UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
| x | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2006
or
| ¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
Commission File Number 000-32085
ALLSCRIPTS HEALTHCARE SOLUTIONS, INC.
(Exact name of registrant as specified in its charter)
| | |
| Delaware | | 36-4392754 |
(State or other jurisdiction of incorporation or organization) | | (I.R.S. Employer Identification No.) |
222 Merchandise Mart Plaza, Suite 2024, Chicago, IL 60654
(Address of principal executive offices and zip code)
(800) 654-0889
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act: None
Securities registered pursuant to Section 12(g) of the Act:
Title of Class
Common Stock, $0.01 par value per share
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes x No ¨
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or section 15(d) of the Exchange Act. Yes ¨ No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding twelve months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ¨
Indicate by check mark whether the Registrant is a large accelerated filer, an accelerated filer, or a non-accelerated filer. See definition of “accelerated filer” and “large accelerated filer” in Rule 12b-2 of the Exchange Act.
| | | | |
Large accelerated filer x | | Accelerated filer ¨ | | Non-accelerated filer ¨ |
Indicate by check mark whether the registrant is a shell company (as defined in Exchange Act Rule 12b-2). Yes ¨ No x
The aggregate market value of the voting and non-voting common stock held by non-affiliates of the registrant as of June 30, 2006, the last business day of the registrant’s most recently completed second fiscal quarter, was approximately $919,350,000.
The number of outstanding shares of the registrant’s Common Stock as of February 15, 2007 was 54,778,049.
Documents Incorporated by Reference: Portions of the Proxy Statement for the 2007 annual stockholders’ meeting are incorporated by reference into Part III.
ALLSCRIPTS HEALTHCARE SOLUTIONS, INC.
TABLE OF CONTENTS TO
2006 ANNUAL REPORT ON FORM 10-K
Allscripts Healthcare Solutions, Inc. was incorporated in the state of Delaware. In this report, “we,” “us,” “our” and “Allscripts” refer to Allscripts Healthcare Solutions, Inc. and its wholly owned subsidiaries as of December 31, 2006, unless the context indicates otherwise. Our trademarks or service marks include Allscripts with logo®, EmSTAT™,Physician Relationship Management Platform™, HealthMatics®, Impact.MD®, Ntierprise®,Physicians Interactive™, TouchChart™, TouchScript®, TouchWorks™, NEPSI™, and eRxNOW™. Other trademarks, service marks and trade names referred to in this report, or documents incorporated or incorporated by reference herein or therein, are the property of their respective owners.
Safe Harbor for Forward-Looking Statements
This report contains forward-looking statements that involve risks and uncertainties, including those discussed under the caption “Risk Factors.” We develop forward-looking statements by combining currently available information with our beliefs and assumptions. These statements relate to future events, including our future performance, and some of these statements can be identified by the use of forward-looking terminology such as “believe,” “expect,” “anticipate,” “intend,” “contemplate,” “seek,” “plan,” “estimate,” “will,” “may,” “should” and the negative or other variations of those terms or comparable terminology or by discussion of strategy, plans or intentions. Forward-looking statements do not guarantee future performance, which may be materially different from that expressed in, or implied by, any such statements. You should not rely upon these statements as facts.
We make these statements under the protection afforded by Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. Because we cannot predict all of the risks and uncertainties that may affect us, or control the ones we do predict, these risks and uncertainties can cause our results to differ materially from the results we express in our forward-looking statements. We undertake no obligation to, and expressly disclaim any such obligation to, update or revise any forward-looking statements to reflect changed assumptions, the occurrence of anticipated or unanticipated events, changes to future results over time or otherwise.
PART I
Item 1. Business
Company Overview
Allscripts Healthcare Solutions, Inc. is a leading provider of clinical software, connectivity and information solutions that physicians use to improve the quality of healthcare. Our businesses provide innovative solutions that inform physicians with just right, just in time information, connect physicians to each other and to the entire community of care, and transform healthcare, improving both the quality and efficiency of care. Our software and related services segment of our business provides clinical software solutions, including electronic health record (“EHR”), electronic prescribing (“e-prescribing”) and document imaging solutions. Our information services segment, through our physicians interactive business unit, provides clinical education and information solutions for physicians and patients, along with physician-patient connectivity solutions through our partnership with Medem, Inc. (“Medem”). Our prepackaged medications segment of our business, provides prepackaged medication fulfillment solutions, which includes both medications and software for dispensing and inventory control.
On March 2, 2006, we acquired A4 Health Systems, Inc. (“A4”), a privately held company and a provider of clinical and practice management solutions to physician practice groups and of certain additional offerings for hospitals. The A4 acquisition allows us to reach new markets: small and mid-sized physician practice groups seeking either a practice management system or a combined EHR and practice management solution, and hospitals seeking emergency department information systems (“EDIS”) or care management solutions. The A4 acquisition enables us to extend our product offerings by allowing us to independently offer an integrated solution that combines our EHR solution with A4’s practice management system. We believe that A4’s EDIS and care management solutions offer a natural connection to our ambulatory applications, facilitating the continuity of care between the acute and ambulatory healthcare settings.
Clinical Solutions
EHR/Practice Management Physician Practice Solutions. EHR solutions automate the collection and management of clinical data, allowing physician practice groups to enter, organize, and effectively utilize secure patient chart information at the point of care. EHR solutions also streamline practice-wide clinical workflow and communication and help physicians manage lab orders, results and other data. EHR solutions can improve healthcare quality and reduce costs by preventing medical errors, reducing paperwork and reducing administrative inefficiencies. Practice management systems automate administrative workflow, including scheduling, patient billing and collection and claims management. Practice management systems improve the efficiency of operations within a physician practice, particularly the financial aspects of the practice related to billing and reimbursement.
Hospital Emergency Department Solutions. Hospital emergency department information systems automate emergency room processes, including patient registration, triage, tracking and reporting. Hospital emergency department information systems enable hospitals to better manage patient flow and emergency department activity.
Care Management Solutions. Hospital care management programs automate processes related to case management, quality management and utilization management. Care management programs help hospitals manage length of stay, billing and claims processing, and patient care resources. The benefits of these solutions to hospitals include enhanced financial performance and improved patient outcomes. We believe there is relatively low penetration of care management solutions in the hospital market, and that there is a significant opportunity for us to penetrate this market.
3
Physicians Interactive
Clinical education and information solutions programs link physicians with pharmaceutical companies, medical product suppliers and health plans through e-mail, surveys, and online interactive programs. These web-based programs, often referred to as e-Detailing, use interactive sessions to provide product information and clinical education to physicians. Pharmaceutical companies leverage e-Detailing to assist in the marketing and sales efforts for their products. We believe that there is a significant opportunity for our clinical education and information solutions within this market. We believe that one of the drivers in this market is the growing need for pharmaceutical companies to communicate with physicians in more efficient and cost-effective ways. As more physicians access online resources, we believe that pharmaceutical companies are increasingly seeking to communicate with physicians directly through this highly effective channel. Our physicians interactive business unit offers electronic marketing and educational programs to pharmaceutical companies, and delivers these programs to a network of physicians nationwide through an interactive web-based platform.
Medication Solutions
The market for the sale of prepackaged medications to physicians for on-site dispensing includes medications distributed for occupational health, workers compensation, urgent care and bariatric facilities. On-site dispensing offers provider organizations an opportunity to improve financial performance by adding an incremental source of revenue and reducing expenses related to prescription transmission, billing and processing. From a patient perspective, the dispensing of medications at the point of care provide an increased level of convenience, privacy and treatment compliance, whether in the physician’s office, at a clinic or at the patient’s place of employment.
Our Competitive Strengths
We believe that the following competitive strengths are the keys to our success:
First-Class Technologies That Enable Industry-Leading Solutions
We have been an innovator in the development and adoption of clinical solutions. We believe our clinical solutions provide the following advantages:
| | • | | Accessibility. Physicians can instantly access our web-based clinical solutions from a variety of locations, including the exam room, hospital, office or home. With our EHR solutions, physicians can easily perform such important tasks as dictation and charge capturing in an offline mode and immediately transfer those files once reconnected to the network. Our solutions run on personal digital assistants, tablet PCs, desktop workstations and other wireless devices. |
| | • | | Connectivity. Our clinical solutions connect physicians to the valuable, objective information they need prior to, during and after the care process, enabling physicians to provide higher quality care and do so more cost effectively. We also provide efficiency to other participants in the care continuum by linking them to the physician. |
| | • | | Paperless Innovation. Our document imaging and scanning solutions allow even the largest organizations to manage information and documentation in a paperless environment and provide optical character recognition technology to rapidly retrieve information within the EHR. |
| | • | | Wireless Leadership. Using wireless handheld devices or desktop workstations, we believe that we have accelerated the use in healthcare of a wireless platform, automating all of the most common physician activities, including prescribing, capturing charges, dictating, ordering lab tests, viewing lab results, providing patient education and taking clinical notes. |
| | • | | Interoperability. Our products are designed to operate with existing installed systems, in both ambulatory and acute settings. |
4
| | • | | Modularity. The ability to implement individual modules of certain of our solutions enables physicians to start with the tools that solve their most pressing needs and provides an opportunity for a rapid return on investment. |
| | • | | Award-Winning and Certified Solutions.Our clinical software solutions have garnered numerous industry accolades and honors. In 2006, the Microsoft Healthcare Users Group (“MS-HUG”) selected Allscripts as “Best in Class” for Ambulatory Care for a second year in a row. Allscripts also earned a first-place award at the 2005 Emerging Technologies Healthcare Innovations Congress (“TETHIC”) Conference, in the category of Technology for the Improvement of Patient Outcomes. We have also won awards at the TEPR (Towards an Electronic Patient Record) Conference in 2005 and 2006. Likewise, Allscripts’ customers have been recognized as HIMMS Physician IT Leader of the Year for results they have achieved in using our solutions. Our TouchWorks EHR and Healthmatics EHR are certified by the Certification Commission for Healthcare Information Technology (“CCHIT”) as meeting CCHIT’s certification standards for functionality, interoperability and security. CCHIT represents the first consensus-based standards for EHRs. |
Significant Installed Base
Over 220 physician practices and 420 hospitals, representing over 4,000 clinics nationwide and including some of the country’s most prestigious medical groups, have selected our EHR solutions. Our significant installed base serves as a referral source for our prospective clients who are interested in purchasing an EHR solution.
Breadth of Product and Service Offering
We are a leading provider of clinical software, connectivity and information solutions that physicians use to improve the delivery and quality of healthcare. Our suite of clinical software solutions includes electronic health records, e-prescribing, and personal health records, encompassing virtually all of the most common functions performed by a physician at the point of care. Our product offerings also include an integrated practice management solution for physician groups, as well as EDIS to hospital emergency departments, and care management solutions to hospitals.
Integrated Solution and Product Offering with IDX
We have a strategic alliance agreement with IDX, a wholly owned subsidiary of General Electric Company (“GE”), which was entered into in January 2001 and amended in January 2006. Under this agreement, we are a preferred provider of ambulatory, point-of-care clinical EHR solutions to IDX’s installed base of medium to large physician practices nationwide. As of December 31, 2006, we had signed contracts with over 125 IDX Clients.
Sales and Marketing
We have experienced sales executives with extensive industry expertise. In the clinical solutions business unit, we primarily sell directly to our customers through our sales force. We also have targeted direct sales forces for our physicians interactive and our medication services business units. As of December 31, 2006, we employed 111 full-time sales and marketing employees.
Products and Services
Clinical Solutions
Our clinical solutions business units provide the following clinical software solutions:
| | • | | TouchWorksElectronic Health Record is an award-winning EHR solution designed to enhance physician productivity using Tablet PCs, wireless handheld devices, or a desktop workstation for the |
5
| | purpose of automating the most common physician activities, including prescribing, dictating, ordering lab tests and viewing results, documenting clinical encounters and capturing charges, among others. TouchWorks has the functionality to handle the complexities of large physician practices, while also addressing the needs of mid-sized physician practice groups. Our Touchworks EHR is certified by CCHIT |
| | • | | TouchWorks PM is a practice management system that streamlines administrative aspects of physician practices, including patient scheduling, electronic remittances, electronic claims submission and electronic statement production. This system also provides multiple resource scheduling, instant reporting and referral tracking. Our electronic data interchange solution facilitates statement management processing, claims management processing, electronic remittances and appointment reminders. |
| | • | | HealthMatics EHRis an electronic health record solution targeted at small to mid-sized physician practice groups. Like our TouchWorks EHR, this solution automates the most common physician activities, such as prescribing, clinical reporting, ordering lab tests and viewing results, and capturing charges. We also offer a disaster recovery solution that safeguards data and provides remote application access in the event of a failure at the primary system site. Our HealthMatics EHR is also certified by CCHIT. |
| | • | | HealthMatics Ntierprise is a practice management system that streamlines administrative aspects of physician practices, including patient scheduling, electronic remittances, electronic claims submission and electronic statement production. This system, which provides the engine for TouchWorks Practice Management, also provides multiple resource scheduling, instant reporting and referral tracking. Our electronic data interchange solution facilitates statement management processing, claims management processing, electronic remittances and appointment reminders. |
| | • | | TouchScript is an e-prescribing solution that physicians can access securely via the Internet to quickly, safely and securely prescribe medications, check for drug interactions, access medication histories, review drug reference information and send prescriptions directly to a pharmacy or mail order facility. TouchScript can be a starting point for medical groups to seamlessly transition over time to a complete EHR. |
| | • | | eRxNOW is an easy-to-use, web-based e-prescribing solution that is safe, secure, requires no downloading and no new hardware. The eRxNOW solution offers all of the functionality of TouchScript in an Application Service Provider (“ASP”) model that is accessible by the Internet on computers, handheld devices and cell phones. The software is being offered free of charge to every prescriber in America in furtherance of the National ePrescribing Patient Safety Initiative, a coalition of companies led by Allscripts. Like TouchScript, eRxNOW can be a starting point for medical groups to seamlessly transition over time to a complete EHR. |
| | • | | Impact.MD provides an electronic repository for all patient record information including patient charts, office notes, lab results, explanation of benefits and referral letters among other paper based documents. As with TouchScript and eRxNOW, Impact.MD can be a starting point for medical groups seeking to seamlessly transition over time to a complete EHR. |
| | • | | HealthMatics ED and EmSTATare emergency department information systems designed to manage patient flow through the emergency department by tracking patient location, activity and outstanding orders and procedures. These solutions guide emergency clinicians in entering consistent, complete and efficient documentation on patients and provide shareable, real-time, mobile access to patient information from registration to discharge. |
| | • | | Canopy is a web-based software solution that streamlines the patient care management process. Canopy automates utilization, case, discharge and quality management processes relating to patient hospital visits. These systems are based on an ASP model designed to provide ease of use and minimal IT staff involvement at the hospital. |
6
Physicians Interactive
Our physicians interactive business unit provides the following key solutions:
| | • | | Physicians Interactive is a web-based solution that connects physicians with pharmaceutical companies, medical device manufacturers and biotech companies. One element of this solution, often referred to as e-Detailing, uses interactive sessions to provide clinical education and information to physicians about medical products and disease states. This promotes more informed decision-making, increased efficiency and ultimately higher quality patient care. Other elements of the Physicians Interactive platform include e-surveys, clinical updates, resource centers, key opinion leader materials and other physician relationship management services. |
| | • | | Physician Relationship Management Platform (“PRMP”)provides pharmaceutical companies with a turnkey system to build an electronic dialogue and manage ongoing relationships with physicians. The PRMP incorporates a full suite of online tools, including campaign management, physician communication and education and sample and rep requests, as well as e-Detailing opportunities. All of these tools are driven through a sophisticated physician-centric database that dynamically delivers customized information according to physician preferences. |
Medication Services
Our medication services business unit provides point-of-care medication management and medical supply solutions for physicians and other healthcare providers. With approximately 10,000 physician customers nationwide, our solutions enable physician groups, including occupational health, workers compensation, urgent care and bariatric facilities, to dispense medications at the point of care. Our medication repackaging solutions offer provider organizations an opportunity to improve financial performance by adding an incremental source of revenue and reducing expenses related to prescription transmission, billing and processing. From a patient perspective, our medication repackaging solutions provide an increased level of convenience, privacy and treatment compliance.
Research and Development
As of December 31, 2006, we had 169 employees in research and development. In addition, we engage the services of approximately 62 dedicated development professionals in India. The primary purposes of our research and development groups are to develop new features and enhancements to our respective solutions, ensure that our solutions comply with continually evolving regulatory requirements and create additional opportunities to connect our systems to the healthcare community.
For the year ended December 31, 2006, we spent approximately 10% of our software and services revenue on related research and product development. Our clinical solutions business unit capitalizes software development costs incurred from the time technological feasibility of the software is established until the software is available for general release. Non-capitalizable research and development costs and other computer software maintenance costs related to software development are expensed as incurred.
Competition
The market for our products and services is fragmented, intensely competitive and is characterized by rapidly evolving industry standards, technology and user needs and the frequent introduction of new products and services. Some of our competitors may be more established, benefit from greater name recognition and have substantially greater financial, technical, and marketing resources than us. We compete on the basis of several factors, including:
| | • | | breadth and depth of services; |
7
| | • | | reliability, accuracy and security; |
| | • | | industry expertise and experience. |
Clinical Solutions
Our industry is intensely competitive and rapidly evolving in terms of both technology and product standards. There are numerous companies that offer EHR and practice management products and the marketplace remains fragmented. We face competition from several types of organizations, including providers of practice management solutions, ambulatory and acute EHR solutions and enterprise-wide application solutions.
Our key competitors in the EHR and practice management markets include Cerner Corporation, eClinicalWorks Inc., Sage Software, Inc., Epic Systems Corporation, GE, iMedica Corporation, McKesson Corporation, Misys Healthcare Systems, Picis, Inc., Quality Systems, Inc., and Wellsoft Corporation. In the hospital market, our key competitors are MedHost, Inc., Picis, Inc. and Wellsoft Corporation.
New safe harbors to the federal Anti-Kickback Statute and corresponding exceptions to the federal Stark law may alter the competitive landscape, as such new safe harbors and exceptions allow hospitals and certain other donors to donate certain items and services used in electronic prescription systems and electronic health records systems. These new safe harbors and exceptions are intended to accelerate the adoption of electronic prescription systems and electronic health records systems, and therefore provide new and attractive opportunities for us to work with hospitals and other donors who wish to provide our clinical solutions to physicians. At the same time, such safe harbors and exceptions may result in increased competition from providers of acute EHR solutions, whose hospital customers may seek to donate their existing acute EHR solutions to physicians for use in ambulatory settings.
Physicians Interactive
We compete with several types of organizations, including clinical information and education providers, such as disease state management companies, full service e-marketing companies, companies who provide e-Detailing software, and the in-house efforts of our clients, including health plans, pharmacy benefit managers and pharmaceutical companies. Our key competitors include Aptilon Inc., Dendrite International, Inc., WebMD Corporation, Lathian Systems, Inc., Quintiles Transnational Corp. and Ventiv Health, Inc.
Medication Services
Our competitors include other medication repackaging service and bulk pharmaceutical distributors. Our key competitors in this segment include Cardinal Health, Inc., DRx (a wholly owned subsidiary of Purkinje, Inc.), McKesson Corporation, PD-Rx Pharmaceuticals, Inc., Pharmapac, Physicians Total Care, Inc., Southwood Pharmaceuticals, Inc. and various other regional distributors.
Strategic Alliances
Our key strategic relationships include the following:
| | • | | IDX. We have a strategic alliance agreement with IDX and GE that was entered into with IDX in January 2001 and amended on January 18, 2006. Under this agreement, we are a preferred provider of ambulatory, point-of-care clinical EHR solutions to IDX’s installed base of medium to large physician practices nationwide. The amended agreement with IDX and GE, which runs through July 2012, supports the ongoing integration and compatibility of the Allscripts and IDX products. We also have the |
8
| | right to offer our own integrated practice management and EHR solution outside of the IDX installed base. IDX sold its ownership interest in Allscripts during the 2006 fiscal year. |
| | • | | Medem.Allscripts has a strategic partnership with Medem, Inc., a physician-patient communications network, founded and governed by the American Medical Association and 45 leading medical societies. Allscripts and Medem collaborate on distribution and expansion of interactive e-health solutions to physicians and their patients, with a focus on secure personal health records for patients, connecting to selected information from Allscripts’ electronic health record and e-prescribing solutions. Medem also provides personal interactive health records for patients, customizable web sites for physician practices with integrated HIPAA-compliant secure email, fee-based online clinical consultation software, and trusted, award-winning clinical content from America’s leading medical societies. We own approximately 1.9% of Medem capital stock and have the ability to increase this ownership to approximately 28.8% (34.1% of voting shares) if we exercise our option to purchase additional equity and exercise our right to convert our notes into additional equity. These percentages give effect to the additional $500,000 convertible secured promissory note funded in February 2006 under a contract signed in November 2005. |
Employees
As of December 31, 2006, we employed 914 persons on a full-time basis, including 241 in customer service and support, 111 in sales and marketing, 33 in production and warehousing, 169 in product development, 226 in product deployment, and 134 in general and administrative. None of our employees is covered by a collective bargaining agreement or is represented by a labor union.
Backlog
At December 31, 2006 and 2005, our aggregate backlog for our software and information services segments totaled approximately $211 million and $91 million, respectively. Approximately $79 million to $84 million of ending 2006 backlog is not expected to be realized during 2007. Our backlog information excludes our prepackaged medications segment due to the short-term nature of a prepackaged medication order and also excludes contracted maintenance beyond a twelve month horizon.
Financial Information About Segments
Financial information about our three segments is described in Part II, Item 7 “Management’s Discussion and Analysis of Financial Condition and Results of Operations.”
Available Information
Our website address is www.allscripts.com. Information on our website is not incorporated by reference herein. Copies of our annual reports on Form 10-K, quarterly reports on Form 10-Q and current reports on Form 8-K and any amendments to those reports, as well as Section 16 reports filed by our insiders, are available free of charge on our website as soon as reasonably practicable after we file the reports with, or furnish the reports to, the Securities and Exchange Commission.
Item 1A. Risk Factors
You should carefully consider the risks and uncertainties described below and other information in this report. These are not the only risks and uncertainties that we face. Additional risks and uncertainties that we do not currently know about or that we currently believe are immaterial may also harm our business operations. If any of these risks or uncertainties occurs, it could have a material adverse effect on our business.
9
Risks Related to Our Business
If physicians and hospitals do not accept our products and services, or delay in deciding whether to purchase our products and services, our business, financial condition and results of operations will be adversely affected.
Our business model depends on our ability to sell our products and services. Acceptance of our products and services requires physicians and hospitals to adopt different behavior patterns and new methods of conducting business and exchanging information. We cannot assure you that physicians and hospitals will integrate our products and services into their workflow or that participants in the healthcare market will accept our products and services as a replacement for traditional methods of conducting healthcare transactions. Achieving market acceptance for our products and services will require substantial sales and marketing efforts and the expenditure of significant financial and other resources to create awareness and demand by participants in the healthcare industry. If we fail to achieve broad acceptance of our products and services by physicians, hospitals and other healthcare industry participants or if we fail to position our services as a preferred method for information management and pharmaceutical healthcare delivery, our business, financial condition and results of operations will be adversely affected.
If we are unable to successfully integrate businesses we acquire, our ability to expand our product and service offerings and our customer base may be limited.
In order to expand our product and service offerings and grow our business by reaching new customers, we may continue to acquire businesses that we believe are complementary. The successful integration of acquired businesses, including A4, is critical to our success. Such acquisitions, including the A4 acquisition, involve numerous risks, including difficulties in the assimilation of the operations, services, products and personnel of the acquired company, the diversion of management’s attention from other business concerns, entry into markets in which we have little or no direct prior experience, the potential loss of the acquired company’s key employees and our inability to maintain the goodwill of the acquired businesses. If we fail to successfully integrate acquired businesses or fail to implement our business strategies with respect to these acquisitions, we may not be able to achieve projected results or support the amount of consideration paid for such acquired businesses.
The successful implementation of our acquisition strategy depends on our ability to identify suitable acquisition candidates, acquire companies on acceptable terms, integrate their operations and technology successfully with our own and maintain the goodwill of the acquired business. We are unable to predict whether or when any prospective acquisition candidate will become available or the likelihood that any acquisition will be completed. Moreover, in pursuing acquisition opportunities, we may compete for acquisition targets with other companies with similar growth strategies. Some of these competitors may be larger and have greater financial and other resources than we have. Competition for these acquisition targets could also result in increased prices of acquisition targets.
Our business will be harmed if we are unable to enter into and maintain relationships with IDX customers.
In 2001, we entered into a 10-year strategic alliance agreement with IDX pursuant to which we and IDX agreed to coordinate product development and align our respective marketing processes. Under this agreement, IDX had granted us the exclusive right to market, sell, license and distribute ambulatory point-of-care and clinical EHR solutions to IDX customers. On January 4, 2006, IDX was acquired by GE and on January 18, 2006, we, IDX and GE amended and restated our strategic alliance agreement. Under this amended agreement, the exclusivity provisions of the original agreement were modified such that, in addition to our solutions, GE may market its Centricity electronic health record ambulatory solution to IDX customers. After July 18, 2007, these exclusivity provisions will terminate and IDX may, but will not be required to, market our solutions to its customers. Further, under the original agreement, we were restricted from providing practice management
10
systems. The amended agreement eliminates these restrictions, except that if we acquire a practice management system, we may not market or provide such system to the existing IDX customer base until July 18, 2007. Therefore, we are unable to market the practice management system acquired in the A4 acquisition to IDX customers until after that date. We have historically generated a significant portion of our bookings from IDX customers. In that regard, approximately 58% and 73% of TouchWorks bookings for the years ended December 31, 2006 and 2005, respectively, were from sales to members of IDX’s customer base. If we are unable to compete effectively against the Centricity product or are otherwise unable to maintain sales to IDX customers at the levels we have historically experienced, our revenues may decrease and our results of operations may be harmed. Additionally, if certain competitors of IDX or GE acquire us prior to July 18, 2007, the above-described restrictions on IDX’s ability to market products competitive to our products will terminate.
Under the amended agreement, we and IDX will continue to cooperate with respect to installation and implementation of one another’s products for common IDX and Allscripts customers and in the provision of customer support services to ensure that such products remain interoperable. If the amended agreement is terminated for any reason, or if IDX and GE were to fail to fulfill their obligations under the amended agreement, we would lose the benefits of the amended agreement, which could harm our business, financial condition and results of operations.
We also have a cross license and software maintenance agreement with IDX pursuant to which we granted IDX a non-exclusive, non-cancelable and non-terminable license to use, market and sublicense certain of our software combined with IDX products, and IDX granted us a non-exclusive, non-cancelable and non-terminable license to use, market and sublicense certain IDX software for use with our products. If the amended agreement is terminated, we will not have access to certain IDX software, harming our ability to integrate our services with IDX systems and provide real-time data synchronization. This may make our systems less desirable to IDX customers and could harm our business, financial condition and results of operations.
It is difficult to predict the sales cycle for our healthcare software solutions and physician education services.
The duration of the sales cycle for our healthcare software solutions and physician education services depends on a number of factors, including the nature and size of the potential customer and the extent of the commitment being made by the potential customer, and is difficult to predict. Our sales and marketing efforts with respect to hospitals and large healthcare organizations generally involve a lengthy sales cycle due to these organizations’ complex decision-making processes. Additionally, in light of increased government involvement in healthcare, and related changes in the operating environment for healthcare organizations, our current and potential customers may react by curtailing or deferring investments, including those for our services. If potential customers take longer than we expect to decide whether to purchase our solutions, our selling expenses could increase and our revenues could decrease, which could harm our business, financial condition and results of operations.
Competition for our employees is intense, and we may not be able to attract and retain the highly skilled employees we need to support our business.
Our ability to provide high-quality services to our clients depends in large part upon our employees’ experience and expertise. We must attract and retain highly qualified personnel with a deep understanding of the healthcare and healthcare information technology industries. We compete with a number of companies for experienced personnel and many of these companies, including clients and competitors, have greater resources than we have and may be able to offer more attractive terms of employment. In addition, we invest significant time and expense in training our employees, which increases their value to clients and competitors who may seek to recruit them and increases the costs of replacing them. If we fail to retain our employees, the quality of our services could diminish and this could have a material adverse effect on our business, financial condition and results of operations.
11
If we lose the services of our key personnel, we may be unable to replace them, and our business, financial condition and results of operations could be adversely affected.
Our success largely depends on the continued skills, experience, efforts and policies of our management and other key personnel and our ability to continue to attract, motivate and retain highly qualified employees. In particular, the services of Glen E. Tullman, our Chairman and Chief Executive Officer, are integral to the execution of our business strategy. If one or more of our key employees leaves our employment, we will have to find a replacement with the combination of skills and attributes necessary to execute our strategy. Because competition for skilled employees is intense, and the process of finding qualified individuals can be lengthy and expensive, we believe that the loss of the services of key personnel could adversely affect our business, financial condition and results of operations. We cannot assure you that we will continue to retain such personnel. We do not maintain keyman insurance for any of our key employees.
If we are unable to successfully introduce new products or services or fail to keep pace with advances in technology, our business, financial condition and results of operations will be adversely affected.
The successful implementation of our business model depends on our ability to adapt to evolving technologies and industry standards and introduce new products and services. We cannot assure you that we will be able to introduce new products on schedule, or at all, or that such products will achieve market acceptance. Moreover, competitors may develop competitive products that could adversely affect our results of operations. A failure by us to introduce planned products or other new products or to introduce these products on schedule could have an adverse effect on our business, financial condition and results of operations.
If we cannot adapt to changing technologies, our products and services may become obsolete, and our business could suffer. Because the Internet and healthcare information markets are characterized by rapid technological change, we may be unable to anticipate changes in our current and potential customers’ requirements that could make our existing technology obsolete. Our success will depend, in part, on our ability to continue to enhance our existing products and services, develop new technology that addresses the increasingly sophisticated and varied needs of our prospective customers, license leading technologies and respond to technological advances and emerging industry standards and practices on a timely and cost-effective basis. The development of our proprietary technology entails significant technical and business risks. We may not be successful in using new technologies effectively or adapting our proprietary technology to evolving customer requirements or emerging industry standards, and, as a result, our business could suffer.
Our business depends in part on and will continue to depend in part on our ability to establish and maintain additional strategic relationships.
To be successful, we must continue to maintain our existing strategic relationships and establish additional strategic relationships with leaders in a number of healthcare and healthcare information technology industry segments. This is critical to our success because we believe that these relationships contribute towards our ability to:
| | • | | extend the reach of our products and services to a larger number of physicians and hospitals and to other participants in the healthcare industry; |
| | • | | develop and deploy new products and services; |
| | • | | further enhance the Allscripts brand; and |
| | • | | generate additional revenue and cash flows. |
Entering into strategic relationships is complicated because strategic partners may decide to compete with us in some or all of our markets. In addition, we may not be able to maintain or establish relationships with key participants in the healthcare industry if we conduct business with their competitors. We depend, in part, on our
12
strategic partners’ ability to generate increased acceptance and use of our products and services. If we lose any of these strategic relationships or fail to establish additional relationships, or if our strategic relationships fail to benefit us as expected, we may not be able to execute our business plan, and our business, financial condition and results of operations may suffer.
Future acquisitions may result in potentially dilutive issuances of equity securities, the incurrence of indebtedness and increased amortization expense.
Future acquisitions may result in potentially dilutive issuances of equity securities. In addition, future acquisitions may result in the incurrence of debt, the assumption of known and unknown liabilities, the write off of software development costs and the amortization of expenses related to intangible assets, all of which could have an adverse effect on our business, financial condition and results of operations. We have taken, and, if an impairment occurs, could take, charges against earnings in connection with acquisitions.
If our products fail to perform properly due to undetected errors or similar problems, our business could suffer.
Complex software such as ours often contains undetected defects or errors. It is possible that such errors may be found after introduction of new software or enhancements to existing software. We continually introduce new solutions and enhancements to our solutions, and, despite testing by us, it is possible that errors might occur in our software. If we detect any errors before we introduce a solution, we might have to delay deployment for an extended period of time while we address the problem. If we do not discover software errors that affect our new or current solutions or enhancements until after they are deployed, we would need to provide enhancements to correct such errors. Errors in our software could result in:
| | • | | harm to our reputation; |
| | • | | delays in commercial release; |
| | • | | product liability claims; |
| | • | | delays in or loss of market acceptance of our solutions; |
| | • | | license terminations or renegotiations; and |
| | • | | unexpected expenses and diversion of resources to remedy errors. |
Furthermore, our customers might use our software together with products from other companies. As a result, when problems occur, it might be difficult to identify the source of the problem. Even when our software does not cause these problems, the existence of these errors might cause us to incur significant costs, divert the attention of our technical personnel from our solution development efforts, impact our reputation and cause significant customer relations problems.
Our future success depends upon our ability to grow, and if we are unable to manage our growth effectively, we may incur unexpected expenses and be unable to meet our customers’ requirements.
We will need to expand our operations if we successfully achieve market acceptance for our products and services. We cannot be certain that our systems, procedures, controls and existing space will be adequate to support expansion of our operations. Our future operating results will depend on the ability of our officers and key employees to manage changing business conditions and to implement and improve our technical, administrative, financial control and reporting systems. We may not be able to expand and upgrade our systems and infrastructure to accommodate these increases. Difficulties in managing any future growth could have a significant negative impact on our business, financial condition and results of operations because we may incur unexpected expenses and be unable to meet our customers’ requirements.
13
Our failure to compete successfully could cause our revenue or market share to decline.
The market for our products and services is fragmented, intensely competitive and is characterized by rapidly evolving industry standards, technology and user needs and the frequent introduction of new products and services. Some of our competitors may be more established, benefit from greater name recognition and have substantially greater financial, technical and marketing resources than us. Moreover, we expect that competition will continue to increase as a result of consolidation in both the information technology and healthcare industries. If one or more of our competitors or potential competitors were to merge or partner with one of our competitors, the change in the competitive landscape could adversely affect our ability to compete effectively. We compete on the basis of several factors, including:
| | • | | breadth and depth of services; |
| | • | | reliability, accuracy and security; |
| | • | | industry expertise and experience. |
Our clinical solutions business unit’s principal competitors include Cerner Corporation, eClinicalWorks Inc., Sage Software, Inc., Epic Systems Corporation, GE, iMedica Corporation, McKesson Corporation, MedHost, Inc., Misys Healthcare Systems, Picis Inc., Quality Systems, Inc. and Wellsoft Corporation. We also face competition from providers of practice management solutions, ambulatory and acute EHR solutions, and enterprise-wide application solutions.
Our physicians interactive business unit’s principal competitors include Aptilon Inc., Dendrite International, Inc., WebMD Corporation, Lathian Systems, Inc., Quintiles Transnational Corp. and Ventiv Health, Inc. We also face competition from clinical information and education providers, such as disease state management companies, full service e-marketing companies, companies who provide electronic detailing software, and the in-house efforts of our clients, including health plans, pharmacy benefit managers, and pharmaceutical companies.
Our medication services business unit’s principal competitors include Cardinal Health, Inc., DRx (a wholly owned subsidiary of Purkinje, Inc.), McKesson Corporation, PD-Rx Pharmaceuticals, Inc., Pharmapac, Physicians Total Care, Inc., Southwood Pharmaceuticals, Inc. and various other regional distributors. We also face competition from providers of other medication repackaging service and bulk pharmaceutical distributors.
There can be no assurance that we will be able to compete successfully against current and future competitors or that the competitive pressures that we face will not materially adversely affect our business, financial condition and results of operations.
Our business depends on our intellectual property rights, and if we are unable to protect them, our competitive position may suffer.
Our business plan is predicated on our proprietary systems and technology and physician education products. Accordingly, protecting our intellectual property rights is critical to our continued success and our ability to maintain our competitive position. We protect our proprietary rights through a combination of trademark, trade secret and copyright law, confidentiality agreements and technical measures. We generally do not have any patents on our technology. We generally enter into non-disclosure agreements with our employees and consultants and limit access to our trade secrets and technology. We cannot assure you that the steps we have taken will prevent misappropriation of our technology. Misappropriation of our intellectual property would have
14
an adverse effect on our competitive position. In addition, we may have to engage in litigation in the future to enforce or protect our intellectual property rights or to defend against claims of invalidity, and we may incur substantial costs and the diversion of management’s time and attention as a result.
If we are deemed to infringe on the proprietary rights of third parties, we could incur unanticipated expense and be prevented from providing our products and services.
We could be subject to intellectual property infringement claims as the number of our competitors grows and our applications’ functionality overlaps with competitive products. While we do not believe that we have infringed or are infringing on any proprietary rights of third parties, we cannot assure you that infringement claims will not be asserted against us or that those claims will be unsuccessful. We could incur substantial costs and diversion of management resources defending any infringement claims. Furthermore, a party making a claim against us could secure a judgment awarding substantial damages, as well as injunctive or other equitable relief that could effectively block our ability to provide products or services. In addition, we cannot assure you that licenses for any intellectual property of third parties that might be required for our products or services will be available on commercially reasonable terms, or at all.
Factors beyond our control could cause interruptions in our operations, which would adversely affect our reputation in the marketplace and our business, financial condition and results of operations.
To succeed, we must be able to operate our systems without interruption. Certain of our communications and information services are provided through our third-party service providers. Our operations are vulnerable to interruption by damage from a variety of sources, many of which are not within our control, including without limitation: (1) power loss and telecommunications failures; (2) software and hardware errors, failures or crashes; (3) computer viruses and similar disruptive problems; and (4) fire, flood and other natural disasters.
Any significant interruptions in our services would damage our reputation in the marketplace and have a negative impact on our business, financial condition and results of operations.
We may be liable for use of data we provide.
We provide data for use by healthcare providers in treating patients. Third-party contractors provide us with most of this data. If this data is incorrect or incomplete, adverse consequences, including death, may occur and give rise to product liability and other claims against us. In addition, certain of our solutions provide applications that relate to patient clinical information, and a court or government agency may take the position that our delivery of health information directly, including through licensed practitioners, or delivery of information by a third party site that a consumer accesses through our websites, exposes us to personal injury liability, or other liability for wrongful delivery or handling of healthcare services or erroneous health information. While we maintain product liability insurance coverage in an amount that we believe is sufficient for our business, we cannot assure you that this coverage will prove to be adequate or will continue to be available on acceptable terms, if at all. A claim brought against us that is uninsured or under-insured could harm our business, financial condition and results of operations. Even unsuccessful claims could result in substantial costs and diversion of management resources.
If our security is breached, we could be subject to liability, and customers could be deterred from using our services.
The difficulty of securely transmitting confidential information over the Internet has been a significant barrier to engaging in sensitive communications over the Internet. Our business relies on using the Internet to transmit confidential information. We believe that any well-publicized compromise of Internet security may deter people from using the Internet for these purposes and from using our system to conduct transactions that involve transmitting confidential healthcare information.
15
It is also possible that third parties could penetrate our network security or otherwise misappropriate patient information and other data. If this happens, our operations could be interrupted, and we could be subject to liability and regulatory action. We may need to devote significant financial and other resources to protect against security breaches or to alleviate problems caused by breaches. We could face financial loss, litigation and other liabilities to the extent that our activities or the activities of third-party contractors involve the storage and transmission of confidential information like patient records or credit information.
If we are unable to obtain additional financing for our future needs, our ability to respond to competitive pressures may be impaired and our business, financial condition and results of operations could be adversely affected.
We cannot be certain that additional financing will be available to us on favorable terms, or at all. If adequate financing is not available or is not available on acceptable terms, our ability to fund our expansion, take advantage of potential acquisition opportunities, develop or enhance services or products, or respond to competitive pressures would be significantly limited.
If our content and service providers fail to perform adequately, our reputation in the marketplace and our business, financial condition and results of operations could be adversely affected.
We depend on independent content and service providers for many of the benefits we provide through our clinical software and our physician education applications and services, including the maintenance of managed care pharmacy guidelines, drug interaction reviews and the routing of transaction data to third-party payers. If our services are interrupted as a result of any problems with our providers, our reputation in the marketplace could be damaged, which would have an adverse effect on our business, financial condition and results of operations. We may have no means of replacing content or services on a timely basis or at all if they are inadequate or in the event of a service interruption or failure.
We also rely on independent content providers for the majority of the clinical, educational and other healthcare information that we provide. In addition, we depend on our content providers to deliver high quality content from reliable sources and to continually upgrade their content in response to demand and evolving healthcare industry trends. If these parties fail to develop and maintain high quality, attractive content, the value of our brand and our business, financial condition and results of operations could be impaired.
If we are forced to reduce our prices, our business, financial condition and results of operations could suffer.
We may be subject to pricing pressures with respect to our future sales arising from various sources, including practices of managed care organizations, Internet pharmacies, including those operating in Canada and other countries outside the United States, and government action affecting pharmaceutical reimbursement under Medicare. Our customers and the other entities with which we have a business relationship are affected by changes in regulations and limitations in governmental spending for Medicare and Medicaid programs. Recent government actions could limit government spending for the Medicare and Medicaid programs, limit payments to hospitals and other providers and increase emphasis on competition and other programs that potentially could have an adverse effect on our customers and the other entities with which we have a business relationship. If our pricing experiences significant downward pressure, our business will be less profitable and our results of operations would be adversely affected. In addition, because cash from sales funds some of our working capital requirements, reduced profitability could require us to raise additional capital sooner than we would otherwise need.
16
If we are unable to maintain existing relationships and create new relationships with managed care payers, our business, financial condition and results of operations will be adversely affected.
We rely on managed care organizations to reimburse our physician customers for prescription medications dispensed in their offices. While many of the leading managed care payers and pharmacy benefit managers currently reimburse our physicians for in-office dispensing, none of these payers is under a long-term obligation to do so. If we are unable to increase the number of managed care payers that reimburse for in-office dispensing, or if some or all of the payers who currently reimburse physicians decline to do so in the future, utilization of our products and services would decrease and, therefore, our business, financial condition and results of operations will be adversely affected.
If we incur costs exceeding our insurance coverage in lawsuits pending against us or that are brought against us in the future, it could adversely affect our business, financial condition and results of operations.
We are a defendant in numerous multi-defendant lawsuits involving the manufacture and sale of dexfenfluramine, fenfluramine and phentermine. In the event we are found liable in any lawsuits filed against us, and if our insurance coverage were inadequate to satisfy these liabilities, it could have an adverse effect on our business, financial condition and results of operations. See “Business—Legal Proceedings.”
If our principal supplier fails or is unable to perform its contract with us, we may be unable to meet our commitments to our customers.
We currently purchase a majority of the medications that we repackage from AmerisourceBergen. If we do not meet certain minimum purchasing requirements, AmerisourceBergen may increase the prices that we pay under this agreement, in which case we would have the option to terminate the agreement. Although we believe that there are a number of other sources of supply of medications, if AmerisourceBergen fails or is unable to perform under our agreement, particularly at certain critical times during the year, we may be unable to meet our commitments to our customers, and our relationships with our customers could suffer.
Our failure to license and integrate third-party technologies could harm our business.
We depend upon licenses for some of the technology used in our solutions from third-party vendors, including Microsoft, and intend to continue licensing technologies from third parties. These technologies might not continue to be available to us on commercially reasonable terms or at all. Most of these licenses can be renewed only by mutual consent and may be terminated if we breach the terms of the license and fail to cure the breach within a specified period of time. Our inability to obtain any of these licenses could delay development until equivalent technology can be identified, licensed and integrated, which would harm our business, financial condition and results of operations.
Most of our third-party licenses are non-exclusive and our competitors may obtain the right to use any of the technology covered by these licenses and use the technology to compete directly with us. Our use of third-party technologies exposes us to increased risks, including, but not limited to, risks associated with the integration of new technology into our solutions, the diversion of our resources from development of our own proprietary technology and our inability to generate revenue from licensed technology sufficient to offset associated acquisition and maintenance costs. In addition, if our vendors choose to discontinue support of the licensed technology in the future or are unsuccessful in their continued research and development efforts, we might not be able to modify or adapt our own solutions.
If we do not maintain and expand our business with our existing customers, our business, financial condition and results of operations could be adversely affected.
Our business model depends on the success of our efforts to sell additional products and services to our existing customers. For example, certain of our clinical solutions business unit customers initially purchase one
17
or a limited number of our modules. These customers might choose not to expand their use of or purchase additional modules. In addition, as we deploy new applications and features for our existing solutions or introduce new solutions and services, our current customers could choose not to purchase these new offerings. If we fail to generate additional business from our current customers, our revenue could grow at a slower rate or even decrease.
Risks Related to Our Industry
We are subject to a number of existing laws, regulations and industry initiatives, non-compliance with certain of which could shut down our operations or otherwise adversely affect our business, financial condition and results of operations, and we are susceptible to a changing regulatory environment.
As a participant in the healthcare industry, our operations and relationships, and those of our customers, are regulated by a number of federal, state and local governmental entities. The impact of this on us is direct, to the extent we are ourselves subject to these laws and regulations, and is also indirect in that, in a number of situations, even though we may not be directly regulated by specific healthcare laws and regulations, our products must be capable of being used by our customers in a manner that complies with those laws and regulations. Inability of our customers to do so could affect the marketability of our products or our compliance with our customer contracts, or even expose us to direct liability on a theory that we had assisted our customers in a violation of healthcare laws or regulations. Because our business relationships with physicians are unique, and the healthcare technology industry as a whole is relatively young, the application of many state and federal regulations to our business operations and to our customers is uncertain. Indeed, there are federal and state fraud and abuse laws, including anti-kickback laws and limitations on physician referrals, and laws related to off-label promotion of prescription drugs that may be directly or indirectly applicable to our operations and relationships or the business practices of our customers. It is possible that a review of our business practices or those of our customers by courts or regulatory authorities could result in a determination that could adversely affect us. In addition, the healthcare regulatory environment may change in a way that restricts our existing operations or our growth. The healthcare industry is expected to continue to undergo significant changes for the foreseeable future, which could have an adverse effect on our business, financial condition and results of operations. We cannot predict the effect of possible future legislation and regulation.
Specific risks include, but are not limited to, risks relating to:
| | • | | Patient Information. As part of the operation of our business, our customers provide to us patient-identifiable medical information related to the prescription drugs that they prescribe and other aspects of patient treatment. Government and industry legislation and rulemaking, especially the Health Insurance Portability and Accountability Act of 1996 (HIPAA), and standards and requirements published by industry groups such as the Joint Commission on Accreditation of Healthcare Organizations, require the use of standard transactions, standard identifiers, security and other standards and requirements for the transmission of certain electronic health information. National standards and procedures under HIPAA include the “Standards for Electronic Transactions and Code Sets” (the Transaction Standards); the “Security Standards” (the Security Standards); and the “Standards for Privacy of Individually Identifiable Health Information” (the Privacy Standards). The Transaction Standards require the use of |
| | specified data coding, formatting and content in all specified “Health Care Transactions” conducted electronically. The Security Standards require the adoption of specified types of security for healthcare information. The Privacy Standards grant a number of rights to individuals as to their identifiable confidential medical information (called Protected Health Information) and restrict the use and disclosure of Protected Health Information by Covered Entities, defined as “health care providers, health care payers, and health care clearinghouses.” Generally, the HIPAA standards directly affect Covered Entities. We have reviewed our activities and believe that we are a Covered Entity to the extent that we maintain a “group health plan” for the benefit of our employees. Such a plan, even if not a separate legal entity from us as its sponsor, is included in the HIPAA definition of Covered Entities. We have taken |
18
| | steps we believe to be appropriate and required to bring our group health plan into compliance with HIPAA. We do not believe that we are a Covered Entity as a health care provider or as a health care clearinghouse; however, the definition of a health care clearinghouse is broad and we cannot offer any assurance that we could not be considered a health care clearinghouse under HIPAA or that, if we are determined to be a healthcare clearinghouse, the consequences would not be adverse to our business, financial condition and results of operations. In addition, the Privacy and Security Standards affect third parties that create, access, or receive Protected Health Information in order to perform a function or activity on behalf of a Covered Entity. Such third parties are called “Business Associates.” Covered Entities must have a written “Business Associate Agreement” with such third parties, containing specified written satisfactory assurances, consistent with the Privacy and Security standards, that the third party will safeguard Protected Health Information that it creates or accesses and will fulfill other material obligations to support the Covered Entity’s own HIPAA compliance. Most of our customers are Covered Entities, and we function in many of our relationships as a Business Associate of those customers. We would face liability under our Business Associate Agreements if we do not comply with our Business Associate obligations. In addition, the federal agencies with enforcement authority have taken the position that a Covered Entity can be subject to HIPAA civil penalties and sanctions for a breach of a Business Associate Agreement. The penalties for a violation of HIPAA by a Covered Entity are significant and could have an adverse impact upon our business, financial condition and results of operations, if such penalties ever were imposed. Additionally, Covered Entities will be required to adopt a unique standard National Provider Identifier (NPI) for use in filing and processing health care claims and other transactions. Subject to the discussion set forth above, we believe that the principal effects of HIPAA are, first, to require that our systems be capable of being operated by our customers in a manner that is compliant with the various HIPAA standards and, second, to require us to enter into and comply with Business Associate Agreements with our Covered Entity customers. For most Covered Entities, the deadlines for compliance with the Privacy Standards and the Transaction Standards occurred in 2003. Covered Entities, with the exception of small health plans (as that term is defined by the Privacy Standards), were required to be in compliance with the Security Standards by April 20, 2005 and to use NPIs in standard transactions no later than the compliance dates, which are May 23, 2007 for all but small health plans and one year later for small health plans. We have policies and procedures that we believe assure compliance with all federal and state confidentiality requirements for the handling of Protected Health Information that we receive and with our obligations under Business Associate Agreements. In particular, we believe that our systems and products are capable of being used by our customers in compliance with the Transaction Standards and Security Standards and are, or will be, capable of being used by our customers in compliance with the NPI requirements. If, however, we do not follow those procedures and policies, or they are not sufficient to prevent the unauthorized disclosure of Protected Health Information, we could be subject to liability, fines and lawsuits, termination of our customer contracts or our operations could be shut down. Moreover, because all HIPAA Standards are subject to change or interpretation and because certain other HIPAA Standards, not discussed above, are not yet published, we cannot predict the full future impact of HIPAA on our business and operations. In the event that the HIPAA standards and compliance requirements change or are interpreted in a way that requires any material change to the way in which we do business, our business, financial condition and results of operations could be adversely affected. Additionally, certain state laws are not preempted by HIPAA and may impose independent obligations upon our customers or us. Additional legislation governing the acquisition, storage and transmission or other dissemination of health record information and other personal information, including social security numbers, has been proposed at both the state and federal level. Such legislation may require holders of such information to implement additional security, reporting or other measures that may require substantial expenditures and may impose liability for a failure to comply with such requirements. In many cases, such proposed state legislation includes provisions that are not preempted by HIPAA. There can be no assurance that changes to state or federal laws will not materially restrict the ability of providers to submit information from patient records using our products and services. |
19
| | • | | Electronic Prescribing. The use of our software by physicians to perform a variety of functions, including electronic prescribing, electronic routing of prescriptions to pharmacies and dispensing, is governed by state and federal law, including fraud and abuse laws. States have differing prescription format requirements, which we have programmed into our software. Many existing laws and regulations, when enacted, did not anticipate methods of e-commerce now being developed. While federal law and the laws of many states permit the electronic transmission of prescription orders, the laws of several states neither specifically permit nor specifically prohibit the practice. Given the rapid growth of electronic transactions in healthcare, and particularly the growth of the Internet, we expect the remaining states to directly address these areas with regulation in the near future. In addition, on November 7, 2005, the Department of Health and Human Services published its final “E-Prescribing and the Prescription Drug Program” regulations (E-Prescribing Regulations). These regulations are required by the Medicare Prescription |
| | | Drug, Improvement, and Modernization Act of 2003 (MMA) and became effective beginning on January 1, 2006. The E-Prescribing Regulations consist of detailed standards and requirements, in addition to the HIPAA standards discussed above, for prescription and other information transmitted electronically in connection with a drug benefit covered by the MMA’s Prescription Drug Benefit. These standards cover not only transactions between prescribers and dispensers for prescriptions but also electronic eligibility and benefits inquiries and drug formulary and benefit coverage information. The standards apply to prescription drug plans participating in the MMA’s Prescription Drug Benefit. Aspects of our clinical products are affected by such regulation because of the need of our customers to comply, as discussed above. Compliance with these regulations could be burdensome, time-consuming and expensive. We also could become subject to future legislation and regulations concerning the development and marketing of healthcare software systems. For example, regulatory authorities such as the U.S. Department of Health and Human Services’ Center for Medicare and Medicaid Services may impose functionality standards with regard to electronic prescribing and EHR technologies. These could increase the cost and time necessary to market new services and could affect us in other respects not presently foreseeable. |
| | • | | Electronic Health Records.A number of important federal and state laws govern the use and content of electronic health record systems, including fraud and abuse laws that may affect the donation of such technology. As a company that provides EHR systems to a variety of providers of healthcare, our systems and services must be designed in a manner that facilitates our customers’ compliance with these laws. Because this is a topic of increasing state and federal regulation, we must continue to monitor legislative and regulatory developments that might affect our business practices as they relate to EHR systems. We cannot predict the content or effect of possible future regulation on our business practices. Also, as described above, our TouchWorks EHR and HealthMatics EHR are certified by CCHIT as meeting CCHIT’s certification standards for functionality, interoperability and security. Our failure to maintain CCHIT certification or otherwise meet industry standards would adversely impact our business. |
| | • | | Claims Transmission. Our system electronically transmits claims for prescription medications dispensed by physicians to patients’ payers for immediate approval and reimbursement. Federal law provides that it is both a civil and a criminal violation for any person to submit, or cause to be submitted, a claim to any payer, including, without limitation, Medicare, Medicaid and all private health plans and managed care plans, seeking payment for any services or products that overbills or bills for items that have not been provided to the patient. We have in place policies and procedures that we believe assure that all claims that are transmitted by our system are accurate and complete, provided that the information given to us by our customers is also accurate and complete. If, however, we do not follow those procedures and policies, or they are not sufficient to prevent inaccurate claims from being submitted, we could be subject to liability. As discussed above, the HIPAA Transaction Standards and the HIPAA Security Standards also affect our claims transmission services, since those services must be structured and provided in a way that supports our customers’ HIPAA compliance obligations. Furthermore, to the extent that there is some type of security breach it could have a material adverse effect. |
20
| | • | | Medical Devices. The U.S. Food and Drug Administration (FDA) has promulgated a draft policy for the regulation of computer software products as medical devices under the 1976 Medical Device Amendments to the Federal Food, Drug and Cosmetic Act. To the extent that computer software is a medical device under the policy, we, as a manufacturer of such products, could be required, depending on the product, to register and list our products with the FDA; notify the FDA and demonstrate substantial equivalence to other products on the market before marketing such products; or obtain FDA approval by demonstrating safety and effectiveness before marketing a product. Depending on the intended use of a device, the FDA could require us to obtain extensive data from clinical studies to demonstrate safety or effectiveness or substantial equivalence. If the FDA requires this data, we would be required to obtain approval of an investigational device exemption before undertaking clinical trials. Clinical trials can take extended periods of time to complete. We cannot provide assurances that the FDA will approve or clear a device after the completion of such trials. In addition, these products would be subject to the Federal Food, Drug and Cosmetic Act’s general controls, including those relating to good manufacturing practices and adverse experience reporting. Although it is not possible to anticipate the final form of the FDA’s policy with regard to computer software, we expect that the FDA is likely to become increasingly active in regulating computer software intended for use in healthcare settings regardless of whether the draft is finalized or changed. The FDA can impose extensive requirements governing pre- and post-market conditions like service investigation, approval, labeling and manufacturing. In addition, the FDA can impose extensive requirements governing development controls and quality assurance processes. |
| | • | | e-Detailing. Our pharmaceutical and medical device clients use physicians interactive e-Detailing programs to provide physicians with valuable and up-to-date information about various medications and medical products, as well as to collect feedback from physician opinion leaders and other experts. Pharmaceutical marketing activities are subject to various federal and state regulatory and compliance initiatives, including an industry-sponsored ethics initiative developed by the Pharmaceutical Research and Manufacturers of America (PhRMA Code) and the final Compliance Program Guidance for Pharmaceutical Manufacturers issued on April 28, 2003 by the HHS Office of Inspector General (OIG). Such initiatives, some of which are required and some of which are voluntary, articulate concerns, recommendations and standards concerning a variety of pharmaceutical product marketing activities and issues, including e-Detailing, kickbacks, discounts, switching arrangements, research/consulting/advisory payments, relationships with other healthcare providers, including physicians, and gifts/entertainment/other remuneration. Additionally, as a sender of electronic mail in connection with some of our educational programs, we are subject to the CAN-SPAM Act of 2003 and other state and federal laws regulating senders of electronic mail for commercial purposes. We believe that our programs and activities comply with applicable federal and state laws and regulations and are consistent with PhRMA Code and OIG initiatives. However, if our physician educational programs were found to be conducted in a manner inconsistent with such federal and state laws, regulations or initiatives, or if we are required to materially change the way in which we do business in order to conform with such laws, regulations and initiatives, our business, financial condition and results of operations would be adversely affected. |
| | • | | Licensure and Physician Dispensing. As a repackager and distributor of drugs, we are subject to regulation by and licensure with the FDA, the Drug Enforcement Agency (DEA) and various state agencies that regulate wholesalers or distributors. Among the regulations applicable to our repackaging operation are the FDA’s “good manufacturing practices.” We are subject to periodic inspections of our facilities by regulatory authorities to confirm that we have policies and procedures in place in order to comply with applicable legal requirements. Because the FDA’s good manufacturing practices were designed to govern the manufacture, rather than the repackaging, of drugs, we face legal uncertainty concerning the application of some aspects of these regulations and of the standards that the FDA will enforce. If we do not maintain all necessary licenses, or the FDA decides to substantially modify the manner in which it has historically enforced its good manufacturing practice regulations against drug repackagers or the FDA or DEA finds any violations during one of their periodic inspections, we could be subject to liability, and our operations could be shut down. In addition to registration/licensure and |
21
| | "good manufacturing practices" compliance issues, federal and certain state laws require recordkeeping and a drug pedigree when a company is involved in the distribution of prescription drugs. Under the pedigree requirements, each person who is engaged in the wholesale distribution of a prescription drug in interstate commerce, who is not the manufacturer or an authorized distributor of record for that drug, must provide to the person who receives the drug, a pedigree for that drug. A drug pedigree is a statement of origin that identifies each prior sale, purchase, or trade of a drug. State laws in this area are not consistent with respect to their requirements, and thus the company needs to carefully monitor legal developments in this area. To the extent we are found to violate any applicable federal or state law related to drug pedigree requirements, any such violation could adversely affect our business. |
| | • | | While physician dispensing of medications for profit is allowed in most states, it is limited in a few states. It is possible that certain states may enact further legislation or regulations prohibiting, restricting or further regulating physician dispensing. Similarly, while in a July 2002 Opinion the American Medical Association’s Council on Ethical and Judicial Affairs (CEJA) provides, in relevant part, that “Physicians may dispense drugs within their office practices provided such dispensing primarily benefits the patient,” the American Medical Association has historically taken inconsistent positions on physician dispensing. Past reports of the CEJA have opposed the in-office sale of health-related products by physicians, and it is possible that the CEJA may in the future oppose the in-office sale of health-related products by physicians. Any such state legislative prohibitions or CEJA opposition of physician dispensing could adversely affect our business, financial condition and results of operations. |
| | • | | Congress enacted significant prohibitions against physician self-referrals in the Omnibus Budget Reconciliation Act of 1993. This law, commonly referred to as “Stark II,” applies to physician dispensing of outpatient prescription drugs that are reimbursable by Medicare or Medicaid. Stark II, however, includes an exception for the provision of in-office ancillary services, including a physician’s dispensing of outpatient prescription drugs, provided that the physician meets specified requirements. We believe that the physicians who use our system or dispense drugs distributed by us are aware of these requirements, but we do not monitor their compliance and have no assurance that the physicians are in material compliance with Stark II. If it were determined that the physicians who use our system or dispense pharmaceuticals purchased from us were not in compliance with Stark II, it could have an adverse effect on our business, financial condition and results of operations. |
| | • | | As a distributor of prescription drugs to physicians, we are subject to the federal anti-kickback statute, which applies to Medicare, Medicaid and other state and federal programs. The federal anti-kickback statute prohibits the solicitation, offer, payment or receipt of remuneration in return for referrals or the purchase, or in return for recommending or arranging for the referral or purchase, of goods, including drugs, covered by the programs. The federal anti-kickback statute provides a number of statutory exceptions and regulatory “safe harbors” for particular types of transactions. We believe that our arrangements with our customers are in material compliance with the anti-kickback statute and relevant safe harbors. Many states have similar fraud and abuse laws, and we believe that we are in material compliance with those laws. If, however, it were determined that we, as a distributor of prescription drugs to physicians, were not in compliance with the federal anti-kickback statute, we could be subject to liability, and our operations could be curtailed. Moreover, if the activities of our customers or other entity with which we have a business relationship were found to constitute a violation of the federal anti-kickback law and we, as a result of the provision of products or services to such customer or entity, were found to have knowingly participated in such activities, we could be subject to sanction or liability under such laws, including civil and/or criminal penalties, as well as exclusion from government health programs. As a result of exclusion from government health programs, neither products nor services could be provided to any beneficiaries of any federal healthcare program. |
22
Increased government involvement in healthcare could adversely affect our business.
U.S. healthcare system reform under the Medicare Prescription Drug, Improvement and Modernization Act of 2003, and other initiatives at both the federal and state level, could increase government involvement in healthcare, lower reimbursement rates and otherwise change the business environment of our customers and the other entities with which we have a business relationship. While no federal price controls are included in the Medicare Prescription Drug, Improvement and Modernization Act, any legislation that reduces physician incentives to dispense medications in their offices could adversely affect physician acceptance of our products. We cannot predict whether or when future healthcare reform initiatives at the federal or state level or other initiatives affecting our business will be proposed, enacted or implemented or what impact those initiatives may have on our business, financial condition or results of operations. Our customers and the other entities with which we have a business relationship could react to these initiatives and the uncertainty surrounding these proposals by curtailing or deferring investments, including those for our products and services. Additionally, government regulation could alter the clinical workflow of physicians, hospitals and other healthcare participants, thereby limiting the utility of our products and services to existing and potential customers and curtailing broad acceptance of our products and services. Further examples of government involvement could include requiring the standardization of technology relating to EHR’s, providing customers with incentives to adopt EHR solutions or developing a low-cost government sponsored EHR solution, such as VistA-Office EHR. Additionally, new safe harbors to the federal Anti-Kickback Statute and corresponding exceptions to the federal Stark law may alter the competitive landscape, as such new safe harbors and exceptions allow hospitals and certain other donors to donate certain items and services used in electronic prescription systems and electronic health records systems. These new safe harbors and exceptions are intended to accelerate the adoption of electronic prescription systems and electronic health records systems, and therefore provide new and attractive opportunities for us to work with hospitals and other donors who wish to provide our clinical solutions to physicians. At the same time, such safe harbors and exceptions may result in increased competition from providers of acute EHR solutions, whose hospital customers may seek to donate their existing acute EHR solutions to physicians for use in ambulatory settings. In addition, the federal government and state governments, including Florida, have imposed or may in the future impose pedigree requirements for pharmaceutical distribution. Our medications business is required to comply with any current regulations relating to pharmaceutical distribution and will be required to comply with any future regulations and such compliance may impose additional costs on our business.
If the electronic healthcare information market fails to develop as quickly as expected, our business, financial condition and results of operations will be adversely affected.
The electronic healthcare information market is in the early stages of development and is rapidly evolving. A number of market entrants have introduced or developed products and services that are competitive with one or more components of the solutions we offer. We expect that additional companies will continue to enter this market. In new and rapidly evolving industries, there is significant uncertainty and risk as to the demand for, and market acceptance of, recently introduced products and services. Because the markets for our products and services are new and evolving, we are not able to predict the size and growth rate of the markets with any certainty. We cannot assure you that markets for our products and services will develop or that, if they do, they will be strong and continue to grow at a sufficient pace. If markets fail to develop, develop more slowly than expected or become saturated with competitors, our business, financial condition and results of operations will be adversely affected.
Consolidation in the healthcare industry could adversely affect our business, financial condition and results of operations.
Many healthcare industry participants are consolidating to create integrated healthcare delivery systems with greater market power. As provider networks and managed care organizations consolidate, thus decreasing the number of market participants, competition to provide products and services like ours will become more intense, and the importance of establishing relationships with key industry participants will become greater. These
23
industry participants may try to use their market power to negotiate price reductions for our products and services. Further, consolidation of management and billing services through integrated delivery systems may decrease demand for our products. If we were forced to reduce our prices, our business would become less profitable unless we were able to achieve corresponding reductions in our expenses.
Risks Related to Our Common Stock
Because of certain features of our outstanding 3.50% convertible senior debentures and anti-takeover provisions under Delaware law and in our organizational documents, a takeover of Allscripts may be difficult, and could prevent investors from obtaining an optimal price for our shares of common stock in the event of a takeover.
We are required to increase the conversion rate on our 3.50% convertible senior debentures that are converted in connection with certain change of control transactions that occur on or prior to July 15, 2009, which effectively increases the cost of a takeover of the company. In addition, in the event of a change of control of the company, subject to certain exceptions, holders of the debentures have the right to require us to repurchase in cash all or any portion of their debentures. These features may in certain circumstances make more difficult or discourage such a takeover. Additionally, certain provisions of Delaware law and our amended and restated certificate of incorporation, as amended, and by-laws could have the effect of making it more difficult for a third party to acquire, or of discouraging a third party from attempting to acquire, control of us. These provisions:
| | • | | authorize the issuance of preferred stock that can be created and issued by our Board of Directors without prior stockholder approval to increase the number of outstanding shares and deter or prevent a takeover attempt; |
| | • | | prohibit common stockholder action by written consent, thereby requiring all common stockholder actions to be taken at a meeting of our common stockholders; |
| | • | | prohibit cumulative voting in the election of directors, which would otherwise enable less than a majority of stockholders to elect director candidates; |
| | • | | limit the ability of stockholders to call special meetings of stockholders; |
| | • | | establish advance notice requirements for nominations for election to our Board of Directors or for proposing matters that can be acted upon by stockholders at stockholder meetings; and |
| | • | | provide for a classified Board of Directors, expanding the time required to change the composition of a majority of directors. |
In addition, we are subject to the anti-takeover provisions of Section 203 of the Delaware General Corporation Law, which could have the effect of delaying or preventing a change in control of us.
Future sales of our common stock in the public market could adversely affect the trading price of our common stock that we may issue and our ability to raise funds in new securities offerings.
Future sales of substantial amounts of our common stock in the public market, or the perception that such sales could occur, could adversely affect prevailing trading prices of our common stock and could impair our ability to raise capital through future offerings of equity or equity-related securities. As of February 15, 2007, we had approximately:
| | • | | 54,778,049 shares of common stock outstanding; |
| | • | | 3,333 shares of common stock reserved for issuance upon exercise of outstanding warrants; |
| | • | | 5,159,904 shares of common stock reserved and available for issuance pursuant to stock options and other awards outstanding under our stock plans at a weighted average exercise price of $8.13 per share; |
24
| | • | | 2,144,686 additional shares of common stock reserved and available for issuance under our stock plans; |
| | • | | 594,304 shares of unvested restricted common stock to employees and directors; and |
| | • | | 7,329,424 shares of common stock reserved for issuance upon conversion of our outstanding 3.50% convertible senior debentures. The number of shares issuable upon conversion of these debentures is subject to adjustment from time to time pursuant to anti-dilution provisions. |
In connection with our acquisition strategy, we may issue shares of our common stock as consideration in other acquisition transactions. We cannot predict the effect, if any, that future sales of shares of common stock or the availability of shares of common stock for future sale will have on the trading price of our common stock.
Our outstanding 3.50% convertible senior debentures are convertible at the option of the holders into shares of our common stock, subject to the certain conditions set forth in the indenture governing these debentures. Any shares of common stock issued on conversion of these debentures and subsequently sold will be freely tradable in the public markets without restriction. In addition, we will be required to repurchase these debentures following certain change in control events relating to us, and the holders of these debentures will have the option to require us to purchase all or a portion of their debentures on July 15, 2009, July 15, 2014 and July 15, 2019. The conversion of these debentures into common stock or the issuance of common stock to pay the purchase price of any such debentures could result in the issuance of a substantial number of shares of our common stock and substantial dilution to our stockholders.
Our issuance of preferred stock could adversely affect holders of our common stock and discourage a takeover.
Our Board of Directors is authorized to issue up to 1,000,000 shares of preferred stock without any action on the part of our stockholders. Our Board of Directors also has the power, without stockholder approval, to set the terms of any series of preferred stock that may be issued, including voting rights (except that shares of preferred stock may not have more than one vote per share), dividend rights, preferences over our common stock with respect to dividends or in the event of a dissolution, liquidation or winding up and other terms. In the event that we issue preferred stock in the future that has preference over our common stock with respect to payment of dividends or upon our liquidation, dissolution or winding up, or if we issue preferred stock that is convertible into our common stock at greater than a one-to-one ratio, the voting and other rights of the holders of our common stock or the market price of our common stock could be adversely affected. In addition, the ability of our Board of Directors to issue shares of preferred stock without any action on the part of our stockholders may impede a takeover of us and prevent a transaction favorable to the holders of our common stock.
Failure to maintain effective internal controls in accordance with Section 404 of the Sarbanes-Oxley Act of 2002 could have an adverse effect on our business and the trading price of our common stock.
If we fail to maintain the adequacy of our internal controls, in accordance with the requirements of Section 404 of the Sarbanes-Oxley Act of 2002 and as such standards are modified, supplemented or amended from time to time, we may not be able to ensure that we can conclude on an ongoing basis that we have effective internal control over financial reporting in accordance with Section 404 of the Sarbanes-Oxley Act of 2002. Failure to achieve and maintain an effective internal control environment could have an adverse effect on the price of our common stock.
The market price of our common stock has been and may continue to be volatile.
The market price of our common stock is volatile and could fluctuate significantly in response to the factors described above and other factors, many of which are beyond our control, including:
| | • | | actual or anticipated variations in our quarterly operating results; |
| | • | | announcements of technological innovations or new services or products by our competitors or us; |
25
| | • | | changes in financial estimates by securities analysts; |
| | • | | conditions and trends in the electronic healthcare information, Internet, e-commerce and pharmaceutical markets; and |
| | • | | general market conditions and other factors. |
In addition, the stock markets, especially the Nasdaq National Market, have experienced extreme price and volume fluctuations that have affected the market prices of equity securities of many technology companies and Internet-related companies in particular. These fluctuations have often been unrelated or disproportionate to operating performance. These broad market factors may materially affect the trading price of our common stock. General economic, political and market conditions such as recessions and interest rate fluctuations may also have an adverse effect on the market price of our common stock. Volatility in the market price for our common stock may result in the filing of securities class action litigation.
Our quarterly operating results may vary.
Our quarterly operating results have varied in the past, and we expect that our quarterly operating results will continue to vary in future periods depending on a number of factors, some of which we have no control over, including customers’ budgetary constraints and internal acceptance procedures, seasonal variances in demand for our products and services, the sales, service and implementation cycles for our clinical software products and physician education products and services, potential downturns in the healthcare market and in economic conditions generally, and other factors described in this “Risk Factors” section. For instance, all other factors aside, sales of our prepackaged medications have historically been highest in the third and fourth quarters. Sales of our software products have also historically been highest in the fourth quarter.
We base our expense levels in part upon our expectations concerning future revenue, and these expense levels are relatively fixed in the short term. If we have lower revenue than expected, we may not be able to reduce our spending in the short term in response. Any shortfall in revenue would have a direct impact on our results of operations. In addition, our product sales cycle for larger sales is lengthy and unpredictable, making it difficult to estimate our future bookings for any given period. If we do not achieve projected booking targets for a given period, securities analysts may change their recommendations on our common stock. For these and other reasons, we may not meet the earnings estimates of securities analysts or investors, and our stock price could suffer.
Conversion of the 3.50% convertible senior debentures will dilute the ownership interest of our stockholders, including holders who had previously converted their debentures.
The conversion of some or all of our 3.50% convertible senior debentures will dilute the ownership interests of our stockholders. Any sales in the public market of the common stock issuable upon such conversion could adversely affect prevailing market prices of our common stock. In addition, the existence of the debentures may encourage short selling by market participants because the conversion of the debentures could depress the price of our common stock.
Item 1B. Unresolved Staff Comments
None.
Item 2. Properties
Our corporate headquarters is located in Chicago, consists of approximately 18,300 square feet and includes corporate administration, finance, education, and some sales and marketing personnel. The corporate headquarters lease expires in February 2015.
26
Our repackaging and operating facilities are located in Libertyville, Illinois, in approximately 62,000 square feet of space under a lease that expires in June 2009. We lease an additional 4,000 square feet of space of repackaging facilities in Grayslake, Illinois, under a lease that expires in June 2007. We also maintain offices for sales, marketing, operations and development efforts in Louisville, Kentucky, with approximately 11,300 square feet under a lease that expires in July 2017; in Port Townsend, Washington, with approximately 2,900 square feet under a lease that expires in March 2007; in Burlington, Vermont, with approximately 31,500 square feet under a lease that expires in December 2014; and beginning February 2007, in Honolulu, HI with approximately 2,000 square feet under a lease that expires November 2010.
As a result of the A4 acquisition on March 2, 2006, we own the former corporate headquarters of A4 in Cary, North Carolina, consisting of approximately 55,000 square feet. We also lease approximately 10,200 square feet of office space in Cary, North Carolina effective November 30, 2006, expiring February 2012; an approximately 7,400 square foot warehouse facility in Morrisville, North Carolina, which expires in September 2010, approximately 4,900 square feet of office space in Austin, Texas under a lease that expires in January 2008, approximately 1,800 square feet of office space in Round Rock, Texas under a lease that expires in October 2007, approximately 15,200 square feet of office space in Nashua, New Hampshire under a lease that expires in October 2008 and approximately 3,000 square feet of office space in Novi, Michigan under a lease that expires in August 2007. We believe that our facilities are adequate for our current operations.
Item 3. Legal Proceedings
We are from time to time involved in litigation incidental to our respective businesses. We are not currently involved in any litigation in which we believe an adverse outcome would have a material adverse effect on our business, financial condition, results of operations or prospects.
Item 4. Submission of Matters to a Vote of Security Holders
None.
27
PART II
(Dollar amounts in thousands, except per share amounts)
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
Public Market for Common Stock
Our common stock is quoted on the Nasdaq National Market under the symbol “MDRX.” The following table sets forth, for the periods indicated, the high and low closing prices per share of the common stock of Allscripts Healthcare Solutions, Inc. for the applicable periods as reported on the Nasdaq National Market.
| | | | |
| | | High | | Low |
Year Ended December 31, 2006 | | | | |
First Quarter | | $19.65 | | $13.85 |
Second Quarter | | $18.74 | | $16.06 |
Third Quarter | | $22.78 | | $16.79 |
Fourth Quarter | | $28.89 | | $21.94 |
| | |
Year Ended December 31, 2005 | | | | |
First Quarter | | $14.97 | | $9.36 |
Second Quarter | | $17.25 | | $12.67 |
Third Quarter | | $18.73 | | $15.97 |
Fourth Quarter | | $17.73 | | $13.17 |
On February 15, 2007, we had approximately 520 holders of record of common stock. We have never declared or paid cash dividends on our common stock. We currently intend to retain all available cash to finance our operations and do not intend to declare or pay cash dividends on our shares of common stock in the foreseeable future. Any future determination to pay cash dividends will be at the discretion of our Board of Directors and will depend upon our results of operations, financial condition, current and anticipated cash needs, contractual restrictions, restrictions imposed by applicable law and other factors that our Board of Directors deems relevant.
There were no repurchases of common stock during the fourth quarter of 2006. However, we did repurchase 1,250 shares of common stock from IDX on March 9, 2006 for $21,078, which was based on 95% of the February 22, 2006 public offering price for our common stock of $17.75.
28
Performance Graph
The following graph shows a comparison of cumulative total returns for Allscripts, the Nasdaq Composite and the Nasdaq Health Services Index from December 31, 2001 through December 31, 2006. The graph assumes an initial investment of $100 and the reinvestment of dividends.
COMPARISON OF 5 YEAR CUMULATIVE TOTAL RETURN*
Among Allscripts Healthcare Solutions, Inc., The NASDAQ Composite Index
And The NASDAQ Health Services Index
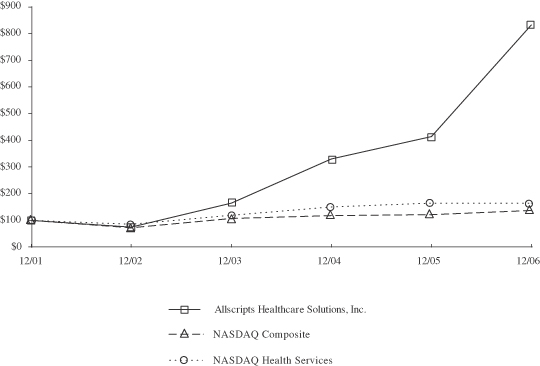
| | * | $100 invested on 12/31/01 in stock or index-including reinvestment of dividends. |
Fiscal year ending December 31.
Cumulative Total Return
| | | | | | | | | | | | |
| | | 12/31/01 | | 12/31/02 | | 12/31/03 | | 12/31/04 | | 12/31/05 | | 12/31/06 |
Allscripts Healthcare Solutions, Inc. | | 100.00 | | 73.77 | | 164.20 | | 329.32 | | 413.58 | | 833.02 |
NASDAQ Composite | | 100.00 | | 71.97 | | 107.18 | | 117.07 | | 120.50 | | 137.02 |
NASDAQ Health Services | | 100.00 | | 85.52 | | 118.76 | | 149.32 | | 164.82 | | 164.88 |
The information in this “Performance Graph” section shall not be deemed to be “soliciting material” or to be “filed” with the Securities and Exchange Commission or subject to Regulation 14A or 14C, or to the liabilities of Section 18 of the Securities Exchange Act of 1934.
29
Item 6. Selected Financial Data
The selected consolidated financial data shown below should be read in conjunction with “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our consolidated financial statements and related notes included elsewhere in this report. The consolidated statements of operations data for the three years ended December 31, 2006, 2005 and 2004 and the consolidated balance sheet data at December 31, 2006 and 2005 are derived from the consolidated financial statements audited by Grant Thornton LLP, which are included elsewhere in this report. The consolidated statements of operations data for the years ended December 31, 2003 and 2002 and the balance sheet data at December 31, 2004, 2003 and 2002 are derived from audited financial statements that are not included in this report. The historical results are not necessarily indicative of results to be expected for any future period.
| | | | | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2006(1) | | | 2005 | | | 2004 | | | 2003(2) | | | 2002(3) | |
| | | (In thousands, except per-share data) | |
Consolidated Statements of Operations Data: | | | | | | | | | | | | | | | |
Revenue | | $227,969 | | | $120,564 | | | $100,770 | | | $85,841 | | | $78,802 | |
Cost of revenue | | 112,031 | | | 65,689 | | | 58,122 | | | 55,169 | | | 58,931 | |
| | | | | | | | | | | | | | | |
Gross profit | | 115,938 | | | 54,875 | | | 42,648 | | | 30,672 | | | 19,871 | |
Operating expenses: | | | | | | | | | | | | | | | |
Selling, general and administrative expenses | | 85,798 | | | 43,908 | | | 37,653 | | | 36,058 | | | 36,412 | |
Amortization of intangibles | | 10,272 | | | 1,744 | | | 1,752 | | | 951 | | | 540 | |
Restructuring and other charges | | — | | | — | | | — | | | — | | | 600 | |
| | | | | | | | | | | | | | | |
Income (loss) from operations | | 19,868 | | | 9,223 | | | 3,243 | | | (6,337 | ) | | (17,681 | ) |
| | | | | |
Interest income | | 3,308 | | | 4,128 | | | 1,675 | | | 1,384 | | | 2,406 | |
Interest expense | | (3,712 | ) | | (3,516 | ) | | (1,717 | ) | | — | | | — | |
Other income (expense), net | | (145 | ) | | (125 | ) | | (93 | ) | | (26 | ) | | 42 | |
| | | | | | | | | | | | | | | |
Income (loss) before income taxes | | 19,319 | | | 9,710 | | | 3,108 | | | (4,979 | ) | | (15,233 | ) |
Income tax expense | | 7,424 | | | — | | | — | | | — | | | — | |
| | | | | | | | | | | | | | | |
Net income (loss) | | $11,895 | | | $9,710 | | | $3,108 | | | ($4,979 | ) | | ($15,233 | ) |
| | | | | | | | | | | | | | | |
Net income (loss) per share—basic | | $0.23 | | | $0.24 | | | $0.08 | | | ($0.13 | ) | | ($0.40 | ) |
| | | | | | | | | | | | | | | |
Net income (loss) per share—diluted | | $0.22 | | | $0.23 | | | $0.07 | | | ($0.13 | ) | | ($0.40 | ) |
| | | | | | | | | | | | | | | |
Weighted-average shares used in computing basic net income (loss) per share | | 51,058 | | | 40,045 | | | 38,979 | | | 38,621 | | | 38,337 | |
| | | | | | | | | | | | | | | |
Weighted-average shares used in computing diluted net income (loss) per share | | 53,367 | | | 43,068 | | | 41,592 | | | 38,621 | | | 38,337 | |
| | | | | | | | | | | | | | | |
Other Operating Data: | | | | | | | | | | | | | | | |
Software and related services revenue | | $173,503 | | | $65,166 | | | $44,121 | | | $28,366 | | | $19,921 | |
Prepackaged medication revenue | | 43,688 | | | 45,609 | | | 44,733 | | | 46,172 | | | 49,298 | |
Information services revenue | | 10,778 | | | 9,789 | | | 11,916 | | | 11,303 | | | 9,583 | |
| | | | | | | | | | | | | | | |
Total revenue | | $227,969 | | | $120,564 | | | $100,770 | | | $85,841 | | | $78,802 | |
| | | | | | | | | | | | | | | |
30
| | | | | | | | | | |
| | | As of December 31, |
| | | 2006 | | 2005 | | 2004 | | 2003 | | 2002 |
Consolidated Balance Sheet Data: | | | | | | | | | | |
Cash, cash equivalents and marketable securities | | $83,038 | | $146,063 | | $128,239 | | $51,309 | | $65,286 |
Working capital | | 82,250 | | 113,317 | | 34,914 | | 17,392 | | 44,426 |
Goodwill and intangible assets, net | | 266,311 | | 22,911 | | 24,546 | | 26,359 | | 4,793 |
Total assets | | 477,610 | | 220,964 | | 194,177 | | 110,392 | | 104,353 |
Long-term debt | | 85,441 | | 82,500 | | 82,500 | | — | | — |
Total stockholders’ equity | | 316,250 | | 98,419 | | 78,693 | | 83,390 | | 85,821 |
(1) | On March 2, 2006, Allscripts completed its acquisition of A4 Health Systems, Inc. (“A4”), whereby Allscripts acquired all of the outstanding equity interests of A4 for aggregate consideration of $227,730 in cash and 3,500 shares of Allscripts common stock. |
(2) | On August 1, 2003, Allscripts acquired 100% of the outstanding common stock of AIC. On August 8, 2003, Allscripts acquired certain assets and assumed certain liabilities of RxCentric. |
(3) | In July 2001, Allscripts announced and began implementation of a restructuring plan. During 2002, Allscripts recorded $414 for severance costs in connection with the departure of the former chief financial officer and an additional charge of $186 for remaining workforce reductions. |
31
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations
The following discussion and analysis should be read together with “Selected Financial Data” and our consolidated financial statements and related notes included elsewhere in this report. This discussion contains certain forward-looking statements that involve risks, uncertainties and assumptions. You should read the cautionary statements made in this report as applying to related forward-looking statements wherever they appear in this report. Our actual results may be materially different from the results we discuss in the forward-looking statements due to certain factors, including those discussed in “Risk Factors” and other sections of this report.
Overview
Allscripts Healthcare Solutions, Inc. is a leading provider of clinical software, connectivity and information solutions that physicians use to improve the quality of healthcare. Our businesses provide innovative solutions that inform physicians with just right, just in time information, connect physicians to each other and to the entire community of care, and transform healthcare, improving both the quality and efficiency of care. We provide clinical software applications, including Electronic Health Record (EHR), practice management, electronic prescribing, Emergency Department Information System (EDIS), hospital care management and document imaging solutions through our clinical solutions businesses. Additionally, we provide clinical education and information solutions for physicians and patients through our physicians interactive business unit, along with physician-patient connectivity solutions through our partnership with Medem. We also provide prepackaged medication fulfillment services through our medication services business unit.
We report our financial results utilizing three business segments: software and related services segment; information services segment; and prepackaged medications segment. The software and related services segment consists of clinical software solutions offered by our clinical solutions business unit, such as Canopy, HealthMatics, TouchWorks, and TouchScript offerings. TouchWorks Electronic Health Record is an award-winning EHR solution designed to enhance physician productivity using Tablet PCs, wireless handheld devices or desktop workstations for the purpose of automating the most common physician activities, including prescribing, dictating, ordering lab tests and viewing results, documenting clinical encounters and capturing charges, among others. TouchWorks Practice Management combines scheduling and financial management tools in a single package with functionality including rules-based appointment scheduling, multi-resource and recurring appointment features, referral and eligibility indicators, and appointment and claims management. TouchWorks EHR and TouchWorks PM, which are offered individually and as a combined solution, both have the functionality to handle the complexities of large physician practice groups with 25 or more physicians.
For physician practice groups with fewer than 25 physicians that are seeking an EHR, a practice management system, or a combined EHR and practice management solution, we offer our HealthMatics EHR, Ntierprise Practice Management and HealthMatics Office, which combines the two offerings into one complete solution for clinical and back-office automation.
TouchScript is an e-prescribing solution that physicians can access securely via the Internet to quickly, safely and securely prescribe medications, check for drug interactions, access medication histories, review drug reference information, and send prescriptions directly to a pharmacy or mail order facility. TouchScript can be a starting point for medical groups to seamlessly transition over time to a complete EHR. Another e-prescribing offering, eRxNOW, is an easy-to-use, web-based solution that is safe, secure, requires no downloading and no new hardware. eRxNOW is accessible by Internet on computers, handheld devices and cell phones and is offered free of charge to every prescriber in America via the National ePrescribing Patient Safety Initiative, a coalition of companies led by Allscripts.
Our offerings for hospitals that are seeking EDIS and care management solutions include HealthMatics ED, EmSTAT and Canopy. HealthMatics ED electronically streamlines processes for large hospital Emergency Departments, including tracking, triage, nurse and physician charting, disposition and reporting. EmSTAT offers
32
similar functionality for streamlining the Emergency Department care process in small hospitals. Canopy is a Web-based solution that streamlines and speeds the patient care management process by automating utilization, case, discharge and quality management processes relating to patient hospital visits.
In our information services segment, our key product offerings are Physicians Interactive and Physician Relationship Management Platform (“PRMP”). Physicians Interactive is a web-based solution that connects physicians with pharmaceutical companies, medical device manufacturers and biotech companies. One element of this solution, often referred to as e-Detailing, uses interactive sessions to provide clinical education and information to physicians about medical products and disease states, which promotes more informed decision-making, increased efficiency and ultimately higher quality patient care. Other elements of the Physicians Interactive platform include e-surveys, clinical updates, resource centers, key opinion leader materials and other physician relationship management services. PRMP provides pharmaceutical companies with a turnkey system to build an electronic dialogue and manage ongoing relationships with physicians. The PRMP incorporates a full suite of online tools, including campaign management, physician communication and education and sample and rep requests, as well as e-Detailing opportunities.
Finally, our prepackaged medications segment is comprised of our medication services business unit. This business unit provides point-of-care medication management and medical supply services and solutions for physicians and other healthcare providers.
The composition of our revenue by segment is as follows:
| | | | | | | | | | | | | | | | |
| | | Quarter Ended |
| | | 2006 | | 2005 |
| | | Dec. 31 | | Sept. 30 | | June 30 | | March 31 | | Dec. 31 | | Sept. 30 | | June 30 | | March 31 |
| | | (Unaudited) |
Software and related services | | $48,910 | | $49,534 | | $46,745 | | $28,314 | | $18,249 | | $16,462 | | $16,145 | | $14,310 |
Prepackaged medications | | 11,232 | | 10,438 | | 10,508 | | 11,510 | | 12,789 | | 11,496 | | 11,489 | | 9,835 |
Information services | | 3,418 | | 2,219 | | 2,761 | | 2,380 | | 3,159 | | 2,680 | | 1,900 | | 2,050 |
| | | | | | | | | | | | | | | | |
Total revenue | | $63,560 | | $62,191 | | $60,014 | | $42,204 | | $34,197 | | $30,638 | | $29,534 | | $26,195 |
| | | | | | | | | | | | | | | | |
Cost of revenue for the software and related services segment consists primarily of salaries, bonuses and benefits of our billable professionals, third-party software costs, hardware costs, capitalized software amortization and other direct engagement costs. Cost of revenue for the prepackaged medications segment consists primarily of the cost of the medications, cost of salaries, bonuses and benefits for repackaging personnel, shipping costs, repackaging facility costs and other costs. Cost of revenue for the information services segment consists primarily of salaries, bonuses and benefits of our program management and program development personnel, third-party program development costs, costs to recruit physicians and other program management costs.
Selling, general and administrative expenses consist primarily of salaries, bonuses and benefits for management and support personnel, commissions, facilities costs, depreciation and amortization, general operating expenses, non-capitalizable product development expenses and selling and marketing expenses. Selling, general and administrative expenses for each segment consist of expenses directly related to that segment. Expenses for the year ended December 31, 2006 include non-recurring integration costs related to the A4 acquisition.
33
Critical Accounting Policies and Estimates
Management’s Discussion and Analysis of Financial Condition and Results of Operations discusses our consolidated financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States of America. The preparation of these financial statements requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenue and expenses during the reporting period.
Management bases its estimates and judgments on historical experience and on various other factors that are believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions. Management believes the following critical accounting policies, among others, affect its more significant judgments and estimates used in the preparation of its consolidated financial statements.
Revenue Recognition
Revenue from software licensing arrangements, where the service element is considered essential to the functionality of the other elements of the arrangement, is accounted for under American Institute of Certified Public Accountants Statement of Position (“SOP”) 81-1, “Accounting for Performance of Construction-Type Contracts and Certain Production-Type Contracts.” Allscripts recognizes such revenue on an input basis using actual hours worked as a percentage of total expected hours required by the arrangement, provided that the fee is fixed and determinable and collection of the receivable is probable. If any such software licensing arrangement is deemed to have extended payment terms, revenue is recognized using the input method but is limited to the amounts due and payable. Maintenance and support revenue from software licensing arrangements is recognized over the term of the applicable support agreement based on vendor-specific objective evidence of fair value of the maintenance and support revenue, which is generally based upon contractual renewal rates.
Revenue from software licensing arrangements where the service element is not considered essential to the functionality of the other elements of the arrangement is accounted for under SOP 97-2, “Software Revenue Recognition,” as amended by SOP 98-9, “Modification of SOP 97-2, Software Revenue Recognition, With Respect to Certain Transactions.” Such revenue is recognized upon shipment of the software or as services are performed, provided that persuasive evidence of an arrangement exists, fees are considered fixed and determinable, and collection of the receivable is considered probable. The revenue recognized for each separate element of a multiple-element software contract is based upon vendor-specific objective evidence of fair value, which is based upon the price the customer is required to pay when the element is sold separately.
Revenue from Allscripts’ sales of pharmaceutical products, net of provisions for estimated returns, is recognized upon shipment of the pharmaceutical products, the point at which the customer takes ownership and assumes risk of loss, when no performance obligations remain and collection of the receivable is probable. Allscripts offers customers the right to return pharmaceutical products under various policies and estimates and maintains reserves for product returns based on historical experience following the provisions of FAS No. 48, “Revenue Recognition When Right of Return Exists.”
Certain of Allscripts’ customer arrangements in its information services segment encompass multiple deliverables. Allscripts accounts for these arrangements in accordance with Emerging Issues Task Force (“EITF”) No. 00-21, “Accounting for Revenue Arrangements with Multiple Deliverables” (“EITF 00-21”). If the deliverables meet the criteria in EITF 00-21, the deliverables are separated into separate units of accounting, and revenue is allocated to the deliverables based on their relative fair values. The criteria specified in EITF 00-21 are that the delivered item has value to the customer on a stand-alone basis, there is objective and reliable evidence of the fair value of the undelivered item, and if the arrangement includes a general right of return
34
relative to the delivered item, delivery or performance of the undelivered item is considered probable and substantially in the control of the vendor. Applicable revenue recognition criteria is considered separately for each separate unit of accounting.
Management applies judgment to ensure appropriate application of EITF 00-21, including value allocation among multiple deliverables, determination of whether undelivered elements are essential to the functionality of delivered elements and timing of revenue recognition, among others. For those arrangements where the deliverables do not qualify as a separate unit of accounting, revenue from all deliverables is treated as one accounting unit and recognized on a straight-line basis over the term of the arrangement. Changes in circumstances and customer data may affect management’s analysis of EITF 00-21 criteria, which may cause Allscripts to adjust upward or downward the amount of revenue recognized under the arrangement.
In accordance with EITF issued Consensus 01-14, “Income Statement Characterization of Reimbursements for ‘Out-of-Pocket’ Expenses Incurred,” revenue includes reimbursable expenses charged to Allscripts’ clients.
Allowance for Doubtful Accounts Receivable
We rely on estimates to determine our bad debt expense and the adequacy of our allowance for doubtful accounts. These estimates are based on our historical experience and the industry in which we operate. If the financial condition of our customers were to deteriorate, resulting in an impairment of their ability to make payments, additional allowances and related bad debt expense may be required.
Inventories
We adjust the value of our inventory downward for estimated obsolescence or unmarketable inventory in an amount equal to the difference between the cost of inventory and the estimated market value based upon assumptions about future demand and market conditions. If actual future demand or market conditions are less favorable than those projected by management, additional inventory write-downs may be required.
Investment in Promissory Note and Minority Interest
Allscripts holds an investment in Medem totaling $3,100 as of December 31, 2006. The investment has been accounted for under the cost basis of accounting and is recorded in other assets in the consolidated balance sheet. The investment consists of a $2,600 note receivable from and a $500 minority interest in Medem. The fair value of the investment is dependent upon the actual financial performance of Medem, its market value, and the volatility inherent in the external markets for this type of investment. In assessing potential impairment of the investment, we consider these factors, as well as the forecasted financial performance of Medem, liquidation preference value of the stock that we hold, and estimated potential for investment recovery. If any of these factors indicate that the investment has become other-than-temporarily impaired, we may have to record an impairment charge.
Goodwill and Intangible Assets
We evaluate the value of intangible assets based upon the present value of the future economic benefits expected to be derived from the assets. We assess the impairment of the identifiable intangibles and goodwill annually, or more frequently if events or changes in circumstances indicate that the asset might be impaired. If we determine that the value of the intangible assets and goodwill may not be recoverable from future cash flows, a write-down of the value of the asset may be required.
We estimate the useful lives of our intangible assets and amortize the value over that estimated life. If the actual useful life is shorter than our estimated useful life, we will amortize the remaining book value over the
35
remaining useful life or the asset may be deemed to be impaired and, accordingly, a write-down of the value of the asset may be required.
Software Capitalization
The carrying value of capitalized software is dependent upon the ability to recover its value through future revenue from the sale of the software. If we determine in the future that the value of the capitalized software could not be recovered, a write-down of the value of the capitalized software to its recoverable value may be required.
We estimate the useful life of our capitalized software and amortize the value over that estimated life. If the actual useful life is shorter than our estimated useful life, we will amortize the remaining book value over the remaining useful life or the asset may be deemed to be impaired and, accordingly, a write-down of the value of the asset may be required.
Income Taxes
Deferred tax assets and liabilities are determined based on the difference between the financial statements and tax basis of assets and liabilities using enacted tax rates in effect for the year in which the differences are expected to reverse. Our provision for income taxes is based on domestic statutory income tax rates in the jurisdictions in which we operate. Significant judgment is required in determining income tax provisions as well as deferred tax asset and liability balances, including the estimation of valuation allowances and the evaluation of tax positions.
As of December 31, 2006, we recognized a net deferred tax asset of $23,522. Net deferred tax assets are primarily comprised of net deductible temporary differences and net operating loss carryforwards that are available to reduce taxable income in future periods. As of December 31, 2006 we did not record a valuation allowance against the net deferred tax asset due to management’s assessment that it was more likely than not that all of the net deferred tax assets will be realized. A valuation allowance is required when it is more likely than not that all or a portion of a deferred tax asset will not be realized. In assessing the need for a valuation allowance, we consider all available positive and negative evidence, including past operating results, projections of future taxable income and the feasibility of ongoing tax planning strategies.
As described in further detail in the Recent Accounting Pronouncements section in Note 2 of Notes to Financial Statements, FASB Interpretation (“FIN”) No. 48 “Accounting for Uncertainty in Income Taxes—an interpretation of FASB Statement 109” is effective beginning January 1, 2007. FIN 48 establishes a single model to address accounting for uncertainty in tax positions. FIN 48 clarifies the accounting for income taxes by prescribing a minimum recognition threshold a tax position is required to meet before being recognized in the financial statements. FIN 48 also provides guidance on derecognition, measurement, classification, interest and penalties, accounting in interim periods, disclosure and transition. FIN 48 is effective for fiscal years beginning after December 15, 2006. Allscripts will adopt FIN 48 as of January 1, 2007 as required. We have not yet completed our evaluation of the impact of adoption on our consolidated financial statements.
36
Results of Operations
The following table shows, for the periods indicated, our results of operations expressed as a percentage of our revenue:
| | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2006 | | | 2005 | | | 2004 | |
Revenue | | 100.0 | % | | 100.0 | % | | 100.0 | % |
Cost of revenue | | 49.1 | | | 54.5 | | | 57.7 | |
| | | | | | | | | |
Gross profit | | 50.9 | | | 45.5 | | | 42.3 | |
Operating expenses: | | | | | | | | | |
Selling, general and administrative expenses | | 37.7 | | | 36.4 | | | 37.4 | |
Amortization of intangibles | | 4.5 | | | 1.5 | | | 1.7 | |
| | | | | | | | | |
Income from operations | | 8.7 | | | 7.6 | | | 3.2 | |
Interest income | | 1.5 | | | 3.4 | | | 1.7 | |
Interest expense | | (1.6 | ) | | (2.9 | ) | | (1.7 | ) |
Other expense, net | | (0.1 | ) | | (0.1 | ) | | (0.1 | ) |
| | | | | | | | | |
Income from operations before income taxes | | 8.5 | | | 8.0 | | | 3.1 | |
Provision for income taxes | | (3.3 | ) | | — | | | — | |
| | | | | | | | | |
Net income | | 5.2 | % | | 8.0 | % | | 3.1 | % |
| | | | | | | | | |
Year Ended December 31, 2006 Compared to Year Ended December 31, 2005
Software and Related Services
Software and related services revenue for the year ended December 31, 2006 increased 166.2%, or $108,337, from $65,166 in 2005 to $173,503 in 2006. Of the increase, a total of $78,444 is attributed to revenue generated by A4 since the March 2, 2006 acquisition. The remaining increase of $29,893 or 45.9% is attributable to an increase in revenue recorded for software and implementation services accounted for under percentage of completion, add-on software sales to existing customers, an increase in related support and maintenance revenue due to the increase in our installed customer base and an increase in hardware revenue.
Gross profit for software and related services for the year ended December 31, 2006 increased 147.6%, or $61,493, from $41,659 in 2005 to $103,152 in 2006. The increase in gross profit is primarily a result of an increase in revenue recorded for software and implementation services accounted for under percentage of completion, add-on software sales to existing customers, an increase in support and maintenance revenue and the contribution of gross profit from A4 since the date of acquisition. Gross profit for software and related services as a percentage of revenue decreased from 63.9% in 2005 to 59.5% in 2006. Allscripts’ gross profit as a percentage of revenue was adversely impacted in 2006 due to the contribution of gross profit from the A4 product line, which tends to have lower margins than our traditional overall software and related services product lines. In addition, the gross margin was negatively affected by an increase of approximately $6,600 in lower margin hardware revenue in 2006, which included one large hardware transaction for $2,000 with a customer in the third quarter of 2006.
Operating expenses for software and related services for the year ended December 31, 2006 increased $34,029 from $23,254 in 2005 to $57,283 in 2006. Of the increase, a total of $27,167 is attributed to A4 operating expenses since the March 2, 2006 acquisition, which includes $247 of stock-based compensation. The remaining increase of $6,862 is primarily the result of an increase of approximately $3,157 in compensation related costs, an increase of $1,173 in commissions, $812 in bad debt expense, $534 in travel related costs, and $846 in stock-based compensation. These increases in operating costs for 2006 reflect the overall growth
37
experienced in the software and services business and also reflect an increase of approximately 36 headcount additions during 2006. A total of $1,093 and $0 was recorded for stock-based compensation in 2006 and 2005, respectively.
We had capitalized software of $7,446 in 2006 and $2,796 for the same period in 2005, which was capitalized pursuant to Statement of Financial Accounting Standard “SFAS” No. 86, “Accounting for Costs of Computer Software to be Sold, Leased or Otherwise Marketed” in our software and related services segment. The increase in capitalized software is largely attributed to the development of TouchWorks version 11 and upgrades to our e-prescribing software.
Prepackaged Medications
Prepackaged medications revenue for the year ended December 31, 2006 decreased 4.2%, or $1,921, from $45,609 in 2005 to $43,688 in 2006. The decrease is primarily due to management’s focus in reducing lower margin revenue from wholesaler customers, which decreased from approximately $13,202 in 2005 to approximately $9,523 in 2006. This $3,679 decrease in wholesaler revenue was partially offset by an increase in prepackaged medications revenue of approximately $1,758, which was primarily driven by new business and an overall increase in average selling price of approximately 5% on a year-over-year basis. Total medication units shipped in 2006 and 2005 remained relatively consistent at 2.4 million units.
Gross profit for prepackaged medications for the year ended December 31, 2006 decreased 1.8%, or $138, from $7,563 in 2005 to $7,425 in 2006. Gross profit as a percentage of revenue increased from 16.6% in 2005 to 17.0% in 2006. The decrease in gross profit is due to the decrease in revenue as noted and the increase in gross profit as a percentage of revenue is due to management’s focus on decreasing lower margin sales to wholesaler customers.
Operating expenses for prepackaged medications for the year ended December 31, 2006 increased $1,298, from $1,826 in 2005 to $3,124 in 2006. The increase is primarily due to an increase in bad debt expense of approximately $875 primarily relating to one wholesaler customer, an increase of approximately $114 in sales commissions, and the remaining increase of $309 represents increased cost for compensation related costs, travel and entertainment expenses, and sales tax related matters.
Information Services
Information services revenue for the year ended December 31, 2006 increased 10.1%, or $989, from $9,789 in 2005 to $10,778 in 2006. This improvement is primarily attributed to the increase in revenue related to the development and hosting of several PRMP solutions in 2006, when compared to the same period in 2005. Revenue from e-detailing sessions remained consistent on a year-over-year basis.
Gross profit for information services decreased 5.2%, or $292, from $5,653 in 2005 to $5,361 in 2006. Gross profit as a percentage of revenue decreased from 57.7% in 2005 to 49.7% in 2006. The decreases in both gross profit and gross profit as a percentage of revenue in 2006 are primarily due to the recognition of non-recurring program early termination fees during 2005, which compares to lower termination fees in 2006. We also recognized a lower e-detail program certificate reward redemption rate in 2005, which resulted in lower cost of revenue when compared to the same period in 2006.
Operating expenses for information services increased 38.0%, or $1,106, from $2,910 in 2005 to $4,016 in 2006. The increase is primarily due to an increase of approximately $460 in compensation related costs due to headcount additions primarily for the platform business, an increase of approximately $213 in sales commissions, a charge of approximately $180 in stock-based compensation costs and an increase in various other operating costs of $253 in 2006. There was no stock-based compensation in 2005.
38
Unallocated Corporate Expenses
Unallocated corporate expenses for the year ended December 31, 2006 increased $13,985, from $17,662 in 2005 to $31,647 in 2006. The increase in 2006 primarily relates to $8,573 in increased intangible asset amortization relating to the A4 acquisition and an increase of approximately $815 in corporate salaries and bonus expense. There was a net increase of $450 in stock-based compensation primarily due to the forfeiture rate adjustment related to the accelerated option vesting during the fourth quarter of 2005. In addition, the increase in corporate costs in 2006 reflects an increase of $811 in facilities expense resulting from expansion of our corporate office and an increase in consulting related costs of $975 for the improvement of our information systems and other related projects. Operating expenses for 2006 included $1,021 in non-recurring integration costs related to the A4 acquisition that was recognized in the first quarter of 2006.
Interest income
Interest income for 2006 decreased 19.9%, or $820, from $4,128 in 2005 to $3,308 in 2006. The decrease in interest income is attributed to a decrease in overall cash and marketable securities resulting from the A4 acquisition and the repurchase of 1,250 shares of Allscripts common stock from IDX Investment Corporation (“IDX”), a subsidiary of General Electric Company (“GE”), partially offset by cash generated from operations and an increase in interest rates during 2006.
Interest expense
Interest expense for 2006 increased 5.6%, or $196, from $3,516 in 2005 to $3,712 in 2006. The increase is primarily due to interest expense incurred on the $3,400 secured promissory note assumed in the A4 acquisition.
Income taxes
As a result of the A4 acquisition in March 2006, management determined under the provisions of SFAS 109, “Accounting for Income Taxes”, that it is more likely than not that Allscripts will generate adequate taxable income for the foreseeable future to realize its deferred tax assets. Accordingly, management reversed all of its $61,284 valuation allowance against goodwill in purchase accounting for the A4 acquisition. In connection with the reversal of its valuation allowance in purchase accounting, approximately $5,656 of net operating losses (“NOL”) were written-off pursuant to Internal Revenue Code Section 382, which imposes an annual limitation on the future utilization of net operating losses. A tax provision of $7,424 and $0 were recorded for the years ended December 31, 2006 and 2005, respectively. As of December 31, 2006 and 2005, the valuation allowance was $0 and $61,284, respectively.
Year Ended December 31, 2005 Compared to Year Ended December 31, 2004
Software and Related Services
Software and related services revenue for the year ended December 31, 2005 increased 47.7%, or $21,045, from $44,121 in 2004 to $65,166 in 2005. The increase is attributable to an increase in our installed customer base, an increase in hardware revenue as a result of obtaining certain large contracts in 2005 and an increase in maintenance revenue, as customers typically continue maintenance with us. We also experienced a number of existing customers converting their subscription contracts into licenses in 2005, and certain existing customers purchasing additional software licenses to resell to local and regional small physician groups.
Gross profit for software and related services for the year ended December 31, 2005 increased 47.4%, or $13,396, from $28,263 in 2004 to $41,659 in 2005. The increase in gross profit is a result of an increase in the overall installed customer base, an increase in hardware revenue, and an increase in maintenance revenue,
39
combined with the continued focus in reducing our costs of implementation and training, and due to a decrease in royalties as a percent of revenue. In addition, the improvement in gross profit during 2005 reflects an increase in the number of customers converting subscription contracts to licenses and the recognition of higher margin revenue associated with add-on license sales to existing customers, including licenses purchased by such customers for resale to external local and regional physician groups. Gross profit for software and related services as a percentage of revenue decreased from 64.1% in 2004 to 63.9% in 2005. This slight decrease is due to a larger percentage of our 2005 revenue being comprised of lower margin hardware sales and due to the recognition of additional capitalized software amortization as a percentage of 2005 revenue.
Operating expenses for software and related services for the year ended December 31, 2005 increased 18.5%, or $3,624, from $19,630 in 2004 to $23,254 in 2005. The increase is primarily the result of an increase in compensation and commissions expense, as we expanded our sales and marketing resources in 2005 to meet increased demand for our EHR solutions, an increase in bad debt expense, which is attributable to our increase in revenue, and due to lower capitalized software costs. We capitalized $2,796 and $3,949 of software development costs for the years ended December 31, 2005 and 2004, respectively, pursuant to Financial Accounting Standards (“FAS”) No. 86, “Accounting for Costs of Computer Software to be Sold, Leased or Otherwise Marketed.” These increases were offset by lower non-capitalizable research and development expense in 2005 compared to 2004.
Prepackaged Medications
Prepackaged medications revenue for the year ended December 31, 2005 increased 2.0%, or $876, from $44,733 in 2004 to $45,609 in 2005. The increase is primarily due to an increase in revenue from wholesale customers from $7,751 in 2004 compared to $13,202 in 2005, as a result of fulfilling available demand, and due to the inclusion of flu vaccine sales in the fourth quarter of 2005, that were not available to us in the fourth quarter of 2004 because of the lack of supply in the United States as a result of manufacturing problems by one of the main flu vaccine suppliers. These increases were offset by lost sales of COX-2 inhibitors that resulted from the Vioxx recall announced on September 30, 2004 and lost sales as a result of Hurricane Katrina.
Gross profit for prepackaged medications for the year ended December 31, 2005 decreased 15.9%, or $1,426, from $8,989 in 2004 to $7,563 in 2005. Gross profit as a percentage of revenue decreased from 20.1% in 2004 to 16.6% in 2005. The decrease in both gross profit and gross profit as a percentage of revenue is due to an overall reduction in the volume of prepackaged medications sold on a year over year basis and an increase in lower margin revenue to wholesale customers.
Operating expenses for prepackaged medications for the year ended December 31, 2005 increased $112, or 6.5%, from $1,714 in 2004 to $1,826 in 2005. The increase is primarily due to an increase in sales and marketing headcount and related salary and benefits.
Information Services
Information services revenue for the year ended December 31, 2005 decreased 17.8%, or $2,127, from $11,916 in 2004 to $9,789 in 2005. The decrease is due to a decline in the number of e-detailing sessions completed during 2005 compared to 2004. The decrease in e-detailing sessions reflects the challenging sales environment experienced in 2004 as a result of the Office of Inspector General highlighting certain issues regarding physician marketing activities by pharmaceutical manufacturers in 2003 and, in some cases, customer cancellations of second and third program waves of a contract. The decrease was offset by revenue recognized from program early termination fees, accelerated development revenue on cancelled programs, and revenue recorded from the development and hosting of a PRMP solution.
Gross profit for information services increased 4.8%, or $257, from $5,396 in 2004 to $5,653 in 2005. Gross profit as a percentage of revenue increased from 45.3% in 2004 to 57.7% in 2005. The increases in both gross profit and gross profit as a percentage of revenue are primarily due to the recognition of additional program early
40
termination fees, the recognition of development revenue on cancelled programs, lower certificate reward redemption rate estimates and revenue from certain higher-margin programs, offset by a lower number of e-detailing programs completed in 2005 compared to 2004.
Operating expenses for information services decreased 7.4%, or $232, from $3,142 in 2004 to $2,910 in 2005. The decrease is primarily the result of lower marketing expenditures, a higher level of capitalizable research and development efforts and lower commission expense, which is reflective of the revenue decrease in the information services segment.
Unallocated Corporate Expenses
Unallocated corporate expenses for the year ended December 31, 2005 increased 18.4%, or $2,743, from $14,919 in 2004 to $17,662 in 2005. The increase is due to an overall increase in corporate salaries expense, which is reflective of an increase in headcount in order to support the growth of our business, standard salary increases, additional marketing expenses and information system consulting expenses related to improvements in our operating systems, offset by a decrease in bad debt expense due to the ability to collect on certain previously determined doubtful accounts. The results of 2005 also reflect a $518 non-cash, stock-based compensation charge in the fourth quarter of 2005 related to the accelerated vesting of certain options. In addition, 2004 results reflect a $400 legal settlement received in first quarter of 2004, which was offset against corporate expenses.
Interest Income
Interest income for the year ended December 31, 2005 increased 146.4%, or $2,453, from $1,675 in 2004 to $4,128 in 2005. The increase is primarily related to interest income earned for the full year of 2005 on the net proceeds received from the issuance of our Senior Convertible Debentures (“Notes”) completed in July 2004 and due to positive cash flow generated in 2005 which resulted in additional investment purchases, as well as more favorable interest rates experienced in 2005 than in 2004. Total net proceeds received from the 2004 offering amounted to $79,612, offset by $11,250, which we used for the repurchase of approximately 1,399 shares of our common stock.
Interest Expense
We incurred $3,516 and $1,717 of interest expense for the years ended December 31, 2005 and 2004, respectively. The increase in interest expense is the result of our Notes being outstanding for the full year of 2005 compared to 2004. In connection with the issuance, we incurred $2,976 of debt issuance costs. Interest expense for both periods includes debt issuance cost amortization expense of $628 and $313 for the years ended December 31, 2005 and 2004, respectively.
Income Taxes
No tax provision or tax benefit for income taxes was recorded for the year ended December 31, 2005 or 2004 due to the fact that any current year income tax liability would be offset by the net operating loss carryforward, which resulted from prior year losses.
41
Selected Quarterly Operating Results
The following table shows our quarterly unaudited consolidated financial information for the eight quarters ended December 31, 2006. We have prepared this information on the same basis as the annual information presented in other sections of this report. In management’s opinion, this information reflects all adjustments, all of which are of a normal recurring nature except the non-cash, stock-based compensation charge incurred in the 4th quarter of 2005, that are necessary for a fair presentation of the results for these periods. The operating results for any quarter should not be relied upon to predict the results for any subsequent period or for the entire fiscal year. You should be aware of possible variances in our future quarterly results. See “Risk Factors—Risks Related to Our Stock—Our quarterly operating results may vary.”
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | Quarter Ended | |
| | | 2006 | | | 2005 | |
| | | Dec. 31 | | | Sept. 30 | | | June 30 | | | March 31 | | | Dec. 31 | | | Sept. 30 | | | June 30 | | | March 31 | |
| | | (unaudited) | |
| Statements of Operations Data: | | | | | | | | | | | | | | | | | | | | | | | | |
Revenue | | $63,560 | | | $62,191 | | | $60,014 | | | $42,204 | | | $34,197 | | | $30,638 | | | $29,534 | | | $26,195 | |
Cost of revenue | | 29,544 | | | 31,666 | | | 28,742 | | | 22,079 | | | 18,630 | | | 17,378 | | | 15,653 | | | 14,028 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Gross profit | | 34,016 | | | 30,525 | | | 31,272 | | | 20,125 | | | 15,567 | | | 13,260 | | | 13,881 | | | 12,167 | |
Operating expenses: | | | | | | | | | | | | | | | | | | | | | | | | |
Selling, general and administrative expenses (a) | | 23,952 | | | 21,916 | | | 23,122 | | | 16,808 | | | 12,068 | | | 10,025 | | | 11,458 | | | 10,357 | |
Amortization of intangibles | | 2,576 | | | 3,045 | | | 3,281 | | | 1,370 | | | 436 | | | 436 | | | 436 | | | 436 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Income from operations | | 7,488 | | | 5,564 | | | 4,869 | | | 1,947 | | | 3,063 | | | 2,799 | | | 1,987 | | | 1,374 | |
Interest income | | 813 | | | 657 | | | 639 | | | 1,199 | | | 1,230 | | | 1,064 | | | 957 | | | 877 | |
Interest expense | | (937 | ) | | (940 | ) | | (940 | ) | | (895 | ) | | (880 | ) | | (880 | ) | | (878 | ) | | (878 | ) |
Other expense, net | | (11 | ) | | (8 | ) | | (8 | ) | | (118 | ) | | (10 | ) | | (43 | ) | | (33 | ) | | (39 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | |
Income before income taxes | | 7,353 | | | 5,273 | | | 4,560 | | | 2,133 | | | 3,403 | | | 2,940 | | | 2,033 | | | 1,334 | |
Income taxes | | 2,870 | | | 2,011 | | | 1,733 | | | 810 | | | — | | | — | | | — | | | — | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Net income | | $4,483 | | | $3,262 | | | $2,827 | | | $1,323 | | | $3,403 | | | $2,940 | | | $2,033 | | | $1,334 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Net income per share—basic | | $0.08 | | | $0.06 | | | $0.05 | | | $0.03 | | | $0.08 | | | $0.07 | | | $0.05 | | | $0.03 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Net income per share—diluted | | $0.08 | | | $0.06 | | | $0.05 | | | $0.03 | | | $0.08 | | | $0.07 | | | $0.05 | | | $0.03 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
| (a) | Includes stock-based compensation expense of $888, $617, $416, $407, $558, and $46 for the three months ended December 31, 2006, September 30, 2006, June 30, 2006, March 31, 2006, December 31, 2005 and September 30, 2005, respectively. |
Our quarterly gross profits improved during 2006 compared to 2005 primarily due to the growth in our higher margin software and related services revenue, the contribution of gross profit from A4 since the date of acquisition and the decrease in lower margin wholesaler revenue in 2006 compared to 2005 in our prepackaged medications segment.
Beginning in the third quarter of 2005, we incurred stock-based compensation expense related to the issuance of restricted stock. In the fourth quarter of 2005, we incurred a $518 non-cash, stock-based compensation charge related to the accelerated vesting of certain options.
42
Liquidity and Capital Resources
At December 31, 2006 and December 31, 2005, our principal sources of liquidity consisted of cash, cash equivalents and marketable securities of $83,038 and $146,063, respectively. The decrease of $63,025 is reflective of the following:
Operating activities
For the year ended December 31, 2006, we generated $27,413 in net cash provided by operations, compared to $16,507 in 2005. This net improvement of $10,906 is due primarily to an increase of $23,312 in net income and related non-cash reconciling adjustments, offset by a net working capital decrease of $12,406.
Investing activities
On March 2, 2006, we acquired all of the outstanding equity interests in A4 for approximately $301,190, of which $227,730 was paid in cash to A4 shareholders and $4,685 was incurred in acquisition-related transaction costs. The remaining $68,775 of consideration was paid through the issuance of 3,500 shares of our common stock (based on the last reported sale price of $19.65 per share of our common stock on the Nasdaq National Market on March 2, 2006). The cash component of the purchase price was offset by $21,742 of cash obtained from the A4 acquisition. A total of $849 of the cash purchase price is unpaid and has been accrued for as of December 31, 2006.
During the year ended December 31, 2006, we used $5,674 of cash for capital expenditures, $9,106 for capitalized software development costs and we funded an additional $500 convertible secured promissory note relating to our investment in Medem, Inc.
Financing activities
On February 28, 2006, we completed our public offering of 8,395 shares of our common stock, which generated approximately $140,675 in net proceeds after deducting underwriting discounts and commissions and transaction costs. Proceeds received from the sale of common stock were used to fund the acquisition of A4.
On March 9, 2006, we repurchased 1,250 shares of Allscripts common stock directly from IDX. We paid $21,078, which is based on 95% of the February 22, 2006 public offering price for our common stock of $17.75.
For the year ended December 31, 2006, we received $14,692 in proceeds from the exercise of stock options and purchases of stock under our employee stock purchase plan.
Allscripts’ working capital decreased by 27.4%, or $31,067, for the year ended December 31, 2006, from $113,317 at December 31, 2005 to $82,250 at December 31, 2006. The decrease is primarily due to a decrease in cash, cash equivalents and short-term marketable securities resulting primarily from funding the net cash component of the A4 acquisition totaling $209,824 and the repurchase of 1,250 shares of common stock from IDX totaling $21,078, offset by the net proceeds of $140,675 received in our common stock offering, $14,692 in proceeds from the exercise of stock options and purchases of stock under our employee stock purchase plan and by cash provided by operating activities. At December 31, 2006, we had an accumulated deficit of $533,805, compared to $545,700 at December 31, 2005.
Future Capital Requirements
We believe that our cash, cash equivalents and marketable securities of $83,038 as of December 31, 2006 and our cash flow from operations will be sufficient to meet the anticipated cash needs of our business for the
43
next twelve months. Our primary needs for cash over the next twelve months will be to fund working capital, service approximately $3,130 in interest payments on our debt instruments, fund capital expenditures, contractual obligations, and investment needs of our current business.
We cannot provide assurance that our actual cash requirements will not be greater than we expect as of the date of this report. We will, from time to time, consider the acquisition of, or investment in, complementary businesses, products, services and technologies, which might impact our liquidity requirements or cause us to issue additional equity or debt securities.
If sources of liquidity are not available or if we cannot generate sufficient cash flow from operations during the next twelve months, we might be required to obtain additional sources of funds through additional operating improvements, capital market transactions, asset sales or financing from third parties, a combination thereof or otherwise. We cannot provide assurance that these additional sources of funds will be available or, if available, would have reasonable terms.
Contractual Obligations, Commitments and Off Balance Sheet Arrangements
We have various contractual obligations, which are recorded as liabilities in our consolidated financial statements. Other items, such as operating lease contract obligations are not recognized as liabilities in our consolidated financial statements but are required to be disclosed.
The following table summarizes our significant contractual obligations as of December 31, 2006 and the effect such obligations are expected to have on our liquidity and cash in future periods assuming all obligations reach maturity:
| | | | | | | | | | |
| | | Total | | 2007 | | 2008-2009 | | 2010-2011 | | 2012+ |
Contractual obligations: | | | | | | | | | | |
3.5% Senior Convertible Debentures (1) | | $82,500 | | $— | | $— | | $— | | $82,500 |
Semi-annual interest due on the 3.5% Senior Convertible Debentures (1) | | 51,252 | | 2,888 | | 5,776 | | 5,776 | | 36,812 |
Development contract (2) | | 14,000 | | 4,908 | | 5,224 | | 3,868 | | — |
7.85% secured promissory note | | 3,199 | | 258 | | 581 | | 679 | | 1,681 |
Monthly interest due on the 7.85% secured promissory note | | 1,259 | | 242 | | 419 | | 321 | | 277 |
Non-cancelable operating leases | | 13,008 | | 2,283 | | 3,990 | | 2,694 | | 4,041 |
Acquisition payment obligations (3) | | 933 | | 933 | | — | | — | | — |
Other contractual obligations | | 1,939 | | 1,609 | | 330 | | — | | — |
| | | | | | | | | | |
Total contractual obligations | | $168,090 | | $13,121 | | $16,320 | | $13,338 | | $125,311 |
| | | | | | | | | | |
| (1) | In July 2004, we completed the private placement of our Notes and are obligated to pay approximately $1,444 in interest payments every six months under the Notes, payable on January 15 and July 15 of each year. These Notes can be converted, in certain circumstances, into approximately 7,300 shares of common stock based upon a conversion price of approximately $11.26 per share, subject to adjustment for certain events. The Notes were convertible during all quarters in 2006 by virtue of the last reported sale price for Allscripts’ common sock having exceeded $14.64 for twenty consecutive days in the 30 trading-day period ending on each fiscal quarter end date. No notes were converted as of December 31, 2006. The timing of our obligation on the Notes may change as it relates to funding interest payments and making a principal payment on the Notes based on whether the holders elect to convert the Notes. In addition, Allscripts may redeem some or all of the Notes for cash any time on or after July 20, 2009 at the Notes’ full principal amount plus accrued and unpaid interest, if any. Holders of the Notes may require Allscripts to repurchase some or all of the Notes on July 15, 2009, 2014 and 2019 or, subject to certain exceptions, upon a change of control of Allscripts. |
44
| (2) | On December 1, 2006, we entered into a $14,000 software content development agreement with a partner to assist in the development of TouchWorks clinical content. The partner will be developing customer content for use within Allscripts solutions by medical professionals. Upon acceptance of contracted deliverables, Allscripts will provide payment for the development efforts over the next five years, with the final deliverable to be completed by September 30, 2011. |
| (3) | As of December 31, 2006, $849 and $84 of the consideration related to the A4 acquisition and the August 2003 Advanced Imaging Concepts, Inc. (“AIC”) acquisition, respectively, had not been paid. Payment on the remaining A4 obligation is expected to be paid in 2007. Payment on the remaining AIC obligation will occur upon the receipt of the required acknowledgement from the AIC stockholders. |
In connection with the corporate facilities lease agreement, Allscripts has provided to the lessor an unconditional irrevocable letter of credit in favor of the lessor in the amount of $500 as security for the full and prompt performance by Allscripts under the lease agreement. The letter of credit may be drawn upon by the lessor and retained, used or applied by lessor for the purpose of curing any monetary default or defaults of Allscripts under the lease. The letter of credit provides for an expiration date of one year from the commencement date of the lease, and will automatically extend for additional successive one-year periods through the term of the lease. As of December 31, 2006 and 2005, no amounts had been drawn on the letter of credit.
We have other letters of credit as security for full and prompt performance under various contractual arrangements totaling $300. As of December 31, 2006 and 2005, no amounts had been drawn on the letters of credit.
Recent Accounting Pronouncements
On January 1, 2006, Allscripts adopted Statement of Financial Accounting Standards No. 123 (Revised), “Share-Based Payment” (“SFAS 123(R)”). SFAS 123(R) requires companies to recognize stock-based compensation expense related to all stock awards issued to employees, including options, in the statement of operations based on their fair values on the date of the grant and after applying an estimated forfeiture rate. The stock-based compensation expense is to be recognized over the period in which an employee is required to provide service in exchange for the stock award. Additionally, for any unvested awards outstanding at the adoption date, SFAS 123(R) requires recognition of compensation expense, after applying a forfeiture rate, over the remaining vesting period of the stock-based award.
Allscripts adopted the modified prospective application transition method as provided by SFAS 123(R). Accordingly, during the year ended December 31, 2006, Allscripts recorded stock-based compensation cost totaling the amount that would have been recognized had the fair value method been applied since the effective date of SFAS 123. Previously reported amounts have not been restated. For the year ended December 31, 2006, the effect on Allscripts’ results of operations of recording stock-based compensation in accordance with SFAS 123(R) was $1,829, or $0.04 and $0.03 per basic and diluted shares, respectively.
Prior to the adoption of SFAS 123(R), our stock-based compensation awards were accounted for under the recognition and measurement provisions of Accounting Principles Board No. 25, “Accounting for Stock Issued to Employees, and Related Interpretations” (“APB 25”). Under the intrinsic value method described in APB 25, no compensation expense was recorded because the exercise price of the employee stock options equaled the market price of the underlying stock on the date of grant. The fair value of stock options granted prior to the adoption of SFAS 123(R) was estimated at the date of grant using the Black-Scholes option-pricing model. No stock options were granted to employees in the twelve months ended December 31, 2006. Allscripts does not expect to grant options to employees in the future, and instead, expects to use awards of restricted stock and restricted stock units as stock-based incentives to employees.
In June 2006, the FASB issued Interpretation No. 48 (FIN 48), Accounting for Uncertainty in Income Taxes. This interpretation clarifies the accounting for uncertainty in income taxes recognized in the financial statements
45
in accordance with Statement of Financial Accounting Standards (SFAS) No. 109, “Accounting for Income Taxes” by prescribing the minimum recognition threshold a tax position is required to meet before being recognized in the financial statements. FIN 48 prescribes a comprehensive model for how a company should recognize, measure, present, and disclose in its financial statements uncertain tax positions that the company has taken or expects to take on a tax return. FIN 48 is effective for fiscal years beginning after December 15, 2006, with the cumulative effect of the change in accounting principle recorded as an adjustment to opening retained earnings. We have not yet completed our evaluation of the impact of adoption on our consolidated financial statements.
In September 2006, the Securities and Exchange Commission (“SEC”) issued Staff Accounting Bulletin No. 108, “Considering the Effects of Prior Year Misstatements when Quantifying Misstatements in Current Year Financial Statements” (“SAB 108”). SAB 108 provides interpretive guidance on how the effects of the carryover or reversal of prior year misstatements should be considered in quantifying a current year misstatement. The SEC staff believes that registrants should quantify errors using both the balance sheet and income statement approach when quantifying a misstatement. We adopted SAB 108 for the year ended December 31, 2006 as required, and the adoption did not have a material effect on our consolidated financial statements.
In September 2006, the Financial Accounting Standards Board (FASB) issued SFAS 157,Fair Value Measurements. SFAS 157 defines fair value, establishes a framework for measuring fair value under generally accepted accounting principles, and expands disclosures about fair value measurements. SFAS 157 clarifies that the fair value is the exchange price in an orderly transaction between market participants to sell the asset or transfer the liability in the market. The standard emphasizes that fair value is a market-based measurement, not an entity-specific measurement and a fair value measurement should therefore be based on the assumptions that market participants would use in pricing the asset or liability. SFAS 157 is effective for financial statements issued for fiscal years beginning after November 15, 2007. The Company is currently evaluating the accounting and disclosure requirements of SFAS 157 and plans to adopt it as required at the beginning of its fiscal year 2008.
Item 7A. Quantitative and Qualitative Disclosures About Market Risk (Dollars in thousands)
As of December 31, 2006, we did not own any derivative financial instruments, but we were exposed to market risks, primarily changes in U.S. interest rates. Our Notes bear a fixed interest rate, and accordingly, the fair market value of the debt is sensitive to changes in interest rates. We have no cash flow or earnings exposure due to market interest rate changes for our fixed debt obligation.
As of December 31, 2006, we had cash, cash equivalents and marketable securities in financial instruments of $83,038. Declines in interest rates over time will reduce our interest income from our investments. Based upon our balance of cash, cash equivalents and marketable securities as of December 31, 2006, a decrease in interest rates of 1.0% would cause a corresponding decrease in our annual interest income of approximately $830.
46
Item 8. Financial Statements and Supplementary Data
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders
Allscripts Healthcare Solutions, Inc.:
We have audited the accompanying consolidated balance sheets of Allscripts Healthcare Solutions, Inc. and subsidiaries (“the Company”) as of December 31, 2006 and 2005, and the related consolidated statements of operations, stockholders’ equity and comprehensive income (loss), and cash flows for the years ended December 31, 2006, 2005 and 2004. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of Allscripts Healthcare Solutions, Inc. as of December 31, 2006 and 2005, and the results of its operations, its changes in stockholders’ equity and comprehensive income (loss) and its cash flows for the years ended December 31, 2006, 2005 and 2004 in conformity with accounting principles generally accepted in the United States of America.
As discussed in Notes 2 and 10 to the consolidated financial statements, effective January 1, 2006, the Company adopted Statement of Financial Accounting Standards Number 123 (revised 2004),Shared-Based Payment.
We have also audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the effectiveness of the Company’s internal control over financial reporting as of December 31, 2006, based on criteria established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission and our report dated March 1, 2007 expressed an unqualified opinion on management’s assessment and the effectiveness of the Company’s internal control over financial reporting.
/s/ GRANT THORNTON LLP
Chicago, Illinois
March 1, 2007
47
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders
Allscripts Healthcare Solutions, Inc.:
We have audited management’s assessment, included in the accompanying Management’s Report on Internal Control Over Financial Reporting appearing under Item 9A, that Allscripts Healthcare Solutions, Inc. and subsidiaries (the Company) (a Delaware Corporation) maintained effective internal control over financial reporting as of December 31, 2006, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). As described in Management’s Report, management excluded from its assessment the internal control over financial reporting for the 2006 acquisition of A4 Health Systems, Inc. (A4) which accounted for approximately 18 percent of consolidated total assets at December 31, 2006 and approximately 34 percent of consolidated net sales for the year then ended. Accordingly, our audit did not include the internal control over financial reporting at A4. The Company’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting. Our responsibility is to express an opinion on management’s assessment and an opinion on the effectiveness of the Company’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, evaluating management’s assessment, testing and evaluating the design and operating effectiveness of internal control, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, management’s assessment that Allscripts Healthcare Solutions, Inc. maintained effective internal control over financial reporting as of December 31, 2006, is fairly stated, in all material respects, based on COSO. Also in our opinion, Allscripts Healthcare Solutions, Inc. maintained, in all material respects, effective internal control over financial reporting as of December 31, 2006, based on COSO.
We have also audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the balance sheets of Allscripts Healthcare Solutions, Inc. as of December 31, 2006 and 2005 and the related statements of operations, stockholders’ equity and comprehensive income (loss), and cash flows for the years the ended December 31, 2006, 2005 and 2004 and our report dated March 1, 2007 expressed an unqualified opinion on those financial statements and included an explanatory paragraph regarding the Company’s adoption of Statement of Financial Accounting Standards (“SFAS”) No. 123(R),Share-Based Payment, in 2006.
/s/ GRANT THORNTON LLP
Chicago, Illinois
March 1, 2007
48
ALLSCRIPTS HEALTHCARE SOLUTIONS, INC.
CONSOLIDATED BALANCE SHEETS
(In thousands, except per share amounts)
| | | | | | |
| | | December 31, | |
| | | 2006 | | | 2005 | |
ASSETS | | | | | | |
Current assets: | | | | | | |
Cash and cash equivalents | | $42,461 | | | $60,905 | |
Marketable securities | | 14,553 | | | 54,408 | |
Accounts receivable, net of allowances of $4,234 and $2,337 in 2006 and 2005, respectively | | 55,579 | | | 29,746 | |
Deferred taxes, net | | 27,437 | | | — | |
Inventories | | 3,247 | | | 2,174 | |
Prepaid expenses and other current assets | | 10,620 | | | 5,811 | |
| | | | | | |
Total current assets | | 153,897 | | | 153,044 | |
Long-term marketable securities | | 26,024 | | | 30,750 | |
Fixed assets, net | | 14,094 | | | 2,753 | |
Software development costs, net | | 12,285 | | | 6,409 | |
Intangible assets, net | | 78,050 | | | 9,151 | |
Goodwill | | 188,261 | | | 13,760 | |
Other assets | | 4,999 | | | 5,097 | |
| | | | | | |
Total assets | | $477,610 | | | $220,964 | |
| | | | | | |
LIABILITIES AND STOCKHOLDERS’ EQUITY | | | | | | |
Current liabilities: | | | | | | |
Accounts payable | | $9,294 | | | $8,630 | |
Accrued expenses | | 17,861 | | | 11,489 | |
Accrued compensation | | 8,685 | | | 2,302 | |
Deferred revenue | | 35,549 | | | 17,306 | |
Current portion of long-term debt | | 258 | | | — | |
| | | | | | |
Total current liabilities | | 71,647 | | | 39,727 | |
Long-term debt | | 85,441 | | | 82,500 | |
Deferred taxes, net | | 3,915 | | | — | |
Other liabilities | | 357 | | | 318 | |
| | | | | | |
Total liabilities | | 161,360 | | | 122,545 | |
Preferred stock: | | | | | | |
Undesignated, $0.01 par value, 1,000 shares authorized, no shares issued and outstanding at December 31, 2006 and 2005 | | — | | | — | |
Common stock: | | | | | | |
$0.01 par value, 150,000 shares authorized; 54,358 issued and outstanding at December 31, 2006; 42,302 shares issued and 40,873 shares outstanding at December 31, 2005 | | 543 | | | 423 | |
Less treasury stock: | | | | | | |
$0.01 par value, 0 and 1,399 shares at December 31, 2006 and 2005, respectively | | — | | | (11,250 | ) |
Additional paid-in capital | | 849,628 | | | 655,980 | |
Unearned compensation | | — | | | (374 | ) |
Accumulated deficit | | (533,805 | ) | | (545,700 | ) |
Accumulated other comprehensive loss | | (116 | ) | | (660 | ) |
| | | | | | |
Total stockholders’ equity | | 316,250 | | | 98,419 | |
| | | | | | |
Total liabilities and stockholders’ equity | | $477,610 | | | $220,964 | |
| | | | | | |
The accompanying notes are an integral part of these consolidated financial statements.
49
ALLSCRIPTS HEALTHCARE SOLUTIONS, INC.
CONSOLIDATED STATEMENTS OF OPERATIONS
(In thousands, except per share amounts)
| | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2006 | | | 2005 | | | 2004 | |
Revenue: | | | | | | | | | |
Software and related services | | $173,503 | | | $65,166 | | | $44,121 | |
Prepackaged medications | | 43,688 | | | 45,609 | | | 44,733 | |
Information services | | 10,778 | | | 9,789 | | | 11,916 | |
| | | | | | | | | |
Total revenue | | 227,969 | | | 120,564 | | | 100,770 | |
Cost of revenue: | | | | | | | | | |
Software and related services | | 70,351 | | | 23,507 | | | 15,858 | |
Prepackaged medications | | 36,263 | | | 38,046 | | | 35,744 | |
Information services | | 5,417 | | | 4,136 | | | 6,520 | |
| | | | | | | | | |
Total cost of revenue | | 112,031 | | | 65,689 | | | 58,122 | |
Gross profit | | 115,938 | | | 54,875 | | | 42,648 | |
Operating expenses: | | | | | | | | | |
Selling, general and administrative expenses | | 85,798 | | | 43,908 | | | 37,653 | |
Amortization of intangibles | | 10,272 | | | 1,744 | | | 1,752 | |
| | | | | | | | | |
Income from operations | | 19,868 | | | 9,223 | | | 3,243 | |
Interest income | | 3,308 | | | 4,128 | | | 1,675 | |
Interest expense | | (3,712 | ) | | (3,516 | ) | | (1,717 | ) |
Other expense, net | | (145 | ) | | (125 | ) | | (93 | ) |
| | | | | | | | | |
Income from operations before income taxes | | 19,319 | | | 9,710 | | | 3,108 | |
| | | | | | | | | |
Provision for income tax | | 7,424 | | | — | | | — | |
| | | | | | | | | |
Net income | | $11,895 | | | $9,710 | | | $3,108 | |
| | | | | | | | | |
Net income per share—basic | | $0.23 | | | $0.24 | | | $0.08 | |
| | | | | | | | | |
Net income per share—diluted | | $0.22 | | | $0.23 | | | $0.07 | |
| | | | | | | | | |
Weighted-average shares outstanding used in computing basic net income per share | | 51,058 | | | 40,045 | | | 38,979 | |
| | | | | | | | | |
Weighted-average shares outstanding used in computing diluted net income per share | | 53,367 | | | 43,068 | | | 41,592 | |
| | | | | | | | | |
The accompanying notes are an integral part of these consolidated financial statements.
50
ALLSCRIPTS HEALTHCARE SOLUTIONS, INC.
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY
AND COMPREHENSIVE INCOME (LOSS)
(In thousands)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Preferred Stock | | Common Stock | | | Additional Paid-In Capital | | | Unearned Compen- sation | | | Treasury Stock | | | Accumulated Deficit | | | Accumulated Other Comprehensive Income (Loss) | | | Total | |
| | | Shares | | Amount | | Shares | | | Amount | | | | | Shares | | | Amount | | | | |
Balance at December 31, 2003 | | — | | $— | | 39,050 | | | $391 | | | $641,415 | | | $— | | | — | | | $— | | | ($558,518 | ) | | $102 | | | $83,390 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Issuance of 1,064 shares of common stock under option agreements | | — | | — | | 1,064 | | | 10 | | | 4,126 | | | — | | | — | | | — | | | — | | | — | | | 4,136 | |
Repurchase of common stock | | — | | — | | — | | | — | | | — | | | — | | | (1,399 | ) | | (11,250 | ) | | — | | | — | | | (11,250 | ) |
Net income | | — | | — | | — | | | — | | | — | | | — | | | — | | | — | | | 3,108 | | | — | | | 3,108 | |
Unrealized loss on marketable securities, net of tax of $0 | | — | | — | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | (691 | ) | | (691 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance at December 31, 2004 | | — | | $— | | 40,114 | | | $401 | | | $645,541 | | | $— | | | (1,399 | ) | | ($11,250 | ) | | ($555,410 | ) | | ($589 | ) | | $78,693 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Issuance of 2,158 shares of common stock under option agreements | | — | | — | | 2,158 | | | 22 | | | 9,460 | | | — | | | — | | | — | | | — | | | — | | | 9,482 | |
Unearned compensation expense related to restricted stock issuance | | — | | — | | 30 | | | — | | | 461 | | | (374 | ) | | — | | | — | | | — | | | — | | | 87 | |
Stock-based compensation expense related to stock option acceleration | | — | | — | | — | | | — | | | 518 | | | — | | | — | | | — | | | — | | | — | | | 518 | |
Net income | | — | | — | | — | | | — | | | — | | | — | | | — | | | — | | | 9,710 | | | — | | | 9,710 | |
Unrealized loss on marketable securities, net of tax of $0 | | — | | — | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | (71 | ) | | (71 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance at December 31, 2005 | | — | | $— | | 42,302 | | | $423 | | | $655,980 | | | ($374 | ) | | (1,399 | ) | | ($11,250 | ) | | ($545,700 | ) | | ($660 | ) | | $98,419 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Issuance of 2,815 shares of common stock under option agreements | | — | | — | | 2,815 | | | 28 | | | 14,349 | | | — | | | — | | | — | | | — | | | — | | | 14,377 | |
Issuance of 10 shares of common stock under restricted stock agreements | | — | | — | | 10 | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | |
Retirement of 30 share certificates of restricted stock | | — | | — | | (30 | ) | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | |
Issuance of 3,500 shares for A4 purchase | | — | | — | | 3,500 | | | 35 | | | 68,740 | | | — | | | — | | | — | | | — | | | — | | | 68,775 | |
Unearned compensation expense related to restricted stock issuance | | — | | — | | — | | | — | | | (374 | ) | | 374 | | | — | | | — | | | — | | | — | | | — | |
Stock-based compensation expense related to stock options and restricted stock issuance | | — | | — | | — | | | — | | | 2,328 | | | — | | | — | | | — | | | — | | | — | | | 2,328 | |
Issuance of 15 shares of common stock under the Employee Stock Purchase Plan | | — | | — | | 15 | | | — | | | 315 | | | — | | | — | | | — | | | — | | | — | | | 315 | |
Issuance of 6,996 shares of common stock under the February 2006 Allscripts share offering | | — | | — | | 6,996 | | | 70 | | | 130,240 | | | — | | | 1,399 | | | 11,250 | | | — | | | — | | | 141,560 | |
Costs incurred related to the February 2006 Allscripts shares offering | | — | | — | | — | | | — | | | (885 | ) | | — | | | — | | | — | | | — | | | — | | | (885 | ) |
Repurchase of 1,250 shares of common stock from General Electric (IDX) | | — | | — | | (1,250 | ) | | (13 | ) | | (21,065 | ) | | — | | | — | | | — | | | — | | | — | | | (21,078 | ) |
Net income | | — | | — | | — | | | — | | | — | | | — | | | — | | | — | | | 11,895 | | | — | | | 11,895 | |
Unrealized loss on marketable securities, net of tax | | — | | — | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | 544 | | | 544 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance at December 31, 2006 | | — | | $— | | 54,358 | | | $543 | | | $849,628 | | | $— | | | — | | | — | | | ($533,805 | ) | | ($116 | ) | | $316,250 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
The accompanying notes are an integral part of these consolidated financial statements.
51
ALLSCRIPTS HEALTHCARE SOLUTIONS, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
(In thousands)
| | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2006 | | | 2005 | | | 2004 | |
Cash flows from operating activities: | | | | | | | | | |
Net income | | $11,895 | | | $9,710 | | | $3,108 | |
Adjustments to reconcile net income to net cash provided by operating activities: | | | | | | | | | |
Depreciation and amortization | | 16,455 | | | 6,528 | | | 4,972 | |
Stock-based compensation expense | | 2,328 | | | 604 | | | — | |
Write-off of capitalized software | | 290 | | | — | | | — | |
Realized (gain) loss on investments | | 145 | | | 51 | | | (3 | ) |
Provision for doubtful accounts | | 3,180 | | | 553 | | | 451 | |
Deferred taxes | | 6,465 | | | — | | | — | |
Changes in operating assets and liabilities: | | | | | | | | | |
Accounts receivable | | (16,510 | ) | | (8,277 | ) | | (4,023 | ) |
Inventories | | 1,291 | | | 198 | | | 877 | |
Prepaid expenses and other assets | | (3,385 | ) | | (1,702 | ) | | 829 | |
Accounts payable | | (1,798 | ) | | 2,649 | | | (101 | ) |
Accrued expenses | | (809 | ) | | 3,686 | | | 2,552 | |
Accrued compensation | | 6,383 | | | (348 | ) | | 406 | |
Accrued restructuring and other charges | | — | | | — | | | (104 | ) |
Deferred revenue | | 1,403 | | | 2,699 | | | 3,648 | |
Other liabilities | | 80 | | | 156 | | | (77 | ) |
| | | | | | | | | |
Net cash provided by operating activities | | 27,413 | | | 16,507 | | | 12,535 | |
Cash flows from investing activities: | | | | | | | | | |
Capital expenditures | | (5,674 | ) | | (1,958 | ) | | (1,623 | ) |
Capitalized software and website development costs | | (9,106 | ) | | (3,186 | ) | | (3,962 | ) |
Purchase of marketable securities | | (29,053 | ) | | (25,907 | ) | | (112,262 | ) |
Maturities of marketable securities | | 74,026 | | | 51,872 | | | 38,299 | |
Investment in promissory note receivable and minority interest | | (500 | ) | | (1,050 | ) | | (1,550 | ) |
Payments for A4 Health Systems, Inc. and related transaction costs (net of $21,742 cash acquired) | | (209,824 | ) | | — | | | — | |
Payments for other acquisitions | | — | | | (1,763 | ) | | (139 | ) |
| | | | | | | | | |
Net cash provided by (used in) investing activities | | (180,131 | ) | | 18,008 | | | (81,237 | ) |
Cash flows from financing activities: | | | | | | | | | |
Payments of capital lease obligations | | (15 | ) | | (64 | ) | | (72 | ) |
Proceeds from exercise of common stock options | | 14,377 | | | 9,482 | | | 4,136 | |
Net proceeds received in issuance of common stock | | 140,675 | | | — | | | — | |
Repurchase of common stock from a related party | | (21,078 | ) | | — | | | — | |
Proceeds from employee stock purchase plan, net | | 315 | | | — | | | — | |
Debt issuance costs | | — | | | — | | | (2,976 | ) |
Issuance of convertible debt | | — | | | — | | | 82,500 | |
Purchase of treasury stock | | — | | | — | | | (11,250 | ) |
| | | | | | | | | |
Net cash provided by financing activities | | 134,274 | | | 9,418 | | | 72,338 | |
| | | | | | | | | |
Net increase (decrease) in cash and cash equivalents | | (18,444 | ) | | 43,933 | | | 3,636 | |
Cash and cash equivalents, beginning of year | | 60,905 | | | 16,972 | | | 13,336 | |
| | | | | | | | | |
Cash and cash equivalents, end of year | | $42,461 | | | $60,905 | | | $16,972 | |
| | | | | | | | | |
The accompanying notes are an integral part of these consolidated financial statements.
52
ALLSCRIPTS HEALTHCARE SOLUTIONS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Dollar and share amounts in thousands, except per-share amounts)
1. Nature of Business
Allscripts Healthcare Solutions, Inc. is a leading provider of clinical software, connectivity and information solutions that physicians use to improve the quality of healthcare. Our businesses provide innovative solutions that inform physicians with just right, just in time information, connect physicians to each other and to the entire community of care, and transform healthcare, improving both the quality and efficiency of care. We provide clinical software applications, including Electronic Health Record (EHR), practice management, electronic prescribing, Emergency Department Information System (EDIS), hospital care management and document imaging solutions through our clinical solutions businesses. Additionally, we provide clinical education and information solutions for physicians and patients through our physicians interactive business unit, along with physician-patient connectivity solutions through our partnership with Medem. We also provide prepackaged medication fulfillment services through our medication services business unit.
We report our financial results utilizing three business segments: software and related services segment; information services segment; and prepackaged medications segment. The software and related services segment consists of clinical software solutions offered by our clinical solutions business unit, such as Canopy, HealthMatics, TouchWorks, and TouchScript offerings. TouchWorks Electronic Health Record is an award-winning EHR solution designed to enhance physician productivity using Tablet PCs, wireless handheld devices or desktop workstations for the purpose of automating the most common physician activities, including prescribing, dictating, ordering lab tests and viewing results, documenting clinical encounters and capturing charges, among others. TouchWorks Practice Management combines scheduling and financial management tools in a single package with functionality including rules-based appointment scheduling, multi-resource and recurring appointment features, referral and eligibility indicators, and appointment and claims management. TouchWorks EHR and TouchWorks PM, which are offered individually and as a combined solution, both have the functionality to handle the complexities of large physician practice groups with 25 or more physicians.
For physician practice groups with fewer than 25 physicians that are seeking an EHR, a practice management system, or a combined EHR and practice management solution, we offer our HealthMatics EHR, Ntierprise Practice Management and HealthMatics Office, which combines the two offerings into one complete solution for clinical and back-office automation.
TouchScript is an e-prescribing solution that physicians can access securely via the Internet to quickly, safely and securely prescribe medications, check for drug interactions, access medication histories, review drug reference information, and send prescriptions directly to a pharmacy or mail order facility. TouchScript can be a starting point for medical groups to seamlessly transition over time to a complete EHR. Another e-prescribing offering, eRxNOW, is an easy-to-use, web-based solution that is safe, secure, requires no downloading and no new hardware. eRxNOW is accessible by Internet on computers, handheld devices and cell phones and is offered free of charge to every prescriber in America via the National ePrescribing Patient Safety Initiative, a coalition of companies led by Allscripts.
Our offerings for hospitals that are seeking EDIS and care management solutions include HealthMatics ED, EmSTAT and Canopy. HealthMatics ED electronically streamlines processes for large hospital Emergency Departments, including tracking, triage, nurse and physician charting, disposition and reporting. EmSTAT offers similar functionality for streamlining the Emergency Department care process in small hospitals. Canopy is a Web-based solution that streamlines and speeds the patient care management process by automating utilization, case, discharge and quality management processes relating to patient hospital visits.
In our information services segment, our key product offerings are Physicians Interactive and Physician Relationship Management Platform (“PRMP”) solutions. Physicians Interactive is a web-based solution that
53
connects physicians with pharmaceutical companies, medical device manufacturers and biotech companies. One element of this solution, often referred to as e-Detailing, uses interactive sessions to provide clinical education and information to physicians about medical products and disease states, which promotes more informed decision-making, increased efficiency and ultimately higher quality patient care. Other elements of the Physicians Interactive platform include e-surveys, clinical updates, resource centers, key opinion leader materials, and other physician relationship management services. Through our partnership with Medem, our TouchWorks solution also provides physicians and patients with a tool for secure online consultations, automated disease management services and personal health records. PRMP solutions provide pharmaceutical companies with a turnkey system to build an electronic dialogue and manage ongoing relationships with physicians. The PRMP solution incorporates a full suite of online tools, including campaign management, physician communication and education and sample and rep requests, as well as e-Detailing opportunities.
Finally, our prepackaged medications segment is comprised of our medication services business unit. This business unit provides point-of-care medication management and medical supply services and solutions for physicians and other healthcare providers.
2. Summary of Significant Accounting Policies
Principles of Consolidation
The consolidated financial statements include the accounts of Allscripts and its wholly owned subsidiaries. All significant intercompany balances and transactions have been eliminated.
Stock-Based Compensation
Effective January 1, 2006, Allscripts adopted the provisions of SFAS No. 123 (Revised), “Share-Based Payment” (“SFAS 123(R)”), which requires the measurement and recognition of compensation expense for all stock-based payment awards made to employees and directors, including employee stock options, based on estimated fair values. Allscripts previously applied Accounting Principles Board Opinion No. 25, “Accounting for Stock Issued to Employees,” (“APB 25”) and related interpretations and provided the pro forma disclosures required by SFAS No. 123, “Accounting for Stock-Based Compensation” (“SFAS 123”), as amended by SFAS No. 148, “Accounting for Stock-Based Compensation – Transition and Disclosures” (“SFAS 148”), both of which were superseded by SFAS 123(R) (See Note 10).
Revenue Recognition
Revenue from software licensing arrangements, where the service element is considered essential to the functionality of the other elements of the arrangement, is accounted for under American Institute of Certified Public Accountants Statement of Position (“SOP”) 81-1, “Accounting for Performance of Construction-Type Contracts and Certain Production-Type Contracts.” Allscripts recognizes revenue on an input basis using actual hours worked as a percentage of total expected hours required by the arrangement, provided that the fee is fixed and determinable and collection of the receivable is probable. Maintenance and support from these agreements is recognized over the term of the support agreement based on vendor-specific objective evidence of fair value of the maintenance revenue, which is generally based upon contractual renewal rates. For agreements that are deemed to have extended payment terms, revenue is recognized using the input method but is limited to the amounts due and payable.
Revenue from software licensing arrangements where the service element is not considered essential to the functionality of the other elements of the arrangement is accounted for under SOP 97-2, “Software Revenue Recognition,” as amended by SOP 98-9, “Modification of SOP 97-2, Software Revenue Recognition, With Respect to Certain Transactions.” Such revenue is recognized upon shipment of the software or as services are performed, provided persuasive evidence of an arrangement exists, fees are considered fixed and determinable, and collection of the receivable is considered probable. The revenue recognized for each separate element of a multiple-element software contract is based upon vendor-specific objective evidence of fair value, which is based upon the price the customer is required to pay when the element is sold separately.
54
Revenue from the prepackaged medications segment, from the sale of medications, net of provisions for estimated returns, is recognized upon shipment of the pharmaceutical products, the point at which the customer takes ownership and assumes risk of loss, when no performance obligations remain and collection of the receivable is probable. Allscripts offers the right of return on pharmaceutical products under various policies and estimates and maintains reserves for product returns based on historical experience following the provisions of FAS No. 48, “Revenue Recognition When Right of Return Exists.”
Certain of our customer arrangements in our information services segment encompass multiple deliverables. We account for these arrangements in accordance with Emerging Issues Task Force (“EITF”) No. 00-21, “Accounting for Revenue Arrangements with Multiple Deliverables” (“EITF 00-21”). If the deliverables meet the criteria in EITF 00-21, the deliverables are separated into separate units of accounting, and revenue is allocated to the deliverables based on their relative fair values. The criteria specified in EITF 00-21 are that the delivered item has value to the customer on a stand-alone basis, there is objective and reliable evidence of the fair value of the undelivered item, and if the arrangement includes a general right of return relative to the delivered item, delivery or performance of the undelivered item is considered probable and substantially in the control of the vendor. Applicable revenue recognition criteria is considered separately for each separate unit of accounting.
Management applies judgment to ensure appropriate application of EITF 00-21, including value allocation among multiple deliverables, determination of whether undelivered elements are essential to the functionality of delivered elements and timing of revenue recognition, among others. For those arrangements where the deliverables do not qualify as a separate unit of accounting, revenue from all deliverables is treated as one accounting unit and recognized on a straight-line basis over the term of the arrangement. Changes in circumstances and customer data may affect management’s analysis of EITF 00-21 criteria, which may cause Allscripts to adjust upward or downward the amount of revenue recognized under the arrangement.
In accordance with EITF issued Consensus 01-14, “Income Statement Characterization of Reimbursements for ‘Out-of-Pocket’ Expenses Incurred,” revenue includes reimbursable expenses charged to our clients.
55
As of December 31, 2006 and 2005, there were $8,942 and $6,668, respectively, of revenue earned on contracts in excess of billings, which are included in the balance of accounts receivable. Billings on contracts where revenue has been earned in excess of billings are expected to occur according to the contract terms. Deferred revenue consisted of the following:
| | | | |
| | | December 31, |
| | | 2006 | | 2005 |
Prepayments and billings in excess of revenue earned on contracts in progress for software and services provided by Allscripts and included in the software and related services segment | | $16,264 | | $11,644 |
Prepayments and billings in excess of revenue earned on contracts in progress for support and maintenance provided by Allscripts and included in the software and related services segment | | 14,676 | | 1,216 |
Prepayments and billings in excess of revenue earned for interactive physician education sessions and related services provided by the Allscripts’ physicians interactive business unit and included in the information services segment | | 4,609 | | 4,446 |
| | | | |
Total deferred revenue | | $35,549 | | $17,306 |
| | | | |
Cash, Cash Equivalents and Marketable Securities
Cash and cash equivalent balances at December 31, 2006 and 2005 consist of cash and highly liquid corporate debt securities with original maturities at the time of purchase of less than 90 days. Allscripts’ cash, cash equivalents, short-term marketable securities and long-term marketable securities are invested in overnight repurchase agreements, money market funds, U.S. and non-U.S. government debt securities, and corporate debt securities. The carrying values of cash and cash equivalents, short-term marketable securities and long-term marketable securities held by Allscripts are as follows:
| | | | |
| | | December 31, |
| | | 2006 | | 2005 |
Cash and cash equivalents: | | | | |
Cash | | $34,314 | | $24,274 |
Money market funds | | 8,147 | | 1,367 |
Corporate debt securities | | — | | 35,264 |
| | | | |
| | 42,461 | | 60,905 |
Short-term marketable securities: | | | | |
U.S. government and agency debt obligations | | — | | 13,151 |
Corporate debt securities | | 14,553 | | 41,257 |
| | | | |
| | 14,553 | | 54,408 |
Long-term marketable securities: | | | | |
U.S. government and agency debt obligations | | 5,027 | | 7,810 |
Corporate debt securities | | 20,997 | | 22,940 |
| | | | |
| | 26,024 | | 30,750 |
| | | | |
Total cash, cash equivalents and marketable securities | | $83,038 | | $146,063 |
| | | | |
The long-term U.S. government and corporate debt securities have contractual maturities ranging from 2 years to 28 years. Management determines the appropriate classification of debt and equity securities at the time of purchase and reevaluates the designation at each balance sheet date. As of December 31, 2006 and 2005, marketable securities were classified as available-for-sale and carried at their fair value, with the unrealized gains
56
and losses reported net-of-tax in a separate component of stockholders’ equity. The components of the net unrealized gain (loss) on marketable securities are as follows:
| | | | | | |
| | | As of December 31, | |
| | | 2006 | | | 2005 | |
Short-term marketable securities: | | | | | | |
Gross unrealized gains | | $3 | | | $14 | |
Gross unrealized losses | | — | | | (258 | ) |
| | | | | | |
Net short-term unrealized gains | | 3 | | | (244 | ) |
Long-term marketable securities: | | | | | | |
Gross unrealized gains | | 3 | | | — | |
Gross unrealized losses | | (122 | ) | | (416 | ) |
| | | | | | |
Net long-term unrealized losses | | (119 | ) | | (416 | ) |
| | | | | | |
Total net unrealized losses on marketable securities | | ($116 | ) | | ($660 | ) |
| | | | | | |
For the years ended December 31, 2006, 2005, and 2004, net realized gains (losses) were ($145), ($51), and $3, respectively.
Realized gains and losses and declines in value determined to be other-than-temporary on available-for-sale securities are included in other expense, net. The cost of securities sold is based on specific identification. Interest and dividends on securities classified as available-for-sale are included in interest income. There were no other-than-temporary declines for the years ended December 31, 2006, 2005, and 2004.
Allowance for Doubtful Accounts Receivable
Accounts receivable are recorded at the invoiced amounts and do not bear interest. The allowance for doubtful accounts is recorded to provide for estimated losses resulting from uncollectible accounts, and is based principally upon specifically identified amounts where collection is deemed doubtful. Additional non-specific allowances are recorded based on historical experience and management’s assessment of a variety of factors related to the general financial condition of Allscripts’ customer base. Allscripts reviews the collectibility of individual accounts and assesses the adequacy of the allowance for doubtful accounts. Account balances are charged off against the allowance after all means of collection have been exhausted and the potential for recovery is considered remote. Allscripts does not have any off-balance-sheet credit exposure related to its customers.
Inventories
Inventories, which consist primarily of medications, are carried at the lower of cost or market with cost being determined using the specific identification method.
Fixed Assets
Fixed assets are stated at cost. Depreciation and amortization are computed on the straight-line method over the estimated useful lives of the related assets. The depreciable life of leasehold improvements is the shorter of the lease term or the useful life. Upon asset retirement or other disposition, cost and the related accumulated depreciation are removed from the accounts, and any gain or loss is included in the consolidated statements of operations. Amounts expended for repairs and maintenance are charged to operations as incurred.
Goodwill and Intangible Assets
Goodwill represents the excess of the costs over the fair value of assets of businesses acquired. Goodwill and intangible assets acquired in a business combination and determined to have an indefinite useful life are not
57
amortized in accordance with FAS No. 142, “Goodwill and Other Intangible Assets” (“FAS 142”), but instead tested for impairment at least annually. FAS 142 also requires that intangible assets with estimable useful lives be amortized over their respective estimated useful lives and reviewed for impairment in accordance with FAS 144, “Accounting for Impairment or Disposal of Long-Lived Assets.”
Allscripts has selected January 1 as the date of its annual impairment test of goodwill. No indicators of impairment were identified as a result of its annual impairment test performed on January 1, 2007.
Intangible assets with estimable useful lives are stated at cost and are amortized using the straight-line method over the remaining estimated economic lives of those assets, including the period being reported on.
Long-Lived Assets and Long-Lived Assets to Be Disposed Of
In accordance with FAS No. 144, “Accounting for the Impairment or Disposal of Long-Lived Assets” (“FAS 144”), Allscripts reviews its long-lived assets and certain identifiable intangible assets for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. Recoverability of assets to be held and used is measured by a comparison of the carrying amount of an asset to future net cash flows expected to be generated by the asset. If assets are considered to be impaired, the impairment to be recognized is measured by the amount by which the carrying amount of the assets exceeds the fair value of the assets.
Investment in Promissory Note and Minority Interest
Allscripts holds an investment in Medem totaling $3,100 and $2,600 as of December 31, 2006 and 2005, respectively. The investment has been accounted for under the cost basis of accounting and is recorded in other assets on the consolidated balance sheets. The investment at December 31, 2006 and 2005 consists of a $2,600 and $2,100 note receivable, respectively, and a $500 minority interest in Medem. The fair value of the investment is dependent upon the actual financial performance of Medem, its market value, and the volatility inherent in the external markets for this type of investment. In assessing potential impairment of the investment, we consider these factors, as well as the forecasted financial performance of Medem, liquidation preference value of the stock that we hold, and estimated potential for investment recovery. If any of these factors indicate that the investment has become other-than-temporarily impaired, an impairment charge would be recorded. At December 31, 2006 and 2005, the investment has not been impaired.
Software Development Costs
Allscripts capitalizes purchased software that is ready for service and software development costs incurred from the time technological feasibility of the software is established until the software is available for general release in accordance with FAS No. 86, “Accounting for the Costs of Computer Software to Be Sold, Leased or Otherwise Marketed.” Research and development costs and other computer software maintenance costs related to software development are expensed as incurred. Upon the establishment of technological feasibility, related software development costs are capitalized. During 2006, 2005, and 2004, software development costs in the amount of $9,106, $3,186, and $3,962, respectively, were capitalized. The unamortized balance of capitalized software at the end of 2006 and 2005 was $12,285 and $6,409, respectively. Upon the availability for general release, Allscripts commences amortization of the software on a product by product basis. Amortization is recorded based upon the greater of the ratio that current gross revenues for a product are to the total of current and anticipated future gross revenues for that product or the straight-line method over the remaining estimated economic life of the product, including the period being reported on, which is estimated to be three years. Amortization of capitalized software development costs amounted to $3,060, $3,047, and $1,719 for 2006, 2005, and 2004, respectively. Software development costs of $10,760, $3,765, and $4,316 have been expensed in 2006, 2005, and 2004, respectively.
58
At each balance sheet date, the unamortized capitalized costs of a software product are compared to the net realizable value of that product. The amount by which the unamortized capitalized costs of a software product exceed the net realizable value of that asset is written off. The net realizable value is the estimated future gross revenues from that product reduced by the estimated future costs of completing and disposing of that product, including the costs of performing maintenance and customer support required to satisfy Allscripts’ responsibility set forth at the time of sale.
Advertising Costs
Advertising costs are expensed as incurred.
Income Taxes
Deferred tax assets or liabilities are established for temporary differences between financial and tax reporting bases and for tax carryforward items and are subsequently adjusted to reflect changes in tax rates expected to be in effect when the temporary differences reverse.
Manufacturer Rebates
Rebates from suppliers are recorded as a reduction of cost of revenue and are generally recognized on an estimated basis upon shipment of the product to customers. The difference between the amount estimated and the amount actually received is reflected prospectively as a change of estimate. These revisions have not been material.
Comprehensive Income
Comprehensive income includes all changes in stockholders’ equity during a period except those resulting from investments by owners and distributions to owners. The components of accumulated other comprehensive income (loss), net of income tax, consist of unrealized losses on Allscripts marketable securities of ($116) and ($660), at December 31, 2006 and 2005, respectively.
The components of comprehensive income are as follows:
| | | | | | | | |
| | | 2006 | | 2005 | | | 2004 | |
Net income | | $11,895 | | $9,710 | | | $3,108 | |
Other comprehensive income: | | | | | | | | |
Unrealized gain (loss) on marketable securities, net of tax | | 544 | | (71 | ) | | (691 | ) |
| | | | | | | | |
Comprehensive income | | $12,439 | | $9,639 | | | $2,417 | |
| | | | | | | | |
Net Income Per Share
Allscripts accounts for net income per share in accordance with FAS No. 128, “Earnings per Share” (“FAS 128”). FAS 128 requires the presentation of “basic” income per share and “diluted” income per share. Basic income per share is computed by dividing the net income by the weighted-average shares of outstanding common stock. For purposes of calculating diluted earnings per share, the denominator includes both the weighted average shares of common stock outstanding and dilutive potential common stock equivalents. Dilutive common stock equivalent shares consist primarily of stock options.
59
The components of diluted weighted average common shares outstanding is as follows:
| | | | | | |
| | | December 31, |
| | | 2006 | | 2005 | | 2004 |
Weighted average shares outstanding: | | | | | | |
Basic | | 51,058 | | 40,045 | | 38,979 |
Effect of dilutive securities | | 2,309 | | 3,023 | | 2,613 |
| | | | | | |
Diluted | | 53,367 | | 43,068 | | 41,592 |
| | | | | | |
On September 30, 2004, the EITF reached a consensus on EITF Issue No. 04-8, “The Effect of Contingently Convertible Debt on Diluted Earnings Per Share” (“EITF 04-8”). Effective December 15, 2004, contingently convertible debt instruments are subject to the if-converted method under FAS 128, “Earnings Per Share,” regardless of the contingent features included in the instrument assuming the shares are not anti-dilutive. Under the provisions of EITF 04-8, the as-if convertible 7,300 shares and interest expense related to Allscripts’ Notes were excluded from years ended December 31, 2006, 2005 and 2004, as the effects were anti-dilutive.
Fair Value of Financial Instruments
Cash, cash equivalents and marketable securities are reported at their fair values in the balance sheets with the corresponding mark-to-market adjustments recorded as other comprehensive income (loss) in stockholders’ equity. The carrying amounts reported in the balance sheets for accounts receivable, investment in Medem, accounts payable, and accrued liabilities approximate their fair values due to the short-term nature of these financial instruments. Allscripts’ notes receivable from Medem, senior convertible debentures, and secured promissory note have interest rates that approximate current market values; therefore, the carrying value of both approximate fair value. Letters of credit fair value amounts are based on fees currently charged on similar agreements.
Risks and Uncertainties
Financial instruments that potentially subject Allscripts to a concentration of credit risk consist of cash, cash equivalents, marketable securities and trade receivables. Allscripts maintains its cash balances with two major commercial banks and its cash equivalents and marketable securities in interest-bearing, investment-grade securities.
Allscripts sells its products and services to healthcare providers. Credit risk with respect to trade receivables is generally diversified due to the large number of customers and their dispersion across the United States. To reduce credit risk, Allscripts performs ongoing credit evaluations of its customers and their payment histories. In general, Allscripts does not require collateral from its customers, but it does enter into advance deposit, security or guarantee agreements, if appropriate. The provision for doubtful accounts aggregated $3,180, $553, and $451 in 2006, 2005, and 2004, respectively.
The majority of revenue is derived from customers located in the United States. All long-lived assets are located in the United States. There were no customers that accounted for greater than 10% of revenue or accounts receivable in 2006, 2005, and 2004.
Allscripts purchases a majority of its drug inventories under a contractual agreement with one wholesaler/distributor, which accounted for approximately 92% of all inventory purchases during the years ended December 2006 and 2005. At December 31, 2006 and 2005, approximately 22% and 66%, respectively, of accounts payable are related to these purchases. Allscripts is exposed to risk of loss of revenue and customers in the event of a breach of contract or nonperformance by this wholesaler/distributor resulting in restriction of or diminished availability of inventory. In addition, if Allscripts does not meet certain minimum purchasing requirements with its primary wholesaler/distributor, it may increase the prices that Allscripts pays under the agreement, in which
60
case Allscripts would have the option to terminate the agreement. However, Allscripts does not anticipate that a breach of contract or any nonperformance will occur. In the event it does, Allscripts believes that there are several other available wholesalers/distributors, which would be able to provide the necessary inventories to Allscripts on a timely basis such that no material loss would occur. As of December 31, 2006, Allscripts believes that it has met all minimum purchase requirements as defined in the agreement with its primary wholesaler/distributor.
Allscripts provides its software customers with a standard product warranty beginning with live use of the software. If a software product is found to have a material defect that causes the product not to operate in accordance with the software specifications, Allscripts will deliver any necessary alterations to the customer.
Use of Estimates
Accounting principles generally accepted in the United States of America require management to make estimates and assumptions that affect the reported amounts of assets and liabilities, the disclosure of contingent assets and liabilities at year end and the reported amounts of revenue and expenses during the year. Actual results could differ from these estimates.
Reclassifications
Certain amounts reported in prior years have been reclassified from what was previously reported to conform to the current year’s presentation.
Recent Accounting Pronouncements
On January 1, 2006, Allscripts adopted Statement of Financial Accounting Standards No. 123 (Revised), “Share-Based Payment” (“SFAS 123(R)”). SFAS 123(R) requires companies to recognize stock-based compensation expense related to all stock awards issued to employees, including options, in the statement of operations based on their fair values on the date of the grant and after applying an estimated forfeiture rate. The stock-based compensation expense is to be recognized over the period in which an employee is required to provide service in exchange for the stock award. Additionally, for any unvested awards outstanding at the adoption date, SFAS 123(R) requires recognition of compensation expense, after applying a forfeiture rate, over the remaining vesting period of the stock-based award.
Allscripts adopted the modified prospective application transition method as provided by SFAS 123(R). Accordingly, during the year ended December 31, 2006, Allscripts recorded stock-based compensation cost totaling the amount that would have been recognized had the fair value method been applied since the effective date of SFAS 123. Previously reported amounts have not been restated. For the year ended December 31, 2006, the effect on Allscripts’ results of operations of recording stock-based compensation in accordance with SFAS 123(R) was $1,829, or $0.03 per diluted share.
Prior to the adoption of SFAS 123(R), our stock-based compensation awards were accounted for under the recognition and measurement provisions of Accounting Principles Board No. 25, “Accounting for Stock Issued to Employees, and Related Interpretations” (“APB 25”). Under the intrinsic value method described in APB 25, no compensation expense was recorded because the exercise price of the employee stock options equaled the market price of the underlying stock on the date of grant. The fair value of stock options granted prior to the adoption of SFAS 123(R) was estimated at the date of grant using the Black-Scholes option-pricing model. No stock options were granted to employees in the twelve months ended December 31, 2006. Allscripts does not expect to grant options to employees in the future, and instead, expects to use awards of restricted stock and restricted stock units as stock-based incentives to employees.
61
In June 2006, the FASB issued Interpretation No. 48 (FIN 48), Accounting for Uncertainty in Income Taxes. This interpretation clarifies the accounting for uncertainty in income taxes recognized in the financial statements in accordance with Statement of Financial Accounting Standards (SFAS) No. 109, “Accounting for Income Taxes” by prescribing the minimum recognition threshold a tax position is required to meet before being recognized in the financial statements. FIN 48 prescribes a comprehensive model for how a company should recognize, measure, present, and disclose in its financial statements uncertain tax positions that the company has taken or expects to take on a tax return. FIN 48 is effective for fiscal years beginning after December 15, 2006, with the cumulative effect of the change in accounting principle recorded as an adjustment to opening retained earnings. We have not yet completed our evaluation of the impact of adoption on our consolidated financial statements.
In September 2006, the Securities and Exchange Commission (“SEC”) issued Staff Accounting Bulletin No. 108, “Considering the Effects of Prior Year Misstatements when Quantifying Misstatements in Current Year Financial Statements” (“SAB 108”). SAB 108 provides interpretive guidance on how the effects of the carryover or reversal of prior year misstatements should be considered in quantifying a current year misstatement. The SEC staff believes that registrants should quantify errors using both the balance sheet and income statement approach when quantifying a misstatement. We adopted SAB 108 for the year ended December 31, 2006 as required, and the adoption did not have a significant effect on our consolidated financial statements.
In September 2006, the Financial Accounting Standards Board (FASB) issued SFAS 157,Fair Value Measurements. SFAS 157 defines fair value, establishes a framework for measuring fair value under generally accepted accounting principles, and expands disclosures about fair value measurements. SFAS 157 clarifies that the fair value is the exchange price in an orderly transaction between market participants to sell the asset or transfer the liability in the market. The standard emphasizes that fair value is a market-based measurement, not an entity-specific measurement and a fair value measurement should therefore be based on the assumptions that market participants would use in pricing the asset or liability. SFAS 157 is effective for financial statements issued for fiscal years beginning after November 15, 2007. The Company is currently evaluating the accounting and disclosure requirements of SFAS 157 and plans to adopt it as required at the beginning of its fiscal year 2008.
3. Business Combinations
On March 2, 2006, Allscripts completed its acquisition of A4 Health Systems, Inc. (“A4”), whereby Allscripts acquired all of the outstanding equity interests of A4 for aggregate consideration of $215,000 in cash and 3,500 shares of Allscripts common stock. An additional payment of approximately $12,730, was made by Allscripts to A4 shareholders in respect of A4’s level of working capital at closing. The A4 acquisition enables Allscripts to reach new markets such as small and mid-sized physician practice groups that seek either an electronic health record (“EHR”) or a combined EHR and practice management system, and hospitals that seek emergency department information systems and care management solutions.
The A4 acquisition has been accounted for as a business combination under Statement of Financial Accounting Standards (“SFAS”) No. 141, “Business Combinations.” The assets acquired and liabilities assumed have been recorded at the date of acquisition at their respective fair values.
62
The results of operations of A4 have been included in the accompanying consolidated statements of operations from the date of the A4 acquisition. The total purchase price for the acquisition is as follows:
| | |
Cash consideration to A4 shareholders (cash payment of $215,000 and additional working capital payment of $12,730) | | $227,730 |
Fair value of Allscripts shares issued to A4 shareholders (3,500 Allscripts common shares at $19.65 per share, the last sale price of Allscripts common stock on March 2, 2006) | | 68,775 |
Acquisition-related transaction costs | | 4,685 |
| | |
Total purchase price | | $301,190 |
| | |
The above purchase price has been allocated to the tangible and intangible assets acquired and liabilities assumed based on management’s estimates of their current fair values. The Company obtained a third party valuation of certain intangible assets. Acquisition-related transaction costs include investment banking fees, loan commitment fees, legal and accounting fees and other external costs directly related to the A4 acquisition.
The purchase price has been allocated as follows:
| | | |
Current assets, including $21,742 of cash acquired in the acquisition | | $37,518 | |
Property and equipment | | 8,791 | |
Intangible assets | | 79,110 | |
Non-current other assets | | 25 | |
Goodwill (before deferred tax adjustment - See Note 8) | | 228,102 | |
Current liabilities, excluding current portion of long term debt | | (26,204 | ) |
Current and long-term debt | | (3,400 | ) |
Deferred tax liabilities, net | | (22,752 | ) |
| | | |
Net assets acquired | | $301,190 | |
| | | |
In connection with the acquisition of A4, management determined under the provisions of SFAS No. 109, “Accounting for Income Taxes” (“SFAS 109”), that it is more likely than not that Allscripts will generate adequate taxable income for the foreseeable future to realize its deferred tax assets. Accordingly, management reversed $61,284 of its valuation allowance against goodwill in purchase accounting for the A4 acquisition.
Goodwill was determined based on the residual difference between the purchase cost and the value assigned to tangible and intangible assets and liabilities, and is not deductible for tax purposes. Of the $79,110 intangible assets acquired, $40,000 was assigned to developed technology rights with a weighted-average useful life of approximately 8 years, $20,800 was assigned to customer relationships with a useful life of 15 years, $15,210 was assigned to registered trade marks with a useful life of 10 years, $1,400 was assigned to A4’s backlog with a useful life of six months, $1,200 was assigned to non-competition agreements with a useful life of 2 years, and $500 was assigned to proprietary technology with a useful life of 5 years. Among the factors that contributed to a purchase price resulting in the recognition of goodwill were A4’s history of profitability and high operating margins, strong sales force and overall employee base, and leadership position in the healthcare information technology market.
63
The following unaudited pro forma information assumes the A4 acquisition occurred on January 1, 2005. These unaudited pro forma results have been prepared for informational purposes only and do not purport to represent what the results of operations would have been had the A4 acquisition occurred as of January 1, 2005, nor of future results of operations. The unaudited pro forma results for the two years ended December 31, 2006 and 2005 are as follows:
| | | | |
| | | 2006 | | 2005 |
Total revenue | | $245,632 | | $193,387 |
Net income | | $10,737 | | $4,931 |
Earnings per share: | | | | |
Basic | | $0.20 | | $0.10 |
Diluted | | $0.19 | | $0.09 |
The unaudited pro forma information for the years ended December 31, 2006 and 2005 include the following adjustments:
| | • | | Increase (decrease) to amortization expense of ($92) and $9,234 for the years ended December 31, 2006 and 2005, respectively, related to management’s estimate of the fair value of intangible assets acquired as a result of the A4 acquisition less the elimination of original amortization recorded by A4. |
| | • | | Decrease to interest income of $1,383 and $3,013 for the years ended December 31, 2006 and 2005, respectively, as a result of lower cash, cash equivalents and marketable securities balances at January 1, 2006 and 2005 as a result of assuming the acquisition of A4 occurred on January 1, 2005. |
| | • | | A transfer from Allscripts’ selling, general and marketing expense of $1,021 from the year ended December 31, 2006 to the year ended December 31, 2005 related to non-recurring A4 integration costs. |
| | • | | An increase (decrease) in revenue of $3,147 and ($2,520) for the years ended December 31, 2006 and 2005, respectively, relating to the timing of deferred revenue purchase accounting adjustments. |
| | • | | A decrease to the tax provision of $722 and $2,979 for the years ended December 31, 2006 and 2005, respectively, to reflect a 38% tax provision on a pro forma basis. |
| | • | | The weighted average number of shares outstanding used for the computation of basic and diluted earnings per share for the years ended December 31, 2006 and 2005 assumes that the issuance of 8,395 shares in connection with Allscripts’ common stock offering completed in February 2006, in order to partially fund the cash portion of the A4 purchase price, and the 3,500 shares issued to A4 shareholders as part of the consideration to acquire A4 occurred on January 1, 2005. |
On August 1, 2003, Allscripts acquired 100% of the outstanding common stock of Advanced Imaging Concepts Inc. (“AIC”), a provider of document imaging, scanning and management software for the medical industry. AIC’s results are included in Allscripts’ results of operations from the date of acquisition. The purchase price of $18,981 included the issuance of 474 common stock options in exchange for existing AIC common stock options, with a value of $1,242, and the granting of an additional 431 common stock options, with a value of $1,000. A purchase price holdback obligation totaling $1,800 was established to provide for certain contingencies and financial items, as defined. The holdback period was for eighteen months following the closing of the acquisition. All of the holdback obligation has been paid as of December 31, 2006, with the exception for $84, which will be paid upon the receipt of required acknowledgment from the AIC stockholders. The holdback obligation of $84 as of December 31, 2006 and 2005 is included in accrued expenses in the consolidated balance sheets.
64
4. Fixed Assets
Fixed assets as of December 31 consist of the following:
| | | | | | | | |
| | | Estimated
Useful Life | | 2006 | | | 2005 | |
Office furniture and equipment | | 2-7 years | | $25,493 | | | $14,973 | |
Service assets | | 2 years | | 2,766 | | | 9,073 | |
Production and warehouse equipment | | 5-7 years | | 1,410 | | | 1,374 | |
Leasehold improvements | | 4-7 years | | 9,552 | | | 2,877 | |
Website development costs | | 2 years | | 397 | | | 397 | |
Buildings | | 2 years | | 5,290 | | | — | |
Land | | — | | 1,377 | | | — | |
Construction in Progress | | — | | 667 | | | 69 | |
| | | | | | | | |
| | | | 46,952 | | | 28,763 | |
Less accumulated depreciation and amortization | | | | (32,858 | ) | | (26,010 | ) |
| | | | | | | | |
Fixed assets, net | | | | $14,094 | | | $2,753 | |
| | | | | | | | |
Depreciation and amortization expense was $3,129, $1,650, and $1,492 in 2006, 2005, and 2004, respectively.
5. Goodwill, Intangible Assets and Impairments
The following table summarizes goodwill and intangible assets by asset class. Goodwill at December 31, 2006, consists of $184,966, $594, and $2,701 related to the software and related services, prepackaged medications and information services segments, respectively. Goodwill at December 31, 2005, consists of $10,465, $594, and $2,701 related to the software and related services, prepackaged medications, and information services segments, respectively. In March 2006, Allscripts acquired A4 Health Systems, resulting in $174,501 of goodwill (after deferred tax adjustment—see Note 8) and $79,110 of intangible assets. The AIC and RxCentric acquisitions resulted in $13,028 of goodwill and $9,369 of intangible assets, collectively.
Goodwill and intangible assets as of December 31 consist of the following:
| | | | | | | | | | | | |
| | | 2006 | | 2005 |
| | | Gross Assets | | Accumulated Amortization | | Intangible Assets, Net | | Gross Assets | | Accumulated Amortization | | Intangible Assets, Net |
Amortized intangible assets | | | | | | | | | | | | |
Proprietary technology | | $60,310 | | $9,156 | | $51,154 | | $4,600 | | $2,184 | | $2,416 |
Customer relationships | | 24,160 | | 3,073 | | 21,087 | | 2,020 | | 519 | | 1,501 |
Strategic agreements | | 12,482 | | 9,373 | | 3,109 | | 4,745 | | 2,211 | | 2,534 |
| | | | | | | | | | | | |
| | 96,952 | | 21,602 | | 75,350 | | 11,365 | | 4,914 | | 6,451 |
| | | | | | | | | | | | |
Unamortized intangible assets | | | | | | | | | | | | |
Registered trademarks | | 2,700 | | — | | 2,700 | | 2,700 | | — | | 2,700 |
Goodwill | | 188,261 | | — | | 188,261 | | 13,760 | | — | | 13,760 |
| | | | | | | | | | | | |
| | 190,961 | | — | | 190,961 | | 16,460 | | — | | 16,460 |
| | | | | | | | | | | | |
Total goodwill and intangible assets | | $287,913 | | $21,602 | | $266,311 | | $27,825 | | $4,914 | | $22,911 |
| | | | | | | | | | | | |
The proprietary technology, customer base and strategic agreement intangible assets are being amortized over their average useful lives. Allscripts recorded amortization expense related to the intangible assets
65
amounting to $10,272 and $1,682 for the years ended December 31, 2006 and 2005, respectively. Estimated amortization expense for the intangible assets that exist as of December 31, 2006 is as follows:
| | |
| | | Year Ended
December 31 |
2007 | | $10,245 |
2008 | | 9,355 |
2009 | | 8,710 |
2010 | | 8,710 |
2011 | | 8,137 |
2012 and thereafter | | 30,193 |
| | |
Total | | $75,350 |
| | |
6. Investment in Promissory Note Receivable and Minority Interest
On August 18, 2004, Allscripts entered into a Convertible Secured Promissory Note Purchase Agreement (“Note Purchase Agreement”) with Medem and certain other investors. Under the Note Purchase Agreement, Allscripts acquired a convertible secured promissory note in the aggregate principal amount of $2,600 (“Promissory Note”) under which Medem may borrow up to $2,600 from Allscripts. The Promissory Note bears interest at an annual rate of 3% and is payable on a quarterly basis. The Promissory Note becomes due and payable upon the earlier to occur of (i) a sale of Medem, as defined in the Note Purchase Agreement, or the filing of a registration statement with the SEC for public offering of any class of securities of Medem (a “Liquidity Event”), and (ii) August 12, 2007. As of December 31, 2006 and 2005, Allscripts had funded $2,600 and $2,100, respectively, under the Note Purchase Agreement. The Promissory Note receivable balance is included in other assets in the consolidated balance sheets as of December 31, 2006 and 2005. In February 2006, Allscripts funded an additional $500 convertible secured promissory note under a contract signed in November 2005 (see Note 15).
At any time on or prior to maturity, Allscripts may convert all (but not a portion) of the Promissory Note into 2,600 shares of Medem’s Series A Common Stock. In connection with the transaction described above, Allscripts entered into a Share Purchase Agreement pursuant to which Allscripts purchased shares of Medem’s Series A Common Stock and shares of Medem’s Series B Common Stock for an aggregate purchase price equal to $500 in cash and is recorded in other assets on the consolidated balance sheets as of December 31, 2006 and 2005. In addition, pursuant to the terms of such agreement, Allscripts has a three-year option to acquire an additional interest in Medem for an aggregate price of $600.
As of December 31, 2006, we owned 3.1% of the voting capital of Medem and 1.9% of the capital stock of Medem. If we convert the entire promissory note and exercise our full option to purchase additional equity in Medem, we will own approximately 34.1% of the voting capital of Medem and 28.8% of the capital stock of Medem. The total investment in the Promissory Note and Share Purchase Agreement totaled 3,100 and $2,600 as of December 31, 2006 and 2005, respectively, and has been accounted for under the cost basis of accounting.
7. Long-Term Debt
In July 2004, Allscripts completed a private placement of $82,500 of 3.50% Senior Convertible Debentures due 2024 (“Notes”). The Notes can be converted, in certain circumstances, into approximately 7,300 shares of common stock based upon a conversion price of approximately $11.26 per share, subject to adjustment for certain events.
The Notes are only convertible under certain circumstances, including: (i) during any fiscal quarter if the closing price of Allscripts’ common stock for at least 20 trading days in the 30 trading-day period ending on the last trading day of the preceding fiscal quarter exceeds $14.64 per share; (ii) if Allscripts calls the Notes for redemption; or (iii) upon the occurrence of certain specified corporate transactions, as defined. Allscripts has the right to deliver common stock, cash or a combination of cash and shares of common stock. The Notes were
66
convertible during all quarters in 2006 by virtue of the last reported sale price for Allscripts’ common stock having exceeded $14.64 for twenty consecutive days in the 30 trading-day period ending on each fiscal quarter end date. No notes were converted as of December 31, 2006. The timing of our obligation on the Notes may change as it relates to funding interest payments and making a principal payment on the Notes based on whether the holders elect to convert the Notes. Upon conversion, Allscripts may redeem some or all of the Notes for cash any time on or after July 20, 2009 at the Notes’ full principal amount plus accrued and unpaid interest, if any. Holders of the Notes may require Allscripts to repurchase some or all of the Notes on July 15, 2009, 2014 and 2019 or, subject to certain exceptions, upon a change of control of Allscripts.
Allscripts received approximately $79,524 in net proceeds from the offering after deduction for issuance costs consisting of underwriting fees and professional expenses. The debt issuance costs of approximately $2,976 have been capitalized as an other asset and is being amortized as interest expense over five years using the effective interest method, through the first date that the holders have the option to require Allscripts to purchase the Notes. In connection with the acquisition of A4, Allscripts assumed a secured promissory note with an aggregate principal amount of $3,400 as of March 2, 2006, maturing on October 31, 2015. The promissory note bears interest at 7.85% per annum, and principal and interest are due monthly. In the event of prepayment in full or in part, Allscripts will be subject to a prepayment fee of 1% or more, as described in the related promissory note agreement, of the amount of principal prepaid on the promissory note. The promissory note is secured by the former corporate facilities of A4 and any lease or rental payments as defined in the related agreements.
Long-term debt outstanding as of December 31, 2006 and 2005 consists of the following:
| | | | |
| | | December 31, |
| | | 2006 | | 2005 |
3.5% Senior convertible debt | | $82,500 | | $82,500 |
| | |
7.85% Secured promissory note | | 3,199 | | — |
| | | | |
Total debt | | 85,699 | | 82,500 |
Less: Current portion of long-term debt | | 258 | | — |
| | | | |
Total long-term debt | | $85,441 | | $82,500 |
| | | | |
The table below presents long-term debt maturities as of December 31, 2006.
| | | | | | | | | |
Fiscal Year | | 3.5% Senior
convertible debt | | 7.85% Secured
promissory note | | Less: Current portion
of long-term debt | | | Total |
2007 | | $— | | $258 | | ($258 | ) | | $— |
2008 | | — | | 279 | | — | | | 279 |
2009 | | — | | 302 | | — | | | 302 |
2010 | | — | | 326 | | — | | | 326 |
2011 and thereafter | | 82,500 | | 2,034 | | — | | | 84,534 |
| | | | | | | | | |
Total | | $82,500 | | $3,199 | | ($258 | ) | | $85,441 |
| | | | | | | | | |
Interest expense for the years ended December 31, 2006 and 2005 both consist of $2,888 in interest expense related to the Notes, and $603 and $628 in debt issuance cost amortization, respectively. Interest expense of $221 for the year ended December 31, 2006 was recorded related to the secured promissory note.
67
8. Income Taxes
The provision for income taxes consists of the following:
| | | | | | |
| | | Year Ended
December 31,
2006 | | Year Ended
December 31,
2005 | | Year Ended
December 31,
2004 |
Current: | | | | | | |
Federal | | $— | | $— | | $— |
State | | 538 | | — | | — |
Deferred: | | | | | | |
Federal | | 5,407 | | — | | — |
State | | 1,479 | | — | | — |
| | | | | | |
| | $7,424 | | $— | | $— |
| | | | | | |
| | | | | | | | | |
| | | 2006 | | | 2005 | | | 2004 | |
U.S. federal statutory tax rate | | 35.0 | % | | 34.0 | % | | 34.0 | % |
Items affecting federal income tax rate: | | | | | | | | | |
State taxes, net of federal benefit | | 4.3 | | | 5.2 | | | 5.5 | |
Expired net operating loss | | — | | | 4.1 | | | 26.8 | |
Deferred tax rate increase | | (1.9 | ) | | — | | | — | |
Other, net | | 1.0 | | | 1.7 | | | 4.0 | |
Valuation allowance | | — | | | (45.0 | ) | | (70.3 | ) |
| | | | | | | | | |
Effective income tax rate | | 38.4 | % | | — | % | | — | % |
| | | | | | | | | |
The tax effects of temporary differences that give rise to significant portions of the deferred tax assets and liabilities for the years ended December 31, 2006 and 2005 are as follows:
| | | | | |
| | | 2006 | | 2005 | |
Deferred tax assets: | | | | | |
Net operating loss carryforwards | | $53,601 | | $65,179 | |
| | |
Allowance for doubtful accounts | | 1,689 | | 899 | |
Fixed assets | | 368 | | 425 | |
Inventory | | 266 | | 221 | |
Stock-based compensation | | 1,152 | | — | |
Accrued compensation | | 1,113 | | — | |
Unrealized losses on marketable securities | | 45 | | 257 | |
Deferred revenue | | 545 | | — | |
Other | | 31 | | 261 | |
| | | | | |
Total deferred tax assets | | 58,810 | | 67,242 | |
Less: valuation allowance | | — | | (61,284 | ) |
| | | | | |
Net deferred tax assets | | 58,810 | | 5,958 | |
Deferred tax liabilities: | | | | | |
Acquired intangibles | | 30,462 | | 3,462 | |
Software development costs | | 4,826 | | 2,496 | |
| | | | | |
Total deferred tax liabilities | | 35,288 | | 5,958 | |
| | | | | |
Net deferred tax assets | | $23,522 | | $— | |
| | | | | |
68
The net deferred tax asset (liability) is classified in the consolidated balance sheet as of December 31, 2006 and 2005 as follows:
| | | | | |
| | | 2006 | | | 2005 |
Current deferred tax assets | | $27,437 | | | $— |
Current deferred tax liabilities | | — | | | — |
| | | | | |
Current deferred tax asset, net | | $27,437 | | | $— |
| | | | | |
Non-current deferred tax assets | | $31,774 | | | $— |
Non-current deferred tax liabilities | | (35,689 | ) | | — |
| | | | | |
Non-current deferred tax liability, net | | ($3,915 | ) | | $— |
| | | | | |
Net deferred tax asset | | $23,522 | | | $— |
| | | | | |
In the consolidated balance sheets, these deferred tax assets and liabilities are classified as either current or non-current based on the classification of the related liability or asset for financial reporting.
In connection with the acquisition of A4, management determined under the provisions of SFAS No. 109, “Accounting for Income Taxes” (“SFAS 109”), that it is more likely than not that Allscripts will generate adequate taxable income for the foreseeable future to realize its deferred tax assets. Accordingly, management reversed its $61,284 valuation allowance against goodwill in purchase accounting for the A4 acquisition. As of December 31, 2006 and 2005 the valuation allowance was $0 and $61,284, respectively. As of December 31, 2006 we did not record a valuation allowance against the net deferred tax asset due to management’s assessment that it was more likely than not that all of the net deferred tax assets will be realized. A valuation allowance is required when it is more likely than not that all or a portion of a deferred tax asset will not be realized. In assessing the need for a valuation allowance, we consider all available positive and negative evidence, including past operating results, projections of future taxable income and the feasibility of ongoing tax planning strategies.
At December 31, 2006, Allscripts had operating loss carryforwards available for federal income tax reporting purposes of approximately $136,459. The operating loss carryforwards expire between the tax years ending 2007 through 2023. Allscripts’ ability to utilize these operating loss carryforwards to offset future taxable income is dependent on a variety of factors, including possible limitations pursuant to Internal Revenue Code Section (IRC) 382. IRC 382 imposes an annual limitation on the future utilization of operating loss carryforwards due to changes in ownership resulting from the issuance of common stock, stock options, warrants and convertible preferred stock.
As described in Note 2, FASB Interpretation (“FIN”) No. 48 “Accounting for Uncertainty in Income Taxes—an interpretation of FASB Statement 109” is effective beginning January 1, 2007. FIN 48 establishes a single model to address accounting for uncertainty in tax positions. FIN 48 clarifies the accounting for income taxes by prescribing a minimum recognition threshold a tax position is required to meet before being recognized in the financial statements. FIN 48 also provides guidance on derecognition, measurement, classification, interest and penalties, accounting in interim periods, disclosure and transition. FIN 48 is effective for fiscal years beginning after December 15, 2006. Allscripts will adopt FIN 48 as of January 1, 2007 as required. We have not yet completed our evaluation of the impact of adoption on our consolidated financial statements.
9. Common Stock
Public Offering of Common Stock
On February 28, 2006, Allscripts completed its offering of 8,395 shares of common stock and received approximately $140,675 in net proceeds, based on a public offering price of $17.75 per share after deducting underwriting discounts and commissions and professional expenses. Of the 8,395 shares issued, 1,399 shares were issued from treasury. All of the net proceeds received from the sale of common stock were used to fund the acquisition of A4.
69
Acquisition of A4 Health Systems, Inc.
On March 2, 2006, Allscripts acquired all of the outstanding equity interests of A4 for aggregate cash consideration of $232,415, which includes $4,685 of acquisition-related transaction costs and a working capital cash payment of $12,370, and stock consideration consisting of the issuance of 3,500 shares of Allscripts common stock.
Repurchase of Common Stock
On March 9, 2006, Allscripts repurchased 1,250 shares of its common stock directly from IDX Investment Corporation, a wholly owned subsidiary of General Electric Company. Allscripts paid $21,078, which is based on 95% of the February 22, 2006 public offering price for our common stock of $17.75.
10. Stock Award Plans
Employee Stock Purchase Plan
The Employee Stock Purchase Plan (“ESPP”) became effective on July 1, 2006 and allows eligible employees to authorize payroll deductions of up to 20% of their base salary to be applied toward the purchase of full shares of common stock on the last day of the offering period. Offering periods under the ESPP are three months in duration and begin on each January 1, April 1, July 1, and October 1. Shares will be purchased on the last day of each offering period at a price of 95% of fair market value of the common stock on such date as reported on Nasdaq. The aggregate number of shares of Allscripts common stock that may be issued under the ESPP may not exceed 250 shares and no one employee may purchase any shares under the ESPP having a collective fair market value greater than $25 in any one calendar year. The shares available for purchase under the ESPP may be drawn from either authorized but previously unissued shares of common stock or from reacquired shares of common stock, including shares purchased by Allscripts in the open market and held as treasury shares. Allscripts will treat the ESPP as a non-compensatory plan in accordance with SFAS No. 123(R). As of December 31, 2006, 15 shares were issued under the ESPP, which resulted in $315 in net proceeds.
Prior to the Adoption of SFAS 123(R)
Prior to the adoption of SFAS 123(R), employee stock-based compensation was not reflected in Allscripts’ net income because all stock options granted under Allscripts’ equity plans had an exercise price equal to the market value of the underlying common stock on the date of grant.
The pro forma disclosures required by SFAS 123 and SFAS 148 for the years ended December 31, 2005 and 2004 are as follows:
| | | | | | |
| | | 2005 | | | 2004 | |
Net income, as reported | | $9,710 | | | $3,108 | |
Stock-based compensation cost related to the issuance of stock awards included in net income (loss), as reported | | 87 | | | — | |
Stock-based compensation cost | | (13,083 | ) | | (16,468 | ) |
| | | | | | |
Pro forma net loss | | ($3,286 | ) | | ($13,360 | ) |
| | | | | | |
Net income per share—basic, as reported | | $0.24 | | | $0.08 | |
| | | | | | |
Net income per share—diluted, as reported | | $0.23 | | | $0.07 | |
| | | | | | |
Pro forma net loss per share—basic and diluted | | ($0.08 | ) | | ($0.34 | ) |
| | | | | | |
Impact of the Adoption of SFAS 123(R)
Allscripts has elected to adopt the modified prospective application transition method as permitted by SFAS 123(R). Accordingly, during the year ended December 31, 2006, Allscripts recorded stock-based
70
compensation cost totaling the amount that would have been recognized had the fair value method been applied since the effective date of SFAS 123. For the year ended December 31, 2006, the effect on Allscripts’ results of operations of recording stock-based compensation in accordance with SFAS 123(R) was as follows:
| | |
| | | 2006 |
Stock-based compensation: | | |
Restricted stock | | $1,540 |
Stock options | | 289 |
| | |
Total stock-based compensation | | $1,829 |
| | |
Effect on net income | | $1,829 |
| | |
Effect on net income per share: | | |
Basic | | $0.04 |
| | |
Diluted | | $0.03 |
| | |
On December 30, 2005, our Board of Directors approved a plan to accelerate vesting of certain options to purchase approximately 1,291 shares of our common stock awarded under our stock plans that were due to fully vest by August 1, 2007. As a result of the acceleration, we recognized an additional, non-cash, non-recurring stock-based compensation expense of approximately $518 in the year ended December 31, 2005, based on an estimated forfeiture rate. During 2006, we calculated the actual stock option forfeiture rate, which resulted in an additional expense of $499 to be recorded in the year ended December 31, 2006. Total stock-based compensation expense of $2,328 was recorded in operating expenses during the year ended December 31, 2006.
In Allscripts’ pro forma disclosures prior to the adoption of SFAS 123(R), Allscripts accounted for forfeitures upon occurrence, and, using this method, Allscripts had an unrecorded deferred stock-based compensation balance related to stock options of $718 before estimated forfeitures as of January 1, 2006. SFAS 123(R) requires forfeitures to be estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates. Pursuant to SFAS 123(R), as of January 1, 2006, Allscripts estimated that the stock-based compensation for options not expected to vest was $154, therefore, the unrecorded deferred stock-based compensation balance related to stock options was adjusted to $564 after estimated forfeitures.
As of December 31, 2006, the unrecorded deferred stock-based compensation balance related to stock options was $185 after estimated forfeitures, and such amount will be recognized over an estimated weighted average amortization period of approximately six months.
No stock-based compensation has been capitalized for the twelve months ended December 31, 2006 or at January 1, 2006, when the provisions of SFAS 123(R) were adopted.
Allscripts did not grant any stock options during the twelve months ended December 31, 2006. The fair value of stock options granted prior to January 1, 2006 was determined using the Black-Scholes option pricing model. The weighted average assumptions used in determining such fair values for the twelve months ended December 31, 2005 are as follows:
| | | |
| | | Twelve Months Ended
December 31, 2005 | |
Risk-free interest rate | | 3.23 | % |
Volatility | | 150 | % |
Dividend rate | | — | % |
Option life (years) | | 2.72 | |
71
The following table summarizes the combined activity with respect to stock options granted under Allscripts’ equity incentive plans during the periods indicated:
| | | | | | | | | |
| | | Options
Outstanding | | | Weighted- Average
Exercise Price | | Options
Exercisable | | Weighted- Average
Exercise Price |
Balance at December 31, 2003 | | 10,303 | | | $6.11 | | 4,602 | | $8.50 |
Options granted | | 2,155 | | | $9.16 | | | | |
Options exercised | | (1,064 | ) | | $3.89 | | | | |
Options forfeited | | (518 | ) | | $8.08 | | | | |
| | | | | | | | | |
Balance at December 31, 2004 | | 10,876 | | | $6.84 | | 6,503 | | $7.86 |
Options granted | | 41 | | | $11.99 | | | | |
Options exercised | | (2,158 | ) | | $4.39 | | | | |
Options forfeited | | (216 | ) | | $10.36 | | | | |
| | | | | | | | | |
Balance at December 31, 2005 | | 8,543 | | | $7.39 | | 8,356 | | $7.38 |
Options granted | | — | | | $— | | | | |
Options exercised | | (2,815 | ) | | $5.11 | | | | |
Options forfeited | | (196 | ) | | $30.46 | | | | |
| | | | | | | | | |
Balance at December 31, 2006 | | 5,532 | | | $7.81 | | 5,485 | | $7.80 |
| | | | | | | | | |
The aggregate intrinsic value of stock options outstanding as of December 31, 2006 was $108,415, which is based on Allscripts’ closing stock price of $26.99 as of December 31, 2006. The intrinsic value of stock options outstanding represents the amount that would have been received by the option holders had all option holders exercised their stock options as of that date. The total number of vested, in-the-money stock options as of December 31, 2006 was 5,485, with an intrinsic value of $107,548.
The total intrinsic value of stock options exercised during the year ended December 31, 2006 was $55,176. The total cash received from employees as a result of employee stock option exercises during the twelve months ended December 31, 2006 was $14,377 net of related taxes. Allscripts settles employee stock option exercises with newly issued common shares.
During the year ended December 31, 2006, management awarded 711 shares of restricted stock to certain employees under the Amended and Restated 1993 Stock Incentive Plan, with an average fair value of $18.77 per share. The awards of restricted stock have an average four-year vesting term. Upon termination of an employee’s employment with Allscripts, any unvested shares of restricted stock will be forfeited. As of December 31, 2006, 711 shares of restricted stock had been awarded, of which 669 were unvested. The fair value of the shares of unvested restricted stock on the date of the grant is amortized ratably over the vesting period. As of December 31, 2006, $8,542 of unearned compensation related to unvested awards of restricted stock was netted against the balance of additional paid in capital and will be recognized over the remaining vesting terms of the awards.
72
Information regarding stock options outstanding at December 31, 2006 is as follows:
| | | | | | | | | | |
Range of
Exercise Prices | | Number of Options Outstanding | | Weighted-Average Remaining Contractual Life (in years) | | Weighted-Average Exercise Price | | Number of Options Exercisable | | Weighted-Average Exercise Price |
$0.06 - $2.80 | | 572 | | 6.36 | | $2.40 | | 572 | | $2.40 |
$3.00 - $3.15 | | 648 | | 4.77 | | $3.13 | | 648 | | $3.13 |
$3.19 - $3.53 | | 1,459 | | 6.47 | | $3.53 | | 1,458 | | $3.53 |
$4.25 - $5.63 | | 667 | | 4.17 | | $5.63 | | 667 | | $5.63 |
$6.40 - $7.73 | | 641 | | 5.88 | | $7.11 | | 614 | | $7.11 |
$9.49 - $10.67 | | 860 | | 7.77 | | $10.27 | | 848 | | $10.27 |
$11.25 - $27.57 | | 350 | | 3.51 | | $17.61 | | 343 | | $17.64 |
$29.65 - $79.75 | | 335 | | 2.93 | | $33.83 | | 335 | | $33.83 |
| | | | | | | | | | |
| | 5,532 | | 5.71 | | $7.81 | | 5,485 | | $7.80 |
| | | | | | | | | | |
11. Commitments
Allscripts conducts its operations from leased premises under several operating leases. Total rent expense from operations was $3,062, $1,489, and $1,326 in 2006, 2005, and 2004, respectively. Rent expense is net of sublease rental income of $145, $36, and $0 in 2006, 2005, and 2004, respectively.
Future minimum rental payments at December 31, 2006 under non-cancelable operating leases, net of sublease rental income of $145 and $145 in 2007 and 2008, respectively, are as follows:
| | |
| | | Year Ending December 31, |
2007 | | $2,138 |
2008 | | 2,117 |
2009 | | 1,728 |
2010 | | 1,370 |
2011 | | 1,324 |
2012 and thereafter | | 4,041 |
| | |
Total future minimum lease payments | | $12,718 |
| | |
In connection with the Allscripts’ lease agreement of its corporate facilities, Allscripts has provided to the lessor an unconditional irrevocable letter of credit in favor of the lessor in the amount of $500 as security for the full and prompt performance by Allscripts under the lease agreement. The letter of credit may be drawn upon by the lessor and retained, used or applied by lessor for the purpose of curing any monetary default or defaults of Allscripts under the lease. The letter of credit provides for an expiration date of one year from the commencement date of the lease, and will automatically extend for additional successive one-year periods through the term of the lease. As of December 31, 2006 and 2005, no amounts had been drawn on the letter of credit.
We have other letters of credit as security for full and prompt performance under various contractual arrangements totaling $300. As of December 31, 2006 and 2005, no amounts had been drawn on the letter of credit.
12. Savings Plan
Allscripts’ employees who meet certain eligibility requirements can participate in Allscripts’ 401(k) Savings and Investment Plan. Under the plan, Allscripts may, at its discretion, match the employee contributions. Allscripts recorded expense related to its matching contributions in 2006, 2005, and 2004 of $862, $361, and $377, respectively.
73
13. Business Segments
FAS No. 131, “Disclosures about Segments of a Business Enterprise and Related Information,” establishes standards for reporting information about operating segments in annual financial statements and requires selected information about operating segments in interim financial reports issued to stockholders. Operating segments are defined as components of an enterprise for which separate financial information is available that is evaluated regularly by the chief operating decision maker in deciding how to allocate resources and in assessing performance.
Allscripts currently organizes its business around groups of similar products, which results in three reportable segments: software and related services; prepackaged medications; and information services. The software and related services segment derives its revenue from the sale and installation of clinical software that provides point-of-care decision support solutions, document imaging solutions, and the resale of related hardware. The prepackaged medications segment derives its revenue from the repackaging, sale, and distribution of medications and medical supplies. The information services segment primarily derives its revenue from the sale of interactive physician education sessions. Allscripts does not report its assets by segment. Allscripts does not allocate interest income, interest expense, other income or income taxes to its operating segments. In addition, Allscripts records corporate selling, general, and administration expenses, amortization of intangibles, restructuring and other related charges in its unallocated corporate costs. These costs are not included in the evaluation of the financial performance of Allscripts’ operating segments.
| | | | | | | | | |
| | | 2006 | | | 2005 | | | 2004 | |
Revenue: | | | | | | | | | |
Software and related services | | $173,503 | | | $65,166 | | | $44,121 | |
Prepackaged medications | | 43,688 | | | 45,609 | | | 44,733 | |
Information services | | 10,778 | | | 9,789 | | | 11,916 | |
| | | | | | | | | |
Total revenue | | $227,969 | | | $120,564 | | | $100,770 | |
| | | | | | | | | |
Income from operations: | | | | | | | | | |
Software and related services | | $45,869 | | | $18,405 | | | $8,633 | |
Prepackaged medications | | 4,301 | | | 5,737 | | | 7,275 | |
Information services | | 1,345 | | | 2,743 | | | 2,254 | |
Unallocated corporate | | (31,647 | ) | | (17,662 | ) | | (14,919 | ) |
| | | | | | | | | |
Income from operations | | 19,868 | | | 9,223 | | | 3,243 | |
Interest income, interest expense, and other income (expense), net | | (549 | ) | | 487 | | | (135 | ) |
| | | | | | | | | |
Income from operations before income taxes | | $19,319 | | | $9,710 | | | $3,108 | |
| | | | | | | | | |
14. Supplemental Disclosure of Cash Flow Information
| | | | | | |
| | | 2006 | | 2005 | | 2004 |
Payment of interest on long-term debt | | $3,149 | | $2,960 | | $— |
Payment of income taxes | | 127 | | 88 | | 7 |
| 15. | Related Party Transactions |
During the year ended December 31, 2006, Allscripts entered into several contracts with Medem for its Interactive Health Record product (“iHealthRecord”) that resulted in payments to Medem of approximately $406 of which $714 is included in prepaid expenses and other current assets in the consolidated balance sheets. There is no accrual to Medem recorded as of December 31, 2006. During the year ended December 31, 2005, Allscripts made payments to Medem totaling $642 and recorded an accrual of $145, of which $648 is included in prepaid expenses and other current assets in the consolidated balance sheets. Allscripts resells the iHealthRecord to
74
Allscripts’ customers and accordingly recorded revenue of $404 and $221 and costs of $281 and $139 for the years ended December 31, 2006 and 2005, respectively. In addition, during the years ended December 31, 2006 and 2005, Allscripts funded $500 and $1,050, respectively, to Medem under the Note Purchase Agreement (see Note 6).
During the second quarter of 2005, Allscripts entered into a service contract with ExactTarget, an email marketing solutions company, of which one of our Board members serves as Chairman of the Board. On July 31, 2006, Allscripts renewed the agreement with ExactTarget. Allscripts has paid Exact Target $56 and $39 during the years ended December 31, 2006 and 2005, respectively, and accrued $24 and $7 for use of ExactTarget’s enterprise software and services as of December 31, 2006 and 2005, respectively.
As part of a 10-year strategic alliance agreement as amended on January 18, 2006, Allscripts is obligated to pay IDX, a wholly owned subsidiary of General Electric, a percentage of Allscripts’ revenue related to IDX customers. Pursuant to this obligation, Allscripts paid IDX approximately $3,266, $2,059, and $1,829 during 2006, 2005, and 2004, respectively. Allscripts also leases office space from IDX and contracted with IDX for certain marketing and consulting services in 2006, 2005 and 2004. Allscripts paid IDX approximately $1,025, $357, and $359 for lease of office space and use of the facility’s infrastructure for the years 2006, 2005, and 2004, respectively, and $13, $47, and $12 for marketing and consulting services for the years 2006, 2005, and 2004, respectively. At December 31, 2006 and 2005, Allscripts had accounts payable and accruals with IDX of $571 and $1,596, respectively.
Allscripts and IDX were also joint parties under certain customer contracts in 2006 and 2005. Allscripts paid IDX approximately $2,075 and $14 for billings on behalf of IDX related to such contracts in 2006 and 2005, respectively. IDX paid us approximately $167 and $904 for billings on our behalf related to such contracts in 2006 and 2005, respectively.
The Chief Executive Officer and Chairman of the Board of A4 prior to Allscripts’ acquisition of A4, became one of our directors in connection with the acquisition of A4. Such director also serves on the Board of Directors of Med3000, Inc. (“Med3000”) and has an ownership interest of approximately 8% in Med3000. Allscripts has a license and distribution agreement with Med3000 pursuant to which Med3000 possesses the right to market, resell and sublicense Allscripts’ electronic health record solutions to its customers. As of December 31, 2006, Med3000 has agreed to purchase from Allscripts approximately $1,650 of hardware, software and related services. For the year ended December 31, 2006, Allscripts recognized $1,062 of revenue under such contracts. As of December 31, 2006, Allscripts had $321 in accounts receivable with Med3000. That same director’s spouse is an owner of Trip Logics, a travel agency used by A4. Allscripts paid $286 to Trip Logics during the year ended December 31, 2006. There are no accounts payable to Trip Logics as of December 31, 2006. That director’s spouse sold the Trip Logics business on January 2, 2007.
Our Chief Executive Officer serves on the Advisory Board of Acquirent, LLC, a telemarketer and a reseller of our e-prescribing solution. During the years ended December 31, 2006 and 2005, we paid $149 and $43, respectively, to this company and had $34 and $28 accrued as of December 31, 2006 and 2005, respectively.
75
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
Board of Directors and Shareholders
Allscripts Healthcare Solutions, Inc.:
We have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated financial statements of Allscripts Healthcare Solutions, Inc. and Subsidiaries referred to in our report dated March 1, 2007, which is included in Item 8 of this form. Our audit was conducted for the purpose of forming an opinion on the basic financial statements taken as a whole. The Valuation and Qualifying Accounts included in Schedule II are presented for purposes of additional analysis and are not a required part of the basic financial statements. This schedule has been subjected to the auditing procedures applied in the audit of the basic financial statements and, in our opinion, is fairly stated in all material respects in relation to the basic financial statements taken as a whole.
/s/ GRANT THORNTON LLP
Chicago, Illinois
March 1, 2007
76
ALLSCRIPTS HEALTHCARE SOLUTIONS, INC.
VALUATION AND QUALIFYING ACCOUNTS
(In thousands)
Schedule II
| | | | | | | | | |
| | | Beginning Balance | | Charged to
Expense | | Deductions | | | Ending Balance |
Allowance for accounts receivable | | | | | | | | | |
Year ended December 31, 2006 | | $2,337 | | 3,180 | | (1,283 | ) | | $4,234 |
Year ended December 31, 2005 | | $3,010 | | 553 | | (1,226 | ) | | $2,337 |
Year ended December 31, 2004 | | $3,128 | | 451 | | (569 | ) | | $3,010 |
| | | | |
| | | Beginning Balance | | Charged to Expense | | Adjustments | | | Ending Balance |
Valuation allowance for deferred tax assets | | | | | | | | | |
Year ended December 31, 2006 | | $61,284 | | — | | (61,284 | ) | | $— |
Year ended December 31, 2005 | | $54,630 | | — | | 6,654 | | | $61,284 |
Year ended December 31, 2004 | | $56,165 | | — | | (1,535 | ) | | $54,630 |
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
None.
Item 9A. Controls and Procedures
Conclusion Regarding the Effectiveness of Disclosure Controls and Procedures
Under the supervision and with the participation of our management, including our principal executive officer and principal financial officer, we conducted an evaluation of our disclosure controls and procedures, as such term is defined under Rule 13a-15(e) promulgated under the Securities Exchange Act of 1934, as amended (the Exchange Act). Based on this evaluation, our principal executive officer and our principal financial officer concluded that our disclosure controls and procedures were effective as of the end of the period covered by this annual report.
Management’s Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Exchange Act Rules 13a-15(f). Allscripts’ internal control system was designed to provide reasonable assurance to Allscripts’ management and Board of Directors regarding the preparation and fair presentation of published financial statements.
All internal control systems, no matter how well designed, have inherent limitations. Therefore, even those systems determined to be effective can provide only reasonable assurance with respect to financial statement preparation and presentation.
Allscripts’ management assessed the effectiveness of the company’s internal control over financial reporting as of December 31, 2006. In making this assessment, it used the criteria set forth inInternal Control — Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission. As allowed by SEC guidance, management excluded from its assessment the March 2, 2006 acquisition of A4, which accounted for approximately 18 percent of consolidated total assets and approximately 34 percent of consolidated total revenue. Based on our assessment, we believe that, as of December 31, 2006, the company’s internal control over financial reporting was effective based on those criteria.
Our management’s assessment of the effectiveness of our internal control over financial reporting as of December 31, 2006 has been audited by Grant Thornton LLP, an independent registered public accounting firm, as stated in their report which is included herein.
There has been no change in our internal control over financial reporting during our most recent fiscal quarter that has materially affected, or is reasonably likely to materially affect, our internal controls over financial reporting.
77
Item 9B. Other Information
None.
78
PART III
Item 10. Directors, Executive Officers and Corporate Governance
Information regarding directors, executive officers and other key employees is included under the captions “Election of Directors” and “Executive Officers” in Allscripts’ proxy statement for the 2007 Annual Meeting of Stockholders and is incorporated by reference herein. Information regarding the audit committee members, any audit committee financial experts and the code of conduct is included under the captions “Meetings and Committees of the Board of Directors” and “Governance—Code of Conduct” in Allscripts’ proxy statement for the 2007 Annual Meeting of Stockholders and is incorporated by reference herein.
Information regarding Section 16(a) reporting compliance is included under the caption “Section 16(a) Beneficial Ownership Reporting Compliance” in Allscripts’ proxy statement for the 2007 Annual Meeting of Stockholders and is incorporated by reference herein.
We have adopted a code of conduct that applies to our directors, officers and employees, including our principal executive officer, principal accounting officer, controller, or persons performing similar functions (the “senior financial officers”). A copy of this code of conduct is posted on the investor relations portion of our website at www.allscripts.com. In the event the code of conduct is revised, or any waiver is granted under the code of conduct with respect to any director, executive officer or senior financial officer, notice of such revision or waiver will be posted on our website.
Item 11. Executive Compensation
Information regarding executive and director compensation in response to this item is included in Allscripts’ proxy statement for the 2007 Annual Meeting of Stockholders and is incorporated by reference herein. Information included under the caption “Compensation Committee Report” in Allscripts’ proxy statement for the 2007 Annual Meeting of Stockholders is incorporated by reference herein; however, this information shall not be deemed to be “soliciting material” or to be “filed” with the Securities and Exchange Commission or subject to Regulation 14A or 14C, or the liabilities of Section 18 of the Securities Exchange Act of 1934.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
Information regarding security ownership is included under the caption “Ownership of Allscripts Common Stock” in Allscripts’ proxy statement for the 2007 Annual Meeting of Stockholders and is incorporated by reference herein.
Information regarding securities authorized for issuance under equity compensation plans is included under the caption “Equity Compensation Plan Information” in Allscripts’ proxy statement for the 2007 Annual Meeting of Stockholders and is incorporated by reference herein.
Item 13. Certain Relationships and Related Transactions and Director Independence
Information regarding certain relationships and related party transactions is included under the caption “Certain Relationships and Related Party Transactions” in Allscripts’ proxy statement for the 2007 Annual Meeting of Stockholders and is incorporated by reference herein. Information included under the caption “Corporate Governance—Director Independence” in Allscripts’ proxy statement for the 2007 Annual Meeting of Stockholders is incorporated by reference herein.
Item 14. Principal Accountant Fees and Services
Information regarding principal accountant fees and services is under the caption “Independent Public Accountants” in Allscripts’ proxy statement for the 2007 Annual Meeting of Stockholders and is incorporated by reference herein.
79
PART IV
Item 15. Exhibits and Financial Statement Schedules
(a)(1) Financial Statements
The following consolidated financial statements of Allscripts Healthcare Solutions, Inc. and its subsidiaries are included in Part II of this report:
| | |
| | | Page |
Report of Independent Registered Public Accounting Firm | | 48 |
| |
Consolidated Balance Sheets as of December 31, 2006 and 2005 | | 49 |
| |
Consolidated Statements of Operations for the years ended December 31, 2006, 2005 and 2004 | | 50 |
| |
Consolidated Statements of Stockholders’ Equity and Comprehensive Income (Loss) for the years ended December 31, 2006, 2005 and 2004 | | 51 |
| |
Consolidated Statements of Cash Flows for the years ended December 31, 2006, 2005 and 2004 | | 52 |
| |
Notes to Consolidated Financial Statements | | 53 |
| |
(a)(2) Financial Statement Schedules | | |
| |
Report of Independent Registered Public Accounting Firm | | 76 |
| |
Schedule II—Valuation and Qualifying Accounts | | 77 |
(a)(3) List of Exhibits
See Index to Exhibits
80
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized, on March 1, 2007.
| | |
| ALLSCRIPTS HEALTHCARE SOLUTIONS, INC. |
| |
| By: | | /s/ GLEN E. TULLMAN |
| | Glen E. Tullman Chief Executive Officer |
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below on March 1, 2007 by the following persons on behalf of the Registrant in the capacities indicated.
| | |
Signature | | Title |
| |
/s/ GLEN E. TULLMAN Glen E. Tullman | | Chairman, Chief Executive Officer, and Director (Principal Executive Officer) |
| |
/s/ WILLIAM J. DAVIS William J. Davis | | Chief Financial Officer (Principal Financial and Accounting Officer) |
| |
/s/ BERNARD GOLDSTEIN Bernard Goldstein | | Director |
| |
/s/ PHILIP D. GREEN Philip D. Green | | Director |
| |
/s/ M. FAZLE HUSAIN M. Fazle Husain | | Director |
| |
/s/ MICHAEL J. KLUGER Michael J. Kluger | | Director |
| |
/s/ ROBERT A. COMPTON Robert A. Compton | | Director |
| |
/s/ M.L. GAMACHE M.L. Gamache | | Director |
| |
/s/ JOHN P. MCCONNELL John P. McConnell | | Director |
81
INDEX TO EXHIBITS
| | | | |
Exhibit Number | | Description | | Reference |
| 2.1 | | Agreement and Plan of Merger, dated as of July 13, 2000, by and among Allscripts Holding, Inc., Allscripts, Inc., Bursar Acquisition, Inc., Bursar Acquisition No. 2, Inc., IDX Systems Corporation and ChannelHealth Incorporated. | | Incorporated herein by reference from the Allscripts, Inc. Current Report on Form 8-K filed on July 27, 2000 |
| | |
| 2.2 | | First Amendment to Agreement and Plan of Merger, entered into as of November 29, 2000, by and among Allscripts Holding, Inc., Allscripts, Inc., Bursar Acquisition, Inc., Bursar Acquisition No. 2, Inc., IDX Systems Corporation and ChannelHealth Incorporated. | | Incorporated herein by reference from the Allscripts Healthcare Solutions, Inc. Registration Statement on Form S-4 as part of Amendment No. 1 filed on December 7, 2000 (SEC file no. 333-49568) |
| | |
| 2.3 | | Agreement of Merger, dated as of January 18, 2006, by and among Allscripts Healthcare Solutions, Inc., Quattro Merger Sub Corp., A4 Health Systems, Inc. and John P. McConnell, in his capacity as Shareholder Representative. | | Incorporated herein by reference from the Allscripts Healthcare Solutions, Inc. Current Report on Form 8-K filed on January 23, 2006 |
| | |
| 3.1 | | Amended and Restated Certificate of Incorporation of Allscripts Healthcare Solutions, Inc. (formerly named Allscripts Holding, Inc.). | | Incorporated herein by reference from the Allscripts Healthcare Solutions, Inc. Registration Statement on Form S-4 as part of Amendment No. 1 filed on December 7, 2000 (SEC file no. 333-49568) |
| | |
| 3.2 | | Certificate of Amendment of Amended and Restated Certificate of Incorporation of Allscripts Healthcare Solutions, Inc. (formerly named Allscripts Holding, Inc.). | | Incorporated herein by reference from the Allscripts Healthcare Solutions, Inc. Registration Statement on Form S-4 as part of Amendment No. 1 filed on December 7, 2000 (SEC file no. 333-49568) |
| | |
| 3.3 | | Certificate of Amendment of Amended and Restated Certificate of Incorporation of Allscripts Healthcare Solutions, Inc. (formerly named Allscripts Holding, Inc.). | | Incorporated herein by reference from the Allscripts Healthcare Solutions, Inc. Registration Statement on Form S-4 as part of Amendment No. 1 filed on December 7, 2000 (SEC file no. 333-49568) |
| | |
| 3.4 | | Bylaws of Allscripts Healthcare Solutions, Inc. (formerly named Allscripts Holding, Inc.). | | Incorporated herein by reference from the Allscripts Healthcare Solutions, Inc. Registration Statement on Form S-4 as part of Amendment No. 1 filed on December 7, 2000 (SEC file no. 333-49568) |
| | |
| 4.1 | | Indenture, dated as of July 6, 2004, between Allscripts Healthcare Solutions, Inc. and LaSalle Bank N.A., as trustee, related to the issuance of 3.50% Convertible Senior Debentures Due 2024. | | Incorporated herein by reference from the Allscripts Healthcare Solutions, Inc. Current Report on Form 8-K filed on July 15, 2004 |
| | |
| 4.2 | | Resale Registration Rights Agreement, dated as of July 6, 2004, between Allscripts Healthcare Solutions, Inc. and Banc of America Securities LLC, as representative of the initial purchasers of the 3.50% Convertible Senior Debentures Due 2024. | | Incorporated herein by reference from the Allscripts Healthcare Solutions, Inc. Current Report on Form 8-K filed on July 15, 2004 |
82
| | | | |
Exhibit Number | | Description | | Reference |
| 10.1† | | Amendment and Restatement of Amended and Restated 1993 Stock Incentive Plan. | | Incorporated herein by reference from Appendix B to the Allscripts Healthcare Solutions, Inc. Proxy Statement relating to its 2005 Annual Meeting of Stockholders, filed on April 28, 2005 |
| | |
| 10.2 | | Twelfth Restated Registration Agreement, dated as of June 18, 1999, by and among Allscripts, Inc., those Holders of Allscripts, Inc. Series A Preferred, Series B Preferred, Series C Preferred, Series D Preferred, Series F Preferred and Series G Preferred listed in Schedule I attached thereto, the Holders of the Extension Guaranty Warrants listed in Schedule II thereto, the Holders of the 1996 Extension Guaranty Warrants listed in Schedule II thereto, those Holders of Common listed in Schedule III thereto, the Holders of Series H Warrants and H Unit Common listed in Schedule IV thereto, the Holders of Extension Series H Warrants listed in Schedule IV thereto, the Holders of I Unit Common listed in Schedule V thereto and the Holders of Debenture Warrants listed in Schedule VI thereto. | | Incorporated herein by reference from the Allscripts, Inc. Registration Statement on Form S-1 as part of Amendment No. 2 filed on June 29, 1999 (SEC file no. 333-78431) |
| | |
| 10.3 | | Industrial Building Lease, dated April 30, 1997, between G2 Limited Partnership and Allscripts, Inc. | | Incorporated herein by reference from the Allscripts, Inc. Registration Statement on Form S-1 filed on May 14, 1999 (SEC file no. 333-78431) |
| | |
| 10.4 | | Lease Agreement between American National Bank and Trust Company of Chicago, as Trustee, and Allscripts, Inc., dated September 1996, as amended December 31, 1999. | | Incorporated herein by reference from the Allscripts, Inc. Registration Statement on Form S-1 as part of Amendment No. 1 filed on February 18, 2000 (SEC file no. 333-95521) |
| | |
| 10.5 | | Second Amendment, dated September 30, 2002, to Lease Agreement between LaSalle Bank National Association (previously American National Bank and Trust Company of Chicago), as Trustee, and Allscripts, Inc. dated September 1996, as amended December 31, 1999. | | Incorporated herein by reference from the Allscripts Healthcare Solutions, Inc. Annual Report on Form 10-K for the fiscal year ended December 31, 2002 |
| | |
| 10.6 | | Lease Agreement, dated as of September 17, 2004, between Allscripts, LLC and Merchandise Mart L.L.C. | | Incorporated herein by reference from the Allscripts Healthcare Solutions, Inc. Annual Report on Form 10-K for the fiscal year ended December 31, 2004 |
| | |
| 10.7 | | First amendment, dated May 17, 2006, to Lease Agreement between Allscripts, LLC, as Tenant and Merchandise Mart L.L.C, as Landlord. | | Filed herewith |
| | |
| 10.8 | | Fourth amendment, dated May 20, 2004, to Lease Agreement between Lincoln Commerce Center Properties, LLC, as Landlord, and Allscripts, LLC, as Tenant. | | Incorporated herein by reference from Allscripts Healthcare Solutions, Inc. Annual Report on Form 10-K for the fiscal year ended December 31, 2004. |
83
| | | | |
Exhibit Number | | Description | | Reference |
| | |
| 10.9† | | Employment Agreement, dated as of July 8, 2002, between Allscripts Healthcare Solutions, Inc. and Glen E. Tullman. | | Incorporated herein by reference from the Allscripts Healthcare Solutions, Inc. Quarterly Report on Form 10-Q for the quarter ended September 30, 2002 |
| | |
| 10.10† | | Amendment, effective July 7, 2006, to Employment Agreement dated as of July 8, 2002 between Allscripts, LLC and Glen E. Tullman. | | Incorporated herein by reference from the Allscripts Healthcare Solutions, Inc. Current Report on Form 8-K filed on July 13, 2006 |
| | |
| 10.11† | | Employment Agreement, dated as of July 8, 2002, between Allscripts Healthcare Solutions, Inc. and Lee A. Shapiro. | | Incorporated herein by reference from the Allscripts Healthcare Solutions, Inc. Quarterly Report on Form 10-Q for the quarter ended September 30, 2002 |
| | |
| 10.12† | | Amendment, effective July 7, 2006, to Employment Agreement dated as of July 8, 2002 between Allscripts, LLC and Lee A. Shapiro. | | Incorporated herein by reference from the Allscripts Healthcare Solutions, Inc. Current Report on Form 8-K filed on July 13, 2006 |
| | |
| 10.13† | | Employment Agreement, dated as of October 8, 2002, between Allscripts Healthcare Solutions, Inc. and William J. Davis. | | Incorporated herein by reference from the Allscripts Healthcare Solutions, Inc. Annual Report on Form 10-K for the fiscal year ended December 31, 2002 |
| | |
| 10.14† | | Amendment, effective July 7, 2006, to Employment Agreement dated as of October 8, 2002 between Allscripts, LLC and William J. Davis. | | Incorporated herein by reference from the Allscripts Healthcare Solutions, Inc. Current Report on Form 8-K filed on July 13, 2006 |
| | |
| 10.15† | | Employment Agreement, dated as of July 8, 2002, between Allscripts Healthcare Solutions, Inc. and Joseph E. Carey. | | Incorporated herein by reference from the Allscripts Healthcare Solutions, Inc. Quarterly Report on Form 10-Q for the quarter ended September 30, 2002 |
| | |
| 10.16† | | Amendment, effective July 7, 2006, to Employment Agreement dated as of July 8, 2002 between Allscripts, LLC and Joseph E. Carey. | | Incorporated herein by reference from the Allscripts Healthcare Solutions, Inc. Current Report on Form 8-K filed on July 13, 2006 |
| | |
| 10.17† | | Employment Agreement, dated as of July 8, 2002, between Allscripts Healthcare Solutions, Inc. and Scott Leisher. | | Incorporated herein by reference from the Allscripts Healthcare Solutions, Inc. Quarterly Report on Form 10-Q for the quarter ended September 30, 2002 |
| | |
| 10.18† | | Amendment, effective January 1, 2005, to Employment Agreement dated as of July 8, 2002 between Allscripts, LLC and Scott Leisher. | | Incorporated herein by reference from the Allscripts Healthcare Solutions, Inc. Annual Report on Form 10-K for the fiscal year ended December 31, 2004 |
| | |
| 10.19† | | Employment Agreement, dated as of January 31, 2003, between Allscripts, Inc. and Laurie McGraw. | | Incorporated herein by reference from the Allscripts Healthcare Solutions, Inc. Annual Report on Form 10-K for the fiscal year ended December 31, 2005 |
| | |
| 10.20† | | Amendment, effective July 7, 2006, to Employment Agreement dated as of January 31, 2003 between Allscripts, Inc. and Laurie McGraw. | | Incorporated herein by reference from the Allscripts Healthcare Solutions, Inc. Current Report on Form 8-K filed on July 13, 2006 |
84
| | | | |
Exhibit Number | | Description | | Reference |
| | |
| 10.21† | | Form of Allscripts Healthcare Solutions, Inc. Nonqualified Incentive Stock Option Agreement. | | Incorporated herein by reference from the Allscripts Healthcare Solutions, Inc. Current Report on Form 8-K filed on January 5, 2005 |
| | |
| 10.22 | | Stock Rights and Restrictions Agreement by and between Allscripts Healthcare Solutions, Inc. and IDX Systems Corporation, dated as of January 8, 2001. | | Incorporated herein by reference from the Allscripts Healthcare Solutions, Inc. Annual Report on Form 10-K for the fiscal year ended December 31, 2001 |
| | |
| 10.23 | | Amended and Restated Strategic Alliance Agreement by and between Allscripts Healthcare Solutions, Inc. and IDX Systems Corporation, dated as of January 18, 2006. | | Incorporated herein by reference from the Allscripts Healthcare Solutions, Inc. Current Report on Form 8-K filed on January 19, 2006 |
| | |
| 10.24 | | Asset Purchase Agreement, dated as of July 13, 2000, by and between ChannelHealth Incorporated and IDX Systems Corporation. | | Incorporated herein by reference from the Allscripts Healthcare Solutions, Inc. Registration Statement on Form S-4 as part of Amendment No. 1 filed on December 7, 2000 (SEC file no. 333-49568) |
| | |
| 10.25 | | Amended and Restated Cross License and Software Maintenance Agreement by and between IDX Systems Corporation and ChannelHealth Incorporated, dated January 8, 2001. | | Incorporated herein by reference from the Allscripts Healthcare Solutions, Inc. Annual Report on Form 10-K for the fiscal year ended December 31, 2001 |
| | |
| 10.26* | | Pharmacy Services Prime Vendor Agreement for Allscripts Healthcare Solutions, Inc., dated as of February 1, 2002, between Allscripts Healthcare Solutions, Inc. and Bergen Brunswig Drug Co. | | Incorporated herein by reference from the Allscripts Healthcare Solutions, Inc. Quarterly Report on Form 10-Q for the quarter ended March 31, 2002 |
| | |
| 10.27 | | First Amendment, dated July 31, 2002, among Allscripts Healthcare Solutions, Inc., Bergen Brunswig Drug Company doing business as Amerisource Bergen and Allscripts, Inc., to Pharmacy Services Prime Vendor Agreement, dated as of February 1, 2002, between Allscripts Healthcare Solutions, Inc. and Bergen Brunswig Drug Company doing business as Amerisource Bergen. | | Incorporated herein by reference from the Allscripts Healthcare Solutions, Inc. Quarterly Report on Form 10-Q for the quarter ended September 30, 2002. |
| | |
| 10.28† | | Allscripts Healthcare Solutions, Inc. 2001 Non-Statutory Stock Option Plan. | | Incorporated herein by reference from the Allscripts Healthcare Solutions, Inc. Annual Report on Form 10-K for the fiscal year ended December 31, 2002 |
| | |
| 10.29† | | Form of Restricted Stock Award Agreement (Directors). | | Incorporated herein by reference from the Allscripts Healthcare Solutions, Inc. Annual Report on Form 10-K for the fiscal year ended December 31, 2005. |
| | |
| 10.30† | | Form of Restricted Stock Award Agreement (Officers and Employees). | | Incorporated herein by reference from the Allscripts Healthcare Solutions, Inc. Annual Report on Form 10-K for the fiscal year ended December 31, 2005. |
85
| | | | |
Exhibit Number | | Description | | Reference |
| | |
| 10.31† | | Amendment to Form of Restricted Stock Award Agreement | | Filed Herewith |
| | |
| 10.32† | | Executive Management Bonus Program 2006 | | Incorporated herein by reference from the Allscripts Healthcare Solutions, Inc. Annual Report on Form 10-K for the fiscal year ended December 31, 2005. |
| | |
| 12.1 | | Statement Regarding Computation of Ratio of Earnings to Fixed Charges | | Filed herewith |
| | |
| 21.1 | | Subsidiaries | | Filed herewith |
| | |
| 23.1 | | Consent of Grant Thornton LLP | | Filed herewith |
| | |
| 31.1 | | Rule 13a-14(a) Certification of Chief Executive Officer | | Filed herewith |
| | |
| 31.2 | | Rule 13a-14(a) Certification of Chief Financial Officer | | Filed herewith |
| | |
| 32.1 | | Section 1350 Certifications of Chief Executive Officer and Chief Financial Officer | | Filed herewith |
| † | Indicates management contract or compensatory plan. |
| * | Portions of this exhibit have been omitted pursuant to the Commission’s grant of confidential treatment. |
86
