions, inversions, translocations, or duplications of nucleotide bases. These changes may result in certain genes becoming overexpressed (excessive protein production), underexpressed (reduced protein production), or silenced altogether, sometimes triggering changes in cellular function. The most common form of variation in humans is called a single nucleotide polymorphism (SNP), which is a base change in a single position in a DNA sequence. Another type of variation, copy number variations (CNVs), occur when there are fewer or more copies of certain genes, segments of a gene, or stretches of DNA. In humans, genetic variation accounts for many of the physical differences we see (e.g., height, hair, eye color, etc.). Genetic variations also can have medical consequences affecting disease susceptibility, including predisposition to complex genetic diseases such as cancer,
diabetes, cardiovascular disease, and Alzheimer’s disease. They can affect individuals’ response to certain drug treatments, causing them to respond well, experience adverse side effects, or not respond at all.
Scientists are studying these variations and their consequences in humans, as well as in a broad range of animals, plants, and microorganisms. Such research takes place in government, university, pharmaceutical, biotechnology, and agrigenomics laboratories around the world, where scientists expand our knowledge of the biological functions essential for life. Beginning at the genetic level, our tools are used to elucidate the relationship between gene sequence and biological processes. Researchers who investigate human and non-human genetic variation to understand the mechanisms of disease are enabling the development of more effective diagnostics and therapeutics. They also provide greater insight into genetic variation in plants (e.g., food and biofuel crops) and animals (e.g., livestock and domestic), enabling improvements in crop yields and animal breeding programs.
By empowering genetic analysis and facilitating a deeper understanding of genetic variation and function, our tools advance disease research, drug development, and the creation of molecular diagnostic tests. We believe that this will trigger a fundamental shift in the practice of medicine and health care, and that the increased emphasis on preventive and predictive molecular medicine will usher in the era of precision health care.
Our Principal Markets
We target the markets and customers outlined below.
Research and Applied
Historically, our core business has been in the life sciences research market. This includes laboratories associated with universities, research centers, and government institutions, along with biotechnology and pharmaceutical companies. Researchers at these institutions use our products and services for basic and translational research across a spectrum of scientific applications, including targeted, exome, and whole-genome sequencing; genetic variation; gene expression; epigenetics; and metagenomics. Next-generation sequencing (NGS) technologies are being adopted due to their ability to cost-effectively sequence large sample sizes quickly and accurately, generating vast amounts of high-quality data. Both private and public funding drive this research, along with global initiatives to characterize genetic variation.
Our products also serve various applied markets including consumer genomics and agrigenomics. For example, in consumer genomics, our customers use our technologies to provide personalized genetic data and analysis to individual consumers. In agrigenomics, government and corporate researchers use our products and services to explore the genetic and biological basis for productivity and nutritional constitution in crops and livestock. Researchers can identify natural and novel genomic variation and deploy genome-wide marker-based applications to accelerate breeding and production of healthier and higher-yielding crops and livestock.
Clinical
We are focused on enabling translational and clinical markets through the introduction of best-in-class sequencing technology. Further, we are developing sample-to-answer solutions to catalyze adoption in the clinical setting, including in reproductive and genetic health and oncology. In reproductive health, our primary focus is driving noninvasive prenatal testing (NIPT) adoption globally through our technology, which identifies fetal chromosomal abnormalities by analyzing cell-free DNA in maternal blood. Our NGS technology is also accelerating rare and undiagnosed disease research to discover the genetic causes of inherited disorders by assessing many genes simultaneously. Using NGS can reduce costs compared to traditional methods of disease diagnosis, which are often expensive and inconclusive while requiring extensive testing.
Cancer is a disease of the genome, and the goal of cancer genomics is to identify genomic changes that transform a normal cell into a cancerous one. Understanding these genomic changes will improve diagnostic accuracy, increase understanding of the prognosis, and enable oncologists to target therapies to individuals. Customers in the translational and clinical oncology markets use our products to perform research that may help identify individuals who are genetically predisposed to cancer and to identify molecular changes in a tumor. We believe that circulating tumor DNA (ctDNA) will become an important clinical tool for managing oncology patients during all stages of tumor progression. Our technology is being used to research the implications of ctDNA in treatment determination, treatment monitoring, minimal residual disease, and asymptomatic screening. For example, GRAIL’s Galleri blood test for early-stage cancer detection is enabled by our sequencing technology.
Our Principal Products, Services, and Technologies
Our unique technology platforms support the scale of experimentation necessary for population-scale studies, genome-wide discovery, target selection, and validation studies (see Figure 1 below). Customers use our products to analyze the genome at all levels of complexity, from targeted panels to whole-genome sequencing. A large and dynamic Illumina user community has published hundreds of thousands of customer-authored scientific papers using our technologies. Through rapid innovation, we are changing the economics of genetic research, enabling projects that were previously considered impossible, and supporting clinical advances towards precision medicine.
Most of our product sales consist of sequencing- and array-based instruments and consumables, which include reagents, flow cells, and library preparation, based on our proprietary technologies. We also perform various services for our customers. In 2024, 2023, and 2022, instrument sales represented 12%, 16%, and 16%, respectively, of total consolidated revenue; consumable sales represented 72%, 68%, and 70%, respectively, of total consolidated revenue; and services represented 16%, 16%, and 14%, respectively, of total consolidated revenue.
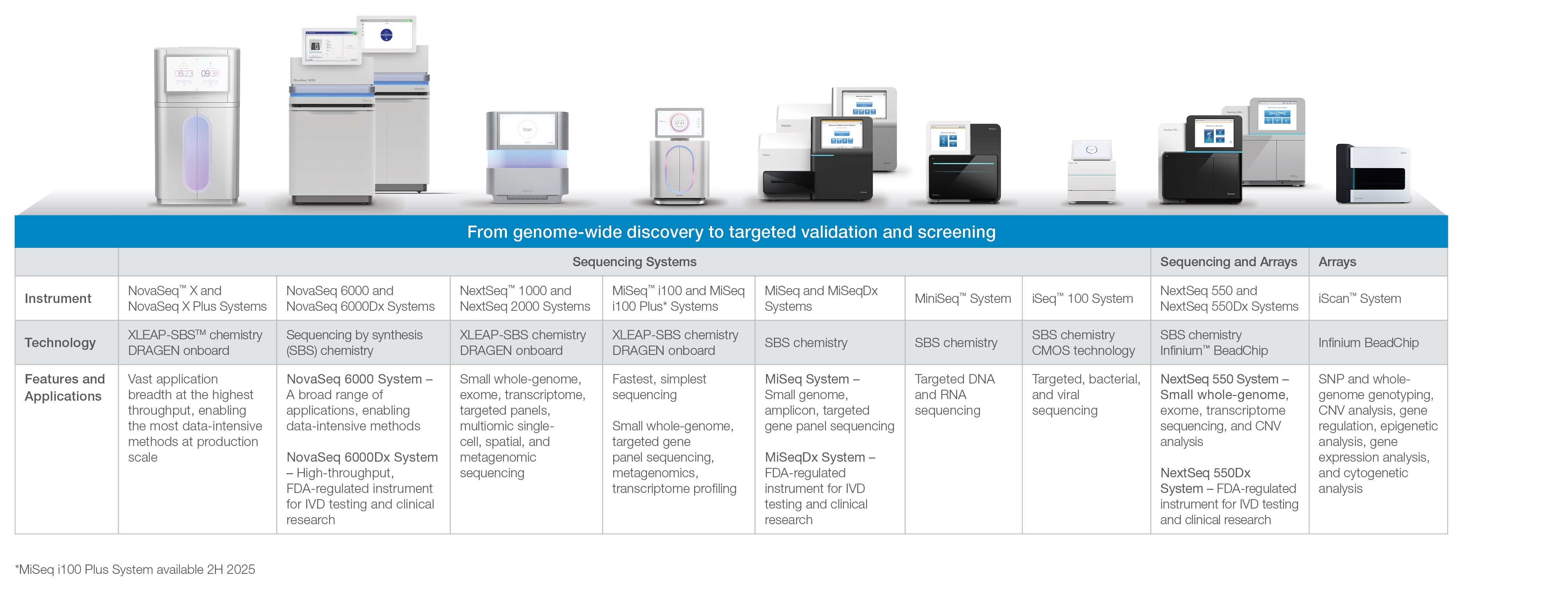
Figure 1: Illumina Platform Overview:
Sequencing
DNA sequencing is the process of determining the order of nucleotide bases (A, C, G, or T) in a DNA sample. Our portfolio of sequencing platforms represents a family of systems that we believe set the standard for productivity, cost-effectiveness, and accuracy among NGS technologies. Customers use our platforms to perform whole-genome, de novo, exome and RNA sequencing, and targeted resequencing of specific gene regions and genes.
Whole-genome sequencing determines the complete DNA sequence of an organism. In de novo sequencing, the goal is to sequence and assemble the genome of that sample without using information from prior sequencing of that species. In targeted resequencing, a portion of the sequence of an organism is compared to a standard or reference sequence from previously sequenced samples to identify genetic variation. Understanding the similarities and differences in DNA sequence between and within species helps us understand the function of the structures encoded in the DNA.
Our DNA sequencing technology is based on our proprietary reversible terminator-based sequencing chemistry, referred to as sequencing by synthesis (SBS) biochemistry. SBS tracks the addition of labeled nucleotides as the DNA chain is copied in a massively parallel fashion. In 2023, we launched XLEAP-SBSTM, a faster, higher quality, and more robust version of our SBS chemistry that delivers the highest level of data accuracy and performance. Our XLEAP-SBS sequencing technology provides researchers with a broad range of applications and the ability to sequence more than 20,000 human genomes per year per instrument.
Our sequencing platforms can generate between 500 megabases (Mb) and 16.0 terabases (Tb) (equivalent to approximately 128 human genomes) of genomic data in a single run, depending on the instrument and application.
There are different price points per gigabase (Gb) for each instrument, and for different applications, which range from small-genome, amplicon, and targeted gene-panel sequencing to population-scale whole human genome sequencing. Since we launched our first sequencing system in 2007, our systems have significantly reduced the cost of sequencing. In 2023, we launched the NovaSeqTM X Plus, a production-scale sequencing system that can sequence a human genome for as little as $200. In 2024, we launched the benchtop MiSeq i100 series, our fastest, simplest sequencing system, featuring room temperature reagents, empowering every lab, everywhere.
Illumina informatics products play a critical role in supporting our sequencing applications and customers’ needs across a range of activities, including sample preparation, instrument control and management, and post-run analysis. Our BaseSpace Informatics Suite integrates directly with our sequencing instruments, allowing customers to manage their biological sample and sequencing runs, process and analyze the raw genomic data, and derive meaningful results. It facilitates data sharing, provides data-storage solutions and streamlines analysis through a growing number of applications developed by us and the bioinformatics community. Our DRAGEN Bio-IT Platform is used for secondary analysis and analyzes sequencing data from a variety of experiment types, including whole genomes, whole exomes, germline and somatic datasets, and RNA sequencing experiments with industry leading accuracy, speed and efficiency. Additionally, Illumina Connected Analytics is an integrated bioinformatics solution that provides a comprehensive, private, cloud-based data platform that empowers customers to manage, analyze, and explore large volumes of multi-omic data in a secure, scalable, and flexible environment.
In 2024, 2023, and 2022, total sequencing revenue comprised 91% of total consolidated revenue for all periods.
Arrays
Arrays are used for a broad range of DNA and RNA analysis applications, including SNP genotyping, CNV analysis, gene expression analysis, and methylation analysis, and enable the detection of millions of known genetic markers on a single array. Arrays are the primary technology used in consumer genomics applications.
Our BeadArray technology combines microscopic beads and a substrate in a proprietary manufacturing process to produce arrays that can perform many assays simultaneously. This facilitates large-scale analysis of genetic variation and biological function in a unique, high-throughput, cost-effective, and flexible manner. Using our BeadArray technology, we achieve high-throughput analysis via a high density of test sites per array and the ability to format arrays in various configurations. To serve the needs of multiple markets and market segments, we can vary the size, shape, and format of the substrate into which the beads self-assemble and create specific bead types for different applications. Our iScan System and our NextSeq 550 System can be used to image arrays.
In 2024, 2023, and 2022, total array revenue comprised 9% of total consolidated revenue for all periods.
Consumables
We have developed various library preparation and sequencing kits to simplify workflows and accelerate analysis. Our sequencing applications include whole-genome sequencing kits, which sequence entire genomes of any size and complexity, and targeted resequencing kits, which can sequence exomes, specific genes, RNA or other genomic regions of interest. In January 2025, we launched Illumina Single Cell 3’ RNA Prep, a simple, end-to-end single cell workflow allowing transcriptome studies of hundreds to millions of cells. Our sequencing kits maximize the ability of our customers to characterize the target genome accurately and are sold in various configurations, addressing a wide range of applications.
Customers use our array-based genotyping consumables for a wide range of analyses, including diverse species, disease-related mutations, and genetic characteristics associated with cancer. Customers can select from a range of human, animal, and agriculturally relevant genome panels or create their own custom arrays to investigate millions of genetic markers targeting any species.
Services
We offer support services to customers who have purchased our products. In addition, we provide whole-genome sequencing, genotyping, NIPT, and product support services. Human whole-genome sequencing services are provided through our CLIA-certified, CAP-accredited laboratory. Using our services, customers can perform whole-genome sequencing projects and microarray projects (including large-scale genotyping studies and whole-genome association studies). We also provide NIPT services through our partner laboratories that direct samples to us on a test send-out basis in our CLIA-certified, CAP-accredited laboratory.
GRAIL
GRAIL’s multi-cancer early detection test, Galleri, is designed as a screening test for adults with an elevated risk for cancer, such as those aged 50 or older, and was commercially launched in 2021 as a laboratory developed test.
Intellectual Property
We have an extensive intellectual property portfolio. As of December 29, 2024, excluding GRAIL which was spun-off on June 24, 2024, we owned or had exclusive licenses to 1,337 issued U.S. patents and 1,119 pending U.S. patent applications and 8,027 issued patents outside the U.S. and 5,668 pending patent applications outside the U.S. Our issued and pending patents cover various aspects of our arrays, assays, oligo synthesis, sequencing technology, instruments, digital microfluidics, software, bioinformatics, and chemical-detection technologies, and our issued patents have terms that expire between 2025 and 2049. We continue to file new patent applications to protect the full range of our technologies. We have filed or have been granted counterparts for many of these patents and applications in foreign countries. We protect our trade secrets, know-how, copyrights, and trademarks. Our success depends in part on obtaining patent protection for our products and processes, preserving trade secrets, patents, obtaining copyrights and trademarks, operating without infringing the proprietary rights of third parties, and acquiring licenses for technology or products. In addition, we invest in technological innovation, and we seek beneficial licensing opportunities to develop and maintain our competitive position. We are party to various exclusive and nonexclusive license agreements and other arrangements with third parties that grant us rights to use key aspects of our sequencing and array technologies, assay methods, chemical detection methods, reagent kits, and scanning equipment. We have additional nonexclusive license agreements with various third parties for other components of our products. In most cases, the agreements remain in effect over the term of the underlying patents, may be terminated at our request without further obligation, and require that we pay customary royalties.
Research and Development
We have historically made substantial investments in research and development and we expect to continue to make investments in research and development during 2025 to support business growth and our innovation pipeline. Our research and development efforts prioritize continuous innovation coupled with product evolution. Research and development expense in 2024, 2023, and 2022 was $1,169 million, $1,354 million, and $1,321 million, respectively.
Marketing and Distribution
We market and distribute our products directly to customers in North America, Europe, Latin America, and the Asia-Pacific region. In addition, we sell through life-science distributors in certain markets within Europe, the Asia-Pacific region, Latin America, the Middle East, and Africa. We expect to continue making commercial investments in 2025 and beyond as we launch new products and expand our potential commercial base in alignment with our strategy.
Manufacturing
We manufacture sequencing and array platforms and reagent kits. In 2024, we continued to increase our manufacturing capability, and we expect to increase our manufacturing capability again in 2025 to meet customer demand. To address increasing product complexity and volume, we continue to automate manufacturing processes to accelerate throughput and improve quality and yield. We are committed to providing medical devices and related services that consistently meet customer and applicable regulatory requirements. We adhere to health and safety standards required by federal, state, and local health ordinances, such as standards for the use, handling, and disposal of hazardous substances. Our key manufacturing and distribution facilities operate under a quality management system certified to ISO 13485.
Raw Materials
Our manufacturing operations require a wide variety of raw materials, electronic and mechanical components, chemical and biochemical materials, and other supplies. Multiple commercial sources provide many of our components and supplies, but there are some raw materials and components that we obtain from single-source suppliers. To manage potential risks arising from single-source suppliers, we believe that, if necessary, we could redesign our products using alternative components or for use with alternative reagents or develop an internal supply capability. In addition, while we attempt to keep our inventory at minimal levels, we purchase incremental inventory as circumstances warrant to protect our supply chain. If the capabilities of our suppliers and component manufacturers are limited or stopped, due to pandemics, disasters, quality, regulatory, or other reasons, it could negatively impact our ability to manufacture our products.
Competition
Although we believe that our products and services provide significant advantages over products and services currently available from other sources, we expect continued intense competition. Our competitors offer products, services, and software for sequencing, SNP genotyping, gene expression, proteomics, and molecular diagnostics markets. In some cases, we compete for the resources our customers allocate for purchasing a wide range of sequencing and non-sequencing products used to analyze genetic variation and associated biological functions, some of which are complementary or adjacent to our own but not directly competitive; in other cases, our products face direct competition as customers choose among sequencing and non-sequencing products that are designed to address the same use case or answer the same biological question. Some of our competitors have, or will have, substantially greater financial, technical, research, artificial intelligence capabilities, and other resources than we do, along with larger, more established marketing, sales, distribution, and service organizations. In addition, they may have greater name recognition than we do in the markets we address, and in some cases a larger installed base of systems. We expect new competitors to emerge and the intensity of competition to increase. To compete effectively, we must scale our organization and infrastructure appropriately and demonstrate that our products have superior throughput, cost, and accuracy.
Segment and Geographic Information
We have one reportable segment, Core Illumina, as of December 29, 2024. Prior to the Spin-Off of GRAIL into a separate, independent publicly traded company on June 24, 2024, our reportable segments included both Core Illumina and GRAIL. See note 12. Segment and Geographic Information for details on our reportable segments. We currently sell our products to a number of customers outside the United States, including customers in other areas of North America, Latin America, Europe, China, and the Asia-Pacific region. Shipments to customers outside the United States totaled $2,084 million, or 48%, of total consolidated revenue in 2024, compared to $2,145 million, or 48%, and $2,294 million, or 50%, in 2023 and 2022, respectively. We consider the U.S. dollar to be the functional currency of our international operations due to the primary activities of our foreign subsidiaries. We expect that sales to international customers will continue to be an important and growing source of revenue. See note 1. Organization and Significant Accounting Policies and note 3. Revenue within the Consolidated Financial Statements section of this report for further information concerning our foreign and domestic operations. Backlog
Our backlog was $657 million and $653 million as of December 29, 2024 and December 31, 2023, respectively, and consists of orders believed to be firm as of the balance sheet date. However, we may allow customers to make product substitutions as we launch new products. The timing of shipments depends on several factors, including agreed upon shipping schedules, which may span multiple quarters, and whether the product is catalog or custom. We expect 78% of our backlog as of December 29, 2024 to be shipped in 2025, 10% in 2026, and the remainder thereafter. Although we generally recognize revenue when control of our products and services is transferred to our customers, some customer contracts might require us to defer revenue recognition beyond the transfer of control.
Properties
The following table, excluding GRAIL which was spun-off on June 24, 2024, summarizes the facilities we leased as of December 29, 2024, including location and size of each principal facility and designated use. We believe our facilities are adequate for our current and near-term needs, and we will be able to locate additional facilities, as needed.
| | | | | | | | | | | | | | | | | | | | |
| Location | | Approximate Square Feet | | Operation | | Lease
Expiration Dates |
| San Diego, CA | | 860,000 | | | Office, Lab, Manufacturing, and Distribution | | 2030 – 2031 |
| Singapore | | 584,000 | | | Office, Lab, Manufacturing, and Distribution | | 2027 – 2037 |
| San Francisco Bay Area, CA | | 269,000 | | | Office, Lab, and Manufacturing | | 2025 – 2033 |
| Cambridge, United Kingdom | | 181,000 | | | Office, Lab, and Manufacturing | | 2025 – 2038 |
| Madison, WI | | 133,000 | | | Office, Lab, and Manufacturing | | 2033 | |
| Eindhoven, the Netherlands | | 90,000 | | | Office and Distribution | | 2036 | |
| China | | 86,000 | | | Office and Lab | | 2026 – 2028 |
| India | | 66,000 | | | Office and Lab | | 2027 – 2029 |
| Other | | 140,000 | | | Office and Lab | | 2025 – 2030 |
Human Capital
To continue as a leader in genomics, we need to harness the world’s best talent and give them the opportunity to thrive. We are committed to attracting, retaining, developing, and supporting our people to enable everyone to fully contribute to our mission and deliver on the transformative power of genomics. We drive innovation by embracing new perspectives and making Illumina a place where everyone can belong. Our key human capital objectives include: attract extraordinary talent, invest in our people, support employee health, safety, and well-being, and engage our people and communities. Additional information is included in our annual Corporate Social Responsibility (CSR) Report, located on our website at www.illumina.com/csr. Information on our website, including the CSR Report, shall not be deemed incorporated by reference into this Annual Report. Our annual CSR Report is guided by the reporting frameworks of the Global Reporting Initiative (GRI), Sustainable Accounting Standards Board (SASB), and the Task Force for Climate related Financial Disclosures (TCFD).
As of December 29, 2024, excluding GRAIL which was spun-off on June 24, 2024, our global workforce was comprised of approximately 8,970 full time employees, 60 part time employees, and 1,340 contingent workers. The regional representation includes approximately 5,250 employees in the Americas, 1,290 employees in Europe, 2,190 employees in Africa, Middle East and Asia, and 300 employees in Greater China. The global voluntary turnover rate for 2024 was 7%. Additional details about our workforce for 2024 will be available in our annual CSR Report, which we expect to publish in May 2025 on our website at www.illumina.com/csr.
Cybersecurity
We recognize the importance of developing, implementing, and maintaining robust cybersecurity measures to safeguard our information systems and protect the confidentiality, integrity, and availability of our data. Our cybersecurity risk management strategy is integrated into our established enterprise risk management program, which includes defined risk, assessment, mitigation, and reporting processes. Our information security team has deployed multiple technical and operational processes to aid in our ability to continuously identify and respond to cybersecurity threats and incidents. Our cybersecurity incident management process includes impact assessment, containment, mitigation and recovery strategies.
In addition to our continuous monitoring of our information systems, we utilize third parties to provide external threat intelligence and evaluation of incident notifications in order to identify potential threats or incidents that could impact us. We also evaluate our cybersecurity program against the National Institute of Standards and Technology’s Cybersecurity Framework. For all suspected cybersecurity incidents, the information security team conducts a preliminary assessment to determine the potential severity and impact extent of the incident and, where appropriate, a materiality assessment is made. Upon a confirmed cybersecurity incident, the information security team initiates an incident response process with goals to contain, respond, recover, protect and minimize any impacts caused by the incident. The response process includes deployment of a variety of short term and long-term technical and procedural actions as appropriate. Further, we have established a third party risk management program to monitor suppliers who have access to our information.
Our Audit Committee, a committee of our Board of Directors, is responsible for governing management’s review and assessment of our cybersecurity and other information technology risks, controls, and procedures, including management’s incident resolution process and any specific cybersecurity issues that could affect the adequacy of our internal controls. Our Chief Information Officer provides regular updates to the Audit Committee and to the Board of Directors, including a review of any security risk events and improvements in our security controls.
Our information security team, under the Chief Information Officer, is led by our Chief Information Security Officer (CISO) and is responsible for assessing and managing risks from cybersecurity threats. Our CISO has over 20 years of information security experience, including as a leader of information security programs at other large enterprises, and is supported by a team of professionals focused on information security. Our information security team regularly meets to review our cybersecurity posture, the broader cybersecurity landscape and any identified cybersecurity incidents. Our information security team has procedures in place for investigating suspected cybersecurity incidents, as well as monitoring cybersecurity risks and ongoing mitigation strategies, the status of prevention, detection, and mitigation controls and any planned future control enhancements.
We believe that risks from prior cybersecurity threats to information systems owned and used by us, including as a result of any previous cybersecurity incidents, have not materially affected our business to date. We can provide no assurance that there will not be incidents in the future or that they will not materially affect us, including our business strategy, results of operations, or financial condition. We maintain a cybersecurity insurance policy which may mitigate certain financial impacts of a cybersecurity incident. Please refer to “Risks Relating to Information Technology Security and Continuity” within the Risk Factors within the Business & Market Information Section of this report. Environmental Matters
As a global corporate citizen, we recognize the importance of the environment to a healthy, sustainable future for our business, our patients, and communities. We are committed to the protection of our employees and the environment with an approach to continuously strengthen our environmental stewardship. We believe that we are in material compliance with current applicable laws and regulations. However, we could be held liable for damages and fines should contamination of the environment or individual exposures to hazardous substances occur. In addition, we cannot predict how changes in these laws and regulations, or the development of new laws and regulations, will affect our business operations or the cost of compliance. Further, regulators are considering, and in some cases have implemented, new environmental disclosure rules. For example, California has recently enacted new climate-related disclosure rules and requirements. The cost of complying with any new disclosure regimes is uncertain. In addition, climate change may impact our business by increasing operating costs due to additional regulatory requirements, physical risks to our facilities, energy limitations, and disruptions to our supply chain. These potential risks are accounted for in our business planning, including investment in renewable energy, reducing energy and water consumption, greenhouse gas emissions, and waste production. As part of our climate action plan, we established emission reduction targets in line with a 1.5 degree pathway, established Net Zero emission commitments by 2050, and had those targets verified by the Sciences Based Target Initiative. Additional information is included in our annual CSR Report located on our website at www.Illumina.com/csr.
Government Regulation
As we expand product lines to address the diagnosis of disease, regulation by governmental authorities in the U.S. and other countries will become an increasingly significant factor in development, testing, production, and marketing activities. Products that we develop in the molecular diagnostic markets, depending on their intended use, may be regulated as medical devices or in vitro diagnostic products (IVDs) by the FDA and comparable agencies in other countries. In the U.S., certain of our products may require FDA clearance following a pre-market notification process, also known as a 510(k) clearance, or premarket approval (PMA) from the FDA. The usually shorter 510(k) clearance process, which we used for the FDA-cleared assays that are run on our FDA-regulated MiSeqDx instrument, generally takes from three to six months after submission, but it can take significantly longer. The longer PMA process, which we used for our FDA-approved TruSight Comprehensive Oncology panel that is run on our NextSeq550 Dx instrument, is typically much more costly and uncertain. It can take from 9 to 18 months after a complete filing, but it can take significantly longer and requires conducting clinical studies that are generally more extensive than those required for 510(k) clearance. All of the products that are currently regulated by the FDA as medical devices and IVDs are also subject to the FDA Quality System Regulation (QSR). Obtaining the requisite regulatory approvals, including the FDA quality system inspections that are required for PMA approval, can be expensive and may involve considerable delay. In the U.S., the products we develop for oncology and non-invasive prenatal testing will be regulated by the PMA process. We cannot be certain which of our other planned molecular diagnostic products will be subject to the shorter 510(k) clearance process, or which of these will need to go through the PMA process.
The regulatory approval process for such products may be significantly delayed, may be significantly more expensive than anticipated, and may conclude without such products being approved by the FDA. Without timely regulatory approval, we will not be able to launch or successfully commercialize such products, which would adversely affect our earnings and competitive position. Many of the products that we are developing are the first of their kind. The regulatory approval pathways for such products do not currently exist and therefore have a high degree of uncertainty.
Changes to the current regulatory framework, including the imposition of additional or new regulations, could arise at any time during the development or marketing of our products. This may negatively affect our ability to obtain or maintain FDA or comparable regulatory clearance or approval of our products. In addition, regulatory agencies may introduce new requirements that may change the regulatory requirements for us or our customers, or both. For example, the final rule published by the FDA in 2024 allows the FDA to regulate laboratory developed tests (LDTs). Under the final rule, our customers will be required to either submit their current test for FDA approval or find an IVD manufacturer to supply them with IVDs.
If our products labeled as “For Research Use Only,” or RUO, are used, or could be used, for the diagnosis of disease, the regulatory requirements related to marketing, selling, and supporting such products could be uncertain. This is true even if such use by our customers occurs without our consent. If the FDA or other regulatory authorities assert that any of our RUO products are subject to regulatory clearance or approval, our business, financial condition, or results of operations could be adversely affected.
Our products sold as medical devices or IVDs in Europe are now regulated under the In Vitro Diagnostics Regulation (EU) 2017/746, the IVDR, that went into full enforcement in May 2022. These regulations include requirements for both presentation and review of performance data and quality-system requirements.
Certain of our products are currently available through laboratories that are certified under the Clinical Laboratory Improvements Amendments (CLIA) of 1988. These products are commonly called “laboratory developed tests,” or LDTs. For a number of years, the FDA has exercised its regulatory enforcement discretion not to regulate LDTs as medical devices if created and used within a single laboratory. However, as mentioned above, the FDA published a final rule in 2024 that phases out the enforcement discretion over a 4 year time period. The final rule is effective as of the 2024 publication. While this rule is being challenged in the U.S. court system, if the final rule stands in its current form, laboratories will be required to submit LDTs for FDA approval and will be required to implement a fully compliant Quality Management System (QMS).
Certification of CLIA laboratories includes standards in the areas of personnel qualifications, administration, and participation in proficiency testing, patient test management, and quality control procedures. CLIA also mandates that, for high complexity labs such as ours, to operate as a lab, we must have an accreditation by an organization recognized by CLIA such as the College of Pathologists (CAP), which we have obtained and must maintain. If we were to lose our CLIA certification or CAP accreditation, our business, financial condition, or results of operations could be adversely affected. In addition, state laboratory licensing and inspection requirements may also apply to our products, which, in some cases, are more stringent than CLIA requirements.
RISK FACTORS
This report and other documents we file with the SEC contain forward-looking statements that are based on current expectations, estimates, forecasts and projections about us, our future performance, our business, our beliefs and our management’s assumptions. These statements are not guarantees of future performance and involve certain risks, uncertainties and assumptions that are difficult to predict. You should carefully consider the risks and uncertainties our business faces. The risks described below are not the only ones we face. Our business is also subject to the risks that affect many other companies, such as employment relations, general economic conditions, geopolitical events and international operations. Further, additional risks not currently known to us or that we currently believe are immaterial may in the future materially and adversely affect our business, operations, liquidity and stock price.
Risks Relating to Our Sales of Products and Services, Marketing and Research and Development
Our success depends upon the continued emergence and growth of markets for analysis of genetic variation, and continued substantial increases in the use of sequencing as the cost of sequencing declines.
The usefulness of our technologies depends in part upon the availability of genetic data and its usefulness in clinical, research, and consumer applications. We are focusing on markets for analysis of genetic variation or biological function, namely sequencing, genotyping, and gene expression profiling. These markets are relatively new and emerging, and they may not develop as quickly as we anticipate, or reach what we expect to be their full potential. Other methods of analysis of genetic variation and biological function may emerge and displace the methods we are developing. In addition, a reduction or delay in research and development budgets and government funding may adversely affect our business. For example, changes in the regulatory environment affecting life sciences and pharmaceutical companies, and reduced allocations to government agencies that fund research and development activities, such as the U.S. National Institutes of Health, or NIH, or targeted cancellations by the U.S. federal government of certain grants or contracts, could adversely affect our business or results of operations.
The introduction of next-generation sequencing technologies, including ours, has reduced the cost of sequencing by a factor of more than 10,000 and reduced the sequencing time per Gb by a factor of approximately 12,000 over the last 20 years. Consequently, demand for sequencing-related products and services has increased substantially as new applications are enabled and more sequencing is done in connection with existing applications. If, as we expect, the cost of sequencing continues to fall, we cannot be sure that the demand for related products and services will increase at least proportionately as new applications are enabled or more sequencing is done in connection with existing applications. In the future, if demand for our products and services due to lower sequencing costs is less than we expect, our business, financial condition, and results of operations will be adversely affected.
Our products may be used to provide genetic information about humans, agricultural crops, other food sources, and other living organisms. The information obtained from our products could be used in a variety of applications, which may have underlying ethical, legal, and social concerns regarding privacy and the appropriate uses of the resulting information, including preimplantation genetic screening of embryos, prenatal genetic testing, genetic engineering or modification of agricultural products, or testing genetic predisposition for certain medical conditions, particularly for those that have no known cure. Our customers’ implementation of our products to provide their own products and services may raise such concerns and affect our own reputation. U.S. and international governmental authorities could, for social or other purposes, call for limits on or regulation of the use of genetic testing or prohibit testing for genetic predisposition to certain conditions, particularly for those that have no known cure. Similarly, such concerns may lead individuals to refuse to use genetics tests, even if permissible. These and other ethical, legal, and social concerns about genetic testing may limit market acceptance of our technology for certain applications or reduce the potential markets for our technology, either of which could have an adverse effect on our business, financial condition, or results of operations.
We face intense competition, which could render our products obsolete, result in significant price reductions, or substantially limit the volume of products that we sell.
We compete with third parties that design, manufacture, and market products and services for analysis of genetic variation and biological function and other applications using a wide range of technologies. We have faced, and expect to continue to face, increased pricing pressure from competitors who offer sequencing products and we have experienced lengthened sales cycles with many customers due to competition. In some cases, we compete for the resources our customers allocate for purchasing a wide range of sequencing and non-sequencing products, some of which are complementary or adjacent to our own but not directly competitive; in other cases, our products face direct
competition as customers choose among sequencing and non-sequencing products that are designed to address the same use case or answer the same biological question. For example, complementary third-party sequencing technologies address use cases to which our products are not as well suited. If we are unable to develop or acquire new technologies that address these complementary sequencing applications, our rate of growth and our ability to grow the overall market for sequencing could be adversely affected.
We anticipate that we will continue to face increased competition as existing companies develop new or improved products and as new companies enter the market with new technologies. One or more of our competitors may render one or more of our technologies obsolete or uneconomical. Some of our competitors have greater financial and personnel resources, broader product lines, more focused product lines, a more established customer base, more experience and broader reach in clinical markets, and more experience in research and development than we do. Furthermore, life sciences, clinical genomics, and pharmaceutical companies, which are our potential customers and strategic partners, could also develop competing products. We believe that customers in our markets display a significant amount of loyalty to their initial supplier of a particular product; therefore, it may be difficult to generate sales to potential customers who have purchased products from competitors. To the extent we are unable to be the first to develop or supply new products, our competitive position may suffer.
The market for clinical and diagnostic products, in particular, is currently limited and highly competitive, with several large companies having significant market share, intellectual property portfolios, and regulatory expertise. For example, the market for noninvasive prenatal testing is rapidly developing, and if our competitors are able to develop and commercialize products superior to or less expensive than ours or are able to obtain regulatory clearances before we do, our business could be adversely impacted. Established clinical and diagnostic companies also have an installed base of instruments in several markets, including clinical and reference laboratories, which could deter acceptance of our products. In addition, some of these companies have formed alliances with genomics companies that provide them access to genetic information that may be incorporated into their diagnostic tests, potentially creating a competitive advantage for them.
China’s Ministry of Commerce has added Illumina to its List of Unreliable Entities, which could result in fines or restrictions on our ability to do business in China and could have a material adverse effect on our revenue and results of operations.
On February 4, 2025, China’s Ministry of Commerce (MOFCOM) announced that it had added Illumina to its List of Unreliable Entities under the Provisions of the List of Unreliable Entities (the UEL Provisions). Under the UEL Provisions, potential penalties for companies placed on the List of Unreliable Entities can include monetary fines, restrictions or prohibitions on the sale of goods in China, engaging in import and export activities related to China, making investments in, or extracting investments from, China, denial of entry of our relevant personnel into China, restrictions or revocation of work permits, stay or residence status of our relevant personnel in China, or other measures.
MOFCOM has not announced what penalties will be imposed on us and we cannot currently predict the duration of our inclusion on the List of Unreliable Entities or any actions that may ultimately be taken by MOFCOM. The decision to place us on the List of Unreliable Entities and any future decision to take action to impose and enforce penalties or restrictions against us could have a material adverse effect on our revenue and results of operations. Furthermore, if, as a result of any such penalties or restrictions, we were to cease entirely or curtail operations in China, we could incur material impairment charges related to any such exit or disposal activities. Our revenue from the Greater China region, which includes China, Taiwan, and Hong Kong, was $308 million in 2024. See note 3. Revenue. If we do not successfully manage the development, manufacturing, and launch of new products or services, including product transitions, our financial results could be adversely affected.
We face risks associated with launching new products and pre-announcing products and services when the products or services have not been fully developed or tested. In addition, we may experience difficulty in managing or forecasting customer reactions, purchasing decisions, transition requirements, or programs with respect to newly-launched products (or products in development), which could adversely affect sales of our existing products. If our products and services are not able to deliver the performance or results expected by our target markets or are not delivered on a timely basis, our reputation and credibility may suffer. If we encounter development challenges or discover errors in our products late in our development cycle, we may delay the product launch date. The expenses or losses associated with unsuccessful product development or launch activities, or a lack of market acceptance of our new products, could adversely affect our business, financial condition, or results of operations.
As we announce future products or integrate new products into our portfolio, such as new instruments or instrument platforms, we face numerous risks relating to product transitions and the evolution of our product portfolio. We may be unable to accurately forecast new product demand and the impact of new products on the demand for current or established products. We may experience challenges relating to managing excess and obsolete inventories, managing new or higher product cost structures, and managing different sales and support requirements. Announcements of currently planned or other new products may cause customers to defer or stop purchasing our current or established products until new products become available. In addition, customers may defer or stop purchasing our current or established products as they assess the features and technological characteristics of new products, as compared to our current or established products, before making a financial commitment.
Our continued growth is dependent on continuously developing and commercializing new products.
Our target markets are characterized by rapid technological change, changes in customer needs, existing and emerging competition, strong price competition, and frequent new product introductions. Accordingly, our continued growth depends on developing and commercializing new products and services, including improving our existing products and services, in order to address evolving market requirements on a timely basis. If we fail to innovate or adequately invest in new technologies, we could lose our competitive position in the markets that we serve.
To the extent that we fail to introduce new and innovative products, or such products are not accepted in the market or suffer significant delays in development, our financial results may suffer. An inability, for technological or other reasons, to successfully develop and introduce new products on a timely basis could reduce our growth rate or otherwise have an adverse effect on our business.
In the past, we have experienced, and are likely to experience in the future, delays in the development and introduction of new products. There can be no assurance that we will keep pace with the rapid rate of change in our markets or that our new products will adequately meet the requirements of the marketplace, achieve market acceptance, or compete successfully with third-party technologies. Some of the factors affecting our ability to develop and successfully commercialize new products and services include:
•the functionality and performance of new and existing products and services;
•the timing of introduction of new products or services relative to competing products and services;
•availability, quality, and price relative to competing products and services;
•scientists’ and customers’ opinions of the utility of new products or services;
•citation of new products or services in published research;
•regulatory trends and approvals; and
•our ability to acquire or otherwise gain access to third party technologies, products, or businesses.
As we develop, market, or sell diagnostic tests, we may encounter delays in receipt, or limits in the amount, of reimbursement approvals and public health funding, which will impact our ability to grow revenues in the healthcare market.
Physicians and patients may not order diagnostic tests that we develop, market, sell, or enable, such as our prenatal tests, unless third-party payors, such as managed care organizations as well as government payors such as Medicare and Medicaid and governmental payors outside of the United States, pay a substantial portion of the test price. Third-party payors are often reluctant to reimburse healthcare providers for the use of medical tests that involve new technologies or provide novel diagnostic information. In addition, third-party payors are increasingly limiting reimbursement coverage for medical diagnostic products and, in many instances, are exerting pressure on diagnostic product suppliers to reduce their prices. Reimbursement by a payor may depend on a number of factors, including a payor's determination that tests using our technologies are: not experimental or investigational; medically necessary; appropriate for the specific patient; cost-effective; supported by peer-reviewed publications; and included in clinical practice guidelines.
Since each third-party payor often makes independent reimbursement decisions and may also make decisions on an individual patient basis, obtaining such approvals is a time-consuming and costly process that requires Illumina and/or our customers to provide scientific and clinical data supporting the clinical benefits of each of our products. As a result, there can be no assurance that reimbursement approvals will be obtained. This process can delay the broad market introduction of new products, and could have a negative effect on our results of operations. As a result, third-party reimbursement may not be consistent or financially adequate to cover the cost of diagnostic products that we develop, market, or sell. This could limit our ability to sell our products or cause us to reduce prices, which would adversely affect our results of operations.
Even if tests are reimbursed, third-party payors may withdraw their coverage policies, cancel their contracts with our customers at any time, review and adjust the rate of reimbursement, require co-payments from patients, or stop paying for tests, which would reduce our revenues. In addition, insurers, including managed care organizations as well as government payors such as Medicare and Medicaid, have increased their efforts to control the cost, utilization, and delivery of healthcare services. These measures have resulted in reduced payment rates and decreased utilization for the clinical laboratory industry. Reductions in the reimbursement rate of payors may occur in the future. Reductions in the prices at which our tests are reimbursed could have a negative impact on our results of operations.
Uncertainties with respect to the development, deployment, and use of artificial intelligence in our business and products may result in harm to our business and reputation.
We have incorporated, and expect to continue to incorporate, artificial intelligence (AI) into our business activities and our product and service offerings. As with many innovations, AI presents risks and challenges that could adversely impact our business. The development, adoption, and use of AI technologies are still in their early stages and ineffective or inadequate AI development or deployment practices could result in unintended consequences. For example, AI algorithms may be flawed or may be based on datasets that are biased or insufficient. In addition, any disruption or failure in the AI functionality we incorporate into our business activities, products or services could adversely impact our business or result in delays or errors in our offerings. Conversely, any failure to successfully develop and deploy AI in our business activities, products and services could adversely affect our competitiveness (particularly if our competitors successfully deploy AI in their businesses, products, and services), and the development and deployment of AI will require additional investment and increase our costs. There also may be real or perceived social harm, unfairness, or other outcomes that undermine public confidence in the use and deployment of AI. Any of the foregoing may result in decreased demand for our products or harm to our reputation, business, financial condition, or results of operations.
The legal and regulatory landscape surrounding AI technologies is rapidly evolving and uncertain, including in the areas of intellectual property, cybersecurity, and privacy and data protection. Compliance with new or changing laws, regulations or industry standards relating to AI may impose significant costs and may limit our ability to develop, deploy, or use AI technologies. Failure to appropriately respond to this evolving landscape may result in legal liability, regulatory action, or brand and reputational harm.
Risks Relating to Supply Chain, Manufacturing, and Quality
We depend on third-party manufacturers and suppliers for some of our products, or sub-assemblies, components, and materials used in our products, and if shipments from these manufacturers or suppliers are delayed or interrupted, or if the quality of the products, components, or materials supplied do not meet our requirements, we may not be able to launch, manufacture, or ship our products in a timely manner, or at all.
The complex nature of our products requires customized, precision-manufactured sub-assemblies, components, and materials that currently are available from a limited number of sources, and, in the case of some sub-assemblies, components, and materials, from only a single source. If deliveries from these vendors are delayed or interrupted for any reason, or if we are otherwise unable to secure a sufficient supply, we may not be able to obtain these sub-assemblies, components, or materials on a timely basis or in sufficient quantities or at satisfactory qualities. We may need to enter into contractual relationships with manufacturers for commercial-scale production of some of our products, in whole or in part, or develop these capabilities internally, and there can be no assurance that we will be able to do this on a timely basis, in sufficient quantities, satisfactory quality, or on commercially reasonable terms. In addition, the lead time needed to establish a relationship with a new supplier, and qualify their supply, can be lengthy, and we may experience delays in meeting demand in the event we must switch to a new supplier. The time and effort required to qualify a new supplier could result in additional costs, diversion of resources, or reduced manufacturing yields, any of which would negatively impact our operating results. Accordingly, we may not be able to establish or maintain reliable, high-volume manufacturing at commercially reasonable costs. In addition, the manufacture or
shipment of our products may be delayed or interrupted if the quality of the products, sub-assemblies, components, or materials supplied by our vendors does not meet our requirements. Current or future social and environmental regulations or critical issues, such as those relating to the sourcing of minerals from conflict-affected areas such as the Democratic Republic of the Congo or the need to eliminate environmentally sensitive materials from our products, could restrict the supply of components and materials used in production or increase our costs. Any delay or interruption to our manufacturing process or in shipping our products could result in lost revenue, which would adversely affect our business, financial condition, or results of operations.
In the past, defects have been discovered in our products, as a result of which we have incurred costs and our products have been subject to recalls. If defects are discovered in our products in the future, we may incur additional unforeseen costs, our products may be subject to recalls, customers may not purchase our products, our reputation may suffer, and ultimately our sales and operating earnings could be negatively impacted.
Our products incorporate complex, precision-manufactured mechanical parts, electrical components, optical components, and fluidics, as well as computer software and complex surface chemistry, biochemistry and reagents, any of which may contain or result in errors or failures, especially when first introduced. In the course of conducting our business, we must adequately address quality issues associated with our products and services, including defects in our engineering, software development, product cybersecurity, design, and manufacturing processes, as well as defects in third-party components included in our products. In addition, new products or enhancements may contain undetected errors or performance problems that, despite testing, are discovered only after commercial shipment. Defects or errors in our products have resulted in shipment holds, product recalls, negative publicity and adverse financial impacts in the past. The costs incurred in correcting any defects or errors may be substantial and could adversely affect our operating margins. Identifying the root cause of quality issues, particularly those affecting reagents and third-party components, may be difficult, which increases the time needed to address quality issues as they arise, and increases the risk that similar problems could recur. Because our products are designed to be used to perform complex genomic analysis and our instruments can be, and often are, connected to the internet, which presents product cybersecurity risk, we expect that our customers will have an increased sensitivity to such defects. If we do not meet applicable regulatory or quality standards, our products may be, and in the past have been, subject to recall, and, under certain circumstances, we may be required to, and have in the past been required to, notify applicable regulatory authorities about a recall. Quality issues may also result in, and have in the past resulted in, additional regulatory and governmental scrutiny. If our products are subject to recall or shipment holds, our reputation, business, financial condition, or results of operations could be adversely affected.
If we are unable to increase our manufacturing or service capacity and develop and maintain operation of our manufacturing or service capability, we may not be able to launch or support our products or services in a timely manner, or at all.
We expect to increase our manufacturing and service capacity to meet the anticipated demand for our products. Although we have consistently increased our manufacturing and service capacity, and we believe we have plans in place sufficient to ensure we have adequate capacity to meet our current business plans, there are uncertainties inherent in expanding our manufacturing and service capabilities, and we may not be able to sufficiently increase our capacity in a timely manner. For example, manufacturing and product quality issues may arise as we increase production rates at our manufacturing facilities and launch new products. Also, we may not manufacture the right product mix to meet customer demand, especially as we introduce new products. As a result, we may experience difficulties in meeting customer, collaborator, and internal demand, in which case we could lose customers or be required to delay new product introductions, and demand for our products could decline. Additionally, in the past, we have experienced variations in manufacturing conditions and quality control issues that have temporarily reduced or suspended production of certain products. Due to the intricate nature of manufacturing complex instruments, consumables, and products that contain DNA and enzymes, we may encounter similar or previously unknown manufacturing difficulties in the future that could significantly reduce production yields, impact our ability to launch or sell these products (or to produce them economically), or prevent us from achieving expected performance levels, any of which could adversely affect our business, financial condition, or results of operations.
An interruption in our ability to manufacture our products or an inability to obtain key components or raw materials due to a catastrophic disaster, infectious disease, or infrastructure failure could adversely affect our business.
We currently manufacture in a limited number of locations. Our manufacturing facilities are located in San Diego and the San Francisco Bay Area in California; Madison, Wisconsin; Cambridge, United Kingdom; and Singapore. These
areas are subject to natural disasters such as earthquakes, wildfires, or floods. If a natural disaster were to damage one of our facilities significantly or if other events, such as the outbreak of a serious infectious disease, were to cause our operations to fail or be significantly curtailed, we may be unable to manufacture our products, provide our services, or develop new products. In addition, if the capabilities of our suppliers and component manufacturers are limited or stopped, due to the outbreak of a serious infectious disease, natural or other disasters, quality, regulatory, or other reasons, it could negatively impact our ability to manufacture our products.
Many of our product manufacturing and distribution processes are automated and are controlled by information management systems, including significant network and storage infrastructure. If either our information management systems or our network or storage infrastructure were to fail for an extended period of time, our ability to manufacture our products on a timely basis could be adversely impacted and we could be prevented from achieving our expected shipments in any given period.
Risks Relating to Our Strategic Collaborations
If we fail to maintain and successfully manage our strategic collaborations, our future results may be adversely impacted.
Strategic collaborations require significant management attention and operational resources. If we are unable to successfully manage or meet milestones related to our strategic collaborations, or if our partners do not perform as we expect, our future results may be adversely impacted. Furthermore, dependence on collaborative arrangements may also subject us to other risks, including:
•we may be required to relinquish important rights, including intellectual property, marketing and distribution rights;
•we may disagree with our partners as to rights to intellectual property, the direction of research programs, or commercialization activities;
•our revenues may be lower than if we were to develop and commercialize such products ourselves;
•a collaboration partner could develop and market a product that is competitive with either products developed under the collaboration or other of our products, either independently or in collaboration with others, including our competitors;
•our partners could become unable or less willing to expend their resources in support of our collaboration;
•collaborations could expose us to additional regulatory risks; and
•we may be unsuccessful at managing multiple simultaneous collaborations.
Moreover, disagreements with a partner or former partner could develop, and any conflict with a partner or former partner could reduce our ability to enter into future collaboration agreements and negatively impact our relationships with one or more existing partners.
Risk Relating to the Protection of Our Intellectual Property
Any inability to effectively protect our proprietary technologies could harm our competitive position.
The proprietary positions of companies developing tools for the life sciences, genomics, forensics, agricultural, and pharmaceutical industries, including our proprietary position, generally are uncertain and involve complex legal and factual questions. Our success depends to a large extent on our ability to develop proprietary products and technologies and to obtain patents and maintain adequate protection of our intellectual property in the United States and other countries. The laws of some foreign countries do not protect proprietary rights to the same extent as the laws of the United States, and many companies have encountered significant challenges in establishing and enforcing their proprietary rights outside of the United States. These challenges can be caused by the absence of rules and methods for the establishment and enforcement of intellectual property rights outside of the United States.
We will be able to protect our proprietary rights from unauthorized use by third parties only to the extent that our proprietary technologies are covered by valid and enforceable patents or are effectively maintained as trade secrets. Any finding that our patents or applications are unenforceable could harm our ability to prevent others from practicing the related technology, and a finding that others have inventorship or ownership rights to our patents and applications could require us to obtain certain rights to practice related technologies, which may not be available on favorable terms, if at all. Furthermore, as issued patents expire, including those related to our sequencing-by-synthesis technology, we may lose some competitive advantage as others develop, market, and sell competing products, which could negatively affect our revenue.
In addition, our existing patents and any future patents we obtain may not be sufficiently broad to prevent others from practicing our technologies or from developing competing products and may therefore fail to provide us with any competitive advantage. We may need to initiate lawsuits to protect or enforce our patents, or litigate against third-party claims, which would be expensive, and, if we lose, may cause us to lose some of our intellectual property rights and reduce our ability to compete in the marketplace. Furthermore, these lawsuits may divert the attention of our management and technical personnel. There is also the risk that others may independently develop similar or alternative technologies or design around our patented technologies. In that regard, certain patent applications in the United States may be maintained in secrecy until the patents issue, and publication of discoveries in the scientific or patent literature tend to lag behind actual discoveries by several months.
We also rely upon trade secrets and proprietary know-how protection for our confidential and proprietary information, and we have taken security measures to protect this information. These measures, however, may not provide adequate protection for our trade secrets, know-how, or other confidential information. Additionally, the use of artificial intelligence (AI) based software is increasingly common. Use of AI based software may lead to the inadvertent release of confidential proprietary information which may impact our ability to realize the benefit of our intellectual property.
Risks Related to Acquisitions, Including the Acquisition of GRAIL
Our acquisitions expose us to risks that could adversely affect our business, and we may not achieve the anticipated benefits of acquisitions of businesses or technologies.
As part of our strategy to develop and identify new products, services, and technologies, we have made, and may continue to make, acquisitions of technologies, products, or businesses. Acquisitions involve numerous risks and operational, financial, and managerial challenges, including the following, any of which could materially and adversely affect our business, financial condition, or results of operations:
•challenges, costs, delays, and uncertainty associated with obtaining any required regulatory approvals;
•difficulties in integrating new operations, technologies, products, and personnel;
•lack of synergies or the inability to realize expected synergies and cost-savings;
•lengthy, expensive, and time and resource-intensive regulatory review processes, the outcomes of which can be unpredictable;
•difficulties in managing geographically dispersed operations;
•underperformance of any acquired technology, product, or business relative to our expectations and the price we paid;
•negative near-term impacts on financial results after an acquisition, including acquisition-related earnings charges;
•the potential loss of key employees, customers, and strategic partners of acquired companies;
•claims by terminated employees and shareholders of acquired companies or other third parties related to the transaction;
•the issuance of dilutive securities, assumption or incurrence of additional debt obligations or expenses, or use of substantial portions of our cash;
•diversion of management’s attention and company resources from existing operations of the business;
•inconsistencies in standards, controls, procedures, and policies;
•the impairment of intangible assets as a result of technological advancements, or worse-than-expected performance of acquired companies; and
•assumption of, or exposure to, known or unknown contingent liabilities or liabilities that are difficult to identify or accurately quantify.
In addition, the successful integration of acquired businesses requires significant efforts and expense across all operational areas, including sales and marketing, research and development, manufacturing, finance, legal, and information technologies. There can be no assurance that any of the acquisitions we make will be successful or will be, or will remain, profitable. Our failure to successfully address the above risks may prevent us from achieving the anticipated benefits from any acquisition in a reasonable time frame, or at all.
On June 24, 2024, we completed the separation of GRAIL into a separate, independent publicly traded company. As of September 6, 2024, all previously disclosed regulatory proceedings in the United States and European Union related to our acquisition of GRAIL (the Acquisition) have come to an end. Litigation, regulation, and other proceedings related to or resulting from the Acquisition have resulted in operational restrictions and increased costs and could result in similar additional future consequences or further result in loss of revenues.
As previously disclosed, the Acquisition was subject to various legal challenges, including by the FTC and European Commission. As a result, we have been a party to a number of regulatory and administrative proceedings regarding the Acquisition.
On June 24, 2024, we completed the separation (the Spin-Off) of GRAIL into a separate, independent publicly traded company as described in note 2. GRAIL Spin-Off within the Consolidated Financial Statements. We incurred significant costs to complete the Spin-Off, including significant legal, financial advisory, regulatory and other professional services fees and additional expenses, and assumed certain liabilities in connection therewith. The Spin-Off also may result in loss of revenue and other adverse effects on our business, financial condition and results of operations. In addition, we have experienced and might continue to experience negative impacts on our stock price. We cannot predict what other adverse consequences to, among other things, our reputation, our relationships with governmental or regulatory authorities, or our ability to successfully complete future transactions, our ability to attract, retain and motivate customers, key personnel and those with whom we conduct business may result. Furthermore, we have and may continue to become subject to stockholder inspection demands under Delaware law, investigations initiated by regulators and law firms, and derivative or other similar litigation that can be expensive, divert management attention and human and financial capital to less productive uses and result in potential reputational damage. The GRAIL acquisition and subsequent litigation resulted in (i) the announcement of an investigation by the SEC and others by law firms of possible securities law violations; (ii) stockholder inspection demands seeking to investigate possible breaches of fiduciary duties, corporate wrongdoing or a lack of independence of the members of the Board, including a complaint filed in the Delaware Court of Chancery seeking to inspect books and records captioned Pavers and Road Builders Benefit Funds v. Illumina, Inc.; (iii) the filing of securities class actions in the United States District Court for the Southern District of California: Kangas v. Illumina, Inc. et al., Roy v. Illumina, Inc. et al., and Louisiana Sheriffs’ Pension & Relief Fund v. Illumina, Inc. et al.; (iv) the filing of a stockholder derivative complaint in the United States District Court for the Southern District of California captioned Warner v. deSouza et al.; (v) the filing of a stockholder derivative complaint in the United States District Court for the District of Delaware captioned Wang v. deSouza et al.; (vi) the filing of two securities class actions in the Superior Court of the State of California, County of San Mateo: Loren Scott Mar v. Illumina, et al. and Scott Zerzanek v. Illumina, Inc. et al.; (vii) the filing of a stockholder derivative and class action complaint captioned Icahn Partners LP, et al. v. deSouza, et al.; (viii) the filing of a stockholder derivative complaint captioned City of Omaha Police and Firefighters Retirement System v. deSouza, et al.; (ix) the filing of a stockholder derivative complaint captioned City of Roseville General Employees Retirement System, et al. v. deSouza, et al.; and (x) the filing of a stockholder derivative complaint captioned Thomas P. DiNapoli v. John Thompson et al.; and (xi) the filing of a stockholder derivative complaint captioned Pavers and Road Builders Benefit Funds v. John Thompson et al. See note 9. Legal Proceedings within the Consolidated Financial Statements for further details. In the event that any of the matters described above
result in one or more adverse judgments or settlements, we may experience an adverse impact on our financial condition, results of operations or stock price.
The Spin-Off could result in substantial tax liability.
We received a private letter ruling from the Internal Revenue Service (the IRS) and a written opinion of tax counsel substantially to the effect that, for U.S. federal income tax purposes, the Spin-Off and certain related transactions qualified for non-recognition of gain and loss under Sections 355 and 368 of the U.S. Internal Revenue Code of 1986, as amended. If the factual assumptions or representations made in the request for the private letter ruling prove to have been inaccurate or incomplete in any material respect, then we will not be able to rely on the ruling. Furthermore, the IRS does not rule on whether a distribution such as the Spin-Off satisfies certain requirements necessary to obtain tax-free treatment under Section 355 of the Code. The private letter ruling was based on representations by us and GRAIL that those requirements were satisfied, and any inaccuracy in those representations could invalidate the ruling.
Additionally, the opinion of tax counsel relied on, among other things, the continuing validity of the private letter ruling and various assumptions and representations as to factual matters made by GRAIL and us which, if inaccurate or incomplete in any material respect, would jeopardize the conclusions reached by such counsel in its opinion. The opinion is not binding on the IRS or the courts, and there can be no assurance that the IRS or the courts would not challenge the conclusions stated in the opinion or that any such challenge would not prevail. If, notwithstanding the private letter ruling and opinion of tax counsel, the IRS determines that the Spin-Off and certain related transactions did not qualify for tax-free treatment for U.S. federal income tax purposes, the resulting tax liability to the Company and its shareholders could be substantial.
Following the Spin-Off, we remain the obligor on the contingent value rights (the CVRs) we issued in connection with the GRAIL Acquisition.
Following the Spin-Off, we remain the obligor on the CVRs and, accordingly, continue to be required to record in our financial statements the estimated future liabilities associated with the CVRs. Since we no longer own GRAIL, it may be more difficult for us to estimate these future liabilities. We also may have difficulty complying with our obligations with respect to the CVRs if we are unable to obtain timely and accurate information from GRAIL.
The Spin-Off could adversely affect the market value of the CVRs.
The business of GRAIL may be adversely affected by the Spin-Off, which could adversely affect the market value of the CVRs.
Risks Relating to Litigation
Litigation, other proceedings, or third-party claims of intellectual property infringement could require us to spend significant time and money and could prevent us from selling our products or services.
Our success depends in part on our non-infringement of the patents or proprietary rights of third parties. Third parties have asserted and may in the future assert that we are employing their proprietary technology without authorization. As we enter new markets or introduce new products, we expect that competitors will likely claim that our products infringe their intellectual property rights as part of a business strategy to impede our successful competition. In addition, third parties may have obtained and may in the future obtain patents allowing them to claim that the use of our technologies infringes these patents. We could incur substantial costs and divert the attention of our management and technical personnel in defending ourselves against any of these claims. Any adverse ruling or perception of an adverse ruling in defending ourselves against these claims could have an adverse impact on our stock price, which may be disproportionate to the actual impact of the ruling itself. Furthermore, parties making claims against us may be able to obtain injunctive or other relief, which effectively could block our ability to develop further, commercialize, or sell products or services, and could result in the award of substantial damages against us. In the event of a successful infringement claim against us, we may be required to pay damages and obtain one or more licenses from third parties or be prohibited from selling certain products or services. In addition, we may be unable to obtain these licenses at a reasonable cost, if at all. We could therefore incur substantial costs related to royalty payments for licenses obtained from third parties, which could negatively affect our gross margins and earnings per share. In addition, we could encounter delays in product introductions while we attempt to develop alternative methods or products. Defense of any lawsuit or failure to obtain any of these licenses on favorable terms could prevent us from commercializing
products, and the prohibition of sale of any of our products or services could adversely affect our ability to grow or maintain profitability.
If product or service liability lawsuits are successfully brought against us, we may face reduced demand for our products and incur significant liabilities.
Our products and services are used for sensitive applications, and we face an inherent risk of exposure to product or service liability claims if our products or services are alleged to have caused harm, resulted in false negatives or false positives, or do not perform in accordance with specifications. Product liability claims filed against us or against third parties to whom we may have an obligation could be costly and time-consuming to defend and result in substantial damages or reputational risk. We cannot be certain that we would be able to successfully defend any product or service liability lawsuit brought against us. Regardless of merit or eventual outcome, product or service liability claims may result in: decreased demand for our products; injury to our reputation; increased product liability insurance costs; costs of related litigation; and substantial monetary awards to plaintiffs.
Although we carry product and service liability insurance, if we become the subject of a successful product or service liability lawsuit, our insurance may not cover all substantial liabilities, which could have an adverse effect on our business, financial condition, or results of operations.
Risks Relating to Government Regulation
Changes in, or failure to comply with, competition laws could adversely affect our business, financial condition, or results of operations.
Governments are actively enforcing competition laws and regulations, and some jurisdictions also allow competitors or consumers to assert claims of anti-competitive conduct. U.S. and foreign antitrust authorities have previously brought enforcement actions and may continue to scrutinize our business (see note 9. Legal Proceedings). Regulators have been asserting expansive and sometimes novel interpretations of the scope of existing competition laws, which reduces predictability with respect to compliance. Further, any new requirements or restrictions, or proposed requirements or restrictions, could result in adverse publicity or fines, whether or not valid or subject to appeal. Governmental agencies and regulators may, among other things, prohibit future acquisitions, divestitures, or combinations we seek to make, impose significant fines or penalties, require divestiture of certain assets, or impose other restrictions that limit or require us to modify our operations, including limitations on our contractual relationships with customers or restrictions on our pricing policies. Such rulings or uncertainty regarding regulatory interpretations of industry business practices may alter the way in which we do business and, therefore, may continue to increase our costs or liabilities or reduce demand for our products, which could adversely affect our business, financial condition, or results of operations.
Antitrust enforcement agencies (including the U.S. Department of Justice (DOJ) and the FTC and their non-U.S. equivalents) may continue to closely scrutinize pricing policies or merger activity, with a particular focus on the healthcare sector, and there can be no assurance that our pricing policies or proposed, completed, or future mergers, acquisitions, and divestitures will not be the subject of an investigation or enforcement action by the DOJ, the FTC, or another antitrust enforcement agency. Changes in antitrust laws globally, or in their interpretation, administration, or enforcement, may limit our future acquisitions, divestitures, operations, and growth.
Our products, if used for the diagnosis of disease, could be subject to government regulation, and the regulatory approval and maintenance process for such products may be expensive, time-consuming, and uncertain both in timing and in outcome. Since our strategy includes an emphasis on increasing our participation in clinical markets, we will be increasingly exposed to these risks.
Our products are not subject to FDA clearance or approval if they are not intended to be used for the diagnosis, treatment or prevention of disease. However, as we implement our strategy to increase our participation in clinical markets by expanding our product line to encompass products that are intended to be used for the diagnosis of disease, such as our FDA-regulated MiSeqDx and NextSeq550Dx, certain of our products will become subject to regulation by the FDA, or comparable international agencies, including requirements for regulatory clearance or approval of such products before they can be marketed. Such regulatory approval processes or clearances may be expensive, time-consuming, and uncertain, and our failure to obtain or comply with such approvals and clearances
could have an adverse effect on our business, financial condition, or operating results. Our failure to obtain such clearance or approval in a timely manner, or our competitors’ success in obtaining clearance or approval before we do for products that are competitive with our planned offerings, may result in material adverse business consequences because the investment and time required to seek and obtain clearance or approval for clinical products are substantial. In addition, changes to the current regulatory framework, including the imposition of additional or new regulations, could arise at any time during the development or marketing of our products, which may negatively affect our ability to obtain or maintain FDA or comparable regulatory approval of our products, if required. Diagnostic products are regulated as medical devices by the FDA and comparable international agencies and may require either clearance from the FDA following the 510(k) pre-market notification process or pre-market approval from the FDA, in each case prior to marketing. Obtaining the requisite regulatory approvals can be expensive and may involve considerable delay. If we fail to obtain, or experience significant delays in obtaining, regulatory approvals for diagnostic products that we develop, we may not be able to launch or successfully commercialize such products in a timely manner, or at all.
In addition, if our products labeled as “For Research Use Only. Not for use in diagnostic procedures,” or RUO, are used, or could be used, for the diagnosis of disease, the regulatory requirements related to marketing, selling, and supporting such products could change or be uncertain, even if such use by our customers is without our consent. If the FDA or other regulatory authorities assert that any of our RUO products are subject to regulatory clearance or approval, our business, financial condition, or results of operations could be adversely affected.
In April 2024, the FDA issued the Final Rule relating to Laboratory Development Tests (LDTs). Newly developed LDT products may be subject to regulatory clearance or approval, and could result in adverse impacts to our business, financial condition, or results of operations.
Certain of our in vitro diagnostic products, or IVDs, are currently available through laboratories that are certified under the Clinical Laboratory Improvements Amendments (CLIA) of 1988. These IVD products are commonly called “laboratory developed tests,” or LDTs.
For a number of years, the FDA has exercised its regulatory enforcement discretion to not regulate LDTs as medical devices if created and used within a single laboratory. On April 29, 2024, the FDA released final regulations under 21 CFR Part 809 under the Federal Food, Drug, and Cosmetic Act (FD&C Act) amending the regulations to make explicit that LDTs offered as IVDs are devices under the FD&C Act including when the manufacturer of the IVD is a laboratory (the LDT Rule).
The LDT Rule also provides that the FDA intends to exercise enforcement discretion with regard to premarket review and most quality system requirements for certain categories of IVDs, including currently marketed IVDs offered as LDTs that were first marketed prior to April 29, 2024. The FDA has included additional enforcement discretion policies within the rule for LDTs approved by the New York State’s Clinical Laboratory Evaluation Program (NYS CLEP).
The majority of revenue from products currently offered by our laboratories do not fall within the scope of the LDT Rule. With one exception, the LDTs currently offered as IVDs by our laboratories that fall within the purview of the LDT Rule are approved by NYS CLEP and were first marketed prior to the release of the LDT Rule.
We cannot predict the specifics of how the FDA intends to implement the Final Rule and uncertainties remain as to whether and how newly developed LDT products that are now require regulatory clearance or approval may impact our business, financial condition, or results of operations.
Risks Relating to Information Technology Security and Continuity
Despite using commercially reasonable measures to secure our systems, networks, and products, security breaches, including with respect to cybersecurity, and other disruptions could compromise our information, products, and services, disrupt our or our customers’ operations, and expose us to liability, which could cause our business and reputation to suffer.
In the ordinary course of our business, we collect sensitive data, including intellectual property, our proprietary business information (and that of our customers), and personally identifiable information of our customers, vendors and employees and store it in our data centers and on our networks. Our customers also collect sensitive data and personally identifiable information using our products. The secure maintenance of information is important to our operations and business strategy. Despite our information systems’ security measures and the security measures built
into our products, our information technology infrastructure and our products may in the future be, and have in the past been, impacted by cyber-attacks, employee error, malfeasance, or other disruptions.
We and users of our products may face, and in the past have faced, cyber-attacks, including from nation state actors or advanced persistent threats who attempt to penetrate our or our customers’ network security, including our data centers; sabotage or otherwise disable our research, products, and services, including instruments at our customers’ sites; misappropriate our or our customers' and partners' proprietary information, which may include personally identifiable information; or cause interruptions of our or our customers’ internal operations, systems and services, including through ransomware attacks. Any such breach could compromise our or our customers’ networks and the information stored there could be accessed, publicly disclosed, lost, or exfiltrated. Any such access, disruption, disclosure, or other loss of information could result in an adverse impact on our or our customers’ business, legal claims or proceedings, liability under laws that protect the privacy of personal information, and damage to our reputation.
Disruption of critical information technology systems could have an adverse effect on our operations, business, customer relations, and financial condition.
Our success depends, in part, on the continued and uninterrupted performance of our IT systems, which are used extensively in virtually all aspects of our business. IT systems may be vulnerable to damage from a variety of sources, including telecommunications or network failures, power loss, natural disasters, human acts, terrorist attacks, computer viruses, computer denial-of-service attacks, ransomware attacks, unauthorized access to customer or employee data or company trade secrets, and other attempts to harm our systems. Certain of our systems are not redundant, and our disaster recovery planning is not sufficient for every eventuality. Further, the development of artificial intelligence is creating unforeseen, more sophisticated attacks. Despite any precautions we may take, such problems could result in, among other consequences, disruption of our operations, which could harm our reputation and financial results.
As we continuously adjust our workflow and business practices and add additional functionality to our enterprise software, problems could arise that we have not foreseen, including interruptions in service, loss of data, inaccurate data, or reduced functionality. Such problems could adversely impact our ability to run our business in a timely manner.
Risk Relating to Public Health Crises
We are unable to predict the extent to which public health crises may adversely impact our business operations and financial performance.
Our global operations expose us to risks associated with public health crises. For example, the COVID-19 pandemic significantly curtailed the movement of people, goods and services worldwide, including in the regions in which we sell our products and services and conduct our business operations. How a future public health crisis may impact our business activity could (1) negatively impact the demand for our products and services, (2) restrict our sales operations, marketing efforts, and customer field support, (3) impede the shipping and delivery of our products to customers (4) disrupt our supply chain, and (5) limit our ability to conduct research and product development and other important business activities. We continue to monitor our operations and applicable government mandates and recommendations, and we have made modifications to our operations because of the COVID-19 pandemic. In the event of a public health crisis, we may incur increased costs and experience delays in sales, purchases, deliveries and other business activities associated with the invocation by customers, suppliers, service providers, and other business partners of contractual provisions they may claim are triggered by such an event. Additionally, concerns over the economic impact of a public health crisis like the COVID-19 pandemic could cause volatility in financial and other capital markets which may adversely impact the fair value of our marketable securities.
General Risk Factors
Doing business internationally, especially in emerging markets, creates operational risk for our business.
Conducting and launching operations on an international scale requires close coordination of activities across multiple jurisdictions and consumes significant management resources. If we fail to coordinate and manage these activities effectively, including the risks noted below, our business, financial condition, or results of operations could be adversely affected. We have sales offices located internationally throughout Europe, the Asia-Pacific region, and
Brazil, as well as manufacturing and research facilities in Singapore and the United Kingdom. Shipments to customers outside the United States comprised 48%, 48%, and 50% of our total revenue in 2024, 2023, and 2022, respectively.
We are subject to the following risks and challenges associated with conducting business globally, particularly in emerging international markets, where we expect a growing proportion of our business to be located:
•longer payment cycles and difficulties in collecting accounts receivable outside of the United States;
•longer sales cycles due to the volume of transactions taking place through public tenders;
•challenges in staffing and managing foreign operations;
•tariffs and other trade barriers;
•lack of consistency, and unexpected changes, in legislative or regulatory requirements of foreign countries into which we sell our products;
•increased risk of governmental and regulatory scrutiny and investigations;
•the burden of complying with a wide variety of foreign laws, regulations, and legal standards;
•operating in locations with a higher incidence of corruption and fraudulent business practices;
•import and export requirements, tariffs, taxes, and other trade barriers;
•weak or no protection of intellectual property rights;
•possible enactment of laws regarding the management of and access to data and public networks and websites;
•potential negative impact of a global health crisis, such as the outbreak of a serious infectious disease, to our commercial or manufacturing operations, including the loss of productivity from our own workforce and consequences of any restrictions on the movement of people or materials;
•possible future limitations on foreign-owned businesses;
•uncertainty regarding, or potential changes in, diplomatic and trade relationships, for example, as a result of the recent change in the U.S. government administration;
•the impact of tariffs recently imposed by the U.S. government and its trading partners in response, other possible tariffs or trade protection measures, import or export licensing requirements, new or different customs duties, trade embargoes and sanctions and other trade barriers;
•significant taxes; and
•general geopolitical risks beyond our control, including political, social and economic instability, changes in diplomatic and trade relations as a result of the recent change in the U.S. government administration or for other reasons, and security concerns in general.
Additionally, we must comply with complex foreign and U.S. laws and regulations, such as the U.S. Foreign Corrupt Practices Act, the U.K. Bribery Act, and other local laws prohibiting corrupt payments to governmental officials, anti-competition regulations and sanctions imposed by the U.S. Office of Foreign Assets Control and other similar laws and regulations. Violations of these laws and regulations could result in fines and penalties, criminal sanctions, restrictions on our business conduct and on our ability to offer our products in one or more countries, and could also materially affect our brand, our ability to attract and retain employees, our international operations, our business and our operating results. Although we have implemented policies and procedures designed to ensure compliance with these laws and regulations, there can be no assurance that our employees, contractors, or agents will not violate our policies.
As we continue to expand our business into multiple international markets, our success will depend, in large part, on our ability to anticipate and effectively manage these and other risks associated with our international operations. Any of these risks could harm our international operations and negatively impact our sales, adversely affecting our business, results of operations, financial condition and growth prospects.
The armed conflict between Russia and Ukraine, the international sanctions imposed on Russia, and the restrictions imposed on exports to Russia will likely continue to negatively affect our business. Armed conflict in the Middle East or elsewhere could also negatively impact us.
As a result of the armed conflict between Russia and Ukraine, doing business in the Ukraine may not be practicable. In addition, the U.S. and other countries have imposed sanctions on Russia, including its major financial institutions and certain other businesses and individuals, as well as restrictions on exports to Russia. These sanctions and export restrictions have increased in magnitude over time. Russia has responded in kind, and the continuation of the conflict may result in additional sanctions and export restrictions being enacted by the U.S. or other countries. The impact of these sanctions and export restrictions, along with the spillover effect of ongoing civil, political and economic disturbances on surrounding areas, has affected our ability to ship products into the region, and has reduced our sales. Sanctions or export restrictions currently prohibit our ability to collect or pay liabilities owed by or to certain Russian entities or to supply products and services, directly or indirectly, into Russia. The impact of the Russia-Ukraine conflict, and armed conflict in the Middle East or elsewhere on general economic conditions is currently unknown and could in the future have a negative effect on our results of operations, cash flows, financial condition or growth prospects.
We are exposed to risks associated with transactions denominated in foreign currency.
During 2024, more than half of our international sales were denominated in foreign currencies, while the majority of our purchases of raw materials were denominated in U.S. dollars. Changes in the value of the relevant currencies may affect the cost of certain items required in our operations. Changes in currency exchange rates may also affect the relative prices at which we are able to sell products in the same market. Our revenues from international customers may be negatively impacted as increases in the U.S. dollar relative to our international customers local currency could make our products more expensive, impacting our ability to compete. Our costs of materials from international suppliers may increase if, in order to continue doing business with us, they raise their prices as the value of the U.S. dollar decreases relative to their local currency. Foreign policies and actions regarding currency valuation could result in actions by the United States and other countries to offset the effects of such fluctuations. Recent global financial conditions have led to a high level of volatility in foreign currency exchange rates and that level of volatility may continue, which could adversely affect our business, financial condition, or results of operations.
We are subject to risks related to taxation in multiple jurisdictions.
We are subject to income taxes in both the United States and numerous foreign jurisdictions. Significant judgments based on interpretations of existing tax laws or regulations are required in determining the provision for income taxes. Our effective income tax rate could be adversely affected by various factors, including, but not limited to, changes in the mix of earnings in tax jurisdictions with different statutory tax rates, changes in the valuation of deferred tax assets and liabilities, changes in existing tax policies, laws, regulations, or rates (including the implementation of global minimum tax rates in certain jurisdictions), changes in the level of non-deductible expenses (including share-based compensation), location of operations, changes in our future levels of research and development spending, mergers and acquisitions, or the result of examinations by various tax authorities. Although we believe our tax estimates are reasonable, if the U.S. Internal Revenue Service or other taxing authority disagrees with the positions taken on our tax returns, we could have additional tax liability, including interest and penalties. If material, payment of such additional amounts upon final adjudication of any disputes could have a material impact on our results of operations and financial position.
Our operating results may vary significantly from period to period.
Our revenue is subject to fluctuations due to the timing of sales of high-value products and services, the effects of new product launches and related promotions, the timing and availability of our customers’ funding, the impact of seasonal spending patterns, the timing and size of research projects our customers perform, changes in overall spending levels in the life sciences industry, and other unpredictable factors that may affect customer ordering patterns. In particular, collaboration agreements and large-scale government funded projects such as population genomic projects are the result of lengthy and complex negotiations, and the timing of revenue recognition in connection with these agreements
and projects may be subject to significant uncertainty because of the long-term nature of development and collaboration projects, as well as sample availability for population genomics projects.
Given the difficulty in predicting the timing and magnitude of sales for our products and services, we may experience quarter-to-quarter fluctuations in revenue resulting in the potential for a sequential decline in quarterly revenue. As a substantial portion of our quarterly revenue is typically recognized in the last month of the quarter with a concentration of orders in the final weeks, our manufacturing and shipping operations may experience increased pressure and demand during the time period shortly before the end of a fiscal quarter; delays related to our manufacturing and shipping operations during this time period could delay the recognition of revenue.
From time to time, we receive large orders that have a significant effect on our operating results in the period in which the order is recognized as revenue. The timing of such orders is difficult to predict, and the timing of revenue recognition from such orders may affect period-to-period changes in net sales. As a result, our operating results could vary materially from quarter-to-quarter based on the receipt of such orders and their ultimate recognition as revenue.
Adverse economic or market conditions may harm our business.
Worsening economic conditions, including inflation, increasing interest rates, decreasing economic activity, volatility in equity and credit markets or other changes in the economic environment, may adversely affect our business, financial condition, or results of operations. For example, we depend on third-party manufacturers and suppliers for some of our products, or sub-assemblies, components, and materials used in our products, and the suppliers of these inputs may seek to raise prices in the current inflationary economic environment. If our costs increase and we are unable to successfully pass along those increased costs to our customers, our revenue and or operating profitability may be adversely affected. In addition, we have a variable-interest-rate credit facility (see note 6. Debt and Other Commitments), under which we have no currently outstanding debt, and we may in the future raise additional debt or refinance existing debt. Our cost of borrowing in the future may be higher than it has been to date because interest rates have risen and may continue to increase. An increased cost of borrowing may adversely affect our financial condition and results of operations.
LEGAL PROCEEDINGS
See discussion of legal proceedings in note 9. Legal Proceedings within the Consolidated Financial Statements section of this report, which is incorporated by reference herein.
MARKET INFORMATION
Our common stock has been quoted on The Nasdaq Global Select Market under the symbol “ILMN” since July 28, 2000. The following table sets forth, for the fiscal periods indicated, the quarterly high and low sales prices per share of our common stock as reported on The Nasdaq Global Select Market.
| | | | | | | | | | | | | | | | | | | | | | | |
| | 2024 | | 2023 |
| | High | | Low | | High | | Low |
| First Quarter | $ | 145.50 | | | $ | 123.54 | | | $ | 238.55 | | | $ | 182.00 | |
| Second Quarter | $ | 136.02 | | | $ | 98.27 | | | $ | 233.42 | | | $ | 181.62 | |
| Third Quarter | $ | 137.18 | | | $ | 103.57 | | | $ | 195.64 | | | $ | 127.37 | |
| Fourth Quarter | $ | 156.66 | | | $ | 125.06 | | | $ | 143.93 | | | $ | 89.00 | |
Stock Performance Graph
The graph below compares the cumulative total stockholder returns on our common stock for the last five fiscal years with the cumulative total stockholder returns on the Nasdaq Composite Index, the Nasdaq Biotechnology Index, and the S&P 500 Index for the same period. The graph assumes that $100 was invested on December 29, 2019 in our common stock and in each index and that all dividends were reinvested. No cash dividends have been declared on our common stock. Stockholder returns over the indicated period should not be considered indicative of future stockholder returns.
Compare 5-Year Cumulative Total Return among Illumina, Nasdaq Composite Index,
Nasdaq Biotechnology Index, and S&P 500 Index
Holders
As of February 7, 2025, we had 497 record holders of our common stock.
Dividends
We have never paid cash dividends and have no present intention to pay cash dividends in the foreseeable future.
SHARE REPURCHASES AND SALES
Purchases of Equity Securities by the Issuer
In August 2024, our Board of Directors authorized a new share repurchase program, which cancels and supersedes all prior and available repurchase authorizations, to repurchase up to $1.5 billion of our outstanding common stock. The repurchases may be completed through open market purchases, pursuant to Rule 10b5-1 or Rule 10b-18, or through an accelerated share repurchase program. We did not repurchase any shares under the prior program during 2024. Shares repurchased in open market transactions pursuant to the new program during 2024 were as follows:
| | | | | | | | | | | | | | | | | | | | | | | |
| In thousands, except price per share |
Total Number
of Shares
Purchased | | Average Price Paid per Share(1) | | Total Number of Shares Purchased as Part of Publicly Announced Program | | Approximate Dollar Value of Shares that May Yet Be Purchased Under the Program |
| Third Quarter | 770 | | | $ | 127.71 | | | 770 | | | $ | 1,401,684 | |
Fourth Quarter (1) | 134 | | | $ | 129.02 | | | 134 | | | $ | 1,384,404 | |
| Total | 904 | | | $ | 127.90 | | | 904 | | | $ | 1,384,404 | |
_____________(1)Average price paid per share excludes the excise tax on share repurchases imposed as part of the Inflation Reduction Act of 2022.
(1) Repurchases during the fourth quarter of 2024 were as follows:
| | | | | | | | | | | | | | | | | | | | | | | |
| In thousands, except price per share |
Total Number
of Shares
Purchased | | Average Price Paid per Share(1) | | Total Number of Shares Purchased as Part of Publicly Announced Program | | Approximate Dollar Value of Shares that May Yet Be Purchased Under the Program |
| September 30, 2024 - October 27, 2024 | 101 | | | $ | 128.72 | | | 101 | | | $ | 1,388,718 | |
| October 28, 2024 - November 24, 2024 | 33 | | | $ | 129.91 | | | 33 | | | $ | 1,384,404 | |
| November 25, 2024 - December 29, 2024 | — | | | $ | — | | | — | | | $ | 1,384,404 | |
| Total | 134 | | | $ | 129.02 | | | 134 | | | $ | 1,384,404 | |
_____________(1)Average price paid per share excludes the excise tax on share repurchases imposed as part of the Inflation Reduction Act of 2022.
Sales of Unregistered Securities
There were no sales of unregistered securities in 2024.
| | |
| MANAGEMENT’S DISCUSSION & ANALYSIS |
Our Management’s Discussion and Analysis (MD&A) will help readers understand our results of operations, financial condition, and cash flow. It is provided in addition to the accompanying consolidated financial statements and notes. This MD&A is organized as follows:
•Management’s Overview and Outlook. High level discussion of our operating results and significant known trends that affect our business.
•Results of Operations. Detailed discussion of our revenues and expenses.
•Liquidity and Capital Resources. Discussion of key aspects of our consolidated statements of cash flows, changes in our financial position, and our financial commitments.
•Critical Accounting Policies and Estimates. Discussion of critical accounting policies and the significant assumptions, estimates, and judgments we make in applying such policies.
•Quantitative and Qualitative Disclosure about Market Risk. Discussion of our financial instruments’ exposure to market risk.
•Recent Accounting Pronouncements. Summary of recent accounting pronouncements applicable to our consolidated financial statements.
This MD&A generally discusses 2024 and 2023 items and year-to-year comparisons between 2024 and 2023. Discussions of 2022 items and year-to-year comparisons between 2023 and 2022 that are not included in this Form 10-K can be found in "Management's Discussion and Analysis of Financial Condition and Results of Operations" in our Annual Report on Form 10-K for the fiscal year ended 2023.
This MD&A discussion contains forward-looking statements that involve risks and uncertainties. See Consideration Regarding Forward-Looking Statements preceding the Business & Market Overview section of this report for additional factors relating to such statements. See Risk Factors within the Business & Market Information section of this report for a discussion of certain risk factors applicable to our business, financial condition, and results of operations. Operating results are not necessarily indicative of results that may occur in future periods.
MANAGEMENT’S OVERVIEW AND OUTLOOK
This overview and outlook provide a high-level discussion of our operating results and significant known trends that affect our business. We believe an understanding of these trends is important to understanding our financial results for the periods being reported herein as well as our future financial performance. This summary is not intended to be exhaustive, nor is it intended to be a substitute for the detailed discussion and analysis provided elsewhere.
About Illumina
Our focus on innovation has established us as a global leader in DNA sequencing and array-based technologies, serving customers in the research, clinical and applied markets. Our products are used for applications in the life sciences, oncology, reproductive health, agriculture and other emerging segments. Our customers include leading genomic research centers, academic institutions, government laboratories, and hospitals, as well as pharmaceutical, biotechnology, commercial molecular diagnostic laboratories, and consumer genomics companies. Our comprehensive line of products addresses the scale of experimentation and breadth of functional analysis to advance disease research, drug development, and the development of molecular tests. This portfolio of leading-edge sequencing and array-based solutions addresses a range of genomic complexity and throughput, enabling researchers and clinical practitioners to select the best solution for their scientific challenge.
On June 24, 2024, we completed the Spin-Off of GRAIL into a separate, independent publicly traded company through the distribution of approximately 85.5% of the outstanding shares of common stock of GRAIL to Illumina stockholders on a pro rata basis. We retained approximately 14.5% of the shares of GRAIL common stock immediately following the Spin-Off. The disposition of GRAIL did not meet the criteria to be reported as a discontinued operation and accordingly, GRAIL’s assets, liabilities, results of operations and cash flows have not been reclassified. In connection with the Spin-Off, Illumina’s stockholders received one share of GRAIL common stock for every six shares of Illumina common stock held on the Record Date. Refer to note 2. GRAIL Spin-Off for further details. We have one reportable segment, Core Illumina, as of December 29, 2024. Prior to the Spin-Off of GRAIL on June 24, 2024, our reportable segments included both Core Illumina and GRAIL. See note 12. Segment and Geographic Information within the Consolidated Financial Statements section of this report for details on our reportable segments. Our financial results have been, and will continue to be, impacted by several significant trends, which are described below. While these trends are important to understanding and evaluating our financial results, this discussion should be read in conjunction with our consolidated financial statements and the notes thereto within the Consolidated Financial Statements section of this report, and the other transactions, events, and trends discussed in Risk Factors within the Business & Market Information section of this report. Financial Overview
Since 2023, macroeconomic factors such as inflation, exchange rate fluctuations and concerns about an economic downturn, competitive challenges in our China region, and the sanctions imposed on Russia as a result of the armed conflict between Russia and Ukraine have impacted both Illumina directly and our customers’ behavior. For example, some customers experienced supply chain pressures that delayed their lab expansions and others are managing inventory and capital more conservatively. We expect these factors to continue to have an impact on our sales and results of operations in 2025, the size and duration of which is significantly uncertain. In February 2025, we were added to China’s Ministry of Commerce List of Unreliable Entities, the implications of which are currently uncertain. See the risk factor “China’s Ministry of Commerce has added Illumina to its List of Unreliable Entities” in Risk Factors within the Business & Market Information section of this report for additional information. During 2024, we made significant progress towards our strategic goals to accelerate growth and expand operating margins. We focused on operational excellence initiatives, to improve productivity and achieve cost savings, and on our capital allocation strategy, including obtaining authorization for a new share repurchase program. We expect to continue to make further progress towards these goals in 2025, including a focus on returning to revenue growth.
Financial highlights for 2024 included the following:
•Revenue decreased 3% in 2024 to $4.4 billion compared to $4.5 billion in 2023 primarily due to a decrease in sequencing instruments revenue, driven by fewer shipments of our high-throughput and mid-throughput instruments, offset by increases in sequencing consumables revenue.
•Gross profit as a percentage of revenue (gross margin) was 65.4% in 2024 compared to 60.9% in 2023. The increase in gross margin was driven primarily by execution of our operational excellence initiatives that continue to deliver cost savings, including freight, and improve productivity, a more favorable revenue mix towards sequencing consumables, and a decrease in warranty and field service costs. This was partially offset by higher strategic partnership revenue that is lower margin. Our gross margin depends on many factors, including: market conditions that may impact our pricing; sales mix changes among consumables, instruments, services, and development and licensing revenue; product mix changes between established products and new products; excess and obsolete inventories; royalties; our cost structure for manufacturing operations relative to volume; freight costs; and product support obligations.
•Loss from operations was $0.8 billion in 2024 compared to $1.1 billion in 2023. The decrease was due to a decrease in operating expense of $119 million and an increase in gross profit of $117 million. The decrease in operating expense included a $476 million favorable impact in legal contingency and settlement, as a result of the European Commission withdrawing, in September 2024, its previously imposed fine, a decrease of $323 million in GRAIL operating expenses, as a result of the Spin-Off in Q2 2024, an increase in the gains recognized on our GRAIL contingent consideration liability of $290 million, and a decrease in restructuring charges of $86 million, offset by an increase in goodwill and intangible impairment of $1,062 million. We continue to focus on our cost reduction initiatives to accelerate progress toward higher margins and create flexibility for further investment in high-growth areas.
•Our effective tax rate was (3.8)% and (3.9)% in 2024 and 2023, respectively. In 2024, the variance from the U.S. federal statutory tax rate of 21% was primarily because of the income tax expense impact of the impairment of goodwill, which is nondeductible for tax purposes, the income tax expense impact of the reversal of the European Commission fine related to the GRAIL acquisition, which is excluded from taxable income, the income tax expense impact of research and development expense capitalization for tax purposes, and the income tax expense impact of GRAIL pre-acquisition net operating losses on GILTI, the utilization of U.S. foreign tax credits, and the Pillar Two global minimum top-up tax. This was partially offset by the mix of earnings in jurisdictions with lower statutory tax rates than the U.S. federal statutory tax rate, such as in Singapore.
•We ended 2024 with cash, cash equivalents, and short-term investments totaling $1,220 million, of which approximately $439 million was held by our foreign subsidiaries.
RESULTS OF OPERATIONS
To enhance comparability, the following table sets forth audited consolidated statement of operations data for 2024, 2023, and 2022, stated as a percentage of total revenue.(1)
| | | | | | | | | | | | | | | | | |
| 2024 | | 2023 | | 2022 |
| Revenue: | | | | | |
| Product revenue | 83.6 | % | | 84.1 | % | | 86.2 | % |
| Service and other revenue | 16.4 | | | 15.9 | | | 13.8 | |
| Total revenue | 100.0 | | | 100.0 | | | 100.0 | |
| Cost of revenue: | | | | | |
| Cost of product revenue | 23.3 | | | 26.1 | | | 25.0 | |
| Cost of service and other revenue | 8.4 | | | 8.7 | | | 6.4 | |
| Amortization of acquired intangible assets | 2.9 | | | 4.3 | | | 3.8 | |
| Total cost of revenue | 34.6 | | | 39.1 | | | 35.2 | |
| Gross profit | 65.4 | | | 60.9 | | | 64.8 | |
| Operating expense: | | | | | |
| Research and development | 26.7 | | | 30.1 | | | 28.8 | |
| Selling, general and administrative | 25.0 | | | 35.8 | | | 28.3 | |
| Goodwill and intangible impairment | 43.2 | | | 18.3 | | | 85.4 | |
| Legal contingency and settlement | (10.4) | | | 0.4 | | | 13.5 | |
| Total operating expense | 84.5 | | | 84.6 | | | 156.0 | |
| Loss from operations | (19.1) | | | (23.7) | | | (91.2) | |
| Other income (expense): | | | | | |
| Interest income | 1.1 | | | 1.3 | | | 0.2 | |
| Interest expense | (2.3) | | | (1.7) | | | (0.6) | |
| Other expense, net | (6.7) | | | (0.7) | | | (3.0) | |
| Total other expense, net | (7.9) | | | (1.1) | | | (3.4) | |
| Loss before income taxes | (27.0) | | | (24.8) | | | (94.6) | |
| Provision for income taxes | 1.0 | | | 1.0 | | | 1.5 | |
| Net loss | (28.0) | % | | (25.8) | % | | (96.1) | % |
_____________
(1)Percentages may not recalculate due to rounding.
Revenue
| | | | | | | | | | | | | | | | | | | | | | | |
| 2024-2023 |
| Dollars in millions | 2024 | | 2023 | | Change | | % Change |
| Core Illumina: | | | | | | | |
| Consumables | $ | 3,169 | | | $ | 3,106 | | | $ | 63 | | | 2 | % |
| Instruments | 501 | | | 706 | | | (205) | | | (29) | |
| Total product revenue | 3,670 | | | 3,812 | | | (142) | | | (4) | |
| Service and other revenue | 662 | | | 626 | | | 36 | | | 6 | |
| Total Core Illumina revenue | 4,332 | | | 4,438 | | | (106) | | | (2) | |
| GRAIL: | | | | | | | |
| Service and other revenue | 55 | | | 93 | | | (38) | | | (41) | |
| Eliminations | (15) | | | (27) | | | 12 | | | (44) | |
| Total consolidated revenue | $ | 4,372 | | | $ | 4,504 | | | $ | (132) | | | (3) | % |
Core Illumina consumables revenue increased in 2024 primarily due to an increase in sequencing consumables revenue of $59 million, driven primarily by an increase in NovaSeq X consumables, partially offset by decreases in consumables across our other high-throughput instruments, as customers transition to NovaSeq X, and across our mid-throughput instruments. Core Illumina instruments revenue decreased in 2024 primarily due to a decrease in sequencing instruments revenue of $203 million, driven by fewer shipments of our high-throughput instruments, following the first year of NovaSeq X shipments in 2023, and fewer shipments of our mid-throughput instruments, as capital and cash flow constraints continue to impact our customer’s purchasing behavior. Core Illumina service and other revenue increased in 2024 primarily due to increased revenue from strategic partnerships and extended maintenance service contracts, partially offset by decreased revenues from genotyping services and development and licensing agreements.
The decrease in GRAIL revenue in 2024 was due to the Spin-Off in Q2 2024.
Gross Margin
| | | | | | | | | | | | | | | | | | | | | | | |
| 2024-2023 |
| Dollars in millions | 2024 | | 2023 | | Change | | % Change |
| Gross profit (loss): | | | | | | | |
| Core Illumina | $ | 2,909 | | $ | 2,856 | | $ | 53 | | | 2 | % |
| GRAIL | (38) | | (96) | | 58 | | | (60) | |
| Eliminations | (10) | | (16) | | 6 | | | (38) | |
| Consolidated gross profit | $ | 2,861 | | $ | 2,744 | | $ | 117 | | | 4 | % |
| | | | | | | |
| Gross margin: | | | | | | | |
| Core Illumina | 67.1 | % | | 64.4 | % | | | | |
| GRAIL | * | | * | | | | |
| Consolidated gross margin | 65.4 | % | | 60.9 | % | | | | |
_____________
*Not meaningful.
The increase in Core Illumina gross margin in 2024 was driven primarily by a favorable impact from the execution of our operational excellence initiatives that continue to deliver cost savings, including freight, and improve productivity, a more favorable revenue mix towards sequencing consumables, and a decrease in warranty and field service costs, partially offset by higher strategic partnership revenue that is lower margin.
The decrease in GRAIL gross loss in 2024 was due to the Spin-Off in Q2 2024.
Operating Expense
| | | | | | | | | | | | | | | | | | | | | | | |
| 2024-2023 |
| Dollars in millions | 2024 | | 2023 | | Change | | % Change |
| Research and development: | | | | | | | |
| Core Illumina | $ | 988 | | | $ | 1,030 | | | $ | (42) | | | (4) | % |
| GRAIL | 189 | | | 338 | | | (149) | | | (44) | |
| Eliminations | (8) | | | (14) | | | 6 | | | (43) | |
| Consolidated research and development | 1,169 | | | 1,354 | | | (185) | | | (14) | |
| | | | | | | |
| Selling, general and administrative: | | | | | | | |
| Core Illumina | 900 | | | 1,248 | | | (348) | | | (28) | |
| GRAIL | 192 | | | 366 | | | (174) | | | (48) | |
| Eliminations | — | | | (2) | | | 2 | | | (100) | |
| Consolidated selling, general and administrative | 1,092 | | | 1,612 | | | (520) | | | (32) | |
| | | | | | | |
| Goodwill and intangible impairment: | | | | | | | |
| Core Illumina | 3 | | | 6 | | | (3) | | | (50) | |
| GRAIL | 1,886 | | | 821 | | | 1,065 | | | 130 | |
| Consolidated goodwill and intangible impairment | 1,889 | | | 827 | | | 1,062 | | | 128 | |
| | | | | | | |
| Legal contingency and settlement: | | | | | | | |
| Core Illumina | (456) | | | 20 | | | (476) | | | (2,380) | |
| | | | | | | |
| Total consolidated operating expense | $ | 3,694 | | | $ | 3,813 | | | $ | (119) | | | (3) | % |
Core Illumina R&D expense decreased by $42 million, or 4%, in 2024 primarily due to decreases in headcount and employee compensation costs, and restructuring charges of $25 million, as we focus on cost reduction initiatives.
Core Illumina SG&A expense decreased by $348 million, or 28%, in 2024 primarily due to an increase in the gains recognized on our GRAIL contingent consideration liability of $290 million, a decrease in proxy contest charges of $30 million, and decreases in restructuring charges of $61 million, facility related costs, and employee compensation costs, as we continue to exit certain of our facilities and focus on our cost reduction initiatives. The decrease was partially offset by an increase in share-based compensation expense related to PSU awards.
The decrease in GRAIL R&D and SG&A expense in 2024 was due to the Spin-Off in Q2 2024.
GRAIL goodwill and intangible impairment in 2024 consisted of goodwill impairment of $1,466 million and IPR&D impairment of $420 million as a result of impairment tests performed in Q2 2024. Core Illumina goodwill and intangible impairment for 2024 consisted of an IPR&D impairment recorded in Q1 2024. GRAIL goodwill and intangible impairment for 2023 consisted of goodwill impairment of $712 million and IPR&D impairment of $109 million as a result of an interim impairment test performed in Q3 2023. See note 5. Goodwill, Intangible Assets, and Acquisitions. Core Illumina legal contingency and settlement in 2024 primarily consisted of a gain recognized in Q3 2024 of $489 million for the reversal of the EC fine accrual, and related accrued interest, following the European Commission’s decision to withdraw its previously imposed fine. Core Illumina legal contingency and settlement for 2023 primarily consisted of an adjustment to our accrual for the fine previously imposed by the European Commission and other patent litigation settlement activity. See note 9. Legal Proceedings for additional details.
Other Income (Expense)
| | | | | | | | | | | | | | | | | | | | | | | |
| 2024-2023 |
| Dollars in millions | 2024 | | 2023 | | Change | | % Change |
| Interest income | $ | 46 | | | $ | 58 | | | $ | (12) | | | (21) | % |
| Interest expense | (100) | | | (77) | | | (23) | | | 30 | |
| Other expense, net | (292) | | | (29) | | | (263) | | | 907 | |
| Total other expense, net | $ | (346) | | | $ | (48) | | | $ | (298) | | | 621 | % |
Total other expense, net primarily relates to the Core Illumina segment.
Interest income consisted primarily of interest on our money market funds, which decreased in 2024 primarily due to a lower cash balance throughout the year, as compared to prior year, partially offset by an increase in average interest rates. Interest expense consisted primarily of interest on our outstanding term debt, including on our delayed draw term loan, and a loss on debt extinguishment of $5 million related to the repayment of our delayed draw term loan. The increase in other expense, net in 2024 was primarily driven by an increase in net losses recognized on our strategic investments of $272 million, which included an unrealized loss of $309 million on our retained investment in GRAIL subsequent to the Spin-Off. This was offset by a favorable net impact related to foreign currency activity, as compared to the prior year, and an increase of $5 million in the gains recognized on our Helix contingent value right, which was settled in Q3 2024.
Provision for Income Taxes
| | | | | | | | | | | | | | | | | | | | | | | |
| 2024-2023 |
| Dollars in millions | 2024 | | 2023 | | Change | | % Change |
| Loss before income taxes | $ | (1,179) | | $ | (1,117) | | $ | (62) | | | 6 | % |
| Provision for income taxes | 44 | | 44 | | — | | | — | |
| Net loss | $ | (1,223) | | $ | (1,161) | | $ | (62) | | | 5 | % |
| Effective tax rate | (3.8)% | | (3.9)% | | | | |
In 2024, the variance from the U.S. federal statutory tax rate of 21% was primarily because of the $308 million income tax expense impact from the impairment of goodwill, which is nondeductible for tax purposes, $90 million income tax expense impact of GRAIL pre-acquisition net operating losses on GILTI, the utilization of U.S. foreign tax credits, and the Pillar Two global minimum top-up tax, and the $52 million income tax expense impact of capitalizing research and development expenses for tax purposes. The income tax rate in 2024 was favorably impacted by the $99 million income tax expense impact of the reversal of the European Commission fine related to the GRAIL acquisition, which is excluded from taxable income, and by the mix of earnings in jurisdictions with lower statutory tax rates than the U.S. federal statutory tax rate, such as in Singapore.
In 2023, the variance from the U.S. federal statutory tax rate of 21% was primarily because of the $149 million income tax expense impact from the impairment of goodwill, which is nondeductible for tax purposes, the $86 million income tax expense impact of capitalizing research and development expense for tax purposes, and the $61 million income tax expense impact of GRAIL pre-acquisition net operating losses on GILTI and the utilization of the U.S. foreign tax credits. The income tax expense in 2023 was also favorably impacted by the mix of earnings in jurisdictions with lower statutory tax rates than the U.S. federal statutory tax rate, such as in Singapore.
Our future effective tax rate may vary from the U.S. federal statutory tax rate due to the mix of earnings in tax jurisdictions with different statutory tax rates and the other factors discussed in the risk factor “We are subject to risks related to taxation in multiple jurisdictions” in Risk Factors within the Business & Market Information section of this report, including future tax legislation that changes existing tax policies, laws, regulations, or rates.
LIQUIDITY AND CAPITAL RESOURCES
As of December 29, 2024, we had $1,127 million in cash and cash equivalents, of which approximately $439 million was held by our foreign subsidiaries. Cash and cash equivalents increased by $79 million from the prior year due primarily to factors described in the “Cash Flow Summary” below. Our primary source of liquidity, other than our holdings of cash, cash equivalents, and investments, has been cash flows from operations and, from time to time, issuances of debt. In 2024, we received net proceeds from the issuance of our 2026 Term Notes of $497 million and repaid our delayed draw term loan of $750 million. Our ability to generate cash from operations, supplemented with the issuance of debt and/or liquidation of our short-term investments, provides us with the financial flexibility we need to meet operating, investing, and financing needs. In connection with the Spin-Off, we derecognized GRAIL’s cash and cash equivalents of $968 million, which included the required Disposal Funding (see note 2. GRAIL Spin-Off). As of December 29, 2024, we had $93 million in short-term investments, comprised of marketable equity securities. In September 2024, the European Commission withdrew its previously imposed fine of €432 million. Accordingly, we reversed the related accrual and recognized a net gain of $481 million in Q3 2024. The guarantees we provided in October 2023 to satisfy the obligation in lieu of cash payment while we appealed the European Commission’s jurisdictional and fine decisions are no longer outstanding. Refer to note 9. Legal Proceedings for additional details. In June 2024, we entered into a 364-day Delayed Draw Credit Facility, which provided us with a senior unsecured term loan credit facility in an aggregate principal amount of up to $750 million. On June 20, 2024, we borrowed $750 million on the Delayed Draw Credit Facility. The delayed draw term loan incurred interest at a rate of 6.7%. On September 9, 2024, we repaid the full principal amount outstanding under the Delayed Draw Credit Facility, as well as accrued interest, in an aggregate amount of $761 million and terminated the Delayed Draw Credit Agreement.
On September 9, 2024, we issued $500 million aggregate principal amount of 2026 Term Notes. We received net proceeds of $497 million, which were used to repay a portion of the outstanding debt under the Delayed Draw Credit Agreement. The 2026 Term Notes, which mature on September 9, 2026, accrue interest at a rate of 4.650% per annum, payable semi-annually on March 9 and September 9 of each year, beginning on March 9, 2025. We may redeem for cash all or any portion of the 2026 Term Notes, at our option, at any time prior to maturity.
In December 2022, we issued $500 million aggregate principal amount of 2025 Term Notes and $500 million aggregate principal amount of 2027 Term Notes. The 2025 Term Notes, which mature on December 12, 2025, and the 2027 Term Notes, which mature on December 13, 2027, accrue interest at a rate of 5.800% and 5.750% per annum, respectively, payable semi-annually in June and December of each year. In March 2021, we issued $500 million aggregate principal amount of 2031 Term Notes, which mature on March 23, 2031 and accrue interest at a rate of 2.550% per annum, payable semi-annually in March and September of each year. We may redeem for cash all or any portion of the 2025, 2027, or 2031 Term Notes, at our option, at any time prior to maturity.
In January 2023, we entered into the Revolving Credit Agreement, which provides us with a $750 million senior unsecured five-year revolving credit facility, including a $40 million sublimit for swingline borrowings and a $50 million sublimit for letters of credit. The credit facility matures, and all amounts outstanding become due and payable in full, on January 4, 2028, subject to two one-year extensions at our option, the consent of the extending lenders, and certain other conditions. As of December 29, 2024, there were no outstanding borrowings.
As of December 29, 2024, the fair value of our contingent consideration liability related to GRAIL was $71 million, of which $70 million was included in other long-term liabilities. The contingent value rights entitle the holders to receive future cash payments on a quarterly basis (Covered Revenue Payments) representing a pro rata portion of certain GRAIL-related revenues (Covered Revenues) each year through August 2033. This reflects a 2.5% payment right to the first $1 billion of revenue each year for 12 years. Revenue above $1 billion each year is subject to a 9% contingent payment right during this same period. In 2024, we paid $1.1 million in aggregate Covered Revenue Payments related to Covered Revenues for the period Q4 2023 through Q3 2024 of $117 million in aggregate.
In August 2024, our Board of Directors authorized a new share repurchase program, which cancels and supersedes all prior and available repurchase authorizations, to repurchase up to $1.5 billion of our outstanding common stock. The repurchases may be completed through open market purchases, pursuant to Rule 10b5-1 or Rule 10b-18, or through an accelerated share repurchase program. Authorizations to repurchase up to $1.4 billion of our outstanding common stock remained available as of December 29, 2024. Subsequent to December 29, 2024 and through February 11, 2025, we repurchased an additional 1.0 million shares of our common stock for $126 million.
We had $3 million (plus recallable distributions of approximately $10 million), $46 million, and $47 million, respectively, remaining in our capital commitments to three venture capital investment funds as of December 29, 2024 that are callable through April 2026, July 2029, and December 2034, respectively.
The impact of the 2017 Tax Cuts and Jobs Act resulted in a one-time transition tax on earnings of certain foreign subsidiaries which we elected to pay in installments. As of December 29, 2024, we owed $39 million, which we expect to pay within the next year.
Our other short-term and long-term material cash requirements, from known contractual obligations as of December 29, 2024, include operating lease liabilities, uncertain tax positions, and amounts due under our executive deferred compensation plan, as discussed in the Consolidated Financial Statements section of this report.
We anticipate that our current cash, cash equivalents, and short-term investments, together with cash provided by operating activities and available borrowing capacity under the Revolving Credit Facility, are sufficient to fund our near-term capital and operating needs for at least the next 12 months. Operating needs include the planned costs to operate our business, including amounts required to fund working capital and capital expenditures. Our primary short-term needs for capital, which are subject to change, may include:
•support of commercialization efforts related to our current and future products;
•acquisitions of equipment and other fixed assets for use in our current and future manufacturing and research and development facilities;
•the continued advancement of research and development efforts;
•potential strategic acquisitions and investments;
•repayment of debt obligations;
•repurchases of our outstanding common stock; and
•the evolving needs of our facilities, including costs of leasing and building out facilities.
We expect that our revenue and results of operations, as well as the status of each of our new product development programs, will significantly impact our cash management decisions. Our future capital requirements and the adequacy of our available funds will depend on many factors, including:
•our ability to successfully commercialize and further develop our technologies and create innovative products in our markets;
•scientific progress in our research and development programs and the magnitude of those programs;
•competing technological and market developments; and
•the need to enter into collaborations with other companies or acquire other companies or technologies to enhance or complement our product and service offerings.
Cash Flow Summary
| | | | | | | | | | | | | | | | | |
| In millions | 2024 | | 2023 | | 2022 |
| Net cash provided by operating activities | $ | 837 | | | $ | 478 | | | $ | 392 | |
| Net cash used in investing activities | (178) | | | (231) | | | (591) | |
| Net cash (used in) provided by financing activities | (570) | | | (1,210) | | | 1,000 | |
| Effect of exchange rate changes on cash and cash equivalents | (10) | | | — | | | (22) | |
| Net increase (decrease) in cash and cash equivalents | $ | 79 | | | $ | (963) | | | $ | 779 | |
Operating Activities
Net cash provided by operating activities in 2024 consisted of a net loss of $1,223 million, plus net adjustments of $2,543 million, less net changes in operating assets and liabilities of $483 million. The primary adjustments to net loss included goodwill and intangible impairment of $1,889 million, share-based compensation expense of $370 million, depreciation and amortization expense of $354 million, net loss on strategic investments of $312 million, and property and equipment and right-of-use asset impairment of $46 million, offset by change in fair value of contingent consideration liabilities of $315 million and deferred income taxes of $112 million. Cash flow impact from changes in net operating assets and liabilities were primarily driven by decreases in accrued liabilities and inventory, offset by increases in accounts receivable, operating lease right-of-use assets and liabilities, net, and other long-term liabilities.
Net cash provided by operating activities in 2023 consisted of net adjustments of $1,729 million, less a net loss of $1,161 million and net changes in operating assets and liabilities of $90 million. The primary adjustments to net loss included goodwill and intangible impairment of $827 million, depreciation and amortization expense of $432 million, share-based compensation of $380 million, property and equipment and right-of-use asset impairment of $100 million, and net loss on strategic investments of $40 million, offset by deferred income taxes of $33 million and change in fair value of contingent consideration liabilities of $24 million. Cash flow impact from changes in net operating assets and liabilities were primarily driven by increases in accounts receivable and inventory and a decrease in accounts payable.
Investing Activities
Net cash used in investing activities totaled $178 million in 2024. We invested $128 million in capital expenditures, net of proceeds received from sales, primarily associated with investments in facilities, paid $81 million for an acquisition, net of cash acquired, and other intangible assets, and purchased strategic investments, net of distributions, of $52 million. This was offset by the receipt of $83 million related to the settlement of our Helix contingent value right.
Net cash used in investing activities totaled $231 million in 2023. We invested $195 million in capital expenditures, primarily associated with our investment in facilities, paid $30 million for an acquisition, net of cash acquired, and other intangible assets, and used $6 million for net purchases of strategic investments.
Financing Activities
Net cash used in financing activities totaled $570 million in 2024. We deconsolidated cash and cash equivalents of $968 million, as a result of the GRAIL Spin-Off, repaid our delayed draw term loan of $750 million, used $116 million to repurchase our common stock, and used $32 million to pay taxes related to net share settlement of equity awards. This was offset by net borrowings on the Delayed Draw Credit Facility of $744 million, net proceeds received from the issuance of our 2026 Term Notes of $497 million, and proceeds received from the sale of shares under our employee stock purchase plan of $56 million.
Net cash used in financing activities totaled $1,210 million in 2023. We repaid our 2023 Term Notes, with an aggregate principal amount of $500 million, repaid our 2023 Convertible Notes, with an aggregate principal amount of $750 million, and used $40 million to pay taxes related to net share settlement of equity awards. This was offset by $67 million received in proceeds from the sale of shares under our employee stock purchase plan and the issuance of common stock through the exercise of stock options.
CRITICAL ACCOUNTING POLICIES AND ESTIMATES
The preparation of financial statements in accordance with U.S. generally accepted accounting principles requires management to make estimates and assumptions that affect the amounts reported in our consolidated financial statements and accompanying notes. Management bases its estimates on historical experience, market and other conditions, and various other assumptions it believes to be reasonable. Although these estimates are based on management’s best knowledge of current events and actions that may impact us in the future, the estimation process is, by its nature, uncertain given that estimates depend on events over which we may not have control.
Though macroeconomic factors such as inflation, exchange rate fluctuations and concerns about an economic downturn present additional uncertainty, we continue to use the best information available to inform our critical accounting estimates. If market and other conditions change from those that we anticipate, our financial statements may be materially affected. In addition, if our assumptions change, we may need to revise our estimates, or take other corrective actions, either of which may also have a material effect on our consolidated financial statements.
We believe the following critical accounting policies and estimates have a higher degree of inherent uncertainty and require our most significant judgments. In addition, had we used estimates different from any of these, our consolidated financial statements could have been materially different from those presented. Members of our senior management have discussed the development and selection of our critical accounting policies and estimates, and our disclosure regarding them, with the audit committee of our board of directors. Our accounting policies are more fully described in note 1. Organization and Significant Accounting Policies in the Consolidated Financial Statements. Revenue Recognition
Our revenue is generated from the sale of products and services. Product revenue consists of sales of instruments and consumables used in genetic analysis. Service and other revenue consists of revenue generated from genotyping and sequencing services, instrument service contracts, development and licensing agreements, and, prior to the Spin-Off of GRAIL on June 24, 2024, cancer detection testing services related to the GRAIL business.
We recognize revenue when control of our products and services is transferred to our customers in an amount that reflects the consideration we expect to receive from our customers in exchange for those products and services. This process involves identifying the contract with a customer, determining the performance obligations in the contract, determining the contract price, allocating the contract price to the distinct performance obligations in the contract, and recognizing revenue when the performance obligations have been satisfied. Revenue recognition for contracts with multiple deliverables is based on the separate satisfaction of each distinct performance obligation within the contract. A performance obligation is considered distinct from other obligations in a contract when it provides a benefit to the customer either on its own or together with other resources that are readily available to the customer and is separately identified in the contract. We consider a performance obligation satisfied once we have transferred control of a good or service to the customer, meaning the customer has the ability to use and obtain the benefit of the good or service. The contract price is allocated to each performance obligation in proportion to its standalone selling price. We determine our best estimate of standalone selling price using average selling prices over a rolling 12-month period coupled with an assessment of current market conditions. If the product or service has no history of sales or if the sales volume is not sufficient, we rely upon prices set by management, adjusted for applicable discounts.
Revenue from product sales is recognized generally upon delivery to the end customer, which is when control of the product is deemed to be transferred. Invoicing typically occurs upon shipment and payment is typically due within 30 days from invoice. In instances where right of payment or transfer of title is contingent upon customer acceptance of the product, revenue is deferred until all acceptance criteria have been met. Revenue from genotyping and sequencing services, including cancer detection testing services related to the GRAIL business prior to the Spin-Off, is recognized when earned, which is generally at the time the genotyping or sequencing analysis data is made available to the customer. Revenue from instrument service contracts is recognized as the services are rendered, typically evenly over the contract term. Revenue from development and licensing agreements generally includes upfront and periodic licensing fees, contract research and development services, or payments for development and regulatory milestones. Revenue for these agreements is recognized when each distinct performance obligation is satisfied.
Revenue is recorded net of discounts, distributor commissions, and sales taxes collected on behalf of governmental authorities. Employee sales commissions are recorded as selling, general and administrative expense when incurred as the amortization period for such costs, if capitalized, would have been one year or less.
In certain markets, products and services are sold to customers through distributors. In most sales through distributors, the product is delivered directly to customers by us. The terms of sales transactions through distributors are consistent with the terms of direct sales to customers.
Inventory Valuation
Inventory is stated at the lower of cost or net realizable value. We regularly review inventory for excess and obsolete products and components, taking into account product life cycles, quality issues, historical experience, and usage forecasts. We record write-downs of inventory for potentially excess, obsolete, or impaired goods in order to state inventory at net realizable value. We make assumptions about future demand, market conditions, and the release of new products that may supersede old ones. However, if actual market conditions are less favorable than anticipated, additional inventory write-downs could be required.
Contingencies
We are involved in various lawsuits and claims arising in the ordinary course of business, including actions with respect to intellectual property, employment, and contractual matters. In connection with these matters, we assess, on a regular basis, the probability and range of possible loss based on the developments in these matters. A liability is recorded in the consolidated financial statements if it is believed to be probable that a loss has been incurred and the amount of the loss can be reasonably estimated. Because litigation is inherently unpredictable and unfavorable resolutions could occur, assessing contingencies is highly subjective and requires judgments about future events. We regularly review outstanding legal matters to determine the adequacy of the liabilities accrued and related disclosures in consideration of many factors, which include, but are not limited to, past history, scientific and other evidence, and the specifics and status of each matter. We may change our estimates if our assessment of the various factors changes and the amount of ultimate loss may differ from our estimates, resulting in a material effect on our business, financial condition, results of operations, and/or cash flows.
Business Combinations
Under the acquisition method of accounting, we allocate the fair value of the total consideration transferred to the tangible and identifiable intangible assets acquired and liabilities assumed based on their estimated fair values on the date of acquisition. The fair values assigned, defined as the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between willing market participants, are based on estimates and assumptions determined by management. These valuations require us to make estimates and assumptions, especially with respect to intangible assets. We record the excess consideration over the aggregate fair value of tangible and intangible assets, net of liabilities assumed, as goodwill. Costs that we incur to complete the business combination, such as legal and other professional fees, are expensed as they are incurred.
In connection with certain acquisitions, contingent consideration can be earned by the sellers upon completion of certain future performance milestones. In these cases, a liability is recorded on the acquisition date, as a component of accrued liabilities and/or other long-term liabilities, for an estimate of the acquisition-date fair value of the contingent consideration. We generally use a Monte Carlo simulation or an income approach to estimate the fair value of contingent consideration. Estimates and assumptions used in a Monte Carlo simulation include forecasted revenues, a revenue risk premium, a revenue volatility estimate, an operational leverage ratio and a counterparty credit spread. An income approach utilizes inputs such as anticipated future cash flows, risk-free adjusted discount rates, and nonperformance risk, as well as management judgment regarding the probability of achieving certain future milestones. Future changes in our estimates could result in expenses or gains. Changes in the fair value of contingent consideration subsequent to the acquisition date are recognized in selling, general and administrative expense.
We typically use a discounted cash flow method to value acquired intangible assets. This method requires management judgment to forecast future operating results and establish residual growth rates and discount factors. The estimates we use to value and amortize intangible assets are consistent with the plans and estimates that we use to manage our business and are based on available historical information and industry estimates and averages. If the subsequent actual results and updated projections of the underlying business activity change compared with the assumptions and projections used to develop these values, we could experience impairment charges. In addition, we have estimated the economic lives of the acquired assets, which are used to calculate depreciation and amortization expense. If our estimates of the economic lives change, depreciation or amortization expense could be accelerated or extended. We capitalize in-process research and development (IPR&D) with an indefinite life until completion or abandonment of the associated research and development efforts. Upon reaching the end of the research and development project (i.e., upon commercialization), the IPR&D asset is amortized over its estimated useful life. If the research and development project is abandoned, the IPR&D asset is expensed in the period of abandonment.
If the initial accounting for a business combination is incomplete by the end of a reporting period that falls within the measurement period (not to exceed one year from the date of acquisition), we report provisional amounts in our consolidated financial statements. During the measurement period, we adjust the provisional amounts recognized at the acquisition date to reflect new information obtained about facts and circumstances that existed as of the acquisition date that, if known, would have affected the measurement of the amounts recognized as of that date. We record these adjustments to the provisional amounts with a corresponding offset to goodwill. Any adjustments identified after the measurement period are recorded in the consolidated statements of operations.
Goodwill and Intangible Assets with Indefinite Lives — Impairment Assessment
Goodwill and other intangible assets with indefinite useful lives (i.e., IPR&D) are not amortized, however they are tested annually for impairment, in the second quarter of our fiscal year, and whenever events or changes in circumstances indicate that it is more likely than not that the fair value is less than the carrying value. Events that would indicate impairment and trigger an interim impairment test include, but are not limited to, current economic and market conditions, including a decline in market capitalization, a significant adverse change in legal factors, business climate or operational performance of the business, and an adverse action or assessment by a regulator.
We perform our goodwill impairment analysis at the reporting unit level, which aligns with our reporting structure and availability of discrete financial information. During the goodwill impairment review, we assess qualitative factors to determine whether it is more likely than not that the fair values of our reporting units are less than the carrying amounts, including goodwill. The qualitative factors include, but are not limited to, macroeconomic conditions, industry and market considerations, and our overall financial performance. If, after assessing the totality of these qualitative factors, we determine that it is not more likely than not that the fair values of our reporting units are less than the carrying amounts, then no additional assessment is deemed necessary. Otherwise, we proceed to compare the estimated fair values of the reporting units with the carrying values, including goodwill. If the carrying amounts of the reporting units exceed the fair values, we record an impairment loss based on the difference. If a quantitative assessment is performed, the evaluation includes management estimates of cash flow projections based on internal future projections and/or use of a market approach by looking at market values of comparable companies. Key assumptions include, but are not limited to, future revenue growth, operating margins, capital expenditures, terminal growth rates and discount rates. We also consider our market capitalization as a part of our analysis. We may elect to bypass the qualitative assessment in a period and proceed to perform the quantitative goodwill impairment test.
The IPR&D impairment test is performed by comparing the fair value of the asset to its carrying amount. When testing indefinite-lived intangibles for impairment, we may assess qualitative factors to determine whether it is more likely than not that the asset is impaired. Alternatively, we may bypass this qualitative assessment and perform a quantitative impairment test. We estimate the fair value of IPR&D using a discounted cash flow model, which requires the use of significant estimates and assumptions, including, but not limited to, estimating the timing of future cash flows, growth rates, and discount rates. If the IPR&D is impaired, the carrying value of the IPR&D is written down to the revised fair value with the related impairment charge recognized in the period in which the impairment occurs.
Intangible Assets and Other Long-Lived Assets — Impairment Assessment
We perform regular reviews to determine if any event has occurred that may indicate that the carrying values of our intangible assets with finite lives and other long-lived assets are impaired. If indicators of impairment exist, we assess the recoverability of the affected assets by determining whether their carrying amounts exceed their undiscounted expected future cash flows. If the affected assets are not recoverable, we estimate the fair value of the assets and record an impairment loss if the carrying value exceeds the fair value. Factors that may indicate potential impairment include a significant decline in our stock price and market capitalization compared to net book value, significant changes in the ability of an asset to generate positive cash flows and the pattern of utilization of a particular asset.
In order to estimate the fair values of identifiable intangible assets with finite lives and other long-lived assets, we estimate the present value of future cash flows from those assets. The key assumptions that we use in our cash flow model are the amount and timing of estimated future cash flows to be generated by the asset over an extended period of time and a rate of return that considers the relative risk of achieving the cash flows, the time value of money, and other factors that a willing market participant would consider. Management judgment is required to estimate the amount and timing of future cash flows and the relative risk of achieving those cash flows.
We review our operating lease right-of-use (ROU) assets for impairment whenever events or changes in circumstances indicate that the carrying value of the ROU asset may not be recoverable. The evaluation is performed at the lowest level for which identifiable cash flows are largely independent of the cash flows of other groups of assets and liabilities. We consider a triggering event to reassess an ROU asset’s asset group to have occurred if we exit a portion of or the full facility or enter into a sublease. Factors that may indicate potential impairment include a significant decrease in the market price of an underlying leased asset group. If we conclude that the carrying value of affected assets will not be recovered, we estimate the fair value of the assets and record an impairment in an amount equal to the excess of the carrying value over the fair value. We estimate the present value of future cash flows from our assets in order to determine the fair value. There is uncertainty in the projected future cash flows used in our impairment review analysis, which requires the use of estimates and assumptions.
Assumptions and estimates about future values and remaining useful lives are complex and often subjective. They can be affected by a variety of factors, including external factors such as industry and economic trends, and internal factors such as changes in our business strategy and our internal forecasts. For example, if our future operating results do not meet current forecasts or if we experience a sustained decline in our market capitalization that is determined to be indicative of a reduction in fair value of our reporting units, we may be required to record future impairment charges for purchased intangible assets with finite lives. Impairment charges could materially decrease our future results of operations and result in lower asset values on our balance sheet.
Income Taxes
Our provision for income taxes, deferred tax assets and liabilities, and reserves for unrecognized tax benefits reflect our best assessment of estimated future taxes to be paid. Judgments and estimates based on interpretations of existing tax laws or regulations in the United States and the numerous foreign jurisdictions where we are subject to income tax are required in determining our provision for income taxes. Changes in tax laws, regulations, or statutory tax rates (including the implementation of global minimum tax rates in certain jurisdictions), and estimates of our future taxable income could impact the deferred tax assets and liabilities provided for in the consolidated financial statements and would require an adjustment to the provision for income taxes.
Deferred tax assets are regularly assessed to determine the likelihood they will be recovered from future taxable income. A valuation allowance is established when we believe it is more likely than not the future realization of all or some of a deferred tax asset will not be achieved. In evaluating our ability to recover deferred tax assets within the jurisdiction which they arise, we consider all available positive and negative evidence. Factors reviewed include the cumulative pre-tax book income for the past three years, scheduled reversals of deferred tax liabilities, our history of earnings and reliability of our forecasts, projections of pre-tax book income over the foreseeable future, and the impact of any feasible and prudent tax planning strategies.
We recognize the impact of a tax position in our consolidated financial statements only if that position is more likely than not of being sustained upon examination by taxing authorities, based on the technical merits of the position. Tax authorities regularly examine our returns in the jurisdictions in which we do business and we regularly assess the tax risk of our return filing positions. Due to the complexity of some of the uncertainties, the ultimate resolution may result in payments that are materially different from our current estimate of the tax liability. These differences, as well as any interest and penalties, will be reflected in the provision for income taxes in the period in which they are determined.
QUANTITATIVE AND QUALITATIVE DISCLOSURE ABOUT MARKET RISK
Interest Rate Risk
Our current investment policy with respect to our cash, cash equivalents and short-term investments focuses on maintaining acceptable levels of interest rate risk and liquidity. To achieve these objectives, our policy allows us to maintain a portfolio of cash equivalents and short-term investments in a variety of securities, including money market funds, U.S. Treasury debt and corporate debt securities. Our policy also limits the amount of credit exposure to any one issuer and type of instrument. Under our current policies, we do not use interest rate derivative instruments to manage exposure to interest rate changes. As of December 29, 2024, our cash equivalents consisted primarily of U.S. government money market funds that invest in very liquid investments, namely, cash, government securities and purchase agreements that are collateralized fully with government securities. U.S. government money market funds provide same day liquidity and have a net asset value of $1.00. We have historically maintained a relatively short average maturity for our investment portfolio, and we believe a hypothetical 100 basis point adverse move in interest rates along the entire interest rate yield curve would not materially affect the fair value of our interest-sensitive financial instruments. Other than convertible notes, we held no debt securities as of December 29, 2024.
In September 2024, we issued $500 million of 4.650% notes due 2026. In December 2022, we issued $500 million of 5.800% notes due 2025 and $500 million of 5.750% notes due 2027. In March 2021, we issued $500 million of 2.550% notes due 2031. We carry the notes at the principal amount, less unamortized discount and debt issuance costs, on our consolidated balance sheets. As the notes have fixed annual interest rates, we do not have economic interest rate exposure or financial statement risk associated with changes in interest rates. The fair value of the notes, however, may fluctuate when interest rates change. See note 6. Debt and Other Commitments for more information. Foreign Currency Exchange Risk
We conduct a portion of our business in currencies other than our U.S. dollar functional currency. These transactions give rise to cash flows and monetary assets and liabilities that are denominated in currencies other than the U.S. dollar; the value of these amounts are exposed to changes in currency exchange rates from the time the transactions are forecasted or originated until the time the cash settlement is converted into U.S. dollars. Our foreign currency exposures are primarily concentrated in the euro, Japanese yen, Australian dollar, Canadian dollar, Singapore dollar, Chinese Yuan Renminbi, and British pound. We use forward exchange contracts to manage these foreign currency risks and to hedge portions of our foreign currency exposure associated with forecasted revenue transactions. We only use derivative financial instruments to reduce foreign currency exchange rate risks; we do not hold any derivative financial instruments for trading or speculative purposes. The counterparties to these forward exchange contracts expose us to credit-related risks in the event of their non-performance. We mitigate this risk by actively monitoring credit ratings and only selecting major financial institutions as counterparties. Additionally, our risk of credit-related loss is limited to the fair value of these financial contracts, which were not material to our financial position.
Our forward exchange contracts used to manage foreign currency risks related to monetary assets and liabilities have terms of one month or less. Realized and unrealized gains or losses on the fair value of these financial contracts are included in the determination of net income (loss), as they have not been designated for hedge accounting. These contracts, which settle monthly, effectively fix the exchange rate at which these specific monetary assets and liabilities will be settled, so that gains or losses on the forward contracts offset the gains or losses from changes in the value of the underlying monetary assets and liabilities. As of December 29, 2024, the total notional amounts of outstanding forward contracts in place for these foreign currency purchases was $477 million. Our forward exchange contracts used to hedge portions of our foreign currency exposure associated with forecasted revenue transactions have terms of up to 24 months. These derivative financial instruments are designated as cash flow hedges. Gains and losses on these financial contracts, which settle monthly, are generally recorded to revenue in the same period the underlying hedged transactions are recorded. As of December 29, 2024, the total notional amounts of outstanding forward contracts in place for these foreign currency purchases was $621 million.
RECENT ACCOUNTING PRONOUNCEMENTS
For a summary of recent accounting pronouncements applicable to our consolidated financial statements refer to note 1. Organization and Significant Accounting Policies within the Consolidated Financial Statements section of this report, which is incorporated herein by reference.
| | |
| CONSOLIDATED FINANCIAL STATEMENTS |
| | | | | |
| INDEX TO CONSOLIDATED FINANCIAL STATEMENTS | Page |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Stockholders and the Board of Directors of Illumina, Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of Illumina, Inc. (the Company) as of December 29, 2024 and December 31, 2023, the related consolidated statements of operations, comprehensive loss, stockholders’ equity and cash flows for each of the three years in the period ended December 29, 2024, and the related notes (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company at December 29, 2024 and December 31, 2023, and the results of its operations and its cash flows for each of the three years in the period ended December 29, 2024, in conformity with U.S. generally accepted accounting principles.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the Company's internal control over financial reporting as of December 29, 2024, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework), and our report dated February 12, 2025 expressed an unqualified opinion thereon.
Basis for Opinion
These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matters
The critical audit matters communicated below are matters arising from the current period audit of the financial statements that were communicated or required to be communicated to the audit committee and that: (1) relate to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit matters below, providing separate opinions on the critical audit matters or on the accounts or disclosures to which they relate.
| | | | | |
| GRAIL Contingent Consideration |
| |
| Description of the Matter | In connection with the August 18, 2021 acquisition of GRAIL, the Company recognized a contingent consideration liability at the estimated fair value on the acquisition date. The Company uses a Monte Carlo simulation model to determine the fair value of the contingent consideration liability each reporting period. As disclosed in Note 4 of the consolidated financial statements, the fair value of the contingent consideration liability as of December 29, 2024 is $71 million. The Company recognized a $315 million gain in the current year as a result of the change in the fair value of the contingent consideration liability.
Auditing the valuation of the contingent consideration liability was complex and required significant auditor judgment due to the estimation uncertainty in evaluating the reasonableness of a significant assumption, which is the revenue risk premium. A significant emphasis is placed on the appropriateness of the estimate considerations used by management to determine the fair value of the GRAIL contingent consideration due to the sensitivity of the fair value to changes in the revenue risk premium. The revenue risk premium is forward-looking and could be affected by future economic and market conditions. |
| | | | | |
| |
How We Addressed the Matter in Our Audit
| We obtained an understanding, evaluated the design, and tested the operating effectiveness of internal controls over the Company's process for determining the fair value of the contingent consideration liability related to GRAIL. This included controls over management’s development of the above-described assumption used in the valuation model applied. In testing the valuation of the contingent consideration liability, we performed audit procedures that included, among others, evaluating the Company’s use of the Monte Carlo simulation model and testing the significant assumption used in the model, as described above. We evaluated the completeness and accuracy of underlying data used in supporting the assumption and estimate. In addition, we involved valuation specialists to assist in evaluating the Company’s use of the Monte Carlo simulation model and selection of the revenue risk premium. Our valuation specialists evaluated the revenue risk premium by comparing it against a range that was independently developed using publicly available market data for comparable entities. |
| |
| Impairment assessment of GRAIL in-process research and development (IPR&D) |
| |
| Description of the Matter | The Company tests indefinite-lived intangible assets for impairment annually, as of May, or more frequently if events or circumstances indicate it is more likely than not that the fair value of an asset is less than its carrying amount. The Company identified a triggering event that occurred in the three months ended June 30, 2024 that required an interim impairment test. GRAIL IPR&D was tested for impairment by comparing its fair value to its carrying value. As disclosed in Note 5 of the consolidated financial statements, as a result of the interim impairment assessment, the Company recorded an impairment loss of $420 million related to GRAIL IPR&D. The carrying value of IPR&D following the impairment assessment was $140 million. The Company divested GRAIL on June 24, 2024. Auditing the Company’s IPR&D impairment assessment was complex and required significant auditor judgment due to the significant estimation uncertainty in determining the fair value of GRAIL IPR&D. Management used an income approach to estimate the fair value of GRAIL IPR&D. A significant emphasis is placed on the appropriateness of the estimate considerations used by management to determine the fair value of GRAIL IPR&D due to the sensitivity of the fair value to the underlying assumptions. The significant assumptions include forecasted revenues for GRAIL IPR&D and the discount rate used to discount future cash flows. These significant assumptions related to the fair value of GRAIL IPR&D are forward-looking and could be affected by future economic and market conditions. |
| |
How We Addressed the Matter in Our Audit
| We obtained an understanding, evaluated the design, and tested the operating effectiveness of internal controls over the Company's process for determining the fair value of GRAIL IPR&D. This included controls over management’s development of the above-described assumptions used in the valuation model applied.
In testing the valuation of GRAIL IPR&D, we performed audit procedures that included, among others, evaluating the Company’s use of the income approach and testing the significant assumptions used in the model, as described above. We evaluated the completeness and accuracy of underlying data used in supporting the assumptions and estimates. We evaluated the reasonableness of projected revenue growth used within the valuation against industry trends, market trends, and other market information. In addition, we involved valuation specialists to assist in evaluating the Company’s use of the income approach and selection of the discount rate. Our valuation specialists evaluated the discount rate by comparing it against a discount rate range that was independently developed using publicly available market data for comparable entities. |
/s/ Ernst & Young LLP
We have served as the Company’s auditor since 2000.
San Diego, California
February 12, 2025
ILLUMINA, INC.
CONSOLIDATED BALANCE SHEETS
(In millions, except par value)
| | | | | | | | | | | |
| December 29,
2024 | | December 31,
2023 |
| ASSETS |
| Current assets: | | | |
| Cash and cash equivalents | $ | 1,127 | | | $ | 1,048 | |
| Short-term investments | 93 | | | 6 | |
| Accounts receivable, net | 735 | | | 734 | |
| Inventory, net | 547 | | | 587 | |
| Prepaid expenses and other current assets | 244 | | | 234 | |
| Total current assets | 2,746 | | | 2,609 | |
| Property and equipment, net | 815 | | | 1,007 | |
| Operating lease right-of-use assets | 419 | | | 544 | |
| Goodwill | 1,113 | | | 2,545 | |
| Intangible assets, net | 295 | | | 2,993 | |
| Deferred tax assets, net | 567 | | | 56 | |
| Other assets | 348 | | | 357 | |
| Total assets | $ | 6,303 | | | $ | 10,111 | |
| LIABILITIES AND STOCKHOLDERS’ EQUITY |
| Current liabilities: | | | |
| Accounts payable | $ | 221 | | | $ | 245 | |
| Accrued liabilities | 827 | | | 1,325 | |
| Term debt, current portion | 499 | | | — | |
| Total current liabilities | 1,547 | | | 1,570 | |
| Operating lease liabilities | 554 | | | 687 | |
| Term debt | 1,490 | | | 1,489 | |
| Other long-term liabilities | 339 | | | 620 | |
| Commitments and contingencies | | | |
| Stockholders’ equity: | | | |
| Preferred stock, $0.01 par value, 10 million shares authorized; no shares issued and outstanding at December 29, 2024 and December 31, 2023 | — | | | — | |
| Common stock, $0.01 par value, 320 million shares authorized; 200 million shares issued and 159 million outstanding at December 29, 2024; 199 million shares issued and 159 million outstanding at December 31, 2023 | 2 | | | 2 | |
| Additional paid-in capital | 7,525 | | | 9,555 | |
| Accumulated other comprehensive income (loss) | 22 | | | (1) | |
| Accumulated deficit | (1,242) | | | (19) | |
| Treasury stock, at cost; 41 million shares and 40 million shares at December 29, 2024 and December 31, 2023, respectively | (3,934) | | | (3,792) | |
| Total stockholders’ equity | 2,373 | | | 5,745 | |
| Total liabilities and stockholders’ equity | $ | 6,303 | | | $ | 10,111 | |
See accompanying notes to consolidated financial statements.
ILLUMINA, INC.
CONSOLIDATED STATEMENTS OF OPERATIONS
(In millions, except per share amounts)
| | | | | | | | | | | | | | | | | |
| | Years Ended |
| December 29,
2024 | | December 31,
2023 | | January 1,
2023 |
| Revenue: | | | | | |
| Product revenue | $ | 3,656 | | | $ | 3,787 | | | $ | 3,953 | |
| Service and other revenue | 716 | | | 717 | | | 631 | |
| Total revenue | 4,372 | | | 4,504 | | | 4,584 | |
| Cost of revenue: | | | | | |
| Cost of product revenue | 1,017 | | | 1,177 | | | 1,144 | |
| Cost of service and other revenue | 367 | | | 392 | | | 295 | |
| Amortization of acquired intangible assets | 127 | | | 191 | | | 173 | |
| Total cost of revenue | 1,511 | | | 1,760 | | | 1,612 | |
| Gross profit | 2,861 | | | 2,744 | | | 2,972 | |
| Operating expense: | | | | | |
| Research and development | 1,169 | | | 1,354 | | | 1,321 | |
| Selling, general and administrative | 1,092 | | | 1,612 | | | 1,297 | |
| Goodwill and intangible impairment | 1,889 | | | 827 | | | 3,914 | |
| Legal contingency and settlement | (456) | | | 20 | | | 619 | |
| Total operating expense | 3,694 | | | 3,813 | | | 7,151 | |
| Loss from operations | (833) | | | (1,069) | | | (4,179) | |
| Other income (expense): | | | | | |
| Interest income | 46 | | | 58 | | | 11 | |
| Interest expense | (100) | | | (77) | | | (26) | |
| Other expense, net | (292) | | | (29) | | | (142) | |
| Total other expense, net | (346) | | | (48) | | | (157) | |
| Loss before income taxes | (1,179) | | | (1,117) | | | (4,336) | |
| Provision for income taxes | 44 | | | 44 | | | 68 | |
| Net loss | $ | (1,223) | | | $ | (1,161) | | | $ | (4,404) | |
| Loss per share: | | | | | |
| Basic | $ | (7.69) | | | $ | (7.34) | | | $ | (28.00) | |
| Diluted | $ | (7.69) | | | $ | (7.34) | | | $ | (28.00) | |
| Shares used in computing loss per share: | | | | | |
| Basic | 159 | | | 158 | | | 157 | |
| Diluted | 159 | | | 158 | | | 157 | |
See accompanying notes to consolidated financial statements.
ILLUMINA, INC.
CONSOLIDATED STATEMENTS OF COMPREHENSIVE LOSS
(In millions)
| | | | | | | | | | | | | | | | | | | | |
| | | Years Ended |
| | | December 29,
2024 | | December 31,
2023 | | January 1,
2023 |
| Net loss | | $ | (1,223) | | | $ | (1,161) | | | $ | (4,404) | |
| Unrealized gain (loss) on cash flow hedges, net of deferred tax | | 23 | | | (4) | | | (14) | |
| Total comprehensive loss | | $ | (1,200) | | | $ | (1,165) | | | $ | (4,418) | |
See accompanying notes to consolidated financial statements.
ILLUMINA, INC.
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY
(In millions)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
|
| | | | | | Accumulated | | Retained | | | | | | |
| | | | | Additional | | Other | | Earnings | | | | | | Total |
| | Common Stock | | Paid-In | | Comprehensive | | (Accumulated | | Treasury Stock | | Stockholders’ |
| | Shares | | Amount | | Capital | | Income (Loss) | | Deficit) | | Shares | | Amount | | Equity |
| Balance as of January 2, 2022 | 197 | | | $ | 2 | | | $ | 8,938 | | | $ | 17 | | | $ | 5,485 | | | (40) | | | $ | (3,702) | | | $ | 10,740 | |
| Net loss | — | | | — | | | — | | | — | | | (4,404) | | | — | | | — | | | (4,404) | |
| Unrealized loss on cash flow hedges, net of deferred tax | — | | | — | | | — | | | (14) | | | — | | | — | | | — | | | (14) | |
| Issuance of common stock, net of repurchases | 1 | | | — | | | 63 | | | — | | | — | | | — | | | (53) | | | 10 | |
| Share-based compensation | — | | | — | | | 299 | | | — | | | — | | | — | | | — | | | 299 | |
| Cumulative-effect adjustment from adoption of ASU 2020-06, net of deferred tax | — | | | — | | | (93) | | | — | | | 61 | | | — | | | — | | | (32) | |
| Balance as of January 1, 2023 | 198 | | | 2 | | | 9,207 | | | 3 | | | 1,142 | | | (40) | | | (3,755) | | | 6,599 | |
| Net loss | — | | | — | | | — | | | — | | | (1,161) | | | — | | | — | | | (1,161) | |
| Unrealized loss on cash flow hedges, net of deferred tax | — | | | — | | | — | | | (4) | | | — | | | — | | | — | | | (4) | |
| Issuance of common stock, net of repurchases | 1 | | | — | | | 64 | | | — | | | — | | | — | | | (37) | | | 27 | |
| Share-based compensation | — | | | — | | | 275 | | | — | | | — | | | — | | | — | | | 275 | |
| Reclassification of liability-classified awards | — | | | — | | | 9 | | | — | | | — | | | — | | | — | | | 9 |
| Balance as of December 31, 2023 | 199 | | | 2 | | | 9,555 | | | (1) | | | (19) | | | (40) | | | (3,792) | | | 5,745 | |
| Net loss | — | | | — | | | — | | | — | | | (1,223) | | | — | | | — | | | (1,223) | |
| Unrealized gain on cash flow hedges, net of deferred tax | — | | | — | | | — | | | 23 | | | — | | | — | | | — | | | 23 | |
| Issuance of common stock, net of repurchases | 1 | | | — | | | 51 | | | — | | | — | | | (1) | | | (142) | | | (91) | |
| Share-based compensation | — | | | — | | | 318 | | | — | | | — | | | — | | | — | | | 318 | |
Spin-Off of GRAIL (see Note 2) | — | | | — | | | (2,399) | | | — | | | — | | | — | | | — | | | (2,399) | |
| Balance as of December 29, 2024 | 200 | | | $ | 2 | | | $ | 7,525 | | | $ | 22 | | | $ | (1,242) | | | (41) | | | $ | (3,934) | | | $ | 2,373 | |
See accompanying notes to consolidated financial statements.
| | | | | | | | | | | | | | | | | |
ILLUMINA, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
(In millions) |
| | Years Ended |
| December 29,
2024 | | December 31,
2023 | | January 1,
2023 |
| Cash flows from operating activities: | | | | | |
| Net loss | $ | (1,223) | | | $ | (1,161) | | | $ | (4,404) | |
| Adjustments to reconcile net loss to net cash provided by operating activities: | | | | | |
| Depreciation expense | 224 | | | 235 | | | 215 | |
| Amortization of intangible assets | 130 | | | 197 | | | 179 | |
| Share-based compensation expense | 370 | | | 380 | | | 366 | |
| Deferred income taxes | (112) | | | (33) | | | (23) | |
| Net losses on strategic investments | 312 | | | 40 | | | 122 | |
| Change in fair value of contingent consideration liabilities | (315) | | | (24) | | | (205) | |
| (Gain) loss on Helix contingent value right | (15) | | | (10) | | | 7 | |
| Goodwill and intangible (IPR&D) impairment | 1,889 | | | 827 | | | 3,914 | |
| Property and equipment and right-of-use asset impairment | 46 | | | 100 | | | 9 | |
| | | | | |
| | | | | |
| Other | 14 | | | 17 | | | 8 | |
| Changes in operating assets and liabilities: | | | | | |
| Accounts receivable | (25) | | | (40) | | | (12) | |
| Inventory | 19 | | | (20) | | | (135) | |
| Prepaid expenses and other current assets | (14) | | | 11 | | | 16 | |
| Operating lease right-of-use assets and liabilities, net | (32) | | | (16) | | | (8) | |
| Other assets | (15) | | | 5 | | | 19 | |
| Accounts payable | (4) | | | (44) | | | (38) | |
| Accrued liabilities | (440) | | | 15 | | | 381 | |
| Other long-term liabilities | 28 | | | (1) | | | (19) | |
| Net cash provided by operating activities | 837 | | | 478 | | | 392 | |
| Cash flows from investing activities: | | | | | |
| Net purchases of property and equipment | (128) | | | (195) | | | (286) | |
| Net purchases of strategic investments | (52) | | | (6) | | | (40) | |
| Cash paid for acquisitions and intangible assets, net of cash acquired | (81) | | | (30) | | | (265) | |
| Cash received for Helix contingent value right | 83 | | | — | | | — | |
| Net cash used in investing activities | (178) | | | (231) | | | (591) | |
| Cash flows from financing activities: | | | | | |
| Proceeds from debt, net of issuance costs | 1,241 | | | (1) | | | 991 | |
| Payments on debt obligations | (750) | | | (1,235) | | | — | |
| Payments on contingent consideration liabilities | (1) | | | (1) | | | — | |
| Proceeds from issuance of common stock | 56 | | | 67 | | | 63 | |
| Taxes paid related to net share settlement of equity awards | (32) | | | (40) | | | (54) | |
| Common stock repurchases | (116) | | | — | | | — | |
| GRAIL cash deconsolidated as a result of spin-off | (968) | | | — | | | — | |
| Net cash (used in) provided by financing activities | (570) | | | (1,210) | | | 1,000 | |
| Effect of exchange rate changes on cash and cash equivalents | (10) | | | — | | | (22) | |
| Net increase (decrease) in cash and cash equivalents | 79 | | | (963) | | | 779 | |
| Cash and cash equivalents at beginning of year | 1,048 | | | 2,011 | | | 1,232 | |
| Cash and cash equivalents at end of year | $ | 1,127 | | | $ | 1,048 | | | $ | 2,011 | |
| Supplemental cash flow information: | | | | | |
| Cash paid for interest | $ | 83 | | | $ | 73 | | | $ | 17 | |
| Cash paid for income taxes | $ | 105 | | | $ | 65 | | | $ | 122 | |
| Cash paid for operating lease liabilities | $ | 132 | | | $ | 123 | | | $ | 112 | |
| Purchases of property and equipment included in accounts payable and accrued liabilities | $ | 4 | | | $ | 12 | | | $ | 16 | |
| GRAIL net assets, excluding cash and cash equivalents, deconsolidated as a result of spin-off | $ | 1,770 | | | $ | — | | | $ | — | |
See accompanying notes to consolidated financial statements.
ILLUMINA, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Unless the context requires otherwise, references in this report to “Illumina,” the “Company,” “we,” “us,” and “our” refer to Illumina, Inc. and its consolidated subsidiaries.
| | |
| 1. ORGANIZATION AND SIGNIFICANT ACCOUNTING POLICIES |
Business OverviewWe are a provider of sequencing- and array-based solutions, serving customers in the research, clinical and applied markets. Our products are used for applications in the life sciences, oncology, reproductive health, agriculture and other emerging segments. Our customers include leading genomic research centers, academic institutions, government laboratories, and hospitals, as well as pharmaceutical, biotechnology, commercial molecular diagnostic laboratories, and consumer genomics companies.
On June 24, 2024, we completed the separation (the Spin-Off) of GRAIL into a new public company through the distribution of 26,547,021 shares of GRAIL common stock to Illumina stockholders on a pro rata basis. The distribution reflected approximately 85.5% of the outstanding common stock of GRAIL as of 5:00 p.m. New York time on June 13, 2024, the record date for the distribution (the Record Date). We retained approximately 14.5% of the shares of GRAIL common stock immediately following the Spin-Off. The disposition of GRAIL did not meet the criteria to be reported as a discontinued operation and accordingly, GRAIL’s assets, liabilities, results of operations and cash flows have not been reclassified. Refer to note 2. GRAIL Spin-Off for additional details. Basis of Presentation
The consolidated financial statements have been prepared in conformity with U.S. generally accepted accounting principles (GAAP) and include our accounts, our wholly-owned subsidiaries, and majority-owned or controlled companies. All intercompany transactions and balances have been eliminated in consolidation. Certain prior period amounts have been reclassified to conform to the current period presentation.
Variable Interest Entities (VIEs)
We evaluate our ownership, contractual and other interests in entities that are not wholly-owned to determine if these entities are VIEs, and, if so, whether we are the primary beneficiary of the VIE. In determining whether we are the primary beneficiary of a VIE and therefore required to consolidate the VIE, we apply a qualitative approach that determines whether we have both (1) the power to direct the activities of the VIE that most significantly impact the VIE’s economic performance and (2) the obligation to absorb losses of, or the rights to receive benefits from, the VIE that could potentially be significant to that VIE. We continuously perform this assessment, as changes to existing relationships or future transactions may result in the consolidation or deconsolidation of a VIE. As of December 29, 2024, there were no VIEs for which we were the primary beneficiary and for which we were required to consolidate.
Use of Estimates
The preparation of the consolidated financial statements requires that management make estimates and assumptions that affect the reported amounts of assets, liabilities, revenues, expenses, and related disclosures of contingent assets and liabilities. Though macroeconomic factors such as inflation, exchange rate fluctuations and concerns about an economic downturn present additional uncertainty, we continue to use the best information available to inform our critical accounting estimates. Actual results could differ from those estimates.
Fiscal Year
Our fiscal year is the 52 or 53 weeks ending the Sunday closest to December 31, with quarters of 13 or 14 weeks ending the Sunday closest to March 31, June 30, September 30, and December 31. References to 2024, 2023, and 2022 refer to fiscal years ended December 29, 2024, December 31, 2023, and January 1, 2023, respectively, which were all 52 weeks.
ILLUMINA, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Functional Currency
The U.S. dollar is the functional currency of our international operations. We re-measure foreign subsidiaries’ monetary assets and liabilities to the U.S. dollar and record the net gains or losses resulting from re-measurement in other expense, net in the consolidated statements of operations.
Concentrations of Risk
Customers
We operate in markets that are highly competitive and rapidly changing. Significant technological changes, shifting customer needs, the emergence of competitive products or services with new capabilities, and other factors could negatively impact our operating results. A portion of our customers consist of university and research institutions that management believes are, to some degree, directly or indirectly supported by the United States Government. A significant change in current research funding, particularly with respect to funding of the U.S. National Institutes of Health or targeted cancellations by the U.S. federal government of certain grants or contracts, could have an adverse impact on future revenues and results of operations.
International sales entail a variety of risks, including currency exchange fluctuations, longer payment cycles, and greater difficulty in accounts receivable collection. We are also subject to general geopolitical risks, such as political, social and economic instability, and changes in diplomatic and trade relations. The risks of international sales are mitigated in part by the extent to which sales are geographically distributed. Shipments to customers outside the United States comprised 48%, 48%, and 50% of total consolidated revenue in 2024, 2023, and 2022, respectively. Customers outside the United States represented 53% and 55% of our gross trade accounts receivable balance as of December 29, 2024 and December 31, 2023, respectively.
We had no customers that provided more than 10% of total consolidated revenue in 2024, 2023, and 2022. We perform regular reviews of customer activity and associated credit risks and do not require collateral or enter into netting arrangements. Historically, we have not experienced significant credit losses from accounts receivable.
Financial Instruments
We are also subject to risks related to our financial instruments, including cash and cash equivalents, investments, and accounts receivable. Most of our cash and cash equivalents as of December 29, 2024 were deposited with U.S. financial institutions, either domestically or with their foreign branches. Our investment policy restricts the amount of credit exposure to any one issuer to 5% of the portfolio or 5% of the total issue size outstanding at the time of purchase and to any one industry sector, as defined by Clearwater Analytics (Industry Sector Report), to 30% of the portfolio at the time of purchase. There is no limit to the percentage of the portfolio that may be maintained in debt securities, U.S. government-sponsored entities, U.S. Treasury securities, and money market funds. Historically, we have not experienced significant credit losses from financial instruments.
Suppliers
We require customized products and components that currently are available from a limited number of sources. We source certain key products and components included in our products from single vendors. Historically, we have not experienced significant issues sourcing materials to build our products.
Segments
We report segment information based on the management approach, which designates the internal reporting used by the Chief Operating Decision Maker (CODM) for making decisions and assessing performance as the source of our reportable segments. Our CODM allocates resources and assesses the performance of segments using information about their revenue and net income (loss). Our CODM does not evaluate our segments using asset information.
ILLUMINA, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Accounting Pronouncements Adopted in 2024
In December 2023, the FASB issued ASU 2023-07, Segment Reporting (Topic 280) - Improvements to Reportable Segment Disclosures. The new standard requires a company to disclose incremental segment information on an annual and interim basis, including significant segment expenses and measures of profit or loss that are regularly provided to the CODM. The standard does not change how an entity identifies its operating segments. The standard was effective for us beginning in fiscal year 2024 and interim periods within fiscal year 2025. We adopted the standard on its effective date in fiscal year 2024 and applied the amendments retrospectively to all prior periods presented in the consolidated financial statements. See note 12. Segment and Geographic Information for additional details. Accounting Pronouncements Adopted in 2022
In August 2020, the FASB issued ASU 2020-06, Debt - Debt with Conversion and Other Options (Subtopic 470-20) and Derivatives and Hedging - Contracts in Entity’s Own Equity (Subtopic 815-40). The new standard reduces the number of accounting models for convertible debt instruments, amends the accounting for certain contracts in an entity’s own equity, and modifies how certain convertible instruments and contracts that may be settled in cash or shares impact the calculation of diluted earnings per share. Specifically, the guidance removes certain accounting models that separate the embedded conversion features from the host contract for convertible instruments and requires the use of the if-converted method to calculate diluted earnings per share. We adopted the standard on its effective date in the first quarter of 2022 using a modified retrospective approach by recognizing a cumulative-effect adjustment to retained earnings on January 3, 2022. As a result of the adoption of ASU 2020-06, we increased retained earnings and decreased additional paid-in capital by $61 million and $93 million, respectively.
Accounting Pronouncements Pending Adoption
In November 2024, the FASB issued ASU 2024-03, Disaggregation of Income Statement Expenses - Income Statement - Reporting Comprehensive Income - Expense Disaggregation Disclosures (Subtopic 220-40). The new standard requires a company to provide disaggregated disclosures, in the notes to the financial statements, of specified categories of expenses that are included in line items on the face of the income statement. The standard is effective for us beginning in fiscal year 2027 and interim periods within fiscal year 2028, with early adoption permitted. The new standard is expected to be applied prospectively, but retrospective application is permitted. We are currently evaluating the impact of ASU 2024-03 on the consolidated financial statements and related disclosures.
In December 2023, the FASB issued ASU 2023-09, Income Taxes (Topic 740) - Improvements to Income Tax Disclosures. The new standard requires a company to expand its existing income tax disclosures, specifically related to the rate reconciliation and income taxes paid. The standard is effective for us beginning in fiscal year 2025, with early adoption permitted, and is expected to be applied prospectively, but retrospective application is permitted. We are currently evaluating the impact of ASU 2023-09 on the consolidated financial statements and related disclosures.
Revenue Recognition
Our revenue is generated from the sale of products and services. Product revenue consists of sales of instruments and consumables used in genetic analysis. Service and other revenue consists of revenue generated from genotyping and sequencing services, instrument service contracts, development and licensing agreements, and, prior to the Spin-Off of GRAIL on June 24, 2024, cancer detection testing services related to the GRAIL business.
ILLUMINA, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
We recognize revenue when control of our products and services is transferred to our customers in an amount that reflects the consideration we expect to receive from our customers in exchange for those products and services. This process involves identifying the contract with a customer, determining the performance obligations in the contract, determining the contract price, allocating the contract price to the distinct performance obligations in the contract, and recognizing revenue when the performance obligations have been satisfied. Revenue recognition for contracts with multiple deliverables is based on the separate satisfaction of each distinct performance obligation within the contract. A performance obligation is considered distinct from other obligations in a contract when it provides a benefit to the customer either on its own or together with other resources that are readily available to the customer and is separately identified in the contract. We consider a performance obligation satisfied once we have transferred control of a good or service to the customer, meaning the customer has the ability to use and obtain the benefit of the good or service. The contract price is allocated to each performance obligation in proportion to its standalone selling price. We determine our best estimate of standalone selling price using average selling prices over a rolling 12-month period coupled with an assessment of current market conditions. If the product or service has no history of sales or if the sales volume is not sufficient, we rely upon prices set by management, adjusted for applicable discounts.
Revenue from product sales is recognized generally upon delivery to the end customer, which is when control of the product is deemed to be transferred. Invoicing typically occurs upon shipment and payment is typically due within 30 days from invoice. In instances where right of payment or transfer of title is contingent upon the customer’s acceptance of the product, revenue is deferred until all acceptance criteria have been met. Revenue from genotyping and sequencing services, including cancer detection testing services related to the GRAIL business, is recognized when earned, which is generally at the time the genotyping or sequencing analysis data is made available to the customer. Revenue from instrument service contracts is recognized as the services are rendered, typically evenly over the contract term. Revenue from development and licensing agreements generally includes upfront and periodic licensing fees, contract research and development services, or payments for development and regulatory milestones. Revenue for these agreements is recognized when each distinct performance obligation is satisfied.
Revenue is recorded net of discounts, distributor commissions, and sales taxes collected on behalf of governmental authorities. Employee sales commissions are recorded as selling, general and administrative expense when incurred as the amortization period for such costs, if capitalized, would have been one year or less.
In certain markets, products and services are sold to customers through distributors. In most sales through distributors, the product is delivered directly to customers by us. The terms of sales transactions through distributors are consistent with the terms of direct sales to customers.
Loss per Share
Basic loss per share is computed based on the weighted average number of common shares outstanding during the period. Diluted loss per share is computed based on the sum of the weighted average number of common shares and potentially dilutive common shares outstanding during the period. In loss periods, basic and diluted loss per share are identical since the effect of potentially dilutive common shares is antidilutive and therefore excluded. Potentially dilutive common shares from equity awards are determined using the average share price for each period under the treasury stock method. In addition, proceeds from exercise of equity awards and the average amount of unrecognized compensation expense for equity awards are assumed to be used to repurchase shares. Potentially dilutive common shares issuable upon conversion of convertible senior notes are determined using the if-converted method.
ILLUMINA, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
The weighted average shares used to calculate basic and diluted loss per share were as follows:
| | | | | | | | | | | | | | | | | |
| | Years Ended |
| In millions | December 29,
2024 | | December 31,
2023 | | January 1,
2023 |
| Weighted average shares used in calculating basic loss per share | 159 | | | 158 | | | 157 | |
| | | | | |
| | | | | |
| | | | | |
| Weighted average shares used in calculating diluted loss per share | 159 | | | 158 | | | 157 | |
| | | | | |
| Antidilutive shares: | | | | | |
| Equity awards | 4 | | | 3 | | | 2 | |
| Convertible senior notes | — | | | 1 | | | 2 | |
| Potentially dilutive shares excluded due to antidilutive effect | 4 | | | 4 | | | 4 | |
Fair Value Measurements
The fair value of assets and liabilities are based on the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. Valuation techniques used to measure fair value maximize use of observable inputs and minimize use of unobservable inputs. We use a fair value hierarchy with three levels of inputs, of which the first two are considered observable and the last unobservable, to measure fair value:
•Level 1 — Quoted prices in active markets for identical assets or liabilities.
•Level 2 — Inputs, other than Level 1, that are observable, either directly or indirectly, such as quoted prices for similar assets or liabilities; quoted prices in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities.
•Level 3 — Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities.
The carrying amounts of financial instruments such as cash and cash equivalents, accounts receivable, prepaid expenses and other current assets, accounts payable, and accrued liabilities approximate the related fair values due to the short-term maturities of these instruments.
Cash Equivalents and Investments
Cash equivalents are comprised of short-term, highly-liquid investments with original maturities of 90 days or less.
We have strategic investments in privately-held companies (non-marketable equity securities) and publicly traded companies (marketable equity securities). Our marketable equity securities are measured at fair value. Our non-marketable equity securities without readily determinable market values are initially measured at cost and adjusted to fair value for observable transactions for identical or similar investments of the same issuer or impairment. Equity investments are classified as current, short-term investments, or noncurrent, recorded in other assets, based on the nature of the securities and their availability for use in current operations. Realized and unrealized gains and losses on our equity investments are recorded in other expense, net in the consolidated statements of operations. Our equity investments are assessed for impairment quarterly. Impairment losses, equal to the difference between the carrying value and the fair value of the investment, are recorded in other expense, net.
We use the equity method to account for investments through which we have the ability to exercise significant influence, but not control, over the investee. Such investments are recorded in other assets, and our share of net income or loss is recognized on a one quarter lag in other expense, net.
ILLUMINA, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Accounts Receivable
Trade accounts receivable are recorded at the net invoice value and are not interest-bearing. Receivables are considered past due based on the contractual payment terms. We reserve a percentage of our trade receivable balance based on collection history and current economic trends that we expect will impact the level of credit losses over the life of our receivables. These reserves are re-evaluated on a regular basis and adjusted, as needed. Once a receivable is deemed to be uncollectible, such balance is charged against the reserve.
Inventory
Inventory is stated at the lower of cost or net realizable value, on a first-in, first-out basis. Inventory includes raw materials and finished goods that may be used in the research and development process, and such items are expensed as consumed or capitalized as property and equipment and depreciated. Inventory write-downs for slow-moving, excess, and obsolete inventories are estimated based on product life cycles, quality issues, historical experience, and usage forecasts.
Property and Equipment
Property and equipment are stated at cost, subject to review for impairment, and depreciated over the estimated useful lives of the assets, using the straight-line method. Depreciation of leasehold improvements is recorded over the shorter of the lease term or the estimated useful life of the related assets. Maintenance and repairs are expensed as incurred. When assets are sold, or otherwise disposed of, the cost and related accumulated depreciation are removed from the accounts and any gain or loss is included in operating expense.
Costs incurred to develop internal-use software during the application development stage are recorded at cost as computer software. Costs incurred in the development of such internal-use software, including external direct costs of materials and services and applicable compensation costs of employees devoted to specific software application development, are capitalized. Costs incurred outside of the application development stage are expensed as incurred.
The estimated useful lives of the major classes of property and equipment are generally as follows:
| | | | | |
| Buildings and leasehold improvements | 4 to 20 years |
| Machinery and equipment | 3 to 5 years |
| Computer hardware and software | 3 to 9 years |
| Furniture and fixtures | 7 years |
Leases
We have various non-cancellable operating lease agreements for office, lab, manufacturing, and distribution facilities. These leases have remaining lease terms of 1 year to 14 years, which represent the non-cancellable periods of the leases and include extension options that we determined are reasonably certain to be exercised. We exclude extension options that are not reasonably certain to be exercised from our lease terms, ranging from 2 years to 20 years. Our lease payments consist primarily of fixed rental payments for the right to use the underlying leased assets over the lease terms, as well as payments for common-area-maintenance and administrative services. We often receive customary incentives from our landlords, such as reimbursements for tenant improvements and rent abatement periods, which effectively reduce the total lease payments owed for these leases. Leases are classified as operating or financing at commencement. As of December 29, 2024, we do not have any financing leases.
ILLUMINA, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Operating lease right-of-use assets and liabilities on our consolidated balance sheets represent the present value of our remaining lease payments over the remaining lease terms, less any impairments recorded for right-of-use assets. We do not allocate lease payments to non-lease components; therefore, fixed payments for common-area-maintenance and administrative services are included in our operating lease right-of-use assets and liabilities. We use our incremental borrowing rate to calculate the present value of our lease payments, as the implicit rates in our leases are not readily determinable. Operating lease costs consist primarily of the fixed lease payments included in our operating lease liabilities and are recorded on a straight-line basis over the lease terms. We sublease certain real estate to third parties and this sublease income is also recorded on a straight-line basis.
Business Combinations
Under the acquisition method of accounting, we allocate the fair value of the total consideration transferred to the tangible and identifiable intangible assets acquired and liabilities assumed based on their estimated fair values on the date of acquisition. These valuations require us to make estimates and assumptions, especially with respect to intangible assets. We record the excess consideration over the aggregate fair value of tangible and intangible assets, net of liabilities assumed, as goodwill. Costs that we incur to complete the business combination, such as legal and other professional fees, are expensed as they are incurred.
In connection with certain acquisitions, contingent consideration can be earned by the sellers upon completion of certain future performance milestones. In these cases, a liability is recorded on the acquisition date, as a component of accrued liabilities and/or other long-term liabilities, for an estimate of the acquisition-date fair value of the contingent consideration. These estimates require management judgment, including probabilities of achieving certain future milestones. Changes in the fair value of the contingent consideration subsequent to the acquisition date are recognized in selling, general and administrative expense in our consolidated statements of operations.
If the initial accounting for a business combination is incomplete by the end of a reporting period that falls within the measurement period (not to exceed a year from the date of acquisition), we report provisional amounts in our financial statements. During the measurement period, we adjust the provisional amounts recognized at the acquisition date to reflect new information obtained about facts and circumstances that existed as of the acquisition date that, if known, would have affected the measurement of the amounts recognized as of that date. We record these adjustments to the provisional amounts with a corresponding offset to goodwill. Any adjustments identified after the measurement period are recorded in the consolidated statements of operations.
Goodwill, Intangible Assets and Other Long-Lived Assets
Assets acquired, including intangible assets and capitalized in-process research and development (IPR&D), and liabilities assumed are measured at fair value as of the acquisition date. Goodwill, which has an indefinite useful life, represents the excess of cost over fair value of the net assets acquired. Intangible assets acquired in a business combination that are used for IPR&D activities are considered indefinite lived until the completion or abandonment of the associated research and development efforts. Upon reaching the end of the relevant research and development project (i.e., upon commercialization), the IPR&D asset is amortized over its estimated useful life. If the relevant research and development project is abandoned, the IPR&D asset is expensed in the period of abandonment.
Goodwill and IPR&D are not amortized; however, they are reviewed for impairment at least annually during the second quarter, or more frequently if an event occurs indicating the potential for impairment. Goodwill and IPR&D are considered to be impaired if the carrying value of the reporting unit or IPR&D asset exceeds its respective fair value.
We perform our goodwill impairment analysis at the reporting unit level, which aligns with our reporting structure and availability of discrete financial information. During the goodwill impairment review, we assess qualitative factors to determine whether it is more likely than not that the fair values of our reporting units are less than the carrying amounts, including goodwill. The qualitative factors include, but are not limited to, macroeconomic conditions, industry and market considerations, and our overall financial performance. If, after assessing the totality of these qualitative factors, we determine that it is not more likely than not that the fair values of our reporting units are less than the carrying amounts, then no additional assessment is deemed necessary. Otherwise, we proceed to compare the estimated fair values of the reporting units with the carrying values, including goodwill. If the carrying amount of a reporting unit exceeds its fair value, we record an impairment loss based on the difference. We may elect to bypass the qualitative assessment in a period and proceed to perform the quantitative goodwill impairment test.
ILLUMINA, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
The IPR&D impairment test is performed by comparing the fair value of the asset to its carrying amount. When testing indefinite-lived intangibles for impairment, we may assess qualitative factors to determine whether it is more likely than not that the asset is impaired. Alternatively, we may bypass this qualitative assessment and perform a quantitative impairment test. If the IPR&D asset is impaired, the carrying value of the IPR&D is written down to the revised fair value with the related impairment charge recognized in the period in which the impairment occurs.
Our identifiable intangible assets with a finite life are typically comprised of acquired developed technologies, licensed technologies, customer relationships, license agreements, and trade names. The cost of identifiable intangible assets with finite lives is generally amortized on a straight-line basis over the assets’ respective estimated useful lives.
We perform regular reviews to determine if any event has occurred that may indicate that intangible assets with finite useful lives and other long-lived assets are potentially impaired. If indicators of impairment exist, an impairment test is performed to assess the recoverability of the affected assets by determining whether the carrying amount of such assets exceeds the undiscounted expected future cash flows. If the affected assets are not recoverable, we estimate the fair value of the assets and record an impairment loss in an amount equal to the excess of the carrying value over the fair value. Factors that may indicate potential impairment include a significant decline in our stock price and market capitalization compared to the net book value, significant changes in the ability of a particular asset to generate positive cash flows for our strategic business objectives, and the pattern of utilization of a particular asset.
We review our operating lease right-of-use assets for impairment whenever events or changes in circumstances indicate the carrying value of the right-of-use asset may not be recoverable. The evaluation is performed at the lowest level for which identifiable cash flows are largely independent of the cash flows of other groups of assets and liabilities. We consider a triggering event to reassess a right-of-use asset’s asset group to have occurred if we exit a portion of or the full facility or enter into a sublease. Factors that may indicate potential impairment include a significant decrease in the market price of an underlying leased asset group. If we conclude the carrying value of affected assets will not be recovered, we estimate the fair value of the assets and record an impairment in an amount equal to the excess of the carrying value over the fair value.
Derivative Financial Instruments
We are exposed to foreign exchange rate risks in the normal course of business and use derivative financial instruments to partially offset this exposure. We do not use derivative financial instruments for speculative or trading purposes. Foreign exchange contracts are carried at fair value in other current assets, other assets, accrued liabilities, or other long-term liabilities, as appropriate, on the consolidated balance sheets. The cash flows associated with such foreign exchange contracts, or derivative financial instruments, are classified as cash flows from operating activities in the consolidated statements of cash flows, which is the same category as the hedged transaction.
We use foreign exchange forward contracts to manage foreign currency risks related to monetary assets and liabilities denominated in currencies other than the U.S. dollar. These derivative financial instruments have terms of one month or less and are not designated as hedging instruments. Changes in fair value of these derivatives are recognized in other expense, net, along with the re-measurement gain or loss on the foreign currency denominated assets or liabilities. As of December 29, 2024, we had foreign exchange forward contracts in place to hedge exposures in the euro, Japanese yen, Australian dollar, Canadian dollar, Singapore dollar, Chinese Yuan Renminbi, and British pound. As of December 29, 2024 and December 31, 2023, the total notional amounts of outstanding forward contracts in place for these foreign currency purchases were $477 million and $926 million, respectively. In September 2024, as a result of the European Commission withdrawing its previously imposed fine, the related forward contracts we previously entered into for a total notional amount of €432 million were terminated.
ILLUMINA, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
We use foreign currency forward contracts to hedge portions of our foreign currency exposure associated with forecasted revenue transactions. These derivative financial instruments have terms up to 24 months and are designated as cash flow hedges. Changes in fair value are recorded as a component of accumulated other comprehensive income (loss) and are reclassified to revenue in the same period the underlying hedged transactions are recorded. We regularly review the effectiveness of our cash flow hedges and consider them to be ineffective if it becomes probable that the forecasted transactions will not occur in the identified period. Changes in fair value of the ineffective portions of our cash flow hedges, if any, are recognized in other expense, net. As of December 29, 2024, we had foreign currency forward contracts in place to hedge exposures associated with forecasted revenue transactions denominated in the euro, Japanese yen, Australian dollar, Canadian dollar, and Chinese Yuan Renminbi. As of December 29, 2024 and December 31, 2023, the total notional amounts of outstanding cash flow hedge contracts in place for these foreign currency purchases were $621 million and $628 million, respectively. We reclassified $15 million, $18 million, and $53 million to revenue in 2024, 2023, and 2022, respectively. As of December 29, 2024, the fair value of foreign currency forward contracts was $27 million, recorded in total assets. As of December 31, 2023, the fair value of foreign currency forward contracts recorded in total assets and total liabilities was $5 million and $9 million, respectively. Estimated gains reported in accumulated other comprehensive income (loss) expected to be reclassified into earnings within the next 12 months are $27 million as of December 29, 2024.
Warranties
We generally provide a one-year warranty on instruments. Additionally, we provide a warranty on consumables through the expiration date, which generally ranges from six to twelve months after the manufacture date. At the time revenue is recognized, an accrual is established for estimated warranty expenses based on historical experience as well as anticipated product performance. We periodically review the warranty reserve for adequacy and adjust the warranty accrual, if necessary, based on actual experience and estimated costs to be incurred. Warranty expense is recorded as a component of cost of product revenue.
Share-Based Compensation
Share-based compensation expense is incurred related to restricted stock, employee stock purchase plan (ESPP), stock options, and, prior to the GRAIL Spin-Off on June 24, 2024, cash-based equity incentive awards. Forfeitures are accounted for, as incurred, as a reversal of share-based compensation expense related to awards that will not vest.
Restricted stock units (RSU) and performance stock units (PSU) are both considered restricted stock. The determination of the amount of share-based compensation expense for our PSU requires the use of certain estimates and assumptions that affect the amount of share-based compensation expense recognized in our consolidated statements of operations. The fair value of restricted stock and performance stock units that do not include a market condition is determined by the closing market price of our common stock on the date of grant. PSU that do not include a market condition represent a right to receive a certain number of shares of common stock based on the achievement of corporate performance goals and continued employment during the vesting period. At each reporting period, we reassess the probability of the achievement of such corporate performance goals and any increase or decrease in share-based compensation expense resulting from an adjustment in the estimated shares to be released is treated as a cumulative catch-up in the period of adjustment. The fair value of performance stock units that include a market condition is determined on the date of grant using a Monte Carlo simulation, which includes assumptions for expected volatility, risk-free interest rate and dividend yield. These unobservable inputs represent a Level 3 measurement because they are supported by little or no market activity and reflect our own assumptions in measuring fair value. Share-based compensation expense is recognized based on the fair value on a straight-line basis over the requisite service periods of the awards. Compensation expense for PSU that include a market condition is recognized over the requisite service period regardless of whether the market conditions are achieved.
ILLUMINA, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
The Black-Scholes-Merton option-pricing model is used to estimate the fair value of stock purchased under our ESPP and stock options granted. The model assumptions include expected volatility, term, dividends, and the risk-free interest rate. The expected volatility is generally determined by weighing the historical and implied volatility of our common stock. The historical volatility is generally commensurate with the estimated expected term. The implied volatility is calculated from the implied market volatility of exchange-traded call options on our common stock. The expected term is generally based on historical forfeiture experience, exercise activity, and on the terms and conditions of the stock awards. The expected dividend yield is determined to be 0% given that we have never declared or paid cash dividends on our common stock and do not anticipate paying such cash dividends. The risk-free interest rate is based upon U.S. Treasury securities with remaining terms similar to the expected term of the share-based awards.
Cash-based equity incentive awards were classified as liability awards, as such awards were to be settled in cash. In connection with the Spin-Off of GRAIL, these awards were assumed by GRAIL. For purposes of valuation and performance measurement of the awards, GRAIL’s stand-alone value calculation, as estimated by GRAIL based on its analysis and on input from independent valuation advisors and analyses, was used. The fair value of the awards was recorded over the respective vesting periods of the awards, with recognition of a corresponding liability recorded in accrued liabilities in the consolidated balance sheets. The awards were remeasured to fair value at each reporting date until the awards were settled, with changes in fair value recognized in share-based compensation expense.
Shipping and Handling Expenses
Shipping and handling expenses are included in cost of product revenue.
Research and Development
Research and development expenses include personnel expenses, contractor fees, facilities-related costs, material costs, and license fees. Expenditures relating to research and development are expensed in the period incurred.
Advertising Costs
Advertising costs are expensed as incurred and were $37 million, $36 million, and $53 million in 2024, 2023, and 2022, respectively.
Restructuring
We measure and accrue liabilities associated with employee separation costs, which primarily consist of severance pay and other separation costs such as outplacement services and benefits, at fair value as of the date the plan is approved and when such costs are reasonably estimable. The fair value measurement of restructuring related liabilities requires certain assumptions and estimates to be made, such as the retention period of certain employees. It is our policy to use the best estimates based on facts and circumstances available at the time of measurement, review the assumptions and estimates periodically, and adjust the liabilities when necessary.
Income Taxes
The provision for income taxes is computed using the asset and liability method, under which deferred tax assets and liabilities are recognized for the expected future tax consequences of temporary differences between the financial reporting and tax bases of assets and liabilities, and for the expected future tax benefit to be derived from tax loss and credit carryforwards. Deferred tax assets and liabilities are determined using the enacted tax rates in effect for the years in which those tax assets are expected to be realized. The effect of a change in tax rates on deferred tax assets and liabilities is recognized in the provision for income taxes in the period that includes the enactment date.
Deferred tax assets are regularly assessed to determine the likelihood they will be recovered from future taxable income. A valuation allowance is established when we believe it is more likely than not the future realization of all or some of a deferred tax asset will not be achieved. In evaluating the ability to recover deferred tax assets within the jurisdiction which they arise, we consider all available positive and negative evidence. Factors reviewed include the cumulative pre-tax book income for the past three years, scheduled reversals of deferred tax liabilities, history of earnings and reliable forecasting, projections of pre-tax book income over the foreseeable future, and the impact of any feasible and prudent tax planning strategies.
ILLUMINA, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
The impact of a tax position is recognized in the consolidated financial statements only if that position is more likely than not of being sustained upon examination by taxing authorities, based on the technical merits of the position. Any interest and penalties related to uncertain tax positions will be reflected in income tax expense.
On June 24, 2024, we completed the Spin-Off of GRAIL into a separate, independent publicly traded company through the distribution of 26,547,021 shares of GRAIL common stock to Illumina stockholders on a pro rata basis. The GRAIL common stock distributed in the Spin-Off consisted of approximately 85.5% of the outstanding common stock of GRAIL as of the Record Date. The Spin-Off was structured as a tax-free spin-off and Illumina stockholders received one share of GRAIL common stock for every six shares of Illumina common stock held on the Record Date. We retained approximately 14.5% of the shares of GRAIL common stock immediately following the Spin-Off. The disposition of GRAIL did not meet the criteria to be reported as a discontinued operation and accordingly, GRAIL’s assets, liabilities, results of operations and cash flows have not been reclassified. As part of the Spin-Off, we contributed to GRAIL an amount, in cash, to cover 2.5 years of GRAIL’s operations (the Disposal Funding), which was determined to be $974 million, less the cash and cash equivalents held by GRAIL.
The carrying amounts of GRAIL’s assets and liabilities included as part of the disposal group were as follows: | | | | | |
| In millions | |
| Cash and cash equivalents | $ | 968 | |
| Accounts receivable, net | 13 | |
| Inventory, net | 22 | |
| Prepaid expenses and other current assets | 27 | |
| Property and equipment, net | 80 | |
| Operating lease right-of-use assets | 74 | |
Intangible assets, net(1) | 2,201 | |
| Other assets | 14 | |
| Accounts payable | (12) | |
| Accrued liabilities | (118) | |
| Operating lease liabilities | (62) | |
| Other long term-liabilities | (469) | |
| GRAIL net assets | $ | 2,738 | |
| Amount of GRAIL net assets recorded to short-term investments | $ | 397 | |
| Amount of GRAIL net assets recorded to additional paid-in capital | $ | 2,341 | |
| |
| Additional adjustments recorded to additional paid-in capital as a result of the GRAIL Spin-Off: | |
Non-contingent indemnification liability (see Note 7) | 1 | |
| Tax adjustment for difference between the book and tax values of our retained investment in GRAIL | 57 | |
| Total recorded to additional paid-in capital as a result of the GRAIL Spin-Off | $ | 2,399 | |
_____________ See note 12. Segment and Geographic Information for GRAIL’s results of operations, prior to the Spin-Off, included in our consolidated statements of operations for the periods presented within. In planning for and executing the Spin-Off, we incurred $53 million and $17 million in separation-related transaction costs in 2024 and 2023, respectively, recognized in selling, general, and administrative expense. The costs primarily related to financial advisory, legal, regulatory and other professional services fees directly related to the Spin-Off.
ILLUMINA, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
In connection with the Spin-Off, Illumina and GRAIL entered into various agreements to effect the Spin-Off and provide a framework for GRAIL’s relationship with Illumina after the Spin-Off, including a separation and distribution agreement, an employee matters agreement, a tax matters agreement, an amended supply and commercialization agreement and a stockholder’s and registration rights agreement (the Agreements). The Agreements determine the treatment of the assets, employees, liabilities and obligations (including certain tax-related assets and liabilities) of Illumina attributable to periods prior to, at and after GRAIL’s separation and also govern certain relationships between Illumina and GRAIL after the Spin-Off.
Our revenue is generated from the sale of products and services. Product revenue consists of sales of instruments and consumables used in genetic analysis. Service and other revenue consists of revenue generated from genotyping and sequencing services, instrument service contracts, development and licensing agreements, and prior to the Spin-Off of GRAIL on June 24, 2024, cancer detection testing services related to the GRAIL business. Revenue by Source
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| 2024 | | 2023 | | 2022 |
| In millions | Sequencing | | Microarray | | Total | | Sequencing | | Microarray | | Total | | Sequencing | | Microarray | | Total |
| Consumables | $ | 2,858 | | | $ | 297 | | | $ | 3,155 | | | $ | 2,790 | | | $ | 293 | | | $ | 3,083 | | | $ | 2,919 | | | $ | 306 | | | $ | 3,225 | |
| Instruments | 484 | | | 17 | | | 501 | | | 685 | | | 19 | | | 704 | | | 709 | | | 19 | | | 728 | |
| Total product revenue | 3,342 | | | 314 | | | 3,656 | | | 3,475 | | | 312 | | | 3,787 | | | 3,628 | | | 325 | | | 3,953 | |
| Service and other revenue | 651 | | | 65 | | | 716 | | | 637 | | | 80 | | | 717 | | | 543 | | | 88 | | | 631 | |
| Total revenue | $ | 3,993 | | | $ | 379 | | | $ | 4,372 | | | $ | 4,112 | | | $ | 392 | | | $ | 4,504 | | | $ | 4,171 | | | $ | 413 | | | $ | 4,584 | |
Revenue by Geographic Area
| | | | | | | | | | | | | | | | | |
| Based on region of destination (in millions) | 2024 | | 2023 | | 2022(1) |
Americas(2) | $ | 2,441 | | | $ | 2,521 | | | $ | 2,479 | |
| Europe | 1,185 | | | 1,140 | | | 1,089 | |
Greater China(3) | 308 | | | 384 | | | 472 | |
Asia-Pacific, Middle East and Africa(4) | 438 | | | 459 | | | 544 | |
| Total revenue | $ | 4,372 | | | $ | 4,504 | | | $ | 4,584 | |
_____________
(1)We implemented a new global commercial structure in Q1 2023 to improve operating efficiencies and better align with local markets. We integrated Asia-Pacific and Japan with emerging markets across the Middle East, Africa, Turkey, and Commonwealth of Independent States (CIS). Beginning in Q1 2023, and going forward, we report regional results for the following regions: Americas, Europe, Greater China, and Asia-Pacific, Middle East and Africa (AMEA). Prior period amounts have been reclassified to conform to this new presentation.
(2)Americas revenue included United States revenue of $2,288 million, $2,359 million, and $2,290 million in 2024, 2023 and 2022, respectively.
(3)Region includes revenue from China, Taiwan, and Hong Kong.
(4)Region includes revenue from Russia and Turkey.
Performance Obligations
We regularly enter into contracts with multiple performance obligations. These contracts are believed to be firm as of the balance sheet date. However, we may allow customers to make product substitutions as we launch new products. The timing of shipments depends on several factors, including agreed upon shipping schedules, which may span multiple quarters. Most performance obligations are generally satisfied within a short time frame, approximately three to six months, after the contract execution date. As of December 29, 2024, the aggregate amount of the transaction price allocated to remaining performance obligations was $657 million, of which approximately 78% is expected to be converted to revenue in 2025, approximately 10% in the following twelve months, and the remainder thereafter.
ILLUMINA, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Contract Assets and Liabilities
Contract assets, which consist of revenue recognized and performance obligations satisfied or partially satisfied in advance of customer billing, as of December 29, 2024 and December 31, 2023, were $16 million and $18 million, respectively, all of which were short-term and recorded in prepaid expenses and other current assets.
Contract liabilities, which consist of deferred revenue and customer deposits, as of December 29, 2024 and December 31, 2023, were $327 million and $329 million, respectively, of which the short-term portions of $260 million and $252 million, respectively, were recorded in accrued liabilities and the remaining long-term portions were recorded in other long-term liabilities. Revenue recorded in 2024 included $245 million of previously deferred revenue that was included in contract liabilities as of December 31, 2023.
| | |
| 4. INVESTMENTS AND FAIR VALUE MEASUREMENTS |
Strategic InvestmentsMarketable Equity Securities
Our short-term investments consist of marketable equity securities. As of December 29, 2024 and December 31, 2023, the fair value of our marketable equity securities totaled $93 million and $6 million, respectively. The increase in our marketable equity securities relates to the investment we retained in GRAIL subsequent to the Spin-Off, which was initially recorded as $397 million, representing 14.5% of GRAIL’s net assets disposed of at Spin-Off. Refer to note 2. GRAIL Spin-Off for details. We recorded an unrealized loss of $309 million in 2024, subsequent to the Spin-Off, based on the fair value of our investment in GRAIL as of December 29, 2024. Gains and (losses) recognized in other expense, net on marketable equity securities were as follows:
| | | | | | | | | | | | | | | | | |
| In millions | 2024 | | 2023 | | 2022 |
| Net (losses) recognized during the period on marketable equity securities | $ | (310) | | | $ | (2) | | | $ | (81) | |
| Less: Net (losses) recognized during the period on marketable equity securities sold during the period | — | | | (2) | | | — | |
| Net unrealized (losses) recognized during the period on marketable equity securities still held at the reporting date | $ | (310) | | | $ | — | | | $ | (81) | |
Non-Marketable Equity Securities
As of December 29, 2024 and December 31, 2023, non-marketable equity securities, without readily determinable fair values, included in other assets, were $26 million and $28 million, respectively.
Venture Funds
We invest in three venture capital investment funds (the Funds), which are accounted for as equity-method investments. The aggregate carrying amount of the Funds, included in other assets, was $201 million and $168 million as of December 29, 2024 and December 31, 2023, respectively. We recorded a net gain of $5 million in 2024, and net losses of $33 million and $25 million in 2023 and 2022, respectively, in other expense, net.
ILLUMINA, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Our commitments to the Funds are as follows:
| | | | | | | | | | | | | | | | | |
| Dollars in millions | Capital commitments | | Callable through date | | Remaining callable as of December 29, 2024(1) |
| Fund I | $ | 100 | | | April 2026 | | $ | 3 | |
| Fund II | $ | 150 | | | July 2029 | | $ | 46 | |
| Fund III | $ | 60 | | | December 2034 | | $ | 47 | |
_____________(1)Fund I also had recallable distributions of approximately $10 million.
Revenue recognized from transactions with our strategic investees was $20 million, $69 million, and $113 million in 2024, 2023, and 2022, respectively.
Fair Value Measurements
The following table presents the hierarchy for assets and liabilities measured at fair value on a recurring basis:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| December 29, 2024 | | December 31, 2023 |
| In millions | Level 1 | | Level 2 | | Level 3 | | Total | | Level 1 | | Level 2 | | Level 3 | | Total |
| Assets: | | | | | | | | | | | | | | | |
| Money market funds (cash equivalents) | $ | 931 | | | $ | — | | | $ | — | | | $ | 931 | | | $ | 774 | | | $ | — | | | $ | — | | | $ | 774 | |
| Marketable equity securities | 93 | | | — | | | — | | | 93 | | | 6 | | | — | | | — | | | 6 | |
| Other investments | — | | | — | | | 17 | | | 17 | | | — | | | — | | | — | | | — | |
| Helix contingent value right | — | | | — | | | — | | | — | | | — | | | — | | | 68 | | | 68 | |
| Deferred compensation plan assets | — | | | 70 | | | — | | | 70 | | | — | | | 61 | | | — | | | 61 | |
| Total assets measured at fair value | $ | 1,024 | | | $ | 70 | | | $ | 17 | | | $ | 1,111 | | | $ | 780 | | | $ | 61 | | | $ | 68 | | | $ | 909 | |
| Liabilities: | | | | | | | | | | | | | | | |
| Contingent consideration liabilities | $ | — | | | $ | — | | | $ | 73 | | | $ | 73 | | | $ | — | | | $ | — | | | $ | 387 | | | $ | 387 | |
| Deferred compensation plan liability | — | | | 65 | | | — | | | 65 | | | — | | | 59 | | | — | | | 59 | |
| Total liabilities measured at fair value | $ | — | | | $ | 65 | | | $ | 73 | | | $ | 138 | | | $ | — | | | $ | 59 | | | $ | 387 | | | $ | 446 | |
Marketable equity securities are measured at fair value based on quoted trade prices in active markets. Other investments, included in other assets, consist of convertible notes, for which we elected the fair value option. Fair value is derived using a probability-weighted scenario approach. Changes in fair value are recognized in other expense, net. Deferred compensation plan assets consist primarily of investments in life insurance contracts carried at cash surrender value, which reflects the net asset value of the underlying publicly traded mutual funds. We corroborate the fair value of our holdings, comparing valuations obtained from our investment service provider to valuations reported by our asset custodians, validating pricing sources and models, and reviewing key model inputs.
ILLUMINA, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Helix Contingent Value Right
In conjunction with the deconsolidation of Helix Holdings I, LLC (Helix) in April 2019, we received a contingent value right with a 7-year term that entitled us to consideration dependent upon the outcome of Helix’s future financing and/or liquidity events. We elected the fair value option to measure the contingent value right received from Helix. Changes in the estimated fair value are recognized in other expense, net. We estimated the fair value of the contingent value right using a Monte Carlo simulation. Estimates and assumptions used in the Monte Carlo simulation included probabilities related to the timing and outcome of future financing and/or liquidity events, assumptions regarding collectability and volatility, and an estimated equity value of Helix. These unobservable inputs represented a Level 3 measurement because they are supported by little or no market activity and reflect our own assumptions in measuring fair value. On July 31, 2024, we received cash of $83 million to settle the contingent value right early.
Changes in the Helix contingent value right were as follows:
| | | | | |
| In millions | |
| Balance as of January 2, 2022 | $ | 65 | |
| Change in estimated fair value | (7) | |
| Balance as of January 1, 2023 | 58 | |
| Change in estimated fair value | 10 | |
| Balance as of December 31, 2023 | 68 | |
| Change in estimated fair value | 15 | |
| Cash received to settle | (83) | |
| Balance as of December 29, 2024 | $ | — | |
Contingent Consideration Liabilities
We reassess the fair value of contingent consideration related to acquisitions on a quarterly basis, with changes in the fair value, subsequent to the acquisition date, recognized in selling, general and administrative expense. The contingent value rights issued as part of the GRAIL acquisition entitle the holders to receive future cash payments on a quarterly basis (Covered Revenue Payments) representing a pro rata portion of certain GRAIL-related revenues (Covered Revenues) each year for a 12-year period (through August 2033). As defined in the Contingent Value Rights Agreement, this will reflect a 2.5% payment right to the first $1 billion of revenue each year for 12 years. Revenue above $1 billion each year will be subject to a 9% contingent payment right during this same period. Covered Revenues for the periods Q4 2023 through Q3 2024, Q4 2022 through Q3 2023, and Q4 2021 through Q3 2022 were $117 million, $85 million, and $42 million, respectively, driven primarily by sales of GRAIL’s Galleri test. Covered Revenue Payments relating to such periods were $1.1 million, $803,000, and $396,000 in 2024, 2023, and 2022, respectively. A portion of the Covered Revenue Payments in 2022 were applied to reimburse us for certain expenses. The fair value of our contingent consideration liability related to GRAIL was $71 million and $387 million as of December 29, 2024 and December 31, 2023, respectively, of which $70 million and $385 million, respectively, was included in other long-term liabilities, with the remaining balances included in accrued liabilities. We use a Monte Carlo simulation to estimate the fair value of the GRAIL contingent consideration liability. Estimates and assumptions used in the Monte Carlo simulation include forecasted revenues for GRAIL, a revenue risk premium, a revenue volatility estimate, an operational leverage ratio and a counterparty credit spread. These unobservable inputs represent a Level 3 measurement because they are supported by little or no market activity and reflect our own assumptions in measuring fair value. Subsequent to the Spin-Off, we no longer have access to GRAIL management’s forecasts. Therefore, we must rely on information made public by GRAIL’s management and, beginning in Q4 2024, information published in analyst reports to estimate forecasted revenues through August 2033. In August 2024, GRAIL publicly announced a corporate restructure, including a reduction in headcount and planned hires and a substantial decrease in certain R&D projects and investments. To estimate the liability as of December 29, 2024, we selected a revenue risk premium of 9%, which was derived from reconciling our forecasted revenues for GRAIL to GRAIL’s market capitalization based on a 60-day trailing average. The significant decrease in the contingent consideration liability from December 31, 2023 was due to a decrease in the forecasted revenues, following revised revenue projections announced by GRAIL in May 2024 and the restructuring announcement in August 2024.
ILLUMINA, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
The assumptions used in estimating the fair value of the contingent consideration liability related to GRAIL are inherently subject to uncertainty and we note that small changes in these assumptions could have a significant impact on the concluded value. For example, an increase or decrease of 20%, in each year, to the forecasted revenues would have resulted in an increase of $17 million and a decrease of $18 million, respectively, in the liability as of December 29, 2024. Additionally, an increase or decrease of 250 basis points to the selected revenue risk premium would have resulted in a decrease of $12 million and an increase of $15 million, respectively. We expect certain levels of volatility in the GRAIL contingent consideration liability are possible in future periods.
Changes in the estimated fair value of our contingent consideration liabilities were as follows:
| | | | | |
| In millions | |
| Balance as of January 2, 2022 | $ | 615 | |
| Acquisition | 2 | |
| Change in estimated fair value | (205) | |
| Balance as of January 1, 2023 | 412 | |
| Change in estimated fair value | (24) | |
| Cash payments | (1) | |
| Balance as of December 31, 2023 | 387 | |
| Acquisition | 2 | |
| Change in estimated fair value | (315) | |
| Cash payments | (1) | |
| Balance as of December 29, 2024 | $ | 73 | |
| | |
| 5. GOODWILL, INTANGIBLE ASSETS, AND ACQUISITIONS |
Goodwill | | | | | |
| In millions | |
Balance as of January 1, 2023(1) | $ | 3,239 | |
| Impairment | (712) | |
| Acquisition | 18 | |
| Balance as of December 31, 2023 | 2,545 | |
| Impairment | (1,466) | |
| Acquisition | 34 | |
| Balance as of December 29, 2024 | $ | 1,113 | |
_____________
(1) The balance as of January 1, 2023 includes accumulated impairment of $3,914 million related to our GRAIL reporting unit.
2024 Impairment of Goodwill
Goodwill is reviewed for impairment annually, during the second quarter of our fiscal year, or more frequently if an event occurs indicating the potential for impairment. In May 2024, we performed our annual goodwill impairment test for our two reporting units: Core Illumina and GRAIL. We performed a quantitative test for both reporting units. GRAIL’s carrying value exceeded its fair value, estimated as $580 million, and we recorded a goodwill impairment of $1,466 million in Q2 2024. There was no impairment for Core Illumina, as its fair value exceeded its carrying value.
ILLUMINA, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
To determine the fair value of GRAIL as of May 2024, we utilized enterprise value estimates of GRAIL, as estimated by investment bankers for purposes of determining pricing for the Spin-Off. Estimates and assumptions used to derive the investment bankers’ enterprise value estimates included estimated revenues for a two year period based on assumed growth rates and implied revenue multiples for comparable companies. These estimates and assumptions represent a Level 3 measurement as they are supported by little or no market activity and reflect our own assumptions in measuring fair value. An increase in estimated enterprise values for GRAIL of 100% would still have resulted in a full impairment of goodwill. In prior periods, we used a combination of both an income (discounted cash flow) and market approach to determine the fair value of GRAIL. The income approach utilized estimated cash flows for GRAIL based on a long-range plan, for a 15 year period, which contemplated FDA approval. Based on this approach, in Q3 2023, we estimated the fair value of GRAIL to be $3.6 billion and using this same approach in Q4 2023 suggested no further decrement in fair value. Initial analyst coverage of GRAIL from December 2023 into the spring of 2024 suggested that GRAIL could be valued between $3 billion and $4 billion. By May 2024, prior to the consummation of the GRAIL Spin-Off, additional information about GRAIL had become available in GRAIL’s amended Form 10 filings and a publicly available management presentation, which included updated disclosure about GRAIL’s business and anticipated near term financial trends. Prior to the consummation of the GRAIL Spin-Off, the amount of GRAIL’s Disposal Funding, $974 million, was also disclosed. Analyst and banker valuation estimates then began to estimate fair values between $400 million and $770 million, consistent with the impairment recorded in Q2 2024.
To determine the fair value of Core Illumina, we used a combination of both an income and market approach consistent with prior periods. The income approach utilized estimated discounted cash flows for the reporting unit, while the market approach utilized comparable company information. Estimates and assumptions used in the income approach included projected cash flows and a discount rate and represent a Level 3 measurement because they are supported by little or no market activity and reflect our own assumptions in measuring fair value.
We evaluated GRAIL’s IPR&D intangible asset for potential impairment, in May 2024, as part of our annual test. We also concluded that the when-issued trading activity for GRAIL’s common stock, in June 2024, represented a triggering event that required an additional impairment test be performed. The carrying value of the IPR&D asset exceeded its estimated fair value and we recorded an impairment of $420 million in Q2 2024. The fair value of GRAIL’s IPR&D was determined by the income approach, using a discounted cash flow model. Estimates and assumptions used in the income approach, which represent a Level 3 measurement, included projected cash flows and a discount rate of 46.5%. The discount rate was derived from reconciling GRAIL’s long-range plan, which contemplated FDA approval and estimated cash flows for a 15 year period, to observed market values of GRAIL based on when-issued trading activity. An increase of 300 basis points to the discount rate used in our analysis would have resulted in additional impairment of $20 million. There is substantial risk inherent in forecasting revenues and spend associated with research and development, including assumptions around the timing and level of resources and investment to be made, which were made more challenging in light of the Spin-Off and related Disposal Funding.
We performed a recoverability test for GRAIL’s definite-lived intangible assets, which included developed technology and trade name, noting no impairment. No impairment was noted for Core Illumina definite-lived intangible assets.
2023 Impairment of Goodwill
In Q3 2023, we concluded that the sustained decrease in the Company’s stock price and overall market capitalization during the quarter was a triggering event indicating the fair values of our reporting units might be less than their carrying amounts and that an interim impairment test was required. Based on our analysis, we concluded GRAIL’s carrying value exceeded its fair value and recorded a goodwill impairment of $712 million, primarily due to the decrease in the Company’s consolidated market capitalization and a higher discount rate selected for the fair value calculation of GRAIL. There was no impairment for Core Illumina, as its fair value exceeded its carrying value.
ILLUMINA, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
We performed our interim goodwill impairment test using a combination of both an income and a market approach to determine the fair value of each reporting unit. The income approach utilized the estimated discounted cash flows for each reporting unit, while the market approach utilized comparable company information. Estimates and assumptions used in the income approach included projected cash flows for both GRAIL and Core Illumina and a discount rate for each reporting unit. Discount rates were determined using a weighted average cost of capital for risk factors specific to each reporting unit and other market and industry data. For GRAIL, the selected discount rate was 24.0%. An increase of 50 to 100 basis points to the discount rate would have resulted in additional impairment of $200 million to $350 million. The estimates and assumptions used in our assessment represent a Level 3 measurement because they are supported by little or no market activity and reflect our own assumptions in measuring fair value. In order to further validate the reasonableness of the fair values concluded for our reporting units, a reconciliation to market capitalization was performed by estimating a reasonable implied control premium and other market factors.
In conjunction with our interim goodwill impairment test, we also evaluated GRAIL’s IPR&D intangible asset for potential impairment. We performed our impairment test by comparing the carrying value of the IPR&D intangible asset to its estimated fair value, which was determined by the income approach, using a discounted cash flow model. Estimates and assumptions used in the income approach, which represent a Level 3 measurement, included projected cash flows and a selected discount rate of 19.0%. Based on our analysis, the carrying value of GRAIL’s IPR&D intangible asset exceeded its estimated fair value and we recorded an impairment of $109 million in Q3 2023, primarily due to a decrease in projected cash flows and a higher discount rate selected for the fair value calculation of the IPR&D asset. We also performed a recoverability test for the definite-lived intangible assets assigned to GRAIL, which included developed technology and trade name, and to Core Illumina and noted no impairment.
In Q4 2023, we concluded, among other events, that our formal announcement to divest GRAIL represented a triggering event that required an additional interim impairment test be performed. As a result of our analysis, no impairment was recorded for Core Illumina or GRAIL. The fair value of GRAIL exceeded its carrying value by approximately $950 million and the selected discount rate used in the analysis was 23.0%. An increase of 100 basis points to the discount rate would still have resulted in no impairment for GRAIL. We also performed a recoverability test for the definite-lived intangible assets assigned to GRAIL and Core Illumina and noted no impairment.
2022 Impairment of Goodwill
On July 13, 2022, the EU General Court ruled that the European Commission had jurisdiction under the EU Merger Regulation to review our acquisition of GRAIL. Additionally, on September 6, 2022, the European Commission issued its decision prohibiting the acquisition. See note 9. Legal Proceedings. These decisions, along with a continued and significant decrease in the Company’s stock price and market capitalization, required us to perform an interim impairment test in Q3 2022. Based on our analysis, we concluded GRAIL’s carrying value exceeded its fair value and recorded a goodwill impairment of $3,914 million, primarily due to the negative impact of capital market conditions and a higher discount rate selected for the fair value calculation of GRAIL. There was no impairment for Core Illumina. We performed our interim goodwill impairment test using a combination of both an income and a market approach to determine the fair value of each reporting unit. The income approach utilized the estimated discounted cash flows for each reporting unit, while the market approach utilized comparable company information. Estimates and assumptions used in the income approach included projected cash flows for both GRAIL and Core Illumina and a discount rate for each reporting unit. Discount rates were determined using a weighted average cost of capital for risk factors specific to each reporting unit and other market and industry data. For GRAIL, the discount rate selected was 22.0%. In order to further validate the reasonableness of the fair values concluded for our reporting units, a reconciliation to market capitalization was performed by estimating a reasonable implied control premium and other market factors.
In conjunction with our interim goodwill impairment test, we also evaluated GRAIL’s IPR&D intangible asset for potential impairment. We performed our impairment test by comparing the carrying value of the IPR&D intangible asset to its estimated fair value, which was determined by the income approach, using a discounted cash flow model. Estimates and assumptions used in the income approach included projected cash flows and a discount rate. Based on our analysis, the carrying value of the IPR&D intangible asset did not exceed its estimated fair value and no impairment was recorded. We also performed a recoverability test for the definite-lived intangible assets assigned to GRAIL, which included developed technology and trade name, and to Core Illumina and noted no impairment.
ILLUMINA, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Intangible Assets
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | December 29, 2024 | | December 31, 2023 |
| In millions | Gross
Carrying
Amount | | Accumulated
Amortization | | Intangible Assets,
Net | | Gross
Carrying
Amount | | Accumulated
Amortization | | Impairment | | Intangible Assets,
Net |
| Developed technologies | $ | 465 | | | $ | (305) | | | $ | 160 | | | $ | 2,807 | | | $ | (585) | | | $ | — | | | $ | 2,222 | |
| Licensed technologies | 234 | | | (114) | | | 120 | | | 274 | | | (133) | | | — | | | 141 | |
| License agreements | 19 | | | (13) | | | 6 | | | 14 | | | (13) | | | — | | | 1 | |
| Customer relationships | 16 | | | (14) | | | 2 | | | 14 | | | (13) | | | — | | | 1 | |
| Database | 12 | | | (5) | | | 7 | | | 12 | | | (3) | | | — | | | 9 | |
| Trade name | 2 | | | (2) | | | — | | | 43 | | | (14) | | | — | | | 29 | |
| Total finite-lived intangible assets, net | 748 | | | (453) | | | 295 | | | 3,164 | | | (761) | | | — | | | 2,403 | |
| In-process research and development (IPR&D) | — | | | — | | | — | | | 705 | | | — | | | (115) | | | 590 | |
| Total intangible assets, net | $ | 748 | | | $ | (453) | | | $ | 295 | | | $ | 3,869 | | | $ | (761) | | | $ | (115) | | | $ | 2,993 | |
The significant decrease in developed technologies, trade name, and IPR&D reflect the GRAIL intangible assets disposed of in connection with the Spin-Off. See note 2. GRAIL Spin-Off for details. Also, in Q1 2024, we placed into service (reflected in developed technologies), with a useful life of 10 years, the $35 million IPR&D intangible asset we acquired in 2021, net of impairments recognized in 2024 and 2023 of $3 million and $6 million, respectively. As a result of the Fluent BioSciences acquisition in Q3 2024, we recorded a developed technology asset of $42 million, with a useful life of 7 years, and a customer relationship asset of $2 million, with a useful life of 11 years. We are still finalizing the allocation of the purchase price as it relates to the completion of certain tax returns. We expect to finalize the valuation as soon as practicable, but no later than one year after the acquisition. As a result of an acquisition in Q4 2023, we recorded a developed technology asset of $19 million, with a useful life of 10 years. We finalized the allocation of the purchase price in Q4 2024 with no material adjustments to provisional amounts.
The estimated future annual amortization of finite-lived intangible assets is shown in the following table. Actual amortization expense to be reported in future periods could differ from these estimates as a result of acquisitions, divestitures, and asset impairments, among other factors.
| | | | | |
| In millions | Estimated Annual Amortization |
| 2025 | $ | 69 | |
| 2026 | 57 | |
| 2027 | 55 | |
| 2028 | 52 | |
| 2029 | 23 | |
| Thereafter | 39 | |
| Total | $ | 295 | |
ILLUMINA, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
| | |
| 6. DEBT AND OTHER COMMITMENTS |
Summary of Term Debt Obligations | | | | | | | | | | | |
| In millions | December 29,
2024 | | December 31,
2023 |
| Principal amount of 2025 Term Notes outstanding | $ | 500 | | | $ | 500 | |
| Principal amount of 2026 Term Notes outstanding | 500 | | | — | |
| Principal amount of 2027 Term Notes outstanding | 500 | | | 500 | |
| Principal amount of 2031 Term Notes outstanding | 500 | | | 500 | |
| Unamortized discounts and debt issuance costs | (11) | | | (11) | |
| Net carrying amount of term debt | 1,989 | | | 1,489 | |
| Less: current portion | 499 | | | — | |
| Term debt, non-current | $ | 1,490 | | | $ | 1,489 | |
| Fair value of term debt outstanding (Level 2) | $ | 1,940 | | | $ | 1,440 | |
Interest expense recognized on our term notes and delayed draw term loan, which included amortization of debt discounts and issuance costs, was $99 million, $74 million, and $21 million in 2024, 2023 and 2022, respectively.
4.650% Term Notes due 2026 (2026 Term Notes)
On September 9, 2024, we issued $500 million aggregate principal amount of 2026 Term Notes. After deducting discounts and issuance costs, we received net proceeds of $497 million, which were used to repay a portion of the outstanding debt under the Delayed Draw Credit Agreement. The 2026 Term Notes, which mature on September 9, 2026, accrue interest at a rate of 4.650% per annum, payable semi-annually on March 9 and September 9 of each year, beginning on March 9, 2025. We may redeem for cash all or any portion of the 2026 Term Notes, at our option, at any time prior to maturity at make-whole premium redemption prices as defined in the form of the notes.
5.800% Term Notes due 2025 (2025 Term Notes) and 5.750% Term Notes due 2027 (2027 Term Notes)
In December 2022, we issued $500 million aggregate principal amount of 2025 Term Notes and $500 million aggregate principal amount of 2027 Term Notes. After deducting discounts and issuance costs, we received net proceeds of $991 million. The 2025 Notes, which mature on December 12, 2025, and the 2027 Notes, which mature on December 13, 2027, accrue interest at a rate of 5.800% and 5.750% per annum, respectively, payable semi-annually. Interest for the 2025 Notes is payable on June 12 and December 12 of each year and interest for the 2027 Notes is payable on June 13 and December 13 of each year, beginning in June 2023.
We may redeem for cash all or any portion of the 2025 or 2027 Term Notes, at our option, at any time prior to maturity. Prior to November 12, 2025 for the 2025 Notes and prior to November 13, 2027 for the 2027 Notes, the notes are redeemable at make-whole premium redemption prices as defined in the applicable forms of note. After November 12, 2025 and November 13, 2027, respectively, the notes are redeemable at a redemption price equal to 100% of the principal to be redeemed, plus accrued and unpaid interest up to, but excluding, the redemption date.
0.550% Term Notes due 2023 (2023 Term Notes) and 2.550% Term Notes due 2031 (2031 Term Notes)
In March 2021, we issued $500 million aggregate principal amount of 2023 Term Notes and $500 million aggregate principal amount of 2031 Term Notes. After deducting discounts and issuance costs, we received net proceeds of $992 million. The 2023 Notes matured and were repaid in cash on March 23, 2023. The 2031 Notes, which mature on March 23, 2031, accrue interest at a rate of 2.550% per annum, payable semi-annually on March 23 and September 23 of each year. We may redeem for cash all or any portion of the 2031 Term Notes, at our option, at any time prior to maturity. Prior to December 23, 2030, the notes are redeemable at make-whole premium redemption prices as defined in the form of the notes. After December 23, 2030, the notes are redeemable at a redemption price equal to 100% of the principal to be redeemed, plus accrued and unpaid interest up to, but excluding, the redemption date.
ILLUMINA, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Delayed Draw Term Loan due 2025
On June 17, 2024, we entered into a 364-day delayed draw credit agreement (the Delayed Draw Credit Agreement), which provided us with a senior unsecured term loan credit facility in an aggregate principal amount of up to $750 million (the Delayed Draw Credit Facility). On June 20, 2024, we borrowed $750 million on the credit facility in order to provide a portion of the Disposal Funding to GRAIL as part of the Spin-Off. The delayed draw term loan incurred interest at a rate of 6.7%. On September 9, 2024, we repaid the full principal outstanding on the Delayed Draw Credit Facility, as well as accrued interest, in an aggregate amount of $761 million and terminated the Delayed Draw Credit Agreement. We recognized a loss on debt extinguishment of $5 million in 2024, included in interest expense in the consolidated statements of operations, related to the write-off of unamortized debt issuance costs.
0% Convertible Senior Notes due 2023 (2023 Convertible Notes)
In August 2018, we issued $750 million aggregate principal amount of 2023 Convertible Notes. The notes were convertible into cash, shares of common stock or a combination of cash and shares of common stock, at our election, based on conversion rates as defined in the indenture. The 2023 Convertible Notes matured on August 15, 2023, at which time the principal was repaid in cash. We did not issue any shares of common stock.
The 2023 Convertible Notes were initially accounted for in accordance with authoritative guidance for convertible debt instruments that may be settled in cash upon conversion. The guidance required the carrying amount of the liability component to be estimated by estimating the fair value of a similar liability that does not have an associated conversion feature. Because at issuance we had no outstanding non-convertible public debt, we determined that market-traded senior, unsecured corporate bonds represented a similar liability without a conversion option. Based on market data available for publicly traded, senior, unsecured corporate bonds issued by companies in our industry, and with similar maturities to the 2023 Convertible Notes, we estimated an implied interest rate of 3.7%, assuming no conversion option. The estimated implied interest rate was applied to the 2023 Convertible Notes, which resulted in a fair value of the liability component in aggregate of $624 million upon issuance, calculated as the present value of implied future payments based on the $750 million aggregate principal amount. The $126 million difference ($93 million, net of tax) between the aggregate principal amount of $750 million and the estimated fair value of the liability component was recorded in additional paid-in capital as the 2023 Convertible Notes were not considered redeemable. As of January 3, 2022, we adopted ASU 2020-06, which removed the requirement to separate the embedded conversion feature from the notes and requires the notes to be accounted for as a single liability measured at amortized cost. Accordingly, we reclassified the unamortized debt discount from additional paid-in capital to convertible senior notes in the consolidated balance sheets on January 3, 2022. This resulted in an increase to retained earnings and a decrease to additional paid-in capital of $61 million and $93 million, respectively.
Interest expense recognized on our 2023 Convertible Notes was immaterial in both 2023 and 2022.
Revolving Credit Agreement
On January 4, 2023, we entered into a new credit agreement (the Revolving Credit Agreement), which provides us with a $750 million senior unsecured five-year revolving credit facility, including a $40 million sublimit for swingline borrowings and a $50 million sublimit for letters of credit (the Revolving Credit Facility). Proceeds of the loans under the Revolving Credit Facility may be used to finance working capital needs and for general corporate purposes. The credit agreement dated as of March 8, 2021 and the commitments thereunder were terminated as of January 4, 2023.
The Revolving Credit Facility matures, and all amounts outstanding become due and payable in full, on January 4, 2028, subject to two one-year extensions at our option, the consent of the extending lenders and certain other conditions. We may prepay amounts borrowed and terminate commitments under the Revolving Credit Facility at any time without premium or penalty. As of December 29, 2024, there were no borrowings or letters of credit outstanding under the credit facility, and we were in compliance with all financial and operating covenants.
ILLUMINA, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Loans under the Revolving Credit Facility will have a variable interest rate based on either the term secured overnight financing rate (SOFR) or the alternate base rate, plus an applicable rate that varies with our debt rating and, in the case of loans bearing interest based on term SOFR, a credit spread adjustment equal to 0.10% per annum. The Revolving Credit Agreement includes an option for us to elect to increase commitments under the credit facility or enter into one or more tranches of term loans in the aggregate principal amount of up to $250 million, subject to consent of the lenders providing the additional commitments or loans and certain other conditions.
The Revolving Credit Agreement contains financial and operating covenants. Pursuant to the Revolving Credit Agreement, we are required to maintain a ratio of total debt to adjusted annual earnings before interest, taxes, depreciation and amortization (EBITDA), calculated based on the four consecutive fiscal quarters ending with the most recent fiscal quarter, of not greater than 3.50 to 1.00 as of the end of each fiscal quarter. Upon the consummation of any Qualified Acquisition (as defined in the Revolving Credit Agreement) and us providing notice to the Administrative Agent, the ratio increases to 4.00 to 1.00 for the fiscal quarter in which the acquisition is consummated and the three consecutive fiscal quarters thereafter. The operating covenants include, among other things, limitations on (i) the incurrence of indebtedness by our subsidiaries, (ii) liens on our and our subsidiaries assets, and (iii) certain fundamental changes and the disposition of assets by us and our subsidiaries. The Credit Agreement contains other customary covenants, representations and warranties, and events of default.
Leases
As of December 29, 2024, the maturities of our operating lease liabilities were as follows:
| | | | | |
| In millions | |
| 2025 | $ | 105 |
| 2026 | 105 |
| 2027 | 103 |
| 2028 | 84 |
| 2029 | 81 |
| Thereafter | 275 |
| Total remaining lease payments | 753 |
| Less: imputed interest | (120) |
| Total operating lease liabilities | 633 |
| Less: current portion | (79) |
| Long-term operating lease liabilities | $ | 554 |
| Weighted-average remaining lease term | 8.0 years |
| Weighted-average discount rate | 4.4 | % |
The components of our lease costs were as follows:
| | | | | | | | | | | | | | | | | |
| In millions | 2024 | | 2023 | | 2022 |
| Operating lease costs | $ | 93 | | | $ | 116 | | | $ | 112 | |
| Sublease income | (19) | | | (20) | | | (20) | |
Variable lease costs(1) | 25 | | | 27 | | | 20 | |
| Total lease costs | $ | 99 | | | $ | 123 | | | $ | 112 | |
_____________
(1)Variable lease costs include non-fixed maintenance charges and property taxes.
ILLUMINA, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Purchase Obligations
In the normal course of business, we enter into agreements to purchase goods or services that are not cancelable without penalty, primarily related to licensing and supply arrangements. For those agreements with variable terms, we do not estimate the total obligation beyond any minimum quantities or pricing as of the reporting date. Licensing agreements under which we commit to minimum royalty payments, some of which are subject to adjustment, may be terminated prior to the expiration of underlying intellectual property under certain circumstances. Annual minimum payments for noncancelable purchase obligations as of December 29, 2024 totaled $212 million, approximately half of which are due within the next twelve months.
The 2015 Stock and Incentive Compensation Plan (the 2015 Stock Plan) and the New Hire Stock and Incentive Plan allow for the issuance of stock options, performance stock options, restricted stock units and awards and performance stock units. In 2023, the Company’s stockholders approved an amended and restated version of the 2015 Stock Plan and increased the maximum number of shares authorized for issuance by 8.0 million shares. In connection with the GRAIL Spin-Off, all unvested RSU and PSU were equitably adjusted pursuant to the plan to preserve their intrinsic value and the number of shares reserved for issuance under the 2015 Stock Plan was increased by 160,000 shares. As of December 29, 2024, approximately 5.4 million shares remained available for future grants under the 2015 Stock Plan. There is no set number of shares reserved for issuance under the New Hire Stock and Incentive Plan. Restricted Stock
We issue restricted stock units (RSU) and performance stock units (PSU), both of which are considered restricted stock. We grant restricted stock pursuant to the 2015 Stock Plan and satisfy such grants through the issuance of either new shares or shares from treasury stock. RSU are share awards that, upon vesting, will deliver to the holder shares of our common stock. RSU generally vest over a four-year period with equal vesting annually. We issue three different PSU awards. We issue PSU for which the number of shares issuable at the end of a three-year performance period is based on our performance relative to specified earnings per share targets (EPS PSU) and, during 2024, we began to issue PSU for which the number of shares issuable at the end of a three-year performance period is based on our performance relative to specified operating margin targets (OM PSU). During 2023, we began to issue PSU with a market condition that vest based on the Company’s relative total shareholder return as compared to a peer group of companies measured over a three-fiscal year performance period (rTSR PSU). Depending on the actual performance over the measurement period, an rTSR PSU award recipient could receive up to 175% of the granted award. Shares issuable under all PSU awards are subject to continued employment through the vesting period.
ILLUMINA, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Restricted stock activity was as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Restricted
Stock Units
(RSU) | | Performance Stock Units (PSU) (1) | | Weighted-Average Grant-
Date Fair Value per Share |
| Units in thousands | | | | RSU | | PSU |
| Outstanding at January 2, 2022 | | 1,130 | | | 328 | | | $ | 345.66 | | | $ | 466.42 | |
| Awarded | | 1,370 | | | (108) | | | $ | 302.52 | | | $ | 479.85 | |
| Vested | | (707) | | | (99) | | | $ | 341.56 | | | $ | 492.55 | |
| Cancelled | | (182) | | | (47) | | | $ | 341.14 | | | $ | 411.78 | |
| Outstanding at January 1, 2023 | | 1,611 | | | 74 | | | $ | 311.23 | | | $ | 446.74 | |
| Awarded | | 2,032 | | | 39 | | | $ | 195.94 | | | $ | 239.98 | |
| Vested | | (987) | | | — | | | $ | 268.08 | | | $ | — | |
| Cancelled | | (458) | | | (113) | | | $ | 253.52 | | | $ | 299.98 | |
| Outstanding at December 31, 2023 | | 2,198 | | | — | | | $ | 236.32 | | | $ | — | |
| Awarded | | 2,788 | | | 729 | | | $ | 133.73 | | | $ | 164.38 | |
| Unvested adjustment for GRAIL Spin-Off | | 107 | | | 12 | | | $ | — | | | $ | — | |
| Vested | | (771) | | | — | | | $ | 249.70 | | | $ | — | |
| Cancelled | | (443) | | | (41) | | | $ | 195.11 | | | $ | 167.68 | |
| Outstanding at December 29, 2024 | | 3,879 | | | 700 | | | $ | 158.60 | | | $ | 164.87 | |
_____________(1)For EPS and OM PSU, the number of units reflect the estimated number of shares to be issued at the end of the performance period. For rTSR PSU, the number of units reflect the estimated number of shares to be issued based on performance as of the current reporting period. Awarded units are presented net of performance adjustments.
Pre-tax intrinsic value and fair value of vested restricted stock was as follows:
| | | | | | | | | | | | | | | | | |
| In millions | 2024 | | 2023 | | 2022 |
| Pre-tax intrinsic value of outstanding restricted stock: | | | | | |
| RSU | $ | 525 | | | $ | 306 | | | $ | 326 | |
| PSU | $ | 95 | | | $ | — | | | $ | 15 | |
| | | | | |
| Fair value of restricted stock vested: | | | | | |
| RSU | $ | 116 | | | $ | 122 | | | $ | 162 | |
| PSU | $ | — | | | $ | — | | | $ | 49 | |
Liability-Classified RSU
In Q1 2023, we granted RSU that were to be settled in cash if stockholder approval to increase our share reserve under the amended and restated 2015 Stock Plan was not obtained. In Q2 2023, the Company’s stockholders approved an amended and restated version of the 2015 Stock Plan and increased the maximum number of shares authorized for issuance. Upon such approval, all RSU previously accounted for as liability-classified awards, approximately 557,000 RSU, were reclassified to stockholders equity and accounted for prospectively as equity awards. There were no RSU liability-classified awards outstanding as of December 29, 2024 or December 31, 2023.
ILLUMINA, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Stock Options
Stock option activity was as follows:
| | | | | | | | | | | | | | | | | | | | | | | |
| Units in thousands | Options | | Weighted-Average
Exercise Price | | Performance Stock Options(1) | | Weighted-Average
Exercise Price |
| Outstanding at January 2, 2022 | 8 | | | $ | 66.42 | | | 17 | | | $ | 85.54 | |
| Granted | 180 | | | $ | 330.25 | | | — | | | $ | — | |
| Exercised | (1) | | | $ | 6.55 | | | — | | | $ | — | |
| Outstanding at January 1, 2023 | 187 | | | $ | 319.72 | | | 17 | | | $ | 85.54 | |
| Exercised | (8) | | | $ | 71.09 | | | (1) | | | $ | 16.69 | |
| Cancelled | (144) | | | $ | 330.25 | | | — | | | $ | — | |
| Outstanding at December 31, 2023 | 35 | | | $ | 330.25 | | | 16 | | | $ | 87.74 | |
| Cancelled | (35) | | | $ | 330.25 | | | (16) | | | $ | 87.74 | |
| Outstanding at December 29, 2024 | — | | | $ | — | | | — | | | $ | — | |
_____________
(1)In connection with the GRAIL acquisition, we issued replacement performance stock options to GRAIL employees in 2021. The number of units reflected awards that had been granted and for which it was assumed to be probable that the underlying performance goals would be achieved. In connection with the GRAIL Spin-Off, all outstanding performance stock options were assumed by GRAIL.
The total intrinsic value of stock options exercised was immaterial in both 2023 and 2022. None exercised in 2024.
Other Liability-Classified Awards
Prior to the GRAIL Spin-Off, we granted cash-based equity incentive awards to GRAIL employees, which were accounted for as liability-classified awards. In connection with the Spin-Off, these awards were assumed by GRAIL. For purposes of valuation and performance measurement of the awards, GRAIL’s stand-alone value calculation, as estimated by GRAIL based on its analysis and on input from independent valuation advisors and analyses, was used. The awards generally had terms of four years and vested in four equal installments on each anniversary of the grant date, subject to continued employment through the vesting period.
ILLUMINA, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Cash-based equity incentive award activity was as follows:
| | | | | |
| In millions | |
| Outstanding at January 2, 2022 | $ | 184 | |
| Granted | 168 | |
| Vested and paid in cash | (41) | |
| Cancelled | (41) | |
| Change in fair value | 23 | |
| Outstanding at January 1, 2023 | 293 | |
| Granted | 116 | |
| Vested and paid in cash | (77) | |
| Cancelled | (32) | |
| Change in fair value | (8) | |
| Outstanding at December 31, 2023 | 292 | |
| Granted | 67 | |
| Vested and paid in cash | (54) | |
| Cancelled | (13) | |
| Change in fair value | (9) | |
Derecognition for GRAIL Spin-Off(1) | (283) | |
| Outstanding at December 29, 2024 | $ | — | |
_____________(1)The estimated liability immediately prior to the Spin-Off, recorded in accrued liabilities, was $53 million, which was disposed of as part of GRAIL’s net assets. See note 2. GRAIL Spin-Off for additional details. We recognized share-based compensation expense on these cash-based equity incentive awards of $52 million in 2024, prior to the Spin-Off, and $95 million and $67 million in 2023 and 2022, respectively.
In connection with the acquisition of GRAIL, we assumed a performance-based award for which vesting was based on GRAIL’s future revenues and had an aggregate potential value of up to $78 million. Prior to the Spin-Off of GRAIL, it was not probable that the performance conditions associated with the award would be achieved and, therefore, no share-based compensation expense was recognized in the consolidated statements of operations. In connection with the Spin-Off, this award was assumed by GRAIL. For a period of 2.5 years following the Spin-Off, we are obligated to indemnify GRAIL for cash payments that become earned and payable related to this award. The indemnification is accounted for in accordance with ASC 460. As of December 29, 2024, we recognized a non-contingent liability of $1 million for this indemnification, with a corresponding charge to additional paid-in capital.
Employee Stock Purchase Plan
The 2000 Employee Stock Purchase Plan, or ESPP, permits eligible employees to purchase common stock at a discount through payroll deductions during defined offering periods. The price at which stock is purchased under the ESPP is equal to 85% of the fair market value of the common stock on the first day of the offering period or purchase date, whichever is lower. The initial offering period commenced in July 2000. During 2024, 2023, and 2022, approximately 0.5 million, 0.4 million, and 0.3 million shares, respectively, were issued under the ESPP. As of December 29, 2024, approximately 12.4 million shares remained available for issuance under the ESPP, which includes an increase of 0.5 million shares pursuant to the terms of the ESPP to account for the GRAIL Spin-Off.
ILLUMINA, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
The assumptions used for the specified reporting periods and the resulting estimates of weighted-average fair value per share for stock purchased under the ESPP were as follows:
| | | | | | | | | | | | | | | | | |
| 2024 | | 2023 | | 2022 |
| Risk-free interest rate | 4.35% - 5.54% | | 0.78% - 5.54% | | 0.06% - 2.98% |
| Expected volatility | 41% - 49% | | 41% - 51% | | 37% - 51% |
| Expected term | 0.5 - 1.1 year | | 0.5 - 1.1 year | | 0.5 - 1.0 year |
| Expected dividends | 0% | | 0% | | 0% |
| Weighted-average grant-date fair value per share | $ | 37.24 | | | $ | 49.87 | | | $ | 50.22 | |
Share Repurchases
In August 2024, our Board of Directors authorized a new share repurchase program, which cancels and supersedes all prior and available repurchase authorizations, to repurchase up to $1.5 billion of our outstanding common stock. The repurchases may be completed through open market purchases, pursuant to Rule 10b5-1 or Rule 10b-18, or through an accelerated share repurchase program. Authorizations to repurchase up to $1.4 billion of our outstanding common stock remained available as of December 29, 2024. We did not repurchase any shares during 2023 or 2022.
Share repurchase activity during 2024 was as follows:
| | | | | |
| In millions, except shares in thousands | |
| Number of shares repurchased | 904 | |
Total cost of shares repurchased(1) | $ | 116 | |
_____________(1)Total cost of shares repurchased includes the 1% excise tax imposed as part of the Inflation Reduction Act of 2022, which is calculated based on share repurchases, net of certain share issuances.
Subsequent to December 29, 2024 and through February 11, 2025, we repurchased an additional 1.0 million shares of our common stock for $126 million.
Share-Based Compensation
Share-based compensation expense, which includes expense for both equity and liability-classified awards, reported in our consolidated statements of operations was as follows:
| | | | | | | | | | | | | | | | | |
| In millions | 2024 | | 2023 | | 2022 |
| Cost of product revenue | $ | 25 | | | $ | 29 | | | $ | 26 | |
| Cost of service and other revenue | 6 | | | 7 | | | 6 | |
| Research and development | 146 | | | 155 | | | 153 | |
| Selling, general and administrative | 194 | | | 189 | | | 181 | |
| Share-based compensation expense, before taxes | 371 | | | 380 | | | 366 | |
| Related income tax benefits | (83) | | | (87) | | | (83) | |
| Share-based compensation expense, net of taxes | $ | 288 | | | $ | 293 | | | $ | 283 | |
As of December 29, 2024, unrecognized compensation cost, related to restricted stock and ESPP shares issued to date, of $565 million was expected to be recognized over a weighted-average period of approximately 2.5 years.
ILLUMINA, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
| | |
| 8. SUPPLEMENTAL BALANCE SHEET DETAILS |
Accounts Receivable | | | | | | | | | | | |
| In millions | December 29,
2024 | | December 31,
2023 |
| Trade accounts receivable, gross | $ | 744 | | | $ | 741 | |
| Allowance for credit losses | (9) | | | (7) | |
| Total accounts receivable, net | $ | 735 | | | $ | 734 | |
Inventory
| | | | | | | | | | | |
| In millions | December 29,
2024 | | December 31,
2023 |
| Raw materials | $ | 225 | | | $ | 276 | |
| Work in process | 404 | | | 402 | |
| Finished goods | 31 | | | 30 | |
| Inventory, gross | 660 | | | 708 | |
| Inventory reserve | (113) | | | (121) | |
| Total inventory, net | $ | 547 | | | $ | 587 | |
Property and Equipment
| | | | | | | | | | | |
| In millions | December 29,
2024 | | December 31,
2023 |
| Leasehold improvements | $ | 772 | | | $ | 803 | |
| Machinery and equipment | 683 | | | 684 | |
| Computer hardware and software | 478 | | | 463 | |
| Furniture and fixtures | 53 | | | 55 | |
| Buildings | 44 | | | 44 | |
| Construction in progress | 39 | | | 96 | |
| Total property and equipment, gross | 2,069 | | | 2,145 | |
| Accumulated depreciation | (1,254) | | | (1,138) | |
| Total property and equipment, net | $ | 815 | | | $ | 1,007 | |
Accrued Liabilities
| | | | | | | | | | | |
| In millions | December 29,
2024 | | December 31,
2023 |
Legal contingencies(1) | $ | 26 | | | $ | 484 | |
| Contract liabilities, current portion | 260 | | | 252 | |
| Accrued compensation expenses | 252 | | | 223 | |
| Accrued taxes payable | 101 | | | 79 | |
| Operating lease liabilities, current portion | 79 | | | 86 | |
| Liability-classified equity incentive awards | — | | | 55 | |
Other, including warranties(2) | 109 | | | 146 | |
| Total accrued liabilities | $ | 827 | | | $ | 1,325 | |
_____________(2)See table below for changes in the reserve for product warranties.
ILLUMINA, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Changes in the reserve for product warranties were as follows:
| | | | | |
| In millions | |
| Balance as of January 2, 2022 | $ | 22 | |
| Additions charged to cost of product revenue | 23 | |
| Repairs and replacements | (27) | |
| Balance as of January 1, 2023 | 18 | |
| Additions charged to cost of product revenue | 42 | |
| Repairs and replacements | (39) | |
| Balance as of December 31, 2023 | 21 | |
| Additions charged to cost of product revenue | 42 | |
| Repairs and replacements | (45) | |
| Balance as of December 29, 2024 | $ | 18 | |
Restructuring
In Q2 2023, we implemented a cost reduction initiative that included workforce reductions, the consolidation of certain facilities and other actions to reduce expenses, all as part of a plan to realign operating expenses while maintaining focus on our innovation roadmap and sustainable long-term growth. In both 2024 and 2023, we recorded restructuring charges primarily consisting of asset impairments related to exit activities at certain of our leased facilities.
A summary of the pre-tax restructuring charges is as follows:
| | | | | | | | | | | | | | | | | |
| In millions | 2024 | | 2023 | | Cumulative charges recorded since inception |
| Employee separation costs | $ | 12 | | | $ | 48 | | | $ | 60 | |
Asset impairment charges(1) | 46 | | | 100 | | | 146 | |
| Other costs | 4 | | | 4 | | | 8 | |
Total restructuring charges(2) | $ | 62 | | | $ | 152 | | | $ | 214 | |
_____________(1)For 2024, relates to impairment of right-of-use assets and leasehold improvements for Foster City campus and other property in San Diego.
For 2023, primarily relates to impairment of right-of-use assets and leasehold improvements for our i3 and Foster City campuses.
(2)For 2024, $59 million was recorded in SG&A expense, $2 million in R&D expense, and remainder in cost of revenue.
For 2023, $122 million was recorded in SG&A expense, $24 million in R&D expense, and remainder in cost of revenue.
Total restructuring charges primarily relate to our Core Illumina segment.
In 2024, we recorded right-of-use asset impairments of $12 million and $19 million related to our campus in Foster City, California and another property in San Diego, California, respectively. In 2023, we recorded right-of-use asset impairments of $38 million and $21 million related to our i3 campus in San Diego and our campus in Foster City, respectively. The impairments were determined by comparing the fair values of the impacted right-of-use assets to the carrying values of the assets as of the impairment measurement date. The fair values of the right-of-use assets were estimated using the discounted future cash flows method, which includes estimates and assumptions for future sublease rental rates that reflect current sublease market conditions, as well as discount rates. The estimates and assumptions used in our assessments represent Level 3 measurements because they are supported by little or no market activity and reflect our own assumptions in measuring fair value. In 2024, we recorded $14 million of leasehold improvement impairments related to our Foster City campus and, in 2023, we recorded $16 million and $22 million of leasehold improvement impairments related to our i3 and Foster City campuses, respectively. The right-of-use asset and leasehold improvement impairments were recognized in selling, general and administrative expense. We continue to evaluate our options for the rest of our Foster City campus, for which, as of December 29, 2024, we had remaining assets, consisting primarily of right-of-use assets and leasehold improvements, of $100 million.
ILLUMINA, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
A summary of the restructuring liability is as follows:
| | | | | | | | | | | | | | | | | |
| In millions | Employee Separation Costs | | Other Costs | | Total |
| Amount recorded in accrued liabilities as of January 1, 2023 | $ | — | | | $ | — | | | $ | — | |
| Expense recorded | 48 | | | 4 | | | 52 | |
| Cash payments | (31) | | | (3) | | | (34) | |
| Amount recorded in accrued liabilities as of December 31, 2023 | 17 | | | 1 | | | 18 | |
| Expense recorded | 12 | | | 4 | | | 16 | |
| Cash payments | (24) | | | (2) | | | (26) | |
| Adjustments to accrual | (3) | | | (1) | | | (4) | |
| Amount recorded in accrued liabilities as of December 29, 2024 | $ | 2 | | | $ | 2 | | | $ | 4 | |
| | | | | |
| | | | | |
We are involved in various lawsuits and claims arising in the ordinary course of business, including actions with respect to intellectual property, employment, and contractual matters. In connection with these matters, we assess, on a regular basis, the probability and range of possible loss based on the developments in these matters. A liability is recorded in the consolidated financial statements if it is believed to be probable that a loss has been incurred and the amount of the loss can be reasonably estimated. Because litigation is inherently unpredictable and unfavorable resolutions could occur, assessing contingencies is highly subjective and requires judgments about future events. We regularly review outstanding legal matters to determine the adequacy of the liabilities accrued and related disclosures in consideration of many factors, which include, but are not limited to, past history, scientific and other evidence, and the specifics and status of each matter. We may change our estimates if our assessment of the various factors changes and the amount of ultimate loss may differ from our estimates, resulting in a material effect on our business, financial condition, results of operations, and/or cash flows. Acquisition of GRAIL
As of September 6, 2024, all previously disclosed regulatory proceedings in the United States and European Union related to our acquisition of GRAIL have been resolved (as further described below).
On April 19, 2021, the European Commission sought and subsequently accepted a request for a referral of the GRAIL acquisition for European Union merger review, submitted by a Member State of the European Union (France), and joined by several other EEA Member States (Belgium, Greece, Iceland, the Netherlands and Norway), under Article 22(1) of Council Regulation (EC) No 139/2004 (the EU Merger Regulation). The European Commission had never solicited referrals to take jurisdiction over an acquisition of a U.S. company that had no revenue in Europe. On April 28, 2021, we filed an action in the General Court of the European Union (the EU General Court) asking for annulment of the European Commission’s assertion of jurisdiction to review the acquisition under Article 22 of the EU Merger Regulation, as the acquisition does not meet the jurisdictional criteria under the EU Merger Regulation or under the national merger control laws of any Member State of the European Union. On July 13, 2022, the EU General Court reached a decision in favor of the European Commission, holding that the European Commission has jurisdiction under the EU Merger Regulation to review the acquisition. On September 22, 2022, we filed an appeal in the Court of Justice of the European Union (the EU Court of Justice) asking for annulment of the EU General Court’s judgment.
On October 12, 2023, the European Commission adopted a decision requiring Illumina to unwind its acquisition of GRAIL (the EC Divestment Decision). On December 17, 2023, we announced that we would divest GRAIL. On June 24, 2024, we completed the separation (the Spin-Off) of GRAIL into a separate, independent publicly traded company through the distribution of approximately 85.5% of the outstanding GRAIL common stock to Illumina stockholders on a pro rata basis.
ILLUMINA, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
On September 3, 2024, the EU Court of Justice ruled in our favor, confirming that the European Commission had unlawfully asserted jurisdiction over our acquisition of GRAIL, and hence annulling the EU General Court’s judgment and the European Commission’s decisions accepting the referral of the GRAIL acquisition for EU merger review (the EU Court of Justice Judgment). The EU Court of Justice Judgment concludes these proceedings and is not subject to further appeals. In view of this judgment, on September 6, 2024, the European Commission issued a decision (the Withdrawal Decision) withdrawing all of its prior decisions, including (1) its July 22, 2021 decision opening an investigation of Illumina’s proposed acquisition of GRAIL, (2) its September 6, 2022 decision prohibiting Illumina’s acquisition of GRAIL, (3) its October 29, 2021 and October 28, 2022 decisions concerning interim measures, (4) the EC Divestment Decision, and (5) its July 12, 2023 decision fining Illumina €432 million and GRAIL for closing the acquisition before approval by the European Commission. The Withdrawal Decision resolves all ongoing regulatory proceedings in the European Union. The European Commission has also been ordered to pay Illumina’s costs incurred in connection with the GRAIL-related proceedings before the EU Court of Justice and the EU General Court.
As a result of the European Commission withdrawing its previously imposed fine, we recognized a net gain of $481 million in Q3 2024. We recognized a gain of $489 million in operating expense, resulting from reversal of the accrued fine and related accrued interest, offset by a loss of $8 million, recognized in other expense, net, for the reversal of associated foreign currency fluctuations. The fine accrued interest at a rate of 5.5% per annum while it was outstanding. The guarantees we provided in October 2023 to satisfy the obligation in lieu of cash payment while we appealed the European Commission’s jurisdictional and fine decisions are no longer outstanding.
On August 15, 2024, the U.S. Federal Trade Commission dismissed the previously disclosed administrative complaint in light of the completion of the Spin-Off.
SEC Inquiry Letter
In July 2023, we were informed that the staff of the SEC was conducting an investigation relating to Illumina and was requesting documents and communications primarily related to Illumina’s acquisition of GRAIL and certain statements and disclosures concerning GRAIL, its products and its acquisition, and related to the conduct and compensation of certain members of Illumina and GRAIL management, among other things. Illumina is cooperating with the SEC in this investigation.
Shareholder Derivative Complaints
On October 17, 2023, a stockholder derivative and class action complaint captioned Icahn Partners LP, et al. v. deSouza, et al., purportedly brought on behalf of Illumina and public holders of Illumina’s common stock, was filed in the Delaware Court of Chancery against certain current and former directors (including our former Chief Executive Officer). We are named as a nominal defendant in the complaint. The lawsuit alleges the named directors breached their fiduciary duties by knowingly causing Illumina to unlawfully close the GRAIL acquisition, concealing material facts related to the GRAIL acquisition and making inadequate disclosures. Before the filing of the complaint, the purported stockholders did not make a demand that our Board of Directors pursue the claims asserted therein. The complaint seeks damages, costs and expenses, including attorney fees, the certification and consolidation of a putative class, the issuance of amended disclosures, the removal of conflicted directors and declaratory and other equitable relief.
On November 1, 2023, the defendants filed a motion to dismiss the complaint, which has not yet been briefed. On the same day, Illumina-joined by the director defendants-moved to strike portions of the complaint that contain improperly included confidential and privileged information. On January 16, 2024, the Court granted the motion to strike. On December 5, 2023, the plaintiffs moved to expedite the proceedings with respect to their direct claims. The director defendants opposed that motion and Illumina joined their opposition. On January 19, 2024, the Court denied plaintiffs’ motion to expedite. On January 23, 2024, the plaintiffs filed a motion for reargument of the Court’s January 16 opinion, which the Court denied on February 19, 2024. On February 29, 2024, the plaintiffs filed an application to the trial court to certify the orders granting the motion to strike and denying the motion for reargument for interlocutory appeal. The Court refused the application on March 20, 2024. On March 14, 2024, the plaintiffs filed an application for interlocutory appeal with the Supreme Court of Delaware, which the Court denied on April 11, 2024.
ILLUMINA, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
On February 26, 2024, a stockholder derivative complaint captioned City of Omaha Police and Firefighters Retirement System v. deSouza, et al., purportedly brought on behalf of Illumina, was filed in the Delaware Court of Chancery against certain current and former directors. On April 16, 2024, a stockholder derivative complaint captioned City of Roseville General Employees Retirement System, et al. v. deSouza, et al., purportedly brought on behalf of Illumina, was filed in the Delaware Court of Chancery against certain current and former directors and officers. On August 16, 2024, a stockholder derivative complaint captioned Thomas P. DiNapoli v. John Thompson et al., purportedly brought on behalf of Illumina, was filed in the Delaware Court of Chancery against certain current and former directors. We are named as a nominal defendant in the complaints. The lawsuits allege the named directors and officers breached their fiduciary duties by knowingly causing Illumina to unlawfully close the GRAIL acquisition. The stockholders previously made requests to inspect certain books and records under Delaware law, and they purport to base their complaint in part on documents obtained from Illumina in response to those requests. Before the filing of the complaint, the purported stockholders did not make a demand that our Board of Directors pursue the claim asserted therein. The complaints seek damages against the individual defendants, costs and expenses, including attorney fees and other equitable relief.
On March 26, 2024, the defendants filed a motion to dismiss the complaint in the lawsuit filed by City of Omaha Police and Firefighters Retirement System. The motion has not yet been briefed. On May 16, 2024, the defendants filed a motion to dismiss the complaint in the lawsuit filed by City of Roseville General Employees Retirement System, et al. The motion has not yet been briefed. On September 16, 2024, the defendants filed a motion to dismiss the complaint in the lawsuit filed by Thomas DiNapoli. The motion has not yet been briefed.
On December 23, 2024, a stockholder derivative complaint captioned The Pavers and Road Builders Benefit Funds v. deSouza, et al., purportedly brought on behalf of Illumina, was filed in the Delaware Court of Chancery against certain current and former directors and officers. Like the complaints described above, the lawsuits allege the named directors and officers breached their fiduciary duties by knowingly causing Illumina to unlawfully close the GRAIL acquisition. The stockholder previously made requests to inspect certain books and records under Delaware law and litigated a books and records action against the Company (described below), and it purports to base its complaint in part on documents obtained from Illumina in response to those requests and that litigation. Before the filing of the complaint, the purported stockholder did not make a demand that our Board of Directors pursue the claim asserted therein. The complaint seeks damages against the individual defendants and other equitable relief.
In light of the fact that these lawsuits are in an early stage, we cannot predict the ultimate outcome of the suits. We deny the allegations in the complaints and intend to vigorously defend the litigations.
On February 21, 2024, a stockholder derivative complaint captioned Elaine Wang, et al. v. deSouza, et al., purportedly brought on behalf of the Company was filed in the United States District Court for the District of Delaware (District of Delaware) against certain current and former directors. The Company was named as a nominal defendant in the complaint. The lawsuit alleged that the named directors breached their fiduciary duties by knowingly causing the Company to unlawfully close the GRAIL acquisition. Before the filing of the complaint, the purported stockholder did not make a demand that our Board of Directors pursue the asserted claims therein. The complaint sought, among other things, restitution to the Company for the alleged damages caused by the named defendants.
On March 8, 2024, a stockholder derivative complaint captioned Michael Warner, et al. v. deSouza, et al., purportedly brought on behalf of Illumina was also filed in the United States District Court for the Southern District of California against certain current and former directors. We were named as a nominal defendant in the complaint. The lawsuit alleged that the named directors breached their fiduciary duties by knowingly causing us to unlawfully close the GRAIL acquisition. Before the filing of the complaint, the purported stockholder did not make a demand that our Board of Directors pursue the asserted claims therein. The complaint sought, among other things, restitution to Illumina for the alleged damages caused by the named defendants. On March 28, 2024, the parties submitted a Joint Motion to Transfer the lawsuit to the District of Delaware, which the Court granted on March 29, 2024, and the Court transferred the lawsuit to the District of Delaware on the same day.
ILLUMINA, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Elaine Wang, et al. v. deSouza, et al. and Michael Warner, et al. v. deSouza, et al. were voluntarily dismissed on April 29, 2024, and May 1, 2024, respectively. On May 1, 2024, Michael Warner sent a litigation demand to our Board of Directors requesting that a civil action for monetary damages be brought by the Board of Directors on behalf of Illumina against officers and directors involved with the GRAIL acquisition. On July 30, 2024, the Board unanimously determined that it was in the best interest of the Company and its shareholders to defer a final decision on the demand given, among other things, the pending stockholder lawsuits described herein and the similarity of issues raised in the demand and those lawsuits. Our Board will monitor the stockholder lawsuits and revisit the demand as warranted as the lawsuits progress. On June 3, 2024, Elaine Wang made requests to inspect certain books and records under Delaware law and the Company sent a production of documents in response to that demand.
On August 21, 2024, an additional stockholder, Jane Davidson, sent a litigation demand to our Board of Directors requesting that a civil action for breaches of fiduciary duty, indemnification, contribution and other appropriate claims be brought by the Board of Directors on behalf of Illumina against officers and directors involved with the GRAIL acquisition. On October 29, 2024, the Board unanimously determined that it was in the best interest of the Company and its shareholders to defer a final decision on the demand given, among other things, the pending stockholder lawsuits described herein and the similarity of issues raised in the demand and those lawsuits. Our Board will monitor the stockholder lawsuits and revisit the demand as warranted as the lawsuits progress.
Securities Class Actions
Federal Securities Class Actions. On November 11, 2023, the first of three securities class action complaints was filed against Illumina and certain of its current and former executive officers in the United States District Court for the Southern District of California. The first-filed case is captioned Kangas v. Illumina, Inc. et al., the second-filed case is captioned Roy v. Illumina, Inc. et al., and the third-filed case is captioned Louisiana Sheriffs’ Pension & Relief Fund v. Illumina, Inc. et al. (collectively, the Actions). The complaints generally allege, among other things, that defendants made materially false and misleading statements and omitted material facts relating to Illumina’s acquisition of GRAIL. The complaints seek unspecified damages, interest, fees, and costs. On January 9, 2024, four movants filed motions to consolidate the Actions and to appoint a lead plaintiff (Lead Plaintiff Motions). On April 11, 2024, the Court issued an order consolidating the Actions into a single action (captioned in re Illumina, Inc. Securities Litigation No. 23-cv-2082-LL-MMP), and appointed Universal-Investment-Gesellschaft mbH, UI BVK Kapitalverwaltungsgesellschaft mbH, and ACATIS Investment Kapitalverwaltungsgesellschaft mbH as lead plaintiffs (the Lead Plaintiffs). On June 21, 2024, the Lead Plaintiffs filed their consolidated amended complaint. The complaint alleges that Illumina and GRAIL and certain of their current and former directors and officers violated Sections 10(b) and 20(a) of the Securities Exchange Act and SEC Rule 10b-5 in connection with Illumina’s acquisition of GRAIL. On September 13, 2024, the Lead Plaintiffs filed a second amended consolidated complaint. On November 12, 2024, the Company and other defendants filed a motion to dismiss the second amended consolidated complaint. On December 20, 2024, the Lead Plaintiffs filed their opposition to the motion to dismiss. The defendants’ final reply brief was filed on February 3, 2025. No hearing date has been set.
State Securities Class Actions. On February 2, 2024, the first of two additional securities class actions was filed against Illumina, certain of its officers and directors, and several other individuals and entities in the Superior Court of the State of California, County of San Mateo, captioned Loren Scott Mar v. Illumina, et al. and Scott Zerzanek v. Illumina, Inc. et al. Both complaints generally allege, among other things, that defendants made materially false and misleading statements and omitted material facts in the November 2020 and February 2021 registration statements and prospectus relating to Illumina’s acquisition of GRAIL. The complaints seek unspecified damages, interest, fees, and costs. On March 29, 2024, the parties to the actions filed a Joint Stipulation to Consolidate the actions and to appoint co-lead counsel for plaintiffs, which the Court granted on April 5, 2024. On August 12, 2024, the Plaintiffs filed their consolidated complaint. On September 6, 2024, Illumina and the other named defendants filed a motion to stay the litigation. On October 4, 2024, the plaintiffs opposed the motion to stay. At a hearing held on December 6, 2024, the Court declined to stay the litigation. The defendants’ demurrer is due February 28, 2025. Plaintiffs’ oppositions to the demurrer are due April 30, 2025, and defendants’ final reply briefs are due May 30, 2025. A hearing is set for June 20, 2025.
In light of the fact that the lawsuits are in an early stage, we cannot predict the ultimate outcome of the suits. We deny the allegations in the complaints and intend to vigorously defend the litigation.
ILLUMINA, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
DOJ Civil Investigative Demand
On January 18, 2024, we received a civil investigative demand (CID) from the U.S. Department of Justice, requiring production of certain documents and information in the course of a False Claims Act investigation to determine whether there is or has been a violation of 31 U.S.C. § 3729. The False Claims Act investigation concerns allegations that the Company caused the submission of false claims to Medicare and other federal government programs because it misrepresented its compliance with cybersecurity requirements to the Food and Drug Administration and other federal agencies that purchase its devices. The Company is cooperating with the government.
Books and Records Action
On February 14, 2024, a stockholder filed a complaint in the Delaware Court of Chancery captioned Pavers and Road Builders Benefit Funds v. Illumina, Inc. seeking to inspect certain books and records related to the GRAIL transaction, including certain materials and minutes from meetings of our Board of Directors, which have been withheld because the Company contends they are non-responsive to the request or subject to the attorney-client privilege. Illumina previously provided documents to the stockholder in response to a demand made by letter under Delaware law, but the stockholder seeks additional and unredacted materials through this action. On March 11, 2024, Illumina filed an answer to the complaint, denying that the stockholder was entitled to inspection. We deny that the stockholder is entitled to review the documents and intend to vigorously defend the litigation. The trial took place on June 7, 2024. On July 16, 2024, the Court issued a decision requiring the Company to produce certain additional documents to plaintiff. On July 26, 2024, the stockholder filed a motion seeking in camera review of certain documents that the Company maintains are not subject to the Court’s July 16, 2024 order to produce documents. The Court granted the motion on August 19, 2024, and the Company filed the documents for in camera review on August 29, 2024. On October 7, 2024, the Court ruled that the Company need not produce the documents subject to the motion for in camera review because they are privileged under the attorney opinion work product doctrine. On December 11, 2024, the Court entered its final order and judgment. On December 23, 2024, the plaintiff in this action filed a derivative complaint, described above.
BGI Genomics Co. Ltd. and its Affiliates
As previously disclosed, we were engaged in litigation in various U.S. jurisdictions with BGI Genomics Co. Ltd (BGI) and certain of its affiliates, including Complete Genomics, Inc. (CGI) since June of 2019. On July 14, 2022, we entered into a Settlement and License Agreement with BGI and CGI (the Agreement). Pursuant to the terms of the Agreement, we agreed to pay CGI a one time payment of $325 million. We allocated the $325 million payment on a relative fair value basis, resulting in $180 million capitalized as an intangible asset in 2022 for the value of the license, which is amortized over a period of 6.5 years on a straight-line basis, $150 million allocated to the release of past damages claimed, and a $5 million gain for damages awarded to us. The fair value of the license was estimated using a discounted cash flow model, which included assumptions for projected revenues covered by the license, an estimated royalty rate and a discount rate. The fair value of the past damages claimed was estimated based on applicable historical revenues and an estimated royalty rate. These inputs represent a Level 3 measurement because they are supported by little or no market activity and reflect our own assumptions in measuring fair value.
Loss before income taxes summarized by region was as follows:
| | | | | | | | | | | | | | | | | |
| In millions | 2024 | | 2023 | | 2022 |
| United States | $ | (1,834) | | | $ | (1,735) | | | $ | (4,942) | |
| Foreign | 655 | | | 618 | | | 606 | |
| Total loss before income taxes | $ | (1,179) | | | $ | (1,117) | | | $ | (4,336) | |
ILLUMINA, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
The provision for income taxes consisted of the following:
| | | | | | | | | | | | | | | | | |
| In millions | 2024 | | 2023 | | 2022 |
| Current: | | | | | |
| Federal | $ | 6 | | | $ | (5) | | | $ | (11) | |
| State | 18 | | | 6 | | | 27 | |
| Foreign | 137 | | | 77 | | | 75 | |
| Total current provision | 161 | | | 78 | | | 91 | |
| Deferred: | | | | | |
| Federal | (59) | | | (13) | | | 40 | |
| State | (56) | | | (26) | | | (47) | |
| Foreign | (2) | | | 5 | | | (16) | |
| Total deferred benefit | (117) | | | (34) | | | (23) | |
| Total tax provision | $ | 44 | | | $ | 44 | | | $ | 68 | |
The provision for income taxes reconciles to the amount computed by applying the federal statutory rate to loss before income taxes as follows:
| | | | | | | | | | | | | | | | | |
| In millions | 2024 | | 2023 | | 2022 |
| Tax at federal statutory rate | $ | (248) | | | $ | (235) | | | $ | (911) | |
| State, net of federal benefit | (38) | | | (16) | | | (9) | |
| Research and other credits | (32) | | | (42) | | | (46) | |
| Change in valuation allowance | 29 | | | 48 | | | 62 | |
| | | | | |
| | | | | |
| Impact of R&D expense capitalization | 52 | | | 86 | | | 87 | |
| Impact of net operating losses on GILTI, U.S. foreign tax credits, and Pillar Two global minimum top-up tax | 90 | | | 61 | | | 60 | |
| Impact of foreign operations | 3 | | | (50) | | | (81) | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| Stock compensation | 16 | | | 31 | | | 20 | |
| | | | | |
| Accrual of European Commission fine | (99) | | | 3 | | | 96 | |
| Goodwill impairment | 308 | | | 149 | | | 822 | |
| Impact of acquisition related items | (46) | | | 8 | | | (27) | |
| Other | 9 | | | 1 | | | (5) | |
| Total tax provision | $ | 44 | | | $ | 44 | | | $ | 68 | |
We have elected to account for the global intangible low-taxed income (GILTI) as a period cost in our consolidated financial statements.
The impact of foreign operations primarily represents the difference between the actual provision for income taxes for our legal entities that operate in jurisdictions that have statutory tax rates that differ from the U.S. federal statutory tax rate of 21%. The most significant tax benefits from foreign operations were from our earnings in Singapore, which had a statutory tax rate of 17% in 2024. The impact of foreign operations also includes the impacts of GILTI, U.S. foreign tax credits, and the Pillar Two global minimum top-up tax of non-U.S. earnings before the tax impact of net operating losses, and uncertain tax positions related to foreign items.
The impact of R&D expense capitalization is primarily the income tax expense impact of capitalizing research and development expenses for tax purposes on GILTI and the utilization of the U.S. foreign tax credits.
ILLUMINA, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
The impact of net operating losses on GILTI, U.S. foreign tax credits, and the Pillar Two global minimum top-up tax is primarily the income tax expense impact of GRAIL pre-acquisition net operating losses on GILTI, the utilization of the U.S. foreign tax credits, and the Pillar Two global minimum top-up tax.
The impact of acquisition related items includes the income tax expense impact of transaction costs, acquisition related compensation, and changes to the contingent value rights associated with the GRAIL acquisition.
Significant components of deferred tax assets and liabilities were as follows:
| | | | | | | | | | | |
| In millions | December 29,
2024 | | December 31,
2023 |
| Deferred tax assets: | | | |
| Net operating losses | $ | 189 | | | $ | 392 | |
| Tax credits | 252 | | | 211 | |
| Other accruals and reserves | 40 | | | 49 | |
| Stock compensation | 34 | | | 15 | |
| | | |
| | | |
| Capitalized U.S. R&D expenses | 181 | | | 158 | |
| Other amortization | 42 | | | 115 | |
| Operating lease liabilities | 112 | | | 146 | |
| Property and equipment | 17 | | | 4 | |
| Investments | 23 | | | 7 | |
| Other | 63 | | | 56 | |
| Total gross deferred tax assets | 953 | | | 1,153 | |
| Valuation allowance on deferred tax assets | (278) | | | (251) | |
| Total deferred tax assets | 675 | | | 902 | |
| Deferred tax liabilities: | | | |
| Purchased intangible amortization | (35) | | | (734) | |
| | | |
| | | |
| Operating lease right-of-use assets | (57) | | | (88) | |
| | | |
| Other | (25) | | | (25) | |
| Total deferred tax liabilities | (117) | | | (847) | |
| Deferred tax assets, net | $ | 558 | | | $ | 55 | |
A valuation allowance is established when it is more likely than not the future realization of all or some of the deferred tax assets will not be achieved. The evaluation of the need for a valuation allowance is performed on a jurisdiction-by-jurisdiction basis and includes a review of all available positive and negative evidence, including operating results and forecasted ranges of future taxable income. Based on the available evidence as of December 29, 2024, we were not able to conclude it is more likely than not certain deferred tax assets will be realized. Therefore, a valuation allowance of $278 million was recorded against certain U.S. and foreign deferred tax assets.
As of December 29, 2024, we had net operating loss carryforwards for federal and state tax purposes of $74 million and $1,872 million, respectively, which will begin to expire in 2036 and 2025, respectively, unless utilized prior. We also had federal and state tax credit carryforwards of $140 million and $239 million, which will begin to expire in 2031 and 2027, respectively, unless utilized prior.
Pursuant to Section 382 and 383 of the Internal Revenue Code, utilization of net operating losses and credits may be subject to annual limitations in the event of any significant future changes in its ownership structure. These annual limitations may result in the expiration of net operating losses and credits prior to utilization. The deferred tax assets as of December 29, 2024 are net of any previous limitations due to Section 382 and 383.
Our manufacturing operations in Singapore operate under various tax holidays and incentives, which will begin to expire in 2028. These tax holidays and incentives resulted in a $33 million, $75 million, and $56 million decrease to the provision for income taxes in 2024, 2023, and 2022, respectively. These tax holidays and incentives resulted in a decrease in diluted loss per share of $0.20, $0.47, and $0.35, in 2024, 2023, and 2022, respectively.
ILLUMINA, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
As of December 29, 2024, we asserted that $1,806 million of foreign earnings would not be indefinitely reinvested, and accordingly, recorded a deferred tax liability of $24 million.
The following table summarizes the gross amount of our uncertain tax positions:
| | | | | | | | | | | | | | | | | |
| In millions | December 29,
2024 | | December 31,
2023 | | January 1,
2023 |
| Balance at beginning of year | $ | 210 | | | $ | 153 | | | $ | 131 | |
| Increases related to prior year tax positions | 2 | | | 27 | | | 12 | |
| Decreases related to prior year tax positions | (2) | | | (2) | | | (3) | |
| Increases related to current year tax positions | 23 | | | 42 | | | 42 | |
| Decreases related to lapse of statute of limitations | (1) | | | (10) | | | (29) | |
| Balance at end of year | $ | 232 | | | $ | 210 | | | $ | 153 | |
Included in the balance of uncertain tax positions as of December 29, 2024 and December 31, 2023, was $202 million and $156 million, respectively, of net unrecognized tax benefits that, if recognized, would reduce the effective income tax rate in future periods.
Any interest and penalties related to uncertain tax positions are reflected in the provision for income taxes. We recognized expense of $6 million and $2 million in 2024 and 2023, respectively, and income of $3 million in 2022, related to potential interest and penalties on uncertain tax positions. We recorded a liability for potential interest and penalties of $13 million and $6 million as of December 29, 2024 and December 31, 2023, respectively.
Tax years 1997 to 2023 remain subject to future examination by the major tax jurisdictions in which we are subject to tax. The Internal Revenue Service completed an examination of the U.S. Corporation Income Tax Returns for tax years 2017, 2018, and 2020. Given the uncertainty of potential adjustments from examination as well as the potential expiration of the statute of limitations, it is reasonably possible that the balance of unrecognized tax benefits could change significantly over the next 12 months. Due to the number of years remaining that are subject to examination, we are unable to estimate the full range of possible adjustments to the balance of gross unrecognized tax benefits.
| | |
| 11. EMPLOYEE BENEFIT PLANS |
Retirement Plan
We have a 401(k) savings plan covering substantially all of our employees in the United States. Our contributions to the plan are discretionary. During 2024, 2023, and 2022, we made matching contributions of $34 million, $36 million, and $30 million, respectively.
Deferred Compensation Plan
The Illumina, Inc. Deferred Compensation Plan (the Plan) allows senior level employees to contribute up to 60% of their base salary and 100% of their variable cash compensation, and members of the board of directors to contribute up to 100% of their director fees and equity awards. Under the Plan, we credit the participants’ contributions with earnings that reflect the performance of certain independent investment funds. On a discretionary basis, we may also make employer contributions to participant accounts in any amount determined by us. The vesting schedules of employer contributions are at the sole discretion of the Compensation Committee. However, all employer contributions shall become 100% vested upon the occurrence of the participant’s disability, death or retirement or a change in control of Illumina. The benefits under this plan are unsecured. Participants are generally eligible to receive payment of their vested benefit at the end of their elected deferral period or after termination of their employment for any reason or at a later date to comply with the restrictions of Section 409A.
ILLUMINA, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
We established a rabbi trust for the benefit of the participants under the Plan and have included the assets of the rabbi trust in other assets in the consolidated balance sheets. As of December 29, 2024 and December 31, 2023, the assets of the trust were $70 million and $61 million, respectively, and our liabilities, included in accrued liabilities, were $65 million and $59 million, respectively. Changes in the values of the assets held by the trust are recorded in other expense, net, and changes in the values of the deferred compensation liabilities are recorded in operating expense.
| | |
| 12. SEGMENT AND GEOGRAPHIC INFORMATION |
Reportable Segment InformationAs of December 29, 2024, we have one reportable segment, Core Illumina. Prior to the Spin-Off of GRAIL, on June 24, 2024, our reportable segments included both Core Illumina and GRAIL. See note 2. GRAIL Spin-Off for details. We continue to disclose certain historical information for GRAIL prior to the Spin-Off. Segment information is consistent with how our Chief Operating Decision Maker (CODM), who is our Chief Executive Officer, reviews financial information, makes operating decisions, allocates resources, and assesses performance. We also consider the way budgets and forecasts are prepared and reviewed and the basis on which executive compensation is determined. Core Illumina: Core Illumina’s products and services serve customers in the research, clinical and applied markets, and enable the adoption of a variety of genomic solutions. Core Illumina sells products and provides services to GRAIL, and vice versa, in accordance with contractual agreements between the entities.
GRAIL: GRAIL is a healthcare company focused on early detection of multiple cancers. Prior to the Spin-Off of GRAIL into a separate, independent public company, GRAIL was required to be held and operated separately and independently from Illumina pursuant to the transitional measures ordered by the European Commission.
Our CODM allocates resources and evaluates business performance based on revenues and net income (loss). Net income (loss) is used in the annual budgeting and monthly forecasting processes and to monitor and assess budgeted/forecasted versus actual results. Our CODM does not evaluate segments using asset information. The accounting policies for segments are the same as those described in the summary of significant accounting policies.
ILLUMINA, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
The following tables present selected financial information with respect to segments for the periods presented:
| | | | | | | | | | | | | | | | | |
| In millions | 2024 | | 2023 | | 2022 |
| Core Illumina: | | | | | |
Revenue(1) | $ | 4,332 | | | $ | 4,438 | | | $ | 4,553 | |
| Less: | | | | | |
| Cost of revenue | 1,424 | | | 1,582 | | | 1,446 | |
| Research and development | 988 | | | 1,030 | | | 1,004 | |
| Selling and marketing | 638 | | | 648 | | | 677 | |
| General and administrative | 262 | | | 600 | | | 326 | |
| Goodwill and intangible impairment | 3 | | | 6 | | | — | |
| Legal contingency and settlement | (456) | | | 20 | | | 619 | |
| | | | | |
| | | | | |
| | | | | |
| Core Illumina income from operations | 1,473 | | | 552 | | | 481 | |
| GRAIL: | | | | | |
| Revenue | 55 | | | 93 | | | 55 | |
Total operating expenses(2) | 2,360 | | | 1,714 | | | 4,712 | |
| Consolidated other expense, net | 346 | | | 48 | | | 157 | |
| Consolidated provision for income taxes | 44 | | | 44 | | | 68 | |
| Intersegment eliminations | (1) | | | — | | | (3) | |
| Consolidated net loss | $ | (1,223) | | | $ | (1,161) | | | $ | (4,404) | |
_____________
(1)Core Illumina revenue for 2024, 2023, and 2022 included intercompany revenue of $15 million, $26 million, and $24 million, respectively.
(2)GRAIL operating expenses are inclusive of cost of revenue, research and development, selling and marketing, general and administrative, and goodwill and intangible impairment for the comparative periods prior to the Spin-Off on June 24, 2024.
| | | | | | | | | | | | | | | | | |
| In millions | 2024 | | 2023 | | 2022 |
| Depreciation and amortization: | | | | | |
| Core Illumina | $ | 280 | | | $ | 273 | | | $ | 240 | |
| GRAIL | 74 | | | 159 | | | 154 | |
| | | | | |
| Consolidated depreciation and amortization | $ | 354 | | | $ | 432 | | | $ | 394 | |
| | | | | |
| Capital expenditures: | | | | | |
| Core Illumina | $ | 137 | | | $ | 183 | | | $ | 262 | |
| GRAIL | 5 | | | 13 | | | 24 | |
| Eliminations | — | | | (1) | | | — | |
| Consolidated capital expenditures | $ | 142 | | | $ | 195 | | | $ | 286 | |
ILLUMINA, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Geographic Data
Long-lived assets, consisting of property and equipment and operating lease right-of-use assets, were as follows:
| | | | | | | | | | | |
| In millions | December 29,
2024 | | December 31,
2023 |
| United States | $ | 750 | | | $ | 1,040 | |
| Singapore | 279 | | | 298 | |
| United Kingdom | 124 | | | 136 | |
| Other countries | 81 | | | 77 | |
| Total long-lived assets, net | $ | 1,234 | | | $ | 1,551 | |
Refer to note 3. Revenue for revenue by geographic area.
CONTROLS AND PROCEDURES
We design our internal controls to provide reasonable assurance that (1) our transactions are properly authorized; (2) our assets are safeguarded against unauthorized or improper use; and (3) our transactions are properly recorded and reported in conformity with U.S. generally accepted accounting principles. We also maintain internal controls and procedures to ensure that we comply with applicable laws and our established financial policies.
During the fourth quarter of 2024, we continued to monitor and evaluate the design and operating effectiveness of key controls. There were no changes in our internal control over financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) of the Exchange Act) that materially affected or are reasonably likely to materially affect internal control over financial reporting.
Our management, under the supervision and with the participation of our chief executive officer (CEO) and chief financial officer (CFO), has evaluated the effectiveness of our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934, as amended (Exchange Act)), as of the end of the period covered by this Annual Report on Form 10-K. Based on such evaluation, our CEO and CFO have concluded that as of December 29, 2024, our disclosure controls and procedures are designed at a reasonable assurance level and are effective to provide reasonable assurance that information we are required to disclose in reports that we file or submit under the Exchange Act is recorded, processed, summarized, and reported within the time periods specified in the rules and forms of the Securities and Exchange Commission (SEC), and that such information is accumulated and communicated to our management, including our CEO and CFO, as appropriate, to allow timely decisions regarding required disclosure.
MANAGEMENT’S REPORT ON INTERNAL CONTROL OVER FINANCIAL REPORTING
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Exchange Act Rules 13a-15(f). Because of its inherent limitations, internal control over financial reporting may not prevent or detect all misstatements. Therefore, even those systems determined to be effective can provide only reasonable assurance with respect to financial statement preparation and presentation.
We conducted an evaluation of the effectiveness of our internal control over financial reporting based on the framework in Internal Control - Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework). Based on this evaluation, our management has concluded that our internal control over financial reporting was effective as of December 29, 2024. The effectiveness of our internal control over financial reporting as of December 29, 2024 has been audited by Ernst & Young LLP, an independent registered public accounting firm, as stated in their report which is included herein.
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Stockholders and the Board of Directors of Illumina, Inc.
Opinion on Internal Control Over Financial Reporting
We have audited Illumina, Inc.’s internal control over financial reporting as of December 29, 2024, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework) (the COSO criteria). In our opinion, Illumina, Inc. (the Company) maintained, in all material respects, effective internal control over financial reporting as of December 29, 2024, based on the COSO criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the consolidated balance sheets of the Company as of December 29, 2024 and December 31, 2023, the related consolidated statements of operations, comprehensive loss, stockholders’ equity and cash flows for each of the three years in the period ended December 29, 2024, and the related notes and our report dated February 12, 2025 expressed an unqualified opinion thereon.
Basis for Opinion
The Company’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying Management’s Report on Internal Control over Financial Reporting. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects.
Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
Definition and Limitations of Internal Control Over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
/s/ Ernst & Young LLP
San Diego, California
February 12, 2025
ADOPTIONS, MODIFICATIONS OR TERMINATIONS OF TRADING PLANS
During the quarterly period ended December 29, 2024, the following directors and officers adopted, modified or terminated 10b5-1 plans:
•On November 6, 2024, Frances Arnold, a member of our Board of Directors, entered in a new arrangement intended to satisfy the affirmative defense conditions of Rule 10b5-1(c). The arrangement terminates on February 27, 2026 and provides for the sale of up to 120 shares.
•On November 7, 2024, Jakob Wedel, our Chief Strategy and Corporate Development Officer, entered in a new arrangement intended to satisfy the affirmative defense conditions of Rule 10b5-1(c). The arrangement terminates on May 29, 2026 and provides for the sale of up to 3,182 shares.
•On November 8, 2024, Scott Davies, our Interim General Counsel, entered in a new arrangement intended to satisfy the affirmative defense conditions of Rule 10b5-1(c). The arrangement terminates on December 16, 2025 and provides for the sale of up to 3,301 shares.
•On November 13, 2024, Patricia Leckman, our Chief People Officer, entered in a new arrangement intended to satisfy the affirmative defense conditions of Rule 10b5-1(c). The arrangement terminates on November 14, 2025 and provides for the sale of up to 1,615 shares.
Other than as disclosed above, during the quarterly period ended December 29, 2024, none of the Company’s directors or officers adopted, modified or terminated any “Rule 10b5-1 trading arrangement” or any “non-Rule 10b5-1 trading arrangement,” in each case as such term is defined in Item 408 of Regulation S-K.
DIRECTORS, EXECUTIVE OFFICERS, AND CORPORATE GOVERNANCE
Directors
Information concerning our directors is incorporated by reference from the section entitled “Proposal One: Election of Directors,” “Information About Directors,” “Director Compensation,” and “Board of Directors and Corporate Governance” to be contained in our definitive Proxy Statement with respect to our 2025 Annual Meeting of Stockholders to be filed with the SEC no later than April 28, 2025.
Executive Officers
Information concerning our executive officers is incorporated by reference from the section entitled “Executive Officers” to be contained in our definitive Proxy Statement with respect to our 2025 Annual Meeting of Stockholders to be filed with the SEC no later than April 28, 2025.
Corporate Governance
Section 16(a) of the Exchange Act
Information concerning compliance with Section 16(a) of the Securities Exchange Act of 1934 is incorporated by reference from the section entitled “Section 16(a) Beneficial Ownership Reporting Compliance” to be contained in our definitive Proxy Statement with respect to our 2025 Annual Meeting of Stockholders to be filed with the SEC no later than April 28, 2025.
Audit Committee Financial Expert
Information concerning the audit committee financial expert as defined by the SEC rules adopted pursuant to the Sarbanes-Oxley Act of 2002 is incorporated by reference from the section entitled “Board of Directors and Corporate Governance” to be contained in our definitive Proxy Statement with respect to our 2025 Annual Meeting of Stockholders to be filed with the SEC no later than April 28, 2025.
Code of Conduct
We have a code of conduct for our directors, officers, and employees, which is available on our website at www.illumina.com in the Corporate Governance portal of the Investor Information section under “Company.” A copy of the Code of Conduct is available in print free of charge to any stockholder who requests a copy. Interested parties may address a written request for a printed copy of the Code of Ethics to: Corporate Secretary, Illumina, Inc., 5200 Illumina Way, San Diego, California 92122. We intend to satisfy the disclosure requirement regarding any amendment to, or a waiver from, a provision of the Code of Ethics for our principal executive officer, principal financial officer, principal accounting officer or controller, or persons performing similar functions, by posting such information on our website. The information on, or that can be accessed from, our website is not incorporated by reference into this report.
EXECUTIVE COMPENSATION
Information concerning executive compensation is incorporated by reference from the sections entitled “Compensation Discussion and Analysis,” “Director Compensation,” and “Executive Compensation” to be contained in our definitive Proxy Statement with respect to our 2025 Annual Meeting of Stockholders to be filed with the SEC no later than April 28, 2025.
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
Information concerning the security ownership of certain beneficial owners and management and information covering securities authorized for issuance under equity compensation plans is incorporated by reference from the sections entitled “Stock Ownership of Principal Stockholders and Management,” “Executive Compensation,” and “Equity Compensation Plan Information” to be contained in our definitive Proxy Statement with respect to our 2025 Annual Meeting of Stockholders to be filed with the SEC no later than April 28, 2025.
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
Information concerning certain relationships and related transactions, and director independence is incorporated by reference from the sections entitled “Proposal One: Election of Directors,” “Information About Directors,” “Director Compensation,” “Executive Compensation,” and “Certain Relationships and Related Party Transactions” to be contained in our definitive Proxy Statement with respect to our 2025 Annual Meeting of Stockholders to be filed with the SEC no later than April 28, 2025.
PRINCIPAL ACCOUNTANT FEES AND SERVICES
Information concerning principal accountant fees and services is incorporated by reference from the sections entitled “Proposal Two: Ratification of Appointment of Independent Registered Public Accounting Firm” and “Independent Registered Public Accountants” to be contained in our definitive Proxy Statement with respect to our 2025 Annual Meeting of Stockholders to be filed with the SEC no later than April 28, 2025.
EXHIBITS, FINANCIAL STATEMENT SCHEDULES
Exhibits
Exhibits listed in the accompanying Index to Exhibits below are filed or incorporated by reference as part of this report.
Financial Statements
Financial Statement Schedules
All financial schedules have been omitted as the required information is not applicable, not material, or because the information required is included in the consolidated financial statements and notes thereto included in the Consolidated Financial Statements section of this report.
Index to Exhibits
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | Incorporated by Reference | | |
| Exhibit | | | | | | | | | | Filing | | Filed |
| Number | | Exhibit Description | | Form | | File Number | | Exhibit | | Date | | Herewith |
| 3.1 | | | | 10-Q | | 001-35406 | | 3.1 | | | 8/11/2022 | | |
| 3.2 | | | | 10-Q | | 001-35406 | | 3.1 | | | 8/7/2024 | | |
| 4.1 | | | | S-1/A | | 333-33922 | | 4.1 | | | 7/3/2000 | | |
| 4.3 | | | | 10-K | | 001-35406 | | 4.5 | | | 2/17/2021 | | |
| 4.4 | | | | S-3 | | 333-54195 | | 4.6 | | | 3/12/2021 | | |
| 4.5 | | | | 8-K | | 001-35406 | | 4.2 | | | 3/22/2021 | | |
| 4.6 | | | | 8-K | | 001-35406 | | 4.1 | | | 8/18/2021 | | |
| 4.7 | | | | 8-K | | 001-35406 | | 4.2 | | | 12/13/2022 | | |
| +10.1 | | | | 10-Q | | 000-30361 | | 10.55 | | | 7/25/2008 | | |
| +10.2 | | | | 10-K | | 000-30361 | | 10.34 | | | 2/26/2009 | | |
| +10.3 | | | | 10-Q | | 001-35406 | | 10.1 | | | 8/10/2023 | | |
| +10.4 | | | | 10-K | | 000-30361 | | 10.7 | | | 2/26/2010 | | |
| 10.5 | | | | 10-Q | | 000-30361 | | 10.5 | | | 5/3/2007 | | |
| +10.6 | | | | 8-K | | 000-30361 | | 99.3 | | | 11/26/2007 | | |
| +10.7 | | | | 8-K | | 000-30361 | | 99.4 | | | 11/26/2007 | | |
| +10.8 | | | | 8-K | | 000-30361 | | 99.5 | | | 11/26/2007 | | |
| +10.9 | | | | 10-K | | 000-30361 | | 10.25 | | | 2/26/2009 | | |
| +10.10 | | | | 10-K | | 000-30361 | | 10.26 | | | 2/26/2009 | | |
| +10.11 | | | | 8-K | | 001-35406 | | 10.1 | | | 2/7/2023 | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| +10.12 | | | | 8-K | | 001-35406 | | 10.4 | | | 2/7/2023 | | |
| +10.13 | | | | 8-K | | 001-35406 | | 10.2 | | | 2/7/2023 | | |
| +10.14 | | | | 8-K | | 001-35406 | | 10.3 | | | 2/7/2023 | | |
| +10.15 | | | | 10-K | | 001-35406 | | 10.15 | | | 2/17/2023 | | |
| 10.16 | | | | 10-Q | | 000-30361 | | 10.41 | | | 5/3/2007 | | |
| 10.17 | | | | 10-Q | | 000-30361 | | 10.42 | | | 5/3/2007 | | |
| 10.18 | | | | 10-Q | | 001-35406 | | 10.1 | | | 5/3/2012 | | |
| 10.19 | | | | 10-K | | 001-35406 | | 10.23 | | | 2/18/2015 | | |
| 10.20 | | | | 10-K | | 001-35406 | | 10.24 | | | 2/18/2015 | | |
| 10.21 | | | | 10-K | | 001-35406 | | 10.18 | | | 2/13/2018 | | |
| +10.22 | | | | 14D-9 | | 005-60457 | | 99(e)(6) | | 2/7/2012 | | |
| 10.23 | | | | 10-K | | 001-35406 | | 10.26 | | | 2/18/2015 | | |
| 10.24 | | | | 10-K | | 001-35406 | | 10.27 | | | 2/18/2015 | | |
| 10.25 | | | | 10-K | | 001-35406 | | 10.22 | | | 2/13/2018 | | |
| 10.26 | | | | 10-K | | 001-35406 | | 10.23 | | | 2/13/2018 | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| 10.27 | | | | 10-K | | 001-35406 | | 10.24 | | | 2/13/2018 | | |
| 10.28 | | | | 10-K | | 001-35406 | | 10.25 | | | 2/11/2020 | | |
| 10.29 | | | | 10-K | | 001-35406 | | 10.25 | | | 2/11/2020 | | |
| 10.30 | | | | 10-Q | | 001-35406 | | 10.1 | | | 10/30/2020 | | |
| 10.31 | | | | 10-Q | | 001-35406 | | 10.1 | | | 7/31/2015 | | |
| 10.32 | | | | 10-K | | 001-35406 | | 10.29 | | | 3/2/2016 | | |
| 10.33 | | | | 10-K | | 001-35406 | | 10.30 | | | 3/2/2016 | | |
| 10.34 | | | | 10-K | | 001-35406 | | 10.28 | | | 2/14/2017 | | |
| 10.35 | | | | 10-K | | 001-35406 | | 10.29 | | | 2/14/2017 | | |
| 10.36 | | | | 10-K | | 001-35406 | | 10.30 | | | 2/14/2017 | | |
| 10.37 | | | | 10-Q | | 001-35406 | | 10.10 | | | 4/25/2018 | | |
| +10.39 | | | | 10-Q | | 001-35406 | | 10.1 | | | 11/5/2021 | | |
| 10.40 | | Credit Agreement, dated as of January 4, 2023, among the Company, as the borrower, the lenders from time to time party thereto, Bank of America, N.A., as administrative agent, an issuing bank and the swingline lender, and the other issuing banks from time to time party thereto | | 8-K | | 001-35406 | | 10.1 | | | 1/4/2023 | | |
| +10.41 | | | | 10-Q | | 001-35406 | | 10.1 | | | 5/3/2024 | | |
| 10.42 | | | | 8-K | | 001-35406 | | 10.1 | | | 6/24/2024 | | |
| 10.43 | | | | 8-K | | 001-35406 | | 10.2 | | | 6/24/2024 | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| 10.44 | | | | 8-K | | 001-35406 | | 10.3 | | | 6/24/2024 | | |
| 10.45 | | | | 8-K | | 001-35406 | | 10.4 | | | 6/24/2024 | | |
| 10.56 | | 364-Day Delayed Draw Credit Agreement, dated as of June 17, 2024, among the Company, as the borrower, the lenders from time to time party thereto and JPMorgan Chase Bank, N.A., as administrative agent Fourth Amendment to the Amended and Restated Supply and Commercialization Agreement, dated June 21, 2024, by and between Illumina, Inc. and GRAIL, LLC* | | 8-K | | 001-35406 | | 10.1 | | | 6/17/2024 | | |
| +10.57 | | | | 10-Q | | 001-35406 | | 10.6 | | | 8/7/2024 | | |
| +10.58 | | | | 10-Q | | 001-35406 | | 10.7 | | | 8/7/2024 | | |
| 10.59 | | | | 8-K | | 001-35406 | | 1.1 | | | 9/9/2024 | | |
| 10.60 | | | | 8-K | | 001-35406 | | 4.2 | | | 9/9/2024 | | |
| +10.61 | | | | 8-K | | 001-35406 | | 10.1 | | | 10/3/2024 | | |
| +10.62 | | | | 8-K | | 001-35406 | | 10.2 | | | 10/3/2024 | | |
| +19.1 | | | | | | | | | | | | X |
| 21.1 | | | | | | | | | | | | X |
| 23.1 | | | | | | | | | | | | X |
| 24.1 | | Power of Attorney (included on the signature page) | | | | | | | | | | X |
| 31.1 | | | | | | | | | | | | X |
| 31.2 | | | | | | | | | | | | X |
| 32.1 | | | | | | | | | | | | X |
| 32.2 | | | | | | | | | | | | X |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| +97.1 | | | | 10-K | | 001-35406 | | 97.1 | | | 2/16/2024 | | |
| 101.INS | | XBRL Instance Document - the instance document does not appear in the Interactive Data File because its XBRL tags are embedded within the Inline XBRL document | | | | | | | | | | X |
| 101.SCH | | XBRL Taxonomy Extension Schema | | | | | | | | | | X |
| 101.CAL | | XBRL Taxonomy Extension Calculation Linkbase | | | | | | | | | | X |
| 101.LAB | | XBRL Taxonomy Extension Label Linkbase | | | | | | | | | | X |
| 101.PRE | | XBRL Taxonomy Extension Presentation Linkbase | | | | | | | | | | X |
| 101.DEF | | XBRL Taxonomy Extension Definition Linkbase | | | | | | | | | | X |
| 104 | | Cover Page Interactive Data File - formatted in Inline XBRL and included as Exhibit 101 | | | | | | | | | | X |
_______________________________________
| | | | | | | | |
| + | | Management contract or corporate plan or arrangement |
| * | | Portions of this exhibit omitted pursuant to Item 601(b)(2) and Item 601(b)(10) of Regulation S-K, as applicable. The Company agrees to furnish a supplemental and unredacted copy of any omitted schedule to the Securities and Exchange Commission upon its request. |
Supplemental Information
No Annual Report to stockholders or proxy materials has been furnished to stockholders as of the date of this report. The Annual Report to stockholders and proxy material will be furnished to our stockholders after the filing of this Annual Report on Form 10-K and we will furnish such material to the SEC at that time.
FORM 10-K CROSS-REFERENCE INDEX
| | | | | | | | |
| | | Page |
| PART I |
| | |
| | |
| Item 1B | Unresolved Staff Comments | None |
| | |
| | |
| | |
| Item 4 | Mine Safety Disclosures | Not Applicable |
| | | |
| PART II |
| | |
| | |
| | |
| | |
| | |
| Item 9 | Changes in and Disagreements with Accountants on Accounting and Financial Disclosure | None |
| | |
| | |
| Item 9C | Disclosure Regarding Foreign Jurisdictions that Prevent Inspections | Not Applicable |
| | | |
| PART III |
| | |
| | |
| | |
| | |
| | |
| | | |
| PART IV |
| | |
| | |
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this Report to be signed on its behalf by the undersigned, thereunto duly authorized, on February 12, 2025.
| | | | | | | | |
| ILLUMINA, INC. |
| | |
| | By: | /s/ JACOB THAYSEN |
| | Jacob Thaysen
Chief Executive Officer |
February 12, 2025
POWER OF ATTORNEY
KNOW ALL PERSONS BY THESE PRESENT, that each person whose signature appears below constitutes and appoints Jacob Thaysen and Ankur Dhingra, and each or any one of them, his or her true and lawful attorney-in-fact and agent, with full power of substitution and resubstitution, for him and in his or her name, place and stead, in any and all capacities, to sign any and all amendments to this Annual Report on Form 10-K, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and thing requisite and necessary to be done in connection therewith, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or any of them, or their, his, or her substitutes or substitute, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this Annual Report on Form 10-K has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| | | | | | | | | | | | | | |
| /s/ JACOB THAYSEN | | Chief Executive Officer, Director
(Principal Executive Officer) | | February 12, 2025 |
| Jacob Thaysen | | | | |
| | | | |
| /s/ ANKUR DHINGRA | | Chief Financial Officer
(Principal Financial Officer) | | February 12, 2025 |
| Ankur Dhingra | | | | |
| | | | |
| /s/ SCOTT ERICKSEN | | Vice President and Chief Accounting Officer
(Principal Accounting Officer) | | February 12, 2025 |
| Scott Ericksen | | | | |
| | | | |
| /s/ STEPHEN P. MACMILLAN | | Independent Chair of the Board of Directors | | February 12, 2025 |
| Stephen P. MacMillan | | | | |
| | | | |
| /s/ FRANCES ARNOLD | | Director | | February 12, 2025 |
| Frances Arnold, Ph.D. | | | | |
| | | | |
| /s/ CAROLINE DORSA | | Director | | February 12, 2025 |
| Caroline Dorsa | | | | |
| | | | |
| /s/ ROBERT S. EPSTEIN | | Director | | February 12, 2025 |
| Robert S. Epstein, M.D. | | | | |
| | | | |
| /s/ SCOTT GOTTLIEB | | Director | | February 12, 2025 |
| Scott Gottlieb, M.D. | | | | |
| | | | |
| /s/ GARY S. GUTHART | | Director | | February 12, 2025 |
| Gary S. Guthart, Ph.D. | | | | |
| | | | |
| /s/ PHILIP SCHILLER | | Director | | February 12, 2025 |
| Philip Schiller | | | | |
| | | | |
| /s/ SUSAN SIEGEL | | Director | | February 12, 2025 |
| Susan Siegel | | | | |
| | | | |
| /s/ ANNA RICHO | | Director | | February 12, 2025 |
| Anna Richo | | | | |
| | | | |
| /s/ SCOTT B. ULLEM | | Director | | February 12, 2025 |
| Scott B. Ullem | | | | |