UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 10-K
| | | |
þ | | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the Fiscal Year Ended December 31, 2005 |
OR |
o | | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to |
Commission File No.: 0-51149
Emageon Inc.
(Exact name of registrant as specified in its charter)
| | | |
| Delaware | | 63-1240138 |
(State or other jurisdiction
of incorporation or organization) | | (I.R.S. Employer Identification No.) |
| | | |
1200 Corporate Drive, Suite 200
Birmingham, Alabama
(Address of principal executive offices) | | 35242
(zip code) |
Registrant’s telephone number, including area code:
(205) 980-9222
Securities registered pursuant to Section 12(b) of the Act:
None
Securities registered pursuant to Section 12(g) of the Act:
Common Stock, $0.001 Par Value Per Share
(Title of Class)
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. YES o NO þ
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. YES o NO þ
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. YES þ NO o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 ofRegulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of thisForm 10-K or any amendment to thisForm 10-K. o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, or a non-accelerated filer.
Large accelerated filer o Accelerated filer o Non-accelerated filer þ
Indicate by check mark whether the registrant is a shell company (as defined inRule 12b-2 of the Act). YES o NO þ
The aggregate market value of the common stock held by non-affiliates of the registrant (which, for purposes hereof, are all holders other than executive officers, directors, and holders of 10% or more of the outstanding common stock of the registrant) as of June 30, 2005 was approximately $208,717,000 based on the closing sale price of such stock as reported by The NASDAQ Stock Market, Inc. on June 30, 2005. The basis of this calculation does not constitute a determination by the registrant that any of the persons referred to in the immediately preceding sentence are affiliates of the registrant.
As of March 23, 2006 there were 20,806,339 shares of Emageon Inc. common stock, $0.001 par value, outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the Company’s Proxy Statement for the May 25, 2006 Annual Meeting of Shareholders are incorporated by reference into Part III.
PART I
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
Some of the statements made under the headings “Business” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and elsewhere in this Annual Report onForm 10-K contain forward-looking statements which reflect our plans, beliefs and current views with respect to, among other things, future events and financial performance. We often identify these forward-looking statements by the use of forward-looking words such as “believe”, “expect”, “potential”, “continue”, “may”, “will”, “should”, “could”, “would”, “seek”, “predict”, “intend”, “plan”, “estimate”, “anticipate” or the negative version of those words or other comparable words. Any forward-looking statements contained in this Annual Report are based upon our historical performance and on current plans, estimates and expectations. The inclusion of this forward-looking information should not be regarded as a representation by us or any other person that the future plans, estimates or expectations contemplated by us will be achieved. Such forward-looking statements are subject to various risks and uncertainties. In addition, there are or will be important factors that could cause our actual results to differ materially from those indicated in these statements. We believe these factors include, but are not limited to, those described in Item 1A of this Annual Report under the caption “Risk Factors”.
These cautionary statements should not be construed as exhaustive and should be read in conjunction with the other cautionary statements that are included in this Annual Report. Moreover, we operate in a continually changing business environment, and new risks and uncertainties emerge from time to time. Management cannot predict these new risks or uncertainties, nor can it assess the impact, if any, that any such risks or uncertainties may have on our business or the extent to which any factor, or combination of factors, may cause actual results to differ from those projected in any forward-looking statement. Accordingly, the risks and uncertainties to which we are subject can be expected to change over time, and we undertake no obligation to update publicly or review the risks or uncertainties described herein. We also undertake no obligation to update publicly or review any of the forward-looking statements made in this Annual Report, whether as a result of new information, future developments or otherwise.
ITEM 1: BUSINESS
Overview
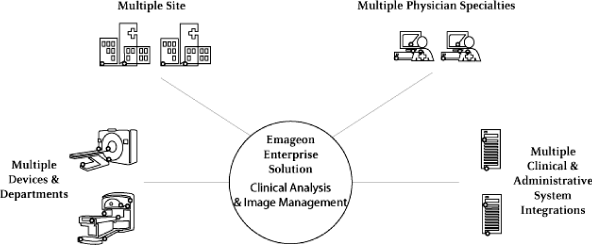
We provide enterprise-level information technology solutions for the clinical analysis and management of digital medical images within health care provider organizations. Our solutions consist of advanced visualization and image management software for multiple medical specialties, comprehensive knowledge tools for cardiology, support services and third-party components. Our web-enabled advanced visualization software provides physicians across the enterprise — in multiple medical specialties and at any network access point — with tools to manipulate and analyze images in two dimensions (“2D”) and three dimensions (“3D”). We enable physicians to better understand internal anatomic structure and pathology, which can improve clinical diagnoses, disease screening and therapy planning. We believe our solutions improve physician productivity and patient care, enhance customer revenue opportunities, automate complex, mission-critical medical imaging workflow, and maximize our customers’ return on investment in capital equipment and clinical information systems.
We sell to multi-hospital networks, community hospitals, physician clinics and diagnostic imaging centers. Health care providers produce growing volumes of medical imaging data that must be analyzed, managed and stored efficiently and cost-effectively. We focus on developing corporate-level relationships with large multi-facility organizations, which can result in substantial cross-selling opportunities and represent an important competitive advantage for us. Since our first commercial implementation in December 2000, we have implemented our solutions at facilities affiliated with some of the largest multi-facility health care providers in the U.S.
As of December 31, 2005, we had $158.0 million in contracted backlog, consisting primarily of fees for contracted future installations and for the support of existing installations, compared with a contracted backlog of $118.2 million at December 31, 2004. We expect to recognize revenue of approximately $73.3 million from
1
our current contracted backlog during fiscal year 2006, $27.0 million during fiscal year 2007, and substantially all of the approximately $57.7 million remaining by 2010.
We were founded in December 1998 as an Alabama corporation and reincorporated in Delaware in January 2000. On February 14, 2005, we completed our initial public offering. We acquired Camtronics Medical Systems, Ltd. (Camtronics) on November 1, 2005.
Our Opportunity
Demand for advanced visualization and image management solutions is growing as the number and size of imaging exams increase due to accelerating physician adoption of advanced imaging, the growing health care needs of an aging U.S. population and the increasing sophistication of imaging devices such as computed tomography (CT), magnetic resonance imaging (MRI), positron emission tomography (PET) and cardiac catheterization. We do not believe existing film-based workflow or department-level Picture Archiving and Communications Systems (PACS) are sufficient to meet this growing demand. Health care providers need digital infrastructure, storage and image management capabilities to alleviate the operating strain created by medical image records.
Frost & Sullivan, a leading health care consulting and research firm, estimated in a 2004 report that the total U.S. medical imaging market, including capital equipment and technology, would reach $16.6 billion in 2008. They report that information technology spending associated with medical imaging accounted for approximately 15% of the total medical imaging market in 2003 and estimate that it will grow at an average compound annual growth rate of 15% from 2002 through 2008. Further, in 2005 Frost & Sullivan forecasted a 16.8% compound annual growth rate in enterprise cardiology PACS from 2004 through 2011.
We believe the rapid expansion in the number and complexity of medical images and the need to automate complex, manual workflow processes are driving health care providers to invest in systems that maximize their return on capital investments in expensive imaging devices and clinical information technology. We facilitate the convergence of imaging technology and clinical automation at the enterprise level by enhancing analysis, integration and automation of medical imaging data. Effective image management can shorten report turnaround times, lower the potential for manual error in data entry and filing, increase staff efficiency, eliminate costs associated with traditional radiological workflow and improve overall diagnostic and clinical quality. We believe the following factors have collectively increased the demand for our solution:
Increasing Number, Size and Complexity of Imaging Exams. The number of imaging exams performed each year is increasing as a result of a number of factors, including increased physician use of advanced imaging as a non-invasive diagnostic and clinical tool, lowered costs of imaging devices and increased health care needs of an aging U.S. population. At the same time, technological advancements are increasing the size and complexity of individual imaging exams. For example, new CT scanners produce 20 times as much data as prior models, with exams consisting of thousands of individual images yielding 500 to 1,000 megabytes of data per exam, versus only 25 to 50 megabytes just three years ago. One modality manufacturer has announced that they plan to have a 256-slice CT scanner on the market by 2008, and that scanner will produce images that are at least ten times the size of the images produced by today’s most advanced scanners. The increasing prevalence of fusion techniques used to combine images from multiple imaging devices increases the complexity of many exams. The rapid growth in the data size of medical images means that medical images also consume a greater share of hospital resources.
Need for Advanced Visualization Tools. Because the output of a cross-sectional imaging device, such as a CT scanner, may consist of thousands of “sliced” 2D images, physicians need sophisticated software tools to model those images in 3D and allow the viewing of a “virtual” patient at all angles. Physicians can benefit from computer-created 3D images and eliminate the need to mentally reconstruct 2D images into a single useful 3D image. This improves diagnostic capabilities, treatment and non-invasive surgical planning. Sophisticated new tools, such as 3D volumetric imaging and volume rendering, maximum intensity projection (MIP), multi-planar reformat (MPR) and surface shading, are increasingly essential to present medical images in a manner that is valuable to the physician for diagnosis and treatment planning. Moreover, some surgical specialists will not perform a complex surgery without first
2
performing pre-operative 3D planning. Hospitals and hospital networks that provide these advanced visualization tools to physicians have the advantage of attracting patient referrals from those physicians that heavily utilize visualization technology in their practices.
Need for Complete Electronic Health Records. The need to improve clinical care and eliminate inefficiencies in existing paper-based methods, including film-based image management, continues to drive investment in clinical information technology. In 2004, the federal government began several initiatives to accelerate information technology adoption rates within the health care system, including the Presidential appointment of a national health care information technology office and a recommitment to the President’s Information Technology Advisory Committee. Health care providers are implementing clinical information systems to automate clinical documentation and integrate patient information into electronic health records. However, these clinical information systems typically lack the sophistication or capability to incorporate digital medical images from radiology modalities, echocardiology, or the cardiac catheterization lab into patient health records. Incomplete electronic health records can result in delayed diagnosis, billing errors and inefficient workflow. A complete electronic health record, which includes all medical images and complete data from cardiac catheterization and echocardiology procedures, enhances the benefits of investment in clinical information technology.
Shortcomings of Film-Based Image Management. Many health care providers still use film to capture medical images from devices such as X-ray machines, which may produce three to four images per typical exam, and CT scanners, which can produce 1,000 images per exam. A film-based system has numerous inefficiencies, including complex exam scheduling, redundant patient data entry, the possibility of misplaced or misfiled notations and case histories, physical films and files that must be copied often or moved among the technologist, the specialist physician and the treating physician, and substantial storage space requirements. Each of these inefficiencies has the potential to increase the total cost per exam.
Limitations of Current Methodologies for Managing Digital Medical Images. Current digital medical image management systems, which correct some of the inefficiencies of film-based imaging, have traditionally consisted of specialized services and technologies tied to specific department-level requirements. For example, a typical PACS installation is a department-level installation with dedicated hardware components primarily designed to address the image storage and distribution needs of a small number of physicians in a single department (e.g., radiology or cardiology, but not both). While PACS may offer substantial automation benefits within such a single department over traditional film-based imaging workflow, they do not offer the full potential of an integrated, enterprise-level digital image management solution and these systems also typically do not integrate with clinical and administrative systems without expensive custom programming. Many hospitals that have embraced image automation have had to purchase multiple PACS and software tools for various departments and imaging devices, which presents integration challenges and requires significant investment. Many PACS were developed prior to the recent growth in the use of 3D imaging techniques and do not easily scale to handle the data volume of current imaging devices. PACS visualization tools are typically limited only to 2D and are not distributable across the network except in a very rudimentary manner.
3
Our Solutions
We provide enterprise-level information technology solutions for the clinical analysis and management of digital medical images within health care provider organizations.
With our solutions, our customers and their constituents, including physicians, technologists and nurses, can improve overall clinical and diagnostic quality and eliminate much of the labor and other costs of dealing with film, disparate department-level information systems, exam scheduling and redundant data entry. We also help to alleviate heavy burdens on a health care provider’s staff by automating medical image workflow for physicians and technologists. We believe our enterprise visual medical system (EVMS) solution provides the benefits of current department-level PACS, including increased automation and better efficiency over traditional film-based methods, with added enterprise-level connectivity and advanced visualization tools that are not available with a typical PACS installation.
We have designed our solutions to offer benefits to the following groups:
| | | |
Group | | Benefits from our Solutions |
| |
| Administration | | • Demonstrable return on investment |
| (CEO, CFO and COO) | | • Better service to physicians |
| | | • Improved staff productivity |
| | | • Improved satisfaction of referring physicians |
| | | • Elimination of many routine, non-productive and non-clinical tasks |
|
| Information Technology | | • Lower total cost of operation |
| (CIO and IT Department) | | • Fault tolerant, redundant and reliable |
| | | • Ease of integration with different clinical information systems |
| | | • Multi-site, standards-based integration |
| | | • Focused, high quality implementation services |
|
| Diagnostic Physicians | | • Integrated, andeasy-to-use visualization tools |
| (Radiologists) | | • Multi-point access to visualization tools and images |
| | | • Productivity gains |
|
| Treating Physicians | | • Availability ofeasy-to-use, specialty specific visualization tools |
| (Cardiologists, Surgeons, etc.) | | • Faster turnaround of information for treatment planning |
| | | • Facilitates collaborative analysis with diagnostic physicians |
| | | • Improved treatment planning |
4
Our solutions offer the following:
Single-Source, Enterprise-Level Image Management. Our solutions provide a single data repository for medical images created by digital imaging devices and related patient data across a single or multi-facility enterprise, whether from radiology, cardiology, pathology, orthopedics, obstetrics, gynecology or other departments. This single repository serves as a central point of workflow and content management for those images. Our solutions catalog, archive and route these images through our software, combining centralized control over sensitive patient imaging records with increased availability to physicians and other authorized users, in multiple medical specialties and at any network access point. Our solutions integrate with our customers’ existing clinical information and administrative systems, serving as the patient’s visual medical record repository, reducing the risk of billing errors and lowering the average cost per exam through automation of complex and manual film-based imaging workflow.
Advanced Visualization Technology. Our solutions quickly deliver web-enabled software toolsets and images to physicians throughout the enterprise for diagnostic analysis and treatment planning. Our advanced visualization software allows physicians to create 2D and 3D views of human anatomy based on the output of imaging devices and to manipulate, navigate within and compare imaging exams in order to better visualize and understand internal anatomic structure and pathology. Improvements in the visualization of medical images can lead to improved clinical diagnosis, disease screening and treatment planning by physicians. Physicians can access our advanced visualization software from any network access point, including home, office or throughout the health care facility. Our intelligent user interface automatically adjusts for the specialty of each physician, the preferences of each user, the type of imaging device used to create the image, and the particular body part and tissue type being examined.
Specialty-Specific Clinical Applications. With our acquisition of Camtronics in November, 2005, we added a full suite of products to enhance capabilities of specialists in the cardiology department. These solutions manage images and clinical data relating to cardiac catheterization, echocardiography, nuclear cardiography, vascular ultrasound, and hemodynamics. We are focused on the development of enhancements and additional functionality to our existing advanced visualization software to further meet the clinical needs of other clinical specialties, including emergency medicine, orthopedics, oncology, pathology, obstetrics, gynecology, and neurology.
Open Standards-Based Software. We believe that our use of open standards has enabled us to design software that stores and manages information faster and with fewer hardware resources than competitive systems, a benefit we believe is becoming increasingly important as the data size of many imaging exams grows. We have designed our software to make full use of the DICOM standard for medical image data. We believe our commitment to open standards, such as DICOM and the standard protocol for the storage of text-based patient information, Health Level 7 (HL7), means that our software will be compatible with new imaging device technologies and other clinical information systems that conform to these standards. We lower our customers’ total costs by eliminating the need for translation to and from non-standard or proprietary communication methods which often require the purchase of additional hardware and software.
Effective Implementation, User Adoption and Support Services. We focus on delivering effective implementation, user adoption and support services as an integral part of our solution. During the implementation phase of our solution, we use proven project management principles, including change management and adult learning techniques, to facilitate rapid and complete adoption by our customer. After implementation, we monitor system and user behaviors and, when appropriate, intervene to make the adjustments we consider necessary to prevent anticipated problems from occurring. Additionally, we use tools that measure the ultimate success of our customers’ implementation, including providing reports on productivity, operating performance and return on investment. We believe our focus on implementation and support services ensures that our customers’ investments in our solutions are well managed and achieve the customers’ financial and operational objectives.
5
Our Strategy
Our goal is to become the industry leader in enterprise-level information technology solutions for the clinical analysis and management of digital medical images. Key elements of our strategy include:
Expand Our Market Share by Attracting New Customers. We believe a full range of health care organizations, from stand-alone imaging centers to multi-site hospital systems, represent a largely underserved market for our solution. Our current base of installed facilities represents a small portion of the prospective customers for our solution. We are expanding our sales and marketing efforts so that we may pursue new customers. As we pursue new customers, we intend to continue focusing our efforts on the large, multi-site health care providers that typically recognize the greatest benefits and fastest return on an investment in our solution and represent the largest individual sales opportunities. We believe our position as a sole source provider of an advanced visualization and image management solution, together with our implementation expertise and our installed base of nationally recognized reference customers will help us attract new customers.
Increase Penetration With Existing Customers. We believe that using our successful relationships with existing multi-facility health care customers to expand our penetration within those organizations and selling additional functionality to our existing installed base are effective ways to increase our operating margins by reducing the average cost of sales and increasing the total revenue from existing customers.
| | |
| | • | Increase Installations with Existing Multi-Facility Customers. As of December 31, 2005, we had customer relationships with 17 multi-facility health care providers that control over 240 hospitals. Our initial contracts with these customers often provide for implementation of the content management functions and sometimes the advanced visualization functions of our EVMS solution at only a portion of the facilities managed by the parent company. We believe there are significant opportunities to expand our installed base at facilities that are part of multi-facility systems in which we have some level of customer relationship with the parent company or with an individual facility within the multi-facility system. |
| |
| | • | Cross-Sell to Existing Customers. We are also in a strong position to sell additional functionality to our existing customers, including advanced visualization tools for image-intensive medical specialties that may not have been part of the initial sale. Typically our initial sale to a customer is for medical content management. As follow-on sales opportunities, we offer our advanced visualization software and additional functionality to radiologists and other specialty groups within the organization. Our offering of the suite of cardiology products we recently obtained in the Camtronics acquisition gives us broader cross-selling opportunities than we had prior to the acquisition. We believe that our excellent customer relationships increase our customers’ comfort in purchasing new products or enhanced functionality from us. |
Enhance Our Product Offerings. We believe developing or acquiring additional functionality for our existing software, including improved advanced visualization products for multiple specialties, such as emergency medicine, orthopedics, oncology, pathology, neurology, obstetrics, and gynecology will further strengthen our position in the market. Further enhancements to our advanced visualization software and our suite of cardiology products should assist us in selling our solution to multi-hospital systems and expanding our existing customer relationships. We also plan to invest further in workflow and integration software to speed integration with existing clinical information systems, including electronic health record systems.
Continue to Deliver Superior Implementation, User Adoption and Customer Support Services. As a single-source provider of advanced visualization and image management solutions, we believe the quality of our implementation, user adoption and support services helps to differentiate us from our competition. We have designed our systems, services and pricing strategies around this belief. We expect to continue to invest in, refine and develop new services to provide our customers with the highest level of services available and to provide us with a base of recurring revenue. We believe delivering superior services will
6
enable us to capture increased market share and enhance our existing customer relationships, thereby increasing our competitiveness.
Maintain Our Open-Standards Focus. We believe our commitment to open standards, such as DICOM and HL7, lowers our development costs, accelerates our time to market, lowers our customers’ total cost of ownership, improves speed and quality of our solution’s integration and differentiates us from our competition. By designing our solution around open standards, we believe we maximize our solution’s integration with our customers’ existing clinical information technology systems and imaging devices, which reduces our customers’ total cost of ownership. We also believe our open-standards model lowers the hardware costs associated with implementing our solution because it enables our customers to use relatively inexpensive,off-the-shelf hardware to visualize, analyze and manipulate images. We plan to continue this commitment, which we believe enables our customers to maximize their return on investment in both current and future imaging devices, computer hardware and clinical information technology systems.
Our Product and Service Offerings
We provide an enterprise-level information technology solution for the clinical analysis and management of digital medical images within health care provider organizations. Our solution consists of advanced visualization and image management software, comprehensive support services and third-party components.
Software
EVMS includes three principal software components: advanced visualization tools, clinical content management and clinical workflow through a dynamic user interface.
Advanced Visualization Toolsconsist of our suite of software tools for the advanced visualization and analysis of digital medical images by physicians and medical professionals. Components include graphics and image processing modules that present information to physicians and medical professionals using relevant multi-specialty tools through a dynamic user interface. Physicians can manipulate 2D and 3D image-related content in a variety of ways including organization, rotation, inversion, magnification and enhancement of images in a collaborative environment for sharing findings with other physicians or medical professionals. These tools help physicians better visualize and understand internal anatomic structure and pathology. Additional benefits include:
| | |
| | • | Sophistication. The software makes use of complex processing techniques such as multi-planar reformat (MPR) and volumetric imaging for 3D imaging applications. |
| |
| | • | Integration. Imaging tools include integrated 2D and 3D viewing methods that can be used simultaneously with the same image or images on the same computer. |
| |
| | • | Ease of Use. The system is intuitive and user-friendly so physicians can easily adapt its use into their current practice patterns. |
| |
| | • | Application to Numerous Clinical Specialties. The user interface automatically adjusts for the type of physician using the system, user preferences, the type of imaging device used to create the image (such as CT, X-ray or MRI), and the particular body part and tissue type being imaged. |
| |
| | • | Platform Compatibility. The system uses a common personal computer graphics standard, allowing off-site physicians to use inexpensive personal computers and permitting the enterprise to make use of lower-priced workstations withoff-the-shelf graphics hardware. |
| |
| | • | Web-enabled. Physicians or other authorized users have secure access to images and advanced visualization tools at any network access point. |
Clinical Content Managementis our image archival and distribution management software. Clinical Content Management supports the DICOM standard for digital medical images enabling a high level of scalability that facilitates fast, efficient access to storage and retrieval of such images in enterprise applications.
7
The system includes auto-routing and predictive capabilities that improve workflow in a clinical environment by performing time intensive tasks in anticipation of their need, thereby minimizing network traffic and facilitating responsiveness across the enterprise.
Clinical Content Management employs a distributed architecture that enables administrative changes without the need to shut down the system, minimizes system memory requirements, increases the speed of access to images through a relational database and provides customized reporting capabilities. In a distributed multi-hospital environment, the system also manages local “caches” at remote sites, which provide local image acquisition and temporary storage for rapid retrieval. Permanent image data is simultaneously stored at the centralized long-term archive. Each remote cache also acts as a proxy server to provide a view of images throughout the enterprise, no matter where the image was originally generated. The use of a local cache ensures that no individual facility is dependent on the wide-area network for the sourcing of locally created images.
Additional benefits include:
| | |
| | • | Multi-Site and Multi-Department. The system permits authorized users to access images from any network access point, including home, office or throughout the health care facility. It handles images created by multiple hospital departments and multiple image devices. |
| |
| | • | Enterprise-Level Scalability. We can install Clinical Content Management as a single facility application or as an enterprise-level solution to support the medical image management needs of large multi-facility health care providers. By using predictive technologies and local caching of images, the system provides optimal network speed and availability. |
| |
| | • | Fault Tolerance. We use an advanced, fault tolerant, high availability configuration on redundant server clusters with redundant storage systems. We support either full backup and recovery or mirrored archives in two different locations, enabling uninterrupted operation in the event of the loss of one archive. |
| |
| | • | Open Standards. Unlike many competitive image management systems, our system has been designed using an open standards architecture that leads to better integration with imaging devices and clinical information systems, improves the speed and reliability of transfers of medical image data and provides a lower total cost of ownership by avoiding unnecessary translation overhead. |
Clinical Workflowis our standards-based software used to manage integration and data migration between our solution and other health information systems throughout the enterprise. We utilize a DICOM imaging device worklist, which automates technologist workflow, prioritizing and managing processes based on other systems such as admissions. In addition, Clinical Workflow includes tools that enable the integration of our solution with electronic health records and other information systems such as voice recognition. The benefits of the system include the rapid and systematic integration of the patient’s digital medical images with the rest of the enterprise’s clinical and administrative information systems without the need for custom programming, integration services, or third-party translation devices.
Our HeartSuite enterprise solutions for cardiology were added to our product offerings as a result of our acquisition of Camtronics on November 1, 2005. Our cardiology solutions include HeartSuite VERICIS, HeartSuite Hemodynamics and HeartSuite CVIS.
HeartSuite VERICIScreates a complete digital record of images and reports for patients in the cardiac catheterization lab, in echocardiography (including specialized applications for pediatric echocardiography), in vascular ultrasound and in nuclear cardiology. Like our EVMS solution, VERICIS is built to conform to DICOM and HL7 standards. Benefits include:
| | |
| | • | Elimination of film and paper-based processes |
| |
| | • | Reduction of redundant tasks, including cine film handling, data entry, archiving and transcription |
| |
| | • | Streamlined access to patient studies and reports |
| |
| | • | Scalable and expandable to meet needs of any size cardiology department |
8
HeartSuite Hemodynamicsis a comprehensive monitoring and data management system that integrates cardiac catheterization lab procedural information into the patient’s cardiac record. HeartSuite Hemodynamics provides functionality for data collection, real-time waveform analysis, inventory control, patient charging and procedure reporting in a single system. Benefits include the ability to mine data and create custom reports that aid in driving improvements in quality and efficiency.
HeartSuite CVISis a web-based system designed to provide the cardiology department with all its information needs in one application. It summarizes the patient cardiovascular state and aggregates all clinical information in one location, tracks patient clinical trends and supports care planning. The system aids in workflow in the department of cardiology by scheduling labs and office encounters, tracking patients while they are in the hospital, capturing and aggregating cardiovascular billing codes. The information in the system is stored in a central database that provides cross-modality statistics for operational, administrative and other business needs.
Service and Support
We believe that our implementation, user adoption and support services differentiate us strategically from our competitors. Large-scale infrastructure information technology installations can present special challenges to an enterprise, regardless of its size or sophistication. We believe that IT projects often fail due to inadequate implementation and support services and believe that our service model better meets the installation and investment objectives of our customers.
Our “Customer Success Program” includes the following components:
| | |
| | • | Adoption Success Management (ASM). ASM is our services program that facilitates rapid and complete adoption by all relevant constituents during the implementation phase, which typically lasts several months. We have designed ASM to maximize the user implementation experience, promote behavioral change at all levels and increase the probability of complete implementation success. |
| |
| | • | Total Solution Management (TSM). TSM is an ongoing set of support services to ensure that our systems are highly available and optimally configured for the users. Through continuous remote monitoring of our solution, we analyze system and user behaviors and, when appropriate, intervene and make the necessary adjustments to prevent anticipated problems from occurring. We provide standard24-hour service and support for our software and any third-party components we provide to the customer. Our standard contracts with customers typically provide for a 99% guarantee of system availability and a 98% guarantee of component availability. Our system availability guarantee covers our solution as a whole, while the component guarantee covers each individual component, as in certain circumstances a component may fail without affecting system availability. |
| |
| | • | Enterprise Performance Monitoring (EPM). EPM is an enterprise-level performance monitoring tool that we use to measure the ultimate success of our customers’ implementation, including full adoption and effective use of the systems we provide. Our EPM service provides the customer with periodic reporting of system performance, operational performance and financial performance metrics to aid in maximizing the return on the customer’s investment in our system. |
We provide revenue mix analysis services, using data combined from hospital information systems and the enterprise’s imaging environment data, to understand and prioritize key equipment and physician revenue producers for a hospital. This analysis can provide critical data to hospital administrators for capital and technology investment planning initiatives. We also provide additional professional information technology services to our customers that have specific needs related to system integrations and interfaces and data migration.
9
Third-Party Components
Our solutions typically include the installation and implementation of platform components that we procure from third parties. We believe that providing third-party components helps us deliver a comprehensive solution that meets the needs of our customers. Some of the third-party components we provide include:
| | |
| | • | Servers. Our software and the database run on a cluster of standard redundant servers. |
| |
| | • | Data Storage. We support industry standard storage configurations, including fault-tolerant RAID (redundant array of independent disks) systems. |
| |
| | • | Backup/Recovery. Our solution typically includes a tape library-based backup and recovery system that provides backup for our database, configuration files and the digital medical images. We also offer an optional configuration with mirrored archives in two locations, enabling uninterrupted operation in the event of the loss of one archive. |
| |
| | • | Workstations and Monitors. Customers typically implement our advanced visualization software using standard personal computer workstations and high-resolution monitors for visualization within the facility. |
| |
| | • | Database. Our software applications operate on Oracletm database technology and other standard relational database applications. |
| |
| | • | Computed Radiography. We offer computed radiography devices, which are manufactured by Eastman Kodak Company and Fujifilm Medical Systems USA, Inc. Computed radiography devices convert analog X-ray images into digital images. |
| |
| | • | We also offer a software toolset for orthopedic surgeons which is licensed from Orthocrat, Ltd. and a voice recognition dictation system from Lanier Worldwide, Inc. |
Our Technology
We believe the following technologies and strategies help us to compete more effectively:
| | |
| | • | Native DICOM Compatibility. We have written our software to exploit the capabilities of the DICOM standard for medical image storage and workflow management, as promulgated by the American College of Radiology and the National Electronic Manufacturer’s Association. DICOM is an industry standard in medical imaging that defines the data elements, communication protocols, storage formats and workflow methods associated with medical imaging data and processes. Our software stores and manages medical images using native DICOM communications, preserves the DICOM information associated with the image and follows DICOM workflow methods. Using native DICOM communication means our solution does not require “translation” devices for converting the DICOM information into a proprietary storage or other format. We believe our commitment to DICOM as the underlying protocol for our software is a competitive advantage, delivering faster streaming, more efficient storage of the image and the ability to integrate our software to new imaging devices that output information using DICOM. |
| |
| | • | Proprietary DICOM-Toolkit. While DICOM is an industry standard protocol for medical image data management and storage, the software toolsets used to process, manage and use DICOM information are generally unique to particular software vendors. Unlike many of our competitors who license DICOM-toolkits from third parties, we have developed and own a DICOM-toolkit that we believe permits us to more rapidly integrate DICOM-based information into our software. We believe that the ownership and continued development of our DICOM-toolkit is a core technology strategy, in part because it reduces reliance on third-party software. |
| |
| | • | Commitment to the IHE Technical Framework. We are a leader in the implementation of the Integrated Healthcare Enterprise (IHE) technical framework. The Radiological Society of North America and the Healthcare Information and Management Systems Society created IHE, which represents a consortium of companies in the radiology and health care information systems fields. The |
10
IHE technical framework is a protocol for the integration of DICOM image information and HL7 text-based patient information. We believe our commitment to IHE helps to ensure that our software integrates seamlessly with HL7-based billing and patient record information systems implemented at our customer sites.
| | |
| | • | Compatibility with the OPEN GL Graphics Standard. Our advanced visualization software performs sophisticated 3D rendering and other graphics intensive functions that provide physicians the ability to view 3D medical images for diagnosis and treatment planning. Historically, workstations and graphics hardware that could perform the advanced rendering and other graphics functions needed for advanced visualization functionality were cost prohibitive for widespread use. Our advanced visualization software uses the OPEN GL graphics standard, which permits our customers, or off-site physicians affiliated with our customers, to purchase inexpensive personal computers and graphics hardware to perform sophisticated image analysis. |
| |
| | • | Component-Based Software Engineering. Our software architecture is based on a component-based services model. Our software development framework supports common and domain specific components that can be plugged in while the system is operating. By building flexible, dynamic, reusable components, we gain great flexibility to add functionality to our system and increase the reliability of our system because we can remedy problems at the component level rather than being forced to address issues throughout the entire application. |
Customers
Our customers range in size from single imaging centers to large multi-facility healthcare networks. As of December 31, 2005, we had installed our EVMS solution in 139 hospitals or other health care facilities, 114 of which are members of multi-facility networks with which we have customer relationships. At December 31, 2005, we had implemented our advanced visualization solution in 66% of our current installed EVMS customer base. While this is a significant increase over the 45% of our installed base that used our advanced visualization software at December 31, 2004, we believe the additional hospitals that are currently using other visualization technology represent a growth opportunity for us. There are also over 300 hospitals where we have installed our HeartSuite solutions in their cardiology departments. Our customers include members of the following multi-facility networks with ten or more facilities: Allina Hospitals and Clinics, Ascension Health, Aurora Health Care, BJC Healthcare, Catholic Healthcare West, Kaiser Foundation Hospitals, Sisters of Mercy Health Systems and Sisters of St. Francis Health Services.
Contracted implementations for Ascension Health constituted 33% of our contracted backlog as of December 31, 2005, compared to 35% as of December 31, 2004.
Sales and Marketing
We use a direct sales model, with sales representatives who have substantial experience in health care-related direct sales. Our sales representatives undergo rigorous training in our products as well as the needs of each constituent group within our potential customers. During our sales cycle for a typical customer we might, at various times, present to the Chief Information Officer, the Director of Radiology or Cardiology, the Chief Financial Officer, the Chief Medical Officer, the Chief Operating Officer and the Chief Executive Officer. We also typically must present to several key physicians representing the specialties that are expected to use our system such as radiologists, cardiologists, emergency room physicians, neurosurgeons and orthopedists. Each of these constituencies may have different priorities and evaluation criteria, and our direct sales representatives must be capable of presenting a compelling business case to each.
Our sales representatives are supported by our sales support and marketing communications team, which provides technical, demonstration, lead generation, market development and proposal assistance.
Research and Development
As of December 31, 2005, we had 150 employees who are primarily dedicated to research and development activities. In addition to our employees, we also utilize contractors and consultants on a routine
11
basis to perform specified research and development activities. We also utilize clinical advisory boards and end-user focus groups that are organized by area of expertise to advise us on the clinical functionality of our solutions. We have focused our research and development mission on the continued evolution of intelligent, fault tolerant, highly scalable image management and visualization systems for mission-critical medical image management applications. We adhere to a philosophy of open standards-based solutions. We believe that we fulfill a critical technology and performance void in current generation departmental PACs systems. We further believe we have designed our visualization platform in a way that enables us to efficiently add new functionality. We are focusing our research and development efforts on:
| | |
| | • | improving physician and technologist workflow; |
| |
| | • | developing and refining visualization capabilities including new 3D and analysis applications; and |
| |
| | • | data storage, retrieval, integration and comparison of past and current images; and |
| |
| | • | extending imaging tools to referring physicians in multiple specialities |
We follow a formal product development process and employ dedicated product development personnel. Under our formal product development process, internal and external (customer) requests for added features or functionality are forwarded to our strategy and architecture team. This team evaluates and prioritizes these potential product enhancements taking into account expected costs, anticipated value to the customer, regulatory requirements, timing and resource availability. After our strategy and architecture team approves these enhancements, our engineering team develops them and subjects them to quality testing and documentation requirements before we make them generally available to our customers.
We invested $4.1 million, $6.0 million and $10.7 million for research and development in 2003, 2004 and 2005, respectively.
Competition
The markets for the digital medical image management and visualization systems that we offer are highly competitive. Many customers purchase products and services from us and from our competitors as well. We compete with companies that fall into four primary categories:
| | |
| | • | companies that manufacture and sell digital imaging devices such as GE Healthcare, Siemens Medical Solutions and Philips Medical Systems, who may integrate some of the functionality provided by our products into their equipment or bundle it with the equipment sale; |
| |
| | • | companies that have traditionally sold imaging films such as Eastman Kodak Company and Fujifilm Medical Systems USA, Inc.; |
| |
| | • | companies that have traditionally sold health care information technology applications such as McKesson Corp. and Cerner Corp.; and |
| |
| | • | a number of smaller companies that sell department-level or cardiology-specific PACS or specialty visualization tools. |
Many of our current and potential competitors have significantly greater name recognition and more established distribution networks and relationships with health care providers. To compete effectively, we often must persuade the prospective customer to separate its purchasing decisions with respect to imaging equipment from its purchasing decisions with respect to archival and visualization tools, because many of our competitors offer imaging devices that they package or bundle with licensed or owned image management applications.
Our ability to compete successfully will depend on a number of factors both within and outside our control, including:
| | |
| | • | product innovation; |
| |
| | • | product quality and performance; |
| |
| | • | customer service and support; |
12
| | |
| | • | the experience of our sales, marketing and service professionals; |
| |
| | • | rapid development of new products and features; |
| |
| | • | price; |
| |
| | • | continued active involvement in the development of DICOM and other standards-based medical communication protocols; and |
| |
| | • | product and policy decisions announced by competitors. |
Intellectual Property
We rely generally on a combination of trade secret and copyright law, employee and third-party nondisclosure agreements and other protective measures to protect intellectual property rights pertaining to all of our software technology. In addition, we have filed patent applications to protect certain aspects of our software technology. To date, one patent has been issued.
As filed in the U.S., Europe, and Japan, our patent applications generally relate to DICOM-type image transmission and, in particular, to methods and apparatus for streaming DICOM-type images via a network. In addition, we have also filed a patent application in the U.S. that generally relates to a method and system for storing, communicating and displaying image data. In particular, this application relates to methods and systems for storing image data on a server, communicating at least a portion of the image data from the server to a client via a network, and displaying images at the client using the communicated data.
We have one device and method patent related to improved quantitative coronary artery analysis. This patent is on file in the U.S. and Canada. This patent, while enforceable, has limited use in our current product offerings and product development efforts.
We have an exclusive, worldwide, royalty-bearing license from the University of Alabama Birmingham (UAB) Research Foundation for certain technology used in our Clinical Content Management software. We pay a nominal royalty for this license.
We do not own all of the software and hardware used in our solution, but we have all of the licenses from third parties we believe are necessary to offer our current solution. As we develop new products and new versions of products, it may be necessary to renegotiate with such third parties to make sure our licenses are complete and valid. In such a case, our existing third-party licensors may not be willing to make the needed licenses available on terms acceptable to us, but we believe in most cases there are alternative vendors from whom we could obtain hardware, other components or any necessary licenses for software.
Emageon®, Camtronics®, Heartsuite, VERICIS® and our logo are our trademarks or service marks. All other trademarks, trade names and service marks appearing in this Annual Report are the property of their respective owners.
Employees
As of December 31, 2005, we had 469 employees, 150 of whom were primarily engaged in research and development, 74 of whom were primarily engaged in sales and marketing, 207 of whom were primarily engaged in providing technical installation and support services and 38 of whom were primarily engaged in administration and finance. With respect to location, 176 of these employees are located at our corporate headquarters in Birmingham, Alabama; 185 of these employees are located at our offices in Hartland and Madison, Wisconsin; 40 of these employees are located at our office in Ottawa, Ontario, Canada; and the remainder of our employees are located at customer locations or in regional support offices. None of our employees is a party to a collective bargaining agreement, and we consider our relationship with our employees to be good.
13
Government Regulation
We market, sell, and distribute our products in the heavily regulated U.S. health care industry. Our business operations and financial arrangements in this industry may be subject to a complex array of federal laws and regulations governing medical devices. We are also subject to laws and regulations governing reimbursement and referrals because our products are used in diagnosing and treating Medicare and Medicaid patients. Moreover, a number of states have adopted their own versions of such laws and regulations, though these may vary significantly from one state to the next. Violation of such federal and state laws and regulations can result in civil and criminal penalties involving substantial fines and imprisonment.
Food and Drug Administration. Our radiology and cardiology PACS, and hemodynamic measurement recording software products are medical devices subject to extensive regulation by the Food and Drug Administration, or FDA, pursuant to the federal Food, Drug, and Cosmetic Act, as amended, or the FDA Act. Each device that we wish to distribute commercially in the U.S., unless otherwise exempt, requires regulatory clearance prior to commercial distribution.
The FDA cleared EVMS, the radiology PACS; Heartsuite VERICIS, the cardiology PACS; and Heartsuite Hemodynamics (formerly known as Physiolog), the hemodynamic measurement recording software, through the 510(k) notification process. We have applied, and will continue to apply for, 510(k) clearance for additional clinical uses of our devices. Clearance under the 510(k) process typically takes 90 days to over a year from the date of a complete filing, depending on the number of questions the FDA has concerning the submission. Some applications may never receive clearance because the FDA raises safety issues or requests additional data that may not be economical to produce. Therefore, there is the risk that FDA clearance for any of our future devices, or for further clinical uses of our existing devices, may be delayed or not cleared. There is also the risk that FDA clearance, once received, may contain more restrictive conditions of use than we would like. Moreover, the FDA is always free to subsequently withdraw any clearance previously granted.
For cases where the 510(k) approval process is not available, the FDA’s other approval process, the pre-market approval process, or PMA, is a more costly, lengthy and uncertain process than the 510(k) process. The PMA application requires human clinical trial data to enable the FDA to evaluate whether the PMA contains sufficient, valid scientific evidence that the device is safe and effective for its intended use. The PMA process generally requires one to several years from the date the applicant submits the device for FDA review, if, in fact, the FDA ever approves the device. Even then, the FDA may condition its approval on stringent limitations regarding the indicated uses for which the device may be marketed. To date, our software and related comprehensive solutions have not required approval under the PMA process. However, we cannot assure you that our products will not require PMA approval in the future, or, in such an event, that such approval would be forthcoming.
The FDA can conduct announced and unannounced inspections of our facilities at any time. We have procedures in place to ensure that protocol is followed in accordance with the FDA guidelines with respect to announced and unannounced inspections. We believe that our manufacturing operations, and those of our suppliers, comply with the FDA’s Quality System Regulations and current good manufacturing practices.
Medical device manufacturers and device user facilities are required to complete Medical Device Reports (MDRs) upon the occurrence of MDR reportable events. For device manufacturers, an MDR reportable event is one about which a manufacturer has received or becomes aware of information that reasonably suggests that one of its marketed devices caused or contributed to a death or serious injury, or has malfunctioned and the device, or a similar device marketed by the manufacturer, would likely cause or contribute to a death or serious injury if the malfunction were to recur. The filing by manufacturers or user facilities of a significant number of MDRs with the FDA could potentially cause the FDA to commence post-marketing investigations, which could revise device labeling, include warnings, restrict use, or could even lead to a withdrawal of marketing clearances or approvals.
Health Canada. Our radiology and cardiology EVMS, and hemodynamic measurement recording software products are medical devices subject to extensive regulation by the Medical Devices Bureau of the Therapeutic Products Directorate (TPD), Health Canada. Health Canada is the Canadian federal regulator
14
responsible for licensing medical devices in accordance with the Food and Drugs Act and Regulations and the Medical Devices Regulations. The TPD applies the Food and Drug Regulations and the Medical Devices Regulations under the authority of the Food and Drugs Act to ensure that the pharmaceutical drugs and medical devices offered for sale in Canada are safe, effective and of high quality. Each device that we wish to distribute commercially in Canada, unless otherwise exempt, requires attainment of the appropriate type of medical device license prior to commercial distribution.
We currently hold licenses to market, sell, and distribute our products in the Canadian health care industry. To date, we have sold only the cardiac PACS and the hemodynamic measurement recording devices in the Canadian marketplace, but our intent is to market all devices for which we hold licenses in the future.
We have procedures in place to ensure that we are compliant with the Canadian Medical Device Regulation as documented in the Food and Drugs Act: Medical Devices Regulations for Canada: SOR/98-282 which includes quality system certificates for ISO 13485:2003, CMDCAS for the classes of our devices.
HIPAA Privacy and Security Regulations. The HIPAA Privacy Rule prohibits a covered entity from using or disclosing an individual’s protected health information unless the use or disclosure is authorized by the individual or is specifically required or permitted under the Privacy Rule. The Privacy Rule has imposed a complex system of requirements on covered entities for complying with this basic standard. Under the Security Rule, covered entities must establish administrative, physical, and technical safeguards to protect the confidentiality, integrity, and availability of electronic protected health information maintained or transmitted by them or by others on their behalf.
The HIPAA Privacy and Security Rules apply directly only to covered entities such as health plans, health care clearinghouses, and health care providers who engage in HIPAA-defined standard electronic transactions. We are not a covered entity, but our customers are. In order to provide to a customer certain services that may involve the use or disclosure of protected health information, the HIPAA Privacy and Security Rules require our customers to enter into “business associate” agreements with us, which must provide adequate written assurances with respect to, among other things, how we will use and disclose the protected health information. In addition to requiring us to provide these adequate written assurances, the business associate agreements with our customers also impose significant privacy and information security requirements on us, and we cannot assure you that we will not in the future be subject to liability in connection with those business associate agreements.
Government Reimbursement. Our customer base consists of health care providers, all of whom are subject to regulation by a number of governmental agencies, including those which administer Medicare and Medicaid programs. Accordingly, our customers are sensitive to legislative and regulatory changes in, and limitations on, the government health care programs and changes in reimbursement. During recent years, there have been numerous federal legislative and administrative actions that have affected the Medicare and Medicaid programs, including past adjustments that have reduced payments to hospitals and other health care providers. For example, in an effort to curb its increasing costs associated with diagnostic imaging, the federal government has recently implemented a percentage reduction applicable to a certain component (i.e., the technical component) of reimbursement for combined diagnostic imaging services under specified circumstances. It is likely that the federal government will consider and could implement future reductions in Medicare reimbursement or other changes that adversely affect our health care customer base. Any such changes could adversely affect our own financial condition by reducing the capital expenditure budgets of our customers.
Fraud and Abuse. A number of federal laws, loosely referred to asfraud-and-abuse laws, are used to prosecute health care providers, physicians and others that fraudulently or wrongfully obtain reimbursement that increases costs to any federal health care program. Given the breadth of these laws and regulations, we cannot assure you that they will not be found applicable to our business or the financial arrangements through which we market, sell, and distribute our products. These include federal anti-kickback and self-referral laws and regulations.
15
| | |
| | • | Anti-Kickback Law. The anti-kickback provisions of the Social Security Act prohibit the exchange of anything of value with the intent to encourage utilization of items or services payable under a federal health care program. Courts have construed the anti-kickback law to mean that a financial arrangement will violate such law if even one of the purposes of one of the parties is to encourage patient referrals or other Medicare/Medicaid business, regardless of whether legitimate purposes also exist for the arrangement. Penalties for federal anti-kickback violations are severe. Conviction can result in up to five years imprisonment, a $25,000 fine per offense, and exclusion from participation under federal health care programs. Violators may also be assessed civil monetary penalties ranging from $10,000 to $50,000 per offense, as well as damage assessments equal to three times the total amount of the kickback. We believe that all of our arrangements with physicians and health care facilities have been fully lawful. But given the broad sweep of the federal anti-kickback law, we cannot assure you that all such arrangements will be found compliant with such law if examined by government regulators, to the extent that such regulators determine that any of our arrangements are subject to such law. |
| |
| | • | Stark Law. The Ethics in Patient Referrals Act, known as the “Stark Law,” also prohibits certain types of referral arrangements between physicians and health care entities. Physicians are prohibited under the original Stark Law, its subsequent Stark II amendment, and the Stark implementing regulations from referring patients for “designated health services” reimbursed under the Medicare and Medicaid programs to entities with which they have a financial relationship or an ownership interest, unless such referrals fall within a Stark exception. Violations of the statute can result in civil monetary penalties of up to $15,000 per improper referral and exclusion from the Medicare and Medicaid programs. We do not believe that our arrangements with physician consultants or other health care providers violate the Stark Law, but we cannot provide assurances to such effect, nor can we assure you that we will not in the future be subject to Stark Law penalties. |
| |
| | • | State Law. Various states have enacted equivalents of the foregoing federal statutory and regulatory provisions. These state law equivalents would apply to items or services reimbursed by any third-party payor, including commercial payors. Many of these laws vary significantly from state to state, rendering compliance a costly and uncertain endeavor. |
Available Information
Our internet website address iswww.emageon.com. We make available free of charge through our website our Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q and Current Reports on Form 8-K, and amendments to those reports, as soon as reasonably practicable after we file them with, or furnish them to, the Securities and Exchange Commission.
Our business involves various risks and uncertainties, some of which are discussed in this section. The information discussed below should be considered carefully with the other information contained in this Annual Report onForm 10-K and the other documents and materials we file with the SEC, as well as news releases and other information we may publicly disseminate from time to time. The risks and uncertainties described below are not the only ones we face. Additional risks and uncertainties not presently known to us, or that we currently believe to be immaterial, may also adversely affect our business. Any of the following risks or uncertainties that develop into actual events could have a materially adverse effect on our business, financial condition or results of operations, or on the market price of our common stock.
| |
| | Our industry includes many large companies that have significantly greater resources and other competitive advantages, and we may not be able to compete successfully against these competitors. |
We compete with large, well-capitalized, multinational corporations such as GE Healthcare, Siemens Medical Solutions, McKesson Corp., and Philips Medical Systems. These competitors have significantly greater brand recognition and more established distribution networks and relationships with health care providers. As our market grows, it may attract other competitors with substantial resources, such as large
16
information technology, or IT, integration companies. Because of their greater resources, many of our existing or potential competitors can respond more quickly to new or emerging technologies or product lines and changes in customer requirements. These companies may also be able to invest more resources in research and development, strategic acquisitions, sales and marketing, and patent prosecution and litigation, and they can also finance capital equipment sales for their customers. In addition, some of our competitors bundle their image management software products with their sales of digital imaging devices at little or no extra cost. This practice may limit our opportunity to compete for customers who are also purchasing these devices. Our ability to market and sell our solution successfully to prospective customers depends, in part, on persuading these customers to separate the purchase of digital imaging devices from the selection and purchase of related software and services. Because we do not and for the foreseeable future will not have the financial resources, technical expertise, marketing, distribution and support capabilities of our competitors, we may not be able to compete successfully against our current and future competitors.
| |
| | We have incurred substantial operating losses in the past and may not be profitable in the future. |
We have incurred substantial operating losses in each fiscal year since our inception in December 1998, and we may continue to incur substantial operating losses in the future. As a result of our operating losses, we had an accumulated deficit of $51.4 million at December 31, 2005. You should not consider our recent growth in quarterly revenue or contracted backlog as necessarily indicative of our future performance. In addition, we expect our sales, marketing, research and development and other operating expenses to increase in the future as we expand our business. If our revenue does not grow to offset these expected increased expenses or if our operating expenses exceed our expectations, we may not be profitable and may incur substantial additional operating losses. Our ability to achieve and maintain annual profitability will depend on, among other things, our ability to market successfully our solution, create new product offerings, respond to competitive developments and attract and retain qualified sales, technical and management employees. Even if we are able to achieve profitability, we may not be able to maintain profitable operations on an annual basis.
| |
| | Our operating results may fluctuate, which makes quarterly results difficult to predict and could cause our stock price to decline or exhibit volatility. |
Our operating results may fluctuate as a result of many factors which are outside our control. Comparing our operating results on aquarter-to-quarter basis may not be meaningful, and you should not rely on our past results as an indication of future performance. Each of the following factors, among others, could cause our operating results to fluctuate from quarter to quarter:
| | |
| | • | Long Sales Cycle: Many of our customers are large organizations with lengthy and unpredictable purchasing processes. Because our solution is a major capital expenditure involving a multi-year commitment, it can take a significant period of time to close a sale. We typically have to educate our prospective customers on the benefits of our solution and obtain approval from senior management. Consolidation in the health care industry may also delay or extend the sales cycle for affected customers. As a result, our solution has a typical sales cycle, from the initial contact to the placing of an order, of six to nine months, and sometimes much longer. This long and unpredictable sales cycle may contribute to substantial fluctuations in our quarterly operating results. |
| |
| | • | Timing of Revenue: A significant portion of our revenue each quarter comes from sales made in prior periods, as we implement our solution and perform services under multi-year maintenance and support agreements with our customers. As a result, a decline in sales, client renewals, or market acceptance of our products in a particular quarter will not necessarily be reflected in revenue in that quarter and may adversely affect our revenue and profitability in future quarters. Moreover, a majority of our customers now purchase perpetual licenses from us. Unlike term licenses, where license revenue and certain implementation fees are recognized over the life of an initial term typically ranging from two to seven years, with perpetual licenses the full software license fee and associated implementation fees are recognized as revenue in the month when all revenue recognition criteria are met. Because revenue recognition may not be achieved in the period expected, our revenue could fluctuate from quarter to quarter solely due to the timing of satisfying our revenue recognition criteria. |
17
| | |
| | • | Implementation Delays: Once we enter into a customer contract, our recognition of revenue from that contract depends, to a significant extent, on the timing of our implementation of the project. Customer implementation schedules may be delayed for reasons beyond our control, such as customer scheduling changes, delays in acceptance testing by customers, unusual integration issues or delays in obtaining equipment from third-party vendors. Delays in the implementation of a particular project may require us to delay the recognition of anticipated revenue from one quarter to another and may contribute to substantial fluctuations in our quarterly operating results. |
Our quarterly results also may fluctuate due to other factors, such as the timing of new product introductions and product enhancements by us or our competitors and changes in the mix of our software and third-party components, which have significantly lower gross margins, included in the systems we sell. If our revenue varies significantly from quarter to quarter, we may have difficulty managing our business, and our quarterly results could fall below expectations of investors and stock market analysts which could cause our stock price to decline or exhibit volatility.
| |
| | Our failure to manage growth effectively may strain our management, personnel and other resources, which could impair our ability to meet customer requirements. |
We have grown very rapidly and must continue to add customers and employees to be successful. Our business could suffer if we fail to manage effectively our growth. From December 31, 2004 to December 31, 2005, our contracted backlog grew from $118.2 million to $158.0 million and the number of our employees increased from 199 to 469, including 212 employees added through our November 2005 acquisition of Camtronics. While it is unlikely that we can continue to grow at this rate, continued growth may significantly strain our management, personnel and other resources. Simultaneously undertaking numerous projects with large multi-site health care providers could also strain our existing resources and cause our implementation and customer service to suffer. This could cause us to fail to satisfy material performance requirements under our contracts which could, under certain circumstances, permit customers to terminate their contracts with us and would adversely affect our reputation.
| |
| | Acquisitions could result in integration difficulties, dilution or other adverse financial consequences. |
In November 2005 we acquired Camtronics, which added a new suite of cardiology tools to our advanced visualization software offering, increased our customer base by over 300 medical facilities, and increased our employee headcount by 212 employees. We may in the future acquire other businesses that we believe are complementary to our business. The pursuit of acquisitions may divert the attention of management and cause us to incur various expenses identifying, investigating and pursuing suitable acquisitions, whether or not they are consummated. If we acquire additional businesses, we may not be able to integrate the acquired operations successfully with our business or we may not achieve the anticipated benefits from the acquired business. If we are unable to integrate any new business successfully, we could be required either to dispose of the acquired operation or to undertake changes to the acquired operations in an effort to integrate them with our business. In either event, our business operations and financial condition could suffer a material adverse effect. Future acquisitions could result in potentially dilutive issuances of our equity securities, the incurrence of debt, contingent liabilities or amortization expenses, or write-offs of goodwill, any of which could harm our financial condition. Acquisition financing, if needed, may not be available on favorable terms.
| |
| | Analogic Corporation, the former owner of Camtronics, identified a material weakness in its internal control over financial reporting due to control deficiencies at Camtronics, and we cannot be certain that such control deficiencies have been fully remediated, or that other control deficiencies will not be identified that will lead us to conclude that a material weakness in our internal control over financial reporting exists. |
A company’s internal control over financial reporting is a process designed by or under the supervision of the chief executive officer and chief financial officer and effected by the company’s board of directors, management, and other personnel to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally
18
accepted accounting principles. A material weakness in internal control over financial reporting is a control deficiency, or combination of control deficiencies, that results in more than a remote likelihood that a material misstatement of the annual or interim financial statements will not be prevented or detected. Analogic Corporation, which sold Camtronics to us on November 1, 2005, reported that as of July 31, 2005 (its fiscal year-end) and as of October 31, 2005, it had a material weakness in its internal control over financial reporting because its review and approval controls over the completeness and accuracy of revenue and deferred revenue under multiple-element software arrangements at its Camtronics subsidiary were ineffective to ensure revenues were recorded in the correct period. Analogic further reported that as of October 31, 2005, it had undertaken numerous remediation measures that had improved the design effectiveness of its internal control over financial reporting with respect to such matters, but that not all of the newly designed controls were operating effectively at such date and not all had operated for a sufficient period of time prior to such date to demonstrate operating effectiveness. In our recently completed review of our internal control over financial reporting we did not identify any material weaknesses of the type previously identified by Analogic at Camtronics prior to its sale to us. However we cannot be certain that the control deficiencies at Camtronics that led to the prior finding of a material weakness have been fully remediated, or that other control deficiencies will not be identified in the future which would lead us to conclude that a material weakness in our internal control over financial reporting exists.
| |
| | We depend on highly specialized personnel, and the loss or failure to identify, hire, motivate and retain additional highly specialized personnel could adversely affect our ability to grow our business. |
Our future success and the execution of our growth strategy depend on our continuing ability to identify, hire, develop, motivate and retain highly specialized personnel for technical and sales positions within our organization. For example, when hiring an advanced visualization software engineer, we generally seek individuals with advanced post-graduate degrees in specialized fields. We also must identify experienced candidates for sales positions who can effectively communicate the cost, clinical and information technology benefits of our products to multiple constituents at our target customers. Our competitors, employers in other industries, academic institutions and governmental entities and organizations also often seek persons with similar qualifications. As a result, we may not be able to identify and hire the personnel we need in a timely manner.
In addition, to hire, motivate and retain these personnel, we believe we must provide them with a competitive compensation package, which may include stock-based incentives, such as restricted stock or stock options. Increases in shares available for issuance under our stock incentive plans generally will require stockholder approval, and our stockholders may not approve future increases. Recent changes in the accounting for stock options may cause us to issue fewer stock options and rely more on restricted stock grants instead, which may be less attractive to potential employees. If this occurs, we may find it more difficult to hire, motivate and retain highly specialized personnel, which could have a material adverse effect on our ability to grow our business.
| |
| | We are dependent on our senior executive management, and the loss of any member of senior executive management may prevent us from managing and growing our businesses effectively. |
Our success depends largely on the continued service of our senior executive management, including Charles A. Jett, Jr., our Chairman, President and Chief Executive Officer, Grady Floyd, our Chief Operating Officer, and W. Randall Pittman, our Chief Financial Officer. We have entered into executive employment agreements with these key members of senior executive management. The terms of these employment agreements are two years for Mr. Jett, 18 months for Mr. Floyd and one year for Mr. Pittman and renew automatically on aday-by-day basis thereafter unless we or the officer give notice to stop the automatic renewal. The loss of any of our senior executive officers could have an adverse impact on our ability to manage and grow our business effectively. We cannot assure you that in such an event we would be able to replace any member of senior executive management in a timely manner, or at all, on acceptable terms.
19
| |
| | The loss of Ascension Health or future major customers could materially and adversely affect our results of operations and financial condition because portions of our future revenues are tied to continuing relationships with significant customers. |
We have historically depended on a small number of customers for a substantial portion of our sales, and we are dependent on Ascension Health for a large portion of the revenue to come from our contracted backlog. Contracted future revenue from Ascension Health was approximately $52 million, or 33%, of our contracted backlog at December 31, 2005. In addition, our future revenue and growth significantly depend on our ability to sell add-on functionality and new products to existing multi-facility customers such as Ascension Health. As a result, the loss of Ascension Health or any other future major customers or their failure to renew maintenance and support agreements with us could have a material adverse effect on our revenue and operating results.
| |
| | Our products are complex and are operated in a wide variety of network configurations, which could result in errors or product failures. |
Because our software is complex, undetected errors, failures or bugs may occur when we first introduce our products or when we release new versions. As we develop product enhancements and extensions, the complexity of our software may increase. Our products often are installed and used in large-scale computing environments with different operating systems, system management software and equipment and networking configurations, any of which may cause errors or failures in our products or may expose undetected errors, failures or bugs in our products. In the past, we have encountered failures in certain of our product offerings after their installation, and we have been required to expend significant resources to repair the problem and sustain the customer relationship. Despite testing by us and by others, errors, failures or bugs may not be found in new products or releases until after general release. The occurrence or existence of such errors, failures or bugs in our products could result in negative publicity, contract cancellations, loss of or delay in market acceptance or claims by customers or others. In addition, if an actual or perceived breach of network security occurs in one of our customers’ medical image storage systems, regardless of whether the breach is attributable to our solution, the market perception of our products and services could be harmed.
| |
| | Changes in our third-party reselling arrangements may affect our revenues and our ability to deliver a complete solution, which may adversely impact our revenue and cause customer dissatisfaction. |
We resell third-party components from numerous companies, including IBM Corporation, EMC Corporation and Eastman Kodak Company, as part of our solution. As the cost of third-party hardware components continues to decline, our revenue from third-party component sales and installation and, consequently, our overall revenue per individual sale may also decline. If we cease selling third-party hardware components as part of our solution or if the vendors of these products, some of whom are also competitors, curtail or delay our ability to resell them as part of our solution, we may be limited in our ability to provide our customers with a complete solution, and our revenue, profit and reputation may decline. Our implementation capabilities and performance also may be adversely affected if our customers are required to obtain the necessary third-party components on their own.
| |
| | We may not be able to respond to changes in our industry, competitive technologies, changes in customer requirements or evolving industry standards, which would result in reduced revenue and profit margins. |
Because our industry is subject to rapid technological change, we must constantly monitor changes in industry standards, customer requirements and other matters. If we fail to anticipate and respond adequately to these changes in a timely manner, our business and operating results could suffer a material adverse effect. Although we currently support emerging industry standards, we cannot assure you that we will be able to conform to future evolving standards in a timely fashion, or that such conformity, if achieved, will benefit our competitive position in the market. In anticipation of new product introductions by us or our competitors, customers could refrain from purchasing our existing products. New products could render certain of our existing products obsolete, or we may fail to develop product enhancements or new products that are accepted by our customers. Furthermore, as the market for our solution matures, we may be subject to pricing pressures,
20
and our revenues and profits may decline. Any of these events could delay or prevent our customers from acquiring our solution or require us to reduce the price of our solution, either of which could lead to a decrease in revenue and profit margins.
| |
| | Our customers depend on third-party reimbursement. A reduction or other change in third-party reimbursements to our customers could negatively affect our business by reducing the demand for our products or adversely impacting our pricing. |
We sell our products to hospitals, clinics, imaging centers and other health care providers which typically bill various third-party payors, such as government health programs, private health insurance plans, managed care organizations and other similar programs. Third-party payors increasingly challenge the prices charged for medical services and, in some instances, have put pressure on service providers to lower their prices or reduce their services. We cannot predict what changes third-party payors will make to their reimbursement methods. Third-party payors can indirectly affect the pricing or relative attractiveness of our products by regulating the maximum amount of reimbursement that they will provide for generating, storing and interpreting medical images. A decline in reimbursements for radiological procedures, for instance, may decrease the amount which physicians, clinics and hospitals are able to recover for such services and may reduce the number and complexity of medical images. A reduction in the use or reimbursement of digital medical images may lead to our customers decreasing their capital investment budgets, which could significantly reduce the demand for our products.
| |
| | If we fail to obtain or maintain necessary FDA clearances for our products, if such clearances are delayed, or if our products are subject to FDA recall, we will be unable to distribute and market some of our products. |
Our advanced visualization software products are subject to FDA regulation of medical devices. Medical devices are a highly regulated class of products. The FDA regulates the development, testing, manufacturing, labeling, promotion and record-keeping procedures for medical devices, including imaging software and systems. The process of obtaining FDA marketing clearance for new products and new applications for existing products can be time consuming and expensive. The FDA has granted us marketing clearance, pursuant to the 510(k) pre-market notification process, for our currently marketed uses of our advanced visualization tools. Before we can market other clinical uses of our advanced visualization tools, generally we must seek 510(k) clearance for the additional clinical uses. We cannot assure you either that the FDA will grant clearance for future uses of our advanced visualization tools, that such clearance will be broad enough to allow all the requested new uses, that such clearance will not be delayed, or that once clearance is obtained, it will not be necessary for us or the FDA to recall one or more of our products. Also, the FDA may not grant clearance with respect to our future products or enhancements, or future FDA reviews may involve delays that could adversely affect our ability to market such future products or enhancements. Moreover, our future products or enhancements may be subject to the FDA’s more lengthy and expensive pre-market approval process if we are unable to demonstrate that such products and enhancements meet the FDA’s requirements regarding similarity to pre-existing approved devices.
Furthermore, it is possible that even if we receive required regulatory clearances and approvals from the FDA to market a given product, these clearances and approvals may include limitations on the indicated uses of the product. Also, the FDA can withdraw product clearances and approvals due to failure to comply with regulatory standards, quality system manufacturing regulations, unapproved manufacturing changes, or if unforeseen problems arise after initial approval. The FDA could also limit or prevent our distribution of products. We might conduct a voluntary recall or the FDA could recall such products if it deems them defective, a health risk, or in violation of FDA regulations. These regulations depend heavily on administrative interpretation, and any such future interpretations could adversely affect us. The FDA may also inspect us and our facilities from time to time, or the facilities of our suppliers, to determine whether we are in compliance with quality system regulations and current good manufacturing practices. If the FDA determines that we are not in compliance with such regulations, it could require us to correct these deficiencies or could suspend the
21
manufacture and sale of the products. The agency could also impose civil penalties, including fines, recall or seize products and, in extreme cases, impose criminal sanctions.
| |
| | If we fail to comply with other potentially applicable health care regulations, we could face substantial penalties, and our business, operations, and financial condition could be adversely impacted. |
We do not deliver health care services directly to patients, control health care referrals, or submit claims to or otherwise bill Medicare, Medicaid, or any other third-party payors. However, we have engaged certain physicians to serve as consultants on our behalf, entered into service agreements and license agreements with health care entities, and had certain of our products evaluated at health care facilities, and some of our health care customers hold warrants to purchase our stock. Because of the breadth of many health care laws and regulations, and their potential impact on our customers, we cannot assure you that such laws and regulations will not apply to our business, either directly or indirectly. We could be subject to health care fraud and patient privacy regulation by both the federal government and the states in which we conduct our business. The regulations that may affect our ability to operate include the following:
| | |
| | • | The Federal Anti-Kickback Statute prohibits the exchange of anything of value with the intent to encourage utilization of services payable under a federal health care program. Courts have construed this statute as being implicated even when only one of the purposes of one of the parties is to encourage patient referrals or other federal health care business, even if legitimate purposes also exist for the arrangement. |
| |
| | • | The Federal Ethics in Patient Referrals Act, known as the Stark Law, prohibits (absent an applicable Stark exception) referrals for designated health services reimbursable under Medicare or Medicaid by a physician to an entity with which the physician, or an immediate family member, has a financial relationship. |
| |
| | • | The Federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, has increased the scope of federalfraud-and-abuse laws by applying them to prohibit fraudulent conduct in connection with any health care benefit program, not only federal health care programs. Although we are not a covered entity that is directly subject to liability under the HIPAA privacy and security standards, we could be impacted by such regulations through contractual relations with those of our customer base who are covered entities. |
| |
| | • | State law equivalents of each of the above federal laws, such as anti-kickback, self-referral, and false claims laws, may apply to items or services reimbursed by any third-party payor (including commercial insurers). State laws governing the privacy of health information in certain circumstances, many of which differ from each other in significant ways and often are not preempted by HIPAA (thus complicating compliance efforts) and some of which may apply to us directly, may also affect our operations. |
If our operations are found to violate any of these laws or other governmental regulations, we may be subject to penalties, including civil and criminal penalties, damages, fines, and the curtailment or restructuring of our operations. Any such occurrences could adversely affect our ability to operate our business and our financial results. Determining such risk is complicated by the fact that many of these laws and regulations have not been fully interpreted by governing regulatory authorities or the courts, and many of the provisions of such laws and regulations are open to a wide range of interpretations. Any action against us for violating such laws or regulations, even if we successfully defend such an action, could cause us to incur significant legal expenses and divert our management’s attention from the operation of our business. Moreover, compliance with applicable federal and state privacy, security, and electronic transaction laws may require us to modify our operations with respect to the handling of patient information. Implementing these modifications may prove costly and time consuming. At this time, we are not able to determine the full consequences to us, including the total cost of compliance, of these various federal and state laws.
22
| |
| | We may not be able to raise additional capital on acceptable terms to fund our operations, develop product enhancements or fund acquisitions, which could adversely affect our growth prospects. |
We expect our cash resources to be sufficient to meet our working capital and capital expenditure needs for the next twelve months. We may need to raise additional funds, however, through public or private financings, strategic relationships or other arrangements in order to:
| | |
| | • | develop new technologies; |
| |
| | • | enhance existing product lines, such as expanding our advanced visualization tools product line to apply to additional clinical specialties; |
| |
| | • | fund additional sales and marketing programs; |
| |
| | • | invest in or acquire complementary businesses, product lines or technologies; or |
| |
| | • | hire additional personnel, particularly to expand sales, marketing, research and development. |
If it becomes necessary to raise additional funds, our ability to operate our business could be adversely affected if we are unable to identify additional sources of capital to fund these activities on acceptable terms.
| |
| | If the market for digital medical imaging products and services does not develop as we expect, our business strategy may be ineffective, and we may not be able to grow our business. |
We operate in a developing industry where customer acceptance and market demand is still evolving. The digital medical imaging solutions market is still developing due to:
| | |
| | • | the availability of high performance computers and storage systems at reduced prices; |
| |
| | • | the continuing development of industry standards for the generation, transmission and storage of medical imaging data; |
| |
| | • | changing dynamics in the health care industry, including consolidation and third-party reimbursement, which are driving increased automation across multiple sites; and |
| |
| | • | changing medical practices, including demand for more and better medical imaging. |
We cannot assure you that this market will continue to develop in the manner we anticipate, that the market will provide growth opportunities for us or that our business strategies will be successful. If the market for digital medical imaging products and services fails to develop as we expect, our business, results of operations and financial condition are likely to be materially and adversely affected.
| |
| | Product liability claims may require us to pay damages, reduce the demand for our products, and harm our reputation. |
Our business exposes us to a risk of product liability claims and other adverse effects of product failures. We provide products that, among other things, assist in clinical decision-making, provide access to patient medical image information and assist in creating patient treatment plans. Although no one has brought a claim against us to date alleging that they suffered damages due to a defect or other failure of any of our products, our customers or their patients may assert claims against us in the future if our software fails to provide accurate and timely information. A product liability claim can cause us to incur significant legal defense costs and adverse publicity regardless of the claim’s merit or eventual outcome. If we are required to pay damages that exceed our insurance coverage to one or more plaintiffs, such payments could significantly harm our financial condition. A product liability claim also could harm our reputation and lead to a decline in revenue. We attempt to limit by contract our liability for damages arising from negligence, errors or mistakes. Despite this precaution, such contract provisions may not be enforceable or may not otherwise protect us from liability for damages. We maintain general liability insurance coverage, including coverage for errors or omissions. However, this coverage may not be sufficient to cover one or more large claims against us or otherwise continue to be available on terms acceptable to us. In addition, the insurer could disclaim coverage as to any future claim.
23
| |
| | If we fail to protect our intellectual property rights, our competitors may take advantage of our ideas to compete more effectively with us. |
We rely on a combination of copyright, trade secret and trademark laws, nondisclosure and confidentiality agreements, and other contractual restrictions to protect our proprietary technology and other intellectual property rights. However, these legal means afford only limited protection and may not adequately protect our rights or permit us to gain or keep any competitive advantage based on our intellectual property. In addition, we have filed patent applications to protect certain aspects of our software technology. However, to date, only one of our patent applications has resulted in the issuance of a patent, and we cannot assure you that these patent applications will result in patents being issued in the U.S., Europe or Japan, or that such patents will be issued in a form that will be advantageous to us. Even if we obtain such patents, they may be challenged, invalidated or circumvented by third parties. We may not be able to prevent the unauthorized disclosure or use of our technical knowledge or other trade secrets by employees. Furthermore, the laws of foreign countries may not protect our intellectual property rights to the same extent as the laws of the U.S. Litigation may be necessary to enforce our intellectual property rights which could result in substantial costs to us and substantial diversion of management attention. If we do not adequately protect our intellectual property, our competitors could use it to enhance their products. Additionally, because we use or include open source software, which is not proprietary, in the components of some of our products, our competitors may freely use such open source software, and in certain circumstances may freely use such components. This could harm our competitive position, decrease our market share or otherwise harm our business.
The prosecution and enforcement of copyrights and patents relating to components licensed or sold to us by third parties is not within our control, and without these components, we may be unable to provide our solution or maintain our technological advantage. If the third-party suppliers of components used by us fail to protect their patents or copyrights or if these components are found to infringe on the rights of another party, the functionality of our products could suffer, and our ability to bring new and existing products to market could be delayed or even prohibited.
| |
| | Our operating results could suffer if we become subject to a protracted infringement claim or litigation or a significant damage award. |
Substantial intellectual property litigation and threats of litigation exist in our industry. We expect that digital image visualization software, image management software and open source software products may become increasingly subject to third-party infringement or other claims as the number of competitors grows and the functionality of products increases. Any claims, with or without merit, could have the following negative consequences:
| | |
| | • | costly litigation and damage awards; |
| |
| | • | diversion of management attention and resources; |
| |
| | • | product sales and distribution delays or suspensions, either temporary or permanent; and |
| |
| | • | the need to enter into royalty or licensing agreements, which may not be available on terms acceptable to us, if at all. |
A successful infringement or other claim against us could result in a substantial damage award and materially harm our financial condition. Our failure or inability to license the infringed or similar technology could prevent us from selling our products and adversely affect our business and financial results.
| |
| | Our directors may not be held personally liable for certain actions, which could discourage stockholder suits against them. |
As permitted by Delaware law, our amended and restated certificate of incorporation provides that our directors shall not be personally liable to us or our stockholders for monetary damages for breach of fiduciary duty as a director, with limited exceptions. These provisions may discourage stockholders from bringing suit against a director for breach of fiduciary duty and may reduce the likelihood of derivative litigation brought by
24
stockholders on our behalf against a director. In addition, we provide for mandatory indemnification of directors and officers to the fullest extent permitted by Delaware law and have entered into indemnification agreements with our directors and officers.
| |
| | Delaware law and certain anti-takeover provisions of our corporate documents could delay or prevent a third party from acquiring us or a change in control even if it would benefit our stockholders. |
Our amended and restated certificate of incorporation and bylaws contain a number of provisions that may delay, deter or inhibit a future acquisition or change in control that is not first approved by our board of directors. This could occur even if our stockholders receive an attractive offer for their shares or if a substantial number or even a majority of our stockholders believe the takeover may be in their best interest. These provisions are intended to encourage any person interested in acquiring us to negotiate with and obtain approval from our board of directors prior to pursuing a transaction. Provisions that could delay, deter or inhibit a future acquisition or change in control include the following:
| | |
| | • | our board of directors may issue 200,000 shares of blank check preferred stock without stockholder approval and that may be substantially dilutive or contain preferences or rights objectionable to an acquiror; |
| |
| | • | our board of directors is comprised of classes of directors with staggered, three-year terms so that only a portion of our directors is subject to election at each annual meeting; |
| |
| | • | our board of directors can amend our bylaws without stockholder approval; |
| |
| | • | stockholders cannot call special meetings of stockholders; |
| |
| | • | stockholders cannot act by written consent; |
| |
| | • | stockholders must give advance notice to nominate directors for election or to submit proposals at stockholder meetings; |
| |
| | • | we may be obligated to make payments under executive employment agreements in the event of a change in control; and |
| |
| | • | some Delaware statutes restrict or prohibit certain transactions with affiliated or interested parties and permit the adoption of “poison pills” without stockholder approval. |
These provisions could also discourage bids for our common stock at a premium and cause the market price of our common stock to decline. In addition, these provisions may also entrench our management by preventing or frustrating any attempt by our stockholders to replace or remove our current management.
| |
| ITEM 1B: | UNRESOLVED STAFF COMMENTS |
Not Applicable.
Our principal offices occupy approximately 40,200 square feet of leased office space in Birmingham, Alabama, under a lease that expires in March 2010 and 79,500 square feet of owned office space including approximately 13 acres of land in Hartland, Wisconsin. We also maintain a research and development facility consisting of approximately 14,400 square feet of leased office space in Madison, Wisconsin, under a lease expiring in January 2013; a research and development and customer support facility consisting of approximately 14,500 square feet of leased office space located in Ottawa, Ontario, under a lease that expires in December 2009; a research and development facility consisting of approximately 2,000 square feet of leased office space in Winter Park, Florida, under a lease expiring in October 2008; and a research and development facility consisting of approximately 2,400 square feet of leased office space located in Hartland, Wisconsin under a lease expiring in April, 2006. We believe our current facilities are adequate for our current needs.
25
| |
| ITEM 3: | LEGAL PROCEEDINGS |
There are no pending material legal proceedings other than ordinary routine litigation incidental to normal business to which the Company is a party or to which any of its properties are subject.
| |
| ITEM 4: | SUBMISSION OF MATTERS TO A VOTE OF SECURITY HOLDERS |
Not applicable.
PART II
| |
| ITEM 5: | MARKET FOR THE REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES |
Our common stock began trading on the Nasdaq National Market under the symbol “EMAG” on February 9, 2005. Prior to such date, there was no established public trading market for our common stock. As of March 23, 2006, the 20,806,339 outstanding shares of common stock were held by 99 holders of record. The closing price per share of our common stock on the Nasdaq National Market on March 23, 2006 was $16.87.
The following table presents the range of share prices for each quarter of 2005:
| | | | | | | | | |
2005 Quarter Ended | | High | | | Low | |
| March 31, 2005 | | $ | 18.50 | | | $ | 14.35 | |
| June 30, 2005 | | $ | 17.29 | | | $ | 13.99 | |
| September 30, 2005 | | $ | 15.00 | | | $ | 11.30 | |
| December 31, 2005 | | $ | 16.47 | | | $ | 12.35 | |
Dividends
We have not declared or paid any cash dividends on our common stock and do not anticipate paying cash dividends on our common stock for the foreseeable future. Instead, we currently intend to retain all future earnings, if any, for use in the operations of our business and to fund future growth. Any future decision to declare and pay dividends will be at the discretion of our board of directors, after taking into account our financial results, capital requirements and other factors it may deem relevant. Covenants in our debt agreements currently prohibit us from paying dividends or making other distributions.
| |
| | Use of Proceeds from Initial Public Offering |
Our initial public offering of common stock was effected through a Registration Statement on Form S-l (File No. 333-120621) that was declared effective by the Securities and Exchange Commission on February 8, 2005, pursuant to which we sold all 5,750,000 shares of our common stock registered. We received net proceeds of approximately $67.2 million from the offering. We used $4.0 million of the net proceeds to repay borrowings outstanding under our subordinated notes on February 18, 2005. We invested the remaining net proceeds, after payment of such subordinated notes, in short-term, investment-grade, interest bearing instruments pending their further use.
During the year ended December 31, 2005, we spent approximately $5.8 million of such net proceeds on capital purchases, substantially all of which was spent on purchases of equipment, and an additional $40 million of the net offering proceeds to acquire all of the outstanding stock of Camtronics Medical Systems, Ltd. on November 1, 2005.
| |
| | Purchases of Equity Securities by the Issuer and Affiliated Purchasers |
We did not repurchase any shares of our common stock during the three month period ended December 31, 2005.
26
| |
| ITEM 6: | SELECTED CONSOLIDATED FINANCIAL DATA |
The following consolidated statements of operations data for the years ended December 31, 2003, 2004 and 2005 and consolidated balance sheet data as of December 31, 2004 and 2005 are derived from our audited consolidated financial statements and related notes, which are included elsewhere in this document. The consolidated statements of operations data for the years ended December 31, 2001 and 2002 and the balance sheet data as of December 31, 2001, 2002 and 2003 are derived from our audited consolidated financial statements that do not appear in this filing. The consolidated selected financial data set forth below should be read in conjunction with our consolidated financial statements and related notes and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” included elsewhere in this filing. Historical results are not necessarily indicative of the results to be expected for any future period.
| | | | | | | | | | | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2001 | | | 2002 | | | 2003 | | | 2004 | | | 2005 | |
| | | (Dollars in thousands, except per share data) | |
| |
Consolidated Statements of Operations Data(1): | | | | | | | | | | | | | | | | | | | | |
| Revenue: | | | | | | | | | | | | | | | | | | | | |
| System sales | | $ | 1,868 | | | $ | 8,437 | | | $ | 17,234 | | | $ | 33,441 | | | $ | 50,041 | |
| Support services | | | 630 | | | | 4,182 | | | | 6,057 | | | | 12,361 | | | | 23,750 | |
| | | | | | | | | | | | | | | | | | | | | |
| Total revenue | | | 2,498 | | | | 12,619 | | | | 23,291 | | | | 45,802 | | | | 73,791 | |
| Cost of revenue: | | | | | | | | | | | | | | | | | | | | |
| System sales | | | 1,031 | | | | 6,316 | | | | 10,227 | | | | 21,452 | | | | 28,316 | |
| Support services | | | 1,396 | | | | 4,040 | | | | 7,493 | | | | 10,728 | | | | 14,648 | |
| | | | | | | | | | | | | | | | | | | | | |
| Total cost of revenue | | | 2,427 | | | | 10,356 | | | | 17,720 | | | | 32,180 | | | | 42,964 | |
| | | | | | | | | | | | | | | | | | | | | |
| Gross profit | | | 71 | | | | 2,263 | | | | 5,571 | | | | 13,622 | | | | 30,827 | |
| Operating expenses: | | | | | | | | | | | | | | | | | | | | |
| Research and development | | | 1,952 | | | | 2,383 | | | | 4,143 | | | | 6,021 | | | | 10,697 | |
| Sales and marketing | | | 4,383 | | | | 4,456 | | | | 6,144 | | | | 9,027 | | | | 11,830 | |
| General and administrative | | | 3,050 | | | | 3,149 | | | | 5,793 | | | | 8,024 | | | | 12,308 | |
| Amortization and write-off of intangible assets related to Camtronics acquisition | | | — | | | | — | | | | — | | | | — | | | | 993 | |
| Integration costs related to Camtronics acquisition | | | — | | | | — | | | | — | | | | — | | | | 244 | |
| Loss on contract from issuance of warrants | | | 550 | | | | — | | | | — | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | | | | | |
| Total operating expenses | | | 9,935 | | | | 9,988 | | | | 16,080 | | | | 23,072 | | | | 36,072 | |
| | | | | | | | | | | | | | | | | | | | | |
| Operating loss | | | (9,864 | ) | | | (7,725 | ) | | | (10,509 | ) | | | (9,450 | ) | | | (5,245 | ) |
| Interest income (expense), net | | | 151 | | | | (601 | ) | | | (850 | ) | | | (1,022 | ) | | | 248 | |
| | | | | | | | | | | | | | | | | | | | | |
| Net loss | | $ | (9,713 | ) | | $ | (8,326 | ) | | $ | (11,359 | ) | | $ | (10,472 | ) | | $ | (4,997 | ) |
| | | | | | | | | | | | | | | | | | | | | |
| Basic and diluted net loss per share data: | | | | | | | | | | | | | | | | | | | | |
| Net loss per share: | | | | | | | | | | | | | | | | | | | | |
| Basic and Diluted | | $ | (7.41 | ) | | $ | (6.38 | ) | | $ | (5.79 | ) | | $ | (4.07 | ) | | $ | (0.28 | ) |
| Weighted average shares: | | | | | | | | | | | | | | | | | | | | |
| Basic and Diluted | | | 1,313,693 | | | | 1,314,238 | | | | 1,973,108 | | | | 2,589,832 | | | | 17,975,083 | |
Selected Cash Flow Data: | | | | | | | | | | | | | | | | | | | | |
| Cash provided by (used in) operations | | $ | (4,280 | ) | | $ | (7,847 | ) | | $ | (2,377 | ) | | $ | 4,959 | | | $ | (1,881 | ) |
27
| | | | | | | | | | | | | | | | | | | | | |
| | | As of December 31, | |
| | | 2001 | | | 2002 | | | 2003 | | | 2004 | | | 2005 | |
| | | (Dollars in thousands) | |
| |
Consolidated Balance Sheet Data: | | | | | | | | | | | | | | | | | | | | |
| Cash and cash equivalents | | $ | 3,399 | | | $ | 2,242 | | | $ | 2,340 | | | $ | 5,995 | | | $ | 15,520 | |
| Marketable securities | | | — | | | | — | | | | — | | | | — | | | | 4,951 | |
| Intangible assets, net | | | — | | | | — | | | | 8,000 | | | | 6,873 | | | | 34,277 | |
| Total assets | | | 15,699 | | | | 24,990 | | | | 29,050 | | | | 41,768 | | | | 117,944 | |
| Total debt and capital lease obligations | | | 2,100 | | | | 10,260 | | | | 8,467 | | | | 9,489 | | | | 3,749 | |
| Redeemable preferred stock | | | 24,269 | | | | 24,326 | | | | 30,282 | | | | 30,348 | | | | — | |
| Total stockholders’ equity (deficit) | | | (17,369 | ) | | | (20,508 | ) | | | (23,535 | ) | | | (32,370 | ) | | | 63,639 | |
Other Data (unaudited, in millions): | | | | | | | | | | | | | | | | | | | | |
| Contracted backlog(2) | | $ | 35.3 | | | $ | 55.4 | | | $ | 82.7 | | | $ | 118.2 | | | $ | 158.0 | |
| | |
| (1) | | On November 1, 2005, we acquired Camtronics Medical Systems, Ltd., and on May 30, 2003, we merged with Ultravisual Medical Systems Corporation. Both the acquisition and the merger were accounted for as purchases under Statement of Financial Accounting Standards No. 141,Business Combinations. Accordingly, the results of operations of Camtronics Medical Systems, Ltd. and Ultravisual Medical Systems Corporation have been included in the accompanying consolidated financial statements since the respective dates of acquisition. For more information, see Note 4 of the notes to our consolidated financial statements. |
| |
| (2) | | We define contracted backlog as the aggregate dollar value of unrecognized revenue from all executed contracts at a given point in time. |
| |
| ITEM 7: | MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
Company Overview
We provide an enterprise-level information technology solution for the clinical analysis and management of digital medical images within multi-hospital networks, community hospitals and diagnostic imaging centers. Our solutions consist of advanced visualization and image management software for multiple medical specialties such as cardiology, radiology and orthopedics, comprehensive support services and third-party components. Our web-enabled advanced visualization software, which is hosted by the customer, provides physicians across the enterprise — in multiple medical specialties and at any network access point — with dynamic tools to manipulate and analyze images in both a 2D perspective and a 3D perspective. With these tools, physicians have the ability to better understand internal anatomic structure and pathology, which can improve clinical diagnoses, disease screening and therapy planning. Our open standards-based solutions are designed to help customers improve staff productivity, enhance revenue opportunities, automate complex medical imaging workflow, lower total cost of ownership and provide better service to physicians and patients.
We were founded in December 1998 as an Alabama corporation and reincorporated in Delaware in January 2000. Since our first commercial implementation in December 2000, we have experienced substantial growth and have implemented our solutions at facilities affiliated with some of the largest multi-facility health care providers in the U.S.
Our fiscal year ends on December 31. References to 2005, for example, refer to the fiscal year ended December 31, 2005.
Results Overview
Total revenue for 2005 was $73.8 million, which represents a 61.1% increase over 2004. The increase was comprised of a 49.6% increase in system sales revenue and a 92.1% increase in support services revenue. Our overall gross margin percentage increased from 29.7% for 2004 to 41.8% for 2005. We achieved gross
28
margin percentages of 43.4% and 38.3% for system sales and support services revenue, respectively, during 2005, compared to 35.9% and 13.2%, respectively, for 2004. Our net loss was $(5.0) million in 2005 compared to a net loss of $(10.5) million in 2004. This improvement was driven by revenue growth, margin expansion and expense control.
As of December 31, 2005, we had $158.0 million in contracted backlog, consisting of fees for contracted future installations and for the support of existing installations, compared with a contracted backlog of $118.2 million at December 31, 2004. We expect to recognize revenue from our current backlog of approximately $73.3 million in 2006 and $27.0 million in 2007. Substantially all of the remaining $57.7 million, which primarily consists of recurring revenue from support services, is expected to be recognized by 2010. Our backlog will decrease as we recognize revenue under existing contracts, and it will increase as we enter into new contracts.
Important Developments
On November 1, 2005, we acquired all the stock of Camtronics Medical Systems, Ltd., based in Hartland, Wisconsin, for $40 million in cash. We acquired all of Camtronics’ assets, including its corporate headquarters campus in Hartland, and all of Camtronics’ liabilities. We expect to substantially complete the integration of this business by the end of the third quarter of 2006.
Camtronics, founded in 1986, is a leading provider of cardiology image and information management systems. Camtronics, which had revenue of $38.1 million for its fiscal year ended July 31, 2005, currently serves a customer base that includes over 300 hospitals. In 2004, Frost and Sullivan, a leading market research firm, named Camtronics as one of the top five cardiology-solution vendors in the United States. Including Camtronics’ customers, our combined organization now has a customer base that includes approximately 600 medical facilities.
On February 14, 2005, we completed our initial public offering of common stock. We sold 5.0 million shares of our common stock at a price of $13.00 per share. On February 18, 2005, our underwriters exercised the over-allotment option to purchase 750,000 additional shares of our common stock at $13.00 per share. Total proceeds from the initial public offering (net of underwriting discount and offering expenses) were $67.2 million.
Our Market
We believe the health information technology market is exhibiting the following trends:
| | |
| | • | Increasing procedure volumes |
| |
| | • | Increasing procedure size |
| |
| | • | Modality “blending”, a layering of studies from two separate modalities for diagnostic and treatment purposes |
| |
| | • | Expanding adoption and use of standards |
| |
| | • | Increasing emphasis by healthcare providers and government agencies on electronic health record integration |
| |
| | • | Body transparency, a new paradigm for navigating through large volumes of information |
The amount of imaging data being generated by health care providers is growing extremely rapidly. This data must be stored and made available for easy retrieval. Increasingly, health care information users want access to the stored data at any time, and in any location. In addition, modalities that provide non-invasive alternatives continue to expand into other clinical domains. Examples include:
| | |
| | • | MR and CT angiography |
| |
| | • | Multi-Detector CT for heart and chest imaging |
29
| | |
| | • | CT/PET Fusion |
| |
| | • | Cardiac catheterization |
One area that has received significant attention is advanced visualization, which uses 3D and other advanced analytic tools as key elements in an enterprise visual medical system. Earlier generation PACS have focused primarily on single departments and have utilized generic 2D tools. A complete enterprise visualization system must not only support full 2D capabilities, but also include 3D tools, integrate easily into other information systems, and adhere to standards. To understand these images, referring physicians need new tools that adapt to their specialty. We expect that clinicians and specialists will soon consider enterprise visualization routine, since these images can be easier to understand and utilize.
Our solutions can be extended to multiple stakeholders throughout the health care enterprise. Our solutions go beyond moving images from point A to point B to effectively distributing multi-specialty tools and clinical content using a web-enabled platform. Our solutions not only manage very complex datasets, but also perform advanced visualization such as 3D reconstruction and analysis within the viewing application, and distribute essential clinical tools through the network.
Significant Events in 2005
During the year ended December 31, 2005, we continued to focus on our core set of strategic goals. In addition to the initial public offering of our stock and our acquisition of Camtronics, we believe the following events were significant with respect to our goals:
| | |
| | • | During February 2005, we entered into a ten-year contract with Sisters of St. Francis Health Services, Inc., an integrated network of 12 hospitals, clinics and associated facilities located in Indiana and Illinois. |
| |
| | • | During March 2005, our quality management system received ISO 13485:2003 certification from the certification body, Lloyd’s Register Quality Assurance, for both the Madison, Wisconsin and Birmingham, Alabama offices. This certification is issued for the design and management of software manufacturing and for services used by healthcare provider organizations for the clinical analysis, management, storage, distribution and visualization of digital medical images and corresponding data. |
| |
| | • | During May 2005, we announced enhancements to our advanced visualization software allowing radiologists and treating physicians to better use volume rendering and 3D navigation in one native, integrated, open-standards package. This improvement means physicians can utilize both 2D and 3D tools without having to resort to third-party software and proprietary hardware to analyze medical images — offering time-savings and increased productivity. |
| |
| | • | During September 2005, we received certification under the Canadian Medical Device Conformity Assessment System recognizing our ISO 13485:2003 status in Canada. We also received our medical device license from Health Canada to allow us to market our products in Canada. We believe that there may be a strong opportunity for business with multi-facility providers throughout the Canadian marketplace. |
| |
| | • | During November 2005, we entered into a contract with Meridian Health, a leading integrated delivery system serving central New Jersey. The agreement with Meridian Health represented our first combined sale of the Emageon Enterprise Visual Medical System with the full suite of Camtronics’ cardiology imaging products. |
| |
| | • | During November 2005, we entered into a contract with The Johns Hopkins Hospital, a world-renowned medical center headquartered in Baltimore, Maryland. |
| |
| | • | Also during November 2005, we entered into an agreement with Orthocrat Ltd. to distribute TraumaCad, Orthocrat’s orthopedic surgical planning application. This agreement allows us to further extend our enterprise vision by offering additional leading edge tools to orthopedic specialists in our customer base. |
30
Change in Financial Position
As noted above, we completed our initial public offering during February 2005. The following significant changes in our financial position occurred as a result of the completion of the initial public offering:
| | |
| | • | We issued 10,843,411 shares of common stock upon the automatic conversion of outstanding shares of preferred stock into shares of common stock. |
| |
| | • | We sold 5,750,000 shares of common stock in connection with the public offering. |
| |
| | • | We issued 537,082 shares of common stock upon exercise of mandatorily redeemable warrants. |
| |
| | • | We received total cash proceeds (net of underwriting discount and offering expenses) of $67.2 million. |
| |
| | • | With a portion of the proceeds, we repaid $4.0 million of our subordinated debt. We also recorded a non-cash interest charge of $0.6 million for the write-off of the debt discount related to the subordinated debt. |
| |
| | • | We invested the remaining proceeds in cash equivalents and short-term marketable securities. |
| |
| | • | In November 2005, we utilized $40 million of the cash proceeds to purchase Camtronics Medical Systems, Ltd. |
As of December 31, 2005, we had 20,628,913 shares of common stock issued, 20,453,156 shares of common stock outstanding, and warrants to purchase 48,986 shares of our common stock outstanding at exercise prices ranging from $3.63 to $5.52 per share.
Sources of Revenue
A typical sale of our solution is comprised of system sales and support services. Revenue from system sales is derived from the licensing of our Advanced Visualization, Clinical Content Management, and Clinical Workflow (collectively referred to as our Enterprise Visual Medical System, or EVMS) and our HeartSuite software products, as well as from sales and integration of third-party components that are required to implement our solution. Support services revenue is derived from fees related to the implementation, training and on-going customer support of our solution.
Our software is comprised of four main components: Advanced Visualization, our suite of software tools for the advanced visualization and analysis of digital medical images; Clinical Content Management, our image archival and distribution management software; Clinical Workflow, our standards-based software used to manage integration and data migration between our solution and other health information systems throughout the enterprise; and HeartSuite, our suite of software tools focused on the cardiology department. Although Clinical Content Management and HeartSuite software products are available collectively as stand-alone applications, we offer our software primarily as an integrated enterprise-level image management solution. License pricing for Advanced Visualization is primarily determined by either number of licenses or number of concurrent users. License pricing for Clinical Content Management and Clinical Workflow is determined based on projected volume and size of image studies to be stored or migrated by the particular customer. License pricing for HeartSuite software products is determined based on the number of workstations purchased. We offer customers our software as perpetual or term licenses, in either case with maintenance and support relating to the software. Term licenses for our software are typically from two to ten years with annual renewals after the initial term. The sale and integration of third-party components typically include servers, data storage, backup and recovery systems, workstations and monitors, database software and computed radiography devices as well as orthopedic templates and dictation systems.
We also derive revenue from the provision of support services, including implementation, project planning, management, design and training services. Our customers typically contract for these support services pursuant to their initial agreements with us. The initial term of these support services under these agreements range from one to ten years, with a typical duration of five years. Upon expiration of the initial term, these agreements typically renew automatically fromyear-to-year thereafter until terminated.
31
Because our solutions represent capital expenditures involving multi-year commitments, it can take a significant period of time to close a sale. Our EVMS solution has a typical sales cycle, from the initial contact to the placing of an order, of six to nine months, and sometimes much longer.
Ascension Health, the largestnot-for-profit hospital system in the United States, is our largest customer. Revenue associated with facilities controlled by Ascension Health accounted for approximately 36% of our total revenue during 2005 and approximately 33% of our total contracted backlog at December 31, 2005. We anticipate that Ascension Health will continue to be a significant customer as we sign order addenda and contracts with additional Ascension Health facilities. In 2003, 2004 and 2005, we had two, one and one customer, respectively, who each accounted for more than 10% of our total revenue.
Cost of Revenue
The cost of our solution is comprised of two elements: the cost of our system sales and the cost of our support services. The cost of system sales consists of the cost of third-party components and the cost of software licenses. The cost of our third-party components consists primarily of direct and indirect expenses related to the purchase, manufacturing, shipment, installation and configuration of our solution. The cost of our software licenses consists primarily of the amortization of acquired software, the amortization of capitalized software costs for internally developed software, and royalties paid for a component of our Clinical Content Management software.
The cost of our support services consists primarily of labor costs and overhead relating to the implementation, installation, training, application support and maintenance of our solution as well as costs related to maintenance of third-party components. The cost of support services revenue varies based upon the productivity of our support services organization as well as costs associated with the use of outside contractors to support internal resources.
We allocate overhead expenses such as rent and occupancy charges and employee benefit costs to all departments based on headcount. As such, general overhead expenses are reflected in cost of support services, as well as in the research and development, sales and marketing and general and administrative expense categories.
We currently own and lease to certain customers, under operating leases, third-party components with a net book value of approximately $3.4 million at December 31, 2005. These components relate to five customer installations completed in 2001 and 2002. We are depreciating these third-party components to cost of support services revenue over the life of the respective contracts. For the years ended December 31, 2003, 2004 and 2005, depreciation related to these third-party components of $2.7 million, $3.4 million and $2.4 million, respectively, was included in cost of support services. The majority of the contracts will expire by the third quarter of 2007. We have not entered into any agreements requiring us to lease third-party components to customers since early 2002 and do not expect to do so in the future. We anticipate that several of these customers will upgrade to our Advanced Visualization software from the existing third-party visualization components that we lease to them.
Gross Profit
Our overall gross profit has improved due to an increase in software and recurring support services revenue derived from our growing installed base of customers. We expect this trend to continue as our installed customer base continues to grow. The gross profit from system sales varies based on several factors, including:
| | |
| | • | actual sales prices negotiated in the contracting process; |
| |
| | • | amount of amortization of acquired software and internally developed capitalized software; |
| |
| | • | costs associated with purchasing and manufacturing third-party components; |
| |
| | • | fluctuations in prices received from third-party component manufacturers and distributors relative to themark-up percentages provided for in customer contracts. |
32
The gross profit from sales of our support services varies based on several factors, including:
| | |
| | • | actual services fees negotiated during the contracting process; |
| |
| | • | productivity of our professional service team; |
| |
| | • | costs of service agreements related to third-party components included in our solution; and |
| |
| | • | costs associated with the use of outside contractors. |
Operating Expenses
Research and Development. Research and development expenses consist primarily of employee-related expenses, allocated overhead, and outside contractors. We have historically focused our research and development efforts on improving the functionality, performance, and integration of our software products. We expect that research and development expenses will increase as we strive to introduce additional products and services.
Sales and Marketing. Sales and marketing expenses consist primarily of employee-related expenses, including travel, marketing programs (which include trade shows, workshops and seminars, corporate communications, other brand building activities, and advertising), allocated overhead and sales commissions. We expect that sales and marketing expenses will increase as we expand our selling and marketing activities associated with existing and new product and service offerings to existing and new customers, build brand awareness and sponsor additional marketing events.
General and Administrative. General and administrative expenses consist primarily of employee-related expenses, professional fees, other corporate expenses and allocated overhead. We expect that general and administrative expenses will increase as we add personnel and incur additional professional fees and insurance costs related to the growth of our business and operations, including additional compliance costs in connection with public company financial reporting requirements.
Depreciation. We depreciate the costs of our tangible capital assets, primarily consisting of building, machinery and equipment, computers and software, leasehold improvements and furniture, on a straight-line basis over the estimated economic life of the asset, which is generally three to 39 years.
Stock-Based Expenses. Our operating expenses and interest expense include the effects of stock-based expenses related to the fair value of common stock options and warrants issued to non-employees and stock option grants to employees in situations where the exercise price was determined to be less than the deemed fair value of our common stock at the date of grant. Ordinarily, we do not issue options with an exercise price below the then-current fair market value. However, certain options awarded prior to the completion of our initial public offering were determined to be below the appropriate fair market value at the time of issuance.
Critical Accounting Policies and Estimates
Our consolidated financial statements are prepared in accordance with accounting principles generally accepted in the United States. The preparation of these consolidated financial statements requires us to make estimates and assumptions that affect the reported amounts of assets, liabilities, revenue, costs and expenses and related disclosures. On an ongoing basis, we evaluate our estimates and assumptions. Our actual results may differ from these estimates.
We believe that, of our significant accounting policies, which are described in Note 2 of the notes to our consolidated financial statements, the following accounting policies involve the greater degree of judgment and complexity. Accordingly, these are the policies we believe are the most critical to aid in fully understanding and evaluating our consolidated financial condition and results of operations.
Revenue Recognition and Deferred Revenue. We derive revenue from two primary sources: (1) system sales, which includes software license revenue and third-party component sales, and (2) support services, which includes fees related to implementation, training, software maintenance, ongoing customer support and third-party component maintenance. While the basis for software license revenue recognition is substantially
33
governed by the provisions of AICPA Statement of Position 97-2,(“SOP 97-2”),Software Revenue Recognition, as amended, in the application of this standard, we exercise judgment and use estimates in connection with the determination of the amount of system sales and support services revenue to be recognized in each accounting period.
We sell software under three types of licenses:
(1) Perpetual licenses: software licensed on a perpetual basis to a customer based on a fixed number of users and/or estimates of annual study volumes with no right to return the licensed software.
(2) Enterprise licenses: software licensed on a perpetual basis to a customer (typically a multi-facility health care provider), as opposed to licensing based on a fixed number of users or on estimates of annual study volumes, with no right to return the licensed software.
(3) Term licenses: software licensed on a term basis according to a fixed number of users and/or estimates of annual study volumes.
Generally, our software license arrangements do not include significant modification or customization of the underlying software and, as a result, we recognize license revenue when: (1) persuasive evidence of an arrangement exists; (2) delivery has occurred; (3) customer payment is deemed fixed or determinable and (4) collection is probable. We assess each of the four criteria as follows:
| | |
| | • | Persuasive evidence of an arrangement exists: It is our customary practice to have a written contract, which is signed by both the customer and us, or a purchase order from those customers that have previously negotiated a standard end-user license arrangement, prior to recognizing revenue on an arrangement. |
| |
| | • | Delivery has occurred: It is our customary practice to obtain acceptance for our software, which is evidenced by written customer acknowledgement. In the event that we grant a customer the right to specified upgrades, we defer recognition of the entire arrangement fee until we deliver the specified upgrades as we have not established vendor-specific objective evidence (VSOE) of fair value for specified upgrades. Specified upgrades include, but are not limited to, future software deliverables that are stated in the customer contract. |
| |
| | • | The customer’s payment is deemed fixed or determinable: We assess whether fees are fixed or determinable and free of contingencies or significant uncertainties at the time of sale and recognize revenue when all other revenue recognition requirements are met. While our standard payment terms are net 30 to 45 days, we have, on a few occasions, extended payment terms beyond 45 days (but none greater than six months) to creditworthy customers. We have established a successful history of collection, without concessions, on these receivables, therefore satisfying the required criteria for revenue recognition. If the fee is determined not to be fixed or determinable, we recognize revenue as the amounts become due and payable. |
| |
| | • | Collection is probable: Likelihood of collection is assessed on acustomer-by-customer basis. Both new and existing customers are subjected to a credit review that evaluates such customer’s financial position and ultimately their ability to pay. For follow-on sales to existing customers, prior payment history is also used to evaluate probability of collection. If it is determined from the outset of the arrangement that collection is not probable based upon our credit review process, revenue is recognized on a cash-collected basis if all other criteria are met. |
We account for software license and non-recurring support services revenue included in multiple element arrangements using the residual method. Under the residual method, the fair value of the undelivered elements (i.e., software maintenance and ongoing support services) based on VSOE of fair value is deferred and the remaining portion of the arrangement fee is allocated to the delivered elements (i.e., software license and non-recurring support services). If evidence of the fair value of one or more of the undelivered services does not exist, revenue is deferred and recognized when delivery of those services occurs or fair value can be established. We determine VSOE of fair value for ongoing support services revenue based upon the renewal rates for the maintenance and ongoing support, which coincide with our pricing model. Significant incremental
34
discounts offered in multiple element arrangements that would be characterized as separate elements are infrequent and are allocated to software license revenue under the residual method.
For term license arrangements, we recognize revenue for the multiple element arrangement over the term of the arrangement beginning in the month after we receive customer acceptance, provided that the other revenue recognition criteria have been met.
Software maintenance services generally include rights to upgrades (when and if available), telephone support, updates and bug fixes. Software maintenance revenue is recognized ratably over the term of the maintenance contract on a straight-line basis when all the revenue recognition requirements are met. We include the first year of software maintenance in the software license fee. We defer this software maintenance fee based on its fair value and recognize it ratably over the first year of the arrangement.
Ongoing support services generally include telephone support related to third-party components as well as quarterly customer metric reporting and other services. Ongoing support service revenue is recognized ratably over the term of the ongoing support services contract on a straight-line basis when all the revenue recognition requirements are met. As it relates to services, the Company may also provide services that vary depending on the scope and complexity requested by the customer. Examples of such services include additional database consulting, system configuration, existing systems interface, and network consulting. These services generally are not deemed to be essential to the functionality of the software. If the Company has VSOE of fair value for the services, the timing of the software license revenue is not impacted, and service revenue is recognized as the services are performed. The Company commonly performs services for which the Company does not have VSOE of fair value; accordingly, the software license revenue is deferred until the services are completed.
The Company recognizes revenue from product sales in accordance with SEC Staff Accounting Bulletin No, 104,Revenue Recognition in Financial Statements. Revenue related to product sales is recognized upon shipment provided that title and risk of loss have passed to the customer, there is persuasive evidence of an arrangement, the sales price is fixed and determinable, collection of the related receivable is reasonably assured and customer acceptance criteria, if any, have been successfully demonstrated. The Company classifies shipping and handling cost in cost of system sales.
When our contracts contain both software and third-party components, we recognize revenue related to third-party components that are stated separately in our contracts according to guidance set forth in Emerging Issues Task Force IssueNo. 00-21,“Accounting for Revenue Arrangements with Multiple Deliverables”(“EITF 00-21”). Third-party component revenue, including hardware sales and hardware maintenance, is recognized in accordance with contractual terms. When we are responsible for installing the third-party components, revenue is recognized when the third-party components are delivered, installed and accepted by the customer. When we are not responsible for installing the third-party components, revenue is recognized when the third-party components are delivered to the customer. We qualify to recognize hardware sales and hardware maintenance underEITF 00-21 as a result of the following factors: 1) our software is not essential to the functionality of the hardware, 2) our customers have the ability to purchase the hardware from other vendors and 3) the purchase price of the hardware and hardware maintenance is separately stated in our contracts. When third-party components and related maintenance are not separately priced in our contracts, we recognize revenue related to the arrangement when all revenue recognition criteria have been met.
The following is a summary of our product warranty and guarantee and our related accounting policies for these agreements:
(1) Our sales agreements with customers generally contain infringement indemnity provisions. Under these agreements, we agree to indemnify, defend and hold harmless the customer in connection with patent, copyright or trade secret infringement claims made by third parties with respect to the customer’s authorized use of our products and services. The indemnity provisions generally provide for our control of any required defense and settlement and cover costs and damages finally awarded against the customer, if any. Our infringement indemnity provisions typically give us the option to make modifications of the product so it is no longer infringing or, if it cannot be corrected, to require the customer to return the product in exchange for a specified payment for loss of use. Our sales agreements with customers
35
sometimes also contain indemnity provisions for death, personal injury or property damage caused by our personnel or contractors in the course of performing services to customers. Under these agreements, we agree to indemnify, defend and hold harmless the customer in connection with death, personal injury and property damage claims made by third parties with respect to actions of our personnel or contractors. The indemnity provisions generally provide for our control of any required defense and settlement and cover costs and damages finally awarded against the customer, if any. The indemnity obligations contained in sales agreements generally have no specified expiration date but typically limit the amount of award covered to a portion of the fees paid by the customer over a portion of the contract term. We have not previously incurred costs to settle claims or pay awards under these indemnification provisions. Accordingly, we have no liabilities recorded for these provisions as of December 31, 2005.
(2) We warrant that our software products will perform in all material respects in accordance with our standard published specifications in effect at the time of delivery of the licensed products to the customer as long as the contract remains in effect. Additionally, we warrant that our services will be performed by qualified personnel in a manner consistent with normally accepted industry standards. We provide for the estimated cost of product and service warranties based on specific warranty claims and claim history. As of December 31, 2005 we have $0.9 million of liabilities recorded for these agreements.
(3) Our standard contracts with customers typically provide for a 99% guarantee of system availability and a 98% guarantee of component availability, with penalty provisions if our solution fails to meet the guarantees. Our 99% system availability guarantee covers our solution as a whole, while the component guarantee covers each individual component, as in certain circumstances a component may fail without affecting system availability. The penalty provisions in our contracts typically allow the customer a reduction in software maintenance fees related to failure to meet guaranteed “uptime” percentages. We calculate these penalties as a percentage of the software maintenance fee and would reduce the amount of the software maintenance fee charged in a specific period for these penalties, if incurred. To date, we have not incurred any penalties associated with these guarantees. Accordingly, we have no liabilities recorded for these agreements as of December 31, 2005.
Billings may not coincide with the recognition of revenue. Unbilled revenue, which is included in accounts receivable in the consolidated balance sheet, occurs when revenue recognition precedes billing to the customer, and arises primarily from sales with predetermined billing schedules. Billings in excess of sales (deferred revenue) occur when billing to the customer precedes revenue recognition, and arise primarily from sales with partial prepayments upon contract execution and from maintenance revenue billed in advance of performance of the maintenance activity. The Company recognizes deferred revenue, as applicable, upon delivery and acceptance of products, as ongoing services are rendered or as other requirements requiring deferral underSOP 97-2 are satisfied. Costs related to deferred revenue are included as an asset in the Company’s consolidated balance sheet and charged to expense when the related deferred revenue is recognized.
The timing of customer acceptances could significantly affect our results of operations during a given period. As noted above, we require written acknowledgement from the customer to evidence that delivery of the products or services has occurred. Delays in the implementation process could negatively affect operations in a given period by increasing volatility in revenue recognition.
Research and Development Costs. Research and development costs are charged to expense as incurred. However, costs incurred for the development of software that will be sold, leased or otherwise marketed are capitalized as incurred after technological feasibility has been established and capitalization ceases when the software is generally available for release. Judgment is involved in determining when technological feasibility is reached. We believe that technological feasibility is reached when we have completed a working model that is ready to be beta-tested at a customer site. These capitalized costs are subject to an ongoing assessment of recoverability based on anticipated future revenue and changes in technologies. Costs deemed not recoverable are charged to expense. Costs that are capitalized primarily consist of direct labor.
36
Amortization of capitalized software development costs begins when the product is available for general release. Amortization is provided on aproduct-by-product basis using the straight-line method over periods not exceeding three years.
Intangible and Other Long-Lived Assets. U.S. generally accepted accounting principles require the purchase method of accounting for all business combinations after June 30, 2001, and that certain acquired intangible assets in a business combination be recognized as assets separate from goodwill. Accordingly, we identified and allocated values to the intangibles based on discounted cash flow analyses and market research, as well as our judgment. Intangibles determined to have an indefinite life are not amortized but are tested for impairment at least annually. We will evaluate intangible assets for impairment on an annual basis and also when impairment indicators are identified. In assessing the recoverability of intangibles, we must make assumptions regarding estimated future cash flows and other factors to determine the fair value of the respective assets. These estimates include forecasted revenue, which is inherently difficult to predict. If these estimates or their related assumptions change in the future, we may be required to record impairment charges for these assets. Historically, intangible assets are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. Property, equipment and intangible assets are amortized over their useful lives. Useful lives of the intangible assets are based on management’s estimates of the periods over which such assets will generate revenue.
Results of Operations
Revenue
Revenue consists of system sales and support services revenue. System sales revenue is comprised of revenue from sales of our software and third party components. Support services revenue is comprised of revenue from up-front professional services such as implementation, adoption and training, as well as ongoing maintenance services. The following table sets forth revenue component data.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Year Ended December 31, | | | Year Ended December 31, | |
| | | 2005 | | | 2004 | | | Change | | | Change (%) | | | 2004 | | | 2003 | | | Change | | | Change (%) | |
| | | (In thousands except percentages) | | | (In thousands except percentages) | |
| |
| System sales | | $ | 50,041 | | | $ | 33,441 | | | $ | 16,600 | | | | 49.6 | % | | $ | 33,441 | | | $ | 17,234 | | | $ | 16,207 | | | | 94.0 | % |
| Percentage of total revenue | | | 67.8 | % | | | 73.0 | % | | | | | | | | | | | 73.0 | % | | | 74.0 | % | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Support services | | $ | 23,750 | | | $ | 12,361 | | | $ | 11,389 | | | | 92.1 | % | | $ | 12,361 | | | $ | 6,057 | | | $ | 6,304 | | | | 104.1 | % |
| Percentage of total revenue | | | 32.2 | % | | | 27.0 | % | | | | | | | | | | | 27.0 | % | | | 26.0 | % | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Total revenue | | $ | 73,791 | | | $ | 45,802 | | | $ | 27,989 | | | | 61.1 | % | | $ | 45,802 | | | $ | 23,291 | | | $ | 22,511 | | | | 96.7 | % |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
The increase in system sales revenue from 2004 to 2005 was attributable to an increase in the number of new and existing customer installations offset by a decrease in the size of these installations, as well as the recognition of $4.5 million of previously deferred revenue. During 2005, we had more acceptances of our software and third party components than in 2004. The average revenue recognized per acceptance (excluding outliers, such as unusually small or large dollar amounts) decreased slightly in 2005 as compared to 2004. As our pricing has not changed significantly from the prior year, the decrease is primarily related to an increase in add-on sales to our existing customer base, which tend to be smaller than initial sales to new customers. Also, during 2005, we recognized system sales revenue of $4.5 million related to two contracts for which we deferred revenue in 2004 as a result of the existence of certain undelivered upgrades. We delivered the additional software features during the second and fourth quarters of 2005 and, as a result of all revenue recognition criteria being met, we recognized the system sales revenue associated with these contracts.
The increase in system sales revenue from 2003 to 2004 was attributable to more health care institutions buying and installing our solution, an increase in average contract value and a shift in our sales from primarily term licenses, in which revenue is recognized ratably over the multiple years covered by the licenses, to primarily perpetual licenses, in which revenue for the license fee is recognized at system acceptance assuming all applicable revenue recognition criteria have been satisfied.
37
The increase in support services revenue from 2004 to 2005 was attributable to an increase in customer installations. Approximately $5.7 million of the increase in support services revenue for 2005 was attributable to an increased number of customers that have implemented our solution and are paying us ongoing support and maintenance fees. Also included in this $5.7 million increase is the recognition of $1.6 million of revenue related to the contracts mentioned above for which we deferred revenue in 2004 as a result of the existence of undelivered upgrades. We delivered the additional software features during the second and fourth quarters of 2005 and, as a result of all revenue recognition criteria being met, we recognized the support services revenue attributable to the previously provided services for which we had deferred support services revenue. The remaining $5.7 million increase was related to the increase in the recognition of non-recurring revenue related to services such as implementation and training for new customers as well as add-on services for existing customers.
The increase in support services revenue from 2003 to 2004 was attributable to an increase in customer installations. Approximately $5.3 million of the increase in support services revenue for 2004, was attributable to an increased number of customers that have implemented our solution and are paying us ongoing support and maintenance fees. The remaining $1.0 million increase was related to the increase in the recognition of non-recurring revenue related to services such as implementation and training for new customers as well as add-on services for existing customers.
In general, the increased number of customer installations was a result of increased customer awareness and acceptance of our products and services with multi-facility healthcare providers. Of the software acceptances received during 2004 and 2005, 84% and 92%, respectively, related to agreements with multi-facility healthcare providers. We expect revenue to continue to increase as we recognize revenue from our existing long-term customer agreements while also recognizing revenue related to new customer agreements.
We also believe that support services as a percentage of total revenue will continue to increase as compared to prior periods as our customer base expands.
Cost of Revenue
Cost of revenue consists of costs associated with system sales and support services revenue. System sales cost of revenue is comprised of the cost of third-party components and the cost of software licenses. Support services cost of revenue is comprised of labor costs and related overhead relating to the implementation, installation, training, application support and maintenance of our solution as well as costs related to maintenance of third-party components. The following table sets forth cost of revenue component data.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Year Ended December 31, | | | Year Ended December 31, | |
| | | 2005 | | | 2004 | | | Change | | | Change (%) | | | 2004 | | | 2003 | | | Change | | | Change (%) | |
| | | (In thousands except percentages) | | | (In thousands except percentages) | |
| |
| Cost of system sales revenue | | $ | 28,316 | | | $ | 21,452 | | | $ | 6,864 | | | | 32.0 | % | | $ | 21,452 | | | $ | 10,227 | | | $ | 11,225 | | | | 109.8 | % |
| Percentage of system sales revenue | | | 56.6 | % | | | 64.1 | % | | | | | | | | | | | 64.1 | % | | | 59.3 | % | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Cost of support services revenue | | $ | 14,648 | | | $ | 10,727 | | | $ | 3,921 | | | | 36.6 | % | | $ | 10,728 | | | $ | 7,493 | | | $ | 3,235 | | | | 43.2 | % |
| Percentage of support services revenue | | | 61.7 | % | | | 86.8 | % | | | | | | | | | | | 86.8 | % | | | 123.7 | % | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Total cost of revenue | | $ | 42,964 | | | $ | 32,179 | | | $ | 10,785 | | | | 33.5 | % | | $ | 32,180 | | | $ | 17,720 | | | $ | 14,460 | | | | 81.6 | % |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Percentage of total revenue | | | 58.2 | % | | | 70.3 | % | | | | | | | | | | | 70.3 | % | | | 76.1 | % | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
The increase in total cost of revenue was attributable to increased purchases of third-party components and, to a lesser extent, increased labor costs, as a result of the increased number and size of new customer installations. The decrease in cost of revenue as a percentage of total revenue was a result of the increase in the number of software acceptances received for which we recognized revenue as compared to the
38
corresponding prior periods. Our costs associated with software licenses as a percentage of total revenue are significantly lower than costs associated with other components of our revenue.
For the years ended December 31, 2005 and 2004, cost of system sales increased by 32.0% and 109.8%, respectively, as compared to 2004 and 2003. These increases were caused by the increased number of health care institutions that acquired and installed our solution. We anticipate that our cost of revenue will continue to increase in absolute dollars as a result of additional purchases of third-party components related to customer installations, which purchases are in turn driven by our increase in customers. Cost of system sales as a percentage of system sales revenue decreased in 2005 and 2004 as compared to 2004 and 2003. A portion of the decrease in 2005 is attributable to the recognition of $4.5 million of previously deferred revenue during the second and fourth quarters of 2005. As a result of the timing of the revenue recognition, there were minimal costs associated with this revenue when it was recognized.
For the years ended December 31, 2005 and 2004, cost of support services increased by 36.6% and 43.2%, respectively, as compared to 2004 and 2003. These increases were caused by an increase in staffing levels in our support services teams as well as increased costs to maintain third-party components due to an increase in volume. Cost of support services as a percentage of support services revenue decreased in 2005 and 2004 presented above as compared to 2004 and 2003. The decrease in cost of support services as a percentage of total support services revenue was a result of efficiencies realized as our customer base grew and the cost of support services was spread over the broader base of customers. Our management team has focused on ensuring the professional services and support departments achieve the benefits associated with efficiencies of scale as a result of our increased customer base. These initiatives include departmental reorganizations, investments in software technologies, use of external consultants to gauge best practices, and use of more cost effective resources.
Gross Margin Percentage
Our gross margin percentage increased from 23.9% of total revenue for 2003 to 29.7% of total revenue for 2004 to 41.8% of total revenue for 2005. These improvements were primarily a result of increased revenue attributable to software license acceptances (earning higher margins than other components of our revenue) as well as efficiencies realized as our customer base has expanded.
Research and Development (R&D)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Year Ended December 31, | | | Year Ended December 31, | |
| | | 2005 | | | 2004 | | | Change | | | Change (%) | | | 2004 | | | 2003 | | | Change | | | Change (%) | |
| | | (In thousands except percentages) | | | (In thousands except percentages) | |
| |
| R&D Expense | | $ | 10,697 | | | $ | 6,021 | | | $ | 4,676 | | | | 77.7 | % | | $ | 6,021 | | | $ | 4,143 | | | $ | 1,878 | | | | 45.3 | % |
| % of Revenue | | | 14.5 | % | | | 13.1 | % | | | | | | | | | | | 13.1 | % | | | 17.8 | % | | | | | | | | |
The increases in research and development expense for the twelve months ended December 31, 2005 and 2004 as compared to corresponding periods in 2004 and 2003, respectively, are mainly attributable to increases in personnel, both through internal growth and growth through acquisitions of other companies. During the twelve months ended December 31, 2005, personnel and related expenses accounted for $3.1 million of the expense growth compared to the corresponding period in 2004. In addition, Camtronics, which we acquired November 1, 2005, contributed $0.8 million of personnel expense during the two months included in 2005 consolidated expenditures. An increase of $1.7 million in research and development cost for the twelve months ended December 31, 2004 from the corresponding period in 2003 is attributable to personnel increases throughout 2004 along with the inclusion of a full year of expense from personnel added through the Ultravisual merger in May 2003. Headcount has increased from 57 employees at December 31, 2004 to 150 employees at December 31, 2005, including the employees added through the Camtronics acquisition. Along with the integration of the research and development teams, the department has reorganized into operating divisions that we believe will provide increased productivity and efficiency during the development phase of a software release and lead to more effective product roadmaps.
39
Increased spending for outside consultants accounted for approximately $0.8 million and $0.2 million of the increases for the twelve months ended December 31, 2005 and December 31, 2004, respectively. A portion of the spending has been focused on enhancing our use of low-cost consultants that are engaged in quality development and service activities in support of an expanding R&D employee base. The remaining spending has been targeted on consultants and developers that assist our personnel in product quality assurance and validation, system framework implementations, and internal network administration.
Investments have been made to develop testing programs and aid development of software releases. Significant expenditures have been made to purchase engineering laboratory equipment and expand our laboratory and testing environments. The additional expenditures are reflected in a growing asset base and increased depreciation expense included in the General and Administrative line item.
Sales and Marketing (S&M)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Year Ended December 31, | | | Year Ended December 31, | |
| | | 2005 | | | 2004 | | | Change | | | Change (%) | | | 2004 | | | 2003 | | | Change | | | Change (%) | |
| | | (In thousands except percentages) | | | (In thousands except percentages) | |
| |
| S&M Expense | | $ | 11,830 | | | $ | 9,027 | | | $ | 2,803 | | | | 31.1 | % | | $ | 9,027 | | | $ | 6,144 | | | $ | 2,883 | | | | 46.9 | % |
| % of Revenue | | | 16.0 | % | | | 19.7 | % | | | | | | | | | | | 19.7 | % | | | 26.4 | % | | | | | | | | |
Continued growth in sales and marketing expense for each of the last two years demonstrates our commitment to extend our product base to current and prospective customers through sales efforts, targeted marketing, and acquisitions. The decrease in sales and marketing expense as a percentage of revenue from 26.4% in 2003 to 16.0% in 2005 is evidence of the efficient use of these expenditures and the leveraging of our sales and marketing efforts in the marketplace.
The additional expenditures have mainly been focused on additional headcount in both direct sales and sales and marketing support teams. Sales and marketing headcount increased to 74 employees at December 31, 2005, from 34 employees at December 31, 2004 and 29 employees at December 31, 2003. Through the Camtronics acquisition, we added ten direct sales representatives focused on selling cardiology products to existing and new customers. The remainder of the additional personnel have augmented our client sales group and marketing support group. The client sales group primarily focuses on support and development of current relationships with large hospitals and hospital networks. The additional increases in marketing support have been focused on expanding the product demonstration team and product marketing teams. The product demonstration team has also been equipped with new tools and technology that have created enhanced avenues for potential customers to evaluate our visualization technology. Accompanying the increased headcount are increased expenditures related to training, communications and technology that have increased the effectiveness and productivity of our sales and marketing personnel. Additional personnel expenses that include associated costs such as travel, training, and communications, but exclude commissions, have added $3.0 million in expenses to the twelve months ended December 31, 2005 compared to 2004 and $1.4 million to the twelve months ended December 31, 2004 compared to 2003.
Commission expense increased $1.1 million from the twelve month period ended December 31, 2003 to the twelve month period ended December 31, 2004. The increase is attributable to the increase in contract signings in 2004. Even though commission expense increased in absolute terms, the decrease in sales and marketing expense as a percentage of revenue noted earlier is the result of a change in our sales commission plans. In late 2003, we changed our sales commission plans so that a portion of the commission payment is linked to customer acceptance. In addition, in 2004 we changed our commission plans so that commission expense is recognized over the periods the payments are earned rather than in the period the payments are paid. Both of these revisions had the effect of delaying payments and expense to future periods.
For the twelve month period ended December 31, 2005 commissions remained relatively stable with the comparable period in 2004. Stability in commission expense even though both contract sales and personnel expense increased is primarily related to the timing and type of contracts closed and accepted during the respective periods. Commission expense for the year ended December 31, 2004 reflected greater than usual commission expense related to the execution of master contracts for large network customers. The twelve
40
months ended December 31, 2005 reflects reduced average payments and expense of commissions related to the execution of contract addenda in association with existing customer master contracts. Commission payments related to the execution of master contract addenda are lower than the payments for new customer contracts, as commissions related to the original master contracts have already been paid or are being paid and expensed on a monthly basis over the period of the master contract initial commitment. Commissions for new contract addenda that are included in the master contract are typically paid based on contract revenue above and beyond the levels in the master contract for that particular site; therefore, although the new contract addenda related to these master contracts represent major new installations, the incremental commission expense associated with these addenda is less than the corresponding level for a similarly sized new contract. In addition, as described earlier, the majority of new hires have filled primarily support roles that have compensation plans with lower commission levels than direct sales personnel. Thus, we have seen significant increases in personnel expenses without significant increases in commission expense.
Direct marketing expense increased during the twelve months ended December 31, 2004 compared to the same period in 2003 by $0.4 million. The increase is related to expenditures targeted to increase awareness of our products in the marketplace. Conversely, direct marketing expense decreased during the twelve months ended December 31, 2005 compared to the same period in 2004 by $0.2 million. The decrease in direct marketing expenses reflects a focused attempt to grow our market presence with a more efficient use of expenditures. We have directed funds towards industry trade shows that provide opportunities for us to display our product to a wider, more knowledgeable customer base through the utilization ofstate-of-the-art booths and exhibits. We have also internally developed and published a periodic industry magazine that promotes industry specific topics while providing brand name awareness.
We believe that all of the efforts discussed above have augmented our reputation in the marketplace and should help deliver continued growth in the future. We expect to increase our sales and marketing expenses at a steady, controlled pace as we hire additional sales and marketing personnel and focus on increasing market awareness of our products and service offerings.
General and Administrative (G&A)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Year Ended December 31, | | | Year Ended December 31, | |
| | | 2005 | | | 2004 | | | Change | | | Change (%) | | | 2004 | | | 2003 | | | Change | | | Change (%) | |
| | | (In thousands except percentages) | | | (In thousands except percentages) | |
| |
| G&A Expense | | $ | 12,308 | | | $ | 8,024 | | | $ | 4,284 | | | | 53.4 | % | | $ | 8,024 | | | $ | 5,793 | | | $ | 2,231 | | | | 38.5 | % |
| % of Revenue | | | 16.7 | % | | | 17.5 | % | | | | | | | | | | | 17.5 | % | | | 24.9 | % | | | | | | | | |
As expected, our general and administrative expense have grown in absolute terms from 2003 to 2004 to 2005. These additional expenditures are in support of our increasing employee base and are a natural result of the growth of our company as a whole. The majority of the increases can be broken down into the following categories:
| | |
| | • | Administrative expense has increased $1.9 million and $0.7 million for the twelve months ended December 31, 2005, and December 31, 2004, as compared to corresponding periods in 2004 and 2003, respectively. The increases occurred in insurance, accounting and legal fees, consultants, and other professional fees mainly as a result of our becoming a public company in 2005 and associated expenses incurred for compliance and regulatory affairs. |
| |
| | • | Depreciation expense has increased $1.1 million and $0.2 million for the twelve months ended December 31, 2005, and December 31, 2004, as compared to corresponding periods in 2004 and 2003, respectively, mainly as a result of increased purchases of employee computer equipment, server equipment for the internal engineering laboratory, and testing equipment. |
| |
| | • | Stock-based compensation has increased $0.7 million and $0.6 million for the twelve months ended December 31, 2005, and December 31, 2004, as compared to corresponding periods in 2004 and 2003, respectively. The majority of stock options with an intrinsic value were issued during 2004 and the beginning of 2005; thus we recorded minimal expense during the first nine months of 2004. Periodic stock-based compensation has remained consistent during 2005 as fewer options have been granted. |
41
| | |
| | • | Personnel expense has increased $0.6 million and $0.5 million for the twelve months ended December 31, 2005, and December 31, 2004, as compared to corresponding periods in 2004 and 2003. New employees have been added in the accounting, human resources and general administrative functions in support of our increased employee base and increased amount of administrative transactions and activity. In addition, associated costs such as travel, communications and rent have increased in conjunction with the growth in our employee base. |
Although our general and administrative expenses have increased as a result of becoming a public company, our general and administrative expenses as a percentage of revenue have decreased as a result of revenue growth. We expect our general and administrative expenses to continue to increase in absolute dollars as a result of becoming a public company and also as a result of our continued growth. Specifically, we expect to incur increased costs associated with accounting, consulting, legal and other professional services, increased insurance costs and increased personnel in our finance, legal and human resources functions. By way of example, although we have not previously been involved in any type of significant litigation, we recognize that most companies from time to time may be the subject of customer or other complaints, and that such complaints could result in litigation. In such a case, it is likely that our external legal expenses would increase.
Amortization and Write-Off of Intangible Assets related to Camtronics Acquisition
During 2005, we estimated that $0.2 million of the purchase price of Camtronics represented acquired in-process research and development (“IPR&D”) that had not yet reached technological feasibility as defined by SFAS No. 86,Accounting for the Cost of Computer Software to Be Sold, Leased or Otherwise Marketed, and had no alternative future use. Accordingly, these amounts were immediately charged to expense upon consummation of the acquisition. The value of the IPR&D was determined with the assistance of an independent third-party appraiser by utilizing a discounted cash flow methodology, focusing on the income-producing capabilities of the in-process technologies and taking into consideration stage of completion, complexity of work to date and to complete, anticipated product development and introduction schedules, forecasted product sales cycles, internal and external risk factors, revenue and operating expense estimates, contributory asset charges, and costs already incurred and the expected costs to complete. The remainder of the expense was related to straight-line amortization of intangible assets related to the acquisition of Camtronics, such as customer relationships and trade names, which are being amortized over periods from one to six years. The estimated asset lives of the amortizable intangible assets are determined based on projected future economic benefits and expected life cycles of the intangible assets.
Integration Costs Related to Camtronics Acquisition
We incurred integration costs of $0.2 million in 2005 as a result of the November 1 acquisition of Camtronics. Integration costs are comprised primarily of costs of transitional employees and other contractors as well as travel costs. We anticipate that integration costs related to this acquisition will result in additional charges in the first half of 2006.
Operating Income (Loss)
For the year ended December 31, 2005, operating loss decreased by $4.2 million as compared to the year ended December 31, 2004. Operating loss as a percentage of total revenue improved from a negative 20.6% for the year ended December 31, 2004 to a negative 7.1% for the year ended December 31, 2005. For the year ended December 31, 2004, operating loss decreased by $1.1 million as compared to the year ended December 31, 2003. Operating loss as a percentage of total revenue decreased from 45.1% for the year ended December 31, 2003 to 20.6% for the year ended December 31, 2004. These improvements are a result of the total revenue increase, increases in gross margin, and the increased leverage in operating costs discussed above.
42
Other Income and Expense
For the year ended December 31, 2005, interest income increased by $1.5 million over 2004. This increase is a result of investing the proceeds from our initial public offering.
For the year ended December 31, 2005, interest expense increased by $0.2 million as compared to 2004 primarily as a result of a non-cash interest charge of $0.6 million for the write-off of subordinated debt discount, offset by a reduction in interest expense from our repayment of $4.0 million of subordinated debt with a portion of the proceeds from our initial public offering.
Quarterly Results of Operations
The following tables set forth selected unaudited quarterly consolidated statement of operations data for the eight most recent quarters. The information for each of these quarters has been prepared on the same basis as the audited consolidated financial statements included in this filing and, in the opinion of management, includes all adjustments necessary for the fair presentation of the results of operations for such periods. This data should be read in conjunction with the audited consolidated financial statements and the related notes included in this filing. These quarterly operating results are not necessarily indicative of our operating results for any future period.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Quarter Ended | |
| | | March 31,
| | | June 30,
| | | September 30,
| | | December 31,
| | | March 31,
| | | June 30,
| | | September 30,
| | | December 31,
| |
| | | 2004 | | | 2004 | | | 2004 | | | 2004 | | | 2005 | | | 2005 | | | 2005 | | | 2005 | |
| | | (Dollars in thousands, except per share data) | |
| |
| Revenue: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| System sales | | $ | 4,909 | | | $ | 8,761 | | | $ | 7,458 | | | $ | 12,313 | | | $ | 7,719 | | | $ | 13,402 | | | $ | 13,490 | | | $ | 15,430 | |
| Support services | | | 2,208 | | | | 3,356 | | | | 2,864 | | | | 3,933 | | | | 3,617 | | | | 5,168 | | | | 6,116 | | | | 8,849 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Total revenue | | | 7,117 | | | | 12,117 | | | | 10,322 | | | | 16,246 | | | | 11,336 | | | | 18,570 | | | | 19,606 | | | | 24,279 | |
| Cost of revenue: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| System sales | | | 3,489 | | | | 4,117 | | | | 5,554 | | | | 8,292 | | | | 4,823 | | | | 5,753 | | | | 7,206 | | | | 10,534 | |
| Support services | | | 2,279 | | | | 2,414 | | | | 2,949 | | | | 3,086 | | | | 3,083 | | | | 3,322 | | | | 3,389 | | | | 4,854 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Total cost of revenue | | | 5,768 | | | | 6,531 | | | | 8,503 | | | | 11,378 | | | | 7,906 | | | | 9,075 | | | | 10,595 | | | | 15,388 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Gross profit | | | 1,349 | | | | 5,586 | | | | 1,819 | | | | 4,868 | | | | 3,430 | | | | 9,495 | | | | 9,011 | | | | 8,891 | |
| Operating expenses: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Research and development | | | 1,270 | | | | 1,303 | | | | 1,581 | | | | 1,867 | | | | 2,385 | | | | 2,533 | | | | 2,364 | | | | 3,415 | |
| Sales and marketing | | | 1,738 | | | | 2,407 | | | | 2,359 | | | | 2,523 | | | | 2,689 | | | | 2,521 | | | | 2,344 | | | | 4,276 | |
| General and administrative | | | 1,741 | | | | 1,832 | | | | 2,079 | | | | 2,372 | | | | 2,555 | | | | 2,876 | | | | 2,838 | | | | 4,039 | |
| Amortization and write-off of intangible assets related to Camtronics acquisition | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 993 | |
| Integration costs related to Camtronics acquisition | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 244 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Total operating expenses | | | 4,749 | | | | 5,542 | | | | 6,019 | | | | 6,762 | | | | 7,629 | | | | 7,930 | | | | 7,546 | | | | 12,967 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Operating income (loss) | | | (3,400 | ) | | | 44 | | | | (4,200 | ) | | | (1,894 | ) | | | (4,199 | ) | | | 1,565 | | | | 1,465 | | | | (4,076 | ) |
| Other expense, net | | | 192 | | | | 188 | | | | 333 | | | | 309 | | | | 619 | | | | (335 | ) | | | (366 | ) | | | (166 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Net income (loss) | | $ | (3,592 | ) | | $ | (144 | ) | | $ | (4,533 | ) | | $ | (2,203 | ) | | $ | (4,818 | ) | | $ | 1,900 | | | $ | 1,831 | | | $ | (3,910 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Net income (loss) per share — basic | | $ | (1.48 | ) | | $ | (0.06 | ) | | $ | (1.69 | ) | | $ | (0.82 | ) | | $ | (0.42 | ) | | $ | 0.09 | | | $ | 0.09 | | | $ | (0.19 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Net income (loss) per share — diluted | | $ | (1.48 | ) | | $ | (0.06 | ) | | $ | (1.69 | ) | | $ | (0.82 | ) | | $ | (0.42 | ) | | $ | 0.09 | | | $ | 0.09 | | | $ | (0.19 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Our operating results have fluctuated from quarter to quarter and are likely to continue to fluctuate for a variety of reasons. We discuss below some of the larger fluctuations in various line items in the table above.
43
Revenue. During the first quarter of each calendar year, the two components of our total revenue have declined from the immediately preceding quarter due to timing issues relating to our sales and installation process. This impact is particularly evident for our system sales revenue. In the past, we have experienced lower sales volumes in the third quarter of each year relative to other quarters. We believe that this is the result of the historical capital expenditure patterns of our customer base. This, in turn, has caused our revenue in the first quarter of the following year to be lower in comparison to the immediately preceding quarter due to the length of our installations and our revenue recognition policies.
Cost of Revenue. Our cost of revenue fluctuates from quarter to quarter as a result of changes in the relative contributions to our revenue from system sales and support services and changes in the productivity of support services personnel.
Operating Expenses. Our sales and marketing expenses fluctuate due to timing of sales. Also, the most significant trade show that we attend occurs within the fourth quarter of each year, increasing our sales and marketing expenses in that quarter.
Operating Income (Loss). Our quarterly operating results are likely to continue to fluctuate. Some important additional factors that could cause our revenue and operating results to fluctuate from quarter to quarter include:
| | |
| | • | our ability to retain and increase sales to existing customers, attract new customers and satisfy our customers’ requirements; |
| |
| | • | our ability to successfully integrate our acquisition of Camtronics; |
| |
| | • | length of the sales cycle for our solution; |
| |
| | • | implementation delays at customer sites, whether within or outside our control; |
| |
| | • | renewal rates for our solutions; |
| |
| | • | changes in our pricing policies; |
| |
| | • | new product introductions and product enhancements by us or our competitors; |
| |
| | • | effectiveness of our sales force; |
| |
| | • | buying and capital budgeting patterns of our customers; |
| |
| | • | our success in selling our solutions to large multi-facility health care providers; |
| |
| | • | technical difficulties or downtime in our solutions; |
| |
| | • | general economic conditions in the U.S.; |
| |
| | • | additional investments in our solutions or operations; and |
| |
| | • | regulatory compliance costs. |
Significant changes in the historical patterns of these factors or the occurrence of unforeseen events could cause our operating results to vary widely. As a result, we believe thatquarter-to-quarter comparisons of our revenue and operating results may not be meaningful and should not be relied upon as indications of future performance.
44
Liquidity and Capital Resources
As of December 31, 2005 and 2004, our net cash position was as follows (in thousands except ratios):
| | | | | | | | | |
| | | December 31 | |
| | | 2005 | | | 2004 | |
| |
| Working capital | | $ | 10,717 | | | $ | (9,404 | ) |
| Current ratio* | | | 1.2:1.0 | | | | 0.7:1.0 | |
| Cash, cash equivalents and marketable securities | | | 20,471 | | | | 5,994 | |
| Short-term borrowings and long-term debt | | | 3,749 | | | | 9,489 | |
| | |
| * | | Current ratio is the ratio of current assets to current liabilities. |
The increase in our working capital, current ratio, and cash, cash equivalents, and marketable securities is due to $67.2 million of net proceeds from the initial public offering of our common stock that was completed in February 2005 offset by our $40 million acquisition of Camtronics in November 2005. The large decrease in short-term borrowings and long-term debt from December 31, 2004 to December 31, 2005 is a result of our repayment of $4.0 million of our subordinated debt with a portion of the proceeds from the initial public offering and scheduled debt retirement on other debt.
Operating Activities
During the year ended December 31, 2005, cash used in operations was $1.9 million, which primarily related to our net loss of $5.0 million and changes in working capital accounts. We experienced significant increases in trade accounts receivable, prepaid expenses and inventory to be sold to customers during the year. Our accounts receivable balance increased as a result of the timing of customer acceptances as well as the timing of new customer contracts. Prepaid expenses increased as a result of the existence of numerous annual hardware maintenance contract renewals for customer third-party components during the period for which expenses are recognized on a monthly basis over the contract periods. Our inventory increased as a result of the timing of acceptances of third party components at customer sites. Our weighted average collection period for accounts receivable as of December 31, 2005 was 56 days compared to 55 days at December 31, 2004. We calculate weighted average collection period based on average days outstanding per customer based on historical experience and then weight these days outstanding based on proportional dollar value of the accounts receivable balance at the end of the period. The changes in other working capital accounts were primarily driven by increased volume of operations and the timing of cash payments.
During the year ended December 31, 2004, cash provided by operations was $5.0 million, which consisted of an increase of $8.5 million from changes in working capital accounts and a decrease of $3.5 million as a result of our net loss, offset by non-cash items. Changes in the working capital accounts primarily related to an increase in accounts receivable, an increase in prepaid expenses, an increase in third-party components to be sold to customers, increases in accounts payable and other accrued expenses and an increase in deferred revenue due to an increased customer base and timing of customer payments. The changes in working capital accounts were primarily driven by increased volume of operations and the timing of cash payments.
During the year ended December 31, 2003, cash used in operations was $2.4 million, which consisted of an increase of $4.5 million from changes in working capital accounts and a decrease of $6.9 million as a result of our net loss, offset by non-cash items. Changes in the working capital accounts primarily related to a decrease in accounts receivable, an increase in prepaid expenses, a decrease in third-party components to be sold to customers, an increase in accrued payroll and related costs and an increase in deferred revenue due to an increased customer base and timing of customer payments. The changes in working capital accounts were primarily driven by increased volume of operations and the timing of cash payments.
Cash provided by and used in operating activities has historically been affected by changes in working capital accounts, primarily deferred revenue, accounts receivable and accrued expenses. Fluctuations within
45
accounts receivable and deferred revenue are primarily related to the timing of billings and associated revenue recognition.
Investing Activities
We used cash of $53.3 million, $2.9 million and $1.0 million for investing activities during 2005, 2004 and 2003, respectively.
We used $7.7 million, $2.9 million and $2.5 million for property and equipment purchases during 2005, 2004 and 2003, respectively. Approximately $5.8 million of the purchases for 2005 related to investments in equipment for internal use, including test equipment for our research and development and quality assurance departments as well as computer equipment and furniture for new and existing personnel. Approximately $2.7 million and $1.4 million of the purchases for 2004 and 2003, respectively, related to computer equipment for new and existing personnel. We anticipate that we will continue to purchase property and equipment for internal use as necessary, consistent with growth of the Company. Approximately $1.9 million, $0.2 million and $1.1 million of the purchases for 2005, 2004 and 2003, respectively, related to investments in equipment located at contracted customer sites. We anticipate that we will incur additional capital expenditures at customer sites as we further standardize and update our hardware platform.
In November 2005, we used $40.4 million to acquire all the stock of Camtronics Medical Systems, Ltd., based in Hartland, Wisconsin. We acquired all of Camtronics’ assets, including its corporate headquarters campus in Hartland, and all of Camtronics’ liabilities.
We used $44.8 million for the purchase of marketable securities and received proceeds of $39.9 million upon the maturity or sale of some of these securities during 2005. The marketable securities consist of U.S. government agency obligations and corporate commercial paper, all with maturities of less than one year.
Financing Activities
Cash provided by financing activities totaled $64.5 million, $1.6 million and $3.5 million for 2005, 2004 and 2003, respectively. The cash provided by financing activities for 2005 resulted primarily from the completion of our initial public offering. This inflow of cash was offset by our $4.0 million repayment of our subordinated debt and other payments on borrowings. During 2005, we also had $0.4 million of restricted cash released from restriction. The cash provided by financing activities for 2004 resulted from proceeds from the issuance of subordinated debt of $4.0 million, which was later repaid upon completion of our initial public offering, offset by payments on existing borrowings and an addition to restricted cash used to secure a letter of credit for an operating lease. The cash provided by financing activities for 2003 resulted from proceeds from the issuance of preferred stock of $5.9 million offset by payments on existing borrowings and a purchase of treasury stock.
The following table summarizes, as of December 31, 2005, the general timing of future payments (including payments of interest) under our outstanding loan agreements, lease agreements and other long-term contractual obligations:
| | | | | | | | | | | | | | | | | | | | | |
| | | Payments Due by Period | |
| | | | | | Less Than
| | | | | | | | | More Than
| |
Contractual Cash Obligations | | Total | | | 1 Year | | | 1-3 Years | | | 3-5 Years | | | 5 Years | |
| | | (Dollars in thousands) | |
| |
| Long-term debt, including interest | | $ | 2,943 | | | $ | 2,181 | | | $ | 762 | | | $ | — | | | $ | — | |
| Capital lease obligations | | | 1,063 | | | | 820 | | | | 243 | | | | — | | | | — | |
| Operating leases | | | 7,292 | | | | 1,419 | | | | 2,951 | | | | 2,057 | | | | 865 | |
| | | | | | | | | | | | | | | | | | | | | |
| Total contractual cash obligations | | $ | 11,298 | | | $ | 4,420 | | | $ | 3,956 | | | $ | 2,057 | | | $ | 865 | |
| | | | | | | | | | | | | | | | | | | | | |
In August 2005, we executed a new operating lease for rental space for our Madison, Wisconsin office. The lease term ends January 31, 2013. Monthly lease payments of $23,468 begin in February 2006 and increase 3% annually on each August 1st until the end of the lease term.
46
In April 2004, we entered into a loan and security agreement with a bank under which we can borrow up to $4.0 million subject to certain restrictions. Interest accrues at the prime rate plus 1.5% to 2.0%, depending on our net income. This line of credit was amended as of July 31, 2004 and expires April 30, 2006 at which time all advances will be due and payable. As of December 31, 2005 we had no outstanding balance under this line of credit. Any borrowings under the agreement are secured by certain assets of the Company, including cash and receivables. We anticipate renewal of this agreement upon its expiration under terms and conditions no less favorable as in the existing agreement.
We believe our existing cash, together with future cash flows from operations and available borrowings under our loan and security agreement, if necessary, will be sufficient to execute our business plan in 2006. However, any projections of future cash inflows and outflows are subject to uncertainty. Our future cash requirements will depend on many factors, including our rate of revenue growth, the expansion of our marketing and sales activities, the timing and extent of spending to support product development efforts and expansion into new territories, the timing of introductions of new products and services, enhancements to existing products and services, the amount and form of consideration we may issue in acquisition or similar transactions, and the continuing market acceptance of our solution. To the extent that our existing cash, together with future cash flows from operations and availability under our loan and security agreement are insufficient to fund our future activities, we may need to raise additional funds through equity or debt financing. Although we are currently not a party to any binding agreement or letter of intent with respect to any other potential investments in, or acquisitions of, complementary businesses, services or technologies, we may enter into these types of arrangements in the future, which could also require us to seek additional equity or debt financing. It is possible that additional funds may not be available on terms favorable to us or at all.
Off-Balance Sheet Arrangements
Except for operating leases entered into for ordinary business purposes, we do not currently have any off-balance sheet arrangements with unconsolidated entities or financial partnerships, or with entities often referred to as structured finance or special purposes entities which would have been established for the purpose of facilitating off-balance sheet arrangements or other contractually narrow or limited purposes. In addition, we do not engage in trading activities involving non-exchange traded contracts. As such, we are not materially exposed to any financing, liquidity, market or credit risk that could arise if we had engaged in these relationships.
Recently Issued Accounting Pronouncements
In December 2004, the FASB issued Statement No. 123 (revised 2004),Share-Based Payment(“SFAS 123R”). SFAS 123R establishes standards for the accounting for transactions in which an entity exchanges its equity instruments for goods or services. It focuses primarily on accounting for transactions in which an entity obtains employee services in share-based payment transactions. SFAS 123R requires publicly-traded companies to measure the cost of employee services received in exchange for an award of equity instruments based on the grant-date fair value of the award and the estimated number of awards that are expected to vest. That cost is to be recognized over the period during which an employee is required to provide service in exchange for the award, which is usually the vesting period. SFAS 123R supersedes APB 25, which the Company has followed for all periods through December 31, 2005. SFAS 123R will be effective for the Company at the beginning of the fiscal first quarter of 2006. SFAS 123R applies to all awards granted after the required effective date and to awards modified, repurchased, or canceled after that date. Compensation cost is recognized on or after the required effective date for the portion of outstanding awards for which the requisite services have not yet been rendered, based on the grant-date fair value of those awards calculated under SFAS 123. For periods before the required effective date, SFAS 123R allows elective adjustment of the financial statements of prior periods on a basis consistent with the pro forma disclosures required for those periods by SFAS 123. Upon adoption in the first quarter of 2006, the Company will not restate prior periods. The Company expects to record additional costs relating to compensation expense as a result of the adoption of SFAS 123R; however, the precise effect of adoption has not been predicted at this time because it will
47
depend on levels of share-based payments granted in the future and the characteristics of our stock on the date of grant, as well as the actual forfeiture rates we experience.
In December 2004, the FASB issued SFAS No. 151,Inventory Costs. SFAS No. 151 clarifies the accounting for inventory when there are abnormal amounts of idle facility expense, freight, handling costs and wasted materials. Under existing U.S. generally accepted accounting principles, items such as idle facility expense, excessive spoilage, double freight and rehandling costs may be “so abnormal” as to require treatment as current period charges rather than recorded as adjustments to the value of the inventory. SFAS No. 151 requires that those items be recognized as current-period charges regardless of whether they meet the criterion of “so abnormal.” In addition, SFAS No. 151 requires that allocation of fixed production overheads to the cost of conversion be based on the normal capacity of the production facilities. The provisions of SFAS No. 151 are effective for inventory costs incurred during fiscal years beginning after June 15, 2005. Earlier application is permitted for inventory costs incurred during fiscal years beginning after the date SFAS No. 151 was issued. The adoption of SFAS No. 151 is not expected to have a material impact on the Company’s financial position or results of operations.
In May 2005, the FASB issued SFAS No. 154,Accounting Changes and Error Corrections, which is a replacement of APB Opinion No. 20,Accounting Changes, and SFAS No. 3,Reporting Changes in Interim Financial Statements. SFAS No. 154 applies to all voluntary changes in accounting principle and changes in the accounting for and reporting of a change in accounting principle. SFAS No. 154 requires that a change in method of depreciation or amortization for long-lived non-financial assets be accounted for as a change in accounting estimate that is effected by a change in accounting principle. SFAS No. 154 is effective for accounting changes and corrections of errors made in fiscal years beginning after December 15, 2005. The Company does not expect that the adoption of SFAS No. 154 will have a material impact on its financial position or results of operations.
| |
| ITEM 7A: | QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK |
Our debt instruments do not expose us to material market risks relating to changes in interest rates. Some of the proceeds of our initial public offering have been invested in short-term, interest-bearing, investment grade securities pending their application. The value of these securities will be subject to interest rate risk and could fall in value if interest rates rise. The effect of a hypothetical one hundred basis point decrease across all interest rates related to our investments would result in an annual decrease of approximately $0.1 million in operating results assuming no further changes in the amount of our investments outstanding at December 31, 2005.
The primary objective of our investment activities is to preserve principal while maximizing the income we receive from our investments without significantly increasing our risk. We invest excess cash principally in U.S. marketable debt securities from a diversified portfolio of institutions with strong credit ratings and in U.S. government and agency bills and notes, and by policy limit the amount of credit exposure at any one institution. These investments are generally not collateralized and mature within one year. Some of the securities we invest in may have market risk. This means that a change in prevailing interest rates may cause the fair value of the principal amount of the investment to fluctuate. To minimize this risk, we schedule our investments to have maturities that coincide with our expected cash flow needs, thus reducing the need to sell or redeem an investment prior to its maturity date. Accordingly, we believe we have no material exposure to interest rate risk arising from our investments.
| |
| ITEM 8: | FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA |
The information required by this item appears beginning onpage F-1 of this report.
| |
| ITEM 9: | CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE |
Not applicable.
48
| |
| ITEM 9A: | CONTROLS AND PROCEDURES |
Evaluation of Disclosure Controls and Procedures
Our management, with the participation of our chief executive officer and chief financial officer, evaluated the effectiveness of our disclosure controls and procedures (as defined inRules 13a-15(e) and15d-15(e) under the Securities Exchange Act of 1934, as amended (the “Exchange Act”)) as of December 31, 2005. Based on this evaluation, our chief executive officer and chief financial officer concluded that, as of December 31, 2005, our disclosure controls and procedures were (1) designed to ensure that material information relating to us is made known to our chief executive officer and chief financial officer by others within our company, particularly during the period in which this report was being prepared and (2) effective, in that they provide reasonable assurance that information required to be disclosed by us in the reports that we file or submit under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the Securities and Exchange Commission’s rules and forms.
Changes in Internal Control over Financial Reporting
No change in our internal control over financial reporting (as defined inRules 13a-15(f) and15d-15(f) under the Exchange Act) occurred during the year ended December 31, 2005 that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
| |
| ITEM 9B: | OTHER INFORMATION |
Not Applicable.
PART III
| |
| ITEM 10: | DIRECTORS AND EXECUTIVE OFFICERS OF THE REGISTRANT |
The information called for by this Item will be contained in our definitive Proxy Statement for our 2006 Annual Meeting of Shareholders and is incorporated herein by reference.
Our board of directors has adopted a code of conduct and code of ethics applicable to our chief executive officer, chief financial officer and senior financial officers, directors, officers and employees in accordance with applicable rules and regulations of the SEC and the Nasdaq National Market. Our code of conduct and code of ethics is available on our website atwww.emageon.com.
��
| |
| ITEM 11: | EXECUTIVE COMPENSATION |
The information called for by this Item will be contained in our definitive Proxy Statement for our 2006 Annual Meeting of Shareholders and is incorporated herein by reference.
| |
| ITEM 12: | SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS |
The information called for by this Item will be contained in our definitive Proxy Statement for our 2006 Annual Meeting of Shareholders and is incorporated herein by reference.
| |
| ITEM 13: | CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS |
The information called for by this Item will be contained in our definitive Proxy Statement for our 2006 Annual Meeting of Shareholders and is incorporated herein by reference.
| |
| ITEM 14: | PRINCIPAL ACCOUNTANT FEES AND SERVICES |
The information called for by this Item will be contained in our definitive Proxy Statement for our 2006 Annual Meeting of Shareholders and is incorporated herein by reference.
49
PART IV
| |
| ITEM 15: | EXHIBITS AND FINANCIAL STATEMENT SCHEDULES |
| | |
| | (a) | The following documents are filed as part of this Annual Report: |
| | | |
| | | Page
|
| | | Number in
|
Description | | Report |
| Report of Independent Registered Public Accounting Firm | | F-2 |
| Consolidated Balance Sheets as of December 31, 2005 and 2004 | | F-3 |
| Consolidated Statements of Operations for the years ended December 31, 2005, 2004 and 2003 | | F-4 |
| Consolidated Statements of Stockholder’s Equity for the years ended December 31, 2005, 2004 and 2003 | | F-5 |
| Consolidated Statements of Cash Flows for the years ended December 31, 2005, 2004 and 2003 | | F-6 |
| Notes to Consolidated Financial Statements | | F-7 |
| |
| 2. | Financial Statement Schedules |
| | | |
| Schedule II — Valuation and Qualifying Accounts and Reserves for the three years ended December 31, 2005 | | Follows page F-29 |
All other schedules are omitted because they are not applicable or the required information is shown in the financial statements or notes thereto.
The following exhibits are required to be filed with this Report by Item 601 of Regulation S-K:
| | | | | |
Exhibit No. | | Description |
| |
| | 2 | .1 | | Agreement and Plan of Merger, dated as of April 30, 2003, by and among Emageon, Inc., Emageon — UV Development Corporation, Ultravisual Medical Systems Corporation and Jeff Rusinow as Stockholders’ Representative (incorporated by reference to Exhibit 2.1 to the Company’s Registration Statement onForm S-1, RegistrationNo. 333-120621, filed on November 19, 2004) |
| | 3 | .1 | | Emageon Inc. Amended and Restated Certificate of Incorporation (incorporated by reference to Exhibit 3.2 to the Company’s Registration Statement onForm S-1/A, RegistrationNo. 333-120621, filed on January 25, 2005) |
| | 3 | .2 | | Emageon Inc. Amended and Restated Bylaws (incorporated by reference to Exhibit 3.4 to the Company’s Registration Statement onForm S-1/A, RegistrationNo. 333-120621, filed on January 25, 2005) |
| | 4 | .1 | | Form of Emageon Inc. common stock certificate (incorporated by reference to Exhibit 3.4 to the Company’s Registration Statement onForm S-1/A, RegistrationNo. 333-120621, filed on February 4, 2005) |
| | 10 | .1# | | Imageon Solutions, Inc. 2000 Equity Incentive Plan (incorporated by reference to Exhibit 10.1 to the Company’s Registration Statement onForm S-1, RegistrationNo. 333-120621, filed on November 19, 2004) |
| | 10 | .2# | | Emageon, Inc. 2000 Equity Compensation Plan (incorporated by reference to Exhibit 10.2 to the Company’s Registration Statement onForm S-1, RegistrationNo. 333-120621, filed on November 19, 2004) |
| | 10 | .3# | | Emageon Inc. 2005 Equity Incentive Plan (incorporated by reference to Exhibit 10.3 to the Company’s Registration Statement onForm S-1/A, RegistrationNo. 333-120621, filed on February 4, 2005) |
| | 10 | .4# | | Emageon Inc. 2005 Non-Employee Director Stock Incentive Plan (incorporated by reference to Exhibit 10.4 to the Company’s Registration Statement onForm S-1/A, RegistrationNo. 333-120621, filed on February 4, 2005) |
50
| | | | | |
Exhibit No. | | Description |
| |
| | 10 | .5# | | Employment Agreement of Charles A. Jett, Jr. (incorporated by reference to Exhibit 10.5 to the Company’s Registration Statement onForm S-1, RegistrationNo. 333-120621, filed on November 19, 2004) |
| | 10 | .6# | | Employment Agreement of Milton G. Silva-Craig (incorporated by reference to Exhibit 10.6 to the Company’s Registration Statement onForm S-1, RegistrationNo. 333-120621, filed on November 19, 2004) |
| | 10 | .7# | | Employment Agreement of W. Randall Pittman (incorporated by reference to Exhibit 10.7 to the Company’s Registration Statement onForm S-1, RegistrationNo. 333-120621, filed on November 19, 2004) |
| | 10 | .8# | | Employment Agreement of Mark A. Gehring (incorporated by reference to Exhibit 10.8 to the Company’s Registration Statement onForm S-1, RegistrationNo. 333-120621, filed on November 19, 2004) |
| | 10 | .9# | | Employment Agreement of Noel D. Gartman (incorporated by reference to Exhibit 10.9 to the Company’s Registration Statement onForm S-1, RegistrationNo. 333-120621, filed on November 19, 2004) |
| | 10 | .10 | | Form of Indemnification Agreement between the Registrant and each of its directors and executive officers (incorporated by reference to Exhibit 10.10 to the Company’s Registration Statement onForm S-1, RegistrationNo. 333-120621, filed on November 19, 2004) |
| | 10 | .11 | | Amended and Restated Registration Rights Agreement, dated as of October 2, 2001, by and among Emageon UV, Inc. and certain stockholders, as amended and joined on May 30, 2003 and June 25, 2003 (incorporated by reference to Exhibit 10.11 to the Company’s Registration Statement onForm S-1, RegistrationNo. 333-120621, filed on November 19, 2004) |
| | 10 | .12† | | Enterprise Agreement, dated as of May 5, 2004, by and between Emageon UV, Inc. and Ascension Health (incorporated by reference to Exhibit 10.12 to the Company’s Registration Statement onForm S-1/A, RegistrationNo. 333-120621, filed on February 8, 2005) |
| | 10 | .13 | | Lease Agreement, dated as of December 20, 2001, by and between Meadow Brook North, L.L.C. and Emageon UV, Inc. (incorporated by reference to Exhibit 10.13 to the Company’s Registration Statement onForm S-1, RegistrationNo. 333-120621, filed on November 19, 2004) |
| | 10 | .13A | | Sixth Amendment to Lease Agreement, dated as of July 23, 2004, by and between Meadow Brook North, L.L.C. and Emageon UV, Inc. (incorporated by reference to Exhibit 10.13A to the Company’s Registration Statement onForm S-1, RegistrationNo. 333-120621, filed on November 19, 2004) |
| | 10 | .14 | | Note and Warrant Purchase Agreement, dated as of June 25, 2004, among Emageon UV, Inc. and Whitecap Alabama Growth Fund I, LLC, Enhanced Alabama Issuer, LLC and Advantage Capital Alabama Partners I, L.P. (incorporated by reference to Exhibit 10.14 to the Company’s Registration Statement onForm S-1/A, RegistrationNo. 333-120621, filed on January 25, 2005) |
| | 10 | .15 | | Emageon, Inc. Amended and Restated Stockholders Agreement, dated as of October 2, 2001, among Emageon, Inc. and the stockholders signatory thereto (incorporated by reference to Exhibit 10.15 to the Company’s Registration Statement onForm S-1/A, RegistrationNo. 333-120621, filed on January 25, 2005) |
| | 10 | .15A | | Emageon, Inc. First Amendment and Joinder to Amended and Restated Stockholders Agreement, dated as of May 30, 2003, among Emageon, Inc and the stockholders signatory thereto (incorporated by reference to Exhibit 10.15A to the Company’s Registration Statement onForm S-1/A, RegistrationNo. 333-120621, filed on January 25, 2005) |
| | 10 | .15B | | Emageon, Inc. Second Amendment and Joinder to Amended and Restated Stockholders Agreement, dated as of June 25, 2003, among Emageon, Inc. and the stockholders signatory thereto (incorporated by reference to Exhibit 10.15B to the Company’s Registration Statement onForm S-1/A, RegistrationNo. 333-120621, filed on January 25, 2005) |
| | 14 | .1 | | Emageon Inc. Code of Ethics (incorporated by reference to Exhibit 14.1 to the Company’s Report on Form 10-K for the year ended December 31, 2004) |
| | 21 | .1* | | Subsidiaries of Emageon Inc. |
| | 23 | .1* | | Consent of Independent Registered Public Accounting Firm |
51
| | | | | |
Exhibit No. | | Description |
| |
| | 31 | .1* | | Certification of Chief Executive Officer pursuant toRule 13a-14(a) orRule 15d-14(a) under the Securities Exchange Act of 1934 |
| | 31 | .2* | | Certification of Chief Financial Officer pursuant toRule 13a-14(a) orRule 15d-14(a) under the Securities Exchange Act of 1934 |
| | 32 | * | | Certification of Chief Executive Officer and Chief Financial Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 |
| | |
| * | | Filed herewith |
| |
| # | | Indicates a management contract or any compensatory plan, contract or arrangement |
| |
| † | | Confidential treatment has been granted for portions of this exhibit |
52
EMAGEON INC.
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
CONTENTS
F-1
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
The Board of Directors and Stockholders
Emageon Inc.
We have audited the accompanying consolidated balance sheets of Emageon Inc. (the Company) as of December 31, 2004 and 2005, and the related consolidated statements of operations, stockholders’ equity (deficit) and cash flows for each of the three years in the period ended December 31, 2005. Our audits also include the financial statement schedule listed in the Index at Item 15(a). These financial statements and schedule are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements and schedule based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. We were not engaged to perform an audit of the Company’s internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of Emageon Inc. at December 31, 2004 and 2005, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2005, in conformity with U.S. generally accepted accounting principles. Also, in our opinion, the related financial statement schedule, when considered in relation to the basic financial statements taken as a whole, presents fairly in all material respects the information set forth therein.
/s/ Ernst & Young LLP
Atlanta, Georgia
March 29, 2006
F-2
EMAGEON INC.
| | | | | | | | | |
| | | December 31, | |
| | | 2004 | | | 2005 | |
| | | (In thousands, except for share data) | |
| |
ASSETS |
| Current assets: | | | | | | | | |
| Cash and cash equivalents | | $ | 5,994 | | | $ | 15,520 | |
| Marketable securities | | | — | | | | 4,951 | |
| Trade accounts receivable, net of allowance for doubtful accounts of $75 and $126 at December 31, 2004 and 2005, respectively | | | 14,557 | | | | 29,261 | |
| Prepaid expenses and other current assets | | | 1,799 | | | | 3,052 | |
| Deferred offering costs | | | 1,326 | | | | — | |
| Inventories | | | 1,422 | | | | 8,031 | |
| | | | | | | | | |
| Total current assets | | | 25,098 | | | | 60,815 | |
| Property and equipment, net | | | 8,832 | | | | 21,433 | |
| Restricted cash | | | 903 | | | | 535 | |
| Other noncurrent assets | | | 62 | | | | 884 | |
| Intangible assets: | | | | | | | | |
| Goodwill | | | 3,755 | | | | 21,079 | |
| Customer relationships, net | | | — | | | | 9,510 | |
| Developed technology, net | | | 2,847 | | | | 3,028 | |
| Capitalized software development costs, net | | | 21 | | | | 231 | |
| Trademark and trade names, net | | | 250 | | | | 429 | |
| | | | | | | | | |
| Total intangible assets | | | 6,873 | | | | 34,277 | |
| | | | | | | | | |
| Total assets | | $ | 41,768 | | | $ | 117,944 | |
| | | | | | | | | |
| |
LIABILITIES, REDEEMABLE PREFERRED STOCK AND STOCKHOLDERS’ EQUITY (DEFICIT) |
| Current liabilities: | | | | | | | | |
| Accounts payable | | $ | 4,658 | | | $ | 13,196 | |
| Accrued payroll and related costs | | | 1,557 | | | | 4,104 | |
| Deferred revenue | | | 21,357 | | | | 25,312 | |
| Other accrued expenses | | | 3,838 | | | | 4,723 | |
| Current portion of long-term debt | | | 2,472 | | | | 2,031 | |
| Current portion of capital lease obligations | | | 620 | | | | 732 | |
| | | | | | | | | |
| Total current liabilities | | | 34,502 | | | | 50,098 | |
| Long-term deferred revenue | | | 2,796 | | | | 3,221 | |
| Deferred tax liability | | | 95 | | | | — | |
| Long-term debt | | | 5,528 | | | | 750 | |
| Capital lease obligations, less current portion | | | 869 | | | | 236 | |
| | | | | | | | | |
| Total liabilities | | | 43,790 | | | | 54,305 | |
| Redeemable preferred stock, $0.001 par value, 64,450,000 shares authorized, 64,007,054 shares issued and outstanding at December 31, 2004 | | | 30,348 | | | | — | |
| Stockholders’ equity (deficit): | | | | | | | | |
| Preferred stock, $0.001 par value, 23,965,000 shares authorized, 19,692,358 shares issued and 18,319,620 shares outstanding at December 31, 2004 | | | 7,306 | | | | — | |
| Common stock, $0.001 par value; 165,050,000 shares authorized, 3,056,181 shares and 20,628,913 shares issued and 2,709,370 shares and 20,453,156 shares outstanding at December 31, 2004 and 2005, respectively | | | 3 | | | | 21 | |
| Additional paid in capital | | | 6,998 | | | | 115,215 | |
| Accumulated other comprehensive income | | | — | | | | 85 | |
| Accumulated deficit | | | (46,402 | ) | | | (51,407 | ) |
| | | | | | | | | |
| | | | (32,095 | ) | | | 63,914 | |
| Treasury stock, 175,757 shares, at cost | | | (275 | ) | | | (275 | ) |
| | | | | | | | | |
| Total stockholders’ equity (deficit) | | | (32,370 | ) | | | 63,639 | |
| | | | | | | | | |
| Total liabilities, redeemable preferred stock and stockholders’ equity | | $ | 41,768 | | | $ | 117,944 | |
| | | | | | | | | |
F-3
EMAGEON INC.
| | | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2003 | | | 2004 | | | 2005 | |
| | | (In thousands, except for share data and per share amounts) | |
| |
| Revenue: | | | | | | | | | | | | |
| System sales | | $ | 17,234 | | | $ | 33,441 | | | $ | 50,041 | |
| Support services | | | 6,057 | | | | 12,361 | | | | 23,750 | |
| | | | | | | | | | | | | |
| Total revenue | | | 23,291 | | | | 45,802 | | | | 73,791 | |
| Cost of revenue: | | | | | | | | | | | | |
| System sales | | | 10,227 | | | | 21,452 | | | | 28,316 | |
| Support services | | | 7,493 | | | | 10,728 | | | | 14,648 | |
| | | | | | | | | | | | | |
| Total cost of revenue | | | 17,720 | | | | 32,180 | | | | 42,964 | |
| | | | | | | | | | | | | |
| Gross profit | | | 5,571 | | | | 13,622 | | | | 30,827 | |
| Operating expenses: | | | | | | | | | | | | |
| Research and development | | | 4,143 | | | | 6,021 | | | | 10,697 | |
| Sales and marketing | | | 6,144 | | | | 9,027 | | | | 11,830 | |
| General and administrative | | | 5,793 | | | | 8,024 | | | | 12,308 | |
| Amortization and write-off of intangible assets related to Camtronics acquisition | | | — | | | | — | | | | 993 | |
| Integration costs related to Camtronics acquisition | | | — | | | | — | | | | 244 | |
| | | | | | | | | | | | | |
| Total operating expenses | | | 16,080 | | | | 23,072 | | | | 36,072 | |
| | | | | | | | | | | | | |
| Operating loss | | | (10,509 | ) | | | (9,450 | ) | | | (5,245 | ) |
| Interest income | | | 14 | | | | 31 | | | | 1,497 | |
| Interest expense | | | (864 | ) | | | (1,053 | ) | | | (1,249 | ) |
| | | | | | | | | | | | | |
| Net loss | | $ | (11,359 | ) | | $ | (10,472 | ) | | $ | (4,997 | ) |
| | | | | | | | | | | | | |
| Net loss per share — basic and diluted | | $ | (5.79 | ) | | $ | (4.07 | ) | | $ | (0.28 | ) |
| | | | | | | | | | | | | |
| Weighted average common stock outstanding — basic and diluted | | | 1,973,108 | | | | 2,589,832 | | | | 17,975,083 | |
| | | | | | | | | | | | | |
F-4
EMAGEON INC.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | Accumulated
| | | | | | | | | Total
| |
| | | Preferred Stock | | | Common Stock | | | Additional
| | | Other
| | | | | | | | | Stockholders’
| |
| | | | | | Carrying
| | | | | | Par
| | | Paid in
| | | Comprehensive
| | | Treasury
| | | Accumulated
| | | Equity
| |
| | | Shares | | | Value | | | Shares | | | Value | | | Capital | | | Income (Loss) | | | Stock | | | Deficit | | | (Deficit) | |
| | | (In thousands, except for share data) | |
| |
| Balance at December 31, 2002 | | | 5,965,000 | | | $ | 1,438 | | | | 1,314,238 | | | $ | 1 | | | $ | 2,490 | | | $ | — | | | $ | — | | | $ | (24,438 | ) | | $ | (20,509 | ) |
| Exercise of stock options | | | — | | | | — | | | | 3,430 | | | | — | | | | 15 | | | | — | | | | — | | | | — | | | | 15 | |
| Common stock, Series D preferred stock and warrants issued in connection with Ultravisual Merger | | | 13,727,358 | | | | 5,868 | | | | 1,715,541 | | | | 2 | | | | 2,789 | | | | — | | | | — | | | | — | | | | 8,659 | |
| Purchase of treasury stock ($1.5675 per share) | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | (275 | ) | | | — | | | | (275 | ) |
| Accretion of redeemable preferred stock | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | (66 | ) | | | (66 | ) |
| Net loss | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | (11,359 | ) | | | (11,359 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Balance at December 31, 2003 | | | 19,692,358 | | | | 7,306 | | | | 3,033,209 | | | | 3 | | | | 5,294 | | | | — | | | | (275 | ) | | | (35,863 | ) | | | (23,535 | ) |
| Exercise of stock options | | | — | | | | — | | | | 22,972 | | | | — | | | | 64 | | | | — | | | | — | | | | — | | | | 64 | |
| Issuance of warrants in connection with subordinated debt and customer sales agreement | | | — | | | | — | | | | — | | | �� | — | | | | 1,046 | | | | — | | | | — | | | | — | | | | 1,046 | |
| Stock based compensation — options | | | — | | | | — | | | | — | | | | — | | | | 594 | | | | — | | | | — | | | | — | | | | 594 | |
| Accretion of redeemable preferred stock | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | (67 | ) | | | (67 | ) |
| Net loss | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | (10,472 | ) | | | (10,472 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Balance at December 31, 2004 | | | 19,692,358 | | | | 7,306 | | | | 3,056,181 | | | | 3 | | | | 6,998 | | | | — | | | | (275 | ) | | | (46,402 | ) | | | (32,370 | ) |
| Exercise of stock options | | | — | | | | — | | | | 417,607 | | | | 1 | | | | 1,292 | | | | — | | | | — | | | | — | | | | 1,293 | |
| Exercise of stock warrants | | | 105,703 | | | | 58 | | | | 24,632 | | | | — | | | | 74 | | | | — | | | | — | | | | — | | | | 132 | |
| Exercise of mandatorily redeemable stock warrants in connection with initial public offering | | | — | | | | — | | | | 537,082 | | | | 1 | | | | 735 | | | | — | | | | — | | | | — | | | | 736 | |
| Proceeds from initial public offering, net of issuance costs | | | — | | | | — | | | | 5,750,000 | | | | 6 | | | | 67,195 | | | | — | | | | — | | | | — | | | | 67,201 | |
| Automatic conversion of non-redeemable preferred stock into common stock in connection with initial public offering | | | (19,798,061 | ) | | | (7,364 | ) | | | 2,402,898 | | | | 2 | | | | 7,362 | | | | — | | | | — | | | | — | | | | — | |
| Automatic conversion of redeemable preferred stock into common stock in connection with initial public offering | | | — | | | | — | | | | 8,440,513 | | | | 8 | | | | 30,348 | | | | — | | | | — | | | | — | | | | 30,356 | |
| Stock based compensation — stock options | | | — | | | | — | | | | — | | | | — | | | | 1,211 | | | | — | | | | — | | | | — | | | | 1,211 | |
| Foreign currency translation adjustments | | | — | | | | — | | | | — | | | | — | | | | — | | | | 93 | | | | — | | | | — | | | | 93 | |
Loss onavailable-for-sale marketable securities | | | — | | | | — | | | | — | | | | — | | | | — | | | | (8 | ) | | | — | | | | — | | | | (8 | ) |
| Accretion of redeemable preferred stock | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | (8 | ) | | | (8 | ) |
| Net loss | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | (4,997 | ) | | | (4,997 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Balance at December 31, 2005 | | | — | | | $ | — | | | | 20,628,913 | | | $ | 21 | | | $ | 115,215 | | | $ | 85 | | | $ | (275 | ) | | $ | (51,407 | ) | | $ | 63,639 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
F-5
EMAGEON INC.
| | | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2003 | | | 2004 | | | 2005 | |
| | | (In thousands) | |
| |
Operating activities | | | | | | | | | | | | |
| Net loss | | $ | (11,359 | ) | | $ | (10,472 | ) | | $ | (4,997 | ) |
| Adjustments to reconcile net loss to net cash (used in)/provided by operating activities: | | | | | | | | | | | | |
| Depreciation | | | 1,087 | | | | 1,430 | | | | 2,970 | |
| Depreciation of property and equipment at contracted customer sites | | | 2,743 | | | | 3,360 | | | | 2,698 | |
| Amortization of developed technology, customer relationships and trade names | | | 486 | | | | 833 | | | | 1,483 | |
| Write off of intangible assets related to Camtronics acquisition, net of tax liability | | | — | | | | — | | | | 403 | |
| Amortization of capitalized software development costs | | | 79 | | | | 327 | | | | 143 | |
| Amortization and write off of subordinated debt discount | | | — | | | | 157 | | | | 646 | |
| Employee stock based compensation expense | | | — | | | | 533 | | | | 1,171 | |
| Other operating activities | | | 42 | | | | 373 | | | | 215 | |
| Changes in operating assets and liabilities, net of acquired companies: | | | | | | | | | | | | |
| Trade accounts receivable | | | 2,589 | | | | (10,233 | ) | | | (7,659 | ) |
| Prepaid expenses and other current assets | | | (788 | ) | | | (2,120 | ) | | | (885 | ) |
| Inventories | | | 657 | | | | (1,137 | ) | | | (3,402 | ) |
| Other noncurrent assets | | | 56 | | | | (59 | ) | | | (726 | ) |
| Accounts payable | | | 13 | | | | 3,243 | | | | 5,775 | |
| Accrued payroll and related costs | | | 688 | | | | 184 | | | | 348 | |
| Other accrued expenses | | | (155 | ) | | | 3,500 | | | | (187 | ) |
| Deferred revenue | | | 1,485 | | | | 15,040 | | | | 123 | |
| | | | | | | | | | | | | |
| Net cash (used in)/provided by operating activities | | | (2,377 | ) | | | 4,959 | | | | (1,881 | ) |
Investing activities | | | | | | | | | | | | |
| Purchases of property and equipment for internal purposes | | | (1,424 | ) | | | (2,655 | ) | | | (5,815 | ) |
| Purchases of third-party components located at contracted customer sites | | | (1,095 | ) | | | (250 | ) | | | (1,865 | ) |
| Purchases of marketable securities | | | — | | | | — | | | | (44,198 | ) |
| Proceeds from sales and maturities of marketable securities | | | — | | | | — | | | | 39,335 | |
| Capitalized software development costs | | | (339 | ) | | | (32 | ) | | | (354 | ) |
| Purchase price of Camtronics, net of cash received | | | — | | | | — | | | | (40,359 | ) |
| Net cash received from Ultravisual merger | | | 1,829 | | | | — | | | | — | |
| | | | | | | | | | | | | |
| Net cash used in investing activities | | | (1,029 | ) | | | (2,937 | ) | | | (53,256 | ) |
Financing activities | | | | | | | | | | | | |
| Proceeds from issuance of common stock, net of issuance costs | | | 15 | | | | 64 | | | | 70,630 | |
| Proceeds from issuance of preferred stock, net of issuance costs | | | 5,889 | | | | — | | | | 58 | |
| Payments on capital lease obligations | | | (514 | ) | | | (577 | ) | | | (642 | ) |
| Proceeds from loans, net of issuance costs | | | — | | | | 3,980 | | | | — | |
| Payments on loans | | | (1,611 | ) | | | (1,739 | ) | | | (5,873 | ) |
| Additions to restricted cash to secure letter of credit | | | — | | | | (96 | ) | | | — | |
| Cash released from restriction | | | — | | | | — | | | | 374 | |
| Purchase of treasury stock | | | (275 | ) | | | — | | | | — | |
| | | | | | | | | | | | | |
| Net cash provided by financing activities | | | 3,504 | | | | 1,632 | | | | 64,547 | |
| | | | | | | | | | | | | |
| Effect of exchange rate changes on cash | | | — | | | | — | | | | 116 | |
| | | | | | | | | | | | | |
| Net increase in cash | | | 98 | | | | 3,654 | | | | 9,526 | |
| Cash at beginning of year | | | 2,242 | | | | 2,340 | | | | 5,994 | |
| | | | | | | | | | | | | |
| Cash at end of year | | $ | 2,340 | | | $ | 5,994 | | | $ | 15,520 | |
| | | | | | | | | | | | | |
| Supplemental disclosure of cash flow: | | | | | | | | | | | | |
| Interest paid | | $ | 897 | | | $ | 863 | | | $ | 1,266 | |
| Assets acquired under capital lease | | $ | 107 | | | $ | — | | | $ | — | |
| Assets acquired through issuance of equity securities | | $ | 8,660 | | | $ | — | | | $ | — | |
F-6
EMAGEON INC.
Years Ended December 31, 2003, 2004 AND 2005
| |
| 1. | Business Description and Background |
Business Description
Emageon Inc., formerly Emageon UV, Inc. (“Emageon” or the “Company”), provides an enterprise-level information technology solution for the clinical analysis and management of digital medical images within health care provider organizations. Emageon’s solution consists of advanced visualization and image management software for multiple medical specialties such as cardiology, radiology and orthopedics, comprehensive support services and third-party components. Emageon’s web-enabled advanced visualization software provides physicians across the enterprise — in multiple medical specialties and at any network access point — with tools to manipulate and analyze images in two dimensions (2D) and three dimensions (3D).
Background
Emageon was incorporated in Delaware on January 3, 2000 as Imageon Solutions, Inc. In June 2000, the Company formally changed its name to Emageon Inc. In May 2003, the Company acquired Ultravisual Medical Systems Corporation, and in November 2005, the Company acquired Camtronics Medical Systems, Ltd., both engaged in businesses similar and complementary to that of the Company. In February 2005, the Company completed its initial public offering of common stock. See Note 4 for details of the Company’s acquisitions and Note 3 for details of the Company’s initial public stock offering.
| |
| 2. | Summary of Significant Accounting Policies |
Principles of Consolidation
The accompanying consolidated financial statements include the accounts of the Company and its wholly-owned subsidiaries. All significant intercompany transactions and balances have been eliminated in consolidation.
Presentation
Unless otherwise noted, all dollar amounts included in the financial statements and notes, except per share data, are denoted in thousands (000’s).
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the U.S. requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amount of revenues and expenses during the reporting period. Actual results could differ from those estimates.
Reclassification
Certain items relating to the prior years have been reclassified to conform to the current year presentation.
Fair value of financial instruments
The Company’s financial instruments consist primarily of cash, cash equivalents, marketable securities, accounts receivable, and accounts payable for which the current carrying amounts approximate fair market values.
F-7
EMAGEON INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Cash and Cash Equivalents
For purposes of financial statement presentation, investments with remaining maturities at acquisition of three months or less are considered to be cash equivalents.
Restricted Cash
In conjunction with two of the secured promissory notes to the finance companies discussed in Note 13, the Company is required to maintain a restricted cash account with a bank of approximately 20% of the note amounts. In conjunction with an operating lease for computer equipment, the Company is required to maintain a certificate of deposit securing a letter of credit with a bank. Both amounts are included in restricted cash.
SecuritiesAvailable-for-Sale
The Company is required to classify debt securities asheld-to-maturity,available-for-sale, or trading. The appropriateness of each classification is reassessed at each reporting date. As of December 31, 2005, the Company classified all debt securities as“available-for-sale”. At December 31, 2005, securitiesavailable-for-sale totaling $4,951 consisted of U.S. Government Agency securities carried at fair market value in accordance with Financial Accounting Standards Board Statement No. 115, “Accounting for Certain Investments in Debt and Equity Securities.”
Trade Accounts Receivable and Allowance for Doubtful Accounts
The Company performs ongoing credit evaluation of its customers’ financial condition and generally does not require collateral. The Company has one customer, Ascension Health, whose hospitals accounted for 36% of the Company’s 2005 revenue. As of December 31, 2005, hospitals controlled by Ascension Health owed the Company approximately $12,200. The Company continuously monitors collections and payments from its customers.
Trade accounts receivable are stated net of an allowance for doubtful accounts, which represents estimated losses resulting from the inability of customers to make required payments. When determining the allowance for doubtful accounts, management takes several factors into consideration, including the overall composition of accounts receivable aging, prior history of accounts receivable write-offs, the type of customer, andday-to-day knowledge of specific customers.
The allowance for doubtful accounts is adjusted when additional information is received that impacts the amount reserved. If circumstances change, the estimates of the recoverability of accounts receivable could be reduced or increased by a material amount. Such a change in estimated recoverability would be accounted for in the period in which the facts that give rise to the change become known. Changes in the allowances for doubtful accounts are recorded as bad debt expense and are included in general and administrative expense in the statements of operations.
As of December 31, 2004 and 2005, unbilled revenue of $302 and $161 is included in accounts receivable.
Inventories
Inventories are stated at the lower of cost or market (net realizable value) using the specific identification and first-in, first-out methods and include materials, labor and manufacturing overhead. The Company periodically reviews its quantities of inventories on hand and compares these amounts to expected usage of each particular product or product line. The Company records as a charge to cost of revenue the amount required to reduce the carrying value of the inventories to estimated net realizable value.
F-8
EMAGEON INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Costs of purchased third-party hardware and software associated with the Company’s customer contracts are included as inventories in the Company’s consolidated balance sheet and charged to cost of system sales once the Company receives customer acceptance and all other relevant revenue recognition criteria are met.
Property and Equipment
Property and equipment used for internal purposes are recorded at cost. Expenditures for property and equipment are capitalized, and minor replacements, maintenance and repairs are charged to expense as incurred. Depreciation is computed using the straight-line method over the related asset’s estimated useful life. Leasehold improvements are amortized over the shorter of their estimated useful lives or the term of the respective leases. The asset cost and related accumulated depreciation or amortization are adjusted upon asset retirement or disposal with the resulting gain or loss, if any, credited or charged to results of operations.
Property and equipment at contracted customer sites are recorded at cost and consist of third-party hardware and software associated with customer contracts. Depreciation is computed using the straight-line method over the lives of the specific customer contracts, which are typically five years.
Assets held under capital leases are recorded at the lower of the net present value of minimum lease payments or the fair value of the leased asset at the inception of the lease. Amortization expense is computed using the straight-line method over the shorter of the estimated useful lives of the assets or the period of the related lease. Amortization of assets under capital leases is included in depreciation expense.
Business Combinations
The company records business combinations in accordance with Statement of Financial Accounting Standard (“SFAS”) No. 141,Business Combinations, and SFAS No. 142,Goodwill and Other Intangible Assets. SFAS No. 141 requires the purchase method of accounting for all business combinations, and that certain acquired intangible assets in a business combination be recognized as assets separate from goodwill. The Company has applied SFAS No. 141 in the allocation of the purchase price of the Camtronics and Ultravisual acquisitions. Accordingly, the company has identified and allocated estimated fair value to the intangibles acquired. SFAS No. 142 requires that goodwill and intangible assets with indefinite useful lives no longer be amortized, but instead be tested for impairment at least annually.
Goodwill, Trademarks and Other Intangible Assets
In accordance with SFAS No. 142, intangible assets are classified into three categories: (1) intangible assets with definite lives subject to amortization; (2) intangible assets with indefinite lives not subject to amortization; and (3) goodwill. Intangible assets with indefinite lives and goodwill are not amortized.
The Company continually evaluates whether events and circumstances have occurred that indicate the carrying value of long-lived assets and certain identifiable intangibles with definite lives may not be recoverable. Recoverability of these assets is evaluated by a comparison of the carrying amount of the asset to future net undiscounted cash flows expected to be generated by the asset. If the carrying amount of an asset exceeds its estimated undiscounted future cash flows, an impairment change is recognized for the excess of the carrying amount over the fair value of the asset. The fair value of the asset or asset group is measured by quoted market prices, if available, or by utilizing present value techniques.
For intangible assets with indefinite lives and goodwill, tests for impairment are performed at least annually or more frequently if events or changes in circumstances indicate that assets might be impaired. The Company is one reporting unit and, consequently, tests its goodwill for impairment as a single amount. The Company’s goodwill impairment test entails calculating the aggregate market value of the Company’s assets,
F-9
EMAGEON INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
including goodwill and other indefinite life intangible assets. If the aggregate market value of the Company’s outstanding securities plus its liabilities is less than the aggregate carrying value of the Company’s assets, including goodwill and other indefinite life intangible assets, the Company would compare the estimated fair value of goodwill to the corresponding book value of goodwill and record an impairment loss to the extent the book value exceeds that estimated fair value. For indefinite-lived assets other than goodwill, the Company records impairment charges equal to the excess of the book value of the asset over its estimated fair value.
The Company determined that its goodwill was not impaired based on its annual tests during the years ended December 31, 2003, 2004 and 2005.
In assessing fair value of intangibles, management must make assumptions regarding estimated future cash flows and other factors. Critical estimates in valuing certain intangible assets include, but are not limited to, future expected cash flows from acquired developed technologies and patents, and discount rates. Management’s estimates of fair value are based upon assumptions believed to be reasonable, but which are inherently uncertain and unpredictable and, as a result, actual results may differ from estimates.
Treasury Stock
Treasury stock is accounted for using the cost method.
Revenue Recognition
Revenue is derived primarily from two sources: system sales, which include software licenses and third-party component sales, and support services, which include fees related to system implementation, user adoption and ongoing customer support services.
Software licenses are sold under both perpetual and term license arrangements ranging in length from two to seven years. The Company typically requires deposits upon the receipt of a signed purchase order or agreement. Deposits are classified as deferred revenue in the Company’s consolidated balance sheet.
The Company accounts for software and support services revenue under the provisions of AICPA Statement of Position 97-2,(“SOP 97-2”),Software Revenue Recognition, as amended. Under this guidance, revenue is recognized when persuasive evidence of an arrangement exists, delivery has occurred or the services have been rendered and accepted by the customer, the price to the customer is fixed or determinable, and collectibility is reasonably assured. The Company considers a signed contract or purchase order to be persuasive evidence of an arrangement. The Company obtains customer acceptance of software and third-party component sales, in the form of written customer acknowledgements. In the event that the Company grants a customer the right to specified upgrades, the Company defers recognition of the entire arrangement fee until the specified upgrades are delivered, as the Company has not established vendor-specific objective evidence (VSOE) of fair value for specified upgrades. Specified upgrades include, but are not limited to, future software deliverables. Payments that extend beyond 30 days from the contract date but that are due within twelve months are generally deemed to be fixed or determinable, based on a successful collection history on such arrangements.
Fees for sales including multiple-element arrangements are allocated to each element of the arrangement based on the relative fair values of the elements. The Company determines the fair value of each element in multi-element arrangements based on VSOE of the fair value for each element. If evidence of fair value of all undelivered elements exists but evidence does not exist for one or more delivered elements, revenue is recognized using the residual method. Under the residual method, the fair value of the undelivered elements is deferred and the remaining portion of the arrangement fee is recognized as revenue. VSOE for the undelivered elements is based on the renewal rates or other objective criteria for maintenance and support services, which coincide with current pricing. The Company may also provide services that vary depending on the scope and
F-10
EMAGEON INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
complexity requested by the customer. Examples of such services include additional database consulting, system configuration, existing systems interface, and network consulting. These services generally are not deemed to be essential to the functionality of the software. If the Company has VSOE of fair value for the services, the timing of software license revenue recognition is not impacted, and service revenue is recognized as the services are performed. If the Company performs services for which VSOE of fair value is not available, software license revenue is deferred until the services are completed.
For term based license arrangements, the Company recognizes revenue for the elements over the term of the arrangement commencing upon customer acceptance, provided that all other revenue recognition criteria have been met.
For perpetual license arrangements, revenue is recognized using the residual method for software license revenue and implementation services commencing upon customer acceptance. The Company generally includes the first year of maintenance in the software license fee. This maintenance fee is deferred based on its fair value and recognized ratably over the first year of the arrangement.
The Company recognizes revenue from product sales in accordance with SEC Staff Accounting Bulletin No, 104,Revenue Recognition in Financial Statements. Revenue related to product sales is recognized upon shipment provided that title and risk of loss have passed to the customer, there is persuasive evidence of an arrangement, the sales price is fixed and determinable, collection of the related receivable is reasonably assured and customer acceptance criteria, if any, have been successfully demonstrated. The Company classifies shipping and handling cost in cost of system sales.
When our contracts contain both software and third-party components, the Company recognizes revenue related to the sale of third-party components according to guidance set forth in Emerging Issues Task Force No.00-21,Accounting for Revenue Arrangements with Multiple Deliverables(“EITF 00-21”). Third-party component revenue is recognized in accordance with contractual terms. When the Company is responsible for installing third-party components, revenue is recognized when the third-party components are delivered, installed and accepted by the customer. When the Company is not responsible for installing third-party components, revenue is recognized when the third-party components are delivered to the customer. Hardware maintenance is marketed under annual and multiyear arrangements, and revenue is recognized ratably over the contracted maintenance term.
Billings may not coincide with the recognition of revenue. Unbilled revenue occurs when revenue recognition precedes billing to the customer, and arises primarily from sales with predetermined billing schedules. Billings in excess of sales (deferred revenue) occur when billing to the customer precedes revenue recognition, and arise primarily from sales with partial prepayments upon contract execution and from maintenance revenue billed in advance of performance of the maintenance activity. The Company recognizes deferred revenue, as applicable, upon delivery and acceptance of products, as ongoing services are rendered or as other requirements requiring deferral underSOP 97-2 are satisfied.
Cost of Revenue
Cost of revenue is comprised of the cost of system sales and the cost of support services.
Cost of system sales consists of the cost of third-party components and the cost of software licenses. The cost of third-party components consists primarily of direct expenses related to the purchase, shipment, installation and configuration of third-party components. The cost of software licenses consists primarily of the amortization of acquired software, the amortization of capitalized software costs for internally developed software, and third-party software royalties.
F-11
EMAGEON INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Cost of support services consists primarily of labor costs and related overhead relating to the implementation, installation, training, application support and maintenance of the Company’s systems as well as costs related to maintenance of third-party components.
The Company expenses its sales commissions and other direct incremental costs related to contract acquisition as the liabilities are incurred, regardless of whether the associated revenue has been recognized.
Customer Indemnity and Warranty Costs
The Company provides for the estimated cost of product warranties at the time revenue is recognized if the customer does not purchase a service contract. Its actual warranty obligations depend upon actual product failure rates and service delivery costs incurred to correct product failures. Should actual product failure rates or service delivery costs differ from the Company’s estimates (which are based on specific warranty claims, historical data and engineering estimates, where applicable), revision to the estimated warranty liability would be required. Such revisions could adversely affect the Company’s operating results.
The Company offers its customers certain indemnities and warranties related to its products as follows:
Customer Indemnity: Sales agreements with customers generally contain infringement indemnity provisions. Under these agreements, the Company agrees to indemnify, defend and hold harmless the customer in connection with patent, copyright or trade secret infringement claims made by third parties with respect to the customer’s authorized use of our products and services. The indemnity provisions generally provide for the Company’s control of any required defense and settlement and cover costs and damages finally awarded against the customer, if any. Infringement indemnity provisions typically give the Company the option to make modifications of the product so it is no longer infringing or, if it cannot be corrected, to require the customer to return the product in exchange for a specified payment for loss of use. Sales agreements with customers generally also contain indemnity provisions for death, personal injury or property damage caused by the Company’s personnel or contractors in the course of performing services for customers. Under these agreements, the Company agrees to indemnify, defend and hold harmless the customer in connection with death, personal injury and property damage claims made by third parties with respect to actions of Company personnel or contractors. The indemnity provisions generally provide for the Company’s control of any required defense and settlement and cover costs and damages finally awarded against the customer, if any. The indemnity obligations contained in sales agreements generally have no specified expiration date but typically limit the amount of award covered to the fees paid by the customer over the contract term. To date, the Company has not incurred any costs to settle claims or pay awards under these indemnification provisions, nor has it been notified of any such claims. Accordingly, there are no liabilities recorded for these provisions as of December 31, 2005.
Product Warranty: The Company warrants that its software products will perform in all material respects in accordance with standard published specifications in effect at the time of delivery of the licensed products to the customer as long as the contract remains in effect. Additionally, the Company warrants that its services will be performed by qualified personnel in a manner consistent with normally accepted industry standards. The Company has a $937 liability recorded for these provisions as of December 31, 2005.
System Availability: Standard contracts with customers typically provide for a guarantee of software availability and third-party component availability, and certain penalty provisions if the Company’s solution fails to meet the guarantee thresholds. The software availability guarantee covers the functionality of software, while the component guarantee covers each individual component, as in certain circumstances a component may fail without affecting system availability. Penalty provisions in these contracts typically allow the customer a reduction in software maintenance fees related to failure to meet
F-12
EMAGEON INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
guaranteed “uptime” percentages. Penalties are calculated as a percentage of the software maintenance fee for the particular component failing to meet the uptime guarantee, and would reduce the amount of the software maintenance fee charged for that component in a specific period for these penalties, if incurred. To date, the Company has not incurred any penalties associated with these guarantees. Accordingly, there is no liability recorded for these provisions as of December 31, 2005.
Income Taxes
The Company accounts for income taxes using the liability method. Deferred income tax assets and liabilities are determined based on differences between financial reporting and tax bases of assets and liabilities and are measured using enacted tax rates and laws. Valuation allowances are established when necessary to reduce deferred tax assets to the amounts that are more likely than not to be realized. The effective tax rate for the year ended December 31, 2005 is zero percent due to a reduction in the valuation allowance, which equaled the tax effect of our taxable loss during the year.
Because the majority of the deferred tax assets relate to net operating loss (NOL) carryforwards that can only be realized if the Company is profitable in future periods, it is uncertain whether the Company will realize any tax benefit related to the net operating loss carryforward. Accordingly, the Company has provided a valuation allowance against the net deferred tax assets. The valuation allowance will remain at the full amount of the deferred tax asset until it is more likely than not that the related tax benefits will be realized through deduction against taxable income during the carryforward period. In the event of certain ownership changes, the Tax Reform Act of 1986 imposes restrictions on the amount of net operating loss and research credit carryforwards that the Company may use in any year. Due to recent stock issuances, it is possible that such limitations could currently apply. The Company has not performed a detailed analysis of its ability to use these net operating loss and research credit carryforwards. However, it is not anticipated that any such analysis would have a material impact on the Company’s financial position or results of operations.
Other Comprehensive Income
SFAS No. 130,Reporting Comprehensive Income, established standards for reporting and displaying other comprehensive income and its components. The Company’s other comprehensive income adjustments consist of foreign currency translation adjustments and unrealized losses onavailable-for-sale marketable securities. Total comprehensive loss for the year ended December 31, 2005 was $4,912.
Computation of Net Loss Per Share
Basic net loss per share is computed using the weighted average common shares outstanding during the period. Diluted net loss per share is computed using the weighted average common shares outstanding and common share equivalents outstanding during the period. Common share equivalents consist of convertible preferred stock, stock warrants, and options to purchase common stock granted to employees and directors of the Company. These common share equivalents were excluded from the computation for periods in which the Company incurred a net loss because they were anti-dilutive.
F-13
EMAGEON INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
The computations for basic and diluted net loss per share for each period are as follows:
| | | | | | | | | | | | | |
| | | For the Year Ended December 31, | |
| | | 2003 | | | 2004 | | | 2005 | |
| |
| Net loss | | $ | (11,359 | ) | | $ | (10,472 | ) | | $ | (4,997 | ) |
| Accretion of redemption value related to redeemable preferred stock | | | (66 | ) | | | (66 | ) | | | (8 | ) |
| | | | | | | | | | | | | |
| Net loss allocable to common stockholders | | $ | (11,425 | ) | | $ | (10,538 | ) | | $ | (5,005 | ) |
| | | | | | | | | | | | | |
| Common stock outstanding at beginning of period | | | 1,314,238 | | | | 2,429,742 | | | | 2,709,370 | |
| Weighted average effect of: | | | | | | | | | | | | |
| Shares issued in connection with the Ultravisual merger | | | 762,114 | | | | — | | | | — | |
| Purchase of treasury shares | | | (104,973 | ) | | | — | | | | — | |
| Release of escrowed common stock | | | — | | | | 151,866 | | | | — | |
| Conversion of preferred stock to common stock | | | — | | | | — | | | | 9,506,552 | |
| Issuance of common stock in initial public offering | | | — | | | | — | | | | 5,032,877 | |
| Issuance of common stock and preferred stock pursuant to stock option and warrant exercises | | | 1,729 | | | | 8,224 | | | | 576,321 | |
| Release of escrowed common stock upon completion of initial public offering | | | — | | | | — | | | | 149,963 | |
| | | | | | | | | | | | | |
| Weighted average number of shares of common stock — basic and diluted | | | 1,973,108 | | | | 2,589,832 | | | | 17,975,083 | |
| | | | | | | | | | | | | |
| Net loss per share — basic and diluted | | $ | (5.79 | ) | | $ | (4.07 | ) | | $ | (0.28 | ) |
| | | | | | | | | | | | | |
Preferred stock convertible into 10,827,403, 10,827,403 and zero shares of common stock for the years ended December 31, 2003, 2004 and 2005, respectively, were not included in the computation of diluted earnings per share because their effect on earnings per share would have been anti-dilutive. Options and warrants to purchase 3,131,649, 3,683,036 and 2,171,361 shares of common stock for the years ended December 31, 2003, 2004 and 2005, respectively, and warrants to purchase 216,138, 216,138 and 51,027 shares of Series D preferred stock for the years ended December 31, 2003, 2004 and 2005, respectively, were not included in the computation of diluted earnings per share because their effect on earnings per share would have been anti-dilutive.
Stock-Based Compensation
The Company recognizes compensation expense on a straight-line basis for its stock-based employee and director compensation plans using the intrinsic value method prescribed in Accounting Principles Board Opinion (“APB”) No. 25,Accounting for Stock Issued to Employees(“APB 25”), and complies with the disclosure provisions of Statement of Financial Accounting Standard (“SFAS”) No. 123,Accounting for Stock-Based Compensation, as amended. Under APB 25, compensation expense of fixed stock options is based on the difference, if any, on the date of the grant between the fair value of the stock and the exercise price of the option. Compensation expense is recognized on a straight-line basis over the vesting period, which is generally three years. The Company recognizes expense for stock-based compensation issued to non-employees and non-directors at fair value in accordance with the provisions of SFAS No. 123 and Emerging Issues Task Force
F-14
EMAGEON INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
(“EITF”) IssueNo. 96-18,Accounting for Equity Instruments That Are Issued to Other Than Employees for Acquiring, or in Conjunction with Selling, Goods or Services. See Note 16.
Had compensation expense for stock-based compensation plans been determined using the fair-value method at the grant date for all employee and director awards using the Black-Scholes pricing model, the Company’s net loss and related net loss per share would have been as follows for the periods indicated:
| | | | | | | | | | | | | |
| | | For the Year Ended December 31, | |
| | | 2003 | | | 2004 | | | 2005 | |
| |
| Actual net loss | | $ | (11,359 | ) | | $ | (10,472 | ) | | $ | (4,997 | ) |
| Deduct: Accretion of redemption value related to redeemable preferred stock | | | (66 | ) | | | (66 | ) | | | (8 | ) |
| Add: Total stock-based employee compensation expense determined under APB 25 | | | — | | | | 533 | | | | 1,171 | |
| Deduct: Total stock-based employee compensation expense determined under fair value method for all awards | | | (64 | ) | | | (801 | ) | | | (2,045 | ) |
| | | | | | | | | | | | | |
| Pro forma net loss allocable to common stockholders | | $ | (11,489 | ) | | $ | (10,806 | ) | | $ | (5,879 | ) |
| | | | | | | | | | | | | |
| Weighted average shares of common stock — basic and diluted | | | 1,973,108 | | | | 2,589,832 | | | | 17,975,083 | |
| | | | | | | | | | | | | |
| Pro forma net loss per share — basic and diluted | | $ | (5.82 | ) | | $ | (4.17 | ) | | $ | (0.33 | ) |
The pro forma effects on the net loss for the periods presented above are not necessarily representative of the effects that may occur in future periods.
Research and Development Costs
Research and development costs are charged to expense as incurred. However, costs incurred for the development of computer software that will be sold, leased or otherwise marketed are capitalized as incurred after technological feasibility of the product has been established, and capitalization ceases when the software is available for general release. These capitalized costs are subject to an ongoing assessment of recoverability based on anticipated future revenues and changes in hardware and software technologies. Costs deemed not recoverable, if any, are charged to expense. Costs that are capitalized primarily consist of direct labor costs.
Amortization of capitalized software development costs begins when the product is available for general release. Amortization is provided on aproduct-by-product basis using the straight-line method, which generates greater period expense than the percent of future revenues method, over periods not exceeding three years and is recorded as cost of system sales.
Sales Commissions
Sales commissions are charged to expense in the periods in which payments of those commissions are earned.
Translation of Foreign Currencies
The assets and liabilities of the Company’s foreign subsidiaries, all of which are located in Canada and whose cash flows are primarily in their local currency, have been translated into U.S. dollars using current exchange rates at each balance sheet date. The operating results of these foreign subsidiaries have been translated at average exchange rates that prevailed during each reporting period. Adjustments resulting from
F-15
EMAGEON INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
translation of foreign currency financial statements are reflected as accumulated other comprehensive income in the consolidated balance sheets.
Exchange gains and losses resulting from foreign currency transactions (transactions denominated in a currency other than that of the entities’ primary cash flow), excluding long-term intercompany receivables and investments, are included in operations in the period in which they occur.
Foreign currency translation and exchange gains and losses have not had, and are not expected to have, a material effect on the results of operations of the Company.
Advertising Expense
The Company expenses advertising costs as they are incurred. Advertising expense for the years ended December 31, 2003, 2004 and 2005 was $195, $191 and $48, respectively.
Recently Issued Accounting Pronouncements
In December 2004, the FASB issued Statement No. 123 (revised 2004),Share-Based Payment(“SFAS 123R”). SFAS 123R establishes standards for the accounting for transactions in which an entity exchanges its equity instruments for goods or services. It focuses primarily on accounting for transactions in which an entity obtains employee services in share-based payment transactions. SFAS 123R requires publicly-traded companies to measure the cost of employee services received in exchange for an award of equity instruments based on the grant-date fair value of the award and the estimated number of awards that are expected to vest. That cost is to be recognized over the period during which an employee is required to provide service in exchange for the award, which is usually the vesting period. SFAS 123R supersedes APB 25, which the Company has followed for all periods through December 31, 2005. SFAS 123R will be effective for the Company at the beginning of the fiscal first quarter of 2006. SFAS 123R applies to all awards granted after the required effective date and to awards modified, repurchased, or canceled after that date. Compensation cost is recognized on or after the required effective date for the portion of outstanding awards for which the requisite services have not yet been rendered, based on the grant-date fair value of those awards calculated under SFAS 123. For periods before the required effective date, SFAS 123R allows elective adjustment of the financial statements of prior periods on a basis consistent with the pro forma disclosures required for those periods by SFAS 123. Upon adoption in the first quarter of 2006, the Company will not restate prior periods. The Company expects to record additional costs relating to compensation expense as a result of the adoption of SFAS 123R; however, the precise effect of adoption has not been predicted at this time because it will depend on levels of share-based payments granted in the future and the characteristics of the Company’s common stock on the date of grant, as well as the actual forfeiture rates experienced.
In December 2004, the FASB issued SFAS No. 151,Inventory Costs. SFAS No. 151 clarifies the accounting for inventory when there are abnormal amounts of idle facility expense, freight, handling costs and wasted materials. Under existing U.S. generally accepted accounting principles (GAAP), items such as idle facility expense, excessive spoilage, double freight and rehandling costs may be “so abnormal” as to require treatment as current period charges rather than recorded as adjustments to the value of the inventory. SFAS No. 151 requires that those items be recognized as current-period charges regardless of whether they meet the criterion of “so abnormal.” In addition, SFAS No. 151 requires that allocation of fixed production overheads to the cost of conversion be based on the normal capacity of the production facilities. The provisions of SFAS No. 151 are effective for inventory costs incurred during fiscal years beginning after June 15, 2005. Earlier application is permitted for inventory costs incurred during fiscal years beginning after the date SFAS No. 151 was issued. The adoption of SFAS No. 151 is not expected to have a material impact on the Company’s financial position or results of operations.
F-16
EMAGEON INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
In May 2005, the FASB issued SFAS No. 154,Accounting Changes and Error Corrections, which is a replacement of APB Opinion No. 20,Accounting Changes, and SFAS No. 3, ReportingChanges in Interim Financial Statements. SFAS No. 154 applies to all voluntary changes in accounting principle and changes in the accounting for and reporting of a change in accounting principle. SFAS No. 154 requires that a change in method of depreciation or amortization for long-lived non-financial assets be accounted for as a change in accounting estimate that is effected by a change in accounting principle. SFAS No. 154 is effective for accounting changes and corrections of errors made in fiscal years beginning after December 15, 2005. The Company does not expect that the adoption of SFAS No. 154 will have a material impact on its financial position, results of operations or cash flows.
| |
| 3. | Initial Public Offering |
On February 14, 2005, the Company completed the initial public offering of its common stock. The Company sold 5,000,000 shares of its common stock at a price of $13.00 per share. On February 18, 2005, the over-allotment option to purchase 750,000 additional shares of common stock was exercised at $13.00 per share. Total proceeds from the initial public offering (net of underwriting discount and offering expenses) were approximately $67,200. In conjunction with the initial public offering, the Company issued 10,843,411 shares of common stock upon the automatic conversion of outstanding shares of preferred stock into shares of common stock. The Company also issued 537,082 shares of common stock upon the required exercise of warrants to purchase common stock upon the closing of the offering. The Company also released the remaining escrow holdback related to the Ultravisual Medical Systems Corporation (“Ultravisual”) merger upon the closing of the offering. Upon completion of the offering, 552,661 of common stock warrants with an exercise price of $0.00825 per share were canceled. As of the close of the initial public offering, the Company had no outstanding warrants to purchase preferred stock.
With a portion of the proceeds from the offering, the Company repaid $4,000 of its subordinated debt on February 18, 2005. Concurrent with this repayment, the Company recorded a non-cash interest charge of $621 for the write-off of debt discount related to the subordinated debt.
| |
| 4. | Acquisitions and Mergers |
Effective November 1, 2005, the Company acquired all of the outstanding capital stock of Camtronics Medical Systems Ltd. (“Camtronics”), for a cash purchase price of $40,359 including expenditures associated with the acquisition and net of $826 of cash acquired. Camtronics develops, manufactures, and markets cardiology image and information management systems.
The Company believes that adding a cardiology solution to its suite of products is particularly important to the Company’s growth. The Company expects to sell its software products to Camtronics’ existing customer base, and vice versa.
F-17
EMAGEON INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
The following table summarizes the estimated fair value of the assets acquired and liabilities assumed at the date of acquisition:
| | | | | |
| Accounts receivable | | $ | 7,101 | |
| Inventories | | | 3,207 | |
| Property, plant and equipment | | | 10,595 | |
| Other current assets | | | 693 | |
| Goodwill | | | 17,325 | |
| Intangible assets: | | | | |
| Customer relationships | | | 10,028 | |
| Developed technology | | | 1,074 | |
| Trade names | | | 501 | |
| In-process technology | | | 248 | |
| | | | | |
| Total assets acquired | | | 50,772 | |
| Accounts payable and other liabilities | | | 5,312 | |
| Accrued expenses | | | 844 | |
| Unearned revenue | | | 4,257 | |
| | | | | |
| Total liabilities assumed | | | 10,413 | |
| | | | | |
| Net assets acquired | | $ | 40,359 | |
| | | | | |
The purchase price was allocated to identified assets and liabilities of Camtronics. A third-party appraisal firm assisted the Company with valuation of the identified intangible assets. The valuation resulted in the allocation of $11,603 to identifiable intangible assets, which will be amortized over periods ranging from one to six years. The valuation also resulted in the identification of $248 of acquired in-process technology costs. This amount was determined by identifying the acquired specific in-process research and development projects that would be continued, and for which (1) technological feasibility had not been established at the acquisition date, (2) there was no alternative future use, and (3) the fair value was estimable with reasonable reliability. Accordingly, this amount was immediately expensed in the consolidated statement of operations upon the acquisition date.
The intangible assets are being amortized on a straight-line basis over lives ranging from one to six years. The estimated asset lives are determined based on projected future economic benefits and expected life cycles of the intangible assets. The amount of the purchase price allocated to customer relationships, developed technology, trade names and in-process technology was determined by an independent appraiser.
F-18
EMAGEON INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
The following unaudited pro forma information shows results of operations for 2004 and 2005 as if the acquisition had occurred at the beginning of 2004 and 2005, respectively. Pro forma results include adjustments for amortization of identified intangible assets, acquired in-process technology, additional interest expense and reduced interest income for cash needed to finance the acquisition. However, pro forma results do not include any anticipated cost savings or other effects of integration. The unaudited pro forma condensed consolidated results of operations are for comparative purposes only and are not necessarily indicative of results that would have occurred had the acquisition occurred as of the beginning of the years presented, nor are they necessarily indicative of future results.
| | | | | | | | | |
| | | For the Year Ended December 31, | |
| | | 2004 | | | 2005 | |
| | | (unaudited) | |
| |
| Revenue | | $ | 99,876 | | | $ | 111,032 | |
| Net loss | | $ | (16,459 | ) | | $ | (11,689 | ) |
| Loss per share — basic and diluted | | $ | (6.38 | ) | | $ | (0.65 | ) |
Effective May 30, 2003, the Company merged with Ultravisual Medical Systems Corporation. Ultravisual had been engaged in the business of developing visualization software for the medical imaging market. The merger with Ultravisual facilitated a strategic expansion of the Company’s product offering in accordance with its growth plan. At the time of the merger, Ultravisual was a software vendor of the Company. The Company issued 1,715,539 shares of common stock valued at $1.5675 per share, 13,727,358 shares of Series D preferred stock valued at $0.4275 per share, and warrants to purchase 552,661 shares of common stock, with an exercise price of $0.000825, exercisable upon sale of the Company. These warrants were canceled as a result of the initial public offering (see Note 3).
Goodwill arising from the acquisition of other businesses includes, but is not limited to, the synergistic value and potential competitive benefits that may be realized by the Company as a result of the acquisitions, any future products that may arise from the related technology, and the skilled and specialized workforce acquired. The amounts assigned to goodwill are not being amortized, but will be tested for impairment at least annually or as circumstances arise that may indicate a potential impairment.
The results of operations of Camtronics and Ultravisual have been included in the Company’s statements of operations since their respective acquisition dates.
Intangible assets are summarized as follows:
| | | | | | | | | | | | | | | | | | | | | |
| | | Weighted
| | | December 31, 2004 | | | December 31, 2005 | |
| | | Average
| | | Gross
| | | | | | Gross
| | | | |
| | | Amortization
| | | Carrying
| | | Accumulated
| | | Carrying
| | | Accumulated
| |
| | | Period (Years) | | | Amount | | | Amortization | | | Amount | | | Amortization | |
| |
| Developed technology | | | 5.0 | | | $ | 4,166 | | | $ | (1,319 | ) | | $ | 5,240 | | | $ | (2,212 | ) |
| Goodwill | | | n/a | | | | 3,755 | | | | — | | | | 21,079 | | | | — | |
| Customer relationships | | | 4.9 | | | | — | | | | — | | | | 10,028 | | | | (518 | ) |
| Trade names | | | 1.2 | | | | — | | | | — | | | | 501 | | | | (72 | ) |
| Capitalized software development costs | | | 1.3 | | | | 445 | | | | (424 | ) | | | 798 | | | | (567 | ) |
| Ultravisual trademark | | | n/a | | | | 250 | | | | — | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | | | | | |
| | | | | | | $ | 8,616 | | | $ | (1,743 | ) | | $ | 37,646 | | | $ | (3,369 | ) |
| | | | | | | | | | | | | | | | | | | | | |
F-19
EMAGEON INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Amortization expense was $565, $1,160 and $2,007 for the years ended December 31, 2003, 2004 and 2005, respectively. The 2005 expense includes the write-off of the Ultravisual trademark and the write-off of the in-process technology acquired in the Camtronics’ acquisition described in Note 4. In accordance with SFAS No. 86,Accounting for the Costs of Computer Software to be Sold, Leased, or Otherwise Marketed, the Company uses the straight-line method of amortization because it generates greater period expense than the percent of future revenues method. Estimated aggregate amortization expense for each of the next five years and beyond is as follows:
| | | | | |
| 2006 | | $ | 4,962 | |
| 2007 | | | 2,573 | |
| 2008 | | | 2,027 | |
| 2009 | | | 1,381 | |
| 2010 and thereafter | | | 2,254 | |
| | | | | |
| Total | | $ | 13,197 | |
| | | | | |
At December 31, 2005, the Company had marketable debt securities that were classified asavailable-for-sale and carried at estimated fair market value, consisting of U.S. government agency securities in the amount of $4,951 and with a maturity date of March 27, 2006.
At December 31, 2005, these securities were classified as a current asset. During the year ended December 31, 2005, the Company recorded $1,399 of interest income and $29 of losses related to its marketable securities. The Company held no marketable securities at December 31, 2004.
Inventories consist of the following components:
| | | | | | | | | |
| | | December 31, | |
| | | 2004 | | | 2005 | |
| |
| Third party components | | $ | — | | | $ | 497 | |
| Work in process | | | — | | | | 345 | |
| Completed systems | | | 1,422 | | | | 7,189 | |
| | | | | | | | | |
| | | $ | 1,422 | | | $ | 8,031 | |
| | | | | | | | | |
F-20
EMAGEON INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
| |
| 8. | Property and Equipment |
The Company’s major classes of property and equipment were as follows:
| | | | | | | | | | | | | |
| | | Estimated
| | | December 31, | |
| | | Useful Lives | | | 2004 | | | 2005 | |
| |
| Land | | | Indefinite | | | $ | — | | | $ | 791 | |
| Buildings and improvements | | | 39 years | | | | — | | | | 5,913 | |
| Machinery and equipment | | | 5 to 7 years | | | | — | | | | 861 | |
| Computers, software and other | | | 3 to 7 years | | | | 5,388 | | | | 12,733 | |
| Furniture and fixtures | | | 3 to 7 years | | | | 1,534 | | | | 1,967 | |
| Leasehold improvements | | | 4 to 5 years | | | | 365 | | | | 1,411 | |
| Third-party components leased to customers under operating leases | | | 5 to 7 years | | | | 11,924 | | | | 13,234 | |
| | | | | | | | | | | | | |
| | | | | | | | 19,211 | | | | 36,910 | |
| Less accumulated depreciation and amortization | | | | | | | (10,379 | ) | | | (15,477 | ) |
| | | | | | | | | | | | | |
| | | | | | | $ | 8,832 | | | $ | 21,433 | |
| | | | | | | | | | | | | |
| |
| 9. | Major Customers and Related Party Transactions |
Revenue associated with hospitals controlled by Ascension Health accounted for approximately 8%, 36% and 36% of total revenue during 2003, 2004 and 2005, respectively. As of December 31, 2005, Ascension Health held warrants to purchase up to 36,424 shares of common stock at an exercise price of $5.52.
In 2003, the Company had two customers who accounted for approximately 14% and 18% of total revenue. In 2004 and 2005, the Company had one customer, Ascension Health, who accounted for more than 10% of total revenue.
In February 1999, the Company entered into a license and royalty agreement with the University of Alabama at Birmingham Research Foundation (“UABRF”) for the right to sell, lease, subscribe, license or sublicense certain technology and know-how as a component or part of its Clinical Content Management software license. Under the terms of the license agreement, the Company paid a licensing fee of $5 and transferred an aggregate of 72,727 shares of common stock to UABRF. The Company is obligated to pay royalties of 5% of gross revenues collected from the sale, lease, subscription, licensing or sublicensing of certain products that are based upon the technology and know-how licensed from UABRF. This royalty percentage declines 1% per year on the anniversary date of the agreement until it reaches 1%. For the royalty years beginning on February 16, 2004, total annual royalty payments are capped at $200. Such royalty payments shall be no less than $5 each calendar year and are not to exceed $2,500 in the aggregate. The Company has paid a total of $104 in connection with this agreement as of December 31, 2005.
| |
| 11. | Defined Contribution Benefit Plans |
The Company has established a 401(k) plan (the “Emageon Plan”) for all eligible employees pursuant to Section 401(k) of the Internal Revenue Code. From inception through December 31, 2005, the Company did not match employee contributions to the Emageon Plan. Effective January 1, 2006, the Company began matching employee contributions to its 401(k) plans at the rate of 50% of employees’ contributions up to 3% of employees’ annual salary.
F-21
EMAGEON INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Effective with the acquisition of Camtronics at November 1, 2005, all eligible former Camtronics employees were given the opportunity to enroll in the Emageon Plan.
On May 30, 2003, the Company adopted the Ultravisual 401(k) plan (the “Ultravisual Plan”) for all former Ultravisual employees. The Ultravisual Plan was established pursuant to Section 401 (k) of the Internal Revenue Code. The Company matched employee contributions to the Ultravisual Plan based on a discretionary percentage of employee contributions determined on an annual basis, with total Company contributions to the Ultravisual Plan of $27 and $10 during the years ended December 31, 2003 and December 31, 2004, respectively. As of April 1, 2004, the Company ceased matching contributions to the Ultravisual Plan. During 2004, the Company dissolved the Ultravisual Plan and all participants were given the opportunity to enroll in the Emageon Plan.
The Company has not had taxable income since incorporation and, therefore, has not paid any income taxes. Significant components of deferred taxes at December 31, 2004 and 2005 are as follows:
| | | | | | | | | |
| | | December 31, | |
| | | 2004 | | | 2005 | |
| Deferred tax assets: | | | | | | | | |
| Net operating loss carryforward | | $ | 15,772 | | | $ | 16,610 | |
| Deferred revenue | | | 1,990 | | | | 3,017 | |
| Reserves and accrued liabilities | | | 1,270 | | | | 809 | |
| Other | | | 232 | | | | 396 | |
| | | | | | | | | |
| | | | 19,264 | | | | 20,832 | |
| | | | | | | | | |
| Deferred tax liabilities: | | | | | | | | |
| Depreciation | | | (632 | ) | | | (231 | ) |
| Developed technology | | | (1,061 | ) | | | (831 | ) |
| Other | | | (95 | ) | | | — | |
| | | | | | | | | |
| | | | (1,788 | ) | | | (1,062 | ) |
| | | | | | | | | |
| Net deferred tax assets | | | 17,476 | | | | 19,770 | |
| Valuation allowance | | | (17,571 | ) | | | (19,770 | ) |
| | | | | | | | | |
| Net deferred tax liability | | $ | (95 | ) | | $ | — | |
| | | | | | | | | |
Based on an updated analysis of the components of deferred taxes, the Company increased net deferred tax assets by $1,202 in 2004. The Company also increased the related valuation allowance by the same amounts. There was no impact on the net deferred tax liability at December 31, 2003, 2004 and 2005 or results of operations for the years then ended.
Because the majority of the deferred tax assets relate to net operating loss (NOL) carryforwards that can only be realized if the Company is profitable in future periods and because the company has never been profitable in the past, it is uncertain whether the Company will realize any tax benefit related to the net operating loss carryforward. Accordingly, the Company has provided a valuation allowance against the net deferred tax assets due to uncertainties as to their ultimate realization. The valuation allowance will remain at the full amount of the deferred tax asset until it is more likely than not that the related tax benefits will be realized through deduction against taxable income during the carryforward period. The net operating loss and research credit carryforwards expire at various times from 2019 through 2026. In the event of certain ownership changes, the Tax Reform Act of 1986 imposes restrictions on the amount of net operating loss and research credit carryforwards that the Company may use in any year. Due to recent stock issuances, it is
F-22
EMAGEON INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
possible that such limitations could currently apply. The Company has not performed a detailed analysis on its ability to use these net operating loss and research credit carryforwards. However, it is not anticipated that any such analysis would have a material impact on the financial position of the Company as a result of offsetting changes in the deferred tax valuation allowance. At December 31, 2005, the Company had net operating loss carryforwards of approximately $48.4 million.
A reconciliation of the income tax benefit computed using the statutory rate of 34% to the tax provision reported in the statements of operations is as follows:
| | | | | | | | | | | | | |
| | | For the Year Ended December 31, | |
| | | 2003 | | | 2004 | | | 2005 | |
| |
| Tax benefit computed at the statutory federal rate | | $ | (3,862 | ) | | $ | (3,561 | ) | | $ | (1,731 | ) |
| State taxes, net of federal tax benefit | | | (341 | ) | | | (314 | ) | | | (227 | ) |
| Increase in tax from: | | | | | | | | | | | | |
| Change in deferred tax valuation allowance | | | 4,123 | | | | 3,899 | | | | 1,591 | |
| Permanent differences | | | 29 | | | | 33 | | | | 184 | |
| Other | | | 51 | | | | (57 | ) | | | 183 | |
| | | | | | | | | | | | | |
| Benefit for income taxes | | $ | — | | | $ | — | | | $ | — | |
| | | | | | | | | | | | | |
The deferred income tax provisions were $3,620, $3,423 and $1,397, respectively, for the years ended December 31, 2003, 2004 and 2005 with respect to federal taxes and $503, $476 and $194, respectively for the years ended December 31, 2003, 2004 and 2005 with respect to state taxes. These amounts were offset entirely in each year by changes in the valuation allowance.
F-23
EMAGEON INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Long-term debt consists of the following:
| | | | | | | | | |
| | | December 31, | |
| | | 2004 | | | 2005 | |
| |
| Secured promissory note payable to finance company in 60 monthly installments of $45, including interest at 10.0%, due in December 2006, secured by hardware and software at customer site with a net book value at December 31, 2005 of $397 | | $ | 967 | | | $ | 508 | |
| Secured promissory note payable to finance company in 60 monthly installments of $38, including interest at 9.88%, due in March 2007, secured by hardware and software at customer site with a net book value at December 31, 2005 of $786 | | | 920 | | | | 536 | |
| Secured promissory note payable to bank in 58 monthly installments of $11, including interest at 4.76%, final payment of $10 in September 2007, secured by hardware and software at customer site with a net book value at December 31, 2005 of $310 | | | 358 | | | | 237 | |
| Secured promissory note payable to bank in 54 monthly installments of $83, including interest at 6.13%, due in June 2007, secured by hardware and software at customer site with a net book value at December 31, 2005 of $1,176 | | | 2,260 | | | | 1,397 | |
| Promissory note to governmental agency payable in 57 monthly installments of $5, including interest at 4.0%, final payment of $4 in October 2007 | | | 156 | | | | 103 | |
| Promissory notes to various purchasers under a subordinated debt agreement, payable in quarterly installments starting June 25, 2005, net of discount of $646 and $0 at December 31, 2004 and 2005 | | | 3,339 | | | | — | |
| | | | | | | | | |
| Total debt | | | 8,000 | | | | 2,781 | |
| Current portion | | | (2,472 | ) | | | (2,031 | ) |
| | | | | | | | | |
| Long-term debt, less current portion | | $ | 5,528 | | | $ | 750 | |
| | | | | | | | | |
Future maturities of long-term debt as of December 31, 2005 are as follows:
| | | | | |
| 2006 | | $ | 2031 | |
| 2007 | | | 750 | |
| | | | | |
| | | $ | 2,781 | |
| | | | | |
The Company entered into a loan and security agreement with a bank dated April 30, 2004, as amended July 31, 2004, under which the Company may borrow up to $4.0 million subject to certain restrictions and covenants, including maintenance of certain minimum levels of tangible net worth and current ratio. Interest accrues at the prime rate plus 1.5% to 2.0%, depending on the Company’s net income. There were no amounts outstanding under this agreement at December 31, 2005 and 2004. Any borrowings under the agreement are secured by certain of the assets of the Company, including its cash and accounts receivable. The agreement expires April 30, 2006, at which time all advances, if any, will be due and payable. The agreement is renewable and the Company believes it will renew the line of credit under terms at least as favorable as the current terms and that the available credit will be adequate to meet any short-term cash needs for the foreseeable future.
F-24
EMAGEON INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
On June 25, 2004, the Company issued $4,000 of promissory notes to three purchasers under a subordinated debt agreement. The agreement incorporates by reference certain of the restrictions contained in the loan and security agreement, as amended, referred to in the preceding paragraph. In connection with the notes, the Company issued 127,589 warrants to purchase common stock at an exercise price of $4.70 per share. The warrants vested upon execution of the subordinated debt agreement. The proceeds from the $4,000 promissory notes were allocated to the carrying value of the notes and the warrants issued based on their relative fair values, resulting in the recognition of a debt discount of $800, which the Company recognized as additional interest expense over the term of the notes using the interest method. Two of the three purchasers of the notes are affiliated with Company stockholders. With a portion of the proceeds from the initial public offering, the Company repaid the promissory notes on February 18, 2005. Concurrent with this repayment, the Company recorded a non-cash interest charge of $621 for the write-off of the debt discount related to the subordinated debt.
| |
| 14. | Capital Lease Obligations |
In July 2002, the Company completed financing of the purchase of third-party hardware and software related to one of its customer sites under a sale-leaseback arrangement with a finance company. The third-party hardware and software was sold to a finance company for $2,648. The transaction has been accounted for as a capital lease, wherein the hardware and software remain on the Company’s books and are depreciated and the obligation of the Company is recorded as debt. The lease has a term of 57 months. The Company has the option to renew the lease at the end of the lease term, the option to prepay the lease after two years and the option to purchase the hardware and software at the end of the lease at its fair value.
The Company has entered into agreements for certain office and computer equipment that are treated for financial reporting purposes as capital leases. For the years ended December 31, 2003, 2004 and 2005, the Company entered into capital lease arrangements for computer equipment totaling $107, $0 and $0, respectively. Accumulated amortization of the leased equipment at December 31, 2003, 2004 and 2005 was $120, $195 and $247, respectively. Amortization of assets under capital leases is included in depreciation expense.
Future minimum lease payments required under capital leases and the present value of the net minimum lease payments as of December 31, 2005, are as follows:
| | | | | |
| 2006 | | $ | 820 | |
| 2007 | | | 229 | |
| 2008 | | | 14 | |
| | | | | |
| Total minimum lease payments | | | 1,063 | |
| Loan closing costs | | | (10 | ) |
| | | | | |
| Net total minimum lease payments | | | 1,053 | |
| Amount representing interest | | | (85 | ) |
| | | | | |
| Present value of net minimum lease payments | | | 968 | |
| Current maturities of capital lease obligations | | | (732 | ) |
| | | | | |
| Long-term capital lease obligations | | $ | 236 | |
| | | | | |
F-25
EMAGEON INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Reverse Stock Split
On January 25, 2005, the Company’s Board of Directors approved a reverse stock split of the Company’s common stock. In a subsequent vote, the Company’s stockholders approved the1-for-8.25 reverse stock split of the outstanding common stock, which was effected February 4, 2005 with the filing of a certificate of amendment to the Company’s Amended and Restated Certificate of Incorporation. The accompanying financial statements give retroactive effect to this reverse stock split for all periods presented.
Preferred Stock
Pursuant to a private placement in January 2000, the Company issued 5,965,000 shares of Series A preferred stock at $0.2514669 per share. The total consideration received was $1,438, net of issuance costs of $61. As of December 31, 2004, there were 5,965,000 Series A shares authorized and outstanding. These shares were converted to common stock at the time of the Company’s initial public offering.
In conjunction with the Ultravisual merger (see Note 3), the Company issued 13,727,358 shares of Series D preferred stock. The shares were valued at $0.4275 per share. As of December 31, 2004, there were 18,000,000 Series D shares authorized, 13,727,358 shares issued and 12,354,620 shares outstanding. These shares were converted to common stock at the time of the Company’s initial public offering.
As of December 31, 2005 the Company is authorized to issue 200,000 shares of preferred stock.
Redeemable Preferred Stock
In the second and third quarter of 2000, the Company issued 22,538,597 shares of Series B redeemable preferred stock at $0.57 per share. The total consideration received was $12,707, net of issuance costs of $140. As of December 31, 2004, there were 17,200,000 Series B shares authorized and 16,885,966 shares outstanding. These shares were converted to common stock at the time of the Company’s initial public offering.
Over the course of fourth quarter 2001 and first quarter 2002, the Company issued a total of 27,433,370 shares of Series C preferred stock at $0.4275 per share. The total consideration received was $11,515, net of issuance costs of $212. As of December 31, 2004, there were 27,500,000 Series C shares authorized and 27,433,370 shares outstanding. These shares were converted to common stock at the time of the Company’s initial public offering.
In conjunction with the closing of the Series C preferred stock private placement, a new class of preferred stock was issued to former Series B preferred stockholders who did not fully participate in the Series C placement. TheSeries B-1 preferred stock has the same rights and privileges as the Series B preferred stock. As of December 31, 2004, there were 5,700,000Series B-1 shares authorized and 5,652,631 shares outstanding. These shares were converted to common stock at the time of the Company’s initial public offering.
Over the course of second and third quarter 2003, the Company issued 14,035,087 shares of Series E preferred stock at $0.4275 per share. The total consideration received was $5,889, net of issuance costs of $111. As of December 31, 2004, there were 14,050,000 shares authorized and 14,035,087 shares outstanding. These shares were converted to common stock at the time of the Company’s initial public offering.
Warrants
During 2003, the Company issued certain warrants in conjunction with the Ultravisual Merger (see note 4). The warrants did not have an expiration date and were canceled as a result of the initial public offering.
F-26
EMAGEON INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
On June 25, 2004, in conjunction with the issuance of $4,000 of promissory notes to various purchasers under a subordinated debt agreement, the Company issued a warrant to purchase 127,589 shares of common stock with an exercise price of $4.70 per share. The warrants vested upon execution of the subordinated debt agreement. The fair value of the warrants issued was $7.80 per share and was estimated using the Black-Scholes method with the following assumptions: fair value of the common stock of $10.725 per share, dividend yield of zero percent, risk-free interest rate of 3.2%, expected volatility of 70.87%,and expected life of four years. These warrants were converted to common stock at the time of the Company’s initial public offering.
In conjunction with a customer agreement signed in May 2004, the Company issued a warrant to purchase 36,424 shares of common stock at an exercise price of $5.52 per share. These warrants vested upon execution of the agreement. The fair value of the warrants issued was $6.76 per share and was estimated using the Black-Scholes method with the following assumptions: fair value of the common stock of $10.725 per share, dividend yield of zero percent, risk-free interest rate of 3.2%, expected volatility of 70.87% and expected life of 2.5 years. The warrants are recorded at a fair value of $246 and classified in prepaid expenses and other current assets in the balance sheet. This amount is being recorded as a sales discount over the life of the agreement.
A summary of warrant activity and related information is as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2003 | | | 2004 | | | 2005 | |
| | | | | | Weighted
| | | | | | Weighted
| | | | | | Weighted
| |
| | | | | | Average
| | | | | | Average
| | | | | | Average
| |
| | | | | | Exercise
| | | | | | Exercise
| | | | | | Exercise
| |
| | | Shares | | | Price | | | Shares | | | Price | | | Shares | | | Price | |
| |
| Common stock warrants at beginning of period | | | 735,760 | | | $ | 5.95 | | | | 1,315,430 | | | $ | 3.39 | | | | 1,479,443 | | | $ | 3.56 | |
| Forfeited or Canceled | | | — | | | | — | | | | — | | | | — | | | | (552,661 | ) | | | 0.01 | |
| Exercised | | | — | | | | — | | | | — | | | | — | | | | (883,981 | ) | | | 5.67 | |
| Granted | | | 579,670 | | | $ | 0.18 | | | | 164,013 | | | $ | 4.88 | | | | 6,185 | | | | 3.63 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Outstanding at end of period | | | 1,315,430 | | | $ | 3.39 | | | | 1,479,443 | | | $ | 3.56 | | | | 48,986 | | | $ | 5.04 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Exercisable at end of period | | | 730,952 | | | $ | 5.81 | | | | 910,116 | | | $ | 5.66 | | | | 48,986 | | | $ | 5.04 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
As of December 31, 2005, common stock warrants outstanding had exercise prices ranging from $3.63 to $5.52 and a weighted average remaining contractual life of 2.5 years.
The Company has established stock option plans (the “Plans”) as a means to attract, motivate and retain key employees and directors. The Compensation Committee of the Board of Directors administers and interprets the Plans and is authorized to grant options to all eligible employees of Emageon and non-employee directors and consultants. The Plans provide for incentive stock options and non-qualified stock options which are, in general, granted under the Plans on such terms and at such prices as determined by the Compensation Committee.
Options granted under the Plans during 2003, 2004 and 2005 generally vest over three to four years and are exercisable for a period of ten years.
F-27
EMAGEON INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
At December 31, 2005, 5.9 million shares of common stock were reserved for issuance under the Plans.
The Company applies APB No. 25,Accounting for Stock Issued to Employees, and related interpretations in accounting for its Plans. Accordingly, no compensation cost has been recognized for fixed stock options granted to employees where the exercise price of the option equals or exceeds the fair value of the underlying common stock on the grant date.
Pro forma information regarding results of operations is required by SFAS No. 123 as if the Company had accounted for its stock options under the fair value method of SFAS No. 123. The fair value of these options was estimated at the date of the grant using the Black-Scholes pricing model using the following assumptions for the years ended December 31, 2003, 2004 and 2005: dividend yield of zero percent, risk-free interest rates of 3.2%, 3.0% and 4.11%, respectively, an expected life of five years and a volatility factor of 70.9%. The required pro forma information is presented in Note 2.
The weighted average grant date fair values of options granted to employees under all stock option plans during the years ended December 31, 2003, 2004 and 2005 were $0.00, $5.86 and $8.53, respectively. During 2003 and 2005, options were granted under these plans at exercise prices greater than or equal to market value of the Company’s stock on the date of grant. During 2004 and 2005, options were granted under these plans at exercise prices less than market value of the Company’s stock on the date of grant.
A summary of stock option activity and related information is detailed below.
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2003 | | | 2004 | | | 2005 | |
| | | | | | Weighted
| | | | | | Weighted
| | | | | | Weighted
| |
| | | | | | Average
| | | | | | Average
| | | | | | Average
| |
| | | | | | Exercise
| | | | | | Exercise
| | | | | | Exercise
| |
| | | Shares | | | Price | | | Shares | | | Price | | | Shares | | | Price | |
| |
| Options at beginning of period | | | 1,299,217 | | | $ | 3.93 | | | | 1,779,527 | | | $ | 4.14 | | | | 2,166,901 | | | $ | 4.46 | |
| Forfeited | | | (15,341 | ) | | | 4.54 | | | | (51,842 | ) | | | 4.26 | | | | (33,517 | ) | | | 4.90 | |
| Exercised | | | (3,432 | ) | | | 4.37 | | | | (22,972 | ) | | | 2.76 | | | | (448,141 | ) | | | 3.76 | |
| Granted | | | 499,083 | | | | 4.70 | | | | 462,188 | | | | 5.85 | | | | 443,317 | | | | 10.74 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Outstanding at end of period | | | 1,779,527 | | | $ | 4.14 | | | | 2,166,901 | | | $ | 4.46 | | | | 2,128,560 | | | $ | 5.91 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Exercisable at end of period | | | 978,280 | | | $ | 3.88 | | | | 1,328,091 | | | $ | 3.94 | | | | 1,262,281 | | | $ | 4.35 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
Further information relating to stock option plans outstanding at December 31, 2005 is as follows:
| | | | | | | | | | | | | | | | | | | | | |
| | | Options Outstanding | | | Options Exercisable | |
| | | | | | Weighted
| | | Weighted
| | | | | | Weighted
| |
| | | | | | Average
| | | Average
| | | | | | Average
| |
| | | | | | Remaining
| | | Exercise
| | | | | | Exercise
| |
Range of Exercise Prices | | Number | | | Contractual Life | | | Price | | | Number | | | Price | |
| |
| $1.73 to $2.07 | | | 205,202 | | | | 5.49 years | | | $ | 1.85 | | | | 205,202 | | | $ | 1.85 | |
| $4.70 to $7.17 | | | 1,648,798 | | | | 6.95 years | | | | 5.25 | | | | 1,057,079 | | | | 4.84 | |
| $12.72 to $14.90 | | | 274,560 | | | | 9.74 years | | | | 12.93 | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | | | | | |
| | | | 2,128,560 | | | | 7.17 years | | | $ | 5.91 | | | | 1,262,281 | | | $ | 4.35 | |
| | | | | | | | | | | | | | | | | | | | | |
F-28
Lessee Arrangements. The Company leases office space and computer equipment under operating leases. The Company recognized rent expense during the years ended December 31, 2003, 2004 and 2005 of $649, $939 and $1,147, respectively. As of December 31, 2005, the amount of operating lease payments in each of the next five years and beyond is as follows:
| | | | | |
| 2006 | | $ | 1,419 | |
| 2007 | | | 1,471 | |
| 2008 | | | 1,480 | |
| 2009 | | | 1,462 | |
| 2010 | | | 595 | |
| 2011 and Beyond | | | 865 | |
| | | | | |
| | | $ | 7,292 | |
| | | | | |
Lessor Arrangements. Revenue associated with rentals under operating leases was approximately $2,004, $2,187 and $2,432 for the years ended December 31, 2003, 2004 and 2005, respectively, and is included in systems sales.
The following is a schedule by year of minimum future rental income under noncancelable operating leases of computer hardware as of December 31, 2005:
| | | | | |
| 2006 | | $ | 2,350 | |
| 2007 | | | 629 | |
| | | | | |
| Total minimum future rentals | | $ | 2,979 | |
| | | | | |
| |
| 18. | Selected Quarterly Financial Data (Unaudited) |
| | | | | | | | | | | | | | | | | |
| | | Quarters | |
| | | First | | | Second | | | Third | | | Fourth | |
| |
2005 | | | | | | | | | | | | | | | | |
| Revenue | | $ | 11,336 | | | $ | 18,570 | | | $ | 19,606 | | | $ | 24,279 | |
| Gross profit | | | 3,430 | | | | 9,495 | | | | 9,011 | | | | 8,891 | |
| Operating income (loss) | | | (4,199 | ) | | | 1,565 | | | | 1,465 | | | | (4,076 | ) |
| Net income (loss) | | $ | (4,818 | ) | | $ | 1,900 | | | $ | 1,831 | | | $ | (3,910 | ) |
| Net income (loss) per share — basic and diluted | | $ | (0.42 | ) | | $ | 0.09 | | | $ | 0.09 | | | $ | (0.19 | ) |
2004 | | | | | | | | | | | | | | | | |
| Revenue | | $ | 7,117 | | | $ | 12,117 | | | $ | 10,322 | | | $ | 16,246 | |
| Gross profit | | | 1,349 | | | | 5,586 | | | | 1,819 | | | | 4,868 | |
| Operating income (loss) | | | (3,400 | ) | | | 44 | | | | (4,200 | ) | | | (1,894 | ) |
| Net loss | | $ | (3,592 | ) | | $ | (144 | ) | | $ | (4,533 | ) | | $ | (2,203 | ) |
| Net loss per share — basic and diluted | | $ | (1.48 | ) | | $ | (0.06 | ) | | $ | (1.69 | ) | | $ | (0.82 | ) |
F-29
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized March 30, 2006.
Emageon Inc.
| | |
| | By: | /s/ Charles A. Jett, Jr. |
Charles A. Jett, Jr.
Chairman, President, and
Chief Executive Officer
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed by the following persons in the capacities indicated on March 30, 2006.
| | | | | |
Signature | | Title |
| |
/s/ Charles A. Jett, Jr.
Charles A. Jett, Jr. | | Chairman of the Board, President and
Chief Executive Officer
(principal executive officer) |
| | | |
/s/ W. Randall Pittman
W. Randall Pittman | | Chief Financial Officer and Treasurer
(principal accounting and financial officer) |
| | | |
/s/ Arthur P. Beattie
Arthur P. Beattie | | Director |
| | | |
/s/ Roddy J.H. Clark
Roddy J.H. Clark | | Director |
| | | |
/s/ Fred C. Goad, Jr.
Fred C. Goad, Jr. | | Director |
| | | |
/s/ Chris H. Horgen
Chris H. Horgen | | Director |
| | | |
/s/ Mylle H. Mangum
Mylle H. Mangum | | Director |
| | | |
/s/ John W. Thompson
John W. Thompson | | Director |
| | | |
/s/ Hugh H. Williamson, III
Hugh H. Williamson, III | | Director |
EMAGEON INC.
SCHEDULE II—VALUATION AND QUALIFYING ACCOUNTS AND RESERVES
(in thousands)
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | Additions | | | | | | Balance at
| |
| | | | | | Balance
| | | Charged to
| | | Charged to
| | | | | | End of
| |
| Description | | | | | Beginning of Period | | | Costs and Expenses | | | Other Accounts | | | Deductions | | | Period | |
| |
| Allowance for doubtful accounts deducted from accounts receivable in the balance sheet | | | 2005 | | | $ | 75 | | | $ | 57 | | | $ | — | | | $ | (6 | )(1) | | $ | 126 | |
| | | | 2004 | | | | 50 | | | | 123 | | | | — | | | | (98 | )(1) | | | 75 | |
| | | | 2003 | | | | — | | | | 104 | | | | — | | | | (54 | )(1) | | | 50 | |
| Valuation allowance deducted from net deferred tax asset in the balance sheet | | | 2005 | | | $ | 17,571 | | | $ | 1,591 | | | $ | 608(2 | ) | | $ | — | | | $ | 19,770 | |
| | | | 2004 | | | | 13,672 | | | | 3,899 | | | | — | | | | — | | | | 17,571 | |
| | | | 2003 | | | | 9,312 | | | | 4,123 | | | | 237(2 | ) | | | — | | | | 13,672 | |
| | |
| (1) | | Uncollectible accounts written off |
| |
| (2) | | Deferred tax assets arising from Ultravisual merger in 2003 and Camtronics acquisition in 2005. |